User login
Aspirin reduces recurrent preeclampsia in real-world study
LAS VEGAS – Using low-dose aspirin during pregnancy significantly reduced the risk of recurrent preeclampsia, according to results of a new study.
“The net benefit of aspirin is substantial,” Mary Catherine Tolcher, MD, the study’s lead author said. “The number needed to treat to prevent one case of recurrent preeclampsia is six... The cost of daily low-dose aspirin for the duration of one pregnancy is about $4.00. Comparatively, the cost to prevent one case of eclampsia is approximately $21,000.”
Dr. Tolcher, a postdoctorate fellow in ob.gyn. at Baylor College of Medicine, Houston, and her colleagues found a total of 417 at-risk women in the institution’s labor and delivery database during the August 2011–June 2016 study period; 284 were identified before the guidelines were implemented in 2014, while 133 women were identified as high-risk after guideline implementation.
While nearly one-third (32.4%) of women with a history of preeclampsia had a recurrence in the before group, the recurrence rate fell to 16.5% in the after group, who had been instructed to take low-dose aspirin in accordance with the guidelines. When the investigators calculated the fully adjusted odds ratio for recurrent preeclampsia, they found a reduction of about 30% in recurrent preeclampsia [aOR, 0.71; 95% confidence interval, 0.52-0.95].
“This decrease is greater than the approximately 10 to 15 percent reduction that has been previously reported in clinical trials, and different from the meta-analysis that prompted the national recommendations,” Dr. Tolcher said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
She and her colleagues hypothesize that the greater effect size may be attributable to limiting the study population to the higher-risk group of women with a history of preeclampsia. Alternatively, she said, aspirin’s pharmacodynamics can differ by race, so racial differences between study populations may also be at play.
One of the causative mechanisms for preeclampsia is thought to be impaired trophoblastic remodeling that impedes development of the low-resistance vascular system of the maternal-fetal unit. “The resulting pathophysiology is multisystemic and includes vascular and endothelial dysfunction, placental ischemia, and an inflammatory and stress response,” Dr. Tolcher said. Overproduction of thromboxanes, she said, plays a role in this process. Aspirin’s inhibition of cyclooxygenase-2 (COX-2) enzymes and thromboxanes is thought to mitigate the vasoconstriction and endothelial dysfunction seen in preeclampsia, she said.
A secondary outcome measure in the study was use of magnesium sulfate, and after guideline implementation “there was a trend toward reduced use of magnesium sulfate, which we reserved for preeclampsia with severe features,” Dr. Tolcher said.
There was no difference in the incidence of preterm deliveries, another secondary outcome measure, after the aspirin guidelines were implemented.
There were some differences in characteristics between the before and after groups: the ratio of Hispanic women with preeclampsia was significantly lower in the after group (P less than .0001). The distribution of method of payment shifted, with more private pay patients, fewer Children’s Health Insurance Program (CHIP) patients, and fewer “other” payment method patients in the after group. Though Dr. Tolcher reported that type 1 diabetes was seen more often in the after group, the rates of any kind of pre-pregnancy diabetes were similar between the groups (6.33% before; 4.26% after).
Potentially confounding variables were controlled by means of logistic regression analysis. Women with multiple gestations were excluded, and only the first pregnancy after a previous episode of preeclampsia was studied.
Otherwise, demographics and other patient characteristics – including rates of chronic hypertension, were similar between the before and after groups. About a quarter of the patients in each group had a history of preterm delivery, and the median age at delivery for both was 38 years.
Within the total group that had recurrent preeclampsia, “maternal age greater than 35, type 2 diabetes, chronic hypertension, and a history of preterm preeclampsia were significantly associated with recurrent preeclampsia,” Dr. Tolcher said.
Study limitations included the retrospective nature, the inclusion of only women who had a prior history of preeclampsia, and the investigators’ inability to determine whether patients were adherent to recommendations for aspirin use. However, the study represents actual clinical use, Dr. Tolcher said, and addresses a real need. “Preeclampsia is responsible for 75,000 maternal deaths annually, and accounts for 15% of the preterm births in the U.S.,” she said.
Dr. Tolcher reported having no relevant financial disclosures.
[email protected]
On Twitter @karioakes
LAS VEGAS – Using low-dose aspirin during pregnancy significantly reduced the risk of recurrent preeclampsia, according to results of a new study.
“The net benefit of aspirin is substantial,” Mary Catherine Tolcher, MD, the study’s lead author said. “The number needed to treat to prevent one case of recurrent preeclampsia is six... The cost of daily low-dose aspirin for the duration of one pregnancy is about $4.00. Comparatively, the cost to prevent one case of eclampsia is approximately $21,000.”
Dr. Tolcher, a postdoctorate fellow in ob.gyn. at Baylor College of Medicine, Houston, and her colleagues found a total of 417 at-risk women in the institution’s labor and delivery database during the August 2011–June 2016 study period; 284 were identified before the guidelines were implemented in 2014, while 133 women were identified as high-risk after guideline implementation.
While nearly one-third (32.4%) of women with a history of preeclampsia had a recurrence in the before group, the recurrence rate fell to 16.5% in the after group, who had been instructed to take low-dose aspirin in accordance with the guidelines. When the investigators calculated the fully adjusted odds ratio for recurrent preeclampsia, they found a reduction of about 30% in recurrent preeclampsia [aOR, 0.71; 95% confidence interval, 0.52-0.95].
“This decrease is greater than the approximately 10 to 15 percent reduction that has been previously reported in clinical trials, and different from the meta-analysis that prompted the national recommendations,” Dr. Tolcher said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
She and her colleagues hypothesize that the greater effect size may be attributable to limiting the study population to the higher-risk group of women with a history of preeclampsia. Alternatively, she said, aspirin’s pharmacodynamics can differ by race, so racial differences between study populations may also be at play.
One of the causative mechanisms for preeclampsia is thought to be impaired trophoblastic remodeling that impedes development of the low-resistance vascular system of the maternal-fetal unit. “The resulting pathophysiology is multisystemic and includes vascular and endothelial dysfunction, placental ischemia, and an inflammatory and stress response,” Dr. Tolcher said. Overproduction of thromboxanes, she said, plays a role in this process. Aspirin’s inhibition of cyclooxygenase-2 (COX-2) enzymes and thromboxanes is thought to mitigate the vasoconstriction and endothelial dysfunction seen in preeclampsia, she said.
A secondary outcome measure in the study was use of magnesium sulfate, and after guideline implementation “there was a trend toward reduced use of magnesium sulfate, which we reserved for preeclampsia with severe features,” Dr. Tolcher said.
There was no difference in the incidence of preterm deliveries, another secondary outcome measure, after the aspirin guidelines were implemented.
There were some differences in characteristics between the before and after groups: the ratio of Hispanic women with preeclampsia was significantly lower in the after group (P less than .0001). The distribution of method of payment shifted, with more private pay patients, fewer Children’s Health Insurance Program (CHIP) patients, and fewer “other” payment method patients in the after group. Though Dr. Tolcher reported that type 1 diabetes was seen more often in the after group, the rates of any kind of pre-pregnancy diabetes were similar between the groups (6.33% before; 4.26% after).
Potentially confounding variables were controlled by means of logistic regression analysis. Women with multiple gestations were excluded, and only the first pregnancy after a previous episode of preeclampsia was studied.
Otherwise, demographics and other patient characteristics – including rates of chronic hypertension, were similar between the before and after groups. About a quarter of the patients in each group had a history of preterm delivery, and the median age at delivery for both was 38 years.
Within the total group that had recurrent preeclampsia, “maternal age greater than 35, type 2 diabetes, chronic hypertension, and a history of preterm preeclampsia were significantly associated with recurrent preeclampsia,” Dr. Tolcher said.
Study limitations included the retrospective nature, the inclusion of only women who had a prior history of preeclampsia, and the investigators’ inability to determine whether patients were adherent to recommendations for aspirin use. However, the study represents actual clinical use, Dr. Tolcher said, and addresses a real need. “Preeclampsia is responsible for 75,000 maternal deaths annually, and accounts for 15% of the preterm births in the U.S.,” she said.
Dr. Tolcher reported having no relevant financial disclosures.
[email protected]
On Twitter @karioakes
LAS VEGAS – Using low-dose aspirin during pregnancy significantly reduced the risk of recurrent preeclampsia, according to results of a new study.
“The net benefit of aspirin is substantial,” Mary Catherine Tolcher, MD, the study’s lead author said. “The number needed to treat to prevent one case of recurrent preeclampsia is six... The cost of daily low-dose aspirin for the duration of one pregnancy is about $4.00. Comparatively, the cost to prevent one case of eclampsia is approximately $21,000.”
Dr. Tolcher, a postdoctorate fellow in ob.gyn. at Baylor College of Medicine, Houston, and her colleagues found a total of 417 at-risk women in the institution’s labor and delivery database during the August 2011–June 2016 study period; 284 were identified before the guidelines were implemented in 2014, while 133 women were identified as high-risk after guideline implementation.
While nearly one-third (32.4%) of women with a history of preeclampsia had a recurrence in the before group, the recurrence rate fell to 16.5% in the after group, who had been instructed to take low-dose aspirin in accordance with the guidelines. When the investigators calculated the fully adjusted odds ratio for recurrent preeclampsia, they found a reduction of about 30% in recurrent preeclampsia [aOR, 0.71; 95% confidence interval, 0.52-0.95].
“This decrease is greater than the approximately 10 to 15 percent reduction that has been previously reported in clinical trials, and different from the meta-analysis that prompted the national recommendations,” Dr. Tolcher said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
She and her colleagues hypothesize that the greater effect size may be attributable to limiting the study population to the higher-risk group of women with a history of preeclampsia. Alternatively, she said, aspirin’s pharmacodynamics can differ by race, so racial differences between study populations may also be at play.
One of the causative mechanisms for preeclampsia is thought to be impaired trophoblastic remodeling that impedes development of the low-resistance vascular system of the maternal-fetal unit. “The resulting pathophysiology is multisystemic and includes vascular and endothelial dysfunction, placental ischemia, and an inflammatory and stress response,” Dr. Tolcher said. Overproduction of thromboxanes, she said, plays a role in this process. Aspirin’s inhibition of cyclooxygenase-2 (COX-2) enzymes and thromboxanes is thought to mitigate the vasoconstriction and endothelial dysfunction seen in preeclampsia, she said.
A secondary outcome measure in the study was use of magnesium sulfate, and after guideline implementation “there was a trend toward reduced use of magnesium sulfate, which we reserved for preeclampsia with severe features,” Dr. Tolcher said.
There was no difference in the incidence of preterm deliveries, another secondary outcome measure, after the aspirin guidelines were implemented.
There were some differences in characteristics between the before and after groups: the ratio of Hispanic women with preeclampsia was significantly lower in the after group (P less than .0001). The distribution of method of payment shifted, with more private pay patients, fewer Children’s Health Insurance Program (CHIP) patients, and fewer “other” payment method patients in the after group. Though Dr. Tolcher reported that type 1 diabetes was seen more often in the after group, the rates of any kind of pre-pregnancy diabetes were similar between the groups (6.33% before; 4.26% after).
Potentially confounding variables were controlled by means of logistic regression analysis. Women with multiple gestations were excluded, and only the first pregnancy after a previous episode of preeclampsia was studied.
Otherwise, demographics and other patient characteristics – including rates of chronic hypertension, were similar between the before and after groups. About a quarter of the patients in each group had a history of preterm delivery, and the median age at delivery for both was 38 years.
Within the total group that had recurrent preeclampsia, “maternal age greater than 35, type 2 diabetes, chronic hypertension, and a history of preterm preeclampsia were significantly associated with recurrent preeclampsia,” Dr. Tolcher said.
Study limitations included the retrospective nature, the inclusion of only women who had a prior history of preeclampsia, and the investigators’ inability to determine whether patients were adherent to recommendations for aspirin use. However, the study represents actual clinical use, Dr. Tolcher said, and addresses a real need. “Preeclampsia is responsible for 75,000 maternal deaths annually, and accounts for 15% of the preterm births in the U.S.,” she said.
Dr. Tolcher reported having no relevant financial disclosures.
[email protected]
On Twitter @karioakes
AT THE PREGNANCY MEETING
Key clinical point:
Major finding: The adjusted odds ratio for recurrent preeclampsia was 0.71 after implementing U.S. Preventive Services Task Force (USPSTF) guidelines for aspirin administration.
Data source: Retrospective cohort study of 17,256 deliveries at a single academic medical center before and after implementation of USPSTF aspirin guidelines, with 417 cases of recurrent preeclampsia identified.
Disclosures: Dr. Tolcher reported having no relevant financial disclosures.
Obstetric perineal trauma prevented with SAFE PASSAGES
LAS VEGAS – Severe obstetric perineal trauma can often be avoided, even in operative deliveries, with the use of a suite of evidence-based interventions, according to findings from two prospective studies.
Collectively, these interventions resulted in significant reductions in third- and fourth-degree perineal lacerations in both military and civilian settings, according to research presented at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Developed by the Military Health System, the SAFE PASSAGES protocol brings together interventions that help achieve a controlled delivery over a relaxed perineum, minimizing risk for maternal obstetric trauma.
The entire SAFE PASSAGES curriculum is available free online.
Military results

In a prospective cohort design, 272,161 deliveries conducted before the 2011 implementation of the SAFE PASSAGES training program were compared with 451,446 postimplementation deliveries. Primary outcome measures were the incidence of third- and fourth-degree perineal lacerations during vaginal deliveries with and without instrumentation.
For vaginal deliveries with instrumentation within one service branch of the military medical system (Service X), implementation of SAFE PASSAGES training was associated with a 63.6% reduction in third- and fourth-degree perineal lacerations, compared with preintervention rates (P less than .001).
The other two services – Service Y and Service Z – received just administrative encouragement and saw a 15.5% reduction and a 12.6% increase in significant obstetric trauma when instrumentation-assisted vaginal deliveries were performed (P = .04 and .30, respectively), according to Merlin Fausett, MD, an ob.gyn. currently in private practice in Missoula, Mont., who led the SAFE PASSAGES efforts before retiring from the Air Force.
For vaginal deliveries performed without instrumentation, the rates also fell for Service X, which saw a 41.8% reduction in third- and fourth-degree perineal lacerations (P less than .002). The other services saw a 16% increase and a 12% decrease with administrative encouragement alone (P = .48 and .08, respectively).
Though the military training program had initially been conducted in person, Dr. Fausett said that the program was switched to web-based simulations because of budget constraints. Efficacy remained high, he said.
Civilian results
When the team-based simulation that formed the core of the military SAFE PASSAGES training was rolled out in a large civilian health care system, similar improvements were seen.
Over an 18-month period, 675 nurses, midwives, and physicians received simulation-based training in the SAFE PASSAGES techniques. Overall, severe perineal laceration rates in the civilian facilities were down by 38.53% after adoption of SAFE PASSAGES.
“We have really achieved a culture shift,” said Emily Marko, MD, an ob.gyn. and clerkship director for the Virginia Commonwealth University School of Medicine Inova Campus in Falls Church, Va. “This really requires the whole delivery team to get involved: the patient, the patient’s support person, the nurses, any midwives or doulas that are there,” she said. “To tell you the truth, this whole program is about paying attention to the perineum, and not rushing delivery.”
Posttraining surveys showed that 95% of providers had changed their practice patterns after training in the delivery strategies.
Interventions
The emphasis in SAFE PASSAGES is to achieve a slow, controlled delivery and to minimize strain on the perineum by means of conditioning, relaxation, and positioning.
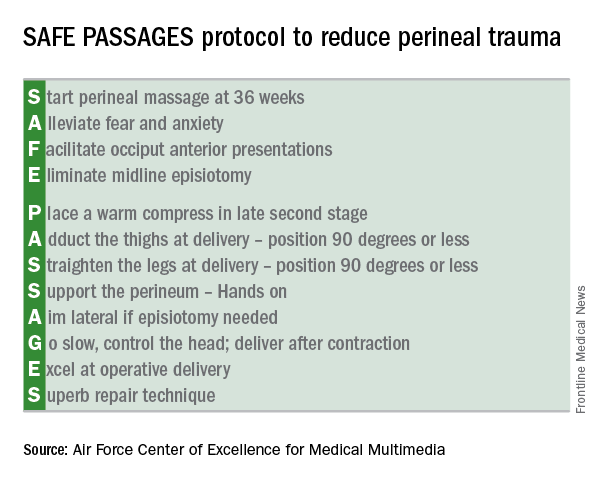
The first intervention is to have pregnant women “start” perineal massage at 36 weeks. Next, providers are urged to “alleviate” maternal fears. Providers are also encouraged to recognize posterior presentations, and to “facilitate” an anterior presentation through rotation. The “E” in SAFE stands for “eliminating” midline episiotomies – one of the more difficult practices to shift, according to both Dr. Fausett and Dr. Marko.
Despite a wealth of evidence showing fewer anal sphincter disruptions and better overall outcomes, it’s been difficult to convince U.S. physicians to adopt the mediolateral episiotomy technique that’s widely adopted in Europe, they said.
The protocol calls for “placing” a warm compress over the perineum during labor to encourage relaxation and stretching. Though prenatal perineal massage is encouraged, Dr. Marko said that intrapartum massage is not, as it’s thought to contribute to edema when performed during labor.
During delivery, leg positioning to reduce stretching of the perineum is also important: The thighs should be “adducted” to 90 degrees or less, and “straightened” to 90 degrees or less as well. Though this can make “a bit of a tight space” for the delivering physician, Dr. Marko said, it really “helps engage the pelvic muscles to support the perineum,” and a few technique adjustments make the position workable.
The perineum should be “supported” during delivery by one hand of the delivering practitioner forming a U shape with the thumb and forefinger, with the first webspace overlying the posterior fourchette. Reinforcing the importance of avoiding a midline episiotomy, the “A” of passages stands for “aiming” lateral when an episiotomy is needed.
During the delivery, the physician should “go” slow, controlling the head and delivering after, rather than during, a contraction.
It’s important to be comfortable with forceps deliveries and vacuum extractions in order to minimize both maternal and fetal trauma; thus, physicians should “excel” at operative delivery, according to the protocol.
The SAFE PASSAGES website includes comprehensive explanations, with graphics and demonstrations using a model, of both forceps and vacuum delivery techniques.
Finally, should a laceration occur, the SAFE PASSAGES website provides detailed explanations of repair techniques, with an emphasis on understanding perineal, vaginal, and anal anatomy so “superb” approximation and repair can be achieved.
Though obstetric trauma may not be life threatening, it’s still associated with significant and persistent morbidity. When perineal and pelvic floor trauma disrupts the anal sphincter, anal incontinence can occur, even after a meticulous attempt at repair. Perineal and pelvic floor trauma can result in a host of urinary and sexual problems as well.
After the intensive training period, Dr. Fausett said, “laceration rates can creep back up if people forget about it and stop paying attention to it. But where it becomes a culture, the rates can stay low. Standardized training can reduce perineal trauma rates without increasing cesarean or neonatal trauma rates,” Dr. Fausett said.
