User login
A survey of liability claims against obstetric providers highlights major areas of contention
An analysis of 882 obstetric claims closed between 2007 and 2014 highlighted 3 common patient allegations:
- a delay in treatment of fetal distress (22%). The term fetal distress remains a common allegation in malpractice claims. Cases in this category most often reflected a delay or failure to act in the face of Category II or III fetal heart-rate tracings.
- improper performance of vaginal delivery (20%). Almost half of the cases in this category involved brachial plexus injuries linked to shoulder dystocia. Patients alleged that improper maneuvers were used to resolve the dystocia. The remainder of cases in this category involved forceps and vacuum extraction deliveries.
- improper management of pregnancy (17%). Among the allegations were a failure to test for fetal abnormalities, failure to recognize complications of pregnancy, and failure to address abnormal findings.
Together, these 3 allegations accounted for 59% of claims. Other allegations included diagnosis-related claims, delay in delivery, improper performance of operative delivery, retained foreign bodies, and improper choice of delivery method.1
The Obstetrics Closed Claims Study findings were released earlier this spring by the Napa, California−based Doctors Company, the nation’s largest physician-owned medical malpractice insurer.1 Susan Mann, MD, a spokesperson for the company, provided expert commentary on the study at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco. (Listen to this accompanying audiocast featuring her comments.) Dr. Mann practices obstetrics and gynecology in Brookline, Massachusetts, and at Beth Israel Deaconess Medical Center in Boston. She is president of the QualBridge Institute, a consulting firm focused on issues of quality and safety.
Top 7 factors contributing to patient injury
The Doctors Company identified specific factors that contributed to patient injury in the closed claims:
1. Selection and management of therapy (34%). Among the issues here were decisions involving augmentation of labor, route of delivery, and the timing of interventions. This factor also related to medications—for example, a failure to order antibiotics for Group A and Group B strep, a failure to order Rho(D) immune globulin for Rh-negative mothers, and a failure to provide magnesium sulfate for women with eclampsia.
2. Patient-assessment issues (32%). The Doctors Company reviewers found that physicians frequently failed to consider information that was available, or overlooked abnormal findings.
3. Technical performance (18%). This factor involved problems associated with known risks of various procedures, such as postpartum hemorrhage and brachial plexus injuries. It also included poor technique.
4. Communication among providers (17%)
5. Patient factors (16%). These factors included a failure to comply with therapy or to show up for appointments.
6. Insufficient or lack of documentation (14%)
7. Communication between patient/family and provider (14%).
“Studying obstetrical medical malpractice claims sheds light on the wide array of problems that may arise during pregnancy and in labor and delivery,” the study authors conclude. “Many of these cases reflect unusual maternal or neonatal conditions that can be diagnosed only with vigilance. Examples include protein deficiencies, clotting abnormalities, placental abruptions, infections, and genetic abnormalities. More common conditions should be identified with close attention to vital signs, laboratory studies, changes to maternal and neonatal conditions, and patient complaints.”
“Obstetric departments must plan for clinical emergencies by developing and maintaining physician and staff competencies through mock drills and simulations that reduce the likelihood of injuries to mothers and their infants,” the study authors conclude.
Tips for reducing malpractice claims in obstetrics
The Obstetrics Closed Claim Study identified a number of “underlying vulnerabilities” that place patients at risk and increase liability for clinicians. The Doctors Company offers the following tips to help reduce these claims:
• Require periodic training and certification for physicians and nurses to maintain competency and facilitate conversations about fetal heart-rate (FHR) tracing interpretation. Both parties should use the same terminology when discussing the strips.
• Use technology that allows physicians to review FHR patterns from remote locations so that physicians and nurses are able to see the same information when discussing next steps.
• When operative vaginal delivery is attempted in the face of a Category III FHR tracing, a contingency team should be available for possible emergent cesarean delivery.
• Foster a culture in which caregivers feel comfortable speaking up if they have a concern. Ensure that the organization has a well-defined escalation guideline.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- The Doctors Company. Obstetrics Closed Claim Study. http://www.thedoctors.com/KnowledgeCenter/PatientSafety/articles/CON_ID_011803. Published April 2015. Accessed May 6, 2015.
An analysis of 882 obstetric claims closed between 2007 and 2014 highlighted 3 common patient allegations:
- a delay in treatment of fetal distress (22%). The term fetal distress remains a common allegation in malpractice claims. Cases in this category most often reflected a delay or failure to act in the face of Category II or III fetal heart-rate tracings.
- improper performance of vaginal delivery (20%). Almost half of the cases in this category involved brachial plexus injuries linked to shoulder dystocia. Patients alleged that improper maneuvers were used to resolve the dystocia. The remainder of cases in this category involved forceps and vacuum extraction deliveries.
- improper management of pregnancy (17%). Among the allegations were a failure to test for fetal abnormalities, failure to recognize complications of pregnancy, and failure to address abnormal findings.
Together, these 3 allegations accounted for 59% of claims. Other allegations included diagnosis-related claims, delay in delivery, improper performance of operative delivery, retained foreign bodies, and improper choice of delivery method.1
The Obstetrics Closed Claims Study findings were released earlier this spring by the Napa, California−based Doctors Company, the nation’s largest physician-owned medical malpractice insurer.1 Susan Mann, MD, a spokesperson for the company, provided expert commentary on the study at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco. (Listen to this accompanying audiocast featuring her comments.) Dr. Mann practices obstetrics and gynecology in Brookline, Massachusetts, and at Beth Israel Deaconess Medical Center in Boston. She is president of the QualBridge Institute, a consulting firm focused on issues of quality and safety.
Top 7 factors contributing to patient injury
The Doctors Company identified specific factors that contributed to patient injury in the closed claims:
1. Selection and management of therapy (34%). Among the issues here were decisions involving augmentation of labor, route of delivery, and the timing of interventions. This factor also related to medications—for example, a failure to order antibiotics for Group A and Group B strep, a failure to order Rho(D) immune globulin for Rh-negative mothers, and a failure to provide magnesium sulfate for women with eclampsia.
2. Patient-assessment issues (32%). The Doctors Company reviewers found that physicians frequently failed to consider information that was available, or overlooked abnormal findings.
3. Technical performance (18%). This factor involved problems associated with known risks of various procedures, such as postpartum hemorrhage and brachial plexus injuries. It also included poor technique.
4. Communication among providers (17%)
5. Patient factors (16%). These factors included a failure to comply with therapy or to show up for appointments.
6. Insufficient or lack of documentation (14%)
7. Communication between patient/family and provider (14%).
“Studying obstetrical medical malpractice claims sheds light on the wide array of problems that may arise during pregnancy and in labor and delivery,” the study authors conclude. “Many of these cases reflect unusual maternal or neonatal conditions that can be diagnosed only with vigilance. Examples include protein deficiencies, clotting abnormalities, placental abruptions, infections, and genetic abnormalities. More common conditions should be identified with close attention to vital signs, laboratory studies, changes to maternal and neonatal conditions, and patient complaints.”
“Obstetric departments must plan for clinical emergencies by developing and maintaining physician and staff competencies through mock drills and simulations that reduce the likelihood of injuries to mothers and their infants,” the study authors conclude.
Tips for reducing malpractice claims in obstetrics
The Obstetrics Closed Claim Study identified a number of “underlying vulnerabilities” that place patients at risk and increase liability for clinicians. The Doctors Company offers the following tips to help reduce these claims:
• Require periodic training and certification for physicians and nurses to maintain competency and facilitate conversations about fetal heart-rate (FHR) tracing interpretation. Both parties should use the same terminology when discussing the strips.
• Use technology that allows physicians to review FHR patterns from remote locations so that physicians and nurses are able to see the same information when discussing next steps.
• When operative vaginal delivery is attempted in the face of a Category III FHR tracing, a contingency team should be available for possible emergent cesarean delivery.
• Foster a culture in which caregivers feel comfortable speaking up if they have a concern. Ensure that the organization has a well-defined escalation guideline.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
An analysis of 882 obstetric claims closed between 2007 and 2014 highlighted 3 common patient allegations:
- a delay in treatment of fetal distress (22%). The term fetal distress remains a common allegation in malpractice claims. Cases in this category most often reflected a delay or failure to act in the face of Category II or III fetal heart-rate tracings.
- improper performance of vaginal delivery (20%). Almost half of the cases in this category involved brachial plexus injuries linked to shoulder dystocia. Patients alleged that improper maneuvers were used to resolve the dystocia. The remainder of cases in this category involved forceps and vacuum extraction deliveries.
- improper management of pregnancy (17%). Among the allegations were a failure to test for fetal abnormalities, failure to recognize complications of pregnancy, and failure to address abnormal findings.
Together, these 3 allegations accounted for 59% of claims. Other allegations included diagnosis-related claims, delay in delivery, improper performance of operative delivery, retained foreign bodies, and improper choice of delivery method.1
The Obstetrics Closed Claims Study findings were released earlier this spring by the Napa, California−based Doctors Company, the nation’s largest physician-owned medical malpractice insurer.1 Susan Mann, MD, a spokesperson for the company, provided expert commentary on the study at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco. (Listen to this accompanying audiocast featuring her comments.) Dr. Mann practices obstetrics and gynecology in Brookline, Massachusetts, and at Beth Israel Deaconess Medical Center in Boston. She is president of the QualBridge Institute, a consulting firm focused on issues of quality and safety.
Top 7 factors contributing to patient injury
The Doctors Company identified specific factors that contributed to patient injury in the closed claims:
1. Selection and management of therapy (34%). Among the issues here were decisions involving augmentation of labor, route of delivery, and the timing of interventions. This factor also related to medications—for example, a failure to order antibiotics for Group A and Group B strep, a failure to order Rho(D) immune globulin for Rh-negative mothers, and a failure to provide magnesium sulfate for women with eclampsia.
2. Patient-assessment issues (32%). The Doctors Company reviewers found that physicians frequently failed to consider information that was available, or overlooked abnormal findings.
3. Technical performance (18%). This factor involved problems associated with known risks of various procedures, such as postpartum hemorrhage and brachial plexus injuries. It also included poor technique.
4. Communication among providers (17%)
5. Patient factors (16%). These factors included a failure to comply with therapy or to show up for appointments.
6. Insufficient or lack of documentation (14%)
7. Communication between patient/family and provider (14%).
“Studying obstetrical medical malpractice claims sheds light on the wide array of problems that may arise during pregnancy and in labor and delivery,” the study authors conclude. “Many of these cases reflect unusual maternal or neonatal conditions that can be diagnosed only with vigilance. Examples include protein deficiencies, clotting abnormalities, placental abruptions, infections, and genetic abnormalities. More common conditions should be identified with close attention to vital signs, laboratory studies, changes to maternal and neonatal conditions, and patient complaints.”
“Obstetric departments must plan for clinical emergencies by developing and maintaining physician and staff competencies through mock drills and simulations that reduce the likelihood of injuries to mothers and their infants,” the study authors conclude.
Tips for reducing malpractice claims in obstetrics
The Obstetrics Closed Claim Study identified a number of “underlying vulnerabilities” that place patients at risk and increase liability for clinicians. The Doctors Company offers the following tips to help reduce these claims:
• Require periodic training and certification for physicians and nurses to maintain competency and facilitate conversations about fetal heart-rate (FHR) tracing interpretation. Both parties should use the same terminology when discussing the strips.
• Use technology that allows physicians to review FHR patterns from remote locations so that physicians and nurses are able to see the same information when discussing next steps.
• When operative vaginal delivery is attempted in the face of a Category III FHR tracing, a contingency team should be available for possible emergent cesarean delivery.
• Foster a culture in which caregivers feel comfortable speaking up if they have a concern. Ensure that the organization has a well-defined escalation guideline.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- The Doctors Company. Obstetrics Closed Claim Study. http://www.thedoctors.com/KnowledgeCenter/PatientSafety/articles/CON_ID_011803. Published April 2015. Accessed May 6, 2015.
Reference
- The Doctors Company. Obstetrics Closed Claim Study. http://www.thedoctors.com/KnowledgeCenter/PatientSafety/articles/CON_ID_011803. Published April 2015. Accessed May 6, 2015.
Mammographic breast density is a strong risk factor for breast cancer
Breast density is a strong, prevalent, and potentially modifiable risk factor for breast cancer, which makes it of special interest to clinicians whose jobs involve breast cancer risk prediction. That was the theme of a talk by Karla Kerlikowske, MD, of the UCSF Helen Diller Family Comprehensive Cancer Center in San Francisco. Dr. Kerlikowske delivered the John I. Brewer Memorial Lecture May 3 at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco.
Mammographic breast density is a radiologic term, Dr. Kerlikowske explained. “The only way to really know someone’s breast density is if they have a mammogram.” The whiter the mammogram, the denser the breast. The darker the mammogram, the fattier the breast.
According to the American College of Radiology, the following 4 categories of breast composition are defined by the “visually estimated” content of fibroglandular-density tissue within the breasts:
A. The breasts are almost entirely fatty.
B. There are scattered areas of fibroglandular density.
C. The breasts are heterogeneously dense, which may obscure small masses.
D. The breasts are extremely dense, which lowers the specificity of mammography.
Categories C and D signify dense breasts, which contain a high degree of collagen, epithelial cells, and stroma. In the United States, more than 25 million women are thought to have dense breasts.
Women who have a family history of breast cancer are more likely to have dense breasts. And women who have dense breasts have an elevated risk of breast cancer. They also have a higher risk of advanced disease, as well as a higher risk of large, high-grade, and lymph node-positive tumors, said Dr. Kerlikowske.
Breast-density legislation is increasing
Twenty-two states now have laws mandating that women found to have heterogeneously dense or extremely dense breasts be notified of their status, said Dr. Kerlikowske. That prompts the question: How should these patients be managed?
Breast density declines with age. Breast density also is influenced by body mass index (BMI). As BMI increases, density declines.
Breast density also can be affected by medications, such as hormone therapy and tamoxifen, Dr. Kerlikowske said.
For example, breast density declines about 1% to 2% per year in postmenopause. In postmenopausal women who take estrogen alone, breast density increases slightly. “But the real increase is for people who take estrogen plus progestin,” said Dr. Kerlikowske. “It’s thought that the progestin component is what increases breast density.” Estrogen-progestin therapy confers the same risk of breast cancer as that faced by a premenopausal woman with dense breasts.
As for tamoxifen, it reduces breast density by 2% to 3% per year in postmenopausal women, Dr. Kerlikowske said. “People who have a decrease of more than 10% in breast density are those who have a reduction in breast cancer.” If a woman doesn’t have that reduction with tamoxifen—about half of women don’t—there is no reduction in breast cancer mortality.
“There’s some thought that you should look at mammograms during the first year of tamoxifen use and, if you don’t see a change, consider switching to another medication,” said Dr. Kerlikowske.
More frequent mammograms and supplemental imaging are options for detecting cancers early. Among the modalities that have been studied in this regard are ultrasonography, tomosynthesis, and breast magnetic resonance imaging (MRI).
“If you do more tests, such as ultrasound, you will definitely find additional lesions,” said Dr. Kerlikowske. “There’s no question. But what are the harms?”
The biopsy rate almost doubles after ultrasonography, compared with mammography. And the number needed to screen to detect cancer is fairly high. For mammography, that number is about 250. For ultrasonography, tomosynthesis, and breast MRI, it is higher.
Tomosynthesis is more cost-effective than supplemental ultrasonography because it decreases the number of false positives, Dr. Kerlikowske said.
What’s the bottom line?
Not every woman with dense breasts is at high risk for breast cancer, said Dr. Kerlikowske. And although breast density is prevalent, it is potentially modifiable.
Nevertheless, breast density confers an elevated risk of breast cancer and can also mask tumors. Women with dense breasts likely should avoid the use of postmenopausal hormone therapy. They also may be candidates for more frequent mammography and/or supplemental imaging.
The Breast Cancer Surveillance Consortium (BCSC) Risk Calculator is the only tool that incorporates breast density. In the works is a new model that also incorporates benign breast disease.
Risk-prediction tool considers density and other factors
A risk-prediction tool from the Breast Cancer Surveillance Consortium (BCSC) is the only model to incorporate breast density. The BCSC Risk Calculator is available free of charge for the iPhone and iPad (an Android version is in the works). The tool takes 5 factors into consideration in estimating a woman’s 5-year risk of developing invasive breast cancer:
• age
• race/ethnicity
• breast density
• family history of breast cancer (first-degree relative)
• personal history of breast biopsy.
The tool is designed for use by health professionals. It is not appropriate for determining risk in women younger than 35 years or older than 79 years; women with a previous diagnosis of breast cancer, lobular carcinoma in situ, ductal carcinoma in situ, or atypical ductal hyperplasia; or women who have undergone breast augmentation. Other risk-prediction models are more appropriate for women with a BRCA mutation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Breast density is a strong, prevalent, and potentially modifiable risk factor for breast cancer, which makes it of special interest to clinicians whose jobs involve breast cancer risk prediction. That was the theme of a talk by Karla Kerlikowske, MD, of the UCSF Helen Diller Family Comprehensive Cancer Center in San Francisco. Dr. Kerlikowske delivered the John I. Brewer Memorial Lecture May 3 at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco.
Mammographic breast density is a radiologic term, Dr. Kerlikowske explained. “The only way to really know someone’s breast density is if they have a mammogram.” The whiter the mammogram, the denser the breast. The darker the mammogram, the fattier the breast.
According to the American College of Radiology, the following 4 categories of breast composition are defined by the “visually estimated” content of fibroglandular-density tissue within the breasts:
A. The breasts are almost entirely fatty.
B. There are scattered areas of fibroglandular density.
C. The breasts are heterogeneously dense, which may obscure small masses.
D. The breasts are extremely dense, which lowers the specificity of mammography.
Categories C and D signify dense breasts, which contain a high degree of collagen, epithelial cells, and stroma. In the United States, more than 25 million women are thought to have dense breasts.
Women who have a family history of breast cancer are more likely to have dense breasts. And women who have dense breasts have an elevated risk of breast cancer. They also have a higher risk of advanced disease, as well as a higher risk of large, high-grade, and lymph node-positive tumors, said Dr. Kerlikowske.
Breast-density legislation is increasing
Twenty-two states now have laws mandating that women found to have heterogeneously dense or extremely dense breasts be notified of their status, said Dr. Kerlikowske. That prompts the question: How should these patients be managed?
Breast density declines with age. Breast density also is influenced by body mass index (BMI). As BMI increases, density declines.
Breast density also can be affected by medications, such as hormone therapy and tamoxifen, Dr. Kerlikowske said.
For example, breast density declines about 1% to 2% per year in postmenopause. In postmenopausal women who take estrogen alone, breast density increases slightly. “But the real increase is for people who take estrogen plus progestin,” said Dr. Kerlikowske. “It’s thought that the progestin component is what increases breast density.” Estrogen-progestin therapy confers the same risk of breast cancer as that faced by a premenopausal woman with dense breasts.
As for tamoxifen, it reduces breast density by 2% to 3% per year in postmenopausal women, Dr. Kerlikowske said. “People who have a decrease of more than 10% in breast density are those who have a reduction in breast cancer.” If a woman doesn’t have that reduction with tamoxifen—about half of women don’t—there is no reduction in breast cancer mortality.
“There’s some thought that you should look at mammograms during the first year of tamoxifen use and, if you don’t see a change, consider switching to another medication,” said Dr. Kerlikowske.
More frequent mammograms and supplemental imaging are options for detecting cancers early. Among the modalities that have been studied in this regard are ultrasonography, tomosynthesis, and breast magnetic resonance imaging (MRI).
“If you do more tests, such as ultrasound, you will definitely find additional lesions,” said Dr. Kerlikowske. “There’s no question. But what are the harms?”
The biopsy rate almost doubles after ultrasonography, compared with mammography. And the number needed to screen to detect cancer is fairly high. For mammography, that number is about 250. For ultrasonography, tomosynthesis, and breast MRI, it is higher.
Tomosynthesis is more cost-effective than supplemental ultrasonography because it decreases the number of false positives, Dr. Kerlikowske said.
What’s the bottom line?
Not every woman with dense breasts is at high risk for breast cancer, said Dr. Kerlikowske. And although breast density is prevalent, it is potentially modifiable.
Nevertheless, breast density confers an elevated risk of breast cancer and can also mask tumors. Women with dense breasts likely should avoid the use of postmenopausal hormone therapy. They also may be candidates for more frequent mammography and/or supplemental imaging.
The Breast Cancer Surveillance Consortium (BCSC) Risk Calculator is the only tool that incorporates breast density. In the works is a new model that also incorporates benign breast disease.
Risk-prediction tool considers density and other factors
A risk-prediction tool from the Breast Cancer Surveillance Consortium (BCSC) is the only model to incorporate breast density. The BCSC Risk Calculator is available free of charge for the iPhone and iPad (an Android version is in the works). The tool takes 5 factors into consideration in estimating a woman’s 5-year risk of developing invasive breast cancer:
• age
• race/ethnicity
• breast density
• family history of breast cancer (first-degree relative)
• personal history of breast biopsy.
The tool is designed for use by health professionals. It is not appropriate for determining risk in women younger than 35 years or older than 79 years; women with a previous diagnosis of breast cancer, lobular carcinoma in situ, ductal carcinoma in situ, or atypical ductal hyperplasia; or women who have undergone breast augmentation. Other risk-prediction models are more appropriate for women with a BRCA mutation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Breast density is a strong, prevalent, and potentially modifiable risk factor for breast cancer, which makes it of special interest to clinicians whose jobs involve breast cancer risk prediction. That was the theme of a talk by Karla Kerlikowske, MD, of the UCSF Helen Diller Family Comprehensive Cancer Center in San Francisco. Dr. Kerlikowske delivered the John I. Brewer Memorial Lecture May 3 at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco.
Mammographic breast density is a radiologic term, Dr. Kerlikowske explained. “The only way to really know someone’s breast density is if they have a mammogram.” The whiter the mammogram, the denser the breast. The darker the mammogram, the fattier the breast.
According to the American College of Radiology, the following 4 categories of breast composition are defined by the “visually estimated” content of fibroglandular-density tissue within the breasts:
A. The breasts are almost entirely fatty.
B. There are scattered areas of fibroglandular density.
C. The breasts are heterogeneously dense, which may obscure small masses.
D. The breasts are extremely dense, which lowers the specificity of mammography.
Categories C and D signify dense breasts, which contain a high degree of collagen, epithelial cells, and stroma. In the United States, more than 25 million women are thought to have dense breasts.
Women who have a family history of breast cancer are more likely to have dense breasts. And women who have dense breasts have an elevated risk of breast cancer. They also have a higher risk of advanced disease, as well as a higher risk of large, high-grade, and lymph node-positive tumors, said Dr. Kerlikowske.
Breast-density legislation is increasing
Twenty-two states now have laws mandating that women found to have heterogeneously dense or extremely dense breasts be notified of their status, said Dr. Kerlikowske. That prompts the question: How should these patients be managed?
Breast density declines with age. Breast density also is influenced by body mass index (BMI). As BMI increases, density declines.
Breast density also can be affected by medications, such as hormone therapy and tamoxifen, Dr. Kerlikowske said.
For example, breast density declines about 1% to 2% per year in postmenopause. In postmenopausal women who take estrogen alone, breast density increases slightly. “But the real increase is for people who take estrogen plus progestin,” said Dr. Kerlikowske. “It’s thought that the progestin component is what increases breast density.” Estrogen-progestin therapy confers the same risk of breast cancer as that faced by a premenopausal woman with dense breasts.
As for tamoxifen, it reduces breast density by 2% to 3% per year in postmenopausal women, Dr. Kerlikowske said. “People who have a decrease of more than 10% in breast density are those who have a reduction in breast cancer.” If a woman doesn’t have that reduction with tamoxifen—about half of women don’t—there is no reduction in breast cancer mortality.
“There’s some thought that you should look at mammograms during the first year of tamoxifen use and, if you don’t see a change, consider switching to another medication,” said Dr. Kerlikowske.
More frequent mammograms and supplemental imaging are options for detecting cancers early. Among the modalities that have been studied in this regard are ultrasonography, tomosynthesis, and breast magnetic resonance imaging (MRI).
“If you do more tests, such as ultrasound, you will definitely find additional lesions,” said Dr. Kerlikowske. “There’s no question. But what are the harms?”
The biopsy rate almost doubles after ultrasonography, compared with mammography. And the number needed to screen to detect cancer is fairly high. For mammography, that number is about 250. For ultrasonography, tomosynthesis, and breast MRI, it is higher.
Tomosynthesis is more cost-effective than supplemental ultrasonography because it decreases the number of false positives, Dr. Kerlikowske said.
What’s the bottom line?
Not every woman with dense breasts is at high risk for breast cancer, said Dr. Kerlikowske. And although breast density is prevalent, it is potentially modifiable.
Nevertheless, breast density confers an elevated risk of breast cancer and can also mask tumors. Women with dense breasts likely should avoid the use of postmenopausal hormone therapy. They also may be candidates for more frequent mammography and/or supplemental imaging.
The Breast Cancer Surveillance Consortium (BCSC) Risk Calculator is the only tool that incorporates breast density. In the works is a new model that also incorporates benign breast disease.
Risk-prediction tool considers density and other factors
A risk-prediction tool from the Breast Cancer Surveillance Consortium (BCSC) is the only model to incorporate breast density. The BCSC Risk Calculator is available free of charge for the iPhone and iPad (an Android version is in the works). The tool takes 5 factors into consideration in estimating a woman’s 5-year risk of developing invasive breast cancer:
• age
• race/ethnicity
• breast density
• family history of breast cancer (first-degree relative)
• personal history of breast biopsy.
The tool is designed for use by health professionals. It is not appropriate for determining risk in women younger than 35 years or older than 79 years; women with a previous diagnosis of breast cancer, lobular carcinoma in situ, ductal carcinoma in situ, or atypical ductal hyperplasia; or women who have undergone breast augmentation. Other risk-prediction models are more appropriate for women with a BRCA mutation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
ACOG, SMFM, and others address safety concerns in labor and delivery
At least half of all cases of maternal morbidity and mortality could be prevented, or so studies suggest.1,2
The main stumbling block?
Faulty communication.
That’s the word from the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, the American College of Nurse-Midwives, and the Association of Women’s Health, Obstetric and Neonatal Nurses.3
In a joint “blueprint” to transform communication and enhance the safety culture in intrapartum care, these organizations, led by Audrey Lyndon, PhD, RN, FAAN, from the University of California, San Francisco, School of Nursing, describe the extent of the problem, steps that various team members can take to improve safety, notable success stories, and communication strategies.3 In this article, the joint blueprint is summarized, with a focus on steps obstetricians can take to improve the intrapartum safety culture.
