User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
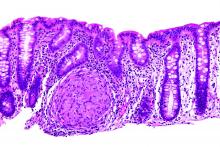
Fecal calprotectin tops CRP as Crohn’s marker
Stool calprotectin correlates with severity of small-bowel Crohn’s disease, as measured against balloon-assisted enteroscopy and computed tomography enterography, according to a review reported in the January issue of Clinical Gastroenterology and Hepatology of 89 patients at Toho University in Chiba, Japan.
Although the correlation was moderate, the findings suggest that fecal calprotectin (FC), with additional work, might turn out to be a good biomarker for tracking small-bowel Crohn’s disease (CD) and its response to tumor necrosis factor blockers. “Currently, it is not widely accepted that FC relates to disease activity in patients with small-intestinal CD,” said investigators led by Tsunetaka Arai of Toho University’s division of gastroenterology and hepatology (Clin Gastroenterol Hepatol. 2016 Aug 23. doi: 10.1016/j.cgh.2016.08.015).
Gastroenterologists need a decent biomarker for small-bowel Crohn’s because old-school endoscopy falls short. Adhesions and strictures block endoscopes, and sometimes scopes simply can’t reach the disease site.
Balloon-assisted enteroscopy (BAE) and computed tomography enterography (CTE) have emerged in recent years as alternatives, but, even so, the need persists for a noninvasive and inexpensive biomarker that’s better than the current standard of C-reactive protein (CRP), which can be thrown off by systemic inflammation, among other problems. The Toho investigators “believe that FC could be a relevant surrogate marker of disease activity in small-bowel CD.” Stool calprotectin paralleled disease activity in their study, while “neither the CDAI [CD activity index] score nor serum CRP showed similar correlation,” they said.
However, elevations in FC – a calcium- and zinc-binding protein released when neutrophils, monocytes, and macrophages inflame the intestinal mucosa – was independent of CD location, which signals the need for further investigation.
Meanwhile, the decent correlation between FC and CTE in the study “should [also] mean that” they could be used together to reliably define mucosal healing. CTE on its own “showed good correlation” with BAE; a CTE score/segment less than 2 [was] associated with endoscopic mucosal healing” on BAE, the investigators said.
The study subjects were an average of 32 years old, and had CD for 9 years; most were men. They had highly active disease at their first endoscopy (average CDAI of 120 points), and an average CRP of 1.09 mg/dL. Twenty-seven patients (30.3%) had small-bowel CD, 50 (56.2%) had ileocolonic CD, and 12 (13.5%) had colonic CD.
They all had endoscopic exams, BAE, and FC stool testing; those with strictures (17) went on to CTE; CTE detected every lesion despite the strictures.
The authors had no conflicts of interest.
Stool calprotectin correlates with severity of small-bowel Crohn’s disease, as measured against balloon-assisted enteroscopy and computed tomography enterography, according to a review reported in the January issue of Clinical Gastroenterology and Hepatology of 89 patients at Toho University in Chiba, Japan.
Although the correlation was moderate, the findings suggest that fecal calprotectin (FC), with additional work, might turn out to be a good biomarker for tracking small-bowel Crohn’s disease (CD) and its response to tumor necrosis factor blockers. “Currently, it is not widely accepted that FC relates to disease activity in patients with small-intestinal CD,” said investigators led by Tsunetaka Arai of Toho University’s division of gastroenterology and hepatology (Clin Gastroenterol Hepatol. 2016 Aug 23. doi: 10.1016/j.cgh.2016.08.015).
Gastroenterologists need a decent biomarker for small-bowel Crohn’s because old-school endoscopy falls short. Adhesions and strictures block endoscopes, and sometimes scopes simply can’t reach the disease site.
Balloon-assisted enteroscopy (BAE) and computed tomography enterography (CTE) have emerged in recent years as alternatives, but, even so, the need persists for a noninvasive and inexpensive biomarker that’s better than the current standard of C-reactive protein (CRP), which can be thrown off by systemic inflammation, among other problems. The Toho investigators “believe that FC could be a relevant surrogate marker of disease activity in small-bowel CD.” Stool calprotectin paralleled disease activity in their study, while “neither the CDAI [CD activity index] score nor serum CRP showed similar correlation,” they said.
However, elevations in FC – a calcium- and zinc-binding protein released when neutrophils, monocytes, and macrophages inflame the intestinal mucosa – was independent of CD location, which signals the need for further investigation.
Meanwhile, the decent correlation between FC and CTE in the study “should [also] mean that” they could be used together to reliably define mucosal healing. CTE on its own “showed good correlation” with BAE; a CTE score/segment less than 2 [was] associated with endoscopic mucosal healing” on BAE, the investigators said.
The study subjects were an average of 32 years old, and had CD for 9 years; most were men. They had highly active disease at their first endoscopy (average CDAI of 120 points), and an average CRP of 1.09 mg/dL. Twenty-seven patients (30.3%) had small-bowel CD, 50 (56.2%) had ileocolonic CD, and 12 (13.5%) had colonic CD.
They all had endoscopic exams, BAE, and FC stool testing; those with strictures (17) went on to CTE; CTE detected every lesion despite the strictures.
The authors had no conflicts of interest.
Stool calprotectin correlates with severity of small-bowel Crohn’s disease, as measured against balloon-assisted enteroscopy and computed tomography enterography, according to a review reported in the January issue of Clinical Gastroenterology and Hepatology of 89 patients at Toho University in Chiba, Japan.
Although the correlation was moderate, the findings suggest that fecal calprotectin (FC), with additional work, might turn out to be a good biomarker for tracking small-bowel Crohn’s disease (CD) and its response to tumor necrosis factor blockers. “Currently, it is not widely accepted that FC relates to disease activity in patients with small-intestinal CD,” said investigators led by Tsunetaka Arai of Toho University’s division of gastroenterology and hepatology (Clin Gastroenterol Hepatol. 2016 Aug 23. doi: 10.1016/j.cgh.2016.08.015).
Gastroenterologists need a decent biomarker for small-bowel Crohn’s because old-school endoscopy falls short. Adhesions and strictures block endoscopes, and sometimes scopes simply can’t reach the disease site.
Balloon-assisted enteroscopy (BAE) and computed tomography enterography (CTE) have emerged in recent years as alternatives, but, even so, the need persists for a noninvasive and inexpensive biomarker that’s better than the current standard of C-reactive protein (CRP), which can be thrown off by systemic inflammation, among other problems. The Toho investigators “believe that FC could be a relevant surrogate marker of disease activity in small-bowel CD.” Stool calprotectin paralleled disease activity in their study, while “neither the CDAI [CD activity index] score nor serum CRP showed similar correlation,” they said.
However, elevations in FC – a calcium- and zinc-binding protein released when neutrophils, monocytes, and macrophages inflame the intestinal mucosa – was independent of CD location, which signals the need for further investigation.
Meanwhile, the decent correlation between FC and CTE in the study “should [also] mean that” they could be used together to reliably define mucosal healing. CTE on its own “showed good correlation” with BAE; a CTE score/segment less than 2 [was] associated with endoscopic mucosal healing” on BAE, the investigators said.
The study subjects were an average of 32 years old, and had CD for 9 years; most were men. They had highly active disease at their first endoscopy (average CDAI of 120 points), and an average CRP of 1.09 mg/dL. Twenty-seven patients (30.3%) had small-bowel CD, 50 (56.2%) had ileocolonic CD, and 12 (13.5%) had colonic CD.
They all had endoscopic exams, BAE, and FC stool testing; those with strictures (17) went on to CTE; CTE detected every lesion despite the strictures.
The authors had no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point:
Major finding: A fecal calprotectin cutoff of 215 mcg/g identified mucosal healing with 82.8% sensitivity, 71.4% specificity, and an AUC of 0.81.
Data source: Review of 89 Crohn’s patients
Disclosures: The investigators had no conflicts of interest.
GOLD: Base COPD treatment on symptoms, exacerbation risk
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has uncoupled spirometry results from the ABCD treatment algorithm; this move marks the organization’s first announcing of major COPD guidance since 2011.
Spirometry now stands apart from GOLD’s ABCD symptom/exacerbation risk score with its own grade, with possibilities ranging from 1 to 4. A forced expiratory volume in 1 second (FEV1) of 80% or more of the predicted value rates a 1; the score degrades to 4 with an FEV1 below 30%.
“In previous GOLD documents, recommendations for management of COPD were based solely on spirometric category. However, there is considerable evidence that the level of FEV1 is a poor descriptor of disease status, and, for this reason, the management of stable COPD based on … disease impact (determined mainly by symptom burden and activity limitation) and future risk of disease progression (especially of exacerbations) is recommended. ... ABCD groups are now proposed to be derived exclusively from patient symptoms and their history of exacerbations,” GOLD said.
The clear focus on symptoms and exacerbations is “the major accomplishment” of the new report, which has been downloaded more than 45,000 times since it’s release, a testament to GOLD’s importance to clinicians trying to help COPD patients.
“We are trying to do a better job of personalizing treatment,” said GOLD board member Gerard Criner, MD, chair and professor of thoracic medicine and surgery at Temple University in Philadelphia.
The change “allows you to plan treatment based on symptoms [even] if you don’t have immediate access to spirometry, and then refine treatment once you have spirometry results. It also allows you to escalate and deescalate treatment because you are not boxed into a letter grade group” forced by spirometry. “You can also take a better look at pharmacologic versus nonpharmacologic therapy” when deciding what to do, he said.
In short, “we think it gives more freedom” to manage patients based on what seems best, Dr. Criner said.
GOLD included an example of how the new assessment can help. “Consider two patients,” it said, both with an FEV1 less than 30% and a COPD Assessment Test result of 18, but one with no exacerbations in the past year and the other with three. Both would have scored a GOLD D in the old system, and been treated similarly.
“However, with the new proposed scheme, the subject with three exacerbations ... would be labeled GOLD [spirometry] grade 4, group D,” and their treatment would focus on exacerbations. The no-exacerbation patient would be classified as GOLD grade 4, group B. Treatment would focus on symptoms. Drugs are still an option, but also lung volume reduction and lung transplant, GOLD said. Spirometry, in other words, is less important than how the patient is doing.
The group incorporated “every major study up to the first week of November” in the new report, Dr. Criner said, so there’s more to consider.
For instance, it’s clear now that patients benefit from home oxygen if they are severely hypoxemic while sitting on the couch watching TV, but not if they desaturate only when they get up and walk around, or come into the clinic to exercise. “We did not” know that in 2011, he said.
GOLD also recommended pulmonary rehabilitation and palliative care when indicated, as well as ongoing evaluation to make sure patients are able to use their inhalers, a major problem in COPD.
GOLD said that group A patients - those with few symptoms and low exacerbation risk - should be offered a bronchodilator. Initial therapy for group B - more symptoms, but low exacerbation risk - and group C - higher exacerbation risk but fewer symptoms - “should consist of a single long-acting bronchodilator. There is no evidence to recommend one class of long-acting bronchodilator over another.”
For group D - highly symptomatic with frequent exacerbations - “we recommend starting therapy with a [long-acting beta-2 agonist]/[long-acting antimuscarinic antagonist] combination,” the group said.
There was no industry involvement in GOLD’s report, but numerous authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on drug company studies. Dr. Criner reported personal payments from Holaria, and research funding and other nonpersonal payments from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Johnson and Johnson, and others.
As health care moves toward individualized care plans for patients, the updated GOLD recommendations enhance the possibility of personalized COPD treatment. This means more symptom-focused treatment for patients and, as Dr. Criner points out, more freedom for providers to manage patients based on what seems best.
As health care moves toward individualized care plans for patients, the updated GOLD recommendations enhance the possibility of personalized COPD treatment. This means more symptom-focused treatment for patients and, as Dr. Criner points out, more freedom for providers to manage patients based on what seems best.
As health care moves toward individualized care plans for patients, the updated GOLD recommendations enhance the possibility of personalized COPD treatment. This means more symptom-focused treatment for patients and, as Dr. Criner points out, more freedom for providers to manage patients based on what seems best.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has uncoupled spirometry results from the ABCD treatment algorithm; this move marks the organization’s first announcing of major COPD guidance since 2011.
Spirometry now stands apart from GOLD’s ABCD symptom/exacerbation risk score with its own grade, with possibilities ranging from 1 to 4. A forced expiratory volume in 1 second (FEV1) of 80% or more of the predicted value rates a 1; the score degrades to 4 with an FEV1 below 30%.
“In previous GOLD documents, recommendations for management of COPD were based solely on spirometric category. However, there is considerable evidence that the level of FEV1 is a poor descriptor of disease status, and, for this reason, the management of stable COPD based on … disease impact (determined mainly by symptom burden and activity limitation) and future risk of disease progression (especially of exacerbations) is recommended. ... ABCD groups are now proposed to be derived exclusively from patient symptoms and their history of exacerbations,” GOLD said.
The clear focus on symptoms and exacerbations is “the major accomplishment” of the new report, which has been downloaded more than 45,000 times since it’s release, a testament to GOLD’s importance to clinicians trying to help COPD patients.
“We are trying to do a better job of personalizing treatment,” said GOLD board member Gerard Criner, MD, chair and professor of thoracic medicine and surgery at Temple University in Philadelphia.
The change “allows you to plan treatment based on symptoms [even] if you don’t have immediate access to spirometry, and then refine treatment once you have spirometry results. It also allows you to escalate and deescalate treatment because you are not boxed into a letter grade group” forced by spirometry. “You can also take a better look at pharmacologic versus nonpharmacologic therapy” when deciding what to do, he said.
In short, “we think it gives more freedom” to manage patients based on what seems best, Dr. Criner said.