Dr. Marko and Dr. Fausett reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
LAS VEGAS – Severe obstetric perineal trauma can often be avoided, even in operative deliveries, with the use of a suite of evidence-based interventions, according to findings from two prospective studies.
Collectively, these interventions resulted in significant reductions in third- and fourth-degree perineal lacerations in both military and civilian settings, according to research presented at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Developed by the Military Health System, the SAFE PASSAGES protocol brings together interventions that help achieve a controlled delivery over a relaxed perineum, minimizing risk for maternal obstetric trauma.
The entire SAFE PASSAGES curriculum is available free online.

Military results

In a prospective cohort design, 272,161 deliveries conducted before the 2011 implementation of the SAFE PASSAGES training program were compared with 451,446 postimplementation deliveries. Primary outcome measures were the incidence of third- and fourth-degree perineal lacerations during vaginal deliveries with and without instrumentation.
For vaginal deliveries with instrumentation within one service branch of the military medical system (Service X), implementation of SAFE PASSAGES training was associated with a 63.6% reduction in third- and fourth-degree perineal lacerations, compared with preintervention rates (P less than .001).
The other two services – Service Y and Service Z – received just administrative encouragement and saw a 15.5% reduction and a 12.6% increase in significant obstetric trauma when instrumentation-assisted vaginal deliveries were performed (P = .04 and .30, respectively), according to Merlin Fausett, MD, an ob.gyn. currently in private practice in Missoula, Mont., who led the SAFE PASSAGES efforts before retiring from the Air Force.
For vaginal deliveries performed without instrumentation, the rates also fell for Service X, which saw a 41.8% reduction in third- and fourth-degree perineal lacerations (P less than .002). The other services saw a 16% increase and a 12% decrease with administrative encouragement alone (P = .48 and .08, respectively).
Though the military training program had initially been conducted in person, Dr. Fausett said that the program was switched to web-based simulations because of budget constraints. Efficacy remained high, he said.
Civilian results
When the team-based simulation that formed the core of the military SAFE PASSAGES training was rolled out in a large civilian health care system, similar improvements were seen.
Over an 18-month period, 675 nurses, midwives, and physicians received simulation-based training in the SAFE PASSAGES techniques. Overall, severe perineal laceration rates in the civilian facilities were down by 38.53% after adoption of SAFE PASSAGES.
“We have really achieved a culture shift,” said Emily Marko, MD, an ob.gyn. and clerkship director for the Virginia Commonwealth University School of Medicine Inova Campus in Falls Church, Va. “This really requires the whole delivery team to get involved: the patient, the patient’s support person, the nurses, any midwives or doulas that are there,” she said. “To tell you the truth, this whole program is about paying attention to the perineum, and not rushing delivery.”
Posttraining surveys showed that 95% of providers had changed their practice patterns after training in the delivery strategies.
Interventions
The emphasis in SAFE PASSAGES is to achieve a slow, controlled delivery and to minimize strain on the perineum by means of conditioning, relaxation, and positioning.
The first intervention is to have pregnant women “start” perineal massage at 36 weeks. Next, providers are urged to “alleviate” maternal fears. Providers are also encouraged to recognize posterior presentations, and to “facilitate” an anterior presentation through rotation. The “E” in SAFE stands for “eliminating” midline episiotomies – one of the more difficult practices to shift, according to both Dr. Fausett and Dr. Marko.
Despite a wealth of evidence showing fewer anal sphincter disruptions and better overall outcomes, it’s been difficult to convince U.S. physicians to adopt the mediolateral episiotomy technique that’s widely adopted in Europe, they said.
The protocol calls for “placing” a warm compress over the perineum during labor to encourage relaxation and stretching. Though prenatal perineal massage is encouraged, Dr. Marko said that intrapartum massage is not, as it’s thought to contribute to edema when performed during labor.
During delivery, leg positioning to reduce stretching of the perineum is also important: The thighs should be “adducted” to 90 degrees or less, and “straightened” to 90 degrees or less as well. Though this can make “a bit of a tight space” for the delivering physician, Dr. Marko said, it really “helps engage the pelvic muscles to support the perineum,” and a few technique adjustments make the position workable.
The perineum should be “supported” during delivery by one hand of the delivering practitioner forming a U shape with the thumb and forefinger, with the first webspace overlying the posterior fourchette. Reinforcing the importance of avoiding a midline episiotomy, the “A” of passages stands for “aiming” lateral when an episiotomy is needed.
During the delivery, the physician should “go” slow, controlling the head and delivering after, rather than during, a contraction.
It’s important to be comfortable with forceps deliveries and vacuum extractions in order to minimize both maternal and fetal trauma; thus, physicians should “excel” at operative delivery, according to the protocol.
The SAFE PASSAGES website includes comprehensive explanations, with graphics and demonstrations using a model, of both forceps and vacuum delivery techniques.
Finally, should a laceration occur, the SAFE PASSAGES website provides detailed explanations of repair techniques, with an emphasis on understanding perineal, vaginal, and anal anatomy so “superb” approximation and repair can be achieved.
Though obstetric trauma may not be life threatening, it’s still associated with significant and persistent morbidity. When perineal and pelvic floor trauma disrupts the anal sphincter, anal incontinence can occur, even after a meticulous attempt at repair. Perineal and pelvic floor trauma can result in a host of urinary and sexual problems as well.
After the intensive training period, Dr. Fausett said, “laceration rates can creep back up if people forget about it and stop paying attention to it. But where it becomes a culture, the rates can stay low. Standardized training can reduce perineal trauma rates without increasing cesarean or neonatal trauma rates,” Dr. Fausett said.
Dr. Marko and Dr. Fausett reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
LAS VEGAS – Severe obstetric perineal trauma can often be avoided, even in operative deliveries, with the use of a suite of evidence-based interventions, according to findings from two prospective studies.
Collectively, these interventions resulted in significant reductions in third- and fourth-degree perineal lacerations in both military and civilian settings, according to research presented at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Developed by the Military Health System, the SAFE PASSAGES protocol brings together interventions that help achieve a controlled delivery over a relaxed perineum, minimizing risk for maternal obstetric trauma.
The entire SAFE PASSAGES curriculum is available free online.

Military results

In a prospective cohort design, 272,161 deliveries conducted before the 2011 implementation of the SAFE PASSAGES training program were compared with 451,446 postimplementation deliveries. Primary outcome measures were the incidence of third- and fourth-degree perineal lacerations during vaginal deliveries with and without instrumentation.
For vaginal deliveries with instrumentation within one service branch of the military medical system (Service X), implementation of SAFE PASSAGES training was associated with a 63.6% reduction in third- and fourth-degree perineal lacerations, compared with preintervention rates (P less than .001).
The other two services – Service Y and Service Z – received just administrative encouragement and saw a 15.5% reduction and a 12.6% increase in significant obstetric trauma when instrumentation-assisted vaginal deliveries were performed (P = .04 and .30, respectively), according to Merlin Fausett, MD, an ob.gyn. currently in private practice in Missoula, Mont., who led the SAFE PASSAGES efforts before retiring from the Air Force.
For vaginal deliveries performed without instrumentation, the rates also fell for Service X, which saw a 41.8% reduction in third- and fourth-degree perineal lacerations (P less than .002). The other services saw a 16% increase and a 12% decrease with administrative encouragement alone (P = .48 and .08, respectively).
Though the military training program had initially been conducted in person, Dr. Fausett said that the program was switched to web-based simulations because of budget constraints. Efficacy remained high, he said.
Civilian results
When the team-based simulation that formed the core of the military SAFE PASSAGES training was rolled out in a large civilian health care system, similar improvements were seen.
Over an 18-month period, 675 nurses, midwives, and physicians received simulation-based training in the SAFE PASSAGES techniques. Overall, severe perineal laceration rates in the civilian facilities were down by 38.53% after adoption of SAFE PASSAGES.
“We have really achieved a culture shift,” said Emily Marko, MD, an ob.gyn. and clerkship director for the Virginia Commonwealth University School of Medicine Inova Campus in Falls Church, Va. “This really requires the whole delivery team to get involved: the patient, the patient’s support person, the nurses, any midwives or doulas that are there,” she said. “To tell you the truth, this whole program is about paying attention to the perineum, and not rushing delivery.”
Posttraining surveys showed that 95% of providers had changed their practice patterns after training in the delivery strategies.
Interventions
The emphasis in SAFE PASSAGES is to achieve a slow, controlled delivery and to minimize strain on the perineum by means of conditioning, relaxation, and positioning.
The first intervention is to have pregnant women “start” perineal massage at 36 weeks. Next, providers are urged to “alleviate” maternal fears. Providers are also encouraged to recognize posterior presentations, and to “facilitate” an anterior presentation through rotation. The “E” in SAFE stands for “eliminating” midline episiotomies – one of the more difficult practices to shift, according to both Dr. Fausett and Dr. Marko.
Despite a wealth of evidence showing fewer anal sphincter disruptions and better overall outcomes, it’s been difficult to convince U.S. physicians to adopt the mediolateral episiotomy technique that’s widely adopted in Europe, they said.
The protocol calls for “placing” a warm compress over the perineum during labor to encourage relaxation and stretching. Though prenatal perineal massage is encouraged, Dr. Marko said that intrapartum massage is not, as it’s thought to contribute to edema when performed during labor.
During delivery, leg positioning to reduce stretching of the perineum is also important: The thighs should be “adducted” to 90 degrees or less, and “straightened” to 90 degrees or less as well. Though this can make “a bit of a tight space” for the delivering physician, Dr. Marko said, it really “helps engage the pelvic muscles to support the perineum,” and a few technique adjustments make the position workable.
The perineum should be “supported” during delivery by one hand of the delivering practitioner forming a U shape with the thumb and forefinger, with the first webspace overlying the posterior fourchette. Reinforcing the importance of avoiding a midline episiotomy, the “A” of passages stands for “aiming” lateral when an episiotomy is needed.
During the delivery, the physician should “go” slow, controlling the head and delivering after, rather than during, a contraction.
It’s important to be comfortable with forceps deliveries and vacuum extractions in order to minimize both maternal and fetal trauma; thus, physicians should “excel” at operative delivery, according to the protocol.
The SAFE PASSAGES website includes comprehensive explanations, with graphics and demonstrations using a model, of both forceps and vacuum delivery techniques.
Finally, should a laceration occur, the SAFE PASSAGES website provides detailed explanations of repair techniques, with an emphasis on understanding perineal, vaginal, and anal anatomy so “superb” approximation and repair can be achieved.
Though obstetric trauma may not be life threatening, it’s still associated with significant and persistent morbidity. When perineal and pelvic floor trauma disrupts the anal sphincter, anal incontinence can occur, even after a meticulous attempt at repair. Perineal and pelvic floor trauma can result in a host of urinary and sexual problems as well.
After the intensive training period, Dr. Fausett said, “laceration rates can creep back up if people forget about it and stop paying attention to it. But where it becomes a culture, the rates can stay low. Standardized training can reduce perineal trauma rates without increasing cesarean or neonatal trauma rates,” Dr. Fausett said.
Dr. Marko and Dr. Fausett reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
AT THE PREGNANCY MEETING
Key clinical point:
Major finding: Third- and fourth-degree perineal lacerations dropped by as much as 64% after SAFE PASSAGES training.
Data source: Prospective cohort study of 723,607 military deliveries; prospective study of 675 providers involved in labor and delivery at four civilian hospitals in a large health care system.
Disclosures: Dr. Marko and Dr. Fausett reported having no conflicts of interest.
Universal cervical length screening reduces preterm birth rate
LAS VEGAS – Universal cervical length screening by transvaginal ultrasound in a low-risk cohort resulted in a significant reduction in spontaneous preterm births.
In a single-center retrospective cohort study of more than 13,000 deliveries, the overall preterm birth rate decreased from 3.8% to 2.4% on implementation of universal cervical length screening (P less than .001).
Screening reduced the numbers of both early and late preterm birth rates. Preterm births occurring earlier than 28 weeks’ gestation dropped from 0.3% to 0.1% (P = .04); preterm births before 34 weeks dropped from 1% to 0.5% (P less than .001); and preterm births before 37 weeks dropped from 2.5% to 1.8% (P = .004).
The data were presented by Alex Argyelan, MD, an ob.gyn. resident at St. Joseph Mercy Hospital–Ann Arbor, Mich., at the Annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Argyelan noted that the current Society for Maternal-Fetal Medicine (SMFM) position regarding cervical length screening is that it should not yet be universally mandated for singleton pregnancies without a prior history of preterm birth. However, a 2016 SMFM guideline stated, “Nonetheless, implementation of such a screening strategy can be viewed as reasonable and can be considered by individual practitioners.” This statement was made, he said, in recognition of the fact that “a sonographic short cervix is a powerful predictor of spontaneous preterm birth,” and that progesterone administration for gravid women with shortening cervixes can, for many, forestall spontaneous preterm labor.
First author Pooja Mittal Green, MD, a maternal-fetal medicine specialist at St. Joseph Mercy Hospital, and her associates looked at preterm delivery data before and after implementation of a universal cervical length screening protocol at a single tertiary referral center. The data collection period spanned from January 2013 to May 2014, with implementation of universal cervical length screening in October 2014.
A total of 13,396 women were included in the study, with a small minority of just 0.5% having experienced a prior spontaneous preterm birth. The mean patient age was just under 30 years; most patients were white, mirroring the demographics of the county where the study took place. Pre- and postintervention patient characteristics did not vary significantly.
Almost all patients agreed to cervical length screening by ultrasound, with the numbers climbing from 93% during the first year after implementing the universal screening protocol, to 99.2% in 2016.
All of the sonographers participating in the study were Cervical Length Education and Review (CLEAR) certified, and the institution’s lead sonographer carried out a ongoing quality assurance program.
A shortened cervix (25 mm or less) was found in a total of 114 women (1.7%) who underwent cervical length screening. According to the protocol, if maternal cervical length was 25 mm or less, women were offered treatment to attempt to stave off preterm delivery, according to the usual standard of care.
For women with no prior history of preterm delivery, treatment was vaginal progesterone. For women who had a prior preterm birth, cervical cerclage was offered, as well as vaginal progesterone if the patient was not already on 17-alpha-hydroxyprogesterone caproate.
The determination whether spontaneous preterm birth had occurred was made by reviewing labor and delivery birth logs, with a subsequent individual chart review to make sure that the delivery happened after either spontaneous labor or preterm premature rupture of membranes.
Only women who received prenatal care at the study institution were included in the study, and women who had not received any prenatal care were excluded.
Dr. Argyelan and his colleagues did not see any significant differences between the preterm and term delivery groups in maternal age, body mass index, or ethnicity.
“Among spontaneous preterm deliveries in low-risk women, the proportion of deliveries before 34 weeks was decreased” after the intervention, said Dr. Argyelan, who reported seeing a decrease from 28% early (less than 34 weeks’ gestation) preterm births before the intervention to 17% after the intervention.
In response to an audience question, Dr. Argyelan noted that his institution charges $186 for an ultrasound examination that includes assessment of cervical length, saying, “We have not had significant issues being reimbursed.”
Another audience member asked what the institution policy had been before the universal screening program was implemented. “Before the universal screening study, there was a policy of screening those with a history of preterm birth; also, if sonographers saw what looked like a short cervix on transabdominal exam, then they would do a transvaginal scan to further assess the cervix,” Dr. Argyelan said.
Dr. Argyelan reported having no financial disclosures.
[email protected]
On Twitter @karioakes
LAS VEGAS – Universal cervical length screening by transvaginal ultrasound in a low-risk cohort resulted in a significant reduction in spontaneous preterm births.
In a single-center retrospective cohort study of more than 13,000 deliveries, the overall preterm birth rate decreased from 3.8% to 2.4% on implementation of universal cervical length screening (P less than .001).
Screening reduced the numbers of both early and late preterm birth rates. Preterm births occurring earlier than 28 weeks’ gestation dropped from 0.3% to 0.1% (P = .04); preterm births before 34 weeks dropped from 1% to 0.5% (P less than .001); and preterm births before 37 weeks dropped from 2.5% to 1.8% (P = .004).
The data were presented by Alex Argyelan, MD, an ob.gyn. resident at St. Joseph Mercy Hospital–Ann Arbor, Mich., at the Annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Argyelan noted that the current Society for Maternal-Fetal Medicine (SMFM) position regarding cervical length screening is that it should not yet be universally mandated for singleton pregnancies without a prior history of preterm birth. However, a 2016 SMFM guideline stated, “Nonetheless, implementation of such a screening strategy can be viewed as reasonable and can be considered by individual practitioners.” This statement was made, he said, in recognition of the fact that “a sonographic short cervix is a powerful predictor of spontaneous preterm birth,” and that progesterone administration for gravid women with shortening cervixes can, for many, forestall spontaneous preterm labor.
First author Pooja Mittal Green, MD, a maternal-fetal medicine specialist at St. Joseph Mercy Hospital, and her associates looked at preterm delivery data before and after implementation of a universal cervical length screening protocol at a single tertiary referral center. The data collection period spanned from January 2013 to May 2014, with implementation of universal cervical length screening in October 2014.
A total of 13,396 women were included in the study, with a small minority of just 0.5% having experienced a prior spontaneous preterm birth. The mean patient age was just under 30 years; most patients were white, mirroring the demographics of the county where the study took place. Pre- and postintervention patient characteristics did not vary significantly.
Almost all patients agreed to cervical length screening by ultrasound, with the numbers climbing from 93% during the first year after implementing the universal screening protocol, to 99.2% in 2016.
All of the sonographers participating in the study were Cervical Length Education and Review (CLEAR) certified, and the institution’s lead sonographer carried out a ongoing quality assurance program.
A shortened cervix (25 mm or less) was found in a total of 114 women (1.7%) who underwent cervical length screening. According to the protocol, if maternal cervical length was 25 mm or less, women were offered treatment to attempt to stave off preterm delivery, according to the usual standard of care.
For women with no prior history of preterm delivery, treatment was vaginal progesterone. For women who had a prior preterm birth, cervical cerclage was offered, as well as vaginal progesterone if the patient was not already on 17-alpha-hydroxyprogesterone caproate.
The determination whether spontaneous preterm birth had occurred was made by reviewing labor and delivery birth logs, with a subsequent individual chart review to make sure that the delivery happened after either spontaneous labor or preterm premature rupture of membranes.
Only women who received prenatal care at the study institution were included in the study, and women who had not received any prenatal care were excluded.
Dr. Argyelan and his colleagues did not see any significant differences between the preterm and term delivery groups in maternal age, body mass index, or ethnicity.
“Among spontaneous preterm deliveries in low-risk women, the proportion of deliveries before 34 weeks was decreased” after the intervention, said Dr. Argyelan, who reported seeing a decrease from 28% early (less than 34 weeks’ gestation) preterm births before the intervention to 17% after the intervention.
