User login
Medicaid expansion predicted to further crowd EDs
CHICAGO – Emergency department visits are projected to increase annually by more than 2 million visits with expansion of Medicaid eligibility under the Affordable Care Act, Christina M. Cutter reported at the annual meeting of the American College of Emergency Physicians.
Similarly, primary care–treatable ED visits – visits that require immediate care but could be treated in an outpatient setting – are projected to increase by nearly 1 million visits nationally among new adult Medicaid beneficiaries.
The projections, based on results from the Oregon Health Insurance Experiment (OHIE), amplify concerns that the nation’s already strained ED system will be further challenged by Medicaid expansion under the ACA, said Ms. Cutter, a medical student at the University of Colorado, Aurora.
To estimate ED utilization by Medicaid beneficiaries following the expansion of Medicaid eligibility under the Affordable Care Act, Ms. Cutter and her colleague Dr. Arjun K. Venkatesh, of Yale University in New Haven, Conn., conducted a cross-sectional analysis of population-level data derived from the American Community Survey, National Hospital Ambulatory Medical Care Survey, and Kaiser Family Foundation.

Initially, they approximated baseline ED visits at the state-level by adults aged 18-64 years with Medicaid. To estimate state and national changes in ED visitation and state-level increases in primary care–treatable conditions, the researchers assumed that all states expanding Medicaid would have increases in ED visits that were proportionate to those observed in the OHIE.
Primary care–treatable ED visits were defined using the N.Y.U. Billings Algorithm. State-level data on measures of emergency care access were obtained from the Centers for Medicare & Medicaid Services Hospital Compare file, AMA Physician Professional file, Health Resources and Sservices Administration Geospatial Data Warehouse, and the American College of Emergency Physicians Emergency Care Environment Report Card. States expanding Medicaid coverage were compared with those not expanding Medicaid.
Based on these measures, the researchers estimated a total of 80,819,573 ED visits in 2012 for the U.S. adult population, of which 25,054,068 were adults with Medicaid. They estimated 8,749,000 additional adult enrollees in the 28 states expanding Medicaid eligibility.
Based on projections using the OHIE, ED visits will increase by 2,143,260 visits in states expanding eligibility and primary care–treatable ED visits will increase by 943,034 nationally among new adult Medicaid beneficiaries, according to the researchers.
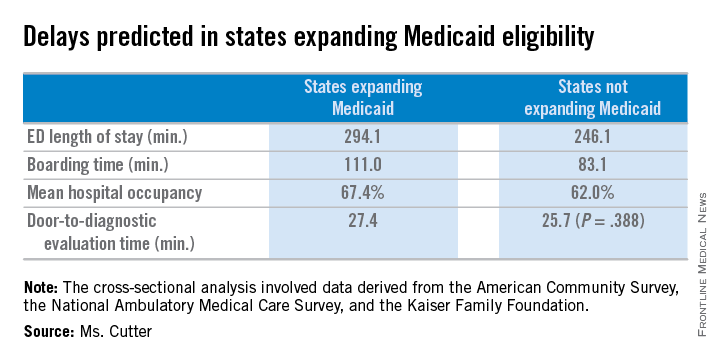
States expanding Medicaid eligibility have longer ED length of stay (294.1 vs. 246.1 minutes, P = .007), longer boarding time (111.0 vs. 83.1 minutes, P = .007), and higher mean hospital occupancy (67.4 vs. 62.0 per 100 staffed beds, P = .012), but no difference in door-to-diagnostic evaluation time (27.4 vs. 25.7 minutes, P = .388).
States expanding eligibility also have fewer EDs (14.8 vs. 24.0/1,000,000 population, P = .004), but are less likely to have shortages of mental health provider shortages (0.6 vs. 1.0 needed full-time equivalents, P = .021) and have no difference in psychiatric beds (24.4 vs. 28.2/100,000 population, P = .557). States expanding Medicaid also have a greater supply of emergency physicians (15 vs. 11.7/100,000 population, P = .004), Ms. Cutter reported.
CHICAGO – Emergency department visits are projected to increase annually by more than 2 million visits with expansion of Medicaid eligibility under the Affordable Care Act, Christina M. Cutter reported at the annual meeting of the American College of Emergency Physicians.
Similarly, primary care–treatable ED visits – visits that require immediate care but could be treated in an outpatient setting – are projected to increase by nearly 1 million visits nationally among new adult Medicaid beneficiaries.
The projections, based on results from the Oregon Health Insurance Experiment (OHIE), amplify concerns that the nation’s already strained ED system will be further challenged by Medicaid expansion under the ACA, said Ms. Cutter, a medical student at the University of Colorado, Aurora.
To estimate ED utilization by Medicaid beneficiaries following the expansion of Medicaid eligibility under the Affordable Care Act, Ms. Cutter and her colleague Dr. Arjun K. Venkatesh, of Yale University in New Haven, Conn., conducted a cross-sectional analysis of population-level data derived from the American Community Survey, National Hospital Ambulatory Medical Care Survey, and Kaiser Family Foundation.

Initially, they approximated baseline ED visits at the state-level by adults aged 18-64 years with Medicaid. To estimate state and national changes in ED visitation and state-level increases in primary care–treatable conditions, the researchers assumed that all states expanding Medicaid would have increases in ED visits that were proportionate to those observed in the OHIE.
Primary care–treatable ED visits were defined using the N.Y.U. Billings Algorithm. State-level data on measures of emergency care access were obtained from the Centers for Medicare & Medicaid Services Hospital Compare file, AMA Physician Professional file, Health Resources and Sservices Administration Geospatial Data Warehouse, and the American College of Emergency Physicians Emergency Care Environment Report Card. States expanding Medicaid coverage were compared with those not expanding Medicaid.
Based on these measures, the researchers estimated a total of 80,819,573 ED visits in 2012 for the U.S. adult population, of which 25,054,068 were adults with Medicaid. They estimated 8,749,000 additional adult enrollees in the 28 states expanding Medicaid eligibility.
Based on projections using the OHIE, ED visits will increase by 2,143,260 visits in states expanding eligibility and primary care–treatable ED visits will increase by 943,034 nationally among new adult Medicaid beneficiaries, according to the researchers.
States expanding Medicaid eligibility have longer ED length of stay (294.1 vs. 246.1 minutes, P = .007), longer boarding time (111.0 vs. 83.1 minutes, P = .007), and higher mean hospital occupancy (67.4 vs. 62.0 per 100 staffed beds, P = .012), but no difference in door-to-diagnostic evaluation time (27.4 vs. 25.7 minutes, P = .388).
States expanding eligibility also have fewer EDs (14.8 vs. 24.0/1,000,000 population, P = .004), but are less likely to have shortages of mental health provider shortages (0.6 vs. 1.0 needed full-time equivalents, P = .021) and have no difference in psychiatric beds (24.4 vs. 28.2/100,000 population, P = .557). States expanding Medicaid also have a greater supply of emergency physicians (15 vs. 11.7/100,000 population, P = .004), Ms. Cutter reported.
CHICAGO – Emergency department visits are projected to increase annually by more than 2 million visits with expansion of Medicaid eligibility under the Affordable Care Act, Christina M. Cutter reported at the annual meeting of the American College of Emergency Physicians.
Similarly, primary care–treatable ED visits – visits that require immediate care but could be treated in an outpatient setting – are projected to increase by nearly 1 million visits nationally among new adult Medicaid beneficiaries.
The projections, based on results from the Oregon Health Insurance Experiment (OHIE), amplify concerns that the nation’s already strained ED system will be further challenged by Medicaid expansion under the ACA, said Ms. Cutter, a medical student at the University of Colorado, Aurora.
To estimate ED utilization by Medicaid beneficiaries following the expansion of Medicaid eligibility under the Affordable Care Act, Ms. Cutter and her colleague Dr. Arjun K. Venkatesh, of Yale University in New Haven, Conn., conducted a cross-sectional analysis of population-level data derived from the American Community Survey, National Hospital Ambulatory Medical Care Survey, and Kaiser Family Foundation.

Initially, they approximated baseline ED visits at the state-level by adults aged 18-64 years with Medicaid. To estimate state and national changes in ED visitation and state-level increases in primary care–treatable conditions, the researchers assumed that all states expanding Medicaid would have increases in ED visits that were proportionate to those observed in the OHIE.
Primary care–treatable ED visits were defined using the N.Y.U. Billings Algorithm. State-level data on measures of emergency care access were obtained from the Centers for Medicare & Medicaid Services Hospital Compare file, AMA Physician Professional file, Health Resources and Sservices Administration Geospatial Data Warehouse, and the American College of Emergency Physicians Emergency Care Environment Report Card. States expanding Medicaid coverage were compared with those not expanding Medicaid.
Based on these measures, the researchers estimated a total of 80,819,573 ED visits in 2012 for the U.S. adult population, of which 25,054,068 were adults with Medicaid. They estimated 8,749,000 additional adult enrollees in the 28 states expanding Medicaid eligibility.
Based on projections using the OHIE, ED visits will increase by 2,143,260 visits in states expanding eligibility and primary care–treatable ED visits will increase by 943,034 nationally among new adult Medicaid beneficiaries, according to the researchers.
States expanding Medicaid eligibility have longer ED length of stay (294.1 vs. 246.1 minutes, P = .007), longer boarding time (111.0 vs. 83.1 minutes, P = .007), and higher mean hospital occupancy (67.4 vs. 62.0 per 100 staffed beds, P = .012), but no difference in door-to-diagnostic evaluation time (27.4 vs. 25.7 minutes, P = .388).
