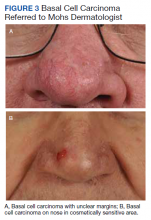
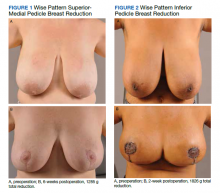
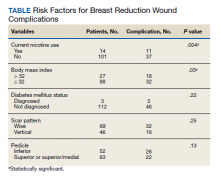
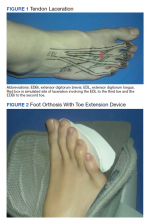
User login
Acquired Factor VIII Deficiency Presenting as Compartment Syndrome
Compartment syndrome occurs when the interstitial tissue pressures within a confined space are elevated to a level at which the arterial perfusion is diminished. Multiple etiologies exist and can be extrinsic (a cast that is too tight or prolonged compression on a limb), iatrogenic (aggressive resuscitation, drug infiltration, arterial puncture, or a spontaneous bleed from anticoagulation), and traumatic (fracture, snake envenomation, circumferential burn, or electrocution). If the compartments are not released, irreversible changes happen to the cells, including nerve and muscle death.1 Definitive management of this emergency requires prompt fasciotomy to decompress the compartment(s).1-3
Case Presentation
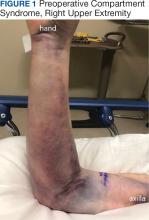
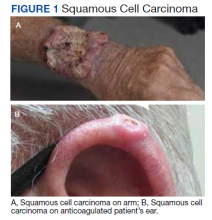
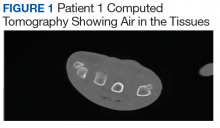
A 76-year-old right-handed woman with a history of chronic obstructive pulmonary disease, hypertension, and hyperlipidemia presented to the emergency department with 2 days of extensive right upper extremity ecchymosis and severe pain that was localized to her forearm (Figure 1). She was taking low-dose aspirin (81 mg/d) for left subclavian stenosis and over-the-counter ginkgo biloba. Leading up to the presentation, the patient was able to perform routine household chores, including yard work, cleaning, and taking care of her cats. Wrist and elbow X-rays were negative for a fracture. An upper extremity ultrasound found no venous occlusion. A computed tomography (CT) angiogram of her arm and chest found diffuse edema around the right elbow and forearm without pulmonary or right upper extremity emboli, fractures, hematoma, abscess, or air in the tissues.
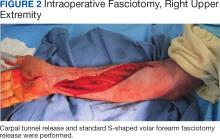
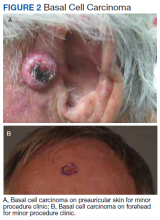
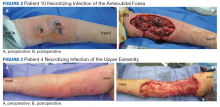
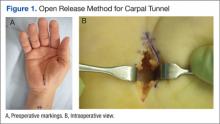
The plastic surgery service was consulted. The patient was found to have a very tense forearm and pain to passive digital extension. The 2-point discrimination and pulses were intact. The patient was diagnosed with compartment syndrome based on the examination alone and gave consent for an emergent forearm and hand fasciotomy. A carpal tunnel release and a standard S-shaped volar forearm fasciotomy release were performed, which provided immediate decompression (Figure 2). The rest of the hand and extremity were soft. Edematous, healthy flexor muscle belly was identified without a hematoma. Most of the forearm wound was left open because the skin could not be reapproximated. Oxidized regenerated cellulose (Surgicel) was placed around the wound edges and the muscle was covered with a nonadherent dressing. Hemoglobin on admission was 12.9 g/dL(reference range, 12 to 16 g/dL). Kidney function was within normal limits. The rest of the complete blood count was unremarkable. Postoperative hemoglobin was 8.6 g/dL. Over the next several days, the patient's skin edges and muscle bellies continued to slowly bleed, and her hemoglobin fell to 5.6 g/dL by postoperative Day 2. The bleeding was managed with topical oxidized regenerated cellulose, thrombin spray, a hemostatic dressing made with kaolin (QuikClot), and a transfusion of 2 units of packed red blood cells.
A hematology consultation was requested. The patient was noted to have an elevated partial thromboplastin time (PTT) since admission measuring between 39.9 to 61.7 seconds (reference range, 26.2 to 37.2 seconds) and a normal prothrombin time test with an international normalized ratio. A PTT measured 17 months prior to admission was within the normal range. She reported no personal or family history of bleeding disorders. Until recently, she had never had easy bruisability. She reported no history of heavy menses or epistaxis. The patient had no children and had never been pregnant. She had tolerated an exploratory laparotomy 40 years prior to admission without bleeding complications and had never required blood transfusions before. A PTT 1:1 mixing study revealed incomplete correction. Subsequent workup included factor VIII (FVIII) activity, factor IX activity, factor XI activity, von Willebrand factor antigen, ristocetin cofactor assay, and von Willebrand factor multimers. FVIII activity was severely reduced at 7.8% (reference, > 54%) with a positive Bethesda assay of 300 to 400 Bodansky units (BU), indicating a strong FVIII inhibitor was present and establishing a diagnosis of acquired hemophilia A. Further workup for secondary causes of acquired hemophilia A including abdominal and pelvic CT, serum protein electrophoresis, and serum free light chains, were negative. She was started on prednisone 1 mg/kg daily and rituximab 375 mg/m2. Her hemoglobin stabilized, and she required no further blood transfusions.
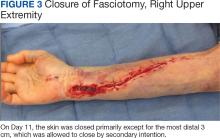
The patient underwent wound closure on postoperative Day 11. At the time of the second surgery, there was still no improvement in her FVIII levels or PTT; therefore, 70 mcg/kg of recombinant coagulation-activated FVII was given just before surgery with no bleeding complications. The skin was closed primarily except for the most distal 3 cm (Figure 3). Due to concerns regarding further bleeding with skin graft, the remaining wound was allowed to close by secondary intention. As a precaution, the wound was covered with oxidized regenerated cellulose and thrombin spray. The patient continued to progress postoperatively without bleeding complications or a need for additional transfusions. She was seen by the hand therapist before and after the second surgery to help with edema management and joint mobility. She completed 4 weekly doses of 375 mg/m² rituximab and prednisone was tapered by 10 mg weekly.
Three weeks after starting treatment, her PTT normalized, and her FVIII increased to 33.7%. The Bethesda assay remained high at 198 BU, although it was lower than at admission. She was discharged home with dressing changes and monthly follow-up appointments. The wounds were fully closed at her 3-month appointment when she proudly demonstrated full digital extension and flexion into her palm.
Discussion
Forearm compartment syndrome is most often caused by fractures—distal radius in adults and supracondylar in children.2 This case initially presented as a diagnostic puzzle to the emergency department due to the patient’s lucid review of several days of nontraumatic injury.
The clinical hallmarks of compartment syndrome are the 5 Ps: pain, pallor, paresthesia, paralysis, and pulselessness. Patients will describe the pain as out of proportion to the nature of the injury; the compartments will be tense and swollen, they will have pain to passive muscle stretch, and sensation will progressively diminish. Distal pulses are the last to go, and permanent tissue damage can still occur when pulses are present.1
Compartment Syndrome
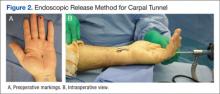
Compartment syndrome is generally a clinical diagnosis; however, in patients who are sedated or uncooperative, or if the clinical findings are equivocal, the examination can be supplemented with intercompartmental pressures using an arterial line transducer system.2 In general, a tissue pressure of 30 mm Hg or a 20- to 30-mm Hg difference between the diastolic and compartment pressures are indications for fasciotomy.1 The hand is treated with an open carpal tunnel release, interosseous muscle release through 2 dorsal hand incisions, and thenar and hypothenar muscle release. The forearm is treated through a curved volar incision that usually decompresses the dorsal compartment, as it did in our patient. If pressures are still high in the forearm, a longitudinal dorsal incision over the mobile wad is necessary. Wounds can be closed primarily days later, left open to close by secondary intention, or reconstructed with skin grafts.2 In our patient, compartment syndrome was isolated to her forearm and the carpal tunnel release was performed prophylactically since it did not add significant time or morbidity to the surgery.
Nontraumatic upper extremity compartment syndrome is rare. A 2021 review of acute nontraumatic upper extremity compartment syndrome found a bleeding disorder as the etiology in 3 cases published in the literature between 1993 and 2016.4 One of these cases was secondary to a known diagnosis of hemophilia A in a teenager.5 Ogrodnik and colleagues described a spontaneous hand hematoma secondary to previously undiagnosed acquired hemophilia A and Waldenström macroglobulinemia.4 Ilyas and colleagues described a spontaneous hematoma in the forearm dorsal compartment in a 67-year-old woman, which presented as compartment syndrome and elevated PTT and led to a diagnosis of acquired FVIII inhibitor. The authors recommended prompt hematology consultation to coordinate treatment once this diagnosis issuspected.6 Compartment syndrome also has been found to develop slowly over weeks in patients with acquired FVIII deficiency, suggesting a high index of suspicion and frequent examinations are needed when patients with known acquired hemophilia A present with a painful extremity.7
Nontraumatic compartment syndrome in the lower extremity in patients with previously undiagnosed acquired hemophilia A has also been described in the literature.8-11 Case reports describe the delay in diagnosis as the patients were originally seen by clinicians for lower extremity pain and swelling within days of presenting to the emergency room with compartment syndrome. Persistent bleeding and abnormal laboratory results prompted further tests and examinations.8,9,11 This underscores the need to be suspicious of this unusual pathology without a history of trauma.
Acquired Hemophilia A
Acquired hemophilia A is an autoimmune disease most often found in older individuals, with a mean age of approximately 70 years.12 It is caused by the spontaneous production of neutralizing immunoglobin autoantibodies that target endogenous FVIII. Many cases are idiopathic; however, up to 50% of cases are associated with underlying autoimmunity, malignancy (especially lymphoproliferative disorders), or pregnancy. It often presents as bleeding that is subcutaneous or in the gastrointestinal system, muscle, retroperitoneal space, or genitourinary system. Unlike congenital hemophilia A, joint bleeding is rare.13
The diagnosis is suspected with an isolated elevated PTT in the absence of other coagulation abnormalities. A 1:1 mixing study will typically show incomplete correction, which suggests the presence of an inhibitor. FVIII activity is reduced, and the FVIII inhibitor is confirmed with the Bethesda assay. Clinically active bleeding is treated with bypassing agents such as recombinant coagulation-activated FVII, activated prothrombin complex concentrates such as anti-inhibitor coagulant complex (FEIBA), or recombinant porcine FVIII.12,14 Not all patients require hemostatic treatment, but close monitoring, education, recognition, and immediate treatment, if needed, are indicated.13 Immunosuppressive therapy (corticosteroids, rituximab, and/or cyclophosphamide) is prescribed to eradicate the antibodies and induce remission.12
Conclusions
An older woman without a preceding trauma was diagnosed with an unusual case of acute compartment syndrome in the forearm. No hematoma was found, but muscle and skin bleeding plus an elevated PTT prompted a hematology workup, and, ultimately, the diagnosis of FVIII inhibitor secondary to acquired hemophilia A.
While a nontraumatic cause of compartment syndrome is rare, it should be considered in differential diagnosis for clinicians who see hand and upper extremity emergencies. An isolated elevated PTT in a patient with a bleed should raise suspicions and trigger immediate further evaluation. Once suspected, multidisciplinary treatment is indicated for immediate and long-term successful outcomes.
Acknowledgments
This manuscript is the result of work supported withresources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. Leversedge FJ, Moore TJ, Peterson BC, Seiler JG 3rd. Compartment syndrome of the upper extremity. J Hand Surg Am. 2011;36:544-559. doi:10.1016/j.jhsa.2010.12.008
2. Kalyani BS, Fisher BE, Roberts CS, Giannoudis PV. Compartment syndrome of the forearm: a systematic review. J Hand Surg Am. 2011;36:535-543. doi:10.1016/j.jhsa.2010.12.007
3. Steadman W, Wu R, Hamilton AT, Richardson MD, Wall CJ. Review article: a comprehensive review of unusual causes of acute limb compartment syndrome. Emerg Med Australas. 2022;34:871-876. doi:10.1111/1742-6723.14098
4. Ogrodnik J, Oliver JD, Cani D, Boczar D, Huayllani MT, Restrepo DJ, et al. Clinical case of acute non-traumatic hand compartment syndrome and systematic review for the upper extremity. Hand (N Y). 2021;16:285-291. doi:10.1177/1558944719856106
5. Kim J, Zelken J, Sacks JM. Case report. Spontaneous forearm compartment syndrome in a boy with hemophilia a: a therapeutic dilemma. Eplasty. 2013:13:e16.
6. Ilyas AM, Wisbeck JM, Shaffer GW, Thoder JJ. Upper extremity compartment syndrome secondary to acquired factor VIII inhibitor. A case report. J Bone Joint Surg Am. 2005;87:1606-1608. doi:10.2106/JBJS.C.01720
7. Adeclat GJ, Hayes M, Amick M, Kahan J, Halim A. Acute forearm compartment syndrome in the setting of acquired hemophilia A. Case Reports Plast Surg Hand Surg. 2022;9:140-144. doi:10.1080/23320885.2022.2071274
8. Abudaqqa RY, Arun KP, Mas AJA, Abushaaban FA. Acute atraumatic compartment syndrome of the thigh due to acquired coagulopathy disorder: a case report in known healthy patient. J Orthop Case Rep. 2021;11:59-62. doi:10.13107/jocr.2021.v11.i08.2366
9. Alidoost M, Conte GA, Chaudry R, Nahum K, Marchesani D. A unique presentation of spontaneous compartment syndrome due to acquired hemophilia A and associated malignancy: case report and literature review. World J Oncol. 2020;11:72-75. doi:10.14740/wjon1260
10. Jentzsch T, Brand-Staufer B, Schäfer FP, Wanner GA, Simmen H-P. Illustrated operative management of spontaneous bleeding and compartment syndrome of the lower extremity in a patient with acquired hemophilia A: a case report. J Med Case Rep. 2014;8:132. doi:10.1186/1752-1947-8-132
11. Pham TV, Sorenson CA, Nable JV. Acquired factor VIII deficiency presenting with compartment syndrome. Am J Emerg Med. 2014;32:195.e1-2. doi:10.1016/j.ajem.2013.09.022
12. Tiede A, Zieger B, Lisman T. Acquired bleeding disorders. Haemophilia. 2022;28(suppl 4):68-76. doi:10.1111/hae.14548
13. Kruse-Jarres R, Kempton CL, Baudo F, Collins PW, Knoebl P, Leissinger CA, et al. Acquired hemophilia A: updated review of evidence and treatment guidance. Am J Hematol. 2017;92:695-705. doi:10.1002/ajh.24777
14. Ilkhchoui Y, Koshkin E, Windsor JJ, Petersen TR, Charles M, Pack JD. Perioperative management of acquired hemophilia A: a case report and review of literature. Anesth Pain Med. 2013;4:e11906. doi:10.5812/aapm.11906
Compartment syndrome occurs when the interstitial tissue pressures within a confined space are elevated to a level at which the arterial perfusion is diminished. Multiple etiologies exist and can be extrinsic (a cast that is too tight or prolonged compression on a limb), iatrogenic (aggressive resuscitation, drug infiltration, arterial puncture, or a spontaneous bleed from anticoagulation), and traumatic (fracture, snake envenomation, circumferential burn, or electrocution). If the compartments are not released, irreversible changes happen to the cells, including nerve and muscle death.1 Definitive management of this emergency requires prompt fasciotomy to decompress the compartment(s).1-3
Case Presentation
A 76-year-old right-handed woman with a history of chronic obstructive pulmonary disease, hypertension, and hyperlipidemia presented to the emergency department with 2 days of extensive right upper extremity ecchymosis and severe pain that was localized to her forearm (Figure 1). She was taking low-dose aspirin (81 mg/d) for left subclavian stenosis and over-the-counter ginkgo biloba. Leading up to the presentation, the patient was able to perform routine household chores, including yard work, cleaning, and taking care of her cats. Wrist and elbow X-rays were negative for a fracture. An upper extremity ultrasound found no venous occlusion. A computed tomography (CT) angiogram of her arm and chest found diffuse edema around the right elbow and forearm without pulmonary or right upper extremity emboli, fractures, hematoma, abscess, or air in the tissues.
The plastic surgery service was consulted. The patient was found to have a very tense forearm and pain to passive digital extension. The 2-point discrimination and pulses were intact. The patient was diagnosed with compartment syndrome based on the examination alone and gave consent for an emergent forearm and hand fasciotomy. A carpal tunnel release and a standard S-shaped volar forearm fasciotomy release were performed, which provided immediate decompression (Figure 2). The rest of the hand and extremity were soft. Edematous, healthy flexor muscle belly was identified without a hematoma. Most of the forearm wound was left open because the skin could not be reapproximated. Oxidized regenerated cellulose (Surgicel) was placed around the wound edges and the muscle was covered with a nonadherent dressing. Hemoglobin on admission was 12.9 g/dL(reference range, 12 to 16 g/dL). Kidney function was within normal limits. The rest of the complete blood count was unremarkable. Postoperative hemoglobin was 8.6 g/dL. Over the next several days, the patient's skin edges and muscle bellies continued to slowly bleed, and her hemoglobin fell to 5.6 g/dL by postoperative Day 2. The bleeding was managed with topical oxidized regenerated cellulose, thrombin spray, a hemostatic dressing made with kaolin (QuikClot), and a transfusion of 2 units of packed red blood cells.
A hematology consultation was requested. The patient was noted to have an elevated partial thromboplastin time (PTT) since admission measuring between 39.9 to 61.7 seconds (reference range, 26.2 to 37.2 seconds) and a normal prothrombin time test with an international normalized ratio. A PTT measured 17 months prior to admission was within the normal range. She reported no personal or family history of bleeding disorders. Until recently, she had never had easy bruisability. She reported no history of heavy menses or epistaxis. The patient had no children and had never been pregnant. She had tolerated an exploratory laparotomy 40 years prior to admission without bleeding complications and had never required blood transfusions before. A PTT 1:1 mixing study revealed incomplete correction. Subsequent workup included factor VIII (FVIII) activity, factor IX activity, factor XI activity, von Willebrand factor antigen, ristocetin cofactor assay, and von Willebrand factor multimers. FVIII activity was severely reduced at 7.8% (reference, > 54%) with a positive Bethesda assay of 300 to 400 Bodansky units (BU), indicating a strong FVIII inhibitor was present and establishing a diagnosis of acquired hemophilia A. Further workup for secondary causes of acquired hemophilia A including abdominal and pelvic CT, serum protein electrophoresis, and serum free light chains, were negative. She was started on prednisone 1 mg/kg daily and rituximab 375 mg/m2. Her hemoglobin stabilized, and she required no further blood transfusions.
The patient underwent wound closure on postoperative Day 11. At the time of the second surgery, there was still no improvement in her FVIII levels or PTT; therefore, 70 mcg/kg of recombinant coagulation-activated FVII was given just before surgery with no bleeding complications. The skin was closed primarily except for the most distal 3 cm (Figure 3). Due to concerns regarding further bleeding with skin graft, the remaining wound was allowed to close by secondary intention. As a precaution, the wound was covered with oxidized regenerated cellulose and thrombin spray. The patient continued to progress postoperatively without bleeding complications or a need for additional transfusions. She was seen by the hand therapist before and after the second surgery to help with edema management and joint mobility. She completed 4 weekly doses of 375 mg/m² rituximab and prednisone was tapered by 10 mg weekly.
Three weeks after starting treatment, her PTT normalized, and her FVIII increased to 33.7%. The Bethesda assay remained high at 198 BU, although it was lower than at admission. She was discharged home with dressing changes and monthly follow-up appointments. The wounds were fully closed at her 3-month appointment when she proudly demonstrated full digital extension and flexion into her palm.
Discussion
Forearm compartment syndrome is most often caused by fractures—distal radius in adults and supracondylar in children.2 This case initially presented as a diagnostic puzzle to the emergency department due to the patient’s lucid review of several days of nontraumatic injury.
The clinical hallmarks of compartment syndrome are the 5 Ps: pain, pallor, paresthesia, paralysis, and pulselessness. Patients will describe the pain as out of proportion to the nature of the injury; the compartments will be tense and swollen, they will have pain to passive muscle stretch, and sensation will progressively diminish. Distal pulses are the last to go, and permanent tissue damage can still occur when pulses are present.1
Compartment Syndrome
Compartment syndrome is generally a clinical diagnosis; however, in patients who are sedated or uncooperative, or if the clinical findings are equivocal, the examination can be supplemented with intercompartmental pressures using an arterial line transducer system.2 In general, a tissue pressure of 30 mm Hg or a 20- to 30-mm Hg difference between the diastolic and compartment pressures are indications for fasciotomy.1 The hand is treated with an open carpal tunnel release, interosseous muscle release through 2 dorsal hand incisions, and thenar and hypothenar muscle release. The forearm is treated through a curved volar incision that usually decompresses the dorsal compartment, as it did in our patient. If pressures are still high in the forearm, a longitudinal dorsal incision over the mobile wad is necessary. Wounds can be closed primarily days later, left open to close by secondary intention, or reconstructed with skin grafts.2 In our patient, compartment syndrome was isolated to her forearm and the carpal tunnel release was performed prophylactically since it did not add significant time or morbidity to the surgery.
Nontraumatic upper extremity compartment syndrome is rare. A 2021 review of acute nontraumatic upper extremity compartment syndrome found a bleeding disorder as the etiology in 3 cases published in the literature between 1993 and 2016.4 One of these cases was secondary to a known diagnosis of hemophilia A in a teenager.5 Ogrodnik and colleagues described a spontaneous hand hematoma secondary to previously undiagnosed acquired hemophilia A and Waldenström macroglobulinemia.4 Ilyas and colleagues described a spontaneous hematoma in the forearm dorsal compartment in a 67-year-old woman, which presented as compartment syndrome and elevated PTT and led to a diagnosis of acquired FVIII inhibitor. The authors recommended prompt hematology consultation to coordinate treatment once this diagnosis issuspected.6 Compartment syndrome also has been found to develop slowly over weeks in patients with acquired FVIII deficiency, suggesting a high index of suspicion and frequent examinations are needed when patients with known acquired hemophilia A present with a painful extremity.7
Nontraumatic compartment syndrome in the lower extremity in patients with previously undiagnosed acquired hemophilia A has also been described in the literature.8-11 Case reports describe the delay in diagnosis as the patients were originally seen by clinicians for lower extremity pain and swelling within days of presenting to the emergency room with compartment syndrome. Persistent bleeding and abnormal laboratory results prompted further tests and examinations.8,9,11 This underscores the need to be suspicious of this unusual pathology without a history of trauma.
Acquired Hemophilia A
Acquired hemophilia A is an autoimmune disease most often found in older individuals, with a mean age of approximately 70 years.12 It is caused by the spontaneous production of neutralizing immunoglobin autoantibodies that target endogenous FVIII. Many cases are idiopathic; however, up to 50% of cases are associated with underlying autoimmunity, malignancy (especially lymphoproliferative disorders), or pregnancy. It often presents as bleeding that is subcutaneous or in the gastrointestinal system, muscle, retroperitoneal space, or genitourinary system. Unlike congenital hemophilia A, joint bleeding is rare.13
The diagnosis is suspected with an isolated elevated PTT in the absence of other coagulation abnormalities. A 1:1 mixing study will typically show incomplete correction, which suggests the presence of an inhibitor. FVIII activity is reduced, and the FVIII inhibitor is confirmed with the Bethesda assay. Clinically active bleeding is treated with bypassing agents such as recombinant coagulation-activated FVII, activated prothrombin complex concentrates such as anti-inhibitor coagulant complex (FEIBA), or recombinant porcine FVIII.12,14 Not all patients require hemostatic treatment, but close monitoring, education, recognition, and immediate treatment, if needed, are indicated.13 Immunosuppressive therapy (corticosteroids, rituximab, and/or cyclophosphamide) is prescribed to eradicate the antibodies and induce remission.12
Conclusions
An older woman without a preceding trauma was diagnosed with an unusual case of acute compartment syndrome in the forearm. No hematoma was found, but muscle and skin bleeding plus an elevated PTT prompted a hematology workup, and, ultimately, the diagnosis of FVIII inhibitor secondary to acquired hemophilia A.
While a nontraumatic cause of compartment syndrome is rare, it should be considered in differential diagnosis for clinicians who see hand and upper extremity emergencies. An isolated elevated PTT in a patient with a bleed should raise suspicions and trigger immediate further evaluation. Once suspected, multidisciplinary treatment is indicated for immediate and long-term successful outcomes.
Acknowledgments
This manuscript is the result of work supported withresources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
Compartment syndrome occurs when the interstitial tissue pressures within a confined space are elevated to a level at which the arterial perfusion is diminished. Multiple etiologies exist and can be extrinsic (a cast that is too tight or prolonged compression on a limb), iatrogenic (aggressive resuscitation, drug infiltration, arterial puncture, or a spontaneous bleed from anticoagulation), and traumatic (fracture, snake envenomation, circumferential burn, or electrocution). If the compartments are not released, irreversible changes happen to the cells, including nerve and muscle death.1 Definitive management of this emergency requires prompt fasciotomy to decompress the compartment(s).1-3
Case Presentation
A 76-year-old right-handed woman with a history of chronic obstructive pulmonary disease, hypertension, and hyperlipidemia presented to the emergency department with 2 days of extensive right upper extremity ecchymosis and severe pain that was localized to her forearm (Figure 1). She was taking low-dose aspirin (81 mg/d) for left subclavian stenosis and over-the-counter ginkgo biloba. Leading up to the presentation, the patient was able to perform routine household chores, including yard work, cleaning, and taking care of her cats. Wrist and elbow X-rays were negative for a fracture. An upper extremity ultrasound found no venous occlusion. A computed tomography (CT) angiogram of her arm and chest found diffuse edema around the right elbow and forearm without pulmonary or right upper extremity emboli, fractures, hematoma, abscess, or air in the tissues.
The plastic surgery service was consulted. The patient was found to have a very tense forearm and pain to passive digital extension. The 2-point discrimination and pulses were intact. The patient was diagnosed with compartment syndrome based on the examination alone and gave consent for an emergent forearm and hand fasciotomy. A carpal tunnel release and a standard S-shaped volar forearm fasciotomy release were performed, which provided immediate decompression (Figure 2). The rest of the hand and extremity were soft. Edematous, healthy flexor muscle belly was identified without a hematoma. Most of the forearm wound was left open because the skin could not be reapproximated. Oxidized regenerated cellulose (Surgicel) was placed around the wound edges and the muscle was covered with a nonadherent dressing. Hemoglobin on admission was 12.9 g/dL(reference range, 12 to 16 g/dL). Kidney function was within normal limits. The rest of the complete blood count was unremarkable. Postoperative hemoglobin was 8.6 g/dL. Over the next several days, the patient's skin edges and muscle bellies continued to slowly bleed, and her hemoglobin fell to 5.6 g/dL by postoperative Day 2. The bleeding was managed with topical oxidized regenerated cellulose, thrombin spray, a hemostatic dressing made with kaolin (QuikClot), and a transfusion of 2 units of packed red blood cells.
A hematology consultation was requested. The patient was noted to have an elevated partial thromboplastin time (PTT) since admission measuring between 39.9 to 61.7 seconds (reference range, 26.2 to 37.2 seconds) and a normal prothrombin time test with an international normalized ratio. A PTT measured 17 months prior to admission was within the normal range. She reported no personal or family history of bleeding disorders. Until recently, she had never had easy bruisability. She reported no history of heavy menses or epistaxis. The patient had no children and had never been pregnant. She had tolerated an exploratory laparotomy 40 years prior to admission without bleeding complications and had never required blood transfusions before. A PTT 1:1 mixing study revealed incomplete correction. Subsequent workup included factor VIII (FVIII) activity, factor IX activity, factor XI activity, von Willebrand factor antigen, ristocetin cofactor assay, and von Willebrand factor multimers. FVIII activity was severely reduced at 7.8% (reference, > 54%) with a positive Bethesda assay of 300 to 400 Bodansky units (BU), indicating a strong FVIII inhibitor was present and establishing a diagnosis of acquired hemophilia A. Further workup for secondary causes of acquired hemophilia A including abdominal and pelvic CT, serum protein electrophoresis, and serum free light chains, were negative. She was started on prednisone 1 mg/kg daily and rituximab 375 mg/m2. Her hemoglobin stabilized, and she required no further blood transfusions.
The patient underwent wound closure on postoperative Day 11. At the time of the second surgery, there was still no improvement in her FVIII levels or PTT; therefore, 70 mcg/kg of recombinant coagulation-activated FVII was given just before surgery with no bleeding complications. The skin was closed primarily except for the most distal 3 cm (Figure 3). Due to concerns regarding further bleeding with skin graft, the remaining wound was allowed to close by secondary intention. As a precaution, the wound was covered with oxidized regenerated cellulose and thrombin spray. The patient continued to progress postoperatively without bleeding complications or a need for additional transfusions. She was seen by the hand therapist before and after the second surgery to help with edema management and joint mobility. She completed 4 weekly doses of 375 mg/m² rituximab and prednisone was tapered by 10 mg weekly.
Three weeks after starting treatment, her PTT normalized, and her FVIII increased to 33.7%. The Bethesda assay remained high at 198 BU, although it was lower than at admission. She was discharged home with dressing changes and monthly follow-up appointments. The wounds were fully closed at her 3-month appointment when she proudly demonstrated full digital extension and flexion into her palm.
Discussion
Forearm compartment syndrome is most often caused by fractures—distal radius in adults and supracondylar in children.2 This case initially presented as a diagnostic puzzle to the emergency department due to the patient’s lucid review of several days of nontraumatic injury.
The clinical hallmarks of compartment syndrome are the 5 Ps: pain, pallor, paresthesia, paralysis, and pulselessness. Patients will describe the pain as out of proportion to the nature of the injury; the compartments will be tense and swollen, they will have pain to passive muscle stretch, and sensation will progressively diminish. Distal pulses are the last to go, and permanent tissue damage can still occur when pulses are present.1
Compartment Syndrome
Compartment syndrome is generally a clinical diagnosis; however, in patients who are sedated or uncooperative, or if the clinical findings are equivocal, the examination can be supplemented with intercompartmental pressures using an arterial line transducer system.2 In general, a tissue pressure of 30 mm Hg or a 20- to 30-mm Hg difference between the diastolic and compartment pressures are indications for fasciotomy.1 The hand is treated with an open carpal tunnel release, interosseous muscle release through 2 dorsal hand incisions, and thenar and hypothenar muscle release. The forearm is treated through a curved volar incision that usually decompresses the dorsal compartment, as it did in our patient. If pressures are still high in the forearm, a longitudinal dorsal incision over the mobile wad is necessary. Wounds can be closed primarily days later, left open to close by secondary intention, or reconstructed with skin grafts.2 In our patient, compartment syndrome was isolated to her forearm and the carpal tunnel release was performed prophylactically since it did not add significant time or morbidity to the surgery.
Nontraumatic upper extremity compartment syndrome is rare. A 2021 review of acute nontraumatic upper extremity compartment syndrome found a bleeding disorder as the etiology in 3 cases published in the literature between 1993 and 2016.4 One of these cases was secondary to a known diagnosis of hemophilia A in a teenager.5 Ogrodnik and colleagues described a spontaneous hand hematoma secondary to previously undiagnosed acquired hemophilia A and Waldenström macroglobulinemia.4 Ilyas and colleagues described a spontaneous hematoma in the forearm dorsal compartment in a 67-year-old woman, which presented as compartment syndrome and elevated PTT and led to a diagnosis of acquired FVIII inhibitor. The authors recommended prompt hematology consultation to coordinate treatment once this diagnosis issuspected.6 Compartment syndrome also has been found to develop slowly over weeks in patients with acquired FVIII deficiency, suggesting a high index of suspicion and frequent examinations are needed when patients with known acquired hemophilia A present with a painful extremity.7
Nontraumatic compartment syndrome in the lower extremity in patients with previously undiagnosed acquired hemophilia A has also been described in the literature.8-11 Case reports describe the delay in diagnosis as the patients were originally seen by clinicians for lower extremity pain and swelling within days of presenting to the emergency room with compartment syndrome. Persistent bleeding and abnormal laboratory results prompted further tests and examinations.8,9,11 This underscores the need to be suspicious of this unusual pathology without a history of trauma.
Acquired Hemophilia A
Acquired hemophilia A is an autoimmune disease most often found in older individuals, with a mean age of approximately 70 years.12 It is caused by the spontaneous production of neutralizing immunoglobin autoantibodies that target endogenous FVIII. Many cases are idiopathic; however, up to 50% of cases are associated with underlying autoimmunity, malignancy (especially lymphoproliferative disorders), or pregnancy. It often presents as bleeding that is subcutaneous or in the gastrointestinal system, muscle, retroperitoneal space, or genitourinary system. Unlike congenital hemophilia A, joint bleeding is rare.13
The diagnosis is suspected with an isolated elevated PTT in the absence of other coagulation abnormalities. A 1:1 mixing study will typically show incomplete correction, which suggests the presence of an inhibitor. FVIII activity is reduced, and the FVIII inhibitor is confirmed with the Bethesda assay. Clinically active bleeding is treated with bypassing agents such as recombinant coagulation-activated FVII, activated prothrombin complex concentrates such as anti-inhibitor coagulant complex (FEIBA), or recombinant porcine FVIII.12,14 Not all patients require hemostatic treatment, but close monitoring, education, recognition, and immediate treatment, if needed, are indicated.13 Immunosuppressive therapy (corticosteroids, rituximab, and/or cyclophosphamide) is prescribed to eradicate the antibodies and induce remission.12
Conclusions
An older woman without a preceding trauma was diagnosed with an unusual case of acute compartment syndrome in the forearm. No hematoma was found, but muscle and skin bleeding plus an elevated PTT prompted a hematology workup, and, ultimately, the diagnosis of FVIII inhibitor secondary to acquired hemophilia A.
While a nontraumatic cause of compartment syndrome is rare, it should be considered in differential diagnosis for clinicians who see hand and upper extremity emergencies. An isolated elevated PTT in a patient with a bleed should raise suspicions and trigger immediate further evaluation. Once suspected, multidisciplinary treatment is indicated for immediate and long-term successful outcomes.
Acknowledgments
This manuscript is the result of work supported withresources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. Leversedge FJ, Moore TJ, Peterson BC, Seiler JG 3rd. Compartment syndrome of the upper extremity. J Hand Surg Am. 2011;36:544-559. doi:10.1016/j.jhsa.2010.12.008
2. Kalyani BS, Fisher BE, Roberts CS, Giannoudis PV. Compartment syndrome of the forearm: a systematic review. J Hand Surg Am. 2011;36:535-543. doi:10.1016/j.jhsa.2010.12.007
3. Steadman W, Wu R, Hamilton AT, Richardson MD, Wall CJ. Review article: a comprehensive review of unusual causes of acute limb compartment syndrome. Emerg Med Australas. 2022;34:871-876. doi:10.1111/1742-6723.14098
4. Ogrodnik J, Oliver JD, Cani D, Boczar D, Huayllani MT, Restrepo DJ, et al. Clinical case of acute non-traumatic hand compartment syndrome and systematic review for the upper extremity. Hand (N Y). 2021;16:285-291. doi:10.1177/1558944719856106
5. Kim J, Zelken J, Sacks JM. Case report. Spontaneous forearm compartment syndrome in a boy with hemophilia a: a therapeutic dilemma. Eplasty. 2013:13:e16.
6. Ilyas AM, Wisbeck JM, Shaffer GW, Thoder JJ. Upper extremity compartment syndrome secondary to acquired factor VIII inhibitor. A case report. J Bone Joint Surg Am. 2005;87:1606-1608. doi:10.2106/JBJS.C.01720
7. Adeclat GJ, Hayes M, Amick M, Kahan J, Halim A. Acute forearm compartment syndrome in the setting of acquired hemophilia A. Case Reports Plast Surg Hand Surg. 2022;9:140-144. doi:10.1080/23320885.2022.2071274
8. Abudaqqa RY, Arun KP, Mas AJA, Abushaaban FA. Acute atraumatic compartment syndrome of the thigh due to acquired coagulopathy disorder: a case report in known healthy patient. J Orthop Case Rep. 2021;11:59-62. doi:10.13107/jocr.2021.v11.i08.2366
9. Alidoost M, Conte GA, Chaudry R, Nahum K, Marchesani D. A unique presentation of spontaneous compartment syndrome due to acquired hemophilia A and associated malignancy: case report and literature review. World J Oncol. 2020;11:72-75. doi:10.14740/wjon1260
10. Jentzsch T, Brand-Staufer B, Schäfer FP, Wanner GA, Simmen H-P. Illustrated operative management of spontaneous bleeding and compartment syndrome of the lower extremity in a patient with acquired hemophilia A: a case report. J Med Case Rep. 2014;8:132. doi:10.1186/1752-1947-8-132
11. Pham TV, Sorenson CA, Nable JV. Acquired factor VIII deficiency presenting with compartment syndrome. Am J Emerg Med. 2014;32:195.e1-2. doi:10.1016/j.ajem.2013.09.022
12. Tiede A, Zieger B, Lisman T. Acquired bleeding disorders. Haemophilia. 2022;28(suppl 4):68-76. doi:10.1111/hae.14548
13. Kruse-Jarres R, Kempton CL, Baudo F, Collins PW, Knoebl P, Leissinger CA, et al. Acquired hemophilia A: updated review of evidence and treatment guidance. Am J Hematol. 2017;92:695-705. doi:10.1002/ajh.24777
14. Ilkhchoui Y, Koshkin E, Windsor JJ, Petersen TR, Charles M, Pack JD. Perioperative management of acquired hemophilia A: a case report and review of literature. Anesth Pain Med. 2013;4:e11906. doi:10.5812/aapm.11906
1. Leversedge FJ, Moore TJ, Peterson BC, Seiler JG 3rd. Compartment syndrome of the upper extremity. J Hand Surg Am. 2011;36:544-559. doi:10.1016/j.jhsa.2010.12.008
2. Kalyani BS, Fisher BE, Roberts CS, Giannoudis PV. Compartment syndrome of the forearm: a systematic review. J Hand Surg Am. 2011;36:535-543. doi:10.1016/j.jhsa.2010.12.007
3. Steadman W, Wu R, Hamilton AT, Richardson MD, Wall CJ. Review article: a comprehensive review of unusual causes of acute limb compartment syndrome. Emerg Med Australas. 2022;34:871-876. doi:10.1111/1742-6723.14098
4. Ogrodnik J, Oliver JD, Cani D, Boczar D, Huayllani MT, Restrepo DJ, et al. Clinical case of acute non-traumatic hand compartment syndrome and systematic review for the upper extremity. Hand (N Y). 2021;16:285-291. doi:10.1177/1558944719856106
5. Kim J, Zelken J, Sacks JM. Case report. Spontaneous forearm compartment syndrome in a boy with hemophilia a: a therapeutic dilemma. Eplasty. 2013:13:e16.
