User login
Fake and Gray Market Drugs Vex U.S. Oncologists
The discovery of fake Avastin and other unauthorized cancer drugs in the United States is putting added pressure on oncologists already struggling with shortages of critical chemotherapy agents and sky-high prices for therapies their patients need but may not be able to afford.
The Food and Drug Administration tracked the counterfeit bevacizumab (Avastin) to at least one foreign supplier and identified 19 medical practices in the United States that purchased unapproved cancer medicines, possibly including the counterfeit.
Discovery of the fake followed an agency warning that the ongoing shortage of injectable cancer drugs "may present an opportunity for unscrupulous individuals to introduce non–FDA-approved products into the drug supply."
At that time, it advised health care providers about the risks of purchasing unapproved injectable cancer medications that are not in short supply from unlicensed sources. The agency named Faslodex (fulvestrant), Neupogen (filgrastim), Rituxan (rituximab) and Herceptin (trastuzumab) – but not Avastin – in the warning.
Subsequently, the United States Attorney’s Office for the Eastern District of Missouri announced that a physician in East St. Louis was indicted on charges of distributing and receiving "misbranded and adulterated" prescription drugs, including Neupogen, Heceptin, and Rituxan. Dr. Abid S. Nisar pled guilty to one misdemeanor count of "misbranding drugs" and will be sentenced on May 25, 2012, according to the government statement.
Impact on Patients Unclear
The widely publicized Avastin case raises two issues for oncologists and patients, according to Dr. J. Leonard Lichtenfeld, deputy chief medical officer for the American Cancer Society.
The "immediate and practical" question concerns the impact on patients who miss their scheduled treatment because of the fake. "Any time that you have a cancer treatment regimen, you’re obviously dealing with a life-threatening illness," he said. "You want to be sure that patients get the right drug at the right time and the right dose. Anything that interrupts that is potentially problematic."
Avastin is "a mainstay of cancer treatment," he noted: a drug that’s used in the treatment of lung cancer, kidney cancer, colon cancer and brain cancer. "If someone is depending on this drug as a lifesaving treatment, and they’ve gotten this counterfeit drug and the disease has progressed that becomes very problematic."
But putting a timetable on the damage is difficult, according to an oncologist whose research team was instrumental in securing approval of Avastin for the treatment of the aggressive brain tumor glioblastoma multiforme (GBM).
"Bevacizumab is the single most active drug for recurrent GBM and may well be the same in newly diagnosed GBM," Dr. Henry S. Friedman, said in an interview.
"One single missed dose isn’t good, but it would be challenging to say that one missed dose was going to have a huge impact. ... Certainly the damage is incremental. The more time that you’re taking the counterfeit drug, the more harm is being done to the patient," observed Dr. Friedman, deputy director of the Preston Robert Tisch Brain Tumor Center at Duke University in Chapel Hill, N.C.
That said, Dr. Friedman added, "One could argue that with cancer, the moment that you give it a chance to resurface, is the moment that you find it explosive. So we’ve had patients in whom we’ve stopped Avastin because we thought their tumor was eradicated, and the tumor has come back within 2 months ... that’s two doses of Avastin that were been missed," he said.
Trust Undermined
For Dr. Lichtenfeld, the undermining of trust is a second, no-less-serious issue arising from the Avastin fraud.
"It’s tough enough to have advanced cancer," he said. "One can only imagine the impact that counterfeit medication can have on destroying the trust of doctors, patients, and families alike in our ability to effectively treat patients with cancer."
In times of drug shortages, oncologists need to be especially wary, Dr. Lichtenfeld said. They should be asking: "What guarantees do you have that the drug that you’re purchasing on the gray market ...are appropriately manufactured by the company and industry in accordance with usual practice?"
The gray market refers to the trade of any commodity – cancer drugs, in this case – through distribution channels which, while legal, are unofficial, unauthorized, or unintended by the original manufacturer.
"It is that chain of custody from the manufacturers to the distributors to the doctor’s office that is truly important," Dr. Lichtenfeld said. "[The Avastin fraud] may put more responsibility on the shoulders of oncology practices to make sure that their drugs are properly sourced."
Dr. Friedman said he was willing to give the physicians in the Avastin case "the benefit of the doubt that their practice was looking for a source of drug that might be less expensive and is passing that on to their patients."
Noting that Avastin is "a really expensive drug" for which many patients do not have coverage, especially when it is being used off label, he added, "I don’t think the physicians should be held accountable at all. ... They had no reason to suspect, just based on the labeling that it really represented someone who was selling garbage."
The Label Tells the Tale
Labeling was an issue in the Missouri case. The U.S. attorney alleged that the physician purchased the prescription drugs from two codefendants employed by Ban Dune Marketing Inc., a business that was located in La Jolla, Calif. The products were not FDA-approved versions for use in the United States; their labeling did not contain National Drug Codes and other legally required information, according to the announcement.
"Some of the drugs contained foreign language labeling, for example Turkish language instructions. Further, these prescription drugs did not come from manufacturing plants that were registered with or inspected by FDA. As such, these prescription drugs were ‘misbranded’ and illegal to receive or provide to patients in the United States," the government said.
In all, the physician was accused of purchasing approximately $352,504 worth of prescription drugs from unlicensed foreign distributors through approximately 47 separate shipments containing 1,138 separate drug units at discounts ranging from 14%-60% of the average wholesale price.
"This indictment reflects the commitment of the FDA’s Office of Criminal Investigations to protect the American public from the harms inherent to misbranded and adulterated drugs. The public must have confidence that their health care providers are receiving and administering drugs that fully comply with U.S. laws," Special Agent in Charge Patrick J. Holland of FDA’s Office of Criminal Investigations, Kansas City Field Office, was quoted in the U.S. Attorney’s announcement.
Dr. Friedman has served as a consultant to and received grants from Genentech.
The discovery of fake Avastin and other unauthorized cancer drugs in the United States is putting added pressure on oncologists already struggling with shortages of critical chemotherapy agents and sky-high prices for therapies their patients need but may not be able to afford.
The Food and Drug Administration tracked the counterfeit bevacizumab (Avastin) to at least one foreign supplier and identified 19 medical practices in the United States that purchased unapproved cancer medicines, possibly including the counterfeit.
Discovery of the fake followed an agency warning that the ongoing shortage of injectable cancer drugs "may present an opportunity for unscrupulous individuals to introduce non–FDA-approved products into the drug supply."
At that time, it advised health care providers about the risks of purchasing unapproved injectable cancer medications that are not in short supply from unlicensed sources. The agency named Faslodex (fulvestrant), Neupogen (filgrastim), Rituxan (rituximab) and Herceptin (trastuzumab) – but not Avastin – in the warning.
Subsequently, the United States Attorney’s Office for the Eastern District of Missouri announced that a physician in East St. Louis was indicted on charges of distributing and receiving "misbranded and adulterated" prescription drugs, including Neupogen, Heceptin, and Rituxan. Dr. Abid S. Nisar pled guilty to one misdemeanor count of "misbranding drugs" and will be sentenced on May 25, 2012, according to the government statement.
Impact on Patients Unclear
The widely publicized Avastin case raises two issues for oncologists and patients, according to Dr. J. Leonard Lichtenfeld, deputy chief medical officer for the American Cancer Society.
The "immediate and practical" question concerns the impact on patients who miss their scheduled treatment because of the fake. "Any time that you have a cancer treatment regimen, you’re obviously dealing with a life-threatening illness," he said. "You want to be sure that patients get the right drug at the right time and the right dose. Anything that interrupts that is potentially problematic."
Avastin is "a mainstay of cancer treatment," he noted: a drug that’s used in the treatment of lung cancer, kidney cancer, colon cancer and brain cancer. "If someone is depending on this drug as a lifesaving treatment, and they’ve gotten this counterfeit drug and the disease has progressed that becomes very problematic."
But putting a timetable on the damage is difficult, according to an oncologist whose research team was instrumental in securing approval of Avastin for the treatment of the aggressive brain tumor glioblastoma multiforme (GBM).
"Bevacizumab is the single most active drug for recurrent GBM and may well be the same in newly diagnosed GBM," Dr. Henry S. Friedman, said in an interview.
"One single missed dose isn’t good, but it would be challenging to say that one missed dose was going to have a huge impact. ... Certainly the damage is incremental. The more time that you’re taking the counterfeit drug, the more harm is being done to the patient," observed Dr. Friedman, deputy director of the Preston Robert Tisch Brain Tumor Center at Duke University in Chapel Hill, N.C.
That said, Dr. Friedman added, "One could argue that with cancer, the moment that you give it a chance to resurface, is the moment that you find it explosive. So we’ve had patients in whom we’ve stopped Avastin because we thought their tumor was eradicated, and the tumor has come back within 2 months ... that’s two doses of Avastin that were been missed," he said.
Trust Undermined
For Dr. Lichtenfeld, the undermining of trust is a second, no-less-serious issue arising from the Avastin fraud.
"It’s tough enough to have advanced cancer," he said. "One can only imagine the impact that counterfeit medication can have on destroying the trust of doctors, patients, and families alike in our ability to effectively treat patients with cancer."
In times of drug shortages, oncologists need to be especially wary, Dr. Lichtenfeld said. They should be asking: "What guarantees do you have that the drug that you’re purchasing on the gray market ...are appropriately manufactured by the company and industry in accordance with usual practice?"
The gray market refers to the trade of any commodity – cancer drugs, in this case – through distribution channels which, while legal, are unofficial, unauthorized, or unintended by the original manufacturer.
"It is that chain of custody from the manufacturers to the distributors to the doctor’s office that is truly important," Dr. Lichtenfeld said. "[The Avastin fraud] may put more responsibility on the shoulders of oncology practices to make sure that their drugs are properly sourced."
Dr. Friedman said he was willing to give the physicians in the Avastin case "the benefit of the doubt that their practice was looking for a source of drug that might be less expensive and is passing that on to their patients."
Noting that Avastin is "a really expensive drug" for which many patients do not have coverage, especially when it is being used off label, he added, "I don’t think the physicians should be held accountable at all. ... They had no reason to suspect, just based on the labeling that it really represented someone who was selling garbage."
The Label Tells the Tale
Labeling was an issue in the Missouri case. The U.S. attorney alleged that the physician purchased the prescription drugs from two codefendants employed by Ban Dune Marketing Inc., a business that was located in La Jolla, Calif. The products were not FDA-approved versions for use in the United States; their labeling did not contain National Drug Codes and other legally required information, according to the announcement.
"Some of the drugs contained foreign language labeling, for example Turkish language instructions. Further, these prescription drugs did not come from manufacturing plants that were registered with or inspected by FDA. As such, these prescription drugs were ‘misbranded’ and illegal to receive or provide to patients in the United States," the government said.
In all, the physician was accused of purchasing approximately $352,504 worth of prescription drugs from unlicensed foreign distributors through approximately 47 separate shipments containing 1,138 separate drug units at discounts ranging from 14%-60% of the average wholesale price.
"This indictment reflects the commitment of the FDA’s Office of Criminal Investigations to protect the American public from the harms inherent to misbranded and adulterated drugs. The public must have confidence that their health care providers are receiving and administering drugs that fully comply with U.S. laws," Special Agent in Charge Patrick J. Holland of FDA’s Office of Criminal Investigations, Kansas City Field Office, was quoted in the U.S. Attorney’s announcement.
Dr. Friedman has served as a consultant to and received grants from Genentech.
The discovery of fake Avastin and other unauthorized cancer drugs in the United States is putting added pressure on oncologists already struggling with shortages of critical chemotherapy agents and sky-high prices for therapies their patients need but may not be able to afford.
The Food and Drug Administration tracked the counterfeit bevacizumab (Avastin) to at least one foreign supplier and identified 19 medical practices in the United States that purchased unapproved cancer medicines, possibly including the counterfeit.
Discovery of the fake followed an agency warning that the ongoing shortage of injectable cancer drugs "may present an opportunity for unscrupulous individuals to introduce non–FDA-approved products into the drug supply."
At that time, it advised health care providers about the risks of purchasing unapproved injectable cancer medications that are not in short supply from unlicensed sources. The agency named Faslodex (fulvestrant), Neupogen (filgrastim), Rituxan (rituximab) and Herceptin (trastuzumab) – but not Avastin – in the warning.
Subsequently, the United States Attorney’s Office for the Eastern District of Missouri announced that a physician in East St. Louis was indicted on charges of distributing and receiving "misbranded and adulterated" prescription drugs, including Neupogen, Heceptin, and Rituxan. Dr. Abid S. Nisar pled guilty to one misdemeanor count of "misbranding drugs" and will be sentenced on May 25, 2012, according to the government statement.
Impact on Patients Unclear
The widely publicized Avastin case raises two issues for oncologists and patients, according to Dr. J. Leonard Lichtenfeld, deputy chief medical officer for the American Cancer Society.
The "immediate and practical" question concerns the impact on patients who miss their scheduled treatment because of the fake. "Any time that you have a cancer treatment regimen, you’re obviously dealing with a life-threatening illness," he said. "You want to be sure that patients get the right drug at the right time and the right dose. Anything that interrupts that is potentially problematic."
Avastin is "a mainstay of cancer treatment," he noted: a drug that’s used in the treatment of lung cancer, kidney cancer, colon cancer and brain cancer. "If someone is depending on this drug as a lifesaving treatment, and they’ve gotten this counterfeit drug and the disease has progressed that becomes very problematic."
But putting a timetable on the damage is difficult, according to an oncologist whose research team was instrumental in securing approval of Avastin for the treatment of the aggressive brain tumor glioblastoma multiforme (GBM).
"Bevacizumab is the single most active drug for recurrent GBM and may well be the same in newly diagnosed GBM," Dr. Henry S. Friedman, said in an interview.
"One single missed dose isn’t good, but it would be challenging to say that one missed dose was going to have a huge impact. ... Certainly the damage is incremental. The more time that you’re taking the counterfeit drug, the more harm is being done to the patient," observed Dr. Friedman, deputy director of the Preston Robert Tisch Brain Tumor Center at Duke University in Chapel Hill, N.C.
That said, Dr. Friedman added, "One could argue that with cancer, the moment that you give it a chance to resurface, is the moment that you find it explosive. So we’ve had patients in whom we’ve stopped Avastin because we thought their tumor was eradicated, and the tumor has come back within 2 months ... that’s two doses of Avastin that were been missed," he said.
Trust Undermined
For Dr. Lichtenfeld, the undermining of trust is a second, no-less-serious issue arising from the Avastin fraud.
"It’s tough enough to have advanced cancer," he said. "One can only imagine the impact that counterfeit medication can have on destroying the trust of doctors, patients, and families alike in our ability to effectively treat patients with cancer."
In times of drug shortages, oncologists need to be especially wary, Dr. Lichtenfeld said. They should be asking: "What guarantees do you have that the drug that you’re purchasing on the gray market ...are appropriately manufactured by the company and industry in accordance with usual practice?"
The gray market refers to the trade of any commodity – cancer drugs, in this case – through distribution channels which, while legal, are unofficial, unauthorized, or unintended by the original manufacturer.
"It is that chain of custody from the manufacturers to the distributors to the doctor’s office that is truly important," Dr. Lichtenfeld said. "[The Avastin fraud] may put more responsibility on the shoulders of oncology practices to make sure that their drugs are properly sourced."
Dr. Friedman said he was willing to give the physicians in the Avastin case "the benefit of the doubt that their practice was looking for a source of drug that might be less expensive and is passing that on to their patients."
Noting that Avastin is "a really expensive drug" for which many patients do not have coverage, especially when it is being used off label, he added, "I don’t think the physicians should be held accountable at all. ... They had no reason to suspect, just based on the labeling that it really represented someone who was selling garbage."
The Label Tells the Tale
Labeling was an issue in the Missouri case. The U.S. attorney alleged that the physician purchased the prescription drugs from two codefendants employed by Ban Dune Marketing Inc., a business that was located in La Jolla, Calif. The products were not FDA-approved versions for use in the United States; their labeling did not contain National Drug Codes and other legally required information, according to the announcement.
"Some of the drugs contained foreign language labeling, for example Turkish language instructions. Further, these prescription drugs did not come from manufacturing plants that were registered with or inspected by FDA. As such, these prescription drugs were ‘misbranded’ and illegal to receive or provide to patients in the United States," the government said.
In all, the physician was accused of purchasing approximately $352,504 worth of prescription drugs from unlicensed foreign distributors through approximately 47 separate shipments containing 1,138 separate drug units at discounts ranging from 14%-60% of the average wholesale price.