States expanding eligibility also have fewer EDs (14.8 vs. 24.0/1,000,000 population, P = .004), but are less likely to have shortages of mental health provider shortages (0.6 vs. 1.0 needed full-time equivalents, P = .021) and have no difference in psychiatric beds (24.4 vs. 28.2/100,000 population, P = .557). States expanding Medicaid also have a greater supply of emergency physicians (15 vs. 11.7/100,000 population, P = .004), Ms. Cutter reported.
AT ACEP 2014
Key clinical point: ED lengths of stay are predicted to become longer as more states expand Medicaid coverage.
Major finding: States expanding Medicaid eligibility have longer mean ED lengths of stay (294.1 vs. 246.1 minutes, P = .007), longer mean boarding time (111.0 vs. 83.1 minutes, P = .007), and higher mean hospital occupancy (67.4 vs. 62.0 per 100 staffed beds, P = .012).
Data source: Historical data from the Oregon Health Insurance Experiment.
Disclosures: Ms. Cutter had no relevant financial disclosures.
High-dose opioids mainly prescribed in offices, not the ED
CHICAGO – When opioids were prescribed for noncancer pain in emergency departments, the doses and numbers of pills prescribed were lower than those prescribed in office-based practices, Dr. Michael D. Menchine reported at the annual meeting of the American College of Emergency Physicians.
One in 400 opioid prescriptions written in the ED were for at least 100 morphine milligram equivalent (MME) daily doses; in office settings, the comparable rate was 1 in 39 prescriptions, based on an analysis of the Medical Expenditure Panel Survey(1997-2011), a nationally representative subsurvey of the annual National Health Interview Survey.
The findings indicate efforts to reduce risky opioid prescribing should focus mainly on office-based and not ED-based settings, said Dr. Menchine of the Leonard D. Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles. They also raise questions about whether emergency physicians are underprescribing opioids.
For their study, the researchers examined where each opioid prescription was generated and used the National Drug Code to determine the exact compounds and doses prescribed, which were converted to MME doses. Patients with ICD-9 codes indicating a history of malignancy were excluded from the study.
The number of high-risk prescriptions, defined as doses of at least 100 MME daily, were identified by source of care for 44,313 unique individuals receiving 164,406 opioid prescriptions during the study period. The mean age of individuals included in the study was 48 years and 63% of the study participants were female.
After researchers adjusted for patient demographic features and diagnosis categories, the average opioid prescription originating from the ED dispensed 44% fewer pills than prescriptions from office visits (95% confidence interval, –0.47 to –0.41; P < .001). On average, the compound prescribed from the ED had 17% lower MME than did those from office visits (95% CI, –0.2 to –0.15; P < .001).
Overall, 1.9% of all opioid prescriptions were for more than 100 MME daily. However, compared with office settings, ED prescriptions were much less likely to be for greater than 100 MME per day (0.26% vs. 2.62% [odds ratio, 0.09; 95% CI 0.05-0.19, P < .001]).
On Twitter @maryjodales
CHICAGO – When opioids were prescribed for noncancer pain in emergency departments, the doses and numbers of pills prescribed were lower than those prescribed in office-based practices, Dr. Michael D. Menchine reported at the annual meeting of the American College of Emergency Physicians.
One in 400 opioid prescriptions written in the ED were for at least 100 morphine milligram equivalent (MME) daily doses; in office settings, the comparable rate was 1 in 39 prescriptions, based on an analysis of the Medical Expenditure Panel Survey(1997-2011), a nationally representative subsurvey of the annual National Health Interview Survey.
The findings indicate efforts to reduce risky opioid prescribing should focus mainly on office-based and not ED-based settings, said Dr. Menchine of the Leonard D. Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles. They also raise questions about whether emergency physicians are underprescribing opioids.
For their study, the researchers examined where each opioid prescription was generated and used the National Drug Code to determine the exact compounds and doses prescribed, which were converted to MME doses. Patients with ICD-9 codes indicating a history of malignancy were excluded from the study.
The number of high-risk prescriptions, defined as doses of at least 100 MME daily, were identified by source of care for 44,313 unique individuals receiving 164,406 opioid prescriptions during the study period. The mean age of individuals included in the study was 48 years and 63% of the study participants were female.
After researchers adjusted for patient demographic features and diagnosis categories, the average opioid prescription originating from the ED dispensed 44% fewer pills than prescriptions from office visits (95% confidence interval, –0.47 to –0.41; P < .001). On average, the compound prescribed from the ED had 17% lower MME than did those from office visits (95% CI, –0.2 to –0.15; P < .001).
Overall, 1.9% of all opioid prescriptions were for more than 100 MME daily. However, compared with office settings, ED prescriptions were much less likely to be for greater than 100 MME per day (0.26% vs. 2.62% [odds ratio, 0.09; 95% CI 0.05-0.19, P < .001]).
On Twitter @maryjodales
CHICAGO – When opioids were prescribed for noncancer pain in emergency departments, the doses and numbers of pills prescribed were lower than those prescribed in office-based practices, Dr. Michael D. Menchine reported at the annual meeting of the American College of Emergency Physicians.
One in 400 opioid prescriptions written in the ED were for at least 100 morphine milligram equivalent (MME) daily doses; in office settings, the comparable rate was 1 in 39 prescriptions, based on an analysis of the Medical Expenditure Panel Survey(1997-2011), a nationally representative subsurvey of the annual National Health Interview Survey.
The findings indicate efforts to reduce risky opioid prescribing should focus mainly on office-based and not ED-based settings, said Dr. Menchine of the Leonard D. Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles. They also raise questions about whether emergency physicians are underprescribing opioids.
For their study, the researchers examined where each opioid prescription was generated and used the National Drug Code to determine the exact compounds and doses prescribed, which were converted to MME doses. Patients with ICD-9 codes indicating a history of malignancy were excluded from the study.
The number of high-risk prescriptions, defined as doses of at least 100 MME daily, were identified by source of care for 44,313 unique individuals receiving 164,406 opioid prescriptions during the study period. The mean age of individuals included in the study was 48 years and 63% of the study participants were female.
After researchers adjusted for patient demographic features and diagnosis categories, the average opioid prescription originating from the ED dispensed 44% fewer pills than prescriptions from office visits (95% confidence interval, –0.47 to –0.41; P < .001). On average, the compound prescribed from the ED had 17% lower MME than did those from office visits (95% CI, –0.2 to –0.15; P < .001).
Overall, 1.9% of all opioid prescriptions were for more than 100 MME daily. However, compared with office settings, ED prescriptions were much less likely to be for greater than 100 MME per day (0.26% vs. 2.62% [odds ratio, 0.09; 95% CI 0.05-0.19, P < .001]).
On Twitter @maryjodales
AT ACEP 2014
Key clinical point: Policy efforts to reduce risky opioid prescribing should focus on office-based settings.
Major finding: High-dose opioids are prescribed for noncancer pain in 1 of 400 opioid prescriptions written in the emergency department and in 1 of 39 office prescriptions.
Data source: An analysis of the Medical Expenditure Panel Survey (1997-2011), a nationally representative subsurvey of the annual National Health Interview Survey.
Disclosures: Dr. Menchine had no relevant financial disclosures.
Hospital admissions account for most emergency care–directed costs
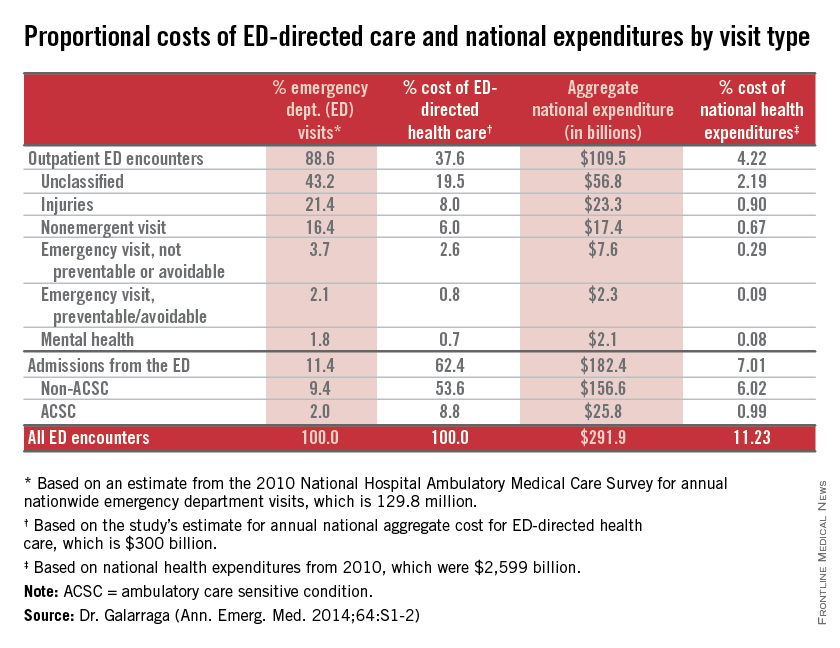
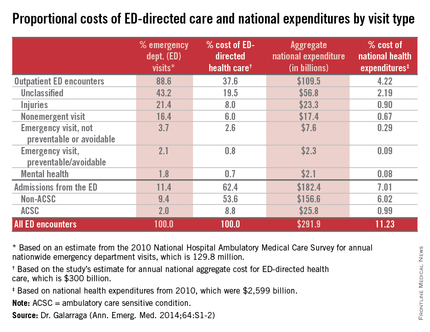
CHICAGO – About 11% of national health expenditures are related to emergency department–directed health care, with 7% of all expenditures from ED admissions and 4% of all expenditures from outpatient ED visits, Dr. Jessica Galarraga reported at the annual meeting of the American College of Emergency Physicians.