6. Ilyas AM, Wisbeck JM, Shaffer GW, Thoder JJ. Upper extremity compartment syndrome secondary to acquired factor VIII inhibitor. A case report. J Bone Joint Surg Am. 2005;87:1606-1608. doi:10.2106/JBJS.C.01720
7. Adeclat GJ, Hayes M, Amick M, Kahan J, Halim A. Acute forearm compartment syndrome in the setting of acquired hemophilia A. Case Reports Plast Surg Hand Surg. 2022;9:140-144. doi:10.1080/23320885.2022.2071274
8. Abudaqqa RY, Arun KP, Mas AJA, Abushaaban FA. Acute atraumatic compartment syndrome of the thigh due to acquired coagulopathy disorder: a case report in known healthy patient. J Orthop Case Rep. 2021;11:59-62. doi:10.13107/jocr.2021.v11.i08.2366
9. Alidoost M, Conte GA, Chaudry R, Nahum K, Marchesani D. A unique presentation of spontaneous compartment syndrome due to acquired hemophilia A and associated malignancy: case report and literature review. World J Oncol. 2020;11:72-75. doi:10.14740/wjon1260
10. Jentzsch T, Brand-Staufer B, Schäfer FP, Wanner GA, Simmen H-P. Illustrated operative management of spontaneous bleeding and compartment syndrome of the lower extremity in a patient with acquired hemophilia A: a case report. J Med Case Rep. 2014;8:132. doi:10.1186/1752-1947-8-132
11. Pham TV, Sorenson CA, Nable JV. Acquired factor VIII deficiency presenting with compartment syndrome. Am J Emerg Med. 2014;32:195.e1-2. doi:10.1016/j.ajem.2013.09.022
12. Tiede A, Zieger B, Lisman T. Acquired bleeding disorders. Haemophilia. 2022;28(suppl 4):68-76. doi:10.1111/hae.14548
13. Kruse-Jarres R, Kempton CL, Baudo F, Collins PW, Knoebl P, Leissinger CA, et al. Acquired hemophilia A: updated review of evidence and treatment guidance. Am J Hematol. 2017;92:695-705. doi:10.1002/ajh.24777
14. Ilkhchoui Y, Koshkin E, Windsor JJ, Petersen TR, Charles M, Pack JD. Perioperative management of acquired hemophilia A: a case report and review of literature. Anesth Pain Med. 2013;4:e11906. doi:10.5812/aapm.11906
Elective Hand Surgery and Antithrombotic Use in Veterans
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
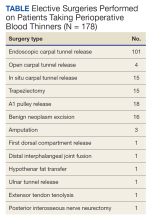
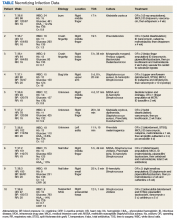
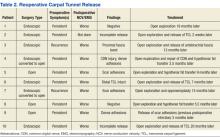
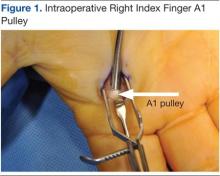
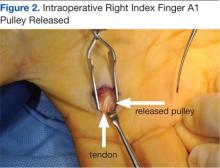
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
Surgical Treatment of Nonmelanoma Skin Cancer in Older Adult Veterans
Skin cancer is the most diagnosed cancer in the United States. Nonmelanoma skin cancers (NMSC), which include basal cell carcinoma and squamous cell carcinoma, are usually cured with removal.1 The incidence of NMSC increases with age and is commonly found in nursing homes and geriatric units. These cancers are not usually metastatic or fatal but can cause local destruction and disfigurement if neglected.2 The current standard of care is to treat diagnosed NMSC; however, the dermatology and geriatric care literature have questioned the logic of treating asymptomatic skin cancers that will not affect a patient’s life expectancy.2-4
Forty-seven percent of the current living veteran population is aged ≥ 65 years.5 Older adult patients are frequently referred to the US Department of Veterans Affairs (VA) surgical service for the treatment of NMSC. The veteran population includes a higher percentage of individuals at an elevated risk of skin cancers (older, White, and male) compared with the general population.6 World War II veterans deployed in regions closer to the equator have been found to have an elevated risk of melanoma and nonmelanoma skin carcinomas.7 A retrospective study of Vietnam veterans exposed to Agent Orange (2,3,7,8-tetrachlorodibenzodioxin) found a significantly higher risk of invasive NMSC in Fitzpatrick skin types I-IV compared with an age-matched subset of the general population.8 Younger veterans who were deployed in Afghanistan and Iraq for Operation Enduring Freedom/Operation Iraqi Freedom worked at more equatorial latitudes than the rest of the US population and may be at increased risk of NMSC. Inadequate sunscreen access, immediate safety concerns, outdoor recreational activities, harsh weather, and insufficient emphasis on sun protection have created a multifactorial challenge for the military population. Riemenschneider and colleagues recommended targeted screening for at-risk veteran patients and prioritizing annual skin cancer screenings during medical mission physical examinations for active military.7
The plastic surgery service regularly receives consults from dermatology, general surgery, and primary care to remove skin cancers on the face, scalp, hands, and forearms. Skin cancer treatment can create serious hardships for older adult patients and their families with multiple appointments for the consult, procedure, and follow-up. Patients are often told to hold their anticoagulant medications when the surgery will be performed on a highly vascular region, such as the scalp or face. This can create wide swings in their laboratory test values and result in life-threatening complications from either bleeding or clotting. The appropriateness of offering surgery to patients with serious comorbidities and a limited life expectancy has been questioned.2-4 The purpose of this study was to measure the morbidity and unrelated 5-year mortality for patients with skin cancer referred to the plastic surgery service to help patients and families make a more informed treatment decision, particularly when the patients are aged > 80 years and have significant life-threatening comorbidities.
Methods
The University of Florida and Malcom Randall VA Medical Center Institutional review board in Gainesville, approved a retrospective review of all consults completed by the plastic surgery service for the treatment of NMSC performed from July 1, 2011 to June 30, 2015. Data collected included age and common life-limiting comorbidities at the time of referral. Morbidities were found on the electronic health record, including coronary artery disease (CAD), congestive heart failure (CHF), cerebral vascular disease (CVD), peripheral vascular disease, dementia, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), tobacco use, diabetes mellitus (DM), liver disease, alcohol use, and obstructive sleep apnea.
Treatment, complications, and 5-year mortality were recorded. A χ2 analysis with P value < .05 was used to determine statistical significance between individual risk factors and 5-year mortality. The relative risk of 5-year mortality was calculated by combining advanced age (aged > 80 years) with the individual comorbidities.
Results
Over 4 years, 800 consults for NMSC were completed by the plastic surgery service. Treatment decisions included 210 excisions (with or without reconstruction) in the operating room, 402 excisions (with or without reconstruction) under local anesthesia in clinic, 55 Mohs surgical dermatology referrals, 21 other service or hospital referrals, and 112 patient who were observed, declined intervention, or died prior to intervention. Five-year mortality was 28.6%. No patients died of NMSC. The median age at consult submission for patients deceased 5 years later was 78 years. Complication rate was 5% and included wound infection, dehiscence, bleeding, or graft loss. Two patients, both deceased within 5 years, had unplanned admissions due to bleeding from either a skin graft donor site or recipient bleeding. Aged ≥ 80 years, CAD, CHF, CVD, peripheral vascular disease, dementia, CKD, COPD, and DM were all found individually to be statistically significant predictors of 5-year mortality (Table 1). Combining aged ≥ 80 years plus CAD, CHF, or dementia all increased the 5-year mortality by a relative risk of > 3 (Table 2).
Discussion
The standard of care is to treat NMSC. Most NMSCs are treated surgically without consideration of patient age or life expectancy.2,4,9,10 A prospective cohort study involving a university-based private practice and a VA medical center in San Francisco found a 22.6% overall 5-year mortality and a 43.3% mortality in the group defined as limited life expectancy (LLE) based on age (≥ 85 years) and medical comorbidities. None died due to the NMSC. Leading cause of death was cardiac, cerebrovascular, and respiratory disease, lung and prostate cancer, and Alzheimer disease. The authors suggested the LLE group may be exposed to wound complications without benefiting from the treatment.4
Another study of 440 patients receiving excision for biopsy-proven facial NMSC at the Roudebush VA Medical Center in Indianapolis, Indiana, found no residual carcinoma in 35.3% of excisions, and in patients aged > 90 years, more than half of the excisions had no residual carcinoma. More than half of the patients aged > 90 years died within 1 year, not as a result of the NMSC. The authors argued for watchful waiting in select patients to maximize comfort and outcomes.10
NMSCs are often asymptomatic and not immediately life threatening. Although NMSCs tend to have a favorable prognosis, studies have found that NMSC may be a marker for other poor health outcomes. A significant increased risk for all-cause mortality was found for patients with a history of SCC, which may be attributed to immune status.11 The aging veteran population has more complex health care needs to be considered when developing surgical treatment plans. These medical problems may limit their life expectancy much sooner than the skin cancer will become symptomatic. We found that individuals aged ≥ 80 years who had CAD, CHF, or dementia had a relative risk of 3 or higher for 5-year mortality. The leading cause of death in the United States in years 2011 to 2015 was heart disease. Alzheimer disease was the sixth leading cause of death in those same years.12-14
Skin cancer excisions do not typically require general anesthesia, deep sedation, or large fluid shifts; however, studies have found that when frail patients undergo low-risk procedures, they tend to have a higher mortality rate than their healthier counterparts.15 Frailty is a concept that identifies patients who are at increased risk of dying in 6 to 60 months due to a decline in their physical reserve. Frail patients have increased rates of perioperative mortality and complications. Various tools have been used to assess the components of physical performance, speed, mobility, nutrition status, mental health, and cognition.16 Frailty screening has been initiated in several VA hospitals, including our own in Gainesville, Florida, with the goal of decreasing postoperative morbidity and mortality in older adult patients.17 The patients are given a 1-page screening assessment that asks about their living situation, medical conditions, nutrition status, cognition, and activities of daily living. The results can trigger the clinician to rethink the surgical plan and mobilize more resources to optimize the patient’s health. This study period precedes the initiative at our institution.
The plastic surgery service’s routine practice is to excise skin cancers in the operating room if sedation or general anesthesia will be needed (Figure 1A), for optimal control of bleeding (Figure 1B) in a patient who cannot safely stop blood thinners, or for excision of a highly vascularized area such as the scalp. Surgery is offered in an office-based setting if the area can be closed primarily, left open to close secondarily, or closed with a small skin graft under local anesthesia only (Figure 2). We prefer treating frail patients in the minor procedure clinic, when possible, to avoid the risks of sedation and the additional preoperative visits and transportation requirements. NMSC with unclear margins (Figure 3A) or in cosmetically sensitive areas where tissue needs to be preserved (Figure 3B) are referred to the Mohs dermatologist. The skin cancers in this study were most frequently found on the face, scalp, hands, and forearms based on referral patterns.
Other treatment options for NMSC include curettage and electrodessication, cryotherapy, and radiation; however, ours is a surgical service and patients are typically referred to us by primary care or dermatology when those are not reasonable or desirable options.18 Published complication rates of patients having skin cancer surgery without age restriction have a rate of 3% to 6%, which is consistent with our study of 5%.19-21 Two bleeding complications that needed to be admitted did not require more than a bedside procedure and neither required transfusions. One patient had been instructed to continue taking coumadin during the perioperative office-based procedure due to a recent carotid stent placement in the setting of a rapidly growing basal cell on an easily accessible location.
The most noted comorbidity in patients with wound complications was found to be DM; however, this was not found to be a statistically significant risk factor for wound complications (P = .10). We do not have a set rule for advising for or against NMSC surgery. We do counsel frail patients and their families that not all cancer is immediately life threatening and will work with them to do whatever makes the most sense to achieve their goals, occasionally accepting positive margins in order to debulk a symptomatic growth. The objective of this paper is to contribute to the discussion of performing invasive procedures on older adult veterans with life-limiting comorbidities. Patients and their families will have different thresholds for what they feel needs intervention, especially if other medical problems are consuming much of their time. We also have the community care referral option for patients whose treatment decisions are being dictated by travel hardships.
Strengths and Limitations
A strength of this study is that the data were obtained from a closed system. Patients tend to stay long-term within the VA and their health record is accessible throughout the country as long as they are seen at a VA facility. Complications, therefore, return to the treating service or primary care, who would route the patient back to the surgeon.
One limitation of the study is that this is a retrospective review from 2011. The authors are limited to data that are recorded in the patient record. Multiple health care professionals saw the patients and notes lack consistency in detail. Size of the lesions were not consistently recorded and did not get logged into our database for that reason.
Conclusions
Treatment of NMSC in older adult patients has a low morbidity but needs to be balanced against a patient and family’s goals when the patient presents with life-limiting comorbidities. An elevated 5-year mortality in patients aged > 80 years with serious unrelated medical conditions is intuitive, but this study may help put treatment plans into perspective for families and health care professionals who want to provide an indicated service while maximizing patient quality of life.
Acknowledgments
This manuscript is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. American Cancer Society. Cancer Facts & Figures 2021. Accessed May 26, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
2. Albert A, Knoll MA, Conti JA, Zbar RIS. Non-melanoma skin cancers in the older patient. Curr Oncol Rep. 2019;21(9):79. Published 2019 Jul 29. doi:10.1007/s11912-019-0828-9
3. Linos E, Chren MM, Stijacic Cenzer I, Covinsky KE. Skin cancer in U.S. elderly adults: does life expectancy play a role in treatment decisions? J Am Geriatr Soc. 2016;64(8):1610-1615. doi:10.1111/jgs.14202
4. Linos E, Parvataneni R, Stuart SE, Boscardin WJ, Landefeld CS, Chren MM. Treatment of nonfatal conditions at the end of life: nonmelanoma skin cancer. JAMA Intern Med. 2013;173(11):1006-1012. doi:10.1001/jamainternmed.2013.639
5. O’Malley KA, Vinson L, Kaiser AP, Sager Z, Hinrichs K. Mental health and aging veterans: how the Veterans Health Administration meets the needs of aging veterans. Public Policy Aging Rep. 2020;30(1):19-23. doi:10.1093/ppar/prz027
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2017. Accessed May 26, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2017.pdf 7. Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78(6):1185-1192. doi:10.1016/j.jaad.2017.11.062
8. Clemens MW, Kochuba AL, Carter ME, Han K, Liu J, Evans K. Association between Agent Orange exposure and nonmelanotic invasive skin cancer: a pilot study. Plast Reconstr Surg. 2014;133(2):432-437. doi:10.1097/01.prs.0000436859.40151.cf
9. Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303-317. doi:10.1016/j.jaad.2018.03.060
10. Chauhan R, Munger BN, Chu MW, et al. Age at diagnosis as a relative contraindication for intervention in facial nonmelanoma skin cancer. JAMA Surg. 2018;153(4):390-392. doi:10.1001/jamasurg.2017.5073
11. Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309(4):243-251. doi:10.1007/s00403-017-1724-5
12. Kochanek KD, Murphy SL, Xu JQ, Arias E. Mortality in the United States, 2013. NCHS Data Brief 178. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db178.htm
13. Xu JQ, Kochanek KD, Murphy SL, Arias E. Mortality in the United States, 2012. NCHS Data Brief 168. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db168.htm
14. Xu JQ, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2015. NCHS Data Brief 267. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db267.htm
15. Varley PR , Borrebach JD, Arya S, et al. Clinical utility of the risk analysis index as a prospective frailty screening tool within a multi-practice, multi-hospital integrated healthcare system. Ann Surg. 2021;274(6):e1230-e1237. doi:10.1097/SLA.0000000000003808
16. Hall DE, Arya S , Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152(2):175-182. doi:10.1001/jamasurg.2016.4202
17. US Department of Veterans Affairs, Health Services Research & Development. Improving healthcare for aging veterans. Updated August 30, 2017. Accessed May 26, 2022. https://www.hsrd.research.va.gov/news/feature/aging0917.cfm
18. Leus AJG, Frie M, Haisma MS, et al. Treatment of keratinocyte carcinoma in elderly patients – a review of the current literature. J Eur Acad Dermatol Venereol. 2020;34(9):1932-1943. doi:10.1111/jdv.16268
19. Amici JM, Rogues AM, Lasheras A, et al. A prospective study of the incidence of complications associated with dermatological surgery. Br J Dermatol. 2005;153(5):967-971. doi:10.1111/j.1365-2133.2005.06861.x
20. Arguello-Guerra L, Vargas-Chandomid E, Díaz-González JM, Méndez-Flores S, Ruelas-Villavicencio A, Domínguez-Cherit J. Incidence of complications in dermatological surgery of melanoma and non-melanoma skin cancer in patients with multiple comorbidity and/or antiplatelet-anticoagulants. Five-year experience in our hospital. Cir Cir. 2019;86(1):15-23. doi:10.24875/CIRUE.M18000003
21. Keith DJ, de Berker DA, Bray AP, Cheung ST, Brain A, Mohd Mustapa MF. British Association of Dermatologists’ national audit on nonmelanoma skin cancer excision, 2014. Clin Exp Dermatol. 2017;42(1):46-53. doi:10.1111/ced.12990
Skin cancer is the most diagnosed cancer in the United States. Nonmelanoma skin cancers (NMSC), which include basal cell carcinoma and squamous cell carcinoma, are usually cured with removal.1 The incidence of NMSC increases with age and is commonly found in nursing homes and geriatric units. These cancers are not usually metastatic or fatal but can cause local destruction and disfigurement if neglected.2 The current standard of care is to treat diagnosed NMSC; however, the dermatology and geriatric care literature have questioned the logic of treating asymptomatic skin cancers that will not affect a patient’s life expectancy.2-4
Forty-seven percent of the current living veteran population is aged ≥ 65 years.5 Older adult patients are frequently referred to the US Department of Veterans Affairs (VA) surgical service for the treatment of NMSC. The veteran population includes a higher percentage of individuals at an elevated risk of skin cancers (older, White, and male) compared with the general population.6 World War II veterans deployed in regions closer to the equator have been found to have an elevated risk of melanoma and nonmelanoma skin carcinomas.7 A retrospective study of Vietnam veterans exposed to Agent Orange (2,3,7,8-tetrachlorodibenzodioxin) found a significantly higher risk of invasive NMSC in Fitzpatrick skin types I-IV compared with an age-matched subset of the general population.8 Younger veterans who were deployed in Afghanistan and Iraq for Operation Enduring Freedom/Operation Iraqi Freedom worked at more equatorial latitudes than the rest of the US population and may be at increased risk of NMSC. Inadequate sunscreen access, immediate safety concerns, outdoor recreational activities, harsh weather, and insufficient emphasis on sun protection have created a multifactorial challenge for the military population. Riemenschneider and colleagues recommended targeted screening for at-risk veteran patients and prioritizing annual skin cancer screenings during medical mission physical examinations for active military.7
The plastic surgery service regularly receives consults from dermatology, general surgery, and primary care to remove skin cancers on the face, scalp, hands, and forearms. Skin cancer treatment can create serious hardships for older adult patients and their families with multiple appointments for the consult, procedure, and follow-up. Patients are often told to hold their anticoagulant medications when the surgery will be performed on a highly vascular region, such as the scalp or face. This can create wide swings in their laboratory test values and result in life-threatening complications from either bleeding or clotting. The appropriateness of offering surgery to patients with serious comorbidities and a limited life expectancy has been questioned.2-4 The purpose of this study was to measure the morbidity and unrelated 5-year mortality for patients with skin cancer referred to the plastic surgery service to help patients and families make a more informed treatment decision, particularly when the patients are aged > 80 years and have significant life-threatening comorbidities.
Methods
The University of Florida and Malcom Randall VA Medical Center Institutional review board in Gainesville, approved a retrospective review of all consults completed by the plastic surgery service for the treatment of NMSC performed from July 1, 2011 to June 30, 2015. Data collected included age and common life-limiting comorbidities at the time of referral. Morbidities were found on the electronic health record, including coronary artery disease (CAD), congestive heart failure (CHF), cerebral vascular disease (CVD), peripheral vascular disease, dementia, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), tobacco use, diabetes mellitus (DM), liver disease, alcohol use, and obstructive sleep apnea.
Treatment, complications, and 5-year mortality were recorded. A χ2 analysis with P value < .05 was used to determine statistical significance between individual risk factors and 5-year mortality. The relative risk of 5-year mortality was calculated by combining advanced age (aged > 80 years) with the individual comorbidities.
Results
Over 4 years, 800 consults for NMSC were completed by the plastic surgery service. Treatment decisions included 210 excisions (with or without reconstruction) in the operating room, 402 excisions (with or without reconstruction) under local anesthesia in clinic, 55 Mohs surgical dermatology referrals, 21 other service or hospital referrals, and 112 patient who were observed, declined intervention, or died prior to intervention. Five-year mortality was 28.6%. No patients died of NMSC. The median age at consult submission for patients deceased 5 years later was 78 years. Complication rate was 5% and included wound infection, dehiscence, bleeding, or graft loss. Two patients, both deceased within 5 years, had unplanned admissions due to bleeding from either a skin graft donor site or recipient bleeding. Aged ≥ 80 years, CAD, CHF, CVD, peripheral vascular disease, dementia, CKD, COPD, and DM were all found individually to be statistically significant predictors of 5-year mortality (Table 1). Combining aged ≥ 80 years plus CAD, CHF, or dementia all increased the 5-year mortality by a relative risk of > 3 (Table 2).
Discussion
The standard of care is to treat NMSC. Most NMSCs are treated surgically without consideration of patient age or life expectancy.2,4,9,10 A prospective cohort study involving a university-based private practice and a VA medical center in San Francisco found a 22.6% overall 5-year mortality and a 43.3% mortality in the group defined as limited life expectancy (LLE) based on age (≥ 85 years) and medical comorbidities. None died due to the NMSC. Leading cause of death was cardiac, cerebrovascular, and respiratory disease, lung and prostate cancer, and Alzheimer disease. The authors suggested the LLE group may be exposed to wound complications without benefiting from the treatment.4
Another study of 440 patients receiving excision for biopsy-proven facial NMSC at the Roudebush VA Medical Center in Indianapolis, Indiana, found no residual carcinoma in 35.3% of excisions, and in patients aged > 90 years, more than half of the excisions had no residual carcinoma. More than half of the patients aged > 90 years died within 1 year, not as a result of the NMSC. The authors argued for watchful waiting in select patients to maximize comfort and outcomes.10
NMSCs are often asymptomatic and not immediately life threatening. Although NMSCs tend to have a favorable prognosis, studies have found that NMSC may be a marker for other poor health outcomes. A significant increased risk for all-cause mortality was found for patients with a history of SCC, which may be attributed to immune status.11 The aging veteran population has more complex health care needs to be considered when developing surgical treatment plans. These medical problems may limit their life expectancy much sooner than the skin cancer will become symptomatic. We found that individuals aged ≥ 80 years who had CAD, CHF, or dementia had a relative risk of 3 or higher for 5-year mortality. The leading cause of death in the United States in years 2011 to 2015 was heart disease. Alzheimer disease was the sixth leading cause of death in those same years.12-14
Skin cancer excisions do not typically require general anesthesia, deep sedation, or large fluid shifts; however, studies have found that when frail patients undergo low-risk procedures, they tend to have a higher mortality rate than their healthier counterparts.15 Frailty is a concept that identifies patients who are at increased risk of dying in 6 to 60 months due to a decline in their physical reserve. Frail patients have increased rates of perioperative mortality and complications. Various tools have been used to assess the components of physical performance, speed, mobility, nutrition status, mental health, and cognition.16 Frailty screening has been initiated in several VA hospitals, including our own in Gainesville, Florida, with the goal of decreasing postoperative morbidity and mortality in older adult patients.17 The patients are given a 1-page screening assessment that asks about their living situation, medical conditions, nutrition status, cognition, and activities of daily living. The results can trigger the clinician to rethink the surgical plan and mobilize more resources to optimize the patient’s health. This study period precedes the initiative at our institution.
The plastic surgery service’s routine practice is to excise skin cancers in the operating room if sedation or general anesthesia will be needed (Figure 1A), for optimal control of bleeding (Figure 1B) in a patient who cannot safely stop blood thinners, or for excision of a highly vascularized area such as the scalp. Surgery is offered in an office-based setting if the area can be closed primarily, left open to close secondarily, or closed with a small skin graft under local anesthesia only (Figure 2). We prefer treating frail patients in the minor procedure clinic, when possible, to avoid the risks of sedation and the additional preoperative visits and transportation requirements. NMSC with unclear margins (Figure 3A) or in cosmetically sensitive areas where tissue needs to be preserved (Figure 3B) are referred to the Mohs dermatologist. The skin cancers in this study were most frequently found on the face, scalp, hands, and forearms based on referral patterns.
Other treatment options for NMSC include curettage and electrodessication, cryotherapy, and radiation; however, ours is a surgical service and patients are typically referred to us by primary care or dermatology when those are not reasonable or desirable options.18 Published complication rates of patients having skin cancer surgery without age restriction have a rate of 3% to 6%, which is consistent with our study of 5%.19-21 Two bleeding complications that needed to be admitted did not require more than a bedside procedure and neither required transfusions. One patient had been instructed to continue taking coumadin during the perioperative office-based procedure due to a recent carotid stent placement in the setting of a rapidly growing basal cell on an easily accessible location.
The most noted comorbidity in patients with wound complications was found to be DM; however, this was not found to be a statistically significant risk factor for wound complications (P = .10). We do not have a set rule for advising for or against NMSC surgery. We do counsel frail patients and their families that not all cancer is immediately life threatening and will work with them to do whatever makes the most sense to achieve their goals, occasionally accepting positive margins in order to debulk a symptomatic growth. The objective of this paper is to contribute to the discussion of performing invasive procedures on older adult veterans with life-limiting comorbidities. Patients and their families will have different thresholds for what they feel needs intervention, especially if other medical problems are consuming much of their time. We also have the community care referral option for patients whose treatment decisions are being dictated by travel hardships.
Strengths and Limitations
A strength of this study is that the data were obtained from a closed system. Patients tend to stay long-term within the VA and their health record is accessible throughout the country as long as they are seen at a VA facility. Complications, therefore, return to the treating service or primary care, who would route the patient back to the surgeon.
One limitation of the study is that this is a retrospective review from 2011. The authors are limited to data that are recorded in the patient record. Multiple health care professionals saw the patients and notes lack consistency in detail. Size of the lesions were not consistently recorded and did not get logged into our database for that reason.
Conclusions
Treatment of NMSC in older adult patients has a low morbidity but needs to be balanced against a patient and family’s goals when the patient presents with life-limiting comorbidities. An elevated 5-year mortality in patients aged > 80 years with serious unrelated medical conditions is intuitive, but this study may help put treatment plans into perspective for families and health care professionals who want to provide an indicated service while maximizing patient quality of life.
Acknowledgments
This manuscript is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
Skin cancer is the most diagnosed cancer in the United States. Nonmelanoma skin cancers (NMSC), which include basal cell carcinoma and squamous cell carcinoma, are usually cured with removal.1 The incidence of NMSC increases with age and is commonly found in nursing homes and geriatric units. These cancers are not usually metastatic or fatal but can cause local destruction and disfigurement if neglected.2 The current standard of care is to treat diagnosed NMSC; however, the dermatology and geriatric care literature have questioned the logic of treating asymptomatic skin cancers that will not affect a patient’s life expectancy.2-4
Forty-seven percent of the current living veteran population is aged ≥ 65 years.5 Older adult patients are frequently referred to the US Department of Veterans Affairs (VA) surgical service for the treatment of NMSC. The veteran population includes a higher percentage of individuals at an elevated risk of skin cancers (older, White, and male) compared with the general population.6 World War II veterans deployed in regions closer to the equator have been found to have an elevated risk of melanoma and nonmelanoma skin carcinomas.7 A retrospective study of Vietnam veterans exposed to Agent Orange (2,3,7,8-tetrachlorodibenzodioxin) found a significantly higher risk of invasive NMSC in Fitzpatrick skin types I-IV compared with an age-matched subset of the general population.8 Younger veterans who were deployed in Afghanistan and Iraq for Operation Enduring Freedom/Operation Iraqi Freedom worked at more equatorial latitudes than the rest of the US population and may be at increased risk of NMSC. Inadequate sunscreen access, immediate safety concerns, outdoor recreational activities, harsh weather, and insufficient emphasis on sun protection have created a multifactorial challenge for the military population. Riemenschneider and colleagues recommended targeted screening for at-risk veteran patients and prioritizing annual skin cancer screenings during medical mission physical examinations for active military.7
The plastic surgery service regularly receives consults from dermatology, general surgery, and primary care to remove skin cancers on the face, scalp, hands, and forearms. Skin cancer treatment can create serious hardships for older adult patients and their families with multiple appointments for the consult, procedure, and follow-up. Patients are often told to hold their anticoagulant medications when the surgery will be performed on a highly vascular region, such as the scalp or face. This can create wide swings in their laboratory test values and result in life-threatening complications from either bleeding or clotting. The appropriateness of offering surgery to patients with serious comorbidities and a limited life expectancy has been questioned.2-4 The purpose of this study was to measure the morbidity and unrelated 5-year mortality for patients with skin cancer referred to the plastic surgery service to help patients and families make a more informed treatment decision, particularly when the patients are aged > 80 years and have significant life-threatening comorbidities.
Methods
The University of Florida and Malcom Randall VA Medical Center Institutional review board in Gainesville, approved a retrospective review of all consults completed by the plastic surgery service for the treatment of NMSC performed from July 1, 2011 to June 30, 2015. Data collected included age and common life-limiting comorbidities at the time of referral. Morbidities were found on the electronic health record, including coronary artery disease (CAD), congestive heart failure (CHF), cerebral vascular disease (CVD), peripheral vascular disease, dementia, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), tobacco use, diabetes mellitus (DM), liver disease, alcohol use, and obstructive sleep apnea.
Treatment, complications, and 5-year mortality were recorded. A χ2 analysis with P value < .05 was used to determine statistical significance between individual risk factors and 5-year mortality. The relative risk of 5-year mortality was calculated by combining advanced age (aged > 80 years) with the individual comorbidities.
Results
Over 4 years, 800 consults for NMSC were completed by the plastic surgery service. Treatment decisions included 210 excisions (with or without reconstruction) in the operating room, 402 excisions (with or without reconstruction) under local anesthesia in clinic, 55 Mohs surgical dermatology referrals, 21 other service or hospital referrals, and 112 patient who were observed, declined intervention, or died prior to intervention. Five-year mortality was 28.6%. No patients died of NMSC. The median age at consult submission for patients deceased 5 years later was 78 years. Complication rate was 5% and included wound infection, dehiscence, bleeding, or graft loss. Two patients, both deceased within 5 years, had unplanned admissions due to bleeding from either a skin graft donor site or recipient bleeding. Aged ≥ 80 years, CAD, CHF, CVD, peripheral vascular disease, dementia, CKD, COPD, and DM were all found individually to be statistically significant predictors of 5-year mortality (Table 1). Combining aged ≥ 80 years plus CAD, CHF, or dementia all increased the 5-year mortality by a relative risk of > 3 (Table 2).
Discussion
The standard of care is to treat NMSC. Most NMSCs are treated surgically without consideration of patient age or life expectancy.2,4,9,10 A prospective cohort study involving a university-based private practice and a VA medical center in San Francisco found a 22.6% overall 5-year mortality and a 43.3% mortality in the group defined as limited life expectancy (LLE) based on age (≥ 85 years) and medical comorbidities. None died due to the NMSC. Leading cause of death was cardiac, cerebrovascular, and respiratory disease, lung and prostate cancer, and Alzheimer disease. The authors suggested the LLE group may be exposed to wound complications without benefiting from the treatment.4
Another study of 440 patients receiving excision for biopsy-proven facial NMSC at the Roudebush VA Medical Center in Indianapolis, Indiana, found no residual carcinoma in 35.3% of excisions, and in patients aged > 90 years, more than half of the excisions had no residual carcinoma. More than half of the patients aged > 90 years died within 1 year, not as a result of the NMSC. The authors argued for watchful waiting in select patients to maximize comfort and outcomes.10
NMSCs are often asymptomatic and not immediately life threatening. Although NMSCs tend to have a favorable prognosis, studies have found that NMSC may be a marker for other poor health outcomes. A significant increased risk for all-cause mortality was found for patients with a history of SCC, which may be attributed to immune status.11 The aging veteran population has more complex health care needs to be considered when developing surgical treatment plans. These medical problems may limit their life expectancy much sooner than the skin cancer will become symptomatic. We found that individuals aged ≥ 80 years who had CAD, CHF, or dementia had a relative risk of 3 or higher for 5-year mortality. The leading cause of death in the United States in years 2011 to 2015 was heart disease. Alzheimer disease was the sixth leading cause of death in those same years.12-14
Skin cancer excisions do not typically require general anesthesia, deep sedation, or large fluid shifts; however, studies have found that when frail patients undergo low-risk procedures, they tend to have a higher mortality rate than their healthier counterparts.15 Frailty is a concept that identifies patients who are at increased risk of dying in 6 to 60 months due to a decline in their physical reserve. Frail patients have increased rates of perioperative mortality and complications. Various tools have been used to assess the components of physical performance, speed, mobility, nutrition status, mental health, and cognition.16 Frailty screening has been initiated in several VA hospitals, including our own in Gainesville, Florida, with the goal of decreasing postoperative morbidity and mortality in older adult patients.17 The patients are given a 1-page screening assessment that asks about their living situation, medical conditions, nutrition status, cognition, and activities of daily living. The results can trigger the clinician to rethink the surgical plan and mobilize more resources to optimize the patient’s health. This study period precedes the initiative at our institution.
The plastic surgery service’s routine practice is to excise skin cancers in the operating room if sedation or general anesthesia will be needed (Figure 1A), for optimal control of bleeding (Figure 1B) in a patient who cannot safely stop blood thinners, or for excision of a highly vascularized area such as the scalp. Surgery is offered in an office-based setting if the area can be closed primarily, left open to close secondarily, or closed with a small skin graft under local anesthesia only (Figure 2). We prefer treating frail patients in the minor procedure clinic, when possible, to avoid the risks of sedation and the additional preoperative visits and transportation requirements. NMSC with unclear margins (Figure 3A) or in cosmetically sensitive areas where tissue needs to be preserved (Figure 3B) are referred to the Mohs dermatologist. The skin cancers in this study were most frequently found on the face, scalp, hands, and forearms based on referral patterns.
Other treatment options for NMSC include curettage and electrodessication, cryotherapy, and radiation; however, ours is a surgical service and patients are typically referred to us by primary care or dermatology when those are not reasonable or desirable options.18 Published complication rates of patients having skin cancer surgery without age restriction have a rate of 3% to 6%, which is consistent with our study of 5%.19-21 Two bleeding complications that needed to be admitted did not require more than a bedside procedure and neither required transfusions. One patient had been instructed to continue taking coumadin during the perioperative office-based procedure due to a recent carotid stent placement in the setting of a rapidly growing basal cell on an easily accessible location.
The most noted comorbidity in patients with wound complications was found to be DM; however, this was not found to be a statistically significant risk factor for wound complications (P = .10). We do not have a set rule for advising for or against NMSC surgery. We do counsel frail patients and their families that not all cancer is immediately life threatening and will work with them to do whatever makes the most sense to achieve their goals, occasionally accepting positive margins in order to debulk a symptomatic growth. The objective of this paper is to contribute to the discussion of performing invasive procedures on older adult veterans with life-limiting comorbidities. Patients and their families will have different thresholds for what they feel needs intervention, especially if other medical problems are consuming much of their time. We also have the community care referral option for patients whose treatment decisions are being dictated by travel hardships.
Strengths and Limitations
A strength of this study is that the data were obtained from a closed system. Patients tend to stay long-term within the VA and their health record is accessible throughout the country as long as they are seen at a VA facility. Complications, therefore, return to the treating service or primary care, who would route the patient back to the surgeon.
One limitation of the study is that this is a retrospective review from 2011. The authors are limited to data that are recorded in the patient record. Multiple health care professionals saw the patients and notes lack consistency in detail. Size of the lesions were not consistently recorded and did not get logged into our database for that reason.
Conclusions
Treatment of NMSC in older adult patients has a low morbidity but needs to be balanced against a patient and family’s goals when the patient presents with life-limiting comorbidities. An elevated 5-year mortality in patients aged > 80 years with serious unrelated medical conditions is intuitive, but this study may help put treatment plans into perspective for families and health care professionals who want to provide an indicated service while maximizing patient quality of life.
