User login
What drugs are effective for periodic limb movement disorder?
CLONAZEPAM improves subjective sleep quality and polysomnogram (PSG) measures of leg movements more than placebo (strength of recommendation [SOR]: B, a small randomized controlled trial [RCT]); temazepam produces similar results (SOR: C, extrapolated from a small comparison trial).
Melatonin and L-dopa consistently improve certain PSG measures, but their effect on subjective sleep quality varies; valproate improves only subjective measures; apomorphine injections reduce limb movements but not awakenings (SOR: C, very small crossover and cohort trials).
Estrogen replacement therapy is ineffective for periodic limb movement disorder (PLMD) associated with menopause (SOR: B, RCT).
Evidence summary
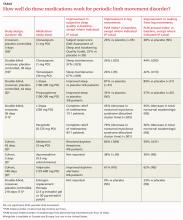
Although PLMD often occurs in association with restless legs syndrome, sleep apnea, narcolepsy, and other sleep disorders, it is itself an intrinsic sleep disorder characterized by stereotyped limb movements and sleep disruption.1 Most treatment studies of PLMD report both subjective and objective measures of sleep quality. Two commonly used objective measures, obtained by PSG, are the periodic leg movement (PLM) index and the PLM arousal index. The TABLE summarizes the evidence of medication trials.
Clonazepam improves subjective sleep measures, leg movements
Three comparative trials evaluated clonazepam against placebo, temazepam, and cognitive behavioral therapy (CBT).1-3 In the placebo-controlled and temazepam trials, clonazepam significantly improved subjective sleep parameters and leg movements.1,2 However, the studies produced conflicting results as to whether clonazepam reduced awakening from limb movements. Both temazepam and clonazepam appeared to be comparably effective; the trial was underpowered to detect a difference between them.
The CBT trial didn’t describe the frequency or duration of CBT clearly.3 It isn’t included in the TABLE.
L-Dopa decreases leg motions, effects on subjective sleep symptoms vary
Two comparison trials evaluated L-dopa (combined with carbidopa). One trial compared L-dopa with propoxyphene and placebo, and the other compared it with pergolide, a bromocriptine agonist available in Canada and Europe.4,5
In both trials, L-dopa consistently reduced leg motions at night but produced a variable response in subjective sleep symptoms and nocturnal waking. Propoxyphene yielded modest improvements in subjective sleep symptoms and nocturnal waking over placebo. The L-dopa–propoxyphene comparison trial was underpowered to allow a statistical comparison between the 2 medications.
Melatonin and valproate produce opposite effects in small studies
Three very small trials recorded symptoms and PSG findings in patients taking melatonin, apomorphine, or valproate, and compared them with the values observed at baseline.6-8 Melatonin significantly improved objective measures, but most patients didn’t feel less sleepy. Valproate produced the opposite effect—no clear PSG improvements, but all study patients felt better. Injected apomorphine reduced limb movements but not awakenings.
Estrogen replacement therapy doesn’t help
An RCT of estrogen replacement therapy for PLMD enrolled postmenopausal women, about half of whom were found to have PLMD.9 The study found estrogen replacement therapy to be ineffective for treating menopause-associated PLMD.
Recommendations
Practice parameters developed by the American Academy of Sleep Medicine state that clonazepam, pergolide, L-dopa (with a decarboxylase inhibitor), oxycodone, and propoxyphene are all reasonable choices for medical treatment of PLMD.10 The practice parameters don’t specify a preference for any of these medications.
1. Saletu M, Anderer P, Saletu-Zyhlarz G, et al. Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo-controlled sleep laboratory studies with clonazepam. Eur Neuropsychopharmacol. 2001;11:153-161.
2. Mitler MM, Browman CP, Menn SJ, et al. Nocturnal myoclonus: treatment efficacy of clonazepam and temazepam. Sleep. 1986;9:385-392.
3. Edinger JD, Fins AI, Sullivan RJ, et al. Comparison of cognitive-behavioral therapy and clonazepam for treating periodic limb movement disorder. Sleep. 1996;19:442-444.
4. Staedt J, Wassmuth F, Ziemann U, et al. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double-blind randomized crossover trial of pergolide versus L-Dopa. J Neural Transm. 1997;104:461-468.
5. Kaplan PW, Allen RP, Buchholz DW, et al. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717-723.
6. Kunz D, Bes F. Exogenous melatonin in periodic limb movement disorder: an open clinical trial and a hypothesis. Sleep. 2001;24:183-187.
7. Haba-Rubio J, Staner L, Cornette F, et al. Acute low single dose of apomorphine reduces periodic limb movements but has no significant effect on sleep arousals: a preliminary report. Neurophysiol Clin. 2003;33:180-184.
8. Ehrenberg BL, Eisensehr I, Corbett KE, et al. Valproate for sleep consolidation in periodic limb movement disorder. J Clin Psychopharmacol. 2000;20:574-578.
9. Polo-Kantola P, Rauhala E, Erkkola R, et al. Estrogen replacement therapy and nocturnal periodic limb movements: a randomized controlled trial. Obstet Gynecol. 2001;97:548-554.
10. Chesson AL, Jr, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Sleep. 1999;22:961-968.
CLONAZEPAM improves subjective sleep quality and polysomnogram (PSG) measures of leg movements more than placebo (strength of recommendation [SOR]: B, a small randomized controlled trial [RCT]); temazepam produces similar results (SOR: C, extrapolated from a small comparison trial).
Melatonin and L-dopa consistently improve certain PSG measures, but their effect on subjective sleep quality varies; valproate improves only subjective measures; apomorphine injections reduce limb movements but not awakenings (SOR: C, very small crossover and cohort trials).
Estrogen replacement therapy is ineffective for periodic limb movement disorder (PLMD) associated with menopause (SOR: B, RCT).
Evidence summary
Although PLMD often occurs in association with restless legs syndrome, sleep apnea, narcolepsy, and other sleep disorders, it is itself an intrinsic sleep disorder characterized by stereotyped limb movements and sleep disruption.1 Most treatment studies of PLMD report both subjective and objective measures of sleep quality. Two commonly used objective measures, obtained by PSG, are the periodic leg movement (PLM) index and the PLM arousal index. The TABLE summarizes the evidence of medication trials.
Clonazepam improves subjective sleep measures, leg movements
Three comparative trials evaluated clonazepam against placebo, temazepam, and cognitive behavioral therapy (CBT).1-3 In the placebo-controlled and temazepam trials, clonazepam significantly improved subjective sleep parameters and leg movements.1,2 However, the studies produced conflicting results as to whether clonazepam reduced awakening from limb movements. Both temazepam and clonazepam appeared to be comparably effective; the trial was underpowered to detect a difference between them.
The CBT trial didn’t describe the frequency or duration of CBT clearly.3 It isn’t included in the TABLE.
L-Dopa decreases leg motions, effects on subjective sleep symptoms vary
Two comparison trials evaluated L-dopa (combined with carbidopa). One trial compared L-dopa with propoxyphene and placebo, and the other compared it with pergolide, a bromocriptine agonist available in Canada and Europe.4,5
In both trials, L-dopa consistently reduced leg motions at night but produced a variable response in subjective sleep symptoms and nocturnal waking. Propoxyphene yielded modest improvements in subjective sleep symptoms and nocturnal waking over placebo. The L-dopa–propoxyphene comparison trial was underpowered to allow a statistical comparison between the 2 medications.
Melatonin and valproate produce opposite effects in small studies
Three very small trials recorded symptoms and PSG findings in patients taking melatonin, apomorphine, or valproate, and compared them with the values observed at baseline.6-8 Melatonin significantly improved objective measures, but most patients didn’t feel less sleepy. Valproate produced the opposite effect—no clear PSG improvements, but all study patients felt better. Injected apomorphine reduced limb movements but not awakenings.
Estrogen replacement therapy doesn’t help
An RCT of estrogen replacement therapy for PLMD enrolled postmenopausal women, about half of whom were found to have PLMD.9 The study found estrogen replacement therapy to be ineffective for treating menopause-associated PLMD.
Recommendations
Practice parameters developed by the American Academy of Sleep Medicine state that clonazepam, pergolide, L-dopa (with a decarboxylase inhibitor), oxycodone, and propoxyphene are all reasonable choices for medical treatment of PLMD.10 The practice parameters don’t specify a preference for any of these medications.
CLONAZEPAM improves subjective sleep quality and polysomnogram (PSG) measures of leg movements more than placebo (strength of recommendation [SOR]: B, a small randomized controlled trial [RCT]); temazepam produces similar results (SOR: C, extrapolated from a small comparison trial).
Melatonin and L-dopa consistently improve certain PSG measures, but their effect on subjective sleep quality varies; valproate improves only subjective measures; apomorphine injections reduce limb movements but not awakenings (SOR: C, very small crossover and cohort trials).
Estrogen replacement therapy is ineffective for periodic limb movement disorder (PLMD) associated with menopause (SOR: B, RCT).
Evidence summary
Although PLMD often occurs in association with restless legs syndrome, sleep apnea, narcolepsy, and other sleep disorders, it is itself an intrinsic sleep disorder characterized by stereotyped limb movements and sleep disruption.1 Most treatment studies of PLMD report both subjective and objective measures of sleep quality. Two commonly used objective measures, obtained by PSG, are the periodic leg movement (PLM) index and the PLM arousal index. The TABLE summarizes the evidence of medication trials.
Clonazepam improves subjective sleep measures, leg movements
Three comparative trials evaluated clonazepam against placebo, temazepam, and cognitive behavioral therapy (CBT).1-3 In the placebo-controlled and temazepam trials, clonazepam significantly improved subjective sleep parameters and leg movements.1,2 However, the studies produced conflicting results as to whether clonazepam reduced awakening from limb movements. Both temazepam and clonazepam appeared to be comparably effective; the trial was underpowered to detect a difference between them.
The CBT trial didn’t describe the frequency or duration of CBT clearly.3 It isn’t included in the TABLE.
L-Dopa decreases leg motions, effects on subjective sleep symptoms vary
Two comparison trials evaluated L-dopa (combined with carbidopa). One trial compared L-dopa with propoxyphene and placebo, and the other compared it with pergolide, a bromocriptine agonist available in Canada and Europe.4,5
In both trials, L-dopa consistently reduced leg motions at night but produced a variable response in subjective sleep symptoms and nocturnal waking. Propoxyphene yielded modest improvements in subjective sleep symptoms and nocturnal waking over placebo. The L-dopa–propoxyphene comparison trial was underpowered to allow a statistical comparison between the 2 medications.
Melatonin and valproate produce opposite effects in small studies
Three very small trials recorded symptoms and PSG findings in patients taking melatonin, apomorphine, or valproate, and compared them with the values observed at baseline.6-8 Melatonin significantly improved objective measures, but most patients didn’t feel less sleepy. Valproate produced the opposite effect—no clear PSG improvements, but all study patients felt better. Injected apomorphine reduced limb movements but not awakenings.
Estrogen replacement therapy doesn’t help
An RCT of estrogen replacement therapy for PLMD enrolled postmenopausal women, about half of whom were found to have PLMD.9 The study found estrogen replacement therapy to be ineffective for treating menopause-associated PLMD.
Recommendations
Practice parameters developed by the American Academy of Sleep Medicine state that clonazepam, pergolide, L-dopa (with a decarboxylase inhibitor), oxycodone, and propoxyphene are all reasonable choices for medical treatment of PLMD.10 The practice parameters don’t specify a preference for any of these medications.
1. Saletu M, Anderer P, Saletu-Zyhlarz G, et al. Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo-controlled sleep laboratory studies with clonazepam. Eur Neuropsychopharmacol. 2001;11:153-161.
2. Mitler MM, Browman CP, Menn SJ, et al. Nocturnal myoclonus: treatment efficacy of clonazepam and temazepam. Sleep. 1986;9:385-392.
3. Edinger JD, Fins AI, Sullivan RJ, et al. Comparison of cognitive-behavioral therapy and clonazepam for treating periodic limb movement disorder. Sleep. 1996;19:442-444.
4. Staedt J, Wassmuth F, Ziemann U, et al. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double-blind randomized crossover trial of pergolide versus L-Dopa. J Neural Transm. 1997;104:461-468.
5. Kaplan PW, Allen RP, Buchholz DW, et al. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717-723.
6. Kunz D, Bes F. Exogenous melatonin in periodic limb movement disorder: an open clinical trial and a hypothesis. Sleep. 2001;24:183-187.
7. Haba-Rubio J, Staner L, Cornette F, et al. Acute low single dose of apomorphine reduces periodic limb movements but has no significant effect on sleep arousals: a preliminary report. Neurophysiol Clin. 2003;33:180-184.
8. Ehrenberg BL, Eisensehr I, Corbett KE, et al. Valproate for sleep consolidation in periodic limb movement disorder. J Clin Psychopharmacol. 2000;20:574-578.
9. Polo-Kantola P, Rauhala E, Erkkola R, et al. Estrogen replacement therapy and nocturnal periodic limb movements: a randomized controlled trial. Obstet Gynecol. 2001;97:548-554.
10. Chesson AL, Jr, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Sleep. 1999;22:961-968.
1. Saletu M, Anderer P, Saletu-Zyhlarz G, et al. Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo-controlled sleep laboratory studies with clonazepam. Eur Neuropsychopharmacol. 2001;11:153-161.
2. Mitler MM, Browman CP, Menn SJ, et al. Nocturnal myoclonus: treatment efficacy of clonazepam and temazepam. Sleep. 1986;9:385-392.
3. Edinger JD, Fins AI, Sullivan RJ, et al. Comparison of cognitive-behavioral therapy and clonazepam for treating periodic limb movement disorder. Sleep. 1996;19:442-444.
4. Staedt J, Wassmuth F, Ziemann U, et al. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double-blind randomized crossover trial of pergolide versus L-Dopa. J Neural Transm. 1997;104:461-468.
5. Kaplan PW, Allen RP, Buchholz DW, et al. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16:717-723.
6. Kunz D, Bes F. Exogenous melatonin in periodic limb movement disorder: an open clinical trial and a hypothesis. Sleep. 2001;24:183-187.
7. Haba-Rubio J, Staner L, Cornette F, et al. Acute low single dose of apomorphine reduces periodic limb movements but has no significant effect on sleep arousals: a preliminary report. Neurophysiol Clin. 2003;33:180-184.
8. Ehrenberg BL, Eisensehr I, Corbett KE, et al. Valproate for sleep consolidation in periodic limb movement disorder. J Clin Psychopharmacol. 2000;20:574-578.
9. Polo-Kantola P, Rauhala E, Erkkola R, et al. Estrogen replacement therapy and nocturnal periodic limb movements: a randomized controlled trial. Obstet Gynecol. 2001;97:548-554.
10. Chesson AL, Jr, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Sleep. 1999;22:961-968.
Evidence-based answers from the Family Physicians Inquiries Network
Medication vs radioablation for Graves’ disease: How do they compare?
THE BENEFITS ARE SIMILAR; the risks vary. Treating Graves’ disease initially with medication or radioablation (or surgery) produces comparable resolution of hyperthyroidism at 2 years (strength of recommendation [SOR]: B, a randomized clinical trial [RCT]). The goal of radio-ablation is lifelong hypothyroidism.
While radioablation doesn’t appear to increase the risk of neoplasia, “theoretical concerns” have led to the recommendation that it not be used for children younger than 5 years (SOR: C, expert opinion).
Radioablation carries a higher risk of thyroid-associated ophthalmopathy (TAO) than medical therapy (SOR: B, an RCT and a lower-quality meta-analysis).
Between 9% and 16% of patients are unable to tolerate medical therapy, mainly because of rash but also because of agranulocytosis (SOR: A, meta-analysis).
Evidence summary
A prospective RCT found that medical therapy, radioablation with iodine-131 (131I), and surgery produced similar control of Graves’ hyperthyroidism in 179 patients.1 Investigators stratified patients by age, assigning younger patients (20-34 years; N=60) to antithyroid medication (methimazole and a β-blocker) for 18 months or subtotal thyroidectomy and older patients (35-55 years; N=119) to 18 months of antithyroid medication, subtotal thyroidectomy, or 131I radioablation.
After 6 weeks, all therapies produced serum triiodothyronine levels of less than 2.5 nmol/L (data extracted from table; no comparison statistic given). Patients were followed for 48 to 121 months (average follow-up time not given). Investigators found no significant differences in sick leave (72 vs 83 days for medical treatment compared with radioablation; no comparison statistic given) or patient satisfaction (95% for both medical treatment and radioablation; no comparison statistic given).
Medication (initially methimazole) was changed in 16% of patients because of adverse effects. More than a third of patients relapsed after medications were stopped (time to relapse 1-57 months); 21% relapsed after a single 131I treatment (time to relapse 5-16 months).
In another study, radioablation outperforms medication
A retrospective case series found that radioablation resolved hyperthyroidism more often than medical therapy among 194 consecutive Saudi Arabian patients (mean age 32 years) diagnosed with Graves’ disease and followed for an average of 50 months.2 One dose of radioiodine (13-15 mCi) cured hyperthyroidism in 83% of patients, whereas 18 months of medical therapy produced remission lasting at least 6 months past the end of therapy in only 26% of patients (no comparison statistic given).
The presence of TAO at diagnosis increased the likelihood of radioablation failure (odds ratio for failure to respond to single dose of radioiodine=6.4; 95% confidence interval [CI], 1.51-24.4; P<.01). A major weakness of the study was that the investigators didn’t describe the medication therapy clearly.
More patients develop TAO after radioablation than medical therapy
An RCT found that radioablation is more commonly associated with development of TAO than medical therapy.3 When investigators randomized 313 patients to receive 131I radioablation or medical therapy for 18 months and followed them for as long as 4 years, more patients receiving radioablation developed TAO (38% compared with 18% for medical therapy, using intention-to-treat analysis; P<.001; number needed to harm [NNH]=5). Twenty-five percent of patients initially receiving medical therapy later underwent radioablation, but these patients didn’t develop TAO at a higher rate.
An earlier meta-analysis of 2 RCTs (N=189) also found an increased risk of TAO with radioablation compared with medical therapy.4 Patients receiving radioablation were more likely to develop TAO (18% vs 4%; relative risk [RR]=4.2; 95% CI, 2.0-8.8; NNH=7) and more likely to develop severe TAO (10% vs 1.6%; RR=4.4; 95% CI, 1.3-15; NNH=12). Adjunctive use of steroids with radioablation didn’t alter the risk of new TAO. However, steroid prophylaxis in patients with preexisting TAO significantly reduced the risk of progression after radioablation (RR=0.03; 95% CI, 0.00-0.24). The authors of the meta-analysis didn’t evaluate the quality of the RCTs.
Despite low neoplasia risk, radioablation isn’t for young children
Expert guidelines state that the goal of radioablation is to induce lifelong hypothyroidism, which is managed with thyroid hormone replacement.5 The risk of neoplasia after radioablation is believed to be low with appropriate dosing. However, based on “theoretical concerns,” experts don’t recommend using radioiodine in children younger than 5 years and advise limited use in children 5 to 10 years of age.5
Medication adverse effects include rashes, transient agranulocytosis
A Cochrane review with 7 RCTs (N=620) describing withdrawal rates for patients receiving medication for Graves’ disease found that 9% to 16% of patients discontinued treatment because of adverse effects.6 Rashes were the most common adverse effect (6%-10% of patients), but as many as 3% of patients developed transient agranulocytosis. In addition, patients on medication need frequent blood tests to monitor for thyroid activity and potential toxicity.
Recommendations
The guidelines of the American Thyroid Association and the American Association of Clinical Endocrinologists state that overt Graves’ hyperthyroidism may be treated with any of the following: 131I radioablation, antithyroid medication, or thyroidectomy.3 Patient characteristics (pregnancy, mild disease, goiter compression symptoms) should help determine the appropriate option in any given case.
1. Törring O, Tallstedt L, Göran W, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine—a prospective, randomized study. J Clin Endocrinol Metab. 1996;81:2986-2993.
2. Alfadda A, Malabu UH, El-Desouki MI, et al. Treatment of Graves’ hyperthyroidism—prognostic factors for outcome. Saudi Med J. 2007;28:225-230.
3. Träisk F, Tallstedt L, Abraham-Nordling M, et al. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700-3707.
4. Acharya SH, Avenell A, Philip S, et al. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review (structured abstract). Clin Endocrinol. 2008;69:943-950.
5. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17:456-520.