"This indictment reflects the commitment of the FDA’s Office of Criminal Investigations to protect the American public from the harms inherent to misbranded and adulterated drugs. The public must have confidence that their health care providers are receiving and administering drugs that fully comply with U.S. laws," Special Agent in Charge Patrick J. Holland of FDA’s Office of Criminal Investigations, Kansas City Field Office, was quoted in the U.S. Attorney’s announcement.
Dr. Friedman has served as a consultant to and received grants from Genentech.
Memory Changes, Hyperglycemia Prompt Statin Label Changes
Physicians can anticipate three key safety changes to the labels of several cholesterol-lowering drugs, according to a Food and Drug Administration announcement on Feb. 28.
The changes affect the following drugs: Lipitor (atorvastatin), Lescol (fluvastatin), Mevacor (lovastatin), Altoprev (lovastatin extended-release), Livalo (pitavastatin), Pravachol (pravastatin), Crestor (rosuvastatin), and Zocor (simvastatin). Three combination products are also included: Advicor (lovastatin/niacin extended-release), Simcor (simvastatin/niacin extended-release), and Vytorin (simvastatin/ezetimibe).
The safety information on the labels will reflect changes with regard to the monitoring of liver enzymes, reports of certain cognitive effects, and reports of hyperglycemia with statin use.
The agency no longer recommends periodic monitoring of liver enzymes. "FDA has concluded that serious liver injury with statins is rare and unpredictable in individual patients, and that routine periodic monitoring of liver enzymes does not appear to be effective in detecting or preventing this rare side effect," according to a press statement. Instead, the agency now recommends that liver enzyme tests be performed before starting statin therapy, and as clinically indicated thereafter.
In addition, labels will now include information about some patients who have experienced memory loss and confusion. The agency noted that these reports generally have not been serious and the patients’ symptoms were reversed by stopping use of the statin. In addition, there have been studies showing that patients being treated with statins may have a small increased risk of increased blood sugar levels and of type 2 diabetes diagnosis. The labels will now warn health care professionals and patients of this potential risk.
More extensive safety changes have been made to the lovastatin label. The changes will note that some medicines may interact with lovastatin, thereby increasing the risk for muscle injury (myopathy/rhabdomyolysis). For example, the use of certain medicines is contraindicated with Mevacor (lovastatin), including drugs used to treat HIV (protease inhibitors) and drugs used to treat certain bacterial and fungal infections.
Health care professionals and patients can report adverse events or side effects related to the use of these products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program online or by downloading a form, or by calling 1-800-332-1088 to request a reporting form, and then completing and returning it to the address on the preaddressed form or submitting it by fax to 1-800-FDA-0178.
For more information on the changes, visit www.fda.gov/Drugs/DrugSafety/ucm293101.htm.
Physicians can anticipate three key safety changes to the labels of several cholesterol-lowering drugs, according to a Food and Drug Administration announcement on Feb. 28.
The changes affect the following drugs: Lipitor (atorvastatin), Lescol (fluvastatin), Mevacor (lovastatin), Altoprev (lovastatin extended-release), Livalo (pitavastatin), Pravachol (pravastatin), Crestor (rosuvastatin), and Zocor (simvastatin). Three combination products are also included: Advicor (lovastatin/niacin extended-release), Simcor (simvastatin/niacin extended-release), and Vytorin (simvastatin/ezetimibe).
The safety information on the labels will reflect changes with regard to the monitoring of liver enzymes, reports of certain cognitive effects, and reports of hyperglycemia with statin use.
The agency no longer recommends periodic monitoring of liver enzymes. "FDA has concluded that serious liver injury with statins is rare and unpredictable in individual patients, and that routine periodic monitoring of liver enzymes does not appear to be effective in detecting or preventing this rare side effect," according to a press statement. Instead, the agency now recommends that liver enzyme tests be performed before starting statin therapy, and as clinically indicated thereafter.
In addition, labels will now include information about some patients who have experienced memory loss and confusion. The agency noted that these reports generally have not been serious and the patients’ symptoms were reversed by stopping use of the statin. In addition, there have been studies showing that patients being treated with statins may have a small increased risk of increased blood sugar levels and of type 2 diabetes diagnosis. The labels will now warn health care professionals and patients of this potential risk.
More extensive safety changes have been made to the lovastatin label. The changes will note that some medicines may interact with lovastatin, thereby increasing the risk for muscle injury (myopathy/rhabdomyolysis). For example, the use of certain medicines is contraindicated with Mevacor (lovastatin), including drugs used to treat HIV (protease inhibitors) and drugs used to treat certain bacterial and fungal infections.
Health care professionals and patients can report adverse events or side effects related to the use of these products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program online or by downloading a form, or by calling 1-800-332-1088 to request a reporting form, and then completing and returning it to the address on the preaddressed form or submitting it by fax to 1-800-FDA-0178.
For more information on the changes, visit www.fda.gov/Drugs/DrugSafety/ucm293101.htm.
Physicians can anticipate three key safety changes to the labels of several cholesterol-lowering drugs, according to a Food and Drug Administration announcement on Feb. 28.
The changes affect the following drugs: Lipitor (atorvastatin), Lescol (fluvastatin), Mevacor (lovastatin), Altoprev (lovastatin extended-release), Livalo (pitavastatin), Pravachol (pravastatin), Crestor (rosuvastatin), and Zocor (simvastatin). Three combination products are also included: Advicor (lovastatin/niacin extended-release), Simcor (simvastatin/niacin extended-release), and Vytorin (simvastatin/ezetimibe).
The safety information on the labels will reflect changes with regard to the monitoring of liver enzymes, reports of certain cognitive effects, and reports of hyperglycemia with statin use.
The agency no longer recommends periodic monitoring of liver enzymes. "FDA has concluded that serious liver injury with statins is rare and unpredictable in individual patients, and that routine periodic monitoring of liver enzymes does not appear to be effective in detecting or preventing this rare side effect," according to a press statement. Instead, the agency now recommends that liver enzyme tests be performed before starting statin therapy, and as clinically indicated thereafter.
In addition, labels will now include information about some patients who have experienced memory loss and confusion. The agency noted that these reports generally have not been serious and the patients’ symptoms were reversed by stopping use of the statin. In addition, there have been studies showing that patients being treated with statins may have a small increased risk of increased blood sugar levels and of type 2 diabetes diagnosis. The labels will now warn health care professionals and patients of this potential risk.
More extensive safety changes have been made to the lovastatin label. The changes will note that some medicines may interact with lovastatin, thereby increasing the risk for muscle injury (myopathy/rhabdomyolysis). For example, the use of certain medicines is contraindicated with Mevacor (lovastatin), including drugs used to treat HIV (protease inhibitors) and drugs used to treat certain bacterial and fungal infections.
Health care professionals and patients can report adverse events or side effects related to the use of these products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program online or by downloading a form, or by calling 1-800-332-1088 to request a reporting form, and then completing and returning it to the address on the preaddressed form or submitting it by fax to 1-800-FDA-0178.
For more information on the changes, visit www.fda.gov/Drugs/DrugSafety/ucm293101.htm.
FDA Tracks Fake Avastin to Foreign Supply Distributed in U.S.
The Food and Drug Administration has tracked counterfeit bevacizumab, the cancer drug marketed as Avastin, to at least one foreign supplier and identified 19 medical practices in the United States that purchased unapproved cancer medicines, possibly including counterfeit bevacizumab.
The FDA said the practices obtained the fake Avastin from Quality Specialty Products (QSP), a foreign supplier also known as Montana Health Care Solutions. QSP products are also distributed by Volunteer Distribution in Gainesboro, Tenn., the agency said.
The FDA has requested that medical practices stop using any remaining products purchased from these suppliers "or any other unapproved foreign source," because the agency cannot ensure the safety or efficacy of any of these unapproved products.
"The Agency is very concerned that these products may cause harm to patients because they are unsafe or ineffective. ... These products may be from unknown sources; have unknown ingredients; and may not have been manufactured, transported or stored under proper conditions required by U.S. law, regulations, and standards," the agency said.
Avastin’s manufacturers announced Feb. 14 that they learned of the distribution of the counterfeit in the United States. "The counterfeit product is not safe or effective and should not be used. Chemical analyses of the counterfeit vials tested to date have confirmed the product does not contain the active ingredients for Avastin," warned F. Hoffmann-La Roche and Genentech.
Avastin, an injectable medicine used to treat many cancers, is administered to patients in clinics, hospitals, and doctors’ offices. The counterfeit version does not contain the monoclonal antibody bevacizumab.
The FDA similarly warned health care professionals and patients about a counterfeit version of Avastin 400mg/16mL, which may have been purchased and used by some medical practices in the United States.
The FDA has since issued letters to 19 U.S. medical practices that purchased unapproved cancer medicines that may include the counterfeit Avastin. It is believed that some product in the United States labeled as Avastin 400 mg/16 mL with the following lot numbers on either the vials or packaging may be counterfeit: B86017, B6011, and B6010.
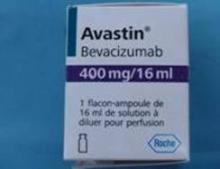
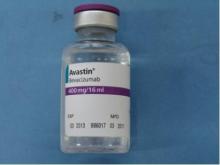
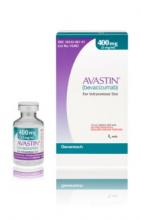
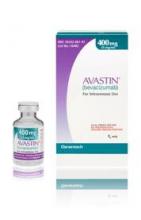
Importantly, the counterfeit product does not look like authentic Avastin. Authentic Avastin’s cartons and vials (approved for use in the United States) have "Genentech" or "Genentech, a member of the Roche Group" printed on the labels. The counterfeit version is labeled as Avastin, manufactured by Roche, which manufactures Avastin for marketing outside of the United States.
In addition, the lot number on the carton and vial should be six digits with no letters; the expiry date is formatted as a three-letter month and four-digit year (such as, JUL 2014); the date of manufacture is not printed on the carton or vial; and all the text on the vial labels, cartons, and package inserts is in English on products approved for sale in the United States.
The fake Avastin scandal comes at a time when many injectable cancer drugs are in short supply – a situation that "may present an opportunity for unscrupulous individuals to introduce non-FDA approved products into the drug supply," the FDA warned on Jan. 13.
At that time, it advised health care providers about the risks of purchasing unapproved injectable cancer medications that are not in short supply from unlicensed sources. The agency named Faslodex (fulvestrant), Neupogen (filgrastim), Rituxan (rituximab) and Herceptin (trastuzumab) – but not Avastin – in the January warning.
"In certain circumstances, the FDA may authorize limited importation of medications that are in short supply. Such medications are imported from approved international sources and distributed in the U.S. through a controlled network, and would not be sold in direct-to-clinic solicitations," the agency advised.
"If the FDA has arranged for limited importation of the foreign version of a medication, information on obtaining that medication will be available on the FDA drug shortages website, often in the form of a ‘Dear Healthcare Professional’ letter."
Based on information to date, the FDA has determined that none of the unapproved cancer medicines that medical practices are known to have received from Volunteer Distribution are in short supply in the United States. FDA-approved versions of these medicines are available in adequate supply to meet current demand.
Medical practices that have obtained unapproved products from foreign sources, in particular from Volunteer Distribution and/or QSP, should stop using them and contact the FDA. These products should be retained and securely stored.
To report suspect counterfeit products and other suspect unapproved products obtained from Volunteer Distribution or QSP/Montana Health Care Solutions or other sources: Call FDA’s Office of Criminal Investigations (OCI) at 800-551-3989, or visit OCI’s website.
For information about this counterfeit medicine, see Roche’s statement.
Click here for more information about counterfeit medicines found in the United States.
The Food and Drug Administration has tracked counterfeit bevacizumab, the cancer drug marketed as Avastin, to at least one foreign supplier and identified 19 medical practices in the United States that purchased unapproved cancer medicines, possibly including counterfeit bevacizumab.
The FDA said the practices obtained the fake Avastin from Quality Specialty Products (QSP), a foreign supplier also known as Montana Health Care Solutions. QSP products are also distributed by Volunteer Distribution in Gainesboro, Tenn., the agency said.
The FDA has requested that medical practices stop using any remaining products purchased from these suppliers "or any other unapproved foreign source," because the agency cannot ensure the safety or efficacy of any of these unapproved products.
"The Agency is very concerned that these products may cause harm to patients because they are unsafe or ineffective. ... These products may be from unknown sources; have unknown ingredients; and may not have been manufactured, transported or stored under proper conditions required by U.S. law, regulations, and standards," the agency said.
Avastin’s manufacturers announced Feb. 14 that they learned of the distribution of the counterfeit in the United States. "The counterfeit product is not safe or effective and should not be used. Chemical analyses of the counterfeit vials tested to date have confirmed the product does not contain the active ingredients for Avastin," warned F. Hoffmann-La Roche and Genentech.
Avastin, an injectable medicine used to treat many cancers, is administered to patients in clinics, hospitals, and doctors’ offices. The counterfeit version does not contain the monoclonal antibody bevacizumab.
The FDA similarly warned health care professionals and patients about a counterfeit version of Avastin 400mg/16mL, which may have been purchased and used by some medical practices in the United States.
The FDA has since issued letters to 19 U.S. medical practices that purchased unapproved cancer medicines that may include the counterfeit Avastin. It is believed that some product in the United States labeled as Avastin 400 mg/16 mL with the following lot numbers on either the vials or packaging may be counterfeit: B86017, B6011, and B6010.
Importantly, the counterfeit product does not look like authentic Avastin. Authentic Avastin’s cartons and vials (approved for use in the United States) have "Genentech" or "Genentech, a member of the Roche Group" printed on the labels. The counterfeit version is labeled as Avastin, manufactured by Roche, which manufactures Avastin for marketing outside of the United States.
In addition, the lot number on the carton and vial should be six digits with no letters; the expiry date is formatted as a three-letter month and four-digit year (such as, JUL 2014); the date of manufacture is not printed on the carton or vial; and all the text on the vial labels, cartons, and package inserts is in English on products approved for sale in the United States.
The fake Avastin scandal comes at a time when many injectable cancer drugs are in short supply – a situation that "may present an opportunity for unscrupulous individuals to introduce non-FDA approved products into the drug supply," the FDA warned on Jan. 13.
At that time, it advised health care providers about the risks of purchasing unapproved injectable cancer medications that are not in short supply from unlicensed sources. The agency named Faslodex (fulvestrant), Neupogen (filgrastim), Rituxan (rituximab) and Herceptin (trastuzumab) – but not Avastin – in the January warning.
"In certain circumstances, the FDA may authorize limited importation of medications that are in short supply. Such medications are imported from approved international sources and distributed in the U.S. through a controlled network, and would not be sold in direct-to-clinic solicitations," the agency advised.
"If the FDA has arranged for limited importation of the foreign version of a medication, information on obtaining that medication will be available on the FDA drug shortages website, often in the form of a ‘Dear Healthcare Professional’ letter."
Based on information to date, the FDA has determined that none of the unapproved cancer medicines that medical practices are known to have received from Volunteer Distribution are in short supply in the United States. FDA-approved versions of these medicines are available in adequate supply to meet current demand.
Medical practices that have obtained unapproved products from foreign sources, in particular from Volunteer Distribution and/or QSP, should stop using them and contact the FDA. These products should be retained and securely stored.
To report suspect counterfeit products and other suspect unapproved products obtained from Volunteer Distribution or QSP/Montana Health Care Solutions or other sources: Call FDA’s Office of Criminal Investigations (OCI) at 800-551-3989, or visit OCI’s website.
For information about this counterfeit medicine, see Roche’s statement.
Click here for more information about counterfeit medicines found in the United States.
The Food and Drug Administration has tracked counterfeit bevacizumab, the cancer drug marketed as Avastin, to at least one foreign supplier and identified 19 medical practices in the United States that purchased unapproved cancer medicines, possibly including counterfeit bevacizumab.
The FDA said the practices obtained the fake Avastin from Quality Specialty Products (QSP), a foreign supplier also known as Montana Health Care Solutions. QSP products are also distributed by Volunteer Distribution in Gainesboro, Tenn., the agency said.
The FDA has requested that medical practices stop using any remaining products purchased from these suppliers "or any other unapproved foreign source," because the agency cannot ensure the safety or efficacy of any of these unapproved products.