GOLD included an example of how the new assessment can help. “Consider two patients,” it said, both with an FEV1 less than 30% and a COPD Assessment Test result of 18, but one with no exacerbations in the past year and the other with three. Both would have scored a GOLD D in the old system, and been treated similarly.
“However, with the new proposed scheme, the subject with three exacerbations ... would be labeled GOLD [spirometry] grade 4, group D,” and their treatment would focus on exacerbations. The no-exacerbation patient would be classified as GOLD grade 4, group B. Treatment would focus on symptoms. Drugs are still an option, but also lung volume reduction and lung transplant, GOLD said. Spirometry, in other words, is less important than how the patient is doing.
The group incorporated “every major study up to the first week of November” in the new report, Dr. Criner said, so there’s more to consider.
For instance, it’s clear now that patients benefit from home oxygen if they are severely hypoxemic while sitting on the couch watching TV, but not if they desaturate only when they get up and walk around, or come into the clinic to exercise. “We did not” know that in 2011, he said.
GOLD also recommended pulmonary rehabilitation and palliative care when indicated, as well as ongoing evaluation to make sure patients are able to use their inhalers, a major problem in COPD.
GOLD said that group A patients - those with few symptoms and low exacerbation risk - should be offered a bronchodilator. Initial therapy for group B - more symptoms, but low exacerbation risk - and group C - higher exacerbation risk but fewer symptoms - “should consist of a single long-acting bronchodilator. There is no evidence to recommend one class of long-acting bronchodilator over another.”
For group D - highly symptomatic with frequent exacerbations - “we recommend starting therapy with a [long-acting beta-2 agonist]/[long-acting antimuscarinic antagonist] combination,” the group said.
There was no industry involvement in GOLD’s report, but numerous authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on drug company studies. Dr. Criner reported personal payments from Holaria, and research funding and other nonpersonal payments from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Johnson and Johnson, and others.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has uncoupled spirometry results from the ABCD treatment algorithm; this move marks the organization’s first announcing of major COPD guidance since 2011.
Spirometry now stands apart from GOLD’s ABCD symptom/exacerbation risk score with its own grade, with possibilities ranging from 1 to 4. A forced expiratory volume in 1 second (FEV1) of 80% or more of the predicted value rates a 1; the score degrades to 4 with an FEV1 below 30%.
“In previous GOLD documents, recommendations for management of COPD were based solely on spirometric category. However, there is considerable evidence that the level of FEV1 is a poor descriptor of disease status, and, for this reason, the management of stable COPD based on … disease impact (determined mainly by symptom burden and activity limitation) and future risk of disease progression (especially of exacerbations) is recommended. ... ABCD groups are now proposed to be derived exclusively from patient symptoms and their history of exacerbations,” GOLD said.
The clear focus on symptoms and exacerbations is “the major accomplishment” of the new report, which has been downloaded more than 45,000 times since it’s release, a testament to GOLD’s importance to clinicians trying to help COPD patients.
“We are trying to do a better job of personalizing treatment,” said GOLD board member Gerard Criner, MD, chair and professor of thoracic medicine and surgery at Temple University in Philadelphia.
The change “allows you to plan treatment based on symptoms [even] if you don’t have immediate access to spirometry, and then refine treatment once you have spirometry results. It also allows you to escalate and deescalate treatment because you are not boxed into a letter grade group” forced by spirometry. “You can also take a better look at pharmacologic versus nonpharmacologic therapy” when deciding what to do, he said.
In short, “we think it gives more freedom” to manage patients based on what seems best, Dr. Criner said.
GOLD included an example of how the new assessment can help. “Consider two patients,” it said, both with an FEV1 less than 30% and a COPD Assessment Test result of 18, but one with no exacerbations in the past year and the other with three. Both would have scored a GOLD D in the old system, and been treated similarly.
“However, with the new proposed scheme, the subject with three exacerbations ... would be labeled GOLD [spirometry] grade 4, group D,” and their treatment would focus on exacerbations. The no-exacerbation patient would be classified as GOLD grade 4, group B. Treatment would focus on symptoms. Drugs are still an option, but also lung volume reduction and lung transplant, GOLD said. Spirometry, in other words, is less important than how the patient is doing.
The group incorporated “every major study up to the first week of November” in the new report, Dr. Criner said, so there’s more to consider.
For instance, it’s clear now that patients benefit from home oxygen if they are severely hypoxemic while sitting on the couch watching TV, but not if they desaturate only when they get up and walk around, or come into the clinic to exercise. “We did not” know that in 2011, he said.
GOLD also recommended pulmonary rehabilitation and palliative care when indicated, as well as ongoing evaluation to make sure patients are able to use their inhalers, a major problem in COPD.
GOLD said that group A patients - those with few symptoms and low exacerbation risk - should be offered a bronchodilator. Initial therapy for group B - more symptoms, but low exacerbation risk - and group C - higher exacerbation risk but fewer symptoms - “should consist of a single long-acting bronchodilator. There is no evidence to recommend one class of long-acting bronchodilator over another.”
For group D - highly symptomatic with frequent exacerbations - “we recommend starting therapy with a [long-acting beta-2 agonist]/[long-acting antimuscarinic antagonist] combination,” the group said.
There was no industry involvement in GOLD’s report, but numerous authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on drug company studies. Dr. Criner reported personal payments from Holaria, and research funding and other nonpersonal payments from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Johnson and Johnson, and others.
VIDEO: No difference between PPI and H2RA for low-dose aspirin gastroprotection
Among patients on low-dose aspirin at risk for recurrent GI bleeding, there were slightly fewer GI bleeds or ulcers when patients were on the proton pump inhibitor rabeprazole (Aciphex) instead of the histamine2-receptor antagonist famotidine (Pepcid), but the difference was not statistically significant according to a study reported in the January issue of Gastroenterology.
SOURCE: American Gastroenterological Association
In a 270-subject, double-blind, randomized trial in Hong Kong and Japan led by Francis Chan, MD, of the Chinese University of Hong Kong, investigators found, “Among high-risk aspirin users, the incidence of recurrent bleeding is comparable with either use of PPI [proton pump inhibitor] or H2RA [H2-receptor antagonist].” However, “since a small difference in efficacy cannot be excluded, PPI probably remains the preferred treatment for long-term protection against upper GI bleeding in high-risk aspirin users” (Gastroenterology. 2016 Sep 15. doi: 10.1053/j.gastro.2016.09.006).
Even so, “our findings suggest that famotidine may be a reasonable alternative option for aspirin users who disfavor long-term PPI therapy,” they said.
Because of concerns about the long-term safety of PPIs, including the association between PPIs and increased risk of serious cardiovascular events in patients on clopidogrel (Plavix), clinicians have been looking for alternatives. The findings reassure that H2RAs are a reasonable choice; many clinicians have already turned to them.
All 270 subjects had previously had endoscopically confirmed ulcer bleeding while on low-dose aspirin (325 mg or less per day). “Considering clinicians will be most concerned with the adequacy of gastroprotective treatment effect in aspirin users with the highest risk, we exclusively enrolled patients with endoscopy-proven upper GI bleeding,” Dr. Chan and his colleagues said.
After the ulcers healed, the subjects resumed aspirin (80 mg) daily and were randomized to either famotidine 40 mg once daily (n = 132) or rabeprazole 20 mg daily (n = 138) for up to 12 months. Helicobacter pylori was eradicated prior to randomization in patients who tested positive. Subjects were evaluated every 2 months, with repeat endoscopy for symptoms of upper GI bleeding or significant drops in hemoglobin, as well as at the end of the study.
During the 12-month study period, upper GI bleeding recurred in one patient receiving rabeprazole (0.7%) and four receiving famotidine (3.1%; P = .16). The composite endpoint of recurrent bleeding or endoscopic ulcers at month 12 was reached by nine patients in the rabeprazole group (7.9%) and 13 receiving famotidine (12.4%; P = .26).
“Our findings indicate that both treatments are comparable in preventing recurrent upper GI bleeding in high-risk aspirin users, although a small difference in efficacy cannot be excluded,” the investigators said.
Over two-thirds of the subjects were men, and the mean age was 73 years. About a quarter in the PPI group and almost 40% in the H2RA group had H. pylori cleared before randomization.
The Research Grant Council of Hong Kong funded the work. Dr. Chan has served as a consultant to Pfizer, Eisai, Takeda, and Otsuka, and has received research grants from Pfizer and lecture fees from Pfizer, AstraZeneca, and Takeda. Several other authors reported similar industry disclosures.
Aspirin is widely used for primary and secondary prophylaxis of cardiovascular disease. Dr. Chan and colleagues present a randomized, controlled trial comparing rabeprazole 20 mg once a day to famotidine 40 mg once a day in preventing recurrent GI hemorrhage and endoscopic ulcers in low-dose (less than 325 mg) aspirin users. The authors conclude that no statistical difference was found between the two agents. The study contrasts with another study from Hong Kong, which found that proton pump inhibitors were more effective.
The authors are to be complimented on this important addition to the literature but the reader should not conclude that H2-receptor antagonists and proton pump inhibitors are equivalent in preventing recurrent bleeding from aspirin-induced ulcers.
Nimish Vakil, MD, AGAF, is clinical professor of medicine at the University of Wisconsin–Madison. He has consulted for Ironwood and AstraZeneca.
Aspirin is widely used for primary and secondary prophylaxis of cardiovascular disease. Dr. Chan and colleagues present a randomized, controlled trial comparing rabeprazole 20 mg once a day to famotidine 40 mg once a day in preventing recurrent GI hemorrhage and endoscopic ulcers in low-dose (less than 325 mg) aspirin users. The authors conclude that no statistical difference was found between the two agents. The study contrasts with another study from Hong Kong, which found that proton pump inhibitors were more effective.
The authors are to be complimented on this important addition to the literature but the reader should not conclude that H2-receptor antagonists and proton pump inhibitors are equivalent in preventing recurrent bleeding from aspirin-induced ulcers.
Nimish Vakil, MD, AGAF, is clinical professor of medicine at the University of Wisconsin–Madison. He has consulted for Ironwood and AstraZeneca.
Aspirin is widely used for primary and secondary prophylaxis of cardiovascular disease. Dr. Chan and colleagues present a randomized, controlled trial comparing rabeprazole 20 mg once a day to famotidine 40 mg once a day in preventing recurrent GI hemorrhage and endoscopic ulcers in low-dose (less than 325 mg) aspirin users. The authors conclude that no statistical difference was found between the two agents. The study contrasts with another study from Hong Kong, which found that proton pump inhibitors were more effective.
The authors are to be complimented on this important addition to the literature but the reader should not conclude that H2-receptor antagonists and proton pump inhibitors are equivalent in preventing recurrent bleeding from aspirin-induced ulcers.
Nimish Vakil, MD, AGAF, is clinical professor of medicine at the University of Wisconsin–Madison. He has consulted for Ironwood and AstraZeneca.
Among patients on low-dose aspirin at risk for recurrent GI bleeding, there were slightly fewer GI bleeds or ulcers when patients were on the proton pump inhibitor rabeprazole (Aciphex) instead of the histamine2-receptor antagonist famotidine (Pepcid), but the difference was not statistically significant according to a study reported in the January issue of Gastroenterology.
SOURCE: American Gastroenterological Association
In a 270-subject, double-blind, randomized trial in Hong Kong and Japan led by Francis Chan, MD, of the Chinese University of Hong Kong, investigators found, “Among high-risk aspirin users, the incidence of recurrent bleeding is comparable with either use of PPI [proton pump inhibitor] or H2RA [H2-receptor antagonist].” However, “since a small difference in efficacy cannot be excluded, PPI probably remains the preferred treatment for long-term protection against upper GI bleeding in high-risk aspirin users” (Gastroenterology. 2016 Sep 15. doi: 10.1053/j.gastro.2016.09.006).
Even so, “our findings suggest that famotidine may be a reasonable alternative option for aspirin users who disfavor long-term PPI therapy,” they said.
Because of concerns about the long-term safety of PPIs, including the association between PPIs and increased risk of serious cardiovascular events in patients on clopidogrel (Plavix), clinicians have been looking for alternatives. The findings reassure that H2RAs are a reasonable choice; many clinicians have already turned to them.
All 270 subjects had previously had endoscopically confirmed ulcer bleeding while on low-dose aspirin (325 mg or less per day). “Considering clinicians will be most concerned with the adequacy of gastroprotective treatment effect in aspirin users with the highest risk, we exclusively enrolled patients with endoscopy-proven upper GI bleeding,” Dr. Chan and his colleagues said.
After the ulcers healed, the subjects resumed aspirin (80 mg) daily and were randomized to either famotidine 40 mg once daily (n = 132) or rabeprazole 20 mg daily (n = 138) for up to 12 months. Helicobacter pylori was eradicated prior to randomization in patients who tested positive. Subjects were evaluated every 2 months, with repeat endoscopy for symptoms of upper GI bleeding or significant drops in hemoglobin, as well as at the end of the study.
During the 12-month study period, upper GI bleeding recurred in one patient receiving rabeprazole (0.7%) and four receiving famotidine (3.1%; P = .16). The composite endpoint of recurrent bleeding or endoscopic ulcers at month 12 was reached by nine patients in the rabeprazole group (7.9%) and 13 receiving famotidine (12.4%; P = .26).
“Our findings indicate that both treatments are comparable in preventing recurrent upper GI bleeding in high-risk aspirin users, although a small difference in efficacy cannot be excluded,” the investigators said.
Over two-thirds of the subjects were men, and the mean age was 73 years. About a quarter in the PPI group and almost 40% in the H2RA group had H. pylori cleared before randomization.
