User login
Pulmonary infarction due to pulmonary embolism
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
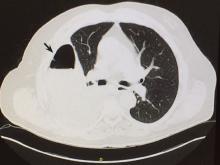
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
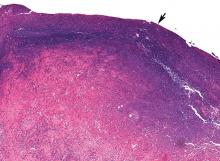
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
Treating and preventing acute exacerbations of COPD
In contrast to stable chronic obstructive pulmonary disease (COPD),1 acute exacerbations of COPD pose special management challenges and can significantly increase the risk of morbidity and death and the cost of care.
This review addresses the definition and diagnosis of COPD exacerbations, disease burden and costs, etiology and pathogenesis, and management and prevention strategies.
DEFINITIONS ARE PROBLEMATIC
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines a COPD exacerbation as “an acute event characterized by a worsening of the patient’s respiratory symptoms that is beyond normal day-to-day variations and leads to a change in medication.”2 It further categorizes acute exacerbations by severity:
- Mild—treated with increased frequency of doses of existing medications
- Moderate—treated with corticosteroids or antibiotics, or both
- Severe—requires hospital utilization (either emergency room treatment or admission).
Although descriptive and useful for retrospective analyses, this current definition poses ambiguities for clinicians. Day-to-day variation in symptoms is not routinely assessed, so deviations from baseline may be difficult to detect. Although clinical tools are available for assessing symptoms in stable and exacerbated states (eg, the COPD assessment test3 and the Exacerbations of Chronic Pulmonary Disease Tool [EXACT]4), they have not been widely adopted in daily practice. Also, according to the current definition, the severity of an exacerbation can be classified only after the course of action is determined, so the severity is not helpful for forming a management strategy at bedside. In addition, physicians may have different thresholds for prescribing antibiotics and corticosteroids.
An earlier definition categorized a COPD exacerbation by the presence of its three cardinal symptoms (ie, increased shortness of breath, sputum volume, and purulence):
- Type I—all three symptoms present
- Type II—two symptoms present
- Type III—one symptom present, accompanied by at least one of the following: upper respiratory tract infection within the past 5 days, unexplained fever, increased wheezing or cough, or 20% increased respiratory rate or heart rate from baseline.
This older definition was successfully used in a prospective clinical trial to identify patients who benefited most from antibiotics for COPD exacerbations.5
Despite these caveats regarding a definition, most clinicians agree on the clinical presentation of a patient with COPD exacerbation: ie, having some combination of shortness of breath, increased sputum volume, and purulence. By the same token, patients with COPD who present with symptoms not typical of an exacerbation should be evaluated for another diagnosis. For instance, Tillie-Leblond et al6 reported that 49 (25%) of 197 patients hospitalized with an “unexplained” exacerbation of COPD were eventually diagnosed with pulmonary embolism.
EXACERBATIONS ARE COSTLY
The care of patients with COPD places a great burden on the healthcare system. Using multiple national databases, Ford et al7 estimated that medical costs in the United States in 2010 attributable to COPD and its complications were $32.1 billion.
The largest component of direct healthcare costs of COPD is exacerbations and subsequent hospitalizations.8 Data from a predominantly Medicare population indicate that the annualized mean COPD-related cost for a patient with no exacerbations was $1,425, compared with $12,765 for a patient with severe exacerbations.9 The investigators estimated that reducing exacerbations from two or more to none could save $5,125 per patient per year.
EXACERBATIONS AFFECT HEALTH BEYOND THE EVENT
COPD exacerbations are associated with a faster decline in lung function,10 reduced quality of life,11 and lost workdays.7 A single exacerbation may cause a decline in lung function and health status that may not return to baseline for several months, particularly if another exacerbation occurs within 6 months.12,13 COPD exacerbations have also been linked to poor clinical outcomes, including death.
In a prospective study in 304 men with COPD followed for 5 years, those who had three or more COPD exacerbations annually were four times as likely to die than patients who did not have an exacerbation.14 Nevertheless, the relationship with mortality may not be causal: Brusselle pointed out in an editorial15 that established mortality predictors for COPD do not include exacerbations, and symptomatic patients with COPD without any history of exacerbations are at greater risk of death than those who are asymptomatic but at high risk for exacerbations.
INFECTION + INFLAMMATION = EXACERBATION
An acute COPD exacerbation can be viewed as an acute inflammatory event superimposed on chronic inflammation associated with COPD. Inflammation in the airways increases resistance to air flow with consequent air trapping. Increased resistance and elastic load due to air trapping place respiratory muscles at a mechanical disadvantage and increase the work of breathing.
Infection starts the process
Infections, particularly bacterial and viral, are thought to be the major instigators of COPD exacerbation, although environmental factors such as air pollution may also play a role.16
Airway inflammation is markedly reduced when bacterial infection is eradicated. But if bacterial colonization continues, inflammatory markers remain elevated despite clinical resolution of the exacerbation.17 Desai et al18 found that patients with COPD and chronic bronchitis with bacterial colonization had a larger symptom burden than patients without colonization, even without an exacerbation.
Allergic profile increases risk
Although most studies indicate that infection is the main cause of exacerbations, clinicians should consider other mechanisms of inflammation on an individual basis. COPD exacerbations may be phenotyped by measuring inflammatory markers, perhaps as a starting point for tailored therapies.
Bafadhel et al19 studied 145 patients with COPD over the course of a year and recorded various biomarkers at baseline and during exacerbations. Exacerbations had an inflammatory profile that was predominantly bacterial in 37%, viral in 10%, and eosinophilic in 17%, and had limited changes in the inflammatory profile in 14%. The remaining episodes were combinations of categories. In another study,20 multivariate analysis conducted in two cohorts with COPD found that patients who had an allergic phenotype had more respiratory symptoms and a higher likelihood of COPD exacerbations.
Frequent COPD exacerbations are increasingly recognized as being associated with an asthma-COPD overlap syndrome, consisting of symptoms of increased airflow variability and incompletely reversible airflow obstruction.21
Inflammation as a marker of frequent exacerbations
Evidence is accumulating that supports systemic inflammation as a marker of frequent exacerbations. The Copenhagen Heart Study tested for baseline plasma C-reactive protein, fibrinogen, and white blood cell count in 6,574 stable patients with COPD.22 After multivariable adjustment, they found a significantly higher likelihood of having a subsequent exacerbation in patients who had all three biomarkers elevated (odds ratio [OR] 3.7, 95% confidence interval [CI] 1.9–7.4), even in patients with milder COPD and those without previous exacerbations.
Past exacerbations predict risk
The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints study23 found that a history of acute COPD exacerbation was the single best predictor of future exacerbations. This risk factor remained stable over 3 years and was present across the severity of COPD, ie, patients at lower GOLD stages who had a history of frequent exacerbations were likely to have exacerbations during follow-up.
EXACERBATION INCREASES CARDIOVASCULAR RISK
COPD exacerbations increase the risk of cardiovascular events, particularly myocardial infarction.24 During hospitalization for acute exacerbation of COPD, markers of myocardial injury and heart failure may be elevated and are a predictor of death.25
Patel et al26 measured arterial stiffness (aortic pulse wave velocity, a validated measure of cardiovascular risk) and cardiac biomarkers (troponin and N-terminal B-type natriuretic peptide) at baseline in 98 patients and longitudinally during and after a COPD exacerbation. In addition to increased levels of cardiac biomarkers, they found a significant rise in arterial stiffness during the exacerbation event without return to baseline levels over 35 days of follow-up. The arterial stiffness increase was related to airway inflammation as measured by sputum interleukin 6, particularly in patients with documented lower respiratory tract infection.
Retrospective analysis suggests a reduced all-cause mortality rate in COPD patients who are treated with beta-blockers.27
Recommendation. We recommend that patients already taking a selective beta-blocker continue to do so during a COPD exacerbation.
OUTPATIENT MANAGEMENT
Treatment with a combination of a corticosteroid, antibiotic, and bronchodilator addresses the underlying pathophysiologic processes of an acute exacerbation: inflammation, infection, and airway trapping.
Short course of a corticosteroid improves outcomes
A 10-day systemic course of a corticosteroid prescribed for COPD exacerbation before discharge from the emergency department was found to offer a small advantage over placebo for reducing treatment failure (unscheduled physician visits, return to emergency room for recurrent symptoms) and improving dyspnea scores and lung function.28 Even just a 3-day course improved measures of respiration (forced expiratory volume in the first second of expiration [FEV1] and arterial oxygenation) at days 3 and 10, and reduced treatment failures compared with placebo.29
Corticosteroid prescription should not be taken lightly, because adverse effects are common. In a systematic review, one adverse effect (hyperglycemia, weight gain, or insomnia) occurred for every five people treated.30
Identifying subgroups of patients most likely to benefit from corticosteroid treatment may be helpful. Corticosteroids may delay improvement in patients without eosinophilic inflammation and hasten recovery in those with more than 2% peripheral eosinophils.31 Siva et al32 found that limiting corticosteroids to patients with sputum eosinophilia reduced corticosteroid use and reduced severe exacerbations compared with standard care.32
Recommendation. For an acute exacerbation, we prescribe a short course of corticosteroids (eg, prednisone 40 mg daily for 5 to 7 days). Tapering dosing is probably unnecessary because adrenal insufficiency is uncommon before 2 weeks of corticosteroid exposure. Clinicians should weigh the merits of tapering (reduced corticosteroid exposure) against patient inconvenience and difficulty following complicated instructions.
Antibiotics help, but exact strategy uncertain
Although antibiotic therapy is one of the three pillars of COPD exacerbation management, the optimal antimicrobial agent, duration of therapy, and which patients will benefit remain areas of controversy and research. Thus far, large trials have been unable to definitely show the superiority of one antibiotic over another.33,34
A 1987 randomized controlled trial5 of antibiotic therapy in acute exacerbation of COPD found the greatest benefit to patients who had all three cardinal symptoms (ie, increased shortness of breath, sputum volume, and purulence), with less marked but still significant improvement in patients with two symptoms. In a 2012 multicenter trial35 patients with mild to moderate COPD experiencing an exacerbation were treated with either combined amoxicillin and clavulanate or placebo if they had one of the three cardinal symptoms. The antibiotic group had a significantly higher clinical cure rate at days 9 to 11 (74.1% vs 59.9%) as well as a longer time until the next exacerbation (233 vs 160 days).
Recommendation. Optimal antibiotic management of COPD exacerbations may also depend on risk factors. For patients with at least two cardinal symptoms, we favor a scheme akin to one proposed for treating community-acquired pneumonia (Table 1).16,36
INPATIENT MANAGEMENT
Corticosteroids improve outcomes
A Department of Veterans Affairs cooperative trial37 randomized 271 patients hospitalized with COPD exacerbation to receive either corticosteroids (intravenous followed by oral) or placebo for either 2 weeks or 8 weeks. Corticosteroid recipients had lower rates of treatment failure at 30 and 90 days, defined as death from any cause, need for mechanical ventilation, readmission, or intensification of pharmacologic therapy. Corticosteroid therapy also reduced hospital length of stay and improved the rate of recovery. The longer corticosteroid course was associated with a higher rate of adverse effects.
Oral corticosteroids not inferior to intravenous
Using the same end point of treatment failure as the Veterans Affairs cooperative trial, deJong et al38 demonstrated that prednisone 60 mg by mouth was not inferior to intravenous prednisone. Neither trial demonstrated a difference in mortality between corticosteroid use and placebo.
Short course of a corticosteroid not inferior to a long course
In 2013, the Reduction in the Use of Corticosteroids in Exacerbated COPD (REDUCE) trial39 randomized 314 patients presenting with an acute COPD exacerbation (92% requiring hospital admission) to oral prednisone 40 mg daily for either 5 days or 14 days. They found that the short course was noninferior in preventing exacerbations over the ensuing 6 months in terms of death and the need for mechanical ventilation.
Recommendation. Our threshold for initiating systemic corticosteroid therapy is lower in hospitalized patients than in outpatients. We recommend the regimen of the REDUCE trial: prednisone 40 mg daily for 5 days.
Corticosteroids for patients on ventilatory support
Severe COPD exacerbations requiring admission to intensive care are a significant source of morbidity and mortality, and the strategy of corticosteroid treatment is still under investigation.
Intravenous corticosteroids are effective. A multicenter trial40 in 354 patients requiring either invasive or noninvasive mechanical ventilation randomized them to treatment with either intravenous methylprednisolone (tapered) or placebo. Treatment was associated with fewer mechanical ventilation days and a lower rate of noninvasive ventilation failure.
Low-dose oral corticosteroids ineffective. In contrast, an open-label trial41 of patients requiring ventilatory support and randomized to either oral prednisone (1 mg/kg for up to 10 days) or usual care found no difference in intensive care length of stay or noninvasive ventilation failure. This study used the oral route and smaller doses, and its open-label design might have introduced bias.
Lower-dose steroids better than high-dose. A 2014 cohort study of 17,239 patients admitted to the ICU with acute exacerbations of COPD evaluated outcomes of treatment with high methylprednisolone dosages (> 240 mg per day) vs lower dosages, using propensity score matching.42 No mortality difference was found between the groups. The lower dosage group (median methylprednisolone dose 100 mg per day) had shorter hospital and intensive care unit stays, shorter duration of noninvasive positive pressure ventilation, less need for insulin therapy, and fewer fungal infections.
Antibiotics for hospitalized patients
Only scarce data are available on the use of antibiotics for patients hospitalized with COPD exacerbation. In a study of patients hospitalized with COPD exacerbations, adding doxycycline to corticosteroids led to better clinical success and cure rates at 10 days compared with placebo, but the primary end point of clinical success at 30 days was not different between the two groups.43
BRONCHODILATORS: A MAINSTAY OF COPD TREATMENT
Bronchodilators are an important part of treatment of COPD exacerbations in inpatient and outpatient settings.
Nebulized beta-2 agonists are given every 1 to 4 hours. Albuterol at a 2.5-mg dose in each nebulization was found to be as effective as 5 mg for length of hospital stay and recovery of lung function in patients with an acute exacerbation of COPD.44
Adding an anticholinergic may help. Nebulized anticholinergics can be given alone or combined with beta-2 agonists. Whether long-acting bronchodilators should be used to manage COPD patients hospitalized with an exacerbation requires further inquiry. In an observational study with historical controls, Drescher and colleagues45 found cost savings and shorter hospital stays if tiotropium (a long-acting anticholinergic) was added to the respiratory care protocol, which also included formoterol (a long-acting beta-2 agonist).
OXYGEN: TITRATED APPROACH SAFER
Oxygen should be supplied during a COPD exacerbation to ensure adequate oxyhemoglobin saturation. Caution is needed to avoid hyperoxemic hypercapnia, particularly in patients with severe COPD and propensity to ventilatory failure. The routine administration of oxygen at high concentrations during a COPD exacerbation has been associated with a higher mortality rate than with a titrated oxygen approach.46 Long-term oxygen treatment started at discharge or as outpatient therapy is associated with reduced hospital admissions and shorter hospital stays for acute exacerbations of COPD.47
VENTILATION SUPPORT
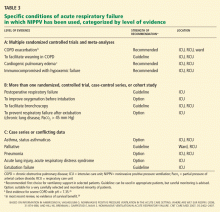
Noninvasive positive-pressure ventilation is a useful adjunct to treatment of COPD exacerbations with evidence of ventilatory failure (ie, acute respiratory acidosis), helping to offset the work of breathing until respiratory system mechanics improve. Keenan et al48 reviewed 15 randomized controlled trials, involving 636 patients, of noninvasive positive-pressure ventilation in the setting of COPD exacerbation. They concluded that noninvasive positive-pressure ventilation reduced the in-hospital mortality rate and length of stay compared with standard therapy. Noninvasive positive-pressure ventilation is most useful in patients with severe COPD exacerbations and acute respiratory acidosis (pH < 7.35).49
Intubation and mechanical ventilation. Although no standards exist for determining which COPD exacerbations may be too severe for noninvasive positive-pressure ventilation, intubation is clearly indicated for impending respiratory failure or hemodynamic instability. Other factors to consider include the greater likelihood of noninvasive positive-pressure ventilation failure in patients with severe respiratory acidosis (pH < 7.25 is associated with a > 50% failure rate) and in those with no improvement in acidosis or respiratory rate during the first hour after initiation of noninvasive positive-pressure ventilation.50
PREVENTING EXACERBATIONS
Recent data indicate that COPD exacerbations can often be prevented (Table 2).
Inhaled pharmacotherapy
Inhaled pharmacotherapeutic agents, singly or in combination, reduce the frequency of COPD exacerbations.
Combined long-acting beta-2 agonist and corticosteroid is better than single-agent therapy. In 2007, the Towards a Revolution in COPD Health (TORCH) trial51 evaluated outpatient therapy in more than 6,000 patients worldwide with either an inhaled long-acting beta-2 agonist (salmeterol), an inhaled corticosteroid (fluticasone), both drugs in combination, or placebo. Patients had baseline prebronchodilator FEV1 of less than 60% and were followed for 3 years. No difference was found between the groups in the primary end point of deaths, but the annualized rate of moderate to severe exacerbations was reduced by 25% in the group that received combination therapy vs placebo. Combination therapy showed superior efficacy over individual drug therapy in preventing exacerbations. Treatment with the inhaled corticosteroid, whether alone or in combination with salmeterol, increased the risk of pneumonia.
A long-acting antimuscarinic agent is better than placebo. In 2008, the Understanding Potential Long-Term Impacts on Function With Tiotropium (UPLIFT) trial52 randomized nearly 6,000 patients with COPD and a postbronchodilator FEV1 of less than 70% to placebo or tiotropium, a long-acting antimuscarinic agent. Tiotropium reduced the exacerbation rate by 14% compared with placebo and improved quality of life.
Antimuscarinics may be better than beta-2 agonists. Head-to-head comparisons suggest that long-acting antimuscarinic agents are preferable to long-acting beta-2 agonists for preventing COPD exacerbations.53,54
Triple therapy: evidence is mixed. For patients with severe symptomatic COPD and frequent exacerbations, triple therapy with a combination of an inhaled long-acting antimuscarinic agent, an inhaled long-acting beta-2 agonist, and an inhaled corticosteroid has been suggested.
Data to support this practice are limited. In the Canadian Optimal Trial,55 the rate of exacerbations was not different between tiotropium alone, tiotropium plus salmeterol, and triple therapy. However, the rate of hospitalization for severe exacerbation was lower with triple therapy than tiotropium alone. A large, retrospective cohort study also supported triple therapy by finding reduced mortality, hospitalizations, and need for oral corticosteroid bursts compared to combination therapy with an inhaled long-acting beta-2 agonist and an inhaled corticosteroid.56
The drawback of triple therapy is an increased incidence of pneumonia associated with combined beta-2 agonist and corticosteroids, most likely due to the corticosteroid component.51 The risk appears to be higher for higher potency corticosteroids, eg, fluticasone.57
In 2014, the Withdrawal of Inhaled Steroids During Optimised Bronchodilator Management (WISDOM) trial58 randomized nearly 2,500 patients with a history of COPD exacerbation receiving triple therapy consisting of tiotropium, salmeterol, and inhaled fluticasone to either continue treatment or withdraw the corticosteroid for 3 months. The investigators defined an annualized exacerbation rate of 1.2 (ie, a 20% increase) as the upper limit of the confidence interval for an acceptable therapeutic margin of noninferiority. The study showed that the risk of moderate to severe exacerbations with combined tiotropium and salmeterol was noninferior to triple therapy.
Nevertheless, caution is advised when removing the corticosteroid component from triple therapy. The trial demonstrated a worsening in overall health status, some reduction in lung function, and a transient increase in severe exacerbations in the withdrawal group. Patients with increased symptom burden at baseline and a history of severe exacerbations may not be optimal candidates for this strategy.
Roflumilast is effective but has side effects
Roflumilast, an oral phosphodiesterase 4 inhibitor, is an anti-inflammatory drug without bronchodilator properties. In randomized controlled trials, the drug was associated with a 17% reduction in acute exacerbations compared with placebo.59
Adding roflumilast to either a long-acting beta-2 agonist or a long-acting antimuscarinic agent resulted in a 6% to 8% further reduction in the proportion of patients with exacerbation.60,61 Martinez et al61 found that roflumilast added to a regimen of a long-acting beta-2 agonist plus an inhaled corticosteroid reduced moderate to severe exacerbations by 14.2%, even in the presence of tiotropium. Compared with placebo, roflumilast treatment reduced exacerbations necessitating hospitalizations by 23.9%.
The FDA has approved oral roflumilast 500 µg once daily to prevent COPD exacerbations.
Roflumilast is frequently associated with side effects, including gastrointestinal symptoms (chiefly diarrhea), weight loss, and psychiatric effects. A benefit-to-harm study in 2014 concluded that using the drug is only favorable for patients who have a high risk of severe exacerbations, ie, those who have a greater than 22% baseline risk of having at least one exacerbation annually.62
Recommendation. Roflumilast should be reserved for patients who have severe COPD with a chronic bronchitis phenotype (ie, with cough and sputum production) and repeated exacerbations despite an optimal regimen of an inhaled corticosteroid, long-acting beta-2 agonist, and long-acting antimuscarinic agent.
Macrolide antibiotics: Role unclear
Macrolide antibiotics have anti-inflammatory and immunomodulatory activities.
Azithromycin: fewer exacerbations but some side effects. A multicenter trial63 in 1,142 COPD patients randomized to either oral azithromycin 250 mg daily or placebo found a 27% reduction in the risk of COPD exacerbation in the intervention arm. No differences were found between the groups in mortality, hospitalizations, emergency department visits, or respiratory failure. Hearing loss and increased macrolide resistance were noted in the intervention arm. In a secondary subgroup analysis,64 no difference in efficacy was found by sex, history of chronic bronchitis, oxygen use, or concomitant COPD treatment.
The COPD: Influence of Macrolides on Exacerbation Frequency in Patients trial65 helped refine patient selection for macrolide therapy. In this single-center study, 92 patients with COPD and at least three exacerbations during the year prior to enrollment were randomized to receive either azithromycin 500 mg three times weekly or placebo. Exacerbations in the intervention group were markedly reduced (42%) with no difference in hospitalization rate.
The place of macrolide antibiotics in the treatment strategy of COPD is unclear, and they are not currently part of the GOLD guidelines. Still unknown is the incremental benefit of adding them to existing preventive regimens, cardiovascular safety, side effects, and potential effects on the resident microbial flora.
Other antibiotics have also been investigated for efficacy in preventing exacerbations.
Moxifloxacin: fewer exacerbations. The Pulsed Moxifloxacin Usage and Its Long-term Impact on the Reduction of Subsequent Exacerbations study66 randomized more than 1,000 patients with stable COPD to receive either moxifloxacin 400 mg or placebo daily for 5 days repeated every 8 weeks for six courses. Frequent assessment during the treatment period and for 6 months afterward revealed a reduced exacerbation rate in the intervention group but without benefit in hospitalization rate, mortality, lung function, or health status.
Recommendation. Azithromycin (either 250 mg daily or 500 mg three times weekly) can be considered for patients who have repeated COPD exacerbations despite an optimal regimen of an inhaled corticosteroid, inhaled long-acting beta-2 agonist, and inhaled long-acting antimuscarinic agent. The need to continue azithromycin should be reassessed yearly.
Mucolytics
Greatest benefit to patients not taking inhaled corticosteroids. Mucolytic agents help clear airway secretions by reducing viscosity. N-acetylcysteine and carbocysteine (not available in the United States) also have antioxidant properties that may counteract oxidant stress associated with acute COPD exacerbations.
The Bronchitis Randomized on NAC Cost-Utility Study (BRONCUS)67 randomized 523 COPD patients to N-acetylcysteine 600 mg daily or placebo. After 3 years of follow-up, no differences were found in the rate of exacerbations, lung function decline, and quality of life. Subgroup analysis suggested a reduction in exacerbations for patients who were not taking inhaled corticosteroids.
The Effect of Carbocisteine on Acute Exacerbation of Chronic Obstructive Pulmonary Disease (PEACE) study randomized more than 700 patients from multiple centers in China who had COPD and a recent history of exacerbations; they found a 25% lower exacerbation rate over 1 year with carbocysteine vs placebo.68 Most of the patients (83%) were not on inhaled corticosteroids, which complemented findings of the BRONCUS trial.
The Effect of High Dose N-acetylcysteine on Air Trapping and Airway Resistance of COPD (HIACE) study randomized 120 patients with stable COPD in a hospital in Hong Kong to either oral N-acetylcysteine (600 mg twice daily) or placebo and found a reduced exacerbation rate of exacerbations. Patients were matched at baseline for inhaled corticosteroid use.69
In 2014, the Twice Daily N-acetylcysteine 600 mg for Exacerbations of Chronic Obstructive Pulmonary Disease (PANTHEON) study70 randomized 1,006 patients from multiple hospitals in China with a history of moderate to severe COPD and exacerbations to receive either N-acetylcysteine 600 mg twice daily or placebo for 1 year. They found a 22% reduction in exacerbations in the treatment group vs placebo.
GOLD guidelines2 recommend mucolytics for patients with severe COPD and exacerbations when inhaled corticosteroids are not available or affordable.
Recommendation. Mucolytics may be useful for patients with difficulty expectorating and with a history of exacerbations despite appropriate inhaled therapy.
OTHER INTERVENTIONS CAN HELP
Pulmonary rehabilitation provides multiple benefits
Pulmonary rehabilitation increases exercise tolerance and reduces symptom burden in patients with stable COPD. It is also a multidisciplinary effort that may help reinforce adherence to medications, enhance COPD education, and provide closer medical surveillance to patients at high risk for recurrent exacerbations.
A small randomized controlled trial71 prescribed pulmonary rehabilitation on discharge for a COPD exacerbation and found sustainable improvements in exercise capacity and health status after 3 months.
In a later study,72 the same group started pulmonary rehabilitation within a week of hospital discharge and found reduced hospital readmissions over a 3-month period.
Smoking cessation is always worth advocating
A large observational cohort study concluded that current smokers were at a higher risk for COPD exacerbations compared with former smokers.73 Although there are no randomized controlled trials that assess the effects of smoking cessation at the time of COPD exacerbation, we recommend seizing the opportunity to implement this important intervention.
Vaccinations: Influenza and pneumococcal
Influenza vaccination is associated with reduced incidence of hospitalization among patients with cardiopulmonary disease.74 A meta-analysis of randomized clinical trials of influenza vaccination for patients with COPD75 reported significantly fewer exacerbations from vaccination, mostly owing to fewer episodes occurring after 3 to 4 weeks, coinciding with anticipated vaccine-induced immune protection. Furumoto and colleagues76 reported an added benefit of combined vaccination with 23-valent pneumococcal polysaccharide vaccine and influenza vaccine in reducing hospital admissions over influenza vaccination alone. We also recommend providing the 13-valent pneumococcal conjugate vaccine to patients with COPD, particularly for those older than 65, consistent with CDC recommendations.77
- Hatipoglu U, Aboussouan LS. Chronic obstructive pulmonary disease: an update for the primary physician. Cleve Clin J Med 2014; 81:373–383.
- Global initiative for chronic obstructive lung disease. Pocket Guide to COPD Diagnosis, Management, and Prevention. A Guide for Health Care Professionals. Updated 2016. http://www.goldcopd.org/uploads/users/files/WatermarkedPocket%20Guide%202016(2).pdf. Accessed March 7, 2016.
- Gupta N, Pinto LM, Morogan A, Bourbeau J. The COPD assessment test: a systematic review. Eur Respir J 2014; 44:873–884.
- Leidy NK, Wilcox TK, Jones PW, et al; EXACT-PRO Study Group. Development of the EXAcerbations of chronic obstructive pulmonary disease tool (EXACT): a patient-reported outcome (PRO) measure. Value Health 2010; 13:965–975.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106:196–204.
- Tillie-Leblond I, Marquette CH, Perez T, et al. Pulmonary embolism in patients with unexplained exacerbation of chronic obstructive pulmonary disease: prevalence and risk factors. Ann Intern Med 2006; 144:390–396.
- Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest 2015; 147:31–45.
- Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD 2010; 7:214–228.
- Pasquale MK, Sun SX, Song F, Hartnett HJ, Stemkowski SA. Impact of exacerbations on health care cost and resource utilization in chronic obstructive pulmonary disease patients with chronic bronchitis from a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis 2012; 7:757–764.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57:847–852.
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157:1418–1422.
- Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161:1608–1613.
- Spencer S, Calverley PM, Sherwood Burge P, Jones PW; ISOLDE Study Group, Inhaled Steroids in Obstructive Lung Disease. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163:122–128.
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60:925–931.
- Brusselle G. Why doesn’t reducing exacerbations decrease COPD mortality? Lancet Respir Med 2014; 2:681–683.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359:2355–2365.
- White AJ, Gompertz S, Bayley DL, et al. Resolution of bronchial inflammation is related to bacterial eradication following treatment of exacerbations of chronic bronchitis. Thorax 2003; 58:680–685.
- Desai H, Eschberger K, Wrona C, et al. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014; 11:303–309.
- Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 2011; 184:662–671.
- Jamieson DB, Matsui EC, Belli A, et al. Effects of allergic phenotype on respiratory symptoms and exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 188:187–192.
- Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 2009; 64:728–735.
- Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA 2013; 309:2353–2361.
- Hurst JR, Vestbo J, Anzueto A, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363:1128–1138.
- Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 2010; 137:1091–1097.
- Chang CL, Robinson SC, Mills GD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax 2011; 66:764–768.
- Patel AR, Kowlessar BS, Donaldson GC, et al. Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 188:1091–1099.
- Dransfield MT, Rowe SM, Johnson JE, Bailey WC, Gerald LB. Use of beta blockers and the risk of death in hospitalised patients with acute exacerbations of COPD. Thorax 2008; 63:301–305.
- Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996; 154:407–412.
- Aaron SD, Vandemheen KL, Hebert P, et al. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease. N Engl J Med 2003; 348:2618–2625.
- Walters JA, Gibson PG, Wood-Baker R, Hannay M, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2009; 1:CD001288.
- Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2012; 186:48–55.
- Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J 2007; 29:906–913.
- Wilson R, Allegra L, Huchon G, et al; MOSAIC Study Group. Short-term and long-term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis. Chest 2004; 125:953–964.
- Wilson R, Anzueto A, Miravitlles M, et al. Moxifloxacin versus amoxicillin/clavulanic acid in outpatient acute exacerbations of COPD: MAESTRAL results. Eur Respir J 2012; 40:17–27.
- Llor C, Moragas A, Hernandez S, Bayona C, Miravitlles M. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:716–723.
- Anzueto A. Primary care management of chronic obstructive pulmonary disease to reduce exacerbations and their consequences. Am J Med Sci 2010; 340:309–318.
- Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999; 340:1941–1947.
- de Jong YP, Uil SM, Grotjohan HP, Postma DS, Kerstjens HA, van den Berg JW. Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double-blind study. Chest 2007; 132:1741–1747.
- Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013; 309:2223–2231.
- Alia I, de la Cal MA, Esteban A, et al. Efficacy of corticosteroid therapy in patients with an acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. Arch Intern Med 2011; 171:1939–1946.
- Abroug F, Ouanes-Besbes L, Fkih-Hassen M, et al. Prednisone in COPD exacerbation requiring ventilatory support: an open-label randomised evaluation. Eur Respir J 2014; 43:717–724.
- Kiser TH, Allen RR, Valuck RJ, Moss M, Vandivier RW. Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 189:1052–1064.
- Daniels JM, Snijders D, de Graaff CS, Vlaspolder F, Jansen HM, Boersma WG. Antibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181:150–157.
- Nair S, Thomas E, Pearson SB, Henry MT. A randomized controlled trial to assess the optimal dose and effect of nebulized albuterol in acute exacerbations of COPD. Chest 2005; 128:48–54.
- Drescher GS, Carnathan BJ, Imus S, Colice GL. Incorporating tiotropium into a respiratory therapist-directed bronchodilator protocol for managing in-patients with COPD exacerbations decreases bronchodilator costs. Respir Care 2008; 53:1678–1684.
- Austin MA, Wills KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ 2010; 341:c5462.
- Ringbaek TJ, Viskum K, Lange P. Does long-term oxygen therapy reduce hospitalisation in hypoxaemic chronic obstructive pulmonary disease? Eur Respir J 2002; 20:38–42.
- Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med 2003; 138:861–870.
- Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest 2008; 133:756–766.
- Sinuff T, Keenan SP; Department of Medicine, McMaster University. Clinical practice guideline for the use of noninvasive positive pressure ventilation in COPD patients with acute respiratory failure. J Crit Care 2004; 19:82–91.
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–789.
- Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359:1543–1554.
- Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011; 364:1093–1103.
- Decramer ML, Chapman KR, Dahl R, et al; INVIGORATE investigators. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med 2013; 1:524–533.
- Aaron SD, Vandemheen KL, Fergusson D, et al; Canadian Thoracic Society/Canadian Respiratory Clinical Research Consortium. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2007; 146:545–555.
- Short PM, Williamson PA, Elder DH, Lipworth SI, Schembri S, Lipworth BJ. The impact of tiotropium on mortality and exacerbations when added to inhaled corticosteroids and long-acting beta-agonist therapy in COPD. Chest 2012; 141:81–86.
- Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 2013; 68:1029–1036.
- Magnussen H, Disse B, Rodriguez-Roisin R, et al; WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med 2014; 371:1285–1294.
- Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009; 374:685–694.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009; 374:695–703.
- Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015; 385:857–866.
- Yu T, Fain K, Boyd CM, et al. Benefits and harms of roflumilast in moderate to severe COPD. Thorax 2014; 69:616–622.
- Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365:689–698.
- Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med 2014; 189:1503–1508.
- Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2014; 2:361–368.
- Sethi S, Jones PW, Theron MS, et al; PULSE Study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res 2010; 11:10.
- Decramer M, Rutten-van Molken M, Dekhuijzen PN, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized On NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet 2005; 365:1552–1560.
- Zheng JP, Kang J, Huang SG, et al. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE study): a randomised placebo-controlled study. Lancet 2008; 371:2013–2018.
- Tse HN, Raiteri L, Wong KY, et al. High-dose N-acetylcysteine in stable COPD: the 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest 2013; 144:106–118.
- Zheng JP, Wen FQ, Bai CX, et al; PANTHEON study group. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med 2014; 2:187–194.
- Man WD, Polkey MI, Donaldson N, Gray BJ, Moxham J. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ 2004; 329:1209.
- Seymour JM, Moore L, Jolley CJ, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010; 65:423–428.
- Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med 2009; 24:457–463.
- Seo YB, Hong KW, Kim IS, et al. Effectiveness of the influenza vaccine at preventing hospitalization due to acute lower respiratory infection and exacerbation of chronic cardiopulmonary disease in Korea during 2010-2011. Vaccine 2013; 31:1426–1430.
- Poole PJ, Chacko E, Wood-Baker RW, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; 1:CD002733.
- Furumoto A, Ohkusa Y, Chen M, et al. Additive effect of pneumococcal vaccine and influenza vaccine on acute exacerbation in patients with chronic lung disease. Vaccine 2008; 26:4284–4289.
- Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822–825.
In contrast to stable chronic obstructive pulmonary disease (COPD),1 acute exacerbations of COPD pose special management challenges and can significantly increase the risk of morbidity and death and the cost of care.
This review addresses the definition and diagnosis of COPD exacerbations, disease burden and costs, etiology and pathogenesis, and management and prevention strategies.
DEFINITIONS ARE PROBLEMATIC
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines a COPD exacerbation as “an acute event characterized by a worsening of the patient’s respiratory symptoms that is beyond normal day-to-day variations and leads to a change in medication.”2 It further categorizes acute exacerbations by severity:
- Mild—treated with increased frequency of doses of existing medications
- Moderate—treated with corticosteroids or antibiotics, or both
- Severe—requires hospital utilization (either emergency room treatment or admission).
Although descriptive and useful for retrospective analyses, this current definition poses ambiguities for clinicians. Day-to-day variation in symptoms is not routinely assessed, so deviations from baseline may be difficult to detect. Although clinical tools are available for assessing symptoms in stable and exacerbated states (eg, the COPD assessment test3 and the Exacerbations of Chronic Pulmonary Disease Tool [EXACT]4), they have not been widely adopted in daily practice. Also, according to the current definition, the severity of an exacerbation can be classified only after the course of action is determined, so the severity is not helpful for forming a management strategy at bedside. In addition, physicians may have different thresholds for prescribing antibiotics and corticosteroids.
An earlier definition categorized a COPD exacerbation by the presence of its three cardinal symptoms (ie, increased shortness of breath, sputum volume, and purulence):
- Type I—all three symptoms present
- Type II—two symptoms present
- Type III—one symptom present, accompanied by at least one of the following: upper respiratory tract infection within the past 5 days, unexplained fever, increased wheezing or cough, or 20% increased respiratory rate or heart rate from baseline.
This older definition was successfully used in a prospective clinical trial to identify patients who benefited most from antibiotics for COPD exacerbations.5
Despite these caveats regarding a definition, most clinicians agree on the clinical presentation of a patient with COPD exacerbation: ie, having some combination of shortness of breath, increased sputum volume, and purulence. By the same token, patients with COPD who present with symptoms not typical of an exacerbation should be evaluated for another diagnosis. For instance, Tillie-Leblond et al6 reported that 49 (25%) of 197 patients hospitalized with an “unexplained” exacerbation of COPD were eventually diagnosed with pulmonary embolism.
EXACERBATIONS ARE COSTLY
The care of patients with COPD places a great burden on the healthcare system. Using multiple national databases, Ford et al7 estimated that medical costs in the United States in 2010 attributable to COPD and its complications were $32.1 billion.
The largest component of direct healthcare costs of COPD is exacerbations and subsequent hospitalizations.8 Data from a predominantly Medicare population indicate that the annualized mean COPD-related cost for a patient with no exacerbations was $1,425, compared with $12,765 for a patient with severe exacerbations.9 The investigators estimated that reducing exacerbations from two or more to none could save $5,125 per patient per year.
EXACERBATIONS AFFECT HEALTH BEYOND THE EVENT
COPD exacerbations are associated with a faster decline in lung function,10 reduced quality of life,11 and lost workdays.7 A single exacerbation may cause a decline in lung function and health status that may not return to baseline for several months, particularly if another exacerbation occurs within 6 months.12,13 COPD exacerbations have also been linked to poor clinical outcomes, including death.
In a prospective study in 304 men with COPD followed for 5 years, those who had three or more COPD exacerbations annually were four times as likely to die than patients who did not have an exacerbation.14 Nevertheless, the relationship with mortality may not be causal: Brusselle pointed out in an editorial15 that established mortality predictors for COPD do not include exacerbations, and symptomatic patients with COPD without any history of exacerbations are at greater risk of death than those who are asymptomatic but at high risk for exacerbations.
INFECTION + INFLAMMATION = EXACERBATION
An acute COPD exacerbation can be viewed as an acute inflammatory event superimposed on chronic inflammation associated with COPD. Inflammation in the airways increases resistance to air flow with consequent air trapping. Increased resistance and elastic load due to air trapping place respiratory muscles at a mechanical disadvantage and increase the work of breathing.
Infection starts the process
Infections, particularly bacterial and viral, are thought to be the major instigators of COPD exacerbation, although environmental factors such as air pollution may also play a role.16
Airway inflammation is markedly reduced when bacterial infection is eradicated. But if bacterial colonization continues, inflammatory markers remain elevated despite clinical resolution of the exacerbation.17 Desai et al18 found that patients with COPD and chronic bronchitis with bacterial colonization had a larger symptom burden than patients without colonization, even without an exacerbation.
Allergic profile increases risk
Although most studies indicate that infection is the main cause of exacerbations, clinicians should consider other mechanisms of inflammation on an individual basis. COPD exacerbations may be phenotyped by measuring inflammatory markers, perhaps as a starting point for tailored therapies.
Bafadhel et al19 studied 145 patients with COPD over the course of a year and recorded various biomarkers at baseline and during exacerbations. Exacerbations had an inflammatory profile that was predominantly bacterial in 37%, viral in 10%, and eosinophilic in 17%, and had limited changes in the inflammatory profile in 14%. The remaining episodes were combinations of categories. In another study,20 multivariate analysis conducted in two cohorts with COPD found that patients who had an allergic phenotype had more respiratory symptoms and a higher likelihood of COPD exacerbations.
Frequent COPD exacerbations are increasingly recognized as being associated with an asthma-COPD overlap syndrome, consisting of symptoms of increased airflow variability and incompletely reversible airflow obstruction.21
Inflammation as a marker of frequent exacerbations
Evidence is accumulating that supports systemic inflammation as a marker of frequent exacerbations. The Copenhagen Heart Study tested for baseline plasma C-reactive protein, fibrinogen, and white blood cell count in 6,574 stable patients with COPD.22 After multivariable adjustment, they found a significantly higher likelihood of having a subsequent exacerbation in patients who had all three biomarkers elevated (odds ratio [OR] 3.7, 95% confidence interval [CI] 1.9–7.4), even in patients with milder COPD and those without previous exacerbations.
Past exacerbations predict risk
The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints study23 found that a history of acute COPD exacerbation was the single best predictor of future exacerbations. This risk factor remained stable over 3 years and was present across the severity of COPD, ie, patients at lower GOLD stages who had a history of frequent exacerbations were likely to have exacerbations during follow-up.
EXACERBATION INCREASES CARDIOVASCULAR RISK
COPD exacerbations increase the risk of cardiovascular events, particularly myocardial infarction.24 During hospitalization for acute exacerbation of COPD, markers of myocardial injury and heart failure may be elevated and are a predictor of death.25
Patel et al26 measured arterial stiffness (aortic pulse wave velocity, a validated measure of cardiovascular risk) and cardiac biomarkers (troponin and N-terminal B-type natriuretic peptide) at baseline in 98 patients and longitudinally during and after a COPD exacerbation. In addition to increased levels of cardiac biomarkers, they found a significant rise in arterial stiffness during the exacerbation event without return to baseline levels over 35 days of follow-up. The arterial stiffness increase was related to airway inflammation as measured by sputum interleukin 6, particularly in patients with documented lower respiratory tract infection.
Retrospective analysis suggests a reduced all-cause mortality rate in COPD patients who are treated with beta-blockers.27
Recommendation. We recommend that patients already taking a selective beta-blocker continue to do so during a COPD exacerbation.
OUTPATIENT MANAGEMENT
Treatment with a combination of a corticosteroid, antibiotic, and bronchodilator addresses the underlying pathophysiologic processes of an acute exacerbation: inflammation, infection, and airway trapping.
Short course of a corticosteroid improves outcomes
A 10-day systemic course of a corticosteroid prescribed for COPD exacerbation before discharge from the emergency department was found to offer a small advantage over placebo for reducing treatment failure (unscheduled physician visits, return to emergency room for recurrent symptoms) and improving dyspnea scores and lung function.28 Even just a 3-day course improved measures of respiration (forced expiratory volume in the first second of expiration [FEV1] and arterial oxygenation) at days 3 and 10, and reduced treatment failures compared with placebo.29
Corticosteroid prescription should not be taken lightly, because adverse effects are common. In a systematic review, one adverse effect (hyperglycemia, weight gain, or insomnia) occurred for every five people treated.30
Identifying subgroups of patients most likely to benefit from corticosteroid treatment may be helpful. Corticosteroids may delay improvement in patients without eosinophilic inflammation and hasten recovery in those with more than 2% peripheral eosinophils.31 Siva et al32 found that limiting corticosteroids to patients with sputum eosinophilia reduced corticosteroid use and reduced severe exacerbations compared with standard care.32
Recommendation. For an acute exacerbation, we prescribe a short course of corticosteroids (eg, prednisone 40 mg daily for 5 to 7 days). Tapering dosing is probably unnecessary because adrenal insufficiency is uncommon before 2 weeks of corticosteroid exposure. Clinicians should weigh the merits of tapering (reduced corticosteroid exposure) against patient inconvenience and difficulty following complicated instructions.
Antibiotics help, but exact strategy uncertain
Although antibiotic therapy is one of the three pillars of COPD exacerbation management, the optimal antimicrobial agent, duration of therapy, and which patients will benefit remain areas of controversy and research. Thus far, large trials have been unable to definitely show the superiority of one antibiotic over another.33,34
A 1987 randomized controlled trial5 of antibiotic therapy in acute exacerbation of COPD found the greatest benefit to patients who had all three cardinal symptoms (ie, increased shortness of breath, sputum volume, and purulence), with less marked but still significant improvement in patients with two symptoms. In a 2012 multicenter trial35 patients with mild to moderate COPD experiencing an exacerbation were treated with either combined amoxicillin and clavulanate or placebo if they had one of the three cardinal symptoms. The antibiotic group had a significantly higher clinical cure rate at days 9 to 11 (74.1% vs 59.9%) as well as a longer time until the next exacerbation (233 vs 160 days).
Recommendation. Optimal antibiotic management of COPD exacerbations may also depend on risk factors. For patients with at least two cardinal symptoms, we favor a scheme akin to one proposed for treating community-acquired pneumonia (Table 1).16,36
INPATIENT MANAGEMENT
Corticosteroids improve outcomes
A Department of Veterans Affairs cooperative trial37 randomized 271 patients hospitalized with COPD exacerbation to receive either corticosteroids (intravenous followed by oral) or placebo for either 2 weeks or 8 weeks. Corticosteroid recipients had lower rates of treatment failure at 30 and 90 days, defined as death from any cause, need for mechanical ventilation, readmission, or intensification of pharmacologic therapy. Corticosteroid therapy also reduced hospital length of stay and improved the rate of recovery. The longer corticosteroid course was associated with a higher rate of adverse effects.
Oral corticosteroids not inferior to intravenous
Using the same end point of treatment failure as the Veterans Affairs cooperative trial, deJong et al38 demonstrated that prednisone 60 mg by mouth was not inferior to intravenous prednisone. Neither trial demonstrated a difference in mortality between corticosteroid use and placebo.
Short course of a corticosteroid not inferior to a long course
In 2013, the Reduction in the Use of Corticosteroids in Exacerbated COPD (REDUCE) trial39 randomized 314 patients presenting with an acute COPD exacerbation (92% requiring hospital admission) to oral prednisone 40 mg daily for either 5 days or 14 days. They found that the short course was noninferior in preventing exacerbations over the ensuing 6 months in terms of death and the need for mechanical ventilation.
