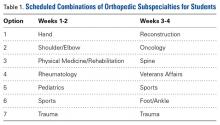
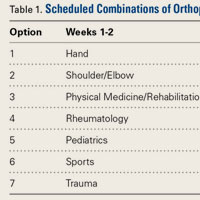
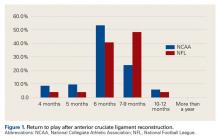
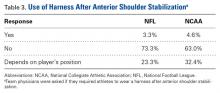
User login
Does Accelerated Physical Therapy After Elective Primary Hip and Knee Arthroplasty Facilitate Early Discharge?
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are among the most effective surgical procedures in modern medicine. Use of primary THA in the United States is projected to increase by 174% by 2030, to 532,000 cases annually, and the estimate for TKA is even greater.1 Hospital length of stay (LOS) accounts for a significant portion of the overall cost of these procedures. Reducing LOS to limit costs without compromising patient safety, satisfaction, and outcomes remains the goal at all joint arthroplasty centers. Rapid-recovery or fast-track clinical pathways limiting opioid use and emphasizing patient education and early (day-of-surgery) mobilization have been shown to reduce LOS without compromising patient outcomes.2-5 Factors correlated with LOS after THA include surgical approach, use of multimodal analgesia, obesity, age, and social situations or living conditions.4,6-10
Our institution recently implemented a protocol in which certified physical therapists provide accelerated (day-of-surgery) physical therapy (PT) for all total joint arthroplasty patients. For the study reported here, we hypothesized that, compared with PT started on postoperative day 1 (POD-1), PT started day of surgery (Day 0) would result in shorter LOS for unilateral primary THA and TKA patients. In addition, we wanted to evaluate any predischarge differences in function, as measured by gait distance, between the groups.
Methods
After obtaining Institutional Review Board approval, we retrospectively evaluated use of the new postoperative protocol (Day 0 PT) for primary THA and TKA patients. We reviewed all cases of primary unilateral THA or TKA performed by a single surgeon over the 12-month period immediately following initiation of the protocol. There were 116 THA cases and 126 TKA cases. Charts were reviewed for patient demographics, intraoperative data, in-hospital course, and PT session notes. Patients who had a PT session at any point on day of surgery were designated the Day 0 group, and patients who had PT starting the next day (POD-1) were designated the Non-Day 0 group. Although the medical records showed that Day 0 PT had been ordered in all cases, not all patients received PT on the day of their surgery; the most common reason was that they returned from postanesthesia care after the physical therapists’ work shift had ended. Another reason was patient noncompliance or unwillingness stemming from the prolonged effects of general anesthesia, diminished mental orientation, excess fatigue, or inadequate pain control. PT sessions after THA and TKA remained consistent over the study period, with twice daily sessions directed at patient mobility, range of motion, and gentle strengthening exercise. PT was performed only with patient consent.
Surgery
A combination of general and spinal anesthesia was used in almost all THA and TKA cases. In <5% of cases, either the patient refused spinal anesthesia, or it was unsuccessful. In addition, tranexamic acid was administered to limit blood loss in all THA and TKA cases. Of the 116 THAs performed over the study period, 3 were excluded (see below). Of the 113 patients included in the study, 88 (77.9%) used a minimally invasive posterolateral approach, 18 (15.9%) a direct anterior approach, and 7 (6.2%) an anterolateral approach. All THAs were performed with conventional instruments and uncemented components. All TKAs were performed with a standard medial parapatellar approach, conventional instruments, and a tourniquet; in each case, the patella was resurfaced, and cemented fixation was used. Drains were not used in any THA or TKA cases. A local anesthetic cocktail (100 mL of 0.25% ropivacaine, 15 mL of 0.5% ropivacaine, and 1 mL of 1:1000 epinephrine) was injected for postoperative analgesia in all THA and TKA cases.
There were 3 important intraoperative findings in the THA Day 0 group: 2 cases of incidental gluteus medius tendon tears requiring repair and 1 case of nondisplaced calcar fracture treated with a cerclage cable. The THA Non-Day 0 group and both TKA groups had no major intraoperative findings.
Physical Therapy
Day-of-surgery PT was ordered for all patients. Patients did not receive formal PT before surgery. The PT protocol consisted of subjective assessment of patient condition, expectations, and goals; lower limb strengthening exercises; and maximum gait training with use of an assistive device as tolerated. Standard hip movement restrictions were ordered for posterolateral approach patients to protect the soft-tissue repair. Continuous passive motion (CPM) was not used during this study period.
Discharge Criteria
Patients were cleared for discharge by a multidisciplinary team using several criteria: no medical condition that would require readmission, intact surgical incision without discharge or concerning erythema, adequate analgesia (oral medications), intact neurovascular examination, and PT goals achieved (independence with bed mobility, transfers, standing balance, and minimum gait distance of 150 feet). Patients who could not be discharged home because of family or occupation issues or because of problems with gait or transfer were referred to skilled nursing or home healthcare. Follow-up for wound assessment and for examination of radiographs and functional range of motion was planned for 2 to 3 weeks after surgery (all patients followed up). Two patients, 1 in the THA Non-Day 0 group and 1 in the TKA Day 0 group, had a mechanical fall 1 day before discharge, but there were no complication-related discharge delays. In addition, there were no readmissions during the first 4 weeks after surgery.
Excluded Patients
Of the 116 THA cases, 113 (63 Day 0, 50 Non-Day 0) were analyzed. To establish homogeneity between groups and remove potential confounding factors, we excluded 4 THA patients (all Non-Day 0) from analysis because of medical complications prolonging LOS. In 1 of these cases, the patient developed respiratory insufficiency and myocardial infarction on POD-3, and critical care support was required (LOS, 16 days). In another case, anticoagulation treatment led to the development of a hip hematoma on POD-9 and to treatment (evacuation) in the operating room (LOS, 14 days). The other 2 cases involved exacerbation of dysphagia from preexisting myasthenia gravis (LOS, 5 days) and Ogilvie syndrome, managed conservatively (LOS, 9 days).
Of the 126 TKA cases, 123 (97 Day 0, 26 Non-Day 0) were analyzed. Three TKA patients were excluded because of prolonged hospitalization for medical reasons: One developed a deep vein thrombosis, 1 acquired Clostridium difficile colitis (history of lung transplantation, multiple immunosuppressive drugs), and 1 developed respiratory insufficiency from asthma exacerbation.
Statistical Analysis
Power analysis (G*Power) was used to determine an appropriate sample size for comparison.11 Given a previously published mean LOS after THA of 4 days, the hypothesized mean LOS reducing that by at least 0.5 day to 3.5 days, a significance level set at 5%, a power of test set at 0.95, and an allocation ratio of 1, a minimum of 23 subjects would be needed in each group to attain a statistically significant difference using the nonparametric Mann-Whitney test. The Shapiro-Wilk test was used to assess data normality. Regarding statistical significance, the Mann-Whitney U test was used for non-normally distributed data, the 2-sided Fisher exact test and χ2 test for qualitative data and contingency, and the 2-tailed, unpaired, independent-samples Student t test for normally distributed data. Data were analyzed with SPSS Statistics for Windows Version 20 (IBM).
Results
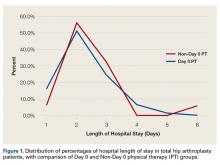
TKA and THA patients had similar demographic profiles, types of anesthesia, operating room and surgery times, surgical approaches, and total number of PT sessions before discharge. Estimated blood loss, however, was significantly (P < .05) higher for Non-Day 0 patients than for Non-Day 0 patients (Table 1).
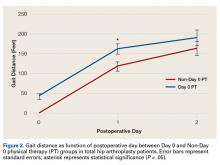
Mean (SD) distance ambulated during first PT session was 2-fold farther (P = .014) for Non-Day 0 patients, 84.1 (10.4) feet, than for Day 0 patients, 42.1 (6.4) feet. On POD-1, mean (SD) gait was significantly (P = .019) longer for Day 0 patients, 162.4 (12.9) feet, than for Non-Day 0 patients, 118 (11.7) feet (Figure 2).
In TKA patients, although mean (SD) distance ambulated tended to be farther for the Day 0 group than for the Non-Day 0 group—114 (12.3) feet on POD-1 and 176 (15.2) feet on POD-2 for Day 0 vs 94 (22.2) feet on POD-1 and 148 (22.1) feet on POD-2 for Non-Day 0—the differences were not statistically significant. In addition, knee arc of motion during first PT session was statistically significantly (P = .3) higher for Day 0 patients, 69.1° (18.7°), than for Non-Day 0 patients, 61.7° (18.8°).
Statistical analysis revealed no difference in LOS based on surgical approach to the hip: 2.4 days for posterolateral (2.2 days for Day 0 and 2.6 days for Non-Day 0; P = .06); 2.1 days for direct anterior (2.1 days for Day 0 and 2.0 days for Non-Day 0; P = .7); and 2.7 days for anterolateral (3.0 days for Day 0 and 2.6 days for Non-Day 0; P = .6).
Discussion
Protocols for PT after THA and TKA remain unstandardized and largely dependent on institutions and surgeons. Factors permitting successful implementation of accelerated rehabilitation include patient motivation, adequate analgesia, and adequate support by physical therapists.12 A potential risk associated with accelerated PT after THA is dislocation, which did not occur in any patient in our Day 0 group. Other risks are increased pain and swelling leading to increased risk of falling and bleeding, which were not observed in our cohort. Although Day 0 PT was ordered in all cases in this study, only 55% of THA patients and 79% of TKA patients received PT the same day as their surgery. The delay can be addressed by making physical therapists’ work shifts more flexible for cases that finish later in the day and by providing preoperative education on the importance of day-of-surgery PT. Dr. Incavo and office staff routinely discuss discharge planning with all patients before surgery, but there was no stimulus protocol or communication to discuss or emphasize LOS with patients before surgery, and there was no questionnaire or survey given to assess patient expectations about PT and discharge.
Our finding of no statistically significant reduction in mean LOS after implementation of accelerated PT for THA or TKA differs from findings in multiple other reports.4,5,13-17 Baseline or control group mean LOS tended to be higher in previous studies3,5,18-23 (3.4-11.4 days) than in our control group (2.5 days) (Table 2).
Conclusion
These results provide useful information for providers who are managing primary THA and TKA cases and seeking continual improvement in postoperative patient care and better resource allocation. Hospitals, particularly those operating in bundled-care environments, are increasingly coming under scrutiny to control costs. Our study results showed that the costs associated with Day 0 PT are justified for THA but not for TKA.
Am J Orthop. 2016;45(6):E337-E342. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Barbieri A, Vanhaecht K, Van Herck P, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32.
3. den Hartog YM, Mathijssen NM, Vehmeijer SB. Reduced length of hospital stay after the introduction of a rapid recovery protocol for primary THA procedures. Acta Orthop. 2013;84(5):444-447.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Robbins CE, Casey D, Bono JV, Murphy SB, Talmo CT, Ward DM. A multidisciplinary total hip arthroplasty protocol with accelerated postoperative rehabilitation: does the patient benefit? Am J Orthop. 2014;43(4):178-181.
6. den Hartog YM, Mathijssen NM, Hannink G, Vehmeijer SB. Which patient characteristics influence length of hospital stay after primary total hip arthroplasty in a ‘fast-track’ setting? Bone Joint J. 2015;97(1):19-23.
7. Forrest G, Fuchs M, Gutierrez A, Girardy J. Factors affecting length of stay and need for rehabilitation after hip and knee arthroplasty. J Arthroplasty. 1998;13(2):186-190.
8. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
9. Sharma V, Morgan PM, Cheng EY. Factors influencing early rehabilitation after THA: a systematic review. Clin Orthop Relat Res. 2009;467(6):1400-1411.
10. Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89(6):1153-1160.
11. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 suppl 3):12-15.
13. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
14. Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82(6):679-684.
15. Husted H, Jensen CM, Solgaard S, Kehlet H. Reduced length of stay following hip and knee arthroplasty in Denmark 2000-2009: from research to implementation. Arch Orthop Trauma Surg. 2012;132(1):101-104.
16. Berger RA, Sanders SA, Thill ES, Sporer SM, Della Valle C. Newer anesthesia and rehabilitation protocols enable outpatient hip replacement in selected patients. Clin Orthop Relat Res. 2009;467(6):1424-1430.
17. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
18. Isaac D, Falode T, Liu P, I’Anson H, Dillow K, Gill P. Accelerated rehabilitation after total knee replacement. Knee. 2005;12(5):346-350.
19. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
20. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation: a quasi-experimental study of 98 patients. Acta Orthop. 2008;79(5):624-630.
21. Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91(4):761-772.
22. Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop. 2008;79(2):149-159.
23. Wellman SS, Murphy AC, Gulcynski D. Murphy SB. Implementation of an accelerated mobilization protocol following primary total hip arthroplasty: impact on length of stay and disposition. Curr Rev Musculoskelet Med. 2011;4(3):84-90.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are among the most effective surgical procedures in modern medicine. Use of primary THA in the United States is projected to increase by 174% by 2030, to 532,000 cases annually, and the estimate for TKA is even greater.1 Hospital length of stay (LOS) accounts for a significant portion of the overall cost of these procedures. Reducing LOS to limit costs without compromising patient safety, satisfaction, and outcomes remains the goal at all joint arthroplasty centers. Rapid-recovery or fast-track clinical pathways limiting opioid use and emphasizing patient education and early (day-of-surgery) mobilization have been shown to reduce LOS without compromising patient outcomes.2-5 Factors correlated with LOS after THA include surgical approach, use of multimodal analgesia, obesity, age, and social situations or living conditions.4,6-10
Our institution recently implemented a protocol in which certified physical therapists provide accelerated (day-of-surgery) physical therapy (PT) for all total joint arthroplasty patients. For the study reported here, we hypothesized that, compared with PT started on postoperative day 1 (POD-1), PT started day of surgery (Day 0) would result in shorter LOS for unilateral primary THA and TKA patients. In addition, we wanted to evaluate any predischarge differences in function, as measured by gait distance, between the groups.
Methods
After obtaining Institutional Review Board approval, we retrospectively evaluated use of the new postoperative protocol (Day 0 PT) for primary THA and TKA patients. We reviewed all cases of primary unilateral THA or TKA performed by a single surgeon over the 12-month period immediately following initiation of the protocol. There were 116 THA cases and 126 TKA cases. Charts were reviewed for patient demographics, intraoperative data, in-hospital course, and PT session notes. Patients who had a PT session at any point on day of surgery were designated the Day 0 group, and patients who had PT starting the next day (POD-1) were designated the Non-Day 0 group. Although the medical records showed that Day 0 PT had been ordered in all cases, not all patients received PT on the day of their surgery; the most common reason was that they returned from postanesthesia care after the physical therapists’ work shift had ended. Another reason was patient noncompliance or unwillingness stemming from the prolonged effects of general anesthesia, diminished mental orientation, excess fatigue, or inadequate pain control. PT sessions after THA and TKA remained consistent over the study period, with twice daily sessions directed at patient mobility, range of motion, and gentle strengthening exercise. PT was performed only with patient consent.
Surgery
A combination of general and spinal anesthesia was used in almost all THA and TKA cases. In <5% of cases, either the patient refused spinal anesthesia, or it was unsuccessful. In addition, tranexamic acid was administered to limit blood loss in all THA and TKA cases. Of the 116 THAs performed over the study period, 3 were excluded (see below). Of the 113 patients included in the study, 88 (77.9%) used a minimally invasive posterolateral approach, 18 (15.9%) a direct anterior approach, and 7 (6.2%) an anterolateral approach. All THAs were performed with conventional instruments and uncemented components. All TKAs were performed with a standard medial parapatellar approach, conventional instruments, and a tourniquet; in each case, the patella was resurfaced, and cemented fixation was used. Drains were not used in any THA or TKA cases. A local anesthetic cocktail (100 mL of 0.25% ropivacaine, 15 mL of 0.5% ropivacaine, and 1 mL of 1:1000 epinephrine) was injected for postoperative analgesia in all THA and TKA cases.
There were 3 important intraoperative findings in the THA Day 0 group: 2 cases of incidental gluteus medius tendon tears requiring repair and 1 case of nondisplaced calcar fracture treated with a cerclage cable. The THA Non-Day 0 group and both TKA groups had no major intraoperative findings.
Physical Therapy
Day-of-surgery PT was ordered for all patients. Patients did not receive formal PT before surgery. The PT protocol consisted of subjective assessment of patient condition, expectations, and goals; lower limb strengthening exercises; and maximum gait training with use of an assistive device as tolerated. Standard hip movement restrictions were ordered for posterolateral approach patients to protect the soft-tissue repair. Continuous passive motion (CPM) was not used during this study period.
Discharge Criteria
Patients were cleared for discharge by a multidisciplinary team using several criteria: no medical condition that would require readmission, intact surgical incision without discharge or concerning erythema, adequate analgesia (oral medications), intact neurovascular examination, and PT goals achieved (independence with bed mobility, transfers, standing balance, and minimum gait distance of 150 feet). Patients who could not be discharged home because of family or occupation issues or because of problems with gait or transfer were referred to skilled nursing or home healthcare. Follow-up for wound assessment and for examination of radiographs and functional range of motion was planned for 2 to 3 weeks after surgery (all patients followed up). Two patients, 1 in the THA Non-Day 0 group and 1 in the TKA Day 0 group, had a mechanical fall 1 day before discharge, but there were no complication-related discharge delays. In addition, there were no readmissions during the first 4 weeks after surgery.
Excluded Patients
Of the 116 THA cases, 113 (63 Day 0, 50 Non-Day 0) were analyzed. To establish homogeneity between groups and remove potential confounding factors, we excluded 4 THA patients (all Non-Day 0) from analysis because of medical complications prolonging LOS. In 1 of these cases, the patient developed respiratory insufficiency and myocardial infarction on POD-3, and critical care support was required (LOS, 16 days). In another case, anticoagulation treatment led to the development of a hip hematoma on POD-9 and to treatment (evacuation) in the operating room (LOS, 14 days). The other 2 cases involved exacerbation of dysphagia from preexisting myasthenia gravis (LOS, 5 days) and Ogilvie syndrome, managed conservatively (LOS, 9 days).
Of the 126 TKA cases, 123 (97 Day 0, 26 Non-Day 0) were analyzed. Three TKA patients were excluded because of prolonged hospitalization for medical reasons: One developed a deep vein thrombosis, 1 acquired Clostridium difficile colitis (history of lung transplantation, multiple immunosuppressive drugs), and 1 developed respiratory insufficiency from asthma exacerbation.
Statistical Analysis
Power analysis (G*Power) was used to determine an appropriate sample size for comparison.11 Given a previously published mean LOS after THA of 4 days, the hypothesized mean LOS reducing that by at least 0.5 day to 3.5 days, a significance level set at 5%, a power of test set at 0.95, and an allocation ratio of 1, a minimum of 23 subjects would be needed in each group to attain a statistically significant difference using the nonparametric Mann-Whitney test. The Shapiro-Wilk test was used to assess data normality. Regarding statistical significance, the Mann-Whitney U test was used for non-normally distributed data, the 2-sided Fisher exact test and χ2 test for qualitative data and contingency, and the 2-tailed, unpaired, independent-samples Student t test for normally distributed data. Data were analyzed with SPSS Statistics for Windows Version 20 (IBM).
Results
TKA and THA patients had similar demographic profiles, types of anesthesia, operating room and surgery times, surgical approaches, and total number of PT sessions before discharge. Estimated blood loss, however, was significantly (P < .05) higher for Non-Day 0 patients than for Non-Day 0 patients (Table 1).
Mean (SD) distance ambulated during first PT session was 2-fold farther (P = .014) for Non-Day 0 patients, 84.1 (10.4) feet, than for Day 0 patients, 42.1 (6.4) feet. On POD-1, mean (SD) gait was significantly (P = .019) longer for Day 0 patients, 162.4 (12.9) feet, than for Non-Day 0 patients, 118 (11.7) feet (Figure 2).
In TKA patients, although mean (SD) distance ambulated tended to be farther for the Day 0 group than for the Non-Day 0 group—114 (12.3) feet on POD-1 and 176 (15.2) feet on POD-2 for Day 0 vs 94 (22.2) feet on POD-1 and 148 (22.1) feet on POD-2 for Non-Day 0—the differences were not statistically significant. In addition, knee arc of motion during first PT session was statistically significantly (P = .3) higher for Day 0 patients, 69.1° (18.7°), than for Non-Day 0 patients, 61.7° (18.8°).
Statistical analysis revealed no difference in LOS based on surgical approach to the hip: 2.4 days for posterolateral (2.2 days for Day 0 and 2.6 days for Non-Day 0; P = .06); 2.1 days for direct anterior (2.1 days for Day 0 and 2.0 days for Non-Day 0; P = .7); and 2.7 days for anterolateral (3.0 days for Day 0 and 2.6 days for Non-Day 0; P = .6).
Discussion
Protocols for PT after THA and TKA remain unstandardized and largely dependent on institutions and surgeons. Factors permitting successful implementation of accelerated rehabilitation include patient motivation, adequate analgesia, and adequate support by physical therapists.12 A potential risk associated with accelerated PT after THA is dislocation, which did not occur in any patient in our Day 0 group. Other risks are increased pain and swelling leading to increased risk of falling and bleeding, which were not observed in our cohort. Although Day 0 PT was ordered in all cases in this study, only 55% of THA patients and 79% of TKA patients received PT the same day as their surgery. The delay can be addressed by making physical therapists’ work shifts more flexible for cases that finish later in the day and by providing preoperative education on the importance of day-of-surgery PT. Dr. Incavo and office staff routinely discuss discharge planning with all patients before surgery, but there was no stimulus protocol or communication to discuss or emphasize LOS with patients before surgery, and there was no questionnaire or survey given to assess patient expectations about PT and discharge.
Our finding of no statistically significant reduction in mean LOS after implementation of accelerated PT for THA or TKA differs from findings in multiple other reports.4,5,13-17 Baseline or control group mean LOS tended to be higher in previous studies3,5,18-23 (3.4-11.4 days) than in our control group (2.5 days) (Table 2).
Conclusion
These results provide useful information for providers who are managing primary THA and TKA cases and seeking continual improvement in postoperative patient care and better resource allocation. Hospitals, particularly those operating in bundled-care environments, are increasingly coming under scrutiny to control costs. Our study results showed that the costs associated with Day 0 PT are justified for THA but not for TKA.
Am J Orthop. 2016;45(6):E337-E342. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are among the most effective surgical procedures in modern medicine. Use of primary THA in the United States is projected to increase by 174% by 2030, to 532,000 cases annually, and the estimate for TKA is even greater.1 Hospital length of stay (LOS) accounts for a significant portion of the overall cost of these procedures. Reducing LOS to limit costs without compromising patient safety, satisfaction, and outcomes remains the goal at all joint arthroplasty centers. Rapid-recovery or fast-track clinical pathways limiting opioid use and emphasizing patient education and early (day-of-surgery) mobilization have been shown to reduce LOS without compromising patient outcomes.2-5 Factors correlated with LOS after THA include surgical approach, use of multimodal analgesia, obesity, age, and social situations or living conditions.4,6-10
Our institution recently implemented a protocol in which certified physical therapists provide accelerated (day-of-surgery) physical therapy (PT) for all total joint arthroplasty patients. For the study reported here, we hypothesized that, compared with PT started on postoperative day 1 (POD-1), PT started day of surgery (Day 0) would result in shorter LOS for unilateral primary THA and TKA patients. In addition, we wanted to evaluate any predischarge differences in function, as measured by gait distance, between the groups.
Methods
After obtaining Institutional Review Board approval, we retrospectively evaluated use of the new postoperative protocol (Day 0 PT) for primary THA and TKA patients. We reviewed all cases of primary unilateral THA or TKA performed by a single surgeon over the 12-month period immediately following initiation of the protocol. There were 116 THA cases and 126 TKA cases. Charts were reviewed for patient demographics, intraoperative data, in-hospital course, and PT session notes. Patients who had a PT session at any point on day of surgery were designated the Day 0 group, and patients who had PT starting the next day (POD-1) were designated the Non-Day 0 group. Although the medical records showed that Day 0 PT had been ordered in all cases, not all patients received PT on the day of their surgery; the most common reason was that they returned from postanesthesia care after the physical therapists’ work shift had ended. Another reason was patient noncompliance or unwillingness stemming from the prolonged effects of general anesthesia, diminished mental orientation, excess fatigue, or inadequate pain control. PT sessions after THA and TKA remained consistent over the study period, with twice daily sessions directed at patient mobility, range of motion, and gentle strengthening exercise. PT was performed only with patient consent.
Surgery
A combination of general and spinal anesthesia was used in almost all THA and TKA cases. In <5% of cases, either the patient refused spinal anesthesia, or it was unsuccessful. In addition, tranexamic acid was administered to limit blood loss in all THA and TKA cases. Of the 116 THAs performed over the study period, 3 were excluded (see below). Of the 113 patients included in the study, 88 (77.9%) used a minimally invasive posterolateral approach, 18 (15.9%) a direct anterior approach, and 7 (6.2%) an anterolateral approach. All THAs were performed with conventional instruments and uncemented components. All TKAs were performed with a standard medial parapatellar approach, conventional instruments, and a tourniquet; in each case, the patella was resurfaced, and cemented fixation was used. Drains were not used in any THA or TKA cases. A local anesthetic cocktail (100 mL of 0.25% ropivacaine, 15 mL of 0.5% ropivacaine, and 1 mL of 1:1000 epinephrine) was injected for postoperative analgesia in all THA and TKA cases.
There were 3 important intraoperative findings in the THA Day 0 group: 2 cases of incidental gluteus medius tendon tears requiring repair and 1 case of nondisplaced calcar fracture treated with a cerclage cable. The THA Non-Day 0 group and both TKA groups had no major intraoperative findings.
Physical Therapy
Day-of-surgery PT was ordered for all patients. Patients did not receive formal PT before surgery. The PT protocol consisted of subjective assessment of patient condition, expectations, and goals; lower limb strengthening exercises; and maximum gait training with use of an assistive device as tolerated. Standard hip movement restrictions were ordered for posterolateral approach patients to protect the soft-tissue repair. Continuous passive motion (CPM) was not used during this study period.
Discharge Criteria
Patients were cleared for discharge by a multidisciplinary team using several criteria: no medical condition that would require readmission, intact surgical incision without discharge or concerning erythema, adequate analgesia (oral medications), intact neurovascular examination, and PT goals achieved (independence with bed mobility, transfers, standing balance, and minimum gait distance of 150 feet). Patients who could not be discharged home because of family or occupation issues or because of problems with gait or transfer were referred to skilled nursing or home healthcare. Follow-up for wound assessment and for examination of radiographs and functional range of motion was planned for 2 to 3 weeks after surgery (all patients followed up). Two patients, 1 in the THA Non-Day 0 group and 1 in the TKA Day 0 group, had a mechanical fall 1 day before discharge, but there were no complication-related discharge delays. In addition, there were no readmissions during the first 4 weeks after surgery.
Excluded Patients
Of the 116 THA cases, 113 (63 Day 0, 50 Non-Day 0) were analyzed. To establish homogeneity between groups and remove potential confounding factors, we excluded 4 THA patients (all Non-Day 0) from analysis because of medical complications prolonging LOS. In 1 of these cases, the patient developed respiratory insufficiency and myocardial infarction on POD-3, and critical care support was required (LOS, 16 days). In another case, anticoagulation treatment led to the development of a hip hematoma on POD-9 and to treatment (evacuation) in the operating room (LOS, 14 days). The other 2 cases involved exacerbation of dysphagia from preexisting myasthenia gravis (LOS, 5 days) and Ogilvie syndrome, managed conservatively (LOS, 9 days).
Of the 126 TKA cases, 123 (97 Day 0, 26 Non-Day 0) were analyzed. Three TKA patients were excluded because of prolonged hospitalization for medical reasons: One developed a deep vein thrombosis, 1 acquired Clostridium difficile colitis (history of lung transplantation, multiple immunosuppressive drugs), and 1 developed respiratory insufficiency from asthma exacerbation.
Statistical Analysis
Power analysis (G*Power) was used to determine an appropriate sample size for comparison.11 Given a previously published mean LOS after THA of 4 days, the hypothesized mean LOS reducing that by at least 0.5 day to 3.5 days, a significance level set at 5%, a power of test set at 0.95, and an allocation ratio of 1, a minimum of 23 subjects would be needed in each group to attain a statistically significant difference using the nonparametric Mann-Whitney test. The Shapiro-Wilk test was used to assess data normality. Regarding statistical significance, the Mann-Whitney U test was used for non-normally distributed data, the 2-sided Fisher exact test and χ2 test for qualitative data and contingency, and the 2-tailed, unpaired, independent-samples Student t test for normally distributed data. Data were analyzed with SPSS Statistics for Windows Version 20 (IBM).
Results
TKA and THA patients had similar demographic profiles, types of anesthesia, operating room and surgery times, surgical approaches, and total number of PT sessions before discharge. Estimated blood loss, however, was significantly (P < .05) higher for Non-Day 0 patients than for Non-Day 0 patients (Table 1).
Mean (SD) distance ambulated during first PT session was 2-fold farther (P = .014) for Non-Day 0 patients, 84.1 (10.4) feet, than for Day 0 patients, 42.1 (6.4) feet. On POD-1, mean (SD) gait was significantly (P = .019) longer for Day 0 patients, 162.4 (12.9) feet, than for Non-Day 0 patients, 118 (11.7) feet (Figure 2).
In TKA patients, although mean (SD) distance ambulated tended to be farther for the Day 0 group than for the Non-Day 0 group—114 (12.3) feet on POD-1 and 176 (15.2) feet on POD-2 for Day 0 vs 94 (22.2) feet on POD-1 and 148 (22.1) feet on POD-2 for Non-Day 0—the differences were not statistically significant. In addition, knee arc of motion during first PT session was statistically significantly (P = .3) higher for Day 0 patients, 69.1° (18.7°), than for Non-Day 0 patients, 61.7° (18.8°).
Statistical analysis revealed no difference in LOS based on surgical approach to the hip: 2.4 days for posterolateral (2.2 days for Day 0 and 2.6 days for Non-Day 0; P = .06); 2.1 days for direct anterior (2.1 days for Day 0 and 2.0 days for Non-Day 0; P = .7); and 2.7 days for anterolateral (3.0 days for Day 0 and 2.6 days for Non-Day 0; P = .6).
Discussion
Protocols for PT after THA and TKA remain unstandardized and largely dependent on institutions and surgeons. Factors permitting successful implementation of accelerated rehabilitation include patient motivation, adequate analgesia, and adequate support by physical therapists.12 A potential risk associated with accelerated PT after THA is dislocation, which did not occur in any patient in our Day 0 group. Other risks are increased pain and swelling leading to increased risk of falling and bleeding, which were not observed in our cohort. Although Day 0 PT was ordered in all cases in this study, only 55% of THA patients and 79% of TKA patients received PT the same day as their surgery. The delay can be addressed by making physical therapists’ work shifts more flexible for cases that finish later in the day and by providing preoperative education on the importance of day-of-surgery PT. Dr. Incavo and office staff routinely discuss discharge planning with all patients before surgery, but there was no stimulus protocol or communication to discuss or emphasize LOS with patients before surgery, and there was no questionnaire or survey given to assess patient expectations about PT and discharge.
Our finding of no statistically significant reduction in mean LOS after implementation of accelerated PT for THA or TKA differs from findings in multiple other reports.4,5,13-17 Baseline or control group mean LOS tended to be higher in previous studies3,5,18-23 (3.4-11.4 days) than in our control group (2.5 days) (Table 2).
Conclusion
These results provide useful information for providers who are managing primary THA and TKA cases and seeking continual improvement in postoperative patient care and better resource allocation. Hospitals, particularly those operating in bundled-care environments, are increasingly coming under scrutiny to control costs. Our study results showed that the costs associated with Day 0 PT are justified for THA but not for TKA.
Am J Orthop. 2016;45(6):E337-E342. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Barbieri A, Vanhaecht K, Van Herck P, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32.
3. den Hartog YM, Mathijssen NM, Vehmeijer SB. Reduced length of hospital stay after the introduction of a rapid recovery protocol for primary THA procedures. Acta Orthop. 2013;84(5):444-447.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Robbins CE, Casey D, Bono JV, Murphy SB, Talmo CT, Ward DM. A multidisciplinary total hip arthroplasty protocol with accelerated postoperative rehabilitation: does the patient benefit? Am J Orthop. 2014;43(4):178-181.
6. den Hartog YM, Mathijssen NM, Hannink G, Vehmeijer SB. Which patient characteristics influence length of hospital stay after primary total hip arthroplasty in a ‘fast-track’ setting? Bone Joint J. 2015;97(1):19-23.
7. Forrest G, Fuchs M, Gutierrez A, Girardy J. Factors affecting length of stay and need for rehabilitation after hip and knee arthroplasty. J Arthroplasty. 1998;13(2):186-190.
8. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
9. Sharma V, Morgan PM, Cheng EY. Factors influencing early rehabilitation after THA: a systematic review. Clin Orthop Relat Res. 2009;467(6):1400-1411.
10. Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89(6):1153-1160.
11. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 suppl 3):12-15.
13. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
14. Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82(6):679-684.
15. Husted H, Jensen CM, Solgaard S, Kehlet H. Reduced length of stay following hip and knee arthroplasty in Denmark 2000-2009: from research to implementation. Arch Orthop Trauma Surg. 2012;132(1):101-104.
16. Berger RA, Sanders SA, Thill ES, Sporer SM, Della Valle C. Newer anesthesia and rehabilitation protocols enable outpatient hip replacement in selected patients. Clin Orthop Relat Res. 2009;467(6):1424-1430.
17. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
18. Isaac D, Falode T, Liu P, I’Anson H, Dillow K, Gill P. Accelerated rehabilitation after total knee replacement. Knee. 2005;12(5):346-350.
19. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
20. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation: a quasi-experimental study of 98 patients. Acta Orthop. 2008;79(5):624-630.
21. Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91(4):761-772.
22. Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop. 2008;79(2):149-159.
23. Wellman SS, Murphy AC, Gulcynski D. Murphy SB. Implementation of an accelerated mobilization protocol following primary total hip arthroplasty: impact on length of stay and disposition. Curr Rev Musculoskelet Med. 2011;4(3):84-90.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Barbieri A, Vanhaecht K, Van Herck P, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32.
3. den Hartog YM, Mathijssen NM, Vehmeijer SB. Reduced length of hospital stay after the introduction of a rapid recovery protocol for primary THA procedures. Acta Orthop. 2013;84(5):444-447.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Robbins CE, Casey D, Bono JV, Murphy SB, Talmo CT, Ward DM. A multidisciplinary total hip arthroplasty protocol with accelerated postoperative rehabilitation: does the patient benefit? Am J Orthop. 2014;43(4):178-181.
6. den Hartog YM, Mathijssen NM, Hannink G, Vehmeijer SB. Which patient characteristics influence length of hospital stay after primary total hip arthroplasty in a ‘fast-track’ setting? Bone Joint J. 2015;97(1):19-23.
7. Forrest G, Fuchs M, Gutierrez A, Girardy J. Factors affecting length of stay and need for rehabilitation after hip and knee arthroplasty. J Arthroplasty. 1998;13(2):186-190.
8. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
9. Sharma V, Morgan PM, Cheng EY. Factors influencing early rehabilitation after THA: a systematic review. Clin Orthop Relat Res. 2009;467(6):1400-1411.
10. Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89(6):1153-1160.
11. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 suppl 3):12-15.
13. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
14. Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82(6):679-684.
15. Husted H, Jensen CM, Solgaard S, Kehlet H. Reduced length of stay following hip and knee arthroplasty in Denmark 2000-2009: from research to implementation. Arch Orthop Trauma Surg. 2012;132(1):101-104.
16. Berger RA, Sanders SA, Thill ES, Sporer SM, Della Valle C. Newer anesthesia and rehabilitation protocols enable outpatient hip replacement in selected patients. Clin Orthop Relat Res. 2009;467(6):1424-1430.
17. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
18. Isaac D, Falode T, Liu P, I’Anson H, Dillow K, Gill P. Accelerated rehabilitation after total knee replacement. Knee. 2005;12(5):346-350.
19. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
20. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation: a quasi-experimental study of 98 patients. Acta Orthop. 2008;79(5):624-630.
21. Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91(4):761-772.
22. Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop. 2008;79(2):149-159.
23. Wellman SS, Murphy AC, Gulcynski D. Murphy SB. Implementation of an accelerated mobilization protocol following primary total hip arthroplasty: impact on length of stay and disposition. Curr Rev Musculoskelet Med. 2011;4(3):84-90.
Glenohumeral Joint Sepsis Caused by Streptococcus mitis: A Case Report
Septic arthritis predominantly involves the weight-bearing joints of the hip and knee, which account for nearly 60% of cases.1 In contrast, the shoulder joint is involved in 10% to 15% of cases, though this number may be higher among intravenous (IV) drug users.2 The most common causative organisms are the Staphylococcus species, followed closely by β-hemolytic streptococci, with these 2 groups accounting for more than 90% of all cases.3 The Streptococcus viridans group belongs to normal oral flora residing predominantly on the surface of teeth. Although well known for its ability to colonize heart valves and frequently cause bacterial endocarditis, this group has rarely been associated with septic arthritis. Furthermore, Streptococcus mitis, a subgroup of S viridans, has been implicated even less commonly.
In this article, we report a case of glenohumeral joint septic arthritis caused by S mitis. To our knowledge, such a case has not been previously reported in the English literature. Given the low virulence of this orally based bacterium, treating physicians must maintain clinical suspicion for the organism in the setting of persistent joint effusion and pain in association with periodontal disease or trauma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand-dominant 54-year-old man presented to Dr. Gruson with complaints of persistent right shoulder pain associated with worsening range of motion (ROM). Three weeks earlier, the patient reported being assaulted and noted progressive swelling about the right shoulder. He denied fevers, chills, or prior shoulder problems. Although his past medical history was remarkable for hepatitis C and diabetes, he was not taking any diabetic medications at that time. A review of systems was remarkable for poor dental hygiene, and the patient was missing several teeth, which he said had been knocked out during the assault. Physical examination revealed diffuse tenderness about the right shoulder and severe pain with all passive movement. The shoulder was pseudoparalyzed. There were no subcutaneous collections, wounds, or ecchymosis about the shoulder. Mild calor was noted on the right shoulder relative to the left. Radiographs of the right shoulder showed no acute osseous abnormalities.
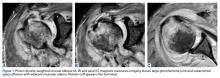
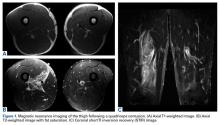
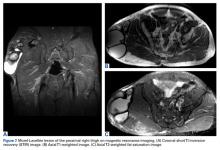
Magnetic resonance imaging (MRI), which was urgently obtained to assess the integrity of the rotator cuff and the location of the effusion, showed a large subacromial and glenohumeral joint effusion as well as diffuse muscular edema (Figures 1A-1C).
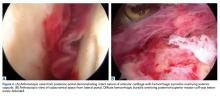
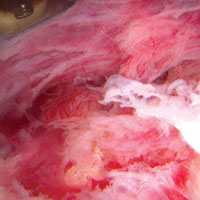
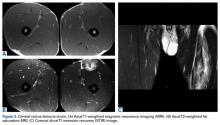
In light of the elevated infection findings of the laboratory tests and the positive culture, urgent arthroscopic irrigation and débridement of the right shoulder were indicated. Given the organism identified, transesophageal echocardiography was performed; there were no valvular vegetations. Creation of the posterior glenohumeral portal resulted in egress of turbid fluid, which was sent for culture. The subacromial space and the glenohumeral joint were thoroughly lavaged and the copious hemorrhagic synovitis débrided (Figures 2A, 2B).
The 8-week course of antibiotics normalized the patient’s ESR to 13 mm/h. Follow-up MRI showed improvement in the soft-tissue edema. Clinically, the patient reported minimal shoulder pain. He was undergoing physical therapy to regain strength and ROM.
Discussion
Staphylococcus aureus is the leading causative organism of septic arthritis, accounting for more than 60% of all cases.4 Conversely, the Streptococcus viridans group is rarely implicated in septic arthritis, accounting for <1% of cases.4S viridans is part of the commensal oral flora and has low virulence. This heterogeneous group is subdivided into S mitis, S salivarius, S anginosus, S mutans, and S bovis. The S mitis group is further subdivided into S sanguinis (formerly known as S sanguis) and S mitis. Infection by an organism of the S viridans group usually occurs on a previously injured focus, and the organism is a causative agent of bacterial endocarditis.5 Reported cases of septic arthritis caused by S viridans have predominantly involved the knee joint—with severe osteoarthritis, poor dental hygiene, and prior IV drug use identified as risk factors.5-7The shoulder joint is seldom involved in septic arthritis; estimated incidence is under 8%.8 Although overall incidence may rise in an increasingly elderly patient population, incidence of shoulder infection remains low.2,9
The main routes for developing septic arthritis include direct inoculation secondary to penetrating trauma or hematologic spread.10 Coatsworth and colleagues11 reported on iatrogenic S mitis septic arthritis of a shoulder arthroplasty during ultrasonography-guided aspiration by a technician who was not wearing a mask. Our institutional policy is to perform joint aspiration under strictly sterile conditions, which were adhered to in the present case. We surmise our patient developed transient bacteremia from the loss of several teeth, particularly given his poor dentition. Yombi and colleagues5 documented 2 cases of septic arthritis caused by Streptococcus gordonii, a relative of S sanguinis. One involved a previously replaced knee, and the other a native knee joint. Other cases of S viridans group septic arthritis have involved the knee,6,7,12,13 the sternoclavicular joint,14-16 and the acromioclavicular joint.17S sanguinis6,7,12,15,16 and S gordonii5 have been implicated in most cases, and an unspeciated S viridans in others.13,14,17 Concomitant periodontal disease has been reported in most cases as well,6,7,12,15 including our patient’s case. In the English-language literature, we found no other reports of S mitis as the causative agent of acute septic glenohumeral joint arthritis from hematogenous spread.
There should be no delay in diagnosing septic arthritis, and infected material should be removed from the joint. In animal models, complete joint destruction occurred only 5 weeks after inoculation with Staphylococcus aureus.10 Garofalo and colleagues18 reported a trend toward improved functional outcomes after earlier operative treatment. The choice of open surgical drainage vs repeat needle aspiration seems to be of little consequence, as both have good long-term outcomes, but open surgical drainage seems to result in better long-term functional ROM.2,9 However, results of a recent study suggested surgical treatment is not always superior to medical treatment for septic arthritis in native joints.19 In some cases involving S viridans species, treatment consisted of a combination of IV antibiotics and onetime or repeat aspiration;6,12-15 treatment in the remaining cases was surgical débridement.5,7,16,17 Given that S viridans is associated with bacterial endocarditis, echocardiography is essential if this organism is to be identified. Medical management and antibiotic treatment should be initiated after consultation with medical and infectious disease specialists.19We have reported a case of septic shoulder caused by S mitis, a low-virulence organism seldom associated with joint infection. The patient’s infection likely resulted from hematogenous spread from the oral cavity (dentition was poor). Urgent aspiration of the joint and baseline infection laboratory tests are recommended. MRI of the shoulder may show an effusion. Urgent arthroscopic irrigation and débridement can yield good clinical outcomes.
Am J Orthop. 2016;45(6):E343-E346. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Mathews CJ, Kingsley G, Field M, et al. Management of septic arthritis: a systematic review. Ann Rheum Dis. 2007;66(4):440-445.
2. Leslie BM, Harris JM 3rd, Driscoll D. Septic arthritis of the shoulder in adults. J Bone Joint Surg Am. 1989;71(10):1516-1522.
3. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology. 2001;40(1):24-30.
4. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61(3):267-269.
5. Yombi J, Belkhir L, Jonckheere S, et al. Streptococcus gordonii septic arthritis: two cases and review of literature. BMC Infect Dis. 2012;12:215.
6. Papaioannides D, Boniatsi L, Korantzopoulos P, Sinapidis D, Giotis C. Acute septic arthritis due to Streptococcus sanguis. Med Princ Pract. 2006;15(1):77-79.
7. Edson RS, Osmon DR, Berry DJ. Septic arthritis due to Streptococcus sanguis. Mayo Clin Proc. 2002;77(7):709-710.
8. Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK health district 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
9. Lossos IS, Yossepowitch O, Kandel L, Yardeni D, Arber N. Septic arthritis of the glenohumeral joint. A report of 11 cases and review of the literature. Medicine. 1998;77(3):177-187.
10. Esterhai JL Jr, Gelb I. Adult septic arthritis. Orthop Clin North Am. 1991;22(3):503-514.
11. Coatsworth NR, Huntington PG, Giuffre B, Kotsiou G. The doctor and the mask: iatrogenic septic arthritis caused by Streptoccocus mitis. Med J Aust. 2013;198(5):285-286.
12. Patrick MR, Lewis D. Short of a length: Streptococcus sanguis knee infection from dental source. Br J Rheumatol. 1992;31(8):569.
13. Barbadillo C, Trujillo A, Cuende E, Mazzucchelli R, Mulero J, Andreu JL. Septic arthritis due to Streptococcus viridans. Clin Exp Rheumatol. 1990;8(5):520-521.
14. Mata P, Molins A, de Oya M. Sternal arthritis caused by Streptococcus viridans in a heroin addict [in Spanish]. Med Clin. 1984;83(16):689.
15. Mandac I, Prkacin I, Sabljar Matovinovic M, Sustercic D. Septic arthritis due to Streptococcus sanguis. Coll Antropol. 2010;34(2):661-664.
16. Nitsche JF, Vaughan JH, Williams G, Curd JG. Septic sternoclavicular arthritis with Pasteurella multocida and Streptococcus sanguis. Arthritis Rheum. 1982;25(4):467-469.
17. Blankstein A, Amsallem JL, Rubenstein E, Horoszowski H, Farin I. Septic arthritis of the acromioclavicular joint. Arch Orthop Trauma Surg. 1985;103(6):417-418.
18. Garofalo R, Flanagin B, Cesari E, Vinci E, Conti M, Castagna A. Destructive septic arthritis of shoulder in adults. Musculoskelet Surg. 2014;98(supp 1):S35-S39.