Based on volume, hospital admissions from the ED were about 11% of all emergency visits; however, they accounted for more than $182 billion or 62% of the costs of ED-directed care, said Dr. Galarraga of the George Washington University in Washington.
Increasingly, the ED is the “front door” for hospital care, as noted in a RAND report published last year.
With ED-directed health care comprising more than 11% of national health expenditures (NHE), it is important for ED providers to work collaboratively with other parts of the health care system to safely reduce health care costs, Dr. Galarraga said.
Potentially avoidable ED-directed encounters, including nonemergent visits, emergent but preventable visits, and ambulatory care sensitive condition (ACSC) admissions, together accounted for 1.8% of NHE. “Given the relative importance of ED admissions over outpatient encounters, making use of the emergency physician’s role as a gatekeeper to admission may be the most effective way to reduce costs,” she said.
Dr. Galarraga and her colleagues examined the costs of ED care and ED-directed health care by visit categories to provide a better understanding of the potential magnitude of cost savings from interventions directed at ED encounters.
|
The 2010 data from the Medical Expenditure Panel Survey (MEPS) were used to determine mean per-visit payments and charges for ED outpatient and inpatient encounters by visit type. National Hospital Ambulatory Medical Care Survey (NHAMCS) and the Healthcare Cost and Utilization Project (HCUP) were used to estimate the aggregate expenditures and proportional costs of NHE by each visit category. Outpatient encounters were categorized based on the New York University (NYU) classification and inpatient encounters by ACSC versus non-ACSC.
Among all encounters involving ED-directed health care, the greatest mean per-visit expenditures were attributable to hospital admissions from the ED, with mean payments of $12,817 and $9,708 for non-ACSC and ACSC admissions, respectively. Nonurgent outpatient ED visits generated the lowest mean per-visit expenditure of $819, even compared with all other outpatient ED encounters.
Dr. Galarraga had no relevant financial disclosures.
[email protected]
On Twitter @maryjodales
CHICAGO – About 11% of national health expenditures are related to emergency department–directed health care, with 7% of all expenditures from ED admissions and 4% of all expenditures from outpatient ED visits, Dr. Jessica Galarraga reported at the annual meeting of the American College of Emergency Physicians.
Based on volume, hospital admissions from the ED were about 11% of all emergency visits; however, they accounted for more than $182 billion or 62% of the costs of ED-directed care, said Dr. Galarraga of the George Washington University in Washington.

Increasingly, the ED is the “front door” for hospital care, as noted in a RAND report published last year.
With ED-directed health care comprising more than 11% of national health expenditures (NHE), it is important for ED providers to work collaboratively with other parts of the health care system to safely reduce health care costs, Dr. Galarraga said.
Potentially avoidable ED-directed encounters, including nonemergent visits, emergent but preventable visits, and ambulatory care sensitive condition (ACSC) admissions, together accounted for 1.8% of NHE. “Given the relative importance of ED admissions over outpatient encounters, making use of the emergency physician’s role as a gatekeeper to admission may be the most effective way to reduce costs,” she said.
Dr. Galarraga and her colleagues examined the costs of ED care and ED-directed health care by visit categories to provide a better understanding of the potential magnitude of cost savings from interventions directed at ED encounters.
|
The 2010 data from the Medical Expenditure Panel Survey (MEPS) were used to determine mean per-visit payments and charges for ED outpatient and inpatient encounters by visit type. National Hospital Ambulatory Medical Care Survey (NHAMCS) and the Healthcare Cost and Utilization Project (HCUP) were used to estimate the aggregate expenditures and proportional costs of NHE by each visit category. Outpatient encounters were categorized based on the New York University (NYU) classification and inpatient encounters by ACSC versus non-ACSC.
Among all encounters involving ED-directed health care, the greatest mean per-visit expenditures were attributable to hospital admissions from the ED, with mean payments of $12,817 and $9,708 for non-ACSC and ACSC admissions, respectively. Nonurgent outpatient ED visits generated the lowest mean per-visit expenditure of $819, even compared with all other outpatient ED encounters.
Dr. Galarraga had no relevant financial disclosures.
[email protected]
On Twitter @maryjodales
CHICAGO – About 11% of national health expenditures are related to emergency department–directed health care, with 7% of all expenditures from ED admissions and 4% of all expenditures from outpatient ED visits, Dr. Jessica Galarraga reported at the annual meeting of the American College of Emergency Physicians.
Based on volume, hospital admissions from the ED were about 11% of all emergency visits; however, they accounted for more than $182 billion or 62% of the costs of ED-directed care, said Dr. Galarraga of the George Washington University in Washington.

Increasingly, the ED is the “front door” for hospital care, as noted in a RAND report published last year.
With ED-directed health care comprising more than 11% of national health expenditures (NHE), it is important for ED providers to work collaboratively with other parts of the health care system to safely reduce health care costs, Dr. Galarraga said.
Potentially avoidable ED-directed encounters, including nonemergent visits, emergent but preventable visits, and ambulatory care sensitive condition (ACSC) admissions, together accounted for 1.8% of NHE. “Given the relative importance of ED admissions over outpatient encounters, making use of the emergency physician’s role as a gatekeeper to admission may be the most effective way to reduce costs,” she said.
Dr. Galarraga and her colleagues examined the costs of ED care and ED-directed health care by visit categories to provide a better understanding of the potential magnitude of cost savings from interventions directed at ED encounters.
|
The 2010 data from the Medical Expenditure Panel Survey (MEPS) were used to determine mean per-visit payments and charges for ED outpatient and inpatient encounters by visit type. National Hospital Ambulatory Medical Care Survey (NHAMCS) and the Healthcare Cost and Utilization Project (HCUP) were used to estimate the aggregate expenditures and proportional costs of NHE by each visit category. Outpatient encounters were categorized based on the New York University (NYU) classification and inpatient encounters by ACSC versus non-ACSC.
Among all encounters involving ED-directed health care, the greatest mean per-visit expenditures were attributable to hospital admissions from the ED, with mean payments of $12,817 and $9,708 for non-ACSC and ACSC admissions, respectively. Nonurgent outpatient ED visits generated the lowest mean per-visit expenditure of $819, even compared with all other outpatient ED encounters.
Dr. Galarraga had no relevant financial disclosures.
[email protected]
On Twitter @maryjodales
AT ACEP14
Key clinical point: Hospital admissions through the ED comprise most emergency medicine costs.
Major finding: About 11% of emergency visits were hospital admissions from the ED, yet they accounted for 62% of the costs of ED-directed care.
Data source: The 2010 data from the Medical Expenditure Panel Survey (MEPS) were used to determine determined mean per-visit payments and charges. The National Hospital Ambulatory Medical Care Survey (NHAMCS) and the Healthcare Cost and Utilization Project (HCUP) were used to estimate the aggregate expenditures and proportional costs by visit category.
Disclosures: Dr. Galarraga had no relevant financial disclosures.
First Ebola-infected patient is diagnosed in the U.S.
The first Ebola-infected patient to be diagnosed in the United States has been hospitalized in Texas, Dr. Thomas Frieden, director of the Centers for Disease Control and Prevention, announced September 30.
The infection was confirmed in a patient who had traveled from Liberia to visit relatives in Texas. The patient traveled from Africa on the 19th, arrived in the United States on the 20th, developed symptoms on the 24th, sought care on the 26th, and was admitted on the 28th to Texas Health Presbyterian Hospital, Dallas. The results of a PCR test confirmed infection with ebolavirus Zaire on the September 30.
Given this progression of infection, none of the passengers traveling on the flight with the patient are at risk for infection, Dr. Frieden emphasized during the teleconference. All contacts with the patient have been identified and will be monitored for 21 days after exposure. “I have no doubt that we will control this case ... and that it will not spread widely. We will stop it here,” he said.
The CDC is working with a broad global coalition, and “we are invested in assuring this disease is controlled in Africa,” he added.
Dr. David Lakey of the Texas Department of State Health Services noted that there are no other suspected cases at this time related to the infected patient. “We are committed to keeping Texas safe. We’re working through this case together.”
Dr. Edward Goodman of the Texas Health Presbyterian Hospital, Dallas, noted that the hospital “has a robust infection control system. We’ve had a plan in place for some time now” for dealing with infection requiring isolation.
“Ironically, we had a staff meeting a week before to discuss this scenario and as a result we were well prepared to deal with this crisis,” he added.
Zachary Thompson, director of Dallas County Health and Human Services, said that contact investigation is underway based on the patient’s travel history and close contacts.
Dr. Frieden deflected questions about the patient’s flights. Ebola is spread only by direct contact, he reiterated, and the patient was incubated with the disease but was not infectious at the time of travel. “Remember, Ebola doesn’t spread until someone gets sick, so we do not believe anyone on that plane is at risk at this time.”
People traveling from epidemic areas are being screened for fever before allowed on planes, he said. But as long as there continue to be cases in Africa, there is risk of spread beyond those countries.
“We are protecting people by making sure we find the contacts (who were exposed at the time of active infection) and by tracing those direct contacts, as well as their contacts, for 21 days.”
Because the initial symptoms of Ebola are nonspecific (fever, muscle pain, weakness, diarrhea/vomiting, hemorrhage/bruising), physicians and emergency physicians in particular should ask about the patient’s history of travel in the last 21 days, he said.
While not revealing any identifying information, Dr. Frieden said that the patient, who is critically ill, was not involved in the medical response to the Ebola epidemic. Virtually any hospital in this country that can do isolation can care for patients infected with Ebola.
