User login
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
Mohs micrographic surgery (MMS)—developed by Dr. Frederic Mohs in the 1930s—is the gold standard for treating various cutaneous malignancies. It provides maximal conservation of uninvolved tissues while producing higher cure rates compared to wide local excision.1,2
We sought to assess the various characteristics that impact patient satisfaction to help Mohs surgeons incorporate relatively simple yet clinically significant practices into their patient encounters. We conducted a systematic literature search of peer-reviewed PubMed articles indexed for MEDLINE from database inception through November 2023 using the terms Mohs micrographic surgery and patient satisfaction. Among the inclusion criteria were studies involving participants having undergone MMS, with objective assessments on patient-reported satisfaction or preferences related to patient education, communication, anxiety-alleviating measures, or QOL in MMS. Studies were excluded if they failed to meet these criteria, were outdated and no longer clinically relevant, or measured unalterable factors with no significant impact on how Mohs surgeons could change clinical practice. Of the 157 nonreplicated studies identified, 34 met inclusion criteria.
Perioperative Patient Communication and Education Techniques
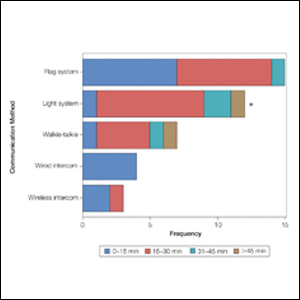
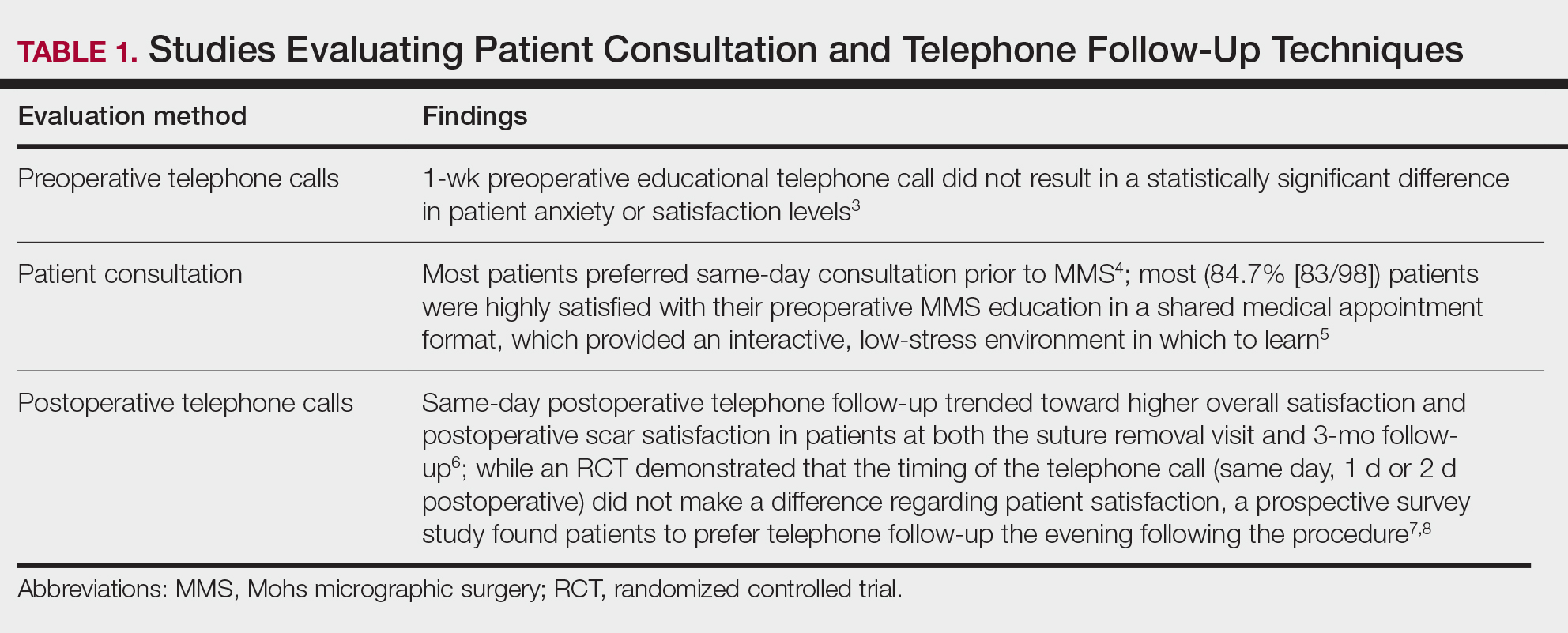
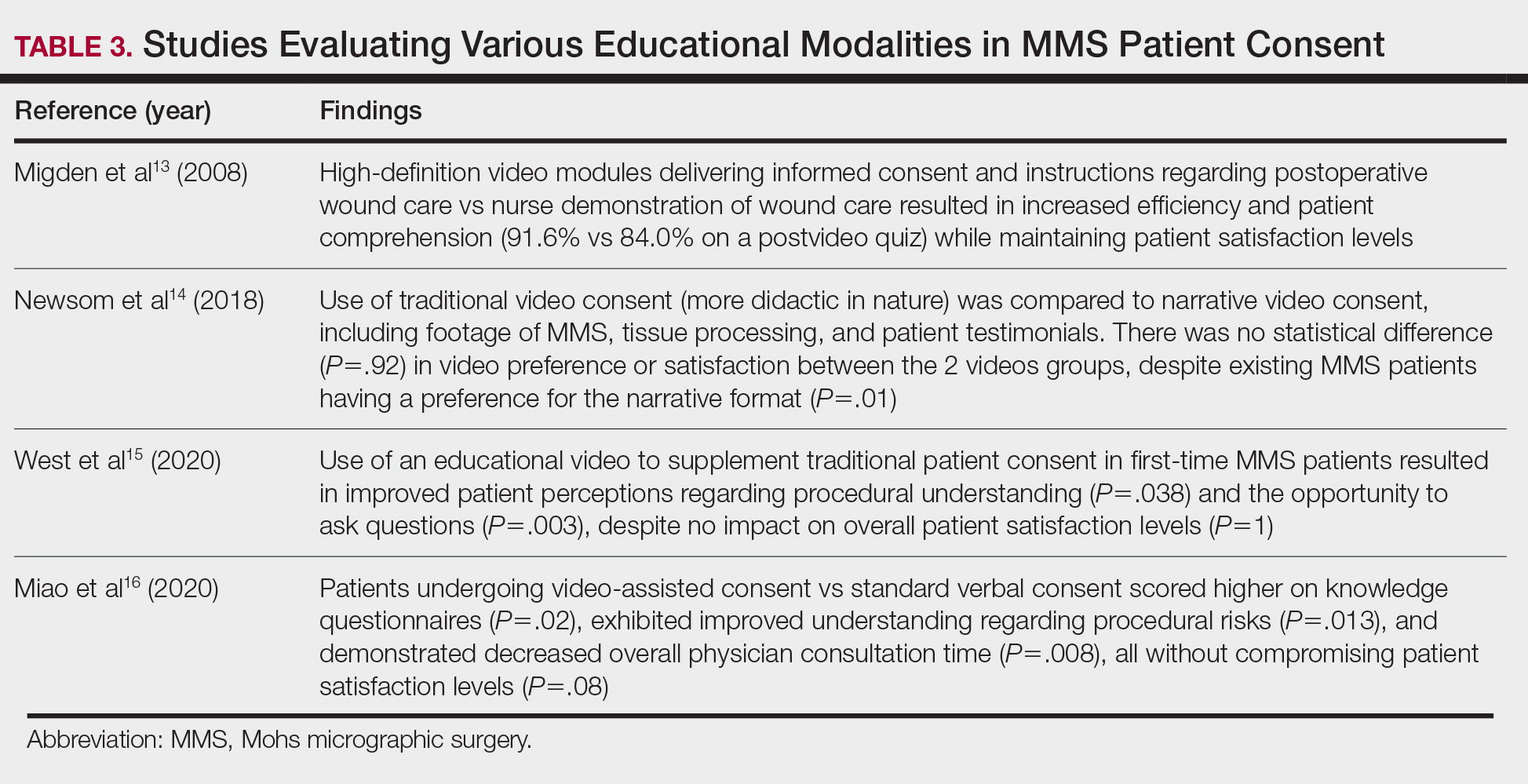
Perioperative Patient Communication—Many studies have evaluated the impact of perioperative patient-provider communication and education on patient satisfaction in those undergoing MMS. Studies focusing on preoperative and postoperative telephone calls, patient consultation formats, and patient-perceived impact of such communication modalities have been well documented (Table 1).3-8 The importance of the patient follow-up after MMS was further supported by a retrospective study concluding that 88.7% (86/97) of patients regarded follow-up visits as important, and 80% (77/97) desired additional follow-up 3 months after MMS.9 Additional studies have highlighted the importance of thorough and open perioperative patient-provider communication during MMS (Table 2).10-12
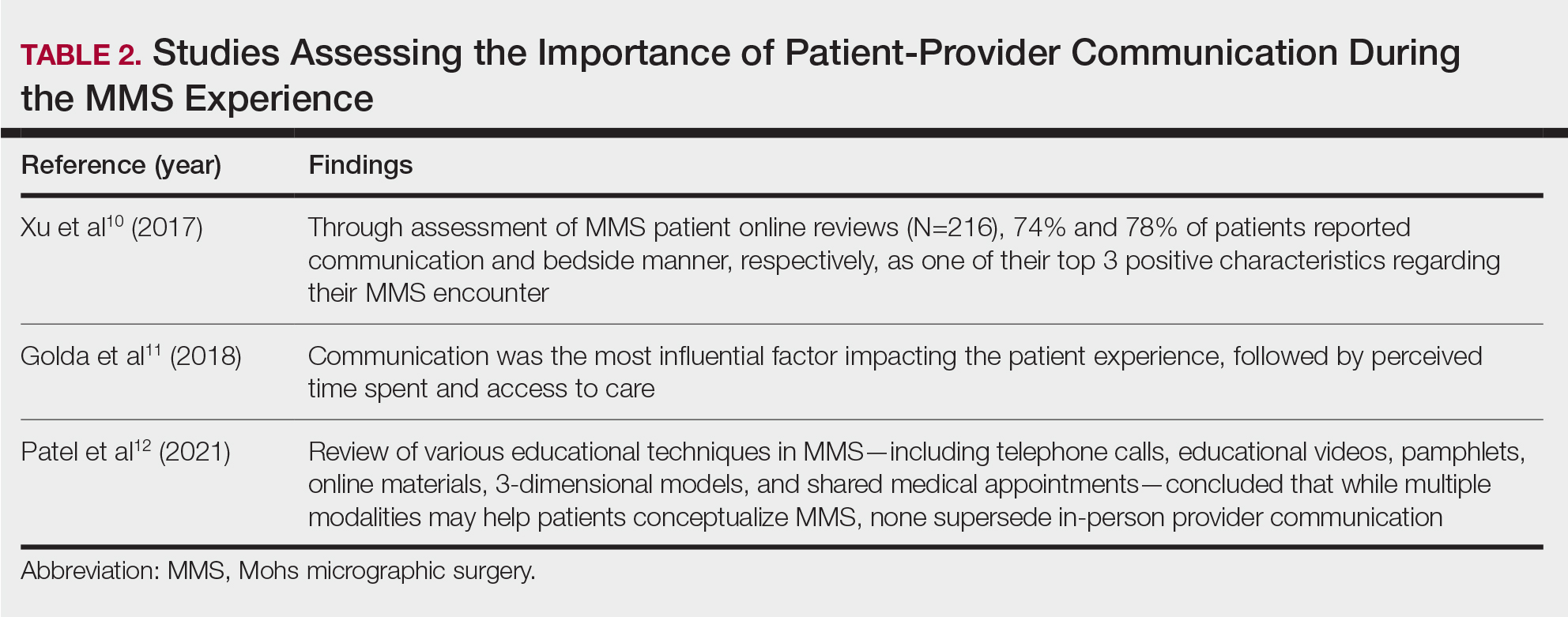
Patient-Education Techniques—Many studies have assessed the use of visual models to aid in patient education on MMS, specifically the preprocedural consent process (Table 3).13-16 Additionally, 2 randomized controlled trials assessing the use of at-home and same-day in-office preoperative educational videos concluded that these interventions increased patient knowledge and confidence regarding procedural risks and benefits, with no statistically significant differences in patient anxiety or satisfaction.17,18
Despite the availability of these educational videos, many patients often turn to online resources for self-education, which is problematic if reader literacy is incongruent with online readability. One study assessing readability of online MMS resources concluded that the most accessed articles exceeded the recommended reading level for adequate patient comprehension.19 A survey studying a wide range of variables related to patient satisfaction (eg, demographics, socioeconomics, health status) in 339 MMS patients found that those who considered themselves more involved in the decision-making process were more satisfied in the short-term, and married patients had even higher long-term satisfaction. Interestingly, this study also concluded that undergoing 3 or more MMS stages was associated with higher short- and long-term satisfaction, likely secondary to perceived effects of increased overall care, medical attention, and time spent with the provider.20
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding patient education and communication13-20:
- Preoperative and same-day postoperative telephone follow-up (TFU) do not show statistically significant impacts on patient satisfaction; however, TFU allows for identification of postoperative concerns and inadequate pain management, which may have downstream effects on long-term perception of the overall patient experience.
- The use of video-assisted consent yields improved patient satisfaction and knowledge, while video content—traditional or didactic—has no impact on satisfaction in new MMS patients.
- The use of at-home or same-day in-office preoperative educational videos can improve procedural knowledge and risk-benefit understanding of MMS while having no impact on satisfaction.
- Bedside manner and effective in-person communication by the provider often takes precedence in the patient experience; however, implementation of additional educational modalities should be considered.
Patient Anxiety and QOL
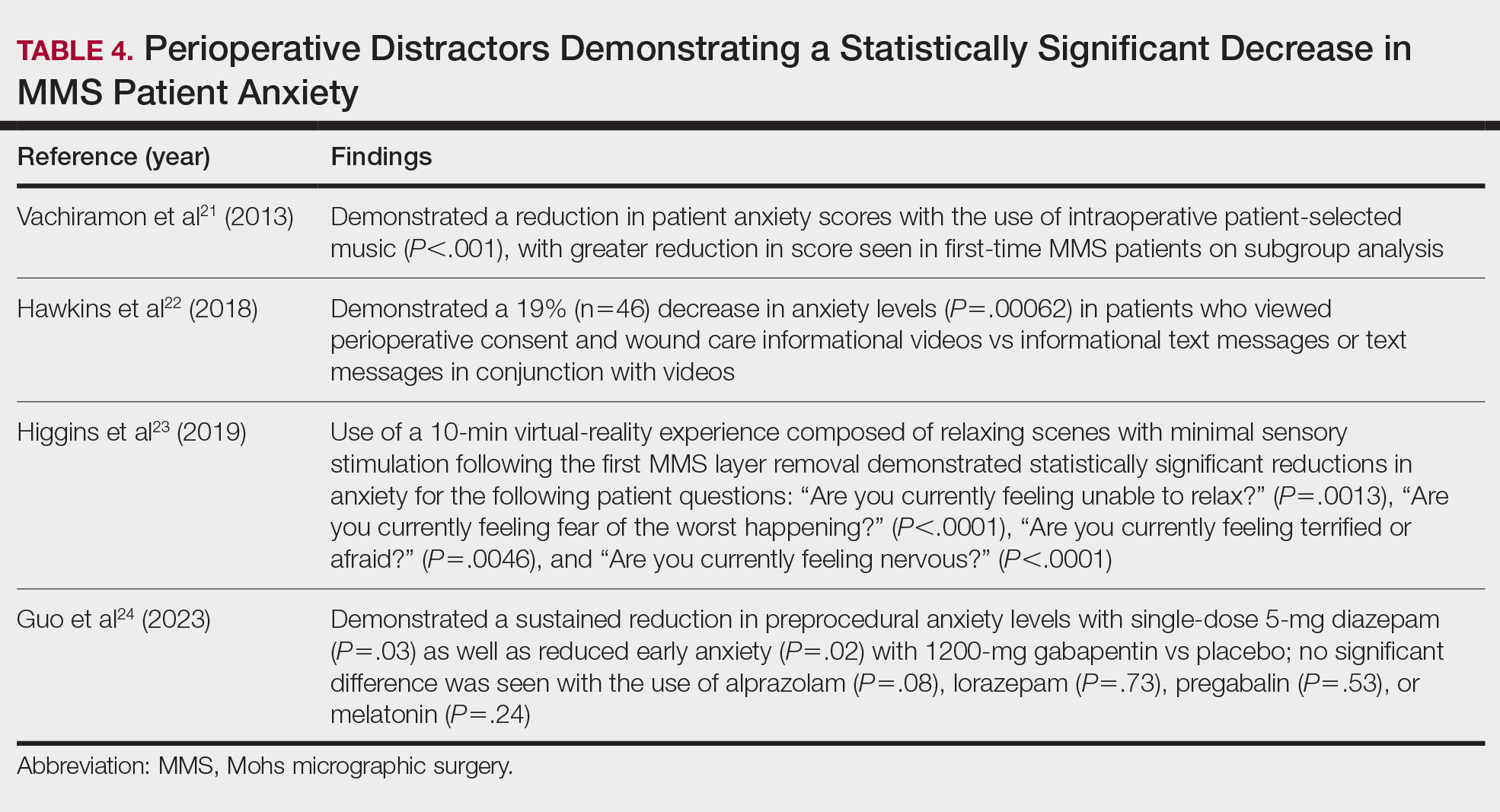
Reducing Patient Anxiety—The use of perioperative distractors to reduce patient anxiety may play an integral role when patients undergo MMS, as there often are prolonged waiting periods between stages when patients may feel increasingly vulnerable or anxious. Table 4 reviews studies on perioperative distractors that showed a statistically significant reduction in MMS patient anxiety.21-24
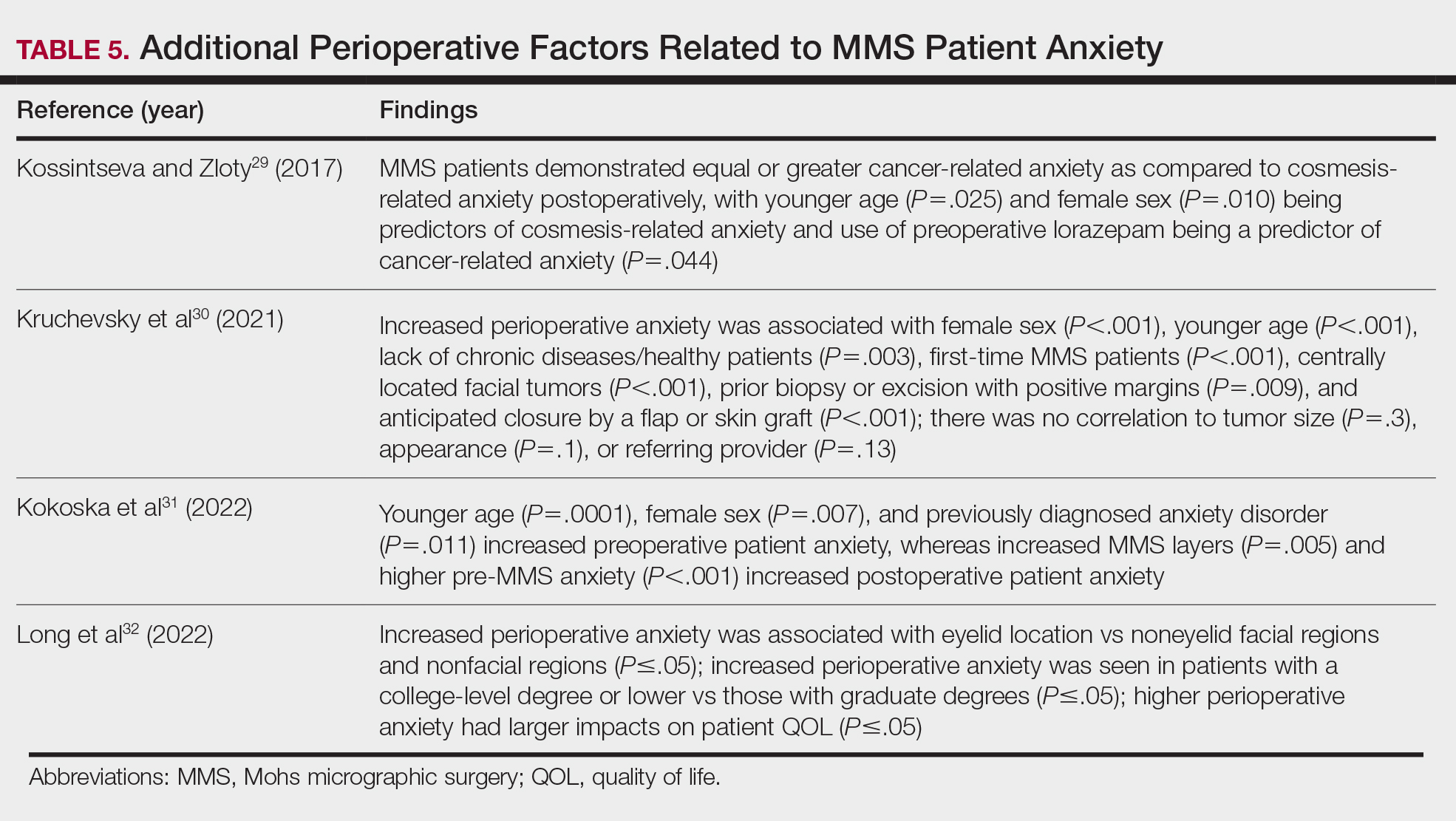
Although not statistically significant, additional studies evaluating the use of intraoperative anxiety-reduction methods in MMS have demonstrated a downtrend in patient anxiety with the following interventions: engaging in small talk with clinic staff, bringing a guest, eating, watching television, communicating surgical expectations with the provider, handholding, use of a stress ball, and use of 3-dimensional educational MMS models.25-27 Similarly, a survey of 73 patients undergoing MMS found that patients tended to enjoy complimentary beverages preprocedurally in the waiting room, reading, speaking with their guest, watching television, or using their telephone during wait times.28 Table 5 lists additional perioperative factors encompassing specific patient and surgical characteristics that help reduce patient anxiety.29-32
Patient QOL—Many methods aimed at decreasing MMS-related patient anxiety often show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience. A prospective observational study of MMS patients noted a statistically significant improvement in patient QOL scores 3 months postsurgery (P=.0007), demonstrating that MMS generally results in positive patient outcomes despite preprocedural anxiety.33 An additional prospective study in MMS patients with nonmelanoma skin cancer concluded that sex, age, and closure type—factors often shown to affect anxiety levels—did not significantly impact patient satisfaction.34 Similarly, high satisfaction levels can be expected among MMS patients undergoing treatment of melanoma in situ, with more than 90% of patients rating their treatment experience a 4 (agree) or 5 (strongly agree) out of 5 in short- and long-term satisfaction assessments (38/41 and 40/42, respectively).35 This assessment, conducted 3 months postoperatively, asked patients to score the statement, “I am completely satisfied with the treatment of my skin problem,” on a scale ranging from 1 (strongly disagree) to 5 (strongly agree).
Lastly, patient perception of their surgeon’s skill may contribute to levels of patient satisfaction. Although suture spacing has not been shown to affect surgical outcomes, it has been demonstrated to impact the patient’s perception of surgical skill and is further supported by a study concluding that closures with 2-mm spacing were ranked significantly lower by patients compared with closures with either 4- or 6-mm spacing (P=.005 and P=.012, respectively).36
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding anxiety-reducing measures and patient-perceived QOL21-36:
- Factors shown to decrease patient anxiety include patient personalized music, virtual-reality experience, perioperative informational videos, and 3-dimensional–printed MMS models.
- Many methods aimed at decreasing MMS-related patient anxiety show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience.
- Higher anxiety can be associated with worse QOL scores in MMS patients, and additional factors that may have a negative impact on anxiety include female sex, younger age, and tumor location on the face.
Conclusion
Many factors affect patient satisfaction in MMS. Increased awareness and acknowledgement of these factors can foster improved clinical practice and patient experience, which can have downstream effects on patient compliance and overall psychosocial and medical well-being. With the movement toward value-based health care, patient satisfaction ratings are likely to play an increasingly important role in physician reimbursement. Adapting one’s practice to include high-quality, time-efficient, patient-centered care goes hand in hand with increasing MMS patient satisfaction. Careful evaluation and scrutiny of one’s current practices while remaining cognizant of patient population, resource availability, and clinical limitations often reveal opportunities for small adjustments that can have a great impact on patient satisfaction. This thorough assessment and review of the published literature aims to assist MMS surgeons in understanding the role that certain factors—(1) perioperative patient communication and education techniques and (2) patient anxiety, QOL, and additional considerations—have on overall satisfaction with MMS. Specific consideration should be placed on the fact that patient satisfaction is multifactorial, and many different interventions can have a positive impact on the overall patient experience.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011; 29:135-139, vii. doi:10.1016/j.det.2011.01.010
