User login
Latest Breakthroughs in Molluscum Contagiosum Therapy
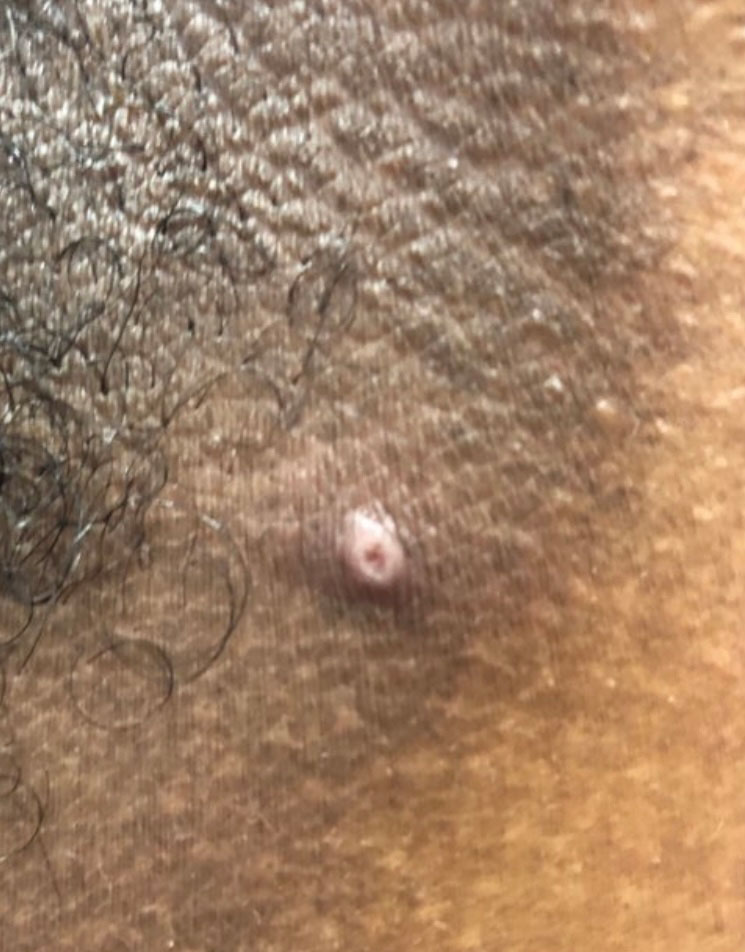
Molluscum contagiosum (ie, molluscum) is a ubiquitous infection caused by the poxvirus molluscum contagiosum virus (MCV). Although skin deep, molluscum shares many factors with the more virulent poxviridae. Moisture and trauma can cause viral material to be released from the pearly papules through a small opening, which also allows entry of bacteria and medications into the lesion. The MCV is transmitted by direct contact with skin or via fomites.1
Molluscum can affect children of any age, with MCV type 1 peaking in toddlers and school-aged children and MCV type 2 after the sexual debut. The prevalence of molluscum has increased since the 1980s. It is stressful for children and caregivers and poses challenges in schools as well as sports such as swimming, wrestling, and karate.1,2
For the first time, we have US Food and Drug Administration (FDA)–approved products to treat MCV infections. Previously, only off-label agents were used. Therefore, we have to contemplate why treatment is important to our patients.
What type of care is required for molluscum?
Counseling is the first and only mandatory treatment, which consists of 3 parts: natural history, risk factors for spread, and options for therapy. The natural history of molluscum in children is early spread, contagion to oneself and others (as high as 60% of sibling co-bathers3), triggering of dermatitis, eventual onset of the beginning-of-the-end (BOTE) sign, and eventually clearance. The natural history in adults is poorly understood.
Early clearance is uncommon; reports have suggested 45.6% to 48.4% of affected patients are clear at 1 year and 69.5% to 72.6% at 1.5 years.4 For many children, especially those with atopic dermatitis (AD), lesions linger and often spread, with many experiencing disease for 3 to 4 years. Fomites such as towels, washcloths, and sponges can transfer the virus and spread lesions; therefore, I advise patients to gently pat their skin dry, wash towels frequently, and avoid sharing bathing equipment.1,3,5 Children and adults with immunosuppression may have a greater number of lesions and more prolonged course of disease, including those with HIV as well as DOC8 and CARD11 mutations.6 The American Academy of Pediatrics (AAP) emphasizes that children should not be excluded from attending child care/school or from swimming in public pools but lesions should be covered.6 Lesions, especially those in the antecubital region, can trigger new-onset AD or AD flares.3 In response, gentle skin care including fragrance-free cleansers and periodic application of moisturizers may ward off AD. Topical corticosteroids are preferred.
Dermatitis in MCV is a great mimicker and can resemble erythema multiforme, Gianotti-Crosti syndrome, impetigo, and AD.1 Superinfection recently has been reported; however, in a retrospective analysis of 56 patients with inflamed lesions secondary to molluscum infection, only 7 had positive bacterial cultures, which supports the idea of the swelling and redness of inflammation as a mimic for infection.7 When true infection does occur, tender, swollen, pus-filled lesions should be lanced and cultured.1,7,8
When should we consider therapy?
Therapy is highly dependent on the child, the caregiver, and the social circumstances.1 More than 80% of parents are anxious about molluscum, and countless children are embarrassed or ashamed.1 Ultimately, an unhappy child merits care. The AAP cites the following as reasons to treat: “(1) alleviate discomfort, including itching; (2) reduce autoinoculation; (3) limit transmission of the virus to close contacts; (4) reduce cosmetic concerns; and (5) prevent secondary infection.”6 For adults, we should consider limitations to intimacy and reduction of sexual transmission risk.6
Treatment can be based on the number of lesions. With a few lesions (<3), therapy is worthwhile if they are unsightly; appear on exposed skin causing embarrassment; and/or are itchy, uncomfortable, or large. In a report of 300 children with molluscum treated with cantharidin, most patients choosing therapy had 10 to 20 lesions, but this was over multiple visits.8 Looking at a 2018 data set of 50 patients (all-comers) with molluscum,3 the mean number of lesions was 10 (median, 7); 3 lesions were 1 SD below, while 14, 17, and 45 were 1, 2, and 3 SDs above, respectively. This data set shows that patients can develop more lesions rapidly, and most children have many visible lesions (N.B. Silverberg, MD, unpublished data).
Because each lesion contains infectious viral particles and patients scratch, more lesions are equated to greater autoinoculation and contagion. In addition to the AAP criteria, treatment can be considered for households with immunocompromised individuals, children at risk for new-onset AD, or those with AD at risk for flare. For patients with 45 lesions or more (3 SDs), clearance is harder to achieve with 2 sessions of in-office therapy, and multiple methods or the addition of immunomodulatory therapeutics should be considered.
Do we have to clear every lesion?
New molluscum lesions may arise until a patient achieves immunity, and they may appear more than a month after inoculation, making it difficult to keep up with the rapid spread. Latency between exposure and lesion development usually is 2 to 7 weeks but may be as long as 6 months, making it difficult to prevent spread.6 Therefore, when we treat, we should not promise full clearance to patients and parents. Rather, we should inform them that new lesions may develop later, and therapy is only effective on visible lesions. In a recent study, a 50% clearance of lesions was the satisfactory threshold for parents, demonstrating that satisfaction is possible with partial clearance.9
What is new in therapeutics for molluscum?
Molluscum therapies are either destructive, immunomodulatory, or antiviral. Two agents now are approved by the FDA for the treatment of molluscum infections.
Berdazimer gel 10.3% is approved for patients 1 year or older, but it is not yet available. This agent has both immunomodulatory and antiviral properties.10 It features a home therapy that is mixed on a small palette, then painted on by the patient or parent once daily for 12 weeks. Study outcomes demonstrated more than 50% lesional clearance.11,12 Complete clearance was achieved in at least 30% of patients.12A proprietary topical version of cantharidin 0.7% in flexible collodion is now FDA approved for patients 2 years and older. This vesicant-triggering iatrogenic is targeted at creating blisters overlying molluscum lesions. It is conceptually similar to older versions but with some enhanced features.5,13,14 This version was used for therapy every 3 weeks for up to 4 sessions in clinical trials. Safety is similar across all body sites treated (nonmucosal and not near the mucosal surfaces) but not for mucosa, the mid face, or eyelids.13 Complete lesion clearance was 46.3% to 54% and statistically greater than placebo (P<.001).14Both agents are well tolerated in children with AD; adverse effects include blistering with cantharidin and dermatitislike symptoms with berdazimer.15,16 These therapies have the advantage of being easy to use.
Final Thoughts
We have entered an era of high-quality molluscum therapy. Patient care involves developing a good knowledge of the agents, incorporating shared decision-making with patients and caregivers, and addressing therapy in the context of comorbid diseases such as AD.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305, E1-E2.
- Thompson AJ, Matinpour K, Hardin J, et al. Molluscum gladiatorum. Dermatol Online J. 2014;20:13030/qt0nj121n1.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357. doi:10.1111/pde.12504
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Molluscum contagiosum. In: Kimberlin DW, Lynfield R, Barnett ED, et al (eds). Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd edition. American Academy of Pediatrics. May 26, 2021. Accessed May 20, 2024. https://publications.aap.org/redbook/book/347/chapter/5754264/Molluscum-Contagiosum
- Gross I, Ben Nachum N, Molho-Pessach V, et al. The molluscum contagiosum BOTE sign—infected or inflamed? Pediatr Dermatol. 2020;37:476-479. doi:10.1111/pde.14124
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507. doi:10.1067/mjd.2000.106370
- Maeda-Chubachi T, McLeod L, Enloe C, et al. Defining clinically meaningful improvement in molluscum contagiosum. J Am Acad Dermatol. 2024;90:443-445. doi:10.1016/j.jaad.2023.10.033
- Guttman-Yassky E, Gallo RL, Pavel AB, et al. A nitric oxide-releasing topical medication as a potential treatment option for atopic dermatitis through antimicrobial and anti-inflammatory activity. J Invest Dermatol. 2020;140:2531-2535.e2. doi:10.1016/j.jid.2020.04.013
- Browning JC, Cartwright M, Thorla I Jr, et al. A patient-centered perspective of molluscum contagiosum as reported by B-SIMPLE4 Clinical Trial patients and caregivers: Global Impression of Change and Exit Interview substudy results. Am J Clin Dermatol. 2023;24:119-133. doi:10.1007/s40257-022-00733-9
- Sugarman JL, Hebert A, Browning JC, et al. Berdazimer gel for molluscum contagiosum: an integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2024;90:299-308. doi:10.1016/j.jaad.2023.09.066
- Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and efficacy of VP-102 (cantharidin, 0.7% w/v) in molluscum contagiosum by body region: post hoc pooled analyses from two phase III randomized trials. J Clin Aesthet Dermatol. 2021;14:42-47.
- Eichenfield LF, McFalda W, Brabec B, et al. Safety and efficacy of VP-102, a proprietary, drug-device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:1315-1323. doi:10.1001/jamadermatol.2020.3238
- Paller AS, Green LJ, Silverberg N, et al. Berdazimer gel for molluscum contagiosum in patients with atopic dermatitis. Pediatr Dermatol.Published online February 27, 2024. doi:10.1111/pde.15575
- Eichenfield L, Hebert A, Mancini A, et al. Therapeutic approaches and special considerations for treating molluscum contagiosum. J Drugs Dermatol. 2021;20:1185-1190. doi:10.36849/jdd.6383
Molluscum contagiosum (ie, molluscum) is a ubiquitous infection caused by the poxvirus molluscum contagiosum virus (MCV). Although skin deep, molluscum shares many factors with the more virulent poxviridae. Moisture and trauma can cause viral material to be released from the pearly papules through a small opening, which also allows entry of bacteria and medications into the lesion. The MCV is transmitted by direct contact with skin or via fomites.1
Molluscum can affect children of any age, with MCV type 1 peaking in toddlers and school-aged children and MCV type 2 after the sexual debut. The prevalence of molluscum has increased since the 1980s. It is stressful for children and caregivers and poses challenges in schools as well as sports such as swimming, wrestling, and karate.1,2
For the first time, we have US Food and Drug Administration (FDA)–approved products to treat MCV infections. Previously, only off-label agents were used. Therefore, we have to contemplate why treatment is important to our patients.
What type of care is required for molluscum?
Counseling is the first and only mandatory treatment, which consists of 3 parts: natural history, risk factors for spread, and options for therapy. The natural history of molluscum in children is early spread, contagion to oneself and others (as high as 60% of sibling co-bathers3), triggering of dermatitis, eventual onset of the beginning-of-the-end (BOTE) sign, and eventually clearance. The natural history in adults is poorly understood.
Early clearance is uncommon; reports have suggested 45.6% to 48.4% of affected patients are clear at 1 year and 69.5% to 72.6% at 1.5 years.4 For many children, especially those with atopic dermatitis (AD), lesions linger and often spread, with many experiencing disease for 3 to 4 years. Fomites such as towels, washcloths, and sponges can transfer the virus and spread lesions; therefore, I advise patients to gently pat their skin dry, wash towels frequently, and avoid sharing bathing equipment.1,3,5 Children and adults with immunosuppression may have a greater number of lesions and more prolonged course of disease, including those with HIV as well as DOC8 and CARD11 mutations.6 The American Academy of Pediatrics (AAP) emphasizes that children should not be excluded from attending child care/school or from swimming in public pools but lesions should be covered.6 Lesions, especially those in the antecubital region, can trigger new-onset AD or AD flares.3 In response, gentle skin care including fragrance-free cleansers and periodic application of moisturizers may ward off AD. Topical corticosteroids are preferred.
Dermatitis in MCV is a great mimicker and can resemble erythema multiforme, Gianotti-Crosti syndrome, impetigo, and AD.1 Superinfection recently has been reported; however, in a retrospective analysis of 56 patients with inflamed lesions secondary to molluscum infection, only 7 had positive bacterial cultures, which supports the idea of the swelling and redness of inflammation as a mimic for infection.7 When true infection does occur, tender, swollen, pus-filled lesions should be lanced and cultured.1,7,8
When should we consider therapy?
Therapy is highly dependent on the child, the caregiver, and the social circumstances.1 More than 80% of parents are anxious about molluscum, and countless children are embarrassed or ashamed.1 Ultimately, an unhappy child merits care. The AAP cites the following as reasons to treat: “(1) alleviate discomfort, including itching; (2) reduce autoinoculation; (3) limit transmission of the virus to close contacts; (4) reduce cosmetic concerns; and (5) prevent secondary infection.”6 For adults, we should consider limitations to intimacy and reduction of sexual transmission risk.6
Treatment can be based on the number of lesions. With a few lesions (<3), therapy is worthwhile if they are unsightly; appear on exposed skin causing embarrassment; and/or are itchy, uncomfortable, or large. In a report of 300 children with molluscum treated with cantharidin, most patients choosing therapy had 10 to 20 lesions, but this was over multiple visits.8 Looking at a 2018 data set of 50 patients (all-comers) with molluscum,3 the mean number of lesions was 10 (median, 7); 3 lesions were 1 SD below, while 14, 17, and 45 were 1, 2, and 3 SDs above, respectively. This data set shows that patients can develop more lesions rapidly, and most children have many visible lesions (N.B. Silverberg, MD, unpublished data).
Because each lesion contains infectious viral particles and patients scratch, more lesions are equated to greater autoinoculation and contagion. In addition to the AAP criteria, treatment can be considered for households with immunocompromised individuals, children at risk for new-onset AD, or those with AD at risk for flare. For patients with 45 lesions or more (3 SDs), clearance is harder to achieve with 2 sessions of in-office therapy, and multiple methods or the addition of immunomodulatory therapeutics should be considered.
Do we have to clear every lesion?
New molluscum lesions may arise until a patient achieves immunity, and they may appear more than a month after inoculation, making it difficult to keep up with the rapid spread. Latency between exposure and lesion development usually is 2 to 7 weeks but may be as long as 6 months, making it difficult to prevent spread.6 Therefore, when we treat, we should not promise full clearance to patients and parents. Rather, we should inform them that new lesions may develop later, and therapy is only effective on visible lesions. In a recent study, a 50% clearance of lesions was the satisfactory threshold for parents, demonstrating that satisfaction is possible with partial clearance.9
What is new in therapeutics for molluscum?
Molluscum therapies are either destructive, immunomodulatory, or antiviral. Two agents now are approved by the FDA for the treatment of molluscum infections.
Berdazimer gel 10.3% is approved for patients 1 year or older, but it is not yet available. This agent has both immunomodulatory and antiviral properties.10 It features a home therapy that is mixed on a small palette, then painted on by the patient or parent once daily for 12 weeks. Study outcomes demonstrated more than 50% lesional clearance.11,12 Complete clearance was achieved in at least 30% of patients.12A proprietary topical version of cantharidin 0.7% in flexible collodion is now FDA approved for patients 2 years and older. This vesicant-triggering iatrogenic is targeted at creating blisters overlying molluscum lesions. It is conceptually similar to older versions but with some enhanced features.5,13,14 This version was used for therapy every 3 weeks for up to 4 sessions in clinical trials. Safety is similar across all body sites treated (nonmucosal and not near the mucosal surfaces) but not for mucosa, the mid face, or eyelids.13 Complete lesion clearance was 46.3% to 54% and statistically greater than placebo (P<.001).14Both agents are well tolerated in children with AD; adverse effects include blistering with cantharidin and dermatitislike symptoms with berdazimer.15,16 These therapies have the advantage of being easy to use.
Final Thoughts
We have entered an era of high-quality molluscum therapy. Patient care involves developing a good knowledge of the agents, incorporating shared decision-making with patients and caregivers, and addressing therapy in the context of comorbid diseases such as AD.
Molluscum contagiosum (ie, molluscum) is a ubiquitous infection caused by the poxvirus molluscum contagiosum virus (MCV). Although skin deep, molluscum shares many factors with the more virulent poxviridae. Moisture and trauma can cause viral material to be released from the pearly papules through a small opening, which also allows entry of bacteria and medications into the lesion. The MCV is transmitted by direct contact with skin or via fomites.1
Molluscum can affect children of any age, with MCV type 1 peaking in toddlers and school-aged children and MCV type 2 after the sexual debut. The prevalence of molluscum has increased since the 1980s. It is stressful for children and caregivers and poses challenges in schools as well as sports such as swimming, wrestling, and karate.1,2
For the first time, we have US Food and Drug Administration (FDA)–approved products to treat MCV infections. Previously, only off-label agents were used. Therefore, we have to contemplate why treatment is important to our patients.
What type of care is required for molluscum?
Counseling is the first and only mandatory treatment, which consists of 3 parts: natural history, risk factors for spread, and options for therapy. The natural history of molluscum in children is early spread, contagion to oneself and others (as high as 60% of sibling co-bathers3), triggering of dermatitis, eventual onset of the beginning-of-the-end (BOTE) sign, and eventually clearance. The natural history in adults is poorly understood.
Early clearance is uncommon; reports have suggested 45.6% to 48.4% of affected patients are clear at 1 year and 69.5% to 72.6% at 1.5 years.4 For many children, especially those with atopic dermatitis (AD), lesions linger and often spread, with many experiencing disease for 3 to 4 years. Fomites such as towels, washcloths, and sponges can transfer the virus and spread lesions; therefore, I advise patients to gently pat their skin dry, wash towels frequently, and avoid sharing bathing equipment.1,3,5 Children and adults with immunosuppression may have a greater number of lesions and more prolonged course of disease, including those with HIV as well as DOC8 and CARD11 mutations.6 The American Academy of Pediatrics (AAP) emphasizes that children should not be excluded from attending child care/school or from swimming in public pools but lesions should be covered.6 Lesions, especially those in the antecubital region, can trigger new-onset AD or AD flares.3 In response, gentle skin care including fragrance-free cleansers and periodic application of moisturizers may ward off AD. Topical corticosteroids are preferred.
Dermatitis in MCV is a great mimicker and can resemble erythema multiforme, Gianotti-Crosti syndrome, impetigo, and AD.1 Superinfection recently has been reported; however, in a retrospective analysis of 56 patients with inflamed lesions secondary to molluscum infection, only 7 had positive bacterial cultures, which supports the idea of the swelling and redness of inflammation as a mimic for infection.7 When true infection does occur, tender, swollen, pus-filled lesions should be lanced and cultured.1,7,8
When should we consider therapy?
Therapy is highly dependent on the child, the caregiver, and the social circumstances.1 More than 80% of parents are anxious about molluscum, and countless children are embarrassed or ashamed.1 Ultimately, an unhappy child merits care. The AAP cites the following as reasons to treat: “(1) alleviate discomfort, including itching; (2) reduce autoinoculation; (3) limit transmission of the virus to close contacts; (4) reduce cosmetic concerns; and (5) prevent secondary infection.”6 For adults, we should consider limitations to intimacy and reduction of sexual transmission risk.6
Treatment can be based on the number of lesions. With a few lesions (<3), therapy is worthwhile if they are unsightly; appear on exposed skin causing embarrassment; and/or are itchy, uncomfortable, or large. In a report of 300 children with molluscum treated with cantharidin, most patients choosing therapy had 10 to 20 lesions, but this was over multiple visits.8 Looking at a 2018 data set of 50 patients (all-comers) with molluscum,3 the mean number of lesions was 10 (median, 7); 3 lesions were 1 SD below, while 14, 17, and 45 were 1, 2, and 3 SDs above, respectively. This data set shows that patients can develop more lesions rapidly, and most children have many visible lesions (N.B. Silverberg, MD, unpublished data).
Because each lesion contains infectious viral particles and patients scratch, more lesions are equated to greater autoinoculation and contagion. In addition to the AAP criteria, treatment can be considered for households with immunocompromised individuals, children at risk for new-onset AD, or those with AD at risk for flare. For patients with 45 lesions or more (3 SDs), clearance is harder to achieve with 2 sessions of in-office therapy, and multiple methods or the addition of immunomodulatory therapeutics should be considered.
Do we have to clear every lesion?
New molluscum lesions may arise until a patient achieves immunity, and they may appear more than a month after inoculation, making it difficult to keep up with the rapid spread. Latency between exposure and lesion development usually is 2 to 7 weeks but may be as long as 6 months, making it difficult to prevent spread.6 Therefore, when we treat, we should not promise full clearance to patients and parents. Rather, we should inform them that new lesions may develop later, and therapy is only effective on visible lesions. In a recent study, a 50% clearance of lesions was the satisfactory threshold for parents, demonstrating that satisfaction is possible with partial clearance.9
What is new in therapeutics for molluscum?
Molluscum therapies are either destructive, immunomodulatory, or antiviral. Two agents now are approved by the FDA for the treatment of molluscum infections.
Berdazimer gel 10.3% is approved for patients 1 year or older, but it is not yet available. This agent has both immunomodulatory and antiviral properties.10 It features a home therapy that is mixed on a small palette, then painted on by the patient or parent once daily for 12 weeks. Study outcomes demonstrated more than 50% lesional clearance.11,12 Complete clearance was achieved in at least 30% of patients.12A proprietary topical version of cantharidin 0.7% in flexible collodion is now FDA approved for patients 2 years and older. This vesicant-triggering iatrogenic is targeted at creating blisters overlying molluscum lesions. It is conceptually similar to older versions but with some enhanced features.5,13,14 This version was used for therapy every 3 weeks for up to 4 sessions in clinical trials. Safety is similar across all body sites treated (nonmucosal and not near the mucosal surfaces) but not for mucosa, the mid face, or eyelids.13 Complete lesion clearance was 46.3% to 54% and statistically greater than placebo (P<.001).14Both agents are well tolerated in children with AD; adverse effects include blistering with cantharidin and dermatitislike symptoms with berdazimer.15,16 These therapies have the advantage of being easy to use.
Final Thoughts
We have entered an era of high-quality molluscum therapy. Patient care involves developing a good knowledge of the agents, incorporating shared decision-making with patients and caregivers, and addressing therapy in the context of comorbid diseases such as AD.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305, E1-E2.
- Thompson AJ, Matinpour K, Hardin J, et al. Molluscum gladiatorum. Dermatol Online J. 2014;20:13030/qt0nj121n1.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357. doi:10.1111/pde.12504
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Molluscum contagiosum. In: Kimberlin DW, Lynfield R, Barnett ED, et al (eds). Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd edition. American Academy of Pediatrics. May 26, 2021. Accessed May 20, 2024. https://publications.aap.org/redbook/book/347/chapter/5754264/Molluscum-Contagiosum
- Gross I, Ben Nachum N, Molho-Pessach V, et al. The molluscum contagiosum BOTE sign—infected or inflamed? Pediatr Dermatol. 2020;37:476-479. doi:10.1111/pde.14124
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507. doi:10.1067/mjd.2000.106370
- Maeda-Chubachi T, McLeod L, Enloe C, et al. Defining clinically meaningful improvement in molluscum contagiosum. J Am Acad Dermatol. 2024;90:443-445. doi:10.1016/j.jaad.2023.10.033
- Guttman-Yassky E, Gallo RL, Pavel AB, et al. A nitric oxide-releasing topical medication as a potential treatment option for atopic dermatitis through antimicrobial and anti-inflammatory activity. J Invest Dermatol. 2020;140:2531-2535.e2. doi:10.1016/j.jid.2020.04.013
- Browning JC, Cartwright M, Thorla I Jr, et al. A patient-centered perspective of molluscum contagiosum as reported by B-SIMPLE4 Clinical Trial patients and caregivers: Global Impression of Change and Exit Interview substudy results. Am J Clin Dermatol. 2023;24:119-133. doi:10.1007/s40257-022-00733-9
- Sugarman JL, Hebert A, Browning JC, et al. Berdazimer gel for molluscum contagiosum: an integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2024;90:299-308. doi:10.1016/j.jaad.2023.09.066
- Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and efficacy of VP-102 (cantharidin, 0.7% w/v) in molluscum contagiosum by body region: post hoc pooled analyses from two phase III randomized trials. J Clin Aesthet Dermatol. 2021;14:42-47.
- Eichenfield LF, McFalda W, Brabec B, et al. Safety and efficacy of VP-102, a proprietary, drug-device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:1315-1323. doi:10.1001/jamadermatol.2020.3238
- Paller AS, Green LJ, Silverberg N, et al. Berdazimer gel for molluscum contagiosum in patients with atopic dermatitis. Pediatr Dermatol.Published online February 27, 2024. doi:10.1111/pde.15575
- Eichenfield L, Hebert A, Mancini A, et al. Therapeutic approaches and special considerations for treating molluscum contagiosum. J Drugs Dermatol. 2021;20:1185-1190. doi:10.36849/jdd.6383
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305, E1-E2.
- Thompson AJ, Matinpour K, Hardin J, et al. Molluscum gladiatorum. Dermatol Online J. 2014;20:13030/qt0nj121n1.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357. doi:10.1111/pde.12504
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Molluscum contagiosum. In: Kimberlin DW, Lynfield R, Barnett ED, et al (eds). Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd edition. American Academy of Pediatrics. May 26, 2021. Accessed May 20, 2024. https://publications.aap.org/redbook/book/347/chapter/5754264/Molluscum-Contagiosum
- Gross I, Ben Nachum N, Molho-Pessach V, et al. The molluscum contagiosum BOTE sign—infected or inflamed? Pediatr Dermatol. 2020;37:476-479. doi:10.1111/pde.14124
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507. doi:10.1067/mjd.2000.106370
- Maeda-Chubachi T, McLeod L, Enloe C, et al. Defining clinically meaningful improvement in molluscum contagiosum. J Am Acad Dermatol. 2024;90:443-445. doi:10.1016/j.jaad.2023.10.033
- Guttman-Yassky E, Gallo RL, Pavel AB, et al. A nitric oxide-releasing topical medication as a potential treatment option for atopic dermatitis through antimicrobial and anti-inflammatory activity. J Invest Dermatol. 2020;140:2531-2535.e2. doi:10.1016/j.jid.2020.04.013
- Browning JC, Cartwright M, Thorla I Jr, et al. A patient-centered perspective of molluscum contagiosum as reported by B-SIMPLE4 Clinical Trial patients and caregivers: Global Impression of Change and Exit Interview substudy results. Am J Clin Dermatol. 2023;24:119-133. doi:10.1007/s40257-022-00733-9
- Sugarman JL, Hebert A, Browning JC, et al. Berdazimer gel for molluscum contagiosum: an integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2024;90:299-308. doi:10.1016/j.jaad.2023.09.066
- Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and efficacy of VP-102 (cantharidin, 0.7% w/v) in molluscum contagiosum by body region: post hoc pooled analyses from two phase III randomized trials. J Clin Aesthet Dermatol. 2021;14:42-47.
- Eichenfield LF, McFalda W, Brabec B, et al. Safety and efficacy of VP-102, a proprietary, drug-device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:1315-1323. doi:10.1001/jamadermatol.2020.3238
- Paller AS, Green LJ, Silverberg N, et al. Berdazimer gel for molluscum contagiosum in patients with atopic dermatitis. Pediatr Dermatol.Published online February 27, 2024. doi:10.1111/pde.15575
- Eichenfield L, Hebert A, Mancini A, et al. Therapeutic approaches and special considerations for treating molluscum contagiosum. J Drugs Dermatol. 2021;20:1185-1190. doi:10.36849/jdd.6383
Mpox Update: Clinical Presentation, Vaccination Guidance, and Management
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
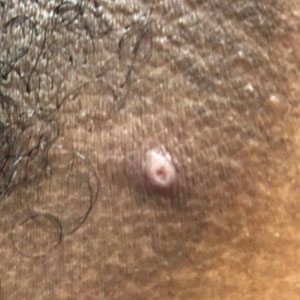
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19
Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19

Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19

Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
Practice Points
- Mpox (monkeypox) lesions typically present as well-circumscribed, painful, umbilicated papules, vesicles, or pustules, with recent cases having a predilection for an anogenital distribution accompanied by systemic viral symptoms.
- Health care workers treating suspected or confirmed cases of mpox should be familiar with current guidelines for controlling the spread of mpox, including proper personal protective equipment (gloves, disposable gowns, N95 or equivalent respirators, and eye protection) and indications for vaccination.
Pediatric Molluscum: An Update
Molluscum contagiosum virus (MCV) infection causes the cutaneous lesions we call molluscum. Molluscum has become common in the last 30 years. Deciding the best course of therapy requires some fundamental understanding about how MCV relates to the following factors: epidemiology, childhood immunity and vaccination, clinical features, comorbidities, and quality of life. Treatment depends on many factors, including presence or absence of atopic dermatitis (AD) and/or pruritus, other symptoms, cosmetic location, and the child’s concern about the lesions. Therapeutics include destructive and immunologic therapies, the latter geared toward increasing immune response.
Epidemiology
Molluscum contagiosum virus is the solo member of the Molluscipoxvirus genus. Infection with MCV causes benign growth or tumors in the skin (ie, molluscum). The infection is slow to clear because the virus reduces the host’s immunity.1,2 Molluscum contagiosum virus is a double-stranded DNA virus that affects keratinocytes and genetically carries the tools for its own replication (ie, DNA-dependent RNA polymerase). The virus has a few subtypes—I/Ia, II, III, and IV—with MCV-I predominating in children and healthy humans and MCV-II in patients with human immunodeficiency virus.1,2 Typing is experimental and is not standardly performed in clinical practice. Molluscum contagiosum virus produces a variety of factors that block the host’s immune response, prolonging infection and preventing erythema and inflammatory response.3
Molluscum contagiosum virus is transmitted through skin-to-skin contact and fomites, including shared towels, bathtubs, spas, bath sponges, and pool equipment.2,4,5 Transmission from household contact and bathing together has been noted in pediatric patients with MCV. Based on the data it can be posited that the lesions are softer when wet and more readily release viral particles or fomites, and fomites may be left on surfaces, especially when a child is wet.6,7 Propensity for infection occurs in patients with AD and in immunosuppressed hosts, including children with human immunodeficiency virus and iatrogenic immunosuppression caused by chemotherapy.1,2,8 Contact sports can increase the risk of transmission, and outbreaks have occurred in pools,5,9 day-care facilities,10 and sports settings.11 Cases of congenital and vertically transmitted molluscum have been documented.12,13 Sexual transmission of MCV may be seen in adolescents who are sexually active. Although child-to-child transmission can occur in the groin area from shared equipment, transmission via sexual abuse also is possible.14 Bargman15 has mentioned the isolated genital location and lack of contact with other infected children as concerning features. Latency of new lesion appearance is anywhere from 1 to 50 days from the date of inoculation; therefore, new lesions are possible and expected even after therapy has been effective in eradicating visible lesions.10 Although clearance has been reported in 6 to 12 months, one pediatric study demonstrated 70% clearance by 1.5 years, suggesting the disease often is more prolonged.16 One-third of children will experience signs of inflammation, such as pruritus and/or erythema. Rare side effects include bacterial superinfection and hypersensitivity.2
One Dutch study from 1994, the largest database survey of children to date, cited a 17% cumulative incidence of molluscum in children by reviewing the data from 103 general practices.17 In a survey and review of molluscum by Braue et al,18 annual rates in populations vary but seem to maximize at approximately 6% to 7%. Sturt et al19 reviewed the prevalence in the indigenous West Sepik section of New Guinea and noted annual incidence rates of 6% in children younger than 10 years (range, 1.8%–10.9%). Epidemics occur and can produce large numbers of cases in a short time period.18 The cumulative prevalence in early childhood may be as high as 22%, as Sturt et al19 observed in children younger than 10 years.
Rising incidence and therefore rising lifetime prevalence appear to have been an issue in the last few decades. Data from the Indian Health Service have demonstrated increases in MCV in Native American children between 2001 and 2005.20 In adults, the data support a steady increase of molluscum from 1988-2007, with a 3-fold increase from 1988-1997 to 1998-2007 in a Spanish study.21 Better population-based data are needed.
Childhood Immunity and Vaccination
Sequence homology between MC133L, a protein of MCV, with vaccinia virus suggests overlapping genes.22 Therefore, it is conceptually possible that the rise in incidence of MCV since the 1980s relates to the loss of herd immunity to variola due to lack of vaccination for smallpox, which has not been offered in the United States since 1972.23 Childhood immunity to MCV varies among studies, but it appears that children do develop antibodies to molluscum in the setting of forming an immune response. Because the rise in molluscum incidence began after the smallpox vaccine was discontinued, the factors appear related; however, the scientific data do not support the theory of a relationship. Mitchell24 has shown that a patient can develop antibodies in response to ground molluscum bodies inoculated into the skin; however, vaccination against molluscum and natural infection do not appear to produce antibodies that would cross-react and protect against other poxviruses, including vaccinia or fowl pox infections.25 Cell-mediated immunity also is required to clear MCV and may account for the inflammatory appearance of lesions as they resolve.26
Demonstrated factors that account for the rise in MCV incidence, aside from alterations in vaccination practices, include spread through sports,9 swimming,11 and AD,7 which have become more commonplace in the United States in the last few decades, supporting the theory that they may be the cause of the increase in childhood MCV infections. Another cause may be the ability of MCV to create factors that stem host immune response.1
Clinical Features
Molluscum lesions have a typical appearance of pearly papules with a central dell. These lesions are lighter to flesh colored and measure 1 to 3 mm.2,4,5 The lesions cluster in the axillae and extremities and average from 10 to 20 per child.6 Lesions clear spontaneously, but new ones will continue to form until immunity is developed. Specific clinical appearances of lesions that are not pearly papules are not infrequent. Table 1 contains a short list of the manifold clinical appearances of molluscum lesions in children.1,2,7,27-35 In particular, certain clinical appearances should be considered. In small children, head and neck lesions resembling milia are not uncommon. Giant or wartlike lesions can appear on the head, neck, or gluteal region in children and are clinical mimics of condyloma or other warts (Figure 1). Giant lesions also can grow in the subcutaneous space and mimic a cyst or abscess.27 Erosive lesions mimicking eczema vaccinatum can be seen (Figure 2), but dermoscopy may demonstrate central dells in some lesions. Other viral processes mimicked include Gianotti Crosti–like lesions (Figure 3) that appear when a papular id reaction forms over the extremities or a localized version in the axilla, mimicking unilateral laterothoracic exanthema.2,36,37 Hypersensitivity reactions are commonly noted with clearance and can be papular or demonstrate swelling and erythema, termed the beginning-of-the-end sign.38


Pruritus, erythema, and swelling can occur with clearance but do not appear in all patients. Addressing pruritus is important to prevent disease spread, as patients are likely to inoculate other areas of the skin with virus when they scratch, and lesion number is reduced with dermatitis interventions.36
Comorbidities
Molluscum lesions can occur in any child; however, the impaired immunologic status and skin barrier in patients with AD is ripe for the extensive spread of lesions that is associated with higher lesion count.36 Children with molluscum infection can experience new-onset dermatitis or triggering of AD flares, especially on the extremities, such as the antecubital and popliteal regions.7 A study of children with MCV infection demonstrated that treatment of active dermatitis reduced spread. The authors mentioned autoinoculation as the mechanism; however, these data also suggest supporting barrier state as a factor in disease spread.36 Superinfection can occur prior to6 or after therapy for lesions,37 but it is unclear if this relates to the underlying atopic diathesis. Children with molluscum have been described to have warts, psoriasis, family history of atopy, diabetes mellitus, and pityriasis alba,7 while immunosuppression of any kind is associated with molluscum and high lesion count or prolonged disease in childhood.1,2
Quality of Life
Children with molluscum who have higher lesion counts appear to be at risk for severe effects on their quality of life. Approximately 10% of children with MCV infection have been documented to have severe impairments on quality of life.39 In my practice, quality of life in children with MCV appears to be affected by many factors (Table 2).7,18,39

Treatments
Proper Skin Care and Treatment of AD
Therapy for AD and/or pruritus appears to limit lesion number in children with MCV and rashes or itch.7,36 I recommend barrier repair agents, including emollients and syndet bar cleansers, to prevent small breaks in the skin that occur with xerosis and AD and that increase itch and risk of spread. Therapy for AD and molluscum dermatitis is similar and overlapping. There is always a concern about the spread of MCV when using topical calcineurin inhibitors. I, therefore, focus the dermatitis therapeutics on topical corticosteroid–based care.6,40
Prevention of Spread
Prevention of spread begins with hygiene interventions. Cobathing is common in children with MCV and should be held off when possible. It is important for the child with MCV to avoid sharing bath towels and equipment23 and having bare skin come in contact with mats in sports. I request that children with MCV wear bathing suits that cover the areas affected.
Reassurance
The most important therapy is reassurance.41 Many parents/guardians are truly unaware that the MCV infection can last for more than a year and therefore worry over normal disease course. When counseled as to the benign course of illness and given instructions on proper skin care, the parent/guardian of a child with MCV will often opt against therapy of uncomplicated cases. On the other hand, there are medical reasons for treatment, and they support the need for intervention (Table 3). Seventy percent of lesions resolve in 1.5 years; however, of the residual infections, some may last as long as 4 years.16 It is not recommended to stop children from attending school because of MCV.