The Research Grant Council of Hong Kong funded the work. Dr. Chan has served as a consultant to Pfizer, Eisai, Takeda, and Otsuka, and has received research grants from Pfizer and lecture fees from Pfizer, AstraZeneca, and Takeda. Several other authors reported similar industry disclosures.
Among patients on low-dose aspirin at risk for recurrent GI bleeding, there were slightly fewer GI bleeds or ulcers when patients were on the proton pump inhibitor rabeprazole (Aciphex) instead of the histamine2-receptor antagonist famotidine (Pepcid), but the difference was not statistically significant according to a study reported in the January issue of Gastroenterology.
SOURCE: American Gastroenterological Association
In a 270-subject, double-blind, randomized trial in Hong Kong and Japan led by Francis Chan, MD, of the Chinese University of Hong Kong, investigators found, “Among high-risk aspirin users, the incidence of recurrent bleeding is comparable with either use of PPI [proton pump inhibitor] or H2RA [H2-receptor antagonist].” However, “since a small difference in efficacy cannot be excluded, PPI probably remains the preferred treatment for long-term protection against upper GI bleeding in high-risk aspirin users” (Gastroenterology. 2016 Sep 15. doi: 10.1053/j.gastro.2016.09.006).
Even so, “our findings suggest that famotidine may be a reasonable alternative option for aspirin users who disfavor long-term PPI therapy,” they said.
Because of concerns about the long-term safety of PPIs, including the association between PPIs and increased risk of serious cardiovascular events in patients on clopidogrel (Plavix), clinicians have been looking for alternatives. The findings reassure that H2RAs are a reasonable choice; many clinicians have already turned to them.
All 270 subjects had previously had endoscopically confirmed ulcer bleeding while on low-dose aspirin (325 mg or less per day). “Considering clinicians will be most concerned with the adequacy of gastroprotective treatment effect in aspirin users with the highest risk, we exclusively enrolled patients with endoscopy-proven upper GI bleeding,” Dr. Chan and his colleagues said.
After the ulcers healed, the subjects resumed aspirin (80 mg) daily and were randomized to either famotidine 40 mg once daily (n = 132) or rabeprazole 20 mg daily (n = 138) for up to 12 months. Helicobacter pylori was eradicated prior to randomization in patients who tested positive. Subjects were evaluated every 2 months, with repeat endoscopy for symptoms of upper GI bleeding or significant drops in hemoglobin, as well as at the end of the study.
During the 12-month study period, upper GI bleeding recurred in one patient receiving rabeprazole (0.7%) and four receiving famotidine (3.1%; P = .16). The composite endpoint of recurrent bleeding or endoscopic ulcers at month 12 was reached by nine patients in the rabeprazole group (7.9%) and 13 receiving famotidine (12.4%; P = .26).
“Our findings indicate that both treatments are comparable in preventing recurrent upper GI bleeding in high-risk aspirin users, although a small difference in efficacy cannot be excluded,” the investigators said.
Over two-thirds of the subjects were men, and the mean age was 73 years. About a quarter in the PPI group and almost 40% in the H2RA group had H. pylori cleared before randomization.
The Research Grant Council of Hong Kong funded the work. Dr. Chan has served as a consultant to Pfizer, Eisai, Takeda, and Otsuka, and has received research grants from Pfizer and lecture fees from Pfizer, AstraZeneca, and Takeda. Several other authors reported similar industry disclosures.
FROM GASTROENTEROLOGY
Key clinical point:
Major finding: During the 12-month study period, upper GI bleeding recurred in one patient receiving rabeprazole (0.7%) and four receiving famotidine (3.1%; P = .16). The composite endpoint of recurrent bleeding or endoscopic ulcers at month 12 was reached by nine patients in the rabeprazole group (7.9%) and 13 receiving famotidine (12.4%; P = .26).
Data source: A 270-subject, double-blind, randomized trial in Hong Kong and Japan.
Disclosures: The Research Grant Council of Hong Kong funded the work. The lead investigator has served as a consultant to Pfizer, Eisai, Takeda, and Otsuka, and has received research grants from Pfizer and lecture fees from Pfizer, AstraZeneca, and Takeda. Several other authors reported similar industry disclosures.
Texas reports local Zika transmission
On Nov. 28, public health officials there reported a case of Zika virus in a Brownsville woman who hadn’t traveled to Mexico or any other area with active Zika transmission. Brownsville sits on the border of Mexico at the state’s southern tip, and is home to Aedes species mosquitoes known to carry the virus. The area had recently been sprayed for mosquitoes.
Zika’s telltale genetic thumbprint was found in the woman’s urine, but her blood was negative, so the virus could no longer be spread from her by mosquito. She was not pregnant. There are no other suspected cases of local transmission, according to Texas officials.
“We knew it was only a matter of time before we saw a Zika case spread by a mosquito in Texas,” John Hellerstedt, MD, commissioner of the Texas Department of State Health Services, said in a statement. “We still don’t believe the virus will become widespread in Texas, but there could be more cases, so people need to protect themselves from mosquito bites, especially in parts of the state that stay relatively warm in the fall and winter.”
The state public health officials recommend testing all pregnant women who have traveled – or who have sexual partners who have traveled – to areas with active Zika transmission. In addition to Mexico, the list includes Southeast Asia, Central and South America, the Caribbean, Cape Verde, and Pacific islands including Tonga, Samoa, and Papua New Guinea.
Texas officials also recommend antibody testing of pregnant women in southern Texas if they have two or more symptoms – fever, itchy rash, joint pain, and eye redness – and anyone statewide with at least three symptoms.
As of Nov. 23, a total of 4,444 Zika cases have been reported to the Centers for Disease Control and Prevention in U.S. states and the District of Columbia. Just 182 of those cases were the result of local spread by mosquitoes in Florida. Puerto Rico has reported nearly 32,000 locally-transmitted cases.
The 257 previously confirmed cases in Texas were all associated with travel. Most were in the Houston and Dallas–Fort Worth areas.
Local and state health officials are working with the CDC to pinpoint how and where the Brownsville infection occurred. Mosquitoes are being trapped, and workers are going door to door to educate people about Zika and request urine samples to screen for infection.
On Nov. 28, public health officials there reported a case of Zika virus in a Brownsville woman who hadn’t traveled to Mexico or any other area with active Zika transmission. Brownsville sits on the border of Mexico at the state’s southern tip, and is home to Aedes species mosquitoes known to carry the virus. The area had recently been sprayed for mosquitoes.
Zika’s telltale genetic thumbprint was found in the woman’s urine, but her blood was negative, so the virus could no longer be spread from her by mosquito. She was not pregnant. There are no other suspected cases of local transmission, according to Texas officials.
“We knew it was only a matter of time before we saw a Zika case spread by a mosquito in Texas,” John Hellerstedt, MD, commissioner of the Texas Department of State Health Services, said in a statement. “We still don’t believe the virus will become widespread in Texas, but there could be more cases, so people need to protect themselves from mosquito bites, especially in parts of the state that stay relatively warm in the fall and winter.”
The state public health officials recommend testing all pregnant women who have traveled – or who have sexual partners who have traveled – to areas with active Zika transmission. In addition to Mexico, the list includes Southeast Asia, Central and South America, the Caribbean, Cape Verde, and Pacific islands including Tonga, Samoa, and Papua New Guinea.
Texas officials also recommend antibody testing of pregnant women in southern Texas if they have two or more symptoms – fever, itchy rash, joint pain, and eye redness – and anyone statewide with at least three symptoms.
As of Nov. 23, a total of 4,444 Zika cases have been reported to the Centers for Disease Control and Prevention in U.S. states and the District of Columbia. Just 182 of those cases were the result of local spread by mosquitoes in Florida. Puerto Rico has reported nearly 32,000 locally-transmitted cases.
The 257 previously confirmed cases in Texas were all associated with travel. Most were in the Houston and Dallas–Fort Worth areas.
Local and state health officials are working with the CDC to pinpoint how and where the Brownsville infection occurred. Mosquitoes are being trapped, and workers are going door to door to educate people about Zika and request urine samples to screen for infection.
On Nov. 28, public health officials there reported a case of Zika virus in a Brownsville woman who hadn’t traveled to Mexico or any other area with active Zika transmission. Brownsville sits on the border of Mexico at the state’s southern tip, and is home to Aedes species mosquitoes known to carry the virus. The area had recently been sprayed for mosquitoes.
Zika’s telltale genetic thumbprint was found in the woman’s urine, but her blood was negative, so the virus could no longer be spread from her by mosquito. She was not pregnant. There are no other suspected cases of local transmission, according to Texas officials.
“We knew it was only a matter of time before we saw a Zika case spread by a mosquito in Texas,” John Hellerstedt, MD, commissioner of the Texas Department of State Health Services, said in a statement. “We still don’t believe the virus will become widespread in Texas, but there could be more cases, so people need to protect themselves from mosquito bites, especially in parts of the state that stay relatively warm in the fall and winter.”
The state public health officials recommend testing all pregnant women who have traveled – or who have sexual partners who have traveled – to areas with active Zika transmission. In addition to Mexico, the list includes Southeast Asia, Central and South America, the Caribbean, Cape Verde, and Pacific islands including Tonga, Samoa, and Papua New Guinea.
Texas officials also recommend antibody testing of pregnant women in southern Texas if they have two or more symptoms – fever, itchy rash, joint pain, and eye redness – and anyone statewide with at least three symptoms.
As of Nov. 23, a total of 4,444 Zika cases have been reported to the Centers for Disease Control and Prevention in U.S. states and the District of Columbia. Just 182 of those cases were the result of local spread by mosquitoes in Florida. Puerto Rico has reported nearly 32,000 locally-transmitted cases.
The 257 previously confirmed cases in Texas were all associated with travel. Most were in the Houston and Dallas–Fort Worth areas.
Local and state health officials are working with the CDC to pinpoint how and where the Brownsville infection occurred. Mosquitoes are being trapped, and workers are going door to door to educate people about Zika and request urine samples to screen for infection.
Increased death rate with platelets for aspirin/clopidogrel GI bleed
Patients with normal platelet counts who have a GI bleed while on antiplatelets were almost six times more likely to die in the hospital if they had a platelet transfusion in a retrospective cohort study from the Yale University in New Haven, Conn.
Ten of the 14 deaths in the 204 transfused patients – versus none of the 3 deaths in the 204 nontransfused patients - were due to bleeding, so it’s possible that the mortality difference was simply because patients with worse bleeding were more likely to get transfused. “On the other hand, the adjusted [odds ratios] for mortality (4.5-6.8 with different sensitivity analyses) [were] large, increasing the likelihood of a cause-and-effect relationship,” said investigators led by gastroenterologist Liam Zakko, MD, now at the Mayo Clinic in Rochester, Minn. (Clin Gastroenterol Hepatol. 2016 Jul 25. doi: 10.1016/j.cgh.2016.07.017).
Current guidelines suggest platelet transfusions are an option for antiplatelet patients with serious GI bleeds, but the Yale team found that they did not reduce rebleeding. “The observation of increased mortality without documentation of clinical benefit suggests a very cautious approach to the use of platelet transfusion. ... We do not support the use of platelet transfusions in patients with GI [bleeds] who are taking antiplatelet agents,” the investigators wrote.
Subjects in the two groups were matched for sex, age, and GI bleed location, and all had platelet counts above 100 × 109/L. Almost everyone was on aspirin for cardiovascular protection, and 30% were on also on clopidogrel.
Just over half in both groups had upper GI bleeds, and about 40% in each group had colonic bleeds. Transfused patients had more-severe bleeding, with overall lower blood pressure and lower hemoglobin; a larger proportion was admitted to the ICU.
On univariate analyses, platelet patients had more cardiovascular events (23% vs. 13%) while in the hospital. They were also more likely to stay in the hospital for more than 4 days (47% vs. 33%) and more likely to die while there (7% vs. 1%). On multivariable analysis, only the greater risk for death during admission remained statistically significant (odds ratio, 5.57; 95% confidence interval, 1.52-27.1). The adjusted odds ratio for recurrent bleeding was not significant.
Four patients in the platelet group died from cardiovascular causes. One patient in the control group had a fatal cardiovascular event.
Although counterintuitive, the authors said that it’s possible that platelet transfusions might actually increase the risk of severe and fatal GI bleeding. “Mechanisms by which platelet transfusion would increase mortality or [GI bleeding]–related mortality are not clear,” but “platelet transfusions are reported to be proinflammatory and alter recipient immunity,” they said.
At least for now, “the most prudent way to manage patients on antiplatelet agents with [GI bleeding] is to follow current evidence-based recommendations,” including early endoscopy, endoscopic hemostatic therapy for high-risk lesions, and intensive proton pump inhibitor therapy in patients with ulcers and high-risk endoscopic features.
“Although not based on high-quality evidence, we believe that hemostatic techniques that do not cause significant tissue damage (e.g., clips rather than thermal devices or sclerosants) should be used in patients on antiplatelet agents, especially if patients are expected to remain on these agents in the future,” they said.
The mean age in the study was 74 years, and about two-thirds of the subjects were men.
The authors had no disclosures.
The management of patients with gastrointestinal bleeding on antithrombotic drugs is a major challenge for gastroenterologists. Unfortunately, the use of aspirin alone has been shown to increase the risk of GI bleed twofold, and the addition of a thienopyridine additionally increases the risk of bleeding twofold. Furthermore, there is no available agent to reverse antiplatelet affects of these drugs, which irreversibly block platelet function for the life of the platelet (8-10 days). Current recommendations for the management of severe GI bleeding in patients receiving antithrombotic therapy include platelet transfusion, including those with a normal platelet count. However, this comes with a price as reversal of platelet function may increase the rate of cardiovascular events.