Recommendation. Our threshold for initiating systemic corticosteroid therapy is lower in hospitalized patients than in outpatients. We recommend the regimen of the REDUCE trial: prednisone 40 mg daily for 5 days.
Corticosteroids for patients on ventilatory support
Severe COPD exacerbations requiring admission to intensive care are a significant source of morbidity and mortality, and the strategy of corticosteroid treatment is still under investigation.
Intravenous corticosteroids are effective. A multicenter trial40 in 354 patients requiring either invasive or noninvasive mechanical ventilation randomized them to treatment with either intravenous methylprednisolone (tapered) or placebo. Treatment was associated with fewer mechanical ventilation days and a lower rate of noninvasive ventilation failure.
Low-dose oral corticosteroids ineffective. In contrast, an open-label trial41 of patients requiring ventilatory support and randomized to either oral prednisone (1 mg/kg for up to 10 days) or usual care found no difference in intensive care length of stay or noninvasive ventilation failure. This study used the oral route and smaller doses, and its open-label design might have introduced bias.
Lower-dose steroids better than high-dose. A 2014 cohort study of 17,239 patients admitted to the ICU with acute exacerbations of COPD evaluated outcomes of treatment with high methylprednisolone dosages (> 240 mg per day) vs lower dosages, using propensity score matching.42 No mortality difference was found between the groups. The lower dosage group (median methylprednisolone dose 100 mg per day) had shorter hospital and intensive care unit stays, shorter duration of noninvasive positive pressure ventilation, less need for insulin therapy, and fewer fungal infections.
Antibiotics for hospitalized patients
Only scarce data are available on the use of antibiotics for patients hospitalized with COPD exacerbation. In a study of patients hospitalized with COPD exacerbations, adding doxycycline to corticosteroids led to better clinical success and cure rates at 10 days compared with placebo, but the primary end point of clinical success at 30 days was not different between the two groups.43
BRONCHODILATORS: A MAINSTAY OF COPD TREATMENT
Bronchodilators are an important part of treatment of COPD exacerbations in inpatient and outpatient settings.
Nebulized beta-2 agonists are given every 1 to 4 hours. Albuterol at a 2.5-mg dose in each nebulization was found to be as effective as 5 mg for length of hospital stay and recovery of lung function in patients with an acute exacerbation of COPD.44
Adding an anticholinergic may help. Nebulized anticholinergics can be given alone or combined with beta-2 agonists. Whether long-acting bronchodilators should be used to manage COPD patients hospitalized with an exacerbation requires further inquiry. In an observational study with historical controls, Drescher and colleagues45 found cost savings and shorter hospital stays if tiotropium (a long-acting anticholinergic) was added to the respiratory care protocol, which also included formoterol (a long-acting beta-2 agonist).
OXYGEN: TITRATED APPROACH SAFER
Oxygen should be supplied during a COPD exacerbation to ensure adequate oxyhemoglobin saturation. Caution is needed to avoid hyperoxemic hypercapnia, particularly in patients with severe COPD and propensity to ventilatory failure. The routine administration of oxygen at high concentrations during a COPD exacerbation has been associated with a higher mortality rate than with a titrated oxygen approach.46 Long-term oxygen treatment started at discharge or as outpatient therapy is associated with reduced hospital admissions and shorter hospital stays for acute exacerbations of COPD.47
VENTILATION SUPPORT
Noninvasive positive-pressure ventilation is a useful adjunct to treatment of COPD exacerbations with evidence of ventilatory failure (ie, acute respiratory acidosis), helping to offset the work of breathing until respiratory system mechanics improve. Keenan et al48 reviewed 15 randomized controlled trials, involving 636 patients, of noninvasive positive-pressure ventilation in the setting of COPD exacerbation. They concluded that noninvasive positive-pressure ventilation reduced the in-hospital mortality rate and length of stay compared with standard therapy. Noninvasive positive-pressure ventilation is most useful in patients with severe COPD exacerbations and acute respiratory acidosis (pH < 7.35).49
Intubation and mechanical ventilation. Although no standards exist for determining which COPD exacerbations may be too severe for noninvasive positive-pressure ventilation, intubation is clearly indicated for impending respiratory failure or hemodynamic instability. Other factors to consider include the greater likelihood of noninvasive positive-pressure ventilation failure in patients with severe respiratory acidosis (pH < 7.25 is associated with a > 50% failure rate) and in those with no improvement in acidosis or respiratory rate during the first hour after initiation of noninvasive positive-pressure ventilation.50
PREVENTING EXACERBATIONS
Recent data indicate that COPD exacerbations can often be prevented (Table 2).
Inhaled pharmacotherapy
Inhaled pharmacotherapeutic agents, singly or in combination, reduce the frequency of COPD exacerbations.
Combined long-acting beta-2 agonist and corticosteroid is better than single-agent therapy. In 2007, the Towards a Revolution in COPD Health (TORCH) trial51 evaluated outpatient therapy in more than 6,000 patients worldwide with either an inhaled long-acting beta-2 agonist (salmeterol), an inhaled corticosteroid (fluticasone), both drugs in combination, or placebo. Patients had baseline prebronchodilator FEV1 of less than 60% and were followed for 3 years. No difference was found between the groups in the primary end point of deaths, but the annualized rate of moderate to severe exacerbations was reduced by 25% in the group that received combination therapy vs placebo. Combination therapy showed superior efficacy over individual drug therapy in preventing exacerbations. Treatment with the inhaled corticosteroid, whether alone or in combination with salmeterol, increased the risk of pneumonia.
A long-acting antimuscarinic agent is better than placebo. In 2008, the Understanding Potential Long-Term Impacts on Function With Tiotropium (UPLIFT) trial52 randomized nearly 6,000 patients with COPD and a postbronchodilator FEV1 of less than 70% to placebo or tiotropium, a long-acting antimuscarinic agent. Tiotropium reduced the exacerbation rate by 14% compared with placebo and improved quality of life.
Antimuscarinics may be better than beta-2 agonists. Head-to-head comparisons suggest that long-acting antimuscarinic agents are preferable to long-acting beta-2 agonists for preventing COPD exacerbations.53,54
Triple therapy: evidence is mixed. For patients with severe symptomatic COPD and frequent exacerbations, triple therapy with a combination of an inhaled long-acting antimuscarinic agent, an inhaled long-acting beta-2 agonist, and an inhaled corticosteroid has been suggested.
Data to support this practice are limited. In the Canadian Optimal Trial,55 the rate of exacerbations was not different between tiotropium alone, tiotropium plus salmeterol, and triple therapy. However, the rate of hospitalization for severe exacerbation was lower with triple therapy than tiotropium alone. A large, retrospective cohort study also supported triple therapy by finding reduced mortality, hospitalizations, and need for oral corticosteroid bursts compared to combination therapy with an inhaled long-acting beta-2 agonist and an inhaled corticosteroid.56
The drawback of triple therapy is an increased incidence of pneumonia associated with combined beta-2 agonist and corticosteroids, most likely due to the corticosteroid component.51 The risk appears to be higher for higher potency corticosteroids, eg, fluticasone.57
In 2014, the Withdrawal of Inhaled Steroids During Optimised Bronchodilator Management (WISDOM) trial58 randomized nearly 2,500 patients with a history of COPD exacerbation receiving triple therapy consisting of tiotropium, salmeterol, and inhaled fluticasone to either continue treatment or withdraw the corticosteroid for 3 months. The investigators defined an annualized exacerbation rate of 1.2 (ie, a 20% increase) as the upper limit of the confidence interval for an acceptable therapeutic margin of noninferiority. The study showed that the risk of moderate to severe exacerbations with combined tiotropium and salmeterol was noninferior to triple therapy.
Nevertheless, caution is advised when removing the corticosteroid component from triple therapy. The trial demonstrated a worsening in overall health status, some reduction in lung function, and a transient increase in severe exacerbations in the withdrawal group. Patients with increased symptom burden at baseline and a history of severe exacerbations may not be optimal candidates for this strategy.
Roflumilast is effective but has side effects
Roflumilast, an oral phosphodiesterase 4 inhibitor, is an anti-inflammatory drug without bronchodilator properties. In randomized controlled trials, the drug was associated with a 17% reduction in acute exacerbations compared with placebo.59
Adding roflumilast to either a long-acting beta-2 agonist or a long-acting antimuscarinic agent resulted in a 6% to 8% further reduction in the proportion of patients with exacerbation.60,61 Martinez et al61 found that roflumilast added to a regimen of a long-acting beta-2 agonist plus an inhaled corticosteroid reduced moderate to severe exacerbations by 14.2%, even in the presence of tiotropium. Compared with placebo, roflumilast treatment reduced exacerbations necessitating hospitalizations by 23.9%.
The FDA has approved oral roflumilast 500 µg once daily to prevent COPD exacerbations.
Roflumilast is frequently associated with side effects, including gastrointestinal symptoms (chiefly diarrhea), weight loss, and psychiatric effects. A benefit-to-harm study in 2014 concluded that using the drug is only favorable for patients who have a high risk of severe exacerbations, ie, those who have a greater than 22% baseline risk of having at least one exacerbation annually.62
Recommendation. Roflumilast should be reserved for patients who have severe COPD with a chronic bronchitis phenotype (ie, with cough and sputum production) and repeated exacerbations despite an optimal regimen of an inhaled corticosteroid, long-acting beta-2 agonist, and long-acting antimuscarinic agent.
Macrolide antibiotics: Role unclear
Macrolide antibiotics have anti-inflammatory and immunomodulatory activities.
Azithromycin: fewer exacerbations but some side effects. A multicenter trial63 in 1,142 COPD patients randomized to either oral azithromycin 250 mg daily or placebo found a 27% reduction in the risk of COPD exacerbation in the intervention arm. No differences were found between the groups in mortality, hospitalizations, emergency department visits, or respiratory failure. Hearing loss and increased macrolide resistance were noted in the intervention arm. In a secondary subgroup analysis,64 no difference in efficacy was found by sex, history of chronic bronchitis, oxygen use, or concomitant COPD treatment.
The COPD: Influence of Macrolides on Exacerbation Frequency in Patients trial65 helped refine patient selection for macrolide therapy. In this single-center study, 92 patients with COPD and at least three exacerbations during the year prior to enrollment were randomized to receive either azithromycin 500 mg three times weekly or placebo. Exacerbations in the intervention group were markedly reduced (42%) with no difference in hospitalization rate.
The place of macrolide antibiotics in the treatment strategy of COPD is unclear, and they are not currently part of the GOLD guidelines. Still unknown is the incremental benefit of adding them to existing preventive regimens, cardiovascular safety, side effects, and potential effects on the resident microbial flora.
Other antibiotics have also been investigated for efficacy in preventing exacerbations.
Moxifloxacin: fewer exacerbations. The Pulsed Moxifloxacin Usage and Its Long-term Impact on the Reduction of Subsequent Exacerbations study66 randomized more than 1,000 patients with stable COPD to receive either moxifloxacin 400 mg or placebo daily for 5 days repeated every 8 weeks for six courses. Frequent assessment during the treatment period and for 6 months afterward revealed a reduced exacerbation rate in the intervention group but without benefit in hospitalization rate, mortality, lung function, or health status.
Recommendation. Azithromycin (either 250 mg daily or 500 mg three times weekly) can be considered for patients who have repeated COPD exacerbations despite an optimal regimen of an inhaled corticosteroid, inhaled long-acting beta-2 agonist, and inhaled long-acting antimuscarinic agent. The need to continue azithromycin should be reassessed yearly.
Mucolytics
Greatest benefit to patients not taking inhaled corticosteroids. Mucolytic agents help clear airway secretions by reducing viscosity. N-acetylcysteine and carbocysteine (not available in the United States) also have antioxidant properties that may counteract oxidant stress associated with acute COPD exacerbations.
The Bronchitis Randomized on NAC Cost-Utility Study (BRONCUS)67 randomized 523 COPD patients to N-acetylcysteine 600 mg daily or placebo. After 3 years of follow-up, no differences were found in the rate of exacerbations, lung function decline, and quality of life. Subgroup analysis suggested a reduction in exacerbations for patients who were not taking inhaled corticosteroids.
The Effect of Carbocisteine on Acute Exacerbation of Chronic Obstructive Pulmonary Disease (PEACE) study randomized more than 700 patients from multiple centers in China who had COPD and a recent history of exacerbations; they found a 25% lower exacerbation rate over 1 year with carbocysteine vs placebo.68 Most of the patients (83%) were not on inhaled corticosteroids, which complemented findings of the BRONCUS trial.
The Effect of High Dose N-acetylcysteine on Air Trapping and Airway Resistance of COPD (HIACE) study randomized 120 patients with stable COPD in a hospital in Hong Kong to either oral N-acetylcysteine (600 mg twice daily) or placebo and found a reduced exacerbation rate of exacerbations. Patients were matched at baseline for inhaled corticosteroid use.69
In 2014, the Twice Daily N-acetylcysteine 600 mg for Exacerbations of Chronic Obstructive Pulmonary Disease (PANTHEON) study70 randomized 1,006 patients from multiple hospitals in China with a history of moderate to severe COPD and exacerbations to receive either N-acetylcysteine 600 mg twice daily or placebo for 1 year. They found a 22% reduction in exacerbations in the treatment group vs placebo.
GOLD guidelines2 recommend mucolytics for patients with severe COPD and exacerbations when inhaled corticosteroids are not available or affordable.
Recommendation. Mucolytics may be useful for patients with difficulty expectorating and with a history of exacerbations despite appropriate inhaled therapy.
OTHER INTERVENTIONS CAN HELP
Pulmonary rehabilitation provides multiple benefits
Pulmonary rehabilitation increases exercise tolerance and reduces symptom burden in patients with stable COPD. It is also a multidisciplinary effort that may help reinforce adherence to medications, enhance COPD education, and provide closer medical surveillance to patients at high risk for recurrent exacerbations.
A small randomized controlled trial71 prescribed pulmonary rehabilitation on discharge for a COPD exacerbation and found sustainable improvements in exercise capacity and health status after 3 months.
In a later study,72 the same group started pulmonary rehabilitation within a week of hospital discharge and found reduced hospital readmissions over a 3-month period.
Smoking cessation is always worth advocating
A large observational cohort study concluded that current smokers were at a higher risk for COPD exacerbations compared with former smokers.73 Although there are no randomized controlled trials that assess the effects of smoking cessation at the time of COPD exacerbation, we recommend seizing the opportunity to implement this important intervention.
Vaccinations: Influenza and pneumococcal
Influenza vaccination is associated with reduced incidence of hospitalization among patients with cardiopulmonary disease.74 A meta-analysis of randomized clinical trials of influenza vaccination for patients with COPD75 reported significantly fewer exacerbations from vaccination, mostly owing to fewer episodes occurring after 3 to 4 weeks, coinciding with anticipated vaccine-induced immune protection. Furumoto and colleagues76 reported an added benefit of combined vaccination with 23-valent pneumococcal polysaccharide vaccine and influenza vaccine in reducing hospital admissions over influenza vaccination alone. We also recommend providing the 13-valent pneumococcal conjugate vaccine to patients with COPD, particularly for those older than 65, consistent with CDC recommendations.77
In contrast to stable chronic obstructive pulmonary disease (COPD),1 acute exacerbations of COPD pose special management challenges and can significantly increase the risk of morbidity and death and the cost of care.
This review addresses the definition and diagnosis of COPD exacerbations, disease burden and costs, etiology and pathogenesis, and management and prevention strategies.
DEFINITIONS ARE PROBLEMATIC
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines a COPD exacerbation as “an acute event characterized by a worsening of the patient’s respiratory symptoms that is beyond normal day-to-day variations and leads to a change in medication.”2 It further categorizes acute exacerbations by severity:
- Mild—treated with increased frequency of doses of existing medications
- Moderate—treated with corticosteroids or antibiotics, or both
- Severe—requires hospital utilization (either emergency room treatment or admission).
Although descriptive and useful for retrospective analyses, this current definition poses ambiguities for clinicians. Day-to-day variation in symptoms is not routinely assessed, so deviations from baseline may be difficult to detect. Although clinical tools are available for assessing symptoms in stable and exacerbated states (eg, the COPD assessment test3 and the Exacerbations of Chronic Pulmonary Disease Tool [EXACT]4), they have not been widely adopted in daily practice. Also, according to the current definition, the severity of an exacerbation can be classified only after the course of action is determined, so the severity is not helpful for forming a management strategy at bedside. In addition, physicians may have different thresholds for prescribing antibiotics and corticosteroids.
An earlier definition categorized a COPD exacerbation by the presence of its three cardinal symptoms (ie, increased shortness of breath, sputum volume, and purulence):
- Type I—all three symptoms present
- Type II—two symptoms present
- Type III—one symptom present, accompanied by at least one of the following: upper respiratory tract infection within the past 5 days, unexplained fever, increased wheezing or cough, or 20% increased respiratory rate or heart rate from baseline.
This older definition was successfully used in a prospective clinical trial to identify patients who benefited most from antibiotics for COPD exacerbations.5
Despite these caveats regarding a definition, most clinicians agree on the clinical presentation of a patient with COPD exacerbation: ie, having some combination of shortness of breath, increased sputum volume, and purulence. By the same token, patients with COPD who present with symptoms not typical of an exacerbation should be evaluated for another diagnosis. For instance, Tillie-Leblond et al6 reported that 49 (25%) of 197 patients hospitalized with an “unexplained” exacerbation of COPD were eventually diagnosed with pulmonary embolism.
EXACERBATIONS ARE COSTLY
The care of patients with COPD places a great burden on the healthcare system. Using multiple national databases, Ford et al7 estimated that medical costs in the United States in 2010 attributable to COPD and its complications were $32.1 billion.
The largest component of direct healthcare costs of COPD is exacerbations and subsequent hospitalizations.8 Data from a predominantly Medicare population indicate that the annualized mean COPD-related cost for a patient with no exacerbations was $1,425, compared with $12,765 for a patient with severe exacerbations.9 The investigators estimated that reducing exacerbations from two or more to none could save $5,125 per patient per year.
EXACERBATIONS AFFECT HEALTH BEYOND THE EVENT
COPD exacerbations are associated with a faster decline in lung function,10 reduced quality of life,11 and lost workdays.7 A single exacerbation may cause a decline in lung function and health status that may not return to baseline for several months, particularly if another exacerbation occurs within 6 months.12,13 COPD exacerbations have also been linked to poor clinical outcomes, including death.
In a prospective study in 304 men with COPD followed for 5 years, those who had three or more COPD exacerbations annually were four times as likely to die than patients who did not have an exacerbation.14 Nevertheless, the relationship with mortality may not be causal: Brusselle pointed out in an editorial15 that established mortality predictors for COPD do not include exacerbations, and symptomatic patients with COPD without any history of exacerbations are at greater risk of death than those who are asymptomatic but at high risk for exacerbations.
INFECTION + INFLAMMATION = EXACERBATION
An acute COPD exacerbation can be viewed as an acute inflammatory event superimposed on chronic inflammation associated with COPD. Inflammation in the airways increases resistance to air flow with consequent air trapping. Increased resistance and elastic load due to air trapping place respiratory muscles at a mechanical disadvantage and increase the work of breathing.
Infection starts the process
Infections, particularly bacterial and viral, are thought to be the major instigators of COPD exacerbation, although environmental factors such as air pollution may also play a role.16
Airway inflammation is markedly reduced when bacterial infection is eradicated. But if bacterial colonization continues, inflammatory markers remain elevated despite clinical resolution of the exacerbation.17 Desai et al18 found that patients with COPD and chronic bronchitis with bacterial colonization had a larger symptom burden than patients without colonization, even without an exacerbation.
Allergic profile increases risk
Although most studies indicate that infection is the main cause of exacerbations, clinicians should consider other mechanisms of inflammation on an individual basis. COPD exacerbations may be phenotyped by measuring inflammatory markers, perhaps as a starting point for tailored therapies.
Bafadhel et al19 studied 145 patients with COPD over the course of a year and recorded various biomarkers at baseline and during exacerbations. Exacerbations had an inflammatory profile that was predominantly bacterial in 37%, viral in 10%, and eosinophilic in 17%, and had limited changes in the inflammatory profile in 14%. The remaining episodes were combinations of categories. In another study,20 multivariate analysis conducted in two cohorts with COPD found that patients who had an allergic phenotype had more respiratory symptoms and a higher likelihood of COPD exacerbations.
Frequent COPD exacerbations are increasingly recognized as being associated with an asthma-COPD overlap syndrome, consisting of symptoms of increased airflow variability and incompletely reversible airflow obstruction.21
Inflammation as a marker of frequent exacerbations
Evidence is accumulating that supports systemic inflammation as a marker of frequent exacerbations. The Copenhagen Heart Study tested for baseline plasma C-reactive protein, fibrinogen, and white blood cell count in 6,574 stable patients with COPD.22 After multivariable adjustment, they found a significantly higher likelihood of having a subsequent exacerbation in patients who had all three biomarkers elevated (odds ratio [OR] 3.7, 95% confidence interval [CI] 1.9–7.4), even in patients with milder COPD and those without previous exacerbations.
Past exacerbations predict risk
The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints study23 found that a history of acute COPD exacerbation was the single best predictor of future exacerbations. This risk factor remained stable over 3 years and was present across the severity of COPD, ie, patients at lower GOLD stages who had a history of frequent exacerbations were likely to have exacerbations during follow-up.
EXACERBATION INCREASES CARDIOVASCULAR RISK
COPD exacerbations increase the risk of cardiovascular events, particularly myocardial infarction.24 During hospitalization for acute exacerbation of COPD, markers of myocardial injury and heart failure may be elevated and are a predictor of death.25
Patel et al26 measured arterial stiffness (aortic pulse wave velocity, a validated measure of cardiovascular risk) and cardiac biomarkers (troponin and N-terminal B-type natriuretic peptide) at baseline in 98 patients and longitudinally during and after a COPD exacerbation. In addition to increased levels of cardiac biomarkers, they found a significant rise in arterial stiffness during the exacerbation event without return to baseline levels over 35 days of follow-up. The arterial stiffness increase was related to airway inflammation as measured by sputum interleukin 6, particularly in patients with documented lower respiratory tract infection.
Retrospective analysis suggests a reduced all-cause mortality rate in COPD patients who are treated with beta-blockers.27
Recommendation. We recommend that patients already taking a selective beta-blocker continue to do so during a COPD exacerbation.
OUTPATIENT MANAGEMENT
Treatment with a combination of a corticosteroid, antibiotic, and bronchodilator addresses the underlying pathophysiologic processes of an acute exacerbation: inflammation, infection, and airway trapping.
Short course of a corticosteroid improves outcomes
A 10-day systemic course of a corticosteroid prescribed for COPD exacerbation before discharge from the emergency department was found to offer a small advantage over placebo for reducing treatment failure (unscheduled physician visits, return to emergency room for recurrent symptoms) and improving dyspnea scores and lung function.28 Even just a 3-day course improved measures of respiration (forced expiratory volume in the first second of expiration [FEV1] and arterial oxygenation) at days 3 and 10, and reduced treatment failures compared with placebo.29
Corticosteroid prescription should not be taken lightly, because adverse effects are common. In a systematic review, one adverse effect (hyperglycemia, weight gain, or insomnia) occurred for every five people treated.30
Identifying subgroups of patients most likely to benefit from corticosteroid treatment may be helpful. Corticosteroids may delay improvement in patients without eosinophilic inflammation and hasten recovery in those with more than 2% peripheral eosinophils.31 Siva et al32 found that limiting corticosteroids to patients with sputum eosinophilia reduced corticosteroid use and reduced severe exacerbations compared with standard care.32
Recommendation. For an acute exacerbation, we prescribe a short course of corticosteroids (eg, prednisone 40 mg daily for 5 to 7 days). Tapering dosing is probably unnecessary because adrenal insufficiency is uncommon before 2 weeks of corticosteroid exposure. Clinicians should weigh the merits of tapering (reduced corticosteroid exposure) against patient inconvenience and difficulty following complicated instructions.
Antibiotics help, but exact strategy uncertain
Although antibiotic therapy is one of the three pillars of COPD exacerbation management, the optimal antimicrobial agent, duration of therapy, and which patients will benefit remain areas of controversy and research. Thus far, large trials have been unable to definitely show the superiority of one antibiotic over another.33,34
A 1987 randomized controlled trial5 of antibiotic therapy in acute exacerbation of COPD found the greatest benefit to patients who had all three cardinal symptoms (ie, increased shortness of breath, sputum volume, and purulence), with less marked but still significant improvement in patients with two symptoms. In a 2012 multicenter trial35 patients with mild to moderate COPD experiencing an exacerbation were treated with either combined amoxicillin and clavulanate or placebo if they had one of the three cardinal symptoms. The antibiotic group had a significantly higher clinical cure rate at days 9 to 11 (74.1% vs 59.9%) as well as a longer time until the next exacerbation (233 vs 160 days).
Recommendation. Optimal antibiotic management of COPD exacerbations may also depend on risk factors. For patients with at least two cardinal symptoms, we favor a scheme akin to one proposed for treating community-acquired pneumonia (Table 1).16,36
INPATIENT MANAGEMENT
Corticosteroids improve outcomes
A Department of Veterans Affairs cooperative trial37 randomized 271 patients hospitalized with COPD exacerbation to receive either corticosteroids (intravenous followed by oral) or placebo for either 2 weeks or 8 weeks. Corticosteroid recipients had lower rates of treatment failure at 30 and 90 days, defined as death from any cause, need for mechanical ventilation, readmission, or intensification of pharmacologic therapy. Corticosteroid therapy also reduced hospital length of stay and improved the rate of recovery. The longer corticosteroid course was associated with a higher rate of adverse effects.
Oral corticosteroids not inferior to intravenous
Using the same end point of treatment failure as the Veterans Affairs cooperative trial, deJong et al38 demonstrated that prednisone 60 mg by mouth was not inferior to intravenous prednisone. Neither trial demonstrated a difference in mortality between corticosteroid use and placebo.
Short course of a corticosteroid not inferior to a long course
In 2013, the Reduction in the Use of Corticosteroids in Exacerbated COPD (REDUCE) trial39 randomized 314 patients presenting with an acute COPD exacerbation (92% requiring hospital admission) to oral prednisone 40 mg daily for either 5 days or 14 days. They found that the short course was noninferior in preventing exacerbations over the ensuing 6 months in terms of death and the need for mechanical ventilation.
Recommendation. Our threshold for initiating systemic corticosteroid therapy is lower in hospitalized patients than in outpatients. We recommend the regimen of the REDUCE trial: prednisone 40 mg daily for 5 days.
Corticosteroids for patients on ventilatory support
Severe COPD exacerbations requiring admission to intensive care are a significant source of morbidity and mortality, and the strategy of corticosteroid treatment is still under investigation.
Intravenous corticosteroids are effective. A multicenter trial40 in 354 patients requiring either invasive or noninvasive mechanical ventilation randomized them to treatment with either intravenous methylprednisolone (tapered) or placebo. Treatment was associated with fewer mechanical ventilation days and a lower rate of noninvasive ventilation failure.
Low-dose oral corticosteroids ineffective. In contrast, an open-label trial41 of patients requiring ventilatory support and randomized to either oral prednisone (1 mg/kg for up to 10 days) or usual care found no difference in intensive care length of stay or noninvasive ventilation failure. This study used the oral route and smaller doses, and its open-label design might have introduced bias.
Lower-dose steroids better than high-dose. A 2014 cohort study of 17,239 patients admitted to the ICU with acute exacerbations of COPD evaluated outcomes of treatment with high methylprednisolone dosages (> 240 mg per day) vs lower dosages, using propensity score matching.42 No mortality difference was found between the groups. The lower dosage group (median methylprednisolone dose 100 mg per day) had shorter hospital and intensive care unit stays, shorter duration of noninvasive positive pressure ventilation, less need for insulin therapy, and fewer fungal infections.
Antibiotics for hospitalized patients
Only scarce data are available on the use of antibiotics for patients hospitalized with COPD exacerbation. In a study of patients hospitalized with COPD exacerbations, adding doxycycline to corticosteroids led to better clinical success and cure rates at 10 days compared with placebo, but the primary end point of clinical success at 30 days was not different between the two groups.43
BRONCHODILATORS: A MAINSTAY OF COPD TREATMENT
Bronchodilators are an important part of treatment of COPD exacerbations in inpatient and outpatient settings.
Nebulized beta-2 agonists are given every 1 to 4 hours. Albuterol at a 2.5-mg dose in each nebulization was found to be as effective as 5 mg for length of hospital stay and recovery of lung function in patients with an acute exacerbation of COPD.44
Adding an anticholinergic may help. Nebulized anticholinergics can be given alone or combined with beta-2 agonists. Whether long-acting bronchodilators should be used to manage COPD patients hospitalized with an exacerbation requires further inquiry. In an observational study with historical controls, Drescher and colleagues45 found cost savings and shorter hospital stays if tiotropium (a long-acting anticholinergic) was added to the respiratory care protocol, which also included formoterol (a long-acting beta-2 agonist).
OXYGEN: TITRATED APPROACH SAFER
Oxygen should be supplied during a COPD exacerbation to ensure adequate oxyhemoglobin saturation. Caution is needed to avoid hyperoxemic hypercapnia, particularly in patients with severe COPD and propensity to ventilatory failure. The routine administration of oxygen at high concentrations during a COPD exacerbation has been associated with a higher mortality rate than with a titrated oxygen approach.46 Long-term oxygen treatment started at discharge or as outpatient therapy is associated with reduced hospital admissions and shorter hospital stays for acute exacerbations of COPD.47
VENTILATION SUPPORT
Noninvasive positive-pressure ventilation is a useful adjunct to treatment of COPD exacerbations with evidence of ventilatory failure (ie, acute respiratory acidosis), helping to offset the work of breathing until respiratory system mechanics improve. Keenan et al48 reviewed 15 randomized controlled trials, involving 636 patients, of noninvasive positive-pressure ventilation in the setting of COPD exacerbation. They concluded that noninvasive positive-pressure ventilation reduced the in-hospital mortality rate and length of stay compared with standard therapy. Noninvasive positive-pressure ventilation is most useful in patients with severe COPD exacerbations and acute respiratory acidosis (pH < 7.35).49
Intubation and mechanical ventilation. Although no standards exist for determining which COPD exacerbations may be too severe for noninvasive positive-pressure ventilation, intubation is clearly indicated for impending respiratory failure or hemodynamic instability. Other factors to consider include the greater likelihood of noninvasive positive-pressure ventilation failure in patients with severe respiratory acidosis (pH < 7.25 is associated with a > 50% failure rate) and in those with no improvement in acidosis or respiratory rate during the first hour after initiation of noninvasive positive-pressure ventilation.50
PREVENTING EXACERBATIONS
Recent data indicate that COPD exacerbations can often be prevented (Table 2).
Inhaled pharmacotherapy
Inhaled pharmacotherapeutic agents, singly or in combination, reduce the frequency of COPD exacerbations.
Combined long-acting beta-2 agonist and corticosteroid is better than single-agent therapy. In 2007, the Towards a Revolution in COPD Health (TORCH) trial51 evaluated outpatient therapy in more than 6,000 patients worldwide with either an inhaled long-acting beta-2 agonist (salmeterol), an inhaled corticosteroid (fluticasone), both drugs in combination, or placebo. Patients had baseline prebronchodilator FEV1 of less than 60% and were followed for 3 years. No difference was found between the groups in the primary end point of deaths, but the annualized rate of moderate to severe exacerbations was reduced by 25% in the group that received combination therapy vs placebo. Combination therapy showed superior efficacy over individual drug therapy in preventing exacerbations. Treatment with the inhaled corticosteroid, whether alone or in combination with salmeterol, increased the risk of pneumonia.
A long-acting antimuscarinic agent is better than placebo. In 2008, the Understanding Potential Long-Term Impacts on Function With Tiotropium (UPLIFT) trial52 randomized nearly 6,000 patients with COPD and a postbronchodilator FEV1 of less than 70% to placebo or tiotropium, a long-acting antimuscarinic agent. Tiotropium reduced the exacerbation rate by 14% compared with placebo and improved quality of life.
Antimuscarinics may be better than beta-2 agonists. Head-to-head comparisons suggest that long-acting antimuscarinic agents are preferable to long-acting beta-2 agonists for preventing COPD exacerbations.53,54
Triple therapy: evidence is mixed. For patients with severe symptomatic COPD and frequent exacerbations, triple therapy with a combination of an inhaled long-acting antimuscarinic agent, an inhaled long-acting beta-2 agonist, and an inhaled corticosteroid has been suggested.
Data to support this practice are limited. In the Canadian Optimal Trial,55 the rate of exacerbations was not different between tiotropium alone, tiotropium plus salmeterol, and triple therapy. However, the rate of hospitalization for severe exacerbation was lower with triple therapy than tiotropium alone. A large, retrospective cohort study also supported triple therapy by finding reduced mortality, hospitalizations, and need for oral corticosteroid bursts compared to combination therapy with an inhaled long-acting beta-2 agonist and an inhaled corticosteroid.56
The drawback of triple therapy is an increased incidence of pneumonia associated with combined beta-2 agonist and corticosteroids, most likely due to the corticosteroid component.51 The risk appears to be higher for higher potency corticosteroids, eg, fluticasone.57
In 2014, the Withdrawal of Inhaled Steroids During Optimised Bronchodilator Management (WISDOM) trial58 randomized nearly 2,500 patients with a history of COPD exacerbation receiving triple therapy consisting of tiotropium, salmeterol, and inhaled fluticasone to either continue treatment or withdraw the corticosteroid for 3 months. The investigators defined an annualized exacerbation rate of 1.2 (ie, a 20% increase) as the upper limit of the confidence interval for an acceptable therapeutic margin of noninferiority. The study showed that the risk of moderate to severe exacerbations with combined tiotropium and salmeterol was noninferior to triple therapy.
Nevertheless, caution is advised when removing the corticosteroid component from triple therapy. The trial demonstrated a worsening in overall health status, some reduction in lung function, and a transient increase in severe exacerbations in the withdrawal group. Patients with increased symptom burden at baseline and a history of severe exacerbations may not be optimal candidates for this strategy.
Roflumilast is effective but has side effects
Roflumilast, an oral phosphodiesterase 4 inhibitor, is an anti-inflammatory drug without bronchodilator properties. In randomized controlled trials, the drug was associated with a 17% reduction in acute exacerbations compared with placebo.59
Adding roflumilast to either a long-acting beta-2 agonist or a long-acting antimuscarinic agent resulted in a 6% to 8% further reduction in the proportion of patients with exacerbation.60,61 Martinez et al61 found that roflumilast added to a regimen of a long-acting beta-2 agonist plus an inhaled corticosteroid reduced moderate to severe exacerbations by 14.2%, even in the presence of tiotropium. Compared with placebo, roflumilast treatment reduced exacerbations necessitating hospitalizations by 23.9%.
The FDA has approved oral roflumilast 500 µg once daily to prevent COPD exacerbations.
Roflumilast is frequently associated with side effects, including gastrointestinal symptoms (chiefly diarrhea), weight loss, and psychiatric effects. A benefit-to-harm study in 2014 concluded that using the drug is only favorable for patients who have a high risk of severe exacerbations, ie, those who have a greater than 22% baseline risk of having at least one exacerbation annually.62
Recommendation. Roflumilast should be reserved for patients who have severe COPD with a chronic bronchitis phenotype (ie, with cough and sputum production) and repeated exacerbations despite an optimal regimen of an inhaled corticosteroid, long-acting beta-2 agonist, and long-acting antimuscarinic agent.
Macrolide antibiotics: Role unclear
Macrolide antibiotics have anti-inflammatory and immunomodulatory activities.
Azithromycin: fewer exacerbations but some side effects. A multicenter trial63 in 1,142 COPD patients randomized to either oral azithromycin 250 mg daily or placebo found a 27% reduction in the risk of COPD exacerbation in the intervention arm. No differences were found between the groups in mortality, hospitalizations, emergency department visits, or respiratory failure. Hearing loss and increased macrolide resistance were noted in the intervention arm. In a secondary subgroup analysis,64 no difference in efficacy was found by sex, history of chronic bronchitis, oxygen use, or concomitant COPD treatment.
The COPD: Influence of Macrolides on Exacerbation Frequency in Patients trial65 helped refine patient selection for macrolide therapy. In this single-center study, 92 patients with COPD and at least three exacerbations during the year prior to enrollment were randomized to receive either azithromycin 500 mg three times weekly or placebo. Exacerbations in the intervention group were markedly reduced (42%) with no difference in hospitalization rate.
The place of macrolide antibiotics in the treatment strategy of COPD is unclear, and they are not currently part of the GOLD guidelines. Still unknown is the incremental benefit of adding them to existing preventive regimens, cardiovascular safety, side effects, and potential effects on the resident microbial flora.
Other antibiotics have also been investigated for efficacy in preventing exacerbations.
Moxifloxacin: fewer exacerbations. The Pulsed Moxifloxacin Usage and Its Long-term Impact on the Reduction of Subsequent Exacerbations study66 randomized more than 1,000 patients with stable COPD to receive either moxifloxacin 400 mg or placebo daily for 5 days repeated every 8 weeks for six courses. Frequent assessment during the treatment period and for 6 months afterward revealed a reduced exacerbation rate in the intervention group but without benefit in hospitalization rate, mortality, lung function, or health status.
Recommendation. Azithromycin (either 250 mg daily or 500 mg three times weekly) can be considered for patients who have repeated COPD exacerbations despite an optimal regimen of an inhaled corticosteroid, inhaled long-acting beta-2 agonist, and inhaled long-acting antimuscarinic agent. The need to continue azithromycin should be reassessed yearly.
Mucolytics
Greatest benefit to patients not taking inhaled corticosteroids. Mucolytic agents help clear airway secretions by reducing viscosity. N-acetylcysteine and carbocysteine (not available in the United States) also have antioxidant properties that may counteract oxidant stress associated with acute COPD exacerbations.
The Bronchitis Randomized on NAC Cost-Utility Study (BRONCUS)67 randomized 523 COPD patients to N-acetylcysteine 600 mg daily or placebo. After 3 years of follow-up, no differences were found in the rate of exacerbations, lung function decline, and quality of life. Subgroup analysis suggested a reduction in exacerbations for patients who were not taking inhaled corticosteroids.
The Effect of Carbocisteine on Acute Exacerbation of Chronic Obstructive Pulmonary Disease (PEACE) study randomized more than 700 patients from multiple centers in China who had COPD and a recent history of exacerbations; they found a 25% lower exacerbation rate over 1 year with carbocysteine vs placebo.68 Most of the patients (83%) were not on inhaled corticosteroids, which complemented findings of the BRONCUS trial.
The Effect of High Dose N-acetylcysteine on Air Trapping and Airway Resistance of COPD (HIACE) study randomized 120 patients with stable COPD in a hospital in Hong Kong to either oral N-acetylcysteine (600 mg twice daily) or placebo and found a reduced exacerbation rate of exacerbations. Patients were matched at baseline for inhaled corticosteroid use.69
In 2014, the Twice Daily N-acetylcysteine 600 mg for Exacerbations of Chronic Obstructive Pulmonary Disease (PANTHEON) study70 randomized 1,006 patients from multiple hospitals in China with a history of moderate to severe COPD and exacerbations to receive either N-acetylcysteine 600 mg twice daily or placebo for 1 year. They found a 22% reduction in exacerbations in the treatment group vs placebo.
GOLD guidelines2 recommend mucolytics for patients with severe COPD and exacerbations when inhaled corticosteroids are not available or affordable.
Recommendation. Mucolytics may be useful for patients with difficulty expectorating and with a history of exacerbations despite appropriate inhaled therapy.
OTHER INTERVENTIONS CAN HELP
Pulmonary rehabilitation provides multiple benefits
Pulmonary rehabilitation increases exercise tolerance and reduces symptom burden in patients with stable COPD. It is also a multidisciplinary effort that may help reinforce adherence to medications, enhance COPD education, and provide closer medical surveillance to patients at high risk for recurrent exacerbations.
A small randomized controlled trial71 prescribed pulmonary rehabilitation on discharge for a COPD exacerbation and found sustainable improvements in exercise capacity and health status after 3 months.
In a later study,72 the same group started pulmonary rehabilitation within a week of hospital discharge and found reduced hospital readmissions over a 3-month period.
Smoking cessation is always worth advocating
A large observational cohort study concluded that current smokers were at a higher risk for COPD exacerbations compared with former smokers.73 Although there are no randomized controlled trials that assess the effects of smoking cessation at the time of COPD exacerbation, we recommend seizing the opportunity to implement this important intervention.
Vaccinations: Influenza and pneumococcal
Influenza vaccination is associated with reduced incidence of hospitalization among patients with cardiopulmonary disease.74 A meta-analysis of randomized clinical trials of influenza vaccination for patients with COPD75 reported significantly fewer exacerbations from vaccination, mostly owing to fewer episodes occurring after 3 to 4 weeks, coinciding with anticipated vaccine-induced immune protection. Furumoto and colleagues76 reported an added benefit of combined vaccination with 23-valent pneumococcal polysaccharide vaccine and influenza vaccine in reducing hospital admissions over influenza vaccination alone. We also recommend providing the 13-valent pneumococcal conjugate vaccine to patients with COPD, particularly for those older than 65, consistent with CDC recommendations.77
- Hatipoglu U, Aboussouan LS. Chronic obstructive pulmonary disease: an update for the primary physician. Cleve Clin J Med 2014; 81:373–383.
- Global initiative for chronic obstructive lung disease. Pocket Guide to COPD Diagnosis, Management, and Prevention. A Guide for Health Care Professionals. Updated 2016. http://www.goldcopd.org/uploads/users/files/WatermarkedPocket%20Guide%202016(2).pdf. Accessed March 7, 2016.
- Gupta N, Pinto LM, Morogan A, Bourbeau J. The COPD assessment test: a systematic review. Eur Respir J 2014; 44:873–884.
- Leidy NK, Wilcox TK, Jones PW, et al; EXACT-PRO Study Group. Development of the EXAcerbations of chronic obstructive pulmonary disease tool (EXACT): a patient-reported outcome (PRO) measure. Value Health 2010; 13:965–975.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106:196–204.
- Tillie-Leblond I, Marquette CH, Perez T, et al. Pulmonary embolism in patients with unexplained exacerbation of chronic obstructive pulmonary disease: prevalence and risk factors. Ann Intern Med 2006; 144:390–396.
- Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest 2015; 147:31–45.
- Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD 2010; 7:214–228.
- Pasquale MK, Sun SX, Song F, Hartnett HJ, Stemkowski SA. Impact of exacerbations on health care cost and resource utilization in chronic obstructive pulmonary disease patients with chronic bronchitis from a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis 2012; 7:757–764.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57:847–852.
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157:1418–1422.
- Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161:1608–1613.
- Spencer S, Calverley PM, Sherwood Burge P, Jones PW; ISOLDE Study Group, Inhaled Steroids in Obstructive Lung Disease. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163:122–128.
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60:925–931.
- Brusselle G. Why doesn’t reducing exacerbations decrease COPD mortality? Lancet Respir Med 2014; 2:681–683.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359:2355–2365.
- White AJ, Gompertz S, Bayley DL, et al. Resolution of bronchial inflammation is related to bacterial eradication following treatment of exacerbations of chronic bronchitis. Thorax 2003; 58:680–685.
- Desai H, Eschberger K, Wrona C, et al. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014; 11:303–309.
- Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 2011; 184:662–671.
- Jamieson DB, Matsui EC, Belli A, et al. Effects of allergic phenotype on respiratory symptoms and exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 188:187–192.
- Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 2009; 64:728–735.
- Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA 2013; 309:2353–2361.
- Hurst JR, Vestbo J, Anzueto A, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363:1128–1138.
- Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 2010; 137:1091–1097.
- Chang CL, Robinson SC, Mills GD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax 2011; 66:764–768.
- Patel AR, Kowlessar BS, Donaldson GC, et al. Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 188:1091–1099.
- Dransfield MT, Rowe SM, Johnson JE, Bailey WC, Gerald LB. Use of beta blockers and the risk of death in hospitalised patients with acute exacerbations of COPD. Thorax 2008; 63:301–305.
- Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996; 154:407–412.
- Aaron SD, Vandemheen KL, Hebert P, et al. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease. N Engl J Med 2003; 348:2618–2625.
- Walters JA, Gibson PG, Wood-Baker R, Hannay M, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2009; 1:CD001288.
- Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2012; 186:48–55.
- Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J 2007; 29:906–913.
- Wilson R, Allegra L, Huchon G, et al; MOSAIC Study Group. Short-term and long-term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis. Chest 2004; 125:953–964.
- Wilson R, Anzueto A, Miravitlles M, et al. Moxifloxacin versus amoxicillin/clavulanic acid in outpatient acute exacerbations of COPD: MAESTRAL results. Eur Respir J 2012; 40:17–27.
- Llor C, Moragas A, Hernandez S, Bayona C, Miravitlles M. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:716–723.
- Anzueto A. Primary care management of chronic obstructive pulmonary disease to reduce exacerbations and their consequences. Am J Med Sci 2010; 340:309–318.
- Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999; 340:1941–1947.
- de Jong YP, Uil SM, Grotjohan HP, Postma DS, Kerstjens HA, van den Berg JW. Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double-blind study. Chest 2007; 132:1741–1747.
- Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013; 309:2223–2231.
- Alia I, de la Cal MA, Esteban A, et al. Efficacy of corticosteroid therapy in patients with an acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. Arch Intern Med 2011; 171:1939–1946.
- Abroug F, Ouanes-Besbes L, Fkih-Hassen M, et al. Prednisone in COPD exacerbation requiring ventilatory support: an open-label randomised evaluation. Eur Respir J 2014; 43:717–724.
- Kiser TH, Allen RR, Valuck RJ, Moss M, Vandivier RW. Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 189:1052–1064.
- Daniels JM, Snijders D, de Graaff CS, Vlaspolder F, Jansen HM, Boersma WG. Antibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181:150–157.
- Nair S, Thomas E, Pearson SB, Henry MT. A randomized controlled trial to assess the optimal dose and effect of nebulized albuterol in acute exacerbations of COPD. Chest 2005; 128:48–54.