Interventional Therapy
Therapeutics of MCV include destructive therapies in office (ie, cantharidin, cryotherapy, curettage, trichloroacetic acid, and glycolic acid) and at-home therapies (ie, topical retinoids, nitric oxide releasers)(eTable).2,5,6,42-58 When there are many lesions or spread is noted, immunotherapies can be used, including topical imiquimod, oral cimetidine, and intralesional Candida antigen.2,4,7 Pulsed dye laser cuts off the lesion vascular supply, while cidofovir is directly antiviral both topically and systemically, the latter reserved for severe cases in immunosuppressed adults.59 Head-to-head studies of cantharidin, curettage, topical peeling agents, and imiquimod demonstrated better satisfaction and fewer office visits with topical anesthetic and curettage on the first visit. Side effects were greatest for salicylic acid and glycolic acid; therefore, these agents are less desirable.42
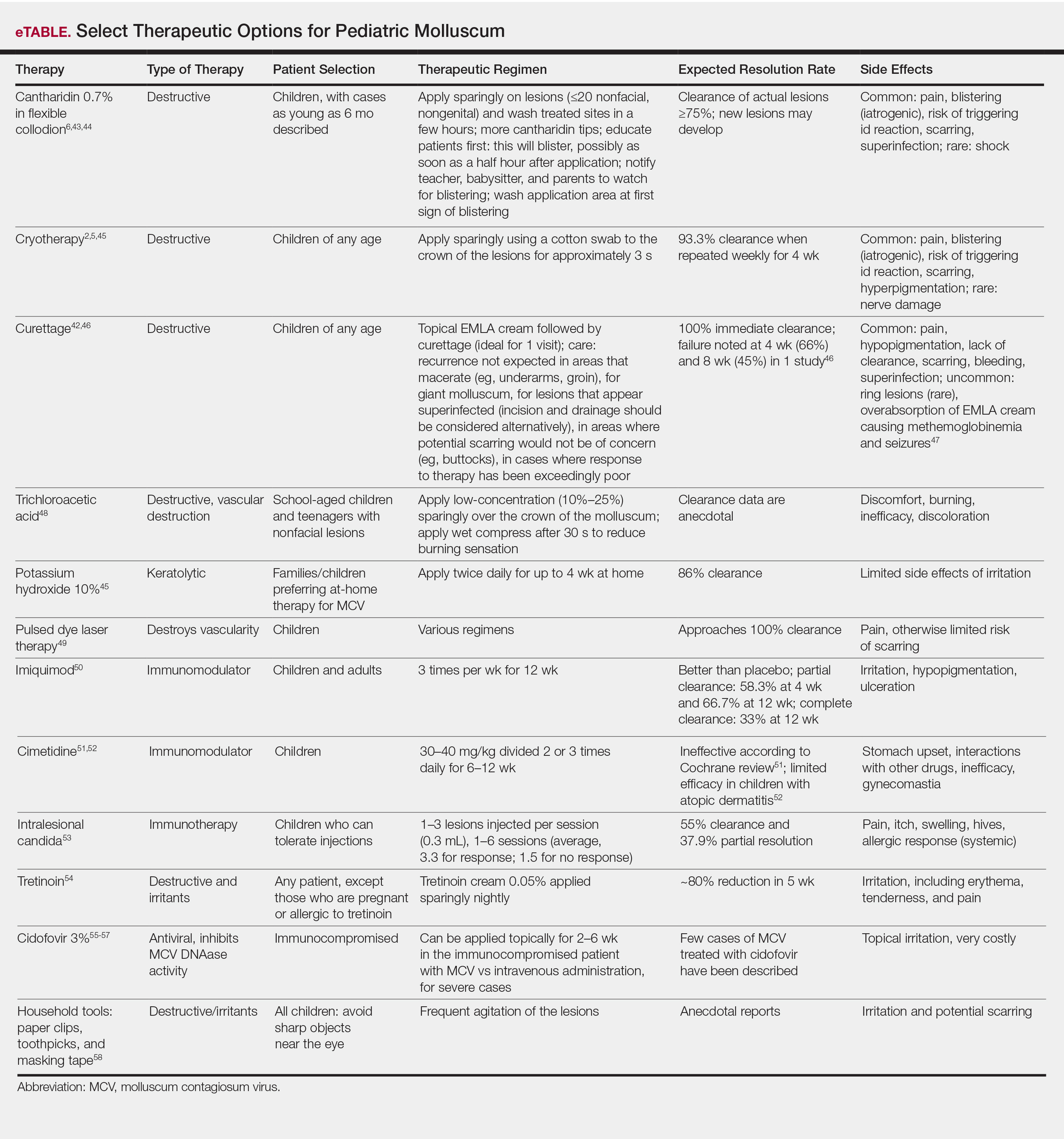
Conclusion
Molluscum is a cutaneous viral infection that is common in children and has associated morbidities, including AD, pruritus, poor quality of life in some cases, and risk of contagion. Addressing the disease includes understanding its natural history and explaining it to parents/guardians. Therapeutics can be offered in cases where need is demonstrated, such as with lesions that spread and cause discomfort. Choice of therapeutics depends on the practitioner’s experience, the child’s clinical appearance, availability of therapy, and review of options with the parents/guardians. When avoidance of intervention is desired, barrier enhancement and treatment of symptomatic dermatitis are still beneficial, as are household (eg, not sharing towels) and activity (eg, adhesive bandages over active lesions) interventions to reduce transmission.
- Shisler JL. Immune evasion strategies of molluscum contagiosum virus. Adv Virus Res. 2015;92:201-252.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Moss B, Shisler JL, Xiang Y, et al. Immune-defense molecules of molluscum contagiosum virus, a human poxvirus. Trends Microbiol. 2000;8:473-477.
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Ajithkumar VT, Sasidharanpillai S, Muhammed K, et al. Disseminated molluscum contagiosum following chemotherapy: a therapeutic challenge. Indian J Dermatol Venereol Leprol. 2017;83:516.
- Oren B, Wende SO. An outbreak of molluscum contagiosum in a kibbutz. Infection. 1991;19:159-161.
- Molluscum contagiosum. Healthy Children website. https://www.healthychildren.org/English/health-issues/conditions/skin/Pages/Molluscum-Contagiosum.aspx. Updated November 21, 2015. Accessed October 16, 2019.
- Peterson AR, Nash E, Anderson BJ. Infectious disease in contact sports. Sports Health. 2019;11:47-58.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Mendiratta V, Agarwal S, Chander R. Reappraisal of sexually transmitted infections in children: a hospital-based study from an urban area. Indian J Sex Transm Dis AIDS. 2014;35:25-28.
- Bargman H. Genital molluscum contagiosum in children: evidence of sexual abuse? CMAJ. 1986;135:432-433.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Koning S, Bruijnzeels MA, van Suijlekom-Smit LW, et al. Molluscum contagiosum in Dutch general practice. Br J Gen Pract. 1994;44:417-419.
- Braue A, Ross G, Varigos G, et al. Epidemiology and impact of childhood molluscum contagiosum: a case series and critical review of the literature. Pediatr Dermatol. 2005;22:287-294.
- Sturt RJ, Muller HK, Francis GD. Molluscum contagiosum in villages of the West Sepik District of New Guinea. Med J Aust. 1971;2:751-754.
- Reynolds MG, Homan RC, Yorita Christensen KL, et al. The incidence of molluscum contagiosum among American Indians and Alaska Natives. PLoS One. 2009;4:e5255.
- Villa L, Varela JA, Otero L, et al. Molluscum contagiosum: a 20-year study in a sexually transmitted infections unit. Sex Transm Dis. 2010;37:423-424.
- Watanabe T, Morikawa S, Suzuki K, et al. Two major antigenic polypeptides of molluscum contagiosum virus. J Infect Dis. 1998;177:284-292.
- Vaccine basics. Centers for Disease Control and Prevention website. https://www.cdc.gov/smallpox/vaccine-basics/index.html. Updated July 12, 2017. Accessed October 16, 2019.
- Mitchell JC. Observations on the virus of molluscum contagiosum. Br J Exp Pathol. 1953;34:44-49.
- Konya J, Thompson CH. Molluscum contagiosum virus: antibody responses in patients with clinical lesions and its sero-epidemiology in a representative Australian population. J Infect Dis. 1999;179:701-704.
- Steffen C, Markman JA. Spontaneous disappearance of molluscum contagiosum. Arch Dermatol. 1980;116:923-924.
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73.
- Persechino S, Abruzzese C, Caperchi C, et al. Condyloma acuminata and mollusca contagiosa: a giant manifestation in a patient with lupus. Skinmed. 2014;12:310-311.
- Kim SK, Do JE, Kang HY, et al. Giant molluscum contagiosum of immunocompetent children occurring on the anogenital area. Eur J Dermatol. 2007;17:537-538.
- Alam MS, Shrirao N. Giant molluscum contagiosum presenting as lid neoplasm in an immunocompetent child. Dermatol Online J. 2016;22. pii:13030/qt56v567gn.
- Krishnamurthy J, Nagappa DK. The cytology of molluscum contagiosum mimicking skin adnexal tumor. J Cytol. 2010;27:74-75.
- Baek YS, Oh CH, Song HJ, et al. Asymmetrical periflexural exanthem of childhood with concurrence of molluscum contagiosum infection. Clin Exp Dermatol. 2011;36:676-677.
- Lee HJ, Kwon JA, Kim JW. Erythema multiforme-like molluscum dermatitis. Acta Derm Venereol. 2002;82:217-218.
- Lee YB, Choi HJ, Park HJ, et al. Two cases of erythema multiforme associated with molluscum contagiosum. Int J Dermatol. 2009;48:659-660.
- Vasily DB, Bhatia SG. Erythema annulare centrifugum and molluscum contagiosum.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653.
- Olsen JR, Gallagher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Goksugur N, Ozbostanci B, Goksugur SB. Molluscum contagiosum infection associated with pimecrolimus use in pityriasis alba. Pediatr Dermatol. 2007;24:E63-E65.
- Lee R, Schwartz RA. Pediatric molluscum contagiosum: reflections on the last challenging poxvirus infection, part 1. Cutis. 2010;86:230-236.
- Hanna D, Hatami A, Powell J, et al. A prospective randomized trial comparing the efficacy and adverse effects of four recognized treatments of molluscum contagiosum in children. Pediatr Dermatol. 2006;23:574-579.
- Coloe Dosal J, Stewart PW, Lin JA, et al. Cantharidin for the treatment of molluscum contagiosum: a prospective, double-blinded, placebo-controlled trial. Pediatr Dermatol. 2014;31:440-449.
- Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
- Handjani F, Behazin E, Sadati MS. Comparison of 10% potassium hydroxide solution versus cryotherapy in the treatment of molluscum contagiosum: an open randomized clinical trial. J Dermatolog Treat. 2014;25:249-250.
- Simonart T, De Maertelaer V. Curettage treatment for molluscum contagiosum: a follow-up survey study. Br J Dermatol. 2008;159:1144-1147.
- Cho YS, Chung BY, Park CW, et al. Seizures and methemoglobinemia after topical application of eutectic mixture of lidocaine and prilocaine on a 3.5-year-old child with molluscum contagiosum and atopic dermatitis. Pediatr Dermatol. 2016;33:E284-E285.
- Bard S, Shiman MI, Bellman B, et al. Treatment of facial molluscum contagiosum with trichloroacetic acid. Pediatr Dermatol. 2009;26:425-426.
- Griffith RD, Yazdani Abyaneh MA, Falto-Aizpurua L, et al. Pulsed dye laser therapy for molluscum contagiosum: a systematic review. J Drugs Dermatol. 2014;13:1349-1352.
- Theos AU, Cummins R, Silverberg NB, et al. Effectiveness of imiquimod cream 5% for treating childhood molluscum contagiosum in a double-blind, randomized pilot trial. Cutis. 2004;74:134-138, 141-142.
- van der Wouden JC, Menke J, Gajadin S, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2006:CD004767.
- Cunningham BB, Paller AS, Garzon M. Inefficacy of oral cimetidine for nonatopic children with molluscum contagiosum. Pediatr Dermatol. 1998;15:71-72.
- Enns LL, Evans MS. Intralesional immunotherapy with Candida antigen for the treatment of molluscum contagiosum in children. Pediatr Dermatol. 2011;28:254-258.
- Rajouria EA, Amatya A, Karn D. Comparative study of 5% potassium hydroxide solution versus 0.05% tretinoin cream for molluscum contagiosum in children. Kathmandu Univ Med J (KUMJ). 2011;9:291-294.
- Briand S, Milpied B, Navas D, et al. 1% topical cidofovir used as last alternative to treat viral infections. J Eur Acad Dermatol Venereol. 2008;22:249-250.
- Zabawski EJ Jr, Cockerell CJ. Topical cidofovir for molluscum contagiosum in children. Pediatr Dermatol. 1999;16:414-415.
- Watanabe T. Cidofovir diphosphate inhibits molluscum contagiosum virus DNA polymerase activity. J Invest Dermatol. 2008;128:1327-1329.
- Lindau MS, Munar MY. Use of duct tape occlusion in the treatment of recurrent molluscum contagiosum. Pediatr Dermatol. 2004;21:609.
- Silverberg N. Pediatric molluscum contagiosum: optimal treatment strategies. Paediatr Drugs. 2003;5:505-512.
Molluscum contagiosum virus (MCV) infection causes the cutaneous lesions we call molluscum. Molluscum has become common in the last 30 years. Deciding the best course of therapy requires some fundamental understanding about how MCV relates to the following factors: epidemiology, childhood immunity and vaccination, clinical features, comorbidities, and quality of life. Treatment depends on many factors, including presence or absence of atopic dermatitis (AD) and/or pruritus, other symptoms, cosmetic location, and the child’s concern about the lesions. Therapeutics include destructive and immunologic therapies, the latter geared toward increasing immune response.
Epidemiology
Molluscum contagiosum virus is the solo member of the Molluscipoxvirus genus. Infection with MCV causes benign growth or tumors in the skin (ie, molluscum). The infection is slow to clear because the virus reduces the host’s immunity.1,2 Molluscum contagiosum virus is a double-stranded DNA virus that affects keratinocytes and genetically carries the tools for its own replication (ie, DNA-dependent RNA polymerase). The virus has a few subtypes—I/Ia, II, III, and IV—with MCV-I predominating in children and healthy humans and MCV-II in patients with human immunodeficiency virus.1,2 Typing is experimental and is not standardly performed in clinical practice. Molluscum contagiosum virus produces a variety of factors that block the host’s immune response, prolonging infection and preventing erythema and inflammatory response.3
Molluscum contagiosum virus is transmitted through skin-to-skin contact and fomites, including shared towels, bathtubs, spas, bath sponges, and pool equipment.2,4,5 Transmission from household contact and bathing together has been noted in pediatric patients with MCV. Based on the data it can be posited that the lesions are softer when wet and more readily release viral particles or fomites, and fomites may be left on surfaces, especially when a child is wet.6,7 Propensity for infection occurs in patients with AD and in immunosuppressed hosts, including children with human immunodeficiency virus and iatrogenic immunosuppression caused by chemotherapy.1,2,8 Contact sports can increase the risk of transmission, and outbreaks have occurred in pools,5,9 day-care facilities,10 and sports settings.11 Cases of congenital and vertically transmitted molluscum have been documented.12,13 Sexual transmission of MCV may be seen in adolescents who are sexually active. Although child-to-child transmission can occur in the groin area from shared equipment, transmission via sexual abuse also is possible.14 Bargman15 has mentioned the isolated genital location and lack of contact with other infected children as concerning features. Latency of new lesion appearance is anywhere from 1 to 50 days from the date of inoculation; therefore, new lesions are possible and expected even after therapy has been effective in eradicating visible lesions.10 Although clearance has been reported in 6 to 12 months, one pediatric study demonstrated 70% clearance by 1.5 years, suggesting the disease often is more prolonged.16 One-third of children will experience signs of inflammation, such as pruritus and/or erythema. Rare side effects include bacterial superinfection and hypersensitivity.2
One Dutch study from 1994, the largest database survey of children to date, cited a 17% cumulative incidence of molluscum in children by reviewing the data from 103 general practices.17 In a survey and review of molluscum by Braue et al,18 annual rates in populations vary but seem to maximize at approximately 6% to 7%. Sturt et al19 reviewed the prevalence in the indigenous West Sepik section of New Guinea and noted annual incidence rates of 6% in children younger than 10 years (range, 1.8%–10.9%). Epidemics occur and can produce large numbers of cases in a short time period.18 The cumulative prevalence in early childhood may be as high as 22%, as Sturt et al19 observed in children younger than 10 years.
Rising incidence and therefore rising lifetime prevalence appear to have been an issue in the last few decades. Data from the Indian Health Service have demonstrated increases in MCV in Native American children between 2001 and 2005.20 In adults, the data support a steady increase of molluscum from 1988-2007, with a 3-fold increase from 1988-1997 to 1998-2007 in a Spanish study.21 Better population-based data are needed.
Childhood Immunity and Vaccination
Sequence homology between MC133L, a protein of MCV, with vaccinia virus suggests overlapping genes.22 Therefore, it is conceptually possible that the rise in incidence of MCV since the 1980s relates to the loss of herd immunity to variola due to lack of vaccination for smallpox, which has not been offered in the United States since 1972.23 Childhood immunity to MCV varies among studies, but it appears that children do develop antibodies to molluscum in the setting of forming an immune response. Because the rise in molluscum incidence began after the smallpox vaccine was discontinued, the factors appear related; however, the scientific data do not support the theory of a relationship. Mitchell24 has shown that a patient can develop antibodies in response to ground molluscum bodies inoculated into the skin; however, vaccination against molluscum and natural infection do not appear to produce antibodies that would cross-react and protect against other poxviruses, including vaccinia or fowl pox infections.25 Cell-mediated immunity also is required to clear MCV and may account for the inflammatory appearance of lesions as they resolve.26
Demonstrated factors that account for the rise in MCV incidence, aside from alterations in vaccination practices, include spread through sports,9 swimming,11 and AD,7 which have become more commonplace in the United States in the last few decades, supporting the theory that they may be the cause of the increase in childhood MCV infections. Another cause may be the ability of MCV to create factors that stem host immune response.1
Clinical Features
Molluscum lesions have a typical appearance of pearly papules with a central dell. These lesions are lighter to flesh colored and measure 1 to 3 mm.2,4,5 The lesions cluster in the axillae and extremities and average from 10 to 20 per child.6 Lesions clear spontaneously, but new ones will continue to form until immunity is developed. Specific clinical appearances of lesions that are not pearly papules are not infrequent. Table 1 contains a short list of the manifold clinical appearances of molluscum lesions in children.1,2,7,27-35 In particular, certain clinical appearances should be considered. In small children, head and neck lesions resembling milia are not uncommon. Giant or wartlike lesions can appear on the head, neck, or gluteal region in children and are clinical mimics of condyloma or other warts (Figure 1). Giant lesions also can grow in the subcutaneous space and mimic a cyst or abscess.27 Erosive lesions mimicking eczema vaccinatum can be seen (Figure 2), but dermoscopy may demonstrate central dells in some lesions. Other viral processes mimicked include Gianotti Crosti–like lesions (Figure 3) that appear when a papular id reaction forms over the extremities or a localized version in the axilla, mimicking unilateral laterothoracic exanthema.2,36,37 Hypersensitivity reactions are commonly noted with clearance and can be papular or demonstrate swelling and erythema, termed the beginning-of-the-end sign.38




Pruritus, erythema, and swelling can occur with clearance but do not appear in all patients. Addressing pruritus is important to prevent disease spread, as patients are likely to inoculate other areas of the skin with virus when they scratch, and lesion number is reduced with dermatitis interventions.36
Comorbidities
Molluscum lesions can occur in any child; however, the impaired immunologic status and skin barrier in patients with AD is ripe for the extensive spread of lesions that is associated with higher lesion count.36 Children with molluscum infection can experience new-onset dermatitis or triggering of AD flares, especially on the extremities, such as the antecubital and popliteal regions.7 A study of children with MCV infection demonstrated that treatment of active dermatitis reduced spread. The authors mentioned autoinoculation as the mechanism; however, these data also suggest supporting barrier state as a factor in disease spread.36 Superinfection can occur prior to6 or after therapy for lesions,37 but it is unclear if this relates to the underlying atopic diathesis. Children with molluscum have been described to have warts, psoriasis, family history of atopy, diabetes mellitus, and pityriasis alba,7 while immunosuppression of any kind is associated with molluscum and high lesion count or prolonged disease in childhood.1,2
Quality of Life
Children with molluscum who have higher lesion counts appear to be at risk for severe effects on their quality of life. Approximately 10% of children with MCV infection have been documented to have severe impairments on quality of life.39 In my practice, quality of life in children with MCV appears to be affected by many factors (Table 2).7,18,39

Treatments
Proper Skin Care and Treatment of AD
Therapy for AD and/or pruritus appears to limit lesion number in children with MCV and rashes or itch.7,36 I recommend barrier repair agents, including emollients and syndet bar cleansers, to prevent small breaks in the skin that occur with xerosis and AD and that increase itch and risk of spread. Therapy for AD and molluscum dermatitis is similar and overlapping. There is always a concern about the spread of MCV when using topical calcineurin inhibitors. I, therefore, focus the dermatitis therapeutics on topical corticosteroid–based care.6,40
Prevention of Spread
Prevention of spread begins with hygiene interventions. Cobathing is common in children with MCV and should be held off when possible. It is important for the child with MCV to avoid sharing bath towels and equipment23 and having bare skin come in contact with mats in sports. I request that children with MCV wear bathing suits that cover the areas affected.
Reassurance
The most important therapy is reassurance.41 Many parents/guardians are truly unaware that the MCV infection can last for more than a year and therefore worry over normal disease course. When counseled as to the benign course of illness and given instructions on proper skin care, the parent/guardian of a child with MCV will often opt against therapy of uncomplicated cases. On the other hand, there are medical reasons for treatment, and they support the need for intervention (Table 3). Seventy percent of lesions resolve in 1.5 years; however, of the residual infections, some may last as long as 4 years.16 It is not recommended to stop children from attending school because of MCV.

Interventional Therapy
Therapeutics of MCV include destructive therapies in office (ie, cantharidin, cryotherapy, curettage, trichloroacetic acid, and glycolic acid) and at-home therapies (ie, topical retinoids, nitric oxide releasers)(eTable).2,5,6,42-58 When there are many lesions or spread is noted, immunotherapies can be used, including topical imiquimod, oral cimetidine, and intralesional Candida antigen.2,4,7 Pulsed dye laser cuts off the lesion vascular supply, while cidofovir is directly antiviral both topically and systemically, the latter reserved for severe cases in immunosuppressed adults.59 Head-to-head studies of cantharidin, curettage, topical peeling agents, and imiquimod demonstrated better satisfaction and fewer office visits with topical anesthetic and curettage on the first visit. Side effects were greatest for salicylic acid and glycolic acid; therefore, these agents are less desirable.42

Conclusion
Molluscum is a cutaneous viral infection that is common in children and has associated morbidities, including AD, pruritus, poor quality of life in some cases, and risk of contagion. Addressing the disease includes understanding its natural history and explaining it to parents/guardians. Therapeutics can be offered in cases where need is demonstrated, such as with lesions that spread and cause discomfort. Choice of therapeutics depends on the practitioner’s experience, the child’s clinical appearance, availability of therapy, and review of options with the parents/guardians. When avoidance of intervention is desired, barrier enhancement and treatment of symptomatic dermatitis are still beneficial, as are household (eg, not sharing towels) and activity (eg, adhesive bandages over active lesions) interventions to reduce transmission.
Molluscum contagiosum virus (MCV) infection causes the cutaneous lesions we call molluscum. Molluscum has become common in the last 30 years. Deciding the best course of therapy requires some fundamental understanding about how MCV relates to the following factors: epidemiology, childhood immunity and vaccination, clinical features, comorbidities, and quality of life. Treatment depends on many factors, including presence or absence of atopic dermatitis (AD) and/or pruritus, other symptoms, cosmetic location, and the child’s concern about the lesions. Therapeutics include destructive and immunologic therapies, the latter geared toward increasing immune response.
Epidemiology
Molluscum contagiosum virus is the solo member of the Molluscipoxvirus genus. Infection with MCV causes benign growth or tumors in the skin (ie, molluscum). The infection is slow to clear because the virus reduces the host’s immunity.1,2 Molluscum contagiosum virus is a double-stranded DNA virus that affects keratinocytes and genetically carries the tools for its own replication (ie, DNA-dependent RNA polymerase). The virus has a few subtypes—I/Ia, II, III, and IV—with MCV-I predominating in children and healthy humans and MCV-II in patients with human immunodeficiency virus.1,2 Typing is experimental and is not standardly performed in clinical practice. Molluscum contagiosum virus produces a variety of factors that block the host’s immune response, prolonging infection and preventing erythema and inflammatory response.3
Molluscum contagiosum virus is transmitted through skin-to-skin contact and fomites, including shared towels, bathtubs, spas, bath sponges, and pool equipment.2,4,5 Transmission from household contact and bathing together has been noted in pediatric patients with MCV. Based on the data it can be posited that the lesions are softer when wet and more readily release viral particles or fomites, and fomites may be left on surfaces, especially when a child is wet.6,7 Propensity for infection occurs in patients with AD and in immunosuppressed hosts, including children with human immunodeficiency virus and iatrogenic immunosuppression caused by chemotherapy.1,2,8 Contact sports can increase the risk of transmission, and outbreaks have occurred in pools,5,9 day-care facilities,10 and sports settings.11 Cases of congenital and vertically transmitted molluscum have been documented.12,13 Sexual transmission of MCV may be seen in adolescents who are sexually active. Although child-to-child transmission can occur in the groin area from shared equipment, transmission via sexual abuse also is possible.14 Bargman15 has mentioned the isolated genital location and lack of contact with other infected children as concerning features. Latency of new lesion appearance is anywhere from 1 to 50 days from the date of inoculation; therefore, new lesions are possible and expected even after therapy has been effective in eradicating visible lesions.10 Although clearance has been reported in 6 to 12 months, one pediatric study demonstrated 70% clearance by 1.5 years, suggesting the disease often is more prolonged.16 One-third of children will experience signs of inflammation, such as pruritus and/or erythema. Rare side effects include bacterial superinfection and hypersensitivity.2
One Dutch study from 1994, the largest database survey of children to date, cited a 17% cumulative incidence of molluscum in children by reviewing the data from 103 general practices.17 In a survey and review of molluscum by Braue et al,18 annual rates in populations vary but seem to maximize at approximately 6% to 7%. Sturt et al19 reviewed the prevalence in the indigenous West Sepik section of New Guinea and noted annual incidence rates of 6% in children younger than 10 years (range, 1.8%–10.9%). Epidemics occur and can produce large numbers of cases in a short time period.18 The cumulative prevalence in early childhood may be as high as 22%, as Sturt et al19 observed in children younger than 10 years.
Rising incidence and therefore rising lifetime prevalence appear to have been an issue in the last few decades. Data from the Indian Health Service have demonstrated increases in MCV in Native American children between 2001 and 2005.20 In adults, the data support a steady increase of molluscum from 1988-2007, with a 3-fold increase from 1988-1997 to 1998-2007 in a Spanish study.21 Better population-based data are needed.
Childhood Immunity and Vaccination
Sequence homology between MC133L, a protein of MCV, with vaccinia virus suggests overlapping genes.22 Therefore, it is conceptually possible that the rise in incidence of MCV since the 1980s relates to the loss of herd immunity to variola due to lack of vaccination for smallpox, which has not been offered in the United States since 1972.23 Childhood immunity to MCV varies among studies, but it appears that children do develop antibodies to molluscum in the setting of forming an immune response. Because the rise in molluscum incidence began after the smallpox vaccine was discontinued, the factors appear related; however, the scientific data do not support the theory of a relationship. Mitchell24 has shown that a patient can develop antibodies in response to ground molluscum bodies inoculated into the skin; however, vaccination against molluscum and natural infection do not appear to produce antibodies that would cross-react and protect against other poxviruses, including vaccinia or fowl pox infections.25 Cell-mediated immunity also is required to clear MCV and may account for the inflammatory appearance of lesions as they resolve.26
Demonstrated factors that account for the rise in MCV incidence, aside from alterations in vaccination practices, include spread through sports,9 swimming,11 and AD,7 which have become more commonplace in the United States in the last few decades, supporting the theory that they may be the cause of the increase in childhood MCV infections. Another cause may be the ability of MCV to create factors that stem host immune response.1
Clinical Features
Molluscum lesions have a typical appearance of pearly papules with a central dell. These lesions are lighter to flesh colored and measure 1 to 3 mm.2,4,5 The lesions cluster in the axillae and extremities and average from 10 to 20 per child.6 Lesions clear spontaneously, but new ones will continue to form until immunity is developed. Specific clinical appearances of lesions that are not pearly papules are not infrequent. Table 1 contains a short list of the manifold clinical appearances of molluscum lesions in children.1,2,7,27-35 In particular, certain clinical appearances should be considered. In small children, head and neck lesions resembling milia are not uncommon. Giant or wartlike lesions can appear on the head, neck, or gluteal region in children and are clinical mimics of condyloma or other warts (Figure 1). Giant lesions also can grow in the subcutaneous space and mimic a cyst or abscess.27 Erosive lesions mimicking eczema vaccinatum can be seen (Figure 2), but dermoscopy may demonstrate central dells in some lesions. Other viral processes mimicked include Gianotti Crosti–like lesions (Figure 3) that appear when a papular id reaction forms over the extremities or a localized version in the axilla, mimicking unilateral laterothoracic exanthema.2,36,37 Hypersensitivity reactions are commonly noted with clearance and can be papular or demonstrate swelling and erythema, termed the beginning-of-the-end sign.38




Pruritus, erythema, and swelling can occur with clearance but do not appear in all patients. Addressing pruritus is important to prevent disease spread, as patients are likely to inoculate other areas of the skin with virus when they scratch, and lesion number is reduced with dermatitis interventions.36
Comorbidities
Molluscum lesions can occur in any child; however, the impaired immunologic status and skin barrier in patients with AD is ripe for the extensive spread of lesions that is associated with higher lesion count.36 Children with molluscum infection can experience new-onset dermatitis or triggering of AD flares, especially on the extremities, such as the antecubital and popliteal regions.7 A study of children with MCV infection demonstrated that treatment of active dermatitis reduced spread. The authors mentioned autoinoculation as the mechanism; however, these data also suggest supporting barrier state as a factor in disease spread.36 Superinfection can occur prior to6 or after therapy for lesions,37 but it is unclear if this relates to the underlying atopic diathesis. Children with molluscum have been described to have warts, psoriasis, family history of atopy, diabetes mellitus, and pityriasis alba,7 while immunosuppression of any kind is associated with molluscum and high lesion count or prolonged disease in childhood.1,2
Quality of Life
Children with molluscum who have higher lesion counts appear to be at risk for severe effects on their quality of life. Approximately 10% of children with MCV infection have been documented to have severe impairments on quality of life.39 In my practice, quality of life in children with MCV appears to be affected by many factors (Table 2).7,18,39

Treatments
Proper Skin Care and Treatment of AD
Therapy for AD and/or pruritus appears to limit lesion number in children with MCV and rashes or itch.7,36 I recommend barrier repair agents, including emollients and syndet bar cleansers, to prevent small breaks in the skin that occur with xerosis and AD and that increase itch and risk of spread. Therapy for AD and molluscum dermatitis is similar and overlapping. There is always a concern about the spread of MCV when using topical calcineurin inhibitors. I, therefore, focus the dermatitis therapeutics on topical corticosteroid–based care.6,40
Prevention of Spread
Prevention of spread begins with hygiene interventions. Cobathing is common in children with MCV and should be held off when possible. It is important for the child with MCV to avoid sharing bath towels and equipment23 and having bare skin come in contact with mats in sports. I request that children with MCV wear bathing suits that cover the areas affected.
Reassurance
The most important therapy is reassurance.41 Many parents/guardians are truly unaware that the MCV infection can last for more than a year and therefore worry over normal disease course. When counseled as to the benign course of illness and given instructions on proper skin care, the parent/guardian of a child with MCV will often opt against therapy of uncomplicated cases. On the other hand, there are medical reasons for treatment, and they support the need for intervention (Table 3). Seventy percent of lesions resolve in 1.5 years; however, of the residual infections, some may last as long as 4 years.16 It is not recommended to stop children from attending school because of MCV.