Acknowledgments
This manuscript is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. American Cancer Society. Cancer Facts & Figures 2021. Accessed May 26, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
2. Albert A, Knoll MA, Conti JA, Zbar RIS. Non-melanoma skin cancers in the older patient. Curr Oncol Rep. 2019;21(9):79. Published 2019 Jul 29. doi:10.1007/s11912-019-0828-9
3. Linos E, Chren MM, Stijacic Cenzer I, Covinsky KE. Skin cancer in U.S. elderly adults: does life expectancy play a role in treatment decisions? J Am Geriatr Soc. 2016;64(8):1610-1615. doi:10.1111/jgs.14202
4. Linos E, Parvataneni R, Stuart SE, Boscardin WJ, Landefeld CS, Chren MM. Treatment of nonfatal conditions at the end of life: nonmelanoma skin cancer. JAMA Intern Med. 2013;173(11):1006-1012. doi:10.1001/jamainternmed.2013.639
5. O’Malley KA, Vinson L, Kaiser AP, Sager Z, Hinrichs K. Mental health and aging veterans: how the Veterans Health Administration meets the needs of aging veterans. Public Policy Aging Rep. 2020;30(1):19-23. doi:10.1093/ppar/prz027
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2017. Accessed May 26, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2017.pdf 7. Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78(6):1185-1192. doi:10.1016/j.jaad.2017.11.062
8. Clemens MW, Kochuba AL, Carter ME, Han K, Liu J, Evans K. Association between Agent Orange exposure and nonmelanotic invasive skin cancer: a pilot study. Plast Reconstr Surg. 2014;133(2):432-437. doi:10.1097/01.prs.0000436859.40151.cf
9. Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303-317. doi:10.1016/j.jaad.2018.03.060
10. Chauhan R, Munger BN, Chu MW, et al. Age at diagnosis as a relative contraindication for intervention in facial nonmelanoma skin cancer. JAMA Surg. 2018;153(4):390-392. doi:10.1001/jamasurg.2017.5073
11. Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309(4):243-251. doi:10.1007/s00403-017-1724-5
12. Kochanek KD, Murphy SL, Xu JQ, Arias E. Mortality in the United States, 2013. NCHS Data Brief 178. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db178.htm
13. Xu JQ, Kochanek KD, Murphy SL, Arias E. Mortality in the United States, 2012. NCHS Data Brief 168. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db168.htm
14. Xu JQ, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2015. NCHS Data Brief 267. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db267.htm
15. Varley PR , Borrebach JD, Arya S, et al. Clinical utility of the risk analysis index as a prospective frailty screening tool within a multi-practice, multi-hospital integrated healthcare system. Ann Surg. 2021;274(6):e1230-e1237. doi:10.1097/SLA.0000000000003808
16. Hall DE, Arya S , Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152(2):175-182. doi:10.1001/jamasurg.2016.4202
17. US Department of Veterans Affairs, Health Services Research & Development. Improving healthcare for aging veterans. Updated August 30, 2017. Accessed May 26, 2022. https://www.hsrd.research.va.gov/news/feature/aging0917.cfm
18. Leus AJG, Frie M, Haisma MS, et al. Treatment of keratinocyte carcinoma in elderly patients – a review of the current literature. J Eur Acad Dermatol Venereol. 2020;34(9):1932-1943. doi:10.1111/jdv.16268
19. Amici JM, Rogues AM, Lasheras A, et al. A prospective study of the incidence of complications associated with dermatological surgery. Br J Dermatol. 2005;153(5):967-971. doi:10.1111/j.1365-2133.2005.06861.x
20. Arguello-Guerra L, Vargas-Chandomid E, Díaz-González JM, Méndez-Flores S, Ruelas-Villavicencio A, Domínguez-Cherit J. Incidence of complications in dermatological surgery of melanoma and non-melanoma skin cancer in patients with multiple comorbidity and/or antiplatelet-anticoagulants. Five-year experience in our hospital. Cir Cir. 2019;86(1):15-23. doi:10.24875/CIRUE.M18000003
21. Keith DJ, de Berker DA, Bray AP, Cheung ST, Brain A, Mohd Mustapa MF. British Association of Dermatologists’ national audit on nonmelanoma skin cancer excision, 2014. Clin Exp Dermatol. 2017;42(1):46-53. doi:10.1111/ced.12990
1. American Cancer Society. Cancer Facts & Figures 2021. Accessed May 26, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
2. Albert A, Knoll MA, Conti JA, Zbar RIS. Non-melanoma skin cancers in the older patient. Curr Oncol Rep. 2019;21(9):79. Published 2019 Jul 29. doi:10.1007/s11912-019-0828-9
3. Linos E, Chren MM, Stijacic Cenzer I, Covinsky KE. Skin cancer in U.S. elderly adults: does life expectancy play a role in treatment decisions? J Am Geriatr Soc. 2016;64(8):1610-1615. doi:10.1111/jgs.14202
4. Linos E, Parvataneni R, Stuart SE, Boscardin WJ, Landefeld CS, Chren MM. Treatment of nonfatal conditions at the end of life: nonmelanoma skin cancer. JAMA Intern Med. 2013;173(11):1006-1012. doi:10.1001/jamainternmed.2013.639
5. O’Malley KA, Vinson L, Kaiser AP, Sager Z, Hinrichs K. Mental health and aging veterans: how the Veterans Health Administration meets the needs of aging veterans. Public Policy Aging Rep. 2020;30(1):19-23. doi:10.1093/ppar/prz027
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2017. Accessed May 26, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2017.pdf 7. Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78(6):1185-1192. doi:10.1016/j.jaad.2017.11.062
8. Clemens MW, Kochuba AL, Carter ME, Han K, Liu J, Evans K. Association between Agent Orange exposure and nonmelanotic invasive skin cancer: a pilot study. Plast Reconstr Surg. 2014;133(2):432-437. doi:10.1097/01.prs.0000436859.40151.cf
9. Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303-317. doi:10.1016/j.jaad.2018.03.060
10. Chauhan R, Munger BN, Chu MW, et al. Age at diagnosis as a relative contraindication for intervention in facial nonmelanoma skin cancer. JAMA Surg. 2018;153(4):390-392. doi:10.1001/jamasurg.2017.5073
11. Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309(4):243-251. doi:10.1007/s00403-017-1724-5
12. Kochanek KD, Murphy SL, Xu JQ, Arias E. Mortality in the United States, 2013. NCHS Data Brief 178. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db178.htm
13. Xu JQ, Kochanek KD, Murphy SL, Arias E. Mortality in the United States, 2012. NCHS Data Brief 168. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db168.htm
14. Xu JQ, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2015. NCHS Data Brief 267. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db267.htm
15. Varley PR , Borrebach JD, Arya S, et al. Clinical utility of the risk analysis index as a prospective frailty screening tool within a multi-practice, multi-hospital integrated healthcare system. Ann Surg. 2021;274(6):e1230-e1237. doi:10.1097/SLA.0000000000003808
16. Hall DE, Arya S , Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152(2):175-182. doi:10.1001/jamasurg.2016.4202
17. US Department of Veterans Affairs, Health Services Research & Development. Improving healthcare for aging veterans. Updated August 30, 2017. Accessed May 26, 2022. https://www.hsrd.research.va.gov/news/feature/aging0917.cfm
18. Leus AJG, Frie M, Haisma MS, et al. Treatment of keratinocyte carcinoma in elderly patients – a review of the current literature. J Eur Acad Dermatol Venereol. 2020;34(9):1932-1943. doi:10.1111/jdv.16268
19. Amici JM, Rogues AM, Lasheras A, et al. A prospective study of the incidence of complications associated with dermatological surgery. Br J Dermatol. 2005;153(5):967-971. doi:10.1111/j.1365-2133.2005.06861.x
20. Arguello-Guerra L, Vargas-Chandomid E, Díaz-González JM, Méndez-Flores S, Ruelas-Villavicencio A, Domínguez-Cherit J. Incidence of complications in dermatological surgery of melanoma and non-melanoma skin cancer in patients with multiple comorbidity and/or antiplatelet-anticoagulants. Five-year experience in our hospital. Cir Cir. 2019;86(1):15-23. doi:10.24875/CIRUE.M18000003
21. Keith DJ, de Berker DA, Bray AP, Cheung ST, Brain A, Mohd Mustapa MF. British Association of Dermatologists’ national audit on nonmelanoma skin cancer excision, 2014. Clin Exp Dermatol. 2017;42(1):46-53. doi:10.1111/ced.12990
Twenty Years of Breast Reduction Surgery at a Veterans Affairs Medical Center
Women make up an estimated 10% of the veteran population.1 The US Department of Veterans Affairs (VA) projected that there would be an increase of 18,000 female veterans per year for 10 years based on 2015 data. The number of women veterans enrolled in the VA health care increased from 397,024 to 729,989 (83.9%) between 2005 and 2015.2 This rise in the number of enrolled women veterans also increased the demand for female-specific health care services, such as breast reduction surgery, a reconstructive procedure provided at the Malcom Randall VA Medical Center (MRVAMC) federal teaching hospital in Gainesville, Florida.
Patients who experience symptomatic macromastia will report a history of neck and shoulder pain, shoulder grooving from bra straps, inframammary intertrigo, difficulty finding clothes that fit, and discomfort participating in sports. For the treatment of symptomatic macromastia, patients report a high satisfaction rate after breast reduction surgery.3-5 Unfortunately, the complications from the surgery can significantly disrupt a woman’s life due to previously unplanned hospital admissions, clinic appointments, wound care, time off work, and poor aesthetic outcome. Faculty awareness of a large number of complications for patients after breast reduction surgery prompted the MRVAMC Plastic Surgery Service to establish a stricter surgical screening protocol using body mass index (BMI) values and negative nicotine status to help patients be healthier and reduce the potential risk before offering surgery. A medical literature search did not find an existing study on veteran-specific breast reduction surgery.
Methods
The University of Florida and North Florida/South Georgia Veterans Health System Institutional Review Board approved a retrospective chart review of all breast reduction surgeries performed at MRVAMC over a 20-year period (July 1, 2000-June 30, 2020). Electronic health records were queried for all primary bilateral breast reduction surgeries performed for symptomatic macromastia using Current Procedural Terminology code 19318. Potentially modifiable or predictable risk factors for wound complications were recorded: nicotine status, BMI, diabetes mellitus (DM) status, skin incision pattern, and pedicle location. Skin incision patterns were either vertical (periareolar plus a vertical scar from the areola to the inframammary fold) or traditional Wise pattern (also known as anchor pattern: periareolar scar, vertical scar to inframammary fold, plus a horizontal scar along the inframammary fold) as seen in Figures 1 and 2. The pedicle is the source of blood supply to the nipple, which was documented as either from the inferior aspect or the superior or superior/medial aspect.
For this study, the blood supply from the superior and superior/medial was logged in the same category. Records were reviewed 3 months after surgery for documentation of local wound complications, such as hematoma, infection, wound breakdown, skin necrosis, and nipple necrosis. Major complications were defined as requiring an unplanned hospital admission or urgent return to the operating room. A χ2 test using a P value of < .05 was used to determine statistical significance between the incidence of wound complications and the individually identifiable variables.
Results
One hundred fifteen bilateral breast reduction surgeries were performed at MRVAMC over a 20-year period. Patient median age was 43 years. Median combined specimen weight was 1272 g. Forty-eight (41.7%) wound complications were documented, including 8 (7%) major complications. Most complications were identified in the first 7 years of the study before the new protocol and consult template became active. The new template resulted in the local complication rate dropping from 62% (July 2000-June 2007) to 26% (July 2007-June 2020). BMI > 32 (P = .03) and active nicotine use (P = .004) were found to be statistically significant independent risk factors for wound complications. Median BMI for all patients was 30. DM status (P = .22), skin incision pattern (P = .25), and pedicle location (P = .13) were not found to be predictors of wound complications (Table). There was no significant change in the incidence of major complications before and after the new protocols were enforced.
Discussion
Breast reduction surgery is an elective reconstructive option to treat symptomatic macromastia. There are several accepted ways to do the reduction surgical procedure where the blood supply (pedicle) to the nipple can vary and the visible scars can be in a horizontal, vertical, or Wise pattern. Technique is usually based on surgeon training, comfort, and preference. There are several known complications specific to this operation that include asymmetry, changes in nipple sensation, unattractive scars, diminished ability to breastfeed, and wound complications.5-7 Wound complications include seroma, hematoma, dehiscence, infection, wound breakdown, skin necrosis, and nipple necrosis.
This study focused on wound complications with the objective of identifying and modifying risk factors. Two known risk factors documented in the literature, nicotine use and obesity, already had been addressed by our service, and results were known anecdotally but had not been previously verified. This study also looked at other potential risk factors, including the pedicle location, skin incision, and DM status.
Residents or fellows participated in all the surgeries. An outcome analysis from The American College of Surgeons National Surgical Quality Improvement Program database from 2005 to 2011 found that resident participation was associated with morbidity, including wound complications.8 This study was performed at a federal hospital with a complexity level 1a rating, which is designated based on the highest level of patient volume, risk, teaching, research, intensive care unit beds, and specialty services.9 The hospital is closely affiliated with a level 1 trauma center and teaching hospital; therefore, resident and fellow participation is not a modifiable risk factor.
This study did not find an increased risk of wound complications in patients with DM, which has been found to be an independent risk factor in a prior study.10 DM status was indicated in only 3 histories, and they all had perioperative hemoglobin A1c levels < 8%. There is documentation of patients receiving perioperative antibiotics in 99 out of 116 of the surgical records; however, we did not include this in the analysis because the operative reports from the first year of the study were incomplete.
Smoking is a known risk factor for local wound complications in breast reduction surgery.10-15 The VA has a smoking cessation program through its mental health service that provides counseling and medication treatment options, including nicotine replacement, bupropion, and varenicline. We require patients to be at least 4 weeks nicotine free before surgery, which has been previously recommended in the literature.16
Existing studies that compare the traditional Wise pattern/inferior pedicle with vertical pattern/superior medial pedicle did not find an increased risk of wound complications.17-19 Our study separated the different incisions from the pedicle because the surgical technique among the different surgeons in the study varied, where sometimes the traditional Wise pattern was combined with the less traditional superior-medial pedicle. We did not find a statistical difference when comparing the incisions and pedicle location, which suggests that the incision type and source of blood supply to the nipple are not the determining factors for wound complications in the early postoperative period.
Obesity is a known risk factor for local wound complications.12,13,15,20-22 Studies have shown that patients who are obese benefit from breast reduction surgery; authors have argued against restricting surgery to these higher risk patients.4,23-25 Patients usually report decades of macromastia symptoms at consultation; so, we believe delaying the surgical procedure to get patients to a safer risk profile is in their best interest. We chose a cutoff BMI of 32 as a realistic value rather than 30, which is considered the definition of obesity. Patients at MRVAMC have access to MOVE!, a weight loss management program through primary care. We believe in being reasonable; so if a patient makes a significant improvement in her health but falls short of the required cutoff, we will still consider offering the surgical procedure after a full explanation of the surgical risks.
Wound complications, especially those that require admission or frequent appointments can seriously disrupt a patient’s life, creating unnecessary hardships and expense in time lost from work, travel, and child care. MRVAMC has a catchment area the size of North Carolina; so many of our patients travel hours for their appointments. The added scars and deformity from wound dehiscence and debridement can lead to asymmetry, widened scars, and future revision operations. Multiple clinic appointments for wound care not only impact that individual patient, but also has the effect of limiting access for all patients in a health care environment with high patient volume and limited providers, operating room time, and clinic appointments. As a result, minimizing predictable wound complications benefits the entire system.
Limitations and Strengths
This retrospective review comprised multiple different surgeons, including faculty and trainees, who were involved in the consultation, surgery, and postoperative care of the patients over a 20-year period; therefore, consistency in documentation is lacking. In addition, we were limited to only the information available on the charts. For example, wound size and laterality were not consistently documented. The MRVAMC complication rate was consistent with the current literature (range, 14-52%).12,18,20,24
The major strength of the study is that the veterans tend to stay within the VA, which makes complications easier to identify and follow. Patients who do not present initially to their surgeon due to travel limitations will typically contact their primary care provider or present to their local VA urgent care or emergency department provider, who will route the patient back to the surgical specialty service through the electronic health record.
Conclusions
Breast reduction surgery has a high wound complication rate, which can be predicted and improved on so that patients can receive their indicated surgical procedure with minimal inconvenience and downtime. This review confirms that preoperative weight loss and nicotine cessation were the appropriate focus of the MRVAMC plastic surgery service’s efforts to achieve a safer surgical experience. We will continue to enforce our protocol and encourage patients who are interested in breast reduction surgery and fall outside the requirements to work with their primary care provider on smoking cessation and weight loss through better nutrition and physical activity.
Acknowledgment
This manuscript is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. US Department of Veterans Affairs. Statistics at a glance. Published February 2020. Accessed June 18, 2021. https://www.va.gov/vetdata/docs/Quickfacts/Homepage_slideshow_4_6_20.PDF
2. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Published February 2017. Accessed June 18, 2020. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf
3. Crittenden TA, Watson DI, Ratcliffe J, Griffin PA, Dean NR. Outcomes of breast reduction surgery using the breast-q: a prospective study and comparison with normative data. Plast Reconstr Surg. 2019;144(5):1034-1044. doi:10.1097/PRS.0000000000006114
4. Thoma A, Sprague S, Veltri K, Duku E, Furlong W. A prospective study of patients undergoing breast reduction surgery: health-related quality of life and clinical outcomes. Plast Reconstr Surg. 2007;120(1):13-26. doi:10.1097/01.prs.0000263370.94191.90
5. Nuzzi LC, Firriolo JM, Pike CM, DiVasta AD, Labow BI. Complications and quality of life following reduction mammaplasty in adolescents and young women.Plast Reconstr Surg. 2019;144(3):572-581. doi:10.1097/PRS.0000000000005907
6. Hall-Findlay EJ, Shestak KC. Breast reduction. Plast Reconstr Surg. 2015;136(4):531e-544e. doi:10.1097/PRS.0000000000001622
7. Kraut RY, Brown E, Korownyk C, et al. The impact of breast reduction surgery on breastfeeding: systematic review of observational studies. PLoS One. 2017;12(10):e0186591. doi:10.1371/journal.pone.0186591
8. Fischer JP, Wes AM, Kovach SJ. The impact of surgical resident participation in breast reduction surgery--outcome analysis from the 2005-2011 ACS-NSQIP datasets. J Plast Surg Hand Surg. 2014;48(5):315-321. doi:10.3109/2000656X.2014.882345
9. Site Facility Name and Complexity Summary of VHA Facility. Accessed June 18, 2021. https://www.vendorportal.ecms.va.gov/FBODocumentServer/DocumentServer.aspx?DocumentId=2793591&FileName=VA118-16-R-1059-A00002002.docx
10. Lewin R, Göransson M, Elander A, Thorarinsson A, Lundberg J, Lidén M. Risk factors for complications after breast reduction surgery. J Plast Surg Hand Surg. 2014;48(1):10-14. doi:10.3109/2000656X.2013.791625
11. Cunningham BL, Gear AJ, Kerrigan CL, Collins ED. Analysis of breast reduction complications derived from the BRAVO study. Plast Reconstr Surg. 2005;115(6):1597-1604. doi:10.1097/01.prs.0000160695.33457.db
12. Karamanos E, Wei B, Siddiqui A, Rubinfeld I. Tobacco use and body mass index as predictors of outcomes in patients undergoing breast reduction mammoplasty. Ann Plast Surg. 2015;75(4):383-387. doi:10.1097/SAP.0000000000000192
13. Manahan MA, Buretta KJ, Chang D, Mithani SK, Mallalieu J, Shermak MA. An outcomes analysis of 2142 breast reduction procedures. Ann Plast Surg. 2015;74(3):289-292. doi:10.1097/SAP.0b013e31829d2261
14. Hillam JS, Borsting EA, Chim JH, Thaller SR. Smoking as a risk factor for breast reduction: an analysis of 13,503 cases. J Plast Reconstr Aesthet Surg. 2017;70(6):734-740. doi:10.1016/j.bjps.2016.12.012
15. Zhang MX, Chen CY, Fang QQ, et al. Risk factors for complications after reduction mammoplasty: a meta-analysis. PLoS One. 2016;11(12):e0167746. doi:10.1371/journal.pone.0167746
16. Sørensen LT. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Ann Surg. 2012;255(6):1069-1079.doi:10.1097/SLA.0b013e31824f632d
17. Antony AK, Yegiyants SS, Danielson KK, et al. A matched cohort study of superomedial pedicle vertical scar breast reduction (100 breasts) and traditional inferior pedicle Wise-pattern reduction (100 breasts): an outcomes study over 3 years. Plast Reconstr Surg. 2013;132(5):1068-1076. doi:10.1097/PRS.0b013e3182a48b2d
18. Hunter-Smith DJ, Smoll NR, Marne B, Maung H, Findlay MW. Comparing breast-reduction techniques: time-to-event analysis and recommendations. Aesthetic Plast Surg. 2012;36(3):600-606. doi:10.1007/s00266-011-9860-3
19. Ogunleye AA, Leroux O, Morrison N, Preminger AB. Complications after reduction mammaplasty: a comparison of wise pattern/inferior pedicle and vertical scar/superomedial pedicle. Ann Plast Surg. 2017;79(1):13-16. doi:10.1097/SAP.0000000000001059
20. Bauermeister AJ, Gill K, Zuriarrain A, Earle SA, Newman MI. Reduction mammaplasty with superomedial pedicle technique: a literature review and retrospective analysis of 938 consecutive breast reductions. J Plast Reconstr Aesthet Surg. 2019;72(3):410-418. doi:10.1016/j.bjps.2018.12.004
21. Nelson JA, Fischer JP, Chung CU, et al. Obesity and early complications following reduction mammaplasty: an analysis of 4545 patients from the 2005-2011 NSQIP datasets. J Plast Surg Hand Surg. 2014;48(5):334-339. doi:10.3109/2000656X.2014.886582
22. Kreithen J, Caffee H, Rosenberg J, et al. A comparison of the LeJour and Wise pattern methods of breast reduction. Ann Plast Surg. 2005;54(3):236-241. doi:10.3109/2000656X.2014.886582
23. Güemes A, Pérez E, Sousa R, et al. Quality of life and alleviation of symptoms after breast reduction for macromastia in obese patients: is surgery worth it? Aesthetic Plast Surg. 2016;40(1):62-70. doi:10.1007/s00266-015-0601-x
24. Setälä L, Papp A, Joukainen S, et al. Obesity and complications in breast reduction surgery: are restrictions justified? J Plast Reconstr Aesthet Surg. 2009;62(2):195-199. doi:10.1016/j.bjps.2007.10.043
25. Shah R, Al-Ajam Y, Stott D, Kang N. Obesity in mammaplasty: a study of complications following breast reduction. J Plast Reconstr Aesthet Surg. 2011;64(4):508-514. doi:10.1016/j.bjps.2007.10.043
Women make up an estimated 10% of the veteran population.1 The US Department of Veterans Affairs (VA) projected that there would be an increase of 18,000 female veterans per year for 10 years based on 2015 data. The number of women veterans enrolled in the VA health care increased from 397,024 to 729,989 (83.9%) between 2005 and 2015.2 This rise in the number of enrolled women veterans also increased the demand for female-specific health care services, such as breast reduction surgery, a reconstructive procedure provided at the Malcom Randall VA Medical Center (MRVAMC) federal teaching hospital in Gainesville, Florida.
Patients who experience symptomatic macromastia will report a history of neck and shoulder pain, shoulder grooving from bra straps, inframammary intertrigo, difficulty finding clothes that fit, and discomfort participating in sports. For the treatment of symptomatic macromastia, patients report a high satisfaction rate after breast reduction surgery.3-5 Unfortunately, the complications from the surgery can significantly disrupt a woman’s life due to previously unplanned hospital admissions, clinic appointments, wound care, time off work, and poor aesthetic outcome. Faculty awareness of a large number of complications for patients after breast reduction surgery prompted the MRVAMC Plastic Surgery Service to establish a stricter surgical screening protocol using body mass index (BMI) values and negative nicotine status to help patients be healthier and reduce the potential risk before offering surgery. A medical literature search did not find an existing study on veteran-specific breast reduction surgery.
Methods
The University of Florida and North Florida/South Georgia Veterans Health System Institutional Review Board approved a retrospective chart review of all breast reduction surgeries performed at MRVAMC over a 20-year period (July 1, 2000-June 30, 2020). Electronic health records were queried for all primary bilateral breast reduction surgeries performed for symptomatic macromastia using Current Procedural Terminology code 19318. Potentially modifiable or predictable risk factors for wound complications were recorded: nicotine status, BMI, diabetes mellitus (DM) status, skin incision pattern, and pedicle location. Skin incision patterns were either vertical (periareolar plus a vertical scar from the areola to the inframammary fold) or traditional Wise pattern (also known as anchor pattern: periareolar scar, vertical scar to inframammary fold, plus a horizontal scar along the inframammary fold) as seen in Figures 1 and 2. The pedicle is the source of blood supply to the nipple, which was documented as either from the inferior aspect or the superior or superior/medial aspect.
For this study, the blood supply from the superior and superior/medial was logged in the same category. Records were reviewed 3 months after surgery for documentation of local wound complications, such as hematoma, infection, wound breakdown, skin necrosis, and nipple necrosis. Major complications were defined as requiring an unplanned hospital admission or urgent return to the operating room. A χ2 test using a P value of < .05 was used to determine statistical significance between the incidence of wound complications and the individually identifiable variables.
Results
One hundred fifteen bilateral breast reduction surgeries were performed at MRVAMC over a 20-year period. Patient median age was 43 years. Median combined specimen weight was 1272 g. Forty-eight (41.7%) wound complications were documented, including 8 (7%) major complications. Most complications were identified in the first 7 years of the study before the new protocol and consult template became active. The new template resulted in the local complication rate dropping from 62% (July 2000-June 2007) to 26% (July 2007-June 2020). BMI > 32 (P = .03) and active nicotine use (P = .004) were found to be statistically significant independent risk factors for wound complications. Median BMI for all patients was 30. DM status (P = .22), skin incision pattern (P = .25), and pedicle location (P = .13) were not found to be predictors of wound complications (Table). There was no significant change in the incidence of major complications before and after the new protocols were enforced.
Discussion
Breast reduction surgery is an elective reconstructive option to treat symptomatic macromastia. There are several accepted ways to do the reduction surgical procedure where the blood supply (pedicle) to the nipple can vary and the visible scars can be in a horizontal, vertical, or Wise pattern. Technique is usually based on surgeon training, comfort, and preference. There are several known complications specific to this operation that include asymmetry, changes in nipple sensation, unattractive scars, diminished ability to breastfeed, and wound complications.5-7 Wound complications include seroma, hematoma, dehiscence, infection, wound breakdown, skin necrosis, and nipple necrosis.
This study focused on wound complications with the objective of identifying and modifying risk factors. Two known risk factors documented in the literature, nicotine use and obesity, already had been addressed by our service, and results were known anecdotally but had not been previously verified. This study also looked at other potential risk factors, including the pedicle location, skin incision, and DM status.
Residents or fellows participated in all the surgeries. An outcome analysis from The American College of Surgeons National Surgical Quality Improvement Program database from 2005 to 2011 found that resident participation was associated with morbidity, including wound complications.8 This study was performed at a federal hospital with a complexity level 1a rating, which is designated based on the highest level of patient volume, risk, teaching, research, intensive care unit beds, and specialty services.9 The hospital is closely affiliated with a level 1 trauma center and teaching hospital; therefore, resident and fellow participation is not a modifiable risk factor.
This study did not find an increased risk of wound complications in patients with DM, which has been found to be an independent risk factor in a prior study.10 DM status was indicated in only 3 histories, and they all had perioperative hemoglobin A1c levels < 8%. There is documentation of patients receiving perioperative antibiotics in 99 out of 116 of the surgical records; however, we did not include this in the analysis because the operative reports from the first year of the study were incomplete.
Smoking is a known risk factor for local wound complications in breast reduction surgery.10-15 The VA has a smoking cessation program through its mental health service that provides counseling and medication treatment options, including nicotine replacement, bupropion, and varenicline. We require patients to be at least 4 weeks nicotine free before surgery, which has been previously recommended in the literature.16
Existing studies that compare the traditional Wise pattern/inferior pedicle with vertical pattern/superior medial pedicle did not find an increased risk of wound complications.17-19 Our study separated the different incisions from the pedicle because the surgical technique among the different surgeons in the study varied, where sometimes the traditional Wise pattern was combined with the less traditional superior-medial pedicle. We did not find a statistical difference when comparing the incisions and pedicle location, which suggests that the incision type and source of blood supply to the nipple are not the determining factors for wound complications in the early postoperative period.
Obesity is a known risk factor for local wound complications.12,13,15,20-22 Studies have shown that patients who are obese benefit from breast reduction surgery; authors have argued against restricting surgery to these higher risk patients.4,23-25 Patients usually report decades of macromastia symptoms at consultation; so, we believe delaying the surgical procedure to get patients to a safer risk profile is in their best interest. We chose a cutoff BMI of 32 as a realistic value rather than 30, which is considered the definition of obesity. Patients at MRVAMC have access to MOVE!, a weight loss management program through primary care. We believe in being reasonable; so if a patient makes a significant improvement in her health but falls short of the required cutoff, we will still consider offering the surgical procedure after a full explanation of the surgical risks.
Wound complications, especially those that require admission or frequent appointments can seriously disrupt a patient’s life, creating unnecessary hardships and expense in time lost from work, travel, and child care. MRVAMC has a catchment area the size of North Carolina; so many of our patients travel hours for their appointments. The added scars and deformity from wound dehiscence and debridement can lead to asymmetry, widened scars, and future revision operations. Multiple clinic appointments for wound care not only impact that individual patient, but also has the effect of limiting access for all patients in a health care environment with high patient volume and limited providers, operating room time, and clinic appointments. As a result, minimizing predictable wound complications benefits the entire system.
Limitations and Strengths
This retrospective review comprised multiple different surgeons, including faculty and trainees, who were involved in the consultation, surgery, and postoperative care of the patients over a 20-year period; therefore, consistency in documentation is lacking. In addition, we were limited to only the information available on the charts. For example, wound size and laterality were not consistently documented. The MRVAMC complication rate was consistent with the current literature (range, 14-52%).12,18,20,24
The major strength of the study is that the veterans tend to stay within the VA, which makes complications easier to identify and follow. Patients who do not present initially to their surgeon due to travel limitations will typically contact their primary care provider or present to their local VA urgent care or emergency department provider, who will route the patient back to the surgical specialty service through the electronic health record.
Conclusions
Breast reduction surgery has a high wound complication rate, which can be predicted and improved on so that patients can receive their indicated surgical procedure with minimal inconvenience and downtime. This review confirms that preoperative weight loss and nicotine cessation were the appropriate focus of the MRVAMC plastic surgery service’s efforts to achieve a safer surgical experience. We will continue to enforce our protocol and encourage patients who are interested in breast reduction surgery and fall outside the requirements to work with their primary care provider on smoking cessation and weight loss through better nutrition and physical activity.
Acknowledgment
This manuscript is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
Women make up an estimated 10% of the veteran population.1 The US Department of Veterans Affairs (VA) projected that there would be an increase of 18,000 female veterans per year for 10 years based on 2015 data. The number of women veterans enrolled in the VA health care increased from 397,024 to 729,989 (83.9%) between 2005 and 2015.2 This rise in the number of enrolled women veterans also increased the demand for female-specific health care services, such as breast reduction surgery, a reconstructive procedure provided at the Malcom Randall VA Medical Center (MRVAMC) federal teaching hospital in Gainesville, Florida.
Patients who experience symptomatic macromastia will report a history of neck and shoulder pain, shoulder grooving from bra straps, inframammary intertrigo, difficulty finding clothes that fit, and discomfort participating in sports. For the treatment of symptomatic macromastia, patients report a high satisfaction rate after breast reduction surgery.3-5 Unfortunately, the complications from the surgery can significantly disrupt a woman’s life due to previously unplanned hospital admissions, clinic appointments, wound care, time off work, and poor aesthetic outcome. Faculty awareness of a large number of complications for patients after breast reduction surgery prompted the MRVAMC Plastic Surgery Service to establish a stricter surgical screening protocol using body mass index (BMI) values and negative nicotine status to help patients be healthier and reduce the potential risk before offering surgery. A medical literature search did not find an existing study on veteran-specific breast reduction surgery.
Methods
The University of Florida and North Florida/South Georgia Veterans Health System Institutional Review Board approved a retrospective chart review of all breast reduction surgeries performed at MRVAMC over a 20-year period (July 1, 2000-June 30, 2020). Electronic health records were queried for all primary bilateral breast reduction surgeries performed for symptomatic macromastia using Current Procedural Terminology code 19318. Potentially modifiable or predictable risk factors for wound complications were recorded: nicotine status, BMI, diabetes mellitus (DM) status, skin incision pattern, and pedicle location. Skin incision patterns were either vertical (periareolar plus a vertical scar from the areola to the inframammary fold) or traditional Wise pattern (also known as anchor pattern: periareolar scar, vertical scar to inframammary fold, plus a horizontal scar along the inframammary fold) as seen in Figures 1 and 2. The pedicle is the source of blood supply to the nipple, which was documented as either from the inferior aspect or the superior or superior/medial aspect.
For this study, the blood supply from the superior and superior/medial was logged in the same category. Records were reviewed 3 months after surgery for documentation of local wound complications, such as hematoma, infection, wound breakdown, skin necrosis, and nipple necrosis. Major complications were defined as requiring an unplanned hospital admission or urgent return to the operating room. A χ2 test using a P value of < .05 was used to determine statistical significance between the incidence of wound complications and the individually identifiable variables.
Results
One hundred fifteen bilateral breast reduction surgeries were performed at MRVAMC over a 20-year period. Patient median age was 43 years. Median combined specimen weight was 1272 g. Forty-eight (41.7%) wound complications were documented, including 8 (7%) major complications. Most complications were identified in the first 7 years of the study before the new protocol and consult template became active. The new template resulted in the local complication rate dropping from 62% (July 2000-June 2007) to 26% (July 2007-June 2020). BMI > 32 (P = .03) and active nicotine use (P = .004) were found to be statistically significant independent risk factors for wound complications. Median BMI for all patients was 30. DM status (P = .22), skin incision pattern (P = .25), and pedicle location (P = .13) were not found to be predictors of wound complications (Table). There was no significant change in the incidence of major complications before and after the new protocols were enforced.
Discussion
Breast reduction surgery is an elective reconstructive option to treat symptomatic macromastia. There are several accepted ways to do the reduction surgical procedure where the blood supply (pedicle) to the nipple can vary and the visible scars can be in a horizontal, vertical, or Wise pattern. Technique is usually based on surgeon training, comfort, and preference. There are several known complications specific to this operation that include asymmetry, changes in nipple sensation, unattractive scars, diminished ability to breastfeed, and wound complications.5-7 Wound complications include seroma, hematoma, dehiscence, infection, wound breakdown, skin necrosis, and nipple necrosis.
This study focused on wound complications with the objective of identifying and modifying risk factors. Two known risk factors documented in the literature, nicotine use and obesity, already had been addressed by our service, and results were known anecdotally but had not been previously verified. This study also looked at other potential risk factors, including the pedicle location, skin incision, and DM status.
Residents or fellows participated in all the surgeries. An outcome analysis from The American College of Surgeons National Surgical Quality Improvement Program database from 2005 to 2011 found that resident participation was associated with morbidity, including wound complications.8 This study was performed at a federal hospital with a complexity level 1a rating, which is designated based on the highest level of patient volume, risk, teaching, research, intensive care unit beds, and specialty services.9 The hospital is closely affiliated with a level 1 trauma center and teaching hospital; therefore, resident and fellow participation is not a modifiable risk factor.
This study did not find an increased risk of wound complications in patients with DM, which has been found to be an independent risk factor in a prior study.10 DM status was indicated in only 3 histories, and they all had perioperative hemoglobin A1c levels < 8%. There is documentation of patients receiving perioperative antibiotics in 99 out of 116 of the surgical records; however, we did not include this in the analysis because the operative reports from the first year of the study were incomplete.
Smoking is a known risk factor for local wound complications in breast reduction surgery.10-15 The VA has a smoking cessation program through its mental health service that provides counseling and medication treatment options, including nicotine replacement, bupropion, and varenicline. We require patients to be at least 4 weeks nicotine free before surgery, which has been previously recommended in the literature.16
Existing studies that compare the traditional Wise pattern/inferior pedicle with vertical pattern/superior medial pedicle did not find an increased risk of wound complications.17-19 Our study separated the different incisions from the pedicle because the surgical technique among the different surgeons in the study varied, where sometimes the traditional Wise pattern was combined with the less traditional superior-medial pedicle. We did not find a statistical difference when comparing the incisions and pedicle location, which suggests that the incision type and source of blood supply to the nipple are not the determining factors for wound complications in the early postoperative period.
Obesity is a known risk factor for local wound complications.12,13,15,20-22 Studies have shown that patients who are obese benefit from breast reduction surgery; authors have argued against restricting surgery to these higher risk patients.4,23-25 Patients usually report decades of macromastia symptoms at consultation; so, we believe delaying the surgical procedure to get patients to a safer risk profile is in their best interest. We chose a cutoff BMI of 32 as a realistic value rather than 30, which is considered the definition of obesity. Patients at MRVAMC have access to MOVE!, a weight loss management program through primary care. We believe in being reasonable; so if a patient makes a significant improvement in her health but falls short of the required cutoff, we will still consider offering the surgical procedure after a full explanation of the surgical risks.
Wound complications, especially those that require admission or frequent appointments can seriously disrupt a patient’s life, creating unnecessary hardships and expense in time lost from work, travel, and child care. MRVAMC has a catchment area the size of North Carolina; so many of our patients travel hours for their appointments. The added scars and deformity from wound dehiscence and debridement can lead to asymmetry, widened scars, and future revision operations. Multiple clinic appointments for wound care not only impact that individual patient, but also has the effect of limiting access for all patients in a health care environment with high patient volume and limited providers, operating room time, and clinic appointments. As a result, minimizing predictable wound complications benefits the entire system.