A CDC team is en route to Texas to identify all possible contacts and monitor them for 21 days, including people who may have been exposed to the patient during the 2-day span between the time he initially sought treatment and the time he was admitted. The patient was staying with family members at the time he became ill. “We are erring on the side of safety ... even monitoring those who might not have been in direct contact,” Dr. Frieden said.
“This is a tried and true protocol. I have no doubt that we will stop this case in its tracks in the US.”
The first Ebola-infected patient to be diagnosed in the United States has been hospitalized in Texas, Dr. Thomas Frieden, director of the Centers for Disease Control and Prevention, announced September 30.
The infection was confirmed in a patient who had traveled from Liberia to visit relatives in Texas. The patient traveled from Africa on the 19th, arrived in the United States on the 20th, developed symptoms on the 24th, sought care on the 26th, and was admitted on the 28th to Texas Health Presbyterian Hospital, Dallas. The results of a PCR test confirmed infection with ebolavirus Zaire on the September 30.
Given this progression of infection, none of the passengers traveling on the flight with the patient are at risk for infection, Dr. Frieden emphasized during the teleconference. All contacts with the patient have been identified and will be monitored for 21 days after exposure. “I have no doubt that we will control this case ... and that it will not spread widely. We will stop it here,” he said.
The CDC is working with a broad global coalition, and “we are invested in assuring this disease is controlled in Africa,” he added.
Dr. David Lakey of the Texas Department of State Health Services noted that there are no other suspected cases at this time related to the infected patient. “We are committed to keeping Texas safe. We’re working through this case together.”
Dr. Edward Goodman of the Texas Health Presbyterian Hospital, Dallas, noted that the hospital “has a robust infection control system. We’ve had a plan in place for some time now” for dealing with infection requiring isolation.
“Ironically, we had a staff meeting a week before to discuss this scenario and as a result we were well prepared to deal with this crisis,” he added.
Zachary Thompson, director of Dallas County Health and Human Services, said that contact investigation is underway based on the patient’s travel history and close contacts.
Dr. Frieden deflected questions about the patient’s flights. Ebola is spread only by direct contact, he reiterated, and the patient was incubated with the disease but was not infectious at the time of travel. “Remember, Ebola doesn’t spread until someone gets sick, so we do not believe anyone on that plane is at risk at this time.”
People traveling from epidemic areas are being screened for fever before allowed on planes, he said. But as long as there continue to be cases in Africa, there is risk of spread beyond those countries.
“We are protecting people by making sure we find the contacts (who were exposed at the time of active infection) and by tracing those direct contacts, as well as their contacts, for 21 days.”
Because the initial symptoms of Ebola are nonspecific (fever, muscle pain, weakness, diarrhea/vomiting, hemorrhage/bruising), physicians and emergency physicians in particular should ask about the patient’s history of travel in the last 21 days, he said.
While not revealing any identifying information, Dr. Frieden said that the patient, who is critically ill, was not involved in the medical response to the Ebola epidemic. Virtually any hospital in this country that can do isolation can care for patients infected with Ebola.
A CDC team is en route to Texas to identify all possible contacts and monitor them for 21 days, including people who may have been exposed to the patient during the 2-day span between the time he initially sought treatment and the time he was admitted. The patient was staying with family members at the time he became ill. “We are erring on the side of safety ... even monitoring those who might not have been in direct contact,” Dr. Frieden said.
“This is a tried and true protocol. I have no doubt that we will stop this case in its tracks in the US.”
The first Ebola-infected patient to be diagnosed in the United States has been hospitalized in Texas, Dr. Thomas Frieden, director of the Centers for Disease Control and Prevention, announced September 30.
The infection was confirmed in a patient who had traveled from Liberia to visit relatives in Texas. The patient traveled from Africa on the 19th, arrived in the United States on the 20th, developed symptoms on the 24th, sought care on the 26th, and was admitted on the 28th to Texas Health Presbyterian Hospital, Dallas. The results of a PCR test confirmed infection with ebolavirus Zaire on the September 30.
Given this progression of infection, none of the passengers traveling on the flight with the patient are at risk for infection, Dr. Frieden emphasized during the teleconference. All contacts with the patient have been identified and will be monitored for 21 days after exposure. “I have no doubt that we will control this case ... and that it will not spread widely. We will stop it here,” he said.
The CDC is working with a broad global coalition, and “we are invested in assuring this disease is controlled in Africa,” he added.
Dr. David Lakey of the Texas Department of State Health Services noted that there are no other suspected cases at this time related to the infected patient. “We are committed to keeping Texas safe. We’re working through this case together.”
Dr. Edward Goodman of the Texas Health Presbyterian Hospital, Dallas, noted that the hospital “has a robust infection control system. We’ve had a plan in place for some time now” for dealing with infection requiring isolation.
“Ironically, we had a staff meeting a week before to discuss this scenario and as a result we were well prepared to deal with this crisis,” he added.
Zachary Thompson, director of Dallas County Health and Human Services, said that contact investigation is underway based on the patient’s travel history and close contacts.
Dr. Frieden deflected questions about the patient’s flights. Ebola is spread only by direct contact, he reiterated, and the patient was incubated with the disease but was not infectious at the time of travel. “Remember, Ebola doesn’t spread until someone gets sick, so we do not believe anyone on that plane is at risk at this time.”
People traveling from epidemic areas are being screened for fever before allowed on planes, he said. But as long as there continue to be cases in Africa, there is risk of spread beyond those countries.
“We are protecting people by making sure we find the contacts (who were exposed at the time of active infection) and by tracing those direct contacts, as well as their contacts, for 21 days.”
Because the initial symptoms of Ebola are nonspecific (fever, muscle pain, weakness, diarrhea/vomiting, hemorrhage/bruising), physicians and emergency physicians in particular should ask about the patient’s history of travel in the last 21 days, he said.
While not revealing any identifying information, Dr. Frieden said that the patient, who is critically ill, was not involved in the medical response to the Ebola epidemic. Virtually any hospital in this country that can do isolation can care for patients infected with Ebola.
A CDC team is en route to Texas to identify all possible contacts and monitor them for 21 days, including people who may have been exposed to the patient during the 2-day span between the time he initially sought treatment and the time he was admitted. The patient was staying with family members at the time he became ill. “We are erring on the side of safety ... even monitoring those who might not have been in direct contact,” Dr. Frieden said.
“This is a tried and true protocol. I have no doubt that we will stop this case in its tracks in the US.”
AT A CDC TELECONFERENCE
Ebola: What you need to know, how the U.S. is reacting
The United States is gearing up to deal with Ebola infections at home and abroad.
The epidemic has now resulted in the deaths of more than 2,000 people in Africa. Medical experts have warned that the disease is poised to spread exponentially and that it may become more transmissible. The concern prompted President Obama to pledge military support for logistics and general assistance to international health workers to contain the epidemic.
The fourth Ebola-infected patient has arrived at Emory University hospital in Atlanta for treatment, but no cases have been diagnosed in the United States. For an insiders’ view of how Emory has handled the care of Ebola-infected patients, read our article featuring Dr. Aneesh Mehta.
The Centers for Disease Control and Prevention is constantly monitoring the Ebola epidemic and provides detail about its spread – as well as extensive information about the diagnosis and management of the disease – at a dedicated website, http://www.cdc.gov/vhf/ebola/.
The United States is gearing up to deal with Ebola infections at home and abroad.
The epidemic has now resulted in the deaths of more than 2,000 people in Africa. Medical experts have warned that the disease is poised to spread exponentially and that it may become more transmissible. The concern prompted President Obama to pledge military support for logistics and general assistance to international health workers to contain the epidemic.
The fourth Ebola-infected patient has arrived at Emory University hospital in Atlanta for treatment, but no cases have been diagnosed in the United States. For an insiders’ view of how Emory has handled the care of Ebola-infected patients, read our article featuring Dr. Aneesh Mehta.
The Centers for Disease Control and Prevention is constantly monitoring the Ebola epidemic and provides detail about its spread – as well as extensive information about the diagnosis and management of the disease – at a dedicated website, http://www.cdc.gov/vhf/ebola/.
The United States is gearing up to deal with Ebola infections at home and abroad.
The epidemic has now resulted in the deaths of more than 2,000 people in Africa. Medical experts have warned that the disease is poised to spread exponentially and that it may become more transmissible. The concern prompted President Obama to pledge military support for logistics and general assistance to international health workers to contain the epidemic.
The fourth Ebola-infected patient has arrived at Emory University hospital in Atlanta for treatment, but no cases have been diagnosed in the United States. For an insiders’ view of how Emory has handled the care of Ebola-infected patients, read our article featuring Dr. Aneesh Mehta.
The Centers for Disease Control and Prevention is constantly monitoring the Ebola epidemic and provides detail about its spread – as well as extensive information about the diagnosis and management of the disease – at a dedicated website, http://www.cdc.gov/vhf/ebola/.
Limited Bone Loss With Low-dose Glucocorticoids
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the posttest.
PARIS – Bone loss at 1 year is limited at the low doses of glucocorticoids typically used to treat adults with chronic inflammatory disorders like rheumatoid arthritis, based on a meta-analysis of prospective studies of 1,565 patients with chronic inflammatory diseases (44 studies) and 635 transplant patients (16 studies).
Glucocorticoid treatment at the high doses used in transplantation patients leads to considerable bone loss, especially in the lumbar spine. In contrast, bone loss is limited during glucocorticoid treatment at the lower doses used in chronic inflammatory disease, Dr. Maarten Boers, professor of clinical epidemiology at VU University Medical Center, Amsterdam, reported at the annual European Congress of Rheumatology.