In response to an audience question, Dr. Argyelan noted that his institution charges $186 for an ultrasound examination that includes assessment of cervical length, saying, “We have not had significant issues being reimbursed.”
Another audience member asked what the institution policy had been before the universal screening program was implemented. “Before the universal screening study, there was a policy of screening those with a history of preterm birth; also, if sonographers saw what looked like a short cervix on transabdominal exam, then they would do a transvaginal scan to further assess the cervix,” Dr. Argyelan said.
Dr. Argyelan reported having no financial disclosures.
[email protected]
On Twitter @karioakes
LAS VEGAS – Universal cervical length screening by transvaginal ultrasound in a low-risk cohort resulted in a significant reduction in spontaneous preterm births.
In a single-center retrospective cohort study of more than 13,000 deliveries, the overall preterm birth rate decreased from 3.8% to 2.4% on implementation of universal cervical length screening (P less than .001).
Screening reduced the numbers of both early and late preterm birth rates. Preterm births occurring earlier than 28 weeks’ gestation dropped from 0.3% to 0.1% (P = .04); preterm births before 34 weeks dropped from 1% to 0.5% (P less than .001); and preterm births before 37 weeks dropped from 2.5% to 1.8% (P = .004).
The data were presented by Alex Argyelan, MD, an ob.gyn. resident at St. Joseph Mercy Hospital–Ann Arbor, Mich., at the Annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Argyelan noted that the current Society for Maternal-Fetal Medicine (SMFM) position regarding cervical length screening is that it should not yet be universally mandated for singleton pregnancies without a prior history of preterm birth. However, a 2016 SMFM guideline stated, “Nonetheless, implementation of such a screening strategy can be viewed as reasonable and can be considered by individual practitioners.” This statement was made, he said, in recognition of the fact that “a sonographic short cervix is a powerful predictor of spontaneous preterm birth,” and that progesterone administration for gravid women with shortening cervixes can, for many, forestall spontaneous preterm labor.
First author Pooja Mittal Green, MD, a maternal-fetal medicine specialist at St. Joseph Mercy Hospital, and her associates looked at preterm delivery data before and after implementation of a universal cervical length screening protocol at a single tertiary referral center. The data collection period spanned from January 2013 to May 2014, with implementation of universal cervical length screening in October 2014.
A total of 13,396 women were included in the study, with a small minority of just 0.5% having experienced a prior spontaneous preterm birth. The mean patient age was just under 30 years; most patients were white, mirroring the demographics of the county where the study took place. Pre- and postintervention patient characteristics did not vary significantly.
Almost all patients agreed to cervical length screening by ultrasound, with the numbers climbing from 93% during the first year after implementing the universal screening protocol, to 99.2% in 2016.
All of the sonographers participating in the study were Cervical Length Education and Review (CLEAR) certified, and the institution’s lead sonographer carried out a ongoing quality assurance program.
A shortened cervix (25 mm or less) was found in a total of 114 women (1.7%) who underwent cervical length screening. According to the protocol, if maternal cervical length was 25 mm or less, women were offered treatment to attempt to stave off preterm delivery, according to the usual standard of care.
For women with no prior history of preterm delivery, treatment was vaginal progesterone. For women who had a prior preterm birth, cervical cerclage was offered, as well as vaginal progesterone if the patient was not already on 17-alpha-hydroxyprogesterone caproate.
The determination whether spontaneous preterm birth had occurred was made by reviewing labor and delivery birth logs, with a subsequent individual chart review to make sure that the delivery happened after either spontaneous labor or preterm premature rupture of membranes.
Only women who received prenatal care at the study institution were included in the study, and women who had not received any prenatal care were excluded.
Dr. Argyelan and his colleagues did not see any significant differences between the preterm and term delivery groups in maternal age, body mass index, or ethnicity.
“Among spontaneous preterm deliveries in low-risk women, the proportion of deliveries before 34 weeks was decreased” after the intervention, said Dr. Argyelan, who reported seeing a decrease from 28% early (less than 34 weeks’ gestation) preterm births before the intervention to 17% after the intervention.
In response to an audience question, Dr. Argyelan noted that his institution charges $186 for an ultrasound examination that includes assessment of cervical length, saying, “We have not had significant issues being reimbursed.”
Another audience member asked what the institution policy had been before the universal screening program was implemented. “Before the universal screening study, there was a policy of screening those with a history of preterm birth; also, if sonographers saw what looked like a short cervix on transabdominal exam, then they would do a transvaginal scan to further assess the cervix,” Dr. Argyelan said.
Dr. Argyelan reported having no financial disclosures.
[email protected]
On Twitter @karioakes
AT THE ANNUAL PREGNANCY MEETING
Key clinical point:
Major finding: Preterm births dropped from 3.8% to 2.4% (P less than .001) after implementing a universal cervical length screening program.
Data source: Retrospective single-site cohort study of 13,396 singleton pregnancies.
Disclosures: Dr. Argyelan reported having no financial disclosures.
Complex congenital heart conditions call for complex care in pregnancy
A new scientific statement from the American Heart Association (AHA) brings together recommendations for management of pregnancy for women with serious congenital heart disease. The 38-page document addresses a wide range of complex congenital heart conditions, presenting a newly unified set of recommendations for care that ranges from preconception counseling, through pregnancy, labor, and delivery, to the postpartum period.
Caring for women with complex congenital heart lesions is becoming more commonplace, as more infants undergo successful repairs of previously-unsurvivable cardiac anomalies. “More moms with congenital heart disease are showing up pregnant, having survived the tumultuous peripartum and neonatal period, and are now facing a new set of risks in pregnancy,” Michael Foley, MD, chair of the department of obstetrics and gynecology at the University of Arizona, Phoenix, said in an interview.
Joseph Kay, MD, a cardiologist and professor of medicine and pediatrics at the University of Colorado, Aurora, said that one big benefit of the new scientific statement is having a single reference point for care of these patients. “The scientific statement brings all of the information about caring for these patients together into one document. This will be a very valuable resource for trainees to get a sense of what’s important; it also represents a platform for new programs to understand the scope of services needed,” said Dr. Kay in an interview.
The document provides a thorough review of the physiologic changes of pregnancy and the intrapartum and postpartum periods, noting that the heterogeneity of congenital heart disease means that women who have different lesions carry different risks in pregnancy.
Examples of lesions presenting intermediate risk include most arrhythmias (category II), hypertrophic cardiomyopathy, and a repaired coarctation (both category II-III). The most severe lesions carry a contraindication for pregnancy; the WHO guidelines suggest discussing termination should women with a category IV lesion become pregnant. Severe mitral stenosis, severe symptomatic aortic stenosis, and severe systemic ventricular dysfunction all place women into category IV.
Beginning with pregnancy risk category III, the WHO guidelines recommend intensive cardiac and obstetric monitoring throughout pregnancy, childbirth, and the puerperium. Several maternal-fetal medicine specialists interviewed all agreed that an interdisciplinary team is a must for good obstetric care in this population.
How interdisciplinary care plays out can depend on geography and facility-dependent resources. Dr. Kay said that his facility is the referral site for pregnant women with complex congenital lesions in an area that spans the Canadian and the Mexican borders from north to south, and ranges from parts of Kansas to eastern Montana from east to west. Still, Dr. Kay said that even for patients with lower-risk lesions, “We will see patients at least once, at approximately the midpoint of pregnancy, and again during the third trimester if possible.” The specifics of care depend on “the nature of the lesion and the complexity of the disease,” said Dr. Kay.
In his facility, said Dr. Kay, telemetry is available for all of the labor and delivery unit beds. This means that the mother and infant can usually stay together and receive postpartum nursing and lactation care from a skilled staff.
In no circumstances should ob.gyns. go it alone, said Dr. Foley. “The conversation with the ob.gyn. needs to be about comanaging these patients, at the very least. Even the most learned maternal-fetal medicine specialist needs to be working with a cardiologist and an anesthesiologist to create a delivery plan that includes pain management, fluid management, and consideration for intrapartum hemodynamic monitoring,” he said.
And the team needs to be in place long before delivery, Dr. Foley pointed out. “In many hospitals, the care delivery gap may be the inability to have this consistent proactive approach. You can’t expect the best outcomes when you have to hurriedly assemble an unfamiliar ad hoc team when a woman with congenital heart disease presents in labor. Despite their best intentions, inconsistent team members may not have the knowledge and experience to provide the safest care for these patients,” he said.
Though an individualized labor and delivery plan is a must, and a multispecialty team should be assembled, maternal congenital heart disease doesn’t necessarily consign a woman to cesarean delivery. “Most women can and should have a vaginal delivery. It’s safer for them. If a natural delivery may increase risk of issues, we may consider a facilitated second stage of labor with epidural anesthesia and forceps- or vacuum-assisted delivery,” said Dr. Kay.
It’s important to understand the nuances of an individual patient’s health and risk status, said Dr. Norton. “A simplified view is often bad. It’s not the case that ‘it’s always better to deliver’ or ‘it’s always better to have a cesarean delivery.’”
Especially for women who need anticoagulation or who may have lesions that put them at great risk should pregnancy occur, preconception counseling is a vital part of their care, and guidance in the scientific statement can help specialists avoid the complications that can occur in the absence of evidence-based treatment. Said Dr. Kay, “I have seen an unfortunate case or two of patients whose anticoagulation was stopped or changed, contrary to guidelines, and who suffered strokes. I hope more people will see this document.”
Ms. Canobbio echoed the sentiment: “You don’t want to have to backpedal once a young woman presents with a pregnancy. Appropriate contraceptive counseling needs to be part of the conversation.”
One key concept underscored in the scientific statement is that elevated risk persists into the postpartum period. “Following delivery, the mother is still at risk for an extended period of time. The greatest risk for mortality in these patients is post delivery, when a large volume of blood is expelled from the uterus back into the maternal circulation,” said Ms. Canobbio. “These women need close follow-up; we can’t say they are home free until several weeks to 2 months after delivery. The need for vigilance and surveillance continues.”
Since the scientific statement is not a new set of guidelines, but rather a compilation of currently existing reference documents, the authors noted that management differences may exist in some cases, but did not assign greater value to one practice than another. “We addressed that there are differences between the European and the American guidelines. For example, with regard to anticoagulation, both would agree to use Lovenox [enoxaparin], but the difference is whether it should be used for the entire pregnancy or for parts of the pregnancy,” said Ms. Canobbio.
Looking forward, more women with complex congenital heart disease will bear children, but their future is not certain. Said Ms. Canobbio: “The data are growing that if the patient is clinically stable at the time of pregnancy, it’s likely we can get them through safely. What’s not yet known is whether the burden of pregnancy in a woman who is otherwise healthy will shorten her lifespan. However, early data are promising, and it’s looking like these women can fare well.”
Topics covered in the scientific statement include:
- Defining which patients are at increased risk in pregnancy.
- Physiological adaptations of pregnancy, the puerperium, and the postpartum period, with an emphasis on hemodynamic changes.
- Assessment and evaluation in the preconception and early prenatal periods.
- Pregnancy management, including appropriate testing.
- Medications in pregnancy, including a table of common cardiac drugs and their pregnancy categories and lactation risks.
- Breakdown of suggested prenatal care by trimester.
- Intrapartum care, including indications for fluid management, ECG and hemodynamic monitoring, and management of the second stage of delivery.
- Postpartum care, with attention to the very rapid increase in blood volume and concomitant leap in stroke volume and cardiac output.
- Considerations when choosing contraceptive method.
- Cardiac complications seen in pregnancy, including arrhythmias, managing mechanical valves and anticoagulation, heart failure, and cyanosis.
- Indications for and risks associated with interventional therapies during pregnancy.
- Detailed discussion of management of pregnancy for women with specific lesions.
None of the members of the writing committee for the scientific statement had relevant disclosures. Dr. Foley and Dr. Kay reported no disclosures. Dr. Norton reported that she has received research funding from Natera and Ultragenyx.
[email protected]
On Twitter @karioakes
A new scientific statement from the American Heart Association (AHA) brings together recommendations for management of pregnancy for women with serious congenital heart disease. The 38-page document addresses a wide range of complex congenital heart conditions, presenting a newly unified set of recommendations for care that ranges from preconception counseling, through pregnancy, labor, and delivery, to the postpartum period.
Caring for women with complex congenital heart lesions is becoming more commonplace, as more infants undergo successful repairs of previously-unsurvivable cardiac anomalies. “More moms with congenital heart disease are showing up pregnant, having survived the tumultuous peripartum and neonatal period, and are now facing a new set of risks in pregnancy,” Michael Foley, MD, chair of the department of obstetrics and gynecology at the University of Arizona, Phoenix, said in an interview.
Joseph Kay, MD, a cardiologist and professor of medicine and pediatrics at the University of Colorado, Aurora, said that one big benefit of the new scientific statement is having a single reference point for care of these patients. “The scientific statement brings all of the information about caring for these patients together into one document. This will be a very valuable resource for trainees to get a sense of what’s important; it also represents a platform for new programs to understand the scope of services needed,” said Dr. Kay in an interview.
The document provides a thorough review of the physiologic changes of pregnancy and the intrapartum and postpartum periods, noting that the heterogeneity of congenital heart disease means that women who have different lesions carry different risks in pregnancy.
Examples of lesions presenting intermediate risk include most arrhythmias (category II), hypertrophic cardiomyopathy, and a repaired coarctation (both category II-III). The most severe lesions carry a contraindication for pregnancy; the WHO guidelines suggest discussing termination should women with a category IV lesion become pregnant. Severe mitral stenosis, severe symptomatic aortic stenosis, and severe systemic ventricular dysfunction all place women into category IV.
Beginning with pregnancy risk category III, the WHO guidelines recommend intensive cardiac and obstetric monitoring throughout pregnancy, childbirth, and the puerperium. Several maternal-fetal medicine specialists interviewed all agreed that an interdisciplinary team is a must for good obstetric care in this population.
How interdisciplinary care plays out can depend on geography and facility-dependent resources. Dr. Kay said that his facility is the referral site for pregnant women with complex congenital lesions in an area that spans the Canadian and the Mexican borders from north to south, and ranges from parts of Kansas to eastern Montana from east to west. Still, Dr. Kay said that even for patients with lower-risk lesions, “We will see patients at least once, at approximately the midpoint of pregnancy, and again during the third trimester if possible.” The specifics of care depend on “the nature of the lesion and the complexity of the disease,” said Dr. Kay.
In his facility, said Dr. Kay, telemetry is available for all of the labor and delivery unit beds. This means that the mother and infant can usually stay together and receive postpartum nursing and lactation care from a skilled staff.
In no circumstances should ob.gyns. go it alone, said Dr. Foley. “The conversation with the ob.gyn. needs to be about comanaging these patients, at the very least. Even the most learned maternal-fetal medicine specialist needs to be working with a cardiologist and an anesthesiologist to create a delivery plan that includes pain management, fluid management, and consideration for intrapartum hemodynamic monitoring,” he said.
And the team needs to be in place long before delivery, Dr. Foley pointed out. “In many hospitals, the care delivery gap may be the inability to have this consistent proactive approach. You can’t expect the best outcomes when you have to hurriedly assemble an unfamiliar ad hoc team when a woman with congenital heart disease presents in labor. Despite their best intentions, inconsistent team members may not have the knowledge and experience to provide the safest care for these patients,” he said.
Though an individualized labor and delivery plan is a must, and a multispecialty team should be assembled, maternal congenital heart disease doesn’t necessarily consign a woman to cesarean delivery. “Most women can and should have a vaginal delivery. It’s safer for them. If a natural delivery may increase risk of issues, we may consider a facilitated second stage of labor with epidural anesthesia and forceps- or vacuum-assisted delivery,” said Dr. Kay.
It’s important to understand the nuances of an individual patient’s health and risk status, said Dr. Norton. “A simplified view is often bad. It’s not the case that ‘it’s always better to deliver’ or ‘it’s always better to have a cesarean delivery.’”
Especially for women who need anticoagulation or who may have lesions that put them at great risk should pregnancy occur, preconception counseling is a vital part of their care, and guidance in the scientific statement can help specialists avoid the complications that can occur in the absence of evidence-based treatment. Said Dr. Kay, “I have seen an unfortunate case or two of patients whose anticoagulation was stopped or changed, contrary to guidelines, and who suffered strokes. I hope more people will see this document.”
Ms. Canobbio echoed the sentiment: “You don’t want to have to backpedal once a young woman presents with a pregnancy. Appropriate contraceptive counseling needs to be part of the conversation.”
One key concept underscored in the scientific statement is that elevated risk persists into the postpartum period. “Following delivery, the mother is still at risk for an extended period of time. The greatest risk for mortality in these patients is post delivery, when a large volume of blood is expelled from the uterus back into the maternal circulation,” said Ms. Canobbio. “These women need close follow-up; we can’t say they are home free until several weeks to 2 months after delivery. The need for vigilance and surveillance continues.”
Since the scientific statement is not a new set of guidelines, but rather a compilation of currently existing reference documents, the authors noted that management differences may exist in some cases, but did not assign greater value to one practice than another. “We addressed that there are differences between the European and the American guidelines. For example, with regard to anticoagulation, both would agree to use Lovenox [enoxaparin], but the difference is whether it should be used for the entire pregnancy or for parts of the pregnancy,” said Ms. Canobbio.
Looking forward, more women with complex congenital heart disease will bear children, but their future is not certain. Said Ms. Canobbio: “The data are growing that if the patient is clinically stable at the time of pregnancy, it’s likely we can get them through safely. What’s not yet known is whether the burden of pregnancy in a woman who is otherwise healthy will shorten her lifespan. However, early data are promising, and it’s looking like these women can fare well.”
Topics covered in the scientific statement include:
- Defining which patients are at increased risk in pregnancy.
- Physiological adaptations of pregnancy, the puerperium, and the postpartum period, with an emphasis on hemodynamic changes.
- Assessment and evaluation in the preconception and early prenatal periods.
- Pregnancy management, including appropriate testing.
- Medications in pregnancy, including a table of common cardiac drugs and their pregnancy categories and lactation risks.
- Breakdown of suggested prenatal care by trimester.
- Intrapartum care, including indications for fluid management, ECG and hemodynamic monitoring, and management of the second stage of delivery.
- Postpartum care, with attention to the very rapid increase in blood volume and concomitant leap in stroke volume and cardiac output.
- Considerations when choosing contraceptive method.
- Cardiac complications seen in pregnancy, including arrhythmias, managing mechanical valves and anticoagulation, heart failure, and cyanosis.
- Indications for and risks associated with interventional therapies during pregnancy.
- Detailed discussion of management of pregnancy for women with specific lesions.
None of the members of the writing committee for the scientific statement had relevant disclosures. Dr. Foley and Dr. Kay reported no disclosures. Dr. Norton reported that she has received research funding from Natera and Ultragenyx.
[email protected]
On Twitter @karioakes
A new scientific statement from the American Heart Association (AHA) brings together recommendations for management of pregnancy for women with serious congenital heart disease. The 38-page document addresses a wide range of complex congenital heart conditions, presenting a newly unified set of recommendations for care that ranges from preconception counseling, through pregnancy, labor, and delivery, to the postpartum period.