Limitations and Strengths
This retrospective review comprised multiple different surgeons, including faculty and trainees, who were involved in the consultation, surgery, and postoperative care of the patients over a 20-year period; therefore, consistency in documentation is lacking. In addition, we were limited to only the information available on the charts. For example, wound size and laterality were not consistently documented. The MRVAMC complication rate was consistent with the current literature (range, 14-52%).12,18,20,24
The major strength of the study is that the veterans tend to stay within the VA, which makes complications easier to identify and follow. Patients who do not present initially to their surgeon due to travel limitations will typically contact their primary care provider or present to their local VA urgent care or emergency department provider, who will route the patient back to the surgical specialty service through the electronic health record.
Conclusions
Breast reduction surgery has a high wound complication rate, which can be predicted and improved on so that patients can receive their indicated surgical procedure with minimal inconvenience and downtime. This review confirms that preoperative weight loss and nicotine cessation were the appropriate focus of the MRVAMC plastic surgery service’s efforts to achieve a safer surgical experience. We will continue to enforce our protocol and encourage patients who are interested in breast reduction surgery and fall outside the requirements to work with their primary care provider on smoking cessation and weight loss through better nutrition and physical activity.
Acknowledgment
This manuscript is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. US Department of Veterans Affairs. Statistics at a glance. Published February 2020. Accessed June 18, 2021. https://www.va.gov/vetdata/docs/Quickfacts/Homepage_slideshow_4_6_20.PDF
2. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Published February 2017. Accessed June 18, 2020. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf
3. Crittenden TA, Watson DI, Ratcliffe J, Griffin PA, Dean NR. Outcomes of breast reduction surgery using the breast-q: a prospective study and comparison with normative data. Plast Reconstr Surg. 2019;144(5):1034-1044. doi:10.1097/PRS.0000000000006114
4. Thoma A, Sprague S, Veltri K, Duku E, Furlong W. A prospective study of patients undergoing breast reduction surgery: health-related quality of life and clinical outcomes. Plast Reconstr Surg. 2007;120(1):13-26. doi:10.1097/01.prs.0000263370.94191.90
5. Nuzzi LC, Firriolo JM, Pike CM, DiVasta AD, Labow BI. Complications and quality of life following reduction mammaplasty in adolescents and young women.Plast Reconstr Surg. 2019;144(3):572-581. doi:10.1097/PRS.0000000000005907
6. Hall-Findlay EJ, Shestak KC. Breast reduction. Plast Reconstr Surg. 2015;136(4):531e-544e. doi:10.1097/PRS.0000000000001622
7. Kraut RY, Brown E, Korownyk C, et al. The impact of breast reduction surgery on breastfeeding: systematic review of observational studies. PLoS One. 2017;12(10):e0186591. doi:10.1371/journal.pone.0186591
8. Fischer JP, Wes AM, Kovach SJ. The impact of surgical resident participation in breast reduction surgery--outcome analysis from the 2005-2011 ACS-NSQIP datasets. J Plast Surg Hand Surg. 2014;48(5):315-321. doi:10.3109/2000656X.2014.882345
9. Site Facility Name and Complexity Summary of VHA Facility. Accessed June 18, 2021. https://www.vendorportal.ecms.va.gov/FBODocumentServer/DocumentServer.aspx?DocumentId=2793591&FileName=VA118-16-R-1059-A00002002.docx
10. Lewin R, Göransson M, Elander A, Thorarinsson A, Lundberg J, Lidén M. Risk factors for complications after breast reduction surgery. J Plast Surg Hand Surg. 2014;48(1):10-14. doi:10.3109/2000656X.2013.791625
11. Cunningham BL, Gear AJ, Kerrigan CL, Collins ED. Analysis of breast reduction complications derived from the BRAVO study. Plast Reconstr Surg. 2005;115(6):1597-1604. doi:10.1097/01.prs.0000160695.33457.db
12. Karamanos E, Wei B, Siddiqui A, Rubinfeld I. Tobacco use and body mass index as predictors of outcomes in patients undergoing breast reduction mammoplasty. Ann Plast Surg. 2015;75(4):383-387. doi:10.1097/SAP.0000000000000192
13. Manahan MA, Buretta KJ, Chang D, Mithani SK, Mallalieu J, Shermak MA. An outcomes analysis of 2142 breast reduction procedures. Ann Plast Surg. 2015;74(3):289-292. doi:10.1097/SAP.0b013e31829d2261
14. Hillam JS, Borsting EA, Chim JH, Thaller SR. Smoking as a risk factor for breast reduction: an analysis of 13,503 cases. J Plast Reconstr Aesthet Surg. 2017;70(6):734-740. doi:10.1016/j.bjps.2016.12.012
15. Zhang MX, Chen CY, Fang QQ, et al. Risk factors for complications after reduction mammoplasty: a meta-analysis. PLoS One. 2016;11(12):e0167746. doi:10.1371/journal.pone.0167746
16. Sørensen LT. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Ann Surg. 2012;255(6):1069-1079.doi:10.1097/SLA.0b013e31824f632d
17. Antony AK, Yegiyants SS, Danielson KK, et al. A matched cohort study of superomedial pedicle vertical scar breast reduction (100 breasts) and traditional inferior pedicle Wise-pattern reduction (100 breasts): an outcomes study over 3 years. Plast Reconstr Surg. 2013;132(5):1068-1076. doi:10.1097/PRS.0b013e3182a48b2d
18. Hunter-Smith DJ, Smoll NR, Marne B, Maung H, Findlay MW. Comparing breast-reduction techniques: time-to-event analysis and recommendations. Aesthetic Plast Surg. 2012;36(3):600-606. doi:10.1007/s00266-011-9860-3
19. Ogunleye AA, Leroux O, Morrison N, Preminger AB. Complications after reduction mammaplasty: a comparison of wise pattern/inferior pedicle and vertical scar/superomedial pedicle. Ann Plast Surg. 2017;79(1):13-16. doi:10.1097/SAP.0000000000001059
20. Bauermeister AJ, Gill K, Zuriarrain A, Earle SA, Newman MI. Reduction mammaplasty with superomedial pedicle technique: a literature review and retrospective analysis of 938 consecutive breast reductions. J Plast Reconstr Aesthet Surg. 2019;72(3):410-418. doi:10.1016/j.bjps.2018.12.004
21. Nelson JA, Fischer JP, Chung CU, et al. Obesity and early complications following reduction mammaplasty: an analysis of 4545 patients from the 2005-2011 NSQIP datasets. J Plast Surg Hand Surg. 2014;48(5):334-339. doi:10.3109/2000656X.2014.886582
22. Kreithen J, Caffee H, Rosenberg J, et al. A comparison of the LeJour and Wise pattern methods of breast reduction. Ann Plast Surg. 2005;54(3):236-241. doi:10.3109/2000656X.2014.886582
23. Güemes A, Pérez E, Sousa R, et al. Quality of life and alleviation of symptoms after breast reduction for macromastia in obese patients: is surgery worth it? Aesthetic Plast Surg. 2016;40(1):62-70. doi:10.1007/s00266-015-0601-x
24. Setälä L, Papp A, Joukainen S, et al. Obesity and complications in breast reduction surgery: are restrictions justified? J Plast Reconstr Aesthet Surg. 2009;62(2):195-199. doi:10.1016/j.bjps.2007.10.043
25. Shah R, Al-Ajam Y, Stott D, Kang N. Obesity in mammaplasty: a study of complications following breast reduction. J Plast Reconstr Aesthet Surg. 2011;64(4):508-514. doi:10.1016/j.bjps.2007.10.043
1. US Department of Veterans Affairs. Statistics at a glance. Published February 2020. Accessed June 18, 2021. https://www.va.gov/vetdata/docs/Quickfacts/Homepage_slideshow_4_6_20.PDF
2. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Published February 2017. Accessed June 18, 2020. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf
3. Crittenden TA, Watson DI, Ratcliffe J, Griffin PA, Dean NR. Outcomes of breast reduction surgery using the breast-q: a prospective study and comparison with normative data. Plast Reconstr Surg. 2019;144(5):1034-1044. doi:10.1097/PRS.0000000000006114
4. Thoma A, Sprague S, Veltri K, Duku E, Furlong W. A prospective study of patients undergoing breast reduction surgery: health-related quality of life and clinical outcomes. Plast Reconstr Surg. 2007;120(1):13-26. doi:10.1097/01.prs.0000263370.94191.90
5. Nuzzi LC, Firriolo JM, Pike CM, DiVasta AD, Labow BI. Complications and quality of life following reduction mammaplasty in adolescents and young women.Plast Reconstr Surg. 2019;144(3):572-581. doi:10.1097/PRS.0000000000005907
6. Hall-Findlay EJ, Shestak KC. Breast reduction. Plast Reconstr Surg. 2015;136(4):531e-544e. doi:10.1097/PRS.0000000000001622
7. Kraut RY, Brown E, Korownyk C, et al. The impact of breast reduction surgery on breastfeeding: systematic review of observational studies. PLoS One. 2017;12(10):e0186591. doi:10.1371/journal.pone.0186591
8. Fischer JP, Wes AM, Kovach SJ. The impact of surgical resident participation in breast reduction surgery--outcome analysis from the 2005-2011 ACS-NSQIP datasets. J Plast Surg Hand Surg. 2014;48(5):315-321. doi:10.3109/2000656X.2014.882345
9. Site Facility Name and Complexity Summary of VHA Facility. Accessed June 18, 2021. https://www.vendorportal.ecms.va.gov/FBODocumentServer/DocumentServer.aspx?DocumentId=2793591&FileName=VA118-16-R-1059-A00002002.docx
10. Lewin R, Göransson M, Elander A, Thorarinsson A, Lundberg J, Lidén M. Risk factors for complications after breast reduction surgery. J Plast Surg Hand Surg. 2014;48(1):10-14. doi:10.3109/2000656X.2013.791625
11. Cunningham BL, Gear AJ, Kerrigan CL, Collins ED. Analysis of breast reduction complications derived from the BRAVO study. Plast Reconstr Surg. 2005;115(6):1597-1604. doi:10.1097/01.prs.0000160695.33457.db
12. Karamanos E, Wei B, Siddiqui A, Rubinfeld I. Tobacco use and body mass index as predictors of outcomes in patients undergoing breast reduction mammoplasty. Ann Plast Surg. 2015;75(4):383-387. doi:10.1097/SAP.0000000000000192
13. Manahan MA, Buretta KJ, Chang D, Mithani SK, Mallalieu J, Shermak MA. An outcomes analysis of 2142 breast reduction procedures. Ann Plast Surg. 2015;74(3):289-292. doi:10.1097/SAP.0b013e31829d2261
14. Hillam JS, Borsting EA, Chim JH, Thaller SR. Smoking as a risk factor for breast reduction: an analysis of 13,503 cases. J Plast Reconstr Aesthet Surg. 2017;70(6):734-740. doi:10.1016/j.bjps.2016.12.012
15. Zhang MX, Chen CY, Fang QQ, et al. Risk factors for complications after reduction mammoplasty: a meta-analysis. PLoS One. 2016;11(12):e0167746. doi:10.1371/journal.pone.0167746
16. Sørensen LT. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Ann Surg. 2012;255(6):1069-1079.doi:10.1097/SLA.0b013e31824f632d
17. Antony AK, Yegiyants SS, Danielson KK, et al. A matched cohort study of superomedial pedicle vertical scar breast reduction (100 breasts) and traditional inferior pedicle Wise-pattern reduction (100 breasts): an outcomes study over 3 years. Plast Reconstr Surg. 2013;132(5):1068-1076. doi:10.1097/PRS.0b013e3182a48b2d
18. Hunter-Smith DJ, Smoll NR, Marne B, Maung H, Findlay MW. Comparing breast-reduction techniques: time-to-event analysis and recommendations. Aesthetic Plast Surg. 2012;36(3):600-606. doi:10.1007/s00266-011-9860-3
19. Ogunleye AA, Leroux O, Morrison N, Preminger AB. Complications after reduction mammaplasty: a comparison of wise pattern/inferior pedicle and vertical scar/superomedial pedicle. Ann Plast Surg. 2017;79(1):13-16. doi:10.1097/SAP.0000000000001059
20. Bauermeister AJ, Gill K, Zuriarrain A, Earle SA, Newman MI. Reduction mammaplasty with superomedial pedicle technique: a literature review and retrospective analysis of 938 consecutive breast reductions. J Plast Reconstr Aesthet Surg. 2019;72(3):410-418. doi:10.1016/j.bjps.2018.12.004
21. Nelson JA, Fischer JP, Chung CU, et al. Obesity and early complications following reduction mammaplasty: an analysis of 4545 patients from the 2005-2011 NSQIP datasets. J Plast Surg Hand Surg. 2014;48(5):334-339. doi:10.3109/2000656X.2014.886582
22. Kreithen J, Caffee H, Rosenberg J, et al. A comparison of the LeJour and Wise pattern methods of breast reduction. Ann Plast Surg. 2005;54(3):236-241. doi:10.3109/2000656X.2014.886582
23. Güemes A, Pérez E, Sousa R, et al. Quality of life and alleviation of symptoms after breast reduction for macromastia in obese patients: is surgery worth it? Aesthetic Plast Surg. 2016;40(1):62-70. doi:10.1007/s00266-015-0601-x
24. Setälä L, Papp A, Joukainen S, et al. Obesity and complications in breast reduction surgery: are restrictions justified? J Plast Reconstr Aesthet Surg. 2009;62(2):195-199. doi:10.1016/j.bjps.2007.10.043
25. Shah R, Al-Ajam Y, Stott D, Kang N. Obesity in mammaplasty: a study of complications following breast reduction. J Plast Reconstr Aesthet Surg. 2011;64(4):508-514. doi:10.1016/j.bjps.2007.10.043
Application of Hand Therapy Extensor Tendon Protocol to Toe Extensor Tendon Rehabilitation
Plastic and orthopedic surgeons worked closely with therapists in military hospitals to rehabilitate soldiers afflicted with upper extremity trauma during World War II. Together, they developed treatment protocols. In 1975, the American Society for Hand Therapists (ASHT) was created during the American Society for Surgery of the Hand meeting. The ASHT application process required case studies, patient logs, and clinical hours, so membership was equivalent to competency. In May 1991, the first hand certification examination took place and designated the first group of certified hand therapists (CHT).1
In the US Department of Veterans Affairs collaboration takes place between different services and communication is facilitated using the electronic heath record. The case presented here is an example of several services (emergency medicine, plastic/hand surgery, and occupational therapy) working together to develop a treatment plan for a condition that often goes undiagnosed or untreated. This article describes an innovative application of hand extensor tendon therapy clinical decision making to rehabilitate foot extensor tendons when the plastic surgery service was called on to work outside its usual comfort zone of the hand and upper extremity. The hand therapist applied hand extensor tendon rehabilitation principles to recover toe extensor lacerations.
Certified hand therapists (CHTs) are key to a successful hand surgery practice. The Plastic Surgery Service at the Malcom Randall VA Medical Center in Gainesville, Florida, relies heavily on the CHTs to optimize patient outcomes. The hand surgery clinic and hand therapy clinics are in the same hospital building, allowing for easy face-to-face communication. Hand therapy students are able to observe cases in the operating room. Immediately after surgery, follow-up consults are scheduled to coordinate postoperative care between the services.
Case Presentation
The next day, the patient was examined in the plastic surgery clinic and found to have a completely lacerated extensor digitorum brevis to the second toe and a completely lacerated extensor digitorum longus to the third toe. These were located proximal to the metatarsal phalangeal joints. Surgery was scheduled for the following week.
In surgery, the tendons were sharply debrided and repaired using a 3.0 Ethibond suture placed in a modified Kessler technique followed by a horizontal mattress for a total of a 4-core repair. This was reinforced with a No. 6 Prolene to the paratendon. The surgery was performed under IV sedation and an ankle block, using 17 minutes of tourniquet time.
On postoperative day 1, the patient was seen in plastic surgery and occupational therapy clinic. The hand therapist modified the hand extensor tendon repair protocol since there was no known protocol for repairs of the foot and toe extensor tendon. The patient was placed in an ankle foot orthosis with a toe extension device created by heating and molding a low-temperature thermoplastic sheet (Figure 2). The toes were boosted into slight hyper extension. This was done to reduce tension across the extensor tendon repair site. All of the toes were held in about 20°of extension, as the extensor digitorum longus (EDL) has a common origin, to aide in adherence of wearing and for comfort. No standing or weight bearing was permitted for 3 weeks.
A wheelchair was issued in lieu of crutches to inhibit the work of toe extension with gait swing-through. Otherwise, the patient would generate tension on the extensor tendon in order for the toes to clear the ground. It was postulated that it would be difficult to turn off the toe extensors while using crutches. Maximal laxity was desired because edema and early scar formation could increase tension on the repair, resulting in rupture if the patient tried to fire the muscle belly even while in passive extension.
The patient kept his appointments and progressed steadily. He started passive toe extension and relaxation once per day for 30 repetitions at 1 week to aide in tendon glide. He started place and hold techniques in toe extension at 3 weeks. This progressed to active extension 50% effort plus active flexion at 4 weeks after surgery, then 75% extension effort plus toe towel crunches at 5 weeks. Toe crunches are toe flexion exercises with a washcloth on the floor with active bending of the toes with light resistance similar to picking up a marble with the toes. He was found to have a third toe extensor lag at that time that was correctible. The patient was actively able to flex and extend the toe independently. The early extension lag was felt to be secondary to edema and scar formation, which, over time are anticipated to resolve and contract and effectively shorten the tendon. Tendon gliding, and scar massage were reviewed. The patient’s last therapy session occurred 7 weeks after surgery, and he was cleared for full activity at 12 weeks. There was no further follow-up as he was planning on back surgery 2 weeks later.
Discussion
The North Florida/South Georgia Veterans Health System is fortunate to have 4 CHTs on staff. CHTs take a 200 question 4 hour certifying exam after being licensed for a minimum of 3 years as a physical or occupational therapist and completing 4,000 hours of direct upper extremity patient experience. Pass rates from 2008 to 2018 ranged from 52% to 68%.3 These clinicians are key to the success of our hand surgery service, utilizing their education and skills on our elective and trauma cases. The hand therapy service applied their knowledge of hand extensor rehabilitation protocols to rehabilitate the patient’s toe extensor in the absence of clear guidelines.
Hand extensor tendon rehabilitation protocols are based on the location of the repair on the hand or forearm. Nine extensor zones are named, distal to proximal, from the distal interphalangeal joints to the proximal forearm (Figure 3). In his review of extensor hallucis longus (EHL) repairs, Al-Qattan described 6 foot-extensor tendon zones, distal to proximal, from the first toe at the insertion of the big toe extensor to the distal leg proximal to the extensor retinaculum (Figure 4).4 Zone 3 is over the metatarsophalangeal joint; zone 5 is under the extensor retinaculum. The extensor tendon repairs described in this report were in dorsal foot zone 4 (proximal to the metatarsophalangeal joint and over the metatarsals), which would be most comparable to hand extensor zone 6 (proximal to the metacarpal phalangeal joint and over the metacarpals).
The EDL originates on the lateral condyle of the tibia and anterior surface of the fibula and the interosseous membrane, passes under the extensor retinaculum, and divides into 4 separate tendons. The 4 tendons split into 3 slips; the central one inserts on the middle phalanx, and the lateral ones insert onto the distal phalanx of the 4 lateral toes, which allows for toe extension.5 The EDL common origin for the muscle belly that serves 4 tendon slips has clinical significance because rehabilitation for one digit will affect the others. Knowledge of the anatomical structures guides the clinical decision making whether it is in the hand or foot. The EDL works synergistically with the extensor digitorum brevis (EDBr) to dorsiflex (extend) the toe phalanges. The EDB originates at the supralateral surface of the calcaneus, lateral talocalcaneal ligament and cruciate crural ligament and inserts at the lateral side of the EDL of second, third, and fourth toes at the level of the metatarsophalangeal joint.6
Repair of lacerated extensor tendons in the foot is the recommended treatment. Chronic extensor lag of the phalanges can result in a claw toe deformity, difficulty controlling the toes when putting on shoes or socks, and catching of the toe on fabric or insoles.7 The extensor tendons are close to the deep and superficial peroneal nerves and to the dorsalis pedis artery, none of which were involved in this case report.
There are case reports and series of EHL repairs that all involves at least 3 weeks of immobilization.4,8,9 The EHL dorsiflexes the big toe. Al-Qattan’s series involved placing K wires across the interphalangeal joint of the big toe and across the metatarsophalangeal joint, which were removed at 6 weeks, in addition to 3.0 polypropylene tendon mattress sutures. All patients in this series healed without tendon rupture or infection. Our PubMed search did not reveal any specific protocol for the EDL or EDB tendons, which are anatomically most comparable to the extensor digitorum communis (EDC) tendons in the hand. The EDC originates at the lateral epicondyle of the humerus, also divides into 4 separate tendons and is responsible for extending the 4 ulnar sided fingers at the metacarpophalangeal joint.10
Tendon repair protocols are a balance between preventing tendon rupture by too aggressive therapy and with preventing tendon adhesions from prolonged immobilization. Orthotic fabrication plays a key early role with blocking possible forces creating unacceptable strain or tension across the surgical repair site. Traditionally, extensor tendon repairs in the hand were immobilized for at least 3 weeks to prevent rupture. This is still the preferred protocol for the patient unwilling or unable to follow instructions. The downside to this method is extension lags, extrinsic tightness, and adhesions that prevent flexion, which can require prolonged therapy or tenolysis surgery to correct.11-13
Early passive motion (EPM) was promoted in the 1980s when studies found better functional outcomes and fewer adhesions. This involved either a dynamic extension splint that relied on elastic bands (Louisville protocol) to keep tension off the repair or the Duran protocol that relied on a static splint and the patient doing the passive exercises with his other uninjured hand. Critics of the EPM protocol point to the costs of the splints and demands of postoperative hand therapy.11
Early active motion (EAM) is the most recent development in hand tendon rehabilitation and starts within days of surgery. Studies have found an earlier regain of total active motion in patients who are mobilized earlier.12 EAM protocols can be divided into controlled active motion (CAM) and relative motion extension splinting (RMES). CAM splints are forearm based and cross more joints. Relative motion splinting is the least restrictive, which makes it less likely that the patient will remove it. Patient friendly splints are ideal because tendon ruptures are often secondary to nonadherence.13 The yoke splint is an example of a RMES, which places the repaired digit in slightly greater extension at the metacarpal phalangeal joint than the other digits (Figure 5), allowing use of the uninjured digits.
The toe extensors do not have the juncturae tendinum connecting the individual EDL tendons to each other, as found between the EDC tendons in the hand. These connective bands can mask a single extensor tendon laceration in the hand when the patient is still able to extend the digit to neutral in the event of a more proximal dorsal hand laceration. A case can be made for closing the skin only in lesser toe extensor injuries in poor surgical candidates because the extensor lag would not be appreciated functionally when wearing shoes. There would be less functional impact when letting a toe extensor go untreated compared with that of a hand extensor. Routine activities such as typing or getting the fingers into a tight pocket could be challenging if hand extensors were untreated. The rehabilitation for toe extensors is more inconvenient when a patient is nonweight bearing, compared with wearing a hand yoke splint.
Conclusion
The case described used an early passive motion protocol without the dynamic splint to rehabilitate the third toe EDL and second toe EDB. This was felt to be the most patient and therapist friendly option, given the previously unchartered territory. The foot orthosis was in stock at the adjacent physical therapy clinic, and the toe booster was created in the hand therapy clinic with readily available supplies. Ideally, one would like to return structures to their anatomic site and control the healing process in the event of a traumatic injury to prevent nonanatomic healing between structures and painful scar adhesions in an area with little subcutaneous tissue. This patient’s tendon repair was still intact at 7 weeks and on his way to recovery, demonstrating good scar management techniques. The risks and benefits to lesser toe tendon repair and recovery would have to be weighed on an individual basis.
Acknowledgments
This project is the result of work supported with resources and use of facilities at the Malcom Randall VA Medical Center in Gainesville, Florida.
1. Hand Therapy Certification Commission. History of HTCC. https://www.htcc.org/consumer-information/about-htcc/history-of-htcc. Accessed November 8, 2019.
2. Coady-Fariborzian L, McGreane A. Comparison of hand emergency triage before and after specialty templates (2007 vs 2012). Hand (N Y). 2015;10(2):215-220.
3. Hand Therapy Certification Commission. Passing rates for the CHT exam. https://www.htcc.org/certify/exam-results/passing-rates. Accessed November 8, 2019.
4. Al-Qattan MM. Surgical treatment and results in 17 cases of open lacerations of the extensor hallucis longus tendon. J Plast Reconstr Aesthet Surg. 2007;60(4):360-367.
5. Wheeless CR. Wheeless’ textbook of orthopaedics: extensor digitorum longus. http://www.wheelessonline.com/ortho/extensor_digitorum_longus. Updated December 8, 2011. Accessed November 8, 2019.
6. Wheeless CR. Wheeless’ textbook of orthopaedics: extensor digitorum brevis. http://www.wheelessonline.com/ortho/extensor_digitorum_brevis. Updated March 4, 2018. Accessed November 8, 2019.
7. Coughlin M, Schon L. Disorders of tendons. https://musculoskeletalkey.com/disorders-of-tendons-2/#s0035. Published August 27, 2016. Accessed November 8, 2019.
8. Bronner S, Ojofeitimi S, Rose D. Repair and rehabilitation of extensor hallucis longus and brevis tendon lacerations in a professional dancer. J Orthop Sports Phys Ther. 2008;38(6):362-370.
9. Wong JC, Daniel JN, Raikin SM. Repair of acute extensor hallucis longus tendon injuries: a retrospective review. Foot Ankle Spec. 2014;7(1):45-51.
10. Wheeless CR. Wheeless’ textbook of orthopaedics: extensor digitorum communis. http://www.wheelessonline.com/ortho/extensor_digitorum_communis. Updated March 4, 2018. Accessed November 8, 2019.
11. Hall B, Lee H, Page R, Rosenwax L, Lee AH. Comparing three postoperative treatment protocols for extensor tendon repair in zones V and VI of the hand. Am J Occup Ther. 2010;64(5):682-688.
12. Wong AL, Wilson M, Girnary S, Nojoomi M, Acharya S, Paul SM. The optimal orthosis and motion protocol for extensor tendon injury in zones IV-VIII: a systematic review. J Hand Ther. 2017;30(4):447-456.
13. Collocott SJ, Kelly E, Ellis RF. Optimal early active mobilisation protocol after extensor tendon repairs in zones V and VI: a systematic review of literature. Hand Ther. 2018;23(1):3-18.
Plastic and orthopedic surgeons worked closely with therapists in military hospitals to rehabilitate soldiers afflicted with upper extremity trauma during World War II. Together, they developed treatment protocols. In 1975, the American Society for Hand Therapists (ASHT) was created during the American Society for Surgery of the Hand meeting. The ASHT application process required case studies, patient logs, and clinical hours, so membership was equivalent to competency. In May 1991, the first hand certification examination took place and designated the first group of certified hand therapists (CHT).1
In the US Department of Veterans Affairs collaboration takes place between different services and communication is facilitated using the electronic heath record. The case presented here is an example of several services (emergency medicine, plastic/hand surgery, and occupational therapy) working together to develop a treatment plan for a condition that often goes undiagnosed or untreated. This article describes an innovative application of hand extensor tendon therapy clinical decision making to rehabilitate foot extensor tendons when the plastic surgery service was called on to work outside its usual comfort zone of the hand and upper extremity. The hand therapist applied hand extensor tendon rehabilitation principles to recover toe extensor lacerations.
Certified hand therapists (CHTs) are key to a successful hand surgery practice. The Plastic Surgery Service at the Malcom Randall VA Medical Center in Gainesville, Florida, relies heavily on the CHTs to optimize patient outcomes. The hand surgery clinic and hand therapy clinics are in the same hospital building, allowing for easy face-to-face communication. Hand therapy students are able to observe cases in the operating room. Immediately after surgery, follow-up consults are scheduled to coordinate postoperative care between the services.
Case Presentation
The next day, the patient was examined in the plastic surgery clinic and found to have a completely lacerated extensor digitorum brevis to the second toe and a completely lacerated extensor digitorum longus to the third toe. These were located proximal to the metatarsal phalangeal joints. Surgery was scheduled for the following week.
In surgery, the tendons were sharply debrided and repaired using a 3.0 Ethibond suture placed in a modified Kessler technique followed by a horizontal mattress for a total of a 4-core repair. This was reinforced with a No. 6 Prolene to the paratendon. The surgery was performed under IV sedation and an ankle block, using 17 minutes of tourniquet time.
On postoperative day 1, the patient was seen in plastic surgery and occupational therapy clinic. The hand therapist modified the hand extensor tendon repair protocol since there was no known protocol for repairs of the foot and toe extensor tendon. The patient was placed in an ankle foot orthosis with a toe extension device created by heating and molding a low-temperature thermoplastic sheet (Figure 2). The toes were boosted into slight hyper extension. This was done to reduce tension across the extensor tendon repair site. All of the toes were held in about 20°of extension, as the extensor digitorum longus (EDL) has a common origin, to aide in adherence of wearing and for comfort. No standing or weight bearing was permitted for 3 weeks.
A wheelchair was issued in lieu of crutches to inhibit the work of toe extension with gait swing-through. Otherwise, the patient would generate tension on the extensor tendon in order for the toes to clear the ground. It was postulated that it would be difficult to turn off the toe extensors while using crutches. Maximal laxity was desired because edema and early scar formation could increase tension on the repair, resulting in rupture if the patient tried to fire the muscle belly even while in passive extension.
The patient kept his appointments and progressed steadily. He started passive toe extension and relaxation once per day for 30 repetitions at 1 week to aide in tendon glide. He started place and hold techniques in toe extension at 3 weeks. This progressed to active extension 50% effort plus active flexion at 4 weeks after surgery, then 75% extension effort plus toe towel crunches at 5 weeks. Toe crunches are toe flexion exercises with a washcloth on the floor with active bending of the toes with light resistance similar to picking up a marble with the toes. He was found to have a third toe extensor lag at that time that was correctible. The patient was actively able to flex and extend the toe independently. The early extension lag was felt to be secondary to edema and scar formation, which, over time are anticipated to resolve and contract and effectively shorten the tendon. Tendon gliding, and scar massage were reviewed. The patient’s last therapy session occurred 7 weeks after surgery, and he was cleared for full activity at 12 weeks. There was no further follow-up as he was planning on back surgery 2 weeks later.
Discussion
The North Florida/South Georgia Veterans Health System is fortunate to have 4 CHTs on staff. CHTs take a 200 question 4 hour certifying exam after being licensed for a minimum of 3 years as a physical or occupational therapist and completing 4,000 hours of direct upper extremity patient experience. Pass rates from 2008 to 2018 ranged from 52% to 68%.3 These clinicians are key to the success of our hand surgery service, utilizing their education and skills on our elective and trauma cases. The hand therapy service applied their knowledge of hand extensor rehabilitation protocols to rehabilitate the patient’s toe extensor in the absence of clear guidelines.
Hand extensor tendon rehabilitation protocols are based on the location of the repair on the hand or forearm. Nine extensor zones are named, distal to proximal, from the distal interphalangeal joints to the proximal forearm (Figure 3). In his review of extensor hallucis longus (EHL) repairs, Al-Qattan described 6 foot-extensor tendon zones, distal to proximal, from the first toe at the insertion of the big toe extensor to the distal leg proximal to the extensor retinaculum (Figure 4).4 Zone 3 is over the metatarsophalangeal joint; zone 5 is under the extensor retinaculum. The extensor tendon repairs described in this report were in dorsal foot zone 4 (proximal to the metatarsophalangeal joint and over the metatarsals), which would be most comparable to hand extensor zone 6 (proximal to the metacarpal phalangeal joint and over the metacarpals).
The EDL originates on the lateral condyle of the tibia and anterior surface of the fibula and the interosseous membrane, passes under the extensor retinaculum, and divides into 4 separate tendons. The 4 tendons split into 3 slips; the central one inserts on the middle phalanx, and the lateral ones insert onto the distal phalanx of the 4 lateral toes, which allows for toe extension.5 The EDL common origin for the muscle belly that serves 4 tendon slips has clinical significance because rehabilitation for one digit will affect the others. Knowledge of the anatomical structures guides the clinical decision making whether it is in the hand or foot. The EDL works synergistically with the extensor digitorum brevis (EDBr) to dorsiflex (extend) the toe phalanges. The EDB originates at the supralateral surface of the calcaneus, lateral talocalcaneal ligament and cruciate crural ligament and inserts at the lateral side of the EDL of second, third, and fourth toes at the level of the metatarsophalangeal joint.6
Repair of lacerated extensor tendons in the foot is the recommended treatment. Chronic extensor lag of the phalanges can result in a claw toe deformity, difficulty controlling the toes when putting on shoes or socks, and catching of the toe on fabric or insoles.7 The extensor tendons are close to the deep and superficial peroneal nerves and to the dorsalis pedis artery, none of which were involved in this case report.
There are case reports and series of EHL repairs that all involves at least 3 weeks of immobilization.4,8,9 The EHL dorsiflexes the big toe. Al-Qattan’s series involved placing K wires across the interphalangeal joint of the big toe and across the metatarsophalangeal joint, which were removed at 6 weeks, in addition to 3.0 polypropylene tendon mattress sutures. All patients in this series healed without tendon rupture or infection. Our PubMed search did not reveal any specific protocol for the EDL or EDB tendons, which are anatomically most comparable to the extensor digitorum communis (EDC) tendons in the hand. The EDC originates at the lateral epicondyle of the humerus, also divides into 4 separate tendons and is responsible for extending the 4 ulnar sided fingers at the metacarpophalangeal joint.10
Tendon repair protocols are a balance between preventing tendon rupture by too aggressive therapy and with preventing tendon adhesions from prolonged immobilization. Orthotic fabrication plays a key early role with blocking possible forces creating unacceptable strain or tension across the surgical repair site. Traditionally, extensor tendon repairs in the hand were immobilized for at least 3 weeks to prevent rupture. This is still the preferred protocol for the patient unwilling or unable to follow instructions. The downside to this method is extension lags, extrinsic tightness, and adhesions that prevent flexion, which can require prolonged therapy or tenolysis surgery to correct.11-13
Early passive motion (EPM) was promoted in the 1980s when studies found better functional outcomes and fewer adhesions. This involved either a dynamic extension splint that relied on elastic bands (Louisville protocol) to keep tension off the repair or the Duran protocol that relied on a static splint and the patient doing the passive exercises with his other uninjured hand. Critics of the EPM protocol point to the costs of the splints and demands of postoperative hand therapy.11
Early active motion (EAM) is the most recent development in hand tendon rehabilitation and starts within days of surgery. Studies have found an earlier regain of total active motion in patients who are mobilized earlier.12 EAM protocols can be divided into controlled active motion (CAM) and relative motion extension splinting (RMES). CAM splints are forearm based and cross more joints. Relative motion splinting is the least restrictive, which makes it less likely that the patient will remove it. Patient friendly splints are ideal because tendon ruptures are often secondary to nonadherence.13 The yoke splint is an example of a RMES, which places the repaired digit in slightly greater extension at the metacarpal phalangeal joint than the other digits (Figure 5), allowing use of the uninjured digits.
The toe extensors do not have the juncturae tendinum connecting the individual EDL tendons to each other, as found between the EDC tendons in the hand. These connective bands can mask a single extensor tendon laceration in the hand when the patient is still able to extend the digit to neutral in the event of a more proximal dorsal hand laceration. A case can be made for closing the skin only in lesser toe extensor injuries in poor surgical candidates because the extensor lag would not be appreciated functionally when wearing shoes. There would be less functional impact when letting a toe extensor go untreated compared with that of a hand extensor. Routine activities such as typing or getting the fingers into a tight pocket could be challenging if hand extensors were untreated. The rehabilitation for toe extensors is more inconvenient when a patient is nonweight bearing, compared with wearing a hand yoke splint.
Conclusion
The case described used an early passive motion protocol without the dynamic splint to rehabilitate the third toe EDL and second toe EDB. This was felt to be the most patient and therapist friendly option, given the previously unchartered territory. The foot orthosis was in stock at the adjacent physical therapy clinic, and the toe booster was created in the hand therapy clinic with readily available supplies. Ideally, one would like to return structures to their anatomic site and control the healing process in the event of a traumatic injury to prevent nonanatomic healing between structures and painful scar adhesions in an area with little subcutaneous tissue. This patient’s tendon repair was still intact at 7 weeks and on his way to recovery, demonstrating good scar management techniques. The risks and benefits to lesser toe tendon repair and recovery would have to be weighed on an individual basis.
Acknowledgments
This project is the result of work supported with resources and use of facilities at the Malcom Randall VA Medical Center in Gainesville, Florida.
Plastic and orthopedic surgeons worked closely with therapists in military hospitals to rehabilitate soldiers afflicted with upper extremity trauma during World War II. Together, they developed treatment protocols. In 1975, the American Society for Hand Therapists (ASHT) was created during the American Society for Surgery of the Hand meeting. The ASHT application process required case studies, patient logs, and clinical hours, so membership was equivalent to competency. In May 1991, the first hand certification examination took place and designated the first group of certified hand therapists (CHT).1
In the US Department of Veterans Affairs collaboration takes place between different services and communication is facilitated using the electronic heath record. The case presented here is an example of several services (emergency medicine, plastic/hand surgery, and occupational therapy) working together to develop a treatment plan for a condition that often goes undiagnosed or untreated. This article describes an innovative application of hand extensor tendon therapy clinical decision making to rehabilitate foot extensor tendons when the plastic surgery service was called on to work outside its usual comfort zone of the hand and upper extremity. The hand therapist applied hand extensor tendon rehabilitation principles to recover toe extensor lacerations.
Certified hand therapists (CHTs) are key to a successful hand surgery practice. The Plastic Surgery Service at the Malcom Randall VA Medical Center in Gainesville, Florida, relies heavily on the CHTs to optimize patient outcomes. The hand surgery clinic and hand therapy clinics are in the same hospital building, allowing for easy face-to-face communication. Hand therapy students are able to observe cases in the operating room. Immediately after surgery, follow-up consults are scheduled to coordinate postoperative care between the services.