19. Ravindran V, Logan I, Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology. 2009;48(10):1320-1322.
Septic arthritis predominantly involves the weight-bearing joints of the hip and knee, which account for nearly 60% of cases.1 In contrast, the shoulder joint is involved in 10% to 15% of cases, though this number may be higher among intravenous (IV) drug users.2 The most common causative organisms are the Staphylococcus species, followed closely by β-hemolytic streptococci, with these 2 groups accounting for more than 90% of all cases.3 The Streptococcus viridans group belongs to normal oral flora residing predominantly on the surface of teeth. Although well known for its ability to colonize heart valves and frequently cause bacterial endocarditis, this group has rarely been associated with septic arthritis. Furthermore, Streptococcus mitis, a subgroup of S viridans, has been implicated even less commonly.
In this article, we report a case of glenohumeral joint septic arthritis caused by S mitis. To our knowledge, such a case has not been previously reported in the English literature. Given the low virulence of this orally based bacterium, treating physicians must maintain clinical suspicion for the organism in the setting of persistent joint effusion and pain in association with periodontal disease or trauma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand-dominant 54-year-old man presented to Dr. Gruson with complaints of persistent right shoulder pain associated with worsening range of motion (ROM). Three weeks earlier, the patient reported being assaulted and noted progressive swelling about the right shoulder. He denied fevers, chills, or prior shoulder problems. Although his past medical history was remarkable for hepatitis C and diabetes, he was not taking any diabetic medications at that time. A review of systems was remarkable for poor dental hygiene, and the patient was missing several teeth, which he said had been knocked out during the assault. Physical examination revealed diffuse tenderness about the right shoulder and severe pain with all passive movement. The shoulder was pseudoparalyzed. There were no subcutaneous collections, wounds, or ecchymosis about the shoulder. Mild calor was noted on the right shoulder relative to the left. Radiographs of the right shoulder showed no acute osseous abnormalities.
Magnetic resonance imaging (MRI), which was urgently obtained to assess the integrity of the rotator cuff and the location of the effusion, showed a large subacromial and glenohumeral joint effusion as well as diffuse muscular edema (Figures 1A-1C).
In light of the elevated infection findings of the laboratory tests and the positive culture, urgent arthroscopic irrigation and débridement of the right shoulder were indicated. Given the organism identified, transesophageal echocardiography was performed; there were no valvular vegetations. Creation of the posterior glenohumeral portal resulted in egress of turbid fluid, which was sent for culture. The subacromial space and the glenohumeral joint were thoroughly lavaged and the copious hemorrhagic synovitis débrided (Figures 2A, 2B).
The 8-week course of antibiotics normalized the patient’s ESR to 13 mm/h. Follow-up MRI showed improvement in the soft-tissue edema. Clinically, the patient reported minimal shoulder pain. He was undergoing physical therapy to regain strength and ROM.
Discussion
Staphylococcus aureus is the leading causative organism of septic arthritis, accounting for more than 60% of all cases.4 Conversely, the Streptococcus viridans group is rarely implicated in septic arthritis, accounting for <1% of cases.4S viridans is part of the commensal oral flora and has low virulence. This heterogeneous group is subdivided into S mitis, S salivarius, S anginosus, S mutans, and S bovis. The S mitis group is further subdivided into S sanguinis (formerly known as S sanguis) and S mitis. Infection by an organism of the S viridans group usually occurs on a previously injured focus, and the organism is a causative agent of bacterial endocarditis.5 Reported cases of septic arthritis caused by S viridans have predominantly involved the knee joint—with severe osteoarthritis, poor dental hygiene, and prior IV drug use identified as risk factors.5-7The shoulder joint is seldom involved in septic arthritis; estimated incidence is under 8%.8 Although overall incidence may rise in an increasingly elderly patient population, incidence of shoulder infection remains low.2,9
The main routes for developing septic arthritis include direct inoculation secondary to penetrating trauma or hematologic spread.10 Coatsworth and colleagues11 reported on iatrogenic S mitis septic arthritis of a shoulder arthroplasty during ultrasonography-guided aspiration by a technician who was not wearing a mask. Our institutional policy is to perform joint aspiration under strictly sterile conditions, which were adhered to in the present case. We surmise our patient developed transient bacteremia from the loss of several teeth, particularly given his poor dentition. Yombi and colleagues5 documented 2 cases of septic arthritis caused by Streptococcus gordonii, a relative of S sanguinis. One involved a previously replaced knee, and the other a native knee joint. Other cases of S viridans group septic arthritis have involved the knee,6,7,12,13 the sternoclavicular joint,14-16 and the acromioclavicular joint.17S sanguinis6,7,12,15,16 and S gordonii5 have been implicated in most cases, and an unspeciated S viridans in others.13,14,17 Concomitant periodontal disease has been reported in most cases as well,6,7,12,15 including our patient’s case. In the English-language literature, we found no other reports of S mitis as the causative agent of acute septic glenohumeral joint arthritis from hematogenous spread.
There should be no delay in diagnosing septic arthritis, and infected material should be removed from the joint. In animal models, complete joint destruction occurred only 5 weeks after inoculation with Staphylococcus aureus.10 Garofalo and colleagues18 reported a trend toward improved functional outcomes after earlier operative treatment. The choice of open surgical drainage vs repeat needle aspiration seems to be of little consequence, as both have good long-term outcomes, but open surgical drainage seems to result in better long-term functional ROM.2,9 However, results of a recent study suggested surgical treatment is not always superior to medical treatment for septic arthritis in native joints.19 In some cases involving S viridans species, treatment consisted of a combination of IV antibiotics and onetime or repeat aspiration;6,12-15 treatment in the remaining cases was surgical débridement.5,7,16,17 Given that S viridans is associated with bacterial endocarditis, echocardiography is essential if this organism is to be identified. Medical management and antibiotic treatment should be initiated after consultation with medical and infectious disease specialists.19We have reported a case of septic shoulder caused by S mitis, a low-virulence organism seldom associated with joint infection. The patient’s infection likely resulted from hematogenous spread from the oral cavity (dentition was poor). Urgent aspiration of the joint and baseline infection laboratory tests are recommended. MRI of the shoulder may show an effusion. Urgent arthroscopic irrigation and débridement can yield good clinical outcomes.
Am J Orthop. 2016;45(6):E343-E346. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Septic arthritis predominantly involves the weight-bearing joints of the hip and knee, which account for nearly 60% of cases.1 In contrast, the shoulder joint is involved in 10% to 15% of cases, though this number may be higher among intravenous (IV) drug users.2 The most common causative organisms are the Staphylococcus species, followed closely by β-hemolytic streptococci, with these 2 groups accounting for more than 90% of all cases.3 The Streptococcus viridans group belongs to normal oral flora residing predominantly on the surface of teeth. Although well known for its ability to colonize heart valves and frequently cause bacterial endocarditis, this group has rarely been associated with septic arthritis. Furthermore, Streptococcus mitis, a subgroup of S viridans, has been implicated even less commonly.
In this article, we report a case of glenohumeral joint septic arthritis caused by S mitis. To our knowledge, such a case has not been previously reported in the English literature. Given the low virulence of this orally based bacterium, treating physicians must maintain clinical suspicion for the organism in the setting of persistent joint effusion and pain in association with periodontal disease or trauma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand-dominant 54-year-old man presented to Dr. Gruson with complaints of persistent right shoulder pain associated with worsening range of motion (ROM). Three weeks earlier, the patient reported being assaulted and noted progressive swelling about the right shoulder. He denied fevers, chills, or prior shoulder problems. Although his past medical history was remarkable for hepatitis C and diabetes, he was not taking any diabetic medications at that time. A review of systems was remarkable for poor dental hygiene, and the patient was missing several teeth, which he said had been knocked out during the assault. Physical examination revealed diffuse tenderness about the right shoulder and severe pain with all passive movement. The shoulder was pseudoparalyzed. There were no subcutaneous collections, wounds, or ecchymosis about the shoulder. Mild calor was noted on the right shoulder relative to the left. Radiographs of the right shoulder showed no acute osseous abnormalities.
Magnetic resonance imaging (MRI), which was urgently obtained to assess the integrity of the rotator cuff and the location of the effusion, showed a large subacromial and glenohumeral joint effusion as well as diffuse muscular edema (Figures 1A-1C).
In light of the elevated infection findings of the laboratory tests and the positive culture, urgent arthroscopic irrigation and débridement of the right shoulder were indicated. Given the organism identified, transesophageal echocardiography was performed; there were no valvular vegetations. Creation of the posterior glenohumeral portal resulted in egress of turbid fluid, which was sent for culture. The subacromial space and the glenohumeral joint were thoroughly lavaged and the copious hemorrhagic synovitis débrided (Figures 2A, 2B).
The 8-week course of antibiotics normalized the patient’s ESR to 13 mm/h. Follow-up MRI showed improvement in the soft-tissue edema. Clinically, the patient reported minimal shoulder pain. He was undergoing physical therapy to regain strength and ROM.
Discussion
Staphylococcus aureus is the leading causative organism of septic arthritis, accounting for more than 60% of all cases.4 Conversely, the Streptococcus viridans group is rarely implicated in septic arthritis, accounting for <1% of cases.4S viridans is part of the commensal oral flora and has low virulence. This heterogeneous group is subdivided into S mitis, S salivarius, S anginosus, S mutans, and S bovis. The S mitis group is further subdivided into S sanguinis (formerly known as S sanguis) and S mitis. Infection by an organism of the S viridans group usually occurs on a previously injured focus, and the organism is a causative agent of bacterial endocarditis.5 Reported cases of septic arthritis caused by S viridans have predominantly involved the knee joint—with severe osteoarthritis, poor dental hygiene, and prior IV drug use identified as risk factors.5-7The shoulder joint is seldom involved in septic arthritis; estimated incidence is under 8%.8 Although overall incidence may rise in an increasingly elderly patient population, incidence of shoulder infection remains low.2,9
The main routes for developing septic arthritis include direct inoculation secondary to penetrating trauma or hematologic spread.10 Coatsworth and colleagues11 reported on iatrogenic S mitis septic arthritis of a shoulder arthroplasty during ultrasonography-guided aspiration by a technician who was not wearing a mask. Our institutional policy is to perform joint aspiration under strictly sterile conditions, which were adhered to in the present case. We surmise our patient developed transient bacteremia from the loss of several teeth, particularly given his poor dentition. Yombi and colleagues5 documented 2 cases of septic arthritis caused by Streptococcus gordonii, a relative of S sanguinis. One involved a previously replaced knee, and the other a native knee joint. Other cases of S viridans group septic arthritis have involved the knee,6,7,12,13 the sternoclavicular joint,14-16 and the acromioclavicular joint.17S sanguinis6,7,12,15,16 and S gordonii5 have been implicated in most cases, and an unspeciated S viridans in others.13,14,17 Concomitant periodontal disease has been reported in most cases as well,6,7,12,15 including our patient’s case. In the English-language literature, we found no other reports of S mitis as the causative agent of acute septic glenohumeral joint arthritis from hematogenous spread.
There should be no delay in diagnosing septic arthritis, and infected material should be removed from the joint. In animal models, complete joint destruction occurred only 5 weeks after inoculation with Staphylococcus aureus.10 Garofalo and colleagues18 reported a trend toward improved functional outcomes after earlier operative treatment. The choice of open surgical drainage vs repeat needle aspiration seems to be of little consequence, as both have good long-term outcomes, but open surgical drainage seems to result in better long-term functional ROM.2,9 However, results of a recent study suggested surgical treatment is not always superior to medical treatment for septic arthritis in native joints.19 In some cases involving S viridans species, treatment consisted of a combination of IV antibiotics and onetime or repeat aspiration;6,12-15 treatment in the remaining cases was surgical débridement.5,7,16,17 Given that S viridans is associated with bacterial endocarditis, echocardiography is essential if this organism is to be identified. Medical management and antibiotic treatment should be initiated after consultation with medical and infectious disease specialists.19We have reported a case of septic shoulder caused by S mitis, a low-virulence organism seldom associated with joint infection. The patient’s infection likely resulted from hematogenous spread from the oral cavity (dentition was poor). Urgent aspiration of the joint and baseline infection laboratory tests are recommended. MRI of the shoulder may show an effusion. Urgent arthroscopic irrigation and débridement can yield good clinical outcomes.
Am J Orthop. 2016;45(6):E343-E346. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Mathews CJ, Kingsley G, Field M, et al. Management of septic arthritis: a systematic review. Ann Rheum Dis. 2007;66(4):440-445.
2. Leslie BM, Harris JM 3rd, Driscoll D. Septic arthritis of the shoulder in adults. J Bone Joint Surg Am. 1989;71(10):1516-1522.
3. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology. 2001;40(1):24-30.
4. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61(3):267-269.
5. Yombi J, Belkhir L, Jonckheere S, et al. Streptococcus gordonii septic arthritis: two cases and review of literature. BMC Infect Dis. 2012;12:215.
6. Papaioannides D, Boniatsi L, Korantzopoulos P, Sinapidis D, Giotis C. Acute septic arthritis due to Streptococcus sanguis. Med Princ Pract. 2006;15(1):77-79.
7. Edson RS, Osmon DR, Berry DJ. Septic arthritis due to Streptococcus sanguis. Mayo Clin Proc. 2002;77(7):709-710.
8. Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK health district 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
9. Lossos IS, Yossepowitch O, Kandel L, Yardeni D, Arber N. Septic arthritis of the glenohumeral joint. A report of 11 cases and review of the literature. Medicine. 1998;77(3):177-187.
10. Esterhai JL Jr, Gelb I. Adult septic arthritis. Orthop Clin North Am. 1991;22(3):503-514.
11. Coatsworth NR, Huntington PG, Giuffre B, Kotsiou G. The doctor and the mask: iatrogenic septic arthritis caused by Streptoccocus mitis. Med J Aust. 2013;198(5):285-286.
12. Patrick MR, Lewis D. Short of a length: Streptococcus sanguis knee infection from dental source. Br J Rheumatol. 1992;31(8):569.
13. Barbadillo C, Trujillo A, Cuende E, Mazzucchelli R, Mulero J, Andreu JL. Septic arthritis due to Streptococcus viridans. Clin Exp Rheumatol. 1990;8(5):520-521.
14. Mata P, Molins A, de Oya M. Sternal arthritis caused by Streptococcus viridans in a heroin addict [in Spanish]. Med Clin. 1984;83(16):689.
15. Mandac I, Prkacin I, Sabljar Matovinovic M, Sustercic D. Septic arthritis due to Streptococcus sanguis. Coll Antropol. 2010;34(2):661-664.
16. Nitsche JF, Vaughan JH, Williams G, Curd JG. Septic sternoclavicular arthritis with Pasteurella multocida and Streptococcus sanguis. Arthritis Rheum. 1982;25(4):467-469.
17. Blankstein A, Amsallem JL, Rubenstein E, Horoszowski H, Farin I. Septic arthritis of the acromioclavicular joint. Arch Orthop Trauma Surg. 1985;103(6):417-418.
18. Garofalo R, Flanagin B, Cesari E, Vinci E, Conti M, Castagna A. Destructive septic arthritis of shoulder in adults. Musculoskelet Surg. 2014;98(supp 1):S35-S39.
19. Ravindran V, Logan I, Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology. 2009;48(10):1320-1322.
1. Mathews CJ, Kingsley G, Field M, et al. Management of septic arthritis: a systematic review. Ann Rheum Dis. 2007;66(4):440-445.
2. Leslie BM, Harris JM 3rd, Driscoll D. Septic arthritis of the shoulder in adults. J Bone Joint Surg Am. 1989;71(10):1516-1522.
3. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology. 2001;40(1):24-30.
4. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61(3):267-269.
5. Yombi J, Belkhir L, Jonckheere S, et al. Streptococcus gordonii septic arthritis: two cases and review of literature. BMC Infect Dis. 2012;12:215.
6. Papaioannides D, Boniatsi L, Korantzopoulos P, Sinapidis D, Giotis C. Acute septic arthritis due to Streptococcus sanguis. Med Princ Pract. 2006;15(1):77-79.
7. Edson RS, Osmon DR, Berry DJ. Septic arthritis due to Streptococcus sanguis. Mayo Clin Proc. 2002;77(7):709-710.
8. Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK health district 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
9. Lossos IS, Yossepowitch O, Kandel L, Yardeni D, Arber N. Septic arthritis of the glenohumeral joint. A report of 11 cases and review of the literature. Medicine. 1998;77(3):177-187.
10. Esterhai JL Jr, Gelb I. Adult septic arthritis. Orthop Clin North Am. 1991;22(3):503-514.
11. Coatsworth NR, Huntington PG, Giuffre B, Kotsiou G. The doctor and the mask: iatrogenic septic arthritis caused by Streptoccocus mitis. Med J Aust. 2013;198(5):285-286.
12. Patrick MR, Lewis D. Short of a length: Streptococcus sanguis knee infection from dental source. Br J Rheumatol. 1992;31(8):569.
13. Barbadillo C, Trujillo A, Cuende E, Mazzucchelli R, Mulero J, Andreu JL. Septic arthritis due to Streptococcus viridans. Clin Exp Rheumatol. 1990;8(5):520-521.
14. Mata P, Molins A, de Oya M. Sternal arthritis caused by Streptococcus viridans in a heroin addict [in Spanish]. Med Clin. 1984;83(16):689.
15. Mandac I, Prkacin I, Sabljar Matovinovic M, Sustercic D. Septic arthritis due to Streptococcus sanguis. Coll Antropol. 2010;34(2):661-664.
16. Nitsche JF, Vaughan JH, Williams G, Curd JG. Septic sternoclavicular arthritis with Pasteurella multocida and Streptococcus sanguis. Arthritis Rheum. 1982;25(4):467-469.
17. Blankstein A, Amsallem JL, Rubenstein E, Horoszowski H, Farin I. Septic arthritis of the acromioclavicular joint. Arch Orthop Trauma Surg. 1985;103(6):417-418.
18. Garofalo R, Flanagin B, Cesari E, Vinci E, Conti M, Castagna A. Destructive septic arthritis of shoulder in adults. Musculoskelet Surg. 2014;98(supp 1):S35-S39.
19. Ravindran V, Logan I, Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology. 2009;48(10):1320-1322.
Fact or Fiction: Is Orthopedic Follow-Up Worse for Patients Who Sustain Penetrating Trauma?
There is a paucity of literature on how mechanism of injury may be associated with patient retention. Failure to attend outpatient clinics is a form of noncompliance and a major obstacle to safe, effective, and efficient healthcare delivery. Noncompliance may lead to increased patient morbidity and carries substantial financial implications for the healthcare system.1,2 In addition to these direct patient and healthcare issues, loss of patient follow-up or the belief of potential loss of follow-up of penetrating trauma patients may also significantly affect research studies. These patients often may be excluded from studies, even if they might otherwise meet inclusion criteria, because of concerns that they are unlikely to follow-up after leaving hospital. Is this myth or fact? To validate or to disprove this selection bias, we conducted a study in which we retrospectively evaluated long bone fractures caused by either penetrating or blunt trauma.
Methods
After obtaining Institutional Review Board approval for this study, we used the trauma database of an American College of Surgeons–verified level I trauma center in a major Midwest metropolitan area to compile a list of all cases of long bone fractures caused by penetrating trauma between 2006 and 2009 (N = 132). Gunshot wounds were the mechanism of injury for the penetrating trauma. We also compiled a list of control cases—long bone fractures caused by blunt trauma in patients demographically matched to the penetrating group patients on sex, race, and age (N = 104) (Table).
We retrospectively performed chart reviews to obtain patient follow-up data 3, 6, 9, and 12 months after injury from penetrating or blunt trauma. Patients scheduled to return on an as-needed basis were considered to have completed follow-up. The 2 groups were also statistically compared with respect to sex, race, age, surgical fixation, and history of tobacco, alcohol, or drug use.
SAS/STAT Version 8 (SAS Institute) was used to test the equality of survival functions (patient retention) for the penetrating and blunt trauma patient groups. A similar comparison was made for the categories of sex, race, and age. Pearson χ2 test was used to compare the 12-month survival rates of the 2 treatment groups across sex and race. Binary logistic regression was used to compare the 12-month survival rates of the 2 treatment groups removing the effect of age. A comparison of the frequency distributions of the 2 treatment groups with respect to alcohol use, tobacco use, drug use, and surgical intervention was also performed. Power analysis showed power of more than 90% in detecting at least a 20% difference in the follow-up rates between the penetrating and blunt trauma groups based on our sample size.
Results
There was no statistically significant difference (P = .736) between the penetrating and blunt trauma patients in terms of follow-up within 1 year after injury. At 1 year, 103 (78%) of the 132 penetrating trauma patients and 83 (80%) of the 104 blunt trauma patients were lost to follow-up (Figure).
Discussion
Trauma outcomes historically have been difficult to determine because of lack of patient follow-up. In a simulation series, Zelle and colleagues3 found that the turning point from significant to nonsignificant varied from 15% to 75% loss of follow-up, thus compromising the validity of a study. They and others have emphasized the importance of establishing research protocols to minimize follow-up loss and eliminate reporting bias, ensure randomization, and report accurate outcomes.3-7
Very few have tried to investigate factors associated with failure to follow up after trauma.1,2,4 Leukhardt and colleagues4 evaluated the medical services that trauma patients follow up with most often. Orthopedic surgery had the largest portion of follow-up visits (37%), followed by the trauma surgery clinic and the emergency department (19% each). The authors also found that penetrating trauma patients were more likely to follow up, though more than 90% of the authors’ patients had blunt trauma. Although our study did not support their finding, it does call into question the commonly held belief that penetrating trauma patients are less likely to follow up, as our study found no difference in follow-up between penetrating and blunt trauma patients.
One of the most interesting findings in this retrospective study is that almost 80% of patients were lost to follow-up regardless of mechanism of injury. Most prospective studies try to reduce loss to follow-up to below 10%. This difference may be attributable to having a dedicated research team and the resources required to ensure follow-up of research patients to improve follow-up beyond baseline values. At our institution, 13 prospective studies (most multicenter) are currently enrolling patients, and the worst loss to follow-up has been 30%. The majority of the studies have loss to follow-up of 15% or less. This low rate represents a significant difference from the 80% “baseline” clinical loss to follow-up for the blunt and penetrating trauma patients treated at our institution, based on the findings of this study. We have been improving follow-up by having dedicated research coordinators call patients to remind them of their appointments (all clinic patients who are not research patients receive a recorded reminder); by having the hospital agree that research patients can be seen without charge (by the facility or the physician), which helps defray costs to the patient; and by excluding patients the principal investigator thinks are unlikely to follow up. Patients unlikely to follow up are routinely excluded by all centers that enroll in prospective studies. Although it is difficult to quantitate, this factor may play a large role in reducing loss to follow-up. Penetrating trauma patients historically routinely biased investigators to exclude them from studies, regardless of whether being considered unlikely to follow-up was an exclusion criterion. Our study results suggest this bias may not be valid.
Our study evaluated the role of mechanism of injury, penetrating or blunt trauma, and the respective orthopedic follow-up. There was no statistically significant difference in the 1-year follow-up rate based on the mechanism of injury. Our study was conducted with a well-matched control group that eliminated potential confounding variables, such as sex, race, age, tobacco use, and alcohol use. Although the prevalence of drug use was higher in the penetrating trauma group, patient retention seemed not to be affected by it. Surprisingly, patient loss to follow-up was extremely high (almost 80%) for both the penetrating and blunt trauma patient groups at the 1-year mark. Our findings call into question the commonly accepted theory that patients with penetrating injuries are less likely to follow up, at least in an academic level I trauma center population. We suggest that the commonly held belief that penetrating trauma patients are less likely to follow up may not be valid and that, when prospective studies are designed, it may not be appropriate to exclude penetrating trauma patients on this basis alone.
The primary limitation of this study is that it was performed at a single institution. Eighty-five percent of blunt trauma patients and 93% of penetrating trauma patients live in the county that is predominantly served by our institution, and electronic medical records from all major hospitals in the metropolitan area are linked, suggesting that the large majority of patients lost to follow-up do not seek further medical care, at least not from local facilities in our metropolitan area. A prospective multicenter study is being designed to help us gain a better understanding of the variables that affect musculoskeletal trauma patient follow-up and learn interventional strategies that can be used to improve patient retention.
Dr. Turner is an Orthopedic Surgeon, Rockwood Clinic, Spokane, Washington. Dr. Turner was a resident at the time the article was written. Dr. Hiatt is an Anesthesia Resident, University of Louisville Department of Anesthesiology and Perioperative Medicine, Louisville, Kentucky. Dr. Mullis is Chief of the Orthopaedic Trauma Service, Eskenazi Health, and Professor & Program Director, Indiana University School of Medicine Department of Orthopaedics, Indianapolis, Indiana.
Acknowledgments: This study was first reported in a poster presentation at the annual meeting of the Orthopaedic Trauma Association, October 2013, Phoenix, Arizona.
The authors gratefully acknowledge and thank Jyoti Sarkar, PhD, for his assistance with statistical analysis and manuscript preparation.
Am J Orthop. 2016;45(6):E331-E334. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Sciberras N, Gregori A, Holt G. The ethical and practical challenges of patient noncompliance in orthopaedic surgery. J Bone Joint Surg Am. 2013;95(9):e61.
2. Sharma H, Crane E, Syme B, Foxworthy M. Non-compliance in orthopaedic surgery and its ethical challenges. Orthop Trauma. 2007;21(4):310-313.
3. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181.
4. Leukhardt WH, Golob JF, McCoy AM, Fadlalla AM, Malangoni MA, Claridge JA. Follow-up disparities after trauma: a real problem for outcomes research. Am J Surg. 2010;199(3):348-352.
5. Shumaker SA, Dugan E, Bowen DJ. Enhancing adherence in randomized controlled clinical trials. Control Clin Trials. 2000;21(5 suppl):226S-232S.
6. Smith JS, Watts HG. Methods for locating missing patients for the purpose of long-term clinical studies. J Bone Joint Surg Am. 1998;80(3):431-438.
7. Sprague S, Leece P, Bhandari M, Tornetta P 3rd, Schemitsch E, Swiontkowski MF; S.P.R.I.N.T. Investigators. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725.
There is a paucity of literature on how mechanism of injury may be associated with patient retention. Failure to attend outpatient clinics is a form of noncompliance and a major obstacle to safe, effective, and efficient healthcare delivery. Noncompliance may lead to increased patient morbidity and carries substantial financial implications for the healthcare system.1,2 In addition to these direct patient and healthcare issues, loss of patient follow-up or the belief of potential loss of follow-up of penetrating trauma patients may also significantly affect research studies. These patients often may be excluded from studies, even if they might otherwise meet inclusion criteria, because of concerns that they are unlikely to follow-up after leaving hospital. Is this myth or fact? To validate or to disprove this selection bias, we conducted a study in which we retrospectively evaluated long bone fractures caused by either penetrating or blunt trauma.
Methods
After obtaining Institutional Review Board approval for this study, we used the trauma database of an American College of Surgeons–verified level I trauma center in a major Midwest metropolitan area to compile a list of all cases of long bone fractures caused by penetrating trauma between 2006 and 2009 (N = 132). Gunshot wounds were the mechanism of injury for the penetrating trauma. We also compiled a list of control cases—long bone fractures caused by blunt trauma in patients demographically matched to the penetrating group patients on sex, race, and age (N = 104) (Table).
We retrospectively performed chart reviews to obtain patient follow-up data 3, 6, 9, and 12 months after injury from penetrating or blunt trauma. Patients scheduled to return on an as-needed basis were considered to have completed follow-up. The 2 groups were also statistically compared with respect to sex, race, age, surgical fixation, and history of tobacco, alcohol, or drug use.
SAS/STAT Version 8 (SAS Institute) was used to test the equality of survival functions (patient retention) for the penetrating and blunt trauma patient groups. A similar comparison was made for the categories of sex, race, and age. Pearson χ2 test was used to compare the 12-month survival rates of the 2 treatment groups across sex and race. Binary logistic regression was used to compare the 12-month survival rates of the 2 treatment groups removing the effect of age. A comparison of the frequency distributions of the 2 treatment groups with respect to alcohol use, tobacco use, drug use, and surgical intervention was also performed. Power analysis showed power of more than 90% in detecting at least a 20% difference in the follow-up rates between the penetrating and blunt trauma groups based on our sample size.
Results
There was no statistically significant difference (P = .736) between the penetrating and blunt trauma patients in terms of follow-up within 1 year after injury. At 1 year, 103 (78%) of the 132 penetrating trauma patients and 83 (80%) of the 104 blunt trauma patients were lost to follow-up (Figure).
Discussion
Trauma outcomes historically have been difficult to determine because of lack of patient follow-up. In a simulation series, Zelle and colleagues3 found that the turning point from significant to nonsignificant varied from 15% to 75% loss of follow-up, thus compromising the validity of a study. They and others have emphasized the importance of establishing research protocols to minimize follow-up loss and eliminate reporting bias, ensure randomization, and report accurate outcomes.3-7
Very few have tried to investigate factors associated with failure to follow up after trauma.1,2,4 Leukhardt and colleagues4 evaluated the medical services that trauma patients follow up with most often. Orthopedic surgery had the largest portion of follow-up visits (37%), followed by the trauma surgery clinic and the emergency department (19% each). The authors also found that penetrating trauma patients were more likely to follow up, though more than 90% of the authors’ patients had blunt trauma. Although our study did not support their finding, it does call into question the commonly held belief that penetrating trauma patients are less likely to follow up, as our study found no difference in follow-up between penetrating and blunt trauma patients.
One of the most interesting findings in this retrospective study is that almost 80% of patients were lost to follow-up regardless of mechanism of injury. Most prospective studies try to reduce loss to follow-up to below 10%. This difference may be attributable to having a dedicated research team and the resources required to ensure follow-up of research patients to improve follow-up beyond baseline values. At our institution, 13 prospective studies (most multicenter) are currently enrolling patients, and the worst loss to follow-up has been 30%. The majority of the studies have loss to follow-up of 15% or less. This low rate represents a significant difference from the 80% “baseline” clinical loss to follow-up for the blunt and penetrating trauma patients treated at our institution, based on the findings of this study. We have been improving follow-up by having dedicated research coordinators call patients to remind them of their appointments (all clinic patients who are not research patients receive a recorded reminder); by having the hospital agree that research patients can be seen without charge (by the facility or the physician), which helps defray costs to the patient; and by excluding patients the principal investigator thinks are unlikely to follow up. Patients unlikely to follow up are routinely excluded by all centers that enroll in prospective studies. Although it is difficult to quantitate, this factor may play a large role in reducing loss to follow-up. Penetrating trauma patients historically routinely biased investigators to exclude them from studies, regardless of whether being considered unlikely to follow-up was an exclusion criterion. Our study results suggest this bias may not be valid.
Our study evaluated the role of mechanism of injury, penetrating or blunt trauma, and the respective orthopedic follow-up. There was no statistically significant difference in the 1-year follow-up rate based on the mechanism of injury. Our study was conducted with a well-matched control group that eliminated potential confounding variables, such as sex, race, age, tobacco use, and alcohol use. Although the prevalence of drug use was higher in the penetrating trauma group, patient retention seemed not to be affected by it. Surprisingly, patient loss to follow-up was extremely high (almost 80%) for both the penetrating and blunt trauma patient groups at the 1-year mark. Our findings call into question the commonly accepted theory that patients with penetrating injuries are less likely to follow up, at least in an academic level I trauma center population. We suggest that the commonly held belief that penetrating trauma patients are less likely to follow up may not be valid and that, when prospective studies are designed, it may not be appropriate to exclude penetrating trauma patients on this basis alone.
The primary limitation of this study is that it was performed at a single institution. Eighty-five percent of blunt trauma patients and 93% of penetrating trauma patients live in the county that is predominantly served by our institution, and electronic medical records from all major hospitals in the metropolitan area are linked, suggesting that the large majority of patients lost to follow-up do not seek further medical care, at least not from local facilities in our metropolitan area. A prospective multicenter study is being designed to help us gain a better understanding of the variables that affect musculoskeletal trauma patient follow-up and learn interventional strategies that can be used to improve patient retention.
Dr. Turner is an Orthopedic Surgeon, Rockwood Clinic, Spokane, Washington. Dr. Turner was a resident at the time the article was written. Dr. Hiatt is an Anesthesia Resident, University of Louisville Department of Anesthesiology and Perioperative Medicine, Louisville, Kentucky. Dr. Mullis is Chief of the Orthopaedic Trauma Service, Eskenazi Health, and Professor & Program Director, Indiana University School of Medicine Department of Orthopaedics, Indianapolis, Indiana.
Acknowledgments: This study was first reported in a poster presentation at the annual meeting of the Orthopaedic Trauma Association, October 2013, Phoenix, Arizona.
The authors gratefully acknowledge and thank Jyoti Sarkar, PhD, for his assistance with statistical analysis and manuscript preparation.
Am J Orthop. 2016;45(6):E331-E334. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
There is a paucity of literature on how mechanism of injury may be associated with patient retention. Failure to attend outpatient clinics is a form of noncompliance and a major obstacle to safe, effective, and efficient healthcare delivery. Noncompliance may lead to increased patient morbidity and carries substantial financial implications for the healthcare system.1,2 In addition to these direct patient and healthcare issues, loss of patient follow-up or the belief of potential loss of follow-up of penetrating trauma patients may also significantly affect research studies. These patients often may be excluded from studies, even if they might otherwise meet inclusion criteria, because of concerns that they are unlikely to follow-up after leaving hospital. Is this myth or fact? To validate or to disprove this selection bias, we conducted a study in which we retrospectively evaluated long bone fractures caused by either penetrating or blunt trauma.
Methods
After obtaining Institutional Review Board approval for this study, we used the trauma database of an American College of Surgeons–verified level I trauma center in a major Midwest metropolitan area to compile a list of all cases of long bone fractures caused by penetrating trauma between 2006 and 2009 (N = 132). Gunshot wounds were the mechanism of injury for the penetrating trauma. We also compiled a list of control cases—long bone fractures caused by blunt trauma in patients demographically matched to the penetrating group patients on sex, race, and age (N = 104) (Table).
We retrospectively performed chart reviews to obtain patient follow-up data 3, 6, 9, and 12 months after injury from penetrating or blunt trauma. Patients scheduled to return on an as-needed basis were considered to have completed follow-up. The 2 groups were also statistically compared with respect to sex, race, age, surgical fixation, and history of tobacco, alcohol, or drug use.
SAS/STAT Version 8 (SAS Institute) was used to test the equality of survival functions (patient retention) for the penetrating and blunt trauma patient groups. A similar comparison was made for the categories of sex, race, and age. Pearson χ2 test was used to compare the 12-month survival rates of the 2 treatment groups across sex and race. Binary logistic regression was used to compare the 12-month survival rates of the 2 treatment groups removing the effect of age. A comparison of the frequency distributions of the 2 treatment groups with respect to alcohol use, tobacco use, drug use, and surgical intervention was also performed. Power analysis showed power of more than 90% in detecting at least a 20% difference in the follow-up rates between the penetrating and blunt trauma groups based on our sample size.
Results
There was no statistically significant difference (P = .736) between the penetrating and blunt trauma patients in terms of follow-up within 1 year after injury. At 1 year, 103 (78%) of the 132 penetrating trauma patients and 83 (80%) of the 104 blunt trauma patients were lost to follow-up (Figure).
Discussion
Trauma outcomes historically have been difficult to determine because of lack of patient follow-up. In a simulation series, Zelle and colleagues3 found that the turning point from significant to nonsignificant varied from 15% to 75% loss of follow-up, thus compromising the validity of a study. They and others have emphasized the importance of establishing research protocols to minimize follow-up loss and eliminate reporting bias, ensure randomization, and report accurate outcomes.3-7
Very few have tried to investigate factors associated with failure to follow up after trauma.1,2,4 Leukhardt and colleagues4 evaluated the medical services that trauma patients follow up with most often. Orthopedic surgery had the largest portion of follow-up visits (37%), followed by the trauma surgery clinic and the emergency department (19% each). The authors also found that penetrating trauma patients were more likely to follow up, though more than 90% of the authors’ patients had blunt trauma. Although our study did not support their finding, it does call into question the commonly held belief that penetrating trauma patients are less likely to follow up, as our study found no difference in follow-up between penetrating and blunt trauma patients.
One of the most interesting findings in this retrospective study is that almost 80% of patients were lost to follow-up regardless of mechanism of injury. Most prospective studies try to reduce loss to follow-up to below 10%. This difference may be attributable to having a dedicated research team and the resources required to ensure follow-up of research patients to improve follow-up beyond baseline values. At our institution, 13 prospective studies (most multicenter) are currently enrolling patients, and the worst loss to follow-up has been 30%. The majority of the studies have loss to follow-up of 15% or less. This low rate represents a significant difference from the 80% “baseline” clinical loss to follow-up for the blunt and penetrating trauma patients treated at our institution, based on the findings of this study. We have been improving follow-up by having dedicated research coordinators call patients to remind them of their appointments (all clinic patients who are not research patients receive a recorded reminder); by having the hospital agree that research patients can be seen without charge (by the facility or the physician), which helps defray costs to the patient; and by excluding patients the principal investigator thinks are unlikely to follow up. Patients unlikely to follow up are routinely excluded by all centers that enroll in prospective studies. Although it is difficult to quantitate, this factor may play a large role in reducing loss to follow-up. Penetrating trauma patients historically routinely biased investigators to exclude them from studies, regardless of whether being considered unlikely to follow-up was an exclusion criterion. Our study results suggest this bias may not be valid.
Our study evaluated the role of mechanism of injury, penetrating or blunt trauma, and the respective orthopedic follow-up. There was no statistically significant difference in the 1-year follow-up rate based on the mechanism of injury. Our study was conducted with a well-matched control group that eliminated potential confounding variables, such as sex, race, age, tobacco use, and alcohol use. Although the prevalence of drug use was higher in the penetrating trauma group, patient retention seemed not to be affected by it. Surprisingly, patient loss to follow-up was extremely high (almost 80%) for both the penetrating and blunt trauma patient groups at the 1-year mark. Our findings call into question the commonly accepted theory that patients with penetrating injuries are less likely to follow up, at least in an academic level I trauma center population. We suggest that the commonly held belief that penetrating trauma patients are less likely to follow up may not be valid and that, when prospective studies are designed, it may not be appropriate to exclude penetrating trauma patients on this basis alone.
The primary limitation of this study is that it was performed at a single institution. Eighty-five percent of blunt trauma patients and 93% of penetrating trauma patients live in the county that is predominantly served by our institution, and electronic medical records from all major hospitals in the metropolitan area are linked, suggesting that the large majority of patients lost to follow-up do not seek further medical care, at least not from local facilities in our metropolitan area. A prospective multicenter study is being designed to help us gain a better understanding of the variables that affect musculoskeletal trauma patient follow-up and learn interventional strategies that can be used to improve patient retention.
Dr. Turner is an Orthopedic Surgeon, Rockwood Clinic, Spokane, Washington. Dr. Turner was a resident at the time the article was written. Dr. Hiatt is an Anesthesia Resident, University of Louisville Department of Anesthesiology and Perioperative Medicine, Louisville, Kentucky. Dr. Mullis is Chief of the Orthopaedic Trauma Service, Eskenazi Health, and Professor & Program Director, Indiana University School of Medicine Department of Orthopaedics, Indianapolis, Indiana.
Acknowledgments: This study was first reported in a poster presentation at the annual meeting of the Orthopaedic Trauma Association, October 2013, Phoenix, Arizona.
The authors gratefully acknowledge and thank Jyoti Sarkar, PhD, for his assistance with statistical analysis and manuscript preparation.
Am J Orthop. 2016;45(6):E331-E334. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Sciberras N, Gregori A, Holt G. The ethical and practical challenges of patient noncompliance in orthopaedic surgery. J Bone Joint Surg Am. 2013;95(9):e61.
2. Sharma H, Crane E, Syme B, Foxworthy M. Non-compliance in orthopaedic surgery and its ethical challenges. Orthop Trauma. 2007;21(4):310-313.
3. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181.
4. Leukhardt WH, Golob JF, McCoy AM, Fadlalla AM, Malangoni MA, Claridge JA. Follow-up disparities after trauma: a real problem for outcomes research. Am J Surg. 2010;199(3):348-352.
5. Shumaker SA, Dugan E, Bowen DJ. Enhancing adherence in randomized controlled clinical trials. Control Clin Trials. 2000;21(5 suppl):226S-232S.
6. Smith JS, Watts HG. Methods for locating missing patients for the purpose of long-term clinical studies. J Bone Joint Surg Am. 1998;80(3):431-438.
7. Sprague S, Leece P, Bhandari M, Tornetta P 3rd, Schemitsch E, Swiontkowski MF; S.P.R.I.N.T. Investigators. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725.
1. Sciberras N, Gregori A, Holt G. The ethical and practical challenges of patient noncompliance in orthopaedic surgery. J Bone Joint Surg Am. 2013;95(9):e61.
2. Sharma H, Crane E, Syme B, Foxworthy M. Non-compliance in orthopaedic surgery and its ethical challenges. Orthop Trauma. 2007;21(4):310-313.
3. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181.
4. Leukhardt WH, Golob JF, McCoy AM, Fadlalla AM, Malangoni MA, Claridge JA. Follow-up disparities after trauma: a real problem for outcomes research. Am J Surg. 2010;199(3):348-352.
5. Shumaker SA, Dugan E, Bowen DJ. Enhancing adherence in randomized controlled clinical trials. Control Clin Trials. 2000;21(5 suppl):226S-232S.
6. Smith JS, Watts HG. Methods for locating missing patients for the purpose of long-term clinical studies. J Bone Joint Surg Am. 1998;80(3):431-438.
7. Sprague S, Leece P, Bhandari M, Tornetta P 3rd, Schemitsch E, Swiontkowski MF; S.P.R.I.N.T. Investigators. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725.
Impact of a Musculoskeletal Clerkship on Orthopedic Surgery Applicant Diversity
As the United States becomes increasingly diverse, with predictions that by 2045 minorities will comprise 50% or more of the population,1 the demographics of the orthopedic surgery population will also likely diversify. It is important that orthopedic surgeons shift in their diversity as well. Lack of diversity in orthopedics (women and racial minorities are underrepresented) relative to the national population and other surgical specialties and their training programs is well documented.2-8
More concerning, the diversity of orthopedic residents does not compare favorably with that of medical school attendees.4,9 The difference suggests the greatest loss of potential diversity occurs during the transition from medical school to residency. A national study demonstrated that instruction in musculoskeletal medicine led to an increase in application rates nationally.10 However, the authors of that study stated they were unexpectedly limited by its large size—they could not validate the accuracy of curriculum data and could not differentiate between a 1-day required experience and a 4-week rotation.
In the present study, which accounted for curricular factors, we compared our medical students’ application rates to orthopedics residencies based on sex and race before and after introduction of a required third-year musculoskeletal clerkship. We hypothesized that making the curriculum a requirement would increase the number of applicants and increase the diversity of applicants in terms of both women and underrepresented minorities. This hypothesis was based on the rationale that these groups might not consider an orthopedics residency without first being directly exposed to orthopedics. We also wanted to determine what factors influenced applicants to choose orthopedic surgery.
Methods
Curriculum
Before 2006, third-year students spent 3 months completing a surgery clerkship. Some students interested in orthopedic surgery would have to wait until their fourth year to complete an elective in orthopedic surgery, and uninterested students would not be exposed at all. Starting in 2006, 1 month of the third-year surgery clerkship was required to be completed in musculoskeletal surgery: orthopedic surgery, plastic surgery, or neurosurgical spine. Plastic surgery was an option, as it exposed students to hand surgery and flap reconstruction.
During the 12-year study period, overall teaching hours in the preclinical curriculum did not change, and there were no other structural changes to the preclinical or clinical curriculum. The orthopedics department increased its faculty from 23 in 2000 to 34 in 2012. Number of female faculty increased from 1 to 3, representing a 4% to 9% increase in department faculty. Throughout the 12 years, there were no underrepresented minority faculty. Total number of residents increased from 26 in 2000 to 30 in 2012. Number of female residents varied year to year, from a low of 3 in the period 2003–2004 to a high of 11 in the period 2009–2010. Number of underrepresented minority residents varied yearly as well, from 1 to 2.
Data Collection
After this study was granted exempt status by our Institutional Review Board, we obtained student data from our registrar. Data included graduation year, self-identified sex and race, exposure to orthopedic surgery during clerkships, and matching residency specialty. National data were obtained from the Electronic Residency Application Service for the periods 2002–2007 and 2009–2012. These data included all US allopathic medical students’ self-identified sex and race, and applied-to primary residency specialty. National data from 2008 and national data on sex differences in orthopedic applications from 2009 were not available.
Graduates who matched into orthopedic surgery were asked to complete an anonymous survey on what influenced their decision to apply to orthopedic surgery and when this decision was made. Our goal with the survey was to substantiate or refute the conclusion that application rates depended on third-year exposure to musculoskeletal medicine.
Statistical Methods
Students were divided into 2 groups: precurriculum (graduated within 7-year period, 2000–2006) and postcurriculum (graduated within 6-year period, 2007–2012). A 2-sample test for proportions was used to compare percentage of total students who applied to orthopedics in each group. In the group of students who applied to orthopedics, we compared precurriculum and postcurriculum proportions of women and underrepresented minorities (non-white, non-Asian). We also compared these proportions with national data (using 2-sample tests for proportions) to determine if any change in diversity of our institution’s applicants was mirroring a national trend. Our definition of underrepresented minority was based on work that showed that the proportion of Asian matriculants in medical school and the proportion of applicants to orthopedics are higher than their respective national proportions.5 Survey data are reported descriptively. Statistical significance was defined with a 2-tailed α of 0.05 for all tests.
Results
Over the 2000–2012 period, 1507 students from our institution successfully applied to residency programs: 792 in the precurriculum group and 715 in the postcurriculum group. Of these students, 91 successfully applied to orthopedic surgery: 48 in the precurriculum group (applied before introduction of the required clerkship) and 43 in the postcurriculum group (applied afterward).
Over the 2002–2012 period, 10,100 US allopathic medical students applied to orthopedic residency programs: 4769 students between 2002 and 2006 and 5331 students between 2007 and 2012.