All patients in the meta-analysis had at least two bone mineral density (BMD) measurements over at least 8 months. None received bisphosphonates or antiresorptive therapies, only vitamin D3 and calcium were allowed.
Glucocorticoid doses ranged from 1 to 16 mg/day (mean 9 mg/day) in the patients with chronic inflammatory diseases and from 6 to 53 mg/day (mean 20 mg/day) in the transplant patients. In those with chronic inflammatory diseases, bone loss at the lumbar spine at 1 year averaged –1.7%. For the patients who had measures of femoral neck bone loss, the average loss was –1.3%. In the transplantation group, average bone loss was much higher at –3.6% in the lumbar spine and –3.1% in the femoral neck.
Among the 44 studies that reported BMD in patients with chronic inflammatory diseases, BMD declined from baseline in the lumbar spine by as much as 6% in 1 study and increased by as much as 2% in another. The 39 studies that also reported changes in femoral neck BMD described a decline as high as 7% and an increase as great as 4%.
"We sought to quantify the ‘pure’ effect of GC [glucocorticoid(s)], because so little high-quality information is available," Dr. Boers explained.
The data analysis in these studies was limited to 1 year, but about two-thirds of the patients with chronic inflammatory disease were on chronic glucocorticoid therapy and almost all of the transplant patients were just starting glucocorticoids, Dr. Boers said.
"On average, the yearly loss in a wide range of doses is limited, but starters have more bone loss. The heterogeneity of studies suggests that factors other than GC dose are the main drivers in determining bone loss. Although the data were not available to study these factors, it appears likely that disease activity is very important and acts as a confounder. In other words, disease activity leads to bone loss, and high GC doses lead to bone loss. Effective treatment of high disease activity requires high GC doses, but the interaction of these factors may lead to bone loss comparable to low disease activity being treated with low doses," he explained in an interview.
Many rheumatoid arthritis patients are initially treated with "bridge" glucocorticoid therapy for a few months, until the effect of methotrexate is established, he said. "We prefer ‘COBRA [combination therapy for rheumatoid arthritis] light,’ where patients are initially treated with a higher dose of 30 mg, rapidly tapered to 7.5 mg/day, and maintained for at least 6 months. Several groups are advising to treat for longer periods, and observational data suggest many patients are kept on chronic therapy for periods longer than 1 year," he said.
Local guidelines differ, but all guidelines agree on the necessity of prophylaxis in high-risk situations and on screening for intermediate-risk patients. Unfortunately, many patients requiring antiresorptive treatment according to the guidelines are still not receiving it, with surveys showing about 70% uptake in patients treated by rheumatologists and only about 30% in patients receiving care from other specialists, he said.
"Given the effects of starting GC therapy in our review, we strongly suggest all patients requiring GC therapy for longer than 3 months at any dose should at least be assessed by DXA scan; postmenopausal women, males age 70 or older, and patients with other risk factors should be treated with antiresorptive agents from the start," Dr. Boers advised.
Dr. Boers his coinvestigators reported having no financial conflicts.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the posttest.
PARIS – Bone loss at 1 year is limited at the low doses of glucocorticoids typically used to treat adults with chronic inflammatory disorders like rheumatoid arthritis, based on a meta-analysis of prospective studies of 1,565 patients with chronic inflammatory diseases (44 studies) and 635 transplant patients (16 studies).
Glucocorticoid treatment at the high doses used in transplantation patients leads to considerable bone loss, especially in the lumbar spine. In contrast, bone loss is limited during glucocorticoid treatment at the lower doses used in chronic inflammatory disease, Dr. Maarten Boers, professor of clinical epidemiology at VU University Medical Center, Amsterdam, reported at the annual European Congress of Rheumatology.
All patients in the meta-analysis had at least two bone mineral density (BMD) measurements over at least 8 months. None received bisphosphonates or antiresorptive therapies, only vitamin D3 and calcium were allowed.
Glucocorticoid doses ranged from 1 to 16 mg/day (mean 9 mg/day) in the patients with chronic inflammatory diseases and from 6 to 53 mg/day (mean 20 mg/day) in the transplant patients. In those with chronic inflammatory diseases, bone loss at the lumbar spine at 1 year averaged –1.7%. For the patients who had measures of femoral neck bone loss, the average loss was –1.3%. In the transplantation group, average bone loss was much higher at –3.6% in the lumbar spine and –3.1% in the femoral neck.
Among the 44 studies that reported BMD in patients with chronic inflammatory diseases, BMD declined from baseline in the lumbar spine by as much as 6% in 1 study and increased by as much as 2% in another. The 39 studies that also reported changes in femoral neck BMD described a decline as high as 7% and an increase as great as 4%.
"We sought to quantify the ‘pure’ effect of GC [glucocorticoid(s)], because so little high-quality information is available," Dr. Boers explained.
The data analysis in these studies was limited to 1 year, but about two-thirds of the patients with chronic inflammatory disease were on chronic glucocorticoid therapy and almost all of the transplant patients were just starting glucocorticoids, Dr. Boers said.
"On average, the yearly loss in a wide range of doses is limited, but starters have more bone loss. The heterogeneity of studies suggests that factors other than GC dose are the main drivers in determining bone loss. Although the data were not available to study these factors, it appears likely that disease activity is very important and acts as a confounder. In other words, disease activity leads to bone loss, and high GC doses lead to bone loss. Effective treatment of high disease activity requires high GC doses, but the interaction of these factors may lead to bone loss comparable to low disease activity being treated with low doses," he explained in an interview.
Many rheumatoid arthritis patients are initially treated with "bridge" glucocorticoid therapy for a few months, until the effect of methotrexate is established, he said. "We prefer ‘COBRA [combination therapy for rheumatoid arthritis] light,’ where patients are initially treated with a higher dose of 30 mg, rapidly tapered to 7.5 mg/day, and maintained for at least 6 months. Several groups are advising to treat for longer periods, and observational data suggest many patients are kept on chronic therapy for periods longer than 1 year," he said.
Local guidelines differ, but all guidelines agree on the necessity of prophylaxis in high-risk situations and on screening for intermediate-risk patients. Unfortunately, many patients requiring antiresorptive treatment according to the guidelines are still not receiving it, with surveys showing about 70% uptake in patients treated by rheumatologists and only about 30% in patients receiving care from other specialists, he said.
"Given the effects of starting GC therapy in our review, we strongly suggest all patients requiring GC therapy for longer than 3 months at any dose should at least be assessed by DXA scan; postmenopausal women, males age 70 or older, and patients with other risk factors should be treated with antiresorptive agents from the start," Dr. Boers advised.
Dr. Boers his coinvestigators reported having no financial conflicts.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the posttest.
PARIS – Bone loss at 1 year is limited at the low doses of glucocorticoids typically used to treat adults with chronic inflammatory disorders like rheumatoid arthritis, based on a meta-analysis of prospective studies of 1,565 patients with chronic inflammatory diseases (44 studies) and 635 transplant patients (16 studies).
Glucocorticoid treatment at the high doses used in transplantation patients leads to considerable bone loss, especially in the lumbar spine. In contrast, bone loss is limited during glucocorticoid treatment at the lower doses used in chronic inflammatory disease, Dr. Maarten Boers, professor of clinical epidemiology at VU University Medical Center, Amsterdam, reported at the annual European Congress of Rheumatology.
All patients in the meta-analysis had at least two bone mineral density (BMD) measurements over at least 8 months. None received bisphosphonates or antiresorptive therapies, only vitamin D3 and calcium were allowed.
Glucocorticoid doses ranged from 1 to 16 mg/day (mean 9 mg/day) in the patients with chronic inflammatory diseases and from 6 to 53 mg/day (mean 20 mg/day) in the transplant patients. In those with chronic inflammatory diseases, bone loss at the lumbar spine at 1 year averaged –1.7%. For the patients who had measures of femoral neck bone loss, the average loss was –1.3%. In the transplantation group, average bone loss was much higher at –3.6% in the lumbar spine and –3.1% in the femoral neck.
Among the 44 studies that reported BMD in patients with chronic inflammatory diseases, BMD declined from baseline in the lumbar spine by as much as 6% in 1 study and increased by as much as 2% in another. The 39 studies that also reported changes in femoral neck BMD described a decline as high as 7% and an increase as great as 4%.
"We sought to quantify the ‘pure’ effect of GC [glucocorticoid(s)], because so little high-quality information is available," Dr. Boers explained.
The data analysis in these studies was limited to 1 year, but about two-thirds of the patients with chronic inflammatory disease were on chronic glucocorticoid therapy and almost all of the transplant patients were just starting glucocorticoids, Dr. Boers said.
"On average, the yearly loss in a wide range of doses is limited, but starters have more bone loss. The heterogeneity of studies suggests that factors other than GC dose are the main drivers in determining bone loss. Although the data were not available to study these factors, it appears likely that disease activity is very important and acts as a confounder. In other words, disease activity leads to bone loss, and high GC doses lead to bone loss. Effective treatment of high disease activity requires high GC doses, but the interaction of these factors may lead to bone loss comparable to low disease activity being treated with low doses," he explained in an interview.
Many rheumatoid arthritis patients are initially treated with "bridge" glucocorticoid therapy for a few months, until the effect of methotrexate is established, he said. "We prefer ‘COBRA [combination therapy for rheumatoid arthritis] light,’ where patients are initially treated with a higher dose of 30 mg, rapidly tapered to 7.5 mg/day, and maintained for at least 6 months. Several groups are advising to treat for longer periods, and observational data suggest many patients are kept on chronic therapy for periods longer than 1 year," he said.