- Drescher GS, Carnathan BJ, Imus S, Colice GL. Incorporating tiotropium into a respiratory therapist-directed bronchodilator protocol for managing in-patients with COPD exacerbations decreases bronchodilator costs. Respir Care 2008; 53:1678–1684.
- Austin MA, Wills KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ 2010; 341:c5462.
- Ringbaek TJ, Viskum K, Lange P. Does long-term oxygen therapy reduce hospitalisation in hypoxaemic chronic obstructive pulmonary disease? Eur Respir J 2002; 20:38–42.
- Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med 2003; 138:861–870.
- Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest 2008; 133:756–766.
- Sinuff T, Keenan SP; Department of Medicine, McMaster University. Clinical practice guideline for the use of noninvasive positive pressure ventilation in COPD patients with acute respiratory failure. J Crit Care 2004; 19:82–91.
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–789.
- Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359:1543–1554.
- Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011; 364:1093–1103.
- Decramer ML, Chapman KR, Dahl R, et al; INVIGORATE investigators. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med 2013; 1:524–533.
- Aaron SD, Vandemheen KL, Fergusson D, et al; Canadian Thoracic Society/Canadian Respiratory Clinical Research Consortium. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2007; 146:545–555.
- Short PM, Williamson PA, Elder DH, Lipworth SI, Schembri S, Lipworth BJ. The impact of tiotropium on mortality and exacerbations when added to inhaled corticosteroids and long-acting beta-agonist therapy in COPD. Chest 2012; 141:81–86.
- Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 2013; 68:1029–1036.
- Magnussen H, Disse B, Rodriguez-Roisin R, et al; WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med 2014; 371:1285–1294.
- Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009; 374:685–694.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009; 374:695–703.
- Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015; 385:857–866.
- Yu T, Fain K, Boyd CM, et al. Benefits and harms of roflumilast in moderate to severe COPD. Thorax 2014; 69:616–622.
- Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365:689–698.
- Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med 2014; 189:1503–1508.
- Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2014; 2:361–368.
- Sethi S, Jones PW, Theron MS, et al; PULSE Study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res 2010; 11:10.
- Decramer M, Rutten-van Molken M, Dekhuijzen PN, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized On NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet 2005; 365:1552–1560.
- Zheng JP, Kang J, Huang SG, et al. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE study): a randomised placebo-controlled study. Lancet 2008; 371:2013–2018.
- Tse HN, Raiteri L, Wong KY, et al. High-dose N-acetylcysteine in stable COPD: the 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest 2013; 144:106–118.
- Zheng JP, Wen FQ, Bai CX, et al; PANTHEON study group. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med 2014; 2:187–194.
- Man WD, Polkey MI, Donaldson N, Gray BJ, Moxham J. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ 2004; 329:1209.
- Seymour JM, Moore L, Jolley CJ, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010; 65:423–428.
- Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med 2009; 24:457–463.
- Seo YB, Hong KW, Kim IS, et al. Effectiveness of the influenza vaccine at preventing hospitalization due to acute lower respiratory infection and exacerbation of chronic cardiopulmonary disease in Korea during 2010-2011. Vaccine 2013; 31:1426–1430.
- Poole PJ, Chacko E, Wood-Baker RW, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; 1:CD002733.
- Furumoto A, Ohkusa Y, Chen M, et al. Additive effect of pneumococcal vaccine and influenza vaccine on acute exacerbation in patients with chronic lung disease. Vaccine 2008; 26:4284–4289.
- Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822–825.
- Hatipoglu U, Aboussouan LS. Chronic obstructive pulmonary disease: an update for the primary physician. Cleve Clin J Med 2014; 81:373–383.
- Global initiative for chronic obstructive lung disease. Pocket Guide to COPD Diagnosis, Management, and Prevention. A Guide for Health Care Professionals. Updated 2016. http://www.goldcopd.org/uploads/users/files/WatermarkedPocket%20Guide%202016(2).pdf. Accessed March 7, 2016.
- Gupta N, Pinto LM, Morogan A, Bourbeau J. The COPD assessment test: a systematic review. Eur Respir J 2014; 44:873–884.
- Leidy NK, Wilcox TK, Jones PW, et al; EXACT-PRO Study Group. Development of the EXAcerbations of chronic obstructive pulmonary disease tool (EXACT): a patient-reported outcome (PRO) measure. Value Health 2010; 13:965–975.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106:196–204.
- Tillie-Leblond I, Marquette CH, Perez T, et al. Pulmonary embolism in patients with unexplained exacerbation of chronic obstructive pulmonary disease: prevalence and risk factors. Ann Intern Med 2006; 144:390–396.
- Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest 2015; 147:31–45.
- Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD 2010; 7:214–228.
- Pasquale MK, Sun SX, Song F, Hartnett HJ, Stemkowski SA. Impact of exacerbations on health care cost and resource utilization in chronic obstructive pulmonary disease patients with chronic bronchitis from a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis 2012; 7:757–764.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57:847–852.
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157:1418–1422.
- Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161:1608–1613.
- Spencer S, Calverley PM, Sherwood Burge P, Jones PW; ISOLDE Study Group, Inhaled Steroids in Obstructive Lung Disease. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163:122–128.
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60:925–931.
- Brusselle G. Why doesn’t reducing exacerbations decrease COPD mortality? Lancet Respir Med 2014; 2:681–683.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359:2355–2365.
- White AJ, Gompertz S, Bayley DL, et al. Resolution of bronchial inflammation is related to bacterial eradication following treatment of exacerbations of chronic bronchitis. Thorax 2003; 58:680–685.
- Desai H, Eschberger K, Wrona C, et al. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014; 11:303–309.
- Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 2011; 184:662–671.
- Jamieson DB, Matsui EC, Belli A, et al. Effects of allergic phenotype on respiratory symptoms and exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 188:187–192.
- Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 2009; 64:728–735.
- Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA 2013; 309:2353–2361.
- Hurst JR, Vestbo J, Anzueto A, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363:1128–1138.
- Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 2010; 137:1091–1097.
- Chang CL, Robinson SC, Mills GD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax 2011; 66:764–768.
- Patel AR, Kowlessar BS, Donaldson GC, et al. Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 188:1091–1099.
- Dransfield MT, Rowe SM, Johnson JE, Bailey WC, Gerald LB. Use of beta blockers and the risk of death in hospitalised patients with acute exacerbations of COPD. Thorax 2008; 63:301–305.
- Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996; 154:407–412.
- Aaron SD, Vandemheen KL, Hebert P, et al. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease. N Engl J Med 2003; 348:2618–2625.
- Walters JA, Gibson PG, Wood-Baker R, Hannay M, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2009; 1:CD001288.
- Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2012; 186:48–55.
- Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J 2007; 29:906–913.
- Wilson R, Allegra L, Huchon G, et al; MOSAIC Study Group. Short-term and long-term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis. Chest 2004; 125:953–964.
- Wilson R, Anzueto A, Miravitlles M, et al. Moxifloxacin versus amoxicillin/clavulanic acid in outpatient acute exacerbations of COPD: MAESTRAL results. Eur Respir J 2012; 40:17–27.
- Llor C, Moragas A, Hernandez S, Bayona C, Miravitlles M. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:716–723.
- Anzueto A. Primary care management of chronic obstructive pulmonary disease to reduce exacerbations and their consequences. Am J Med Sci 2010; 340:309–318.
- Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999; 340:1941–1947.
- de Jong YP, Uil SM, Grotjohan HP, Postma DS, Kerstjens HA, van den Berg JW. Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double-blind study. Chest 2007; 132:1741–1747.
- Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013; 309:2223–2231.
- Alia I, de la Cal MA, Esteban A, et al. Efficacy of corticosteroid therapy in patients with an acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. Arch Intern Med 2011; 171:1939–1946.
- Abroug F, Ouanes-Besbes L, Fkih-Hassen M, et al. Prednisone in COPD exacerbation requiring ventilatory support: an open-label randomised evaluation. Eur Respir J 2014; 43:717–724.
- Kiser TH, Allen RR, Valuck RJ, Moss M, Vandivier RW. Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 189:1052–1064.
- Daniels JM, Snijders D, de Graaff CS, Vlaspolder F, Jansen HM, Boersma WG. Antibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181:150–157.
- Nair S, Thomas E, Pearson SB, Henry MT. A randomized controlled trial to assess the optimal dose and effect of nebulized albuterol in acute exacerbations of COPD. Chest 2005; 128:48–54.
- Drescher GS, Carnathan BJ, Imus S, Colice GL. Incorporating tiotropium into a respiratory therapist-directed bronchodilator protocol for managing in-patients with COPD exacerbations decreases bronchodilator costs. Respir Care 2008; 53:1678–1684.
- Austin MA, Wills KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ 2010; 341:c5462.
- Ringbaek TJ, Viskum K, Lange P. Does long-term oxygen therapy reduce hospitalisation in hypoxaemic chronic obstructive pulmonary disease? Eur Respir J 2002; 20:38–42.
- Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med 2003; 138:861–870.
- Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest 2008; 133:756–766.
- Sinuff T, Keenan SP; Department of Medicine, McMaster University. Clinical practice guideline for the use of noninvasive positive pressure ventilation in COPD patients with acute respiratory failure. J Crit Care 2004; 19:82–91.
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–789.
- Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359:1543–1554.
- Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011; 364:1093–1103.
- Decramer ML, Chapman KR, Dahl R, et al; INVIGORATE investigators. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med 2013; 1:524–533.
- Aaron SD, Vandemheen KL, Fergusson D, et al; Canadian Thoracic Society/Canadian Respiratory Clinical Research Consortium. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2007; 146:545–555.
- Short PM, Williamson PA, Elder DH, Lipworth SI, Schembri S, Lipworth BJ. The impact of tiotropium on mortality and exacerbations when added to inhaled corticosteroids and long-acting beta-agonist therapy in COPD. Chest 2012; 141:81–86.
- Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 2013; 68:1029–1036.
- Magnussen H, Disse B, Rodriguez-Roisin R, et al; WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med 2014; 371:1285–1294.
- Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009; 374:685–694.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009; 374:695–703.
- Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015; 385:857–866.
- Yu T, Fain K, Boyd CM, et al. Benefits and harms of roflumilast in moderate to severe COPD. Thorax 2014; 69:616–622.
- Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365:689–698.
- Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med 2014; 189:1503–1508.
- Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2014; 2:361–368.
- Sethi S, Jones PW, Theron MS, et al; PULSE Study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res 2010; 11:10.
- Decramer M, Rutten-van Molken M, Dekhuijzen PN, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized On NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet 2005; 365:1552–1560.
- Zheng JP, Kang J, Huang SG, et al. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE study): a randomised placebo-controlled study. Lancet 2008; 371:2013–2018.
- Tse HN, Raiteri L, Wong KY, et al. High-dose N-acetylcysteine in stable COPD: the 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest 2013; 144:106–118.
- Zheng JP, Wen FQ, Bai CX, et al; PANTHEON study group. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med 2014; 2:187–194.
- Man WD, Polkey MI, Donaldson N, Gray BJ, Moxham J. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ 2004; 329:1209.
- Seymour JM, Moore L, Jolley CJ, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010; 65:423–428.
- Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med 2009; 24:457–463.
- Seo YB, Hong KW, Kim IS, et al. Effectiveness of the influenza vaccine at preventing hospitalization due to acute lower respiratory infection and exacerbation of chronic cardiopulmonary disease in Korea during 2010-2011. Vaccine 2013; 31:1426–1430.
- Poole PJ, Chacko E, Wood-Baker RW, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; 1:CD002733.
- Furumoto A, Ohkusa Y, Chen M, et al. Additive effect of pneumococcal vaccine and influenza vaccine on acute exacerbation in patients with chronic lung disease. Vaccine 2008; 26:4284–4289.
- Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822–825.
KEY POINTS
- COPD exacerbations usually start with an infection.
- A short course of corticosteroids (eg, prednisone 40 mg daily for 5 to 7 days) improves outcomes with low risk.
- The choice of antibiotic depends on severity and frequency of exacerbations and the patient’s age and condition.
- Inhaled albuterol 2.5 mg, every 1 to 4 hours, should be prescribed with or without a nebulized anticholinergic.
- Ventilation support is important for patients with acute respiratory acidosis (pH < 7.35).
- Exacerbations can be prevented with some combination of inhaled agents (long-acting beta-2 agonist, corticosteroid, long-acting antimuscarinic), roflumilast (an oral phosphodiesterase inhibitor), and a mucolytic, depending on the patient’s needs.
Chronic obstructive pulmonary disease: An update for the primary physician
Chronic obstructive pulmonary disease (COPD) has seen several changes in its assessment and treatment in recent years, reflecting advances in our understanding of this common and serious disease.
This review updates busy practitioners on the major advances, including new assessment tools and new therapies.
COMMON AND INCREASING
COPD is the third leading cause of death in the United States, behind heart disease and cancer,1 and of the top five (the others being stroke and accidents), it is the only one that increased in incidence between 2007 and 2010.2 The 11th leading cause of disability-adjusted life years worldwide in 2002, COPD is projected to become the seventh by the year 2030.3
CHARACTERIZED BY OBSTRUCTION
COPD is characterized by persistent and progressive airflow obstruction associated with chronic airway inflammation in response to noxious particles and gases. Disease of the small airways (inflammation, mucus plugging, and fibrosis) and parenchymal destruction (emphysema) limit the flow of air.
COPD is diagnosed by spirometry—specifically, a ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) of less than 0.7 after a bronchodilator is given. The severity of airflow limitation is revealed by the FEV1 as a percent of the predicted value.
Cigarette smoking is the major cause of COPD, but the prevalence of COPD is 6.6% in people who have never smoked, and one-fourth of COPD patients in the United States have never smoked.4
GOLDEN GOALS: FEWER SYMPTOMS, LOWER RISK
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) periodically issues evidence-based statements on how to prevent and treat COPD.
In its 2013 update,5 GOLD suggested two goals: improving symptoms and reducing the risk of death, exacerbations, progression of disease, and treatment-related adverse effects. The latter goal—reducing risk—is relatively new.
Exacerbations are acute inflammatory events superimposed on chronic inflammation. The inflammation is often brought on by infection6 and increases the risk of death7 and the risk of a faster decline in lung function.8
Exacerbations may characterize a phenotype of COPD. The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) analyzed the frequency of COPD exacerbations and associated factors in 2,138 patients with COPD over a period of 3 years.9 Although patients with more severe obstruction tended to have more exacerbations, some patients appeared susceptible to exacerbations irrespective of the severity of obstruction. The best predictor of exacerbations was a history of exacerbations.
HOW DO I ASSESS A PATIENT WITH COPD ON PRESENTATION?
Markers of airflow obstruction such as the FEV1 do not correlate strongly with exertional capacity and health status in patients with COPD.10,11
The BODE index (body mass index, obstruction, dyspnea score, and exercise oximetry) takes into account the multidimensional nature of COPD. It performs better than the FEV1 in predicting the risk of death.12 The propensity for exacerbations and comorbidities further modulates outcome.
Assessing symptoms
The modified British Medical Research Council (mMRC) dyspnea scale, based on work by Fletcher in 1952,13 has five grades, numbered 0 through 4:
- Grade 0—Breathless with strenuous exercise only
- Grade 1—Breathless when hurrying on level ground or walking up a slight hill
- Grade 2—Walks slower than people of the same age on level ground because of shortness of breath or has to stop when walking at own pace on level ground
- Grade 3—Stops for breath after walking about 100 yards or after a few minutes on level ground
- Grade 4—Too breathless to leave the house or breathless when dressing or undressing.
Grade 2 or higher separates symptomatic from asymptomatic COPD.
The COPD Assessment Test (CAT) (www.catestonline.org) is a proprietary questionnaire. Patients use a 6-point scale (numbered 0 though 5) to rate eight symptoms (cough, mucus production, chest tightness, shortness of breath on exertion, limitations in home activities, lack of confidence leaving the home, poor sleep, and lack of energy). A total score of 10 or higher is abnormal.
Four GOLD groups
The new GOLD guidelines (Table 1)5 define four groups of patients according to their severity of airflow obstruction, symptoms, and exacerbation history:
- Group A—fewer symptoms, low risk: Fewer symptoms (“less symptoms,” as worded in the guidelines) means a CAT score less than 10 or an mMRC grade less than 2; “low risk” means no more than one exacerbation per year and an FEV1 of at least 50%
- Group B—more symptoms, low risk: “More symptoms” means a CAT score of 10 or more or an mMRC grade of 2 or more
- Group C—fewer symptoms, high risk: “High risk” means two or more exacerbations per year or an FEV1 less than 50%
- Group D—more symptoms, high risk.
Thus, a patient with an FEV1 of 60% (moderate airflow limitation) who has had one exacerbation during the past year and a CAT score of 8 would be in group A. In contrast, a patient who has an FEV1 of 40% (severe airflow limitation), no history of exacerbations, and a CAT score of 20 would be in group D.
Updated GOLD guidelines suggest utilizing a stepwise approach to treatment, akin to asthma management guidelines, based on patient grouping.5
How accurate is the new GOLD system?
Although practical and suited for use in primary care, the new GOLD system is arbitrary and has not been thoroughly studied, and may therefore need refinement.
Lange et al14 compared the new GOLD system with the previous one in 6,628 patients with COPD. As anticipated, the new system was better at predicting exacerbations, as it incorporates a history of exacerbations in stratification. The presence of symptoms (as determined by an mMRC grade ≥ 2) was a marker of mortality risk that distinguished group A from group B, and group C from group D. Surprisingly, the rate of death was higher in group B (more symptoms, low risk) than in group C (fewer symptoms, high risk).
Notably, most patients in group C qualified for this group because of the severity of airflow obstruction, not because of a history of exacerbations. Therefore, patients whose symptoms are out of proportion to the severity of obstruction may be at higher risk of death, possibly because of comorbidities such as cardiovascular disease.15 Patients who qualified for groups C and D by having both a history of frequent exacerbations (≥ 2 per year) and symptoms rather than either one alone had a higher risk of death in 3 years.
Similarly, the symptom-assessment tool that is used—ie, the mMRC grade or the CAT score—also makes a difference.
The Health-Related Quality of Life in COPD in Europe Study16 retrospectively analyzed data from 1,817 patients to determine whether the cutoff points for symptoms as assessed by mMRC grade and CAT score were equivalent. Although the mMRC grade correlated well with overall health status, the cutoff mMRC grade of 2 or higher did not correspond to a CAT score of 10 or higher, classifying patients with health status impairment as asymptomatic (mean weighted kappa 0.626). The two tools agreed much better when the cutoff was set at an mMRC grade of 1 or higher (mean weighted kappa 0.792).16
Although assessment schemes continue to evolve as data accumulate, we believe the new system is a welcome initiative that reflects the changing notions of COPD.
Comorbidities matter
Another shift is the recognition that certain comorbidities increase the risk of death. In 1,664 patients with COPD who were followed for 51 months, 12 distinct comorbidities were associated with a higher risk of death after multivariate analysis.17
The COTE index (COPD-Specific Comorbidity Test) is based on these findings. It awards points as follows:
- 6 points for cancer of the lung, esophagus, pancreas, or breast, or for anxiety
- 2 points for all other cancers, liver cirrhosis, atrial fibrillation or flutter, diabetes with neuropathy, or pulmonary fibrosis
- 1 point for congestive heart failure, gastric or duodenal ulcer, or coronary artery disease.
A COTE index score of 4 or higher was associated with a risk of death 2.2 times higher in each quartile of the BODE index.
We strongly recommend being aware of comorbidities in COPD patients, particularly when symptoms are out of proportion to the severity of obstruction.
SHOULD I USE ANTIBIOTICS TO TREAT ALL COPD EXACERBATIONS?
Infections are thought to cause more than 80% of acute exacerbations of COPD.
Anthonisen et al,18 in a landmark trial, found broad-spectrum antibiotics to be most helpful if the patient had at least two of the three cardinal symptoms of COPD exacerbation (ie, shortness of breath, increase in sputum volume, and sputum purulence). Antibiotics decreased the rate of treatment failure and led to a more rapid clinical resolution of exacerbation. However, they did not help patients who had milder exacerbations.
Antibiotics may nevertheless have a role in ambulatory patients with mild to moderate COPD who present with exacerbations characterized by one or more cardinal symptoms.
Llor et al,19 in a multicenter randomized double-blind placebo-controlled trial in Spain, concluded that amoxicillin clavulanate (Augmentin) led to higher clinical cure rates and longer time to the next exacerbation in these patients. Most of the benefit was in patients with more symptoms, consistent with the results of the study by Anthonisen et al.18
There is also strong evidence to support the use of antibiotics in addition to systemic corticosteroids in hospitalized patients with acute exacerbations of COPD. A 7-day course of doxycycline (Vibramycin) added to a standard regimen of corticosteroids was associated with higher rates of clinical and microbiological cure on day 10 of the exacerbation.20 In a large retrospective cohort study in 84,621 hospitalized patients with COPD exacerbations, fewer of those who received antibiotics needed mechanical ventilation, died, or were readmitted.21 Although sicker patients received antibiotics more frequently, their mortality rate was lower than in those who did not receive antibiotics, who were presumably less sick.
A meta-analysis confirmed the salutary effect of antibiotics in inpatients and particularly those admitted to the intensive care unit.22 Mortality rates and hospital length of stay were not affected in patients who were not in intensive care.
Biomarkers such as procalcitonin might help reduce the unnecessary use of antibiotics. Stolz et al23 conducted a randomized controlled trial in which they based the decision to give antibiotics on a threshold procalcitonin level of at least 1 μg/L in hospitalized patients with COPD exacerbation. The rate of antibiotic use was reduced by more than 40% in the procalcitonin group without any difference in clinical outcomes, 6-month exacerbation rate, or rehospitalization compared with controls. Nonstandardized procalcitonin assays are a possible barrier to the widespread adoption of this threshold.
Comment. In general, we recommend antibiotics for hospitalized patients with COPD exacerbation and look forward to confirmatory data that support the use of biomarkers. For outpatients, we find the Anthonisen criteria useful for decision-making at the point of care.
ARE THERE ANY NEW INTERVENTIONS TO PREVENT COPD EXACERBATIONS?
Macrolides
Macrolides have a proven role in managing chronic suppurative respiratory diseases such as cystic fibrosis24 and diffuse panbronchiolitis.25 Since they are beneficial at lower doses than those used to treat infection, the mechanism may be anti-inflammatory rather than antimicrobial.
Albert et al26 assigned 1,142 patients who had had a COPD exacerbation within a year before enrollment or who were on home oxygen therapy to receive azithromycin (Zithromax) 250 mg daily or placebo.25 The azithromycin group had fewer acute exacerbations (hazard ratio 0.73, 95% CI 0.63–0.84, P < .001), and more patients in the azithromycin group achieved clinically significant improvements in quality of life, ie, a reduction in the St. George’s Respiratory Questionnaire (SGRQ) score of at least 4 points (43% vs 36%, P = .03). Adverse events that were more common in the azithromycin group were hearing loss (25% vs 20%) and macrolide-resistant strains in nasopharyngeal secretions (81% vs 41%). In subgroup analysis, the benefit in terms of reducing exacerbations was greater in patients over age 65, patients on home oxygen, and patients with moderate or severe obstruction compared with those with very severe obstruction.
Comment. Macrolides are a valuable addition to the agents available for preventing COPD exacerbation (Table 2), but their role is still uncertain. Potential topics of research are whether these drugs have a role in patients already on preventive regimens, whether they would have a greater effect in distinct patient populations (eg, patients who have two or more exacerbations per year), and whether their broader use would lead to a change in the resident flora in the community.
Clinicians should exercise caution in the use of azithromycin in light of recent concern about associated cardiac morbidity and death. All patients should undergo electrocardiography to assess the QTc interval before starting treatment, as in the trial by Albert et al.26
Phosphodiesterase inhibitors
Roflumilast (Daliresp) is an oral phosphodiesterase 4 inhibitor approved for treating exacerbations and symptoms of chronic bronchitis in patients with severe COPD (Table 3). Phosphodiesterase 4, one of the 11 isoforms of the enzyme, is found in immune and inflammatory cells and promotes inflammatory responses. Roflumilast has anti-inflammatory properties but no acute bronchodilatory effect.27 Several phase 3 trials found the compound to have beneficial effects.
Calverley et al28 performed two placebo-controlled double-blind trials in outpatients with the clinical diagnosis of COPD who had chronic cough; increased sputum production; at least one recorded exacerbation requiring corticosteroids or hospitalization, or both; and an FEV1 of 50% or less. Patients were randomized to receive roflumilast 500 μg once a day (n = 1,537) or placebo (n = 1,554) for 1 year. The rate of moderate to severe exacerbations was 1.17 per year with roflumilast vs 1.37 with placebo (P < .0003). Adverse events were significantly more common with roflumilast and were related to the known side effects of the drug, namely, diarrhea, weight loss, decreased appetite, and nausea.
Fabbri et al29 performed two other placebo-controlled double-blind multicenter trials, studying the combinations of roflumilast with salmeterol (Serevent) and roflumilast with tiotropium (Spiriva) compared with placebo in 1,676 patients with COPD who had post-bronchodilator FEV1 values of 40% to 70% of predicted. The mean prebronchodilator FEV1 improved by 49 mL (P < .0001) in the salmeterol-plus-roflumilast trial and by 80 mL (P < .0001) in the tiotropium-plus-roflumilast trial compared with placebo. Fewer patients on roflumilast had exacerbations of any severity in both trials (risk ratio 0.82, P = .0419 and risk ratio 0.75, P = .0169, respectively).
No trial has yet addressed whether roflumilast is better than the combination of a long-acting muscarinic antagonist and a beta agonist, or whether roflumilast can be substituted for inhaled corticosteroids in a new triple-therapy combination. Clinicians should also be aware of psychiatric side effects of roflumilast, which include depression and, possibly, suicide.
ARE THERE ANY NEW BRONCHODILATORS FOR PATIENTS WITH COPD?
Long-acting muscarinic antagonists
Reversible airflow obstruction and mucus secretion are determined by the vagal cholinergic tone in patients with COPD.30 Antagonism of cholinergic (muscarinic) receptors results in bronchodilation and reduction in mucus production. Consequently, inhaled anticholinergic agents are the first-line therapy for COPD (Table 4).
Tiotropium bromide is a long-acting antimuscarinic approved in 2002 by the US Food and Drug Administration (FDA). The UPLIFT trial (Understanding Potential Long-Term Impacts on Function With Tiotropium)31 enrolled 5,993 patients with a mean FEV1 of 48% of predicted. Over a 4-year follow-up, significant improvements in mean FEV1 values (ranging from 87 mL to 103 mL before bronchodilation and 47 mL to 65 mL after bronchodilation, P < .001) in the tiotropium group were observed compared with placebo. The rate of the primary end point—the rate of decline in mean FEV1—was not different between tiotropium and placebo. However, there were important salutary effects in multiple clinical end points in the tiotropium group. Health-related quality of life as measured by the SGRQ improved in a clinically significant manner (> 4 points) in favor of tiotropium in a higher proportion of patients (45% vs 36%, P < .001). Tiotropium reduced the number of exacerbations per patient year (0.73 ± 0.02 vs 0.85 ± 0.02, RR = 0.86 (95% CI 0.81–0.91), P < .001) and the risk of respiratory failure (RR = 0.67, 95% CI 0.51–0.89). There were no significant differences in the risk of myocardial infarction, stroke, or pneumonia.
Aclidinium bromide (Tudorza Pressair) is a long-acting antimuscarinic recently approved by the FDA. Compared with tiotropium, it has a slightly faster onset of action and a considerably shorter half-life (29 hours vs 64 hours).32,33 Its dosage is 400 μg twice daily by inhalation. It provides sustained bronchodilation over 24 hours and may have a favorable side-effect profile, because it undergoes rapid hydrolysis in human plasma.34
ACCORD COPD I35 and ATTAIN,36 two phase 3 trials in patients with moderate-to severe COPD, found that twice-daily aclidinium was associated with statistically and clinically significant (> 100 mL) improvements in trough and peak FEV1 compared with placebo. Health status (assessed by SGRQ) and dyspnea (assessed by transitional dyspnea index) also improved significantly. However, improvements beyond minimum clinically significant thresholds were achieved only with 400 μg twice-daily dosing.
To date, no study has evaluated the impact of aclidinium on COPD exacerbation as a primary end point. Fewer moderate to severe exacerbations were reported in an earlier 52-week study of once-daily aclidinium (ACCLAIM COPD II) but not in ACCLAIM COPD I.37
Aclidinium may offer an advantage over tiotropium in patients who have nocturnal symptoms. Twice-daily aclidinium 400 μg was associated with superior FEV1 area-under-the-curve values compared with placebo and tiotropium, the difference mostly owing to improved nocturnal profile.38
Long-acting beta-2 agonists
Stimulation of airway beta-2 receptors relaxes smooth muscles and consequently dilates bronchioles via a cyclic adenosine monophosphate-dependent pathway.39
Short-acting beta-2 agonists such as albuterol and terbutaline have long been used as rescue medications for obstructive lung disease. Long-acting beta-2 agonists provide sustained bronchodilation and are therefore more efficacious as maintenance medications. Salmeterol, formoterol (Foradil), and arformoterol (Brovana) are long-acting beta-2 agonists in clinical use that are taken twice daily.
Clinical studies indicate that use of long-acting beta-2 agonists leads to significant improvements in FEV1,40–42 dynamic hyperinflation, exercise tolerance,43,44 and dyspnea.45,46 These drugs have also been associated with significant improvements in health-related quality of life and in the frequency of exacerbations.47–49
In patients with asthma, long-acting beta agonists may increase the risk of death.50 In contrast, in patients with COPD, they appear to offer a survival advantage when used in combination with inhaled corticosteroids,51 and some argue that this benefit is entirely from the long-acting beta agonist (a 17% reduction in mortality) rather than the inhaled corticosteroid (0% reduction in mortality).52
Indacaterol (Arcapta), approved in July 2011, is the first once-daily beta agonist or “ultra-long-acting” beta agonist (Table 5). Possibly because it has a high affinity for the lipid raft domain of the cell membrane where beta-2 receptors are coupled to second messengers,53 the drug has a 24-hour duration of action.
In patients with COPD, inhaled indacaterol 150 μg once daily improved airflow obstruction and health status as measured by SGRQ compared with salmeterol 50 μg twice daily and placebo.54 At the higher dose of 300 μg daily, the 52-week INVOLVE trial55 demonstrated early and more sustained improvement in FEV1 compared with placebo and formoterol. In this study, a lower exacerbation rate than with placebo was also noted. The drug has also shown equivalent bronchodilator efficacy at 150 μg and 300 μg daily dosing compared with tiotropium.56
The benefits of a longer-acting bronchodilator such as indacaterol are likely mediated by smoothing out airway bronchomotor tone over 24 hours without the dips seen with shorter-acting agents and by improvement of the FEV1 trough before the subsequent dose is due, aptly named “pharmacologic stenting.”57 Once-daily dosing should also foster better adherence. The safety profile appears excellent with no increase in cardiovascular or cerebrovascular events compared with placebo.58
The FDA approved the 75-μg daily dose instead of the higher doses used in the studies mentioned above. This decision was based on the observation that there appeared to be a flattened dose-response in patients with more severe COPD, with no further improvement in trough FEV1 at higher doses.59
DOES VITAMIN D SUPPLEMENTATION HAVE A ROLE IN COPD MANAGEMENT?
Vitamin D is vital for calcium and phosphate metabolism and bone health. Low vitamin D levels are associated with diminished leg strength and falls in the elderly.60 Osteoporosis, preventable with vitamin D and calcium supplementation, is linked to thoracic vertebral fracture and consequent reduced lung function.61,62
Patients with COPD are at higher risk of vitamin D deficiency, and more so if they also are obese, have advanced airflow obstruction, are depressed, or smoke.62 Therefore, there are sound reasons to look for vitamin D deficiency in patients with COPD and to treat it if the 25-hydroxyvitamin D level is less than 10 ng/ mL (Table 6).
Vitamin D may also have antimicrobial and immunomodulatory effects.63 Since COPD exacerbations are frequently caused by infection, it was hypothesized that vitamin D supplementation might reduce the rate of exacerbations.
In a study in 182 patients with moderate to very severe COPD and a history of recent exacerbations, high-dose vitamin D supplementation (100,000 IU) was given every 4 weeks for 1 year.64 There were no differences in the time to first exacerbation, in the rate of exacerbation, hospitalization, or death, or in quality of life between the placebo and intervention groups. However, subgroup analysis indicated that, in those with severe vitamin D deficiency at baseline, the exacerbation rate was reduced by more than 40%.
Comment. We recommend screening for vitamin D deficiency in patients with COPD. Supplementation is appropriate in those with low levels, but data indicate no role in those with normal levels.
WHAT ARE THE NONPHARMACOLOGIC APPROACHES TO COPD TREATMENT?
Noninvasive positive-pressure ventilation
Nocturnal noninvasive positive-pressure ventilation may be beneficial in patients with severe COPD, daytime hypercapnia, and nocturnal hypoventilation, particularly if higher inspiratory pressures are selected (Table 7).65,66
For instance, a randomized controlled trial of noninvasive positive-pressure ventilation plus long-term oxygen therapy compared with long-term oxygen therapy alone in hypercapnic COPD demonstrated a survival benefit in favor of ventilation (hazard ratio 0.6).67
In another randomized trial,68 settings that aimed to maximally reduce Paco2 (mean inspiratory positive airway pressure 29 cm H2O with a backup rate of 17.5/min) were compared with low-intensity positive airway pressure (mean inspiratory positive airway pressure 14 cm H2O, backup rate 8/min). The high inspiratory pressures increased the daily use of ventilation by 3.6 hours per day and improved exercise-related dyspnea, daytime Paco2, FEV1, vital capacity, and health-related quality of life66 without disrupting sleep quality.68
Caveats are that acclimation to the high pressures was achieved in the hospital, and the high pressures were associated with a significant increase in air leaks.66
Comments. Whether high-pressure noninvasive positive-pressure ventilation can be routinely implemented and adopted in the outpatient setting, and whether it is associated with a survival advantage remains to be determined. The advantages of noninvasive positive-pressure ventilation in the setting of hypercapnic COPD appear to augment those of pulmonary rehabilitation, with improved quality of life, gas exchange, and exercise tolerance, and a slower decline of lung function.69
Pulmonary rehabilitation
Pulmonary rehabilitation is a multidisciplinary approach to managing COPD (Table 8).
Patients participate in three to five supervised sessions per week, each lasting 3 to 4 hours, for 6 to 12 weeks. Less-frequent sessions may not be effective. For instance, in a randomized trial, exercising twice a week was not enough.70 Additionally, a program lasting longer than 12 weeks produced more sustained benefits than shorter programs.71
A key component is an exercise protocol centered on the lower extremities (walking, cycling, treadmill), with progressive exercise intensity to a target of about 60% to 80% of the maximal exercise tolerance,72 though more modest targets of about 50% can also be beneficial.73
Exercise should be tailored to the desired outcome. For instance, training of the upper arms may help with activities of daily living. In one study, unsupported (against gravity) arm training improved upper-extremity function more than supported arm training (by ergometer).74 Ventilatory muscle training is less common, as most randomized trials have not shown conclusive evidence of benefit. Current guidelines do not recommend routine inspiratory muscle training.71
Even though indices of pulmonary function do not improve after an exercise program, randomized trials have shown that pulmonary rehabilitation improves exercise capacity, dyspnea, and health-related quality of life; improves cost-effectiveness of health care utilization; and provides psychosocial benefits that often exceed those of other therapies. Although there is no significant evidence of whether pulmonary rehabilitation improves survival in patients with COPD,71 an observational study documented improvements in BODE scores as well as a reduction in respiratory mortality rates in patients undergoing pulmonary rehabilitation.75
A limitation of pulmonary rehabilitation is that endurance and psychological and cognitive function decline significantly if exercise is not maintained. However, the role of a maintenance program is uncertain, with long-term benefits considered modest.71
Lung-volume reduction surgery
Lung-volume reduction consists of surgical wedge resections of emphysematous areas of the lung (Table 9).
The National Emphysema Treatment Trial76 randomized 1,218 patients to undergo either lung-volume reduction surgery or maximal medical therapy. Surgery improved survival, quality of life, and dyspnea in patients with upper-lobe emphysema and a low exercise capacity (corresponding to < 40 watts for men or < 25 watts for women in the maximal power achieved on cycle ergometry). While conferring no survival benefit in patients with upper-lobe-predominant emphysema and high exercise capacity, this surgery is likely to improve exercise capacity and quality of life in this subset of patients.
Importantly, the procedure is associated with a lower survival rate in patients with an FEV1 lower than 20%, homogeneous emphysema, a diffusing capacity of the lung for carbon monoxide lower than 20%, non-upper-lobe emphysema, or high baseline exercise capacity.
The proposed mechanisms of improvement of lung function include placing the diaphragm in a position with better mechanical advantage, reducing overall lung volume, better size-matching between the lungs and chest cavity, and restoring elastic recoil.76,77
Ongoing trials aim to replicate the success of lung-volume reduction using nonsurgical bronchoscopic techniques with one-way valves, coils, biologic sealants, thermal ablation, and airway stents.
- Miniño AM, Xu J, Kochanek KD. Deaths: preliminary data for 2008. National Vital Statistics Reports 2010; 59:1–52. http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_02.pdf. Accessed April 10, 2014.
- Murphy SL, Xu J, Kochanek KD. Deaths: preliminary data from 2010. National Vital Statistics Reports 2012; 60:1–51. http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf. Accessed April 10, 2014.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3:e442.
- Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest 2005; 128:1239–1244.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). http://www.goldcopd.org/Guidelines/guidelines-resources.html. Accessed April 10, 2014.
- Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006; 173:1114–1121.
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60:925–931.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57:847–852.
- Hurst JR, Vestbo J, Anzueto A, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363:1128–1138.
- Cooper CB. The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med 2006; 119(suppl 1):21–31.
- Jones PW. Issues concerning health-related quality of life in COPD. Chest 1995; 107(suppl):187S–193S.
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:1005–1012.
- Fletcher CM. The clinical diagnosis of pulmonary emphysema—an experimental study. J Royal Soc Med 1952; 45:577–584.
- Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med 2012; 186:975–981.
- Hurst J. Phenotype-based care in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:935–936.
- Jones PW, Adamek L, Nadeau G, Banik N. Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification. Eur Respir J 2013; 42:647–654.
- Divo M, Cote C, de Torres JP, et al; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:155–161.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106:196–204.
- Llor C, Moragas A, Hernández S, Bayona C, Miravitlles M. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:716–723.
- Daniels JM, Snijders D, de Graaff CS, Vlaspolder F, Jansen HM, Boersma WG. Antibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181:150–157.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA 2010; 303:2035–2042.
- Vollenweider DJ, Jarrett H, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; 12:CD010257.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007; 131:9–19.
- Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 2002; 57:212–216.
- Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med 1998; 157:1829–1832.
- Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365:689–698.
- Gross NJ, Giembycz MA, Rennard SI. Treatment of chronic obstructive pulmonary disease with roflumilast, a new phosphodiesterase 4 inhibitor. COPD 2010; 7:141–153.
- Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009; 374:685–694.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with long acting bronchodilators: two randomised clinical trials. Lancet 2009; 374:695–703.
- Gross NJ, Skorodin MS. Anticholinergic, antimuscarinic bronchodilators. Am Rev Respir Dis 1984; 129:856–870.
- Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359:1543–1554.
- Cazzola M. Aclidinium bromide, a novel long-acting muscarinic M3 antagonist for the treatment of COPD. Curr Opin Investig Drugs 2009; 10:482–490.
- Gavaldà A, Miralpeix M, Ramos I, et al. Characterization of aclidinium bromide, a novel inhaled muscarinic antagonist, with long duration of action and a favorable pharmacological profile. J Pharmacol Exp Ther 2009; 331:740–751.
- Alagha K, Bourdin A, Tummino C, Chanez P. An update on the efficacy and safety of aclidinium bromide in patients with COPD. Ther Adv Respir Dis 2011; 5:19–28.
- Kerwin EM, D’Urzo AD, Gelb AF, Lakkis H, Garcia Gil E, Caracta CF; ACCORD I study investigators. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD 2012; 9:90–101.
- Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J 2012; 40:830–836.
- Jones PW, Rennard SI, Agusti A, et al. Efficacy and safety of once-daily aclidinium in chronic obstructive pulmonary disease. Respir Res 2011; 12:55.
- Fuhr R, Magnussen H, Sarem K, et al. Efficacy of aclidinium bromide 400 μg twice daily compared with placebo and tiotropium in patients with moderate to severe COPD. Chest 2012; 141:745–752.
- Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res 2010; 11:149.
- Mahler DA, Donohue JF, Barbee RA, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999; 115:957–965.
- Campbell M, Eliraz A, Johansson G, et al. Formoterol for maintenance and as-needed treatment of chronic obstructive pulmonary disease. Respir Med 2005; 99:1511–1520.
- Hanrahan JP, Hanania NA, Calhoun WJ, Sahn SA, Sciarappa K, Baumgartner RA. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD 2008; 5:25–34.
- Neder JA, Fuld JP, Overend T, et al. Effects of formoterol on exercise tolerance in severely disabled patients with COPD. Respir Med 2007; 101:2056–2064.
- O’Donnell DE, Voduc N, Fitzpatrick M, Webb KA. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur Respir J 2004; 24:86–94.
- Rennard SI, Anderson W, ZuWallack R, et al. Use of a long-acting inhaled beta 2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163:1087–1092.
- Schultze-Werninghaus G. Multicenter 1-year trial on formoterol, a new long-acting beta 2-agonist, in chronic obstructive airway disease. Lung 1990; 168(suppl):83–89.
- Rodrigo GJ, Nannini LJ, Rodríguez-Roisin R. Safety of long-acting beta-agonists in stable COPD: a systematic review. Chest 2008; 133:1079–1087.
- Baker WL, Baker EL, Coleman CI. Pharmacologic treatments for chronic obstructive pulmonary disease: a mixed-treatment comparison meta-analysis. Pharmacotherapy 2009; 29:891–905.
- Rodrigo GJ, Castro-Rodriguez JA, Plaza V. Safety and efficacy of combined long-acting beta-agonists and inhaled corticosteroids vs long-acting beta-agonists monotherapy for stable COPD: a systematic review. Chest 2009; 136:1029–1038.
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006; 129:15–26.
- Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–789.
- Suissa S, Ernst P, Vandemheen KL, Aaron SD. Methodological issues in therapeutic trials of COPD. Eur Respir J 2008; 31:927–933.
- Lombardi D, Cuenoud B, Krämer SD. Lipid membrane interactions of indacaterol and salmeterol: do they influence their pharmacological properties? Eur J Pharm Sci 2009; 38:533–547.
- Kornmann O, Dahl R, Centanni S, et al; INLIGHT-2 (Indacaterol Efficacy Evaluation Using 150-μg Doses with COPD Patients) study investigators. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J 2011; 37:273–279.
- Dahl R, Chung KF, Buhl R, et al; INVOLVE (INdacaterol: Value in COPD: Longer Term Validation of Efficacy and Safety) Study Investigators. Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax 2010; 65:473–479.
- Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 2010; 182:155–162.
- Beeh KM, Beier J. The short, the long and the “ultra-long”: why duration of bronchodilator action matters in chronic obstructive pulmonary disease. Adv Ther 2010; 27:150–159.
- Worth H, Chung KF, Felser JM, Hu H, Rueegg P. Cardio- and cerebrovascular safety of indacaterol vs formoterol, salmeterol, tiotropium and placebo in COPD. Respir Med 2011; 105:571–579.
- Chowdhury BA, Seymour SM, Michele TM, Durmowicz AG, Liu D, Rosebraugh CJ. The risks and benefits of indacaterol--the FDA’s review. N Engl J Med 2011; 365:2247–2249.
- Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA 2004; 291:1999–2006.
- Leech JA, Dulberg C, Kellie S, Pattee L, Gay J. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis 1990; 141:68–71.
- Persson LJ, Aanerud M, Hiemstra PS, Hardie JA, Bakke PS, Eagan TM. Chronic obstructive pulmonary disease is associated with low levels of vitamin D. PLoS One 2012; 7:e38934.
- Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med 2009; 179:630–636.
- Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2012; 156:105–114.
- Casanova C, Celli BR, Tost L, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 2000; 118:1582–1590.
- Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010; 65:303–308.
- McEvoy RD, Pierce RJ, Hillman D, et al; Australian trial of non-invasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64:561–566.
- Dreher M, Ekkernkamp E, Walterspacher S, et al. Noninvasive ventilation in COPD: impact of inspiratory pressure levels on sleep quality. Chest 2011; 140:939–945.
- Duiverman ML, Wempe JB, Bladder G, et al. Two-year home-based nocturnal noninvasive ventilation added to rehabilitation in chronic obstructive pulmonary disease patients: a randomized controlled trial. Respir Res 2011; 12:112.
- Ringbaek TJ, Broendum E, Hemmingsen L, et al. Rehabilitation of patients with chronic obstructive pulmonary disease. Exercise twice a week is not sufficient! Respir Med 2000; 94:150–154.
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007; 131(suppl):4S–42S.
- Vallet G, Ahmaïdi S, Serres I, et al. Comparison of two training programmes in chronic airway limitation patients: standardized versus individualized protocols. Eur Respir J 1997; 10:114–122.
- Vogiatzis I, Williamson AF, Miles J, Taylor IK. Physiological response to moderate exercise workloads in a pulmonary rehabilitation program in patients with varying degrees of airflow obstruction. Chest 1999; 116:1200–1207.
- Martinez FJ, Vogel PD, Dupont DN, Stanopoulos I, Gray A, Beamis JF. Supported arm exercise vs unsupported arm exercise in the rehabilitation of patients with severe chronic airflow obstruction. Chest 1993; 103:1397–1402.
- Cote CG, Celli BR. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J 2005; 26:630–636.
- Fishman A, Martinez F, Naunheim K, et al; National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348:2059–2073.
- Naunheim KS, Wood DE, Mohsenifar Z, et al; National Emphysema Treatment Trial Research Group. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg 2006; 82:431–443.
Chronic obstructive pulmonary disease (COPD) has seen several changes in its assessment and treatment in recent years, reflecting advances in our understanding of this common and serious disease.
