User login
CDC Issues Guidelines on Antiviral Use for Influenza
Children younger than 1 year of age may be given oseltamivir for influenza treatment and prophylaxis despite the expiration of the Food and Drug Administration’s emergency authorization allowing use of the drug in that age group. The Centers for Disease Control and Prevention issued new guidelines Jan. 21.
In an interview, the CDC’s Dr. Tim Uyeki said, "ACIP [the Advisory Committee on Immunization Practices] and CDC are recommending use of oseltamivir for treatment or chemoprophylaxis in children less than 1 year of age with suspected or confirmed influenza, because of the high risk for complications – including serious complications – in children less than 1 year of age, as well as the fact that the 2009 H1N1 virus continues to circulate worldwide including in the U.S."
The FDA issued its emergency use authorization during the 2009-2010 pandemic of influenza A(H1N1). The authorization expired in June 2010.
While encouraging the use of the neuraminidase inhibitors oseltamivir (Tamiflu) and zanamivir (Relenza), the new guidelines emphasize that the antivirals amantadine (Symmetrel) and rimantadine (Flumadine) should not be used for influenza. Those drugs are inactive against influenza B, and the currently circulating strains of influenza A have developed resistance.
In another significant change, the guidelines now emphasize that it’s permissible to treat individuals with influenza who are at low risk of complications with oseltamivir and zanamivir.
"We never said, ‘Don’t treat persons who are not hospitalized and not high risk,’ " said Dr. Uyeki, a pediatrician and medical epidemiologist. "The emphasis on high-risk patients and hospitalized patients might have been interpreted as, ‘Don’t treat persons with mild, uncomplicated illness who were previously healthy.’ "
The guidelines encourage physicians to rely on their clinical judgment in making treatment decisions regarding patients with suspected or confirmed influenza. Knowledge of the locally prevalent influenza strains as well as local patterns of antiviral resistance should inform that judgment.
In other influenza news:
• The CDC reports in its latest update that eight children have died from influenza in the United States so far this season. In comparison, there were 282 pediatric deaths during the full 2009-2010 season and 133 during the 2008-2009 season. The CDC continues to find no evidence of resistance to oseltamivir and zanamivir by any influenza strain currently circulating. In the week ending Jan. 8, 2010, 11 states were reporting widespread influenza activity (Alabama, Arizona, Connecticut, Kentucky, Louisiana, Maryland, Nevada, New York, North Carolina, Tennessee, and Virginia). Another 17 states were reporting regional influenza activity (Colorado, Florida, Georgia, Illinois, Indiana, Kansas, Maine, Massachusetts, Mississippi, Missouri, New Hampshire, New Jersey, Ohio, Oklahoma, Pennsylvania, South Carolina, and Texas). The remaining states were reporting only local or sporadic influenza activity.
• In its latest influenza update, the World Health Organization reports that influenza cases are continuing to increase in North America, and that the primary strain is influenza A(H3N2). In the United Kingdom, severe and fatal cases of influenza A(H1N1) have increased compared with 2 weeks ago, and 25% of all intensive care beds are occupied by influenza patients. Severe disease associated with influenza A(H1N1) and, to a lesser extent, influenza B are also increasing throughout the European continent and the Middle East. On the other hand, tropical countries throughout the world and temperate countries in the Southern Hemisphere are reporting very little influenza circulation.
Children younger than 1 year of age may be given oseltamivir for influenza treatment and prophylaxis despite the expiration of the Food and Drug Administration’s emergency authorization allowing use of the drug in that age group. The Centers for Disease Control and Prevention issued new guidelines Jan. 21.
In an interview, the CDC’s Dr. Tim Uyeki said, "ACIP [the Advisory Committee on Immunization Practices] and CDC are recommending use of oseltamivir for treatment or chemoprophylaxis in children less than 1 year of age with suspected or confirmed influenza, because of the high risk for complications – including serious complications – in children less than 1 year of age, as well as the fact that the 2009 H1N1 virus continues to circulate worldwide including in the U.S."
The FDA issued its emergency use authorization during the 2009-2010 pandemic of influenza A(H1N1). The authorization expired in June 2010.
While encouraging the use of the neuraminidase inhibitors oseltamivir (Tamiflu) and zanamivir (Relenza), the new guidelines emphasize that the antivirals amantadine (Symmetrel) and rimantadine (Flumadine) should not be used for influenza. Those drugs are inactive against influenza B, and the currently circulating strains of influenza A have developed resistance.
In another significant change, the guidelines now emphasize that it’s permissible to treat individuals with influenza who are at low risk of complications with oseltamivir and zanamivir.
"We never said, ‘Don’t treat persons who are not hospitalized and not high risk,’ " said Dr. Uyeki, a pediatrician and medical epidemiologist. "The emphasis on high-risk patients and hospitalized patients might have been interpreted as, ‘Don’t treat persons with mild, uncomplicated illness who were previously healthy.’ "
The guidelines encourage physicians to rely on their clinical judgment in making treatment decisions regarding patients with suspected or confirmed influenza. Knowledge of the locally prevalent influenza strains as well as local patterns of antiviral resistance should inform that judgment.
In other influenza news:
• The CDC reports in its latest update that eight children have died from influenza in the United States so far this season. In comparison, there were 282 pediatric deaths during the full 2009-2010 season and 133 during the 2008-2009 season. The CDC continues to find no evidence of resistance to oseltamivir and zanamivir by any influenza strain currently circulating. In the week ending Jan. 8, 2010, 11 states were reporting widespread influenza activity (Alabama, Arizona, Connecticut, Kentucky, Louisiana, Maryland, Nevada, New York, North Carolina, Tennessee, and Virginia). Another 17 states were reporting regional influenza activity (Colorado, Florida, Georgia, Illinois, Indiana, Kansas, Maine, Massachusetts, Mississippi, Missouri, New Hampshire, New Jersey, Ohio, Oklahoma, Pennsylvania, South Carolina, and Texas). The remaining states were reporting only local or sporadic influenza activity.
• In its latest influenza update, the World Health Organization reports that influenza cases are continuing to increase in North America, and that the primary strain is influenza A(H3N2). In the United Kingdom, severe and fatal cases of influenza A(H1N1) have increased compared with 2 weeks ago, and 25% of all intensive care beds are occupied by influenza patients. Severe disease associated with influenza A(H1N1) and, to a lesser extent, influenza B are also increasing throughout the European continent and the Middle East. On the other hand, tropical countries throughout the world and temperate countries in the Southern Hemisphere are reporting very little influenza circulation.