Caring for women with complex congenital heart lesions is becoming more commonplace, as more infants undergo successful repairs of previously-unsurvivable cardiac anomalies. “More moms with congenital heart disease are showing up pregnant, having survived the tumultuous peripartum and neonatal period, and are now facing a new set of risks in pregnancy,” Michael Foley, MD, chair of the department of obstetrics and gynecology at the University of Arizona, Phoenix, said in an interview.
Joseph Kay, MD, a cardiologist and professor of medicine and pediatrics at the University of Colorado, Aurora, said that one big benefit of the new scientific statement is having a single reference point for care of these patients. “The scientific statement brings all of the information about caring for these patients together into one document. This will be a very valuable resource for trainees to get a sense of what’s important; it also represents a platform for new programs to understand the scope of services needed,” said Dr. Kay in an interview.
The document provides a thorough review of the physiologic changes of pregnancy and the intrapartum and postpartum periods, noting that the heterogeneity of congenital heart disease means that women who have different lesions carry different risks in pregnancy.
Examples of lesions presenting intermediate risk include most arrhythmias (category II), hypertrophic cardiomyopathy, and a repaired coarctation (both category II-III). The most severe lesions carry a contraindication for pregnancy; the WHO guidelines suggest discussing termination should women with a category IV lesion become pregnant. Severe mitral stenosis, severe symptomatic aortic stenosis, and severe systemic ventricular dysfunction all place women into category IV.
Beginning with pregnancy risk category III, the WHO guidelines recommend intensive cardiac and obstetric monitoring throughout pregnancy, childbirth, and the puerperium. Several maternal-fetal medicine specialists interviewed all agreed that an interdisciplinary team is a must for good obstetric care in this population.
How interdisciplinary care plays out can depend on geography and facility-dependent resources. Dr. Kay said that his facility is the referral site for pregnant women with complex congenital lesions in an area that spans the Canadian and the Mexican borders from north to south, and ranges from parts of Kansas to eastern Montana from east to west. Still, Dr. Kay said that even for patients with lower-risk lesions, “We will see patients at least once, at approximately the midpoint of pregnancy, and again during the third trimester if possible.” The specifics of care depend on “the nature of the lesion and the complexity of the disease,” said Dr. Kay.
In his facility, said Dr. Kay, telemetry is available for all of the labor and delivery unit beds. This means that the mother and infant can usually stay together and receive postpartum nursing and lactation care from a skilled staff.
In no circumstances should ob.gyns. go it alone, said Dr. Foley. “The conversation with the ob.gyn. needs to be about comanaging these patients, at the very least. Even the most learned maternal-fetal medicine specialist needs to be working with a cardiologist and an anesthesiologist to create a delivery plan that includes pain management, fluid management, and consideration for intrapartum hemodynamic monitoring,” he said.
And the team needs to be in place long before delivery, Dr. Foley pointed out. “In many hospitals, the care delivery gap may be the inability to have this consistent proactive approach. You can’t expect the best outcomes when you have to hurriedly assemble an unfamiliar ad hoc team when a woman with congenital heart disease presents in labor. Despite their best intentions, inconsistent team members may not have the knowledge and experience to provide the safest care for these patients,” he said.
Though an individualized labor and delivery plan is a must, and a multispecialty team should be assembled, maternal congenital heart disease doesn’t necessarily consign a woman to cesarean delivery. “Most women can and should have a vaginal delivery. It’s safer for them. If a natural delivery may increase risk of issues, we may consider a facilitated second stage of labor with epidural anesthesia and forceps- or vacuum-assisted delivery,” said Dr. Kay.
It’s important to understand the nuances of an individual patient’s health and risk status, said Dr. Norton. “A simplified view is often bad. It’s not the case that ‘it’s always better to deliver’ or ‘it’s always better to have a cesarean delivery.’”
Especially for women who need anticoagulation or who may have lesions that put them at great risk should pregnancy occur, preconception counseling is a vital part of their care, and guidance in the scientific statement can help specialists avoid the complications that can occur in the absence of evidence-based treatment. Said Dr. Kay, “I have seen an unfortunate case or two of patients whose anticoagulation was stopped or changed, contrary to guidelines, and who suffered strokes. I hope more people will see this document.”
Ms. Canobbio echoed the sentiment: “You don’t want to have to backpedal once a young woman presents with a pregnancy. Appropriate contraceptive counseling needs to be part of the conversation.”
One key concept underscored in the scientific statement is that elevated risk persists into the postpartum period. “Following delivery, the mother is still at risk for an extended period of time. The greatest risk for mortality in these patients is post delivery, when a large volume of blood is expelled from the uterus back into the maternal circulation,” said Ms. Canobbio. “These women need close follow-up; we can’t say they are home free until several weeks to 2 months after delivery. The need for vigilance and surveillance continues.”
Since the scientific statement is not a new set of guidelines, but rather a compilation of currently existing reference documents, the authors noted that management differences may exist in some cases, but did not assign greater value to one practice than another. “We addressed that there are differences between the European and the American guidelines. For example, with regard to anticoagulation, both would agree to use Lovenox [enoxaparin], but the difference is whether it should be used for the entire pregnancy or for parts of the pregnancy,” said Ms. Canobbio.
Looking forward, more women with complex congenital heart disease will bear children, but their future is not certain. Said Ms. Canobbio: “The data are growing that if the patient is clinically stable at the time of pregnancy, it’s likely we can get them through safely. What’s not yet known is whether the burden of pregnancy in a woman who is otherwise healthy will shorten her lifespan. However, early data are promising, and it’s looking like these women can fare well.”
Topics covered in the scientific statement include:
- Defining which patients are at increased risk in pregnancy.
- Physiological adaptations of pregnancy, the puerperium, and the postpartum period, with an emphasis on hemodynamic changes.
- Assessment and evaluation in the preconception and early prenatal periods.
- Pregnancy management, including appropriate testing.
- Medications in pregnancy, including a table of common cardiac drugs and their pregnancy categories and lactation risks.
- Breakdown of suggested prenatal care by trimester.
- Intrapartum care, including indications for fluid management, ECG and hemodynamic monitoring, and management of the second stage of delivery.
- Postpartum care, with attention to the very rapid increase in blood volume and concomitant leap in stroke volume and cardiac output.
- Considerations when choosing contraceptive method.
- Cardiac complications seen in pregnancy, including arrhythmias, managing mechanical valves and anticoagulation, heart failure, and cyanosis.
- Indications for and risks associated with interventional therapies during pregnancy.
- Detailed discussion of management of pregnancy for women with specific lesions.
None of the members of the writing committee for the scientific statement had relevant disclosures. Dr. Foley and Dr. Kay reported no disclosures. Dr. Norton reported that she has received research funding from Natera and Ultragenyx.
[email protected]
On Twitter @karioakes
Reports of new-onset joint pain differ after starting vedolizumab
Two recent reports that provide opposing evidence about the potential for inflammatory bowel disease patients to develop articular manifestations after starting vedolizumab raise questions for future studies to answer in regard to a plausible mechanism for the adverse event and its relative importance.
The two reports, one a case series of 5 patients with inflammatory bowel disease (IBD) who developed articular manifestations after beginning vedolizumab (Entyvio) and the other a prospective cohort study of 53 patients with IBD who started vedolizumab without any subsequent cases of induction or flare of arthritis and/or sacroiliitis, came to somewhat different conclusions about the beneficial or paradoxical effects of vedolizumab’s blockade of the alpha4beta7 receptor on articular manifestations of IBD.
The five-patient cases series reported by Gaëlle Varkas, MD, a doctoral student at the University of Ghent, Belgium, and her colleagues consisted of five IBD patients, aged 26-50 years, who developed either new onset or an exacerbation of sacroiliitis or arthritis soon after starting vedolizumab. All but one of the patients was female (Ann Rheum Dis. 2016 Nov 29. doi: 10.1136/annrheumdis-2016-210233). In these patients, the investigators said, vedolizumab did not “seem to show any efficacy in and might even induce arthritis and/or sacroiliitis.”
The first was a 50-year-old woman who had progressive back pain with MRI-confirmed bilateral sacroiliitis about 2 months after beginning vedolizumab. The second patient, a 28-year-old woman with no previous history of spondyloarthropathy, had lower limb pain, a painful left shoulder, and arthritis of one wrist. Ultrasound examination confirmed intercarpal effusions and synovial hyperproliferation.
The third patient was 30 years old and male. He had both ankylosing spondylitis and Crohn’s disease, and experienced arthralgias, elevated C-reactive protein, and MRI-confirmed axial skeletal inflammation 4 weeks after starting vedolizumab.
The fourth patient was a 47-year-old woman with no previous history of spondyloarthropathy who developed MRI-confirmed sacroiliitis after beginning vedolizumab. The fifth patient, a 26-year-old woman, developed polyarticular joint pain after starting vedolizumab. Examination of this patient showed synovitis and enthesitis of multiple joints of the appendicular skeleton.
In discussion, Dr. Varkas and her colleagues noted that “one of the many hypotheses is that integrins and adhesion molecules play a role in the interception of recirculating activated lymphocytes between the gut and the synovial membrane due to the inhibition of the alpha4beta7 integrin homing at the level of the gut.” However, the investigators also acknowledged that other hypotheses may also account for their findings. “Alternatively, in the presence of vedolizumab, cellular recruitment may be mediated by yet to be determined adhesion molecules. This recirculation theory might explain the short mean interval of 64 days between vedolizumab initiation and the expression of symptoms.”
Another group, publishing its prospective cohort study in a letter, had different findings (Ann Rheum Dis. 2017 Jan 17. doi: 10.1136/annrheumdis-2016-211011).
“Although the hypotheses proposed by the authors to explain such events sounds reasonable,” wrote Ambrogio Orlando, MD, and his coauthors, their experience of the effect of vedolizumab on spondyloarthritis differed.
In the report on 53 patients who began treatment with vedolizumab at Villa Sofia-Cervello Hospital, Palermo, Italy, where Dr. Orlando and his associates work, almost all (96%) had been steroid dependent and 81% had been treated with at least one tumor necrosis factor inhibitor. About two-thirds had completed the induction phase of vedolizumab treatment during follow-up, which lasted a mean of 2.6 months. Of the 14 patients (26%) who had active IBD-associated spondyloarthropathy when starting vedolizumab, 6 (46.2%) saw “a sharp clinical benefit after the initiation of vedolizumab,” wrote Dr. Orlando and his colleagues. Five of these six patients experienced clinical remission of gut symptoms by 12 weeks of therapy.
Dr. Orlando and his colleagues wrote that “our preliminary prospective data indicate a potential benefit of vedolizumab on IBD-associated spondyloarthropathy.”
Looking for mechanistic reasons for this apparent benefit, Dr. Orlando and his collaborators wrote that “the previous demonstration of alpha4beta7 in the joint and the recent evidence of the upregulation of mucosal vascular address in cell adhesion molecule (MadCAM-1) in the high endothelial venules of bone marrow in patients with active axial SpA seem to strengthen the hypothesis of a beneficial rather than a paradoxical effect of alpha4beta7 blockade on articular manifestations of IBD.”
Two authors of the case series reported relationships with multiple pharmaceutical companies, as did Dr. Orlando and two other authors of the letter describing the prospective study.
[email protected]
On Twitter @karioakes
Vedolizumab is gut-selective, and therefore a question that’s been raised is whether it would uncover extra-intestinal manifestations of inflammatory bowel disease (IBD).
When extra-intestinal manifestations of IBD occur with new treatments, we try to discern whether there is still active disease in the bowel. If the bowel is responding, we try to determine if the extra-intestinal symptoms are occurring in parallel to the bowel disease or if they represent a unique side effect of the medication.
In February 2017, at ECCO [the annual meeting of the European Crohn’s and Colitis Organisation], we will be presenting a post hoc analysis of the data from the vedolizumab pivotal clinical trial that examines whether joint pain was independently associated with administration of vedolizumab.
The individual case reports of joint pain with vedolizumab have not limited our using the drug for the patients who need it. It’s a matter of weighing risks and benefits, and the safety profile of this medication is overall so good that we don’t hesitate to use it. In our clinic, we have treated more than 400 IBD patients with vedolizumab, and I can only recall one patient who had to stop using it due to joint pain.
David Rubin, MD, is professor of medicine and chief of the gastroenterology, hepatology, and nutrition section of the University of Chicago. He reported that he is a consultant for and has received grant support from Takeda Pharmaceuticals. These remarks were drawn from an interview.
Vedolizumab is gut-selective, and therefore a question that’s been raised is whether it would uncover extra-intestinal manifestations of inflammatory bowel disease (IBD).
When extra-intestinal manifestations of IBD occur with new treatments, we try to discern whether there is still active disease in the bowel. If the bowel is responding, we try to determine if the extra-intestinal symptoms are occurring in parallel to the bowel disease or if they represent a unique side effect of the medication.
In February 2017, at ECCO [the annual meeting of the European Crohn’s and Colitis Organisation], we will be presenting a post hoc analysis of the data from the vedolizumab pivotal clinical trial that examines whether joint pain was independently associated with administration of vedolizumab.
The individual case reports of joint pain with vedolizumab have not limited our using the drug for the patients who need it. It’s a matter of weighing risks and benefits, and the safety profile of this medication is overall so good that we don’t hesitate to use it. In our clinic, we have treated more than 400 IBD patients with vedolizumab, and I can only recall one patient who had to stop using it due to joint pain.
David Rubin, MD, is professor of medicine and chief of the gastroenterology, hepatology, and nutrition section of the University of Chicago. He reported that he is a consultant for and has received grant support from Takeda Pharmaceuticals. These remarks were drawn from an interview.
Vedolizumab is gut-selective, and therefore a question that’s been raised is whether it would uncover extra-intestinal manifestations of inflammatory bowel disease (IBD).
When extra-intestinal manifestations of IBD occur with new treatments, we try to discern whether there is still active disease in the bowel. If the bowel is responding, we try to determine if the extra-intestinal symptoms are occurring in parallel to the bowel disease or if they represent a unique side effect of the medication.
In February 2017, at ECCO [the annual meeting of the European Crohn’s and Colitis Organisation], we will be presenting a post hoc analysis of the data from the vedolizumab pivotal clinical trial that examines whether joint pain was independently associated with administration of vedolizumab.
The individual case reports of joint pain with vedolizumab have not limited our using the drug for the patients who need it. It’s a matter of weighing risks and benefits, and the safety profile of this medication is overall so good that we don’t hesitate to use it. In our clinic, we have treated more than 400 IBD patients with vedolizumab, and I can only recall one patient who had to stop using it due to joint pain.
David Rubin, MD, is professor of medicine and chief of the gastroenterology, hepatology, and nutrition section of the University of Chicago. He reported that he is a consultant for and has received grant support from Takeda Pharmaceuticals. These remarks were drawn from an interview.
Two recent reports that provide opposing evidence about the potential for inflammatory bowel disease patients to develop articular manifestations after starting vedolizumab raise questions for future studies to answer in regard to a plausible mechanism for the adverse event and its relative importance.
The two reports, one a case series of 5 patients with inflammatory bowel disease (IBD) who developed articular manifestations after beginning vedolizumab (Entyvio) and the other a prospective cohort study of 53 patients with IBD who started vedolizumab without any subsequent cases of induction or flare of arthritis and/or sacroiliitis, came to somewhat different conclusions about the beneficial or paradoxical effects of vedolizumab’s blockade of the alpha4beta7 receptor on articular manifestations of IBD.
The five-patient cases series reported by Gaëlle Varkas, MD, a doctoral student at the University of Ghent, Belgium, and her colleagues consisted of five IBD patients, aged 26-50 years, who developed either new onset or an exacerbation of sacroiliitis or arthritis soon after starting vedolizumab. All but one of the patients was female (Ann Rheum Dis. 2016 Nov 29. doi: 10.1136/annrheumdis-2016-210233). In these patients, the investigators said, vedolizumab did not “seem to show any efficacy in and might even induce arthritis and/or sacroiliitis.”
The first was a 50-year-old woman who had progressive back pain with MRI-confirmed bilateral sacroiliitis about 2 months after beginning vedolizumab. The second patient, a 28-year-old woman with no previous history of spondyloarthropathy, had lower limb pain, a painful left shoulder, and arthritis of one wrist. Ultrasound examination confirmed intercarpal effusions and synovial hyperproliferation.
The third patient was 30 years old and male. He had both ankylosing spondylitis and Crohn’s disease, and experienced arthralgias, elevated C-reactive protein, and MRI-confirmed axial skeletal inflammation 4 weeks after starting vedolizumab.
The fourth patient was a 47-year-old woman with no previous history of spondyloarthropathy who developed MRI-confirmed sacroiliitis after beginning vedolizumab. The fifth patient, a 26-year-old woman, developed polyarticular joint pain after starting vedolizumab. Examination of this patient showed synovitis and enthesitis of multiple joints of the appendicular skeleton.
In discussion, Dr. Varkas and her colleagues noted that “one of the many hypotheses is that integrins and adhesion molecules play a role in the interception of recirculating activated lymphocytes between the gut and the synovial membrane due to the inhibition of the alpha4beta7 integrin homing at the level of the gut.” However, the investigators also acknowledged that other hypotheses may also account for their findings. “Alternatively, in the presence of vedolizumab, cellular recruitment may be mediated by yet to be determined adhesion molecules. This recirculation theory might explain the short mean interval of 64 days between vedolizumab initiation and the expression of symptoms.”
Another group, publishing its prospective cohort study in a letter, had different findings (Ann Rheum Dis. 2017 Jan 17. doi: 10.1136/annrheumdis-2016-211011).
“Although the hypotheses proposed by the authors to explain such events sounds reasonable,” wrote Ambrogio Orlando, MD, and his coauthors, their experience of the effect of vedolizumab on spondyloarthritis differed.
In the report on 53 patients who began treatment with vedolizumab at Villa Sofia-Cervello Hospital, Palermo, Italy, where Dr. Orlando and his associates work, almost all (96%) had been steroid dependent and 81% had been treated with at least one tumor necrosis factor inhibitor. About two-thirds had completed the induction phase of vedolizumab treatment during follow-up, which lasted a mean of 2.6 months. Of the 14 patients (26%) who had active IBD-associated spondyloarthropathy when starting vedolizumab, 6 (46.2%) saw “a sharp clinical benefit after the initiation of vedolizumab,” wrote Dr. Orlando and his colleagues. Five of these six patients experienced clinical remission of gut symptoms by 12 weeks of therapy.
Dr. Orlando and his colleagues wrote that “our preliminary prospective data indicate a potential benefit of vedolizumab on IBD-associated spondyloarthropathy.”
Looking for mechanistic reasons for this apparent benefit, Dr. Orlando and his collaborators wrote that “the previous demonstration of alpha4beta7 in the joint and the recent evidence of the upregulation of mucosal vascular address in cell adhesion molecule (MadCAM-1) in the high endothelial venules of bone marrow in patients with active axial SpA seem to strengthen the hypothesis of a beneficial rather than a paradoxical effect of alpha4beta7 blockade on articular manifestations of IBD.”