"The Agency is very concerned that these products may cause harm to patients because they are unsafe or ineffective. ... These products may be from unknown sources; have unknown ingredients; and may not have been manufactured, transported or stored under proper conditions required by U.S. law, regulations, and standards," the agency said.
Avastin’s manufacturers announced Feb. 14 that they learned of the distribution of the counterfeit in the United States. "The counterfeit product is not safe or effective and should not be used. Chemical analyses of the counterfeit vials tested to date have confirmed the product does not contain the active ingredients for Avastin," warned F. Hoffmann-La Roche and Genentech.
Avastin, an injectable medicine used to treat many cancers, is administered to patients in clinics, hospitals, and doctors’ offices. The counterfeit version does not contain the monoclonal antibody bevacizumab.
The FDA similarly warned health care professionals and patients about a counterfeit version of Avastin 400mg/16mL, which may have been purchased and used by some medical practices in the United States.
The FDA has since issued letters to 19 U.S. medical practices that purchased unapproved cancer medicines that may include the counterfeit Avastin. It is believed that some product in the United States labeled as Avastin 400 mg/16 mL with the following lot numbers on either the vials or packaging may be counterfeit: B86017, B6011, and B6010.
Importantly, the counterfeit product does not look like authentic Avastin. Authentic Avastin’s cartons and vials (approved for use in the United States) have "Genentech" or "Genentech, a member of the Roche Group" printed on the labels. The counterfeit version is labeled as Avastin, manufactured by Roche, which manufactures Avastin for marketing outside of the United States.
In addition, the lot number on the carton and vial should be six digits with no letters; the expiry date is formatted as a three-letter month and four-digit year (such as, JUL 2014); the date of manufacture is not printed on the carton or vial; and all the text on the vial labels, cartons, and package inserts is in English on products approved for sale in the United States.
The fake Avastin scandal comes at a time when many injectable cancer drugs are in short supply – a situation that "may present an opportunity for unscrupulous individuals to introduce non-FDA approved products into the drug supply," the FDA warned on Jan. 13.
At that time, it advised health care providers about the risks of purchasing unapproved injectable cancer medications that are not in short supply from unlicensed sources. The agency named Faslodex (fulvestrant), Neupogen (filgrastim), Rituxan (rituximab) and Herceptin (trastuzumab) – but not Avastin – in the January warning.
"In certain circumstances, the FDA may authorize limited importation of medications that are in short supply. Such medications are imported from approved international sources and distributed in the U.S. through a controlled network, and would not be sold in direct-to-clinic solicitations," the agency advised.
"If the FDA has arranged for limited importation of the foreign version of a medication, information on obtaining that medication will be available on the FDA drug shortages website, often in the form of a ‘Dear Healthcare Professional’ letter."
Based on information to date, the FDA has determined that none of the unapproved cancer medicines that medical practices are known to have received from Volunteer Distribution are in short supply in the United States. FDA-approved versions of these medicines are available in adequate supply to meet current demand.
Medical practices that have obtained unapproved products from foreign sources, in particular from Volunteer Distribution and/or QSP, should stop using them and contact the FDA. These products should be retained and securely stored.
To report suspect counterfeit products and other suspect unapproved products obtained from Volunteer Distribution or QSP/Montana Health Care Solutions or other sources: Call FDA’s Office of Criminal Investigations (OCI) at 800-551-3989, or visit OCI’s website.
For information about this counterfeit medicine, see Roche’s statement.
Click here for more information about counterfeit medicines found in the United States.
Experts Can't Pin Down Best Carotid Atherosclerosis Treatments
There are still no clear answers about the best treatments to use to prevent stroke in patients with carotid artery stenosis, even with the input of a Medicare advisory panel of experts.
The Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) voted yesterday that they are only moderately confident that there is adequate evidence to determine which interventions – carotid artery endarterectomy (CEA), carotid artery stenting (CAS), or best medical treatment (BMT) – to use for different patient populations within the overall Medicare population. There’s unlikely to be any consensus in the near future as patient populations, detection, and treatments evolve.
"If there was ever a moving target problem in assessing health care interventions, this was one. The epidemiology is changing, the patient population is changing accordingly, [and] all of the interventions continue to change. We’ve not been keeping up in our data collection for the safety and effectiveness of these interventions as they continue to evolve," commented committee chair, Clifford Goodman, Ph.D. "This means that to the extent that CMS is going to revisit its coverage decision making over time, this is an ongoing data collection issue. You’re not done collecting these data; you may not ever be done collecting these data as long as the patient population continues to change and as long as we have very innovative people improving these interventions."
The panel was asked to vote on several questions regarding their confidence level that there is adequate evidence to identify specific patient populations and in some cases to select the best treatment option. Most scenarios garnered an "intermediate" confidence level. Confidence ran high only for the lowest-risk patients.
The panel voted that they had moderate confidence that the evidence is adequate to determine whether:
• Patients who are asymptomatic for carotid atherosclerosis can be identified as being at high risk for stroke in either cerebral hemisphere.
• Patients who are considering carotid revascularization can be identified as being at high risk for adverse events from CEA.
• Either CAS or CEA is the favored treatment strategy compared to BMT alone in patients with symptomatic carotid atherosclerosis and narrowing (at least 50% by angiography or at least 70% by ultrasound) who are not generally at high risk for adverse events from CEA.
• CAS, CEA, or BMT is the favored treatment strategy to decrease stroke or death for patients with asymptomatic carotid atherosclerosis.
In addition, for patients with symptomatic carotid atherosclerosis, who are not generally at high risk for adverse events from CEA, the panel had intermediate confidence that CEA is the favored treatment strategy to decrease stroke and death. For patients with asymptomatic carotid atherosclerosis, the panel had intermediate to high confidence that BMT is the favored treatment strategy to decrease stroke and death.
However, the panel was less confident that for patients with asymptomatic carotid atherosclerosis but with carotid narrowing – who are not generally considered at high risk for adverse events from CEA – that there is adequate evidence to determine whether or not either CAS or CEA is the favored strategy compared to BMT alone to decrease stroke or death in the Medicare population. The panel had very low confidence that there is adequate evidence to determine whether carotid artery screening of asymptomatic people decreases stroke or death.
The panel primarily based its decisions on the results of a handful of randomized, controlled trials with different patient populations, at different time periods, using different criteria to assess efficacy and safety. The results of the most recent of these – the CREST trial (Carotid Revascularization Endarterectomy vs. Stenting Trial) – were announced in 2010. The overall safety and efficacy of the CAS and CEA at 4 years were considered equivocal in terms of benefits for both men and for women, for patients who had previously had a stroke, and for those who had not (N. Engl. J. Med. 2010;363:11-23).
However, differences were seen between the two procedures for heart attacks and strokes. More heart attacks occurred with CEA – 2.3% vs. 1.1% – than for CAS, while more strokes occurred in the stenting group – 4.1% vs. 2.3% –than for CEA in the weeks following the procedure. There was also a difference in outcomes based on patient age. At approximately age 69 and younger, stenting results were slightly better, with CAS benefits increasing for decreasing patient age. Conversely, for patients older than 70, CEA results were slightly superior to stenting, with larger benefits for surgery with older patient age.
Instead of answering the question of which technique has better outcomes and safety, the trial left proponents of each technique still arguing.
In addition, BMT has not been assessed in clinical trials for many years and has evolved substantially since that time with the development and growing use of statins, antiplatelet medications, beta-blockers, ACE inhibitors, calcium channel blockers, and others.
What is lacking is a randomized, controlled trial pitting all three treatments against one another in a head-to-head comparison of the most current techniques, the panel noted repeatedly.
"I think that drawing conclusions from indirect comparisons of things that were done in the past is incredibly hazardous and we need to have a contemporary direct comparison to know what the right thing to do is," argued panel member Dr. Larry B. Goldstein, who is a professor of neurology and director of the Duke Stroke Center in Chapel Hill, N.C.
Both surgery and CAS received a class I recommendation in guidelines published by the American Heart Association and other organizations in 2011 (J. Am. Coll. Cardiol. 2011;57:1002-44), but the level of evidence was stronger for CEA (level A) than for CAS (level B).
Planning is in the works for a possible CREST II trial in asymptomatic patients intended to compare intervention (with either CEA or CAS) vs. medical treatment alone. Many panel members expressed the need for this trial to proceed.
Twelve members of the panel reported that they did not have any relevant financial relationships. Dr. Spence reported receiving speaking fees from several pharmaceutical companies, Dr. Curtis reported that he owns stock in Medtronic and receives grant support from Boston Scientific, industry representative Dr. Peter Juhn works for Medco Health Solutions, and committee chair Dr. Clifford Goodman is the senior vice president at the Lewin Group, which has ties to both government and industry.
There are still no clear answers about the best treatments to use to prevent stroke in patients with carotid artery stenosis, even with the input of a Medicare advisory panel of experts.
The Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) voted yesterday that they are only moderately confident that there is adequate evidence to determine which interventions – carotid artery endarterectomy (CEA), carotid artery stenting (CAS), or best medical treatment (BMT) – to use for different patient populations within the overall Medicare population. There’s unlikely to be any consensus in the near future as patient populations, detection, and treatments evolve.
"If there was ever a moving target problem in assessing health care interventions, this was one. The epidemiology is changing, the patient population is changing accordingly, [and] all of the interventions continue to change. We’ve not been keeping up in our data collection for the safety and effectiveness of these interventions as they continue to evolve," commented committee chair, Clifford Goodman, Ph.D. "This means that to the extent that CMS is going to revisit its coverage decision making over time, this is an ongoing data collection issue. You’re not done collecting these data; you may not ever be done collecting these data as long as the patient population continues to change and as long as we have very innovative people improving these interventions."
The panel was asked to vote on several questions regarding their confidence level that there is adequate evidence to identify specific patient populations and in some cases to select the best treatment option. Most scenarios garnered an "intermediate" confidence level. Confidence ran high only for the lowest-risk patients.
The panel voted that they had moderate confidence that the evidence is adequate to determine whether:
• Patients who are asymptomatic for carotid atherosclerosis can be identified as being at high risk for stroke in either cerebral hemisphere.
• Patients who are considering carotid revascularization can be identified as being at high risk for adverse events from CEA.
• Either CAS or CEA is the favored treatment strategy compared to BMT alone in patients with symptomatic carotid atherosclerosis and narrowing (at least 50% by angiography or at least 70% by ultrasound) who are not generally at high risk for adverse events from CEA.
• CAS, CEA, or BMT is the favored treatment strategy to decrease stroke or death for patients with asymptomatic carotid atherosclerosis.
In addition, for patients with symptomatic carotid atherosclerosis, who are not generally at high risk for adverse events from CEA, the panel had intermediate confidence that CEA is the favored treatment strategy to decrease stroke and death. For patients with asymptomatic carotid atherosclerosis, the panel had intermediate to high confidence that BMT is the favored treatment strategy to decrease stroke and death.
However, the panel was less confident that for patients with asymptomatic carotid atherosclerosis but with carotid narrowing – who are not generally considered at high risk for adverse events from CEA – that there is adequate evidence to determine whether or not either CAS or CEA is the favored strategy compared to BMT alone to decrease stroke or death in the Medicare population. The panel had very low confidence that there is adequate evidence to determine whether carotid artery screening of asymptomatic people decreases stroke or death.
The panel primarily based its decisions on the results of a handful of randomized, controlled trials with different patient populations, at different time periods, using different criteria to assess efficacy and safety. The results of the most recent of these – the CREST trial (Carotid Revascularization Endarterectomy vs. Stenting Trial) – were announced in 2010. The overall safety and efficacy of the CAS and CEA at 4 years were considered equivocal in terms of benefits for both men and for women, for patients who had previously had a stroke, and for those who had not (N. Engl. J. Med. 2010;363:11-23).
However, differences were seen between the two procedures for heart attacks and strokes. More heart attacks occurred with CEA – 2.3% vs. 1.1% – than for CAS, while more strokes occurred in the stenting group – 4.1% vs. 2.3% –than for CEA in the weeks following the procedure. There was also a difference in outcomes based on patient age. At approximately age 69 and younger, stenting results were slightly better, with CAS benefits increasing for decreasing patient age. Conversely, for patients older than 70, CEA results were slightly superior to stenting, with larger benefits for surgery with older patient age.
Instead of answering the question of which technique has better outcomes and safety, the trial left proponents of each technique still arguing.
In addition, BMT has not been assessed in clinical trials for many years and has evolved substantially since that time with the development and growing use of statins, antiplatelet medications, beta-blockers, ACE inhibitors, calcium channel blockers, and others.
What is lacking is a randomized, controlled trial pitting all three treatments against one another in a head-to-head comparison of the most current techniques, the panel noted repeatedly.
"I think that drawing conclusions from indirect comparisons of things that were done in the past is incredibly hazardous and we need to have a contemporary direct comparison to know what the right thing to do is," argued panel member Dr. Larry B. Goldstein, who is a professor of neurology and director of the Duke Stroke Center in Chapel Hill, N.C.
Both surgery and CAS received a class I recommendation in guidelines published by the American Heart Association and other organizations in 2011 (J. Am. Coll. Cardiol. 2011;57:1002-44), but the level of evidence was stronger for CEA (level A) than for CAS (level B).
Planning is in the works for a possible CREST II trial in asymptomatic patients intended to compare intervention (with either CEA or CAS) vs. medical treatment alone. Many panel members expressed the need for this trial to proceed.
Twelve members of the panel reported that they did not have any relevant financial relationships. Dr. Spence reported receiving speaking fees from several pharmaceutical companies, Dr. Curtis reported that he owns stock in Medtronic and receives grant support from Boston Scientific, industry representative Dr. Peter Juhn works for Medco Health Solutions, and committee chair Dr. Clifford Goodman is the senior vice president at the Lewin Group, which has ties to both government and industry.
There are still no clear answers about the best treatments to use to prevent stroke in patients with carotid artery stenosis, even with the input of a Medicare advisory panel of experts.
The Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) voted yesterday that they are only moderately confident that there is adequate evidence to determine which interventions – carotid artery endarterectomy (CEA), carotid artery stenting (CAS), or best medical treatment (BMT) – to use for different patient populations within the overall Medicare population. There’s unlikely to be any consensus in the near future as patient populations, detection, and treatments evolve.
"If there was ever a moving target problem in assessing health care interventions, this was one. The epidemiology is changing, the patient population is changing accordingly, [and] all of the interventions continue to change. We’ve not been keeping up in our data collection for the safety and effectiveness of these interventions as they continue to evolve," commented committee chair, Clifford Goodman, Ph.D. "This means that to the extent that CMS is going to revisit its coverage decision making over time, this is an ongoing data collection issue. You’re not done collecting these data; you may not ever be done collecting these data as long as the patient population continues to change and as long as we have very innovative people improving these interventions."
The panel was asked to vote on several questions regarding their confidence level that there is adequate evidence to identify specific patient populations and in some cases to select the best treatment option. Most scenarios garnered an "intermediate" confidence level. Confidence ran high only for the lowest-risk patients.
The panel voted that they had moderate confidence that the evidence is adequate to determine whether:
• Patients who are asymptomatic for carotid atherosclerosis can be identified as being at high risk for stroke in either cerebral hemisphere.
• Patients who are considering carotid revascularization can be identified as being at high risk for adverse events from CEA.
• Either CAS or CEA is the favored treatment strategy compared to BMT alone in patients with symptomatic carotid atherosclerosis and narrowing (at least 50% by angiography or at least 70% by ultrasound) who are not generally at high risk for adverse events from CEA.
• CAS, CEA, or BMT is the favored treatment strategy to decrease stroke or death for patients with asymptomatic carotid atherosclerosis.
In addition, for patients with symptomatic carotid atherosclerosis, who are not generally at high risk for adverse events from CEA, the panel had intermediate confidence that CEA is the favored treatment strategy to decrease stroke and death. For patients with asymptomatic carotid atherosclerosis, the panel had intermediate to high confidence that BMT is the favored treatment strategy to decrease stroke and death.
However, the panel was less confident that for patients with asymptomatic carotid atherosclerosis but with carotid narrowing – who are not generally considered at high risk for adverse events from CEA – that there is adequate evidence to determine whether or not either CAS or CEA is the favored strategy compared to BMT alone to decrease stroke or death in the Medicare population. The panel had very low confidence that there is adequate evidence to determine whether carotid artery screening of asymptomatic people decreases stroke or death.