6. Abraham P, Avenell A, McGeoch SC, et al. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Syst Rev. 2010;(1):CD003420.
THE BENEFITS ARE SIMILAR; the risks vary. Treating Graves’ disease initially with medication or radioablation (or surgery) produces comparable resolution of hyperthyroidism at 2 years (strength of recommendation [SOR]: B, a randomized clinical trial [RCT]). The goal of radio-ablation is lifelong hypothyroidism.
While radioablation doesn’t appear to increase the risk of neoplasia, “theoretical concerns” have led to the recommendation that it not be used for children younger than 5 years (SOR: C, expert opinion).
Radioablation carries a higher risk of thyroid-associated ophthalmopathy (TAO) than medical therapy (SOR: B, an RCT and a lower-quality meta-analysis).
Between 9% and 16% of patients are unable to tolerate medical therapy, mainly because of rash but also because of agranulocytosis (SOR: A, meta-analysis).
Evidence summary
A prospective RCT found that medical therapy, radioablation with iodine-131 (131I), and surgery produced similar control of Graves’ hyperthyroidism in 179 patients.1 Investigators stratified patients by age, assigning younger patients (20-34 years; N=60) to antithyroid medication (methimazole and a β-blocker) for 18 months or subtotal thyroidectomy and older patients (35-55 years; N=119) to 18 months of antithyroid medication, subtotal thyroidectomy, or 131I radioablation.
After 6 weeks, all therapies produced serum triiodothyronine levels of less than 2.5 nmol/L (data extracted from table; no comparison statistic given). Patients were followed for 48 to 121 months (average follow-up time not given). Investigators found no significant differences in sick leave (72 vs 83 days for medical treatment compared with radioablation; no comparison statistic given) or patient satisfaction (95% for both medical treatment and radioablation; no comparison statistic given).
Medication (initially methimazole) was changed in 16% of patients because of adverse effects. More than a third of patients relapsed after medications were stopped (time to relapse 1-57 months); 21% relapsed after a single 131I treatment (time to relapse 5-16 months).
In another study, radioablation outperforms medication
A retrospective case series found that radioablation resolved hyperthyroidism more often than medical therapy among 194 consecutive Saudi Arabian patients (mean age 32 years) diagnosed with Graves’ disease and followed for an average of 50 months.2 One dose of radioiodine (13-15 mCi) cured hyperthyroidism in 83% of patients, whereas 18 months of medical therapy produced remission lasting at least 6 months past the end of therapy in only 26% of patients (no comparison statistic given).
The presence of TAO at diagnosis increased the likelihood of radioablation failure (odds ratio for failure to respond to single dose of radioiodine=6.4; 95% confidence interval [CI], 1.51-24.4; P<.01). A major weakness of the study was that the investigators didn’t describe the medication therapy clearly.
More patients develop TAO after radioablation than medical therapy
An RCT found that radioablation is more commonly associated with development of TAO than medical therapy.3 When investigators randomized 313 patients to receive 131I radioablation or medical therapy for 18 months and followed them for as long as 4 years, more patients receiving radioablation developed TAO (38% compared with 18% for medical therapy, using intention-to-treat analysis; P<.001; number needed to harm [NNH]=5). Twenty-five percent of patients initially receiving medical therapy later underwent radioablation, but these patients didn’t develop TAO at a higher rate.
An earlier meta-analysis of 2 RCTs (N=189) also found an increased risk of TAO with radioablation compared with medical therapy.4 Patients receiving radioablation were more likely to develop TAO (18% vs 4%; relative risk [RR]=4.2; 95% CI, 2.0-8.8; NNH=7) and more likely to develop severe TAO (10% vs 1.6%; RR=4.4; 95% CI, 1.3-15; NNH=12). Adjunctive use of steroids with radioablation didn’t alter the risk of new TAO. However, steroid prophylaxis in patients with preexisting TAO significantly reduced the risk of progression after radioablation (RR=0.03; 95% CI, 0.00-0.24). The authors of the meta-analysis didn’t evaluate the quality of the RCTs.
Despite low neoplasia risk, radioablation isn’t for young children
Expert guidelines state that the goal of radioablation is to induce lifelong hypothyroidism, which is managed with thyroid hormone replacement.5 The risk of neoplasia after radioablation is believed to be low with appropriate dosing. However, based on “theoretical concerns,” experts don’t recommend using radioiodine in children younger than 5 years and advise limited use in children 5 to 10 years of age.5
Medication adverse effects include rashes, transient agranulocytosis
A Cochrane review with 7 RCTs (N=620) describing withdrawal rates for patients receiving medication for Graves’ disease found that 9% to 16% of patients discontinued treatment because of adverse effects.6 Rashes were the most common adverse effect (6%-10% of patients), but as many as 3% of patients developed transient agranulocytosis. In addition, patients on medication need frequent blood tests to monitor for thyroid activity and potential toxicity.
Recommendations
The guidelines of the American Thyroid Association and the American Association of Clinical Endocrinologists state that overt Graves’ hyperthyroidism may be treated with any of the following: 131I radioablation, antithyroid medication, or thyroidectomy.3 Patient characteristics (pregnancy, mild disease, goiter compression symptoms) should help determine the appropriate option in any given case.
THE BENEFITS ARE SIMILAR; the risks vary. Treating Graves’ disease initially with medication or radioablation (or surgery) produces comparable resolution of hyperthyroidism at 2 years (strength of recommendation [SOR]: B, a randomized clinical trial [RCT]). The goal of radio-ablation is lifelong hypothyroidism.
While radioablation doesn’t appear to increase the risk of neoplasia, “theoretical concerns” have led to the recommendation that it not be used for children younger than 5 years (SOR: C, expert opinion).
Radioablation carries a higher risk of thyroid-associated ophthalmopathy (TAO) than medical therapy (SOR: B, an RCT and a lower-quality meta-analysis).
Between 9% and 16% of patients are unable to tolerate medical therapy, mainly because of rash but also because of agranulocytosis (SOR: A, meta-analysis).
Evidence summary
A prospective RCT found that medical therapy, radioablation with iodine-131 (131I), and surgery produced similar control of Graves’ hyperthyroidism in 179 patients.1 Investigators stratified patients by age, assigning younger patients (20-34 years; N=60) to antithyroid medication (methimazole and a β-blocker) for 18 months or subtotal thyroidectomy and older patients (35-55 years; N=119) to 18 months of antithyroid medication, subtotal thyroidectomy, or 131I radioablation.
After 6 weeks, all therapies produced serum triiodothyronine levels of less than 2.5 nmol/L (data extracted from table; no comparison statistic given). Patients were followed for 48 to 121 months (average follow-up time not given). Investigators found no significant differences in sick leave (72 vs 83 days for medical treatment compared with radioablation; no comparison statistic given) or patient satisfaction (95% for both medical treatment and radioablation; no comparison statistic given).
Medication (initially methimazole) was changed in 16% of patients because of adverse effects. More than a third of patients relapsed after medications were stopped (time to relapse 1-57 months); 21% relapsed after a single 131I treatment (time to relapse 5-16 months).
In another study, radioablation outperforms medication
A retrospective case series found that radioablation resolved hyperthyroidism more often than medical therapy among 194 consecutive Saudi Arabian patients (mean age 32 years) diagnosed with Graves’ disease and followed for an average of 50 months.2 One dose of radioiodine (13-15 mCi) cured hyperthyroidism in 83% of patients, whereas 18 months of medical therapy produced remission lasting at least 6 months past the end of therapy in only 26% of patients (no comparison statistic given).
The presence of TAO at diagnosis increased the likelihood of radioablation failure (odds ratio for failure to respond to single dose of radioiodine=6.4; 95% confidence interval [CI], 1.51-24.4; P<.01). A major weakness of the study was that the investigators didn’t describe the medication therapy clearly.
More patients develop TAO after radioablation than medical therapy
An RCT found that radioablation is more commonly associated with development of TAO than medical therapy.3 When investigators randomized 313 patients to receive 131I radioablation or medical therapy for 18 months and followed them for as long as 4 years, more patients receiving radioablation developed TAO (38% compared with 18% for medical therapy, using intention-to-treat analysis; P<.001; number needed to harm [NNH]=5). Twenty-five percent of patients initially receiving medical therapy later underwent radioablation, but these patients didn’t develop TAO at a higher rate.
An earlier meta-analysis of 2 RCTs (N=189) also found an increased risk of TAO with radioablation compared with medical therapy.4 Patients receiving radioablation were more likely to develop TAO (18% vs 4%; relative risk [RR]=4.2; 95% CI, 2.0-8.8; NNH=7) and more likely to develop severe TAO (10% vs 1.6%; RR=4.4; 95% CI, 1.3-15; NNH=12). Adjunctive use of steroids with radioablation didn’t alter the risk of new TAO. However, steroid prophylaxis in patients with preexisting TAO significantly reduced the risk of progression after radioablation (RR=0.03; 95% CI, 0.00-0.24). The authors of the meta-analysis didn’t evaluate the quality of the RCTs.
Despite low neoplasia risk, radioablation isn’t for young children
Expert guidelines state that the goal of radioablation is to induce lifelong hypothyroidism, which is managed with thyroid hormone replacement.5 The risk of neoplasia after radioablation is believed to be low with appropriate dosing. However, based on “theoretical concerns,” experts don’t recommend using radioiodine in children younger than 5 years and advise limited use in children 5 to 10 years of age.5
Medication adverse effects include rashes, transient agranulocytosis
A Cochrane review with 7 RCTs (N=620) describing withdrawal rates for patients receiving medication for Graves’ disease found that 9% to 16% of patients discontinued treatment because of adverse effects.6 Rashes were the most common adverse effect (6%-10% of patients), but as many as 3% of patients developed transient agranulocytosis. In addition, patients on medication need frequent blood tests to monitor for thyroid activity and potential toxicity.
Recommendations
The guidelines of the American Thyroid Association and the American Association of Clinical Endocrinologists state that overt Graves’ hyperthyroidism may be treated with any of the following: 131I radioablation, antithyroid medication, or thyroidectomy.3 Patient characteristics (pregnancy, mild disease, goiter compression symptoms) should help determine the appropriate option in any given case.
1. Törring O, Tallstedt L, Göran W, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine—a prospective, randomized study. J Clin Endocrinol Metab. 1996;81:2986-2993.
2. Alfadda A, Malabu UH, El-Desouki MI, et al. Treatment of Graves’ hyperthyroidism—prognostic factors for outcome. Saudi Med J. 2007;28:225-230.
3. Träisk F, Tallstedt L, Abraham-Nordling M, et al. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700-3707.
4. Acharya SH, Avenell A, Philip S, et al. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review (structured abstract). Clin Endocrinol. 2008;69:943-950.
5. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17:456-520.
6. Abraham P, Avenell A, McGeoch SC, et al. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Syst Rev. 2010;(1):CD003420.
1. Törring O, Tallstedt L, Göran W, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine—a prospective, randomized study. J Clin Endocrinol Metab. 1996;81:2986-2993.
2. Alfadda A, Malabu UH, El-Desouki MI, et al. Treatment of Graves’ hyperthyroidism—prognostic factors for outcome. Saudi Med J. 2007;28:225-230.
3. Träisk F, Tallstedt L, Abraham-Nordling M, et al. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700-3707.
4. Acharya SH, Avenell A, Philip S, et al. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review (structured abstract). Clin Endocrinol. 2008;69:943-950.
5. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17:456-520.
6. Abraham P, Avenell A, McGeoch SC, et al. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Syst Rev. 2010;(1):CD003420.
Evidence-based answers from the Family Physicians Inquiries Network
How does smoking in the home affect children with asthma?
CHILDREN WITH ASTHMA who are exposed to smoking in the home are likely to have more severe asthma symptoms, more asthma-related doctor visits (strength of recommendation [SOR]: B, a preponderance of evidence from heterogeneous cohort studies), and a poorer response to asthma therapy (SOR: B, 1 small cohort study) than unexposed children.
Evidence summary
A systematic review from the US Surgeon General’s office of studies addressing the relationship between secondhand smoke exposure and asthma severity in children from 0 to 18 years of age found that children with asthma who were exposed to secondhand smoke had “greater disease severity” than unexposed children.1 The studies—including 8 prospective and retrospective cohort studies (N=6095), one case-control study (N=149), and 11 uncontrolled case series (N=2932)—were performed in the United States, Canada, the United Kingdom, Sweden, Singapore, South Africa, Kenya, and Nigeria.
Investigators found a significant worsening of asthma caused by secondhand smoke in 6 of 11 clinic-based studies and 2 of 9 population-based studies. Children with asthma who were exposed to secondhand smoke had more doctor visits, more frequent flares, and higher disease severity scores than children who weren’t exposed. Heterogeneity among the studies prevented a meta-analysis of data on severity of asthma.
Where there’s smoke, there are worse health outcomes
Three of 4 subsequent cohort studies found poorer health outcomes among children with asthma who were exposed to smoking than children who weren’t. The first study, of 523 children 4 to 16 years of age with physician-diagnosed asthma, correlated smoke exposure, as indicated by serum cotinine levels, with pulmonary function tests and clinical outcomes.2 Children with high serum cotinine levels (>0.63 mg/mL) were more likely to have asthma symptoms monthly or more often, as reported by the family (adjusted odds ratio [OR]=2.7; 95% confidence interval [CI], 1.1-6.5), than children with low cotinine levels (<0.116 ng/mL). High cotinine levels weren’t associated with significant changes in forced expiratory volume in one second, decreased school attendance, or increased physician visits.
Another study of 438 children ages 2 to 12 years with physician-diagnosed asthma and at least one parent who smoked, correlated salivary cotinine levels with the likelihood of contacting a physician for asthma symptoms.3 Children with high salivary cotinine levels (>4.5 ng/mL) had higher asthma-related physician contact rates than children with low cotinine levels (≤2 ng/mL) (incidence rate ratio=1.2; 95% CI, 1.1-1.4).
A third study evaluated asthma treatment response in 167 children from families throughout France who were 6 to 12 years of age and recently diagnosed with mild or moderate persistent asthma.4 Investigators performed pulmonary function tests and collected data on symptoms every 4 months for 3 years. Children who lived with someone who smoked were less likely to have controlled asthma symptoms (OR=0.34; 95% CI, 0.13–0.91).
The fourth study, of 126 urban children ages 6 to 12 years with physician-diagnosed asthma and in-home smoke exposure, correlated urinary cotinine levels and rates of clinical illness. It found no significant differences in parent-reported illness between children with higher urinary cotinine levels and children with lower levels.5
Recommendations
The National Asthma Education and Prevention Program Expert Panel recommends that physicians ask patients about their smoking status and refer adults who have children with asthma to smoking cessation programs.6 The panel further recommends that clinicians advise people with asthma to avoid smoking and limit exposure to environmental tobacco smoke.
1. Respiratory effects in children from exposure to second hand smoke. In: United States Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006;355-375.
2. Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409-415.
3. Crombie IK, Wright A, Irvine L, et al. Does passive smoking increase the frequency of health service contacts in children with asthma? Thorax. 2001;56:9-12.
4. Soussan D, Liard R, Zureik M, et al. Treatment compliance, passive smoking, and asthma control: a three-year cohort study. Arch Dis Child. 2003;88:229-233.
5. Butz AM, Breysse P, Rand C, et al. Household smoking behavior: effects on indoor air quality and health of urban children with asthma. Matern Child Health J. 2011;15:460-468.
6. Control of environmental factors and comorbid conditions that affect asthma. In: National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007;165-212.
CHILDREN WITH ASTHMA who are exposed to smoking in the home are likely to have more severe asthma symptoms, more asthma-related doctor visits (strength of recommendation [SOR]: B, a preponderance of evidence from heterogeneous cohort studies), and a poorer response to asthma therapy (SOR: B, 1 small cohort study) than unexposed children.
Evidence summary
A systematic review from the US Surgeon General’s office of studies addressing the relationship between secondhand smoke exposure and asthma severity in children from 0 to 18 years of age found that children with asthma who were exposed to secondhand smoke had “greater disease severity” than unexposed children.1 The studies—including 8 prospective and retrospective cohort studies (N=6095), one case-control study (N=149), and 11 uncontrolled case series (N=2932)—were performed in the United States, Canada, the United Kingdom, Sweden, Singapore, South Africa, Kenya, and Nigeria.
Investigators found a significant worsening of asthma caused by secondhand smoke in 6 of 11 clinic-based studies and 2 of 9 population-based studies. Children with asthma who were exposed to secondhand smoke had more doctor visits, more frequent flares, and higher disease severity scores than children who weren’t exposed. Heterogeneity among the studies prevented a meta-analysis of data on severity of asthma.
Where there’s smoke, there are worse health outcomes
Three of 4 subsequent cohort studies found poorer health outcomes among children with asthma who were exposed to smoking than children who weren’t. The first study, of 523 children 4 to 16 years of age with physician-diagnosed asthma, correlated smoke exposure, as indicated by serum cotinine levels, with pulmonary function tests and clinical outcomes.2 Children with high serum cotinine levels (>0.63 mg/mL) were more likely to have asthma symptoms monthly or more often, as reported by the family (adjusted odds ratio [OR]=2.7; 95% confidence interval [CI], 1.1-6.5), than children with low cotinine levels (<0.116 ng/mL). High cotinine levels weren’t associated with significant changes in forced expiratory volume in one second, decreased school attendance, or increased physician visits.
Another study of 438 children ages 2 to 12 years with physician-diagnosed asthma and at least one parent who smoked, correlated salivary cotinine levels with the likelihood of contacting a physician for asthma symptoms.3 Children with high salivary cotinine levels (>4.5 ng/mL) had higher asthma-related physician contact rates than children with low cotinine levels (≤2 ng/mL) (incidence rate ratio=1.2; 95% CI, 1.1-1.4).
A third study evaluated asthma treatment response in 167 children from families throughout France who were 6 to 12 years of age and recently diagnosed with mild or moderate persistent asthma.4 Investigators performed pulmonary function tests and collected data on symptoms every 4 months for 3 years. Children who lived with someone who smoked were less likely to have controlled asthma symptoms (OR=0.34; 95% CI, 0.13–0.91).
The fourth study, of 126 urban children ages 6 to 12 years with physician-diagnosed asthma and in-home smoke exposure, correlated urinary cotinine levels and rates of clinical illness. It found no significant differences in parent-reported illness between children with higher urinary cotinine levels and children with lower levels.5
Recommendations
The National Asthma Education and Prevention Program Expert Panel recommends that physicians ask patients about their smoking status and refer adults who have children with asthma to smoking cessation programs.6 The panel further recommends that clinicians advise people with asthma to avoid smoking and limit exposure to environmental tobacco smoke.
CHILDREN WITH ASTHMA who are exposed to smoking in the home are likely to have more severe asthma symptoms, more asthma-related doctor visits (strength of recommendation [SOR]: B, a preponderance of evidence from heterogeneous cohort studies), and a poorer response to asthma therapy (SOR: B, 1 small cohort study) than unexposed children.
Evidence summary
A systematic review from the US Surgeon General’s office of studies addressing the relationship between secondhand smoke exposure and asthma severity in children from 0 to 18 years of age found that children with asthma who were exposed to secondhand smoke had “greater disease severity” than unexposed children.1 The studies—including 8 prospective and retrospective cohort studies (N=6095), one case-control study (N=149), and 11 uncontrolled case series (N=2932)—were performed in the United States, Canada, the United Kingdom, Sweden, Singapore, South Africa, Kenya, and Nigeria.
Investigators found a significant worsening of asthma caused by secondhand smoke in 6 of 11 clinic-based studies and 2 of 9 population-based studies. Children with asthma who were exposed to secondhand smoke had more doctor visits, more frequent flares, and higher disease severity scores than children who weren’t exposed. Heterogeneity among the studies prevented a meta-analysis of data on severity of asthma.
Where there’s smoke, there are worse health outcomes
Three of 4 subsequent cohort studies found poorer health outcomes among children with asthma who were exposed to smoking than children who weren’t. The first study, of 523 children 4 to 16 years of age with physician-diagnosed asthma, correlated smoke exposure, as indicated by serum cotinine levels, with pulmonary function tests and clinical outcomes.2 Children with high serum cotinine levels (>0.63 mg/mL) were more likely to have asthma symptoms monthly or more often, as reported by the family (adjusted odds ratio [OR]=2.7; 95% confidence interval [CI], 1.1-6.5), than children with low cotinine levels (<0.116 ng/mL). High cotinine levels weren’t associated with significant changes in forced expiratory volume in one second, decreased school attendance, or increased physician visits.