Children younger than 1 year of age may be given oseltamivir for influenza treatment and prophylaxis despite the expiration of the Food and Drug Administration’s emergency authorization allowing use of the drug in that age group. The Centers for Disease Control and Prevention issued new guidelines Jan. 21.
In an interview, the CDC’s Dr. Tim Uyeki said, "ACIP [the Advisory Committee on Immunization Practices] and CDC are recommending use of oseltamivir for treatment or chemoprophylaxis in children less than 1 year of age with suspected or confirmed influenza, because of the high risk for complications – including serious complications – in children less than 1 year of age, as well as the fact that the 2009 H1N1 virus continues to circulate worldwide including in the U.S."
The FDA issued its emergency use authorization during the 2009-2010 pandemic of influenza A(H1N1). The authorization expired in June 2010.
While encouraging the use of the neuraminidase inhibitors oseltamivir (Tamiflu) and zanamivir (Relenza), the new guidelines emphasize that the antivirals amantadine (Symmetrel) and rimantadine (Flumadine) should not be used for influenza. Those drugs are inactive against influenza B, and the currently circulating strains of influenza A have developed resistance.
In another significant change, the guidelines now emphasize that it’s permissible to treat individuals with influenza who are at low risk of complications with oseltamivir and zanamivir.
"We never said, ‘Don’t treat persons who are not hospitalized and not high risk,’ " said Dr. Uyeki, a pediatrician and medical epidemiologist. "The emphasis on high-risk patients and hospitalized patients might have been interpreted as, ‘Don’t treat persons with mild, uncomplicated illness who were previously healthy.’ "
The guidelines encourage physicians to rely on their clinical judgment in making treatment decisions regarding patients with suspected or confirmed influenza. Knowledge of the locally prevalent influenza strains as well as local patterns of antiviral resistance should inform that judgment.
In other influenza news:
• The CDC reports in its latest update that eight children have died from influenza in the United States so far this season. In comparison, there were 282 pediatric deaths during the full 2009-2010 season and 133 during the 2008-2009 season. The CDC continues to find no evidence of resistance to oseltamivir and zanamivir by any influenza strain currently circulating. In the week ending Jan. 8, 2010, 11 states were reporting widespread influenza activity (Alabama, Arizona, Connecticut, Kentucky, Louisiana, Maryland, Nevada, New York, North Carolina, Tennessee, and Virginia). Another 17 states were reporting regional influenza activity (Colorado, Florida, Georgia, Illinois, Indiana, Kansas, Maine, Massachusetts, Mississippi, Missouri, New Hampshire, New Jersey, Ohio, Oklahoma, Pennsylvania, South Carolina, and Texas). The remaining states were reporting only local or sporadic influenza activity.
• In its latest influenza update, the World Health Organization reports that influenza cases are continuing to increase in North America, and that the primary strain is influenza A(H3N2). In the United Kingdom, severe and fatal cases of influenza A(H1N1) have increased compared with 2 weeks ago, and 25% of all intensive care beds are occupied by influenza patients. Severe disease associated with influenza A(H1N1) and, to a lesser extent, influenza B are also increasing throughout the European continent and the Middle East. On the other hand, tropical countries throughout the world and temperate countries in the Southern Hemisphere are reporting very little influenza circulation.
FDA Approves New Head Lice Treatment
The Food and Drug administration has approved a new topical treatment for head lice in children and adults.
Natroba Topical Suspension (spinosad 0.9%), proved more effective than Nix (permethrin 1%) when compared directly in clinical trials. In two trials involving a total of 1,038 children and adults, after one or two applications of spinosad, 85% and 87% of patients were lice free, compared with 45% and 43% of patients receiving permethrin (Pediatrics 2009;124:e389-95).
Another advantage of spinosad, according to its labeling, is that it doesn’t require combing to be effective. A single, 10-minute application of spinosad is followed by a warm-water rinse. If desired, a fine tooth comb can be used to remove dead lice and nits from the hair. A second application is permissible if the patient continues to harbor live lice 7 days later.
Investigators noted no serious adverse events in the trials, and only a small number of mild to moderate adverse events. The most common were application site erythema (seen in 6.8% of the patients given spinosad), ocular hyperemia (in 3.3% of patients), and application site irritation (in 1.5% of patients).
Spinosad works by causing neuronal excitation in insects. After a period of hyperexcitation, lice become paralyzed and die.
The FDA approval covered the use of spinosad in adults and children aged 4 years and older. The agency said that it is important not to use spinosad in infants younger than age 6 months. The product contains benzyl alcohol, which can cause serious reactions and even death in infants.
The clinical trials reported in Pediatrics were sponsored by ParaPRO, the manufacturer of Natroba. Two of the coauthors received research funding from ParaPRO, and two others served as consultants to the company.
Natroba Topical Suspension, spinosad 0.9%, Nix permethrin 1%, spinosad, nits,
The Food and Drug administration has approved a new topical treatment for head lice in children and adults.
Natroba Topical Suspension (spinosad 0.9%), proved more effective than Nix (permethrin 1%) when compared directly in clinical trials. In two trials involving a total of 1,038 children and adults, after one or two applications of spinosad, 85% and 87% of patients were lice free, compared with 45% and 43% of patients receiving permethrin (Pediatrics 2009;124:e389-95).
Another advantage of spinosad, according to its labeling, is that it doesn’t require combing to be effective. A single, 10-minute application of spinosad is followed by a warm-water rinse. If desired, a fine tooth comb can be used to remove dead lice and nits from the hair. A second application is permissible if the patient continues to harbor live lice 7 days later.
Investigators noted no serious adverse events in the trials, and only a small number of mild to moderate adverse events. The most common were application site erythema (seen in 6.8% of the patients given spinosad), ocular hyperemia (in 3.3% of patients), and application site irritation (in 1.5% of patients).
Spinosad works by causing neuronal excitation in insects. After a period of hyperexcitation, lice become paralyzed and die.
The FDA approval covered the use of spinosad in adults and children aged 4 years and older. The agency said that it is important not to use spinosad in infants younger than age 6 months. The product contains benzyl alcohol, which can cause serious reactions and even death in infants.
The clinical trials reported in Pediatrics were sponsored by ParaPRO, the manufacturer of Natroba. Two of the coauthors received research funding from ParaPRO, and two others served as consultants to the company.