Scope of the problem
A study of more than 3,282 physicians, midwives, and registered nurses produced a troubling statistic: More than 90% of respondents said that they had “witnessed shortcuts, missing competencies, disrespect, or performance problems” during the preceding year of practice.4 Few of these clinicians reported that they had discussed their concerns with the parties involved.
A second study of 1,932 clinicians found that 34% of physicians, 40% of midwives, and 56% of registered nurses had witnessed patients being put at risk within the preceding 2 years by other team members’ inattentiveness or lack of responsiveness.5
These findings suggest that health care providers often witness weak links in intrapartum safety but do not always address or report them. Among the reasons team members may be hesitant to speak up when they perceive a potential problem:
- feelings of resignation or inability to change the situation
- fear of retribution or ridicule
- fear of interpersonal or intrateam conflict.
Although Lyndon and colleagues acknowledge that it is impossible to eliminate adverse outcomes entirely or completely eradicate human error, they argue that significant improvements can be made by adopting a number of manageable strategies.
Recommended strategies
Lyndon and colleagues describe some of the challenges of effective communication in a health care setting:
Lyndon and colleagues go on to mention a number of strategies to improve communication, boost safety, and reduce medical errors.
1. Remember that the patient is part of the team
The patient and her family play a key role in identifying the potential for harm during labor and delivery, Lyndon and colleagues assert. They should be considered members of the intrapartum team, care should be patient-focused, and any communications from the patient should not only be heard but fully considered. In fact, explicit elicitation of her experience and concerns is recommended.
2. Consider that you might be part of the problem
It is human nature to attribute a communication problem to the other people involved, rather than take responsibility for it oneself. One potential solution to this mindset is team training, where all members are encouraged to communicate clearly and listen attentively. Organizations that have been successful at improving their culture of safety have implemented such training, as well as the use of checklists, training in fetal heart-rate monitoring, formation of a patient safety committee, external review of safety practices, and designation of a key clinician to lead the safety program and oversee team training.
3. Structure handoffs
The team should standardize handoffs so that they occur smoothly and all channels of communication remain open and clear.
“Having structured formats for debriefing and handoffs are steps in the right direction, but solving the problem of communication breakdowns is more complicated than standardizing the flow and format of information transfer,” Lyndon and colleagues assert. “Indeed, solving communication breakdowns is a matter of individual, group, organizational, and professional responsibility for creating and sustaining an environment of mutual respect, curiosity, and accountability for behavior and performance.”3
4. Learn to communicate responsibly
“Differences of opinion about clinical assessments, goals of care, and the pathway to optimal outcomes are bound to occur with some regularity in the dynamic environment of labor and delivery,” note Lyndon and colleagues. “Every person has the responsibility to contribute to improving how we relate to and communicate with each other. Collectively, we must create environments in which every team member (woman, family member, physician, midwife, nurse, unit clerk, patient care assistant, or scrub tech) is comfortable expressing and discussing concerns about safety or performance, is encouraged to do so, and has the support of the team to articulate the rationale for and urgency of the concern without fear of put-downs, retribution, or receiving poor-quality care.”3
5. Be persistent and proactive
When team members have differing expectations and communication styles, useful approaches include structured communication tools such as situation, background, assessment, recommendation (SBAR); structured handoffs; board rounds; huddles; attentive listening; and explicit elicitation of the patient’s concerns and desires.3
If someone fails to pay attention to a concern you raise, be persistent about restating that concern until you elicit a response.
If someone exhibits disruptive behavior, point to or establish a code of conduct that clearly describes professional behavior.
If there is a difference of opinion on patient management, such as fetal monitoring and interpretation, conduct regular case reviews and standardize a plan for notification of complications.
6. If you’re a team leader, set clear goals
Then ask team members what will be needed to achieve the outcomes desired.
“Team leaders need to develop outstanding skills for listening and eliciting feedback and cross-monitoring (being aware of each other’s actions and performance) from other team members,” note Lyndon and colleagues.
7. Increase public awareness of safety concepts
When these concepts and best practices are made known to the public, women and families become “empowered” to speak up when they have concerns about care.
And when they do speak up, it pays to listen.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Geller SE, Rosenberg D, Cox SM, et al. The continuum of maternal morbidity and mortality: factors associated with severity. Am J Obstet Gynecol. 2004;191(3):939–944.
2. Mitchell C, Lawton E, Morton C, McCain C, Holtby S, Main E. California Pregnancy-Associated Mortality Review: mixed methods approach for improved case identification, cause of death analyses and translation of findings. Matern Child Health J. 2014;18(3):518–526.
3. Lyndon A, Johnson MC, Bingham D, et al. Transforming communication and safety culture in intrapartum care: a multi-organization blueprint. Obstet Gynecol. 2015;125(5):1049–1055.
4. Maxfield DG, Lyndon A, Kennedy HP, O’Keeffe DF, Ziatnik MG. Confronting safety gaps across labor and delivery teams. Am J Obstet Gynecol. 2013;209(5):402–408.e3.
5. Lyndon A, Zlatnik MG, Maxfield DG, Lewis A, McMillan C, Kennedy HP. Contributions of clinical disconnections and unresolved conflict to failures in intrapartum safety. J Obstet Gynecol Neonatal Nurs. 2014;43(1):2–12.
At least half of all cases of maternal morbidity and mortality could be prevented, or so studies suggest.1,2
The main stumbling block?
Faulty communication.
That’s the word from the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, the American College of Nurse-Midwives, and the Association of Women’s Health, Obstetric and Neonatal Nurses.3
In a joint “blueprint” to transform communication and enhance the safety culture in intrapartum care, these organizations, led by Audrey Lyndon, PhD, RN, FAAN, from the University of California, San Francisco, School of Nursing, describe the extent of the problem, steps that various team members can take to improve safety, notable success stories, and communication strategies.3 In this article, the joint blueprint is summarized, with a focus on steps obstetricians can take to improve the intrapartum safety culture.
Scope of the problem
A study of more than 3,282 physicians, midwives, and registered nurses produced a troubling statistic: More than 90% of respondents said that they had “witnessed shortcuts, missing competencies, disrespect, or performance problems” during the preceding year of practice.4 Few of these clinicians reported that they had discussed their concerns with the parties involved.
A second study of 1,932 clinicians found that 34% of physicians, 40% of midwives, and 56% of registered nurses had witnessed patients being put at risk within the preceding 2 years by other team members’ inattentiveness or lack of responsiveness.5
These findings suggest that health care providers often witness weak links in intrapartum safety but do not always address or report them. Among the reasons team members may be hesitant to speak up when they perceive a potential problem:
- feelings of resignation or inability to change the situation
- fear of retribution or ridicule
- fear of interpersonal or intrateam conflict.
Although Lyndon and colleagues acknowledge that it is impossible to eliminate adverse outcomes entirely or completely eradicate human error, they argue that significant improvements can be made by adopting a number of manageable strategies.
Recommended strategies
Lyndon and colleagues describe some of the challenges of effective communication in a health care setting:
Lyndon and colleagues go on to mention a number of strategies to improve communication, boost safety, and reduce medical errors.
1. Remember that the patient is part of the team
The patient and her family play a key role in identifying the potential for harm during labor and delivery, Lyndon and colleagues assert. They should be considered members of the intrapartum team, care should be patient-focused, and any communications from the patient should not only be heard but fully considered. In fact, explicit elicitation of her experience and concerns is recommended.
2. Consider that you might be part of the problem
It is human nature to attribute a communication problem to the other people involved, rather than take responsibility for it oneself. One potential solution to this mindset is team training, where all members are encouraged to communicate clearly and listen attentively. Organizations that have been successful at improving their culture of safety have implemented such training, as well as the use of checklists, training in fetal heart-rate monitoring, formation of a patient safety committee, external review of safety practices, and designation of a key clinician to lead the safety program and oversee team training.
3. Structure handoffs
The team should standardize handoffs so that they occur smoothly and all channels of communication remain open and clear.
“Having structured formats for debriefing and handoffs are steps in the right direction, but solving the problem of communication breakdowns is more complicated than standardizing the flow and format of information transfer,” Lyndon and colleagues assert. “Indeed, solving communication breakdowns is a matter of individual, group, organizational, and professional responsibility for creating and sustaining an environment of mutual respect, curiosity, and accountability for behavior and performance.”3
4. Learn to communicate responsibly
“Differences of opinion about clinical assessments, goals of care, and the pathway to optimal outcomes are bound to occur with some regularity in the dynamic environment of labor and delivery,” note Lyndon and colleagues. “Every person has the responsibility to contribute to improving how we relate to and communicate with each other. Collectively, we must create environments in which every team member (woman, family member, physician, midwife, nurse, unit clerk, patient care assistant, or scrub tech) is comfortable expressing and discussing concerns about safety or performance, is encouraged to do so, and has the support of the team to articulate the rationale for and urgency of the concern without fear of put-downs, retribution, or receiving poor-quality care.”3
5. Be persistent and proactive
When team members have differing expectations and communication styles, useful approaches include structured communication tools such as situation, background, assessment, recommendation (SBAR); structured handoffs; board rounds; huddles; attentive listening; and explicit elicitation of the patient’s concerns and desires.3
If someone fails to pay attention to a concern you raise, be persistent about restating that concern until you elicit a response.
If someone exhibits disruptive behavior, point to or establish a code of conduct that clearly describes professional behavior.
If there is a difference of opinion on patient management, such as fetal monitoring and interpretation, conduct regular case reviews and standardize a plan for notification of complications.
6. If you’re a team leader, set clear goals
Then ask team members what will be needed to achieve the outcomes desired.
“Team leaders need to develop outstanding skills for listening and eliciting feedback and cross-monitoring (being aware of each other’s actions and performance) from other team members,” note Lyndon and colleagues.
7. Increase public awareness of safety concepts
When these concepts and best practices are made known to the public, women and families become “empowered” to speak up when they have concerns about care.
And when they do speak up, it pays to listen.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
At least half of all cases of maternal morbidity and mortality could be prevented, or so studies suggest.1,2
The main stumbling block?
Faulty communication.
That’s the word from the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, the American College of Nurse-Midwives, and the Association of Women’s Health, Obstetric and Neonatal Nurses.3
In a joint “blueprint” to transform communication and enhance the safety culture in intrapartum care, these organizations, led by Audrey Lyndon, PhD, RN, FAAN, from the University of California, San Francisco, School of Nursing, describe the extent of the problem, steps that various team members can take to improve safety, notable success stories, and communication strategies.3 In this article, the joint blueprint is summarized, with a focus on steps obstetricians can take to improve the intrapartum safety culture.
Scope of the problem
A study of more than 3,282 physicians, midwives, and registered nurses produced a troubling statistic: More than 90% of respondents said that they had “witnessed shortcuts, missing competencies, disrespect, or performance problems” during the preceding year of practice.4 Few of these clinicians reported that they had discussed their concerns with the parties involved.
A second study of 1,932 clinicians found that 34% of physicians, 40% of midwives, and 56% of registered nurses had witnessed patients being put at risk within the preceding 2 years by other team members’ inattentiveness or lack of responsiveness.5
These findings suggest that health care providers often witness weak links in intrapartum safety but do not always address or report them. Among the reasons team members may be hesitant to speak up when they perceive a potential problem:
- feelings of resignation or inability to change the situation
- fear of retribution or ridicule
- fear of interpersonal or intrateam conflict.
Although Lyndon and colleagues acknowledge that it is impossible to eliminate adverse outcomes entirely or completely eradicate human error, they argue that significant improvements can be made by adopting a number of manageable strategies.
Recommended strategies
Lyndon and colleagues describe some of the challenges of effective communication in a health care setting:
Lyndon and colleagues go on to mention a number of strategies to improve communication, boost safety, and reduce medical errors.
1. Remember that the patient is part of the team
The patient and her family play a key role in identifying the potential for harm during labor and delivery, Lyndon and colleagues assert. They should be considered members of the intrapartum team, care should be patient-focused, and any communications from the patient should not only be heard but fully considered. In fact, explicit elicitation of her experience and concerns is recommended.
2. Consider that you might be part of the problem
It is human nature to attribute a communication problem to the other people involved, rather than take responsibility for it oneself. One potential solution to this mindset is team training, where all members are encouraged to communicate clearly and listen attentively. Organizations that have been successful at improving their culture of safety have implemented such training, as well as the use of checklists, training in fetal heart-rate monitoring, formation of a patient safety committee, external review of safety practices, and designation of a key clinician to lead the safety program and oversee team training.
3. Structure handoffs
The team should standardize handoffs so that they occur smoothly and all channels of communication remain open and clear.
“Having structured formats for debriefing and handoffs are steps in the right direction, but solving the problem of communication breakdowns is more complicated than standardizing the flow and format of information transfer,” Lyndon and colleagues assert. “Indeed, solving communication breakdowns is a matter of individual, group, organizational, and professional responsibility for creating and sustaining an environment of mutual respect, curiosity, and accountability for behavior and performance.”3
4. Learn to communicate responsibly
“Differences of opinion about clinical assessments, goals of care, and the pathway to optimal outcomes are bound to occur with some regularity in the dynamic environment of labor and delivery,” note Lyndon and colleagues. “Every person has the responsibility to contribute to improving how we relate to and communicate with each other. Collectively, we must create environments in which every team member (woman, family member, physician, midwife, nurse, unit clerk, patient care assistant, or scrub tech) is comfortable expressing and discussing concerns about safety or performance, is encouraged to do so, and has the support of the team to articulate the rationale for and urgency of the concern without fear of put-downs, retribution, or receiving poor-quality care.”3
5. Be persistent and proactive
When team members have differing expectations and communication styles, useful approaches include structured communication tools such as situation, background, assessment, recommendation (SBAR); structured handoffs; board rounds; huddles; attentive listening; and explicit elicitation of the patient’s concerns and desires.3
If someone fails to pay attention to a concern you raise, be persistent about restating that concern until you elicit a response.
If someone exhibits disruptive behavior, point to or establish a code of conduct that clearly describes professional behavior.
If there is a difference of opinion on patient management, such as fetal monitoring and interpretation, conduct regular case reviews and standardize a plan for notification of complications.
6. If you’re a team leader, set clear goals
Then ask team members what will be needed to achieve the outcomes desired.
“Team leaders need to develop outstanding skills for listening and eliciting feedback and cross-monitoring (being aware of each other’s actions and performance) from other team members,” note Lyndon and colleagues.
7. Increase public awareness of safety concepts
When these concepts and best practices are made known to the public, women and families become “empowered” to speak up when they have concerns about care.
And when they do speak up, it pays to listen.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Geller SE, Rosenberg D, Cox SM, et al. The continuum of maternal morbidity and mortality: factors associated with severity. Am J Obstet Gynecol. 2004;191(3):939–944.
2. Mitchell C, Lawton E, Morton C, McCain C, Holtby S, Main E. California Pregnancy-Associated Mortality Review: mixed methods approach for improved case identification, cause of death analyses and translation of findings. Matern Child Health J. 2014;18(3):518–526.
3. Lyndon A, Johnson MC, Bingham D, et al. Transforming communication and safety culture in intrapartum care: a multi-organization blueprint. Obstet Gynecol. 2015;125(5):1049–1055.
4. Maxfield DG, Lyndon A, Kennedy HP, O’Keeffe DF, Ziatnik MG. Confronting safety gaps across labor and delivery teams. Am J Obstet Gynecol. 2013;209(5):402–408.e3.
5. Lyndon A, Zlatnik MG, Maxfield DG, Lewis A, McMillan C, Kennedy HP. Contributions of clinical disconnections and unresolved conflict to failures in intrapartum safety. J Obstet Gynecol Neonatal Nurs. 2014;43(1):2–12.
1. Geller SE, Rosenberg D, Cox SM, et al. The continuum of maternal morbidity and mortality: factors associated with severity. Am J Obstet Gynecol. 2004;191(3):939–944.
2. Mitchell C, Lawton E, Morton C, McCain C, Holtby S, Main E. California Pregnancy-Associated Mortality Review: mixed methods approach for improved case identification, cause of death analyses and translation of findings. Matern Child Health J. 2014;18(3):518–526.
3. Lyndon A, Johnson MC, Bingham D, et al. Transforming communication and safety culture in intrapartum care: a multi-organization blueprint. Obstet Gynecol. 2015;125(5):1049–1055.
4. Maxfield DG, Lyndon A, Kennedy HP, O’Keeffe DF, Ziatnik MG. Confronting safety gaps across labor and delivery teams. Am J Obstet Gynecol. 2013;209(5):402–408.e3.
5. Lyndon A, Zlatnik MG, Maxfield DG, Lewis A, McMillan C, Kennedy HP. Contributions of clinical disconnections and unresolved conflict to failures in intrapartum safety. J Obstet Gynecol Neonatal Nurs. 2014;43(1):2–12.
Clinicians are adept at estimating uterine size prior to benign hysterectomy
In a poster presented at the 2015 ACOG Annual Clinical Meeting in San Francisco, Neal Marc Lonky, MD, and colleagues from the Southern California Permanente Group assessed the clinical acumen of physicians in estimating uterine size prior to elective hysterectomy for benign indications. They found that the correlation between estimates and actual uterine weight was 0.79 (P<.001), with a very low conversion rate for the surgery.1
Lonky and colleagues collected preoperative uterine estimates and actual specimen weights prospectively for 1,079 cases of benign hysterectomy. The surgeries were performed by 186 primary surgeons and assistant surgeons at 10 Kaiser Permanente Southern California medical centers. Surgeons based the route of hysterectomy on estimates of uterine size, which were calculated using bimanual examination, ultrasonography, or both. Linear regression was used to measure and compare the relationship between estimated uterine size and the pelvic specimen weight.
Uterine size estimates ranged from 4 cm to 40 cm, and specimen weights ranged from 2 g to 4,607 g. The mean (SD) estimate of uterine size was 11.7 (4.43) cm, and the mean actual specimen weight was 334.6 (401.42) g.
The mean age of women in the sample was 47.2 (8.35) years. Overall, 379 women (35.1%) were Hispanic, 325 (30.1%) were non-Hispanic white, 281 (26.0%) were non-Hispanic black, and 81 (7.5%) were Asian/Pacific Islander. The mean body mass index (BMI) was 30.0 (6.37) kg/m2, with a range of 16.8 to 67.9 kg/m2.
“This is real world research,” said Dr. Lonky. “It’s called comparative effectiveness research. Basically, all patients who are undergoing the procedure are entered in the registry, and the clinical acumen of the physician—either using or not using ultrasound—is assessed.”
“We looked at whether or not we had a bias toward one patient age group, race/ethnicity, BMI, or estimated uterine size. But there were no clusters, so this was truly a random distribution,” said Dr. Lonky.
“These findings may be population-specific to my group of doctors,” he added. “They should be replicated in other settings. It may be that residents are not going to be as linear.”
Reference
- Lonky NM, Chiu V, Mohan Y. Clinical utility of the estimation of uterine size in planning hysterectomy approach. Obstet Gynecol. 2015;125(5 suppl):19S.
In a poster presented at the 2015 ACOG Annual Clinical Meeting in San Francisco, Neal Marc Lonky, MD, and colleagues from the Southern California Permanente Group assessed the clinical acumen of physicians in estimating uterine size prior to elective hysterectomy for benign indications. They found that the correlation between estimates and actual uterine weight was 0.79 (P<.001), with a very low conversion rate for the surgery.1
Lonky and colleagues collected preoperative uterine estimates and actual specimen weights prospectively for 1,079 cases of benign hysterectomy. The surgeries were performed by 186 primary surgeons and assistant surgeons at 10 Kaiser Permanente Southern California medical centers. Surgeons based the route of hysterectomy on estimates of uterine size, which were calculated using bimanual examination, ultrasonography, or both. Linear regression was used to measure and compare the relationship between estimated uterine size and the pelvic specimen weight.
Uterine size estimates ranged from 4 cm to 40 cm, and specimen weights ranged from 2 g to 4,607 g. The mean (SD) estimate of uterine size was 11.7 (4.43) cm, and the mean actual specimen weight was 334.6 (401.42) g.
The mean age of women in the sample was 47.2 (8.35) years. Overall, 379 women (35.1%) were Hispanic, 325 (30.1%) were non-Hispanic white, 281 (26.0%) were non-Hispanic black, and 81 (7.5%) were Asian/Pacific Islander. The mean body mass index (BMI) was 30.0 (6.37) kg/m2, with a range of 16.8 to 67.9 kg/m2.
“This is real world research,” said Dr. Lonky. “It’s called comparative effectiveness research. Basically, all patients who are undergoing the procedure are entered in the registry, and the clinical acumen of the physician—either using or not using ultrasound—is assessed.”
“We looked at whether or not we had a bias toward one patient age group, race/ethnicity, BMI, or estimated uterine size. But there were no clusters, so this was truly a random distribution,” said Dr. Lonky.
“These findings may be population-specific to my group of doctors,” he added. “They should be replicated in other settings. It may be that residents are not going to be as linear.”
In a poster presented at the 2015 ACOG Annual Clinical Meeting in San Francisco, Neal Marc Lonky, MD, and colleagues from the Southern California Permanente Group assessed the clinical acumen of physicians in estimating uterine size prior to elective hysterectomy for benign indications. They found that the correlation between estimates and actual uterine weight was 0.79 (P<.001), with a very low conversion rate for the surgery.1
Lonky and colleagues collected preoperative uterine estimates and actual specimen weights prospectively for 1,079 cases of benign hysterectomy. The surgeries were performed by 186 primary surgeons and assistant surgeons at 10 Kaiser Permanente Southern California medical centers. Surgeons based the route of hysterectomy on estimates of uterine size, which were calculated using bimanual examination, ultrasonography, or both. Linear regression was used to measure and compare the relationship between estimated uterine size and the pelvic specimen weight.
Uterine size estimates ranged from 4 cm to 40 cm, and specimen weights ranged from 2 g to 4,607 g. The mean (SD) estimate of uterine size was 11.7 (4.43) cm, and the mean actual specimen weight was 334.6 (401.42) g.
The mean age of women in the sample was 47.2 (8.35) years. Overall, 379 women (35.1%) were Hispanic, 325 (30.1%) were non-Hispanic white, 281 (26.0%) were non-Hispanic black, and 81 (7.5%) were Asian/Pacific Islander. The mean body mass index (BMI) was 30.0 (6.37) kg/m2, with a range of 16.8 to 67.9 kg/m2.
“This is real world research,” said Dr. Lonky. “It’s called comparative effectiveness research. Basically, all patients who are undergoing the procedure are entered in the registry, and the clinical acumen of the physician—either using or not using ultrasound—is assessed.”
“We looked at whether or not we had a bias toward one patient age group, race/ethnicity, BMI, or estimated uterine size. But there were no clusters, so this was truly a random distribution,” said Dr. Lonky.
“These findings may be population-specific to my group of doctors,” he added. “They should be replicated in other settings. It may be that residents are not going to be as linear.”
Reference
- Lonky NM, Chiu V, Mohan Y. Clinical utility of the estimation of uterine size in planning hysterectomy approach. Obstet Gynecol. 2015;125(5 suppl):19S.
Reference
- Lonky NM, Chiu V, Mohan Y. Clinical utility of the estimation of uterine size in planning hysterectomy approach. Obstet Gynecol. 2015;125(5 suppl):19S.
Endometriosis and pain: Expert answers to 6 questions targeting your management options
Endometriosis has always posed a treatment challenge. Take the early 19th Century, for example, before the widespread advent of surgery, when the disease was managed by applying leeches to the cervix. In fact, as Nezhat and colleagues note in their comprehensive survey of the 4,000-year history of endometriosis, “leeches were considered a mainstay in treating any condition associated with menstruation.”1
Fast forward to the 21st Century, and the picture is a lot clearer, though still not crystal clear. The optimal approach to endometriosis depends on numerous factors, foremost among them the chief complaint of the patient—pain or infertility (or both).
In this article—Part 2 of a 3-part series on endometriosis—the focus is on medical and surgical management of pain. Six experts address such questions as when is laparoscopy indicated, who is best qualified to treat endometriosis, is excision or ablation of lesions preferred, what is the role of hysterectomy in eliminating pain, and what to do about the problem of recurrence.
In Part 3, to be published in the June 2015 issue of OBG Management, endometriosis-associated infertility will be the topic of discussion.
In Part 1, 7 experts answer crucial questions on the diagnosis of endometriosis.
For a detailed look at the pathophysiology of endometriosis-associated pain, see “Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain,” by Kenneth A. Levey, MD, MPH, in this issue.
1. What are the options for empiric therapy?
One reason for the diagnostic delay for endometriosis, which still averages about 6 years, is that definitive diagnosis is achieved only through laparoscopic investigation and histologic confirmation. For many women who experience pain thought to be associated with endometriosis, however, clinicians begin empiric treatment with medical agents as a way to avert the need for surgery, if at all possible.
“There is no cure for endometriosis,” says John R. Lue, MD, MPH, “but there are many ways that endometriosis can be treated” and the impact of the disease reduced in a patient’s life. Dr. Lue is Associate Professor and Chief of the Section of General Obstetrics and Gynecology and Medical Director of Women’s Ambulatory Services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
Among the medical and hormonal management options:
- Nonsteroidal anti-inflammatory drugs (NSAIDs), often used with combined oral contraceptives (OCs). NSAIDs are not a long-term treatment option because of their effect on cyclo-oxygenase (COX) 1 and 2 enzymes, says Dr. Lue. COX-1 protects the gastrointestinal (GI) system, and prolonged use of NSAIDs can cause adverse GI effects.
- Cyclic combined OCs “are recommended as first-line therapy in the absence of contraindications,” says Dr. Lue, and are often used in combination with NSAIDs. However, the failure rate may be as high as 20% to 25%.2 “If pain persists after a trial of 3 to 6 months of cyclic OCs, one can consider switching to continuous low-dose combined OCs for an additional 6 months,” says Dr. Lue. When combined OCs were compared with placebo in the treatment of dysmenorrhea, they reduced baseline pain scores by 45% to 52%, compared with 14% to 17% for placebo (P<.001).2 They also reduced the volume of endometriomas by 48%, compared with 32% for placebo (P = .04). According to Linda C. Giudice, MD, PhD, “In women with severe dysmenorrhea who have been treated with cyclic combined OCs, a switch to continuous combined OCs reduced pain scores by 58% within 6 months and by 75% at 2 years” (P<.001).2 Dr. Giudice is the Robert B. Jaffe, MD, Endowed Professor in the Reproductive Sciences and Chair of Obstetrics, Gynecology, and Reproductive Sciences at the University of California, San Francisco.