- Leslie DF, Greenway HT. Mohs micrographic surgery for skin cancer. Australas J Dermatol. 1991;32:159-164. doi:10.1111/j.1440 -0960.1991.tb01783.x
- Sobanko JF, Da Silva D, Chiesa Fuxench ZC, et al. Preoperative telephone consultation does not decrease patient anxiety before Mohs micrographic surgery. J Am Acad Dermatol. 2017;76:519-526. doi:10.1016/j.jaad.2016.09.027
- Sharon VR, Armstrong AW, Jim On SC, et al. Separate- versus same-day preoperative consultation in dermatologic surgery: a patient-centered investigation in an academic practice. Dermatol Surg. 2013;39:240-247. doi:10.1111/dsu.12083
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344. doi:10.1016/j.jaad.2014.10.022
- Vance S, Fontecilla N, Samie FH, et al. Effect of postoperative telephone calls on patient satisfaction and scar satisfaction after Mohs micrographic surgery. Dermatol Surg. 2019;45:1459-1464. doi:10.1097/DSS.0000000000001913
- Hafiji J, Salmon P, Hussain W. Patient satisfaction with post-operative telephone calls after Mohs micrographic surgery: a New Zealand and U.K. experience. Br J Dermatol. 2012;167:570-574. doi:10.1111 /j.1365-2133.2012.11011.x
- Bednarek R, Jonak C, Golda N. Optimal timing of postoperative patient telephone calls after Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2021;85:220-221. doi:10.1016 /j.jaad.2020.07.106
- Sharon VR, Armstrong AW, Jim-On S, et al. Postoperative preferences in cutaneous surgery: a patient-centered investigation from an academic dermatologic surgery practice. Dermatol Surg. 2013;39:773-778. doi:10.1111/dsu.12136
- Xu S, Atanelov Z, Bhatia AC. Online patient-reported reviews of Mohs micrographic surgery: qualitative analysis of positive and negative experiences. Cutis. 2017;99:E25-E29.
- Golda N, Beeson S, Kohli N, et al. Recommendations for improving the patient experience in specialty encounters. J Am Acad Dermatol. 2018;78:653-659. doi:10.1016/j.jaad.2017.05.040
- Patel P, Malik K, Khachemoune A. Patient education in Mohs surgery: a review and critical evaluation of techniques. Arch Dermatol Res. 2021;313:217-224. doi:10.1007/s00403-020-02119-5
- Migden M, Chavez-Frazier A, Nguyen T. The use of high definition video modules for delivery of informed consent and wound care education in the Mohs surgery unit. Semin Cutan Med Surg. 2008;27:89-93. doi:10.1016/j.sder.2008.02.001
- Newsom E, Lee E, Rossi A, et al. Modernizing the Mohs surgery consultation: instituting a video module for improved patient education and satisfaction. Dermatol Surg. 2018;44:778-784. doi:10.1097/DSS.0000000000001473
- West L, Srivastava D, Goldberg LH, et al. Multimedia technology used to supplement patient consent for Mohs micrographic surgery. Dermatol Surg. 2020;46:586-590. doi:10.1097/DSS.0000000000002134
- Miao Y, Venning VL, Mallitt KA, et al. A randomized controlled trial comparing video-assisted informed consent with standard consent for Mohs micrographic surgery. JAAD Int. 2020;1:13-20. doi:10.1016 /j.jdin.2020.03.005
- Mann J, Li L, Kulakov E, et al. Home viewing of educational video improves patient understanding of Mohs micrographic surgery. Clin Exp Dermatol. 2022;47:93-97. doi:10.1111/ced.14845
- Delcambre M, Haynes D, Hajar T, et al. Using a multimedia tool for informed consent in Mohs surgery: a randomized trial measuring effects on patient anxiety, knowledge, and satisfaction. Dermatol Surg. 2020;46:591-598. doi:10.1097/DSS.0000000000002213
- Vargas CR, DePry J, Lee BT, et al. The readability of online patient information about Mohs micrographic surgery. Dermatol Surg. 2016;42:1135-1141. doi:10.1097/DSS.0000000000000866
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Hawkins SD, Koch SB, Williford PM, et al. Web app- and text message-based patient education in Mohs micrographic surgery-a randomized controlled trial. Dermatol Surg. 2018;44:924-932. doi:10.1097/DSS.0000000000001489
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient-a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097 /DSS.0000000000001854
- Guo D, Zloty DM, Kossintseva I. Efficacy and safety of anxiolytics in Mohs micrographic surgery: a randomized, double-blinded, placebo-controlled trial. Dermatol Surg. 2023;49:989-994. doi:10.1097 /DSS.0000000000003905
- Locke MC, Wilkerson EC, Mistur RL, et al. 2015 Arte Poster Competition first place winner: assessing the correlation between patient anxiety and satisfaction for Mohs surgery. J Drugs Dermatol. 2015;14:1070-1072.
- Yanes AF, Weil A, Furlan KC, et al. Effect of stress ball use or hand-holding on anxiety during skin cancer excision: a randomized clinical trial. JAMA Dermatol. 2018;154:1045-1049. doi:10.1001 /jamadermatol.2018.1783
- Biro M, Kim I, Huynh A, et al. The use of 3-dimensionally printed models to optimize patient education and alleviate perioperative anxiety in Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2019;81:1339-1345. doi:10.1016/j.jaad.2019.05.085
- Ali FR, Al-Niaimi F, Craythorne EE, et al. Patient satisfaction and the waiting room in Mohs surgery: appropriate prewarning may abrogate boredom. J Eur Acad Dermatol Venereol. 2017;31:e337-e338.
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035.
- Kruchevsky D, Hirth J, Capucha T, et al. Triggers of preoperative anxiety in patients undergoing Mohs micrographic surgery. Dermatol Surg. 2021;47:1110-1112.
- Kokoska RE, Szeto MD, Steadman L, et al. Analysis of factors contributing to perioperative Mohs micrographic surgery anxiety: patient survey study at an academic center. Dermatol Surg. 2022;48:1279-1282.
- Long J, Rajabi-Estarabadi A, Levin A, et al. Perioperative anxiety associated with Mohs micrographic surgery: a survey-based study. Dermatol Surg. 2022;48:711-715.
- Zhang J, Miller CJ, O’Malley V, et al. Patient quality of life fluctuates before and after Mohs micrographic surgery: a longitudinal assessment of the patient experience. J Am Acad Dermatol. 2018;78:1060-1067.
- Lee EB, Ford A, Clarey D, et al. Patient outcomes and satisfaction after Mohs micrographic surgery in patients with nonmelanoma skin cancer. Dermatol Sur. 2021;47:1190-1194.
- Condie D, West L, Hynan LS, et al. Patient satisfaction with Mohs surgery for melanoma in situ. Dermatol Surg. 2021;47:288-290.
- Arshanapalli A, Tra n JM, Aylward JL, et al. The effect of suture spacing on patient perception of surgical skill. J Am Acad Dermatol. 2021;84:735-736.
Mohs micrographic surgery (MMS)—developed by Dr. Frederic Mohs in the 1930s—is the gold standard for treating various cutaneous malignancies. It provides maximal conservation of uninvolved tissues while producing higher cure rates compared to wide local excision.1,2
We sought to assess the various characteristics that impact patient satisfaction to help Mohs surgeons incorporate relatively simple yet clinically significant practices into their patient encounters. We conducted a systematic literature search of peer-reviewed PubMed articles indexed for MEDLINE from database inception through November 2023 using the terms Mohs micrographic surgery and patient satisfaction. Among the inclusion criteria were studies involving participants having undergone MMS, with objective assessments on patient-reported satisfaction or preferences related to patient education, communication, anxiety-alleviating measures, or QOL in MMS. Studies were excluded if they failed to meet these criteria, were outdated and no longer clinically relevant, or measured unalterable factors with no significant impact on how Mohs surgeons could change clinical practice. Of the 157 nonreplicated studies identified, 34 met inclusion criteria.
Perioperative Patient Communication and Education Techniques
Perioperative Patient Communication—Many studies have evaluated the impact of perioperative patient-provider communication and education on patient satisfaction in those undergoing MMS. Studies focusing on preoperative and postoperative telephone calls, patient consultation formats, and patient-perceived impact of such communication modalities have been well documented (Table 1).3-8 The importance of the patient follow-up after MMS was further supported by a retrospective study concluding that 88.7% (86/97) of patients regarded follow-up visits as important, and 80% (77/97) desired additional follow-up 3 months after MMS.9 Additional studies have highlighted the importance of thorough and open perioperative patient-provider communication during MMS (Table 2).10-12


