User login
Atopic Dermatitis Prevention and Treatment
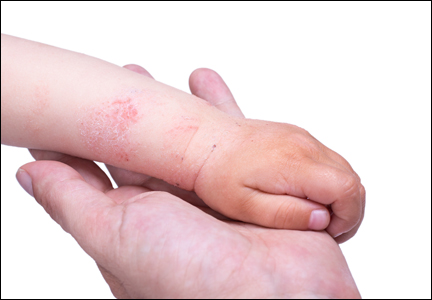
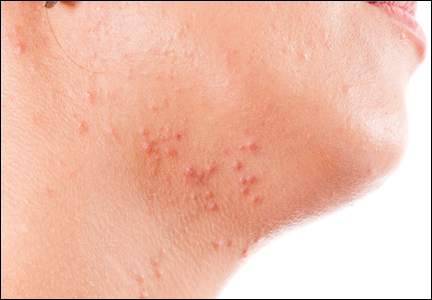
Atopic dermatitis (AD) is a disease that finally is coming of age in dermatology research. New topical agents and systemic biologic agents offer patients with AD other options for medical management. This article provides a practical review of prevention strategies and treatment guidelines for AD.
PREVENTION
Prevention strategies for AD have been largely unsuccessful in the past, which may relate to factors such as prenatal triggers.1 However, some newer interventional studies have shown some promise in AD prevention in specific settings. For example, a randomized trial of infants in the United States and United Kingdom at high risk for AD (ie, family history of atopy) reported that the AD risk was reduced by 50% when patients were treated with at least once-daily application of full-body emollients for 6 months (beginning by 3 weeks of life).2 The strategy of daily application of emollients for avoidance of AD in infants with a family history of AD is reasonable but may not offer lifetime prevention, and the benefit in children not from AD families is unknown.
Other trials to prevent AD have included usage of dust avoidance and dust covers for mattresses. This strategy showed modest benefit in reducing the incidence of atopic diatheses in the first year3 but did not gain endorsement by the most recent guidelines of the American Academy of Dermatology (AAD).4
Prenatal and postnatal (maternal and child) supplementation of Lactobacillus rhamnosus has shown promise in prevention.5 The exact regimen likely makes an impact on efficacy. An early study showed the usage of probiotics (eg, Lactobacillus reuteri) prenatally in pregnant women and postnatally in infants resulted in no reduction in occurrence of AD and possible reduction in IgE-associated AD.6 Kalliomäki et al7 demonstrated that L rhamnosus GG alone reduced AD by half in at-risk infants in a double-blind, placebo-controlled trial. On the other hand, Taylor et al8 performed a study of probiotic supplementation in which patients at high risk for AD developed higher rates of allergen sensitization. The most successful recent trial involved the randomization of 415 pregnant women to receive interventions from 36 weeks’ gestation until 3 months postpartum.9 The intervention was a randomized comparison of milk without probiotics versus a blend of probiotic milk containing L rhamnosus GG, Lactobacillus acidophilus La-5, and Bifidobacterium animalis subsp lactis Bb-12. At 6 years of age, 81 babies who consumed probiotic milk and 82 babies who consumed milk without probiotics were available for testing. The strategy caused a statistically significant reduction in AD in the complete case analysis (odds ratio, 0.48; 95% confidence interval, 0.25-0.92; P=.027; number needed to treat, 6). Sadly, other allergic diseases were not prevented in this study.9
MANAGEMENT OF AD
There currently is no cure or perfected prevention technique for AD. As a result, therapy focuses on avoiding triggers and alleviating symptoms.10 Recent guidelines from the AAD state that“[t]he ultimate judgment regarding the propriety of any specific therapy must be made by the physician and the patient in light of all the circumstances presented by the individual patient, and the known variability and biologic behavior of the disease.”11 Skin-directed therapies are the first line of treatment including emollients, gentle skin care, and topical medicaments. In AD, therapies are needed to reduce disease activity and flare severity, clear flares, and provide relief.
Parental education and written eczema action plans are recommended to help patients and parents/guardians follow recommended regimens12; Tollefson and Bruckner13 for the American Academy of Pediatrics provide an action plan to guide the care of children with atopic dermatitis that is simple, but many others exist online. The eczema action plan usually provides information on how to bathe and what to do when the skin is actively inflamed.
In 2014, a 4-part series of guidelines of care for the management of AD was published by the AAD, replacing prior guidelines.4,11,14,15 The following sections review some of the important parameters of care highlighted in these management guidelines.
Psychological Support
Appropriate psychological support for AD patients can be sought through counselors, therapists, psychiatrists, and support groups such as the National Eczema Association (https://nationaleczema.org/).
Education
Education is the leading form of medical therapy in patients with AD. Eczema schools are popular in Europe and are just beginning to form in the United States (http://tuh.templehealth.org/content/eczema_school.htm), which can be helpful to educate caregivers and patients with AD. Patient resources online and through support groups with an online presence, in-person meetings, and patient/family conventions can be helpful to AD patients. Often, an initial office visit with a dermatologist involves a review of avoidance of triggers, usage of gentle skin care including bland emollients, and therapeutic regimens for disease activity. This form of verbal education is to be paired with an eczema action plan, a written document that allows individuals to reference recommendations and share information with other caregivers.12,13,16
Emollients and Gentle Skin Care
Gentle skin care regimens, which includes the usage of synthetic cleansers with a low pH to help maintain the acidity (acid mantle) of the skin, seek to reduce irritation and have been rated as level IA (highest level) in recent AAD guidelines.14 Although bathing frequency has been emphasized in the guidelines, AD severity as reflected by SCORAD (SCORing Atopic Dermatitis) was not different for daily bathing versus twice weekly.17 The American Academy of Pediatrics recommended a skin care regimen of bathing every 2 to 3 days in lukewarm water for 10 to 15 minutes, followed by application of emollients that are fragrance free and have few preservatives.13 Topical emollients with additives such as colloidal oatmeal, avenanthramides, or ceramides can be used to enhance the skin barrier and are well tolerated in all age groups.18,19 Despite enhanced emollients, the therapy of AD still requires usage of prescription or over-the-counter TCs and/or topical calcineurin inhibitors (TCIs) in many cases.20
Topical Medication
Children have a relatively higher body surface area–to-weight ratio, allowing for greater potential absorption of topical medicaments and potential side effects from absorption. Types of vehicle, cost, site of application, and availability may impact patient and physician preference in choice of therapeutic topical agent.14
Topical Corticosteroids
Topical corticosteroids (TCs) are the mainstay of treatment for AD and have been used for more than 60 years.14,20 Topical corticosteroids provide anti-inflammatory effects on T cells, monocytes, and macrophages, producing altered cytokine activity locally. Topical corticosteroids inhibit collagen synthesis, potentially causing skin atrophy. They also inhibit IL-1, IL-2, IL-6, IFN-α, and tumor necrosis factor α.21 Topical corticosteroids are classified as class I (ultra-high potency) to class VII (low potency). In children, low-potency TCs generally are applied to the face, intertriginous areas, groin, and genitalia, and mid-potency corticosteroids are applied to the body, arms, and legs. An even higher-strength agent can be prescribed as a rescue medication in severe cases. After clearance with once- or twice-daily therapy, twice-weekly usage can benefit disease activity.22 Topical corticosteroids reduce inflammation as well as Staphylococcus aureus load through inhibition of cytokines that inhibit antimicrobial peptides. Topical corticosteroids have been endorsed as level IA evidence therapy by the AAD guidelines.14
Topical corticosteroids, particularly prolonged usage of mid- to high-potency products, have been associated with side effects such as skin atrophy, striae, telangiectases, hypopigmentation, rosacea, acneiform eruptions, focal hypertrichosis, perioral dermatitis, and acne23; potential systemic side effects include hypothalamic-pituitary-adrenal axis suppression, cataracts, glaucoma (with periocular application), Cushing syndrome, hyperglycemia, hypertension,23 and growth retardation.14 Long-term corticosteroid therapy is associated with tachyphylaxis and potential rebound of disease with discontinuation.24 Based on the potential risk of side effects with TCs, the least potent product for the shortest time needed is recommended, with special care for thin skin. Discontinuation when clearance occurs is advised. Allergy to TCs and/or vehicle ingredients such as propylene glycol should be suspected in severe unremitting cases.14 A recent registry review of children screened for contact dermatitis demonstrated that children with AD had higher sensitization to the steroid tixocortol pivalate.25
Topical Calcineurin Inhibitors
Topical calcineurin inhibitors include pimecrolimus cream 1%, which is approved for mild to moderate AD in adults and children 2 years and older, and tacrolimus ointment 0.03% and 0.1%, which are approved for moderate to severe AD in adults and children aged 2 to 15 years (0.03% formulation only). Topical calcineurin inhibitors can be used as second-line agents in AD in patients who have inadequate response to TCs or who may not be able to use TCs due to the disease site.10,13,14 Guidelines from the AAD also have endorsed TCIs as level IA evidence for steroid-sparing agents.
Concerns about the reporting of cancers and lymphomas prompted the US Food and Drug Administration to issue a black box warning on TCIs more than 10 years ago. Pimecrolimus, which has little absorption and no notable immunosuppressive effects, has been used without detrimental effect on vaccination and delayed-type hypersensitivities, but many decades of data are lacking.10,13,14,17,26-29 Topical calcineurin inhibitors can be used as steroid-sparing agents in lieu of corticosteroids in specific locations such as the face and eyelids and for long-term suppressive therapy twice weekly.30 Intermittent usage and cycling with corticosteroids is advisable,28 but usage intermittently beyond 1 year has not been evaluated.
Topical calcineurin inhibitors are recommended as effective for acute and chronic AD. Their use as maintenance therapy in adults and children, for AD recalcitrant to steroids, for AD in sensitive areas, for steroid-induced atrophy, and for long-term uninterrupted topical steroid usage carries a level IA evidence recommendation. Furthermore, the AAD guidelines have recommended TCIs as steroid-sparing agents with level IA evidence and off-label use of TCIs in children younger than 2 years with level IA evidence. Pretreatment with TCs to reduce stinging has level IIB evidence. Usage for flare prevention is level IA evidence. Routine blood monitoring of TCI-treated patients was not recommended; in fact, the AAD guidelines provided this recommendation as level IA evidence against routine laboratory monitoring of TCI-treated patients.14
Topical Antibiotics
Topical antibiotics are indicated for the therapy of impetigo and can be used in the setting of impetiginized AD in conjunction with TCs. Recent AAD guidelines suggested against routine usage of topical antistaphylococcal agents as level IA evidence.14 There is one study supporting usage of topical mupirocin in addition to TCs to heal children with eczema area and severity index scores more than 7 more rapidly in the first week of AD therapy, but in the same study, additive benefit was not demonstrated in AD beyond the first week.31 There also are data supporting usage of intranasal mupirocin adjunctively with bleach baths in patients with moderate to severe AD, which was rated as level IIB evidence in the AAD guidelines.14,32 There are limited data on the long-term utility of topical anti-infectives in AD. The risks of long-term usage could include resistance formation to agents such as mupirocin, contact dermatitis, and lack of efficacy.
Additional Therapeutics
Wet Wraps
Penetration through the stratum corneum is needed for drug activity in AD. Penetration can be enhanced using wet wrap therapy or using ointments, which produce higher relative potency.13 Wet wraps overlying a dilute topical medicament have been described as effective in AD and are recommended in AAD guidelines as level IIB evidence.14 Different wet wrap techniques can be used, including wet pajamas covered by dry pajamas or saline-soaked gauze wrapped around the affected areas and then dry gauze applied over the wet gauze. The methodology used should be tailored to the patient as well as to whether the individual is an inpatient or outpatient.
Bleach Baths
Dilute sodium hypochlorite solution 0.005% (one-quarter cup bleach in 20 gallons of water) has been demonstrated to be beneficial in reduction of disease activity in AD patients with recurrent bacterial infections.32 This simple technique in addition to intranasal mupirocin can reduce AD severity and improve quality of life and is the only ongoing S aureus therapeutic management endorsed by the AAD guidelines for the management of AD.14,32
Topical and Oral Delivery
Antihistamines
Topical antihistamines are ineffective in AD. Oral antihistamines can be used to reduce pruritus and are most effective when given as sedating agents for sleep enhancement but may be given as nonsedating agents for patients with concomitant allergic disorders such as allergic rhinoconjunctivitis. Paradoxical hyperreactivity with sedating antihistamines is not uncommon in small children, and sedating antihistamine usage should be discontinued in these instances.13 Parents of children with AD have reported giving the child antihistamines to sleep was helpful, as well as putting on creams, using special clothes (eg, all cotton), and keeping the room cool.33 There is level IIIC evidence against use of systemic antihistamines and level IIA evidence for sedating and nonsedating, according to the AAD guidelines.14
Systemic Therapeutics
Oral therapeutics range from oral antihistamines to oral antibiotics and immunosuppressive medications. Oral antibiotics (level IIB evidence) are reserved for superinfected AD, which is not easily defined for the following reasons: there is no consensus definition of superinfected AD; the majority of active AD lesions when cultured will demonstrate S aureus growth; and most AD lesions ooze, thereby creating the appearance of superinfection. In real-world practice, superinfection can be diagnosed based on the presence of pustules; furuncles; or signs of infection such as tracking erythema, tenderness, severe erosions, or maceration. Clinical judgment is always required.
The immunosuppressive medications used in AD include leukotriene inhibitors, which rarely are effective for AD.34 More effective systemic agents for AD include cyclosporine (level I to IIB evidence), azathioprine (level IIB evidence), mycophenolate mofetil (level IIIC evidence), and methotrexate (level IIB evidence). These agents are indicated for pediatric or adult patients when topical agents and/or phototherapy have failed.15 Monitoring these agents for side effects includes ongoing evaluation for renal and liver toxicity. Short courses (ie, 6 months) are preferred to minimize side effects.35
Dupilumab, an injectable AD therapy, is approved in the United States. This agent is injected every 2 weeks and binds to the IL-4Rα shared by IL-4 and IL-13. In 4 weeks of monotherapy, 85% of adult patients treated had 50% or greater clearance.36 Recently published consensus opinion from the International Eczema Council recommends assessment of a variety of factors before initiating systemic therapy including comorbid illnesses such as contact allergy, trigger avoidance, superinfection, and impact on quality of life.37
Oral Corticosteroids
Systemic corticosteroids clear patients quickly but provide no sustained improvement; in fact, many patients rebound or have tachyphylaxis. Although short-term corticosteroid usage can break the itch-scratch cycle, long-term usage is associated with osteoporosis, Cushing syndrome, and aseptic necrosis of the femoral head. Decreased linear growth will occur during therapy in children; therefore, systemic steroids are not recommended in children with AD, except for additional or comorbid conditions (eg, asthma or contact dermatitis).4
Phototherapy
Phototherapy has been recommended in the AAD guidelines as a second-line treatment after failure of first-line agents (ie, TCIs and TCs) for clearance and or maintenance and should be tailored to the patient’s skin tone by an experienced physician. Narrowband UVB phototherapy may act through the suppression of T-cell activity in the skin and possibly via suppression of staphylococcal superantigens; however, many phototherapy types have been described for AD.38,39 Usage can be effective in school-aged children and teenagers but may be limited due to school attendance. Phototherapy was graded as level IIB evidence in the AAD guidelines.15 Side effects include aggravation of AD by exposure to heat and UV light, actinic damage, tenderness, erythema, pruritus, burning, and stinging. Lentigines; skin cancers (melanoma and nonmelanoma); folliculitis; and ocular toxicity, especially cataracts, can occur.15 Children younger than 6 years will find it difficult to stand in a phototherapy booth and may be poor candidates.15,38,39
Complementary and Alternative Medicine
Complementary and alternative medicine (CAM) also has been used for AD in the United States. In a review of the 2007 National Health Interview Survey of 9417 children aged 0 to 17 years, CAM was used for AD by 0.99% of children. Some CAM techniques were associated with worsening severity of AD, including herbal therapy, vitamins, homeopathic agents, diet, and movement techniques.40 Usage of Chinese herbal medications for AD can be associated with liver toxicity.41 Only one CAM therapy—massage therapy—has some mild supportive data.42
Allergen Avoidance and Diet
Bronsnick et al43 discussed the possible benefit of prenatal and postnatal probiotics for prevention of AD, which were not supported in the AAD guidelines for management of AD4; postnatal prebiotic supplementation; and exclusive breastfeeding and/or supplementation with hydrolyzed formula in at-risk children. Elimination diets for children and mothers were not recommended. The authors found no beneficial role of supplements including vitamin D, selenium, fish oil, borage oil, and zinc sulfate.43
A National Institute of Allergy and Infectious Diseases consensus group recommended avoidance of proven but not random elimination of food allergens in AD, asthma, and/or eosinophilic esophagitis.44 Restricted maternal diet was not recommended, and breastfeeding exclusively for the first 4 to 6 months was recommended. Hydrolyzed formulas were suggested as a possible preventive strategy in at-risk infants as a breastfeeding alternative, with cost of these formulas being a problem.44
In children younger than 5 years, food allergy screening for the most common allergens (eg, milk, eggs, peanuts, wheat, soy) should be considered in children with persistent unremitting dermatitis and/or known food challenge–induced reactions.4 Conservative measures to avoid house dust mite exposure in known sensitized individuals including dust covers for pillows and mattresses may be beneficial.4,45
Emerging Therapies
Recently approved therapies include better-targeted agents that appear to have a reasonable safety profile and may fulfill unmet needs in AD care. Of these agents, crisaborole, a topical boron-based phosphodiesterase 4 inhibitor, was approved in December 2016 for mild to moderate AD in patients 2 years and older.Topically, this agent seems to be efficacious in the absence of notable carcinogenicity.46
The systemic (injectable) biologic agent dupilumab was approved in March 2017 for moderate to severe AD. Phase 3 studies in adults with AD showed excellent success in adults with moderate to severe AD.37 This agent is a monoclonal antibody targeted at blockade of the crucial atopic inflammatory triggering pathway via blockade of the IL-4A receptor site, targeting IL-4 and IL-13 activity.36,47 There are many medications in the pipeline, which Renert-Yuval and Guttman-Yassky48 review. However, an overview of the landscape demonstrates that Janus kinase (JAK) inhibitors49 and biologic medications in addition to dupilumab affecting targeted inflammatory cascades in AD are in development. In particular, the JAK inhibitors appear promising due to availability both as oral and topical agents.49
Need for Ongoing Care and Monitoring
Atopic dermatitis is a chronic inflammatory skin disorder with a genetic basis. Once initiated, the process of AD may persist throughout the patient’s life and become a systemic disorder with comorbidities including sleep disturbance, reduced quality of life, and cardiovascular disease.50 Ongoing management of AD includes topical reduction in irritants and triggers, topical medicaments, and management of pruritus and infections. At this time, emollients and irritant avoidance paired with judicious topical medicaments including TCs and second-line or site-specific (eg, eyelids) usage of TCIs or phosphodiesterase 4 inhibitors remain the backbone of therapy. Ongoing review of therapeutics for associated morbidities is underway, which may guide future therapeutic interventions into AD. The future of prevention and therapy look bright, but time will tell.
- Kelleher M, Dunn-Galvin A, Hourihane JO, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015;135:930-935.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Tsitoura S, Nestoridou K, Botis P, et al. Randomized trial to prevent sensitization to mite allergens in toddlers and preschoolers by allergen reduction and education: one-year results. Arch Pediatr Adolesc Med. 2002;156:1021-1027.
- Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218-1233.
- Foolad N, Brezinski EA, Chase EP, et al. Effect of nutrient supplementation on atopic dermatitis in children: a systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013;149:350-355.
- Abrahamsson TR, Jakobsson T, Böttcher MF, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1174-1180.
- Kalliomäki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076-1079.
- Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184-191.
- Simpson MR, Dotterud CK, Storrø O, et al. Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol. 2015;15:13. doi:10.1186/s12895-015-0030-1.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Silverberg NB. Creating an action plan for eczema patients. Cutis. 2015;96:362-363.
- Tollefson MM, Bruckner AL; Section on Dermatology. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Shi VY, Nanda S, Lee K, et al. Improving patient education with an eczema action plan: a randomized controlled trial. JAMA Dermatol. 2013;149:481-483.
- Koutroulis I, Petrova K, Kratimenos P, et al. Frequency of bathing in the management of atopic dermatitis: to bathe or not to bathe? Clin Pediatr (Phila). 2014;53:677-681.
- Fowler JF, Nebus J, Wallo W, et al. Colloidal oatmeal formulations as adjunct treatments in atopic dermatitis. J Drugs Dermatol. 2012;11:804-807.
- Fowler J Jr, Silverberg N. Active naturals have a key role in atopic dermatitis. Semin Cutan Med Surg. 2008;27:8-10.
- Eichenfield LF. Consensus guidelines in diagnosis and treatment of atopic dermatitis. Allergy. 2004;59:86-92.
- Nghiem P, Pearson G, Langley RG. Tacrolimus and pimecrolimus: from clever prokaryotes to inhibiting calcineurin and treating atopic dermatitis. J Am Acad Dermatol. 2002;46:228-241.
- Schmitt J. Commentary: eczema and cancer risk. Br J Dermatol. 2011;165:463-464.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takahashi-Ando N, Jones MA, Fujisawa S, et al. Patient-reported outcomes after discontinuation of long-term topical corticosteroid treatment for atopic dermatitis: a targeted cross-sectional survey. Drug Healthc Patient Saf. 2015;7:57-62.
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric contact dermatitis registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770.
- Werfel T. Topical use of pimecrolimus in atopic dermatitis: update on the safety and efficacy. J Dtsch Dermatol Ges. 2009;7:739-742.
- Wahn U, Bos JD, Goodfield M, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics. 2002;110(1, pt 1):E2.
- Berger TG, Duvic M, Van Voorhees AS, et al; American Academy of Dermatology Association Task Force. The use of topical calcineurin inhibitors in dermatology: safety concerns. report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Thaçi D, Reitamo S, Gonzalez Ensenat MA, et al. Proactive disease management with 0.03% tacrolimus ointment for children with atopic dermatitis: results of a randomized, multicentre, comparative study. Br J Dermatol. 2008;159:1348-1356.
- Gong JQ, Lin L, Lin T, et al. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. Br J Dermatol. 2006;155:680-687.
- Huang JT, Abrams M, Tlougan B, et al. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123:E808-E814.
- Reid P, Lewis-Jones MS. Sleep difficulties and their management in preschoolers with atopic eczema. Clin Exp Dermatol. 1995;20:38-41.
- Silverberg NB, Paller AS. Leukotriene receptor antagonists are ineffective for severe atopic dermatitis. J Am Acad Dermatol. 2004;50:485-486.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. New York, NY: Elsevier Saunders; 2013.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Simpson EL, Bruin-Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? recommendations from an expert panel of the International Eczema Council [published online August 10, 2017]. J Am Acad Dermatol. doi:10.1016/j.jaad.2017.06.042.
- Veith W, DeLeo V, Silverberg N. Medical phototherapy in childhood skin diseases. Minerva Pediatr. 2011;63:327-333.
- Song E, Reja D, Silverberg N, et al. Phototherapy: kids are not just little people. Clin Dermatol. 2015;33:672-680.
- Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25:246-254.
- Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol. 2015;89:851-865.
- Schachner L, Field T, Hernandez-Reif M, et al. Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr Dermatol. 1998;15:390-395.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
- Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. Nutr Res. 2011;31:61-75.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Hanifin JM, Chan SC, Cheng JB, et al. Type phosphodiesterase inhibitors have clinical and in vitro anti-inflammatory effects in atopic dermatitis. J Invest Dermatol. 1996;107:51-56.
- Boguniewicz M, Leung DY. Targeted therapy for allergic diseases: at the intersection of cutting-edge science and clinical practice. J Allergy Clin Immunol. 2015;135:354-356.
- Renert-Yuval Y, Guttman-Yassky E. Systemic therapies in atopic dermatitis: the pipeline. Clin Dermatol. 2017;35:387-397.
- Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76:736-744.
- Brunner PM, Silverberg JI, Guttman-Yassky E, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol. 2017;137:18-25.
Atopic dermatitis (AD) is a disease that finally is coming of age in dermatology research. New topical agents and systemic biologic agents offer patients with AD other options for medical management. This article provides a practical review of prevention strategies and treatment guidelines for AD.
PREVENTION
Prevention strategies for AD have been largely unsuccessful in the past, which may relate to factors such as prenatal triggers.1 However, some newer interventional studies have shown some promise in AD prevention in specific settings. For example, a randomized trial of infants in the United States and United Kingdom at high risk for AD (ie, family history of atopy) reported that the AD risk was reduced by 50% when patients were treated with at least once-daily application of full-body emollients for 6 months (beginning by 3 weeks of life).2 The strategy of daily application of emollients for avoidance of AD in infants with a family history of AD is reasonable but may not offer lifetime prevention, and the benefit in children not from AD families is unknown.
Other trials to prevent AD have included usage of dust avoidance and dust covers for mattresses. This strategy showed modest benefit in reducing the incidence of atopic diatheses in the first year3 but did not gain endorsement by the most recent guidelines of the American Academy of Dermatology (AAD).4
Prenatal and postnatal (maternal and child) supplementation of Lactobacillus rhamnosus has shown promise in prevention.5 The exact regimen likely makes an impact on efficacy. An early study showed the usage of probiotics (eg, Lactobacillus reuteri) prenatally in pregnant women and postnatally in infants resulted in no reduction in occurrence of AD and possible reduction in IgE-associated AD.6 Kalliomäki et al7 demonstrated that L rhamnosus GG alone reduced AD by half in at-risk infants in a double-blind, placebo-controlled trial. On the other hand, Taylor et al8 performed a study of probiotic supplementation in which patients at high risk for AD developed higher rates of allergen sensitization. The most successful recent trial involved the randomization of 415 pregnant women to receive interventions from 36 weeks’ gestation until 3 months postpartum.9 The intervention was a randomized comparison of milk without probiotics versus a blend of probiotic milk containing L rhamnosus GG, Lactobacillus acidophilus La-5, and Bifidobacterium animalis subsp lactis Bb-12. At 6 years of age, 81 babies who consumed probiotic milk and 82 babies who consumed milk without probiotics were available for testing. The strategy caused a statistically significant reduction in AD in the complete case analysis (odds ratio, 0.48; 95% confidence interval, 0.25-0.92; P=.027; number needed to treat, 6). Sadly, other allergic diseases were not prevented in this study.9
MANAGEMENT OF AD
There currently is no cure or perfected prevention technique for AD. As a result, therapy focuses on avoiding triggers and alleviating symptoms.10 Recent guidelines from the AAD state that“[t]he ultimate judgment regarding the propriety of any specific therapy must be made by the physician and the patient in light of all the circumstances presented by the individual patient, and the known variability and biologic behavior of the disease.”11 Skin-directed therapies are the first line of treatment including emollients, gentle skin care, and topical medicaments. In AD, therapies are needed to reduce disease activity and flare severity, clear flares, and provide relief.
Parental education and written eczema action plans are recommended to help patients and parents/guardians follow recommended regimens12; Tollefson and Bruckner13 for the American Academy of Pediatrics provide an action plan to guide the care of children with atopic dermatitis that is simple, but many others exist online. The eczema action plan usually provides information on how to bathe and what to do when the skin is actively inflamed.
In 2014, a 4-part series of guidelines of care for the management of AD was published by the AAD, replacing prior guidelines.4,11,14,15 The following sections review some of the important parameters of care highlighted in these management guidelines.
Psychological Support
Appropriate psychological support for AD patients can be sought through counselors, therapists, psychiatrists, and support groups such as the National Eczema Association (https://nationaleczema.org/).
Education
Education is the leading form of medical therapy in patients with AD. Eczema schools are popular in Europe and are just beginning to form in the United States (http://tuh.templehealth.org/content/eczema_school.htm), which can be helpful to educate caregivers and patients with AD. Patient resources online and through support groups with an online presence, in-person meetings, and patient/family conventions can be helpful to AD patients. Often, an initial office visit with a dermatologist involves a review of avoidance of triggers, usage of gentle skin care including bland emollients, and therapeutic regimens for disease activity. This form of verbal education is to be paired with an eczema action plan, a written document that allows individuals to reference recommendations and share information with other caregivers.12,13,16
Emollients and Gentle Skin Care
Gentle skin care regimens, which includes the usage of synthetic cleansers with a low pH to help maintain the acidity (acid mantle) of the skin, seek to reduce irritation and have been rated as level IA (highest level) in recent AAD guidelines.14 Although bathing frequency has been emphasized in the guidelines, AD severity as reflected by SCORAD (SCORing Atopic Dermatitis) was not different for daily bathing versus twice weekly.17 The American Academy of Pediatrics recommended a skin care regimen of bathing every 2 to 3 days in lukewarm water for 10 to 15 minutes, followed by application of emollients that are fragrance free and have few preservatives.13 Topical emollients with additives such as colloidal oatmeal, avenanthramides, or ceramides can be used to enhance the skin barrier and are well tolerated in all age groups.18,19 Despite enhanced emollients, the therapy of AD still requires usage of prescription or over-the-counter TCs and/or topical calcineurin inhibitors (TCIs) in many cases.20
Topical Medication
Children have a relatively higher body surface area–to-weight ratio, allowing for greater potential absorption of topical medicaments and potential side effects from absorption. Types of vehicle, cost, site of application, and availability may impact patient and physician preference in choice of therapeutic topical agent.14
Topical Corticosteroids
Topical corticosteroids (TCs) are the mainstay of treatment for AD and have been used for more than 60 years.14,20 Topical corticosteroids provide anti-inflammatory effects on T cells, monocytes, and macrophages, producing altered cytokine activity locally. Topical corticosteroids inhibit collagen synthesis, potentially causing skin atrophy. They also inhibit IL-1, IL-2, IL-6, IFN-α, and tumor necrosis factor α.21 Topical corticosteroids are classified as class I (ultra-high potency) to class VII (low potency). In children, low-potency TCs generally are applied to the face, intertriginous areas, groin, and genitalia, and mid-potency corticosteroids are applied to the body, arms, and legs. An even higher-strength agent can be prescribed as a rescue medication in severe cases. After clearance with once- or twice-daily therapy, twice-weekly usage can benefit disease activity.22 Topical corticosteroids reduce inflammation as well as Staphylococcus aureus load through inhibition of cytokines that inhibit antimicrobial peptides. Topical corticosteroids have been endorsed as level IA evidence therapy by the AAD guidelines.14
Topical corticosteroids, particularly prolonged usage of mid- to high-potency products, have been associated with side effects such as skin atrophy, striae, telangiectases, hypopigmentation, rosacea, acneiform eruptions, focal hypertrichosis, perioral dermatitis, and acne23; potential systemic side effects include hypothalamic-pituitary-adrenal axis suppression, cataracts, glaucoma (with periocular application), Cushing syndrome, hyperglycemia, hypertension,23 and growth retardation.14 Long-term corticosteroid therapy is associated with tachyphylaxis and potential rebound of disease with discontinuation.24 Based on the potential risk of side effects with TCs, the least potent product for the shortest time needed is recommended, with special care for thin skin. Discontinuation when clearance occurs is advised. Allergy to TCs and/or vehicle ingredients such as propylene glycol should be suspected in severe unremitting cases.14 A recent registry review of children screened for contact dermatitis demonstrated that children with AD had higher sensitization to the steroid tixocortol pivalate.25
Topical Calcineurin Inhibitors
Topical calcineurin inhibitors include pimecrolimus cream 1%, which is approved for mild to moderate AD in adults and children 2 years and older, and tacrolimus ointment 0.03% and 0.1%, which are approved for moderate to severe AD in adults and children aged 2 to 15 years (0.03% formulation only). Topical calcineurin inhibitors can be used as second-line agents in AD in patients who have inadequate response to TCs or who may not be able to use TCs due to the disease site.10,13,14 Guidelines from the AAD also have endorsed TCIs as level IA evidence for steroid-sparing agents.
Concerns about the reporting of cancers and lymphomas prompted the US Food and Drug Administration to issue a black box warning on TCIs more than 10 years ago. Pimecrolimus, which has little absorption and no notable immunosuppressive effects, has been used without detrimental effect on vaccination and delayed-type hypersensitivities, but many decades of data are lacking.10,13,14,17,26-29 Topical calcineurin inhibitors can be used as steroid-sparing agents in lieu of corticosteroids in specific locations such as the face and eyelids and for long-term suppressive therapy twice weekly.30 Intermittent usage and cycling with corticosteroids is advisable,28 but usage intermittently beyond 1 year has not been evaluated.
Topical calcineurin inhibitors are recommended as effective for acute and chronic AD. Their use as maintenance therapy in adults and children, for AD recalcitrant to steroids, for AD in sensitive areas, for steroid-induced atrophy, and for long-term uninterrupted topical steroid usage carries a level IA evidence recommendation. Furthermore, the AAD guidelines have recommended TCIs as steroid-sparing agents with level IA evidence and off-label use of TCIs in children younger than 2 years with level IA evidence. Pretreatment with TCs to reduce stinging has level IIB evidence. Usage for flare prevention is level IA evidence. Routine blood monitoring of TCI-treated patients was not recommended; in fact, the AAD guidelines provided this recommendation as level IA evidence against routine laboratory monitoring of TCI-treated patients.14
Topical Antibiotics
Topical antibiotics are indicated for the therapy of impetigo and can be used in the setting of impetiginized AD in conjunction with TCs. Recent AAD guidelines suggested against routine usage of topical antistaphylococcal agents as level IA evidence.14 There is one study supporting usage of topical mupirocin in addition to TCs to heal children with eczema area and severity index scores more than 7 more rapidly in the first week of AD therapy, but in the same study, additive benefit was not demonstrated in AD beyond the first week.31 There also are data supporting usage of intranasal mupirocin adjunctively with bleach baths in patients with moderate to severe AD, which was rated as level IIB evidence in the AAD guidelines.14,32 There are limited data on the long-term utility of topical anti-infectives in AD. The risks of long-term usage could include resistance formation to agents such as mupirocin, contact dermatitis, and lack of efficacy.
Additional Therapeutics
Wet Wraps
Penetration through the stratum corneum is needed for drug activity in AD. Penetration can be enhanced using wet wrap therapy or using ointments, which produce higher relative potency.13 Wet wraps overlying a dilute topical medicament have been described as effective in AD and are recommended in AAD guidelines as level IIB evidence.14 Different wet wrap techniques can be used, including wet pajamas covered by dry pajamas or saline-soaked gauze wrapped around the affected areas and then dry gauze applied over the wet gauze. The methodology used should be tailored to the patient as well as to whether the individual is an inpatient or outpatient.
Bleach Baths
Dilute sodium hypochlorite solution 0.005% (one-quarter cup bleach in 20 gallons of water) has been demonstrated to be beneficial in reduction of disease activity in AD patients with recurrent bacterial infections.32 This simple technique in addition to intranasal mupirocin can reduce AD severity and improve quality of life and is the only ongoing S aureus therapeutic management endorsed by the AAD guidelines for the management of AD.14,32
Topical and Oral Delivery
Antihistamines
Topical antihistamines are ineffective in AD. Oral antihistamines can be used to reduce pruritus and are most effective when given as sedating agents for sleep enhancement but may be given as nonsedating agents for patients with concomitant allergic disorders such as allergic rhinoconjunctivitis. Paradoxical hyperreactivity with sedating antihistamines is not uncommon in small children, and sedating antihistamine usage should be discontinued in these instances.13 Parents of children with AD have reported giving the child antihistamines to sleep was helpful, as well as putting on creams, using special clothes (eg, all cotton), and keeping the room cool.33 There is level IIIC evidence against use of systemic antihistamines and level IIA evidence for sedating and nonsedating, according to the AAD guidelines.14
Systemic Therapeutics
Oral therapeutics range from oral antihistamines to oral antibiotics and immunosuppressive medications. Oral antibiotics (level IIB evidence) are reserved for superinfected AD, which is not easily defined for the following reasons: there is no consensus definition of superinfected AD; the majority of active AD lesions when cultured will demonstrate S aureus growth; and most AD lesions ooze, thereby creating the appearance of superinfection. In real-world practice, superinfection can be diagnosed based on the presence of pustules; furuncles; or signs of infection such as tracking erythema, tenderness, severe erosions, or maceration. Clinical judgment is always required.
The immunosuppressive medications used in AD include leukotriene inhibitors, which rarely are effective for AD.34 More effective systemic agents for AD include cyclosporine (level I to IIB evidence), azathioprine (level IIB evidence), mycophenolate mofetil (level IIIC evidence), and methotrexate (level IIB evidence). These agents are indicated for pediatric or adult patients when topical agents and/or phototherapy have failed.15 Monitoring these agents for side effects includes ongoing evaluation for renal and liver toxicity. Short courses (ie, 6 months) are preferred to minimize side effects.35
Dupilumab, an injectable AD therapy, is approved in the United States. This agent is injected every 2 weeks and binds to the IL-4Rα shared by IL-4 and IL-13. In 4 weeks of monotherapy, 85% of adult patients treated had 50% or greater clearance.36 Recently published consensus opinion from the International Eczema Council recommends assessment of a variety of factors before initiating systemic therapy including comorbid illnesses such as contact allergy, trigger avoidance, superinfection, and impact on quality of life.37
Oral Corticosteroids
Systemic corticosteroids clear patients quickly but provide no sustained improvement; in fact, many patients rebound or have tachyphylaxis. Although short-term corticosteroid usage can break the itch-scratch cycle, long-term usage is associated with osteoporosis, Cushing syndrome, and aseptic necrosis of the femoral head. Decreased linear growth will occur during therapy in children; therefore, systemic steroids are not recommended in children with AD, except for additional or comorbid conditions (eg, asthma or contact dermatitis).4
Phototherapy
Phototherapy has been recommended in the AAD guidelines as a second-line treatment after failure of first-line agents (ie, TCIs and TCs) for clearance and or maintenance and should be tailored to the patient’s skin tone by an experienced physician. Narrowband UVB phototherapy may act through the suppression of T-cell activity in the skin and possibly via suppression of staphylococcal superantigens; however, many phototherapy types have been described for AD.38,39 Usage can be effective in school-aged children and teenagers but may be limited due to school attendance. Phototherapy was graded as level IIB evidence in the AAD guidelines.15 Side effects include aggravation of AD by exposure to heat and UV light, actinic damage, tenderness, erythema, pruritus, burning, and stinging. Lentigines; skin cancers (melanoma and nonmelanoma); folliculitis; and ocular toxicity, especially cataracts, can occur.15 Children younger than 6 years will find it difficult to stand in a phototherapy booth and may be poor candidates.15,38,39
Complementary and Alternative Medicine
Complementary and alternative medicine (CAM) also has been used for AD in the United States. In a review of the 2007 National Health Interview Survey of 9417 children aged 0 to 17 years, CAM was used for AD by 0.99% of children. Some CAM techniques were associated with worsening severity of AD, including herbal therapy, vitamins, homeopathic agents, diet, and movement techniques.40 Usage of Chinese herbal medications for AD can be associated with liver toxicity.41 Only one CAM therapy—massage therapy—has some mild supportive data.42
Allergen Avoidance and Diet
Bronsnick et al43 discussed the possible benefit of prenatal and postnatal probiotics for prevention of AD, which were not supported in the AAD guidelines for management of AD4; postnatal prebiotic supplementation; and exclusive breastfeeding and/or supplementation with hydrolyzed formula in at-risk children. Elimination diets for children and mothers were not recommended. The authors found no beneficial role of supplements including vitamin D, selenium, fish oil, borage oil, and zinc sulfate.43
A National Institute of Allergy and Infectious Diseases consensus group recommended avoidance of proven but not random elimination of food allergens in AD, asthma, and/or eosinophilic esophagitis.44 Restricted maternal diet was not recommended, and breastfeeding exclusively for the first 4 to 6 months was recommended. Hydrolyzed formulas were suggested as a possible preventive strategy in at-risk infants as a breastfeeding alternative, with cost of these formulas being a problem.44
In children younger than 5 years, food allergy screening for the most common allergens (eg, milk, eggs, peanuts, wheat, soy) should be considered in children with persistent unremitting dermatitis and/or known food challenge–induced reactions.4 Conservative measures to avoid house dust mite exposure in known sensitized individuals including dust covers for pillows and mattresses may be beneficial.4,45
Emerging Therapies
Recently approved therapies include better-targeted agents that appear to have a reasonable safety profile and may fulfill unmet needs in AD care. Of these agents, crisaborole, a topical boron-based phosphodiesterase 4 inhibitor, was approved in December 2016 for mild to moderate AD in patients 2 years and older.Topically, this agent seems to be efficacious in the absence of notable carcinogenicity.46
The systemic (injectable) biologic agent dupilumab was approved in March 2017 for moderate to severe AD. Phase 3 studies in adults with AD showed excellent success in adults with moderate to severe AD.37 This agent is a monoclonal antibody targeted at blockade of the crucial atopic inflammatory triggering pathway via blockade of the IL-4A receptor site, targeting IL-4 and IL-13 activity.36,47 There are many medications in the pipeline, which Renert-Yuval and Guttman-Yassky48 review. However, an overview of the landscape demonstrates that Janus kinase (JAK) inhibitors49 and biologic medications in addition to dupilumab affecting targeted inflammatory cascades in AD are in development. In particular, the JAK inhibitors appear promising due to availability both as oral and topical agents.49
Need for Ongoing Care and Monitoring
Atopic dermatitis is a chronic inflammatory skin disorder with a genetic basis. Once initiated, the process of AD may persist throughout the patient’s life and become a systemic disorder with comorbidities including sleep disturbance, reduced quality of life, and cardiovascular disease.50 Ongoing management of AD includes topical reduction in irritants and triggers, topical medicaments, and management of pruritus and infections. At this time, emollients and irritant avoidance paired with judicious topical medicaments including TCs and second-line or site-specific (eg, eyelids) usage of TCIs or phosphodiesterase 4 inhibitors remain the backbone of therapy. Ongoing review of therapeutics for associated morbidities is underway, which may guide future therapeutic interventions into AD. The future of prevention and therapy look bright, but time will tell.
Atopic dermatitis (AD) is a disease that finally is coming of age in dermatology research. New topical agents and systemic biologic agents offer patients with AD other options for medical management. This article provides a practical review of prevention strategies and treatment guidelines for AD.
PREVENTION
Prevention strategies for AD have been largely unsuccessful in the past, which may relate to factors such as prenatal triggers.1 However, some newer interventional studies have shown some promise in AD prevention in specific settings. For example, a randomized trial of infants in the United States and United Kingdom at high risk for AD (ie, family history of atopy) reported that the AD risk was reduced by 50% when patients were treated with at least once-daily application of full-body emollients for 6 months (beginning by 3 weeks of life).2 The strategy of daily application of emollients for avoidance of AD in infants with a family history of AD is reasonable but may not offer lifetime prevention, and the benefit in children not from AD families is unknown.
Other trials to prevent AD have included usage of dust avoidance and dust covers for mattresses. This strategy showed modest benefit in reducing the incidence of atopic diatheses in the first year3 but did not gain endorsement by the most recent guidelines of the American Academy of Dermatology (AAD).4
Prenatal and postnatal (maternal and child) supplementation of Lactobacillus rhamnosus has shown promise in prevention.5 The exact regimen likely makes an impact on efficacy. An early study showed the usage of probiotics (eg, Lactobacillus reuteri) prenatally in pregnant women and postnatally in infants resulted in no reduction in occurrence of AD and possible reduction in IgE-associated AD.6 Kalliomäki et al7 demonstrated that L rhamnosus GG alone reduced AD by half in at-risk infants in a double-blind, placebo-controlled trial. On the other hand, Taylor et al8 performed a study of probiotic supplementation in which patients at high risk for AD developed higher rates of allergen sensitization. The most successful recent trial involved the randomization of 415 pregnant women to receive interventions from 36 weeks’ gestation until 3 months postpartum.9 The intervention was a randomized comparison of milk without probiotics versus a blend of probiotic milk containing L rhamnosus GG, Lactobacillus acidophilus La-5, and Bifidobacterium animalis subsp lactis Bb-12. At 6 years of age, 81 babies who consumed probiotic milk and 82 babies who consumed milk without probiotics were available for testing. The strategy caused a statistically significant reduction in AD in the complete case analysis (odds ratio, 0.48; 95% confidence interval, 0.25-0.92; P=.027; number needed to treat, 6). Sadly, other allergic diseases were not prevented in this study.9
MANAGEMENT OF AD
There currently is no cure or perfected prevention technique for AD. As a result, therapy focuses on avoiding triggers and alleviating symptoms.10 Recent guidelines from the AAD state that“[t]he ultimate judgment regarding the propriety of any specific therapy must be made by the physician and the patient in light of all the circumstances presented by the individual patient, and the known variability and biologic behavior of the disease.”11 Skin-directed therapies are the first line of treatment including emollients, gentle skin care, and topical medicaments. In AD, therapies are needed to reduce disease activity and flare severity, clear flares, and provide relief.