- Depot medroxyprogesterone acetate (DMPA) or the levonorgestrel-releasing intrauterine system (LNG-IUS). These agents suppress the hypothalamic-pituitary-ovarian (HPO) axis to different degrees. DMPA suppresses the HPO completely, preventing ovulation. The LNG-IUS does not fully suppress the HPO but acts directly on endometrial tissue, with antiproliferative effects on eutopic and endometriotic implants, says Dr. Lue. The LNG-IUS also is effective at suppressing disease after surgical treatment, says Dr. Giudice.2
- Gonadotropin-releasing hormone (GnRH) agonist therapy, with estrogen and/or progestin add-back therapy to temper the associated loss in bone mineral density, “may be effective—if only temporarily—as it inhibits the HPO axis and blocks ovarian function, thereby greatly reducing systemic estrogen levels and inducing artificial menopause,” says Dr. Lue.
- Norethindrone acetate, a synthetic progestational agent, is occasionally used as empiric therapy for endometriosis because of its ability to inhibit ovulation. It has antiandrogenic and antiestrogenic effects.
- Aromatase inhibitors. Dr. Lue points to considerable evidence that endometriotic implants are an autocrine source of estrogen.3 “This locally produced estrogen results from overexpression of the enzyme P450 aromatase by endometriotic tissue,” he says. Consequently, in postmenopausal women, “aromatase inhibitors may be used orally in a daily pill form to curtail endometriotic implant production of estrogen and subsequent implant growth.”4 In women of reproductive age, aromatase inhibitors are combined with an HPO-suppressive therapy, such as norethindrone acetate. These strategies represent off-label use of aromatase inhibitors.
- Danazol, a synthetic androgen, has been used in the past to treat dysmenorrhea and dyspareunia. Because of its severe androgenic effects, however, it is not widely used today.
“For those using medical approaches, endometriosis-related pain may be reduced by using hormonal treatments to modify reproductive tract events, thereby decreasing local peritoneal inflammation and cytokine production,” says Pamela Stratton, MD. Because endometriosis is a “central sensitivity syndrome,” multidisciplinary approaches may be beneficial to treat myofascial dysfunction and sensitization, such as physical therapy. “Chronic pain conditions that overlap with endometriosis-associated pain, such as migraines, irritable bowel syndrome, or painful bladder syndrome should be identified and treated. Mood changes of depression and anxiety common to women with endometriosis-associated pain also warrant treatment,” she says.
Dr. Stratton is Chief of the Gynecology Consult Service, Program in Reproductive and Adult Endocrinology, at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
2. When is laparoscopy indicated?
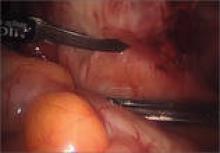
When medical and hormonal treatments fail to control a patient’s pain, laparoscopy is indicated to confirm the diagnosis of endometriosis. During that procedure, it is also advisable to treat any endometriosis that is present, provided the surgeon is highly experienced in such treatment.
Proper treatment is preferable—even if it requires expert consultation. “No treatment and referral to a more experienced surgeon are better than incomplete treatment by an inexperienced surgeon,” says Ceana Nezhat, MD. “Not all GYN surgeons have the expertise to treat advanced endometriosis.” Dr. Nezhat is Director of the Nezhat Medical Center and Medical Director of Training and Education at Northside Hospital, both in Atlanta, Georgia.
Dr. Stratton agrees about the importance of thorough treatment of endometriosis at the time of diagnostic laparoscopy. “At the laparoscopy, the patient benefits if all potential sources of pain are investigated and addressed.” At surgery, the surgeon should look for and treat any lesions suspicious for endometriosis, as well as any other finding that might contribute to pain, she says. “For example, routinely inspecting the appendix for endometriosis or other lesions, and removing affected appendices is reasonable; also, lysis and, where possible, excision of adhesions is an important strategy.”
If a medical approach fails for a patient, “then surgery is indicated to confirm the diagnosis and treat the disease,” agrees Tommaso Falcone, MD.
“Surgery is very effective in treating the pain associated with endometriosis,” Dr. Falcone continues. Randomized clinical trials have shown that up to 90% of patients who obtain pain relief from surgery will have an effect lasting 1 year.6 If patients do not get relief, then the association of the pain with endometriosis should be questioned and other causes searched.” Dr. Falcone is Professor and Chair of Obstetrics and Gynecology at the Cleveland Clinic in Cleveland, Ohio.
The most common anatomic sites of implants
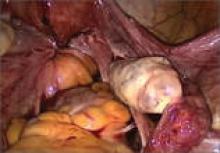
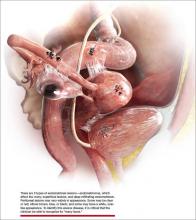
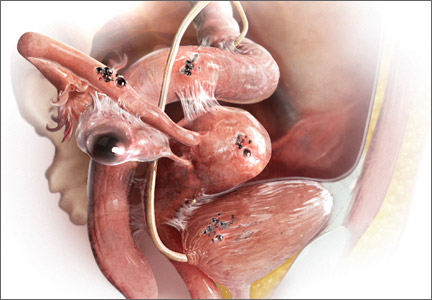
“The most common accepted theory for pathogenesis of endometriosis suggests that implants develop when debris from retrograde menstruation attaches to the pelvic peritoneum,” says Dr. Stratton.7 “Thus, the vast majority of lesions occur in the dependent portions of the pelvis, which include the ovarian fossae (posterior broad ligament under the ovaries), cul de sac, and the uterosacral ligaments.8 The bladder peritoneum, ovarian surface, uterine peritoneal surface, fallopian tube, and pelvic sidewall are also frequent sites. The colon and appendix are less common sites, and small bowel lesions are rare.”
“However, pain location does not correlate with lesion location,” Dr. Stratton notes. “For this reason, the goal at surgery is to treat all lesions, even ones that are not in sites of pain.”
3. How should disease be staged?
Most surgeons with expertise in treating endometriosis attempt to stage the disease at the time of initial laparoscopy, even though a patient’s pain does not always correlate with the stage of disease.
“The staging system for endometriosis is a means to systematically catalogue where lesions are located,” says Dr. Stratton.
The most commonly used classification system was developed by the American Society for Reproductive Medicine (ASRM). It takes into account such characteristics as how deep an implant lies, the extent to which it obliterates the posterior cul de sac, and the presence and extent of adhesions. Although the classification system is broken down into 4 stages ranging from minimal to severe disease, it is fairly complex. For example, it assigns a score for each lesion as well as the size and location of that lesion, notes Dr. Stratton. The presence of an endometrioma automatically renders the disease as stage III or IV, and an obliterated cul de sac means the endometriosis is graded as stage IV.
“This system enables us to communicate with each other about patients and may guide future surgeries for assessment of lesion recurrence or the planning of treatment for lesions the surgeon was unable to treat at an initial surgery,” says Dr. Stratton.
“Women with uterosacral nodularity, fixed pelvic organs, or severe pain with endometriomas may have deep infiltrating lesions. These lesions, in particular, are not captured well with the current staging system,” says Dr. Stratton. Because they appear to be innervated, “the greatest benefit to the patient is achieved by completely excising these lesions.” Preoperative imaging may help confirm the existence, location, and extent of these deep lesions and help the surgeon plan her approach “based on clinical and imaging findings.”
“Severity of pain or duration of surgical effect does not correlate with stage or extent of disease,” Dr. Stratton says.9 “In fact, patients with the least amount of disease noted at surgery experience pain sooner, suggesting that the central nervous system may have been remodeled prior to surgery or that the pain is in part due to some other cause.10 This observation underscores the principle that, while endometriosis may initiate pain, the pain experience is determined by engagement of the central nervous system.”
For more information on the ASRM revised classification of endometriosis, go to http://www.fertstert.org/article/S0015-0282(97)81391-X/pdf.
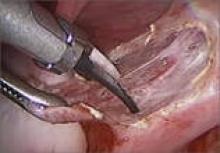
4. Which is preferable—excision or ablation?
In a prospective, randomized, double-blind study, Healey and colleagues compared pain levels following laparoscopic treatment of endometriosis with either excision or ablation. Preoperatively, women in the study completed a questionnaire rating various types of pain using visual analogue scales. They then were randomly assigned to treatment of endometriosis via excision or ablation. Postoperatively, they again completed a questionnaire about pain levels at 3, 6, 9, and 12 months. Investigators found no significant difference in pain scores at 12 months.11
Five-year follow-up of the same population yielded slightly different findings, however. Although there was a reduction in all pain scores at 5 years in both the excision and ablation groups, a significantly greater reduction in dyspareunia was observed in the excision group at 5 years.12
In an editorial accompanying the 5-year follow-up data, Dr. Falcone and a coauthor called excision versus ablation of ovarian, bowel, and peritoneal endometriosis one of the “great debates” in the surgical management of endometriosis.13
“When there is deep involvement of adjacent organs, there is general consensus that excision is best for optimal surgical outcome,” they write. “However, for disease involving the peritoneum alone, there are proponents for either option.”13
“This is a very controversial issue,” says Dr. Falcone, “and the debate can sometimes be somewhat inflammatory…. It is hard to understand how a comparative trial could even be accomplished between excision and ablation,” he adds. “In my experience, deep disease typically occurs on the pelvic sidewall over the ureter or in the cul de sac on the bowel or infiltrating the bladder peritoneum. Therefore, ablation would increase the risk of damaging any of these structures. With superficial disease away from critical structures, it should be fine to ablate. Everywhere else and with deep disease you need to excise or leave disease behind.”
“Endometriomas are a special situation,” Dr. Falcone says. “Excision of the cyst has been shown in randomized controlled trials (RCTs) to be associated with less risk of recurrence.14 Therefore, it should be the treatment of choice. However, in patients interested in future fertility, we must take into consideration the potential damage to ovarian reserve associated with excision.”
Endometriosis of the ovaries has unique manifestations. “My approach to ovarian cysts depends on their classification,” says Dr. Nezhat.15 In general, primary endometriomas (Type 1) are small, superficial cysts that contain dark “chocolate” fluid. They tend to be firmly adherent to the ovarian tissue and difficult to remove surgically.
Secondary endometriomas (Type 2) are follicular or luteal cysts that have been involved or invaded by cortical endometriotic implants or by primary endometrioma. Secondary endometriomas are further classified by the relationship between cortical endometriosis and the cyst wall. Type 2A endometriomas are usually large, with a capsule that is easily separated from ovarian tissue. Type 2B endometriomas have some features of functional cysts but show deep involvement with surface endometriosis. Type 2C endometriomas are similar, showing extensive surface endometrial implants but with deep penetration of the endometriosis into the cyst wall.15
“For Type 1 endometriomas, I biopsy the cyst to ensure the lesion is benign, then vaporize the endometrioma,” Dr. Nezhat says. “In cases of type 2A and 2B endometriomas, the cyst capsule is easily enucleated and removed. Type 2C endometriomas are biopsied as well and then I proceed with vaporizing the fibrotic area with a low-power energy source, such as neutral argon plasma, avoiding excessive coagulation and thermal injury.” Recent literature supports the idea of evaluation and biopsy of fibrotic endometriomas to confirm benign conditions, followed by ablation without compromising ovarian function.16
“Excision and ablation both have indications,” Dr. Nezhat asserts. “It depends on the location and depth of penetration of implants, as well as the patient’s ultimate goal. For example, if the patient desires future fertility and has endometriosis on the ovary, removal by excision could damage ovarian function. The same holds true for endometriosis on the fallopian tubes. It’s better in such cases to ablate.”
“Ablation is different from coagulation, which is not recommended,” Dr. Nezhat explains. “Ablation vaporizes the diseased area layer by layer, like peeling an onion, until the disease is eradicated. It is similar to dermatological skin resurfacing. Vaporization is preferable for endometriosis on the tubes and ovaries in patients who desire pregnancy. The choice between excision and ablation depends on the location, depth of penetration, and the patient’s desire for fertility.”
Either way, and regardless of the primary indication for surgery—pain versus infertility—a minimally invasive gynecologic surgeon is expected to have ability in performing both techniques, Dr. Nezhat says.
The following videos have been provided by AAGL SurgeryU to compliment the content of this article regarding endometriosis. You can watch these videos, and more than 1,500 others, at AAGL.org/surgeryu. Laparoscopic excision of stage IV endometriosis Einarsson JI This case, originally presented as a SurgeryU live event, features a 41-year-old woman (G3P1) with a 3-year history of left-sided pelvic pain, deep dyspareunia, constipation, and dysmenorrhea. She also has infertility and is planning an IVF treatment shortly. On examination she was noted to have significant rectovaginal tenderness and nodularity. A pelvic MRI demonstrated a 3-cm irregular mass extending from the cervix into the cul-de-sac up to the left lateral pelvic sidewall. Abdominal wall endometriosis Hawkins E, Patzkowsky K, Lopez J This video demonstrates a typical presentation of abdominal wall endometriosis (AWE), also known as subcutaneous endometriosis or scar endometriosis. It is important for gynecologists to be familiar with this more uncommon form of the disease and its management. This video also demonstrates surgical management of advanced AWE involving the subcutaneous tissue, fascia, and rectus muscle. Laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain Pendergrass M This video depicts the laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain. The patient underwent menarche at age 11 and developed cyclic pelvic pain 6 months later. Due to the severity of the pain she has been unable to attend school for the past 2 months, and has stopped participating in sports. A diagnostic laparoscopy revealed red/brown superficial endometriosis lesions on the peritoneum in the posterior cul de sac, bilateral uterosacral ligaments, and bilateral broad ligaments. |
5. Is hysterectomy definitive treatment for pain?
“Not necessarily,” says Dr. Nezhat. “Hysterectomy by itself doesn’t take care of endometriosis unless the patient has adenomyosis. If a patient has endometriosis, the first step is complete treatment of the disease to restore the anatomy. Then the next step might be hysterectomy to give a better long-term result, especially in cases of adenomyosis. Removal of the ovaries at the time of hysterectomy has to be individualized.”
“The implication that hysterectomy ‘cures’ endometriosis is false yet is stated in some textbooks,” says Dr. Nezhat. “Even at the time of hysterectomy, the first step should be complete treatment of endometriosis and restoration of anatomy, followed by the hysterectomy. Leaving endometriosis behind, believing it will go away by itself or not cause future issues, is a gross misperception.”
Removal of the ovaries at hysterectomy?
“There are few comparative studies on the long-term follow-up of patients who have undergone hysterectomy with or without removal of both ovaries,” says Dr. Falcone. “The conventional dogma has been that, in women undergoing definitive surgery for endometriosis, both ovaries should be removed, even if they are normal. I personally believe that this was the case because hysterectomy was often performed without excision of the endometriosis. So the uterus was removed and disease was left behind. In these cases, recurrent symptoms were due to persistent disease.”
“We reported our experience at the Cleveland Clinic with a 7-year follow-up,” Dr. Falcone continues. “Hysterectomy was performed with excision of all visible disease. Ovaries were conserved if normal and removed if they had disease. We looked at the reoperation-free frequency over time. In women undergoing hysterectomy with excision of visible disease but ovarian preservation, the reoperation-free percentages at 2, 5, and 7 years were 95%, 86%, and 77%, respectively, versus 96%, 91%, and 91% in those without ovarian preservation. So, overall, there was an advantage over time for removal of the ovaries. However, in the subset of women between ages 30 and 39 years, there was no difference in the long-term recurrence rate if the ovaries were left in. For this reason, in women under 40, we recommend keeping normal ovaries if all disease is removed.”17
6. Can the risk of postoperative recurrence be reduced?
“The main problem with surgery is the recurrence rate,” Dr. Falcone says. “Studies have shown that the recurrence rate of pain at 7 years may be as high as 50%.”17 Furthermore, “the recurrence of pain may not be associated with visualized endometriosis at laparoscopy.”
“Incomplete removal of lesions may be associated with an increase in pain after surgery,” says Dr. Stratton.18 “Incomplete removal of lesions may occur because of varying technical skill or specific lesion characteristics. The lesions may be difficult to remove because of their location. Lesions may not be recognized because their appearance can vary from subtle (red or clear or white) to classic (blue-black). The depth of the lesion may not be appreciated until surgery is under way and a surgeon may not be adequately prepared to treat deep lesions when they are identified.”
Another reason pain may persist or recur after surgery for endometriosis: Adenomyosis.19 “Adenomyosis appears as either diffuse or focal thickening of the junctional zone between the endometrium and myometrium of the uterus on T2 weighted magnetic resonance imaging (MRI),” says Dr. Stratton. “After excision of endometriosis, chronic pelvic pain is significantly more likely to persist in women who have a junctional zone thickness of more than 11 mm on MRI,” she says.
The frequent recurrence of pain after surgery for endometriosis means that the disease is a long-term challenge.
“Pelvic pain caused by endometriosis is a chronic problem that requires a multiyear management plan, involving both surgery and hormonal therapy,” says Robert L. Barbieri, MD. “To reduce the number of surgical procedures in the lifetime of a woman with endometriosis and pain, I suggest hormonal medical therapy following conservative surgery for endometriosis.”
“Definitive surgery, such as hysterectomy or hysterectomy plus bilateral salpingo-oophorectomy (BSO) typicallyresults in prolonged symptom relief,” Dr. Barbieri says. “Following hysterectomy, hormonal therapy may not be needed. Following BSO, low-dose hormonal therapy is often needed to reduce the severity of menopausal symptoms.”
Dr. Barbieri is Editor in Chief of OBG Management; Chair of Obstetrics and Gynecology at Brigham and Women’s Hospital in Boston, Massachusetts; and the Kate Macy Ladd Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School in Boston.
After surgical treatment of endometriosis associated with pain, Dr. Barbieri presents the patient with the following menu of hormonal options:
- no hormonal therapy
- estrogen-progestin contraceptives, either cyclic or continuous
- the LNG-IUS
- norethindrone acetate 5 mg daily
- DMPA 150 mg every 3 months
- leuprolide acetate depot 3.75 mg intramuscularly monthly
- nafarelin nasal spray 200 µg twice a day
- danazol 200 mg twice a day.
“I explain the side effects common with each approach and have the patient select what she determines to be her best option,” says Dr. Barbieri. “In my experience, conservative surgery followed by hormonal therapy is effective in more than 75% of women.”
“The evidence to support postoperative hormonal therapy is modest,” Dr. Barbieri notes. “The best evidence is available for use of the LNG-IUS, estrogen-progestin contraceptives, and GnRH agonists.”20–22
In addition, “major professional societies have highlighted the option of postoperative hormonal therapy to reduce the risk of recurrent pain and repetitive surgical procedures in the future,” Dr. Barbieri says.23,24
When pain recurs after surgery for endometriosis, it pays to consider what type of pain it is, says Dr. Barbieri.
“There are 2 major types of pain—nociceptive and neuropathic,” he says. “Nociceptive pain is caused by an injury, acute or chronic. Neuropathic pain is caused by ‘activation’ of neural circuits, sometimes in the absence of an ongoing injury. Many women with endometriosis and chronic pain have both nociceptive and neuropathic pain. Consequently, it is important to consider the use of a multidisciplinary pain practice in the management of chronic pain syndromes. Multidisciplinary pain practices have special expertise in the management of neuropathic pain. Standard conservative surgical intervention is unlikely to improve pain caused by neuropathic mechanisms. Likewise, opioid analgesics are not recommended for the treatment of neuropathic pain.”
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6S):S1–S62.
2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398.
3. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis: a review. Fertil Steril. 2012;98(6):1370–1379.
4. Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87.
5. Chwalisz K, Garg R, Brenner RM, Schubert G, Elger W. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann N Y Acad Sci. 2002;955:373–393, 396–406.
6. Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031.
7. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
8. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632–1636.
9. Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118(2 pt 1):223–230.
10. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346.
11. Healey M, Ang WC, Cheng C. Surgical treatment of endometriosis: a prospective randomized double-blinded trial comparing excision and ablation. Fertil Steril. 2010;94(7):2536–2540.
12. Healey M, Chang C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. JMIG. 2014;21(6):999–1004.
13. Falcone T, Wilson JR. Surgical management of endometriosis: excision or ablation. JMIG. 2014;21(6):969.
14. Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
15. Nezhat C, Nezhat F, Nezhat CH, Seidman D. Classification of endometriosis: improving the classification of endometriotic ovarian cysts. Hum Reprod 1994;9(12):2212–2216.
16. Roman H, Auber M, Mokdad C, et al. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertil Steril. 2011;96(6):1396–1400.
17. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285–1292.
18. McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147(1–3):255–264.
19. Parker JD, Leondires M, Sinaii N, Premkumar A, Nieman LK, Stratton P. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86(3):711–715.
20. Abou-Setta AM, Al-Inany HG, Farquar CM. Levonorgestrel-releasing intrauterine device for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(1):CD005072.
21. Seracchioli R, Mabrouk M, Manuzzi L, et al. Postoperative use of oral contraceptive pills for prevention of anatomic relapse or symptom recurrence following surgery. Hum Reprod. 2009;24(11):2729–2735.
22. Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril. 1997;68(5):860–864.
23. Practice Committee of the American Society for Reproductive Medicine. Treatment of pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927–935.
24. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
Endometriosis has always posed a treatment challenge. Take the early 19th Century, for example, before the widespread advent of surgery, when the disease was managed by applying leeches to the cervix. In fact, as Nezhat and colleagues note in their comprehensive survey of the 4,000-year history of endometriosis, “leeches were considered a mainstay in treating any condition associated with menstruation.”1
Fast forward to the 21st Century, and the picture is a lot clearer, though still not crystal clear. The optimal approach to endometriosis depends on numerous factors, foremost among them the chief complaint of the patient—pain or infertility (or both).
In this article—Part 2 of a 3-part series on endometriosis—the focus is on medical and surgical management of pain. Six experts address such questions as when is laparoscopy indicated, who is best qualified to treat endometriosis, is excision or ablation of lesions preferred, what is the role of hysterectomy in eliminating pain, and what to do about the problem of recurrence.
In Part 3, to be published in the June 2015 issue of OBG Management, endometriosis-associated infertility will be the topic of discussion.
In Part 1, 7 experts answer crucial questions on the diagnosis of endometriosis.
For a detailed look at the pathophysiology of endometriosis-associated pain, see “Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain,” by Kenneth A. Levey, MD, MPH, in this issue.
1. What are the options for empiric therapy?
One reason for the diagnostic delay for endometriosis, which still averages about 6 years, is that definitive diagnosis is achieved only through laparoscopic investigation and histologic confirmation. For many women who experience pain thought to be associated with endometriosis, however, clinicians begin empiric treatment with medical agents as a way to avert the need for surgery, if at all possible.
“There is no cure for endometriosis,” says John R. Lue, MD, MPH, “but there are many ways that endometriosis can be treated” and the impact of the disease reduced in a patient’s life. Dr. Lue is Associate Professor and Chief of the Section of General Obstetrics and Gynecology and Medical Director of Women’s Ambulatory Services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
Among the medical and hormonal management options:
- Nonsteroidal anti-inflammatory drugs (NSAIDs), often used with combined oral contraceptives (OCs). NSAIDs are not a long-term treatment option because of their effect on cyclo-oxygenase (COX) 1 and 2 enzymes, says Dr. Lue. COX-1 protects the gastrointestinal (GI) system, and prolonged use of NSAIDs can cause adverse GI effects.
- Cyclic combined OCs “are recommended as first-line therapy in the absence of contraindications,” says Dr. Lue, and are often used in combination with NSAIDs. However, the failure rate may be as high as 20% to 25%.2 “If pain persists after a trial of 3 to 6 months of cyclic OCs, one can consider switching to continuous low-dose combined OCs for an additional 6 months,” says Dr. Lue. When combined OCs were compared with placebo in the treatment of dysmenorrhea, they reduced baseline pain scores by 45% to 52%, compared with 14% to 17% for placebo (P<.001).2 They also reduced the volume of endometriomas by 48%, compared with 32% for placebo (P = .04). According to Linda C. Giudice, MD, PhD, “In women with severe dysmenorrhea who have been treated with cyclic combined OCs, a switch to continuous combined OCs reduced pain scores by 58% within 6 months and by 75% at 2 years” (P<.001).2 Dr. Giudice is the Robert B. Jaffe, MD, Endowed Professor in the Reproductive Sciences and Chair of Obstetrics, Gynecology, and Reproductive Sciences at the University of California, San Francisco.
- Depot medroxyprogesterone acetate (DMPA) or the levonorgestrel-releasing intrauterine system (LNG-IUS). These agents suppress the hypothalamic-pituitary-ovarian (HPO) axis to different degrees. DMPA suppresses the HPO completely, preventing ovulation. The LNG-IUS does not fully suppress the HPO but acts directly on endometrial tissue, with antiproliferative effects on eutopic and endometriotic implants, says Dr. Lue. The LNG-IUS also is effective at suppressing disease after surgical treatment, says Dr. Giudice.2
- Gonadotropin-releasing hormone (GnRH) agonist therapy, with estrogen and/or progestin add-back therapy to temper the associated loss in bone mineral density, “may be effective—if only temporarily—as it inhibits the HPO axis and blocks ovarian function, thereby greatly reducing systemic estrogen levels and inducing artificial menopause,” says Dr. Lue.
- Norethindrone acetate, a synthetic progestational agent, is occasionally used as empiric therapy for endometriosis because of its ability to inhibit ovulation. It has antiandrogenic and antiestrogenic effects.
- Aromatase inhibitors. Dr. Lue points to considerable evidence that endometriotic implants are an autocrine source of estrogen.3 “This locally produced estrogen results from overexpression of the enzyme P450 aromatase by endometriotic tissue,” he says. Consequently, in postmenopausal women, “aromatase inhibitors may be used orally in a daily pill form to curtail endometriotic implant production of estrogen and subsequent implant growth.”4 In women of reproductive age, aromatase inhibitors are combined with an HPO-suppressive therapy, such as norethindrone acetate. These strategies represent off-label use of aromatase inhibitors.
- Danazol, a synthetic androgen, has been used in the past to treat dysmenorrhea and dyspareunia. Because of its severe androgenic effects, however, it is not widely used today.
“For those using medical approaches, endometriosis-related pain may be reduced by using hormonal treatments to modify reproductive tract events, thereby decreasing local peritoneal inflammation and cytokine production,” says Pamela Stratton, MD. Because endometriosis is a “central sensitivity syndrome,” multidisciplinary approaches may be beneficial to treat myofascial dysfunction and sensitization, such as physical therapy. “Chronic pain conditions that overlap with endometriosis-associated pain, such as migraines, irritable bowel syndrome, or painful bladder syndrome should be identified and treated. Mood changes of depression and anxiety common to women with endometriosis-associated pain also warrant treatment,” she says.