Zakko et al. performed a retrospective case-control study evaluating the role of platelet transfusion in patients presenting with GI bleeding. Patients were matched by age, sex, and the location of the GI bleed. Most patients included in the study were on low-dose aspirin and almost a third of the patients were taking both aspirin and a thienopyridine. Patients receiving platelet transfusions appeared to have more severe GI bleeding compared with matched controls, as patients receiving transfusion were more likely to have been hypotensive, tachycardic, have a low hemoglobin level, and require treatment in the intensive care unit (72% vs. 28%, P less than .0001). Patients receiving platelet transfusions were also more likely than matched controls to have recurrent GI bleeding as well as major cardiovascular adverse events, including myocardial infarction and inpatient death. After adjusting for patient characteristics, patients receiving platelet transfusions were more likely to have an increased risk of death (adjusted OR, 5.57; 95% CI, 1.52-27.1). The authors conclude that “the use of platelet transfusions in patients with GI bleeding who are taking antiplatelet agents without thrombocytopenia did not reduce rebleeding but was associated with higher mortality.”
Currently, there is no convincing evidence to support platelet transfusion in patients with bleeding on aspirin and/or a thienopyridine. Because the majority of the deaths were due to GI bleeding and not cardiovascular events, the observed increase in adverse events in patients receiving platelet transfusions likely reflects more severe GI bleeding in patients receiving platelet transfusions than in controls. We should avoid platelet transfusions and focus our management on achieving adequate resuscitation, use of proton pump inhibitors for patients with high-risk ulcers, and early endoscopy with endoscopic therapy for high-risk lesions.
John R. Saltzman, MD, AGAF, is director of endoscopy, Brigham and Women’s Hospital, professor of medicine, Harvard Medical School, Boston. He has no conflicts of interest.
The management of patients with gastrointestinal bleeding on antithrombotic drugs is a major challenge for gastroenterologists. Unfortunately, the use of aspirin alone has been shown to increase the risk of GI bleed twofold, and the addition of a thienopyridine additionally increases the risk of bleeding twofold. Furthermore, there is no available agent to reverse antiplatelet affects of these drugs, which irreversibly block platelet function for the life of the platelet (8-10 days). Current recommendations for the management of severe GI bleeding in patients receiving antithrombotic therapy include platelet transfusion, including those with a normal platelet count. However, this comes with a price as reversal of platelet function may increase the rate of cardiovascular events.
Zakko et al. performed a retrospective case-control study evaluating the role of platelet transfusion in patients presenting with GI bleeding. Patients were matched by age, sex, and the location of the GI bleed. Most patients included in the study were on low-dose aspirin and almost a third of the patients were taking both aspirin and a thienopyridine. Patients receiving platelet transfusions appeared to have more severe GI bleeding compared with matched controls, as patients receiving transfusion were more likely to have been hypotensive, tachycardic, have a low hemoglobin level, and require treatment in the intensive care unit (72% vs. 28%, P less than .0001). Patients receiving platelet transfusions were also more likely than matched controls to have recurrent GI bleeding as well as major cardiovascular adverse events, including myocardial infarction and inpatient death. After adjusting for patient characteristics, patients receiving platelet transfusions were more likely to have an increased risk of death (adjusted OR, 5.57; 95% CI, 1.52-27.1). The authors conclude that “the use of platelet transfusions in patients with GI bleeding who are taking antiplatelet agents without thrombocytopenia did not reduce rebleeding but was associated with higher mortality.”
Currently, there is no convincing evidence to support platelet transfusion in patients with bleeding on aspirin and/or a thienopyridine. Because the majority of the deaths were due to GI bleeding and not cardiovascular events, the observed increase in adverse events in patients receiving platelet transfusions likely reflects more severe GI bleeding in patients receiving platelet transfusions than in controls. We should avoid platelet transfusions and focus our management on achieving adequate resuscitation, use of proton pump inhibitors for patients with high-risk ulcers, and early endoscopy with endoscopic therapy for high-risk lesions.
John R. Saltzman, MD, AGAF, is director of endoscopy, Brigham and Women’s Hospital, professor of medicine, Harvard Medical School, Boston. He has no conflicts of interest.
The management of patients with gastrointestinal bleeding on antithrombotic drugs is a major challenge for gastroenterologists. Unfortunately, the use of aspirin alone has been shown to increase the risk of GI bleed twofold, and the addition of a thienopyridine additionally increases the risk of bleeding twofold. Furthermore, there is no available agent to reverse antiplatelet affects of these drugs, which irreversibly block platelet function for the life of the platelet (8-10 days). Current recommendations for the management of severe GI bleeding in patients receiving antithrombotic therapy include platelet transfusion, including those with a normal platelet count. However, this comes with a price as reversal of platelet function may increase the rate of cardiovascular events.
Zakko et al. performed a retrospective case-control study evaluating the role of platelet transfusion in patients presenting with GI bleeding. Patients were matched by age, sex, and the location of the GI bleed. Most patients included in the study were on low-dose aspirin and almost a third of the patients were taking both aspirin and a thienopyridine. Patients receiving platelet transfusions appeared to have more severe GI bleeding compared with matched controls, as patients receiving transfusion were more likely to have been hypotensive, tachycardic, have a low hemoglobin level, and require treatment in the intensive care unit (72% vs. 28%, P less than .0001). Patients receiving platelet transfusions were also more likely than matched controls to have recurrent GI bleeding as well as major cardiovascular adverse events, including myocardial infarction and inpatient death. After adjusting for patient characteristics, patients receiving platelet transfusions were more likely to have an increased risk of death (adjusted OR, 5.57; 95% CI, 1.52-27.1). The authors conclude that “the use of platelet transfusions in patients with GI bleeding who are taking antiplatelet agents without thrombocytopenia did not reduce rebleeding but was associated with higher mortality.”
Currently, there is no convincing evidence to support platelet transfusion in patients with bleeding on aspirin and/or a thienopyridine. Because the majority of the deaths were due to GI bleeding and not cardiovascular events, the observed increase in adverse events in patients receiving platelet transfusions likely reflects more severe GI bleeding in patients receiving platelet transfusions than in controls. We should avoid platelet transfusions and focus our management on achieving adequate resuscitation, use of proton pump inhibitors for patients with high-risk ulcers, and early endoscopy with endoscopic therapy for high-risk lesions.
John R. Saltzman, MD, AGAF, is director of endoscopy, Brigham and Women’s Hospital, professor of medicine, Harvard Medical School, Boston. He has no conflicts of interest.
Patients with normal platelet counts who have a GI bleed while on antiplatelets were almost six times more likely to die in the hospital if they had a platelet transfusion in a retrospective cohort study from the Yale University in New Haven, Conn.
Ten of the 14 deaths in the 204 transfused patients – versus none of the 3 deaths in the 204 nontransfused patients - were due to bleeding, so it’s possible that the mortality difference was simply because patients with worse bleeding were more likely to get transfused. “On the other hand, the adjusted [odds ratios] for mortality (4.5-6.8 with different sensitivity analyses) [were] large, increasing the likelihood of a cause-and-effect relationship,” said investigators led by gastroenterologist Liam Zakko, MD, now at the Mayo Clinic in Rochester, Minn. (Clin Gastroenterol Hepatol. 2016 Jul 25. doi: 10.1016/j.cgh.2016.07.017).
Current guidelines suggest platelet transfusions are an option for antiplatelet patients with serious GI bleeds, but the Yale team found that they did not reduce rebleeding. “The observation of increased mortality without documentation of clinical benefit suggests a very cautious approach to the use of platelet transfusion. ... We do not support the use of platelet transfusions in patients with GI [bleeds] who are taking antiplatelet agents,” the investigators wrote.
Subjects in the two groups were matched for sex, age, and GI bleed location, and all had platelet counts above 100 × 109/L. Almost everyone was on aspirin for cardiovascular protection, and 30% were on also on clopidogrel.
Just over half in both groups had upper GI bleeds, and about 40% in each group had colonic bleeds. Transfused patients had more-severe bleeding, with overall lower blood pressure and lower hemoglobin; a larger proportion was admitted to the ICU.
On univariate analyses, platelet patients had more cardiovascular events (23% vs. 13%) while in the hospital. They were also more likely to stay in the hospital for more than 4 days (47% vs. 33%) and more likely to die while there (7% vs. 1%). On multivariable analysis, only the greater risk for death during admission remained statistically significant (odds ratio, 5.57; 95% confidence interval, 1.52-27.1). The adjusted odds ratio for recurrent bleeding was not significant.
Four patients in the platelet group died from cardiovascular causes. One patient in the control group had a fatal cardiovascular event.
Although counterintuitive, the authors said that it’s possible that platelet transfusions might actually increase the risk of severe and fatal GI bleeding. “Mechanisms by which platelet transfusion would increase mortality or [GI bleeding]–related mortality are not clear,” but “platelet transfusions are reported to be proinflammatory and alter recipient immunity,” they said.
At least for now, “the most prudent way to manage patients on antiplatelet agents with [GI bleeding] is to follow current evidence-based recommendations,” including early endoscopy, endoscopic hemostatic therapy for high-risk lesions, and intensive proton pump inhibitor therapy in patients with ulcers and high-risk endoscopic features.
“Although not based on high-quality evidence, we believe that hemostatic techniques that do not cause significant tissue damage (e.g., clips rather than thermal devices or sclerosants) should be used in patients on antiplatelet agents, especially if patients are expected to remain on these agents in the future,” they said.
The mean age in the study was 74 years, and about two-thirds of the subjects were men.
The authors had no disclosures.
Patients with normal platelet counts who have a GI bleed while on antiplatelets were almost six times more likely to die in the hospital if they had a platelet transfusion in a retrospective cohort study from the Yale University in New Haven, Conn.
Ten of the 14 deaths in the 204 transfused patients – versus none of the 3 deaths in the 204 nontransfused patients - were due to bleeding, so it’s possible that the mortality difference was simply because patients with worse bleeding were more likely to get transfused. “On the other hand, the adjusted [odds ratios] for mortality (4.5-6.8 with different sensitivity analyses) [were] large, increasing the likelihood of a cause-and-effect relationship,” said investigators led by gastroenterologist Liam Zakko, MD, now at the Mayo Clinic in Rochester, Minn. (Clin Gastroenterol Hepatol. 2016 Jul 25. doi: 10.1016/j.cgh.2016.07.017).
Current guidelines suggest platelet transfusions are an option for antiplatelet patients with serious GI bleeds, but the Yale team found that they did not reduce rebleeding. “The observation of increased mortality without documentation of clinical benefit suggests a very cautious approach to the use of platelet transfusion. ... We do not support the use of platelet transfusions in patients with GI [bleeds] who are taking antiplatelet agents,” the investigators wrote.
Subjects in the two groups were matched for sex, age, and GI bleed location, and all had platelet counts above 100 × 109/L. Almost everyone was on aspirin for cardiovascular protection, and 30% were on also on clopidogrel.
Just over half in both groups had upper GI bleeds, and about 40% in each group had colonic bleeds. Transfused patients had more-severe bleeding, with overall lower blood pressure and lower hemoglobin; a larger proportion was admitted to the ICU.
On univariate analyses, platelet patients had more cardiovascular events (23% vs. 13%) while in the hospital. They were also more likely to stay in the hospital for more than 4 days (47% vs. 33%) and more likely to die while there (7% vs. 1%). On multivariable analysis, only the greater risk for death during admission remained statistically significant (odds ratio, 5.57; 95% confidence interval, 1.52-27.1). The adjusted odds ratio for recurrent bleeding was not significant.
Four patients in the platelet group died from cardiovascular causes. One patient in the control group had a fatal cardiovascular event.
Although counterintuitive, the authors said that it’s possible that platelet transfusions might actually increase the risk of severe and fatal GI bleeding. “Mechanisms by which platelet transfusion would increase mortality or [GI bleeding]–related mortality are not clear,” but “platelet transfusions are reported to be proinflammatory and alter recipient immunity,” they said.
At least for now, “the most prudent way to manage patients on antiplatelet agents with [GI bleeding] is to follow current evidence-based recommendations,” including early endoscopy, endoscopic hemostatic therapy for high-risk lesions, and intensive proton pump inhibitor therapy in patients with ulcers and high-risk endoscopic features.
“Although not based on high-quality evidence, we believe that hemostatic techniques that do not cause significant tissue damage (e.g., clips rather than thermal devices or sclerosants) should be used in patients on antiplatelet agents, especially if patients are expected to remain on these agents in the future,” they said.
The mean age in the study was 74 years, and about two-thirds of the subjects were men.
The authors had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point:
Major finding: Compared with those not transfused, the risk for death during admission remained statistically significant on multivariate analysis (OR, 5.57; 95% CI, 1.52-27.1).
Data source: Retrospective cohort study of 408 GI bleed patients
Disclosures: The authors had no disclosures.
Survey: Primary care needs opioid alternatives
Almost a third of doctors blamed overprescribing for the opioid crisis, according to a survey of 225 U.S. primary care, emergency department, and pain management physicians by InCrowd, an online physician survey company.
Respondents said their own and other physicians’ overprescribing is the single biggest factor fueling the leap in opioid abuse over the past 5 years.
In short, the survey pointed out what front-line doctors think needs to be fixed as the nation combats prescription opioid abuse and the subsequent heroin epidemic. Their insights “should be a rallying cry” for changes in 2017, said epidemiologist Diane Hayes, PhD, president and cofounder of InCrowd.