Parental education and written eczema action plans are recommended to help patients and parents/guardians follow recommended regimens12; Tollefson and Bruckner13 for the American Academy of Pediatrics provide an action plan to guide the care of children with atopic dermatitis that is simple, but many others exist online. The eczema action plan usually provides information on how to bathe and what to do when the skin is actively inflamed.
In 2014, a 4-part series of guidelines of care for the management of AD was published by the AAD, replacing prior guidelines.4,11,14,15 The following sections review some of the important parameters of care highlighted in these management guidelines.
Psychological Support
Appropriate psychological support for AD patients can be sought through counselors, therapists, psychiatrists, and support groups such as the National Eczema Association (https://nationaleczema.org/).
Education
Education is the leading form of medical therapy in patients with AD. Eczema schools are popular in Europe and are just beginning to form in the United States (http://tuh.templehealth.org/content/eczema_school.htm), which can be helpful to educate caregivers and patients with AD. Patient resources online and through support groups with an online presence, in-person meetings, and patient/family conventions can be helpful to AD patients. Often, an initial office visit with a dermatologist involves a review of avoidance of triggers, usage of gentle skin care including bland emollients, and therapeutic regimens for disease activity. This form of verbal education is to be paired with an eczema action plan, a written document that allows individuals to reference recommendations and share information with other caregivers.12,13,16
Emollients and Gentle Skin Care
Gentle skin care regimens, which includes the usage of synthetic cleansers with a low pH to help maintain the acidity (acid mantle) of the skin, seek to reduce irritation and have been rated as level IA (highest level) in recent AAD guidelines.14 Although bathing frequency has been emphasized in the guidelines, AD severity as reflected by SCORAD (SCORing Atopic Dermatitis) was not different for daily bathing versus twice weekly.17 The American Academy of Pediatrics recommended a skin care regimen of bathing every 2 to 3 days in lukewarm water for 10 to 15 minutes, followed by application of emollients that are fragrance free and have few preservatives.13 Topical emollients with additives such as colloidal oatmeal, avenanthramides, or ceramides can be used to enhance the skin barrier and are well tolerated in all age groups.18,19 Despite enhanced emollients, the therapy of AD still requires usage of prescription or over-the-counter TCs and/or topical calcineurin inhibitors (TCIs) in many cases.20
Topical Medication
Children have a relatively higher body surface area–to-weight ratio, allowing for greater potential absorption of topical medicaments and potential side effects from absorption. Types of vehicle, cost, site of application, and availability may impact patient and physician preference in choice of therapeutic topical agent.14
Topical Corticosteroids
Topical corticosteroids (TCs) are the mainstay of treatment for AD and have been used for more than 60 years.14,20 Topical corticosteroids provide anti-inflammatory effects on T cells, monocytes, and macrophages, producing altered cytokine activity locally. Topical corticosteroids inhibit collagen synthesis, potentially causing skin atrophy. They also inhibit IL-1, IL-2, IL-6, IFN-α, and tumor necrosis factor α.21 Topical corticosteroids are classified as class I (ultra-high potency) to class VII (low potency). In children, low-potency TCs generally are applied to the face, intertriginous areas, groin, and genitalia, and mid-potency corticosteroids are applied to the body, arms, and legs. An even higher-strength agent can be prescribed as a rescue medication in severe cases. After clearance with once- or twice-daily therapy, twice-weekly usage can benefit disease activity.22 Topical corticosteroids reduce inflammation as well as Staphylococcus aureus load through inhibition of cytokines that inhibit antimicrobial peptides. Topical corticosteroids have been endorsed as level IA evidence therapy by the AAD guidelines.14
Topical corticosteroids, particularly prolonged usage of mid- to high-potency products, have been associated with side effects such as skin atrophy, striae, telangiectases, hypopigmentation, rosacea, acneiform eruptions, focal hypertrichosis, perioral dermatitis, and acne23; potential systemic side effects include hypothalamic-pituitary-adrenal axis suppression, cataracts, glaucoma (with periocular application), Cushing syndrome, hyperglycemia, hypertension,23 and growth retardation.14 Long-term corticosteroid therapy is associated with tachyphylaxis and potential rebound of disease with discontinuation.24 Based on the potential risk of side effects with TCs, the least potent product for the shortest time needed is recommended, with special care for thin skin. Discontinuation when clearance occurs is advised. Allergy to TCs and/or vehicle ingredients such as propylene glycol should be suspected in severe unremitting cases.14 A recent registry review of children screened for contact dermatitis demonstrated that children with AD had higher sensitization to the steroid tixocortol pivalate.25
Topical Calcineurin Inhibitors
Topical calcineurin inhibitors include pimecrolimus cream 1%, which is approved for mild to moderate AD in adults and children 2 years and older, and tacrolimus ointment 0.03% and 0.1%, which are approved for moderate to severe AD in adults and children aged 2 to 15 years (0.03% formulation only). Topical calcineurin inhibitors can be used as second-line agents in AD in patients who have inadequate response to TCs or who may not be able to use TCs due to the disease site.10,13,14 Guidelines from the AAD also have endorsed TCIs as level IA evidence for steroid-sparing agents.
Concerns about the reporting of cancers and lymphomas prompted the US Food and Drug Administration to issue a black box warning on TCIs more than 10 years ago. Pimecrolimus, which has little absorption and no notable immunosuppressive effects, has been used without detrimental effect on vaccination and delayed-type hypersensitivities, but many decades of data are lacking.10,13,14,17,26-29 Topical calcineurin inhibitors can be used as steroid-sparing agents in lieu of corticosteroids in specific locations such as the face and eyelids and for long-term suppressive therapy twice weekly.30 Intermittent usage and cycling with corticosteroids is advisable,28 but usage intermittently beyond 1 year has not been evaluated.
Topical calcineurin inhibitors are recommended as effective for acute and chronic AD. Their use as maintenance therapy in adults and children, for AD recalcitrant to steroids, for AD in sensitive areas, for steroid-induced atrophy, and for long-term uninterrupted topical steroid usage carries a level IA evidence recommendation. Furthermore, the AAD guidelines have recommended TCIs as steroid-sparing agents with level IA evidence and off-label use of TCIs in children younger than 2 years with level IA evidence. Pretreatment with TCs to reduce stinging has level IIB evidence. Usage for flare prevention is level IA evidence. Routine blood monitoring of TCI-treated patients was not recommended; in fact, the AAD guidelines provided this recommendation as level IA evidence against routine laboratory monitoring of TCI-treated patients.14
Topical Antibiotics
Topical antibiotics are indicated for the therapy of impetigo and can be used in the setting of impetiginized AD in conjunction with TCs. Recent AAD guidelines suggested against routine usage of topical antistaphylococcal agents as level IA evidence.14 There is one study supporting usage of topical mupirocin in addition to TCs to heal children with eczema area and severity index scores more than 7 more rapidly in the first week of AD therapy, but in the same study, additive benefit was not demonstrated in AD beyond the first week.31 There also are data supporting usage of intranasal mupirocin adjunctively with bleach baths in patients with moderate to severe AD, which was rated as level IIB evidence in the AAD guidelines.14,32 There are limited data on the long-term utility of topical anti-infectives in AD. The risks of long-term usage could include resistance formation to agents such as mupirocin, contact dermatitis, and lack of efficacy.
Additional Therapeutics
Wet Wraps
Penetration through the stratum corneum is needed for drug activity in AD. Penetration can be enhanced using wet wrap therapy or using ointments, which produce higher relative potency.13 Wet wraps overlying a dilute topical medicament have been described as effective in AD and are recommended in AAD guidelines as level IIB evidence.14 Different wet wrap techniques can be used, including wet pajamas covered by dry pajamas or saline-soaked gauze wrapped around the affected areas and then dry gauze applied over the wet gauze. The methodology used should be tailored to the patient as well as to whether the individual is an inpatient or outpatient.
Bleach Baths
Dilute sodium hypochlorite solution 0.005% (one-quarter cup bleach in 20 gallons of water) has been demonstrated to be beneficial in reduction of disease activity in AD patients with recurrent bacterial infections.32 This simple technique in addition to intranasal mupirocin can reduce AD severity and improve quality of life and is the only ongoing S aureus therapeutic management endorsed by the AAD guidelines for the management of AD.14,32
Topical and Oral Delivery
Antihistamines
Topical antihistamines are ineffective in AD. Oral antihistamines can be used to reduce pruritus and are most effective when given as sedating agents for sleep enhancement but may be given as nonsedating agents for patients with concomitant allergic disorders such as allergic rhinoconjunctivitis. Paradoxical hyperreactivity with sedating antihistamines is not uncommon in small children, and sedating antihistamine usage should be discontinued in these instances.13 Parents of children with AD have reported giving the child antihistamines to sleep was helpful, as well as putting on creams, using special clothes (eg, all cotton), and keeping the room cool.33 There is level IIIC evidence against use of systemic antihistamines and level IIA evidence for sedating and nonsedating, according to the AAD guidelines.14
Systemic Therapeutics
Oral therapeutics range from oral antihistamines to oral antibiotics and immunosuppressive medications. Oral antibiotics (level IIB evidence) are reserved for superinfected AD, which is not easily defined for the following reasons: there is no consensus definition of superinfected AD; the majority of active AD lesions when cultured will demonstrate S aureus growth; and most AD lesions ooze, thereby creating the appearance of superinfection. In real-world practice, superinfection can be diagnosed based on the presence of pustules; furuncles; or signs of infection such as tracking erythema, tenderness, severe erosions, or maceration. Clinical judgment is always required.
The immunosuppressive medications used in AD include leukotriene inhibitors, which rarely are effective for AD.34 More effective systemic agents for AD include cyclosporine (level I to IIB evidence), azathioprine (level IIB evidence), mycophenolate mofetil (level IIIC evidence), and methotrexate (level IIB evidence). These agents are indicated for pediatric or adult patients when topical agents and/or phototherapy have failed.15 Monitoring these agents for side effects includes ongoing evaluation for renal and liver toxicity. Short courses (ie, 6 months) are preferred to minimize side effects.35
Dupilumab, an injectable AD therapy, is approved in the United States. This agent is injected every 2 weeks and binds to the IL-4Rα shared by IL-4 and IL-13. In 4 weeks of monotherapy, 85% of adult patients treated had 50% or greater clearance.36 Recently published consensus opinion from the International Eczema Council recommends assessment of a variety of factors before initiating systemic therapy including comorbid illnesses such as contact allergy, trigger avoidance, superinfection, and impact on quality of life.37
Oral Corticosteroids
Systemic corticosteroids clear patients quickly but provide no sustained improvement; in fact, many patients rebound or have tachyphylaxis. Although short-term corticosteroid usage can break the itch-scratch cycle, long-term usage is associated with osteoporosis, Cushing syndrome, and aseptic necrosis of the femoral head. Decreased linear growth will occur during therapy in children; therefore, systemic steroids are not recommended in children with AD, except for additional or comorbid conditions (eg, asthma or contact dermatitis).4
Phototherapy
Phototherapy has been recommended in the AAD guidelines as a second-line treatment after failure of first-line agents (ie, TCIs and TCs) for clearance and or maintenance and should be tailored to the patient’s skin tone by an experienced physician. Narrowband UVB phototherapy may act through the suppression of T-cell activity in the skin and possibly via suppression of staphylococcal superantigens; however, many phototherapy types have been described for AD.38,39 Usage can be effective in school-aged children and teenagers but may be limited due to school attendance. Phototherapy was graded as level IIB evidence in the AAD guidelines.15 Side effects include aggravation of AD by exposure to heat and UV light, actinic damage, tenderness, erythema, pruritus, burning, and stinging. Lentigines; skin cancers (melanoma and nonmelanoma); folliculitis; and ocular toxicity, especially cataracts, can occur.15 Children younger than 6 years will find it difficult to stand in a phototherapy booth and may be poor candidates.15,38,39
Complementary and Alternative Medicine
Complementary and alternative medicine (CAM) also has been used for AD in the United States. In a review of the 2007 National Health Interview Survey of 9417 children aged 0 to 17 years, CAM was used for AD by 0.99% of children. Some CAM techniques were associated with worsening severity of AD, including herbal therapy, vitamins, homeopathic agents, diet, and movement techniques.40 Usage of Chinese herbal medications for AD can be associated with liver toxicity.41 Only one CAM therapy—massage therapy—has some mild supportive data.42
Allergen Avoidance and Diet
Bronsnick et al43 discussed the possible benefit of prenatal and postnatal probiotics for prevention of AD, which were not supported in the AAD guidelines for management of AD4; postnatal prebiotic supplementation; and exclusive breastfeeding and/or supplementation with hydrolyzed formula in at-risk children. Elimination diets for children and mothers were not recommended. The authors found no beneficial role of supplements including vitamin D, selenium, fish oil, borage oil, and zinc sulfate.43
A National Institute of Allergy and Infectious Diseases consensus group recommended avoidance of proven but not random elimination of food allergens in AD, asthma, and/or eosinophilic esophagitis.44 Restricted maternal diet was not recommended, and breastfeeding exclusively for the first 4 to 6 months was recommended. Hydrolyzed formulas were suggested as a possible preventive strategy in at-risk infants as a breastfeeding alternative, with cost of these formulas being a problem.44
In children younger than 5 years, food allergy screening for the most common allergens (eg, milk, eggs, peanuts, wheat, soy) should be considered in children with persistent unremitting dermatitis and/or known food challenge–induced reactions.4 Conservative measures to avoid house dust mite exposure in known sensitized individuals including dust covers for pillows and mattresses may be beneficial.4,45
Emerging Therapies
Recently approved therapies include better-targeted agents that appear to have a reasonable safety profile and may fulfill unmet needs in AD care. Of these agents, crisaborole, a topical boron-based phosphodiesterase 4 inhibitor, was approved in December 2016 for mild to moderate AD in patients 2 years and older.Topically, this agent seems to be efficacious in the absence of notable carcinogenicity.46
The systemic (injectable) biologic agent dupilumab was approved in March 2017 for moderate to severe AD. Phase 3 studies in adults with AD showed excellent success in adults with moderate to severe AD.37 This agent is a monoclonal antibody targeted at blockade of the crucial atopic inflammatory triggering pathway via blockade of the IL-4A receptor site, targeting IL-4 and IL-13 activity.36,47 There are many medications in the pipeline, which Renert-Yuval and Guttman-Yassky48 review. However, an overview of the landscape demonstrates that Janus kinase (JAK) inhibitors49 and biologic medications in addition to dupilumab affecting targeted inflammatory cascades in AD are in development. In particular, the JAK inhibitors appear promising due to availability both as oral and topical agents.49
Need for Ongoing Care and Monitoring
Atopic dermatitis is a chronic inflammatory skin disorder with a genetic basis. Once initiated, the process of AD may persist throughout the patient’s life and become a systemic disorder with comorbidities including sleep disturbance, reduced quality of life, and cardiovascular disease.50 Ongoing management of AD includes topical reduction in irritants and triggers, topical medicaments, and management of pruritus and infections. At this time, emollients and irritant avoidance paired with judicious topical medicaments including TCs and second-line or site-specific (eg, eyelids) usage of TCIs or phosphodiesterase 4 inhibitors remain the backbone of therapy. Ongoing review of therapeutics for associated morbidities is underway, which may guide future therapeutic interventions into AD. The future of prevention and therapy look bright, but time will tell.
- Kelleher M, Dunn-Galvin A, Hourihane JO, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015;135:930-935.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Tsitoura S, Nestoridou K, Botis P, et al. Randomized trial to prevent sensitization to mite allergens in toddlers and preschoolers by allergen reduction and education: one-year results. Arch Pediatr Adolesc Med. 2002;156:1021-1027.
- Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218-1233.
- Foolad N, Brezinski EA, Chase EP, et al. Effect of nutrient supplementation on atopic dermatitis in children: a systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013;149:350-355.
- Abrahamsson TR, Jakobsson T, Böttcher MF, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1174-1180.
- Kalliomäki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076-1079.
- Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184-191.
- Simpson MR, Dotterud CK, Storrø O, et al. Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol. 2015;15:13. doi:10.1186/s12895-015-0030-1.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Silverberg NB. Creating an action plan for eczema patients. Cutis. 2015;96:362-363.
- Tollefson MM, Bruckner AL; Section on Dermatology. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Shi VY, Nanda S, Lee K, et al. Improving patient education with an eczema action plan: a randomized controlled trial. JAMA Dermatol. 2013;149:481-483.
- Koutroulis I, Petrova K, Kratimenos P, et al. Frequency of bathing in the management of atopic dermatitis: to bathe or not to bathe? Clin Pediatr (Phila). 2014;53:677-681.
- Fowler JF, Nebus J, Wallo W, et al. Colloidal oatmeal formulations as adjunct treatments in atopic dermatitis. J Drugs Dermatol. 2012;11:804-807.
- Fowler J Jr, Silverberg N. Active naturals have a key role in atopic dermatitis. Semin Cutan Med Surg. 2008;27:8-10.
- Eichenfield LF. Consensus guidelines in diagnosis and treatment of atopic dermatitis. Allergy. 2004;59:86-92.
- Nghiem P, Pearson G, Langley RG. Tacrolimus and pimecrolimus: from clever prokaryotes to inhibiting calcineurin and treating atopic dermatitis. J Am Acad Dermatol. 2002;46:228-241.
- Schmitt J. Commentary: eczema and cancer risk. Br J Dermatol. 2011;165:463-464.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takahashi-Ando N, Jones MA, Fujisawa S, et al. Patient-reported outcomes after discontinuation of long-term topical corticosteroid treatment for atopic dermatitis: a targeted cross-sectional survey. Drug Healthc Patient Saf. 2015;7:57-62.
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric contact dermatitis registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770.
- Werfel T. Topical use of pimecrolimus in atopic dermatitis: update on the safety and efficacy. J Dtsch Dermatol Ges. 2009;7:739-742.
- Wahn U, Bos JD, Goodfield M, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics. 2002;110(1, pt 1):E2.
- Berger TG, Duvic M, Van Voorhees AS, et al; American Academy of Dermatology Association Task Force. The use of topical calcineurin inhibitors in dermatology: safety concerns. report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Thaçi D, Reitamo S, Gonzalez Ensenat MA, et al. Proactive disease management with 0.03% tacrolimus ointment for children with atopic dermatitis: results of a randomized, multicentre, comparative study. Br J Dermatol. 2008;159:1348-1356.
- Gong JQ, Lin L, Lin T, et al. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. Br J Dermatol. 2006;155:680-687.
- Huang JT, Abrams M, Tlougan B, et al. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123:E808-E814.
- Reid P, Lewis-Jones MS. Sleep difficulties and their management in preschoolers with atopic eczema. Clin Exp Dermatol. 1995;20:38-41.
- Silverberg NB, Paller AS. Leukotriene receptor antagonists are ineffective for severe atopic dermatitis. J Am Acad Dermatol. 2004;50:485-486.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. New York, NY: Elsevier Saunders; 2013.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Simpson EL, Bruin-Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? recommendations from an expert panel of the International Eczema Council [published online August 10, 2017]. J Am Acad Dermatol. doi:10.1016/j.jaad.2017.06.042.
- Veith W, DeLeo V, Silverberg N. Medical phototherapy in childhood skin diseases. Minerva Pediatr. 2011;63:327-333.
- Song E, Reja D, Silverberg N, et al. Phototherapy: kids are not just little people. Clin Dermatol. 2015;33:672-680.
- Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25:246-254.
- Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol. 2015;89:851-865.
- Schachner L, Field T, Hernandez-Reif M, et al. Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr Dermatol. 1998;15:390-395.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
- Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. Nutr Res. 2011;31:61-75.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Hanifin JM, Chan SC, Cheng JB, et al. Type phosphodiesterase inhibitors have clinical and in vitro anti-inflammatory effects in atopic dermatitis. J Invest Dermatol. 1996;107:51-56.
- Boguniewicz M, Leung DY. Targeted therapy for allergic diseases: at the intersection of cutting-edge science and clinical practice. J Allergy Clin Immunol. 2015;135:354-356.
- Renert-Yuval Y, Guttman-Yassky E. Systemic therapies in atopic dermatitis: the pipeline. Clin Dermatol. 2017;35:387-397.
- Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76:736-744.
- Brunner PM, Silverberg JI, Guttman-Yassky E, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol. 2017;137:18-25.
- Kelleher M, Dunn-Galvin A, Hourihane JO, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015;135:930-935.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Tsitoura S, Nestoridou K, Botis P, et al. Randomized trial to prevent sensitization to mite allergens in toddlers and preschoolers by allergen reduction and education: one-year results. Arch Pediatr Adolesc Med. 2002;156:1021-1027.
- Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218-1233.
- Foolad N, Brezinski EA, Chase EP, et al. Effect of nutrient supplementation on atopic dermatitis in children: a systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013;149:350-355.
- Abrahamsson TR, Jakobsson T, Böttcher MF, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1174-1180.
- Kalliomäki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076-1079.
- Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184-191.
- Simpson MR, Dotterud CK, Storrø O, et al. Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol. 2015;15:13. doi:10.1186/s12895-015-0030-1.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Silverberg NB. Creating an action plan for eczema patients. Cutis. 2015;96:362-363.
- Tollefson MM, Bruckner AL; Section on Dermatology. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Shi VY, Nanda S, Lee K, et al. Improving patient education with an eczema action plan: a randomized controlled trial. JAMA Dermatol. 2013;149:481-483.
- Koutroulis I, Petrova K, Kratimenos P, et al. Frequency of bathing in the management of atopic dermatitis: to bathe or not to bathe? Clin Pediatr (Phila). 2014;53:677-681.
- Fowler JF, Nebus J, Wallo W, et al. Colloidal oatmeal formulations as adjunct treatments in atopic dermatitis. J Drugs Dermatol. 2012;11:804-807.
- Fowler J Jr, Silverberg N. Active naturals have a key role in atopic dermatitis. Semin Cutan Med Surg. 2008;27:8-10.
- Eichenfield LF. Consensus guidelines in diagnosis and treatment of atopic dermatitis. Allergy. 2004;59:86-92.
- Nghiem P, Pearson G, Langley RG. Tacrolimus and pimecrolimus: from clever prokaryotes to inhibiting calcineurin and treating atopic dermatitis. J Am Acad Dermatol. 2002;46:228-241.
- Schmitt J. Commentary: eczema and cancer risk. Br J Dermatol. 2011;165:463-464.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takahashi-Ando N, Jones MA, Fujisawa S, et al. Patient-reported outcomes after discontinuation of long-term topical corticosteroid treatment for atopic dermatitis: a targeted cross-sectional survey. Drug Healthc Patient Saf. 2015;7:57-62.
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric contact dermatitis registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770.
- Werfel T. Topical use of pimecrolimus in atopic dermatitis: update on the safety and efficacy. J Dtsch Dermatol Ges. 2009;7:739-742.
- Wahn U, Bos JD, Goodfield M, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics. 2002;110(1, pt 1):E2.
- Berger TG, Duvic M, Van Voorhees AS, et al; American Academy of Dermatology Association Task Force. The use of topical calcineurin inhibitors in dermatology: safety concerns. report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Thaçi D, Reitamo S, Gonzalez Ensenat MA, et al. Proactive disease management with 0.03% tacrolimus ointment for children with atopic dermatitis: results of a randomized, multicentre, comparative study. Br J Dermatol. 2008;159:1348-1356.
- Gong JQ, Lin L, Lin T, et al. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. Br J Dermatol. 2006;155:680-687.
- Huang JT, Abrams M, Tlougan B, et al. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123:E808-E814.
- Reid P, Lewis-Jones MS. Sleep difficulties and their management in preschoolers with atopic eczema. Clin Exp Dermatol. 1995;20:38-41.
- Silverberg NB, Paller AS. Leukotriene receptor antagonists are ineffective for severe atopic dermatitis. J Am Acad Dermatol. 2004;50:485-486.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. New York, NY: Elsevier Saunders; 2013.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Simpson EL, Bruin-Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? recommendations from an expert panel of the International Eczema Council [published online August 10, 2017]. J Am Acad Dermatol. doi:10.1016/j.jaad.2017.06.042.
- Veith W, DeLeo V, Silverberg N. Medical phototherapy in childhood skin diseases. Minerva Pediatr. 2011;63:327-333.
- Song E, Reja D, Silverberg N, et al. Phototherapy: kids are not just little people. Clin Dermatol. 2015;33:672-680.
- Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25:246-254.
- Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol. 2015;89:851-865.
- Schachner L, Field T, Hernandez-Reif M, et al. Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr Dermatol. 1998;15:390-395.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
- Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. Nutr Res. 2011;31:61-75.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Hanifin JM, Chan SC, Cheng JB, et al. Type phosphodiesterase inhibitors have clinical and in vitro anti-inflammatory effects in atopic dermatitis. J Invest Dermatol. 1996;107:51-56.
- Boguniewicz M, Leung DY. Targeted therapy for allergic diseases: at the intersection of cutting-edge science and clinical practice. J Allergy Clin Immunol. 2015;135:354-356.
- Renert-Yuval Y, Guttman-Yassky E. Systemic therapies in atopic dermatitis: the pipeline. Clin Dermatol. 2017;35:387-397.
- Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76:736-744.
- Brunner PM, Silverberg JI, Guttman-Yassky E, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol. 2017;137:18-25.
Practice Points
- Prevention of atopic dermatitis is desired in high-risk settings (ie, 1 or more relatives with atopy).
- Emollient therapy from early infancy has been described as one method.
- Other forms of disease prevention have not yet been adequately developed.
Recent Controversies in Pediatric Dermatology: The Usage of General Anesthesia in Young Children
Clinicians who have attempted to perform an in-office procedure on infants or young children will recognize the difficulties that arise from the developmental inability to cooperate with procedures.1 Potential problems mentioned in the literature include but are not limited to anxiety, which is identified in all age groups of patients undergoing dermatologic procedures2; limitation of pain control3; and poor outcomes due to movement by the patient.1 In one author’s experience (N.B.S.), anxious and scared children can potentially cause injury to themselves, parents/guardians, and health care professionals by flailing and kicking; children are flexible and can wriggle out of even fine grips, and some children, especially toddlers, can be strong.
The usage of topical anesthetics can only give superficial anesthesia. They can ostensibly reduce pain and are useful for anesthesia of curettage, but their use is limited in infants and young children by the minimal amount of drug that is safe for application, as risks of absorption include methemoglobinemia and seizure
General anesthesia seems to be the best alternative due to associated amnesia of the events occurring including pain; immobilization and ability to produce more accurate biopsy sampling; better immobilization leading to superior cosmetic results; and reduced risk to patients, parents/guardians, and health care professionals from a flailing child. In the field of pediatric dermatology, general anesthesia often is used for excision of larger lesions and cosmetic repairs. Operating room privileges are not always easy to obtain, but many pediatric dermatologists take advantage of outpatient surgical centers associated with their medical center. A retrospective review of 226 children receiving 681 procedures at a single institution documented low rates of complications.1
If it was that easy, most children would be anesthetized with general anesthesia. However, there are risks associated with general anesthesia. Parents/guardians often will do what they can to avoid risk and may therefore refuse general anesthesia, but it is not completely avoidable in complicated skin disease. Despite the risks, the benefit is present in a major anomaly correction such as a cleft palate in a 6-month-old but may not be there for the treatment of a wart. When procedures are nonessential or may be conducted without anesthesia, avoidance of general anesthesia is reasonable and a combination of topical and local infiltrative anesthesia can help. In the American Academy of Dermatology guidelines on in-office anesthesia, Kouba et al5 states: “Topical agents are recommended as a first-line method of anesthesia for the repair of dermal lacerations in children and for other minor dermatologic procedures, including curettage. For skin biopsy, excision, or other cases where topical agents alone are insufficient, adjunctive use of topical anesthesia to lessen the discomfort of infiltrative anesthetic should be considered.”
A new controversy recently has emerged concerning the potential risks of anesthesia on neurocognitive development in infants and young children. These concerns regardingthe labeling changes of anesthetic and sedation drugs by the US Food and Drug Administration (FDA) in December 2016 specifically focused on these risks in children younger than 3 years with prolonged (>3 hours) and repeated exposures; however, this kind of exposure is unlikely with standard pediatric dermatologic procedures.6-9
There is compelling evidence from animal studies that exposure to all anesthetic agents in clinical use induces neurotoxicity and long-term adverse neurobehavioral deficits; however, whether these findings are applicable in human infants is unknown.6-9 Most of the studies in humans showing adverse outcomes have been retrospective observational studies subject to multiple sources of bias. Two recent large clinical studies—the GAS (General Anaesthesia compared to Spinal anaesthesia) trial10 and the PANDA (Pediatric Anesthesia and Neurodevelopment Assessment) study11—have shown no evidence of abnormal neurocognitive effects with a single brief exposure before 3 years of age (PANDA) or during infancy (GAS) in otherwise-healthy children.10,11
It is important to note that the FDA labeling change warning specifically stated that “[c]onsistent with animal studies, recent human data suggest that a single, relatively short exposure to general anesthetic and sedation drugs in infants or toddlers is unlikely to have negative effects on behavior or learning.” Moreover, the FDA emphasized that “Surgeries or procedures in children younger than 3 years should not be delayed or avoided when medically necessary.”12 Taking these points into consideration, we should offer our patients in-office care when possible and postpone elective procedures when advisable but proceed when necessary for our patients’ physical and emotional health.
- Juern AM, Cassidy LD, Lyon VB. More evidence confirming the safety of general anesthesia in pediatric dermatologic surgery. Pediatr Dermatol. 2010;27:355-360.
- Gerwels JW, Bezzant JL, Le Maire L, et al. Oral transmucosal fentanyl citrate premedication in patients undergoing outpatient dermatologic procedures. J Dermatol Surg Oncol. 1994;20:823-826.
- D’Acunto C, Raone B, Neri I, et al. Outpatient pediatric dermatologic surgery: experience in 296 patients. Pediatr Dermatol. 2015;32:424-426.
- Gunter JB. Benefit and risks of local anesthetics in infants and children. Paediatr Drugs. 2002;4:649-672.
- Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery [published online March 4, 2016]. J Am Acad Dermatol. 2016;74:1201-1219.
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876-882.
- Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834-841.
- Raper J, Alvarado MC, Murphy KL, et al. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084-1092.
- Davidson AJ. Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatric Anaesthesia. 2011;21:716-721.
- Davidson AJ, Disma N, de Graaff JC, et al; GAS consortium. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239-250.
- Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312-2320.
- General anesthetic and sedation drugs: drug safety communication—new warnings for young children and pregnant women. US Food and Drug Administration website. https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm533195.htm. Published December 14, 2016. Accessed July 25, 2017.
Clinicians who have attempted to perform an in-office procedure on infants or young children will recognize the difficulties that arise from the developmental inability to cooperate with procedures.1 Potential problems mentioned in the literature include but are not limited to anxiety, which is identified in all age groups of patients undergoing dermatologic procedures2; limitation of pain control3; and poor outcomes due to movement by the patient.1 In one author’s experience (N.B.S.), anxious and scared children can potentially cause injury to themselves, parents/guardians, and health care professionals by flailing and kicking; children are flexible and can wriggle out of even fine grips, and some children, especially toddlers, can be strong.
The usage of topical anesthetics can only give superficial anesthesia. They can ostensibly reduce pain and are useful for anesthesia of curettage, but their use is limited in infants and young children by the minimal amount of drug that is safe for application, as risks of absorption include methemoglobinemia and seizure
General anesthesia seems to be the best alternative due to associated amnesia of the events occurring including pain; immobilization and ability to produce more accurate biopsy sampling; better immobilization leading to superior cosmetic results; and reduced risk to patients, parents/guardians, and health care professionals from a flailing child. In the field of pediatric dermatology, general anesthesia often is used for excision of larger lesions and cosmetic repairs. Operating room privileges are not always easy to obtain, but many pediatric dermatologists take advantage of outpatient surgical centers associated with their medical center. A retrospective review of 226 children receiving 681 procedures at a single institution documented low rates of complications.1
If it was that easy, most children would be anesthetized with general anesthesia. However, there are risks associated with general anesthesia. Parents/guardians often will do what they can to avoid risk and may therefore refuse general anesthesia, but it is not completely avoidable in complicated skin disease. Despite the risks, the benefit is present in a major anomaly correction such as a cleft palate in a 6-month-old but may not be there for the treatment of a wart. When procedures are nonessential or may be conducted without anesthesia, avoidance of general anesthesia is reasonable and a combination of topical and local infiltrative anesthesia can help. In the American Academy of Dermatology guidelines on in-office anesthesia, Kouba et al5 states: “Topical agents are recommended as a first-line method of anesthesia for the repair of dermal lacerations in children and for other minor dermatologic procedures, including curettage. For skin biopsy, excision, or other cases where topical agents alone are insufficient, adjunctive use of topical anesthesia to lessen the discomfort of infiltrative anesthetic should be considered.”
A new controversy recently has emerged concerning the potential risks of anesthesia on neurocognitive development in infants and young children. These concerns regardingthe labeling changes of anesthetic and sedation drugs by the US Food and Drug Administration (FDA) in December 2016 specifically focused on these risks in children younger than 3 years with prolonged (>3 hours) and repeated exposures; however, this kind of exposure is unlikely with standard pediatric dermatologic procedures.6-9
There is compelling evidence from animal studies that exposure to all anesthetic agents in clinical use induces neurotoxicity and long-term adverse neurobehavioral deficits; however, whether these findings are applicable in human infants is unknown.6-9 Most of the studies in humans showing adverse outcomes have been retrospective observational studies subject to multiple sources of bias. Two recent large clinical studies—the GAS (General Anaesthesia compared to Spinal anaesthesia) trial10 and the PANDA (Pediatric Anesthesia and Neurodevelopment Assessment) study11—have shown no evidence of abnormal neurocognitive effects with a single brief exposure before 3 years of age (PANDA) or during infancy (GAS) in otherwise-healthy children.10,11
It is important to note that the FDA labeling change warning specifically stated that “[c]onsistent with animal studies, recent human data suggest that a single, relatively short exposure to general anesthetic and sedation drugs in infants or toddlers is unlikely to have negative effects on behavior or learning.” Moreover, the FDA emphasized that “Surgeries or procedures in children younger than 3 years should not be delayed or avoided when medically necessary.”12 Taking these points into consideration, we should offer our patients in-office care when possible and postpone elective procedures when advisable but proceed when necessary for our patients’ physical and emotional health.
Clinicians who have attempted to perform an in-office procedure on infants or young children will recognize the difficulties that arise from the developmental inability to cooperate with procedures.1 Potential problems mentioned in the literature include but are not limited to anxiety, which is identified in all age groups of patients undergoing dermatologic procedures2; limitation of pain control3; and poor outcomes due to movement by the patient.1 In one author’s experience (N.B.S.), anxious and scared children can potentially cause injury to themselves, parents/guardians, and health care professionals by flailing and kicking; children are flexible and can wriggle out of even fine grips, and some children, especially toddlers, can be strong.
The usage of topical anesthetics can only give superficial anesthesia. They can ostensibly reduce pain and are useful for anesthesia of curettage, but their use is limited in infants and young children by the minimal amount of drug that is safe for application, as risks of absorption include methemoglobinemia and seizure
General anesthesia seems to be the best alternative due to associated amnesia of the events occurring including pain; immobilization and ability to produce more accurate biopsy sampling; better immobilization leading to superior cosmetic results; and reduced risk to patients, parents/guardians, and health care professionals from a flailing child. In the field of pediatric dermatology, general anesthesia often is used for excision of larger lesions and cosmetic repairs. Operating room privileges are not always easy to obtain, but many pediatric dermatologists take advantage of outpatient surgical centers associated with their medical center. A retrospective review of 226 children receiving 681 procedures at a single institution documented low rates of complications.1
If it was that easy, most children would be anesthetized with general anesthesia. However, there are risks associated with general anesthesia. Parents/guardians often will do what they can to avoid risk and may therefore refuse general anesthesia, but it is not completely avoidable in complicated skin disease. Despite the risks, the benefit is present in a major anomaly correction such as a cleft palate in a 6-month-old but may not be there for the treatment of a wart. When procedures are nonessential or may be conducted without anesthesia, avoidance of general anesthesia is reasonable and a combination of topical and local infiltrative anesthesia can help. In the American Academy of Dermatology guidelines on in-office anesthesia, Kouba et al5 states: “Topical agents are recommended as a first-line method of anesthesia for the repair of dermal lacerations in children and for other minor dermatologic procedures, including curettage. For skin biopsy, excision, or other cases where topical agents alone are insufficient, adjunctive use of topical anesthesia to lessen the discomfort of infiltrative anesthetic should be considered.”
A new controversy recently has emerged concerning the potential risks of anesthesia on neurocognitive development in infants and young children. These concerns regardingthe labeling changes of anesthetic and sedation drugs by the US Food and Drug Administration (FDA) in December 2016 specifically focused on these risks in children younger than 3 years with prolonged (>3 hours) and repeated exposures; however, this kind of exposure is unlikely with standard pediatric dermatologic procedures.6-9
There is compelling evidence from animal studies that exposure to all anesthetic agents in clinical use induces neurotoxicity and long-term adverse neurobehavioral deficits; however, whether these findings are applicable in human infants is unknown.6-9 Most of the studies in humans showing adverse outcomes have been retrospective observational studies subject to multiple sources of bias. Two recent large clinical studies—the GAS (General Anaesthesia compared to Spinal anaesthesia) trial10 and the PANDA (Pediatric Anesthesia and Neurodevelopment Assessment) study11—have shown no evidence of abnormal neurocognitive effects with a single brief exposure before 3 years of age (PANDA) or during infancy (GAS) in otherwise-healthy children.10,11
It is important to note that the FDA labeling change warning specifically stated that “[c]onsistent with animal studies, recent human data suggest that a single, relatively short exposure to general anesthetic and sedation drugs in infants or toddlers is unlikely to have negative effects on behavior or learning.” Moreover, the FDA emphasized that “Surgeries or procedures in children younger than 3 years should not be delayed or avoided when medically necessary.”12 Taking these points into consideration, we should offer our patients in-office care when possible and postpone elective procedures when advisable but proceed when necessary for our patients’ physical and emotional health.
- Juern AM, Cassidy LD, Lyon VB. More evidence confirming the safety of general anesthesia in pediatric dermatologic surgery. Pediatr Dermatol. 2010;27:355-360.
- Gerwels JW, Bezzant JL, Le Maire L, et al. Oral transmucosal fentanyl citrate premedication in patients undergoing outpatient dermatologic procedures. J Dermatol Surg Oncol. 1994;20:823-826.
- D’Acunto C, Raone B, Neri I, et al. Outpatient pediatric dermatologic surgery: experience in 296 patients. Pediatr Dermatol. 2015;32:424-426.
- Gunter JB. Benefit and risks of local anesthetics in infants and children. Paediatr Drugs. 2002;4:649-672.
- Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery [published online March 4, 2016]. J Am Acad Dermatol. 2016;74:1201-1219.
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876-882.
- Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834-841.
- Raper J, Alvarado MC, Murphy KL, et al. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084-1092.
- Davidson AJ. Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatric Anaesthesia. 2011;21:716-721.
- Davidson AJ, Disma N, de Graaff JC, et al; GAS consortium. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239-250.
- Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312-2320.
- General anesthetic and sedation drugs: drug safety communication—new warnings for young children and pregnant women. US Food and Drug Administration website. https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm533195.htm. Published December 14, 2016. Accessed July 25, 2017.
- Juern AM, Cassidy LD, Lyon VB. More evidence confirming the safety of general anesthesia in pediatric dermatologic surgery. Pediatr Dermatol. 2010;27:355-360.
- Gerwels JW, Bezzant JL, Le Maire L, et al. Oral transmucosal fentanyl citrate premedication in patients undergoing outpatient dermatologic procedures. J Dermatol Surg Oncol. 1994;20:823-826.
- D’Acunto C, Raone B, Neri I, et al. Outpatient pediatric dermatologic surgery: experience in 296 patients. Pediatr Dermatol. 2015;32:424-426.
- Gunter JB. Benefit and risks of local anesthetics in infants and children. Paediatr Drugs. 2002;4:649-672.
- Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery [published online March 4, 2016]. J Am Acad Dermatol. 2016;74:1201-1219.
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876-882.
- Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834-841.
- Raper J, Alvarado MC, Murphy KL, et al. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084-1092.
- Davidson AJ. Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatric Anaesthesia. 2011;21:716-721.
- Davidson AJ, Disma N, de Graaff JC, et al; GAS consortium. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239-250.
- Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312-2320.
- General anesthetic and sedation drugs: drug safety communication—new warnings for young children and pregnant women. US Food and Drug Administration website. https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm533195.htm. Published December 14, 2016. Accessed July 25, 2017.
Vitiligo Patients Experience Barriers in Accessing Care
Vitiligo is a disorder typified by loss of pigmentation. Worldwide estimates of disease demonstrate 0.4% to 2% prevalence.1 Vitiligo generally is felt to be an autoimmune disorder with a complex multifactorial inheritance.2 Therapeutic options for vitiligo are largely off label and include topical corticosteroids, topical calcineurin inhibitors, narrowband UVB (NB-UVB) light phototherapy, and excimer (308 nm) laser therapy.3,4 Therapies for vitiligo are time consuming, as most topical therapies require twice-daily application. Additionally, many patients require 2 or more topical therapies due to involvement of both the head and neck as well as other body sites.3,4 Generalized disease often is treated with NB-UVB therapy 3 times weekly in-office visits, while excimer laser therapy is used for limited disease resistant to topical agents.3,4
Many barriers to good outcomes and care exist for patients with vitiligo.5 Patients may experience reduced quality of life and/or sexual dysfunction because of vitiligo lesions. The purpose of this pilot study was to identify barriers to access of care in vitiligo patients.
Methods
A survey was designed and then reviewed for unclear wording by members of the local vitiligo support group at Mount Sinai St. Luke’s-Roosevelt Hospital and Beth Israel Medical Centers (New York, New York). Linguistic revision and clarifications were added to the survey to correct identified communication problems. The survey was then posted using an Internet-based survey software. Links to the survey were sent via email to 107 individuals in a LISTSERV comprising Vitiligo Support International members who participated in a New York City support group (led by C.G. and N.B.S.). Only 1 email was used per household and only individuals 18 years or older could participate. These individuals were asked to complete a deidentified, 82-question, institutional review board–reviewed and exempted survey addressing issues affecting delivery and receipt of medical care for vitiligo.
Data were analyzed using the χ2 test, analysis of variance, or Student t test depending on the type of variable (categorical vs continuous). Fisher exact or Wilcoxon-Mann-Whitney tests were used when distributional assumptions were not met. A type I error rate (α=.05) was used to determine statistical significance. All analyses were performed using SAS 9.3 software.
Results
Respondents
The survey was completed by 81% (n=87) of individuals. The mean (SD) age of the treated patients about whom the respondents communicated was 33 (16) years and 71% (n=62) were women. The majority of respondents (64 [74%]) reported their race as white, followed by African American/black (12 [14%]), Hispanic (7 [8%]), and Asian (4 [5%]). Twenty-nine percent (22/76) of respondents reported a family income of less than $50,000 per year, 34% (26/76) reported an income of $50,000 to $100,000, and 37% (28/76) reported an income greater than $100,000, while 11 respondents did not report income.
Number of Physicians Seen
Respondents had reportedly seen an average (SD) number of 2 (1) physicians in the past/present before being offered any therapy for vitiligo and only 37% (32/87) of respondents reported being offered therapy by the first physician they saw. The number of physicians seen did not have a statistical relationship with years with vitiligo (ie, disease duration), sex, race, age of onset, income level, or number of sites affected.
Number of Sites Affected
The survey identified the following 23 sites affected by vitiligo: scalp, forehead, eyelids, lips, nose, cheeks, chin, neck, chest, stomach, back, upper arms, forearms, hands, wrists, fingers, genitalia, buttocks, thighs, calves/shins, ankles, feet, and toes. The average (SD) number of sites affected was 12 (6). The number of sites affected was correlated to the recommendation for phototherapy, while the recommendation for excimer laser therapy was inversely associated with the number of sites affected. The median number of sites affected for those who were not prescribed phototherapy was 10 (interquartile range [IQR]=9; P=.05); the median number of sites affected for those who were prescribed phototherapy was 15 (IQR=11). The association between the number of sites affected and whether the patient proceeded with phototherapy was not statistically significant. The need for phototherapy was not related to years with vitiligo (ie, disease duration), sex, or race.
Excimer laser therapy was prescribed more often to patients with fewer sites affected (median of 9 [IQR=3] vs median of 15 [IQR=9]; P=.04). Respondents who had fewer sites affected were on average more likely to proceed with excimer laser therapy (median of 8 [IQR=4] vs median of 11 [IQR=5]). The association between the number of sites affected and whether the patient proceeded with excimer laser therapy was not statistically significant.