Before the musculoskeletal clerkship was required, 317 (40%) of the 792 precurriculum students were exposed to orthopedics during their third year. During this period, 42 of the 48 orthopedic surgery applicants completed an orthopedic surgery rotation during their third year of medical school. After the clerkship was required, 465 (65%) of the 715 postcurriculum students were exposed to orthopedics during their third year, including all 43 orthopedic surgery applicants (100% of students were exposed to musculoskeletal surgery, including plastic surgery and neurologic spine). The 25% increase in exposure to orthopedic surgery during the third year was statistically significant (P < .0001), but there was no resultant increase in overall percentage of students applying to orthopedic residencies (6% in each case; P = .98).
Over the 12-year study period, the proportion of female medical students at our institution declined from 50% (395/792) to 46% (328/715) (P = .13). However, there was an 81% relative increase, from 17% (8/48) before introduction of the clerkship to 30% (13/43) afterward, in the proportion of female applicants to orthopedic surgery. This contrasted with national data showing the percentage of female applicants to orthopedic surgery remained stable from 2002–2006 (14%, 675/4758) to 2007–2012 (15%, 643/4277). Before the clerkship was required, the proportion of female applicants from our institution was similar to national rates (P = .50). Afterward, our institution produced a significantly higher proportion of female applicants compared with the national proportion (P = .026).
Over the 12-year period, our self-identified underrepresented minority medical student population increased significantly (P = .02), from 13% (103/792) to 17% (124/715). The relative proportion of underrepresented minority orthopedic surgery applicants increased by 101%, from 10% (5/48) before the clerkship was required to 21% (9/43) afterward. Nationally, over the same period, underrepresented minorities’ orthopedic surgery application rates increased significantly (P < .001), from 16% (763/4769) to 19% (1002/5331). The proportion of underrepresented racial minorities that applied did not differ significantly between our institution and nationally for the years either before (P = .97) or after (P = .68) introduction of the curriculum.
Surveys were completed by 58 (64%) of 91 graduates (21 women, 70 men). Respondents’ characteristics are listed in Table 4.
Discussion
Orthopedic surgery needs a more diverse workforce11-17 in order to better mirror the population served, bring care to underserved areas,18-26 and provide better training environments.27 Several hypotheses about the lack of diversity have been posited: stereotypes about the specialty,28-31 lack of interest among minority medical students, and lack of exposure to the specialty.5,6,32,33
Lack of exposure deserves scrutiny, as a large proportion of medical students who choose to apply to orthopedic surgery make their decision before entering medical school, which is not typical.33 Such a finding suggests that exposure to orthopedic surgery is lacking, especially given that an orthopedic surgery rotation is usually not required during the clinical years. The idea that increased exposure to orthopedics affects application patterns is logical, as clinical exposure has been shown to play a role in medical students’ choice of specialty.34
Exposure helps in several key areas. Firsthand experience can help dispel stereotypes, such as the idea that success in orthopedic surgery depends on physical strength and that only former athletes pursue orthopedics.28-31 Authors have also reported on a perceived negative bias against women: Orthopedics is an “old boys’ network”; women will not fit in and need not apply; the orthopedic lifestyle is difficult and not conducive to a satisfying personal life.9 Requiring exposure ensures that all students, but especially women, can gain firsthand experience that can show these stereotypes to be false. Beyond dispelling these stereotypes, exposure to orthopedic surgery is essential for women, as studies have shown that clinical rotations play a larger role in determining specialty choice for women compared to men,33 and this would be particularly critical for specialties they may not be initially considering.
A national study found that requiring an orthopedic/musculoskeletal clerkship led to a 12% relative increase in the application rate, from 5.1% to 5.7%, and to an increase in applicant diversity (race, sex).10 However, the investigators could not determine individual reasons for specialty choice or the exact nature of each institution’s musculoskeletal curriculum. Confirming these factors, we found an 81% increase in number of female applicants and a 101% increase in number of underrepresented minority applicants after introduction of the required third-year musculoskeletal surgery clerkship at our institution.
We were unable to replicate the 12% relative increase in the overall application rate; our orthopedic surgery match rate remained 6%. Our findings cannot directly explain this, but we have several hypotheses. First, whereas other studies measured the application rate, we measured the successful match rate, given our data structure. This difference in data definition could account for some of the discrepancy. Second, we did not account for individuals’ academic success, and career counseling is paramount in decisions regarding residency specialties. It is possible we are substituting qualified female and underrepresented minority candidates for less-than-qualified male applicants. Third, the 25% increase in medical student exposure to orthopedic surgery led to a corresponding increase in number of orthopedic faculty providing undergraduate medical education. Some of these faculty could have been inexperienced in undergraduate medical education, and thus the teaching environment may not have been optimal.
Our study had several limitations. First, our institution has limited racial diversity. Over the past 12 years, only 15% of our students have been underrepresented minorities. (Nationally, the proportion is closer to 18%.) This may have limited the ability of our orthopedic rotation to affect the proportion of underrepresented minority applicants. Second, this study involved medical students at only one institution, which limits generalizability of findings. Third, we were unable to obtain records specifying which faculty and residents interacted with which medical students, and the increased number of female faculty and residents coinciding with the curriculum change may also be a factor. However, we expect that, without the curriculum change, these students would have had smaller odds of interacting with these potential female role models in orthopedics, negating any affect they may have had. Last, although we contacted former students to ask about their reasons for choosing the orthopedics residency, those findings are limited by a potential respondent selection bias.
The qualities and characteristics of successful orthopedic surgeons, as presented in both medical and lay cultures, are subject to numerous stereotypes. By increasing medical student exposure to orthopedics during the third year of medical school, we are giving a larger proportion of our students direct clinical experience in a field they may not have been considering. This exposure allows students to interact with mentors who can be positive role models—orthopedic surgeons who are dispelling stereotypes. By increasing medical student exposure and reaching students who may not have been considering orthopedics, we have increased diversity among our applicants. Third-year medical students’ exposure to orthopedic surgery is essential in promoting a more diverse workforce.
Am J Orthop. 2016;45(6):E347-E351. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. US Census Bureau. 2012 National Population Projections: Summary Tables. http://www.census.gov/population/projections/data/national/2012/summarytables.html. Accessed April 15, 2013.
2. Blakemore LC, Hall JM, Biermann JS. Women in surgical residency training programs. J Bone Joint Surg Am. 2003;85(12):2477-2480.
3. Day CS, Lage DE, Ahn CS. Diversity based on race, ethnicity, and sex between academic orthopaedic surgery and other specialties: a comparative study. J Bone Joint Surg Am. 2010;92(13):2328-2335.
4. Lewis VO, Scherl SA, O’Connor MI. Women in orthopaedics—way behind the number curve. J Bone Joint Surg Am. 2012;94(5):e30.
5. Okike K, Utuk ME, White AA. Racial and ethnic diversity in orthopaedic surgery residency programs. J Bone Joint Surg Am. 2011;93(18):e107.
6. Salsberg ES, Grover A, Simon MA, Frick SL, Kuremsky MA, Goodman DC. An AOA critical issue. Future physician workforce requirements: implications for orthopaedic surgery education. J Bone Joint Surg Am. 2008;90(5):1143-1159.
7. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the US 2008. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009.
8. White AA 3rd. Alfred R. Shands, Jr., lecture: our humanitarian orthopaedic opportunity. J Bone Joint Surg Am. 2002;84(3):478-484.
9. Templeton K, Wood VJ, Haynes R. Women and minorities in orthopaedic residency programs. J Am Acad Orthop Surg. 2007;15(suppl 1):S37-S41.
10. Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86(10):2335-2338.
11. Dykes DC, White AA. Getting to equal: strategies to understand and eliminate general and orthopaedic healthcare disparities. Clin Orthop Relat Res. 2009;467(10):2598-2605.
12. Gebhardt MC. Improving diversity in orthopaedic residency programs. J Am Acad Orthop Surg. 2007;15(suppl 1):S49-S50.
13. Hammond RA. The moral imperatives for diversity. Clin Orthop Relat Res. 1999;(362):102-106.
14. Lindsey RW. The role of the department chair in promoting diversity. J Am Acad Orthop Surg. 2007;15(suppl 1):S65-S69.
15. Satcher RL. African Americans and orthopaedic surgery. A resident’s perspective. Clin Orthop Relat Res. 1999;(362):114-116.
16. White AA. Justifications and needs for diversity in orthopaedics. Clin Orthop Relat Res. 1999;(362):22-33.
17. White AA. Resident selection: are we putting the cart before the horse? Clin Orthop Relat Res. 2002;(399):255-259.
18. Dominick KL, Baker TA. Racial and ethnic differences in osteoarthritis: prevalence, outcomes, and medical care. Ethn Dis. 2004;14(4):558-566.
19. Furstenberg AL, Mezey MD. Differences in outcome between black and white elderly hip fracture patients. J Chronic Dis. 1987;40(10):931-938.
20. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
21. Komaromy M, Grumbach K, Drake M, et al. The role of black and Hispanic physicians in providing health care for underserved populations. N Engl J Med. 1996;334(20):1305-1310.
22. Moy E, Bartman BA. Physician race and care of minority and medically indigent patients. JAMA. 1995;273(19):1515-1520.
23. Nelson CL. Disparities in orthopaedic surgical intervention. J Am Acad Orthop Surg. 2007;15(suppl 1):S13-S17.
24. Rowley DL, Jenkins BC, Frazier E. Utilization of joint arthroplasty: racial and ethnic disparities in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S43-S48.
25. Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med. 2003;349(14):1350-1359.
26. Steel N, Clark A, Lang LA, Wallace RB, Melzer D. Racial disparities in receipt of hip and knee joint replacements are not explained by need: the Health and Retirement Study 1998-2004. J Gerontol A Biol Sci Med Sci. 2008;63(6):629-634.
27. Whitla DK, Orfield G, Silen W, Teperow C, Howard C, Reede J. Educational benefits of diversity in medical school: a survey of students. Acad Med. 2003;78(5):460-466.
28. Barrett DS. Are orthopaedic surgeons gorillas? Br Med J. 1988;297(6664):1638-1639.
29. Brenkel IJ, Pearse M, Gregg PJ. A “cracking” complication of hemiarthroplasty of the hip. Br Med J. 1986;293(6562):1648.
30. Fox JS, Bell GR, Sweeney PJ. Are orthopaedic surgeons really gorillas? Br Med J. 1990;301(6766):1425-1426.
31. Subramanian P, Kantharuban S, Subramanian V, Willis-Owen SA, Willis-Owen CA. Orthopaedic surgeons: as strong as an ox and almost twice as clever? Multicentre prospective comparative study. Br Med J. 2011;343:d7506.
32. Baldwin K, Namdari S, Bowers A, Keenan MA, Levin LS, Ahn J. Factors affecting interest in orthopedics among female medical students: a prospective analysis. Orthopedics. 2011;34(12):e919-e932.
33. Johnson AL, Sharma J, Chinchilli VM, et al. Why do medical students choose orthopaedics as a career? J Bone Joint Surg Am. 2012;94(11):e78.
34. Wilson FC. Teaching by residents. Clin Orthop Relat Res. 2007;(454):247-250.
As the United States becomes increasingly diverse, with predictions that by 2045 minorities will comprise 50% or more of the population,1 the demographics of the orthopedic surgery population will also likely diversify. It is important that orthopedic surgeons shift in their diversity as well. Lack of diversity in orthopedics (women and racial minorities are underrepresented) relative to the national population and other surgical specialties and their training programs is well documented.2-8
More concerning, the diversity of orthopedic residents does not compare favorably with that of medical school attendees.4,9 The difference suggests the greatest loss of potential diversity occurs during the transition from medical school to residency. A national study demonstrated that instruction in musculoskeletal medicine led to an increase in application rates nationally.10 However, the authors of that study stated they were unexpectedly limited by its large size—they could not validate the accuracy of curriculum data and could not differentiate between a 1-day required experience and a 4-week rotation.
In the present study, which accounted for curricular factors, we compared our medical students’ application rates to orthopedics residencies based on sex and race before and after introduction of a required third-year musculoskeletal clerkship. We hypothesized that making the curriculum a requirement would increase the number of applicants and increase the diversity of applicants in terms of both women and underrepresented minorities. This hypothesis was based on the rationale that these groups might not consider an orthopedics residency without first being directly exposed to orthopedics. We also wanted to determine what factors influenced applicants to choose orthopedic surgery.
Methods
Curriculum
Before 2006, third-year students spent 3 months completing a surgery clerkship. Some students interested in orthopedic surgery would have to wait until their fourth year to complete an elective in orthopedic surgery, and uninterested students would not be exposed at all. Starting in 2006, 1 month of the third-year surgery clerkship was required to be completed in musculoskeletal surgery: orthopedic surgery, plastic surgery, or neurosurgical spine. Plastic surgery was an option, as it exposed students to hand surgery and flap reconstruction.
During the 12-year study period, overall teaching hours in the preclinical curriculum did not change, and there were no other structural changes to the preclinical or clinical curriculum. The orthopedics department increased its faculty from 23 in 2000 to 34 in 2012. Number of female faculty increased from 1 to 3, representing a 4% to 9% increase in department faculty. Throughout the 12 years, there were no underrepresented minority faculty. Total number of residents increased from 26 in 2000 to 30 in 2012. Number of female residents varied year to year, from a low of 3 in the period 2003–2004 to a high of 11 in the period 2009–2010. Number of underrepresented minority residents varied yearly as well, from 1 to 2.
Data Collection
After this study was granted exempt status by our Institutional Review Board, we obtained student data from our registrar. Data included graduation year, self-identified sex and race, exposure to orthopedic surgery during clerkships, and matching residency specialty. National data were obtained from the Electronic Residency Application Service for the periods 2002–2007 and 2009–2012. These data included all US allopathic medical students’ self-identified sex and race, and applied-to primary residency specialty. National data from 2008 and national data on sex differences in orthopedic applications from 2009 were not available.
Graduates who matched into orthopedic surgery were asked to complete an anonymous survey on what influenced their decision to apply to orthopedic surgery and when this decision was made. Our goal with the survey was to substantiate or refute the conclusion that application rates depended on third-year exposure to musculoskeletal medicine.
Statistical Methods
Students were divided into 2 groups: precurriculum (graduated within 7-year period, 2000–2006) and postcurriculum (graduated within 6-year period, 2007–2012). A 2-sample test for proportions was used to compare percentage of total students who applied to orthopedics in each group. In the group of students who applied to orthopedics, we compared precurriculum and postcurriculum proportions of women and underrepresented minorities (non-white, non-Asian). We also compared these proportions with national data (using 2-sample tests for proportions) to determine if any change in diversity of our institution’s applicants was mirroring a national trend. Our definition of underrepresented minority was based on work that showed that the proportion of Asian matriculants in medical school and the proportion of applicants to orthopedics are higher than their respective national proportions.5 Survey data are reported descriptively. Statistical significance was defined with a 2-tailed α of 0.05 for all tests.
Results
Over the 2000–2012 period, 1507 students from our institution successfully applied to residency programs: 792 in the precurriculum group and 715 in the postcurriculum group. Of these students, 91 successfully applied to orthopedic surgery: 48 in the precurriculum group (applied before introduction of the required clerkship) and 43 in the postcurriculum group (applied afterward).
Over the 2002–2012 period, 10,100 US allopathic medical students applied to orthopedic residency programs: 4769 students between 2002 and 2006 and 5331 students between 2007 and 2012.
Before the musculoskeletal clerkship was required, 317 (40%) of the 792 precurriculum students were exposed to orthopedics during their third year. During this period, 42 of the 48 orthopedic surgery applicants completed an orthopedic surgery rotation during their third year of medical school. After the clerkship was required, 465 (65%) of the 715 postcurriculum students were exposed to orthopedics during their third year, including all 43 orthopedic surgery applicants (100% of students were exposed to musculoskeletal surgery, including plastic surgery and neurologic spine). The 25% increase in exposure to orthopedic surgery during the third year was statistically significant (P < .0001), but there was no resultant increase in overall percentage of students applying to orthopedic residencies (6% in each case; P = .98).
Over the 12-year study period, the proportion of female medical students at our institution declined from 50% (395/792) to 46% (328/715) (P = .13). However, there was an 81% relative increase, from 17% (8/48) before introduction of the clerkship to 30% (13/43) afterward, in the proportion of female applicants to orthopedic surgery. This contrasted with national data showing the percentage of female applicants to orthopedic surgery remained stable from 2002–2006 (14%, 675/4758) to 2007–2012 (15%, 643/4277). Before the clerkship was required, the proportion of female applicants from our institution was similar to national rates (P = .50). Afterward, our institution produced a significantly higher proportion of female applicants compared with the national proportion (P = .026).
Over the 12-year period, our self-identified underrepresented minority medical student population increased significantly (P = .02), from 13% (103/792) to 17% (124/715). The relative proportion of underrepresented minority orthopedic surgery applicants increased by 101%, from 10% (5/48) before the clerkship was required to 21% (9/43) afterward. Nationally, over the same period, underrepresented minorities’ orthopedic surgery application rates increased significantly (P < .001), from 16% (763/4769) to 19% (1002/5331). The proportion of underrepresented racial minorities that applied did not differ significantly between our institution and nationally for the years either before (P = .97) or after (P = .68) introduction of the curriculum.
Surveys were completed by 58 (64%) of 91 graduates (21 women, 70 men). Respondents’ characteristics are listed in Table 4.
Discussion
Orthopedic surgery needs a more diverse workforce11-17 in order to better mirror the population served, bring care to underserved areas,18-26 and provide better training environments.27 Several hypotheses about the lack of diversity have been posited: stereotypes about the specialty,28-31 lack of interest among minority medical students, and lack of exposure to the specialty.5,6,32,33
Lack of exposure deserves scrutiny, as a large proportion of medical students who choose to apply to orthopedic surgery make their decision before entering medical school, which is not typical.33 Such a finding suggests that exposure to orthopedic surgery is lacking, especially given that an orthopedic surgery rotation is usually not required during the clinical years. The idea that increased exposure to orthopedics affects application patterns is logical, as clinical exposure has been shown to play a role in medical students’ choice of specialty.34
Exposure helps in several key areas. Firsthand experience can help dispel stereotypes, such as the idea that success in orthopedic surgery depends on physical strength and that only former athletes pursue orthopedics.28-31 Authors have also reported on a perceived negative bias against women: Orthopedics is an “old boys’ network”; women will not fit in and need not apply; the orthopedic lifestyle is difficult and not conducive to a satisfying personal life.9 Requiring exposure ensures that all students, but especially women, can gain firsthand experience that can show these stereotypes to be false. Beyond dispelling these stereotypes, exposure to orthopedic surgery is essential for women, as studies have shown that clinical rotations play a larger role in determining specialty choice for women compared to men,33 and this would be particularly critical for specialties they may not be initially considering.
A national study found that requiring an orthopedic/musculoskeletal clerkship led to a 12% relative increase in the application rate, from 5.1% to 5.7%, and to an increase in applicant diversity (race, sex).10 However, the investigators could not determine individual reasons for specialty choice or the exact nature of each institution’s musculoskeletal curriculum. Confirming these factors, we found an 81% increase in number of female applicants and a 101% increase in number of underrepresented minority applicants after introduction of the required third-year musculoskeletal surgery clerkship at our institution.
We were unable to replicate the 12% relative increase in the overall application rate; our orthopedic surgery match rate remained 6%. Our findings cannot directly explain this, but we have several hypotheses. First, whereas other studies measured the application rate, we measured the successful match rate, given our data structure. This difference in data definition could account for some of the discrepancy. Second, we did not account for individuals’ academic success, and career counseling is paramount in decisions regarding residency specialties. It is possible we are substituting qualified female and underrepresented minority candidates for less-than-qualified male applicants. Third, the 25% increase in medical student exposure to orthopedic surgery led to a corresponding increase in number of orthopedic faculty providing undergraduate medical education. Some of these faculty could have been inexperienced in undergraduate medical education, and thus the teaching environment may not have been optimal.
Our study had several limitations. First, our institution has limited racial diversity. Over the past 12 years, only 15% of our students have been underrepresented minorities. (Nationally, the proportion is closer to 18%.) This may have limited the ability of our orthopedic rotation to affect the proportion of underrepresented minority applicants. Second, this study involved medical students at only one institution, which limits generalizability of findings. Third, we were unable to obtain records specifying which faculty and residents interacted with which medical students, and the increased number of female faculty and residents coinciding with the curriculum change may also be a factor. However, we expect that, without the curriculum change, these students would have had smaller odds of interacting with these potential female role models in orthopedics, negating any affect they may have had. Last, although we contacted former students to ask about their reasons for choosing the orthopedics residency, those findings are limited by a potential respondent selection bias.
The qualities and characteristics of successful orthopedic surgeons, as presented in both medical and lay cultures, are subject to numerous stereotypes. By increasing medical student exposure to orthopedics during the third year of medical school, we are giving a larger proportion of our students direct clinical experience in a field they may not have been considering. This exposure allows students to interact with mentors who can be positive role models—orthopedic surgeons who are dispelling stereotypes. By increasing medical student exposure and reaching students who may not have been considering orthopedics, we have increased diversity among our applicants. Third-year medical students’ exposure to orthopedic surgery is essential in promoting a more diverse workforce.
Am J Orthop. 2016;45(6):E347-E351. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
As the United States becomes increasingly diverse, with predictions that by 2045 minorities will comprise 50% or more of the population,1 the demographics of the orthopedic surgery population will also likely diversify. It is important that orthopedic surgeons shift in their diversity as well. Lack of diversity in orthopedics (women and racial minorities are underrepresented) relative to the national population and other surgical specialties and their training programs is well documented.2-8
More concerning, the diversity of orthopedic residents does not compare favorably with that of medical school attendees.4,9 The difference suggests the greatest loss of potential diversity occurs during the transition from medical school to residency. A national study demonstrated that instruction in musculoskeletal medicine led to an increase in application rates nationally.10 However, the authors of that study stated they were unexpectedly limited by its large size—they could not validate the accuracy of curriculum data and could not differentiate between a 1-day required experience and a 4-week rotation.
In the present study, which accounted for curricular factors, we compared our medical students’ application rates to orthopedics residencies based on sex and race before and after introduction of a required third-year musculoskeletal clerkship. We hypothesized that making the curriculum a requirement would increase the number of applicants and increase the diversity of applicants in terms of both women and underrepresented minorities. This hypothesis was based on the rationale that these groups might not consider an orthopedics residency without first being directly exposed to orthopedics. We also wanted to determine what factors influenced applicants to choose orthopedic surgery.
Methods
Curriculum
Before 2006, third-year students spent 3 months completing a surgery clerkship. Some students interested in orthopedic surgery would have to wait until their fourth year to complete an elective in orthopedic surgery, and uninterested students would not be exposed at all. Starting in 2006, 1 month of the third-year surgery clerkship was required to be completed in musculoskeletal surgery: orthopedic surgery, plastic surgery, or neurosurgical spine. Plastic surgery was an option, as it exposed students to hand surgery and flap reconstruction.
During the 12-year study period, overall teaching hours in the preclinical curriculum did not change, and there were no other structural changes to the preclinical or clinical curriculum. The orthopedics department increased its faculty from 23 in 2000 to 34 in 2012. Number of female faculty increased from 1 to 3, representing a 4% to 9% increase in department faculty. Throughout the 12 years, there were no underrepresented minority faculty. Total number of residents increased from 26 in 2000 to 30 in 2012. Number of female residents varied year to year, from a low of 3 in the period 2003–2004 to a high of 11 in the period 2009–2010. Number of underrepresented minority residents varied yearly as well, from 1 to 2.
Data Collection
After this study was granted exempt status by our Institutional Review Board, we obtained student data from our registrar. Data included graduation year, self-identified sex and race, exposure to orthopedic surgery during clerkships, and matching residency specialty. National data were obtained from the Electronic Residency Application Service for the periods 2002–2007 and 2009–2012. These data included all US allopathic medical students’ self-identified sex and race, and applied-to primary residency specialty. National data from 2008 and national data on sex differences in orthopedic applications from 2009 were not available.
Graduates who matched into orthopedic surgery were asked to complete an anonymous survey on what influenced their decision to apply to orthopedic surgery and when this decision was made. Our goal with the survey was to substantiate or refute the conclusion that application rates depended on third-year exposure to musculoskeletal medicine.
Statistical Methods
Students were divided into 2 groups: precurriculum (graduated within 7-year period, 2000–2006) and postcurriculum (graduated within 6-year period, 2007–2012). A 2-sample test for proportions was used to compare percentage of total students who applied to orthopedics in each group. In the group of students who applied to orthopedics, we compared precurriculum and postcurriculum proportions of women and underrepresented minorities (non-white, non-Asian). We also compared these proportions with national data (using 2-sample tests for proportions) to determine if any change in diversity of our institution’s applicants was mirroring a national trend. Our definition of underrepresented minority was based on work that showed that the proportion of Asian matriculants in medical school and the proportion of applicants to orthopedics are higher than their respective national proportions.5 Survey data are reported descriptively. Statistical significance was defined with a 2-tailed α of 0.05 for all tests.
Results
Over the 2000–2012 period, 1507 students from our institution successfully applied to residency programs: 792 in the precurriculum group and 715 in the postcurriculum group. Of these students, 91 successfully applied to orthopedic surgery: 48 in the precurriculum group (applied before introduction of the required clerkship) and 43 in the postcurriculum group (applied afterward).
Over the 2002–2012 period, 10,100 US allopathic medical students applied to orthopedic residency programs: 4769 students between 2002 and 2006 and 5331 students between 2007 and 2012.
Before the musculoskeletal clerkship was required, 317 (40%) of the 792 precurriculum students were exposed to orthopedics during their third year. During this period, 42 of the 48 orthopedic surgery applicants completed an orthopedic surgery rotation during their third year of medical school. After the clerkship was required, 465 (65%) of the 715 postcurriculum students were exposed to orthopedics during their third year, including all 43 orthopedic surgery applicants (100% of students were exposed to musculoskeletal surgery, including plastic surgery and neurologic spine). The 25% increase in exposure to orthopedic surgery during the third year was statistically significant (P < .0001), but there was no resultant increase in overall percentage of students applying to orthopedic residencies (6% in each case; P = .98).
Over the 12-year study period, the proportion of female medical students at our institution declined from 50% (395/792) to 46% (328/715) (P = .13). However, there was an 81% relative increase, from 17% (8/48) before introduction of the clerkship to 30% (13/43) afterward, in the proportion of female applicants to orthopedic surgery. This contrasted with national data showing the percentage of female applicants to orthopedic surgery remained stable from 2002–2006 (14%, 675/4758) to 2007–2012 (15%, 643/4277). Before the clerkship was required, the proportion of female applicants from our institution was similar to national rates (P = .50). Afterward, our institution produced a significantly higher proportion of female applicants compared with the national proportion (P = .026).
Over the 12-year period, our self-identified underrepresented minority medical student population increased significantly (P = .02), from 13% (103/792) to 17% (124/715). The relative proportion of underrepresented minority orthopedic surgery applicants increased by 101%, from 10% (5/48) before the clerkship was required to 21% (9/43) afterward. Nationally, over the same period, underrepresented minorities’ orthopedic surgery application rates increased significantly (P < .001), from 16% (763/4769) to 19% (1002/5331). The proportion of underrepresented racial minorities that applied did not differ significantly between our institution and nationally for the years either before (P = .97) or after (P = .68) introduction of the curriculum.
Surveys were completed by 58 (64%) of 91 graduates (21 women, 70 men). Respondents’ characteristics are listed in Table 4.
Discussion
Orthopedic surgery needs a more diverse workforce11-17 in order to better mirror the population served, bring care to underserved areas,18-26 and provide better training environments.27 Several hypotheses about the lack of diversity have been posited: stereotypes about the specialty,28-31 lack of interest among minority medical students, and lack of exposure to the specialty.5,6,32,33
Lack of exposure deserves scrutiny, as a large proportion of medical students who choose to apply to orthopedic surgery make their decision before entering medical school, which is not typical.33 Such a finding suggests that exposure to orthopedic surgery is lacking, especially given that an orthopedic surgery rotation is usually not required during the clinical years. The idea that increased exposure to orthopedics affects application patterns is logical, as clinical exposure has been shown to play a role in medical students’ choice of specialty.34
Exposure helps in several key areas. Firsthand experience can help dispel stereotypes, such as the idea that success in orthopedic surgery depends on physical strength and that only former athletes pursue orthopedics.28-31 Authors have also reported on a perceived negative bias against women: Orthopedics is an “old boys’ network”; women will not fit in and need not apply; the orthopedic lifestyle is difficult and not conducive to a satisfying personal life.9 Requiring exposure ensures that all students, but especially women, can gain firsthand experience that can show these stereotypes to be false. Beyond dispelling these stereotypes, exposure to orthopedic surgery is essential for women, as studies have shown that clinical rotations play a larger role in determining specialty choice for women compared to men,33 and this would be particularly critical for specialties they may not be initially considering.
A national study found that requiring an orthopedic/musculoskeletal clerkship led to a 12% relative increase in the application rate, from 5.1% to 5.7%, and to an increase in applicant diversity (race, sex).10 However, the investigators could not determine individual reasons for specialty choice or the exact nature of each institution’s musculoskeletal curriculum. Confirming these factors, we found an 81% increase in number of female applicants and a 101% increase in number of underrepresented minority applicants after introduction of the required third-year musculoskeletal surgery clerkship at our institution.
We were unable to replicate the 12% relative increase in the overall application rate; our orthopedic surgery match rate remained 6%. Our findings cannot directly explain this, but we have several hypotheses. First, whereas other studies measured the application rate, we measured the successful match rate, given our data structure. This difference in data definition could account for some of the discrepancy. Second, we did not account for individuals’ academic success, and career counseling is paramount in decisions regarding residency specialties. It is possible we are substituting qualified female and underrepresented minority candidates for less-than-qualified male applicants. Third, the 25% increase in medical student exposure to orthopedic surgery led to a corresponding increase in number of orthopedic faculty providing undergraduate medical education. Some of these faculty could have been inexperienced in undergraduate medical education, and thus the teaching environment may not have been optimal.
Our study had several limitations. First, our institution has limited racial diversity. Over the past 12 years, only 15% of our students have been underrepresented minorities. (Nationally, the proportion is closer to 18%.) This may have limited the ability of our orthopedic rotation to affect the proportion of underrepresented minority applicants. Second, this study involved medical students at only one institution, which limits generalizability of findings. Third, we were unable to obtain records specifying which faculty and residents interacted with which medical students, and the increased number of female faculty and residents coinciding with the curriculum change may also be a factor. However, we expect that, without the curriculum change, these students would have had smaller odds of interacting with these potential female role models in orthopedics, negating any affect they may have had. Last, although we contacted former students to ask about their reasons for choosing the orthopedics residency, those findings are limited by a potential respondent selection bias.
The qualities and characteristics of successful orthopedic surgeons, as presented in both medical and lay cultures, are subject to numerous stereotypes. By increasing medical student exposure to orthopedics during the third year of medical school, we are giving a larger proportion of our students direct clinical experience in a field they may not have been considering. This exposure allows students to interact with mentors who can be positive role models—orthopedic surgeons who are dispelling stereotypes. By increasing medical student exposure and reaching students who may not have been considering orthopedics, we have increased diversity among our applicants. Third-year medical students’ exposure to orthopedic surgery is essential in promoting a more diverse workforce.
Am J Orthop. 2016;45(6):E347-E351. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. US Census Bureau. 2012 National Population Projections: Summary Tables. http://www.census.gov/population/projections/data/national/2012/summarytables.html. Accessed April 15, 2013.
2. Blakemore LC, Hall JM, Biermann JS. Women in surgical residency training programs. J Bone Joint Surg Am. 2003;85(12):2477-2480.
3. Day CS, Lage DE, Ahn CS. Diversity based on race, ethnicity, and sex between academic orthopaedic surgery and other specialties: a comparative study. J Bone Joint Surg Am. 2010;92(13):2328-2335.
4. Lewis VO, Scherl SA, O’Connor MI. Women in orthopaedics—way behind the number curve. J Bone Joint Surg Am. 2012;94(5):e30.
5. Okike K, Utuk ME, White AA. Racial and ethnic diversity in orthopaedic surgery residency programs. J Bone Joint Surg Am. 2011;93(18):e107.
6. Salsberg ES, Grover A, Simon MA, Frick SL, Kuremsky MA, Goodman DC. An AOA critical issue. Future physician workforce requirements: implications for orthopaedic surgery education. J Bone Joint Surg Am. 2008;90(5):1143-1159.
7. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the US 2008. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009.
8. White AA 3rd. Alfred R. Shands, Jr., lecture: our humanitarian orthopaedic opportunity. J Bone Joint Surg Am. 2002;84(3):478-484.
9. Templeton K, Wood VJ, Haynes R. Women and minorities in orthopaedic residency programs. J Am Acad Orthop Surg. 2007;15(suppl 1):S37-S41.
10. Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86(10):2335-2338.
11. Dykes DC, White AA. Getting to equal: strategies to understand and eliminate general and orthopaedic healthcare disparities. Clin Orthop Relat Res. 2009;467(10):2598-2605.
12. Gebhardt MC. Improving diversity in orthopaedic residency programs. J Am Acad Orthop Surg. 2007;15(suppl 1):S49-S50.
13. Hammond RA. The moral imperatives for diversity. Clin Orthop Relat Res. 1999;(362):102-106.
14. Lindsey RW. The role of the department chair in promoting diversity. J Am Acad Orthop Surg. 2007;15(suppl 1):S65-S69.
15. Satcher RL. African Americans and orthopaedic surgery. A resident’s perspective. Clin Orthop Relat Res. 1999;(362):114-116.
16. White AA. Justifications and needs for diversity in orthopaedics. Clin Orthop Relat Res. 1999;(362):22-33.
17. White AA. Resident selection: are we putting the cart before the horse? Clin Orthop Relat Res. 2002;(399):255-259.
18. Dominick KL, Baker TA. Racial and ethnic differences in osteoarthritis: prevalence, outcomes, and medical care. Ethn Dis. 2004;14(4):558-566.
19. Furstenberg AL, Mezey MD. Differences in outcome between black and white elderly hip fracture patients. J Chronic Dis. 1987;40(10):931-938.
20. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
21. Komaromy M, Grumbach K, Drake M, et al. The role of black and Hispanic physicians in providing health care for underserved populations. N Engl J Med. 1996;334(20):1305-1310.
22. Moy E, Bartman BA. Physician race and care of minority and medically indigent patients. JAMA. 1995;273(19):1515-1520.
23. Nelson CL. Disparities in orthopaedic surgical intervention. J Am Acad Orthop Surg. 2007;15(suppl 1):S13-S17.
24. Rowley DL, Jenkins BC, Frazier E. Utilization of joint arthroplasty: racial and ethnic disparities in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S43-S48.
25. Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med. 2003;349(14):1350-1359.
26. Steel N, Clark A, Lang LA, Wallace RB, Melzer D. Racial disparities in receipt of hip and knee joint replacements are not explained by need: the Health and Retirement Study 1998-2004. J Gerontol A Biol Sci Med Sci. 2008;63(6):629-634.
27. Whitla DK, Orfield G, Silen W, Teperow C, Howard C, Reede J. Educational benefits of diversity in medical school: a survey of students. Acad Med. 2003;78(5):460-466.
28. Barrett DS. Are orthopaedic surgeons gorillas? Br Med J. 1988;297(6664):1638-1639.
29. Brenkel IJ, Pearse M, Gregg PJ. A “cracking” complication of hemiarthroplasty of the hip. Br Med J. 1986;293(6562):1648.
30. Fox JS, Bell GR, Sweeney PJ. Are orthopaedic surgeons really gorillas? Br Med J. 1990;301(6766):1425-1426.
31. Subramanian P, Kantharuban S, Subramanian V, Willis-Owen SA, Willis-Owen CA. Orthopaedic surgeons: as strong as an ox and almost twice as clever? Multicentre prospective comparative study. Br Med J. 2011;343:d7506.
32. Baldwin K, Namdari S, Bowers A, Keenan MA, Levin LS, Ahn J. Factors affecting interest in orthopedics among female medical students: a prospective analysis. Orthopedics. 2011;34(12):e919-e932.
33. Johnson AL, Sharma J, Chinchilli VM, et al. Why do medical students choose orthopaedics as a career? J Bone Joint Surg Am. 2012;94(11):e78.
34. Wilson FC. Teaching by residents. Clin Orthop Relat Res. 2007;(454):247-250.
1. US Census Bureau. 2012 National Population Projections: Summary Tables. http://www.census.gov/population/projections/data/national/2012/summarytables.html. Accessed April 15, 2013.
2. Blakemore LC, Hall JM, Biermann JS. Women in surgical residency training programs. J Bone Joint Surg Am. 2003;85(12):2477-2480.
3. Day CS, Lage DE, Ahn CS. Diversity based on race, ethnicity, and sex between academic orthopaedic surgery and other specialties: a comparative study. J Bone Joint Surg Am. 2010;92(13):2328-2335.
4. Lewis VO, Scherl SA, O’Connor MI. Women in orthopaedics—way behind the number curve. J Bone Joint Surg Am. 2012;94(5):e30.
5. Okike K, Utuk ME, White AA. Racial and ethnic diversity in orthopaedic surgery residency programs. J Bone Joint Surg Am. 2011;93(18):e107.
6. Salsberg ES, Grover A, Simon MA, Frick SL, Kuremsky MA, Goodman DC. An AOA critical issue. Future physician workforce requirements: implications for orthopaedic surgery education. J Bone Joint Surg Am. 2008;90(5):1143-1159.
7. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the US 2008. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009.
8. White AA 3rd. Alfred R. Shands, Jr., lecture: our humanitarian orthopaedic opportunity. J Bone Joint Surg Am. 2002;84(3):478-484.
9. Templeton K, Wood VJ, Haynes R. Women and minorities in orthopaedic residency programs. J Am Acad Orthop Surg. 2007;15(suppl 1):S37-S41.
10. Bernstein J, Dicaprio MR, Mehta S. The relationship between required medical school instruction in musculoskeletal medicine and application rates to orthopaedic surgery residency programs. J Bone Joint Surg Am. 2004;86(10):2335-2338.
11. Dykes DC, White AA. Getting to equal: strategies to understand and eliminate general and orthopaedic healthcare disparities. Clin Orthop Relat Res. 2009;467(10):2598-2605.
12. Gebhardt MC. Improving diversity in orthopaedic residency programs. J Am Acad Orthop Surg. 2007;15(suppl 1):S49-S50.
13. Hammond RA. The moral imperatives for diversity. Clin Orthop Relat Res. 1999;(362):102-106.
14. Lindsey RW. The role of the department chair in promoting diversity. J Am Acad Orthop Surg. 2007;15(suppl 1):S65-S69.
15. Satcher RL. African Americans and orthopaedic surgery. A resident’s perspective. Clin Orthop Relat Res. 1999;(362):114-116.
16. White AA. Justifications and needs for diversity in orthopaedics. Clin Orthop Relat Res. 1999;(362):22-33.
17. White AA. Resident selection: are we putting the cart before the horse? Clin Orthop Relat Res. 2002;(399):255-259.
18. Dominick KL, Baker TA. Racial and ethnic differences in osteoarthritis: prevalence, outcomes, and medical care. Ethn Dis. 2004;14(4):558-566.
19. Furstenberg AL, Mezey MD. Differences in outcome between black and white elderly hip fracture patients. J Chronic Dis. 1987;40(10):931-938.
20. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
21. Komaromy M, Grumbach K, Drake M, et al. The role of black and Hispanic physicians in providing health care for underserved populations. N Engl J Med. 1996;334(20):1305-1310.
22. Moy E, Bartman BA. Physician race and care of minority and medically indigent patients. JAMA. 1995;273(19):1515-1520.
23. Nelson CL. Disparities in orthopaedic surgical intervention. J Am Acad Orthop Surg. 2007;15(suppl 1):S13-S17.
24. Rowley DL, Jenkins BC, Frazier E. Utilization of joint arthroplasty: racial and ethnic disparities in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S43-S48.
25. Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med. 2003;349(14):1350-1359.
26. Steel N, Clark A, Lang LA, Wallace RB, Melzer D. Racial disparities in receipt of hip and knee joint replacements are not explained by need: the Health and Retirement Study 1998-2004. J Gerontol A Biol Sci Med Sci. 2008;63(6):629-634.
27. Whitla DK, Orfield G, Silen W, Teperow C, Howard C, Reede J. Educational benefits of diversity in medical school: a survey of students. Acad Med. 2003;78(5):460-466.
28. Barrett DS. Are orthopaedic surgeons gorillas? Br Med J. 1988;297(6664):1638-1639.
29. Brenkel IJ, Pearse M, Gregg PJ. A “cracking” complication of hemiarthroplasty of the hip. Br Med J. 1986;293(6562):1648.
30. Fox JS, Bell GR, Sweeney PJ. Are orthopaedic surgeons really gorillas? Br Med J. 1990;301(6766):1425-1426.
31. Subramanian P, Kantharuban S, Subramanian V, Willis-Owen SA, Willis-Owen CA. Orthopaedic surgeons: as strong as an ox and almost twice as clever? Multicentre prospective comparative study. Br Med J. 2011;343:d7506.
32. Baldwin K, Namdari S, Bowers A, Keenan MA, Levin LS, Ahn J. Factors affecting interest in orthopedics among female medical students: a prospective analysis. Orthopedics. 2011;34(12):e919-e932.
33. Johnson AL, Sharma J, Chinchilli VM, et al. Why do medical students choose orthopaedics as a career? J Bone Joint Surg Am. 2012;94(11):e78.
34. Wilson FC. Teaching by residents. Clin Orthop Relat Res. 2007;(454):247-250.
Direct-to-Consumer Marketing: Implications for Patient Care and Orthopedic Education
Direct-to-consumer marketing (DTCM) is the promotion of health-related products or services directly to patients. Although this topic is not new to orthopedics, several emerging trends hold troubling implications for patients as well as orthopedic surgeons, particularly surgeons in training.
Orthopedics DTCM most commonly involves television and print advertisements. Supporters contend DTCM is an empowering educational tool that increases awareness of medical ailments and encourages patients to seek treatment. Opponents point to inaccuracies and misleading claims. Bhattacharyya and colleagues1 found that about half the claims in orthopedic print advertisements were not supported by clinical evidence. Woloshin and colleagues2 found that information in DTCM was vague and often was designed to act on the emotions. Patients misled by these claims and innately seeking improvement could present with unreasonable expectations and difficult discussions that can be detrimental to the patient–physician relationship.3Given changing patient demographics and the information revolution, the effects of DTCM likely will continue to grow. Total joint arthroplasty (TJA), which represents Medicare’s largest expenditure,4 is a classic example. Today’s TJA patients are younger, more active, and better educated, and they live longer, have higher expectations, and are more reliant on the media.5 Television is no longer our main medium—the internet is the source of healthcare education for 70% of adults in the United States.6Healthcare reform has also brought significant changes in the delivery of DTCM. In an era of competition for market share brought by increased demand and decreased reimbursement, DTCM has evolved into sales pitches by hospitals and physicians. Robotic joint replacement, minimally invasive surgery (MIS), use of the anterior hip approach, use of sex-specific or high-flexion knee implants, and other practices have become popular marketing tools for surgery centers competing for new patients. As a result, patients often present not only with a complaint but with a request for a particular procedure.4,5 Labovitch and colleagues7 found that 70% of MIS information on the internet was produced by hospitals and private medical groups, and only 6% was produced by industry. Although the vast majority of the sources reported on the advantages of MIS, only 15% explained patient eligibility, and a mere 9% supplied references for examination of peer-reviewed data. Another unfortunate consequence of DCTM is “physician shopping.” Bozic and colleagues4 found that patients exposed to DCTM were more likely to demand a specific surgery, approach, or implant and were less open to alternatives; in addition, they saw more than one surgeon before deciding on joint arthroplasty.
The effects of DTCM on resident and fellowship training require serious consideration. An emphasis on technology has come at the expense of learning the science and art of orthopedics.8 Physicians in training are pressured both to produce more and to use whichever specific technique or product a patient requests.4 Similarly, orthopedic surgeons are seeing job advertisements that read, “Training in robotic surgery or anterior approach is preferred.” Employer pressure can have profound implications for residents and fellows, who may feel compelled to learn these techniques. To a large degree, residents and fellows learn by accompanying their mentors and closely observing their decision-making processes and interactions with patients. Decisions regarding fellowships should not be influenced by surgical techniques or implant choices but by the quality and breadth of clinical experience.
DTCM likely will continue to shape all aspects of care. Claims made by physicians and hospitals are especially troubling because patients trust these sources. We face the challenge of reaffirming our commitment to patients and orthopedic surgeons. As the leader in musculoskeletal education, the American Academy of Orthopaedic Surgeons (AAOS) not only must provide educational material that is compatible with current technological media but must address current controversies and misleading claims. Toward that end, AAOS can expand its patient website, OrthoInfo, to include information on new technologies and surgical techniques pertaining to each musculoskeletal condition. Educating the public about risk factors for poor surgical outcomes is equally important in order to moderate unrealistic expectations and stimulate discussions on risks involved in unnecessary or potentially harmful technologies. The American Association of Hip and Knee Surgeons (AAHKS) has already embarked on this approach. Orthopedic surgeons should continue to abide by the standards of professionalism—maintaining the tenet of “First do no harm,” resisting the temptations of consumerism, and giving patients accurate information. Taking these measures may help reduce physician shopping and strengthen the patient–physician relationship. We physicians are the guardians of patients’ well-being. We also owe it to orthopedic surgeons in training to provide well-balanced, unbiased education. The focus of training should not be on techniques for gaining market edge but on learning evidence-based medicine and surgical principles. In our burdened healthcare system, curbing DTCM has the potential to decrease unnecessary use of resources and improve the quality of education and patient care.
Am J Orthop. 2016;45(6):E335-E336. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Bhattacharyya T, Tornetta P 3rd, Healy WL, Einhorn TA. The validity of claims made in orthopaedic print advertisements. J Bone Joint Surgery Am. 2003;85(7):1224-1228.
2. Woloshin S, Schwartz LM, Tremmel J, Welch HG. Direct-to-consumer advertisements for prescription drugs: what are Americans being sold? Lancet. 2001;358(9288):1141-1146.
3. Robinson AR, Hohmann KB, Rifkin JI, et al. Direct-to-consumer pharmaceutical advertising: physician and public opinion and potential effects on the physician-patient relationship. Arch Intern Med. 2004;164(4):427-432.
4. Bozic KJ, Smith AR, Hariri S, et al. The 2007 ABJS Marshall Urist award: the impact of direct-to-consumer advertising in orthopaedics. Clin Orthop Relat Res. 2007;(458):202-219.