Two authors of the case series reported relationships with multiple pharmaceutical companies, as did Dr. Orlando and two other authors of the letter describing the prospective study.
[email protected]
On Twitter @karioakes
Two recent reports that provide opposing evidence about the potential for inflammatory bowel disease patients to develop articular manifestations after starting vedolizumab raise questions for future studies to answer in regard to a plausible mechanism for the adverse event and its relative importance.
The two reports, one a case series of 5 patients with inflammatory bowel disease (IBD) who developed articular manifestations after beginning vedolizumab (Entyvio) and the other a prospective cohort study of 53 patients with IBD who started vedolizumab without any subsequent cases of induction or flare of arthritis and/or sacroiliitis, came to somewhat different conclusions about the beneficial or paradoxical effects of vedolizumab’s blockade of the alpha4beta7 receptor on articular manifestations of IBD.
The five-patient cases series reported by Gaëlle Varkas, MD, a doctoral student at the University of Ghent, Belgium, and her colleagues consisted of five IBD patients, aged 26-50 years, who developed either new onset or an exacerbation of sacroiliitis or arthritis soon after starting vedolizumab. All but one of the patients was female (Ann Rheum Dis. 2016 Nov 29. doi: 10.1136/annrheumdis-2016-210233). In these patients, the investigators said, vedolizumab did not “seem to show any efficacy in and might even induce arthritis and/or sacroiliitis.”
The first was a 50-year-old woman who had progressive back pain with MRI-confirmed bilateral sacroiliitis about 2 months after beginning vedolizumab. The second patient, a 28-year-old woman with no previous history of spondyloarthropathy, had lower limb pain, a painful left shoulder, and arthritis of one wrist. Ultrasound examination confirmed intercarpal effusions and synovial hyperproliferation.
The third patient was 30 years old and male. He had both ankylosing spondylitis and Crohn’s disease, and experienced arthralgias, elevated C-reactive protein, and MRI-confirmed axial skeletal inflammation 4 weeks after starting vedolizumab.
The fourth patient was a 47-year-old woman with no previous history of spondyloarthropathy who developed MRI-confirmed sacroiliitis after beginning vedolizumab. The fifth patient, a 26-year-old woman, developed polyarticular joint pain after starting vedolizumab. Examination of this patient showed synovitis and enthesitis of multiple joints of the appendicular skeleton.
In discussion, Dr. Varkas and her colleagues noted that “one of the many hypotheses is that integrins and adhesion molecules play a role in the interception of recirculating activated lymphocytes between the gut and the synovial membrane due to the inhibition of the alpha4beta7 integrin homing at the level of the gut.” However, the investigators also acknowledged that other hypotheses may also account for their findings. “Alternatively, in the presence of vedolizumab, cellular recruitment may be mediated by yet to be determined adhesion molecules. This recirculation theory might explain the short mean interval of 64 days between vedolizumab initiation and the expression of symptoms.”
Another group, publishing its prospective cohort study in a letter, had different findings (Ann Rheum Dis. 2017 Jan 17. doi: 10.1136/annrheumdis-2016-211011).
“Although the hypotheses proposed by the authors to explain such events sounds reasonable,” wrote Ambrogio Orlando, MD, and his coauthors, their experience of the effect of vedolizumab on spondyloarthritis differed.
In the report on 53 patients who began treatment with vedolizumab at Villa Sofia-Cervello Hospital, Palermo, Italy, where Dr. Orlando and his associates work, almost all (96%) had been steroid dependent and 81% had been treated with at least one tumor necrosis factor inhibitor. About two-thirds had completed the induction phase of vedolizumab treatment during follow-up, which lasted a mean of 2.6 months. Of the 14 patients (26%) who had active IBD-associated spondyloarthropathy when starting vedolizumab, 6 (46.2%) saw “a sharp clinical benefit after the initiation of vedolizumab,” wrote Dr. Orlando and his colleagues. Five of these six patients experienced clinical remission of gut symptoms by 12 weeks of therapy.
Dr. Orlando and his colleagues wrote that “our preliminary prospective data indicate a potential benefit of vedolizumab on IBD-associated spondyloarthropathy.”
Looking for mechanistic reasons for this apparent benefit, Dr. Orlando and his collaborators wrote that “the previous demonstration of alpha4beta7 in the joint and the recent evidence of the upregulation of mucosal vascular address in cell adhesion molecule (MadCAM-1) in the high endothelial venules of bone marrow in patients with active axial SpA seem to strengthen the hypothesis of a beneficial rather than a paradoxical effect of alpha4beta7 blockade on articular manifestations of IBD.”
Two authors of the case series reported relationships with multiple pharmaceutical companies, as did Dr. Orlando and two other authors of the letter describing the prospective study.
[email protected]
On Twitter @karioakes
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Imaging-confirmed arthritis or sacroiliitis after starting vedolizumab was seen in a case series of 5 patients; a prospective study of 53 patients saw zero new-onset cases of joint pain.
Data source: Case series of 5 IBD patients starting vedolizumab, and prospective surveillance at another facility of 53 IBD patients receiving vedolizumab.
Disclosures: Two authors of the case series reported multiple relationships with pharmaceutical companies, as did three authors of a letter describing a prospective study.
Radiosurgery found not superior to open surgery for temporal lobe epilepsy
HOUSTON – Despite enrollment difficulties that limited the study, a recently completed randomized trial comparing radiosurgery with open lobectomy to treat temporal lobe epilepsy offers some guidance for patients and their physicians.
Radiosurgery’s noninferiority to open lobectomy couldn’t be shown from the ROSE (Radiosurgery or Open Surgery for Epilepsy) trial, but language deficits were similar – and quite small – by 3 years after either procedure. Expected visual field deficits were similar in each procedure as well. However, since the trial didn’t reach its target enrollment, several primary outcome measures could not be fully assessed.
On the face of it, radiosurgery has significant appeal. Although open resective surgery is effective, there’s still some risk of infection and blood loss, and neuropsychological changes as well as other focal neurologic deficits are seen. Still, the study saw many challenges, but the largest, according to the investigators, was in recruitment. “Patients like to choose,” said Nicholas M. Barbaro, MD, chair of the department of neurosurgery at Indiana University, Indianapolis. Dr. Barbaro, one of several ROSE coinvestigators who presented the study findings at the annual meeting of the American Epilepsy Society, noted that if patients felt that lobectomy was the best choice, then there would be no incentive to enter a trial where they might be randomized to radiosurgery. Also, he said, some patients might be reluctant to be irradiated, fearing short-term or long-term toxicity.
Trial hypotheses and protocols
The ROSE trial aimed to show that stereotactic radiosurgery (SRS) would not be inferior to anterior temporal lobectomy (ATL) in achieving a seizure-free state by months 25-36 post procedure. The lag to response after radiosurgery is about 1 year; seizure freedom, defined as 12 consecutive months with no seizures, was assessed from months 25 to 36 of the study for the primary outcome of seizure freedom.
Investigators also hypothesized that fewer SRS patients would have significant reductions in measures of language function; further, they predicted that patients in both treatment arms would experience improvements in quality of life (QOL), and that QOL would improve as seizure freedom increased. Finally, the trial sought to show that SRS was cost effective, compared with ATL, with the marginal cost-utility ratio dropping below $50,000 per quality-adjusted life-year (QALY).
Patients in the ATL arm received a standard “Spencer” ATL, with adequacy of resection assessed by MRI performed 3 months after surgery. An inadequate resection would have been classified as an adverse event, but all ATL patients had an adequate resection by study criteria, and all those whose histopathology was available (n = 20) had some hippocampal sclerosis.
Patients in the SRS arm had the amygdala and anterior 2 cm of the hippocampus, as well as the adjacent parahippocampal gyrus, irradiated. This resulted in a total treatment volume ranging from 5.5 to 7.5 cc. Patients received 4 Gy to the 50% isodose line, and treatment could involve an unlimited number of isocenters. The brain stem could receive no more than 10 Gy and the optic nerve and chiasm no more than 8 Gy. All treatment plans were cleared by the ROSE steering committee. The SRS patients had some variation in dose and volumes treated, but all were within the approved limits of the study.
Trial outcomes
As expected, the surgery arm achieved rapid seizure remission, while the SRS arm saw a steady increase in seizure-free numbers beginning at about 12 months after surgery. During study months 25-36, 78% of the ATL arm and 52% of the SRS arm were seizure free. “The null hypothesis of inferiority of SRS was not rejected,” said Mark Quigg, MD, professor of neurology at the University of Virginia, Charlottesville.
Most patients in both groups had no or minimal changes in verbal memory, with no significant differences between the groups at 36 months after treatment.
QOL measures improved rapidly for those who received open surgery, and more slowly for those in the radiosurgery arm, a pattern “consistent with the known association between improved seizure control and quality of life,” said John Langfitt, PhD, a neuropsychologist and professor of neurology and psychiatry at the University of Rochester (N.Y.). However, the study was underpowered to show noninferiority of SRS for QOL measures at 36 months.
“There was a preliminary trend toward reduced health care use over time in the open surgery arm,” said Dr. Langfitt, again noting that the earlier seizure control achieved in surgery reduced health care utilization for that group sooner than for the SRS group. “The power may be limited by sample size and the tendency of utilization to be highly skewed,” he said.
Also as expected, the ATL arm saw early surgery-related adverse events such as scalp wound infections, subdural hematomas, and deep vein thromboses. These were infrequent overall. In contrast, the SRS group saw more cerebral edema–related adverse events during months 9-18, with headaches, new neurologic deficits, and transient seizure exacerbation.
All but three patients received postoperative visual field testing. Of the patients receiving SRS, 34% (10 of 29) had an upper superior quadrant visual field defect, as did 42% (11 of 26) of patients in the ATL arm.
Since the primary treating surgeon and neurologist could not be blinded as to study arm, another neurologist who was blinded was responsible for assessing the outcome measures, and also could identify adverse events. The trial’s steering committee was also blinded to ongoing outcomes.
Pilot study results
A pilot study had previously found that SRS was comparable to the efficacy that had been seen in larger, prospective trials of open surgery, with about two-thirds of patients seizure free at 36 months. Although most patients experienced brief exacerbation of auras or complex partial seizures after radiosurgery, visual field defects were similar to those experienced by patients undergoing standard ATL. Overall, neuropsychological outcomes for those undergoing SRS in the pilot were good, with a low incidence of declines in language and verbal memory function of the dominant hemisphere, and no short-term affective changes were seen. SRS patients who were seizure free after the procedure experienced a significant improvement in QOL.
The promising pilot results contrasted with the limited findings of the ROSE study. In regard to seizure freedom in ROSE, said Dr. Quigg, “The data appear to show that radiosurgery is inferior to ATL, but the low power of the study means that we cannot conclude this with sufficient confidence. Nor can we conclude that the two treatments are noninferior.”
The study was partially funded by Elekta, the manufacturer of the Gamma Knife radiosurgery device used in the study. Dr. Barbaro reported no other disclosures. Dr. Langfitt reported being a consultant for Monteris. Dr. Quigg reported being an investigator for several antiepileptic drug trials sponsored by pharmaceutical companies.
[email protected]
On Twitter @karioakes
HOUSTON – Despite enrollment difficulties that limited the study, a recently completed randomized trial comparing radiosurgery with open lobectomy to treat temporal lobe epilepsy offers some guidance for patients and their physicians.
Radiosurgery’s noninferiority to open lobectomy couldn’t be shown from the ROSE (Radiosurgery or Open Surgery for Epilepsy) trial, but language deficits were similar – and quite small – by 3 years after either procedure. Expected visual field deficits were similar in each procedure as well. However, since the trial didn’t reach its target enrollment, several primary outcome measures could not be fully assessed.
On the face of it, radiosurgery has significant appeal. Although open resective surgery is effective, there’s still some risk of infection and blood loss, and neuropsychological changes as well as other focal neurologic deficits are seen. Still, the study saw many challenges, but the largest, according to the investigators, was in recruitment. “Patients like to choose,” said Nicholas M. Barbaro, MD, chair of the department of neurosurgery at Indiana University, Indianapolis. Dr. Barbaro, one of several ROSE coinvestigators who presented the study findings at the annual meeting of the American Epilepsy Society, noted that if patients felt that lobectomy was the best choice, then there would be no incentive to enter a trial where they might be randomized to radiosurgery. Also, he said, some patients might be reluctant to be irradiated, fearing short-term or long-term toxicity.
Trial hypotheses and protocols
The ROSE trial aimed to show that stereotactic radiosurgery (SRS) would not be inferior to anterior temporal lobectomy (ATL) in achieving a seizure-free state by months 25-36 post procedure. The lag to response after radiosurgery is about 1 year; seizure freedom, defined as 12 consecutive months with no seizures, was assessed from months 25 to 36 of the study for the primary outcome of seizure freedom.
Investigators also hypothesized that fewer SRS patients would have significant reductions in measures of language function; further, they predicted that patients in both treatment arms would experience improvements in quality of life (QOL), and that QOL would improve as seizure freedom increased. Finally, the trial sought to show that SRS was cost effective, compared with ATL, with the marginal cost-utility ratio dropping below $50,000 per quality-adjusted life-year (QALY).
Patients in the ATL arm received a standard “Spencer” ATL, with adequacy of resection assessed by MRI performed 3 months after surgery. An inadequate resection would have been classified as an adverse event, but all ATL patients had an adequate resection by study criteria, and all those whose histopathology was available (n = 20) had some hippocampal sclerosis.
Patients in the SRS arm had the amygdala and anterior 2 cm of the hippocampus, as well as the adjacent parahippocampal gyrus, irradiated. This resulted in a total treatment volume ranging from 5.5 to 7.5 cc. Patients received 4 Gy to the 50% isodose line, and treatment could involve an unlimited number of isocenters. The brain stem could receive no more than 10 Gy and the optic nerve and chiasm no more than 8 Gy. All treatment plans were cleared by the ROSE steering committee. The SRS patients had some variation in dose and volumes treated, but all were within the approved limits of the study.
Trial outcomes
As expected, the surgery arm achieved rapid seizure remission, while the SRS arm saw a steady increase in seizure-free numbers beginning at about 12 months after surgery. During study months 25-36, 78% of the ATL arm and 52% of the SRS arm were seizure free. “The null hypothesis of inferiority of SRS was not rejected,” said Mark Quigg, MD, professor of neurology at the University of Virginia, Charlottesville.
Most patients in both groups had no or minimal changes in verbal memory, with no significant differences between the groups at 36 months after treatment.
QOL measures improved rapidly for those who received open surgery, and more slowly for those in the radiosurgery arm, a pattern “consistent with the known association between improved seizure control and quality of life,” said John Langfitt, PhD, a neuropsychologist and professor of neurology and psychiatry at the University of Rochester (N.Y.). However, the study was underpowered to show noninferiority of SRS for QOL measures at 36 months.
“There was a preliminary trend toward reduced health care use over time in the open surgery arm,” said Dr. Langfitt, again noting that the earlier seizure control achieved in surgery reduced health care utilization for that group sooner than for the SRS group. “The power may be limited by sample size and the tendency of utilization to be highly skewed,” he said.
Also as expected, the ATL arm saw early surgery-related adverse events such as scalp wound infections, subdural hematomas, and deep vein thromboses. These were infrequent overall. In contrast, the SRS group saw more cerebral edema–related adverse events during months 9-18, with headaches, new neurologic deficits, and transient seizure exacerbation.
All but three patients received postoperative visual field testing. Of the patients receiving SRS, 34% (10 of 29) had an upper superior quadrant visual field defect, as did 42% (11 of 26) of patients in the ATL arm.
Since the primary treating surgeon and neurologist could not be blinded as to study arm, another neurologist who was blinded was responsible for assessing the outcome measures, and also could identify adverse events. The trial’s steering committee was also blinded to ongoing outcomes.
Pilot study results
A pilot study had previously found that SRS was comparable to the efficacy that had been seen in larger, prospective trials of open surgery, with about two-thirds of patients seizure free at 36 months. Although most patients experienced brief exacerbation of auras or complex partial seizures after radiosurgery, visual field defects were similar to those experienced by patients undergoing standard ATL. Overall, neuropsychological outcomes for those undergoing SRS in the pilot were good, with a low incidence of declines in language and verbal memory function of the dominant hemisphere, and no short-term affective changes were seen. SRS patients who were seizure free after the procedure experienced a significant improvement in QOL.
The promising pilot results contrasted with the limited findings of the ROSE study. In regard to seizure freedom in ROSE, said Dr. Quigg, “The data appear to show that radiosurgery is inferior to ATL, but the low power of the study means that we cannot conclude this with sufficient confidence. Nor can we conclude that the two treatments are noninferior.”
The study was partially funded by Elekta, the manufacturer of the Gamma Knife radiosurgery device used in the study. Dr. Barbaro reported no other disclosures. Dr. Langfitt reported being a consultant for Monteris. Dr. Quigg reported being an investigator for several antiepileptic drug trials sponsored by pharmaceutical companies.
[email protected]
On Twitter @karioakes
HOUSTON – Despite enrollment difficulties that limited the study, a recently completed randomized trial comparing radiosurgery with open lobectomy to treat temporal lobe epilepsy offers some guidance for patients and their physicians.
Radiosurgery’s noninferiority to open lobectomy couldn’t be shown from the ROSE (Radiosurgery or Open Surgery for Epilepsy) trial, but language deficits were similar – and quite small – by 3 years after either procedure. Expected visual field deficits were similar in each procedure as well. However, since the trial didn’t reach its target enrollment, several primary outcome measures could not be fully assessed.
On the face of it, radiosurgery has significant appeal. Although open resective surgery is effective, there’s still some risk of infection and blood loss, and neuropsychological changes as well as other focal neurologic deficits are seen. Still, the study saw many challenges, but the largest, according to the investigators, was in recruitment. “Patients like to choose,” said Nicholas M. Barbaro, MD, chair of the department of neurosurgery at Indiana University, Indianapolis. Dr. Barbaro, one of several ROSE coinvestigators who presented the study findings at the annual meeting of the American Epilepsy Society, noted that if patients felt that lobectomy was the best choice, then there would be no incentive to enter a trial where they might be randomized to radiosurgery. Also, he said, some patients might be reluctant to be irradiated, fearing short-term or long-term toxicity.
Trial hypotheses and protocols
The ROSE trial aimed to show that stereotactic radiosurgery (SRS) would not be inferior to anterior temporal lobectomy (ATL) in achieving a seizure-free state by months 25-36 post procedure. The lag to response after radiosurgery is about 1 year; seizure freedom, defined as 12 consecutive months with no seizures, was assessed from months 25 to 36 of the study for the primary outcome of seizure freedom.