Access to Topical Medications
Forty-one percent (36/87) of respondents reported difficulty accessing 1 or more topical therapies. Of 52 respondents who were prescribed a topical corticosteroid, 12 (23%) reported difficulty accessing therapy. Of 67 respondents who were prescribed a topical calcineurin inhibitor, 27 (40%) reported difficulty accessing medication (tacrolimus, n=17; pimecrolimus, n=10). Calcipotriene prescription coverage was not specifically addressed in this survey, as it usually is a second-line or adjunctive medication. Difficulty getting topical tacrolimus but not topical corticosteroids was associated with female sex (P=.03) but was not associated with race, income level, or level of education. Difficulty obtaining medication was not related to race, sex, level of education, or income level.
Consequences of Phototherapy
Twenty-three of 34 respondents (68%) who were told they required phototherapy actually received phototherapy and reported paying $38 weekly (IQR=$75). The majority of patients who proceeded with phototherapy lived (17/23 [74%]) or worked (16/23 [70%]) within 20 minutes of the therapy center. Self-reported response to phototherapy was good to very good in 65% (15/23) of respondents and no response in 30% (7/23); only 1 respondent reported worsening vitiligo. Sixty percent (15/25) of respondents said they were not satisfied with phototherapy. Respondents who were satisfied with the outcome of phototherapy had on average fewer sites affected by vitiligo (mean [SD], 10 [8]; P=.05). The association with other demographic and economic parameters (eg, sex, race, level of education, income level) was not statistically significant. Proceeding with phototherapy was not related to race, sex, level of education, or income level.
When questioned how many aspects of daily life (eg, work, home, school) were affected by phototherapy, 40% (35/87) of respondents reported that more than one life parameter was disturbed. Thirty-five percent (8/23) of respondents who received phototherapy reported that it affected their daily life “quite a bit” or “severely.” More respondents were likely to report that the therapy interfered with their life “somewhat,” “quite a bit,” or “severely” (76% [19/25]; 95% confidence interval, 55%-92%; P=.01) rather than “not at all” or “a little.”
Excimer Laser
Nine of 17 respondents (53%) who were recommended to undergo excimer laser therapy actually received therapy and reported paying $100 weekly (IQR=$60).
There was a trend toward significance of excimer usage being associated with lower age quartile (0–20 years)(P=.0553) and income more than $100,000 (P=.0788), neither of which reached statistical significance.
Insurance Coverage
Respondents were offered 7 answer options regarding the reason for noncoverage of topical calcineurin inhibitors. They were allowed to pick more than one reason where appropriate. For individuals who were prescribed topical tacrolimus but did not receive drug (n=17), the following reasons were cited: “no insurance coverage for the medication” (59% [10/17]), “your deductible was too high” (24% [4/17]), “prior authorization failed to produce coverage of the medication” (24% [4/17]), “your copay was prohibitively expensive” (24% [4/17]), “you were uncomfortable with the medication’s side effects” (18% [3/17]), “the tube was too small to cover your skin affected areas” (12% [2/17]), and “other” (29% [5/17]). Three patients selected 3 or more reasons, 8 patients selected 2 reasons, and 5 patients selected one reason.
Comment
It has been reported that patients with vitiligo may have difficulty related to treatment compliance for a variety of reasons.5 We identified notable barriers that arise for some, if not all, patients with vitiligo in the United States at some point in their care, including interference with other aspects of daily life, lack of coverage by current health insurance provider, and high out-of-pocket expenses, in addition to the negative effects of vitiligo on quality of life that have already been reported.6,7 These barriers are not a function of race/ethnicity, income level, or age of onset, but they may be impacted, as in the case of tacrolimus, by female sex. It is clear that, based on this study’s numbers, many patients will be unable to receive and/or comply with recommended treatment plans.
A limitation of this analysis is the study population, a select group of patients who had not been prescribed all the therapies in question. The sample size may not be large enough to demonstrate differences between level of education, race, or income level; however, even with a sample size of 87 respondents, the barriers to access of care are prominent. Larger population-based surveys would potentially tease out patterns of barriers not apparent with a smaller sample. No data were generated specific to calcipotriene, and this medication was not specified as a write-in agent on open question by any respondents; therefore, access to topical calcipotriene cannot be projected from this study. Phototherapy was queried as a nonspecific term and the breakdown of NB-UVB versus psoralen plus UVA was not available for this survey. Data suggesting a burden of socioeconomic barriers have been reported for atopic dermatitis8 and psoriasis,9 which corroborate the need for greater research in the field of access to care in dermatology.
Despite some advancement in the care of vitiligo, patients often are unable to access preferred or recommended treatment modalities. Standard recommendations for care are initial usage of calcineurin inhibitors for facial involvement and topical high-potency corticosteroids for involvement of the body.3,4 Based on this survey, it would seem that many patients are not able to receive the standard of care. Similarly, NB-UVB phototherapy and excimer laser therapy are recommended for widespread vitiligo and lesions unresponsive to topical care. It would seem that almost half of our respondents did not have access to one or more of the recommended therapies. Barriers to care may have substantial clinical and psychological outcomes, which were not evaluated in this study but merit future research.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206-1212.
- Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676-680.
- Silverberg NB. Pediatric vitiligo. Pediatr Clin North Am. 2014;61:347-366.
- Taieb A, Alomar A, Böhm M, et al, Vitiligo European Task Force (VETF); European Academy of Dermatology and Venereology (EADV); Union Europénne des Médecins Spécialistes (UEMS). Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168:5-19.
- Abraham S, Raghavan P. Myths and facts about vitiligo: an epidemiological study. Indian J Pharm Sci. 2015;77:8-13.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Hamilton MP, Ntais D, Griffiths CE, et al. Psoriasis treatment and management—a systematic review of full economic evaluations. Br J Dermatol. 2015;172:574-583.
Vitiligo is a disorder typified by loss of pigmentation. Worldwide estimates of disease demonstrate 0.4% to 2% prevalence.1 Vitiligo generally is felt to be an autoimmune disorder with a complex multifactorial inheritance.2 Therapeutic options for vitiligo are largely off label and include topical corticosteroids, topical calcineurin inhibitors, narrowband UVB (NB-UVB) light phototherapy, and excimer (308 nm) laser therapy.3,4 Therapies for vitiligo are time consuming, as most topical therapies require twice-daily application. Additionally, many patients require 2 or more topical therapies due to involvement of both the head and neck as well as other body sites.3,4 Generalized disease often is treated with NB-UVB therapy 3 times weekly in-office visits, while excimer laser therapy is used for limited disease resistant to topical agents.3,4
Many barriers to good outcomes and care exist for patients with vitiligo.5 Patients may experience reduced quality of life and/or sexual dysfunction because of vitiligo lesions. The purpose of this pilot study was to identify barriers to access of care in vitiligo patients.
Methods
A survey was designed and then reviewed for unclear wording by members of the local vitiligo support group at Mount Sinai St. Luke’s-Roosevelt Hospital and Beth Israel Medical Centers (New York, New York). Linguistic revision and clarifications were added to the survey to correct identified communication problems. The survey was then posted using an Internet-based survey software. Links to the survey were sent via email to 107 individuals in a LISTSERV comprising Vitiligo Support International members who participated in a New York City support group (led by C.G. and N.B.S.). Only 1 email was used per household and only individuals 18 years or older could participate. These individuals were asked to complete a deidentified, 82-question, institutional review board–reviewed and exempted survey addressing issues affecting delivery and receipt of medical care for vitiligo.
Data were analyzed using the χ2 test, analysis of variance, or Student t test depending on the type of variable (categorical vs continuous). Fisher exact or Wilcoxon-Mann-Whitney tests were used when distributional assumptions were not met. A type I error rate (α=.05) was used to determine statistical significance. All analyses were performed using SAS 9.3 software.
Results
Respondents
The survey was completed by 81% (n=87) of individuals. The mean (SD) age of the treated patients about whom the respondents communicated was 33 (16) years and 71% (n=62) were women. The majority of respondents (64 [74%]) reported their race as white, followed by African American/black (12 [14%]), Hispanic (7 [8%]), and Asian (4 [5%]). Twenty-nine percent (22/76) of respondents reported a family income of less than $50,000 per year, 34% (26/76) reported an income of $50,000 to $100,000, and 37% (28/76) reported an income greater than $100,000, while 11 respondents did not report income.
Number of Physicians Seen
Respondents had reportedly seen an average (SD) number of 2 (1) physicians in the past/present before being offered any therapy for vitiligo and only 37% (32/87) of respondents reported being offered therapy by the first physician they saw. The number of physicians seen did not have a statistical relationship with years with vitiligo (ie, disease duration), sex, race, age of onset, income level, or number of sites affected.
Number of Sites Affected
The survey identified the following 23 sites affected by vitiligo: scalp, forehead, eyelids, lips, nose, cheeks, chin, neck, chest, stomach, back, upper arms, forearms, hands, wrists, fingers, genitalia, buttocks, thighs, calves/shins, ankles, feet, and toes. The average (SD) number of sites affected was 12 (6). The number of sites affected was correlated to the recommendation for phototherapy, while the recommendation for excimer laser therapy was inversely associated with the number of sites affected. The median number of sites affected for those who were not prescribed phototherapy was 10 (interquartile range [IQR]=9; P=.05); the median number of sites affected for those who were prescribed phototherapy was 15 (IQR=11). The association between the number of sites affected and whether the patient proceeded with phototherapy was not statistically significant. The need for phototherapy was not related to years with vitiligo (ie, disease duration), sex, or race.
Excimer laser therapy was prescribed more often to patients with fewer sites affected (median of 9 [IQR=3] vs median of 15 [IQR=9]; P=.04). Respondents who had fewer sites affected were on average more likely to proceed with excimer laser therapy (median of 8 [IQR=4] vs median of 11 [IQR=5]). The association between the number of sites affected and whether the patient proceeded with excimer laser therapy was not statistically significant.
Access to Topical Medications
Forty-one percent (36/87) of respondents reported difficulty accessing 1 or more topical therapies. Of 52 respondents who were prescribed a topical corticosteroid, 12 (23%) reported difficulty accessing therapy. Of 67 respondents who were prescribed a topical calcineurin inhibitor, 27 (40%) reported difficulty accessing medication (tacrolimus, n=17; pimecrolimus, n=10). Calcipotriene prescription coverage was not specifically addressed in this survey, as it usually is a second-line or adjunctive medication. Difficulty getting topical tacrolimus but not topical corticosteroids was associated with female sex (P=.03) but was not associated with race, income level, or level of education. Difficulty obtaining medication was not related to race, sex, level of education, or income level.
Consequences of Phototherapy
Twenty-three of 34 respondents (68%) who were told they required phototherapy actually received phototherapy and reported paying $38 weekly (IQR=$75). The majority of patients who proceeded with phototherapy lived (17/23 [74%]) or worked (16/23 [70%]) within 20 minutes of the therapy center. Self-reported response to phototherapy was good to very good in 65% (15/23) of respondents and no response in 30% (7/23); only 1 respondent reported worsening vitiligo. Sixty percent (15/25) of respondents said they were not satisfied with phototherapy. Respondents who were satisfied with the outcome of phototherapy had on average fewer sites affected by vitiligo (mean [SD], 10 [8]; P=.05). The association with other demographic and economic parameters (eg, sex, race, level of education, income level) was not statistically significant. Proceeding with phototherapy was not related to race, sex, level of education, or income level.
When questioned how many aspects of daily life (eg, work, home, school) were affected by phototherapy, 40% (35/87) of respondents reported that more than one life parameter was disturbed. Thirty-five percent (8/23) of respondents who received phototherapy reported that it affected their daily life “quite a bit” or “severely.” More respondents were likely to report that the therapy interfered with their life “somewhat,” “quite a bit,” or “severely” (76% [19/25]; 95% confidence interval, 55%-92%; P=.01) rather than “not at all” or “a little.”
Excimer Laser
Nine of 17 respondents (53%) who were recommended to undergo excimer laser therapy actually received therapy and reported paying $100 weekly (IQR=$60).
There was a trend toward significance of excimer usage being associated with lower age quartile (0–20 years)(P=.0553) and income more than $100,000 (P=.0788), neither of which reached statistical significance.
Insurance Coverage
Respondents were offered 7 answer options regarding the reason for noncoverage of topical calcineurin inhibitors. They were allowed to pick more than one reason where appropriate. For individuals who were prescribed topical tacrolimus but did not receive drug (n=17), the following reasons were cited: “no insurance coverage for the medication” (59% [10/17]), “your deductible was too high” (24% [4/17]), “prior authorization failed to produce coverage of the medication” (24% [4/17]), “your copay was prohibitively expensive” (24% [4/17]), “you were uncomfortable with the medication’s side effects” (18% [3/17]), “the tube was too small to cover your skin affected areas” (12% [2/17]), and “other” (29% [5/17]). Three patients selected 3 or more reasons, 8 patients selected 2 reasons, and 5 patients selected one reason.
Comment
It has been reported that patients with vitiligo may have difficulty related to treatment compliance for a variety of reasons.5 We identified notable barriers that arise for some, if not all, patients with vitiligo in the United States at some point in their care, including interference with other aspects of daily life, lack of coverage by current health insurance provider, and high out-of-pocket expenses, in addition to the negative effects of vitiligo on quality of life that have already been reported.6,7 These barriers are not a function of race/ethnicity, income level, or age of onset, but they may be impacted, as in the case of tacrolimus, by female sex. It is clear that, based on this study’s numbers, many patients will be unable to receive and/or comply with recommended treatment plans.
A limitation of this analysis is the study population, a select group of patients who had not been prescribed all the therapies in question. The sample size may not be large enough to demonstrate differences between level of education, race, or income level; however, even with a sample size of 87 respondents, the barriers to access of care are prominent. Larger population-based surveys would potentially tease out patterns of barriers not apparent with a smaller sample. No data were generated specific to calcipotriene, and this medication was not specified as a write-in agent on open question by any respondents; therefore, access to topical calcipotriene cannot be projected from this study. Phototherapy was queried as a nonspecific term and the breakdown of NB-UVB versus psoralen plus UVA was not available for this survey. Data suggesting a burden of socioeconomic barriers have been reported for atopic dermatitis8 and psoriasis,9 which corroborate the need for greater research in the field of access to care in dermatology.
Despite some advancement in the care of vitiligo, patients often are unable to access preferred or recommended treatment modalities. Standard recommendations for care are initial usage of calcineurin inhibitors for facial involvement and topical high-potency corticosteroids for involvement of the body.3,4 Based on this survey, it would seem that many patients are not able to receive the standard of care. Similarly, NB-UVB phototherapy and excimer laser therapy are recommended for widespread vitiligo and lesions unresponsive to topical care. It would seem that almost half of our respondents did not have access to one or more of the recommended therapies. Barriers to care may have substantial clinical and psychological outcomes, which were not evaluated in this study but merit future research.
Vitiligo is a disorder typified by loss of pigmentation. Worldwide estimates of disease demonstrate 0.4% to 2% prevalence.1 Vitiligo generally is felt to be an autoimmune disorder with a complex multifactorial inheritance.2 Therapeutic options for vitiligo are largely off label and include topical corticosteroids, topical calcineurin inhibitors, narrowband UVB (NB-UVB) light phototherapy, and excimer (308 nm) laser therapy.3,4 Therapies for vitiligo are time consuming, as most topical therapies require twice-daily application. Additionally, many patients require 2 or more topical therapies due to involvement of both the head and neck as well as other body sites.3,4 Generalized disease often is treated with NB-UVB therapy 3 times weekly in-office visits, while excimer laser therapy is used for limited disease resistant to topical agents.3,4
Many barriers to good outcomes and care exist for patients with vitiligo.5 Patients may experience reduced quality of life and/or sexual dysfunction because of vitiligo lesions. The purpose of this pilot study was to identify barriers to access of care in vitiligo patients.
Methods
A survey was designed and then reviewed for unclear wording by members of the local vitiligo support group at Mount Sinai St. Luke’s-Roosevelt Hospital and Beth Israel Medical Centers (New York, New York). Linguistic revision and clarifications were added to the survey to correct identified communication problems. The survey was then posted using an Internet-based survey software. Links to the survey were sent via email to 107 individuals in a LISTSERV comprising Vitiligo Support International members who participated in a New York City support group (led by C.G. and N.B.S.). Only 1 email was used per household and only individuals 18 years or older could participate. These individuals were asked to complete a deidentified, 82-question, institutional review board–reviewed and exempted survey addressing issues affecting delivery and receipt of medical care for vitiligo.
Data were analyzed using the χ2 test, analysis of variance, or Student t test depending on the type of variable (categorical vs continuous). Fisher exact or Wilcoxon-Mann-Whitney tests were used when distributional assumptions were not met. A type I error rate (α=.05) was used to determine statistical significance. All analyses were performed using SAS 9.3 software.
Results
Respondents
The survey was completed by 81% (n=87) of individuals. The mean (SD) age of the treated patients about whom the respondents communicated was 33 (16) years and 71% (n=62) were women. The majority of respondents (64 [74%]) reported their race as white, followed by African American/black (12 [14%]), Hispanic (7 [8%]), and Asian (4 [5%]). Twenty-nine percent (22/76) of respondents reported a family income of less than $50,000 per year, 34% (26/76) reported an income of $50,000 to $100,000, and 37% (28/76) reported an income greater than $100,000, while 11 respondents did not report income.
Number of Physicians Seen
Respondents had reportedly seen an average (SD) number of 2 (1) physicians in the past/present before being offered any therapy for vitiligo and only 37% (32/87) of respondents reported being offered therapy by the first physician they saw. The number of physicians seen did not have a statistical relationship with years with vitiligo (ie, disease duration), sex, race, age of onset, income level, or number of sites affected.
Number of Sites Affected
The survey identified the following 23 sites affected by vitiligo: scalp, forehead, eyelids, lips, nose, cheeks, chin, neck, chest, stomach, back, upper arms, forearms, hands, wrists, fingers, genitalia, buttocks, thighs, calves/shins, ankles, feet, and toes. The average (SD) number of sites affected was 12 (6). The number of sites affected was correlated to the recommendation for phototherapy, while the recommendation for excimer laser therapy was inversely associated with the number of sites affected. The median number of sites affected for those who were not prescribed phototherapy was 10 (interquartile range [IQR]=9; P=.05); the median number of sites affected for those who were prescribed phototherapy was 15 (IQR=11). The association between the number of sites affected and whether the patient proceeded with phototherapy was not statistically significant. The need for phototherapy was not related to years with vitiligo (ie, disease duration), sex, or race.
Excimer laser therapy was prescribed more often to patients with fewer sites affected (median of 9 [IQR=3] vs median of 15 [IQR=9]; P=.04). Respondents who had fewer sites affected were on average more likely to proceed with excimer laser therapy (median of 8 [IQR=4] vs median of 11 [IQR=5]). The association between the number of sites affected and whether the patient proceeded with excimer laser therapy was not statistically significant.
Access to Topical Medications
Forty-one percent (36/87) of respondents reported difficulty accessing 1 or more topical therapies. Of 52 respondents who were prescribed a topical corticosteroid, 12 (23%) reported difficulty accessing therapy. Of 67 respondents who were prescribed a topical calcineurin inhibitor, 27 (40%) reported difficulty accessing medication (tacrolimus, n=17; pimecrolimus, n=10). Calcipotriene prescription coverage was not specifically addressed in this survey, as it usually is a second-line or adjunctive medication. Difficulty getting topical tacrolimus but not topical corticosteroids was associated with female sex (P=.03) but was not associated with race, income level, or level of education. Difficulty obtaining medication was not related to race, sex, level of education, or income level.
Consequences of Phototherapy
Twenty-three of 34 respondents (68%) who were told they required phototherapy actually received phototherapy and reported paying $38 weekly (IQR=$75). The majority of patients who proceeded with phototherapy lived (17/23 [74%]) or worked (16/23 [70%]) within 20 minutes of the therapy center. Self-reported response to phototherapy was good to very good in 65% (15/23) of respondents and no response in 30% (7/23); only 1 respondent reported worsening vitiligo. Sixty percent (15/25) of respondents said they were not satisfied with phototherapy. Respondents who were satisfied with the outcome of phototherapy had on average fewer sites affected by vitiligo (mean [SD], 10 [8]; P=.05). The association with other demographic and economic parameters (eg, sex, race, level of education, income level) was not statistically significant. Proceeding with phototherapy was not related to race, sex, level of education, or income level.
When questioned how many aspects of daily life (eg, work, home, school) were affected by phototherapy, 40% (35/87) of respondents reported that more than one life parameter was disturbed. Thirty-five percent (8/23) of respondents who received phototherapy reported that it affected their daily life “quite a bit” or “severely.” More respondents were likely to report that the therapy interfered with their life “somewhat,” “quite a bit,” or “severely” (76% [19/25]; 95% confidence interval, 55%-92%; P=.01) rather than “not at all” or “a little.”
Excimer Laser
Nine of 17 respondents (53%) who were recommended to undergo excimer laser therapy actually received therapy and reported paying $100 weekly (IQR=$60).
There was a trend toward significance of excimer usage being associated with lower age quartile (0–20 years)(P=.0553) and income more than $100,000 (P=.0788), neither of which reached statistical significance.
Insurance Coverage
Respondents were offered 7 answer options regarding the reason for noncoverage of topical calcineurin inhibitors. They were allowed to pick more than one reason where appropriate. For individuals who were prescribed topical tacrolimus but did not receive drug (n=17), the following reasons were cited: “no insurance coverage for the medication” (59% [10/17]), “your deductible was too high” (24% [4/17]), “prior authorization failed to produce coverage of the medication” (24% [4/17]), “your copay was prohibitively expensive” (24% [4/17]), “you were uncomfortable with the medication’s side effects” (18% [3/17]), “the tube was too small to cover your skin affected areas” (12% [2/17]), and “other” (29% [5/17]). Three patients selected 3 or more reasons, 8 patients selected 2 reasons, and 5 patients selected one reason.
Comment
It has been reported that patients with vitiligo may have difficulty related to treatment compliance for a variety of reasons.5 We identified notable barriers that arise for some, if not all, patients with vitiligo in the United States at some point in their care, including interference with other aspects of daily life, lack of coverage by current health insurance provider, and high out-of-pocket expenses, in addition to the negative effects of vitiligo on quality of life that have already been reported.6,7 These barriers are not a function of race/ethnicity, income level, or age of onset, but they may be impacted, as in the case of tacrolimus, by female sex. It is clear that, based on this study’s numbers, many patients will be unable to receive and/or comply with recommended treatment plans.
A limitation of this analysis is the study population, a select group of patients who had not been prescribed all the therapies in question. The sample size may not be large enough to demonstrate differences between level of education, race, or income level; however, even with a sample size of 87 respondents, the barriers to access of care are prominent. Larger population-based surveys would potentially tease out patterns of barriers not apparent with a smaller sample. No data were generated specific to calcipotriene, and this medication was not specified as a write-in agent on open question by any respondents; therefore, access to topical calcipotriene cannot be projected from this study. Phototherapy was queried as a nonspecific term and the breakdown of NB-UVB versus psoralen plus UVA was not available for this survey. Data suggesting a burden of socioeconomic barriers have been reported for atopic dermatitis8 and psoriasis,9 which corroborate the need for greater research in the field of access to care in dermatology.
Despite some advancement in the care of vitiligo, patients often are unable to access preferred or recommended treatment modalities. Standard recommendations for care are initial usage of calcineurin inhibitors for facial involvement and topical high-potency corticosteroids for involvement of the body.3,4 Based on this survey, it would seem that many patients are not able to receive the standard of care. Similarly, NB-UVB phototherapy and excimer laser therapy are recommended for widespread vitiligo and lesions unresponsive to topical care. It would seem that almost half of our respondents did not have access to one or more of the recommended therapies. Barriers to care may have substantial clinical and psychological outcomes, which were not evaluated in this study but merit future research.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206-1212.
- Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676-680.
- Silverberg NB. Pediatric vitiligo. Pediatr Clin North Am. 2014;61:347-366.
- Taieb A, Alomar A, Böhm M, et al, Vitiligo European Task Force (VETF); European Academy of Dermatology and Venereology (EADV); Union Europénne des Médecins Spécialistes (UEMS). Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168:5-19.
- Abraham S, Raghavan P. Myths and facts about vitiligo: an epidemiological study. Indian J Pharm Sci. 2015;77:8-13.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Hamilton MP, Ntais D, Griffiths CE, et al. Psoriasis treatment and management—a systematic review of full economic evaluations. Br J Dermatol. 2015;172:574-583.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206-1212.
- Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676-680.
- Silverberg NB. Pediatric vitiligo. Pediatr Clin North Am. 2014;61:347-366.
- Taieb A, Alomar A, Böhm M, et al, Vitiligo European Task Force (VETF); European Academy of Dermatology and Venereology (EADV); Union Europénne des Médecins Spécialistes (UEMS). Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168:5-19.
- Abraham S, Raghavan P. Myths and facts about vitiligo: an epidemiological study. Indian J Pharm Sci. 2015;77:8-13.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Hamilton MP, Ntais D, Griffiths CE, et al. Psoriasis treatment and management—a systematic review of full economic evaluations. Br J Dermatol. 2015;172:574-583.
Practice Points
- Patients with vitiligo may experience difficulty receiving the care prescribed to them.
- It is best to identify barriers such as work schedule or distance before recommending a treatment plan.
Pediatric Rosacea
Rosacea is a chronic skin disease characterized by flushing, erythema, telangiectasia, papules, and pustules in the central face region.1 It most often affects middle-aged women (age range, 30–50 years).2 Rosacea is rare in the pediatric population, especially before puberty.3 There are 3 subtypes of pediatric rosacea: vascular, papulopustular, and ocular. Phymatous/rhinophymatous rosacea is only seen in the adult population.3 Recommendations for the management of pediatric rosacea heavily rely on data from retrospective chart reviews and case series.
Etiology of Pediatric Rosacea
Rosacea is thought to be a consequence of vasomotor instability in both adults and children. A family history of rosacea is sometimes reported in patients with pediatric rosacea.4 Patients often are sensitive to heat, sunlight, topical corticosteroids, spicy foods, hot liquids, and certain soaps and cleansers.1,3,4 In a review of the literature by Vemuri et al,5 the various reported triggers of rosacea include harsh climates that damage the blood vessels and dermal connective tissue, defects in the endothelium and dermal matrix, perivascular inflammation, orally ingested chemicals, changes in the flora of the hair follicles, excessive antimicrobial peptides, and the presence of free radicals. Overall, it is unclear which of these factors are triggers of pediatric rosacea.
The molecular basis of rosacea has been elucidated. It is well known that rosacea patients have higher levels of cathelicidins in the facial skin. Furthermore, they appear to have different processed forms of cathelicidin peptides compared to adults without rosacea, possibly due to changes in posttranslational processing.6 One such peptide, cathelicidin LL-37, also has been implicated in atopic dermatitis7 and psoriasis.8 Its role in rosacea appears to be multifaceted. Cathelicidin LL-37 helps to attract neutrophils, monocytes, and T lymphocytes, and also has antimicrobial properties; therefore, it plays a role in both the innate and adaptive immune systems.9 Cathelicidin LL-37 also has been implicated in inducing angiogenesis10 and suppressing dermal fibroblasts.11
Muto et al12 found that there is an increased number of mast cells in the dermis of patients with rosacea. Mast cells contribute to vasodilation, angiogenesis, and the recruitment of other inflammatory cells.12 Importantly, human mast cells are a source of cathelicidins including cathelicidin LL-37; these proteins play a vital role in the antimicrobial capabilities of mast cells.13
Clinical Presentation and Comorbidities
Vascular rosacea presents with characteristic flushing and erythema, which lasts more than a few minutes as compared to physiologic erythema,1 and sometimes telangiectasia is seen.3 The cheeks, chin, and nasolabial folds are most commonly involved.2 In papulopustular rosacea, papules and pustules are seen overlying the erythema.1,3 Open and closed comedones also have been documented in case reports but are not commonly seen.2 Pediatric rosacea often begins with flushing of the face and then progresses to the development of papules and pustules.4
Ocular rosacea can occur with or without cutaneous findings. In a retrospective study of 20 pediatric patients (aged 1–15 years), 11 (55%) patients had both ocular and cutaneous rosacea, 3 (15%) only had ocular symptoms, and 6 (30%) only had cutaneous symptoms. The most common form of rosacea in this study was papulopustular rosacea.14 Ocular symptoms often are bilateral15 and can include blepharitis, conjunctival injection, recurrent chalazion, conjunctivitis,2 and less commonly corneal ulceration and scarring.16 Patients also may report photophobia or a foreign body sensation.17 Importantly, ocular symptoms often precede the cutaneous symptoms and can delay the diagnosis of rosacea,14,16,18 as these symptoms often are misdiagnosed as viral or bacterial infections.15 Fortunately, ocular disease responds well to treatment if diagnosed early.
Weston and Morelli19 conducted a retrospective study of 106 children (46 males; 60 females) 13 years and younger with steroid rosacea; 29 children were younger than 3 years. A family history of rosacea was present in 20% of participants, and prior use of class 7 steroids was reported in 54%, whereas only 3% had used class 1 topical steroids. Ninety-eight participants had perinasal involvement, 94 had perioral involvement, and 44 had periorbital involvement of the lower eyelids.19
Rosacea fulminans (also known as pyoderma faciale) is a rare acute-onset eruption typically found in young women in their 20s and 30s.20 Rosacea fulminans is characterized by papules, pustules, nodules, cysts, draining sinuses, communicating sinus tracts, and less commonly comedones.20,21 The skin can appear erythematous, cyanotic, or dull red.21 Most of the lesions are found on the face, particularly on the forehead, cheeks, nose, and chin,21 but lesions on the chest and back have been documented in adult patients.20 In an examination of prior case series, most patients were otherwise healthy. There are case reports documenting rosacea fulminans in teenagers,20 but the youngest patient recorded was an otherwise healthy 3-year-old girl who developed a sudden onset of erythematous papules, pustules, cysts, and purulent discharging sinuses on the cheeks that spread to the chin, perioral, and paranasal areas.21
Differential Diagnosis
Rosacea is rare in children, so other papulopustular disorders must be ruled out, including acne vulgaris, periorificial/perioral dermatitis, sarcoidosis, systemic lupus erythematosus, steroid-induced rosacea, ataxia telangiectasia, and demodicosis.
Acne vulgaris commonly presents in older adolescents and teenagers with open and closed comedones, inflammatory papules, and pustules.2 Intense facial flushing and telangiectasia usually is not seen.
In perioral dermatitis, skin lesions often are clustered around the mouth, nose, and eyes. Typically there are no telangiectases or ocular complications.3 Facial flushing and telangiectases are uncommon, except in steroid-induced perioral dermatitis.2
The cutaneous findings of sarcoidosis include red-brown papules on the face and lips, and patients also may have ocular involvement such as uveitis and iritis.3 However, there are typically other systemic findings such as pulmonary symptoms, weight loss, fatigue, lethargy, fever, and erythema nodosum.2,3 Chest radiograph findings (eg, bilateral hilar lym-phadenopathy), ophthalmologic examination, and laboratory data (eg, elevated alkaline phosphate and/or elevated angiotensin-converting enzyme) can help confirm or rule out the diagnosis of sarcoidosis.2,3
Unlike systemic lupus erythematosus, patients with rosacea will have involvement of sun-protected areas of the skin. Patients with systemic lupus erythematosus typically report arthralgia and severe photosensitivity and will have elevated antinuclear antibody titers. Skin biopsies and immunofluorescence can help confirm the diagnosis.3 Importantly, some patients with rosacea will have a positive lupus band test.22,23
Steroid-induced rosacea typically occurs 2 weeks after discontinuing therapy with topical fluorinated glucocorticosteroids.24 Children present with monomorphic papules, pustules, and telangiectases4 on the eyelids and lateral face as opposed to the central face regions.24
Ataxia telangiectasia can present with telangiectases, skin atrophy, café au lait spots, and premature graying.25 A 15-year-old adolescent girl with ataxia telangiectasia presented with granulomatous acne rosacea that improved after 4 weeks of treatment with isotretinoin 0.5 mg/kg daily. The lesions cleared almost completely after 5 months.25
Demodicosis is a disorder of the pilosebaceous units caused by the human Demodex mite.26 It typically involves the periorificial regions in adults and the elderly population. Patients can present with fine, white-yellow, scaly changes of the sebaceous hair follicles, with minimal erythema and inflammation. Papules and pustules also can be present.26
Diagnosis and Histopathology
Because rosacea is rare in children, it is important to thoroughly evaluate other possible diagnoses. The diagnosis of pediatric rosacea is clinical and biopsies are rarely performed. Laboratory tests such as cultures generally are not useful.
Marks and Harcourt-Webster27 reviewed the biopsies of 108 adult patients with rosacea. The biopsies of patients with predominantly erythema and telangiectasia showed evidence of vascular dilatation with a perivascular infiltrate composed predominantly of lymphocytes and 39 specimens that were compared to controls showed more solar elastosis. Biopsies of papular rosacea contained inflammatory infiltrates in the upper and mid dermis composed primarily of lymphocytes and histiocytes. In some patients, neutrophils, plasma cells, and giant cells also were observed. Hair follicle abnormalities were present in 20% of the biopsies, with 19% showing evidence of the Demodex mite. Vascular dilatation also was common. Overall, common findings included lymphohistiocytic infiltrates around the blood vessels of the upper dermis, dilated vessels, edema, elastosis, and disorganization of connective tissue in the upper dermis.
Helm et al28 reviewed histopathologic patterns from 53 patients with granulomatous rosacea. Findings included a mixed lymphohistiocytic infiltrate (predominantly lymphocytic in 40% of patients and predominantly histiocytic with occasional giant cells in 34% of patients), epithelioid granulomas (11% of patients), and epithelioid granulomas with caseation necrosis (11% of patients).
The histopathology of rosacea fulminans is characterized by dense perivascular and periadnexal infiltrates composed of granulocytes, eosinophils, and epithelioid granulomas, as well as panniculitis.20
Treatment and Clinical Outcomes
Certain lifestyle recommendations are integral components of disease management, including avoidance of triggers such as extreme temperatures, hot drinks, spicy food, and topical agents that could be irritating (especially topical corticosteroids).29 Patients should be advised to use daily sunscreen containing physical blockers such as titanium dioxide or zinc oxide. Teenagers should avoid the use of cosmetics and makeup, especially products containing sodium lauryl sulfate, menthol, and camphor. Daily use of emollients can help some patients.29
There are both topical and systemic therapies available for pediatric rosacea; however, most of the data are based on the use of these treatments in the adult population. Patients with mild to moderate disease often can be managed using topical agents. Metronidazole (0.75% cream, 1% gel, or 0.75% lotion) has been studied extensively in adult patients, and when used once daily for 12 weeks, it has been able to control moderate to severe disease.30,31 In one study conducted in adult patients, topical metronidazole was able to maintain remission in adults who had previously been treated with a combination of oral tetracycline and metronidazole gel.31 Sodium sulfacetamide 10%–sulfur 5% (cleanser or lotion) has been successful in adult patients and often is used in combination with other therapies such as topical metronidazole.32-34 Azelaic acid cream 20%,35 benzoyl peroxide (wash or gel),29 topical clindamycin,36 topical erythromycin,29,37 tacrolimus ointment 0.1%,38 and tretinoin cream also have been studied in adults.3,39 Several of these topical agents can cause irritation on application (eg, metronidazole, sulfur-based agents, azelaic acid, benzoyl peroxide, erythromycin, tretinoin).3
The use of systemic treatments in pediatric patients is heavily based on case reports and case series.2,14,16,40 Therapies have included tetracycline (500 mg twice daily tapered to 250 mg daily),29 minocycline (50–100 mg twice daily), doxycycline (50–100 mg twice daily or 4 times daily), erythromycin (30–50 mg/kg daily), clarithromycin (15 mg/kg twice daily for 4 weeks and then daily for 4 weeks), and azithromycin (5–10 mg/kg daily).3 Tetracycline antibiotics should not be used in children 8 years or younger.
In a case series by Drolet and Paller,2 an 11-year-old girl was treated with tetracycline 500 mg (later tapered to 250 mg daily) and metronidazole gel 0.75%, both used twice daily. Previously, she had not responded to topical steroids, tretinoin cream 0.05%, benzoyl peroxide 5%, or systemic prednisone. After 6 weeks of treatment, the pustules and chalazion had resolved and she had only minimal erythema of the skin and conjunctiva. Sixteen months after the start of treatment, a regimen of tetracycline 250 mg daily and metronidazole gel resulted in disease clearance on the face.2
A 9-year-old girl with concurrent systemic lupus erythematosus was treated with tetracycline 250 mg and topical erythromycin 2%, both used twice daily.2 After 4 weeks her face was clear. Four months later she developed new telangiectases and topical erythromycin was replaced with topical metronidazole. Eventually the dose of tetracycline was reduced to 250 mg daily.2
An 11-year-old boy with likely granulomatous rosacea was treated with erythromycin 250 mg 4 times daily, alclometasone dipropionate cream 0.05% twice daily, and topical clindamycin twice daily.2 Marked improvement was noticed after 3 weeks of treatment. Metronidazole gel 0.75% was added and 3 months later the patient’s face was clear, without evidence of scarring. The dose of erythromycin was later reduced to 500 mg daily, and eventually the patient experienced clearance with the use of metronidazole gel daily.2
In another case series, 4 female patients (age range, 4–12 years) were treated with systemic erythromycin 20 mg/kg daily (ocular involvement only) or doxycycline 2.2 mg/kg daily used in two 12-year-old patients with ocular and cutaneous involvement for at least 12 months. All 4 patients showed considerable improvement within 1 month and remained free of disease throughout a mean follow-up period of 25.5 months.40
As evidenced by these case reports, there is a wide array of treatments that have been used for pediatric rosacea. Although there are no formal evidence-based guidelines, there are certain considerations that must be taken into account when choosing treatment plans. Doxycycline and minocycline are known to cause less gastrointestinal upset than tetracycline with similar efficacy.41 Importantly, the tetracyclines are contraindicated in children younger than 9 years, as they can cause teeth staining and possibly affect skeletal growth.3,4 When used in older children (age range, 9–12 years), patients must be advised not to take their medication with calcium or antacids.3 Clarithromycin and azithromycin tend to have fewer gastrointestinal side effects than erythromycin. Erythromycin and other macrolides can be used in children of all ages and in patients who are allergic to tetracyclines.3
Children with mild ocular symptoms often can control their disease with bacitracin and topical ocular antibiotics such as erythromycin.2,15 For patients who require systemic antibiotics, various tetracyclines and macrolides have been used with success.2,14-16,40
Adults with rosacea fulminans can require treatment with isotretinoin, oral antibiotics, and topical or even systemic corticosteroids.42 The 3-year-old girl with rosacea fulminans initially was treated with oral erythromycin (250 mg 4 times daily), oral prednisolone (0.5 mg/kg daily tapered over 2 weeks), fluocinolone acetonide cream 0.025%, and warm compresses with only moderate improvement.21 She was then started on oral isotretinoin (0.75 mg/kg daily) and within 4 weeks marked improvement was noted. After 8 weeks, the lesions had disappeared completely with only a few pitted scars remaining. Isotretinoin was continued for 24 weeks. One year after completion of treatment, she was still disease free.21
Weston and Morelli19 recommended the following treatment regimen for children with steroid rosacea: abrupt cessation of topical steroid use (as opposed to gradual withdrawal) and initiation of oral erythromycin stearate (30 mg/kg daily) in 2 daily doses for 4 weeks. Children who were unable to tolerate erythromycin (n=6) were told to use topical clindamycin phosphate twice daily for 4 weeks. Within 3 weeks 22% of patients had resolution, while 86% had resolution within 4 weeks. All of the patients cleared within 8 weeks. Importantly, there was no significant difference in duration of time until clearance between children who used the oral antibiotic or topical antibiotic.19
Conclusion
We know that the skin of rosacea patients contains higher levels of cathelicidins, which have been implicated in amplifying and contributing to the inflammatory response in several ways. Mast cells, which are a source of cathelicidins, also are increased in the skin of these patients. Children can present with vascular rosacea (characterized by flushing, erythema, and/or telangiectasia), papulopustular rosacea, or ocular rosacea. Common ocular symptoms include blepharitis, conjunctivitis, and recurrent chalazion. It is important to refer pediatric rosacea patients with ocular symptoms to an ophthalmologist to prevent ocular sequelae.
Rosacea is a clinical diagnosis but biopsy can be performed to rule out other diagnoses. Treatment consists of lifestyle modifications such as avoiding known triggers and the use of topical and/or oral agents. Common topical therapies include metronidazole and erythromycin. Systemic antibiotics include tetracycline, doxycycline, minocycline, azithromycin, and erythromycin. Some children are able to taper systemic agents and maintain disease control with topical therapy, while others may need to continue a low-dose antibiotic. Although flares can be controlled within weeks to months, rosacea is a chronic disorder and childhood rosacea tends to persist into adulthood.
- Crawford GH, Pelle MT, James WD. Rosacea: i. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Drolet B, Paller AS. Childhood rosacea. Pediatr Dermatol. 1992;9:22-26.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Lacz NL, Schwartz RA. Rosacea in the pediatric population. Cutis. 2004;74:99-103.
- Vemuri RC, Gundamaraju R, Sekaran SD, et al. Major pathophysiological correlations of rosacea: a complete clinical appraisal. Int J Med Sci. 2015;12:387-396.
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
- Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151-1160.
- Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564-569.
- De Y, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069-1074.
- Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665-1672.
- Park HJ, Cho DH, Kim HJ, et al. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL-37. J Invest Dermatol. 2009;129:843-850.
- Muto Y, Wang Z, Vanderberghe M, et al. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J Invest Dermatol. 2014;134:2728-2736.
- Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274-2278.
- Leoni S, Mesplie N, Aitali F, et al. Metronidazole: alternative treatment for ocular and cutaneous rosacea in the pediatric population [in French]. J Fr Ophthalmol. 2011;34:703-710.
- Nazir SA, Murphy S, Siatkowski RM, et al. Ocular rosacea in childhood. Am J Ophthalmol. 2004;137:138-144.
- Miguel AI, Salgado MB, Lisboa MS, et al. Pediatric ocular rosacea: 2 cases. Eur J Ophthalmol. 2012;22:664-666.
- Stone DU, Chodosh J. Ocular rosacea: an update on pathogenesis and therapy. Curr Opin Ophthalmol. 2004;15:499-502.
- Mavrakanas N, Schutz JS, Dosso AA. Pediatric ocular rosacea [published online March 22, 2010]. J Pediatr Ophthalmol Strabismus. 2010;47:117-120.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Plewig G, Jansen T, Kligman AM. Pyoderma faciale. a review and report of 20 additional cases: is it rosacea? Arch Dermatol. 1992;128:1611-1617.
- Firooz A, Firoozabadi MR, Dowlati Y. Rosacea fulminans (pyoderma faciale): successful treatment of a 3-year-old girl with oral isotretinoin. Int J Dermatol. 2001;40:203-205.
- Baart de la Faille H, Baart de la F-K. Immunofluorescent studies of the skin in rosacea. Dermatologica. 1969;139:49-54.
- Wilkin JK. Rosacea. Int J Dermatol. 1983;22:393-400.
- Franco HL, Weston WL. Steroid rosacea in children. Pediatrics. 1979;64:36-38.
- Cantarutti N, Claps A, Angelino G, et al. Multi-drugs resistant acne rosacea in a child affected by ataxia-telangiectasia: successful treatment with isotretinoin. Ital J Pediatr. 2015;41:23.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Marks R, Harcourt-Webster J. Histopathology of rosacea. Arch Dermatol. 1969;100:683-691.
- Helm KF, Menz J, Gibson LE, et al. A clinical and histopathologic study of granulomatous rosacea. J Am Acad Dermatol. 1991;25(6, pt 1):1038-1043.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512; quiz 513-494.
- Dahl MV, Jarratt M, Kaplan D, et al. Once-daily topical metronidazole cream formulations in the treatment of the papules and pustules of rosacea. J Am Acad Dermatol. 2001;45:723-730.
- Dahl MV, Katz HI, Krueger GG, et al. Topical metronidazole maintains remissions of rosacea. Arch Dermatol. 1998;134:679-683.
- Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
- Del Rosso JQ. Evaluating the role of topical therapies in the management of rosacea: focus on combination sodium sulfacetamide and sulfur formulations. Cutis. 2004;73(1 suppl):29-33.
- Del Rosso JQ. A status report on the medical management of rosacea: focus on topical therapies. Cutis. 2002;70:271-275.
- Maddin S. A comparison of topical azelaic acid 20% cream and topical metronidazole 0.75% cream in the treatment of patients with papulopustular rosacea. J Am Acad Dermatol. 1999;40(6, pt 1):961-965.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Mills OH Jr, Kligman AM. Letter: topically applied erythromycin in rosacea. Arch Dermatol. 1976;112:553-554.
- Hengge UR. Off-label indications for topical tacrolimus [in German]. Hautarzt. 2013;64:752-756.