Case Presentation
The next day, the patient was examined in the plastic surgery clinic and found to have a completely lacerated extensor digitorum brevis to the second toe and a completely lacerated extensor digitorum longus to the third toe. These were located proximal to the metatarsal phalangeal joints. Surgery was scheduled for the following week.
In surgery, the tendons were sharply debrided and repaired using a 3.0 Ethibond suture placed in a modified Kessler technique followed by a horizontal mattress for a total of a 4-core repair. This was reinforced with a No. 6 Prolene to the paratendon. The surgery was performed under IV sedation and an ankle block, using 17 minutes of tourniquet time.
On postoperative day 1, the patient was seen in plastic surgery and occupational therapy clinic. The hand therapist modified the hand extensor tendon repair protocol since there was no known protocol for repairs of the foot and toe extensor tendon. The patient was placed in an ankle foot orthosis with a toe extension device created by heating and molding a low-temperature thermoplastic sheet (Figure 2). The toes were boosted into slight hyper extension. This was done to reduce tension across the extensor tendon repair site. All of the toes were held in about 20°of extension, as the extensor digitorum longus (EDL) has a common origin, to aide in adherence of wearing and for comfort. No standing or weight bearing was permitted for 3 weeks.
A wheelchair was issued in lieu of crutches to inhibit the work of toe extension with gait swing-through. Otherwise, the patient would generate tension on the extensor tendon in order for the toes to clear the ground. It was postulated that it would be difficult to turn off the toe extensors while using crutches. Maximal laxity was desired because edema and early scar formation could increase tension on the repair, resulting in rupture if the patient tried to fire the muscle belly even while in passive extension.
The patient kept his appointments and progressed steadily. He started passive toe extension and relaxation once per day for 30 repetitions at 1 week to aide in tendon glide. He started place and hold techniques in toe extension at 3 weeks. This progressed to active extension 50% effort plus active flexion at 4 weeks after surgery, then 75% extension effort plus toe towel crunches at 5 weeks. Toe crunches are toe flexion exercises with a washcloth on the floor with active bending of the toes with light resistance similar to picking up a marble with the toes. He was found to have a third toe extensor lag at that time that was correctible. The patient was actively able to flex and extend the toe independently. The early extension lag was felt to be secondary to edema and scar formation, which, over time are anticipated to resolve and contract and effectively shorten the tendon. Tendon gliding, and scar massage were reviewed. The patient’s last therapy session occurred 7 weeks after surgery, and he was cleared for full activity at 12 weeks. There was no further follow-up as he was planning on back surgery 2 weeks later.
Discussion
The North Florida/South Georgia Veterans Health System is fortunate to have 4 CHTs on staff. CHTs take a 200 question 4 hour certifying exam after being licensed for a minimum of 3 years as a physical or occupational therapist and completing 4,000 hours of direct upper extremity patient experience. Pass rates from 2008 to 2018 ranged from 52% to 68%.3 These clinicians are key to the success of our hand surgery service, utilizing their education and skills on our elective and trauma cases. The hand therapy service applied their knowledge of hand extensor rehabilitation protocols to rehabilitate the patient’s toe extensor in the absence of clear guidelines.
Hand extensor tendon rehabilitation protocols are based on the location of the repair on the hand or forearm. Nine extensor zones are named, distal to proximal, from the distal interphalangeal joints to the proximal forearm (Figure 3). In his review of extensor hallucis longus (EHL) repairs, Al-Qattan described 6 foot-extensor tendon zones, distal to proximal, from the first toe at the insertion of the big toe extensor to the distal leg proximal to the extensor retinaculum (Figure 4).4 Zone 3 is over the metatarsophalangeal joint; zone 5 is under the extensor retinaculum. The extensor tendon repairs described in this report were in dorsal foot zone 4 (proximal to the metatarsophalangeal joint and over the metatarsals), which would be most comparable to hand extensor zone 6 (proximal to the metacarpal phalangeal joint and over the metacarpals).
The EDL originates on the lateral condyle of the tibia and anterior surface of the fibula and the interosseous membrane, passes under the extensor retinaculum, and divides into 4 separate tendons. The 4 tendons split into 3 slips; the central one inserts on the middle phalanx, and the lateral ones insert onto the distal phalanx of the 4 lateral toes, which allows for toe extension.5 The EDL common origin for the muscle belly that serves 4 tendon slips has clinical significance because rehabilitation for one digit will affect the others. Knowledge of the anatomical structures guides the clinical decision making whether it is in the hand or foot. The EDL works synergistically with the extensor digitorum brevis (EDBr) to dorsiflex (extend) the toe phalanges. The EDB originates at the supralateral surface of the calcaneus, lateral talocalcaneal ligament and cruciate crural ligament and inserts at the lateral side of the EDL of second, third, and fourth toes at the level of the metatarsophalangeal joint.6
Repair of lacerated extensor tendons in the foot is the recommended treatment. Chronic extensor lag of the phalanges can result in a claw toe deformity, difficulty controlling the toes when putting on shoes or socks, and catching of the toe on fabric or insoles.7 The extensor tendons are close to the deep and superficial peroneal nerves and to the dorsalis pedis artery, none of which were involved in this case report.
There are case reports and series of EHL repairs that all involves at least 3 weeks of immobilization.4,8,9 The EHL dorsiflexes the big toe. Al-Qattan’s series involved placing K wires across the interphalangeal joint of the big toe and across the metatarsophalangeal joint, which were removed at 6 weeks, in addition to 3.0 polypropylene tendon mattress sutures. All patients in this series healed without tendon rupture or infection. Our PubMed search did not reveal any specific protocol for the EDL or EDB tendons, which are anatomically most comparable to the extensor digitorum communis (EDC) tendons in the hand. The EDC originates at the lateral epicondyle of the humerus, also divides into 4 separate tendons and is responsible for extending the 4 ulnar sided fingers at the metacarpophalangeal joint.10
Tendon repair protocols are a balance between preventing tendon rupture by too aggressive therapy and with preventing tendon adhesions from prolonged immobilization. Orthotic fabrication plays a key early role with blocking possible forces creating unacceptable strain or tension across the surgical repair site. Traditionally, extensor tendon repairs in the hand were immobilized for at least 3 weeks to prevent rupture. This is still the preferred protocol for the patient unwilling or unable to follow instructions. The downside to this method is extension lags, extrinsic tightness, and adhesions that prevent flexion, which can require prolonged therapy or tenolysis surgery to correct.11-13
Early passive motion (EPM) was promoted in the 1980s when studies found better functional outcomes and fewer adhesions. This involved either a dynamic extension splint that relied on elastic bands (Louisville protocol) to keep tension off the repair or the Duran protocol that relied on a static splint and the patient doing the passive exercises with his other uninjured hand. Critics of the EPM protocol point to the costs of the splints and demands of postoperative hand therapy.11
Early active motion (EAM) is the most recent development in hand tendon rehabilitation and starts within days of surgery. Studies have found an earlier regain of total active motion in patients who are mobilized earlier.12 EAM protocols can be divided into controlled active motion (CAM) and relative motion extension splinting (RMES). CAM splints are forearm based and cross more joints. Relative motion splinting is the least restrictive, which makes it less likely that the patient will remove it. Patient friendly splints are ideal because tendon ruptures are often secondary to nonadherence.13 The yoke splint is an example of a RMES, which places the repaired digit in slightly greater extension at the metacarpal phalangeal joint than the other digits (Figure 5), allowing use of the uninjured digits.
The toe extensors do not have the juncturae tendinum connecting the individual EDL tendons to each other, as found between the EDC tendons in the hand. These connective bands can mask a single extensor tendon laceration in the hand when the patient is still able to extend the digit to neutral in the event of a more proximal dorsal hand laceration. A case can be made for closing the skin only in lesser toe extensor injuries in poor surgical candidates because the extensor lag would not be appreciated functionally when wearing shoes. There would be less functional impact when letting a toe extensor go untreated compared with that of a hand extensor. Routine activities such as typing or getting the fingers into a tight pocket could be challenging if hand extensors were untreated. The rehabilitation for toe extensors is more inconvenient when a patient is nonweight bearing, compared with wearing a hand yoke splint.
Conclusion
The case described used an early passive motion protocol without the dynamic splint to rehabilitate the third toe EDL and second toe EDB. This was felt to be the most patient and therapist friendly option, given the previously unchartered territory. The foot orthosis was in stock at the adjacent physical therapy clinic, and the toe booster was created in the hand therapy clinic with readily available supplies. Ideally, one would like to return structures to their anatomic site and control the healing process in the event of a traumatic injury to prevent nonanatomic healing between structures and painful scar adhesions in an area with little subcutaneous tissue. This patient’s tendon repair was still intact at 7 weeks and on his way to recovery, demonstrating good scar management techniques. The risks and benefits to lesser toe tendon repair and recovery would have to be weighed on an individual basis.
Acknowledgments
This project is the result of work supported with resources and use of facilities at the Malcom Randall VA Medical Center in Gainesville, Florida.
1. Hand Therapy Certification Commission. History of HTCC. https://www.htcc.org/consumer-information/about-htcc/history-of-htcc. Accessed November 8, 2019.
2. Coady-Fariborzian L, McGreane A. Comparison of hand emergency triage before and after specialty templates (2007 vs 2012). Hand (N Y). 2015;10(2):215-220.
3. Hand Therapy Certification Commission. Passing rates for the CHT exam. https://www.htcc.org/certify/exam-results/passing-rates. Accessed November 8, 2019.
4. Al-Qattan MM. Surgical treatment and results in 17 cases of open lacerations of the extensor hallucis longus tendon. J Plast Reconstr Aesthet Surg. 2007;60(4):360-367.
5. Wheeless CR. Wheeless’ textbook of orthopaedics: extensor digitorum longus. http://www.wheelessonline.com/ortho/extensor_digitorum_longus. Updated December 8, 2011. Accessed November 8, 2019.
6. Wheeless CR. Wheeless’ textbook of orthopaedics: extensor digitorum brevis. http://www.wheelessonline.com/ortho/extensor_digitorum_brevis. Updated March 4, 2018. Accessed November 8, 2019.
7. Coughlin M, Schon L. Disorders of tendons. https://musculoskeletalkey.com/disorders-of-tendons-2/#s0035. Published August 27, 2016. Accessed November 8, 2019.
8. Bronner S, Ojofeitimi S, Rose D. Repair and rehabilitation of extensor hallucis longus and brevis tendon lacerations in a professional dancer. J Orthop Sports Phys Ther. 2008;38(6):362-370.
9. Wong JC, Daniel JN, Raikin SM. Repair of acute extensor hallucis longus tendon injuries: a retrospective review. Foot Ankle Spec. 2014;7(1):45-51.
10. Wheeless CR. Wheeless’ textbook of orthopaedics: extensor digitorum communis. http://www.wheelessonline.com/ortho/extensor_digitorum_communis. Updated March 4, 2018. Accessed November 8, 2019.
11. Hall B, Lee H, Page R, Rosenwax L, Lee AH. Comparing three postoperative treatment protocols for extensor tendon repair in zones V and VI of the hand. Am J Occup Ther. 2010;64(5):682-688.
12. Wong AL, Wilson M, Girnary S, Nojoomi M, Acharya S, Paul SM. The optimal orthosis and motion protocol for extensor tendon injury in zones IV-VIII: a systematic review. J Hand Ther. 2017;30(4):447-456.
13. Collocott SJ, Kelly E, Ellis RF. Optimal early active mobilisation protocol after extensor tendon repairs in zones V and VI: a systematic review of literature. Hand Ther. 2018;23(1):3-18.
1. Hand Therapy Certification Commission. History of HTCC. https://www.htcc.org/consumer-information/about-htcc/history-of-htcc. Accessed November 8, 2019.
2. Coady-Fariborzian L, McGreane A. Comparison of hand emergency triage before and after specialty templates (2007 vs 2012). Hand (N Y). 2015;10(2):215-220.
3. Hand Therapy Certification Commission. Passing rates for the CHT exam. https://www.htcc.org/certify/exam-results/passing-rates. Accessed November 8, 2019.
4. Al-Qattan MM. Surgical treatment and results in 17 cases of open lacerations of the extensor hallucis longus tendon. J Plast Reconstr Aesthet Surg. 2007;60(4):360-367.
5. Wheeless CR. Wheeless’ textbook of orthopaedics: extensor digitorum longus. http://www.wheelessonline.com/ortho/extensor_digitorum_longus. Updated December 8, 2011. Accessed November 8, 2019.
6. Wheeless CR. Wheeless’ textbook of orthopaedics: extensor digitorum brevis. http://www.wheelessonline.com/ortho/extensor_digitorum_brevis. Updated March 4, 2018. Accessed November 8, 2019.
7. Coughlin M, Schon L. Disorders of tendons. https://musculoskeletalkey.com/disorders-of-tendons-2/#s0035. Published August 27, 2016. Accessed November 8, 2019.
8. Bronner S, Ojofeitimi S, Rose D. Repair and rehabilitation of extensor hallucis longus and brevis tendon lacerations in a professional dancer. J Orthop Sports Phys Ther. 2008;38(6):362-370.
9. Wong JC, Daniel JN, Raikin SM. Repair of acute extensor hallucis longus tendon injuries: a retrospective review. Foot Ankle Spec. 2014;7(1):45-51.
10. Wheeless CR. Wheeless’ textbook of orthopaedics: extensor digitorum communis. http://www.wheelessonline.com/ortho/extensor_digitorum_communis. Updated March 4, 2018. Accessed November 8, 2019.
11. Hall B, Lee H, Page R, Rosenwax L, Lee AH. Comparing three postoperative treatment protocols for extensor tendon repair in zones V and VI of the hand. Am J Occup Ther. 2010;64(5):682-688.
12. Wong AL, Wilson M, Girnary S, Nojoomi M, Acharya S, Paul SM. The optimal orthosis and motion protocol for extensor tendon injury in zones IV-VIII: a systematic review. J Hand Ther. 2017;30(4):447-456.
13. Collocott SJ, Kelly E, Ellis RF. Optimal early active mobilisation protocol after extensor tendon repairs in zones V and VI: a systematic review of literature. Hand Ther. 2018;23(1):3-18.
Necrotizing Infection of the Upper Extremity: A Veterans Affairs Medical Center Experience (2008-2017)
Necrotizing infection of the extremity is a rare but potentially lethal diagnosis with a mortality rate in the range of 17% to 35%.1-4 The plastic surgery service at the Malcom Randall Veterans Affairs Medical Center (MRVAMC) treats all hand emergencies, including upper extremity infection, in the North Florida/South Georgia Veterans Heath System. There has been a well-coordinated emergency hand care system in place for several years that includes specialty templates on the electronic health record, pre-existing urgent clinic appointments, and single service surgical specialty care.5 This facilitates a fluid line of communication between primary care, emergency department (ED) providers, and surgical specialties. The objective of the study was to evaluate our identification, treatment, and outcome of these serious infections.
Methods
The MRVAMC Institutional Review Board approved a retrospective review of necrotizing infection of the upper extremity treated at the facility by the plastic surgery service. Surgical cases over a 9-year period (June 5, 2008-June 5, 2017) were identified by CPT (current procedural technology) codes for amputation and/or debridement of the upper extremity. The charts were reviewed for evidence of necrotizing infection by clinical description or pathology report. The patients’ age, sex, etiology, comorbidities from their problem list, vitals, and laboratory results were recorded upon arrival at the hospital. Time from presentation to surgery, treatment, and outcomes were recorded.
Results
Ten patients were treated for necrotizing infection of the upper extremity over a 9-year period; all were men with an average age of 64 years. Etiologies included nail biting, “bug bites,” crush injuries, burns, suspected IV drug use, and unknown. Nine of 10 patients had diabetes mellitus (DM). Most did not show evidence of hemodynamic instability on hospital arrival (Table). One patient was hypotensive with a mean arterial blood pressure < 65 mm Hg, 2 had heart rates > 100 beats/min, 1 patient had a temperature > 38° C, and 7 had elevated white blood cell (WBC) counts ranging from 11 to 24 k/cmm. Two undiagnosed patients with DM (patients 1 and 8) expressed no complaints of pain and presented with blood glucose > 450 mg/dL with hemoglobin A1c levels > 12%.
Infectious disease and critical care services were involved in the treatment of several cases when requested. A computed tomography (CT) scan was used in 2 of the patients (patients 1 and 4) to assist in the diagnosis (Figure 1).
Seven patients out of 10 were treated with surgery within 24 hours on hospital arrival. The severity of the pathology was not initially recognized in 2 of the patients earlier in the review. A third patient resisted surgical treatment until the second hospital day. Four patients had from 1 to 3 digital amputations, 2 patients had wrist disarticulations, and 1 had a distal forearm amputation.
Antibiotics were managed by critical care, hospitalist, or infectious disease services and adjusted once final cultures were returned (Table).
Discussion
Necrotizing infection of the upper extremity is a rare pathology with a substantial risk of amputation and mortality that requires a high index of suspicion and expeditious referral to a hand surgeon. It is well accepted that the key to survival is prompt surgical debridement of all necrotic tissue, ideally within 24 hours of hospital arrival.2-4,6 Death is usually secondary to sepsis.3 The classic presentation of pain out of proportion to exam, hypotension, erythema, skin necrosis, elevated WBC count, and fever may not be present and can delay diagnosis.1-4,6
DM is the most common comorbidity, and reviews have found the disease occurs more often in males, both which are consistent with our study.1-3 Diabetic infections have been found to be more likely to present as necrotizing infection than are nondiabetic infections and be at a higher risk for amputation.7 The patients with the wrist disarticulations and forearm amputation had DM. A minor trauma can be a portal for infection, which can be monomicrobial or polymicrobial.1,4 Once the diagnosis is suspected, prompt resuscitation, surgical debridement, IV antibiotics, and early intensive care are lifesaving. Hyperbaric oxygen is not available at MRVAMC and was not pursued as a transfer request due to its controversial benefit.6
There were no perioperative 30-day mortalities over a 9-year period in patients identified as having necrotizing infection of the upper extremity. This is attributed to an aggressive and well-coordinated, multisystem approach involving emergency, surgical, anesthesia, intensive care, and infectious disease services.
The hand trauma triage system in place at MRVAMC was started in 2008 and presented at the 38th Annual VA Surgeons Meeting in New Haven, Connecticut. The process starts at the level of the ED, urgent care or primary care provider and facilitates rapid access to subspecialty care by reducing unnecessary phone calls and appointment wait times.
All hand emergencies are covered by the plastic surgery service rather than the traditional split coverage between orthopedics and plastic surgery. This provides consistency and continuity for the patients and staff. The electronic health record consult template gives specific instructions to contact the on-call plastic surgeon. The resident/fellow gets called if patient is in-house, and faculty is called if the patient is outside the main hospital. The requesting provider gets instructions on treatment and follow-up. Clinic profiles have appointments reserved for urgent consults during the first hour so that patients can be sent to pre-anesthesia clinic or hand therapy, depending on the diagnosis. This triage system increased our hand trauma volume by a multiple of 6 between 2008 and 2012 but cut the appointment wait time > 1 week by half, as a percentage of consults, and did not significantly increase after-hour use of the operating room. The number of faculty and trainees stayed the same.
We did find that speed to diagnosis for necrotizing infection is an area that can be improved on with a higher clinical suspicion. There is a learning curve to the diagnosis and treatment, which can be prolonged when the index cases do not present themselves often and the patients do not appear in distress. This argues for consistency in hand-specific trauma coverage. The patients were most often initially seen by the resident and examined by a faculty member within hours. There were 4 different plastic surgery faculty involved in these cases, and they all included resident participation before, during, and after surgery. Debridement consists of wide local excision to bleeding tissue. Author review of the operative notes found the numbers of trips to the operating room for debridement can be reduced as the surgeon becomes more confident in the diagnosis and management, resulting in less “whittling” and a more definitive debridement, resulting in a faster recovery.
The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) is a tool that helps to distinguish necrotizing infection from other forms of soft tissue infection by using a point system for laboratory values that include C-reactive protein (CRP), white blood count, hemoglobin, sodium, creatinine, and glucose values.8 We do not routinely request CRP results, but 1 of the 2 patients (patient 9) who had the full complement of laboratory tests would have met high-risk criteria. The diagnostic accuracy of this tool has been questioned9; however, the authors welcome any method that can rapidly and noninvasively assist in getting the patient proper attention.
The patients were not seen for long-term follow-up, but some did return to the main hospital or clinic for other pathology and were pleased to show off their grip strength after a 3-ray amputation (patient 1) and aesthetics after upper arm and forearm debridement and skin graft reconstruction (patient 4, Figure 4).
A single-ray amputation can be expected to result in a loss of grip and pinch strength, about 43.3% and 33.6%, respectively; however, given the alternative of further loss of life or limb, this was considered a reasonable trade-off.10 One wrist disarticulation and the forearm amputation were seen by amputee clinic for prosthetic fitting many months after the amputations once the wounds were healed and edema had subsided.
Conclusion
A well-coordinated multidisciplinary effort was the key to successful identification and treatment of this serious life- and limb-threatening infection at our institution. We did identify room for improvement in making an earlier diagnosis and performing a more aggressive first debridement.
Acknowledgments
This project is the result of work supported with resources and use of facilities at the Malcom Randall VA Medical Center in Gainesville, Florida.
1. Angoules AG, Kontakis G, Drakoulakis E, Vrentzos G, Granick MS, Giannoudis PV. Necrotizing fasciitis of upper and lower limb: a systemic review. Injury. 2007;38(suppl 5):S19-S26.
2. Chauhan A, Wigton MD, Palmer BA. Necrotizing fasciitis. J Hand Surg Am. 2014;39(8):1598-1601.
3. Cheng NC, SU YM, Kuo YS, Tai HC, Tang YB. Factors affecting the mortality of necrotizing fasciitis involving the upper extremities. Surg Today. 2008;38(12):1108-1113.
4. Sunderland IR, Friedrich JB. Predictors of mortality and limb loss in necrotizing soft tissue infections of the upper extremity. J Hand Surg Am. 2009;34(10):1900-1901.
5. Coady-Fariborzian L, McGreane A. Comparison of hand emergency triage before and after specialty templates (2007 vs 2012). Hand (N Y). 2015;10(2):215-220.
6. Stevens D, Bryant A. Necrotizing soft-tissue infections. N Engl J Med. 2017;377(23):2253-2265.
7. Sharma K, Pan D, Friedman J, Yu JL, Mull A, Moore AM. Quantifying the effect of diabetes on surgical hand and forearm infections. J Hand Surg Am. 2018;43(2):105-114.
8. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
9. Fernando SM, Tran A, Cheng W, et al. Necrotizing soft tissue infection: diagnostic accuracy of physical examination, imaging, and LRINEC score: a systematic review and meta-analysis. Ann Surg. 2019;269(1):58-65. 10. Bhat AK, Acharya AM, Narayanakurup JK, Kumar B, Nagpal PS, Kamath A. Functional and cosmetic outcome of single-digit ray amputation in hand. Musculoskelet Surg. 2017;101(3):275-281.
Necrotizing infection of the extremity is a rare but potentially lethal diagnosis with a mortality rate in the range of 17% to 35%.1-4 The plastic surgery service at the Malcom Randall Veterans Affairs Medical Center (MRVAMC) treats all hand emergencies, including upper extremity infection, in the North Florida/South Georgia Veterans Heath System. There has been a well-coordinated emergency hand care system in place for several years that includes specialty templates on the electronic health record, pre-existing urgent clinic appointments, and single service surgical specialty care.5 This facilitates a fluid line of communication between primary care, emergency department (ED) providers, and surgical specialties. The objective of the study was to evaluate our identification, treatment, and outcome of these serious infections.
Methods
The MRVAMC Institutional Review Board approved a retrospective review of necrotizing infection of the upper extremity treated at the facility by the plastic surgery service. Surgical cases over a 9-year period (June 5, 2008-June 5, 2017) were identified by CPT (current procedural technology) codes for amputation and/or debridement of the upper extremity. The charts were reviewed for evidence of necrotizing infection by clinical description or pathology report. The patients’ age, sex, etiology, comorbidities from their problem list, vitals, and laboratory results were recorded upon arrival at the hospital. Time from presentation to surgery, treatment, and outcomes were recorded.
Results
Ten patients were treated for necrotizing infection of the upper extremity over a 9-year period; all were men with an average age of 64 years. Etiologies included nail biting, “bug bites,” crush injuries, burns, suspected IV drug use, and unknown. Nine of 10 patients had diabetes mellitus (DM). Most did not show evidence of hemodynamic instability on hospital arrival (Table). One patient was hypotensive with a mean arterial blood pressure < 65 mm Hg, 2 had heart rates > 100 beats/min, 1 patient had a temperature > 38° C, and 7 had elevated white blood cell (WBC) counts ranging from 11 to 24 k/cmm. Two undiagnosed patients with DM (patients 1 and 8) expressed no complaints of pain and presented with blood glucose > 450 mg/dL with hemoglobin A1c levels > 12%.
Infectious disease and critical care services were involved in the treatment of several cases when requested. A computed tomography (CT) scan was used in 2 of the patients (patients 1 and 4) to assist in the diagnosis (Figure 1).
Seven patients out of 10 were treated with surgery within 24 hours on hospital arrival. The severity of the pathology was not initially recognized in 2 of the patients earlier in the review. A third patient resisted surgical treatment until the second hospital day. Four patients had from 1 to 3 digital amputations, 2 patients had wrist disarticulations, and 1 had a distal forearm amputation.
Antibiotics were managed by critical care, hospitalist, or infectious disease services and adjusted once final cultures were returned (Table).
Discussion
Necrotizing infection of the upper extremity is a rare pathology with a substantial risk of amputation and mortality that requires a high index of suspicion and expeditious referral to a hand surgeon. It is well accepted that the key to survival is prompt surgical debridement of all necrotic tissue, ideally within 24 hours of hospital arrival.2-4,6 Death is usually secondary to sepsis.3 The classic presentation of pain out of proportion to exam, hypotension, erythema, skin necrosis, elevated WBC count, and fever may not be present and can delay diagnosis.1-4,6
DM is the most common comorbidity, and reviews have found the disease occurs more often in males, both which are consistent with our study.1-3 Diabetic infections have been found to be more likely to present as necrotizing infection than are nondiabetic infections and be at a higher risk for amputation.7 The patients with the wrist disarticulations and forearm amputation had DM. A minor trauma can be a portal for infection, which can be monomicrobial or polymicrobial.1,4 Once the diagnosis is suspected, prompt resuscitation, surgical debridement, IV antibiotics, and early intensive care are lifesaving. Hyperbaric oxygen is not available at MRVAMC and was not pursued as a transfer request due to its controversial benefit.6
There were no perioperative 30-day mortalities over a 9-year period in patients identified as having necrotizing infection of the upper extremity. This is attributed to an aggressive and well-coordinated, multisystem approach involving emergency, surgical, anesthesia, intensive care, and infectious disease services.
The hand trauma triage system in place at MRVAMC was started in 2008 and presented at the 38th Annual VA Surgeons Meeting in New Haven, Connecticut. The process starts at the level of the ED, urgent care or primary care provider and facilitates rapid access to subspecialty care by reducing unnecessary phone calls and appointment wait times.
All hand emergencies are covered by the plastic surgery service rather than the traditional split coverage between orthopedics and plastic surgery. This provides consistency and continuity for the patients and staff. The electronic health record consult template gives specific instructions to contact the on-call plastic surgeon. The resident/fellow gets called if patient is in-house, and faculty is called if the patient is outside the main hospital. The requesting provider gets instructions on treatment and follow-up. Clinic profiles have appointments reserved for urgent consults during the first hour so that patients can be sent to pre-anesthesia clinic or hand therapy, depending on the diagnosis. This triage system increased our hand trauma volume by a multiple of 6 between 2008 and 2012 but cut the appointment wait time > 1 week by half, as a percentage of consults, and did not significantly increase after-hour use of the operating room. The number of faculty and trainees stayed the same.
We did find that speed to diagnosis for necrotizing infection is an area that can be improved on with a higher clinical suspicion. There is a learning curve to the diagnosis and treatment, which can be prolonged when the index cases do not present themselves often and the patients do not appear in distress. This argues for consistency in hand-specific trauma coverage. The patients were most often initially seen by the resident and examined by a faculty member within hours. There were 4 different plastic surgery faculty involved in these cases, and they all included resident participation before, during, and after surgery. Debridement consists of wide local excision to bleeding tissue. Author review of the operative notes found the numbers of trips to the operating room for debridement can be reduced as the surgeon becomes more confident in the diagnosis and management, resulting in less “whittling” and a more definitive debridement, resulting in a faster recovery.
The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) is a tool that helps to distinguish necrotizing infection from other forms of soft tissue infection by using a point system for laboratory values that include C-reactive protein (CRP), white blood count, hemoglobin, sodium, creatinine, and glucose values.8 We do not routinely request CRP results, but 1 of the 2 patients (patient 9) who had the full complement of laboratory tests would have met high-risk criteria. The diagnostic accuracy of this tool has been questioned9; however, the authors welcome any method that can rapidly and noninvasively assist in getting the patient proper attention.
The patients were not seen for long-term follow-up, but some did return to the main hospital or clinic for other pathology and were pleased to show off their grip strength after a 3-ray amputation (patient 1) and aesthetics after upper arm and forearm debridement and skin graft reconstruction (patient 4, Figure 4).
A single-ray amputation can be expected to result in a loss of grip and pinch strength, about 43.3% and 33.6%, respectively; however, given the alternative of further loss of life or limb, this was considered a reasonable trade-off.10 One wrist disarticulation and the forearm amputation were seen by amputee clinic for prosthetic fitting many months after the amputations once the wounds were healed and edema had subsided.
Conclusion
A well-coordinated multidisciplinary effort was the key to successful identification and treatment of this serious life- and limb-threatening infection at our institution. We did identify room for improvement in making an earlier diagnosis and performing a more aggressive first debridement.
Acknowledgments
This project is the result of work supported with resources and use of facilities at the Malcom Randall VA Medical Center in Gainesville, Florida.
Necrotizing infection of the extremity is a rare but potentially lethal diagnosis with a mortality rate in the range of 17% to 35%.1-4 The plastic surgery service at the Malcom Randall Veterans Affairs Medical Center (MRVAMC) treats all hand emergencies, including upper extremity infection, in the North Florida/South Georgia Veterans Heath System. There has been a well-coordinated emergency hand care system in place for several years that includes specialty templates on the electronic health record, pre-existing urgent clinic appointments, and single service surgical specialty care.5 This facilitates a fluid line of communication between primary care, emergency department (ED) providers, and surgical specialties. The objective of the study was to evaluate our identification, treatment, and outcome of these serious infections.
Methods
The MRVAMC Institutional Review Board approved a retrospective review of necrotizing infection of the upper extremity treated at the facility by the plastic surgery service. Surgical cases over a 9-year period (June 5, 2008-June 5, 2017) were identified by CPT (current procedural technology) codes for amputation and/or debridement of the upper extremity. The charts were reviewed for evidence of necrotizing infection by clinical description or pathology report. The patients’ age, sex, etiology, comorbidities from their problem list, vitals, and laboratory results were recorded upon arrival at the hospital. Time from presentation to surgery, treatment, and outcomes were recorded.
Results
Ten patients were treated for necrotizing infection of the upper extremity over a 9-year period; all were men with an average age of 64 years. Etiologies included nail biting, “bug bites,” crush injuries, burns, suspected IV drug use, and unknown. Nine of 10 patients had diabetes mellitus (DM). Most did not show evidence of hemodynamic instability on hospital arrival (Table). One patient was hypotensive with a mean arterial blood pressure < 65 mm Hg, 2 had heart rates > 100 beats/min, 1 patient had a temperature > 38° C, and 7 had elevated white blood cell (WBC) counts ranging from 11 to 24 k/cmm. Two undiagnosed patients with DM (patients 1 and 8) expressed no complaints of pain and presented with blood glucose > 450 mg/dL with hemoglobin A1c levels > 12%.
Infectious disease and critical care services were involved in the treatment of several cases when requested. A computed tomography (CT) scan was used in 2 of the patients (patients 1 and 4) to assist in the diagnosis (Figure 1).
Seven patients out of 10 were treated with surgery within 24 hours on hospital arrival. The severity of the pathology was not initially recognized in 2 of the patients earlier in the review. A third patient resisted surgical treatment until the second hospital day. Four patients had from 1 to 3 digital amputations, 2 patients had wrist disarticulations, and 1 had a distal forearm amputation.
Antibiotics were managed by critical care, hospitalist, or infectious disease services and adjusted once final cultures were returned (Table).
Discussion
Necrotizing infection of the upper extremity is a rare pathology with a substantial risk of amputation and mortality that requires a high index of suspicion and expeditious referral to a hand surgeon. It is well accepted that the key to survival is prompt surgical debridement of all necrotic tissue, ideally within 24 hours of hospital arrival.2-4,6 Death is usually secondary to sepsis.3 The classic presentation of pain out of proportion to exam, hypotension, erythema, skin necrosis, elevated WBC count, and fever may not be present and can delay diagnosis.1-4,6
DM is the most common comorbidity, and reviews have found the disease occurs more often in males, both which are consistent with our study.1-3 Diabetic infections have been found to be more likely to present as necrotizing infection than are nondiabetic infections and be at a higher risk for amputation.7 The patients with the wrist disarticulations and forearm amputation had DM. A minor trauma can be a portal for infection, which can be monomicrobial or polymicrobial.1,4 Once the diagnosis is suspected, prompt resuscitation, surgical debridement, IV antibiotics, and early intensive care are lifesaving. Hyperbaric oxygen is not available at MRVAMC and was not pursued as a transfer request due to its controversial benefit.6
There were no perioperative 30-day mortalities over a 9-year period in patients identified as having necrotizing infection of the upper extremity. This is attributed to an aggressive and well-coordinated, multisystem approach involving emergency, surgical, anesthesia, intensive care, and infectious disease services.
The hand trauma triage system in place at MRVAMC was started in 2008 and presented at the 38th Annual VA Surgeons Meeting in New Haven, Connecticut. The process starts at the level of the ED, urgent care or primary care provider and facilitates rapid access to subspecialty care by reducing unnecessary phone calls and appointment wait times.
All hand emergencies are covered by the plastic surgery service rather than the traditional split coverage between orthopedics and plastic surgery. This provides consistency and continuity for the patients and staff. The electronic health record consult template gives specific instructions to contact the on-call plastic surgeon. The resident/fellow gets called if patient is in-house, and faculty is called if the patient is outside the main hospital. The requesting provider gets instructions on treatment and follow-up. Clinic profiles have appointments reserved for urgent consults during the first hour so that patients can be sent to pre-anesthesia clinic or hand therapy, depending on the diagnosis. This triage system increased our hand trauma volume by a multiple of 6 between 2008 and 2012 but cut the appointment wait time > 1 week by half, as a percentage of consults, and did not significantly increase after-hour use of the operating room. The number of faculty and trainees stayed the same.
We did find that speed to diagnosis for necrotizing infection is an area that can be improved on with a higher clinical suspicion. There is a learning curve to the diagnosis and treatment, which can be prolonged when the index cases do not present themselves often and the patients do not appear in distress. This argues for consistency in hand-specific trauma coverage. The patients were most often initially seen by the resident and examined by a faculty member within hours. There were 4 different plastic surgery faculty involved in these cases, and they all included resident participation before, during, and after surgery. Debridement consists of wide local excision to bleeding tissue. Author review of the operative notes found the numbers of trips to the operating room for debridement can be reduced as the surgeon becomes more confident in the diagnosis and management, resulting in less “whittling” and a more definitive debridement, resulting in a faster recovery.
The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) is a tool that helps to distinguish necrotizing infection from other forms of soft tissue infection by using a point system for laboratory values that include C-reactive protein (CRP), white blood count, hemoglobin, sodium, creatinine, and glucose values.8 We do not routinely request CRP results, but 1 of the 2 patients (patient 9) who had the full complement of laboratory tests would have met high-risk criteria. The diagnostic accuracy of this tool has been questioned9; however, the authors welcome any method that can rapidly and noninvasively assist in getting the patient proper attention.
The patients were not seen for long-term follow-up, but some did return to the main hospital or clinic for other pathology and were pleased to show off their grip strength after a 3-ray amputation (patient 1) and aesthetics after upper arm and forearm debridement and skin graft reconstruction (patient 4, Figure 4).
A single-ray amputation can be expected to result in a loss of grip and pinch strength, about 43.3% and 33.6%, respectively; however, given the alternative of further loss of life or limb, this was considered a reasonable trade-off.10 One wrist disarticulation and the forearm amputation were seen by amputee clinic for prosthetic fitting many months after the amputations once the wounds were healed and edema had subsided.
Conclusion
A well-coordinated multidisciplinary effort was the key to successful identification and treatment of this serious life- and limb-threatening infection at our institution. We did identify room for improvement in making an earlier diagnosis and performing a more aggressive first debridement.
Acknowledgments
This project is the result of work supported with resources and use of facilities at the Malcom Randall VA Medical Center in Gainesville, Florida.
1. Angoules AG, Kontakis G, Drakoulakis E, Vrentzos G, Granick MS, Giannoudis PV. Necrotizing fasciitis of upper and lower limb: a systemic review. Injury. 2007;38(suppl 5):S19-S26.
2. Chauhan A, Wigton MD, Palmer BA. Necrotizing fasciitis. J Hand Surg Am. 2014;39(8):1598-1601.
3. Cheng NC, SU YM, Kuo YS, Tai HC, Tang YB. Factors affecting the mortality of necrotizing fasciitis involving the upper extremities. Surg Today. 2008;38(12):1108-1113.
4. Sunderland IR, Friedrich JB. Predictors of mortality and limb loss in necrotizing soft tissue infections of the upper extremity. J Hand Surg Am. 2009;34(10):1900-1901.
5. Coady-Fariborzian L, McGreane A. Comparison of hand emergency triage before and after specialty templates (2007 vs 2012). Hand (N Y). 2015;10(2):215-220.