Dr. Stratton is Chief of the Gynecology Consult Service, Program in Reproductive and Adult Endocrinology, at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
2. When is laparoscopy indicated?
When medical and hormonal treatments fail to control a patient’s pain, laparoscopy is indicated to confirm the diagnosis of endometriosis. During that procedure, it is also advisable to treat any endometriosis that is present, provided the surgeon is highly experienced in such treatment.
Proper treatment is preferable—even if it requires expert consultation. “No treatment and referral to a more experienced surgeon are better than incomplete treatment by an inexperienced surgeon,” says Ceana Nezhat, MD. “Not all GYN surgeons have the expertise to treat advanced endometriosis.” Dr. Nezhat is Director of the Nezhat Medical Center and Medical Director of Training and Education at Northside Hospital, both in Atlanta, Georgia.
Dr. Stratton agrees about the importance of thorough treatment of endometriosis at the time of diagnostic laparoscopy. “At the laparoscopy, the patient benefits if all potential sources of pain are investigated and addressed.” At surgery, the surgeon should look for and treat any lesions suspicious for endometriosis, as well as any other finding that might contribute to pain, she says. “For example, routinely inspecting the appendix for endometriosis or other lesions, and removing affected appendices is reasonable; also, lysis and, where possible, excision of adhesions is an important strategy.”
If a medical approach fails for a patient, “then surgery is indicated to confirm the diagnosis and treat the disease,” agrees Tommaso Falcone, MD.
“Surgery is very effective in treating the pain associated with endometriosis,” Dr. Falcone continues. Randomized clinical trials have shown that up to 90% of patients who obtain pain relief from surgery will have an effect lasting 1 year.6 If patients do not get relief, then the association of the pain with endometriosis should be questioned and other causes searched.” Dr. Falcone is Professor and Chair of Obstetrics and Gynecology at the Cleveland Clinic in Cleveland, Ohio.
The most common anatomic sites of implants
“The most common accepted theory for pathogenesis of endometriosis suggests that implants develop when debris from retrograde menstruation attaches to the pelvic peritoneum,” says Dr. Stratton.7 “Thus, the vast majority of lesions occur in the dependent portions of the pelvis, which include the ovarian fossae (posterior broad ligament under the ovaries), cul de sac, and the uterosacral ligaments.8 The bladder peritoneum, ovarian surface, uterine peritoneal surface, fallopian tube, and pelvic sidewall are also frequent sites. The colon and appendix are less common sites, and small bowel lesions are rare.”
“However, pain location does not correlate with lesion location,” Dr. Stratton notes. “For this reason, the goal at surgery is to treat all lesions, even ones that are not in sites of pain.”
3. How should disease be staged?
Most surgeons with expertise in treating endometriosis attempt to stage the disease at the time of initial laparoscopy, even though a patient’s pain does not always correlate with the stage of disease.
“The staging system for endometriosis is a means to systematically catalogue where lesions are located,” says Dr. Stratton.
The most commonly used classification system was developed by the American Society for Reproductive Medicine (ASRM). It takes into account such characteristics as how deep an implant lies, the extent to which it obliterates the posterior cul de sac, and the presence and extent of adhesions. Although the classification system is broken down into 4 stages ranging from minimal to severe disease, it is fairly complex. For example, it assigns a score for each lesion as well as the size and location of that lesion, notes Dr. Stratton. The presence of an endometrioma automatically renders the disease as stage III or IV, and an obliterated cul de sac means the endometriosis is graded as stage IV.
“This system enables us to communicate with each other about patients and may guide future surgeries for assessment of lesion recurrence or the planning of treatment for lesions the surgeon was unable to treat at an initial surgery,” says Dr. Stratton.
“Women with uterosacral nodularity, fixed pelvic organs, or severe pain with endometriomas may have deep infiltrating lesions. These lesions, in particular, are not captured well with the current staging system,” says Dr. Stratton. Because they appear to be innervated, “the greatest benefit to the patient is achieved by completely excising these lesions.” Preoperative imaging may help confirm the existence, location, and extent of these deep lesions and help the surgeon plan her approach “based on clinical and imaging findings.”
“Severity of pain or duration of surgical effect does not correlate with stage or extent of disease,” Dr. Stratton says.9 “In fact, patients with the least amount of disease noted at surgery experience pain sooner, suggesting that the central nervous system may have been remodeled prior to surgery or that the pain is in part due to some other cause.10 This observation underscores the principle that, while endometriosis may initiate pain, the pain experience is determined by engagement of the central nervous system.”
For more information on the ASRM revised classification of endometriosis, go to http://www.fertstert.org/article/S0015-0282(97)81391-X/pdf.
4. Which is preferable—excision or ablation?
In a prospective, randomized, double-blind study, Healey and colleagues compared pain levels following laparoscopic treatment of endometriosis with either excision or ablation. Preoperatively, women in the study completed a questionnaire rating various types of pain using visual analogue scales. They then were randomly assigned to treatment of endometriosis via excision or ablation. Postoperatively, they again completed a questionnaire about pain levels at 3, 6, 9, and 12 months. Investigators found no significant difference in pain scores at 12 months.11
Five-year follow-up of the same population yielded slightly different findings, however. Although there was a reduction in all pain scores at 5 years in both the excision and ablation groups, a significantly greater reduction in dyspareunia was observed in the excision group at 5 years.12
In an editorial accompanying the 5-year follow-up data, Dr. Falcone and a coauthor called excision versus ablation of ovarian, bowel, and peritoneal endometriosis one of the “great debates” in the surgical management of endometriosis.13
“When there is deep involvement of adjacent organs, there is general consensus that excision is best for optimal surgical outcome,” they write. “However, for disease involving the peritoneum alone, there are proponents for either option.”13
“This is a very controversial issue,” says Dr. Falcone, “and the debate can sometimes be somewhat inflammatory…. It is hard to understand how a comparative trial could even be accomplished between excision and ablation,” he adds. “In my experience, deep disease typically occurs on the pelvic sidewall over the ureter or in the cul de sac on the bowel or infiltrating the bladder peritoneum. Therefore, ablation would increase the risk of damaging any of these structures. With superficial disease away from critical structures, it should be fine to ablate. Everywhere else and with deep disease you need to excise or leave disease behind.”
“Endometriomas are a special situation,” Dr. Falcone says. “Excision of the cyst has been shown in randomized controlled trials (RCTs) to be associated with less risk of recurrence.14 Therefore, it should be the treatment of choice. However, in patients interested in future fertility, we must take into consideration the potential damage to ovarian reserve associated with excision.”
Endometriosis of the ovaries has unique manifestations. “My approach to ovarian cysts depends on their classification,” says Dr. Nezhat.15 In general, primary endometriomas (Type 1) are small, superficial cysts that contain dark “chocolate” fluid. They tend to be firmly adherent to the ovarian tissue and difficult to remove surgically.
Secondary endometriomas (Type 2) are follicular or luteal cysts that have been involved or invaded by cortical endometriotic implants or by primary endometrioma. Secondary endometriomas are further classified by the relationship between cortical endometriosis and the cyst wall. Type 2A endometriomas are usually large, with a capsule that is easily separated from ovarian tissue. Type 2B endometriomas have some features of functional cysts but show deep involvement with surface endometriosis. Type 2C endometriomas are similar, showing extensive surface endometrial implants but with deep penetration of the endometriosis into the cyst wall.15
“For Type 1 endometriomas, I biopsy the cyst to ensure the lesion is benign, then vaporize the endometrioma,” Dr. Nezhat says. “In cases of type 2A and 2B endometriomas, the cyst capsule is easily enucleated and removed. Type 2C endometriomas are biopsied as well and then I proceed with vaporizing the fibrotic area with a low-power energy source, such as neutral argon plasma, avoiding excessive coagulation and thermal injury.” Recent literature supports the idea of evaluation and biopsy of fibrotic endometriomas to confirm benign conditions, followed by ablation without compromising ovarian function.16
“Excision and ablation both have indications,” Dr. Nezhat asserts. “It depends on the location and depth of penetration of implants, as well as the patient’s ultimate goal. For example, if the patient desires future fertility and has endometriosis on the ovary, removal by excision could damage ovarian function. The same holds true for endometriosis on the fallopian tubes. It’s better in such cases to ablate.”
“Ablation is different from coagulation, which is not recommended,” Dr. Nezhat explains. “Ablation vaporizes the diseased area layer by layer, like peeling an onion, until the disease is eradicated. It is similar to dermatological skin resurfacing. Vaporization is preferable for endometriosis on the tubes and ovaries in patients who desire pregnancy. The choice between excision and ablation depends on the location, depth of penetration, and the patient’s desire for fertility.”
Either way, and regardless of the primary indication for surgery—pain versus infertility—a minimally invasive gynecologic surgeon is expected to have ability in performing both techniques, Dr. Nezhat says.
The following videos have been provided by AAGL SurgeryU to compliment the content of this article regarding endometriosis. You can watch these videos, and more than 1,500 others, at AAGL.org/surgeryu. Laparoscopic excision of stage IV endometriosis Einarsson JI This case, originally presented as a SurgeryU live event, features a 41-year-old woman (G3P1) with a 3-year history of left-sided pelvic pain, deep dyspareunia, constipation, and dysmenorrhea. She also has infertility and is planning an IVF treatment shortly. On examination she was noted to have significant rectovaginal tenderness and nodularity. A pelvic MRI demonstrated a 3-cm irregular mass extending from the cervix into the cul-de-sac up to the left lateral pelvic sidewall. Abdominal wall endometriosis Hawkins E, Patzkowsky K, Lopez J This video demonstrates a typical presentation of abdominal wall endometriosis (AWE), also known as subcutaneous endometriosis or scar endometriosis. It is important for gynecologists to be familiar with this more uncommon form of the disease and its management. This video also demonstrates surgical management of advanced AWE involving the subcutaneous tissue, fascia, and rectus muscle. Laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain Pendergrass M This video depicts the laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain. The patient underwent menarche at age 11 and developed cyclic pelvic pain 6 months later. Due to the severity of the pain she has been unable to attend school for the past 2 months, and has stopped participating in sports. A diagnostic laparoscopy revealed red/brown superficial endometriosis lesions on the peritoneum in the posterior cul de sac, bilateral uterosacral ligaments, and bilateral broad ligaments. |
5. Is hysterectomy definitive treatment for pain?
“Not necessarily,” says Dr. Nezhat. “Hysterectomy by itself doesn’t take care of endometriosis unless the patient has adenomyosis. If a patient has endometriosis, the first step is complete treatment of the disease to restore the anatomy. Then the next step might be hysterectomy to give a better long-term result, especially in cases of adenomyosis. Removal of the ovaries at the time of hysterectomy has to be individualized.”
“The implication that hysterectomy ‘cures’ endometriosis is false yet is stated in some textbooks,” says Dr. Nezhat. “Even at the time of hysterectomy, the first step should be complete treatment of endometriosis and restoration of anatomy, followed by the hysterectomy. Leaving endometriosis behind, believing it will go away by itself or not cause future issues, is a gross misperception.”
Removal of the ovaries at hysterectomy?
“There are few comparative studies on the long-term follow-up of patients who have undergone hysterectomy with or without removal of both ovaries,” says Dr. Falcone. “The conventional dogma has been that, in women undergoing definitive surgery for endometriosis, both ovaries should be removed, even if they are normal. I personally believe that this was the case because hysterectomy was often performed without excision of the endometriosis. So the uterus was removed and disease was left behind. In these cases, recurrent symptoms were due to persistent disease.”
“We reported our experience at the Cleveland Clinic with a 7-year follow-up,” Dr. Falcone continues. “Hysterectomy was performed with excision of all visible disease. Ovaries were conserved if normal and removed if they had disease. We looked at the reoperation-free frequency over time. In women undergoing hysterectomy with excision of visible disease but ovarian preservation, the reoperation-free percentages at 2, 5, and 7 years were 95%, 86%, and 77%, respectively, versus 96%, 91%, and 91% in those without ovarian preservation. So, overall, there was an advantage over time for removal of the ovaries. However, in the subset of women between ages 30 and 39 years, there was no difference in the long-term recurrence rate if the ovaries were left in. For this reason, in women under 40, we recommend keeping normal ovaries if all disease is removed.”17
6. Can the risk of postoperative recurrence be reduced?
“The main problem with surgery is the recurrence rate,” Dr. Falcone says. “Studies have shown that the recurrence rate of pain at 7 years may be as high as 50%.”17 Furthermore, “the recurrence of pain may not be associated with visualized endometriosis at laparoscopy.”
“Incomplete removal of lesions may be associated with an increase in pain after surgery,” says Dr. Stratton.18 “Incomplete removal of lesions may occur because of varying technical skill or specific lesion characteristics. The lesions may be difficult to remove because of their location. Lesions may not be recognized because their appearance can vary from subtle (red or clear or white) to classic (blue-black). The depth of the lesion may not be appreciated until surgery is under way and a surgeon may not be adequately prepared to treat deep lesions when they are identified.”
Another reason pain may persist or recur after surgery for endometriosis: Adenomyosis.19 “Adenomyosis appears as either diffuse or focal thickening of the junctional zone between the endometrium and myometrium of the uterus on T2 weighted magnetic resonance imaging (MRI),” says Dr. Stratton. “After excision of endometriosis, chronic pelvic pain is significantly more likely to persist in women who have a junctional zone thickness of more than 11 mm on MRI,” she says.
The frequent recurrence of pain after surgery for endometriosis means that the disease is a long-term challenge.
“Pelvic pain caused by endometriosis is a chronic problem that requires a multiyear management plan, involving both surgery and hormonal therapy,” says Robert L. Barbieri, MD. “To reduce the number of surgical procedures in the lifetime of a woman with endometriosis and pain, I suggest hormonal medical therapy following conservative surgery for endometriosis.”
“Definitive surgery, such as hysterectomy or hysterectomy plus bilateral salpingo-oophorectomy (BSO) typicallyresults in prolonged symptom relief,” Dr. Barbieri says. “Following hysterectomy, hormonal therapy may not be needed. Following BSO, low-dose hormonal therapy is often needed to reduce the severity of menopausal symptoms.”
Dr. Barbieri is Editor in Chief of OBG Management; Chair of Obstetrics and Gynecology at Brigham and Women’s Hospital in Boston, Massachusetts; and the Kate Macy Ladd Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School in Boston.
After surgical treatment of endometriosis associated with pain, Dr. Barbieri presents the patient with the following menu of hormonal options:
- no hormonal therapy
- estrogen-progestin contraceptives, either cyclic or continuous
- the LNG-IUS
- norethindrone acetate 5 mg daily
- DMPA 150 mg every 3 months
- leuprolide acetate depot 3.75 mg intramuscularly monthly
- nafarelin nasal spray 200 µg twice a day
- danazol 200 mg twice a day.
“I explain the side effects common with each approach and have the patient select what she determines to be her best option,” says Dr. Barbieri. “In my experience, conservative surgery followed by hormonal therapy is effective in more than 75% of women.”
“The evidence to support postoperative hormonal therapy is modest,” Dr. Barbieri notes. “The best evidence is available for use of the LNG-IUS, estrogen-progestin contraceptives, and GnRH agonists.”20–22
In addition, “major professional societies have highlighted the option of postoperative hormonal therapy to reduce the risk of recurrent pain and repetitive surgical procedures in the future,” Dr. Barbieri says.23,24
When pain recurs after surgery for endometriosis, it pays to consider what type of pain it is, says Dr. Barbieri.
“There are 2 major types of pain—nociceptive and neuropathic,” he says. “Nociceptive pain is caused by an injury, acute or chronic. Neuropathic pain is caused by ‘activation’ of neural circuits, sometimes in the absence of an ongoing injury. Many women with endometriosis and chronic pain have both nociceptive and neuropathic pain. Consequently, it is important to consider the use of a multidisciplinary pain practice in the management of chronic pain syndromes. Multidisciplinary pain practices have special expertise in the management of neuropathic pain. Standard conservative surgical intervention is unlikely to improve pain caused by neuropathic mechanisms. Likewise, opioid analgesics are not recommended for the treatment of neuropathic pain.”
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometriosis has always posed a treatment challenge. Take the early 19th Century, for example, before the widespread advent of surgery, when the disease was managed by applying leeches to the cervix. In fact, as Nezhat and colleagues note in their comprehensive survey of the 4,000-year history of endometriosis, “leeches were considered a mainstay in treating any condition associated with menstruation.”1
Fast forward to the 21st Century, and the picture is a lot clearer, though still not crystal clear. The optimal approach to endometriosis depends on numerous factors, foremost among them the chief complaint of the patient—pain or infertility (or both).
In this article—Part 2 of a 3-part series on endometriosis—the focus is on medical and surgical management of pain. Six experts address such questions as when is laparoscopy indicated, who is best qualified to treat endometriosis, is excision or ablation of lesions preferred, what is the role of hysterectomy in eliminating pain, and what to do about the problem of recurrence.
In Part 3, to be published in the June 2015 issue of OBG Management, endometriosis-associated infertility will be the topic of discussion.
In Part 1, 7 experts answer crucial questions on the diagnosis of endometriosis.
For a detailed look at the pathophysiology of endometriosis-associated pain, see “Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain,” by Kenneth A. Levey, MD, MPH, in this issue.
1. What are the options for empiric therapy?
One reason for the diagnostic delay for endometriosis, which still averages about 6 years, is that definitive diagnosis is achieved only through laparoscopic investigation and histologic confirmation. For many women who experience pain thought to be associated with endometriosis, however, clinicians begin empiric treatment with medical agents as a way to avert the need for surgery, if at all possible.
“There is no cure for endometriosis,” says John R. Lue, MD, MPH, “but there are many ways that endometriosis can be treated” and the impact of the disease reduced in a patient’s life. Dr. Lue is Associate Professor and Chief of the Section of General Obstetrics and Gynecology and Medical Director of Women’s Ambulatory Services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
Among the medical and hormonal management options:
- Nonsteroidal anti-inflammatory drugs (NSAIDs), often used with combined oral contraceptives (OCs). NSAIDs are not a long-term treatment option because of their effect on cyclo-oxygenase (COX) 1 and 2 enzymes, says Dr. Lue. COX-1 protects the gastrointestinal (GI) system, and prolonged use of NSAIDs can cause adverse GI effects.
- Cyclic combined OCs “are recommended as first-line therapy in the absence of contraindications,” says Dr. Lue, and are often used in combination with NSAIDs. However, the failure rate may be as high as 20% to 25%.2 “If pain persists after a trial of 3 to 6 months of cyclic OCs, one can consider switching to continuous low-dose combined OCs for an additional 6 months,” says Dr. Lue. When combined OCs were compared with placebo in the treatment of dysmenorrhea, they reduced baseline pain scores by 45% to 52%, compared with 14% to 17% for placebo (P<.001).2 They also reduced the volume of endometriomas by 48%, compared with 32% for placebo (P = .04). According to Linda C. Giudice, MD, PhD, “In women with severe dysmenorrhea who have been treated with cyclic combined OCs, a switch to continuous combined OCs reduced pain scores by 58% within 6 months and by 75% at 2 years” (P<.001).2 Dr. Giudice is the Robert B. Jaffe, MD, Endowed Professor in the Reproductive Sciences and Chair of Obstetrics, Gynecology, and Reproductive Sciences at the University of California, San Francisco.
- Depot medroxyprogesterone acetate (DMPA) or the levonorgestrel-releasing intrauterine system (LNG-IUS). These agents suppress the hypothalamic-pituitary-ovarian (HPO) axis to different degrees. DMPA suppresses the HPO completely, preventing ovulation. The LNG-IUS does not fully suppress the HPO but acts directly on endometrial tissue, with antiproliferative effects on eutopic and endometriotic implants, says Dr. Lue. The LNG-IUS also is effective at suppressing disease after surgical treatment, says Dr. Giudice.2
- Gonadotropin-releasing hormone (GnRH) agonist therapy, with estrogen and/or progestin add-back therapy to temper the associated loss in bone mineral density, “may be effective—if only temporarily—as it inhibits the HPO axis and blocks ovarian function, thereby greatly reducing systemic estrogen levels and inducing artificial menopause,” says Dr. Lue.
- Norethindrone acetate, a synthetic progestational agent, is occasionally used as empiric therapy for endometriosis because of its ability to inhibit ovulation. It has antiandrogenic and antiestrogenic effects.
- Aromatase inhibitors. Dr. Lue points to considerable evidence that endometriotic implants are an autocrine source of estrogen.3 “This locally produced estrogen results from overexpression of the enzyme P450 aromatase by endometriotic tissue,” he says. Consequently, in postmenopausal women, “aromatase inhibitors may be used orally in a daily pill form to curtail endometriotic implant production of estrogen and subsequent implant growth.”4 In women of reproductive age, aromatase inhibitors are combined with an HPO-suppressive therapy, such as norethindrone acetate. These strategies represent off-label use of aromatase inhibitors.
- Danazol, a synthetic androgen, has been used in the past to treat dysmenorrhea and dyspareunia. Because of its severe androgenic effects, however, it is not widely used today.
“For those using medical approaches, endometriosis-related pain may be reduced by using hormonal treatments to modify reproductive tract events, thereby decreasing local peritoneal inflammation and cytokine production,” says Pamela Stratton, MD. Because endometriosis is a “central sensitivity syndrome,” multidisciplinary approaches may be beneficial to treat myofascial dysfunction and sensitization, such as physical therapy. “Chronic pain conditions that overlap with endometriosis-associated pain, such as migraines, irritable bowel syndrome, or painful bladder syndrome should be identified and treated. Mood changes of depression and anxiety common to women with endometriosis-associated pain also warrant treatment,” she says.
Dr. Stratton is Chief of the Gynecology Consult Service, Program in Reproductive and Adult Endocrinology, at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
2. When is laparoscopy indicated?
When medical and hormonal treatments fail to control a patient’s pain, laparoscopy is indicated to confirm the diagnosis of endometriosis. During that procedure, it is also advisable to treat any endometriosis that is present, provided the surgeon is highly experienced in such treatment.
Proper treatment is preferable—even if it requires expert consultation. “No treatment and referral to a more experienced surgeon are better than incomplete treatment by an inexperienced surgeon,” says Ceana Nezhat, MD. “Not all GYN surgeons have the expertise to treat advanced endometriosis.” Dr. Nezhat is Director of the Nezhat Medical Center and Medical Director of Training and Education at Northside Hospital, both in Atlanta, Georgia.
Dr. Stratton agrees about the importance of thorough treatment of endometriosis at the time of diagnostic laparoscopy. “At the laparoscopy, the patient benefits if all potential sources of pain are investigated and addressed.” At surgery, the surgeon should look for and treat any lesions suspicious for endometriosis, as well as any other finding that might contribute to pain, she says. “For example, routinely inspecting the appendix for endometriosis or other lesions, and removing affected appendices is reasonable; also, lysis and, where possible, excision of adhesions is an important strategy.”
If a medical approach fails for a patient, “then surgery is indicated to confirm the diagnosis and treat the disease,” agrees Tommaso Falcone, MD.
“Surgery is very effective in treating the pain associated with endometriosis,” Dr. Falcone continues. Randomized clinical trials have shown that up to 90% of patients who obtain pain relief from surgery will have an effect lasting 1 year.6 If patients do not get relief, then the association of the pain with endometriosis should be questioned and other causes searched.” Dr. Falcone is Professor and Chair of Obstetrics and Gynecology at the Cleveland Clinic in Cleveland, Ohio.
The most common anatomic sites of implants
“The most common accepted theory for pathogenesis of endometriosis suggests that implants develop when debris from retrograde menstruation attaches to the pelvic peritoneum,” says Dr. Stratton.7 “Thus, the vast majority of lesions occur in the dependent portions of the pelvis, which include the ovarian fossae (posterior broad ligament under the ovaries), cul de sac, and the uterosacral ligaments.8 The bladder peritoneum, ovarian surface, uterine peritoneal surface, fallopian tube, and pelvic sidewall are also frequent sites. The colon and appendix are less common sites, and small bowel lesions are rare.”
“However, pain location does not correlate with lesion location,” Dr. Stratton notes. “For this reason, the goal at surgery is to treat all lesions, even ones that are not in sites of pain.”
3. How should disease be staged?
Most surgeons with expertise in treating endometriosis attempt to stage the disease at the time of initial laparoscopy, even though a patient’s pain does not always correlate with the stage of disease.
“The staging system for endometriosis is a means to systematically catalogue where lesions are located,” says Dr. Stratton.
The most commonly used classification system was developed by the American Society for Reproductive Medicine (ASRM). It takes into account such characteristics as how deep an implant lies, the extent to which it obliterates the posterior cul de sac, and the presence and extent of adhesions. Although the classification system is broken down into 4 stages ranging from minimal to severe disease, it is fairly complex. For example, it assigns a score for each lesion as well as the size and location of that lesion, notes Dr. Stratton. The presence of an endometrioma automatically renders the disease as stage III or IV, and an obliterated cul de sac means the endometriosis is graded as stage IV.
“This system enables us to communicate with each other about patients and may guide future surgeries for assessment of lesion recurrence or the planning of treatment for lesions the surgeon was unable to treat at an initial surgery,” says Dr. Stratton.
“Women with uterosacral nodularity, fixed pelvic organs, or severe pain with endometriomas may have deep infiltrating lesions. These lesions, in particular, are not captured well with the current staging system,” says Dr. Stratton. Because they appear to be innervated, “the greatest benefit to the patient is achieved by completely excising these lesions.” Preoperative imaging may help confirm the existence, location, and extent of these deep lesions and help the surgeon plan her approach “based on clinical and imaging findings.”
“Severity of pain or duration of surgical effect does not correlate with stage or extent of disease,” Dr. Stratton says.9 “In fact, patients with the least amount of disease noted at surgery experience pain sooner, suggesting that the central nervous system may have been remodeled prior to surgery or that the pain is in part due to some other cause.10 This observation underscores the principle that, while endometriosis may initiate pain, the pain experience is determined by engagement of the central nervous system.”
For more information on the ASRM revised classification of endometriosis, go to http://www.fertstert.org/article/S0015-0282(97)81391-X/pdf.