The panel primarily based its decisions on the results of a handful of randomized, controlled trials with different patient populations, at different time periods, using different criteria to assess efficacy and safety. The results of the most recent of these – the CREST trial (Carotid Revascularization Endarterectomy vs. Stenting Trial) – were announced in 2010. The overall safety and efficacy of the CAS and CEA at 4 years were considered equivocal in terms of benefits for both men and for women, for patients who had previously had a stroke, and for those who had not (N. Engl. J. Med. 2010;363:11-23).
However, differences were seen between the two procedures for heart attacks and strokes. More heart attacks occurred with CEA – 2.3% vs. 1.1% – than for CAS, while more strokes occurred in the stenting group – 4.1% vs. 2.3% –than for CEA in the weeks following the procedure. There was also a difference in outcomes based on patient age. At approximately age 69 and younger, stenting results were slightly better, with CAS benefits increasing for decreasing patient age. Conversely, for patients older than 70, CEA results were slightly superior to stenting, with larger benefits for surgery with older patient age.
Instead of answering the question of which technique has better outcomes and safety, the trial left proponents of each technique still arguing.
In addition, BMT has not been assessed in clinical trials for many years and has evolved substantially since that time with the development and growing use of statins, antiplatelet medications, beta-blockers, ACE inhibitors, calcium channel blockers, and others.
What is lacking is a randomized, controlled trial pitting all three treatments against one another in a head-to-head comparison of the most current techniques, the panel noted repeatedly.
"I think that drawing conclusions from indirect comparisons of things that were done in the past is incredibly hazardous and we need to have a contemporary direct comparison to know what the right thing to do is," argued panel member Dr. Larry B. Goldstein, who is a professor of neurology and director of the Duke Stroke Center in Chapel Hill, N.C.
Both surgery and CAS received a class I recommendation in guidelines published by the American Heart Association and other organizations in 2011 (J. Am. Coll. Cardiol. 2011;57:1002-44), but the level of evidence was stronger for CEA (level A) than for CAS (level B).
Planning is in the works for a possible CREST II trial in asymptomatic patients intended to compare intervention (with either CEA or CAS) vs. medical treatment alone. Many panel members expressed the need for this trial to proceed.
Twelve members of the panel reported that they did not have any relevant financial relationships. Dr. Spence reported receiving speaking fees from several pharmaceutical companies, Dr. Curtis reported that he owns stock in Medtronic and receives grant support from Boston Scientific, industry representative Dr. Peter Juhn works for Medco Health Solutions, and committee chair Dr. Clifford Goodman is the senior vice president at the Lewin Group, which has ties to both government and industry.
FROM A MEETING OF THE MEDICARE EVIDENCE DEVELOPMENT AND COVERAGE ADVISORY COMMITTEE
Watch for Counterfeit Cancer Drug Avastin
The makers of bevacizumab, the cancer drug marketed as Avastin, announced Feb. 14 that they have learned that a counterfeit product has been distributed in the United States.
"The counterfeit product is not safe or effective and should not be used. Chemical analyses of the counterfeit vials tested to date have confirmed the product does not contain the active ingredients for Avastin," said makers F. Hoffmann-La Roche and Genentech in a press release.
The companies are working with the Food and Drug Administration and law enforcement to determine the source of the counterfeit drug and prevent its further distribution.
It is believed that some product in the United States labeled as Avastin 400 mg/16 mL with the following lot numbers on either the vials or packaging may be counterfeit: B86017, B6011, and B6010.
Importantly, the counterfeit product does not look like authentic Avastin.
Authentic Avastin’s cartons and vials (approved for use in the United States) have "Genentech" or "Genentech, a member of the Roche Group" printed on the labels. In addition, the lot number on the carton and vial should be six digits with no letters; the expiry date is formatted as a three-letter month and four-digit year (e.g., JUL 2014); the date of manufacture is not printed on the carton or vial; and all the text on the vial labels, cartons, and package inserts is English.
Health care providers who suspect that products in their possession may be counterfeit should immediately contact the FDA’s Office of Criminal Investigations (OCI) at 1-800-551-3989 or Genentech’s Product Quality Assurance Department at 1-800-334-0290.
If a patient is experiencing any side effects that a health care provider thinks may be related to Avastin or that are different from those commonly associated with Avastin, the health care provider should immediately call the FDA’s MedWatch Program (1-800-FDA-1088) or Genentech’s Drug Safety Department at 1-888-835-2555.
Avastin is approved in the United States for the first- and second-line treatment of metastatic colorectal cancer in combination with intravenous 5-fluorouracil-based chemotherapy; first-line treatment of unresectable, locally advanced, recurrent or metastatic, nonsquamous, non–small cell lung cancer in combination with carboplatin and paclitaxel; treatment of metastatic renal cell carcinoma in combination with interferon-alfa; and treatment of glioblastoma, as a single agent for adult patients with progressive disease following prior therapy.
The makers of bevacizumab, the cancer drug marketed as Avastin, announced Feb. 14 that they have learned that a counterfeit product has been distributed in the United States.
"The counterfeit product is not safe or effective and should not be used. Chemical analyses of the counterfeit vials tested to date have confirmed the product does not contain the active ingredients for Avastin," said makers F. Hoffmann-La Roche and Genentech in a press release.
The companies are working with the Food and Drug Administration and law enforcement to determine the source of the counterfeit drug and prevent its further distribution.
It is believed that some product in the United States labeled as Avastin 400 mg/16 mL with the following lot numbers on either the vials or packaging may be counterfeit: B86017, B6011, and B6010.
Importantly, the counterfeit product does not look like authentic Avastin.
Authentic Avastin’s cartons and vials (approved for use in the United States) have "Genentech" or "Genentech, a member of the Roche Group" printed on the labels. In addition, the lot number on the carton and vial should be six digits with no letters; the expiry date is formatted as a three-letter month and four-digit year (e.g., JUL 2014); the date of manufacture is not printed on the carton or vial; and all the text on the vial labels, cartons, and package inserts is English.
Health care providers who suspect that products in their possession may be counterfeit should immediately contact the FDA’s Office of Criminal Investigations (OCI) at 1-800-551-3989 or Genentech’s Product Quality Assurance Department at 1-800-334-0290.
If a patient is experiencing any side effects that a health care provider thinks may be related to Avastin or that are different from those commonly associated with Avastin, the health care provider should immediately call the FDA’s MedWatch Program (1-800-FDA-1088) or Genentech’s Drug Safety Department at 1-888-835-2555.
Avastin is approved in the United States for the first- and second-line treatment of metastatic colorectal cancer in combination with intravenous 5-fluorouracil-based chemotherapy; first-line treatment of unresectable, locally advanced, recurrent or metastatic, nonsquamous, non–small cell lung cancer in combination with carboplatin and paclitaxel; treatment of metastatic renal cell carcinoma in combination with interferon-alfa; and treatment of glioblastoma, as a single agent for adult patients with progressive disease following prior therapy.
The makers of bevacizumab, the cancer drug marketed as Avastin, announced Feb. 14 that they have learned that a counterfeit product has been distributed in the United States.
"The counterfeit product is not safe or effective and should not be used. Chemical analyses of the counterfeit vials tested to date have confirmed the product does not contain the active ingredients for Avastin," said makers F. Hoffmann-La Roche and Genentech in a press release.
The companies are working with the Food and Drug Administration and law enforcement to determine the source of the counterfeit drug and prevent its further distribution.
It is believed that some product in the United States labeled as Avastin 400 mg/16 mL with the following lot numbers on either the vials or packaging may be counterfeit: B86017, B6011, and B6010.
Importantly, the counterfeit product does not look like authentic Avastin.
Authentic Avastin’s cartons and vials (approved for use in the United States) have "Genentech" or "Genentech, a member of the Roche Group" printed on the labels. In addition, the lot number on the carton and vial should be six digits with no letters; the expiry date is formatted as a three-letter month and four-digit year (e.g., JUL 2014); the date of manufacture is not printed on the carton or vial; and all the text on the vial labels, cartons, and package inserts is English.
Health care providers who suspect that products in their possession may be counterfeit should immediately contact the FDA’s Office of Criminal Investigations (OCI) at 1-800-551-3989 or Genentech’s Product Quality Assurance Department at 1-800-334-0290.
If a patient is experiencing any side effects that a health care provider thinks may be related to Avastin or that are different from those commonly associated with Avastin, the health care provider should immediately call the FDA’s MedWatch Program (1-800-FDA-1088) or Genentech’s Drug Safety Department at 1-888-835-2555.
Avastin is approved in the United States for the first- and second-line treatment of metastatic colorectal cancer in combination with intravenous 5-fluorouracil-based chemotherapy; first-line treatment of unresectable, locally advanced, recurrent or metastatic, nonsquamous, non–small cell lung cancer in combination with carboplatin and paclitaxel; treatment of metastatic renal cell carcinoma in combination with interferon-alfa; and treatment of glioblastoma, as a single agent for adult patients with progressive disease following prior therapy.
Race and Sex Skew Congenital Heart Surgery Outcomes
FT. LAUDERDALE, FLA. – Sex and race appear to play a role in outcomes following congenital heart surgery in children and adolescents, according to a new analysis of data from almost 21,000 patients.
Black patients had significantly greater rates of mortality and complications and a significantly longer length of postoperative stay than other races, while female patients had a significantly shorter length of stay than males, Dr. Daniel J. DiBardino reported at the annual meeting of the Society of Thoracic Surgeons.
"The analysis of demographic and clinical data from nearly 21,000 patients in the congenital heart surgery database revealed important associations between gender, race, and outcome," said Dr. DiBardino, who is a cardiac surgeon at the Blair E. Batson Children’s Hospital in Jackson, Miss. Dr. DiBardino’s study was chosen as a 2011 Richard E. Clark Paper by the Society of Thoracic Surgeons.
The researchers used data from the Society of Thoracic Surgeons Congenital Heart Surgery Database (STS-CHSD). Patients were included in the analysis if they were less than 18 years of age and had undergone cardiac surgery between 2007 and 2009. Exclusion criteria included centers with more than 15% of data missing for key variables and centers with very small samples (fewer than five cases).
Data collection included demographics (age, sex, weight, and race) and preoperative data (noncardiac/genetic abnormalities and STS-defined risk factors). Race was classified as white, black, Hispanic, and other. Operations were classified by STAT Mortality category, which is "a complexity stratification tool based on empiric data from 80,000 cases in STS and EACTS (European Association for Cardio-Thoracic Surgery) databases," said Dr. DiBardino.
The researchers looked at hospital mortality, postoperative length of stay, and complications. Multivariable analyses included dichotomous variables (mortality, complications) and a continuous variable (postoperative length of stay). Models were adjusted for age, weight, noncardiac/genetic abnormalities, any other STS preoperative risk factor, and STAT Mortality category.
In all, 20,399 patients were included from 49 centers. Of these, 54% were male. In terms of race, 55% were white, 17% were black, 16% were Hispanic, and 12% were other.
Based on unadjusted outcomes, there were no differences between the sexes for in-hospital mortality or complications. However, females had significantly shorter postoperative stays. In terms of race, white patients had significantly lower mortality, shorter length of stay, and fewer complications than any of the other racial groups.
In the adjusted multivariate analysis, there was no difference for mortality between the sexes. However, black patients had a significantly greater mortality risk with an odds ratio of 1.67.
Females did have a significantly shorter mean length of stay – 0.8 fewer days. In terms of race, black patients had a significantly longer mean length of stay by 2.4 hospital days, compared with white patients. Hispanic patients also had a significantly longer mean length of stay by almost 1 hospital day.
There was no difference between the sexes in terms of the occurrence of complications. In terms of race, "black patients experienced significantly more complications than other races with an odds ratio of 1.15," said Dr. DiBardino.
The study is unique with the respect to the use of multivariable models. The researchers measured the association of sex and race with outcomes within each center and then combined the results, in order to mitigate the potential center effects. "Our results cannot be explained by the possibility that patients of certain races might be disproportionately treated at centers with poorer outcomes in general."
The evaluation of complex relationships between clinical variables and socioeconomic and other factors affecting health care remains a significant challenge. Since some pertinent socioeconomic data are not collected in the STS-CHSD, an analysis of a linked data set, which capitalizes on the strengths of both the CHSD and those of an administrative claims data set may be the next logical step, said Dr. DiBardino.
Dr. DiBardino and his coinvestigators reported that they have no relevant disclosures.
FT. LAUDERDALE, FLA. – Sex and race appear to play a role in outcomes following congenital heart surgery in children and adolescents, according to a new analysis of data from almost 21,000 patients.
Black patients had significantly greater rates of mortality and complications and a significantly longer length of postoperative stay than other races, while female patients had a significantly shorter length of stay than males, Dr. Daniel J. DiBardino reported at the annual meeting of the Society of Thoracic Surgeons.
"The analysis of demographic and clinical data from nearly 21,000 patients in the congenital heart surgery database revealed important associations between gender, race, and outcome," said Dr. DiBardino, who is a cardiac surgeon at the Blair E. Batson Children’s Hospital in Jackson, Miss. Dr. DiBardino’s study was chosen as a 2011 Richard E. Clark Paper by the Society of Thoracic Surgeons.
The researchers used data from the Society of Thoracic Surgeons Congenital Heart Surgery Database (STS-CHSD). Patients were included in the analysis if they were less than 18 years of age and had undergone cardiac surgery between 2007 and 2009. Exclusion criteria included centers with more than 15% of data missing for key variables and centers with very small samples (fewer than five cases).
Data collection included demographics (age, sex, weight, and race) and preoperative data (noncardiac/genetic abnormalities and STS-defined risk factors). Race was classified as white, black, Hispanic, and other. Operations were classified by STAT Mortality category, which is "a complexity stratification tool based on empiric data from 80,000 cases in STS and EACTS (European Association for Cardio-Thoracic Surgery) databases," said Dr. DiBardino.
The researchers looked at hospital mortality, postoperative length of stay, and complications. Multivariable analyses included dichotomous variables (mortality, complications) and a continuous variable (postoperative length of stay). Models were adjusted for age, weight, noncardiac/genetic abnormalities, any other STS preoperative risk factor, and STAT Mortality category.
In all, 20,399 patients were included from 49 centers. Of these, 54% were male. In terms of race, 55% were white, 17% were black, 16% were Hispanic, and 12% were other.
Based on unadjusted outcomes, there were no differences between the sexes for in-hospital mortality or complications. However, females had significantly shorter postoperative stays. In terms of race, white patients had significantly lower mortality, shorter length of stay, and fewer complications than any of the other racial groups.
In the adjusted multivariate analysis, there was no difference for mortality between the sexes. However, black patients had a significantly greater mortality risk with an odds ratio of 1.67.
Females did have a significantly shorter mean length of stay – 0.8 fewer days. In terms of race, black patients had a significantly longer mean length of stay by 2.4 hospital days, compared with white patients. Hispanic patients also had a significantly longer mean length of stay by almost 1 hospital day.
There was no difference between the sexes in terms of the occurrence of complications. In terms of race, "black patients experienced significantly more complications than other races with an odds ratio of 1.15," said Dr. DiBardino.
The study is unique with the respect to the use of multivariable models. The researchers measured the association of sex and race with outcomes within each center and then combined the results, in order to mitigate the potential center effects. "Our results cannot be explained by the possibility that patients of certain races might be disproportionately treated at centers with poorer outcomes in general."
The evaluation of complex relationships between clinical variables and socioeconomic and other factors affecting health care remains a significant challenge. Since some pertinent socioeconomic data are not collected in the STS-CHSD, an analysis of a linked data set, which capitalizes on the strengths of both the CHSD and those of an administrative claims data set may be the next logical step, said Dr. DiBardino.
Dr. DiBardino and his coinvestigators reported that they have no relevant disclosures.
FT. LAUDERDALE, FLA. – Sex and race appear to play a role in outcomes following congenital heart surgery in children and adolescents, according to a new analysis of data from almost 21,000 patients.
Black patients had significantly greater rates of mortality and complications and a significantly longer length of postoperative stay than other races, while female patients had a significantly shorter length of stay than males, Dr. Daniel J. DiBardino reported at the annual meeting of the Society of Thoracic Surgeons.
"The analysis of demographic and clinical data from nearly 21,000 patients in the congenital heart surgery database revealed important associations between gender, race, and outcome," said Dr. DiBardino, who is a cardiac surgeon at the Blair E. Batson Children’s Hospital in Jackson, Miss. Dr. DiBardino’s study was chosen as a 2011 Richard E. Clark Paper by the Society of Thoracic Surgeons.