Making pain the “fifth vital sign” and allowing patients to downgrade doctors on surveys if they don’t refill narcotic prescriptions compounded the situation. Lengthy waits for specialists with better pain options, many of whom are not covered by Medicaid or the Affordable Care Act, also added to the problem, survey respondents said.
“We’re caught in the middle” between the Joint Commission on Accreditation of Healthcare Organization’s fifth vital sign and overprescribing, a primary care doctor said.
Seventy-three percent of survey respondents said that they want opioid alternatives. They’re tired of trying to get the job done with NSAIDs, physical therapy, and exercise. About half recommend behavioral health interventions, while 20% recommend vitamin and herbal supplements. Only 10% recommend medical marijuana, probably because most U.S. patients can’t get it.
Meanwhile, the respondents said they want opioid prescribing hemmed in. Almost two-thirds wanted refill limits and more frequent refill evaluations, and many agreed that there needs to be a weaning protocol before the drugs are even started. Some wanted to limit advertising.
Easton Jackson, MD, a primary care physician in West Valley City, Utah, who answered the survey, helped make the answers real by sharing his thoughts.
“We need to recognize that ... people don’t set out to get addicted to opioids ... We need to educate [patients] and assist them with their expectations. They need to understand that they’re going to have pain from surgery and injuries. Our goal isn’t to make them pain free. It’s to manage their pain,” he said.
“We as physicians need to write for fewer pills and in lower doses. We need to see our patients back sooner. If it’s not working, stop increasing the dose and instead taper the patient off the medication. We need to be familiar with the adjuvant therapies. As easy as it is to say, ‘send them all to the pain specialist,’ there simply aren’t enough of them around,” Dr. Easton said.
Physician respondents to InCrowd’s opioid survey have practiced an average of 25 years, and were scattered around the United States. They filled out the four-question survey during Oct. 27-28, 2016. They signed up to receive and answer InCrowd’s questions, and were paid nominally for their time.
Half (50%) of respondents estimated that they prescribed opioids to fewer than 10% of their patients, while 38% said they prescribed to less than half of their patients, and 12% estimated they prescribed opioids to more than half of their patients.
Almost a third of doctors blamed overprescribing for the opioid crisis, according to a survey of 225 U.S. primary care, emergency department, and pain management physicians by InCrowd, an online physician survey company.
Respondents said their own and other physicians’ overprescribing is the single biggest factor fueling the leap in opioid abuse over the past 5 years.
In short, the survey pointed out what front-line doctors think needs to be fixed as the nation combats prescription opioid abuse and the subsequent heroin epidemic. Their insights “should be a rallying cry” for changes in 2017, said epidemiologist Diane Hayes, PhD, president and cofounder of InCrowd.
Making pain the “fifth vital sign” and allowing patients to downgrade doctors on surveys if they don’t refill narcotic prescriptions compounded the situation. Lengthy waits for specialists with better pain options, many of whom are not covered by Medicaid or the Affordable Care Act, also added to the problem, survey respondents said.
“We’re caught in the middle” between the Joint Commission on Accreditation of Healthcare Organization’s fifth vital sign and overprescribing, a primary care doctor said.
Seventy-three percent of survey respondents said that they want opioid alternatives. They’re tired of trying to get the job done with NSAIDs, physical therapy, and exercise. About half recommend behavioral health interventions, while 20% recommend vitamin and herbal supplements. Only 10% recommend medical marijuana, probably because most U.S. patients can’t get it.
Meanwhile, the respondents said they want opioid prescribing hemmed in. Almost two-thirds wanted refill limits and more frequent refill evaluations, and many agreed that there needs to be a weaning protocol before the drugs are even started. Some wanted to limit advertising.
Easton Jackson, MD, a primary care physician in West Valley City, Utah, who answered the survey, helped make the answers real by sharing his thoughts.
“We need to recognize that ... people don’t set out to get addicted to opioids ... We need to educate [patients] and assist them with their expectations. They need to understand that they’re going to have pain from surgery and injuries. Our goal isn’t to make them pain free. It’s to manage their pain,” he said.
“We as physicians need to write for fewer pills and in lower doses. We need to see our patients back sooner. If it’s not working, stop increasing the dose and instead taper the patient off the medication. We need to be familiar with the adjuvant therapies. As easy as it is to say, ‘send them all to the pain specialist,’ there simply aren’t enough of them around,” Dr. Easton said.
Physician respondents to InCrowd’s opioid survey have practiced an average of 25 years, and were scattered around the United States. They filled out the four-question survey during Oct. 27-28, 2016. They signed up to receive and answer InCrowd’s questions, and were paid nominally for their time.
Half (50%) of respondents estimated that they prescribed opioids to fewer than 10% of their patients, while 38% said they prescribed to less than half of their patients, and 12% estimated they prescribed opioids to more than half of their patients.
Almost a third of doctors blamed overprescribing for the opioid crisis, according to a survey of 225 U.S. primary care, emergency department, and pain management physicians by InCrowd, an online physician survey company.
Respondents said their own and other physicians’ overprescribing is the single biggest factor fueling the leap in opioid abuse over the past 5 years.
In short, the survey pointed out what front-line doctors think needs to be fixed as the nation combats prescription opioid abuse and the subsequent heroin epidemic. Their insights “should be a rallying cry” for changes in 2017, said epidemiologist Diane Hayes, PhD, president and cofounder of InCrowd.
Making pain the “fifth vital sign” and allowing patients to downgrade doctors on surveys if they don’t refill narcotic prescriptions compounded the situation. Lengthy waits for specialists with better pain options, many of whom are not covered by Medicaid or the Affordable Care Act, also added to the problem, survey respondents said.
“We’re caught in the middle” between the Joint Commission on Accreditation of Healthcare Organization’s fifth vital sign and overprescribing, a primary care doctor said.
Seventy-three percent of survey respondents said that they want opioid alternatives. They’re tired of trying to get the job done with NSAIDs, physical therapy, and exercise. About half recommend behavioral health interventions, while 20% recommend vitamin and herbal supplements. Only 10% recommend medical marijuana, probably because most U.S. patients can’t get it.
Meanwhile, the respondents said they want opioid prescribing hemmed in. Almost two-thirds wanted refill limits and more frequent refill evaluations, and many agreed that there needs to be a weaning protocol before the drugs are even started. Some wanted to limit advertising.
Easton Jackson, MD, a primary care physician in West Valley City, Utah, who answered the survey, helped make the answers real by sharing his thoughts.
“We need to recognize that ... people don’t set out to get addicted to opioids ... We need to educate [patients] and assist them with their expectations. They need to understand that they’re going to have pain from surgery and injuries. Our goal isn’t to make them pain free. It’s to manage their pain,” he said.
“We as physicians need to write for fewer pills and in lower doses. We need to see our patients back sooner. If it’s not working, stop increasing the dose and instead taper the patient off the medication. We need to be familiar with the adjuvant therapies. As easy as it is to say, ‘send them all to the pain specialist,’ there simply aren’t enough of them around,” Dr. Easton said.
Physician respondents to InCrowd’s opioid survey have practiced an average of 25 years, and were scattered around the United States. They filled out the four-question survey during Oct. 27-28, 2016. They signed up to receive and answer InCrowd’s questions, and were paid nominally for their time.
Half (50%) of respondents estimated that they prescribed opioids to fewer than 10% of their patients, while 38% said they prescribed to less than half of their patients, and 12% estimated they prescribed opioids to more than half of their patients.
Kaiser experience: A helping hand reduces COPD readmissions
LOS ANGELES – With a handful of common-sense steps, the Kaiser Permanente Los Angeles Medical Center reduced 30-day hospital readmissions for chronic obstructive pulmonary disease (COPD) from 17.4/1,000 in Dec. 2013 to 11.9/1,000 in Dec. 2015.
The 57 readmissions avoided in 2015 saved the medical center $700,359, according to a report at the annual meeting of the American College of Chest Physicians.
The quality improvement project – dubbed KP Breath – started in 2013 after staff realized their COPD readmission rates were significantly higher than other area hospitals, and likely to increase. “We knew we had a problem, and that if we did not address it, it was going to be out of control,” Mr. Cam said. There was also the risk of Centers for Medicare & Medicaid Services penalties for COPD readmissions.
Mr. Cam and his colleagues discovered several problems. “Leaving the hospital, [COPD patients] didn’t know what medication was for what, or their medication schedule. They didn’t know how to use their inhalers, and didn’t understand what the disease process was all about, and what it was doing to them,” he said.
There was little continuity of care after discharge; many patients didn’t even have a pulmonologist. Essentially, COPD patients were lost to follow-up until they returned to the emergency department with another exacerbation.
A rapid Plan, Do, Study, Act cycle was the first step; it identified solutions that would work based on COPD management guidelines and published studies. “They were all things that have been shown to reduce rehospitalizations,” said pulmonologist Luis Moreta-Sainz, MD, another key project member.
The team staggered their changes over 2 years. Pulmonary consults for acute exacerbation admissions shot up, and respiratory therapists started to stop by to educate almost every COPD patient about medication use, trigger avoidance, and other matters. Patients began watching educational videos from their bed.
Changes were made after discharge, too. “We felt strongly that pulmonary rehabilitation needed to be an integral part of care, and that patients had to be connected to the pulmonary clinic,” Dr. Moreta-Sainz said.
Patients were booked for a pulmonologist at the clinic soon after they left the hospital, and greeted there by their COPD navigator – a respiratory therapist operating at the top of their license – who bridged the gap between inpatient and outpatient care and oversaw their case, helping with medical, psychosocial, and palliative needs.
Patients were also channeled into pulmonary rehab, three sessions per week for 6-8 weeks, with additional sessions as needed. The outpatient education emphasized and expanded the inpatient lessons, and patients exercised on treadmills and other equipment. They learned how to use resistance bands at home to increase upper body strength and decrease disability. Kaiser increased the number of weekly pulmonary rehab slots from 8 to 64 to make it happen.
After rehab, patients were offered a pedometer to measure how many steps they walked, and a phone number to report it each day. Those who participated got a call from the navigator when they fell below targets.
It has all made a huge difference. Dr. Moreta-Sainz said he’d like to add in-home visits and family support groups, so caregivers know what to do if things head south.
The work was funded by Kaiser; Dr. Moreta-Sainz and Mr. Cam have no disclosures.
[email protected]
On a recent morning, two of my scheduled clinic patients were “no-shows.” Both of them were patients with COPD that I had recently cared for in-hospital for an exacerbation. While I know that snow may have played a role, there are other barriers to care, including lack of access to transportation, poor health literacy, and no effective health insurance.
On a recent morning, two of my scheduled clinic patients were “no-shows.” Both of them were patients with COPD that I had recently cared for in-hospital for an exacerbation. While I know that snow may have played a role, there are other barriers to care, including lack of access to transportation, poor health literacy, and no effective health insurance.
On a recent morning, two of my scheduled clinic patients were “no-shows.” Both of them were patients with COPD that I had recently cared for in-hospital for an exacerbation. While I know that snow may have played a role, there are other barriers to care, including lack of access to transportation, poor health literacy, and no effective health insurance.
LOS ANGELES – With a handful of common-sense steps, the Kaiser Permanente Los Angeles Medical Center reduced 30-day hospital readmissions for chronic obstructive pulmonary disease (COPD) from 17.4/1,000 in Dec. 2013 to 11.9/1,000 in Dec. 2015.
The 57 readmissions avoided in 2015 saved the medical center $700,359, according to a report at the annual meeting of the American College of Chest Physicians.
The quality improvement project – dubbed KP Breath – started in 2013 after staff realized their COPD readmission rates were significantly higher than other area hospitals, and likely to increase. “We knew we had a problem, and that if we did not address it, it was going to be out of control,” Mr. Cam said. There was also the risk of Centers for Medicare & Medicaid Services penalties for COPD readmissions.
Mr. Cam and his colleagues discovered several problems. “Leaving the hospital, [COPD patients] didn’t know what medication was for what, or their medication schedule. They didn’t know how to use their inhalers, and didn’t understand what the disease process was all about, and what it was doing to them,” he said.
There was little continuity of care after discharge; many patients didn’t even have a pulmonologist. Essentially, COPD patients were lost to follow-up until they returned to the emergency department with another exacerbation.
A rapid Plan, Do, Study, Act cycle was the first step; it identified solutions that would work based on COPD management guidelines and published studies. “They were all things that have been shown to reduce rehospitalizations,” said pulmonologist Luis Moreta-Sainz, MD, another key project member.
The team staggered their changes over 2 years. Pulmonary consults for acute exacerbation admissions shot up, and respiratory therapists started to stop by to educate almost every COPD patient about medication use, trigger avoidance, and other matters. Patients began watching educational videos from their bed.
Changes were made after discharge, too. “We felt strongly that pulmonary rehabilitation needed to be an integral part of care, and that patients had to be connected to the pulmonary clinic,” Dr. Moreta-Sainz said.
Patients were booked for a pulmonologist at the clinic soon after they left the hospital, and greeted there by their COPD navigator – a respiratory therapist operating at the top of their license – who bridged the gap between inpatient and outpatient care and oversaw their case, helping with medical, psychosocial, and palliative needs.
Patients were also channeled into pulmonary rehab, three sessions per week for 6-8 weeks, with additional sessions as needed. The outpatient education emphasized and expanded the inpatient lessons, and patients exercised on treadmills and other equipment. They learned how to use resistance bands at home to increase upper body strength and decrease disability. Kaiser increased the number of weekly pulmonary rehab slots from 8 to 64 to make it happen.
After rehab, patients were offered a pedometer to measure how many steps they walked, and a phone number to report it each day. Those who participated got a call from the navigator when they fell below targets.