Interventional Therapy
Therapeutics of MCV include destructive therapies in office (ie, cantharidin, cryotherapy, curettage, trichloroacetic acid, and glycolic acid) and at-home therapies (ie, topical retinoids, nitric oxide releasers)(eTable).2,5,6,42-58 When there are many lesions or spread is noted, immunotherapies can be used, including topical imiquimod, oral cimetidine, and intralesional Candida antigen.2,4,7 Pulsed dye laser cuts off the lesion vascular supply, while cidofovir is directly antiviral both topically and systemically, the latter reserved for severe cases in immunosuppressed adults.59 Head-to-head studies of cantharidin, curettage, topical peeling agents, and imiquimod demonstrated better satisfaction and fewer office visits with topical anesthetic and curettage on the first visit. Side effects were greatest for salicylic acid and glycolic acid; therefore, these agents are less desirable.42

Conclusion
Molluscum is a cutaneous viral infection that is common in children and has associated morbidities, including AD, pruritus, poor quality of life in some cases, and risk of contagion. Addressing the disease includes understanding its natural history and explaining it to parents/guardians. Therapeutics can be offered in cases where need is demonstrated, such as with lesions that spread and cause discomfort. Choice of therapeutics depends on the practitioner’s experience, the child’s clinical appearance, availability of therapy, and review of options with the parents/guardians. When avoidance of intervention is desired, barrier enhancement and treatment of symptomatic dermatitis are still beneficial, as are household (eg, not sharing towels) and activity (eg, adhesive bandages over active lesions) interventions to reduce transmission.
- Shisler JL. Immune evasion strategies of molluscum contagiosum virus. Adv Virus Res. 2015;92:201-252.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Moss B, Shisler JL, Xiang Y, et al. Immune-defense molecules of molluscum contagiosum virus, a human poxvirus. Trends Microbiol. 2000;8:473-477.
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Ajithkumar VT, Sasidharanpillai S, Muhammed K, et al. Disseminated molluscum contagiosum following chemotherapy: a therapeutic challenge. Indian J Dermatol Venereol Leprol. 2017;83:516.
- Oren B, Wende SO. An outbreak of molluscum contagiosum in a kibbutz. Infection. 1991;19:159-161.
- Molluscum contagiosum. Healthy Children website. https://www.healthychildren.org/English/health-issues/conditions/skin/Pages/Molluscum-Contagiosum.aspx. Updated November 21, 2015. Accessed October 16, 2019.
- Peterson AR, Nash E, Anderson BJ. Infectious disease in contact sports. Sports Health. 2019;11:47-58.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Mendiratta V, Agarwal S, Chander R. Reappraisal of sexually transmitted infections in children: a hospital-based study from an urban area. Indian J Sex Transm Dis AIDS. 2014;35:25-28.
- Bargman H. Genital molluscum contagiosum in children: evidence of sexual abuse? CMAJ. 1986;135:432-433.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Koning S, Bruijnzeels MA, van Suijlekom-Smit LW, et al. Molluscum contagiosum in Dutch general practice. Br J Gen Pract. 1994;44:417-419.
- Braue A, Ross G, Varigos G, et al. Epidemiology and impact of childhood molluscum contagiosum: a case series and critical review of the literature. Pediatr Dermatol. 2005;22:287-294.
- Sturt RJ, Muller HK, Francis GD. Molluscum contagiosum in villages of the West Sepik District of New Guinea. Med J Aust. 1971;2:751-754.
- Reynolds MG, Homan RC, Yorita Christensen KL, et al. The incidence of molluscum contagiosum among American Indians and Alaska Natives. PLoS One. 2009;4:e5255.
- Villa L, Varela JA, Otero L, et al. Molluscum contagiosum: a 20-year study in a sexually transmitted infections unit. Sex Transm Dis. 2010;37:423-424.
- Watanabe T, Morikawa S, Suzuki K, et al. Two major antigenic polypeptides of molluscum contagiosum virus. J Infect Dis. 1998;177:284-292.
- Vaccine basics. Centers for Disease Control and Prevention website. https://www.cdc.gov/smallpox/vaccine-basics/index.html. Updated July 12, 2017. Accessed October 16, 2019.
- Mitchell JC. Observations on the virus of molluscum contagiosum. Br J Exp Pathol. 1953;34:44-49.
- Konya J, Thompson CH. Molluscum contagiosum virus: antibody responses in patients with clinical lesions and its sero-epidemiology in a representative Australian population. J Infect Dis. 1999;179:701-704.
- Steffen C, Markman JA. Spontaneous disappearance of molluscum contagiosum. Arch Dermatol. 1980;116:923-924.
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73.
- Persechino S, Abruzzese C, Caperchi C, et al. Condyloma acuminata and mollusca contagiosa: a giant manifestation in a patient with lupus. Skinmed. 2014;12:310-311.
- Kim SK, Do JE, Kang HY, et al. Giant molluscum contagiosum of immunocompetent children occurring on the anogenital area. Eur J Dermatol. 2007;17:537-538.
- Alam MS, Shrirao N. Giant molluscum contagiosum presenting as lid neoplasm in an immunocompetent child. Dermatol Online J. 2016;22. pii:13030/qt56v567gn.
- Krishnamurthy J, Nagappa DK. The cytology of molluscum contagiosum mimicking skin adnexal tumor. J Cytol. 2010;27:74-75.
- Baek YS, Oh CH, Song HJ, et al. Asymmetrical periflexural exanthem of childhood with concurrence of molluscum contagiosum infection. Clin Exp Dermatol. 2011;36:676-677.
- Lee HJ, Kwon JA, Kim JW. Erythema multiforme-like molluscum dermatitis. Acta Derm Venereol. 2002;82:217-218.
- Lee YB, Choi HJ, Park HJ, et al. Two cases of erythema multiforme associated with molluscum contagiosum. Int J Dermatol. 2009;48:659-660.
- Vasily DB, Bhatia SG. Erythema annulare centrifugum and molluscum contagiosum.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653.
- Olsen JR, Gallagher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Goksugur N, Ozbostanci B, Goksugur SB. Molluscum contagiosum infection associated with pimecrolimus use in pityriasis alba. Pediatr Dermatol. 2007;24:E63-E65.
- Lee R, Schwartz RA. Pediatric molluscum contagiosum: reflections on the last challenging poxvirus infection, part 1. Cutis. 2010;86:230-236.
- Hanna D, Hatami A, Powell J, et al. A prospective randomized trial comparing the efficacy and adverse effects of four recognized treatments of molluscum contagiosum in children. Pediatr Dermatol. 2006;23:574-579.
- Coloe Dosal J, Stewart PW, Lin JA, et al. Cantharidin for the treatment of molluscum contagiosum: a prospective, double-blinded, placebo-controlled trial. Pediatr Dermatol. 2014;31:440-449.
- Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
- Handjani F, Behazin E, Sadati MS. Comparison of 10% potassium hydroxide solution versus cryotherapy in the treatment of molluscum contagiosum: an open randomized clinical trial. J Dermatolog Treat. 2014;25:249-250.
- Simonart T, De Maertelaer V. Curettage treatment for molluscum contagiosum: a follow-up survey study. Br J Dermatol. 2008;159:1144-1147.
- Cho YS, Chung BY, Park CW, et al. Seizures and methemoglobinemia after topical application of eutectic mixture of lidocaine and prilocaine on a 3.5-year-old child with molluscum contagiosum and atopic dermatitis. Pediatr Dermatol. 2016;33:E284-E285.
- Bard S, Shiman MI, Bellman B, et al. Treatment of facial molluscum contagiosum with trichloroacetic acid. Pediatr Dermatol. 2009;26:425-426.
- Griffith RD, Yazdani Abyaneh MA, Falto-Aizpurua L, et al. Pulsed dye laser therapy for molluscum contagiosum: a systematic review. J Drugs Dermatol. 2014;13:1349-1352.
- Theos AU, Cummins R, Silverberg NB, et al. Effectiveness of imiquimod cream 5% for treating childhood molluscum contagiosum in a double-blind, randomized pilot trial. Cutis. 2004;74:134-138, 141-142.
- van der Wouden JC, Menke J, Gajadin S, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2006:CD004767.
- Cunningham BB, Paller AS, Garzon M. Inefficacy of oral cimetidine for nonatopic children with molluscum contagiosum. Pediatr Dermatol. 1998;15:71-72.
- Enns LL, Evans MS. Intralesional immunotherapy with Candida antigen for the treatment of molluscum contagiosum in children. Pediatr Dermatol. 2011;28:254-258.
- Rajouria EA, Amatya A, Karn D. Comparative study of 5% potassium hydroxide solution versus 0.05% tretinoin cream for molluscum contagiosum in children. Kathmandu Univ Med J (KUMJ). 2011;9:291-294.
- Briand S, Milpied B, Navas D, et al. 1% topical cidofovir used as last alternative to treat viral infections. J Eur Acad Dermatol Venereol. 2008;22:249-250.
- Zabawski EJ Jr, Cockerell CJ. Topical cidofovir for molluscum contagiosum in children. Pediatr Dermatol. 1999;16:414-415.
- Watanabe T. Cidofovir diphosphate inhibits molluscum contagiosum virus DNA polymerase activity. J Invest Dermatol. 2008;128:1327-1329.
- Lindau MS, Munar MY. Use of duct tape occlusion in the treatment of recurrent molluscum contagiosum. Pediatr Dermatol. 2004;21:609.
- Silverberg N. Pediatric molluscum contagiosum: optimal treatment strategies. Paediatr Drugs. 2003;5:505-512.
- Shisler JL. Immune evasion strategies of molluscum contagiosum virus. Adv Virus Res. 2015;92:201-252.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Moss B, Shisler JL, Xiang Y, et al. Immune-defense molecules of molluscum contagiosum virus, a human poxvirus. Trends Microbiol. 2000;8:473-477.
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Choong KY, Roberts LJ. Molluscum contagiosum, swimming and bathing: a clinical analysis. Australas J Dermatol. 1999;40:89-92.
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Ajithkumar VT, Sasidharanpillai S, Muhammed K, et al. Disseminated molluscum contagiosum following chemotherapy: a therapeutic challenge. Indian J Dermatol Venereol Leprol. 2017;83:516.
- Oren B, Wende SO. An outbreak of molluscum contagiosum in a kibbutz. Infection. 1991;19:159-161.
- Molluscum contagiosum. Healthy Children website. https://www.healthychildren.org/English/health-issues/conditions/skin/Pages/Molluscum-Contagiosum.aspx. Updated November 21, 2015. Accessed October 16, 2019.
- Peterson AR, Nash E, Anderson BJ. Infectious disease in contact sports. Sports Health. 2019;11:47-58.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Mendiratta V, Agarwal S, Chander R. Reappraisal of sexually transmitted infections in children: a hospital-based study from an urban area. Indian J Sex Transm Dis AIDS. 2014;35:25-28.
- Bargman H. Genital molluscum contagiosum in children: evidence of sexual abuse? CMAJ. 1986;135:432-433.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Koning S, Bruijnzeels MA, van Suijlekom-Smit LW, et al. Molluscum contagiosum in Dutch general practice. Br J Gen Pract. 1994;44:417-419.
- Braue A, Ross G, Varigos G, et al. Epidemiology and impact of childhood molluscum contagiosum: a case series and critical review of the literature. Pediatr Dermatol. 2005;22:287-294.
- Sturt RJ, Muller HK, Francis GD. Molluscum contagiosum in villages of the West Sepik District of New Guinea. Med J Aust. 1971;2:751-754.
- Reynolds MG, Homan RC, Yorita Christensen KL, et al. The incidence of molluscum contagiosum among American Indians and Alaska Natives. PLoS One. 2009;4:e5255.
- Villa L, Varela JA, Otero L, et al. Molluscum contagiosum: a 20-year study in a sexually transmitted infections unit. Sex Transm Dis. 2010;37:423-424.
- Watanabe T, Morikawa S, Suzuki K, et al. Two major antigenic polypeptides of molluscum contagiosum virus. J Infect Dis. 1998;177:284-292.
- Vaccine basics. Centers for Disease Control and Prevention website. https://www.cdc.gov/smallpox/vaccine-basics/index.html. Updated July 12, 2017. Accessed October 16, 2019.
- Mitchell JC. Observations on the virus of molluscum contagiosum. Br J Exp Pathol. 1953;34:44-49.
- Konya J, Thompson CH. Molluscum contagiosum virus: antibody responses in patients with clinical lesions and its sero-epidemiology in a representative Australian population. J Infect Dis. 1999;179:701-704.
- Steffen C, Markman JA. Spontaneous disappearance of molluscum contagiosum. Arch Dermatol. 1980;116:923-924.
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73.
- Persechino S, Abruzzese C, Caperchi C, et al. Condyloma acuminata and mollusca contagiosa: a giant manifestation in a patient with lupus. Skinmed. 2014;12:310-311.
- Kim SK, Do JE, Kang HY, et al. Giant molluscum contagiosum of immunocompetent children occurring on the anogenital area. Eur J Dermatol. 2007;17:537-538.
- Alam MS, Shrirao N. Giant molluscum contagiosum presenting as lid neoplasm in an immunocompetent child. Dermatol Online J. 2016;22. pii:13030/qt56v567gn.
- Krishnamurthy J, Nagappa DK. The cytology of molluscum contagiosum mimicking skin adnexal tumor. J Cytol. 2010;27:74-75.
- Baek YS, Oh CH, Song HJ, et al. Asymmetrical periflexural exanthem of childhood with concurrence of molluscum contagiosum infection. Clin Exp Dermatol. 2011;36:676-677.
- Lee HJ, Kwon JA, Kim JW. Erythema multiforme-like molluscum dermatitis. Acta Derm Venereol. 2002;82:217-218.
- Lee YB, Choi HJ, Park HJ, et al. Two cases of erythema multiforme associated with molluscum contagiosum. Int J Dermatol. 2009;48:659-660.
- Vasily DB, Bhatia SG. Erythema annulare centrifugum and molluscum contagiosum.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653.
- Olsen JR, Gallagher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Goksugur N, Ozbostanci B, Goksugur SB. Molluscum contagiosum infection associated with pimecrolimus use in pityriasis alba. Pediatr Dermatol. 2007;24:E63-E65.
- Lee R, Schwartz RA. Pediatric molluscum contagiosum: reflections on the last challenging poxvirus infection, part 1. Cutis. 2010;86:230-236.
- Hanna D, Hatami A, Powell J, et al. A prospective randomized trial comparing the efficacy and adverse effects of four recognized treatments of molluscum contagiosum in children. Pediatr Dermatol. 2006;23:574-579.
- Coloe Dosal J, Stewart PW, Lin JA, et al. Cantharidin for the treatment of molluscum contagiosum: a prospective, double-blinded, placebo-controlled trial. Pediatr Dermatol. 2014;31:440-449.
- Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
- Handjani F, Behazin E, Sadati MS. Comparison of 10% potassium hydroxide solution versus cryotherapy in the treatment of molluscum contagiosum: an open randomized clinical trial. J Dermatolog Treat. 2014;25:249-250.
- Simonart T, De Maertelaer V. Curettage treatment for molluscum contagiosum: a follow-up survey study. Br J Dermatol. 2008;159:1144-1147.
- Cho YS, Chung BY, Park CW, et al. Seizures and methemoglobinemia after topical application of eutectic mixture of lidocaine and prilocaine on a 3.5-year-old child with molluscum contagiosum and atopic dermatitis. Pediatr Dermatol. 2016;33:E284-E285.
- Bard S, Shiman MI, Bellman B, et al. Treatment of facial molluscum contagiosum with trichloroacetic acid. Pediatr Dermatol. 2009;26:425-426.
- Griffith RD, Yazdani Abyaneh MA, Falto-Aizpurua L, et al. Pulsed dye laser therapy for molluscum contagiosum: a systematic review. J Drugs Dermatol. 2014;13:1349-1352.
- Theos AU, Cummins R, Silverberg NB, et al. Effectiveness of imiquimod cream 5% for treating childhood molluscum contagiosum in a double-blind, randomized pilot trial. Cutis. 2004;74:134-138, 141-142.
- van der Wouden JC, Menke J, Gajadin S, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2006:CD004767.
- Cunningham BB, Paller AS, Garzon M. Inefficacy of oral cimetidine for nonatopic children with molluscum contagiosum. Pediatr Dermatol. 1998;15:71-72.
- Enns LL, Evans MS. Intralesional immunotherapy with Candida antigen for the treatment of molluscum contagiosum in children. Pediatr Dermatol. 2011;28:254-258.
- Rajouria EA, Amatya A, Karn D. Comparative study of 5% potassium hydroxide solution versus 0.05% tretinoin cream for molluscum contagiosum in children. Kathmandu Univ Med J (KUMJ). 2011;9:291-294.
- Briand S, Milpied B, Navas D, et al. 1% topical cidofovir used as last alternative to treat viral infections. J Eur Acad Dermatol Venereol. 2008;22:249-250.
- Zabawski EJ Jr, Cockerell CJ. Topical cidofovir for molluscum contagiosum in children. Pediatr Dermatol. 1999;16:414-415.
- Watanabe T. Cidofovir diphosphate inhibits molluscum contagiosum virus DNA polymerase activity. J Invest Dermatol. 2008;128:1327-1329.
- Lindau MS, Munar MY. Use of duct tape occlusion in the treatment of recurrent molluscum contagiosum. Pediatr Dermatol. 2004;21:609.
- Silverberg N. Pediatric molluscum contagiosum: optimal treatment strategies. Paediatr Drugs. 2003;5:505-512.
Practice Points
- Molluscum appears as pearly papules with a central dell (ie, umbilicated).
- Caused by a poxvirus, the disease is very contagious and transferred via skin-to-skin contact or fomites.
- One-third of children with molluscum will develop symptoms of local erythema, swelling, or pruritus.
- Diagnosis usually is clinical.
- Children are primarily managed through observation; however, cantharidin, cryotherapy, or curettage can be used for symptomatic or cosmetically concerning lesions.
Pediatric Warts: Update on Interventions
The definition of warts is variable, largely reflecting their manifold appearance, biologic potential, and public health concerns. One vernacular dictionary defines warts as:
Small, benign growths caused by a vital infection of the skin or mucous membrane. The virus infects the surface layer. The viruses that cause warts are members of the human papilloma virus (HPV) family. Warts are not cancerous but some strains of HPV, usually not associated with warts, have been linked with cancer formation. Warts are contagious from person to person and from one area of the body to another on the same person.1
The World Health Organization defines warts by their structural components as:
Human papillomavirus (HPV) is a small, non-enveloped deoxyribonucleic acid (DNA) virus that infects skin or mucosal cells. The circular, double-stranded viral genome is approximately 8-kb in length. The genome encodes for 6 early proteins responsible for virus replication and 2 late proteins, L1 and L2, which are the viral structural proteins.2
In pediatric and adolescent dermatology, warts often are defined by their location and morphology; for example, facial warts typically are flat, minimally hyperkeratotic, or filiform, wherein the base is narrow and the lesion is tall, growing at a 90° angle to the surface of the skin. On the arms and legs, warts usually present as round to oval papules with overlying thick hyperkeratosis and/or callosity.3,4 Common warts usually are flesh colored or lighter, and heavily pigmented lesions should be evaluated dermoscopically for a pigment network and biopsied when pigment is present.5
In this article, a successful paradigm for management of pediatric warts is provided with enhanced outcomes based on further insight into the disease course and patient selection.
Epidemiology of Pediatric Warts
There are more than 200 types of human papillomaviruses (HPV), with more than 100 oncogenic types. There is quite a bit of homology by species and genus that contributes to cross-immunity and similar behavior between certain types of HPV. The lifetime incidence of warts is very high. Approximately 30% of children develop a wart.6 A review of the 2007 National Health Interview Survey of 9417 children demonstrated a steady increase in prevalence of warts from 1 to 2 years of age to 7 to 8 years of age, with a peak at 9 to 10 years of age and a plateau at 11 to 17 years of age. Warts were most common in non-Hispanic white children and less common in black children.7 In an in-person survey of 12,370 individuals aged 18 to 74 years from 5 European countries, warts were the most common physician-diagnosed (27.3%) and self-reported (41.0%) dermatologic condition. Warts are more common in Northern countries (eg, Netherlands, Germany).8 Children with atopic dermatitis have a higher risk of developing warts and extracutaneous infections. In one study, children with warts and atopic dermatitis had a higher number of infections and food allergies and higher incidence of asthma and hay fever than either condition alone.9
Clinical Presentation of Warts
Warts usually present as common, palmoplantar, flat, or filiform in childhood, but variations by age are common (eFigure). The common and palmoplantar variants often are caused by HPV types 1 and 2.4,5 In infancy, vertically transmitted HPV infections can cause juvenile-onset respiratory papillomatosis or vertically transmitted condyloma. Juvenile-onset respiratory papillomatosis refers to upper respiratory papillomas that are difficult to eliminate and has been associated with exfoliated cervical cell testing with 18.1% (13/72) typed HPV-positive, which allows neonates to be exposed to HPV in the upper respiratory tract in utero.10
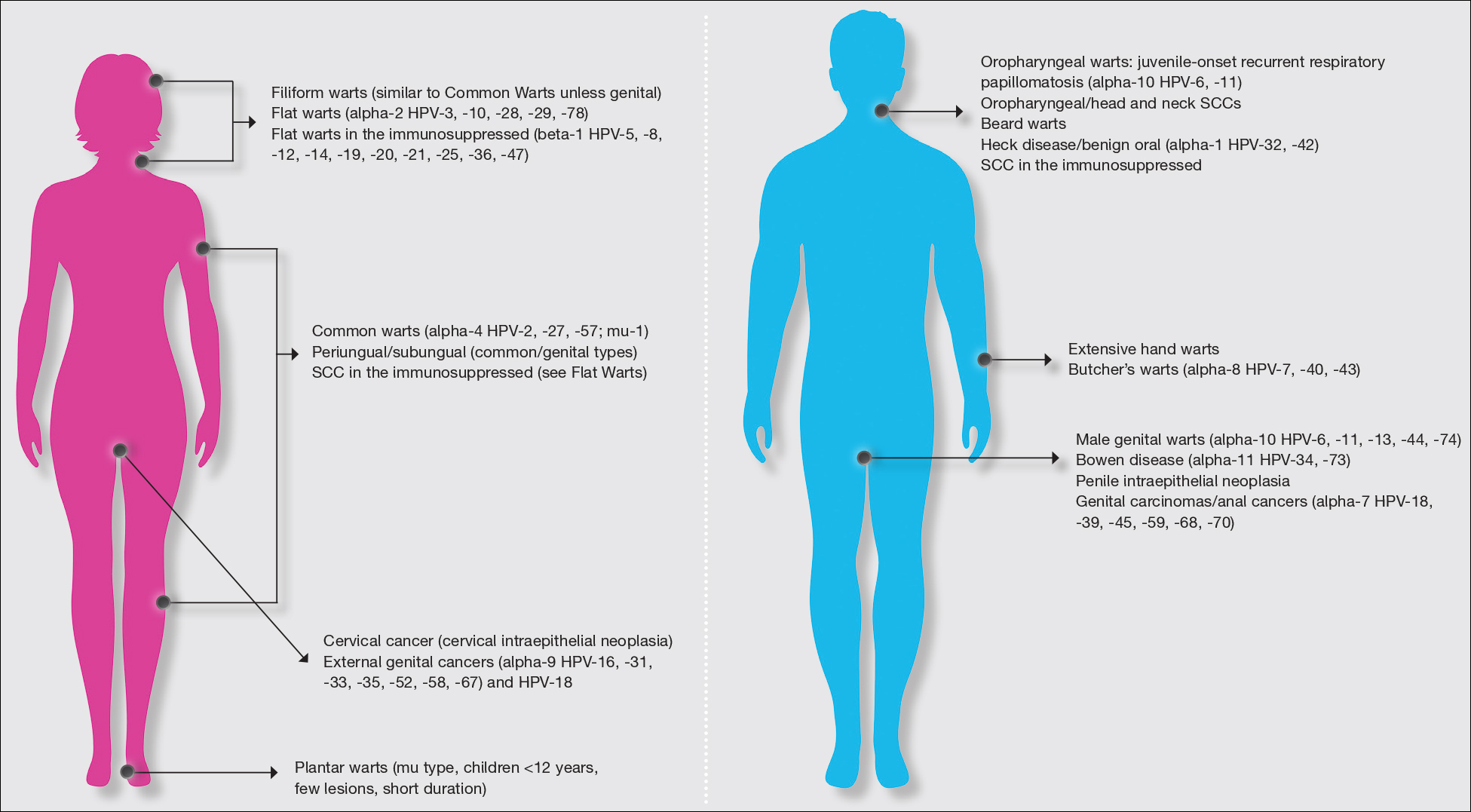
Vertically transmitted condyloma is a difficult topic. Much data supports the vertical transmission of condyloma as the leading cause of condyloma in small children; however, a reasonable amount of caution is needed in this patient population. In cases suspicious for sexual abuse as well as those presenting in children 4 years and older, formal household evaluation by a sexual abuse clinic and mandatory reporting is needed. Anywhere from 2.6% to 32% of cases of genital warts in children have been reported to be caused by sexual abuse.11-13 Therefore, most investigators have recommended careful review of the patient’s history and socioeconomic circumstances as well as a thorough physical examination. Mandatory reporting of suspected child sexual abuse is required in suspicious cases. Because HPV type 16 has been found in vertically transmitted cases, concern for long-term oncogenesis exists.11-13
Adolescents generally present with lesions on the hands and feet. Plantar warts often are caused by HPV types from the alpha genus. Subtypes noted in plantar warts include HPV types 1a, 2, 27, 57, and 65.14 By 15 years of age, genital HPV becomes a common adolescent infection, persisting into adulthood.15 When studied, genital HPV often is subclinical or latent and often is preventable through vaccination. High-risk oncogenic alpha-genus HPV types can immortalize human keratinocytes. When HPV types 11, 16, 18, and 31 are compared, HPV-18 has the highest oncogenic potential based on colony-stimulating potential.16 Vaccination with the 9-valent HPV vaccine is recommended in adolescence due to the concern for exposures to both low-potential (HPV types 6 and 11) and high-potential (HPV types 16 and 18) oncogenic HPV types. Data strongly support the benefit of 9-valent HPV vaccination in the prevention of sexually transmitted HPV in both males and females.17
Contagion of HPV is easy due to its excellent survival of fomites on surfaces, which generally is how warts are transferred in gym or pool settings where individuals who walk barefoot in changing rooms are almost twice as likely to contract plantar warts (odds ratio, 1.97 [95% CI, 1.39%-2.79%]).18 In another case series, walking barefoot, using a swimming pool, and having a household contact with warts were the leading risk factors for contraction of warts in children younger than 13 years.19 Children often transfer warts from site to site as well as to siblings and other close contacts. Skin-to-skin contact is responsible for sexual transmission of warts, and surface transmission occurs via fomites. Entry of the virus often occurs through small breaks in the skin. Other modes of transmission include orogenital.20
Therapeutic Options
Although the nuances of each available treatment for pediatric warts are beyond the scope of this article, the main core of therapy is 1 of 3 approaches: (1) observation, (2) over-the-counter salicylic acid therapy, and (3) in-office cryotherapy. Observation is an affirmed style of therapy for warts, as it is expected that two-thirds of warts will spontaneously resolve in 2 years and three-quarters will resolve in 3 years.4,5 Condyloma in children has been responsive to therapies such as cryotherapy and imiquimod,13 but spontaneous clearance in 5 years has been noted in 76% of children,21 which is linked to development of spontaneous immune response in most individuals.
Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; irritant; vascular destructive; and nitric oxide releasing (eTable).
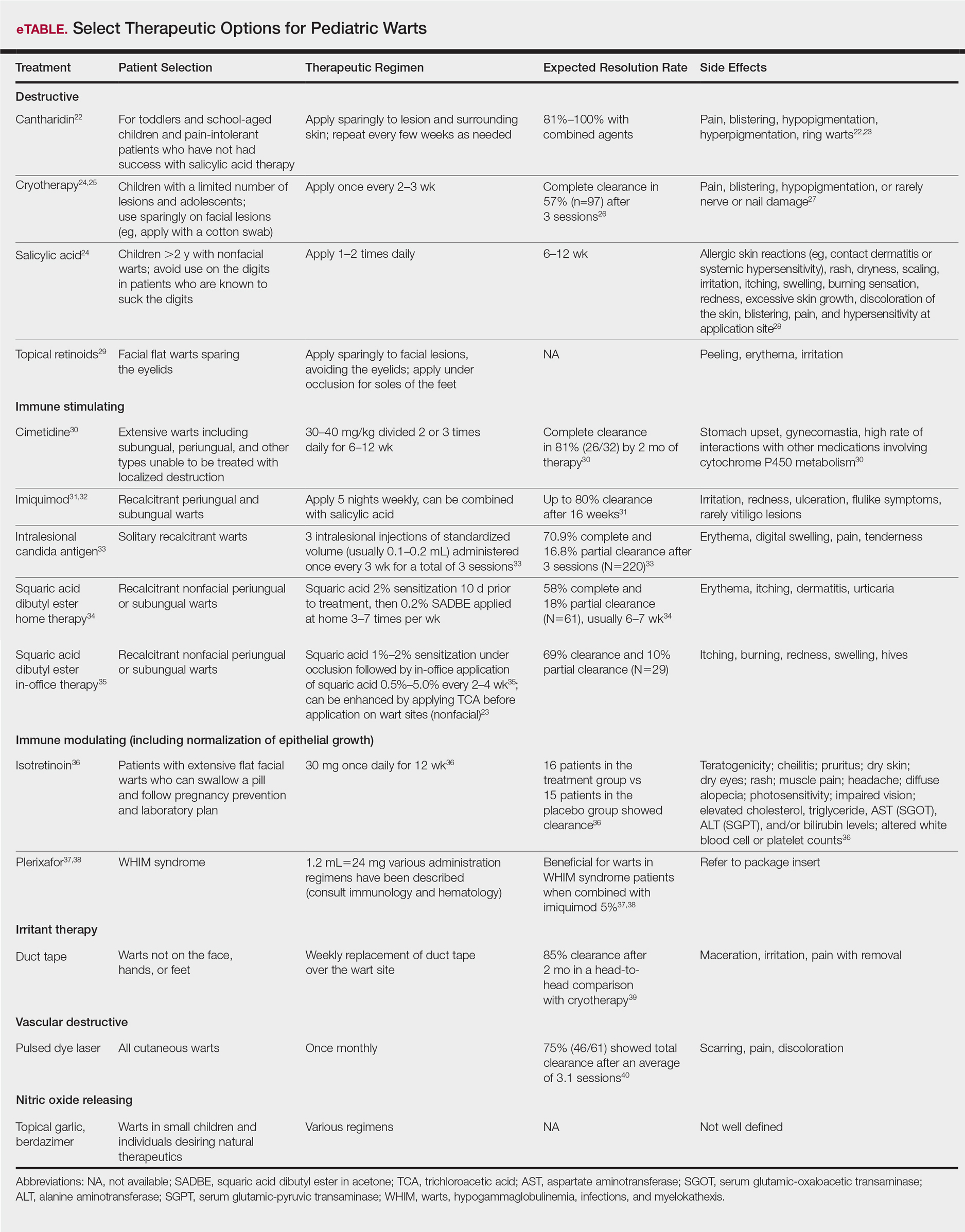
Destructive Therapies
Destructive therapies for warts often are implemented in cases of disfigurement, discomfort/pain, and/or spreading, as well as to control contagion. According to a 2001 Cochrane review, salicylic acid has the best evidence of all therapeutics for the clearance of warts compared to placebo.24 On the other hand, aggressive cryotherapy and combined salicylic acid and cryotherapy had the best evidence in their favor in a 2011 meta-analysis by Kwok et al.25 Both salicylic acid and cryotherapy are considered destructive therapies. A recent meta-analysis of cantharidin, another destructive therapy, showed that local cantharidin alone as well as in combination with salicylic acid and podophyllotoxin showed good efficacy for warts; however, increased caution should be exerted with the combination regimen in young children due to a potential increase in the side-effect profile (eg, severe blistering).22 Other destructive agents such as topical retinoids can only peel surface layers of the skin and therefore are limited to flat facial warts, which are not expected to have an extensive hyperkeratotic layer; however, with occlusion, agents such as adapalene gel 0.1% can be used even on plantar warts with some efficacy.29
Immune-Stimulating Therapies
Immune stimulants often are used to treat warts in children and adolescents who have many lesions, a prolonged disease course, disfigurement, and/or subungual localizations, as well as in those who have been treated with multiple destructive methods without success. Topical imiquimod and oral cimetidine are readily available, while squaric acid (at-home or in-office therapy) and intralesional candida antigen can be used in offices that carry these agents. Topical imiquimod has been reported to achieve success in genital warts in children,13 with good efficacy in recalcitrant, periungual, and subungual warts when used for up to 16 weeks.31 In one randomized clinical trial, imiquimod cream 5% combined with salicylic acid 15% was applied to warts for 6 to 10 hours for 5 consecutive days per week versus cryotherapy with liquid nitrogen every 2 weeks for a maximum of 3 months. At the end of the study period, 81.1% (30/37) of participants treated with imiquimod and salicylic acid showed clearance of their warts versus 67.3% (33/49) of those treated with cryotherapy.32
Oral cimetidine has been reported to be successful in treating recalcitrant warts in more than 80% of children when dosed at 30 to 40 mg/kg 3 times daily, requiring 6 to 12 weeks to achieve clearance. Side effects of oral cimetidine include many cytochrome P450 interactions; gynecomastia, which limits usage in teenaged males; and stomach upset.30
Treatment of recalcitrant pediatric warts with intralesional candida antigen has been associated with side effects consistent with delayed-type hypersensitivity reactions. Injections should be administered once monthly, with a minimum of 3 cycles if not effective and up to 6 cycles where partial efficacy is noted. In a retrospective review of 220 cases, 70.9% of children showed complete clearance and 16.8% had partial response.33 However, the treatment may be limited in children by fear of needles.
Squaric acid dibutyl ester is a universal allergen that is not mutagenic on Ames testing and causes milder allergy symptoms than the mutagenic dinitrochlorobenzene and less erythema and pruritus than diphencyclopropenone. Squaric acid dibutyl ester home therapy was evaluated in 61 children with at least one nonfacial wart.34 Application began with squaric acid dibutyl ester in acetone (SADBE) 2% sensitization on the arm followed by at-home application of SADBE 0.2% three to seven times weekly for a minimum of 2 months to determine benefit and for 3 to 4 months as needed; however, average response was 7 weeks. The average complete clearance was 58% and partial clearance was 18%. Side effects included erythema and mild itching as well as urticaria in one case.34 In-office SADBE also has been evaluated in children. In a case series that included 29 children sensitized with SADBE 1% to 2% under occlusion followed by once monthly application of SADBE 0.5% to 5.0% to their warts, 69% clearance and 10% partial clearance was noted after a little more than 4 months of treatment.35 One retrospective review compared combination SADBE, trichloroacetic acid (TCA), and cantharidin both alone and in combination as duos (eg, SADBE and TCA) or trios (SADBE, TCA, and cantharidin).23 Of the 74 children whose medical charts were reviewed, the addition of pretreatment of warts with TCA 50% prior to in-office sensitization and monthly in-office application of SADBE increased treatment response to 100% with an average 2.45 months of therapy, whereas no enhancement was noted with cantharidin. Therefore, it appears that there may be enhanced immune reactivity when TCA pretreatment of warts is performed.23
Immune-Modulating Therapies (Including Normalization of Epithelial Growth)
The most novel immunologic therapy for warts is plerixafor, an agent used to treat WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome, which has been linked to heterozygous gain of function mutations in the chemokine receptor CXCR4 (located on 2q22). In WHIM syndrome, the mutated CXCR4 is more sensitive to CXCL12 activation. Plerixafor is a selective reversible antagonist that blocks the capacity of the chemokine CXCL12 to sustain the permanent activation of CXCR4.37 Combination therapy with plerixafor and topical imiquimod has resulted in wart improvement in WHIM syndrome patients in a small series.38
Oral isotretinoin has been described to be efficacious over placebo at a dosage of 30 mg daily for 12 weeks and can be used in teenagers but requires standard monitoring.36
Irritant Therapies
Duct tape is a classic agent that produces maceration and irritation of warts. Application of duct tape over warts has been described in cycles of 6 days on, 1 day off with weekly repetition for a few months but usually not on the palms or soles due to difficulty maintaining occlusive tape in these locations over an extended period of time. In one trial, 85% (22/26) of duct tape–treated cases cleared versus 60% (15/25) of cryotherapy-treated cases over a 2-month maximum therapeutic period.39
Vascular Destructive Therapies
The pulsed dye laser is a classic modality that induces localized destruction of blood supply to warts in children. A case series of 61 children treated with the pulsed dye laser revealed 75% overall clearance in an average of 3.1 sessions. The usage of this therapy often is limited to institutions where the technology is readily available for usage.40
Nitric Oxide–Releasing Therapies
Nitric oxide release may increase local blood flow, thereby increasing immune response, or may have a primary mechanism of antimicrobial activity, which is why these agents have been investigated for wart treatment. Topical garlic has been described anecdotally as a therapy for thin childhood warts with the putative mechanism being nitric oxide release.42 A new investigational drug recently has had phase 2 data published. Berdazimer sodium plus carboxymethyl cellulose hydrogel has demonstrated benefit in adult warts, but data in children is lacking.41
Therapeutic Ladder for Childhood Warts
The therapeutic ladder (Table) for childhood warts starts with first doing no harm. Although many parents are disturbed by their child’s condition, the natural history of resolution is spontaneous and therefore no therapy is required in many cases. The child and his/her caregivers should be engaged to determine if he/she is emotionally disturbed or uncomfortable with their lesions and to address any fears and concerns that some children may experience (eg, contagion risk, pain with ambulation, ostracism). For example, children with hand warts may report that other children will not hold their hand while in line at school. Prominent facial lesions can be particularly problematic for children due to teasing and bullying.
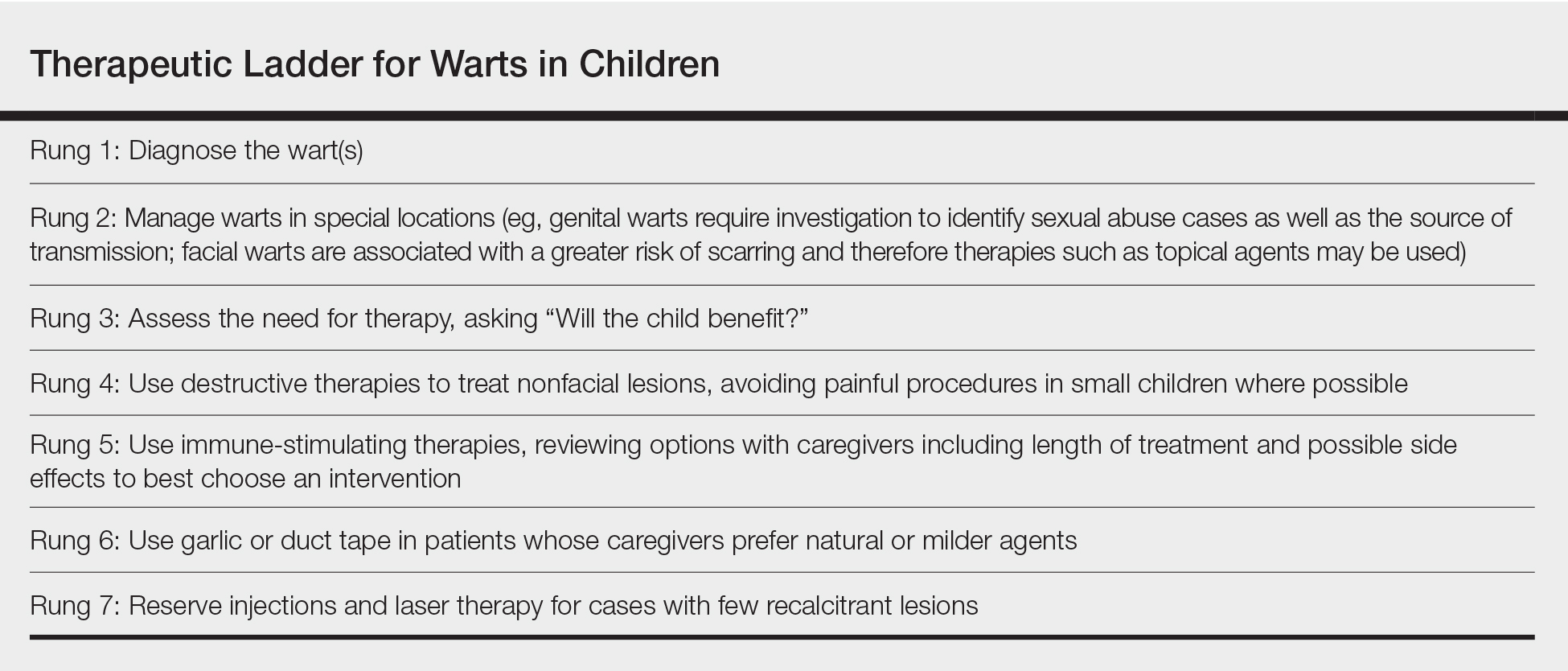
Conclusion
Warts are a common infection in childhood caused by the ubiquitous HPV virus. Therapeutic options abound, but most cases are either ignored or treated with over-the-counter salicylic acid or in-office cryotherapy. The decision to employ alternative therapeutic options requires agreement by the child, his/her caregiver, and the treating physician and can be tailored to suit the desires and needs of the child. Whether or not therapy is offered, spontaneous clearance is frequently seen in common warts. On the other hand, genital warts are associated with later conversion to malignancies of the genital tract; therefore, encouragement of HPV vaccination is needed in the adolescent population to best ensure long-term genital health.
1. Warts. https://medical-dictionary.thefreedictionary.com/warts. Accessed November 30, 2018.
2. Human papillomavirus. WHO website. http://www.who.int/biologicals/areas/human_papillomavirus/en. Accessed December 3, 2018.
3. Silverberg NB. Human papillomavirus infections in children. Curr Opin Pediatr. 2004;16:402-409.
4. Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
5. Silverberg NB, McCuaig CC. Melanoma in childhood: changing our mind-set. Cutis. 2013;92:217-218.
6. Bruggink SC, Eekhof JA, Egberts PF, et al. Warts transmitted in families and schools: a prospective cohort. Pediatrics. 2013;131:928-934.
7. Silverberg JI, Silverberg NB. The U.S. prevalence of common warts in childhood: a population-based study. J Invest Dermatol. 2013;133:2788-2790.
8. Svensson A, Ofenloch RF, Bruze M, et al. Br J Dermatol. 2018;178:1111-1118.
9. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047.
10. Smith EM, Johnson SR, Cripe TP, et al. Perinatal vertical transmission of human papillomavirus and subsequent development of respiratory tract papillomatosis. Ann Otol Rhinol Laryngol. 1991;100:479-483.
11. Costa-Silva M, Azevedo F, Lisboa C. Anogenital warts in children: analysis of a cohort of 34 prepubertal children. Pediatr Dermatol. 2018;35:E325-E327.
12. Marcoux D, Nadeau K, McCuaig C, et al. Pediatric anogenital warts: a 7-year review of children referred to a tertiary-care hospital in Montreal, Canada. Pediatr Dermatol. 2006;23:199-207.
13. Stefanaki C, Barkas G, Valari M, et al. Condylomata acuminata in children. Pediatr Infect Dis J. 2012;31:422-424.
14. dePlanell-Mas E, Martinez-Garriga B, Zalacain AJ, et al. Human papillomaviruses genotyping in plantar warts. J Med Virol. 2017;89:902-907.
15. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187-193.
16. Lace MJ, Anson JR, Klingelhutz AJ, et al. Human papillomavirus (HPV) type 18 induces extended growth in primary human cervical, tonsillar, or foreskin keratinocytes more effectively than other high-risk mucosal HPVs. J Virol. 2009;83:11784-11794.
17. Sudenga SL, Ingles DJ, Pierce Campbell CM, et al. Genital human papillomavirus infection progression to external genital lesions: the HIM study. Eur Urol. 2016;69:166-173.
18. Rigo MV, Martínez Campillo F, Verdú M, et al. Risk factors linked to the transmission of papilloma virus in the school environment [in Spanish]. Alicante, 1999. Aten Primaria. 2003;31:415-420.
19. Al-Mutairi N, AlKhalaf M. Mucocutaneous warts in children: clinical presentations, risk factors, and response to treatment. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:69-72.
20. Clarke J, Terry RM, Lacey CJ. A study to estimate the prevalence of upper respiratory tract papillomatosis in patients with genital warts. Int J STD AIDS. 1991;2:114-115.
21. Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39:951-955.
22. Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
23. Silverberg JI, Silverberg NB. Adjunctive trichloroacetic acid therapy enhances squaric acid response to verruca vulgaris. J Drugs Dermatol. 2012;11:1228-1230.
24. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001:CD001781.
25. Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233-246.
26. Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950;72:153-155.
27. Caravati CM Jr, Wood BT, Richardson DR. Onychodystrophies secondary to liquid nitrogen cryotherapy. Arch Dermatol. 1969;100:441-442.
28. Duofilm [package insert]. Sligo, Ireland: Stiefel Laboratories (Ireland) Ltd; 2016.
29. Gupta R, Gupta S. Topical adapalene in the treatment of plantar warts: randomized comparative open trial in comparison with cryo-therapy. Indian J Dermatol. 2015;60:102.
30. Orlow SJ, Paller A. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1993;28(5 pt 1):794-796.
31. Micali G, Dall’Oglio F, Nasca MR. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous warts. J Dermatolog Treat. 2003;14:233-236.
32. Stefanaki C, Lagogiani I, Kouris A, et al. Cryotherapy versus imiquimod 5% cream combined with a keratolytic lotion in cutaneous warts in children: a randomized study. J Dermatolog Treat. 2016;27:80-82.
33. Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M, et al. Intralesional Candida antigen immunotherapy for the treatment of recalcitrant and multiple warts in children. Pediatr Dermatol. 2015;32:797-801.
34. Silverberg NB, Lim JK, Paller AS, et al. Squaric acid immunotherapy for warts in children. J Am Acad Dermatol. 2000;42(5 pt 1):803-808.
35. Lee AN, Mallory SB. Contact immunotherapy with squaric acid dibutylester for the treatment of recalcitrant warts. J Am Acad Dermatol. 1999;41:595-599.
36. Olguin-García MG, Jurado-Santa Cruz F, Peralta-Pedrero ML, et al. A double-blind, randomized, placebo-controlled trial of oral isotretinoin in the treatment of recalcitrant facial flat warts. J Dermatolog Treat. 2015;26:78-82.
37. Badolato R, Donadieu J; WHIM Research Group. How I treat warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Blood. 2017;130:2491-2498.
38. McDermott DH, Liu Q, Velez D, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308-2316.
39. Focht DR 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
40. Sethuraman G, Richards KA, Hiremagalore RN, et al. Effectiveness of pulsed dye laser in the treatment of recalcitrant warts in children. Dermatol Surg. 2010;36:58-65.
41. Tyring SK, Rosen T, Berman B, et al. A phase 2 controlled study of SB206, a topical nitric oxide-releasing drug for extragenital wart treatment. J Drugs Dermatol. 2018;17:1100-1105.
42. Silverberg NB. Garlic cloves for verruca vulgaris. Pediatr Dermatol. 2002;19:183.
The definition of warts is variable, largely reflecting their manifold appearance, biologic potential, and public health concerns. One vernacular dictionary defines warts as:
Small, benign growths caused by a vital infection of the skin or mucous membrane. The virus infects the surface layer. The viruses that cause warts are members of the human papilloma virus (HPV) family. Warts are not cancerous but some strains of HPV, usually not associated with warts, have been linked with cancer formation. Warts are contagious from person to person and from one area of the body to another on the same person.1
The World Health Organization defines warts by their structural components as:
Human papillomavirus (HPV) is a small, non-enveloped deoxyribonucleic acid (DNA) virus that infects skin or mucosal cells. The circular, double-stranded viral genome is approximately 8-kb in length. The genome encodes for 6 early proteins responsible for virus replication and 2 late proteins, L1 and L2, which are the viral structural proteins.2
In pediatric and adolescent dermatology, warts often are defined by their location and morphology; for example, facial warts typically are flat, minimally hyperkeratotic, or filiform, wherein the base is narrow and the lesion is tall, growing at a 90° angle to the surface of the skin. On the arms and legs, warts usually present as round to oval papules with overlying thick hyperkeratosis and/or callosity.3,4 Common warts usually are flesh colored or lighter, and heavily pigmented lesions should be evaluated dermoscopically for a pigment network and biopsied when pigment is present.5
In this article, a successful paradigm for management of pediatric warts is provided with enhanced outcomes based on further insight into the disease course and patient selection.
Epidemiology of Pediatric Warts
There are more than 200 types of human papillomaviruses (HPV), with more than 100 oncogenic types. There is quite a bit of homology by species and genus that contributes to cross-immunity and similar behavior between certain types of HPV. The lifetime incidence of warts is very high. Approximately 30% of children develop a wart.6 A review of the 2007 National Health Interview Survey of 9417 children demonstrated a steady increase in prevalence of warts from 1 to 2 years of age to 7 to 8 years of age, with a peak at 9 to 10 years of age and a plateau at 11 to 17 years of age. Warts were most common in non-Hispanic white children and less common in black children.7 In an in-person survey of 12,370 individuals aged 18 to 74 years from 5 European countries, warts were the most common physician-diagnosed (27.3%) and self-reported (41.0%) dermatologic condition. Warts are more common in Northern countries (eg, Netherlands, Germany).8 Children with atopic dermatitis have a higher risk of developing warts and extracutaneous infections. In one study, children with warts and atopic dermatitis had a higher number of infections and food allergies and higher incidence of asthma and hay fever than either condition alone.9
Clinical Presentation of Warts
Warts usually present as common, palmoplantar, flat, or filiform in childhood, but variations by age are common (eFigure). The common and palmoplantar variants often are caused by HPV types 1 and 2.4,5 In infancy, vertically transmitted HPV infections can cause juvenile-onset respiratory papillomatosis or vertically transmitted condyloma. Juvenile-onset respiratory papillomatosis refers to upper respiratory papillomas that are difficult to eliminate and has been associated with exfoliated cervical cell testing with 18.1% (13/72) typed HPV-positive, which allows neonates to be exposed to HPV in the upper respiratory tract in utero.10