Another study of 438 children ages 2 to 12 years with physician-diagnosed asthma and at least one parent who smoked, correlated salivary cotinine levels with the likelihood of contacting a physician for asthma symptoms.3 Children with high salivary cotinine levels (>4.5 ng/mL) had higher asthma-related physician contact rates than children with low cotinine levels (≤2 ng/mL) (incidence rate ratio=1.2; 95% CI, 1.1-1.4).
A third study evaluated asthma treatment response in 167 children from families throughout France who were 6 to 12 years of age and recently diagnosed with mild or moderate persistent asthma.4 Investigators performed pulmonary function tests and collected data on symptoms every 4 months for 3 years. Children who lived with someone who smoked were less likely to have controlled asthma symptoms (OR=0.34; 95% CI, 0.13–0.91).
The fourth study, of 126 urban children ages 6 to 12 years with physician-diagnosed asthma and in-home smoke exposure, correlated urinary cotinine levels and rates of clinical illness. It found no significant differences in parent-reported illness between children with higher urinary cotinine levels and children with lower levels.5
Recommendations
The National Asthma Education and Prevention Program Expert Panel recommends that physicians ask patients about their smoking status and refer adults who have children with asthma to smoking cessation programs.6 The panel further recommends that clinicians advise people with asthma to avoid smoking and limit exposure to environmental tobacco smoke.
1. Respiratory effects in children from exposure to second hand smoke. In: United States Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006;355-375.
2. Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409-415.
3. Crombie IK, Wright A, Irvine L, et al. Does passive smoking increase the frequency of health service contacts in children with asthma? Thorax. 2001;56:9-12.
4. Soussan D, Liard R, Zureik M, et al. Treatment compliance, passive smoking, and asthma control: a three-year cohort study. Arch Dis Child. 2003;88:229-233.
5. Butz AM, Breysse P, Rand C, et al. Household smoking behavior: effects on indoor air quality and health of urban children with asthma. Matern Child Health J. 2011;15:460-468.
6. Control of environmental factors and comorbid conditions that affect asthma. In: National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007;165-212.
1. Respiratory effects in children from exposure to second hand smoke. In: United States Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006;355-375.
2. Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409-415.
3. Crombie IK, Wright A, Irvine L, et al. Does passive smoking increase the frequency of health service contacts in children with asthma? Thorax. 2001;56:9-12.
4. Soussan D, Liard R, Zureik M, et al. Treatment compliance, passive smoking, and asthma control: a three-year cohort study. Arch Dis Child. 2003;88:229-233.
5. Butz AM, Breysse P, Rand C, et al. Household smoking behavior: effects on indoor air quality and health of urban children with asthma. Matern Child Health J. 2011;15:460-468.
6. Control of environmental factors and comorbid conditions that affect asthma. In: National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007;165-212.
Evidence-based answers from the Family Physicians Inquiries Network
How effective—and safe—are systemic steroids for acute low back pain?
SHORT COURSES OF SYSTEMIC STEROIDS ARE LIKELY SAFE, but they are ineffective. A single dose of intramuscular (IM) or intravenous (IV) methylprednisolone doesn’t improve long-term pain scores in patients with low back pain and sciatica and produces conflicting effects on function. Oral prednisone (9-day taper) doesn’t improve pain or function in patients with back pain and sciatica. A single IM dose of methylprednisolone doesn’t improve pain scores or function in patients with back pain without sciatica (strength of recommendation: B, randomized controlled trials [RCTs]).
No trials of corticosteroids for back pain reported an increase in adverse outcomes, but studies were small, and only short-term (1 month) follow-up data are available.
Evidence summary
A double-blind RCT of 82 patients who reported to an emergency department with acute low back pain and sciatica compared the efficacy of a single IM dose of 160 mg methylprednisolone with placebo.1 Sciatica was confirmed with a positive straight leg test. All patients were given an instruction sheet and a small supply of naproxen and oxycodone with acetaminophen. The primary outcome was change in pain score, rated on a 0-to-10 visual analog scale (VAS).
Pain scores dropped in both groups over time. The magnitude of the change was not significantly greater in the steroid group (at 1 week: mean difference=1.1; 95% confidence interval [CI], –0.5 to 2.8; at 1 month: mean difference=1.3; 95% CI, –0.5 to 2.7).
Among secondary outcomes at 1 month, 19% of the methylprednisolone group reported continued functional disability compared with 49% of the placebo group (absolute difference=30%; 95% CI, 9-49; P=.007). Analgesic use in the previous 24 hours was similar for both groups (22% with steroid injection vs 43% with placebo; P=.06). There were no reports of gastrointestinal bleeding, osteonecrosis, infection, or serious hyperglycemia.
The same applies to back pain without sciatica
Another double-blind RCT of 87 patients evaluated IM methylprednisolone for acute low back pain of less than 1 week duration without sciatica.2 Patients received a single IM dose of 160 mg methylprednisolone or placebo. Both groups were given an instruction sheet and a small supply of naproxen and oxycodone with acetaminophen. The primary outcome was change in pain score on a 0-to-10 VAS.
Pain scores dropped in both groups over time, but the reduction wasn’t significantly larger in the steroid group (at 1 week: mean difference=0.6; 95% CI, –0.9 to 2.2; at 1 month: mean difference=0.6; 95% CI, –1.0 to 2.2). At 1 month, neither functional status nor “medication use in the preceding 24 hours” differed between the 2 groups.
The most common adverse effects were upper gastrointestinal complaints, drowsiness, and weakness. Adverse effect rates were comparable for the 2 groups and believed to be caused by the naproxen and oxycodone all patients received.
Relief of sciatica with IV steroids is short-lived
A double-blind RCT evaluated the efficacy of a single IV dose of 500 mg methylprednisolone or placebo for 65 patients with leg sciatica (with or without back pain) associated with imaging-confirmed lumbar disk disease.3 The primary outcome was reduction in sciatic leg pain during the first 3 days after injection as measured on a 100-mm VAS. All patients received standard pain medication and physical therapy.
At day 1, 48% of the methylprednisolone group and 28% of the placebo group showed a decrease on the VAS for sciatic pain of 20 mm or more (P=.04; number needed to treat=5). Pain measurements at 2, 3, 10, and 30 days found no significant difference between the groups, however. Nor did the groups differ significantly in functional status or medication use. The study didn’t assess adverse events.
Oral prednisone relieves back pain with sciatica no better than placebo
A double-blind RCT compared an oral prednisone taper (60 mg, 40 mg, and 20 mg each for 3 days) with placebo for treating 27 patients with acute low back pain and sciatica.4 All patients received nonsteroidal anti-inflammatory drugs (NSAIDs) and narcotics for pain control, directions to engage in activity as tolerated, and a referral for physical therapy. Outcomes were evaluated weekly for 1 month, then monthly for 5 months.
Pain scores, functional ability, and medication use didn’t differ significantly between the 2 groups. Steroid injections were later given to 15% of the oral steroid group and 43% of the control group, but the difference in outcomes wasn’t statistically significant. Investigators didn’t assess adverse events.
Recommendations
The joint guidelines of the American College of Physicians and the American Pain Society recommend acetaminophen and NSAIDs as first-line treatments for back pain and back pain with sciatica.5 The guidelines advise against using systemic corticosteroids because “they have not been shown to be more effective than placebo.”
1. Friedman BW, Esses D, Solorzano C, et al. A randomized placebo-controlled trial of single-dose IM corticosteroid for radicular low back pain. Spine. 2008;33:E624-E629.
2. Friedman BW, Holden L, Esses D, et al. Parenteral corticosteroids for emergency department patients with nonradicular low back pain. J Emerg Med. 2006;31:365-370.
3. Finckh A, Zufferey P, Schurch MA, et al. Short-term efficacy of intravenous pulse glucocorticoids in acute discogenic sciatica: a randomized controlled trial. Spine. 2006;31:377-381.
4. Holve RL, Barkan H. Oral steroids in initial treatment of acute sciatica. J Am Board Fam Med. 2008;21:469-474.
5. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
SHORT COURSES OF SYSTEMIC STEROIDS ARE LIKELY SAFE, but they are ineffective. A single dose of intramuscular (IM) or intravenous (IV) methylprednisolone doesn’t improve long-term pain scores in patients with low back pain and sciatica and produces conflicting effects on function. Oral prednisone (9-day taper) doesn’t improve pain or function in patients with back pain and sciatica. A single IM dose of methylprednisolone doesn’t improve pain scores or function in patients with back pain without sciatica (strength of recommendation: B, randomized controlled trials [RCTs]).
No trials of corticosteroids for back pain reported an increase in adverse outcomes, but studies were small, and only short-term (1 month) follow-up data are available.
Evidence summary
A double-blind RCT of 82 patients who reported to an emergency department with acute low back pain and sciatica compared the efficacy of a single IM dose of 160 mg methylprednisolone with placebo.1 Sciatica was confirmed with a positive straight leg test. All patients were given an instruction sheet and a small supply of naproxen and oxycodone with acetaminophen. The primary outcome was change in pain score, rated on a 0-to-10 visual analog scale (VAS).
Pain scores dropped in both groups over time. The magnitude of the change was not significantly greater in the steroid group (at 1 week: mean difference=1.1; 95% confidence interval [CI], –0.5 to 2.8; at 1 month: mean difference=1.3; 95% CI, –0.5 to 2.7).
Among secondary outcomes at 1 month, 19% of the methylprednisolone group reported continued functional disability compared with 49% of the placebo group (absolute difference=30%; 95% CI, 9-49; P=.007). Analgesic use in the previous 24 hours was similar for both groups (22% with steroid injection vs 43% with placebo; P=.06). There were no reports of gastrointestinal bleeding, osteonecrosis, infection, or serious hyperglycemia.
The same applies to back pain without sciatica
Another double-blind RCT of 87 patients evaluated IM methylprednisolone for acute low back pain of less than 1 week duration without sciatica.2 Patients received a single IM dose of 160 mg methylprednisolone or placebo. Both groups were given an instruction sheet and a small supply of naproxen and oxycodone with acetaminophen. The primary outcome was change in pain score on a 0-to-10 VAS.
Pain scores dropped in both groups over time, but the reduction wasn’t significantly larger in the steroid group (at 1 week: mean difference=0.6; 95% CI, –0.9 to 2.2; at 1 month: mean difference=0.6; 95% CI, –1.0 to 2.2). At 1 month, neither functional status nor “medication use in the preceding 24 hours” differed between the 2 groups.
The most common adverse effects were upper gastrointestinal complaints, drowsiness, and weakness. Adverse effect rates were comparable for the 2 groups and believed to be caused by the naproxen and oxycodone all patients received.
Relief of sciatica with IV steroids is short-lived
A double-blind RCT evaluated the efficacy of a single IV dose of 500 mg methylprednisolone or placebo for 65 patients with leg sciatica (with or without back pain) associated with imaging-confirmed lumbar disk disease.3 The primary outcome was reduction in sciatic leg pain during the first 3 days after injection as measured on a 100-mm VAS. All patients received standard pain medication and physical therapy.
At day 1, 48% of the methylprednisolone group and 28% of the placebo group showed a decrease on the VAS for sciatic pain of 20 mm or more (P=.04; number needed to treat=5). Pain measurements at 2, 3, 10, and 30 days found no significant difference between the groups, however. Nor did the groups differ significantly in functional status or medication use. The study didn’t assess adverse events.
Oral prednisone relieves back pain with sciatica no better than placebo
A double-blind RCT compared an oral prednisone taper (60 mg, 40 mg, and 20 mg each for 3 days) with placebo for treating 27 patients with acute low back pain and sciatica.4 All patients received nonsteroidal anti-inflammatory drugs (NSAIDs) and narcotics for pain control, directions to engage in activity as tolerated, and a referral for physical therapy. Outcomes were evaluated weekly for 1 month, then monthly for 5 months.
Pain scores, functional ability, and medication use didn’t differ significantly between the 2 groups. Steroid injections were later given to 15% of the oral steroid group and 43% of the control group, but the difference in outcomes wasn’t statistically significant. Investigators didn’t assess adverse events.
Recommendations
The joint guidelines of the American College of Physicians and the American Pain Society recommend acetaminophen and NSAIDs as first-line treatments for back pain and back pain with sciatica.5 The guidelines advise against using systemic corticosteroids because “they have not been shown to be more effective than placebo.”
SHORT COURSES OF SYSTEMIC STEROIDS ARE LIKELY SAFE, but they are ineffective. A single dose of intramuscular (IM) or intravenous (IV) methylprednisolone doesn’t improve long-term pain scores in patients with low back pain and sciatica and produces conflicting effects on function. Oral prednisone (9-day taper) doesn’t improve pain or function in patients with back pain and sciatica. A single IM dose of methylprednisolone doesn’t improve pain scores or function in patients with back pain without sciatica (strength of recommendation: B, randomized controlled trials [RCTs]).
No trials of corticosteroids for back pain reported an increase in adverse outcomes, but studies were small, and only short-term (1 month) follow-up data are available.
Evidence summary
A double-blind RCT of 82 patients who reported to an emergency department with acute low back pain and sciatica compared the efficacy of a single IM dose of 160 mg methylprednisolone with placebo.1 Sciatica was confirmed with a positive straight leg test. All patients were given an instruction sheet and a small supply of naproxen and oxycodone with acetaminophen. The primary outcome was change in pain score, rated on a 0-to-10 visual analog scale (VAS).
Pain scores dropped in both groups over time. The magnitude of the change was not significantly greater in the steroid group (at 1 week: mean difference=1.1; 95% confidence interval [CI], –0.5 to 2.8; at 1 month: mean difference=1.3; 95% CI, –0.5 to 2.7).
Among secondary outcomes at 1 month, 19% of the methylprednisolone group reported continued functional disability compared with 49% of the placebo group (absolute difference=30%; 95% CI, 9-49; P=.007). Analgesic use in the previous 24 hours was similar for both groups (22% with steroid injection vs 43% with placebo; P=.06). There were no reports of gastrointestinal bleeding, osteonecrosis, infection, or serious hyperglycemia.
The same applies to back pain without sciatica
Another double-blind RCT of 87 patients evaluated IM methylprednisolone for acute low back pain of less than 1 week duration without sciatica.2 Patients received a single IM dose of 160 mg methylprednisolone or placebo. Both groups were given an instruction sheet and a small supply of naproxen and oxycodone with acetaminophen. The primary outcome was change in pain score on a 0-to-10 VAS.
Pain scores dropped in both groups over time, but the reduction wasn’t significantly larger in the steroid group (at 1 week: mean difference=0.6; 95% CI, –0.9 to 2.2; at 1 month: mean difference=0.6; 95% CI, –1.0 to 2.2). At 1 month, neither functional status nor “medication use in the preceding 24 hours” differed between the 2 groups.
The most common adverse effects were upper gastrointestinal complaints, drowsiness, and weakness. Adverse effect rates were comparable for the 2 groups and believed to be caused by the naproxen and oxycodone all patients received.
Relief of sciatica with IV steroids is short-lived
A double-blind RCT evaluated the efficacy of a single IV dose of 500 mg methylprednisolone or placebo for 65 patients with leg sciatica (with or without back pain) associated with imaging-confirmed lumbar disk disease.3 The primary outcome was reduction in sciatic leg pain during the first 3 days after injection as measured on a 100-mm VAS. All patients received standard pain medication and physical therapy.
At day 1, 48% of the methylprednisolone group and 28% of the placebo group showed a decrease on the VAS for sciatic pain of 20 mm or more (P=.04; number needed to treat=5). Pain measurements at 2, 3, 10, and 30 days found no significant difference between the groups, however. Nor did the groups differ significantly in functional status or medication use. The study didn’t assess adverse events.
Oral prednisone relieves back pain with sciatica no better than placebo
A double-blind RCT compared an oral prednisone taper (60 mg, 40 mg, and 20 mg each for 3 days) with placebo for treating 27 patients with acute low back pain and sciatica.4 All patients received nonsteroidal anti-inflammatory drugs (NSAIDs) and narcotics for pain control, directions to engage in activity as tolerated, and a referral for physical therapy. Outcomes were evaluated weekly for 1 month, then monthly for 5 months.
Pain scores, functional ability, and medication use didn’t differ significantly between the 2 groups. Steroid injections were later given to 15% of the oral steroid group and 43% of the control group, but the difference in outcomes wasn’t statistically significant. Investigators didn’t assess adverse events.
Recommendations
The joint guidelines of the American College of Physicians and the American Pain Society recommend acetaminophen and NSAIDs as first-line treatments for back pain and back pain with sciatica.5 The guidelines advise against using systemic corticosteroids because “they have not been shown to be more effective than placebo.”
1. Friedman BW, Esses D, Solorzano C, et al. A randomized placebo-controlled trial of single-dose IM corticosteroid for radicular low back pain. Spine. 2008;33:E624-E629.
2. Friedman BW, Holden L, Esses D, et al. Parenteral corticosteroids for emergency department patients with nonradicular low back pain. J Emerg Med. 2006;31:365-370.
3. Finckh A, Zufferey P, Schurch MA, et al. Short-term efficacy of intravenous pulse glucocorticoids in acute discogenic sciatica: a randomized controlled trial. Spine. 2006;31:377-381.
4. Holve RL, Barkan H. Oral steroids in initial treatment of acute sciatica. J Am Board Fam Med. 2008;21:469-474.
5. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
1. Friedman BW, Esses D, Solorzano C, et al. A randomized placebo-controlled trial of single-dose IM corticosteroid for radicular low back pain. Spine. 2008;33:E624-E629.
2. Friedman BW, Holden L, Esses D, et al. Parenteral corticosteroids for emergency department patients with nonradicular low back pain. J Emerg Med. 2006;31:365-370.
3. Finckh A, Zufferey P, Schurch MA, et al. Short-term efficacy of intravenous pulse glucocorticoids in acute discogenic sciatica: a randomized controlled trial. Spine. 2006;31:377-381.
4. Holve RL, Barkan H. Oral steroids in initial treatment of acute sciatica. J Am Board Fam Med. 2008;21:469-474.
5. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
Evidence-based answers from the Family Physicians Inquiries Network
When is the best time to clamp the umbilical cord after routine vaginal delivery?
SOMETIME BETWEEN 30 SECONDS AND 2 MINUTES after delivery appears to be the best interval. In term infants, delayed clamping (waiting 1 or 2 minutes or until the cord stops pulsating) improves hemoglobin and ferritin levels, but slightly increases the risk of neonatal jaundice requiring phototherapy (strength of recommendation [SOR]: A, meta-analysis).
In preterm infants less than 37 weeks of age, cord clamping between 30 and 120 seconds after delivery reduces the need for blood transfusion (number needed to treat [NNT]=4) and frequency of intraventricular hemorrhage (NNT=8) compared with clamping in less than 20 seconds (SOR: A, meta-analyses).
Evidence summary
A 2008 Cochrane meta-analysis reviewed 11 randomized controlled trials (RCTs), enrolling more than 2900 women who had term vaginal deliveries, that compared early cord clamping (ECC) with delayed cord clamping (DCC).1 All of the trials defined ECC as clamping less than 1 minute after birth. DCC was variously defined as clamping after 1 minute, after 2 minutes, or after the cord stopped pulsating.
DCC was associated with increased newborn hemoglobin values (weighted mean difference [WMD]=2.2 g/dL; 95% confidence interval [CI], 0.3-4.1) and increased mean ferritin levels that persisted for as long as 6 months (WMD=12 mcg/L; 95% CI, 4.1-20). However, significantly fewer infants who underwent ECC required phototherapy for jaundice (relative risk [RR]=0.59; 95% CI, 0.38-0.92; NNT=45).
This meta-analysis was limited by variations in the definition of DCC, the level at which newborns were held in relation to the placenta (above, below, or level with), and the use of uterotonics. These limitations also apply to the other systematic reviews discussed here.
But is hyperbilirubinemia significant?
A 2007 meta-analysis of 15 clinical trials (8 randomized and 7 nonrandomized) with 1001 term infants in the DCC group and 911 in the ECC group found results similar to the Cochrane review.2 When compared with ECC, delayed clamping at least 2 minutes after birth was associated with significantly higher hematocrit (WMD=3.7%; 95% CI, 2-4), ferritin (WMD=18 mcg/L; 95% CI, 17-19), and stored iron (WMD=20 mg; 95% CI, 8-32), as well as decreased risk of anemia (RR=0.5; 95% CI, 0.4-0.7).