4. Which is preferable—excision or ablation?
In a prospective, randomized, double-blind study, Healey and colleagues compared pain levels following laparoscopic treatment of endometriosis with either excision or ablation. Preoperatively, women in the study completed a questionnaire rating various types of pain using visual analogue scales. They then were randomly assigned to treatment of endometriosis via excision or ablation. Postoperatively, they again completed a questionnaire about pain levels at 3, 6, 9, and 12 months. Investigators found no significant difference in pain scores at 12 months.11
Five-year follow-up of the same population yielded slightly different findings, however. Although there was a reduction in all pain scores at 5 years in both the excision and ablation groups, a significantly greater reduction in dyspareunia was observed in the excision group at 5 years.12
In an editorial accompanying the 5-year follow-up data, Dr. Falcone and a coauthor called excision versus ablation of ovarian, bowel, and peritoneal endometriosis one of the “great debates” in the surgical management of endometriosis.13
“When there is deep involvement of adjacent organs, there is general consensus that excision is best for optimal surgical outcome,” they write. “However, for disease involving the peritoneum alone, there are proponents for either option.”13
“This is a very controversial issue,” says Dr. Falcone, “and the debate can sometimes be somewhat inflammatory…. It is hard to understand how a comparative trial could even be accomplished between excision and ablation,” he adds. “In my experience, deep disease typically occurs on the pelvic sidewall over the ureter or in the cul de sac on the bowel or infiltrating the bladder peritoneum. Therefore, ablation would increase the risk of damaging any of these structures. With superficial disease away from critical structures, it should be fine to ablate. Everywhere else and with deep disease you need to excise or leave disease behind.”
“Endometriomas are a special situation,” Dr. Falcone says. “Excision of the cyst has been shown in randomized controlled trials (RCTs) to be associated with less risk of recurrence.14 Therefore, it should be the treatment of choice. However, in patients interested in future fertility, we must take into consideration the potential damage to ovarian reserve associated with excision.”
Endometriosis of the ovaries has unique manifestations. “My approach to ovarian cysts depends on their classification,” says Dr. Nezhat.15 In general, primary endometriomas (Type 1) are small, superficial cysts that contain dark “chocolate” fluid. They tend to be firmly adherent to the ovarian tissue and difficult to remove surgically.
Secondary endometriomas (Type 2) are follicular or luteal cysts that have been involved or invaded by cortical endometriotic implants or by primary endometrioma. Secondary endometriomas are further classified by the relationship between cortical endometriosis and the cyst wall. Type 2A endometriomas are usually large, with a capsule that is easily separated from ovarian tissue. Type 2B endometriomas have some features of functional cysts but show deep involvement with surface endometriosis. Type 2C endometriomas are similar, showing extensive surface endometrial implants but with deep penetration of the endometriosis into the cyst wall.15
“For Type 1 endometriomas, I biopsy the cyst to ensure the lesion is benign, then vaporize the endometrioma,” Dr. Nezhat says. “In cases of type 2A and 2B endometriomas, the cyst capsule is easily enucleated and removed. Type 2C endometriomas are biopsied as well and then I proceed with vaporizing the fibrotic area with a low-power energy source, such as neutral argon plasma, avoiding excessive coagulation and thermal injury.” Recent literature supports the idea of evaluation and biopsy of fibrotic endometriomas to confirm benign conditions, followed by ablation without compromising ovarian function.16
“Excision and ablation both have indications,” Dr. Nezhat asserts. “It depends on the location and depth of penetration of implants, as well as the patient’s ultimate goal. For example, if the patient desires future fertility and has endometriosis on the ovary, removal by excision could damage ovarian function. The same holds true for endometriosis on the fallopian tubes. It’s better in such cases to ablate.”
“Ablation is different from coagulation, which is not recommended,” Dr. Nezhat explains. “Ablation vaporizes the diseased area layer by layer, like peeling an onion, until the disease is eradicated. It is similar to dermatological skin resurfacing. Vaporization is preferable for endometriosis on the tubes and ovaries in patients who desire pregnancy. The choice between excision and ablation depends on the location, depth of penetration, and the patient’s desire for fertility.”
Either way, and regardless of the primary indication for surgery—pain versus infertility—a minimally invasive gynecologic surgeon is expected to have ability in performing both techniques, Dr. Nezhat says.
The following videos have been provided by AAGL SurgeryU to compliment the content of this article regarding endometriosis. You can watch these videos, and more than 1,500 others, at AAGL.org/surgeryu. Laparoscopic excision of stage IV endometriosis Einarsson JI This case, originally presented as a SurgeryU live event, features a 41-year-old woman (G3P1) with a 3-year history of left-sided pelvic pain, deep dyspareunia, constipation, and dysmenorrhea. She also has infertility and is planning an IVF treatment shortly. On examination she was noted to have significant rectovaginal tenderness and nodularity. A pelvic MRI demonstrated a 3-cm irregular mass extending from the cervix into the cul-de-sac up to the left lateral pelvic sidewall. Abdominal wall endometriosis Hawkins E, Patzkowsky K, Lopez J This video demonstrates a typical presentation of abdominal wall endometriosis (AWE), also known as subcutaneous endometriosis or scar endometriosis. It is important for gynecologists to be familiar with this more uncommon form of the disease and its management. This video also demonstrates surgical management of advanced AWE involving the subcutaneous tissue, fascia, and rectus muscle. Laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain Pendergrass M This video depicts the laparoscopic excision of endometriosis in a 14-year-old patient with chronic pelvic pain. The patient underwent menarche at age 11 and developed cyclic pelvic pain 6 months later. Due to the severity of the pain she has been unable to attend school for the past 2 months, and has stopped participating in sports. A diagnostic laparoscopy revealed red/brown superficial endometriosis lesions on the peritoneum in the posterior cul de sac, bilateral uterosacral ligaments, and bilateral broad ligaments. |
5. Is hysterectomy definitive treatment for pain?
“Not necessarily,” says Dr. Nezhat. “Hysterectomy by itself doesn’t take care of endometriosis unless the patient has adenomyosis. If a patient has endometriosis, the first step is complete treatment of the disease to restore the anatomy. Then the next step might be hysterectomy to give a better long-term result, especially in cases of adenomyosis. Removal of the ovaries at the time of hysterectomy has to be individualized.”
“The implication that hysterectomy ‘cures’ endometriosis is false yet is stated in some textbooks,” says Dr. Nezhat. “Even at the time of hysterectomy, the first step should be complete treatment of endometriosis and restoration of anatomy, followed by the hysterectomy. Leaving endometriosis behind, believing it will go away by itself or not cause future issues, is a gross misperception.”
Removal of the ovaries at hysterectomy?
“There are few comparative studies on the long-term follow-up of patients who have undergone hysterectomy with or without removal of both ovaries,” says Dr. Falcone. “The conventional dogma has been that, in women undergoing definitive surgery for endometriosis, both ovaries should be removed, even if they are normal. I personally believe that this was the case because hysterectomy was often performed without excision of the endometriosis. So the uterus was removed and disease was left behind. In these cases, recurrent symptoms were due to persistent disease.”
“We reported our experience at the Cleveland Clinic with a 7-year follow-up,” Dr. Falcone continues. “Hysterectomy was performed with excision of all visible disease. Ovaries were conserved if normal and removed if they had disease. We looked at the reoperation-free frequency over time. In women undergoing hysterectomy with excision of visible disease but ovarian preservation, the reoperation-free percentages at 2, 5, and 7 years were 95%, 86%, and 77%, respectively, versus 96%, 91%, and 91% in those without ovarian preservation. So, overall, there was an advantage over time for removal of the ovaries. However, in the subset of women between ages 30 and 39 years, there was no difference in the long-term recurrence rate if the ovaries were left in. For this reason, in women under 40, we recommend keeping normal ovaries if all disease is removed.”17
6. Can the risk of postoperative recurrence be reduced?
“The main problem with surgery is the recurrence rate,” Dr. Falcone says. “Studies have shown that the recurrence rate of pain at 7 years may be as high as 50%.”17 Furthermore, “the recurrence of pain may not be associated with visualized endometriosis at laparoscopy.”
“Incomplete removal of lesions may be associated with an increase in pain after surgery,” says Dr. Stratton.18 “Incomplete removal of lesions may occur because of varying technical skill or specific lesion characteristics. The lesions may be difficult to remove because of their location. Lesions may not be recognized because their appearance can vary from subtle (red or clear or white) to classic (blue-black). The depth of the lesion may not be appreciated until surgery is under way and a surgeon may not be adequately prepared to treat deep lesions when they are identified.”
Another reason pain may persist or recur after surgery for endometriosis: Adenomyosis.19 “Adenomyosis appears as either diffuse or focal thickening of the junctional zone between the endometrium and myometrium of the uterus on T2 weighted magnetic resonance imaging (MRI),” says Dr. Stratton. “After excision of endometriosis, chronic pelvic pain is significantly more likely to persist in women who have a junctional zone thickness of more than 11 mm on MRI,” she says.
The frequent recurrence of pain after surgery for endometriosis means that the disease is a long-term challenge.
“Pelvic pain caused by endometriosis is a chronic problem that requires a multiyear management plan, involving both surgery and hormonal therapy,” says Robert L. Barbieri, MD. “To reduce the number of surgical procedures in the lifetime of a woman with endometriosis and pain, I suggest hormonal medical therapy following conservative surgery for endometriosis.”
“Definitive surgery, such as hysterectomy or hysterectomy plus bilateral salpingo-oophorectomy (BSO) typicallyresults in prolonged symptom relief,” Dr. Barbieri says. “Following hysterectomy, hormonal therapy may not be needed. Following BSO, low-dose hormonal therapy is often needed to reduce the severity of menopausal symptoms.”
Dr. Barbieri is Editor in Chief of OBG Management; Chair of Obstetrics and Gynecology at Brigham and Women’s Hospital in Boston, Massachusetts; and the Kate Macy Ladd Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School in Boston.
After surgical treatment of endometriosis associated with pain, Dr. Barbieri presents the patient with the following menu of hormonal options:
- no hormonal therapy
- estrogen-progestin contraceptives, either cyclic or continuous
- the LNG-IUS
- norethindrone acetate 5 mg daily
- DMPA 150 mg every 3 months
- leuprolide acetate depot 3.75 mg intramuscularly monthly
- nafarelin nasal spray 200 µg twice a day
- danazol 200 mg twice a day.
“I explain the side effects common with each approach and have the patient select what she determines to be her best option,” says Dr. Barbieri. “In my experience, conservative surgery followed by hormonal therapy is effective in more than 75% of women.”
“The evidence to support postoperative hormonal therapy is modest,” Dr. Barbieri notes. “The best evidence is available for use of the LNG-IUS, estrogen-progestin contraceptives, and GnRH agonists.”20–22
In addition, “major professional societies have highlighted the option of postoperative hormonal therapy to reduce the risk of recurrent pain and repetitive surgical procedures in the future,” Dr. Barbieri says.23,24
When pain recurs after surgery for endometriosis, it pays to consider what type of pain it is, says Dr. Barbieri.
“There are 2 major types of pain—nociceptive and neuropathic,” he says. “Nociceptive pain is caused by an injury, acute or chronic. Neuropathic pain is caused by ‘activation’ of neural circuits, sometimes in the absence of an ongoing injury. Many women with endometriosis and chronic pain have both nociceptive and neuropathic pain. Consequently, it is important to consider the use of a multidisciplinary pain practice in the management of chronic pain syndromes. Multidisciplinary pain practices have special expertise in the management of neuropathic pain. Standard conservative surgical intervention is unlikely to improve pain caused by neuropathic mechanisms. Likewise, opioid analgesics are not recommended for the treatment of neuropathic pain.”
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6S):S1–S62.
2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398.
3. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis: a review. Fertil Steril. 2012;98(6):1370–1379.
4. Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87.
5. Chwalisz K, Garg R, Brenner RM, Schubert G, Elger W. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann N Y Acad Sci. 2002;955:373–393, 396–406.
6. Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031.
7. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
8. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632–1636.
9. Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118(2 pt 1):223–230.
10. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346.
11. Healey M, Ang WC, Cheng C. Surgical treatment of endometriosis: a prospective randomized double-blinded trial comparing excision and ablation. Fertil Steril. 2010;94(7):2536–2540.
12. Healey M, Chang C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. JMIG. 2014;21(6):999–1004.
13. Falcone T, Wilson JR. Surgical management of endometriosis: excision or ablation. JMIG. 2014;21(6):969.
14. Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
15. Nezhat C, Nezhat F, Nezhat CH, Seidman D. Classification of endometriosis: improving the classification of endometriotic ovarian cysts. Hum Reprod 1994;9(12):2212–2216.
16. Roman H, Auber M, Mokdad C, et al. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertil Steril. 2011;96(6):1396–1400.
17. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285–1292.
18. McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147(1–3):255–264.
19. Parker JD, Leondires M, Sinaii N, Premkumar A, Nieman LK, Stratton P. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86(3):711–715.
20. Abou-Setta AM, Al-Inany HG, Farquar CM. Levonorgestrel-releasing intrauterine device for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(1):CD005072.
21. Seracchioli R, Mabrouk M, Manuzzi L, et al. Postoperative use of oral contraceptive pills for prevention of anatomic relapse or symptom recurrence following surgery. Hum Reprod. 2009;24(11):2729–2735.
22. Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril. 1997;68(5):860–864.
23. Practice Committee of the American Society for Reproductive Medicine. Treatment of pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927–935.
24. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
1. Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6S):S1–S62.
2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398.
3. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis: a review. Fertil Steril. 2012;98(6):1370–1379.
4. Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87.
5. Chwalisz K, Garg R, Brenner RM, Schubert G, Elger W. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann N Y Acad Sci. 2002;955:373–393, 396–406.
6. Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031.
7. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
8. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632–1636.
9. Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118(2 pt 1):223–230.
10. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346.
11. Healey M, Ang WC, Cheng C. Surgical treatment of endometriosis: a prospective randomized double-blinded trial comparing excision and ablation. Fertil Steril. 2010;94(7):2536–2540.
12. Healey M, Chang C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. JMIG. 2014;21(6):999–1004.
13. Falcone T, Wilson JR. Surgical management of endometriosis: excision or ablation. JMIG. 2014;21(6):969.
14. Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
15. Nezhat C, Nezhat F, Nezhat CH, Seidman D. Classification of endometriosis: improving the classification of endometriotic ovarian cysts. Hum Reprod 1994;9(12):2212–2216.
16. Roman H, Auber M, Mokdad C, et al. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertil Steril. 2011;96(6):1396–1400.
17. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285–1292.
18. McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147(1–3):255–264.
19. Parker JD, Leondires M, Sinaii N, Premkumar A, Nieman LK, Stratton P. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86(3):711–715.
20. Abou-Setta AM, Al-Inany HG, Farquar CM. Levonorgestrel-releasing intrauterine device for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(1):CD005072.
21. Seracchioli R, Mabrouk M, Manuzzi L, et al. Postoperative use of oral contraceptive pills for prevention of anatomic relapse or symptom recurrence following surgery. Hum Reprod. 2009;24(11):2729–2735.
22. Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril. 1997;68(5):860–864.
23. Practice Committee of the American Society for Reproductive Medicine. Treatment of pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927–935.
24. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
Endometriosis: 3 intraoperative videos
The following videos have been provided by AAGL SurgeryU to compliment the content of this article regarding endometriosis. You can watch these videos, and more than 1,500 others, at AAGL.org/surgeryu.
Endometriosis: Expert answers to 7 crucial questions on diagnosis
CASE Is her chronic pelvic pain caused by endometriosis?
M.L. is a 32-year-old nulliparous woman who is referred to your office by her primary care provider for chronic pelvic pain. She reports severe dysmenorrhea as her main symptom, but she also mentions dyspareunia. She says these symptoms have been present for several years but have increased in intensity gradually. She asks what you consider to be the most likely diagnosis.
What potential diagnoses do you mention to her? And how do you identify the cause of her pain?
Although endometriosis—the presence of endometrial tissue outside the uterus—affects at least 5 million women of reproductive age in the United States alone, it can be a challenging diagnosis for several reasons.
“Endometriosis is a great masquerader,” says Linda Giudice, MD, PhD. “It presents with a variety of pain patterns, intensities, and triggers. It can also involve symptoms that overlap those of other disorders, including disorders of the gastrointestinal and urinary tracts.”
Although endometriosis falls within the differential diagnosis of chronic pelvic pain, “it is usually not high on the list in the primary care setting (adult and adolescent),” Dr. Giudice adds. She is the Robert B. Jaffe, MD, Endowed Professor in the reproductive sciences and chair of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco.
John R. Lue, MD, MPH, an author of the most recent practice bulletin on endometriosis from the American College of Obstetricians and Gynecologists,1 sees the situation similarly.
“The main challenge in the diagnosis of endometriosis is that its presentation mimics other causes of chronic pelvic pain,” he says. “Pelvic pain due to endometriosis is usually chronic (lasting ≥6 months). It is associated with dysmenorrhea in 50% to 90% of cases, as well as with dyspareunia, deep pelvic pain, and lower abdominal pain with or without back and loin pain. The pain can occur unpredictably and intermittently throughout the menstrual cycle or it can be continuous. In addition, it can be dull, throbbing, or sharp and may be exacerbated by physical activity.2,3 Up to 20% of women with endometriosis have concurrent pain conditions.”4 Dr. Lue is associate professor, chief of the section of general obstetrics and gynecology, and medical director of women’s ambulatory services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
Among other diseases of the female pelvis that have relatively similar presentation, Dr. Lue adds, are pathologies of the:
- uterus (adenomyosis, fibroids)
- fallopian tube (hydrosalpinx)
- ovaries (ovarian cysts)
- bladder (interstitial cystitis)
- bowel (irritable bowel syndrome)
- musculoskeletal system (piriformis syndrome).
Before pelvic pain is attributed to endometriosis, he says, the provider should rule out bowel, bladder, musculoskeletal, and psychiatric causes.
This article focuses on 7 questions, the answers of which are critical to narrowing in on the diagnosis of endometriosis, including essential factors to consider in the patient history, imaging and other diagnostic tools, and considerations in surgical exploration. In the second and third installments of this in-depth series on endometriosis, pain and infertility will be the respective subjects of investigation.
Several theories explain the “why” of endometriosis
A dominant theory is that peritoneal endometrial implants arise from retrograde menstruation, during which endometrial tissue passes through the fallopian tubes into the pelvis, says John R. Lue, MD, MPH. Dr. Lue is associate professor and chief of the section of general obstetrics and gynecology and medical director of women’s ambulatory services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
“Additional theories include immune dysfunction that interferes with clearing of endometrial lesions in the pelvis, as well as genetic alterations that lead to growth dysregulation,” he says.1 “These theories all have merit, and it is likely that the pathogenesis of endometriosis is multifactorial.”
Another strong theory involves the homeobox (HOX) genes, “which mediate embryonic development,” says Dr. Lue.2,3 “These genes are translated into transcription factors that regulate downstream genes necessary for growth and differentiation. It has been demonstrated that HOX genes play an analogous role in endometrial development during the adult menstrual cycle.4 HOX gene expression regulates the growth and development of the human endometrium.5 The expression of HOX genes A10 and A11 varies in response to sex steroids during the menstrual cycle, with dramatic upregulation in the mid-secretory phase,” says Dr. Lue. Recent studies suggest that these genes “play a major role” in endometriosis.6
“Since ovarian endometriomas are clonal and lesions usually have genetic mutations, such somatic mutations with subsequent growth dysregulation may also be etiologic factors,” says Dr. Lue.1,7,8 “Disease at distant sites may be caused by lymphatic or hematogenous spread or metaplastic transformation.”
References
1. Giudice LC, Swierz LM, Burney RO. Edometriosis. In: Jameson JL, DeGroot LJ, eds. Endocrinology. 6th ed. New York, NY: Elsevier; 2010:2356–2370.
2. Krumlauf R. Hox genes in vertebrate development. Cell. 1992;78(2):191–201.
3. McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68(2):283–302.
4. Taylor H, Igarashi P, Olive D, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab. 1999;84(3):1129–1135.
5. Taylor H, Vanden Heuvel GB, Igarashi P. A conserved HOX axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the HOXA cluster genes. Biol Reprod. 1997;57(6):1338–1345.
6. Taylor HS, Bagot C, Kardana A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14(5):1328–1331.
7. Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis. Fertil Steril. 2008;90(5 suppl):S260–S269.
8. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
1. Why such a long delay in diagnosis?
Investigators exploring the length of time between a patient’s presentation with symptoms and diagnosis have found it to be particularly long for endometriosis, ranging from 6 to 11 years.
Because endometriosis is usually not high on the list of differential diagnoses for chronic pelvic pain in the primary care setting, a patient may not be referred to a gynecologist unless those symptoms include severe dysmenorrhea, dyspareunia, or similar findings. Once the referral is made, the gynecologist “will usually try contraceptive steroids, nonsteroidal anti-inflammatory drugs, or second-line progestins before a diagnosis is made,” says Dr. Giudice.5
The delay in diagnosis “is astounding,” she adds, “and has its roots in empiric medical therapies and a combination of patients fearing a diagnosis of cancer and reluctance of gynecologists to perform laparoscopy on adolescents.”6
Another possible cause of diagnostic delay: Some adolescent girls may not realize when their pain is severe. Because they may have always experienced a high degree of pain since menarche, they may assume it to be a normal aspect of womanhood and delay seeking help, says Pamela Stratton, MD, chief of the gynecology consult service at the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
2. Have any biomarkers proved to be useful diagnostic tools?
Any biomarker proven to reliably identify endometriosis would be a boon to medicine, as it would provide a noninvasive or minimally invasive alternative to diagnostic laparoscopy, the current gold standard. Regrettably, the search for such a biomarker has produced “disappointing results,” says Dr. Giudice.
“Recent systematic reviews of all proposed endometriosis-related biomarkers over the last 25 years in serum, plasma, urine, and endometrium could not identify an unequivocally clinically useful biomarker or panel of biomarkers,” she notes.7,8 “This is due mainly to low numbers of subjects, small populations for validations, cycle/hormonal- and disease stage-dependence, poorly defined controls, and low sensitivity and specificity.”
One hopeful development: “Whole genome transcriptomics of archived endometrial tissue and machine learning found several classifiers to diagnose and stage endometriosis with high accuracy that were validated on an independent sample set,” says Dr. Giudice.9 “However, these data now warrant a prospective, multisite study for further validation.”
Environmental factors, estrogen, and endometriosis
“There is increasing evidence that in utero and also adult exposures to endocrine-disrupting chemicals (EDCs) play a role in the pathogenesis and progression of disease,” says Linda Giudice, MD, PhD, the Robert B. Jaffe, MD, Endowed Professor in the reproductive sciences and chair of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco.
“For example, the Nurses’ Health Study II, a prospective cohort study of more than 80,000 women, revealed that daughters exposed to diethylstilbestrol (DES) had an 80% increased risk (odds ratio [OR], 1.8; 95% confidence interval [CI], 1.2–2.8) of developing endometriosis,” she says.1
“Also, dioxin (TCDD) exposure in rats in utero on gestational day 8 increased the size of endometriosis lesions when combined with an adult exposure,” Dr. Giudice says.2 Although we do not know the precise mechanisms underlying in utero events that result in disease onset as a teen or adult, abnormal programming of the female reproductive tract by EDCs and similar agents is believed to play a role, she says.3
Because estrogen is essential for endometriosis lesions and associated symptoms to progress, “EDCs that have either estrogenic activity or interfere with estrogen metabolism or action, or both, have been proposed as contributors to progression of disease. Abundant animal data using nonhuman primate and rodent models and exposures to organochlorines and other EDCs support this hypothesis,” Dr. Giudice says.4
“The weight of human evidence of associations of EDCs and the risk of endometriosis in adult women depends on the class of endocrine disrupter,” Dr. Giudice continues. “Strongest correlations are with polychlorobiphenyls (PCBs), where 10 of 12 studies found significant odds of disease and circulating or omental fat concentrations of these compounds. PCBs inhibit peripheral natural killer cell activity and interleukin 1b and 12 production, relevant to the immune component of endometriosis progression.”
“Significant risk has also been associated with organochlorines (in three of three studies) and perfluorochemicals. In contrast, data linking endometriosis with exposures to dioxins, phthalates, and bisphenol A are equivocal, with some studies finding significant odds ratios and others failing to find significant correlations. Interestingly, dioxins have a significant association with deep infiltrating endometriotic lesions.”5
References
1. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Michels KB, Hunter DJ. In utero exposures and the incidence of endometriosis. Fertil Steril. 2004;82(6):1501–1508.
2. Cummings AM, Hedge JM, Birnbaum LS. Effects on prenatal exposure to TCDD on the promotion of endometriotic lesion growth by TCDD in adult female rats and mice. Toxicol Sci. 1999;52(1):45–49.
3. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
4. Crain DA, Janssen SJ, Edwards TM, et al. Female reproductive disorders: the roles of endocrine disrupting compounds and developmental timing. Fertil Steril. 2008;90(4):911–940.
5. Heilier JF, Donnez J, Nackers F, et al. Environmental and host-associated risk factors in endometriosis and deep endometriotic nodules: a matched case-control study. Environ Res. 2007;103(1):121–129.
3. What aspects of the patient history are key?
Dr. Stratton recommends that clinicians begin their evaluation of the patient with pain by asking her to describe that pain—how long she has had it, when it occurs, and which areas are affected.
“Most women with endometriosis-associated pain have chronic pelvic pain,” Dr. Stratton continues.5 “Up to 90% of those have dysmenorrhea or cyclic pain with menses.”10 In addition, women with endometriosis “commonly report having pain with any bleeding or spotting. About 30% of women diagnosed with endometriosis initially present to their gynecologist with dyspareunia.”11
“Episodic pain with menses may become more constant, lasting for many days of the month,” says Dr. Stratton. “Women with dyschezia or dysuria may have endometriosis lesions associated with the bowel or bladder, respectively.12 When women with these symptoms do not have lesions on bowel or bladder, these pain symptoms may occur because of higher peritoneal hormone and inflammatory factor levels or because adjacent organs share the neural networks.”
Dr. Giudice views the history similarly.
“I believe listening to the patient is essential in evaluating the possibility of her having endometriosis. This involves asking her to describe where her pain is, grading it on a scale of 1 to 10, identifying when in her cycle it occurs, and learning what makes it better or worse,” she says.
“It also is important to assess the quality of the pain,” Dr. Giudice says. “Does it radiate, does it limit her daily activities, does it interfere with her relationships, intercourse, work, school? Is it associated with bowel movements, urination, other pain syndromes?”
“Having a pain questionnaire is a great help so that patients have a chance to reflect on these and other questions that help to frame the pain associated with endometriosis when they come for consultation,” she adds.
By determining if pain is associated with menstruation or spotting, the clinician is better informed about the value of menstrual suppression, says Dr. Stratton. “Determining what makes the pain better or worse can help define triggers which, if treated, can decrease the likelihood of episodes of pain.”
“A detailed history of any medical or surgical treatments and their outcome is helpful in guiding future treatment,” she adds. “While hormonal therapy has been a mainstay of treatment, in some women, some hormonal treatments may worsen pain or have unacceptable side effects like worsening depression or anxiety. In addition, some pain—especially that associated with deep lesions—may be relieved by surgical treatment13,14; pain that worsened after surgery may suggest neural damage.”