This review updates busy practitioners on the major advances, including new assessment tools and new therapies.
COMMON AND INCREASING
COPD is the third leading cause of death in the United States, behind heart disease and cancer,1 and of the top five (the others being stroke and accidents), it is the only one that increased in incidence between 2007 and 2010.2 The 11th leading cause of disability-adjusted life years worldwide in 2002, COPD is projected to become the seventh by the year 2030.3
CHARACTERIZED BY OBSTRUCTION
COPD is characterized by persistent and progressive airflow obstruction associated with chronic airway inflammation in response to noxious particles and gases. Disease of the small airways (inflammation, mucus plugging, and fibrosis) and parenchymal destruction (emphysema) limit the flow of air.
COPD is diagnosed by spirometry—specifically, a ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) of less than 0.7 after a bronchodilator is given. The severity of airflow limitation is revealed by the FEV1 as a percent of the predicted value.
Cigarette smoking is the major cause of COPD, but the prevalence of COPD is 6.6% in people who have never smoked, and one-fourth of COPD patients in the United States have never smoked.4
GOLDEN GOALS: FEWER SYMPTOMS, LOWER RISK
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) periodically issues evidence-based statements on how to prevent and treat COPD.
In its 2013 update,5 GOLD suggested two goals: improving symptoms and reducing the risk of death, exacerbations, progression of disease, and treatment-related adverse effects. The latter goal—reducing risk—is relatively new.
Exacerbations are acute inflammatory events superimposed on chronic inflammation. The inflammation is often brought on by infection6 and increases the risk of death7 and the risk of a faster decline in lung function.8
Exacerbations may characterize a phenotype of COPD. The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) analyzed the frequency of COPD exacerbations and associated factors in 2,138 patients with COPD over a period of 3 years.9 Although patients with more severe obstruction tended to have more exacerbations, some patients appeared susceptible to exacerbations irrespective of the severity of obstruction. The best predictor of exacerbations was a history of exacerbations.
HOW DO I ASSESS A PATIENT WITH COPD ON PRESENTATION?
Markers of airflow obstruction such as the FEV1 do not correlate strongly with exertional capacity and health status in patients with COPD.10,11
The BODE index (body mass index, obstruction, dyspnea score, and exercise oximetry) takes into account the multidimensional nature of COPD. It performs better than the FEV1 in predicting the risk of death.12 The propensity for exacerbations and comorbidities further modulates outcome.
Assessing symptoms
The modified British Medical Research Council (mMRC) dyspnea scale, based on work by Fletcher in 1952,13 has five grades, numbered 0 through 4:
- Grade 0—Breathless with strenuous exercise only
- Grade 1—Breathless when hurrying on level ground or walking up a slight hill
- Grade 2—Walks slower than people of the same age on level ground because of shortness of breath or has to stop when walking at own pace on level ground
- Grade 3—Stops for breath after walking about 100 yards or after a few minutes on level ground
- Grade 4—Too breathless to leave the house or breathless when dressing or undressing.
Grade 2 or higher separates symptomatic from asymptomatic COPD.
The COPD Assessment Test (CAT) (www.catestonline.org) is a proprietary questionnaire. Patients use a 6-point scale (numbered 0 though 5) to rate eight symptoms (cough, mucus production, chest tightness, shortness of breath on exertion, limitations in home activities, lack of confidence leaving the home, poor sleep, and lack of energy). A total score of 10 or higher is abnormal.
Four GOLD groups
The new GOLD guidelines (Table 1)5 define four groups of patients according to their severity of airflow obstruction, symptoms, and exacerbation history:
- Group A—fewer symptoms, low risk: Fewer symptoms (“less symptoms,” as worded in the guidelines) means a CAT score less than 10 or an mMRC grade less than 2; “low risk” means no more than one exacerbation per year and an FEV1 of at least 50%
- Group B—more symptoms, low risk: “More symptoms” means a CAT score of 10 or more or an mMRC grade of 2 or more
- Group C—fewer symptoms, high risk: “High risk” means two or more exacerbations per year or an FEV1 less than 50%
- Group D—more symptoms, high risk.
Thus, a patient with an FEV1 of 60% (moderate airflow limitation) who has had one exacerbation during the past year and a CAT score of 8 would be in group A. In contrast, a patient who has an FEV1 of 40% (severe airflow limitation), no history of exacerbations, and a CAT score of 20 would be in group D.
Updated GOLD guidelines suggest utilizing a stepwise approach to treatment, akin to asthma management guidelines, based on patient grouping.5
How accurate is the new GOLD system?
Although practical and suited for use in primary care, the new GOLD system is arbitrary and has not been thoroughly studied, and may therefore need refinement.
Lange et al14 compared the new GOLD system with the previous one in 6,628 patients with COPD. As anticipated, the new system was better at predicting exacerbations, as it incorporates a history of exacerbations in stratification. The presence of symptoms (as determined by an mMRC grade ≥ 2) was a marker of mortality risk that distinguished group A from group B, and group C from group D. Surprisingly, the rate of death was higher in group B (more symptoms, low risk) than in group C (fewer symptoms, high risk).
Notably, most patients in group C qualified for this group because of the severity of airflow obstruction, not because of a history of exacerbations. Therefore, patients whose symptoms are out of proportion to the severity of obstruction may be at higher risk of death, possibly because of comorbidities such as cardiovascular disease.15 Patients who qualified for groups C and D by having both a history of frequent exacerbations (≥ 2 per year) and symptoms rather than either one alone had a higher risk of death in 3 years.
Similarly, the symptom-assessment tool that is used—ie, the mMRC grade or the CAT score—also makes a difference.
The Health-Related Quality of Life in COPD in Europe Study16 retrospectively analyzed data from 1,817 patients to determine whether the cutoff points for symptoms as assessed by mMRC grade and CAT score were equivalent. Although the mMRC grade correlated well with overall health status, the cutoff mMRC grade of 2 or higher did not correspond to a CAT score of 10 or higher, classifying patients with health status impairment as asymptomatic (mean weighted kappa 0.626). The two tools agreed much better when the cutoff was set at an mMRC grade of 1 or higher (mean weighted kappa 0.792).16
Although assessment schemes continue to evolve as data accumulate, we believe the new system is a welcome initiative that reflects the changing notions of COPD.
Comorbidities matter
Another shift is the recognition that certain comorbidities increase the risk of death. In 1,664 patients with COPD who were followed for 51 months, 12 distinct comorbidities were associated with a higher risk of death after multivariate analysis.17
The COTE index (COPD-Specific Comorbidity Test) is based on these findings. It awards points as follows:
- 6 points for cancer of the lung, esophagus, pancreas, or breast, or for anxiety
- 2 points for all other cancers, liver cirrhosis, atrial fibrillation or flutter, diabetes with neuropathy, or pulmonary fibrosis
- 1 point for congestive heart failure, gastric or duodenal ulcer, or coronary artery disease.
A COTE index score of 4 or higher was associated with a risk of death 2.2 times higher in each quartile of the BODE index.
We strongly recommend being aware of comorbidities in COPD patients, particularly when symptoms are out of proportion to the severity of obstruction.
SHOULD I USE ANTIBIOTICS TO TREAT ALL COPD EXACERBATIONS?
Infections are thought to cause more than 80% of acute exacerbations of COPD.
Anthonisen et al,18 in a landmark trial, found broad-spectrum antibiotics to be most helpful if the patient had at least two of the three cardinal symptoms of COPD exacerbation (ie, shortness of breath, increase in sputum volume, and sputum purulence). Antibiotics decreased the rate of treatment failure and led to a more rapid clinical resolution of exacerbation. However, they did not help patients who had milder exacerbations.
Antibiotics may nevertheless have a role in ambulatory patients with mild to moderate COPD who present with exacerbations characterized by one or more cardinal symptoms.
Llor et al,19 in a multicenter randomized double-blind placebo-controlled trial in Spain, concluded that amoxicillin clavulanate (Augmentin) led to higher clinical cure rates and longer time to the next exacerbation in these patients. Most of the benefit was in patients with more symptoms, consistent with the results of the study by Anthonisen et al.18
There is also strong evidence to support the use of antibiotics in addition to systemic corticosteroids in hospitalized patients with acute exacerbations of COPD. A 7-day course of doxycycline (Vibramycin) added to a standard regimen of corticosteroids was associated with higher rates of clinical and microbiological cure on day 10 of the exacerbation.20 In a large retrospective cohort study in 84,621 hospitalized patients with COPD exacerbations, fewer of those who received antibiotics needed mechanical ventilation, died, or were readmitted.21 Although sicker patients received antibiotics more frequently, their mortality rate was lower than in those who did not receive antibiotics, who were presumably less sick.
A meta-analysis confirmed the salutary effect of antibiotics in inpatients and particularly those admitted to the intensive care unit.22 Mortality rates and hospital length of stay were not affected in patients who were not in intensive care.
Biomarkers such as procalcitonin might help reduce the unnecessary use of antibiotics. Stolz et al23 conducted a randomized controlled trial in which they based the decision to give antibiotics on a threshold procalcitonin level of at least 1 μg/L in hospitalized patients with COPD exacerbation. The rate of antibiotic use was reduced by more than 40% in the procalcitonin group without any difference in clinical outcomes, 6-month exacerbation rate, or rehospitalization compared with controls. Nonstandardized procalcitonin assays are a possible barrier to the widespread adoption of this threshold.
Comment. In general, we recommend antibiotics for hospitalized patients with COPD exacerbation and look forward to confirmatory data that support the use of biomarkers. For outpatients, we find the Anthonisen criteria useful for decision-making at the point of care.
ARE THERE ANY NEW INTERVENTIONS TO PREVENT COPD EXACERBATIONS?
Macrolides
Macrolides have a proven role in managing chronic suppurative respiratory diseases such as cystic fibrosis24 and diffuse panbronchiolitis.25 Since they are beneficial at lower doses than those used to treat infection, the mechanism may be anti-inflammatory rather than antimicrobial.
Albert et al26 assigned 1,142 patients who had had a COPD exacerbation within a year before enrollment or who were on home oxygen therapy to receive azithromycin (Zithromax) 250 mg daily or placebo.25 The azithromycin group had fewer acute exacerbations (hazard ratio 0.73, 95% CI 0.63–0.84, P < .001), and more patients in the azithromycin group achieved clinically significant improvements in quality of life, ie, a reduction in the St. George’s Respiratory Questionnaire (SGRQ) score of at least 4 points (43% vs 36%, P = .03). Adverse events that were more common in the azithromycin group were hearing loss (25% vs 20%) and macrolide-resistant strains in nasopharyngeal secretions (81% vs 41%). In subgroup analysis, the benefit in terms of reducing exacerbations was greater in patients over age 65, patients on home oxygen, and patients with moderate or severe obstruction compared with those with very severe obstruction.
Comment. Macrolides are a valuable addition to the agents available for preventing COPD exacerbation (Table 2), but their role is still uncertain. Potential topics of research are whether these drugs have a role in patients already on preventive regimens, whether they would have a greater effect in distinct patient populations (eg, patients who have two or more exacerbations per year), and whether their broader use would lead to a change in the resident flora in the community.
Clinicians should exercise caution in the use of azithromycin in light of recent concern about associated cardiac morbidity and death. All patients should undergo electrocardiography to assess the QTc interval before starting treatment, as in the trial by Albert et al.26
Phosphodiesterase inhibitors
Roflumilast (Daliresp) is an oral phosphodiesterase 4 inhibitor approved for treating exacerbations and symptoms of chronic bronchitis in patients with severe COPD (Table 3). Phosphodiesterase 4, one of the 11 isoforms of the enzyme, is found in immune and inflammatory cells and promotes inflammatory responses. Roflumilast has anti-inflammatory properties but no acute bronchodilatory effect.27 Several phase 3 trials found the compound to have beneficial effects.
Calverley et al28 performed two placebo-controlled double-blind trials in outpatients with the clinical diagnosis of COPD who had chronic cough; increased sputum production; at least one recorded exacerbation requiring corticosteroids or hospitalization, or both; and an FEV1 of 50% or less. Patients were randomized to receive roflumilast 500 μg once a day (n = 1,537) or placebo (n = 1,554) for 1 year. The rate of moderate to severe exacerbations was 1.17 per year with roflumilast vs 1.37 with placebo (P < .0003). Adverse events were significantly more common with roflumilast and were related to the known side effects of the drug, namely, diarrhea, weight loss, decreased appetite, and nausea.
Fabbri et al29 performed two other placebo-controlled double-blind multicenter trials, studying the combinations of roflumilast with salmeterol (Serevent) and roflumilast with tiotropium (Spiriva) compared with placebo in 1,676 patients with COPD who had post-bronchodilator FEV1 values of 40% to 70% of predicted. The mean prebronchodilator FEV1 improved by 49 mL (P < .0001) in the salmeterol-plus-roflumilast trial and by 80 mL (P < .0001) in the tiotropium-plus-roflumilast trial compared with placebo. Fewer patients on roflumilast had exacerbations of any severity in both trials (risk ratio 0.82, P = .0419 and risk ratio 0.75, P = .0169, respectively).
No trial has yet addressed whether roflumilast is better than the combination of a long-acting muscarinic antagonist and a beta agonist, or whether roflumilast can be substituted for inhaled corticosteroids in a new triple-therapy combination. Clinicians should also be aware of psychiatric side effects of roflumilast, which include depression and, possibly, suicide.
ARE THERE ANY NEW BRONCHODILATORS FOR PATIENTS WITH COPD?
Long-acting muscarinic antagonists
Reversible airflow obstruction and mucus secretion are determined by the vagal cholinergic tone in patients with COPD.30 Antagonism of cholinergic (muscarinic) receptors results in bronchodilation and reduction in mucus production. Consequently, inhaled anticholinergic agents are the first-line therapy for COPD (Table 4).
Tiotropium bromide is a long-acting antimuscarinic approved in 2002 by the US Food and Drug Administration (FDA). The UPLIFT trial (Understanding Potential Long-Term Impacts on Function With Tiotropium)31 enrolled 5,993 patients with a mean FEV1 of 48% of predicted. Over a 4-year follow-up, significant improvements in mean FEV1 values (ranging from 87 mL to 103 mL before bronchodilation and 47 mL to 65 mL after bronchodilation, P < .001) in the tiotropium group were observed compared with placebo. The rate of the primary end point—the rate of decline in mean FEV1—was not different between tiotropium and placebo. However, there were important salutary effects in multiple clinical end points in the tiotropium group. Health-related quality of life as measured by the SGRQ improved in a clinically significant manner (> 4 points) in favor of tiotropium in a higher proportion of patients (45% vs 36%, P < .001). Tiotropium reduced the number of exacerbations per patient year (0.73 ± 0.02 vs 0.85 ± 0.02, RR = 0.86 (95% CI 0.81–0.91), P < .001) and the risk of respiratory failure (RR = 0.67, 95% CI 0.51–0.89). There were no significant differences in the risk of myocardial infarction, stroke, or pneumonia.
Aclidinium bromide (Tudorza Pressair) is a long-acting antimuscarinic recently approved by the FDA. Compared with tiotropium, it has a slightly faster onset of action and a considerably shorter half-life (29 hours vs 64 hours).32,33 Its dosage is 400 μg twice daily by inhalation. It provides sustained bronchodilation over 24 hours and may have a favorable side-effect profile, because it undergoes rapid hydrolysis in human plasma.34
ACCORD COPD I35 and ATTAIN,36 two phase 3 trials in patients with moderate-to severe COPD, found that twice-daily aclidinium was associated with statistically and clinically significant (> 100 mL) improvements in trough and peak FEV1 compared with placebo. Health status (assessed by SGRQ) and dyspnea (assessed by transitional dyspnea index) also improved significantly. However, improvements beyond minimum clinically significant thresholds were achieved only with 400 μg twice-daily dosing.
To date, no study has evaluated the impact of aclidinium on COPD exacerbation as a primary end point. Fewer moderate to severe exacerbations were reported in an earlier 52-week study of once-daily aclidinium (ACCLAIM COPD II) but not in ACCLAIM COPD I.37
Aclidinium may offer an advantage over tiotropium in patients who have nocturnal symptoms. Twice-daily aclidinium 400 μg was associated with superior FEV1 area-under-the-curve values compared with placebo and tiotropium, the difference mostly owing to improved nocturnal profile.38
Long-acting beta-2 agonists
Stimulation of airway beta-2 receptors relaxes smooth muscles and consequently dilates bronchioles via a cyclic adenosine monophosphate-dependent pathway.39
Short-acting beta-2 agonists such as albuterol and terbutaline have long been used as rescue medications for obstructive lung disease. Long-acting beta-2 agonists provide sustained bronchodilation and are therefore more efficacious as maintenance medications. Salmeterol, formoterol (Foradil), and arformoterol (Brovana) are long-acting beta-2 agonists in clinical use that are taken twice daily.
Clinical studies indicate that use of long-acting beta-2 agonists leads to significant improvements in FEV1,40–42 dynamic hyperinflation, exercise tolerance,43,44 and dyspnea.45,46 These drugs have also been associated with significant improvements in health-related quality of life and in the frequency of exacerbations.47–49
In patients with asthma, long-acting beta agonists may increase the risk of death.50 In contrast, in patients with COPD, they appear to offer a survival advantage when used in combination with inhaled corticosteroids,51 and some argue that this benefit is entirely from the long-acting beta agonist (a 17% reduction in mortality) rather than the inhaled corticosteroid (0% reduction in mortality).52
Indacaterol (Arcapta), approved in July 2011, is the first once-daily beta agonist or “ultra-long-acting” beta agonist (Table 5). Possibly because it has a high affinity for the lipid raft domain of the cell membrane where beta-2 receptors are coupled to second messengers,53 the drug has a 24-hour duration of action.
In patients with COPD, inhaled indacaterol 150 μg once daily improved airflow obstruction and health status as measured by SGRQ compared with salmeterol 50 μg twice daily and placebo.54 At the higher dose of 300 μg daily, the 52-week INVOLVE trial55 demonstrated early and more sustained improvement in FEV1 compared with placebo and formoterol. In this study, a lower exacerbation rate than with placebo was also noted. The drug has also shown equivalent bronchodilator efficacy at 150 μg and 300 μg daily dosing compared with tiotropium.56
The benefits of a longer-acting bronchodilator such as indacaterol are likely mediated by smoothing out airway bronchomotor tone over 24 hours without the dips seen with shorter-acting agents and by improvement of the FEV1 trough before the subsequent dose is due, aptly named “pharmacologic stenting.”57 Once-daily dosing should also foster better adherence. The safety profile appears excellent with no increase in cardiovascular or cerebrovascular events compared with placebo.58
The FDA approved the 75-μg daily dose instead of the higher doses used in the studies mentioned above. This decision was based on the observation that there appeared to be a flattened dose-response in patients with more severe COPD, with no further improvement in trough FEV1 at higher doses.59
DOES VITAMIN D SUPPLEMENTATION HAVE A ROLE IN COPD MANAGEMENT?
Vitamin D is vital for calcium and phosphate metabolism and bone health. Low vitamin D levels are associated with diminished leg strength and falls in the elderly.60 Osteoporosis, preventable with vitamin D and calcium supplementation, is linked to thoracic vertebral fracture and consequent reduced lung function.61,62
Patients with COPD are at higher risk of vitamin D deficiency, and more so if they also are obese, have advanced airflow obstruction, are depressed, or smoke.62 Therefore, there are sound reasons to look for vitamin D deficiency in patients with COPD and to treat it if the 25-hydroxyvitamin D level is less than 10 ng/ mL (Table 6).
Vitamin D may also have antimicrobial and immunomodulatory effects.63 Since COPD exacerbations are frequently caused by infection, it was hypothesized that vitamin D supplementation might reduce the rate of exacerbations.
In a study in 182 patients with moderate to very severe COPD and a history of recent exacerbations, high-dose vitamin D supplementation (100,000 IU) was given every 4 weeks for 1 year.64 There were no differences in the time to first exacerbation, in the rate of exacerbation, hospitalization, or death, or in quality of life between the placebo and intervention groups. However, subgroup analysis indicated that, in those with severe vitamin D deficiency at baseline, the exacerbation rate was reduced by more than 40%.
Comment. We recommend screening for vitamin D deficiency in patients with COPD. Supplementation is appropriate in those with low levels, but data indicate no role in those with normal levels.
WHAT ARE THE NONPHARMACOLOGIC APPROACHES TO COPD TREATMENT?
Noninvasive positive-pressure ventilation
Nocturnal noninvasive positive-pressure ventilation may be beneficial in patients with severe COPD, daytime hypercapnia, and nocturnal hypoventilation, particularly if higher inspiratory pressures are selected (Table 7).65,66
For instance, a randomized controlled trial of noninvasive positive-pressure ventilation plus long-term oxygen therapy compared with long-term oxygen therapy alone in hypercapnic COPD demonstrated a survival benefit in favor of ventilation (hazard ratio 0.6).67
In another randomized trial,68 settings that aimed to maximally reduce Paco2 (mean inspiratory positive airway pressure 29 cm H2O with a backup rate of 17.5/min) were compared with low-intensity positive airway pressure (mean inspiratory positive airway pressure 14 cm H2O, backup rate 8/min). The high inspiratory pressures increased the daily use of ventilation by 3.6 hours per day and improved exercise-related dyspnea, daytime Paco2, FEV1, vital capacity, and health-related quality of life66 without disrupting sleep quality.68
Caveats are that acclimation to the high pressures was achieved in the hospital, and the high pressures were associated with a significant increase in air leaks.66
Comments. Whether high-pressure noninvasive positive-pressure ventilation can be routinely implemented and adopted in the outpatient setting, and whether it is associated with a survival advantage remains to be determined. The advantages of noninvasive positive-pressure ventilation in the setting of hypercapnic COPD appear to augment those of pulmonary rehabilitation, with improved quality of life, gas exchange, and exercise tolerance, and a slower decline of lung function.69
Pulmonary rehabilitation
Pulmonary rehabilitation is a multidisciplinary approach to managing COPD (Table 8).
Patients participate in three to five supervised sessions per week, each lasting 3 to 4 hours, for 6 to 12 weeks. Less-frequent sessions may not be effective. For instance, in a randomized trial, exercising twice a week was not enough.70 Additionally, a program lasting longer than 12 weeks produced more sustained benefits than shorter programs.71
A key component is an exercise protocol centered on the lower extremities (walking, cycling, treadmill), with progressive exercise intensity to a target of about 60% to 80% of the maximal exercise tolerance,72 though more modest targets of about 50% can also be beneficial.73
Exercise should be tailored to the desired outcome. For instance, training of the upper arms may help with activities of daily living. In one study, unsupported (against gravity) arm training improved upper-extremity function more than supported arm training (by ergometer).74 Ventilatory muscle training is less common, as most randomized trials have not shown conclusive evidence of benefit. Current guidelines do not recommend routine inspiratory muscle training.71
Even though indices of pulmonary function do not improve after an exercise program, randomized trials have shown that pulmonary rehabilitation improves exercise capacity, dyspnea, and health-related quality of life; improves cost-effectiveness of health care utilization; and provides psychosocial benefits that often exceed those of other therapies. Although there is no significant evidence of whether pulmonary rehabilitation improves survival in patients with COPD,71 an observational study documented improvements in BODE scores as well as a reduction in respiratory mortality rates in patients undergoing pulmonary rehabilitation.75
A limitation of pulmonary rehabilitation is that endurance and psychological and cognitive function decline significantly if exercise is not maintained. However, the role of a maintenance program is uncertain, with long-term benefits considered modest.71
Lung-volume reduction surgery
Lung-volume reduction consists of surgical wedge resections of emphysematous areas of the lung (Table 9).
The National Emphysema Treatment Trial76 randomized 1,218 patients to undergo either lung-volume reduction surgery or maximal medical therapy. Surgery improved survival, quality of life, and dyspnea in patients with upper-lobe emphysema and a low exercise capacity (corresponding to < 40 watts for men or < 25 watts for women in the maximal power achieved on cycle ergometry). While conferring no survival benefit in patients with upper-lobe-predominant emphysema and high exercise capacity, this surgery is likely to improve exercise capacity and quality of life in this subset of patients.
Importantly, the procedure is associated with a lower survival rate in patients with an FEV1 lower than 20%, homogeneous emphysema, a diffusing capacity of the lung for carbon monoxide lower than 20%, non-upper-lobe emphysema, or high baseline exercise capacity.
The proposed mechanisms of improvement of lung function include placing the diaphragm in a position with better mechanical advantage, reducing overall lung volume, better size-matching between the lungs and chest cavity, and restoring elastic recoil.76,77
Ongoing trials aim to replicate the success of lung-volume reduction using nonsurgical bronchoscopic techniques with one-way valves, coils, biologic sealants, thermal ablation, and airway stents.
Chronic obstructive pulmonary disease (COPD) has seen several changes in its assessment and treatment in recent years, reflecting advances in our understanding of this common and serious disease.
This review updates busy practitioners on the major advances, including new assessment tools and new therapies.
COMMON AND INCREASING
COPD is the third leading cause of death in the United States, behind heart disease and cancer,1 and of the top five (the others being stroke and accidents), it is the only one that increased in incidence between 2007 and 2010.2 The 11th leading cause of disability-adjusted life years worldwide in 2002, COPD is projected to become the seventh by the year 2030.3
CHARACTERIZED BY OBSTRUCTION
COPD is characterized by persistent and progressive airflow obstruction associated with chronic airway inflammation in response to noxious particles and gases. Disease of the small airways (inflammation, mucus plugging, and fibrosis) and parenchymal destruction (emphysema) limit the flow of air.
COPD is diagnosed by spirometry—specifically, a ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) of less than 0.7 after a bronchodilator is given. The severity of airflow limitation is revealed by the FEV1 as a percent of the predicted value.
Cigarette smoking is the major cause of COPD, but the prevalence of COPD is 6.6% in people who have never smoked, and one-fourth of COPD patients in the United States have never smoked.4
GOLDEN GOALS: FEWER SYMPTOMS, LOWER RISK
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) periodically issues evidence-based statements on how to prevent and treat COPD.
In its 2013 update,5 GOLD suggested two goals: improving symptoms and reducing the risk of death, exacerbations, progression of disease, and treatment-related adverse effects. The latter goal—reducing risk—is relatively new.
Exacerbations are acute inflammatory events superimposed on chronic inflammation. The inflammation is often brought on by infection6 and increases the risk of death7 and the risk of a faster decline in lung function.8
Exacerbations may characterize a phenotype of COPD. The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) analyzed the frequency of COPD exacerbations and associated factors in 2,138 patients with COPD over a period of 3 years.9 Although patients with more severe obstruction tended to have more exacerbations, some patients appeared susceptible to exacerbations irrespective of the severity of obstruction. The best predictor of exacerbations was a history of exacerbations.
HOW DO I ASSESS A PATIENT WITH COPD ON PRESENTATION?
Markers of airflow obstruction such as the FEV1 do not correlate strongly with exertional capacity and health status in patients with COPD.10,11
The BODE index (body mass index, obstruction, dyspnea score, and exercise oximetry) takes into account the multidimensional nature of COPD. It performs better than the FEV1 in predicting the risk of death.12 The propensity for exacerbations and comorbidities further modulates outcome.
Assessing symptoms
The modified British Medical Research Council (mMRC) dyspnea scale, based on work by Fletcher in 1952,13 has five grades, numbered 0 through 4:
- Grade 0—Breathless with strenuous exercise only
- Grade 1—Breathless when hurrying on level ground or walking up a slight hill
- Grade 2—Walks slower than people of the same age on level ground because of shortness of breath or has to stop when walking at own pace on level ground
- Grade 3—Stops for breath after walking about 100 yards or after a few minutes on level ground
- Grade 4—Too breathless to leave the house or breathless when dressing or undressing.
Grade 2 or higher separates symptomatic from asymptomatic COPD.
The COPD Assessment Test (CAT) (www.catestonline.org) is a proprietary questionnaire. Patients use a 6-point scale (numbered 0 though 5) to rate eight symptoms (cough, mucus production, chest tightness, shortness of breath on exertion, limitations in home activities, lack of confidence leaving the home, poor sleep, and lack of energy). A total score of 10 or higher is abnormal.
Four GOLD groups
The new GOLD guidelines (Table 1)5 define four groups of patients according to their severity of airflow obstruction, symptoms, and exacerbation history:
- Group A—fewer symptoms, low risk: Fewer symptoms (“less symptoms,” as worded in the guidelines) means a CAT score less than 10 or an mMRC grade less than 2; “low risk” means no more than one exacerbation per year and an FEV1 of at least 50%
- Group B—more symptoms, low risk: “More symptoms” means a CAT score of 10 or more or an mMRC grade of 2 or more
- Group C—fewer symptoms, high risk: “High risk” means two or more exacerbations per year or an FEV1 less than 50%
- Group D—more symptoms, high risk.
Thus, a patient with an FEV1 of 60% (moderate airflow limitation) who has had one exacerbation during the past year and a CAT score of 8 would be in group A. In contrast, a patient who has an FEV1 of 40% (severe airflow limitation), no history of exacerbations, and a CAT score of 20 would be in group D.
Updated GOLD guidelines suggest utilizing a stepwise approach to treatment, akin to asthma management guidelines, based on patient grouping.5
How accurate is the new GOLD system?
Although practical and suited for use in primary care, the new GOLD system is arbitrary and has not been thoroughly studied, and may therefore need refinement.
Lange et al14 compared the new GOLD system with the previous one in 6,628 patients with COPD. As anticipated, the new system was better at predicting exacerbations, as it incorporates a history of exacerbations in stratification. The presence of symptoms (as determined by an mMRC grade ≥ 2) was a marker of mortality risk that distinguished group A from group B, and group C from group D. Surprisingly, the rate of death was higher in group B (more symptoms, low risk) than in group C (fewer symptoms, high risk).
Notably, most patients in group C qualified for this group because of the severity of airflow obstruction, not because of a history of exacerbations. Therefore, patients whose symptoms are out of proportion to the severity of obstruction may be at higher risk of death, possibly because of comorbidities such as cardiovascular disease.15 Patients who qualified for groups C and D by having both a history of frequent exacerbations (≥ 2 per year) and symptoms rather than either one alone had a higher risk of death in 3 years.
Similarly, the symptom-assessment tool that is used—ie, the mMRC grade or the CAT score—also makes a difference.
The Health-Related Quality of Life in COPD in Europe Study16 retrospectively analyzed data from 1,817 patients to determine whether the cutoff points for symptoms as assessed by mMRC grade and CAT score were equivalent. Although the mMRC grade correlated well with overall health status, the cutoff mMRC grade of 2 or higher did not correspond to a CAT score of 10 or higher, classifying patients with health status impairment as asymptomatic (mean weighted kappa 0.626). The two tools agreed much better when the cutoff was set at an mMRC grade of 1 or higher (mean weighted kappa 0.792).16
Although assessment schemes continue to evolve as data accumulate, we believe the new system is a welcome initiative that reflects the changing notions of COPD.
Comorbidities matter
Another shift is the recognition that certain comorbidities increase the risk of death. In 1,664 patients with COPD who were followed for 51 months, 12 distinct comorbidities were associated with a higher risk of death after multivariate analysis.17
The COTE index (COPD-Specific Comorbidity Test) is based on these findings. It awards points as follows:
- 6 points for cancer of the lung, esophagus, pancreas, or breast, or for anxiety
- 2 points for all other cancers, liver cirrhosis, atrial fibrillation or flutter, diabetes with neuropathy, or pulmonary fibrosis
- 1 point for congestive heart failure, gastric or duodenal ulcer, or coronary artery disease.
A COTE index score of 4 or higher was associated with a risk of death 2.2 times higher in each quartile of the BODE index.
We strongly recommend being aware of comorbidities in COPD patients, particularly when symptoms are out of proportion to the severity of obstruction.
SHOULD I USE ANTIBIOTICS TO TREAT ALL COPD EXACERBATIONS?
Infections are thought to cause more than 80% of acute exacerbations of COPD.
Anthonisen et al,18 in a landmark trial, found broad-spectrum antibiotics to be most helpful if the patient had at least two of the three cardinal symptoms of COPD exacerbation (ie, shortness of breath, increase in sputum volume, and sputum purulence). Antibiotics decreased the rate of treatment failure and led to a more rapid clinical resolution of exacerbation. However, they did not help patients who had milder exacerbations.
Antibiotics may nevertheless have a role in ambulatory patients with mild to moderate COPD who present with exacerbations characterized by one or more cardinal symptoms.
Llor et al,19 in a multicenter randomized double-blind placebo-controlled trial in Spain, concluded that amoxicillin clavulanate (Augmentin) led to higher clinical cure rates and longer time to the next exacerbation in these patients. Most of the benefit was in patients with more symptoms, consistent with the results of the study by Anthonisen et al.18
There is also strong evidence to support the use of antibiotics in addition to systemic corticosteroids in hospitalized patients with acute exacerbations of COPD. A 7-day course of doxycycline (Vibramycin) added to a standard regimen of corticosteroids was associated with higher rates of clinical and microbiological cure on day 10 of the exacerbation.20 In a large retrospective cohort study in 84,621 hospitalized patients with COPD exacerbations, fewer of those who received antibiotics needed mechanical ventilation, died, or were readmitted.21 Although sicker patients received antibiotics more frequently, their mortality rate was lower than in those who did not receive antibiotics, who were presumably less sick.
A meta-analysis confirmed the salutary effect of antibiotics in inpatients and particularly those admitted to the intensive care unit.22 Mortality rates and hospital length of stay were not affected in patients who were not in intensive care.
Biomarkers such as procalcitonin might help reduce the unnecessary use of antibiotics. Stolz et al23 conducted a randomized controlled trial in which they based the decision to give antibiotics on a threshold procalcitonin level of at least 1 μg/L in hospitalized patients with COPD exacerbation. The rate of antibiotic use was reduced by more than 40% in the procalcitonin group without any difference in clinical outcomes, 6-month exacerbation rate, or rehospitalization compared with controls. Nonstandardized procalcitonin assays are a possible barrier to the widespread adoption of this threshold.
Comment. In general, we recommend antibiotics for hospitalized patients with COPD exacerbation and look forward to confirmatory data that support the use of biomarkers. For outpatients, we find the Anthonisen criteria useful for decision-making at the point of care.
ARE THERE ANY NEW INTERVENTIONS TO PREVENT COPD EXACERBATIONS?
Macrolides
Macrolides have a proven role in managing chronic suppurative respiratory diseases such as cystic fibrosis24 and diffuse panbronchiolitis.25 Since they are beneficial at lower doses than those used to treat infection, the mechanism may be anti-inflammatory rather than antimicrobial.
Albert et al26 assigned 1,142 patients who had had a COPD exacerbation within a year before enrollment or who were on home oxygen therapy to receive azithromycin (Zithromax) 250 mg daily or placebo.25 The azithromycin group had fewer acute exacerbations (hazard ratio 0.73, 95% CI 0.63–0.84, P < .001), and more patients in the azithromycin group achieved clinically significant improvements in quality of life, ie, a reduction in the St. George’s Respiratory Questionnaire (SGRQ) score of at least 4 points (43% vs 36%, P = .03). Adverse events that were more common in the azithromycin group were hearing loss (25% vs 20%) and macrolide-resistant strains in nasopharyngeal secretions (81% vs 41%). In subgroup analysis, the benefit in terms of reducing exacerbations was greater in patients over age 65, patients on home oxygen, and patients with moderate or severe obstruction compared with those with very severe obstruction.
Comment. Macrolides are a valuable addition to the agents available for preventing COPD exacerbation (Table 2), but their role is still uncertain. Potential topics of research are whether these drugs have a role in patients already on preventive regimens, whether they would have a greater effect in distinct patient populations (eg, patients who have two or more exacerbations per year), and whether their broader use would lead to a change in the resident flora in the community.
Clinicians should exercise caution in the use of azithromycin in light of recent concern about associated cardiac morbidity and death. All patients should undergo electrocardiography to assess the QTc interval before starting treatment, as in the trial by Albert et al.26
Phosphodiesterase inhibitors
Roflumilast (Daliresp) is an oral phosphodiesterase 4 inhibitor approved for treating exacerbations and symptoms of chronic bronchitis in patients with severe COPD (Table 3). Phosphodiesterase 4, one of the 11 isoforms of the enzyme, is found in immune and inflammatory cells and promotes inflammatory responses. Roflumilast has anti-inflammatory properties but no acute bronchodilatory effect.27 Several phase 3 trials found the compound to have beneficial effects.
Calverley et al28 performed two placebo-controlled double-blind trials in outpatients with the clinical diagnosis of COPD who had chronic cough; increased sputum production; at least one recorded exacerbation requiring corticosteroids or hospitalization, or both; and an FEV1 of 50% or less. Patients were randomized to receive roflumilast 500 μg once a day (n = 1,537) or placebo (n = 1,554) for 1 year. The rate of moderate to severe exacerbations was 1.17 per year with roflumilast vs 1.37 with placebo (P < .0003). Adverse events were significantly more common with roflumilast and were related to the known side effects of the drug, namely, diarrhea, weight loss, decreased appetite, and nausea.
Fabbri et al29 performed two other placebo-controlled double-blind multicenter trials, studying the combinations of roflumilast with salmeterol (Serevent) and roflumilast with tiotropium (Spiriva) compared with placebo in 1,676 patients with COPD who had post-bronchodilator FEV1 values of 40% to 70% of predicted. The mean prebronchodilator FEV1 improved by 49 mL (P < .0001) in the salmeterol-plus-roflumilast trial and by 80 mL (P < .0001) in the tiotropium-plus-roflumilast trial compared with placebo. Fewer patients on roflumilast had exacerbations of any severity in both trials (risk ratio 0.82, P = .0419 and risk ratio 0.75, P = .0169, respectively).
No trial has yet addressed whether roflumilast is better than the combination of a long-acting muscarinic antagonist and a beta agonist, or whether roflumilast can be substituted for inhaled corticosteroids in a new triple-therapy combination. Clinicians should also be aware of psychiatric side effects of roflumilast, which include depression and, possibly, suicide.
ARE THERE ANY NEW BRONCHODILATORS FOR PATIENTS WITH COPD?
Long-acting muscarinic antagonists
Reversible airflow obstruction and mucus secretion are determined by the vagal cholinergic tone in patients with COPD.30 Antagonism of cholinergic (muscarinic) receptors results in bronchodilation and reduction in mucus production. Consequently, inhaled anticholinergic agents are the first-line therapy for COPD (Table 4).
Tiotropium bromide is a long-acting antimuscarinic approved in 2002 by the US Food and Drug Administration (FDA). The UPLIFT trial (Understanding Potential Long-Term Impacts on Function With Tiotropium)31 enrolled 5,993 patients with a mean FEV1 of 48% of predicted. Over a 4-year follow-up, significant improvements in mean FEV1 values (ranging from 87 mL to 103 mL before bronchodilation and 47 mL to 65 mL after bronchodilation, P < .001) in the tiotropium group were observed compared with placebo. The rate of the primary end point—the rate of decline in mean FEV1—was not different between tiotropium and placebo. However, there were important salutary effects in multiple clinical end points in the tiotropium group. Health-related quality of life as measured by the SGRQ improved in a clinically significant manner (> 4 points) in favor of tiotropium in a higher proportion of patients (45% vs 36%, P < .001). Tiotropium reduced the number of exacerbations per patient year (0.73 ± 0.02 vs 0.85 ± 0.02, RR = 0.86 (95% CI 0.81–0.91), P < .001) and the risk of respiratory failure (RR = 0.67, 95% CI 0.51–0.89). There were no significant differences in the risk of myocardial infarction, stroke, or pneumonia.
Aclidinium bromide (Tudorza Pressair) is a long-acting antimuscarinic recently approved by the FDA. Compared with tiotropium, it has a slightly faster onset of action and a considerably shorter half-life (29 hours vs 64 hours).32,33 Its dosage is 400 μg twice daily by inhalation. It provides sustained bronchodilation over 24 hours and may have a favorable side-effect profile, because it undergoes rapid hydrolysis in human plasma.34
ACCORD COPD I35 and ATTAIN,36 two phase 3 trials in patients with moderate-to severe COPD, found that twice-daily aclidinium was associated with statistically and clinically significant (> 100 mL) improvements in trough and peak FEV1 compared with placebo. Health status (assessed by SGRQ) and dyspnea (assessed by transitional dyspnea index) also improved significantly. However, improvements beyond minimum clinically significant thresholds were achieved only with 400 μg twice-daily dosing.
To date, no study has evaluated the impact of aclidinium on COPD exacerbation as a primary end point. Fewer moderate to severe exacerbations were reported in an earlier 52-week study of once-daily aclidinium (ACCLAIM COPD II) but not in ACCLAIM COPD I.37
Aclidinium may offer an advantage over tiotropium in patients who have nocturnal symptoms. Twice-daily aclidinium 400 μg was associated with superior FEV1 area-under-the-curve values compared with placebo and tiotropium, the difference mostly owing to improved nocturnal profile.38
Long-acting beta-2 agonists
Stimulation of airway beta-2 receptors relaxes smooth muscles and consequently dilates bronchioles via a cyclic adenosine monophosphate-dependent pathway.39
Short-acting beta-2 agonists such as albuterol and terbutaline have long been used as rescue medications for obstructive lung disease. Long-acting beta-2 agonists provide sustained bronchodilation and are therefore more efficacious as maintenance medications. Salmeterol, formoterol (Foradil), and arformoterol (Brovana) are long-acting beta-2 agonists in clinical use that are taken twice daily.
Clinical studies indicate that use of long-acting beta-2 agonists leads to significant improvements in FEV1,40–42 dynamic hyperinflation, exercise tolerance,43,44 and dyspnea.45,46 These drugs have also been associated with significant improvements in health-related quality of life and in the frequency of exacerbations.47–49
In patients with asthma, long-acting beta agonists may increase the risk of death.50 In contrast, in patients with COPD, they appear to offer a survival advantage when used in combination with inhaled corticosteroids,51 and some argue that this benefit is entirely from the long-acting beta agonist (a 17% reduction in mortality) rather than the inhaled corticosteroid (0% reduction in mortality).52
Indacaterol (Arcapta), approved in July 2011, is the first once-daily beta agonist or “ultra-long-acting” beta agonist (Table 5). Possibly because it has a high affinity for the lipid raft domain of the cell membrane where beta-2 receptors are coupled to second messengers,53 the drug has a 24-hour duration of action.
In patients with COPD, inhaled indacaterol 150 μg once daily improved airflow obstruction and health status as measured by SGRQ compared with salmeterol 50 μg twice daily and placebo.54 At the higher dose of 300 μg daily, the 52-week INVOLVE trial55 demonstrated early and more sustained improvement in FEV1 compared with placebo and formoterol. In this study, a lower exacerbation rate than with placebo was also noted. The drug has also shown equivalent bronchodilator efficacy at 150 μg and 300 μg daily dosing compared with tiotropium.56
The benefits of a longer-acting bronchodilator such as indacaterol are likely mediated by smoothing out airway bronchomotor tone over 24 hours without the dips seen with shorter-acting agents and by improvement of the FEV1 trough before the subsequent dose is due, aptly named “pharmacologic stenting.”57 Once-daily dosing should also foster better adherence. The safety profile appears excellent with no increase in cardiovascular or cerebrovascular events compared with placebo.58
The FDA approved the 75-μg daily dose instead of the higher doses used in the studies mentioned above. This decision was based on the observation that there appeared to be a flattened dose-response in patients with more severe COPD, with no further improvement in trough FEV1 at higher doses.59
DOES VITAMIN D SUPPLEMENTATION HAVE A ROLE IN COPD MANAGEMENT?
Vitamin D is vital for calcium and phosphate metabolism and bone health. Low vitamin D levels are associated with diminished leg strength and falls in the elderly.60 Osteoporosis, preventable with vitamin D and calcium supplementation, is linked to thoracic vertebral fracture and consequent reduced lung function.61,62
Patients with COPD are at higher risk of vitamin D deficiency, and more so if they also are obese, have advanced airflow obstruction, are depressed, or smoke.62 Therefore, there are sound reasons to look for vitamin D deficiency in patients with COPD and to treat it if the 25-hydroxyvitamin D level is less than 10 ng/ mL (Table 6).
Vitamin D may also have antimicrobial and immunomodulatory effects.63 Since COPD exacerbations are frequently caused by infection, it was hypothesized that vitamin D supplementation might reduce the rate of exacerbations.
In a study in 182 patients with moderate to very severe COPD and a history of recent exacerbations, high-dose vitamin D supplementation (100,000 IU) was given every 4 weeks for 1 year.64 There were no differences in the time to first exacerbation, in the rate of exacerbation, hospitalization, or death, or in quality of life between the placebo and intervention groups. However, subgroup analysis indicated that, in those with severe vitamin D deficiency at baseline, the exacerbation rate was reduced by more than 40%.
Comment. We recommend screening for vitamin D deficiency in patients with COPD. Supplementation is appropriate in those with low levels, but data indicate no role in those with normal levels.
WHAT ARE THE NONPHARMACOLOGIC APPROACHES TO COPD TREATMENT?
Noninvasive positive-pressure ventilation
Nocturnal noninvasive positive-pressure ventilation may be beneficial in patients with severe COPD, daytime hypercapnia, and nocturnal hypoventilation, particularly if higher inspiratory pressures are selected (Table 7).65,66
For instance, a randomized controlled trial of noninvasive positive-pressure ventilation plus long-term oxygen therapy compared with long-term oxygen therapy alone in hypercapnic COPD demonstrated a survival benefit in favor of ventilation (hazard ratio 0.6).67
In another randomized trial,68 settings that aimed to maximally reduce Paco2 (mean inspiratory positive airway pressure 29 cm H2O with a backup rate of 17.5/min) were compared with low-intensity positive airway pressure (mean inspiratory positive airway pressure 14 cm H2O, backup rate 8/min). The high inspiratory pressures increased the daily use of ventilation by 3.6 hours per day and improved exercise-related dyspnea, daytime Paco2, FEV1, vital capacity, and health-related quality of life66 without disrupting sleep quality.68
Caveats are that acclimation to the high pressures was achieved in the hospital, and the high pressures were associated with a significant increase in air leaks.66
Comments. Whether high-pressure noninvasive positive-pressure ventilation can be routinely implemented and adopted in the outpatient setting, and whether it is associated with a survival advantage remains to be determined. The advantages of noninvasive positive-pressure ventilation in the setting of hypercapnic COPD appear to augment those of pulmonary rehabilitation, with improved quality of life, gas exchange, and exercise tolerance, and a slower decline of lung function.69
Pulmonary rehabilitation
Pulmonary rehabilitation is a multidisciplinary approach to managing COPD (Table 8).