The Food and Drug administration has approved a new topical treatment for head lice in children and adults.
Natroba Topical Suspension (spinosad 0.9%), proved more effective than Nix (permethrin 1%) when compared directly in clinical trials. In two trials involving a total of 1,038 children and adults, after one or two applications of spinosad, 85% and 87% of patients were lice free, compared with 45% and 43% of patients receiving permethrin (Pediatrics 2009;124:e389-95).
Another advantage of spinosad, according to its labeling, is that it doesn’t require combing to be effective. A single, 10-minute application of spinosad is followed by a warm-water rinse. If desired, a fine tooth comb can be used to remove dead lice and nits from the hair. A second application is permissible if the patient continues to harbor live lice 7 days later.
Investigators noted no serious adverse events in the trials, and only a small number of mild to moderate adverse events. The most common were application site erythema (seen in 6.8% of the patients given spinosad), ocular hyperemia (in 3.3% of patients), and application site irritation (in 1.5% of patients).
Spinosad works by causing neuronal excitation in insects. After a period of hyperexcitation, lice become paralyzed and die.
The FDA approval covered the use of spinosad in adults and children aged 4 years and older. The agency said that it is important not to use spinosad in infants younger than age 6 months. The product contains benzyl alcohol, which can cause serious reactions and even death in infants.
The clinical trials reported in Pediatrics were sponsored by ParaPRO, the manufacturer of Natroba. Two of the coauthors received research funding from ParaPRO, and two others served as consultants to the company.
Natroba Topical Suspension, spinosad 0.9%, Nix permethrin 1%, spinosad, nits,
Natroba Topical Suspension, spinosad 0.9%, Nix permethrin 1%, spinosad, nits,
FROM THE FOOD AND DRUG ADMINISTRATION
FDA Announces Voluntary Recall of Hydrocodone Bitartrate/Acetaminophen and Phenobarbital Products
The Food and Drug Administration announced Feb. 5 a voluntary, nationwide recall of certain lots of hydrocodone bitartrate and acetaminophen tablets and also certain lots of phenobarbital tablets. The tablets were manufactured by Qualitest Pharmaceuticals, a subsidiary of Endo Pharmaceuticals.
A single bottle of hydrocodone bitartrate and acetaminophen tablets was incorrectly labeled as phenobarbital. As a result, the company is recalling the following products:
• Hydrocodone bitartrate and acetaminophen tablets (USP 10 mg/500 mg, NDC 0603-3888-20, 60 count). The affected lot numbers are T150G10B, T120J10E, and T023M10A.
• Phenobarbital tablets (USP 32.4 mg, NDC 0603-5166-32, 1,000 count). The affected lot numbers are T150G10B, T120J10E, and T023M10A.
The company distributed the lots widely throughout the United States, including Puerto Rico, between Sept. 21 and Dec. 29, 2010.
No injuries have been reported.
The Food and Drug Administration announced Feb. 5 a voluntary, nationwide recall of certain lots of hydrocodone bitartrate and acetaminophen tablets and also certain lots of phenobarbital tablets. The tablets were manufactured by Qualitest Pharmaceuticals, a subsidiary of Endo Pharmaceuticals.
A single bottle of hydrocodone bitartrate and acetaminophen tablets was incorrectly labeled as phenobarbital. As a result, the company is recalling the following products:
• Hydrocodone bitartrate and acetaminophen tablets (USP 10 mg/500 mg, NDC 0603-3888-20, 60 count). The affected lot numbers are T150G10B, T120J10E, and T023M10A.
• Phenobarbital tablets (USP 32.4 mg, NDC 0603-5166-32, 1,000 count). The affected lot numbers are T150G10B, T120J10E, and T023M10A.
The company distributed the lots widely throughout the United States, including Puerto Rico, between Sept. 21 and Dec. 29, 2010.
No injuries have been reported.
The Food and Drug Administration announced Feb. 5 a voluntary, nationwide recall of certain lots of hydrocodone bitartrate and acetaminophen tablets and also certain lots of phenobarbital tablets. The tablets were manufactured by Qualitest Pharmaceuticals, a subsidiary of Endo Pharmaceuticals.
A single bottle of hydrocodone bitartrate and acetaminophen tablets was incorrectly labeled as phenobarbital. As a result, the company is recalling the following products:
• Hydrocodone bitartrate and acetaminophen tablets (USP 10 mg/500 mg, NDC 0603-3888-20, 60 count). The affected lot numbers are T150G10B, T120J10E, and T023M10A.
• Phenobarbital tablets (USP 32.4 mg, NDC 0603-5166-32, 1,000 count). The affected lot numbers are T150G10B, T120J10E, and T023M10A.
The company distributed the lots widely throughout the United States, including Puerto Rico, between Sept. 21 and Dec. 29, 2010.
No injuries have been reported.
FDA Announces Voluntary Recall of Hydrocodone Bitartrate/Acetaminophen and Phenobarbital Products
The Food and Drug Administration announced Feb. 5 a voluntary, nationwide recall of certain lots of hydrocodone bitartrate and acetaminophen tablets and also certain lots of phenobarbital tablets. The tablets were manufactured by Qualitest Pharmaceuticals, a subsidiary of Endo Pharmaceuticals.
A single bottle of hydrocodone bitartrate and acetaminophen tablets was incorrectly labeled as phenobarbital. As a result, the company is recalling the following products:
• Hydrocodone bitartrate and acetaminophen tablets (USP 10 mg/500 mg, NDC 0603-3888-20, 60 count). The affected lot numbers are T150G10B, T120J10E, and T023M10A.
• Phenobarbital tablets (USP 32.4 mg, NDC 0603-5166-32, 1,000 count). The affected lot numbers are T150G10B, T120J10E, and T023M10A.
The company distributed the lots widely throughout the United States, including Puerto Rico, between Sept. 21 and Dec. 29, 2010.
No injuries have been reported.
The Food and Drug Administration announced Feb. 5 a voluntary, nationwide recall of certain lots of hydrocodone bitartrate and acetaminophen tablets and also certain lots of phenobarbital tablets. The tablets were manufactured by Qualitest Pharmaceuticals, a subsidiary of Endo Pharmaceuticals.
A single bottle of hydrocodone bitartrate and acetaminophen tablets was incorrectly labeled as phenobarbital. As a result, the company is recalling the following products:
• Hydrocodone bitartrate and acetaminophen tablets (USP 10 mg/500 mg, NDC 0603-3888-20, 60 count). The affected lot numbers are T150G10B, T120J10E, and T023M10A.