Patient-Education Techniques—Many studies have assessed the use of visual models to aid in patient education on MMS, specifically the preprocedural consent process (Table 3).13-16 Additionally, 2 randomized controlled trials assessing the use of at-home and same-day in-office preoperative educational videos concluded that these interventions increased patient knowledge and confidence regarding procedural risks and benefits, with no statistically significant differences in patient anxiety or satisfaction.17,18

Despite the availability of these educational videos, many patients often turn to online resources for self-education, which is problematic if reader literacy is incongruent with online readability. One study assessing readability of online MMS resources concluded that the most accessed articles exceeded the recommended reading level for adequate patient comprehension.19 A survey studying a wide range of variables related to patient satisfaction (eg, demographics, socioeconomics, health status) in 339 MMS patients found that those who considered themselves more involved in the decision-making process were more satisfied in the short-term, and married patients had even higher long-term satisfaction. Interestingly, this study also concluded that undergoing 3 or more MMS stages was associated with higher short- and long-term satisfaction, likely secondary to perceived effects of increased overall care, medical attention, and time spent with the provider.20
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding patient education and communication13-20:
- Preoperative and same-day postoperative telephone follow-up (TFU) do not show statistically significant impacts on patient satisfaction; however, TFU allows for identification of postoperative concerns and inadequate pain management, which may have downstream effects on long-term perception of the overall patient experience.
- The use of video-assisted consent yields improved patient satisfaction and knowledge, while video content—traditional or didactic—has no impact on satisfaction in new MMS patients.
- The use of at-home or same-day in-office preoperative educational videos can improve procedural knowledge and risk-benefit understanding of MMS while having no impact on satisfaction.
- Bedside manner and effective in-person communication by the provider often takes precedence in the patient experience; however, implementation of additional educational modalities should be considered.
Patient Anxiety and QOL
Reducing Patient Anxiety—The use of perioperative distractors to reduce patient anxiety may play an integral role when patients undergo MMS, as there often are prolonged waiting periods between stages when patients may feel increasingly vulnerable or anxious. Table 4 reviews studies on perioperative distractors that showed a statistically significant reduction in MMS patient anxiety.21-24

Although not statistically significant, additional studies evaluating the use of intraoperative anxiety-reduction methods in MMS have demonstrated a downtrend in patient anxiety with the following interventions: engaging in small talk with clinic staff, bringing a guest, eating, watching television, communicating surgical expectations with the provider, handholding, use of a stress ball, and use of 3-dimensional educational MMS models.25-27 Similarly, a survey of 73 patients undergoing MMS found that patients tended to enjoy complimentary beverages preprocedurally in the waiting room, reading, speaking with their guest, watching television, or using their telephone during wait times.28 Table 5 lists additional perioperative factors encompassing specific patient and surgical characteristics that help reduce patient anxiety.29-32

Patient QOL—Many methods aimed at decreasing MMS-related patient anxiety often show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience. A prospective observational study of MMS patients noted a statistically significant improvement in patient QOL scores 3 months postsurgery (P=.0007), demonstrating that MMS generally results in positive patient outcomes despite preprocedural anxiety.33 An additional prospective study in MMS patients with nonmelanoma skin cancer concluded that sex, age, and closure type—factors often shown to affect anxiety levels—did not significantly impact patient satisfaction.34 Similarly, high satisfaction levels can be expected among MMS patients undergoing treatment of melanoma in situ, with more than 90% of patients rating their treatment experience a 4 (agree) or 5 (strongly agree) out of 5 in short- and long-term satisfaction assessments (38/41 and 40/42, respectively).35 This assessment, conducted 3 months postoperatively, asked patients to score the statement, “I am completely satisfied with the treatment of my skin problem,” on a scale ranging from 1 (strongly disagree) to 5 (strongly agree).
Lastly, patient perception of their surgeon’s skill may contribute to levels of patient satisfaction. Although suture spacing has not been shown to affect surgical outcomes, it has been demonstrated to impact the patient’s perception of surgical skill and is further supported by a study concluding that closures with 2-mm spacing were ranked significantly lower by patients compared with closures with either 4- or 6-mm spacing (P=.005 and P=.012, respectively).36
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding anxiety-reducing measures and patient-perceived QOL21-36:
- Factors shown to decrease patient anxiety include patient personalized music, virtual-reality experience, perioperative informational videos, and 3-dimensional–printed MMS models.
- Many methods aimed at decreasing MMS-related patient anxiety show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience.
- Higher anxiety can be associated with worse QOL scores in MMS patients, and additional factors that may have a negative impact on anxiety include female sex, younger age, and tumor location on the face.
Conclusion
Many factors affect patient satisfaction in MMS. Increased awareness and acknowledgement of these factors can foster improved clinical practice and patient experience, which can have downstream effects on patient compliance and overall psychosocial and medical well-being. With the movement toward value-based health care, patient satisfaction ratings are likely to play an increasingly important role in physician reimbursement. Adapting one’s practice to include high-quality, time-efficient, patient-centered care goes hand in hand with increasing MMS patient satisfaction. Careful evaluation and scrutiny of one’s current practices while remaining cognizant of patient population, resource availability, and clinical limitations often reveal opportunities for small adjustments that can have a great impact on patient satisfaction. This thorough assessment and review of the published literature aims to assist MMS surgeons in understanding the role that certain factors—(1) perioperative patient communication and education techniques and (2) patient anxiety, QOL, and additional considerations—have on overall satisfaction with MMS. Specific consideration should be placed on the fact that patient satisfaction is multifactorial, and many different interventions can have a positive impact on the overall patient experience.
Mohs micrographic surgery (MMS)—developed by Dr. Frederic Mohs in the 1930s—is the gold standard for treating various cutaneous malignancies. It provides maximal conservation of uninvolved tissues while producing higher cure rates compared to wide local excision.1,2
We sought to assess the various characteristics that impact patient satisfaction to help Mohs surgeons incorporate relatively simple yet clinically significant practices into their patient encounters. We conducted a systematic literature search of peer-reviewed PubMed articles indexed for MEDLINE from database inception through November 2023 using the terms Mohs micrographic surgery and patient satisfaction. Among the inclusion criteria were studies involving participants having undergone MMS, with objective assessments on patient-reported satisfaction or preferences related to patient education, communication, anxiety-alleviating measures, or QOL in MMS. Studies were excluded if they failed to meet these criteria, were outdated and no longer clinically relevant, or measured unalterable factors with no significant impact on how Mohs surgeons could change clinical practice. Of the 157 nonreplicated studies identified, 34 met inclusion criteria.
Perioperative Patient Communication and Education Techniques
Perioperative Patient Communication—Many studies have evaluated the impact of perioperative patient-provider communication and education on patient satisfaction in those undergoing MMS. Studies focusing on preoperative and postoperative telephone calls, patient consultation formats, and patient-perceived impact of such communication modalities have been well documented (Table 1).3-8 The importance of the patient follow-up after MMS was further supported by a retrospective study concluding that 88.7% (86/97) of patients regarded follow-up visits as important, and 80% (77/97) desired additional follow-up 3 months after MMS.9 Additional studies have highlighted the importance of thorough and open perioperative patient-provider communication during MMS (Table 2).10-12


Patient-Education Techniques—Many studies have assessed the use of visual models to aid in patient education on MMS, specifically the preprocedural consent process (Table 3).13-16 Additionally, 2 randomized controlled trials assessing the use of at-home and same-day in-office preoperative educational videos concluded that these interventions increased patient knowledge and confidence regarding procedural risks and benefits, with no statistically significant differences in patient anxiety or satisfaction.17,18

Despite the availability of these educational videos, many patients often turn to online resources for self-education, which is problematic if reader literacy is incongruent with online readability. One study assessing readability of online MMS resources concluded that the most accessed articles exceeded the recommended reading level for adequate patient comprehension.19 A survey studying a wide range of variables related to patient satisfaction (eg, demographics, socioeconomics, health status) in 339 MMS patients found that those who considered themselves more involved in the decision-making process were more satisfied in the short-term, and married patients had even higher long-term satisfaction. Interestingly, this study also concluded that undergoing 3 or more MMS stages was associated with higher short- and long-term satisfaction, likely secondary to perceived effects of increased overall care, medical attention, and time spent with the provider.20
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding patient education and communication13-20:
- Preoperative and same-day postoperative telephone follow-up (TFU) do not show statistically significant impacts on patient satisfaction; however, TFU allows for identification of postoperative concerns and inadequate pain management, which may have downstream effects on long-term perception of the overall patient experience.
- The use of video-assisted consent yields improved patient satisfaction and knowledge, while video content—traditional or didactic—has no impact on satisfaction in new MMS patients.
- The use of at-home or same-day in-office preoperative educational videos can improve procedural knowledge and risk-benefit understanding of MMS while having no impact on satisfaction.
- Bedside manner and effective in-person communication by the provider often takes precedence in the patient experience; however, implementation of additional educational modalities should be considered.
Patient Anxiety and QOL
Reducing Patient Anxiety—The use of perioperative distractors to reduce patient anxiety may play an integral role when patients undergo MMS, as there often are prolonged waiting periods between stages when patients may feel increasingly vulnerable or anxious. Table 4 reviews studies on perioperative distractors that showed a statistically significant reduction in MMS patient anxiety.21-24

Although not statistically significant, additional studies evaluating the use of intraoperative anxiety-reduction methods in MMS have demonstrated a downtrend in patient anxiety with the following interventions: engaging in small talk with clinic staff, bringing a guest, eating, watching television, communicating surgical expectations with the provider, handholding, use of a stress ball, and use of 3-dimensional educational MMS models.25-27 Similarly, a survey of 73 patients undergoing MMS found that patients tended to enjoy complimentary beverages preprocedurally in the waiting room, reading, speaking with their guest, watching television, or using their telephone during wait times.28 Table 5 lists additional perioperative factors encompassing specific patient and surgical characteristics that help reduce patient anxiety.29-32