Patients participate in three to five supervised sessions per week, each lasting 3 to 4 hours, for 6 to 12 weeks. Less-frequent sessions may not be effective. For instance, in a randomized trial, exercising twice a week was not enough.70 Additionally, a program lasting longer than 12 weeks produced more sustained benefits than shorter programs.71
A key component is an exercise protocol centered on the lower extremities (walking, cycling, treadmill), with progressive exercise intensity to a target of about 60% to 80% of the maximal exercise tolerance,72 though more modest targets of about 50% can also be beneficial.73
Exercise should be tailored to the desired outcome. For instance, training of the upper arms may help with activities of daily living. In one study, unsupported (against gravity) arm training improved upper-extremity function more than supported arm training (by ergometer).74 Ventilatory muscle training is less common, as most randomized trials have not shown conclusive evidence of benefit. Current guidelines do not recommend routine inspiratory muscle training.71
Even though indices of pulmonary function do not improve after an exercise program, randomized trials have shown that pulmonary rehabilitation improves exercise capacity, dyspnea, and health-related quality of life; improves cost-effectiveness of health care utilization; and provides psychosocial benefits that often exceed those of other therapies. Although there is no significant evidence of whether pulmonary rehabilitation improves survival in patients with COPD,71 an observational study documented improvements in BODE scores as well as a reduction in respiratory mortality rates in patients undergoing pulmonary rehabilitation.75
A limitation of pulmonary rehabilitation is that endurance and psychological and cognitive function decline significantly if exercise is not maintained. However, the role of a maintenance program is uncertain, with long-term benefits considered modest.71
Lung-volume reduction surgery
Lung-volume reduction consists of surgical wedge resections of emphysematous areas of the lung (Table 9).
The National Emphysema Treatment Trial76 randomized 1,218 patients to undergo either lung-volume reduction surgery or maximal medical therapy. Surgery improved survival, quality of life, and dyspnea in patients with upper-lobe emphysema and a low exercise capacity (corresponding to < 40 watts for men or < 25 watts for women in the maximal power achieved on cycle ergometry). While conferring no survival benefit in patients with upper-lobe-predominant emphysema and high exercise capacity, this surgery is likely to improve exercise capacity and quality of life in this subset of patients.
Importantly, the procedure is associated with a lower survival rate in patients with an FEV1 lower than 20%, homogeneous emphysema, a diffusing capacity of the lung for carbon monoxide lower than 20%, non-upper-lobe emphysema, or high baseline exercise capacity.
The proposed mechanisms of improvement of lung function include placing the diaphragm in a position with better mechanical advantage, reducing overall lung volume, better size-matching between the lungs and chest cavity, and restoring elastic recoil.76,77
Ongoing trials aim to replicate the success of lung-volume reduction using nonsurgical bronchoscopic techniques with one-way valves, coils, biologic sealants, thermal ablation, and airway stents.
- Miniño AM, Xu J, Kochanek KD. Deaths: preliminary data for 2008. National Vital Statistics Reports 2010; 59:1–52. http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_02.pdf. Accessed April 10, 2014.
- Murphy SL, Xu J, Kochanek KD. Deaths: preliminary data from 2010. National Vital Statistics Reports 2012; 60:1–51. http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf. Accessed April 10, 2014.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3:e442.
- Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest 2005; 128:1239–1244.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). http://www.goldcopd.org/Guidelines/guidelines-resources.html. Accessed April 10, 2014.
- Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006; 173:1114–1121.
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60:925–931.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57:847–852.
- Hurst JR, Vestbo J, Anzueto A, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363:1128–1138.
- Cooper CB. The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med 2006; 119(suppl 1):21–31.
- Jones PW. Issues concerning health-related quality of life in COPD. Chest 1995; 107(suppl):187S–193S.
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:1005–1012.
- Fletcher CM. The clinical diagnosis of pulmonary emphysema—an experimental study. J Royal Soc Med 1952; 45:577–584.
- Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med 2012; 186:975–981.
- Hurst J. Phenotype-based care in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:935–936.
- Jones PW, Adamek L, Nadeau G, Banik N. Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification. Eur Respir J 2013; 42:647–654.
- Divo M, Cote C, de Torres JP, et al; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:155–161.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106:196–204.
- Llor C, Moragas A, Hernández S, Bayona C, Miravitlles M. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:716–723.
- Daniels JM, Snijders D, de Graaff CS, Vlaspolder F, Jansen HM, Boersma WG. Antibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181:150–157.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA 2010; 303:2035–2042.
- Vollenweider DJ, Jarrett H, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; 12:CD010257.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007; 131:9–19.
- Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 2002; 57:212–216.
- Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med 1998; 157:1829–1832.
- Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365:689–698.
- Gross NJ, Giembycz MA, Rennard SI. Treatment of chronic obstructive pulmonary disease with roflumilast, a new phosphodiesterase 4 inhibitor. COPD 2010; 7:141–153.
- Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009; 374:685–694.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with long acting bronchodilators: two randomised clinical trials. Lancet 2009; 374:695–703.
- Gross NJ, Skorodin MS. Anticholinergic, antimuscarinic bronchodilators. Am Rev Respir Dis 1984; 129:856–870.
- Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359:1543–1554.
- Cazzola M. Aclidinium bromide, a novel long-acting muscarinic M3 antagonist for the treatment of COPD. Curr Opin Investig Drugs 2009; 10:482–490.
- Gavaldà A, Miralpeix M, Ramos I, et al. Characterization of aclidinium bromide, a novel inhaled muscarinic antagonist, with long duration of action and a favorable pharmacological profile. J Pharmacol Exp Ther 2009; 331:740–751.
- Alagha K, Bourdin A, Tummino C, Chanez P. An update on the efficacy and safety of aclidinium bromide in patients with COPD. Ther Adv Respir Dis 2011; 5:19–28.
- Kerwin EM, D’Urzo AD, Gelb AF, Lakkis H, Garcia Gil E, Caracta CF; ACCORD I study investigators. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD 2012; 9:90–101.
- Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J 2012; 40:830–836.
- Jones PW, Rennard SI, Agusti A, et al. Efficacy and safety of once-daily aclidinium in chronic obstructive pulmonary disease. Respir Res 2011; 12:55.
- Fuhr R, Magnussen H, Sarem K, et al. Efficacy of aclidinium bromide 400 μg twice daily compared with placebo and tiotropium in patients with moderate to severe COPD. Chest 2012; 141:745–752.
- Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res 2010; 11:149.
- Mahler DA, Donohue JF, Barbee RA, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999; 115:957–965.
- Campbell M, Eliraz A, Johansson G, et al. Formoterol for maintenance and as-needed treatment of chronic obstructive pulmonary disease. Respir Med 2005; 99:1511–1520.
- Hanrahan JP, Hanania NA, Calhoun WJ, Sahn SA, Sciarappa K, Baumgartner RA. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD 2008; 5:25–34.
- Neder JA, Fuld JP, Overend T, et al. Effects of formoterol on exercise tolerance in severely disabled patients with COPD. Respir Med 2007; 101:2056–2064.
- O’Donnell DE, Voduc N, Fitzpatrick M, Webb KA. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur Respir J 2004; 24:86–94.
- Rennard SI, Anderson W, ZuWallack R, et al. Use of a long-acting inhaled beta 2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163:1087–1092.
- Schultze-Werninghaus G. Multicenter 1-year trial on formoterol, a new long-acting beta 2-agonist, in chronic obstructive airway disease. Lung 1990; 168(suppl):83–89.
- Rodrigo GJ, Nannini LJ, Rodríguez-Roisin R. Safety of long-acting beta-agonists in stable COPD: a systematic review. Chest 2008; 133:1079–1087.
- Baker WL, Baker EL, Coleman CI. Pharmacologic treatments for chronic obstructive pulmonary disease: a mixed-treatment comparison meta-analysis. Pharmacotherapy 2009; 29:891–905.
- Rodrigo GJ, Castro-Rodriguez JA, Plaza V. Safety and efficacy of combined long-acting beta-agonists and inhaled corticosteroids vs long-acting beta-agonists monotherapy for stable COPD: a systematic review. Chest 2009; 136:1029–1038.
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006; 129:15–26.
- Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–789.
- Suissa S, Ernst P, Vandemheen KL, Aaron SD. Methodological issues in therapeutic trials of COPD. Eur Respir J 2008; 31:927–933.
- Lombardi D, Cuenoud B, Krämer SD. Lipid membrane interactions of indacaterol and salmeterol: do they influence their pharmacological properties? Eur J Pharm Sci 2009; 38:533–547.
- Kornmann O, Dahl R, Centanni S, et al; INLIGHT-2 (Indacaterol Efficacy Evaluation Using 150-μg Doses with COPD Patients) study investigators. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J 2011; 37:273–279.
- Dahl R, Chung KF, Buhl R, et al; INVOLVE (INdacaterol: Value in COPD: Longer Term Validation of Efficacy and Safety) Study Investigators. Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax 2010; 65:473–479.
- Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 2010; 182:155–162.
- Beeh KM, Beier J. The short, the long and the “ultra-long”: why duration of bronchodilator action matters in chronic obstructive pulmonary disease. Adv Ther 2010; 27:150–159.
- Worth H, Chung KF, Felser JM, Hu H, Rueegg P. Cardio- and cerebrovascular safety of indacaterol vs formoterol, salmeterol, tiotropium and placebo in COPD. Respir Med 2011; 105:571–579.
- Chowdhury BA, Seymour SM, Michele TM, Durmowicz AG, Liu D, Rosebraugh CJ. The risks and benefits of indacaterol--the FDA’s review. N Engl J Med 2011; 365:2247–2249.
- Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA 2004; 291:1999–2006.
- Leech JA, Dulberg C, Kellie S, Pattee L, Gay J. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis 1990; 141:68–71.
- Persson LJ, Aanerud M, Hiemstra PS, Hardie JA, Bakke PS, Eagan TM. Chronic obstructive pulmonary disease is associated with low levels of vitamin D. PLoS One 2012; 7:e38934.
- Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med 2009; 179:630–636.
- Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2012; 156:105–114.
- Casanova C, Celli BR, Tost L, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 2000; 118:1582–1590.
- Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010; 65:303–308.
- McEvoy RD, Pierce RJ, Hillman D, et al; Australian trial of non-invasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64:561–566.
- Dreher M, Ekkernkamp E, Walterspacher S, et al. Noninvasive ventilation in COPD: impact of inspiratory pressure levels on sleep quality. Chest 2011; 140:939–945.
- Duiverman ML, Wempe JB, Bladder G, et al. Two-year home-based nocturnal noninvasive ventilation added to rehabilitation in chronic obstructive pulmonary disease patients: a randomized controlled trial. Respir Res 2011; 12:112.
- Ringbaek TJ, Broendum E, Hemmingsen L, et al. Rehabilitation of patients with chronic obstructive pulmonary disease. Exercise twice a week is not sufficient! Respir Med 2000; 94:150–154.
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007; 131(suppl):4S–42S.
- Vallet G, Ahmaïdi S, Serres I, et al. Comparison of two training programmes in chronic airway limitation patients: standardized versus individualized protocols. Eur Respir J 1997; 10:114–122.
- Vogiatzis I, Williamson AF, Miles J, Taylor IK. Physiological response to moderate exercise workloads in a pulmonary rehabilitation program in patients with varying degrees of airflow obstruction. Chest 1999; 116:1200–1207.
- Martinez FJ, Vogel PD, Dupont DN, Stanopoulos I, Gray A, Beamis JF. Supported arm exercise vs unsupported arm exercise in the rehabilitation of patients with severe chronic airflow obstruction. Chest 1993; 103:1397–1402.
- Cote CG, Celli BR. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J 2005; 26:630–636.
- Fishman A, Martinez F, Naunheim K, et al; National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348:2059–2073.
- Naunheim KS, Wood DE, Mohsenifar Z, et al; National Emphysema Treatment Trial Research Group. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg 2006; 82:431–443.
- Miniño AM, Xu J, Kochanek KD. Deaths: preliminary data for 2008. National Vital Statistics Reports 2010; 59:1–52. http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_02.pdf. Accessed April 10, 2014.
- Murphy SL, Xu J, Kochanek KD. Deaths: preliminary data from 2010. National Vital Statistics Reports 2012; 60:1–51. http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf. Accessed April 10, 2014.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3:e442.
- Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest 2005; 128:1239–1244.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). http://www.goldcopd.org/Guidelines/guidelines-resources.html. Accessed April 10, 2014.
- Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006; 173:1114–1121.
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60:925–931.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57:847–852.
- Hurst JR, Vestbo J, Anzueto A, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363:1128–1138.
- Cooper CB. The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med 2006; 119(suppl 1):21–31.
- Jones PW. Issues concerning health-related quality of life in COPD. Chest 1995; 107(suppl):187S–193S.
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:1005–1012.
- Fletcher CM. The clinical diagnosis of pulmonary emphysema—an experimental study. J Royal Soc Med 1952; 45:577–584.
- Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med 2012; 186:975–981.
- Hurst J. Phenotype-based care in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:935–936.
- Jones PW, Adamek L, Nadeau G, Banik N. Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification. Eur Respir J 2013; 42:647–654.
- Divo M, Cote C, de Torres JP, et al; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:155–161.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106:196–204.
- Llor C, Moragas A, Hernández S, Bayona C, Miravitlles M. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:716–723.
- Daniels JM, Snijders D, de Graaff CS, Vlaspolder F, Jansen HM, Boersma WG. Antibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181:150–157.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA 2010; 303:2035–2042.
- Vollenweider DJ, Jarrett H, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; 12:CD010257.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007; 131:9–19.
- Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 2002; 57:212–216.
- Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med 1998; 157:1829–1832.
- Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365:689–698.
- Gross NJ, Giembycz MA, Rennard SI. Treatment of chronic obstructive pulmonary disease with roflumilast, a new phosphodiesterase 4 inhibitor. COPD 2010; 7:141–153.
- Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009; 374:685–694.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with long acting bronchodilators: two randomised clinical trials. Lancet 2009; 374:695–703.
- Gross NJ, Skorodin MS. Anticholinergic, antimuscarinic bronchodilators. Am Rev Respir Dis 1984; 129:856–870.
- Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359:1543–1554.
- Cazzola M. Aclidinium bromide, a novel long-acting muscarinic M3 antagonist for the treatment of COPD. Curr Opin Investig Drugs 2009; 10:482–490.
- Gavaldà A, Miralpeix M, Ramos I, et al. Characterization of aclidinium bromide, a novel inhaled muscarinic antagonist, with long duration of action and a favorable pharmacological profile. J Pharmacol Exp Ther 2009; 331:740–751.
- Alagha K, Bourdin A, Tummino C, Chanez P. An update on the efficacy and safety of aclidinium bromide in patients with COPD. Ther Adv Respir Dis 2011; 5:19–28.
- Kerwin EM, D’Urzo AD, Gelb AF, Lakkis H, Garcia Gil E, Caracta CF; ACCORD I study investigators. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD 2012; 9:90–101.
- Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J 2012; 40:830–836.
- Jones PW, Rennard SI, Agusti A, et al. Efficacy and safety of once-daily aclidinium in chronic obstructive pulmonary disease. Respir Res 2011; 12:55.
- Fuhr R, Magnussen H, Sarem K, et al. Efficacy of aclidinium bromide 400 μg twice daily compared with placebo and tiotropium in patients with moderate to severe COPD. Chest 2012; 141:745–752.
- Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res 2010; 11:149.
- Mahler DA, Donohue JF, Barbee RA, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999; 115:957–965.
- Campbell M, Eliraz A, Johansson G, et al. Formoterol for maintenance and as-needed treatment of chronic obstructive pulmonary disease. Respir Med 2005; 99:1511–1520.
- Hanrahan JP, Hanania NA, Calhoun WJ, Sahn SA, Sciarappa K, Baumgartner RA. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD 2008; 5:25–34.
- Neder JA, Fuld JP, Overend T, et al. Effects of formoterol on exercise tolerance in severely disabled patients with COPD. Respir Med 2007; 101:2056–2064.
- O’Donnell DE, Voduc N, Fitzpatrick M, Webb KA. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur Respir J 2004; 24:86–94.
- Rennard SI, Anderson W, ZuWallack R, et al. Use of a long-acting inhaled beta 2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163:1087–1092.
- Schultze-Werninghaus G. Multicenter 1-year trial on formoterol, a new long-acting beta 2-agonist, in chronic obstructive airway disease. Lung 1990; 168(suppl):83–89.
- Rodrigo GJ, Nannini LJ, Rodríguez-Roisin R. Safety of long-acting beta-agonists in stable COPD: a systematic review. Chest 2008; 133:1079–1087.
- Baker WL, Baker EL, Coleman CI. Pharmacologic treatments for chronic obstructive pulmonary disease: a mixed-treatment comparison meta-analysis. Pharmacotherapy 2009; 29:891–905.
- Rodrigo GJ, Castro-Rodriguez JA, Plaza V. Safety and efficacy of combined long-acting beta-agonists and inhaled corticosteroids vs long-acting beta-agonists monotherapy for stable COPD: a systematic review. Chest 2009; 136:1029–1038.
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006; 129:15–26.
- Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–789.
- Suissa S, Ernst P, Vandemheen KL, Aaron SD. Methodological issues in therapeutic trials of COPD. Eur Respir J 2008; 31:927–933.
- Lombardi D, Cuenoud B, Krämer SD. Lipid membrane interactions of indacaterol and salmeterol: do they influence their pharmacological properties? Eur J Pharm Sci 2009; 38:533–547.
- Kornmann O, Dahl R, Centanni S, et al; INLIGHT-2 (Indacaterol Efficacy Evaluation Using 150-μg Doses with COPD Patients) study investigators. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J 2011; 37:273–279.
- Dahl R, Chung KF, Buhl R, et al; INVOLVE (INdacaterol: Value in COPD: Longer Term Validation of Efficacy and Safety) Study Investigators. Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax 2010; 65:473–479.
- Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 2010; 182:155–162.
- Beeh KM, Beier J. The short, the long and the “ultra-long”: why duration of bronchodilator action matters in chronic obstructive pulmonary disease. Adv Ther 2010; 27:150–159.
- Worth H, Chung KF, Felser JM, Hu H, Rueegg P. Cardio- and cerebrovascular safety of indacaterol vs formoterol, salmeterol, tiotropium and placebo in COPD. Respir Med 2011; 105:571–579.
- Chowdhury BA, Seymour SM, Michele TM, Durmowicz AG, Liu D, Rosebraugh CJ. The risks and benefits of indacaterol--the FDA’s review. N Engl J Med 2011; 365:2247–2249.
- Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA 2004; 291:1999–2006.
- Leech JA, Dulberg C, Kellie S, Pattee L, Gay J. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis 1990; 141:68–71.
- Persson LJ, Aanerud M, Hiemstra PS, Hardie JA, Bakke PS, Eagan TM. Chronic obstructive pulmonary disease is associated with low levels of vitamin D. PLoS One 2012; 7:e38934.
- Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med 2009; 179:630–636.
- Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2012; 156:105–114.
- Casanova C, Celli BR, Tost L, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 2000; 118:1582–1590.
- Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010; 65:303–308.
- McEvoy RD, Pierce RJ, Hillman D, et al; Australian trial of non-invasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64:561–566.
- Dreher M, Ekkernkamp E, Walterspacher S, et al. Noninvasive ventilation in COPD: impact of inspiratory pressure levels on sleep quality. Chest 2011; 140:939–945.
- Duiverman ML, Wempe JB, Bladder G, et al. Two-year home-based nocturnal noninvasive ventilation added to rehabilitation in chronic obstructive pulmonary disease patients: a randomized controlled trial. Respir Res 2011; 12:112.
- Ringbaek TJ, Broendum E, Hemmingsen L, et al. Rehabilitation of patients with chronic obstructive pulmonary disease. Exercise twice a week is not sufficient! Respir Med 2000; 94:150–154.
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007; 131(suppl):4S–42S.
- Vallet G, Ahmaïdi S, Serres I, et al. Comparison of two training programmes in chronic airway limitation patients: standardized versus individualized protocols. Eur Respir J 1997; 10:114–122.
- Vogiatzis I, Williamson AF, Miles J, Taylor IK. Physiological response to moderate exercise workloads in a pulmonary rehabilitation program in patients with varying degrees of airflow obstruction. Chest 1999; 116:1200–1207.
- Martinez FJ, Vogel PD, Dupont DN, Stanopoulos I, Gray A, Beamis JF. Supported arm exercise vs unsupported arm exercise in the rehabilitation of patients with severe chronic airflow obstruction. Chest 1993; 103:1397–1402.
- Cote CG, Celli BR. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J 2005; 26:630–636.
- Fishman A, Martinez F, Naunheim K, et al; National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348:2059–2073.
- Naunheim KS, Wood DE, Mohsenifar Z, et al; National Emphysema Treatment Trial Research Group. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg 2006; 82:431–443.
KEY POINTS
- A new COPD classification scheme is based on severity, symptoms, and exacerbations.
- Azithromycin 250 mg daily prevents exacerbations of COPD in those at high risk.
- Long-acting muscarinic antagonists such as aclidinium and tiotropium are first-line therapy.
- Relatively new options include roflumilast, an oral phosphodiesterase inhibitor, and indacaterol, an ultra-long-acting beta agonist that is taken once daily.
- Nondrug interventions include pulmonary rehabilitation, vitamin D supplementation, noninvasive positive-pressure ventilation, and lung-volume reduction surgery.
Noninvasive positive pressure ventilation for stable outpatients: CPAP and beyond
Noninvasive positive pressure ventilation (NIPPV) is any form of positive ventilatory support applied without an endotracheal tube, including continuous positive airway pressure (CPAP).1 The role of NIPPV in acute care has been discussed in an earlier review in the Cleveland Clinic Journal of Medicine.2
NIPPV is also used at night in outpatients with stable chronic conditions, first used in the 1980s in the treatment of obstructive sleep apnea3 and neuromuscular diseases,4 and since then in several other conditions including sleep disorders associated with congestive heart failure (including sleep apnea and the Cheyne-Stokes respiration-central sleep apnea syndrome), chronic obstructive pulmonary disease (COPD), and the obesity-hypoventilation syndrome.
In this review, we discuss the different types of NIPPV, the specific conditions in which they can be used, and the indications, recommendations, and evidence supporting the efficacy of NIPPV in outpatients.
THE TYPES OF NIPPV AND THEIR USES
Although the conditions for which different types of NIPPV can be used overlap significantly, each type has general indications and different goals of treatment. This section begins with types of NIPPV that are predominantly used to treat sleep-disordered breathing, and then proceeds to those predominantly used for conditions associated with hypoventilation and hypercapnia.
Continuous positive airway pressure
CPAP, currently the most widely used form of NIPPV, applies a constant level of positive pressure at the airway opening during spontaneous breathing.
CPAP is commonly used to treat obstructive sleep-disordered breathing, with the goals of improving daytime sleepiness and reducing cardiovascular risk. It has also been used to treat sleep-disordered breathing associated with congestive heart failure.
CPAP’s role in the support of ventilation is limited and indirect. For instance, it has been used in the obesity-hypoventilation syndrome and in the “overlap” syndrome (in which both sleep apnea and COPD coexist). However, its benefits in those conditions are probably derived in large part from correction of underlying obstructive sleep apnea.
The mechanisms of action of CPAP include:
- Preventing intermittent narrowing and collapse of the airway in patients with obstructive sleep apnea-hypopnea syndrome by acting as a pneumatic splint during sleep3,5,6
- Counteracting auto-positive end-expiratory pressure, thereby reducing respiratory muscle load, reducing the work of breathing, and lowering daytime Paco2 in patients with coexistent COPD and obstructive sleep apnea-hypopnea syndrome (the overlap syndrome)7–9
- Improving lung function (particularly the functional residual capacity) and daytime gas exchange in obstructive sleep apnea-hypopnea syndrome 10
- Improving left ventricular systolic function in patients with heart failure coexisting with obstructive sleep apnea-hypopnea syndrome.11,12
Auto-CPAP is delivered via a self-titrating CPAP device, which uses algorithms to detect variations in the degree of obstruction and consequently adjusts the pressure level to restore normal breathing. Auto-CPAP therefore compensates for factors that modify the upper airway collapsibility, such as body posture during sleep, stage of sleep, use of alcohol, and drugs that affect upper airway muscle tone.13
Although one of the premises of using auto-CPAP is that it improves the patient’s satisfaction and compliance, several studies found it to be no more effective than fixed CPAP for treating obstructive sleep apnea-hypopnea syndrome.14–16 Current guidelines of the American Academy of Sleep Medicine do not recommend self-titrating CPAP devices to diagnose obstructive sleep apnea or to treat patients with cardiopulmonary disorders or other conditions in which nocturnal desaturation may be unrelated to obstructive events.17
Adaptive servo-ventilation
Adaptive servo-ventilation was developed for Cheyne-Stokes respiration-central sleep apnea syndrome in patients with congestive heart failure, who may have periods of crescendo-decrescendo change in tidal volume (Cheyne-Stokes respiration) with possible intercalated episodes of central apnea or hypopnea. It is also applied in patients with the complex sleep apnea syndrome.
Adaptive servo-ventilation devices are usually set at an expiratory positive airway pressure (EPAP) level sufficient to control obstructive sleep apnea. The device then automatically adjusts the pressure support for each inspiration, within a prespecified range, to maintain a moving-target ventilation set at 90% of the patient’s recent average ventilation. The aim is to stabilize breathing and reduce respiratory alkalosis, which can trigger apnea reentry cycles.18
Bilevel positive airway pressure
Bilevel positive airway pressure (bilevel PAP) can be of use in sleep-disordered breathing (including cases associated with congestive heart failure), but it is predominantly applied in conditions associated with hypoventilation.
Bilevel PAP devices deliver a higher pressure during inspiration (inspiratory positive airway pressure, or IPAP) and a lower pressure during expiration (EPAP). The gradient between IPAP and EPAP is of key importance in maintaining alveolar ventilation and reducing Paco2. The IPAP acts as pressure support to augment the patient’s effort, maintain adequate alveolar ventilation, unload respiratory muscles, decrease the work of breathing, and control obstructive hypopnea, whereas EPAP is set to maintain upper airway patency, control obstructive apnea, improve functional residual capacity, and prevent microatelectasis.
Although there is no evidence that bilevel PAP is better adhered to or more effective than CPAP, current guidelines propose it as an option for patients who require high pressures to control obstructive sleep apnea-hypopnea syndrome or for those who cannot tolerate exhaling against a high fixed CPAP pressure.19
Other, more-common uses of bilevel PAP are to treat coexisting central sleep apnea or hypoventilation,19 the obesity-hypoventilation syndrome with residual alveolar hypoventilation despite CPAP and control of concomitant obstructive sleep apnea-hypopnea syndrome,5,20 severe stable COPD with significant nocturnal hypoventilation and daytime hypercarbia,21 and restrictive pulmonary diseases.21
Average volume-assured pressure support
CONDITIONS IN WHICH NOCTURNAL NIPPV IS USED IN OUT PATIENTS
Obstructive sleep apnea-hypopnea syndrome
Obstructive sleep apnea-hypopnea syndrome is estimated to affect 2% of women and 4% of men.25 It is characterized by recurrent episodes of partial (hypopnea) or complete (apnea) upper airway obstruction during sleep despite ongoing inspiratory efforts, with associated episodes of arousal or desaturation or both. Corresponding symptoms include excessive daytime sleepiness, choking and gasping during sleep, recurrent awakenings from sleep, unrefreshing sleep, daytime fatigue, and impaired concentration that is not explained by other factors.26
Current understanding of the pathophysiology of obstructive sleep apnea implicates an impairment in the balance between factors that promote collapsibility of the airway (including obesity and anatomic issues such as the volume of the soft-tissue structures surrounding the upper airway) and the compensatory neuromuscular response.27,28
Long-standing obstructive sleep apnea-hypopnea syndrome has been linked in prospective studies to the development of hypertension,29–31 coronary artery disease,32,33 increased coagulation,34,35 and stroke or death from any cause.36,37 It is also associated with a greater rate and severity of motor vehicular accidents,38 greater health care utilization, impaired work performance, and occupational injuries.39
Strong evidence exists that NIPPV (most commonly CPAP) is beneficial in obstructive sleep apnea-hypopnea syndrome, improving sleep quality, sleepiness, cognitive impairment, and quality of life,40,41 decreasing motor vehicle accidents,42 lowering blood pressure, 43,44 and decreasing the rates of myocardial infarction,32 stroke,32 and death.45
The American Academy of Sleep Medicine recommends CPAP as an optional adjunctive therapy to lower blood pressure in patients with obstructive sleep apnea-hypopnea syndrome with concomitant hypertension.19 This is supported by a recent study that suggested that CPAP may have additional benefits on blood pressure in a subgroup of patients with uncontrolled hypertension while on antihypertensive medications.46 Indications with Medicare guidelines for reimbursement of CPAP devices are summarized in Table 2.
Complex sleep apnea syndrome
The complex sleep apnea syndrome is characterized by the emergence of significant central sleep apnea or Cheyne-Stokes respiration after obstructive events have been brought under control with NIPPV in patients who initially appear to have obstructive sleep apnea-hypopnea syndrome. A retrospective study of patients with the complex sleep apnea syndrome who continued their NIPPV use until a subsequent polysomnographic study and NIPPV titration showed that the syndrome may resolve spontaneously, but that 46% of patients had a persistently elevated apnea-hypopnea index with central apnea activity.47
Restoration of upper airway patency by CPAP and dysregulation or delayed adaptation of chemosensitive ventilatory control to a changing Paco2 level may be a key pathophysiologic mechanism of the complex sleep apnea syndrome.48 In this mechanism, increased ventilation from restored airway patency and from an increase in the slope of the ventilatory response may intermittently draw the Paco2 to below the hypocapnic apneic threshold and trigger episodes of central apnea.48
The ideal NIPPV device for use in the complex sleep apnea syndrome should be able to provide enough pressure to resolve the obstructive sleep apnea-hypopnea syndrome while maintaining proper ventilatory support during central apnea episodes without fluctuations of Paco2, which could further worsen the dysregulated ventilatory control. Currently, adaptive servo-ventilation appears to be superior to bilevel PAP and CPAP in the management of the complex sleep apnea syndrome.49,50
Sleep disturbances associated with cardiac dysfunction
There are specific indications for NIPPV modes in the setting of respiratory sleep disturbances associated with heart failure.
Obstructive sleep apnea in congestive heart failure. The prevalence of obstructive sleep apnea in patients with impaired left ventricular ejection fraction is 11% to 53%.51 Obstructive sleep apnea-hypopnea syndrome can worsen congestive heart failure by causing a periodic increase in negative intrathoracic pressure from breathing against an occluded airway, by raising arterial blood pressure, and causing tachycardia from sympathetic nervous system stimulation from hypoxia, hypercarbia, and arousals.52,53 Both heart failure and sleep apnea contribute in an additive manner to the increased sympathetic nervous activity.54
Fortunately, treatment with CPAP has been found to reduce systolic blood pressure and improve left ventricular systolic function in medically treated patients with heart failure and coexisting obstructive sleep apnea.11,12 Furthermore, in a randomized trial in patients with stable congestive heart failure and newly diagnosed obstructive sleep apnea-hypopnea syndrome, a greater improvement in cardiac function was observed in patients on bilevel PAP than in those on CPAP.55 The authors speculated that bilevel PAP might provide more unloading of the respiratory muscles, reduce the work of breathing more, and result in less positive intrathoracic pressure than with CPAP, and that the higher intrathoracic pressure with CPAP could reduce the left ventricular ejection fraction in patients with low filling pressures (pulmonary capillary wedge pressure < 12 mm Hg) and low baseline left ventricular ejection fractions (< 30%).55
Whether these interventions reduce the mortality rate is uncertain. In a prospective nonrandomized study, 9 (24%) of 37 patients who had heart failure with untreated obstructive sleep apnea died, compared with no deaths in 14 treated patients (P = .07).56
Cheyne-Stokes respiration with central sleep apnea in congestive heart failure. A related but different situation is central apnea associated with congestive heart failure.
There are several pathophysiologic mechanisms of Cheyne-Stokes respiration with central sleep apnea. Specifically, the elevation of left ventricular filling pressures, end-diastolic volumes, and pulmonary congestion generate hyperventilation, chronic hypocapnia, and increased chemoreceptor responsiveness, which contribute to the development of central apnea by promoting a decrease in the Paco2 during sleep to below the hypocapnic apneic threshold.57,58 Additionally, an increase in circulation time may result in periodicity of breathing and hyperpnea.59 Obstructive events can then occur at the end of the central events corresponding with the nadir of the inspiratory drive.60,61
The Canadian Continuous Positive Airway Pressure for Patients With Central Sleep Apnea and Heart Failure (CANPAP) trial, a randomized trial of CPAP in this clinical setting, showed that compared with optimal medical therapy alone, CPAP plus optimal medical therapy improved the ejection fraction, reduced central sleep apnea, improved nocturnal oxygenation, and improved the 6-minute walking distance, but without a survival benefit.62 The disappointing survival results from CANPAP have to be interpreted in the context that CPAP may have failed to control the central apnea in some patients, such that the mean apnea-hypopnea index in treated patients (19 events/hour) remained above the entry criterion for recruitment (15 events/hour). In a post hoc analysis of this study, the heart-transplantation-free survival rate was significantly greater in the subgroup of patients in whom CPAP effectively suppressed central sleep apnea (< 15 events/hour).63
Other NIPPV modes such as bilevel PAP with backup rate and adaptive servo-ventilation have been shown in some studies to be superior to CPAP in controlling respiratory events, with adaptive servo-ventilation being the most effective in controlling central, mixed, and complex sleep apnea in this setting. 49,50 Whether more effective resolution of obstructive and central events with these treatment modes translates into improved mortality rates and transplantation-free survival rates remains to be determined.
Obesity-hypoventilation syndrome
Obesity-hypoventilation syndrome refers to daytime hypercapnia (Paco2 > 45 mm Hg) in obese people when no other cause of hypoventilation is present.
The prevalence of obesity-hypoventilation syndrome among patients with obstructive sleep apnea-hypopnea syndrome is 20% to 30% and is greater in extremely obese patients (body mass index > 40 kg/m2).64 However, about 10% of patients with obesity-hypoventilation syndrome do not have obstructive sleep apnea-hypopnea syndrome.64 Additionally, nocturnal hypoxemia and diurnal hypercapnia persist in about 40% of patients with obesity-hypoventilation syndrome after CPAP eliminates their sleep apnea.65 Therefore, factors other than sleep apnea contribute to the development of obesity-hypoventilation syndrome, and in a meta-analysis, factors associated with daytime hypercapnia included, in addition to body mass index and the apnea-hypopnea index, mean overnight oxygen saturation and severity of restrictive pulmonary function.66 Predictors of success with CPAP include better spirometric findings, a higher apnea-hypopnea index, and adequate oxygenation.67,68
Bilevel PAP therapy can be tried in patients in whom CPAP by itself fails. In a study of patients with obesity-hypoventilation syndrome in whom initial CPAP treatment failed, average volume-assured pressure support lowered Paco2 compared to bilevel PAP alone, but did not further improve oxygenation, sleep quality, or quality of life.69
Restrictive pulmonary diseases
Neuromuscular diseases and thoracic cage abnormalities. Noninvasive ventilation has been used in patients with progressive neuromuscular disorders or severe thoracic cage abnormalities, with recognized benefits including an improved survival rate and improved quality of life.70,71 However, NIPPV is used in only 9% of patients with amyotrophic lateral sclerosis when clearly indicated.72 The indications and Medicare guidelines for reimbursement of NIPPV (with or without a backup rate) in this setting are shown in Table 2.
Potential contraindications to starting NIPPV in this population include upper airway obstruction, failure to clear secretions despite optimal noninvasive support, inability to achieve a mask fit, and intolerance of the intervention.73,74
The mechanisms of benefit of NIPPV in these settings include improvements in daytime blood gas levels (including hypercapnia75), a reduction in the oxygen cost of breathing,76 an increase in the ventilatory response to carbon dioxide,75 and improved lung compliance.77
Chronic hypercapnic failure due to severe COPD
The use of NIPPV in chronic COPD is less well established than in patients with exacerbations of COPD,78 and limitations in its use are reflected in the more stringent Medicare indications for NIPPV in this setting (Table 2).
A particular subset of patients with stable COPD who may benefit from NIPPV includes those with daytime hypercapnia and super-imposed nocturnal hypoventilation.78 The potential benefits of NIPPV in these patients include improved daytime and nocturnal gas exchange, increased sleep duration, and improved quality of life.78 Additionally, a recent randomized controlled trial of NIPPV plus long-term oxygen therapy compared with oxygen therapy alone in patients with severe COPD and a Paco2 greater than 46 mm Hg demonstrated a survival benefit in favor of adding NIPPV (hazard ratio 0.6).79
However, that study also found no reduction in hospitalization rates, an apparent worsening in general and mental health (as reflected on the 36-Item Short Form Health Survey or SF-36, a quality-of-life questionnaire), as well as increased confusion and bewilderment (reflected on the Profile of Mood States scale).79 These potentially deleterious effects may explain why adherence to NIPPV is low in patients with stable COPD: only 37% to 57% of patients continued to use it in several reported studies.79–81
A level of inspiratory pressure support that is insufficient to reduce hypercapnia may account for the low adherence rate and worsened quality of life in such patients. For instance, in a randomized trial,82 compared with low-intensity NIPPV (mean IPAP 14 cm H2O, backup rate 8 per minute), settings that aimed to maximally reduce Paco2 (mean IPAP 29 cm H2O with a backup rate of 17.5 per minute) increased the daily use of NIPPV by 3.6 hours/day and improved exercise-related dyspnea, daytime Paco2, forced expiratory volume in 1 second (FEV1), vital capacity, and health-related quality of life.
The overlap syndrome was first described by Flenley in 1985 as a combination of chronic respiratory disease (more generally limited to COPD) and obstructive sleep apnea-hypopnea syndrome.83 Epidemiologic studies do not consistently show a higher incidence of obstructive sleep apnea-hypopnea syndrome in patients with COPD, but the exaggerated oxygen desaturation during sleep in patients with this combination increases the risk of hypoxemia, hypercapnia, and pulmonary hypertension. 84 In addition, there was evidence of higher risks of death and of hospitalization for COPD in patients with the overlap syndrome. 85 NIPPV is the main treatment for obstructive sleep apnea-hypopnea syndrome with or without COPD.
A recent study by Marin et al85 showed that CPAP was associated with improved survival and decreased hospitalization in patients with the overlap syndrome. However, polysomnography or nocturnal oximetry while on NIPPV alone must be done, as additional nocturnal oxygen therapy may be warranted when significant chronic respiratory illness coexists with sleep apnea.
Acknowledgment: The authors gratefully acknowledge the contribution of Scott Marlow, RRT, to Table 2 of this review.
- Organized jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Française, and approved by ATS Board of Directors, December 2000. International Consensus Conferences in Intensive Care Medicine: noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 2001; 163:283–291.
- Aboussouan LS, Ricaurte B. Noninvasive positive pressure ventilation: increasing use in acute care. Cleve Clin J Med 2010; 77:307–316.
- Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983; 128:177–181.
- Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease. Positive-pressure ventilation through a nose mask. Am Rev Respir Dis 1987; 135:148–152.
- Berger KI, Ayappa I, Chatr-Amontri B, et al. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest 2001; 120:1231–1238.
- Olson AL, Zwillich C. The obesity hypoventilation syndrome. Am J Med 2005; 118:948–956.
- de Miguel J, Cabello J, Sánchez-Alarcos JM, Alvarez-Sala R, Espinós D, Alvarez-Sala JL. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath 2002; 6:3–10.
- Mezzanotte WS, Tangel DJ, Fox AM, Ballard RD, White DP. Nocturnal nasal continuous positive airway pressure in patients with chronic obstructive pulmonary disease. Influence on waking respiratory muscle function. Chest 1994; 106:1100–1108.
- Petrof BJ, Legaré M, Goldberg P, Milic-Emili J, Gottfried SB. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1990; 141:281–289.
- Verbraecken J, Willemen M, De Cock W, Van de Heyning P, De Backer WA. Continuous positive airway pressure and lung inflation in sleep apnea patients. Respiration 2001; 68:357–364.
- Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003; 348:1233–1241.
- Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 2004; 169:361–366.
- Malhotra A, Trinder J, Fogel R, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep 2004; 27:1105–1112.
- Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep 2004; 27:249–253.
- Nolan GM, Ryan S, O’Connor TM, McNicholas WT. Comparison of three auto-adjusting positive pressure devices in patients with sleep apnoea. Eur Respir J 2006; 28:159–164.
- Meurice JC, Cornette A, Philip-Joet F, et al; ANTADIR “PPC” Working Group. Evaluation of autoCPAP devices in home treatment of sleep apnea/hypopnea syndrome. Sleep Med 2007; 8:695–703.
- Morgenthaler TI, Aurora RN, Brown T, et al; Standards of Practice Committee of the AASM; American Academy of Sleep Medicine. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep 2008; 31:141–147.
- Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med 2001; 164:614–619.
- Kushida CA, Littner MR, Hirshkowitz M, et al; American Academy of Sleep Medicine. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep 2006; 29:375–380.
- Schäfer H, Ewig S, Hasper E, Lüderitz B. Failure of CPAP therapy in obstructive sleep apnoea syndrome: predictive factors and treatment with bilevel-positive airway pressure. Respir Med 1998; 92:208–215.
- Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation—a consensus conference report. Chest 1999; 116:521–534.
- Aboussouan LS, Khan SU, Meeker DP, Stelmach K, Mitsumoto H. Effect of noninvasive positive-pressure ventilation on survival in amyotrophic lateral sclerosis. Ann Intern Med 1997; 127:450–453.
- Köhnlein T, Welte T, Tan LB, Elliott MW. Assisted ventilation for heart failure patients with Cheyne-Stokes respiration. Eur Respir J 2002; 20:934–941.
- Johnson KG, Johnson DC. Bilevel positive airway pressure worsens central apneas during sleep. Chest 2005; 128:2141–2150.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999; 22:667–689.
- Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest 2007; 132:325–337.
- Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 2003; 168:522–530.
- Hedner J, Bengtsson-Boström K, Peker Y, Grote L, Råstam L, Lindblad U. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J 2006; 27:564–570.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J 2006; 28:596–602.
- Guardiola JJ, Matheson PJ, Clavijo LC, Wilson MA, Fletcher EC. Hypercoagulability in patients with obstructive sleep apnea. Sleep Med 2001; 2:517–523.
- von Känel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest 2007; 131:733–739.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2010; Epub ahead of print.
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005; 353:2034–2041.
- Mulgrew AT, Nasvadi G, Butt A, et al. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnoea/hypopnoea. Thorax 2008; 63:536–541.
- AlGhanim N, Comondore VR, Fleetham J, Marra CA, Ayas NT. The economic impact of obstructive sleep apnea. Lung 2008; 186:7–12.
- Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006; 3:CD001106.
- Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003; 163:565–571.
- George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001; 56:508–512.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107:68–73.
- Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med 2001; 163:344–348.
- Campos-Rodriguez F, Peña-Griñan N, Reyes-Nuñez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest 2005; 128:624–633.
- Pepin JL, Tamisier R, Barone-Rochette G, Launois SH, Levy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive sleep apnea patients. Am J Respir Crit Care Med 2010; Epub ahead of print.
- Kuzniar TJ, Pusalavidyasagar S, Gay PC, Morgenthaler TI. Natural course of complex sleep apnea—a retrospective study. Sleep Breath 2008; 12:135–139.
- Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep 2006; 29:1203–1209.
- Allam JS, Olson EJ, Gay PC, Morgenthaler TI. Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes. Chest 2007; 132:1839–1846.
- Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep 2007; 30:468–475.
- Bordier P. Sleep apnoea in patients with heart failure. Part I: diagnosis, definitions, prevalence, pathophysiology and haemodynamic consequences. Arch Cardiovasc Dis 2009; 102:651–661.
- Romero-Corral A, Somers VK, Pellikka PA, et al. Decreased right and left ventricular myocardial performance in obstructive sleep apnea. Chest 2007; 132:1863–1870.
- Solin P, Kaye DM, Little PJ, Bergin P, Richardson M, Naughton MT. Impact of sleep apnea on sympathetic nervous system activity in heart failure. Chest 2003; 123:1119–1126.
- Floras JS. Should sleep apnoea be a specific target of therapy in chronic heart failure? Heart 2009; 95:1041–1046.
- Khayat RN, Abraham WT, Patt B, Roy M, Hua K, Jarjoura D. Cardiac effects of continuous and bilevel positive airway pressure for patients with heart failure and obstructive sleep apnea: a pilot study. Chest 2008; 134:1162–1168.
- Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007; 49:1625–1631.
- Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med 1999; 341:949–954.
- Tkacova R, Hall MJ, Liu PP, Fitzgerald FS, Bradley TD. Left ventricular volume in patients with heart failure and Cheyne-Stokes respiration during sleep. Am J Respir Crit Care Med 1997; 156:1549–1555.
- Lorenzi-Filho G, Rankin F, Bies I, Douglas Bradley T. Effects of inhaled carbon dioxide and oxygen on Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med 1999; 159:1490–1498.
- Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol 1995; 78:1806–1815.
- Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol 1986; 61:1438–1443.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353:2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115:3173–3180.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007; 11:117–124.
- Banerjee D, Yee BJ, Piper AJ, Zwillich CW, Grunstein RR. Obesity hypoventilation syndrome: hypoxemia during continuous positive airway pressure. Chest 2007; 131:1678–1684.
- Kaw R, Hernandez AV, Walker E, Aboussouan L, Mokhlesi B. Determinants of hypercapnia in obese patients with obstructive sleep apnea: a systematic review and metaanalysis of cohort studies. Chest 2009; 136:787–796.