Local guidelines differ, but all guidelines agree on the necessity of prophylaxis in high-risk situations and on screening for intermediate-risk patients. Unfortunately, many patients requiring antiresorptive treatment according to the guidelines are still not receiving it, with surveys showing about 70% uptake in patients treated by rheumatologists and only about 30% in patients receiving care from other specialists, he said.
"Given the effects of starting GC therapy in our review, we strongly suggest all patients requiring GC therapy for longer than 3 months at any dose should at least be assessed by DXA scan; postmenopausal women, males age 70 or older, and patients with other risk factors should be treated with antiresorptive agents from the start," Dr. Boers advised.
Dr. Boers his coinvestigators reported having no financial conflicts.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
AT THE EULAR CONGRESS 2014
Respiratory disease in unaccompanied children from Central America
At least 16 of nearly 54,000 unaccompanied children from Central America have been hospitalized with respiratory diseases, Dr. Edith N. Nyangoma and her associates reported August 15 in Morbidity and Mortality Weekly Report (63(32);698-99).
The cases were in persons aged 14–17 years, and diagnoses included laboratory-confirmed pneumococcal pneumonia with laboratory-confirmed influenza (three cases) and without laboratory-confirmed influenza (four cases), influenza pneumonia (one case), and pneumonia with no identified etiology (eight cases).
Five patients experienced septic shock requiring intensive care. No case was fatal. All six cases for which pneumococcal isolates were available were identified as serotype 5, a serotype included in the 13-valent pneumococcal conjugate vaccine (PCV13) (Prevnar-13, Pfizer). Of the 16 patients identified in this cluster, 11 were tested for influenza viruses; four (36%) were positive (two for influenza A[H1N1]pdm09, one for influenza B, and one for influenza A by rapid test), according to the report.
In response to the findings, officials at the Centers for Disease Control have recommended that all unaccompanied children residing in temporary or standard shelters receive influenza vaccine and PCV13 in addition to routinely recommended vaccines. Among the 2,000 children thus far vaccinated in four affected shelters, there have been no serious adverse events.
Routine annual influenza vaccination is recommended for all persons in the United States who are at least age 6 months, but school-aged children in Central America generally are not targeted for vaccination.
PCV13 is routinely given in the United States at age 2–59 months. It is recommended for the older unaccompanied children because of the unexpected number of pneumococcal pneumonia cases occurring in the context of crowded conditions.
Additional information about the ongoing humanitarian and public health response is available at http://emergency.cdc.gov/children/unaccompanied/index.asp.
At least 16 of nearly 54,000 unaccompanied children from Central America have been hospitalized with respiratory diseases, Dr. Edith N. Nyangoma and her associates reported August 15 in Morbidity and Mortality Weekly Report (63(32);698-99).
The cases were in persons aged 14–17 years, and diagnoses included laboratory-confirmed pneumococcal pneumonia with laboratory-confirmed influenza (three cases) and without laboratory-confirmed influenza (four cases), influenza pneumonia (one case), and pneumonia with no identified etiology (eight cases).
Five patients experienced septic shock requiring intensive care. No case was fatal. All six cases for which pneumococcal isolates were available were identified as serotype 5, a serotype included in the 13-valent pneumococcal conjugate vaccine (PCV13) (Prevnar-13, Pfizer). Of the 16 patients identified in this cluster, 11 were tested for influenza viruses; four (36%) were positive (two for influenza A[H1N1]pdm09, one for influenza B, and one for influenza A by rapid test), according to the report.
In response to the findings, officials at the Centers for Disease Control have recommended that all unaccompanied children residing in temporary or standard shelters receive influenza vaccine and PCV13 in addition to routinely recommended vaccines. Among the 2,000 children thus far vaccinated in four affected shelters, there have been no serious adverse events.
Routine annual influenza vaccination is recommended for all persons in the United States who are at least age 6 months, but school-aged children in Central America generally are not targeted for vaccination.
PCV13 is routinely given in the United States at age 2–59 months. It is recommended for the older unaccompanied children because of the unexpected number of pneumococcal pneumonia cases occurring in the context of crowded conditions.
Additional information about the ongoing humanitarian and public health response is available at http://emergency.cdc.gov/children/unaccompanied/index.asp.
At least 16 of nearly 54,000 unaccompanied children from Central America have been hospitalized with respiratory diseases, Dr. Edith N. Nyangoma and her associates reported August 15 in Morbidity and Mortality Weekly Report (63(32);698-99).
The cases were in persons aged 14–17 years, and diagnoses included laboratory-confirmed pneumococcal pneumonia with laboratory-confirmed influenza (three cases) and without laboratory-confirmed influenza (four cases), influenza pneumonia (one case), and pneumonia with no identified etiology (eight cases).
Five patients experienced septic shock requiring intensive care. No case was fatal. All six cases for which pneumococcal isolates were available were identified as serotype 5, a serotype included in the 13-valent pneumococcal conjugate vaccine (PCV13) (Prevnar-13, Pfizer). Of the 16 patients identified in this cluster, 11 were tested for influenza viruses; four (36%) were positive (two for influenza A[H1N1]pdm09, one for influenza B, and one for influenza A by rapid test), according to the report.
In response to the findings, officials at the Centers for Disease Control have recommended that all unaccompanied children residing in temporary or standard shelters receive influenza vaccine and PCV13 in addition to routinely recommended vaccines. Among the 2,000 children thus far vaccinated in four affected shelters, there have been no serious adverse events.
Routine annual influenza vaccination is recommended for all persons in the United States who are at least age 6 months, but school-aged children in Central America generally are not targeted for vaccination.
PCV13 is routinely given in the United States at age 2–59 months. It is recommended for the older unaccompanied children because of the unexpected number of pneumococcal pneumonia cases occurring in the context of crowded conditions.
Additional information about the ongoing humanitarian and public health response is available at http://emergency.cdc.gov/children/unaccompanied/index.asp.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Meta-analysis finds limited bone loss with low-dose glucocorticoids
PARIS – Bone loss at 1 year is limited at the low doses of glucocorticoids typically used to treat adults with chronic inflammatory disorders like rheumatoid arthritis, based on a meta-analysis of prospective studies of 1,565 patients with chronic inflammatory diseases (44 studies) and 635 transplant patients (16 studies).
Glucocorticoid treatment at the high doses used in transplantation patients leads to considerable bone loss, especially in the lumbar spine. In contrast, bone loss is limited during glucocorticoid treatment at the lower doses used in chronic inflammatory disease, Dr. Maarten Boers, professor of clinical epidemiology at VU University Medical Center, Amsterdam, reported at the annual European Congress of Rheumatology.
All patients in the meta-analysis had at least two bone mineral density (BMD) measurements over at least 8 months. None received bisphosphonates or antiresorptive therapies, only vitamin D3 and calcium were allowed.
Glucocorticoid doses ranged from 1 to 16 mg/day (mean 9 mg/day) in the patients with chronic inflammatory diseases and from 6 to 53 mg/day (mean 20 mg/day) in the transplant patients. In those with chronic inflammatory diseases, bone loss at the lumbar spine at 1 year averaged –1.7%. For the patients who had measures of femoral neck bone loss, the average loss was –1.3%. In the transplantation group, average bone loss was much higher at –3.6% in the lumbar spine and –3.1% in the femoral neck.
Among the 44 studies that reported BMD in patients with chronic inflammatory diseases, BMD declined from baseline in the lumbar spine by as much as 6% in 1 study and increased by as much as 2% in another. The 39 studies that also reported changes in femoral neck BMD described a decline as high as 7% and an increase as great as 4%.
"We sought to quantify the ‘pure’ effect of GC [glucocorticoid(s)], because so little high-quality information is available," Dr. Boers explained.
The data analysis in these studies was limited to 1 year, but about two-thirds of the patients with chronic inflammatory disease were on chronic glucocorticoid therapy and almost all of the transplant patients were just starting glucocorticoids, Dr. Boers said.
"On average, the yearly loss in a wide range of doses is limited, but starters have more bone loss. The heterogeneity of studies suggests that factors other than GC dose are the main drivers in determining bone loss. Although the data were not available to study these factors, it appears likely that disease activity is very important and acts as a confounder. In other words, disease activity leads to bone loss, and high GC doses lead to bone loss. Effective treatment of high disease activity requires high GC doses, but the interaction of these factors may lead to bone loss comparable to low disease activity being treated with low doses," he explained in an interview.
Many rheumatoid arthritis patients are initially treated with "bridge" glucocorticoid therapy for a few months, until the effect of methotrexate is established, he said. "We prefer ‘COBRA [combination therapy for rheumatoid arthritis] light,’ where patients are initially treated with a higher dose of 30 mg, rapidly tapered to 7.5 mg/day, and maintained for at least 6 months. Several groups are advising to treat for longer periods, and observational data suggest many patients are kept on chronic therapy for periods longer than 1 year," he said.
Local guidelines differ, but all guidelines agree on the necessity of prophylaxis in high-risk situations and on screening for intermediate-risk patients. Unfortunately, many patients requiring antiresorptive treatment according to the guidelines are still not receiving it, with surveys showing about 70% uptake in patients treated by rheumatologists and only about 30% in patients receiving care from other specialists, he said.
"Given the effects of starting GC therapy in our review, we strongly suggest all patients requiring GC therapy for longer than 3 months at any dose should at least be assessed by DXA scan; postmenopausal women, males age 70 or older, and patients with other risk factors should be treated with antiresorptive agents from the start," Dr. Boers advised.
Dr. Boers his coinvestigators reported having no financial conflicts.