The researchers used data from the Society of Thoracic Surgeons Congenital Heart Surgery Database (STS-CHSD). Patients were included in the analysis if they were less than 18 years of age and had undergone cardiac surgery between 2007 and 2009. Exclusion criteria included centers with more than 15% of data missing for key variables and centers with very small samples (fewer than five cases).
Data collection included demographics (age, sex, weight, and race) and preoperative data (noncardiac/genetic abnormalities and STS-defined risk factors). Race was classified as white, black, Hispanic, and other. Operations were classified by STAT Mortality category, which is "a complexity stratification tool based on empiric data from 80,000 cases in STS and EACTS (European Association for Cardio-Thoracic Surgery) databases," said Dr. DiBardino.
The researchers looked at hospital mortality, postoperative length of stay, and complications. Multivariable analyses included dichotomous variables (mortality, complications) and a continuous variable (postoperative length of stay). Models were adjusted for age, weight, noncardiac/genetic abnormalities, any other STS preoperative risk factor, and STAT Mortality category.
In all, 20,399 patients were included from 49 centers. Of these, 54% were male. In terms of race, 55% were white, 17% were black, 16% were Hispanic, and 12% were other.
Based on unadjusted outcomes, there were no differences between the sexes for in-hospital mortality or complications. However, females had significantly shorter postoperative stays. In terms of race, white patients had significantly lower mortality, shorter length of stay, and fewer complications than any of the other racial groups.
In the adjusted multivariate analysis, there was no difference for mortality between the sexes. However, black patients had a significantly greater mortality risk with an odds ratio of 1.67.
Females did have a significantly shorter mean length of stay – 0.8 fewer days. In terms of race, black patients had a significantly longer mean length of stay by 2.4 hospital days, compared with white patients. Hispanic patients also had a significantly longer mean length of stay by almost 1 hospital day.
There was no difference between the sexes in terms of the occurrence of complications. In terms of race, "black patients experienced significantly more complications than other races with an odds ratio of 1.15," said Dr. DiBardino.
The study is unique with the respect to the use of multivariable models. The researchers measured the association of sex and race with outcomes within each center and then combined the results, in order to mitigate the potential center effects. "Our results cannot be explained by the possibility that patients of certain races might be disproportionately treated at centers with poorer outcomes in general."
The evaluation of complex relationships between clinical variables and socioeconomic and other factors affecting health care remains a significant challenge. Since some pertinent socioeconomic data are not collected in the STS-CHSD, an analysis of a linked data set, which capitalizes on the strengths of both the CHSD and those of an administrative claims data set may be the next logical step, said Dr. DiBardino.
Dr. DiBardino and his coinvestigators reported that they have no relevant disclosures.
FROM THE ANNUAL MEETING OF THE SOCIETY OF THORACIC SURGEONS
Major Finding: In adjusted multivariate analyses, black patients had a significantly greater mortality risk (67%), a significantly longer mean length of stay by 2.4 hospital days, and a significantly greater risk of complications (15%). Female patients had a significantly shorter mean length of stay – 0.8 fewer days.
Data Source: The retrospective review included 20,399 patients younger than 18 years from 49 centers, collected in the Society of Thoracic Surgeons Congenital Heart Surgery Database.
Disclosures: Dr. DiBardino and his coinvestigators reported that they have no relevant disclosures.
Transaortic Approach Works for TAVI With Sapien
FT. LAUDERDALE, FLA. – A transaortic approach may be a useful alternative to the transapical or transfemoral approach when performing transcatheter aortic valve implantation (TAVI), according to a retrospective review of European cases presented at the annual meeting of the Society of Thoracic Surgeons.
Procedural success was achieved in 157 of 158 cases in which surgeons used the transaortic approach to place the Sapien valve with the Ascendra delivery system (both developed by Edwards Lifesciences), Dr. Vinayak Bapat reported.
He provided the results of a retrospective analysis of European multicenter experience with transaortic TAVI. So far, this approach has been encouraging, despite the high-risk population. Perhaps more importantly, no strokes occurred with the transaortic approach, while strokes are a complication associated with the transapical approach.
To date, more than 250 procedures have been performed in Europe. All patients were unsuitable for the transfemoral approach. Dr. Bapat analyzed 158 cases from 10 centers.
More patients were female (61%); mean age was 80 years. Roughly a quarter of patients had diabetes and almost 40% had peripheral vascular disease. The mean valve area was 0.60 cm2 and peak gradient was 67 mm Hg.
Thirteen percent of patients had previously undergone coronary artery bypass graft (CABG) surgery and 37% had chronic obstructive pulmonary disease. Twelve percent of patients had an ejection fraction (EF) less than 30%, 23% had an EF of 30%-50%, and almost two-thirds had an EF of more than 50%. Most patients (87%) had a mini-sternotomy performed, 9% had a mini-thoracotomy, and 4% had a sternotomy (these patients also had off-pump CABG and then had a transaortic TAVI).
Dr. Bapat assessed the cases using procedural end points from the Valve Academic Research Consortium (VARC).
Procedural success was achieved in all but one case, in which the left interior mammary artery was damaged. The surgical team proceeded with a femoral/femoral bypass but the patient died on the table.
In terms of device success, the device was placed in the correct position in all other patients (157). In terms of the prosthetic valve performance, a mean postoperative peak gradient of 11 mm Hg was achieved. Lastly, only one valve was deployed in each of these patients. In terms of device size, 47% received a 23-mm device, another 47% received a 26-mm device, and 6% received a 29-mm device.
All-cause mortality at 30 days was 7%. One patient died due to bleeding from a left interior mammary artery tear, six died of renal insufficiency, one died of aortic dissection, and three died of respiratory failure.
Importantly, no major strokes or periprocedural MIs occurred. Two patients had bleeding that required reoperation; eight patients had renal failure that required dialysis, and four patients had perivalvular leaks of at least grade 2.
The transaortic approach mimics the transfemoral approach but goes through the aorta, avoiding the aortic arch. Embolic protection can be used. In addition, aortic purse-string sutures are safer and have no effect on the left ventricle, he said.
This approach can be performed through either a mini-sternotomy or a mini-thoracotomy. The approach depends on the relationship of the sternum and aorta. A mini-sternotomy can go through either the second or third space. "It is used for aortas, which are midline, in obese patients, and in obese patients with poor lung function," said Dr. Bapat. A mini-thoracotomy is performed through the second space. "I think that it’s important not to excise the second costal cartilage because you want to keep this minimally invasive and less painful." This approach is preferred if the aorta is horizontal, on the right side, or if it’s a redo with patent graft.
In contrast, using the transapical approach, acute and chronic left ventricular complications can occur. There is also an effect on respiratory function because the route is through the left chest. A large incision is commonly needed for obese patients.
"I think that transaortic gives you better control, it’s familiar to surgeons, [and] valve crossing is no longer a challenge because you’re working as a heart team...cardiopulmonary bypass – whether on an elective or emergency basis is very easy because the aorta is right in front of you. Finally, conversion – if there is a complication – to full sternotomy is easy," said Dr. Bapat, who is a consultant cardiac surgeon at St. Thomas Hospital in London.
Dr. Bapat reported that he is a consultant for Edwards Lifesciences and Medtronic Inc. All of his coauthors reported significant relationships with Edwards, either as proctors or consultants.
FT. LAUDERDALE, FLA. – A transaortic approach may be a useful alternative to the transapical or transfemoral approach when performing transcatheter aortic valve implantation (TAVI), according to a retrospective review of European cases presented at the annual meeting of the Society of Thoracic Surgeons.
Procedural success was achieved in 157 of 158 cases in which surgeons used the transaortic approach to place the Sapien valve with the Ascendra delivery system (both developed by Edwards Lifesciences), Dr. Vinayak Bapat reported.
He provided the results of a retrospective analysis of European multicenter experience with transaortic TAVI. So far, this approach has been encouraging, despite the high-risk population. Perhaps more importantly, no strokes occurred with the transaortic approach, while strokes are a complication associated with the transapical approach.
To date, more than 250 procedures have been performed in Europe. All patients were unsuitable for the transfemoral approach. Dr. Bapat analyzed 158 cases from 10 centers.
More patients were female (61%); mean age was 80 years. Roughly a quarter of patients had diabetes and almost 40% had peripheral vascular disease. The mean valve area was 0.60 cm2 and peak gradient was 67 mm Hg.
Thirteen percent of patients had previously undergone coronary artery bypass graft (CABG) surgery and 37% had chronic obstructive pulmonary disease. Twelve percent of patients had an ejection fraction (EF) less than 30%, 23% had an EF of 30%-50%, and almost two-thirds had an EF of more than 50%. Most patients (87%) had a mini-sternotomy performed, 9% had a mini-thoracotomy, and 4% had a sternotomy (these patients also had off-pump CABG and then had a transaortic TAVI).
Dr. Bapat assessed the cases using procedural end points from the Valve Academic Research Consortium (VARC).
Procedural success was achieved in all but one case, in which the left interior mammary artery was damaged. The surgical team proceeded with a femoral/femoral bypass but the patient died on the table.
In terms of device success, the device was placed in the correct position in all other patients (157). In terms of the prosthetic valve performance, a mean postoperative peak gradient of 11 mm Hg was achieved. Lastly, only one valve was deployed in each of these patients. In terms of device size, 47% received a 23-mm device, another 47% received a 26-mm device, and 6% received a 29-mm device.
All-cause mortality at 30 days was 7%. One patient died due to bleeding from a left interior mammary artery tear, six died of renal insufficiency, one died of aortic dissection, and three died of respiratory failure.
Importantly, no major strokes or periprocedural MIs occurred. Two patients had bleeding that required reoperation; eight patients had renal failure that required dialysis, and four patients had perivalvular leaks of at least grade 2.
The transaortic approach mimics the transfemoral approach but goes through the aorta, avoiding the aortic arch. Embolic protection can be used. In addition, aortic purse-string sutures are safer and have no effect on the left ventricle, he said.
This approach can be performed through either a mini-sternotomy or a mini-thoracotomy. The approach depends on the relationship of the sternum and aorta. A mini-sternotomy can go through either the second or third space. "It is used for aortas, which are midline, in obese patients, and in obese patients with poor lung function," said Dr. Bapat. A mini-thoracotomy is performed through the second space. "I think that it’s important not to excise the second costal cartilage because you want to keep this minimally invasive and less painful." This approach is preferred if the aorta is horizontal, on the right side, or if it’s a redo with patent graft.
In contrast, using the transapical approach, acute and chronic left ventricular complications can occur. There is also an effect on respiratory function because the route is through the left chest. A large incision is commonly needed for obese patients.
"I think that transaortic gives you better control, it’s familiar to surgeons, [and] valve crossing is no longer a challenge because you’re working as a heart team...cardiopulmonary bypass – whether on an elective or emergency basis is very easy because the aorta is right in front of you. Finally, conversion – if there is a complication – to full sternotomy is easy," said Dr. Bapat, who is a consultant cardiac surgeon at St. Thomas Hospital in London.
Dr. Bapat reported that he is a consultant for Edwards Lifesciences and Medtronic Inc. All of his coauthors reported significant relationships with Edwards, either as proctors or consultants.
FT. LAUDERDALE, FLA. – A transaortic approach may be a useful alternative to the transapical or transfemoral approach when performing transcatheter aortic valve implantation (TAVI), according to a retrospective review of European cases presented at the annual meeting of the Society of Thoracic Surgeons.
Procedural success was achieved in 157 of 158 cases in which surgeons used the transaortic approach to place the Sapien valve with the Ascendra delivery system (both developed by Edwards Lifesciences), Dr. Vinayak Bapat reported.
He provided the results of a retrospective analysis of European multicenter experience with transaortic TAVI. So far, this approach has been encouraging, despite the high-risk population. Perhaps more importantly, no strokes occurred with the transaortic approach, while strokes are a complication associated with the transapical approach.
To date, more than 250 procedures have been performed in Europe. All patients were unsuitable for the transfemoral approach. Dr. Bapat analyzed 158 cases from 10 centers.
More patients were female (61%); mean age was 80 years. Roughly a quarter of patients had diabetes and almost 40% had peripheral vascular disease. The mean valve area was 0.60 cm2 and peak gradient was 67 mm Hg.
Thirteen percent of patients had previously undergone coronary artery bypass graft (CABG) surgery and 37% had chronic obstructive pulmonary disease. Twelve percent of patients had an ejection fraction (EF) less than 30%, 23% had an EF of 30%-50%, and almost two-thirds had an EF of more than 50%. Most patients (87%) had a mini-sternotomy performed, 9% had a mini-thoracotomy, and 4% had a sternotomy (these patients also had off-pump CABG and then had a transaortic TAVI).
Dr. Bapat assessed the cases using procedural end points from the Valve Academic Research Consortium (VARC).
Procedural success was achieved in all but one case, in which the left interior mammary artery was damaged. The surgical team proceeded with a femoral/femoral bypass but the patient died on the table.
In terms of device success, the device was placed in the correct position in all other patients (157). In terms of the prosthetic valve performance, a mean postoperative peak gradient of 11 mm Hg was achieved. Lastly, only one valve was deployed in each of these patients. In terms of device size, 47% received a 23-mm device, another 47% received a 26-mm device, and 6% received a 29-mm device.
All-cause mortality at 30 days was 7%. One patient died due to bleeding from a left interior mammary artery tear, six died of renal insufficiency, one died of aortic dissection, and three died of respiratory failure.
Importantly, no major strokes or periprocedural MIs occurred. Two patients had bleeding that required reoperation; eight patients had renal failure that required dialysis, and four patients had perivalvular leaks of at least grade 2.
The transaortic approach mimics the transfemoral approach but goes through the aorta, avoiding the aortic arch. Embolic protection can be used. In addition, aortic purse-string sutures are safer and have no effect on the left ventricle, he said.
This approach can be performed through either a mini-sternotomy or a mini-thoracotomy. The approach depends on the relationship of the sternum and aorta. A mini-sternotomy can go through either the second or third space. "It is used for aortas, which are midline, in obese patients, and in obese patients with poor lung function," said Dr. Bapat. A mini-thoracotomy is performed through the second space. "I think that it’s important not to excise the second costal cartilage because you want to keep this minimally invasive and less painful." This approach is preferred if the aorta is horizontal, on the right side, or if it’s a redo with patent graft.
In contrast, using the transapical approach, acute and chronic left ventricular complications can occur. There is also an effect on respiratory function because the route is through the left chest. A large incision is commonly needed for obese patients.
"I think that transaortic gives you better control, it’s familiar to surgeons, [and] valve crossing is no longer a challenge because you’re working as a heart team...cardiopulmonary bypass – whether on an elective or emergency basis is very easy because the aorta is right in front of you. Finally, conversion – if there is a complication – to full sternotomy is easy," said Dr. Bapat, who is a consultant cardiac surgeon at St. Thomas Hospital in London.
Dr. Bapat reported that he is a consultant for Edwards Lifesciences and Medtronic Inc. All of his coauthors reported significant relationships with Edwards, either as proctors or consultants.
FROM THE ANNUAL MEETING OF THE SOCIETY OF THORACIC SURGEONS
Major Finding: Procedural success was achieved in all but one case, in which surgeons used the transaortic approach to place the Sapien valve. All-cause mortality at 30 days was 7% and no strokes occurred.
Data Source: The findings come from a retrospective analysis of 158 European cases using transaortic transcathether aortic valve implantation.
Disclosures: Dr. Bapat reported that he is a consultant for Edwards Lifesciences and Medtronic Inc. All of his coauthors reported significant relationships with Edwards, either as proctors or consultants.
CABG Beats Stents for Long-Term Outcomes in High-Risk Patients
FT. LAUDERDALE, FLA. – Coronary artery bypass graft surgery shows a clear long-term survival advantage in certain high-risk groups over percutaneous coronary intervention, based on results of the largest study of real-world data so far.
The survival advantage for a composite high-risk group – including patients aged 75 years and older, patients with diabetes, those with ejection fractions (EF) less than 50%, and those with a glomerular filtration rate (GFR) less than 60 mL/min per 1.73 m2 – was 28% at 4 years, Dr. Fred H. Edwards reported at the annual meeting of the Society of Thoracic Surgeons.
The findings come from the ASCERT (The American College of Cardiology Foundation – The Society of Thoracic Surgeons Collaboration on the Comparative Effectiveness of Revascularization Strategies) study, in which researchers compared catheter- and surgery-based procedures using the existing ACC and STS databases, as well as the Centers for Medicare and Medicaid Services 100% denominator file data. The study was designed to identify specific patient characteristics that favor one mode of treatment over the other. The study is also supported by the National Heart, Lung, and Blood Institute.