• Phenobarbital tablets (USP 32.4 mg, NDC 0603-5166-32, 1,000 count). The affected lot numbers are T150G10B, T120J10E, and T023M10A.
The company distributed the lots widely throughout the United States, including Puerto Rico, between Sept. 21 and Dec. 29, 2010.
No injuries have been reported.
The Food and Drug Administration announced Feb. 5 a voluntary, nationwide recall of certain lots of hydrocodone bitartrate and acetaminophen tablets and also certain lots of phenobarbital tablets. The tablets were manufactured by Qualitest Pharmaceuticals, a subsidiary of Endo Pharmaceuticals.
A single bottle of hydrocodone bitartrate and acetaminophen tablets was incorrectly labeled as phenobarbital. As a result, the company is recalling the following products:
• Hydrocodone bitartrate and acetaminophen tablets (USP 10 mg/500 mg, NDC 0603-3888-20, 60 count). The affected lot numbers are T150G10B, T120J10E, and T023M10A.
• Phenobarbital tablets (USP 32.4 mg, NDC 0603-5166-32, 1,000 count). The affected lot numbers are T150G10B, T120J10E, and T023M10A.
The company distributed the lots widely throughout the United States, including Puerto Rico, between Sept. 21 and Dec. 29, 2010.
No injuries have been reported.
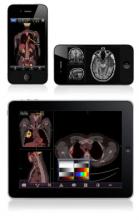
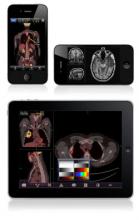
FDA Clears iPhone/iPad App
The Food and Drug Administration on Feb. 4 gave clearance to an application for Apple’s iPad and iPhone that will allow physicians to review radiology images in the absence of a standard workstation.
The FDA cleared the app, named Mobile MIM, for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and nuclear medicine technology such as positron emission tomography.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image’s luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine whether he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site that the Mobile MIM should be available in Apple’s App Store the week of Feb. 7.
The Food and Drug Administration on Feb. 4 gave clearance to an application for Apple’s iPad and iPhone that will allow physicians to review radiology images in the absence of a standard workstation.
The FDA cleared the app, named Mobile MIM, for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and nuclear medicine technology such as positron emission tomography.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image’s luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine whether he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site that the Mobile MIM should be available in Apple’s App Store the week of Feb. 7.
The Food and Drug Administration on Feb. 4 gave clearance to an application for Apple’s iPad and iPhone that will allow physicians to review radiology images in the absence of a standard workstation.
The FDA cleared the app, named Mobile MIM, for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and nuclear medicine technology such as positron emission tomography.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image’s luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine whether he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site that the Mobile MIM should be available in Apple’s App Store the week of Feb. 7.
FROM THE FOOD AND DRUG ADMINISTRATION
FDA Clears iPhone/iPad App
The Food and Drug Administration on Feb. 4 gave clearance to an application for Apple’s iPad and iPhone that will allow physicians to review radiology images in the absence of a standard workstation.
The FDA cleared the app, named Mobile MIM, for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and nuclear medicine technology such as positron emission tomography.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image’s luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine whether he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site that the Mobile MIM should be available in Apple’s App Store the week of Feb. 7.
The Food and Drug Administration on Feb. 4 gave clearance to an application for Apple’s iPad and iPhone that will allow physicians to review radiology images in the absence of a standard workstation.
The FDA cleared the app, named Mobile MIM, for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and nuclear medicine technology such as positron emission tomography.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image’s luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine whether he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site that the Mobile MIM should be available in Apple’s App Store the week of Feb. 7.
The Food and Drug Administration on Feb. 4 gave clearance to an application for Apple’s iPad and iPhone that will allow physicians to review radiology images in the absence of a standard workstation.
The FDA cleared the app, named Mobile MIM, for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and nuclear medicine technology such as positron emission tomography.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image’s luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine whether he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site that the Mobile MIM should be available in Apple’s App Store the week of Feb. 7.
FROM THE FOOD AND DRUG ADMINISTRATION
This Week in Influenza
Almost 46% of the pediatric patients hospitalized with influenza during the 2010-2011 flu season so far and 17% of the hospitalized adult patients had no known underlying medical conditions, according to data reported by the Centers for Disease Control and Prevention.
Among pediatric hospitalized patients, the most common underlying conditions were asthma (seen in 20.6% of cases), cardiovascular disease, (6.1%), metabolic disorder (6.1%), neurologic disease (4.6%), and immune suppression (3.8%). Among adult hospitalized patients metabolic disorder was the most common underlying condition (seen in 32.2% of patients) followed by cardiovascular disease (27.8%), asthma (20.3%), chronic lung disease (18.6%), immune suppression (16.5%), and renal disease (16.5%).
In other influenza news:
• During the third week of 2011, 25 states reported widespread influenza outbreaks and another 16 reported regional outbreaks to the CDC. Four states and the District of Columbia reported local outbreaks. Another four states plus Puerto Rico and the U.S. Virgin Islands reported sporadic outbreaks. Of all the states and territories, only Guam reported that it had no influenza activity. (California did not report its influenza activity during this period.)
• In a study published Jan. 31 in PLoS ONE, a team of researchers from the Food and Drug Administration demonstrated that during the 2009-2010 influenza season, the trivalent seasonal flu vaccine provided a moderate amount of protection against 2009 pandemic influenza A(H1N1). As judged by hemagglutination inhibition assays (HAI), 24% of adults and 36% of elderly subjects who received only the seasonal flu vaccine achieved a seroprotective HAI titer against one of the main strains of pandemic influenza A(H1N1), compared with 4% and 7%, respectively, before vaccination. "A moderate boost on cross-reactive response to a novel influenza virus by seasonal vaccination may not lead to a complete protection against a pandemic, but might reduce the burden of infections substantially in affected subjects," the investigators wrote. "Ours and others’ studies suggest that annual seasonal vaccination plays an important role in protecting the public not only against seasonal flu but also a pandemic"(PLoS ONE 2011;6:e16650 [doi:10.1371/journal.pone.0016650]).