- Pérez de Llano LA, Golpe R, Piquer MO, et al. Clinical heterogeneity among patients with obesity hypoventilation syndrome: therapeutic implications. Respiration 2008; 75:34–39.
- Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax 2008; 63:395–401.
- Storre JH, Seuthe B, Fiechter R, et al. Average volume-assured pressure support in obesity hypoventilation: a randomized crossover trial. Chest 2006; 130:815–821.
- Aboussouan LS, Khan SU, Banerjee M, Arroliga AC, Mitsumoto H. Objective measures of the efficacy of noninvasive positive-pressure ventilation in amyotrophic lateral sclerosis. Muscle Nerve 2001; 24:403–409.
- Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol 2006; 5:140–147.
- Miller RG, Anderson F, Brooks BR, Mitsumoto H, Bradley WG, Ringel SP; ALS CARE Study Group. Outcomes research in amyotrophic lateral sclerosis: lessons learned from the amyotrophic lateral sclerosis clinical assessment, research, and education database. Ann Neurol 2009; 65(suppl 1):S24–S28.
- Bach JR. Amyotrophic lateral sclerosis: prolongation of life by noninvasive respiratory AIDS. Chest 2002; 122:92–98.
- Perrin C, Unterborn JN, Ambrosio CD, Hill NS. Pulmonary complications of chronic neuromuscular diseases and their management. Muscle Nerve 2004; 29:5–27.
- Nickol AH, Hart N, Hopkinson NS, Moxham J, Simonds A, Polkey MI. Mechanisms of improvement of respiratory failure in patients with restrictive thoracic disease treated with non-invasive ventilation. Thorax 2005; 60:754–760.
- Barle H, Söderberg P, Haegerstrand C, Markström A. Bi-level positive airway pressure ventilation reduces the oxygen cost of breathing in long-standing post-polio patients on invasive home mechanical ventilation. Acta Anaesthesiol Scand 2005; 49:197–202.
- Lechtzin N, Shade D, Clawson L, Wiener CM. Supramaximal inflation improves lung compliance in subjects with amyotrophic lateral sclerosis. Chest 2006; 129:1322–1329.
- Hill NS. Noninvasive ventilation for chronic obstructive pulmonary disease. Respir Care 2004; 49:72–87;
- McEvoy RD, Pierce RJ, Hillman D, et al; Australian trial of noninvasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64:561–566.
- Criner GJ, Brennan K, Travaline JM, Kreimer D. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest 1999; 116:667–675.
- Strumpf DA, Millman RP, Carlisle CC, et al. Nocturnal positive-pressure ventilation via nasal mask in patients with severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1991; 144:1234–1239.
- Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010; 65:303–308.
- Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med 1985; 6:651–661.
- Weitzenblum E, Chaouat A, Kessler R, Canuet M. Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5:237–241.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea. The overlap syndrome. Am J Respir Crit Care Med 2010; Epub ahead of print.
Noninvasive positive pressure ventilation (NIPPV) is any form of positive ventilatory support applied without an endotracheal tube, including continuous positive airway pressure (CPAP).1 The role of NIPPV in acute care has been discussed in an earlier review in the Cleveland Clinic Journal of Medicine.2
NIPPV is also used at night in outpatients with stable chronic conditions, first used in the 1980s in the treatment of obstructive sleep apnea3 and neuromuscular diseases,4 and since then in several other conditions including sleep disorders associated with congestive heart failure (including sleep apnea and the Cheyne-Stokes respiration-central sleep apnea syndrome), chronic obstructive pulmonary disease (COPD), and the obesity-hypoventilation syndrome.
In this review, we discuss the different types of NIPPV, the specific conditions in which they can be used, and the indications, recommendations, and evidence supporting the efficacy of NIPPV in outpatients.
THE TYPES OF NIPPV AND THEIR USES
Although the conditions for which different types of NIPPV can be used overlap significantly, each type has general indications and different goals of treatment. This section begins with types of NIPPV that are predominantly used to treat sleep-disordered breathing, and then proceeds to those predominantly used for conditions associated with hypoventilation and hypercapnia.
Continuous positive airway pressure
CPAP, currently the most widely used form of NIPPV, applies a constant level of positive pressure at the airway opening during spontaneous breathing.
CPAP is commonly used to treat obstructive sleep-disordered breathing, with the goals of improving daytime sleepiness and reducing cardiovascular risk. It has also been used to treat sleep-disordered breathing associated with congestive heart failure.
CPAP’s role in the support of ventilation is limited and indirect. For instance, it has been used in the obesity-hypoventilation syndrome and in the “overlap” syndrome (in which both sleep apnea and COPD coexist). However, its benefits in those conditions are probably derived in large part from correction of underlying obstructive sleep apnea.
The mechanisms of action of CPAP include:
- Preventing intermittent narrowing and collapse of the airway in patients with obstructive sleep apnea-hypopnea syndrome by acting as a pneumatic splint during sleep3,5,6
- Counteracting auto-positive end-expiratory pressure, thereby reducing respiratory muscle load, reducing the work of breathing, and lowering daytime Paco2 in patients with coexistent COPD and obstructive sleep apnea-hypopnea syndrome (the overlap syndrome)7–9
- Improving lung function (particularly the functional residual capacity) and daytime gas exchange in obstructive sleep apnea-hypopnea syndrome 10
- Improving left ventricular systolic function in patients with heart failure coexisting with obstructive sleep apnea-hypopnea syndrome.11,12
Auto-CPAP is delivered via a self-titrating CPAP device, which uses algorithms to detect variations in the degree of obstruction and consequently adjusts the pressure level to restore normal breathing. Auto-CPAP therefore compensates for factors that modify the upper airway collapsibility, such as body posture during sleep, stage of sleep, use of alcohol, and drugs that affect upper airway muscle tone.13
Although one of the premises of using auto-CPAP is that it improves the patient’s satisfaction and compliance, several studies found it to be no more effective than fixed CPAP for treating obstructive sleep apnea-hypopnea syndrome.14–16 Current guidelines of the American Academy of Sleep Medicine do not recommend self-titrating CPAP devices to diagnose obstructive sleep apnea or to treat patients with cardiopulmonary disorders or other conditions in which nocturnal desaturation may be unrelated to obstructive events.17
Adaptive servo-ventilation
Adaptive servo-ventilation was developed for Cheyne-Stokes respiration-central sleep apnea syndrome in patients with congestive heart failure, who may have periods of crescendo-decrescendo change in tidal volume (Cheyne-Stokes respiration) with possible intercalated episodes of central apnea or hypopnea. It is also applied in patients with the complex sleep apnea syndrome.
Adaptive servo-ventilation devices are usually set at an expiratory positive airway pressure (EPAP) level sufficient to control obstructive sleep apnea. The device then automatically adjusts the pressure support for each inspiration, within a prespecified range, to maintain a moving-target ventilation set at 90% of the patient’s recent average ventilation. The aim is to stabilize breathing and reduce respiratory alkalosis, which can trigger apnea reentry cycles.18
Bilevel positive airway pressure
Bilevel positive airway pressure (bilevel PAP) can be of use in sleep-disordered breathing (including cases associated with congestive heart failure), but it is predominantly applied in conditions associated with hypoventilation.
Bilevel PAP devices deliver a higher pressure during inspiration (inspiratory positive airway pressure, or IPAP) and a lower pressure during expiration (EPAP). The gradient between IPAP and EPAP is of key importance in maintaining alveolar ventilation and reducing Paco2. The IPAP acts as pressure support to augment the patient’s effort, maintain adequate alveolar ventilation, unload respiratory muscles, decrease the work of breathing, and control obstructive hypopnea, whereas EPAP is set to maintain upper airway patency, control obstructive apnea, improve functional residual capacity, and prevent microatelectasis.
Although there is no evidence that bilevel PAP is better adhered to or more effective than CPAP, current guidelines propose it as an option for patients who require high pressures to control obstructive sleep apnea-hypopnea syndrome or for those who cannot tolerate exhaling against a high fixed CPAP pressure.19
Other, more-common uses of bilevel PAP are to treat coexisting central sleep apnea or hypoventilation,19 the obesity-hypoventilation syndrome with residual alveolar hypoventilation despite CPAP and control of concomitant obstructive sleep apnea-hypopnea syndrome,5,20 severe stable COPD with significant nocturnal hypoventilation and daytime hypercarbia,21 and restrictive pulmonary diseases.21
Average volume-assured pressure support
CONDITIONS IN WHICH NOCTURNAL NIPPV IS USED IN OUT PATIENTS
Obstructive sleep apnea-hypopnea syndrome
Obstructive sleep apnea-hypopnea syndrome is estimated to affect 2% of women and 4% of men.25 It is characterized by recurrent episodes of partial (hypopnea) or complete (apnea) upper airway obstruction during sleep despite ongoing inspiratory efforts, with associated episodes of arousal or desaturation or both. Corresponding symptoms include excessive daytime sleepiness, choking and gasping during sleep, recurrent awakenings from sleep, unrefreshing sleep, daytime fatigue, and impaired concentration that is not explained by other factors.26
Current understanding of the pathophysiology of obstructive sleep apnea implicates an impairment in the balance between factors that promote collapsibility of the airway (including obesity and anatomic issues such as the volume of the soft-tissue structures surrounding the upper airway) and the compensatory neuromuscular response.27,28
Long-standing obstructive sleep apnea-hypopnea syndrome has been linked in prospective studies to the development of hypertension,29–31 coronary artery disease,32,33 increased coagulation,34,35 and stroke or death from any cause.36,37 It is also associated with a greater rate and severity of motor vehicular accidents,38 greater health care utilization, impaired work performance, and occupational injuries.39
Strong evidence exists that NIPPV (most commonly CPAP) is beneficial in obstructive sleep apnea-hypopnea syndrome, improving sleep quality, sleepiness, cognitive impairment, and quality of life,40,41 decreasing motor vehicle accidents,42 lowering blood pressure, 43,44 and decreasing the rates of myocardial infarction,32 stroke,32 and death.45
The American Academy of Sleep Medicine recommends CPAP as an optional adjunctive therapy to lower blood pressure in patients with obstructive sleep apnea-hypopnea syndrome with concomitant hypertension.19 This is supported by a recent study that suggested that CPAP may have additional benefits on blood pressure in a subgroup of patients with uncontrolled hypertension while on antihypertensive medications.46 Indications with Medicare guidelines for reimbursement of CPAP devices are summarized in Table 2.
Complex sleep apnea syndrome
The complex sleep apnea syndrome is characterized by the emergence of significant central sleep apnea or Cheyne-Stokes respiration after obstructive events have been brought under control with NIPPV in patients who initially appear to have obstructive sleep apnea-hypopnea syndrome. A retrospective study of patients with the complex sleep apnea syndrome who continued their NIPPV use until a subsequent polysomnographic study and NIPPV titration showed that the syndrome may resolve spontaneously, but that 46% of patients had a persistently elevated apnea-hypopnea index with central apnea activity.47
Restoration of upper airway patency by CPAP and dysregulation or delayed adaptation of chemosensitive ventilatory control to a changing Paco2 level may be a key pathophysiologic mechanism of the complex sleep apnea syndrome.48 In this mechanism, increased ventilation from restored airway patency and from an increase in the slope of the ventilatory response may intermittently draw the Paco2 to below the hypocapnic apneic threshold and trigger episodes of central apnea.48
The ideal NIPPV device for use in the complex sleep apnea syndrome should be able to provide enough pressure to resolve the obstructive sleep apnea-hypopnea syndrome while maintaining proper ventilatory support during central apnea episodes without fluctuations of Paco2, which could further worsen the dysregulated ventilatory control. Currently, adaptive servo-ventilation appears to be superior to bilevel PAP and CPAP in the management of the complex sleep apnea syndrome.49,50
Sleep disturbances associated with cardiac dysfunction
There are specific indications for NIPPV modes in the setting of respiratory sleep disturbances associated with heart failure.
Obstructive sleep apnea in congestive heart failure. The prevalence of obstructive sleep apnea in patients with impaired left ventricular ejection fraction is 11% to 53%.51 Obstructive sleep apnea-hypopnea syndrome can worsen congestive heart failure by causing a periodic increase in negative intrathoracic pressure from breathing against an occluded airway, by raising arterial blood pressure, and causing tachycardia from sympathetic nervous system stimulation from hypoxia, hypercarbia, and arousals.52,53 Both heart failure and sleep apnea contribute in an additive manner to the increased sympathetic nervous activity.54
Fortunately, treatment with CPAP has been found to reduce systolic blood pressure and improve left ventricular systolic function in medically treated patients with heart failure and coexisting obstructive sleep apnea.11,12 Furthermore, in a randomized trial in patients with stable congestive heart failure and newly diagnosed obstructive sleep apnea-hypopnea syndrome, a greater improvement in cardiac function was observed in patients on bilevel PAP than in those on CPAP.55 The authors speculated that bilevel PAP might provide more unloading of the respiratory muscles, reduce the work of breathing more, and result in less positive intrathoracic pressure than with CPAP, and that the higher intrathoracic pressure with CPAP could reduce the left ventricular ejection fraction in patients with low filling pressures (pulmonary capillary wedge pressure < 12 mm Hg) and low baseline left ventricular ejection fractions (< 30%).55
Whether these interventions reduce the mortality rate is uncertain. In a prospective nonrandomized study, 9 (24%) of 37 patients who had heart failure with untreated obstructive sleep apnea died, compared with no deaths in 14 treated patients (P = .07).56
Cheyne-Stokes respiration with central sleep apnea in congestive heart failure. A related but different situation is central apnea associated with congestive heart failure.
There are several pathophysiologic mechanisms of Cheyne-Stokes respiration with central sleep apnea. Specifically, the elevation of left ventricular filling pressures, end-diastolic volumes, and pulmonary congestion generate hyperventilation, chronic hypocapnia, and increased chemoreceptor responsiveness, which contribute to the development of central apnea by promoting a decrease in the Paco2 during sleep to below the hypocapnic apneic threshold.57,58 Additionally, an increase in circulation time may result in periodicity of breathing and hyperpnea.59 Obstructive events can then occur at the end of the central events corresponding with the nadir of the inspiratory drive.60,61
The Canadian Continuous Positive Airway Pressure for Patients With Central Sleep Apnea and Heart Failure (CANPAP) trial, a randomized trial of CPAP in this clinical setting, showed that compared with optimal medical therapy alone, CPAP plus optimal medical therapy improved the ejection fraction, reduced central sleep apnea, improved nocturnal oxygenation, and improved the 6-minute walking distance, but without a survival benefit.62 The disappointing survival results from CANPAP have to be interpreted in the context that CPAP may have failed to control the central apnea in some patients, such that the mean apnea-hypopnea index in treated patients (19 events/hour) remained above the entry criterion for recruitment (15 events/hour). In a post hoc analysis of this study, the heart-transplantation-free survival rate was significantly greater in the subgroup of patients in whom CPAP effectively suppressed central sleep apnea (< 15 events/hour).63
Other NIPPV modes such as bilevel PAP with backup rate and adaptive servo-ventilation have been shown in some studies to be superior to CPAP in controlling respiratory events, with adaptive servo-ventilation being the most effective in controlling central, mixed, and complex sleep apnea in this setting. 49,50 Whether more effective resolution of obstructive and central events with these treatment modes translates into improved mortality rates and transplantation-free survival rates remains to be determined.
Obesity-hypoventilation syndrome
Obesity-hypoventilation syndrome refers to daytime hypercapnia (Paco2 > 45 mm Hg) in obese people when no other cause of hypoventilation is present.
The prevalence of obesity-hypoventilation syndrome among patients with obstructive sleep apnea-hypopnea syndrome is 20% to 30% and is greater in extremely obese patients (body mass index > 40 kg/m2).64 However, about 10% of patients with obesity-hypoventilation syndrome do not have obstructive sleep apnea-hypopnea syndrome.64 Additionally, nocturnal hypoxemia and diurnal hypercapnia persist in about 40% of patients with obesity-hypoventilation syndrome after CPAP eliminates their sleep apnea.65 Therefore, factors other than sleep apnea contribute to the development of obesity-hypoventilation syndrome, and in a meta-analysis, factors associated with daytime hypercapnia included, in addition to body mass index and the apnea-hypopnea index, mean overnight oxygen saturation and severity of restrictive pulmonary function.66 Predictors of success with CPAP include better spirometric findings, a higher apnea-hypopnea index, and adequate oxygenation.67,68
Bilevel PAP therapy can be tried in patients in whom CPAP by itself fails. In a study of patients with obesity-hypoventilation syndrome in whom initial CPAP treatment failed, average volume-assured pressure support lowered Paco2 compared to bilevel PAP alone, but did not further improve oxygenation, sleep quality, or quality of life.69
Restrictive pulmonary diseases
Neuromuscular diseases and thoracic cage abnormalities. Noninvasive ventilation has been used in patients with progressive neuromuscular disorders or severe thoracic cage abnormalities, with recognized benefits including an improved survival rate and improved quality of life.70,71 However, NIPPV is used in only 9% of patients with amyotrophic lateral sclerosis when clearly indicated.72 The indications and Medicare guidelines for reimbursement of NIPPV (with or without a backup rate) in this setting are shown in Table 2.
Potential contraindications to starting NIPPV in this population include upper airway obstruction, failure to clear secretions despite optimal noninvasive support, inability to achieve a mask fit, and intolerance of the intervention.73,74
The mechanisms of benefit of NIPPV in these settings include improvements in daytime blood gas levels (including hypercapnia75), a reduction in the oxygen cost of breathing,76 an increase in the ventilatory response to carbon dioxide,75 and improved lung compliance.77
Chronic hypercapnic failure due to severe COPD
The use of NIPPV in chronic COPD is less well established than in patients with exacerbations of COPD,78 and limitations in its use are reflected in the more stringent Medicare indications for NIPPV in this setting (Table 2).
A particular subset of patients with stable COPD who may benefit from NIPPV includes those with daytime hypercapnia and super-imposed nocturnal hypoventilation.78 The potential benefits of NIPPV in these patients include improved daytime and nocturnal gas exchange, increased sleep duration, and improved quality of life.78 Additionally, a recent randomized controlled trial of NIPPV plus long-term oxygen therapy compared with oxygen therapy alone in patients with severe COPD and a Paco2 greater than 46 mm Hg demonstrated a survival benefit in favor of adding NIPPV (hazard ratio 0.6).79
However, that study also found no reduction in hospitalization rates, an apparent worsening in general and mental health (as reflected on the 36-Item Short Form Health Survey or SF-36, a quality-of-life questionnaire), as well as increased confusion and bewilderment (reflected on the Profile of Mood States scale).79 These potentially deleterious effects may explain why adherence to NIPPV is low in patients with stable COPD: only 37% to 57% of patients continued to use it in several reported studies.79–81
A level of inspiratory pressure support that is insufficient to reduce hypercapnia may account for the low adherence rate and worsened quality of life in such patients. For instance, in a randomized trial,82 compared with low-intensity NIPPV (mean IPAP 14 cm H2O, backup rate 8 per minute), settings that aimed to maximally reduce Paco2 (mean IPAP 29 cm H2O with a backup rate of 17.5 per minute) increased the daily use of NIPPV by 3.6 hours/day and improved exercise-related dyspnea, daytime Paco2, forced expiratory volume in 1 second (FEV1), vital capacity, and health-related quality of life.
The overlap syndrome was first described by Flenley in 1985 as a combination of chronic respiratory disease (more generally limited to COPD) and obstructive sleep apnea-hypopnea syndrome.83 Epidemiologic studies do not consistently show a higher incidence of obstructive sleep apnea-hypopnea syndrome in patients with COPD, but the exaggerated oxygen desaturation during sleep in patients with this combination increases the risk of hypoxemia, hypercapnia, and pulmonary hypertension. 84 In addition, there was evidence of higher risks of death and of hospitalization for COPD in patients with the overlap syndrome. 85 NIPPV is the main treatment for obstructive sleep apnea-hypopnea syndrome with or without COPD.
A recent study by Marin et al85 showed that CPAP was associated with improved survival and decreased hospitalization in patients with the overlap syndrome. However, polysomnography or nocturnal oximetry while on NIPPV alone must be done, as additional nocturnal oxygen therapy may be warranted when significant chronic respiratory illness coexists with sleep apnea.
Acknowledgment: The authors gratefully acknowledge the contribution of Scott Marlow, RRT, to Table 2 of this review.
Noninvasive positive pressure ventilation (NIPPV) is any form of positive ventilatory support applied without an endotracheal tube, including continuous positive airway pressure (CPAP).1 The role of NIPPV in acute care has been discussed in an earlier review in the Cleveland Clinic Journal of Medicine.2
NIPPV is also used at night in outpatients with stable chronic conditions, first used in the 1980s in the treatment of obstructive sleep apnea3 and neuromuscular diseases,4 and since then in several other conditions including sleep disorders associated with congestive heart failure (including sleep apnea and the Cheyne-Stokes respiration-central sleep apnea syndrome), chronic obstructive pulmonary disease (COPD), and the obesity-hypoventilation syndrome.
In this review, we discuss the different types of NIPPV, the specific conditions in which they can be used, and the indications, recommendations, and evidence supporting the efficacy of NIPPV in outpatients.
THE TYPES OF NIPPV AND THEIR USES
Although the conditions for which different types of NIPPV can be used overlap significantly, each type has general indications and different goals of treatment. This section begins with types of NIPPV that are predominantly used to treat sleep-disordered breathing, and then proceeds to those predominantly used for conditions associated with hypoventilation and hypercapnia.
Continuous positive airway pressure
CPAP, currently the most widely used form of NIPPV, applies a constant level of positive pressure at the airway opening during spontaneous breathing.
CPAP is commonly used to treat obstructive sleep-disordered breathing, with the goals of improving daytime sleepiness and reducing cardiovascular risk. It has also been used to treat sleep-disordered breathing associated with congestive heart failure.
CPAP’s role in the support of ventilation is limited and indirect. For instance, it has been used in the obesity-hypoventilation syndrome and in the “overlap” syndrome (in which both sleep apnea and COPD coexist). However, its benefits in those conditions are probably derived in large part from correction of underlying obstructive sleep apnea.
The mechanisms of action of CPAP include:
- Preventing intermittent narrowing and collapse of the airway in patients with obstructive sleep apnea-hypopnea syndrome by acting as a pneumatic splint during sleep3,5,6
- Counteracting auto-positive end-expiratory pressure, thereby reducing respiratory muscle load, reducing the work of breathing, and lowering daytime Paco2 in patients with coexistent COPD and obstructive sleep apnea-hypopnea syndrome (the overlap syndrome)7–9
- Improving lung function (particularly the functional residual capacity) and daytime gas exchange in obstructive sleep apnea-hypopnea syndrome 10
- Improving left ventricular systolic function in patients with heart failure coexisting with obstructive sleep apnea-hypopnea syndrome.11,12
Auto-CPAP is delivered via a self-titrating CPAP device, which uses algorithms to detect variations in the degree of obstruction and consequently adjusts the pressure level to restore normal breathing. Auto-CPAP therefore compensates for factors that modify the upper airway collapsibility, such as body posture during sleep, stage of sleep, use of alcohol, and drugs that affect upper airway muscle tone.13
Although one of the premises of using auto-CPAP is that it improves the patient’s satisfaction and compliance, several studies found it to be no more effective than fixed CPAP for treating obstructive sleep apnea-hypopnea syndrome.14–16 Current guidelines of the American Academy of Sleep Medicine do not recommend self-titrating CPAP devices to diagnose obstructive sleep apnea or to treat patients with cardiopulmonary disorders or other conditions in which nocturnal desaturation may be unrelated to obstructive events.17
Adaptive servo-ventilation
Adaptive servo-ventilation was developed for Cheyne-Stokes respiration-central sleep apnea syndrome in patients with congestive heart failure, who may have periods of crescendo-decrescendo change in tidal volume (Cheyne-Stokes respiration) with possible intercalated episodes of central apnea or hypopnea. It is also applied in patients with the complex sleep apnea syndrome.
Adaptive servo-ventilation devices are usually set at an expiratory positive airway pressure (EPAP) level sufficient to control obstructive sleep apnea. The device then automatically adjusts the pressure support for each inspiration, within a prespecified range, to maintain a moving-target ventilation set at 90% of the patient’s recent average ventilation. The aim is to stabilize breathing and reduce respiratory alkalosis, which can trigger apnea reentry cycles.18
Bilevel positive airway pressure
Bilevel positive airway pressure (bilevel PAP) can be of use in sleep-disordered breathing (including cases associated with congestive heart failure), but it is predominantly applied in conditions associated with hypoventilation.
Bilevel PAP devices deliver a higher pressure during inspiration (inspiratory positive airway pressure, or IPAP) and a lower pressure during expiration (EPAP). The gradient between IPAP and EPAP is of key importance in maintaining alveolar ventilation and reducing Paco2. The IPAP acts as pressure support to augment the patient’s effort, maintain adequate alveolar ventilation, unload respiratory muscles, decrease the work of breathing, and control obstructive hypopnea, whereas EPAP is set to maintain upper airway patency, control obstructive apnea, improve functional residual capacity, and prevent microatelectasis.
Although there is no evidence that bilevel PAP is better adhered to or more effective than CPAP, current guidelines propose it as an option for patients who require high pressures to control obstructive sleep apnea-hypopnea syndrome or for those who cannot tolerate exhaling against a high fixed CPAP pressure.19
Other, more-common uses of bilevel PAP are to treat coexisting central sleep apnea or hypoventilation,19 the obesity-hypoventilation syndrome with residual alveolar hypoventilation despite CPAP and control of concomitant obstructive sleep apnea-hypopnea syndrome,5,20 severe stable COPD with significant nocturnal hypoventilation and daytime hypercarbia,21 and restrictive pulmonary diseases.21
Average volume-assured pressure support
CONDITIONS IN WHICH NOCTURNAL NIPPV IS USED IN OUT PATIENTS
Obstructive sleep apnea-hypopnea syndrome
Obstructive sleep apnea-hypopnea syndrome is estimated to affect 2% of women and 4% of men.25 It is characterized by recurrent episodes of partial (hypopnea) or complete (apnea) upper airway obstruction during sleep despite ongoing inspiratory efforts, with associated episodes of arousal or desaturation or both. Corresponding symptoms include excessive daytime sleepiness, choking and gasping during sleep, recurrent awakenings from sleep, unrefreshing sleep, daytime fatigue, and impaired concentration that is not explained by other factors.26
Current understanding of the pathophysiology of obstructive sleep apnea implicates an impairment in the balance between factors that promote collapsibility of the airway (including obesity and anatomic issues such as the volume of the soft-tissue structures surrounding the upper airway) and the compensatory neuromuscular response.27,28
Long-standing obstructive sleep apnea-hypopnea syndrome has been linked in prospective studies to the development of hypertension,29–31 coronary artery disease,32,33 increased coagulation,34,35 and stroke or death from any cause.36,37 It is also associated with a greater rate and severity of motor vehicular accidents,38 greater health care utilization, impaired work performance, and occupational injuries.39
Strong evidence exists that NIPPV (most commonly CPAP) is beneficial in obstructive sleep apnea-hypopnea syndrome, improving sleep quality, sleepiness, cognitive impairment, and quality of life,40,41 decreasing motor vehicle accidents,42 lowering blood pressure, 43,44 and decreasing the rates of myocardial infarction,32 stroke,32 and death.45
The American Academy of Sleep Medicine recommends CPAP as an optional adjunctive therapy to lower blood pressure in patients with obstructive sleep apnea-hypopnea syndrome with concomitant hypertension.19 This is supported by a recent study that suggested that CPAP may have additional benefits on blood pressure in a subgroup of patients with uncontrolled hypertension while on antihypertensive medications.46 Indications with Medicare guidelines for reimbursement of CPAP devices are summarized in Table 2.
Complex sleep apnea syndrome
The complex sleep apnea syndrome is characterized by the emergence of significant central sleep apnea or Cheyne-Stokes respiration after obstructive events have been brought under control with NIPPV in patients who initially appear to have obstructive sleep apnea-hypopnea syndrome. A retrospective study of patients with the complex sleep apnea syndrome who continued their NIPPV use until a subsequent polysomnographic study and NIPPV titration showed that the syndrome may resolve spontaneously, but that 46% of patients had a persistently elevated apnea-hypopnea index with central apnea activity.47
Restoration of upper airway patency by CPAP and dysregulation or delayed adaptation of chemosensitive ventilatory control to a changing Paco2 level may be a key pathophysiologic mechanism of the complex sleep apnea syndrome.48 In this mechanism, increased ventilation from restored airway patency and from an increase in the slope of the ventilatory response may intermittently draw the Paco2 to below the hypocapnic apneic threshold and trigger episodes of central apnea.48
The ideal NIPPV device for use in the complex sleep apnea syndrome should be able to provide enough pressure to resolve the obstructive sleep apnea-hypopnea syndrome while maintaining proper ventilatory support during central apnea episodes without fluctuations of Paco2, which could further worsen the dysregulated ventilatory control. Currently, adaptive servo-ventilation appears to be superior to bilevel PAP and CPAP in the management of the complex sleep apnea syndrome.49,50
Sleep disturbances associated with cardiac dysfunction
There are specific indications for NIPPV modes in the setting of respiratory sleep disturbances associated with heart failure.
Obstructive sleep apnea in congestive heart failure. The prevalence of obstructive sleep apnea in patients with impaired left ventricular ejection fraction is 11% to 53%.51 Obstructive sleep apnea-hypopnea syndrome can worsen congestive heart failure by causing a periodic increase in negative intrathoracic pressure from breathing against an occluded airway, by raising arterial blood pressure, and causing tachycardia from sympathetic nervous system stimulation from hypoxia, hypercarbia, and arousals.52,53 Both heart failure and sleep apnea contribute in an additive manner to the increased sympathetic nervous activity.54
Fortunately, treatment with CPAP has been found to reduce systolic blood pressure and improve left ventricular systolic function in medically treated patients with heart failure and coexisting obstructive sleep apnea.11,12 Furthermore, in a randomized trial in patients with stable congestive heart failure and newly diagnosed obstructive sleep apnea-hypopnea syndrome, a greater improvement in cardiac function was observed in patients on bilevel PAP than in those on CPAP.55 The authors speculated that bilevel PAP might provide more unloading of the respiratory muscles, reduce the work of breathing more, and result in less positive intrathoracic pressure than with CPAP, and that the higher intrathoracic pressure with CPAP could reduce the left ventricular ejection fraction in patients with low filling pressures (pulmonary capillary wedge pressure < 12 mm Hg) and low baseline left ventricular ejection fractions (< 30%).55
Whether these interventions reduce the mortality rate is uncertain. In a prospective nonrandomized study, 9 (24%) of 37 patients who had heart failure with untreated obstructive sleep apnea died, compared with no deaths in 14 treated patients (P = .07).56
Cheyne-Stokes respiration with central sleep apnea in congestive heart failure. A related but different situation is central apnea associated with congestive heart failure.
There are several pathophysiologic mechanisms of Cheyne-Stokes respiration with central sleep apnea. Specifically, the elevation of left ventricular filling pressures, end-diastolic volumes, and pulmonary congestion generate hyperventilation, chronic hypocapnia, and increased chemoreceptor responsiveness, which contribute to the development of central apnea by promoting a decrease in the Paco2 during sleep to below the hypocapnic apneic threshold.57,58 Additionally, an increase in circulation time may result in periodicity of breathing and hyperpnea.59 Obstructive events can then occur at the end of the central events corresponding with the nadir of the inspiratory drive.60,61
The Canadian Continuous Positive Airway Pressure for Patients With Central Sleep Apnea and Heart Failure (CANPAP) trial, a randomized trial of CPAP in this clinical setting, showed that compared with optimal medical therapy alone, CPAP plus optimal medical therapy improved the ejection fraction, reduced central sleep apnea, improved nocturnal oxygenation, and improved the 6-minute walking distance, but without a survival benefit.62 The disappointing survival results from CANPAP have to be interpreted in the context that CPAP may have failed to control the central apnea in some patients, such that the mean apnea-hypopnea index in treated patients (19 events/hour) remained above the entry criterion for recruitment (15 events/hour). In a post hoc analysis of this study, the heart-transplantation-free survival rate was significantly greater in the subgroup of patients in whom CPAP effectively suppressed central sleep apnea (< 15 events/hour).63
Other NIPPV modes such as bilevel PAP with backup rate and adaptive servo-ventilation have been shown in some studies to be superior to CPAP in controlling respiratory events, with adaptive servo-ventilation being the most effective in controlling central, mixed, and complex sleep apnea in this setting. 49,50 Whether more effective resolution of obstructive and central events with these treatment modes translates into improved mortality rates and transplantation-free survival rates remains to be determined.
Obesity-hypoventilation syndrome
Obesity-hypoventilation syndrome refers to daytime hypercapnia (Paco2 > 45 mm Hg) in obese people when no other cause of hypoventilation is present.
The prevalence of obesity-hypoventilation syndrome among patients with obstructive sleep apnea-hypopnea syndrome is 20% to 30% and is greater in extremely obese patients (body mass index > 40 kg/m2).64 However, about 10% of patients with obesity-hypoventilation syndrome do not have obstructive sleep apnea-hypopnea syndrome.64 Additionally, nocturnal hypoxemia and diurnal hypercapnia persist in about 40% of patients with obesity-hypoventilation syndrome after CPAP eliminates their sleep apnea.65 Therefore, factors other than sleep apnea contribute to the development of obesity-hypoventilation syndrome, and in a meta-analysis, factors associated with daytime hypercapnia included, in addition to body mass index and the apnea-hypopnea index, mean overnight oxygen saturation and severity of restrictive pulmonary function.66 Predictors of success with CPAP include better spirometric findings, a higher apnea-hypopnea index, and adequate oxygenation.67,68
Bilevel PAP therapy can be tried in patients in whom CPAP by itself fails. In a study of patients with obesity-hypoventilation syndrome in whom initial CPAP treatment failed, average volume-assured pressure support lowered Paco2 compared to bilevel PAP alone, but did not further improve oxygenation, sleep quality, or quality of life.69
Restrictive pulmonary diseases
Neuromuscular diseases and thoracic cage abnormalities. Noninvasive ventilation has been used in patients with progressive neuromuscular disorders or severe thoracic cage abnormalities, with recognized benefits including an improved survival rate and improved quality of life.70,71 However, NIPPV is used in only 9% of patients with amyotrophic lateral sclerosis when clearly indicated.72 The indications and Medicare guidelines for reimbursement of NIPPV (with or without a backup rate) in this setting are shown in Table 2.
Potential contraindications to starting NIPPV in this population include upper airway obstruction, failure to clear secretions despite optimal noninvasive support, inability to achieve a mask fit, and intolerance of the intervention.73,74
The mechanisms of benefit of NIPPV in these settings include improvements in daytime blood gas levels (including hypercapnia75), a reduction in the oxygen cost of breathing,76 an increase in the ventilatory response to carbon dioxide,75 and improved lung compliance.77
Chronic hypercapnic failure due to severe COPD
The use of NIPPV in chronic COPD is less well established than in patients with exacerbations of COPD,78 and limitations in its use are reflected in the more stringent Medicare indications for NIPPV in this setting (Table 2).
A particular subset of patients with stable COPD who may benefit from NIPPV includes those with daytime hypercapnia and super-imposed nocturnal hypoventilation.78 The potential benefits of NIPPV in these patients include improved daytime and nocturnal gas exchange, increased sleep duration, and improved quality of life.78 Additionally, a recent randomized controlled trial of NIPPV plus long-term oxygen therapy compared with oxygen therapy alone in patients with severe COPD and a Paco2 greater than 46 mm Hg demonstrated a survival benefit in favor of adding NIPPV (hazard ratio 0.6).79
However, that study also found no reduction in hospitalization rates, an apparent worsening in general and mental health (as reflected on the 36-Item Short Form Health Survey or SF-36, a quality-of-life questionnaire), as well as increased confusion and bewilderment (reflected on the Profile of Mood States scale).79 These potentially deleterious effects may explain why adherence to NIPPV is low in patients with stable COPD: only 37% to 57% of patients continued to use it in several reported studies.79–81
A level of inspiratory pressure support that is insufficient to reduce hypercapnia may account for the low adherence rate and worsened quality of life in such patients. For instance, in a randomized trial,82 compared with low-intensity NIPPV (mean IPAP 14 cm H2O, backup rate 8 per minute), settings that aimed to maximally reduce Paco2 (mean IPAP 29 cm H2O with a backup rate of 17.5 per minute) increased the daily use of NIPPV by 3.6 hours/day and improved exercise-related dyspnea, daytime Paco2, forced expiratory volume in 1 second (FEV1), vital capacity, and health-related quality of life.
The overlap syndrome was first described by Flenley in 1985 as a combination of chronic respiratory disease (more generally limited to COPD) and obstructive sleep apnea-hypopnea syndrome.83 Epidemiologic studies do not consistently show a higher incidence of obstructive sleep apnea-hypopnea syndrome in patients with COPD, but the exaggerated oxygen desaturation during sleep in patients with this combination increases the risk of hypoxemia, hypercapnia, and pulmonary hypertension. 84 In addition, there was evidence of higher risks of death and of hospitalization for COPD in patients with the overlap syndrome. 85 NIPPV is the main treatment for obstructive sleep apnea-hypopnea syndrome with or without COPD.
A recent study by Marin et al85 showed that CPAP was associated with improved survival and decreased hospitalization in patients with the overlap syndrome. However, polysomnography or nocturnal oximetry while on NIPPV alone must be done, as additional nocturnal oxygen therapy may be warranted when significant chronic respiratory illness coexists with sleep apnea.
Acknowledgment: The authors gratefully acknowledge the contribution of Scott Marlow, RRT, to Table 2 of this review.
- Organized jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Française, and approved by ATS Board of Directors, December 2000. International Consensus Conferences in Intensive Care Medicine: noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 2001; 163:283–291.
- Aboussouan LS, Ricaurte B. Noninvasive positive pressure ventilation: increasing use in acute care. Cleve Clin J Med 2010; 77:307–316.
- Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983; 128:177–181.
- Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease. Positive-pressure ventilation through a nose mask. Am Rev Respir Dis 1987; 135:148–152.
- Berger KI, Ayappa I, Chatr-Amontri B, et al. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest 2001; 120:1231–1238.
- Olson AL, Zwillich C. The obesity hypoventilation syndrome. Am J Med 2005; 118:948–956.
- de Miguel J, Cabello J, Sánchez-Alarcos JM, Alvarez-Sala R, Espinós D, Alvarez-Sala JL. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath 2002; 6:3–10.
- Mezzanotte WS, Tangel DJ, Fox AM, Ballard RD, White DP. Nocturnal nasal continuous positive airway pressure in patients with chronic obstructive pulmonary disease. Influence on waking respiratory muscle function. Chest 1994; 106:1100–1108.
- Petrof BJ, Legaré M, Goldberg P, Milic-Emili J, Gottfried SB. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1990; 141:281–289.
- Verbraecken J, Willemen M, De Cock W, Van de Heyning P, De Backer WA. Continuous positive airway pressure and lung inflation in sleep apnea patients. Respiration 2001; 68:357–364.
- Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003; 348:1233–1241.
- Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 2004; 169:361–366.
- Malhotra A, Trinder J, Fogel R, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep 2004; 27:1105–1112.
- Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep 2004; 27:249–253.
- Nolan GM, Ryan S, O’Connor TM, McNicholas WT. Comparison of three auto-adjusting positive pressure devices in patients with sleep apnoea. Eur Respir J 2006; 28:159–164.
- Meurice JC, Cornette A, Philip-Joet F, et al; ANTADIR “PPC” Working Group. Evaluation of autoCPAP devices in home treatment of sleep apnea/hypopnea syndrome. Sleep Med 2007; 8:695–703.
- Morgenthaler TI, Aurora RN, Brown T, et al; Standards of Practice Committee of the AASM; American Academy of Sleep Medicine. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep 2008; 31:141–147.
- Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med 2001; 164:614–619.
- Kushida CA, Littner MR, Hirshkowitz M, et al; American Academy of Sleep Medicine. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep 2006; 29:375–380.
- Schäfer H, Ewig S, Hasper E, Lüderitz B. Failure of CPAP therapy in obstructive sleep apnoea syndrome: predictive factors and treatment with bilevel-positive airway pressure. Respir Med 1998; 92:208–215.
- Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation—a consensus conference report. Chest 1999; 116:521–534.
- Aboussouan LS, Khan SU, Meeker DP, Stelmach K, Mitsumoto H. Effect of noninvasive positive-pressure ventilation on survival in amyotrophic lateral sclerosis. Ann Intern Med 1997; 127:450–453.
- Köhnlein T, Welte T, Tan LB, Elliott MW. Assisted ventilation for heart failure patients with Cheyne-Stokes respiration. Eur Respir J 2002; 20:934–941.
- Johnson KG, Johnson DC. Bilevel positive airway pressure worsens central apneas during sleep. Chest 2005; 128:2141–2150.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999; 22:667–689.
- Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest 2007; 132:325–337.
- Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 2003; 168:522–530.
- Hedner J, Bengtsson-Boström K, Peker Y, Grote L, Råstam L, Lindblad U. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J 2006; 27:564–570.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J 2006; 28:596–602.
- Guardiola JJ, Matheson PJ, Clavijo LC, Wilson MA, Fletcher EC. Hypercoagulability in patients with obstructive sleep apnea. Sleep Med 2001; 2:517–523.
- von Känel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest 2007; 131:733–739.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2010; Epub ahead of print.
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005; 353:2034–2041.
- Mulgrew AT, Nasvadi G, Butt A, et al. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnoea/hypopnoea. Thorax 2008; 63:536–541.
- AlGhanim N, Comondore VR, Fleetham J, Marra CA, Ayas NT. The economic impact of obstructive sleep apnea. Lung 2008; 186:7–12.
- Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006; 3:CD001106.
- Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003; 163:565–571.
- George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001; 56:508–512.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107:68–73.
- Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med 2001; 163:344–348.
- Campos-Rodriguez F, Peña-Griñan N, Reyes-Nuñez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest 2005; 128:624–633.
- Pepin JL, Tamisier R, Barone-Rochette G, Launois SH, Levy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive sleep apnea patients. Am J Respir Crit Care Med 2010; Epub ahead of print.
- Kuzniar TJ, Pusalavidyasagar S, Gay PC, Morgenthaler TI. Natural course of complex sleep apnea—a retrospective study. Sleep Breath 2008; 12:135–139.
- Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep 2006; 29:1203–1209.
- Allam JS, Olson EJ, Gay PC, Morgenthaler TI. Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes. Chest 2007; 132:1839–1846.
- Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep 2007; 30:468–475.
- Bordier P. Sleep apnoea in patients with heart failure. Part I: diagnosis, definitions, prevalence, pathophysiology and haemodynamic consequences. Arch Cardiovasc Dis 2009; 102:651–661.
- Romero-Corral A, Somers VK, Pellikka PA, et al. Decreased right and left ventricular myocardial performance in obstructive sleep apnea. Chest 2007; 132:1863–1870.
- Solin P, Kaye DM, Little PJ, Bergin P, Richardson M, Naughton MT. Impact of sleep apnea on sympathetic nervous system activity in heart failure. Chest 2003; 123:1119–1126.
- Floras JS. Should sleep apnoea be a specific target of therapy in chronic heart failure? Heart 2009; 95:1041–1046.
- Khayat RN, Abraham WT, Patt B, Roy M, Hua K, Jarjoura D. Cardiac effects of continuous and bilevel positive airway pressure for patients with heart failure and obstructive sleep apnea: a pilot study. Chest 2008; 134:1162–1168.
- Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007; 49:1625–1631.
- Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med 1999; 341:949–954.
- Tkacova R, Hall MJ, Liu PP, Fitzgerald FS, Bradley TD. Left ventricular volume in patients with heart failure and Cheyne-Stokes respiration during sleep. Am J Respir Crit Care Med 1997; 156:1549–1555.
- Lorenzi-Filho G, Rankin F, Bies I, Douglas Bradley T. Effects of inhaled carbon dioxide and oxygen on Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med 1999; 159:1490–1498.
- Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol 1995; 78:1806–1815.
- Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol 1986; 61:1438–1443.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353:2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115:3173–3180.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007; 11:117–124.
- Banerjee D, Yee BJ, Piper AJ, Zwillich CW, Grunstein RR. Obesity hypoventilation syndrome: hypoxemia during continuous positive airway pressure. Chest 2007; 131:1678–1684.
- Kaw R, Hernandez AV, Walker E, Aboussouan L, Mokhlesi B. Determinants of hypercapnia in obese patients with obstructive sleep apnea: a systematic review and metaanalysis of cohort studies. Chest 2009; 136:787–796.
- Pérez de Llano LA, Golpe R, Piquer MO, et al. Clinical heterogeneity among patients with obesity hypoventilation syndrome: therapeutic implications. Respiration 2008; 75:34–39.
- Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax 2008; 63:395–401.
- Storre JH, Seuthe B, Fiechter R, et al. Average volume-assured pressure support in obesity hypoventilation: a randomized crossover trial. Chest 2006; 130:815–821.
- Aboussouan LS, Khan SU, Banerjee M, Arroliga AC, Mitsumoto H. Objective measures of the efficacy of noninvasive positive-pressure ventilation in amyotrophic lateral sclerosis. Muscle Nerve 2001; 24:403–409.
- Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol 2006; 5:140–147.
- Miller RG, Anderson F, Brooks BR, Mitsumoto H, Bradley WG, Ringel SP; ALS CARE Study Group. Outcomes research in amyotrophic lateral sclerosis: lessons learned from the amyotrophic lateral sclerosis clinical assessment, research, and education database. Ann Neurol 2009; 65(suppl 1):S24–S28.
- Bach JR. Amyotrophic lateral sclerosis: prolongation of life by noninvasive respiratory AIDS. Chest 2002; 122:92–98.
- Perrin C, Unterborn JN, Ambrosio CD, Hill NS. Pulmonary complications of chronic neuromuscular diseases and their management. Muscle Nerve 2004; 29:5–27.
- Nickol AH, Hart N, Hopkinson NS, Moxham J, Simonds A, Polkey MI. Mechanisms of improvement of respiratory failure in patients with restrictive thoracic disease treated with non-invasive ventilation. Thorax 2005; 60:754–760.