Patient QOL—Many methods aimed at decreasing MMS-related patient anxiety often show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience. A prospective observational study of MMS patients noted a statistically significant improvement in patient QOL scores 3 months postsurgery (P=.0007), demonstrating that MMS generally results in positive patient outcomes despite preprocedural anxiety.33 An additional prospective study in MMS patients with nonmelanoma skin cancer concluded that sex, age, and closure type—factors often shown to affect anxiety levels—did not significantly impact patient satisfaction.34 Similarly, high satisfaction levels can be expected among MMS patients undergoing treatment of melanoma in situ, with more than 90% of patients rating their treatment experience a 4 (agree) or 5 (strongly agree) out of 5 in short- and long-term satisfaction assessments (38/41 and 40/42, respectively).35 This assessment, conducted 3 months postoperatively, asked patients to score the statement, “I am completely satisfied with the treatment of my skin problem,” on a scale ranging from 1 (strongly disagree) to 5 (strongly agree).
Lastly, patient perception of their surgeon’s skill may contribute to levels of patient satisfaction. Although suture spacing has not been shown to affect surgical outcomes, it has been demonstrated to impact the patient’s perception of surgical skill and is further supported by a study concluding that closures with 2-mm spacing were ranked significantly lower by patients compared with closures with either 4- or 6-mm spacing (P=.005 and P=.012, respectively).36
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding anxiety-reducing measures and patient-perceived QOL21-36:
- Factors shown to decrease patient anxiety include patient personalized music, virtual-reality experience, perioperative informational videos, and 3-dimensional–printed MMS models.
- Many methods aimed at decreasing MMS-related patient anxiety show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience.
- Higher anxiety can be associated with worse QOL scores in MMS patients, and additional factors that may have a negative impact on anxiety include female sex, younger age, and tumor location on the face.
Conclusion
Many factors affect patient satisfaction in MMS. Increased awareness and acknowledgement of these factors can foster improved clinical practice and patient experience, which can have downstream effects on patient compliance and overall psychosocial and medical well-being. With the movement toward value-based health care, patient satisfaction ratings are likely to play an increasingly important role in physician reimbursement. Adapting one’s practice to include high-quality, time-efficient, patient-centered care goes hand in hand with increasing MMS patient satisfaction. Careful evaluation and scrutiny of one’s current practices while remaining cognizant of patient population, resource availability, and clinical limitations often reveal opportunities for small adjustments that can have a great impact on patient satisfaction. This thorough assessment and review of the published literature aims to assist MMS surgeons in understanding the role that certain factors—(1) perioperative patient communication and education techniques and (2) patient anxiety, QOL, and additional considerations—have on overall satisfaction with MMS. Specific consideration should be placed on the fact that patient satisfaction is multifactorial, and many different interventions can have a positive impact on the overall patient experience.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011; 29:135-139, vii. doi:10.1016/j.det.2011.01.010
- Leslie DF, Greenway HT. Mohs micrographic surgery for skin cancer. Australas J Dermatol. 1991;32:159-164. doi:10.1111/j.1440 -0960.1991.tb01783.x
- Sobanko JF, Da Silva D, Chiesa Fuxench ZC, et al. Preoperative telephone consultation does not decrease patient anxiety before Mohs micrographic surgery. J Am Acad Dermatol. 2017;76:519-526. doi:10.1016/j.jaad.2016.09.027
- Sharon VR, Armstrong AW, Jim On SC, et al. Separate- versus same-day preoperative consultation in dermatologic surgery: a patient-centered investigation in an academic practice. Dermatol Surg. 2013;39:240-247. doi:10.1111/dsu.12083
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344. doi:10.1016/j.jaad.2014.10.022
- Vance S, Fontecilla N, Samie FH, et al. Effect of postoperative telephone calls on patient satisfaction and scar satisfaction after Mohs micrographic surgery. Dermatol Surg. 2019;45:1459-1464. doi:10.1097/DSS.0000000000001913
- Hafiji J, Salmon P, Hussain W. Patient satisfaction with post-operative telephone calls after Mohs micrographic surgery: a New Zealand and U.K. experience. Br J Dermatol. 2012;167:570-574. doi:10.1111 /j.1365-2133.2012.11011.x
- Bednarek R, Jonak C, Golda N. Optimal timing of postoperative patient telephone calls after Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2021;85:220-221. doi:10.1016 /j.jaad.2020.07.106
- Sharon VR, Armstrong AW, Jim-On S, et al. Postoperative preferences in cutaneous surgery: a patient-centered investigation from an academic dermatologic surgery practice. Dermatol Surg. 2013;39:773-778. doi:10.1111/dsu.12136
- Xu S, Atanelov Z, Bhatia AC. Online patient-reported reviews of Mohs micrographic surgery: qualitative analysis of positive and negative experiences. Cutis. 2017;99:E25-E29.
- Golda N, Beeson S, Kohli N, et al. Recommendations for improving the patient experience in specialty encounters. J Am Acad Dermatol. 2018;78:653-659. doi:10.1016/j.jaad.2017.05.040
- Patel P, Malik K, Khachemoune A. Patient education in Mohs surgery: a review and critical evaluation of techniques. Arch Dermatol Res. 2021;313:217-224. doi:10.1007/s00403-020-02119-5
- Migden M, Chavez-Frazier A, Nguyen T. The use of high definition video modules for delivery of informed consent and wound care education in the Mohs surgery unit. Semin Cutan Med Surg. 2008;27:89-93. doi:10.1016/j.sder.2008.02.001
- Newsom E, Lee E, Rossi A, et al. Modernizing the Mohs surgery consultation: instituting a video module for improved patient education and satisfaction. Dermatol Surg. 2018;44:778-784. doi:10.1097/DSS.0000000000001473
- West L, Srivastava D, Goldberg LH, et al. Multimedia technology used to supplement patient consent for Mohs micrographic surgery. Dermatol Surg. 2020;46:586-590. doi:10.1097/DSS.0000000000002134
- Miao Y, Venning VL, Mallitt KA, et al. A randomized controlled trial comparing video-assisted informed consent with standard consent for Mohs micrographic surgery. JAAD Int. 2020;1:13-20. doi:10.1016 /j.jdin.2020.03.005
- Mann J, Li L, Kulakov E, et al. Home viewing of educational video improves patient understanding of Mohs micrographic surgery. Clin Exp Dermatol. 2022;47:93-97. doi:10.1111/ced.14845
- Delcambre M, Haynes D, Hajar T, et al. Using a multimedia tool for informed consent in Mohs surgery: a randomized trial measuring effects on patient anxiety, knowledge, and satisfaction. Dermatol Surg. 2020;46:591-598. doi:10.1097/DSS.0000000000002213
- Vargas CR, DePry J, Lee BT, et al. The readability of online patient information about Mohs micrographic surgery. Dermatol Surg. 2016;42:1135-1141. doi:10.1097/DSS.0000000000000866
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Hawkins SD, Koch SB, Williford PM, et al. Web app- and text message-based patient education in Mohs micrographic surgery-a randomized controlled trial. Dermatol Surg. 2018;44:924-932. doi:10.1097/DSS.0000000000001489
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient-a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097 /DSS.0000000000001854
- Guo D, Zloty DM, Kossintseva I. Efficacy and safety of anxiolytics in Mohs micrographic surgery: a randomized, double-blinded, placebo-controlled trial. Dermatol Surg. 2023;49:989-994. doi:10.1097 /DSS.0000000000003905
- Locke MC, Wilkerson EC, Mistur RL, et al. 2015 Arte Poster Competition first place winner: assessing the correlation between patient anxiety and satisfaction for Mohs surgery. J Drugs Dermatol. 2015;14:1070-1072.
- Yanes AF, Weil A, Furlan KC, et al. Effect of stress ball use or hand-holding on anxiety during skin cancer excision: a randomized clinical trial. JAMA Dermatol. 2018;154:1045-1049. doi:10.1001 /jamadermatol.2018.1783
- Biro M, Kim I, Huynh A, et al. The use of 3-dimensionally printed models to optimize patient education and alleviate perioperative anxiety in Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2019;81:1339-1345. doi:10.1016/j.jaad.2019.05.085
- Ali FR, Al-Niaimi F, Craythorne EE, et al. Patient satisfaction and the waiting room in Mohs surgery: appropriate prewarning may abrogate boredom. J Eur Acad Dermatol Venereol. 2017;31:e337-e338.
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035.
- Kruchevsky D, Hirth J, Capucha T, et al. Triggers of preoperative anxiety in patients undergoing Mohs micrographic surgery. Dermatol Surg. 2021;47:1110-1112.
- Kokoska RE, Szeto MD, Steadman L, et al. Analysis of factors contributing to perioperative Mohs micrographic surgery anxiety: patient survey study at an academic center. Dermatol Surg. 2022;48:1279-1282.
- Long J, Rajabi-Estarabadi A, Levin A, et al. Perioperative anxiety associated with Mohs micrographic surgery: a survey-based study. Dermatol Surg. 2022;48:711-715.
- Zhang J, Miller CJ, O’Malley V, et al. Patient quality of life fluctuates before and after Mohs micrographic surgery: a longitudinal assessment of the patient experience. J Am Acad Dermatol. 2018;78:1060-1067.
- Lee EB, Ford A, Clarey D, et al. Patient outcomes and satisfaction after Mohs micrographic surgery in patients with nonmelanoma skin cancer. Dermatol Sur. 2021;47:1190-1194.
- Condie D, West L, Hynan LS, et al. Patient satisfaction with Mohs surgery for melanoma in situ. Dermatol Surg. 2021;47:288-290.
- Arshanapalli A, Tra n JM, Aylward JL, et al. The effect of suture spacing on patient perception of surgical skill. J Am Acad Dermatol. 2021;84:735-736.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011; 29:135-139, vii. doi:10.1016/j.det.2011.01.010
- Leslie DF, Greenway HT. Mohs micrographic surgery for skin cancer. Australas J Dermatol. 1991;32:159-164. doi:10.1111/j.1440 -0960.1991.tb01783.x
- Sobanko JF, Da Silva D, Chiesa Fuxench ZC, et al. Preoperative telephone consultation does not decrease patient anxiety before Mohs micrographic surgery. J Am Acad Dermatol. 2017;76:519-526. doi:10.1016/j.jaad.2016.09.027
- Sharon VR, Armstrong AW, Jim On SC, et al. Separate- versus same-day preoperative consultation in dermatologic surgery: a patient-centered investigation in an academic practice. Dermatol Surg. 2013;39:240-247. doi:10.1111/dsu.12083
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344. doi:10.1016/j.jaad.2014.10.022
- Vance S, Fontecilla N, Samie FH, et al. Effect of postoperative telephone calls on patient satisfaction and scar satisfaction after Mohs micrographic surgery. Dermatol Surg. 2019;45:1459-1464. doi:10.1097/DSS.0000000000001913
- Hafiji J, Salmon P, Hussain W. Patient satisfaction with post-operative telephone calls after Mohs micrographic surgery: a New Zealand and U.K. experience. Br J Dermatol. 2012;167:570-574. doi:10.1111 /j.1365-2133.2012.11011.x
- Bednarek R, Jonak C, Golda N. Optimal timing of postoperative patient telephone calls after Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2021;85:220-221. doi:10.1016 /j.jaad.2020.07.106
- Sharon VR, Armstrong AW, Jim-On S, et al. Postoperative preferences in cutaneous surgery: a patient-centered investigation from an academic dermatologic surgery practice. Dermatol Surg. 2013;39:773-778. doi:10.1111/dsu.12136
- Xu S, Atanelov Z, Bhatia AC. Online patient-reported reviews of Mohs micrographic surgery: qualitative analysis of positive and negative experiences. Cutis. 2017;99:E25-E29.
- Golda N, Beeson S, Kohli N, et al. Recommendations for improving the patient experience in specialty encounters. J Am Acad Dermatol. 2018;78:653-659. doi:10.1016/j.jaad.2017.05.040
- Patel P, Malik K, Khachemoune A. Patient education in Mohs surgery: a review and critical evaluation of techniques. Arch Dermatol Res. 2021;313:217-224. doi:10.1007/s00403-020-02119-5
- Migden M, Chavez-Frazier A, Nguyen T. The use of high definition video modules for delivery of informed consent and wound care education in the Mohs surgery unit. Semin Cutan Med Surg. 2008;27:89-93. doi:10.1016/j.sder.2008.02.001
- Newsom E, Lee E, Rossi A, et al. Modernizing the Mohs surgery consultation: instituting a video module for improved patient education and satisfaction. Dermatol Surg. 2018;44:778-784. doi:10.1097/DSS.0000000000001473
- West L, Srivastava D, Goldberg LH, et al. Multimedia technology used to supplement patient consent for Mohs micrographic surgery. Dermatol Surg. 2020;46:586-590. doi:10.1097/DSS.0000000000002134
- Miao Y, Venning VL, Mallitt KA, et al. A randomized controlled trial comparing video-assisted informed consent with standard consent for Mohs micrographic surgery. JAAD Int. 2020;1:13-20. doi:10.1016 /j.jdin.2020.03.005
- Mann J, Li L, Kulakov E, et al. Home viewing of educational video improves patient understanding of Mohs micrographic surgery. Clin Exp Dermatol. 2022;47:93-97. doi:10.1111/ced.14845
- Delcambre M, Haynes D, Hajar T, et al. Using a multimedia tool for informed consent in Mohs surgery: a randomized trial measuring effects on patient anxiety, knowledge, and satisfaction. Dermatol Surg. 2020;46:591-598. doi:10.1097/DSS.0000000000002213
- Vargas CR, DePry J, Lee BT, et al. The readability of online patient information about Mohs micrographic surgery. Dermatol Surg. 2016;42:1135-1141. doi:10.1097/DSS.0000000000000866
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Hawkins SD, Koch SB, Williford PM, et al. Web app- and text message-based patient education in Mohs micrographic surgery-a randomized controlled trial. Dermatol Surg. 2018;44:924-932. doi:10.1097/DSS.0000000000001489
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient-a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097 /DSS.0000000000001854
- Guo D, Zloty DM, Kossintseva I. Efficacy and safety of anxiolytics in Mohs micrographic surgery: a randomized, double-blinded, placebo-controlled trial. Dermatol Surg. 2023;49:989-994. doi:10.1097 /DSS.0000000000003905
- Locke MC, Wilkerson EC, Mistur RL, et al. 2015 Arte Poster Competition first place winner: assessing the correlation between patient anxiety and satisfaction for Mohs surgery. J Drugs Dermatol. 2015;14:1070-1072.
- Yanes AF, Weil A, Furlan KC, et al. Effect of stress ball use or hand-holding on anxiety during skin cancer excision: a randomized clinical trial. JAMA Dermatol. 2018;154:1045-1049. doi:10.1001 /jamadermatol.2018.1783
- Biro M, Kim I, Huynh A, et al. The use of 3-dimensionally printed models to optimize patient education and alleviate perioperative anxiety in Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2019;81:1339-1345. doi:10.1016/j.jaad.2019.05.085
- Ali FR, Al-Niaimi F, Craythorne EE, et al. Patient satisfaction and the waiting room in Mohs surgery: appropriate prewarning may abrogate boredom. J Eur Acad Dermatol Venereol. 2017;31:e337-e338.
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035.
- Kruchevsky D, Hirth J, Capucha T, et al. Triggers of preoperative anxiety in patients undergoing Mohs micrographic surgery. Dermatol Surg. 2021;47:1110-1112.