• A study of an H1N1 pandemic that began in an elementary school in April and May of 2009 and spread to a semirural community in Pennsylvania allowed investigators to quantify the role that social networks play in the spread of respiratory disease. The investigators – from Imperial College London, the CDC, and the Pennsylvania Department of Health – determined that sitting next to or being a playmate of a child who developed influenza did not increase the risk of transmission. However, the division of children into classes and grades did significantly affect the spread of the virus. Boys were more likely to spread the virus to other boys, and girls were more likely to spread the virus to other girls. The study was published online Jan. 31 (Proc. Natl. Acad. Sci. 2011 [doi:10.1073/pnas.1008895108]).
• According to reports by Russia’s Itar-Tass news agency, authorities have closed more than 1,500 public and private schools serving grades 1-8 in Moscow for a full week beginning Jan. 31 because of a widespread influenza outbreak. During the last week in January, about 91,000 individuals in Russia’s capital were on sick leave from influenza and other respiratory illnesses, with children making up more than 50% of that number.
Almost 46% of the pediatric patients hospitalized with influenza during the 2010-2011 flu season so far and 17% of the hospitalized adult patients had no known underlying medical conditions, according to data reported by the Centers for Disease Control and Prevention.
Among pediatric hospitalized patients, the most common underlying conditions were asthma (seen in 20.6% of cases), cardiovascular disease, (6.1%), metabolic disorder (6.1%), neurologic disease (4.6%), and immune suppression (3.8%). Among adult hospitalized patients metabolic disorder was the most common underlying condition (seen in 32.2% of patients) followed by cardiovascular disease (27.8%), asthma (20.3%), chronic lung disease (18.6%), immune suppression (16.5%), and renal disease (16.5%).
In other influenza news:
• During the third week of 2011, 25 states reported widespread influenza outbreaks and another 16 reported regional outbreaks to the CDC. Four states and the District of Columbia reported local outbreaks. Another four states plus Puerto Rico and the U.S. Virgin Islands reported sporadic outbreaks. Of all the states and territories, only Guam reported that it had no influenza activity. (California did not report its influenza activity during this period.)
• In a study published Jan. 31 in PLoS ONE, a team of researchers from the Food and Drug Administration demonstrated that during the 2009-2010 influenza season, the trivalent seasonal flu vaccine provided a moderate amount of protection against 2009 pandemic influenza A(H1N1). As judged by hemagglutination inhibition assays (HAI), 24% of adults and 36% of elderly subjects who received only the seasonal flu vaccine achieved a seroprotective HAI titer against one of the main strains of pandemic influenza A(H1N1), compared with 4% and 7%, respectively, before vaccination. "A moderate boost on cross-reactive response to a novel influenza virus by seasonal vaccination may not lead to a complete protection against a pandemic, but might reduce the burden of infections substantially in affected subjects," the investigators wrote. "Ours and others’ studies suggest that annual seasonal vaccination plays an important role in protecting the public not only against seasonal flu but also a pandemic"(PLoS ONE 2011;6:e16650 [doi:10.1371/journal.pone.0016650]).
• A study of an H1N1 pandemic that began in an elementary school in April and May of 2009 and spread to a semirural community in Pennsylvania allowed investigators to quantify the role that social networks play in the spread of respiratory disease. The investigators – from Imperial College London, the CDC, and the Pennsylvania Department of Health – determined that sitting next to or being a playmate of a child who developed influenza did not increase the risk of transmission. However, the division of children into classes and grades did significantly affect the spread of the virus. Boys were more likely to spread the virus to other boys, and girls were more likely to spread the virus to other girls. The study was published online Jan. 31 (Proc. Natl. Acad. Sci. 2011 [doi:10.1073/pnas.1008895108]).
• According to reports by Russia’s Itar-Tass news agency, authorities have closed more than 1,500 public and private schools serving grades 1-8 in Moscow for a full week beginning Jan. 31 because of a widespread influenza outbreak. During the last week in January, about 91,000 individuals in Russia’s capital were on sick leave from influenza and other respiratory illnesses, with children making up more than 50% of that number.
Almost 46% of the pediatric patients hospitalized with influenza during the 2010-2011 flu season so far and 17% of the hospitalized adult patients had no known underlying medical conditions, according to data reported by the Centers for Disease Control and Prevention.
Among pediatric hospitalized patients, the most common underlying conditions were asthma (seen in 20.6% of cases), cardiovascular disease, (6.1%), metabolic disorder (6.1%), neurologic disease (4.6%), and immune suppression (3.8%). Among adult hospitalized patients metabolic disorder was the most common underlying condition (seen in 32.2% of patients) followed by cardiovascular disease (27.8%), asthma (20.3%), chronic lung disease (18.6%), immune suppression (16.5%), and renal disease (16.5%).
In other influenza news:
• During the third week of 2011, 25 states reported widespread influenza outbreaks and another 16 reported regional outbreaks to the CDC. Four states and the District of Columbia reported local outbreaks. Another four states plus Puerto Rico and the U.S. Virgin Islands reported sporadic outbreaks. Of all the states and territories, only Guam reported that it had no influenza activity. (California did not report its influenza activity during this period.)
• In a study published Jan. 31 in PLoS ONE, a team of researchers from the Food and Drug Administration demonstrated that during the 2009-2010 influenza season, the trivalent seasonal flu vaccine provided a moderate amount of protection against 2009 pandemic influenza A(H1N1). As judged by hemagglutination inhibition assays (HAI), 24% of adults and 36% of elderly subjects who received only the seasonal flu vaccine achieved a seroprotective HAI titer against one of the main strains of pandemic influenza A(H1N1), compared with 4% and 7%, respectively, before vaccination. "A moderate boost on cross-reactive response to a novel influenza virus by seasonal vaccination may not lead to a complete protection against a pandemic, but might reduce the burden of infections substantially in affected subjects," the investigators wrote. "Ours and others’ studies suggest that annual seasonal vaccination plays an important role in protecting the public not only against seasonal flu but also a pandemic"(PLoS ONE 2011;6:e16650 [doi:10.1371/journal.pone.0016650]).
• A study of an H1N1 pandemic that began in an elementary school in April and May of 2009 and spread to a semirural community in Pennsylvania allowed investigators to quantify the role that social networks play in the spread of respiratory disease. The investigators – from Imperial College London, the CDC, and the Pennsylvania Department of Health – determined that sitting next to or being a playmate of a child who developed influenza did not increase the risk of transmission. However, the division of children into classes and grades did significantly affect the spread of the virus. Boys were more likely to spread the virus to other boys, and girls were more likely to spread the virus to other girls. The study was published online Jan. 31 (Proc. Natl. Acad. Sci. 2011 [doi:10.1073/pnas.1008895108]).