Vertically transmitted condyloma is a difficult topic. Much data supports the vertical transmission of condyloma as the leading cause of condyloma in small children; however, a reasonable amount of caution is needed in this patient population. In cases suspicious for sexual abuse as well as those presenting in children 4 years and older, formal household evaluation by a sexual abuse clinic and mandatory reporting is needed. Anywhere from 2.6% to 32% of cases of genital warts in children have been reported to be caused by sexual abuse.11-13 Therefore, most investigators have recommended careful review of the patient’s history and socioeconomic circumstances as well as a thorough physical examination. Mandatory reporting of suspected child sexual abuse is required in suspicious cases. Because HPV type 16 has been found in vertically transmitted cases, concern for long-term oncogenesis exists.11-13
Adolescents generally present with lesions on the hands and feet. Plantar warts often are caused by HPV types from the alpha genus. Subtypes noted in plantar warts include HPV types 1a, 2, 27, 57, and 65.14 By 15 years of age, genital HPV becomes a common adolescent infection, persisting into adulthood.15 When studied, genital HPV often is subclinical or latent and often is preventable through vaccination. High-risk oncogenic alpha-genus HPV types can immortalize human keratinocytes. When HPV types 11, 16, 18, and 31 are compared, HPV-18 has the highest oncogenic potential based on colony-stimulating potential.16 Vaccination with the 9-valent HPV vaccine is recommended in adolescence due to the concern for exposures to both low-potential (HPV types 6 and 11) and high-potential (HPV types 16 and 18) oncogenic HPV types. Data strongly support the benefit of 9-valent HPV vaccination in the prevention of sexually transmitted HPV in both males and females.17
Contagion of HPV is easy due to its excellent survival of fomites on surfaces, which generally is how warts are transferred in gym or pool settings where individuals who walk barefoot in changing rooms are almost twice as likely to contract plantar warts (odds ratio, 1.97 [95% CI, 1.39%-2.79%]).18 In another case series, walking barefoot, using a swimming pool, and having a household contact with warts were the leading risk factors for contraction of warts in children younger than 13 years.19 Children often transfer warts from site to site as well as to siblings and other close contacts. Skin-to-skin contact is responsible for sexual transmission of warts, and surface transmission occurs via fomites. Entry of the virus often occurs through small breaks in the skin. Other modes of transmission include orogenital.20
Therapeutic Options
Although the nuances of each available treatment for pediatric warts are beyond the scope of this article, the main core of therapy is 1 of 3 approaches: (1) observation, (2) over-the-counter salicylic acid therapy, and (3) in-office cryotherapy. Observation is an affirmed style of therapy for warts, as it is expected that two-thirds of warts will spontaneously resolve in 2 years and three-quarters will resolve in 3 years.4,5 Condyloma in children has been responsive to therapies such as cryotherapy and imiquimod,13 but spontaneous clearance in 5 years has been noted in 76% of children,21 which is linked to development of spontaneous immune response in most individuals.
Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; irritant; vascular destructive; and nitric oxide releasing (eTable).

Destructive Therapies
Destructive therapies for warts often are implemented in cases of disfigurement, discomfort/pain, and/or spreading, as well as to control contagion. According to a 2001 Cochrane review, salicylic acid has the best evidence of all therapeutics for the clearance of warts compared to placebo.24 On the other hand, aggressive cryotherapy and combined salicylic acid and cryotherapy had the best evidence in their favor in a 2011 meta-analysis by Kwok et al.25 Both salicylic acid and cryotherapy are considered destructive therapies. A recent meta-analysis of cantharidin, another destructive therapy, showed that local cantharidin alone as well as in combination with salicylic acid and podophyllotoxin showed good efficacy for warts; however, increased caution should be exerted with the combination regimen in young children due to a potential increase in the side-effect profile (eg, severe blistering).22 Other destructive agents such as topical retinoids can only peel surface layers of the skin and therefore are limited to flat facial warts, which are not expected to have an extensive hyperkeratotic layer; however, with occlusion, agents such as adapalene gel 0.1% can be used even on plantar warts with some efficacy.29
Immune-Stimulating Therapies
Immune stimulants often are used to treat warts in children and adolescents who have many lesions, a prolonged disease course, disfigurement, and/or subungual localizations, as well as in those who have been treated with multiple destructive methods without success. Topical imiquimod and oral cimetidine are readily available, while squaric acid (at-home or in-office therapy) and intralesional candida antigen can be used in offices that carry these agents. Topical imiquimod has been reported to achieve success in genital warts in children,13 with good efficacy in recalcitrant, periungual, and subungual warts when used for up to 16 weeks.31 In one randomized clinical trial, imiquimod cream 5% combined with salicylic acid 15% was applied to warts for 6 to 10 hours for 5 consecutive days per week versus cryotherapy with liquid nitrogen every 2 weeks for a maximum of 3 months. At the end of the study period, 81.1% (30/37) of participants treated with imiquimod and salicylic acid showed clearance of their warts versus 67.3% (33/49) of those treated with cryotherapy.32
Oral cimetidine has been reported to be successful in treating recalcitrant warts in more than 80% of children when dosed at 30 to 40 mg/kg 3 times daily, requiring 6 to 12 weeks to achieve clearance. Side effects of oral cimetidine include many cytochrome P450 interactions; gynecomastia, which limits usage in teenaged males; and stomach upset.30
Treatment of recalcitrant pediatric warts with intralesional candida antigen has been associated with side effects consistent with delayed-type hypersensitivity reactions. Injections should be administered once monthly, with a minimum of 3 cycles if not effective and up to 6 cycles where partial efficacy is noted. In a retrospective review of 220 cases, 70.9% of children showed complete clearance and 16.8% had partial response.33 However, the treatment may be limited in children by fear of needles.
Squaric acid dibutyl ester is a universal allergen that is not mutagenic on Ames testing and causes milder allergy symptoms than the mutagenic dinitrochlorobenzene and less erythema and pruritus than diphencyclopropenone. Squaric acid dibutyl ester home therapy was evaluated in 61 children with at least one nonfacial wart.34 Application began with squaric acid dibutyl ester in acetone (SADBE) 2% sensitization on the arm followed by at-home application of SADBE 0.2% three to seven times weekly for a minimum of 2 months to determine benefit and for 3 to 4 months as needed; however, average response was 7 weeks. The average complete clearance was 58% and partial clearance was 18%. Side effects included erythema and mild itching as well as urticaria in one case.34 In-office SADBE also has been evaluated in children. In a case series that included 29 children sensitized with SADBE 1% to 2% under occlusion followed by once monthly application of SADBE 0.5% to 5.0% to their warts, 69% clearance and 10% partial clearance was noted after a little more than 4 months of treatment.35 One retrospective review compared combination SADBE, trichloroacetic acid (TCA), and cantharidin both alone and in combination as duos (eg, SADBE and TCA) or trios (SADBE, TCA, and cantharidin).23 Of the 74 children whose medical charts were reviewed, the addition of pretreatment of warts with TCA 50% prior to in-office sensitization and monthly in-office application of SADBE increased treatment response to 100% with an average 2.45 months of therapy, whereas no enhancement was noted with cantharidin. Therefore, it appears that there may be enhanced immune reactivity when TCA pretreatment of warts is performed.23
Immune-Modulating Therapies (Including Normalization of Epithelial Growth)
The most novel immunologic therapy for warts is plerixafor, an agent used to treat WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome, which has been linked to heterozygous gain of function mutations in the chemokine receptor CXCR4 (located on 2q22). In WHIM syndrome, the mutated CXCR4 is more sensitive to CXCL12 activation. Plerixafor is a selective reversible antagonist that blocks the capacity of the chemokine CXCL12 to sustain the permanent activation of CXCR4.37 Combination therapy with plerixafor and topical imiquimod has resulted in wart improvement in WHIM syndrome patients in a small series.38
Oral isotretinoin has been described to be efficacious over placebo at a dosage of 30 mg daily for 12 weeks and can be used in teenagers but requires standard monitoring.36
Irritant Therapies
Duct tape is a classic agent that produces maceration and irritation of warts. Application of duct tape over warts has been described in cycles of 6 days on, 1 day off with weekly repetition for a few months but usually not on the palms or soles due to difficulty maintaining occlusive tape in these locations over an extended period of time. In one trial, 85% (22/26) of duct tape–treated cases cleared versus 60% (15/25) of cryotherapy-treated cases over a 2-month maximum therapeutic period.39
Vascular Destructive Therapies
The pulsed dye laser is a classic modality that induces localized destruction of blood supply to warts in children. A case series of 61 children treated with the pulsed dye laser revealed 75% overall clearance in an average of 3.1 sessions. The usage of this therapy often is limited to institutions where the technology is readily available for usage.40
Nitric Oxide–Releasing Therapies
Nitric oxide release may increase local blood flow, thereby increasing immune response, or may have a primary mechanism of antimicrobial activity, which is why these agents have been investigated for wart treatment. Topical garlic has been described anecdotally as a therapy for thin childhood warts with the putative mechanism being nitric oxide release.42 A new investigational drug recently has had phase 2 data published. Berdazimer sodium plus carboxymethyl cellulose hydrogel has demonstrated benefit in adult warts, but data in children is lacking.41
Therapeutic Ladder for Childhood Warts
The therapeutic ladder (Table) for childhood warts starts with first doing no harm. Although many parents are disturbed by their child’s condition, the natural history of resolution is spontaneous and therefore no therapy is required in many cases. The child and his/her caregivers should be engaged to determine if he/she is emotionally disturbed or uncomfortable with their lesions and to address any fears and concerns that some children may experience (eg, contagion risk, pain with ambulation, ostracism). For example, children with hand warts may report that other children will not hold their hand while in line at school. Prominent facial lesions can be particularly problematic for children due to teasing and bullying.

Conclusion
Warts are a common infection in childhood caused by the ubiquitous HPV virus. Therapeutic options abound, but most cases are either ignored or treated with over-the-counter salicylic acid or in-office cryotherapy. The decision to employ alternative therapeutic options requires agreement by the child, his/her caregiver, and the treating physician and can be tailored to suit the desires and needs of the child. Whether or not therapy is offered, spontaneous clearance is frequently seen in common warts. On the other hand, genital warts are associated with later conversion to malignancies of the genital tract; therefore, encouragement of HPV vaccination is needed in the adolescent population to best ensure long-term genital health.
The definition of warts is variable, largely reflecting their manifold appearance, biologic potential, and public health concerns. One vernacular dictionary defines warts as:
Small, benign growths caused by a vital infection of the skin or mucous membrane. The virus infects the surface layer. The viruses that cause warts are members of the human papilloma virus (HPV) family. Warts are not cancerous but some strains of HPV, usually not associated with warts, have been linked with cancer formation. Warts are contagious from person to person and from one area of the body to another on the same person.1
The World Health Organization defines warts by their structural components as:
Human papillomavirus (HPV) is a small, non-enveloped deoxyribonucleic acid (DNA) virus that infects skin or mucosal cells. The circular, double-stranded viral genome is approximately 8-kb in length. The genome encodes for 6 early proteins responsible for virus replication and 2 late proteins, L1 and L2, which are the viral structural proteins.2
In pediatric and adolescent dermatology, warts often are defined by their location and morphology; for example, facial warts typically are flat, minimally hyperkeratotic, or filiform, wherein the base is narrow and the lesion is tall, growing at a 90° angle to the surface of the skin. On the arms and legs, warts usually present as round to oval papules with overlying thick hyperkeratosis and/or callosity.3,4 Common warts usually are flesh colored or lighter, and heavily pigmented lesions should be evaluated dermoscopically for a pigment network and biopsied when pigment is present.5
In this article, a successful paradigm for management of pediatric warts is provided with enhanced outcomes based on further insight into the disease course and patient selection.
Epidemiology of Pediatric Warts
There are more than 200 types of human papillomaviruses (HPV), with more than 100 oncogenic types. There is quite a bit of homology by species and genus that contributes to cross-immunity and similar behavior between certain types of HPV. The lifetime incidence of warts is very high. Approximately 30% of children develop a wart.6 A review of the 2007 National Health Interview Survey of 9417 children demonstrated a steady increase in prevalence of warts from 1 to 2 years of age to 7 to 8 years of age, with a peak at 9 to 10 years of age and a plateau at 11 to 17 years of age. Warts were most common in non-Hispanic white children and less common in black children.7 In an in-person survey of 12,370 individuals aged 18 to 74 years from 5 European countries, warts were the most common physician-diagnosed (27.3%) and self-reported (41.0%) dermatologic condition. Warts are more common in Northern countries (eg, Netherlands, Germany).8 Children with atopic dermatitis have a higher risk of developing warts and extracutaneous infections. In one study, children with warts and atopic dermatitis had a higher number of infections and food allergies and higher incidence of asthma and hay fever than either condition alone.9
Clinical Presentation of Warts
Warts usually present as common, palmoplantar, flat, or filiform in childhood, but variations by age are common (eFigure). The common and palmoplantar variants often are caused by HPV types 1 and 2.4,5 In infancy, vertically transmitted HPV infections can cause juvenile-onset respiratory papillomatosis or vertically transmitted condyloma. Juvenile-onset respiratory papillomatosis refers to upper respiratory papillomas that are difficult to eliminate and has been associated with exfoliated cervical cell testing with 18.1% (13/72) typed HPV-positive, which allows neonates to be exposed to HPV in the upper respiratory tract in utero.10

Vertically transmitted condyloma is a difficult topic. Much data supports the vertical transmission of condyloma as the leading cause of condyloma in small children; however, a reasonable amount of caution is needed in this patient population. In cases suspicious for sexual abuse as well as those presenting in children 4 years and older, formal household evaluation by a sexual abuse clinic and mandatory reporting is needed. Anywhere from 2.6% to 32% of cases of genital warts in children have been reported to be caused by sexual abuse.11-13 Therefore, most investigators have recommended careful review of the patient’s history and socioeconomic circumstances as well as a thorough physical examination. Mandatory reporting of suspected child sexual abuse is required in suspicious cases. Because HPV type 16 has been found in vertically transmitted cases, concern for long-term oncogenesis exists.11-13
Adolescents generally present with lesions on the hands and feet. Plantar warts often are caused by HPV types from the alpha genus. Subtypes noted in plantar warts include HPV types 1a, 2, 27, 57, and 65.14 By 15 years of age, genital HPV becomes a common adolescent infection, persisting into adulthood.15 When studied, genital HPV often is subclinical or latent and often is preventable through vaccination. High-risk oncogenic alpha-genus HPV types can immortalize human keratinocytes. When HPV types 11, 16, 18, and 31 are compared, HPV-18 has the highest oncogenic potential based on colony-stimulating potential.16 Vaccination with the 9-valent HPV vaccine is recommended in adolescence due to the concern for exposures to both low-potential (HPV types 6 and 11) and high-potential (HPV types 16 and 18) oncogenic HPV types. Data strongly support the benefit of 9-valent HPV vaccination in the prevention of sexually transmitted HPV in both males and females.17
Contagion of HPV is easy due to its excellent survival of fomites on surfaces, which generally is how warts are transferred in gym or pool settings where individuals who walk barefoot in changing rooms are almost twice as likely to contract plantar warts (odds ratio, 1.97 [95% CI, 1.39%-2.79%]).18 In another case series, walking barefoot, using a swimming pool, and having a household contact with warts were the leading risk factors for contraction of warts in children younger than 13 years.19 Children often transfer warts from site to site as well as to siblings and other close contacts. Skin-to-skin contact is responsible for sexual transmission of warts, and surface transmission occurs via fomites. Entry of the virus often occurs through small breaks in the skin. Other modes of transmission include orogenital.20
Therapeutic Options
Although the nuances of each available treatment for pediatric warts are beyond the scope of this article, the main core of therapy is 1 of 3 approaches: (1) observation, (2) over-the-counter salicylic acid therapy, and (3) in-office cryotherapy. Observation is an affirmed style of therapy for warts, as it is expected that two-thirds of warts will spontaneously resolve in 2 years and three-quarters will resolve in 3 years.4,5 Condyloma in children has been responsive to therapies such as cryotherapy and imiquimod,13 but spontaneous clearance in 5 years has been noted in 76% of children,21 which is linked to development of spontaneous immune response in most individuals.
Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; irritant; vascular destructive; and nitric oxide releasing (eTable).

Destructive Therapies
Destructive therapies for warts often are implemented in cases of disfigurement, discomfort/pain, and/or spreading, as well as to control contagion. According to a 2001 Cochrane review, salicylic acid has the best evidence of all therapeutics for the clearance of warts compared to placebo.24 On the other hand, aggressive cryotherapy and combined salicylic acid and cryotherapy had the best evidence in their favor in a 2011 meta-analysis by Kwok et al.25 Both salicylic acid and cryotherapy are considered destructive therapies. A recent meta-analysis of cantharidin, another destructive therapy, showed that local cantharidin alone as well as in combination with salicylic acid and podophyllotoxin showed good efficacy for warts; however, increased caution should be exerted with the combination regimen in young children due to a potential increase in the side-effect profile (eg, severe blistering).22 Other destructive agents such as topical retinoids can only peel surface layers of the skin and therefore are limited to flat facial warts, which are not expected to have an extensive hyperkeratotic layer; however, with occlusion, agents such as adapalene gel 0.1% can be used even on plantar warts with some efficacy.29
Immune-Stimulating Therapies
Immune stimulants often are used to treat warts in children and adolescents who have many lesions, a prolonged disease course, disfigurement, and/or subungual localizations, as well as in those who have been treated with multiple destructive methods without success. Topical imiquimod and oral cimetidine are readily available, while squaric acid (at-home or in-office therapy) and intralesional candida antigen can be used in offices that carry these agents. Topical imiquimod has been reported to achieve success in genital warts in children,13 with good efficacy in recalcitrant, periungual, and subungual warts when used for up to 16 weeks.31 In one randomized clinical trial, imiquimod cream 5% combined with salicylic acid 15% was applied to warts for 6 to 10 hours for 5 consecutive days per week versus cryotherapy with liquid nitrogen every 2 weeks for a maximum of 3 months. At the end of the study period, 81.1% (30/37) of participants treated with imiquimod and salicylic acid showed clearance of their warts versus 67.3% (33/49) of those treated with cryotherapy.32
Oral cimetidine has been reported to be successful in treating recalcitrant warts in more than 80% of children when dosed at 30 to 40 mg/kg 3 times daily, requiring 6 to 12 weeks to achieve clearance. Side effects of oral cimetidine include many cytochrome P450 interactions; gynecomastia, which limits usage in teenaged males; and stomach upset.30
Treatment of recalcitrant pediatric warts with intralesional candida antigen has been associated with side effects consistent with delayed-type hypersensitivity reactions. Injections should be administered once monthly, with a minimum of 3 cycles if not effective and up to 6 cycles where partial efficacy is noted. In a retrospective review of 220 cases, 70.9% of children showed complete clearance and 16.8% had partial response.33 However, the treatment may be limited in children by fear of needles.
Squaric acid dibutyl ester is a universal allergen that is not mutagenic on Ames testing and causes milder allergy symptoms than the mutagenic dinitrochlorobenzene and less erythema and pruritus than diphencyclopropenone. Squaric acid dibutyl ester home therapy was evaluated in 61 children with at least one nonfacial wart.34 Application began with squaric acid dibutyl ester in acetone (SADBE) 2% sensitization on the arm followed by at-home application of SADBE 0.2% three to seven times weekly for a minimum of 2 months to determine benefit and for 3 to 4 months as needed; however, average response was 7 weeks. The average complete clearance was 58% and partial clearance was 18%. Side effects included erythema and mild itching as well as urticaria in one case.34 In-office SADBE also has been evaluated in children. In a case series that included 29 children sensitized with SADBE 1% to 2% under occlusion followed by once monthly application of SADBE 0.5% to 5.0% to their warts, 69% clearance and 10% partial clearance was noted after a little more than 4 months of treatment.35 One retrospective review compared combination SADBE, trichloroacetic acid (TCA), and cantharidin both alone and in combination as duos (eg, SADBE and TCA) or trios (SADBE, TCA, and cantharidin).23 Of the 74 children whose medical charts were reviewed, the addition of pretreatment of warts with TCA 50% prior to in-office sensitization and monthly in-office application of SADBE increased treatment response to 100% with an average 2.45 months of therapy, whereas no enhancement was noted with cantharidin. Therefore, it appears that there may be enhanced immune reactivity when TCA pretreatment of warts is performed.23
Immune-Modulating Therapies (Including Normalization of Epithelial Growth)
The most novel immunologic therapy for warts is plerixafor, an agent used to treat WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome, which has been linked to heterozygous gain of function mutations in the chemokine receptor CXCR4 (located on 2q22). In WHIM syndrome, the mutated CXCR4 is more sensitive to CXCL12 activation. Plerixafor is a selective reversible antagonist that blocks the capacity of the chemokine CXCL12 to sustain the permanent activation of CXCR4.37 Combination therapy with plerixafor and topical imiquimod has resulted in wart improvement in WHIM syndrome patients in a small series.38
Oral isotretinoin has been described to be efficacious over placebo at a dosage of 30 mg daily for 12 weeks and can be used in teenagers but requires standard monitoring.36
Irritant Therapies
Duct tape is a classic agent that produces maceration and irritation of warts. Application of duct tape over warts has been described in cycles of 6 days on, 1 day off with weekly repetition for a few months but usually not on the palms or soles due to difficulty maintaining occlusive tape in these locations over an extended period of time. In one trial, 85% (22/26) of duct tape–treated cases cleared versus 60% (15/25) of cryotherapy-treated cases over a 2-month maximum therapeutic period.39
Vascular Destructive Therapies
The pulsed dye laser is a classic modality that induces localized destruction of blood supply to warts in children. A case series of 61 children treated with the pulsed dye laser revealed 75% overall clearance in an average of 3.1 sessions. The usage of this therapy often is limited to institutions where the technology is readily available for usage.40
Nitric Oxide–Releasing Therapies
Nitric oxide release may increase local blood flow, thereby increasing immune response, or may have a primary mechanism of antimicrobial activity, which is why these agents have been investigated for wart treatment. Topical garlic has been described anecdotally as a therapy for thin childhood warts with the putative mechanism being nitric oxide release.42 A new investigational drug recently has had phase 2 data published. Berdazimer sodium plus carboxymethyl cellulose hydrogel has demonstrated benefit in adult warts, but data in children is lacking.41
Therapeutic Ladder for Childhood Warts
The therapeutic ladder (Table) for childhood warts starts with first doing no harm. Although many parents are disturbed by their child’s condition, the natural history of resolution is spontaneous and therefore no therapy is required in many cases. The child and his/her caregivers should be engaged to determine if he/she is emotionally disturbed or uncomfortable with their lesions and to address any fears and concerns that some children may experience (eg, contagion risk, pain with ambulation, ostracism). For example, children with hand warts may report that other children will not hold their hand while in line at school. Prominent facial lesions can be particularly problematic for children due to teasing and bullying.