It has all made a huge difference. Dr. Moreta-Sainz said he’d like to add in-home visits and family support groups, so caregivers know what to do if things head south.
The work was funded by Kaiser; Dr. Moreta-Sainz and Mr. Cam have no disclosures.
[email protected]
LOS ANGELES – With a handful of common-sense steps, the Kaiser Permanente Los Angeles Medical Center reduced 30-day hospital readmissions for chronic obstructive pulmonary disease (COPD) from 17.4/1,000 in Dec. 2013 to 11.9/1,000 in Dec. 2015.
The 57 readmissions avoided in 2015 saved the medical center $700,359, according to a report at the annual meeting of the American College of Chest Physicians.
The quality improvement project – dubbed KP Breath – started in 2013 after staff realized their COPD readmission rates were significantly higher than other area hospitals, and likely to increase. “We knew we had a problem, and that if we did not address it, it was going to be out of control,” Mr. Cam said. There was also the risk of Centers for Medicare & Medicaid Services penalties for COPD readmissions.
Mr. Cam and his colleagues discovered several problems. “Leaving the hospital, [COPD patients] didn’t know what medication was for what, or their medication schedule. They didn’t know how to use their inhalers, and didn’t understand what the disease process was all about, and what it was doing to them,” he said.
There was little continuity of care after discharge; many patients didn’t even have a pulmonologist. Essentially, COPD patients were lost to follow-up until they returned to the emergency department with another exacerbation.
A rapid Plan, Do, Study, Act cycle was the first step; it identified solutions that would work based on COPD management guidelines and published studies. “They were all things that have been shown to reduce rehospitalizations,” said pulmonologist Luis Moreta-Sainz, MD, another key project member.
The team staggered their changes over 2 years. Pulmonary consults for acute exacerbation admissions shot up, and respiratory therapists started to stop by to educate almost every COPD patient about medication use, trigger avoidance, and other matters. Patients began watching educational videos from their bed.
Changes were made after discharge, too. “We felt strongly that pulmonary rehabilitation needed to be an integral part of care, and that patients had to be connected to the pulmonary clinic,” Dr. Moreta-Sainz said.
Patients were booked for a pulmonologist at the clinic soon after they left the hospital, and greeted there by their COPD navigator – a respiratory therapist operating at the top of their license – who bridged the gap between inpatient and outpatient care and oversaw their case, helping with medical, psychosocial, and palliative needs.
Patients were also channeled into pulmonary rehab, three sessions per week for 6-8 weeks, with additional sessions as needed. The outpatient education emphasized and expanded the inpatient lessons, and patients exercised on treadmills and other equipment. They learned how to use resistance bands at home to increase upper body strength and decrease disability. Kaiser increased the number of weekly pulmonary rehab slots from 8 to 64 to make it happen.
After rehab, patients were offered a pedometer to measure how many steps they walked, and a phone number to report it each day. Those who participated got a call from the navigator when they fell below targets.
It has all made a huge difference. Dr. Moreta-Sainz said he’d like to add in-home visits and family support groups, so caregivers know what to do if things head south.
The work was funded by Kaiser; Dr. Moreta-Sainz and Mr. Cam have no disclosures.
[email protected]
AT CHEST 2016
USPSTF: Expand statin use beyond lipids to CVD risk
Use of statins for the primary prevention of cardiovascular disease should be based on overall cardiovascular disease risk, not necessarily blood lipid levels, according to new recommendations from the United States Preventive Services Task Force.
Despite widespread use in the elderly, USPSTF also said there’s insufficient evidence to recommend starting statins for cardiovascular disease (CVD) prevention in patients 76 years or older (JAMA. 2016 Nov;316[19]:1997-2007).
USPSTF now recommends low- to moderate-dose statins for primary prevention in adults 40-75 years old who have at least one CVD risk factor – dyslipidemia, diabetes, hypertension, or smoking – and a 10-year CVD event risk of at least 10% (B grade). It also recommended “selectively” offering low- to moderate-dose statins if patients have a 7.5%-10% risk (C grade). The task force recommends using the online American College of Cardiology/American Heart Association risk calculator.
A B grade denotes that USPSTF recommends the service as one where there is “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.” With a C grade, the task force recommends that the service be provided subject to professional judgment and patient preferences.
The new advices from USPSTF is consistent with the task force’s 2015 draft proposals, and is based on a pooled analysis of 19 randomized trials in 71,344 adults at risk for CVD but without prior events; 17 of the studies were sponsored at least in part by the companies that make statins. The median duration of follow-up was 3 years. Trials included atorvastatin (Lipitor) and six other statins from 1994-2016.
Few of the 19 trials enrolled patients older than 75 years, so “the balance of benefits and harms of initiating statin use for” primary prevention in elderly adults “cannot be determined.” The task force did not make a recommendation either way, and called for further investigation.
For younger patients, the scales tipped toward benefit when the group found no overall increased risk of cancer, liver damage, diabetes, or cognitive problems with statins for primary prevention. “Although muscle pain, soreness, or weakness are commonly reported with statin use, there were no statistically significant differences between the intervention and control groups for myalgia, myopathy, or rhabdomyolysis,” according to the task force.
Across the 19 trials, statin therapy was associated with a statistically significant 14% reduction in all-cause mortality; 31% reduction in cardiovascular mortality; 29% reduction in stroke; and a 36% reduction in myocardial infarction; 250 patients needed to be treated to prevent one death from any cause after 1-6 years, and 233 to prevent one cardiovascular death after 2-6 years.
The recommendations call for low to moderate doses because that’s what most of the studies used, and there were no clear benefits when trials stratified patients by dose.
USPSTF cautioned that “reliance on a risk calculator ... alone as a basis for prevention may be problematic, given its possible overestimation of risk in some populations” and noted that the benefits “of statin use may be linear according to a patient’s absolute risk level, and any cut points used are only population estimates of benefits.”
The recommendations do not pertain to adults with very high CVD risk, such as those with familial hypercholesterolemia or an LDL level greater than 190mg/dL, since they were excluded from primary prevention trials.
While the USPSTF was careful in its evidence review, “ the limitations of the evidence were not considered sufficiently, given the serious concerns about the harms of statins for primary prevention,” Rita Redberg, MD, and Mitchell Katz, MD, wrote in an editorial accompanying the recommendations.
“USPSTF also did not have access to patient-level data; they had to rely on peer-reviewed published reports,” according to the editorialists. “The actual trial data are largely held by the Cholesterol Treatment Trialists’ Collaboration on behalf of industry sponsors and have not been made available to other researchers, despite multiple requests over many years.” Further, many of the trials were industry sponsored.
Using the USPSTF data, only 2% of patients who take statins for 5 years will avoid a myocardial infarction; virtually all (98%) will not experience any benefit. At the same time, 5%-20% will experience side effects including rhabdomyolysis, cognitive dysfunction, and increased risk of diabetes (JAMA. 2016 Nov;316[19]:1979-81).
There are unintended consequences of widespread statin use in healthy persons, Dr. Redberg and Dr. Katz wrote. People taking statins are more likely to become obese and more sedentary over time, likely because they mistakenly think they do not need to eat a healthy diet and exercise.
The USPSTF analysis was funded by the Agency for Healthcare Research and Quality. Task force lead Kirsten Bibbins-Domingo, MD, PhD, of the University of California, San Francisco, has advised the Institute for Clinical and Economic Review, which is partly funded by industry, on the cost-effectiveness of lipid-lowering drugs. Another member reported comparing how well they worked. The other 15 task force members had no disclosures.
Dare cardiovascular experts consider a future in which there are near-universal statin recommendations for middle-aged adults?
[USPSTF analysis suggests] that statin recommendations could be based primarily on a patient’s underlying CVD risk rather than on his or her cholesterol level. Disseminating a treatment strategy based largely on CVD risk alone has been a difficult message for the clinical community to accept and implement.
Nearly a generation of physicians has considered high-cholesterol levels, rather than generalized CVD risk, the target for statin treatment. Only a minority of physicians consistently use complex, risk-based probabilistic calculations to determine therapy.
Several key questions deserve careful consideration. Should LDL be considered in treatment recommendations beyond CVD risk? The decision to use absolute risk to guide statin recommendations is based on the finding that the relative risk reduction seen with statin therapy is independent of baseline risk; thus, those with the highest absolute baseline CVD risk experience the greatest reduction in CVD events.
However, the relative risk reduction of lipid-lowering therapy is also proportional to mmol/dL reduction in LDL level. This supports the contention that those with the highest baseline LDL levels should benefit the most from treatment because they have the most potential decline in LDL with intervention. One way to reconcile these findings is to incorporate both LDL levels and CVD risk into treatment recommendations, as has been done in the European guidelines. This approach recognizes that the relative benefit of statins is proportional to LDL lowering but that the absolute treatment benefit is largely driven by baseline risk.
From the resource perspective, the vast majority of statins are now available as generic products and require limited monitoring, leading to quite modest therapeutic costs. For patients in the gray area not covered by the guidelines, clinicians should be cautioned against adopting either a “treat none” or a “treat all” strategy. Rather, gaps in the evidence provide opportunities for clinicians to practice the art of medicine and engage with patients in shared decision-making regarding strategies for CVD prevention.
Ann Marie Navar, MD, PhD, and Eric Peterson, MD, MPH, are both at the Duke Clinical Research Institute in Durham, N.C. Dr. Navar reported funding from Regeneron and Sanofi. Dr. Peterson reported funding from Merck, Sanofi, Regeneron, and AstraZeneca. They made their comments in an editorial to the USPSTF report (JAMA. 2016 Nov;316[19]:1981-3).
Dare cardiovascular experts consider a future in which there are near-universal statin recommendations for middle-aged adults?
[USPSTF analysis suggests] that statin recommendations could be based primarily on a patient’s underlying CVD risk rather than on his or her cholesterol level. Disseminating a treatment strategy based largely on CVD risk alone has been a difficult message for the clinical community to accept and implement.
Nearly a generation of physicians has considered high-cholesterol levels, rather than generalized CVD risk, the target for statin treatment. Only a minority of physicians consistently use complex, risk-based probabilistic calculations to determine therapy.
Several key questions deserve careful consideration. Should LDL be considered in treatment recommendations beyond CVD risk? The decision to use absolute risk to guide statin recommendations is based on the finding that the relative risk reduction seen with statin therapy is independent of baseline risk; thus, those with the highest absolute baseline CVD risk experience the greatest reduction in CVD events.
However, the relative risk reduction of lipid-lowering therapy is also proportional to mmol/dL reduction in LDL level. This supports the contention that those with the highest baseline LDL levels should benefit the most from treatment because they have the most potential decline in LDL with intervention. One way to reconcile these findings is to incorporate both LDL levels and CVD risk into treatment recommendations, as has been done in the European guidelines. This approach recognizes that the relative benefit of statins is proportional to LDL lowering but that the absolute treatment benefit is largely driven by baseline risk.
From the resource perspective, the vast majority of statins are now available as generic products and require limited monitoring, leading to quite modest therapeutic costs. For patients in the gray area not covered by the guidelines, clinicians should be cautioned against adopting either a “treat none” or a “treat all” strategy. Rather, gaps in the evidence provide opportunities for clinicians to practice the art of medicine and engage with patients in shared decision-making regarding strategies for CVD prevention.
Ann Marie Navar, MD, PhD, and Eric Peterson, MD, MPH, are both at the Duke Clinical Research Institute in Durham, N.C. Dr. Navar reported funding from Regeneron and Sanofi. Dr. Peterson reported funding from Merck, Sanofi, Regeneron, and AstraZeneca. They made their comments in an editorial to the USPSTF report (JAMA. 2016 Nov;316[19]:1981-3).
Dare cardiovascular experts consider a future in which there are near-universal statin recommendations for middle-aged adults?
[USPSTF analysis suggests] that statin recommendations could be based primarily on a patient’s underlying CVD risk rather than on his or her cholesterol level. Disseminating a treatment strategy based largely on CVD risk alone has been a difficult message for the clinical community to accept and implement.
Nearly a generation of physicians has considered high-cholesterol levels, rather than generalized CVD risk, the target for statin treatment. Only a minority of physicians consistently use complex, risk-based probabilistic calculations to determine therapy.
Several key questions deserve careful consideration. Should LDL be considered in treatment recommendations beyond CVD risk? The decision to use absolute risk to guide statin recommendations is based on the finding that the relative risk reduction seen with statin therapy is independent of baseline risk; thus, those with the highest absolute baseline CVD risk experience the greatest reduction in CVD events.
However, the relative risk reduction of lipid-lowering therapy is also proportional to mmol/dL reduction in LDL level. This supports the contention that those with the highest baseline LDL levels should benefit the most from treatment because they have the most potential decline in LDL with intervention. One way to reconcile these findings is to incorporate both LDL levels and CVD risk into treatment recommendations, as has been done in the European guidelines. This approach recognizes that the relative benefit of statins is proportional to LDL lowering but that the absolute treatment benefit is largely driven by baseline risk.
From the resource perspective, the vast majority of statins are now available as generic products and require limited monitoring, leading to quite modest therapeutic costs. For patients in the gray area not covered by the guidelines, clinicians should be cautioned against adopting either a “treat none” or a “treat all” strategy. Rather, gaps in the evidence provide opportunities for clinicians to practice the art of medicine and engage with patients in shared decision-making regarding strategies for CVD prevention.
Ann Marie Navar, MD, PhD, and Eric Peterson, MD, MPH, are both at the Duke Clinical Research Institute in Durham, N.C. Dr. Navar reported funding from Regeneron and Sanofi. Dr. Peterson reported funding from Merck, Sanofi, Regeneron, and AstraZeneca. They made their comments in an editorial to the USPSTF report (JAMA. 2016 Nov;316[19]:1981-3).