“As there is an engagement of the central nervous system, endometriosis is considered a central sensitivity syndrome in which women may also have other sites of pain,” Dr. Stratton says. “Thus, obtaining a history about current symptoms or prior diagnosis of irritable bowel syndrome, interstitial cystitis/painful bladder, migraines, fibromyalgia, or chronic fatigue syndrome is beneficial.10,15–17 Facilitating treatment for these comorbidities is a key principle in helping women with endometriosis-associated pain, as any condition that triggers or perpetuates pain warrants treatment.”
4. What should the physical exam entail?
“An abdominal exam and a pelvic exam are essential in evaluating pain in a woman when endometriosis is suspected,” says Dr. Giudice. “Sometimes the latter is challenging in young teens and can be deferred.” Overall, however, “the pelvic exam can give insight into pain triggers, adnexal masses (possible endometriomas) and mobility of pelvic organs. A rectovaginal exam is important in evaluating deep infiltrating disease and to gauge the pelvic pain landscape overall. In addition, palpating the pelvic floor musculature is important to distinguish pelvic floor muscle spasm from endometriosis pain.”
“The challenge for clinicians is to think beyond the endometrial implants, taking into account multiple factors that influence pain perception,” says Dr. Stratton. During the examination, the clinician should begin by mapping the regions of pain in the abdomen and back, “distinguishing musculoskeletal pain from deep pain. Determining whether pains are focused or diffuse is also important.”
Dr. Stratton recommends that the routine pelvic exam be modified because a standard bimanual exam “confuses pain signals from the pelvic floor, abdominal wall, bladder, and other viscera. For this reason, a pain-oriented assessment is mandatory.”
Begin with a single digital examination to map tender areas, Dr. Stratton advises. Then consider the size, shape, and mobility of reproductive and pelvic organs. “A bimanual exam will help identify adnexal masses like endometriomas,” she says.
Endometriomas usually are not associated with pain, she adds, but “they are associated with deep infiltrating lesions. Nodularity along the uterosacral ligaments, limited reproductive organ mobility, and thickening of the rectovaginal septum also suggest deep infiltrating lesions. Importantly, deep infiltrating lesions are the lesion type most associated with pain.”18,19
5. Is imaging useful in the diagnosis of endometriosis?
Laparoscopy remains the gold standard for diagnosis of endometriosis, observes Steven R. Goldstein, MD. Visualization of endometriotic implants at the time of surgery—with histologic assessment—offers definitive confirmation of the diagnosis. The physical examination, too, can offer a strong suggestion of endometriosis, he says. Dr. Goldstein is professor of obstetrics and gynecology at New York University School of Medicine and director of gynecologic ultrasound and co-director of bone densitometry at New York University Medical Center in New York City. He serves on the OBG Management Board of Editors.
“In the past, the pelvic examination and history often were the sine qua non for patients with pain,” Dr. Goldstein says. “Extreme dysmenorrhea and pain between periods, especially with intercourse, defecation, and exercise, all increased the suspicion of endometriosis. People used to talk about feeling nodularity in the uterosacral ligaments and finding decreased mobility of pelvic structures—but I don’t have any question that the skill of today’s gynecologists in doing a bimanual pelvic exam is a fraction of what it was in years gone by because they haven’t had the necessity of experience. The first thing they do if there’s any question is they send the patient for an ultrasound.”
Of course, ultrasound can be especially helpful in identifying endometriomas—sometimes called “chocolate cysts”—in the ovary. Endometriomas can have a solid appearance on ultrasound, says Dr. Goldstein, because the fluid they contain (dried blood) is sonolucent or pure black on ultrasound, similar to amniotic fluid or the fluid seen in the bladder. “This ‘chocolate’ fluid contained in endometriomas is homogeneous, particulate, and very monotonous in its appearance, in contrast to the internal echoes observed in hemorrhagic corpus lutea, which are very cobweblike and can sometimes mimic papillary projections,” he adds.
“What’s absolutely essential when imaging a suspected endometrioma by ultrasound is that there be no evidence of any blood flow contained within that structure. Because it’s dried blood, it shouldn’t have any vascularity. If you see blood flow inside what you would call an endometrioma, you need to rethink your diagnosis,” he says.
In some cases, a supposed endometriomalacks a black, sonolucent appearance, but “the clinician often can tell that it’s a cystic structure by the very bright posterior wall—what we call posterior wall acoustic enhancement—even though the interior of the structure may appear sort of grayish or whitish rather than the pure black of a simple cyst. It’s still fluid-filled,” Dr. Goldstein says.
In some instances, even endometriotic nodules can be imaged by ultrasound, he adds. “There’s an increasing body of literature that suggests that, if you look carefully in people with deep infiltrating endometriosis, you can often see solid-appearing nodules in the rectovaginal septum or between the uterus and bladder. With the kind of resolution that we now have with the vaginal probe, some of these nodules can be seen. That’s somewhat new, and it’s a function of 2 things—people looking for endometriosis and the better resolution of more modern equipment.”
Dr. Goldstein believes that magnetic resonance imaging (MRI) is “almost never” indicated in the diagnosis of endometriosis. A more helpful approach would be a consultative ultrasound with someone with more experience. However, when that is not available, or “in areas where you have excellent backup in terms of pelvic MRI, that may be the way to go. I don’t think so,” he demurs, “and some of my colleagues would be very upset at the thought of needing to use MRI to diagnose endometriosis. But in the occasional confusing or difficult case, depending on the quality of the referral pattern you have, it might make sense,” he says.
6. When is diagnostic laparoscopy clearly indicated?
Dr. Giudice believes that laparoscopy—with the intention to treat endometriosis, if present—“is essential when first-line medical therapy fails or when pain is acute and severe.”5
Dr. Stratton concurs. “Any woman with chronic pain wants to know what is causing the pain,” she says. Therefore, “women report a benefit from knowing that their pain is associated with endometriosis.6 However, diagnostic laparoscopy alone, with the sole purpose of determining the presence of endometriosis but not treating the lesions, is no longer performed, as it poses little benefit to the patient other than peace of mind.”
“The general trend in the United States has been to first use hormonal treatments when the diagnosis of endometriosis is suspected, prior to performing surgery,” Dr. Stratton says.1 In many cases, by using cyclic combined hormonal contraceptives to reduce menstrual flow or “suppressing menstruation with continuous combined hormonal contraceptives,” gonadotropin-releasing hormone analogues (combined with progestin to prevent bone loss), “or continuous progestin alone may be effective in decreasing pain. Not surprisingly, these hormonal approaches are effective for any chronic pelvic pain, even for women who do not have the surgical diagnosis of endometriosis.”20
“When the first-line approach to chronic pelvic pain is hormonal treatment, laparoscopy is considered when these medical treatments have failed to control the pain or are poorly tolerated, or when the diagnosis of endometriosis is in question,” Dr. Stratton says.
“Laparoscopy to treat endometriomas is indicated if an endometrioma is enlarging, measures more than 4 cm in diameter, or if the diagnosis of an ovarian mass is in question,” she explains. “While surgeons have previously been aggressive in removing endometriomas, this practice may have negative consequences on ovarian function. Because endometriomas are pseudocysts, removing them completely leads to the removal of viable ovarian tissue and may diminish ovarian reserve.”21,22
7. What is the surgical appearance of endometriosis?
Dr. Giudice returns to the enigmatic nature of endometriosis in addressing this question, mentioning its “many faces” at the time of surgery. “It is imperative that the surgeon recognize the disease in its many forms,” she says. “Also, it is especially helpful at the time of surgery if suspected lesions are biopsied and sent to pathology to have the diagnosis made unequivocally.”5
As for the surgical appearance of endometriosis, Dr. Stratton notes that there are 3 types of lesions—“superficial lesions, deep infiltrating lesions, and endometriomas. Endometriomas occur almost exclusively in the ovary and are pseudocysts without an identifiable cystic lining. They vary in dimension from a few millimeters to several centimeters.”
“Superficial peritoneal endometriosis lesions have a variable appearance, with some lesions being clear or red, some brown, blue or black, and some having a white appearance, like a scar,” says Dr. Stratton. “Endometriosis can be diagnosed on histologic examination of any of these lesion types.
“Overall, single-color lesions have similar frequencies of biopsy-confirmed endometriosis (59% to 62%),” she says.23 “These lesion appearances likely represent different stages of development of endometriosis, with red or clear lesions occurring first, soon after endometrial tissue implantation; black, blue, or brown lesions occurring later, in response to the hormones varying in the menstrual cycle; and white lesions occurring as the lesions age. Deep infiltrating lesions generally have blue/black or white features.”
“Wide, deep, multiple-color lesions in the cul-de-sac, ovarian fossa or uterosacral ligaments are most likely endometriosis,” Dr. Stratton adds.23 Only lesions with multiple colors have a significantly higher percentage of positive biopsies (76%). Importantly, over half of women with only subtle lesions (small red or white lesions) have endometriosis.
CASE Resolved
You tell the patient that endometriosis is one of the possible diagnoses for her chronic pelvic pain, and you take a focused history. During a pelvic examination, you observe that her right ovary lacks mobility, and you map a number of trigger points for her pain. Transvaginal ultrasound results suggest the presence of nodules in the rectovaginal septum. You begin empiric treatment with continuous combined hormonal contraceptives to suppress menstruation. On her next visit, the patient reports reduced but still bothersome pain. Laparoscopy reveals a 2-cm endometrioma in the right ovary and deep infiltrating lesions in the cul-de-sac. The endometrioma is resected. Histology confirms the diagnosis of endometriosis.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. American College of Obstetricians and Gynecologists. Practice Bulletin #114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223–236.
2. Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154(1):39–43.
3. Taylor HS, Bagot C, Kardana A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14(5):1328–1331.
4. Berkley KJ, Stratton P. Mechanisms: lessons from translational studies of endometriosis. In: Giamberardino MA, ed. Visceral Pain: Clinical, Pathophysiological and Therapeutic Aspects. Oxford, UK: Oxford University Press; 2009:39–50.
5. Giudice LC. Clinical practice: endometriosis. N Engl J Med. 2010;362(25):2389–2398.
6. Ballard K, Lowton K, Wright J. What’s the delay: a qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296–1301.
7. May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010;16(6):651–674.
8. May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011;17(5):637–653.
9. Tamaresis JS, Irwin JC, Goldfien GA, et al. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155(12):4986–4999.
10. Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715–2724.
11. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677–2685.
12. Lafay Pillet MC, Huchon C, Santulli P, Borghese B, Chapron C, Fauconnier A. A clinical score can predict associated deep infiltrating endometriosis before surgery for an endometrioma. Hum Reprod. 2014;29(8):1666–1676.
13. Healey M, Cheng C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. J Minim Invasive Gynecol. 2014;21(6):999–1004.
14. Anaf V, El Nakadi I, De Moor V, Chapron C, Pistofidis G, Noel JC. Increased nerve density in deep infiltrating endometriotic nodules. Gynecol Obstet Invest. 2011;71(2):112–117.
15. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346.
16. Karp BI, Sinaii N, Nieman LK, Silberstein SD, Stratton P. Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril. 2011;95(3):895–899.
17. Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86(3):272–280.
18. Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595–606.
19. Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266–271.
20. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93(1):51–58.
21. Muzii L, Di Tucci C, Di Feliciantonio M, Marchetti C, Perniola G, Panici PB. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: a systematic review and meta-analysis. Hum Reprod. 2014;29(10):2190–2198.
22. Muzii L, Luciano AA, Zupi E, Panici PB. Effect of surgery for endometrioma on ovarian function: a different point of view. J Minim Invasive Gynecol. 2014;21(4):531–533.
23. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632–1636.
CASE Is her chronic pelvic pain caused by endometriosis?
M.L. is a 32-year-old nulliparous woman who is referred to your office by her primary care provider for chronic pelvic pain. She reports severe dysmenorrhea as her main symptom, but she also mentions dyspareunia. She says these symptoms have been present for several years but have increased in intensity gradually. She asks what you consider to be the most likely diagnosis.
What potential diagnoses do you mention to her? And how do you identify the cause of her pain?
Although endometriosis—the presence of endometrial tissue outside the uterus—affects at least 5 million women of reproductive age in the United States alone, it can be a challenging diagnosis for several reasons.
“Endometriosis is a great masquerader,” says Linda Giudice, MD, PhD. “It presents with a variety of pain patterns, intensities, and triggers. It can also involve symptoms that overlap those of other disorders, including disorders of the gastrointestinal and urinary tracts.”
Although endometriosis falls within the differential diagnosis of chronic pelvic pain, “it is usually not high on the list in the primary care setting (adult and adolescent),” Dr. Giudice adds. She is the Robert B. Jaffe, MD, Endowed Professor in the reproductive sciences and chair of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco.
John R. Lue, MD, MPH, an author of the most recent practice bulletin on endometriosis from the American College of Obstetricians and Gynecologists,1 sees the situation similarly.
“The main challenge in the diagnosis of endometriosis is that its presentation mimics other causes of chronic pelvic pain,” he says. “Pelvic pain due to endometriosis is usually chronic (lasting ≥6 months). It is associated with dysmenorrhea in 50% to 90% of cases, as well as with dyspareunia, deep pelvic pain, and lower abdominal pain with or without back and loin pain. The pain can occur unpredictably and intermittently throughout the menstrual cycle or it can be continuous. In addition, it can be dull, throbbing, or sharp and may be exacerbated by physical activity.2,3 Up to 20% of women with endometriosis have concurrent pain conditions.”4 Dr. Lue is associate professor, chief of the section of general obstetrics and gynecology, and medical director of women’s ambulatory services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
Among other diseases of the female pelvis that have relatively similar presentation, Dr. Lue adds, are pathologies of the:
- uterus (adenomyosis, fibroids)
- fallopian tube (hydrosalpinx)
- ovaries (ovarian cysts)
- bladder (interstitial cystitis)
- bowel (irritable bowel syndrome)
- musculoskeletal system (piriformis syndrome).
Before pelvic pain is attributed to endometriosis, he says, the provider should rule out bowel, bladder, musculoskeletal, and psychiatric causes.
This article focuses on 7 questions, the answers of which are critical to narrowing in on the diagnosis of endometriosis, including essential factors to consider in the patient history, imaging and other diagnostic tools, and considerations in surgical exploration. In the second and third installments of this in-depth series on endometriosis, pain and infertility will be the respective subjects of investigation.
Several theories explain the “why” of endometriosis
A dominant theory is that peritoneal endometrial implants arise from retrograde menstruation, during which endometrial tissue passes through the fallopian tubes into the pelvis, says John R. Lue, MD, MPH. Dr. Lue is associate professor and chief of the section of general obstetrics and gynecology and medical director of women’s ambulatory services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
“Additional theories include immune dysfunction that interferes with clearing of endometrial lesions in the pelvis, as well as genetic alterations that lead to growth dysregulation,” he says.1 “These theories all have merit, and it is likely that the pathogenesis of endometriosis is multifactorial.”
Another strong theory involves the homeobox (HOX) genes, “which mediate embryonic development,” says Dr. Lue.2,3 “These genes are translated into transcription factors that regulate downstream genes necessary for growth and differentiation. It has been demonstrated that HOX genes play an analogous role in endometrial development during the adult menstrual cycle.4 HOX gene expression regulates the growth and development of the human endometrium.5 The expression of HOX genes A10 and A11 varies in response to sex steroids during the menstrual cycle, with dramatic upregulation in the mid-secretory phase,” says Dr. Lue. Recent studies suggest that these genes “play a major role” in endometriosis.6
“Since ovarian endometriomas are clonal and lesions usually have genetic mutations, such somatic mutations with subsequent growth dysregulation may also be etiologic factors,” says Dr. Lue.1,7,8 “Disease at distant sites may be caused by lymphatic or hematogenous spread or metaplastic transformation.”
References
1. Giudice LC, Swierz LM, Burney RO. Edometriosis. In: Jameson JL, DeGroot LJ, eds. Endocrinology. 6th ed. New York, NY: Elsevier; 2010:2356–2370.
2. Krumlauf R. Hox genes in vertebrate development. Cell. 1992;78(2):191–201.
3. McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68(2):283–302.
4. Taylor H, Igarashi P, Olive D, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab. 1999;84(3):1129–1135.
5. Taylor H, Vanden Heuvel GB, Igarashi P. A conserved HOX axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the HOXA cluster genes. Biol Reprod. 1997;57(6):1338–1345.
6. Taylor HS, Bagot C, Kardana A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14(5):1328–1331.
7. Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis. Fertil Steril. 2008;90(5 suppl):S260–S269.
8. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
1. Why such a long delay in diagnosis?
Investigators exploring the length of time between a patient’s presentation with symptoms and diagnosis have found it to be particularly long for endometriosis, ranging from 6 to 11 years.
Because endometriosis is usually not high on the list of differential diagnoses for chronic pelvic pain in the primary care setting, a patient may not be referred to a gynecologist unless those symptoms include severe dysmenorrhea, dyspareunia, or similar findings. Once the referral is made, the gynecologist “will usually try contraceptive steroids, nonsteroidal anti-inflammatory drugs, or second-line progestins before a diagnosis is made,” says Dr. Giudice.5
The delay in diagnosis “is astounding,” she adds, “and has its roots in empiric medical therapies and a combination of patients fearing a diagnosis of cancer and reluctance of gynecologists to perform laparoscopy on adolescents.”6
Another possible cause of diagnostic delay: Some adolescent girls may not realize when their pain is severe. Because they may have always experienced a high degree of pain since menarche, they may assume it to be a normal aspect of womanhood and delay seeking help, says Pamela Stratton, MD, chief of the gynecology consult service at the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
2. Have any biomarkers proved to be useful diagnostic tools?
Any biomarker proven to reliably identify endometriosis would be a boon to medicine, as it would provide a noninvasive or minimally invasive alternative to diagnostic laparoscopy, the current gold standard. Regrettably, the search for such a biomarker has produced “disappointing results,” says Dr. Giudice.
“Recent systematic reviews of all proposed endometriosis-related biomarkers over the last 25 years in serum, plasma, urine, and endometrium could not identify an unequivocally clinically useful biomarker or panel of biomarkers,” she notes.7,8 “This is due mainly to low numbers of subjects, small populations for validations, cycle/hormonal- and disease stage-dependence, poorly defined controls, and low sensitivity and specificity.”
One hopeful development: “Whole genome transcriptomics of archived endometrial tissue and machine learning found several classifiers to diagnose and stage endometriosis with high accuracy that were validated on an independent sample set,” says Dr. Giudice.9 “However, these data now warrant a prospective, multisite study for further validation.”
Environmental factors, estrogen, and endometriosis
“There is increasing evidence that in utero and also adult exposures to endocrine-disrupting chemicals (EDCs) play a role in the pathogenesis and progression of disease,” says Linda Giudice, MD, PhD, the Robert B. Jaffe, MD, Endowed Professor in the reproductive sciences and chair of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco.
“For example, the Nurses’ Health Study II, a prospective cohort study of more than 80,000 women, revealed that daughters exposed to diethylstilbestrol (DES) had an 80% increased risk (odds ratio [OR], 1.8; 95% confidence interval [CI], 1.2–2.8) of developing endometriosis,” she says.1
“Also, dioxin (TCDD) exposure in rats in utero on gestational day 8 increased the size of endometriosis lesions when combined with an adult exposure,” Dr. Giudice says.2 Although we do not know the precise mechanisms underlying in utero events that result in disease onset as a teen or adult, abnormal programming of the female reproductive tract by EDCs and similar agents is believed to play a role, she says.3
Because estrogen is essential for endometriosis lesions and associated symptoms to progress, “EDCs that have either estrogenic activity or interfere with estrogen metabolism or action, or both, have been proposed as contributors to progression of disease. Abundant animal data using nonhuman primate and rodent models and exposures to organochlorines and other EDCs support this hypothesis,” Dr. Giudice says.4
“The weight of human evidence of associations of EDCs and the risk of endometriosis in adult women depends on the class of endocrine disrupter,” Dr. Giudice continues. “Strongest correlations are with polychlorobiphenyls (PCBs), where 10 of 12 studies found significant odds of disease and circulating or omental fat concentrations of these compounds. PCBs inhibit peripheral natural killer cell activity and interleukin 1b and 12 production, relevant to the immune component of endometriosis progression.”
“Significant risk has also been associated with organochlorines (in three of three studies) and perfluorochemicals. In contrast, data linking endometriosis with exposures to dioxins, phthalates, and bisphenol A are equivocal, with some studies finding significant odds ratios and others failing to find significant correlations. Interestingly, dioxins have a significant association with deep infiltrating endometriotic lesions.”5
References
1. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Michels KB, Hunter DJ. In utero exposures and the incidence of endometriosis. Fertil Steril. 2004;82(6):1501–1508.
2. Cummings AM, Hedge JM, Birnbaum LS. Effects on prenatal exposure to TCDD on the promotion of endometriotic lesion growth by TCDD in adult female rats and mice. Toxicol Sci. 1999;52(1):45–49.
3. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
4. Crain DA, Janssen SJ, Edwards TM, et al. Female reproductive disorders: the roles of endocrine disrupting compounds and developmental timing. Fertil Steril. 2008;90(4):911–940.
5. Heilier JF, Donnez J, Nackers F, et al. Environmental and host-associated risk factors in endometriosis and deep endometriotic nodules: a matched case-control study. Environ Res. 2007;103(1):121–129.
3. What aspects of the patient history are key?
Dr. Stratton recommends that clinicians begin their evaluation of the patient with pain by asking her to describe that pain—how long she has had it, when it occurs, and which areas are affected.
“Most women with endometriosis-associated pain have chronic pelvic pain,” Dr. Stratton continues.5 “Up to 90% of those have dysmenorrhea or cyclic pain with menses.”10 In addition, women with endometriosis “commonly report having pain with any bleeding or spotting. About 30% of women diagnosed with endometriosis initially present to their gynecologist with dyspareunia.”11
“Episodic pain with menses may become more constant, lasting for many days of the month,” says Dr. Stratton. “Women with dyschezia or dysuria may have endometriosis lesions associated with the bowel or bladder, respectively.12 When women with these symptoms do not have lesions on bowel or bladder, these pain symptoms may occur because of higher peritoneal hormone and inflammatory factor levels or because adjacent organs share the neural networks.”
Dr. Giudice views the history similarly.
“I believe listening to the patient is essential in evaluating the possibility of her having endometriosis. This involves asking her to describe where her pain is, grading it on a scale of 1 to 10, identifying when in her cycle it occurs, and learning what makes it better or worse,” she says.
“It also is important to assess the quality of the pain,” Dr. Giudice says. “Does it radiate, does it limit her daily activities, does it interfere with her relationships, intercourse, work, school? Is it associated with bowel movements, urination, other pain syndromes?”
“Having a pain questionnaire is a great help so that patients have a chance to reflect on these and other questions that help to frame the pain associated with endometriosis when they come for consultation,” she adds.
By determining if pain is associated with menstruation or spotting, the clinician is better informed about the value of menstrual suppression, says Dr. Stratton. “Determining what makes the pain better or worse can help define triggers which, if treated, can decrease the likelihood of episodes of pain.”
“A detailed history of any medical or surgical treatments and their outcome is helpful in guiding future treatment,” she adds. “While hormonal therapy has been a mainstay of treatment, in some women, some hormonal treatments may worsen pain or have unacceptable side effects like worsening depression or anxiety. In addition, some pain—especially that associated with deep lesions—may be relieved by surgical treatment13,14; pain that worsened after surgery may suggest neural damage.”
“As there is an engagement of the central nervous system, endometriosis is considered a central sensitivity syndrome in which women may also have other sites of pain,” Dr. Stratton says. “Thus, obtaining a history about current symptoms or prior diagnosis of irritable bowel syndrome, interstitial cystitis/painful bladder, migraines, fibromyalgia, or chronic fatigue syndrome is beneficial.10,15–17 Facilitating treatment for these comorbidities is a key principle in helping women with endometriosis-associated pain, as any condition that triggers or perpetuates pain warrants treatment.”
4. What should the physical exam entail?
“An abdominal exam and a pelvic exam are essential in evaluating pain in a woman when endometriosis is suspected,” says Dr. Giudice. “Sometimes the latter is challenging in young teens and can be deferred.” Overall, however, “the pelvic exam can give insight into pain triggers, adnexal masses (possible endometriomas) and mobility of pelvic organs. A rectovaginal exam is important in evaluating deep infiltrating disease and to gauge the pelvic pain landscape overall. In addition, palpating the pelvic floor musculature is important to distinguish pelvic floor muscle spasm from endometriosis pain.”
“The challenge for clinicians is to think beyond the endometrial implants, taking into account multiple factors that influence pain perception,” says Dr. Stratton. During the examination, the clinician should begin by mapping the regions of pain in the abdomen and back, “distinguishing musculoskeletal pain from deep pain. Determining whether pains are focused or diffuse is also important.”
Dr. Stratton recommends that the routine pelvic exam be modified because a standard bimanual exam “confuses pain signals from the pelvic floor, abdominal wall, bladder, and other viscera. For this reason, a pain-oriented assessment is mandatory.”
Begin with a single digital examination to map tender areas, Dr. Stratton advises. Then consider the size, shape, and mobility of reproductive and pelvic organs. “A bimanual exam will help identify adnexal masses like endometriomas,” she says.
Endometriomas usually are not associated with pain, she adds, but “they are associated with deep infiltrating lesions. Nodularity along the uterosacral ligaments, limited reproductive organ mobility, and thickening of the rectovaginal septum also suggest deep infiltrating lesions. Importantly, deep infiltrating lesions are the lesion type most associated with pain.”18,19
5. Is imaging useful in the diagnosis of endometriosis?
Laparoscopy remains the gold standard for diagnosis of endometriosis, observes Steven R. Goldstein, MD. Visualization of endometriotic implants at the time of surgery—with histologic assessment—offers definitive confirmation of the diagnosis. The physical examination, too, can offer a strong suggestion of endometriosis, he says. Dr. Goldstein is professor of obstetrics and gynecology at New York University School of Medicine and director of gynecologic ultrasound and co-director of bone densitometry at New York University Medical Center in New York City. He serves on the OBG Management Board of Editors.
“In the past, the pelvic examination and history often were the sine qua non for patients with pain,” Dr. Goldstein says. “Extreme dysmenorrhea and pain between periods, especially with intercourse, defecation, and exercise, all increased the suspicion of endometriosis. People used to talk about feeling nodularity in the uterosacral ligaments and finding decreased mobility of pelvic structures—but I don’t have any question that the skill of today’s gynecologists in doing a bimanual pelvic exam is a fraction of what it was in years gone by because they haven’t had the necessity of experience. The first thing they do if there’s any question is they send the patient for an ultrasound.”