Conclusion
Warts are a common infection in childhood caused by the ubiquitous HPV virus. Therapeutic options abound, but most cases are either ignored or treated with over-the-counter salicylic acid or in-office cryotherapy. The decision to employ alternative therapeutic options requires agreement by the child, his/her caregiver, and the treating physician and can be tailored to suit the desires and needs of the child. Whether or not therapy is offered, spontaneous clearance is frequently seen in common warts. On the other hand, genital warts are associated with later conversion to malignancies of the genital tract; therefore, encouragement of HPV vaccination is needed in the adolescent population to best ensure long-term genital health.
1. Warts. https://medical-dictionary.thefreedictionary.com/warts. Accessed November 30, 2018.
2. Human papillomavirus. WHO website. http://www.who.int/biologicals/areas/human_papillomavirus/en. Accessed December 3, 2018.
3. Silverberg NB. Human papillomavirus infections in children. Curr Opin Pediatr. 2004;16:402-409.
4. Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
5. Silverberg NB, McCuaig CC. Melanoma in childhood: changing our mind-set. Cutis. 2013;92:217-218.
6. Bruggink SC, Eekhof JA, Egberts PF, et al. Warts transmitted in families and schools: a prospective cohort. Pediatrics. 2013;131:928-934.
7. Silverberg JI, Silverberg NB. The U.S. prevalence of common warts in childhood: a population-based study. J Invest Dermatol. 2013;133:2788-2790.
8. Svensson A, Ofenloch RF, Bruze M, et al. Br J Dermatol. 2018;178:1111-1118.
9. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047.
10. Smith EM, Johnson SR, Cripe TP, et al. Perinatal vertical transmission of human papillomavirus and subsequent development of respiratory tract papillomatosis. Ann Otol Rhinol Laryngol. 1991;100:479-483.
11. Costa-Silva M, Azevedo F, Lisboa C. Anogenital warts in children: analysis of a cohort of 34 prepubertal children. Pediatr Dermatol. 2018;35:E325-E327.
12. Marcoux D, Nadeau K, McCuaig C, et al. Pediatric anogenital warts: a 7-year review of children referred to a tertiary-care hospital in Montreal, Canada. Pediatr Dermatol. 2006;23:199-207.
13. Stefanaki C, Barkas G, Valari M, et al. Condylomata acuminata in children. Pediatr Infect Dis J. 2012;31:422-424.
14. dePlanell-Mas E, Martinez-Garriga B, Zalacain AJ, et al. Human papillomaviruses genotyping in plantar warts. J Med Virol. 2017;89:902-907.
15. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187-193.
16. Lace MJ, Anson JR, Klingelhutz AJ, et al. Human papillomavirus (HPV) type 18 induces extended growth in primary human cervical, tonsillar, or foreskin keratinocytes more effectively than other high-risk mucosal HPVs. J Virol. 2009;83:11784-11794.
17. Sudenga SL, Ingles DJ, Pierce Campbell CM, et al. Genital human papillomavirus infection progression to external genital lesions: the HIM study. Eur Urol. 2016;69:166-173.
18. Rigo MV, Martínez Campillo F, Verdú M, et al. Risk factors linked to the transmission of papilloma virus in the school environment [in Spanish]. Alicante, 1999. Aten Primaria. 2003;31:415-420.
19. Al-Mutairi N, AlKhalaf M. Mucocutaneous warts in children: clinical presentations, risk factors, and response to treatment. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:69-72.
20. Clarke J, Terry RM, Lacey CJ. A study to estimate the prevalence of upper respiratory tract papillomatosis in patients with genital warts. Int J STD AIDS. 1991;2:114-115.
21. Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39:951-955.
22. Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
23. Silverberg JI, Silverberg NB. Adjunctive trichloroacetic acid therapy enhances squaric acid response to verruca vulgaris. J Drugs Dermatol. 2012;11:1228-1230.
24. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001:CD001781.
25. Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233-246.
26. Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950;72:153-155.
27. Caravati CM Jr, Wood BT, Richardson DR. Onychodystrophies secondary to liquid nitrogen cryotherapy. Arch Dermatol. 1969;100:441-442.
28. Duofilm [package insert]. Sligo, Ireland: Stiefel Laboratories (Ireland) Ltd; 2016.
29. Gupta R, Gupta S. Topical adapalene in the treatment of plantar warts: randomized comparative open trial in comparison with cryo-therapy. Indian J Dermatol. 2015;60:102.
30. Orlow SJ, Paller A. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1993;28(5 pt 1):794-796.
31. Micali G, Dall’Oglio F, Nasca MR. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous warts. J Dermatolog Treat. 2003;14:233-236.
32. Stefanaki C, Lagogiani I, Kouris A, et al. Cryotherapy versus imiquimod 5% cream combined with a keratolytic lotion in cutaneous warts in children: a randomized study. J Dermatolog Treat. 2016;27:80-82.
33. Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M, et al. Intralesional Candida antigen immunotherapy for the treatment of recalcitrant and multiple warts in children. Pediatr Dermatol. 2015;32:797-801.
34. Silverberg NB, Lim JK, Paller AS, et al. Squaric acid immunotherapy for warts in children. J Am Acad Dermatol. 2000;42(5 pt 1):803-808.
35. Lee AN, Mallory SB. Contact immunotherapy with squaric acid dibutylester for the treatment of recalcitrant warts. J Am Acad Dermatol. 1999;41:595-599.
36. Olguin-García MG, Jurado-Santa Cruz F, Peralta-Pedrero ML, et al. A double-blind, randomized, placebo-controlled trial of oral isotretinoin in the treatment of recalcitrant facial flat warts. J Dermatolog Treat. 2015;26:78-82.
37. Badolato R, Donadieu J; WHIM Research Group. How I treat warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Blood. 2017;130:2491-2498.
38. McDermott DH, Liu Q, Velez D, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308-2316.
39. Focht DR 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
40. Sethuraman G, Richards KA, Hiremagalore RN, et al. Effectiveness of pulsed dye laser in the treatment of recalcitrant warts in children. Dermatol Surg. 2010;36:58-65.
41. Tyring SK, Rosen T, Berman B, et al. A phase 2 controlled study of SB206, a topical nitric oxide-releasing drug for extragenital wart treatment. J Drugs Dermatol. 2018;17:1100-1105.
42. Silverberg NB. Garlic cloves for verruca vulgaris. Pediatr Dermatol. 2002;19:183.
1. Warts. https://medical-dictionary.thefreedictionary.com/warts. Accessed November 30, 2018.
2. Human papillomavirus. WHO website. http://www.who.int/biologicals/areas/human_papillomavirus/en. Accessed December 3, 2018.
3. Silverberg NB. Human papillomavirus infections in children. Curr Opin Pediatr. 2004;16:402-409.
4. Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
5. Silverberg NB, McCuaig CC. Melanoma in childhood: changing our mind-set. Cutis. 2013;92:217-218.
6. Bruggink SC, Eekhof JA, Egberts PF, et al. Warts transmitted in families and schools: a prospective cohort. Pediatrics. 2013;131:928-934.
7. Silverberg JI, Silverberg NB. The U.S. prevalence of common warts in childhood: a population-based study. J Invest Dermatol. 2013;133:2788-2790.
8. Svensson A, Ofenloch RF, Bruze M, et al. Br J Dermatol. 2018;178:1111-1118.
9. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047.
10. Smith EM, Johnson SR, Cripe TP, et al. Perinatal vertical transmission of human papillomavirus and subsequent development of respiratory tract papillomatosis. Ann Otol Rhinol Laryngol. 1991;100:479-483.
11. Costa-Silva M, Azevedo F, Lisboa C. Anogenital warts in children: analysis of a cohort of 34 prepubertal children. Pediatr Dermatol. 2018;35:E325-E327.
12. Marcoux D, Nadeau K, McCuaig C, et al. Pediatric anogenital warts: a 7-year review of children referred to a tertiary-care hospital in Montreal, Canada. Pediatr Dermatol. 2006;23:199-207.
13. Stefanaki C, Barkas G, Valari M, et al. Condylomata acuminata in children. Pediatr Infect Dis J. 2012;31:422-424.
14. dePlanell-Mas E, Martinez-Garriga B, Zalacain AJ, et al. Human papillomaviruses genotyping in plantar warts. J Med Virol. 2017;89:902-907.
15. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187-193.
16. Lace MJ, Anson JR, Klingelhutz AJ, et al. Human papillomavirus (HPV) type 18 induces extended growth in primary human cervical, tonsillar, or foreskin keratinocytes more effectively than other high-risk mucosal HPVs. J Virol. 2009;83:11784-11794.
17. Sudenga SL, Ingles DJ, Pierce Campbell CM, et al. Genital human papillomavirus infection progression to external genital lesions: the HIM study. Eur Urol. 2016;69:166-173.
18. Rigo MV, Martínez Campillo F, Verdú M, et al. Risk factors linked to the transmission of papilloma virus in the school environment [in Spanish]. Alicante, 1999. Aten Primaria. 2003;31:415-420.
19. Al-Mutairi N, AlKhalaf M. Mucocutaneous warts in children: clinical presentations, risk factors, and response to treatment. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:69-72.
20. Clarke J, Terry RM, Lacey CJ. A study to estimate the prevalence of upper respiratory tract papillomatosis in patients with genital warts. Int J STD AIDS. 1991;2:114-115.
21. Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39:951-955.
22. Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
23. Silverberg JI, Silverberg NB. Adjunctive trichloroacetic acid therapy enhances squaric acid response to verruca vulgaris. J Drugs Dermatol. 2012;11:1228-1230.
24. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001:CD001781.
25. Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233-246.
26. Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950;72:153-155.
27. Caravati CM Jr, Wood BT, Richardson DR. Onychodystrophies secondary to liquid nitrogen cryotherapy. Arch Dermatol. 1969;100:441-442.
28. Duofilm [package insert]. Sligo, Ireland: Stiefel Laboratories (Ireland) Ltd; 2016.
29. Gupta R, Gupta S. Topical adapalene in the treatment of plantar warts: randomized comparative open trial in comparison with cryo-therapy. Indian J Dermatol. 2015;60:102.
30. Orlow SJ, Paller A. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1993;28(5 pt 1):794-796.
31. Micali G, Dall’Oglio F, Nasca MR. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous warts. J Dermatolog Treat. 2003;14:233-236.
32. Stefanaki C, Lagogiani I, Kouris A, et al. Cryotherapy versus imiquimod 5% cream combined with a keratolytic lotion in cutaneous warts in children: a randomized study. J Dermatolog Treat. 2016;27:80-82.
33. Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M, et al. Intralesional Candida antigen immunotherapy for the treatment of recalcitrant and multiple warts in children. Pediatr Dermatol. 2015;32:797-801.
34. Silverberg NB, Lim JK, Paller AS, et al. Squaric acid immunotherapy for warts in children. J Am Acad Dermatol. 2000;42(5 pt 1):803-808.
35. Lee AN, Mallory SB. Contact immunotherapy with squaric acid dibutylester for the treatment of recalcitrant warts. J Am Acad Dermatol. 1999;41:595-599.
36. Olguin-García MG, Jurado-Santa Cruz F, Peralta-Pedrero ML, et al. A double-blind, randomized, placebo-controlled trial of oral isotretinoin in the treatment of recalcitrant facial flat warts. J Dermatolog Treat. 2015;26:78-82.
37. Badolato R, Donadieu J; WHIM Research Group. How I treat warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Blood. 2017;130:2491-2498.
38. McDermott DH, Liu Q, Velez D, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308-2316.
39. Focht DR 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
40. Sethuraman G, Richards KA, Hiremagalore RN, et al. Effectiveness of pulsed dye laser in the treatment of recalcitrant warts in children. Dermatol Surg. 2010;36:58-65.
41. Tyring SK, Rosen T, Berman B, et al. A phase 2 controlled study of SB206, a topical nitric oxide-releasing drug for extragenital wart treatment. J Drugs Dermatol. 2018;17:1100-1105.
42. Silverberg NB. Garlic cloves for verruca vulgaris. Pediatr Dermatol. 2002;19:183.
Practice Points
- Warts are caused by infection with the human papillomavirus.
- Warts are extremely common in all age groups, but risk factors and types of lesions vary by age and location of lesions.
- Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; vascular destructive; irritant; and nitric oxide releasing.
Pediatric Dermatology Workforce Shortage Explained
The Society for Pediatric Dermatology (SPD) was established in 1975, and the pediatric dermatology workforce shortage began shortly after. In 1986, Honig and Burke1 reported that opportunities in pediatric dermatology were limited and that pediatric dermatologists were predominantly located in larger teaching hospitals and selected private practice settings; furthermore, only approximately 20% had patient populations comprising more than 75% children.1 Positive changes have occurred since that time, with more practitioners dedicated to pediatric dermatology and increased opportunities within the specialty. The SPD has expanded to a thriving group of collegial pediatric dermatologists now topping 1200 members worldwide.
Although the SPD has strongly influenced practice development in pediatric dermatology, there are fewer than 300 board-certified pediatric dermatologists in the United States and approximately double that number of pediatric dermatology practitioners. The deficiency is glaring based on the national population alone. The US Census Bureau reported 325,719,178 individuals living in the United States (as of July 1, 2017).2 With approximately 75 million children in the United States and estimates that 22.8% of the population is younger than 18 years,3 there currently is 1 pediatric dermatologist for every 120,000 children or more.
As if the numbers alone were not adequate, a number of publications have addressed the benefits of pediatric dermatologists in both dermatology and pediatrics training and furthermore in pediatric care. A 2004 survey of dermatology program directors and chairpersons regarding the issue of the pediatric dermatology workforce shortage revealed that 45 of 94 (47.9%) programs employed a pediatric dermatologist and 24 (25.5%) had been looking to hire one for more than a year.4 Although more pediatric dermatologists have joined the workforce, it is not surprising that programs with no pediatric dermatologists want them. First, pediatric dermatologists dramatically improve the quality of training with regard to pediatric dermatology education and can increase dermatology residents’ comfort level with children. In a survey of a group of graduating third-year dermatology residents, dermatology residency program directors, and pediatric dermatology fellowship program directors by Nijhawan et al,5 residents who were trained in a program with one or more full-time pediatric dermatologists were more likely to feel competent treating children and to feel satisfied with their training program’s pediatric dermatology curriculum than residents without contact with a full-time pediatric dermatologist (50.0% vs 5.9% [P=.002] and 85.3% vs 52.9% [P<.001], respectively). The availability of a pediatric dermatology fellowship further enhanced satisfaction. Residents in programs with no full-time pediatric dermatologist on staff were more likely to be somewhat or extremely dissatisfied with their pediatric dermatology training. Residency program directors were more satisfied with their curriculums when there was one or more pediatric dermatologist on staff (P<.01).5
Programs with pediatric dermatologists also offer easy access to a mentor in the field. In a 2010 survey of pediatric dermatologists (published in 2014), Admani et al6 reported that 84% (91/109) of respondents (board-certified pediatric dermatologists) cited mentorship as the most important factor influencing their career choice. Exposure to the specialty was noted as a key motivating factor. In my opinion, the actual inclusion of a pediatric dermatology fellowship, whether the position is filled or not, appears to increase the chances of expansion and retention in the field.
Furthermore, due to the outpatient burden of skin disease in a pediatrics practice, providing pediatric trainees with contact with a pediatric dermatologist is needed.
As if there was not enough evidence that pediatric dermatologists are in high demand, SPD pediatric dermatology workforce surveys from the last 5 years, which will soon be updated, show similar indications.7,10 Fogel and Teng11 showed that 60% of surveyed pediatric dermatologists (N=226) were academic and 81% were salaried. Unlike previous data,1 the investigators showed that children constituted 79.5% of respondents’ patient populations.
For the medical student or resident seeking a career in pediatric dermatology, it appears that finding and working on projects with mentors likely is the key to stepping in the field. From my own experience, pediatric dermatologists are extremely friendly and open to supporting career development in earnest students. Reach out to potential mentors months before starting desired electives, as you are competing with other students and pediatrics, dermatology, and emergency medicine residents. Joining and attending meetings of the SPD is a great way to find direction in this friendly and collegial field. Additionally, pediatric dermatology sessions at the annual meetings of the American Academy of Dermatology are a wonderful way to experience the excitement of the field. As a pediatric dermatologist in practice for almost 2 decades, I can honestly say that the field is always intellectually stimulating and evolving rapidly through enhanced understanding of disease pathogenesis, genetics, and therapeutics. Helping children and their parents/guardians never gets boring.
The solution to improving the size and accessibility of the pediatric dermatology workforce is not simple and likely starts from the bottom up. More than 75% of pediatric dermatologists favor implementing systems to encourage medical students to pursue a career in pediatric dermatology.7 Increasing resident exposure to dedicated pediatric dermatology training time enhances satisfaction.5 Increased funding of fellowships can help these students and residents meet their goals. Current fellowship training programs now total 36, but not all approved institutions have been able to support a postgraduate year 5 (PGY-5) or higher fellow, and in my experience some institutions have avoided adding a fellow due to lack of funding internally. The average pediatric dermatologist earns $100,000 less than colleagues who treat adults, which is an impediment to the expansion of the field.10 This disparity may chase away practitioners, especially those with medical school debt. Debt forgiveness programs, enhanced practice development, and better base pay for pediatric dermatologists could positively impact growth in this specialty. Dermatology and pediatrics training programs need to dedicate more money and developmental support for pediatric dermatologists as a way to invest in the quality of pediatric dermatology education for their trainees. By recognizing the true value of the academic contributions of pediatric dermatologists, dermatology residency programs can invest in producing trainees with greater aplomb and acumen in pediatric dermatology.
- Honig PJ, Burke L. The subspecialty of pediatric dermatology. J Am Acad Dermatol. 1986;15:123-126.
- United States Census Bureau. QuickFacts. https://www.census.gov/quickfacts/fact/table/US/PST045217#PST045217. Accessed October 19, 2018.
- An aging nation: projected number of children and older adults. United States Census Bureau website. https://www.census.gov/library/visualizations/2018/comm/historic-first.html. Published March 13, 2018. Accessed October 9, 2018.
- Hester EJ, McNealy KM, Kelloff JN, et al. Demand outstrips supply of US pediatric dermatologists: results from a national survey. J Am Acad Dermatol. 2004;50:431-434.
- Nijhawan RI, Mazza JM, Silverberg NB. Pediatric dermatology training survey of United States dermatology residency programs. Pediatr Dermatol. 2014;31:131-137.
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-375.
- 2014 Society for Pediatric Dermatology Peds Derm Training Survey. Society for Pediatric Dermatology website. https://pedsderm.net/site/assets/files/8639/06b-peds_training_survey_responses_final.pdf. Accessed October 9, 2018.
- ABD approved pediatric dermatology fellowship programs. Society for Pediatric Dermatology website. https://pedsderm.net/training/fellowships/abd-approved-pediatric-dermatology-fellowship-programs/. Accessed October 9, 2018.
- Prindaville B, Simon SD, Horii KA. Dermatology-related outpatient visits by children: implications for workforce and pediatric education. J Am Acad Dermatol. 2016;75:228-229.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present and future [published online July 21, 2014]. Pediatr Dermatol. 2015;32:1-12.
- Fogel AL, Teng JM. The US pediatric dermatology workforce: an assessment of productivity and practice patterns. Pediatr Dermatol. 2015;32:825-829.
The Society for Pediatric Dermatology (SPD) was established in 1975, and the pediatric dermatology workforce shortage began shortly after. In 1986, Honig and Burke1 reported that opportunities in pediatric dermatology were limited and that pediatric dermatologists were predominantly located in larger teaching hospitals and selected private practice settings; furthermore, only approximately 20% had patient populations comprising more than 75% children.1 Positive changes have occurred since that time, with more practitioners dedicated to pediatric dermatology and increased opportunities within the specialty. The SPD has expanded to a thriving group of collegial pediatric dermatologists now topping 1200 members worldwide.
Although the SPD has strongly influenced practice development in pediatric dermatology, there are fewer than 300 board-certified pediatric dermatologists in the United States and approximately double that number of pediatric dermatology practitioners. The deficiency is glaring based on the national population alone. The US Census Bureau reported 325,719,178 individuals living in the United States (as of July 1, 2017).2 With approximately 75 million children in the United States and estimates that 22.8% of the population is younger than 18 years,3 there currently is 1 pediatric dermatologist for every 120,000 children or more.
As if the numbers alone were not adequate, a number of publications have addressed the benefits of pediatric dermatologists in both dermatology and pediatrics training and furthermore in pediatric care. A 2004 survey of dermatology program directors and chairpersons regarding the issue of the pediatric dermatology workforce shortage revealed that 45 of 94 (47.9%) programs employed a pediatric dermatologist and 24 (25.5%) had been looking to hire one for more than a year.4 Although more pediatric dermatologists have joined the workforce, it is not surprising that programs with no pediatric dermatologists want them. First, pediatric dermatologists dramatically improve the quality of training with regard to pediatric dermatology education and can increase dermatology residents’ comfort level with children. In a survey of a group of graduating third-year dermatology residents, dermatology residency program directors, and pediatric dermatology fellowship program directors by Nijhawan et al,5 residents who were trained in a program with one or more full-time pediatric dermatologists were more likely to feel competent treating children and to feel satisfied with their training program’s pediatric dermatology curriculum than residents without contact with a full-time pediatric dermatologist (50.0% vs 5.9% [P=.002] and 85.3% vs 52.9% [P<.001], respectively). The availability of a pediatric dermatology fellowship further enhanced satisfaction. Residents in programs with no full-time pediatric dermatologist on staff were more likely to be somewhat or extremely dissatisfied with their pediatric dermatology training. Residency program directors were more satisfied with their curriculums when there was one or more pediatric dermatologist on staff (P<.01).5
Programs with pediatric dermatologists also offer easy access to a mentor in the field. In a 2010 survey of pediatric dermatologists (published in 2014), Admani et al6 reported that 84% (91/109) of respondents (board-certified pediatric dermatologists) cited mentorship as the most important factor influencing their career choice. Exposure to the specialty was noted as a key motivating factor. In my opinion, the actual inclusion of a pediatric dermatology fellowship, whether the position is filled or not, appears to increase the chances of expansion and retention in the field.
Furthermore, due to the outpatient burden of skin disease in a pediatrics practice, providing pediatric trainees with contact with a pediatric dermatologist is needed.
As if there was not enough evidence that pediatric dermatologists are in high demand, SPD pediatric dermatology workforce surveys from the last 5 years, which will soon be updated, show similar indications.7,10 Fogel and Teng11 showed that 60% of surveyed pediatric dermatologists (N=226) were academic and 81% were salaried. Unlike previous data,1 the investigators showed that children constituted 79.5% of respondents’ patient populations.
For the medical student or resident seeking a career in pediatric dermatology, it appears that finding and working on projects with mentors likely is the key to stepping in the field. From my own experience, pediatric dermatologists are extremely friendly and open to supporting career development in earnest students. Reach out to potential mentors months before starting desired electives, as you are competing with other students and pediatrics, dermatology, and emergency medicine residents. Joining and attending meetings of the SPD is a great way to find direction in this friendly and collegial field. Additionally, pediatric dermatology sessions at the annual meetings of the American Academy of Dermatology are a wonderful way to experience the excitement of the field. As a pediatric dermatologist in practice for almost 2 decades, I can honestly say that the field is always intellectually stimulating and evolving rapidly through enhanced understanding of disease pathogenesis, genetics, and therapeutics. Helping children and their parents/guardians never gets boring.
The solution to improving the size and accessibility of the pediatric dermatology workforce is not simple and likely starts from the bottom up. More than 75% of pediatric dermatologists favor implementing systems to encourage medical students to pursue a career in pediatric dermatology.7 Increasing resident exposure to dedicated pediatric dermatology training time enhances satisfaction.5 Increased funding of fellowships can help these students and residents meet their goals. Current fellowship training programs now total 36, but not all approved institutions have been able to support a postgraduate year 5 (PGY-5) or higher fellow, and in my experience some institutions have avoided adding a fellow due to lack of funding internally. The average pediatric dermatologist earns $100,000 less than colleagues who treat adults, which is an impediment to the expansion of the field.10 This disparity may chase away practitioners, especially those with medical school debt. Debt forgiveness programs, enhanced practice development, and better base pay for pediatric dermatologists could positively impact growth in this specialty. Dermatology and pediatrics training programs need to dedicate more money and developmental support for pediatric dermatologists as a way to invest in the quality of pediatric dermatology education for their trainees. By recognizing the true value of the academic contributions of pediatric dermatologists, dermatology residency programs can invest in producing trainees with greater aplomb and acumen in pediatric dermatology.
The Society for Pediatric Dermatology (SPD) was established in 1975, and the pediatric dermatology workforce shortage began shortly after. In 1986, Honig and Burke1 reported that opportunities in pediatric dermatology were limited and that pediatric dermatologists were predominantly located in larger teaching hospitals and selected private practice settings; furthermore, only approximately 20% had patient populations comprising more than 75% children.1 Positive changes have occurred since that time, with more practitioners dedicated to pediatric dermatology and increased opportunities within the specialty. The SPD has expanded to a thriving group of collegial pediatric dermatologists now topping 1200 members worldwide.
Although the SPD has strongly influenced practice development in pediatric dermatology, there are fewer than 300 board-certified pediatric dermatologists in the United States and approximately double that number of pediatric dermatology practitioners. The deficiency is glaring based on the national population alone. The US Census Bureau reported 325,719,178 individuals living in the United States (as of July 1, 2017).2 With approximately 75 million children in the United States and estimates that 22.8% of the population is younger than 18 years,3 there currently is 1 pediatric dermatologist for every 120,000 children or more.
As if the numbers alone were not adequate, a number of publications have addressed the benefits of pediatric dermatologists in both dermatology and pediatrics training and furthermore in pediatric care. A 2004 survey of dermatology program directors and chairpersons regarding the issue of the pediatric dermatology workforce shortage revealed that 45 of 94 (47.9%) programs employed a pediatric dermatologist and 24 (25.5%) had been looking to hire one for more than a year.4 Although more pediatric dermatologists have joined the workforce, it is not surprising that programs with no pediatric dermatologists want them. First, pediatric dermatologists dramatically improve the quality of training with regard to pediatric dermatology education and can increase dermatology residents’ comfort level with children. In a survey of a group of graduating third-year dermatology residents, dermatology residency program directors, and pediatric dermatology fellowship program directors by Nijhawan et al,5 residents who were trained in a program with one or more full-time pediatric dermatologists were more likely to feel competent treating children and to feel satisfied with their training program’s pediatric dermatology curriculum than residents without contact with a full-time pediatric dermatologist (50.0% vs 5.9% [P=.002] and 85.3% vs 52.9% [P<.001], respectively). The availability of a pediatric dermatology fellowship further enhanced satisfaction. Residents in programs with no full-time pediatric dermatologist on staff were more likely to be somewhat or extremely dissatisfied with their pediatric dermatology training. Residency program directors were more satisfied with their curriculums when there was one or more pediatric dermatologist on staff (P<.01).5
Programs with pediatric dermatologists also offer easy access to a mentor in the field. In a 2010 survey of pediatric dermatologists (published in 2014), Admani et al6 reported that 84% (91/109) of respondents (board-certified pediatric dermatologists) cited mentorship as the most important factor influencing their career choice. Exposure to the specialty was noted as a key motivating factor. In my opinion, the actual inclusion of a pediatric dermatology fellowship, whether the position is filled or not, appears to increase the chances of expansion and retention in the field.
Furthermore, due to the outpatient burden of skin disease in a pediatrics practice, providing pediatric trainees with contact with a pediatric dermatologist is needed.
As if there was not enough evidence that pediatric dermatologists are in high demand, SPD pediatric dermatology workforce surveys from the last 5 years, which will soon be updated, show similar indications.7,10 Fogel and Teng11 showed that 60% of surveyed pediatric dermatologists (N=226) were academic and 81% were salaried. Unlike previous data,1 the investigators showed that children constituted 79.5% of respondents’ patient populations.
For the medical student or resident seeking a career in pediatric dermatology, it appears that finding and working on projects with mentors likely is the key to stepping in the field. From my own experience, pediatric dermatologists are extremely friendly and open to supporting career development in earnest students. Reach out to potential mentors months before starting desired electives, as you are competing with other students and pediatrics, dermatology, and emergency medicine residents. Joining and attending meetings of the SPD is a great way to find direction in this friendly and collegial field. Additionally, pediatric dermatology sessions at the annual meetings of the American Academy of Dermatology are a wonderful way to experience the excitement of the field. As a pediatric dermatologist in practice for almost 2 decades, I can honestly say that the field is always intellectually stimulating and evolving rapidly through enhanced understanding of disease pathogenesis, genetics, and therapeutics. Helping children and their parents/guardians never gets boring.
The solution to improving the size and accessibility of the pediatric dermatology workforce is not simple and likely starts from the bottom up. More than 75% of pediatric dermatologists favor implementing systems to encourage medical students to pursue a career in pediatric dermatology.7 Increasing resident exposure to dedicated pediatric dermatology training time enhances satisfaction.5 Increased funding of fellowships can help these students and residents meet their goals. Current fellowship training programs now total 36, but not all approved institutions have been able to support a postgraduate year 5 (PGY-5) or higher fellow, and in my experience some institutions have avoided adding a fellow due to lack of funding internally. The average pediatric dermatologist earns $100,000 less than colleagues who treat adults, which is an impediment to the expansion of the field.10 This disparity may chase away practitioners, especially those with medical school debt. Debt forgiveness programs, enhanced practice development, and better base pay for pediatric dermatologists could positively impact growth in this specialty. Dermatology and pediatrics training programs need to dedicate more money and developmental support for pediatric dermatologists as a way to invest in the quality of pediatric dermatology education for their trainees. By recognizing the true value of the academic contributions of pediatric dermatologists, dermatology residency programs can invest in producing trainees with greater aplomb and acumen in pediatric dermatology.
- Honig PJ, Burke L. The subspecialty of pediatric dermatology. J Am Acad Dermatol. 1986;15:123-126.
- United States Census Bureau. QuickFacts. https://www.census.gov/quickfacts/fact/table/US/PST045217#PST045217. Accessed October 19, 2018.
- An aging nation: projected number of children and older adults. United States Census Bureau website. https://www.census.gov/library/visualizations/2018/comm/historic-first.html. Published March 13, 2018. Accessed October 9, 2018.
- Hester EJ, McNealy KM, Kelloff JN, et al. Demand outstrips supply of US pediatric dermatologists: results from a national survey. J Am Acad Dermatol. 2004;50:431-434.
- Nijhawan RI, Mazza JM, Silverberg NB. Pediatric dermatology training survey of United States dermatology residency programs. Pediatr Dermatol. 2014;31:131-137.
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-375.
- 2014 Society for Pediatric Dermatology Peds Derm Training Survey. Society for Pediatric Dermatology website. https://pedsderm.net/site/assets/files/8639/06b-peds_training_survey_responses_final.pdf. Accessed October 9, 2018.
- ABD approved pediatric dermatology fellowship programs. Society for Pediatric Dermatology website. https://pedsderm.net/training/fellowships/abd-approved-pediatric-dermatology-fellowship-programs/. Accessed October 9, 2018.
- Prindaville B, Simon SD, Horii KA. Dermatology-related outpatient visits by children: implications for workforce and pediatric education. J Am Acad Dermatol. 2016;75:228-229.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present and future [published online July 21, 2014]. Pediatr Dermatol. 2015;32:1-12.
- Fogel AL, Teng JM. The US pediatric dermatology workforce: an assessment of productivity and practice patterns. Pediatr Dermatol. 2015;32:825-829.
- Honig PJ, Burke L. The subspecialty of pediatric dermatology. J Am Acad Dermatol. 1986;15:123-126.
- United States Census Bureau. QuickFacts. https://www.census.gov/quickfacts/fact/table/US/PST045217#PST045217. Accessed October 19, 2018.
- An aging nation: projected number of children and older adults. United States Census Bureau website. https://www.census.gov/library/visualizations/2018/comm/historic-first.html. Published March 13, 2018. Accessed October 9, 2018.
- Hester EJ, McNealy KM, Kelloff JN, et al. Demand outstrips supply of US pediatric dermatologists: results from a national survey. J Am Acad Dermatol. 2004;50:431-434.
- Nijhawan RI, Mazza JM, Silverberg NB. Pediatric dermatology training survey of United States dermatology residency programs. Pediatr Dermatol. 2014;31:131-137.
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-375.
- 2014 Society for Pediatric Dermatology Peds Derm Training Survey. Society for Pediatric Dermatology website. https://pedsderm.net/site/assets/files/8639/06b-peds_training_survey_responses_final.pdf. Accessed October 9, 2018.
- ABD approved pediatric dermatology fellowship programs. Society for Pediatric Dermatology website. https://pedsderm.net/training/fellowships/abd-approved-pediatric-dermatology-fellowship-programs/. Accessed October 9, 2018.
- Prindaville B, Simon SD, Horii KA. Dermatology-related outpatient visits by children: implications for workforce and pediatric education. J Am Acad Dermatol. 2016;75:228-229.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present and future [published online July 21, 2014]. Pediatr Dermatol. 2015;32:1-12.
- Fogel AL, Teng JM. The US pediatric dermatology workforce: an assessment of productivity and practice patterns. Pediatr Dermatol. 2015;32:825-829.
Molluscum Contagiosum Virus Infection Can Trigger Atopic Dermatitis Disease Onset or Flare
Molluscum contagiosum virus (MCV) is a common pediatric viral infection of the skin and/or mucous membranes.1 It has been noted in increasingly younger patient populations, ranging from congenital cases resulting from perinatal/vertical transmission to transmission from cobathing and pool usage.2,3
An association between MCV infection and atopic dermatitis (AD) has been reported to be caused by a predisposition to prolonged and severe cutaneous viral infections.4 However, the exact nature of the relationship between MCV and AD is unknown.
The purpose of this study was to identify pediatric patients with AD onset or flare of AD triggered by MCV infection as well as to characterize the setting under which MCV may trigger AD onset or flares in children.
Methods
Medical records for 50 children with prior or current MCV infection who presented sequentially to an outpatient pediatric dermatology practice over a 1-month period were identified. Institutional review board approval was obtained.
Results
The age range of the 50 patients with MCV infection was 1 to 13 years, with an average age of 3.6 years at the onset of infection (reported by parents/guardians) and 4.5 years at presentation to the pediatric dermatology office (Table 1).
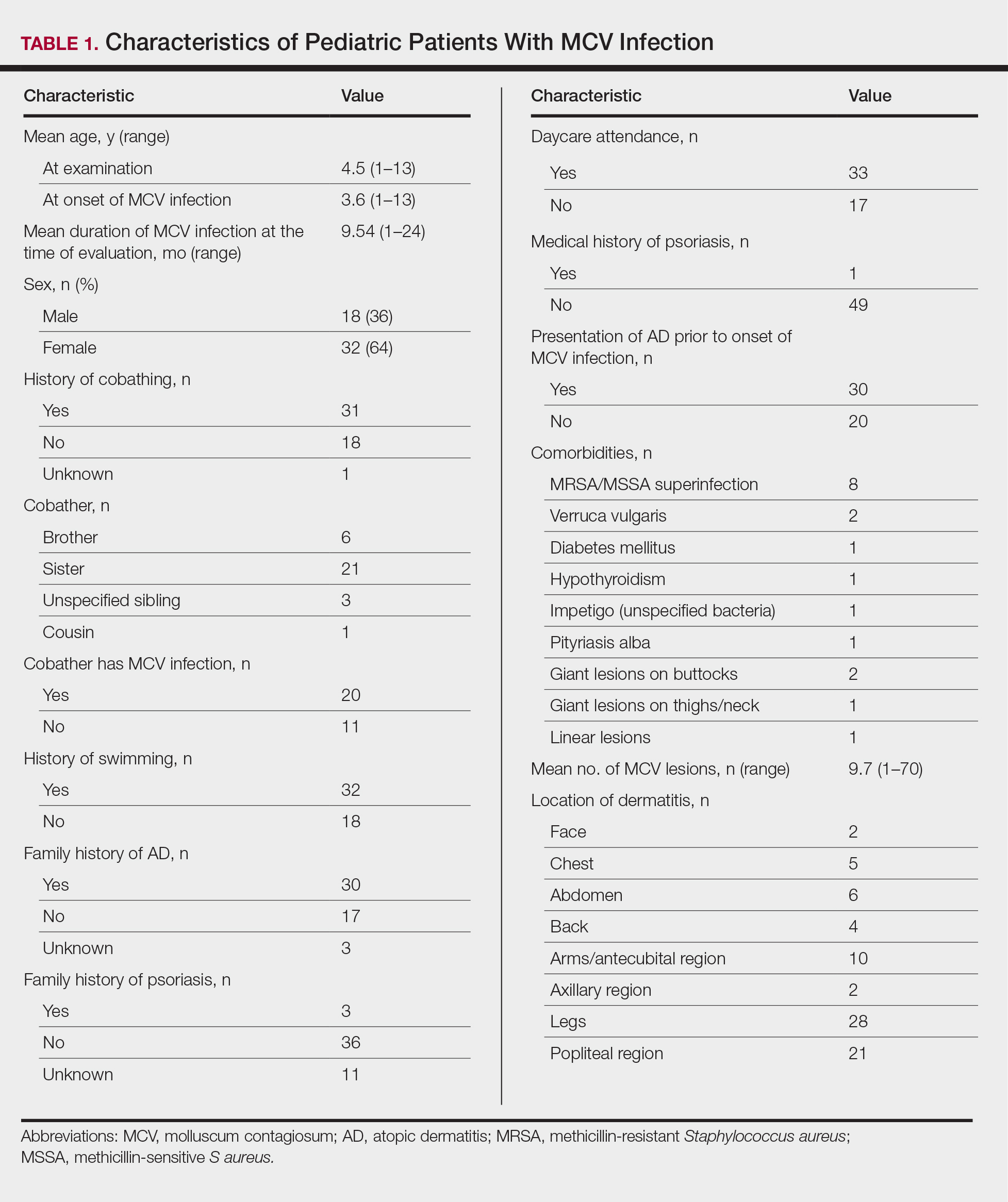
The role of cobathing is unknown; however, 62% (31/50) of patients previously or currently cobathed at home, suggesting it may be a risk factor for MCV infection. An association of MCV lesions in the popliteal region trended toward being more likely with cobathing, but the association was not statistically significant.
Children with AD onset triggered by MCV infection statistically were more likely to have flexural localization of MCV and AD lesions and were statistically more likely to have a family history of AD (P<.04)(Table 2). Children with AD flares triggered by MCV infection were more likely to have MCV and AD lesions of the popliteal region and legs (P<.05)(Figure) and family history of AD (P<.04)(Table 3). Location of MCV lesions on the upper and lower extremities, buttocks, and genitalia were more likely to be associated with presence of any dermatitis than facial and/or truncal lesions (P<.05). Treatment of the MCV infection did not appear to impact the course of AD when present, but prospective interventions would be needed to assess this issue.
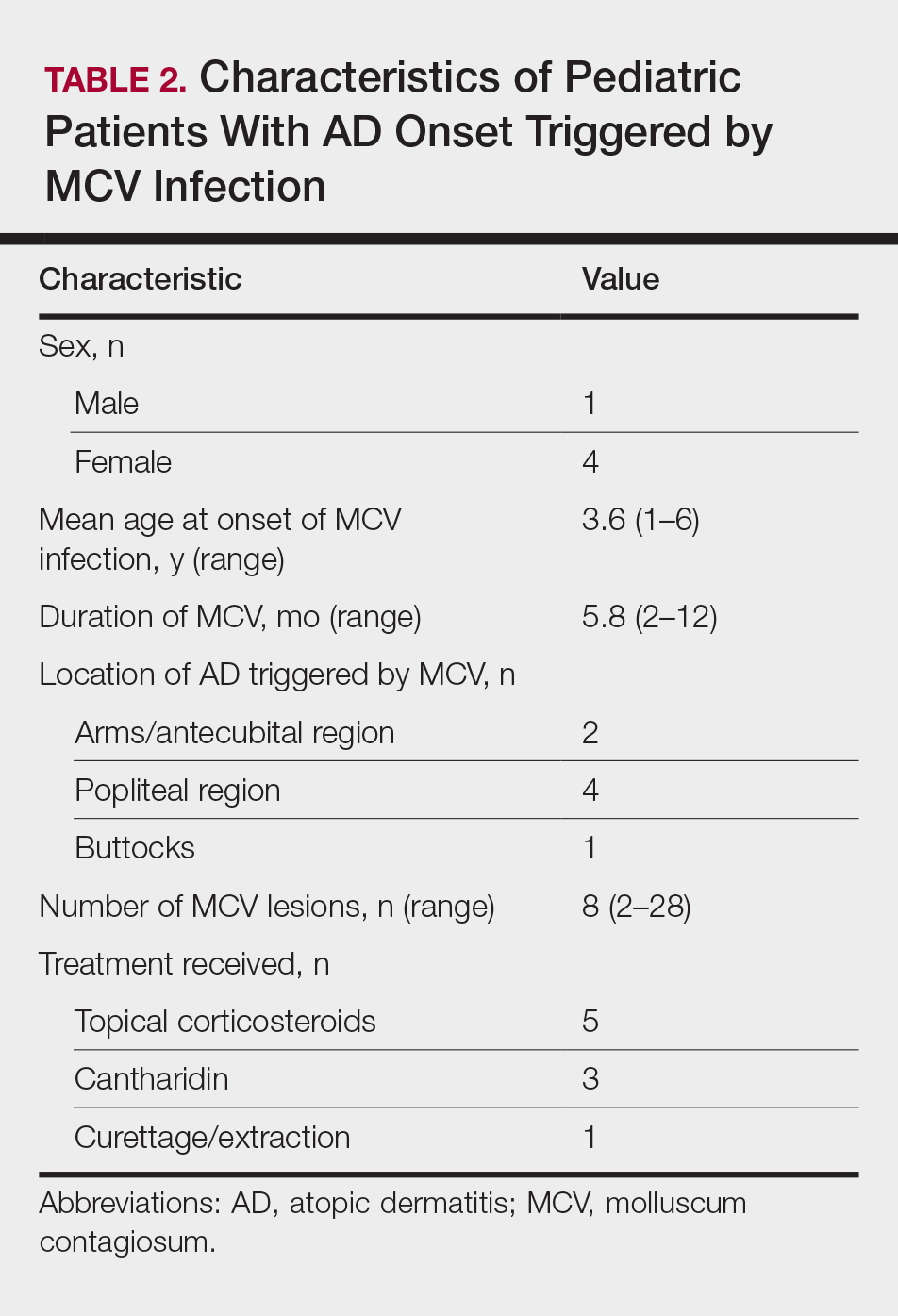
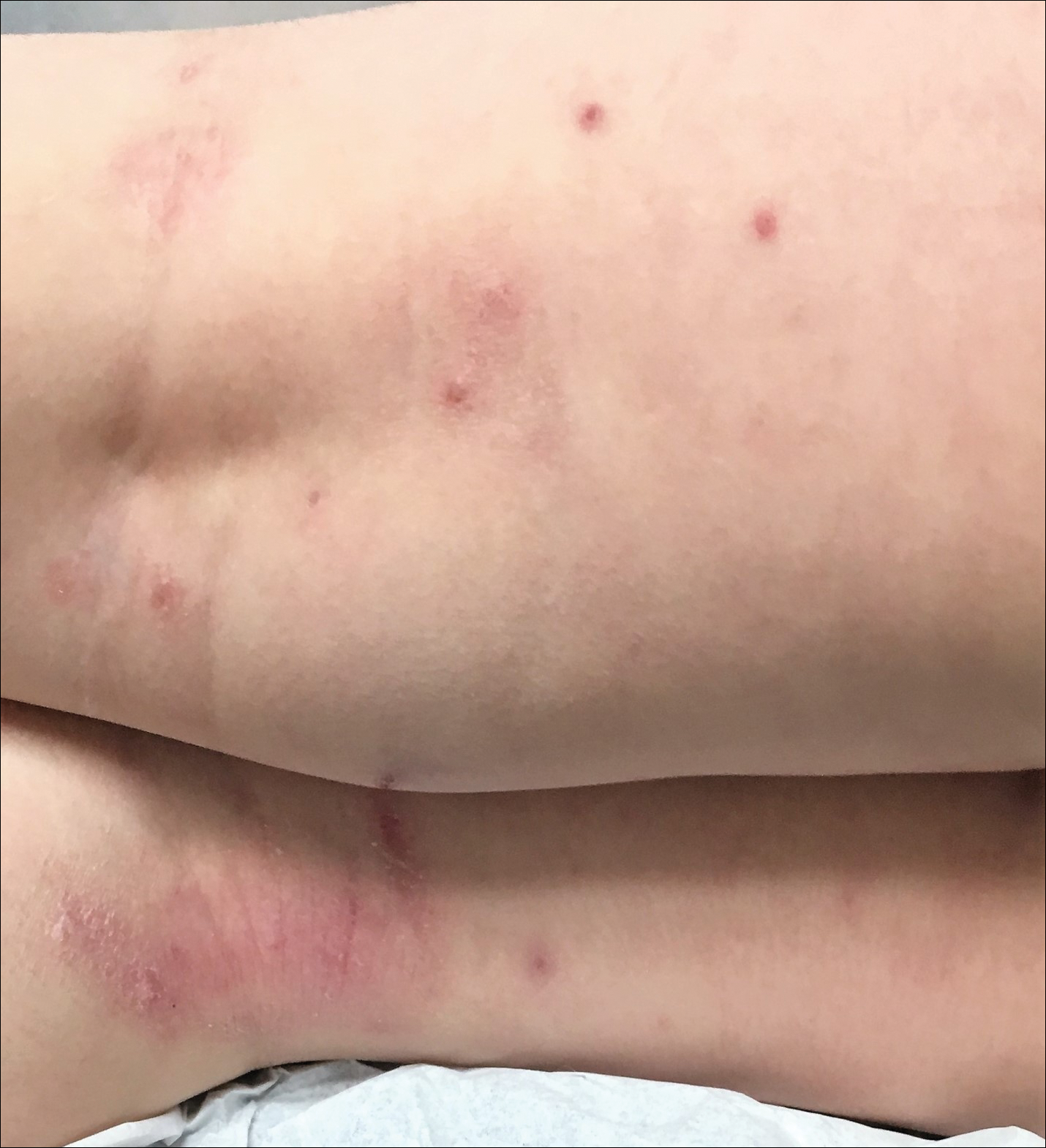
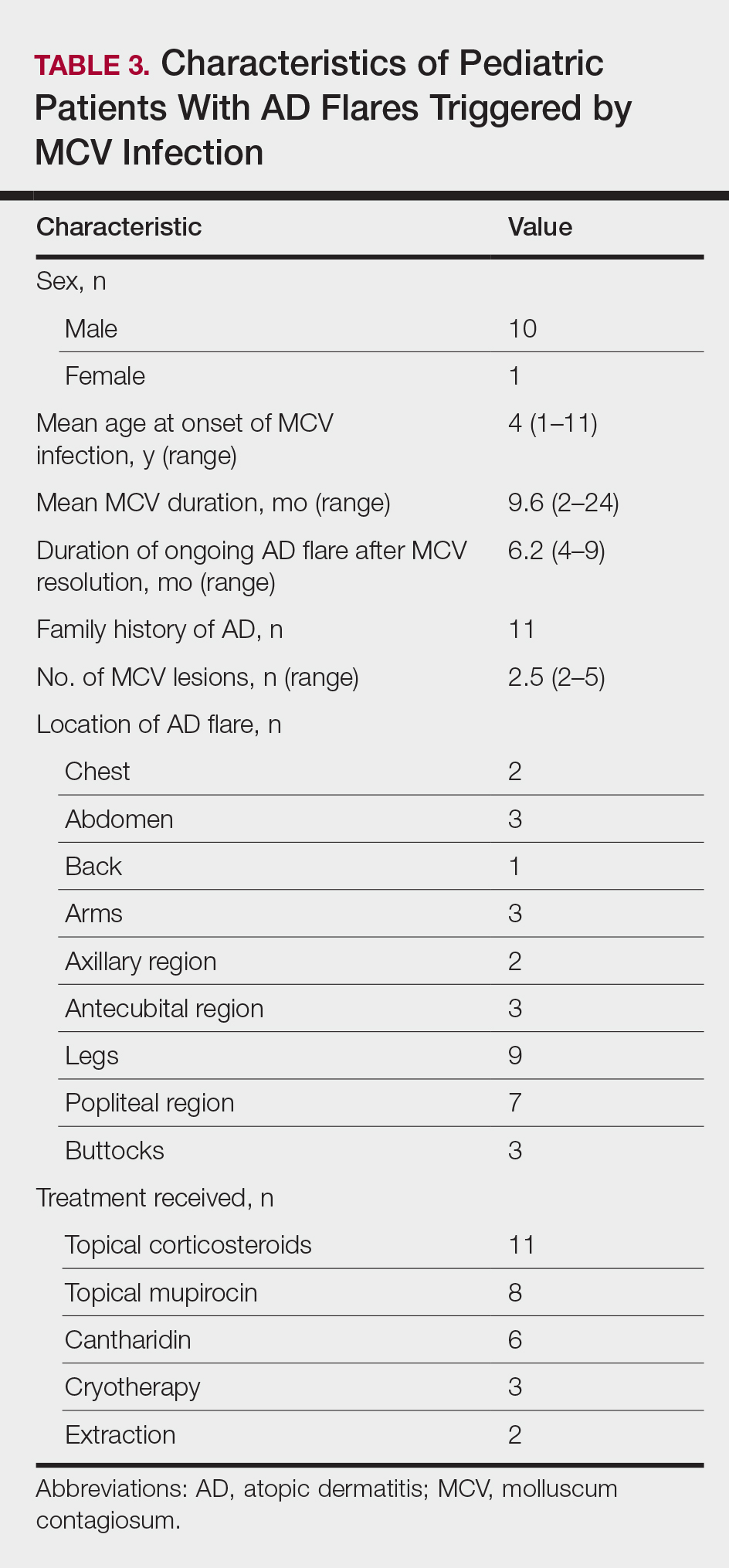
Superinfection with methicillin-resistant and methicillin-sensitive Staphylococcus aureus as well as atypical giant lesions of the intertriginous neck, inner thighs, and buttocks also were noted, but AD was uncommon in these cases. Given the limited number of cases, statistical significance could not be assessed.
Comment
Cutaneous infections with Malassezia have been postulated to trigger AD in infancy,1 while systemic viral infections such as varicella-zoster virus may be protective against AD when acquired in younger children.7 It appears that MCV infection in young children (eg, 3 years or younger) with specific localization to the flexural areas has the potential to trigger AD in susceptible hosts. Larger studies are needed to chart the long-term disease course of AD in these children. Due to the small size of this study, it is unclear if the rise of MCV infections since the 1980s has contributed to increased AD.8 Susceptible children appear to have a family history of AD and localization of MCV lesions on the legs, buttocks, and antecubital region. Atopic dermatitis risk appears to be highest when MCV lesions are localized to intertriginous or flexural locations.
In addition to triggering the onset of AD, MCV infection also can trigger persistent flaring of AD, especially in the popliteal region and legs. Atopic dermatitis flares can occur at any age, but they appear to cluster in preschoolers and typically are not prevented by AD or MCV treatments; however, randomized trials are needed to identify if early intervention of MCV has a preventive benefit on AD onset or flares, and longer-term observation is needed to identify true disease course modification. Reduction of the number of MCV lesions previously has been demonstrated with institution of topical corticosteroid therapy.6 Therefore, institution of atopic skin care generally is advisable in the setting of MCV infection. Future studies should address the potential use of interventions to prevent the triggering of AD onset or flares in the setting of MCV infection in children.5
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Oriel JD. The increase in molluscum contagiosum. Br Med J (Clin Res Ed). 1987;294:74.
Molluscum contagiosum virus (MCV) is a common pediatric viral infection of the skin and/or mucous membranes.1 It has been noted in increasingly younger patient populations, ranging from congenital cases resulting from perinatal/vertical transmission to transmission from cobathing and pool usage.2,3
An association between MCV infection and atopic dermatitis (AD) has been reported to be caused by a predisposition to prolonged and severe cutaneous viral infections.4 However, the exact nature of the relationship between MCV and AD is unknown.
The purpose of this study was to identify pediatric patients with AD onset or flare of AD triggered by MCV infection as well as to characterize the setting under which MCV may trigger AD onset or flares in children.
Methods
Medical records for 50 children with prior or current MCV infection who presented sequentially to an outpatient pediatric dermatology practice over a 1-month period were identified. Institutional review board approval was obtained.
Results
The age range of the 50 patients with MCV infection was 1 to 13 years, with an average age of 3.6 years at the onset of infection (reported by parents/guardians) and 4.5 years at presentation to the pediatric dermatology office (Table 1).