- Ertl GA, Levine N, Kligman AM. A comparison of the efficacy of topical tretinoin and low-dose oral isotretinoin in rosacea. Arch Dermatol. 1994;130:319-324.
- Cetinkaya A, Akova YA. Pediatric ocular acne rosacea: long-term treatment with systemic antibiotics. Am J Ophthalmol. 2006;142:816-821.
- Maibach H. Second-generation tetracyclines, a dermatologic overview: clinical uses and pharmacology. Cutis. 1991;48:411-417.
- Jansen T, Plewig G, Kligman AM. Diagnosis and treatment of rosacea fulminans. Dermatology. 1994;188:251-254.
Rosacea is a chronic skin disease characterized by flushing, erythema, telangiectasia, papules, and pustules in the central face region.1 It most often affects middle-aged women (age range, 30–50 years).2 Rosacea is rare in the pediatric population, especially before puberty.3 There are 3 subtypes of pediatric rosacea: vascular, papulopustular, and ocular. Phymatous/rhinophymatous rosacea is only seen in the adult population.3 Recommendations for the management of pediatric rosacea heavily rely on data from retrospective chart reviews and case series.
Etiology of Pediatric Rosacea
Rosacea is thought to be a consequence of vasomotor instability in both adults and children. A family history of rosacea is sometimes reported in patients with pediatric rosacea.4 Patients often are sensitive to heat, sunlight, topical corticosteroids, spicy foods, hot liquids, and certain soaps and cleansers.1,3,4 In a review of the literature by Vemuri et al,5 the various reported triggers of rosacea include harsh climates that damage the blood vessels and dermal connective tissue, defects in the endothelium and dermal matrix, perivascular inflammation, orally ingested chemicals, changes in the flora of the hair follicles, excessive antimicrobial peptides, and the presence of free radicals. Overall, it is unclear which of these factors are triggers of pediatric rosacea.
The molecular basis of rosacea has been elucidated. It is well known that rosacea patients have higher levels of cathelicidins in the facial skin. Furthermore, they appear to have different processed forms of cathelicidin peptides compared to adults without rosacea, possibly due to changes in posttranslational processing.6 One such peptide, cathelicidin LL-37, also has been implicated in atopic dermatitis7 and psoriasis.8 Its role in rosacea appears to be multifaceted. Cathelicidin LL-37 helps to attract neutrophils, monocytes, and T lymphocytes, and also has antimicrobial properties; therefore, it plays a role in both the innate and adaptive immune systems.9 Cathelicidin LL-37 also has been implicated in inducing angiogenesis10 and suppressing dermal fibroblasts.11
Muto et al12 found that there is an increased number of mast cells in the dermis of patients with rosacea. Mast cells contribute to vasodilation, angiogenesis, and the recruitment of other inflammatory cells.12 Importantly, human mast cells are a source of cathelicidins including cathelicidin LL-37; these proteins play a vital role in the antimicrobial capabilities of mast cells.13
Clinical Presentation and Comorbidities
Vascular rosacea presents with characteristic flushing and erythema, which lasts more than a few minutes as compared to physiologic erythema,1 and sometimes telangiectasia is seen.3 The cheeks, chin, and nasolabial folds are most commonly involved.2 In papulopustular rosacea, papules and pustules are seen overlying the erythema.1,3 Open and closed comedones also have been documented in case reports but are not commonly seen.2 Pediatric rosacea often begins with flushing of the face and then progresses to the development of papules and pustules.4
Ocular rosacea can occur with or without cutaneous findings. In a retrospective study of 20 pediatric patients (aged 1–15 years), 11 (55%) patients had both ocular and cutaneous rosacea, 3 (15%) only had ocular symptoms, and 6 (30%) only had cutaneous symptoms. The most common form of rosacea in this study was papulopustular rosacea.14 Ocular symptoms often are bilateral15 and can include blepharitis, conjunctival injection, recurrent chalazion, conjunctivitis,2 and less commonly corneal ulceration and scarring.16 Patients also may report photophobia or a foreign body sensation.17 Importantly, ocular symptoms often precede the cutaneous symptoms and can delay the diagnosis of rosacea,14,16,18 as these symptoms often are misdiagnosed as viral or bacterial infections.15 Fortunately, ocular disease responds well to treatment if diagnosed early.
Weston and Morelli19 conducted a retrospective study of 106 children (46 males; 60 females) 13 years and younger with steroid rosacea; 29 children were younger than 3 years. A family history of rosacea was present in 20% of participants, and prior use of class 7 steroids was reported in 54%, whereas only 3% had used class 1 topical steroids. Ninety-eight participants had perinasal involvement, 94 had perioral involvement, and 44 had periorbital involvement of the lower eyelids.19
Rosacea fulminans (also known as pyoderma faciale) is a rare acute-onset eruption typically found in young women in their 20s and 30s.20 Rosacea fulminans is characterized by papules, pustules, nodules, cysts, draining sinuses, communicating sinus tracts, and less commonly comedones.20,21 The skin can appear erythematous, cyanotic, or dull red.21 Most of the lesions are found on the face, particularly on the forehead, cheeks, nose, and chin,21 but lesions on the chest and back have been documented in adult patients.20 In an examination of prior case series, most patients were otherwise healthy. There are case reports documenting rosacea fulminans in teenagers,20 but the youngest patient recorded was an otherwise healthy 3-year-old girl who developed a sudden onset of erythematous papules, pustules, cysts, and purulent discharging sinuses on the cheeks that spread to the chin, perioral, and paranasal areas.21
Differential Diagnosis
Rosacea is rare in children, so other papulopustular disorders must be ruled out, including acne vulgaris, periorificial/perioral dermatitis, sarcoidosis, systemic lupus erythematosus, steroid-induced rosacea, ataxia telangiectasia, and demodicosis.
Acne vulgaris commonly presents in older adolescents and teenagers with open and closed comedones, inflammatory papules, and pustules.2 Intense facial flushing and telangiectasia usually is not seen.
In perioral dermatitis, skin lesions often are clustered around the mouth, nose, and eyes. Typically there are no telangiectases or ocular complications.3 Facial flushing and telangiectases are uncommon, except in steroid-induced perioral dermatitis.2
The cutaneous findings of sarcoidosis include red-brown papules on the face and lips, and patients also may have ocular involvement such as uveitis and iritis.3 However, there are typically other systemic findings such as pulmonary symptoms, weight loss, fatigue, lethargy, fever, and erythema nodosum.2,3 Chest radiograph findings (eg, bilateral hilar lym-phadenopathy), ophthalmologic examination, and laboratory data (eg, elevated alkaline phosphate and/or elevated angiotensin-converting enzyme) can help confirm or rule out the diagnosis of sarcoidosis.2,3
Unlike systemic lupus erythematosus, patients with rosacea will have involvement of sun-protected areas of the skin. Patients with systemic lupus erythematosus typically report arthralgia and severe photosensitivity and will have elevated antinuclear antibody titers. Skin biopsies and immunofluorescence can help confirm the diagnosis.3 Importantly, some patients with rosacea will have a positive lupus band test.22,23
Steroid-induced rosacea typically occurs 2 weeks after discontinuing therapy with topical fluorinated glucocorticosteroids.24 Children present with monomorphic papules, pustules, and telangiectases4 on the eyelids and lateral face as opposed to the central face regions.24
Ataxia telangiectasia can present with telangiectases, skin atrophy, café au lait spots, and premature graying.25 A 15-year-old adolescent girl with ataxia telangiectasia presented with granulomatous acne rosacea that improved after 4 weeks of treatment with isotretinoin 0.5 mg/kg daily. The lesions cleared almost completely after 5 months.25
Demodicosis is a disorder of the pilosebaceous units caused by the human Demodex mite.26 It typically involves the periorificial regions in adults and the elderly population. Patients can present with fine, white-yellow, scaly changes of the sebaceous hair follicles, with minimal erythema and inflammation. Papules and pustules also can be present.26
Diagnosis and Histopathology
Because rosacea is rare in children, it is important to thoroughly evaluate other possible diagnoses. The diagnosis of pediatric rosacea is clinical and biopsies are rarely performed. Laboratory tests such as cultures generally are not useful.
Marks and Harcourt-Webster27 reviewed the biopsies of 108 adult patients with rosacea. The biopsies of patients with predominantly erythema and telangiectasia showed evidence of vascular dilatation with a perivascular infiltrate composed predominantly of lymphocytes and 39 specimens that were compared to controls showed more solar elastosis. Biopsies of papular rosacea contained inflammatory infiltrates in the upper and mid dermis composed primarily of lymphocytes and histiocytes. In some patients, neutrophils, plasma cells, and giant cells also were observed. Hair follicle abnormalities were present in 20% of the biopsies, with 19% showing evidence of the Demodex mite. Vascular dilatation also was common. Overall, common findings included lymphohistiocytic infiltrates around the blood vessels of the upper dermis, dilated vessels, edema, elastosis, and disorganization of connective tissue in the upper dermis.
Helm et al28 reviewed histopathologic patterns from 53 patients with granulomatous rosacea. Findings included a mixed lymphohistiocytic infiltrate (predominantly lymphocytic in 40% of patients and predominantly histiocytic with occasional giant cells in 34% of patients), epithelioid granulomas (11% of patients), and epithelioid granulomas with caseation necrosis (11% of patients).
The histopathology of rosacea fulminans is characterized by dense perivascular and periadnexal infiltrates composed of granulocytes, eosinophils, and epithelioid granulomas, as well as panniculitis.20
Treatment and Clinical Outcomes
Certain lifestyle recommendations are integral components of disease management, including avoidance of triggers such as extreme temperatures, hot drinks, spicy food, and topical agents that could be irritating (especially topical corticosteroids).29 Patients should be advised to use daily sunscreen containing physical blockers such as titanium dioxide or zinc oxide. Teenagers should avoid the use of cosmetics and makeup, especially products containing sodium lauryl sulfate, menthol, and camphor. Daily use of emollients can help some patients.29
There are both topical and systemic therapies available for pediatric rosacea; however, most of the data are based on the use of these treatments in the adult population. Patients with mild to moderate disease often can be managed using topical agents. Metronidazole (0.75% cream, 1% gel, or 0.75% lotion) has been studied extensively in adult patients, and when used once daily for 12 weeks, it has been able to control moderate to severe disease.30,31 In one study conducted in adult patients, topical metronidazole was able to maintain remission in adults who had previously been treated with a combination of oral tetracycline and metronidazole gel.31 Sodium sulfacetamide 10%–sulfur 5% (cleanser or lotion) has been successful in adult patients and often is used in combination with other therapies such as topical metronidazole.32-34 Azelaic acid cream 20%,35 benzoyl peroxide (wash or gel),29 topical clindamycin,36 topical erythromycin,29,37 tacrolimus ointment 0.1%,38 and tretinoin cream also have been studied in adults.3,39 Several of these topical agents can cause irritation on application (eg, metronidazole, sulfur-based agents, azelaic acid, benzoyl peroxide, erythromycin, tretinoin).3
The use of systemic treatments in pediatric patients is heavily based on case reports and case series.2,14,16,40 Therapies have included tetracycline (500 mg twice daily tapered to 250 mg daily),29 minocycline (50–100 mg twice daily), doxycycline (50–100 mg twice daily or 4 times daily), erythromycin (30–50 mg/kg daily), clarithromycin (15 mg/kg twice daily for 4 weeks and then daily for 4 weeks), and azithromycin (5–10 mg/kg daily).3 Tetracycline antibiotics should not be used in children 8 years or younger.
In a case series by Drolet and Paller,2 an 11-year-old girl was treated with tetracycline 500 mg (later tapered to 250 mg daily) and metronidazole gel 0.75%, both used twice daily. Previously, she had not responded to topical steroids, tretinoin cream 0.05%, benzoyl peroxide 5%, or systemic prednisone. After 6 weeks of treatment, the pustules and chalazion had resolved and she had only minimal erythema of the skin and conjunctiva. Sixteen months after the start of treatment, a regimen of tetracycline 250 mg daily and metronidazole gel resulted in disease clearance on the face.2
A 9-year-old girl with concurrent systemic lupus erythematosus was treated with tetracycline 250 mg and topical erythromycin 2%, both used twice daily.2 After 4 weeks her face was clear. Four months later she developed new telangiectases and topical erythromycin was replaced with topical metronidazole. Eventually the dose of tetracycline was reduced to 250 mg daily.2
An 11-year-old boy with likely granulomatous rosacea was treated with erythromycin 250 mg 4 times daily, alclometasone dipropionate cream 0.05% twice daily, and topical clindamycin twice daily.2 Marked improvement was noticed after 3 weeks of treatment. Metronidazole gel 0.75% was added and 3 months later the patient’s face was clear, without evidence of scarring. The dose of erythromycin was later reduced to 500 mg daily, and eventually the patient experienced clearance with the use of metronidazole gel daily.2
In another case series, 4 female patients (age range, 4–12 years) were treated with systemic erythromycin 20 mg/kg daily (ocular involvement only) or doxycycline 2.2 mg/kg daily used in two 12-year-old patients with ocular and cutaneous involvement for at least 12 months. All 4 patients showed considerable improvement within 1 month and remained free of disease throughout a mean follow-up period of 25.5 months.40
As evidenced by these case reports, there is a wide array of treatments that have been used for pediatric rosacea. Although there are no formal evidence-based guidelines, there are certain considerations that must be taken into account when choosing treatment plans. Doxycycline and minocycline are known to cause less gastrointestinal upset than tetracycline with similar efficacy.41 Importantly, the tetracyclines are contraindicated in children younger than 9 years, as they can cause teeth staining and possibly affect skeletal growth.3,4 When used in older children (age range, 9–12 years), patients must be advised not to take their medication with calcium or antacids.3 Clarithromycin and azithromycin tend to have fewer gastrointestinal side effects than erythromycin. Erythromycin and other macrolides can be used in children of all ages and in patients who are allergic to tetracyclines.3
Children with mild ocular symptoms often can control their disease with bacitracin and topical ocular antibiotics such as erythromycin.2,15 For patients who require systemic antibiotics, various tetracyclines and macrolides have been used with success.2,14-16,40
Adults with rosacea fulminans can require treatment with isotretinoin, oral antibiotics, and topical or even systemic corticosteroids.42 The 3-year-old girl with rosacea fulminans initially was treated with oral erythromycin (250 mg 4 times daily), oral prednisolone (0.5 mg/kg daily tapered over 2 weeks), fluocinolone acetonide cream 0.025%, and warm compresses with only moderate improvement.21 She was then started on oral isotretinoin (0.75 mg/kg daily) and within 4 weeks marked improvement was noted. After 8 weeks, the lesions had disappeared completely with only a few pitted scars remaining. Isotretinoin was continued for 24 weeks. One year after completion of treatment, she was still disease free.21
Weston and Morelli19 recommended the following treatment regimen for children with steroid rosacea: abrupt cessation of topical steroid use (as opposed to gradual withdrawal) and initiation of oral erythromycin stearate (30 mg/kg daily) in 2 daily doses for 4 weeks. Children who were unable to tolerate erythromycin (n=6) were told to use topical clindamycin phosphate twice daily for 4 weeks. Within 3 weeks 22% of patients had resolution, while 86% had resolution within 4 weeks. All of the patients cleared within 8 weeks. Importantly, there was no significant difference in duration of time until clearance between children who used the oral antibiotic or topical antibiotic.19
Conclusion
We know that the skin of rosacea patients contains higher levels of cathelicidins, which have been implicated in amplifying and contributing to the inflammatory response in several ways. Mast cells, which are a source of cathelicidins, also are increased in the skin of these patients. Children can present with vascular rosacea (characterized by flushing, erythema, and/or telangiectasia), papulopustular rosacea, or ocular rosacea. Common ocular symptoms include blepharitis, conjunctivitis, and recurrent chalazion. It is important to refer pediatric rosacea patients with ocular symptoms to an ophthalmologist to prevent ocular sequelae.
Rosacea is a clinical diagnosis but biopsy can be performed to rule out other diagnoses. Treatment consists of lifestyle modifications such as avoiding known triggers and the use of topical and/or oral agents. Common topical therapies include metronidazole and erythromycin. Systemic antibiotics include tetracycline, doxycycline, minocycline, azithromycin, and erythromycin. Some children are able to taper systemic agents and maintain disease control with topical therapy, while others may need to continue a low-dose antibiotic. Although flares can be controlled within weeks to months, rosacea is a chronic disorder and childhood rosacea tends to persist into adulthood.
Rosacea is a chronic skin disease characterized by flushing, erythema, telangiectasia, papules, and pustules in the central face region.1 It most often affects middle-aged women (age range, 30–50 years).2 Rosacea is rare in the pediatric population, especially before puberty.3 There are 3 subtypes of pediatric rosacea: vascular, papulopustular, and ocular. Phymatous/rhinophymatous rosacea is only seen in the adult population.3 Recommendations for the management of pediatric rosacea heavily rely on data from retrospective chart reviews and case series.
Etiology of Pediatric Rosacea
Rosacea is thought to be a consequence of vasomotor instability in both adults and children. A family history of rosacea is sometimes reported in patients with pediatric rosacea.4 Patients often are sensitive to heat, sunlight, topical corticosteroids, spicy foods, hot liquids, and certain soaps and cleansers.1,3,4 In a review of the literature by Vemuri et al,5 the various reported triggers of rosacea include harsh climates that damage the blood vessels and dermal connective tissue, defects in the endothelium and dermal matrix, perivascular inflammation, orally ingested chemicals, changes in the flora of the hair follicles, excessive antimicrobial peptides, and the presence of free radicals. Overall, it is unclear which of these factors are triggers of pediatric rosacea.
The molecular basis of rosacea has been elucidated. It is well known that rosacea patients have higher levels of cathelicidins in the facial skin. Furthermore, they appear to have different processed forms of cathelicidin peptides compared to adults without rosacea, possibly due to changes in posttranslational processing.6 One such peptide, cathelicidin LL-37, also has been implicated in atopic dermatitis7 and psoriasis.8 Its role in rosacea appears to be multifaceted. Cathelicidin LL-37 helps to attract neutrophils, monocytes, and T lymphocytes, and also has antimicrobial properties; therefore, it plays a role in both the innate and adaptive immune systems.9 Cathelicidin LL-37 also has been implicated in inducing angiogenesis10 and suppressing dermal fibroblasts.11
Muto et al12 found that there is an increased number of mast cells in the dermis of patients with rosacea. Mast cells contribute to vasodilation, angiogenesis, and the recruitment of other inflammatory cells.12 Importantly, human mast cells are a source of cathelicidins including cathelicidin LL-37; these proteins play a vital role in the antimicrobial capabilities of mast cells.13
Clinical Presentation and Comorbidities
Vascular rosacea presents with characteristic flushing and erythema, which lasts more than a few minutes as compared to physiologic erythema,1 and sometimes telangiectasia is seen.3 The cheeks, chin, and nasolabial folds are most commonly involved.2 In papulopustular rosacea, papules and pustules are seen overlying the erythema.1,3 Open and closed comedones also have been documented in case reports but are not commonly seen.2 Pediatric rosacea often begins with flushing of the face and then progresses to the development of papules and pustules.4
Ocular rosacea can occur with or without cutaneous findings. In a retrospective study of 20 pediatric patients (aged 1–15 years), 11 (55%) patients had both ocular and cutaneous rosacea, 3 (15%) only had ocular symptoms, and 6 (30%) only had cutaneous symptoms. The most common form of rosacea in this study was papulopustular rosacea.14 Ocular symptoms often are bilateral15 and can include blepharitis, conjunctival injection, recurrent chalazion, conjunctivitis,2 and less commonly corneal ulceration and scarring.16 Patients also may report photophobia or a foreign body sensation.17 Importantly, ocular symptoms often precede the cutaneous symptoms and can delay the diagnosis of rosacea,14,16,18 as these symptoms often are misdiagnosed as viral or bacterial infections.15 Fortunately, ocular disease responds well to treatment if diagnosed early.
Weston and Morelli19 conducted a retrospective study of 106 children (46 males; 60 females) 13 years and younger with steroid rosacea; 29 children were younger than 3 years. A family history of rosacea was present in 20% of participants, and prior use of class 7 steroids was reported in 54%, whereas only 3% had used class 1 topical steroids. Ninety-eight participants had perinasal involvement, 94 had perioral involvement, and 44 had periorbital involvement of the lower eyelids.19
Rosacea fulminans (also known as pyoderma faciale) is a rare acute-onset eruption typically found in young women in their 20s and 30s.20 Rosacea fulminans is characterized by papules, pustules, nodules, cysts, draining sinuses, communicating sinus tracts, and less commonly comedones.20,21 The skin can appear erythematous, cyanotic, or dull red.21 Most of the lesions are found on the face, particularly on the forehead, cheeks, nose, and chin,21 but lesions on the chest and back have been documented in adult patients.20 In an examination of prior case series, most patients were otherwise healthy. There are case reports documenting rosacea fulminans in teenagers,20 but the youngest patient recorded was an otherwise healthy 3-year-old girl who developed a sudden onset of erythematous papules, pustules, cysts, and purulent discharging sinuses on the cheeks that spread to the chin, perioral, and paranasal areas.21
Differential Diagnosis
Rosacea is rare in children, so other papulopustular disorders must be ruled out, including acne vulgaris, periorificial/perioral dermatitis, sarcoidosis, systemic lupus erythematosus, steroid-induced rosacea, ataxia telangiectasia, and demodicosis.
Acne vulgaris commonly presents in older adolescents and teenagers with open and closed comedones, inflammatory papules, and pustules.2 Intense facial flushing and telangiectasia usually is not seen.
In perioral dermatitis, skin lesions often are clustered around the mouth, nose, and eyes. Typically there are no telangiectases or ocular complications.3 Facial flushing and telangiectases are uncommon, except in steroid-induced perioral dermatitis.2
The cutaneous findings of sarcoidosis include red-brown papules on the face and lips, and patients also may have ocular involvement such as uveitis and iritis.3 However, there are typically other systemic findings such as pulmonary symptoms, weight loss, fatigue, lethargy, fever, and erythema nodosum.2,3 Chest radiograph findings (eg, bilateral hilar lym-phadenopathy), ophthalmologic examination, and laboratory data (eg, elevated alkaline phosphate and/or elevated angiotensin-converting enzyme) can help confirm or rule out the diagnosis of sarcoidosis.2,3
Unlike systemic lupus erythematosus, patients with rosacea will have involvement of sun-protected areas of the skin. Patients with systemic lupus erythematosus typically report arthralgia and severe photosensitivity and will have elevated antinuclear antibody titers. Skin biopsies and immunofluorescence can help confirm the diagnosis.3 Importantly, some patients with rosacea will have a positive lupus band test.22,23
Steroid-induced rosacea typically occurs 2 weeks after discontinuing therapy with topical fluorinated glucocorticosteroids.24 Children present with monomorphic papules, pustules, and telangiectases4 on the eyelids and lateral face as opposed to the central face regions.24
Ataxia telangiectasia can present with telangiectases, skin atrophy, café au lait spots, and premature graying.25 A 15-year-old adolescent girl with ataxia telangiectasia presented with granulomatous acne rosacea that improved after 4 weeks of treatment with isotretinoin 0.5 mg/kg daily. The lesions cleared almost completely after 5 months.25
Demodicosis is a disorder of the pilosebaceous units caused by the human Demodex mite.26 It typically involves the periorificial regions in adults and the elderly population. Patients can present with fine, white-yellow, scaly changes of the sebaceous hair follicles, with minimal erythema and inflammation. Papules and pustules also can be present.26
Diagnosis and Histopathology
Because rosacea is rare in children, it is important to thoroughly evaluate other possible diagnoses. The diagnosis of pediatric rosacea is clinical and biopsies are rarely performed. Laboratory tests such as cultures generally are not useful.
Marks and Harcourt-Webster27 reviewed the biopsies of 108 adult patients with rosacea. The biopsies of patients with predominantly erythema and telangiectasia showed evidence of vascular dilatation with a perivascular infiltrate composed predominantly of lymphocytes and 39 specimens that were compared to controls showed more solar elastosis. Biopsies of papular rosacea contained inflammatory infiltrates in the upper and mid dermis composed primarily of lymphocytes and histiocytes. In some patients, neutrophils, plasma cells, and giant cells also were observed. Hair follicle abnormalities were present in 20% of the biopsies, with 19% showing evidence of the Demodex mite. Vascular dilatation also was common. Overall, common findings included lymphohistiocytic infiltrates around the blood vessels of the upper dermis, dilated vessels, edema, elastosis, and disorganization of connective tissue in the upper dermis.
Helm et al28 reviewed histopathologic patterns from 53 patients with granulomatous rosacea. Findings included a mixed lymphohistiocytic infiltrate (predominantly lymphocytic in 40% of patients and predominantly histiocytic with occasional giant cells in 34% of patients), epithelioid granulomas (11% of patients), and epithelioid granulomas with caseation necrosis (11% of patients).
The histopathology of rosacea fulminans is characterized by dense perivascular and periadnexal infiltrates composed of granulocytes, eosinophils, and epithelioid granulomas, as well as panniculitis.20
Treatment and Clinical Outcomes
Certain lifestyle recommendations are integral components of disease management, including avoidance of triggers such as extreme temperatures, hot drinks, spicy food, and topical agents that could be irritating (especially topical corticosteroids).29 Patients should be advised to use daily sunscreen containing physical blockers such as titanium dioxide or zinc oxide. Teenagers should avoid the use of cosmetics and makeup, especially products containing sodium lauryl sulfate, menthol, and camphor. Daily use of emollients can help some patients.29
There are both topical and systemic therapies available for pediatric rosacea; however, most of the data are based on the use of these treatments in the adult population. Patients with mild to moderate disease often can be managed using topical agents. Metronidazole (0.75% cream, 1% gel, or 0.75% lotion) has been studied extensively in adult patients, and when used once daily for 12 weeks, it has been able to control moderate to severe disease.30,31 In one study conducted in adult patients, topical metronidazole was able to maintain remission in adults who had previously been treated with a combination of oral tetracycline and metronidazole gel.31 Sodium sulfacetamide 10%–sulfur 5% (cleanser or lotion) has been successful in adult patients and often is used in combination with other therapies such as topical metronidazole.32-34 Azelaic acid cream 20%,35 benzoyl peroxide (wash or gel),29 topical clindamycin,36 topical erythromycin,29,37 tacrolimus ointment 0.1%,38 and tretinoin cream also have been studied in adults.3,39 Several of these topical agents can cause irritation on application (eg, metronidazole, sulfur-based agents, azelaic acid, benzoyl peroxide, erythromycin, tretinoin).3
The use of systemic treatments in pediatric patients is heavily based on case reports and case series.2,14,16,40 Therapies have included tetracycline (500 mg twice daily tapered to 250 mg daily),29 minocycline (50–100 mg twice daily), doxycycline (50–100 mg twice daily or 4 times daily), erythromycin (30–50 mg/kg daily), clarithromycin (15 mg/kg twice daily for 4 weeks and then daily for 4 weeks), and azithromycin (5–10 mg/kg daily).3 Tetracycline antibiotics should not be used in children 8 years or younger.
In a case series by Drolet and Paller,2 an 11-year-old girl was treated with tetracycline 500 mg (later tapered to 250 mg daily) and metronidazole gel 0.75%, both used twice daily. Previously, she had not responded to topical steroids, tretinoin cream 0.05%, benzoyl peroxide 5%, or systemic prednisone. After 6 weeks of treatment, the pustules and chalazion had resolved and she had only minimal erythema of the skin and conjunctiva. Sixteen months after the start of treatment, a regimen of tetracycline 250 mg daily and metronidazole gel resulted in disease clearance on the face.2
A 9-year-old girl with concurrent systemic lupus erythematosus was treated with tetracycline 250 mg and topical erythromycin 2%, both used twice daily.2 After 4 weeks her face was clear. Four months later she developed new telangiectases and topical erythromycin was replaced with topical metronidazole. Eventually the dose of tetracycline was reduced to 250 mg daily.2
An 11-year-old boy with likely granulomatous rosacea was treated with erythromycin 250 mg 4 times daily, alclometasone dipropionate cream 0.05% twice daily, and topical clindamycin twice daily.2 Marked improvement was noticed after 3 weeks of treatment. Metronidazole gel 0.75% was added and 3 months later the patient’s face was clear, without evidence of scarring. The dose of erythromycin was later reduced to 500 mg daily, and eventually the patient experienced clearance with the use of metronidazole gel daily.2
In another case series, 4 female patients (age range, 4–12 years) were treated with systemic erythromycin 20 mg/kg daily (ocular involvement only) or doxycycline 2.2 mg/kg daily used in two 12-year-old patients with ocular and cutaneous involvement for at least 12 months. All 4 patients showed considerable improvement within 1 month and remained free of disease throughout a mean follow-up period of 25.5 months.40
As evidenced by these case reports, there is a wide array of treatments that have been used for pediatric rosacea. Although there are no formal evidence-based guidelines, there are certain considerations that must be taken into account when choosing treatment plans. Doxycycline and minocycline are known to cause less gastrointestinal upset than tetracycline with similar efficacy.41 Importantly, the tetracyclines are contraindicated in children younger than 9 years, as they can cause teeth staining and possibly affect skeletal growth.3,4 When used in older children (age range, 9–12 years), patients must be advised not to take their medication with calcium or antacids.3 Clarithromycin and azithromycin tend to have fewer gastrointestinal side effects than erythromycin. Erythromycin and other macrolides can be used in children of all ages and in patients who are allergic to tetracyclines.3
Children with mild ocular symptoms often can control their disease with bacitracin and topical ocular antibiotics such as erythromycin.2,15 For patients who require systemic antibiotics, various tetracyclines and macrolides have been used with success.2,14-16,40
Adults with rosacea fulminans can require treatment with isotretinoin, oral antibiotics, and topical or even systemic corticosteroids.42 The 3-year-old girl with rosacea fulminans initially was treated with oral erythromycin (250 mg 4 times daily), oral prednisolone (0.5 mg/kg daily tapered over 2 weeks), fluocinolone acetonide cream 0.025%, and warm compresses with only moderate improvement.21 She was then started on oral isotretinoin (0.75 mg/kg daily) and within 4 weeks marked improvement was noted. After 8 weeks, the lesions had disappeared completely with only a few pitted scars remaining. Isotretinoin was continued for 24 weeks. One year after completion of treatment, she was still disease free.21
Weston and Morelli19 recommended the following treatment regimen for children with steroid rosacea: abrupt cessation of topical steroid use (as opposed to gradual withdrawal) and initiation of oral erythromycin stearate (30 mg/kg daily) in 2 daily doses for 4 weeks. Children who were unable to tolerate erythromycin (n=6) were told to use topical clindamycin phosphate twice daily for 4 weeks. Within 3 weeks 22% of patients had resolution, while 86% had resolution within 4 weeks. All of the patients cleared within 8 weeks. Importantly, there was no significant difference in duration of time until clearance between children who used the oral antibiotic or topical antibiotic.19
Conclusion
We know that the skin of rosacea patients contains higher levels of cathelicidins, which have been implicated in amplifying and contributing to the inflammatory response in several ways. Mast cells, which are a source of cathelicidins, also are increased in the skin of these patients. Children can present with vascular rosacea (characterized by flushing, erythema, and/or telangiectasia), papulopustular rosacea, or ocular rosacea. Common ocular symptoms include blepharitis, conjunctivitis, and recurrent chalazion. It is important to refer pediatric rosacea patients with ocular symptoms to an ophthalmologist to prevent ocular sequelae.
Rosacea is a clinical diagnosis but biopsy can be performed to rule out other diagnoses. Treatment consists of lifestyle modifications such as avoiding known triggers and the use of topical and/or oral agents. Common topical therapies include metronidazole and erythromycin. Systemic antibiotics include tetracycline, doxycycline, minocycline, azithromycin, and erythromycin. Some children are able to taper systemic agents and maintain disease control with topical therapy, while others may need to continue a low-dose antibiotic. Although flares can be controlled within weeks to months, rosacea is a chronic disorder and childhood rosacea tends to persist into adulthood.
- Crawford GH, Pelle MT, James WD. Rosacea: i. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Drolet B, Paller AS. Childhood rosacea. Pediatr Dermatol. 1992;9:22-26.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Lacz NL, Schwartz RA. Rosacea in the pediatric population. Cutis. 2004;74:99-103.
- Vemuri RC, Gundamaraju R, Sekaran SD, et al. Major pathophysiological correlations of rosacea: a complete clinical appraisal. Int J Med Sci. 2015;12:387-396.
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
- Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151-1160.
- Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564-569.
- De Y, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069-1074.
- Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665-1672.
- Park HJ, Cho DH, Kim HJ, et al. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL-37. J Invest Dermatol. 2009;129:843-850.
- Muto Y, Wang Z, Vanderberghe M, et al. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J Invest Dermatol. 2014;134:2728-2736.
- Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274-2278.
- Leoni S, Mesplie N, Aitali F, et al. Metronidazole: alternative treatment for ocular and cutaneous rosacea in the pediatric population [in French]. J Fr Ophthalmol. 2011;34:703-710.
- Nazir SA, Murphy S, Siatkowski RM, et al. Ocular rosacea in childhood. Am J Ophthalmol. 2004;137:138-144.
- Miguel AI, Salgado MB, Lisboa MS, et al. Pediatric ocular rosacea: 2 cases. Eur J Ophthalmol. 2012;22:664-666.
- Stone DU, Chodosh J. Ocular rosacea: an update on pathogenesis and therapy. Curr Opin Ophthalmol. 2004;15:499-502.
- Mavrakanas N, Schutz JS, Dosso AA. Pediatric ocular rosacea [published online March 22, 2010]. J Pediatr Ophthalmol Strabismus. 2010;47:117-120.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Plewig G, Jansen T, Kligman AM. Pyoderma faciale. a review and report of 20 additional cases: is it rosacea? Arch Dermatol. 1992;128:1611-1617.
- Firooz A, Firoozabadi MR, Dowlati Y. Rosacea fulminans (pyoderma faciale): successful treatment of a 3-year-old girl with oral isotretinoin. Int J Dermatol. 2001;40:203-205.
- Baart de la Faille H, Baart de la F-K. Immunofluorescent studies of the skin in rosacea. Dermatologica. 1969;139:49-54.
- Wilkin JK. Rosacea. Int J Dermatol. 1983;22:393-400.
- Franco HL, Weston WL. Steroid rosacea in children. Pediatrics. 1979;64:36-38.
- Cantarutti N, Claps A, Angelino G, et al. Multi-drugs resistant acne rosacea in a child affected by ataxia-telangiectasia: successful treatment with isotretinoin. Ital J Pediatr. 2015;41:23.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Marks R, Harcourt-Webster J. Histopathology of rosacea. Arch Dermatol. 1969;100:683-691.
- Helm KF, Menz J, Gibson LE, et al. A clinical and histopathologic study of granulomatous rosacea. J Am Acad Dermatol. 1991;25(6, pt 1):1038-1043.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512; quiz 513-494.
- Dahl MV, Jarratt M, Kaplan D, et al. Once-daily topical metronidazole cream formulations in the treatment of the papules and pustules of rosacea. J Am Acad Dermatol. 2001;45:723-730.
- Dahl MV, Katz HI, Krueger GG, et al. Topical metronidazole maintains remissions of rosacea. Arch Dermatol. 1998;134:679-683.
- Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
- Del Rosso JQ. Evaluating the role of topical therapies in the management of rosacea: focus on combination sodium sulfacetamide and sulfur formulations. Cutis. 2004;73(1 suppl):29-33.
- Del Rosso JQ. A status report on the medical management of rosacea: focus on topical therapies. Cutis. 2002;70:271-275.
- Maddin S. A comparison of topical azelaic acid 20% cream and topical metronidazole 0.75% cream in the treatment of patients with papulopustular rosacea. J Am Acad Dermatol. 1999;40(6, pt 1):961-965.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Mills OH Jr, Kligman AM. Letter: topically applied erythromycin in rosacea. Arch Dermatol. 1976;112:553-554.
- Hengge UR. Off-label indications for topical tacrolimus [in German]. Hautarzt. 2013;64:752-756.
- Ertl GA, Levine N, Kligman AM. A comparison of the efficacy of topical tretinoin and low-dose oral isotretinoin in rosacea. Arch Dermatol. 1994;130:319-324.
- Cetinkaya A, Akova YA. Pediatric ocular acne rosacea: long-term treatment with systemic antibiotics. Am J Ophthalmol. 2006;142:816-821.
- Maibach H. Second-generation tetracyclines, a dermatologic overview: clinical uses and pharmacology. Cutis. 1991;48:411-417.
- Jansen T, Plewig G, Kligman AM. Diagnosis and treatment of rosacea fulminans. Dermatology. 1994;188:251-254.
- Crawford GH, Pelle MT, James WD. Rosacea: i. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Drolet B, Paller AS. Childhood rosacea. Pediatr Dermatol. 1992;9:22-26.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Lacz NL, Schwartz RA. Rosacea in the pediatric population. Cutis. 2004;74:99-103.
- Vemuri RC, Gundamaraju R, Sekaran SD, et al. Major pathophysiological correlations of rosacea: a complete clinical appraisal. Int J Med Sci. 2015;12:387-396.
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
- Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151-1160.
- Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564-569.
- De Y, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069-1074.
- Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665-1672.
- Park HJ, Cho DH, Kim HJ, et al. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL-37. J Invest Dermatol. 2009;129:843-850.
- Muto Y, Wang Z, Vanderberghe M, et al. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J Invest Dermatol. 2014;134:2728-2736.
- Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274-2278.
- Leoni S, Mesplie N, Aitali F, et al. Metronidazole: alternative treatment for ocular and cutaneous rosacea in the pediatric population [in French]. J Fr Ophthalmol. 2011;34:703-710.
- Nazir SA, Murphy S, Siatkowski RM, et al. Ocular rosacea in childhood. Am J Ophthalmol. 2004;137:138-144.
- Miguel AI, Salgado MB, Lisboa MS, et al. Pediatric ocular rosacea: 2 cases. Eur J Ophthalmol. 2012;22:664-666.
- Stone DU, Chodosh J. Ocular rosacea: an update on pathogenesis and therapy. Curr Opin Ophthalmol. 2004;15:499-502.
- Mavrakanas N, Schutz JS, Dosso AA. Pediatric ocular rosacea [published online March 22, 2010]. J Pediatr Ophthalmol Strabismus. 2010;47:117-120.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Plewig G, Jansen T, Kligman AM. Pyoderma faciale. a review and report of 20 additional cases: is it rosacea? Arch Dermatol. 1992;128:1611-1617.
- Firooz A, Firoozabadi MR, Dowlati Y. Rosacea fulminans (pyoderma faciale): successful treatment of a 3-year-old girl with oral isotretinoin. Int J Dermatol. 2001;40:203-205.
- Baart de la Faille H, Baart de la F-K. Immunofluorescent studies of the skin in rosacea. Dermatologica. 1969;139:49-54.
- Wilkin JK. Rosacea. Int J Dermatol. 1983;22:393-400.
- Franco HL, Weston WL. Steroid rosacea in children. Pediatrics. 1979;64:36-38.
- Cantarutti N, Claps A, Angelino G, et al. Multi-drugs resistant acne rosacea in a child affected by ataxia-telangiectasia: successful treatment with isotretinoin. Ital J Pediatr. 2015;41:23.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Marks R, Harcourt-Webster J. Histopathology of rosacea. Arch Dermatol. 1969;100:683-691.
- Helm KF, Menz J, Gibson LE, et al. A clinical and histopathologic study of granulomatous rosacea. J Am Acad Dermatol. 1991;25(6, pt 1):1038-1043.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512; quiz 513-494.
- Dahl MV, Jarratt M, Kaplan D, et al. Once-daily topical metronidazole cream formulations in the treatment of the papules and pustules of rosacea. J Am Acad Dermatol. 2001;45:723-730.
- Dahl MV, Katz HI, Krueger GG, et al. Topical metronidazole maintains remissions of rosacea. Arch Dermatol. 1998;134:679-683.
- Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
- Del Rosso JQ. Evaluating the role of topical therapies in the management of rosacea: focus on combination sodium sulfacetamide and sulfur formulations. Cutis. 2004;73(1 suppl):29-33.
- Del Rosso JQ. A status report on the medical management of rosacea: focus on topical therapies. Cutis. 2002;70:271-275.
- Maddin S. A comparison of topical azelaic acid 20% cream and topical metronidazole 0.75% cream in the treatment of patients with papulopustular rosacea. J Am Acad Dermatol. 1999;40(6, pt 1):961-965.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Mills OH Jr, Kligman AM. Letter: topically applied erythromycin in rosacea. Arch Dermatol. 1976;112:553-554.
- Hengge UR. Off-label indications for topical tacrolimus [in German]. Hautarzt. 2013;64:752-756.
- Ertl GA, Levine N, Kligman AM. A comparison of the efficacy of topical tretinoin and low-dose oral isotretinoin in rosacea. Arch Dermatol. 1994;130:319-324.
- Cetinkaya A, Akova YA. Pediatric ocular acne rosacea: long-term treatment with systemic antibiotics. Am J Ophthalmol. 2006;142:816-821.
- Maibach H. Second-generation tetracyclines, a dermatologic overview: clinical uses and pharmacology. Cutis. 1991;48:411-417.
- Jansen T, Plewig G, Kligman AM. Diagnosis and treatment of rosacea fulminans. Dermatology. 1994;188:251-254.
Practice Points
- Although rosacea is largely a diagnosis of adults, it also can begin in childhood and adolescence.
- Ocular rosacea and papulopustular disease are common clinical findings in younger patients.
- Usage of topical metronidazole and age-appropriate oral antibiotics are the mainstay of management.
Atopic Dermatitis in Children
A Practical Overview of Pediatric Atopic Dermatitis, Part 3: Differential Diagnosis, Comorbidities, and Measurement of Disease Burden
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
Practice Points
- Atopic dermatitis (AD) has a variety of comorbidities including psychosocial disorders, obesity, and infection.
- A variety of skin conditions can mimic AD.
- Atopic dermatitis can be complicated by coinfections.
A Practical Overview of Pediatric Atopic Dermatitis, Part 2: Triggers and Grading
Atopic dermatitis (AD) may be triggered by viral infections, food allergens, weather, and other causes, and it may trigger an inflammatory progression known as the atopic march. This article reviews research on triggers of pediatric AD so that dermatologists may discuss trigger avoidance with patients and guardians. Other factors affecting AD development include genetics and hygiene. Grading of AD also is discussed.
The Atopic March
The persistence of AD in untreated skin can trigger an inflammatory progression called the atopic march in which food and environmental allergies as well as asthma may occur progressively due to ongoing inflammatory triggering.1 In a study of asthma and food allergy reporting and management in public schools in Chicago, Illinois, food allergies were seen in 9.3% of asthmatic students (n=18,000), and 40.1% of food allergic students (n=4000) had asthma.2 An observational study by Flohr et al3 in London, England, included 619 exclusively breastfed infants who were recruited at 3 months of age. The investigators determined that food sensitization was unrelated to the presence of filaggrin mutations, type of eczema (flexural vs nonflexural), and transepidermal water loss but was associated with AD severity as determined by SCORAD (SCORing Atopic Dermatitis), a composite score of AD that includes pruritus as a factor in severity. Other AD associations included 3 leading food allergens: eggs, milk, and peanuts. No association with cod, wheat, or sesame allergy was noted. The investigators concluded that AD and AD severity were the leading skin-related risk factors for food allergies and therefore food allergy development in breastfed infants was probably mediated by cutaneous antigen-presenting cells.3
The skin has been documented to react to contact with known food allergens4 and is known to be a route of allergic sensitization to allergens such as fragrance in patients with AD.5,6 Two phenotypes of eczema that have been associated with asthma development are severe AD disease and multiple environmental allergies, supporting the theory of the atopic march.7 There also is evidence that release of danger-associated proteins from an impaired barrier also may trigger asthma.8 An analysis of the 2007 National Survey of Children’s Health, a population-based study of91,642 children aged 0 to 17 years, showed that children with AD had a higher prevalence of comorbid asthma (25.1% vs 12.3%), hay fever (34.4% vs 14.3%), and food allergies (15.1% vs 3.6%) compared to children without AD.9 A recent article provided detailed information on how food and diet interplay with AD.10
Triggers of Disease Flares
Triggers are the leading source of AD flare initiation, and avoidance of triggers is an important mechanism by which patients can control disease activity. Despite the best skin care and trigger avoidance, disease flares occur, sometimes due to ongoing inflammation and other times due to inability to prevent flares such as heat and humidity. A survey of patients with AD in Spain identified the following triggers: cosmetic products, clothing, mites, detergents/soaps, and temperature changes.11 In childhood, wool also is a known trigger of AD.12 Viral infections including respiratory syncytial virus may trigger the first onset of AD.13 Patients with AD may become allergic to fragrance and metals causing disease exacerbation on exposure.14,15 Food allergens contribute to approximately 40% of cases of AD in infancy but are not the cause of AD. The best evidence for improvement of AD with food allergen avoidance exists for egg white allergy.16 Food avoidance programs should be developed in conjunction with an allergist, as it is no longer advised in many cases to completely withdraw foods; therefore, an allergist has to assess the level of allergic severity and the risk-benefit ratio of food avoidance or introduction.17 Emotional stressors, heat, and humidity, as well as indoor heating in the winter months, can cause AD flares.18
A study by Silverberg et al19 provided evidence of climate influences on the US prevalence of childhood eczema using a merged analysis of the 2007 National Survey of Children’s Health and the 2006-2007 National Climate Data Center and Weather Service. Results showed that eczema prevalence was significantly lower when associated with higher annual relative humidity (P=.01), UV index (P<.0001), and highest-quartile air temperature (P=.002).19 The Pediatric Eczema Elective Registry also showed that warm, humid, and high-sun-exposure climates are associated with poorly controlled eczema in affected patients.20 The association of eczema with latitude as well as its negative association with mean annual outdoor temperature has been described by Weiland et al21 in the ISAAC (International Study of Asthma and Allergies in Childhood) study. Long airplane flights in low humidity can trigger eczema in adults. Climate has been postulated to affect eczema through alterations in filaggrin and skin barrier function.22 Indoor temperature and humidity regulation may be used adjunctively for daily flare prevention.