• According to reports by Russia’s Itar-Tass news agency, authorities have closed more than 1,500 public and private schools serving grades 1-8 in Moscow for a full week beginning Jan. 31 because of a widespread influenza outbreak. During the last week in January, about 91,000 individuals in Russia’s capital were on sick leave from influenza and other respiratory illnesses, with children making up more than 50% of that number.
This Week in Influenza
Almost 46% of the pediatric patients hospitalized with influenza during the 2010-2011 flu season so far and 17% of the hospitalized adult patients had no known underlying medical conditions, according to data reported by the Centers for Disease Control and Prevention.
Among pediatric hospitalized patients, the most common underlying conditions were asthma (seen in 20.6% of cases), cardiovascular disease, (6.1%), metabolic disorder (6.1%), neurologic disease (4.6%), and immune suppression (3.8%). Among adult hospitalized patients metabolic disorder was the most common underlying condition (seen in 32.2% of patients) followed by cardiovascular disease (27.8%), asthma (20.3%), chronic lung disease (18.6%), immune suppression (16.5%), and renal disease (16.5%).
In other influenza news:
• During the third week of 2011, 25 states reported widespread influenza outbreaks and another 16 reported regional outbreaks to the CDC. Four states and the District of Columbia reported local outbreaks. Another four states plus Puerto Rico and the U.S. Virgin Islands reported sporadic outbreaks. Of all the states and territories, only Guam reported that it had no influenza activity. (California did not report its influenza activity during this period.)
• In a study published Jan. 31 in PLoS ONE, a team of researchers from the Food and Drug Administration demonstrated that during the 2009-2010 influenza season, the trivalent seasonal flu vaccine provided a moderate amount of protection against 2009 pandemic influenza A(H1N1). As judged by hemagglutination inhibition assays (HAI), 24% of adults and 36% of elderly subjects who received only the seasonal flu vaccine achieved a seroprotective HAI titer against one of the main strains of pandemic influenza A(H1N1), compared with 4% and 7%, respectively, before vaccination. "A moderate boost on cross-reactive response to a novel influenza virus by seasonal vaccination may not lead to a complete protection against a pandemic, but might reduce the burden of infections substantially in affected subjects," the investigators wrote. "Ours and others’ studies suggest that annual seasonal vaccination plays an important role in protecting the public not only against seasonal flu but also a pandemic"(PLoS ONE 2011;6:e16650 [doi:10.1371/journal.pone.0016650]).
• A study of an H1N1 pandemic that began in an elementary school in April and May of 2009 and spread to a semirural community in Pennsylvania allowed investigators to quantify the role that social networks play in the spread of respiratory disease. The investigators – from Imperial College London, the CDC, and the Pennsylvania Department of Health – determined that sitting next to or being a playmate of a child who developed influenza did not increase the risk of transmission. However, the division of children into classes and grades did significantly affect the spread of the virus. Boys were more likely to spread the virus to other boys, and girls were more likely to spread the virus to other girls. The study was published online Jan. 31 (Proc. Natl. Acad. Sci. 2011 [doi:10.1073/pnas.1008895108]).
• According to reports by Russia’s Itar-Tass news agency, authorities have closed more than 1,500 public and private schools serving grades 1-8 in Moscow for a full week beginning Jan. 31 because of a widespread influenza outbreak. During the last week in January, about 91,000 individuals in Russia’s capital were on sick leave from influenza and other respiratory illnesses, with children making up more than 50% of that number.
Almost 46% of the pediatric patients hospitalized with influenza during the 2010-2011 flu season so far and 17% of the hospitalized adult patients had no known underlying medical conditions, according to data reported by the Centers for Disease Control and Prevention.
Among pediatric hospitalized patients, the most common underlying conditions were asthma (seen in 20.6% of cases), cardiovascular disease, (6.1%), metabolic disorder (6.1%), neurologic disease (4.6%), and immune suppression (3.8%). Among adult hospitalized patients metabolic disorder was the most common underlying condition (seen in 32.2% of patients) followed by cardiovascular disease (27.8%), asthma (20.3%), chronic lung disease (18.6%), immune suppression (16.5%), and renal disease (16.5%).
In other influenza news:
• During the third week of 2011, 25 states reported widespread influenza outbreaks and another 16 reported regional outbreaks to the CDC. Four states and the District of Columbia reported local outbreaks. Another four states plus Puerto Rico and the U.S. Virgin Islands reported sporadic outbreaks. Of all the states and territories, only Guam reported that it had no influenza activity. (California did not report its influenza activity during this period.)
• In a study published Jan. 31 in PLoS ONE, a team of researchers from the Food and Drug Administration demonstrated that during the 2009-2010 influenza season, the trivalent seasonal flu vaccine provided a moderate amount of protection against 2009 pandemic influenza A(H1N1). As judged by hemagglutination inhibition assays (HAI), 24% of adults and 36% of elderly subjects who received only the seasonal flu vaccine achieved a seroprotective HAI titer against one of the main strains of pandemic influenza A(H1N1), compared with 4% and 7%, respectively, before vaccination. "A moderate boost on cross-reactive response to a novel influenza virus by seasonal vaccination may not lead to a complete protection against a pandemic, but might reduce the burden of infections substantially in affected subjects," the investigators wrote. "Ours and others’ studies suggest that annual seasonal vaccination plays an important role in protecting the public not only against seasonal flu but also a pandemic"(PLoS ONE 2011;6:e16650 [doi:10.1371/journal.pone.0016650]).
• A study of an H1N1 pandemic that began in an elementary school in April and May of 2009 and spread to a semirural community in Pennsylvania allowed investigators to quantify the role that social networks play in the spread of respiratory disease. The investigators – from Imperial College London, the CDC, and the Pennsylvania Department of Health – determined that sitting next to or being a playmate of a child who developed influenza did not increase the risk of transmission. However, the division of children into classes and grades did significantly affect the spread of the virus. Boys were more likely to spread the virus to other boys, and girls were more likely to spread the virus to other girls. The study was published online Jan. 31 (Proc. Natl. Acad. Sci. 2011 [doi:10.1073/pnas.1008895108]).
• According to reports by Russia’s Itar-Tass news agency, authorities have closed more than 1,500 public and private schools serving grades 1-8 in Moscow for a full week beginning Jan. 31 because of a widespread influenza outbreak. During the last week in January, about 91,000 individuals in Russia’s capital were on sick leave from influenza and other respiratory illnesses, with children making up more than 50% of that number.