Infants in the DCC group had an increased risk of asymptomatic polycythemia (RR=3.9; 95% CI, 1.0-15). Delayed clamping was also associated with an increased rate of phototherapy for hyperbilirubinemia that didn’t reach statistical significance, although the confidence interval was wide (RR=1.78; 95% CI, 0.71-4.46).
Early clamping poses risks for preterm infants
A 2008 meta-analysis (using Cochrane methodology) identified 10 RCTs enrolling 454 infants born at less than 37 weeks’ gestation.3 ECC was defined as less than 20 seconds after delivery and DCC as greater than 30 seconds (and up to 120 seconds).
The review found ECC to be inferior to DCC. Early clamping was associated with an increased risk of transfusion for anemia (3 studies, 112 patients; RR=2.1; 95% CI, 1.2-3.3; NNT=4), increased number of blood transfusions (4 studies, 170 patients; WMD=1.2; 95% CI, 0.52-1.8), and increased rate of intraventricular hemorrhage (RR=1.9; 95% CI, 1.3-2.8; NNT=8).
Recommendations
The World Health Organization (WHO) recommends against clamping the umbilical cord any earlier than is necessary to apply traction to the placenta in the active management of the third state of labor.4 (WHO estimates this would normally take around 3 minutes.) Early clamping may be required if the baby is asphyxiated and needs immediate resuscitation.
The Society of Obstetricians and Gynecologists of Canada recommends delaying cord clamping by at least 60 seconds in premature newborns (<37 weeks’ gestation) to reduce the risk of intraventricular hemorrhage and the need for transfusion.5 For term newborns, the Society advises clinicians to weigh the increased risk of neonatal jaundice against the benefit of greater iron stores on a case-by-case basis.
1. McDonald SJ, Middleton P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2008;(2):CD004074.-
2. Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA. 2007;297:1241-1252.
3. Rabe H, Reynolds G, Diaz-Rossello J. A systematic review and meta-analysis of a brief delay in clamping the umbilical cord of preterm infants. Neonatology. 2008;93:138-144.
4. Abalos E. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes: RHL commentary (last revised March 2, 2009). In: The WHO Reproductive Health Library; Geneva, Switzerland: World Health Organization. Available at: http://apps.who.int/rhl/pregnancy_childbirth/childbirth/3rd_stage/cd004074_abalose_com/en/index.html. Accessed July 21, 2010.
5. Leduc D, Senikas V, Lalonde AB, et al. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can. 2009;31:980-993.
SOMETIME BETWEEN 30 SECONDS AND 2 MINUTES after delivery appears to be the best interval. In term infants, delayed clamping (waiting 1 or 2 minutes or until the cord stops pulsating) improves hemoglobin and ferritin levels, but slightly increases the risk of neonatal jaundice requiring phototherapy (strength of recommendation [SOR]: A, meta-analysis).
In preterm infants less than 37 weeks of age, cord clamping between 30 and 120 seconds after delivery reduces the need for blood transfusion (number needed to treat [NNT]=4) and frequency of intraventricular hemorrhage (NNT=8) compared with clamping in less than 20 seconds (SOR: A, meta-analyses).
Evidence summary
A 2008 Cochrane meta-analysis reviewed 11 randomized controlled trials (RCTs), enrolling more than 2900 women who had term vaginal deliveries, that compared early cord clamping (ECC) with delayed cord clamping (DCC).1 All of the trials defined ECC as clamping less than 1 minute after birth. DCC was variously defined as clamping after 1 minute, after 2 minutes, or after the cord stopped pulsating.
DCC was associated with increased newborn hemoglobin values (weighted mean difference [WMD]=2.2 g/dL; 95% confidence interval [CI], 0.3-4.1) and increased mean ferritin levels that persisted for as long as 6 months (WMD=12 mcg/L; 95% CI, 4.1-20). However, significantly fewer infants who underwent ECC required phototherapy for jaundice (relative risk [RR]=0.59; 95% CI, 0.38-0.92; NNT=45).
This meta-analysis was limited by variations in the definition of DCC, the level at which newborns were held in relation to the placenta (above, below, or level with), and the use of uterotonics. These limitations also apply to the other systematic reviews discussed here.
But is hyperbilirubinemia significant?
A 2007 meta-analysis of 15 clinical trials (8 randomized and 7 nonrandomized) with 1001 term infants in the DCC group and 911 in the ECC group found results similar to the Cochrane review.2 When compared with ECC, delayed clamping at least 2 minutes after birth was associated with significantly higher hematocrit (WMD=3.7%; 95% CI, 2-4), ferritin (WMD=18 mcg/L; 95% CI, 17-19), and stored iron (WMD=20 mg; 95% CI, 8-32), as well as decreased risk of anemia (RR=0.5; 95% CI, 0.4-0.7).
Infants in the DCC group had an increased risk of asymptomatic polycythemia (RR=3.9; 95% CI, 1.0-15). Delayed clamping was also associated with an increased rate of phototherapy for hyperbilirubinemia that didn’t reach statistical significance, although the confidence interval was wide (RR=1.78; 95% CI, 0.71-4.46).
Early clamping poses risks for preterm infants
A 2008 meta-analysis (using Cochrane methodology) identified 10 RCTs enrolling 454 infants born at less than 37 weeks’ gestation.3 ECC was defined as less than 20 seconds after delivery and DCC as greater than 30 seconds (and up to 120 seconds).
The review found ECC to be inferior to DCC. Early clamping was associated with an increased risk of transfusion for anemia (3 studies, 112 patients; RR=2.1; 95% CI, 1.2-3.3; NNT=4), increased number of blood transfusions (4 studies, 170 patients; WMD=1.2; 95% CI, 0.52-1.8), and increased rate of intraventricular hemorrhage (RR=1.9; 95% CI, 1.3-2.8; NNT=8).
Recommendations
The World Health Organization (WHO) recommends against clamping the umbilical cord any earlier than is necessary to apply traction to the placenta in the active management of the third state of labor.4 (WHO estimates this would normally take around 3 minutes.) Early clamping may be required if the baby is asphyxiated and needs immediate resuscitation.
The Society of Obstetricians and Gynecologists of Canada recommends delaying cord clamping by at least 60 seconds in premature newborns (<37 weeks’ gestation) to reduce the risk of intraventricular hemorrhage and the need for transfusion.5 For term newborns, the Society advises clinicians to weigh the increased risk of neonatal jaundice against the benefit of greater iron stores on a case-by-case basis.
SOMETIME BETWEEN 30 SECONDS AND 2 MINUTES after delivery appears to be the best interval. In term infants, delayed clamping (waiting 1 or 2 minutes or until the cord stops pulsating) improves hemoglobin and ferritin levels, but slightly increases the risk of neonatal jaundice requiring phototherapy (strength of recommendation [SOR]: A, meta-analysis).
In preterm infants less than 37 weeks of age, cord clamping between 30 and 120 seconds after delivery reduces the need for blood transfusion (number needed to treat [NNT]=4) and frequency of intraventricular hemorrhage (NNT=8) compared with clamping in less than 20 seconds (SOR: A, meta-analyses).
Evidence summary
A 2008 Cochrane meta-analysis reviewed 11 randomized controlled trials (RCTs), enrolling more than 2900 women who had term vaginal deliveries, that compared early cord clamping (ECC) with delayed cord clamping (DCC).1 All of the trials defined ECC as clamping less than 1 minute after birth. DCC was variously defined as clamping after 1 minute, after 2 minutes, or after the cord stopped pulsating.
DCC was associated with increased newborn hemoglobin values (weighted mean difference [WMD]=2.2 g/dL; 95% confidence interval [CI], 0.3-4.1) and increased mean ferritin levels that persisted for as long as 6 months (WMD=12 mcg/L; 95% CI, 4.1-20). However, significantly fewer infants who underwent ECC required phototherapy for jaundice (relative risk [RR]=0.59; 95% CI, 0.38-0.92; NNT=45).
This meta-analysis was limited by variations in the definition of DCC, the level at which newborns were held in relation to the placenta (above, below, or level with), and the use of uterotonics. These limitations also apply to the other systematic reviews discussed here.
But is hyperbilirubinemia significant?
A 2007 meta-analysis of 15 clinical trials (8 randomized and 7 nonrandomized) with 1001 term infants in the DCC group and 911 in the ECC group found results similar to the Cochrane review.2 When compared with ECC, delayed clamping at least 2 minutes after birth was associated with significantly higher hematocrit (WMD=3.7%; 95% CI, 2-4), ferritin (WMD=18 mcg/L; 95% CI, 17-19), and stored iron (WMD=20 mg; 95% CI, 8-32), as well as decreased risk of anemia (RR=0.5; 95% CI, 0.4-0.7).
Infants in the DCC group had an increased risk of asymptomatic polycythemia (RR=3.9; 95% CI, 1.0-15). Delayed clamping was also associated with an increased rate of phototherapy for hyperbilirubinemia that didn’t reach statistical significance, although the confidence interval was wide (RR=1.78; 95% CI, 0.71-4.46).
Early clamping poses risks for preterm infants
A 2008 meta-analysis (using Cochrane methodology) identified 10 RCTs enrolling 454 infants born at less than 37 weeks’ gestation.3 ECC was defined as less than 20 seconds after delivery and DCC as greater than 30 seconds (and up to 120 seconds).
The review found ECC to be inferior to DCC. Early clamping was associated with an increased risk of transfusion for anemia (3 studies, 112 patients; RR=2.1; 95% CI, 1.2-3.3; NNT=4), increased number of blood transfusions (4 studies, 170 patients; WMD=1.2; 95% CI, 0.52-1.8), and increased rate of intraventricular hemorrhage (RR=1.9; 95% CI, 1.3-2.8; NNT=8).
Recommendations
The World Health Organization (WHO) recommends against clamping the umbilical cord any earlier than is necessary to apply traction to the placenta in the active management of the third state of labor.4 (WHO estimates this would normally take around 3 minutes.) Early clamping may be required if the baby is asphyxiated and needs immediate resuscitation.
The Society of Obstetricians and Gynecologists of Canada recommends delaying cord clamping by at least 60 seconds in premature newborns (<37 weeks’ gestation) to reduce the risk of intraventricular hemorrhage and the need for transfusion.5 For term newborns, the Society advises clinicians to weigh the increased risk of neonatal jaundice against the benefit of greater iron stores on a case-by-case basis.
1. McDonald SJ, Middleton P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2008;(2):CD004074.-
2. Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA. 2007;297:1241-1252.
3. Rabe H, Reynolds G, Diaz-Rossello J. A systematic review and meta-analysis of a brief delay in clamping the umbilical cord of preterm infants. Neonatology. 2008;93:138-144.
4. Abalos E. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes: RHL commentary (last revised March 2, 2009). In: The WHO Reproductive Health Library; Geneva, Switzerland: World Health Organization. Available at: http://apps.who.int/rhl/pregnancy_childbirth/childbirth/3rd_stage/cd004074_abalose_com/en/index.html. Accessed July 21, 2010.
5. Leduc D, Senikas V, Lalonde AB, et al. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can. 2009;31:980-993.
1. McDonald SJ, Middleton P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2008;(2):CD004074.-
2. Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA. 2007;297:1241-1252.
3. Rabe H, Reynolds G, Diaz-Rossello J. A systematic review and meta-analysis of a brief delay in clamping the umbilical cord of preterm infants. Neonatology. 2008;93:138-144.
4. Abalos E. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes: RHL commentary (last revised March 2, 2009). In: The WHO Reproductive Health Library; Geneva, Switzerland: World Health Organization. Available at: http://apps.who.int/rhl/pregnancy_childbirth/childbirth/3rd_stage/cd004074_abalose_com/en/index.html. Accessed July 21, 2010.
5. Leduc D, Senikas V, Lalonde AB, et al. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can. 2009;31:980-993.
Evidence-based answers from the Family Physicians Inquiries Network
What is the prognosis for patients with chronic urticaria?
THE PROGNOSIS FOR CHRONIC URTICARIA IN PRIMARY CARE IS UNKNOWN; studies in dermatology clinics in multiple countries report complete resolution in approximately one-third of patients with idiopathic chronic urticaria over 1 to 5 years and partial improvement in another third. Patients younger than 30 years with more severe symptoms, or symptoms with physical causes, fared less well (strength of recommendation: B, cohort studies).
Evidence summary
A prospective cohort study of 220 patients from an outpatient dermatology center in Amsterdam investigated the natural course of chronic urticaria and angioedema.1 Researchers categorized patients according to subtypes: idiopathic urticaria-angioedema, idiopathic urticaria, idiopathic angioedema, physical and idiopathic urticaria, and physical urticaria only.
The duration of symptoms at enrollment wasn’t reported. Therapy wasn’t controlled and was composed of oral antihistamines, steroids, and other drugs.
One year after enrollment, 35% of patients had complete resolution of symptoms. Resolution rates ranged from a high of 59.6% in patients with idiopathic urticaria-angioedema to a low of 16.4% in patients who had urticaria with a physical cause.
A study finds 1-year control or improvement in chronic urticaria
Another prospective cohort study from an outpatient dermatology center in Brazil evaluated 125 patients with chronic urticaria-angioedema.2 Participants were predominantly adults 20 to 40 years of age, with a mean duration of symptoms of 45 months.
Most patients had idiopathic disease (78%), but some had parasitic and skin infections, medication sensitivities, thyroid disease, and other problems that could contribute to skin hyperreactivity. Therapeutic interventions for underlying conditions or angioedema-urticaria weren’t controlled or reported.
One year after presentation, 58.4% of patients had symptoms “under control,” 31.7% were improved, and 8.9% were unchanged. One patient’s symptoms worsened.
Urticaria is less severe in patients older than 30 years
A prospective cohort study followed 62 patients with urticaria caused by cold from a tertiary referral center in Greece.3 The mean age at presentation was 42 years and the mean duration of symptoms was 10 years. The study followed patients for a mean of 9 years. Therapeutic interventions weren’t controlled or reported
Overall, 29% of patients experienced resolution of symptoms, 41.9% noted improvement, and 29% experienced worsening of symptoms. The mean time to resolution was 5.6 years. The study also found that chronic urticaria was less severe if patients developed the condition after 30 years of age.
Worst prognosis found in patients with cold-related urticaria
A retrospective cohort study identified 544 cases of chronic urticaria and angioedema in 22 years of records from a tertiary referral center in the Netherlands.4 The mean age at presentation was 35 years; patients had been symptomatic an average of 5 years. All patients were sent a questionnaire to fill out; 372 questionnaires were returned.
At 5 years after presentation, symptoms resolved in 29% of patients; at 10 years, the number of resolved cases increased to 44%. Patients with cold-related urticaria had the worst prognosis.
Zafirlukast has no effect
An RCT with a study group of 137 patients (mean age 41 years) compared the effectiveness of zafirlukast vs placebo for treating chronic urticaria symptoms. Zafirlukast showed no significant benefit over placebo; symptoms resolved or improved in 41.3% of all patients after 12 weeks.5
Recommendations
Habif’s Clinical Dermatology states that chronic urticaria:
- may last for months or years
- may be subject to lengthy and often un-rewarding evaluation
- resolves spontaneously in most cases.6
1. Kozel MM, Mekkes JR, Bossuyt PM, et al. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45:387-391.
2. Silvares MR, Coelho KI, Dalben I, et al. Sociodemographic and clinical characteristics, causal factors and evolution of a group of patients with chronic urticaria-angioedema. Sao Paulo Med J. 2007;125:281-285.
3. Katsarou-Katsari A, Makris M, Lagogianni E, et al. Clinical features and natural history of acquired cold urticaria in a tertiary referral hospital: a 10-year prospective study. J Eur Acad Dermatol Venereol. 2008;22:1405-1411.
4. van der Valk PG, Moret G, Kiemeney LA. The natural history of chronic urticaria and angioedema in patients visiting a tertiary referral centre. Br J Dermatol. 2003;146:110-113.
5. Reimers A, Pichler C, Helbing A, et al. Zafirlukast has no beneficial effects in the treatment of chronic urticaria. Clin Exp Allergy. 2002;32:1763-1768.
6. Habif TP. Urticaria and angioedema. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004:129–161.
THE PROGNOSIS FOR CHRONIC URTICARIA IN PRIMARY CARE IS UNKNOWN; studies in dermatology clinics in multiple countries report complete resolution in approximately one-third of patients with idiopathic chronic urticaria over 1 to 5 years and partial improvement in another third. Patients younger than 30 years with more severe symptoms, or symptoms with physical causes, fared less well (strength of recommendation: B, cohort studies).
Evidence summary
A prospective cohort study of 220 patients from an outpatient dermatology center in Amsterdam investigated the natural course of chronic urticaria and angioedema.1 Researchers categorized patients according to subtypes: idiopathic urticaria-angioedema, idiopathic urticaria, idiopathic angioedema, physical and idiopathic urticaria, and physical urticaria only.
The duration of symptoms at enrollment wasn’t reported. Therapy wasn’t controlled and was composed of oral antihistamines, steroids, and other drugs.
One year after enrollment, 35% of patients had complete resolution of symptoms. Resolution rates ranged from a high of 59.6% in patients with idiopathic urticaria-angioedema to a low of 16.4% in patients who had urticaria with a physical cause.
A study finds 1-year control or improvement in chronic urticaria
Another prospective cohort study from an outpatient dermatology center in Brazil evaluated 125 patients with chronic urticaria-angioedema.2 Participants were predominantly adults 20 to 40 years of age, with a mean duration of symptoms of 45 months.
Most patients had idiopathic disease (78%), but some had parasitic and skin infections, medication sensitivities, thyroid disease, and other problems that could contribute to skin hyperreactivity. Therapeutic interventions for underlying conditions or angioedema-urticaria weren’t controlled or reported.
One year after presentation, 58.4% of patients had symptoms “under control,” 31.7% were improved, and 8.9% were unchanged. One patient’s symptoms worsened.
Urticaria is less severe in patients older than 30 years
A prospective cohort study followed 62 patients with urticaria caused by cold from a tertiary referral center in Greece.3 The mean age at presentation was 42 years and the mean duration of symptoms was 10 years. The study followed patients for a mean of 9 years. Therapeutic interventions weren’t controlled or reported
Overall, 29% of patients experienced resolution of symptoms, 41.9% noted improvement, and 29% experienced worsening of symptoms. The mean time to resolution was 5.6 years. The study also found that chronic urticaria was less severe if patients developed the condition after 30 years of age.
Worst prognosis found in patients with cold-related urticaria
A retrospective cohort study identified 544 cases of chronic urticaria and angioedema in 22 years of records from a tertiary referral center in the Netherlands.4 The mean age at presentation was 35 years; patients had been symptomatic an average of 5 years. All patients were sent a questionnaire to fill out; 372 questionnaires were returned.
At 5 years after presentation, symptoms resolved in 29% of patients; at 10 years, the number of resolved cases increased to 44%. Patients with cold-related urticaria had the worst prognosis.
Zafirlukast has no effect
An RCT with a study group of 137 patients (mean age 41 years) compared the effectiveness of zafirlukast vs placebo for treating chronic urticaria symptoms. Zafirlukast showed no significant benefit over placebo; symptoms resolved or improved in 41.3% of all patients after 12 weeks.5
Recommendations
Habif’s Clinical Dermatology states that chronic urticaria:
- may last for months or years
- may be subject to lengthy and often un-rewarding evaluation
- resolves spontaneously in most cases.6
THE PROGNOSIS FOR CHRONIC URTICARIA IN PRIMARY CARE IS UNKNOWN; studies in dermatology clinics in multiple countries report complete resolution in approximately one-third of patients with idiopathic chronic urticaria over 1 to 5 years and partial improvement in another third. Patients younger than 30 years with more severe symptoms, or symptoms with physical causes, fared less well (strength of recommendation: B, cohort studies).
Evidence summary
A prospective cohort study of 220 patients from an outpatient dermatology center in Amsterdam investigated the natural course of chronic urticaria and angioedema.1 Researchers categorized patients according to subtypes: idiopathic urticaria-angioedema, idiopathic urticaria, idiopathic angioedema, physical and idiopathic urticaria, and physical urticaria only.
The duration of symptoms at enrollment wasn’t reported. Therapy wasn’t controlled and was composed of oral antihistamines, steroids, and other drugs.