- Barle H, Söderberg P, Haegerstrand C, Markström A. Bi-level positive airway pressure ventilation reduces the oxygen cost of breathing in long-standing post-polio patients on invasive home mechanical ventilation. Acta Anaesthesiol Scand 2005; 49:197–202.
- Lechtzin N, Shade D, Clawson L, Wiener CM. Supramaximal inflation improves lung compliance in subjects with amyotrophic lateral sclerosis. Chest 2006; 129:1322–1329.
- Hill NS. Noninvasive ventilation for chronic obstructive pulmonary disease. Respir Care 2004; 49:72–87;
- McEvoy RD, Pierce RJ, Hillman D, et al; Australian trial of noninvasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64:561–566.
- Criner GJ, Brennan K, Travaline JM, Kreimer D. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest 1999; 116:667–675.
- Strumpf DA, Millman RP, Carlisle CC, et al. Nocturnal positive-pressure ventilation via nasal mask in patients with severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1991; 144:1234–1239.
- Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010; 65:303–308.
- Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med 1985; 6:651–661.
- Weitzenblum E, Chaouat A, Kessler R, Canuet M. Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5:237–241.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea. The overlap syndrome. Am J Respir Crit Care Med 2010; Epub ahead of print.
- Organized jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Française, and approved by ATS Board of Directors, December 2000. International Consensus Conferences in Intensive Care Medicine: noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 2001; 163:283–291.
- Aboussouan LS, Ricaurte B. Noninvasive positive pressure ventilation: increasing use in acute care. Cleve Clin J Med 2010; 77:307–316.
- Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983; 128:177–181.
- Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease. Positive-pressure ventilation through a nose mask. Am Rev Respir Dis 1987; 135:148–152.
- Berger KI, Ayappa I, Chatr-Amontri B, et al. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest 2001; 120:1231–1238.
- Olson AL, Zwillich C. The obesity hypoventilation syndrome. Am J Med 2005; 118:948–956.
- de Miguel J, Cabello J, Sánchez-Alarcos JM, Alvarez-Sala R, Espinós D, Alvarez-Sala JL. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath 2002; 6:3–10.
- Mezzanotte WS, Tangel DJ, Fox AM, Ballard RD, White DP. Nocturnal nasal continuous positive airway pressure in patients with chronic obstructive pulmonary disease. Influence on waking respiratory muscle function. Chest 1994; 106:1100–1108.
- Petrof BJ, Legaré M, Goldberg P, Milic-Emili J, Gottfried SB. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1990; 141:281–289.
- Verbraecken J, Willemen M, De Cock W, Van de Heyning P, De Backer WA. Continuous positive airway pressure and lung inflation in sleep apnea patients. Respiration 2001; 68:357–364.
- Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003; 348:1233–1241.
- Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 2004; 169:361–366.
- Malhotra A, Trinder J, Fogel R, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep 2004; 27:1105–1112.
- Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep 2004; 27:249–253.
- Nolan GM, Ryan S, O’Connor TM, McNicholas WT. Comparison of three auto-adjusting positive pressure devices in patients with sleep apnoea. Eur Respir J 2006; 28:159–164.
- Meurice JC, Cornette A, Philip-Joet F, et al; ANTADIR “PPC” Working Group. Evaluation of autoCPAP devices in home treatment of sleep apnea/hypopnea syndrome. Sleep Med 2007; 8:695–703.
- Morgenthaler TI, Aurora RN, Brown T, et al; Standards of Practice Committee of the AASM; American Academy of Sleep Medicine. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep 2008; 31:141–147.
- Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med 2001; 164:614–619.
- Kushida CA, Littner MR, Hirshkowitz M, et al; American Academy of Sleep Medicine. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep 2006; 29:375–380.
- Schäfer H, Ewig S, Hasper E, Lüderitz B. Failure of CPAP therapy in obstructive sleep apnoea syndrome: predictive factors and treatment with bilevel-positive airway pressure. Respir Med 1998; 92:208–215.
- Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation—a consensus conference report. Chest 1999; 116:521–534.
- Aboussouan LS, Khan SU, Meeker DP, Stelmach K, Mitsumoto H. Effect of noninvasive positive-pressure ventilation on survival in amyotrophic lateral sclerosis. Ann Intern Med 1997; 127:450–453.
- Köhnlein T, Welte T, Tan LB, Elliott MW. Assisted ventilation for heart failure patients with Cheyne-Stokes respiration. Eur Respir J 2002; 20:934–941.
- Johnson KG, Johnson DC. Bilevel positive airway pressure worsens central apneas during sleep. Chest 2005; 128:2141–2150.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999; 22:667–689.
- Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest 2007; 132:325–337.
- Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 2003; 168:522–530.
- Hedner J, Bengtsson-Boström K, Peker Y, Grote L, Råstam L, Lindblad U. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J 2006; 27:564–570.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J 2006; 28:596–602.
- Guardiola JJ, Matheson PJ, Clavijo LC, Wilson MA, Fletcher EC. Hypercoagulability in patients with obstructive sleep apnea. Sleep Med 2001; 2:517–523.
- von Känel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest 2007; 131:733–739.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2010; Epub ahead of print.
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005; 353:2034–2041.
- Mulgrew AT, Nasvadi G, Butt A, et al. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnoea/hypopnoea. Thorax 2008; 63:536–541.
- AlGhanim N, Comondore VR, Fleetham J, Marra CA, Ayas NT. The economic impact of obstructive sleep apnea. Lung 2008; 186:7–12.
- Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006; 3:CD001106.
- Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003; 163:565–571.
- George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001; 56:508–512.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107:68–73.
- Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med 2001; 163:344–348.
- Campos-Rodriguez F, Peña-Griñan N, Reyes-Nuñez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest 2005; 128:624–633.
- Pepin JL, Tamisier R, Barone-Rochette G, Launois SH, Levy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive sleep apnea patients. Am J Respir Crit Care Med 2010; Epub ahead of print.
- Kuzniar TJ, Pusalavidyasagar S, Gay PC, Morgenthaler TI. Natural course of complex sleep apnea—a retrospective study. Sleep Breath 2008; 12:135–139.
- Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep 2006; 29:1203–1209.
- Allam JS, Olson EJ, Gay PC, Morgenthaler TI. Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes. Chest 2007; 132:1839–1846.
- Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep 2007; 30:468–475.
- Bordier P. Sleep apnoea in patients with heart failure. Part I: diagnosis, definitions, prevalence, pathophysiology and haemodynamic consequences. Arch Cardiovasc Dis 2009; 102:651–661.
- Romero-Corral A, Somers VK, Pellikka PA, et al. Decreased right and left ventricular myocardial performance in obstructive sleep apnea. Chest 2007; 132:1863–1870.
- Solin P, Kaye DM, Little PJ, Bergin P, Richardson M, Naughton MT. Impact of sleep apnea on sympathetic nervous system activity in heart failure. Chest 2003; 123:1119–1126.
- Floras JS. Should sleep apnoea be a specific target of therapy in chronic heart failure? Heart 2009; 95:1041–1046.
- Khayat RN, Abraham WT, Patt B, Roy M, Hua K, Jarjoura D. Cardiac effects of continuous and bilevel positive airway pressure for patients with heart failure and obstructive sleep apnea: a pilot study. Chest 2008; 134:1162–1168.
- Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007; 49:1625–1631.
- Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med 1999; 341:949–954.
- Tkacova R, Hall MJ, Liu PP, Fitzgerald FS, Bradley TD. Left ventricular volume in patients with heart failure and Cheyne-Stokes respiration during sleep. Am J Respir Crit Care Med 1997; 156:1549–1555.
- Lorenzi-Filho G, Rankin F, Bies I, Douglas Bradley T. Effects of inhaled carbon dioxide and oxygen on Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med 1999; 159:1490–1498.
- Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol 1995; 78:1806–1815.
- Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol 1986; 61:1438–1443.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353:2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115:3173–3180.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007; 11:117–124.
- Banerjee D, Yee BJ, Piper AJ, Zwillich CW, Grunstein RR. Obesity hypoventilation syndrome: hypoxemia during continuous positive airway pressure. Chest 2007; 131:1678–1684.
- Kaw R, Hernandez AV, Walker E, Aboussouan L, Mokhlesi B. Determinants of hypercapnia in obese patients with obstructive sleep apnea: a systematic review and metaanalysis of cohort studies. Chest 2009; 136:787–796.
- Pérez de Llano LA, Golpe R, Piquer MO, et al. Clinical heterogeneity among patients with obesity hypoventilation syndrome: therapeutic implications. Respiration 2008; 75:34–39.
- Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax 2008; 63:395–401.
- Storre JH, Seuthe B, Fiechter R, et al. Average volume-assured pressure support in obesity hypoventilation: a randomized crossover trial. Chest 2006; 130:815–821.
- Aboussouan LS, Khan SU, Banerjee M, Arroliga AC, Mitsumoto H. Objective measures of the efficacy of noninvasive positive-pressure ventilation in amyotrophic lateral sclerosis. Muscle Nerve 2001; 24:403–409.
- Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol 2006; 5:140–147.
- Miller RG, Anderson F, Brooks BR, Mitsumoto H, Bradley WG, Ringel SP; ALS CARE Study Group. Outcomes research in amyotrophic lateral sclerosis: lessons learned from the amyotrophic lateral sclerosis clinical assessment, research, and education database. Ann Neurol 2009; 65(suppl 1):S24–S28.
- Bach JR. Amyotrophic lateral sclerosis: prolongation of life by noninvasive respiratory AIDS. Chest 2002; 122:92–98.
- Perrin C, Unterborn JN, Ambrosio CD, Hill NS. Pulmonary complications of chronic neuromuscular diseases and their management. Muscle Nerve 2004; 29:5–27.
- Nickol AH, Hart N, Hopkinson NS, Moxham J, Simonds A, Polkey MI. Mechanisms of improvement of respiratory failure in patients with restrictive thoracic disease treated with non-invasive ventilation. Thorax 2005; 60:754–760.
- Barle H, Söderberg P, Haegerstrand C, Markström A. Bi-level positive airway pressure ventilation reduces the oxygen cost of breathing in long-standing post-polio patients on invasive home mechanical ventilation. Acta Anaesthesiol Scand 2005; 49:197–202.
- Lechtzin N, Shade D, Clawson L, Wiener CM. Supramaximal inflation improves lung compliance in subjects with amyotrophic lateral sclerosis. Chest 2006; 129:1322–1329.
- Hill NS. Noninvasive ventilation for chronic obstructive pulmonary disease. Respir Care 2004; 49:72–87;
- McEvoy RD, Pierce RJ, Hillman D, et al; Australian trial of noninvasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64:561–566.
- Criner GJ, Brennan K, Travaline JM, Kreimer D. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest 1999; 116:667–675.
- Strumpf DA, Millman RP, Carlisle CC, et al. Nocturnal positive-pressure ventilation via nasal mask in patients with severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1991; 144:1234–1239.
- Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010; 65:303–308.
- Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med 1985; 6:651–661.
- Weitzenblum E, Chaouat A, Kessler R, Canuet M. Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5:237–241.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea. The overlap syndrome. Am J Respir Crit Care Med 2010; Epub ahead of print.
KEY POINTS
- In sleep apnea, NIPPV has both short-term benefits such as improved daytime alertness and reduced fatigue, and long-term benefits such as a reduced cardiovascular risk.
- The potential development of complex sleep apnea with NIPPV may be managed by using lower pressures, by continued treatment (more than half of cases improve over time), and by advanced options such as adaptive servo-ventilation.
- In patients with concomitant obstructive sleep apnea and congestive heart failure, NIPPV, particularly bilevel positive airway pressure, improves blood pressure and left ventricular function, though it is not clear whether it has a survival benefit.
Noninvasive positive pressure ventilation: Increasing use in acute care
Noninvasive positive pressure ventilation (NIPPV)—delivered via a tight-fitting mask rather than via an endotracheal tube or tracheostomy—is one of the most important advances in the management of acute respiratory failure to emerge in the past 2 decades. It is now recommended as the first choice for ventilatory support in selected patients, such as those with exacerbations of chronic obstructive pulmonary disease (COPD) or with cardiogenic pulmonary edema.1–3 In fact, some authors suggest that using NIPPV in more than 20% of COPD patients is a characteristic of respiratory care departments that are “avid for change”4—change being a good thing.
However, NIPPV has not been universally accepted, with wide variations in its utilization. In a 2006 survey, it was being used in only 33% of patients with COPD or congestive heart failure, for which it might be indicated. 5 Some potential reasons for the low rate are that physicians do not know about it, respiratory therapists are not sufficiently trained in it, and hospitals lack the equipment to do it.5
Our goal in this review is to familiarize the reader with how NIPPV has evolved and with its indications and contraindications in specific acute care conditions.
FROM A VACUUM CLEANER TO THE INTENSIVE CARE UNIT
NIPPV appears to have been first tried in 1870 by Chaussier, who used a bag and face mask to resuscitate neonates.6
In 1936, Poulton and Oxon7 described their “pulmonary plus pressure machine,” which used a vacuum cleaner blower and a mask to increase the alveolar pressure and thus counteract the increased intrapulmonary pressure in patients with heart failure, pulmonary edema, Cheyne-Stokes breathing, and asthma.
In the 1940s, intermittent positive pressure breathing devices were developed for use in high-altitude aviation. Motley, Werko, and Cournand8,9 subsequently used these devices to treat acute respiratory failure in pneumonia, pulmonary edema, near-drowning, Guillain-Barré syndrome, and acute severe asthma.
Although NIPPV was shown to be effective for acute conditions, invasive ventilation became preferred, particularly as blood gas analysis and ventilator technologies simultaneously matured, spurred at least in part by the polio epidemics of the 1950s.10
NIPPV reemerged in the 1980s for use in chronic conditions. First, continuous positive airway pressure (CPAP) came into use for obstructive sleep apnea,11 followed by noninvasive positive-pressure volume ventilation in neuromuscular diseases.12 Bilevel positive pressure devices (ie, with separate inspiratory and expiratory pressures) soon followed, again initially for obstructive sleep apnea13 and then for diverse neuromuscular diseases.14
NIPPV is now a mainstream therapy for diverse conditions in acute and chronic care.3 One reason we now use it in acute conditions is to avoid the complications associated with intubation.
Some clinicians initially resisted using NIPPV, concerned that it demanded too much of the nurses’ time15 and was costly.16 However, in a 1997 study in patients with COPD and acute respiratory failure, Nava et al17 found that NIPPV was no more expensive and no more demanding of staff resources than invasive mechanical ventilation in the first 48 hours of ventilation. Further, after the first few days of ventilation, NIPPV put fewer time demands on physicians and nurses than did invasive mechanical ventilation.
THREE MODES: CPAP, PRESSURE-LIMITED, VOLUME-LIMITED
The term “noninvasive ventilation” generally encompasses various forms of positive pressure ventilation. However, negative pressure ventilation, in the form of diaphragm pacing, may regain a foothold in the devices used for respiratory support.18 We therefore favor the term “NIPPV” in this review.
NIPPV IN ACUTE RESPIRATORY FAILURE
The main reasons to use NIPPV instead of invasive ventilation in acute care are to avoid the complications of invasive ventilation, to improve outcomes (eg, reduce mortality rates, decrease hospital length of stay), and to decrease the cost of care.
NIPPV is the standard of care for acute exacerbations of COPD
In a meta-analysis of eight randomized controlled trials,24 the specific advantages of NIPPV compared with usual care in acute exacerbations of COPD included:
- A lower risk of treatment failure, defined as death, need for intubation, or inability to tolerate the treatment (relative risk [RR] 0.51, number needed to treat [NNT] to prevent one treatment failure = 5)
- A lower risk of intubation (RR 0.43, NNT = 5)
- A lower mortality rate (RR 0.41, NNT = 8)
- A lower risk of complications (RR 0.32, NNT = 3)
- A shorter hospital length of stay (by about 3 days).
Mechanisms by which NIPPV may impart these benefits include reducing the work of breathing, unloading the respiratory muscles, lessening diaphragmatic pressure swings, reducing the respiratory rate, eliminating diaphragmatic work, and counteracting the threshold loading effects of auto-positive end-expiratory pressure (auto-PEEP).24–26
Also, if a patient with COPD is intubated, NIPPV seems to help after the tube is removed, preventing postextubation respiratory failure and facilitating weaning from invasive ventilation.27 These topics are discussed below.
A Cochrane systematic review24 concluded that NIPPV should be tried early in the course of respiratory failure, before severe acidosis develops. The patients in the studies in this review all had partial pressure of arterial carbon dioxide (Paco2) levels greater than 45 mm Hg.
In patients with severe respiratory acidosis (pH < 7.25), NIPPV failure rates are greater than 50%. However, trying NIPPV may still be justified, even in the presence of hypercapnic encephalopathy, as long as no other indications for invasive support and facilities for prompt endotracheal intubation are available. 1
However, in another systematic review,26 in patients with mild COPD exacerbations (pH > 7.35), NIPPV was no more effective than standard medical therapy in preventing acute respiratory failure, preventing death, or reducing length of hospitalization. Moreover, nearly 50% of the patients could not tolerate NIPPV.
Rapid improvement in cardiogenic pulmonary edema, but possibly no lower mortality rate
The Three Interventions in Cardiogenic Pulmonary Oedema (3CPO) trial,28 with 1,156 patients, was the largest randomized trial to compare NIPPV and standard oxygen therapy for acute pulmonary edema. It found that NIPPV (either CPAP or noninvasive intermittent positive pressure ventilation) was significantly better than standard oxygen therapy (through a variable-delivery oxygen mask with a reservoir) in the first hour of treatment in terms of the dyspnea score, heart rate, acidosis, and hypercapnia. However, there were no significant differences between groups in the 7- or 30-day mortality rates, the rates of intubation, rates of admission to the critical care unit, or in the mean length of hospital stay.
In contrast, several smaller randomized trials and meta-analyses showed lower intubation and mortality rates with NIPPV.29,30 Factors that may account for those differences include a much lower intubation rate in the 3CPO trial (2.9% overall, compared with 20% with conventional therapy in other trials), a higher mortality rate in the 3CPO trial, and methodologic differences (eg, patients for whom standard therapy failed in the 3CPO trial received rescue NIPPV).
If NIPPV is beneficial in cardiogenic pulmonary edema, the mechanisms are probably its favorable hemodynamic effects and its positive end-expiratory pressure (PEEP) effect on flooded alveoli. Specifically, positive intrathoracic pressure can be expected to reduce both preload and afterload, with improvement in the cardiac index and reduced work of breathing. 31,32
Notwithstanding the possible lack of impact of NIPPV on death or intubation rates in this setting, the intervention rapidly improves dyspnea and respiratory and metabolic abnormalities and should be considered for treatment of cardiogenic pulmonary edema associated with severe respiratory distress. A subgroup in which the NIPPV may reduce intubation rates is those with hypercapnia.33 A concern that NIPPV may increase the rate of myocardial infarction34 was not confirmed in the 3CPO trial.28 Interestingly, there were no differences in outcomes between CPAP and noninvasive intermittent positive pressure ventilation in this setting.28,34,35
Immunocompromised patients with acute respiratory failure
A particular challenge of NIPPV in immunocompromised patients, particularly compared with its use in COPD exacerbation or cardiogenic pulmonary edema, is that the underlying pathophysiology of respiratory dysfunction in immunocompromised patients may not be readily reversible. Therefore, its application in this group may need to follow clearly defined indications.
In one trial,20 inclusion criteria were:
- Immune suppression (due to neutropenia after chemotherapy or bone marrow transplantation, immunosuppressive drugs for organ transplantation, corticosteroids, cytotoxic therapy for nonmalignant conditions, or the acquired immunodeficiency syndrome)
- Persistent pulmonary infiltrates
- Fever (temperature > 38.3°C; 100.9°F)
- A respiratory rate greater than 30 breaths per minute
- Severe dyspnea at rest
- Early hypoxemic acute respiratory failure, defined as a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (Pao2/Fio2 ratio) less than 200 while on oxygen.
Compared with patients who received conventional treatment, fewer of those randomized to additional intermittent noninvasive ventilation had to be intubated (46% vs 77%, P = .03), suffered serious complications (50% vs 81%, P = .02), or died in the intensive care unit (38% vs 69%, P = .03) or in the hospital (50% vs 81%, P = .02).
Similarly, in a randomized trial in 40 patients with acute respiratory failure after solid organ transplantation, more patients in the NIPPV group than in the control group had an improvement in the Pao2/Fio2 ratio within the first hour (70% vs 25%, P = .004) or a sustained improvement in the Pao2/Fio2 ratio (60% vs 25%, P = .03); fewer of them needed endotracheal intubation (20% vs 70%, P = .002); fewer of them died of complications (20% vs 50%, P = .05); they had a shorter length of stay in the intensive care unit (mean 5.5 vs 9 days, P = .03); and fewer of them died in the intensive care unit (20% vs 50%, P = .05). There was, however, no difference in the overall hospital mortality rate.36
MAY NOT HELP AFTER EXTUBATION, EXCEPT IN SPECIFIC CASES
NIPPV has been used to treat respiratory failure after extubation,22,37 to prevent acute respiratory failure after failure of weaning,38–41 and to support breathing in patients who failed a trial of spontaneous breathing.42–45
Unfortunately, the evidence for using NIPPV in respiratory failure after extubation, including unplanned extubation, appears to be unfavorable, except possibly in patients with chronic pulmonary disease (particularly COPD and possibly obesity) and hypercapnia. An international consensus report stated that NIPPV should be considered in patients with hypercapnic respiratory insufficiency, especially those with COPD, to shorten the duration of intubation, but that it should not be routinely used in extubation respiratory failure.46
Treatment of respiratory failure after extubation
Two recent randomized controlled trials compared NIPPV and standard care in patients who met the criteria for readiness for extubation but who developed respiratory failure after mechanical ventilation was discontinued. 22,37 Those two studies showed a longer time to reintubation for patients randomized to NIPPV but no differences in the rate of reintubation between the two groups and no difference in the lengths of stay in the intensive care unit.
Of greater concern, one study showed a higher rate of death in the intensive care unit in the NIPPV group than in the standard therapy group (25% vs 14%, respectively).22 This finding suggests that NIPPV delayed necessary reintubation in patients developing respiratory failure after extubation, with a consequent risk of fatal complications.
Prevention of respiratory failure after extubation
Other studies used NIPPV to prevent respiratory failure after extubation rather than wait to apply it after respiratory failure developed.38–41
Nava et al,40 in a trial in patients successfully weaned but considered to be at risk of reintubation, found that fewer of those randomized to NIPPV had to be reintubated than those who received standard care (8% vs 24%), and 10% fewer of them died in the intensive care unit. Risk factors for reintubation (and therefore eligibility criteria for this trial) included a Paco2 higher than 45 mm Hg, more than one consecutive failure of weaning, chronic heart failure, other comorbidity, weak cough, or stridor.
Extubated patients are a heterogeneous group, so if some subgroups benefit from a transition to NIPPV after extubation, it will be important to identify them. For instance, a subgroup analysis of a study by Ferrer et al38 indicated the survival benefit of NIPPV after extubation was limited to patients with chronic respiratory disorders and hypercapnia during a trial of spontaneous breathing.
In a subsequent successful test of this hypothesis, a randomized trial showed that the early use of noninvasive ventilation in patients with hypercapnia after a trial of spontaneous breathing and with chronic respiratory disorders (COPD, chronic bronchitis, bronchiectasis, obesity-hypoventilation, sequelae of tuberculosis, chest wall deformity, or chronic persistent asthma) reduced the risk of respiratory failure after extubation and the risk of death within the first 90 days.39
Others in which this approach may be helpful are obese patients who have high Paco2 levels. Compared with historical controls, 62 patients with a body mass index greater than 35 kg/m2 who received NIPPV in the 48 hours after extubation had a lower rate of respiratory failure, shorter lengths of stay in the intensive care unit and hospital, and, in the subgroup with hypercapnia, a lower hospital mortality rate.41
NIPPV to facilitate weaning
In several studies, mechanically ventilated patients who had failed a trial of spontaneous breathing were randomized to undergo either accelerated weaning, extubation, and NIPPV or conventional weaning with pressure support via mechanical ventilation.42–46 Most patients developed hypercapnia during the spontaneous breathing trials, and most of the patients had COPD.
A meta-analysis47 of the randomized trials of this approach concluded that, compared with continued invasive ventilation, NIPPV decreased the risk of death (relative risk 0.41) and of ventilator-associated pneumonia (relative risk 0.28) and reduced the total duration of mechanical ventilation by a weighted mean difference of 7.33 days. The benefits appeared to be most significant in patients with COPD.
NIPPV IN ASTHMA AND STATUS ASTHMATICUS
Noninvasive ventilation is an attractive alternative to intubation for patients with status asthmaticus, given the challenges and conflicting demands of maintaining ventilation despite severe airway obstruction.
In a 1996 prospective study of 17 episodes of asthma associated with acute respiratory failure, Meduri et al48 showed that NIPPV could progressively improve the pH and the Paco2 over 12 to 24 hours and reduce the respiratory rate.
In a subsequent controlled trial, Soroksky et al49 randomized 30 patients presenting to an emergency room with a severe asthma attack to NIPPV with conventional therapy vs conventional therapy only. The study group had a significantly greater increase in the forced expiratory volume in 1 second compared with the control group (54% vs 29%, respectively) and a lower hospitalization rate (18% vs 63%).
Another randomized trial of NIPPV, in patients with status asthmaticus presenting to an emergency room, was prematurely terminated due to a physician treatment bias that favored NIPPV.50 The preliminary results of that study showed a 7.3% higher intubation rate in the control group than in the NIPPV group, along with trends toward a lower intubation rate, a shorter length of hospital stay, and lower hospital charges in the NIPPV group.
Despite these initial favorable results, a Cochrane review concluded that the use of NIPPV in patients with status asthmaticus is controversial.51 NIPPV can be tried in selected patients such as those with mild to moderate respiratory distress (respiratory rate greater than 25 breaths per minute, use of accessory muscles to breathe, difficulty speaking), an arterial pH of 7.25 to 7.35, and a Paco2 of 45 to 55 mm Hg.52 Patients with impending respiratory failure or the inability to protect the airway should probably not be considered for NIPPV.52
IN ACUTE LUNG INJURY AND ACUTE RESPIRATORY DISTRESS SYNDROME
The most challenging application of NIPPV may be in patients with acute lung injury and the acute respiratory distress syndrome.
Initial trials of NIPPV in this setting have been disappointing, and a meta-analysis of the topic concluded that NIPPV was unlikely to have any significant benefit.53 An earlier study that used CPAP in patients with acute respiratory failure predominantly due to acute lung injury showed early physiologic improvements but no reduction in the need for intubation, no improvement in outcomes, and a higher rate of adverse events, including cardiac arrest, in those randomized to CPAP.54
A subsequent observational cohort specifically identified shock, metabolic acidosis, and severe hypoxemia as predictors of NIPPV failure.55
A more recent prospective study demonstrated that NIPPV improved gas exchange and obviated intubation in 54% of patients, with a consequent reduction in ventilator-associated pneumonia and a lower rate of death in the intensive care unit.56 A Simplified Acute Physiology Score (SAPS) II greater than 34 and a Pao2/Fio2 ratio less than 175 after 1 hour of NIPPV were identified as predicting that NIPPV would fail.56
MISCELLANEOUS APPLICATIONS
The more widespread use of NIPPV has encouraged its use in other acute situations, including during procedures such as percutaneous endoscopic gastrostomy (PEG)57,58 or bronchoscopy,59,60 for palliative use in patients listed as “do-not-intubate,”61–63 and for oxygenation before intubation.64
NIPPV during PEG tube insertion
NIPPV during PEG tube placement is particularly useful for patients with neuromuscular diseases who are at a combined risk of aspiration, poor oral intake, and respiratory failure during procedures. The experience with patients with amyotrophic lateral sclerosis58 and Duchenne muscular dystrophy57 indicates that even patients at high risk of respiratory failure during procedures can be successfully managed with NIPPV. The most recent practice parameters for patients with amyotrophic lateral sclerosis propose that patients with dysphagia may be exposed to less risk if the PEG procedure is performed when the forced vital capacity is greater than 50% of predicted.65
In randomized trials of CPAP59 or pressure-support NIPPV60 in high-risk hypoxemic patients who needed diagnostic bronchoscopy, patients in the intervention groups fared better than those who received oxygen alone, with better oxygenation during and after the procedure and a lower risk of postprocedure respiratory failure. Improved hemodynamics with a lower mean heart rate and a stable mean arterial pressure were also reported in one of those studies.60
Palliative use in ‘do-not-intubate’ patients
In patients who decline intubation, NIPPV appears to be most effective in reversing acute respiratory failure and improving mortality rates in those with COPD or with cardiogenic pulmonary edema.61,62 Controversy surrounding the use of NIPPV in “do-not-intubate” patients, particularly as a potentially uncomfortable life support technique, has been addressed by a task force of the Society of Critical Care Medicine, which recommends that it be applied only after careful discussion of goals of care and parameters of treatment with patients and their families.63
Oxygenation before intubation
In a prospective randomized study of oxygenation before rapid-sequence intubation via either a nonrebreather bag-valve mask or NIPPV, the NIPPV group had a higher oxygen saturation rate before, during, and after the intubation procedure.64
Acknowledgment: The authors wish to thank Jodith Janes of the Cleveland Clinic Alumni Library for her help with reference citations and with locating articles.
- Ambrosino N, Vagheggini G. Noninvasive positive pressure ventilation in the acute care setting: where are we? Eur Respir J 2008; 31:874–886.
- Hill NS, Brennan J, Garpestad E, Nava S. Noninvasive ventilation in acute respiratory failure. Crit Care Med 2007; 35:2402–2407.
- Nava S, Navalesi P, Conti G. Time of non-invasive ventilation. Intensive Care Med 2006; 32:361–370.
- Stoller JK, Kester L, Roberts VT, et al; An analysis of features of respiratory therapy departments that are avid for change. Respir Care 2008; 53:871–884.
- Maheshwari V, Paioli D, Rothaar R, Hill NS. Utilization of noninvasive ventilation in acute care hospitals: a regional survey. Chest 2006; 129:1226–1233.
- Obladen M. History of neonatal resuscitation. Part 1: Artificial ventilation. Neonatology 2008; 94:144–149.
- Poulton EP, Oxon DM. Left-sided heart failure with pulmonary oedema: its treatment with the “pulmonary plus” pressure machine. Lancet 1936; 228:981–983.
- Motley HL, Werko L. Observations on the clinical use of intermittent positive pressure. J Aviat Med 1947; 18:417–435.
- Cournand A, Motley HL. Physiological studies of the effects of intermittent positive pressure breathing on cardiac output in man. Am J Physiol 1948; 152:162–174.
- Severinghaus JW, Astrup P, Murray JF. Blood gas analysis and critical care medicine. Am J Respir Crit Care Med 1998; 157:S114–S122.
- Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983; 128:177–181.
- Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease. Positive-pressure ventilation through a nose mask. Am Rev Respir Dis 1987; 135:148–152.
- Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal mask. Physiologic and clinical implications. Chest 1990; 98:317–324.
- Bach JR. Mechanical exsufflation, noninvasive ventilation, and new strategies for pulmonary rehabilitation and sleep disordered breathing. Bull N Y Acad Med 1992; 68:321–340.
- Chevrolet JC, Jolliet P, Abajo B, Toussi A, Louis M. Nasal positive pressure ventilation in patients with acute respiratory failure. Difficult and time-consuming procedure for nurses. Chest 1991; 100:775–782.
- Criner GJ, Kreimer DT, Tomaselli M, Pierson W, Evans D. Financial implications of noninvasive positive pressure ventilation (NPPV). Chest 1995; 108:475–481.
- Nava S, Evangelisti I, Rampulla C, Compagnoni ML, Fracchia C, Rubini F. Human and financial costs of noninvasive mechanical ventilation in patients affected by COPD and acute respiratory failure. Chest 1997; 111:1631–1638.
- DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Mortimer JT. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest 2005; 127:671–678.
- Antonelli M, Conti G. Noninvasive positive pressure ventilation as treatment for acute respiratory failure in critically ill patients. Crit Care 2000; 4:15–22.
- Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med 2001; 344:481–487.
- L’Her E, Deye N, Lellouche F, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med 2005; 172:1112–1118.
- Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004; 350:2452–2460.
- Hill NS. Noninvasive positive pressure ventilation for respiratory failure caused by exacerbations of chronic obstructive pulmonary disease: a standard of care? Crit Care 2003; 7:400–401.
- Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ 2003; 326:185–187.
- Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995; 333:817–822.
- Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med 2003; 138:861–870.
- Epstein SK. Noninvasive ventilation to shorten the duration of mechanical ventilation. Respir Care 2009; 54:198–208.
- Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J; 3CPO Trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med 2008; 359:142–151.
- Collins SP, Mielniczuk LM, Whittingham HA, Boseley ME, Schramm DR, Storrow AB. The use of noninvasive ventilation in emergency department patients with acute cardiogenic pulmonary edema: a systematic review. Ann Emerg Med 2006; 48:260–269.
- Masip J, Roque M, Sánchez B, Fernández R, Subirana M, Expósito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta-analysis. JAMA 2005; 294:3124–3130.
- Baratz DM, Westbrook PR, Shah PK, Mohsenifar Z. Effect of nasal continuous positive airway pressure on cardiac output and oxygen delivery in patients with congestive heart failure. Chest 1992; 102:1397–1401.
- Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation 1995; 91:1725–1731.
- Nava S, Carbone G, DiBattista N, et al. Noninvasive ventilation in cardiogenic pulmonary edema: a multicenter randomized trial. Am J Respir Crit Care Med 2003; 168:1432–1437.
- Mehta S, Jay GD, Woolard RH, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med 1997; 25:620–628.
- Ho KM, Wong K. A comparison of continuous and bi-level positive airway pressure non-invasive ventilation in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Crit Care 2006; 10:R49.
- Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA 2000; 283:235–241.
- Keenan SP, Powers C, McCormack DG, Block G. Noninvasive positive-pressure ventilation for postextubation respiratory distress: a randomized controlled trial. JAMA 2002; 287:3238–3244.
- Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med 2006; 173:164–170.
- Ferrer M, Sellarés J, Valencia M, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet 2009; 374:1082–1088.
- Nava S, Gregoretti C, Fanfulla F, et al. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med 2005; 33:2465–2470.
- El-Solh AA, Aquilina A, Pineda L, Dhanvantri V, Grant B, Bouquin P. Noninvasive ventilation for prevention of post-extubation respiratory failure in obese patients. Eur Respir J 2006; 28:588–595.
- Ferrer M, Esquinas A, Arancibia F, et al. Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med 2003; 168:70–76.
- Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med 1999; 160:86–92.
- Nava S, Ambrosino N, Clini E, et al. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann Intern Med 1998; 128:721–728.
- Trevisan CE, Vieira SR; Research Group in Mechanical Ventilation Weaning. Noninvasive mechanical ventilation may be useful in treating patients who fail weaning from invasive mechanical ventilation: a randomized clinical trial. Crit Care 2008; 12:R51.
- Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J 2007; 29:1033–1056.
- Burns KE, Adhikari NK, Meade MO. A meta-analysis of noninvasive weaning to facilitate liberation from mechanical ventilation. Can J Anaesth 2006; 53:305–315.
- Meduri GU, Cook TR, Turner RE, Cohen M, Leeper KV. Noninvasive positive pressure ventilation in status asthmaticus. Chest 1996; 110:767–774.
- Soroksky A, Stav D, Shpirer I. A pilot prospective, randomized, placebo-controlled trial of bilevel positive airway pressure in acute asthmatic attack. Chest 2003; 123:1018–1025.
- Holley MT, Morrissey TK, Seaberg DC, Afessa B, Wears RL. Ethical dilemmas in a randomized trial of asthma treatment: can Bayesian statistical analysis explain the results? Acad Emerg Med 2001; 8:1128–1135.
- Ram FS, Wellington S, Rowe BH, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev 2005;CD004360.
- Medoff BD. Invasive and noninvasive ventilation in patients with asthma. Respir Care 2008; 53:740–748.
- Agarwal R, Reddy C, Aggarwal AN, Gupta D. Is there a role for noninvasive ventilation in acute respiratory distress syndrome? A meta-analysis. Respir Med 2006; 100:2235–2238.
- Delclaux C, L’Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA 2000; 284:2352–2360.
- Rana S, Jenad H, Gay PC, Buck CF, Hubmayr RD, Gajic O. Failure of non-invasive ventilation in patients with acute lung injury: observational cohort study. Crit Care 2006; 10:R79.
- Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med 2007; 35:18–25.
- Birnkrant DJ, Ferguson RD, Martin JE, Gordon GJ. Noninvasive ventilation during gastrostomy tube placement in patients with severe Duchenne muscular dystrophy: case reports and review of the literature. Pediatr Pulmonol 2006; 41:188–193.
- Boitano LJ, Jordan T, Benditt JO. Noninvasive ventilation allows gastrostomy tube placement in patients with advanced ALS. Neurology 2001; 56:413–414.
- Maitre B, Jaber S, Maggiore SM, et al. Continuous positive airway pressure during fiberoptic bronchoscopy in hypoxemic patients. A randomized double-blind study using a new device. Am J Respir Crit Care Med 2000; 162:1063–1067.
- Antonelli M, Conti G, Rocco M, et al. Noninvasive positive-pressure ventilation vs conventional oxygen supplementation in hypoxemic patients undergoing diagnostic bronchoscopy. Chest 2002; 121:1149–1154.
- Levy M, Tanios MA, Nelson D, et al. Outcomes of patients with do-not-intubate orders treated with noninvasive ventilation. Crit Care Med 2004; 32:2002–2007.
- Schettino G, Altobelli N, Kacmarek RM. Noninvasive positive pressure ventilation reverses acute respiratory failure in select “do-not-intubate” patients. Crit Care Med 2005; 33:1976–1982.
- Curtis JR, Cook DJ, Sinuff T, et al; Society of Critical Care Medicine Palliative Noninvasive Positive Ventilation Task Force. Noninvasive positive pressure ventilation in critical and palliative care settings: understanding the goals of therapy. Crit Care Med 2007; 35:932–939.
- Baillard C, Fosse JP, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med 2006; 174:171–177.
- Miller RG, Jackson CE, Kasarskis EJ, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009; 73:1218–1226.
Noninvasive positive pressure ventilation (NIPPV)—delivered via a tight-fitting mask rather than via an endotracheal tube or tracheostomy—is one of the most important advances in the management of acute respiratory failure to emerge in the past 2 decades. It is now recommended as the first choice for ventilatory support in selected patients, such as those with exacerbations of chronic obstructive pulmonary disease (COPD) or with cardiogenic pulmonary edema.1–3 In fact, some authors suggest that using NIPPV in more than 20% of COPD patients is a characteristic of respiratory care departments that are “avid for change”4—change being a good thing.
However, NIPPV has not been universally accepted, with wide variations in its utilization. In a 2006 survey, it was being used in only 33% of patients with COPD or congestive heart failure, for which it might be indicated. 5 Some potential reasons for the low rate are that physicians do not know about it, respiratory therapists are not sufficiently trained in it, and hospitals lack the equipment to do it.5
Our goal in this review is to familiarize the reader with how NIPPV has evolved and with its indications and contraindications in specific acute care conditions.
FROM A VACUUM CLEANER TO THE INTENSIVE CARE UNIT
NIPPV appears to have been first tried in 1870 by Chaussier, who used a bag and face mask to resuscitate neonates.6
In 1936, Poulton and Oxon7 described their “pulmonary plus pressure machine,” which used a vacuum cleaner blower and a mask to increase the alveolar pressure and thus counteract the increased intrapulmonary pressure in patients with heart failure, pulmonary edema, Cheyne-Stokes breathing, and asthma.
In the 1940s, intermittent positive pressure breathing devices were developed for use in high-altitude aviation. Motley, Werko, and Cournand8,9 subsequently used these devices to treat acute respiratory failure in pneumonia, pulmonary edema, near-drowning, Guillain-Barré syndrome, and acute severe asthma.
Although NIPPV was shown to be effective for acute conditions, invasive ventilation became preferred, particularly as blood gas analysis and ventilator technologies simultaneously matured, spurred at least in part by the polio epidemics of the 1950s.10
NIPPV reemerged in the 1980s for use in chronic conditions. First, continuous positive airway pressure (CPAP) came into use for obstructive sleep apnea,11 followed by noninvasive positive-pressure volume ventilation in neuromuscular diseases.12 Bilevel positive pressure devices (ie, with separate inspiratory and expiratory pressures) soon followed, again initially for obstructive sleep apnea13 and then for diverse neuromuscular diseases.14
NIPPV is now a mainstream therapy for diverse conditions in acute and chronic care.3 One reason we now use it in acute conditions is to avoid the complications associated with intubation.
Some clinicians initially resisted using NIPPV, concerned that it demanded too much of the nurses’ time15 and was costly.16 However, in a 1997 study in patients with COPD and acute respiratory failure, Nava et al17 found that NIPPV was no more expensive and no more demanding of staff resources than invasive mechanical ventilation in the first 48 hours of ventilation. Further, after the first few days of ventilation, NIPPV put fewer time demands on physicians and nurses than did invasive mechanical ventilation.
THREE MODES: CPAP, PRESSURE-LIMITED, VOLUME-LIMITED
The term “noninvasive ventilation” generally encompasses various forms of positive pressure ventilation. However, negative pressure ventilation, in the form of diaphragm pacing, may regain a foothold in the devices used for respiratory support.18 We therefore favor the term “NIPPV” in this review.
NIPPV IN ACUTE RESPIRATORY FAILURE
The main reasons to use NIPPV instead of invasive ventilation in acute care are to avoid the complications of invasive ventilation, to improve outcomes (eg, reduce mortality rates, decrease hospital length of stay), and to decrease the cost of care.
NIPPV is the standard of care for acute exacerbations of COPD
In a meta-analysis of eight randomized controlled trials,24 the specific advantages of NIPPV compared with usual care in acute exacerbations of COPD included:
- A lower risk of treatment failure, defined as death, need for intubation, or inability to tolerate the treatment (relative risk [RR] 0.51, number needed to treat [NNT] to prevent one treatment failure = 5)
- A lower risk of intubation (RR 0.43, NNT = 5)
- A lower mortality rate (RR 0.41, NNT = 8)
- A lower risk of complications (RR 0.32, NNT = 3)
- A shorter hospital length of stay (by about 3 days).
Mechanisms by which NIPPV may impart these benefits include reducing the work of breathing, unloading the respiratory muscles, lessening diaphragmatic pressure swings, reducing the respiratory rate, eliminating diaphragmatic work, and counteracting the threshold loading effects of auto-positive end-expiratory pressure (auto-PEEP).24–26
Also, if a patient with COPD is intubated, NIPPV seems to help after the tube is removed, preventing postextubation respiratory failure and facilitating weaning from invasive ventilation.27 These topics are discussed below.
A Cochrane systematic review24 concluded that NIPPV should be tried early in the course of respiratory failure, before severe acidosis develops. The patients in the studies in this review all had partial pressure of arterial carbon dioxide (Paco2) levels greater than 45 mm Hg.
In patients with severe respiratory acidosis (pH < 7.25), NIPPV failure rates are greater than 50%. However, trying NIPPV may still be justified, even in the presence of hypercapnic encephalopathy, as long as no other indications for invasive support and facilities for prompt endotracheal intubation are available. 1
However, in another systematic review,26 in patients with mild COPD exacerbations (pH > 7.35), NIPPV was no more effective than standard medical therapy in preventing acute respiratory failure, preventing death, or reducing length of hospitalization. Moreover, nearly 50% of the patients could not tolerate NIPPV.
Rapid improvement in cardiogenic pulmonary edema, but possibly no lower mortality rate
The Three Interventions in Cardiogenic Pulmonary Oedema (3CPO) trial,28 with 1,156 patients, was the largest randomized trial to compare NIPPV and standard oxygen therapy for acute pulmonary edema. It found that NIPPV (either CPAP or noninvasive intermittent positive pressure ventilation) was significantly better than standard oxygen therapy (through a variable-delivery oxygen mask with a reservoir) in the first hour of treatment in terms of the dyspnea score, heart rate, acidosis, and hypercapnia. However, there were no significant differences between groups in the 7- or 30-day mortality rates, the rates of intubation, rates of admission to the critical care unit, or in the mean length of hospital stay.
In contrast, several smaller randomized trials and meta-analyses showed lower intubation and mortality rates with NIPPV.29,30 Factors that may account for those differences include a much lower intubation rate in the 3CPO trial (2.9% overall, compared with 20% with conventional therapy in other trials), a higher mortality rate in the 3CPO trial, and methodologic differences (eg, patients for whom standard therapy failed in the 3CPO trial received rescue NIPPV).
If NIPPV is beneficial in cardiogenic pulmonary edema, the mechanisms are probably its favorable hemodynamic effects and its positive end-expiratory pressure (PEEP) effect on flooded alveoli. Specifically, positive intrathoracic pressure can be expected to reduce both preload and afterload, with improvement in the cardiac index and reduced work of breathing. 31,32
Notwithstanding the possible lack of impact of NIPPV on death or intubation rates in this setting, the intervention rapidly improves dyspnea and respiratory and metabolic abnormalities and should be considered for treatment of cardiogenic pulmonary edema associated with severe respiratory distress. A subgroup in which the NIPPV may reduce intubation rates is those with hypercapnia.33 A concern that NIPPV may increase the rate of myocardial infarction34 was not confirmed in the 3CPO trial.28 Interestingly, there were no differences in outcomes between CPAP and noninvasive intermittent positive pressure ventilation in this setting.28,34,35
Immunocompromised patients with acute respiratory failure
A particular challenge of NIPPV in immunocompromised patients, particularly compared with its use in COPD exacerbation or cardiogenic pulmonary edema, is that the underlying pathophysiology of respiratory dysfunction in immunocompromised patients may not be readily reversible. Therefore, its application in this group may need to follow clearly defined indications.