The ACC and the STS both have large registries containing detailed clinical information on millions of procedures. However, the information in these databases extends to only 1 month after the procedure. The researchers linked this short-term clinical information with the administrative data registry from the CMS to provide long-term mortality, rehospitalization, and resource utilization outcomes. The 3- to 5-year outcomes after coronary artery bypass graft (CABG) surgery are being compared with those after percutaneous coronary intervention (PCI) – primarily using drug-eluting coronary stents, from the STS and ACC databases, respectively. In addition to survival, researchers are assessing the need for additional procedures and hospitalizations, new cardiac disease conditions, and the medications being taken at various points in time after the coronary artery procedure.
Patients in this CMS population were aged at least 65 years with two- to three-vessel disease. Patients with either single-vessel disease or left-main disease were excluded. Emergent procedures were also excluded.
"So what’s different about this [study]? Well, the N [number] is huge. We’ve got a population that is actually 10 times greater than the sum total of all patients ever having been enrolled in randomized [revascularization] trials," said Dr. Edwards, who is a professor of surgery and medical director of cardiothoracic surgery at the University of Florida/Shands Jacksonville, as well as chairman of the STS National Database. In addition to the size of this observational study, the focus is on real-world experience with a true national scope.
For the head-to-head comparison, data from both the STS and ACC databases were linked to data from the CMS. A propensity score – the probability of having CABG – was calculated for each patient, and clinically important subgroups were identified before the files were linked. The propensity scores and inverse weighting were used to calculate adjusted survival curves. "Then we compared the survival for coronary bypass and percutaneous intervention for groups having very similar characteristics," said Dr. Edwards.
High- and Low-Risk Groups Identified
This analysis included a total of 189,793 patients, of which 103,549 received PCI. Dr. Edwards presented the survival results for high-risk subgroups; the overall results will be presented at the ACC’s Annual Scientific Session in March, he said.
High-risk subgroups include patients who were aged 75 years and older, had diabetes, had EFs less than 50%, and had a GFR less than 60 mL/min per 1.73 m2.
For those aged 75 years and older, the mortality risk ratio at 4 years was 0.78 favoring surgery. Correspondingly, the survival advantage in this group for surgery was 22%. For patients with three-vessel disease, the survival advantage at 4 years was 25%. Patients with insulin-dependent diabetes had a 28% survival advantage at 4 years with CABG, compared with PCI. For patients with EFs less than 50%, the survival advantage with surgery was 30% at 4 years.
However, there appears to be a survival advantage with PCI in these groups at up to 1 year of follow-up. "We should keep in mind that in many of these subgroups, the survival with percutaneous intervention is better than surgery in that first 6-10 months after the procedure. The reason for that, of course, is the procedural mortality," Dr. Edwards said.
They also defined a low-risk population (about 20% of the total population). They looked at survival advantages at years 1-4. "I think this is important because it illustrates that surgery really does start to declare its advantage in year 1 to year 2. Then it looks like it begins to plateau off a little bit," he said. "Still, at 4 years for both high-risk and low-risk patients, you’ve got more than a 25% survival advantage for surgery."
He noted that "this is a Medicare population that I’ve presented, so we would be on shaky ground if we tried to extrapolate these results to a global population."
He concluded by saying that "the results should improve the quality of care for patients with coronary disease, and it should clarify the indications for intervention in the subgroups that we’ve presented here. Ideally, it would minimize overuse and underuse, so that we could really provide optimal care for patients with coronary disease."
Prediction Models Gleaned From Data
During the same presentation, Dr. David M. Shahian reported on long-term prediction models of death and nonfatal events for both CABG and PCI. "Longer term outcomes are clearly going to be necessary if we’re really going to determine the true comparative effectiveness of these various strategies," he said.
In this analysis, the researchers looked at all isolated CABG patients at STS-participating hospitals who were discharged between the beginning of 2002 and the end of 2007. STS procedural records were linked to CMS claims and denominator files.
The final study cohort included 348,341 CABG patients at 917 sites. Follow-up was carried through 2008 (median follow-up, 4 years). Long-term variables were based on those from short-term CABG models and clinical experience. Separate hazard ratios were estimated for each of these variables for four time intervals: 0-30 days, 31-80 days, 181-730 days, and more than 2 years. The researchers focused on main effects for this model.
Kaplan-Meier estimated mortality rates for CABG were 3% at 30 days, 6% at 180 days, 8% at 1 year, 11% at 2 years, and 23% at 3 years. Predicted mortality rates were superimposable with observed mortality rates, said Dr. Shahian, who is a cardiothoracic surgeon at Harvard Medical School in Boston. Dr. Shahian is also the chair of the STS Adult Cardiac Surgery Database and the STS Quality Measurement Task Force.
"We did observe the obesity paradox here. It’s the frail, almost cachectic individuals, who do the worst, while the more obese individuals tend to do better over time," he said. In addition, EF appears to be protective, with the greater the EF, the lower the long-term mortality.
However, smoking increases risk over time, as does diabetes. Immunosuppresive therapy has a stable and substantial negative effect over time.
The impact of some predictors changed over time. For example, patients with an acute MI have an increased initial mortality risk, which becomes generally insignificant over 1-2 years. In addition, early reoperation, shock, and emergency status have high up-front risks that decrease over time. However, preoperative atrial fibrillation progressively increases risk over time, he said.
"Among hospital survivors, higher ejection fraction and higher [body mass index] are protective at all time periods. A past history of stroke ... [and] chronic lung-disease immunosuppression have a persistent and negative impact on survival. Smoking, diabetes, dialysis-dependent renal failure – their negative impact increases over time. ... Some early important risk factors, like shock, emergency status, and reoperation are not predictors of late outcomes."
Dr. Edwards, who is the principal investigator of the ASCERT trial, reported that he is a consultant and/or on the advisory board for Humana. Dr. Shahian reported that he has no relevant financial relationships. However, several of their collaborators reported financial ties to several pharmaceutical or device manufacturers, including Boston Scientific and Medtronic Inc.
FT. LAUDERDALE, FLA. – Coronary artery bypass graft surgery shows a clear long-term survival advantage in certain high-risk groups over percutaneous coronary intervention, based on results of the largest study of real-world data so far.
The survival advantage for a composite high-risk group – including patients aged 75 years and older, patients with diabetes, those with ejection fractions (EF) less than 50%, and those with a glomerular filtration rate (GFR) less than 60 mL/min per 1.73 m2 – was 28% at 4 years, Dr. Fred H. Edwards reported at the annual meeting of the Society of Thoracic Surgeons.
The findings come from the ASCERT (The American College of Cardiology Foundation – The Society of Thoracic Surgeons Collaboration on the Comparative Effectiveness of Revascularization Strategies) study, in which researchers compared catheter- and surgery-based procedures using the existing ACC and STS databases, as well as the Centers for Medicare and Medicaid Services 100% denominator file data. The study was designed to identify specific patient characteristics that favor one mode of treatment over the other. The study is also supported by the National Heart, Lung, and Blood Institute.
The ACC and the STS both have large registries containing detailed clinical information on millions of procedures. However, the information in these databases extends to only 1 month after the procedure. The researchers linked this short-term clinical information with the administrative data registry from the CMS to provide long-term mortality, rehospitalization, and resource utilization outcomes. The 3- to 5-year outcomes after coronary artery bypass graft (CABG) surgery are being compared with those after percutaneous coronary intervention (PCI) – primarily using drug-eluting coronary stents, from the STS and ACC databases, respectively. In addition to survival, researchers are assessing the need for additional procedures and hospitalizations, new cardiac disease conditions, and the medications being taken at various points in time after the coronary artery procedure.
Patients in this CMS population were aged at least 65 years with two- to three-vessel disease. Patients with either single-vessel disease or left-main disease were excluded. Emergent procedures were also excluded.
"So what’s different about this [study]? Well, the N [number] is huge. We’ve got a population that is actually 10 times greater than the sum total of all patients ever having been enrolled in randomized [revascularization] trials," said Dr. Edwards, who is a professor of surgery and medical director of cardiothoracic surgery at the University of Florida/Shands Jacksonville, as well as chairman of the STS National Database. In addition to the size of this observational study, the focus is on real-world experience with a true national scope.
For the head-to-head comparison, data from both the STS and ACC databases were linked to data from the CMS. A propensity score – the probability of having CABG – was calculated for each patient, and clinically important subgroups were identified before the files were linked. The propensity scores and inverse weighting were used to calculate adjusted survival curves. "Then we compared the survival for coronary bypass and percutaneous intervention for groups having very similar characteristics," said Dr. Edwards.
High- and Low-Risk Groups Identified
This analysis included a total of 189,793 patients, of which 103,549 received PCI. Dr. Edwards presented the survival results for high-risk subgroups; the overall results will be presented at the ACC’s Annual Scientific Session in March, he said.
High-risk subgroups include patients who were aged 75 years and older, had diabetes, had EFs less than 50%, and had a GFR less than 60 mL/min per 1.73 m2.
For those aged 75 years and older, the mortality risk ratio at 4 years was 0.78 favoring surgery. Correspondingly, the survival advantage in this group for surgery was 22%. For patients with three-vessel disease, the survival advantage at 4 years was 25%. Patients with insulin-dependent diabetes had a 28% survival advantage at 4 years with CABG, compared with PCI. For patients with EFs less than 50%, the survival advantage with surgery was 30% at 4 years.
However, there appears to be a survival advantage with PCI in these groups at up to 1 year of follow-up. "We should keep in mind that in many of these subgroups, the survival with percutaneous intervention is better than surgery in that first 6-10 months after the procedure. The reason for that, of course, is the procedural mortality," Dr. Edwards said.
They also defined a low-risk population (about 20% of the total population). They looked at survival advantages at years 1-4. "I think this is important because it illustrates that surgery really does start to declare its advantage in year 1 to year 2. Then it looks like it begins to plateau off a little bit," he said. "Still, at 4 years for both high-risk and low-risk patients, you’ve got more than a 25% survival advantage for surgery."
He noted that "this is a Medicare population that I’ve presented, so we would be on shaky ground if we tried to extrapolate these results to a global population."
He concluded by saying that "the results should improve the quality of care for patients with coronary disease, and it should clarify the indications for intervention in the subgroups that we’ve presented here. Ideally, it would minimize overuse and underuse, so that we could really provide optimal care for patients with coronary disease."
Prediction Models Gleaned From Data
During the same presentation, Dr. David M. Shahian reported on long-term prediction models of death and nonfatal events for both CABG and PCI. "Longer term outcomes are clearly going to be necessary if we’re really going to determine the true comparative effectiveness of these various strategies," he said.
In this analysis, the researchers looked at all isolated CABG patients at STS-participating hospitals who were discharged between the beginning of 2002 and the end of 2007. STS procedural records were linked to CMS claims and denominator files.
The final study cohort included 348,341 CABG patients at 917 sites. Follow-up was carried through 2008 (median follow-up, 4 years). Long-term variables were based on those from short-term CABG models and clinical experience. Separate hazard ratios were estimated for each of these variables for four time intervals: 0-30 days, 31-80 days, 181-730 days, and more than 2 years. The researchers focused on main effects for this model.
Kaplan-Meier estimated mortality rates for CABG were 3% at 30 days, 6% at 180 days, 8% at 1 year, 11% at 2 years, and 23% at 3 years. Predicted mortality rates were superimposable with observed mortality rates, said Dr. Shahian, who is a cardiothoracic surgeon at Harvard Medical School in Boston. Dr. Shahian is also the chair of the STS Adult Cardiac Surgery Database and the STS Quality Measurement Task Force.
"We did observe the obesity paradox here. It’s the frail, almost cachectic individuals, who do the worst, while the more obese individuals tend to do better over time," he said. In addition, EF appears to be protective, with the greater the EF, the lower the long-term mortality.
However, smoking increases risk over time, as does diabetes. Immunosuppresive therapy has a stable and substantial negative effect over time.
The impact of some predictors changed over time. For example, patients with an acute MI have an increased initial mortality risk, which becomes generally insignificant over 1-2 years. In addition, early reoperation, shock, and emergency status have high up-front risks that decrease over time. However, preoperative atrial fibrillation progressively increases risk over time, he said.
"Among hospital survivors, higher ejection fraction and higher [body mass index] are protective at all time periods. A past history of stroke ... [and] chronic lung-disease immunosuppression have a persistent and negative impact on survival. Smoking, diabetes, dialysis-dependent renal failure – their negative impact increases over time. ... Some early important risk factors, like shock, emergency status, and reoperation are not predictors of late outcomes."
Dr. Edwards, who is the principal investigator of the ASCERT trial, reported that he is a consultant and/or on the advisory board for Humana. Dr. Shahian reported that he has no relevant financial relationships. However, several of their collaborators reported financial ties to several pharmaceutical or device manufacturers, including Boston Scientific and Medtronic Inc.
FT. LAUDERDALE, FLA. – Coronary artery bypass graft surgery shows a clear long-term survival advantage in certain high-risk groups over percutaneous coronary intervention, based on results of the largest study of real-world data so far.
The survival advantage for a composite high-risk group – including patients aged 75 years and older, patients with diabetes, those with ejection fractions (EF) less than 50%, and those with a glomerular filtration rate (GFR) less than 60 mL/min per 1.73 m2 – was 28% at 4 years, Dr. Fred H. Edwards reported at the annual meeting of the Society of Thoracic Surgeons.
The findings come from the ASCERT (The American College of Cardiology Foundation – The Society of Thoracic Surgeons Collaboration on the Comparative Effectiveness of Revascularization Strategies) study, in which researchers compared catheter- and surgery-based procedures using the existing ACC and STS databases, as well as the Centers for Medicare and Medicaid Services 100% denominator file data. The study was designed to identify specific patient characteristics that favor one mode of treatment over the other. The study is also supported by the National Heart, Lung, and Blood Institute.
The ACC and the STS both have large registries containing detailed clinical information on millions of procedures. However, the information in these databases extends to only 1 month after the procedure. The researchers linked this short-term clinical information with the administrative data registry from the CMS to provide long-term mortality, rehospitalization, and resource utilization outcomes. The 3- to 5-year outcomes after coronary artery bypass graft (CABG) surgery are being compared with those after percutaneous coronary intervention (PCI) – primarily using drug-eluting coronary stents, from the STS and ACC databases, respectively. In addition to survival, researchers are assessing the need for additional procedures and hospitalizations, new cardiac disease conditions, and the medications being taken at various points in time after the coronary artery procedure.
Patients in this CMS population were aged at least 65 years with two- to three-vessel disease. Patients with either single-vessel disease or left-main disease were excluded. Emergent procedures were also excluded.
"So what’s different about this [study]? Well, the N [number] is huge. We’ve got a population that is actually 10 times greater than the sum total of all patients ever having been enrolled in randomized [revascularization] trials," said Dr. Edwards, who is a professor of surgery and medical director of cardiothoracic surgery at the University of Florida/Shands Jacksonville, as well as chairman of the STS National Database. In addition to the size of this observational study, the focus is on real-world experience with a true national scope.
For the head-to-head comparison, data from both the STS and ACC databases were linked to data from the CMS. A propensity score – the probability of having CABG – was calculated for each patient, and clinically important subgroups were identified before the files were linked. The propensity scores and inverse weighting were used to calculate adjusted survival curves. "Then we compared the survival for coronary bypass and percutaneous intervention for groups having very similar characteristics," said Dr. Edwards.
High- and Low-Risk Groups Identified
This analysis included a total of 189,793 patients, of which 103,549 received PCI. Dr. Edwards presented the survival results for high-risk subgroups; the overall results will be presented at the ACC’s Annual Scientific Session in March, he said.
High-risk subgroups include patients who were aged 75 years and older, had diabetes, had EFs less than 50%, and had a GFR less than 60 mL/min per 1.73 m2.
For those aged 75 years and older, the mortality risk ratio at 4 years was 0.78 favoring surgery. Correspondingly, the survival advantage in this group for surgery was 22%. For patients with three-vessel disease, the survival advantage at 4 years was 25%. Patients with insulin-dependent diabetes had a 28% survival advantage at 4 years with CABG, compared with PCI. For patients with EFs less than 50%, the survival advantage with surgery was 30% at 4 years.
However, there appears to be a survival advantage with PCI in these groups at up to 1 year of follow-up. "We should keep in mind that in many of these subgroups, the survival with percutaneous intervention is better than surgery in that first 6-10 months after the procedure. The reason for that, of course, is the procedural mortality," Dr. Edwards said.