The role of cobathing is unknown; however, 62% (31/50) of patients previously or currently cobathed at home, suggesting it may be a risk factor for MCV infection. An association of MCV lesions in the popliteal region trended toward being more likely with cobathing, but the association was not statistically significant.
Children with AD onset triggered by MCV infection statistically were more likely to have flexural localization of MCV and AD lesions and were statistically more likely to have a family history of AD (P<.04)(Table 2). Children with AD flares triggered by MCV infection were more likely to have MCV and AD lesions of the popliteal region and legs (P<.05)(Figure) and family history of AD (P<.04)(Table 3). Location of MCV lesions on the upper and lower extremities, buttocks, and genitalia were more likely to be associated with presence of any dermatitis than facial and/or truncal lesions (P<.05). Treatment of the MCV infection did not appear to impact the course of AD when present, but prospective interventions would be needed to assess this issue.



Superinfection with methicillin-resistant and methicillin-sensitive Staphylococcus aureus as well as atypical giant lesions of the intertriginous neck, inner thighs, and buttocks also were noted, but AD was uncommon in these cases. Given the limited number of cases, statistical significance could not be assessed.
Comment
Cutaneous infections with Malassezia have been postulated to trigger AD in infancy,1 while systemic viral infections such as varicella-zoster virus may be protective against AD when acquired in younger children.7 It appears that MCV infection in young children (eg, 3 years or younger) with specific localization to the flexural areas has the potential to trigger AD in susceptible hosts. Larger studies are needed to chart the long-term disease course of AD in these children. Due to the small size of this study, it is unclear if the rise of MCV infections since the 1980s has contributed to increased AD.8 Susceptible children appear to have a family history of AD and localization of MCV lesions on the legs, buttocks, and antecubital region. Atopic dermatitis risk appears to be highest when MCV lesions are localized to intertriginous or flexural locations.
In addition to triggering the onset of AD, MCV infection also can trigger persistent flaring of AD, especially in the popliteal region and legs. Atopic dermatitis flares can occur at any age, but they appear to cluster in preschoolers and typically are not prevented by AD or MCV treatments; however, randomized trials are needed to identify if early intervention of MCV has a preventive benefit on AD onset or flares, and longer-term observation is needed to identify true disease course modification. Reduction of the number of MCV lesions previously has been demonstrated with institution of topical corticosteroid therapy.6 Therefore, institution of atopic skin care generally is advisable in the setting of MCV infection. Future studies should address the potential use of interventions to prevent the triggering of AD onset or flares in the setting of MCV infection in children.5
Molluscum contagiosum virus (MCV) is a common pediatric viral infection of the skin and/or mucous membranes.1 It has been noted in increasingly younger patient populations, ranging from congenital cases resulting from perinatal/vertical transmission to transmission from cobathing and pool usage.2,3
An association between MCV infection and atopic dermatitis (AD) has been reported to be caused by a predisposition to prolonged and severe cutaneous viral infections.4 However, the exact nature of the relationship between MCV and AD is unknown.
The purpose of this study was to identify pediatric patients with AD onset or flare of AD triggered by MCV infection as well as to characterize the setting under which MCV may trigger AD onset or flares in children.
Methods
Medical records for 50 children with prior or current MCV infection who presented sequentially to an outpatient pediatric dermatology practice over a 1-month period were identified. Institutional review board approval was obtained.
Results
The age range of the 50 patients with MCV infection was 1 to 13 years, with an average age of 3.6 years at the onset of infection (reported by parents/guardians) and 4.5 years at presentation to the pediatric dermatology office (Table 1).

The role of cobathing is unknown; however, 62% (31/50) of patients previously or currently cobathed at home, suggesting it may be a risk factor for MCV infection. An association of MCV lesions in the popliteal region trended toward being more likely with cobathing, but the association was not statistically significant.
Children with AD onset triggered by MCV infection statistically were more likely to have flexural localization of MCV and AD lesions and were statistically more likely to have a family history of AD (P<.04)(Table 2). Children with AD flares triggered by MCV infection were more likely to have MCV and AD lesions of the popliteal region and legs (P<.05)(Figure) and family history of AD (P<.04)(Table 3). Location of MCV lesions on the upper and lower extremities, buttocks, and genitalia were more likely to be associated with presence of any dermatitis than facial and/or truncal lesions (P<.05). Treatment of the MCV infection did not appear to impact the course of AD when present, but prospective interventions would be needed to assess this issue.