One year after enrollment, 35% of patients had complete resolution of symptoms. Resolution rates ranged from a high of 59.6% in patients with idiopathic urticaria-angioedema to a low of 16.4% in patients who had urticaria with a physical cause.
A study finds 1-year control or improvement in chronic urticaria
Another prospective cohort study from an outpatient dermatology center in Brazil evaluated 125 patients with chronic urticaria-angioedema.2 Participants were predominantly adults 20 to 40 years of age, with a mean duration of symptoms of 45 months.
Most patients had idiopathic disease (78%), but some had parasitic and skin infections, medication sensitivities, thyroid disease, and other problems that could contribute to skin hyperreactivity. Therapeutic interventions for underlying conditions or angioedema-urticaria weren’t controlled or reported.
One year after presentation, 58.4% of patients had symptoms “under control,” 31.7% were improved, and 8.9% were unchanged. One patient’s symptoms worsened.
Urticaria is less severe in patients older than 30 years
A prospective cohort study followed 62 patients with urticaria caused by cold from a tertiary referral center in Greece.3 The mean age at presentation was 42 years and the mean duration of symptoms was 10 years. The study followed patients for a mean of 9 years. Therapeutic interventions weren’t controlled or reported
Overall, 29% of patients experienced resolution of symptoms, 41.9% noted improvement, and 29% experienced worsening of symptoms. The mean time to resolution was 5.6 years. The study also found that chronic urticaria was less severe if patients developed the condition after 30 years of age.
Worst prognosis found in patients with cold-related urticaria
A retrospective cohort study identified 544 cases of chronic urticaria and angioedema in 22 years of records from a tertiary referral center in the Netherlands.4 The mean age at presentation was 35 years; patients had been symptomatic an average of 5 years. All patients were sent a questionnaire to fill out; 372 questionnaires were returned.
At 5 years after presentation, symptoms resolved in 29% of patients; at 10 years, the number of resolved cases increased to 44%. Patients with cold-related urticaria had the worst prognosis.
Zafirlukast has no effect
An RCT with a study group of 137 patients (mean age 41 years) compared the effectiveness of zafirlukast vs placebo for treating chronic urticaria symptoms. Zafirlukast showed no significant benefit over placebo; symptoms resolved or improved in 41.3% of all patients after 12 weeks.5
Recommendations
Habif’s Clinical Dermatology states that chronic urticaria:
- may last for months or years
- may be subject to lengthy and often un-rewarding evaluation
- resolves spontaneously in most cases.6
1. Kozel MM, Mekkes JR, Bossuyt PM, et al. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45:387-391.
2. Silvares MR, Coelho KI, Dalben I, et al. Sociodemographic and clinical characteristics, causal factors and evolution of a group of patients with chronic urticaria-angioedema. Sao Paulo Med J. 2007;125:281-285.
3. Katsarou-Katsari A, Makris M, Lagogianni E, et al. Clinical features and natural history of acquired cold urticaria in a tertiary referral hospital: a 10-year prospective study. J Eur Acad Dermatol Venereol. 2008;22:1405-1411.
4. van der Valk PG, Moret G, Kiemeney LA. The natural history of chronic urticaria and angioedema in patients visiting a tertiary referral centre. Br J Dermatol. 2003;146:110-113.
5. Reimers A, Pichler C, Helbing A, et al. Zafirlukast has no beneficial effects in the treatment of chronic urticaria. Clin Exp Allergy. 2002;32:1763-1768.
6. Habif TP. Urticaria and angioedema. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004:129–161.
1. Kozel MM, Mekkes JR, Bossuyt PM, et al. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45:387-391.
2. Silvares MR, Coelho KI, Dalben I, et al. Sociodemographic and clinical characteristics, causal factors and evolution of a group of patients with chronic urticaria-angioedema. Sao Paulo Med J. 2007;125:281-285.
3. Katsarou-Katsari A, Makris M, Lagogianni E, et al. Clinical features and natural history of acquired cold urticaria in a tertiary referral hospital: a 10-year prospective study. J Eur Acad Dermatol Venereol. 2008;22:1405-1411.
4. van der Valk PG, Moret G, Kiemeney LA. The natural history of chronic urticaria and angioedema in patients visiting a tertiary referral centre. Br J Dermatol. 2003;146:110-113.
5. Reimers A, Pichler C, Helbing A, et al. Zafirlukast has no beneficial effects in the treatment of chronic urticaria. Clin Exp Allergy. 2002;32:1763-1768.
6. Habif TP. Urticaria and angioedema. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004:129–161.
Evidence-based answers from the Family Physicians Inquiries Network
Do intra-articular steroid injections affect glycemic control in patients with diabetes?
YES, BUT THE CLINICAL IMPORTANCE IS MINIMAL. A single intra-articular steroid injection into the knee produces acute hyperglycemia for 2 or 3 days in patients with diabetes who otherwise have good glucose control (strength of recommendation [SOR]: B, small cohort studies).
Intra-articular steroid injections into the shoulder may briefly raise postprandial (but not mean) glucose levels with larger and repeated doses (SOR: C, extrapolated from heterogenous and mixed cohort studies).
Evidence summary
Two prospective cohort studies evaluated the effect on glycemic control of a single glucocorticoid injection into the knee of patients with controlled type 2 diabetes (glycosylated hemoglobin A1c <7.0%). The first enrolled 9 patients with symptomatic osteoarthritis of the knee unresponsive to 3 months of nonsteroidal anti-inflammatory drugs (NSAIDs).1 All received a 50-mg injection of methylprednisolone acetate after maximal aspiration of any joint fluid. No changes were made to the diabetes care regimen, including medication, diet, or exercise prescriptions.
With self-monitoring 6 times a day during the week after the injection, 7 patients showed an increase over baseline blood glucose levels of more than 2 standard deviations; values typically rose above 300 mg/dL. Peak blood sugar elevation occurred 5 to 84 hours after injection; the hyperglycemic effect lasted for 2 or 3 days.
In a second cohort study, 6 patients received a knee injection of 1 mL Celestone Chronodose (3 mg betamethasone acetate and 3 mg betamethasone sodium phosphate, comparable in anti-inflammatory and glucocorticoid potency to 32 mg methylprednisolone acetate).2 Patients monitored their blood glucose 6 times a day for 1 week; investigators measured fructosamine levels (a measure of intermediate-term glucose control) at baseline and again 2 weeks after injection.
The injection produced hyperglycemia in all participants, with peak blood glucose levels ranging from 251 to 430 mg/dL and time to peak glucose usually less than 6 hours. Fructosamine levels didn’t change significantly.
No change in glucose after a single shoulder injection
Two studies evaluated the effect of a single shoulder injection. One prospective cohort study included 18 patients with diabetes (type not specified, mean A1c=7.6%).3 All had shoulder pain unresponsive to NSAIDs for more than a month, had not changed diabetes medications within the preceding 2 weeks, and had not had steroid therapy within the preceding 3 months.
All patients received a single injection containing 35 mg methylprednisolone acetate into the anterior glenohumeral joint. They monitored their blood glucose levels 6 times a day for 1 week and had a fructosamine level drawn before injection and 2 weeks afterward. The injection produced no significant change in mean blood glucose or fructosamine levels.
But repeated shoulder injections raise postprandial glucose
In contrast, another prospective cohort study followed 11 patients (8 with diabetes) who received 3 injections of 3.75 mg cortivazol (comparable to 50 mg methylprednisolone acetate) at 3-day intervals into 1 shoulder joint.4 Investigators checked fasting and postprandial glucose levels before the first injection and on post-treatment days 1, 7, and 21.
The shoulder injections elevated post-prandial glucose levels (from 170±60 mg/dL at baseline to 258±100 mg/dL on day 1 and 252±87 mg/dL on day 7; P<.05 for both comparisons). Mean fasting glucose levels didn’t change, however.
Recommendations
The American Academy of Orthopedic Surgeons treatment guidelines for osteoarthritis of the knee don’t discuss possible adverse effects from steroid injections.5 The American Diabetes Association makes no recommendations regarding steroid injections in patients with diabetes.
1. Habib GS, Bashir M, Jabbour A. Increased blood glucose levels following intra-articular injection of methylprednisolone acetate in patients with controlled diabetes and symptomatic osteoarthritis of the knee. Ann Rheum Dis. 2008;67:1790-1791.
2. Habib G, Safia A. The effect of intra-articular injection of beta-methasone acetate/betamethasone sodium phosphate on blood glucose levels in controlled diabetic patients with symptomatic osteoarthritis of the knee. Clin Rheumatol. 2009;28:85-87.
3. Habib GS, Abu-Ahmad R. Lack of effect of corticosteroid injection at the shoulder joint on blood glucose levels in diabetic patients. Clin Rheumatol. 2007;26:566-568.
4. Younes M, Neffati F, Touzi M, et al. Systemic effects of epidural and intra-articular glucocorticoid injections in diabetic and non-diabetic patients. Joint Bone Spine. 2007;74:472-476.
5. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee (non-arthoplasty). Available at: www.aaos.org/research/guidelines/OAKguideline.pdf. Accessed July 2, 2009.
YES, BUT THE CLINICAL IMPORTANCE IS MINIMAL. A single intra-articular steroid injection into the knee produces acute hyperglycemia for 2 or 3 days in patients with diabetes who otherwise have good glucose control (strength of recommendation [SOR]: B, small cohort studies).
Intra-articular steroid injections into the shoulder may briefly raise postprandial (but not mean) glucose levels with larger and repeated doses (SOR: C, extrapolated from heterogenous and mixed cohort studies).
Evidence summary
Two prospective cohort studies evaluated the effect on glycemic control of a single glucocorticoid injection into the knee of patients with controlled type 2 diabetes (glycosylated hemoglobin A1c <7.0%). The first enrolled 9 patients with symptomatic osteoarthritis of the knee unresponsive to 3 months of nonsteroidal anti-inflammatory drugs (NSAIDs).1 All received a 50-mg injection of methylprednisolone acetate after maximal aspiration of any joint fluid. No changes were made to the diabetes care regimen, including medication, diet, or exercise prescriptions.
With self-monitoring 6 times a day during the week after the injection, 7 patients showed an increase over baseline blood glucose levels of more than 2 standard deviations; values typically rose above 300 mg/dL. Peak blood sugar elevation occurred 5 to 84 hours after injection; the hyperglycemic effect lasted for 2 or 3 days.
In a second cohort study, 6 patients received a knee injection of 1 mL Celestone Chronodose (3 mg betamethasone acetate and 3 mg betamethasone sodium phosphate, comparable in anti-inflammatory and glucocorticoid potency to 32 mg methylprednisolone acetate).2 Patients monitored their blood glucose 6 times a day for 1 week; investigators measured fructosamine levels (a measure of intermediate-term glucose control) at baseline and again 2 weeks after injection.
The injection produced hyperglycemia in all participants, with peak blood glucose levels ranging from 251 to 430 mg/dL and time to peak glucose usually less than 6 hours. Fructosamine levels didn’t change significantly.
No change in glucose after a single shoulder injection
Two studies evaluated the effect of a single shoulder injection. One prospective cohort study included 18 patients with diabetes (type not specified, mean A1c=7.6%).3 All had shoulder pain unresponsive to NSAIDs for more than a month, had not changed diabetes medications within the preceding 2 weeks, and had not had steroid therapy within the preceding 3 months.
All patients received a single injection containing 35 mg methylprednisolone acetate into the anterior glenohumeral joint. They monitored their blood glucose levels 6 times a day for 1 week and had a fructosamine level drawn before injection and 2 weeks afterward. The injection produced no significant change in mean blood glucose or fructosamine levels.
But repeated shoulder injections raise postprandial glucose
In contrast, another prospective cohort study followed 11 patients (8 with diabetes) who received 3 injections of 3.75 mg cortivazol (comparable to 50 mg methylprednisolone acetate) at 3-day intervals into 1 shoulder joint.4 Investigators checked fasting and postprandial glucose levels before the first injection and on post-treatment days 1, 7, and 21.
The shoulder injections elevated post-prandial glucose levels (from 170±60 mg/dL at baseline to 258±100 mg/dL on day 1 and 252±87 mg/dL on day 7; P<.05 for both comparisons). Mean fasting glucose levels didn’t change, however.
Recommendations
The American Academy of Orthopedic Surgeons treatment guidelines for osteoarthritis of the knee don’t discuss possible adverse effects from steroid injections.5 The American Diabetes Association makes no recommendations regarding steroid injections in patients with diabetes.
YES, BUT THE CLINICAL IMPORTANCE IS MINIMAL. A single intra-articular steroid injection into the knee produces acute hyperglycemia for 2 or 3 days in patients with diabetes who otherwise have good glucose control (strength of recommendation [SOR]: B, small cohort studies).
Intra-articular steroid injections into the shoulder may briefly raise postprandial (but not mean) glucose levels with larger and repeated doses (SOR: C, extrapolated from heterogenous and mixed cohort studies).
Evidence summary
Two prospective cohort studies evaluated the effect on glycemic control of a single glucocorticoid injection into the knee of patients with controlled type 2 diabetes (glycosylated hemoglobin A1c <7.0%). The first enrolled 9 patients with symptomatic osteoarthritis of the knee unresponsive to 3 months of nonsteroidal anti-inflammatory drugs (NSAIDs).1 All received a 50-mg injection of methylprednisolone acetate after maximal aspiration of any joint fluid. No changes were made to the diabetes care regimen, including medication, diet, or exercise prescriptions.
With self-monitoring 6 times a day during the week after the injection, 7 patients showed an increase over baseline blood glucose levels of more than 2 standard deviations; values typically rose above 300 mg/dL. Peak blood sugar elevation occurred 5 to 84 hours after injection; the hyperglycemic effect lasted for 2 or 3 days.
In a second cohort study, 6 patients received a knee injection of 1 mL Celestone Chronodose (3 mg betamethasone acetate and 3 mg betamethasone sodium phosphate, comparable in anti-inflammatory and glucocorticoid potency to 32 mg methylprednisolone acetate).2 Patients monitored their blood glucose 6 times a day for 1 week; investigators measured fructosamine levels (a measure of intermediate-term glucose control) at baseline and again 2 weeks after injection.
The injection produced hyperglycemia in all participants, with peak blood glucose levels ranging from 251 to 430 mg/dL and time to peak glucose usually less than 6 hours. Fructosamine levels didn’t change significantly.
No change in glucose after a single shoulder injection
Two studies evaluated the effect of a single shoulder injection. One prospective cohort study included 18 patients with diabetes (type not specified, mean A1c=7.6%).3 All had shoulder pain unresponsive to NSAIDs for more than a month, had not changed diabetes medications within the preceding 2 weeks, and had not had steroid therapy within the preceding 3 months.
All patients received a single injection containing 35 mg methylprednisolone acetate into the anterior glenohumeral joint. They monitored their blood glucose levels 6 times a day for 1 week and had a fructosamine level drawn before injection and 2 weeks afterward. The injection produced no significant change in mean blood glucose or fructosamine levels.
But repeated shoulder injections raise postprandial glucose
In contrast, another prospective cohort study followed 11 patients (8 with diabetes) who received 3 injections of 3.75 mg cortivazol (comparable to 50 mg methylprednisolone acetate) at 3-day intervals into 1 shoulder joint.4 Investigators checked fasting and postprandial glucose levels before the first injection and on post-treatment days 1, 7, and 21.
The shoulder injections elevated post-prandial glucose levels (from 170±60 mg/dL at baseline to 258±100 mg/dL on day 1 and 252±87 mg/dL on day 7; P<.05 for both comparisons). Mean fasting glucose levels didn’t change, however.
Recommendations
The American Academy of Orthopedic Surgeons treatment guidelines for osteoarthritis of the knee don’t discuss possible adverse effects from steroid injections.5 The American Diabetes Association makes no recommendations regarding steroid injections in patients with diabetes.
1. Habib GS, Bashir M, Jabbour A. Increased blood glucose levels following intra-articular injection of methylprednisolone acetate in patients with controlled diabetes and symptomatic osteoarthritis of the knee. Ann Rheum Dis. 2008;67:1790-1791.
2. Habib G, Safia A. The effect of intra-articular injection of beta-methasone acetate/betamethasone sodium phosphate on blood glucose levels in controlled diabetic patients with symptomatic osteoarthritis of the knee. Clin Rheumatol. 2009;28:85-87.
3. Habib GS, Abu-Ahmad R. Lack of effect of corticosteroid injection at the shoulder joint on blood glucose levels in diabetic patients. Clin Rheumatol. 2007;26:566-568.
4. Younes M, Neffati F, Touzi M, et al. Systemic effects of epidural and intra-articular glucocorticoid injections in diabetic and non-diabetic patients. Joint Bone Spine. 2007;74:472-476.
5. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee (non-arthoplasty). Available at: www.aaos.org/research/guidelines/OAKguideline.pdf. Accessed July 2, 2009.
1. Habib GS, Bashir M, Jabbour A. Increased blood glucose levels following intra-articular injection of methylprednisolone acetate in patients with controlled diabetes and symptomatic osteoarthritis of the knee. Ann Rheum Dis. 2008;67:1790-1791.
2. Habib G, Safia A. The effect of intra-articular injection of beta-methasone acetate/betamethasone sodium phosphate on blood glucose levels in controlled diabetic patients with symptomatic osteoarthritis of the knee. Clin Rheumatol. 2009;28:85-87.
3. Habib GS, Abu-Ahmad R. Lack of effect of corticosteroid injection at the shoulder joint on blood glucose levels in diabetic patients. Clin Rheumatol. 2007;26:566-568.
4. Younes M, Neffati F, Touzi M, et al. Systemic effects of epidural and intra-articular glucocorticoid injections in diabetic and non-diabetic patients. Joint Bone Spine. 2007;74:472-476.
5. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee (non-arthoplasty). Available at: www.aaos.org/research/guidelines/OAKguideline.pdf. Accessed July 2, 2009.
Evidence-based answers from the Family Physicians Inquiries Network
What’s the best approach to diagnosing food allergies in infants?
A WELL-DESIGNED ORAL FOOD CHALLENGE (OFC) is the most reliable diagnostic test for infants whose clinical history and physical examination suggest a specific food allergy (strength of recommendation [SOR]: C, consensus guidelines).
Serum-specific immunoglobulin E (IgE), atopy patch testing (APT), and skin prick testing (SPT) are all alternatives to OFC, but the likelihood ratios are not robust and the tests vary widely in sensitivity and specificity to different allergens. For diagnosing egg and milk allergies, larger wheal sizes with SPT are more predictive of a positive OFC (SOR: C, extrapolated from cohort studies evaluating mixed populations of infants, children, and teenagers).
Evidence summary
The American Academy of Allergy, Asthma and Immunology (AAAAI) states that a double-blind, placebo-controlled OFC is the best test for diagnosing infants clinically suspected of having food allergies (that is, who develop gastrointestinal symptoms after eating a specific food). However, performing an OFC in an infant is often difficult and potentially dangerous, especially if a severe allergy is suspected; testing also may eliminate nutritious foods, such as milk and eggs, from the infant’s diet.1 For these reasons, physicians have sought simpler alternatives to the OFC.
Comparisons of serum and skin testing with OFCs produce variable, weak results
Two large cohort studies compared OFCs with serum and skin testing in infants, children, and teenagers. Overall, serum and skin testing didn’t produce robust results and the results varied with the antigen (TABLE).
TABLE
How allergy tests in infants and children compare with an oral food challenge
| Test* | Sensitivity (%) | Specificity (%) | LR+ | LR- |
|---|---|---|---|---|
| Milk | ||||
| IgE2 | 83 | 53 | 1.8 | 0.32 |
| SPT3 | 85 | 70 | 2.8 | 0.21 |
| APT3 | 31 | 95 | 6.2 | 0.73 |
| Egg | ||||
| IgE2 | 97 | 51 | 2.0 | 0.59 |
| SPT3 | 93 | 54 | 2.0 | 0.13 |
| APT3 | 41 | 87 | 3.2 | 0.68 |
| Wheat | ||||
| IgE2 | 79 | 38 | 1.3 | 0.55 |
| SPT3 | 75 | 64 | 2.1 | 0.39 |
| APT3 | 27 | 89 | 2.5 | 0.82 |
| Soy | ||||
| IgE2 | 69 | 50 | 1.4 | 0.31 |
| SPT3 | 29 | 85 | 1.9 | 0.84 |
| APT3 | 23 | 86 | 1.6 | 0.90 |
| APT, atopy patch test; IgE, serum immunoglobulin E; LR+, positive likelihood ratio; LR–, negative likelihood ratio; SPT, skin prick test. *Positive tests were defined as follows: IgE=serum level >0.35 kU/L (detection limit of assay). SPT=wheal ≥3 mm. APT=erythema with skin surface change. | ||||
In 1 study, researchers compared specific serum IgE levels with OFC results for 4 foods—milk, eggs, wheat, and soy—in 501 consecutive pediatric patients referred to an allergy ward based on clinical or parental suspicion of food allergy. Children ranged in age from 1 month to 16 years (median age 13 months); results for infants were not provided separately. Eighty-eight percent of the children were atopic. Investigators measured serum IgE (using the Pharmacia CAP-system fluorescence enzyme immunoassay) before administering the OFCs.