Investigators also hypothesized that fewer SRS patients would have significant reductions in measures of language function; further, they predicted that patients in both treatment arms would experience improvements in quality of life (QOL), and that QOL would improve as seizure freedom increased. Finally, the trial sought to show that SRS was cost effective, compared with ATL, with the marginal cost-utility ratio dropping below $50,000 per quality-adjusted life-year (QALY).
Patients in the ATL arm received a standard “Spencer” ATL, with adequacy of resection assessed by MRI performed 3 months after surgery. An inadequate resection would have been classified as an adverse event, but all ATL patients had an adequate resection by study criteria, and all those whose histopathology was available (n = 20) had some hippocampal sclerosis.
Patients in the SRS arm had the amygdala and anterior 2 cm of the hippocampus, as well as the adjacent parahippocampal gyrus, irradiated. This resulted in a total treatment volume ranging from 5.5 to 7.5 cc. Patients received 4 Gy to the 50% isodose line, and treatment could involve an unlimited number of isocenters. The brain stem could receive no more than 10 Gy and the optic nerve and chiasm no more than 8 Gy. All treatment plans were cleared by the ROSE steering committee. The SRS patients had some variation in dose and volumes treated, but all were within the approved limits of the study.
Trial outcomes
As expected, the surgery arm achieved rapid seizure remission, while the SRS arm saw a steady increase in seizure-free numbers beginning at about 12 months after surgery. During study months 25-36, 78% of the ATL arm and 52% of the SRS arm were seizure free. “The null hypothesis of inferiority of SRS was not rejected,” said Mark Quigg, MD, professor of neurology at the University of Virginia, Charlottesville.
Most patients in both groups had no or minimal changes in verbal memory, with no significant differences between the groups at 36 months after treatment.
QOL measures improved rapidly for those who received open surgery, and more slowly for those in the radiosurgery arm, a pattern “consistent with the known association between improved seizure control and quality of life,” said John Langfitt, PhD, a neuropsychologist and professor of neurology and psychiatry at the University of Rochester (N.Y.). However, the study was underpowered to show noninferiority of SRS for QOL measures at 36 months.
“There was a preliminary trend toward reduced health care use over time in the open surgery arm,” said Dr. Langfitt, again noting that the earlier seizure control achieved in surgery reduced health care utilization for that group sooner than for the SRS group. “The power may be limited by sample size and the tendency of utilization to be highly skewed,” he said.
Also as expected, the ATL arm saw early surgery-related adverse events such as scalp wound infections, subdural hematomas, and deep vein thromboses. These were infrequent overall. In contrast, the SRS group saw more cerebral edema–related adverse events during months 9-18, with headaches, new neurologic deficits, and transient seizure exacerbation.
All but three patients received postoperative visual field testing. Of the patients receiving SRS, 34% (10 of 29) had an upper superior quadrant visual field defect, as did 42% (11 of 26) of patients in the ATL arm.
Since the primary treating surgeon and neurologist could not be blinded as to study arm, another neurologist who was blinded was responsible for assessing the outcome measures, and also could identify adverse events. The trial’s steering committee was also blinded to ongoing outcomes.
Pilot study results
A pilot study had previously found that SRS was comparable to the efficacy that had been seen in larger, prospective trials of open surgery, with about two-thirds of patients seizure free at 36 months. Although most patients experienced brief exacerbation of auras or complex partial seizures after radiosurgery, visual field defects were similar to those experienced by patients undergoing standard ATL. Overall, neuropsychological outcomes for those undergoing SRS in the pilot were good, with a low incidence of declines in language and verbal memory function of the dominant hemisphere, and no short-term affective changes were seen. SRS patients who were seizure free after the procedure experienced a significant improvement in QOL.
The promising pilot results contrasted with the limited findings of the ROSE study. In regard to seizure freedom in ROSE, said Dr. Quigg, “The data appear to show that radiosurgery is inferior to ATL, but the low power of the study means that we cannot conclude this with sufficient confidence. Nor can we conclude that the two treatments are noninferior.”
The study was partially funded by Elekta, the manufacturer of the Gamma Knife radiosurgery device used in the study. Dr. Barbaro reported no other disclosures. Dr. Langfitt reported being a consultant for Monteris. Dr. Quigg reported being an investigator for several antiepileptic drug trials sponsored by pharmaceutical companies.
[email protected]
On Twitter @karioakes
AT AES 2016
Key clinical point:
Major finding: During study months 25-36, 78% of the ATL arm and 52% of the SRS arm were seizure free.
Data source: Trial of 58 patients with temporal lobe epilepsy randomized to receive ATL or SRS.
Disclosures: The study was partially funded by Elekta, the manufacturer of the Gamma Knife radiosurgery device used in the study. Several of the presenting ROSE steering committee members reported financial relationships with pharmaceutical companies.
Children with infantile spasms or nonsyndromic epilepsy achieve similar outcomes
HOUSTON – Infants and children who had epilepsy that was not identified as being part of a syndrome fared slightly worse in developmental outcomes and pharmacoresistance than did those with West syndrome/infantile spasms, Dravet syndrome, or another type of syndromic epilepsy, according to a prospective multisite study.
But in the study of 775 patients from 17 American pediatric epilepsy centers, early age at diagnosis was associated with greater mortality, greater risk of developmental decline, and greater pharmacoresistance, regardless of seizure type.
Lead study author Anne Berg, PhD, and her colleagues wrote that they were surprised to see that children in their study population with nonsyndromic epilepsy (NSE) were slightly more likely to have pharmacoresistant seizures (PR).
Further, although logistic regression analysis showed that seizure etiology, younger age at onset, and PR all had independent contributions to developmental decline, “West/IS was not convincingly associated with developmental decline,” they said.
In a poster session at the annual meeting of the American Epilepsy Society, Dr. Berg, an epidemiologist and research professor of pediatric neurology at Northwestern University, Chicago, presented the findings of a study that examined outcomes for infants and children diagnosed with epilepsy.
Patients were prospectively identified during a 3-year period from 2012 to 2015. Patients were eligible if their epilepsy began before their third birthday, and if the epilepsy was initially diagnosed at one of the participating centers. Patient data were evaluated for seizure and developmental outcomes if the patient was followed for at least 6 months after diagnosis.
Of the 775 patients initially recruited, 367 (47.3%) were girls. The mean age of epilepsy onset (which usually meant age at first unprovoked seizure) was 11.1 months (standard deviation, 9.4). Most patients (n = 509; 65.7%) were diagnosed with epilepsy before the age of 1 year. Just 115 patients (14.8%) received their epilepsy diagnosis when they were older than 2 years.
A key outcome investigated by Dr. Berg and her colleagues was pharmacoresistance, identified as lack of seizure control (i.e., at least a 3-month seizure-free period) after trying two appropriate medications. Other outcome measures included tracking whether patients developed West/IS, and whether West/IS evolved into other seizure types. The investigators also tracked developmental delay after epilepsy diagnosis and collected data about deaths among participating patients.
About a quarter (27%) of patients had persistent PR; these were more likely to occur in children who were younger at the onset of epilepsy. PR were more common when seizures began before the age of 1 year, occurring in 30% of this patient population, whereas 20% of patients with seizure onset happening after 1 year of age had PR (P = .0008).
Other findings from the study revealed that infants whose NSE had an etiology of focal cortical dysplasia or of an acquired insult such as trauma were more than twice as likely to have their seizures evolve into WS/IS.
Of 214 children whose initial presentation was WS/IS, 49 (23%) developed new seizure types. Most of these (47 of 49) were infants. Patients with WS/IS due to tuberous sclerosis complex, infectious causes, hypoxic-ischemic encephalopathy, and cephalic brain disorders were more likely to develop new seizure types.
“At initial presentation of epilepsy, children with West/IS were more likely already to have developmental delay than children with other syndromes or NSE,” they said.
Of the 22 patient deaths that occurred during the study, all but 1 occurred in infants younger than 1 year. None of the deaths occurred in typically-developing children with unknown epilepsy etiology.
“West/IS is the only early life epilepsy with consensus guidelines for treatment,” noted Dr. Berg and her coauthors, speculating that the guidelines might contribute to the slightly better outcomes observed for this population in their study.
However, although some groups of infants and children in the study fared slightly better than others, “[F]or the most part, there are no clearly ‘low’ risk groups,” Dr. Berg and her colleagues said. “Our findings highlight that most, if not all, early life epilepsies pose serious risk for poor outcomes and are equally deserving of concerted efforts.”
Dr. Berg reported no relevant financial disclosures. The study was funded by the Pediatric Epilepsy Research Foundation, and conducted through the Pediatric Epilepsy Research Consortium.
[email protected]
On Twitter @karioakes
HOUSTON – Infants and children who had epilepsy that was not identified as being part of a syndrome fared slightly worse in developmental outcomes and pharmacoresistance than did those with West syndrome/infantile spasms, Dravet syndrome, or another type of syndromic epilepsy, according to a prospective multisite study.
But in the study of 775 patients from 17 American pediatric epilepsy centers, early age at diagnosis was associated with greater mortality, greater risk of developmental decline, and greater pharmacoresistance, regardless of seizure type.
Lead study author Anne Berg, PhD, and her colleagues wrote that they were surprised to see that children in their study population with nonsyndromic epilepsy (NSE) were slightly more likely to have pharmacoresistant seizures (PR).
Further, although logistic regression analysis showed that seizure etiology, younger age at onset, and PR all had independent contributions to developmental decline, “West/IS was not convincingly associated with developmental decline,” they said.
In a poster session at the annual meeting of the American Epilepsy Society, Dr. Berg, an epidemiologist and research professor of pediatric neurology at Northwestern University, Chicago, presented the findings of a study that examined outcomes for infants and children diagnosed with epilepsy.
Patients were prospectively identified during a 3-year period from 2012 to 2015. Patients were eligible if their epilepsy began before their third birthday, and if the epilepsy was initially diagnosed at one of the participating centers. Patient data were evaluated for seizure and developmental outcomes if the patient was followed for at least 6 months after diagnosis.
Of the 775 patients initially recruited, 367 (47.3%) were girls. The mean age of epilepsy onset (which usually meant age at first unprovoked seizure) was 11.1 months (standard deviation, 9.4). Most patients (n = 509; 65.7%) were diagnosed with epilepsy before the age of 1 year. Just 115 patients (14.8%) received their epilepsy diagnosis when they were older than 2 years.
A key outcome investigated by Dr. Berg and her colleagues was pharmacoresistance, identified as lack of seizure control (i.e., at least a 3-month seizure-free period) after trying two appropriate medications. Other outcome measures included tracking whether patients developed West/IS, and whether West/IS evolved into other seizure types. The investigators also tracked developmental delay after epilepsy diagnosis and collected data about deaths among participating patients.
About a quarter (27%) of patients had persistent PR; these were more likely to occur in children who were younger at the onset of epilepsy. PR were more common when seizures began before the age of 1 year, occurring in 30% of this patient population, whereas 20% of patients with seizure onset happening after 1 year of age had PR (P = .0008).
Other findings from the study revealed that infants whose NSE had an etiology of focal cortical dysplasia or of an acquired insult such as trauma were more than twice as likely to have their seizures evolve into WS/IS.
Of 214 children whose initial presentation was WS/IS, 49 (23%) developed new seizure types. Most of these (47 of 49) were infants. Patients with WS/IS due to tuberous sclerosis complex, infectious causes, hypoxic-ischemic encephalopathy, and cephalic brain disorders were more likely to develop new seizure types.
“At initial presentation of epilepsy, children with West/IS were more likely already to have developmental delay than children with other syndromes or NSE,” they said.
Of the 22 patient deaths that occurred during the study, all but 1 occurred in infants younger than 1 year. None of the deaths occurred in typically-developing children with unknown epilepsy etiology.
“West/IS is the only early life epilepsy with consensus guidelines for treatment,” noted Dr. Berg and her coauthors, speculating that the guidelines might contribute to the slightly better outcomes observed for this population in their study.
However, although some groups of infants and children in the study fared slightly better than others, “[F]or the most part, there are no clearly ‘low’ risk groups,” Dr. Berg and her colleagues said. “Our findings highlight that most, if not all, early life epilepsies pose serious risk for poor outcomes and are equally deserving of concerted efforts.”
Dr. Berg reported no relevant financial disclosures. The study was funded by the Pediatric Epilepsy Research Foundation, and conducted through the Pediatric Epilepsy Research Consortium.
[email protected]
On Twitter @karioakes
HOUSTON – Infants and children who had epilepsy that was not identified as being part of a syndrome fared slightly worse in developmental outcomes and pharmacoresistance than did those with West syndrome/infantile spasms, Dravet syndrome, or another type of syndromic epilepsy, according to a prospective multisite study.
But in the study of 775 patients from 17 American pediatric epilepsy centers, early age at diagnosis was associated with greater mortality, greater risk of developmental decline, and greater pharmacoresistance, regardless of seizure type.
Lead study author Anne Berg, PhD, and her colleagues wrote that they were surprised to see that children in their study population with nonsyndromic epilepsy (NSE) were slightly more likely to have pharmacoresistant seizures (PR).
Further, although logistic regression analysis showed that seizure etiology, younger age at onset, and PR all had independent contributions to developmental decline, “West/IS was not convincingly associated with developmental decline,” they said.
In a poster session at the annual meeting of the American Epilepsy Society, Dr. Berg, an epidemiologist and research professor of pediatric neurology at Northwestern University, Chicago, presented the findings of a study that examined outcomes for infants and children diagnosed with epilepsy.
Patients were prospectively identified during a 3-year period from 2012 to 2015. Patients were eligible if their epilepsy began before their third birthday, and if the epilepsy was initially diagnosed at one of the participating centers. Patient data were evaluated for seizure and developmental outcomes if the patient was followed for at least 6 months after diagnosis.
Of the 775 patients initially recruited, 367 (47.3%) were girls. The mean age of epilepsy onset (which usually meant age at first unprovoked seizure) was 11.1 months (standard deviation, 9.4). Most patients (n = 509; 65.7%) were diagnosed with epilepsy before the age of 1 year. Just 115 patients (14.8%) received their epilepsy diagnosis when they were older than 2 years.
A key outcome investigated by Dr. Berg and her colleagues was pharmacoresistance, identified as lack of seizure control (i.e., at least a 3-month seizure-free period) after trying two appropriate medications. Other outcome measures included tracking whether patients developed West/IS, and whether West/IS evolved into other seizure types. The investigators also tracked developmental delay after epilepsy diagnosis and collected data about deaths among participating patients.
About a quarter (27%) of patients had persistent PR; these were more likely to occur in children who were younger at the onset of epilepsy. PR were more common when seizures began before the age of 1 year, occurring in 30% of this patient population, whereas 20% of patients with seizure onset happening after 1 year of age had PR (P = .0008).
Other findings from the study revealed that infants whose NSE had an etiology of focal cortical dysplasia or of an acquired insult such as trauma were more than twice as likely to have their seizures evolve into WS/IS.
Of 214 children whose initial presentation was WS/IS, 49 (23%) developed new seizure types. Most of these (47 of 49) were infants. Patients with WS/IS due to tuberous sclerosis complex, infectious causes, hypoxic-ischemic encephalopathy, and cephalic brain disorders were more likely to develop new seizure types.
“At initial presentation of epilepsy, children with West/IS were more likely already to have developmental delay than children with other syndromes or NSE,” they said.
Of the 22 patient deaths that occurred during the study, all but 1 occurred in infants younger than 1 year. None of the deaths occurred in typically-developing children with unknown epilepsy etiology.
“West/IS is the only early life epilepsy with consensus guidelines for treatment,” noted Dr. Berg and her coauthors, speculating that the guidelines might contribute to the slightly better outcomes observed for this population in their study.
However, although some groups of infants and children in the study fared slightly better than others, “[F]or the most part, there are no clearly ‘low’ risk groups,” Dr. Berg and her colleagues said. “Our findings highlight that most, if not all, early life epilepsies pose serious risk for poor outcomes and are equally deserving of concerted efforts.”
Dr. Berg reported no relevant financial disclosures. The study was funded by the Pediatric Epilepsy Research Foundation, and conducted through the Pediatric Epilepsy Research Consortium.
[email protected]
On Twitter @karioakes
AT AES 2016
Key clinical point:
Major finding: Infantile spasms was not independently associated with worse developmental decline compared with nonsyndromic epilepsy.
Data source: Prospective study of 775 infants and children with epilepsy.
Disclosures: Dr. Berg reported no relevant financial disclosures. The study was funded by the Pediatric Epilepsy Research Foundation, and conducted through the Pediatric Epilepsy Research Consortium.
Sorafenib survival benefit in HCC depends on etiology
The impact of sorafenib in treating patients with advanced hepatocellular cancer may be dependent on their hepatitis status, according to a meta-analysis of patient-level data from three large prospective clinical trials using sorafenib as the control treatment.
The analysis indicated that sorafenib had a favorable effect on overall survival for hepatocellular carcinoma patients who were positive for hepatitis C (HCV), but negative for hepatitis B (HBV) only.
For HCV-positive/HBV-negative patients with advanced unresectable hepatocellular carcinoma (aHCC) who received sorafenib, median unadjusted survival was 12.6 months, compared to 10.2 months for patients who received other treatments, yielding a log hazard ratio of –0.27 (95% confidence interval [CI] –0.46 to –0.06).
Though the study did not shed light on the reasons for this difference in overall survival (OS), the results were seen consistently in data from all trials, and sorafenib did not confer any significant survival benefit for individuals who were not HCV positive and HBV negative. “Irrespective of the mechanism, our data suggest that in future trials in aHCC, particularly where sorafenib is the control arm, there should be stratification according to etiology,” wrote Richard Jackson, MSc, and his coauthors (J Clin Oncol. 2017 Jan 3:JCO2016695197 [Epub ahead of print]).
Mr. Jackson, a medical statistician with the Institute of Translational Medicine at the University of Liverpool, England, and his collaborators examined data from 3,256 patients with aHCC. Of these, 1,643 (50%) received sorafenib. The remainder was aggregated into an “other treatment” group, pooling data from patients who received brivanib, sunitinib, and linifanib.
All patients were also divided into four etiologic subgroups: HBV-negative/HCV-negative; HBV-negative/HCV-positive; HBV-positive/HCV-negative; and HBV-positive/HCV-positive. Mr. Jackson and his colleagues then examined the pooled data to see what effect treatment type (sorafenib versus pooled comparator treatments) had on overall survival for each etiologic subgroup. They found no statistically significant survival benefit for sorafenib in the three etiologic subgroups that were not HCV positive/HBV negative, though the data showed a statistically insignificant trend favoring sorafenib. Race, when examined as a potential confounding factor, was not associated with a difference in OS benefit for sorafenib.
The sponsors of three large clinical trials of treatments for aHCC that used sorafenib as the control arm provided deidentified patient-level data to the study’s authors, who then undertook an individual patient data (IPD) meta-analysis. “IPD meta-analyses have a major advantage over aggregate meta-analyses in that they ensure consistent analytic techniques and allow for detailed inspection of interaction of subgroup effects that are not available in published evidence,” wrote Mr. Jackson and his coauthors.