In one trial,20 inclusion criteria were:
- Immune suppression (due to neutropenia after chemotherapy or bone marrow transplantation, immunosuppressive drugs for organ transplantation, corticosteroids, cytotoxic therapy for nonmalignant conditions, or the acquired immunodeficiency syndrome)
- Persistent pulmonary infiltrates
- Fever (temperature > 38.3°C; 100.9°F)
- A respiratory rate greater than 30 breaths per minute
- Severe dyspnea at rest
- Early hypoxemic acute respiratory failure, defined as a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (Pao2/Fio2 ratio) less than 200 while on oxygen.
Compared with patients who received conventional treatment, fewer of those randomized to additional intermittent noninvasive ventilation had to be intubated (46% vs 77%, P = .03), suffered serious complications (50% vs 81%, P = .02), or died in the intensive care unit (38% vs 69%, P = .03) or in the hospital (50% vs 81%, P = .02).
Similarly, in a randomized trial in 40 patients with acute respiratory failure after solid organ transplantation, more patients in the NIPPV group than in the control group had an improvement in the Pao2/Fio2 ratio within the first hour (70% vs 25%, P = .004) or a sustained improvement in the Pao2/Fio2 ratio (60% vs 25%, P = .03); fewer of them needed endotracheal intubation (20% vs 70%, P = .002); fewer of them died of complications (20% vs 50%, P = .05); they had a shorter length of stay in the intensive care unit (mean 5.5 vs 9 days, P = .03); and fewer of them died in the intensive care unit (20% vs 50%, P = .05). There was, however, no difference in the overall hospital mortality rate.36
MAY NOT HELP AFTER EXTUBATION, EXCEPT IN SPECIFIC CASES
NIPPV has been used to treat respiratory failure after extubation,22,37 to prevent acute respiratory failure after failure of weaning,38–41 and to support breathing in patients who failed a trial of spontaneous breathing.42–45
Unfortunately, the evidence for using NIPPV in respiratory failure after extubation, including unplanned extubation, appears to be unfavorable, except possibly in patients with chronic pulmonary disease (particularly COPD and possibly obesity) and hypercapnia. An international consensus report stated that NIPPV should be considered in patients with hypercapnic respiratory insufficiency, especially those with COPD, to shorten the duration of intubation, but that it should not be routinely used in extubation respiratory failure.46
Treatment of respiratory failure after extubation
Two recent randomized controlled trials compared NIPPV and standard care in patients who met the criteria for readiness for extubation but who developed respiratory failure after mechanical ventilation was discontinued. 22,37 Those two studies showed a longer time to reintubation for patients randomized to NIPPV but no differences in the rate of reintubation between the two groups and no difference in the lengths of stay in the intensive care unit.
Of greater concern, one study showed a higher rate of death in the intensive care unit in the NIPPV group than in the standard therapy group (25% vs 14%, respectively).22 This finding suggests that NIPPV delayed necessary reintubation in patients developing respiratory failure after extubation, with a consequent risk of fatal complications.
Prevention of respiratory failure after extubation
Other studies used NIPPV to prevent respiratory failure after extubation rather than wait to apply it after respiratory failure developed.38–41
Nava et al,40 in a trial in patients successfully weaned but considered to be at risk of reintubation, found that fewer of those randomized to NIPPV had to be reintubated than those who received standard care (8% vs 24%), and 10% fewer of them died in the intensive care unit. Risk factors for reintubation (and therefore eligibility criteria for this trial) included a Paco2 higher than 45 mm Hg, more than one consecutive failure of weaning, chronic heart failure, other comorbidity, weak cough, or stridor.
Extubated patients are a heterogeneous group, so if some subgroups benefit from a transition to NIPPV after extubation, it will be important to identify them. For instance, a subgroup analysis of a study by Ferrer et al38 indicated the survival benefit of NIPPV after extubation was limited to patients with chronic respiratory disorders and hypercapnia during a trial of spontaneous breathing.
In a subsequent successful test of this hypothesis, a randomized trial showed that the early use of noninvasive ventilation in patients with hypercapnia after a trial of spontaneous breathing and with chronic respiratory disorders (COPD, chronic bronchitis, bronchiectasis, obesity-hypoventilation, sequelae of tuberculosis, chest wall deformity, or chronic persistent asthma) reduced the risk of respiratory failure after extubation and the risk of death within the first 90 days.39
Others in which this approach may be helpful are obese patients who have high Paco2 levels. Compared with historical controls, 62 patients with a body mass index greater than 35 kg/m2 who received NIPPV in the 48 hours after extubation had a lower rate of respiratory failure, shorter lengths of stay in the intensive care unit and hospital, and, in the subgroup with hypercapnia, a lower hospital mortality rate.41
NIPPV to facilitate weaning
In several studies, mechanically ventilated patients who had failed a trial of spontaneous breathing were randomized to undergo either accelerated weaning, extubation, and NIPPV or conventional weaning with pressure support via mechanical ventilation.42–46 Most patients developed hypercapnia during the spontaneous breathing trials, and most of the patients had COPD.
A meta-analysis47 of the randomized trials of this approach concluded that, compared with continued invasive ventilation, NIPPV decreased the risk of death (relative risk 0.41) and of ventilator-associated pneumonia (relative risk 0.28) and reduced the total duration of mechanical ventilation by a weighted mean difference of 7.33 days. The benefits appeared to be most significant in patients with COPD.
NIPPV IN ASTHMA AND STATUS ASTHMATICUS
Noninvasive ventilation is an attractive alternative to intubation for patients with status asthmaticus, given the challenges and conflicting demands of maintaining ventilation despite severe airway obstruction.
In a 1996 prospective study of 17 episodes of asthma associated with acute respiratory failure, Meduri et al48 showed that NIPPV could progressively improve the pH and the Paco2 over 12 to 24 hours and reduce the respiratory rate.
In a subsequent controlled trial, Soroksky et al49 randomized 30 patients presenting to an emergency room with a severe asthma attack to NIPPV with conventional therapy vs conventional therapy only. The study group had a significantly greater increase in the forced expiratory volume in 1 second compared with the control group (54% vs 29%, respectively) and a lower hospitalization rate (18% vs 63%).
Another randomized trial of NIPPV, in patients with status asthmaticus presenting to an emergency room, was prematurely terminated due to a physician treatment bias that favored NIPPV.50 The preliminary results of that study showed a 7.3% higher intubation rate in the control group than in the NIPPV group, along with trends toward a lower intubation rate, a shorter length of hospital stay, and lower hospital charges in the NIPPV group.
Despite these initial favorable results, a Cochrane review concluded that the use of NIPPV in patients with status asthmaticus is controversial.51 NIPPV can be tried in selected patients such as those with mild to moderate respiratory distress (respiratory rate greater than 25 breaths per minute, use of accessory muscles to breathe, difficulty speaking), an arterial pH of 7.25 to 7.35, and a Paco2 of 45 to 55 mm Hg.52 Patients with impending respiratory failure or the inability to protect the airway should probably not be considered for NIPPV.52
IN ACUTE LUNG INJURY AND ACUTE RESPIRATORY DISTRESS SYNDROME
The most challenging application of NIPPV may be in patients with acute lung injury and the acute respiratory distress syndrome.
Initial trials of NIPPV in this setting have been disappointing, and a meta-analysis of the topic concluded that NIPPV was unlikely to have any significant benefit.53 An earlier study that used CPAP in patients with acute respiratory failure predominantly due to acute lung injury showed early physiologic improvements but no reduction in the need for intubation, no improvement in outcomes, and a higher rate of adverse events, including cardiac arrest, in those randomized to CPAP.54
A subsequent observational cohort specifically identified shock, metabolic acidosis, and severe hypoxemia as predictors of NIPPV failure.55
A more recent prospective study demonstrated that NIPPV improved gas exchange and obviated intubation in 54% of patients, with a consequent reduction in ventilator-associated pneumonia and a lower rate of death in the intensive care unit.56 A Simplified Acute Physiology Score (SAPS) II greater than 34 and a Pao2/Fio2 ratio less than 175 after 1 hour of NIPPV were identified as predicting that NIPPV would fail.56
MISCELLANEOUS APPLICATIONS
The more widespread use of NIPPV has encouraged its use in other acute situations, including during procedures such as percutaneous endoscopic gastrostomy (PEG)57,58 or bronchoscopy,59,60 for palliative use in patients listed as “do-not-intubate,”61–63 and for oxygenation before intubation.64
NIPPV during PEG tube insertion
NIPPV during PEG tube placement is particularly useful for patients with neuromuscular diseases who are at a combined risk of aspiration, poor oral intake, and respiratory failure during procedures. The experience with patients with amyotrophic lateral sclerosis58 and Duchenne muscular dystrophy57 indicates that even patients at high risk of respiratory failure during procedures can be successfully managed with NIPPV. The most recent practice parameters for patients with amyotrophic lateral sclerosis propose that patients with dysphagia may be exposed to less risk if the PEG procedure is performed when the forced vital capacity is greater than 50% of predicted.65
In randomized trials of CPAP59 or pressure-support NIPPV60 in high-risk hypoxemic patients who needed diagnostic bronchoscopy, patients in the intervention groups fared better than those who received oxygen alone, with better oxygenation during and after the procedure and a lower risk of postprocedure respiratory failure. Improved hemodynamics with a lower mean heart rate and a stable mean arterial pressure were also reported in one of those studies.60
Palliative use in ‘do-not-intubate’ patients
In patients who decline intubation, NIPPV appears to be most effective in reversing acute respiratory failure and improving mortality rates in those with COPD or with cardiogenic pulmonary edema.61,62 Controversy surrounding the use of NIPPV in “do-not-intubate” patients, particularly as a potentially uncomfortable life support technique, has been addressed by a task force of the Society of Critical Care Medicine, which recommends that it be applied only after careful discussion of goals of care and parameters of treatment with patients and their families.63
Oxygenation before intubation
In a prospective randomized study of oxygenation before rapid-sequence intubation via either a nonrebreather bag-valve mask or NIPPV, the NIPPV group had a higher oxygen saturation rate before, during, and after the intubation procedure.64
Acknowledgment: The authors wish to thank Jodith Janes of the Cleveland Clinic Alumni Library for her help with reference citations and with locating articles.
Noninvasive positive pressure ventilation (NIPPV)—delivered via a tight-fitting mask rather than via an endotracheal tube or tracheostomy—is one of the most important advances in the management of acute respiratory failure to emerge in the past 2 decades. It is now recommended as the first choice for ventilatory support in selected patients, such as those with exacerbations of chronic obstructive pulmonary disease (COPD) or with cardiogenic pulmonary edema.1–3 In fact, some authors suggest that using NIPPV in more than 20% of COPD patients is a characteristic of respiratory care departments that are “avid for change”4—change being a good thing.
However, NIPPV has not been universally accepted, with wide variations in its utilization. In a 2006 survey, it was being used in only 33% of patients with COPD or congestive heart failure, for which it might be indicated. 5 Some potential reasons for the low rate are that physicians do not know about it, respiratory therapists are not sufficiently trained in it, and hospitals lack the equipment to do it.5
Our goal in this review is to familiarize the reader with how NIPPV has evolved and with its indications and contraindications in specific acute care conditions.
FROM A VACUUM CLEANER TO THE INTENSIVE CARE UNIT
NIPPV appears to have been first tried in 1870 by Chaussier, who used a bag and face mask to resuscitate neonates.6
In 1936, Poulton and Oxon7 described their “pulmonary plus pressure machine,” which used a vacuum cleaner blower and a mask to increase the alveolar pressure and thus counteract the increased intrapulmonary pressure in patients with heart failure, pulmonary edema, Cheyne-Stokes breathing, and asthma.
In the 1940s, intermittent positive pressure breathing devices were developed for use in high-altitude aviation. Motley, Werko, and Cournand8,9 subsequently used these devices to treat acute respiratory failure in pneumonia, pulmonary edema, near-drowning, Guillain-Barré syndrome, and acute severe asthma.
Although NIPPV was shown to be effective for acute conditions, invasive ventilation became preferred, particularly as blood gas analysis and ventilator technologies simultaneously matured, spurred at least in part by the polio epidemics of the 1950s.10
NIPPV reemerged in the 1980s for use in chronic conditions. First, continuous positive airway pressure (CPAP) came into use for obstructive sleep apnea,11 followed by noninvasive positive-pressure volume ventilation in neuromuscular diseases.12 Bilevel positive pressure devices (ie, with separate inspiratory and expiratory pressures) soon followed, again initially for obstructive sleep apnea13 and then for diverse neuromuscular diseases.14
NIPPV is now a mainstream therapy for diverse conditions in acute and chronic care.3 One reason we now use it in acute conditions is to avoid the complications associated with intubation.
Some clinicians initially resisted using NIPPV, concerned that it demanded too much of the nurses’ time15 and was costly.16 However, in a 1997 study in patients with COPD and acute respiratory failure, Nava et al17 found that NIPPV was no more expensive and no more demanding of staff resources than invasive mechanical ventilation in the first 48 hours of ventilation. Further, after the first few days of ventilation, NIPPV put fewer time demands on physicians and nurses than did invasive mechanical ventilation.
THREE MODES: CPAP, PRESSURE-LIMITED, VOLUME-LIMITED
The term “noninvasive ventilation” generally encompasses various forms of positive pressure ventilation. However, negative pressure ventilation, in the form of diaphragm pacing, may regain a foothold in the devices used for respiratory support.18 We therefore favor the term “NIPPV” in this review.
NIPPV IN ACUTE RESPIRATORY FAILURE
The main reasons to use NIPPV instead of invasive ventilation in acute care are to avoid the complications of invasive ventilation, to improve outcomes (eg, reduce mortality rates, decrease hospital length of stay), and to decrease the cost of care.
NIPPV is the standard of care for acute exacerbations of COPD
In a meta-analysis of eight randomized controlled trials,24 the specific advantages of NIPPV compared with usual care in acute exacerbations of COPD included:
- A lower risk of treatment failure, defined as death, need for intubation, or inability to tolerate the treatment (relative risk [RR] 0.51, number needed to treat [NNT] to prevent one treatment failure = 5)
- A lower risk of intubation (RR 0.43, NNT = 5)
- A lower mortality rate (RR 0.41, NNT = 8)
- A lower risk of complications (RR 0.32, NNT = 3)
- A shorter hospital length of stay (by about 3 days).
Mechanisms by which NIPPV may impart these benefits include reducing the work of breathing, unloading the respiratory muscles, lessening diaphragmatic pressure swings, reducing the respiratory rate, eliminating diaphragmatic work, and counteracting the threshold loading effects of auto-positive end-expiratory pressure (auto-PEEP).24–26
Also, if a patient with COPD is intubated, NIPPV seems to help after the tube is removed, preventing postextubation respiratory failure and facilitating weaning from invasive ventilation.27 These topics are discussed below.
A Cochrane systematic review24 concluded that NIPPV should be tried early in the course of respiratory failure, before severe acidosis develops. The patients in the studies in this review all had partial pressure of arterial carbon dioxide (Paco2) levels greater than 45 mm Hg.
In patients with severe respiratory acidosis (pH < 7.25), NIPPV failure rates are greater than 50%. However, trying NIPPV may still be justified, even in the presence of hypercapnic encephalopathy, as long as no other indications for invasive support and facilities for prompt endotracheal intubation are available. 1
However, in another systematic review,26 in patients with mild COPD exacerbations (pH > 7.35), NIPPV was no more effective than standard medical therapy in preventing acute respiratory failure, preventing death, or reducing length of hospitalization. Moreover, nearly 50% of the patients could not tolerate NIPPV.
Rapid improvement in cardiogenic pulmonary edema, but possibly no lower mortality rate
The Three Interventions in Cardiogenic Pulmonary Oedema (3CPO) trial,28 with 1,156 patients, was the largest randomized trial to compare NIPPV and standard oxygen therapy for acute pulmonary edema. It found that NIPPV (either CPAP or noninvasive intermittent positive pressure ventilation) was significantly better than standard oxygen therapy (through a variable-delivery oxygen mask with a reservoir) in the first hour of treatment in terms of the dyspnea score, heart rate, acidosis, and hypercapnia. However, there were no significant differences between groups in the 7- or 30-day mortality rates, the rates of intubation, rates of admission to the critical care unit, or in the mean length of hospital stay.
In contrast, several smaller randomized trials and meta-analyses showed lower intubation and mortality rates with NIPPV.29,30 Factors that may account for those differences include a much lower intubation rate in the 3CPO trial (2.9% overall, compared with 20% with conventional therapy in other trials), a higher mortality rate in the 3CPO trial, and methodologic differences (eg, patients for whom standard therapy failed in the 3CPO trial received rescue NIPPV).
If NIPPV is beneficial in cardiogenic pulmonary edema, the mechanisms are probably its favorable hemodynamic effects and its positive end-expiratory pressure (PEEP) effect on flooded alveoli. Specifically, positive intrathoracic pressure can be expected to reduce both preload and afterload, with improvement in the cardiac index and reduced work of breathing. 31,32
Notwithstanding the possible lack of impact of NIPPV on death or intubation rates in this setting, the intervention rapidly improves dyspnea and respiratory and metabolic abnormalities and should be considered for treatment of cardiogenic pulmonary edema associated with severe respiratory distress. A subgroup in which the NIPPV may reduce intubation rates is those with hypercapnia.33 A concern that NIPPV may increase the rate of myocardial infarction34 was not confirmed in the 3CPO trial.28 Interestingly, there were no differences in outcomes between CPAP and noninvasive intermittent positive pressure ventilation in this setting.28,34,35
Immunocompromised patients with acute respiratory failure
A particular challenge of NIPPV in immunocompromised patients, particularly compared with its use in COPD exacerbation or cardiogenic pulmonary edema, is that the underlying pathophysiology of respiratory dysfunction in immunocompromised patients may not be readily reversible. Therefore, its application in this group may need to follow clearly defined indications.
In one trial,20 inclusion criteria were:
- Immune suppression (due to neutropenia after chemotherapy or bone marrow transplantation, immunosuppressive drugs for organ transplantation, corticosteroids, cytotoxic therapy for nonmalignant conditions, or the acquired immunodeficiency syndrome)
- Persistent pulmonary infiltrates
- Fever (temperature > 38.3°C; 100.9°F)
- A respiratory rate greater than 30 breaths per minute
- Severe dyspnea at rest
- Early hypoxemic acute respiratory failure, defined as a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (Pao2/Fio2 ratio) less than 200 while on oxygen.
Compared with patients who received conventional treatment, fewer of those randomized to additional intermittent noninvasive ventilation had to be intubated (46% vs 77%, P = .03), suffered serious complications (50% vs 81%, P = .02), or died in the intensive care unit (38% vs 69%, P = .03) or in the hospital (50% vs 81%, P = .02).
Similarly, in a randomized trial in 40 patients with acute respiratory failure after solid organ transplantation, more patients in the NIPPV group than in the control group had an improvement in the Pao2/Fio2 ratio within the first hour (70% vs 25%, P = .004) or a sustained improvement in the Pao2/Fio2 ratio (60% vs 25%, P = .03); fewer of them needed endotracheal intubation (20% vs 70%, P = .002); fewer of them died of complications (20% vs 50%, P = .05); they had a shorter length of stay in the intensive care unit (mean 5.5 vs 9 days, P = .03); and fewer of them died in the intensive care unit (20% vs 50%, P = .05). There was, however, no difference in the overall hospital mortality rate.36
MAY NOT HELP AFTER EXTUBATION, EXCEPT IN SPECIFIC CASES
NIPPV has been used to treat respiratory failure after extubation,22,37 to prevent acute respiratory failure after failure of weaning,38–41 and to support breathing in patients who failed a trial of spontaneous breathing.42–45
Unfortunately, the evidence for using NIPPV in respiratory failure after extubation, including unplanned extubation, appears to be unfavorable, except possibly in patients with chronic pulmonary disease (particularly COPD and possibly obesity) and hypercapnia. An international consensus report stated that NIPPV should be considered in patients with hypercapnic respiratory insufficiency, especially those with COPD, to shorten the duration of intubation, but that it should not be routinely used in extubation respiratory failure.46
Treatment of respiratory failure after extubation
Two recent randomized controlled trials compared NIPPV and standard care in patients who met the criteria for readiness for extubation but who developed respiratory failure after mechanical ventilation was discontinued. 22,37 Those two studies showed a longer time to reintubation for patients randomized to NIPPV but no differences in the rate of reintubation between the two groups and no difference in the lengths of stay in the intensive care unit.
Of greater concern, one study showed a higher rate of death in the intensive care unit in the NIPPV group than in the standard therapy group (25% vs 14%, respectively).22 This finding suggests that NIPPV delayed necessary reintubation in patients developing respiratory failure after extubation, with a consequent risk of fatal complications.
Prevention of respiratory failure after extubation
Other studies used NIPPV to prevent respiratory failure after extubation rather than wait to apply it after respiratory failure developed.38–41
Nava et al,40 in a trial in patients successfully weaned but considered to be at risk of reintubation, found that fewer of those randomized to NIPPV had to be reintubated than those who received standard care (8% vs 24%), and 10% fewer of them died in the intensive care unit. Risk factors for reintubation (and therefore eligibility criteria for this trial) included a Paco2 higher than 45 mm Hg, more than one consecutive failure of weaning, chronic heart failure, other comorbidity, weak cough, or stridor.
Extubated patients are a heterogeneous group, so if some subgroups benefit from a transition to NIPPV after extubation, it will be important to identify them. For instance, a subgroup analysis of a study by Ferrer et al38 indicated the survival benefit of NIPPV after extubation was limited to patients with chronic respiratory disorders and hypercapnia during a trial of spontaneous breathing.
In a subsequent successful test of this hypothesis, a randomized trial showed that the early use of noninvasive ventilation in patients with hypercapnia after a trial of spontaneous breathing and with chronic respiratory disorders (COPD, chronic bronchitis, bronchiectasis, obesity-hypoventilation, sequelae of tuberculosis, chest wall deformity, or chronic persistent asthma) reduced the risk of respiratory failure after extubation and the risk of death within the first 90 days.39
Others in which this approach may be helpful are obese patients who have high Paco2 levels. Compared with historical controls, 62 patients with a body mass index greater than 35 kg/m2 who received NIPPV in the 48 hours after extubation had a lower rate of respiratory failure, shorter lengths of stay in the intensive care unit and hospital, and, in the subgroup with hypercapnia, a lower hospital mortality rate.41
NIPPV to facilitate weaning
In several studies, mechanically ventilated patients who had failed a trial of spontaneous breathing were randomized to undergo either accelerated weaning, extubation, and NIPPV or conventional weaning with pressure support via mechanical ventilation.42–46 Most patients developed hypercapnia during the spontaneous breathing trials, and most of the patients had COPD.
A meta-analysis47 of the randomized trials of this approach concluded that, compared with continued invasive ventilation, NIPPV decreased the risk of death (relative risk 0.41) and of ventilator-associated pneumonia (relative risk 0.28) and reduced the total duration of mechanical ventilation by a weighted mean difference of 7.33 days. The benefits appeared to be most significant in patients with COPD.
NIPPV IN ASTHMA AND STATUS ASTHMATICUS
Noninvasive ventilation is an attractive alternative to intubation for patients with status asthmaticus, given the challenges and conflicting demands of maintaining ventilation despite severe airway obstruction.
In a 1996 prospective study of 17 episodes of asthma associated with acute respiratory failure, Meduri et al48 showed that NIPPV could progressively improve the pH and the Paco2 over 12 to 24 hours and reduce the respiratory rate.
In a subsequent controlled trial, Soroksky et al49 randomized 30 patients presenting to an emergency room with a severe asthma attack to NIPPV with conventional therapy vs conventional therapy only. The study group had a significantly greater increase in the forced expiratory volume in 1 second compared with the control group (54% vs 29%, respectively) and a lower hospitalization rate (18% vs 63%).
Another randomized trial of NIPPV, in patients with status asthmaticus presenting to an emergency room, was prematurely terminated due to a physician treatment bias that favored NIPPV.50 The preliminary results of that study showed a 7.3% higher intubation rate in the control group than in the NIPPV group, along with trends toward a lower intubation rate, a shorter length of hospital stay, and lower hospital charges in the NIPPV group.
Despite these initial favorable results, a Cochrane review concluded that the use of NIPPV in patients with status asthmaticus is controversial.51 NIPPV can be tried in selected patients such as those with mild to moderate respiratory distress (respiratory rate greater than 25 breaths per minute, use of accessory muscles to breathe, difficulty speaking), an arterial pH of 7.25 to 7.35, and a Paco2 of 45 to 55 mm Hg.52 Patients with impending respiratory failure or the inability to protect the airway should probably not be considered for NIPPV.52
IN ACUTE LUNG INJURY AND ACUTE RESPIRATORY DISTRESS SYNDROME
The most challenging application of NIPPV may be in patients with acute lung injury and the acute respiratory distress syndrome.
Initial trials of NIPPV in this setting have been disappointing, and a meta-analysis of the topic concluded that NIPPV was unlikely to have any significant benefit.53 An earlier study that used CPAP in patients with acute respiratory failure predominantly due to acute lung injury showed early physiologic improvements but no reduction in the need for intubation, no improvement in outcomes, and a higher rate of adverse events, including cardiac arrest, in those randomized to CPAP.54
A subsequent observational cohort specifically identified shock, metabolic acidosis, and severe hypoxemia as predictors of NIPPV failure.55
A more recent prospective study demonstrated that NIPPV improved gas exchange and obviated intubation in 54% of patients, with a consequent reduction in ventilator-associated pneumonia and a lower rate of death in the intensive care unit.56 A Simplified Acute Physiology Score (SAPS) II greater than 34 and a Pao2/Fio2 ratio less than 175 after 1 hour of NIPPV were identified as predicting that NIPPV would fail.56
MISCELLANEOUS APPLICATIONS
The more widespread use of NIPPV has encouraged its use in other acute situations, including during procedures such as percutaneous endoscopic gastrostomy (PEG)57,58 or bronchoscopy,59,60 for palliative use in patients listed as “do-not-intubate,”61–63 and for oxygenation before intubation.64
NIPPV during PEG tube insertion
NIPPV during PEG tube placement is particularly useful for patients with neuromuscular diseases who are at a combined risk of aspiration, poor oral intake, and respiratory failure during procedures. The experience with patients with amyotrophic lateral sclerosis58 and Duchenne muscular dystrophy57 indicates that even patients at high risk of respiratory failure during procedures can be successfully managed with NIPPV. The most recent practice parameters for patients with amyotrophic lateral sclerosis propose that patients with dysphagia may be exposed to less risk if the PEG procedure is performed when the forced vital capacity is greater than 50% of predicted.65
In randomized trials of CPAP59 or pressure-support NIPPV60 in high-risk hypoxemic patients who needed diagnostic bronchoscopy, patients in the intervention groups fared better than those who received oxygen alone, with better oxygenation during and after the procedure and a lower risk of postprocedure respiratory failure. Improved hemodynamics with a lower mean heart rate and a stable mean arterial pressure were also reported in one of those studies.60
Palliative use in ‘do-not-intubate’ patients
In patients who decline intubation, NIPPV appears to be most effective in reversing acute respiratory failure and improving mortality rates in those with COPD or with cardiogenic pulmonary edema.61,62 Controversy surrounding the use of NIPPV in “do-not-intubate” patients, particularly as a potentially uncomfortable life support technique, has been addressed by a task force of the Society of Critical Care Medicine, which recommends that it be applied only after careful discussion of goals of care and parameters of treatment with patients and their families.63
Oxygenation before intubation
In a prospective randomized study of oxygenation before rapid-sequence intubation via either a nonrebreather bag-valve mask or NIPPV, the NIPPV group had a higher oxygen saturation rate before, during, and after the intubation procedure.64
Acknowledgment: The authors wish to thank Jodith Janes of the Cleveland Clinic Alumni Library for her help with reference citations and with locating articles.
- Ambrosino N, Vagheggini G. Noninvasive positive pressure ventilation in the acute care setting: where are we? Eur Respir J 2008; 31:874–886.
- Hill NS, Brennan J, Garpestad E, Nava S. Noninvasive ventilation in acute respiratory failure. Crit Care Med 2007; 35:2402–2407.
- Nava S, Navalesi P, Conti G. Time of non-invasive ventilation. Intensive Care Med 2006; 32:361–370.
- Stoller JK, Kester L, Roberts VT, et al; An analysis of features of respiratory therapy departments that are avid for change. Respir Care 2008; 53:871–884.
- Maheshwari V, Paioli D, Rothaar R, Hill NS. Utilization of noninvasive ventilation in acute care hospitals: a regional survey. Chest 2006; 129:1226–1233.
- Obladen M. History of neonatal resuscitation. Part 1: Artificial ventilation. Neonatology 2008; 94:144–149.
- Poulton EP, Oxon DM. Left-sided heart failure with pulmonary oedema: its treatment with the “pulmonary plus” pressure machine. Lancet 1936; 228:981–983.
- Motley HL, Werko L. Observations on the clinical use of intermittent positive pressure. J Aviat Med 1947; 18:417–435.
- Cournand A, Motley HL. Physiological studies of the effects of intermittent positive pressure breathing on cardiac output in man. Am J Physiol 1948; 152:162–174.
- Severinghaus JW, Astrup P, Murray JF. Blood gas analysis and critical care medicine. Am J Respir Crit Care Med 1998; 157:S114–S122.
- Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983; 128:177–181.
- Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease. Positive-pressure ventilation through a nose mask. Am Rev Respir Dis 1987; 135:148–152.
- Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal mask. Physiologic and clinical implications. Chest 1990; 98:317–324.
- Bach JR. Mechanical exsufflation, noninvasive ventilation, and new strategies for pulmonary rehabilitation and sleep disordered breathing. Bull N Y Acad Med 1992; 68:321–340.
- Chevrolet JC, Jolliet P, Abajo B, Toussi A, Louis M. Nasal positive pressure ventilation in patients with acute respiratory failure. Difficult and time-consuming procedure for nurses. Chest 1991; 100:775–782.
- Criner GJ, Kreimer DT, Tomaselli M, Pierson W, Evans D. Financial implications of noninvasive positive pressure ventilation (NPPV). Chest 1995; 108:475–481.
- Nava S, Evangelisti I, Rampulla C, Compagnoni ML, Fracchia C, Rubini F. Human and financial costs of noninvasive mechanical ventilation in patients affected by COPD and acute respiratory failure. Chest 1997; 111:1631–1638.
- DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Mortimer JT. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest 2005; 127:671–678.
- Antonelli M, Conti G. Noninvasive positive pressure ventilation as treatment for acute respiratory failure in critically ill patients. Crit Care 2000; 4:15–22.
- Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med 2001; 344:481–487.
- L’Her E, Deye N, Lellouche F, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med 2005; 172:1112–1118.
- Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004; 350:2452–2460.
- Hill NS. Noninvasive positive pressure ventilation for respiratory failure caused by exacerbations of chronic obstructive pulmonary disease: a standard of care? Crit Care 2003; 7:400–401.
- Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ 2003; 326:185–187.
- Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995; 333:817–822.
- Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med 2003; 138:861–870.
- Epstein SK. Noninvasive ventilation to shorten the duration of mechanical ventilation. Respir Care 2009; 54:198–208.
- Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J; 3CPO Trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med 2008; 359:142–151.
- Collins SP, Mielniczuk LM, Whittingham HA, Boseley ME, Schramm DR, Storrow AB. The use of noninvasive ventilation in emergency department patients with acute cardiogenic pulmonary edema: a systematic review. Ann Emerg Med 2006; 48:260–269.
- Masip J, Roque M, Sánchez B, Fernández R, Subirana M, Expósito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta-analysis. JAMA 2005; 294:3124–3130.
- Baratz DM, Westbrook PR, Shah PK, Mohsenifar Z. Effect of nasal continuous positive airway pressure on cardiac output and oxygen delivery in patients with congestive heart failure. Chest 1992; 102:1397–1401.
- Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation 1995; 91:1725–1731.
- Nava S, Carbone G, DiBattista N, et al. Noninvasive ventilation in cardiogenic pulmonary edema: a multicenter randomized trial. Am J Respir Crit Care Med 2003; 168:1432–1437.
- Mehta S, Jay GD, Woolard RH, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med 1997; 25:620–628.
- Ho KM, Wong K. A comparison of continuous and bi-level positive airway pressure non-invasive ventilation in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Crit Care 2006; 10:R49.
- Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA 2000; 283:235–241.
- Keenan SP, Powers C, McCormack DG, Block G. Noninvasive positive-pressure ventilation for postextubation respiratory distress: a randomized controlled trial. JAMA 2002; 287:3238–3244.
- Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med 2006; 173:164–170.
- Ferrer M, Sellarés J, Valencia M, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet 2009; 374:1082–1088.
- Nava S, Gregoretti C, Fanfulla F, et al. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med 2005; 33:2465–2470.
- El-Solh AA, Aquilina A, Pineda L, Dhanvantri V, Grant B, Bouquin P. Noninvasive ventilation for prevention of post-extubation respiratory failure in obese patients. Eur Respir J 2006; 28:588–595.
- Ferrer M, Esquinas A, Arancibia F, et al. Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med 2003; 168:70–76.
- Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med 1999; 160:86–92.
- Nava S, Ambrosino N, Clini E, et al. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann Intern Med 1998; 128:721–728.
- Trevisan CE, Vieira SR; Research Group in Mechanical Ventilation Weaning. Noninvasive mechanical ventilation may be useful in treating patients who fail weaning from invasive mechanical ventilation: a randomized clinical trial. Crit Care 2008; 12:R51.
- Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J 2007; 29:1033–1056.
- Burns KE, Adhikari NK, Meade MO. A meta-analysis of noninvasive weaning to facilitate liberation from mechanical ventilation. Can J Anaesth 2006; 53:305–315.
- Meduri GU, Cook TR, Turner RE, Cohen M, Leeper KV. Noninvasive positive pressure ventilation in status asthmaticus. Chest 1996; 110:767–774.
- Soroksky A, Stav D, Shpirer I. A pilot prospective, randomized, placebo-controlled trial of bilevel positive airway pressure in acute asthmatic attack. Chest 2003; 123:1018–1025.
- Holley MT, Morrissey TK, Seaberg DC, Afessa B, Wears RL. Ethical dilemmas in a randomized trial of asthma treatment: can Bayesian statistical analysis explain the results? Acad Emerg Med 2001; 8:1128–1135.
- Ram FS, Wellington S, Rowe BH, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev 2005;CD004360.
- Medoff BD. Invasive and noninvasive ventilation in patients with asthma. Respir Care 2008; 53:740–748.
- Agarwal R, Reddy C, Aggarwal AN, Gupta D. Is there a role for noninvasive ventilation in acute respiratory distress syndrome? A meta-analysis. Respir Med 2006; 100:2235–2238.
- Delclaux C, L’Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA 2000; 284:2352–2360.
- Rana S, Jenad H, Gay PC, Buck CF, Hubmayr RD, Gajic O. Failure of non-invasive ventilation in patients with acute lung injury: observational cohort study. Crit Care 2006; 10:R79.
- Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med 2007; 35:18–25.
- Birnkrant DJ, Ferguson RD, Martin JE, Gordon GJ. Noninvasive ventilation during gastrostomy tube placement in patients with severe Duchenne muscular dystrophy: case reports and review of the literature. Pediatr Pulmonol 2006; 41:188–193.
- Boitano LJ, Jordan T, Benditt JO. Noninvasive ventilation allows gastrostomy tube placement in patients with advanced ALS. Neurology 2001; 56:413–414.
- Maitre B, Jaber S, Maggiore SM, et al. Continuous positive airway pressure during fiberoptic bronchoscopy in hypoxemic patients. A randomized double-blind study using a new device. Am J Respir Crit Care Med 2000; 162:1063–1067.
- Antonelli M, Conti G, Rocco M, et al. Noninvasive positive-pressure ventilation vs conventional oxygen supplementation in hypoxemic patients undergoing diagnostic bronchoscopy. Chest 2002; 121:1149–1154.
- Levy M, Tanios MA, Nelson D, et al. Outcomes of patients with do-not-intubate orders treated with noninvasive ventilation. Crit Care Med 2004; 32:2002–2007.
- Schettino G, Altobelli N, Kacmarek RM. Noninvasive positive pressure ventilation reverses acute respiratory failure in select “do-not-intubate” patients. Crit Care Med 2005; 33:1976–1982.
- Curtis JR, Cook DJ, Sinuff T, et al; Society of Critical Care Medicine Palliative Noninvasive Positive Ventilation Task Force. Noninvasive positive pressure ventilation in critical and palliative care settings: understanding the goals of therapy. Crit Care Med 2007; 35:932–939.
- Baillard C, Fosse JP, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med 2006; 174:171–177.
- Miller RG, Jackson CE, Kasarskis EJ, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009; 73:1218–1226.
- Ambrosino N, Vagheggini G. Noninvasive positive pressure ventilation in the acute care setting: where are we? Eur Respir J 2008; 31:874–886.
- Hill NS, Brennan J, Garpestad E, Nava S. Noninvasive ventilation in acute respiratory failure. Crit Care Med 2007; 35:2402–2407.
- Nava S, Navalesi P, Conti G. Time of non-invasive ventilation. Intensive Care Med 2006; 32:361–370.
- Stoller JK, Kester L, Roberts VT, et al; An analysis of features of respiratory therapy departments that are avid for change. Respir Care 2008; 53:871–884.
- Maheshwari V, Paioli D, Rothaar R, Hill NS. Utilization of noninvasive ventilation in acute care hospitals: a regional survey. Chest 2006; 129:1226–1233.
- Obladen M. History of neonatal resuscitation. Part 1: Artificial ventilation. Neonatology 2008; 94:144–149.
- Poulton EP, Oxon DM. Left-sided heart failure with pulmonary oedema: its treatment with the “pulmonary plus” pressure machine. Lancet 1936; 228:981–983.
- Motley HL, Werko L. Observations on the clinical use of intermittent positive pressure. J Aviat Med 1947; 18:417–435.
- Cournand A, Motley HL. Physiological studies of the effects of intermittent positive pressure breathing on cardiac output in man. Am J Physiol 1948; 152:162–174.
- Severinghaus JW, Astrup P, Murray JF. Blood gas analysis and critical care medicine. Am J Respir Crit Care Med 1998; 157:S114–S122.
- Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983; 128:177–181.
- Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease. Positive-pressure ventilation through a nose mask. Am Rev Respir Dis 1987; 135:148–152.
- Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal mask. Physiologic and clinical implications. Chest 1990; 98:317–324.
- Bach JR. Mechanical exsufflation, noninvasive ventilation, and new strategies for pulmonary rehabilitation and sleep disordered breathing. Bull N Y Acad Med 1992; 68:321–340.
- Chevrolet JC, Jolliet P, Abajo B, Toussi A, Louis M. Nasal positive pressure ventilation in patients with acute respiratory failure. Difficult and time-consuming procedure for nurses. Chest 1991; 100:775–782.
- Criner GJ, Kreimer DT, Tomaselli M, Pierson W, Evans D. Financial implications of noninvasive positive pressure ventilation (NPPV). Chest 1995; 108:475–481.
- Nava S, Evangelisti I, Rampulla C, Compagnoni ML, Fracchia C, Rubini F. Human and financial costs of noninvasive mechanical ventilation in patients affected by COPD and acute respiratory failure. Chest 1997; 111:1631–1638.
- DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Mortimer JT. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest 2005; 127:671–678.
- Antonelli M, Conti G. Noninvasive positive pressure ventilation as treatment for acute respiratory failure in critically ill patients. Crit Care 2000; 4:15–22.
- Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med 2001; 344:481–487.
- L’Her E, Deye N, Lellouche F, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med 2005; 172:1112–1118.
- Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004; 350:2452–2460.
- Hill NS. Noninvasive positive pressure ventilation for respiratory failure caused by exacerbations of chronic obstructive pulmonary disease: a standard of care? Crit Care 2003; 7:400–401.
- Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ 2003; 326:185–187.
- Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995; 333:817–822.
- Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med 2003; 138:861–870.
- Epstein SK. Noninvasive ventilation to shorten the duration of mechanical ventilation. Respir Care 2009; 54:198–208.
- Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J; 3CPO Trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med 2008; 359:142–151.
- Collins SP, Mielniczuk LM, Whittingham HA, Boseley ME, Schramm DR, Storrow AB. The use of noninvasive ventilation in emergency department patients with acute cardiogenic pulmonary edema: a systematic review. Ann Emerg Med 2006; 48:260–269.
- Masip J, Roque M, Sánchez B, Fernández R, Subirana M, Expósito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta-analysis. JAMA 2005; 294:3124–3130.
- Baratz DM, Westbrook PR, Shah PK, Mohsenifar Z. Effect of nasal continuous positive airway pressure on cardiac output and oxygen delivery in patients with congestive heart failure. Chest 1992; 102:1397–1401.
- Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation 1995; 91:1725–1731.
- Nava S, Carbone G, DiBattista N, et al. Noninvasive ventilation in cardiogenic pulmonary edema: a multicenter randomized trial. Am J Respir Crit Care Med 2003; 168:1432–1437.
- Mehta S, Jay GD, Woolard RH, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med 1997; 25:620–628.
- Ho KM, Wong K. A comparison of continuous and bi-level positive airway pressure non-invasive ventilation in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Crit Care 2006; 10:R49.
- Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA 2000; 283:235–241.
- Keenan SP, Powers C, McCormack DG, Block G. Noninvasive positive-pressure ventilation for postextubation respiratory distress: a randomized controlled trial. JAMA 2002; 287:3238–3244.
- Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med 2006; 173:164–170.
- Ferrer M, Sellarés J, Valencia M, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet 2009; 374:1082–1088.
- Nava S, Gregoretti C, Fanfulla F, et al. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med 2005; 33:2465–2470.
- El-Solh AA, Aquilina A, Pineda L, Dhanvantri V, Grant B, Bouquin P. Noninvasive ventilation for prevention of post-extubation respiratory failure in obese patients. Eur Respir J 2006; 28:588–595.
- Ferrer M, Esquinas A, Arancibia F, et al. Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med 2003; 168:70–76.
- Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med 1999; 160:86–92.
- Nava S, Ambrosino N, Clini E, et al. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann Intern Med 1998; 128:721–728.
- Trevisan CE, Vieira SR; Research Group in Mechanical Ventilation Weaning. Noninvasive mechanical ventilation may be useful in treating patients who fail weaning from invasive mechanical ventilation: a randomized clinical trial. Crit Care 2008; 12:R51.
- Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J 2007; 29:1033–1056.
- Burns KE, Adhikari NK, Meade MO. A meta-analysis of noninvasive weaning to facilitate liberation from mechanical ventilation. Can J Anaesth 2006; 53:305–315.
- Meduri GU, Cook TR, Turner RE, Cohen M, Leeper KV. Noninvasive positive pressure ventilation in status asthmaticus. Chest 1996; 110:767–774.
- Soroksky A, Stav D, Shpirer I. A pilot prospective, randomized, placebo-controlled trial of bilevel positive airway pressure in acute asthmatic attack. Chest 2003; 123:1018–1025.
- Holley MT, Morrissey TK, Seaberg DC, Afessa B, Wears RL. Ethical dilemmas in a randomized trial of asthma treatment: can Bayesian statistical analysis explain the results? Acad Emerg Med 2001; 8:1128–1135.
- Ram FS, Wellington S, Rowe BH, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev 2005;CD004360.
- Medoff BD. Invasive and noninvasive ventilation in patients with asthma. Respir Care 2008; 53:740–748.
- Agarwal R, Reddy C, Aggarwal AN, Gupta D. Is there a role for noninvasive ventilation in acute respiratory distress syndrome? A meta-analysis. Respir Med 2006; 100:2235–2238.
- Delclaux C, L’Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA 2000; 284:2352–2360.
- Rana S, Jenad H, Gay PC, Buck CF, Hubmayr RD, Gajic O. Failure of non-invasive ventilation in patients with acute lung injury: observational cohort study. Crit Care 2006; 10:R79.
- Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med 2007; 35:18–25.
- Birnkrant DJ, Ferguson RD, Martin JE, Gordon GJ. Noninvasive ventilation during gastrostomy tube placement in patients with severe Duchenne muscular dystrophy: case reports and review of the literature. Pediatr Pulmonol 2006; 41:188–193.
- Boitano LJ, Jordan T, Benditt JO. Noninvasive ventilation allows gastrostomy tube placement in patients with advanced ALS. Neurology 2001; 56:413–414.
- Maitre B, Jaber S, Maggiore SM, et al. Continuous positive airway pressure during fiberoptic bronchoscopy in hypoxemic patients. A randomized double-blind study using a new device. Am J Respir Crit Care Med 2000; 162:1063–1067.
- Antonelli M, Conti G, Rocco M, et al. Noninvasive positive-pressure ventilation vs conventional oxygen supplementation in hypoxemic patients undergoing diagnostic bronchoscopy. Chest 2002; 121:1149–1154.
- Levy M, Tanios MA, Nelson D, et al. Outcomes of patients with do-not-intubate orders treated with noninvasive ventilation. Crit Care Med 2004; 32:2002–2007.
- Schettino G, Altobelli N, Kacmarek RM. Noninvasive positive pressure ventilation reverses acute respiratory failure in select “do-not-intubate” patients. Crit Care Med 2005; 33:1976–1982.
- Curtis JR, Cook DJ, Sinuff T, et al; Society of Critical Care Medicine Palliative Noninvasive Positive Ventilation Task Force. Noninvasive positive pressure ventilation in critical and palliative care settings: understanding the goals of therapy. Crit Care Med 2007; 35:932–939.
- Baillard C, Fosse JP, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med 2006; 174:171–177.
- Miller RG, Jackson CE, Kasarskis EJ, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009; 73:1218–1226.
KEY POINTS
- The advantages of NIPPV over invasive ventilation are that it preserves normal physiologic functions such as coughing, swallowing, feeding, and speech and avoids the risks of tracheal and laryngeal injury and respiratory tract infections.
- The best level of evidence for the efficacy of NIPPV is in acute hypercarbic or hypoxemic respiratory failure during exacerbations of chronic obstructive pulmonary disease, in cardiogenic pulmonary edema, and in immunocompromised patients.
- NIPPV should not be applied indiscriminately for lessestablished indications (such as in unconscious patients, respiratory failure after extubation, acute lung injury, or acute respiratory distress syndrome), in severe hypoxemia or acidemia, or after failure to improve dyspnea or gas exchange. The use of NIPPV in these situations may delay a necessary intubation and increase the risks of such a delay, including death.