Of course, ultrasound can be especially helpful in identifying endometriomas—sometimes called “chocolate cysts”—in the ovary. Endometriomas can have a solid appearance on ultrasound, says Dr. Goldstein, because the fluid they contain (dried blood) is sonolucent or pure black on ultrasound, similar to amniotic fluid or the fluid seen in the bladder. “This ‘chocolate’ fluid contained in endometriomas is homogeneous, particulate, and very monotonous in its appearance, in contrast to the internal echoes observed in hemorrhagic corpus lutea, which are very cobweblike and can sometimes mimic papillary projections,” he adds.
“What’s absolutely essential when imaging a suspected endometrioma by ultrasound is that there be no evidence of any blood flow contained within that structure. Because it’s dried blood, it shouldn’t have any vascularity. If you see blood flow inside what you would call an endometrioma, you need to rethink your diagnosis,” he says.
In some cases, a supposed endometriomalacks a black, sonolucent appearance, but “the clinician often can tell that it’s a cystic structure by the very bright posterior wall—what we call posterior wall acoustic enhancement—even though the interior of the structure may appear sort of grayish or whitish rather than the pure black of a simple cyst. It’s still fluid-filled,” Dr. Goldstein says.
In some instances, even endometriotic nodules can be imaged by ultrasound, he adds. “There’s an increasing body of literature that suggests that, if you look carefully in people with deep infiltrating endometriosis, you can often see solid-appearing nodules in the rectovaginal septum or between the uterus and bladder. With the kind of resolution that we now have with the vaginal probe, some of these nodules can be seen. That’s somewhat new, and it’s a function of 2 things—people looking for endometriosis and the better resolution of more modern equipment.”
Dr. Goldstein believes that magnetic resonance imaging (MRI) is “almost never” indicated in the diagnosis of endometriosis. A more helpful approach would be a consultative ultrasound with someone with more experience. However, when that is not available, or “in areas where you have excellent backup in terms of pelvic MRI, that may be the way to go. I don’t think so,” he demurs, “and some of my colleagues would be very upset at the thought of needing to use MRI to diagnose endometriosis. But in the occasional confusing or difficult case, depending on the quality of the referral pattern you have, it might make sense,” he says.
6. When is diagnostic laparoscopy clearly indicated?
Dr. Giudice believes that laparoscopy—with the intention to treat endometriosis, if present—“is essential when first-line medical therapy fails or when pain is acute and severe.”5
Dr. Stratton concurs. “Any woman with chronic pain wants to know what is causing the pain,” she says. Therefore, “women report a benefit from knowing that their pain is associated with endometriosis.6 However, diagnostic laparoscopy alone, with the sole purpose of determining the presence of endometriosis but not treating the lesions, is no longer performed, as it poses little benefit to the patient other than peace of mind.”
“The general trend in the United States has been to first use hormonal treatments when the diagnosis of endometriosis is suspected, prior to performing surgery,” Dr. Stratton says.1 In many cases, by using cyclic combined hormonal contraceptives to reduce menstrual flow or “suppressing menstruation with continuous combined hormonal contraceptives,” gonadotropin-releasing hormone analogues (combined with progestin to prevent bone loss), “or continuous progestin alone may be effective in decreasing pain. Not surprisingly, these hormonal approaches are effective for any chronic pelvic pain, even for women who do not have the surgical diagnosis of endometriosis.”20
“When the first-line approach to chronic pelvic pain is hormonal treatment, laparoscopy is considered when these medical treatments have failed to control the pain or are poorly tolerated, or when the diagnosis of endometriosis is in question,” Dr. Stratton says.
“Laparoscopy to treat endometriomas is indicated if an endometrioma is enlarging, measures more than 4 cm in diameter, or if the diagnosis of an ovarian mass is in question,” she explains. “While surgeons have previously been aggressive in removing endometriomas, this practice may have negative consequences on ovarian function. Because endometriomas are pseudocysts, removing them completely leads to the removal of viable ovarian tissue and may diminish ovarian reserve.”21,22
7. What is the surgical appearance of endometriosis?
Dr. Giudice returns to the enigmatic nature of endometriosis in addressing this question, mentioning its “many faces” at the time of surgery. “It is imperative that the surgeon recognize the disease in its many forms,” she says. “Also, it is especially helpful at the time of surgery if suspected lesions are biopsied and sent to pathology to have the diagnosis made unequivocally.”5
As for the surgical appearance of endometriosis, Dr. Stratton notes that there are 3 types of lesions—“superficial lesions, deep infiltrating lesions, and endometriomas. Endometriomas occur almost exclusively in the ovary and are pseudocysts without an identifiable cystic lining. They vary in dimension from a few millimeters to several centimeters.”
“Superficial peritoneal endometriosis lesions have a variable appearance, with some lesions being clear or red, some brown, blue or black, and some having a white appearance, like a scar,” says Dr. Stratton. “Endometriosis can be diagnosed on histologic examination of any of these lesion types.
“Overall, single-color lesions have similar frequencies of biopsy-confirmed endometriosis (59% to 62%),” she says.23 “These lesion appearances likely represent different stages of development of endometriosis, with red or clear lesions occurring first, soon after endometrial tissue implantation; black, blue, or brown lesions occurring later, in response to the hormones varying in the menstrual cycle; and white lesions occurring as the lesions age. Deep infiltrating lesions generally have blue/black or white features.”
“Wide, deep, multiple-color lesions in the cul-de-sac, ovarian fossa or uterosacral ligaments are most likely endometriosis,” Dr. Stratton adds.23 Only lesions with multiple colors have a significantly higher percentage of positive biopsies (76%). Importantly, over half of women with only subtle lesions (small red or white lesions) have endometriosis.
CASE Resolved
You tell the patient that endometriosis is one of the possible diagnoses for her chronic pelvic pain, and you take a focused history. During a pelvic examination, you observe that her right ovary lacks mobility, and you map a number of trigger points for her pain. Transvaginal ultrasound results suggest the presence of nodules in the rectovaginal septum. You begin empiric treatment with continuous combined hormonal contraceptives to suppress menstruation. On her next visit, the patient reports reduced but still bothersome pain. Laparoscopy reveals a 2-cm endometrioma in the right ovary and deep infiltrating lesions in the cul-de-sac. The endometrioma is resected. Histology confirms the diagnosis of endometriosis.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Is her chronic pelvic pain caused by endometriosis?
M.L. is a 32-year-old nulliparous woman who is referred to your office by her primary care provider for chronic pelvic pain. She reports severe dysmenorrhea as her main symptom, but she also mentions dyspareunia. She says these symptoms have been present for several years but have increased in intensity gradually. She asks what you consider to be the most likely diagnosis.
What potential diagnoses do you mention to her? And how do you identify the cause of her pain?
Although endometriosis—the presence of endometrial tissue outside the uterus—affects at least 5 million women of reproductive age in the United States alone, it can be a challenging diagnosis for several reasons.
“Endometriosis is a great masquerader,” says Linda Giudice, MD, PhD. “It presents with a variety of pain patterns, intensities, and triggers. It can also involve symptoms that overlap those of other disorders, including disorders of the gastrointestinal and urinary tracts.”
Although endometriosis falls within the differential diagnosis of chronic pelvic pain, “it is usually not high on the list in the primary care setting (adult and adolescent),” Dr. Giudice adds. She is the Robert B. Jaffe, MD, Endowed Professor in the reproductive sciences and chair of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco.
John R. Lue, MD, MPH, an author of the most recent practice bulletin on endometriosis from the American College of Obstetricians and Gynecologists,1 sees the situation similarly.
“The main challenge in the diagnosis of endometriosis is that its presentation mimics other causes of chronic pelvic pain,” he says. “Pelvic pain due to endometriosis is usually chronic (lasting ≥6 months). It is associated with dysmenorrhea in 50% to 90% of cases, as well as with dyspareunia, deep pelvic pain, and lower abdominal pain with or without back and loin pain. The pain can occur unpredictably and intermittently throughout the menstrual cycle or it can be continuous. In addition, it can be dull, throbbing, or sharp and may be exacerbated by physical activity.2,3 Up to 20% of women with endometriosis have concurrent pain conditions.”4 Dr. Lue is associate professor, chief of the section of general obstetrics and gynecology, and medical director of women’s ambulatory services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
Among other diseases of the female pelvis that have relatively similar presentation, Dr. Lue adds, are pathologies of the:
- uterus (adenomyosis, fibroids)
- fallopian tube (hydrosalpinx)
- ovaries (ovarian cysts)
- bladder (interstitial cystitis)
- bowel (irritable bowel syndrome)
- musculoskeletal system (piriformis syndrome).
Before pelvic pain is attributed to endometriosis, he says, the provider should rule out bowel, bladder, musculoskeletal, and psychiatric causes.
This article focuses on 7 questions, the answers of which are critical to narrowing in on the diagnosis of endometriosis, including essential factors to consider in the patient history, imaging and other diagnostic tools, and considerations in surgical exploration. In the second and third installments of this in-depth series on endometriosis, pain and infertility will be the respective subjects of investigation.
Several theories explain the “why” of endometriosis
A dominant theory is that peritoneal endometrial implants arise from retrograde menstruation, during which endometrial tissue passes through the fallopian tubes into the pelvis, says John R. Lue, MD, MPH. Dr. Lue is associate professor and chief of the section of general obstetrics and gynecology and medical director of women’s ambulatory services at the Medical College of Georgia and Georgia Regents University in Augusta, Georgia.
“Additional theories include immune dysfunction that interferes with clearing of endometrial lesions in the pelvis, as well as genetic alterations that lead to growth dysregulation,” he says.1 “These theories all have merit, and it is likely that the pathogenesis of endometriosis is multifactorial.”
Another strong theory involves the homeobox (HOX) genes, “which mediate embryonic development,” says Dr. Lue.2,3 “These genes are translated into transcription factors that regulate downstream genes necessary for growth and differentiation. It has been demonstrated that HOX genes play an analogous role in endometrial development during the adult menstrual cycle.4 HOX gene expression regulates the growth and development of the human endometrium.5 The expression of HOX genes A10 and A11 varies in response to sex steroids during the menstrual cycle, with dramatic upregulation in the mid-secretory phase,” says Dr. Lue. Recent studies suggest that these genes “play a major role” in endometriosis.6
“Since ovarian endometriomas are clonal and lesions usually have genetic mutations, such somatic mutations with subsequent growth dysregulation may also be etiologic factors,” says Dr. Lue.1,7,8 “Disease at distant sites may be caused by lymphatic or hematogenous spread or metaplastic transformation.”
References
1. Giudice LC, Swierz LM, Burney RO. Edometriosis. In: Jameson JL, DeGroot LJ, eds. Endocrinology. 6th ed. New York, NY: Elsevier; 2010:2356–2370.
2. Krumlauf R. Hox genes in vertebrate development. Cell. 1992;78(2):191–201.
3. McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68(2):283–302.
4. Taylor H, Igarashi P, Olive D, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab. 1999;84(3):1129–1135.
5. Taylor H, Vanden Heuvel GB, Igarashi P. A conserved HOX axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the HOXA cluster genes. Biol Reprod. 1997;57(6):1338–1345.
6. Taylor HS, Bagot C, Kardana A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14(5):1328–1331.
7. Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis. Fertil Steril. 2008;90(5 suppl):S260–S269.
8. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
1. Why such a long delay in diagnosis?
Investigators exploring the length of time between a patient’s presentation with symptoms and diagnosis have found it to be particularly long for endometriosis, ranging from 6 to 11 years.
Because endometriosis is usually not high on the list of differential diagnoses for chronic pelvic pain in the primary care setting, a patient may not be referred to a gynecologist unless those symptoms include severe dysmenorrhea, dyspareunia, or similar findings. Once the referral is made, the gynecologist “will usually try contraceptive steroids, nonsteroidal anti-inflammatory drugs, or second-line progestins before a diagnosis is made,” says Dr. Giudice.5
The delay in diagnosis “is astounding,” she adds, “and has its roots in empiric medical therapies and a combination of patients fearing a diagnosis of cancer and reluctance of gynecologists to perform laparoscopy on adolescents.”6
Another possible cause of diagnostic delay: Some adolescent girls may not realize when their pain is severe. Because they may have always experienced a high degree of pain since menarche, they may assume it to be a normal aspect of womanhood and delay seeking help, says Pamela Stratton, MD, chief of the gynecology consult service at the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
2. Have any biomarkers proved to be useful diagnostic tools?
Any biomarker proven to reliably identify endometriosis would be a boon to medicine, as it would provide a noninvasive or minimally invasive alternative to diagnostic laparoscopy, the current gold standard. Regrettably, the search for such a biomarker has produced “disappointing results,” says Dr. Giudice.
“Recent systematic reviews of all proposed endometriosis-related biomarkers over the last 25 years in serum, plasma, urine, and endometrium could not identify an unequivocally clinically useful biomarker or panel of biomarkers,” she notes.7,8 “This is due mainly to low numbers of subjects, small populations for validations, cycle/hormonal- and disease stage-dependence, poorly defined controls, and low sensitivity and specificity.”
One hopeful development: “Whole genome transcriptomics of archived endometrial tissue and machine learning found several classifiers to diagnose and stage endometriosis with high accuracy that were validated on an independent sample set,” says Dr. Giudice.9 “However, these data now warrant a prospective, multisite study for further validation.”
Environmental factors, estrogen, and endometriosis
“There is increasing evidence that in utero and also adult exposures to endocrine-disrupting chemicals (EDCs) play a role in the pathogenesis and progression of disease,” says Linda Giudice, MD, PhD, the Robert B. Jaffe, MD, Endowed Professor in the reproductive sciences and chair of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco.
“For example, the Nurses’ Health Study II, a prospective cohort study of more than 80,000 women, revealed that daughters exposed to diethylstilbestrol (DES) had an 80% increased risk (odds ratio [OR], 1.8; 95% confidence interval [CI], 1.2–2.8) of developing endometriosis,” she says.1
“Also, dioxin (TCDD) exposure in rats in utero on gestational day 8 increased the size of endometriosis lesions when combined with an adult exposure,” Dr. Giudice says.2 Although we do not know the precise mechanisms underlying in utero events that result in disease onset as a teen or adult, abnormal programming of the female reproductive tract by EDCs and similar agents is believed to play a role, she says.3
Because estrogen is essential for endometriosis lesions and associated symptoms to progress, “EDCs that have either estrogenic activity or interfere with estrogen metabolism or action, or both, have been proposed as contributors to progression of disease. Abundant animal data using nonhuman primate and rodent models and exposures to organochlorines and other EDCs support this hypothesis,” Dr. Giudice says.4
“The weight of human evidence of associations of EDCs and the risk of endometriosis in adult women depends on the class of endocrine disrupter,” Dr. Giudice continues. “Strongest correlations are with polychlorobiphenyls (PCBs), where 10 of 12 studies found significant odds of disease and circulating or omental fat concentrations of these compounds. PCBs inhibit peripheral natural killer cell activity and interleukin 1b and 12 production, relevant to the immune component of endometriosis progression.”
“Significant risk has also been associated with organochlorines (in three of three studies) and perfluorochemicals. In contrast, data linking endometriosis with exposures to dioxins, phthalates, and bisphenol A are equivocal, with some studies finding significant odds ratios and others failing to find significant correlations. Interestingly, dioxins have a significant association with deep infiltrating endometriotic lesions.”5
References
1. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Michels KB, Hunter DJ. In utero exposures and the incidence of endometriosis. Fertil Steril. 2004;82(6):1501–1508.
2. Cummings AM, Hedge JM, Birnbaum LS. Effects on prenatal exposure to TCDD on the promotion of endometriotic lesion growth by TCDD in adult female rats and mice. Toxicol Sci. 1999;52(1):45–49.
3. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279.
4. Crain DA, Janssen SJ, Edwards TM, et al. Female reproductive disorders: the roles of endocrine disrupting compounds and developmental timing. Fertil Steril. 2008;90(4):911–940.
5. Heilier JF, Donnez J, Nackers F, et al. Environmental and host-associated risk factors in endometriosis and deep endometriotic nodules: a matched case-control study. Environ Res. 2007;103(1):121–129.
3. What aspects of the patient history are key?
Dr. Stratton recommends that clinicians begin their evaluation of the patient with pain by asking her to describe that pain—how long she has had it, when it occurs, and which areas are affected.
“Most women with endometriosis-associated pain have chronic pelvic pain,” Dr. Stratton continues.5 “Up to 90% of those have dysmenorrhea or cyclic pain with menses.”10 In addition, women with endometriosis “commonly report having pain with any bleeding or spotting. About 30% of women diagnosed with endometriosis initially present to their gynecologist with dyspareunia.”11
“Episodic pain with menses may become more constant, lasting for many days of the month,” says Dr. Stratton. “Women with dyschezia or dysuria may have endometriosis lesions associated with the bowel or bladder, respectively.12 When women with these symptoms do not have lesions on bowel or bladder, these pain symptoms may occur because of higher peritoneal hormone and inflammatory factor levels or because adjacent organs share the neural networks.”
Dr. Giudice views the history similarly.
“I believe listening to the patient is essential in evaluating the possibility of her having endometriosis. This involves asking her to describe where her pain is, grading it on a scale of 1 to 10, identifying when in her cycle it occurs, and learning what makes it better or worse,” she says.
“It also is important to assess the quality of the pain,” Dr. Giudice says. “Does it radiate, does it limit her daily activities, does it interfere with her relationships, intercourse, work, school? Is it associated with bowel movements, urination, other pain syndromes?”
“Having a pain questionnaire is a great help so that patients have a chance to reflect on these and other questions that help to frame the pain associated with endometriosis when they come for consultation,” she adds.
By determining if pain is associated with menstruation or spotting, the clinician is better informed about the value of menstrual suppression, says Dr. Stratton. “Determining what makes the pain better or worse can help define triggers which, if treated, can decrease the likelihood of episodes of pain.”
“A detailed history of any medical or surgical treatments and their outcome is helpful in guiding future treatment,” she adds. “While hormonal therapy has been a mainstay of treatment, in some women, some hormonal treatments may worsen pain or have unacceptable side effects like worsening depression or anxiety. In addition, some pain—especially that associated with deep lesions—may be relieved by surgical treatment13,14; pain that worsened after surgery may suggest neural damage.”
“As there is an engagement of the central nervous system, endometriosis is considered a central sensitivity syndrome in which women may also have other sites of pain,” Dr. Stratton says. “Thus, obtaining a history about current symptoms or prior diagnosis of irritable bowel syndrome, interstitial cystitis/painful bladder, migraines, fibromyalgia, or chronic fatigue syndrome is beneficial.10,15–17 Facilitating treatment for these comorbidities is a key principle in helping women with endometriosis-associated pain, as any condition that triggers or perpetuates pain warrants treatment.”
4. What should the physical exam entail?
“An abdominal exam and a pelvic exam are essential in evaluating pain in a woman when endometriosis is suspected,” says Dr. Giudice. “Sometimes the latter is challenging in young teens and can be deferred.” Overall, however, “the pelvic exam can give insight into pain triggers, adnexal masses (possible endometriomas) and mobility of pelvic organs. A rectovaginal exam is important in evaluating deep infiltrating disease and to gauge the pelvic pain landscape overall. In addition, palpating the pelvic floor musculature is important to distinguish pelvic floor muscle spasm from endometriosis pain.”
“The challenge for clinicians is to think beyond the endometrial implants, taking into account multiple factors that influence pain perception,” says Dr. Stratton. During the examination, the clinician should begin by mapping the regions of pain in the abdomen and back, “distinguishing musculoskeletal pain from deep pain. Determining whether pains are focused or diffuse is also important.”
Dr. Stratton recommends that the routine pelvic exam be modified because a standard bimanual exam “confuses pain signals from the pelvic floor, abdominal wall, bladder, and other viscera. For this reason, a pain-oriented assessment is mandatory.”
Begin with a single digital examination to map tender areas, Dr. Stratton advises. Then consider the size, shape, and mobility of reproductive and pelvic organs. “A bimanual exam will help identify adnexal masses like endometriomas,” she says.
Endometriomas usually are not associated with pain, she adds, but “they are associated with deep infiltrating lesions. Nodularity along the uterosacral ligaments, limited reproductive organ mobility, and thickening of the rectovaginal septum also suggest deep infiltrating lesions. Importantly, deep infiltrating lesions are the lesion type most associated with pain.”18,19
5. Is imaging useful in the diagnosis of endometriosis?
Laparoscopy remains the gold standard for diagnosis of endometriosis, observes Steven R. Goldstein, MD. Visualization of endometriotic implants at the time of surgery—with histologic assessment—offers definitive confirmation of the diagnosis. The physical examination, too, can offer a strong suggestion of endometriosis, he says. Dr. Goldstein is professor of obstetrics and gynecology at New York University School of Medicine and director of gynecologic ultrasound and co-director of bone densitometry at New York University Medical Center in New York City. He serves on the OBG Management Board of Editors.
“In the past, the pelvic examination and history often were the sine qua non for patients with pain,” Dr. Goldstein says. “Extreme dysmenorrhea and pain between periods, especially with intercourse, defecation, and exercise, all increased the suspicion of endometriosis. People used to talk about feeling nodularity in the uterosacral ligaments and finding decreased mobility of pelvic structures—but I don’t have any question that the skill of today’s gynecologists in doing a bimanual pelvic exam is a fraction of what it was in years gone by because they haven’t had the necessity of experience. The first thing they do if there’s any question is they send the patient for an ultrasound.”
Of course, ultrasound can be especially helpful in identifying endometriomas—sometimes called “chocolate cysts”—in the ovary. Endometriomas can have a solid appearance on ultrasound, says Dr. Goldstein, because the fluid they contain (dried blood) is sonolucent or pure black on ultrasound, similar to amniotic fluid or the fluid seen in the bladder. “This ‘chocolate’ fluid contained in endometriomas is homogeneous, particulate, and very monotonous in its appearance, in contrast to the internal echoes observed in hemorrhagic corpus lutea, which are very cobweblike and can sometimes mimic papillary projections,” he adds.
“What’s absolutely essential when imaging a suspected endometrioma by ultrasound is that there be no evidence of any blood flow contained within that structure. Because it’s dried blood, it shouldn’t have any vascularity. If you see blood flow inside what you would call an endometrioma, you need to rethink your diagnosis,” he says.
In some cases, a supposed endometriomalacks a black, sonolucent appearance, but “the clinician often can tell that it’s a cystic structure by the very bright posterior wall—what we call posterior wall acoustic enhancement—even though the interior of the structure may appear sort of grayish or whitish rather than the pure black of a simple cyst. It’s still fluid-filled,” Dr. Goldstein says.
In some instances, even endometriotic nodules can be imaged by ultrasound, he adds. “There’s an increasing body of literature that suggests that, if you look carefully in people with deep infiltrating endometriosis, you can often see solid-appearing nodules in the rectovaginal septum or between the uterus and bladder. With the kind of resolution that we now have with the vaginal probe, some of these nodules can be seen. That’s somewhat new, and it’s a function of 2 things—people looking for endometriosis and the better resolution of more modern equipment.”
Dr. Goldstein believes that magnetic resonance imaging (MRI) is “almost never” indicated in the diagnosis of endometriosis. A more helpful approach would be a consultative ultrasound with someone with more experience. However, when that is not available, or “in areas where you have excellent backup in terms of pelvic MRI, that may be the way to go. I don’t think so,” he demurs, “and some of my colleagues would be very upset at the thought of needing to use MRI to diagnose endometriosis. But in the occasional confusing or difficult case, depending on the quality of the referral pattern you have, it might make sense,” he says.
6. When is diagnostic laparoscopy clearly indicated?
Dr. Giudice believes that laparoscopy—with the intention to treat endometriosis, if present—“is essential when first-line medical therapy fails or when pain is acute and severe.”5
Dr. Stratton concurs. “Any woman with chronic pain wants to know what is causing the pain,” she says. Therefore, “women report a benefit from knowing that their pain is associated with endometriosis.6 However, diagnostic laparoscopy alone, with the sole purpose of determining the presence of endometriosis but not treating the lesions, is no longer performed, as it poses little benefit to the patient other than peace of mind.”
“The general trend in the United States has been to first use hormonal treatments when the diagnosis of endometriosis is suspected, prior to performing surgery,” Dr. Stratton says.1 In many cases, by using cyclic combined hormonal contraceptives to reduce menstrual flow or “suppressing menstruation with continuous combined hormonal contraceptives,” gonadotropin-releasing hormone analogues (combined with progestin to prevent bone loss), “or continuous progestin alone may be effective in decreasing pain. Not surprisingly, these hormonal approaches are effective for any chronic pelvic pain, even for women who do not have the surgical diagnosis of endometriosis.”20
“When the first-line approach to chronic pelvic pain is hormonal treatment, laparoscopy is considered when these medical treatments have failed to control the pain or are poorly tolerated, or when the diagnosis of endometriosis is in question,” Dr. Stratton says.
“Laparoscopy to treat endometriomas is indicated if an endometrioma is enlarging, measures more than 4 cm in diameter, or if the diagnosis of an ovarian mass is in question,” she explains. “While surgeons have previously been aggressive in removing endometriomas, this practice may have negative consequences on ovarian function. Because endometriomas are pseudocysts, removing them completely leads to the removal of viable ovarian tissue and may diminish ovarian reserve.”21,22
7. What is the surgical appearance of endometriosis?
Dr. Giudice returns to the enigmatic nature of endometriosis in addressing this question, mentioning its “many faces” at the time of surgery. “It is imperative that the surgeon recognize the disease in its many forms,” she says. “Also, it is especially helpful at the time of surgery if suspected lesions are biopsied and sent to pathology to have the diagnosis made unequivocally.”5
As for the surgical appearance of endometriosis, Dr. Stratton notes that there are 3 types of lesions—“superficial lesions, deep infiltrating lesions, and endometriomas. Endometriomas occur almost exclusively in the ovary and are pseudocysts without an identifiable cystic lining. They vary in dimension from a few millimeters to several centimeters.”
“Superficial peritoneal endometriosis lesions have a variable appearance, with some lesions being clear or red, some brown, blue or black, and some having a white appearance, like a scar,” says Dr. Stratton. “Endometriosis can be diagnosed on histologic examination of any of these lesion types.
“Overall, single-color lesions have similar frequencies of biopsy-confirmed endometriosis (59% to 62%),” she says.23 “These lesion appearances likely represent different stages of development of endometriosis, with red or clear lesions occurring first, soon after endometrial tissue implantation; black, blue, or brown lesions occurring later, in response to the hormones varying in the menstrual cycle; and white lesions occurring as the lesions age. Deep infiltrating lesions generally have blue/black or white features.”