- Kokoska RE, Szeto MD, Steadman L, et al. Analysis of factors contributing to perioperative Mohs micrographic surgery anxiety: patient survey study at an academic center. Dermatol Surg. 2022;48:1279-1282.
- Long J, Rajabi-Estarabadi A, Levin A, et al. Perioperative anxiety associated with Mohs micrographic surgery: a survey-based study. Dermatol Surg. 2022;48:711-715.
- Zhang J, Miller CJ, O’Malley V, et al. Patient quality of life fluctuates before and after Mohs micrographic surgery: a longitudinal assessment of the patient experience. J Am Acad Dermatol. 2018;78:1060-1067.
- Lee EB, Ford A, Clarey D, et al. Patient outcomes and satisfaction after Mohs micrographic surgery in patients with nonmelanoma skin cancer. Dermatol Sur. 2021;47:1190-1194.
- Condie D, West L, Hynan LS, et al. Patient satisfaction with Mohs surgery for melanoma in situ. Dermatol Surg. 2021;47:288-290.
- Arshanapalli A, Tra n JM, Aylward JL, et al. The effect of suture spacing on patient perception of surgical skill. J Am Acad Dermatol. 2021;84:735-736.
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
PRACTICE POINTS
- When patients are treated with Mohs micrographic surgery (MMS), thorough in-person dialogue augmented by pre- and same-day telephone follow-ups can help them feel heard and better supported, even though follow-up calls alone may not drive satisfaction scores.
- Increased awareness and implementation of the various factors influencing patient satisfaction and quality of life in MMS can enhance clinical practice and improve patient experiences, with potential impacts on compliance, psychosocial well-being, medical outcomes, and physician reimbursement.
- Patient satisfaction and procedural understanding can be improved with video and visual-based education. Anxiety-reducing methods help lower perioperative stress.
Erythematous and Ulcerated Plaque on the Left Temple
The Diagnosis: Primary Cutaneous Carcinosarcoma
The immunohistochemical findings supported an epithelial component consistent with moderately differentiated squamous cell carcinoma (SCC) and a mesenchymal component with features consistent with a sarcoma. Consequently, the lesion was diagnosed as a primary cutaneous carcinosarcoma (PCCS).
Primary cutaneous carcinosarcoma is a rare biphasic neoplasm consisting of malignant epithelial (carcinoma) and mesenchymal (sarcoma) components.1 Primary cutaneous carcinosarcomas are uncommon, poorly understood, primary cutaneous tumors.2,3 Characteristic of this tumor, cytokeratins highlight the epithelial component while vimentin highlights the mesenchymal component.4 Histologically, the sarcomatous components of PCCS often are highly variable, with an absence of transitional areas within the epithelial component, which frequently resembles basal cell carcinoma and/ or SCC.5-7 Primary cutaneous carcinosarcoma favors areas of chronic UV radiation exposure, particularly on the head and neck. Most tumors present with a slowly growing, polypoid, flesh-colored to erythematous nodule due to the infiltrative mesenchymal component.7 Primary cutaneous carcinosarcoma primarily is diagnosed in elderly patients, with the majority of cases diagnosed in the eighth or ninth decades of life (range, 32–98 years).1,8 Men appear to be twice as likely to be diagnosed with a PCCS compared to women.1 Primary cutaneous carcinosarcomas are recognized as aggressive tumors with a high propensity to metastasize and recur locally, necessitating early diagnosis and treatment.4 Accurate diagnosis of PCCSs can be challenging due to the biphasic nature of the neoplasm as well as poor differentiation or unequal proportions of the epithelial and mesenchymal components.5 Additionally, overlapping diagnostic criteria coupled with vague demarcation between soft-tissue sarcomas and distinct carcinomas also may contribute to a delay in diagnosis.9 Treatment is achieved surgically by complete wide resection, with no evidence to support the use of adjuvant or neoadjuvant external beam radiation therapy. Due to the small number of reported cases, no treatment recommendations currently exist.1
Surgical management with wide local excision has been disappointing, with recurrence rates reported as high as 33%.6 Primary cutaneous carcinosarcoma has an estimated overall recurrence rate of 19% and a 5-year disease-free rate of 50%.10 Risk factors associated with poorer prognosis include tumors with adnexal subtype, age less than 65 years, rapid tumor growth, a tumor greater than 20 mm at presentation, and a long-standing tumor lasting up to 30 years.2,4 Although wide local excision and Mohs micrographic surgery (MMS) both have been utilized successfully, MMS has been shown to result in a cure rate of greater than 98%.6
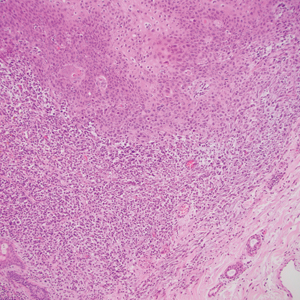
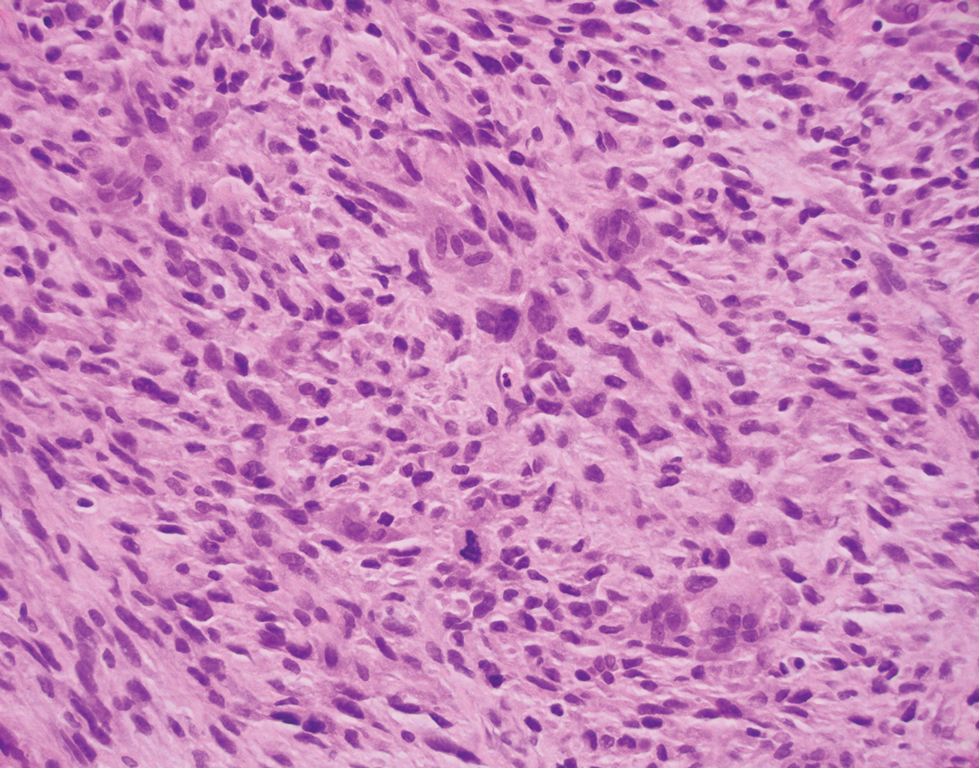
Atypical fibroxanthoma (AFX) is a cutaneous tumor of fibrohistiocytic mesenchymal origin that typically manifests on sun-damaged skin in elderly individuals. Clinically, it presents as a rapidly growing neoplasm that often ulcerates and bleeds. These heterogenous neoplasms have several distinct characteristics, including dense cellularity with disorganized, large, pleomorphic, and atypical-appearing spindle-shaped cells arising in the upper layers of the dermis, often disseminating into the reticular dermis and occasionally into the subcutaneous fat (Figure 1). The neoplastic cells often exhibit hyperchromic and irregular nuclei, multinucleated giant cells, and atypical mitotic figures. In most cases, negative immunohistochemical staining with SOX-10, S-100, cytokeratins, desmin, and caldesmon will allow pathologists to differentiate between AFX and other common tumors on the differential diagnosis, such as SCC, melanoma, and leiomyosarcoma. CD10 and procollagen type 1 are positive antigenic markers in AFX, but they are not specific. The standard treatment of AFX includes wide local excision or MMS for superior margin control.11
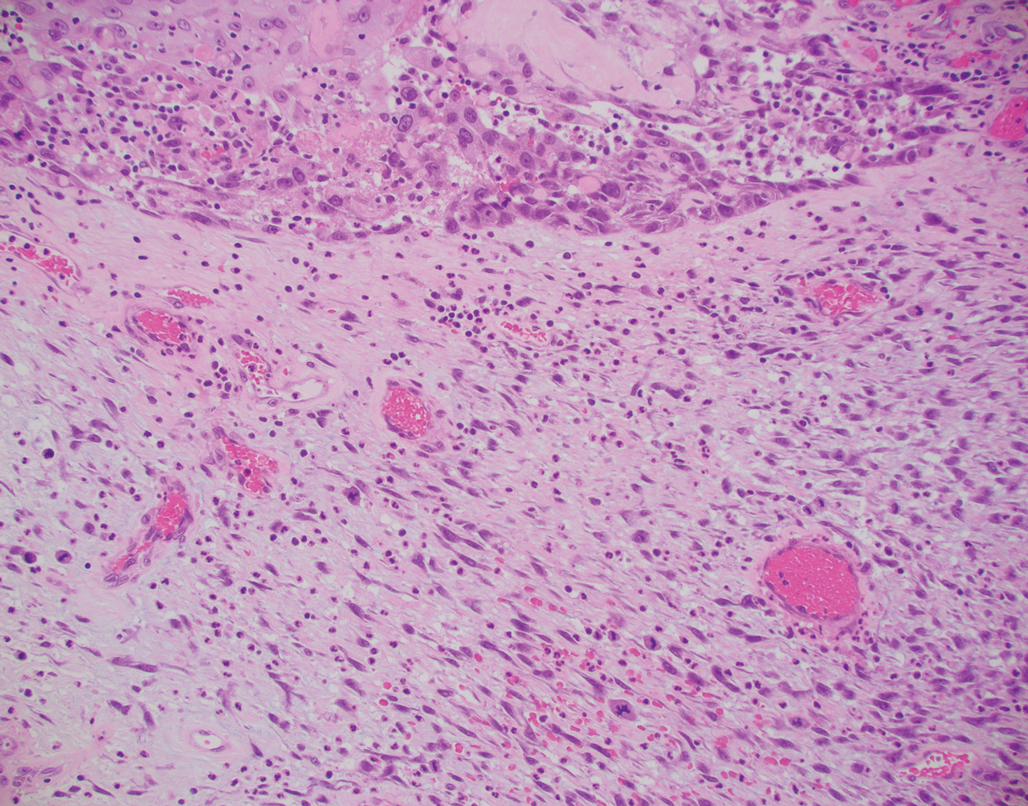
Spindle cell SCC presents as a raised or exophytic nodule, often with spontaneous bleeding and central ulceration. It usually presents on sun-damaged skin or in individuals with a history of ionizing radiation. Histologically, it is characterized by atypical spindleshaped keratinocytes in the dermis existing as single cells or cohesive nests along with keratin pearls (Figure 2). The atypical spindle cells may comprise the entire tumor or only a small portion. The use of immunohistochemical markers often is required to establish a definitive diagnosis. Spindle cell SCC stains positively, albeit frequently focally, for p63, p40, and high-molecular-weight cytokeratins such as cytokeratin 5/6, while S-100 protein, SOX-10, MART-1/Melan-A, and muscle-specific actin stains typically are negative. Wide local excision or MMS is recommended for treatment of these lesions.12
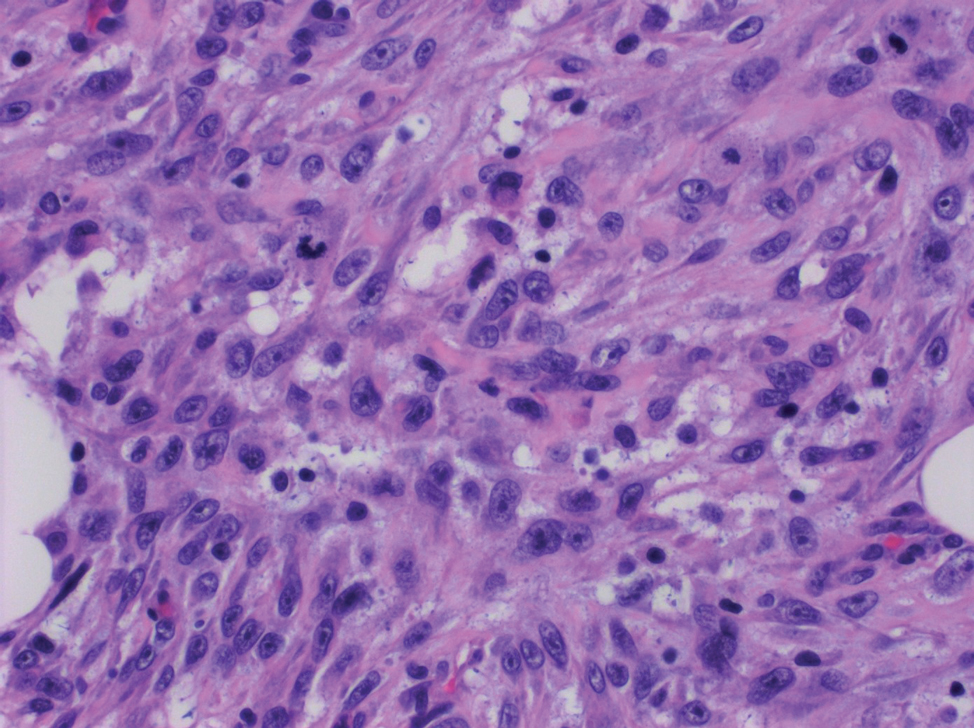
Primary cutaneous myoepithelial carcinomas are uncommon neoplasms of myoepithelial differentiation. Clinically, they often arise as soft nodular lesions on the head, neck, and lower extremities with a bimodal age distribution (50 years). Histologically cutaneous myoepithelial tumors are well-differentiated, dermal-based nodules without connection to the overlying epidermis (Figure 3). The myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show variability in cell growth patterns. One of the most common growth patterns is oval to round cells forming cords and chains in a chondromyxoid stroma. Most cases display an immunophenotyped co-expression of an epithelial cytokeratin and S-100 protein. Myoepithelial markers also may be present, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin. Surgical removal with wide local excision or MMS is essential.13
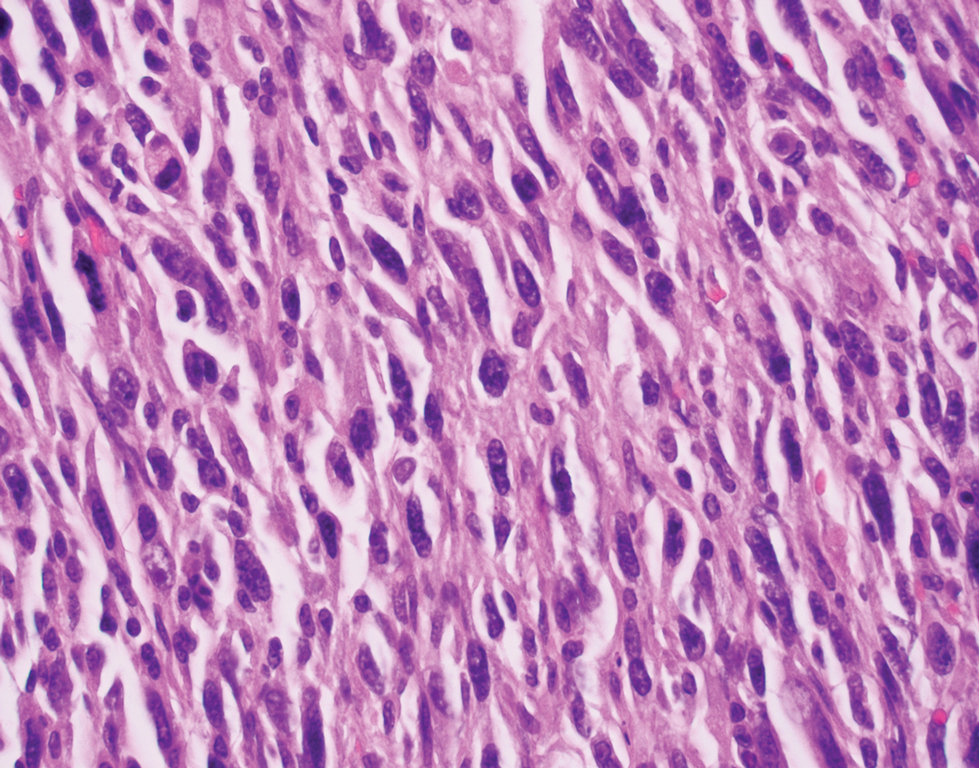
Leiomyosarcoma (LMS) is a tumor that originates from smooth muscle and rarely develops in the dermis.14 Pleomorphic LMS is a morphologic variant of LMS that has a low propensity to metastasize but commonly exhibits local recurrence.15 Leiomyosarcoma can present in any age group but most commonly manifests in individuals aged 50 to 70 years. Clinically, LMS presents as a firm solitary nodule with a smooth pink surface or a more exophytic tumor with a reddish or brown color on the extensor surface of the lower limbs; it is less common on the scalp and face.14 Histologically, most cases of pleomorphic LMS show small foci of fascicles consisting of smooth muscle tumor cells in addition to cellular pleomorphism (Figure 4).15 Many of these cells demonstrate a clear perinuclear vacuole that generally is appreciated in neoplastic smooth muscle cells.14 Pleomorphic LMS typically stains positively for at least one smooth muscle marker including desmin, h-caldesmon, muscle-specific actin, α-smooth muscle actin, or smooth muscle myosin in the leiomyosarcomatous fascicular areas.16 Complete surgical excision is the treatment of choice, and the best results are obtained with MMS.14
- Syme-Grant J, Syme-Grant NJ, Motta L, et al. Are primary cutaneous carcinosarcomas underdiagnosed? five cases and a review of the literature. J Plast Reconstr Aesthet Surg. 2006;59:1402-1408.
- Bourgeault E, Alain J, Gagne E. Primary cutaneous carcinosarcoma of the basal cell subtype should be treated as a high-risk basal cell carcinoma. J Cutan Med Surg. 2015;19:407-411.
- West L, Srivastava D. Cutaneous carcinosarcoma of the medial canthus discovered on Mohs debulk analysis. Dermatol Surg. 2019;45:1700-1702.
- Kwan JM, Satter EK. Carcinosarcoma: a primary cutaneous tumor with biphasic differentiation. Cutis. 2013;92:247-249.
- Suh KY, Lacouture M, Gerami P. p63 in primary cutaneous carcinosarcoma. Am J Dermatopathol. 2007;29:374‐377.
- Ruiz-Villaverde R, Aneiros-Fernandez J. Primary cutaneous carcinosarcoma: a cutaneous neoplasm with an exceptional presentation. Sultan Qaboos Univ Med J. 2018;18:E114-E115.
- Smart CN, Pucci RA, Binder SW, et al. Cutaneous carcinosarcoma with myoepithelial differentiation: immunohistochemical and cytogenetic analysis of a case presenting in an unusual location. Am J Dermatopathol. 2009;31:715‐717.
- Clark JJ, Bowen AR, Bowen GM, et al. Cutaneous carcinosarcoma: a series of six cases and a review of the literature. J Cutan Pathol. 2017;44:34‐44.
- Müller CS, Pföhler C, Schiekofer C, et al. Primary cutaneous carcinosarcomas: a morphological histogenetic concept revisited. Am J Dermatopathol. 2014;36:328‐339.
- Bellew S, Del Rosso JQ, Mobini N. Primary carcinosarcoma of the ear: case report and review of the literature. J Clin Aesthet Dermatol. 2009;2:33‐35.
- Hong SH, Hong SJ, Lee Y, et al. Primary cutaneous carcinosarcoma of the shoulder: case report with literature review. Dermatol Surg. 2013;39:338-340.
- Soleymani T, Aasi SZ, Novoa R, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: updates on classification and management. Dermatol Clin. 2019;37:253-259.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525.
- Johnson GE, Stevens K, Morrison AO, et al. Cutaneous myoepithelial carcinoma with disseminated metastases. Cutis. 2017;99:E19-E26.
- Llombart B, Serra-Guillén C, Requena C, et al. Leiomyosarcoma and pleomorphic dermal sarcoma: guidelines for diagnosis and treatment. Actas Dermosifiliogr. 2019;110:4-11.
- Oda Y, Miyajima K, Kawaguchi K, et al. Pleomorphic leiomyosarcoma: clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am J Surg Pathol. 2001;25:1030-1038.
The Diagnosis: Primary Cutaneous Carcinosarcoma
The immunohistochemical findings supported an epithelial component consistent with moderately differentiated squamous cell carcinoma (SCC) and a mesenchymal component with features consistent with a sarcoma. Consequently, the lesion was diagnosed as a primary cutaneous carcinosarcoma (PCCS).
Primary cutaneous carcinosarcoma is a rare biphasic neoplasm consisting of malignant epithelial (carcinoma) and mesenchymal (sarcoma) components.1 Primary cutaneous carcinosarcomas are uncommon, poorly understood, primary cutaneous tumors.2,3 Characteristic of this tumor, cytokeratins highlight the epithelial component while vimentin highlights the mesenchymal component.4 Histologically, the sarcomatous components of PCCS often are highly variable, with an absence of transitional areas within the epithelial component, which frequently resembles basal cell carcinoma and/ or SCC.5-7 Primary cutaneous carcinosarcoma favors areas of chronic UV radiation exposure, particularly on the head and neck. Most tumors present with a slowly growing, polypoid, flesh-colored to erythematous nodule due to the infiltrative mesenchymal component.7 Primary cutaneous carcinosarcoma primarily is diagnosed in elderly patients, with the majority of cases diagnosed in the eighth or ninth decades of life (range, 32–98 years).1,8 Men appear to be twice as likely to be diagnosed with a PCCS compared to women.1 Primary cutaneous carcinosarcomas are recognized as aggressive tumors with a high propensity to metastasize and recur locally, necessitating early diagnosis and treatment.4 Accurate diagnosis of PCCSs can be challenging due to the biphasic nature of the neoplasm as well as poor differentiation or unequal proportions of the epithelial and mesenchymal components.5 Additionally, overlapping diagnostic criteria coupled with vague demarcation between soft-tissue sarcomas and distinct carcinomas also may contribute to a delay in diagnosis.9 Treatment is achieved surgically by complete wide resection, with no evidence to support the use of adjuvant or neoadjuvant external beam radiation therapy. Due to the small number of reported cases, no treatment recommendations currently exist.1
Surgical management with wide local excision has been disappointing, with recurrence rates reported as high as 33%.6 Primary cutaneous carcinosarcoma has an estimated overall recurrence rate of 19% and a 5-year disease-free rate of 50%.10 Risk factors associated with poorer prognosis include tumors with adnexal subtype, age less than 65 years, rapid tumor growth, a tumor greater than 20 mm at presentation, and a long-standing tumor lasting up to 30 years.2,4 Although wide local excision and Mohs micrographic surgery (MMS) both have been utilized successfully, MMS has been shown to result in a cure rate of greater than 98%.6
Atypical fibroxanthoma (AFX) is a cutaneous tumor of fibrohistiocytic mesenchymal origin that typically manifests on sun-damaged skin in elderly individuals. Clinically, it presents as a rapidly growing neoplasm that often ulcerates and bleeds. These heterogenous neoplasms have several distinct characteristics, including dense cellularity with disorganized, large, pleomorphic, and atypical-appearing spindle-shaped cells arising in the upper layers of the dermis, often disseminating into the reticular dermis and occasionally into the subcutaneous fat (Figure 1). The neoplastic cells often exhibit hyperchromic and irregular nuclei, multinucleated giant cells, and atypical mitotic figures. In most cases, negative immunohistochemical staining with SOX-10, S-100, cytokeratins, desmin, and caldesmon will allow pathologists to differentiate between AFX and other common tumors on the differential diagnosis, such as SCC, melanoma, and leiomyosarcoma. CD10 and procollagen type 1 are positive antigenic markers in AFX, but they are not specific. The standard treatment of AFX includes wide local excision or MMS for superior margin control.11