Use of statins for the primary prevention of cardiovascular disease should be based on overall cardiovascular disease risk, not necessarily blood lipid levels, according to new recommendations from the United States Preventive Services Task Force.
Despite widespread use in the elderly, USPSTF also said there’s insufficient evidence to recommend starting statins for cardiovascular disease (CVD) prevention in patients 76 years or older (JAMA. 2016 Nov;316[19]:1997-2007).
USPSTF now recommends low- to moderate-dose statins for primary prevention in adults 40-75 years old who have at least one CVD risk factor – dyslipidemia, diabetes, hypertension, or smoking – and a 10-year CVD event risk of at least 10% (B grade). It also recommended “selectively” offering low- to moderate-dose statins if patients have a 7.5%-10% risk (C grade). The task force recommends using the online American College of Cardiology/American Heart Association risk calculator.
A B grade denotes that USPSTF recommends the service as one where there is “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.” With a C grade, the task force recommends that the service be provided subject to professional judgment and patient preferences.
The new advices from USPSTF is consistent with the task force’s 2015 draft proposals, and is based on a pooled analysis of 19 randomized trials in 71,344 adults at risk for CVD but without prior events; 17 of the studies were sponsored at least in part by the companies that make statins. The median duration of follow-up was 3 years. Trials included atorvastatin (Lipitor) and six other statins from 1994-2016.
Few of the 19 trials enrolled patients older than 75 years, so “the balance of benefits and harms of initiating statin use for” primary prevention in elderly adults “cannot be determined.” The task force did not make a recommendation either way, and called for further investigation.
For younger patients, the scales tipped toward benefit when the group found no overall increased risk of cancer, liver damage, diabetes, or cognitive problems with statins for primary prevention. “Although muscle pain, soreness, or weakness are commonly reported with statin use, there were no statistically significant differences between the intervention and control groups for myalgia, myopathy, or rhabdomyolysis,” according to the task force.
Across the 19 trials, statin therapy was associated with a statistically significant 14% reduction in all-cause mortality; 31% reduction in cardiovascular mortality; 29% reduction in stroke; and a 36% reduction in myocardial infarction; 250 patients needed to be treated to prevent one death from any cause after 1-6 years, and 233 to prevent one cardiovascular death after 2-6 years.
The recommendations call for low to moderate doses because that’s what most of the studies used, and there were no clear benefits when trials stratified patients by dose.
USPSTF cautioned that “reliance on a risk calculator ... alone as a basis for prevention may be problematic, given its possible overestimation of risk in some populations” and noted that the benefits “of statin use may be linear according to a patient’s absolute risk level, and any cut points used are only population estimates of benefits.”
The recommendations do not pertain to adults with very high CVD risk, such as those with familial hypercholesterolemia or an LDL level greater than 190mg/dL, since they were excluded from primary prevention trials.
While the USPSTF was careful in its evidence review, “ the limitations of the evidence were not considered sufficiently, given the serious concerns about the harms of statins for primary prevention,” Rita Redberg, MD, and Mitchell Katz, MD, wrote in an editorial accompanying the recommendations.
“USPSTF also did not have access to patient-level data; they had to rely on peer-reviewed published reports,” according to the editorialists. “The actual trial data are largely held by the Cholesterol Treatment Trialists’ Collaboration on behalf of industry sponsors and have not been made available to other researchers, despite multiple requests over many years.” Further, many of the trials were industry sponsored.
Using the USPSTF data, only 2% of patients who take statins for 5 years will avoid a myocardial infarction; virtually all (98%) will not experience any benefit. At the same time, 5%-20% will experience side effects including rhabdomyolysis, cognitive dysfunction, and increased risk of diabetes (JAMA. 2016 Nov;316[19]:1979-81).
There are unintended consequences of widespread statin use in healthy persons, Dr. Redberg and Dr. Katz wrote. People taking statins are more likely to become obese and more sedentary over time, likely because they mistakenly think they do not need to eat a healthy diet and exercise.
The USPSTF analysis was funded by the Agency for Healthcare Research and Quality. Task force lead Kirsten Bibbins-Domingo, MD, PhD, of the University of California, San Francisco, has advised the Institute for Clinical and Economic Review, which is partly funded by industry, on the cost-effectiveness of lipid-lowering drugs. Another member reported comparing how well they worked. The other 15 task force members had no disclosures.
Use of statins for the primary prevention of cardiovascular disease should be based on overall cardiovascular disease risk, not necessarily blood lipid levels, according to new recommendations from the United States Preventive Services Task Force.
Despite widespread use in the elderly, USPSTF also said there’s insufficient evidence to recommend starting statins for cardiovascular disease (CVD) prevention in patients 76 years or older (JAMA. 2016 Nov;316[19]:1997-2007).
USPSTF now recommends low- to moderate-dose statins for primary prevention in adults 40-75 years old who have at least one CVD risk factor – dyslipidemia, diabetes, hypertension, or smoking – and a 10-year CVD event risk of at least 10% (B grade). It also recommended “selectively” offering low- to moderate-dose statins if patients have a 7.5%-10% risk (C grade). The task force recommends using the online American College of Cardiology/American Heart Association risk calculator.
A B grade denotes that USPSTF recommends the service as one where there is “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.” With a C grade, the task force recommends that the service be provided subject to professional judgment and patient preferences.
The new advices from USPSTF is consistent with the task force’s 2015 draft proposals, and is based on a pooled analysis of 19 randomized trials in 71,344 adults at risk for CVD but without prior events; 17 of the studies were sponsored at least in part by the companies that make statins. The median duration of follow-up was 3 years. Trials included atorvastatin (Lipitor) and six other statins from 1994-2016.
Few of the 19 trials enrolled patients older than 75 years, so “the balance of benefits and harms of initiating statin use for” primary prevention in elderly adults “cannot be determined.” The task force did not make a recommendation either way, and called for further investigation.
For younger patients, the scales tipped toward benefit when the group found no overall increased risk of cancer, liver damage, diabetes, or cognitive problems with statins for primary prevention. “Although muscle pain, soreness, or weakness are commonly reported with statin use, there were no statistically significant differences between the intervention and control groups for myalgia, myopathy, or rhabdomyolysis,” according to the task force.
Across the 19 trials, statin therapy was associated with a statistically significant 14% reduction in all-cause mortality; 31% reduction in cardiovascular mortality; 29% reduction in stroke; and a 36% reduction in myocardial infarction; 250 patients needed to be treated to prevent one death from any cause after 1-6 years, and 233 to prevent one cardiovascular death after 2-6 years.
The recommendations call for low to moderate doses because that’s what most of the studies used, and there were no clear benefits when trials stratified patients by dose.
USPSTF cautioned that “reliance on a risk calculator ... alone as a basis for prevention may be problematic, given its possible overestimation of risk in some populations” and noted that the benefits “of statin use may be linear according to a patient’s absolute risk level, and any cut points used are only population estimates of benefits.”
The recommendations do not pertain to adults with very high CVD risk, such as those with familial hypercholesterolemia or an LDL level greater than 190mg/dL, since they were excluded from primary prevention trials.
While the USPSTF was careful in its evidence review, “ the limitations of the evidence were not considered sufficiently, given the serious concerns about the harms of statins for primary prevention,” Rita Redberg, MD, and Mitchell Katz, MD, wrote in an editorial accompanying the recommendations.
“USPSTF also did not have access to patient-level data; they had to rely on peer-reviewed published reports,” according to the editorialists. “The actual trial data are largely held by the Cholesterol Treatment Trialists’ Collaboration on behalf of industry sponsors and have not been made available to other researchers, despite multiple requests over many years.” Further, many of the trials were industry sponsored.
Using the USPSTF data, only 2% of patients who take statins for 5 years will avoid a myocardial infarction; virtually all (98%) will not experience any benefit. At the same time, 5%-20% will experience side effects including rhabdomyolysis, cognitive dysfunction, and increased risk of diabetes (JAMA. 2016 Nov;316[19]:1979-81).
There are unintended consequences of widespread statin use in healthy persons, Dr. Redberg and Dr. Katz wrote. People taking statins are more likely to become obese and more sedentary over time, likely because they mistakenly think they do not need to eat a healthy diet and exercise.
The USPSTF analysis was funded by the Agency for Healthcare Research and Quality. Task force lead Kirsten Bibbins-Domingo, MD, PhD, of the University of California, San Francisco, has advised the Institute for Clinical and Economic Review, which is partly funded by industry, on the cost-effectiveness of lipid-lowering drugs. Another member reported comparing how well they worked. The other 15 task force members had no disclosures.
NCCN: Deliver vincristine by mini IV drip bag
Always dilute chemotherapy agent vincristine and administer it by mini IV-drip bag, instead of syringe, urges the National Comprehensive Cancer Network in a new campaign.
The goal of “Just Bag It” is to prevent a rare but uniformly fatal medical error – administering vincristine to the spinal fluid. When syringes are side by side – one with vincristine for IV push, another with a chemotherapeutic agent meant for push into the spinal fluid – it is just too easy to make a mistake. When administered intrathecally, vincristine causes ascending paralysis, neurological defects, and eventually death.
Despite all the warning labels and checks, “this still happens,” Marc Stewart, MD, cochair of the National Comprehensive Cancer Network (NCCN) Best Practices Committee, as well as medical director of the Seattle Cancer Care Alliance and professor of medicine at the University of Washington, said at a press conference.
Mini IV-drip bag administration will make it “virtually impossible. No physician would hook the bag up to a needle in someone’s spine” and even if they did, there wouldn’t be enough pressure in the bag to push vincristine in, he said.
The group has encouraged drip-bag delivery of vincristine for years, but only about half of hospitals have adopted the policy. The mistake happens so rarely – about 125 cases since the 1960s – “that the motivation for change is just not there.” Until somebody like NCCN calls it out in a high-profile campaign, “it’s not high on the radar screen,” Dr. Stewart said. It should be a relatively easy fix because bagging vincristine is not more costly. In general, the cost difference versus syringe “is going to be pennies,” he said.
“We challenge all medical centers, hospitals, and oncology practices around the nation and the world to implement this medication safety policy so this error never occurs again,” NCCN Chief Executive Officer Robert Carlson, MD, said in a press release. A medical oncologist, he witnessed the death of a 21-year-old patient after an intrathecal vincristine injection in 2005.
“Some health care providers may associate the use of an IV bag with a heightened risk of extravasation, but research shows that the risk of extravasation is extremely low (less than 0.05%) regardless of how vincristine is administered,” the press release noted.
Vincristine is widely used in treating patients with leukemia or lymphoma.
The safety of intravenous administration of vincristine has been a long-standing concern for anyone who participates in the management of patients with hematologic malignancies. As we all know, accidental intrathecal administration of vincristine is uniformly fatal.
At many centers, including ours, policies related to intravenous infusion of vesicants via a peripheral line have made the implementation of the safety recommendations difficult. It is not surprising that only 50% of hospitals surveyed by NCCN have fully implemented the mini-bag recommendation given the concern for extravasation. However, the newest ONS guidelines for vesicant administration allow for short-term infusions via a peripheral line. For our center, this support has been instrumental in allowing us to move to a practice with the recommended mini-bags. The NCCN “Just Bag It” campaign will likely help to move institutions such as ours to be in compliance with this important safety initiative.
Donna Capozzi, PharmD, is associate director of ambulatory services in the department of pharmacy at the Hospital of the University of Pennsylvania Perelman Center for Advanced Medicine in Philadelphia. She is on the editorial advisory board of Hematology News, a publication of this news company.
The safety of intravenous administration of vincristine has been a long-standing concern for anyone who participates in the management of patients with hematologic malignancies. As we all know, accidental intrathecal administration of vincristine is uniformly fatal.
At many centers, including ours, policies related to intravenous infusion of vesicants via a peripheral line have made the implementation of the safety recommendations difficult. It is not surprising that only 50% of hospitals surveyed by NCCN have fully implemented the mini-bag recommendation given the concern for extravasation. However, the newest ONS guidelines for vesicant administration allow for short-term infusions via a peripheral line. For our center, this support has been instrumental in allowing us to move to a practice with the recommended mini-bags. The NCCN “Just Bag It” campaign will likely help to move institutions such as ours to be in compliance with this important safety initiative.
Donna Capozzi, PharmD, is associate director of ambulatory services in the department of pharmacy at the Hospital of the University of Pennsylvania Perelman Center for Advanced Medicine in Philadelphia. She is on the editorial advisory board of Hematology News, a publication of this news company.
The safety of intravenous administration of vincristine has been a long-standing concern for anyone who participates in the management of patients with hematologic malignancies. As we all know, accidental intrathecal administration of vincristine is uniformly fatal.
At many centers, including ours, policies related to intravenous infusion of vesicants via a peripheral line have made the implementation of the safety recommendations difficult. It is not surprising that only 50% of hospitals surveyed by NCCN have fully implemented the mini-bag recommendation given the concern for extravasation. However, the newest ONS guidelines for vesicant administration allow for short-term infusions via a peripheral line. For our center, this support has been instrumental in allowing us to move to a practice with the recommended mini-bags. The NCCN “Just Bag It” campaign will likely help to move institutions such as ours to be in compliance with this important safety initiative.
Donna Capozzi, PharmD, is associate director of ambulatory services in the department of pharmacy at the Hospital of the University of Pennsylvania Perelman Center for Advanced Medicine in Philadelphia. She is on the editorial advisory board of Hematology News, a publication of this news company.
Always dilute chemotherapy agent vincristine and administer it by mini IV-drip bag, instead of syringe, urges the National Comprehensive Cancer Network in a new campaign.