5. Mason JB. The new demands by patients in the modern era of total joint arthroplasty: a point of view. Clin Orthop Relat Res. 2008;466(1):146-152.
6. Weinstein SL. Words from a “wise old hand”—guideposts for the future. Professor Stuart L. Weinstein. Iowa Orthop J. 2008;28:94-97.
7. Labovitch RS, Bozic KJ, Hansen E. An evaluation of information available on the internet regarding minimally invasive hip arthroplasty. J Arthroplasty. 2006;21(1):1-5.
8. Buckwalter JA. Advancing the science and art of orthopaedics. Lessons from history. J Bone Joint Surg Am. 2000;82(12):1782-1803.
Direct-to-consumer marketing (DTCM) is the promotion of health-related products or services directly to patients. Although this topic is not new to orthopedics, several emerging trends hold troubling implications for patients as well as orthopedic surgeons, particularly surgeons in training.
Orthopedics DTCM most commonly involves television and print advertisements. Supporters contend DTCM is an empowering educational tool that increases awareness of medical ailments and encourages patients to seek treatment. Opponents point to inaccuracies and misleading claims. Bhattacharyya and colleagues1 found that about half the claims in orthopedic print advertisements were not supported by clinical evidence. Woloshin and colleagues2 found that information in DTCM was vague and often was designed to act on the emotions. Patients misled by these claims and innately seeking improvement could present with unreasonable expectations and difficult discussions that can be detrimental to the patient–physician relationship.3Given changing patient demographics and the information revolution, the effects of DTCM likely will continue to grow. Total joint arthroplasty (TJA), which represents Medicare’s largest expenditure,4 is a classic example. Today’s TJA patients are younger, more active, and better educated, and they live longer, have higher expectations, and are more reliant on the media.5 Television is no longer our main medium—the internet is the source of healthcare education for 70% of adults in the United States.6Healthcare reform has also brought significant changes in the delivery of DTCM. In an era of competition for market share brought by increased demand and decreased reimbursement, DTCM has evolved into sales pitches by hospitals and physicians. Robotic joint replacement, minimally invasive surgery (MIS), use of the anterior hip approach, use of sex-specific or high-flexion knee implants, and other practices have become popular marketing tools for surgery centers competing for new patients. As a result, patients often present not only with a complaint but with a request for a particular procedure.4,5 Labovitch and colleagues7 found that 70% of MIS information on the internet was produced by hospitals and private medical groups, and only 6% was produced by industry. Although the vast majority of the sources reported on the advantages of MIS, only 15% explained patient eligibility, and a mere 9% supplied references for examination of peer-reviewed data. Another unfortunate consequence of DCTM is “physician shopping.” Bozic and colleagues4 found that patients exposed to DCTM were more likely to demand a specific surgery, approach, or implant and were less open to alternatives; in addition, they saw more than one surgeon before deciding on joint arthroplasty.
The effects of DTCM on resident and fellowship training require serious consideration. An emphasis on technology has come at the expense of learning the science and art of orthopedics.8 Physicians in training are pressured both to produce more and to use whichever specific technique or product a patient requests.4 Similarly, orthopedic surgeons are seeing job advertisements that read, “Training in robotic surgery or anterior approach is preferred.” Employer pressure can have profound implications for residents and fellows, who may feel compelled to learn these techniques. To a large degree, residents and fellows learn by accompanying their mentors and closely observing their decision-making processes and interactions with patients. Decisions regarding fellowships should not be influenced by surgical techniques or implant choices but by the quality and breadth of clinical experience.
DTCM likely will continue to shape all aspects of care. Claims made by physicians and hospitals are especially troubling because patients trust these sources. We face the challenge of reaffirming our commitment to patients and orthopedic surgeons. As the leader in musculoskeletal education, the American Academy of Orthopaedic Surgeons (AAOS) not only must provide educational material that is compatible with current technological media but must address current controversies and misleading claims. Toward that end, AAOS can expand its patient website, OrthoInfo, to include information on new technologies and surgical techniques pertaining to each musculoskeletal condition. Educating the public about risk factors for poor surgical outcomes is equally important in order to moderate unrealistic expectations and stimulate discussions on risks involved in unnecessary or potentially harmful technologies. The American Association of Hip and Knee Surgeons (AAHKS) has already embarked on this approach. Orthopedic surgeons should continue to abide by the standards of professionalism—maintaining the tenet of “First do no harm,” resisting the temptations of consumerism, and giving patients accurate information. Taking these measures may help reduce physician shopping and strengthen the patient–physician relationship. We physicians are the guardians of patients’ well-being. We also owe it to orthopedic surgeons in training to provide well-balanced, unbiased education. The focus of training should not be on techniques for gaining market edge but on learning evidence-based medicine and surgical principles. In our burdened healthcare system, curbing DTCM has the potential to decrease unnecessary use of resources and improve the quality of education and patient care.
Am J Orthop. 2016;45(6):E335-E336. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Direct-to-consumer marketing (DTCM) is the promotion of health-related products or services directly to patients. Although this topic is not new to orthopedics, several emerging trends hold troubling implications for patients as well as orthopedic surgeons, particularly surgeons in training.
Orthopedics DTCM most commonly involves television and print advertisements. Supporters contend DTCM is an empowering educational tool that increases awareness of medical ailments and encourages patients to seek treatment. Opponents point to inaccuracies and misleading claims. Bhattacharyya and colleagues1 found that about half the claims in orthopedic print advertisements were not supported by clinical evidence. Woloshin and colleagues2 found that information in DTCM was vague and often was designed to act on the emotions. Patients misled by these claims and innately seeking improvement could present with unreasonable expectations and difficult discussions that can be detrimental to the patient–physician relationship.3Given changing patient demographics and the information revolution, the effects of DTCM likely will continue to grow. Total joint arthroplasty (TJA), which represents Medicare’s largest expenditure,4 is a classic example. Today’s TJA patients are younger, more active, and better educated, and they live longer, have higher expectations, and are more reliant on the media.5 Television is no longer our main medium—the internet is the source of healthcare education for 70% of adults in the United States.6Healthcare reform has also brought significant changes in the delivery of DTCM. In an era of competition for market share brought by increased demand and decreased reimbursement, DTCM has evolved into sales pitches by hospitals and physicians. Robotic joint replacement, minimally invasive surgery (MIS), use of the anterior hip approach, use of sex-specific or high-flexion knee implants, and other practices have become popular marketing tools for surgery centers competing for new patients. As a result, patients often present not only with a complaint but with a request for a particular procedure.4,5 Labovitch and colleagues7 found that 70% of MIS information on the internet was produced by hospitals and private medical groups, and only 6% was produced by industry. Although the vast majority of the sources reported on the advantages of MIS, only 15% explained patient eligibility, and a mere 9% supplied references for examination of peer-reviewed data. Another unfortunate consequence of DCTM is “physician shopping.” Bozic and colleagues4 found that patients exposed to DCTM were more likely to demand a specific surgery, approach, or implant and were less open to alternatives; in addition, they saw more than one surgeon before deciding on joint arthroplasty.
The effects of DTCM on resident and fellowship training require serious consideration. An emphasis on technology has come at the expense of learning the science and art of orthopedics.8 Physicians in training are pressured both to produce more and to use whichever specific technique or product a patient requests.4 Similarly, orthopedic surgeons are seeing job advertisements that read, “Training in robotic surgery or anterior approach is preferred.” Employer pressure can have profound implications for residents and fellows, who may feel compelled to learn these techniques. To a large degree, residents and fellows learn by accompanying their mentors and closely observing their decision-making processes and interactions with patients. Decisions regarding fellowships should not be influenced by surgical techniques or implant choices but by the quality and breadth of clinical experience.
DTCM likely will continue to shape all aspects of care. Claims made by physicians and hospitals are especially troubling because patients trust these sources. We face the challenge of reaffirming our commitment to patients and orthopedic surgeons. As the leader in musculoskeletal education, the American Academy of Orthopaedic Surgeons (AAOS) not only must provide educational material that is compatible with current technological media but must address current controversies and misleading claims. Toward that end, AAOS can expand its patient website, OrthoInfo, to include information on new technologies and surgical techniques pertaining to each musculoskeletal condition. Educating the public about risk factors for poor surgical outcomes is equally important in order to moderate unrealistic expectations and stimulate discussions on risks involved in unnecessary or potentially harmful technologies. The American Association of Hip and Knee Surgeons (AAHKS) has already embarked on this approach. Orthopedic surgeons should continue to abide by the standards of professionalism—maintaining the tenet of “First do no harm,” resisting the temptations of consumerism, and giving patients accurate information. Taking these measures may help reduce physician shopping and strengthen the patient–physician relationship. We physicians are the guardians of patients’ well-being. We also owe it to orthopedic surgeons in training to provide well-balanced, unbiased education. The focus of training should not be on techniques for gaining market edge but on learning evidence-based medicine and surgical principles. In our burdened healthcare system, curbing DTCM has the potential to decrease unnecessary use of resources and improve the quality of education and patient care.
Am J Orthop. 2016;45(6):E335-E336. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Bhattacharyya T, Tornetta P 3rd, Healy WL, Einhorn TA. The validity of claims made in orthopaedic print advertisements. J Bone Joint Surgery Am. 2003;85(7):1224-1228.
2. Woloshin S, Schwartz LM, Tremmel J, Welch HG. Direct-to-consumer advertisements for prescription drugs: what are Americans being sold? Lancet. 2001;358(9288):1141-1146.
3. Robinson AR, Hohmann KB, Rifkin JI, et al. Direct-to-consumer pharmaceutical advertising: physician and public opinion and potential effects on the physician-patient relationship. Arch Intern Med. 2004;164(4):427-432.
4. Bozic KJ, Smith AR, Hariri S, et al. The 2007 ABJS Marshall Urist award: the impact of direct-to-consumer advertising in orthopaedics. Clin Orthop Relat Res. 2007;(458):202-219.
5. Mason JB. The new demands by patients in the modern era of total joint arthroplasty: a point of view. Clin Orthop Relat Res. 2008;466(1):146-152.
6. Weinstein SL. Words from a “wise old hand”—guideposts for the future. Professor Stuart L. Weinstein. Iowa Orthop J. 2008;28:94-97.
7. Labovitch RS, Bozic KJ, Hansen E. An evaluation of information available on the internet regarding minimally invasive hip arthroplasty. J Arthroplasty. 2006;21(1):1-5.
8. Buckwalter JA. Advancing the science and art of orthopaedics. Lessons from history. J Bone Joint Surg Am. 2000;82(12):1782-1803.
1. Bhattacharyya T, Tornetta P 3rd, Healy WL, Einhorn TA. The validity of claims made in orthopaedic print advertisements. J Bone Joint Surgery Am. 2003;85(7):1224-1228.
2. Woloshin S, Schwartz LM, Tremmel J, Welch HG. Direct-to-consumer advertisements for prescription drugs: what are Americans being sold? Lancet. 2001;358(9288):1141-1146.
3. Robinson AR, Hohmann KB, Rifkin JI, et al. Direct-to-consumer pharmaceutical advertising: physician and public opinion and potential effects on the physician-patient relationship. Arch Intern Med. 2004;164(4):427-432.
4. Bozic KJ, Smith AR, Hariri S, et al. The 2007 ABJS Marshall Urist award: the impact of direct-to-consumer advertising in orthopaedics. Clin Orthop Relat Res. 2007;(458):202-219.
5. Mason JB. The new demands by patients in the modern era of total joint arthroplasty: a point of view. Clin Orthop Relat Res. 2008;466(1):146-152.
6. Weinstein SL. Words from a “wise old hand”—guideposts for the future. Professor Stuart L. Weinstein. Iowa Orthop J. 2008;28:94-97.
7. Labovitch RS, Bozic KJ, Hansen E. An evaluation of information available on the internet regarding minimally invasive hip arthroplasty. J Arthroplasty. 2006;21(1):1-5.
8. Buckwalter JA. Advancing the science and art of orthopaedics. Lessons from history. J Bone Joint Surg Am. 2000;82(12):1782-1803.
High-Grade Articular, Bursal, and Intratendinous Partial-Thickness Rotator Cuff Tears: A Retrospective Study Comparing Functional Outcomes After Completion and Repair
The Ellman1 classification of partial-thickness rotator cuff tears (PTRCTs) is based on tear location or subtype (A, articular; B, bursal; C, intratendinous) and tear depth (grade 1, <3 mm; grade 2, 3-6 mm; grade 3, >6 mm). Ruotolo and colleagues2 reported that the medial-lateral insertion width of the supraspinatus averaged 12.1 mm, and most authors have indicated that tear depth of 6 mm or more represents 50% tendon thickness. Therefore, Ellman grade 3 tears are considered high-grade (>50% thickness).
Advancements in shoulder arthroscopy, imaging modalities, and clinical research have helped refine our understanding of PTRCTs. Classic teaching based on the retrospective study by Weber3 calls for simple débridement of low-grade (<50%) tears and repair of tears thicker than 50%. According to this standard, Ellman grade 1 and 2 tears should be débrided and grade 3 tears repaired. However, Cordasco and colleagues4 provided evidence supporting an algorithm reformation based on tear location. In their study, results of simple débridement were significantly worse for Ellman grade 2B PTRCTs than for 2A tears, suggesting low-grade bursal tears should also be repaired. Although their study supported a change in operative management for grade 2 tears, to our knowledge no one has investigated the need for differing surgical treatments for grade 3 subtypes based on tear location.
Several studies have demonstrated the efficacy of arthroscopic completion and repair for high-grade PTRCTs of the supraspinatus.5-7 Although all these studies addressed articular- and bursal-sided tears, there has been relative silence with respect to the intratendinous subtype. One explanation is that these tears, given their interstitial nature, pose diagnostic challenges. Histologic research has also shown that they can exist in combination with other tears.8 Despite such challenges, these tears are well documented. They were identified in the seminal study by Ellman1 and were the most common PTRCTs encountered in a well-known cadaveric study (N = 249).9,10 More recently, in 2011, a radiologic study using magnetic resonance arthrography found that 33.8% of PTRCTs were intratendinous (N = 68).11 That study also documented the case of a nonoperatively treated intratendinous tear that progressed to a full-thickness tear within about 6 months.11 Given these facts, it was important for the current PTRCT debate to include an intratendinous group when investigating treatment algorithms for grade 3 tears. Although results of the present study may continue reformation of the 50% algorithm, we hypothesized that arthroscopic completion and repair of all grade 3 PTRCTs will be equally effective, regardless of tear location.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the operative reports of a fellowship-trained shoulder surgeon for the period 2008–2010. Patients who underwent arthroscopic completion and repair of a supraspinatus tendon PTRCT were identified. Preoperative identification of PTRCT was made on the basis of physical examination and magnetic resonance imaging (MRI) findings (Figures 1–3).
Patients with low-grade PTRCTs of the supraspinatus, identified at time of arthroscopy, were excluded, as were patients with tears that extended into other rotator cuff tendons and patients with previous rotator cuff repair, glenohumeral instability, or adhesive capsulitis.
During the initial appointment, each patient completed a standard questionnaire that included standardized subjective scales evaluating pain and function. A fellowship-trained surgeon then took the patient’s history and performed a physical examination. Postoperative clinical outcome was determined at a minimum of 12 months. Clinical outcomes were assessed with 3 validated outcome measures: visual analog scale (VAS) score, American Shoulder and Elbow Surgeons (ASES) score, and Constant score.
Surgical Procedure and Rehabilitation
All procedures were performed with the patient under general anesthesia with or without an interscalene block. The patient was positioned in the upright beach-chair position. Diagnostic arthroscopy was used to assess the rotator cuff and associated pathologic conditions. If impingement was noted, subacromial decompression was performed. An acromioplasty was limited to removal of osteophytic bone. Distal clavicle excision and biceps tenotomy or tenodesis were performed if preoperative evaluation warranted these procedures.
The rotator cuff was assessed from the articular and bursal sides. For articular PTRCTs, a tagging suture was used to identify the lesion from the bursal side. Bursal-sided tears were probed to assess thinning of the tendon and determine tear grade. If preoperative MRI findings suggested an intratendinous tear, a probe was used to confirm thinning of the tendon. An arthroscopic shaver was then carefully used to débride the capsule on either side of the tendon at the location of the suspected tear. The shaver inevitably penetrated the capsule and entered the tear, where any degenerative tissue was further débrided (Figure 4).
After the PTRCT was completed to full thickness, the rotator cuff footprint on the greater tuberosity was débrided to bleeding cortical bone. Depending on tear length, 1 or 2 Bio-Corkscrew absorbable suture anchors (Arthrex) with 2 No. 2 FiberWire sutures (Arthrex) were then placed in the tuberosity 3 to 5 mm lateral to the articular margin. An arthroscopic suture passer was used to move the 2 sutures through the rotator cuff, such that one was placed in the horizontal mattress and the other was placed in a simple fashion deep to the horizontal mattress. The sutures were then tied with a modified Roeder knot.
A standardized postoperative protocol was used for all patients starting within the first week after surgery. Passive range of motion (ROM) was performed for the first 6 weeks after surgery and was advanced to include active ROM from 6 to 8 weeks after surgery. Strengthening was initiated 8 weeks after surgery.
Statistical Analysis
Power analysis demonstrated that a sample size of 20 in each group was adequate for detecting a medium to large effect size with 80% power. Wilcoxon signed rank test was used to compare the preoperative and postoperative scores for each outcome measure, and analysis of variance (ANOVA) was used to compare the amount of improvement for each of the 3 PTRCT subtypes. Paired t test was used to compare preoperative and postoperative ROM values, and unpaired t tests were used to determine the impact of corticosteroid injections and preoperative PT. For statistical analysis, patients were divided into 2 groups (yes, no) regarding injections and 2 groups (yes, no) regarding PT. Last, multiple linear regression analyses were performed for each outcome measure to determine the impact of potential confounders. Covariates included symptom duration, etiology, age, injection, PT, tear location, percentage of tendon torn (medial-lateral), and tear length (anterior-posterior). P < .05 was considered significant.
Results
Patient Sample and Demographics
Sixty-seven patients underwent arthroscopic repair of a PTRCT—22 grade 3A, 23 grade 3B, and 22 grade 3C. In each of the 3 groups, 20 patients returned for end-of-healing evaluation. Thus, the study population consisted of 60 patients (60 shoulders). The 7 patients who did not return for end-of-healing evaluation or who could not be contacted were excluded from the study.
Table 1 summarizes the key patient demographics. Of the 60 patients, 35 were men and 25 were women.
Range of Motion
The sample as a whole exhibited statistically significant improvement in active ROM (Table 2).
Operative Findings
Operative findings included mean tear thickness of 74% for the sample as a whole and mean anterior-to-posterior tear length of 10.7 mm overall. There was very little variance among the articular, bursal, and intratendinous means with respect to percentage of tear thickness (78.3%, 75.0%, and 68.8%, respectively) and anterior-to-posterior tear thickness (11.5 mm, 11.4 mm, and 9.1 mm, respectively). Each of the 6 tears (3 bursal, 2 articular, 1 intratendinous) that were longer than 15 mm required 2 anchors. Fifty-nine repairs (98%) involved subacromial decompression, 38 (63%) involved acromioclavicular resection, 18 (30%) involved débridement of the superior labrum anterior-to-posterior (SLAP), and 12 (20%) involved biceps tenodesis/tenotomy.
Outcome Measures
In the study population as a whole, and in all 3 tear subtypes, postoperative improvement in VAS, ASES, and Constant scores was statistically significant (Table 3).
Multiple linear regression analyses showed that etiology, symptom duration, and steroid injection were the primary predictors of each outcome. After the other variables were adjusted for, injection (vs noninjection) seemed to be associated with more improvement in ASES (P = .0061), VAS (P = .020), and Constant (P = .067) scores. Insidious (vs traumatic) etiology was significantly associated with more improvement in ASES scores (P = .033) and VAS scores (P = .014) but not Constant scores (P = .50). Longer time from symptom onset to surgery was associated with less improvement, though the coefficient was not statistically significant in any of the models at P = .05. The other possible covariates had no significant impact on outcomes.
Complications
There were no intraoperative or postoperative complications, and there were no incidents of recurrent rotator cuff tear or postoperative stiffness.
Discussion
We investigated the effectiveness of arthroscopic completion and repair of Ellman grade 3 PTRCTs by comparing the functional outcomes for each subtype. Although several studies have analyzed results of PTRCT repair, they all either omitted intratendinous tears or were not grade-specific. In a systematic review, Strauss and colleagues13 discussed 4 PTRCT outcome studies4,6,14,15 in which only articular- and bursal-sided tears were addressed. Of these studies, only 1 (Kamath and colleagues6) focused on grade 3 lesions, and the number of bursal tears was insufficient for comparison with the articular tear group. Cordasco and colleagues4 limited their study to grade 1 and 2 tears but did not include intratendinous lesions.
In other research, Itoi and Tabata16 distinguished among the 3 subtypes but did not measure grade. As we did in our study, Deutsch5 focused on grade 3 lesions and used the completion-and-repair method, but he did not include intratendinous tears. Porat and colleagues17 reviewed grade 3 completion-and-repair results but did not compare them by subtype. Last, Uchiyama and colleagues18 reported strong outcomes for intratendinous tears but did not measure grade and used various surgical methods.
These studies have made important contributions to the ongoing PTRCT discussion, but debate about appropriate operative management persists. To limit the influence of external variables and provide the most exhaustive evidence regarding current PTRCT treatment algorithms, we designed the present study to consider outcomes with all 3 Ellman subtypes, only grade 3 lesions of the supraspinatus, only 1 surgical method, and consistent techniques of only 1 fellowship-trained shoulder surgeon.
Results of this chart review confirmed the findings of other grade 3 PTRCT repair studies. For instance, Koh and colleagues15 reported excellent results of 38 grade 3B PTRCTs completed to full thickness and repaired. Specifically, their mean ASES and Constant scores improved 34.1 and 23.7 points, respectively. These results are similar to our ASES and Constant score improvements—38.9 and 24.7 points for the group as a whole and 36 and 25.1 points for the grade 3B cohort. In addition, our ASES scores are nearly identical to the preoperative (46.1) and postoperative (82.1) ASES scores found by Kamath and colleagues.6 Although the mean ASES and VAS score improvements reported by Deutsch5 (51 and 5.7 points, respectively) were slightly better than ours, these results are still comparable and support completion and repair.
Although results of the study by Cordasco and colleagues4 support differing surgical treatments of grade 2 tears based on location, the present findings support the established 50% algorithm for all 3 high-grade PTRCTs. The completion-and-repair method not only produced significant improvements for each PTRCT subtype, but, importantly, there was no significant difference among those outcomes. Unlike previous results for grade 2 tears, the present results confirmed the established algorithm for grade 3 tears.
Our multiple linear regression analyses suggested that etiology, longer duration of symptoms, and steroid injections each had a strong impact on outcomes. The literature on these preoperative factors is often conflicting, and our results continue the trend. For instance, in a study of acute rotator cuff tears, Petersen and Murphy19 studied acute rotator cuff tears and also found tear size had no significant effect on functional outcomes. However, contrary to our findings, they did not find symptom duration to be a significant predictor of results. Also contrary to our findings, Oh and colleagues20 found age and tear size to be significant influences on outcomes for full-thickness tears. The strong correlation of preoperative steroid injection and better outcomes is novel and warrants further investigation.
In this study, we investigated the effectiveness of the completion-and-repair method in treating Ellman grade 3 PTRCTs. Although our findings validate this surgical technique, we acknowledge alternative approaches to high-grade PTRCTs. For instance, the transtendon method, which does not convert PTRCTs to full thickness, has also shown good clinical outcomes.21-23 In fact, the preoperative and postoperative VAS measures used in our study are nearly identical to those used in an Ellman grade 3A transtendon repair study.1 However, we agree with Porat and colleagues17 that the remaining, intact cuff material of PTRCTs is degenerative and may result in poor fixation, increased pain, or retear. In addition, nonoperative treatment typically is attempted before surgery, though little evidence is reported for success specifically in high-grade PTRCTs. One study found that 91% of PTRCT patients were still satisfied 4 years after nonoperative treatment, but it was noted that many of the tears were low-grade.24 To continue an evidence-based discussion on the more effective treatment, we invite advocates of alternative approaches to conduct a similar study on all 3 Ellman grade 3 subtypes.
Study Limitations
Concomitant procedures were not uniform among all patients and therefore may have affected some outcome measurements. Subacromial decompression was nearly universal, as it was performed for surgical visualization in 98% of patients. The additional procedures were also deemed necessary based on the preoperative assessment and arthroscopic findings. Although these procedures may have influenced outcome measurements, similar studies regularly include them as well.5-7,17 Our minimum 12-month follow-up could be considered a restriction, as other studies have cited a 2-year follow-up threshold.5-7 However, Strauss and colleagues13 endorsed a 12-month standard in their systematic review. Last, about 10% (7/67) of our initial patients were lost to follow-up; this percentage, however, is comparable to what has been reported in other PTRCT studies.4-6,14,15,21,22
Conclusion
Our study findings validate use of the current algorithm for Ellman grade 3 PTRCTs of the supraspinatus and advocate their completion and repair, regardless of tear location.
Acknowledgment: The authors thank Lisa Rein, MS, and Sergey Tarima, PhD, of the Division of Biostatistics, Medical College of Wisconsin, for their help in data analysis and manuscript preparation.
Am J Orthop. 2016;45(5):E254-E260. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res. 1990;(254):64-74.
2. Ruotolo C, Fow JE, Nottage WM. The supraspinatus footprint: an anatomic study of the supraspinatus insertion. Arthroscopy. 2004;20(3):246-249.
3. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
4. Cordasco FA, Backer M, Craig EV, Klein D, Warren RF. The partial-thickness rotator cuff tear: is acromioplasty without repair sufficient? Am J Sports Med. 2002;30(2):257-260.
5. Deutsch A. Arthroscopic repair of partial-thickness tears of the rotator cuff. J Shoulder Elbow Surg. 2007;16(2):193-201.
6. Kamath G, Galatz LM, Keener JD, Teefey S, Middleton W, Yamaguchi K. Tendon integrity and functional outcome after arthroscopic repair of high-grade partial-thickness supraspinatus tears. J Bone Joint Surg Am. 2009;91(5):1055-1062.
7. Park JY, Yoo MJ, Kim MH. Comparison of surgical outcome between bursal and articular partial thickness rotator cuff tears. Orthopedics. 2003;26(4):387-390.
8. Fukuda H, Hamada K, Nakajima T, Tomonaga A. Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clin Orthop Relat Res. 1994;(304):60-67.
9. Fukuda H, Mikasa M, Yamanaka K. Incomplete thickness rotator cuff tears diagnosed by subacromial bursography. Clin Orthop Relat Res. 1987;(223):51-58.
10. Yamanaka K, Fukuda H, Hamada K, Mikasa M. Incomplete thickness tears of the rotator cuff [abstract]. Orthop Surg Traumatol (Toyko). 1983;26:713.
11. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21(7):1477-1484.
12. Nakagawa S, Yoneda M, Mizuno N, Hayashida K, Mae T, Take Y. Throwing shoulder injury involving the anterior rotator cuff: concealed tears not as uncommon as previously thought. Arthroscopy. 2006;22(12):1298-1303.
13. Strauss EJ, Salata MJ, Kercher J, et al. Multimedia article. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580.
14. Kartus J, Kartus C, Rostgard-Christensen L, Sernert N, Read J, Perko M. Long-term clinical and ultrasound evaluation after arthroscopic acromioplasty in patients with partial rotator cuff tears. Arthroscopy. 2006;22(1):44-49.
15. Koh KH, Shon MS, Lim TK, Yoo JC. Clinical and magnetic resonance imaging results of arthroscopic full-layer repair of bursal-side partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(8):1660-1667.
16. Itoi E, Tabata S. Incomplete rotator cuff tears. Results of operative treatment. Clin Orthop Relat Res. 1992;(284):128-135.
17. Porat S, Nottage WM, Fouse MN. Repair of partial thickness rotator cuff tears: a retrospective review with minimum two-year follow-up. J Shoulder Elbow Surg. 2008;17(5):729-731.
18. Uchiyama Y, Hamada K, Khruekarnchana P, et al. Surgical treatment of confirmed intratendinous rotator cuff tears: retrospective analysis after an average of eight years of follow-up. J Shoulder Elbow Surg. 2010;19(6):837-846.
19. Petersen SA, Murphy TP. The timing of rotator cuff repair for the restoration of function. J Shoulder Elbow Surg. 2011;20(1):62-68.
20. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
21. Castagna A, Delle Rose G, Conti M, Snyder SJ, Borroni M, Garofalo R. Predictive factors of subtle residual shoulder symptoms after transtendinous arthroscopic cuff repair: a clinical study. Am J Sports Med. 2009;37(1):103-108.
22. Castricini R, Panfoli N, Nittoli R, Spurio S, Pirani O. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the supraspinatus: results at 2 years. Chir Organi Mov. 2009;93(suppl 1):S49-S54.
23. Spencer EE Jr. Partial-thickness articular surface rotator cuff tears: an all-inside repair technique. Clin Orthop Relat Res. 2010;468(6):1514-1520.
24. Denkers M, Pletsch K, Boorman R, Hollinshead R, Lo IKY. Partial thickness rotator cuff tears: observe or operative. In: Proceedings of the American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
The Ellman1 classification of partial-thickness rotator cuff tears (PTRCTs) is based on tear location or subtype (A, articular; B, bursal; C, intratendinous) and tear depth (grade 1, <3 mm; grade 2, 3-6 mm; grade 3, >6 mm). Ruotolo and colleagues2 reported that the medial-lateral insertion width of the supraspinatus averaged 12.1 mm, and most authors have indicated that tear depth of 6 mm or more represents 50% tendon thickness. Therefore, Ellman grade 3 tears are considered high-grade (>50% thickness).
Advancements in shoulder arthroscopy, imaging modalities, and clinical research have helped refine our understanding of PTRCTs. Classic teaching based on the retrospective study by Weber3 calls for simple débridement of low-grade (<50%) tears and repair of tears thicker than 50%. According to this standard, Ellman grade 1 and 2 tears should be débrided and grade 3 tears repaired. However, Cordasco and colleagues4 provided evidence supporting an algorithm reformation based on tear location. In their study, results of simple débridement were significantly worse for Ellman grade 2B PTRCTs than for 2A tears, suggesting low-grade bursal tears should also be repaired. Although their study supported a change in operative management for grade 2 tears, to our knowledge no one has investigated the need for differing surgical treatments for grade 3 subtypes based on tear location.
Several studies have demonstrated the efficacy of arthroscopic completion and repair for high-grade PTRCTs of the supraspinatus.5-7 Although all these studies addressed articular- and bursal-sided tears, there has been relative silence with respect to the intratendinous subtype. One explanation is that these tears, given their interstitial nature, pose diagnostic challenges. Histologic research has also shown that they can exist in combination with other tears.8 Despite such challenges, these tears are well documented. They were identified in the seminal study by Ellman1 and were the most common PTRCTs encountered in a well-known cadaveric study (N = 249).9,10 More recently, in 2011, a radiologic study using magnetic resonance arthrography found that 33.8% of PTRCTs were intratendinous (N = 68).11 That study also documented the case of a nonoperatively treated intratendinous tear that progressed to a full-thickness tear within about 6 months.11 Given these facts, it was important for the current PTRCT debate to include an intratendinous group when investigating treatment algorithms for grade 3 tears. Although results of the present study may continue reformation of the 50% algorithm, we hypothesized that arthroscopic completion and repair of all grade 3 PTRCTs will be equally effective, regardless of tear location.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the operative reports of a fellowship-trained shoulder surgeon for the period 2008–2010. Patients who underwent arthroscopic completion and repair of a supraspinatus tendon PTRCT were identified. Preoperative identification of PTRCT was made on the basis of physical examination and magnetic resonance imaging (MRI) findings (Figures 1–3).
Patients with low-grade PTRCTs of the supraspinatus, identified at time of arthroscopy, were excluded, as were patients with tears that extended into other rotator cuff tendons and patients with previous rotator cuff repair, glenohumeral instability, or adhesive capsulitis.
During the initial appointment, each patient completed a standard questionnaire that included standardized subjective scales evaluating pain and function. A fellowship-trained surgeon then took the patient’s history and performed a physical examination. Postoperative clinical outcome was determined at a minimum of 12 months. Clinical outcomes were assessed with 3 validated outcome measures: visual analog scale (VAS) score, American Shoulder and Elbow Surgeons (ASES) score, and Constant score.
Surgical Procedure and Rehabilitation
All procedures were performed with the patient under general anesthesia with or without an interscalene block. The patient was positioned in the upright beach-chair position. Diagnostic arthroscopy was used to assess the rotator cuff and associated pathologic conditions. If impingement was noted, subacromial decompression was performed. An acromioplasty was limited to removal of osteophytic bone. Distal clavicle excision and biceps tenotomy or tenodesis were performed if preoperative evaluation warranted these procedures.
The rotator cuff was assessed from the articular and bursal sides. For articular PTRCTs, a tagging suture was used to identify the lesion from the bursal side. Bursal-sided tears were probed to assess thinning of the tendon and determine tear grade. If preoperative MRI findings suggested an intratendinous tear, a probe was used to confirm thinning of the tendon. An arthroscopic shaver was then carefully used to débride the capsule on either side of the tendon at the location of the suspected tear. The shaver inevitably penetrated the capsule and entered the tear, where any degenerative tissue was further débrided (Figure 4).
After the PTRCT was completed to full thickness, the rotator cuff footprint on the greater tuberosity was débrided to bleeding cortical bone. Depending on tear length, 1 or 2 Bio-Corkscrew absorbable suture anchors (Arthrex) with 2 No. 2 FiberWire sutures (Arthrex) were then placed in the tuberosity 3 to 5 mm lateral to the articular margin. An arthroscopic suture passer was used to move the 2 sutures through the rotator cuff, such that one was placed in the horizontal mattress and the other was placed in a simple fashion deep to the horizontal mattress. The sutures were then tied with a modified Roeder knot.
A standardized postoperative protocol was used for all patients starting within the first week after surgery. Passive range of motion (ROM) was performed for the first 6 weeks after surgery and was advanced to include active ROM from 6 to 8 weeks after surgery. Strengthening was initiated 8 weeks after surgery.
Statistical Analysis
Power analysis demonstrated that a sample size of 20 in each group was adequate for detecting a medium to large effect size with 80% power. Wilcoxon signed rank test was used to compare the preoperative and postoperative scores for each outcome measure, and analysis of variance (ANOVA) was used to compare the amount of improvement for each of the 3 PTRCT subtypes. Paired t test was used to compare preoperative and postoperative ROM values, and unpaired t tests were used to determine the impact of corticosteroid injections and preoperative PT. For statistical analysis, patients were divided into 2 groups (yes, no) regarding injections and 2 groups (yes, no) regarding PT. Last, multiple linear regression analyses were performed for each outcome measure to determine the impact of potential confounders. Covariates included symptom duration, etiology, age, injection, PT, tear location, percentage of tendon torn (medial-lateral), and tear length (anterior-posterior). P < .05 was considered significant.
Results
Patient Sample and Demographics
Sixty-seven patients underwent arthroscopic repair of a PTRCT—22 grade 3A, 23 grade 3B, and 22 grade 3C. In each of the 3 groups, 20 patients returned for end-of-healing evaluation. Thus, the study population consisted of 60 patients (60 shoulders). The 7 patients who did not return for end-of-healing evaluation or who could not be contacted were excluded from the study.
Table 1 summarizes the key patient demographics. Of the 60 patients, 35 were men and 25 were women.
Range of Motion
The sample as a whole exhibited statistically significant improvement in active ROM (Table 2).
Operative Findings
Operative findings included mean tear thickness of 74% for the sample as a whole and mean anterior-to-posterior tear length of 10.7 mm overall. There was very little variance among the articular, bursal, and intratendinous means with respect to percentage of tear thickness (78.3%, 75.0%, and 68.8%, respectively) and anterior-to-posterior tear thickness (11.5 mm, 11.4 mm, and 9.1 mm, respectively). Each of the 6 tears (3 bursal, 2 articular, 1 intratendinous) that were longer than 15 mm required 2 anchors. Fifty-nine repairs (98%) involved subacromial decompression, 38 (63%) involved acromioclavicular resection, 18 (30%) involved débridement of the superior labrum anterior-to-posterior (SLAP), and 12 (20%) involved biceps tenodesis/tenotomy.
Outcome Measures
In the study population as a whole, and in all 3 tear subtypes, postoperative improvement in VAS, ASES, and Constant scores was statistically significant (Table 3).
Multiple linear regression analyses showed that etiology, symptom duration, and steroid injection were the primary predictors of each outcome. After the other variables were adjusted for, injection (vs noninjection) seemed to be associated with more improvement in ASES (P = .0061), VAS (P = .020), and Constant (P = .067) scores. Insidious (vs traumatic) etiology was significantly associated with more improvement in ASES scores (P = .033) and VAS scores (P = .014) but not Constant scores (P = .50). Longer time from symptom onset to surgery was associated with less improvement, though the coefficient was not statistically significant in any of the models at P = .05. The other possible covariates had no significant impact on outcomes.
Complications
There were no intraoperative or postoperative complications, and there were no incidents of recurrent rotator cuff tear or postoperative stiffness.
Discussion
We investigated the effectiveness of arthroscopic completion and repair of Ellman grade 3 PTRCTs by comparing the functional outcomes for each subtype. Although several studies have analyzed results of PTRCT repair, they all either omitted intratendinous tears or were not grade-specific. In a systematic review, Strauss and colleagues13 discussed 4 PTRCT outcome studies4,6,14,15 in which only articular- and bursal-sided tears were addressed. Of these studies, only 1 (Kamath and colleagues6) focused on grade 3 lesions, and the number of bursal tears was insufficient for comparison with the articular tear group. Cordasco and colleagues4 limited their study to grade 1 and 2 tears but did not include intratendinous lesions.
In other research, Itoi and Tabata16 distinguished among the 3 subtypes but did not measure grade. As we did in our study, Deutsch5 focused on grade 3 lesions and used the completion-and-repair method, but he did not include intratendinous tears. Porat and colleagues17 reviewed grade 3 completion-and-repair results but did not compare them by subtype. Last, Uchiyama and colleagues18 reported strong outcomes for intratendinous tears but did not measure grade and used various surgical methods.
These studies have made important contributions to the ongoing PTRCT discussion, but debate about appropriate operative management persists. To limit the influence of external variables and provide the most exhaustive evidence regarding current PTRCT treatment algorithms, we designed the present study to consider outcomes with all 3 Ellman subtypes, only grade 3 lesions of the supraspinatus, only 1 surgical method, and consistent techniques of only 1 fellowship-trained shoulder surgeon.
Results of this chart review confirmed the findings of other grade 3 PTRCT repair studies. For instance, Koh and colleagues15 reported excellent results of 38 grade 3B PTRCTs completed to full thickness and repaired. Specifically, their mean ASES and Constant scores improved 34.1 and 23.7 points, respectively. These results are similar to our ASES and Constant score improvements—38.9 and 24.7 points for the group as a whole and 36 and 25.1 points for the grade 3B cohort. In addition, our ASES scores are nearly identical to the preoperative (46.1) and postoperative (82.1) ASES scores found by Kamath and colleagues.6 Although the mean ASES and VAS score improvements reported by Deutsch5 (51 and 5.7 points, respectively) were slightly better than ours, these results are still comparable and support completion and repair.
Although results of the study by Cordasco and colleagues4 support differing surgical treatments of grade 2 tears based on location, the present findings support the established 50% algorithm for all 3 high-grade PTRCTs. The completion-and-repair method not only produced significant improvements for each PTRCT subtype, but, importantly, there was no significant difference among those outcomes. Unlike previous results for grade 2 tears, the present results confirmed the established algorithm for grade 3 tears.
Our multiple linear regression analyses suggested that etiology, longer duration of symptoms, and steroid injections each had a strong impact on outcomes. The literature on these preoperative factors is often conflicting, and our results continue the trend. For instance, in a study of acute rotator cuff tears, Petersen and Murphy19 studied acute rotator cuff tears and also found tear size had no significant effect on functional outcomes. However, contrary to our findings, they did not find symptom duration to be a significant predictor of results. Also contrary to our findings, Oh and colleagues20 found age and tear size to be significant influences on outcomes for full-thickness tears. The strong correlation of preoperative steroid injection and better outcomes is novel and warrants further investigation.
In this study, we investigated the effectiveness of the completion-and-repair method in treating Ellman grade 3 PTRCTs. Although our findings validate this surgical technique, we acknowledge alternative approaches to high-grade PTRCTs. For instance, the transtendon method, which does not convert PTRCTs to full thickness, has also shown good clinical outcomes.21-23 In fact, the preoperative and postoperative VAS measures used in our study are nearly identical to those used in an Ellman grade 3A transtendon repair study.1 However, we agree with Porat and colleagues17 that the remaining, intact cuff material of PTRCTs is degenerative and may result in poor fixation, increased pain, or retear. In addition, nonoperative treatment typically is attempted before surgery, though little evidence is reported for success specifically in high-grade PTRCTs. One study found that 91% of PTRCT patients were still satisfied 4 years after nonoperative treatment, but it was noted that many of the tears were low-grade.24 To continue an evidence-based discussion on the more effective treatment, we invite advocates of alternative approaches to conduct a similar study on all 3 Ellman grade 3 subtypes.
Study Limitations
Concomitant procedures were not uniform among all patients and therefore may have affected some outcome measurements. Subacromial decompression was nearly universal, as it was performed for surgical visualization in 98% of patients. The additional procedures were also deemed necessary based on the preoperative assessment and arthroscopic findings. Although these procedures may have influenced outcome measurements, similar studies regularly include them as well.5-7,17 Our minimum 12-month follow-up could be considered a restriction, as other studies have cited a 2-year follow-up threshold.5-7 However, Strauss and colleagues13 endorsed a 12-month standard in their systematic review. Last, about 10% (7/67) of our initial patients were lost to follow-up; this percentage, however, is comparable to what has been reported in other PTRCT studies.4-6,14,15,21,22
Conclusion
Our study findings validate use of the current algorithm for Ellman grade 3 PTRCTs of the supraspinatus and advocate their completion and repair, regardless of tear location.
Acknowledgment: The authors thank Lisa Rein, MS, and Sergey Tarima, PhD, of the Division of Biostatistics, Medical College of Wisconsin, for their help in data analysis and manuscript preparation.
Am J Orthop. 2016;45(5):E254-E260. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The Ellman1 classification of partial-thickness rotator cuff tears (PTRCTs) is based on tear location or subtype (A, articular; B, bursal; C, intratendinous) and tear depth (grade 1, <3 mm; grade 2, 3-6 mm; grade 3, >6 mm). Ruotolo and colleagues2 reported that the medial-lateral insertion width of the supraspinatus averaged 12.1 mm, and most authors have indicated that tear depth of 6 mm or more represents 50% tendon thickness. Therefore, Ellman grade 3 tears are considered high-grade (>50% thickness).
Advancements in shoulder arthroscopy, imaging modalities, and clinical research have helped refine our understanding of PTRCTs. Classic teaching based on the retrospective study by Weber3 calls for simple débridement of low-grade (<50%) tears and repair of tears thicker than 50%. According to this standard, Ellman grade 1 and 2 tears should be débrided and grade 3 tears repaired. However, Cordasco and colleagues4 provided evidence supporting an algorithm reformation based on tear location. In their study, results of simple débridement were significantly worse for Ellman grade 2B PTRCTs than for 2A tears, suggesting low-grade bursal tears should also be repaired. Although their study supported a change in operative management for grade 2 tears, to our knowledge no one has investigated the need for differing surgical treatments for grade 3 subtypes based on tear location.
Several studies have demonstrated the efficacy of arthroscopic completion and repair for high-grade PTRCTs of the supraspinatus.5-7 Although all these studies addressed articular- and bursal-sided tears, there has been relative silence with respect to the intratendinous subtype. One explanation is that these tears, given their interstitial nature, pose diagnostic challenges. Histologic research has also shown that they can exist in combination with other tears.8 Despite such challenges, these tears are well documented. They were identified in the seminal study by Ellman1 and were the most common PTRCTs encountered in a well-known cadaveric study (N = 249).9,10 More recently, in 2011, a radiologic study using magnetic resonance arthrography found that 33.8% of PTRCTs were intratendinous (N = 68).11 That study also documented the case of a nonoperatively treated intratendinous tear that progressed to a full-thickness tear within about 6 months.11 Given these facts, it was important for the current PTRCT debate to include an intratendinous group when investigating treatment algorithms for grade 3 tears. Although results of the present study may continue reformation of the 50% algorithm, we hypothesized that arthroscopic completion and repair of all grade 3 PTRCTs will be equally effective, regardless of tear location.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the operative reports of a fellowship-trained shoulder surgeon for the period 2008–2010. Patients who underwent arthroscopic completion and repair of a supraspinatus tendon PTRCT were identified. Preoperative identification of PTRCT was made on the basis of physical examination and magnetic resonance imaging (MRI) findings (Figures 1–3).
Patients with low-grade PTRCTs of the supraspinatus, identified at time of arthroscopy, were excluded, as were patients with tears that extended into other rotator cuff tendons and patients with previous rotator cuff repair, glenohumeral instability, or adhesive capsulitis.
During the initial appointment, each patient completed a standard questionnaire that included standardized subjective scales evaluating pain and function. A fellowship-trained surgeon then took the patient’s history and performed a physical examination. Postoperative clinical outcome was determined at a minimum of 12 months. Clinical outcomes were assessed with 3 validated outcome measures: visual analog scale (VAS) score, American Shoulder and Elbow Surgeons (ASES) score, and Constant score.
Surgical Procedure and Rehabilitation
All procedures were performed with the patient under general anesthesia with or without an interscalene block. The patient was positioned in the upright beach-chair position. Diagnostic arthroscopy was used to assess the rotator cuff and associated pathologic conditions. If impingement was noted, subacromial decompression was performed. An acromioplasty was limited to removal of osteophytic bone. Distal clavicle excision and biceps tenotomy or tenodesis were performed if preoperative evaluation warranted these procedures.