Superinfection with methicillin-resistant and methicillin-sensitive Staphylococcus aureus as well as atypical giant lesions of the intertriginous neck, inner thighs, and buttocks also were noted, but AD was uncommon in these cases. Given the limited number of cases, statistical significance could not be assessed.
Comment
Cutaneous infections with Malassezia have been postulated to trigger AD in infancy,1 while systemic viral infections such as varicella-zoster virus may be protective against AD when acquired in younger children.7 It appears that MCV infection in young children (eg, 3 years or younger) with specific localization to the flexural areas has the potential to trigger AD in susceptible hosts. Larger studies are needed to chart the long-term disease course of AD in these children. Due to the small size of this study, it is unclear if the rise of MCV infections since the 1980s has contributed to increased AD.8 Susceptible children appear to have a family history of AD and localization of MCV lesions on the legs, buttocks, and antecubital region. Atopic dermatitis risk appears to be highest when MCV lesions are localized to intertriginous or flexural locations.
In addition to triggering the onset of AD, MCV infection also can trigger persistent flaring of AD, especially in the popliteal region and legs. Atopic dermatitis flares can occur at any age, but they appear to cluster in preschoolers and typically are not prevented by AD or MCV treatments; however, randomized trials are needed to identify if early intervention of MCV has a preventive benefit on AD onset or flares, and longer-term observation is needed to identify true disease course modification. Reduction of the number of MCV lesions previously has been demonstrated with institution of topical corticosteroid therapy.6 Therefore, institution of atopic skin care generally is advisable in the setting of MCV infection. Future studies should address the potential use of interventions to prevent the triggering of AD onset or flares in the setting of MCV infection in children.5
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Oriel JD. The increase in molluscum contagiosum. Br Med J (Clin Res Ed). 1987;294:74.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Oriel JD. The increase in molluscum contagiosum. Br Med J (Clin Res Ed). 1987;294:74.
Practice Points
- Molluscum contagiosum virus (MCV) infection appears to aggravate atopic dermatitis (AD) symptoms in a subset of pediatric patients.
- In susceptible children, the first onset of AD symptoms can occur during the course of MCV infection.
Atopic Dermatitis Pipeline
Just when you might have thought dermatologic therapies were peaking, along came another banner year in atopic dermatitis (AD). Last year we saw the landmark launch of dupilumab, the first US Food and Drug Administration (FDA)–approved biologic therapy for AD. Dupilumab addresses a novel mechanism of AD in adults by blocking IL-4 and IL-13, which both play a central role in the type 2 helper T cell (TH2) axis on the dual development of barrier-impaired skin and aberrant immune response including IgE to cutaneous aggravating agents with resultant inflammation. Additional information has shown direct effects to reduce itch in AD.1 A 12-week study of dupilumab monotherapy showed that 85% (47/55) of treated patients had at least a 50% reduction in Eczema Area and Severity Index (EASI) score and 40% (22/55) were clear or almost clear on the investigator global assessment. With concomitant corticosteroid therapy, 100% of patients achieved EASI-50.2 Also notable, 2017 ushered in the appearance of a novel iteration of the 30-year-old concept of phosphodiesterase inhibition with the approval of the topical agent crisaborole for AD treatment in patients 2 years and older, which has been shown to be effective in both children and adults.3,4 However, despite these leaps of advancement in the care of AD, by no means has the condition been cured.
Atopic dermatitis has remained an incurable disease due to many factors: (1) variable immunologic and environmental triggers and patient disease course; (2) intolerance to therapeutic agents, including an enhanced sense of stinging and/or reactivity; (3) poor access to novel therapies among underserved patient populations; (4) lack of available data and information on variable treatment response by ethnicity and race; and (5) the absence of biologic treatments for severe childhood AD to modify long-term recurrence and progression of atopy, which is probably the most important issue, as the majority of AD cases start in children 5 years and younger.
Instituting a treatment today to provide children with disease-free skin for a lifetime truly is the Holy Grail in pediatric dermatology. To aid in the progress toward this goal, a deeper understanding of the manifestation of pediatric versus adult AD is now being investigated. It is clear that with adult chronicity, type 1 helper T cell (TH1) axis activity and prolonged defects are triggered in barrier maturation; however, recent data have started to demonstrate that the youngest patients have different issues in lipid maturation and lack TH1 activation. In particular, fatty acyl-CoA reductase 2 and fatty acid 2-hydroxylase is preferentially downregulated in children.5 It appears that the young immune system may be ripe for immune modification, which previously has been demonstrated with wild-type viral infections of varicella in children.6 However, future research will focus on what kind of tweaks to the immune system are required.
To encapsulate the AD pipeline, we will review drug trials that are in active recruitment as well as recently published data, which constitute an exciting group full of modifications of current therapies and agents with novel mechanisms of action.
Therapies targeting new mechanisms of action include Janus kinase (JAK) inhibitors, which have shown promising results for alopecia areata and vitiligo vulgaris. These agents may create selective modification of the immune system and are being tested topically and orally (Clinicaltrials.gov identifier NCT03011892).
Another mechanism that currently is being studied includes a topical IL-4 and IL-13 inhibitor, which would hopefully mimic the efficacy of dupilumab, antioxidant therapies, and antimicrobials (NCT03351777, NCT03381625, NCT02910011).
Data on the outcome of a phase 3 trial of dupilumab in adolescents has been released but not yet published by the manufacturer and shows promising results in children aged 12 to 17 years, both in reduction of EASI score and in achieving clear or almost clear skin.11 Interestingly, limited data available from a press release reported similar results with dupilumab injection every 2 weeks versus every 4 weeks, which may give alternative dosing regimens in this age group once approved11; however, publication has yet to occur for the latter data.
Other mechanistic agents include blockade of cytokines and interleukins, particularly those involved in type 2 helper T cell (TH2) activity, such as thymic stromal lymphopoietin (a cytokine), as well as targeted single inhibition of IL-4, IL-5, IL-13, and IL-31 and/or their receptors. Nemolizumab, an anti–IL-31 receptor A antibody, is showing promise in the control of AD-associated itch and reduction in EASI
The future of AD therapy is anyone’s guess. Having entered the biologic era with dupilumab, we have a high bar set for efficacy and safety of AD therapies, yet there remains a core group of AD patients who have not yet achieved clearance or refuse injectables; therefore, adjunctive or alternative therapeutics are still needed. Furthermore, we still have not identified who will best benefit long-term from systemic intervention and how to best effect long-term disease control with biologics or novel agents, and choosing the therapy based on patient disease characteristics or serotyping has not yet come of age. It is exciting to think about what next year will bring!
- Xu X, Zheng Y, Zhang X, et al. Efficacy and safety of dupilumab for the treatment of moderate-to-severe atopic dermatitis in adults. Oncotarget. 2017;8:108480-108491.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Murrell D, Gebauer K, Spelman L, et al. Crisaborole topical ointment, 2% in adults with atopic dermatitis: a phase 2a, vehicle-controlled, proof-of-concept study. J Drugs Dermatol. 2015;14:1108-1112.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Brunner PM, Israel A, Zhang N, et al. Early-onset pediatric atopic dermatitis is characterized by TH2/TH17/TH22-centered inflammation and lipid alterations. J Allergy Clin Immunol. 2018;141:2094-2106.
- Silverberg JI, Kleiman E, Silverberg NB, et al. Chickenpox in childhood is associated with decreased atopic disorders, IgE, allergic sensitization, and leukocyte subsets. Pediatr Allergy Immunol. 2012;23:50-58.
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? Allergy Clin Immunol. 2017;140:633-643.
- Renert-Yuval Y, Guttman-Yassky E. Systemic therapies in atopic dermatitis: the pipeline. Clin Dermatol. 2017;35:387-397.
- Bissonnette R, Papp KA, Poulin Y, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol. 2016;175:902-911.
- Guttman-Yassky E, Silverberg JI, Nemoto O, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study [published online February 1, 2018]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2018.01.018.
- Dupixent (dupilumab) showed positive phase 3 results in adolescents with inadequately controlled moderate-to-severe atopic dermatitis [press release]. Tarrytown, NY: Sanofi; May 16, 2018. https://www.prnewswire.com/news-releases/dupixent-dupilumab-showed-positive-phase-3-results-in-adolescents-with-inadequately-controlled-moderate-to-severe-atopic-dermatitis-300649146.html. Accessed July 11, 2018.
- Ruzicka T, Hanifin JM, Furue M, et al. Anti–interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med. 2017;376:826-835.
Just when you might have thought dermatologic therapies were peaking, along came another banner year in atopic dermatitis (AD). Last year we saw the landmark launch of dupilumab, the first US Food and Drug Administration (FDA)–approved biologic therapy for AD. Dupilumab addresses a novel mechanism of AD in adults by blocking IL-4 and IL-13, which both play a central role in the type 2 helper T cell (TH2) axis on the dual development of barrier-impaired skin and aberrant immune response including IgE to cutaneous aggravating agents with resultant inflammation. Additional information has shown direct effects to reduce itch in AD.1 A 12-week study of dupilumab monotherapy showed that 85% (47/55) of treated patients had at least a 50% reduction in Eczema Area and Severity Index (EASI) score and 40% (22/55) were clear or almost clear on the investigator global assessment. With concomitant corticosteroid therapy, 100% of patients achieved EASI-50.2 Also notable, 2017 ushered in the appearance of a novel iteration of the 30-year-old concept of phosphodiesterase inhibition with the approval of the topical agent crisaborole for AD treatment in patients 2 years and older, which has been shown to be effective in both children and adults.3,4 However, despite these leaps of advancement in the care of AD, by no means has the condition been cured.
Atopic dermatitis has remained an incurable disease due to many factors: (1) variable immunologic and environmental triggers and patient disease course; (2) intolerance to therapeutic agents, including an enhanced sense of stinging and/or reactivity; (3) poor access to novel therapies among underserved patient populations; (4) lack of available data and information on variable treatment response by ethnicity and race; and (5) the absence of biologic treatments for severe childhood AD to modify long-term recurrence and progression of atopy, which is probably the most important issue, as the majority of AD cases start in children 5 years and younger.
Instituting a treatment today to provide children with disease-free skin for a lifetime truly is the Holy Grail in pediatric dermatology. To aid in the progress toward this goal, a deeper understanding of the manifestation of pediatric versus adult AD is now being investigated. It is clear that with adult chronicity, type 1 helper T cell (TH1) axis activity and prolonged defects are triggered in barrier maturation; however, recent data have started to demonstrate that the youngest patients have different issues in lipid maturation and lack TH1 activation. In particular, fatty acyl-CoA reductase 2 and fatty acid 2-hydroxylase is preferentially downregulated in children.5 It appears that the young immune system may be ripe for immune modification, which previously has been demonstrated with wild-type viral infections of varicella in children.6 However, future research will focus on what kind of tweaks to the immune system are required.
To encapsulate the AD pipeline, we will review drug trials that are in active recruitment as well as recently published data, which constitute an exciting group full of modifications of current therapies and agents with novel mechanisms of action.
Therapies targeting new mechanisms of action include Janus kinase (JAK) inhibitors, which have shown promising results for alopecia areata and vitiligo vulgaris. These agents may create selective modification of the immune system and are being tested topically and orally (Clinicaltrials.gov identifier NCT03011892).
Another mechanism that currently is being studied includes a topical IL-4 and IL-13 inhibitor, which would hopefully mimic the efficacy of dupilumab, antioxidant therapies, and antimicrobials (NCT03351777, NCT03381625, NCT02910011).
Data on the outcome of a phase 3 trial of dupilumab in adolescents has been released but not yet published by the manufacturer and shows promising results in children aged 12 to 17 years, both in reduction of EASI score and in achieving clear or almost clear skin.11 Interestingly, limited data available from a press release reported similar results with dupilumab injection every 2 weeks versus every 4 weeks, which may give alternative dosing regimens in this age group once approved11; however, publication has yet to occur for the latter data.
Other mechanistic agents include blockade of cytokines and interleukins, particularly those involved in type 2 helper T cell (TH2) activity, such as thymic stromal lymphopoietin (a cytokine), as well as targeted single inhibition of IL-4, IL-5, IL-13, and IL-31 and/or their receptors. Nemolizumab, an anti–IL-31 receptor A antibody, is showing promise in the control of AD-associated itch and reduction in EASI
The future of AD therapy is anyone’s guess. Having entered the biologic era with dupilumab, we have a high bar set for efficacy and safety of AD therapies, yet there remains a core group of AD patients who have not yet achieved clearance or refuse injectables; therefore, adjunctive or alternative therapeutics are still needed. Furthermore, we still have not identified who will best benefit long-term from systemic intervention and how to best effect long-term disease control with biologics or novel agents, and choosing the therapy based on patient disease characteristics or serotyping has not yet come of age. It is exciting to think about what next year will bring!
Just when you might have thought dermatologic therapies were peaking, along came another banner year in atopic dermatitis (AD). Last year we saw the landmark launch of dupilumab, the first US Food and Drug Administration (FDA)–approved biologic therapy for AD. Dupilumab addresses a novel mechanism of AD in adults by blocking IL-4 and IL-13, which both play a central role in the type 2 helper T cell (TH2) axis on the dual development of barrier-impaired skin and aberrant immune response including IgE to cutaneous aggravating agents with resultant inflammation. Additional information has shown direct effects to reduce itch in AD.1 A 12-week study of dupilumab monotherapy showed that 85% (47/55) of treated patients had at least a 50% reduction in Eczema Area and Severity Index (EASI) score and 40% (22/55) were clear or almost clear on the investigator global assessment. With concomitant corticosteroid therapy, 100% of patients achieved EASI-50.2 Also notable, 2017 ushered in the appearance of a novel iteration of the 30-year-old concept of phosphodiesterase inhibition with the approval of the topical agent crisaborole for AD treatment in patients 2 years and older, which has been shown to be effective in both children and adults.3,4 However, despite these leaps of advancement in the care of AD, by no means has the condition been cured.
Atopic dermatitis has remained an incurable disease due to many factors: (1) variable immunologic and environmental triggers and patient disease course; (2) intolerance to therapeutic agents, including an enhanced sense of stinging and/or reactivity; (3) poor access to novel therapies among underserved patient populations; (4) lack of available data and information on variable treatment response by ethnicity and race; and (5) the absence of biologic treatments for severe childhood AD to modify long-term recurrence and progression of atopy, which is probably the most important issue, as the majority of AD cases start in children 5 years and younger.
Instituting a treatment today to provide children with disease-free skin for a lifetime truly is the Holy Grail in pediatric dermatology. To aid in the progress toward this goal, a deeper understanding of the manifestation of pediatric versus adult AD is now being investigated. It is clear that with adult chronicity, type 1 helper T cell (TH1) axis activity and prolonged defects are triggered in barrier maturation; however, recent data have started to demonstrate that the youngest patients have different issues in lipid maturation and lack TH1 activation. In particular, fatty acyl-CoA reductase 2 and fatty acid 2-hydroxylase is preferentially downregulated in children.5 It appears that the young immune system may be ripe for immune modification, which previously has been demonstrated with wild-type viral infections of varicella in children.6 However, future research will focus on what kind of tweaks to the immune system are required.
To encapsulate the AD pipeline, we will review drug trials that are in active recruitment as well as recently published data, which constitute an exciting group full of modifications of current therapies and agents with novel mechanisms of action.
Therapies targeting new mechanisms of action include Janus kinase (JAK) inhibitors, which have shown promising results for alopecia areata and vitiligo vulgaris. These agents may create selective modification of the immune system and are being tested topically and orally (Clinicaltrials.gov identifier NCT03011892).
Another mechanism that currently is being studied includes a topical IL-4 and IL-13 inhibitor, which would hopefully mimic the efficacy of dupilumab, antioxidant therapies, and antimicrobials (NCT03351777, NCT03381625, NCT02910011).
Data on the outcome of a phase 3 trial of dupilumab in adolescents has been released but not yet published by the manufacturer and shows promising results in children aged 12 to 17 years, both in reduction of EASI score and in achieving clear or almost clear skin.11 Interestingly, limited data available from a press release reported similar results with dupilumab injection every 2 weeks versus every 4 weeks, which may give alternative dosing regimens in this age group once approved11; however, publication has yet to occur for the latter data.
Other mechanistic agents include blockade of cytokines and interleukins, particularly those involved in type 2 helper T cell (TH2) activity, such as thymic stromal lymphopoietin (a cytokine), as well as targeted single inhibition of IL-4, IL-5, IL-13, and IL-31 and/or their receptors. Nemolizumab, an anti–IL-31 receptor A antibody, is showing promise in the control of AD-associated itch and reduction in EASI
The future of AD therapy is anyone’s guess. Having entered the biologic era with dupilumab, we have a high bar set for efficacy and safety of AD therapies, yet there remains a core group of AD patients who have not yet achieved clearance or refuse injectables; therefore, adjunctive or alternative therapeutics are still needed. Furthermore, we still have not identified who will best benefit long-term from systemic intervention and how to best effect long-term disease control with biologics or novel agents, and choosing the therapy based on patient disease characteristics or serotyping has not yet come of age. It is exciting to think about what next year will bring!
- Xu X, Zheng Y, Zhang X, et al. Efficacy and safety of dupilumab for the treatment of moderate-to-severe atopic dermatitis in adults. Oncotarget. 2017;8:108480-108491.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Murrell D, Gebauer K, Spelman L, et al. Crisaborole topical ointment, 2% in adults with atopic dermatitis: a phase 2a, vehicle-controlled, proof-of-concept study. J Drugs Dermatol. 2015;14:1108-1112.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Brunner PM, Israel A, Zhang N, et al. Early-onset pediatric atopic dermatitis is characterized by TH2/TH17/TH22-centered inflammation and lipid alterations. J Allergy Clin Immunol. 2018;141:2094-2106.
- Silverberg JI, Kleiman E, Silverberg NB, et al. Chickenpox in childhood is associated with decreased atopic disorders, IgE, allergic sensitization, and leukocyte subsets. Pediatr Allergy Immunol. 2012;23:50-58.
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? Allergy Clin Immunol. 2017;140:633-643.
- Renert-Yuval Y, Guttman-Yassky E. Systemic therapies in atopic dermatitis: the pipeline. Clin Dermatol. 2017;35:387-397.
- Bissonnette R, Papp KA, Poulin Y, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol. 2016;175:902-911.
- Guttman-Yassky E, Silverberg JI, Nemoto O, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study [published online February 1, 2018]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2018.01.018.
- Dupixent (dupilumab) showed positive phase 3 results in adolescents with inadequately controlled moderate-to-severe atopic dermatitis [press release]. Tarrytown, NY: Sanofi; May 16, 2018. https://www.prnewswire.com/news-releases/dupixent-dupilumab-showed-positive-phase-3-results-in-adolescents-with-inadequately-controlled-moderate-to-severe-atopic-dermatitis-300649146.html. Accessed July 11, 2018.
- Ruzicka T, Hanifin JM, Furue M, et al. Anti–interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med. 2017;376:826-835.
- Xu X, Zheng Y, Zhang X, et al. Efficacy and safety of dupilumab for the treatment of moderate-to-severe atopic dermatitis in adults. Oncotarget. 2017;8:108480-108491.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Murrell D, Gebauer K, Spelman L, et al. Crisaborole topical ointment, 2% in adults with atopic dermatitis: a phase 2a, vehicle-controlled, proof-of-concept study. J Drugs Dermatol. 2015;14:1108-1112.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Brunner PM, Israel A, Zhang N, et al. Early-onset pediatric atopic dermatitis is characterized by TH2/TH17/TH22-centered inflammation and lipid alterations. J Allergy Clin Immunol. 2018;141:2094-2106.
- Silverberg JI, Kleiman E, Silverberg NB, et al. Chickenpox in childhood is associated with decreased atopic disorders, IgE, allergic sensitization, and leukocyte subsets. Pediatr Allergy Immunol. 2012;23:50-58.
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? Allergy Clin Immunol. 2017;140:633-643.
- Renert-Yuval Y, Guttman-Yassky E. Systemic therapies in atopic dermatitis: the pipeline. Clin Dermatol. 2017;35:387-397.
- Bissonnette R, Papp KA, Poulin Y, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol. 2016;175:902-911.
- Guttman-Yassky E, Silverberg JI, Nemoto O, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study [published online February 1, 2018]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2018.01.018.
- Dupixent (dupilumab) showed positive phase 3 results in adolescents with inadequately controlled moderate-to-severe atopic dermatitis [press release]. Tarrytown, NY: Sanofi; May 16, 2018. https://www.prnewswire.com/news-releases/dupixent-dupilumab-showed-positive-phase-3-results-in-adolescents-with-inadequately-controlled-moderate-to-severe-atopic-dermatitis-300649146.html. Accessed July 11, 2018.
- Ruzicka T, Hanifin JM, Furue M, et al. Anti–interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med. 2017;376:826-835.
Pediatric Periorificial Dermatitis
Perioral dermatitis is an acneform eruption presenting with erythematous papules, vesicles, and rarely pustules clustered around the orifices of the face. 1 Lesions may be found near the eyes, mouth, and nose but typically spare the vermilion border of the lips. 2 Nguyen and Eichenfield 3 preferred the term periorificial dermatitis (POD), which has since been adopted by others. 4 Patients may report pruritus, but there generally are no systemic symptoms unless patients have comorbid conditions such as atopic dermatitis. 5 Although this condition has been well examined in the literature on adults, data in the pediatric population are far more limited, consisting of case series and retrospective chart reviews. In 1979, Wilkinson et al 6 published a study of more than 200 patients with perioral dermatitis, but only 15 patients younger than 12 years were included.
Etiology
Although the exact pathogenesis of POD is unknown, a common denominator among many patients is prior exposure to topical corticosteroids.3,7-9 Periorificial dermatitis also has been linked to the use of systemic corticosteroids in pediatric patients.10 The exact relationship between steroid use and dermatitis is unknown; it may be related to a change in the flora of hair follicles and in particular an association with fusiform bacteria–rich conditions.11 Aside from steroid exposure, POD has been associated with the use of physical sunscreen in pediatric patients with dry skin,12 rosin in chewing gum,13 and inhaled corticosteroids in those with asthma.14 In one case, a 15-year-old adolescent girl developed POD and swelling of the lips after 2 years of playing a flute made of cocus wood.15,16
Epidemiology
Comorbidities and Family History
Goel et al17 (N=222) reported the following comorbidities associated with pediatric POD: atopic dermatitis (29.3%), asthma (14.9%), and allergies (9.9%). Steroid exposure was noted in 58.1% of patients.17 Similarly, Nguyen and Eichenfield3 (N=79) found that the most common comorbidities were atopic dermatitis (14%), keratosis pilaris (14%), viral infections (14%), acne (10%), and seborrheic dermatitis (10%). Family history of atopy was noted in 55% of patients and family history of rosacea was noted in 3%. In a case series of 11 pediatric patients, 3 (27%) had keratosis pilaris, 7 (64%) had a family history of atopy, and 2 (18%) had a family history of rosacea.8 Weston and Morelli9 found a much higher incidence of familial rosacea (20%) in 106 children with steroid rosacea.
Clinical Presentation
Periorificial dermatitis generally presents with small, pink- to flesh-colored papules in a perioral, periocular, and perinasal distribution. Although many patients are white, a particularly prominent variant has been noted in black children with papules that may be hyperpigmented.18 In a 2006 chart review in 79 pediatric POD patients aged 6 months to 18 years, Nguyen and Eichenfield3 reported that 92% (73/79) of patients presented for a facial rash with an average duration ranging from 2 weeks to 4 years.
Boeck et al19 described 7 pediatric patients with perioral dermatitis. Six (86%) patients had perioral lesions, and 6 (86%) had previously been treated with moderate- to high-potency topical corticosteroids. Skin prick tests were negative in 6 (86%) patients.19 In one case report, a 6-year-old boy did not present with the classic acneform lesions but rather sharply demarcated eczematous patches around the eyes, nose, and mouth. The rash began to fade after 2 weeks of using metronidazole gel 1%, and after 4 months he was only left with mild hyperpigmentation.4
Periorificial dermatitis was once thought to be a juvenile form of rosacea.5 In 1972, Savin et al8 described 11 pediatric patients with “rosacea-like” facial flushing, papules, pustules, and scaling over the cheeks, forehead, and chin. In some patients, the eyelids also were involved. At least 8 patients had been using potent topical corticosteroids and had noticed exacerbation of their skin lesions after stopping therapy.8
Variants of POD
Several other variants of POD have been described in pediatric patients including childhood granulomatous periorificial dermatitis (CGPD)(also known as facial Afro-Caribbean [childhood] eruption) and lupus miliaris disseminatus faciei. Childhood granulomatous periorificial dermatitis presents in prepubertal children as dome-shaped, red to yellow-brown, monomorphous papules around the eyes, nose, and mouth; there are no systemic findings.20,21 It occurs equally in males and females and is more commonly seen in dark-skinned patients. Childhood granulomatous periorificial dermatitis usually resolves within a few months to years but may be associated with blepharitis or conjunctivitis.20 Urbatsch et al20 analyzed extrafacial lesions in 8 patients (aged 2–12 years) with CGPD. Lesions were found on the trunk (38% [3/8]), neck (25% [2/8]), ears (25% [2/8]), extremities (50% [4/8]), labia majora (38% [3/8]), and abdomen (13% [1/8]). In addition, 2 (25% [2/8]) patients had blepharitis.20
Lupus miliaris disseminatus faciei, which occurs in adolescents and adults, commonly involves the eyelids and central areas of the face such as the nose and upper lips. Patients typically present with erythematous or flesh-colored papules.1
Diagnosis
Diagnosis of POD is made clinically based on the observation of papules (and sometimes pustules) around the orifices of the face, sparing the vermilion border, together with a lack of comedones.17 Laboratory tests are not useful.5 Biopsies rarely are performed, and the results mimic those of rosacea, demonstrating a perifollicular lymphohistiocytic infiltrate, epithelioid cells, and occasionally giant cells.5,22,23 Early papular lesions can show mild acanthosis, epidermal edema, and parakeratosis.23 Biopsies in patients with CGPD reveal noncaseating perifollicular granulomas.20
Treatment and Clinical Outcome
Although topical corticosteroids can improve facial lesions in pediatric POD, the eruption often rebounds when therapy is discontinued.1 One therapy frequently used in adults is oral tetracyclines; however, these agents must not be used in patients younger than 9 years due to potential dental staining.4 The standards are either topical metronidazole twice daily with clearance in 3 to 8 weeks or oral erythromycin.7
In the review conducted by Goel et al,17 treatment included azithromycin (44.6%), topical metronidazole (42.3%), sodium sulfacetamide lotion (35.6%), oral antibiotic monotherapy (15.3%), topical agent monotherapy (44.6%), and combined oral and topical agent therapy (40.1%). Of those patients who presented for a follow-up visit (59%), 72% of cases resolved and 10.7% showed some improvement. For those patients who returned for follow-up, the average duration until symptom resolution was approximately 4 months. The most common side effects were pigmentation changes (1.8%), worsening of symptoms (1.8%), gastrointestinal upset (0.9%), irritant dermatitis (0.9%), and xerosis (0.5%).17
Changes were made to the treatment plans for 16 patients, most often due to inadequate treatment response.17 Five patients treated with sodium sulfacetamide lotion also were started on oral azithromycin. Four patients treated with oral antibiotics were given a topical agent (metronidazole or sodium sulfacetamide lotion). Other modifications included replacing sodium sulfacetamide lotion with topical metronidazole and an oral antibiotic (azithromycin or doxycycline, n=3), adjusting the doses of oral or topical medications (n=2), adding tacrolimus (n=1), and replacing topical metronidazole with sodium sulfacetamide lotion (n=1). Of the patients who underwent a change in treatment plan, 5 experienced symptom recurrence, 4 had mild improvement, and 1 patient had no improvement. Six patients were lost to follow-up.17
In the study conducted by Nguyen and Eichenfield,3 follow-up visits occurred approximately 3 months after the first visit.
In the case series by Boeck et al,19 all patients were started on metronidazole gel 1% applied once daily for the first week, and then twice daily until the lesions resolved. All patients showed improvement after 4 to 6 weeks, and eventually the disease cleared between 3 and 6 months. All patients were still symptom free during a 2-year observation period.19
Manders and Lucky7 described 14 patients with POD (aged 9 months to 6.5 years). Eight patients used only metronidazole gel 0.75%, while 5 used the gel in combination with topical corticosteroids (21% [3/14]), oral erythromycin (7% [1/14]), or topical erythromycin (7% [1/14]); 1 patient remained on hydrocortisone 1% and cleared. Patients responded well within 1 to 8 weeks and were symptom free for up to 16 months. Mid- to high-potency steroids were discontinued in all patients.7
In some pediatric patients with CGPD, recovery occurs faster with the use of oral macrolides or tetracyclines, either alone or in combination with topical antibiotics or sulfur-based lotions.20 Extrafacial lesions associated with CGPD do not appear to negatively impact treatment response or duration of disease. In the review conducted by Urbatsch et al,20 7 of 8 (88%) CGPD patients with extrafacial lesions were treated with oral agents including erythromycin, hydroxychloroquine, cyclosporine, minocycline, and azithromycin. Most of these patients also were using topical agents such as triamcinolone acetonide, desonide, metronidazole, and erythromycin. The time to resolution ranged from several weeks to 6 months.20
Weston and Morelli9 described a treatment regimen for steroid rosacea. The study included data on 106 children (60 females, 46 males) who had been exposed to mostly class 7 low-potency agents. All patients were advised to immediately stop topical steroid therapy without gradual withdrawal and to begin oral erythromycin stearate 30 mg/kg daily in 2 doses per day for 4 weeks. Patients who were unable to tolerate erythromycin were advised to use topical clindamycin phosphate twice daily for 4 weeks (n=6). Eighty-six percent of patients showed resolution within 4 weeks, and 100% showed clearance by 8 weeks. Twenty-two percent of patients had clearance within 3 weeks. There was no difference in the duration until resolution for those who had used oral or topical antibiotics.9 A different study suggested that low-potency topical steroids can be used to control inflammation when weaning patients off of strong steroids.5
Differential Diagnosis
The differential diagnosis should include acne vulgaris, allergic contact dermatitis, irritant contact dermatitis, seborrheic dermatitis, impetigo, dermatophyte infection, rosacea, and angiofibromas.4
Acne vulgaris commonly is found in older adolescents, and unlike POD, it will present with open or closed comedones.2 In patients aged 1 to 7 years, acne is a reason to consider endocrine evaluation. Allergic contact dermatitis is extremely pruritic, and the lesions often are papulovesicular with active weeping or crusting. Patients with irritant contact dermatitis often report burning and pain, and papules and pustules typically are absent. A thorough history can help rule out allergic or irritant contact dermatitis. Seborrheic dermatitis presents with erythema and scaling of the scalp, eyebrows, and nasolabial folds; it tends to spare the perioral regions and also lacks papules.2 The lesions of impetigo typically have a yellow-brown exudate, which forms a honey-colored crust.24 Tinea faciei, unlike the other tinea infections, can have an extremely variable presentation. Lesions usually begin as scaly macules that develop raised borders with central hypopigmentation, but papules, vesicles, and crusts can be seen.25 Potassium hydroxide
Conclusion
Diagnosis of POD is clinical and rests upon the finding of erythematous papules on the face near the eyes, mouth, and nose. Extrafacial lesions also have been described, particularly in pediatric patients with CGPD. Many patients will report a history of atopic dermatitis and asthma. Therapy for POD includes both topical and systemic agents. For those with mild disease, topical metronidazole commonly is used. For patients requiring oral antibiotics, tetracyclines or macrolides can be prescribed based on the age of the patient. Many pediatric patients who begin with both oral and topical agents can later be maintained on topical therapy, sometimes with a low-dose oral antibiotic. Periorificial dermatitis has an excellent prognosis and most pediatric patients show marked improvement within weeks to months.
- Tempark T, Shwayder TA. Perioral dermatitis: a review of the condition with special attention to treatment options. Am J Clin Dermatol. 2014;15:101-113.
- McFarland SL, Polcari IC. Morphology-based diagnosis of acneiform eruptions. Pediatr Ann. 2015;44:E188-E193.
- Nguyen V, Eichenfield LF. Periorificial dermatitis in children and adolescents. J Am Acad Dermatol. 2006;55:781-785.
- Kihiczak GG, Cruz MA, Schwartz RA. Periorificial dermatitis in children: an update and description of a child with striking features. Int J Dermatol. 2009;48:304-306.
- Laude TA, Salvemini JN. Perioral dermatitis in children. Sem Cutan Med Surg. 1999;18:206-209.
- Wilkinson DS, Kirton V, Wilkinson JD. Perioral dermatitis: a 12-year review. Br J Dermatol. 1979;101:245-257.
- Manders SM, Lucky AW. Perioral dermatitis in childhood. J Am Acad Dermatol. 1992;27(5 pt 1):688-692.
- Savin JA, Alexander S, Marks R. A rosacea-like eruption of children. Br J Dermatol. 1972;87:425-429.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Clementson B, Smidt AC. Periorificial dermatitis due to systemic corticosteroids in children: report of two cases. Pediatr Dermatol. 2012;29:331-332.
- Takiwaki H, Tsuda H, Arase S, et al. Differences between intrafollicular microorganism profiles in perioral and seborrhoeic dermatitis. Clin Exp Dermatol. 2003;28:531-534.
- Abeck D, Geisenfelder B, Brandt O. Physical sunscreens with high sun protection factor may cause perioral dermatitis in children. J Dtsch Dermatol Ges. 2009;7:701-703.
- Satyawan I, Oranje AP, van Joost T. Perioral dermatitis in a child due to rosin in chewing gum. Contact Dermatitis. 1990;22:182-183.
- Dubus JC, Marguet C, Deschildre A, et al. Local side-effects of inhaled corticosteroids in asthmatic children: influence of drug, dose, age, and device. Allergy. 2001;56:944-948.
- Hausen BM, Bruhn G, Koenig WA. New hydroxyisoflavans as contact sensitizers in cocus wood Brya ebenus DC (Fabaceae). Contact Dermatitis. 1991;25:149-155.
- Dirschka T, Weber K, Tronnier H. Topical cosmetics and perioral dermatitis. J Dtsch Dermatol Ges. 2004;2:194-199.
- Goel NS, Burkhart CN, Morrell DS. Pediatric periorificial dermatitis: clinical course and treatment outcomes in 222 patients. Pediatr Dermatol. 2015;32:333-336.
- Cribier B, Lieber-Mbomeyo A, Lipsker D. Clinical and histological study of a case of facial Afro-Caribbean childhood eruption (FACE) [in French][published online July 23, 2008]. Ann Dermatol Venerol. 2008;135:663-667.
- Boeck K, Abeck D, Werfel S, et al. Perioral dermatitis in children—clinical presentation, pathogenesis-related factors and response to topical metronidazole. Dermatology. 1997;195:235-238.
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Ramelet AA, Delacrétaz J. Histopathologic study of perioral dermatitis [in French]. Dermatologica. 1981;163:361-369.
- Ljubojevi´c S, Lipozenci´c J, Turci´c P. Perioral dermatitis. Acta Dermatovenerol Croat. 2008;16:96-100.
- Nichols RL, Florman S. Clinical presentations of soft-tissue infections and surgical site infections. Clin Infect Dis. 2001;33(suppl 2):S84-S93.
- Lin RL, Szepietowski JC, Schwartz RA. Tinea faciei, an often deceptive facial eruption. Int J Dermatol. 2004;43:437-440.
Perioral dermatitis is an acneform eruption presenting with erythematous papules, vesicles, and rarely pustules clustered around the orifices of the face. 1 Lesions may be found near the eyes, mouth, and nose but typically spare the vermilion border of the lips. 2 Nguyen and Eichenfield 3 preferred the term periorificial dermatitis (POD), which has since been adopted by others. 4 Patients may report pruritus, but there generally are no systemic symptoms unless patients have comorbid conditions such as atopic dermatitis. 5 Although this condition has been well examined in the literature on adults, data in the pediatric population are far more limited, consisting of case series and retrospective chart reviews. In 1979, Wilkinson et al 6 published a study of more than 200 patients with perioral dermatitis, but only 15 patients younger than 12 years were included.
Etiology
Although the exact pathogenesis of POD is unknown, a common denominator among many patients is prior exposure to topical corticosteroids.3,7-9 Periorificial dermatitis also has been linked to the use of systemic corticosteroids in pediatric patients.10 The exact relationship between steroid use and dermatitis is unknown; it may be related to a change in the flora of hair follicles and in particular an association with fusiform bacteria–rich conditions.11 Aside from steroid exposure, POD has been associated with the use of physical sunscreen in pediatric patients with dry skin,12 rosin in chewing gum,13 and inhaled corticosteroids in those with asthma.14 In one case, a 15-year-old adolescent girl developed POD and swelling of the lips after 2 years of playing a flute made of cocus wood.15,16
Epidemiology
Comorbidities and Family History
Goel et al17 (N=222) reported the following comorbidities associated with pediatric POD: atopic dermatitis (29.3%), asthma (14.9%), and allergies (9.9%). Steroid exposure was noted in 58.1% of patients.17 Similarly, Nguyen and Eichenfield3 (N=79) found that the most common comorbidities were atopic dermatitis (14%), keratosis pilaris (14%), viral infections (14%), acne (10%), and seborrheic dermatitis (10%). Family history of atopy was noted in 55% of patients and family history of rosacea was noted in 3%. In a case series of 11 pediatric patients, 3 (27%) had keratosis pilaris, 7 (64%) had a family history of atopy, and 2 (18%) had a family history of rosacea.8 Weston and Morelli9 found a much higher incidence of familial rosacea (20%) in 106 children with steroid rosacea.
Clinical Presentation
Periorificial dermatitis generally presents with small, pink- to flesh-colored papules in a perioral, periocular, and perinasal distribution. Although many patients are white, a particularly prominent variant has been noted in black children with papules that may be hyperpigmented.18 In a 2006 chart review in 79 pediatric POD patients aged 6 months to 18 years, Nguyen and Eichenfield3 reported that 92% (73/79) of patients presented for a facial rash with an average duration ranging from 2 weeks to 4 years.
Boeck et al19 described 7 pediatric patients with perioral dermatitis. Six (86%) patients had perioral lesions, and 6 (86%) had previously been treated with moderate- to high-potency topical corticosteroids. Skin prick tests were negative in 6 (86%) patients.19 In one case report, a 6-year-old boy did not present with the classic acneform lesions but rather sharply demarcated eczematous patches around the eyes, nose, and mouth. The rash began to fade after 2 weeks of using metronidazole gel 1%, and after 4 months he was only left with mild hyperpigmentation.4
Periorificial dermatitis was once thought to be a juvenile form of rosacea.5 In 1972, Savin et al8 described 11 pediatric patients with “rosacea-like” facial flushing, papules, pustules, and scaling over the cheeks, forehead, and chin. In some patients, the eyelids also were involved. At least 8 patients had been using potent topical corticosteroids and had noticed exacerbation of their skin lesions after stopping therapy.8
Variants of POD
Several other variants of POD have been described in pediatric patients including childhood granulomatous periorificial dermatitis (CGPD)(also known as facial Afro-Caribbean [childhood] eruption) and lupus miliaris disseminatus faciei. Childhood granulomatous periorificial dermatitis presents in prepubertal children as dome-shaped, red to yellow-brown, monomorphous papules around the eyes, nose, and mouth; there are no systemic findings.20,21 It occurs equally in males and females and is more commonly seen in dark-skinned patients. Childhood granulomatous periorificial dermatitis usually resolves within a few months to years but may be associated with blepharitis or conjunctivitis.20 Urbatsch et al20 analyzed extrafacial lesions in 8 patients (aged 2–12 years) with CGPD. Lesions were found on the trunk (38% [3/8]), neck (25% [2/8]), ears (25% [2/8]), extremities (50% [4/8]), labia majora (38% [3/8]), and abdomen (13% [1/8]). In addition, 2 (25% [2/8]) patients had blepharitis.20
Lupus miliaris disseminatus faciei, which occurs in adolescents and adults, commonly involves the eyelids and central areas of the face such as the nose and upper lips. Patients typically present with erythematous or flesh-colored papules.1
Diagnosis
Diagnosis of POD is made clinically based on the observation of papules (and sometimes pustules) around the orifices of the face, sparing the vermilion border, together with a lack of comedones.17 Laboratory tests are not useful.5 Biopsies rarely are performed, and the results mimic those of rosacea, demonstrating a perifollicular lymphohistiocytic infiltrate, epithelioid cells, and occasionally giant cells.5,22,23 Early papular lesions can show mild acanthosis, epidermal edema, and parakeratosis.23 Biopsies in patients with CGPD reveal noncaseating perifollicular granulomas.20
Treatment and Clinical Outcome
Although topical corticosteroids can improve facial lesions in pediatric POD, the eruption often rebounds when therapy is discontinued.1 One therapy frequently used in adults is oral tetracyclines; however, these agents must not be used in patients younger than 9 years due to potential dental staining.4 The standards are either topical metronidazole twice daily with clearance in 3 to 8 weeks or oral erythromycin.7
In the review conducted by Goel et al,17 treatment included azithromycin (44.6%), topical metronidazole (42.3%), sodium sulfacetamide lotion (35.6%), oral antibiotic monotherapy (15.3%), topical agent monotherapy (44.6%), and combined oral and topical agent therapy (40.1%). Of those patients who presented for a follow-up visit (59%), 72% of cases resolved and 10.7% showed some improvement. For those patients who returned for follow-up, the average duration until symptom resolution was approximately 4 months. The most common side effects were pigmentation changes (1.8%), worsening of symptoms (1.8%), gastrointestinal upset (0.9%), irritant dermatitis (0.9%), and xerosis (0.5%).17
Changes were made to the treatment plans for 16 patients, most often due to inadequate treatment response.17 Five patients treated with sodium sulfacetamide lotion also were started on oral azithromycin. Four patients treated with oral antibiotics were given a topical agent (metronidazole or sodium sulfacetamide lotion). Other modifications included replacing sodium sulfacetamide lotion with topical metronidazole and an oral antibiotic (azithromycin or doxycycline, n=3), adjusting the doses of oral or topical medications (n=2), adding tacrolimus (n=1), and replacing topical metronidazole with sodium sulfacetamide lotion (n=1). Of the patients who underwent a change in treatment plan, 5 experienced symptom recurrence, 4 had mild improvement, and 1 patient had no improvement. Six patients were lost to follow-up.17
In the study conducted by Nguyen and Eichenfield,3 follow-up visits occurred approximately 3 months after the first visit.
In the case series by Boeck et al,19 all patients were started on metronidazole gel 1% applied once daily for the first week, and then twice daily until the lesions resolved. All patients showed improvement after 4 to 6 weeks, and eventually the disease cleared between 3 and 6 months. All patients were still symptom free during a 2-year observation period.19
Manders and Lucky7 described 14 patients with POD (aged 9 months to 6.5 years). Eight patients used only metronidazole gel 0.75%, while 5 used the gel in combination with topical corticosteroids (21% [3/14]), oral erythromycin (7% [1/14]), or topical erythromycin (7% [1/14]); 1 patient remained on hydrocortisone 1% and cleared. Patients responded well within 1 to 8 weeks and were symptom free for up to 16 months. Mid- to high-potency steroids were discontinued in all patients.7
In some pediatric patients with CGPD, recovery occurs faster with the use of oral macrolides or tetracyclines, either alone or in combination with topical antibiotics or sulfur-based lotions.20 Extrafacial lesions associated with CGPD do not appear to negatively impact treatment response or duration of disease. In the review conducted by Urbatsch et al,20 7 of 8 (88%) CGPD patients with extrafacial lesions were treated with oral agents including erythromycin, hydroxychloroquine, cyclosporine, minocycline, and azithromycin. Most of these patients also were using topical agents such as triamcinolone acetonide, desonide, metronidazole, and erythromycin. The time to resolution ranged from several weeks to 6 months.20
Weston and Morelli9 described a treatment regimen for steroid rosacea. The study included data on 106 children (60 females, 46 males) who had been exposed to mostly class 7 low-potency agents. All patients were advised to immediately stop topical steroid therapy without gradual withdrawal and to begin oral erythromycin stearate 30 mg/kg daily in 2 doses per day for 4 weeks. Patients who were unable to tolerate erythromycin were advised to use topical clindamycin phosphate twice daily for 4 weeks (n=6). Eighty-six percent of patients showed resolution within 4 weeks, and 100% showed clearance by 8 weeks. Twenty-two percent of patients had clearance within 3 weeks. There was no difference in the duration until resolution for those who had used oral or topical antibiotics.9 A different study suggested that low-potency topical steroids can be used to control inflammation when weaning patients off of strong steroids.5
Differential Diagnosis
The differential diagnosis should include acne vulgaris, allergic contact dermatitis, irritant contact dermatitis, seborrheic dermatitis, impetigo, dermatophyte infection, rosacea, and angiofibromas.4
Acne vulgaris commonly is found in older adolescents, and unlike POD, it will present with open or closed comedones.2 In patients aged 1 to 7 years, acne is a reason to consider endocrine evaluation. Allergic contact dermatitis is extremely pruritic, and the lesions often are papulovesicular with active weeping or crusting. Patients with irritant contact dermatitis often report burning and pain, and papules and pustules typically are absent. A thorough history can help rule out allergic or irritant contact dermatitis. Seborrheic dermatitis presents with erythema and scaling of the scalp, eyebrows, and nasolabial folds; it tends to spare the perioral regions and also lacks papules.2 The lesions of impetigo typically have a yellow-brown exudate, which forms a honey-colored crust.24 Tinea faciei, unlike the other tinea infections, can have an extremely variable presentation. Lesions usually begin as scaly macules that develop raised borders with central hypopigmentation, but papules, vesicles, and crusts can be seen.25 Potassium hydroxide
Conclusion
Diagnosis of POD is clinical and rests upon the finding of erythematous papules on the face near the eyes, mouth, and nose. Extrafacial lesions also have been described, particularly in pediatric patients with CGPD. Many patients will report a history of atopic dermatitis and asthma. Therapy for POD includes both topical and systemic agents. For those with mild disease, topical metronidazole commonly is used. For patients requiring oral antibiotics, tetracyclines or macrolides can be prescribed based on the age of the patient. Many pediatric patients who begin with both oral and topical agents can later be maintained on topical therapy, sometimes with a low-dose oral antibiotic. Periorificial dermatitis has an excellent prognosis and most pediatric patients show marked improvement within weeks to months.
Perioral dermatitis is an acneform eruption presenting with erythematous papules, vesicles, and rarely pustules clustered around the orifices of the face. 1 Lesions may be found near the eyes, mouth, and nose but typically spare the vermilion border of the lips. 2 Nguyen and Eichenfield 3 preferred the term periorificial dermatitis (POD), which has since been adopted by others. 4 Patients may report pruritus, but there generally are no systemic symptoms unless patients have comorbid conditions such as atopic dermatitis. 5 Although this condition has been well examined in the literature on adults, data in the pediatric population are far more limited, consisting of case series and retrospective chart reviews. In 1979, Wilkinson et al 6 published a study of more than 200 patients with perioral dermatitis, but only 15 patients younger than 12 years were included.
Etiology
Although the exact pathogenesis of POD is unknown, a common denominator among many patients is prior exposure to topical corticosteroids.3,7-9 Periorificial dermatitis also has been linked to the use of systemic corticosteroids in pediatric patients.10 The exact relationship between steroid use and dermatitis is unknown; it may be related to a change in the flora of hair follicles and in particular an association with fusiform bacteria–rich conditions.11 Aside from steroid exposure, POD has been associated with the use of physical sunscreen in pediatric patients with dry skin,12 rosin in chewing gum,13 and inhaled corticosteroids in those with asthma.14 In one case, a 15-year-old adolescent girl developed POD and swelling of the lips after 2 years of playing a flute made of cocus wood.15,16
Epidemiology
Comorbidities and Family History