Of 992 OFCs performed, 445 (45%) were positive (defined as producing urticaria, angioedema, wheezing, vomiting, diarrhea, abdominal pain, shock, or exacerbation of eczema). Most OFCs (73%) were double-blind placebo-controlled, but investigators performed open OFCs in infants and in children with a history of immediate allergic reactions. Investigators retrospectively analyzed serum-specific IgE levels for the 4 food antigens and compared them with the results of the OFCs. Positive likelihood ratios (LR+) for IgE testing ranged from 1.3 to 2.0 for the 4 antigens; negative likelihood ratios (LR–) ranged from 0.31 to 0.59.2
The second cohort study compared APT and SPT with OFCs for the same 4 food antigens (milk, eggs, wheat, soy) in 437 children. The study population comprised consecutive referrals to a pediatric immunology department based on either parental suspicion of a food allergy or a positive IgE test. The children ranged in age from 3 months to 14 years (median age 13 months); results for infants weren’t provided separately. Ninety percent of the children were atopic. Investigators performed APTs and SPTs for the 4 food antigens on all children and OFCs only for foods that were clinically suspect (total OFCs=873).
As in the previous study, investigators performed open OFCs (23%) in infants and children with a history of immediate reaction. A positive APT was defined by erythema with infiltration or papules, and a positive SPT by a wheal ≥3 mm. For the SPT, the LR+ ranged from 1.9 to 2.8 for the 4 antigens, and the LR–ranged from 0.13 (for egg) to 0.84. For the APT, the LR+ ranged from 1.6 to 6.2 (for milk), whereas the LR– was of little value, ranging from 0.68 to 0.90.3
For milk and eggs, the larger the wheal, the more sensitive the skin test
The size of the wheal may increase the sensitivity of the SPT in some situations. A cohort study similar to the ones described previously compared SPT with OFCs in children with possible food allergies and found that a large SPT wheal was highly correlated with OFC-confirmed allergy to milk and eggs.4
Investigators recruited 385 children—3 months to 14 years of age (median age 22 months), results for infants not provided separately—from consecutive referrals to a pediatric immunology department. Most children (87%) were atopic. Investigators performed SPTs, followed by OFCs for milk, egg, wheat, and soy allergens. Overall, 312 (43%) of the OFCs were positive. Wheals measuring ≥13 mm for eggs and ≥12.5 mm for milk correlated well with OFC results (95% positive predictive value). Wheal sizes for wheat and soy were poorly predictive, however.4
No validation yet for new techniques to improve accuracy and safety
New techniques to improve the accuracy and safety of allergy testing have yet to be validated clinically. One cohort study of 58 children that used fresh fruit or vegetable preparations for SPT instead of commonly used commercial extracts found added sensitivity.5 Another cohort study of 142 children allowed suspect foods to contact only the labial mucosa in order to reduce the risk of systemic reactions (1 case of anaphylaxis occurred nevertheless).6
Recommendations
The AAAAI guidelines state that history and physical examination help determine that food is causing symptoms and that an OFC is diagnostic of food allergy (but risks and benefits must be considered, including the possibility of severe adverse reaction).1 The guidelines note that other available tests, including food-specific IgE and skin tests, are not specific enough for screening but may be used when a particular food allergy is clinically suspected.
1. American College of Allergy, Asthma, and Immunology. Food allergy: a practice parameter. Ann Allergy Asthma Immunol. 2006;96(3 suppl 2):S1-S68.
2. Celik-Bilgili S, Mehl A, Verstege A, et al. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy. 2005;35:268-273.
3. Mehl A, Rolinck-Werninghaus C, Staden U, et al. The atopy patch test in the diagnostic workup of suspected food-related symptoms in children. J Allergy Clin Immunol. 2006;118:923-929.
4. Verstege A, Mehl A, Rolinck-Werninghaus C, et al. The predictive value of the skin prick test weal size for the outcome of oral food challenges. Clin Exp Allergy. 2005;35:1220-1226.
5. Cantani A, Micera M. The prick by prick test is safe and reliable in 58 children with atopic dermatitis and food allergy. Eur Rev Med Pharm Sci. 2006;10:115-120.
6. Rance F, Dutau G. Labial food challenge in children with food allergy. Pediatr Allergy Immunol. 1997;8:41-44.
A WELL-DESIGNED ORAL FOOD CHALLENGE (OFC) is the most reliable diagnostic test for infants whose clinical history and physical examination suggest a specific food allergy (strength of recommendation [SOR]: C, consensus guidelines).
Serum-specific immunoglobulin E (IgE), atopy patch testing (APT), and skin prick testing (SPT) are all alternatives to OFC, but the likelihood ratios are not robust and the tests vary widely in sensitivity and specificity to different allergens. For diagnosing egg and milk allergies, larger wheal sizes with SPT are more predictive of a positive OFC (SOR: C, extrapolated from cohort studies evaluating mixed populations of infants, children, and teenagers).
Evidence summary
The American Academy of Allergy, Asthma and Immunology (AAAAI) states that a double-blind, placebo-controlled OFC is the best test for diagnosing infants clinically suspected of having food allergies (that is, who develop gastrointestinal symptoms after eating a specific food). However, performing an OFC in an infant is often difficult and potentially dangerous, especially if a severe allergy is suspected; testing also may eliminate nutritious foods, such as milk and eggs, from the infant’s diet.1 For these reasons, physicians have sought simpler alternatives to the OFC.
Comparisons of serum and skin testing with OFCs produce variable, weak results
Two large cohort studies compared OFCs with serum and skin testing in infants, children, and teenagers. Overall, serum and skin testing didn’t produce robust results and the results varied with the antigen (TABLE).
TABLE
How allergy tests in infants and children compare with an oral food challenge
| Test* | Sensitivity (%) | Specificity (%) | LR+ | LR- |
|---|---|---|---|---|
| Milk | ||||
| IgE2 | 83 | 53 | 1.8 | 0.32 |
| SPT3 | 85 | 70 | 2.8 | 0.21 |
| APT3 | 31 | 95 | 6.2 | 0.73 |
| Egg | ||||
| IgE2 | 97 | 51 | 2.0 | 0.59 |
| SPT3 | 93 | 54 | 2.0 | 0.13 |
| APT3 | 41 | 87 | 3.2 | 0.68 |
| Wheat | ||||
| IgE2 | 79 | 38 | 1.3 | 0.55 |
| SPT3 | 75 | 64 | 2.1 | 0.39 |
| APT3 | 27 | 89 | 2.5 | 0.82 |
| Soy | ||||
| IgE2 | 69 | 50 | 1.4 | 0.31 |
| SPT3 | 29 | 85 | 1.9 | 0.84 |
| APT3 | 23 | 86 | 1.6 | 0.90 |
| APT, atopy patch test; IgE, serum immunoglobulin E; LR+, positive likelihood ratio; LR–, negative likelihood ratio; SPT, skin prick test. *Positive tests were defined as follows: IgE=serum level >0.35 kU/L (detection limit of assay). SPT=wheal ≥3 mm. APT=erythema with skin surface change. | ||||
In 1 study, researchers compared specific serum IgE levels with OFC results for 4 foods—milk, eggs, wheat, and soy—in 501 consecutive pediatric patients referred to an allergy ward based on clinical or parental suspicion of food allergy. Children ranged in age from 1 month to 16 years (median age 13 months); results for infants were not provided separately. Eighty-eight percent of the children were atopic. Investigators measured serum IgE (using the Pharmacia CAP-system fluorescence enzyme immunoassay) before administering the OFCs.
Of 992 OFCs performed, 445 (45%) were positive (defined as producing urticaria, angioedema, wheezing, vomiting, diarrhea, abdominal pain, shock, or exacerbation of eczema). Most OFCs (73%) were double-blind placebo-controlled, but investigators performed open OFCs in infants and in children with a history of immediate allergic reactions. Investigators retrospectively analyzed serum-specific IgE levels for the 4 food antigens and compared them with the results of the OFCs. Positive likelihood ratios (LR+) for IgE testing ranged from 1.3 to 2.0 for the 4 antigens; negative likelihood ratios (LR–) ranged from 0.31 to 0.59.2
The second cohort study compared APT and SPT with OFCs for the same 4 food antigens (milk, eggs, wheat, soy) in 437 children. The study population comprised consecutive referrals to a pediatric immunology department based on either parental suspicion of a food allergy or a positive IgE test. The children ranged in age from 3 months to 14 years (median age 13 months); results for infants weren’t provided separately. Ninety percent of the children were atopic. Investigators performed APTs and SPTs for the 4 food antigens on all children and OFCs only for foods that were clinically suspect (total OFCs=873).
As in the previous study, investigators performed open OFCs (23%) in infants and children with a history of immediate reaction. A positive APT was defined by erythema with infiltration or papules, and a positive SPT by a wheal ≥3 mm. For the SPT, the LR+ ranged from 1.9 to 2.8 for the 4 antigens, and the LR–ranged from 0.13 (for egg) to 0.84. For the APT, the LR+ ranged from 1.6 to 6.2 (for milk), whereas the LR– was of little value, ranging from 0.68 to 0.90.3
For milk and eggs, the larger the wheal, the more sensitive the skin test
The size of the wheal may increase the sensitivity of the SPT in some situations. A cohort study similar to the ones described previously compared SPT with OFCs in children with possible food allergies and found that a large SPT wheal was highly correlated with OFC-confirmed allergy to milk and eggs.4
Investigators recruited 385 children—3 months to 14 years of age (median age 22 months), results for infants not provided separately—from consecutive referrals to a pediatric immunology department. Most children (87%) were atopic. Investigators performed SPTs, followed by OFCs for milk, egg, wheat, and soy allergens. Overall, 312 (43%) of the OFCs were positive. Wheals measuring ≥13 mm for eggs and ≥12.5 mm for milk correlated well with OFC results (95% positive predictive value). Wheal sizes for wheat and soy were poorly predictive, however.4
No validation yet for new techniques to improve accuracy and safety
New techniques to improve the accuracy and safety of allergy testing have yet to be validated clinically. One cohort study of 58 children that used fresh fruit or vegetable preparations for SPT instead of commonly used commercial extracts found added sensitivity.5 Another cohort study of 142 children allowed suspect foods to contact only the labial mucosa in order to reduce the risk of systemic reactions (1 case of anaphylaxis occurred nevertheless).6
Recommendations
The AAAAI guidelines state that history and physical examination help determine that food is causing symptoms and that an OFC is diagnostic of food allergy (but risks and benefits must be considered, including the possibility of severe adverse reaction).1 The guidelines note that other available tests, including food-specific IgE and skin tests, are not specific enough for screening but may be used when a particular food allergy is clinically suspected.
A WELL-DESIGNED ORAL FOOD CHALLENGE (OFC) is the most reliable diagnostic test for infants whose clinical history and physical examination suggest a specific food allergy (strength of recommendation [SOR]: C, consensus guidelines).
Serum-specific immunoglobulin E (IgE), atopy patch testing (APT), and skin prick testing (SPT) are all alternatives to OFC, but the likelihood ratios are not robust and the tests vary widely in sensitivity and specificity to different allergens. For diagnosing egg and milk allergies, larger wheal sizes with SPT are more predictive of a positive OFC (SOR: C, extrapolated from cohort studies evaluating mixed populations of infants, children, and teenagers).
Evidence summary
The American Academy of Allergy, Asthma and Immunology (AAAAI) states that a double-blind, placebo-controlled OFC is the best test for diagnosing infants clinically suspected of having food allergies (that is, who develop gastrointestinal symptoms after eating a specific food). However, performing an OFC in an infant is often difficult and potentially dangerous, especially if a severe allergy is suspected; testing also may eliminate nutritious foods, such as milk and eggs, from the infant’s diet.1 For these reasons, physicians have sought simpler alternatives to the OFC.
Comparisons of serum and skin testing with OFCs produce variable, weak results
Two large cohort studies compared OFCs with serum and skin testing in infants, children, and teenagers. Overall, serum and skin testing didn’t produce robust results and the results varied with the antigen (TABLE).
TABLE
How allergy tests in infants and children compare with an oral food challenge
| Test* | Sensitivity (%) | Specificity (%) | LR+ | LR- |
|---|---|---|---|---|
| Milk | ||||
| IgE2 | 83 | 53 | 1.8 | 0.32 |
| SPT3 | 85 | 70 | 2.8 | 0.21 |
| APT3 | 31 | 95 | 6.2 | 0.73 |
| Egg | ||||
| IgE2 | 97 | 51 | 2.0 | 0.59 |
| SPT3 | 93 | 54 | 2.0 | 0.13 |
| APT3 | 41 | 87 | 3.2 | 0.68 |
| Wheat | ||||
| IgE2 | 79 | 38 | 1.3 | 0.55 |
| SPT3 | 75 | 64 | 2.1 | 0.39 |
| APT3 | 27 | 89 | 2.5 | 0.82 |
| Soy | ||||
| IgE2 | 69 | 50 | 1.4 | 0.31 |
| SPT3 | 29 | 85 | 1.9 | 0.84 |
| APT3 | 23 | 86 | 1.6 | 0.90 |
| APT, atopy patch test; IgE, serum immunoglobulin E; LR+, positive likelihood ratio; LR–, negative likelihood ratio; SPT, skin prick test. *Positive tests were defined as follows: IgE=serum level >0.35 kU/L (detection limit of assay). SPT=wheal ≥3 mm. APT=erythema with skin surface change. | ||||
In 1 study, researchers compared specific serum IgE levels with OFC results for 4 foods—milk, eggs, wheat, and soy—in 501 consecutive pediatric patients referred to an allergy ward based on clinical or parental suspicion of food allergy. Children ranged in age from 1 month to 16 years (median age 13 months); results for infants were not provided separately. Eighty-eight percent of the children were atopic. Investigators measured serum IgE (using the Pharmacia CAP-system fluorescence enzyme immunoassay) before administering the OFCs.
Of 992 OFCs performed, 445 (45%) were positive (defined as producing urticaria, angioedema, wheezing, vomiting, diarrhea, abdominal pain, shock, or exacerbation of eczema). Most OFCs (73%) were double-blind placebo-controlled, but investigators performed open OFCs in infants and in children with a history of immediate allergic reactions. Investigators retrospectively analyzed serum-specific IgE levels for the 4 food antigens and compared them with the results of the OFCs. Positive likelihood ratios (LR+) for IgE testing ranged from 1.3 to 2.0 for the 4 antigens; negative likelihood ratios (LR–) ranged from 0.31 to 0.59.2
The second cohort study compared APT and SPT with OFCs for the same 4 food antigens (milk, eggs, wheat, soy) in 437 children. The study population comprised consecutive referrals to a pediatric immunology department based on either parental suspicion of a food allergy or a positive IgE test. The children ranged in age from 3 months to 14 years (median age 13 months); results for infants weren’t provided separately. Ninety percent of the children were atopic. Investigators performed APTs and SPTs for the 4 food antigens on all children and OFCs only for foods that were clinically suspect (total OFCs=873).
As in the previous study, investigators performed open OFCs (23%) in infants and children with a history of immediate reaction. A positive APT was defined by erythema with infiltration or papules, and a positive SPT by a wheal ≥3 mm. For the SPT, the LR+ ranged from 1.9 to 2.8 for the 4 antigens, and the LR–ranged from 0.13 (for egg) to 0.84. For the APT, the LR+ ranged from 1.6 to 6.2 (for milk), whereas the LR– was of little value, ranging from 0.68 to 0.90.3
For milk and eggs, the larger the wheal, the more sensitive the skin test
The size of the wheal may increase the sensitivity of the SPT in some situations. A cohort study similar to the ones described previously compared SPT with OFCs in children with possible food allergies and found that a large SPT wheal was highly correlated with OFC-confirmed allergy to milk and eggs.4
Investigators recruited 385 children—3 months to 14 years of age (median age 22 months), results for infants not provided separately—from consecutive referrals to a pediatric immunology department. Most children (87%) were atopic. Investigators performed SPTs, followed by OFCs for milk, egg, wheat, and soy allergens. Overall, 312 (43%) of the OFCs were positive. Wheals measuring ≥13 mm for eggs and ≥12.5 mm for milk correlated well with OFC results (95% positive predictive value). Wheal sizes for wheat and soy were poorly predictive, however.4
No validation yet for new techniques to improve accuracy and safety
New techniques to improve the accuracy and safety of allergy testing have yet to be validated clinically. One cohort study of 58 children that used fresh fruit or vegetable preparations for SPT instead of commonly used commercial extracts found added sensitivity.5 Another cohort study of 142 children allowed suspect foods to contact only the labial mucosa in order to reduce the risk of systemic reactions (1 case of anaphylaxis occurred nevertheless).6
Recommendations
The AAAAI guidelines state that history and physical examination help determine that food is causing symptoms and that an OFC is diagnostic of food allergy (but risks and benefits must be considered, including the possibility of severe adverse reaction).1 The guidelines note that other available tests, including food-specific IgE and skin tests, are not specific enough for screening but may be used when a particular food allergy is clinically suspected.
1. American College of Allergy, Asthma, and Immunology. Food allergy: a practice parameter. Ann Allergy Asthma Immunol. 2006;96(3 suppl 2):S1-S68.
2. Celik-Bilgili S, Mehl A, Verstege A, et al. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy. 2005;35:268-273.
3. Mehl A, Rolinck-Werninghaus C, Staden U, et al. The atopy patch test in the diagnostic workup of suspected food-related symptoms in children. J Allergy Clin Immunol. 2006;118:923-929.
4. Verstege A, Mehl A, Rolinck-Werninghaus C, et al. The predictive value of the skin prick test weal size for the outcome of oral food challenges. Clin Exp Allergy. 2005;35:1220-1226.
5. Cantani A, Micera M. The prick by prick test is safe and reliable in 58 children with atopic dermatitis and food allergy. Eur Rev Med Pharm Sci. 2006;10:115-120.
6. Rance F, Dutau G. Labial food challenge in children with food allergy. Pediatr Allergy Immunol. 1997;8:41-44.
1. American College of Allergy, Asthma, and Immunology. Food allergy: a practice parameter. Ann Allergy Asthma Immunol. 2006;96(3 suppl 2):S1-S68.
2. Celik-Bilgili S, Mehl A, Verstege A, et al. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy. 2005;35:268-273.
3. Mehl A, Rolinck-Werninghaus C, Staden U, et al. The atopy patch test in the diagnostic workup of suspected food-related symptoms in children. J Allergy Clin Immunol. 2006;118:923-929.
4. Verstege A, Mehl A, Rolinck-Werninghaus C, et al. The predictive value of the skin prick test weal size for the outcome of oral food challenges. Clin Exp Allergy. 2005;35:1220-1226.
5. Cantani A, Micera M. The prick by prick test is safe and reliable in 58 children with atopic dermatitis and food allergy. Eur Rev Med Pharm Sci. 2006;10:115-120.
6. Rance F, Dutau G. Labial food challenge in children with food allergy. Pediatr Allergy Immunol. 1997;8:41-44.
Evidence-based answers from the Family Physicians Inquiries Network
Do hyaluronic acid injections relieve OA knee pain?
Yes, hyaluronic acid (HA) injections relieve pain more than placebo. The effect is small but similar to results from oral nonsteroidal anti-inflammatory drugs (NSAIDs) or steroid injection (strength of recommendation ([SOR]: B, conflicting meta-analyses). The various HA products all appear to be equally effective in reducing pain (SOR: B, randomized clinical trials [RCTs]). Data concerning the effect of HA on functional ability are conflicting.