In discussing their findings, the researchers called for more of the data-sharing that allowed their IPD meta-analysis, citing a proposal on the topic from the International Committee of Medical Journal Editors. “[O]ur study showed how the benefits of access to completed trial data are not necessarily confined to reanalysis of the original hypothesis tested by the trial. Here, by a meta-analysis, we arrive at an answer to a question that was not considered when the trials were conceived and could not have been answered by any of the trials individually,” they wrote.
The study data were provided by Bristol-Myers Squibb, Pfizer, and AbbVie from studies they sponsored. Mr. Jackson reported no conflicts of interest.
[email protected]
On Twitter @karioakes
The impact of sorafenib in treating patients with advanced hepatocellular cancer may be dependent on their hepatitis status, according to a meta-analysis of patient-level data from three large prospective clinical trials using sorafenib as the control treatment.
The analysis indicated that sorafenib had a favorable effect on overall survival for hepatocellular carcinoma patients who were positive for hepatitis C (HCV), but negative for hepatitis B (HBV) only.
For HCV-positive/HBV-negative patients with advanced unresectable hepatocellular carcinoma (aHCC) who received sorafenib, median unadjusted survival was 12.6 months, compared to 10.2 months for patients who received other treatments, yielding a log hazard ratio of –0.27 (95% confidence interval [CI] –0.46 to –0.06).
Though the study did not shed light on the reasons for this difference in overall survival (OS), the results were seen consistently in data from all trials, and sorafenib did not confer any significant survival benefit for individuals who were not HCV positive and HBV negative. “Irrespective of the mechanism, our data suggest that in future trials in aHCC, particularly where sorafenib is the control arm, there should be stratification according to etiology,” wrote Richard Jackson, MSc, and his coauthors (J Clin Oncol. 2017 Jan 3:JCO2016695197 [Epub ahead of print]).
Mr. Jackson, a medical statistician with the Institute of Translational Medicine at the University of Liverpool, England, and his collaborators examined data from 3,256 patients with aHCC. Of these, 1,643 (50%) received sorafenib. The remainder was aggregated into an “other treatment” group, pooling data from patients who received brivanib, sunitinib, and linifanib.
All patients were also divided into four etiologic subgroups: HBV-negative/HCV-negative; HBV-negative/HCV-positive; HBV-positive/HCV-negative; and HBV-positive/HCV-positive. Mr. Jackson and his colleagues then examined the pooled data to see what effect treatment type (sorafenib versus pooled comparator treatments) had on overall survival for each etiologic subgroup. They found no statistically significant survival benefit for sorafenib in the three etiologic subgroups that were not HCV positive/HBV negative, though the data showed a statistically insignificant trend favoring sorafenib. Race, when examined as a potential confounding factor, was not associated with a difference in OS benefit for sorafenib.
The sponsors of three large clinical trials of treatments for aHCC that used sorafenib as the control arm provided deidentified patient-level data to the study’s authors, who then undertook an individual patient data (IPD) meta-analysis. “IPD meta-analyses have a major advantage over aggregate meta-analyses in that they ensure consistent analytic techniques and allow for detailed inspection of interaction of subgroup effects that are not available in published evidence,” wrote Mr. Jackson and his coauthors.
In discussing their findings, the researchers called for more of the data-sharing that allowed their IPD meta-analysis, citing a proposal on the topic from the International Committee of Medical Journal Editors. “[O]ur study showed how the benefits of access to completed trial data are not necessarily confined to reanalysis of the original hypothesis tested by the trial. Here, by a meta-analysis, we arrive at an answer to a question that was not considered when the trials were conceived and could not have been answered by any of the trials individually,” they wrote.
The study data were provided by Bristol-Myers Squibb, Pfizer, and AbbVie from studies they sponsored. Mr. Jackson reported no conflicts of interest.
[email protected]
On Twitter @karioakes
The impact of sorafenib in treating patients with advanced hepatocellular cancer may be dependent on their hepatitis status, according to a meta-analysis of patient-level data from three large prospective clinical trials using sorafenib as the control treatment.
The analysis indicated that sorafenib had a favorable effect on overall survival for hepatocellular carcinoma patients who were positive for hepatitis C (HCV), but negative for hepatitis B (HBV) only.
For HCV-positive/HBV-negative patients with advanced unresectable hepatocellular carcinoma (aHCC) who received sorafenib, median unadjusted survival was 12.6 months, compared to 10.2 months for patients who received other treatments, yielding a log hazard ratio of –0.27 (95% confidence interval [CI] –0.46 to –0.06).
Though the study did not shed light on the reasons for this difference in overall survival (OS), the results were seen consistently in data from all trials, and sorafenib did not confer any significant survival benefit for individuals who were not HCV positive and HBV negative. “Irrespective of the mechanism, our data suggest that in future trials in aHCC, particularly where sorafenib is the control arm, there should be stratification according to etiology,” wrote Richard Jackson, MSc, and his coauthors (J Clin Oncol. 2017 Jan 3:JCO2016695197 [Epub ahead of print]).
Mr. Jackson, a medical statistician with the Institute of Translational Medicine at the University of Liverpool, England, and his collaborators examined data from 3,256 patients with aHCC. Of these, 1,643 (50%) received sorafenib. The remainder was aggregated into an “other treatment” group, pooling data from patients who received brivanib, sunitinib, and linifanib.
All patients were also divided into four etiologic subgroups: HBV-negative/HCV-negative; HBV-negative/HCV-positive; HBV-positive/HCV-negative; and HBV-positive/HCV-positive. Mr. Jackson and his colleagues then examined the pooled data to see what effect treatment type (sorafenib versus pooled comparator treatments) had on overall survival for each etiologic subgroup. They found no statistically significant survival benefit for sorafenib in the three etiologic subgroups that were not HCV positive/HBV negative, though the data showed a statistically insignificant trend favoring sorafenib. Race, when examined as a potential confounding factor, was not associated with a difference in OS benefit for sorafenib.
The sponsors of three large clinical trials of treatments for aHCC that used sorafenib as the control arm provided deidentified patient-level data to the study’s authors, who then undertook an individual patient data (IPD) meta-analysis. “IPD meta-analyses have a major advantage over aggregate meta-analyses in that they ensure consistent analytic techniques and allow for detailed inspection of interaction of subgroup effects that are not available in published evidence,” wrote Mr. Jackson and his coauthors.
In discussing their findings, the researchers called for more of the data-sharing that allowed their IPD meta-analysis, citing a proposal on the topic from the International Committee of Medical Journal Editors. “[O]ur study showed how the benefits of access to completed trial data are not necessarily confined to reanalysis of the original hypothesis tested by the trial. Here, by a meta-analysis, we arrive at an answer to a question that was not considered when the trials were conceived and could not have been answered by any of the trials individually,” they wrote.
The study data were provided by Bristol-Myers Squibb, Pfizer, and AbbVie from studies they sponsored. Mr. Jackson reported no conflicts of interest.
[email protected]
On Twitter @karioakes
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: Median unadjusted survival time for HCV-positive/HBV-negative patients was 12.6 months on sorafenib, compared to 10.2 months for these patients who received other treatments.
Data source: Individual patient data meta-analysis of three randomized phase III clinical trials, examining data from 3,256 patients.
Disclosures: The study data were provided by Bristol-Myers Squibb, Pfizer, and AbbVie. Mr. Jackson reported no conflicts of interest.
Infantile spasms diagnosis, treatment too often delayed
HOUSTON – The appropriate evaluation and treatment of infantile spasms often comes after significant delays that are largely attributable to lack of recognition of the condition, according to recent survey results.
“Parental suspicions that ‘something was wrong’ were often discounted by health care providers, and survey respondents frequently reported that health care providers (including neurologists) were unfamiliar with infantile spasms [IS],” wrote Johnson Lay and his collaborators, in presenting results of the recently-completed Assessment of Symptoms and Specialists in Infantile Spasms (ASSIST) study at the annual meeting of the American Epilepsy Society. It’s critically important not to discount parental concerns, senior author Shaun Hussain, MD, said in an interview about the study, which surveyed parents of children with IS: “Sometimes we get in the habit of reassuring patients too much.”
On average, a pediatrician will see two cases of infantile spasms in a career of caring for children, Dr. Hussain said. These characteristically brief episodes last just a second or so, and frequently come in clusters, during or after which crying is common. Though the classic presentation of IS is a brief drop of the head with accompanying elevation of the shoulders – and perhaps a lifting of the arms – there’s wide variation in presentation. “Infantile spasms can present in a lot of different ways; babies haven’t read the neurology textbooks,” Dr. Hussain said. A curated collection of videos showing the variety of infantile spasms is available on the Infantile Spasms Project website.
Mr. Lay and his collaborators surveyed the parents of 100 children with IS in order to understand and describe their experiences in having their child receive the correct diagnosis and treatment. Also, they sought to determine whether socioeconomic factors affected prompt access to care. Dr. Lay completed the study with Dr. Hussain and other coauthors after graduating from UCLA in 2015.
The study, presented during a poster session at the meeting, measured the time between the parents’ first identification of IS and their contact with an “effective provider.” A provider was deemed effective if he or she correctly diagnosed IS and prescribed a first-line therapy: adrenocorticotropin-releasing hormone (ACTH), corticosteroids, or vigabatrin (Sabril).
Of the 100 children whose families were surveyed, 29% were seen by an effective provider within a week of the onset of IS. The median time from the onset of IS to the patient’s being seen by an effective provider was 24.5 days, with a wide range seen in the population surveyed (interquartile range, 0-110.5 days). This exceeds the 7 days that has been identified as the goal cumulative latency to treatment, Mr. Lay and his coauthors said.
The median age of onset of IS was 6.6 months in the study population, 45% of whom were female.
A total of 64 patients were first seen for IS concerns by a provider who was not a neurologist. Of those, the cumulative latency to seeing the first effective provider was over 100 days, with some patients’ wait time stretching to over 200 days.
The survey asked about socioeconomic factors, including income, health insurance status, proximity to specialty care, ethnicity, and parental language preference. Of these, only a non-English parental language preference was associated with increased time to seeing an effective provider (hazard ratio, 0.37; 95% confidence interval, 0.20-0.68; P = .002).
“These results suggest that a simple lack of awareness of infantile spasms among health care providers may be responsible for potentially catastrophic delays in IS care,” wrote Mr. Lay and his coauthors. Dr. Hussain concurred: “The clear driver of treatment delay is the failure of health care providers to recognize the appearance, importance, and urgency of infantile spasms. Delay in diagnosis and treatment of infantile spasms is common, costly – to both patients and the health care system – and almost entirely preventable.”
The Elsie and Isaac Fogelman Endowment, the Hughes Family Foundation, and the UCLA Children’s Discovery and Innovation Institute funded the study. Dr. Hussain has received research support from and been on the advisory boards of several pharmaceutical companies. Mr. Lay reported no disclosures.
[email protected]
On Twitter @karioakes
HOUSTON – The appropriate evaluation and treatment of infantile spasms often comes after significant delays that are largely attributable to lack of recognition of the condition, according to recent survey results.
“Parental suspicions that ‘something was wrong’ were often discounted by health care providers, and survey respondents frequently reported that health care providers (including neurologists) were unfamiliar with infantile spasms [IS],” wrote Johnson Lay and his collaborators, in presenting results of the recently-completed Assessment of Symptoms and Specialists in Infantile Spasms (ASSIST) study at the annual meeting of the American Epilepsy Society. It’s critically important not to discount parental concerns, senior author Shaun Hussain, MD, said in an interview about the study, which surveyed parents of children with IS: “Sometimes we get in the habit of reassuring patients too much.”
On average, a pediatrician will see two cases of infantile spasms in a career of caring for children, Dr. Hussain said. These characteristically brief episodes last just a second or so, and frequently come in clusters, during or after which crying is common. Though the classic presentation of IS is a brief drop of the head with accompanying elevation of the shoulders – and perhaps a lifting of the arms – there’s wide variation in presentation. “Infantile spasms can present in a lot of different ways; babies haven’t read the neurology textbooks,” Dr. Hussain said. A curated collection of videos showing the variety of infantile spasms is available on the Infantile Spasms Project website.
Mr. Lay and his collaborators surveyed the parents of 100 children with IS in order to understand and describe their experiences in having their child receive the correct diagnosis and treatment. Also, they sought to determine whether socioeconomic factors affected prompt access to care. Dr. Lay completed the study with Dr. Hussain and other coauthors after graduating from UCLA in 2015.
The study, presented during a poster session at the meeting, measured the time between the parents’ first identification of IS and their contact with an “effective provider.” A provider was deemed effective if he or she correctly diagnosed IS and prescribed a first-line therapy: adrenocorticotropin-releasing hormone (ACTH), corticosteroids, or vigabatrin (Sabril).
Of the 100 children whose families were surveyed, 29% were seen by an effective provider within a week of the onset of IS. The median time from the onset of IS to the patient’s being seen by an effective provider was 24.5 days, with a wide range seen in the population surveyed (interquartile range, 0-110.5 days). This exceeds the 7 days that has been identified as the goal cumulative latency to treatment, Mr. Lay and his coauthors said.
The median age of onset of IS was 6.6 months in the study population, 45% of whom were female.
A total of 64 patients were first seen for IS concerns by a provider who was not a neurologist. Of those, the cumulative latency to seeing the first effective provider was over 100 days, with some patients’ wait time stretching to over 200 days.
The survey asked about socioeconomic factors, including income, health insurance status, proximity to specialty care, ethnicity, and parental language preference. Of these, only a non-English parental language preference was associated with increased time to seeing an effective provider (hazard ratio, 0.37; 95% confidence interval, 0.20-0.68; P = .002).
“These results suggest that a simple lack of awareness of infantile spasms among health care providers may be responsible for potentially catastrophic delays in IS care,” wrote Mr. Lay and his coauthors. Dr. Hussain concurred: “The clear driver of treatment delay is the failure of health care providers to recognize the appearance, importance, and urgency of infantile spasms. Delay in diagnosis and treatment of infantile spasms is common, costly – to both patients and the health care system – and almost entirely preventable.”
The Elsie and Isaac Fogelman Endowment, the Hughes Family Foundation, and the UCLA Children’s Discovery and Innovation Institute funded the study. Dr. Hussain has received research support from and been on the advisory boards of several pharmaceutical companies. Mr. Lay reported no disclosures.
[email protected]
On Twitter @karioakes
HOUSTON – The appropriate evaluation and treatment of infantile spasms often comes after significant delays that are largely attributable to lack of recognition of the condition, according to recent survey results.
“Parental suspicions that ‘something was wrong’ were often discounted by health care providers, and survey respondents frequently reported that health care providers (including neurologists) were unfamiliar with infantile spasms [IS],” wrote Johnson Lay and his collaborators, in presenting results of the recently-completed Assessment of Symptoms and Specialists in Infantile Spasms (ASSIST) study at the annual meeting of the American Epilepsy Society. It’s critically important not to discount parental concerns, senior author Shaun Hussain, MD, said in an interview about the study, which surveyed parents of children with IS: “Sometimes we get in the habit of reassuring patients too much.”
On average, a pediatrician will see two cases of infantile spasms in a career of caring for children, Dr. Hussain said. These characteristically brief episodes last just a second or so, and frequently come in clusters, during or after which crying is common. Though the classic presentation of IS is a brief drop of the head with accompanying elevation of the shoulders – and perhaps a lifting of the arms – there’s wide variation in presentation. “Infantile spasms can present in a lot of different ways; babies haven’t read the neurology textbooks,” Dr. Hussain said. A curated collection of videos showing the variety of infantile spasms is available on the Infantile Spasms Project website.
Mr. Lay and his collaborators surveyed the parents of 100 children with IS in order to understand and describe their experiences in having their child receive the correct diagnosis and treatment. Also, they sought to determine whether socioeconomic factors affected prompt access to care. Dr. Lay completed the study with Dr. Hussain and other coauthors after graduating from UCLA in 2015.
The study, presented during a poster session at the meeting, measured the time between the parents’ first identification of IS and their contact with an “effective provider.” A provider was deemed effective if he or she correctly diagnosed IS and prescribed a first-line therapy: adrenocorticotropin-releasing hormone (ACTH), corticosteroids, or vigabatrin (Sabril).
Of the 100 children whose families were surveyed, 29% were seen by an effective provider within a week of the onset of IS. The median time from the onset of IS to the patient’s being seen by an effective provider was 24.5 days, with a wide range seen in the population surveyed (interquartile range, 0-110.5 days). This exceeds the 7 days that has been identified as the goal cumulative latency to treatment, Mr. Lay and his coauthors said.
The median age of onset of IS was 6.6 months in the study population, 45% of whom were female.
A total of 64 patients were first seen for IS concerns by a provider who was not a neurologist. Of those, the cumulative latency to seeing the first effective provider was over 100 days, with some patients’ wait time stretching to over 200 days.
The survey asked about socioeconomic factors, including income, health insurance status, proximity to specialty care, ethnicity, and parental language preference. Of these, only a non-English parental language preference was associated with increased time to seeing an effective provider (hazard ratio, 0.37; 95% confidence interval, 0.20-0.68; P = .002).
“These results suggest that a simple lack of awareness of infantile spasms among health care providers may be responsible for potentially catastrophic delays in IS care,” wrote Mr. Lay and his coauthors. Dr. Hussain concurred: “The clear driver of treatment delay is the failure of health care providers to recognize the appearance, importance, and urgency of infantile spasms. Delay in diagnosis and treatment of infantile spasms is common, costly – to both patients and the health care system – and almost entirely preventable.”
The Elsie and Isaac Fogelman Endowment, the Hughes Family Foundation, and the UCLA Children’s Discovery and Innovation Institute funded the study. Dr. Hussain has received research support from and been on the advisory boards of several pharmaceutical companies. Mr. Lay reported no disclosures.
[email protected]
On Twitter @karioakes
AT AES 2016
Key clinical point:
Major finding: The cumulative latency to seeing an effective provider was over 100 days for IS patients initially seen by a nonneurologist.
Data source: Survey of parent(s) of 100 children with infantile spasms.
Disclosures: The Elsie and Isaac Fogelman Endowment, the Hughes Family Foundation, and the UCLA Children’s Discovery and Innovation Institute funded the study. Dr. Hussain has received research support from and been on the advisory boards of several pharmaceutical companies. Mr. Lay reported no disclosures.
Lysosomal acid lipase replacement corrects rare genetic cause of liver failure, atherosclerosis
BOSTON – Lysosomal acid lipase deficiency (LAL-D), a rare genetic cause of marked dyslipidemia that causes early multisystem organ damage, was effectively treated by a human recombinant enzyme to replace deficient lysosomal acid lipase, and the replacement enzyme was well tolerated over a 76-week trial.
LAL-D, when it begins in infancy, is usually fatal within the first year.
When LAL-D occurs later in life, it’s believed to be an “underappreciated cause of fibrosis, cirrhosis, severe dyslipidemia, and early-onset atherosclerosis,” according to Katryn Furuya, MD, and the coauthors of a poster presentation given at the annual meeting of the American Association for the Study of Liver Diseases.