The rotator cuff was assessed from the articular and bursal sides. For articular PTRCTs, a tagging suture was used to identify the lesion from the bursal side. Bursal-sided tears were probed to assess thinning of the tendon and determine tear grade. If preoperative MRI findings suggested an intratendinous tear, a probe was used to confirm thinning of the tendon. An arthroscopic shaver was then carefully used to débride the capsule on either side of the tendon at the location of the suspected tear. The shaver inevitably penetrated the capsule and entered the tear, where any degenerative tissue was further débrided (Figure 4).
After the PTRCT was completed to full thickness, the rotator cuff footprint on the greater tuberosity was débrided to bleeding cortical bone. Depending on tear length, 1 or 2 Bio-Corkscrew absorbable suture anchors (Arthrex) with 2 No. 2 FiberWire sutures (Arthrex) were then placed in the tuberosity 3 to 5 mm lateral to the articular margin. An arthroscopic suture passer was used to move the 2 sutures through the rotator cuff, such that one was placed in the horizontal mattress and the other was placed in a simple fashion deep to the horizontal mattress. The sutures were then tied with a modified Roeder knot.
A standardized postoperative protocol was used for all patients starting within the first week after surgery. Passive range of motion (ROM) was performed for the first 6 weeks after surgery and was advanced to include active ROM from 6 to 8 weeks after surgery. Strengthening was initiated 8 weeks after surgery.
Statistical Analysis
Power analysis demonstrated that a sample size of 20 in each group was adequate for detecting a medium to large effect size with 80% power. Wilcoxon signed rank test was used to compare the preoperative and postoperative scores for each outcome measure, and analysis of variance (ANOVA) was used to compare the amount of improvement for each of the 3 PTRCT subtypes. Paired t test was used to compare preoperative and postoperative ROM values, and unpaired t tests were used to determine the impact of corticosteroid injections and preoperative PT. For statistical analysis, patients were divided into 2 groups (yes, no) regarding injections and 2 groups (yes, no) regarding PT. Last, multiple linear regression analyses were performed for each outcome measure to determine the impact of potential confounders. Covariates included symptom duration, etiology, age, injection, PT, tear location, percentage of tendon torn (medial-lateral), and tear length (anterior-posterior). P < .05 was considered significant.
Results
Patient Sample and Demographics
Sixty-seven patients underwent arthroscopic repair of a PTRCT—22 grade 3A, 23 grade 3B, and 22 grade 3C. In each of the 3 groups, 20 patients returned for end-of-healing evaluation. Thus, the study population consisted of 60 patients (60 shoulders). The 7 patients who did not return for end-of-healing evaluation or who could not be contacted were excluded from the study.
Table 1 summarizes the key patient demographics. Of the 60 patients, 35 were men and 25 were women.
Range of Motion
The sample as a whole exhibited statistically significant improvement in active ROM (Table 2).
Operative Findings
Operative findings included mean tear thickness of 74% for the sample as a whole and mean anterior-to-posterior tear length of 10.7 mm overall. There was very little variance among the articular, bursal, and intratendinous means with respect to percentage of tear thickness (78.3%, 75.0%, and 68.8%, respectively) and anterior-to-posterior tear thickness (11.5 mm, 11.4 mm, and 9.1 mm, respectively). Each of the 6 tears (3 bursal, 2 articular, 1 intratendinous) that were longer than 15 mm required 2 anchors. Fifty-nine repairs (98%) involved subacromial decompression, 38 (63%) involved acromioclavicular resection, 18 (30%) involved débridement of the superior labrum anterior-to-posterior (SLAP), and 12 (20%) involved biceps tenodesis/tenotomy.
Outcome Measures
In the study population as a whole, and in all 3 tear subtypes, postoperative improvement in VAS, ASES, and Constant scores was statistically significant (Table 3).
Multiple linear regression analyses showed that etiology, symptom duration, and steroid injection were the primary predictors of each outcome. After the other variables were adjusted for, injection (vs noninjection) seemed to be associated with more improvement in ASES (P = .0061), VAS (P = .020), and Constant (P = .067) scores. Insidious (vs traumatic) etiology was significantly associated with more improvement in ASES scores (P = .033) and VAS scores (P = .014) but not Constant scores (P = .50). Longer time from symptom onset to surgery was associated with less improvement, though the coefficient was not statistically significant in any of the models at P = .05. The other possible covariates had no significant impact on outcomes.
Complications
There were no intraoperative or postoperative complications, and there were no incidents of recurrent rotator cuff tear or postoperative stiffness.
Discussion
We investigated the effectiveness of arthroscopic completion and repair of Ellman grade 3 PTRCTs by comparing the functional outcomes for each subtype. Although several studies have analyzed results of PTRCT repair, they all either omitted intratendinous tears or were not grade-specific. In a systematic review, Strauss and colleagues13 discussed 4 PTRCT outcome studies4,6,14,15 in which only articular- and bursal-sided tears were addressed. Of these studies, only 1 (Kamath and colleagues6) focused on grade 3 lesions, and the number of bursal tears was insufficient for comparison with the articular tear group. Cordasco and colleagues4 limited their study to grade 1 and 2 tears but did not include intratendinous lesions.
In other research, Itoi and Tabata16 distinguished among the 3 subtypes but did not measure grade. As we did in our study, Deutsch5 focused on grade 3 lesions and used the completion-and-repair method, but he did not include intratendinous tears. Porat and colleagues17 reviewed grade 3 completion-and-repair results but did not compare them by subtype. Last, Uchiyama and colleagues18 reported strong outcomes for intratendinous tears but did not measure grade and used various surgical methods.
These studies have made important contributions to the ongoing PTRCT discussion, but debate about appropriate operative management persists. To limit the influence of external variables and provide the most exhaustive evidence regarding current PTRCT treatment algorithms, we designed the present study to consider outcomes with all 3 Ellman subtypes, only grade 3 lesions of the supraspinatus, only 1 surgical method, and consistent techniques of only 1 fellowship-trained shoulder surgeon.
Results of this chart review confirmed the findings of other grade 3 PTRCT repair studies. For instance, Koh and colleagues15 reported excellent results of 38 grade 3B PTRCTs completed to full thickness and repaired. Specifically, their mean ASES and Constant scores improved 34.1 and 23.7 points, respectively. These results are similar to our ASES and Constant score improvements—38.9 and 24.7 points for the group as a whole and 36 and 25.1 points for the grade 3B cohort. In addition, our ASES scores are nearly identical to the preoperative (46.1) and postoperative (82.1) ASES scores found by Kamath and colleagues.6 Although the mean ASES and VAS score improvements reported by Deutsch5 (51 and 5.7 points, respectively) were slightly better than ours, these results are still comparable and support completion and repair.
Although results of the study by Cordasco and colleagues4 support differing surgical treatments of grade 2 tears based on location, the present findings support the established 50% algorithm for all 3 high-grade PTRCTs. The completion-and-repair method not only produced significant improvements for each PTRCT subtype, but, importantly, there was no significant difference among those outcomes. Unlike previous results for grade 2 tears, the present results confirmed the established algorithm for grade 3 tears.
Our multiple linear regression analyses suggested that etiology, longer duration of symptoms, and steroid injections each had a strong impact on outcomes. The literature on these preoperative factors is often conflicting, and our results continue the trend. For instance, in a study of acute rotator cuff tears, Petersen and Murphy19 studied acute rotator cuff tears and also found tear size had no significant effect on functional outcomes. However, contrary to our findings, they did not find symptom duration to be a significant predictor of results. Also contrary to our findings, Oh and colleagues20 found age and tear size to be significant influences on outcomes for full-thickness tears. The strong correlation of preoperative steroid injection and better outcomes is novel and warrants further investigation.
In this study, we investigated the effectiveness of the completion-and-repair method in treating Ellman grade 3 PTRCTs. Although our findings validate this surgical technique, we acknowledge alternative approaches to high-grade PTRCTs. For instance, the transtendon method, which does not convert PTRCTs to full thickness, has also shown good clinical outcomes.21-23 In fact, the preoperative and postoperative VAS measures used in our study are nearly identical to those used in an Ellman grade 3A transtendon repair study.1 However, we agree with Porat and colleagues17 that the remaining, intact cuff material of PTRCTs is degenerative and may result in poor fixation, increased pain, or retear. In addition, nonoperative treatment typically is attempted before surgery, though little evidence is reported for success specifically in high-grade PTRCTs. One study found that 91% of PTRCT patients were still satisfied 4 years after nonoperative treatment, but it was noted that many of the tears were low-grade.24 To continue an evidence-based discussion on the more effective treatment, we invite advocates of alternative approaches to conduct a similar study on all 3 Ellman grade 3 subtypes.
Study Limitations
Concomitant procedures were not uniform among all patients and therefore may have affected some outcome measurements. Subacromial decompression was nearly universal, as it was performed for surgical visualization in 98% of patients. The additional procedures were also deemed necessary based on the preoperative assessment and arthroscopic findings. Although these procedures may have influenced outcome measurements, similar studies regularly include them as well.5-7,17 Our minimum 12-month follow-up could be considered a restriction, as other studies have cited a 2-year follow-up threshold.5-7 However, Strauss and colleagues13 endorsed a 12-month standard in their systematic review. Last, about 10% (7/67) of our initial patients were lost to follow-up; this percentage, however, is comparable to what has been reported in other PTRCT studies.4-6,14,15,21,22
Conclusion
Our study findings validate use of the current algorithm for Ellman grade 3 PTRCTs of the supraspinatus and advocate their completion and repair, regardless of tear location.
Acknowledgment: The authors thank Lisa Rein, MS, and Sergey Tarima, PhD, of the Division of Biostatistics, Medical College of Wisconsin, for their help in data analysis and manuscript preparation.
Am J Orthop. 2016;45(5):E254-E260. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res. 1990;(254):64-74.
2. Ruotolo C, Fow JE, Nottage WM. The supraspinatus footprint: an anatomic study of the supraspinatus insertion. Arthroscopy. 2004;20(3):246-249.
3. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
4. Cordasco FA, Backer M, Craig EV, Klein D, Warren RF. The partial-thickness rotator cuff tear: is acromioplasty without repair sufficient? Am J Sports Med. 2002;30(2):257-260.
5. Deutsch A. Arthroscopic repair of partial-thickness tears of the rotator cuff. J Shoulder Elbow Surg. 2007;16(2):193-201.
6. Kamath G, Galatz LM, Keener JD, Teefey S, Middleton W, Yamaguchi K. Tendon integrity and functional outcome after arthroscopic repair of high-grade partial-thickness supraspinatus tears. J Bone Joint Surg Am. 2009;91(5):1055-1062.
7. Park JY, Yoo MJ, Kim MH. Comparison of surgical outcome between bursal and articular partial thickness rotator cuff tears. Orthopedics. 2003;26(4):387-390.
8. Fukuda H, Hamada K, Nakajima T, Tomonaga A. Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clin Orthop Relat Res. 1994;(304):60-67.
9. Fukuda H, Mikasa M, Yamanaka K. Incomplete thickness rotator cuff tears diagnosed by subacromial bursography. Clin Orthop Relat Res. 1987;(223):51-58.
10. Yamanaka K, Fukuda H, Hamada K, Mikasa M. Incomplete thickness tears of the rotator cuff [abstract]. Orthop Surg Traumatol (Toyko). 1983;26:713.
11. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21(7):1477-1484.
12. Nakagawa S, Yoneda M, Mizuno N, Hayashida K, Mae T, Take Y. Throwing shoulder injury involving the anterior rotator cuff: concealed tears not as uncommon as previously thought. Arthroscopy. 2006;22(12):1298-1303.
13. Strauss EJ, Salata MJ, Kercher J, et al. Multimedia article. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580.
14. Kartus J, Kartus C, Rostgard-Christensen L, Sernert N, Read J, Perko M. Long-term clinical and ultrasound evaluation after arthroscopic acromioplasty in patients with partial rotator cuff tears. Arthroscopy. 2006;22(1):44-49.
15. Koh KH, Shon MS, Lim TK, Yoo JC. Clinical and magnetic resonance imaging results of arthroscopic full-layer repair of bursal-side partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(8):1660-1667.
16. Itoi E, Tabata S. Incomplete rotator cuff tears. Results of operative treatment. Clin Orthop Relat Res. 1992;(284):128-135.
17. Porat S, Nottage WM, Fouse MN. Repair of partial thickness rotator cuff tears: a retrospective review with minimum two-year follow-up. J Shoulder Elbow Surg. 2008;17(5):729-731.
18. Uchiyama Y, Hamada K, Khruekarnchana P, et al. Surgical treatment of confirmed intratendinous rotator cuff tears: retrospective analysis after an average of eight years of follow-up. J Shoulder Elbow Surg. 2010;19(6):837-846.
19. Petersen SA, Murphy TP. The timing of rotator cuff repair for the restoration of function. J Shoulder Elbow Surg. 2011;20(1):62-68.
20. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
21. Castagna A, Delle Rose G, Conti M, Snyder SJ, Borroni M, Garofalo R. Predictive factors of subtle residual shoulder symptoms after transtendinous arthroscopic cuff repair: a clinical study. Am J Sports Med. 2009;37(1):103-108.
22. Castricini R, Panfoli N, Nittoli R, Spurio S, Pirani O. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the supraspinatus: results at 2 years. Chir Organi Mov. 2009;93(suppl 1):S49-S54.
23. Spencer EE Jr. Partial-thickness articular surface rotator cuff tears: an all-inside repair technique. Clin Orthop Relat Res. 2010;468(6):1514-1520.
24. Denkers M, Pletsch K, Boorman R, Hollinshead R, Lo IKY. Partial thickness rotator cuff tears: observe or operative. In: Proceedings of the American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
1. Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res. 1990;(254):64-74.
2. Ruotolo C, Fow JE, Nottage WM. The supraspinatus footprint: an anatomic study of the supraspinatus insertion. Arthroscopy. 2004;20(3):246-249.
3. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
4. Cordasco FA, Backer M, Craig EV, Klein D, Warren RF. The partial-thickness rotator cuff tear: is acromioplasty without repair sufficient? Am J Sports Med. 2002;30(2):257-260.
5. Deutsch A. Arthroscopic repair of partial-thickness tears of the rotator cuff. J Shoulder Elbow Surg. 2007;16(2):193-201.
6. Kamath G, Galatz LM, Keener JD, Teefey S, Middleton W, Yamaguchi K. Tendon integrity and functional outcome after arthroscopic repair of high-grade partial-thickness supraspinatus tears. J Bone Joint Surg Am. 2009;91(5):1055-1062.
7. Park JY, Yoo MJ, Kim MH. Comparison of surgical outcome between bursal and articular partial thickness rotator cuff tears. Orthopedics. 2003;26(4):387-390.
8. Fukuda H, Hamada K, Nakajima T, Tomonaga A. Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clin Orthop Relat Res. 1994;(304):60-67.
9. Fukuda H, Mikasa M, Yamanaka K. Incomplete thickness rotator cuff tears diagnosed by subacromial bursography. Clin Orthop Relat Res. 1987;(223):51-58.
10. Yamanaka K, Fukuda H, Hamada K, Mikasa M. Incomplete thickness tears of the rotator cuff [abstract]. Orthop Surg Traumatol (Toyko). 1983;26:713.
11. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21(7):1477-1484.
12. Nakagawa S, Yoneda M, Mizuno N, Hayashida K, Mae T, Take Y. Throwing shoulder injury involving the anterior rotator cuff: concealed tears not as uncommon as previously thought. Arthroscopy. 2006;22(12):1298-1303.
13. Strauss EJ, Salata MJ, Kercher J, et al. Multimedia article. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580.
14. Kartus J, Kartus C, Rostgard-Christensen L, Sernert N, Read J, Perko M. Long-term clinical and ultrasound evaluation after arthroscopic acromioplasty in patients with partial rotator cuff tears. Arthroscopy. 2006;22(1):44-49.
15. Koh KH, Shon MS, Lim TK, Yoo JC. Clinical and magnetic resonance imaging results of arthroscopic full-layer repair of bursal-side partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(8):1660-1667.
16. Itoi E, Tabata S. Incomplete rotator cuff tears. Results of operative treatment. Clin Orthop Relat Res. 1992;(284):128-135.
17. Porat S, Nottage WM, Fouse MN. Repair of partial thickness rotator cuff tears: a retrospective review with minimum two-year follow-up. J Shoulder Elbow Surg. 2008;17(5):729-731.
18. Uchiyama Y, Hamada K, Khruekarnchana P, et al. Surgical treatment of confirmed intratendinous rotator cuff tears: retrospective analysis after an average of eight years of follow-up. J Shoulder Elbow Surg. 2010;19(6):837-846.
19. Petersen SA, Murphy TP. The timing of rotator cuff repair for the restoration of function. J Shoulder Elbow Surg. 2011;20(1):62-68.
20. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
21. Castagna A, Delle Rose G, Conti M, Snyder SJ, Borroni M, Garofalo R. Predictive factors of subtle residual shoulder symptoms after transtendinous arthroscopic cuff repair: a clinical study. Am J Sports Med. 2009;37(1):103-108.
22. Castricini R, Panfoli N, Nittoli R, Spurio S, Pirani O. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the supraspinatus: results at 2 years. Chir Organi Mov. 2009;93(suppl 1):S49-S54.
23. Spencer EE Jr. Partial-thickness articular surface rotator cuff tears: an all-inside repair technique. Clin Orthop Relat Res. 2010;468(6):1514-1520.
24. Denkers M, Pletsch K, Boorman R, Hollinshead R, Lo IKY. Partial thickness rotator cuff tears: observe or operative. In: Proceedings of the American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
Up in Arms: Bilateral Luxatio Erecta Fracture-Dislocations
Unilateral inferior shoulder dislocation (luxatio erecta) is uncommon, accounting for only 0.5% of all shoulder dislocations.1 Bilateral luxatio erecta is extremely rare, having been described fewer than 20 times in the literature. The most common etiology is hyperabduction causing the humerus to lever on the acromion; less common is axial loading onto a fully abducted arm and an extended elbow.2 Hyperabduction can occur when a person grabs an object in an attempt to stop a fall, as occurred in the present case. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man with a trauma injury presented to our emergency department. For his open right elbow fracture, emergency medical services had given him fentanyl en route, and when he arrived he was less responsive. As the patient reported, he had been on a scaffold 16 feet high when it began to give way. He jumped for another scaffold, 3 to 4 feet away, but came up short and, in an attempt to stop himself from falling, grabbed onto it with arms extended and above his head. His hands and arms were immediately pulled up in full extension. When both shoulders became dislocated, he could not hold on and fell to the ground, landing on a buttock. He did not lose consciousness.
Physical examination revealed both arms abducted at the shoulder, and elbows extended (Figure 1).
Radiographs confirmed the diagnosis and showed bilateral nondisplaced proximal humeral fractures of the greater tuberosity (Figure 2).
For the shoulder reductions, we administered propofol for conscious sedation and fentanyl for analgesia. Then, a sheet was wrapped supraclavicular and pulled across the torso inferiorly to allow countertraction when pulling the arm superiorly on the axial line. Another countertraction sheet was placed on the opposite side. For each arm, the countertraction was pulled inferiorly when the arm was pulled superiorly, both on the longitudinal plane. The arm was then gently rotated in adduction until reduction was achieved.
The right shoulder reduced relatively easily. The left shoulder reduced into an anterior dislocation—a relatively uncommon outcome in in-line traction attempts.3 (Reduction into anterior dislocation can also be a desired result in a specific technique of 2-step reduction, as described by Nho and colleagues.4) The patient’s anterior dislocation was then easily reduced into anatomical position with use of the Kocher technique of arm adduction with elbow flexion, followed by external rotation, and then finally into anatomical position with internal rotation.5 Both arms were then immobilized in full adduction with bilateral slings. The patient was admitted for further treatment of multiple fractures of the arms and vertebrae.
He was discharged in bilateral shoulder slings to an extended-care facility for physical therapy. One month after discharge, he could not elevate his arms and had minimal use of them. Two weeks later, magnetic resonance imaging showed a “comminuted greater tuberosity fracture with new displacement of fragments involving the attachment of the supraspinatus and infraspinatus; posterior subluxation of the glenohumeral joint with evidence of posterior and anterior labral tears; and large glenohumeral joint effusion.” The patient opted for surgical repair and underwent left shoulder arthroscopy with extensive débridement, open rotator cuff repair, open greater tuberosity reduction and internal fixation, and open biceps tenodesis. He was then discharged back to an extended-care facility to continue rehabilitation. One and a half months after surgery, he started the physical therapy phase of the massive rotator cuff repair protocol. He declined reverse total shoulder arthroplasty (RTSA).
Four and a half months after injury (3 months after surgery), the left shoulder demonstrated 20° of flexion and 70° to 110° of abduction (external rotation not tested), and the right shoulder demonstrated 30° of flexion and 70° to 110° of abduction (external rotation not tested). He had no instability and no lag with good external rotation.
Six months after injury, the patient still could not lift his arms above his head. He likely would not be able to do so without RTSA, which he again declined. He continued physical therapy and clinical follow-ups.
Discussion
Although inferior shoulder dislocations are rare, they carry a higher rate of complications, most of which our patient experienced. Our patient had bilateral humeral head fractures, which occur in 80% of cases.6 Postreduction CT showed the degree of his fractures (Figure 3).
Our patient also had reduced sensation in the axillary nerve distribution, which occurs in 60% of inferior dislocations.6 Axillary nerve injuries produce numbness in the lateral arm or posterior shoulder and weakness with shoulder flexion, abduction, and external rotation.7 In our patient’s case, sensation returned after reduction, which is typical (most patients have a positive prognosis).8 As the shoulder dislocates inferiorly, the humeral head tears the glenohumeral capsule inferiorly, which can damage the axillary artery. This artery becomes the brachial and eventually the radial and ulnar arteries, which can have decreased or absent pulses with injury.
Inferior dislocations are also associated with abundant soft-tissue injuries, including torn rotator cuff, shoulder capsule avulsion, and disruption of adjacent muscles (supraspinatus, infraspinatus, teres minor, subscapularis, pectoralis major).9Luxatio erecta is relatively easy to diagnose given the unmistakable arm positioning. The key for the physician is first to assess for the many possible complications, then to administer the proper sedation and analgesia for reduction, and finally to reassess for complications.
Am J Orthop. 2016;45(6):E328-E330. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Camarda L, Martorana U, D’Arienzo M. A case of bilateral luxatio erecta. J Orthop Traumatol. 2009;10(2):97-99.
2. Musmeci E, Gaspari D, Sandri A, Regis D, Bartolozzi P. Bilateral luxatio erecta humeri associated with a unilateral brachial plexus and bilateral rotator cuff injuries: a case report. J Orthop Trauma. 2008;22(7):498-500.
3. Lam AC, Shih RD. Luxatio erecta complicated by anterior shoulder dislocation during reduction. West J Emerg Med. 2010;11(1):28-30.
4. Nho SJ, Dodson CC, Bardzik KF, Brophy RH, Domb BG, MacGillivray JD. The two-step maneuver for closed reduction of inferior glenohumeral dislocation (luxatio erecta to anterior dislocation to reduction). J Orthop Trauma. 2006;20(5):354-357.
5. Beattie TF, Steedman DJ, McGowan A, Robertson CE. A comparison of the Milch and Kocher techniques for acute anterior dislocation of the shoulder. Injury. 1986;17(5):349-352.
6. Mallon WJ, Bassett FH 3rd, Goldner RD. Luxatio erecta: the inferior glenohumeral dislocation. J Orthop Trauma. 1990;4(1):19-24.
7. Miller T. Peripheral nerve injuries at the shoulder. J Manipulative Physiol Ther. 1998;6(4):170-183.
8. Groh GI, Wirth MA, Rockwood CA Jr. Results of treatment of luxatio erecta (inferior shoulder dislocation). J Shoulder Elbow Surg. 2010;19(3):423-426.
9. Garcia R, Ponsky T, Brody F, Long J. Bilateral luxatio erecta complicated by venous thrombosis. J Trauma. 2006;60(5):1132-1134.
Unilateral inferior shoulder dislocation (luxatio erecta) is uncommon, accounting for only 0.5% of all shoulder dislocations.1 Bilateral luxatio erecta is extremely rare, having been described fewer than 20 times in the literature. The most common etiology is hyperabduction causing the humerus to lever on the acromion; less common is axial loading onto a fully abducted arm and an extended elbow.2 Hyperabduction can occur when a person grabs an object in an attempt to stop a fall, as occurred in the present case. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man with a trauma injury presented to our emergency department. For his open right elbow fracture, emergency medical services had given him fentanyl en route, and when he arrived he was less responsive. As the patient reported, he had been on a scaffold 16 feet high when it began to give way. He jumped for another scaffold, 3 to 4 feet away, but came up short and, in an attempt to stop himself from falling, grabbed onto it with arms extended and above his head. His hands and arms were immediately pulled up in full extension. When both shoulders became dislocated, he could not hold on and fell to the ground, landing on a buttock. He did not lose consciousness.
Physical examination revealed both arms abducted at the shoulder, and elbows extended (Figure 1).
Radiographs confirmed the diagnosis and showed bilateral nondisplaced proximal humeral fractures of the greater tuberosity (Figure 2).
For the shoulder reductions, we administered propofol for conscious sedation and fentanyl for analgesia. Then, a sheet was wrapped supraclavicular and pulled across the torso inferiorly to allow countertraction when pulling the arm superiorly on the axial line. Another countertraction sheet was placed on the opposite side. For each arm, the countertraction was pulled inferiorly when the arm was pulled superiorly, both on the longitudinal plane. The arm was then gently rotated in adduction until reduction was achieved.
The right shoulder reduced relatively easily. The left shoulder reduced into an anterior dislocation—a relatively uncommon outcome in in-line traction attempts.3 (Reduction into anterior dislocation can also be a desired result in a specific technique of 2-step reduction, as described by Nho and colleagues.4) The patient’s anterior dislocation was then easily reduced into anatomical position with use of the Kocher technique of arm adduction with elbow flexion, followed by external rotation, and then finally into anatomical position with internal rotation.5 Both arms were then immobilized in full adduction with bilateral slings. The patient was admitted for further treatment of multiple fractures of the arms and vertebrae.
He was discharged in bilateral shoulder slings to an extended-care facility for physical therapy. One month after discharge, he could not elevate his arms and had minimal use of them. Two weeks later, magnetic resonance imaging showed a “comminuted greater tuberosity fracture with new displacement of fragments involving the attachment of the supraspinatus and infraspinatus; posterior subluxation of the glenohumeral joint with evidence of posterior and anterior labral tears; and large glenohumeral joint effusion.” The patient opted for surgical repair and underwent left shoulder arthroscopy with extensive débridement, open rotator cuff repair, open greater tuberosity reduction and internal fixation, and open biceps tenodesis. He was then discharged back to an extended-care facility to continue rehabilitation. One and a half months after surgery, he started the physical therapy phase of the massive rotator cuff repair protocol. He declined reverse total shoulder arthroplasty (RTSA).
Four and a half months after injury (3 months after surgery), the left shoulder demonstrated 20° of flexion and 70° to 110° of abduction (external rotation not tested), and the right shoulder demonstrated 30° of flexion and 70° to 110° of abduction (external rotation not tested). He had no instability and no lag with good external rotation.
Six months after injury, the patient still could not lift his arms above his head. He likely would not be able to do so without RTSA, which he again declined. He continued physical therapy and clinical follow-ups.
Discussion
Although inferior shoulder dislocations are rare, they carry a higher rate of complications, most of which our patient experienced. Our patient had bilateral humeral head fractures, which occur in 80% of cases.6 Postreduction CT showed the degree of his fractures (Figure 3).
Our patient also had reduced sensation in the axillary nerve distribution, which occurs in 60% of inferior dislocations.6 Axillary nerve injuries produce numbness in the lateral arm or posterior shoulder and weakness with shoulder flexion, abduction, and external rotation.7 In our patient’s case, sensation returned after reduction, which is typical (most patients have a positive prognosis).8 As the shoulder dislocates inferiorly, the humeral head tears the glenohumeral capsule inferiorly, which can damage the axillary artery. This artery becomes the brachial and eventually the radial and ulnar arteries, which can have decreased or absent pulses with injury.
Inferior dislocations are also associated with abundant soft-tissue injuries, including torn rotator cuff, shoulder capsule avulsion, and disruption of adjacent muscles (supraspinatus, infraspinatus, teres minor, subscapularis, pectoralis major).9Luxatio erecta is relatively easy to diagnose given the unmistakable arm positioning. The key for the physician is first to assess for the many possible complications, then to administer the proper sedation and analgesia for reduction, and finally to reassess for complications.
Am J Orthop. 2016;45(6):E328-E330. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Unilateral inferior shoulder dislocation (luxatio erecta) is uncommon, accounting for only 0.5% of all shoulder dislocations.1 Bilateral luxatio erecta is extremely rare, having been described fewer than 20 times in the literature. The most common etiology is hyperabduction causing the humerus to lever on the acromion; less common is axial loading onto a fully abducted arm and an extended elbow.2 Hyperabduction can occur when a person grabs an object in an attempt to stop a fall, as occurred in the present case. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man with a trauma injury presented to our emergency department. For his open right elbow fracture, emergency medical services had given him fentanyl en route, and when he arrived he was less responsive. As the patient reported, he had been on a scaffold 16 feet high when it began to give way. He jumped for another scaffold, 3 to 4 feet away, but came up short and, in an attempt to stop himself from falling, grabbed onto it with arms extended and above his head. His hands and arms were immediately pulled up in full extension. When both shoulders became dislocated, he could not hold on and fell to the ground, landing on a buttock. He did not lose consciousness.
Physical examination revealed both arms abducted at the shoulder, and elbows extended (Figure 1).
Radiographs confirmed the diagnosis and showed bilateral nondisplaced proximal humeral fractures of the greater tuberosity (Figure 2).
For the shoulder reductions, we administered propofol for conscious sedation and fentanyl for analgesia. Then, a sheet was wrapped supraclavicular and pulled across the torso inferiorly to allow countertraction when pulling the arm superiorly on the axial line. Another countertraction sheet was placed on the opposite side. For each arm, the countertraction was pulled inferiorly when the arm was pulled superiorly, both on the longitudinal plane. The arm was then gently rotated in adduction until reduction was achieved.
The right shoulder reduced relatively easily. The left shoulder reduced into an anterior dislocation—a relatively uncommon outcome in in-line traction attempts.3 (Reduction into anterior dislocation can also be a desired result in a specific technique of 2-step reduction, as described by Nho and colleagues.4) The patient’s anterior dislocation was then easily reduced into anatomical position with use of the Kocher technique of arm adduction with elbow flexion, followed by external rotation, and then finally into anatomical position with internal rotation.5 Both arms were then immobilized in full adduction with bilateral slings. The patient was admitted for further treatment of multiple fractures of the arms and vertebrae.
He was discharged in bilateral shoulder slings to an extended-care facility for physical therapy. One month after discharge, he could not elevate his arms and had minimal use of them. Two weeks later, magnetic resonance imaging showed a “comminuted greater tuberosity fracture with new displacement of fragments involving the attachment of the supraspinatus and infraspinatus; posterior subluxation of the glenohumeral joint with evidence of posterior and anterior labral tears; and large glenohumeral joint effusion.” The patient opted for surgical repair and underwent left shoulder arthroscopy with extensive débridement, open rotator cuff repair, open greater tuberosity reduction and internal fixation, and open biceps tenodesis. He was then discharged back to an extended-care facility to continue rehabilitation. One and a half months after surgery, he started the physical therapy phase of the massive rotator cuff repair protocol. He declined reverse total shoulder arthroplasty (RTSA).
Four and a half months after injury (3 months after surgery), the left shoulder demonstrated 20° of flexion and 70° to 110° of abduction (external rotation not tested), and the right shoulder demonstrated 30° of flexion and 70° to 110° of abduction (external rotation not tested). He had no instability and no lag with good external rotation.
Six months after injury, the patient still could not lift his arms above his head. He likely would not be able to do so without RTSA, which he again declined. He continued physical therapy and clinical follow-ups.
Discussion
Although inferior shoulder dislocations are rare, they carry a higher rate of complications, most of which our patient experienced. Our patient had bilateral humeral head fractures, which occur in 80% of cases.6 Postreduction CT showed the degree of his fractures (Figure 3).
Our patient also had reduced sensation in the axillary nerve distribution, which occurs in 60% of inferior dislocations.6 Axillary nerve injuries produce numbness in the lateral arm or posterior shoulder and weakness with shoulder flexion, abduction, and external rotation.7 In our patient’s case, sensation returned after reduction, which is typical (most patients have a positive prognosis).8 As the shoulder dislocates inferiorly, the humeral head tears the glenohumeral capsule inferiorly, which can damage the axillary artery. This artery becomes the brachial and eventually the radial and ulnar arteries, which can have decreased or absent pulses with injury.
Inferior dislocations are also associated with abundant soft-tissue injuries, including torn rotator cuff, shoulder capsule avulsion, and disruption of adjacent muscles (supraspinatus, infraspinatus, teres minor, subscapularis, pectoralis major).9Luxatio erecta is relatively easy to diagnose given the unmistakable arm positioning. The key for the physician is first to assess for the many possible complications, then to administer the proper sedation and analgesia for reduction, and finally to reassess for complications.
Am J Orthop. 2016;45(6):E328-E330. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Camarda L, Martorana U, D’Arienzo M. A case of bilateral luxatio erecta. J Orthop Traumatol. 2009;10(2):97-99.
2. Musmeci E, Gaspari D, Sandri A, Regis D, Bartolozzi P. Bilateral luxatio erecta humeri associated with a unilateral brachial plexus and bilateral rotator cuff injuries: a case report. J Orthop Trauma. 2008;22(7):498-500.
3. Lam AC, Shih RD. Luxatio erecta complicated by anterior shoulder dislocation during reduction. West J Emerg Med. 2010;11(1):28-30.
4. Nho SJ, Dodson CC, Bardzik KF, Brophy RH, Domb BG, MacGillivray JD. The two-step maneuver for closed reduction of inferior glenohumeral dislocation (luxatio erecta to anterior dislocation to reduction). J Orthop Trauma. 2006;20(5):354-357.
5. Beattie TF, Steedman DJ, McGowan A, Robertson CE. A comparison of the Milch and Kocher techniques for acute anterior dislocation of the shoulder. Injury. 1986;17(5):349-352.
6. Mallon WJ, Bassett FH 3rd, Goldner RD. Luxatio erecta: the inferior glenohumeral dislocation. J Orthop Trauma. 1990;4(1):19-24.
7. Miller T. Peripheral nerve injuries at the shoulder. J Manipulative Physiol Ther. 1998;6(4):170-183.
8. Groh GI, Wirth MA, Rockwood CA Jr. Results of treatment of luxatio erecta (inferior shoulder dislocation). J Shoulder Elbow Surg. 2010;19(3):423-426.
9. Garcia R, Ponsky T, Brody F, Long J. Bilateral luxatio erecta complicated by venous thrombosis. J Trauma. 2006;60(5):1132-1134.
1. Camarda L, Martorana U, D’Arienzo M. A case of bilateral luxatio erecta. J Orthop Traumatol. 2009;10(2):97-99.
2. Musmeci E, Gaspari D, Sandri A, Regis D, Bartolozzi P. Bilateral luxatio erecta humeri associated with a unilateral brachial plexus and bilateral rotator cuff injuries: a case report. J Orthop Trauma. 2008;22(7):498-500.
3. Lam AC, Shih RD. Luxatio erecta complicated by anterior shoulder dislocation during reduction. West J Emerg Med. 2010;11(1):28-30.
4. Nho SJ, Dodson CC, Bardzik KF, Brophy RH, Domb BG, MacGillivray JD. The two-step maneuver for closed reduction of inferior glenohumeral dislocation (luxatio erecta to anterior dislocation to reduction). J Orthop Trauma. 2006;20(5):354-357.
5. Beattie TF, Steedman DJ, McGowan A, Robertson CE. A comparison of the Milch and Kocher techniques for acute anterior dislocation of the shoulder. Injury. 1986;17(5):349-352.
6. Mallon WJ, Bassett FH 3rd, Goldner RD. Luxatio erecta: the inferior glenohumeral dislocation. J Orthop Trauma. 1990;4(1):19-24.
7. Miller T. Peripheral nerve injuries at the shoulder. J Manipulative Physiol Ther. 1998;6(4):170-183.
8. Groh GI, Wirth MA, Rockwood CA Jr. Results of treatment of luxatio erecta (inferior shoulder dislocation). J Shoulder Elbow Surg. 2010;19(3):423-426.
9. Garcia R, Ponsky T, Brody F, Long J. Bilateral luxatio erecta complicated by venous thrombosis. J Trauma. 2006;60(5):1132-1134.
Historical Patterns and Variation in Treatment of Injuries in NFL (National Football League) Players and NCAA (National Collegiate Athletic Association) Division I Football Players
Among National Football League (NFL) and National Collegiate Athletic Association (NCAA) team physicians, there is no consensus on the management of various injuries. At national and regional meetings, the management of football injuries often is debated.
Given the high level of interest in the treatment of elite football players, we wanted to determine treatment patterns by surveying orthopedic team physicians. We conducted a study to determine the demographics of NFL and NCAA team physicians and to identify patterns and variations in the management of common injuries in these groups of elite football players.
Materials and Methods
The study was reviewed by an Institutional Review Board before data collection and was classified as exempt. The study population consisted of head orthopedic team physicians for NFL teams and NCAA Division I universities. The survey (Appendix),
Chi-square tests were used to determine significant differences between groups. P < .05 was considered statistically significant.
Results
Responses were received from 31 (97%) of the 32 NFL and 111 (93%) of the 119 NCAA team physicians. The 2 groups’ surveys were identical with the exception of question 3, regarding NFL division or NCAA conference.
Team Physician Demographics
All survey respondents were the head orthopedic physicians for their teams. Seventy-one percent were the head team physicians as well; another 25% named a primary care physician as the head team physician. Thirty-nine percent of the NFL team physicians had been a team physician at the NFL level for more than 15 years, and 58% of the NCAA team physicians had been a team physician at the Division I level for more than 15 years. Eighty-one percent of NFL and 66% of NCAA team physicians had fellowship training in sports medicine. For away games, 10% of NFL vs 65% of NCAA teams traveled with 2 physicians; 90% of NFL and 28% of NCAA teams traveled with 3 or more physicians.
Only a small percentage of respondents (NFL, 10%; NCAA, 14%) indicated they had received advertising in exchange for services. Most respondents (NFL, 93%; NCAA, 89%) did not pay to provide team coverage. In contrast, 97% of NFL vs only 31% of NCAA physicians indicated they received a monetary stipend for providing orthopedic coverage.
Anterior Cruciate Ligament Reconstructions
Eighty-seven percent of NFL and 67% of NCAA respondents indicated that patellar tendon autograft was their preferred graft choice (Table 1).
Anterior Shoulder Dislocations (Without Bony Bankart)
Sling use after reduction of anterior shoulder dislocation was varied, with most physicians using a sling 2 weeks or less (Table 2).
Acromioclavicular Joint Injuries
Roughly two-thirds of respondents (NFL, 60%; NCAA, 69%) indicated that, during a game, they managed acute acromioclavicular (AC) joint injuries (type I/II) with injection of a local anesthetic that allowed return to play. In addition, a majority (NFL, 90%; NCAA, 87%) indicated they gave such athletes pregame injections that allowed them to play. About half the physicians (NFL, 57%; NCAA, 52%) injected the AC joint with cortisone during the acute/subacute period (<1 month) to decrease inflammation.
No significant difference was found between the 2 groups in terms of proportion of surgeons electing to treat type III AC joint injuries operatively versus nonoperatively (Table 4).
Medial Collateral Ligament Injuries
There was a significant (P < .0001) difference in use of prophylactic bracing for medial collateral ligament (MCL) injuries (NFL, 28%; NCAA, 89%).
Posterior Cruciate Ligament Injuries
The percentage of physicians who allowed athletes to return to play after a grade I/II posterior cruciate ligament (PCL) injury was significantly (P = .01) higher in NFL physicians (22%) than in NCAA physicians (7%). The amount of time varied up to more than 4 weeks (Figure 4).
Elbow Ulnar Collateral Ligament Tears
A majority of respondents indicated they would treat a complete elbow ulnar collateral ligament (UCL) tear in a quarterback; a much smaller percentage preferred operative repair in athletes playing other positions (Table 6).
Thumb Ulnar Collateral Ligament Tears
For athletes with in-season thumb UCL tears, 63% of NFL and 54% of NCAA physicians indicated they cast the thumb and allowed return to play. Others recommended operative repair and either cast the thumb and allowed return to play (NFL, 30%; NCAA, 41%) or let the thumb heal before allowing return to play (NFL, 7%; NCAA, 5%).
Fifth Metatarsal Fractures
For a large majority of physicians (NFL, 100%; NCAA, 94%), the preferred treatment for fifth metatarsal fractures was screw fixation.
Tibia Fractures
In the 5-year period before the survey, 43% of NFL and 75% of NCAA physicians managed at least one tibia fracture (P < .001) (Figure 7).
Ketorolac Injections
Intramuscular ketorolac injections were frequently given to elite football players, significantly (P < .01) more so in the NFL (93%) than in the NCAA (62%). The average number of injections varied among physicians, though a significantly (P < .0001) higher percentage of NFL (79%) than NCAA (13%) physicians gave 5 or more injections per game.
Discussion
This survey on managing common injuries in elite football players had an overall response rate of 94%. All NFL divisions and NCAA conferences were represented in physicians’ responses. Ninety percent of NFL and 65% of NCAA head team physicians were orthopedists. These findings differ from those of Stockard1 (1997), who surveyed athletic directors at Division I schools and reported 45% of head team physicians were family medicine-trained and 41% were orthopedists.
Given the high visibility of team coverage and the economics of college football’s highest division, one might expect team physicians to receive financial remuneration. This was not the case, according to our survey: Only 30% of physicians received a monetary stipend for team coverage, and only 14% received advertising in exchange for their services. Twelve NCAA team physicians indicated they pay to be allowed to provide team coverage.
Injury Management
Anterior Cruciate Ligament Injuries. For NFL and NCAA team physicians, the preferred graft choice for ACL reconstruction was patellar tendon autograft. This finding is similar to what Erickson and colleagues2 reported from a survey of NFL and NCAA team physicians: 86% of surgeons preferred bone–patellar tendon–bone (BPTB) autograft. However, only 1 surgeon (0.7%) in that study, vs 16% in ours, preferred allograft. Allograft use may be somewhat controversial, as relevant data on competitive athletes are lacking, and it has been shown that the graft rupture rate3 is higher for BPTB allograft than for BPTB autograft in young patients. However, much of the data on higher failure rates with use of allograft in young patients4,5 has appeared since our data were collected.
Our return-to-play data are similar to data from other studies.2,6 According to our survey, the most common length of time from ACL reconstruction to return to football was 6 months, and 94% of team physicians allowed return to football by 9 months. In the survey by Erickson and colleagues,2 55% of surgeons waited a minimum of 6 months before returning athletes to play, and only 12% waited at least 9 months. In the study by Bradley and colleagues6 (2002), 84% of surgeons waited at least 6 months before returning athletes to play. Of note, we found a significantly higher percentage of NCAA football players than NFL players returning within 6 months after surgery. The difference may be attributable to a more cautious approach being taken with NFL players, whereas most NCAA players are limited in the time remaining in their football careers and want to return to the playing field as soon as possible.
Shoulder Dislocations. Responses to the 5 survey questions on anterior shoulder dislocation showed little consensus with respect to management. The exception pertained to use of a harness for in-season return to play with a dislocation—92% of physicians preferred management with a harness. Of note, 7 of 10 team surgeons performed anterior stabilization through an arthroscopic approach. Despite historical recommendations to perform open anterior stabilization in collision athletes, NFL and NCAA physicians’ practice patterns have evolved.7 Although return to contact activity was varied among responses, 94% of physicians allowed return to contact within 6 months.
Acromioclavicular Joint Injuries. For college football players, AC joint injuries are the most common shoulder injuries.8 In the NFL Combine, the incidence of AC joint injuries was 15.7 per 100 players.8 Several studies have cited favorable results with nonoperative management of type III AC joint injuries.9-12 Nonoperative management was the preferred treatment in our study as well, yet 26% of surgeons still preferred operative treatment in quarterbacks. Opinions about operative repair of type III injuries in overhead athletes vary,13 but nonoperative management clearly is the preferred method for elite football players. A 2013 study by Lynch and colleagues14 found that only 2 of 40 NFL players with type III AC joint injuries underwent surgery.
For type I and II AC joint injuries that occur during a game, more than two-thirds of the NCAA team physicians in our study favored injecting a local anesthetic to reduce pain and allow return to play in the same game. An even larger majority indicated they gave a pregame injection of an anesthetic to allow play. Similar use of injections for AC joint injuries has been reported in Australian-rules football and rugby.15Medial Collateral Ligament Injuries. Whether bracing is prophylactic against MCL injuries is controversial.16 Some studies have found it effective.17,18 According to our survey, 89% of Division I football teams used prophylactic knee bracing, mainly in offensive linemen but frequently in defensive linemen, too. No schools used bracing in athletes who played skill positions, except quarterbacks. Six schools used bracing on a quarterback’s front leg.
The percentage of teams that used prophylactic MCL bracing was significantly higher in the NCAA than in the NFL. NCAA team physicians generally have more control over players and therefore can implement widespread use of this bracing.
Posterior Cruciate Ligament Injuries. These injuries are infrequent. According to Parolie and Bergfeld,19 only 2% of college football players at the NFL Combine had a PCL injury. Treatment in athletes remains controversial. Our survey showed physicians’ willingness to return players to competition within 4 weeks after grade I/II PCL injuries. There is no consensus on management or on postinjury bracing. In operative cases, however, the preferred graft is allograft, and the preferred repair method is the arthroscopic single-bundle technique. These findings mirror those of a 2004 survey of the Herodicus Society by Dennis and colleagues.20 Elbow Ulnar Collateral Ligament Tears. In throwing athletes with UCL tears, operative treatment has been recommended.21,22 A majority of our survey respondents preferred operative treatment for quarterbacks. However, operative treatment is still controversial, and quarterbacks differ from baseball players in their throwing motions and in the stresses acting on the UCLs during throwing. Two systematic reviews of UCL reconstruction have affirmed the positive outcomes of operative treatment in throwing athletes.21,22 However, most of the studies covered by these reviews focused on baseball players. In athletes playing positions other than quarterback, these injuries were typically treated nonoperatively.