PARIS – Bone loss at 1 year is limited at the low doses of glucocorticoids typically used to treat adults with chronic inflammatory disorders like rheumatoid arthritis, based on a meta-analysis of prospective studies of 1,565 patients with chronic inflammatory diseases (44 studies) and 635 transplant patients (16 studies).
Glucocorticoid treatment at the high doses used in transplantation patients leads to considerable bone loss, especially in the lumbar spine. In contrast, bone loss is limited during glucocorticoid treatment at the lower doses used in chronic inflammatory disease, Dr. Maarten Boers, professor of clinical epidemiology at VU University Medical Center, Amsterdam, reported at the annual European Congress of Rheumatology.
All patients in the meta-analysis had at least two bone mineral density (BMD) measurements over at least 8 months. None received bisphosphonates or antiresorptive therapies, only vitamin D3 and calcium were allowed.
Glucocorticoid doses ranged from 1 to 16 mg/day (mean 9 mg/day) in the patients with chronic inflammatory diseases and from 6 to 53 mg/day (mean 20 mg/day) in the transplant patients. In those with chronic inflammatory diseases, bone loss at the lumbar spine at 1 year averaged –1.7%. For the patients who had measures of femoral neck bone loss, the average loss was –1.3%. In the transplantation group, average bone loss was much higher at –3.6% in the lumbar spine and –3.1% in the femoral neck.
Among the 44 studies that reported BMD in patients with chronic inflammatory diseases, BMD declined from baseline in the lumbar spine by as much as 6% in 1 study and increased by as much as 2% in another. The 39 studies that also reported changes in femoral neck BMD described a decline as high as 7% and an increase as great as 4%.
"We sought to quantify the ‘pure’ effect of GC [glucocorticoid(s)], because so little high-quality information is available," Dr. Boers explained.
The data analysis in these studies was limited to 1 year, but about two-thirds of the patients with chronic inflammatory disease were on chronic glucocorticoid therapy and almost all of the transplant patients were just starting glucocorticoids, Dr. Boers said.
"On average, the yearly loss in a wide range of doses is limited, but starters have more bone loss. The heterogeneity of studies suggests that factors other than GC dose are the main drivers in determining bone loss. Although the data were not available to study these factors, it appears likely that disease activity is very important and acts as a confounder. In other words, disease activity leads to bone loss, and high GC doses lead to bone loss. Effective treatment of high disease activity requires high GC doses, but the interaction of these factors may lead to bone loss comparable to low disease activity being treated with low doses," he explained in an interview.
Many rheumatoid arthritis patients are initially treated with "bridge" glucocorticoid therapy for a few months, until the effect of methotrexate is established, he said. "We prefer ‘COBRA [combination therapy for rheumatoid arthritis] light,’ where patients are initially treated with a higher dose of 30 mg, rapidly tapered to 7.5 mg/day, and maintained for at least 6 months. Several groups are advising to treat for longer periods, and observational data suggest many patients are kept on chronic therapy for periods longer than 1 year," he said.
Local guidelines differ, but all guidelines agree on the necessity of prophylaxis in high-risk situations and on screening for intermediate-risk patients. Unfortunately, many patients requiring antiresorptive treatment according to the guidelines are still not receiving it, with surveys showing about 70% uptake in patients treated by rheumatologists and only about 30% in patients receiving care from other specialists, he said.
"Given the effects of starting GC therapy in our review, we strongly suggest all patients requiring GC therapy for longer than 3 months at any dose should at least be assessed by DXA scan; postmenopausal women, males age 70 or older, and patients with other risk factors should be treated with antiresorptive agents from the start," Dr. Boers advised.
Dr. Boers his coinvestigators reported having no financial conflicts.
PARIS – Bone loss at 1 year is limited at the low doses of glucocorticoids typically used to treat adults with chronic inflammatory disorders like rheumatoid arthritis, based on a meta-analysis of prospective studies of 1,565 patients with chronic inflammatory diseases (44 studies) and 635 transplant patients (16 studies).
Glucocorticoid treatment at the high doses used in transplantation patients leads to considerable bone loss, especially in the lumbar spine. In contrast, bone loss is limited during glucocorticoid treatment at the lower doses used in chronic inflammatory disease, Dr. Maarten Boers, professor of clinical epidemiology at VU University Medical Center, Amsterdam, reported at the annual European Congress of Rheumatology.
All patients in the meta-analysis had at least two bone mineral density (BMD) measurements over at least 8 months. None received bisphosphonates or antiresorptive therapies, only vitamin D3 and calcium were allowed.
Glucocorticoid doses ranged from 1 to 16 mg/day (mean 9 mg/day) in the patients with chronic inflammatory diseases and from 6 to 53 mg/day (mean 20 mg/day) in the transplant patients. In those with chronic inflammatory diseases, bone loss at the lumbar spine at 1 year averaged –1.7%. For the patients who had measures of femoral neck bone loss, the average loss was –1.3%. In the transplantation group, average bone loss was much higher at –3.6% in the lumbar spine and –3.1% in the femoral neck.
Among the 44 studies that reported BMD in patients with chronic inflammatory diseases, BMD declined from baseline in the lumbar spine by as much as 6% in 1 study and increased by as much as 2% in another. The 39 studies that also reported changes in femoral neck BMD described a decline as high as 7% and an increase as great as 4%.
"We sought to quantify the ‘pure’ effect of GC [glucocorticoid(s)], because so little high-quality information is available," Dr. Boers explained.
The data analysis in these studies was limited to 1 year, but about two-thirds of the patients with chronic inflammatory disease were on chronic glucocorticoid therapy and almost all of the transplant patients were just starting glucocorticoids, Dr. Boers said.
"On average, the yearly loss in a wide range of doses is limited, but starters have more bone loss. The heterogeneity of studies suggests that factors other than GC dose are the main drivers in determining bone loss. Although the data were not available to study these factors, it appears likely that disease activity is very important and acts as a confounder. In other words, disease activity leads to bone loss, and high GC doses lead to bone loss. Effective treatment of high disease activity requires high GC doses, but the interaction of these factors may lead to bone loss comparable to low disease activity being treated with low doses," he explained in an interview.
Many rheumatoid arthritis patients are initially treated with "bridge" glucocorticoid therapy for a few months, until the effect of methotrexate is established, he said. "We prefer ‘COBRA [combination therapy for rheumatoid arthritis] light,’ where patients are initially treated with a higher dose of 30 mg, rapidly tapered to 7.5 mg/day, and maintained for at least 6 months. Several groups are advising to treat for longer periods, and observational data suggest many patients are kept on chronic therapy for periods longer than 1 year," he said.
Local guidelines differ, but all guidelines agree on the necessity of prophylaxis in high-risk situations and on screening for intermediate-risk patients. Unfortunately, many patients requiring antiresorptive treatment according to the guidelines are still not receiving it, with surveys showing about 70% uptake in patients treated by rheumatologists and only about 30% in patients receiving care from other specialists, he said.
"Given the effects of starting GC therapy in our review, we strongly suggest all patients requiring GC therapy for longer than 3 months at any dose should at least be assessed by DXA scan; postmenopausal women, males age 70 or older, and patients with other risk factors should be treated with antiresorptive agents from the start," Dr. Boers advised.
Dr. Boers his coinvestigators reported having no financial conflicts.
AT THE EULAR CONGRESS 2014
Key clinical point: One-year bone loss is limited during glucocorticoid treatment at the lower doses used in chronic inflammatory disease.
Major finding: In those with chronic inflammatory diseases, bone loss at the lumbar spine at 1 year averaged –1.7%. For the patients who had measures of femoral neck bone loss, the average loss was –1.3%.
Data source: A meta-analysis of prospective studies of 1,565 patients with chronic inflammatory diseases (44 studies) and 635 transplant patients (16 studies).
Disclosures: Dr. Boers his coinvestigators reported having no financial conflicts.
Obesity may drive symptom severity in rheumatoid arthritis
PARIS – The higher disease activity scores seen in obese patients with rheumatoid arthritis may be driven by the proinflammatory state that is associated with obesity, Dr. Christopher Sparks reported in a press conference at the annual European Congress of Rheumatology.
Although obese RA patients tend to have less radiographic joint damage than do normal-weight RA patients, they have comparable DAS (disease activity score) 28. With the clinical focus on treat-to-target, an approach guided by DAS scores, obese RA patients may be getting more aggressive treatment. The finding may explain why obese patients with RA have better outcomes than normal-weight and thin RA patients, said Dr. Sparks, a clinical research fellow at the University of Liverpool, England.
Dr. Sparks and his colleagues used an international RA database to examine two patient subgroups: those diagnosed with RA in the previous year and those with longstanding RA. The 3,534 patients were stratified by their body mass index into five groups: underweight (less than 18.5 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), obese (30-34.9 kg/m2), and obese II (35 kg/m2 or more). In the 1,981 patients with longstanding disease, median disease duration was about 7 years; the other 1,553 patients had disease duration of 1 year or less.
About 73% of the each cohort was female, and the distribution of BMI measures was similar for the two cohorts.
The groups were compared by BMI and RA disease measures that included DAS28, erythrocyte sedimentation rate, tender joint count; swollen joint count; and visual analog scale disease activity.
After adjusting for RA risk factors, obesity (BMI of 28 kg/m2 or greater) was significantly associated with a DAS28 exceeding 5.1, an elevated erythrocyte sedimentation rate, high tender joint count, and high visual analog scale score. For instance, compared with normal weight and overweight patients, underweight and both groups of obese patients were 1.5-2.2 times as likely to have a DAS28 exceeding 5.1, Dr. Sparks reported.