They also defined a low-risk population (about 20% of the total population). They looked at survival advantages at years 1-4. "I think this is important because it illustrates that surgery really does start to declare its advantage in year 1 to year 2. Then it looks like it begins to plateau off a little bit," he said. "Still, at 4 years for both high-risk and low-risk patients, you’ve got more than a 25% survival advantage for surgery."
He noted that "this is a Medicare population that I’ve presented, so we would be on shaky ground if we tried to extrapolate these results to a global population."
He concluded by saying that "the results should improve the quality of care for patients with coronary disease, and it should clarify the indications for intervention in the subgroups that we’ve presented here. Ideally, it would minimize overuse and underuse, so that we could really provide optimal care for patients with coronary disease."
Prediction Models Gleaned From Data
During the same presentation, Dr. David M. Shahian reported on long-term prediction models of death and nonfatal events for both CABG and PCI. "Longer term outcomes are clearly going to be necessary if we’re really going to determine the true comparative effectiveness of these various strategies," he said.
In this analysis, the researchers looked at all isolated CABG patients at STS-participating hospitals who were discharged between the beginning of 2002 and the end of 2007. STS procedural records were linked to CMS claims and denominator files.
The final study cohort included 348,341 CABG patients at 917 sites. Follow-up was carried through 2008 (median follow-up, 4 years). Long-term variables were based on those from short-term CABG models and clinical experience. Separate hazard ratios were estimated for each of these variables for four time intervals: 0-30 days, 31-80 days, 181-730 days, and more than 2 years. The researchers focused on main effects for this model.
Kaplan-Meier estimated mortality rates for CABG were 3% at 30 days, 6% at 180 days, 8% at 1 year, 11% at 2 years, and 23% at 3 years. Predicted mortality rates were superimposable with observed mortality rates, said Dr. Shahian, who is a cardiothoracic surgeon at Harvard Medical School in Boston. Dr. Shahian is also the chair of the STS Adult Cardiac Surgery Database and the STS Quality Measurement Task Force.
"We did observe the obesity paradox here. It’s the frail, almost cachectic individuals, who do the worst, while the more obese individuals tend to do better over time," he said. In addition, EF appears to be protective, with the greater the EF, the lower the long-term mortality.
However, smoking increases risk over time, as does diabetes. Immunosuppresive therapy has a stable and substantial negative effect over time.
The impact of some predictors changed over time. For example, patients with an acute MI have an increased initial mortality risk, which becomes generally insignificant over 1-2 years. In addition, early reoperation, shock, and emergency status have high up-front risks that decrease over time. However, preoperative atrial fibrillation progressively increases risk over time, he said.
"Among hospital survivors, higher ejection fraction and higher [body mass index] are protective at all time periods. A past history of stroke ... [and] chronic lung-disease immunosuppression have a persistent and negative impact on survival. Smoking, diabetes, dialysis-dependent renal failure – their negative impact increases over time. ... Some early important risk factors, like shock, emergency status, and reoperation are not predictors of late outcomes."
Dr. Edwards, who is the principal investigator of the ASCERT trial, reported that he is a consultant and/or on the advisory board for Humana. Dr. Shahian reported that he has no relevant financial relationships. However, several of their collaborators reported financial ties to several pharmaceutical or device manufacturers, including Boston Scientific and Medtronic Inc.
FROM THE ANNUAL MEETING OF THE SOCIETY OF THORACIC SURGEONS
Major Finding: The survival advantage of CABG over PCI for a composite high-risk group (patients aged 75 years and older, patients with diabetes, those with EFs less than 50%, and those with a GFR less than 60 mL/min per 1.73 m2) was 28% at 4 years.
Data Source: Almost 190,000 patients in the ASCERT study, in which researchers compared catheter- andsurgery-based procedures using the existing ACC and STS databases,as well as the Centers for Medicare and Medicaid Services 100%denominator file data.
Disclosures: The study was sponsored by the National Heart, Lung, and Blood Institute. Dr. Edwards reported that he is a consultant and/or on the advisory board for Humana. Dr. Shahian reported that he has no relevant financial relationships. Several of their collaborators reported financial ties to several pharmaceutical or device manufacturers, including Boston Scientific and Medtronic.
Experts Can't Pin Down Best Carotid Atherosclerosis Treatments
There are still no clear answers about the best treatments to use to prevent stroke in patients with carotid artery stenosis, even with the input of a Medicare advisory panel of experts.
The Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) voted yesterday that they are only moderately confident that there is adequate evidence to determine which interventions – carotid artery endarterectomy (CEA), carotid artery stenting (CAS), or best medical treatment (BMT) – to use for different patient populations within the overall Medicare population. There’s unlikely to be any consensus in the near future as patient populations, detection, and treatments evolve.
"If there was ever a moving target problem in assessing health care interventions, this was one. The epidemiology is changing, the patient population is changing accordingly, [and] all of the interventions continue to change. We’ve not been keeping up in our data collection for the safety and effectiveness of these interventions as they continue to evolve," commented committee chair, Clifford Goodman, Ph.D. "This means that to the extent that CMS is going to revisit its coverage decision making over time, this is an ongoing data collection issue. You’re not done collecting these data; you may not ever be done collecting these data as long as the patient population continues to change and as long as we have very innovative people improving these interventions."
The panel was asked to vote on several questions regarding their confidence level that there is adequate evidence to identify specific patient populations and in some cases to select the best treatment option. Most scenarios garnered an "intermediate" confidence level. Confidence ran high only for the lowest-risk patients.
The panel voted that they had moderate confidence that the evidence is adequate to determine whether:
• Patients who are asymptomatic for carotid atherosclerosis can be identified as being at high risk for stroke in either cerebral hemisphere.
• Patients who are considering carotid revascularization can be identified as being at high risk for adverse events from CEA.
• Either CAS or CEA is the favored treatment strategy compared to BMT alone in patients with symptomatic carotid atherosclerosis and narrowing (at least 50% by angiography or at least 70% by ultrasound) who are not generally at high risk for adverse events from CEA.
• CAS, CEA, or BMT is the favored treatment strategy to decrease stroke or death for patients with asymptomatic carotid atherosclerosis.
In addition, for patients with symptomatic carotid atherosclerosis, who are not generally at high risk for adverse events from CEA, the panel had intermediate confidence that CEA is the favored treatment strategy to decrease stroke and death. For patients with asymptomatic carotid atherosclerosis, the panel had intermediate to high confidence that BMT is the favored treatment strategy to decrease stroke and death.
However, the panel was less confident that for patients with asymptomatic carotid atherosclerosis but with carotid narrowing – who are not generally considered at high risk for adverse events from CEA – that there is adequate evidence to determine whether or not either CAS or CEA is the favored strategy compared to BMT alone to decrease stroke or death in the Medicare population. The panel had very low confidence that there is adequate evidence to determine whether carotid artery screening of asymptomatic people decreases stroke or death.
The panel primarily based its decisions on the results of a handful of randomized, controlled trials with different patient populations, at different time periods, using different criteria to assess efficacy and safety. The results of the most recent of these – the CREST trial (Carotid Revascularization Endarterectomy vs. Stenting Trial) – were announced in 2010. The overall safety and efficacy of the CAS and CEA at 4 years were considered equivocal in terms of benefits for both men and for women, for patients who had previously had a stroke, and for those who had not (N. Engl. J. Med. 2010;363:11-23).
However, differences were seen between the two procedures for heart attacks and strokes. More heart attacks occurred with CEA – 2.3% vs. 1.1% – than for CAS, while more strokes occurred in the stenting group – 4.1% vs. 2.3% –than for CEA in the weeks following the procedure. There was also a difference in outcomes based on patient age. At approximately age 69 and younger, stenting results were slightly better, with CAS benefits increasing for decreasing patient age. Conversely, for patients older than 70, CEA results were slightly superior to stenting, with larger benefits for surgery with older patient age.
Instead of answering the question of which technique has better outcomes and safety, the trial left proponents of each technique still arguing.
In addition, BMT has not been assessed in clinical trials for many years and has evolved substantially since that time with the development and growing use of statins, antiplatelet medications, beta-blockers, ACE inhibitors, calcium channel blockers, and others.
What is lacking is a randomized, controlled trial pitting all three treatments against one another in a head-to-head comparison of the most current techniques, the panel noted repeatedly.
"I think that drawing conclusions from indirect comparisons of things that were done in the past is incredibly hazardous and we need to have a contemporary direct comparison to know what the right thing to do is," argued panel member Dr. Larry B. Goldstein, who is a professor of neurology and director of the Duke Stroke Center in Chapel Hill, N.C.
Both surgery and CAS received a class I recommendation in guidelines published by the American Heart Association and other organizations in 2011 (J. Am. Coll. Cardiol. 2011;57:1002-44), but the level of evidence was stronger for CEA (level A) than for CAS (level B).
Planning is in the works for a possible CREST II trial in asymptomatic patients intended to compare intervention (with either CEA or CAS) vs. medical treatment alone. Many panel members expressed the need for this trial to proceed.
Twelve members of the panel reported that they did not have any relevant financial relationships. Dr. Spence reported receiving speaking fees from several pharmaceutical companies, Dr. Curtis reported that he owns stock in Medtronic and receives grant support from Boston Scientific, industry representative Dr. Peter Juhn works for Medco Health Solutions, and committee chair Dr. Clifford Goodman is the senior vice president at the Lewin Group, which has ties to both government and industry.
There are still no clear answers about the best treatments to use to prevent stroke in patients with carotid artery stenosis, even with the input of a Medicare advisory panel of experts.
The Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) voted yesterday that they are only moderately confident that there is adequate evidence to determine which interventions – carotid artery endarterectomy (CEA), carotid artery stenting (CAS), or best medical treatment (BMT) – to use for different patient populations within the overall Medicare population. There’s unlikely to be any consensus in the near future as patient populations, detection, and treatments evolve.
"If there was ever a moving target problem in assessing health care interventions, this was one. The epidemiology is changing, the patient population is changing accordingly, [and] all of the interventions continue to change. We’ve not been keeping up in our data collection for the safety and effectiveness of these interventions as they continue to evolve," commented committee chair, Clifford Goodman, Ph.D. "This means that to the extent that CMS is going to revisit its coverage decision making over time, this is an ongoing data collection issue. You’re not done collecting these data; you may not ever be done collecting these data as long as the patient population continues to change and as long as we have very innovative people improving these interventions."
The panel was asked to vote on several questions regarding their confidence level that there is adequate evidence to identify specific patient populations and in some cases to select the best treatment option. Most scenarios garnered an "intermediate" confidence level. Confidence ran high only for the lowest-risk patients.
The panel voted that they had moderate confidence that the evidence is adequate to determine whether:
• Patients who are asymptomatic for carotid atherosclerosis can be identified as being at high risk for stroke in either cerebral hemisphere.
• Patients who are considering carotid revascularization can be identified as being at high risk for adverse events from CEA.
• Either CAS or CEA is the favored treatment strategy compared to BMT alone in patients with symptomatic carotid atherosclerosis and narrowing (at least 50% by angiography or at least 70% by ultrasound) who are not generally at high risk for adverse events from CEA.
• CAS, CEA, or BMT is the favored treatment strategy to decrease stroke or death for patients with asymptomatic carotid atherosclerosis.
In addition, for patients with symptomatic carotid atherosclerosis, who are not generally at high risk for adverse events from CEA, the panel had intermediate confidence that CEA is the favored treatment strategy to decrease stroke and death. For patients with asymptomatic carotid atherosclerosis, the panel had intermediate to high confidence that BMT is the favored treatment strategy to decrease stroke and death.
However, the panel was less confident that for patients with asymptomatic carotid atherosclerosis but with carotid narrowing – who are not generally considered at high risk for adverse events from CEA – that there is adequate evidence to determine whether or not either CAS or CEA is the favored strategy compared to BMT alone to decrease stroke or death in the Medicare population. The panel had very low confidence that there is adequate evidence to determine whether carotid artery screening of asymptomatic people decreases stroke or death.
The panel primarily based its decisions on the results of a handful of randomized, controlled trials with different patient populations, at different time periods, using different criteria to assess efficacy and safety. The results of the most recent of these – the CREST trial (Carotid Revascularization Endarterectomy vs. Stenting Trial) – were announced in 2010. The overall safety and efficacy of the CAS and CEA at 4 years were considered equivocal in terms of benefits for both men and for women, for patients who had previously had a stroke, and for those who had not (N. Engl. J. Med. 2010;363:11-23).
However, differences were seen between the two procedures for heart attacks and strokes. More heart attacks occurred with CEA – 2.3% vs. 1.1% – than for CAS, while more strokes occurred in the stenting group – 4.1% vs. 2.3% –than for CEA in the weeks following the procedure. There was also a difference in outcomes based on patient age. At approximately age 69 and younger, stenting results were slightly better, with CAS benefits increasing for decreasing patient age. Conversely, for patients older than 70, CEA results were slightly superior to stenting, with larger benefits for surgery with older patient age.
Instead of answering the question of which technique has better outcomes and safety, the trial left proponents of each technique still arguing.
In addition, BMT has not been assessed in clinical trials for many years and has evolved substantially since that time with the development and growing use of statins, antiplatelet medications, beta-blockers, ACE inhibitors, calcium channel blockers, and others.
What is lacking is a randomized, controlled trial pitting all three treatments against one another in a head-to-head comparison of the most current techniques, the panel noted repeatedly.
"I think that drawing conclusions from indirect comparisons of things that were done in the past is incredibly hazardous and we need to have a contemporary direct comparison to know what the right thing to do is," argued panel member Dr. Larry B. Goldstein, who is a professor of neurology and director of the Duke Stroke Center in Chapel Hill, N.C.
Both surgery and CAS received a class I recommendation in guidelines published by the American Heart Association and other organizations in 2011 (J. Am. Coll. Cardiol. 2011;57:1002-44), but the level of evidence was stronger for CEA (level A) than for CAS (level B).
Planning is in the works for a possible CREST II trial in asymptomatic patients intended to compare intervention (with either CEA or CAS) vs. medical treatment alone. Many panel members expressed the need for this trial to proceed.
Twelve members of the panel reported that they did not have any relevant financial relationships. Dr. Spence reported receiving speaking fees from several pharmaceutical companies, Dr. Curtis reported that he owns stock in Medtronic and receives grant support from Boston Scientific, industry representative Dr. Peter Juhn works for Medco Health Solutions, and committee chair Dr. Clifford Goodman is the senior vice president at the Lewin Group, which has ties to both government and industry.
There are still no clear answers about the best treatments to use to prevent stroke in patients with carotid artery stenosis, even with the input of a Medicare advisory panel of experts.
The Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) voted yesterday that they are only moderately confident that there is adequate evidence to determine which interventions – carotid artery endarterectomy (CEA), carotid artery stenting (CAS), or best medical treatment (BMT) – to use for different patient populations within the overall Medicare population. There’s unlikely to be any consensus in the near future as patient populations, detection, and treatments evolve.
"If there was ever a moving target problem in assessing health care interventions, this was one. The epidemiology is changing, the patient population is changing accordingly, [and] all of the interventions continue to change. We’ve not been keeping up in our data collection for the safety and effectiveness of these interventions as they continue to evolve," commented committee chair, Clifford Goodman, Ph.D. "This means that to the extent that CMS is going to revisit its coverage decision making over time, this is an ongoing data collection issue. You’re not done collecting these data; you may not ever be done collecting these data as long as the patient population continues to change and as long as we have very innovative people improving these interventions."
The panel was asked to vote on several questions regarding their confidence level that there is adequate evidence to identify specific patient populations and in some cases to select the best treatment option. Most scenarios garnered an "intermediate" confidence level. Confidence ran high only for the lowest-risk patients.
The panel voted that they had moderate confidence that the evidence is adequate to determine whether:
• Patients who are asymptomatic for carotid atherosclerosis can be identified as being at high risk for stroke in either cerebral hemisphere.
• Patients who are considering carotid revascularization can be identified as being at high risk for adverse events from CEA.
• Either CAS or CEA is the favored treatment strategy compared to BMT alone in patients with symptomatic carotid atherosclerosis and narrowing (at least 50% by angiography or at least 70% by ultrasound) who are not generally at high risk for adverse events from CEA.
• CAS, CEA, or BMT is the favored treatment strategy to decrease stroke or death for patients with asymptomatic carotid atherosclerosis.