Spindle cell SCC presents as a raised or exophytic nodule, often with spontaneous bleeding and central ulceration. It usually presents on sun-damaged skin or in individuals with a history of ionizing radiation. Histologically, it is characterized by atypical spindleshaped keratinocytes in the dermis existing as single cells or cohesive nests along with keratin pearls (Figure 2). The atypical spindle cells may comprise the entire tumor or only a small portion. The use of immunohistochemical markers often is required to establish a definitive diagnosis. Spindle cell SCC stains positively, albeit frequently focally, for p63, p40, and high-molecular-weight cytokeratins such as cytokeratin 5/6, while S-100 protein, SOX-10, MART-1/Melan-A, and muscle-specific actin stains typically are negative. Wide local excision or MMS is recommended for treatment of these lesions.12

Primary cutaneous myoepithelial carcinomas are uncommon neoplasms of myoepithelial differentiation. Clinically, they often arise as soft nodular lesions on the head, neck, and lower extremities with a bimodal age distribution (50 years). Histologically cutaneous myoepithelial tumors are well-differentiated, dermal-based nodules without connection to the overlying epidermis (Figure 3). The myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show variability in cell growth patterns. One of the most common growth patterns is oval to round cells forming cords and chains in a chondromyxoid stroma. Most cases display an immunophenotyped co-expression of an epithelial cytokeratin and S-100 protein. Myoepithelial markers also may be present, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin. Surgical removal with wide local excision or MMS is essential.13

Leiomyosarcoma (LMS) is a tumor that originates from smooth muscle and rarely develops in the dermis.14 Pleomorphic LMS is a morphologic variant of LMS that has a low propensity to metastasize but commonly exhibits local recurrence.15 Leiomyosarcoma can present in any age group but most commonly manifests in individuals aged 50 to 70 years. Clinically, LMS presents as a firm solitary nodule with a smooth pink surface or a more exophytic tumor with a reddish or brown color on the extensor surface of the lower limbs; it is less common on the scalp and face.14 Histologically, most cases of pleomorphic LMS show small foci of fascicles consisting of smooth muscle tumor cells in addition to cellular pleomorphism (Figure 4).15 Many of these cells demonstrate a clear perinuclear vacuole that generally is appreciated in neoplastic smooth muscle cells.14 Pleomorphic LMS typically stains positively for at least one smooth muscle marker including desmin, h-caldesmon, muscle-specific actin, α-smooth muscle actin, or smooth muscle myosin in the leiomyosarcomatous fascicular areas.16 Complete surgical excision is the treatment of choice, and the best results are obtained with MMS.14

The Diagnosis: Primary Cutaneous Carcinosarcoma
The immunohistochemical findings supported an epithelial component consistent with moderately differentiated squamous cell carcinoma (SCC) and a mesenchymal component with features consistent with a sarcoma. Consequently, the lesion was diagnosed as a primary cutaneous carcinosarcoma (PCCS).
Primary cutaneous carcinosarcoma is a rare biphasic neoplasm consisting of malignant epithelial (carcinoma) and mesenchymal (sarcoma) components.1 Primary cutaneous carcinosarcomas are uncommon, poorly understood, primary cutaneous tumors.2,3 Characteristic of this tumor, cytokeratins highlight the epithelial component while vimentin highlights the mesenchymal component.4 Histologically, the sarcomatous components of PCCS often are highly variable, with an absence of transitional areas within the epithelial component, which frequently resembles basal cell carcinoma and/ or SCC.5-7 Primary cutaneous carcinosarcoma favors areas of chronic UV radiation exposure, particularly on the head and neck. Most tumors present with a slowly growing, polypoid, flesh-colored to erythematous nodule due to the infiltrative mesenchymal component.7 Primary cutaneous carcinosarcoma primarily is diagnosed in elderly patients, with the majority of cases diagnosed in the eighth or ninth decades of life (range, 32–98 years).1,8 Men appear to be twice as likely to be diagnosed with a PCCS compared to women.1 Primary cutaneous carcinosarcomas are recognized as aggressive tumors with a high propensity to metastasize and recur locally, necessitating early diagnosis and treatment.4 Accurate diagnosis of PCCSs can be challenging due to the biphasic nature of the neoplasm as well as poor differentiation or unequal proportions of the epithelial and mesenchymal components.5 Additionally, overlapping diagnostic criteria coupled with vague demarcation between soft-tissue sarcomas and distinct carcinomas also may contribute to a delay in diagnosis.9 Treatment is achieved surgically by complete wide resection, with no evidence to support the use of adjuvant or neoadjuvant external beam radiation therapy. Due to the small number of reported cases, no treatment recommendations currently exist.1
Surgical management with wide local excision has been disappointing, with recurrence rates reported as high as 33%.6 Primary cutaneous carcinosarcoma has an estimated overall recurrence rate of 19% and a 5-year disease-free rate of 50%.10 Risk factors associated with poorer prognosis include tumors with adnexal subtype, age less than 65 years, rapid tumor growth, a tumor greater than 20 mm at presentation, and a long-standing tumor lasting up to 30 years.2,4 Although wide local excision and Mohs micrographic surgery (MMS) both have been utilized successfully, MMS has been shown to result in a cure rate of greater than 98%.6
Atypical fibroxanthoma (AFX) is a cutaneous tumor of fibrohistiocytic mesenchymal origin that typically manifests on sun-damaged skin in elderly individuals. Clinically, it presents as a rapidly growing neoplasm that often ulcerates and bleeds. These heterogenous neoplasms have several distinct characteristics, including dense cellularity with disorganized, large, pleomorphic, and atypical-appearing spindle-shaped cells arising in the upper layers of the dermis, often disseminating into the reticular dermis and occasionally into the subcutaneous fat (Figure 1). The neoplastic cells often exhibit hyperchromic and irregular nuclei, multinucleated giant cells, and atypical mitotic figures. In most cases, negative immunohistochemical staining with SOX-10, S-100, cytokeratins, desmin, and caldesmon will allow pathologists to differentiate between AFX and other common tumors on the differential diagnosis, such as SCC, melanoma, and leiomyosarcoma. CD10 and procollagen type 1 are positive antigenic markers in AFX, but they are not specific. The standard treatment of AFX includes wide local excision or MMS for superior margin control.11

Spindle cell SCC presents as a raised or exophytic nodule, often with spontaneous bleeding and central ulceration. It usually presents on sun-damaged skin or in individuals with a history of ionizing radiation. Histologically, it is characterized by atypical spindleshaped keratinocytes in the dermis existing as single cells or cohesive nests along with keratin pearls (Figure 2). The atypical spindle cells may comprise the entire tumor or only a small portion. The use of immunohistochemical markers often is required to establish a definitive diagnosis. Spindle cell SCC stains positively, albeit frequently focally, for p63, p40, and high-molecular-weight cytokeratins such as cytokeratin 5/6, while S-100 protein, SOX-10, MART-1/Melan-A, and muscle-specific actin stains typically are negative. Wide local excision or MMS is recommended for treatment of these lesions.12

Primary cutaneous myoepithelial carcinomas are uncommon neoplasms of myoepithelial differentiation. Clinically, they often arise as soft nodular lesions on the head, neck, and lower extremities with a bimodal age distribution (50 years). Histologically cutaneous myoepithelial tumors are well-differentiated, dermal-based nodules without connection to the overlying epidermis (Figure 3). The myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show variability in cell growth patterns. One of the most common growth patterns is oval to round cells forming cords and chains in a chondromyxoid stroma. Most cases display an immunophenotyped co-expression of an epithelial cytokeratin and S-100 protein. Myoepithelial markers also may be present, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin. Surgical removal with wide local excision or MMS is essential.13

Leiomyosarcoma (LMS) is a tumor that originates from smooth muscle and rarely develops in the dermis.14 Pleomorphic LMS is a morphologic variant of LMS that has a low propensity to metastasize but commonly exhibits local recurrence.15 Leiomyosarcoma can present in any age group but most commonly manifests in individuals aged 50 to 70 years. Clinically, LMS presents as a firm solitary nodule with a smooth pink surface or a more exophytic tumor with a reddish or brown color on the extensor surface of the lower limbs; it is less common on the scalp and face.14 Histologically, most cases of pleomorphic LMS show small foci of fascicles consisting of smooth muscle tumor cells in addition to cellular pleomorphism (Figure 4).15 Many of these cells demonstrate a clear perinuclear vacuole that generally is appreciated in neoplastic smooth muscle cells.14 Pleomorphic LMS typically stains positively for at least one smooth muscle marker including desmin, h-caldesmon, muscle-specific actin, α-smooth muscle actin, or smooth muscle myosin in the leiomyosarcomatous fascicular areas.16 Complete surgical excision is the treatment of choice, and the best results are obtained with MMS.14

- Syme-Grant J, Syme-Grant NJ, Motta L, et al. Are primary cutaneous carcinosarcomas underdiagnosed? five cases and a review of the literature. J Plast Reconstr Aesthet Surg. 2006;59:1402-1408.
- Bourgeault E, Alain J, Gagne E. Primary cutaneous carcinosarcoma of the basal cell subtype should be treated as a high-risk basal cell carcinoma. J Cutan Med Surg. 2015;19:407-411.
- West L, Srivastava D. Cutaneous carcinosarcoma of the medial canthus discovered on Mohs debulk analysis. Dermatol Surg. 2019;45:1700-1702.
- Kwan JM, Satter EK. Carcinosarcoma: a primary cutaneous tumor with biphasic differentiation. Cutis. 2013;92:247-249.
- Suh KY, Lacouture M, Gerami P. p63 in primary cutaneous carcinosarcoma. Am J Dermatopathol. 2007;29:374‐377.
- Ruiz-Villaverde R, Aneiros-Fernandez J. Primary cutaneous carcinosarcoma: a cutaneous neoplasm with an exceptional presentation. Sultan Qaboos Univ Med J. 2018;18:E114-E115.
- Smart CN, Pucci RA, Binder SW, et al. Cutaneous carcinosarcoma with myoepithelial differentiation: immunohistochemical and cytogenetic analysis of a case presenting in an unusual location. Am J Dermatopathol. 2009;31:715‐717.
- Clark JJ, Bowen AR, Bowen GM, et al. Cutaneous carcinosarcoma: a series of six cases and a review of the literature. J Cutan Pathol. 2017;44:34‐44.
- Müller CS, Pföhler C, Schiekofer C, et al. Primary cutaneous carcinosarcomas: a morphological histogenetic concept revisited. Am J Dermatopathol. 2014;36:328‐339.
- Bellew S, Del Rosso JQ, Mobini N. Primary carcinosarcoma of the ear: case report and review of the literature. J Clin Aesthet Dermatol. 2009;2:33‐35.
- Hong SH, Hong SJ, Lee Y, et al. Primary cutaneous carcinosarcoma of the shoulder: case report with literature review. Dermatol Surg. 2013;39:338-340.
- Soleymani T, Aasi SZ, Novoa R, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: updates on classification and management. Dermatol Clin. 2019;37:253-259.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525.
- Johnson GE, Stevens K, Morrison AO, et al. Cutaneous myoepithelial carcinoma with disseminated metastases. Cutis. 2017;99:E19-E26.
- Llombart B, Serra-Guillén C, Requena C, et al. Leiomyosarcoma and pleomorphic dermal sarcoma: guidelines for diagnosis and treatment. Actas Dermosifiliogr. 2019;110:4-11.
- Oda Y, Miyajima K, Kawaguchi K, et al. Pleomorphic leiomyosarcoma: clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am J Surg Pathol. 2001;25:1030-1038.
- Syme-Grant J, Syme-Grant NJ, Motta L, et al. Are primary cutaneous carcinosarcomas underdiagnosed? five cases and a review of the literature. J Plast Reconstr Aesthet Surg. 2006;59:1402-1408.
- Bourgeault E, Alain J, Gagne E. Primary cutaneous carcinosarcoma of the basal cell subtype should be treated as a high-risk basal cell carcinoma. J Cutan Med Surg. 2015;19:407-411.
- West L, Srivastava D. Cutaneous carcinosarcoma of the medial canthus discovered on Mohs debulk analysis. Dermatol Surg. 2019;45:1700-1702.
- Kwan JM, Satter EK. Carcinosarcoma: a primary cutaneous tumor with biphasic differentiation. Cutis. 2013;92:247-249.
- Suh KY, Lacouture M, Gerami P. p63 in primary cutaneous carcinosarcoma. Am J Dermatopathol. 2007;29:374‐377.
- Ruiz-Villaverde R, Aneiros-Fernandez J. Primary cutaneous carcinosarcoma: a cutaneous neoplasm with an exceptional presentation. Sultan Qaboos Univ Med J. 2018;18:E114-E115.
- Smart CN, Pucci RA, Binder SW, et al. Cutaneous carcinosarcoma with myoepithelial differentiation: immunohistochemical and cytogenetic analysis of a case presenting in an unusual location. Am J Dermatopathol. 2009;31:715‐717.
- Clark JJ, Bowen AR, Bowen GM, et al. Cutaneous carcinosarcoma: a series of six cases and a review of the literature. J Cutan Pathol. 2017;44:34‐44.
- Müller CS, Pföhler C, Schiekofer C, et al. Primary cutaneous carcinosarcomas: a morphological histogenetic concept revisited. Am J Dermatopathol. 2014;36:328‐339.
- Bellew S, Del Rosso JQ, Mobini N. Primary carcinosarcoma of the ear: case report and review of the literature. J Clin Aesthet Dermatol. 2009;2:33‐35.
- Hong SH, Hong SJ, Lee Y, et al. Primary cutaneous carcinosarcoma of the shoulder: case report with literature review. Dermatol Surg. 2013;39:338-340.
- Soleymani T, Aasi SZ, Novoa R, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: updates on classification and management. Dermatol Clin. 2019;37:253-259.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525.
- Johnson GE, Stevens K, Morrison AO, et al. Cutaneous myoepithelial carcinoma with disseminated metastases. Cutis. 2017;99:E19-E26.
- Llombart B, Serra-Guillén C, Requena C, et al. Leiomyosarcoma and pleomorphic dermal sarcoma: guidelines for diagnosis and treatment. Actas Dermosifiliogr. 2019;110:4-11.
- Oda Y, Miyajima K, Kawaguchi K, et al. Pleomorphic leiomyosarcoma: clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am J Surg Pathol. 2001;25:1030-1038.
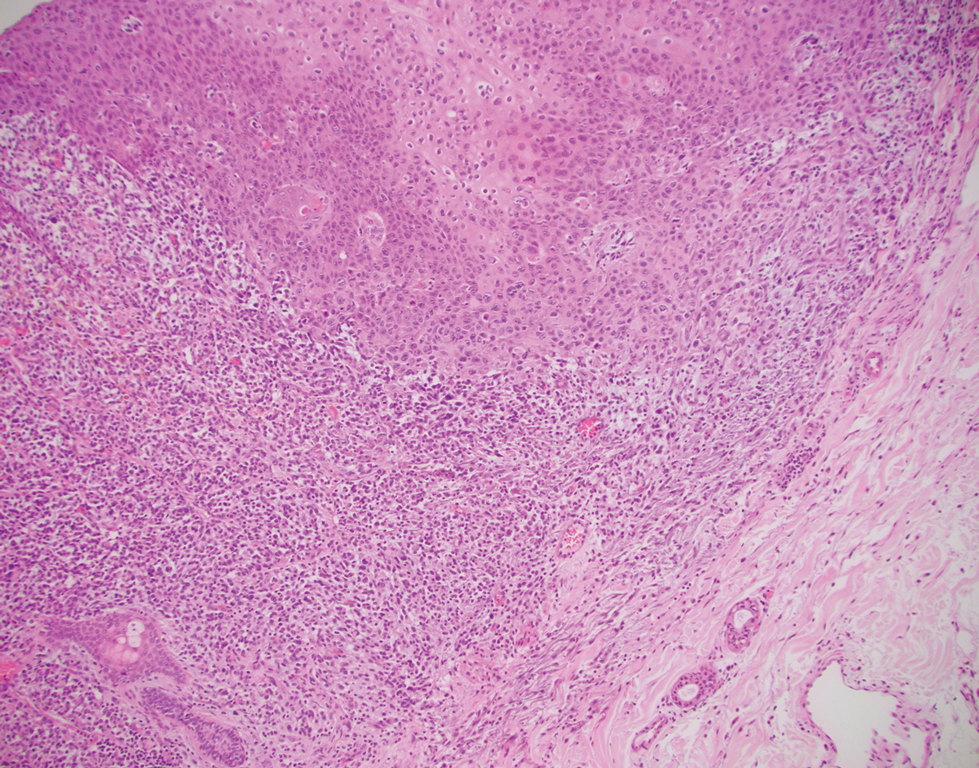
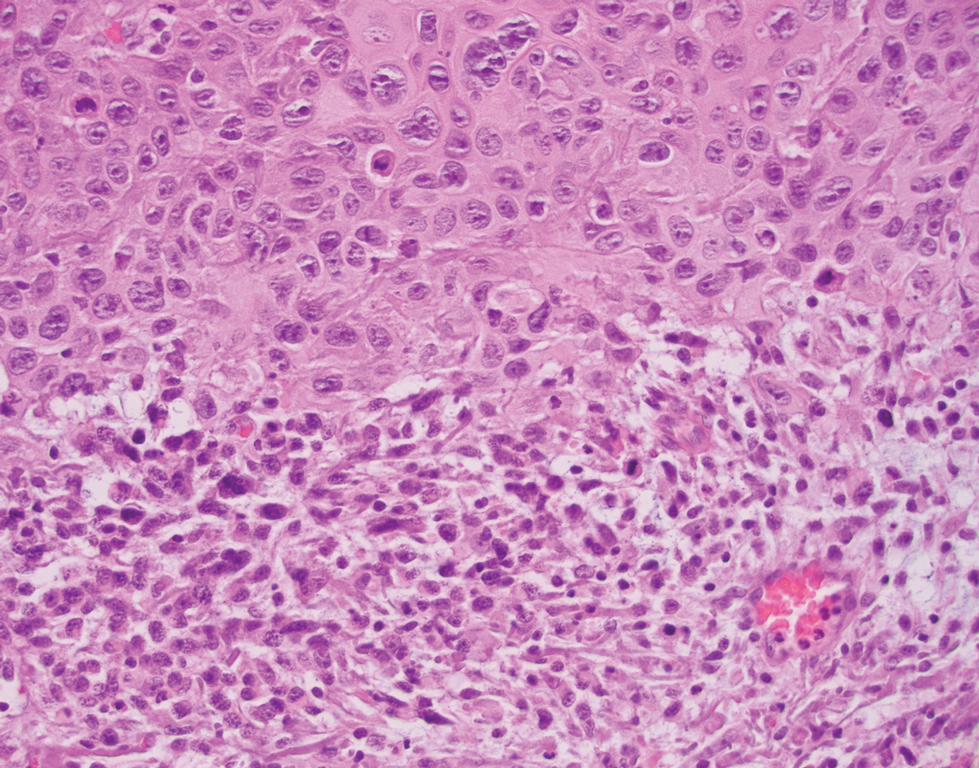
A 72-year-old man with a history of nonmelanoma skin cancer and lung transplant maintained on stable doses of prednisone and tacrolimus presented with a 1.3×1.8-cm, slow-growing, well-demarcated, ulcerated, erythematous plaque with overlying serous crust on the left temple of 6 months’ duration. No cervical or axillary lymphadenopathy was appreciated on physical examination. A biopsy was performed followed by Mohs micrographic surgery. Microscopic examination of the debulking specimen revealed atypical spindle cells in the papillary and reticular dermis radiating from a central focus of a moderately differentiated squamous cell carcinoma. The squamous cells stained positive for cytokeratin 5/6, pankeratin, and p40, while the spindle cells stained positive only for vimentin.
Communication Strategies in Mohs Micrographic Surgery: A Survey of Methods, Time Savings, and Perceived Patient Satisfaction
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.
Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.
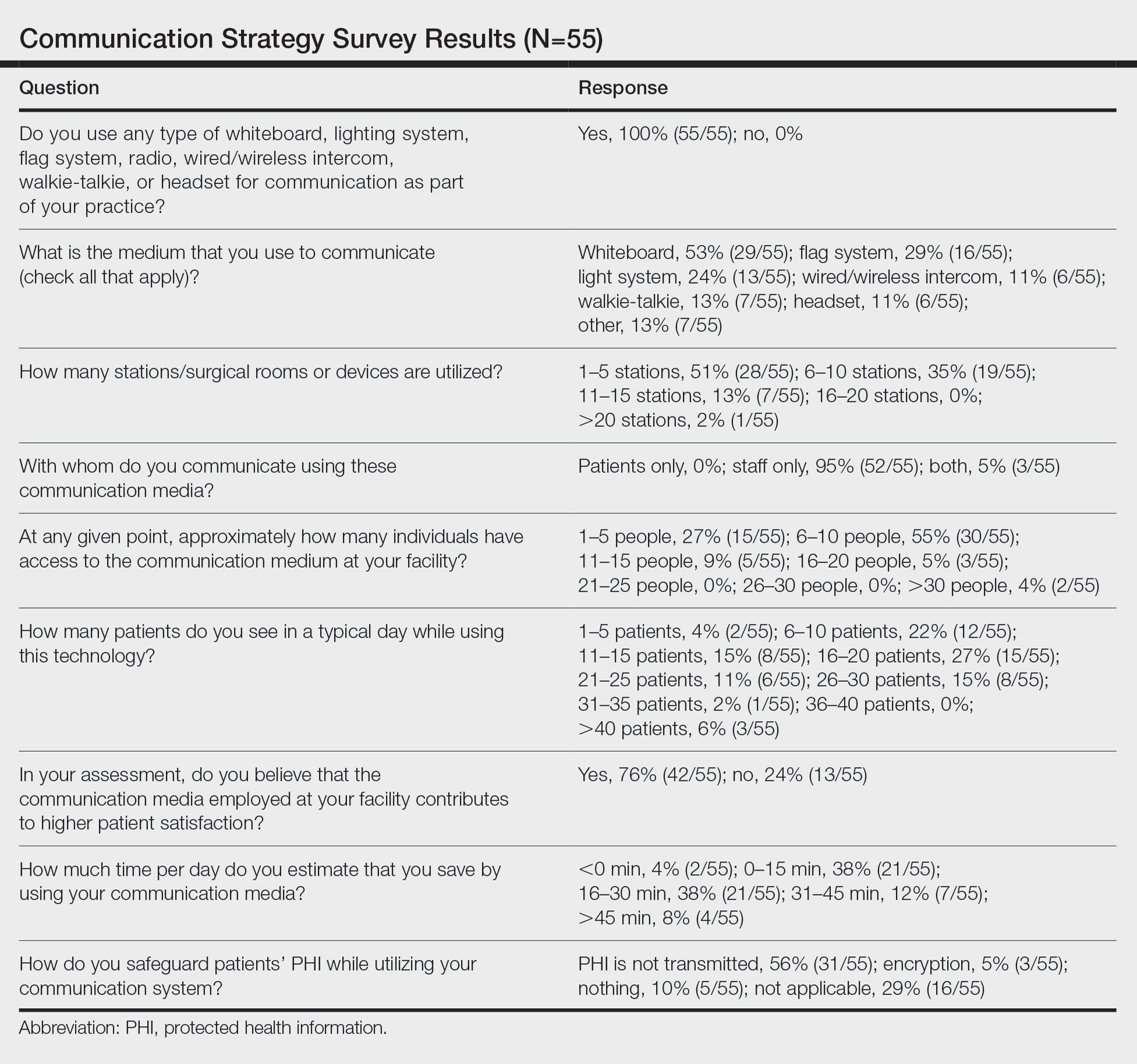
Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.

Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.

Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.

Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.

Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
Practice Points
- There are limited studies evaluating the efficacy of different communication methods in Mohs micrographic surgery (MMS) clinics.
- This study suggests that incorporation of a light-based system into an MMS clinic improves workplace efficiency.
Guarding against nonmelanoma skin cancer in solid organ transplant recipients
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.
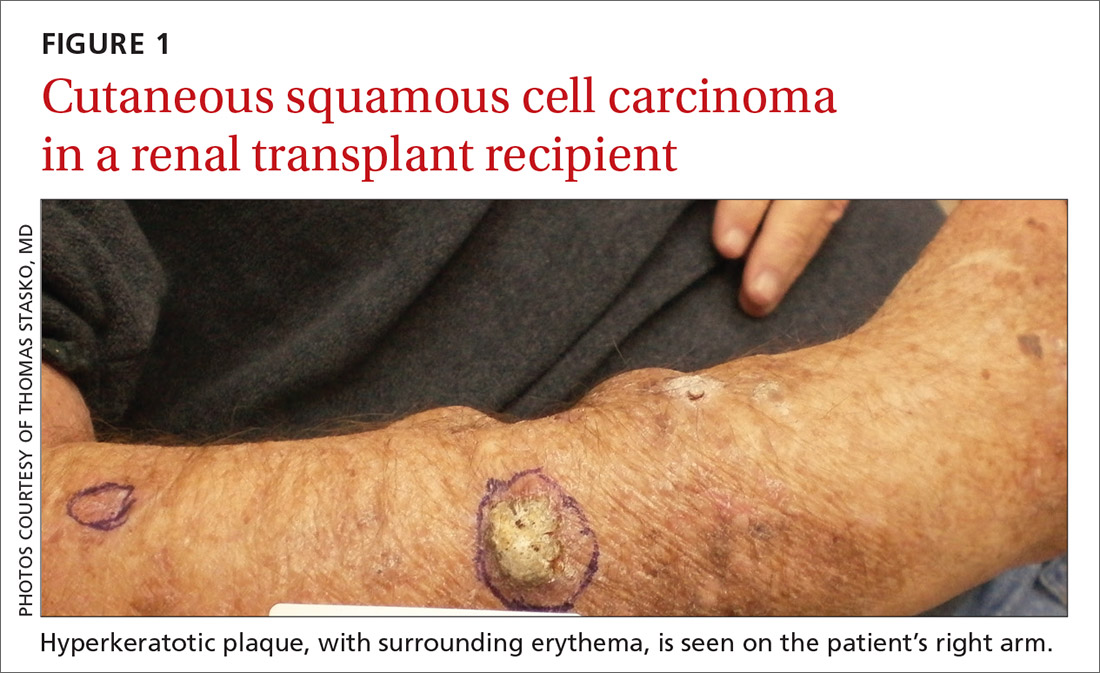
NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8
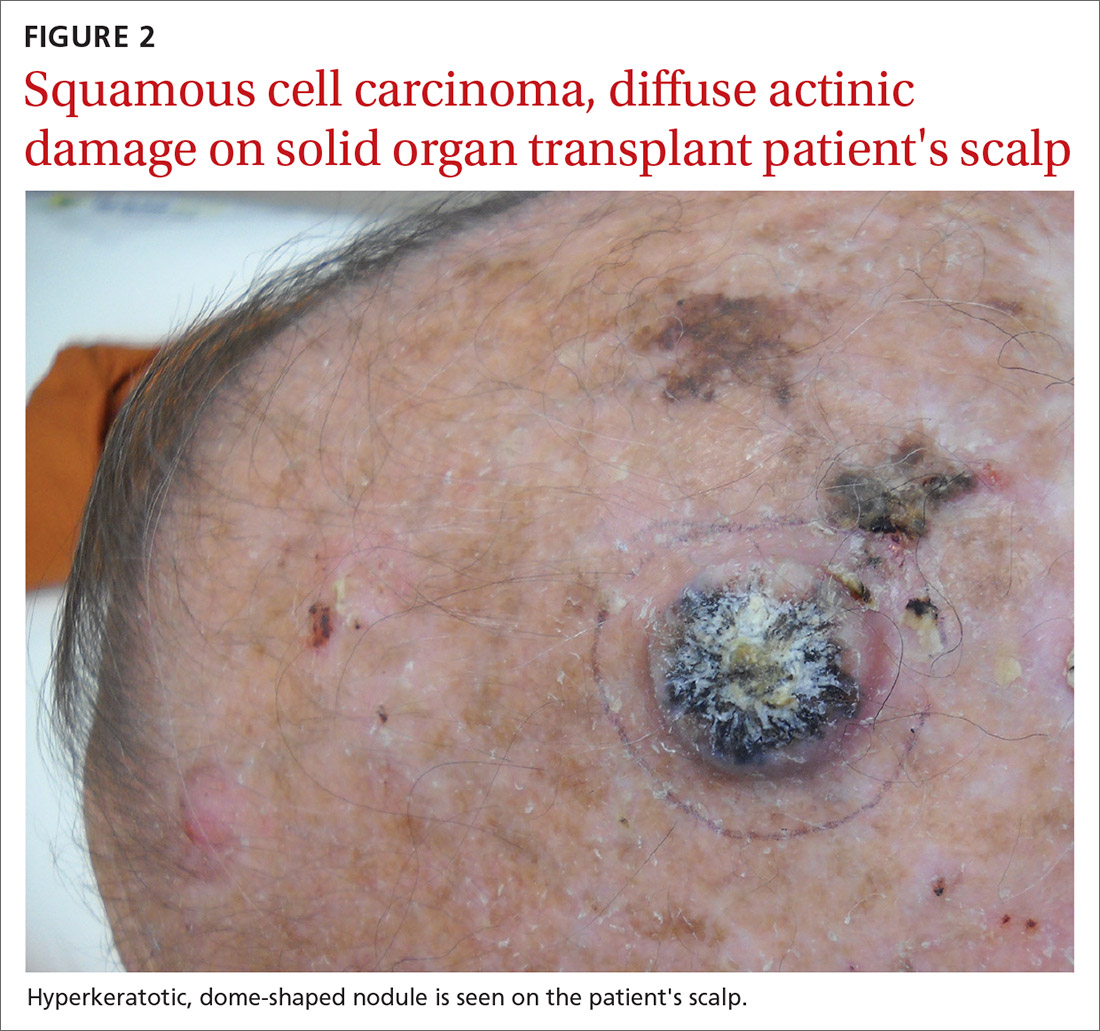
The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22
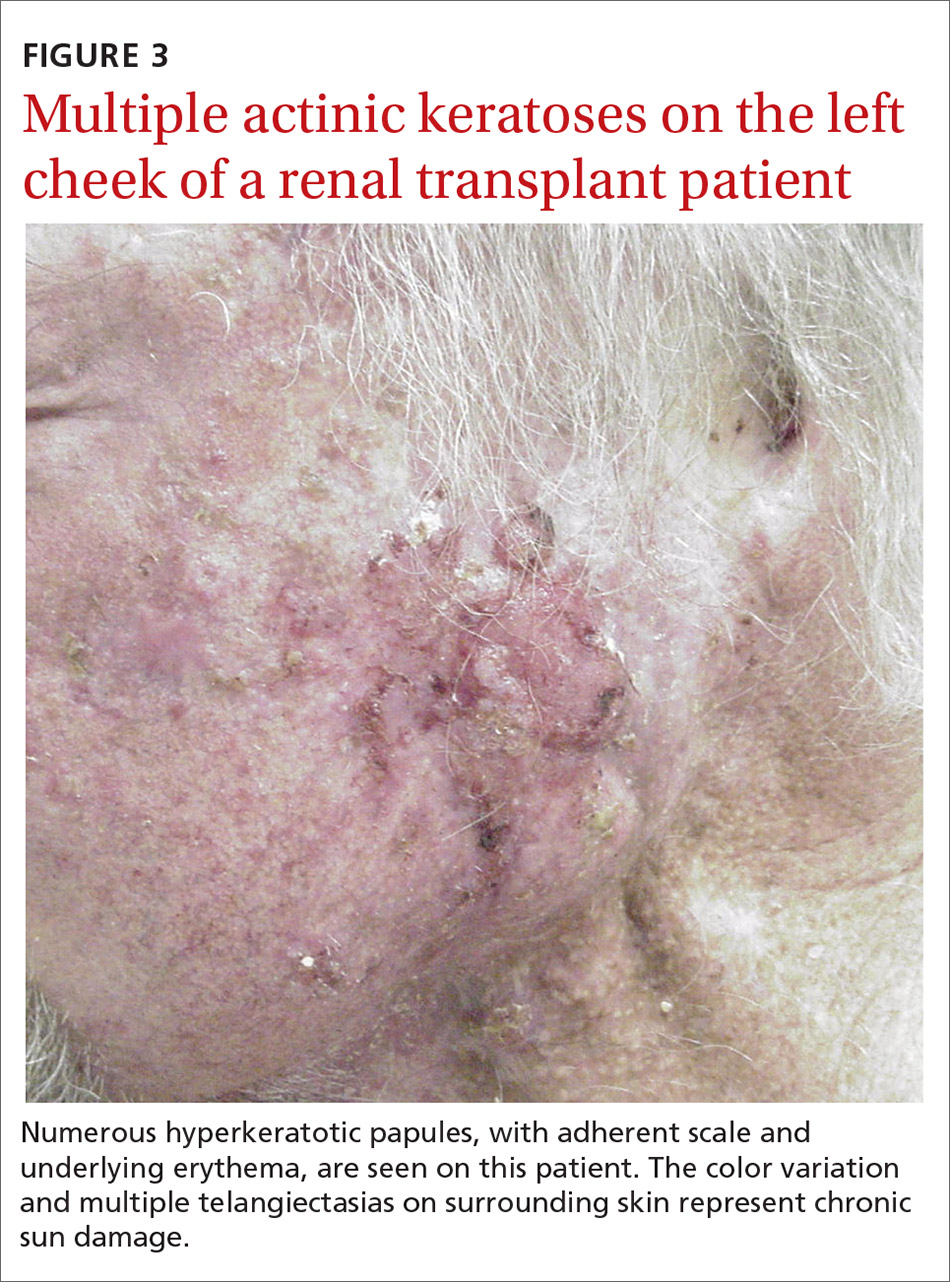
Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15
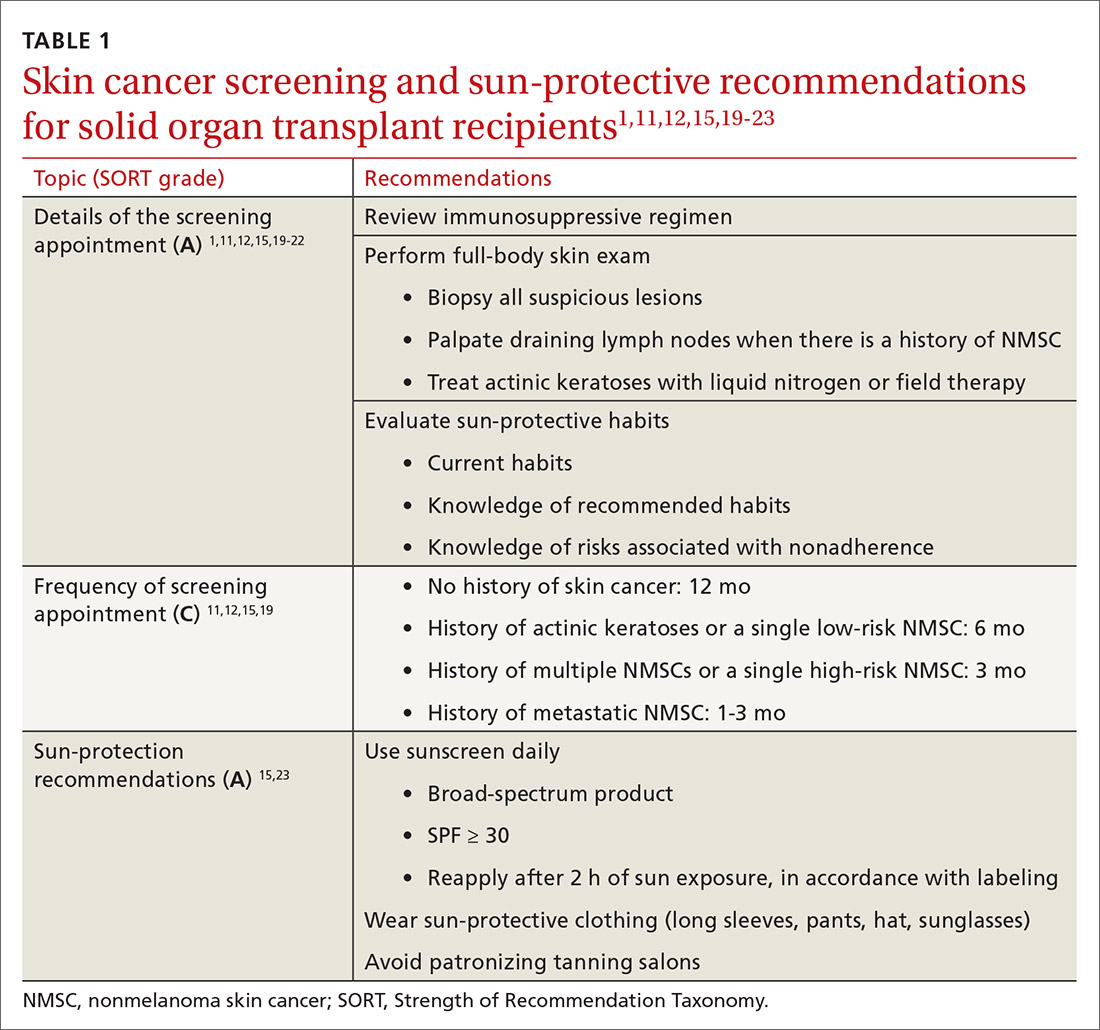
SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27
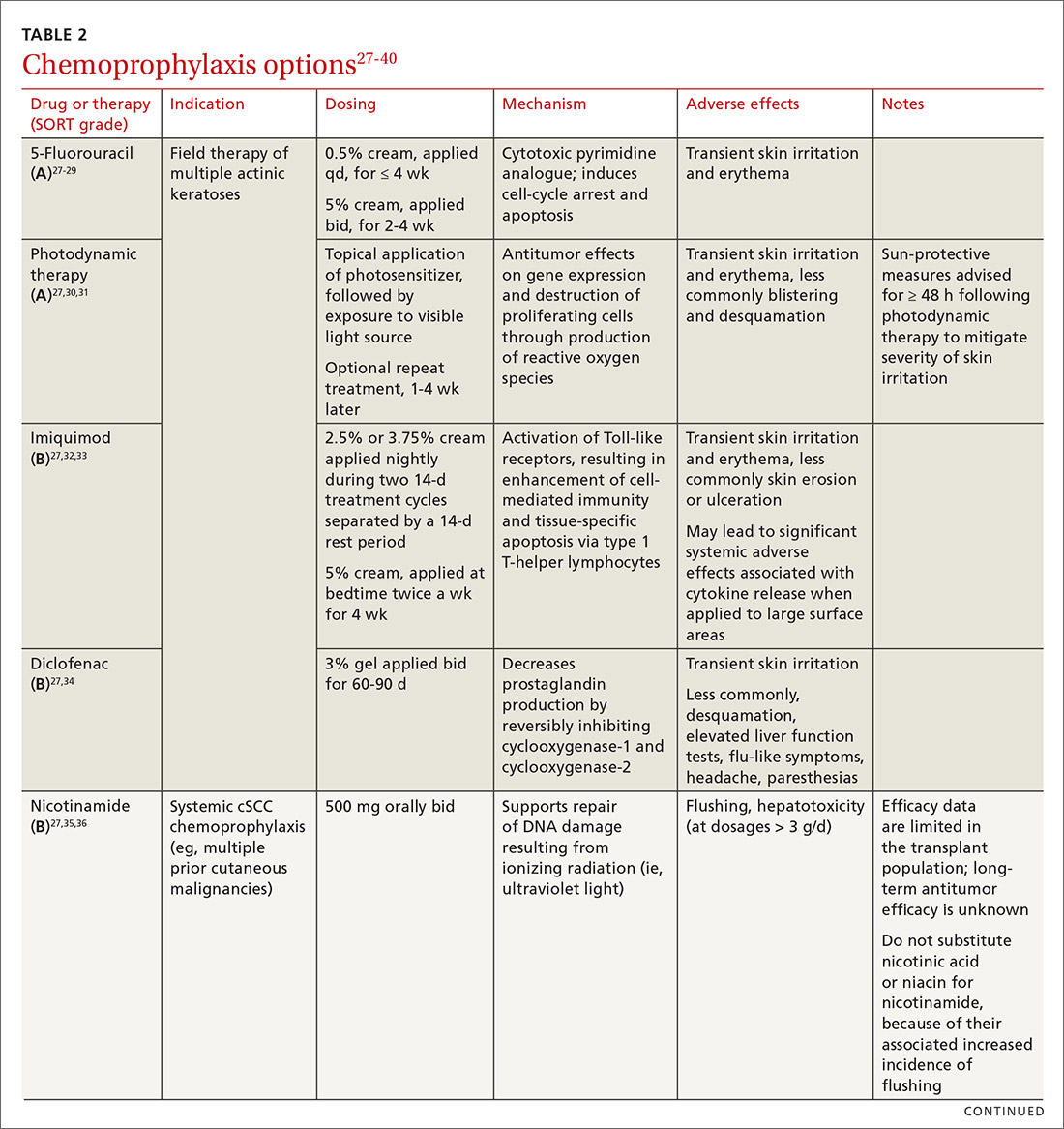
5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27
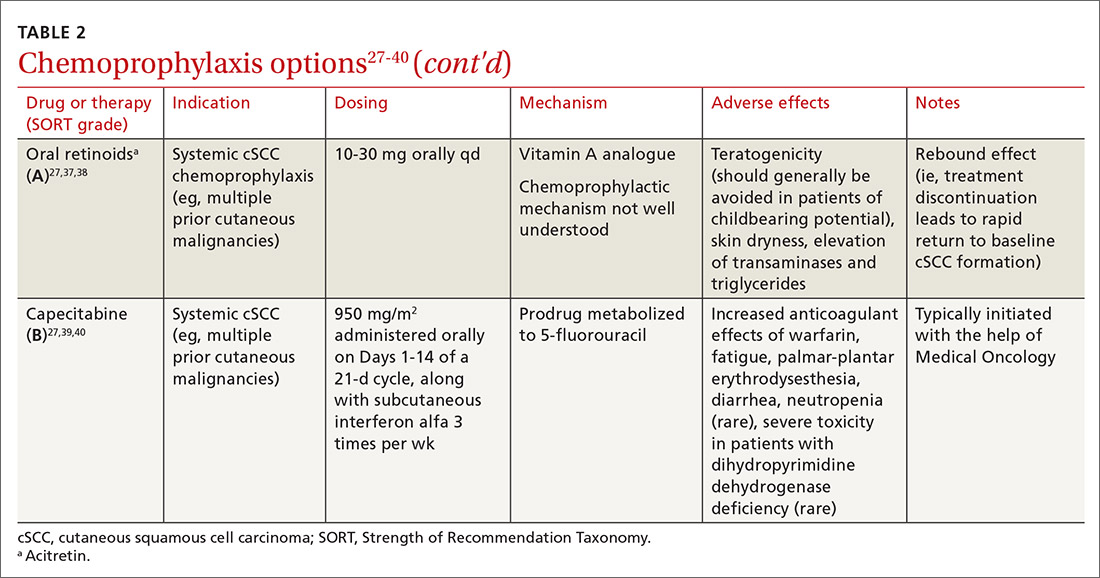
PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3
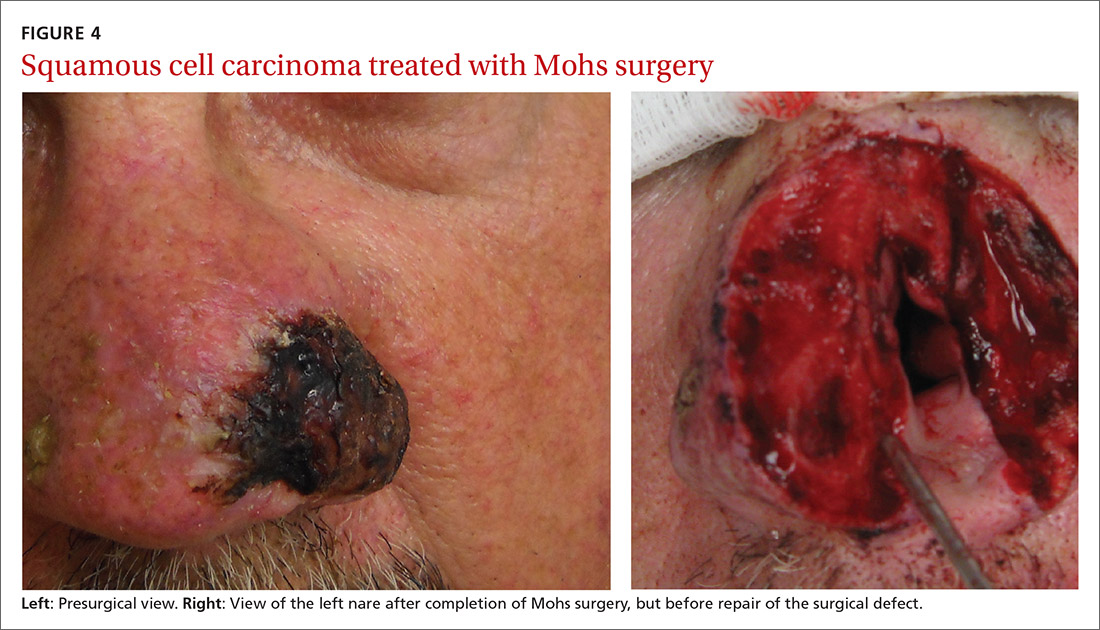
Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)
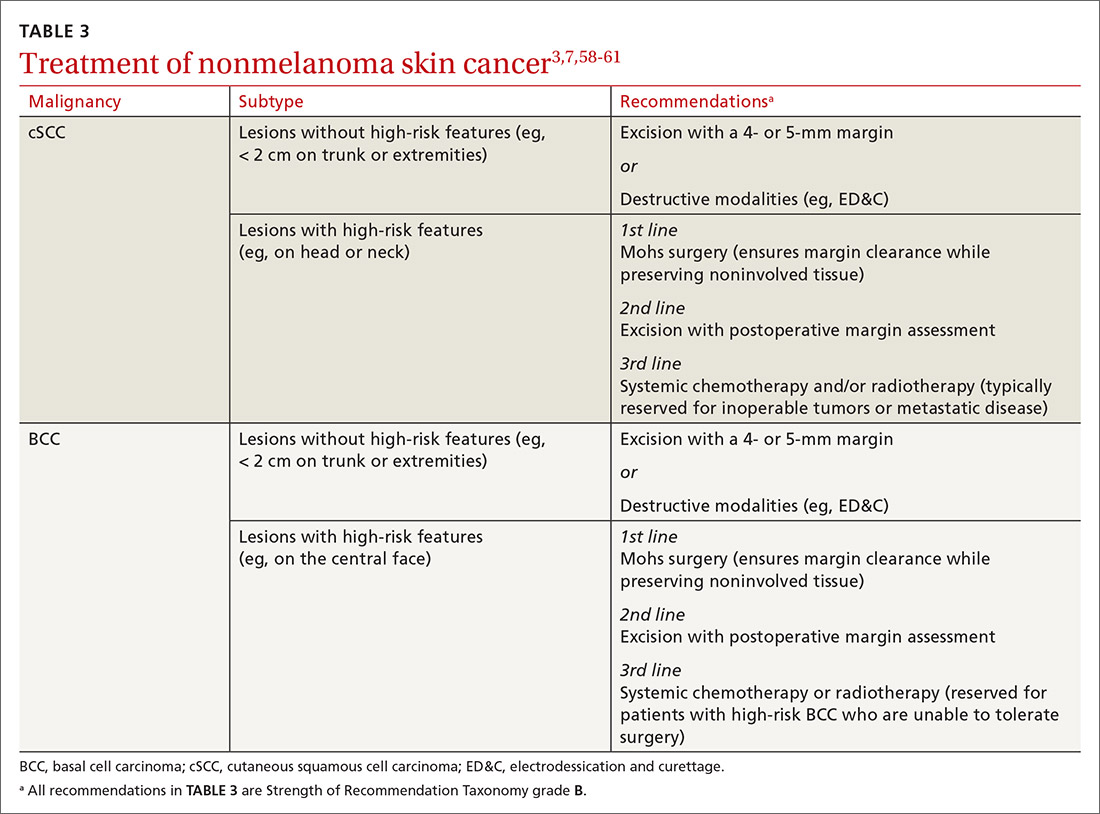
Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; [email protected]
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.

NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8

The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22

Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15

SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27

5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27

PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3

Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)

Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; [email protected]
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.

NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8

The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22

Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15

SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27

5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27

PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3

Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)

Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; [email protected]
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
PRACTICE RECOMMENDATIONS
› Conduct a full-body skin examination at least once annually for solid organ transplant recipients. C
› Encourage daily use of broad-spectrum SPF ≥ 30 sunscreen and sun-protective clothing (long sleeves, pants, wide-brimmed hats) for these patients. A
› Consider chemoprophylactic agents for patients at especially high risk of nonmelanoma skin cancer. A
› Treat nonmelanoma skin cancer in a solid organ transplant recipient aggressively because of their increased risk of recurrence, local invasion, and metastasis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series