6. Stevens D, Bryant A. Necrotizing soft-tissue infections. N Engl J Med. 2017;377(23):2253-2265.
7. Sharma K, Pan D, Friedman J, Yu JL, Mull A, Moore AM. Quantifying the effect of diabetes on surgical hand and forearm infections. J Hand Surg Am. 2018;43(2):105-114.
8. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
9. Fernando SM, Tran A, Cheng W, et al. Necrotizing soft tissue infection: diagnostic accuracy of physical examination, imaging, and LRINEC score: a systematic review and meta-analysis. Ann Surg. 2019;269(1):58-65. 10. Bhat AK, Acharya AM, Narayanakurup JK, Kumar B, Nagpal PS, Kamath A. Functional and cosmetic outcome of single-digit ray amputation in hand. Musculoskelet Surg. 2017;101(3):275-281.
1. Angoules AG, Kontakis G, Drakoulakis E, Vrentzos G, Granick MS, Giannoudis PV. Necrotizing fasciitis of upper and lower limb: a systemic review. Injury. 2007;38(suppl 5):S19-S26.
2. Chauhan A, Wigton MD, Palmer BA. Necrotizing fasciitis. J Hand Surg Am. 2014;39(8):1598-1601.
3. Cheng NC, SU YM, Kuo YS, Tai HC, Tang YB. Factors affecting the mortality of necrotizing fasciitis involving the upper extremities. Surg Today. 2008;38(12):1108-1113.
4. Sunderland IR, Friedrich JB. Predictors of mortality and limb loss in necrotizing soft tissue infections of the upper extremity. J Hand Surg Am. 2009;34(10):1900-1901.
5. Coady-Fariborzian L, McGreane A. Comparison of hand emergency triage before and after specialty templates (2007 vs 2012). Hand (N Y). 2015;10(2):215-220.
6. Stevens D, Bryant A. Necrotizing soft-tissue infections. N Engl J Med. 2017;377(23):2253-2265.
7. Sharma K, Pan D, Friedman J, Yu JL, Mull A, Moore AM. Quantifying the effect of diabetes on surgical hand and forearm infections. J Hand Surg Am. 2018;43(2):105-114.
8. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
9. Fernando SM, Tran A, Cheng W, et al. Necrotizing soft tissue infection: diagnostic accuracy of physical examination, imaging, and LRINEC score: a systematic review and meta-analysis. Ann Surg. 2019;269(1):58-65. 10. Bhat AK, Acharya AM, Narayanakurup JK, Kumar B, Nagpal PS, Kamath A. Functional and cosmetic outcome of single-digit ray amputation in hand. Musculoskelet Surg. 2017;101(3):275-281.
Assessment of Free Flap Breast Reconstructions
Free flap autologous breast reconstruction is an excellent surgical option for breast reconstruction in select patients. A free flap involves moving skin, fat, and/or muscle from a distant part of the body, based on a named blood supply (pedicle), and attaching it to another blood supply adjacent to the acquired defect. This procedure is particularly useful in areas where local tissue supply is lacking in volume or is damaged due to trauma or radiation. These reconstructions are performed largely in high-volume centers outside the VA because of the required specialized level of surgical training, manpower, and nursing support.1 The Malcom Randall VAMC in Gainesville, Florida, started offering autologous free flap breast reconstruction as an option to select patients in October 2012.
The Malcom Randall VAMC operating room (OR) does not operate 24/7, and the system has limited available OR time and surgical staff compared with the volume of patients requesting care.2 Operative planning for free flap autologous breast reconstruction must occur months ahead of surgery to balance the system limitations with the ability to offer the highest level of care. Planning includes strict patient selection, preoperative imaging, practice runs with OR staff, use of venous couplers, and frequent intensive care unit (ICU) staff in-services. Planning also includes the need to keep surgeries within the allocated OR time to avoid shift changes during critical periods. Frequent and early communication occurs between the surgical scheduler, OR nurses, and the anesthesia and critical care teams.
Studies have found that the best chance of flap salvage in the event of a thrombotic event is a rapid return to the OR.3 It is essential to minimize the risk of emergent returns to the OR because it is not staffed throughout the night. Patient risk factors for perioperative vascular complications include hypercoagulable disorders, peripheral vascular disease, use of the superficial epigastric system, and smoking.4-7
A PubMed search for free flap reconstruction solely within the VA over the past 20 years found 1 article discussing the use of free flaps in head and neck reconstruction which demonstrated an impressive success rate of 93%.8
The object of this study was to assess free flap breast reconstruction results at the Malcolm Randall VAMC to determine whether it is a realistic treatment to offer in the federal system.
Methods
The Malcolm Randall Institutional Review Board approved a retrospective chart review of all autologous free flap breast reconstructions using CPT code 19364, performed from October 2012 to June 2016. Medical records of patients who had a free flap breast reconstruction were queried during that period. Patient age; comorbidities listed on the electronic medical record “problem list;” body mass index (BMI); type of reconstruction (delayed vs immediate); length of surgery; length of stay; and complications over a 30-day period were recorded (Table). The authors looked for documentation of preoperative imaging and unplanned returns to the OR within the 30-day period.
Of 3 full-time VA plastic surgeons on staff during the study period, 2 surgeons had advanced fellowship training in either microsurgery or hand and microsurgery. Plastic surgery fellows and general surgery interns participated in the surgeries and postoperative care. The service had 1 dedicated advanced practice registered nurse involved in the surgical scheduling and perioperative care.
Results
A total of 11 abdominally based free flap breast reconstructions—6 muscle-sparing transverse rectus abdominus musculocutaneous (TRAM) and 5 deep inferior epigastric perforator (DIEP) flaps—were performed in 8 patients during the study period (Figures 1A, 1B, 1C, and 1D). Patient ages ranged from 31 to 58 years with a mean of 45.6 years. Six patients had preoperative computer tomography angiography (CTA) to define the location of the abdominal wall perforators. One muscle-sparing free flap was performed immediately after mastectomy; the other free flaps were performed as delayed reconstructions. Body mass index ranged from 24 to 35, with a mean of 30. All patients reported no tobacco use during the consultation; however, 1 patient later admitted to chewing tobacco. No urinary cotinine confirmation was requested. Two patients had 1 free flap reconstruction and 1 pedicle TRAM. This bilateral combination has been recently described in the literature and was chosen as a reasonable option to balance limited resources with abdominal wall morbidity.9 Operating room time ranged from 7 hours 50 minutes to 13 hours 3 minutes. All patients went to the ICU for hourly flap monitoring.
Length of stay ranged from 4 to 7 days, with a mean of 4.5 days. The longest stay was for a patient who had immediate reconstruction using a pedicle TRAM and muscle-sparing free TRAM. She was not a DIEP candidate because poor perforator quality had been noted during preoperative imaging.
Six patients had documentation of postoperative wound complications. One patient returned to the OR on the elective schedule 3 weeks postoperatively for a partial flap debridement. Her tissue transfer was > 1,000 g, and she required a matching reduction on the other side. There were no complete flap losses or postoperative thrombotic events; no cases went back to the OR emergently.
Discussion
With the number of women veterans steadily increasing, the number of patients in need of breast cancer surgery, including reconstruction, will rise in the VA.10 Fortunately, breast reconstruction is an elective procedure. Immediate breast reconstruction is a popular option because patients can combine surgeries and potentially avoid 2 recovery periods, and a better aesthetic outcome is possible because the skin does not have time to contract. Although immediate reconstruction has been increasing in popularity, it is associated with a higher complication rate.11 Further, reconstruction can be jeopardized if the oncologic plan is changed in the early postoperative period.
Positive margins found after an autologous reconstruction result in a more complicated postoperative course and a higher rate of wound complications.12 Unexpected radiation therapy after autologous reconstruction can severely distort a tissue flap because of fat necrosis, fibrosis, and contraction.13,14 From a practical perspective in the federal system, it is very difficult to coordinate 2 surgeons’ schedules when the system is already struggling to keep up with demand. Splitting the ablative and reconstructive surgery allows the urgent problem (cancer) to be addressed first, ensuring clear margins and allowing the patient to recover and consider all reconstructive options without feeling time pressure.
A large tertiary care center will have staff and equipment redundancy, but this study had to consider limitations in resources. The preoperative lead time allows the ICU to arrange a bed for hourly flap checks and for in-servicing new nursing staff on free flap monitoring. This was well received, and patients gave positive feedback on the staff. The OR schedulers can schedule nurses and techs who are familiar with the microscope and microsurgery instruments. The micro sets were opened, and the microscope powered on for practice runs a week before the procedures to insure no broken or missing instruments.
High-procedure volume would logically improve efficiency. Although the VA is not likely to become a tertiary center for breast reconstruction, the findings of other high-volume microsurgeons can be applied to improve speed and limit complications. Efforts to limit the OR time included use of preoperative imaging and intraoperative venous couplers. Venous couplers can result in shorter OR time, fewer returns to the OR, and excellent patency rates.15,16 One microsurgeon performed his surgery using only loupe-assisted vision (x 3.5), without use of the microscope. Pannucci and colleagues have recommended this as a way to improve access and OR efficiency.17 Use of the CTA has been found to decrease the rate of partial flap necrosis and improve speed of surgery.18-20
Careful patient selection allowed a hospital stay that averaged 4.5 days and minimized risks for return to the OR. Only patients who were nonsmokers were offered the surgery. Average BMI was 30 to prevent the known operative risks in breast surgery patients who are morbidly obese.21-23 No patients had a history of thromboembolic disease. Most patients were discharged home from the ICU. They eventually returned for elective revisions, second stages, and balancing procedures.
Conclusion
Free flap breast reconstruction can be offered as a treatment option with appropriate patient selection and planning. The most efficient way to provide this procedure within the federal system and to minimize the risk of flap loss and complications is by offering delayed reconstruction, obtaining preoperative CTA imaging, utilizing venous couplers, and frequently communicating with all involved practitioners from the OR to the ICU. This small study provides a good starting point to illustrate that tertiary-care reconstructive surgery can be offered to veterans within the federal system.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. Tuggle CT, Patel A, Broer N, Persing JA, Sosa JA, Au AF. Increased hospital volume is associated with improved outcomes following abdominal-based breast reconstruction. J Plast Surg Hand Surg. 2014;48(6):382-388.
2. Shulkin DJ. Beyond the VA crisis — becoming a high-performance network. N Engl J Med. 2016;374(11):1003-1005.
3. Novakovic D, Patel RS, Goldstein DP, Gullane PJ. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol. 2009;1:33.
4. Davison SP, Kessler CM, Al-Attar A. Microvascular free flap failure caused by unrecognized hypercoagulability. Plast Reconstr Surg. 2009;124(2):490-495.
5. Masoomi H, Clark EG, Paydar KZ, et al. Predictive risk factors of free flap thrombosis in breast reconstructive surgery. Microsurgery. 2014;34(8):589-594.
6. O’Neill AC, Haykal S, Bagher S, Zhong T, Hofer S. Predictors and consequences of intraoperative microvascular problems in autologous breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69(10):1349-1355.
7. Sanati-Mehrizy P, Massengburg BB, Rozehnal JM, Ignargiola MJ, Hernandez Rosa J, Taub PJ. Risk factors leading to free flap failure: analysis from the national surgical quality improvement program database. J Craniofac Surg. 2016;27(8):1956-1964.
8. Myers LL, Sumer BD, Defatta RJ, Minhajuddin A. Free tissue transfer reconstruction of the head and neck at a Veterans Affairs hospital. Head Neck. 2008;30(8):1007-1011.
9. Roslan EJ, Kelly EG, Zain MA, Basiron NH, Imran FH. Immediate simultaneous bilateral breast reconstruction with deep inferior epigastric (DIEP) flap and pedicled transverse rectus abdominis musculocutaneous (TRAM) pedicle flap. Med J Malaysia. 2017;72(1):85-87.
10. Leong M, Chike-Obi CJ, Basu CB, Lee EL, Albo D, Netscher DT. Effective breast reconstruction in female veterans. Am J Surg. 2009;198(5):658-663.
11. Kwok AC, Goodwin IA, Ying J, Agarwal JP. National trends and complication rates after bilateral mastectomy and immediate breast reconstruction from 2005 to 2012. Am J Surg. 2015;210(3):512-516.
12. Ochoa O, Theoharis C, Pisano S, et al. Positive margin re-excision following immediate autologous breast reconstruction: morbidity, cosmetic outcome, and oncologic significance. Aesthet Surg J. 2017; [Epub ahead of print.]
13. Garvey PB, Clemens MW, Hoy AE, et al. Muscle-sparing TRAM flap does not protect breast reconstruction from post-mastectomy radiation damage compared to DIEP flap. Plast Reconstr Surg. 2014;133(2):223-233.
14. Kronowitz SJ. Current status of autologous tissue-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130(2):282-292.
15. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, et al. The microvascular anastomotic coupler for venous anastomoses in free flap breast reconstruction improves outcomes. Gland Surg. 2016;5(2):88-92.
16. Jandali S, Wu LC, Vega SJ, Kovach SJ, Serletti JM. 1000 consecutive venous anastomoses using the microvascular anastomotic coupler in breast reconstruction. Plast Reconstr Surg. 2010;125(3):792-798.
17. Pannucci CJ, Basta MN, Kovach SJ, Kanchwala SK, Wu LC, Serletti JM. Loupes-only microsurgery is a safe alternative to the operating microscope: an analysis of 1,649 consecutive free flap breast reconstruction. J Reconstr Microsurg. 2015;31(9):636-642.
18. Teunis T, Heerma van Voss MR, Kon M, van Maurik JF. CT-angiography prior to DIEP flap reconstruction: a systemic review and meta-analysis. Microsurgery. 2013;33(6):496-502.
19. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, Band B, Ramakrishnan VV, Griffiths M. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surgery. 2016;5(2):93-98.
20. Malhotra A, Chhaya N, Nsiah-Sarbeng P, Mosahebi A. CT-guided deep inferior epigastric perforator (DIEP) flap localization—better for the patient, the surgeon, and the hospital. Clin Radiol. 2013;68(2):131-138.
21. Ilonzo N, Tsang A, Tsantes S, Estabrook A, Thu Ma AM. Breast reconstruction after mastectomy: a ten-year analysis of trends and immediate postoperative outcomes. Breast. 2017;32:7-12.
22. McAllister P, Teo L, Chin K, Makubate B, Alexander Munnoch D. Bilateral breast reconstruction with abdominal free flaps: a single centre, single surgeon retrospective review of 55 consecutive patients. Plast Surg Int. 2016;2016:6085624.
23. Myung Y, Heo CY. Relationship between obesity and surgical complications after reduction mammoplasty: a systemic literature review and meta-analysis. Aesthet Surg J. 2017;37(3):308-315.
Free flap autologous breast reconstruction is an excellent surgical option for breast reconstruction in select patients. A free flap involves moving skin, fat, and/or muscle from a distant part of the body, based on a named blood supply (pedicle), and attaching it to another blood supply adjacent to the acquired defect. This procedure is particularly useful in areas where local tissue supply is lacking in volume or is damaged due to trauma or radiation. These reconstructions are performed largely in high-volume centers outside the VA because of the required specialized level of surgical training, manpower, and nursing support.1 The Malcom Randall VAMC in Gainesville, Florida, started offering autologous free flap breast reconstruction as an option to select patients in October 2012.
The Malcom Randall VAMC operating room (OR) does not operate 24/7, and the system has limited available OR time and surgical staff compared with the volume of patients requesting care.2 Operative planning for free flap autologous breast reconstruction must occur months ahead of surgery to balance the system limitations with the ability to offer the highest level of care. Planning includes strict patient selection, preoperative imaging, practice runs with OR staff, use of venous couplers, and frequent intensive care unit (ICU) staff in-services. Planning also includes the need to keep surgeries within the allocated OR time to avoid shift changes during critical periods. Frequent and early communication occurs between the surgical scheduler, OR nurses, and the anesthesia and critical care teams.
Studies have found that the best chance of flap salvage in the event of a thrombotic event is a rapid return to the OR.3 It is essential to minimize the risk of emergent returns to the OR because it is not staffed throughout the night. Patient risk factors for perioperative vascular complications include hypercoagulable disorders, peripheral vascular disease, use of the superficial epigastric system, and smoking.4-7
A PubMed search for free flap reconstruction solely within the VA over the past 20 years found 1 article discussing the use of free flaps in head and neck reconstruction which demonstrated an impressive success rate of 93%.8
The object of this study was to assess free flap breast reconstruction results at the Malcolm Randall VAMC to determine whether it is a realistic treatment to offer in the federal system.
Methods
The Malcolm Randall Institutional Review Board approved a retrospective chart review of all autologous free flap breast reconstructions using CPT code 19364, performed from October 2012 to June 2016. Medical records of patients who had a free flap breast reconstruction were queried during that period. Patient age; comorbidities listed on the electronic medical record “problem list;” body mass index (BMI); type of reconstruction (delayed vs immediate); length of surgery; length of stay; and complications over a 30-day period were recorded (Table). The authors looked for documentation of preoperative imaging and unplanned returns to the OR within the 30-day period.
Of 3 full-time VA plastic surgeons on staff during the study period, 2 surgeons had advanced fellowship training in either microsurgery or hand and microsurgery. Plastic surgery fellows and general surgery interns participated in the surgeries and postoperative care. The service had 1 dedicated advanced practice registered nurse involved in the surgical scheduling and perioperative care.
Results
A total of 11 abdominally based free flap breast reconstructions—6 muscle-sparing transverse rectus abdominus musculocutaneous (TRAM) and 5 deep inferior epigastric perforator (DIEP) flaps—were performed in 8 patients during the study period (Figures 1A, 1B, 1C, and 1D). Patient ages ranged from 31 to 58 years with a mean of 45.6 years. Six patients had preoperative computer tomography angiography (CTA) to define the location of the abdominal wall perforators. One muscle-sparing free flap was performed immediately after mastectomy; the other free flaps were performed as delayed reconstructions. Body mass index ranged from 24 to 35, with a mean of 30. All patients reported no tobacco use during the consultation; however, 1 patient later admitted to chewing tobacco. No urinary cotinine confirmation was requested. Two patients had 1 free flap reconstruction and 1 pedicle TRAM. This bilateral combination has been recently described in the literature and was chosen as a reasonable option to balance limited resources with abdominal wall morbidity.9 Operating room time ranged from 7 hours 50 minutes to 13 hours 3 minutes. All patients went to the ICU for hourly flap monitoring.
Length of stay ranged from 4 to 7 days, with a mean of 4.5 days. The longest stay was for a patient who had immediate reconstruction using a pedicle TRAM and muscle-sparing free TRAM. She was not a DIEP candidate because poor perforator quality had been noted during preoperative imaging.
Six patients had documentation of postoperative wound complications. One patient returned to the OR on the elective schedule 3 weeks postoperatively for a partial flap debridement. Her tissue transfer was > 1,000 g, and she required a matching reduction on the other side. There were no complete flap losses or postoperative thrombotic events; no cases went back to the OR emergently.
Discussion
With the number of women veterans steadily increasing, the number of patients in need of breast cancer surgery, including reconstruction, will rise in the VA.10 Fortunately, breast reconstruction is an elective procedure. Immediate breast reconstruction is a popular option because patients can combine surgeries and potentially avoid 2 recovery periods, and a better aesthetic outcome is possible because the skin does not have time to contract. Although immediate reconstruction has been increasing in popularity, it is associated with a higher complication rate.11 Further, reconstruction can be jeopardized if the oncologic plan is changed in the early postoperative period.
Positive margins found after an autologous reconstruction result in a more complicated postoperative course and a higher rate of wound complications.12 Unexpected radiation therapy after autologous reconstruction can severely distort a tissue flap because of fat necrosis, fibrosis, and contraction.13,14 From a practical perspective in the federal system, it is very difficult to coordinate 2 surgeons’ schedules when the system is already struggling to keep up with demand. Splitting the ablative and reconstructive surgery allows the urgent problem (cancer) to be addressed first, ensuring clear margins and allowing the patient to recover and consider all reconstructive options without feeling time pressure.
A large tertiary care center will have staff and equipment redundancy, but this study had to consider limitations in resources. The preoperative lead time allows the ICU to arrange a bed for hourly flap checks and for in-servicing new nursing staff on free flap monitoring. This was well received, and patients gave positive feedback on the staff. The OR schedulers can schedule nurses and techs who are familiar with the microscope and microsurgery instruments. The micro sets were opened, and the microscope powered on for practice runs a week before the procedures to insure no broken or missing instruments.
High-procedure volume would logically improve efficiency. Although the VA is not likely to become a tertiary center for breast reconstruction, the findings of other high-volume microsurgeons can be applied to improve speed and limit complications. Efforts to limit the OR time included use of preoperative imaging and intraoperative venous couplers. Venous couplers can result in shorter OR time, fewer returns to the OR, and excellent patency rates.15,16 One microsurgeon performed his surgery using only loupe-assisted vision (x 3.5), without use of the microscope. Pannucci and colleagues have recommended this as a way to improve access and OR efficiency.17 Use of the CTA has been found to decrease the rate of partial flap necrosis and improve speed of surgery.18-20
Careful patient selection allowed a hospital stay that averaged 4.5 days and minimized risks for return to the OR. Only patients who were nonsmokers were offered the surgery. Average BMI was 30 to prevent the known operative risks in breast surgery patients who are morbidly obese.21-23 No patients had a history of thromboembolic disease. Most patients were discharged home from the ICU. They eventually returned for elective revisions, second stages, and balancing procedures.
Conclusion
Free flap breast reconstruction can be offered as a treatment option with appropriate patient selection and planning. The most efficient way to provide this procedure within the federal system and to minimize the risk of flap loss and complications is by offering delayed reconstruction, obtaining preoperative CTA imaging, utilizing venous couplers, and frequently communicating with all involved practitioners from the OR to the ICU. This small study provides a good starting point to illustrate that tertiary-care reconstructive surgery can be offered to veterans within the federal system.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
Free flap autologous breast reconstruction is an excellent surgical option for breast reconstruction in select patients. A free flap involves moving skin, fat, and/or muscle from a distant part of the body, based on a named blood supply (pedicle), and attaching it to another blood supply adjacent to the acquired defect. This procedure is particularly useful in areas where local tissue supply is lacking in volume or is damaged due to trauma or radiation. These reconstructions are performed largely in high-volume centers outside the VA because of the required specialized level of surgical training, manpower, and nursing support.1 The Malcom Randall VAMC in Gainesville, Florida, started offering autologous free flap breast reconstruction as an option to select patients in October 2012.
The Malcom Randall VAMC operating room (OR) does not operate 24/7, and the system has limited available OR time and surgical staff compared with the volume of patients requesting care.2 Operative planning for free flap autologous breast reconstruction must occur months ahead of surgery to balance the system limitations with the ability to offer the highest level of care. Planning includes strict patient selection, preoperative imaging, practice runs with OR staff, use of venous couplers, and frequent intensive care unit (ICU) staff in-services. Planning also includes the need to keep surgeries within the allocated OR time to avoid shift changes during critical periods. Frequent and early communication occurs between the surgical scheduler, OR nurses, and the anesthesia and critical care teams.
Studies have found that the best chance of flap salvage in the event of a thrombotic event is a rapid return to the OR.3 It is essential to minimize the risk of emergent returns to the OR because it is not staffed throughout the night. Patient risk factors for perioperative vascular complications include hypercoagulable disorders, peripheral vascular disease, use of the superficial epigastric system, and smoking.4-7
A PubMed search for free flap reconstruction solely within the VA over the past 20 years found 1 article discussing the use of free flaps in head and neck reconstruction which demonstrated an impressive success rate of 93%.8
The object of this study was to assess free flap breast reconstruction results at the Malcolm Randall VAMC to determine whether it is a realistic treatment to offer in the federal system.
Methods
The Malcolm Randall Institutional Review Board approved a retrospective chart review of all autologous free flap breast reconstructions using CPT code 19364, performed from October 2012 to June 2016. Medical records of patients who had a free flap breast reconstruction were queried during that period. Patient age; comorbidities listed on the electronic medical record “problem list;” body mass index (BMI); type of reconstruction (delayed vs immediate); length of surgery; length of stay; and complications over a 30-day period were recorded (Table). The authors looked for documentation of preoperative imaging and unplanned returns to the OR within the 30-day period.
Of 3 full-time VA plastic surgeons on staff during the study period, 2 surgeons had advanced fellowship training in either microsurgery or hand and microsurgery. Plastic surgery fellows and general surgery interns participated in the surgeries and postoperative care. The service had 1 dedicated advanced practice registered nurse involved in the surgical scheduling and perioperative care.
Results
A total of 11 abdominally based free flap breast reconstructions—6 muscle-sparing transverse rectus abdominus musculocutaneous (TRAM) and 5 deep inferior epigastric perforator (DIEP) flaps—were performed in 8 patients during the study period (Figures 1A, 1B, 1C, and 1D). Patient ages ranged from 31 to 58 years with a mean of 45.6 years. Six patients had preoperative computer tomography angiography (CTA) to define the location of the abdominal wall perforators. One muscle-sparing free flap was performed immediately after mastectomy; the other free flaps were performed as delayed reconstructions. Body mass index ranged from 24 to 35, with a mean of 30. All patients reported no tobacco use during the consultation; however, 1 patient later admitted to chewing tobacco. No urinary cotinine confirmation was requested. Two patients had 1 free flap reconstruction and 1 pedicle TRAM. This bilateral combination has been recently described in the literature and was chosen as a reasonable option to balance limited resources with abdominal wall morbidity.9 Operating room time ranged from 7 hours 50 minutes to 13 hours 3 minutes. All patients went to the ICU for hourly flap monitoring.
Length of stay ranged from 4 to 7 days, with a mean of 4.5 days. The longest stay was for a patient who had immediate reconstruction using a pedicle TRAM and muscle-sparing free TRAM. She was not a DIEP candidate because poor perforator quality had been noted during preoperative imaging.
Six patients had documentation of postoperative wound complications. One patient returned to the OR on the elective schedule 3 weeks postoperatively for a partial flap debridement. Her tissue transfer was > 1,000 g, and she required a matching reduction on the other side. There were no complete flap losses or postoperative thrombotic events; no cases went back to the OR emergently.
Discussion
With the number of women veterans steadily increasing, the number of patients in need of breast cancer surgery, including reconstruction, will rise in the VA.10 Fortunately, breast reconstruction is an elective procedure. Immediate breast reconstruction is a popular option because patients can combine surgeries and potentially avoid 2 recovery periods, and a better aesthetic outcome is possible because the skin does not have time to contract. Although immediate reconstruction has been increasing in popularity, it is associated with a higher complication rate.11 Further, reconstruction can be jeopardized if the oncologic plan is changed in the early postoperative period.
Positive margins found after an autologous reconstruction result in a more complicated postoperative course and a higher rate of wound complications.12 Unexpected radiation therapy after autologous reconstruction can severely distort a tissue flap because of fat necrosis, fibrosis, and contraction.13,14 From a practical perspective in the federal system, it is very difficult to coordinate 2 surgeons’ schedules when the system is already struggling to keep up with demand. Splitting the ablative and reconstructive surgery allows the urgent problem (cancer) to be addressed first, ensuring clear margins and allowing the patient to recover and consider all reconstructive options without feeling time pressure.
A large tertiary care center will have staff and equipment redundancy, but this study had to consider limitations in resources. The preoperative lead time allows the ICU to arrange a bed for hourly flap checks and for in-servicing new nursing staff on free flap monitoring. This was well received, and patients gave positive feedback on the staff. The OR schedulers can schedule nurses and techs who are familiar with the microscope and microsurgery instruments. The micro sets were opened, and the microscope powered on for practice runs a week before the procedures to insure no broken or missing instruments.
High-procedure volume would logically improve efficiency. Although the VA is not likely to become a tertiary center for breast reconstruction, the findings of other high-volume microsurgeons can be applied to improve speed and limit complications. Efforts to limit the OR time included use of preoperative imaging and intraoperative venous couplers. Venous couplers can result in shorter OR time, fewer returns to the OR, and excellent patency rates.15,16 One microsurgeon performed his surgery using only loupe-assisted vision (x 3.5), without use of the microscope. Pannucci and colleagues have recommended this as a way to improve access and OR efficiency.17 Use of the CTA has been found to decrease the rate of partial flap necrosis and improve speed of surgery.18-20
Careful patient selection allowed a hospital stay that averaged 4.5 days and minimized risks for return to the OR. Only patients who were nonsmokers were offered the surgery. Average BMI was 30 to prevent the known operative risks in breast surgery patients who are morbidly obese.21-23 No patients had a history of thromboembolic disease. Most patients were discharged home from the ICU. They eventually returned for elective revisions, second stages, and balancing procedures.
Conclusion
Free flap breast reconstruction can be offered as a treatment option with appropriate patient selection and planning. The most efficient way to provide this procedure within the federal system and to minimize the risk of flap loss and complications is by offering delayed reconstruction, obtaining preoperative CTA imaging, utilizing venous couplers, and frequently communicating with all involved practitioners from the OR to the ICU. This small study provides a good starting point to illustrate that tertiary-care reconstructive surgery can be offered to veterans within the federal system.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. Tuggle CT, Patel A, Broer N, Persing JA, Sosa JA, Au AF. Increased hospital volume is associated with improved outcomes following abdominal-based breast reconstruction. J Plast Surg Hand Surg. 2014;48(6):382-388.
2. Shulkin DJ. Beyond the VA crisis — becoming a high-performance network. N Engl J Med. 2016;374(11):1003-1005.
3. Novakovic D, Patel RS, Goldstein DP, Gullane PJ. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol. 2009;1:33.
4. Davison SP, Kessler CM, Al-Attar A. Microvascular free flap failure caused by unrecognized hypercoagulability. Plast Reconstr Surg. 2009;124(2):490-495.
5. Masoomi H, Clark EG, Paydar KZ, et al. Predictive risk factors of free flap thrombosis in breast reconstructive surgery. Microsurgery. 2014;34(8):589-594.
6. O’Neill AC, Haykal S, Bagher S, Zhong T, Hofer S. Predictors and consequences of intraoperative microvascular problems in autologous breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69(10):1349-1355.
7. Sanati-Mehrizy P, Massengburg BB, Rozehnal JM, Ignargiola MJ, Hernandez Rosa J, Taub PJ. Risk factors leading to free flap failure: analysis from the national surgical quality improvement program database. J Craniofac Surg. 2016;27(8):1956-1964.
8. Myers LL, Sumer BD, Defatta RJ, Minhajuddin A. Free tissue transfer reconstruction of the head and neck at a Veterans Affairs hospital. Head Neck. 2008;30(8):1007-1011.
9. Roslan EJ, Kelly EG, Zain MA, Basiron NH, Imran FH. Immediate simultaneous bilateral breast reconstruction with deep inferior epigastric (DIEP) flap and pedicled transverse rectus abdominis musculocutaneous (TRAM) pedicle flap. Med J Malaysia. 2017;72(1):85-87.
10. Leong M, Chike-Obi CJ, Basu CB, Lee EL, Albo D, Netscher DT. Effective breast reconstruction in female veterans. Am J Surg. 2009;198(5):658-663.
11. Kwok AC, Goodwin IA, Ying J, Agarwal JP. National trends and complication rates after bilateral mastectomy and immediate breast reconstruction from 2005 to 2012. Am J Surg. 2015;210(3):512-516.
12. Ochoa O, Theoharis C, Pisano S, et al. Positive margin re-excision following immediate autologous breast reconstruction: morbidity, cosmetic outcome, and oncologic significance. Aesthet Surg J. 2017; [Epub ahead of print.]
13. Garvey PB, Clemens MW, Hoy AE, et al. Muscle-sparing TRAM flap does not protect breast reconstruction from post-mastectomy radiation damage compared to DIEP flap. Plast Reconstr Surg. 2014;133(2):223-233.
14. Kronowitz SJ. Current status of autologous tissue-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130(2):282-292.
15. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, et al. The microvascular anastomotic coupler for venous anastomoses in free flap breast reconstruction improves outcomes. Gland Surg. 2016;5(2):88-92.
16. Jandali S, Wu LC, Vega SJ, Kovach SJ, Serletti JM. 1000 consecutive venous anastomoses using the microvascular anastomotic coupler in breast reconstruction. Plast Reconstr Surg. 2010;125(3):792-798.
17. Pannucci CJ, Basta MN, Kovach SJ, Kanchwala SK, Wu LC, Serletti JM. Loupes-only microsurgery is a safe alternative to the operating microscope: an analysis of 1,649 consecutive free flap breast reconstruction. J Reconstr Microsurg. 2015;31(9):636-642.
18. Teunis T, Heerma van Voss MR, Kon M, van Maurik JF. CT-angiography prior to DIEP flap reconstruction: a systemic review and meta-analysis. Microsurgery. 2013;33(6):496-502.
19. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, Band B, Ramakrishnan VV, Griffiths M. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surgery. 2016;5(2):93-98.
20. Malhotra A, Chhaya N, Nsiah-Sarbeng P, Mosahebi A. CT-guided deep inferior epigastric perforator (DIEP) flap localization—better for the patient, the surgeon, and the hospital. Clin Radiol. 2013;68(2):131-138.
21. Ilonzo N, Tsang A, Tsantes S, Estabrook A, Thu Ma AM. Breast reconstruction after mastectomy: a ten-year analysis of trends and immediate postoperative outcomes. Breast. 2017;32:7-12.
22. McAllister P, Teo L, Chin K, Makubate B, Alexander Munnoch D. Bilateral breast reconstruction with abdominal free flaps: a single centre, single surgeon retrospective review of 55 consecutive patients. Plast Surg Int. 2016;2016:6085624.
23. Myung Y, Heo CY. Relationship between obesity and surgical complications after reduction mammoplasty: a systemic literature review and meta-analysis. Aesthet Surg J. 2017;37(3):308-315.
1. Tuggle CT, Patel A, Broer N, Persing JA, Sosa JA, Au AF. Increased hospital volume is associated with improved outcomes following abdominal-based breast reconstruction. J Plast Surg Hand Surg. 2014;48(6):382-388.
2. Shulkin DJ. Beyond the VA crisis — becoming a high-performance network. N Engl J Med. 2016;374(11):1003-1005.
3. Novakovic D, Patel RS, Goldstein DP, Gullane PJ. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol. 2009;1:33.
4. Davison SP, Kessler CM, Al-Attar A. Microvascular free flap failure caused by unrecognized hypercoagulability. Plast Reconstr Surg. 2009;124(2):490-495.
5. Masoomi H, Clark EG, Paydar KZ, et al. Predictive risk factors of free flap thrombosis in breast reconstructive surgery. Microsurgery. 2014;34(8):589-594.
6. O’Neill AC, Haykal S, Bagher S, Zhong T, Hofer S. Predictors and consequences of intraoperative microvascular problems in autologous breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69(10):1349-1355.
7. Sanati-Mehrizy P, Massengburg BB, Rozehnal JM, Ignargiola MJ, Hernandez Rosa J, Taub PJ. Risk factors leading to free flap failure: analysis from the national surgical quality improvement program database. J Craniofac Surg. 2016;27(8):1956-1964.
8. Myers LL, Sumer BD, Defatta RJ, Minhajuddin A. Free tissue transfer reconstruction of the head and neck at a Veterans Affairs hospital. Head Neck. 2008;30(8):1007-1011.
9. Roslan EJ, Kelly EG, Zain MA, Basiron NH, Imran FH. Immediate simultaneous bilateral breast reconstruction with deep inferior epigastric (DIEP) flap and pedicled transverse rectus abdominis musculocutaneous (TRAM) pedicle flap. Med J Malaysia. 2017;72(1):85-87.
10. Leong M, Chike-Obi CJ, Basu CB, Lee EL, Albo D, Netscher DT. Effective breast reconstruction in female veterans. Am J Surg. 2009;198(5):658-663.
11. Kwok AC, Goodwin IA, Ying J, Agarwal JP. National trends and complication rates after bilateral mastectomy and immediate breast reconstruction from 2005 to 2012. Am J Surg. 2015;210(3):512-516.
12. Ochoa O, Theoharis C, Pisano S, et al. Positive margin re-excision following immediate autologous breast reconstruction: morbidity, cosmetic outcome, and oncologic significance. Aesthet Surg J. 2017; [Epub ahead of print.]
13. Garvey PB, Clemens MW, Hoy AE, et al. Muscle-sparing TRAM flap does not protect breast reconstruction from post-mastectomy radiation damage compared to DIEP flap. Plast Reconstr Surg. 2014;133(2):223-233.
14. Kronowitz SJ. Current status of autologous tissue-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130(2):282-292.
15. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, et al. The microvascular anastomotic coupler for venous anastomoses in free flap breast reconstruction improves outcomes. Gland Surg. 2016;5(2):88-92.
16. Jandali S, Wu LC, Vega SJ, Kovach SJ, Serletti JM. 1000 consecutive venous anastomoses using the microvascular anastomotic coupler in breast reconstruction. Plast Reconstr Surg. 2010;125(3):792-798.
17. Pannucci CJ, Basta MN, Kovach SJ, Kanchwala SK, Wu LC, Serletti JM. Loupes-only microsurgery is a safe alternative to the operating microscope: an analysis of 1,649 consecutive free flap breast reconstruction. J Reconstr Microsurg. 2015;31(9):636-642.
18. Teunis T, Heerma van Voss MR, Kon M, van Maurik JF. CT-angiography prior to DIEP flap reconstruction: a systemic review and meta-analysis. Microsurgery. 2013;33(6):496-502.
19. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, Band B, Ramakrishnan VV, Griffiths M. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surgery. 2016;5(2):93-98.
20. Malhotra A, Chhaya N, Nsiah-Sarbeng P, Mosahebi A. CT-guided deep inferior epigastric perforator (DIEP) flap localization—better for the patient, the surgeon, and the hospital. Clin Radiol. 2013;68(2):131-138.