Evidence summary
A 2005 meta-analysis evaluated the effectiveness of HA injections for osteoarthritis of the knee compared with saline placebo. Researchers identified 22 studies of 8 HA products that used the common end point of pain with movement.1 (TABLE 1 lists FDA-approved HA products available in the United States.2) A decrease in pain of 15% was deemed clinically meaningful.
Compared with placebo, the mean difference in pain scores with HA products was -4% (95% confidence interval [CI], -9% to 1%) after 2 to 6 weeks; -4% (95% CI, -8% to -1%) after 10 to 14 weeks; and -7% (95% CI, -12% to -2%) after 22 to 30 weeks. The authors note that the small measured effect of HA was magnified by trials that didn’t report intention-to-treat results. The effect of HA was also larger in studies that didn’t conceal allocation. A weakness of the analysis was its inability to assess potential differences between HA products.
In this 2005 meta-analysis, HA injection didn’t improve knee function in any time interval. But in a Cochrane meta-analysis conducted the following year, HA was found to have positive results.3
TABLE 1
FDA-approved hyaluronic acid products
| TRADE NAME | COMPOSITION | MOLECULAR WEIGHT (×106 DALTONS) | COST PER INJECTION* (3-5 DOSES) | SOURCE |
|---|---|---|---|---|
| Hyalgan | Sodium hyaluronate | 0.5-0.7 | $138 | Avian |
| Supartz | Sodium hyaluronate | 0.6-1.2 | $136 | Avian |
| Euflexxa | Sodium hyaluronate | 2.4-3.6 | $133 | Bacterial |
| Orthovisc | Hyaluronin | 1.0-2.9 | $238 | Avian |
| Synvisc | Hylan G-F 20 | 6.0 | $230 | Avian |
HA relieves pain about as much as NSAIDs
The comprehensive 2006 Cochrane meta-analysis reviewed single- and double-blinded RCTs that evaluated the effect of 12 HA products on osteoarthritis of the knee.3 Studies compared HA products with placebo (40), intra-articular steroids (10), NSAIDs (6), physical therapy (3), exercise (2), and each other (15). Efficacy data for different products couldn’t be combined because the studies measured different sets of outcomes at different time points.
Overall, the authors concluded that HA injections effectively reduced pain scores, with the largest benefit occurring within 5 to 13 weeks (TABLE 2). The authors also noted that the reductions in pain with HA injections, although generally small, were comparable to oral NSAID therapy and intra-articular corticosteroids. The trials reported few adverse events.
Two RCTs show no difference in efficacy among HA products
The Cochrane review determined that not enough evidence existed to evaluate HA products against each other. Two subsequent RCTs compared HA products and found no differences in efficacy. One study compared Synvisc, Orthovisc, and Ostenil therapy in 660 patients over 6 months.4 The other compared Synvisc with Euflexxa in 321 patients over 3 months.5 Notably, 8% of patients in this study who used Synvisc developed an effusion, compared with 0.6% of patients who used Euflexxa (P=.0015).
Recommendations
A 2007 report from the Agency for Healthcare Research and Quality (AHRQ) states that “viscosupplementation trials generally report positive effects on pain and function scores compared with placebo, but the evidence on clinical benefit is uncertain.”6
The 2007 guidelines of the Institute for Clinical Systems Improvement note that synthetic hyaluronates “may be effective” in selected patients with mild to moderate degenerative joint disease (based on evidence of middle quality on a 3-tier grading system).7
The American Academy of Orthopaedic Surgeons 2008 guideline on osteoarthritis of the knee indicates that, based on the AHRQ report,6 it cannot recommend for or against the use of intra-articular hyaluronic acid for mild to moderate symptomatic OA of the knee.8
TABLE 2
How HA products affect pain measures
| AT 1-4 WEKS | AT 5-13 WEKS | |||||
|---|---|---|---|---|---|---|
| PRODUCT | COMPARATOR | OUTCOME | N | PERCENT CHANGE VS COMPARATOR* (95% CI) | N | PERCENT CHANGE VS COMPARATOR* (95% CI) |
| HA/hyalin | Placebo | Pain on weight bearing | 2542 | -8 (-11 to -4) | 2090 | -13 (-18 to -8) |
| Hyalgan | Placebo | Pain on weight bearing | 1398 | -6 (-11 to -1) | 1095 | -9 (-14 to -4) |
| Synvisc | Placebo | Pain on weight bearing | 481 | -13 (-20 to -5) | 155 | -10 (-14 to -5) |
| Suplasyn | Placebo | WOMAC pain | 53 | NS | (Data not available) | |
| Durolane | Placebo | WOMAC pain | 346 | 4 (0 to 7) | 436 | NS |
| Orthovisc | Placebo | WOMAC pain | 110 | -12 (-13 to -10) | 69 | -5 (-7 to -4) |
| HA/hyalin | NSAID | Pain after walking | 333 | NS | (Data not available) | |
| Hyalgan | NSAID | Pain after 50-foot walk | 279 | NS | 140 | NS |
| Synvisc | NSAID | Pain at rest | (Data not available) | 57 | NS | |
| Suplasyn | NSAID | Pain after walking | 54 | NS | (Data not available) | |
| Hyalgan | Methylprednisolone | Spontaneous pain | 170 | NS | 170 | -8 (-13 to -3) |
| Synvisc | Triamcinolone | WOMAC pain, walking | (Data not available) | 215 | -10 (-17 to -3) | |
| Orthovisc | Methylprednisolone | Pain, walking | 55 | NS | 55 | -18 (-29 to -8) |
| Us medications Supartz and Euflexxa were not included in the Cochrane review. | ||||||
| CI, confidence interval; HA, hyaluronic acid; NS, not statistically significant; NSAID, nonsteroidal anti-inflammatory drug; WOMAC, Western Ontario and MacMaster universities Osteoarthritis Index. | ||||||
| *Negative values favor HA products. | ||||||
| Source: Bellamy n, et al. Cochrane Database Syst. Rev. 2006.3 | ||||||
1. Arrich J, Piribauer F, Mad P, et al. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ 2005;172:1239-1242.
2. Waddell DD. viscosupplementation with hyaluronans for osteoarthritis of the knee: clinical efficacy and economic implications. Drugs Aging 2007;24:629-642.
3. Bellamy N, Campbell J, Robinson V, et al. viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.
4. Jüni P, Reichenbach S, Trelle S, et al. efficacy and safety of intra-articular hylan or hyaluronic acids for osteoarthritis of the knee: a randomized controlled trial. Arthritis Rheum. 2007;54:3610-3619.
5. Kirchner M, Marshall D. A double-blind, randomized, controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14:154-162.
6. Samson DJ, Grant MD, Ratko TA, et al. Treatment of primary and secondary osteoarthritis of the knee. evidence report/Technology Assessment no. 157 (prepared by Blue Cross and Blue shield Association Technology evaluation Center evidence-based Practice Center under Contract no. 290-02-0026). AHrq Publication no. 07-e012. rockville, Md: Agency for Healthcare research and quality. september 2007. Available at: www.ahrq.gov/clinic/tp/oakneetp.htm. Accessed February 11, 2009.
7. Institute for Clinical systems Improvement. Diagnosis and treatment of adult degenerative joint disease (DJD)/osteoarthritis (OA) of the knee. Bloomington, Minn: Institute for Clinical systems Improvement; 2007.
8. American Academy of Orthopaedic surgeons. Clinical Practice Guideline: Treatment of Osteoarthritis of the Knee (Non-Arthroplasty). rosemont, Ill: American Academy of Orthopaedic surgeons; 2008. Available at: www.aaos.org/research/guidelines/GuidelineOAKnee.asp. Accessed April 21, 2009.
Yes, hyaluronic acid (HA) injections relieve pain more than placebo. The effect is small but similar to results from oral nonsteroidal anti-inflammatory drugs (NSAIDs) or steroid injection (strength of recommendation ([SOR]: B, conflicting meta-analyses). The various HA products all appear to be equally effective in reducing pain (SOR: B, randomized clinical trials [RCTs]). Data concerning the effect of HA on functional ability are conflicting.
Evidence summary
A 2005 meta-analysis evaluated the effectiveness of HA injections for osteoarthritis of the knee compared with saline placebo. Researchers identified 22 studies of 8 HA products that used the common end point of pain with movement.1 (TABLE 1 lists FDA-approved HA products available in the United States.2) A decrease in pain of 15% was deemed clinically meaningful.
Compared with placebo, the mean difference in pain scores with HA products was -4% (95% confidence interval [CI], -9% to 1%) after 2 to 6 weeks; -4% (95% CI, -8% to -1%) after 10 to 14 weeks; and -7% (95% CI, -12% to -2%) after 22 to 30 weeks. The authors note that the small measured effect of HA was magnified by trials that didn’t report intention-to-treat results. The effect of HA was also larger in studies that didn’t conceal allocation. A weakness of the analysis was its inability to assess potential differences between HA products.
In this 2005 meta-analysis, HA injection didn’t improve knee function in any time interval. But in a Cochrane meta-analysis conducted the following year, HA was found to have positive results.3
TABLE 1
FDA-approved hyaluronic acid products
| TRADE NAME | COMPOSITION | MOLECULAR WEIGHT (×106 DALTONS) | COST PER INJECTION* (3-5 DOSES) | SOURCE |
|---|---|---|---|---|
| Hyalgan | Sodium hyaluronate | 0.5-0.7 | $138 | Avian |
| Supartz | Sodium hyaluronate | 0.6-1.2 | $136 | Avian |
| Euflexxa | Sodium hyaluronate | 2.4-3.6 | $133 | Bacterial |
| Orthovisc | Hyaluronin | 1.0-2.9 | $238 | Avian |
| Synvisc | Hylan G-F 20 | 6.0 | $230 | Avian |
HA relieves pain about as much as NSAIDs
The comprehensive 2006 Cochrane meta-analysis reviewed single- and double-blinded RCTs that evaluated the effect of 12 HA products on osteoarthritis of the knee.3 Studies compared HA products with placebo (40), intra-articular steroids (10), NSAIDs (6), physical therapy (3), exercise (2), and each other (15). Efficacy data for different products couldn’t be combined because the studies measured different sets of outcomes at different time points.
Overall, the authors concluded that HA injections effectively reduced pain scores, with the largest benefit occurring within 5 to 13 weeks (TABLE 2). The authors also noted that the reductions in pain with HA injections, although generally small, were comparable to oral NSAID therapy and intra-articular corticosteroids. The trials reported few adverse events.
Two RCTs show no difference in efficacy among HA products
The Cochrane review determined that not enough evidence existed to evaluate HA products against each other. Two subsequent RCTs compared HA products and found no differences in efficacy. One study compared Synvisc, Orthovisc, and Ostenil therapy in 660 patients over 6 months.4 The other compared Synvisc with Euflexxa in 321 patients over 3 months.5 Notably, 8% of patients in this study who used Synvisc developed an effusion, compared with 0.6% of patients who used Euflexxa (P=.0015).
Recommendations
A 2007 report from the Agency for Healthcare Research and Quality (AHRQ) states that “viscosupplementation trials generally report positive effects on pain and function scores compared with placebo, but the evidence on clinical benefit is uncertain.”6
The 2007 guidelines of the Institute for Clinical Systems Improvement note that synthetic hyaluronates “may be effective” in selected patients with mild to moderate degenerative joint disease (based on evidence of middle quality on a 3-tier grading system).7
The American Academy of Orthopaedic Surgeons 2008 guideline on osteoarthritis of the knee indicates that, based on the AHRQ report,6 it cannot recommend for or against the use of intra-articular hyaluronic acid for mild to moderate symptomatic OA of the knee.8
TABLE 2
How HA products affect pain measures
| AT 1-4 WEKS | AT 5-13 WEKS | |||||
|---|---|---|---|---|---|---|
| PRODUCT | COMPARATOR | OUTCOME | N | PERCENT CHANGE VS COMPARATOR* (95% CI) | N | PERCENT CHANGE VS COMPARATOR* (95% CI) |
| HA/hyalin | Placebo | Pain on weight bearing | 2542 | -8 (-11 to -4) | 2090 | -13 (-18 to -8) |
| Hyalgan | Placebo | Pain on weight bearing | 1398 | -6 (-11 to -1) | 1095 | -9 (-14 to -4) |
| Synvisc | Placebo | Pain on weight bearing | 481 | -13 (-20 to -5) | 155 | -10 (-14 to -5) |
| Suplasyn | Placebo | WOMAC pain | 53 | NS | (Data not available) | |
| Durolane | Placebo | WOMAC pain | 346 | 4 (0 to 7) | 436 | NS |
| Orthovisc | Placebo | WOMAC pain | 110 | -12 (-13 to -10) | 69 | -5 (-7 to -4) |
| HA/hyalin | NSAID | Pain after walking | 333 | NS | (Data not available) | |
| Hyalgan | NSAID | Pain after 50-foot walk | 279 | NS | 140 | NS |
| Synvisc | NSAID | Pain at rest | (Data not available) | 57 | NS | |
| Suplasyn | NSAID | Pain after walking | 54 | NS | (Data not available) | |
| Hyalgan | Methylprednisolone | Spontaneous pain | 170 | NS | 170 | -8 (-13 to -3) |
| Synvisc | Triamcinolone | WOMAC pain, walking | (Data not available) | 215 | -10 (-17 to -3) | |
| Orthovisc | Methylprednisolone | Pain, walking | 55 | NS | 55 | -18 (-29 to -8) |
| Us medications Supartz and Euflexxa were not included in the Cochrane review. | ||||||
| CI, confidence interval; HA, hyaluronic acid; NS, not statistically significant; NSAID, nonsteroidal anti-inflammatory drug; WOMAC, Western Ontario and MacMaster universities Osteoarthritis Index. | ||||||
| *Negative values favor HA products. | ||||||
| Source: Bellamy n, et al. Cochrane Database Syst. Rev. 2006.3 | ||||||
Yes, hyaluronic acid (HA) injections relieve pain more than placebo. The effect is small but similar to results from oral nonsteroidal anti-inflammatory drugs (NSAIDs) or steroid injection (strength of recommendation ([SOR]: B, conflicting meta-analyses). The various HA products all appear to be equally effective in reducing pain (SOR: B, randomized clinical trials [RCTs]). Data concerning the effect of HA on functional ability are conflicting.
Evidence summary
A 2005 meta-analysis evaluated the effectiveness of HA injections for osteoarthritis of the knee compared with saline placebo. Researchers identified 22 studies of 8 HA products that used the common end point of pain with movement.1 (TABLE 1 lists FDA-approved HA products available in the United States.2) A decrease in pain of 15% was deemed clinically meaningful.
Compared with placebo, the mean difference in pain scores with HA products was -4% (95% confidence interval [CI], -9% to 1%) after 2 to 6 weeks; -4% (95% CI, -8% to -1%) after 10 to 14 weeks; and -7% (95% CI, -12% to -2%) after 22 to 30 weeks. The authors note that the small measured effect of HA was magnified by trials that didn’t report intention-to-treat results. The effect of HA was also larger in studies that didn’t conceal allocation. A weakness of the analysis was its inability to assess potential differences between HA products.
In this 2005 meta-analysis, HA injection didn’t improve knee function in any time interval. But in a Cochrane meta-analysis conducted the following year, HA was found to have positive results.3
TABLE 1
FDA-approved hyaluronic acid products
| TRADE NAME | COMPOSITION | MOLECULAR WEIGHT (×106 DALTONS) | COST PER INJECTION* (3-5 DOSES) | SOURCE |
|---|---|---|---|---|
| Hyalgan | Sodium hyaluronate | 0.5-0.7 | $138 | Avian |
| Supartz | Sodium hyaluronate | 0.6-1.2 | $136 | Avian |
| Euflexxa | Sodium hyaluronate | 2.4-3.6 | $133 | Bacterial |
| Orthovisc | Hyaluronin | 1.0-2.9 | $238 | Avian |
| Synvisc | Hylan G-F 20 | 6.0 | $230 | Avian |
HA relieves pain about as much as NSAIDs
The comprehensive 2006 Cochrane meta-analysis reviewed single- and double-blinded RCTs that evaluated the effect of 12 HA products on osteoarthritis of the knee.3 Studies compared HA products with placebo (40), intra-articular steroids (10), NSAIDs (6), physical therapy (3), exercise (2), and each other (15). Efficacy data for different products couldn’t be combined because the studies measured different sets of outcomes at different time points.
Overall, the authors concluded that HA injections effectively reduced pain scores, with the largest benefit occurring within 5 to 13 weeks (TABLE 2). The authors also noted that the reductions in pain with HA injections, although generally small, were comparable to oral NSAID therapy and intra-articular corticosteroids. The trials reported few adverse events.
Two RCTs show no difference in efficacy among HA products
The Cochrane review determined that not enough evidence existed to evaluate HA products against each other. Two subsequent RCTs compared HA products and found no differences in efficacy. One study compared Synvisc, Orthovisc, and Ostenil therapy in 660 patients over 6 months.4 The other compared Synvisc with Euflexxa in 321 patients over 3 months.5 Notably, 8% of patients in this study who used Synvisc developed an effusion, compared with 0.6% of patients who used Euflexxa (P=.0015).
Recommendations
A 2007 report from the Agency for Healthcare Research and Quality (AHRQ) states that “viscosupplementation trials generally report positive effects on pain and function scores compared with placebo, but the evidence on clinical benefit is uncertain.”6
The 2007 guidelines of the Institute for Clinical Systems Improvement note that synthetic hyaluronates “may be effective” in selected patients with mild to moderate degenerative joint disease (based on evidence of middle quality on a 3-tier grading system).7
The American Academy of Orthopaedic Surgeons 2008 guideline on osteoarthritis of the knee indicates that, based on the AHRQ report,6 it cannot recommend for or against the use of intra-articular hyaluronic acid for mild to moderate symptomatic OA of the knee.8
TABLE 2
How HA products affect pain measures
| AT 1-4 WEKS | AT 5-13 WEKS | |||||
|---|---|---|---|---|---|---|
| PRODUCT | COMPARATOR | OUTCOME | N | PERCENT CHANGE VS COMPARATOR* (95% CI) | N | PERCENT CHANGE VS COMPARATOR* (95% CI) |
| HA/hyalin | Placebo | Pain on weight bearing | 2542 | -8 (-11 to -4) | 2090 | -13 (-18 to -8) |
| Hyalgan | Placebo | Pain on weight bearing | 1398 | -6 (-11 to -1) | 1095 | -9 (-14 to -4) |
| Synvisc | Placebo | Pain on weight bearing | 481 | -13 (-20 to -5) | 155 | -10 (-14 to -5) |
| Suplasyn | Placebo | WOMAC pain | 53 | NS | (Data not available) | |
| Durolane | Placebo | WOMAC pain | 346 | 4 (0 to 7) | 436 | NS |
| Orthovisc | Placebo | WOMAC pain | 110 | -12 (-13 to -10) | 69 | -5 (-7 to -4) |
| HA/hyalin | NSAID | Pain after walking | 333 | NS | (Data not available) | |
| Hyalgan | NSAID | Pain after 50-foot walk | 279 | NS | 140 | NS |
| Synvisc | NSAID | Pain at rest | (Data not available) | 57 | NS | |
| Suplasyn | NSAID | Pain after walking | 54 | NS | (Data not available) | |
| Hyalgan | Methylprednisolone | Spontaneous pain | 170 | NS | 170 | -8 (-13 to -3) |
| Synvisc | Triamcinolone | WOMAC pain, walking | (Data not available) | 215 | -10 (-17 to -3) | |
| Orthovisc | Methylprednisolone | Pain, walking | 55 | NS | 55 | -18 (-29 to -8) |
| Us medications Supartz and Euflexxa were not included in the Cochrane review. | ||||||
| CI, confidence interval; HA, hyaluronic acid; NS, not statistically significant; NSAID, nonsteroidal anti-inflammatory drug; WOMAC, Western Ontario and MacMaster universities Osteoarthritis Index. | ||||||
| *Negative values favor HA products. | ||||||
| Source: Bellamy n, et al. Cochrane Database Syst. Rev. 2006.3 | ||||||
1. Arrich J, Piribauer F, Mad P, et al. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ 2005;172:1239-1242.
2. Waddell DD. viscosupplementation with hyaluronans for osteoarthritis of the knee: clinical efficacy and economic implications. Drugs Aging 2007;24:629-642.
3. Bellamy N, Campbell J, Robinson V, et al. viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.
4. Jüni P, Reichenbach S, Trelle S, et al. efficacy and safety of intra-articular hylan or hyaluronic acids for osteoarthritis of the knee: a randomized controlled trial. Arthritis Rheum. 2007;54:3610-3619.