In addition, for patients with symptomatic carotid atherosclerosis, who are not generally at high risk for adverse events from CEA, the panel had intermediate confidence that CEA is the favored treatment strategy to decrease stroke and death. For patients with asymptomatic carotid atherosclerosis, the panel had intermediate to high confidence that BMT is the favored treatment strategy to decrease stroke and death.
However, the panel was less confident that for patients with asymptomatic carotid atherosclerosis but with carotid narrowing – who are not generally considered at high risk for adverse events from CEA – that there is adequate evidence to determine whether or not either CAS or CEA is the favored strategy compared to BMT alone to decrease stroke or death in the Medicare population. The panel had very low confidence that there is adequate evidence to determine whether carotid artery screening of asymptomatic people decreases stroke or death.
The panel primarily based its decisions on the results of a handful of randomized, controlled trials with different patient populations, at different time periods, using different criteria to assess efficacy and safety. The results of the most recent of these – the CREST trial (Carotid Revascularization Endarterectomy vs. Stenting Trial) – were announced in 2010. The overall safety and efficacy of the CAS and CEA at 4 years were considered equivocal in terms of benefits for both men and for women, for patients who had previously had a stroke, and for those who had not (N. Engl. J. Med. 2010;363:11-23).
However, differences were seen between the two procedures for heart attacks and strokes. More heart attacks occurred with CEA – 2.3% vs. 1.1% – than for CAS, while more strokes occurred in the stenting group – 4.1% vs. 2.3% –than for CEA in the weeks following the procedure. There was also a difference in outcomes based on patient age. At approximately age 69 and younger, stenting results were slightly better, with CAS benefits increasing for decreasing patient age. Conversely, for patients older than 70, CEA results were slightly superior to stenting, with larger benefits for surgery with older patient age.
Instead of answering the question of which technique has better outcomes and safety, the trial left proponents of each technique still arguing.
In addition, BMT has not been assessed in clinical trials for many years and has evolved substantially since that time with the development and growing use of statins, antiplatelet medications, beta-blockers, ACE inhibitors, calcium channel blockers, and others.
What is lacking is a randomized, controlled trial pitting all three treatments against one another in a head-to-head comparison of the most current techniques, the panel noted repeatedly.
"I think that drawing conclusions from indirect comparisons of things that were done in the past is incredibly hazardous and we need to have a contemporary direct comparison to know what the right thing to do is," argued panel member Dr. Larry B. Goldstein, who is a professor of neurology and director of the Duke Stroke Center in Chapel Hill, N.C.
Both surgery and CAS received a class I recommendation in guidelines published by the American Heart Association and other organizations in 2011 (J. Am. Coll. Cardiol. 2011;57:1002-44), but the level of evidence was stronger for CEA (level A) than for CAS (level B).
Planning is in the works for a possible CREST II trial in asymptomatic patients intended to compare intervention (with either CEA or CAS) vs. medical treatment alone. Many panel members expressed the need for this trial to proceed.
Twelve members of the panel reported that they did not have any relevant financial relationships. Dr. Spence reported receiving speaking fees from several pharmaceutical companies, Dr. Curtis reported that he owns stock in Medtronic and receives grant support from Boston Scientific, industry representative Dr. Peter Juhn works for Medco Health Solutions, and committee chair Dr. Clifford Goodman is the senior vice president at the Lewin Group, which has ties to both government and industry.
FROM A MEETING OF THE MEDICARE EVIDENCE DEVELOPMENT AND COVERAGE ADVISORY COMMITTEE
School Staff Can Aid Treatment of Psychotic Children
School staff can play an important role in helping children with psychotic disorders live more normal lives, according to Dr. Jonathan R. Stevens and Dr. Jefferson B. Prince.
"School interventions often involve optimizing the environment to minimize undue stress to the patient, which increases vulnerability to psychotic episodes, and to match the level of stimulation with the patient’s level of arousal. Across conditions contributing to psychosis, school staff efforts to identify precipitants surrounding episodes or deterioration can be useful in determining appropriate home and classroom expectations," they wrote in the January issue of Child and Adolescent Psychiatric Clinics of North America.
They noted several early warning signs of psychosis that may present at school: changes in thinking; mood; behavior; physicality; social changes, including withdrawal or isolation from friends and family; changes in functioning; students’ perception; and persistent false beliefs (delusions), suspicion, and paranoia.
"Although data on psychosocial treatments in youth psychotic disorders in schools are scant, psychosocial treatment remain a mainstay of the overall treatment plan, and school staff have an essential role in caring for a child with psychosis," wrote Dr. Stevens and Dr. Prince, both of the pediatric inpatient psychiatry unit of MassGeneral for Children at North Shore in Lynn, Mass (Child Adolesc. Psychiatric Clin. N. Am. 2012;21:187-200 [doi:10.1016/j.chc.2011.09.008]).
In the school setting, the goals of psychosocial interventions are largely to anticipate problems and to develop strategies that better allow the student to cope. Interventions include developing trust and rapport among the student, family, treatment team, and school staff; developing strategies that support and enhance reality testing for the student; developing strategies that support academic, psychological, and social adjustment; developing a monitoring system to reduce the likelihood of relapse and to respond if it occurs; identifying barriers to treatment; and supporting the student’s autonomy, relatedness, and competency.
"Parents and other caregivers can help identify the patient’s progression toward psychosis, which may provide specific topics for school staff to ‘check in’ with the patient to monitor the patient’s reality testing," the researchers wrote. Strategies should address both positive (delusions, hallucinations) and negative (withdrawal, decreased interactions) symptoms. Treatment for positive symptoms may include anchoring activities with others as part of their daily routine. "When a patient with psychotic symptoms is agitated, distressed, or unsure of what is and is not ‘real,’ simplifying the environment, decreasing expectations, and diminishing stimulation are often required," they wrote. For negative symptoms, regular social interactions with others and structured familiar activities can help diminish isolation. These interactions need to be carefully configured and supported by staff because these students may be inclined to withdraw or avoid interactions.
Psychosocial treatments for students with psychosis typically involve either multimodal treatment programs or specific psychosocial interventions. Multimodal treatments programs can provide a comprehensive array of service, such as community outreach/early detection efforts; inpatient and outpatient treatment; individual, group, or family therapy; and case management.
Specific psychosocial interventions have been studied, including individual cognitive behavioral therapy, group programming, and family interventions. "Providing youth suffering psychosis with ‘cognitively oriented’ individual psychotherapy appears to help these youth adjust to their condition, resume usual developmental tasks, as well as improve symptoms of anxiety and depression," wrote Dr. Stevens and Dr. Prince. CBT can help students target specific thoughts and beliefs that intensify their symptoms and compromise their function at school.
In particular, a "safe and private space" can be designated, which allows the student to decompress initially as practice with CBT techniques can then more easily be internalized and generalized. The more specific and frequent the practice sessions are, the more likely they will take hold and be helpful to the student.
Family and school intervention programs can emphasize psychoeducation, the need to identify warning signs, stress management techniques, the importance of attributing maladaptive behavior to the illness rather than to "bad behaviors" or "laziness," communication skills training, and the reduction of high expressed emotion, the researchers noted.
Dr. Stevens and Dr. Prince outlined specific tasks for the school team, teachers and support staff, and administrators. The school team needs to stay connected; stay positive; remember that children dealing with psychosis are children first, and often unable to understand their perceptions or why they are changing so differently from peers; and refer children who show early warning signs to mental health clinicians.
Teachers and support staff should break tasks down into smaller steps, minimize distractions, and have a plan to redirect the student to help him or her return to the task at hand; give short, concise directions; assist with planning and organizational skills; provide modifications and accommodations to the school; and provide support with social problems.
School administrators should provide professional development for staff, collaborate with parents/caregivers and community resources, and modify the student’s school day based on waxing and waning of symptoms during the school year.
The authors reported that they have no conflicts to disclose.
School staff can play an important role in helping children with psychotic disorders live more normal lives, according to Dr. Jonathan R. Stevens and Dr. Jefferson B. Prince.
"School interventions often involve optimizing the environment to minimize undue stress to the patient, which increases vulnerability to psychotic episodes, and to match the level of stimulation with the patient’s level of arousal. Across conditions contributing to psychosis, school staff efforts to identify precipitants surrounding episodes or deterioration can be useful in determining appropriate home and classroom expectations," they wrote in the January issue of Child and Adolescent Psychiatric Clinics of North America.
They noted several early warning signs of psychosis that may present at school: changes in thinking; mood; behavior; physicality; social changes, including withdrawal or isolation from friends and family; changes in functioning; students’ perception; and persistent false beliefs (delusions), suspicion, and paranoia.
"Although data on psychosocial treatments in youth psychotic disorders in schools are scant, psychosocial treatment remain a mainstay of the overall treatment plan, and school staff have an essential role in caring for a child with psychosis," wrote Dr. Stevens and Dr. Prince, both of the pediatric inpatient psychiatry unit of MassGeneral for Children at North Shore in Lynn, Mass (Child Adolesc. Psychiatric Clin. N. Am. 2012;21:187-200 [doi:10.1016/j.chc.2011.09.008]).
In the school setting, the goals of psychosocial interventions are largely to anticipate problems and to develop strategies that better allow the student to cope. Interventions include developing trust and rapport among the student, family, treatment team, and school staff; developing strategies that support and enhance reality testing for the student; developing strategies that support academic, psychological, and social adjustment; developing a monitoring system to reduce the likelihood of relapse and to respond if it occurs; identifying barriers to treatment; and supporting the student’s autonomy, relatedness, and competency.
"Parents and other caregivers can help identify the patient’s progression toward psychosis, which may provide specific topics for school staff to ‘check in’ with the patient to monitor the patient’s reality testing," the researchers wrote. Strategies should address both positive (delusions, hallucinations) and negative (withdrawal, decreased interactions) symptoms. Treatment for positive symptoms may include anchoring activities with others as part of their daily routine. "When a patient with psychotic symptoms is agitated, distressed, or unsure of what is and is not ‘real,’ simplifying the environment, decreasing expectations, and diminishing stimulation are often required," they wrote. For negative symptoms, regular social interactions with others and structured familiar activities can help diminish isolation. These interactions need to be carefully configured and supported by staff because these students may be inclined to withdraw or avoid interactions.
Psychosocial treatments for students with psychosis typically involve either multimodal treatment programs or specific psychosocial interventions. Multimodal treatments programs can provide a comprehensive array of service, such as community outreach/early detection efforts; inpatient and outpatient treatment; individual, group, or family therapy; and case management.
Specific psychosocial interventions have been studied, including individual cognitive behavioral therapy, group programming, and family interventions. "Providing youth suffering psychosis with ‘cognitively oriented’ individual psychotherapy appears to help these youth adjust to their condition, resume usual developmental tasks, as well as improve symptoms of anxiety and depression," wrote Dr. Stevens and Dr. Prince. CBT can help students target specific thoughts and beliefs that intensify their symptoms and compromise their function at school.
In particular, a "safe and private space" can be designated, which allows the student to decompress initially as practice with CBT techniques can then more easily be internalized and generalized. The more specific and frequent the practice sessions are, the more likely they will take hold and be helpful to the student.
Family and school intervention programs can emphasize psychoeducation, the need to identify warning signs, stress management techniques, the importance of attributing maladaptive behavior to the illness rather than to "bad behaviors" or "laziness," communication skills training, and the reduction of high expressed emotion, the researchers noted.
Dr. Stevens and Dr. Prince outlined specific tasks for the school team, teachers and support staff, and administrators. The school team needs to stay connected; stay positive; remember that children dealing with psychosis are children first, and often unable to understand their perceptions or why they are changing so differently from peers; and refer children who show early warning signs to mental health clinicians.
Teachers and support staff should break tasks down into smaller steps, minimize distractions, and have a plan to redirect the student to help him or her return to the task at hand; give short, concise directions; assist with planning and organizational skills; provide modifications and accommodations to the school; and provide support with social problems.
School administrators should provide professional development for staff, collaborate with parents/caregivers and community resources, and modify the student’s school day based on waxing and waning of symptoms during the school year.
The authors reported that they have no conflicts to disclose.
School staff can play an important role in helping children with psychotic disorders live more normal lives, according to Dr. Jonathan R. Stevens and Dr. Jefferson B. Prince.
"School interventions often involve optimizing the environment to minimize undue stress to the patient, which increases vulnerability to psychotic episodes, and to match the level of stimulation with the patient’s level of arousal. Across conditions contributing to psychosis, school staff efforts to identify precipitants surrounding episodes or deterioration can be useful in determining appropriate home and classroom expectations," they wrote in the January issue of Child and Adolescent Psychiatric Clinics of North America.
They noted several early warning signs of psychosis that may present at school: changes in thinking; mood; behavior; physicality; social changes, including withdrawal or isolation from friends and family; changes in functioning; students’ perception; and persistent false beliefs (delusions), suspicion, and paranoia.
"Although data on psychosocial treatments in youth psychotic disorders in schools are scant, psychosocial treatment remain a mainstay of the overall treatment plan, and school staff have an essential role in caring for a child with psychosis," wrote Dr. Stevens and Dr. Prince, both of the pediatric inpatient psychiatry unit of MassGeneral for Children at North Shore in Lynn, Mass (Child Adolesc. Psychiatric Clin. N. Am. 2012;21:187-200 [doi:10.1016/j.chc.2011.09.008]).
In the school setting, the goals of psychosocial interventions are largely to anticipate problems and to develop strategies that better allow the student to cope. Interventions include developing trust and rapport among the student, family, treatment team, and school staff; developing strategies that support and enhance reality testing for the student; developing strategies that support academic, psychological, and social adjustment; developing a monitoring system to reduce the likelihood of relapse and to respond if it occurs; identifying barriers to treatment; and supporting the student’s autonomy, relatedness, and competency.
"Parents and other caregivers can help identify the patient’s progression toward psychosis, which may provide specific topics for school staff to ‘check in’ with the patient to monitor the patient’s reality testing," the researchers wrote. Strategies should address both positive (delusions, hallucinations) and negative (withdrawal, decreased interactions) symptoms. Treatment for positive symptoms may include anchoring activities with others as part of their daily routine. "When a patient with psychotic symptoms is agitated, distressed, or unsure of what is and is not ‘real,’ simplifying the environment, decreasing expectations, and diminishing stimulation are often required," they wrote. For negative symptoms, regular social interactions with others and structured familiar activities can help diminish isolation. These interactions need to be carefully configured and supported by staff because these students may be inclined to withdraw or avoid interactions.
Psychosocial treatments for students with psychosis typically involve either multimodal treatment programs or specific psychosocial interventions. Multimodal treatments programs can provide a comprehensive array of service, such as community outreach/early detection efforts; inpatient and outpatient treatment; individual, group, or family therapy; and case management.
Specific psychosocial interventions have been studied, including individual cognitive behavioral therapy, group programming, and family interventions. "Providing youth suffering psychosis with ‘cognitively oriented’ individual psychotherapy appears to help these youth adjust to their condition, resume usual developmental tasks, as well as improve symptoms of anxiety and depression," wrote Dr. Stevens and Dr. Prince. CBT can help students target specific thoughts and beliefs that intensify their symptoms and compromise their function at school.
In particular, a "safe and private space" can be designated, which allows the student to decompress initially as practice with CBT techniques can then more easily be internalized and generalized. The more specific and frequent the practice sessions are, the more likely they will take hold and be helpful to the student.
Family and school intervention programs can emphasize psychoeducation, the need to identify warning signs, stress management techniques, the importance of attributing maladaptive behavior to the illness rather than to "bad behaviors" or "laziness," communication skills training, and the reduction of high expressed emotion, the researchers noted.
Dr. Stevens and Dr. Prince outlined specific tasks for the school team, teachers and support staff, and administrators. The school team needs to stay connected; stay positive; remember that children dealing with psychosis are children first, and often unable to understand their perceptions or why they are changing so differently from peers; and refer children who show early warning signs to mental health clinicians.
Teachers and support staff should break tasks down into smaller steps, minimize distractions, and have a plan to redirect the student to help him or her return to the task at hand; give short, concise directions; assist with planning and organizational skills; provide modifications and accommodations to the school; and provide support with social problems.
School administrators should provide professional development for staff, collaborate with parents/caregivers and community resources, and modify the student’s school day based on waxing and waning of symptoms during the school year.
The authors reported that they have no conflicts to disclose.
FROM CHILD AND ADOLESCENT PSYCHIATRIC CLINICS OF NORTH AMERICA