21. Ilonzo N, Tsang A, Tsantes S, Estabrook A, Thu Ma AM. Breast reconstruction after mastectomy: a ten-year analysis of trends and immediate postoperative outcomes. Breast. 2017;32:7-12.
22. McAllister P, Teo L, Chin K, Makubate B, Alexander Munnoch D. Bilateral breast reconstruction with abdominal free flaps: a single centre, single surgeon retrospective review of 55 consecutive patients. Plast Surg Int. 2016;2016:6085624.
23. Myung Y, Heo CY. Relationship between obesity and surgical complications after reduction mammoplasty: a systemic literature review and meta-analysis. Aesthet Surg J. 2017;37(3):308-315.
Comparison of Carpal Tunnel Release Methods and Complications
Carpal tunnel release is one of the most common hand surgeries performed at the North Florida/South Georgia Veterans Health System (NFSGVHS). Depending on surgeon experience and comfort level, surgeries are performed through either the traditional open method or the endoscopic method, single or double port (Figures 1 and 2). The advantage of the endoscopic method is faster recovery and return to work; however, the endoscopic method requires more expensive equipment and a steeper learning curve for surgeons. Complications are uncommon but can create unsatisfactory patient experiences because of costly lost workdays and long travel distances to the medical facility.
The purpose of this study was to compare the endoscopic method with the open carpal tunnel release method to determine whether there was an increased complication risk. Researchers anticipated that this information would help surgeons better inform patients of operative risks and prompt changes in NFSGVHS treatment plans to improve the quality of veteran care.
Methods
An Institutional Review Board- approved (#647-2011) retrospective review was done of patients who had carpal tunnel surgery performed by the NFSGVHS plastic surgery service from January 1, 2005, to December 31, 2010. Surgeries included in the review took place at the Malcom Randall VAMC in Gainesville and at the Lake City VAMC, both in Florida. Most of the surgeries included in the study were performed by a resident or fellow under the supervision of an attending physician. Eight different attending surgeons staffed the operations. Seven were board-certified or board-eligible plastic surgeons, 2 had advanced hand fellowship training, and 1 was a general surgeon with hand fellowship training. All hand fellowship-trained surgeons were in their first year of practice at the time of the study.
Only primary carpal tunnel releases were included in the study. Exclusion criteria included patients who were operated on by a service section other than the plastic surgery service (orthopedics or neurosurgery) and hands on which other procedures were performed during the same operation. Charts were reviewed for up to 1 year post surgery. Complications that required intervention were recorded. Researchers did not include pillar tenderness or an increase in occupational therapy visits as complications, due to the wide variety of patient tolerance to postoperative pain and varying motivation to return to work and daily routine.
Methods of release were endoscopic, open, or endoscopic converted to open. All but 6 of the completed endoscopic surgeries were performed using the double port Chow technique. The other 6 endoscopic surgeries were performed using the single port Agee technique at the distal wrist crease. There were 3 endoscopic converted to open cases that were performed using a single port, proximally-based technique in the midpalm. This method was abandoned after 3 unsuccessful endoscopic attempts, 1 resulting in digital nerve injury despite interactive cadaver labs prior to operative experience.
Endoscopic surgeries converted to open were recorded as open surgeries, because the patients had the full invasive experience. Researchers used the chi-square test and P value < .05 to compare the different methods of carpal tunnel release with identified complications.
Results and Complications
A total of 584 hands belonging to 452 patients were included in the study. Patients included 395 men and 57 women aged from 33 to 91 years. There were 271 endoscopic releases, 228 open releases, and 85 endoscopic converted to open releases. The NFSGVHS conversion rate was 23.7%. Complications in the converted cases (n = 4) were included in the open release results.
There were 40 complications in 38 hands. The overall complication rate was 6.5%. Complications noted were tendonitis presenting as De Quervain disease or trigger finger (9 endoscopic surgeries; 6 open surgeries), infection (2 endoscopic surgeries; 6 open surgeries), wound dehiscence (5 open surgeries), nerve injury (1 open surgery), respiratory distress (1 endoscopic), complex regional pain syndrome (1 open surgery), and scheduled returns to the operating room (OR) for recurrent, ongoing, or worsening symptoms (5 endoscopic surgeries; 5 open surgeries). Complications with an n > 1 were evaluated for statistical significance with P value < .05 (Table 1).
The NFSGVHS study had 10 patients return to the OR for open exploration (Table 2). Nine of these patients went back to the OR based on symptoms consistent with nerve conduction studies that had deteriorated compared with their preoperative studies. One endoscopic case was brought back to the OR for a suspected nerve injury without nerve conduction studies. Findings during reoperation included scar adhesions, incomplete release of ligaments, digital nerve injury, and negative explorations.
Two hypothenar fat transfers were performed to prevent scar adhesions in cases that had originally been open releases.1 Two of the open cases were endoscopic converted to open cases. One went back to the OR with a suspected nerve injury. Dense adhesions and an injured common digital nerve were identified and repaired. The second converted case that went back to the OR had a suspected, but unconfirmed, nerve injury to the motor branch. The diagnosis and treatment were delayed for more than a year due to the patient having other pressing medical and family concerns. An exploration found significant scar adhesions, and an opponensplasty was performed.
One patient had respiratory insufficiency secondary to chemical pneumonitis. The patient was sedated during an endoscopic carpal tunnel release, aspirated, and kept intubated in the intensive care unit until the morning after surgery.
An early complex regional pain syndrome diagnosis was made in a patient with underlying neuropathy and a preoperative “profound” median neuropathies diagnosis at the wrist with underlying peripheral neuropathy found on nerve conduction studies. The patient experienced an unusual amount of postoperative pain and edema after an uncomplicated open carpal tunnel release. This was treated with rapid intervention using anti- inflammatories and hand therapy. The patient also started a regimen of skin care, edema management, neuroreeducation, and contrast baths. Symptoms responded within a week.
There were 12 wound complications: 10 in open and 2 in endoscopic surgeries. Total wound complications were equally split between patients with and without diabetes. Infection and dehiscence were noted. Sutures were removed an average of 9.6 days after surgery in the patients whose wounds broke down. A statistically significant relationship was found only between the open method of release and wound dehiscence (P < .05).
There was no statistically significant difference in the overall complication rate in the NFSGVHS population when comparing endoscopic with open carpal tunnel release or when comparing the risk of postoperative tendonitis, wound infection, or return to the OR.
Discussion
Carpal tunnel syndrome was documented by James Paget in mid-19th century in reference to a distal radius fracture.2 It is the most common peripheral nerve compression, with an incidence ranging from 1 to 3 cases per 1,000 subjects per year and a prevalence of 50 cases per 1,000 subjects per year.3 In an active-duty U.S. military population, the incidence of carpal tunnel syndrome is 3.98 per 1,000 person years.4
Related: Risk Factors for Postoperative Complications in Trigger Finger Release
The endoscopic method of release was first introduced in 1989 by Okutsu and colleagues.5 About 500,000 carpal tunnel releases are now performed in the U.S. every year, with 50,000 performed endoscopically.3 There were 185 carpal tunnel releases (56 endoscopic and 129 open) performed at the NFSGVHS in 2012.6 The minimally invasive procedure was designed to preserve the overlying skin and fascia, promoting an earlier return to work and daily activities. This is particularly relevant for manual workers who desire rapid return of grip strength. Multiple published reports have found more rapid recovery based on a reduction in scar tenderness, increase in grip strength, or return to work.7-13 Patients seem to have equivalent results over the long term, ranging from 3 months to 1 year.7,8,13-15 Return to work was not evaluated in this study, because many patients were either retired or not working steadily.
The endoscopic method was criticized after its introduction due to its potential increase in major structural injury to the median nerve, ulnar nerve, palmar arch, ulnar artery, or flexor tendons.16 A meta-analysis found improved outcomes but a statistically significant higher complication rate in endoscopic, compared with open release (2.2% in endoscopic vs 1.2% in open).16 Referral patterns have found iatrogenic nerve injury in patients referred by surgeons without formal hand fellowship training.17 There is a wide variety of background training for surgeons who may offer carpal tunnel release, including plastic surgery, orthopedics, general surgery, and neurosurgery.
Major structural injuries were reported by hand surgeons using both open and endoscopic methods in a questionnaire sent to members of the American Society for Surgery of the Hand, indicating that either approach demands respect.18 A large review of the literature from 1966 to 2001 by Benson and colleagues found that the endoscopic approach was not more likely to produce injury to tendons, arteries, or nerves compared with the open approach and actually had a lower rate of structural damage (0.49% vs 0.19%).19 Researchers who conducted this study confirmed one common digital nerve injury in an endoscopic converted to open technique, using a distally-based port with the blade not being deployed via the endoscopic method. The endoscopic method has been found to have a higher rate of reversible nerve injury (neuropraxia) compared with the open technique.7,10,19
The NFSGVHS results found a higher rate of wound dehiscence. More frequent wound site complications, particularly infection, hypertrophic scar, and scar tenderness have been noted using the open method.3,8,20 This is probably due to the deeper and slightly larger incision used for the open method compared with the smaller and shallower incisions used for the endoscopic release.
There is the inevitable learning curve for the endoscopic release due to the more complicated nature of the procedure. The NFSGVHS conversion rate was 23.7% over the 5-year period from 2005 to 2010. All 3 fellowship- trained hand surgeons were in their first year of practice at the time of the study, so the authors anticipate a lower conversion rate in forthcoming studies. The NFSGVHS researchers did not consider converting to an open technique to be a complication and believe it is appropriate to teach plastic surgery residents and fellows to have a low threshold to convert when visualization is not optimal and the potential for significant injury exists. The learning curve and a higher conversion rate have been acknowledged by Beck and colleagues with no increase in morbidity.21
The authors anticipated finding an increased rate of tendonitis in the endoscopic method, as found by Goshtasby and colleagues, where trigger finger was found more frequently in the endoscopic patients.22 The NFSGVHS study found that the number of patients presenting for steroid injections to treat postoperative tendonitis in the hand and wrist was not statistically significant when comparing the 2 surgical methods of release (3.3% in endoscopic vs 1.9% in open; P = .28).
The NFSGVHS rate of return to the OR within a year of surgery was 1.7%. The researchers from NFSGHVS anticipated a higher rate of return to the OR for ongoing symptoms secondary to incomplete release of the transverse carpal ligament. Published studies have found an intact retinaculum to be a cause of persistent symptoms when smaller incisions are used.23,24 Five endoscopic cases and 5 open cases eventually returned to the OR for carpal tunnel exploration. Two of the patients were classified as recurrent, because they had improvement of symptoms initially but presented > 6 months later with new symptoms. Eight of the patients were classified as persistent, because they did not have an extended period of relief of preoperative symptoms (Table 2).25 There was no statistically significant difference in return to the OR in the 2 study groups. The NFSGVHS researchers did note a trend in more incomplete nerve releases in the endoscopic group and more scar adhesions as the etiology of symptoms in the open group who went back to surgery.
Published studies have found no difference in overall complication rates when comparing the open with the endoscopic method of release, which is consistent with NFSGVHS data.8,11,12,26
A limitation of the current retrospective study is the large number of providers who both operated on the patients and documented their postoperative findings. The strength of the study is that VA patients tend to stay within the VISN for their health care so postoperative problems will be identified and routed to the plastic surgery service for evaluation and treatment.
Clinical implications for the NFSGVHS practice are that surgeons can confidently offer both the open and endoscopic surgeries without an overall risk of increased complications to patients. Patients who are identified as higher risk for wound dehiscence, such as those who place an unusual amount of pressure on their palms due to assisted walking devices or are at a higher risk of falling onto the surgical site, will be steered toward an endoscopic surgery. The NFSGVHS began a splinting protocol in the early postoperative period that was not previously used on those select patients who have open carpal tunnel releases.
Conclusion
Wound dehiscence was the only statistically significant complication found in the NFSGVHS veteran population when comparing open with endoscopic carpal tunnel release. This can potentially be prevented in future patients by delaying the removal of sutures and prolonging the use of a protective dressing in patients who undergo open release. There was not a statistically significant increase in overall complications when using the minimally invasive method of release, which is consistent with existing literature.
Acknowledgement
This material is the result of work supported with resources and the use of facilities at the Malcom Randall VAMC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Chrysopoulo MT, Greenberg JA, Kleinman WB. The hypothenar fat pad transposition flap: a modified surgical technique. Tech Hand Up Extrem Surg. 2006;10(3):150-156.
2. Paget J. Lectures on Surgical Pathology Delivered at the Royal College of Surgeons of England. London, England: Longman, Green, Brown, and Longmans; 1853.
3. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43(4):431-437.
4. Wolf JM, Mountcastle S, Owens BD. Incidence of carpal tunnel syndrome in the US military population. Hand (NY). 2009;4(3):289-293.
5. Okutsu I, Ninomiya S, Takatori Y, Ugawa Y. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
6. U.S. Department of Veterans Affairs. Health Information Systems and Technology Architecture Database, Ambulatory Surgical Case Load Report, 2012. Accessed March 14, 2013.
7. Larsen MB, Sørensen AI, Crone KL, Weis T, Boeckstyns ME. Carpal tunnel release: a randomized comparison of three surgical methods. J Hand Surg Eur Vol. 2013;38(6):646-650.
8. Malhotra R, Kiran EK, Dua A, Mallinath SG, Bhan S. Endoscopic versus open carpal tunnel release: a short-term comparative study. Indian J Orthop. 2007;41(1):57-61.
9. Sabesan VJ, Pedrotty D, Urbaniak JR, Aldridge JM 3rd. An evidence-based review of a single surgeon’s experience with endoscopic carpal tunnel release. J Surg Orthop Adv. 2012;21(3):117-121.
10. Thoma A, Veltri K, Haines T, Duku E. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
11. Tian Y, Zhao H, Wang T. Prospective comparison of endoscopic and open surgical methods for carpal tunnel syndrome. Chin Med Sci J. 2007;22(2):104-107.
12. Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84-A(7):1107-1115.
13. Vasiliadis HS, Xenakis TA, Mitsionis G, Paschos N, Georgoulis A. Endoscopic versus open carpal tunnel release. Arthroscopy. 2010:26(1):26-33.
14. Macdermid JC, Richards RS, Roth JH, Ross DC, King GJ. Endoscopic versus open carpal tunnel release: a randomized trial. J Hand Surg Am. 2003;28(3):475-480.
15. Aslani HR, Alizadeh K, Eajazi A, et al. Comparison of carpal tunnel release with three different techniques. Clin Neurol Neurosurg. 2012;114(7):965-968.
16. Kohanzadeh S, Herrera FA, Dobke M. Outcomes of open and endoscopic carpal tunnel release: a meta-analysis. Hand (NY). 2012;7(3):247-251.
17. Azari KK, Spiess AM, Buterbaugh GA, Imbriglia JE. Major nerve injuries associated with carpal tunnel release. Plast Reconstr Surg. 2007;119(6):1977-1978.
18. Palmer AK, Toivonen DA. Complications of endoscopic and open carpal tunnel release. J Hand Surg Am. 1999;24(3):561-565.
19. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
20. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
21. Beck JD, Deegan JH, Rhoades D, Klena JC. Results of endoscopic carpal tunnel release relative to surgeon experience with the Agee technique. J Hand Surg Am. 2011;36(1):61-64.
22. Goshtasby PH, Wheeler DR, Moy OJ. Risk factors for trigger finger occurrence after carpal tunnel release. Hand Surg. 2010;15(2):81-87.
23. Assmus H, Dombert T, Staub F. Reoperations for CTS because of recurrence or for correction [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):306-311.
24. Frik A, Baumeister RG. Re-intervention after carpal tunnel release [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):312-316.
25. Jones NF, Ahn HC, Eo S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast Reconstr Surg. 2012;129(3):683-692.
26. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002:84(3):375-379.
Carpal tunnel release is one of the most common hand surgeries performed at the North Florida/South Georgia Veterans Health System (NFSGVHS). Depending on surgeon experience and comfort level, surgeries are performed through either the traditional open method or the endoscopic method, single or double port (Figures 1 and 2). The advantage of the endoscopic method is faster recovery and return to work; however, the endoscopic method requires more expensive equipment and a steeper learning curve for surgeons. Complications are uncommon but can create unsatisfactory patient experiences because of costly lost workdays and long travel distances to the medical facility.
The purpose of this study was to compare the endoscopic method with the open carpal tunnel release method to determine whether there was an increased complication risk. Researchers anticipated that this information would help surgeons better inform patients of operative risks and prompt changes in NFSGVHS treatment plans to improve the quality of veteran care.
Methods
An Institutional Review Board- approved (#647-2011) retrospective review was done of patients who had carpal tunnel surgery performed by the NFSGVHS plastic surgery service from January 1, 2005, to December 31, 2010. Surgeries included in the review took place at the Malcom Randall VAMC in Gainesville and at the Lake City VAMC, both in Florida. Most of the surgeries included in the study were performed by a resident or fellow under the supervision of an attending physician. Eight different attending surgeons staffed the operations. Seven were board-certified or board-eligible plastic surgeons, 2 had advanced hand fellowship training, and 1 was a general surgeon with hand fellowship training. All hand fellowship-trained surgeons were in their first year of practice at the time of the study.
Only primary carpal tunnel releases were included in the study. Exclusion criteria included patients who were operated on by a service section other than the plastic surgery service (orthopedics or neurosurgery) and hands on which other procedures were performed during the same operation. Charts were reviewed for up to 1 year post surgery. Complications that required intervention were recorded. Researchers did not include pillar tenderness or an increase in occupational therapy visits as complications, due to the wide variety of patient tolerance to postoperative pain and varying motivation to return to work and daily routine.
Methods of release were endoscopic, open, or endoscopic converted to open. All but 6 of the completed endoscopic surgeries were performed using the double port Chow technique. The other 6 endoscopic surgeries were performed using the single port Agee technique at the distal wrist crease. There were 3 endoscopic converted to open cases that were performed using a single port, proximally-based technique in the midpalm. This method was abandoned after 3 unsuccessful endoscopic attempts, 1 resulting in digital nerve injury despite interactive cadaver labs prior to operative experience.
Endoscopic surgeries converted to open were recorded as open surgeries, because the patients had the full invasive experience. Researchers used the chi-square test and P value < .05 to compare the different methods of carpal tunnel release with identified complications.
Results and Complications
A total of 584 hands belonging to 452 patients were included in the study. Patients included 395 men and 57 women aged from 33 to 91 years. There were 271 endoscopic releases, 228 open releases, and 85 endoscopic converted to open releases. The NFSGVHS conversion rate was 23.7%. Complications in the converted cases (n = 4) were included in the open release results.
There were 40 complications in 38 hands. The overall complication rate was 6.5%. Complications noted were tendonitis presenting as De Quervain disease or trigger finger (9 endoscopic surgeries; 6 open surgeries), infection (2 endoscopic surgeries; 6 open surgeries), wound dehiscence (5 open surgeries), nerve injury (1 open surgery), respiratory distress (1 endoscopic), complex regional pain syndrome (1 open surgery), and scheduled returns to the operating room (OR) for recurrent, ongoing, or worsening symptoms (5 endoscopic surgeries; 5 open surgeries). Complications with an n > 1 were evaluated for statistical significance with P value < .05 (Table 1).
The NFSGVHS study had 10 patients return to the OR for open exploration (Table 2). Nine of these patients went back to the OR based on symptoms consistent with nerve conduction studies that had deteriorated compared with their preoperative studies. One endoscopic case was brought back to the OR for a suspected nerve injury without nerve conduction studies. Findings during reoperation included scar adhesions, incomplete release of ligaments, digital nerve injury, and negative explorations.
Two hypothenar fat transfers were performed to prevent scar adhesions in cases that had originally been open releases.1 Two of the open cases were endoscopic converted to open cases. One went back to the OR with a suspected nerve injury. Dense adhesions and an injured common digital nerve were identified and repaired. The second converted case that went back to the OR had a suspected, but unconfirmed, nerve injury to the motor branch. The diagnosis and treatment were delayed for more than a year due to the patient having other pressing medical and family concerns. An exploration found significant scar adhesions, and an opponensplasty was performed.
One patient had respiratory insufficiency secondary to chemical pneumonitis. The patient was sedated during an endoscopic carpal tunnel release, aspirated, and kept intubated in the intensive care unit until the morning after surgery.
An early complex regional pain syndrome diagnosis was made in a patient with underlying neuropathy and a preoperative “profound” median neuropathies diagnosis at the wrist with underlying peripheral neuropathy found on nerve conduction studies. The patient experienced an unusual amount of postoperative pain and edema after an uncomplicated open carpal tunnel release. This was treated with rapid intervention using anti- inflammatories and hand therapy. The patient also started a regimen of skin care, edema management, neuroreeducation, and contrast baths. Symptoms responded within a week.
There were 12 wound complications: 10 in open and 2 in endoscopic surgeries. Total wound complications were equally split between patients with and without diabetes. Infection and dehiscence were noted. Sutures were removed an average of 9.6 days after surgery in the patients whose wounds broke down. A statistically significant relationship was found only between the open method of release and wound dehiscence (P < .05).
There was no statistically significant difference in the overall complication rate in the NFSGVHS population when comparing endoscopic with open carpal tunnel release or when comparing the risk of postoperative tendonitis, wound infection, or return to the OR.
Discussion
Carpal tunnel syndrome was documented by James Paget in mid-19th century in reference to a distal radius fracture.2 It is the most common peripheral nerve compression, with an incidence ranging from 1 to 3 cases per 1,000 subjects per year and a prevalence of 50 cases per 1,000 subjects per year.3 In an active-duty U.S. military population, the incidence of carpal tunnel syndrome is 3.98 per 1,000 person years.4
Related: Risk Factors for Postoperative Complications in Trigger Finger Release
The endoscopic method of release was first introduced in 1989 by Okutsu and colleagues.5 About 500,000 carpal tunnel releases are now performed in the U.S. every year, with 50,000 performed endoscopically.3 There were 185 carpal tunnel releases (56 endoscopic and 129 open) performed at the NFSGVHS in 2012.6 The minimally invasive procedure was designed to preserve the overlying skin and fascia, promoting an earlier return to work and daily activities. This is particularly relevant for manual workers who desire rapid return of grip strength. Multiple published reports have found more rapid recovery based on a reduction in scar tenderness, increase in grip strength, or return to work.7-13 Patients seem to have equivalent results over the long term, ranging from 3 months to 1 year.7,8,13-15 Return to work was not evaluated in this study, because many patients were either retired or not working steadily.
The endoscopic method was criticized after its introduction due to its potential increase in major structural injury to the median nerve, ulnar nerve, palmar arch, ulnar artery, or flexor tendons.16 A meta-analysis found improved outcomes but a statistically significant higher complication rate in endoscopic, compared with open release (2.2% in endoscopic vs 1.2% in open).16 Referral patterns have found iatrogenic nerve injury in patients referred by surgeons without formal hand fellowship training.17 There is a wide variety of background training for surgeons who may offer carpal tunnel release, including plastic surgery, orthopedics, general surgery, and neurosurgery.
Major structural injuries were reported by hand surgeons using both open and endoscopic methods in a questionnaire sent to members of the American Society for Surgery of the Hand, indicating that either approach demands respect.18 A large review of the literature from 1966 to 2001 by Benson and colleagues found that the endoscopic approach was not more likely to produce injury to tendons, arteries, or nerves compared with the open approach and actually had a lower rate of structural damage (0.49% vs 0.19%).19 Researchers who conducted this study confirmed one common digital nerve injury in an endoscopic converted to open technique, using a distally-based port with the blade not being deployed via the endoscopic method. The endoscopic method has been found to have a higher rate of reversible nerve injury (neuropraxia) compared with the open technique.7,10,19
The NFSGVHS results found a higher rate of wound dehiscence. More frequent wound site complications, particularly infection, hypertrophic scar, and scar tenderness have been noted using the open method.3,8,20 This is probably due to the deeper and slightly larger incision used for the open method compared with the smaller and shallower incisions used for the endoscopic release.
There is the inevitable learning curve for the endoscopic release due to the more complicated nature of the procedure. The NFSGVHS conversion rate was 23.7% over the 5-year period from 2005 to 2010. All 3 fellowship- trained hand surgeons were in their first year of practice at the time of the study, so the authors anticipate a lower conversion rate in forthcoming studies. The NFSGVHS researchers did not consider converting to an open technique to be a complication and believe it is appropriate to teach plastic surgery residents and fellows to have a low threshold to convert when visualization is not optimal and the potential for significant injury exists. The learning curve and a higher conversion rate have been acknowledged by Beck and colleagues with no increase in morbidity.21
The authors anticipated finding an increased rate of tendonitis in the endoscopic method, as found by Goshtasby and colleagues, where trigger finger was found more frequently in the endoscopic patients.22 The NFSGVHS study found that the number of patients presenting for steroid injections to treat postoperative tendonitis in the hand and wrist was not statistically significant when comparing the 2 surgical methods of release (3.3% in endoscopic vs 1.9% in open; P = .28).
The NFSGVHS rate of return to the OR within a year of surgery was 1.7%. The researchers from NFSGHVS anticipated a higher rate of return to the OR for ongoing symptoms secondary to incomplete release of the transverse carpal ligament. Published studies have found an intact retinaculum to be a cause of persistent symptoms when smaller incisions are used.23,24 Five endoscopic cases and 5 open cases eventually returned to the OR for carpal tunnel exploration. Two of the patients were classified as recurrent, because they had improvement of symptoms initially but presented > 6 months later with new symptoms. Eight of the patients were classified as persistent, because they did not have an extended period of relief of preoperative symptoms (Table 2).25 There was no statistically significant difference in return to the OR in the 2 study groups. The NFSGVHS researchers did note a trend in more incomplete nerve releases in the endoscopic group and more scar adhesions as the etiology of symptoms in the open group who went back to surgery.
Published studies have found no difference in overall complication rates when comparing the open with the endoscopic method of release, which is consistent with NFSGVHS data.8,11,12,26
A limitation of the current retrospective study is the large number of providers who both operated on the patients and documented their postoperative findings. The strength of the study is that VA patients tend to stay within the VISN for their health care so postoperative problems will be identified and routed to the plastic surgery service for evaluation and treatment.
Clinical implications for the NFSGVHS practice are that surgeons can confidently offer both the open and endoscopic surgeries without an overall risk of increased complications to patients. Patients who are identified as higher risk for wound dehiscence, such as those who place an unusual amount of pressure on their palms due to assisted walking devices or are at a higher risk of falling onto the surgical site, will be steered toward an endoscopic surgery. The NFSGVHS began a splinting protocol in the early postoperative period that was not previously used on those select patients who have open carpal tunnel releases.
Conclusion
Wound dehiscence was the only statistically significant complication found in the NFSGVHS veteran population when comparing open with endoscopic carpal tunnel release. This can potentially be prevented in future patients by delaying the removal of sutures and prolonging the use of a protective dressing in patients who undergo open release. There was not a statistically significant increase in overall complications when using the minimally invasive method of release, which is consistent with existing literature.
Acknowledgement
This material is the result of work supported with resources and the use of facilities at the Malcom Randall VAMC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Carpal tunnel release is one of the most common hand surgeries performed at the North Florida/South Georgia Veterans Health System (NFSGVHS). Depending on surgeon experience and comfort level, surgeries are performed through either the traditional open method or the endoscopic method, single or double port (Figures 1 and 2). The advantage of the endoscopic method is faster recovery and return to work; however, the endoscopic method requires more expensive equipment and a steeper learning curve for surgeons. Complications are uncommon but can create unsatisfactory patient experiences because of costly lost workdays and long travel distances to the medical facility.
The purpose of this study was to compare the endoscopic method with the open carpal tunnel release method to determine whether there was an increased complication risk. Researchers anticipated that this information would help surgeons better inform patients of operative risks and prompt changes in NFSGVHS treatment plans to improve the quality of veteran care.
Methods
An Institutional Review Board- approved (#647-2011) retrospective review was done of patients who had carpal tunnel surgery performed by the NFSGVHS plastic surgery service from January 1, 2005, to December 31, 2010. Surgeries included in the review took place at the Malcom Randall VAMC in Gainesville and at the Lake City VAMC, both in Florida. Most of the surgeries included in the study were performed by a resident or fellow under the supervision of an attending physician. Eight different attending surgeons staffed the operations. Seven were board-certified or board-eligible plastic surgeons, 2 had advanced hand fellowship training, and 1 was a general surgeon with hand fellowship training. All hand fellowship-trained surgeons were in their first year of practice at the time of the study.
Only primary carpal tunnel releases were included in the study. Exclusion criteria included patients who were operated on by a service section other than the plastic surgery service (orthopedics or neurosurgery) and hands on which other procedures were performed during the same operation. Charts were reviewed for up to 1 year post surgery. Complications that required intervention were recorded. Researchers did not include pillar tenderness or an increase in occupational therapy visits as complications, due to the wide variety of patient tolerance to postoperative pain and varying motivation to return to work and daily routine.
Methods of release were endoscopic, open, or endoscopic converted to open. All but 6 of the completed endoscopic surgeries were performed using the double port Chow technique. The other 6 endoscopic surgeries were performed using the single port Agee technique at the distal wrist crease. There were 3 endoscopic converted to open cases that were performed using a single port, proximally-based technique in the midpalm. This method was abandoned after 3 unsuccessful endoscopic attempts, 1 resulting in digital nerve injury despite interactive cadaver labs prior to operative experience.
Endoscopic surgeries converted to open were recorded as open surgeries, because the patients had the full invasive experience. Researchers used the chi-square test and P value < .05 to compare the different methods of carpal tunnel release with identified complications.
Results and Complications
A total of 584 hands belonging to 452 patients were included in the study. Patients included 395 men and 57 women aged from 33 to 91 years. There were 271 endoscopic releases, 228 open releases, and 85 endoscopic converted to open releases. The NFSGVHS conversion rate was 23.7%. Complications in the converted cases (n = 4) were included in the open release results.
There were 40 complications in 38 hands. The overall complication rate was 6.5%. Complications noted were tendonitis presenting as De Quervain disease or trigger finger (9 endoscopic surgeries; 6 open surgeries), infection (2 endoscopic surgeries; 6 open surgeries), wound dehiscence (5 open surgeries), nerve injury (1 open surgery), respiratory distress (1 endoscopic), complex regional pain syndrome (1 open surgery), and scheduled returns to the operating room (OR) for recurrent, ongoing, or worsening symptoms (5 endoscopic surgeries; 5 open surgeries). Complications with an n > 1 were evaluated for statistical significance with P value < .05 (Table 1).
The NFSGVHS study had 10 patients return to the OR for open exploration (Table 2). Nine of these patients went back to the OR based on symptoms consistent with nerve conduction studies that had deteriorated compared with their preoperative studies. One endoscopic case was brought back to the OR for a suspected nerve injury without nerve conduction studies. Findings during reoperation included scar adhesions, incomplete release of ligaments, digital nerve injury, and negative explorations.
Two hypothenar fat transfers were performed to prevent scar adhesions in cases that had originally been open releases.1 Two of the open cases were endoscopic converted to open cases. One went back to the OR with a suspected nerve injury. Dense adhesions and an injured common digital nerve were identified and repaired. The second converted case that went back to the OR had a suspected, but unconfirmed, nerve injury to the motor branch. The diagnosis and treatment were delayed for more than a year due to the patient having other pressing medical and family concerns. An exploration found significant scar adhesions, and an opponensplasty was performed.
One patient had respiratory insufficiency secondary to chemical pneumonitis. The patient was sedated during an endoscopic carpal tunnel release, aspirated, and kept intubated in the intensive care unit until the morning after surgery.
An early complex regional pain syndrome diagnosis was made in a patient with underlying neuropathy and a preoperative “profound” median neuropathies diagnosis at the wrist with underlying peripheral neuropathy found on nerve conduction studies. The patient experienced an unusual amount of postoperative pain and edema after an uncomplicated open carpal tunnel release. This was treated with rapid intervention using anti- inflammatories and hand therapy. The patient also started a regimen of skin care, edema management, neuroreeducation, and contrast baths. Symptoms responded within a week.
There were 12 wound complications: 10 in open and 2 in endoscopic surgeries. Total wound complications were equally split between patients with and without diabetes. Infection and dehiscence were noted. Sutures were removed an average of 9.6 days after surgery in the patients whose wounds broke down. A statistically significant relationship was found only between the open method of release and wound dehiscence (P < .05).
There was no statistically significant difference in the overall complication rate in the NFSGVHS population when comparing endoscopic with open carpal tunnel release or when comparing the risk of postoperative tendonitis, wound infection, or return to the OR.
Discussion
Carpal tunnel syndrome was documented by James Paget in mid-19th century in reference to a distal radius fracture.2 It is the most common peripheral nerve compression, with an incidence ranging from 1 to 3 cases per 1,000 subjects per year and a prevalence of 50 cases per 1,000 subjects per year.3 In an active-duty U.S. military population, the incidence of carpal tunnel syndrome is 3.98 per 1,000 person years.4
Related: Risk Factors for Postoperative Complications in Trigger Finger Release
The endoscopic method of release was first introduced in 1989 by Okutsu and colleagues.5 About 500,000 carpal tunnel releases are now performed in the U.S. every year, with 50,000 performed endoscopically.3 There were 185 carpal tunnel releases (56 endoscopic and 129 open) performed at the NFSGVHS in 2012.6 The minimally invasive procedure was designed to preserve the overlying skin and fascia, promoting an earlier return to work and daily activities. This is particularly relevant for manual workers who desire rapid return of grip strength. Multiple published reports have found more rapid recovery based on a reduction in scar tenderness, increase in grip strength, or return to work.7-13 Patients seem to have equivalent results over the long term, ranging from 3 months to 1 year.7,8,13-15 Return to work was not evaluated in this study, because many patients were either retired or not working steadily.
The endoscopic method was criticized after its introduction due to its potential increase in major structural injury to the median nerve, ulnar nerve, palmar arch, ulnar artery, or flexor tendons.16 A meta-analysis found improved outcomes but a statistically significant higher complication rate in endoscopic, compared with open release (2.2% in endoscopic vs 1.2% in open).16 Referral patterns have found iatrogenic nerve injury in patients referred by surgeons without formal hand fellowship training.17 There is a wide variety of background training for surgeons who may offer carpal tunnel release, including plastic surgery, orthopedics, general surgery, and neurosurgery.
Major structural injuries were reported by hand surgeons using both open and endoscopic methods in a questionnaire sent to members of the American Society for Surgery of the Hand, indicating that either approach demands respect.18 A large review of the literature from 1966 to 2001 by Benson and colleagues found that the endoscopic approach was not more likely to produce injury to tendons, arteries, or nerves compared with the open approach and actually had a lower rate of structural damage (0.49% vs 0.19%).19 Researchers who conducted this study confirmed one common digital nerve injury in an endoscopic converted to open technique, using a distally-based port with the blade not being deployed via the endoscopic method. The endoscopic method has been found to have a higher rate of reversible nerve injury (neuropraxia) compared with the open technique.7,10,19
The NFSGVHS results found a higher rate of wound dehiscence. More frequent wound site complications, particularly infection, hypertrophic scar, and scar tenderness have been noted using the open method.3,8,20 This is probably due to the deeper and slightly larger incision used for the open method compared with the smaller and shallower incisions used for the endoscopic release.
There is the inevitable learning curve for the endoscopic release due to the more complicated nature of the procedure. The NFSGVHS conversion rate was 23.7% over the 5-year period from 2005 to 2010. All 3 fellowship- trained hand surgeons were in their first year of practice at the time of the study, so the authors anticipate a lower conversion rate in forthcoming studies. The NFSGVHS researchers did not consider converting to an open technique to be a complication and believe it is appropriate to teach plastic surgery residents and fellows to have a low threshold to convert when visualization is not optimal and the potential for significant injury exists. The learning curve and a higher conversion rate have been acknowledged by Beck and colleagues with no increase in morbidity.21
The authors anticipated finding an increased rate of tendonitis in the endoscopic method, as found by Goshtasby and colleagues, where trigger finger was found more frequently in the endoscopic patients.22 The NFSGVHS study found that the number of patients presenting for steroid injections to treat postoperative tendonitis in the hand and wrist was not statistically significant when comparing the 2 surgical methods of release (3.3% in endoscopic vs 1.9% in open; P = .28).
The NFSGVHS rate of return to the OR within a year of surgery was 1.7%. The researchers from NFSGHVS anticipated a higher rate of return to the OR for ongoing symptoms secondary to incomplete release of the transverse carpal ligament. Published studies have found an intact retinaculum to be a cause of persistent symptoms when smaller incisions are used.23,24 Five endoscopic cases and 5 open cases eventually returned to the OR for carpal tunnel exploration. Two of the patients were classified as recurrent, because they had improvement of symptoms initially but presented > 6 months later with new symptoms. Eight of the patients were classified as persistent, because they did not have an extended period of relief of preoperative symptoms (Table 2).25 There was no statistically significant difference in return to the OR in the 2 study groups. The NFSGVHS researchers did note a trend in more incomplete nerve releases in the endoscopic group and more scar adhesions as the etiology of symptoms in the open group who went back to surgery.
Published studies have found no difference in overall complication rates when comparing the open with the endoscopic method of release, which is consistent with NFSGVHS data.8,11,12,26
A limitation of the current retrospective study is the large number of providers who both operated on the patients and documented their postoperative findings. The strength of the study is that VA patients tend to stay within the VISN for their health care so postoperative problems will be identified and routed to the plastic surgery service for evaluation and treatment.
Clinical implications for the NFSGVHS practice are that surgeons can confidently offer both the open and endoscopic surgeries without an overall risk of increased complications to patients. Patients who are identified as higher risk for wound dehiscence, such as those who place an unusual amount of pressure on their palms due to assisted walking devices or are at a higher risk of falling onto the surgical site, will be steered toward an endoscopic surgery. The NFSGVHS began a splinting protocol in the early postoperative period that was not previously used on those select patients who have open carpal tunnel releases.
Conclusion
Wound dehiscence was the only statistically significant complication found in the NFSGVHS veteran population when comparing open with endoscopic carpal tunnel release. This can potentially be prevented in future patients by delaying the removal of sutures and prolonging the use of a protective dressing in patients who undergo open release. There was not a statistically significant increase in overall complications when using the minimally invasive method of release, which is consistent with existing literature.