Almost 46% of the pediatric patients hospitalized with influenza during the 2010-2011 flu season so far and 17% of the hospitalized adult patients had no known underlying medical conditions, according to data reported by the Centers for Disease Control and Prevention.
Among pediatric hospitalized patients, the most common underlying conditions were asthma (seen in 20.6% of cases), cardiovascular disease, (6.1%), metabolic disorder (6.1%), neurologic disease (4.6%), and immune suppression (3.8%). Among adult hospitalized patients metabolic disorder was the most common underlying condition (seen in 32.2% of patients) followed by cardiovascular disease (27.8%), asthma (20.3%), chronic lung disease (18.6%), immune suppression (16.5%), and renal disease (16.5%).
In other influenza news:
• During the third week of 2011, 25 states reported widespread influenza outbreaks and another 16 reported regional outbreaks to the CDC. Four states and the District of Columbia reported local outbreaks. Another four states plus Puerto Rico and the U.S. Virgin Islands reported sporadic outbreaks. Of all the states and territories, only Guam reported that it had no influenza activity. (California did not report its influenza activity during this period.)
• In a study published Jan. 31 in PLoS ONE, a team of researchers from the Food and Drug Administration demonstrated that during the 2009-2010 influenza season, the trivalent seasonal flu vaccine provided a moderate amount of protection against 2009 pandemic influenza A(H1N1). As judged by hemagglutination inhibition assays (HAI), 24% of adults and 36% of elderly subjects who received only the seasonal flu vaccine achieved a seroprotective HAI titer against one of the main strains of pandemic influenza A(H1N1), compared with 4% and 7%, respectively, before vaccination. "A moderate boost on cross-reactive response to a novel influenza virus by seasonal vaccination may not lead to a complete protection against a pandemic, but might reduce the burden of infections substantially in affected subjects," the investigators wrote. "Ours and others’ studies suggest that annual seasonal vaccination plays an important role in protecting the public not only against seasonal flu but also a pandemic"(PLoS ONE 2011;6:e16650 [doi:10.1371/journal.pone.0016650]).
• A study of an H1N1 pandemic that began in an elementary school in April and May of 2009 and spread to a semirural community in Pennsylvania allowed investigators to quantify the role that social networks play in the spread of respiratory disease. The investigators – from Imperial College London, the CDC, and the Pennsylvania Department of Health – determined that sitting next to or being a playmate of a child who developed influenza did not increase the risk of transmission. However, the division of children into classes and grades did significantly affect the spread of the virus. Boys were more likely to spread the virus to other boys, and girls were more likely to spread the virus to other girls. The study was published online Jan. 31 (Proc. Natl. Acad. Sci. 2011 [doi:10.1073/pnas.1008895108]).
• According to reports by Russia’s Itar-Tass news agency, authorities have closed more than 1,500 public and private schools serving grades 1-8 in Moscow for a full week beginning Jan. 31 because of a widespread influenza outbreak. During the last week in January, about 91,000 individuals in Russia’s capital were on sick leave from influenza and other respiratory illnesses, with children making up more than 50% of that number.
Birth Rate for U.S. Teens Falls to Lowest Level
The birth rate for U.S. teens aged 15-19 years fell to the lowest level since recording began in 1940, according to new data for 2009.
The 2009 teen birth rate was 39.1 births per 1,000 teens, down 6% from the 2008 rate of 41.5 births per 1,000, according to the report by the CDC National Center for Health Statistics. The 2009 rate was 37% lower than in 1991, the peak year for teen births. The CDC's annual report is based on virtually 100% of vital records collected in the 50 U.S. states, the District of Columbia, and U.S. territories. The report is available at www.cdc.gov/nchs
Overall fertility also fell in 2009 to 66.7 births per 1,000 women aged 15-44 years, compared with 68.6 per 1,000 women in 2008. The CDC's preliminary estimate of births in 2009 was 4,131,019, 3% less than 2008. Early data through June 2010 suggest that the decline in fertility has continued, according to the report.
Fertility rates increased in only one age group: women aged 40-44 years. In that group, the 2009 rate was 10.1 births per 1,000 women, up 3% from the 2008 figure and the highest rate since 1967.
The rate of preterm births declined for the third straight year, to 12.2% of all births in 2009. The rate of cesarean deliveries rose to a record high of 32.9% in 2009, up from 32.3% in 2008.
The low birth weight rate remained unchanged at about 8.2% between 2008 and 2009.
The CDC also reported the total fertility rate (TFR) – an estimate of the number of births that a hypothetical group of 1,000 women would have over their lifetimes, based on the age-specific rates of a particular year. The TFR for 2009 was 2,007.5, down 4% from the rate in 2008. This is the largest decline in TFR since 1973. The 2008 and 2009 rates were both below the replacement rate of 2,100 births per 1,000 women. The U.S. TFR was below replacement for every year between 1972 and 2005 and above replacement in 2006 and 2007.
The birth rate for U.S. teens aged 15-19 years fell to the lowest level since recording began in 1940, according to new data for 2009.
The 2009 teen birth rate was 39.1 births per 1,000 teens, down 6% from the 2008 rate of 41.5 births per 1,000, according to the report by the CDC National Center for Health Statistics. The 2009 rate was 37% lower than in 1991, the peak year for teen births. The CDC's annual report is based on virtually 100% of vital records collected in the 50 U.S. states, the District of Columbia, and U.S. territories. The report is available at www.cdc.gov/nchs
Overall fertility also fell in 2009 to 66.7 births per 1,000 women aged 15-44 years, compared with 68.6 per 1,000 women in 2008. The CDC's preliminary estimate of births in 2009 was 4,131,019, 3% less than 2008. Early data through June 2010 suggest that the decline in fertility has continued, according to the report.
Fertility rates increased in only one age group: women aged 40-44 years. In that group, the 2009 rate was 10.1 births per 1,000 women, up 3% from the 2008 figure and the highest rate since 1967.
The rate of preterm births declined for the third straight year, to 12.2% of all births in 2009. The rate of cesarean deliveries rose to a record high of 32.9% in 2009, up from 32.3% in 2008.
The low birth weight rate remained unchanged at about 8.2% between 2008 and 2009.