The goal of “Just Bag It” is to prevent a rare but uniformly fatal medical error – administering vincristine to the spinal fluid. When syringes are side by side – one with vincristine for IV push, another with a chemotherapeutic agent meant for push into the spinal fluid – it is just too easy to make a mistake. When administered intrathecally, vincristine causes ascending paralysis, neurological defects, and eventually death.
Despite all the warning labels and checks, “this still happens,” Marc Stewart, MD, cochair of the National Comprehensive Cancer Network (NCCN) Best Practices Committee, as well as medical director of the Seattle Cancer Care Alliance and professor of medicine at the University of Washington, said at a press conference.
Mini IV-drip bag administration will make it “virtually impossible. No physician would hook the bag up to a needle in someone’s spine” and even if they did, there wouldn’t be enough pressure in the bag to push vincristine in, he said.
The group has encouraged drip-bag delivery of vincristine for years, but only about half of hospitals have adopted the policy. The mistake happens so rarely – about 125 cases since the 1960s – “that the motivation for change is just not there.” Until somebody like NCCN calls it out in a high-profile campaign, “it’s not high on the radar screen,” Dr. Stewart said. It should be a relatively easy fix because bagging vincristine is not more costly. In general, the cost difference versus syringe “is going to be pennies,” he said.
“We challenge all medical centers, hospitals, and oncology practices around the nation and the world to implement this medication safety policy so this error never occurs again,” NCCN Chief Executive Officer Robert Carlson, MD, said in a press release. A medical oncologist, he witnessed the death of a 21-year-old patient after an intrathecal vincristine injection in 2005.
“Some health care providers may associate the use of an IV bag with a heightened risk of extravasation, but research shows that the risk of extravasation is extremely low (less than 0.05%) regardless of how vincristine is administered,” the press release noted.
Vincristine is widely used in treating patients with leukemia or lymphoma.
Always dilute chemotherapy agent vincristine and administer it by mini IV-drip bag, instead of syringe, urges the National Comprehensive Cancer Network in a new campaign.
The goal of “Just Bag It” is to prevent a rare but uniformly fatal medical error – administering vincristine to the spinal fluid. When syringes are side by side – one with vincristine for IV push, another with a chemotherapeutic agent meant for push into the spinal fluid – it is just too easy to make a mistake. When administered intrathecally, vincristine causes ascending paralysis, neurological defects, and eventually death.
Despite all the warning labels and checks, “this still happens,” Marc Stewart, MD, cochair of the National Comprehensive Cancer Network (NCCN) Best Practices Committee, as well as medical director of the Seattle Cancer Care Alliance and professor of medicine at the University of Washington, said at a press conference.
Mini IV-drip bag administration will make it “virtually impossible. No physician would hook the bag up to a needle in someone’s spine” and even if they did, there wouldn’t be enough pressure in the bag to push vincristine in, he said.
The group has encouraged drip-bag delivery of vincristine for years, but only about half of hospitals have adopted the policy. The mistake happens so rarely – about 125 cases since the 1960s – “that the motivation for change is just not there.” Until somebody like NCCN calls it out in a high-profile campaign, “it’s not high on the radar screen,” Dr. Stewart said. It should be a relatively easy fix because bagging vincristine is not more costly. In general, the cost difference versus syringe “is going to be pennies,” he said.
“We challenge all medical centers, hospitals, and oncology practices around the nation and the world to implement this medication safety policy so this error never occurs again,” NCCN Chief Executive Officer Robert Carlson, MD, said in a press release. A medical oncologist, he witnessed the death of a 21-year-old patient after an intrathecal vincristine injection in 2005.
“Some health care providers may associate the use of an IV bag with a heightened risk of extravasation, but research shows that the risk of extravasation is extremely low (less than 0.05%) regardless of how vincristine is administered,” the press release noted.
Vincristine is widely used in treating patients with leukemia or lymphoma.
Combine qSOFA and SIRS for best sepsis score
LOS ANGELES – Instead of replacing the Systemic Inflammatory Response Syndrome (SIRS) score with the new quick Sequential Organ Failure Assessment (qSOFA) score to identify severe sepsis patients, it might be best to use both, according to two studies presented at the American College of Chest Physicians annual meeting.
The gold standard 3rd International Consensus Definitions for Sepsis and Septic Shock Task Force recently introduced qSOFA to replace SIRS, in part because SIRS is too sensitive. With criteria that include a temperature above 38° C; a heart rate above 90 bpm, and a respiratory rate above 20 breaths per minute, it’s possible to score positive on SIRS by walking up a flight of stairs, audience members at the study presentations noted.
The first study at the meeting session – a prospective cohort of 152 patients scored by both systems within 8 hours of ICU admission at the New York–Presbyterian Hospital – found that qSOFA was slightly better at predicting in-hospital mortality and ICU-free days, but no better than SIRS at predicting ventilator- or organ failure–free days.
However, of the 36% of patients (55) who met only one of the three qSOFA criteria - a respiratory rate of 22 breaths per minute, altered mental status, or a systolic blood pressure of 100 mg Hg or less - 6% (3) died in the hospital. Of those patients, two-thirds (2) were SIRS positive, meaning that they met two or more SIRS criteria.
“Having a borderline qSOFA of 1 point, which is considered negative, with the addition of having SIRS criteria, should raise concerns that patients need further evaluation. SIRS criteria should not be [entirely] discarded” in favor of qSOFA, said lead investigator Eli Finkelsztein, MD, of the New York–Presbyterian Hospital in New York City
The second study – a review of 6,811 severe sepsis/septic shock patients scored by both systems within 3 hours of emergency department admission at the University of Kansas Hospital emergency department in Kansas City – found that the two scores performed largely the same when it came to predicting ICU admission and 30-day mortality, but that people who met two or more criteria in both systems were of special concern.
Twenty-five percent of patients (1,713) scored 2 or more on both SIRS and qSOFA. These patients were more likely to be admitted to the ICU and be readmitted to the hospital after a month, compared with those patients who were positive in only one scoring system or negative in both. Additional factors associated with these patients were that they had the longest ICU and hospital lengths of stay. Two hundred (12%) of these patients scoring 2 or more on both SIRS and qSOFA died within 30 days.
“SIRS criteria continue to be more sensitive at identifying severe sepsis, but they are equally as accurate [as qSOFA criteria] at predicting adverse patient outcomes,” said lead investigator and Kansas University medical student Amanda Deis.
SIRS and qSOFA take only a few seconds to assess at the bedside. Using both builds “a clinical picture,” she said.
There was no industry funding for the work, and the investigators had no relevant financial disclosures.
Everybody got fed up with SIRS because it’s overly sensitive, but now we’ve swung in the other direction. It’s absolutely true that qSOFA is more specific, but one of the presenters had a 6% rate of qSOFA missing sick patients.
We want to be somewhere in the middle in terms of not missing too many of these cases. I thought 6% was reasonable, but others may not.
Zaza Cohen, MD, is the director of critical care at Mountainside Hospital in Montclair, N.J. He moderated - but was not involved with - the two studies.
Everybody got fed up with SIRS because it’s overly sensitive, but now we’ve swung in the other direction. It’s absolutely true that qSOFA is more specific, but one of the presenters had a 6% rate of qSOFA missing sick patients.
We want to be somewhere in the middle in terms of not missing too many of these cases. I thought 6% was reasonable, but others may not.
Zaza Cohen, MD, is the director of critical care at Mountainside Hospital in Montclair, N.J. He moderated - but was not involved with - the two studies.
Everybody got fed up with SIRS because it’s overly sensitive, but now we’ve swung in the other direction. It’s absolutely true that qSOFA is more specific, but one of the presenters had a 6% rate of qSOFA missing sick patients.
We want to be somewhere in the middle in terms of not missing too many of these cases. I thought 6% was reasonable, but others may not.
Zaza Cohen, MD, is the director of critical care at Mountainside Hospital in Montclair, N.J. He moderated - but was not involved with - the two studies.
LOS ANGELES – Instead of replacing the Systemic Inflammatory Response Syndrome (SIRS) score with the new quick Sequential Organ Failure Assessment (qSOFA) score to identify severe sepsis patients, it might be best to use both, according to two studies presented at the American College of Chest Physicians annual meeting.
The gold standard 3rd International Consensus Definitions for Sepsis and Septic Shock Task Force recently introduced qSOFA to replace SIRS, in part because SIRS is too sensitive. With criteria that include a temperature above 38° C; a heart rate above 90 bpm, and a respiratory rate above 20 breaths per minute, it’s possible to score positive on SIRS by walking up a flight of stairs, audience members at the study presentations noted.
The first study at the meeting session – a prospective cohort of 152 patients scored by both systems within 8 hours of ICU admission at the New York–Presbyterian Hospital – found that qSOFA was slightly better at predicting in-hospital mortality and ICU-free days, but no better than SIRS at predicting ventilator- or organ failure–free days.
However, of the 36% of patients (55) who met only one of the three qSOFA criteria - a respiratory rate of 22 breaths per minute, altered mental status, or a systolic blood pressure of 100 mg Hg or less - 6% (3) died in the hospital. Of those patients, two-thirds (2) were SIRS positive, meaning that they met two or more SIRS criteria.
“Having a borderline qSOFA of 1 point, which is considered negative, with the addition of having SIRS criteria, should raise concerns that patients need further evaluation. SIRS criteria should not be [entirely] discarded” in favor of qSOFA, said lead investigator Eli Finkelsztein, MD, of the New York–Presbyterian Hospital in New York City
The second study – a review of 6,811 severe sepsis/septic shock patients scored by both systems within 3 hours of emergency department admission at the University of Kansas Hospital emergency department in Kansas City – found that the two scores performed largely the same when it came to predicting ICU admission and 30-day mortality, but that people who met two or more criteria in both systems were of special concern.
Twenty-five percent of patients (1,713) scored 2 or more on both SIRS and qSOFA. These patients were more likely to be admitted to the ICU and be readmitted to the hospital after a month, compared with those patients who were positive in only one scoring system or negative in both. Additional factors associated with these patients were that they had the longest ICU and hospital lengths of stay. Two hundred (12%) of these patients scoring 2 or more on both SIRS and qSOFA died within 30 days.
“SIRS criteria continue to be more sensitive at identifying severe sepsis, but they are equally as accurate [as qSOFA criteria] at predicting adverse patient outcomes,” said lead investigator and Kansas University medical student Amanda Deis.
SIRS and qSOFA take only a few seconds to assess at the bedside. Using both builds “a clinical picture,” she said.
There was no industry funding for the work, and the investigators had no relevant financial disclosures.
LOS ANGELES – Instead of replacing the Systemic Inflammatory Response Syndrome (SIRS) score with the new quick Sequential Organ Failure Assessment (qSOFA) score to identify severe sepsis patients, it might be best to use both, according to two studies presented at the American College of Chest Physicians annual meeting.
The gold standard 3rd International Consensus Definitions for Sepsis and Septic Shock Task Force recently introduced qSOFA to replace SIRS, in part because SIRS is too sensitive. With criteria that include a temperature above 38° C; a heart rate above 90 bpm, and a respiratory rate above 20 breaths per minute, it’s possible to score positive on SIRS by walking up a flight of stairs, audience members at the study presentations noted.
The first study at the meeting session – a prospective cohort of 152 patients scored by both systems within 8 hours of ICU admission at the New York–Presbyterian Hospital – found that qSOFA was slightly better at predicting in-hospital mortality and ICU-free days, but no better than SIRS at predicting ventilator- or organ failure–free days.
However, of the 36% of patients (55) who met only one of the three qSOFA criteria - a respiratory rate of 22 breaths per minute, altered mental status, or a systolic blood pressure of 100 mg Hg or less - 6% (3) died in the hospital. Of those patients, two-thirds (2) were SIRS positive, meaning that they met two or more SIRS criteria.
“Having a borderline qSOFA of 1 point, which is considered negative, with the addition of having SIRS criteria, should raise concerns that patients need further evaluation. SIRS criteria should not be [entirely] discarded” in favor of qSOFA, said lead investigator Eli Finkelsztein, MD, of the New York–Presbyterian Hospital in New York City
The second study – a review of 6,811 severe sepsis/septic shock patients scored by both systems within 3 hours of emergency department admission at the University of Kansas Hospital emergency department in Kansas City – found that the two scores performed largely the same when it came to predicting ICU admission and 30-day mortality, but that people who met two or more criteria in both systems were of special concern.
Twenty-five percent of patients (1,713) scored 2 or more on both SIRS and qSOFA. These patients were more likely to be admitted to the ICU and be readmitted to the hospital after a month, compared with those patients who were positive in only one scoring system or negative in both. Additional factors associated with these patients were that they had the longest ICU and hospital lengths of stay. Two hundred (12%) of these patients scoring 2 or more on both SIRS and qSOFA died within 30 days.
“SIRS criteria continue to be more sensitive at identifying severe sepsis, but they are equally as accurate [as qSOFA criteria] at predicting adverse patient outcomes,” said lead investigator and Kansas University medical student Amanda Deis.
SIRS and qSOFA take only a few seconds to assess at the bedside. Using both builds “a clinical picture,” she said.
There was no industry funding for the work, and the investigators had no relevant financial disclosures.
AT CHEST 2016
Key clinical point:
Major finding: Of the 36% of patients who met only one of the three qSOFA criteria, 6% died in the hospital. Of those patients, two-thirds were SIRS positive, meaning that they met two or more SIRS criteria.
Data source: Two studies of almost 7,000 septic patients.
Disclosures: There was no industry funding for the work, and the investigators had no relevant financial disclosures.