Genetics and AD
Of 762 infants in a birth cohort with a parent with atopy in Cincinnati, Ohio, 39% developed eczema by the age of 3 years. Single nucleotide polymorphisms of IL-4Rα 175 V and CD14-159 C/T were linked to greater eczema risk at 2 to 3 years of age.23 Monozygotic twins have a concordance rate of 0.72 to 0.86 versus 0.21 to 0.23 in dizygotic twins, demonstrating a strong genetic component in the development of AD.24 Linkage to AD has been positively made to the epidermal differentiation complex on human chromosome 1q21, which contains the genes for filaggrin and other proteins such as loricrin. Other genes linked to AD include the serine protease inhibitor SPINK5 (serine peptidase inhibitor, Kazal type 5) implicated in Netherton syndrome (triad of ichthyosis linearis circumflexa, bamboo hair, and atopic disorders); RANTES (regulated on activation, normal T-expressed, and secreted), which has been associated with severity of AD; IL-4; and IL-13.5,25,26
The Hygiene Hypothesis
Atopic dermatitis is more common in wealthy developed countries, leading some to believe that hygiene and relative reduction in illness via vaccination have contributed to the rise of AD prevalence in developed nations.13,27 There currently is evidence demonstrating that wild-type varicella infection confers long-standing protection against AD and mediates reduced total IgE and peripheral blood lymphocytes.27
Grading of AD
Grading of AD is a subject of controversy, as there currently are no uniform grading scales.28 A recent outcomes group attempted to determine the best scale for disease monitoring. Schmitt et al29 presented the Harmonizing Outcome Measures for Eczema (HOME) roadmap, which was intended to determine a core outcome set for eczema; however, because these outcome measurements have not yet been standardized, only the eczema assessment and severity index (EASI) scoring system meets criteria for standardization. In clinical practice, physicians often assign mild, moderate, or severe labeling based on their general sense of the disease extent using an investigator global assessment score.28
The EASI score is a well-validated composite score of AD severity based on 4 body regions: (1) head and neck, (2) trunk (including genital area), (3) upper limbs, and (4) lower limbs (including buttocks). The total area of involvement in each region is graded on a scale of 0 to 6, and AD severity is graded as a composite of 4 parameters (ranked on a scale of 0–3), including redness (erythema, inflammation), thickness (induration, papulation, swelling [acute eczema]), scratching (excoriation), and lichenification (prurigo nodules [chronic eczema]). The surface area of each region relative to body size is used as a multiplying factor, resulting in the following severity strata: 0=clear; 0.1–1.0=almost clear; 1.1–7.0=mild; 7.1–21.0=moderate; 21.1–50.0=severe; 50.1–72.0=very severe (κ=0.75).30-32 The six area, six sign AD (SASSAD) score32,33 is a similar score without adjustment for body surface area by region.34
An older, now less frequently used eczema score is the SCORAD, which addressed surface area by rule of nines and severity of 6 features—redness, swelling, oozing/crusting, scratch marks, skin thickening (lichenification), dryness (assessed in an area with no inflammation)—by region on a scale of 0 to 3. A subjective symptom parameter for itching and sleeplessness helped highlight that these comorbidities are important in gauging disease activity and impact on a child’s life.35
Natural History of AD
The clinical dogma has been that AD would improve with age, with reduction at grade school entry and perhaps full disappearance in adulthood; however, 3 recent surveys have suggested otherwise. The ISAAC group has found prevalence of AD in wealthy developed countries among children aged 6 to 7 years to be at a consistent increase.36 A US-based survey from the National Health Interview Survey showed a 1-year prevalence of 10.2% of active AD in adults and 9.8% when occupational dermatitis was excluded.37 Halvorsen et al38 demonstrated that eczema prevalence is 9.7% in individuals aged 18 to 19 years.
A prospective trial of eighth graders followed from 1995 to 2010 demonstrated that AD persisted in 50% at school age. Persistent eczema into adulthood was associated with early-onset childhood allergic rhinitis and hand eczema.39 In a cohort of hand eczema patients (N=368), 28% had AD and 39% had an atopic illness.40 An association with allergic contact dermatitis and increased IgE to Malassezia furfur was further associated.41
Conclusion
The role of triggers and allergens in disease activity in AD is an important consideration in children with AD and requires ongoing consideration with age and varied exposures. Understanding the grading of AD is important in evaluating clinical trial data. The natural history of AD has changed, which is important for the practitioner to note when counseling patients and guardians.
- Li M. Current evidence of epidermal barrier dysfunction and thymic stromal lymphopoietin in the atopic march. Eur Respir Rev. 2014;23:292-298.
- Gupta RS, Rivkina V, DeSantiago-Cardenas L, et al. Asthma and food allergy management in Chicago public schools. Pediatrics. 2014;134:729-736.
- Flohr C, Perkin M, Logan K, et al. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol. 2014;134:345-350.
- Silverberg NB. Food, glorious food. Cutis. 2011;87:267-268.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affecting the association between atopic dermatitis and contact sensitization. Allergy. 2014;69:28-36.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA Cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Ortiz de Frutos FJ, Torrelo A, de Lucas R, et al. Patient perspectives on triggers, adherence to medical recommendations, and disease control in atopic dermatitis: the DATOP study. Actas Dermosifiliogr. 2014;105:487-496.
- Ricci G, Patrizi A, Bellini F, et al. Use of textiles in atopic dermatitis: care of atopic dermatitis. Curr Probl Dermatol. 2006;33:127-143.
- Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
- Aquino M, Fonacier L. The role of contact dermatitis in patients with atopic dermatitis. J Allergy Clin Immunol Pract. 2014;2:382-387.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Martorell A, Alonso E, Boné J, et al. Position document: IgE-mediated allergy to egg protein. Allergol Immunopathol (Madr). 2013;41:320-336.
- Sicherer SH. Early introduction of peanut to infants at high allergic risk can reduce peanut allergy at age 5 years [published online September 17, 2015]. Evid Based Med. 2015;20:204.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Weiland SK, Hüsing A, Strachan DP, et al. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med. 2004;61:609-615.
- Langan SM, Irvine AD. Childhood eczema and the importance of the physical environment. J Invest Dermatol. 2013;133:1706-1709.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Brown SJ, McLean WH. Eczema genetics: current state of knowledge and future goals. J Invest Dermatol. 2009;129:543-552.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Futamura M, Leshem YA, Thomas KS, et al. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74:288-294.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J Invest Dermatol. 2015;135:24-30.
- Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11-18.
- Leshem YA, Hajar T, Hanifin JM, et al. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172:1353-1357.
- Barbier N, Paul C, Luger T, et al. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol. 2004;150:96-102.
- Berth-Jones J. Six area, six sign atopic dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol. 1996;135(suppl 48):25-30.
- Zhao CY, Tran AQ, Lazo-Dizon JP, et al. A pilot comparison study of four clinician-rated atopic dermatitis severity scales. Br J Dermatol. 2015;173:488-497.
- Kunz B, Oranje AP, Labrèze L, et al. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10-19.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence, and comorbidities. Allergy. 2015;70:836-845.
- Rystedt I. Atopic background in patients with occupational hand eczema. Contact Dermatitis. 1985;12:247-254.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70:836-845.
Atopic dermatitis (AD) may be triggered by viral infections, food allergens, weather, and other causes, and it may trigger an inflammatory progression known as the atopic march. This article reviews research on triggers of pediatric AD so that dermatologists may discuss trigger avoidance with patients and guardians. Other factors affecting AD development include genetics and hygiene. Grading of AD also is discussed.
The Atopic March
The persistence of AD in untreated skin can trigger an inflammatory progression called the atopic march in which food and environmental allergies as well as asthma may occur progressively due to ongoing inflammatory triggering.1 In a study of asthma and food allergy reporting and management in public schools in Chicago, Illinois, food allergies were seen in 9.3% of asthmatic students (n=18,000), and 40.1% of food allergic students (n=4000) had asthma.2 An observational study by Flohr et al3 in London, England, included 619 exclusively breastfed infants who were recruited at 3 months of age. The investigators determined that food sensitization was unrelated to the presence of filaggrin mutations, type of eczema (flexural vs nonflexural), and transepidermal water loss but was associated with AD severity as determined by SCORAD (SCORing Atopic Dermatitis), a composite score of AD that includes pruritus as a factor in severity. Other AD associations included 3 leading food allergens: eggs, milk, and peanuts. No association with cod, wheat, or sesame allergy was noted. The investigators concluded that AD and AD severity were the leading skin-related risk factors for food allergies and therefore food allergy development in breastfed infants was probably mediated by cutaneous antigen-presenting cells.3
The skin has been documented to react to contact with known food allergens4 and is known to be a route of allergic sensitization to allergens such as fragrance in patients with AD.5,6 Two phenotypes of eczema that have been associated with asthma development are severe AD disease and multiple environmental allergies, supporting the theory of the atopic march.7 There also is evidence that release of danger-associated proteins from an impaired barrier also may trigger asthma.8 An analysis of the 2007 National Survey of Children’s Health, a population-based study of91,642 children aged 0 to 17 years, showed that children with AD had a higher prevalence of comorbid asthma (25.1% vs 12.3%), hay fever (34.4% vs 14.3%), and food allergies (15.1% vs 3.6%) compared to children without AD.9 A recent article provided detailed information on how food and diet interplay with AD.10
Triggers of Disease Flares
Triggers are the leading source of AD flare initiation, and avoidance of triggers is an important mechanism by which patients can control disease activity. Despite the best skin care and trigger avoidance, disease flares occur, sometimes due to ongoing inflammation and other times due to inability to prevent flares such as heat and humidity. A survey of patients with AD in Spain identified the following triggers: cosmetic products, clothing, mites, detergents/soaps, and temperature changes.11 In childhood, wool also is a known trigger of AD.12 Viral infections including respiratory syncytial virus may trigger the first onset of AD.13 Patients with AD may become allergic to fragrance and metals causing disease exacerbation on exposure.14,15 Food allergens contribute to approximately 40% of cases of AD in infancy but are not the cause of AD. The best evidence for improvement of AD with food allergen avoidance exists for egg white allergy.16 Food avoidance programs should be developed in conjunction with an allergist, as it is no longer advised in many cases to completely withdraw foods; therefore, an allergist has to assess the level of allergic severity and the risk-benefit ratio of food avoidance or introduction.17 Emotional stressors, heat, and humidity, as well as indoor heating in the winter months, can cause AD flares.18
A study by Silverberg et al19 provided evidence of climate influences on the US prevalence of childhood eczema using a merged analysis of the 2007 National Survey of Children’s Health and the 2006-2007 National Climate Data Center and Weather Service. Results showed that eczema prevalence was significantly lower when associated with higher annual relative humidity (P=.01), UV index (P<.0001), and highest-quartile air temperature (P=.002).19 The Pediatric Eczema Elective Registry also showed that warm, humid, and high-sun-exposure climates are associated with poorly controlled eczema in affected patients.20 The association of eczema with latitude as well as its negative association with mean annual outdoor temperature has been described by Weiland et al21 in the ISAAC (International Study of Asthma and Allergies in Childhood) study. Long airplane flights in low humidity can trigger eczema in adults. Climate has been postulated to affect eczema through alterations in filaggrin and skin barrier function.22 Indoor temperature and humidity regulation may be used adjunctively for daily flare prevention.
Genetics and AD
Of 762 infants in a birth cohort with a parent with atopy in Cincinnati, Ohio, 39% developed eczema by the age of 3 years. Single nucleotide polymorphisms of IL-4Rα 175 V and CD14-159 C/T were linked to greater eczema risk at 2 to 3 years of age.23 Monozygotic twins have a concordance rate of 0.72 to 0.86 versus 0.21 to 0.23 in dizygotic twins, demonstrating a strong genetic component in the development of AD.24 Linkage to AD has been positively made to the epidermal differentiation complex on human chromosome 1q21, which contains the genes for filaggrin and other proteins such as loricrin. Other genes linked to AD include the serine protease inhibitor SPINK5 (serine peptidase inhibitor, Kazal type 5) implicated in Netherton syndrome (triad of ichthyosis linearis circumflexa, bamboo hair, and atopic disorders); RANTES (regulated on activation, normal T-expressed, and secreted), which has been associated with severity of AD; IL-4; and IL-13.5,25,26
The Hygiene Hypothesis
Atopic dermatitis is more common in wealthy developed countries, leading some to believe that hygiene and relative reduction in illness via vaccination have contributed to the rise of AD prevalence in developed nations.13,27 There currently is evidence demonstrating that wild-type varicella infection confers long-standing protection against AD and mediates reduced total IgE and peripheral blood lymphocytes.27
Grading of AD
Grading of AD is a subject of controversy, as there currently are no uniform grading scales.28 A recent outcomes group attempted to determine the best scale for disease monitoring. Schmitt et al29 presented the Harmonizing Outcome Measures for Eczema (HOME) roadmap, which was intended to determine a core outcome set for eczema; however, because these outcome measurements have not yet been standardized, only the eczema assessment and severity index (EASI) scoring system meets criteria for standardization. In clinical practice, physicians often assign mild, moderate, or severe labeling based on their general sense of the disease extent using an investigator global assessment score.28
The EASI score is a well-validated composite score of AD severity based on 4 body regions: (1) head and neck, (2) trunk (including genital area), (3) upper limbs, and (4) lower limbs (including buttocks). The total area of involvement in each region is graded on a scale of 0 to 6, and AD severity is graded as a composite of 4 parameters (ranked on a scale of 0–3), including redness (erythema, inflammation), thickness (induration, papulation, swelling [acute eczema]), scratching (excoriation), and lichenification (prurigo nodules [chronic eczema]). The surface area of each region relative to body size is used as a multiplying factor, resulting in the following severity strata: 0=clear; 0.1–1.0=almost clear; 1.1–7.0=mild; 7.1–21.0=moderate; 21.1–50.0=severe; 50.1–72.0=very severe (κ=0.75).30-32 The six area, six sign AD (SASSAD) score32,33 is a similar score without adjustment for body surface area by region.34
An older, now less frequently used eczema score is the SCORAD, which addressed surface area by rule of nines and severity of 6 features—redness, swelling, oozing/crusting, scratch marks, skin thickening (lichenification), dryness (assessed in an area with no inflammation)—by region on a scale of 0 to 3. A subjective symptom parameter for itching and sleeplessness helped highlight that these comorbidities are important in gauging disease activity and impact on a child’s life.35
Natural History of AD
The clinical dogma has been that AD would improve with age, with reduction at grade school entry and perhaps full disappearance in adulthood; however, 3 recent surveys have suggested otherwise. The ISAAC group has found prevalence of AD in wealthy developed countries among children aged 6 to 7 years to be at a consistent increase.36 A US-based survey from the National Health Interview Survey showed a 1-year prevalence of 10.2% of active AD in adults and 9.8% when occupational dermatitis was excluded.37 Halvorsen et al38 demonstrated that eczema prevalence is 9.7% in individuals aged 18 to 19 years.
A prospective trial of eighth graders followed from 1995 to 2010 demonstrated that AD persisted in 50% at school age. Persistent eczema into adulthood was associated with early-onset childhood allergic rhinitis and hand eczema.39 In a cohort of hand eczema patients (N=368), 28% had AD and 39% had an atopic illness.40 An association with allergic contact dermatitis and increased IgE to Malassezia furfur was further associated.41
Conclusion
The role of triggers and allergens in disease activity in AD is an important consideration in children with AD and requires ongoing consideration with age and varied exposures. Understanding the grading of AD is important in evaluating clinical trial data. The natural history of AD has changed, which is important for the practitioner to note when counseling patients and guardians.
Atopic dermatitis (AD) may be triggered by viral infections, food allergens, weather, and other causes, and it may trigger an inflammatory progression known as the atopic march. This article reviews research on triggers of pediatric AD so that dermatologists may discuss trigger avoidance with patients and guardians. Other factors affecting AD development include genetics and hygiene. Grading of AD also is discussed.
The Atopic March
The persistence of AD in untreated skin can trigger an inflammatory progression called the atopic march in which food and environmental allergies as well as asthma may occur progressively due to ongoing inflammatory triggering.1 In a study of asthma and food allergy reporting and management in public schools in Chicago, Illinois, food allergies were seen in 9.3% of asthmatic students (n=18,000), and 40.1% of food allergic students (n=4000) had asthma.2 An observational study by Flohr et al3 in London, England, included 619 exclusively breastfed infants who were recruited at 3 months of age. The investigators determined that food sensitization was unrelated to the presence of filaggrin mutations, type of eczema (flexural vs nonflexural), and transepidermal water loss but was associated with AD severity as determined by SCORAD (SCORing Atopic Dermatitis), a composite score of AD that includes pruritus as a factor in severity. Other AD associations included 3 leading food allergens: eggs, milk, and peanuts. No association with cod, wheat, or sesame allergy was noted. The investigators concluded that AD and AD severity were the leading skin-related risk factors for food allergies and therefore food allergy development in breastfed infants was probably mediated by cutaneous antigen-presenting cells.3
The skin has been documented to react to contact with known food allergens4 and is known to be a route of allergic sensitization to allergens such as fragrance in patients with AD.5,6 Two phenotypes of eczema that have been associated with asthma development are severe AD disease and multiple environmental allergies, supporting the theory of the atopic march.7 There also is evidence that release of danger-associated proteins from an impaired barrier also may trigger asthma.8 An analysis of the 2007 National Survey of Children’s Health, a population-based study of91,642 children aged 0 to 17 years, showed that children with AD had a higher prevalence of comorbid asthma (25.1% vs 12.3%), hay fever (34.4% vs 14.3%), and food allergies (15.1% vs 3.6%) compared to children without AD.9 A recent article provided detailed information on how food and diet interplay with AD.10
Triggers of Disease Flares
Triggers are the leading source of AD flare initiation, and avoidance of triggers is an important mechanism by which patients can control disease activity. Despite the best skin care and trigger avoidance, disease flares occur, sometimes due to ongoing inflammation and other times due to inability to prevent flares such as heat and humidity. A survey of patients with AD in Spain identified the following triggers: cosmetic products, clothing, mites, detergents/soaps, and temperature changes.11 In childhood, wool also is a known trigger of AD.12 Viral infections including respiratory syncytial virus may trigger the first onset of AD.13 Patients with AD may become allergic to fragrance and metals causing disease exacerbation on exposure.14,15 Food allergens contribute to approximately 40% of cases of AD in infancy but are not the cause of AD. The best evidence for improvement of AD with food allergen avoidance exists for egg white allergy.16 Food avoidance programs should be developed in conjunction with an allergist, as it is no longer advised in many cases to completely withdraw foods; therefore, an allergist has to assess the level of allergic severity and the risk-benefit ratio of food avoidance or introduction.17 Emotional stressors, heat, and humidity, as well as indoor heating in the winter months, can cause AD flares.18
A study by Silverberg et al19 provided evidence of climate influences on the US prevalence of childhood eczema using a merged analysis of the 2007 National Survey of Children’s Health and the 2006-2007 National Climate Data Center and Weather Service. Results showed that eczema prevalence was significantly lower when associated with higher annual relative humidity (P=.01), UV index (P<.0001), and highest-quartile air temperature (P=.002).19 The Pediatric Eczema Elective Registry also showed that warm, humid, and high-sun-exposure climates are associated with poorly controlled eczema in affected patients.20 The association of eczema with latitude as well as its negative association with mean annual outdoor temperature has been described by Weiland et al21 in the ISAAC (International Study of Asthma and Allergies in Childhood) study. Long airplane flights in low humidity can trigger eczema in adults. Climate has been postulated to affect eczema through alterations in filaggrin and skin barrier function.22 Indoor temperature and humidity regulation may be used adjunctively for daily flare prevention.
Genetics and AD
Of 762 infants in a birth cohort with a parent with atopy in Cincinnati, Ohio, 39% developed eczema by the age of 3 years. Single nucleotide polymorphisms of IL-4Rα 175 V and CD14-159 C/T were linked to greater eczema risk at 2 to 3 years of age.23 Monozygotic twins have a concordance rate of 0.72 to 0.86 versus 0.21 to 0.23 in dizygotic twins, demonstrating a strong genetic component in the development of AD.24 Linkage to AD has been positively made to the epidermal differentiation complex on human chromosome 1q21, which contains the genes for filaggrin and other proteins such as loricrin. Other genes linked to AD include the serine protease inhibitor SPINK5 (serine peptidase inhibitor, Kazal type 5) implicated in Netherton syndrome (triad of ichthyosis linearis circumflexa, bamboo hair, and atopic disorders); RANTES (regulated on activation, normal T-expressed, and secreted), which has been associated with severity of AD; IL-4; and IL-13.5,25,26
The Hygiene Hypothesis
Atopic dermatitis is more common in wealthy developed countries, leading some to believe that hygiene and relative reduction in illness via vaccination have contributed to the rise of AD prevalence in developed nations.13,27 There currently is evidence demonstrating that wild-type varicella infection confers long-standing protection against AD and mediates reduced total IgE and peripheral blood lymphocytes.27
Grading of AD
Grading of AD is a subject of controversy, as there currently are no uniform grading scales.28 A recent outcomes group attempted to determine the best scale for disease monitoring. Schmitt et al29 presented the Harmonizing Outcome Measures for Eczema (HOME) roadmap, which was intended to determine a core outcome set for eczema; however, because these outcome measurements have not yet been standardized, only the eczema assessment and severity index (EASI) scoring system meets criteria for standardization. In clinical practice, physicians often assign mild, moderate, or severe labeling based on their general sense of the disease extent using an investigator global assessment score.28
The EASI score is a well-validated composite score of AD severity based on 4 body regions: (1) head and neck, (2) trunk (including genital area), (3) upper limbs, and (4) lower limbs (including buttocks). The total area of involvement in each region is graded on a scale of 0 to 6, and AD severity is graded as a composite of 4 parameters (ranked on a scale of 0–3), including redness (erythema, inflammation), thickness (induration, papulation, swelling [acute eczema]), scratching (excoriation), and lichenification (prurigo nodules [chronic eczema]). The surface area of each region relative to body size is used as a multiplying factor, resulting in the following severity strata: 0=clear; 0.1–1.0=almost clear; 1.1–7.0=mild; 7.1–21.0=moderate; 21.1–50.0=severe; 50.1–72.0=very severe (κ=0.75).30-32 The six area, six sign AD (SASSAD) score32,33 is a similar score without adjustment for body surface area by region.34
An older, now less frequently used eczema score is the SCORAD, which addressed surface area by rule of nines and severity of 6 features—redness, swelling, oozing/crusting, scratch marks, skin thickening (lichenification), dryness (assessed in an area with no inflammation)—by region on a scale of 0 to 3. A subjective symptom parameter for itching and sleeplessness helped highlight that these comorbidities are important in gauging disease activity and impact on a child’s life.35
Natural History of AD
The clinical dogma has been that AD would improve with age, with reduction at grade school entry and perhaps full disappearance in adulthood; however, 3 recent surveys have suggested otherwise. The ISAAC group has found prevalence of AD in wealthy developed countries among children aged 6 to 7 years to be at a consistent increase.36 A US-based survey from the National Health Interview Survey showed a 1-year prevalence of 10.2% of active AD in adults and 9.8% when occupational dermatitis was excluded.37 Halvorsen et al38 demonstrated that eczema prevalence is 9.7% in individuals aged 18 to 19 years.
A prospective trial of eighth graders followed from 1995 to 2010 demonstrated that AD persisted in 50% at school age. Persistent eczema into adulthood was associated with early-onset childhood allergic rhinitis and hand eczema.39 In a cohort of hand eczema patients (N=368), 28% had AD and 39% had an atopic illness.40 An association with allergic contact dermatitis and increased IgE to Malassezia furfur was further associated.41
Conclusion
The role of triggers and allergens in disease activity in AD is an important consideration in children with AD and requires ongoing consideration with age and varied exposures. Understanding the grading of AD is important in evaluating clinical trial data. The natural history of AD has changed, which is important for the practitioner to note when counseling patients and guardians.
- Li M. Current evidence of epidermal barrier dysfunction and thymic stromal lymphopoietin in the atopic march. Eur Respir Rev. 2014;23:292-298.
- Gupta RS, Rivkina V, DeSantiago-Cardenas L, et al. Asthma and food allergy management in Chicago public schools. Pediatrics. 2014;134:729-736.
- Flohr C, Perkin M, Logan K, et al. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol. 2014;134:345-350.
- Silverberg NB. Food, glorious food. Cutis. 2011;87:267-268.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affecting the association between atopic dermatitis and contact sensitization. Allergy. 2014;69:28-36.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA Cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Ortiz de Frutos FJ, Torrelo A, de Lucas R, et al. Patient perspectives on triggers, adherence to medical recommendations, and disease control in atopic dermatitis: the DATOP study. Actas Dermosifiliogr. 2014;105:487-496.
- Ricci G, Patrizi A, Bellini F, et al. Use of textiles in atopic dermatitis: care of atopic dermatitis. Curr Probl Dermatol. 2006;33:127-143.
- Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
- Aquino M, Fonacier L. The role of contact dermatitis in patients with atopic dermatitis. J Allergy Clin Immunol Pract. 2014;2:382-387.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Martorell A, Alonso E, Boné J, et al. Position document: IgE-mediated allergy to egg protein. Allergol Immunopathol (Madr). 2013;41:320-336.
- Sicherer SH. Early introduction of peanut to infants at high allergic risk can reduce peanut allergy at age 5 years [published online September 17, 2015]. Evid Based Med. 2015;20:204.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Weiland SK, Hüsing A, Strachan DP, et al. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med. 2004;61:609-615.
- Langan SM, Irvine AD. Childhood eczema and the importance of the physical environment. J Invest Dermatol. 2013;133:1706-1709.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Brown SJ, McLean WH. Eczema genetics: current state of knowledge and future goals. J Invest Dermatol. 2009;129:543-552.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Futamura M, Leshem YA, Thomas KS, et al. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74:288-294.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J Invest Dermatol. 2015;135:24-30.
- Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11-18.
- Leshem YA, Hajar T, Hanifin JM, et al. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172:1353-1357.
- Barbier N, Paul C, Luger T, et al. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol. 2004;150:96-102.
- Berth-Jones J. Six area, six sign atopic dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol. 1996;135(suppl 48):25-30.
- Zhao CY, Tran AQ, Lazo-Dizon JP, et al. A pilot comparison study of four clinician-rated atopic dermatitis severity scales. Br J Dermatol. 2015;173:488-497.
- Kunz B, Oranje AP, Labrèze L, et al. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10-19.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence, and comorbidities. Allergy. 2015;70:836-845.
- Rystedt I. Atopic background in patients with occupational hand eczema. Contact Dermatitis. 1985;12:247-254.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70:836-845.
- Li M. Current evidence of epidermal barrier dysfunction and thymic stromal lymphopoietin in the atopic march. Eur Respir Rev. 2014;23:292-298.
- Gupta RS, Rivkina V, DeSantiago-Cardenas L, et al. Asthma and food allergy management in Chicago public schools. Pediatrics. 2014;134:729-736.
- Flohr C, Perkin M, Logan K, et al. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol. 2014;134:345-350.
- Silverberg NB. Food, glorious food. Cutis. 2011;87:267-268.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affecting the association between atopic dermatitis and contact sensitization. Allergy. 2014;69:28-36.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA Cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Ortiz de Frutos FJ, Torrelo A, de Lucas R, et al. Patient perspectives on triggers, adherence to medical recommendations, and disease control in atopic dermatitis: the DATOP study. Actas Dermosifiliogr. 2014;105:487-496.
- Ricci G, Patrizi A, Bellini F, et al. Use of textiles in atopic dermatitis: care of atopic dermatitis. Curr Probl Dermatol. 2006;33:127-143.
- Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
- Aquino M, Fonacier L. The role of contact dermatitis in patients with atopic dermatitis. J Allergy Clin Immunol Pract. 2014;2:382-387.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Martorell A, Alonso E, Boné J, et al. Position document: IgE-mediated allergy to egg protein. Allergol Immunopathol (Madr). 2013;41:320-336.
- Sicherer SH. Early introduction of peanut to infants at high allergic risk can reduce peanut allergy at age 5 years [published online September 17, 2015]. Evid Based Med. 2015;20:204.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Weiland SK, Hüsing A, Strachan DP, et al. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med. 2004;61:609-615.
- Langan SM, Irvine AD. Childhood eczema and the importance of the physical environment. J Invest Dermatol. 2013;133:1706-1709.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Brown SJ, McLean WH. Eczema genetics: current state of knowledge and future goals. J Invest Dermatol. 2009;129:543-552.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Futamura M, Leshem YA, Thomas KS, et al. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74:288-294.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J Invest Dermatol. 2015;135:24-30.
- Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11-18.
- Leshem YA, Hajar T, Hanifin JM, et al. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172:1353-1357.
- Barbier N, Paul C, Luger T, et al. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol. 2004;150:96-102.
- Berth-Jones J. Six area, six sign atopic dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol. 1996;135(suppl 48):25-30.
- Zhao CY, Tran AQ, Lazo-Dizon JP, et al. A pilot comparison study of four clinician-rated atopic dermatitis severity scales. Br J Dermatol. 2015;173:488-497.
- Kunz B, Oranje AP, Labrèze L, et al. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10-19.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence, and comorbidities. Allergy. 2015;70:836-845.
- Rystedt I. Atopic background in patients with occupational hand eczema. Contact Dermatitis. 1985;12:247-254.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70:836-845.
Practice Points
- Atopic dermatitis (AD) can be triggered by viral infections, weather, and food allergens.
- The scoring of AD is largely used experimentally and includes the eczema assessment and severity index; the SCORAD (SCORing Atopic Dermatitis); and the six area, six sign AD (SASSAD) scores.
- There is a strong genetic contribution to the development of AD.
- Children with AD may have persistent disease into adulthood in half of cases.
A Practical Overview of Pediatric Atopic Dermatitis, Part 1: Epidemiology and Pathogenesis
Atopic dermatitis (AD), or eczema, is the leading dermatologic diagnosis worldwide and is vexing to patients due to the itchiness of the rash. It is the leading cause of skin disease burden worldwide with a prevalence of 229,761,000 reported cases in 2010, presenting largely in preadolescence but also persisting through adulthood.1 Using the children’s life quality index, it has been demonstrated that AD has a greater impact on health-related quality of life than renal disease and cystic fibrosis.2 The overall burden of AD includes stress on the patient and his/her family as well as financial burdens that have been estimated to be similar to that of type 1 diabetes mellitus.3
Epidemiology of AD
The worldwide prevalence of AD varies by country and age group surveyed, with a higher prevalence in wealthy developed nations (eg, the United States) compared to poorer developing nations.4 Efforts to identify prevalence data for AD in the United States have been approached through a variety of strategies. A group in Oregon estimated the prevalence of AD in children aged 5 to 9 years to be 17.2% via a survey of parents (N=1465) and 11.8% with doctor-diagnosed eczema. In the same study, the question “Has a doctor ever said that your child has eczema?” was found to have a 91.3% predictive correlation.5 Analysis of the 2003 National Survey of Children’s Health demonstrated the overall US prevalence of pediatric AD to be 10.7% in 102,353 children 17 years or younger, with a range of 8.7% to 18.1% by region.6
In its evaluation of the worldwide prevalence of AD, the International Study of Asthma and Allergies in Childhood ranked the United States 17th.7,8 The prevalence of AD in developed countries such as the United States is fluid and is expected to increase if the trends from the last 20 years remain true. In an assessment of the National Health Interview Survey data from 1997 to 2011 based on responses to the question, “During the past 12 months, has your child had eczema or any kind of skin allergy?”, the Centers for Disease Control and Prevention identified an increase in the prevalence of AD in patients aged 0 to 17 years from 7.4% in 1997-1999 to 12.5% in 2009-2011.9 Rising prevalence seems to be paired with rising incidence in the total number of severe intractable cases, reduced clearance at the approach of grade school, or cases persisting into adulthood.
Racial Disparity in AD
Racial disparity worldwide and migration are thought to contribute to the prevalence of and therapeutic need for AD. For example, in the United Kingdom, the prevalence of AD in London-born Afro-Caribbean children versus white children (total cross-section, N=693 [junior school children]) was 16.3% and 8.7%, respectively.10 In the United States, black children were more likely to have AD than white children (odds ratio, 1.7).6 Asian and black children also were more likely to present to a physician for treatment of AD than white children.6,10-13
Definition and Diagnostic Considerations
According to Hanifin,14 “Eczema represents a family of inflammatory skin conditions characterized by pruritic, papulovesicular, sometimes weeping dermatitis. All demonstrate the histological hallmark of spongiosis, which helps to distinguish the eczemas from papulosquamous diseases such as psoriasis.”14 Atopic dermatitis is a variant of eczema; however, most laymen identify eczema and AD as being one and the same.
The Hanifin and Rajka15 criteria are the major diagnostic criteria for AD but are difficult to use in clinical practice. Three of the following 4 major criteria are needed for diagnosis: (1) pruritus, which is present universally; (2) typical morphology and distribution; (3) chronic or chronically relapsing dermatitis; and (4) personal and/or family history of atopy. Additionally, 3 of the following 23 minor criteria are needed for diagnosis: xerosis; ichthyosis vulgaris, palmar hyperlinearity, or keratosis pilaris; positive skin prick test; elevated serum IgE level; early age of onset; tendency toward cutaneous infections or impaired cell-mediated immunity; tendency toward nonspecific hand or foot dermatitis; nipple eczema; cheilitis; recurrent conjunctivitis; Dennie-Morgan fold (infraorbital fold); keratoconus; anterior subcapsular cataracts; orbital darkening; facial pallor or facial erythema; pityriasis alba; anterior neck folds; itching when sweating; intolerance to wool and lipid solvents; perifollicular accentuation; skin reactions from ingested foods or by food contact; environmental or emotional factors; and lesional/nonlesional white dermographism or delayed blanch.15-17
More pragmatic streamlined diagnostic criteria were established by Eichenfield et al.18 According to these guidelines, essential features for AD include pruritus and eczema. Important features seen in most cases and adding support to the diagnosis include early age of onset, atopy, and xerosis.18 In clinical practice, diagnosis is often made based on a pruritic relapsing condition in typical locations including the face, neck, and extensor surfaces in infants and children.
Age Considerations
Diagnosis of AD is made by 5 years of age in 85% to 90% of children who will develop the disease and by age 1 year in 60% to 65%.6,19,20 Atopic dermatitis will persist into adulthood in up to one-third of children.21,22 Infantile AD is characterized by erythematous, oozing, excoriated plaques on the cheeks (sparing the nose), scalp, trunk, and extensor surfaces. Pruritus is always seen in AD and can be a source of morbidity.16-18 Seborrheic dermatitis may complicate or overlap with AD in infancy.22
By 2 years of age, most children who are going to develop AD begin to show disease signs of childhood AD characterized by flexural lesions and lesions on the neck and in the postauricular area with sparing of the diaper area.23 Adult AD often presents as eczema of the hands and/or feet. Hand eczema in adulthood is correlated with a prior history of childhood hand eczema and/or childhood AD as well as wet work and caring for small children.24 Children with skin of color may manifest with follicular eczema as their primary disease phenotype. Facial and eyelid dermatitis are more common in Asian females, infants, and teenagers.12,25 Other disease phenotypes that are common in patients with skin of color include lichenoid AD and postinflammatory hypopigmentation.12
Pathogenesis of AD
There are 2 theories on the pathogenesis of AD known as the inside-out and outside-in hypotheses.26 The inside-out hypothesis suggests that allergic triggering leads to a weakened skin barrier that furthers allergen introduction and presentation, while the outside-in hypothesis suggests that the skin barrier is weakened in AD and allows for the presentation of allergens. Both theories have validity and biologic basis, and both may in fact be true in certain individuals.26
The Skin Barrier: An Overview
The skin barrier is a complex set of factors present and functional at birth that seal the keratinocytes and the interkeratinocyte space so that the skin can perform key processes and functions including retention of fluid, exclusion of allergens, protection from UV light and solvents, and prevention of pathogen entry (eg, infections).27-29 The superficial stratum corneum or the cornified envelope consists of keratinocytes with intercellular stripes of hydrophobic and hydrophilic substances formed by various intercellular lipids, largely ceramides, cholesterol, and free fatty acids.30,31 Keratinocytes are the first responders to a variety of environmental insults with the production of IL-18, RANTES (regulated on activation, normal T-expressed, and presumably secreted), granulocyte-macrophage colony-stimulating factor, and thymic stromal lymphopoietin. These inflammatory substances produce acute and chronic inflammation, mast cell reactivity, and T-cell activation.14 Corneodesmosins link the keratinocytes. Peptidases released will cleave the corneodesmosins and allow normal desquamation or shedding of surface skin, which is replaced by division of stem cells in the basal layer.29
The stratum granulosum is the layer beneath the stratum corneum that co-contributes to barrier activity. The stratum granulosum is absent or reduced histologically in ichthyosis vulgaris,32 a form of skin dryness linked to filaggrin mutations and AD. Filaggrin breakdown creates natural moisturizing factor, a series of hygroscopic compounds that attract water into the skin.33 Histidine, a filaggrin breakdown product, is used by urocanic acid to process UV light insults.34 Filaggrin also contributes to other barrier functions including pH and stratum corneum cohesion as well as paracellular permeability of the stratum corneum. Tight junctions in the stratum granulosum include claudin-1 and claudin-6 and provide another barrier feature.29
The skin barrier is composed of lipids and keratinocytes. Ceramides, which represent one type of lipids, are reduced in AD, causing alteration in the lamellar pattern35 and increased transepidermal water loss. Furthermore, the stratum corneum is thickened in AD, possibly in response to trauma, and hydration is reduced.36 Filaggrin (chromosome arm 1q21.3) is formed from the 400-kDa+ precursor profilaggrin through dephosphorylation and cleavage, and it performs an essential function in the skin barrier through its differential cleavage and breakdown as well as release of natural moisturizing factor and other compounds.37 Filaggrin mutations are linked to AD and ichthyosis vulgaris; however, barrier defects as evidenced by transepidermal water loss in the absence of filaggrin mutation are sufficient to allow for sensitization to allergens through the skin.29 Filaggrin mutations have been associated with AD development and vary in prevalence worldwide. In the United Kingdom, a prevalence study of filaggrin mutations in patients aged 7 to 9 years (N=792) demonstrated an 18.4% carrier rate in AD patients versus 12.9% in controls.34 A similar study in Sweden (N=3301) showed carrier rates of 13.5% versus 6.5%, respectively.38 Although filaggrin mutations are lower in black patients,39 ceramide content may be reduced in this population, demonstrating that a variety of skin barrier defects can result in AD. Carriers of filaggrin mutations are more likely to have eczema on skin exposed to environmental factors (eg, face, hands).40
Barrier Defects Contributing to AD
The breakdown of the stratum corneum allows for antigen presentation to Langerhans cells, the dendritic antigen-presenting cells of the skin. Breaks in the stratum corneum may occur from scratching. These macroscopic breaks are large, whereas the breaks that otherwise occur due to barrier breakdown may be more microscopic in nature. Scratching causes aggravation of the helper T cell (TH2) response.29 For example, it allows the dendritic ends of Langerhans cells to be exposed to antigens. The dendritic ends capture allergens through IgE (may be elevated in AD29), which is bound to the high-affinity FCER1 receptors on Langerhans cells. Rather than causing a type I hypersensitivity reaction, these Langerhans cells are activated and move to the lymph nodes where they present antigen and initiate a cascade of proinflammatory activity. This TH2 cascade includes release of cytokines such as IL-2, IL-4, IL-8, IL-10, tumor necrosis factor α, and IFN-γ.26,29
Transepidermal water loss and barrier dysfunction contribute to disease activity and facilitate food/environmental allergen sensitization by allowing increased penetration of allergens through the skin to be presented by Langerhans cells to TH1 cells (sensitization phase). The Langerhans cells can reach their dendritic ends through tight junctions and into the stratum corneum, allowing them to reach surface allergens when the barrier is impaired. Ultimate expansion to systemic allergy (effector phase) occurs when dendritic cells move to draining lymph nodes, causing antigen presentation to CD4 and/or CD8 cells. Langerhans cells and dendritic cell sensitization through the weakened skin is believed to be the basis or role of barrier disruption as a trigger of atopic diseases, including AD and food and environmental allergies.
Many different forms of barrier disruption can cause a TH2 response in AD. The TH2 response triggers a constellation of proinflammatory activities including release of IL-4, associated with eosinophilia and elevated IgE levels, the latter being minor criterion in the diagnosis of AD.15 One mechanism by which the TH2 response is elicited may be the release of molecules such as danger-associated molecule patterns that may elicit recruitment of other inflammatory cells. Helper T cell (TH2) activity also can worsen barrier defects through IL-4 and IL-13 release, which can reduce filaggrin expression,29,41 and can aggravate barrier dysfunction in AD.
Inflammatory activation in AD also may involve inflammatory dendritic epidermal cells (IDECs). The IDECs can be tolerogenic or immunogenic mature phenotypes. The IDECs activate helper T cells (TH1), which may contribute to long-term AD activity.
Conclusion
Atopic dermatitis is a common skin condition worldwide and is characterized by the hallmark of pruritus and features that include a typical pattern, history of atopy (personal or family), and usually xerosis and early disease onset. Barrier dysfunction and immune dysregulation are prominent in AD, both of which aggravate the other and may encourage increased development of allergies and other forms of atopy over time.
1. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
2. Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
3. Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
4. Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
5. Laughter D, Istvan JA, Tofte SJ, et al. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649-655.
6. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
7. Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-1258.
8. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225-1232.
9. Hansen TE, Evjenth B, Holt J. Increasing prevalence of asthma, allergic rhinoconjunctivitis and eczema among schoolchildren: three surveys during the period 1985-2008. Acta Paediatr. 2013;102:47-52.
10. Williams HC, Pembroke AC, Forsdyke H, et al. London-born black Caribbean children are at increased risk of atopic dermatitis. J Am Acad Dermatol. 1995;32:212-217.
11. Horii KA, Simon SD, Liu DY, et al. Atopic dermatitis in children in the United States, 1997-2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics. 2007;120:e527-e534.
12. Silverberg NB. Eczematous diseases. In: Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity. New York, NY: Springer; 2012:69-88.
13. Gupta J, Grube E, Ericksen MB, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725-730.
14. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
15. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980;92:44-47.
16. Queille-Roussel C, Raynaud F, Saurat JH. A prospective computerized study of 500 cases of atopic dermatitis in childhood. I. Initial analysis of 250 parameters. Acta Derm Venereol Suppl (Stockh). 1985;114:87-92.
17. Böhme M, Svensson A, Kull I, et al. Hanifin’s and Rajka’s minor criteria for atopic dermatitis: which do 2-year-olds exhibit? J Am Acad Dermatol. 2000;43:785-792.
18. Eichenfield LF, Hanifin JM, Luger TA, et al. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol. 2003;49:1088-1095.
19. Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
20. Perkin MR, Strachan DP, Williams HC, et al. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr Allergy Immunol. 2004;15:221-229.
21. Ellis CN, Mancini AJ, Paller AS, et al. Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg. 2012;31(suppl 2):S18-S22.
22. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
23. Meding B, Wrangsjö K, Järvholm B. Hand eczema extent and morphology—association and influence on long-term prognosis. J Invest Dermatol. 2007;127:2147-2151.
24. Mortz CG, Bindslev-Jensen C, Andersen KE. Hand eczema in The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS): prevalence, incidence and risk factors from adolescence to adulthood [published online August 7, 2014]. Br J Dermatol. 2014;171:313-323.
25. Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
26. Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
27. Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care [published online December 8, 2014]. Clin Dermatol. 2015;33:271-280.
28. Miyagaki T, Sugaya M. Recent advances in atopic dermatitis and psoriasis: genetic background, barrier function, and therapeutic targets. J Dermatol Sci. 2015;78:89-94.
29. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
30. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
31. Janssens M, van Smeden J, Gooris GS, et al. Lamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczema. J Invest Dermatol. 2011;131:2136-2138.
32. Fitch N, Segool R, Ferenczy A, et al. Dominant ichthyosis vulgaris with an ultrastructurally normal granular layer. Clin Genet. 1976;9:71-76.
33. Chandar P, Nole G, Johnson AW. Understanding natural moisturizing mechanisms: implications for moisturizer technology. Cutis. 2009;84(suppl 1):2-15.
34. Brown SJ, Relton CL, Liao H, et al. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol. 2008;121:940-946.
35. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
36. Nemoto-Hasebe I, Akiyama M, Nomura T, et al. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009;129:682-689.
37. Hoste E, Kemperman P, Devos M, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233-2241.
38. Ballardini N, Kull I, Söderhäll C, et al. Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol. 2013;168:588-594.
39. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
40. Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
41. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
Atopic dermatitis (AD), or eczema, is the leading dermatologic diagnosis worldwide and is vexing to patients due to the itchiness of the rash. It is the leading cause of skin disease burden worldwide with a prevalence of 229,761,000 reported cases in 2010, presenting largely in preadolescence but also persisting through adulthood.1 Using the children’s life quality index, it has been demonstrated that AD has a greater impact on health-related quality of life than renal disease and cystic fibrosis.2 The overall burden of AD includes stress on the patient and his/her family as well as financial burdens that have been estimated to be similar to that of type 1 diabetes mellitus.3
Epidemiology of AD
The worldwide prevalence of AD varies by country and age group surveyed, with a higher prevalence in wealthy developed nations (eg, the United States) compared to poorer developing nations.4 Efforts to identify prevalence data for AD in the United States have been approached through a variety of strategies. A group in Oregon estimated the prevalence of AD in children aged 5 to 9 years to be 17.2% via a survey of parents (N=1465) and 11.8% with doctor-diagnosed eczema. In the same study, the question “Has a doctor ever said that your child has eczema?” was found to have a 91.3% predictive correlation.5 Analysis of the 2003 National Survey of Children’s Health demonstrated the overall US prevalence of pediatric AD to be 10.7% in 102,353 children 17 years or younger, with a range of 8.7% to 18.1% by region.6
In its evaluation of the worldwide prevalence of AD, the International Study of Asthma and Allergies in Childhood ranked the United States 17th.7,8 The prevalence of AD in developed countries such as the United States is fluid and is expected to increase if the trends from the last 20 years remain true. In an assessment of the National Health Interview Survey data from 1997 to 2011 based on responses to the question, “During the past 12 months, has your child had eczema or any kind of skin allergy?”, the Centers for Disease Control and Prevention identified an increase in the prevalence of AD in patients aged 0 to 17 years from 7.4% in 1997-1999 to 12.5% in 2009-2011.9 Rising prevalence seems to be paired with rising incidence in the total number of severe intractable cases, reduced clearance at the approach of grade school, or cases persisting into adulthood.
Racial Disparity in AD
Racial disparity worldwide and migration are thought to contribute to the prevalence of and therapeutic need for AD. For example, in the United Kingdom, the prevalence of AD in London-born Afro-Caribbean children versus white children (total cross-section, N=693 [junior school children]) was 16.3% and 8.7%, respectively.10 In the United States, black children were more likely to have AD than white children (odds ratio, 1.7).6 Asian and black children also were more likely to present to a physician for treatment of AD than white children.6,10-13
Definition and Diagnostic Considerations
According to Hanifin,14 “Eczema represents a family of inflammatory skin conditions characterized by pruritic, papulovesicular, sometimes weeping dermatitis. All demonstrate the histological hallmark of spongiosis, which helps to distinguish the eczemas from papulosquamous diseases such as psoriasis.”14 Atopic dermatitis is a variant of eczema; however, most laymen identify eczema and AD as being one and the same.
The Hanifin and Rajka15 criteria are the major diagnostic criteria for AD but are difficult to use in clinical practice. Three of the following 4 major criteria are needed for diagnosis: (1) pruritus, which is present universally; (2) typical morphology and distribution; (3) chronic or chronically relapsing dermatitis; and (4) personal and/or family history of atopy. Additionally, 3 of the following 23 minor criteria are needed for diagnosis: xerosis; ichthyosis vulgaris, palmar hyperlinearity, or keratosis pilaris; positive skin prick test; elevated serum IgE level; early age of onset; tendency toward cutaneous infections or impaired cell-mediated immunity; tendency toward nonspecific hand or foot dermatitis; nipple eczema; cheilitis; recurrent conjunctivitis; Dennie-Morgan fold (infraorbital fold); keratoconus; anterior subcapsular cataracts; orbital darkening; facial pallor or facial erythema; pityriasis alba; anterior neck folds; itching when sweating; intolerance to wool and lipid solvents; perifollicular accentuation; skin reactions from ingested foods or by food contact; environmental or emotional factors; and lesional/nonlesional white dermographism or delayed blanch.15-17
More pragmatic streamlined diagnostic criteria were established by Eichenfield et al.18 According to these guidelines, essential features for AD include pruritus and eczema. Important features seen in most cases and adding support to the diagnosis include early age of onset, atopy, and xerosis.18 In clinical practice, diagnosis is often made based on a pruritic relapsing condition in typical locations including the face, neck, and extensor surfaces in infants and children.
Age Considerations
Diagnosis of AD is made by 5 years of age in 85% to 90% of children who will develop the disease and by age 1 year in 60% to 65%.6,19,20 Atopic dermatitis will persist into adulthood in up to one-third of children.21,22 Infantile AD is characterized by erythematous, oozing, excoriated plaques on the cheeks (sparing the nose), scalp, trunk, and extensor surfaces. Pruritus is always seen in AD and can be a source of morbidity.16-18 Seborrheic dermatitis may complicate or overlap with AD in infancy.22
By 2 years of age, most children who are going to develop AD begin to show disease signs of childhood AD characterized by flexural lesions and lesions on the neck and in the postauricular area with sparing of the diaper area.23 Adult AD often presents as eczema of the hands and/or feet. Hand eczema in adulthood is correlated with a prior history of childhood hand eczema and/or childhood AD as well as wet work and caring for small children.24 Children with skin of color may manifest with follicular eczema as their primary disease phenotype. Facial and eyelid dermatitis are more common in Asian females, infants, and teenagers.12,25 Other disease phenotypes that are common in patients with skin of color include lichenoid AD and postinflammatory hypopigmentation.12
Pathogenesis of AD
There are 2 theories on the pathogenesis of AD known as the inside-out and outside-in hypotheses.26 The inside-out hypothesis suggests that allergic triggering leads to a weakened skin barrier that furthers allergen introduction and presentation, while the outside-in hypothesis suggests that the skin barrier is weakened in AD and allows for the presentation of allergens. Both theories have validity and biologic basis, and both may in fact be true in certain individuals.26
The Skin Barrier: An Overview
The skin barrier is a complex set of factors present and functional at birth that seal the keratinocytes and the interkeratinocyte space so that the skin can perform key processes and functions including retention of fluid, exclusion of allergens, protection from UV light and solvents, and prevention of pathogen entry (eg, infections).27-29 The superficial stratum corneum or the cornified envelope consists of keratinocytes with intercellular stripes of hydrophobic and hydrophilic substances formed by various intercellular lipids, largely ceramides, cholesterol, and free fatty acids.30,31 Keratinocytes are the first responders to a variety of environmental insults with the production of IL-18, RANTES (regulated on activation, normal T-expressed, and presumably secreted), granulocyte-macrophage colony-stimulating factor, and thymic stromal lymphopoietin. These inflammatory substances produce acute and chronic inflammation, mast cell reactivity, and T-cell activation.14 Corneodesmosins link the keratinocytes. Peptidases released will cleave the corneodesmosins and allow normal desquamation or shedding of surface skin, which is replaced by division of stem cells in the basal layer.29
The stratum granulosum is the layer beneath the stratum corneum that co-contributes to barrier activity. The stratum granulosum is absent or reduced histologically in ichthyosis vulgaris,32 a form of skin dryness linked to filaggrin mutations and AD. Filaggrin breakdown creates natural moisturizing factor, a series of hygroscopic compounds that attract water into the skin.33 Histidine, a filaggrin breakdown product, is used by urocanic acid to process UV light insults.34 Filaggrin also contributes to other barrier functions including pH and stratum corneum cohesion as well as paracellular permeability of the stratum corneum. Tight junctions in the stratum granulosum include claudin-1 and claudin-6 and provide another barrier feature.29
The skin barrier is composed of lipids and keratinocytes. Ceramides, which represent one type of lipids, are reduced in AD, causing alteration in the lamellar pattern35 and increased transepidermal water loss. Furthermore, the stratum corneum is thickened in AD, possibly in response to trauma, and hydration is reduced.36 Filaggrin (chromosome arm 1q21.3) is formed from the 400-kDa+ precursor profilaggrin through dephosphorylation and cleavage, and it performs an essential function in the skin barrier through its differential cleavage and breakdown as well as release of natural moisturizing factor and other compounds.37 Filaggrin mutations are linked to AD and ichthyosis vulgaris; however, barrier defects as evidenced by transepidermal water loss in the absence of filaggrin mutation are sufficient to allow for sensitization to allergens through the skin.29 Filaggrin mutations have been associated with AD development and vary in prevalence worldwide. In the United Kingdom, a prevalence study of filaggrin mutations in patients aged 7 to 9 years (N=792) demonstrated an 18.4% carrier rate in AD patients versus 12.9% in controls.34 A similar study in Sweden (N=3301) showed carrier rates of 13.5% versus 6.5%, respectively.38 Although filaggrin mutations are lower in black patients,39 ceramide content may be reduced in this population, demonstrating that a variety of skin barrier defects can result in AD. Carriers of filaggrin mutations are more likely to have eczema on skin exposed to environmental factors (eg, face, hands).40
Barrier Defects Contributing to AD
The breakdown of the stratum corneum allows for antigen presentation to Langerhans cells, the dendritic antigen-presenting cells of the skin. Breaks in the stratum corneum may occur from scratching. These macroscopic breaks are large, whereas the breaks that otherwise occur due to barrier breakdown may be more microscopic in nature. Scratching causes aggravation of the helper T cell (TH2) response.29 For example, it allows the dendritic ends of Langerhans cells to be exposed to antigens. The dendritic ends capture allergens through IgE (may be elevated in AD29), which is bound to the high-affinity FCER1 receptors on Langerhans cells. Rather than causing a type I hypersensitivity reaction, these Langerhans cells are activated and move to the lymph nodes where they present antigen and initiate a cascade of proinflammatory activity. This TH2 cascade includes release of cytokines such as IL-2, IL-4, IL-8, IL-10, tumor necrosis factor α, and IFN-γ.26,29
Transepidermal water loss and barrier dysfunction contribute to disease activity and facilitate food/environmental allergen sensitization by allowing increased penetration of allergens through the skin to be presented by Langerhans cells to TH1 cells (sensitization phase). The Langerhans cells can reach their dendritic ends through tight junctions and into the stratum corneum, allowing them to reach surface allergens when the barrier is impaired. Ultimate expansion to systemic allergy (effector phase) occurs when dendritic cells move to draining lymph nodes, causing antigen presentation to CD4 and/or CD8 cells. Langerhans cells and dendritic cell sensitization through the weakened skin is believed to be the basis or role of barrier disruption as a trigger of atopic diseases, including AD and food and environmental allergies.
Many different forms of barrier disruption can cause a TH2 response in AD. The TH2 response triggers a constellation of proinflammatory activities including release of IL-4, associated with eosinophilia and elevated IgE levels, the latter being minor criterion in the diagnosis of AD.15 One mechanism by which the TH2 response is elicited may be the release of molecules such as danger-associated molecule patterns that may elicit recruitment of other inflammatory cells. Helper T cell (TH2) activity also can worsen barrier defects through IL-4 and IL-13 release, which can reduce filaggrin expression,29,41 and can aggravate barrier dysfunction in AD.
Inflammatory activation in AD also may involve inflammatory dendritic epidermal cells (IDECs). The IDECs can be tolerogenic or immunogenic mature phenotypes. The IDECs activate helper T cells (TH1), which may contribute to long-term AD activity.
Conclusion
Atopic dermatitis is a common skin condition worldwide and is characterized by the hallmark of pruritus and features that include a typical pattern, history of atopy (personal or family), and usually xerosis and early disease onset. Barrier dysfunction and immune dysregulation are prominent in AD, both of which aggravate the other and may encourage increased development of allergies and other forms of atopy over time.
Atopic dermatitis (AD), or eczema, is the leading dermatologic diagnosis worldwide and is vexing to patients due to the itchiness of the rash. It is the leading cause of skin disease burden worldwide with a prevalence of 229,761,000 reported cases in 2010, presenting largely in preadolescence but also persisting through adulthood.1 Using the children’s life quality index, it has been demonstrated that AD has a greater impact on health-related quality of life than renal disease and cystic fibrosis.2 The overall burden of AD includes stress on the patient and his/her family as well as financial burdens that have been estimated to be similar to that of type 1 diabetes mellitus.3
Epidemiology of AD
The worldwide prevalence of AD varies by country and age group surveyed, with a higher prevalence in wealthy developed nations (eg, the United States) compared to poorer developing nations.4 Efforts to identify prevalence data for AD in the United States have been approached through a variety of strategies. A group in Oregon estimated the prevalence of AD in children aged 5 to 9 years to be 17.2% via a survey of parents (N=1465) and 11.8% with doctor-diagnosed eczema. In the same study, the question “Has a doctor ever said that your child has eczema?” was found to have a 91.3% predictive correlation.5 Analysis of the 2003 National Survey of Children’s Health demonstrated the overall US prevalence of pediatric AD to be 10.7% in 102,353 children 17 years or younger, with a range of 8.7% to 18.1% by region.6
In its evaluation of the worldwide prevalence of AD, the International Study of Asthma and Allergies in Childhood ranked the United States 17th.7,8 The prevalence of AD in developed countries such as the United States is fluid and is expected to increase if the trends from the last 20 years remain true. In an assessment of the National Health Interview Survey data from 1997 to 2011 based on responses to the question, “During the past 12 months, has your child had eczema or any kind of skin allergy?”, the Centers for Disease Control and Prevention identified an increase in the prevalence of AD in patients aged 0 to 17 years from 7.4% in 1997-1999 to 12.5% in 2009-2011.9 Rising prevalence seems to be paired with rising incidence in the total number of severe intractable cases, reduced clearance at the approach of grade school, or cases persisting into adulthood.
Racial Disparity in AD
Racial disparity worldwide and migration are thought to contribute to the prevalence of and therapeutic need for AD. For example, in the United Kingdom, the prevalence of AD in London-born Afro-Caribbean children versus white children (total cross-section, N=693 [junior school children]) was 16.3% and 8.7%, respectively.10 In the United States, black children were more likely to have AD than white children (odds ratio, 1.7).6 Asian and black children also were more likely to present to a physician for treatment of AD than white children.6,10-13
Definition and Diagnostic Considerations
According to Hanifin,14 “Eczema represents a family of inflammatory skin conditions characterized by pruritic, papulovesicular, sometimes weeping dermatitis. All demonstrate the histological hallmark of spongiosis, which helps to distinguish the eczemas from papulosquamous diseases such as psoriasis.”14 Atopic dermatitis is a variant of eczema; however, most laymen identify eczema and AD as being one and the same.
The Hanifin and Rajka15 criteria are the major diagnostic criteria for AD but are difficult to use in clinical practice. Three of the following 4 major criteria are needed for diagnosis: (1) pruritus, which is present universally; (2) typical morphology and distribution; (3) chronic or chronically relapsing dermatitis; and (4) personal and/or family history of atopy. Additionally, 3 of the following 23 minor criteria are needed for diagnosis: xerosis; ichthyosis vulgaris, palmar hyperlinearity, or keratosis pilaris; positive skin prick test; elevated serum IgE level; early age of onset; tendency toward cutaneous infections or impaired cell-mediated immunity; tendency toward nonspecific hand or foot dermatitis; nipple eczema; cheilitis; recurrent conjunctivitis; Dennie-Morgan fold (infraorbital fold); keratoconus; anterior subcapsular cataracts; orbital darkening; facial pallor or facial erythema; pityriasis alba; anterior neck folds; itching when sweating; intolerance to wool and lipid solvents; perifollicular accentuation; skin reactions from ingested foods or by food contact; environmental or emotional factors; and lesional/nonlesional white dermographism or delayed blanch.15-17
More pragmatic streamlined diagnostic criteria were established by Eichenfield et al.18 According to these guidelines, essential features for AD include pruritus and eczema. Important features seen in most cases and adding support to the diagnosis include early age of onset, atopy, and xerosis.18 In clinical practice, diagnosis is often made based on a pruritic relapsing condition in typical locations including the face, neck, and extensor surfaces in infants and children.
Age Considerations
Diagnosis of AD is made by 5 years of age in 85% to 90% of children who will develop the disease and by age 1 year in 60% to 65%.6,19,20 Atopic dermatitis will persist into adulthood in up to one-third of children.21,22 Infantile AD is characterized by erythematous, oozing, excoriated plaques on the cheeks (sparing the nose), scalp, trunk, and extensor surfaces. Pruritus is always seen in AD and can be a source of morbidity.16-18 Seborrheic dermatitis may complicate or overlap with AD in infancy.22
By 2 years of age, most children who are going to develop AD begin to show disease signs of childhood AD characterized by flexural lesions and lesions on the neck and in the postauricular area with sparing of the diaper area.23 Adult AD often presents as eczema of the hands and/or feet. Hand eczema in adulthood is correlated with a prior history of childhood hand eczema and/or childhood AD as well as wet work and caring for small children.24 Children with skin of color may manifest with follicular eczema as their primary disease phenotype. Facial and eyelid dermatitis are more common in Asian females, infants, and teenagers.12,25 Other disease phenotypes that are common in patients with skin of color include lichenoid AD and postinflammatory hypopigmentation.12
Pathogenesis of AD
There are 2 theories on the pathogenesis of AD known as the inside-out and outside-in hypotheses.26 The inside-out hypothesis suggests that allergic triggering leads to a weakened skin barrier that furthers allergen introduction and presentation, while the outside-in hypothesis suggests that the skin barrier is weakened in AD and allows for the presentation of allergens. Both theories have validity and biologic basis, and both may in fact be true in certain individuals.26
The Skin Barrier: An Overview
The skin barrier is a complex set of factors present and functional at birth that seal the keratinocytes and the interkeratinocyte space so that the skin can perform key processes and functions including retention of fluid, exclusion of allergens, protection from UV light and solvents, and prevention of pathogen entry (eg, infections).27-29 The superficial stratum corneum or the cornified envelope consists of keratinocytes with intercellular stripes of hydrophobic and hydrophilic substances formed by various intercellular lipids, largely ceramides, cholesterol, and free fatty acids.30,31 Keratinocytes are the first responders to a variety of environmental insults with the production of IL-18, RANTES (regulated on activation, normal T-expressed, and presumably secreted), granulocyte-macrophage colony-stimulating factor, and thymic stromal lymphopoietin. These inflammatory substances produce acute and chronic inflammation, mast cell reactivity, and T-cell activation.14 Corneodesmosins link the keratinocytes. Peptidases released will cleave the corneodesmosins and allow normal desquamation or shedding of surface skin, which is replaced by division of stem cells in the basal layer.29
The stratum granulosum is the layer beneath the stratum corneum that co-contributes to barrier activity. The stratum granulosum is absent or reduced histologically in ichthyosis vulgaris,32 a form of skin dryness linked to filaggrin mutations and AD. Filaggrin breakdown creates natural moisturizing factor, a series of hygroscopic compounds that attract water into the skin.33 Histidine, a filaggrin breakdown product, is used by urocanic acid to process UV light insults.34 Filaggrin also contributes to other barrier functions including pH and stratum corneum cohesion as well as paracellular permeability of the stratum corneum. Tight junctions in the stratum granulosum include claudin-1 and claudin-6 and provide another barrier feature.29
The skin barrier is composed of lipids and keratinocytes. Ceramides, which represent one type of lipids, are reduced in AD, causing alteration in the lamellar pattern35 and increased transepidermal water loss. Furthermore, the stratum corneum is thickened in AD, possibly in response to trauma, and hydration is reduced.36 Filaggrin (chromosome arm 1q21.3) is formed from the 400-kDa+ precursor profilaggrin through dephosphorylation and cleavage, and it performs an essential function in the skin barrier through its differential cleavage and breakdown as well as release of natural moisturizing factor and other compounds.37 Filaggrin mutations are linked to AD and ichthyosis vulgaris; however, barrier defects as evidenced by transepidermal water loss in the absence of filaggrin mutation are sufficient to allow for sensitization to allergens through the skin.29 Filaggrin mutations have been associated with AD development and vary in prevalence worldwide. In the United Kingdom, a prevalence study of filaggrin mutations in patients aged 7 to 9 years (N=792) demonstrated an 18.4% carrier rate in AD patients versus 12.9% in controls.34 A similar study in Sweden (N=3301) showed carrier rates of 13.5% versus 6.5%, respectively.38 Although filaggrin mutations are lower in black patients,39 ceramide content may be reduced in this population, demonstrating that a variety of skin barrier defects can result in AD. Carriers of filaggrin mutations are more likely to have eczema on skin exposed to environmental factors (eg, face, hands).40
Barrier Defects Contributing to AD
The breakdown of the stratum corneum allows for antigen presentation to Langerhans cells, the dendritic antigen-presenting cells of the skin. Breaks in the stratum corneum may occur from scratching. These macroscopic breaks are large, whereas the breaks that otherwise occur due to barrier breakdown may be more microscopic in nature. Scratching causes aggravation of the helper T cell (TH2) response.29 For example, it allows the dendritic ends of Langerhans cells to be exposed to antigens. The dendritic ends capture allergens through IgE (may be elevated in AD29), which is bound to the high-affinity FCER1 receptors on Langerhans cells. Rather than causing a type I hypersensitivity reaction, these Langerhans cells are activated and move to the lymph nodes where they present antigen and initiate a cascade of proinflammatory activity. This TH2 cascade includes release of cytokines such as IL-2, IL-4, IL-8, IL-10, tumor necrosis factor α, and IFN-γ.26,29
Transepidermal water loss and barrier dysfunction contribute to disease activity and facilitate food/environmental allergen sensitization by allowing increased penetration of allergens through the skin to be presented by Langerhans cells to TH1 cells (sensitization phase). The Langerhans cells can reach their dendritic ends through tight junctions and into the stratum corneum, allowing them to reach surface allergens when the barrier is impaired. Ultimate expansion to systemic allergy (effector phase) occurs when dendritic cells move to draining lymph nodes, causing antigen presentation to CD4 and/or CD8 cells. Langerhans cells and dendritic cell sensitization through the weakened skin is believed to be the basis or role of barrier disruption as a trigger of atopic diseases, including AD and food and environmental allergies.
Many different forms of barrier disruption can cause a TH2 response in AD. The TH2 response triggers a constellation of proinflammatory activities including release of IL-4, associated with eosinophilia and elevated IgE levels, the latter being minor criterion in the diagnosis of AD.15 One mechanism by which the TH2 response is elicited may be the release of molecules such as danger-associated molecule patterns that may elicit recruitment of other inflammatory cells. Helper T cell (TH2) activity also can worsen barrier defects through IL-4 and IL-13 release, which can reduce filaggrin expression,29,41 and can aggravate barrier dysfunction in AD.
Inflammatory activation in AD also may involve inflammatory dendritic epidermal cells (IDECs). The IDECs can be tolerogenic or immunogenic mature phenotypes. The IDECs activate helper T cells (TH1), which may contribute to long-term AD activity.
Conclusion
Atopic dermatitis is a common skin condition worldwide and is characterized by the hallmark of pruritus and features that include a typical pattern, history of atopy (personal or family), and usually xerosis and early disease onset. Barrier dysfunction and immune dysregulation are prominent in AD, both of which aggravate the other and may encourage increased development of allergies and other forms of atopy over time.
1. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
2. Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
3. Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
4. Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
5. Laughter D, Istvan JA, Tofte SJ, et al. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649-655.
6. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
7. Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-1258.
8. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225-1232.
9. Hansen TE, Evjenth B, Holt J. Increasing prevalence of asthma, allergic rhinoconjunctivitis and eczema among schoolchildren: three surveys during the period 1985-2008. Acta Paediatr. 2013;102:47-52.
10. Williams HC, Pembroke AC, Forsdyke H, et al. London-born black Caribbean children are at increased risk of atopic dermatitis. J Am Acad Dermatol. 1995;32:212-217.
11. Horii KA, Simon SD, Liu DY, et al. Atopic dermatitis in children in the United States, 1997-2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics. 2007;120:e527-e534.
12. Silverberg NB. Eczematous diseases. In: Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity. New York, NY: Springer; 2012:69-88.
13. Gupta J, Grube E, Ericksen MB, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725-730.
14. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
15. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980;92:44-47.
16. Queille-Roussel C, Raynaud F, Saurat JH. A prospective computerized study of 500 cases of atopic dermatitis in childhood. I. Initial analysis of 250 parameters. Acta Derm Venereol Suppl (Stockh). 1985;114:87-92.
17. Böhme M, Svensson A, Kull I, et al. Hanifin’s and Rajka’s minor criteria for atopic dermatitis: which do 2-year-olds exhibit? J Am Acad Dermatol. 2000;43:785-792.
18. Eichenfield LF, Hanifin JM, Luger TA, et al. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol. 2003;49:1088-1095.
19. Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
20. Perkin MR, Strachan DP, Williams HC, et al. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr Allergy Immunol. 2004;15:221-229.
21. Ellis CN, Mancini AJ, Paller AS, et al. Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg. 2012;31(suppl 2):S18-S22.
22. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
23. Meding B, Wrangsjö K, Järvholm B. Hand eczema extent and morphology—association and influence on long-term prognosis. J Invest Dermatol. 2007;127:2147-2151.
24. Mortz CG, Bindslev-Jensen C, Andersen KE. Hand eczema in The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS): prevalence, incidence and risk factors from adolescence to adulthood [published online August 7, 2014]. Br J Dermatol. 2014;171:313-323.
25. Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
26. Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
27. Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care [published online December 8, 2014]. Clin Dermatol. 2015;33:271-280.
28. Miyagaki T, Sugaya M. Recent advances in atopic dermatitis and psoriasis: genetic background, barrier function, and therapeutic targets. J Dermatol Sci. 2015;78:89-94.
29. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
30. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
31. Janssens M, van Smeden J, Gooris GS, et al. Lamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczema. J Invest Dermatol. 2011;131:2136-2138.
32. Fitch N, Segool R, Ferenczy A, et al. Dominant ichthyosis vulgaris with an ultrastructurally normal granular layer. Clin Genet. 1976;9:71-76.
33. Chandar P, Nole G, Johnson AW. Understanding natural moisturizing mechanisms: implications for moisturizer technology. Cutis. 2009;84(suppl 1):2-15.
34. Brown SJ, Relton CL, Liao H, et al. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol. 2008;121:940-946.
35. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
36. Nemoto-Hasebe I, Akiyama M, Nomura T, et al. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009;129:682-689.
37. Hoste E, Kemperman P, Devos M, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233-2241.
38. Ballardini N, Kull I, Söderhäll C, et al. Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol. 2013;168:588-594.
39. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
40. Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
41. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
1. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
2. Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
3. Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
4. Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
5. Laughter D, Istvan JA, Tofte SJ, et al. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649-655.
6. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
7. Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-1258.
8. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225-1232.
9. Hansen TE, Evjenth B, Holt J. Increasing prevalence of asthma, allergic rhinoconjunctivitis and eczema among schoolchildren: three surveys during the period 1985-2008. Acta Paediatr. 2013;102:47-52.
10. Williams HC, Pembroke AC, Forsdyke H, et al. London-born black Caribbean children are at increased risk of atopic dermatitis. J Am Acad Dermatol. 1995;32:212-217.
11. Horii KA, Simon SD, Liu DY, et al. Atopic dermatitis in children in the United States, 1997-2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics. 2007;120:e527-e534.
12. Silverberg NB. Eczematous diseases. In: Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity. New York, NY: Springer; 2012:69-88.
13. Gupta J, Grube E, Ericksen MB, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725-730.
14. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
15. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980;92:44-47.
16. Queille-Roussel C, Raynaud F, Saurat JH. A prospective computerized study of 500 cases of atopic dermatitis in childhood. I. Initial analysis of 250 parameters. Acta Derm Venereol Suppl (Stockh). 1985;114:87-92.
17. Böhme M, Svensson A, Kull I, et al. Hanifin’s and Rajka’s minor criteria for atopic dermatitis: which do 2-year-olds exhibit? J Am Acad Dermatol. 2000;43:785-792.
18. Eichenfield LF, Hanifin JM, Luger TA, et al. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol. 2003;49:1088-1095.
19. Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
20. Perkin MR, Strachan DP, Williams HC, et al. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr Allergy Immunol. 2004;15:221-229.
21. Ellis CN, Mancini AJ, Paller AS, et al. Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg. 2012;31(suppl 2):S18-S22.
22. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
23. Meding B, Wrangsjö K, Järvholm B. Hand eczema extent and morphology—association and influence on long-term prognosis. J Invest Dermatol. 2007;127:2147-2151.
24. Mortz CG, Bindslev-Jensen C, Andersen KE. Hand eczema in The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS): prevalence, incidence and risk factors from adolescence to adulthood [published online August 7, 2014]. Br J Dermatol. 2014;171:313-323.
25. Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
26. Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
27. Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care [published online December 8, 2014]. Clin Dermatol. 2015;33:271-280.
28. Miyagaki T, Sugaya M. Recent advances in atopic dermatitis and psoriasis: genetic background, barrier function, and therapeutic targets. J Dermatol Sci. 2015;78:89-94.
29. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
30. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
31. Janssens M, van Smeden J, Gooris GS, et al. Lamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczema. J Invest Dermatol. 2011;131:2136-2138.
32. Fitch N, Segool R, Ferenczy A, et al. Dominant ichthyosis vulgaris with an ultrastructurally normal granular layer. Clin Genet. 1976;9:71-76.
33. Chandar P, Nole G, Johnson AW. Understanding natural moisturizing mechanisms: implications for moisturizer technology. Cutis. 2009;84(suppl 1):2-15.
34. Brown SJ, Relton CL, Liao H, et al. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol. 2008;121:940-946.
35. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
36. Nemoto-Hasebe I, Akiyama M, Nomura T, et al. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009;129:682-689.
37. Hoste E, Kemperman P, Devos M, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233-2241.
38. Ballardini N, Kull I, Söderhäll C, et al. Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol. 2013;168:588-594.
39. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
40. Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
41. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
Practice Points
- The impact of atopic dermatitis (AD) on health-related quality of life mimics that of chronic childhood illnesses such as cystic fibrosis.
- The prevalence of pediatric AD in the United States is estimated at more than 10% of children, with a 1.7 increased odds ratio in black children.
- Diagnosis generally is made based on the presence of a pruritic eczematous eruption with typical morphology and a personal and/or family history of atopy.
- Atopic dermatitis is caused by a complex interplay of skin barrier dysfunction and immune tendency toward allergy development.
Diet and Atopic Dermatitis
Atopic dermatitis (AD) is the leading diagnosis among pediatric dermatologists,1 and this condition is commonly seen worldwide by dermatologists and allergists.2 There is a widespread misconception held by many patients and their guardians who believe that AD is caused by a food allergy.3 Although AD is related to and part of the atopic complex of disorders associated with food allergies, the role of diet in AD is not well defined. Previously it was recommended to delay early exposure to foods, but now it is recommended to do the opposite in certain situations. In fact, delaying exposure to certain types of foods can increase the likelihood of food allergies (eg, early exposure to peanut butter lowers the statistical chance of developing peanut allergies). This article reviews recent data on the role of diet in AD regarding disease activity as well as new and emerging data on dietary modifications for prevention and intervention. Emerging data on the relationship between AD and food allergies also are presented.
Pathogenesis of AD
The skin barrier plays a vital role in the prevention of pathogens, allergen exposure, and sensitization. There is no solitary root cause of AD, rather it is a combination of inflammation and barrier dysfunction associated with allergic diathesis (eg, atopy). Many patients with AD, especially those with persistent disease, have an intrinsic barrier dysfunction as part of the root cause of their illness, which may be caused by genetically mediated filaggrin defects or alternative barrier dysfunction such as decreased ceramide content that predisposes to percutaneous and mucosal sensitization.4,5 Another source of percutaneous exposure to allergens is macroscopic breaks in the skin caused by scratching, which allows dendritic termini of Langerhans cells to be exposed to percutaneous antigens4,6 through binding to high-affinity IgE receptors.
Langerhans cells exposed to allergens can trigger either an immediate or delayed-type (type I or type II) reaction (sensitization phase) in the lymph node causing inflammatory activation (elicitation). Inflammatory activity in AD is broad and complex and includes the release of IL-4, elevated IgE levels, and eosinophilia, which trigger the helper T cell TH2 and TH17 cascade of cytokines, including IL-2, IL-4, IL-5, IL-8, IL-10, IL-13, IL-17α, tumor necrosis factor α, and IFN-γ,7-9 with the latter worsening barrier defect via downregulation of intercellular substances (eg, filaggrin) and intercellular adhesion expression (eg, claudin 1).6,7,10
Atopic dermatitis does not exist in isolation. The barrier dysfunction associated with AD allows for sensitization to allergens, including those found in food and/or the environment. The atopic march, which occurs via barrier abnormalities facilitating sensitization, can result in further atopy, such as food allergies, environmental allergies, asthma, and eosinophilic esophagitis.11
|
AD and Food Allergies
Many patients and guardians believe AD is caused by a food allergy and that diet restrictions will resolve the disease. Although the latter is not true, in reality many patients with AD do have food allergies. Approximately 40% of infants and young children with moderate to severe AD and 8% of the general population of children will manifest a specific IgE-based food allergy. Food-specific IgE can be triggered or exacerbated by AD through the induction of hives, cutaneous activation of mast cells, increased “spontaneous” basophil histamine release, and food-related lymphocyte-proliferative responses measurable by food patch testing.12 Allergists generally recommend avoidance of or use of heavily denatured food (in the case of a milk/egg allergy) in the setting of documented IgE-mediated allergens.13 Food allergies in AD can manifest with flares, hives, pruritus, and/or other cutaneous symptoms in the absence of flaring AD disease.
Guidelines from the American Academy of Dermatology (AAD)(Table) for the management of AD have recently recommended testing for food allergies in children younger than 5 years who have intractable AD or known food-induced reactions.14 This technique will largely identify children at risk for anaphylaxis but may not yield information contributing to AD improvement. Furthermore, withdrawal of allergens with known IgE-mediated response was classified by the AAD as having consistent good-quality patient-oriented evidence, and asking about allergic reactions as well as acting on a reported allergic history had inconsistent or limited-quality patient-oriented evidence. It is believed that atopy can progress, or march, into a food and/or environmental allergy at any point in life; therefore, testing for a food allergy should be considered in all patients with recent onset of severe and/or persistent AD and/or food-aggravated AD due to a lifetime risk of sensitization.14,15 A food introduction plan may require collaboration with an allergist, especially in high-risk patients (eg, those with known food reactions, family history of food allergies, severe atopy).
Prevention of AD Through Dietary Modification
The National Institute of Allergy and Infectious Diseases consensus group published guidelines on food allergies that affect AD management, including avoidance of proven allergens but not random elimination of food allergens in AD; the group identifies AD and family history of AD as risk factors for food allergies.16 The best data in support of avoidance of documented food allergens to reduce AD severity has been found for egg white allergy and avoidance. Active egg allergy also is linked to staphylococcal superantigen IgE sensitizations,17 but the reason for the link is not yet clear. For the pediatric population, exclusive breastfeeding until 4 to 6 months of age and introduction of solids within the first 4 to 6 months as well as avoidance of maternal dietary restriction during pregnancy and lactation was further endorsed, with use of hydrolyzed formulas as an alternative to exclusive breastfeeding in infants who are not exclusively breastfed (cost permitting).16,18
A Cochrane review of maternal dietary restrictions during pregnancy found no benefit of maternal prenatal dietary restriction on AD prevalence in the first 18 months of life but did note an association with lower mean gestational weight.19
There is currently an effort to produce foods, such as soybeans and corn, that are genetically modified to reduce exposure to the allergenic component, but it is possible that when large-scale challenges occur, these foods also will be allergenic.20,21 In the case of a modified apple, some promising reduction in allergy symptoms has been reported.22 Although genetically modified foods may benefit children with food allergies in the future, they are a source of some controversy.
Complementary and Alternative Medicine
The AAD guidelines do not recommend complementary and alternative medicine (CAM) to treat AD,14 but it remains a commonly used therapy in the United States. A 2014 analysis of data from the 2007 US-based national health interview survey of 9417 children (age range, 0–17 years) demonstrated that 46.9% of children used 1 or more CAM, of which 0.99% used CAM specifically for AD. In this study, herbal therapy, vitamins, homeopathy, diet, and movement techniques were associated with increased prevalence of AD.23 Although some herbals have been shown to be beneficial in AD,24 hepatotoxicity has been reported with some herbal therapies.25 Complementary techniques with evidence-based support include massage therapy,26 relocation to an alternative climate, acupuncture that rivals cetirizine in efficacy, and supportive nutritional advice.24,27
Factors Affecting the Incidence of AD
Atopic dermatitis is of greater prevalence in children in developed wealthy nations such as the United States, supporting the role of enhanced hygiene and overall good health through vaccination as a possible contributor to the rise in AD prevalence in the last 4 decades.28,29 Alternatively, viruses such as respiratory syncytial virus may trigger AD, suggesting vaccination against the virus may reduce the risk for AD.30 Overall, vaccination improves life expectancy and should be conducted on schedule without reservation. Other aspects of hygiene that could conceptually affect prevalence of AD are raw food ingestion and the effects of foodborne microbes on the intestinal microbiome in relationship to AD development. Probiotics have been tested for this purpose.
Probiotics and prebiotics have been theorized to work through a reduction in inflammation; these agents have some evidence in their favor, but they were not endorsed in the AAD guidelines14 despite showing promise in meta-analysis. In particular prenatal and postnatal (maternal and child) supplementation of Lactobacillus rhamnosus shows promise.31-33 Food elimination diets and supplements including vitamin D, selenium, fish oil, borage oil, and zinc were not found to be beneficial and were not recommended in the AAD guidelines.14,34
Percutaneous exposure to peanuts, possibly in household dust, may be the mechanism of peanut sensitization in AD27 via an inherent adjuvant effect of peanut protein.28 The recent LEAP (Learning Early About Peanut Allergy) trial randomized 530 infants aged 4 to 11 months to peanut-avoidant versus peanut-exposed diets for 60 months. The results showed statistically reduced (approximately one-twelfth of the risk) peanut allergy even in infants known to be sensitized (approximately one-third of the risk).35 It is now recommended in countries with a high prevalence of peanut allergies to introduce peanuts to an infant’s diet between 4 and 11 months of age (evidence level 1 [highest level of evidence]), with referral to an allergist for introduction in known sensitization cases and severe AD.36 In the setting of known or documented peanut allergy and for evaluation of potential food allergies, an allergist should be consulted.
Other interventions have been described as promising in mouse models. Those supplements include Lithospermum erythrorhizon,37Platycodon grandiflorus,38Hypsizygus marmoreous,39 fortified ginseng extract,40 polyunsaturated fatty acids,41 and galactooligosaccharide.42 Prebiotic oligosaccharides also are promising for early prevention of AD symptoms in infants, but otherwise these agents have remained largely untested in AD.43 None of these therapies have been endorsed by the AAD, and the long-term safety and efficacy in humans remains to be proven.
Risks of Dietary Restriction
Dietary restrictions in treating AD can have negative consequences, including reduced birth weight when initiated in pregnancy,19 osteomalacia from vitamin D deficiency,44 and nutritional deficiencies (eg, calcium, phosphorus, iron, vitamin K, vitamin D, zinc, vitamin A, B1, B2, B6, niacin, cholesterol, and/or vitamin C deficiencies).45 Excess dietary intake of vegetables in individuals with extensive food allergies can result in carotenemia.46 Protein-restricted diets from use of rice milk or dietary protein restriction can result in kwashiorkorlike protein malnutrition and marasmus.47-49 Nutritional counseling and/or supplementation is recommended for patients with food-restricted diets.
Avoiding Fragrance in Food
Food intolerance often is reported by AD patients. In allergies, food intolerance refers to side effects such as gastrointestinal symptoms; in dermatology, food intolerance can include itching, systemic flares of allergic contact dermatitis (eg, fragrance allergy), or true IgE-mediated allergies such as oral allergy syndrome. Oral allergy syndrome (pollen-food allergy syndrome) is an epitope-spread phenomenon related to an allergy to tree pollen, causing broad allergy to specific groups of fruits and nuts.50 Food triggers in AD include kiwi, milk, apple, tomato, citrus fruits, tree nuts, and peanuts. Oral allergy syndrome is common in food-sensitive AD patients (51.2%) followed by gastrointestinal symptoms (23.5%) and worsening AD (11.4%).51 Sensitization to fragrance can cross-react with foods (eg, balsam of Peru and tomatoes).52 A tomato allergy can be detected either by a skin-prick test or a food patch test in this setting.53 An allergist should be consulted if oral allergy syndrome is suspected.
Conclusion
Food allergies are more common in AD patients and patients should be referred to an allergist for evaluation and management. Strict dietary practice is not recommended, while avoiding proven food allergens in AD could be beneficial. Dermatologists should be aware that patients with dietary restrictions may lack key nutrients, manifesting with nutritional deficiencies in the skin; therefore, nutrition counseling may be needed in the most severe AD/allergy patients. This field is evolving; therefore, ongoing study and evaluation of interventions as they relate to AD will be needed to assess best practices for diet in AD over time.
1. Schachner L, Ling NS, Press S. A statistical analysis of a pediatric dermatology clinic. Pediatr Dermatol. 1983;1:157-164.
2. Kiprono SK, Muchunu JW, Masenga JE. Skin diseases in pediatric patients attending a tertiary dermatology hospital in Northern Tanzania: a cross-sectional study. BMC Dermatol. 2015;15:16.
3. Wensink M, Timmer C, Brand PL. Atopic dermatitis in infants not caused by food allergy [in Dutch]. Ned Tijdschr Geneeskd. 2008;152:4-9.
4. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132(3, pt 2):949-963.
5. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
6. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
7. Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
8. Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
9. Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
10. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
11. Cianferoni A, Spergel J. Eosinophilic esophagitis: a comprehensive review [published online July 22, 2015]. Clin Rev Allergy Immunol. doi:10.1111/all.12846.
12. Sicherer SH, Sampson HA. Food hypersensitivity and atopic dermatitis; pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 1999;104(3, pt 2):S114-S122.
13. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.
14. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218-1233.
15. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the epidermal differentiation complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
16. Burks AW, Jones SM, Boyce JA, et al. NIAID-sponsored 2010 guidelines for managing food allergy: applications in the pediatric population. Pediatrics. 2011;128:955-965.
17. Ong PY. Association between egg and staphylococcal superantigen IgE sensitizations in atopic dermatitis. Allergy Asthma Proc. 2014;35:346-348.
18. Botteman M, Detzel P. Cost-effectiveness of partially hydrolyzed whey protein formula in the primary prevention of atopic dermatitis in high-risk urban infants in Southeast Asia. Ann Nutr Metab. 2015;66(suppl 1):26-32.
19. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;9:CD000133.
20. Yum HY, Lee SY, Lee KE, et al. Genetically modified and wild soybeans: an immunologic comparison. Allergy Asthma Proc. 2005;26:210-216.
21. Mathur C, Kathuria PC, Dahiya P, et al. Lack of detectable allergenicity in genetically modified maize containing “Cry” proteins as compared to native maize based on in silico & in vitro analysis. PLoS One. 2015;10:e0117340.
22. Dubois AE, Pagliarani G, Brouwer RM, et al. First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1-silenced apples cause fewer allergy symptoms than the wild-type cultivar [published online July 24, 2015]. Allergy. 2015;70:1406-1412.
23. Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25:246-254.
24. Pfab F, Schalock PC, Napadow V, et al. Complementary integrative approach for treating pruritus. Dermatol Ther. 2013;26:149-156.
25. Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol. 2015;89:851-865.
26. Schachner L, Field T, Hernandez-Reif M, et al. Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr Dermatol. 1998;15:390-395.
27. Pfab F, Schalock PC, Napadow V, et al. Acupuncture for allergic disease therapy–the current state of evidence. Expert Rev Clin Immunol. 2014;10:831-841.
28. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
29. Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
30. Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
31. Foolad N, Brezinski EA, Chase EP, et al. Effect of nutrient supplementation on atopic dermatitis in children: a systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013;149:350-355.
32. Kalliomäki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076-1079.
33. Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184-191.
34. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I: atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
35. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
36. Fleischer DM, Sicherer S, Greenhawt M, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants [published online October 2015]. Allergy. 2015;70:1193-1195.
37. Kim J, Cho Y. Gromwell (Lithospermum erythrorhizon) supplementation enhances epidermal levels of cera-mides, glucosylceramides, β-glucocerebrosidase, and acidicsphingomyelinase in NC/Nga mice. J Med Food. 2013;16:927-933.
38. Choi JH, Jin SW, Han EH, et al. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-κB and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine. 2014;21:1053-1061.
39. Kim T, Park K, Jung HS, et al. Evaluation of anti-atopic dermatitis activity of Hypsizigus marmoreus extract. Phytother Res. 2014;28:1539-1546.
40. Kim JR, Choi J, Kim J, et al. 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol-fortified ginseng extract attenuates the development of atopic dermatitis-like symptoms in NC/Nga mice. J Ethnopharmacol. 2014;151:365-371.
41. Weise C, Ernst D, van Tol EA, et al. Dietary polyunsaturated fatty acids and non-digestible oligosaccharides reduce dermatitis in mice. Pediatr Allergy Immunol. 2013;24:361-367.
42. Tanabe S, Hochi S. Oral administration of a galactooligosaccharide preparation inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. Int J Mol Med. 2010;25:331-336.
43. Arslanoglu S, Moro GE, Boehm G, et al. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J Biol Regul Homeost Agents. 2012;26(3 suppl):49-59.
44. Shikino K, Ikusaka M, Yamashita T. Vitamin D-deficient osteomalacia due to excessive self-restrictions for atopic dermatitis [published online July 4, 2014] . BMJ Case Rep.
45. Kim J, Kwon J, Noh G, et al. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract. 2013;7:488-494.
46. Silverberg NB, Lee-Wong M. Generalized yellow discoloration of the skin. Cutis. 2014;93:E11-E12.
47. Hon KL, Nip SY, Cheung KL. A tragic case of atopic eczema: malnutrition and infections despite multivitamins and supplements. Iran J Allergy Asthma Immunol. 2012;11:267-270.
48. Diamanti A, Pedicelli S, D’Argenio P, et al. Iatrogenic kwashiorkor in three infants on a diet of rice beverages. Pediatr Allergy Immunol. 2011;22:878-879.
49. Pillai K, Acharya S. Iatrogenic kwashiorkar. Indian Pediatr. 2010;47:540-541.
50. Price A, Ramachandran S, Smith GP, et al. Oral allergy syndrome (pollen-food allergy syndrome). Dermatitis. 2015;26:78-88.
51. Mattila L, Kilpeläinen M, Terho EO, et al. Food hypersensitivity among Finnish university students: association with atopic diseases. Clin Exp Allergy. 2003;33:600-606.
52. Paulsen E, Christensen LP, Andersen KE. Tomato contact dermatitis. Contact Dermatitis. 2012;67:321-327.
53. Di Leo E, Nettis E, Cardinale F, et al. Tomato atopy patch test in adult atopic dermatitis: diagnostic value and comparison among different methods. Allergy. 2009;64:659-663.
Atopic dermatitis (AD) is the leading diagnosis among pediatric dermatologists,1 and this condition is commonly seen worldwide by dermatologists and allergists.2 There is a widespread misconception held by many patients and their guardians who believe that AD is caused by a food allergy.3 Although AD is related to and part of the atopic complex of disorders associated with food allergies, the role of diet in AD is not well defined. Previously it was recommended to delay early exposure to foods, but now it is recommended to do the opposite in certain situations. In fact, delaying exposure to certain types of foods can increase the likelihood of food allergies (eg, early exposure to peanut butter lowers the statistical chance of developing peanut allergies). This article reviews recent data on the role of diet in AD regarding disease activity as well as new and emerging data on dietary modifications for prevention and intervention. Emerging data on the relationship between AD and food allergies also are presented.
Pathogenesis of AD
The skin barrier plays a vital role in the prevention of pathogens, allergen exposure, and sensitization. There is no solitary root cause of AD, rather it is a combination of inflammation and barrier dysfunction associated with allergic diathesis (eg, atopy). Many patients with AD, especially those with persistent disease, have an intrinsic barrier dysfunction as part of the root cause of their illness, which may be caused by genetically mediated filaggrin defects or alternative barrier dysfunction such as decreased ceramide content that predisposes to percutaneous and mucosal sensitization.4,5 Another source of percutaneous exposure to allergens is macroscopic breaks in the skin caused by scratching, which allows dendritic termini of Langerhans cells to be exposed to percutaneous antigens4,6 through binding to high-affinity IgE receptors.
Langerhans cells exposed to allergens can trigger either an immediate or delayed-type (type I or type II) reaction (sensitization phase) in the lymph node causing inflammatory activation (elicitation). Inflammatory activity in AD is broad and complex and includes the release of IL-4, elevated IgE levels, and eosinophilia, which trigger the helper T cell TH2 and TH17 cascade of cytokines, including IL-2, IL-4, IL-5, IL-8, IL-10, IL-13, IL-17α, tumor necrosis factor α, and IFN-γ,7-9 with the latter worsening barrier defect via downregulation of intercellular substances (eg, filaggrin) and intercellular adhesion expression (eg, claudin 1).6,7,10
Atopic dermatitis does not exist in isolation. The barrier dysfunction associated with AD allows for sensitization to allergens, including those found in food and/or the environment. The atopic march, which occurs via barrier abnormalities facilitating sensitization, can result in further atopy, such as food allergies, environmental allergies, asthma, and eosinophilic esophagitis.11
|
AD and Food Allergies
Many patients and guardians believe AD is caused by a food allergy and that diet restrictions will resolve the disease. Although the latter is not true, in reality many patients with AD do have food allergies. Approximately 40% of infants and young children with moderate to severe AD and 8% of the general population of children will manifest a specific IgE-based food allergy. Food-specific IgE can be triggered or exacerbated by AD through the induction of hives, cutaneous activation of mast cells, increased “spontaneous” basophil histamine release, and food-related lymphocyte-proliferative responses measurable by food patch testing.12 Allergists generally recommend avoidance of or use of heavily denatured food (in the case of a milk/egg allergy) in the setting of documented IgE-mediated allergens.13 Food allergies in AD can manifest with flares, hives, pruritus, and/or other cutaneous symptoms in the absence of flaring AD disease.
Guidelines from the American Academy of Dermatology (AAD)(Table) for the management of AD have recently recommended testing for food allergies in children younger than 5 years who have intractable AD or known food-induced reactions.14 This technique will largely identify children at risk for anaphylaxis but may not yield information contributing to AD improvement. Furthermore, withdrawal of allergens with known IgE-mediated response was classified by the AAD as having consistent good-quality patient-oriented evidence, and asking about allergic reactions as well as acting on a reported allergic history had inconsistent or limited-quality patient-oriented evidence. It is believed that atopy can progress, or march, into a food and/or environmental allergy at any point in life; therefore, testing for a food allergy should be considered in all patients with recent onset of severe and/or persistent AD and/or food-aggravated AD due to a lifetime risk of sensitization.14,15 A food introduction plan may require collaboration with an allergist, especially in high-risk patients (eg, those with known food reactions, family history of food allergies, severe atopy).
Prevention of AD Through Dietary Modification
The National Institute of Allergy and Infectious Diseases consensus group published guidelines on food allergies that affect AD management, including avoidance of proven allergens but not random elimination of food allergens in AD; the group identifies AD and family history of AD as risk factors for food allergies.16 The best data in support of avoidance of documented food allergens to reduce AD severity has been found for egg white allergy and avoidance. Active egg allergy also is linked to staphylococcal superantigen IgE sensitizations,17 but the reason for the link is not yet clear. For the pediatric population, exclusive breastfeeding until 4 to 6 months of age and introduction of solids within the first 4 to 6 months as well as avoidance of maternal dietary restriction during pregnancy and lactation was further endorsed, with use of hydrolyzed formulas as an alternative to exclusive breastfeeding in infants who are not exclusively breastfed (cost permitting).16,18
A Cochrane review of maternal dietary restrictions during pregnancy found no benefit of maternal prenatal dietary restriction on AD prevalence in the first 18 months of life but did note an association with lower mean gestational weight.19
There is currently an effort to produce foods, such as soybeans and corn, that are genetically modified to reduce exposure to the allergenic component, but it is possible that when large-scale challenges occur, these foods also will be allergenic.20,21 In the case of a modified apple, some promising reduction in allergy symptoms has been reported.22 Although genetically modified foods may benefit children with food allergies in the future, they are a source of some controversy.
Complementary and Alternative Medicine
The AAD guidelines do not recommend complementary and alternative medicine (CAM) to treat AD,14 but it remains a commonly used therapy in the United States. A 2014 analysis of data from the 2007 US-based national health interview survey of 9417 children (age range, 0–17 years) demonstrated that 46.9% of children used 1 or more CAM, of which 0.99% used CAM specifically for AD. In this study, herbal therapy, vitamins, homeopathy, diet, and movement techniques were associated with increased prevalence of AD.23 Although some herbals have been shown to be beneficial in AD,24 hepatotoxicity has been reported with some herbal therapies.25 Complementary techniques with evidence-based support include massage therapy,26 relocation to an alternative climate, acupuncture that rivals cetirizine in efficacy, and supportive nutritional advice.24,27
Factors Affecting the Incidence of AD
Atopic dermatitis is of greater prevalence in children in developed wealthy nations such as the United States, supporting the role of enhanced hygiene and overall good health through vaccination as a possible contributor to the rise in AD prevalence in the last 4 decades.28,29 Alternatively, viruses such as respiratory syncytial virus may trigger AD, suggesting vaccination against the virus may reduce the risk for AD.30 Overall, vaccination improves life expectancy and should be conducted on schedule without reservation. Other aspects of hygiene that could conceptually affect prevalence of AD are raw food ingestion and the effects of foodborne microbes on the intestinal microbiome in relationship to AD development. Probiotics have been tested for this purpose.
Probiotics and prebiotics have been theorized to work through a reduction in inflammation; these agents have some evidence in their favor, but they were not endorsed in the AAD guidelines14 despite showing promise in meta-analysis. In particular prenatal and postnatal (maternal and child) supplementation of Lactobacillus rhamnosus shows promise.31-33 Food elimination diets and supplements including vitamin D, selenium, fish oil, borage oil, and zinc were not found to be beneficial and were not recommended in the AAD guidelines.14,34
Percutaneous exposure to peanuts, possibly in household dust, may be the mechanism of peanut sensitization in AD27 via an inherent adjuvant effect of peanut protein.28 The recent LEAP (Learning Early About Peanut Allergy) trial randomized 530 infants aged 4 to 11 months to peanut-avoidant versus peanut-exposed diets for 60 months. The results showed statistically reduced (approximately one-twelfth of the risk) peanut allergy even in infants known to be sensitized (approximately one-third of the risk).35 It is now recommended in countries with a high prevalence of peanut allergies to introduce peanuts to an infant’s diet between 4 and 11 months of age (evidence level 1 [highest level of evidence]), with referral to an allergist for introduction in known sensitization cases and severe AD.36 In the setting of known or documented peanut allergy and for evaluation of potential food allergies, an allergist should be consulted.
Other interventions have been described as promising in mouse models. Those supplements include Lithospermum erythrorhizon,37Platycodon grandiflorus,38Hypsizygus marmoreous,39 fortified ginseng extract,40 polyunsaturated fatty acids,41 and galactooligosaccharide.42 Prebiotic oligosaccharides also are promising for early prevention of AD symptoms in infants, but otherwise these agents have remained largely untested in AD.43 None of these therapies have been endorsed by the AAD, and the long-term safety and efficacy in humans remains to be proven.
Risks of Dietary Restriction
Dietary restrictions in treating AD can have negative consequences, including reduced birth weight when initiated in pregnancy,19 osteomalacia from vitamin D deficiency,44 and nutritional deficiencies (eg, calcium, phosphorus, iron, vitamin K, vitamin D, zinc, vitamin A, B1, B2, B6, niacin, cholesterol, and/or vitamin C deficiencies).45 Excess dietary intake of vegetables in individuals with extensive food allergies can result in carotenemia.46 Protein-restricted diets from use of rice milk or dietary protein restriction can result in kwashiorkorlike protein malnutrition and marasmus.47-49 Nutritional counseling and/or supplementation is recommended for patients with food-restricted diets.
Avoiding Fragrance in Food
Food intolerance often is reported by AD patients. In allergies, food intolerance refers to side effects such as gastrointestinal symptoms; in dermatology, food intolerance can include itching, systemic flares of allergic contact dermatitis (eg, fragrance allergy), or true IgE-mediated allergies such as oral allergy syndrome. Oral allergy syndrome (pollen-food allergy syndrome) is an epitope-spread phenomenon related to an allergy to tree pollen, causing broad allergy to specific groups of fruits and nuts.50 Food triggers in AD include kiwi, milk, apple, tomato, citrus fruits, tree nuts, and peanuts. Oral allergy syndrome is common in food-sensitive AD patients (51.2%) followed by gastrointestinal symptoms (23.5%) and worsening AD (11.4%).51 Sensitization to fragrance can cross-react with foods (eg, balsam of Peru and tomatoes).52 A tomato allergy can be detected either by a skin-prick test or a food patch test in this setting.53 An allergist should be consulted if oral allergy syndrome is suspected.
Conclusion
Food allergies are more common in AD patients and patients should be referred to an allergist for evaluation and management. Strict dietary practice is not recommended, while avoiding proven food allergens in AD could be beneficial. Dermatologists should be aware that patients with dietary restrictions may lack key nutrients, manifesting with nutritional deficiencies in the skin; therefore, nutrition counseling may be needed in the most severe AD/allergy patients. This field is evolving; therefore, ongoing study and evaluation of interventions as they relate to AD will be needed to assess best practices for diet in AD over time.
Atopic dermatitis (AD) is the leading diagnosis among pediatric dermatologists,1 and this condition is commonly seen worldwide by dermatologists and allergists.2 There is a widespread misconception held by many patients and their guardians who believe that AD is caused by a food allergy.3 Although AD is related to and part of the atopic complex of disorders associated with food allergies, the role of diet in AD is not well defined. Previously it was recommended to delay early exposure to foods, but now it is recommended to do the opposite in certain situations. In fact, delaying exposure to certain types of foods can increase the likelihood of food allergies (eg, early exposure to peanut butter lowers the statistical chance of developing peanut allergies). This article reviews recent data on the role of diet in AD regarding disease activity as well as new and emerging data on dietary modifications for prevention and intervention. Emerging data on the relationship between AD and food allergies also are presented.
Pathogenesis of AD
The skin barrier plays a vital role in the prevention of pathogens, allergen exposure, and sensitization. There is no solitary root cause of AD, rather it is a combination of inflammation and barrier dysfunction associated with allergic diathesis (eg, atopy). Many patients with AD, especially those with persistent disease, have an intrinsic barrier dysfunction as part of the root cause of their illness, which may be caused by genetically mediated filaggrin defects or alternative barrier dysfunction such as decreased ceramide content that predisposes to percutaneous and mucosal sensitization.4,5 Another source of percutaneous exposure to allergens is macroscopic breaks in the skin caused by scratching, which allows dendritic termini of Langerhans cells to be exposed to percutaneous antigens4,6 through binding to high-affinity IgE receptors.
Langerhans cells exposed to allergens can trigger either an immediate or delayed-type (type I or type II) reaction (sensitization phase) in the lymph node causing inflammatory activation (elicitation). Inflammatory activity in AD is broad and complex and includes the release of IL-4, elevated IgE levels, and eosinophilia, which trigger the helper T cell TH2 and TH17 cascade of cytokines, including IL-2, IL-4, IL-5, IL-8, IL-10, IL-13, IL-17α, tumor necrosis factor α, and IFN-γ,7-9 with the latter worsening barrier defect via downregulation of intercellular substances (eg, filaggrin) and intercellular adhesion expression (eg, claudin 1).6,7,10
Atopic dermatitis does not exist in isolation. The barrier dysfunction associated with AD allows for sensitization to allergens, including those found in food and/or the environment. The atopic march, which occurs via barrier abnormalities facilitating sensitization, can result in further atopy, such as food allergies, environmental allergies, asthma, and eosinophilic esophagitis.11
|
AD and Food Allergies
Many patients and guardians believe AD is caused by a food allergy and that diet restrictions will resolve the disease. Although the latter is not true, in reality many patients with AD do have food allergies. Approximately 40% of infants and young children with moderate to severe AD and 8% of the general population of children will manifest a specific IgE-based food allergy. Food-specific IgE can be triggered or exacerbated by AD through the induction of hives, cutaneous activation of mast cells, increased “spontaneous” basophil histamine release, and food-related lymphocyte-proliferative responses measurable by food patch testing.12 Allergists generally recommend avoidance of or use of heavily denatured food (in the case of a milk/egg allergy) in the setting of documented IgE-mediated allergens.13 Food allergies in AD can manifest with flares, hives, pruritus, and/or other cutaneous symptoms in the absence of flaring AD disease.
Guidelines from the American Academy of Dermatology (AAD)(Table) for the management of AD have recently recommended testing for food allergies in children younger than 5 years who have intractable AD or known food-induced reactions.14 This technique will largely identify children at risk for anaphylaxis but may not yield information contributing to AD improvement. Furthermore, withdrawal of allergens with known IgE-mediated response was classified by the AAD as having consistent good-quality patient-oriented evidence, and asking about allergic reactions as well as acting on a reported allergic history had inconsistent or limited-quality patient-oriented evidence. It is believed that atopy can progress, or march, into a food and/or environmental allergy at any point in life; therefore, testing for a food allergy should be considered in all patients with recent onset of severe and/or persistent AD and/or food-aggravated AD due to a lifetime risk of sensitization.14,15 A food introduction plan may require collaboration with an allergist, especially in high-risk patients (eg, those with known food reactions, family history of food allergies, severe atopy).
Prevention of AD Through Dietary Modification
The National Institute of Allergy and Infectious Diseases consensus group published guidelines on food allergies that affect AD management, including avoidance of proven allergens but not random elimination of food allergens in AD; the group identifies AD and family history of AD as risk factors for food allergies.16 The best data in support of avoidance of documented food allergens to reduce AD severity has been found for egg white allergy and avoidance. Active egg allergy also is linked to staphylococcal superantigen IgE sensitizations,17 but the reason for the link is not yet clear. For the pediatric population, exclusive breastfeeding until 4 to 6 months of age and introduction of solids within the first 4 to 6 months as well as avoidance of maternal dietary restriction during pregnancy and lactation was further endorsed, with use of hydrolyzed formulas as an alternative to exclusive breastfeeding in infants who are not exclusively breastfed (cost permitting).16,18
A Cochrane review of maternal dietary restrictions during pregnancy found no benefit of maternal prenatal dietary restriction on AD prevalence in the first 18 months of life but did note an association with lower mean gestational weight.19
There is currently an effort to produce foods, such as soybeans and corn, that are genetically modified to reduce exposure to the allergenic component, but it is possible that when large-scale challenges occur, these foods also will be allergenic.20,21 In the case of a modified apple, some promising reduction in allergy symptoms has been reported.22 Although genetically modified foods may benefit children with food allergies in the future, they are a source of some controversy.
Complementary and Alternative Medicine
The AAD guidelines do not recommend complementary and alternative medicine (CAM) to treat AD,14 but it remains a commonly used therapy in the United States. A 2014 analysis of data from the 2007 US-based national health interview survey of 9417 children (age range, 0–17 years) demonstrated that 46.9% of children used 1 or more CAM, of which 0.99% used CAM specifically for AD. In this study, herbal therapy, vitamins, homeopathy, diet, and movement techniques were associated with increased prevalence of AD.23 Although some herbals have been shown to be beneficial in AD,24 hepatotoxicity has been reported with some herbal therapies.25 Complementary techniques with evidence-based support include massage therapy,26 relocation to an alternative climate, acupuncture that rivals cetirizine in efficacy, and supportive nutritional advice.24,27
Factors Affecting the Incidence of AD
Atopic dermatitis is of greater prevalence in children in developed wealthy nations such as the United States, supporting the role of enhanced hygiene and overall good health through vaccination as a possible contributor to the rise in AD prevalence in the last 4 decades.28,29 Alternatively, viruses such as respiratory syncytial virus may trigger AD, suggesting vaccination against the virus may reduce the risk for AD.30 Overall, vaccination improves life expectancy and should be conducted on schedule without reservation. Other aspects of hygiene that could conceptually affect prevalence of AD are raw food ingestion and the effects of foodborne microbes on the intestinal microbiome in relationship to AD development. Probiotics have been tested for this purpose.
Probiotics and prebiotics have been theorized to work through a reduction in inflammation; these agents have some evidence in their favor, but they were not endorsed in the AAD guidelines14 despite showing promise in meta-analysis. In particular prenatal and postnatal (maternal and child) supplementation of Lactobacillus rhamnosus shows promise.31-33 Food elimination diets and supplements including vitamin D, selenium, fish oil, borage oil, and zinc were not found to be beneficial and were not recommended in the AAD guidelines.14,34
Percutaneous exposure to peanuts, possibly in household dust, may be the mechanism of peanut sensitization in AD27 via an inherent adjuvant effect of peanut protein.28 The recent LEAP (Learning Early About Peanut Allergy) trial randomized 530 infants aged 4 to 11 months to peanut-avoidant versus peanut-exposed diets for 60 months. The results showed statistically reduced (approximately one-twelfth of the risk) peanut allergy even in infants known to be sensitized (approximately one-third of the risk).35 It is now recommended in countries with a high prevalence of peanut allergies to introduce peanuts to an infant’s diet between 4 and 11 months of age (evidence level 1 [highest level of evidence]), with referral to an allergist for introduction in known sensitization cases and severe AD.36 In the setting of known or documented peanut allergy and for evaluation of potential food allergies, an allergist should be consulted.
Other interventions have been described as promising in mouse models. Those supplements include Lithospermum erythrorhizon,37Platycodon grandiflorus,38Hypsizygus marmoreous,39 fortified ginseng extract,40 polyunsaturated fatty acids,41 and galactooligosaccharide.42 Prebiotic oligosaccharides also are promising for early prevention of AD symptoms in infants, but otherwise these agents have remained largely untested in AD.43 None of these therapies have been endorsed by the AAD, and the long-term safety and efficacy in humans remains to be proven.
Risks of Dietary Restriction
Dietary restrictions in treating AD can have negative consequences, including reduced birth weight when initiated in pregnancy,19 osteomalacia from vitamin D deficiency,44 and nutritional deficiencies (eg, calcium, phosphorus, iron, vitamin K, vitamin D, zinc, vitamin A, B1, B2, B6, niacin, cholesterol, and/or vitamin C deficiencies).45 Excess dietary intake of vegetables in individuals with extensive food allergies can result in carotenemia.46 Protein-restricted diets from use of rice milk or dietary protein restriction can result in kwashiorkorlike protein malnutrition and marasmus.47-49 Nutritional counseling and/or supplementation is recommended for patients with food-restricted diets.
Avoiding Fragrance in Food
Food intolerance often is reported by AD patients. In allergies, food intolerance refers to side effects such as gastrointestinal symptoms; in dermatology, food intolerance can include itching, systemic flares of allergic contact dermatitis (eg, fragrance allergy), or true IgE-mediated allergies such as oral allergy syndrome. Oral allergy syndrome (pollen-food allergy syndrome) is an epitope-spread phenomenon related to an allergy to tree pollen, causing broad allergy to specific groups of fruits and nuts.50 Food triggers in AD include kiwi, milk, apple, tomato, citrus fruits, tree nuts, and peanuts. Oral allergy syndrome is common in food-sensitive AD patients (51.2%) followed by gastrointestinal symptoms (23.5%) and worsening AD (11.4%).51 Sensitization to fragrance can cross-react with foods (eg, balsam of Peru and tomatoes).52 A tomato allergy can be detected either by a skin-prick test or a food patch test in this setting.53 An allergist should be consulted if oral allergy syndrome is suspected.
Conclusion
Food allergies are more common in AD patients and patients should be referred to an allergist for evaluation and management. Strict dietary practice is not recommended, while avoiding proven food allergens in AD could be beneficial. Dermatologists should be aware that patients with dietary restrictions may lack key nutrients, manifesting with nutritional deficiencies in the skin; therefore, nutrition counseling may be needed in the most severe AD/allergy patients. This field is evolving; therefore, ongoing study and evaluation of interventions as they relate to AD will be needed to assess best practices for diet in AD over time.
1. Schachner L, Ling NS, Press S. A statistical analysis of a pediatric dermatology clinic. Pediatr Dermatol. 1983;1:157-164.
2. Kiprono SK, Muchunu JW, Masenga JE. Skin diseases in pediatric patients attending a tertiary dermatology hospital in Northern Tanzania: a cross-sectional study. BMC Dermatol. 2015;15:16.
3. Wensink M, Timmer C, Brand PL. Atopic dermatitis in infants not caused by food allergy [in Dutch]. Ned Tijdschr Geneeskd. 2008;152:4-9.
4. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132(3, pt 2):949-963.
5. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
6. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
7. Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
8. Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
9. Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
10. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
11. Cianferoni A, Spergel J. Eosinophilic esophagitis: a comprehensive review [published online July 22, 2015]. Clin Rev Allergy Immunol. doi:10.1111/all.12846.
12. Sicherer SH, Sampson HA. Food hypersensitivity and atopic dermatitis; pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 1999;104(3, pt 2):S114-S122.
13. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.
14. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218-1233.
15. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the epidermal differentiation complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
16. Burks AW, Jones SM, Boyce JA, et al. NIAID-sponsored 2010 guidelines for managing food allergy: applications in the pediatric population. Pediatrics. 2011;128:955-965.
17. Ong PY. Association between egg and staphylococcal superantigen IgE sensitizations in atopic dermatitis. Allergy Asthma Proc. 2014;35:346-348.
18. Botteman M, Detzel P. Cost-effectiveness of partially hydrolyzed whey protein formula in the primary prevention of atopic dermatitis in high-risk urban infants in Southeast Asia. Ann Nutr Metab. 2015;66(suppl 1):26-32.
19. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;9:CD000133.
20. Yum HY, Lee SY, Lee KE, et al. Genetically modified and wild soybeans: an immunologic comparison. Allergy Asthma Proc. 2005;26:210-216.
21. Mathur C, Kathuria PC, Dahiya P, et al. Lack of detectable allergenicity in genetically modified maize containing “Cry” proteins as compared to native maize based on in silico & in vitro analysis. PLoS One. 2015;10:e0117340.
22. Dubois AE, Pagliarani G, Brouwer RM, et al. First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1-silenced apples cause fewer allergy symptoms than the wild-type cultivar [published online July 24, 2015]. Allergy. 2015;70:1406-1412.
23. Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25:246-254.
24. Pfab F, Schalock PC, Napadow V, et al. Complementary integrative approach for treating pruritus. Dermatol Ther. 2013;26:149-156.
25. Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol. 2015;89:851-865.
26. Schachner L, Field T, Hernandez-Reif M, et al. Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr Dermatol. 1998;15:390-395.
27. Pfab F, Schalock PC, Napadow V, et al. Acupuncture for allergic disease therapy–the current state of evidence. Expert Rev Clin Immunol. 2014;10:831-841.
28. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
29. Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
30. Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
31. Foolad N, Brezinski EA, Chase EP, et al. Effect of nutrient supplementation on atopic dermatitis in children: a systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013;149:350-355.
32. Kalliomäki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076-1079.
33. Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184-191.
34. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I: atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
35. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
36. Fleischer DM, Sicherer S, Greenhawt M, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants [published online October 2015]. Allergy. 2015;70:1193-1195.
37. Kim J, Cho Y. Gromwell (Lithospermum erythrorhizon) supplementation enhances epidermal levels of cera-mides, glucosylceramides, β-glucocerebrosidase, and acidicsphingomyelinase in NC/Nga mice. J Med Food. 2013;16:927-933.
38. Choi JH, Jin SW, Han EH, et al. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-κB and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine. 2014;21:1053-1061.
39. Kim T, Park K, Jung HS, et al. Evaluation of anti-atopic dermatitis activity of Hypsizigus marmoreus extract. Phytother Res. 2014;28:1539-1546.
40. Kim JR, Choi J, Kim J, et al. 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol-fortified ginseng extract attenuates the development of atopic dermatitis-like symptoms in NC/Nga mice. J Ethnopharmacol. 2014;151:365-371.
41. Weise C, Ernst D, van Tol EA, et al. Dietary polyunsaturated fatty acids and non-digestible oligosaccharides reduce dermatitis in mice. Pediatr Allergy Immunol. 2013;24:361-367.
42. Tanabe S, Hochi S. Oral administration of a galactooligosaccharide preparation inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. Int J Mol Med. 2010;25:331-336.
43. Arslanoglu S, Moro GE, Boehm G, et al. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J Biol Regul Homeost Agents. 2012;26(3 suppl):49-59.
44. Shikino K, Ikusaka M, Yamashita T. Vitamin D-deficient osteomalacia due to excessive self-restrictions for atopic dermatitis [published online July 4, 2014] . BMJ Case Rep.
45. Kim J, Kwon J, Noh G, et al. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract. 2013;7:488-494.
46. Silverberg NB, Lee-Wong M. Generalized yellow discoloration of the skin. Cutis. 2014;93:E11-E12.
47. Hon KL, Nip SY, Cheung KL. A tragic case of atopic eczema: malnutrition and infections despite multivitamins and supplements. Iran J Allergy Asthma Immunol. 2012;11:267-270.
48. Diamanti A, Pedicelli S, D’Argenio P, et al. Iatrogenic kwashiorkor in three infants on a diet of rice beverages. Pediatr Allergy Immunol. 2011;22:878-879.
49. Pillai K, Acharya S. Iatrogenic kwashiorkar. Indian Pediatr. 2010;47:540-541.
50. Price A, Ramachandran S, Smith GP, et al. Oral allergy syndrome (pollen-food allergy syndrome). Dermatitis. 2015;26:78-88.
51. Mattila L, Kilpeläinen M, Terho EO, et al. Food hypersensitivity among Finnish university students: association with atopic diseases. Clin Exp Allergy. 2003;33:600-606.
52. Paulsen E, Christensen LP, Andersen KE. Tomato contact dermatitis. Contact Dermatitis. 2012;67:321-327.
53. Di Leo E, Nettis E, Cardinale F, et al. Tomato atopy patch test in adult atopic dermatitis: diagnostic value and comparison among different methods. Allergy. 2009;64:659-663.
1. Schachner L, Ling NS, Press S. A statistical analysis of a pediatric dermatology clinic. Pediatr Dermatol. 1983;1:157-164.
2. Kiprono SK, Muchunu JW, Masenga JE. Skin diseases in pediatric patients attending a tertiary dermatology hospital in Northern Tanzania: a cross-sectional study. BMC Dermatol. 2015;15:16.
3. Wensink M, Timmer C, Brand PL. Atopic dermatitis in infants not caused by food allergy [in Dutch]. Ned Tijdschr Geneeskd. 2008;152:4-9.
4. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132(3, pt 2):949-963.
5. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
6. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
7. Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
8. Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
9. Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
10. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
11. Cianferoni A, Spergel J. Eosinophilic esophagitis: a comprehensive review [published online July 22, 2015]. Clin Rev Allergy Immunol. doi:10.1111/all.12846.
12. Sicherer SH, Sampson HA. Food hypersensitivity and atopic dermatitis; pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 1999;104(3, pt 2):S114-S122.
13. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.
14. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218-1233.
15. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the epidermal differentiation complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
16. Burks AW, Jones SM, Boyce JA, et al. NIAID-sponsored 2010 guidelines for managing food allergy: applications in the pediatric population. Pediatrics. 2011;128:955-965.
17. Ong PY. Association between egg and staphylococcal superantigen IgE sensitizations in atopic dermatitis. Allergy Asthma Proc. 2014;35:346-348.
18. Botteman M, Detzel P. Cost-effectiveness of partially hydrolyzed whey protein formula in the primary prevention of atopic dermatitis in high-risk urban infants in Southeast Asia. Ann Nutr Metab. 2015;66(suppl 1):26-32.
19. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;9:CD000133.
20. Yum HY, Lee SY, Lee KE, et al. Genetically modified and wild soybeans: an immunologic comparison. Allergy Asthma Proc. 2005;26:210-216.
21. Mathur C, Kathuria PC, Dahiya P, et al. Lack of detectable allergenicity in genetically modified maize containing “Cry” proteins as compared to native maize based on in silico & in vitro analysis. PLoS One. 2015;10:e0117340.
22. Dubois AE, Pagliarani G, Brouwer RM, et al. First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1-silenced apples cause fewer allergy symptoms than the wild-type cultivar [published online July 24, 2015]. Allergy. 2015;70:1406-1412.
23. Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25:246-254.
24. Pfab F, Schalock PC, Napadow V, et al. Complementary integrative approach for treating pruritus. Dermatol Ther. 2013;26:149-156.
25. Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol. 2015;89:851-865.
26. Schachner L, Field T, Hernandez-Reif M, et al. Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr Dermatol. 1998;15:390-395.
27. Pfab F, Schalock PC, Napadow V, et al. Acupuncture for allergic disease therapy–the current state of evidence. Expert Rev Clin Immunol. 2014;10:831-841.
28. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
29. Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
30. Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
31. Foolad N, Brezinski EA, Chase EP, et al. Effect of nutrient supplementation on atopic dermatitis in children: a systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013;149:350-355.
32. Kalliomäki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076-1079.
33. Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184-191.
34. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I: atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
35. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
36. Fleischer DM, Sicherer S, Greenhawt M, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants [published online October 2015]. Allergy. 2015;70:1193-1195.
37. Kim J, Cho Y. Gromwell (Lithospermum erythrorhizon) supplementation enhances epidermal levels of cera-mides, glucosylceramides, β-glucocerebrosidase, and acidicsphingomyelinase in NC/Nga mice. J Med Food. 2013;16:927-933.
38. Choi JH, Jin SW, Han EH, et al. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-κB and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine. 2014;21:1053-1061.
39. Kim T, Park K, Jung HS, et al. Evaluation of anti-atopic dermatitis activity of Hypsizigus marmoreus extract. Phytother Res. 2014;28:1539-1546.
40. Kim JR, Choi J, Kim J, et al. 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol-fortified ginseng extract attenuates the development of atopic dermatitis-like symptoms in NC/Nga mice. J Ethnopharmacol. 2014;151:365-371.
41. Weise C, Ernst D, van Tol EA, et al. Dietary polyunsaturated fatty acids and non-digestible oligosaccharides reduce dermatitis in mice. Pediatr Allergy Immunol. 2013;24:361-367.
42. Tanabe S, Hochi S. Oral administration of a galactooligosaccharide preparation inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. Int J Mol Med. 2010;25:331-336.
43. Arslanoglu S, Moro GE, Boehm G, et al. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J Biol Regul Homeost Agents. 2012;26(3 suppl):49-59.
44. Shikino K, Ikusaka M, Yamashita T. Vitamin D-deficient osteomalacia due to excessive self-restrictions for atopic dermatitis [published online July 4, 2014] . BMJ Case Rep.
45. Kim J, Kwon J, Noh G, et al. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract. 2013;7:488-494.
46. Silverberg NB, Lee-Wong M. Generalized yellow discoloration of the skin. Cutis. 2014;93:E11-E12.
47. Hon KL, Nip SY, Cheung KL. A tragic case of atopic eczema: malnutrition and infections despite multivitamins and supplements. Iran J Allergy Asthma Immunol. 2012;11:267-270.
48. Diamanti A, Pedicelli S, D’Argenio P, et al. Iatrogenic kwashiorkor in three infants on a diet of rice beverages. Pediatr Allergy Immunol. 2011;22:878-879.
49. Pillai K, Acharya S. Iatrogenic kwashiorkar. Indian Pediatr. 2010;47:540-541.
50. Price A, Ramachandran S, Smith GP, et al. Oral allergy syndrome (pollen-food allergy syndrome). Dermatitis. 2015;26:78-88.
51. Mattila L, Kilpeläinen M, Terho EO, et al. Food hypersensitivity among Finnish university students: association with atopic diseases. Clin Exp Allergy. 2003;33:600-606.
52. Paulsen E, Christensen LP, Andersen KE. Tomato contact dermatitis. Contact Dermatitis. 2012;67:321-327.
53. Di Leo E, Nettis E, Cardinale F, et al. Tomato atopy patch test in adult atopic dermatitis: diagnostic value and comparison among different methods. Allergy. 2009;64:659-663.
Practice Points
- Test children younger than 5 years with moderate to severe atopic dermatitis (AD) for food allergies if they have persistently severe AD or known food-induced reactions.
- Food elimination diets are not recommended for management of AD.
- There is not enough evidence supporting the use of complementary and alternative medicine, probiotics/prebiotics, or supplements for the treatment of AD.
Status Report From the American Acne & Rosacea Society on Medical Management of Acne in Adult Women, Part 3: Oral Therapies
Selection of oral agents for treatment of AV in adult women is dependent on multiple factors including the patient’s age, medication history, child-bearing potential, clinical presentation, and treatment preference following a discussion of the anticipated benefits versus potential risks.1,2 In patients with the mixed inflammatory and comedonal clinical pattern of AV, oral antibiotics can be used concurrently with topical therapies when moderate to severe inflammatory lesions are noted.3,4 However, many adult women who had AV as teenagers have already utilized oral antibiotic therapies in the past and often are interested in alternative options, express concerns regarding antibiotic resistance, report a history of antibiotic-associated yeast infections or other side effects, and/or encounter issues related to drug-drug interactions.3,5-8 Oral hormonal therapies such as combination oral contraceptives (COCs) or spironolactone often are utilized to treat adult women with AV, sometimes in combination with each other or other agents. Combination oral contraceptives appear to be especially effective in the management of the U-shaped clinical pattern or predominantly inflammatory, late-onset AV.1,5,9,10 Potential warnings, contraindications, adverse effects, and drug-drug interactions are important to keep in mind when considering the use of oral hormonal therapies.8-10 Oral isotretinoin, which should be prescribed with strict adherence to the iPLEDGE™ program (https://www.ipledgeprogram.com/), remains a viable option for cases of severe nodular AV and selected cases of refractory inflammatory AV, especially when scarring and/or marked psychosocial distress are noted.1,2,5,11 Although it is recognized that adult women with AV typically present with either a mixed inflammatory and comedonal or U-shaped clinical pattern predominantly involving the lower face and anterolateral neck, the available data do not adequately differentiate the relative responsiveness of these clinical patterns to specific therapeutic agents.
Combination Oral Contraceptives
Combination oral contraceptives are commonly used to treat AV in adult women, including those without and those with measurable androgen excess (eg, polycystic ovary syndrome [PCOS]). Combination oral contraceptives contain ethinyl estradiol and a progestational agent (eg, progestin); the latter varies in terms of its nonselective receptor interactions and the relative magnitude or absence of androgenic effects.10,12,13 Although some COCs are approved by the US Food and Drug Administration (FDA) for AV, there is little data available to determine the comparative efficacy among these and other COCs.10,14 When choosing a COC for treatment of AV, it is best to select an agent whose effectiveness is supported by evidence from clinical studies.10,15
Mechanisms of Action
The reported mechanisms of action for COCs include inhibition of ovarian androgen production and ovulation through gonadotropin suppression; upregulated synthesis of sex hormone–binding globulin, which decreases free testosterone levels through receptor binding; and inhibition of 5α-reductase (by some progestins), which reduces conversion of testosterone to dihydrotestosterone, the active derivative that induces androgenic effects at peripheral target tissues.10,13,16,17
Therapeutic Benefits
Use of COCs to treat AV in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception. Multiple monotherapy studies have demonstrated the efficacy of COCs in the treatment of AV on the face and trunk.4,10,12,15,17,18 It may take a minimum of 3 monthly cycles of use before acne lesion counts begin to appreciably decrease.12,15,19-21 Initiating COC therapy during menstruation ensures the absence of pregnancy. Combination oral contraceptives may be used with other topical and oral therapies for AV.2,3,9,10 Potential ancillary benefits of COCs include normalization of the menstrual cycle; reduced premenstrual dysphoric disorder symptoms; and reduced risk of endometrial cancer (approximately 50%), ovarian cancer (approximately 40%), and colorectal cancer.22-24
Risks and Contraindications
It is important to consider the potential risks associated with the use of COCs, especially in women with AV who are not seeking a method of contraception. Side effects of COCs can include nausea, breast tenderness, breakthrough bleeding, and weight gain.25,26 Potential adverse associations of COCs are described in the Table. The major potential vascular associations include venous thromboembolism, myocardial infarction, and cerebrovascular accident, all of which are influenced by concurrent factors such as a history of smoking, age (≥35 years), and hypertension.27-32 It is recommended that blood pressure be measured before initiating COC therapy as part of the general examination.33
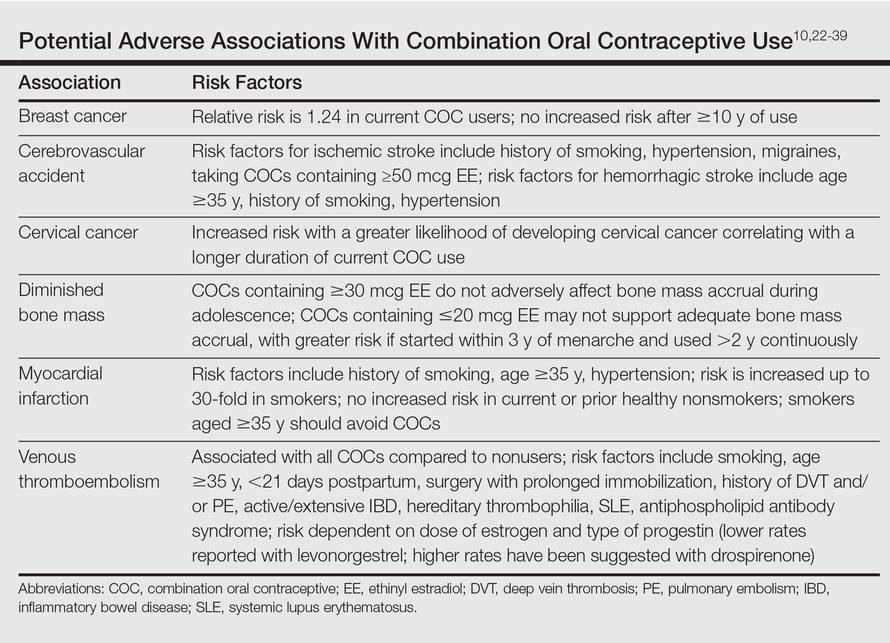
The potential increase in breast cancer risk appears to be low, while the cervical cancer risk is reported to increase relative to the duration of use.34-37 This latter observation may be due to the greater likelihood of unprotected sex in women using a COC and exposure to multiple sexual partners in some cases, which may increase the likelihood of oncogenic human papillomavirus infection of the cervix. If a dermatologist elects to prescribe a COC to treat AV, it has been suggested that the patient also consult with her general practitioner or gynecologist to undergo pelvic and breast examinations and a Papanicolaou test.33 The recommendation for initial screening for cervical cancer is within 3 years of initiation of sexual intercourse or by 21 years of age, whichever is first.33,38,39
Combination oral contraceptives are not ideal for all adult women with AV. Absolute contraindications are pregnancy and history of thromboembolic, cardiac, or hepatic disease; in women aged 35 years and older who smoke, relative contraindications include hypertension, diabetes, migraines, breastfeeding, and current breast or liver cancer.33 In adult women with AV who have relative contra-indications but are likely to benefit from the use of a COC when other options are limited or not viable, consultation with a gynecologist is prudent. Other than rifamycin antibiotics (eg, rifampin) and griseofulvin, there is no definitive evidence that oral antibiotics (eg, tetracycline) or oral antifungal agents reduce the contraceptive efficacy of COCs, although cautions remain in print within some approved package inserts.8
Spironolactone
Available since 1957, spironolactone is an oral aldos-terone antagonist and potassium-sparing diuretic used to treat hypertension and congestive heart failure.9 Recognition of its antiandrogenic effects led to its use in dermatology to treat certain dermatologic disorders in women (eg, hirsutism, alopecia, AV).1,4,5,9,10 Spironolactone is not approved for AV by the FDA; therefore, available data from multiple independent studies and retrospective analyses that have been collectively reviewed support its efficacy when used as both monotherapy or in combination with other agents in adult women with AV, especially those with a U-shaped pattern and/or late-onset AV.9,40-43
Mechanism of Action
Spironolactone inhibits sebaceous gland activity through peripheral androgen receptor blockade, inhibition of 5α-reductase, decrease in androgen production, and increase in sex hormone–binding globulin.9,10,40
Therapeutic Benefits
Good to excellent improvement of AV in women, many of whom are postadolescent, has ranged from 66% to 100% in published reports9,40-43; however, inclusion and exclusion criteria, dosing regimens, and concomitant therapies were not usually controlled. Spironolactone has been used to treat AV in adult women as monotherapy or in combination with topical agents, oral antibiotics, and COCs.9,40-42 Additionally, dose-ranging studies have not been completed with spironolactone for AV.9,40 The suggested dose range is 50 mg to 200 mg daily; however, it usually is best to start at 50 mg daily and increase to 100 mg daily if clinical response is not adequate after 2 to 3 months. The gastrointestinal (GI) absorption of spironolactone is increased when ingested with a high-fat meal.9,10
Once effective control of AV is achieved, it is optimal to use the lowest dose needed to continue reasonable suppression of new AV lesions. There is no defined end point for spironolactone use in AV, with or without concurrent PCOS, as many adult women usually continue treatment with low-dose therapy because they experience marked flaring shortly after the drug is stopped.9
Risks and Contraindications
Side effects associated with spironolactone are dose related and include increased diuresis, migraines, menstrual irregularities, breast tenderness, gynecomastia, fatigue, and dizziness.9,10,40-44 Side effects (particularly menstrual irregularities and breast tenderness) are more common at doses higher than 100 mg daily, especially when used as monotherapy without concurrent use of a COC.9,40
Spironolactone-associated hyperkalemia is most clinically relevant in patients on higher doses (eg, 100–200 mg daily), in those with renal impairment and/or congestive heart failure, and when used concurrently with certain other medications. In any patient on spironolactone, the risk of clinically relevant hyperkalemia may be increased by coingestion of potassium supplements, potassium-based salt substitutes, potassium-sparing diuretics (eg, amiloride, triamterene); aldosterone antagonists and angiotensin-converting enzyme inhibitors (eg, lisinopril, benazepril); angiotensin II receptor blockers (eg, losartan, valsartan); and tri-methoprim (with or without sulfamethoxazole).8,9,40,45 Spironolactone may also increase serum levels of lithium or digoxin.9,40,45,46 For management of AV, it is best that spironolactone be avoided in patients taking any of these medications.9
In healthy adult women with AV who are not on medications or supplements that interact adversely with spironolactone, there is no definitive recommendation regarding monitoring of serum potassium levels during treatment with spironolactone, and it has been suggested that monitoring serum potassium levels in this subgroup is not necessary.47 However, each clinician is advised to choose whether or not they wish to obtain baseline and/or periodic serum potassium levels when prescribing spironolactone for AV based on their degree of comfort and the patient’s history. Baseline and periodic blood testing to evaluate serum electrolytes and renal function are reasonable, especially as adult women with AV are usually treated with spironolactone over a prolonged period of time.9
The FDA black box warning for spironolactone states that it is tumorigenic in chronic toxicity studies in rats and refers to exposures 25- to 100-fold higher than those administered to humans.9,48 Although continued vigilance is warranted, evaluation of large populations of women treated with spironolactone do not suggest an association with increased risk of breast cancer.49,50
Spironolactone is a category C drug and thus should be avoided during pregnancy, primarily due to animal data suggesting risks of hypospadias and feminization in male fetuses.9 Importantly, there is an absence of reports linking exposure during pregnancy with congenital defects in humans, including in 2 known cases of high-dose exposures for maternal Bartter syndrome.9
The active metabolite, canrenone, is known to be present in breast milk at 0.2% of the maternal daily dose, but breastfeeding is generally believed to be safe with spironolactone based on evidence to date.9
Oral Antibiotics
Oral antibiotic therapy may be used in combination with a topical regimen to treat AV in adult women, keeping in mind some important caveats.1-7 For instance, monotherapy with oral antibiotics should be avoided, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.3,4 A therapeutic exit plan also is suggested when prescribing oral antibiotics to limit treatment to 3 to 4 months, if possible, to help mitigate the emergence of antibiotic-resistant bacteria (eg, staphylococci and streptococci).3-5,51
Tetracyclines, especially doxycycline and minocycline, are the most commonly prescribed agents. Doxycycline use warrants patient education on measures to limit the risks of esophageal and GI side effects and phototoxicity; enteric-coated and small tablet formulations have been shown to reduce GI side effects, especially when administered with food.3,52-55 In addition to vestibular side effects and hyperpigmentation, minocycline may be associated with rare but potentially severe adverse reactions such as drug hypersensitivity syndrome, autoimmune hepatitis, and lupus-like syndrome, which are reported more commonly in women.5,52,54 Vestibular side effects have been shown to decrease with use of extended-release tablets with weight-based dosing.53
Oral Isotretinoin
Oral isotretinoin is well established as highly effective for treatment of severe, recalcitrant AV, including nodular acne on the face and trunk.4,56 Currently available oral isotretinoins are branded generic formulations based on the pharmacokinetic profile of the original brand (Accutane [Roche Pharmaceuticals]) and with the use of Lidose Technology (Absorica [Cipher Pharmaceuticals]), which substantially increases GI absorption of isotretinoin in the absence of ingestion with a high-calorie, high-fat meal.57 The short- and long-term efficacy, dosing regimens, safety considerations, and serious teratogenic risks for oral isotretinoin are well published.4,56-58 Importantly, oral isotretinoin must be prescribed with strict adherence to the federally mandated iPLEDGE risk management program.