Thumb Ulnar Collateral Ligament Tears. Our survey respondents differed in their opinions on treating thumb UCL tears. About half recommended cast treatment, and the other half recommended operative treatment. Previous data suggest that delaying surgical treatment may be deleterious to the eventual outcome.23,24Fifth Metatarsal Fractures. For fifth metatarsal fractures, screw fixation was preferred by 90% of our survey respondents—vs 73% of NFL team physicians in a 2004 study by Low and colleagues.25 What remains controversial is the length of time before return to play. Our most frequent response was 4 to 6 weeks, and 46% of our respondents indicated they would wait 7 weeks or longer. These times differ significantly from what Low and colleagues25 reported: 86% of their physicians allowed return to competition after 6 to 12 weeks.
Tibia Fractures. Management of tibia fractures in US football players has not been reported. Chang and colleagues26 described 24 tibial shaft fractures in UK soccer players. Eleven fractures (~50%) were treated with intramedullary nails, 2 with plating, and 11 with conservative management. All players returned to activity, the operative group at 23.3 weeks and the nonoperative group at 27.6 weeks. Our respondents reported treating at least 150 tibial shaft fractures in the 5-year period before our survey, demonstrating the incidence and importance of this type of injury. A vast majority of team surgeons (96%) opted for treatment with intramedullary nailing. This choice may reflect an ability to return to play earlier—the ability to move the knee and maintain strength in the legs. Some have suggested it is important to remove the nail before the player returns to the football field, but this was not common practice among our groups of team surgeons. Other studies have not found any advantage to tibial nail removal.27Ketorolac Injections. Authors have described using ketorolac for the treatment of acute or pregame pain in professional football players.28-30 According to a 2000 survey, 93% of NFL teams used intramuscular ketorolac, and on average 15 players per team were treated, primarily on game day. Our survey found frequent use of ketorolac, with almost two-thirds of team orthopedists indicating pregame use. Ketorolac use was popular, particularly because of its effect in reducing postoperative pain and its potent effect in reducing pain on game day. However, injections by football team physicians have declined significantly in recent years, ever since an NFL Physician Society task force published recommendations on ketorolac use.31
Conclusion
There is a wide variety of patterns in treating athletes who play football at the highest levels of competition. Our findings can initiate further discussion on these topics and assist orthopedists providing game coverage at all levels of play in their decision-making process by helping to define the standard of care for their injured players.
Am J Orthop. 2016;45(6):E319-E327. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Stockard AR. Team physician preferences at National Collegiate Athletic Association Division I universities. J Am Osteopath Assoc. 1997;97(2):89-95.
2. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
3. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
4. Bottoni CR, Smith EL, Shaha J, et al. Autograft versus allograft anterior cruciate ligament reconstruction: a prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43(10):2501-2509.
5. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
6. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
7. Rhee YG, Ha JH, Cho NS. Anterior shoulder stabilization in collision athletes: arthroscopic versus open Bankart repair. Am J Sports Med. 2006;34(6):979-985.
8. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine—trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
9. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc. 2006;14(4):237-245.
10. Mazzocca AD, Arciero RA, Bicos J. Evaluation and treatment of acromioclavicular joint injuries. Am J Sports Med. 2007;35(2):316-329.
11. Schlegel TF, Burks RT, Marcus RL, Dunn HK. A prospective evaluation of untreated acute grade III acromioclavicular separations. Am J Sports Med. 2001;29(6):699-703.
12. Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;(455):38-44.
13. Kraeutler MJ, Williams GR Jr, Cohen SB, et al. Inter- and intraobserver reliability of the radiographic diagnosis and treatment of acromioclavicular joint separations. Orthopedics. 2012;35(10):e1483-e1487.
14. Lynch TS, Saltzman MD, Ghodasra JH, Bilimoria KY, Bowen MK, Nuber GW. Acromioclavicular joint injuries in the National Football League: epidemiology and management. Am J Sports Med. 2013;41(12):2904-2908.
15. Orchard JW. Benefits and risks of using local anaesthetic for pain relief to allow early return to play in professional football. Br J Sports Med. 2002;36(3):209-213.
16. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
17. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Effectiveness of preventive braces. Am J Sports Med. 1994;22(1):12-18.
18. Sitler M, Ryan J, Hopkinson W, et al. The efficacy of a prophylactic knee brace to reduce knee injuries in football. A prospective, randomized study at West Point. Am J Sports Med. 1990;18(3):310-315.
19. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
20. Dennis MG, Fox JA, Alford JW, Hayden JK, Bach BR Jr. Posterior cruciate ligament reconstruction: current trends. J Knee Surg. 2004;17(3):133-139.
21. Purcell DB, Matava MJ, Wright RW. Ulnar collateral ligament reconstruction: a systematic review. Clin Orthop Relat Res. 2007;(455):72-77.
22. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
23. Fricker R, Hintermann B. Skier’s thumb. Treatment, prevention and recommendations. Sports Med. 1995;19(1):73-79.
24. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
25. Low K, Noblin JD, Browne JE, Barnthouse CD, Scott AR. Jones fractures in the elite football player. J Surg Orthop Adv. 2004;13(3):156-160.
26. Chang WR, Kapasi Z, Daisley S, Leach WJ. Tibial shaft fractures in football players. J Orthop Surg Res. 2007;2:11.
27. Karladani AH, Ericsson PA, Granhed H, Karlsson L, Nyberg P. Tibial intramedullary nails—should they be removed? A retrospective study of 71 patients. Acta Orthop. 2007;78(5):668-671.
28. Eichner ER. Intramuscular ketorolac injections: the pregame Toradol parade. Curr Sports Med Rep. 2012;11(4):169-170.
29. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1(5):396-404.
30. Powell ET, Tokish JM, Hawkins RJ. Toradol use in the athletic population. Curr Sports Med Rep. 2002;1(4):191.
31. Matava M, Brater DC, Gritter N, et al. Recommendations of the National Football League physician society task force on the use of toradol® ketorolac in the National Football League. Sports Health. 2012;4(5):377-383.
Among National Football League (NFL) and National Collegiate Athletic Association (NCAA) team physicians, there is no consensus on the management of various injuries. At national and regional meetings, the management of football injuries often is debated.
Given the high level of interest in the treatment of elite football players, we wanted to determine treatment patterns by surveying orthopedic team physicians. We conducted a study to determine the demographics of NFL and NCAA team physicians and to identify patterns and variations in the management of common injuries in these groups of elite football players.
Materials and Methods
The study was reviewed by an Institutional Review Board before data collection and was classified as exempt. The study population consisted of head orthopedic team physicians for NFL teams and NCAA Division I universities. The survey (Appendix),
Chi-square tests were used to determine significant differences between groups. P < .05 was considered statistically significant.
Results
Responses were received from 31 (97%) of the 32 NFL and 111 (93%) of the 119 NCAA team physicians. The 2 groups’ surveys were identical with the exception of question 3, regarding NFL division or NCAA conference.
Team Physician Demographics
All survey respondents were the head orthopedic physicians for their teams. Seventy-one percent were the head team physicians as well; another 25% named a primary care physician as the head team physician. Thirty-nine percent of the NFL team physicians had been a team physician at the NFL level for more than 15 years, and 58% of the NCAA team physicians had been a team physician at the Division I level for more than 15 years. Eighty-one percent of NFL and 66% of NCAA team physicians had fellowship training in sports medicine. For away games, 10% of NFL vs 65% of NCAA teams traveled with 2 physicians; 90% of NFL and 28% of NCAA teams traveled with 3 or more physicians.
Only a small percentage of respondents (NFL, 10%; NCAA, 14%) indicated they had received advertising in exchange for services. Most respondents (NFL, 93%; NCAA, 89%) did not pay to provide team coverage. In contrast, 97% of NFL vs only 31% of NCAA physicians indicated they received a monetary stipend for providing orthopedic coverage.
Anterior Cruciate Ligament Reconstructions
Eighty-seven percent of NFL and 67% of NCAA respondents indicated that patellar tendon autograft was their preferred graft choice (Table 1).
Anterior Shoulder Dislocations (Without Bony Bankart)
Sling use after reduction of anterior shoulder dislocation was varied, with most physicians using a sling 2 weeks or less (Table 2).
Acromioclavicular Joint Injuries
Roughly two-thirds of respondents (NFL, 60%; NCAA, 69%) indicated that, during a game, they managed acute acromioclavicular (AC) joint injuries (type I/II) with injection of a local anesthetic that allowed return to play. In addition, a majority (NFL, 90%; NCAA, 87%) indicated they gave such athletes pregame injections that allowed them to play. About half the physicians (NFL, 57%; NCAA, 52%) injected the AC joint with cortisone during the acute/subacute period (<1 month) to decrease inflammation.
No significant difference was found between the 2 groups in terms of proportion of surgeons electing to treat type III AC joint injuries operatively versus nonoperatively (Table 4).
Medial Collateral Ligament Injuries
There was a significant (P < .0001) difference in use of prophylactic bracing for medial collateral ligament (MCL) injuries (NFL, 28%; NCAA, 89%).
Posterior Cruciate Ligament Injuries
The percentage of physicians who allowed athletes to return to play after a grade I/II posterior cruciate ligament (PCL) injury was significantly (P = .01) higher in NFL physicians (22%) than in NCAA physicians (7%). The amount of time varied up to more than 4 weeks (Figure 4).
Elbow Ulnar Collateral Ligament Tears
A majority of respondents indicated they would treat a complete elbow ulnar collateral ligament (UCL) tear in a quarterback; a much smaller percentage preferred operative repair in athletes playing other positions (Table 6).
Thumb Ulnar Collateral Ligament Tears
For athletes with in-season thumb UCL tears, 63% of NFL and 54% of NCAA physicians indicated they cast the thumb and allowed return to play. Others recommended operative repair and either cast the thumb and allowed return to play (NFL, 30%; NCAA, 41%) or let the thumb heal before allowing return to play (NFL, 7%; NCAA, 5%).
Fifth Metatarsal Fractures
For a large majority of physicians (NFL, 100%; NCAA, 94%), the preferred treatment for fifth metatarsal fractures was screw fixation.
Tibia Fractures
In the 5-year period before the survey, 43% of NFL and 75% of NCAA physicians managed at least one tibia fracture (P < .001) (Figure 7).
Ketorolac Injections
Intramuscular ketorolac injections were frequently given to elite football players, significantly (P < .01) more so in the NFL (93%) than in the NCAA (62%). The average number of injections varied among physicians, though a significantly (P < .0001) higher percentage of NFL (79%) than NCAA (13%) physicians gave 5 or more injections per game.
Discussion
This survey on managing common injuries in elite football players had an overall response rate of 94%. All NFL divisions and NCAA conferences were represented in physicians’ responses. Ninety percent of NFL and 65% of NCAA head team physicians were orthopedists. These findings differ from those of Stockard1 (1997), who surveyed athletic directors at Division I schools and reported 45% of head team physicians were family medicine-trained and 41% were orthopedists.
Given the high visibility of team coverage and the economics of college football’s highest division, one might expect team physicians to receive financial remuneration. This was not the case, according to our survey: Only 30% of physicians received a monetary stipend for team coverage, and only 14% received advertising in exchange for their services. Twelve NCAA team physicians indicated they pay to be allowed to provide team coverage.
Injury Management
Anterior Cruciate Ligament Injuries. For NFL and NCAA team physicians, the preferred graft choice for ACL reconstruction was patellar tendon autograft. This finding is similar to what Erickson and colleagues2 reported from a survey of NFL and NCAA team physicians: 86% of surgeons preferred bone–patellar tendon–bone (BPTB) autograft. However, only 1 surgeon (0.7%) in that study, vs 16% in ours, preferred allograft. Allograft use may be somewhat controversial, as relevant data on competitive athletes are lacking, and it has been shown that the graft rupture rate3 is higher for BPTB allograft than for BPTB autograft in young patients. However, much of the data on higher failure rates with use of allograft in young patients4,5 has appeared since our data were collected.
Our return-to-play data are similar to data from other studies.2,6 According to our survey, the most common length of time from ACL reconstruction to return to football was 6 months, and 94% of team physicians allowed return to football by 9 months. In the survey by Erickson and colleagues,2 55% of surgeons waited a minimum of 6 months before returning athletes to play, and only 12% waited at least 9 months. In the study by Bradley and colleagues6 (2002), 84% of surgeons waited at least 6 months before returning athletes to play. Of note, we found a significantly higher percentage of NCAA football players than NFL players returning within 6 months after surgery. The difference may be attributable to a more cautious approach being taken with NFL players, whereas most NCAA players are limited in the time remaining in their football careers and want to return to the playing field as soon as possible.
Shoulder Dislocations. Responses to the 5 survey questions on anterior shoulder dislocation showed little consensus with respect to management. The exception pertained to use of a harness for in-season return to play with a dislocation—92% of physicians preferred management with a harness. Of note, 7 of 10 team surgeons performed anterior stabilization through an arthroscopic approach. Despite historical recommendations to perform open anterior stabilization in collision athletes, NFL and NCAA physicians’ practice patterns have evolved.7 Although return to contact activity was varied among responses, 94% of physicians allowed return to contact within 6 months.
Acromioclavicular Joint Injuries. For college football players, AC joint injuries are the most common shoulder injuries.8 In the NFL Combine, the incidence of AC joint injuries was 15.7 per 100 players.8 Several studies have cited favorable results with nonoperative management of type III AC joint injuries.9-12 Nonoperative management was the preferred treatment in our study as well, yet 26% of surgeons still preferred operative treatment in quarterbacks. Opinions about operative repair of type III injuries in overhead athletes vary,13 but nonoperative management clearly is the preferred method for elite football players. A 2013 study by Lynch and colleagues14 found that only 2 of 40 NFL players with type III AC joint injuries underwent surgery.
For type I and II AC joint injuries that occur during a game, more than two-thirds of the NCAA team physicians in our study favored injecting a local anesthetic to reduce pain and allow return to play in the same game. An even larger majority indicated they gave a pregame injection of an anesthetic to allow play. Similar use of injections for AC joint injuries has been reported in Australian-rules football and rugby.15Medial Collateral Ligament Injuries. Whether bracing is prophylactic against MCL injuries is controversial.16 Some studies have found it effective.17,18 According to our survey, 89% of Division I football teams used prophylactic knee bracing, mainly in offensive linemen but frequently in defensive linemen, too. No schools used bracing in athletes who played skill positions, except quarterbacks. Six schools used bracing on a quarterback’s front leg.
The percentage of teams that used prophylactic MCL bracing was significantly higher in the NCAA than in the NFL. NCAA team physicians generally have more control over players and therefore can implement widespread use of this bracing.
Posterior Cruciate Ligament Injuries. These injuries are infrequent. According to Parolie and Bergfeld,19 only 2% of college football players at the NFL Combine had a PCL injury. Treatment in athletes remains controversial. Our survey showed physicians’ willingness to return players to competition within 4 weeks after grade I/II PCL injuries. There is no consensus on management or on postinjury bracing. In operative cases, however, the preferred graft is allograft, and the preferred repair method is the arthroscopic single-bundle technique. These findings mirror those of a 2004 survey of the Herodicus Society by Dennis and colleagues.20 Elbow Ulnar Collateral Ligament Tears. In throwing athletes with UCL tears, operative treatment has been recommended.21,22 A majority of our survey respondents preferred operative treatment for quarterbacks. However, operative treatment is still controversial, and quarterbacks differ from baseball players in their throwing motions and in the stresses acting on the UCLs during throwing. Two systematic reviews of UCL reconstruction have affirmed the positive outcomes of operative treatment in throwing athletes.21,22 However, most of the studies covered by these reviews focused on baseball players. In athletes playing positions other than quarterback, these injuries were typically treated nonoperatively.
Thumb Ulnar Collateral Ligament Tears. Our survey respondents differed in their opinions on treating thumb UCL tears. About half recommended cast treatment, and the other half recommended operative treatment. Previous data suggest that delaying surgical treatment may be deleterious to the eventual outcome.23,24Fifth Metatarsal Fractures. For fifth metatarsal fractures, screw fixation was preferred by 90% of our survey respondents—vs 73% of NFL team physicians in a 2004 study by Low and colleagues.25 What remains controversial is the length of time before return to play. Our most frequent response was 4 to 6 weeks, and 46% of our respondents indicated they would wait 7 weeks or longer. These times differ significantly from what Low and colleagues25 reported: 86% of their physicians allowed return to competition after 6 to 12 weeks.
Tibia Fractures. Management of tibia fractures in US football players has not been reported. Chang and colleagues26 described 24 tibial shaft fractures in UK soccer players. Eleven fractures (~50%) were treated with intramedullary nails, 2 with plating, and 11 with conservative management. All players returned to activity, the operative group at 23.3 weeks and the nonoperative group at 27.6 weeks. Our respondents reported treating at least 150 tibial shaft fractures in the 5-year period before our survey, demonstrating the incidence and importance of this type of injury. A vast majority of team surgeons (96%) opted for treatment with intramedullary nailing. This choice may reflect an ability to return to play earlier—the ability to move the knee and maintain strength in the legs. Some have suggested it is important to remove the nail before the player returns to the football field, but this was not common practice among our groups of team surgeons. Other studies have not found any advantage to tibial nail removal.27Ketorolac Injections. Authors have described using ketorolac for the treatment of acute or pregame pain in professional football players.28-30 According to a 2000 survey, 93% of NFL teams used intramuscular ketorolac, and on average 15 players per team were treated, primarily on game day. Our survey found frequent use of ketorolac, with almost two-thirds of team orthopedists indicating pregame use. Ketorolac use was popular, particularly because of its effect in reducing postoperative pain and its potent effect in reducing pain on game day. However, injections by football team physicians have declined significantly in recent years, ever since an NFL Physician Society task force published recommendations on ketorolac use.31
Conclusion
There is a wide variety of patterns in treating athletes who play football at the highest levels of competition. Our findings can initiate further discussion on these topics and assist orthopedists providing game coverage at all levels of play in their decision-making process by helping to define the standard of care for their injured players.
Am J Orthop. 2016;45(6):E319-E327. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Among National Football League (NFL) and National Collegiate Athletic Association (NCAA) team physicians, there is no consensus on the management of various injuries. At national and regional meetings, the management of football injuries often is debated.
Given the high level of interest in the treatment of elite football players, we wanted to determine treatment patterns by surveying orthopedic team physicians. We conducted a study to determine the demographics of NFL and NCAA team physicians and to identify patterns and variations in the management of common injuries in these groups of elite football players.
Materials and Methods
The study was reviewed by an Institutional Review Board before data collection and was classified as exempt. The study population consisted of head orthopedic team physicians for NFL teams and NCAA Division I universities. The survey (Appendix),
Chi-square tests were used to determine significant differences between groups. P < .05 was considered statistically significant.
Results
Responses were received from 31 (97%) of the 32 NFL and 111 (93%) of the 119 NCAA team physicians. The 2 groups’ surveys were identical with the exception of question 3, regarding NFL division or NCAA conference.
Team Physician Demographics
All survey respondents were the head orthopedic physicians for their teams. Seventy-one percent were the head team physicians as well; another 25% named a primary care physician as the head team physician. Thirty-nine percent of the NFL team physicians had been a team physician at the NFL level for more than 15 years, and 58% of the NCAA team physicians had been a team physician at the Division I level for more than 15 years. Eighty-one percent of NFL and 66% of NCAA team physicians had fellowship training in sports medicine. For away games, 10% of NFL vs 65% of NCAA teams traveled with 2 physicians; 90% of NFL and 28% of NCAA teams traveled with 3 or more physicians.
Only a small percentage of respondents (NFL, 10%; NCAA, 14%) indicated they had received advertising in exchange for services. Most respondents (NFL, 93%; NCAA, 89%) did not pay to provide team coverage. In contrast, 97% of NFL vs only 31% of NCAA physicians indicated they received a monetary stipend for providing orthopedic coverage.
Anterior Cruciate Ligament Reconstructions
Eighty-seven percent of NFL and 67% of NCAA respondents indicated that patellar tendon autograft was their preferred graft choice (Table 1).
Anterior Shoulder Dislocations (Without Bony Bankart)
Sling use after reduction of anterior shoulder dislocation was varied, with most physicians using a sling 2 weeks or less (Table 2).
Acromioclavicular Joint Injuries
Roughly two-thirds of respondents (NFL, 60%; NCAA, 69%) indicated that, during a game, they managed acute acromioclavicular (AC) joint injuries (type I/II) with injection of a local anesthetic that allowed return to play. In addition, a majority (NFL, 90%; NCAA, 87%) indicated they gave such athletes pregame injections that allowed them to play. About half the physicians (NFL, 57%; NCAA, 52%) injected the AC joint with cortisone during the acute/subacute period (<1 month) to decrease inflammation.
No significant difference was found between the 2 groups in terms of proportion of surgeons electing to treat type III AC joint injuries operatively versus nonoperatively (Table 4).
Medial Collateral Ligament Injuries
There was a significant (P < .0001) difference in use of prophylactic bracing for medial collateral ligament (MCL) injuries (NFL, 28%; NCAA, 89%).
Posterior Cruciate Ligament Injuries
The percentage of physicians who allowed athletes to return to play after a grade I/II posterior cruciate ligament (PCL) injury was significantly (P = .01) higher in NFL physicians (22%) than in NCAA physicians (7%). The amount of time varied up to more than 4 weeks (Figure 4).
Elbow Ulnar Collateral Ligament Tears
A majority of respondents indicated they would treat a complete elbow ulnar collateral ligament (UCL) tear in a quarterback; a much smaller percentage preferred operative repair in athletes playing other positions (Table 6).
Thumb Ulnar Collateral Ligament Tears
For athletes with in-season thumb UCL tears, 63% of NFL and 54% of NCAA physicians indicated they cast the thumb and allowed return to play. Others recommended operative repair and either cast the thumb and allowed return to play (NFL, 30%; NCAA, 41%) or let the thumb heal before allowing return to play (NFL, 7%; NCAA, 5%).
Fifth Metatarsal Fractures
For a large majority of physicians (NFL, 100%; NCAA, 94%), the preferred treatment for fifth metatarsal fractures was screw fixation.
Tibia Fractures
In the 5-year period before the survey, 43% of NFL and 75% of NCAA physicians managed at least one tibia fracture (P < .001) (Figure 7).
Ketorolac Injections
Intramuscular ketorolac injections were frequently given to elite football players, significantly (P < .01) more so in the NFL (93%) than in the NCAA (62%). The average number of injections varied among physicians, though a significantly (P < .0001) higher percentage of NFL (79%) than NCAA (13%) physicians gave 5 or more injections per game.
Discussion
This survey on managing common injuries in elite football players had an overall response rate of 94%. All NFL divisions and NCAA conferences were represented in physicians’ responses. Ninety percent of NFL and 65% of NCAA head team physicians were orthopedists. These findings differ from those of Stockard1 (1997), who surveyed athletic directors at Division I schools and reported 45% of head team physicians were family medicine-trained and 41% were orthopedists.
Given the high visibility of team coverage and the economics of college football’s highest division, one might expect team physicians to receive financial remuneration. This was not the case, according to our survey: Only 30% of physicians received a monetary stipend for team coverage, and only 14% received advertising in exchange for their services. Twelve NCAA team physicians indicated they pay to be allowed to provide team coverage.
Injury Management
Anterior Cruciate Ligament Injuries. For NFL and NCAA team physicians, the preferred graft choice for ACL reconstruction was patellar tendon autograft. This finding is similar to what Erickson and colleagues2 reported from a survey of NFL and NCAA team physicians: 86% of surgeons preferred bone–patellar tendon–bone (BPTB) autograft. However, only 1 surgeon (0.7%) in that study, vs 16% in ours, preferred allograft. Allograft use may be somewhat controversial, as relevant data on competitive athletes are lacking, and it has been shown that the graft rupture rate3 is higher for BPTB allograft than for BPTB autograft in young patients. However, much of the data on higher failure rates with use of allograft in young patients4,5 has appeared since our data were collected.
Our return-to-play data are similar to data from other studies.2,6 According to our survey, the most common length of time from ACL reconstruction to return to football was 6 months, and 94% of team physicians allowed return to football by 9 months. In the survey by Erickson and colleagues,2 55% of surgeons waited a minimum of 6 months before returning athletes to play, and only 12% waited at least 9 months. In the study by Bradley and colleagues6 (2002), 84% of surgeons waited at least 6 months before returning athletes to play. Of note, we found a significantly higher percentage of NCAA football players than NFL players returning within 6 months after surgery. The difference may be attributable to a more cautious approach being taken with NFL players, whereas most NCAA players are limited in the time remaining in their football careers and want to return to the playing field as soon as possible.
Shoulder Dislocations. Responses to the 5 survey questions on anterior shoulder dislocation showed little consensus with respect to management. The exception pertained to use of a harness for in-season return to play with a dislocation—92% of physicians preferred management with a harness. Of note, 7 of 10 team surgeons performed anterior stabilization through an arthroscopic approach. Despite historical recommendations to perform open anterior stabilization in collision athletes, NFL and NCAA physicians’ practice patterns have evolved.7 Although return to contact activity was varied among responses, 94% of physicians allowed return to contact within 6 months.
Acromioclavicular Joint Injuries. For college football players, AC joint injuries are the most common shoulder injuries.8 In the NFL Combine, the incidence of AC joint injuries was 15.7 per 100 players.8 Several studies have cited favorable results with nonoperative management of type III AC joint injuries.9-12 Nonoperative management was the preferred treatment in our study as well, yet 26% of surgeons still preferred operative treatment in quarterbacks. Opinions about operative repair of type III injuries in overhead athletes vary,13 but nonoperative management clearly is the preferred method for elite football players. A 2013 study by Lynch and colleagues14 found that only 2 of 40 NFL players with type III AC joint injuries underwent surgery.
For type I and II AC joint injuries that occur during a game, more than two-thirds of the NCAA team physicians in our study favored injecting a local anesthetic to reduce pain and allow return to play in the same game. An even larger majority indicated they gave a pregame injection of an anesthetic to allow play. Similar use of injections for AC joint injuries has been reported in Australian-rules football and rugby.15Medial Collateral Ligament Injuries. Whether bracing is prophylactic against MCL injuries is controversial.16 Some studies have found it effective.17,18 According to our survey, 89% of Division I football teams used prophylactic knee bracing, mainly in offensive linemen but frequently in defensive linemen, too. No schools used bracing in athletes who played skill positions, except quarterbacks. Six schools used bracing on a quarterback’s front leg.
The percentage of teams that used prophylactic MCL bracing was significantly higher in the NCAA than in the NFL. NCAA team physicians generally have more control over players and therefore can implement widespread use of this bracing.
Posterior Cruciate Ligament Injuries. These injuries are infrequent. According to Parolie and Bergfeld,19 only 2% of college football players at the NFL Combine had a PCL injury. Treatment in athletes remains controversial. Our survey showed physicians’ willingness to return players to competition within 4 weeks after grade I/II PCL injuries. There is no consensus on management or on postinjury bracing. In operative cases, however, the preferred graft is allograft, and the preferred repair method is the arthroscopic single-bundle technique. These findings mirror those of a 2004 survey of the Herodicus Society by Dennis and colleagues.20 Elbow Ulnar Collateral Ligament Tears. In throwing athletes with UCL tears, operative treatment has been recommended.21,22 A majority of our survey respondents preferred operative treatment for quarterbacks. However, operative treatment is still controversial, and quarterbacks differ from baseball players in their throwing motions and in the stresses acting on the UCLs during throwing. Two systematic reviews of UCL reconstruction have affirmed the positive outcomes of operative treatment in throwing athletes.21,22 However, most of the studies covered by these reviews focused on baseball players. In athletes playing positions other than quarterback, these injuries were typically treated nonoperatively.
Thumb Ulnar Collateral Ligament Tears. Our survey respondents differed in their opinions on treating thumb UCL tears. About half recommended cast treatment, and the other half recommended operative treatment. Previous data suggest that delaying surgical treatment may be deleterious to the eventual outcome.23,24Fifth Metatarsal Fractures. For fifth metatarsal fractures, screw fixation was preferred by 90% of our survey respondents—vs 73% of NFL team physicians in a 2004 study by Low and colleagues.25 What remains controversial is the length of time before return to play. Our most frequent response was 4 to 6 weeks, and 46% of our respondents indicated they would wait 7 weeks or longer. These times differ significantly from what Low and colleagues25 reported: 86% of their physicians allowed return to competition after 6 to 12 weeks.
Tibia Fractures. Management of tibia fractures in US football players has not been reported. Chang and colleagues26 described 24 tibial shaft fractures in UK soccer players. Eleven fractures (~50%) were treated with intramedullary nails, 2 with plating, and 11 with conservative management. All players returned to activity, the operative group at 23.3 weeks and the nonoperative group at 27.6 weeks. Our respondents reported treating at least 150 tibial shaft fractures in the 5-year period before our survey, demonstrating the incidence and importance of this type of injury. A vast majority of team surgeons (96%) opted for treatment with intramedullary nailing. This choice may reflect an ability to return to play earlier—the ability to move the knee and maintain strength in the legs. Some have suggested it is important to remove the nail before the player returns to the football field, but this was not common practice among our groups of team surgeons. Other studies have not found any advantage to tibial nail removal.27Ketorolac Injections. Authors have described using ketorolac for the treatment of acute or pregame pain in professional football players.28-30 According to a 2000 survey, 93% of NFL teams used intramuscular ketorolac, and on average 15 players per team were treated, primarily on game day. Our survey found frequent use of ketorolac, with almost two-thirds of team orthopedists indicating pregame use. Ketorolac use was popular, particularly because of its effect in reducing postoperative pain and its potent effect in reducing pain on game day. However, injections by football team physicians have declined significantly in recent years, ever since an NFL Physician Society task force published recommendations on ketorolac use.31
Conclusion
There is a wide variety of patterns in treating athletes who play football at the highest levels of competition. Our findings can initiate further discussion on these topics and assist orthopedists providing game coverage at all levels of play in their decision-making process by helping to define the standard of care for their injured players.
Am J Orthop. 2016;45(6):E319-E327. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Stockard AR. Team physician preferences at National Collegiate Athletic Association Division I universities. J Am Osteopath Assoc. 1997;97(2):89-95.
2. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
3. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
4. Bottoni CR, Smith EL, Shaha J, et al. Autograft versus allograft anterior cruciate ligament reconstruction: a prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43(10):2501-2509.
5. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
6. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
7. Rhee YG, Ha JH, Cho NS. Anterior shoulder stabilization in collision athletes: arthroscopic versus open Bankart repair. Am J Sports Med. 2006;34(6):979-985.
8. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine—trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
9. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc. 2006;14(4):237-245.
10. Mazzocca AD, Arciero RA, Bicos J. Evaluation and treatment of acromioclavicular joint injuries. Am J Sports Med. 2007;35(2):316-329.
11. Schlegel TF, Burks RT, Marcus RL, Dunn HK. A prospective evaluation of untreated acute grade III acromioclavicular separations. Am J Sports Med. 2001;29(6):699-703.
12. Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;(455):38-44.
13. Kraeutler MJ, Williams GR Jr, Cohen SB, et al. Inter- and intraobserver reliability of the radiographic diagnosis and treatment of acromioclavicular joint separations. Orthopedics. 2012;35(10):e1483-e1487.
14. Lynch TS, Saltzman MD, Ghodasra JH, Bilimoria KY, Bowen MK, Nuber GW. Acromioclavicular joint injuries in the National Football League: epidemiology and management. Am J Sports Med. 2013;41(12):2904-2908.
15. Orchard JW. Benefits and risks of using local anaesthetic for pain relief to allow early return to play in professional football. Br J Sports Med. 2002;36(3):209-213.
16. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
17. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Effectiveness of preventive braces. Am J Sports Med. 1994;22(1):12-18.
18. Sitler M, Ryan J, Hopkinson W, et al. The efficacy of a prophylactic knee brace to reduce knee injuries in football. A prospective, randomized study at West Point. Am J Sports Med. 1990;18(3):310-315.
19. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
20. Dennis MG, Fox JA, Alford JW, Hayden JK, Bach BR Jr. Posterior cruciate ligament reconstruction: current trends. J Knee Surg. 2004;17(3):133-139.
21. Purcell DB, Matava MJ, Wright RW. Ulnar collateral ligament reconstruction: a systematic review. Clin Orthop Relat Res. 2007;(455):72-77.
22. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
23. Fricker R, Hintermann B. Skier’s thumb. Treatment, prevention and recommendations. Sports Med. 1995;19(1):73-79.
24. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
25. Low K, Noblin JD, Browne JE, Barnthouse CD, Scott AR. Jones fractures in the elite football player. J Surg Orthop Adv. 2004;13(3):156-160.
26. Chang WR, Kapasi Z, Daisley S, Leach WJ. Tibial shaft fractures in football players. J Orthop Surg Res. 2007;2:11.
27. Karladani AH, Ericsson PA, Granhed H, Karlsson L, Nyberg P. Tibial intramedullary nails—should they be removed? A retrospective study of 71 patients. Acta Orthop. 2007;78(5):668-671.
28. Eichner ER. Intramuscular ketorolac injections: the pregame Toradol parade. Curr Sports Med Rep. 2012;11(4):169-170.
29. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1(5):396-404.
30. Powell ET, Tokish JM, Hawkins RJ. Toradol use in the athletic population. Curr Sports Med Rep. 2002;1(4):191.
31. Matava M, Brater DC, Gritter N, et al. Recommendations of the National Football League physician society task force on the use of toradol® ketorolac in the National Football League. Sports Health. 2012;4(5):377-383.
1. Stockard AR. Team physician preferences at National Collegiate Athletic Association Division I universities. J Am Osteopath Assoc. 1997;97(2):89-95.
2. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
3. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
4. Bottoni CR, Smith EL, Shaha J, et al. Autograft versus allograft anterior cruciate ligament reconstruction: a prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43(10):2501-2509.
5. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
6. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
7. Rhee YG, Ha JH, Cho NS. Anterior shoulder stabilization in collision athletes: arthroscopic versus open Bankart repair. Am J Sports Med. 2006;34(6):979-985.
8. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine—trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
9. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc. 2006;14(4):237-245.
10. Mazzocca AD, Arciero RA, Bicos J. Evaluation and treatment of acromioclavicular joint injuries. Am J Sports Med. 2007;35(2):316-329.
11. Schlegel TF, Burks RT, Marcus RL, Dunn HK. A prospective evaluation of untreated acute grade III acromioclavicular separations. Am J Sports Med. 2001;29(6):699-703.
12. Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;(455):38-44.
13. Kraeutler MJ, Williams GR Jr, Cohen SB, et al. Inter- and intraobserver reliability of the radiographic diagnosis and treatment of acromioclavicular joint separations. Orthopedics. 2012;35(10):e1483-e1487.
14. Lynch TS, Saltzman MD, Ghodasra JH, Bilimoria KY, Bowen MK, Nuber GW. Acromioclavicular joint injuries in the National Football League: epidemiology and management. Am J Sports Med. 2013;41(12):2904-2908.
15. Orchard JW. Benefits and risks of using local anaesthetic for pain relief to allow early return to play in professional football. Br J Sports Med. 2002;36(3):209-213.
16. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
17. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Effectiveness of preventive braces. Am J Sports Med. 1994;22(1):12-18.
18. Sitler M, Ryan J, Hopkinson W, et al. The efficacy of a prophylactic knee brace to reduce knee injuries in football. A prospective, randomized study at West Point. Am J Sports Med. 1990;18(3):310-315.
19. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
20. Dennis MG, Fox JA, Alford JW, Hayden JK, Bach BR Jr. Posterior cruciate ligament reconstruction: current trends. J Knee Surg. 2004;17(3):133-139.
21. Purcell DB, Matava MJ, Wright RW. Ulnar collateral ligament reconstruction: a systematic review. Clin Orthop Relat Res. 2007;(455):72-77.
22. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
23. Fricker R, Hintermann B. Skier’s thumb. Treatment, prevention and recommendations. Sports Med. 1995;19(1):73-79.
24. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
25. Low K, Noblin JD, Browne JE, Barnthouse CD, Scott AR. Jones fractures in the elite football player. J Surg Orthop Adv. 2004;13(3):156-160.
26. Chang WR, Kapasi Z, Daisley S, Leach WJ. Tibial shaft fractures in football players. J Orthop Surg Res. 2007;2:11.
27. Karladani AH, Ericsson PA, Granhed H, Karlsson L, Nyberg P. Tibial intramedullary nails—should they be removed? A retrospective study of 71 patients. Acta Orthop. 2007;78(5):668-671.
28. Eichner ER. Intramuscular ketorolac injections: the pregame Toradol parade. Curr Sports Med Rep. 2012;11(4):169-170.
29. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1(5):396-404.
30. Powell ET, Tokish JM, Hawkins RJ. Toradol use in the athletic population. Curr Sports Med Rep. 2002;1(4):191.
31. Matava M, Brater DC, Gritter N, et al. Recommendations of the National Football League physician society task force on the use of toradol® ketorolac in the National Football League. Sports Health. 2012;4(5):377-383.
Thigh Injuries in American Football
American football has the highest injury rate of any team sport in the United States at the high school, collegiate, and professional levels.1-3 Muscle strains and contusions constitute a large proportion of football injuries. For example, at the high school level, muscle strains comprise 12% to 24% of all injuries;2 at the collegiate level, they account for approximately 20% of all practice injuries, with nearly half of all strains occurring within the thigh.1,4 Among a single National Football League (NFL) team, Feeley and colleagues5 reported that muscle strains accounted for 46% of practice and 22% of preseason game injuries. The hamstrings, followed by the quadriceps, are the most commonly strained muscle groups among both professional and amateur athletes,5,6 with hamstring and quadriceps injuries making up approximately 13% of all injuries among NFL players.7 Given the relatively large surface area and muscle volume of the anterior and posterior thigh, as well as the activities and maneuvers necessitated by the various football positions, it is not surprising that the thigh is frequently involved in football-related injuries.
The purpose of this review is to describe the clinical manifestations of thigh-related soft-tissue injuries seen in football players. Two of these conditions—muscle strains and contusions—are relatively common, while a third condition—the Morel-Lavallée lesion—is a rare, yet relevant injury that warrants discussion.
Quadriceps Contusion
Pathophysiology
Contusion to the quadriceps muscle is a common injury in contact sports generally resulting from a direct blow from a helmet, knee, or shoulder.8 Bleeding within the musculature causes swelling, pain, stiffness, and limitation of quadriceps excursion, ultimately resulting in loss of knee flexion and an inability to run or squat. The injury is typically confined to a single quadriceps muscle.8 The use of thigh padding, though helpful, does not completely eliminate the risk of this injury.
History and Physical Examination
Immediately after injury, the athlete may complain only of thigh pain. However, swelling, pain, and diminished range of knee motion may develop within the first 24 hours depending on the severity of injury and how quickly treatment is instituted.8 Jackson and Feagin9 developed an injury grading system for quadriceps contusions based on the limitation of knee flexion observed (Table 1).
Imaging
A quadriceps contusion is a clinical diagnosis based on a typical history and physical examination; therefore, advanced imaging usually does not need to be obtained except to gauge the severity of injury, to rule out concurrent injuries (ie, tendon rupture), and to identify the presence of a hematoma that may necessitate aspiration. Plain radiographs are typically unremarkable in the acute setting. Appearance on magnetic resonance imaging (MRI) varies by injury severity, with increased signal throughout the affected muscle belly and a diffuse, feathery appearance centered at the point of impact on short TI inversion recovery (STIR) and T2-weighted images reflecting edema and possibly hematoma (Figures 1A-1C).8,11
Treatment
Treatment of a quadriceps contusion is nonoperative and consists of a 3-phase recovery.10 The first phase lasts approximately 2 days and consists of rest, ice, compression, and elevation (RICE) to limit hemorrhage. The knee should be rested in a flexed position to maintain quadriceps muscle fiber length in order to promote muscle compression and limit knee stiffness. For severe contusions in which there is a question of an acute thigh compartment syndrome, compression should be avoided with appropriate treatment based on typical symptoms and intra-compartmental pressure measurement.12 Nonsteroidal anti-inflammatory drugs (NSAIDs) may be administered to diminish pain as well as the risk of myositis ossificans. While there is no data on the efficacy of NSAIDs in preventing myositis ossificans following quadriceps contusions, both COX-2 selective (ie, celecoxib) and nonselective (ie, naproxen, indomethacin) COX inhibitors have been demonstrated to significantly reduce the incidence of heterotopic ossification following hip surgery—a condition occurring from a similar pathophysiologic process as myositis ossificans.13-17 However, this class of drugs should not be given any sooner than 48 to 72 hours after injury to decrease further bleeding risk, given its inhibitory effect on platelet function.18 Narcotic pain medications are rarely required.
The second phase focuses on restoring active and passive knee and hip flexion and begins when permitted by pain.8 Icing, pain control, and physical therapy modalities are also continued in order to reduce pain and swelling as knee motion is progressed. The third phase begins once full range of knee and hip motion is restored and consists of quadriceps strengthening and functional rehabilitation of the lower extremity.8,19 Return to athletic activities and eventually competition should take place when a full, painless range of motion is restored and strength returns to baseline. Isokinetic strength testing may be utilized to more accurately assess strength and endurance. Noncontact, position-specific drills are incorporated as clinical improvement allows. A full recovery should be expected within 4 weeks of injury, with faster resolution and return to play seen in less severe contusions depending on the athlete’s position.8 Continued quadriceps stretching is recommended to prevent recurrence once the athlete returns to play. A protective hard shell may also be utilized both during rehabilitation as well as once the athlete returns to play in order to protect the thigh from reinjury, which may increase the risk of myositis ossificans.8
Complications
A prolonged recovery or persistent symptoms should alert the treating physician to the possibility of complications, including myositis ossificans.8,20 Myositis ossificans typically results from moderate to severe contusions, which may present initially as a painful, indurated mass that later becomes quite firm. This mass may be seen on plain radiographs as early as 2 to 4 weeks following injury if the athlete complains of persistent pain or a palpable thigh mass (Figure 2).9
Mani-Babu and colleagues23 reported a case of a 14-year-old male football player who sustained a quadriceps contusion after a direct blow from an opponent’s helmet to the lateral thigh. Persistent pain and limitation of motion at 2 months follow-up prompted imaging studies that demonstrated myositis ossificans. The patient was treated with intravenous pamidronate (a bisphosphonate) twice over a 3-month period and demonstrated a full recovery within 5 months.
Acute compartment syndrome of the thigh has also been reported following severe quadriceps contusions, with the majority occurring in the anterior compartment.12,24-28 When injury from blunt trauma extends into and disrupts the muscular layer adjacent to the femur, vascular disruption can cause hematoma formation, muscle edema, and significant swelling, thereby increasing intracompartmental pressure. The relatively large volume of the anterior thigh compartment and lack of a rigid deep fascial envelope may be protective from the development of compartment syndrome compared to other sites.28 It can be difficult to distinguish a severe contusion from a compartment syndrome, as both can occur from the same mechanism and have similar presenting signs and symptoms. Signs of a compartment syndrome include pain out of proportion to the injury that is aggravated by passive stretch of the quadriceps muscles, an increasingly firm muscle compartment to palpation, and neurovascular deficits.29 Both acute compartment syndrome and a severe contusion may present with significant pain, inability to bear weight, tense swelling, tenderness to palpation, and pain with passive knee flexion.24 While the successful conservative treatment of athletes with acute compartment syndrome of the thigh has been reported, it is important to closely monitor the patient’s condition and consider intracompartmental pressure monitoring if the patient’s clinical condition deteriorates.12 An acute fasciotomy should be strongly considered when intracompartmental pressures are within 30 mm Hg of diastolic pressure.24-27 Fortunately, it is highly uncommon for thigh compartment pressure to rise to this level. Percutaneous compartment decompression using liposuction equipment or a large cannula has been described to decrease intracompartmental pressure, potentially expediting recovery and minimizing morbidity.18 Interestingly, reports of fasciotomies for acute thigh compartment syndrome following closed athletic injuries have not described necrotic or non-contractile muscle typical of an acute compartment syndrome, calling into question the need for fasciotomy following closed blunt athletic trauma to the thigh.18
Quadriceps Strain
Pathophysiology
Acute quadriceps strains occur during sudden forceful eccentric contraction of the extensor mechanism. Occasionally, in the absence of a clear mechanism, these injuries mistakenly appear as a contusion resulting from a direct blow to the thigh.30,31 The rectus femoris is the most frequently strained quadriceps muscle due, in part, to its superficial location and predominance of type II muscle fibers, which are more likely to be strained.11,32 Although classically described as occurring along the distal portion of the rectus femoris at the musculotendinous junction, quadriceps strains most commonly occur at the mid to proximal aspect of the rectus femoris.30,33 The quadriceps muscle complex crosses 2 joints and, as a result, is more predisposed to eccentric injury than mono-articular muscles.34 We have had a subset of complete myotendinous tears of the rectus femoris that occur in the plant leg of placekickers that result in significant disability.
Risk Factors
Quadriceps and thigh injuries comprise approximately 4.5% of injuries among NFL players.7 Several risk factors for quadriceps strains have been described. In a study of Australian Rules football players, Orchard35 demonstrated that for all muscle strains, the strongest risk factor was a recent history of the same injury, with the next strongest risk factor being a past history of the same injury. Increasing age was found to be a risk factor for hamstring strains but not quadriceps strains. Muscle fatigue may also contribute to injury susceptibility.36
History and Physical Examination
Injuries typically occur during kicking, jumping, or a sudden change in direction while running.30 Athletes may localize pain anywhere along the quadriceps muscle, although strains most commonly occur at the proximal to mid portion of the rectus femoris.30,33 The grading system for quadriceps strains described by Kary30 is based on level of pain, quadriceps strength, and the presence or absence of a palpable defect (Table 2).
The athlete typically walks with an antalgic gait. Visible swelling and/or ecchymosis may be present depending on when the athlete is seen, as ecchymosis may develop within the first 24 hours of injury. The examiner should palpate along the entire length of the injured muscle. High-grade strains or complete tears may present with a bulge or defect in the muscle belly, but in most cases no defect will be palpable. There may be loss of knee flexion similar to a quadriceps contusion. Strength testing should be performed in both the sitting and prone position with the hip both flexed and extended to assess resisted knee extension strength.30 Loss of strength is proportional to the degree of injury.