Acknowledgement
This material is the result of work supported with resources and the use of facilities at the Malcom Randall VAMC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Chrysopoulo MT, Greenberg JA, Kleinman WB. The hypothenar fat pad transposition flap: a modified surgical technique. Tech Hand Up Extrem Surg. 2006;10(3):150-156.
2. Paget J. Lectures on Surgical Pathology Delivered at the Royal College of Surgeons of England. London, England: Longman, Green, Brown, and Longmans; 1853.
3. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43(4):431-437.
4. Wolf JM, Mountcastle S, Owens BD. Incidence of carpal tunnel syndrome in the US military population. Hand (NY). 2009;4(3):289-293.
5. Okutsu I, Ninomiya S, Takatori Y, Ugawa Y. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
6. U.S. Department of Veterans Affairs. Health Information Systems and Technology Architecture Database, Ambulatory Surgical Case Load Report, 2012. Accessed March 14, 2013.
7. Larsen MB, Sørensen AI, Crone KL, Weis T, Boeckstyns ME. Carpal tunnel release: a randomized comparison of three surgical methods. J Hand Surg Eur Vol. 2013;38(6):646-650.
8. Malhotra R, Kiran EK, Dua A, Mallinath SG, Bhan S. Endoscopic versus open carpal tunnel release: a short-term comparative study. Indian J Orthop. 2007;41(1):57-61.
9. Sabesan VJ, Pedrotty D, Urbaniak JR, Aldridge JM 3rd. An evidence-based review of a single surgeon’s experience with endoscopic carpal tunnel release. J Surg Orthop Adv. 2012;21(3):117-121.
10. Thoma A, Veltri K, Haines T, Duku E. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
11. Tian Y, Zhao H, Wang T. Prospective comparison of endoscopic and open surgical methods for carpal tunnel syndrome. Chin Med Sci J. 2007;22(2):104-107.
12. Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84-A(7):1107-1115.
13. Vasiliadis HS, Xenakis TA, Mitsionis G, Paschos N, Georgoulis A. Endoscopic versus open carpal tunnel release. Arthroscopy. 2010:26(1):26-33.
14. Macdermid JC, Richards RS, Roth JH, Ross DC, King GJ. Endoscopic versus open carpal tunnel release: a randomized trial. J Hand Surg Am. 2003;28(3):475-480.
15. Aslani HR, Alizadeh K, Eajazi A, et al. Comparison of carpal tunnel release with three different techniques. Clin Neurol Neurosurg. 2012;114(7):965-968.
16. Kohanzadeh S, Herrera FA, Dobke M. Outcomes of open and endoscopic carpal tunnel release: a meta-analysis. Hand (NY). 2012;7(3):247-251.
17. Azari KK, Spiess AM, Buterbaugh GA, Imbriglia JE. Major nerve injuries associated with carpal tunnel release. Plast Reconstr Surg. 2007;119(6):1977-1978.
18. Palmer AK, Toivonen DA. Complications of endoscopic and open carpal tunnel release. J Hand Surg Am. 1999;24(3):561-565.
19. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
20. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
21. Beck JD, Deegan JH, Rhoades D, Klena JC. Results of endoscopic carpal tunnel release relative to surgeon experience with the Agee technique. J Hand Surg Am. 2011;36(1):61-64.
22. Goshtasby PH, Wheeler DR, Moy OJ. Risk factors for trigger finger occurrence after carpal tunnel release. Hand Surg. 2010;15(2):81-87.
23. Assmus H, Dombert T, Staub F. Reoperations for CTS because of recurrence or for correction [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):306-311.
24. Frik A, Baumeister RG. Re-intervention after carpal tunnel release [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):312-316.
25. Jones NF, Ahn HC, Eo S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast Reconstr Surg. 2012;129(3):683-692.
26. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002:84(3):375-379.
1. Chrysopoulo MT, Greenberg JA, Kleinman WB. The hypothenar fat pad transposition flap: a modified surgical technique. Tech Hand Up Extrem Surg. 2006;10(3):150-156.
2. Paget J. Lectures on Surgical Pathology Delivered at the Royal College of Surgeons of England. London, England: Longman, Green, Brown, and Longmans; 1853.
3. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43(4):431-437.
4. Wolf JM, Mountcastle S, Owens BD. Incidence of carpal tunnel syndrome in the US military population. Hand (NY). 2009;4(3):289-293.
5. Okutsu I, Ninomiya S, Takatori Y, Ugawa Y. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
6. U.S. Department of Veterans Affairs. Health Information Systems and Technology Architecture Database, Ambulatory Surgical Case Load Report, 2012. Accessed March 14, 2013.
7. Larsen MB, Sørensen AI, Crone KL, Weis T, Boeckstyns ME. Carpal tunnel release: a randomized comparison of three surgical methods. J Hand Surg Eur Vol. 2013;38(6):646-650.
8. Malhotra R, Kiran EK, Dua A, Mallinath SG, Bhan S. Endoscopic versus open carpal tunnel release: a short-term comparative study. Indian J Orthop. 2007;41(1):57-61.
9. Sabesan VJ, Pedrotty D, Urbaniak JR, Aldridge JM 3rd. An evidence-based review of a single surgeon’s experience with endoscopic carpal tunnel release. J Surg Orthop Adv. 2012;21(3):117-121.
10. Thoma A, Veltri K, Haines T, Duku E. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
11. Tian Y, Zhao H, Wang T. Prospective comparison of endoscopic and open surgical methods for carpal tunnel syndrome. Chin Med Sci J. 2007;22(2):104-107.
12. Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84-A(7):1107-1115.
13. Vasiliadis HS, Xenakis TA, Mitsionis G, Paschos N, Georgoulis A. Endoscopic versus open carpal tunnel release. Arthroscopy. 2010:26(1):26-33.
14. Macdermid JC, Richards RS, Roth JH, Ross DC, King GJ. Endoscopic versus open carpal tunnel release: a randomized trial. J Hand Surg Am. 2003;28(3):475-480.
15. Aslani HR, Alizadeh K, Eajazi A, et al. Comparison of carpal tunnel release with three different techniques. Clin Neurol Neurosurg. 2012;114(7):965-968.
16. Kohanzadeh S, Herrera FA, Dobke M. Outcomes of open and endoscopic carpal tunnel release: a meta-analysis. Hand (NY). 2012;7(3):247-251.
17. Azari KK, Spiess AM, Buterbaugh GA, Imbriglia JE. Major nerve injuries associated with carpal tunnel release. Plast Reconstr Surg. 2007;119(6):1977-1978.
18. Palmer AK, Toivonen DA. Complications of endoscopic and open carpal tunnel release. J Hand Surg Am. 1999;24(3):561-565.
19. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
20. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
21. Beck JD, Deegan JH, Rhoades D, Klena JC. Results of endoscopic carpal tunnel release relative to surgeon experience with the Agee technique. J Hand Surg Am. 2011;36(1):61-64.
22. Goshtasby PH, Wheeler DR, Moy OJ. Risk factors for trigger finger occurrence after carpal tunnel release. Hand Surg. 2010;15(2):81-87.
23. Assmus H, Dombert T, Staub F. Reoperations for CTS because of recurrence or for correction [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):306-311.
24. Frik A, Baumeister RG. Re-intervention after carpal tunnel release [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):312-316.
25. Jones NF, Ahn HC, Eo S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast Reconstr Surg. 2012;129(3):683-692.
26. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002:84(3):375-379.
Risk Factors for Postoperative Complications in Trigger Finger Release
Stenosing tenosynovitis, or trigger finger, is a pathology commonly referred to the plastic and hand surgery service of the North Florida/South Georgia Veterans Health System (NFSGVHS). Patients usually present to their primary care provider with symptoms of the finger being temporarily locked or stuck in the flexed position. This can be a painful problem due to the size mismatch between the flexor tendon and the pulley under which it glides.
Patients are typically referred to surgery after failing ≥ 1 attempt at nonoperative management. The surgery is relatively quick and straightforward; however, postoperative complications can lead to an unexpected costly and lengthy recovery. The objective of this study was to identify potential risk factors that can predispose patients to postoperative complications so that those risk factors may be better anticipated and modified, if possible.
Methods
A retrospective chart review of trigger finger release surgery was performed on-site at the Malcom Randall VAMC in Gainesville, Florida, from January 2005 to December 2010 to identify risk factors associated with postoperative complications. The study was approved by both the NFSGVHS Internal Review Board and the University of Florida Institutional Review Board. Patients who underwent surgery exclusively for ≥ 1 trigger fingers by the plastic surgery service were included in the study.
The surgery involves making an incision over the affected A1 pulley in the hand (Figure 1) and sharply releasing it (Figure 2) under direct vision. Potential risk factors for postoperative complications were recorded. These risk factors included smoking status, diabetic status, type of incision, and number of digits released during the surgical procedure.
Results
Ninety-eight digits (on 81 hands) were identified as meeting inclusion criteria. Surgeries were performed using a longitudinal (43), transverse (48), oblique (5), or Brunner (2) incision. There were 10 complications: cellulitis (3), pyogenic flexor tenosynovitis (3), scar adhesion (1), delayed healing (2), and incomplete release (1). The overall complication rate was 10.2%. The authors compared risk factors with complications, using the chi square test and a determining of P < .05.
Related: Making the Case for Minimally Invasive Surgery
There was no link found between overall postoperative complications and diabetic status, incision type, or smoking status. There was a statistically significant link between diabetic patients and the incidence of postoperative infection (P = .002) and between 2 digits operated on during the same surgery and postoperative infection (P = .027)
Discussion
The routine practice of the NFSGVHS hand clinic is to offer a steroid injection as the initial treatment for trigger finger. Health care providers (HCPs) allow no more than 3 injections to the same digit to avoid the rare but potentially serious complication of a tendon rupture.1 Due to the large NFSGVHS catchment area, wait time for elective trigger finger surgery is several months. This 3-injection plan has been well received by patients and referring providers due to these wait times. However, a recent article by Kerrigan and Stanwix concluded that the most cost-efficient treatment strategy is 2 steroid injections before surgery.2
More often than not, trigger finger release is a short, outpatient surgery with a quick recovery. To minimize the risk of stiffness and scar adhesions, the NFSGVHS practice is to refer all postoperative hand cases for ≥ 1 hand therapy appointment on the same day as their first postoperative visit.
Cost Estimates
When complications occur, they can be costly to patients due to both time spent away from home and work and additional expenses. When the current procedural terminology (CPT) codes are run through the VistA integrated billing system, based on the VHA Chief Business Office Reasonable Charges, a complication can more than double the charges associated with A1 pulley surgery.
A flexor sheath incision and drainage (I+D) (CPT 26020) charges $8,935.35 (facility charge, $6,911.95 plus professional fee, $2,023.40), compared with open trigger finger release (CPT 26055) at $8,365.66 (facility charge, $6,911.95 plus professional fee, $1,453.71). According to a conversation with the finance service officer at NFSGVHS (2/11/2014), the anesthesia bill ($490.56/15 min), anticipated level 3 emergency department visits (facility charge, $889.22 plus professional fee $493.40), and inpatient stays (daily floor bed $786.19) can make an infectious complication costly.
Trigger finger can also be released percutaneously. This is a reasonable option that avoids the operating room, but NFSGVHS surgeons prefer the open surgery due to concerns for tendon and nerve injury that can result from a blind sweep of the needle.3,4
Related: Prevention of Venous Thromboembolism After Total Joint Replacement
Existing studies found complications for trigger finger release ranging from 1% to 31%.5,6 Wound complications and joint stiffness are known complications.5-7 In this study, 60% of the complications were infections, and 80% of the complications were wound complications. Six of 8 patients with wound-healing complications received perioperative antibiotics. Three patients returned to the operating room for an I+D of the flexor sheath. The results showed a statistically significant link between > 1 digit treated at the same surgery and postoperative complications (P = .027). A PubMed search revealed no existing hand literature with this association.
Risk Factors
Diabetes, tobacco use, type of incision, and number of digits treated were assessed as risk factors for complications after trigger finger surgery. Nicotine is widely accepted as increasing the risk for wound complications.8 Almost 20% of the U.S. population smokes, compared with 22% of the VA population and 32% of active-duty military personnel.9 One in 4 veterans has been diagnosed with diabetes, a well-known predisposing factor in delayed wound healing and infection.10,11 No prior studies were found comparing type of incision or multiple digits treated as complications risk factors.
There is also a well-known association between trigger finger and diabetes. Chronic hyperglycemia results in the accumulation of collagen within tendon sheaths due to impairment of collagen breakdown. Patients with diabetes tend to present with multiple digit involvement and respond less favorably to steroid injections compared with patients without diabetes.12 Wound healing is also impaired in patients with diabetes. All 6 wound infections in this study were in patients with diabetes. Proposed etiologies for wound-healing complications include pathologic angiogenesis, impaired fibroblast proliferation and migration, impaired circulation, decreased oxygenation, and a defective immune response to the injured site.13
Trigger finger may develop in multiple digits. Once surgery has been planned for 1 digit, patients may request surgery on another digit on the same hand that has not had an attempt at nonoperative intervention. The NFSGVHS plastic surgeons have raised the threshold to offer multiple surgical procedures on the same hand at the same operative visit to minimize recovery time and number of visits, particularly when patients are travelling long distances. This may be less convenient; however, the overall cost to the patient and the health care system in the event of a complication is significant. Plastic surgery providers also run an alcohol prep pad over the incision site to prevent inoculation of the flexor sheath during suture removal.
Current recommendations to ameliorate the postoperative risks to the patient and costs to the system include endorsing a more conservative approach to treating trigger finger than was previously practiced at NFSGVHS. The known, less favorable response of patients with diabetes to steroid injections plus their elevated risk of postoperative infection create a catch-22 for the treatment plan. Given the low risk of a single steroid injection to the flexor sheath, this procedure is still recommended as a first-line treatment.
Related: Experience Tells in Hip Arthroplasty
During the 5-year study there was a lower threshold for surgical management and for treatment of multiple digits during the same surgery than the one currently practiced, with an overall consensus of the hospital’s HCPs. The authors recommend that all patients start with a steroid injection before committing to surgery. Patients with diabetes are informed that the injection will cause a temporary rise in their blood glucose.14 If they are resistant to the injection, high-dose oral nonsteroidal anti-inflammatory drugs and/or proximal interphalangeal joint splinting is ordered.
Verification of A1C values showing better chronic management of blood sugar is a procedure HCPs from the NFSGVHS will begin to follow. Preoperative A1C values between 6.5% and 8% in patients known to have diabetes has been recommended.15 A1C values > 7% have been found to be an independent risk factor for stenosing tenosynovitis.16 The total number of trigger finger surgeries may drop with the benefit of improved utilization of resources.
Conclusion
The authors found a statistically significant association between postoperative infection and 2 patient populations: patients with diabetes (P = .002) and patients having > 1 digit released during the same surgery (P = .027). This outcome suggests using caution when offering A1 pulley release in select patient populations.
Acknowledgement
Justine Pierson, BS, research coordinator at University of Florida, for statistical analysis. Funding is through salary.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Yamada K, Masuko, T, Iwasaki N. Rupture of the flexor digitorum profundus tendon after injections of insoluble steroid for a trigger finger. J Hand Surg Eur. 2011;36(1):77-78.
2. Kerrigan CL, Stanwix MG. Using evidence to minimize the cost of trigger finger care. J Hand Surg Am. 2009;34(6):997-1005.
3. Habbu R, Putnam MD, Adams JE. Percutaneous Release of the A1 pulley: a cadaver study. J Hand Surg Am. 2012;37(11):2273-2277.
4. Guler F, Kose O, Ercan EC, Turan A, Canbora K. Open vs percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36(10):e1290-e1294.
5. Lim M-H, Lim K-K, Rasheed MZ, Narayana S, Tan B-H. Outcome of open trigger digit release. J Hand Surg Eur. 2007;32(4):457-479.
6. Will R, Lubahn J. Complications of open trigger finger release. J Hand Surg Am. 2010;35(4):594-596.
7. Lee WT, Chong AK. Outcome study of open trigger digit release. J Hand Surg Eur. 2011;36(4):339.
8. Rinker B. The evils of nicotine: An evidence-based guide to smoking and plastic surgery. Ann Plast Surg. 2013;70(5):599-605.
9. Bondurant S, Wedge R, eds. Combating Tobacco Use in Military and Veteran Populations. Washington, DC: The National Academies; 2009.
10. Shilling AM, Raphael J. Diabetes, hyperglycemia, and infections. Best Pract Res Clin Anaesthesiol. 2008;22(3):519-535.
11. Kuppersmith J, Francis J, Kerr E, et al. Advancing evidence-based care for diabetes: Lessons from the Veterans Health Administration. Health Aff. 2007;26(2):156-158.
12. Brown E, Genoway KA. Impact of diabetes on outcomes in hand surgery. J Hand Surg Am. 2011;36(12):2067-2072.
13. Francis-Goforth KN, Harken AH, Saba JD. Normalization of diabetic wound healing. Surgery. 2010;147(3):446-449.
14. Wang AA, Hutchinson DT. The effect of corticosteroid injection for trigger finger on blood glucose level in diabetic patients. J Hand Surg Am. 2006;31(6):979-981.
15. Underwood P, Askari R, Hurwitz S, Chamarthi B, Garg R. Preoperative A1C and Clinical Outcomes in patients with diabetes undergoing major noncardiac surgical procedures. Diabetes Care. 2014; 37(3): 611-616.
16. Vance MC, Tucker JJ, Harness NG. The association of hemoglobin A1c with the prevalence of stenosing tenosynovitis. J Hand Surg Am. 2012;37(9):1765-1769.
Stenosing tenosynovitis, or trigger finger, is a pathology commonly referred to the plastic and hand surgery service of the North Florida/South Georgia Veterans Health System (NFSGVHS). Patients usually present to their primary care provider with symptoms of the finger being temporarily locked or stuck in the flexed position. This can be a painful problem due to the size mismatch between the flexor tendon and the pulley under which it glides.
Patients are typically referred to surgery after failing ≥ 1 attempt at nonoperative management. The surgery is relatively quick and straightforward; however, postoperative complications can lead to an unexpected costly and lengthy recovery. The objective of this study was to identify potential risk factors that can predispose patients to postoperative complications so that those risk factors may be better anticipated and modified, if possible.
Methods
A retrospective chart review of trigger finger release surgery was performed on-site at the Malcom Randall VAMC in Gainesville, Florida, from January 2005 to December 2010 to identify risk factors associated with postoperative complications. The study was approved by both the NFSGVHS Internal Review Board and the University of Florida Institutional Review Board. Patients who underwent surgery exclusively for ≥ 1 trigger fingers by the plastic surgery service were included in the study.
The surgery involves making an incision over the affected A1 pulley in the hand (Figure 1) and sharply releasing it (Figure 2) under direct vision. Potential risk factors for postoperative complications were recorded. These risk factors included smoking status, diabetic status, type of incision, and number of digits released during the surgical procedure.
Results
Ninety-eight digits (on 81 hands) were identified as meeting inclusion criteria. Surgeries were performed using a longitudinal (43), transverse (48), oblique (5), or Brunner (2) incision. There were 10 complications: cellulitis (3), pyogenic flexor tenosynovitis (3), scar adhesion (1), delayed healing (2), and incomplete release (1). The overall complication rate was 10.2%. The authors compared risk factors with complications, using the chi square test and a determining of P < .05.
Related: Making the Case for Minimally Invasive Surgery
There was no link found between overall postoperative complications and diabetic status, incision type, or smoking status. There was a statistically significant link between diabetic patients and the incidence of postoperative infection (P = .002) and between 2 digits operated on during the same surgery and postoperative infection (P = .027)
Discussion
The routine practice of the NFSGVHS hand clinic is to offer a steroid injection as the initial treatment for trigger finger. Health care providers (HCPs) allow no more than 3 injections to the same digit to avoid the rare but potentially serious complication of a tendon rupture.1 Due to the large NFSGVHS catchment area, wait time for elective trigger finger surgery is several months. This 3-injection plan has been well received by patients and referring providers due to these wait times. However, a recent article by Kerrigan and Stanwix concluded that the most cost-efficient treatment strategy is 2 steroid injections before surgery.2
More often than not, trigger finger release is a short, outpatient surgery with a quick recovery. To minimize the risk of stiffness and scar adhesions, the NFSGVHS practice is to refer all postoperative hand cases for ≥ 1 hand therapy appointment on the same day as their first postoperative visit.
Cost Estimates
When complications occur, they can be costly to patients due to both time spent away from home and work and additional expenses. When the current procedural terminology (CPT) codes are run through the VistA integrated billing system, based on the VHA Chief Business Office Reasonable Charges, a complication can more than double the charges associated with A1 pulley surgery.
A flexor sheath incision and drainage (I+D) (CPT 26020) charges $8,935.35 (facility charge, $6,911.95 plus professional fee, $2,023.40), compared with open trigger finger release (CPT 26055) at $8,365.66 (facility charge, $6,911.95 plus professional fee, $1,453.71). According to a conversation with the finance service officer at NFSGVHS (2/11/2014), the anesthesia bill ($490.56/15 min), anticipated level 3 emergency department visits (facility charge, $889.22 plus professional fee $493.40), and inpatient stays (daily floor bed $786.19) can make an infectious complication costly.
Trigger finger can also be released percutaneously. This is a reasonable option that avoids the operating room, but NFSGVHS surgeons prefer the open surgery due to concerns for tendon and nerve injury that can result from a blind sweep of the needle.3,4
Related: Prevention of Venous Thromboembolism After Total Joint Replacement
Existing studies found complications for trigger finger release ranging from 1% to 31%.5,6 Wound complications and joint stiffness are known complications.5-7 In this study, 60% of the complications were infections, and 80% of the complications were wound complications. Six of 8 patients with wound-healing complications received perioperative antibiotics. Three patients returned to the operating room for an I+D of the flexor sheath. The results showed a statistically significant link between > 1 digit treated at the same surgery and postoperative complications (P = .027). A PubMed search revealed no existing hand literature with this association.
Risk Factors
Diabetes, tobacco use, type of incision, and number of digits treated were assessed as risk factors for complications after trigger finger surgery. Nicotine is widely accepted as increasing the risk for wound complications.8 Almost 20% of the U.S. population smokes, compared with 22% of the VA population and 32% of active-duty military personnel.9 One in 4 veterans has been diagnosed with diabetes, a well-known predisposing factor in delayed wound healing and infection.10,11 No prior studies were found comparing type of incision or multiple digits treated as complications risk factors.
There is also a well-known association between trigger finger and diabetes. Chronic hyperglycemia results in the accumulation of collagen within tendon sheaths due to impairment of collagen breakdown. Patients with diabetes tend to present with multiple digit involvement and respond less favorably to steroid injections compared with patients without diabetes.12 Wound healing is also impaired in patients with diabetes. All 6 wound infections in this study were in patients with diabetes. Proposed etiologies for wound-healing complications include pathologic angiogenesis, impaired fibroblast proliferation and migration, impaired circulation, decreased oxygenation, and a defective immune response to the injured site.13
Trigger finger may develop in multiple digits. Once surgery has been planned for 1 digit, patients may request surgery on another digit on the same hand that has not had an attempt at nonoperative intervention. The NFSGVHS plastic surgeons have raised the threshold to offer multiple surgical procedures on the same hand at the same operative visit to minimize recovery time and number of visits, particularly when patients are travelling long distances. This may be less convenient; however, the overall cost to the patient and the health care system in the event of a complication is significant. Plastic surgery providers also run an alcohol prep pad over the incision site to prevent inoculation of the flexor sheath during suture removal.
Current recommendations to ameliorate the postoperative risks to the patient and costs to the system include endorsing a more conservative approach to treating trigger finger than was previously practiced at NFSGVHS. The known, less favorable response of patients with diabetes to steroid injections plus their elevated risk of postoperative infection create a catch-22 for the treatment plan. Given the low risk of a single steroid injection to the flexor sheath, this procedure is still recommended as a first-line treatment.
Related: Experience Tells in Hip Arthroplasty
During the 5-year study there was a lower threshold for surgical management and for treatment of multiple digits during the same surgery than the one currently practiced, with an overall consensus of the hospital’s HCPs. The authors recommend that all patients start with a steroid injection before committing to surgery. Patients with diabetes are informed that the injection will cause a temporary rise in their blood glucose.14 If they are resistant to the injection, high-dose oral nonsteroidal anti-inflammatory drugs and/or proximal interphalangeal joint splinting is ordered.
Verification of A1C values showing better chronic management of blood sugar is a procedure HCPs from the NFSGVHS will begin to follow. Preoperative A1C values between 6.5% and 8% in patients known to have diabetes has been recommended.15 A1C values > 7% have been found to be an independent risk factor for stenosing tenosynovitis.16 The total number of trigger finger surgeries may drop with the benefit of improved utilization of resources.
Conclusion
The authors found a statistically significant association between postoperative infection and 2 patient populations: patients with diabetes (P = .002) and patients having > 1 digit released during the same surgery (P = .027). This outcome suggests using caution when offering A1 pulley release in select patient populations.
Acknowledgement
Justine Pierson, BS, research coordinator at University of Florida, for statistical analysis. Funding is through salary.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Stenosing tenosynovitis, or trigger finger, is a pathology commonly referred to the plastic and hand surgery service of the North Florida/South Georgia Veterans Health System (NFSGVHS). Patients usually present to their primary care provider with symptoms of the finger being temporarily locked or stuck in the flexed position. This can be a painful problem due to the size mismatch between the flexor tendon and the pulley under which it glides.
Patients are typically referred to surgery after failing ≥ 1 attempt at nonoperative management. The surgery is relatively quick and straightforward; however, postoperative complications can lead to an unexpected costly and lengthy recovery. The objective of this study was to identify potential risk factors that can predispose patients to postoperative complications so that those risk factors may be better anticipated and modified, if possible.
Methods
A retrospective chart review of trigger finger release surgery was performed on-site at the Malcom Randall VAMC in Gainesville, Florida, from January 2005 to December 2010 to identify risk factors associated with postoperative complications. The study was approved by both the NFSGVHS Internal Review Board and the University of Florida Institutional Review Board. Patients who underwent surgery exclusively for ≥ 1 trigger fingers by the plastic surgery service were included in the study.
The surgery involves making an incision over the affected A1 pulley in the hand (Figure 1) and sharply releasing it (Figure 2) under direct vision. Potential risk factors for postoperative complications were recorded. These risk factors included smoking status, diabetic status, type of incision, and number of digits released during the surgical procedure.
Results
Ninety-eight digits (on 81 hands) were identified as meeting inclusion criteria. Surgeries were performed using a longitudinal (43), transverse (48), oblique (5), or Brunner (2) incision. There were 10 complications: cellulitis (3), pyogenic flexor tenosynovitis (3), scar adhesion (1), delayed healing (2), and incomplete release (1). The overall complication rate was 10.2%. The authors compared risk factors with complications, using the chi square test and a determining of P < .05.
Related: Making the Case for Minimally Invasive Surgery
There was no link found between overall postoperative complications and diabetic status, incision type, or smoking status. There was a statistically significant link between diabetic patients and the incidence of postoperative infection (P = .002) and between 2 digits operated on during the same surgery and postoperative infection (P = .027)
Discussion
The routine practice of the NFSGVHS hand clinic is to offer a steroid injection as the initial treatment for trigger finger. Health care providers (HCPs) allow no more than 3 injections to the same digit to avoid the rare but potentially serious complication of a tendon rupture.1 Due to the large NFSGVHS catchment area, wait time for elective trigger finger surgery is several months. This 3-injection plan has been well received by patients and referring providers due to these wait times. However, a recent article by Kerrigan and Stanwix concluded that the most cost-efficient treatment strategy is 2 steroid injections before surgery.2
More often than not, trigger finger release is a short, outpatient surgery with a quick recovery. To minimize the risk of stiffness and scar adhesions, the NFSGVHS practice is to refer all postoperative hand cases for ≥ 1 hand therapy appointment on the same day as their first postoperative visit.
Cost Estimates
When complications occur, they can be costly to patients due to both time spent away from home and work and additional expenses. When the current procedural terminology (CPT) codes are run through the VistA integrated billing system, based on the VHA Chief Business Office Reasonable Charges, a complication can more than double the charges associated with A1 pulley surgery.
A flexor sheath incision and drainage (I+D) (CPT 26020) charges $8,935.35 (facility charge, $6,911.95 plus professional fee, $2,023.40), compared with open trigger finger release (CPT 26055) at $8,365.66 (facility charge, $6,911.95 plus professional fee, $1,453.71). According to a conversation with the finance service officer at NFSGVHS (2/11/2014), the anesthesia bill ($490.56/15 min), anticipated level 3 emergency department visits (facility charge, $889.22 plus professional fee $493.40), and inpatient stays (daily floor bed $786.19) can make an infectious complication costly.
Trigger finger can also be released percutaneously. This is a reasonable option that avoids the operating room, but NFSGVHS surgeons prefer the open surgery due to concerns for tendon and nerve injury that can result from a blind sweep of the needle.3,4
Related: Prevention of Venous Thromboembolism After Total Joint Replacement
Existing studies found complications for trigger finger release ranging from 1% to 31%.5,6 Wound complications and joint stiffness are known complications.5-7 In this study, 60% of the complications were infections, and 80% of the complications were wound complications. Six of 8 patients with wound-healing complications received perioperative antibiotics. Three patients returned to the operating room for an I+D of the flexor sheath. The results showed a statistically significant link between > 1 digit treated at the same surgery and postoperative complications (P = .027). A PubMed search revealed no existing hand literature with this association.
Risk Factors
Diabetes, tobacco use, type of incision, and number of digits treated were assessed as risk factors for complications after trigger finger surgery. Nicotine is widely accepted as increasing the risk for wound complications.8 Almost 20% of the U.S. population smokes, compared with 22% of the VA population and 32% of active-duty military personnel.9 One in 4 veterans has been diagnosed with diabetes, a well-known predisposing factor in delayed wound healing and infection.10,11 No prior studies were found comparing type of incision or multiple digits treated as complications risk factors.
There is also a well-known association between trigger finger and diabetes. Chronic hyperglycemia results in the accumulation of collagen within tendon sheaths due to impairment of collagen breakdown. Patients with diabetes tend to present with multiple digit involvement and respond less favorably to steroid injections compared with patients without diabetes.12 Wound healing is also impaired in patients with diabetes. All 6 wound infections in this study were in patients with diabetes. Proposed etiologies for wound-healing complications include pathologic angiogenesis, impaired fibroblast proliferation and migration, impaired circulation, decreased oxygenation, and a defective immune response to the injured site.13
Trigger finger may develop in multiple digits. Once surgery has been planned for 1 digit, patients may request surgery on another digit on the same hand that has not had an attempt at nonoperative intervention. The NFSGVHS plastic surgeons have raised the threshold to offer multiple surgical procedures on the same hand at the same operative visit to minimize recovery time and number of visits, particularly when patients are travelling long distances. This may be less convenient; however, the overall cost to the patient and the health care system in the event of a complication is significant. Plastic surgery providers also run an alcohol prep pad over the incision site to prevent inoculation of the flexor sheath during suture removal.
Current recommendations to ameliorate the postoperative risks to the patient and costs to the system include endorsing a more conservative approach to treating trigger finger than was previously practiced at NFSGVHS. The known, less favorable response of patients with diabetes to steroid injections plus their elevated risk of postoperative infection create a catch-22 for the treatment plan. Given the low risk of a single steroid injection to the flexor sheath, this procedure is still recommended as a first-line treatment.
Related: Experience Tells in Hip Arthroplasty
During the 5-year study there was a lower threshold for surgical management and for treatment of multiple digits during the same surgery than the one currently practiced, with an overall consensus of the hospital’s HCPs. The authors recommend that all patients start with a steroid injection before committing to surgery. Patients with diabetes are informed that the injection will cause a temporary rise in their blood glucose.14 If they are resistant to the injection, high-dose oral nonsteroidal anti-inflammatory drugs and/or proximal interphalangeal joint splinting is ordered.
Verification of A1C values showing better chronic management of blood sugar is a procedure HCPs from the NFSGVHS will begin to follow. Preoperative A1C values between 6.5% and 8% in patients known to have diabetes has been recommended.15 A1C values > 7% have been found to be an independent risk factor for stenosing tenosynovitis.16 The total number of trigger finger surgeries may drop with the benefit of improved utilization of resources.
Conclusion
The authors found a statistically significant association between postoperative infection and 2 patient populations: patients with diabetes (P = .002) and patients having > 1 digit released during the same surgery (P = .027). This outcome suggests using caution when offering A1 pulley release in select patient populations.
Acknowledgement
Justine Pierson, BS, research coordinator at University of Florida, for statistical analysis. Funding is through salary.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Yamada K, Masuko, T, Iwasaki N. Rupture of the flexor digitorum profundus tendon after injections of insoluble steroid for a trigger finger. J Hand Surg Eur. 2011;36(1):77-78.
2. Kerrigan CL, Stanwix MG. Using evidence to minimize the cost of trigger finger care. J Hand Surg Am. 2009;34(6):997-1005.
3. Habbu R, Putnam MD, Adams JE. Percutaneous Release of the A1 pulley: a cadaver study. J Hand Surg Am. 2012;37(11):2273-2277.
4. Guler F, Kose O, Ercan EC, Turan A, Canbora K. Open vs percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36(10):e1290-e1294.
5. Lim M-H, Lim K-K, Rasheed MZ, Narayana S, Tan B-H. Outcome of open trigger digit release. J Hand Surg Eur. 2007;32(4):457-479.
6. Will R, Lubahn J. Complications of open trigger finger release. J Hand Surg Am. 2010;35(4):594-596.
7. Lee WT, Chong AK. Outcome study of open trigger digit release. J Hand Surg Eur. 2011;36(4):339.
8. Rinker B. The evils of nicotine: An evidence-based guide to smoking and plastic surgery. Ann Plast Surg. 2013;70(5):599-605.
9. Bondurant S, Wedge R, eds. Combating Tobacco Use in Military and Veteran Populations. Washington, DC: The National Academies; 2009.
10. Shilling AM, Raphael J. Diabetes, hyperglycemia, and infections. Best Pract Res Clin Anaesthesiol. 2008;22(3):519-535.
11. Kuppersmith J, Francis J, Kerr E, et al. Advancing evidence-based care for diabetes: Lessons from the Veterans Health Administration. Health Aff. 2007;26(2):156-158.
12. Brown E, Genoway KA. Impact of diabetes on outcomes in hand surgery. J Hand Surg Am. 2011;36(12):2067-2072.
13. Francis-Goforth KN, Harken AH, Saba JD. Normalization of diabetic wound healing. Surgery. 2010;147(3):446-449.
14. Wang AA, Hutchinson DT. The effect of corticosteroid injection for trigger finger on blood glucose level in diabetic patients. J Hand Surg Am. 2006;31(6):979-981.
15. Underwood P, Askari R, Hurwitz S, Chamarthi B, Garg R. Preoperative A1C and Clinical Outcomes in patients with diabetes undergoing major noncardiac surgical procedures. Diabetes Care. 2014; 37(3): 611-616.
16. Vance MC, Tucker JJ, Harness NG. The association of hemoglobin A1c with the prevalence of stenosing tenosynovitis. J Hand Surg Am. 2012;37(9):1765-1769.
1. Yamada K, Masuko, T, Iwasaki N. Rupture of the flexor digitorum profundus tendon after injections of insoluble steroid for a trigger finger. J Hand Surg Eur. 2011;36(1):77-78.
2. Kerrigan CL, Stanwix MG. Using evidence to minimize the cost of trigger finger care. J Hand Surg Am. 2009;34(6):997-1005.
3. Habbu R, Putnam MD, Adams JE. Percutaneous Release of the A1 pulley: a cadaver study. J Hand Surg Am. 2012;37(11):2273-2277.
4. Guler F, Kose O, Ercan EC, Turan A, Canbora K. Open vs percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36(10):e1290-e1294.
5. Lim M-H, Lim K-K, Rasheed MZ, Narayana S, Tan B-H. Outcome of open trigger digit release. J Hand Surg Eur. 2007;32(4):457-479.
6. Will R, Lubahn J. Complications of open trigger finger release. J Hand Surg Am. 2010;35(4):594-596.
7. Lee WT, Chong AK. Outcome study of open trigger digit release. J Hand Surg Eur. 2011;36(4):339.
8. Rinker B. The evils of nicotine: An evidence-based guide to smoking and plastic surgery. Ann Plast Surg. 2013;70(5):599-605.
9. Bondurant S, Wedge R, eds. Combating Tobacco Use in Military and Veteran Populations. Washington, DC: The National Academies; 2009.
10. Shilling AM, Raphael J. Diabetes, hyperglycemia, and infections. Best Pract Res Clin Anaesthesiol. 2008;22(3):519-535.
11. Kuppersmith J, Francis J, Kerr E, et al. Advancing evidence-based care for diabetes: Lessons from the Veterans Health Administration. Health Aff. 2007;26(2):156-158.
12. Brown E, Genoway KA. Impact of diabetes on outcomes in hand surgery. J Hand Surg Am. 2011;36(12):2067-2072.
13. Francis-Goforth KN, Harken AH, Saba JD. Normalization of diabetic wound healing. Surgery. 2010;147(3):446-449.
14. Wang AA, Hutchinson DT. The effect of corticosteroid injection for trigger finger on blood glucose level in diabetic patients. J Hand Surg Am. 2006;31(6):979-981.
15. Underwood P, Askari R, Hurwitz S, Chamarthi B, Garg R. Preoperative A1C and Clinical Outcomes in patients with diabetes undergoing major noncardiac surgical procedures. Diabetes Care. 2014; 37(3): 611-616.
16. Vance MC, Tucker JJ, Harness NG. The association of hemoglobin A1c with the prevalence of stenosing tenosynovitis. J Hand Surg Am. 2012;37(9):1765-1769.