Low-dose oral isotretinoin therapy (<0.5 mg/kg–1 mg/kg daily) administered over several months longer than conventional regimens (ie, 16–20 weeks) has been suggested with demonstrated efficacy.57 However, this approach is not optimal due to the lack of established sustained clearance of AV after discontinuation of therapy and the greater potential for exposure to isotretinoin during pregnancy. Recurrences of AV do occur after completion of isotretinoin therapy, especially if cumulative systemic exposure to the drug during the initial course of treatment was inadequate.56,57
Oral isotretinoin has been shown to be effective in AV in adult women with or without PCOS with 0.5 mg/kg to 1 mg/kg daily and a total cumulative exposure of 120 mg/kg to 150 mg/kg.59 In one study, the presence of PCOS and greater number of nodules at baseline were predictive of a higher risk of relapse during the second year posttreatment.59
Conclusion
All oral therapies that are used to treat AV in adult women warrant individual consideration of possible benefits versus risks. Careful attention to possible side effects, patient-related risk factors, and potential drug-drug interactions is important. End points of therapy are not well established, with the exception of oral isotretinoin therapy. Clinicians must use their judgment in each case along with obtaining feedback from patients regarding the selection of therapy after a discussion of the available options.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
- Del Rosso JQ, Kim G. Optimizing use of oral antibiotics in acne vulgaris. Dermatol Clin. 2009;27:33-42.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:125-133.
- Del Rosso JQ. Oral antibiotic drug interactions of clinical significance to dermatologists. Dermatol Clin. 2009;27:91-94.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
- American Academy of Dermatology. Position statement on isotretinoin. AAD Web site. https://www.aad.org /Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Updated November 13, 2010. Accessed October 28, 2015.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. June 2012;7:CD004425.
- Sitruk-Ware R. Pharmacology of different progestogens: the special case of drospirenone. Climacteric. 2005;8 (suppl 3):4-12.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for the treatment of acne. Cochrane Database Syst Rev. July 2012;7:CD004425.
- Thiboutot D, Archer DF, Lemay A, et al. A randomized, controlled trial of a low-dose contraceptive containing 20 microg of ethinyl estradiol and 100 microg of levonogestrel for acne treatment. Fertil Steril. 2001;76:461-468.
- Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
- Rabe T, Kowald A, Ortmann J, et al. Inhibition of skin 5-alpha reductase by oral contraceptive progestins in vitro. Gynecol Endocrinol. 2000;14:223-230.
- Palli MB, Reyes-Habito CM, Lima XT, et al. A single-center, randomized double-blind, parallel-group study to examine the safety and efficacy of 3mg drospirenone/0.02mg ethinyl estradiol compared with placebo in the treatment of moderate truncal acne vulgaris. J Drugs Dermatol. 2013;12:633-637.
- Koltun W, Maloney JM, Marr J, et al. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 μg plus drospirenone 3 mg administered in a 24/4 regimen: a pooled analysis. Eur J Obstet Gynecol Reprod Biol. 2011;155:171-175.
- Maloney JM, Dietze P, Watson D, et al. A randomized controlled trial of a low-dose combined oral contraceptive containing 3 mg drospirenone plus 20 μg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self-assessment. J Drugs Dermatol. 2009;8:837-844.
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-μg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Maguire K, Westhoff C. The state of hormonal contraception today: established and emerging noncontraceptive health benefits. Am J Obstet Gynecol. 2011;205 (suppl 4):S4-S8.
- Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980;302:551-554.
- Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol. 2013;68:1022-1029.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008;4:CD003987.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
- Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119:1039-1044.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ. 2011;342:d2151.
- US Food and Drug Administration Office of Surveillance and Epidemiology. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs /Drug Safety/UCM277384.pdf. Accessed October 28, 2015.
- The American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
- World Health Organization. Cardiovascular Disease and Steroid Hormone Contraception: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1998. Technical Report Series 877.
- Frangos JE, Alavian CN, Kimball AB. Acne and oral contraceptives: update on women’s health screening guidelines. J Am Acad Dermatol. 2008;58:781-786.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931-1943.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609-1621.
- Agostino H, Di Meglio G. Low-dose oral contraceptives in adolescents: how low can you go? J Pediatr Adolesc Gynecol. 2010;23:195-201.
- Buzney E, Sheu J, Buzney C, et al. Polycystic ovary syndrome: a review for dermatologists: part II. Treatment. J Am Acad Dermatol. 2014;71:859.e1-859.e15.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- Sawaya ME, Somani N. Antiandrogens and androgen inhibitors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:361-374.
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Sato K, Matsumoto D, Iizuka F, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in Asians. Aesth Plast Surg. 2006;30:689-694.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Stockley I. Antihypertensive drug interactions. In: Stockley I, ed. Drug Interactions. 5th ed. London, United Kingdom: Pharmaceutical Press; 1999:335-347.
- Antoniou T, Gomes T, Mamdani MM, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ. 2011;343:d5228.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Aldactone [package insert]. New York, NY: Pfizer Inc; 2008.
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:e4447.
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24:330-334.
- Kim S, Michaels BD, Kim GK, et al. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:61-97.
- Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: pharmacokinetic and pharmacodynamics perspectives. J Clin Aesthet Dermatol. 2011;4:40-47.
- Del Rosso JQ. Oral antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:113-124.
- Del Rosso JQ. Oral doxycycline in the management of acne vulgaris: current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. 2015;8:19-26.
- Osofsky MG, Strauss JS. Isotretinoin. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:134-145.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
- Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:252-268.
- Cakir GA, Erdogan FG, Gurler A. Isotretinoin treatment in nodulocystic acne with and without polycystic ovary syndrome: efficacy and determinants of relapse. Int J Dermatol. 2013;52:371-376.
Selection of oral agents for treatment of AV in adult women is dependent on multiple factors including the patient’s age, medication history, child-bearing potential, clinical presentation, and treatment preference following a discussion of the anticipated benefits versus potential risks.1,2 In patients with the mixed inflammatory and comedonal clinical pattern of AV, oral antibiotics can be used concurrently with topical therapies when moderate to severe inflammatory lesions are noted.3,4 However, many adult women who had AV as teenagers have already utilized oral antibiotic therapies in the past and often are interested in alternative options, express concerns regarding antibiotic resistance, report a history of antibiotic-associated yeast infections or other side effects, and/or encounter issues related to drug-drug interactions.3,5-8 Oral hormonal therapies such as combination oral contraceptives (COCs) or spironolactone often are utilized to treat adult women with AV, sometimes in combination with each other or other agents. Combination oral contraceptives appear to be especially effective in the management of the U-shaped clinical pattern or predominantly inflammatory, late-onset AV.1,5,9,10 Potential warnings, contraindications, adverse effects, and drug-drug interactions are important to keep in mind when considering the use of oral hormonal therapies.8-10 Oral isotretinoin, which should be prescribed with strict adherence to the iPLEDGE™ program (https://www.ipledgeprogram.com/), remains a viable option for cases of severe nodular AV and selected cases of refractory inflammatory AV, especially when scarring and/or marked psychosocial distress are noted.1,2,5,11 Although it is recognized that adult women with AV typically present with either a mixed inflammatory and comedonal or U-shaped clinical pattern predominantly involving the lower face and anterolateral neck, the available data do not adequately differentiate the relative responsiveness of these clinical patterns to specific therapeutic agents.
Combination Oral Contraceptives
Combination oral contraceptives are commonly used to treat AV in adult women, including those without and those with measurable androgen excess (eg, polycystic ovary syndrome [PCOS]). Combination oral contraceptives contain ethinyl estradiol and a progestational agent (eg, progestin); the latter varies in terms of its nonselective receptor interactions and the relative magnitude or absence of androgenic effects.10,12,13 Although some COCs are approved by the US Food and Drug Administration (FDA) for AV, there is little data available to determine the comparative efficacy among these and other COCs.10,14 When choosing a COC for treatment of AV, it is best to select an agent whose effectiveness is supported by evidence from clinical studies.10,15
Mechanisms of Action
The reported mechanisms of action for COCs include inhibition of ovarian androgen production and ovulation through gonadotropin suppression; upregulated synthesis of sex hormone–binding globulin, which decreases free testosterone levels through receptor binding; and inhibition of 5α-reductase (by some progestins), which reduces conversion of testosterone to dihydrotestosterone, the active derivative that induces androgenic effects at peripheral target tissues.10,13,16,17
Therapeutic Benefits
Use of COCs to treat AV in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception. Multiple monotherapy studies have demonstrated the efficacy of COCs in the treatment of AV on the face and trunk.4,10,12,15,17,18 It may take a minimum of 3 monthly cycles of use before acne lesion counts begin to appreciably decrease.12,15,19-21 Initiating COC therapy during menstruation ensures the absence of pregnancy. Combination oral contraceptives may be used with other topical and oral therapies for AV.2,3,9,10 Potential ancillary benefits of COCs include normalization of the menstrual cycle; reduced premenstrual dysphoric disorder symptoms; and reduced risk of endometrial cancer (approximately 50%), ovarian cancer (approximately 40%), and colorectal cancer.22-24
Risks and Contraindications
It is important to consider the potential risks associated with the use of COCs, especially in women with AV who are not seeking a method of contraception. Side effects of COCs can include nausea, breast tenderness, breakthrough bleeding, and weight gain.25,26 Potential adverse associations of COCs are described in the Table. The major potential vascular associations include venous thromboembolism, myocardial infarction, and cerebrovascular accident, all of which are influenced by concurrent factors such as a history of smoking, age (≥35 years), and hypertension.27-32 It is recommended that blood pressure be measured before initiating COC therapy as part of the general examination.33

The potential increase in breast cancer risk appears to be low, while the cervical cancer risk is reported to increase relative to the duration of use.34-37 This latter observation may be due to the greater likelihood of unprotected sex in women using a COC and exposure to multiple sexual partners in some cases, which may increase the likelihood of oncogenic human papillomavirus infection of the cervix. If a dermatologist elects to prescribe a COC to treat AV, it has been suggested that the patient also consult with her general practitioner or gynecologist to undergo pelvic and breast examinations and a Papanicolaou test.33 The recommendation for initial screening for cervical cancer is within 3 years of initiation of sexual intercourse or by 21 years of age, whichever is first.33,38,39
Combination oral contraceptives are not ideal for all adult women with AV. Absolute contraindications are pregnancy and history of thromboembolic, cardiac, or hepatic disease; in women aged 35 years and older who smoke, relative contraindications include hypertension, diabetes, migraines, breastfeeding, and current breast or liver cancer.33 In adult women with AV who have relative contra-indications but are likely to benefit from the use of a COC when other options are limited or not viable, consultation with a gynecologist is prudent. Other than rifamycin antibiotics (eg, rifampin) and griseofulvin, there is no definitive evidence that oral antibiotics (eg, tetracycline) or oral antifungal agents reduce the contraceptive efficacy of COCs, although cautions remain in print within some approved package inserts.8
Spironolactone
Available since 1957, spironolactone is an oral aldos-terone antagonist and potassium-sparing diuretic used to treat hypertension and congestive heart failure.9 Recognition of its antiandrogenic effects led to its use in dermatology to treat certain dermatologic disorders in women (eg, hirsutism, alopecia, AV).1,4,5,9,10 Spironolactone is not approved for AV by the FDA; therefore, available data from multiple independent studies and retrospective analyses that have been collectively reviewed support its efficacy when used as both monotherapy or in combination with other agents in adult women with AV, especially those with a U-shaped pattern and/or late-onset AV.9,40-43
Mechanism of Action
Spironolactone inhibits sebaceous gland activity through peripheral androgen receptor blockade, inhibition of 5α-reductase, decrease in androgen production, and increase in sex hormone–binding globulin.9,10,40
Therapeutic Benefits
Good to excellent improvement of AV in women, many of whom are postadolescent, has ranged from 66% to 100% in published reports9,40-43; however, inclusion and exclusion criteria, dosing regimens, and concomitant therapies were not usually controlled. Spironolactone has been used to treat AV in adult women as monotherapy or in combination with topical agents, oral antibiotics, and COCs.9,40-42 Additionally, dose-ranging studies have not been completed with spironolactone for AV.9,40 The suggested dose range is 50 mg to 200 mg daily; however, it usually is best to start at 50 mg daily and increase to 100 mg daily if clinical response is not adequate after 2 to 3 months. The gastrointestinal (GI) absorption of spironolactone is increased when ingested with a high-fat meal.9,10
Once effective control of AV is achieved, it is optimal to use the lowest dose needed to continue reasonable suppression of new AV lesions. There is no defined end point for spironolactone use in AV, with or without concurrent PCOS, as many adult women usually continue treatment with low-dose therapy because they experience marked flaring shortly after the drug is stopped.9
Risks and Contraindications
Side effects associated with spironolactone are dose related and include increased diuresis, migraines, menstrual irregularities, breast tenderness, gynecomastia, fatigue, and dizziness.9,10,40-44 Side effects (particularly menstrual irregularities and breast tenderness) are more common at doses higher than 100 mg daily, especially when used as monotherapy without concurrent use of a COC.9,40
Spironolactone-associated hyperkalemia is most clinically relevant in patients on higher doses (eg, 100–200 mg daily), in those with renal impairment and/or congestive heart failure, and when used concurrently with certain other medications. In any patient on spironolactone, the risk of clinically relevant hyperkalemia may be increased by coingestion of potassium supplements, potassium-based salt substitutes, potassium-sparing diuretics (eg, amiloride, triamterene); aldosterone antagonists and angiotensin-converting enzyme inhibitors (eg, lisinopril, benazepril); angiotensin II receptor blockers (eg, losartan, valsartan); and tri-methoprim (with or without sulfamethoxazole).8,9,40,45 Spironolactone may also increase serum levels of lithium or digoxin.9,40,45,46 For management of AV, it is best that spironolactone be avoided in patients taking any of these medications.9
In healthy adult women with AV who are not on medications or supplements that interact adversely with spironolactone, there is no definitive recommendation regarding monitoring of serum potassium levels during treatment with spironolactone, and it has been suggested that monitoring serum potassium levels in this subgroup is not necessary.47 However, each clinician is advised to choose whether or not they wish to obtain baseline and/or periodic serum potassium levels when prescribing spironolactone for AV based on their degree of comfort and the patient’s history. Baseline and periodic blood testing to evaluate serum electrolytes and renal function are reasonable, especially as adult women with AV are usually treated with spironolactone over a prolonged period of time.9
The FDA black box warning for spironolactone states that it is tumorigenic in chronic toxicity studies in rats and refers to exposures 25- to 100-fold higher than those administered to humans.9,48 Although continued vigilance is warranted, evaluation of large populations of women treated with spironolactone do not suggest an association with increased risk of breast cancer.49,50
Spironolactone is a category C drug and thus should be avoided during pregnancy, primarily due to animal data suggesting risks of hypospadias and feminization in male fetuses.9 Importantly, there is an absence of reports linking exposure during pregnancy with congenital defects in humans, including in 2 known cases of high-dose exposures for maternal Bartter syndrome.9
The active metabolite, canrenone, is known to be present in breast milk at 0.2% of the maternal daily dose, but breastfeeding is generally believed to be safe with spironolactone based on evidence to date.9
Oral Antibiotics
Oral antibiotic therapy may be used in combination with a topical regimen to treat AV in adult women, keeping in mind some important caveats.1-7 For instance, monotherapy with oral antibiotics should be avoided, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.3,4 A therapeutic exit plan also is suggested when prescribing oral antibiotics to limit treatment to 3 to 4 months, if possible, to help mitigate the emergence of antibiotic-resistant bacteria (eg, staphylococci and streptococci).3-5,51
Tetracyclines, especially doxycycline and minocycline, are the most commonly prescribed agents. Doxycycline use warrants patient education on measures to limit the risks of esophageal and GI side effects and phototoxicity; enteric-coated and small tablet formulations have been shown to reduce GI side effects, especially when administered with food.3,52-55 In addition to vestibular side effects and hyperpigmentation, minocycline may be associated with rare but potentially severe adverse reactions such as drug hypersensitivity syndrome, autoimmune hepatitis, and lupus-like syndrome, which are reported more commonly in women.5,52,54 Vestibular side effects have been shown to decrease with use of extended-release tablets with weight-based dosing.53
Oral Isotretinoin
Oral isotretinoin is well established as highly effective for treatment of severe, recalcitrant AV, including nodular acne on the face and trunk.4,56 Currently available oral isotretinoins are branded generic formulations based on the pharmacokinetic profile of the original brand (Accutane [Roche Pharmaceuticals]) and with the use of Lidose Technology (Absorica [Cipher Pharmaceuticals]), which substantially increases GI absorption of isotretinoin in the absence of ingestion with a high-calorie, high-fat meal.57 The short- and long-term efficacy, dosing regimens, safety considerations, and serious teratogenic risks for oral isotretinoin are well published.4,56-58 Importantly, oral isotretinoin must be prescribed with strict adherence to the federally mandated iPLEDGE risk management program.
Low-dose oral isotretinoin therapy (<0.5 mg/kg–1 mg/kg daily) administered over several months longer than conventional regimens (ie, 16–20 weeks) has been suggested with demonstrated efficacy.57 However, this approach is not optimal due to the lack of established sustained clearance of AV after discontinuation of therapy and the greater potential for exposure to isotretinoin during pregnancy. Recurrences of AV do occur after completion of isotretinoin therapy, especially if cumulative systemic exposure to the drug during the initial course of treatment was inadequate.56,57
Oral isotretinoin has been shown to be effective in AV in adult women with or without PCOS with 0.5 mg/kg to 1 mg/kg daily and a total cumulative exposure of 120 mg/kg to 150 mg/kg.59 In one study, the presence of PCOS and greater number of nodules at baseline were predictive of a higher risk of relapse during the second year posttreatment.59
Conclusion
All oral therapies that are used to treat AV in adult women warrant individual consideration of possible benefits versus risks. Careful attention to possible side effects, patient-related risk factors, and potential drug-drug interactions is important. End points of therapy are not well established, with the exception of oral isotretinoin therapy. Clinicians must use their judgment in each case along with obtaining feedback from patients regarding the selection of therapy after a discussion of the available options.
Selection of oral agents for treatment of AV in adult women is dependent on multiple factors including the patient’s age, medication history, child-bearing potential, clinical presentation, and treatment preference following a discussion of the anticipated benefits versus potential risks.1,2 In patients with the mixed inflammatory and comedonal clinical pattern of AV, oral antibiotics can be used concurrently with topical therapies when moderate to severe inflammatory lesions are noted.3,4 However, many adult women who had AV as teenagers have already utilized oral antibiotic therapies in the past and often are interested in alternative options, express concerns regarding antibiotic resistance, report a history of antibiotic-associated yeast infections or other side effects, and/or encounter issues related to drug-drug interactions.3,5-8 Oral hormonal therapies such as combination oral contraceptives (COCs) or spironolactone often are utilized to treat adult women with AV, sometimes in combination with each other or other agents. Combination oral contraceptives appear to be especially effective in the management of the U-shaped clinical pattern or predominantly inflammatory, late-onset AV.1,5,9,10 Potential warnings, contraindications, adverse effects, and drug-drug interactions are important to keep in mind when considering the use of oral hormonal therapies.8-10 Oral isotretinoin, which should be prescribed with strict adherence to the iPLEDGE™ program (https://www.ipledgeprogram.com/), remains a viable option for cases of severe nodular AV and selected cases of refractory inflammatory AV, especially when scarring and/or marked psychosocial distress are noted.1,2,5,11 Although it is recognized that adult women with AV typically present with either a mixed inflammatory and comedonal or U-shaped clinical pattern predominantly involving the lower face and anterolateral neck, the available data do not adequately differentiate the relative responsiveness of these clinical patterns to specific therapeutic agents.
Combination Oral Contraceptives
Combination oral contraceptives are commonly used to treat AV in adult women, including those without and those with measurable androgen excess (eg, polycystic ovary syndrome [PCOS]). Combination oral contraceptives contain ethinyl estradiol and a progestational agent (eg, progestin); the latter varies in terms of its nonselective receptor interactions and the relative magnitude or absence of androgenic effects.10,12,13 Although some COCs are approved by the US Food and Drug Administration (FDA) for AV, there is little data available to determine the comparative efficacy among these and other COCs.10,14 When choosing a COC for treatment of AV, it is best to select an agent whose effectiveness is supported by evidence from clinical studies.10,15
Mechanisms of Action
The reported mechanisms of action for COCs include inhibition of ovarian androgen production and ovulation through gonadotropin suppression; upregulated synthesis of sex hormone–binding globulin, which decreases free testosterone levels through receptor binding; and inhibition of 5α-reductase (by some progestins), which reduces conversion of testosterone to dihydrotestosterone, the active derivative that induces androgenic effects at peripheral target tissues.10,13,16,17
Therapeutic Benefits
Use of COCs to treat AV in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception. Multiple monotherapy studies have demonstrated the efficacy of COCs in the treatment of AV on the face and trunk.4,10,12,15,17,18 It may take a minimum of 3 monthly cycles of use before acne lesion counts begin to appreciably decrease.12,15,19-21 Initiating COC therapy during menstruation ensures the absence of pregnancy. Combination oral contraceptives may be used with other topical and oral therapies for AV.2,3,9,10 Potential ancillary benefits of COCs include normalization of the menstrual cycle; reduced premenstrual dysphoric disorder symptoms; and reduced risk of endometrial cancer (approximately 50%), ovarian cancer (approximately 40%), and colorectal cancer.22-24
Risks and Contraindications
It is important to consider the potential risks associated with the use of COCs, especially in women with AV who are not seeking a method of contraception. Side effects of COCs can include nausea, breast tenderness, breakthrough bleeding, and weight gain.25,26 Potential adverse associations of COCs are described in the Table. The major potential vascular associations include venous thromboembolism, myocardial infarction, and cerebrovascular accident, all of which are influenced by concurrent factors such as a history of smoking, age (≥35 years), and hypertension.27-32 It is recommended that blood pressure be measured before initiating COC therapy as part of the general examination.33

The potential increase in breast cancer risk appears to be low, while the cervical cancer risk is reported to increase relative to the duration of use.34-37 This latter observation may be due to the greater likelihood of unprotected sex in women using a COC and exposure to multiple sexual partners in some cases, which may increase the likelihood of oncogenic human papillomavirus infection of the cervix. If a dermatologist elects to prescribe a COC to treat AV, it has been suggested that the patient also consult with her general practitioner or gynecologist to undergo pelvic and breast examinations and a Papanicolaou test.33 The recommendation for initial screening for cervical cancer is within 3 years of initiation of sexual intercourse or by 21 years of age, whichever is first.33,38,39
Combination oral contraceptives are not ideal for all adult women with AV. Absolute contraindications are pregnancy and history of thromboembolic, cardiac, or hepatic disease; in women aged 35 years and older who smoke, relative contraindications include hypertension, diabetes, migraines, breastfeeding, and current breast or liver cancer.33 In adult women with AV who have relative contra-indications but are likely to benefit from the use of a COC when other options are limited or not viable, consultation with a gynecologist is prudent. Other than rifamycin antibiotics (eg, rifampin) and griseofulvin, there is no definitive evidence that oral antibiotics (eg, tetracycline) or oral antifungal agents reduce the contraceptive efficacy of COCs, although cautions remain in print within some approved package inserts.8
Spironolactone
Available since 1957, spironolactone is an oral aldos-terone antagonist and potassium-sparing diuretic used to treat hypertension and congestive heart failure.9 Recognition of its antiandrogenic effects led to its use in dermatology to treat certain dermatologic disorders in women (eg, hirsutism, alopecia, AV).1,4,5,9,10 Spironolactone is not approved for AV by the FDA; therefore, available data from multiple independent studies and retrospective analyses that have been collectively reviewed support its efficacy when used as both monotherapy or in combination with other agents in adult women with AV, especially those with a U-shaped pattern and/or late-onset AV.9,40-43
Mechanism of Action
Spironolactone inhibits sebaceous gland activity through peripheral androgen receptor blockade, inhibition of 5α-reductase, decrease in androgen production, and increase in sex hormone–binding globulin.9,10,40
Therapeutic Benefits
Good to excellent improvement of AV in women, many of whom are postadolescent, has ranged from 66% to 100% in published reports9,40-43; however, inclusion and exclusion criteria, dosing regimens, and concomitant therapies were not usually controlled. Spironolactone has been used to treat AV in adult women as monotherapy or in combination with topical agents, oral antibiotics, and COCs.9,40-42 Additionally, dose-ranging studies have not been completed with spironolactone for AV.9,40 The suggested dose range is 50 mg to 200 mg daily; however, it usually is best to start at 50 mg daily and increase to 100 mg daily if clinical response is not adequate after 2 to 3 months. The gastrointestinal (GI) absorption of spironolactone is increased when ingested with a high-fat meal.9,10
Once effective control of AV is achieved, it is optimal to use the lowest dose needed to continue reasonable suppression of new AV lesions. There is no defined end point for spironolactone use in AV, with or without concurrent PCOS, as many adult women usually continue treatment with low-dose therapy because they experience marked flaring shortly after the drug is stopped.9
Risks and Contraindications
Side effects associated with spironolactone are dose related and include increased diuresis, migraines, menstrual irregularities, breast tenderness, gynecomastia, fatigue, and dizziness.9,10,40-44 Side effects (particularly menstrual irregularities and breast tenderness) are more common at doses higher than 100 mg daily, especially when used as monotherapy without concurrent use of a COC.9,40
Spironolactone-associated hyperkalemia is most clinically relevant in patients on higher doses (eg, 100–200 mg daily), in those with renal impairment and/or congestive heart failure, and when used concurrently with certain other medications. In any patient on spironolactone, the risk of clinically relevant hyperkalemia may be increased by coingestion of potassium supplements, potassium-based salt substitutes, potassium-sparing diuretics (eg, amiloride, triamterene); aldosterone antagonists and angiotensin-converting enzyme inhibitors (eg, lisinopril, benazepril); angiotensin II receptor blockers (eg, losartan, valsartan); and tri-methoprim (with or without sulfamethoxazole).8,9,40,45 Spironolactone may also increase serum levels of lithium or digoxin.9,40,45,46 For management of AV, it is best that spironolactone be avoided in patients taking any of these medications.9
In healthy adult women with AV who are not on medications or supplements that interact adversely with spironolactone, there is no definitive recommendation regarding monitoring of serum potassium levels during treatment with spironolactone, and it has been suggested that monitoring serum potassium levels in this subgroup is not necessary.47 However, each clinician is advised to choose whether or not they wish to obtain baseline and/or periodic serum potassium levels when prescribing spironolactone for AV based on their degree of comfort and the patient’s history. Baseline and periodic blood testing to evaluate serum electrolytes and renal function are reasonable, especially as adult women with AV are usually treated with spironolactone over a prolonged period of time.9
The FDA black box warning for spironolactone states that it is tumorigenic in chronic toxicity studies in rats and refers to exposures 25- to 100-fold higher than those administered to humans.9,48 Although continued vigilance is warranted, evaluation of large populations of women treated with spironolactone do not suggest an association with increased risk of breast cancer.49,50
Spironolactone is a category C drug and thus should be avoided during pregnancy, primarily due to animal data suggesting risks of hypospadias and feminization in male fetuses.9 Importantly, there is an absence of reports linking exposure during pregnancy with congenital defects in humans, including in 2 known cases of high-dose exposures for maternal Bartter syndrome.9
The active metabolite, canrenone, is known to be present in breast milk at 0.2% of the maternal daily dose, but breastfeeding is generally believed to be safe with spironolactone based on evidence to date.9
Oral Antibiotics
Oral antibiotic therapy may be used in combination with a topical regimen to treat AV in adult women, keeping in mind some important caveats.1-7 For instance, monotherapy with oral antibiotics should be avoided, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.3,4 A therapeutic exit plan also is suggested when prescribing oral antibiotics to limit treatment to 3 to 4 months, if possible, to help mitigate the emergence of antibiotic-resistant bacteria (eg, staphylococci and streptococci).3-5,51
Tetracyclines, especially doxycycline and minocycline, are the most commonly prescribed agents. Doxycycline use warrants patient education on measures to limit the risks of esophageal and GI side effects and phototoxicity; enteric-coated and small tablet formulations have been shown to reduce GI side effects, especially when administered with food.3,52-55 In addition to vestibular side effects and hyperpigmentation, minocycline may be associated with rare but potentially severe adverse reactions such as drug hypersensitivity syndrome, autoimmune hepatitis, and lupus-like syndrome, which are reported more commonly in women.5,52,54 Vestibular side effects have been shown to decrease with use of extended-release tablets with weight-based dosing.53
Oral Isotretinoin
Oral isotretinoin is well established as highly effective for treatment of severe, recalcitrant AV, including nodular acne on the face and trunk.4,56 Currently available oral isotretinoins are branded generic formulations based on the pharmacokinetic profile of the original brand (Accutane [Roche Pharmaceuticals]) and with the use of Lidose Technology (Absorica [Cipher Pharmaceuticals]), which substantially increases GI absorption of isotretinoin in the absence of ingestion with a high-calorie, high-fat meal.57 The short- and long-term efficacy, dosing regimens, safety considerations, and serious teratogenic risks for oral isotretinoin are well published.4,56-58 Importantly, oral isotretinoin must be prescribed with strict adherence to the federally mandated iPLEDGE risk management program.
Low-dose oral isotretinoin therapy (<0.5 mg/kg–1 mg/kg daily) administered over several months longer than conventional regimens (ie, 16–20 weeks) has been suggested with demonstrated efficacy.57 However, this approach is not optimal due to the lack of established sustained clearance of AV after discontinuation of therapy and the greater potential for exposure to isotretinoin during pregnancy. Recurrences of AV do occur after completion of isotretinoin therapy, especially if cumulative systemic exposure to the drug during the initial course of treatment was inadequate.56,57
Oral isotretinoin has been shown to be effective in AV in adult women with or without PCOS with 0.5 mg/kg to 1 mg/kg daily and a total cumulative exposure of 120 mg/kg to 150 mg/kg.59 In one study, the presence of PCOS and greater number of nodules at baseline were predictive of a higher risk of relapse during the second year posttreatment.59
Conclusion
All oral therapies that are used to treat AV in adult women warrant individual consideration of possible benefits versus risks. Careful attention to possible side effects, patient-related risk factors, and potential drug-drug interactions is important. End points of therapy are not well established, with the exception of oral isotretinoin therapy. Clinicians must use their judgment in each case along with obtaining feedback from patients regarding the selection of therapy after a discussion of the available options.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
- Del Rosso JQ, Kim G. Optimizing use of oral antibiotics in acne vulgaris. Dermatol Clin. 2009;27:33-42.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:125-133.
- Del Rosso JQ. Oral antibiotic drug interactions of clinical significance to dermatologists. Dermatol Clin. 2009;27:91-94.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
- American Academy of Dermatology. Position statement on isotretinoin. AAD Web site. https://www.aad.org /Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Updated November 13, 2010. Accessed October 28, 2015.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. June 2012;7:CD004425.
- Sitruk-Ware R. Pharmacology of different progestogens: the special case of drospirenone. Climacteric. 2005;8 (suppl 3):4-12.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for the treatment of acne. Cochrane Database Syst Rev. July 2012;7:CD004425.
- Thiboutot D, Archer DF, Lemay A, et al. A randomized, controlled trial of a low-dose contraceptive containing 20 microg of ethinyl estradiol and 100 microg of levonogestrel for acne treatment. Fertil Steril. 2001;76:461-468.
- Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
- Rabe T, Kowald A, Ortmann J, et al. Inhibition of skin 5-alpha reductase by oral contraceptive progestins in vitro. Gynecol Endocrinol. 2000;14:223-230.
- Palli MB, Reyes-Habito CM, Lima XT, et al. A single-center, randomized double-blind, parallel-group study to examine the safety and efficacy of 3mg drospirenone/0.02mg ethinyl estradiol compared with placebo in the treatment of moderate truncal acne vulgaris. J Drugs Dermatol. 2013;12:633-637.
- Koltun W, Maloney JM, Marr J, et al. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 μg plus drospirenone 3 mg administered in a 24/4 regimen: a pooled analysis. Eur J Obstet Gynecol Reprod Biol. 2011;155:171-175.
- Maloney JM, Dietze P, Watson D, et al. A randomized controlled trial of a low-dose combined oral contraceptive containing 3 mg drospirenone plus 20 μg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self-assessment. J Drugs Dermatol. 2009;8:837-844.
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-μg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Maguire K, Westhoff C. The state of hormonal contraception today: established and emerging noncontraceptive health benefits. Am J Obstet Gynecol. 2011;205 (suppl 4):S4-S8.
- Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980;302:551-554.
- Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol. 2013;68:1022-1029.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008;4:CD003987.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
- Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119:1039-1044.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ. 2011;342:d2151.
- US Food and Drug Administration Office of Surveillance and Epidemiology. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs /Drug Safety/UCM277384.pdf. Accessed October 28, 2015.
- The American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
- World Health Organization. Cardiovascular Disease and Steroid Hormone Contraception: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1998. Technical Report Series 877.
- Frangos JE, Alavian CN, Kimball AB. Acne and oral contraceptives: update on women’s health screening guidelines. J Am Acad Dermatol. 2008;58:781-786.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931-1943.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609-1621.
- Agostino H, Di Meglio G. Low-dose oral contraceptives in adolescents: how low can you go? J Pediatr Adolesc Gynecol. 2010;23:195-201.
- Buzney E, Sheu J, Buzney C, et al. Polycystic ovary syndrome: a review for dermatologists: part II. Treatment. J Am Acad Dermatol. 2014;71:859.e1-859.e15.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- Sawaya ME, Somani N. Antiandrogens and androgen inhibitors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:361-374.
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Sato K, Matsumoto D, Iizuka F, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in Asians. Aesth Plast Surg. 2006;30:689-694.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Stockley I. Antihypertensive drug interactions. In: Stockley I, ed. Drug Interactions. 5th ed. London, United Kingdom: Pharmaceutical Press; 1999:335-347.
- Antoniou T, Gomes T, Mamdani MM, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ. 2011;343:d5228.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Aldactone [package insert]. New York, NY: Pfizer Inc; 2008.
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:e4447.
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24:330-334.
- Kim S, Michaels BD, Kim GK, et al. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:61-97.
- Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: pharmacokinetic and pharmacodynamics perspectives. J Clin Aesthet Dermatol. 2011;4:40-47.
- Del Rosso JQ. Oral antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:113-124.
- Del Rosso JQ. Oral doxycycline in the management of acne vulgaris: current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. 2015;8:19-26.
- Osofsky MG, Strauss JS. Isotretinoin. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:134-145.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
- Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:252-268.
- Cakir GA, Erdogan FG, Gurler A. Isotretinoin treatment in nodulocystic acne with and without polycystic ovary syndrome: efficacy and determinants of relapse. Int J Dermatol. 2013;52:371-376.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
- Del Rosso JQ, Kim G. Optimizing use of oral antibiotics in acne vulgaris. Dermatol Clin. 2009;27:33-42.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:125-133.
- Del Rosso JQ. Oral antibiotic drug interactions of clinical significance to dermatologists. Dermatol Clin. 2009;27:91-94.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
- American Academy of Dermatology. Position statement on isotretinoin. AAD Web site. https://www.aad.org /Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Updated November 13, 2010. Accessed October 28, 2015.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. June 2012;7:CD004425.
- Sitruk-Ware R. Pharmacology of different progestogens: the special case of drospirenone. Climacteric. 2005;8 (suppl 3):4-12.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for the treatment of acne. Cochrane Database Syst Rev. July 2012;7:CD004425.
- Thiboutot D, Archer DF, Lemay A, et al. A randomized, controlled trial of a low-dose contraceptive containing 20 microg of ethinyl estradiol and 100 microg of levonogestrel for acne treatment. Fertil Steril. 2001;76:461-468.
- Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
- Rabe T, Kowald A, Ortmann J, et al. Inhibition of skin 5-alpha reductase by oral contraceptive progestins in vitro. Gynecol Endocrinol. 2000;14:223-230.
- Palli MB, Reyes-Habito CM, Lima XT, et al. A single-center, randomized double-blind, parallel-group study to examine the safety and efficacy of 3mg drospirenone/0.02mg ethinyl estradiol compared with placebo in the treatment of moderate truncal acne vulgaris. J Drugs Dermatol. 2013;12:633-637.
- Koltun W, Maloney JM, Marr J, et al. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 μg plus drospirenone 3 mg administered in a 24/4 regimen: a pooled analysis. Eur J Obstet Gynecol Reprod Biol. 2011;155:171-175.
- Maloney JM, Dietze P, Watson D, et al. A randomized controlled trial of a low-dose combined oral contraceptive containing 3 mg drospirenone plus 20 μg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self-assessment. J Drugs Dermatol. 2009;8:837-844.
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-μg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Maguire K, Westhoff C. The state of hormonal contraception today: established and emerging noncontraceptive health benefits. Am J Obstet Gynecol. 2011;205 (suppl 4):S4-S8.
- Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980;302:551-554.
- Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol. 2013;68:1022-1029.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008;4:CD003987.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
- Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119:1039-1044.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ. 2011;342:d2151.
- US Food and Drug Administration Office of Surveillance and Epidemiology. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs /Drug Safety/UCM277384.pdf. Accessed October 28, 2015.
- The American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
- World Health Organization. Cardiovascular Disease and Steroid Hormone Contraception: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1998. Technical Report Series 877.
- Frangos JE, Alavian CN, Kimball AB. Acne and oral contraceptives: update on women’s health screening guidelines. J Am Acad Dermatol. 2008;58:781-786.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931-1943.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609-1621.
- Agostino H, Di Meglio G. Low-dose oral contraceptives in adolescents: how low can you go? J Pediatr Adolesc Gynecol. 2010;23:195-201.
- Buzney E, Sheu J, Buzney C, et al. Polycystic ovary syndrome: a review for dermatologists: part II. Treatment. J Am Acad Dermatol. 2014;71:859.e1-859.e15.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- Sawaya ME, Somani N. Antiandrogens and androgen inhibitors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:361-374.
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Sato K, Matsumoto D, Iizuka F, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in Asians. Aesth Plast Surg. 2006;30:689-694.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Stockley I. Antihypertensive drug interactions. In: Stockley I, ed. Drug Interactions. 5th ed. London, United Kingdom: Pharmaceutical Press; 1999:335-347.
- Antoniou T, Gomes T, Mamdani MM, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ. 2011;343:d5228.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Aldactone [package insert]. New York, NY: Pfizer Inc; 2008.
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:e4447.
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24:330-334.
- Kim S, Michaels BD, Kim GK, et al. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:61-97.
- Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: pharmacokinetic and pharmacodynamics perspectives. J Clin Aesthet Dermatol. 2011;4:40-47.
- Del Rosso JQ. Oral antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:113-124.
- Del Rosso JQ. Oral doxycycline in the management of acne vulgaris: current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. 2015;8:19-26.
- Osofsky MG, Strauss JS. Isotretinoin. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:134-145.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
- Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:252-268.
- Cakir GA, Erdogan FG, Gurler A. Isotretinoin treatment in nodulocystic acne with and without polycystic ovary syndrome: efficacy and determinants of relapse. Int J Dermatol. 2013;52:371-376.
Practice Points
- Use of combination oral contraceptives to treat acne vulgaris (AV) in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception.
- Spironolactone is widely accepted as an oral agent that can be effective in treating adult women with AV and may be used in combination with other therapies.
- Monotherapy with oral antibiotics should be avoided in the treatment of adult women with AV, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.
- Oral isotretinoin use in adult women with AV warrants strict adherence to pregnancy prevention measures and requirements set forth by the federally mandated iPLEDGE™ risk management program.