Imaging
While most quadriceps strains are adequately diagnosed clinically without the need for imaging studies, ultrasound or MRI can be used to evaluate for partial or complete rupture.30,33 In milder cases, MRI usually demonstrates interstitial edema and hemorrhage with a feathery appearance on STIR and T2-weighted imaging (Figures 3A-3C).11
Treatment
Acute treatment of quadriceps strains focuses on minimizing bleeding using the principles of RICE treatment.37 NSAIDs may be used immediately to assist with pain control.30 COX-2-specific NSAIDs are preferred due to their lack of any inhibitory effect on platelet function in order to reduce the risk of further bleeding within the muscle compartment. For the first 24 to 72 hours following injury, the quadriceps should be maintained relatively immobilized to prevent further injury.38 High-grade injuries might necessitate crutches for ambulatory assistance.
Depending on injury severity, the active phase of treatment usually begins within 5 days of injury and consists of stretching and knee/hip range of motion. An active warm-up should precede rehabilitation exercises to activate neural pathways within the muscle and improve muscle elasticity.38 Ballistic stretching should be avoided to prevent additional injury to the muscle fibers. Strengthening should proceed when the athlete recovers a pain-free range of motion. When isometric exercises can be completed at increasing degrees of knee flexion, isotonic exercises may be implemented into the rehabilitation program.30 Return to football can be considered when the athlete has recovered knee and hip range of motion, is pain-free, and has near-normal strength compared to the contralateral side. The athlete should also perform satisfactorily in simulated position-specific activities in a noncontact fashion prior to return to full competition.30
Hamstring Strain
Pathophysiology
Hamstring strains are the most common noncontact injuries in football resulting from excessive muscle stretching during eccentric contraction generally occurring at the musculotendinous junction.5,39 Because the hamstrings cross both the hip and knee, simultaneous hip flexion and knee extension results in maximal lengthening, making them most vulnerable to injury at the terminal swing phase of gait just prior to heel strike.39-42 The long head of the biceps femoris undergoes the greatest stretch, reaching 110% of resting length during terminal swing phase and is the most commonly injured hamstring muscle.43,44 Injury occurs when the force of eccentric contraction, and resulting muscle strain, exceeds the mechanical limits of the tissue.42,45 It remains to be shown whether hamstring strains occur as a result of accumulated microscopic muscle damage or secondary to a single event that exceeds the mechanical limits of the muscle.42
Epidemiology and Risk Factors
The majority of hamstring strains are sustained during noncontact activities, with most athletes citing sprinting as the activity at the time of injury.3 Approximately 93% of injuries occur during noncontact activities among defensive backs and wide receivers.3 Hamstring strains are the second-most common injury among NFL players, comprising approximately 9% of all injuries,5,7 with 16% to 31% of these injuries associated with recurrence.3,5,35,46 Using the NFL’s Injury Surveillance System, Elliott and colleagues3 reported 1716 hamstring strains over a 10-year period (1989-1998). Fifty-one percent of hamstring strains occurred during the 7-week preseason, with a greater than 4-fold increased injury rate noted during the preseason compared to the 16-week regular season. An increased incidence in the preseason is partially attributable to relative deconditioning over the offseason. Defensive backs, wide receivers, and special teams players accounted for the majority of injured players, suggesting that speed position players and those who must “backpedal” (run backwards) are at an increased risk for injury.
Several risk factors for hamstring strain have been described, including prior injury, older age, quadriceps-hamstring strength imbalances, limited hip and knee flexibility, and fatigue.39,42,47 Inadequate rehabilitation and premature return to competition are also likely important factors predisposing to recurrent injury.39,48
History and Physical Examination
The majority of hamstring strains occur in the acute setting when the player experiences the sudden onset of pain in the posterior thigh during strenuous exercise, most commonly while sprinting.39 The injury typically occurs in the early or late stage of practice or competition due, in part, to inadequate warm-up or fatigue. The athlete may describe an audible pop and an inability to continue play, depending on injury severity.
Physical examination may demonstrate palpable induration and tenderness immediately or shortly after injury. In the setting of severe strains, there can be significant thigh swelling and ecchymosis, and in complete ruptures, a palpable defect.39 The affected muscle should be palpated along its entire length, and is best performed prone with the knee flexed to 90° as well as with the knee partially extended to place it under mild tension. Injury severity can be assessed by determining the restriction of passive knee extension while the athlete is lying supine with the hip flexed to 90°. The severity of hamstring strains varies from minor damage of a few myofibers without loss of structural integrity to complete muscle rupture.
Imaging
Similar to other muscle strains, hamstring strains are a clinical diagnosis and generally do not necessitate advanced imaging studies except to assess the degree of damage (ie, partial vs complete rupture) and to rule out other injuries, especially if the athlete fails to respond to treatment. Plain radiographs in acute cases are usually unremarkable. However, more severe injuries may go on to develop myositis ossificans similar to quadriceps soft tissue injuries (Figure 5).
Treatment
Most hamstring strains respond to conservative treatment, with operative intervention rarely indicated except for proximal or distal tendon avulsions.39 Like other muscle strains, initial management consists of RICE. COX-2-selective NSAIDs are preferred initially following injury. During a brief period of immobilization, the leg should be extended as much as tolerated to maximize muscle length, limit hematoma formation, and reduce the risk of contracture.39 Controlled mobilization should begin as soon as tolerated by the athlete.39 Isometric exercises and a stretching program should be started early in the rehabilitation period, with isotonic exercises added as motion and pain improve. Active stretching should be initiated and progressed to passive, static stretching as guided by pain.
The late phase of rehabilitation and long-term conditioning protocols should incorporate eccentric training once the athlete is pain-free, performing isotonic and isokinetic exercises. Eccentric exercises best strengthen the hamstrings at their most susceptible point, prepares the athlete for functional activities, and minimizes the risk of reinjury,3,50,51 Elliot and colleagues3 reported an order of magnitude decrease in hamstring injuries in high-risk athletes with identifiable hamstring muscle weakness after implementing an eccentric strengthening program and progressive sprint training. Similarly, in a large cohort of elite soccer players, correction of strength deficits in players with prior hamstring injuries led to similar rates of injury compared to athletes without strength deficits or prior injury.52 Those athletes with persistent weakness who did not undergo rehabilitation had significantly higher rates of reinjury.
Various injections containing local anesthetics, corticosteroids, platelet-rich plasma (PRP), and other substances have been administered to football players following acute muscle strains in an effort to alleviate pain and safely return the athlete to competition. Some practitioners have been reluctant to administer injections (especially those containing corticosteroids) due to a potentially increased risk of tendinopathy or rupture.31 Drakos and colleagues53 reported their outcomes following muscle and ligament strains treated with combined corticosteroid and local anesthetic injections on one NFL team. While quadriceps and hamstring strains were associated with the most missed games among all muscle strains, these injections resulted in no adverse events or progression of injury severity. Similarly, Levine and colleagues 51 administered intramuscular corticosteroid injections to 58 NFL players with high-grade hamstring injuries that had a palpable defect within the muscle belly. They reported no complications or strength deficits at final examination. In a case-control study, Rettig and colleagues46 administered PRP injections under ultrasound guidance in 5 NFL players with hamstring injuries. Compared to players treated with a focused rehabilitation program only, there were no significant differences in recovery or return to play.
The decision to return to play should be based on a clinical assessment considering pain, strength, motion, and flexibility. Player position should also be considered. Return-to-play guidelines describing the appropriate progression through rehabilitation and return to sport have been described and can be used as a template for the rehabilitation of football players.54 It should be noted that primary hamstring strains are associated with decreased athletic performance and an increased risk of more severe reinjury after return to sport.55,56
Morel-Lavallée Lesion
Pathophysiology
Morel-Lavallée lesions (MLLs) are uncommon football injuries, but often occur in the thigh.57,58 An MLL is a posttraumatic soft tissue injury in which deforming forces of pressure and shear cause a closed, soft tissue degloving injury; in this injury, the skin and subcutaneous tissues are separated from the underlying fascia, disrupting perforating blood vessels. The resulting space between the fascia and subcutaneous tissue fills with blood, lymphatics, and necrotic fat, resulting in a hematoma/seroma that can be a nidus for bacterial infection.58 The most common anatomic regions are the anterior distal thigh and lateral hip. Both of these areas are commonly involved in both direct contact and shear forces following a fall to the ground.
History and Physical Examination
Athletes with MLLs typically present with the insidious onset of a fluid collection within the thigh following a fall to the ground, usually while sliding or diving on the playing surface.57,58 The fluid collection can be associated with thigh tightness and may extend distally into the suprapatellar region or proximally over the greater trochanter. Thigh swelling, ecchymosis, and palpable fluctuance are seen in most cases. Progressive increases in pain and thigh swelling may be seen in severe injuries, but thigh compartments generally remain soft and nontender. Signs and symptoms of an MLL do not typically manifest immediately following the athletic event. Tejwani and colleagues58 reported a case series of MLLs of the knee in 27 NFL players from a single team over a 14-year period, with an average of 3 days between injury and evaluation by the medical staff. The mechanism of injury was a shearing blow from the knee striking the playing surface in 81% of cases and direct contact to the knee from another player in 19% of cases; all cases occurred in game situations. No affected players were wearing kneepads at the time of injury.
Imaging
Plain radiography may reveal a noncalcified soft tissue mass over the involved area and is not usually helpful except to rule out an underlying fracture. The appearance of an MLL on ultrasound is nonspecific and variable, often described as anechoic, hypoechoic, or hyperechoic depending on the presence of hemolymphatic fluid sedimentation and varying amounts of internal fat debris. MRI is the imaging modality of choice and typically shows a well-defined oval or fusiform, fluid-filled mass with tapering margins blending with adjacent fascial planes.
Treatment
Similar to quadriceps contusions, treatment goals for MLLs are evacuation of the fluid collection, prevention of fluid recurrence, a full range of active knee flexion, and prompt return to play.57,58 Initial treatment for smaller lesions consists of cryotherapy, compression wrapping of the involved area, and immediate active and passive range of motion of the hip and knee. While MLLs were traditionally treated with serial open debridements, less invasive approaches—including elastic compression, aspiration, percutaneous irrigation with debridement and suction drainage, or liposuction and drainage followed by suction therapy—have been recently described.57,58,60,61 Less invasive approaches aim to minimize soft tissue dissection and disruption of the vascular supply while accelerating rehabilitation. The presence of a surrounding capsule on MRI makes conservative or minimally invasive approaches less likely to be successful and may necessitate an open procedure.62 Antibiotics should be used preoperatively due to the presence of a dead space containing necrotic debris that makes infection a potential complication. While elite contact athletes can expect to return to competition long before complete resolution of an MLL, there is a risk of further delamination and lesion expansion due to re-injury prior to compete healing.
Tejwani and colleagues58 performed aspiration at the area of palpable fluctuance in the thigh or suprapatellar region using a 14-gauge needle in those athletes who failed to improve with conservative treatments alone. Mean time to resolution of the fluid collection was 16 days following aspiration. Fifty-two percent of the athletes were successfully treated with cryotherapy, compression, and motion exercises alone; 48% were treated with at least one aspiration, with a mean of 2.7 aspirations per knee. In 11% of cases that failed to resolve after multiple aspirations, doxycycline sclerodesis was performed immediately following an aspiration. Patients treated with sclerodesis had no return of the fluid collection and returned to play the following day.
Matava and colleagues57 described the case of an NFL player who sustained a closed MLL of the lateral hip while diving onto an artificial turf surface attempting to catch a pass. Despite immediate thigh pain and swelling, he was able to continue play. Immediately following the game, the player was examined and had a tense thigh with ecchymosis extending into the trochanteric region. Aspiration of the fluctuant area was unsuccessful. Progressive increases in pain and thigh swelling prompted hospital admission. Percutaneous irrigation and debridement was performed as described by Tseng and Tornetta.61 A suction drain was placed within the residual dead space, and constant wall suction was applied in addition to hip compression using a spica
Conclusion
Quadriceps and hamstring injuries occur frequently in football and are generally treated conservatively. While return to competition following hamstring strains is relatively quick, a high rate of injury recurrence highlights the importance of targeted rehabilitation and conditioning. Rarely, complications from quadriceps contusions, including acute compartment syndrome and myositis ossificans, may require operative intervention if unresponsive to conservative treatment. MLLs are rare in sports, but usually involve the thigh when they occur in football players. Team physicians must maintain a heightened degree of awareness of this injury as it may require operative intervention.
Acknowledgements: The authors would like to thank Jonathon Baker, MD and David Rubin, MD for their assistance in providing radiographic images for this paper.
Am J Orthop. 2016;45(6):E308-E318. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
2. Rechel JA, Yard EE, Comstock RD. An epidemiologic comparison of high school sports injuries sustained in practice and competition. J Athl Train. 2008;43(2):197-204.
3. Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39(4):843-850.
4. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
5. Feeley BT, Kennelly S, Barnes RP, et al. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med. 2008;36(8):1597-1603.
6. Garrett WE Jr. Muscle strain injuries. Am J Sports Med. 1996;24(6 Suppl):S2-S8.
7. Lawrence DW, Hutchison MG, Comper P. Descriptive epidemiology of musculoskeletal injuries and concussions in the National Football League, 2012-2014. Orthop J Sports Med. 2015;3(5):2325967115583653.
8. Diaz JA, Fischer DA, Rettig AC, Davis TJ, Shelbourne KD. Severe quadriceps muscle contusions in athletes. A report of three cases. Am J Sports Med. 2003;31(2):289-293.
9. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
10. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
11. Bencardino JT, Rosenberg ZS, Brown RR, Hassankhani A, Lustrin ES, Beltran J. Traumatic musculotendinous injuries of the knee: diagnosis with MR imaging. Radiographics. 2000;20 Spec No:S103-S120.
12. Robinson D, On E, Halperin N. Anterior compartment syndrome of the thigh in athletes--indications for conservative treatment. J Trauma. 1992;32(2):183-186.
13. Beckmann JT, Wylie JD, Kapron AL, Hanson JA, Maak TG, Aoki SK. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med. 2014;42(6):1359-1364.
14. Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43(3):346-353.
15. Beckmann JT, Wylie JD, Potter MQ, Maak TG, Greene TH, Aoki SK. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double-blind randomized placebo-controlled trial. J Bone Joint Surg Am. 2015;97(24):2032-2037.
16. Yeung M, Jamshidi S, Horner N, Simunovic N, Karlsson J, Ayeni OR. Efficacy of nonsteroidal anti-inflammatory drug prophylaxis for heterotrophic ossification in hip arthroscopy: a systematic review. Arthroscopy. 2016;32(3):519-525.
17. Goyal K, Pettis CR, Bancroft AE, Wasyliw CW, Scherer KF. Myositis ossificans in the thigh of a lacrosse player. Orthopedics. 2015;38(8):468,515-518.
18. Cooper DE. Severe quadriceps muscle contusions in athletes. Am J Sports Med. 2004;32(3):820.
19. Bonsell S, Freudigman PT, Moore HA. Quadriceps muscle contusion resulting in osteomyelitis of the femur in a high school football player. A case report. Am J Sports Med. 2001;29(6):818-820.
20. Rothwell AG. Quadriceps hematoma. A prospective clinical study. Clin Orthop Relat Res. 1982;(171):97-103.
21. Armfield DR, Kim DH, Towers JD, Bradley JP, Robertson DD. Sports-related muscle injury in the lower extremity. Clin Sports Med. 2006;25(4):803-842.
22. Lipscomb AB, Thomas ED, Johnston RK. Treatment of myositis ossificans traumatica in athletes. Am J Sports Med. 1976;4(3):111-120.
23. Mani-Babu S, Wolman R, Keen R. Quadriceps traumatic myositis ossificans in a football player: management with intravenous pamidronate. Clin J Sport Med. 2014;24(5):e56-e58.
24. McCaffrey DD, Clarke J, Bunn J, McCormack MJ. Acute compartment syndrome of the anterior thigh in the absence of fracture secondary to sporting trauma. J Trauma. 2009;66(4):1238-1242.
25. Klasson SC, Vander Schilden JL. Acute anterior thigh compartment syndrome complicating quadriceps hematoma. Two case reports and review of the literature. Orthop Rev. 1990;19(5):421-427.
26. Rooser B. Quadriceps contusion with compartment syndrome. Evacuation of hematoma in 2 cases. Acta Orthop Scand. 1987;58(2):170-172.
27. Rooser B, Bengtson S, Hagglund G. Acute compartment syndrome from anterior thigh muscle contusion: a report of eight cases. J Orthop Trauma. 1991;5(1):57-59.
28. Schwartz JT Jr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh. A spectrum of injury. J Bone Joint Surg Am. 1989;71(3):392-400.
29. Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625-632.
30. Kary JM. Diagnosis and management of quadriceps strains and contusions. Curr Rev Musculoskelet Med. 2010;3(1-4):26-31.
31. Boublik M, Schlegel TF, Koonce RC, Genuario JW, Kinkartz JD. Quadriceps tendon injuries in national football league players. Am J Sports Med. 2013;41(8):1841-1846.
32. Palmer WE, Kuong SJ, Elmadbouh HM. MR imaging of myotendinous strain. AJR Am J Roentgenol. 1999;173(3):703-709.
33. Cross TM, Gibbs N, Houang MT, Cameron M. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32(3):710-719.
34. Hughes C 4th, Hasselman CT, Best TM, Martinez S, Garrett WE Jr. Incomplete, intrasubstance strain injuries of the rectus femoris muscle. Am J Sports Med. 1995;23(4):500-506.
35. Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 2001;29(3):300-303.36. Mair SD, Seaber AV, Glisson RR, Garrett WE, Jr. The role of fatigue in susceptibility to acute muscle strain injury. Am J Sports Med. 1996;24(2):137-143.
37. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261.
38. Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-764.
39. Clanton TO, Coupe KJ. Hamstring strains in athletes: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(4):237-248.
40. Novacheck TF. The biomechanics of running. Gait Posture. 1998;7(1):77-95.
41. Yu B, Queen RM, Abbey AN, Liu Y, Moorman CT, Garrett WE. Hamstring muscle kinematics and activation during overground sprinting. J Biomech. 2008;41(15):3121-3126.
42. Opar DA, Williams MD, Shield AJ. Hamstring strain injuries: factors that lead to injury and re-injury. Sports Med. 2012;42(3):209-226.
43. Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35(2):197-206.
44. Thelen DG, Chumanov ES, Hoerth DM, et al. Hamstring muscle kinematics during treadmill sprinting. Med Sci Sports Exerc. 2005;37(1):108-114.
45. Chumanov ES, Heiderscheit BC, Thelen DG. The effect of speed and influence of individual muscles on hamstring mechanics during the swing phase of sprinting. J Biomech. 2007;40(16):3555-3562.
46. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
47. Zvijac JE, Toriscelli TA, Merrick S, Kiebzak GM. Isokinetic concentric quadriceps and hamstring strength variables from the NFL Scouting Combine are not predictive of hamstring injury in first-year professional football players. Am J Sports Med. 2013;41(7):1511-1518.
48. Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl):5S-16S.
49. Zarins B, Ciullo JV. Acute muscle and tendon injuries in athletes. Clin Sports Med. 1983;2(1):167-182.
50. Arnason A, Andersen TE, Holme I, Engebretsen L, Bahr R. Prevention of hamstring strains in elite soccer: an intervention study. Scand J Med Sci Sports. 2008;18(1):40-48.
51. Levine WN, Bergfeld JA, Tessendorf W, Moorman CT 3rd. Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28(3):297-300.
52. Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med. 2008;36(8):1469-1475.
53. Drakos M, Birmingham P, Delos D, et al. Corticosteroid and anesthetic injections for muscle strains and ligament sprains in the NFL. HSS J. 2014;10(2):136-142.
54. Worrell TW. Factors associated with hamstring injuries. An approach to treatment and preventative measures. Sports Med. 1994;17(5):338-345.
55. Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34(8):1297-1306.
56. Verrall GM, Kalairajah Y, Slavotinek JP, Spriggins AJ. Assessment of player performance following return to sport after hamstring muscle strain injury. J Sci Med Sport. 2006;9(1-2):87-90.
57. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-lavallee lesion in a professional american football player. Am J Orthop. 2010;39(3):144-147.
58. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med. 2007;35(7):1162-1167.
59. Mellado JM, Bencardino JT. Morel-Lavallee lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am. 2005;13(4):775-782.
60. Harma A, Inan M, Ertem K. [The Morel-Lavallee lesion: a conservative approach to closed degloving injuries]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
61. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
62. Gilbert BC, Bui-Mansfield LT, Dejong S. MRI of a Morel-Lavellee lesion. AJR Am J Roentgenol. 2004;182(5):1347-1348.
American football has the highest injury rate of any team sport in the United States at the high school, collegiate, and professional levels.1-3 Muscle strains and contusions constitute a large proportion of football injuries. For example, at the high school level, muscle strains comprise 12% to 24% of all injuries;2 at the collegiate level, they account for approximately 20% of all practice injuries, with nearly half of all strains occurring within the thigh.1,4 Among a single National Football League (NFL) team, Feeley and colleagues5 reported that muscle strains accounted for 46% of practice and 22% of preseason game injuries. The hamstrings, followed by the quadriceps, are the most commonly strained muscle groups among both professional and amateur athletes,5,6 with hamstring and quadriceps injuries making up approximately 13% of all injuries among NFL players.7 Given the relatively large surface area and muscle volume of the anterior and posterior thigh, as well as the activities and maneuvers necessitated by the various football positions, it is not surprising that the thigh is frequently involved in football-related injuries.
The purpose of this review is to describe the clinical manifestations of thigh-related soft-tissue injuries seen in football players. Two of these conditions—muscle strains and contusions—are relatively common, while a third condition—the Morel-Lavallée lesion—is a rare, yet relevant injury that warrants discussion.
Quadriceps Contusion
Pathophysiology
Contusion to the quadriceps muscle is a common injury in contact sports generally resulting from a direct blow from a helmet, knee, or shoulder.8 Bleeding within the musculature causes swelling, pain, stiffness, and limitation of quadriceps excursion, ultimately resulting in loss of knee flexion and an inability to run or squat. The injury is typically confined to a single quadriceps muscle.8 The use of thigh padding, though helpful, does not completely eliminate the risk of this injury.
History and Physical Examination
Immediately after injury, the athlete may complain only of thigh pain. However, swelling, pain, and diminished range of knee motion may develop within the first 24 hours depending on the severity of injury and how quickly treatment is instituted.8 Jackson and Feagin9 developed an injury grading system for quadriceps contusions based on the limitation of knee flexion observed (Table 1).
Imaging
A quadriceps contusion is a clinical diagnosis based on a typical history and physical examination; therefore, advanced imaging usually does not need to be obtained except to gauge the severity of injury, to rule out concurrent injuries (ie, tendon rupture), and to identify the presence of a hematoma that may necessitate aspiration. Plain radiographs are typically unremarkable in the acute setting. Appearance on magnetic resonance imaging (MRI) varies by injury severity, with increased signal throughout the affected muscle belly and a diffuse, feathery appearance centered at the point of impact on short TI inversion recovery (STIR) and T2-weighted images reflecting edema and possibly hematoma (Figures 1A-1C).8,11
Treatment
Treatment of a quadriceps contusion is nonoperative and consists of a 3-phase recovery.10 The first phase lasts approximately 2 days and consists of rest, ice, compression, and elevation (RICE) to limit hemorrhage. The knee should be rested in a flexed position to maintain quadriceps muscle fiber length in order to promote muscle compression and limit knee stiffness. For severe contusions in which there is a question of an acute thigh compartment syndrome, compression should be avoided with appropriate treatment based on typical symptoms and intra-compartmental pressure measurement.12 Nonsteroidal anti-inflammatory drugs (NSAIDs) may be administered to diminish pain as well as the risk of myositis ossificans. While there is no data on the efficacy of NSAIDs in preventing myositis ossificans following quadriceps contusions, both COX-2 selective (ie, celecoxib) and nonselective (ie, naproxen, indomethacin) COX inhibitors have been demonstrated to significantly reduce the incidence of heterotopic ossification following hip surgery—a condition occurring from a similar pathophysiologic process as myositis ossificans.13-17 However, this class of drugs should not be given any sooner than 48 to 72 hours after injury to decrease further bleeding risk, given its inhibitory effect on platelet function.18 Narcotic pain medications are rarely required.
The second phase focuses on restoring active and passive knee and hip flexion and begins when permitted by pain.8 Icing, pain control, and physical therapy modalities are also continued in order to reduce pain and swelling as knee motion is progressed. The third phase begins once full range of knee and hip motion is restored and consists of quadriceps strengthening and functional rehabilitation of the lower extremity.8,19 Return to athletic activities and eventually competition should take place when a full, painless range of motion is restored and strength returns to baseline. Isokinetic strength testing may be utilized to more accurately assess strength and endurance. Noncontact, position-specific drills are incorporated as clinical improvement allows. A full recovery should be expected within 4 weeks of injury, with faster resolution and return to play seen in less severe contusions depending on the athlete’s position.8 Continued quadriceps stretching is recommended to prevent recurrence once the athlete returns to play. A protective hard shell may also be utilized both during rehabilitation as well as once the athlete returns to play in order to protect the thigh from reinjury, which may increase the risk of myositis ossificans.8
Complications
A prolonged recovery or persistent symptoms should alert the treating physician to the possibility of complications, including myositis ossificans.8,20 Myositis ossificans typically results from moderate to severe contusions, which may present initially as a painful, indurated mass that later becomes quite firm. This mass may be seen on plain radiographs as early as 2 to 4 weeks following injury if the athlete complains of persistent pain or a palpable thigh mass (Figure 2).9
Mani-Babu and colleagues23 reported a case of a 14-year-old male football player who sustained a quadriceps contusion after a direct blow from an opponent’s helmet to the lateral thigh. Persistent pain and limitation of motion at 2 months follow-up prompted imaging studies that demonstrated myositis ossificans. The patient was treated with intravenous pamidronate (a bisphosphonate) twice over a 3-month period and demonstrated a full recovery within 5 months.
Acute compartment syndrome of the thigh has also been reported following severe quadriceps contusions, with the majority occurring in the anterior compartment.12,24-28 When injury from blunt trauma extends into and disrupts the muscular layer adjacent to the femur, vascular disruption can cause hematoma formation, muscle edema, and significant swelling, thereby increasing intracompartmental pressure. The relatively large volume of the anterior thigh compartment and lack of a rigid deep fascial envelope may be protective from the development of compartment syndrome compared to other sites.28 It can be difficult to distinguish a severe contusion from a compartment syndrome, as both can occur from the same mechanism and have similar presenting signs and symptoms. Signs of a compartment syndrome include pain out of proportion to the injury that is aggravated by passive stretch of the quadriceps muscles, an increasingly firm muscle compartment to palpation, and neurovascular deficits.29 Both acute compartment syndrome and a severe contusion may present with significant pain, inability to bear weight, tense swelling, tenderness to palpation, and pain with passive knee flexion.24 While the successful conservative treatment of athletes with acute compartment syndrome of the thigh has been reported, it is important to closely monitor the patient’s condition and consider intracompartmental pressure monitoring if the patient’s clinical condition deteriorates.12 An acute fasciotomy should be strongly considered when intracompartmental pressures are within 30 mm Hg of diastolic pressure.24-27 Fortunately, it is highly uncommon for thigh compartment pressure to rise to this level. Percutaneous compartment decompression using liposuction equipment or a large cannula has been described to decrease intracompartmental pressure, potentially expediting recovery and minimizing morbidity.18 Interestingly, reports of fasciotomies for acute thigh compartment syndrome following closed athletic injuries have not described necrotic or non-contractile muscle typical of an acute compartment syndrome, calling into question the need for fasciotomy following closed blunt athletic trauma to the thigh.18
Quadriceps Strain
Pathophysiology
Acute quadriceps strains occur during sudden forceful eccentric contraction of the extensor mechanism. Occasionally, in the absence of a clear mechanism, these injuries mistakenly appear as a contusion resulting from a direct blow to the thigh.30,31 The rectus femoris is the most frequently strained quadriceps muscle due, in part, to its superficial location and predominance of type II muscle fibers, which are more likely to be strained.11,32 Although classically described as occurring along the distal portion of the rectus femoris at the musculotendinous junction, quadriceps strains most commonly occur at the mid to proximal aspect of the rectus femoris.30,33 The quadriceps muscle complex crosses 2 joints and, as a result, is more predisposed to eccentric injury than mono-articular muscles.34 We have had a subset of complete myotendinous tears of the rectus femoris that occur in the plant leg of placekickers that result in significant disability.
Risk Factors
Quadriceps and thigh injuries comprise approximately 4.5% of injuries among NFL players.7 Several risk factors for quadriceps strains have been described. In a study of Australian Rules football players, Orchard35 demonstrated that for all muscle strains, the strongest risk factor was a recent history of the same injury, with the next strongest risk factor being a past history of the same injury. Increasing age was found to be a risk factor for hamstring strains but not quadriceps strains. Muscle fatigue may also contribute to injury susceptibility.36
History and Physical Examination
Injuries typically occur during kicking, jumping, or a sudden change in direction while running.30 Athletes may localize pain anywhere along the quadriceps muscle, although strains most commonly occur at the proximal to mid portion of the rectus femoris.30,33 The grading system for quadriceps strains described by Kary30 is based on level of pain, quadriceps strength, and the presence or absence of a palpable defect (Table 2).
The athlete typically walks with an antalgic gait. Visible swelling and/or ecchymosis may be present depending on when the athlete is seen, as ecchymosis may develop within the first 24 hours of injury. The examiner should palpate along the entire length of the injured muscle. High-grade strains or complete tears may present with a bulge or defect in the muscle belly, but in most cases no defect will be palpable. There may be loss of knee flexion similar to a quadriceps contusion. Strength testing should be performed in both the sitting and prone position with the hip both flexed and extended to assess resisted knee extension strength.30 Loss of strength is proportional to the degree of injury.
Imaging
While most quadriceps strains are adequately diagnosed clinically without the need for imaging studies, ultrasound or MRI can be used to evaluate for partial or complete rupture.30,33 In milder cases, MRI usually demonstrates interstitial edema and hemorrhage with a feathery appearance on STIR and T2-weighted imaging (Figures 3A-3C).11
Treatment
Acute treatment of quadriceps strains focuses on minimizing bleeding using the principles of RICE treatment.37 NSAIDs may be used immediately to assist with pain control.30 COX-2-specific NSAIDs are preferred due to their lack of any inhibitory effect on platelet function in order to reduce the risk of further bleeding within the muscle compartment. For the first 24 to 72 hours following injury, the quadriceps should be maintained relatively immobilized to prevent further injury.38 High-grade injuries might necessitate crutches for ambulatory assistance.
Depending on injury severity, the active phase of treatment usually begins within 5 days of injury and consists of stretching and knee/hip range of motion. An active warm-up should precede rehabilitation exercises to activate neural pathways within the muscle and improve muscle elasticity.38 Ballistic stretching should be avoided to prevent additional injury to the muscle fibers. Strengthening should proceed when the athlete recovers a pain-free range of motion. When isometric exercises can be completed at increasing degrees of knee flexion, isotonic exercises may be implemented into the rehabilitation program.30 Return to football can be considered when the athlete has recovered knee and hip range of motion, is pain-free, and has near-normal strength compared to the contralateral side. The athlete should also perform satisfactorily in simulated position-specific activities in a noncontact fashion prior to return to full competition.30
Hamstring Strain
Pathophysiology
Hamstring strains are the most common noncontact injuries in football resulting from excessive muscle stretching during eccentric contraction generally occurring at the musculotendinous junction.5,39 Because the hamstrings cross both the hip and knee, simultaneous hip flexion and knee extension results in maximal lengthening, making them most vulnerable to injury at the terminal swing phase of gait just prior to heel strike.39-42 The long head of the biceps femoris undergoes the greatest stretch, reaching 110% of resting length during terminal swing phase and is the most commonly injured hamstring muscle.43,44 Injury occurs when the force of eccentric contraction, and resulting muscle strain, exceeds the mechanical limits of the tissue.42,45 It remains to be shown whether hamstring strains occur as a result of accumulated microscopic muscle damage or secondary to a single event that exceeds the mechanical limits of the muscle.42
Epidemiology and Risk Factors
The majority of hamstring strains are sustained during noncontact activities, with most athletes citing sprinting as the activity at the time of injury.3 Approximately 93% of injuries occur during noncontact activities among defensive backs and wide receivers.3 Hamstring strains are the second-most common injury among NFL players, comprising approximately 9% of all injuries,5,7 with 16% to 31% of these injuries associated with recurrence.3,5,35,46 Using the NFL’s Injury Surveillance System, Elliott and colleagues3 reported 1716 hamstring strains over a 10-year period (1989-1998). Fifty-one percent of hamstring strains occurred during the 7-week preseason, with a greater than 4-fold increased injury rate noted during the preseason compared to the 16-week regular season. An increased incidence in the preseason is partially attributable to relative deconditioning over the offseason. Defensive backs, wide receivers, and special teams players accounted for the majority of injured players, suggesting that speed position players and those who must “backpedal” (run backwards) are at an increased risk for injury.
Several risk factors for hamstring strain have been described, including prior injury, older age, quadriceps-hamstring strength imbalances, limited hip and knee flexibility, and fatigue.39,42,47 Inadequate rehabilitation and premature return to competition are also likely important factors predisposing to recurrent injury.39,48
History and Physical Examination
The majority of hamstring strains occur in the acute setting when the player experiences the sudden onset of pain in the posterior thigh during strenuous exercise, most commonly while sprinting.39 The injury typically occurs in the early or late stage of practice or competition due, in part, to inadequate warm-up or fatigue. The athlete may describe an audible pop and an inability to continue play, depending on injury severity.
Physical examination may demonstrate palpable induration and tenderness immediately or shortly after injury. In the setting of severe strains, there can be significant thigh swelling and ecchymosis, and in complete ruptures, a palpable defect.39 The affected muscle should be palpated along its entire length, and is best performed prone with the knee flexed to 90° as well as with the knee partially extended to place it under mild tension. Injury severity can be assessed by determining the restriction of passive knee extension while the athlete is lying supine with the hip flexed to 90°. The severity of hamstring strains varies from minor damage of a few myofibers without loss of structural integrity to complete muscle rupture.
Imaging
Similar to other muscle strains, hamstring strains are a clinical diagnosis and generally do not necessitate advanced imaging studies except to assess the degree of damage (ie, partial vs complete rupture) and to rule out other injuries, especially if the athlete fails to respond to treatment. Plain radiographs in acute cases are usually unremarkable. However, more severe injuries may go on to develop myositis ossificans similar to quadriceps soft tissue injuries (Figure 5).
Treatment
Most hamstring strains respond to conservative treatment, with operative intervention rarely indicated except for proximal or distal tendon avulsions.39 Like other muscle strains, initial management consists of RICE. COX-2-selective NSAIDs are preferred initially following injury. During a brief period of immobilization, the leg should be extended as much as tolerated to maximize muscle length, limit hematoma formation, and reduce the risk of contracture.39 Controlled mobilization should begin as soon as tolerated by the athlete.39 Isometric exercises and a stretching program should be started early in the rehabilitation period, with isotonic exercises added as motion and pain improve. Active stretching should be initiated and progressed to passive, static stretching as guided by pain.
The late phase of rehabilitation and long-term conditioning protocols should incorporate eccentric training once the athlete is pain-free, performing isotonic and isokinetic exercises. Eccentric exercises best strengthen the hamstrings at their most susceptible point, prepares the athlete for functional activities, and minimizes the risk of reinjury,3,50,51 Elliot and colleagues3 reported an order of magnitude decrease in hamstring injuries in high-risk athletes with identifiable hamstring muscle weakness after implementing an eccentric strengthening program and progressive sprint training. Similarly, in a large cohort of elite soccer players, correction of strength deficits in players with prior hamstring injuries led to similar rates of injury compared to athletes without strength deficits or prior injury.52 Those athletes with persistent weakness who did not undergo rehabilitation had significantly higher rates of reinjury.
Various injections containing local anesthetics, corticosteroids, platelet-rich plasma (PRP), and other substances have been administered to football players following acute muscle strains in an effort to alleviate pain and safely return the athlete to competition. Some practitioners have been reluctant to administer injections (especially those containing corticosteroids) due to a potentially increased risk of tendinopathy or rupture.31 Drakos and colleagues53 reported their outcomes following muscle and ligament strains treated with combined corticosteroid and local anesthetic injections on one NFL team. While quadriceps and hamstring strains were associated with the most missed games among all muscle strains, these injections resulted in no adverse events or progression of injury severity. Similarly, Levine and colleagues 51 administered intramuscular corticosteroid injections to 58 NFL players with high-grade hamstring injuries that had a palpable defect within the muscle belly. They reported no complications or strength deficits at final examination. In a case-control study, Rettig and colleagues46 administered PRP injections under ultrasound guidance in 5 NFL players with hamstring injuries. Compared to players treated with a focused rehabilitation program only, there were no significant differences in recovery or return to play.
The decision to return to play should be based on a clinical assessment considering pain, strength, motion, and flexibility. Player position should also be considered. Return-to-play guidelines describing the appropriate progression through rehabilitation and return to sport have been described and can be used as a template for the rehabilitation of football players.54 It should be noted that primary hamstring strains are associated with decreased athletic performance and an increased risk of more severe reinjury after return to sport.55,56
Morel-Lavallée Lesion
Pathophysiology
Morel-Lavallée lesions (MLLs) are uncommon football injuries, but often occur in the thigh.57,58 An MLL is a posttraumatic soft tissue injury in which deforming forces of pressure and shear cause a closed, soft tissue degloving injury; in this injury, the skin and subcutaneous tissues are separated from the underlying fascia, disrupting perforating blood vessels. The resulting space between the fascia and subcutaneous tissue fills with blood, lymphatics, and necrotic fat, resulting in a hematoma/seroma that can be a nidus for bacterial infection.58 The most common anatomic regions are the anterior distal thigh and lateral hip. Both of these areas are commonly involved in both direct contact and shear forces following a fall to the ground.
History and Physical Examination
Athletes with MLLs typically present with the insidious onset of a fluid collection within the thigh following a fall to the ground, usually while sliding or diving on the playing surface.57,58 The fluid collection can be associated with thigh tightness and may extend distally into the suprapatellar region or proximally over the greater trochanter. Thigh swelling, ecchymosis, and palpable fluctuance are seen in most cases. Progressive increases in pain and thigh swelling may be seen in severe injuries, but thigh compartments generally remain soft and nontender. Signs and symptoms of an MLL do not typically manifest immediately following the athletic event. Tejwani and colleagues58 reported a case series of MLLs of the knee in 27 NFL players from a single team over a 14-year period, with an average of 3 days between injury and evaluation by the medical staff. The mechanism of injury was a shearing blow from the knee striking the playing surface in 81% of cases and direct contact to the knee from another player in 19% of cases; all cases occurred in game situations. No affected players were wearing kneepads at the time of injury.
Imaging
Plain radiography may reveal a noncalcified soft tissue mass over the involved area and is not usually helpful except to rule out an underlying fracture. The appearance of an MLL on ultrasound is nonspecific and variable, often described as anechoic, hypoechoic, or hyperechoic depending on the presence of hemolymphatic fluid sedimentation and varying amounts of internal fat debris. MRI is the imaging modality of choice and typically shows a well-defined oval or fusiform, fluid-filled mass with tapering margins blending with adjacent fascial planes.
Treatment
Similar to quadriceps contusions, treatment goals for MLLs are evacuation of the fluid collection, prevention of fluid recurrence, a full range of active knee flexion, and prompt return to play.57,58 Initial treatment for smaller lesions consists of cryotherapy, compression wrapping of the involved area, and immediate active and passive range of motion of the hip and knee. While MLLs were traditionally treated with serial open debridements, less invasive approaches—including elastic compression, aspiration, percutaneous irrigation with debridement and suction drainage, or liposuction and drainage followed by suction therapy—have been recently described.57,58,60,61 Less invasive approaches aim to minimize soft tissue dissection and disruption of the vascular supply while accelerating rehabilitation. The presence of a surrounding capsule on MRI makes conservative or minimally invasive approaches less likely to be successful and may necessitate an open procedure.62 Antibiotics should be used preoperatively due to the presence of a dead space containing necrotic debris that makes infection a potential complication. While elite contact athletes can expect to return to competition long before complete resolution of an MLL, there is a risk of further delamination and lesion expansion due to re-injury prior to compete healing.
Tejwani and colleagues58 performed aspiration at the area of palpable fluctuance in the thigh or suprapatellar region using a 14-gauge needle in those athletes who failed to improve with conservative treatments alone. Mean time to resolution of the fluid collection was 16 days following aspiration. Fifty-two percent of the athletes were successfully treated with cryotherapy, compression, and motion exercises alone; 48% were treated with at least one aspiration, with a mean of 2.7 aspirations per knee. In 11% of cases that failed to resolve after multiple aspirations, doxycycline sclerodesis was performed immediately following an aspiration. Patients treated with sclerodesis had no return of the fluid collection and returned to play the following day.
Matava and colleagues57 described the case of an NFL player who sustained a closed MLL of the lateral hip while diving onto an artificial turf surface attempting to catch a pass. Despite immediate thigh pain and swelling, he was able to continue play. Immediately following the game, the player was examined and had a tense thigh with ecchymosis extending into the trochanteric region. Aspiration of the fluctuant area was unsuccessful. Progressive increases in pain and thigh swelling prompted hospital admission. Percutaneous irrigation and debridement was performed as described by Tseng and Tornetta.61 A suction drain was placed within the residual dead space, and constant wall suction was applied in addition to hip compression using a spica
Conclusion
Quadriceps and hamstring injuries occur frequently in football and are generally treated conservatively. While return to competition following hamstring strains is relatively quick, a high rate of injury recurrence highlights the importance of targeted rehabilitation and conditioning. Rarely, complications from quadriceps contusions, including acute compartment syndrome and myositis ossificans, may require operative intervention if unresponsive to conservative treatment. MLLs are rare in sports, but usually involve the thigh when they occur in football players. Team physicians must maintain a heightened degree of awareness of this injury as it may require operative intervention.
Acknowledgements: The authors would like to thank Jonathon Baker, MD and David Rubin, MD for their assistance in providing radiographic images for this paper.
Am J Orthop. 2016;45(6):E308-E318. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
American football has the highest injury rate of any team sport in the United States at the high school, collegiate, and professional levels.1-3 Muscle strains and contusions constitute a large proportion of football injuries. For example, at the high school level, muscle strains comprise 12% to 24% of all injuries;2 at the collegiate level, they account for approximately 20% of all practice injuries, with nearly half of all strains occurring within the thigh.1,4 Among a single National Football League (NFL) team, Feeley and colleagues5 reported that muscle strains accounted for 46% of practice and 22% of preseason game injuries. The hamstrings, followed by the quadriceps, are the most commonly strained muscle groups among both professional and amateur athletes,5,6 with hamstring and quadriceps injuries making up approximately 13% of all injuries among NFL players.7 Given the relatively large surface area and muscle volume of the anterior and posterior thigh, as well as the activities and maneuvers necessitated by the various football positions, it is not surprising that the thigh is frequently involved in football-related injuries.
The purpose of this review is to describe the clinical manifestations of thigh-related soft-tissue injuries seen in football players. Two of these conditions—muscle strains and contusions—are relatively common, while a third condition—the Morel-Lavallée lesion—is a rare, yet relevant injury that warrants discussion.
Quadriceps Contusion
Pathophysiology
Contusion to the quadriceps muscle is a common injury in contact sports generally resulting from a direct blow from a helmet, knee, or shoulder.8 Bleeding within the musculature causes swelling, pain, stiffness, and limitation of quadriceps excursion, ultimately resulting in loss of knee flexion and an inability to run or squat. The injury is typically confined to a single quadriceps muscle.8 The use of thigh padding, though helpful, does not completely eliminate the risk of this injury.
History and Physical Examination
Immediately after injury, the athlete may complain only of thigh pain. However, swelling, pain, and diminished range of knee motion may develop within the first 24 hours depending on the severity of injury and how quickly treatment is instituted.8 Jackson and Feagin9 developed an injury grading system for quadriceps contusions based on the limitation of knee flexion observed (Table 1).
Imaging
A quadriceps contusion is a clinical diagnosis based on a typical history and physical examination; therefore, advanced imaging usually does not need to be obtained except to gauge the severity of injury, to rule out concurrent injuries (ie, tendon rupture), and to identify the presence of a hematoma that may necessitate aspiration. Plain radiographs are typically unremarkable in the acute setting. Appearance on magnetic resonance imaging (MRI) varies by injury severity, with increased signal throughout the affected muscle belly and a diffuse, feathery appearance centered at the point of impact on short TI inversion recovery (STIR) and T2-weighted images reflecting edema and possibly hematoma (Figures 1A-1C).8,11
Treatment
Treatment of a quadriceps contusion is nonoperative and consists of a 3-phase recovery.10 The first phase lasts approximately 2 days and consists of rest, ice, compression, and elevation (RICE) to limit hemorrhage. The knee should be rested in a flexed position to maintain quadriceps muscle fiber length in order to promote muscle compression and limit knee stiffness. For severe contusions in which there is a question of an acute thigh compartment syndrome, compression should be avoided with appropriate treatment based on typical symptoms and intra-compartmental pressure measurement.12 Nonsteroidal anti-inflammatory drugs (NSAIDs) may be administered to diminish pain as well as the risk of myositis ossificans. While there is no data on the efficacy of NSAIDs in preventing myositis ossificans following quadriceps contusions, both COX-2 selective (ie, celecoxib) and nonselective (ie, naproxen, indomethacin) COX inhibitors have been demonstrated to significantly reduce the incidence of heterotopic ossification following hip surgery—a condition occurring from a similar pathophysiologic process as myositis ossificans.13-17 However, this class of drugs should not be given any sooner than 48 to 72 hours after injury to decrease further bleeding risk, given its inhibitory effect on platelet function.18 Narcotic pain medications are rarely required.