5. Kirchner M, Marshall D. A double-blind, randomized, controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14:154-162.
6. Samson DJ, Grant MD, Ratko TA, et al. Treatment of primary and secondary osteoarthritis of the knee. evidence report/Technology Assessment no. 157 (prepared by Blue Cross and Blue shield Association Technology evaluation Center evidence-based Practice Center under Contract no. 290-02-0026). AHrq Publication no. 07-e012. rockville, Md: Agency for Healthcare research and quality. september 2007. Available at: www.ahrq.gov/clinic/tp/oakneetp.htm. Accessed February 11, 2009.
7. Institute for Clinical systems Improvement. Diagnosis and treatment of adult degenerative joint disease (DJD)/osteoarthritis (OA) of the knee. Bloomington, Minn: Institute for Clinical systems Improvement; 2007.
8. American Academy of Orthopaedic surgeons. Clinical Practice Guideline: Treatment of Osteoarthritis of the Knee (Non-Arthroplasty). rosemont, Ill: American Academy of Orthopaedic surgeons; 2008. Available at: www.aaos.org/research/guidelines/GuidelineOAKnee.asp. Accessed April 21, 2009.
1. Arrich J, Piribauer F, Mad P, et al. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ 2005;172:1239-1242.
2. Waddell DD. viscosupplementation with hyaluronans for osteoarthritis of the knee: clinical efficacy and economic implications. Drugs Aging 2007;24:629-642.
3. Bellamy N, Campbell J, Robinson V, et al. viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.
4. Jüni P, Reichenbach S, Trelle S, et al. efficacy and safety of intra-articular hylan or hyaluronic acids for osteoarthritis of the knee: a randomized controlled trial. Arthritis Rheum. 2007;54:3610-3619.
5. Kirchner M, Marshall D. A double-blind, randomized, controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14:154-162.
6. Samson DJ, Grant MD, Ratko TA, et al. Treatment of primary and secondary osteoarthritis of the knee. evidence report/Technology Assessment no. 157 (prepared by Blue Cross and Blue shield Association Technology evaluation Center evidence-based Practice Center under Contract no. 290-02-0026). AHrq Publication no. 07-e012. rockville, Md: Agency for Healthcare research and quality. september 2007. Available at: www.ahrq.gov/clinic/tp/oakneetp.htm. Accessed February 11, 2009.
7. Institute for Clinical systems Improvement. Diagnosis and treatment of adult degenerative joint disease (DJD)/osteoarthritis (OA) of the knee. Bloomington, Minn: Institute for Clinical systems Improvement; 2007.
8. American Academy of Orthopaedic surgeons. Clinical Practice Guideline: Treatment of Osteoarthritis of the Knee (Non-Arthroplasty). rosemont, Ill: American Academy of Orthopaedic surgeons; 2008. Available at: www.aaos.org/research/guidelines/GuidelineOAKnee.asp. Accessed April 21, 2009.
Evidence-based answers from the Family Physicians Inquiries Network
Does reducing smoking in the home protect children from the effects of second-hand smoke?
Yes, taking this step helps asthmatic children, and may even help nonasthmatic children. In families of asthmatic children, education to reduce exposure to secondhand smoke leads to fewer medical visits (strength of recommendation [SOR]: B, a single randomized, controlled trial). The effects of educating families of nonasthmatic children about secondhand smoke are not known, but parents who smoke outside expose their children to much less nicotine than parents who smoke in the house (SOR: B, cohort studies and cross-sectional surveys).
Evidence summary
Parent education reduces clinic visits for asthmatic children
A 2001 trial randomized 81 families with a smoking parent and an asthmatic child between 3 and 12 years of age to 3 sessions of behavioral and educational counseling or usual care at an outpatient asthma clinic.1 Parental education included information on second-hand smoke, basic asthma education, and feedback about urine cotinine levels (a marker of nicotine absorption). Behavioral counseling focused on reducing second-hand smoke exposure by caregivers.
The education group had a significantly reduced risk of 2 or more asthma-related clinic visits in the following 12 months compared with usual care (odds ratio=0.32; P=.03; number needed to treat=5). No significant decrease was noted in mean urine cotinine levels between groups (adjusted mean difference=-0.38 ng/mg favoring education; P=.26).
A similar trial that measured changes in urine cotinine randomized 91 families with a smoking parent and an asthmatic child into 3 groups:2
- A control group received usual care (regular office visits at an asthma clinic and medication management)
- A monitoring group used a parental smoking diary and a children’s asthma symptom diary
- A counseling group received 5 counseling sessions and also kept diaries. An environmental monitor in the home was used to assess exposure to secondhand smoke.
In the counseling group, 21.4% of patients (6 of 28) maintained 0% exposure throughout the 30-month trial period compared with 3.6% and 3.8% in the monitoring and control groups, respectively (P<.05 for comparison of counseling group to monitoring and control).
Banning indoor smoking sharply cuts nicotine exposure
No data are available on education about second-hand smoke in families with nonasthmatic children. However, strong evidence suggests that smoking outside the house reduces exposure generally.
A 2003 cross-sectional survey of 164 households in the United Kingdom with at least 1 smoking parent and 1 bottle-fed infant looked for a correlation between strategies to reduce second-hand smoke and urine cotinine-to-creatinine ratios in the infants.3 Parents were classified into 3 groups according to whether they maintained a strict ban on smoking in the home, a less strict ban (smoking at home but not near the infant), or no ban.
The mean infant urinary cotinine-to-creatinine ratio was 2.43 in the no-ban group and 2.61 in the less-strict ban group (difference not significant). The combined mean for these 2 groups—2.58—was significantly higher than the mean of 1.26 in the strictest group (P<.001).
A later study recruited a convenience sample of 49 interested families with a smoking mother and a nonbreastfeeding infant between 2 and 12 months of age.4 Families were classified by smoking history into one of 3 groups: nonsmoking households, smoking households where efforts were made to limit smoke exposure, and smoking households where no efforts were made to limit exposure. Urine samples were obtained 3 times over 1 week. Urine cotinine levels in infants averaged 0.33 ng/mL in nonsmoking households, 2.47 ng/mL in smoking households with limited exposure, and 15.47 ng/mL in smoking households with unlimited exposure (P<.001 for all comparisons).
A case-control study that recruited families with asthmatic and nonasthmatic children assessed the effectiveness of parental behaviors to reduce second-hand smoke in 182 households with 1 smoking parent and a child between 6 and 12 years of age.5 Researchers measured room air nicotine and salivary cotinine concentrations.
The nicotine levels on children’s belts and in their bedrooms and the family room were approximately 3 log units lower in houses with strict smoking bans compared with households with any degree of indoor smoking (P<.0001). Similarly, salivary cotinine levels were approximately 4 log units lower in children of households with indoor smoking bans (P<.0001).
Recommendations
The United States Preventive Services Task Force (USPSTF) strongly recommends that physicians help all smoking adults to quit.6 The American Academy of Family Physicians endorses the USPSTF position and further advises that smoking parents be counseled about the health effects of environmental tobacco smoke on their children.7
The American Academy of Pediatrics8 and the Veterans Administration9 recommend urging parents to stop smoking to prevent serious health implications for their children; they further encourage pediatric clinicians to offer parents advice on quitting in order to limit children’s exposure to second-hand smoke.
1. Wilson SR, Yamada EG, Sudhaker R, et al. A controlled trial of an environmental tobacco smoke reduction intervention in low-income children with asthma. Chest. 2001;120:1709-1722.
2. Wahlgren DR, Hovell MF, Meltzer SB, et al. Reduction of environmental tobacco smoke exposure in asthmatic children: a 2-year follow-up. Chest. 1997;111:81-88.
3. Blackburn C, Spencer N, Bonas S, et al. Effect of strategies to reduce exposure of infants to environmental tobacco smoke in the home: cross sectional survey. BMJ. 2003;327:257-261.
4. Matt GE, Quintana PJ, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control. 2004;13:29-37.
5. Wambolt FS, Balkissoon RC, Rankin AE, et al. Correlates of household smoking bans in low-income families of children with and without asthma. Fam Process. 2008;47:81-94.
6. US Preventive Services Task Force. Counseling to prevent tobacco use and tobacco-caused disease. Rockville, Md: Agency for Healthcare Research and Quality; 2003. Available at: www.ahrq.gov/clinic/uspstf/uspstbac.htm. Accessed september 11, 2008.
7. AAFP Summary of Recommendations for Clinical Preventive Services. Revision 6.3. Leawood, Kan: American Academy of Family Physicians (AAFP); 2007.
8. American Academy of Pediatrics. Tobacco’s toll: implications for the pediatrician. Pediatrics. 2001;107:794-798.
9. Veterans Administration Department of Defense VA/ DOD clinical practice guideline for the management of tobacco use. Washington, DC: Department of Veteran Affairs; 2004:81.
Yes, taking this step helps asthmatic children, and may even help nonasthmatic children. In families of asthmatic children, education to reduce exposure to secondhand smoke leads to fewer medical visits (strength of recommendation [SOR]: B, a single randomized, controlled trial). The effects of educating families of nonasthmatic children about secondhand smoke are not known, but parents who smoke outside expose their children to much less nicotine than parents who smoke in the house (SOR: B, cohort studies and cross-sectional surveys).
Evidence summary
Parent education reduces clinic visits for asthmatic children
A 2001 trial randomized 81 families with a smoking parent and an asthmatic child between 3 and 12 years of age to 3 sessions of behavioral and educational counseling or usual care at an outpatient asthma clinic.1 Parental education included information on second-hand smoke, basic asthma education, and feedback about urine cotinine levels (a marker of nicotine absorption). Behavioral counseling focused on reducing second-hand smoke exposure by caregivers.
The education group had a significantly reduced risk of 2 or more asthma-related clinic visits in the following 12 months compared with usual care (odds ratio=0.32; P=.03; number needed to treat=5). No significant decrease was noted in mean urine cotinine levels between groups (adjusted mean difference=-0.38 ng/mg favoring education; P=.26).
A similar trial that measured changes in urine cotinine randomized 91 families with a smoking parent and an asthmatic child into 3 groups:2
- A control group received usual care (regular office visits at an asthma clinic and medication management)
- A monitoring group used a parental smoking diary and a children’s asthma symptom diary
- A counseling group received 5 counseling sessions and also kept diaries. An environmental monitor in the home was used to assess exposure to secondhand smoke.
In the counseling group, 21.4% of patients (6 of 28) maintained 0% exposure throughout the 30-month trial period compared with 3.6% and 3.8% in the monitoring and control groups, respectively (P<.05 for comparison of counseling group to monitoring and control).
Banning indoor smoking sharply cuts nicotine exposure
No data are available on education about second-hand smoke in families with nonasthmatic children. However, strong evidence suggests that smoking outside the house reduces exposure generally.
A 2003 cross-sectional survey of 164 households in the United Kingdom with at least 1 smoking parent and 1 bottle-fed infant looked for a correlation between strategies to reduce second-hand smoke and urine cotinine-to-creatinine ratios in the infants.3 Parents were classified into 3 groups according to whether they maintained a strict ban on smoking in the home, a less strict ban (smoking at home but not near the infant), or no ban.
The mean infant urinary cotinine-to-creatinine ratio was 2.43 in the no-ban group and 2.61 in the less-strict ban group (difference not significant). The combined mean for these 2 groups—2.58—was significantly higher than the mean of 1.26 in the strictest group (P<.001).
A later study recruited a convenience sample of 49 interested families with a smoking mother and a nonbreastfeeding infant between 2 and 12 months of age.4 Families were classified by smoking history into one of 3 groups: nonsmoking households, smoking households where efforts were made to limit smoke exposure, and smoking households where no efforts were made to limit exposure. Urine samples were obtained 3 times over 1 week. Urine cotinine levels in infants averaged 0.33 ng/mL in nonsmoking households, 2.47 ng/mL in smoking households with limited exposure, and 15.47 ng/mL in smoking households with unlimited exposure (P<.001 for all comparisons).
A case-control study that recruited families with asthmatic and nonasthmatic children assessed the effectiveness of parental behaviors to reduce second-hand smoke in 182 households with 1 smoking parent and a child between 6 and 12 years of age.5 Researchers measured room air nicotine and salivary cotinine concentrations.
The nicotine levels on children’s belts and in their bedrooms and the family room were approximately 3 log units lower in houses with strict smoking bans compared with households with any degree of indoor smoking (P<.0001). Similarly, salivary cotinine levels were approximately 4 log units lower in children of households with indoor smoking bans (P<.0001).
Recommendations
The United States Preventive Services Task Force (USPSTF) strongly recommends that physicians help all smoking adults to quit.6 The American Academy of Family Physicians endorses the USPSTF position and further advises that smoking parents be counseled about the health effects of environmental tobacco smoke on their children.7
The American Academy of Pediatrics8 and the Veterans Administration9 recommend urging parents to stop smoking to prevent serious health implications for their children; they further encourage pediatric clinicians to offer parents advice on quitting in order to limit children’s exposure to second-hand smoke.
Yes, taking this step helps asthmatic children, and may even help nonasthmatic children. In families of asthmatic children, education to reduce exposure to secondhand smoke leads to fewer medical visits (strength of recommendation [SOR]: B, a single randomized, controlled trial). The effects of educating families of nonasthmatic children about secondhand smoke are not known, but parents who smoke outside expose their children to much less nicotine than parents who smoke in the house (SOR: B, cohort studies and cross-sectional surveys).
Evidence summary
Parent education reduces clinic visits for asthmatic children
A 2001 trial randomized 81 families with a smoking parent and an asthmatic child between 3 and 12 years of age to 3 sessions of behavioral and educational counseling or usual care at an outpatient asthma clinic.1 Parental education included information on second-hand smoke, basic asthma education, and feedback about urine cotinine levels (a marker of nicotine absorption). Behavioral counseling focused on reducing second-hand smoke exposure by caregivers.
The education group had a significantly reduced risk of 2 or more asthma-related clinic visits in the following 12 months compared with usual care (odds ratio=0.32; P=.03; number needed to treat=5). No significant decrease was noted in mean urine cotinine levels between groups (adjusted mean difference=-0.38 ng/mg favoring education; P=.26).
A similar trial that measured changes in urine cotinine randomized 91 families with a smoking parent and an asthmatic child into 3 groups:2
- A control group received usual care (regular office visits at an asthma clinic and medication management)
- A monitoring group used a parental smoking diary and a children’s asthma symptom diary
- A counseling group received 5 counseling sessions and also kept diaries. An environmental monitor in the home was used to assess exposure to secondhand smoke.
In the counseling group, 21.4% of patients (6 of 28) maintained 0% exposure throughout the 30-month trial period compared with 3.6% and 3.8% in the monitoring and control groups, respectively (P<.05 for comparison of counseling group to monitoring and control).
Banning indoor smoking sharply cuts nicotine exposure
No data are available on education about second-hand smoke in families with nonasthmatic children. However, strong evidence suggests that smoking outside the house reduces exposure generally.
A 2003 cross-sectional survey of 164 households in the United Kingdom with at least 1 smoking parent and 1 bottle-fed infant looked for a correlation between strategies to reduce second-hand smoke and urine cotinine-to-creatinine ratios in the infants.3 Parents were classified into 3 groups according to whether they maintained a strict ban on smoking in the home, a less strict ban (smoking at home but not near the infant), or no ban.
The mean infant urinary cotinine-to-creatinine ratio was 2.43 in the no-ban group and 2.61 in the less-strict ban group (difference not significant). The combined mean for these 2 groups—2.58—was significantly higher than the mean of 1.26 in the strictest group (P<.001).
A later study recruited a convenience sample of 49 interested families with a smoking mother and a nonbreastfeeding infant between 2 and 12 months of age.4 Families were classified by smoking history into one of 3 groups: nonsmoking households, smoking households where efforts were made to limit smoke exposure, and smoking households where no efforts were made to limit exposure. Urine samples were obtained 3 times over 1 week. Urine cotinine levels in infants averaged 0.33 ng/mL in nonsmoking households, 2.47 ng/mL in smoking households with limited exposure, and 15.47 ng/mL in smoking households with unlimited exposure (P<.001 for all comparisons).
A case-control study that recruited families with asthmatic and nonasthmatic children assessed the effectiveness of parental behaviors to reduce second-hand smoke in 182 households with 1 smoking parent and a child between 6 and 12 years of age.5 Researchers measured room air nicotine and salivary cotinine concentrations.
The nicotine levels on children’s belts and in their bedrooms and the family room were approximately 3 log units lower in houses with strict smoking bans compared with households with any degree of indoor smoking (P<.0001). Similarly, salivary cotinine levels were approximately 4 log units lower in children of households with indoor smoking bans (P<.0001).
Recommendations
The United States Preventive Services Task Force (USPSTF) strongly recommends that physicians help all smoking adults to quit.6 The American Academy of Family Physicians endorses the USPSTF position and further advises that smoking parents be counseled about the health effects of environmental tobacco smoke on their children.7
The American Academy of Pediatrics8 and the Veterans Administration9 recommend urging parents to stop smoking to prevent serious health implications for their children; they further encourage pediatric clinicians to offer parents advice on quitting in order to limit children’s exposure to second-hand smoke.
1. Wilson SR, Yamada EG, Sudhaker R, et al. A controlled trial of an environmental tobacco smoke reduction intervention in low-income children with asthma. Chest. 2001;120:1709-1722.
2. Wahlgren DR, Hovell MF, Meltzer SB, et al. Reduction of environmental tobacco smoke exposure in asthmatic children: a 2-year follow-up. Chest. 1997;111:81-88.
3. Blackburn C, Spencer N, Bonas S, et al. Effect of strategies to reduce exposure of infants to environmental tobacco smoke in the home: cross sectional survey. BMJ. 2003;327:257-261.
4. Matt GE, Quintana PJ, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control. 2004;13:29-37.
5. Wambolt FS, Balkissoon RC, Rankin AE, et al. Correlates of household smoking bans in low-income families of children with and without asthma. Fam Process. 2008;47:81-94.
6. US Preventive Services Task Force. Counseling to prevent tobacco use and tobacco-caused disease. Rockville, Md: Agency for Healthcare Research and Quality; 2003. Available at: www.ahrq.gov/clinic/uspstf/uspstbac.htm. Accessed september 11, 2008.
7. AAFP Summary of Recommendations for Clinical Preventive Services. Revision 6.3. Leawood, Kan: American Academy of Family Physicians (AAFP); 2007.
8. American Academy of Pediatrics. Tobacco’s toll: implications for the pediatrician. Pediatrics. 2001;107:794-798.
9. Veterans Administration Department of Defense VA/ DOD clinical practice guideline for the management of tobacco use. Washington, DC: Department of Veteran Affairs; 2004:81.
1. Wilson SR, Yamada EG, Sudhaker R, et al. A controlled trial of an environmental tobacco smoke reduction intervention in low-income children with asthma. Chest. 2001;120:1709-1722.
2. Wahlgren DR, Hovell MF, Meltzer SB, et al. Reduction of environmental tobacco smoke exposure in asthmatic children: a 2-year follow-up. Chest. 1997;111:81-88.
3. Blackburn C, Spencer N, Bonas S, et al. Effect of strategies to reduce exposure of infants to environmental tobacco smoke in the home: cross sectional survey. BMJ. 2003;327:257-261.
4. Matt GE, Quintana PJ, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control. 2004;13:29-37.
5. Wambolt FS, Balkissoon RC, Rankin AE, et al. Correlates of household smoking bans in low-income families of children with and without asthma. Fam Process. 2008;47:81-94.
6. US Preventive Services Task Force. Counseling to prevent tobacco use and tobacco-caused disease. Rockville, Md: Agency for Healthcare Research and Quality; 2003. Available at: www.ahrq.gov/clinic/uspstf/uspstbac.htm. Accessed september 11, 2008.
7. AAFP Summary of Recommendations for Clinical Preventive Services. Revision 6.3. Leawood, Kan: American Academy of Family Physicians (AAFP); 2007.
8. American Academy of Pediatrics. Tobacco’s toll: implications for the pediatrician. Pediatrics. 2001;107:794-798.
9. Veterans Administration Department of Defense VA/ DOD clinical practice guideline for the management of tobacco use. Washington, DC: Department of Veteran Affairs; 2004:81.
Evidence-based answers from the Family Physicians Inquiries Network