He had no financial disclosures.
PARIS – The higher disease activity scores seen in obese patients with rheumatoid arthritis may be driven by the proinflammatory state that is associated with obesity, Dr. Christopher Sparks reported in a press conference at the annual European Congress of Rheumatology.
Although obese RA patients tend to have less radiographic joint damage than do normal-weight RA patients, they have comparable DAS (disease activity score) 28. With the clinical focus on treat-to-target, an approach guided by DAS scores, obese RA patients may be getting more aggressive treatment. The finding may explain why obese patients with RA have better outcomes than normal-weight and thin RA patients, said Dr. Sparks, a clinical research fellow at the University of Liverpool, England.
Dr. Sparks and his colleagues used an international RA database to examine two patient subgroups: those diagnosed with RA in the previous year and those with longstanding RA. The 3,534 patients were stratified by their body mass index into five groups: underweight (less than 18.5 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), obese (30-34.9 kg/m2), and obese II (35 kg/m2 or more). In the 1,981 patients with longstanding disease, median disease duration was about 7 years; the other 1,553 patients had disease duration of 1 year or less.
About 73% of the each cohort was female, and the distribution of BMI measures was similar for the two cohorts.
The groups were compared by BMI and RA disease measures that included DAS28, erythrocyte sedimentation rate, tender joint count; swollen joint count; and visual analog scale disease activity.
After adjusting for RA risk factors, obesity (BMI of 28 kg/m2 or greater) was significantly associated with a DAS28 exceeding 5.1, an elevated erythrocyte sedimentation rate, high tender joint count, and high visual analog scale score. For instance, compared with normal weight and overweight patients, underweight and both groups of obese patients were 1.5-2.2 times as likely to have a DAS28 exceeding 5.1, Dr. Sparks reported.
He had no financial disclosures.
PARIS – The higher disease activity scores seen in obese patients with rheumatoid arthritis may be driven by the proinflammatory state that is associated with obesity, Dr. Christopher Sparks reported in a press conference at the annual European Congress of Rheumatology.
Although obese RA patients tend to have less radiographic joint damage than do normal-weight RA patients, they have comparable DAS (disease activity score) 28. With the clinical focus on treat-to-target, an approach guided by DAS scores, obese RA patients may be getting more aggressive treatment. The finding may explain why obese patients with RA have better outcomes than normal-weight and thin RA patients, said Dr. Sparks, a clinical research fellow at the University of Liverpool, England.
Dr. Sparks and his colleagues used an international RA database to examine two patient subgroups: those diagnosed with RA in the previous year and those with longstanding RA. The 3,534 patients were stratified by their body mass index into five groups: underweight (less than 18.5 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), obese (30-34.9 kg/m2), and obese II (35 kg/m2 or more). In the 1,981 patients with longstanding disease, median disease duration was about 7 years; the other 1,553 patients had disease duration of 1 year or less.
About 73% of the each cohort was female, and the distribution of BMI measures was similar for the two cohorts.
The groups were compared by BMI and RA disease measures that included DAS28, erythrocyte sedimentation rate, tender joint count; swollen joint count; and visual analog scale disease activity.
After adjusting for RA risk factors, obesity (BMI of 28 kg/m2 or greater) was significantly associated with a DAS28 exceeding 5.1, an elevated erythrocyte sedimentation rate, high tender joint count, and high visual analog scale score. For instance, compared with normal weight and overweight patients, underweight and both groups of obese patients were 1.5-2.2 times as likely to have a DAS28 exceeding 5.1, Dr. Sparks reported.
He had no financial disclosures.
AT THE EULAR CONGRESS 2014
Key clinical point: Obesity and underweight status are associated with worse symptoms of RA.
Major finding: Compared with normal weight and overweight patients, underweight and both groups of obese patients were 1.5-2.2 times as likely to have a DAS28 exceeding 5.1.
Data source: An international RA database sample of 3,534 patients.
Disclosures: Dr. Sparks had no financial disclosures.
Obesity may drive symptom severity in rheumatoid arthritis
PARIS – The higher disease activity scores seen in obese patients with rheumatoid arthritis may be driven by the proinflammatory state that is associated with obesity, Dr. Christopher Sparks reported in a press conference at the annual European Congress of Rheumatology.
Although obese RA patients tend to have less radiographic joint damage than do normal-weight RA patients, they have comparable DAS (disease activity score) 28. With the clinical focus on treat-to-target, an approach guided by DAS scores, obese RA patients may be getting more aggressive treatment. The finding may explain why obese patients with RA have better outcomes than normal-weight and thin RA patients, said Dr. Sparks, a clinical research fellow at the University of Liverpool, England.
Dr. Sparks and his colleagues used an international RA database to examine two patient subgroups: those diagnosed with RA in the previous year and those with longstanding RA. The 3,534 patients were stratified by their body mass index into five groups: underweight (less than 18.5 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), obese (30-34.9 kg/m2), and obese II (35 kg/m2 or more). In the 1,981 patients with longstanding disease, median disease duration was about 7 years; the other 1,553 patients had disease duration of 1 year or less.
About 73% of the each cohort was female, and the distribution of BMI measures was similar for the two cohorts.
The groups were compared by BMI and RA disease measures that included DAS28, erythrocyte sedimentation rate, tender joint count; swollen joint count; and visual analog scale disease activity.
After adjusting for RA risk factors, obesity (BMI of 28 kg/m2 or greater) was significantly associated with a DAS28 exceeding 5.1, an elevated erythrocyte sedimentation rate, high tender joint count, and high visual analog scale score. For instance, compared with normal weight and overweight patients, underweight and both groups of obese patients were 1.5-2.2 times as likely to have a DAS28 exceeding 5.1, Dr. Sparks reported.
He had no financial disclosures.
PARIS – The higher disease activity scores seen in obese patients with rheumatoid arthritis may be driven by the proinflammatory state that is associated with obesity, Dr. Christopher Sparks reported in a press conference at the annual European Congress of Rheumatology.
Although obese RA patients tend to have less radiographic joint damage than do normal-weight RA patients, they have comparable DAS (disease activity score) 28. With the clinical focus on treat-to-target, an approach guided by DAS scores, obese RA patients may be getting more aggressive treatment. The finding may explain why obese patients with RA have better outcomes than normal-weight and thin RA patients, said Dr. Sparks, a clinical research fellow at the University of Liverpool, England.
Dr. Sparks and his colleagues used an international RA database to examine two patient subgroups: those diagnosed with RA in the previous year and those with longstanding RA. The 3,534 patients were stratified by their body mass index into five groups: underweight (less than 18.5 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), obese (30-34.9 kg/m2), and obese II (35 kg/m2 or more). In the 1,981 patients with longstanding disease, median disease duration was about 7 years; the other 1,553 patients had disease duration of 1 year or less.
About 73% of the each cohort was female, and the distribution of BMI measures was similar for the two cohorts.
The groups were compared by BMI and RA disease measures that included DAS28, erythrocyte sedimentation rate, tender joint count; swollen joint count; and visual analog scale disease activity.
After adjusting for RA risk factors, obesity (BMI of 28 kg/m2 or greater) was significantly associated with a DAS28 exceeding 5.1, an elevated erythrocyte sedimentation rate, high tender joint count, and high visual analog scale score. For instance, compared with normal weight and overweight patients, underweight and both groups of obese patients were 1.5-2.2 times as likely to have a DAS28 exceeding 5.1, Dr. Sparks reported.
He had no financial disclosures.
PARIS – The higher disease activity scores seen in obese patients with rheumatoid arthritis may be driven by the proinflammatory state that is associated with obesity, Dr. Christopher Sparks reported in a press conference at the annual European Congress of Rheumatology.
Although obese RA patients tend to have less radiographic joint damage than do normal-weight RA patients, they have comparable DAS (disease activity score) 28. With the clinical focus on treat-to-target, an approach guided by DAS scores, obese RA patients may be getting more aggressive treatment. The finding may explain why obese patients with RA have better outcomes than normal-weight and thin RA patients, said Dr. Sparks, a clinical research fellow at the University of Liverpool, England.
Dr. Sparks and his colleagues used an international RA database to examine two patient subgroups: those diagnosed with RA in the previous year and those with longstanding RA. The 3,534 patients were stratified by their body mass index into five groups: underweight (less than 18.5 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), obese (30-34.9 kg/m2), and obese II (35 kg/m2 or more). In the 1,981 patients with longstanding disease, median disease duration was about 7 years; the other 1,553 patients had disease duration of 1 year or less.
About 73% of the each cohort was female, and the distribution of BMI measures was similar for the two cohorts.
The groups were compared by BMI and RA disease measures that included DAS28, erythrocyte sedimentation rate, tender joint count; swollen joint count; and visual analog scale disease activity.
After adjusting for RA risk factors, obesity (BMI of 28 kg/m2 or greater) was significantly associated with a DAS28 exceeding 5.1, an elevated erythrocyte sedimentation rate, high tender joint count, and high visual analog scale score. For instance, compared with normal weight and overweight patients, underweight and both groups of obese patients were 1.5-2.2 times as likely to have a DAS28 exceeding 5.1, Dr. Sparks reported.
He had no financial disclosures.
AT THE EULAR CONGRESS 2014
Key clinical point: Obesity and underweight status are associated with worse symptoms of RA.
Major finding: Compared with normal weight and overweight patients, underweight and both groups of obese patients were 1.5-2.2 times as likely to have a DAS28 exceeding 5.1.
Data source: An international RA database sample of 3,534 patients.
Disclosures: Dr. Sparks had no financial disclosures.