The CDC also reported the total fertility rate (TFR) – an estimate of the number of births that a hypothetical group of 1,000 women would have over their lifetimes, based on the age-specific rates of a particular year. The TFR for 2009 was 2,007.5, down 4% from the rate in 2008. This is the largest decline in TFR since 1973. The 2008 and 2009 rates were both below the replacement rate of 2,100 births per 1,000 women. The U.S. TFR was below replacement for every year between 1972 and 2005 and above replacement in 2006 and 2007.
The birth rate for U.S. teens aged 15-19 years fell to the lowest level since recording began in 1940, according to new data for 2009.
The 2009 teen birth rate was 39.1 births per 1,000 teens, down 6% from the 2008 rate of 41.5 births per 1,000, according to the report by the CDC National Center for Health Statistics. The 2009 rate was 37% lower than in 1991, the peak year for teen births. The CDC's annual report is based on virtually 100% of vital records collected in the 50 U.S. states, the District of Columbia, and U.S. territories. The report is available at www.cdc.gov/nchs
Overall fertility also fell in 2009 to 66.7 births per 1,000 women aged 15-44 years, compared with 68.6 per 1,000 women in 2008. The CDC's preliminary estimate of births in 2009 was 4,131,019, 3% less than 2008. Early data through June 2010 suggest that the decline in fertility has continued, according to the report.
Fertility rates increased in only one age group: women aged 40-44 years. In that group, the 2009 rate was 10.1 births per 1,000 women, up 3% from the 2008 figure and the highest rate since 1967.
The rate of preterm births declined for the third straight year, to 12.2% of all births in 2009. The rate of cesarean deliveries rose to a record high of 32.9% in 2009, up from 32.3% in 2008.
The low birth weight rate remained unchanged at about 8.2% between 2008 and 2009.
The CDC also reported the total fertility rate (TFR) – an estimate of the number of births that a hypothetical group of 1,000 women would have over their lifetimes, based on the age-specific rates of a particular year. The TFR for 2009 was 2,007.5, down 4% from the rate in 2008. This is the largest decline in TFR since 1973. The 2008 and 2009 rates were both below the replacement rate of 2,100 births per 1,000 women. The U.S. TFR was below replacement for every year between 1972 and 2005 and above replacement in 2006 and 2007.
From the Centers For Disease Control and Prevention
Daily Omega-3 Fatty Acids Benefit HF Patients
Twelve months of daily doses of omega-3 fatty acids resulted in substantial improvements in chronic heart failure, according to a randomized, placebo-controlled study of 133 patients with mild to moderate chronic heart failure caused by nonischemic dilated cardiomyopathy.
The study demonstrated improvements in left ventricular ejection fraction, peak oxygen uptake (VO2), exercise duration, and New York Heart Association functional class among patients taking about 5 g of omega-3 polyunsaturated fatty acids (PUFAs) daily for 1 month followed by another 11 months of 2-g daily doses (J. Am. Coll. Cardiol. 2011;57 [doi:10.1016/j.jacc.2010.11.017]).
“These beneficial effects suggest that omega-3 PUFAs may favorably affect cardiac remodeling and the decline of myocardial function in patients with [heart failure (HF)] and may account for the reduction in cardiovascular hospitalizations and hospitalizations for HF observed in our study,” wrote Dr. Savina Nodari of the University of Brescia [Italy], and colleagues. Whether omega-3 PUFAs exert similar effects in patients with other types of HF or with more advanced HF remains to be verified, they added.
The study was funded by the University of Brescia, Brescia, Italy. One of the study's coauthors (Dr. Mihai Gheorghiade of Northwestern University, Chicago) acknowledged consulting for, and receiving travel funds from, a number of pharmaceutical and device manufacturers. The other coauthors stated that they had no conflicts.
Twelve months of daily doses of omega-3 fatty acids resulted in substantial improvements in chronic heart failure, according to a randomized, placebo-controlled study of 133 patients with mild to moderate chronic heart failure caused by nonischemic dilated cardiomyopathy.
The study demonstrated improvements in left ventricular ejection fraction, peak oxygen uptake (VO2), exercise duration, and New York Heart Association functional class among patients taking about 5 g of omega-3 polyunsaturated fatty acids (PUFAs) daily for 1 month followed by another 11 months of 2-g daily doses (J. Am. Coll. Cardiol. 2011;57 [doi:10.1016/j.jacc.2010.11.017]).
“These beneficial effects suggest that omega-3 PUFAs may favorably affect cardiac remodeling and the decline of myocardial function in patients with [heart failure (HF)] and may account for the reduction in cardiovascular hospitalizations and hospitalizations for HF observed in our study,” wrote Dr. Savina Nodari of the University of Brescia [Italy], and colleagues. Whether omega-3 PUFAs exert similar effects in patients with other types of HF or with more advanced HF remains to be verified, they added.
The study was funded by the University of Brescia, Brescia, Italy. One of the study's coauthors (Dr. Mihai Gheorghiade of Northwestern University, Chicago) acknowledged consulting for, and receiving travel funds from, a number of pharmaceutical and device manufacturers. The other coauthors stated that they had no conflicts.
Twelve months of daily doses of omega-3 fatty acids resulted in substantial improvements in chronic heart failure, according to a randomized, placebo-controlled study of 133 patients with mild to moderate chronic heart failure caused by nonischemic dilated cardiomyopathy.
The study demonstrated improvements in left ventricular ejection fraction, peak oxygen uptake (VO2), exercise duration, and New York Heart Association functional class among patients taking about 5 g of omega-3 polyunsaturated fatty acids (PUFAs) daily for 1 month followed by another 11 months of 2-g daily doses (J. Am. Coll. Cardiol. 2011;57 [doi:10.1016/j.jacc.2010.11.017]).
“These beneficial effects suggest that omega-3 PUFAs may favorably affect cardiac remodeling and the decline of myocardial function in patients with [heart failure (HF)] and may account for the reduction in cardiovascular hospitalizations and hospitalizations for HF observed in our study,” wrote Dr. Savina Nodari of the University of Brescia [Italy], and colleagues. Whether omega-3 PUFAs exert similar effects in patients with other types of HF or with more advanced HF remains to be verified, they added.
The study was funded by the University of Brescia, Brescia, Italy. One of the study's coauthors (Dr. Mihai Gheorghiade of Northwestern University, Chicago) acknowledged consulting for, and receiving travel funds from, a number of pharmaceutical and device manufacturers. The other coauthors stated that they had no conflicts.