The second phase focuses on restoring active and passive knee and hip flexion and begins when permitted by pain.8 Icing, pain control, and physical therapy modalities are also continued in order to reduce pain and swelling as knee motion is progressed. The third phase begins once full range of knee and hip motion is restored and consists of quadriceps strengthening and functional rehabilitation of the lower extremity.8,19 Return to athletic activities and eventually competition should take place when a full, painless range of motion is restored and strength returns to baseline. Isokinetic strength testing may be utilized to more accurately assess strength and endurance. Noncontact, position-specific drills are incorporated as clinical improvement allows. A full recovery should be expected within 4 weeks of injury, with faster resolution and return to play seen in less severe contusions depending on the athlete’s position.8 Continued quadriceps stretching is recommended to prevent recurrence once the athlete returns to play. A protective hard shell may also be utilized both during rehabilitation as well as once the athlete returns to play in order to protect the thigh from reinjury, which may increase the risk of myositis ossificans.8
Complications
A prolonged recovery or persistent symptoms should alert the treating physician to the possibility of complications, including myositis ossificans.8,20 Myositis ossificans typically results from moderate to severe contusions, which may present initially as a painful, indurated mass that later becomes quite firm. This mass may be seen on plain radiographs as early as 2 to 4 weeks following injury if the athlete complains of persistent pain or a palpable thigh mass (Figure 2).9
Mani-Babu and colleagues23 reported a case of a 14-year-old male football player who sustained a quadriceps contusion after a direct blow from an opponent’s helmet to the lateral thigh. Persistent pain and limitation of motion at 2 months follow-up prompted imaging studies that demonstrated myositis ossificans. The patient was treated with intravenous pamidronate (a bisphosphonate) twice over a 3-month period and demonstrated a full recovery within 5 months.
Acute compartment syndrome of the thigh has also been reported following severe quadriceps contusions, with the majority occurring in the anterior compartment.12,24-28 When injury from blunt trauma extends into and disrupts the muscular layer adjacent to the femur, vascular disruption can cause hematoma formation, muscle edema, and significant swelling, thereby increasing intracompartmental pressure. The relatively large volume of the anterior thigh compartment and lack of a rigid deep fascial envelope may be protective from the development of compartment syndrome compared to other sites.28 It can be difficult to distinguish a severe contusion from a compartment syndrome, as both can occur from the same mechanism and have similar presenting signs and symptoms. Signs of a compartment syndrome include pain out of proportion to the injury that is aggravated by passive stretch of the quadriceps muscles, an increasingly firm muscle compartment to palpation, and neurovascular deficits.29 Both acute compartment syndrome and a severe contusion may present with significant pain, inability to bear weight, tense swelling, tenderness to palpation, and pain with passive knee flexion.24 While the successful conservative treatment of athletes with acute compartment syndrome of the thigh has been reported, it is important to closely monitor the patient’s condition and consider intracompartmental pressure monitoring if the patient’s clinical condition deteriorates.12 An acute fasciotomy should be strongly considered when intracompartmental pressures are within 30 mm Hg of diastolic pressure.24-27 Fortunately, it is highly uncommon for thigh compartment pressure to rise to this level. Percutaneous compartment decompression using liposuction equipment or a large cannula has been described to decrease intracompartmental pressure, potentially expediting recovery and minimizing morbidity.18 Interestingly, reports of fasciotomies for acute thigh compartment syndrome following closed athletic injuries have not described necrotic or non-contractile muscle typical of an acute compartment syndrome, calling into question the need for fasciotomy following closed blunt athletic trauma to the thigh.18
Quadriceps Strain
Pathophysiology
Acute quadriceps strains occur during sudden forceful eccentric contraction of the extensor mechanism. Occasionally, in the absence of a clear mechanism, these injuries mistakenly appear as a contusion resulting from a direct blow to the thigh.30,31 The rectus femoris is the most frequently strained quadriceps muscle due, in part, to its superficial location and predominance of type II muscle fibers, which are more likely to be strained.11,32 Although classically described as occurring along the distal portion of the rectus femoris at the musculotendinous junction, quadriceps strains most commonly occur at the mid to proximal aspect of the rectus femoris.30,33 The quadriceps muscle complex crosses 2 joints and, as a result, is more predisposed to eccentric injury than mono-articular muscles.34 We have had a subset of complete myotendinous tears of the rectus femoris that occur in the plant leg of placekickers that result in significant disability.
Risk Factors
Quadriceps and thigh injuries comprise approximately 4.5% of injuries among NFL players.7 Several risk factors for quadriceps strains have been described. In a study of Australian Rules football players, Orchard35 demonstrated that for all muscle strains, the strongest risk factor was a recent history of the same injury, with the next strongest risk factor being a past history of the same injury. Increasing age was found to be a risk factor for hamstring strains but not quadriceps strains. Muscle fatigue may also contribute to injury susceptibility.36
History and Physical Examination
Injuries typically occur during kicking, jumping, or a sudden change in direction while running.30 Athletes may localize pain anywhere along the quadriceps muscle, although strains most commonly occur at the proximal to mid portion of the rectus femoris.30,33 The grading system for quadriceps strains described by Kary30 is based on level of pain, quadriceps strength, and the presence or absence of a palpable defect (Table 2).
The athlete typically walks with an antalgic gait. Visible swelling and/or ecchymosis may be present depending on when the athlete is seen, as ecchymosis may develop within the first 24 hours of injury. The examiner should palpate along the entire length of the injured muscle. High-grade strains or complete tears may present with a bulge or defect in the muscle belly, but in most cases no defect will be palpable. There may be loss of knee flexion similar to a quadriceps contusion. Strength testing should be performed in both the sitting and prone position with the hip both flexed and extended to assess resisted knee extension strength.30 Loss of strength is proportional to the degree of injury.
Imaging
While most quadriceps strains are adequately diagnosed clinically without the need for imaging studies, ultrasound or MRI can be used to evaluate for partial or complete rupture.30,33 In milder cases, MRI usually demonstrates interstitial edema and hemorrhage with a feathery appearance on STIR and T2-weighted imaging (Figures 3A-3C).11
Treatment
Acute treatment of quadriceps strains focuses on minimizing bleeding using the principles of RICE treatment.37 NSAIDs may be used immediately to assist with pain control.30 COX-2-specific NSAIDs are preferred due to their lack of any inhibitory effect on platelet function in order to reduce the risk of further bleeding within the muscle compartment. For the first 24 to 72 hours following injury, the quadriceps should be maintained relatively immobilized to prevent further injury.38 High-grade injuries might necessitate crutches for ambulatory assistance.
Depending on injury severity, the active phase of treatment usually begins within 5 days of injury and consists of stretching and knee/hip range of motion. An active warm-up should precede rehabilitation exercises to activate neural pathways within the muscle and improve muscle elasticity.38 Ballistic stretching should be avoided to prevent additional injury to the muscle fibers. Strengthening should proceed when the athlete recovers a pain-free range of motion. When isometric exercises can be completed at increasing degrees of knee flexion, isotonic exercises may be implemented into the rehabilitation program.30 Return to football can be considered when the athlete has recovered knee and hip range of motion, is pain-free, and has near-normal strength compared to the contralateral side. The athlete should also perform satisfactorily in simulated position-specific activities in a noncontact fashion prior to return to full competition.30
Hamstring Strain
Pathophysiology
Hamstring strains are the most common noncontact injuries in football resulting from excessive muscle stretching during eccentric contraction generally occurring at the musculotendinous junction.5,39 Because the hamstrings cross both the hip and knee, simultaneous hip flexion and knee extension results in maximal lengthening, making them most vulnerable to injury at the terminal swing phase of gait just prior to heel strike.39-42 The long head of the biceps femoris undergoes the greatest stretch, reaching 110% of resting length during terminal swing phase and is the most commonly injured hamstring muscle.43,44 Injury occurs when the force of eccentric contraction, and resulting muscle strain, exceeds the mechanical limits of the tissue.42,45 It remains to be shown whether hamstring strains occur as a result of accumulated microscopic muscle damage or secondary to a single event that exceeds the mechanical limits of the muscle.42
Epidemiology and Risk Factors
The majority of hamstring strains are sustained during noncontact activities, with most athletes citing sprinting as the activity at the time of injury.3 Approximately 93% of injuries occur during noncontact activities among defensive backs and wide receivers.3 Hamstring strains are the second-most common injury among NFL players, comprising approximately 9% of all injuries,5,7 with 16% to 31% of these injuries associated with recurrence.3,5,35,46 Using the NFL’s Injury Surveillance System, Elliott and colleagues3 reported 1716 hamstring strains over a 10-year period (1989-1998). Fifty-one percent of hamstring strains occurred during the 7-week preseason, with a greater than 4-fold increased injury rate noted during the preseason compared to the 16-week regular season. An increased incidence in the preseason is partially attributable to relative deconditioning over the offseason. Defensive backs, wide receivers, and special teams players accounted for the majority of injured players, suggesting that speed position players and those who must “backpedal” (run backwards) are at an increased risk for injury.
Several risk factors for hamstring strain have been described, including prior injury, older age, quadriceps-hamstring strength imbalances, limited hip and knee flexibility, and fatigue.39,42,47 Inadequate rehabilitation and premature return to competition are also likely important factors predisposing to recurrent injury.39,48
History and Physical Examination
The majority of hamstring strains occur in the acute setting when the player experiences the sudden onset of pain in the posterior thigh during strenuous exercise, most commonly while sprinting.39 The injury typically occurs in the early or late stage of practice or competition due, in part, to inadequate warm-up or fatigue. The athlete may describe an audible pop and an inability to continue play, depending on injury severity.
Physical examination may demonstrate palpable induration and tenderness immediately or shortly after injury. In the setting of severe strains, there can be significant thigh swelling and ecchymosis, and in complete ruptures, a palpable defect.39 The affected muscle should be palpated along its entire length, and is best performed prone with the knee flexed to 90° as well as with the knee partially extended to place it under mild tension. Injury severity can be assessed by determining the restriction of passive knee extension while the athlete is lying supine with the hip flexed to 90°. The severity of hamstring strains varies from minor damage of a few myofibers without loss of structural integrity to complete muscle rupture.
Imaging
Similar to other muscle strains, hamstring strains are a clinical diagnosis and generally do not necessitate advanced imaging studies except to assess the degree of damage (ie, partial vs complete rupture) and to rule out other injuries, especially if the athlete fails to respond to treatment. Plain radiographs in acute cases are usually unremarkable. However, more severe injuries may go on to develop myositis ossificans similar to quadriceps soft tissue injuries (Figure 5).
Treatment
Most hamstring strains respond to conservative treatment, with operative intervention rarely indicated except for proximal or distal tendon avulsions.39 Like other muscle strains, initial management consists of RICE. COX-2-selective NSAIDs are preferred initially following injury. During a brief period of immobilization, the leg should be extended as much as tolerated to maximize muscle length, limit hematoma formation, and reduce the risk of contracture.39 Controlled mobilization should begin as soon as tolerated by the athlete.39 Isometric exercises and a stretching program should be started early in the rehabilitation period, with isotonic exercises added as motion and pain improve. Active stretching should be initiated and progressed to passive, static stretching as guided by pain.
The late phase of rehabilitation and long-term conditioning protocols should incorporate eccentric training once the athlete is pain-free, performing isotonic and isokinetic exercises. Eccentric exercises best strengthen the hamstrings at their most susceptible point, prepares the athlete for functional activities, and minimizes the risk of reinjury,3,50,51 Elliot and colleagues3 reported an order of magnitude decrease in hamstring injuries in high-risk athletes with identifiable hamstring muscle weakness after implementing an eccentric strengthening program and progressive sprint training. Similarly, in a large cohort of elite soccer players, correction of strength deficits in players with prior hamstring injuries led to similar rates of injury compared to athletes without strength deficits or prior injury.52 Those athletes with persistent weakness who did not undergo rehabilitation had significantly higher rates of reinjury.
Various injections containing local anesthetics, corticosteroids, platelet-rich plasma (PRP), and other substances have been administered to football players following acute muscle strains in an effort to alleviate pain and safely return the athlete to competition. Some practitioners have been reluctant to administer injections (especially those containing corticosteroids) due to a potentially increased risk of tendinopathy or rupture.31 Drakos and colleagues53 reported their outcomes following muscle and ligament strains treated with combined corticosteroid and local anesthetic injections on one NFL team. While quadriceps and hamstring strains were associated with the most missed games among all muscle strains, these injections resulted in no adverse events or progression of injury severity. Similarly, Levine and colleagues 51 administered intramuscular corticosteroid injections to 58 NFL players with high-grade hamstring injuries that had a palpable defect within the muscle belly. They reported no complications or strength deficits at final examination. In a case-control study, Rettig and colleagues46 administered PRP injections under ultrasound guidance in 5 NFL players with hamstring injuries. Compared to players treated with a focused rehabilitation program only, there were no significant differences in recovery or return to play.
The decision to return to play should be based on a clinical assessment considering pain, strength, motion, and flexibility. Player position should also be considered. Return-to-play guidelines describing the appropriate progression through rehabilitation and return to sport have been described and can be used as a template for the rehabilitation of football players.54 It should be noted that primary hamstring strains are associated with decreased athletic performance and an increased risk of more severe reinjury after return to sport.55,56
Morel-Lavallée Lesion
Pathophysiology
Morel-Lavallée lesions (MLLs) are uncommon football injuries, but often occur in the thigh.57,58 An MLL is a posttraumatic soft tissue injury in which deforming forces of pressure and shear cause a closed, soft tissue degloving injury; in this injury, the skin and subcutaneous tissues are separated from the underlying fascia, disrupting perforating blood vessels. The resulting space between the fascia and subcutaneous tissue fills with blood, lymphatics, and necrotic fat, resulting in a hematoma/seroma that can be a nidus for bacterial infection.58 The most common anatomic regions are the anterior distal thigh and lateral hip. Both of these areas are commonly involved in both direct contact and shear forces following a fall to the ground.
History and Physical Examination
Athletes with MLLs typically present with the insidious onset of a fluid collection within the thigh following a fall to the ground, usually while sliding or diving on the playing surface.57,58 The fluid collection can be associated with thigh tightness and may extend distally into the suprapatellar region or proximally over the greater trochanter. Thigh swelling, ecchymosis, and palpable fluctuance are seen in most cases. Progressive increases in pain and thigh swelling may be seen in severe injuries, but thigh compartments generally remain soft and nontender. Signs and symptoms of an MLL do not typically manifest immediately following the athletic event. Tejwani and colleagues58 reported a case series of MLLs of the knee in 27 NFL players from a single team over a 14-year period, with an average of 3 days between injury and evaluation by the medical staff. The mechanism of injury was a shearing blow from the knee striking the playing surface in 81% of cases and direct contact to the knee from another player in 19% of cases; all cases occurred in game situations. No affected players were wearing kneepads at the time of injury.
Imaging
Plain radiography may reveal a noncalcified soft tissue mass over the involved area and is not usually helpful except to rule out an underlying fracture. The appearance of an MLL on ultrasound is nonspecific and variable, often described as anechoic, hypoechoic, or hyperechoic depending on the presence of hemolymphatic fluid sedimentation and varying amounts of internal fat debris. MRI is the imaging modality of choice and typically shows a well-defined oval or fusiform, fluid-filled mass with tapering margins blending with adjacent fascial planes.
Treatment
Similar to quadriceps contusions, treatment goals for MLLs are evacuation of the fluid collection, prevention of fluid recurrence, a full range of active knee flexion, and prompt return to play.57,58 Initial treatment for smaller lesions consists of cryotherapy, compression wrapping of the involved area, and immediate active and passive range of motion of the hip and knee. While MLLs were traditionally treated with serial open debridements, less invasive approaches—including elastic compression, aspiration, percutaneous irrigation with debridement and suction drainage, or liposuction and drainage followed by suction therapy—have been recently described.57,58,60,61 Less invasive approaches aim to minimize soft tissue dissection and disruption of the vascular supply while accelerating rehabilitation. The presence of a surrounding capsule on MRI makes conservative or minimally invasive approaches less likely to be successful and may necessitate an open procedure.62 Antibiotics should be used preoperatively due to the presence of a dead space containing necrotic debris that makes infection a potential complication. While elite contact athletes can expect to return to competition long before complete resolution of an MLL, there is a risk of further delamination and lesion expansion due to re-injury prior to compete healing.
Tejwani and colleagues58 performed aspiration at the area of palpable fluctuance in the thigh or suprapatellar region using a 14-gauge needle in those athletes who failed to improve with conservative treatments alone. Mean time to resolution of the fluid collection was 16 days following aspiration. Fifty-two percent of the athletes were successfully treated with cryotherapy, compression, and motion exercises alone; 48% were treated with at least one aspiration, with a mean of 2.7 aspirations per knee. In 11% of cases that failed to resolve after multiple aspirations, doxycycline sclerodesis was performed immediately following an aspiration. Patients treated with sclerodesis had no return of the fluid collection and returned to play the following day.
Matava and colleagues57 described the case of an NFL player who sustained a closed MLL of the lateral hip while diving onto an artificial turf surface attempting to catch a pass. Despite immediate thigh pain and swelling, he was able to continue play. Immediately following the game, the player was examined and had a tense thigh with ecchymosis extending into the trochanteric region. Aspiration of the fluctuant area was unsuccessful. Progressive increases in pain and thigh swelling prompted hospital admission. Percutaneous irrigation and debridement was performed as described by Tseng and Tornetta.61 A suction drain was placed within the residual dead space, and constant wall suction was applied in addition to hip compression using a spica
Conclusion
Quadriceps and hamstring injuries occur frequently in football and are generally treated conservatively. While return to competition following hamstring strains is relatively quick, a high rate of injury recurrence highlights the importance of targeted rehabilitation and conditioning. Rarely, complications from quadriceps contusions, including acute compartment syndrome and myositis ossificans, may require operative intervention if unresponsive to conservative treatment. MLLs are rare in sports, but usually involve the thigh when they occur in football players. Team physicians must maintain a heightened degree of awareness of this injury as it may require operative intervention.
Acknowledgements: The authors would like to thank Jonathon Baker, MD and David Rubin, MD for their assistance in providing radiographic images for this paper.
Am J Orthop. 2016;45(6):E308-E318. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
2. Rechel JA, Yard EE, Comstock RD. An epidemiologic comparison of high school sports injuries sustained in practice and competition. J Athl Train. 2008;43(2):197-204.
3. Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39(4):843-850.
4. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
5. Feeley BT, Kennelly S, Barnes RP, et al. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med. 2008;36(8):1597-1603.
6. Garrett WE Jr. Muscle strain injuries. Am J Sports Med. 1996;24(6 Suppl):S2-S8.
7. Lawrence DW, Hutchison MG, Comper P. Descriptive epidemiology of musculoskeletal injuries and concussions in the National Football League, 2012-2014. Orthop J Sports Med. 2015;3(5):2325967115583653.
8. Diaz JA, Fischer DA, Rettig AC, Davis TJ, Shelbourne KD. Severe quadriceps muscle contusions in athletes. A report of three cases. Am J Sports Med. 2003;31(2):289-293.
9. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
10. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
11. Bencardino JT, Rosenberg ZS, Brown RR, Hassankhani A, Lustrin ES, Beltran J. Traumatic musculotendinous injuries of the knee: diagnosis with MR imaging. Radiographics. 2000;20 Spec No:S103-S120.
12. Robinson D, On E, Halperin N. Anterior compartment syndrome of the thigh in athletes--indications for conservative treatment. J Trauma. 1992;32(2):183-186.
13. Beckmann JT, Wylie JD, Kapron AL, Hanson JA, Maak TG, Aoki SK. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med. 2014;42(6):1359-1364.
14. Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43(3):346-353.
15. Beckmann JT, Wylie JD, Potter MQ, Maak TG, Greene TH, Aoki SK. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double-blind randomized placebo-controlled trial. J Bone Joint Surg Am. 2015;97(24):2032-2037.
16. Yeung M, Jamshidi S, Horner N, Simunovic N, Karlsson J, Ayeni OR. Efficacy of nonsteroidal anti-inflammatory drug prophylaxis for heterotrophic ossification in hip arthroscopy: a systematic review. Arthroscopy. 2016;32(3):519-525.
17. Goyal K, Pettis CR, Bancroft AE, Wasyliw CW, Scherer KF. Myositis ossificans in the thigh of a lacrosse player. Orthopedics. 2015;38(8):468,515-518.
18. Cooper DE. Severe quadriceps muscle contusions in athletes. Am J Sports Med. 2004;32(3):820.
19. Bonsell S, Freudigman PT, Moore HA. Quadriceps muscle contusion resulting in osteomyelitis of the femur in a high school football player. A case report. Am J Sports Med. 2001;29(6):818-820.
20. Rothwell AG. Quadriceps hematoma. A prospective clinical study. Clin Orthop Relat Res. 1982;(171):97-103.
21. Armfield DR, Kim DH, Towers JD, Bradley JP, Robertson DD. Sports-related muscle injury in the lower extremity. Clin Sports Med. 2006;25(4):803-842.
22. Lipscomb AB, Thomas ED, Johnston RK. Treatment of myositis ossificans traumatica in athletes. Am J Sports Med. 1976;4(3):111-120.
23. Mani-Babu S, Wolman R, Keen R. Quadriceps traumatic myositis ossificans in a football player: management with intravenous pamidronate. Clin J Sport Med. 2014;24(5):e56-e58.
24. McCaffrey DD, Clarke J, Bunn J, McCormack MJ. Acute compartment syndrome of the anterior thigh in the absence of fracture secondary to sporting trauma. J Trauma. 2009;66(4):1238-1242.
25. Klasson SC, Vander Schilden JL. Acute anterior thigh compartment syndrome complicating quadriceps hematoma. Two case reports and review of the literature. Orthop Rev. 1990;19(5):421-427.
26. Rooser B. Quadriceps contusion with compartment syndrome. Evacuation of hematoma in 2 cases. Acta Orthop Scand. 1987;58(2):170-172.
27. Rooser B, Bengtson S, Hagglund G. Acute compartment syndrome from anterior thigh muscle contusion: a report of eight cases. J Orthop Trauma. 1991;5(1):57-59.
28. Schwartz JT Jr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh. A spectrum of injury. J Bone Joint Surg Am. 1989;71(3):392-400.
29. Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625-632.
30. Kary JM. Diagnosis and management of quadriceps strains and contusions. Curr Rev Musculoskelet Med. 2010;3(1-4):26-31.
31. Boublik M, Schlegel TF, Koonce RC, Genuario JW, Kinkartz JD. Quadriceps tendon injuries in national football league players. Am J Sports Med. 2013;41(8):1841-1846.
32. Palmer WE, Kuong SJ, Elmadbouh HM. MR imaging of myotendinous strain. AJR Am J Roentgenol. 1999;173(3):703-709.
33. Cross TM, Gibbs N, Houang MT, Cameron M. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32(3):710-719.
34. Hughes C 4th, Hasselman CT, Best TM, Martinez S, Garrett WE Jr. Incomplete, intrasubstance strain injuries of the rectus femoris muscle. Am J Sports Med. 1995;23(4):500-506.
35. Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 2001;29(3):300-303.36. Mair SD, Seaber AV, Glisson RR, Garrett WE, Jr. The role of fatigue in susceptibility to acute muscle strain injury. Am J Sports Med. 1996;24(2):137-143.
37. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261.
38. Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-764.
39. Clanton TO, Coupe KJ. Hamstring strains in athletes: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(4):237-248.
40. Novacheck TF. The biomechanics of running. Gait Posture. 1998;7(1):77-95.
41. Yu B, Queen RM, Abbey AN, Liu Y, Moorman CT, Garrett WE. Hamstring muscle kinematics and activation during overground sprinting. J Biomech. 2008;41(15):3121-3126.
42. Opar DA, Williams MD, Shield AJ. Hamstring strain injuries: factors that lead to injury and re-injury. Sports Med. 2012;42(3):209-226.
43. Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35(2):197-206.
44. Thelen DG, Chumanov ES, Hoerth DM, et al. Hamstring muscle kinematics during treadmill sprinting. Med Sci Sports Exerc. 2005;37(1):108-114.
45. Chumanov ES, Heiderscheit BC, Thelen DG. The effect of speed and influence of individual muscles on hamstring mechanics during the swing phase of sprinting. J Biomech. 2007;40(16):3555-3562.
46. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
47. Zvijac JE, Toriscelli TA, Merrick S, Kiebzak GM. Isokinetic concentric quadriceps and hamstring strength variables from the NFL Scouting Combine are not predictive of hamstring injury in first-year professional football players. Am J Sports Med. 2013;41(7):1511-1518.
48. Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl):5S-16S.
49. Zarins B, Ciullo JV. Acute muscle and tendon injuries in athletes. Clin Sports Med. 1983;2(1):167-182.
50. Arnason A, Andersen TE, Holme I, Engebretsen L, Bahr R. Prevention of hamstring strains in elite soccer: an intervention study. Scand J Med Sci Sports. 2008;18(1):40-48.
51. Levine WN, Bergfeld JA, Tessendorf W, Moorman CT 3rd. Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28(3):297-300.
52. Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med. 2008;36(8):1469-1475.
53. Drakos M, Birmingham P, Delos D, et al. Corticosteroid and anesthetic injections for muscle strains and ligament sprains in the NFL. HSS J. 2014;10(2):136-142.
54. Worrell TW. Factors associated with hamstring injuries. An approach to treatment and preventative measures. Sports Med. 1994;17(5):338-345.
55. Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34(8):1297-1306.
56. Verrall GM, Kalairajah Y, Slavotinek JP, Spriggins AJ. Assessment of player performance following return to sport after hamstring muscle strain injury. J Sci Med Sport. 2006;9(1-2):87-90.
57. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-lavallee lesion in a professional american football player. Am J Orthop. 2010;39(3):144-147.
58. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med. 2007;35(7):1162-1167.
59. Mellado JM, Bencardino JT. Morel-Lavallee lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am. 2005;13(4):775-782.
60. Harma A, Inan M, Ertem K. [The Morel-Lavallee lesion: a conservative approach to closed degloving injuries]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
61. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
62. Gilbert BC, Bui-Mansfield LT, Dejong S. MRI of a Morel-Lavellee lesion. AJR Am J Roentgenol. 2004;182(5):1347-1348.
1. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
2. Rechel JA, Yard EE, Comstock RD. An epidemiologic comparison of high school sports injuries sustained in practice and competition. J Athl Train. 2008;43(2):197-204.
3. Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39(4):843-850.
4. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
5. Feeley BT, Kennelly S, Barnes RP, et al. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med. 2008;36(8):1597-1603.
6. Garrett WE Jr. Muscle strain injuries. Am J Sports Med. 1996;24(6 Suppl):S2-S8.
7. Lawrence DW, Hutchison MG, Comper P. Descriptive epidemiology of musculoskeletal injuries and concussions in the National Football League, 2012-2014. Orthop J Sports Med. 2015;3(5):2325967115583653.
8. Diaz JA, Fischer DA, Rettig AC, Davis TJ, Shelbourne KD. Severe quadriceps muscle contusions in athletes. A report of three cases. Am J Sports Med. 2003;31(2):289-293.
9. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
10. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
11. Bencardino JT, Rosenberg ZS, Brown RR, Hassankhani A, Lustrin ES, Beltran J. Traumatic musculotendinous injuries of the knee: diagnosis with MR imaging. Radiographics. 2000;20 Spec No:S103-S120.
12. Robinson D, On E, Halperin N. Anterior compartment syndrome of the thigh in athletes--indications for conservative treatment. J Trauma. 1992;32(2):183-186.
13. Beckmann JT, Wylie JD, Kapron AL, Hanson JA, Maak TG, Aoki SK. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med. 2014;42(6):1359-1364.
14. Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43(3):346-353.
15. Beckmann JT, Wylie JD, Potter MQ, Maak TG, Greene TH, Aoki SK. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double-blind randomized placebo-controlled trial. J Bone Joint Surg Am. 2015;97(24):2032-2037.
16. Yeung M, Jamshidi S, Horner N, Simunovic N, Karlsson J, Ayeni OR. Efficacy of nonsteroidal anti-inflammatory drug prophylaxis for heterotrophic ossification in hip arthroscopy: a systematic review. Arthroscopy. 2016;32(3):519-525.
17. Goyal K, Pettis CR, Bancroft AE, Wasyliw CW, Scherer KF. Myositis ossificans in the thigh of a lacrosse player. Orthopedics. 2015;38(8):468,515-518.
18. Cooper DE. Severe quadriceps muscle contusions in athletes. Am J Sports Med. 2004;32(3):820.
19. Bonsell S, Freudigman PT, Moore HA. Quadriceps muscle contusion resulting in osteomyelitis of the femur in a high school football player. A case report. Am J Sports Med. 2001;29(6):818-820.
20. Rothwell AG. Quadriceps hematoma. A prospective clinical study. Clin Orthop Relat Res. 1982;(171):97-103.
21. Armfield DR, Kim DH, Towers JD, Bradley JP, Robertson DD. Sports-related muscle injury in the lower extremity. Clin Sports Med. 2006;25(4):803-842.
22. Lipscomb AB, Thomas ED, Johnston RK. Treatment of myositis ossificans traumatica in athletes. Am J Sports Med. 1976;4(3):111-120.
23. Mani-Babu S, Wolman R, Keen R. Quadriceps traumatic myositis ossificans in a football player: management with intravenous pamidronate. Clin J Sport Med. 2014;24(5):e56-e58.
24. McCaffrey DD, Clarke J, Bunn J, McCormack MJ. Acute compartment syndrome of the anterior thigh in the absence of fracture secondary to sporting trauma. J Trauma. 2009;66(4):1238-1242.
25. Klasson SC, Vander Schilden JL. Acute anterior thigh compartment syndrome complicating quadriceps hematoma. Two case reports and review of the literature. Orthop Rev. 1990;19(5):421-427.
26. Rooser B. Quadriceps contusion with compartment syndrome. Evacuation of hematoma in 2 cases. Acta Orthop Scand. 1987;58(2):170-172.
27. Rooser B, Bengtson S, Hagglund G. Acute compartment syndrome from anterior thigh muscle contusion: a report of eight cases. J Orthop Trauma. 1991;5(1):57-59.
28. Schwartz JT Jr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh. A spectrum of injury. J Bone Joint Surg Am. 1989;71(3):392-400.
29. Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625-632.
30. Kary JM. Diagnosis and management of quadriceps strains and contusions. Curr Rev Musculoskelet Med. 2010;3(1-4):26-31.
31. Boublik M, Schlegel TF, Koonce RC, Genuario JW, Kinkartz JD. Quadriceps tendon injuries in national football league players. Am J Sports Med. 2013;41(8):1841-1846.
32. Palmer WE, Kuong SJ, Elmadbouh HM. MR imaging of myotendinous strain. AJR Am J Roentgenol. 1999;173(3):703-709.
33. Cross TM, Gibbs N, Houang MT, Cameron M. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32(3):710-719.
34. Hughes C 4th, Hasselman CT, Best TM, Martinez S, Garrett WE Jr. Incomplete, intrasubstance strain injuries of the rectus femoris muscle. Am J Sports Med. 1995;23(4):500-506.
35. Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 2001;29(3):300-303.36. Mair SD, Seaber AV, Glisson RR, Garrett WE, Jr. The role of fatigue in susceptibility to acute muscle strain injury. Am J Sports Med. 1996;24(2):137-143.
37. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261.
38. Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-764.
39. Clanton TO, Coupe KJ. Hamstring strains in athletes: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(4):237-248.
40. Novacheck TF. The biomechanics of running. Gait Posture. 1998;7(1):77-95.
41. Yu B, Queen RM, Abbey AN, Liu Y, Moorman CT, Garrett WE. Hamstring muscle kinematics and activation during overground sprinting. J Biomech. 2008;41(15):3121-3126.
42. Opar DA, Williams MD, Shield AJ. Hamstring strain injuries: factors that lead to injury and re-injury. Sports Med. 2012;42(3):209-226.
43. Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35(2):197-206.
44. Thelen DG, Chumanov ES, Hoerth DM, et al. Hamstring muscle kinematics during treadmill sprinting. Med Sci Sports Exerc. 2005;37(1):108-114.
45. Chumanov ES, Heiderscheit BC, Thelen DG. The effect of speed and influence of individual muscles on hamstring mechanics during the swing phase of sprinting. J Biomech. 2007;40(16):3555-3562.
46. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
47. Zvijac JE, Toriscelli TA, Merrick S, Kiebzak GM. Isokinetic concentric quadriceps and hamstring strength variables from the NFL Scouting Combine are not predictive of hamstring injury in first-year professional football players. Am J Sports Med. 2013;41(7):1511-1518.
48. Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl):5S-16S.
49. Zarins B, Ciullo JV. Acute muscle and tendon injuries in athletes. Clin Sports Med. 1983;2(1):167-182.
50. Arnason A, Andersen TE, Holme I, Engebretsen L, Bahr R. Prevention of hamstring strains in elite soccer: an intervention study. Scand J Med Sci Sports. 2008;18(1):40-48.
51. Levine WN, Bergfeld JA, Tessendorf W, Moorman CT 3rd. Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28(3):297-300.
52. Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med. 2008;36(8):1469-1475.
53. Drakos M, Birmingham P, Delos D, et al. Corticosteroid and anesthetic injections for muscle strains and ligament sprains in the NFL. HSS J. 2014;10(2):136-142.
54. Worrell TW. Factors associated with hamstring injuries. An approach to treatment and preventative measures. Sports Med. 1994;17(5):338-345.
55. Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34(8):1297-1306.
56. Verrall GM, Kalairajah Y, Slavotinek JP, Spriggins AJ. Assessment of player performance following return to sport after hamstring muscle strain injury. J Sci Med Sport. 2006;9(1-2):87-90.
57. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-lavallee lesion in a professional american football player. Am J Orthop. 2010;39(3):144-147.
58. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med. 2007;35(7):1162-1167.
59. Mellado JM, Bencardino JT. Morel-Lavallee lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am. 2005;13(4):775-782.
60. Harma A, Inan M, Ertem K. [The Morel-Lavallee lesion: a conservative approach to closed degloving injuries]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
61. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
62. Gilbert BC, Bui-Mansfield LT, Dejong S. MRI of a Morel-Lavellee lesion. AJR Am J Roentgenol. 2004;182(5):1347-1348.
Cutaneous Artifactual Disease Represented as Recurrent Toxic Epidermal Necrolysis
To the Editor:
Lyell1 coined the term cutaneous artifactual disease to describe the spectrum of factitious disorders associated with skin presentations. Interestingly, Lyell was the first to name toxic epidermal necrolysis (TEN).2,3 We present a rare case of factitial TEN, a dangerous and life-threatening manifestation of factitial disease.
A 49-year-old homeless man with a history of Stevens-Johnson syndrome (SJS)/TEN from trimethoprim-sulfamethoxazole (TMP-SMX) was admitted on 4 separate occasions over an 18-month period for recurrent exposure to the medication producing SJS/TEN. Originally, this patient was given TMP-SMX for a skin infection and 10 days later presented with 15% body surface area (BSA) involvement of SJS/TEN. He was successfully treated with intravenous immunoglobulin (IVIg) in the burn intensive care unit (BICU) and discharged. Several months later, the patient was given TMP-SMX for a leg infection by a different clinic. He was admitted to the BICU with 40% BSA, treated with IVIg, and survived. Eight months later, the patient was again admitted to the BICU with 30% BSA and treated with IVIg; however, this admission required intubation due to complications secondary to volume resuscitation. He was evaluated by psychiatry and confessed to purposely seeking TMP-SMX, stating that he “liked the food and care in the hospital.” He was diagnosed with factitial disorder and given a referral for further treatment at an outpatient facility. Two months later, the patient was again admitted to the BICU after taking a single dose of TMP-SMX obtained from a “friend.” He had 10% BSA with conjunctival involvement and was again successfully treated with IVIg. He was discharged with the state psychiatric system for further treatment and evaluation.
Factitial disease in dermatology is difficult to diagnose. Its incidence is unknown, as only case reports exist in the literature. In factitial disease, patients “perform self-mutilating and clinically relevant damage to themselves without the direct intention of suicide.”4 Harth et al4 described 3 subcategories of factitious disorders: dermatitis artefacta syndrome, dermatitis para-artefacta syndrome, or malingering. Dermatitis artefacta syndrome is “a dissociated self-injury or behavior where the patient unconsciously simulated disease with intention to be cared for as a patient.”4 Dermatitis para-artefacta syndrome was described as a disorder of impulse control in which a patient will produce or manipulate a specific dermatosis presentation. The patient usually admits to doing it in a semiconscious state. Dermatitis artefacta and dermatitis para-artefacta differ from malingering in that malingering patients knowingly fake symptoms for external gain, which can be monetary or the avoidance of responsibility.4 More familiar examples to dermatologists of factitial disease include factitial panniculitis,1 direct applications of caustic agents to the skin, and excoriations from instruments or fingernails.4,5
This case illustrates the difficult and potentially dangerous nature of factitial disorders, specifically dermatitis para-artefacta syndrome. Our patient was intensely preoccupied with the outcome of being a patient in a hospital. Our patient sought out a medication from multiple providers to produce a deadly and severe life-threatening reaction. If his main intentions were solely to obtain a bed and 3 square meals a day, then malingering would have represented his factitial disease. However, his main intent was to be seen as a patient, and then doted on and cared for by medical professionals in a hospital setting. From this assessment, the patient’s behavior would fall under the factitial disorder of dermatitis para-artefacta syndrome.
Factitious disorders pose immense challenges for diagnosis and treatment. It is prudent for physicians to learn to recognize patterns of history and examination that do not coincide. The first step in treatment is the recognition and early involvement of psychiatry to aid in curbing this behavior. Remission of factitial disorders can be induced with proper diagnosis and treatment. Patients with the highest chance of remission are those with treatment centered on behavioral therapy in conjunction with psychotropic medications.1
- Lyell A. Cutaneous artifactual disease. J Am Acad Dermatol. 1979;1:391-402.
- Palmieri TL, Greenhalgh, DG, Saffle JR, et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J Burn Care Rehab. 2002;23:87-96.
- Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol. 1956;68:355-361.
- Harth W, Gieler U, Kusnir D, et al. Primarily psychogenic dermatoses. In: Clinical Management in Psychodermatology. 1st ed. Leipzog, Germany: Springer-Verlag Berlin Heidelberg; 2009:11-19.
- Sanmartin O, Requena C, Requena L. Factitial panniculitis. Dermatol Clin. 2008;26:519-527.
To the Editor:
Lyell1 coined the term cutaneous artifactual disease to describe the spectrum of factitious disorders associated with skin presentations. Interestingly, Lyell was the first to name toxic epidermal necrolysis (TEN).2,3 We present a rare case of factitial TEN, a dangerous and life-threatening manifestation of factitial disease.
A 49-year-old homeless man with a history of Stevens-Johnson syndrome (SJS)/TEN from trimethoprim-sulfamethoxazole (TMP-SMX) was admitted on 4 separate occasions over an 18-month period for recurrent exposure to the medication producing SJS/TEN. Originally, this patient was given TMP-SMX for a skin infection and 10 days later presented with 15% body surface area (BSA) involvement of SJS/TEN. He was successfully treated with intravenous immunoglobulin (IVIg) in the burn intensive care unit (BICU) and discharged. Several months later, the patient was given TMP-SMX for a leg infection by a different clinic. He was admitted to the BICU with 40% BSA, treated with IVIg, and survived. Eight months later, the patient was again admitted to the BICU with 30% BSA and treated with IVIg; however, this admission required intubation due to complications secondary to volume resuscitation. He was evaluated by psychiatry and confessed to purposely seeking TMP-SMX, stating that he “liked the food and care in the hospital.” He was diagnosed with factitial disorder and given a referral for further treatment at an outpatient facility. Two months later, the patient was again admitted to the BICU after taking a single dose of TMP-SMX obtained from a “friend.” He had 10% BSA with conjunctival involvement and was again successfully treated with IVIg. He was discharged with the state psychiatric system for further treatment and evaluation.
Factitial disease in dermatology is difficult to diagnose. Its incidence is unknown, as only case reports exist in the literature. In factitial disease, patients “perform self-mutilating and clinically relevant damage to themselves without the direct intention of suicide.”4 Harth et al4 described 3 subcategories of factitious disorders: dermatitis artefacta syndrome, dermatitis para-artefacta syndrome, or malingering. Dermatitis artefacta syndrome is “a dissociated self-injury or behavior where the patient unconsciously simulated disease with intention to be cared for as a patient.”4 Dermatitis para-artefacta syndrome was described as a disorder of impulse control in which a patient will produce or manipulate a specific dermatosis presentation. The patient usually admits to doing it in a semiconscious state. Dermatitis artefacta and dermatitis para-artefacta differ from malingering in that malingering patients knowingly fake symptoms for external gain, which can be monetary or the avoidance of responsibility.4 More familiar examples to dermatologists of factitial disease include factitial panniculitis,1 direct applications of caustic agents to the skin, and excoriations from instruments or fingernails.4,5
This case illustrates the difficult and potentially dangerous nature of factitial disorders, specifically dermatitis para-artefacta syndrome. Our patient was intensely preoccupied with the outcome of being a patient in a hospital. Our patient sought out a medication from multiple providers to produce a deadly and severe life-threatening reaction. If his main intentions were solely to obtain a bed and 3 square meals a day, then malingering would have represented his factitial disease. However, his main intent was to be seen as a patient, and then doted on and cared for by medical professionals in a hospital setting. From this assessment, the patient’s behavior would fall under the factitial disorder of dermatitis para-artefacta syndrome.
Factitious disorders pose immense challenges for diagnosis and treatment. It is prudent for physicians to learn to recognize patterns of history and examination that do not coincide. The first step in treatment is the recognition and early involvement of psychiatry to aid in curbing this behavior. Remission of factitial disorders can be induced with proper diagnosis and treatment. Patients with the highest chance of remission are those with treatment centered on behavioral therapy in conjunction with psychotropic medications.1
To the Editor:
Lyell1 coined the term cutaneous artifactual disease to describe the spectrum of factitious disorders associated with skin presentations. Interestingly, Lyell was the first to name toxic epidermal necrolysis (TEN).2,3 We present a rare case of factitial TEN, a dangerous and life-threatening manifestation of factitial disease.
A 49-year-old homeless man with a history of Stevens-Johnson syndrome (SJS)/TEN from trimethoprim-sulfamethoxazole (TMP-SMX) was admitted on 4 separate occasions over an 18-month period for recurrent exposure to the medication producing SJS/TEN. Originally, this patient was given TMP-SMX for a skin infection and 10 days later presented with 15% body surface area (BSA) involvement of SJS/TEN. He was successfully treated with intravenous immunoglobulin (IVIg) in the burn intensive care unit (BICU) and discharged. Several months later, the patient was given TMP-SMX for a leg infection by a different clinic. He was admitted to the BICU with 40% BSA, treated with IVIg, and survived. Eight months later, the patient was again admitted to the BICU with 30% BSA and treated with IVIg; however, this admission required intubation due to complications secondary to volume resuscitation. He was evaluated by psychiatry and confessed to purposely seeking TMP-SMX, stating that he “liked the food and care in the hospital.” He was diagnosed with factitial disorder and given a referral for further treatment at an outpatient facility. Two months later, the patient was again admitted to the BICU after taking a single dose of TMP-SMX obtained from a “friend.” He had 10% BSA with conjunctival involvement and was again successfully treated with IVIg. He was discharged with the state psychiatric system for further treatment and evaluation.
Factitial disease in dermatology is difficult to diagnose. Its incidence is unknown, as only case reports exist in the literature. In factitial disease, patients “perform self-mutilating and clinically relevant damage to themselves without the direct intention of suicide.”4 Harth et al4 described 3 subcategories of factitious disorders: dermatitis artefacta syndrome, dermatitis para-artefacta syndrome, or malingering. Dermatitis artefacta syndrome is “a dissociated self-injury or behavior where the patient unconsciously simulated disease with intention to be cared for as a patient.”4 Dermatitis para-artefacta syndrome was described as a disorder of impulse control in which a patient will produce or manipulate a specific dermatosis presentation. The patient usually admits to doing it in a semiconscious state. Dermatitis artefacta and dermatitis para-artefacta differ from malingering in that malingering patients knowingly fake symptoms for external gain, which can be monetary or the avoidance of responsibility.4 More familiar examples to dermatologists of factitial disease include factitial panniculitis,1 direct applications of caustic agents to the skin, and excoriations from instruments or fingernails.4,5
This case illustrates the difficult and potentially dangerous nature of factitial disorders, specifically dermatitis para-artefacta syndrome. Our patient was intensely preoccupied with the outcome of being a patient in a hospital. Our patient sought out a medication from multiple providers to produce a deadly and severe life-threatening reaction. If his main intentions were solely to obtain a bed and 3 square meals a day, then malingering would have represented his factitial disease. However, his main intent was to be seen as a patient, and then doted on and cared for by medical professionals in a hospital setting. From this assessment, the patient’s behavior would fall under the factitial disorder of dermatitis para-artefacta syndrome.
Factitious disorders pose immense challenges for diagnosis and treatment. It is prudent for physicians to learn to recognize patterns of history and examination that do not coincide. The first step in treatment is the recognition and early involvement of psychiatry to aid in curbing this behavior. Remission of factitial disorders can be induced with proper diagnosis and treatment. Patients with the highest chance of remission are those with treatment centered on behavioral therapy in conjunction with psychotropic medications.1
- Lyell A. Cutaneous artifactual disease. J Am Acad Dermatol. 1979;1:391-402.
- Palmieri TL, Greenhalgh, DG, Saffle JR, et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J Burn Care Rehab. 2002;23:87-96.
- Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol. 1956;68:355-361.
- Harth W, Gieler U, Kusnir D, et al. Primarily psychogenic dermatoses. In: Clinical Management in Psychodermatology. 1st ed. Leipzog, Germany: Springer-Verlag Berlin Heidelberg; 2009:11-19.
- Sanmartin O, Requena C, Requena L. Factitial panniculitis. Dermatol Clin. 2008;26:519-527.
- Lyell A. Cutaneous artifactual disease. J Am Acad Dermatol. 1979;1:391-402.
- Palmieri TL, Greenhalgh, DG, Saffle JR, et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J Burn Care Rehab. 2002;23:87-96.
- Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol. 1956;68:355-361.
- Harth W, Gieler U, Kusnir D, et al. Primarily psychogenic dermatoses. In: Clinical Management in Psychodermatology. 1st ed. Leipzog, Germany: Springer-Verlag Berlin Heidelberg; 2009:11-19.
- Sanmartin O, Requena C, Requena L. Factitial panniculitis. Dermatol Clin. 2008;26:519-527.
Practice Points
- It is important to consider an underlying psychiatric disorder (eg, factitial disorders) in dermatologic patients, even when an exogenous cause can be identified.
- On occasion, dermatologic disease is best treated and prevented with routine psychiatric care and psychotropic therapy.