Goel et al17 (N=222) reported the following comorbidities associated with pediatric POD: atopic dermatitis (29.3%), asthma (14.9%), and allergies (9.9%). Steroid exposure was noted in 58.1% of patients.17 Similarly, Nguyen and Eichenfield3 (N=79) found that the most common comorbidities were atopic dermatitis (14%), keratosis pilaris (14%), viral infections (14%), acne (10%), and seborrheic dermatitis (10%). Family history of atopy was noted in 55% of patients and family history of rosacea was noted in 3%. In a case series of 11 pediatric patients, 3 (27%) had keratosis pilaris, 7 (64%) had a family history of atopy, and 2 (18%) had a family history of rosacea.8 Weston and Morelli9 found a much higher incidence of familial rosacea (20%) in 106 children with steroid rosacea.
Clinical Presentation
Periorificial dermatitis generally presents with small, pink- to flesh-colored papules in a perioral, periocular, and perinasal distribution. Although many patients are white, a particularly prominent variant has been noted in black children with papules that may be hyperpigmented.18 In a 2006 chart review in 79 pediatric POD patients aged 6 months to 18 years, Nguyen and Eichenfield3 reported that 92% (73/79) of patients presented for a facial rash with an average duration ranging from 2 weeks to 4 years.
Boeck et al19 described 7 pediatric patients with perioral dermatitis. Six (86%) patients had perioral lesions, and 6 (86%) had previously been treated with moderate- to high-potency topical corticosteroids. Skin prick tests were negative in 6 (86%) patients.19 In one case report, a 6-year-old boy did not present with the classic acneform lesions but rather sharply demarcated eczematous patches around the eyes, nose, and mouth. The rash began to fade after 2 weeks of using metronidazole gel 1%, and after 4 months he was only left with mild hyperpigmentation.4
Periorificial dermatitis was once thought to be a juvenile form of rosacea.5 In 1972, Savin et al8 described 11 pediatric patients with “rosacea-like” facial flushing, papules, pustules, and scaling over the cheeks, forehead, and chin. In some patients, the eyelids also were involved. At least 8 patients had been using potent topical corticosteroids and had noticed exacerbation of their skin lesions after stopping therapy.8
Variants of POD
Several other variants of POD have been described in pediatric patients including childhood granulomatous periorificial dermatitis (CGPD)(also known as facial Afro-Caribbean [childhood] eruption) and lupus miliaris disseminatus faciei. Childhood granulomatous periorificial dermatitis presents in prepubertal children as dome-shaped, red to yellow-brown, monomorphous papules around the eyes, nose, and mouth; there are no systemic findings.20,21 It occurs equally in males and females and is more commonly seen in dark-skinned patients. Childhood granulomatous periorificial dermatitis usually resolves within a few months to years but may be associated with blepharitis or conjunctivitis.20 Urbatsch et al20 analyzed extrafacial lesions in 8 patients (aged 2–12 years) with CGPD. Lesions were found on the trunk (38% [3/8]), neck (25% [2/8]), ears (25% [2/8]), extremities (50% [4/8]), labia majora (38% [3/8]), and abdomen (13% [1/8]). In addition, 2 (25% [2/8]) patients had blepharitis.20
Lupus miliaris disseminatus faciei, which occurs in adolescents and adults, commonly involves the eyelids and central areas of the face such as the nose and upper lips. Patients typically present with erythematous or flesh-colored papules.1
Diagnosis
Diagnosis of POD is made clinically based on the observation of papules (and sometimes pustules) around the orifices of the face, sparing the vermilion border, together with a lack of comedones.17 Laboratory tests are not useful.5 Biopsies rarely are performed, and the results mimic those of rosacea, demonstrating a perifollicular lymphohistiocytic infiltrate, epithelioid cells, and occasionally giant cells.5,22,23 Early papular lesions can show mild acanthosis, epidermal edema, and parakeratosis.23 Biopsies in patients with CGPD reveal noncaseating perifollicular granulomas.20
Treatment and Clinical Outcome
Although topical corticosteroids can improve facial lesions in pediatric POD, the eruption often rebounds when therapy is discontinued.1 One therapy frequently used in adults is oral tetracyclines; however, these agents must not be used in patients younger than 9 years due to potential dental staining.4 The standards are either topical metronidazole twice daily with clearance in 3 to 8 weeks or oral erythromycin.7
In the review conducted by Goel et al,17 treatment included azithromycin (44.6%), topical metronidazole (42.3%), sodium sulfacetamide lotion (35.6%), oral antibiotic monotherapy (15.3%), topical agent monotherapy (44.6%), and combined oral and topical agent therapy (40.1%). Of those patients who presented for a follow-up visit (59%), 72% of cases resolved and 10.7% showed some improvement. For those patients who returned for follow-up, the average duration until symptom resolution was approximately 4 months. The most common side effects were pigmentation changes (1.8%), worsening of symptoms (1.8%), gastrointestinal upset (0.9%), irritant dermatitis (0.9%), and xerosis (0.5%).17
Changes were made to the treatment plans for 16 patients, most often due to inadequate treatment response.17 Five patients treated with sodium sulfacetamide lotion also were started on oral azithromycin. Four patients treated with oral antibiotics were given a topical agent (metronidazole or sodium sulfacetamide lotion). Other modifications included replacing sodium sulfacetamide lotion with topical metronidazole and an oral antibiotic (azithromycin or doxycycline, n=3), adjusting the doses of oral or topical medications (n=2), adding tacrolimus (n=1), and replacing topical metronidazole with sodium sulfacetamide lotion (n=1). Of the patients who underwent a change in treatment plan, 5 experienced symptom recurrence, 4 had mild improvement, and 1 patient had no improvement. Six patients were lost to follow-up.17
In the study conducted by Nguyen and Eichenfield,3 follow-up visits occurred approximately 3 months after the first visit.
In the case series by Boeck et al,19 all patients were started on metronidazole gel 1% applied once daily for the first week, and then twice daily until the lesions resolved. All patients showed improvement after 4 to 6 weeks, and eventually the disease cleared between 3 and 6 months. All patients were still symptom free during a 2-year observation period.19
Manders and Lucky7 described 14 patients with POD (aged 9 months to 6.5 years). Eight patients used only metronidazole gel 0.75%, while 5 used the gel in combination with topical corticosteroids (21% [3/14]), oral erythromycin (7% [1/14]), or topical erythromycin (7% [1/14]); 1 patient remained on hydrocortisone 1% and cleared. Patients responded well within 1 to 8 weeks and were symptom free for up to 16 months. Mid- to high-potency steroids were discontinued in all patients.7
In some pediatric patients with CGPD, recovery occurs faster with the use of oral macrolides or tetracyclines, either alone or in combination with topical antibiotics or sulfur-based lotions.20 Extrafacial lesions associated with CGPD do not appear to negatively impact treatment response or duration of disease. In the review conducted by Urbatsch et al,20 7 of 8 (88%) CGPD patients with extrafacial lesions were treated with oral agents including erythromycin, hydroxychloroquine, cyclosporine, minocycline, and azithromycin. Most of these patients also were using topical agents such as triamcinolone acetonide, desonide, metronidazole, and erythromycin. The time to resolution ranged from several weeks to 6 months.20
Weston and Morelli9 described a treatment regimen for steroid rosacea. The study included data on 106 children (60 females, 46 males) who had been exposed to mostly class 7 low-potency agents. All patients were advised to immediately stop topical steroid therapy without gradual withdrawal and to begin oral erythromycin stearate 30 mg/kg daily in 2 doses per day for 4 weeks. Patients who were unable to tolerate erythromycin were advised to use topical clindamycin phosphate twice daily for 4 weeks (n=6). Eighty-six percent of patients showed resolution within 4 weeks, and 100% showed clearance by 8 weeks. Twenty-two percent of patients had clearance within 3 weeks. There was no difference in the duration until resolution for those who had used oral or topical antibiotics.9 A different study suggested that low-potency topical steroids can be used to control inflammation when weaning patients off of strong steroids.5
Differential Diagnosis
The differential diagnosis should include acne vulgaris, allergic contact dermatitis, irritant contact dermatitis, seborrheic dermatitis, impetigo, dermatophyte infection, rosacea, and angiofibromas.4
Acne vulgaris commonly is found in older adolescents, and unlike POD, it will present with open or closed comedones.2 In patients aged 1 to 7 years, acne is a reason to consider endocrine evaluation. Allergic contact dermatitis is extremely pruritic, and the lesions often are papulovesicular with active weeping or crusting. Patients with irritant contact dermatitis often report burning and pain, and papules and pustules typically are absent. A thorough history can help rule out allergic or irritant contact dermatitis. Seborrheic dermatitis presents with erythema and scaling of the scalp, eyebrows, and nasolabial folds; it tends to spare the perioral regions and also lacks papules.2 The lesions of impetigo typically have a yellow-brown exudate, which forms a honey-colored crust.24 Tinea faciei, unlike the other tinea infections, can have an extremely variable presentation. Lesions usually begin as scaly macules that develop raised borders with central hypopigmentation, but papules, vesicles, and crusts can be seen.25 Potassium hydroxide
Conclusion
Diagnosis of POD is clinical and rests upon the finding of erythematous papules on the face near the eyes, mouth, and nose. Extrafacial lesions also have been described, particularly in pediatric patients with CGPD. Many patients will report a history of atopic dermatitis and asthma. Therapy for POD includes both topical and systemic agents. For those with mild disease, topical metronidazole commonly is used. For patients requiring oral antibiotics, tetracyclines or macrolides can be prescribed based on the age of the patient. Many pediatric patients who begin with both oral and topical agents can later be maintained on topical therapy, sometimes with a low-dose oral antibiotic. Periorificial dermatitis has an excellent prognosis and most pediatric patients show marked improvement within weeks to months.
- Tempark T, Shwayder TA. Perioral dermatitis: a review of the condition with special attention to treatment options. Am J Clin Dermatol. 2014;15:101-113.
- McFarland SL, Polcari IC. Morphology-based diagnosis of acneiform eruptions. Pediatr Ann. 2015;44:E188-E193.
- Nguyen V, Eichenfield LF. Periorificial dermatitis in children and adolescents. J Am Acad Dermatol. 2006;55:781-785.
- Kihiczak GG, Cruz MA, Schwartz RA. Periorificial dermatitis in children: an update and description of a child with striking features. Int J Dermatol. 2009;48:304-306.
- Laude TA, Salvemini JN. Perioral dermatitis in children. Sem Cutan Med Surg. 1999;18:206-209.
- Wilkinson DS, Kirton V, Wilkinson JD. Perioral dermatitis: a 12-year review. Br J Dermatol. 1979;101:245-257.
- Manders SM, Lucky AW. Perioral dermatitis in childhood. J Am Acad Dermatol. 1992;27(5 pt 1):688-692.
- Savin JA, Alexander S, Marks R. A rosacea-like eruption of children. Br J Dermatol. 1972;87:425-429.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Clementson B, Smidt AC. Periorificial dermatitis due to systemic corticosteroids in children: report of two cases. Pediatr Dermatol. 2012;29:331-332.
- Takiwaki H, Tsuda H, Arase S, et al. Differences between intrafollicular microorganism profiles in perioral and seborrhoeic dermatitis. Clin Exp Dermatol. 2003;28:531-534.
- Abeck D, Geisenfelder B, Brandt O. Physical sunscreens with high sun protection factor may cause perioral dermatitis in children. J Dtsch Dermatol Ges. 2009;7:701-703.
- Satyawan I, Oranje AP, van Joost T. Perioral dermatitis in a child due to rosin in chewing gum. Contact Dermatitis. 1990;22:182-183.
- Dubus JC, Marguet C, Deschildre A, et al. Local side-effects of inhaled corticosteroids in asthmatic children: influence of drug, dose, age, and device. Allergy. 2001;56:944-948.
- Hausen BM, Bruhn G, Koenig WA. New hydroxyisoflavans as contact sensitizers in cocus wood Brya ebenus DC (Fabaceae). Contact Dermatitis. 1991;25:149-155.
- Dirschka T, Weber K, Tronnier H. Topical cosmetics and perioral dermatitis. J Dtsch Dermatol Ges. 2004;2:194-199.
- Goel NS, Burkhart CN, Morrell DS. Pediatric periorificial dermatitis: clinical course and treatment outcomes in 222 patients. Pediatr Dermatol. 2015;32:333-336.
- Cribier B, Lieber-Mbomeyo A, Lipsker D. Clinical and histological study of a case of facial Afro-Caribbean childhood eruption (FACE) [in French][published online July 23, 2008]. Ann Dermatol Venerol. 2008;135:663-667.
- Boeck K, Abeck D, Werfel S, et al. Perioral dermatitis in children—clinical presentation, pathogenesis-related factors and response to topical metronidazole. Dermatology. 1997;195:235-238.
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Ramelet AA, Delacrétaz J. Histopathologic study of perioral dermatitis [in French]. Dermatologica. 1981;163:361-369.
- Ljubojevi´c S, Lipozenci´c J, Turci´c P. Perioral dermatitis. Acta Dermatovenerol Croat. 2008;16:96-100.
- Nichols RL, Florman S. Clinical presentations of soft-tissue infections and surgical site infections. Clin Infect Dis. 2001;33(suppl 2):S84-S93.
- Lin RL, Szepietowski JC, Schwartz RA. Tinea faciei, an often deceptive facial eruption. Int J Dermatol. 2004;43:437-440.
- Tempark T, Shwayder TA. Perioral dermatitis: a review of the condition with special attention to treatment options. Am J Clin Dermatol. 2014;15:101-113.
- McFarland SL, Polcari IC. Morphology-based diagnosis of acneiform eruptions. Pediatr Ann. 2015;44:E188-E193.
- Nguyen V, Eichenfield LF. Periorificial dermatitis in children and adolescents. J Am Acad Dermatol. 2006;55:781-785.
- Kihiczak GG, Cruz MA, Schwartz RA. Periorificial dermatitis in children: an update and description of a child with striking features. Int J Dermatol. 2009;48:304-306.
- Laude TA, Salvemini JN. Perioral dermatitis in children. Sem Cutan Med Surg. 1999;18:206-209.
- Wilkinson DS, Kirton V, Wilkinson JD. Perioral dermatitis: a 12-year review. Br J Dermatol. 1979;101:245-257.
- Manders SM, Lucky AW. Perioral dermatitis in childhood. J Am Acad Dermatol. 1992;27(5 pt 1):688-692.
- Savin JA, Alexander S, Marks R. A rosacea-like eruption of children. Br J Dermatol. 1972;87:425-429.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Clementson B, Smidt AC. Periorificial dermatitis due to systemic corticosteroids in children: report of two cases. Pediatr Dermatol. 2012;29:331-332.
- Takiwaki H, Tsuda H, Arase S, et al. Differences between intrafollicular microorganism profiles in perioral and seborrhoeic dermatitis. Clin Exp Dermatol. 2003;28:531-534.
- Abeck D, Geisenfelder B, Brandt O. Physical sunscreens with high sun protection factor may cause perioral dermatitis in children. J Dtsch Dermatol Ges. 2009;7:701-703.
- Satyawan I, Oranje AP, van Joost T. Perioral dermatitis in a child due to rosin in chewing gum. Contact Dermatitis. 1990;22:182-183.
- Dubus JC, Marguet C, Deschildre A, et al. Local side-effects of inhaled corticosteroids in asthmatic children: influence of drug, dose, age, and device. Allergy. 2001;56:944-948.
- Hausen BM, Bruhn G, Koenig WA. New hydroxyisoflavans as contact sensitizers in cocus wood Brya ebenus DC (Fabaceae). Contact Dermatitis. 1991;25:149-155.
- Dirschka T, Weber K, Tronnier H. Topical cosmetics and perioral dermatitis. J Dtsch Dermatol Ges. 2004;2:194-199.
- Goel NS, Burkhart CN, Morrell DS. Pediatric periorificial dermatitis: clinical course and treatment outcomes in 222 patients. Pediatr Dermatol. 2015;32:333-336.
- Cribier B, Lieber-Mbomeyo A, Lipsker D. Clinical and histological study of a case of facial Afro-Caribbean childhood eruption (FACE) [in French][published online July 23, 2008]. Ann Dermatol Venerol. 2008;135:663-667.
- Boeck K, Abeck D, Werfel S, et al. Perioral dermatitis in children—clinical presentation, pathogenesis-related factors and response to topical metronidazole. Dermatology. 1997;195:235-238.
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Ramelet AA, Delacrétaz J. Histopathologic study of perioral dermatitis [in French]. Dermatologica. 1981;163:361-369.
- Ljubojevi´c S, Lipozenci´c J, Turci´c P. Perioral dermatitis. Acta Dermatovenerol Croat. 2008;16:96-100.
- Nichols RL, Florman S. Clinical presentations of soft-tissue infections and surgical site infections. Clin Infect Dis. 2001;33(suppl 2):S84-S93.
- Lin RL, Szepietowski JC, Schwartz RA. Tinea faciei, an often deceptive facial eruption. Int J Dermatol. 2004;43:437-440.
Practice Points
- Periorificial dermatitis (POD) affects young children and presents as flesh-colored papules around the mouth, nose, and even groin.
- Periorificial dermatitis has been associated with prior use of topical or inhaled steroids.
- Children with POD can be treated with oral erythromycin.
The Atopic Dermatitis Biologic Era Has Begun
Atopic dermatitis (AD) is a vexing multisystem disorder characterized by frequently recurrent, intrusive, and sometimes disabling itch and dermatitis. The itch may be present throughout the day but crescendos at bedtime or 1 to 2 hours after sleep initiation, resulting in disrupted sleep cycles, lack of rest, more hours scratching, daytime somnolence, poor work attendance and performance, and poor school attendance and performance.1
Atopic dermatitis is a lifelong disease that only remits in approximately half of patients.2 There is a need for a disease-specific systemic drug in AD. Phototherapy, cyclosporine, methotrexate, and azathioprine are nonspecific immunosuppressive agents that can be used off label for AD but may or may not be effective.3 Oral or intramuscular corticosteroids are associated with problematic side effects such as weight gain, osteoporosis, fractures, psychological problems, striae, buffalo hump, and steroid withdrawal symptoms and disease aggravation upon withdrawal (ie, flaring to a state worse than prior to steroid initiation).3,4
A biologic medication for AD has been long overdue. Psoriatic biologic medications have been tried in AD with occasional benefit in case reports but no major response in larger trials. Belloni et al5 reviewed early data on off-label usage of biologics approved by the US Food and Drug Administration for psoriasis or other indications applied to AD patients. In their review of cases, they make the point that results are variable and anti-B-cell activity may hold the greatest promise.5 On the other hand, a recent series of 3 patients showed limited response to rituximab in chronic AD,6 while a combination of omalizumab, an anti-IgE medication, and rituximab was helpful in some patients.7 Ultimately, the issue is that nonspecific biologics may or may not address the underlying disease factors in AD. Therefore, there has been a true need for biologic intervention targeted directly at the pathogenic mechanism of AD. Furthermore, the desire for a biologic targeted at AD is paired with the true need to have a medication so targeted that the drug would have little effect on the rest of the immune system, resulting in targeted immunomodulation without secondary risk of infections.
Wait no longer, that era arrived a few months ago with the rapid US Food and Drug Administration approval of dupilumab, an injectable medication used every 2 weeks for the therapy of moderate to severe AD. This fully human monoclonal antibody against the IL-4Rα subunit blocks IL-4 and IL-13, key inflammatory agents in the triggering of production of IgE and eosinophil activation. Even better than the fact that it is targeted are the excellent outcomes in the therapy of moderate to severe AD in adults and the minimal side-effect profile resulting in no requirements for laboratory screening or ongoing monitoring.8
Dupilumab seems to perform well, both clinically and in improving the lives of AD patients. Meta-analysis of trials involving dupilumab has shown improved health-related quality of life outcomes.9,10 Usage of dupilumab alone in clinical trials for 16 weeks (SOLO 1 and SOLO 2) has resulted in stunning reduction in disease severity with a limited side-effect profile, with patients most commonly reporting conjunctivitis.11 In real-world models where dupilumab is added into a regimen of topical corticosteroid usage (LIBERTY AD CHRONOS trial), patients fared even better with the combination, highlighting that this medication may best be used adjunctively to our skin care guidance as dermatologists.12
A new era for AD patients has arrived and we as practitioners are now fortunate to be able to therapeutically reach the worst cases of AD. The new era has only begun with dozens of new agents addressing a variety of interleukin pathways including IL-17 and IL-22 still under development. Ultimately, we hope that ongoing pediatric trials will allow us to glean the role of early disease intervention at the root cause of AD and address our abilities to prevent comorbidities and disease persistence. Will we be able to avert years of disabling disease? The future holds immense hope.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Somanunt S, Chinratanapisit S, Pacharn P, et al. The natural history of atopic dermatitis and its association with Atopic March [published online Dec 12, 2016]. Asian Pac J Allergy Immunol. doi:10.12932/AP0825.
- Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Hajar T, Leshem YA, Hanifin JM, et al; the National Eczema Association Task Force. A systematic review of topical corticosteroid withdrawal ("steroid addiction") in patients with atopic dermatitis and other dermatoses [published online January 13, 2015]. J Am Acad Dermatol. 2015;72:541.e2-549.e2.
- Belloni B, Andres C, Ollert M, et al. Novel immunological approaches in the treatment of atopic eczema. Curr Opin Allergy Clin Immunol. 2008;8:423-427.
- McDonald BS, Jones J, Rustin M. Rituximab as a treatment for severe atopic eczema: failure to improve in three consecutive patients. Clin Exp Dermatol. 2016;41:45-47.
- Sánchez-Ramón S, Eguíluz-Gracia I, Rodríguez-Mazariego ME, et al. Sequential combined therapy with omalizumab and rituximab: a new approach to severe atopic dermatitis. J Investig Allergol Clin Immunol. 2013;23:190-196.
- D'Erme AM, Romanelli M, Chiricozzi A. Spotlight on dupilumab in the treatment of atopic dermatitis: design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:1473-1480.
- Han Y, Chen Y, Liu X, et al. Efficacy and safety of dupilumab for the treatment of adult atopic dermatitis: a meta-analysis of randomized clinical trials [published online May 4, 2017]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2017.04.015.
- Simpson EL. Dupilumab improves general health-related quality-of-life in patients with moderate-to-severe atopic dermatitis: pooled results from two randomized, controlled phase 3 clinical trials. Dermatol Ther (Heidelb). 2017;7:243-248.
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis [published online Sep 30, 2016]. N Engl J Med. 2016;375:2335-2348.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial [published online May 4, 2017]. Lancet. 2017;389:2287-2303.
Atopic dermatitis (AD) is a vexing multisystem disorder characterized by frequently recurrent, intrusive, and sometimes disabling itch and dermatitis. The itch may be present throughout the day but crescendos at bedtime or 1 to 2 hours after sleep initiation, resulting in disrupted sleep cycles, lack of rest, more hours scratching, daytime somnolence, poor work attendance and performance, and poor school attendance and performance.1
Atopic dermatitis is a lifelong disease that only remits in approximately half of patients.2 There is a need for a disease-specific systemic drug in AD. Phototherapy, cyclosporine, methotrexate, and azathioprine are nonspecific immunosuppressive agents that can be used off label for AD but may or may not be effective.3 Oral or intramuscular corticosteroids are associated with problematic side effects such as weight gain, osteoporosis, fractures, psychological problems, striae, buffalo hump, and steroid withdrawal symptoms and disease aggravation upon withdrawal (ie, flaring to a state worse than prior to steroid initiation).3,4
A biologic medication for AD has been long overdue. Psoriatic biologic medications have been tried in AD with occasional benefit in case reports but no major response in larger trials. Belloni et al5 reviewed early data on off-label usage of biologics approved by the US Food and Drug Administration for psoriasis or other indications applied to AD patients. In their review of cases, they make the point that results are variable and anti-B-cell activity may hold the greatest promise.5 On the other hand, a recent series of 3 patients showed limited response to rituximab in chronic AD,6 while a combination of omalizumab, an anti-IgE medication, and rituximab was helpful in some patients.7 Ultimately, the issue is that nonspecific biologics may or may not address the underlying disease factors in AD. Therefore, there has been a true need for biologic intervention targeted directly at the pathogenic mechanism of AD. Furthermore, the desire for a biologic targeted at AD is paired with the true need to have a medication so targeted that the drug would have little effect on the rest of the immune system, resulting in targeted immunomodulation without secondary risk of infections.
Wait no longer, that era arrived a few months ago with the rapid US Food and Drug Administration approval of dupilumab, an injectable medication used every 2 weeks for the therapy of moderate to severe AD. This fully human monoclonal antibody against the IL-4Rα subunit blocks IL-4 and IL-13, key inflammatory agents in the triggering of production of IgE and eosinophil activation. Even better than the fact that it is targeted are the excellent outcomes in the therapy of moderate to severe AD in adults and the minimal side-effect profile resulting in no requirements for laboratory screening or ongoing monitoring.8
Dupilumab seems to perform well, both clinically and in improving the lives of AD patients. Meta-analysis of trials involving dupilumab has shown improved health-related quality of life outcomes.9,10 Usage of dupilumab alone in clinical trials for 16 weeks (SOLO 1 and SOLO 2) has resulted in stunning reduction in disease severity with a limited side-effect profile, with patients most commonly reporting conjunctivitis.11 In real-world models where dupilumab is added into a regimen of topical corticosteroid usage (LIBERTY AD CHRONOS trial), patients fared even better with the combination, highlighting that this medication may best be used adjunctively to our skin care guidance as dermatologists.12
A new era for AD patients has arrived and we as practitioners are now fortunate to be able to therapeutically reach the worst cases of AD. The new era has only begun with dozens of new agents addressing a variety of interleukin pathways including IL-17 and IL-22 still under development. Ultimately, we hope that ongoing pediatric trials will allow us to glean the role of early disease intervention at the root cause of AD and address our abilities to prevent comorbidities and disease persistence. Will we be able to avert years of disabling disease? The future holds immense hope.
Atopic dermatitis (AD) is a vexing multisystem disorder characterized by frequently recurrent, intrusive, and sometimes disabling itch and dermatitis. The itch may be present throughout the day but crescendos at bedtime or 1 to 2 hours after sleep initiation, resulting in disrupted sleep cycles, lack of rest, more hours scratching, daytime somnolence, poor work attendance and performance, and poor school attendance and performance.1
Atopic dermatitis is a lifelong disease that only remits in approximately half of patients.2 There is a need for a disease-specific systemic drug in AD. Phototherapy, cyclosporine, methotrexate, and azathioprine are nonspecific immunosuppressive agents that can be used off label for AD but may or may not be effective.3 Oral or intramuscular corticosteroids are associated with problematic side effects such as weight gain, osteoporosis, fractures, psychological problems, striae, buffalo hump, and steroid withdrawal symptoms and disease aggravation upon withdrawal (ie, flaring to a state worse than prior to steroid initiation).3,4
A biologic medication for AD has been long overdue. Psoriatic biologic medications have been tried in AD with occasional benefit in case reports but no major response in larger trials. Belloni et al5 reviewed early data on off-label usage of biologics approved by the US Food and Drug Administration for psoriasis or other indications applied to AD patients. In their review of cases, they make the point that results are variable and anti-B-cell activity may hold the greatest promise.5 On the other hand, a recent series of 3 patients showed limited response to rituximab in chronic AD,6 while a combination of omalizumab, an anti-IgE medication, and rituximab was helpful in some patients.7 Ultimately, the issue is that nonspecific biologics may or may not address the underlying disease factors in AD. Therefore, there has been a true need for biologic intervention targeted directly at the pathogenic mechanism of AD. Furthermore, the desire for a biologic targeted at AD is paired with the true need to have a medication so targeted that the drug would have little effect on the rest of the immune system, resulting in targeted immunomodulation without secondary risk of infections.
Wait no longer, that era arrived a few months ago with the rapid US Food and Drug Administration approval of dupilumab, an injectable medication used every 2 weeks for the therapy of moderate to severe AD. This fully human monoclonal antibody against the IL-4Rα subunit blocks IL-4 and IL-13, key inflammatory agents in the triggering of production of IgE and eosinophil activation. Even better than the fact that it is targeted are the excellent outcomes in the therapy of moderate to severe AD in adults and the minimal side-effect profile resulting in no requirements for laboratory screening or ongoing monitoring.8
Dupilumab seems to perform well, both clinically and in improving the lives of AD patients. Meta-analysis of trials involving dupilumab has shown improved health-related quality of life outcomes.9,10 Usage of dupilumab alone in clinical trials for 16 weeks (SOLO 1 and SOLO 2) has resulted in stunning reduction in disease severity with a limited side-effect profile, with patients most commonly reporting conjunctivitis.11 In real-world models where dupilumab is added into a regimen of topical corticosteroid usage (LIBERTY AD CHRONOS trial), patients fared even better with the combination, highlighting that this medication may best be used adjunctively to our skin care guidance as dermatologists.12
A new era for AD patients has arrived and we as practitioners are now fortunate to be able to therapeutically reach the worst cases of AD. The new era has only begun with dozens of new agents addressing a variety of interleukin pathways including IL-17 and IL-22 still under development. Ultimately, we hope that ongoing pediatric trials will allow us to glean the role of early disease intervention at the root cause of AD and address our abilities to prevent comorbidities and disease persistence. Will we be able to avert years of disabling disease? The future holds immense hope.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Somanunt S, Chinratanapisit S, Pacharn P, et al. The natural history of atopic dermatitis and its association with Atopic March [published online Dec 12, 2016]. Asian Pac J Allergy Immunol. doi:10.12932/AP0825.
- Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Hajar T, Leshem YA, Hanifin JM, et al; the National Eczema Association Task Force. A systematic review of topical corticosteroid withdrawal ("steroid addiction") in patients with atopic dermatitis and other dermatoses [published online January 13, 2015]. J Am Acad Dermatol. 2015;72:541.e2-549.e2.
- Belloni B, Andres C, Ollert M, et al. Novel immunological approaches in the treatment of atopic eczema. Curr Opin Allergy Clin Immunol. 2008;8:423-427.
- McDonald BS, Jones J, Rustin M. Rituximab as a treatment for severe atopic eczema: failure to improve in three consecutive patients. Clin Exp Dermatol. 2016;41:45-47.
- Sánchez-Ramón S, Eguíluz-Gracia I, Rodríguez-Mazariego ME, et al. Sequential combined therapy with omalizumab and rituximab: a new approach to severe atopic dermatitis. J Investig Allergol Clin Immunol. 2013;23:190-196.
- D'Erme AM, Romanelli M, Chiricozzi A. Spotlight on dupilumab in the treatment of atopic dermatitis: design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:1473-1480.
- Han Y, Chen Y, Liu X, et al. Efficacy and safety of dupilumab for the treatment of adult atopic dermatitis: a meta-analysis of randomized clinical trials [published online May 4, 2017]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2017.04.015.
- Simpson EL. Dupilumab improves general health-related quality-of-life in patients with moderate-to-severe atopic dermatitis: pooled results from two randomized, controlled phase 3 clinical trials. Dermatol Ther (Heidelb). 2017;7:243-248.
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis [published online Sep 30, 2016]. N Engl J Med. 2016;375:2335-2348.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial [published online May 4, 2017]. Lancet. 2017;389:2287-2303.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Somanunt S, Chinratanapisit S, Pacharn P, et al. The natural history of atopic dermatitis and its association with Atopic March [published online Dec 12, 2016]. Asian Pac J Allergy Immunol. doi:10.12932/AP0825.
- Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Hajar T, Leshem YA, Hanifin JM, et al; the National Eczema Association Task Force. A systematic review of topical corticosteroid withdrawal ("steroid addiction") in patients with atopic dermatitis and other dermatoses [published online January 13, 2015]. J Am Acad Dermatol. 2015;72:541.e2-549.e2.
- Belloni B, Andres C, Ollert M, et al. Novel immunological approaches in the treatment of atopic eczema. Curr Opin Allergy Clin Immunol. 2008;8:423-427.
- McDonald BS, Jones J, Rustin M. Rituximab as a treatment for severe atopic eczema: failure to improve in three consecutive patients. Clin Exp Dermatol. 2016;41:45-47.
- Sánchez-Ramón S, Eguíluz-Gracia I, Rodríguez-Mazariego ME, et al. Sequential combined therapy with omalizumab and rituximab: a new approach to severe atopic dermatitis. J Investig Allergol Clin Immunol. 2013;23:190-196.
- D'Erme AM, Romanelli M, Chiricozzi A. Spotlight on dupilumab in the treatment of atopic dermatitis: design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:1473-1480.
- Han Y, Chen Y, Liu X, et al. Efficacy and safety of dupilumab for the treatment of adult atopic dermatitis: a meta-analysis of randomized clinical trials [published online May 4, 2017]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2017.04.015.
- Simpson EL. Dupilumab improves general health-related quality-of-life in patients with moderate-to-severe atopic dermatitis: pooled results from two randomized, controlled phase 3 clinical trials. Dermatol Ther (Heidelb). 2017;7:243-248.
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis [published online Sep 30, 2016]. N Engl J Med. 2016;375:2335-2348.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial [published online May 4, 2017]. Lancet. 2017;389:2287-2303.
Incorporating New Atopic Dermatitis Medications in Your Practice
What advice do you give your patients today?
There is more scientific data supporting educational intervention with an eczema action plan as the core of prevention and therapy. Early institution of emollient therapy is preventive of approximately half of atopic dermatitis (AD) cases. Application of emollients immediately after bathing is best for improvement of skin hydration. The art of medicine is deciding how to pick emollients with patients. It is important to avoid patient's allergens, but ultimately the choice comes down to cold weather petrolatum and warm weather thick lotions or creams.
Therapy must still be individually tailored. Head and neck disease is best treated with nonsteroidal agents including low-strength topical corticosteroids and calcineurin inhibitors that have a black box warning, both of which have a track record of efficacy in the care of AD. A newer option is crisaborole, a topical phosphodiesterase inhibitor, which is an alternative for childhood and adult AD. For the body, any of these agents can be used comfortably, but often a mixture of topical corticosteroids of various strengths is chosen to address different sites of disease. When topical corticosteroids fail, the usage of systemic agents or phototherapy may be appropriate. The new prescription injectable dupilumab is approved for adults with AD and therapies such as these will hopefully soon be available for children with severe disease who need intervention to improve their quality of life.
How have you integrated new medications? How do you deal with side effects?
For all the therapies that truly work for AD, there are still many patients with limited to poor response on standard regimens and I offer them newer options and I also review their old regimens. Many patients believe they will be cured in 1 to 2 weeks and stop ongoing care. Counseling on the recurrent and relapsing nature of AD is important. On the other hand, I have AD patients who believe they had or truly have steroid sensitivity including allergy or withdrawal syndromes. I have seen topical steroid atrophy in this setting due to lack of intermittent discontinuation. Other situations in which topical steroid side effects are common in my practice are in the application sites of the thigh and calf in teenaged girls and the chest in teenaged boys, sites where striae are not uncommon naturally during adolescence. In these settings, confirmation of allergy via patch testing may be helpful and offering nonsteroidal agents can allow for remission of disease. Side effects with nonsteroidal agents are common but usually mild including pruritus, burning, and stinging. It is common for these symptoms to dissipate with time; therefore, preemptive education is vital (ie, stopping and restarting a day later) as well as avoidance of application to recently washed skin and limited application initially. Steroid pretreatment sometimes aids in acceptance of a nonsteroidal agent.
What information do patients want to hear?
Patients and guardians believe there has to be a cure for AD and that it will be dietary in nature. They hope I will provide an avoidance diet that will rapidly clear the disease, which I wish was true. In reality, the nature of current research is such that long-term remissions and possible cure do lie on the horizon but today are not readily available. No one can bypass good skin care and the current treatment paradigm. Withdrawal diets may cause malnourishment in children and should not be undertaken without proof of allergy.
How do you deal with steroid phobia?
Steroid phobia has become a hot topic but has existed since the advent of topical agents. Steroid phobia can cause nonadherence and poor outcomes. In reality, many topical steroidal agents have good testing and approvals in younger children. Fear is a powerful motivator and hard to break. Therefore, parents/guardians may reasonably opt for nonsteroidal care, which is a fine option when it works. Although little data on real-world combination usage of nonsteroidal and steroidal agents exist, combinations in my practice often enhance clearance.
What patient resources do you recommend?
Quoting study data may be beneficial. One of my favorite studies is historic comparative data of hydrocortisone cream 1% and mometasone furoate cream 0.1% in 48 children with moderate to severe AD (Vernon et al). At completion of the study, mometasone performed better in clearance and the only patient who developed hypothalamic-pituitary-adrenal axis suppression was in the hydrocortisone arm. I use this study to explain to parents why a prescription-strength agent may produce better results with fewer side effects.
Online snake oils abound in AD and the sources for solid information I choose are the websites of the National Eczema Association as well as academic organizations such as the American Academy of Dermatology and the Society for Pediatric Dermatology. Membership in support groups and participation can help parents/guardians and children alike and allow access to early clinical trial data. I sometimes ask parents/guardians to review manufacturer websites to specifically look for quoted clinical trial data. Although all clinical trials are not equivalent, many better eczema care manufacturers have numerous clinical trials in support of their agents, which should give a parent some enhanced comfort level.
Suggested Readings
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Juha'sz MLW, Curley RA, Rasmussen A, et al. Systematic review of the topical steroid addiction and steroid withdrawal phenomenon in children diagnosed with atopic dermatitis and treated with topical corticosteroids. J Dermatol Nurses Assoc. In press.
- Mueller SM, Itin P, Vogt DR, et al. Assessment of "corticophobia" as an indicator of non-adherence to topical corticosteroids: a pilot study. J Dermatolog Treat. 2017;28:104-111.
- Shirley M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Silverberg NB, Durán-McKinster C. Special considerations for therapy of pediatric atopic dermatitis. Dermatol Clin. 2017;35:351-363.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. J Am Acad Dermatol. 1991;24:603-607.
What advice do you give your patients today?
There is more scientific data supporting educational intervention with an eczema action plan as the core of prevention and therapy. Early institution of emollient therapy is preventive of approximately half of atopic dermatitis (AD) cases. Application of emollients immediately after bathing is best for improvement of skin hydration. The art of medicine is deciding how to pick emollients with patients. It is important to avoid patient's allergens, but ultimately the choice comes down to cold weather petrolatum and warm weather thick lotions or creams.
Therapy must still be individually tailored. Head and neck disease is best treated with nonsteroidal agents including low-strength topical corticosteroids and calcineurin inhibitors that have a black box warning, both of which have a track record of efficacy in the care of AD. A newer option is crisaborole, a topical phosphodiesterase inhibitor, which is an alternative for childhood and adult AD. For the body, any of these agents can be used comfortably, but often a mixture of topical corticosteroids of various strengths is chosen to address different sites of disease. When topical corticosteroids fail, the usage of systemic agents or phototherapy may be appropriate. The new prescription injectable dupilumab is approved for adults with AD and therapies such as these will hopefully soon be available for children with severe disease who need intervention to improve their quality of life.
How have you integrated new medications? How do you deal with side effects?
For all the therapies that truly work for AD, there are still many patients with limited to poor response on standard regimens and I offer them newer options and I also review their old regimens. Many patients believe they will be cured in 1 to 2 weeks and stop ongoing care. Counseling on the recurrent and relapsing nature of AD is important. On the other hand, I have AD patients who believe they had or truly have steroid sensitivity including allergy or withdrawal syndromes. I have seen topical steroid atrophy in this setting due to lack of intermittent discontinuation. Other situations in which topical steroid side effects are common in my practice are in the application sites of the thigh and calf in teenaged girls and the chest in teenaged boys, sites where striae are not uncommon naturally during adolescence. In these settings, confirmation of allergy via patch testing may be helpful and offering nonsteroidal agents can allow for remission of disease. Side effects with nonsteroidal agents are common but usually mild including pruritus, burning, and stinging. It is common for these symptoms to dissipate with time; therefore, preemptive education is vital (ie, stopping and restarting a day later) as well as avoidance of application to recently washed skin and limited application initially. Steroid pretreatment sometimes aids in acceptance of a nonsteroidal agent.
What information do patients want to hear?
Patients and guardians believe there has to be a cure for AD and that it will be dietary in nature. They hope I will provide an avoidance diet that will rapidly clear the disease, which I wish was true. In reality, the nature of current research is such that long-term remissions and possible cure do lie on the horizon but today are not readily available. No one can bypass good skin care and the current treatment paradigm. Withdrawal diets may cause malnourishment in children and should not be undertaken without proof of allergy.
How do you deal with steroid phobia?
Steroid phobia has become a hot topic but has existed since the advent of topical agents. Steroid phobia can cause nonadherence and poor outcomes. In reality, many topical steroidal agents have good testing and approvals in younger children. Fear is a powerful motivator and hard to break. Therefore, parents/guardians may reasonably opt for nonsteroidal care, which is a fine option when it works. Although little data on real-world combination usage of nonsteroidal and steroidal agents exist, combinations in my practice often enhance clearance.
What patient resources do you recommend?
Quoting study data may be beneficial. One of my favorite studies is historic comparative data of hydrocortisone cream 1% and mometasone furoate cream 0.1% in 48 children with moderate to severe AD (Vernon et al). At completion of the study, mometasone performed better in clearance and the only patient who developed hypothalamic-pituitary-adrenal axis suppression was in the hydrocortisone arm. I use this study to explain to parents why a prescription-strength agent may produce better results with fewer side effects.
Online snake oils abound in AD and the sources for solid information I choose are the websites of the National Eczema Association as well as academic organizations such as the American Academy of Dermatology and the Society for Pediatric Dermatology. Membership in support groups and participation can help parents/guardians and children alike and allow access to early clinical trial data. I sometimes ask parents/guardians to review manufacturer websites to specifically look for quoted clinical trial data. Although all clinical trials are not equivalent, many better eczema care manufacturers have numerous clinical trials in support of their agents, which should give a parent some enhanced comfort level.
Suggested Readings
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Juha'sz MLW, Curley RA, Rasmussen A, et al. Systematic review of the topical steroid addiction and steroid withdrawal phenomenon in children diagnosed with atopic dermatitis and treated with topical corticosteroids. J Dermatol Nurses Assoc. In press.
- Mueller SM, Itin P, Vogt DR, et al. Assessment of "corticophobia" as an indicator of non-adherence to topical corticosteroids: a pilot study. J Dermatolog Treat. 2017;28:104-111.
- Shirley M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Silverberg NB, Durán-McKinster C. Special considerations for therapy of pediatric atopic dermatitis. Dermatol Clin. 2017;35:351-363.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. J Am Acad Dermatol. 1991;24:603-607.
What advice do you give your patients today?
There is more scientific data supporting educational intervention with an eczema action plan as the core of prevention and therapy. Early institution of emollient therapy is preventive of approximately half of atopic dermatitis (AD) cases. Application of emollients immediately after bathing is best for improvement of skin hydration. The art of medicine is deciding how to pick emollients with patients. It is important to avoid patient's allergens, but ultimately the choice comes down to cold weather petrolatum and warm weather thick lotions or creams.
Therapy must still be individually tailored. Head and neck disease is best treated with nonsteroidal agents including low-strength topical corticosteroids and calcineurin inhibitors that have a black box warning, both of which have a track record of efficacy in the care of AD. A newer option is crisaborole, a topical phosphodiesterase inhibitor, which is an alternative for childhood and adult AD. For the body, any of these agents can be used comfortably, but often a mixture of topical corticosteroids of various strengths is chosen to address different sites of disease. When topical corticosteroids fail, the usage of systemic agents or phototherapy may be appropriate. The new prescription injectable dupilumab is approved for adults with AD and therapies such as these will hopefully soon be available for children with severe disease who need intervention to improve their quality of life.
How have you integrated new medications? How do you deal with side effects?
For all the therapies that truly work for AD, there are still many patients with limited to poor response on standard regimens and I offer them newer options and I also review their old regimens. Many patients believe they will be cured in 1 to 2 weeks and stop ongoing care. Counseling on the recurrent and relapsing nature of AD is important. On the other hand, I have AD patients who believe they had or truly have steroid sensitivity including allergy or withdrawal syndromes. I have seen topical steroid atrophy in this setting due to lack of intermittent discontinuation. Other situations in which topical steroid side effects are common in my practice are in the application sites of the thigh and calf in teenaged girls and the chest in teenaged boys, sites where striae are not uncommon naturally during adolescence. In these settings, confirmation of allergy via patch testing may be helpful and offering nonsteroidal agents can allow for remission of disease. Side effects with nonsteroidal agents are common but usually mild including pruritus, burning, and stinging. It is common for these symptoms to dissipate with time; therefore, preemptive education is vital (ie, stopping and restarting a day later) as well as avoidance of application to recently washed skin and limited application initially. Steroid pretreatment sometimes aids in acceptance of a nonsteroidal agent.
What information do patients want to hear?
Patients and guardians believe there has to be a cure for AD and that it will be dietary in nature. They hope I will provide an avoidance diet that will rapidly clear the disease, which I wish was true. In reality, the nature of current research is such that long-term remissions and possible cure do lie on the horizon but today are not readily available. No one can bypass good skin care and the current treatment paradigm. Withdrawal diets may cause malnourishment in children and should not be undertaken without proof of allergy.
How do you deal with steroid phobia?
Steroid phobia has become a hot topic but has existed since the advent of topical agents. Steroid phobia can cause nonadherence and poor outcomes. In reality, many topical steroidal agents have good testing and approvals in younger children. Fear is a powerful motivator and hard to break. Therefore, parents/guardians may reasonably opt for nonsteroidal care, which is a fine option when it works. Although little data on real-world combination usage of nonsteroidal and steroidal agents exist, combinations in my practice often enhance clearance.
What patient resources do you recommend?
Quoting study data may be beneficial. One of my favorite studies is historic comparative data of hydrocortisone cream 1% and mometasone furoate cream 0.1% in 48 children with moderate to severe AD (Vernon et al). At completion of the study, mometasone performed better in clearance and the only patient who developed hypothalamic-pituitary-adrenal axis suppression was in the hydrocortisone arm. I use this study to explain to parents why a prescription-strength agent may produce better results with fewer side effects.
Online snake oils abound in AD and the sources for solid information I choose are the websites of the National Eczema Association as well as academic organizations such as the American Academy of Dermatology and the Society for Pediatric Dermatology. Membership in support groups and participation can help parents/guardians and children alike and allow access to early clinical trial data. I sometimes ask parents/guardians to review manufacturer websites to specifically look for quoted clinical trial data. Although all clinical trials are not equivalent, many better eczema care manufacturers have numerous clinical trials in support of their agents, which should give a parent some enhanced comfort level.
Suggested Readings
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Juha'sz MLW, Curley RA, Rasmussen A, et al. Systematic review of the topical steroid addiction and steroid withdrawal phenomenon in children diagnosed with atopic dermatitis and treated with topical corticosteroids. J Dermatol Nurses Assoc. In press.
- Mueller SM, Itin P, Vogt DR, et al. Assessment of "corticophobia" as an indicator of non-adherence to topical corticosteroids: a pilot study. J Dermatolog Treat. 2017;28:104-111.
- Shirley M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Silverberg NB, Durán-McKinster C. Special considerations for therapy of pediatric atopic dermatitis. Dermatol Clin. 2017;35:351-363.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. J Am Acad Dermatol. 1991;24:603-607.