Supplying the human recombinant lysosomal acid lipase, termed sebelipase alfa (SA), to children and adults with LAL-D over a 76-week period resulted in a reduction in alanine aminotransferase (ALT) for 98% of participants, normalization of ALT for 51% of participants, and normalization of aspartate aminotransferase (AST) levels for 65% of patients. Patients on SA also experienced reductions in serum triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and liver fat and total liver volume. “We’re basically replacing what they’re missing,” senior author Barbara Burton, MD, said in an interview.
“The target tissues that we want to get to are the liver, primarily, but also the spleen and the endothelial cells,” said Dr. Burton, professor of gastroenterology at Northwestern University, Chicago. “So the enzyme gets in and then it clears the accumulated fat, and that leads to a reduction in inflammation in the liver, because you don’t have these enlarged lysosomes that are irritating to the cells.”
The effects were seen in LAL-D patients participating in an open-label extension of a double-blind placebo-controlled trial of SA. Patients in the placebo arm who began receiving SA “experienced marked and sustained improvements in liver and lipid parameters, mirroring those observed in the SA group during the double-blind period,” wrote Dr. Furuya and her coauthors. Dr. Furuya, currently a pediatric gastroenterologist at the Mayo Clinic in Rochester, Minn., was a fellow at the Alfred I. duPont Hospital for Children, Wilmington, Del., at the time of the study.
The ARISE (Acid Lipase Replacement Investigating Safety and Efficacy) study included patients aged 4 years and older with a confirmed LAL-D diagnosis. They had to have a baseline ALT at least 1.5 times the upper limit of normal, and if taking lipid-lowering medications, they had to have been on a stable dose for at least 6 weeks before starting the study, and remain on the stable dose for at least the first 32 weeks of the study.
Patients with a history of hematopoietic or liver transplantation were excluded, as were those with severe liver dysfunction, indicated by a Child-Pugh score falling into class C.
The study began with 66 patients entering a randomized, double-blind, placebo-controlled study in which patients received an every-other-week intravenous infusion of SA 1 mg/kg (n = 36), or placebo (n = 30). Median age of participants was 13 years (range, 4-58 years).
After 22 weeks, this was followed by an open-label extension period, during which patients in both arms were unblinded and received SA 1 mg/kg for the duration of the study period; 65/66 patients entered this phase. This means that patients who were initially given SA during the blinded phase of the trial received a total of 76 weeks of SA, while patients who first received placebo before entering the open-label extension did not have their data analyzed until they had been on SA for a total of 78 weeks. The extra 2 weeks accounted for a crossover period for the placebo arm to enter the open-label phase.
The protocol allowed dose increases up to 3 mg/kg if patients’ AST, ALT, LDL-C, or TG levels remained elevated, or if patients under the age of 18 continued to have low weight-for-age z scores. For patients who had problems tolerating SA, the dose could be reduced to 0.35 kg/mg.
Efficacy outcome measures included the proportion of patients who achieved AST and ALT normalization, and those whose ALT values were reduced (but not necessarily normalized). Other measures included changes in LDL-C, HDL-C, non-HDL-C, and TG; reductions in hepatic fat content and total liver volume were also tracked.
After 76 weeks, LDL-C levels were reduced by a mean 27.5%, from 199 to 142 mg/dL, and non-HDL-C dropped by a mean 26.6%, from 230 to 166 mg/dL.
Liver volume and fat content was assessed by multiecho gradient echo magnetic resonance imaging (MEGE-MRI) performed at baseline, at week 20, and at study week 52, representing 52 weeks of SA treatment for the intervention arm and 30 weeks for the placebo arm of the initial trial.
After 52 weeks of SA exposure, the mean hepatic fat reduction was 20.5%, with 88% of patients having reduced liver fat. Of those with 30 weeks of SA exposure, 88% also had reduced liver fat, with a mean fat reduction of 28%.
Liver volume also dropped, by a mean of 13.2% for those with 52 weeks of SA exposure, with 90% of patients experiencing reduced liver volume. Patients with 30 weeks of SA treatment saw a mean 11.2% reduction in liver volume; 96% of this patient group saw some decrease in liver volume.
Safety outcomes included tracking treatment-emergent adverse events (TEAEs), as well as monitoring participants for anti-drug antibodies and for the development of neutralizing antibodies to SA. The safety outcome measures were assessed for patients with longer SA exposure, ranging from 86 to 152 weeks.
There were no patient discontinuations because of TEAEs, and most events were mild or moderate; the only serious adverse event considered related to treatment was an infusion-associated reaction. This patient was able to restart SA therapy after desensitization.
Anti-drug antibodies showed up in 11% of patients (n = 7), and two of these patients had neutralizing antibodies. The safety profile was not different for the group of patients testing positive for anti-drug antibodies, wrote Dr. Furuya and her coauthors.
Replacing LAL-D has promise for a population whose disease may go long undetected. “The patients are not obvious. They are difficult to diagnose,” said Dr. Burton. “They look normal, and they feel normal in many cases, until they have life-threatening disease,” such as end-stage liver disease or cardiovascular complications, she said. Even if elevated transaminases are found in routine screening, physicians are much more likely to think of the more-common nonalcoholic fatty liver disease (NAFLD) than LAL-D, said Dr. Burton, noting that an MRI won’t clarify the diagnosis, though a liver biopsy will show microvesicular rather than macrovesicular fat distribution in LAL-D.
When the clinical picture doesn’t fit with NAFLD, though, LAL-D should be in the differential, she said, adding that she suspects the actual incidence of LAL-D may be higher than has been reported in the literature.
Dr. Burton reported receiving research support, consulting fees, and honoraria from Alexion Pharmaceuticals – the study sponsor and manufacturer of sebelipase alfa. Dr. Furuya reported no disclosures.
[email protected]
On Twitter @karioakes
BOSTON – Lysosomal acid lipase deficiency (LAL-D), a rare genetic cause of marked dyslipidemia that causes early multisystem organ damage, was effectively treated by a human recombinant enzyme to replace deficient lysosomal acid lipase, and the replacement enzyme was well tolerated over a 76-week trial.
LAL-D, when it begins in infancy, is usually fatal within the first year.
When LAL-D occurs later in life, it’s believed to be an “underappreciated cause of fibrosis, cirrhosis, severe dyslipidemia, and early-onset atherosclerosis,” according to Katryn Furuya, MD, and the coauthors of a poster presentation given at the annual meeting of the American Association for the Study of Liver Diseases.
Supplying the human recombinant lysosomal acid lipase, termed sebelipase alfa (SA), to children and adults with LAL-D over a 76-week period resulted in a reduction in alanine aminotransferase (ALT) for 98% of participants, normalization of ALT for 51% of participants, and normalization of aspartate aminotransferase (AST) levels for 65% of patients. Patients on SA also experienced reductions in serum triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and liver fat and total liver volume. “We’re basically replacing what they’re missing,” senior author Barbara Burton, MD, said in an interview.
“The target tissues that we want to get to are the liver, primarily, but also the spleen and the endothelial cells,” said Dr. Burton, professor of gastroenterology at Northwestern University, Chicago. “So the enzyme gets in and then it clears the accumulated fat, and that leads to a reduction in inflammation in the liver, because you don’t have these enlarged lysosomes that are irritating to the cells.”
The effects were seen in LAL-D patients participating in an open-label extension of a double-blind placebo-controlled trial of SA. Patients in the placebo arm who began receiving SA “experienced marked and sustained improvements in liver and lipid parameters, mirroring those observed in the SA group during the double-blind period,” wrote Dr. Furuya and her coauthors. Dr. Furuya, currently a pediatric gastroenterologist at the Mayo Clinic in Rochester, Minn., was a fellow at the Alfred I. duPont Hospital for Children, Wilmington, Del., at the time of the study.
The ARISE (Acid Lipase Replacement Investigating Safety and Efficacy) study included patients aged 4 years and older with a confirmed LAL-D diagnosis. They had to have a baseline ALT at least 1.5 times the upper limit of normal, and if taking lipid-lowering medications, they had to have been on a stable dose for at least 6 weeks before starting the study, and remain on the stable dose for at least the first 32 weeks of the study.
Patients with a history of hematopoietic or liver transplantation were excluded, as were those with severe liver dysfunction, indicated by a Child-Pugh score falling into class C.
The study began with 66 patients entering a randomized, double-blind, placebo-controlled study in which patients received an every-other-week intravenous infusion of SA 1 mg/kg (n = 36), or placebo (n = 30). Median age of participants was 13 years (range, 4-58 years).
After 22 weeks, this was followed by an open-label extension period, during which patients in both arms were unblinded and received SA 1 mg/kg for the duration of the study period; 65/66 patients entered this phase. This means that patients who were initially given SA during the blinded phase of the trial received a total of 76 weeks of SA, while patients who first received placebo before entering the open-label extension did not have their data analyzed until they had been on SA for a total of 78 weeks. The extra 2 weeks accounted for a crossover period for the placebo arm to enter the open-label phase.
The protocol allowed dose increases up to 3 mg/kg if patients’ AST, ALT, LDL-C, or TG levels remained elevated, or if patients under the age of 18 continued to have low weight-for-age z scores. For patients who had problems tolerating SA, the dose could be reduced to 0.35 kg/mg.
Efficacy outcome measures included the proportion of patients who achieved AST and ALT normalization, and those whose ALT values were reduced (but not necessarily normalized). Other measures included changes in LDL-C, HDL-C, non-HDL-C, and TG; reductions in hepatic fat content and total liver volume were also tracked.
After 76 weeks, LDL-C levels were reduced by a mean 27.5%, from 199 to 142 mg/dL, and non-HDL-C dropped by a mean 26.6%, from 230 to 166 mg/dL.
Liver volume and fat content was assessed by multiecho gradient echo magnetic resonance imaging (MEGE-MRI) performed at baseline, at week 20, and at study week 52, representing 52 weeks of SA treatment for the intervention arm and 30 weeks for the placebo arm of the initial trial.
After 52 weeks of SA exposure, the mean hepatic fat reduction was 20.5%, with 88% of patients having reduced liver fat. Of those with 30 weeks of SA exposure, 88% also had reduced liver fat, with a mean fat reduction of 28%.
Liver volume also dropped, by a mean of 13.2% for those with 52 weeks of SA exposure, with 90% of patients experiencing reduced liver volume. Patients with 30 weeks of SA treatment saw a mean 11.2% reduction in liver volume; 96% of this patient group saw some decrease in liver volume.
Safety outcomes included tracking treatment-emergent adverse events (TEAEs), as well as monitoring participants for anti-drug antibodies and for the development of neutralizing antibodies to SA. The safety outcome measures were assessed for patients with longer SA exposure, ranging from 86 to 152 weeks.
There were no patient discontinuations because of TEAEs, and most events were mild or moderate; the only serious adverse event considered related to treatment was an infusion-associated reaction. This patient was able to restart SA therapy after desensitization.
Anti-drug antibodies showed up in 11% of patients (n = 7), and two of these patients had neutralizing antibodies. The safety profile was not different for the group of patients testing positive for anti-drug antibodies, wrote Dr. Furuya and her coauthors.
Replacing LAL-D has promise for a population whose disease may go long undetected. “The patients are not obvious. They are difficult to diagnose,” said Dr. Burton. “They look normal, and they feel normal in many cases, until they have life-threatening disease,” such as end-stage liver disease or cardiovascular complications, she said. Even if elevated transaminases are found in routine screening, physicians are much more likely to think of the more-common nonalcoholic fatty liver disease (NAFLD) than LAL-D, said Dr. Burton, noting that an MRI won’t clarify the diagnosis, though a liver biopsy will show microvesicular rather than macrovesicular fat distribution in LAL-D.
When the clinical picture doesn’t fit with NAFLD, though, LAL-D should be in the differential, she said, adding that she suspects the actual incidence of LAL-D may be higher than has been reported in the literature.
Dr. Burton reported receiving research support, consulting fees, and honoraria from Alexion Pharmaceuticals – the study sponsor and manufacturer of sebelipase alfa. Dr. Furuya reported no disclosures.
[email protected]
On Twitter @karioakes
BOSTON – Lysosomal acid lipase deficiency (LAL-D), a rare genetic cause of marked dyslipidemia that causes early multisystem organ damage, was effectively treated by a human recombinant enzyme to replace deficient lysosomal acid lipase, and the replacement enzyme was well tolerated over a 76-week trial.
LAL-D, when it begins in infancy, is usually fatal within the first year.
When LAL-D occurs later in life, it’s believed to be an “underappreciated cause of fibrosis, cirrhosis, severe dyslipidemia, and early-onset atherosclerosis,” according to Katryn Furuya, MD, and the coauthors of a poster presentation given at the annual meeting of the American Association for the Study of Liver Diseases.
Supplying the human recombinant lysosomal acid lipase, termed sebelipase alfa (SA), to children and adults with LAL-D over a 76-week period resulted in a reduction in alanine aminotransferase (ALT) for 98% of participants, normalization of ALT for 51% of participants, and normalization of aspartate aminotransferase (AST) levels for 65% of patients. Patients on SA also experienced reductions in serum triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and liver fat and total liver volume. “We’re basically replacing what they’re missing,” senior author Barbara Burton, MD, said in an interview.
“The target tissues that we want to get to are the liver, primarily, but also the spleen and the endothelial cells,” said Dr. Burton, professor of gastroenterology at Northwestern University, Chicago. “So the enzyme gets in and then it clears the accumulated fat, and that leads to a reduction in inflammation in the liver, because you don’t have these enlarged lysosomes that are irritating to the cells.”
The effects were seen in LAL-D patients participating in an open-label extension of a double-blind placebo-controlled trial of SA. Patients in the placebo arm who began receiving SA “experienced marked and sustained improvements in liver and lipid parameters, mirroring those observed in the SA group during the double-blind period,” wrote Dr. Furuya and her coauthors. Dr. Furuya, currently a pediatric gastroenterologist at the Mayo Clinic in Rochester, Minn., was a fellow at the Alfred I. duPont Hospital for Children, Wilmington, Del., at the time of the study.
The ARISE (Acid Lipase Replacement Investigating Safety and Efficacy) study included patients aged 4 years and older with a confirmed LAL-D diagnosis. They had to have a baseline ALT at least 1.5 times the upper limit of normal, and if taking lipid-lowering medications, they had to have been on a stable dose for at least 6 weeks before starting the study, and remain on the stable dose for at least the first 32 weeks of the study.
Patients with a history of hematopoietic or liver transplantation were excluded, as were those with severe liver dysfunction, indicated by a Child-Pugh score falling into class C.
The study began with 66 patients entering a randomized, double-blind, placebo-controlled study in which patients received an every-other-week intravenous infusion of SA 1 mg/kg (n = 36), or placebo (n = 30). Median age of participants was 13 years (range, 4-58 years).
After 22 weeks, this was followed by an open-label extension period, during which patients in both arms were unblinded and received SA 1 mg/kg for the duration of the study period; 65/66 patients entered this phase. This means that patients who were initially given SA during the blinded phase of the trial received a total of 76 weeks of SA, while patients who first received placebo before entering the open-label extension did not have their data analyzed until they had been on SA for a total of 78 weeks. The extra 2 weeks accounted for a crossover period for the placebo arm to enter the open-label phase.
The protocol allowed dose increases up to 3 mg/kg if patients’ AST, ALT, LDL-C, or TG levels remained elevated, or if patients under the age of 18 continued to have low weight-for-age z scores. For patients who had problems tolerating SA, the dose could be reduced to 0.35 kg/mg.
Efficacy outcome measures included the proportion of patients who achieved AST and ALT normalization, and those whose ALT values were reduced (but not necessarily normalized). Other measures included changes in LDL-C, HDL-C, non-HDL-C, and TG; reductions in hepatic fat content and total liver volume were also tracked.
After 76 weeks, LDL-C levels were reduced by a mean 27.5%, from 199 to 142 mg/dL, and non-HDL-C dropped by a mean 26.6%, from 230 to 166 mg/dL.
Liver volume and fat content was assessed by multiecho gradient echo magnetic resonance imaging (MEGE-MRI) performed at baseline, at week 20, and at study week 52, representing 52 weeks of SA treatment for the intervention arm and 30 weeks for the placebo arm of the initial trial.
After 52 weeks of SA exposure, the mean hepatic fat reduction was 20.5%, with 88% of patients having reduced liver fat. Of those with 30 weeks of SA exposure, 88% also had reduced liver fat, with a mean fat reduction of 28%.
Liver volume also dropped, by a mean of 13.2% for those with 52 weeks of SA exposure, with 90% of patients experiencing reduced liver volume. Patients with 30 weeks of SA treatment saw a mean 11.2% reduction in liver volume; 96% of this patient group saw some decrease in liver volume.
Safety outcomes included tracking treatment-emergent adverse events (TEAEs), as well as monitoring participants for anti-drug antibodies and for the development of neutralizing antibodies to SA. The safety outcome measures were assessed for patients with longer SA exposure, ranging from 86 to 152 weeks.
There were no patient discontinuations because of TEAEs, and most events were mild or moderate; the only serious adverse event considered related to treatment was an infusion-associated reaction. This patient was able to restart SA therapy after desensitization.
Anti-drug antibodies showed up in 11% of patients (n = 7), and two of these patients had neutralizing antibodies. The safety profile was not different for the group of patients testing positive for anti-drug antibodies, wrote Dr. Furuya and her coauthors.
Replacing LAL-D has promise for a population whose disease may go long undetected. “The patients are not obvious. They are difficult to diagnose,” said Dr. Burton. “They look normal, and they feel normal in many cases, until they have life-threatening disease,” such as end-stage liver disease or cardiovascular complications, she said. Even if elevated transaminases are found in routine screening, physicians are much more likely to think of the more-common nonalcoholic fatty liver disease (NAFLD) than LAL-D, said Dr. Burton, noting that an MRI won’t clarify the diagnosis, though a liver biopsy will show microvesicular rather than macrovesicular fat distribution in LAL-D.
When the clinical picture doesn’t fit with NAFLD, though, LAL-D should be in the differential, she said, adding that she suspects the actual incidence of LAL-D may be higher than has been reported in the literature.
Dr. Burton reported receiving research support, consulting fees, and honoraria from Alexion Pharmaceuticals – the study sponsor and manufacturer of sebelipase alfa. Dr. Furuya reported no disclosures.
[email protected]
On Twitter @karioakes
AT THE LIVER MEETING 2016
Key clinical point:
Major finding: Of patients with LAL-D who received sebelipase alfa (recombinant LAL), 51% experienced normalization of ALT, and 65% had normalization of AST.
Data source: Open-label extension of randomized, double-blind, placebo-controlled trial of 66 patients with LAL-D.
Disclosures: Dr. Burton reported receiving research support, consulting fees, and honoraria from Alexion Pharmaceuticals – the study sponsor and manufacturer of sebelipase alfa. Dr. Furuya reported no disclosures.


