“Wide, deep, multiple-color lesions in the cul-de-sac, ovarian fossa or uterosacral ligaments are most likely endometriosis,” Dr. Stratton adds.23 Only lesions with multiple colors have a significantly higher percentage of positive biopsies (76%). Importantly, over half of women with only subtle lesions (small red or white lesions) have endometriosis.
CASE Resolved
You tell the patient that endometriosis is one of the possible diagnoses for her chronic pelvic pain, and you take a focused history. During a pelvic examination, you observe that her right ovary lacks mobility, and you map a number of trigger points for her pain. Transvaginal ultrasound results suggest the presence of nodules in the rectovaginal septum. You begin empiric treatment with continuous combined hormonal contraceptives to suppress menstruation. On her next visit, the patient reports reduced but still bothersome pain. Laparoscopy reveals a 2-cm endometrioma in the right ovary and deep infiltrating lesions in the cul-de-sac. The endometrioma is resected. Histology confirms the diagnosis of endometriosis.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. American College of Obstetricians and Gynecologists. Practice Bulletin #114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223–236.
2. Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154(1):39–43.
3. Taylor HS, Bagot C, Kardana A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14(5):1328–1331.
4. Berkley KJ, Stratton P. Mechanisms: lessons from translational studies of endometriosis. In: Giamberardino MA, ed. Visceral Pain: Clinical, Pathophysiological and Therapeutic Aspects. Oxford, UK: Oxford University Press; 2009:39–50.
5. Giudice LC. Clinical practice: endometriosis. N Engl J Med. 2010;362(25):2389–2398.
6. Ballard K, Lowton K, Wright J. What’s the delay: a qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296–1301.
7. May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010;16(6):651–674.
8. May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011;17(5):637–653.
9. Tamaresis JS, Irwin JC, Goldfien GA, et al. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155(12):4986–4999.
10. Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715–2724.
11. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677–2685.
12. Lafay Pillet MC, Huchon C, Santulli P, Borghese B, Chapron C, Fauconnier A. A clinical score can predict associated deep infiltrating endometriosis before surgery for an endometrioma. Hum Reprod. 2014;29(8):1666–1676.
13. Healey M, Cheng C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. J Minim Invasive Gynecol. 2014;21(6):999–1004.
14. Anaf V, El Nakadi I, De Moor V, Chapron C, Pistofidis G, Noel JC. Increased nerve density in deep infiltrating endometriotic nodules. Gynecol Obstet Invest. 2011;71(2):112–117.
15. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346.
16. Karp BI, Sinaii N, Nieman LK, Silberstein SD, Stratton P. Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril. 2011;95(3):895–899.
17. Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86(3):272–280.
18. Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595–606.
19. Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266–271.
20. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93(1):51–58.
21. Muzii L, Di Tucci C, Di Feliciantonio M, Marchetti C, Perniola G, Panici PB. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: a systematic review and meta-analysis. Hum Reprod. 2014;29(10):2190–2198.
22. Muzii L, Luciano AA, Zupi E, Panici PB. Effect of surgery for endometrioma on ovarian function: a different point of view. J Minim Invasive Gynecol. 2014;21(4):531–533.
23. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632–1636.
1. American College of Obstetricians and Gynecologists. Practice Bulletin #114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223–236.
2. Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154(1):39–43.
3. Taylor HS, Bagot C, Kardana A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14(5):1328–1331.
4. Berkley KJ, Stratton P. Mechanisms: lessons from translational studies of endometriosis. In: Giamberardino MA, ed. Visceral Pain: Clinical, Pathophysiological and Therapeutic Aspects. Oxford, UK: Oxford University Press; 2009:39–50.
5. Giudice LC. Clinical practice: endometriosis. N Engl J Med. 2010;362(25):2389–2398.
6. Ballard K, Lowton K, Wright J. What’s the delay: a qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296–1301.
7. May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010;16(6):651–674.
8. May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011;17(5):637–653.
9. Tamaresis JS, Irwin JC, Goldfien GA, et al. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155(12):4986–4999.
10. Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715–2724.
11. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677–2685.
12. Lafay Pillet MC, Huchon C, Santulli P, Borghese B, Chapron C, Fauconnier A. A clinical score can predict associated deep infiltrating endometriosis before surgery for an endometrioma. Hum Reprod. 2014;29(8):1666–1676.
13. Healey M, Cheng C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. J Minim Invasive Gynecol. 2014;21(6):999–1004.
14. Anaf V, El Nakadi I, De Moor V, Chapron C, Pistofidis G, Noel JC. Increased nerve density in deep infiltrating endometriotic nodules. Gynecol Obstet Invest. 2011;71(2):112–117.
15. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346.
16. Karp BI, Sinaii N, Nieman LK, Silberstein SD, Stratton P. Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril. 2011;95(3):895–899.
17. Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86(3):272–280.
18. Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595–606.
19. Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266–271.
20. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93(1):51–58.
21. Muzii L, Di Tucci C, Di Feliciantonio M, Marchetti C, Perniola G, Panici PB. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: a systematic review and meta-analysis. Hum Reprod. 2014;29(10):2190–2198.
22. Muzii L, Luciano AA, Zupi E, Panici PB. Effect of surgery for endometrioma on ovarian function: a different point of view. J Minim Invasive Gynecol. 2014;21(4):531–533.
23. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632–1636.
IN THIS ARTICLE
- The “why” of endometriosis
- Is imaging useful?
- When is diagnostic laparoscopy clearly indicated?
- Environmental factors, estrogen, and endometriosis
Vasomotor symptoms of menopause often persist longer than 7 years
Frequent menopausal vasomotor symptoms (VMS), including hot flashes and night sweats, lasted longer than 7 years during the transition to menopause for more than 50% of women in the Study of Women’s Health Across the Nation (SWAN).1 Among the factors related to a longer duration of VMS:
- younger age
- African American heritage
- lower educational level
- greater perceived stress and symptom sensitivity
- higher depressive symptoms and anxiety at the first report of VMS.
Details of the study
Avis and colleagues analyzed data from SWAN, a multiracial/multiethnic study of women transitioning to menopause that was conducted from February 1996 through April 2013. The analyses included 1,449 women with frequent VMS (ie, occurring at least 6 days in the previous 2 weeks).
Baseline eligibility was age between 42 and 52 years, an intact uterus and at least one ovary, report of a menstrual cycle in the 3 months before screening, absence of pregnancy and lactation, and no use of oral contraceptives or hormone therapy (HT). Women were assessed in person at baseline and approximately annually over the course of the study (mean and maximum follow-up durations were 12.7 and 17.2 years, respectively).
The main outcomes were total VMS duration (in years) and persistence of VMS (in years) beyond the final menstrual period (FMP).
Among the findings:
- The unadjusted median total VMS duration was 7.4 years
- Women who were premenopausal or early perimenopausal when they first reported frequent VMS had the longest total duration of VMS (median, >11.8 years) and longest persistence of VMS beyond the FMP (median, 9.4 years)
- Women who were postmenopausal at the onset of VMS had the shortest total VMS duration after the FMP (median, 3.4 years; P<.001).
- The median total VMS duration varied significantly by race, with African American women reporting the longest total VMS duration (median, 10.1 years) and Japanese and Chinese women reporting the shortest total duration (median, 4.8 and 5.4 years, respectively). Non-Hispanic white women had a median total VMS duration of 6.5 years; among Hispanic women, the median was 8.9 years.
Key takeaway
“These findings can help health-care professionals counsel patients about expectations regarding VMS and assist women in making treatment decisions based on the probability of their VMS persisting,” Avis and colleagues concluded. “In addition, the median total duration of 7.4 years highlights the limitations of guidance recommending short-term HT use and emphasizes the need to identify safe long-term therapies for the treatment of VMS.”
SWAN is the largest and longest longitudinal study to date to report on total duration of VMS and their persistence beyond the FMP.
“More than 50% of midlife women experience frequent VMS, yet clinical guidelines typically underestimate their true duration,” Avis and colleagues observed.
Reference
- Avis NE, Crawford SL, Greendale G, et al; Study of Women’s Health Across the Nation (SWAN). Duration of menopausal symptoms over the menopause transition [published online ahead of print February 16, 2015]. JAMA Intern Med. doi:10.1001/jamainternmed.2014.8063.
Frequent menopausal vasomotor symptoms (VMS), including hot flashes and night sweats, lasted longer than 7 years during the transition to menopause for more than 50% of women in the Study of Women’s Health Across the Nation (SWAN).1 Among the factors related to a longer duration of VMS:
- younger age
- African American heritage
- lower educational level
- greater perceived stress and symptom sensitivity
- higher depressive symptoms and anxiety at the first report of VMS.
Details of the study
Avis and colleagues analyzed data from SWAN, a multiracial/multiethnic study of women transitioning to menopause that was conducted from February 1996 through April 2013. The analyses included 1,449 women with frequent VMS (ie, occurring at least 6 days in the previous 2 weeks).
Baseline eligibility was age between 42 and 52 years, an intact uterus and at least one ovary, report of a menstrual cycle in the 3 months before screening, absence of pregnancy and lactation, and no use of oral contraceptives or hormone therapy (HT). Women were assessed in person at baseline and approximately annually over the course of the study (mean and maximum follow-up durations were 12.7 and 17.2 years, respectively).
The main outcomes were total VMS duration (in years) and persistence of VMS (in years) beyond the final menstrual period (FMP).
Among the findings:
- The unadjusted median total VMS duration was 7.4 years
- Women who were premenopausal or early perimenopausal when they first reported frequent VMS had the longest total duration of VMS (median, >11.8 years) and longest persistence of VMS beyond the FMP (median, 9.4 years)
- Women who were postmenopausal at the onset of VMS had the shortest total VMS duration after the FMP (median, 3.4 years; P<.001).
- The median total VMS duration varied significantly by race, with African American women reporting the longest total VMS duration (median, 10.1 years) and Japanese and Chinese women reporting the shortest total duration (median, 4.8 and 5.4 years, respectively). Non-Hispanic white women had a median total VMS duration of 6.5 years; among Hispanic women, the median was 8.9 years.
Key takeaway
“These findings can help health-care professionals counsel patients about expectations regarding VMS and assist women in making treatment decisions based on the probability of their VMS persisting,” Avis and colleagues concluded. “In addition, the median total duration of 7.4 years highlights the limitations of guidance recommending short-term HT use and emphasizes the need to identify safe long-term therapies for the treatment of VMS.”
SWAN is the largest and longest longitudinal study to date to report on total duration of VMS and their persistence beyond the FMP.
“More than 50% of midlife women experience frequent VMS, yet clinical guidelines typically underestimate their true duration,” Avis and colleagues observed.
Frequent menopausal vasomotor symptoms (VMS), including hot flashes and night sweats, lasted longer than 7 years during the transition to menopause for more than 50% of women in the Study of Women’s Health Across the Nation (SWAN).1 Among the factors related to a longer duration of VMS:
- younger age
- African American heritage
- lower educational level
- greater perceived stress and symptom sensitivity
- higher depressive symptoms and anxiety at the first report of VMS.
Details of the study
Avis and colleagues analyzed data from SWAN, a multiracial/multiethnic study of women transitioning to menopause that was conducted from February 1996 through April 2013. The analyses included 1,449 women with frequent VMS (ie, occurring at least 6 days in the previous 2 weeks).
Baseline eligibility was age between 42 and 52 years, an intact uterus and at least one ovary, report of a menstrual cycle in the 3 months before screening, absence of pregnancy and lactation, and no use of oral contraceptives or hormone therapy (HT). Women were assessed in person at baseline and approximately annually over the course of the study (mean and maximum follow-up durations were 12.7 and 17.2 years, respectively).
The main outcomes were total VMS duration (in years) and persistence of VMS (in years) beyond the final menstrual period (FMP).
Among the findings:
- The unadjusted median total VMS duration was 7.4 years
- Women who were premenopausal or early perimenopausal when they first reported frequent VMS had the longest total duration of VMS (median, >11.8 years) and longest persistence of VMS beyond the FMP (median, 9.4 years)
- Women who were postmenopausal at the onset of VMS had the shortest total VMS duration after the FMP (median, 3.4 years; P<.001).
- The median total VMS duration varied significantly by race, with African American women reporting the longest total VMS duration (median, 10.1 years) and Japanese and Chinese women reporting the shortest total duration (median, 4.8 and 5.4 years, respectively). Non-Hispanic white women had a median total VMS duration of 6.5 years; among Hispanic women, the median was 8.9 years.
Key takeaway
“These findings can help health-care professionals counsel patients about expectations regarding VMS and assist women in making treatment decisions based on the probability of their VMS persisting,” Avis and colleagues concluded. “In addition, the median total duration of 7.4 years highlights the limitations of guidance recommending short-term HT use and emphasizes the need to identify safe long-term therapies for the treatment of VMS.”
SWAN is the largest and longest longitudinal study to date to report on total duration of VMS and their persistence beyond the FMP.
“More than 50% of midlife women experience frequent VMS, yet clinical guidelines typically underestimate their true duration,” Avis and colleagues observed.
Reference
- Avis NE, Crawford SL, Greendale G, et al; Study of Women’s Health Across the Nation (SWAN). Duration of menopausal symptoms over the menopause transition [published online ahead of print February 16, 2015]. JAMA Intern Med. doi:10.1001/jamainternmed.2014.8063.
Reference
- Avis NE, Crawford SL, Greendale G, et al; Study of Women’s Health Across the Nation (SWAN). Duration of menopausal symptoms over the menopause transition [published online ahead of print February 16, 2015]. JAMA Intern Med. doi:10.1001/jamainternmed.2014.8063.
Best practices for the surgical management of adnexal masses in pregnancy
During the 43rd AAGL Global Congress, held November 17–21 in Vancouver, British Columbia, Sarah L. Cohen, MD, MPH, of Brigham and Women’s Hospital in Boston, Massachusetts, stepped attendees through diagnosis and surgical management of adnexal masses in pregnancy, noting the approaches backed by the highest-quality data.
The incidence of adnexal masses in pregnancy is 1 in every 600 live births. A mass can be benign or malignant. Among benign masses found in pregnancy are functional cysts, teratomas, and the corpus luteum.
Work-up
Ultrasound imaging is a valuable component of the work-up, owing to its risk-free nature. Magnetic resonance imaging may be appropriate in selected cases, but gadolinium contrast should be avoided.
In pregnancy, the aim is to limit ionizing radiation to less than 5 to 10 rads to minimize the risk of childhood malignancy/leukemia, with no single imaging study exceeding 5 rads.
Tumor markers may be helpful, but careful interpretation is critical, taking into account the effects of pregnancy itself on CA-125 (which peaks in the first trimester), human chorionic gonadotropin, alpha fetoprotein, inhibin A, and lactate dehydrogenase.
When expectant management may be appropriate
Watchful waiting may be considered for simple cysts less than 6 cm in size, provided the patient is asymptomatic with no signs of malignancy.
Surgery is indicated when the patient is symptomatic, when there is a concern for malignancy, and when a persistent mass exceeds 10 cm in size.
As always, elective surgery is preferable, as emergent surgery in pregnancy is associated with a risk of preterm labor of 22% to 35%.
Optimal timing of surgery
Surgery can be performed safely in any trimester, provided the gynecologist is aware of special concerns. For example, in the first trimester, organogenesis is under way and the corpus luteum is still present. If the corpus luteum is removed, progesterone supplementation is necessary.
When surgery can be postponed to the second trimester, it allows time for possible resolution of the mass.
Mode of surgery
Laparoscopy allows for faster recovery, less pain (and, therefore, lower narcotic exposure to the fetus), and improved maternal ventilation.
Prophylaxis for venous thromboembolism is indicated through the use of pneumatic compression devices and, when appropriate, heparin.
Initial port placement can be performed using a Hassan technique, Veress needle, or optical trocar.
Insufflation pressures of 10 to 15 mm Hg are safe, with intraoperative monitoring of carbon dioxide.
Availability of guidelines
Surgeons should make use of guidelines, when feasible, to guide surgery. For example, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) publishes guidelines on surgery during pregnancy. The American College of Obstetricians and Gynecologists also offers guidelines.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
During the 43rd AAGL Global Congress, held November 17–21 in Vancouver, British Columbia, Sarah L. Cohen, MD, MPH, of Brigham and Women’s Hospital in Boston, Massachusetts, stepped attendees through diagnosis and surgical management of adnexal masses in pregnancy, noting the approaches backed by the highest-quality data.
The incidence of adnexal masses in pregnancy is 1 in every 600 live births. A mass can be benign or malignant. Among benign masses found in pregnancy are functional cysts, teratomas, and the corpus luteum.
Work-up
Ultrasound imaging is a valuable component of the work-up, owing to its risk-free nature. Magnetic resonance imaging may be appropriate in selected cases, but gadolinium contrast should be avoided.
In pregnancy, the aim is to limit ionizing radiation to less than 5 to 10 rads to minimize the risk of childhood malignancy/leukemia, with no single imaging study exceeding 5 rads.
Tumor markers may be helpful, but careful interpretation is critical, taking into account the effects of pregnancy itself on CA-125 (which peaks in the first trimester), human chorionic gonadotropin, alpha fetoprotein, inhibin A, and lactate dehydrogenase.
When expectant management may be appropriate
Watchful waiting may be considered for simple cysts less than 6 cm in size, provided the patient is asymptomatic with no signs of malignancy.
Surgery is indicated when the patient is symptomatic, when there is a concern for malignancy, and when a persistent mass exceeds 10 cm in size.
As always, elective surgery is preferable, as emergent surgery in pregnancy is associated with a risk of preterm labor of 22% to 35%.
Optimal timing of surgery
Surgery can be performed safely in any trimester, provided the gynecologist is aware of special concerns. For example, in the first trimester, organogenesis is under way and the corpus luteum is still present. If the corpus luteum is removed, progesterone supplementation is necessary.
When surgery can be postponed to the second trimester, it allows time for possible resolution of the mass.
Mode of surgery
Laparoscopy allows for faster recovery, less pain (and, therefore, lower narcotic exposure to the fetus), and improved maternal ventilation.
Prophylaxis for venous thromboembolism is indicated through the use of pneumatic compression devices and, when appropriate, heparin.
Initial port placement can be performed using a Hassan technique, Veress needle, or optical trocar.
Insufflation pressures of 10 to 15 mm Hg are safe, with intraoperative monitoring of carbon dioxide.
Availability of guidelines
Surgeons should make use of guidelines, when feasible, to guide surgery. For example, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) publishes guidelines on surgery during pregnancy. The American College of Obstetricians and Gynecologists also offers guidelines.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
During the 43rd AAGL Global Congress, held November 17–21 in Vancouver, British Columbia, Sarah L. Cohen, MD, MPH, of Brigham and Women’s Hospital in Boston, Massachusetts, stepped attendees through diagnosis and surgical management of adnexal masses in pregnancy, noting the approaches backed by the highest-quality data.
The incidence of adnexal masses in pregnancy is 1 in every 600 live births. A mass can be benign or malignant. Among benign masses found in pregnancy are functional cysts, teratomas, and the corpus luteum.
Work-up
Ultrasound imaging is a valuable component of the work-up, owing to its risk-free nature. Magnetic resonance imaging may be appropriate in selected cases, but gadolinium contrast should be avoided.
In pregnancy, the aim is to limit ionizing radiation to less than 5 to 10 rads to minimize the risk of childhood malignancy/leukemia, with no single imaging study exceeding 5 rads.
Tumor markers may be helpful, but careful interpretation is critical, taking into account the effects of pregnancy itself on CA-125 (which peaks in the first trimester), human chorionic gonadotropin, alpha fetoprotein, inhibin A, and lactate dehydrogenase.
When expectant management may be appropriate
Watchful waiting may be considered for simple cysts less than 6 cm in size, provided the patient is asymptomatic with no signs of malignancy.
Surgery is indicated when the patient is symptomatic, when there is a concern for malignancy, and when a persistent mass exceeds 10 cm in size.
As always, elective surgery is preferable, as emergent surgery in pregnancy is associated with a risk of preterm labor of 22% to 35%.
Optimal timing of surgery
Surgery can be performed safely in any trimester, provided the gynecologist is aware of special concerns. For example, in the first trimester, organogenesis is under way and the corpus luteum is still present. If the corpus luteum is removed, progesterone supplementation is necessary.
When surgery can be postponed to the second trimester, it allows time for possible resolution of the mass.
Mode of surgery
Laparoscopy allows for faster recovery, less pain (and, therefore, lower narcotic exposure to the fetus), and improved maternal ventilation.
Prophylaxis for venous thromboembolism is indicated through the use of pneumatic compression devices and, when appropriate, heparin.
Initial port placement can be performed using a Hassan technique, Veress needle, or optical trocar.
Insufflation pressures of 10 to 15 mm Hg are safe, with intraoperative monitoring of carbon dioxide.
Availability of guidelines
Surgeons should make use of guidelines, when feasible, to guide surgery. For example, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) publishes guidelines on surgery during pregnancy. The American College of Obstetricians and Gynecologists also offers guidelines.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
New mobile app assists clinicians in assessing menopausal patients
A new mobile app for iPhone and iPad enables both clinicians and patients to make decisions about menopausal therapies for moderate to severe hot flashes, night sweats, and/or genitourinary symptoms. The app also aids in assessing the patient’s risk of cardiovascular disease, breast cancer, and fracture.

|

|
|
The MenoPro app, developed in association with the North American Menopause Society (NAMS), is available free of charge from Apple. The app is designed to aid in the assessment and management of bothersome menopausal symptoms in women aged 45 and older.
Designed for both clinician and patient
A novel feature of the app is its two modes—one for the clinician and another for the patient. The clinician mode enables risk assessment and decision-making to determine whether hormonal therapy might be indicated and to determine the formulation and dosage of the therapy selected. It also features assessment of the patient’s 10-year risk of cardiovascular disease, her risk of breast cancer using the Gail model, and her fracture risk using the FRAX tool. When hormonal therapies are not appropriate, the app steers the clinician to nonhormonal options.
The patient can make use of the app to learn about her different treatment options, including lifestyle modifications. The app guides her through a self-assessment to gauge how far along she is in the menopausal transition, the severity of her symptoms, and her interest in hormonal or nonhormonal therapy. The app begins by recommending lifestyle changes and behavioral factors that can reduce menopausal symptoms. After a 3-month trial of these modifications, the patient is prompted to visit her health-care provider if further relief is needed.
Only FDA-approved drugs are recommended
“The app is completely up to date in terms of information about the newest medications that have been approved by the US Food and Drug Administration,” says JoAnn E. Manson, MD, DrPH, current chair of the NAMS Scientific Program and a past president of NAMS. Dr. Manson is Chief of the Division of Preventive Medicine at Brigham and Women’s Hospital in Boston. She also is Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health at Harvard Medical School.
“The app focuses on FDA-approved medications, including off-label use of medications that may be commonly prescribed in practice to treat hot flashes, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs),” she says.
“I think another big advantage is that very often clinicians who are managing patients during the menopausal transition or in early menopause may not be thinking that much about cardiovascular risk or even know how to evaluate it or make use of a 10-year risk score. So the app really helps them to become very familiar with the evaluation of cardiovascular risk, breast cancer risk, and fracture risk, and provides them with the resources to make use of the information.”
An algorithm is available within the app
The app is based on an algorithm that can be accessed within the app by choosing the “About” button. Another feature: the clinician can email a summary of the patient’s assessment directly to her, along with links to resources on a variety of relevant topics.
“In the future, there is a plan to have the app available for other mobile phones and tablet devices in addition to the iPhone and iPad,” says Dr. Manson. “We also hope to have it incorporated into electronic health records, where it could be used for clinical decision-making within the record.”
The app is not intended to replace clinical judgment, she adds. “I think clinicians are really familiar with the concept that, when you’re using an app, clinical judgment remains paramount. The app is not going to replace the clinician’s own discernment of what is going on with the patient.”
For detailed information, see an article on the app in the journal Menopause, available at http://www.menopause.org/docs/default-source/professional/our-new-paper.pdf
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
A new mobile app for iPhone and iPad enables both clinicians and patients to make decisions about menopausal therapies for moderate to severe hot flashes, night sweats, and/or genitourinary symptoms. The app also aids in assessing the patient’s risk of cardiovascular disease, breast cancer, and fracture.

|

|

|
The MenoPro app, developed in association with the North American Menopause Society (NAMS), is available free of charge from Apple. The app is designed to aid in the assessment and management of bothersome menopausal symptoms in women aged 45 and older.
Designed for both clinician and patient
A novel feature of the app is its two modes—one for the clinician and another for the patient. The clinician mode enables risk assessment and decision-making to determine whether hormonal therapy might be indicated and to determine the formulation and dosage of the therapy selected. It also features assessment of the patient’s 10-year risk of cardiovascular disease, her risk of breast cancer using the Gail model, and her fracture risk using the FRAX tool. When hormonal therapies are not appropriate, the app steers the clinician to nonhormonal options.
The patient can make use of the app to learn about her different treatment options, including lifestyle modifications. The app guides her through a self-assessment to gauge how far along she is in the menopausal transition, the severity of her symptoms, and her interest in hormonal or nonhormonal therapy. The app begins by recommending lifestyle changes and behavioral factors that can reduce menopausal symptoms. After a 3-month trial of these modifications, the patient is prompted to visit her health-care provider if further relief is needed.
Only FDA-approved drugs are recommended
“The app is completely up to date in terms of information about the newest medications that have been approved by the US Food and Drug Administration,” says JoAnn E. Manson, MD, DrPH, current chair of the NAMS Scientific Program and a past president of NAMS. Dr. Manson is Chief of the Division of Preventive Medicine at Brigham and Women’s Hospital in Boston. She also is Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health at Harvard Medical School.
“The app focuses on FDA-approved medications, including off-label use of medications that may be commonly prescribed in practice to treat hot flashes, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs),” she says.
“I think another big advantage is that very often clinicians who are managing patients during the menopausal transition or in early menopause may not be thinking that much about cardiovascular risk or even know how to evaluate it or make use of a 10-year risk score. So the app really helps them to become very familiar with the evaluation of cardiovascular risk, breast cancer risk, and fracture risk, and provides them with the resources to make use of the information.”
An algorithm is available within the app
The app is based on an algorithm that can be accessed within the app by choosing the “About” button. Another feature: the clinician can email a summary of the patient’s assessment directly to her, along with links to resources on a variety of relevant topics.
“In the future, there is a plan to have the app available for other mobile phones and tablet devices in addition to the iPhone and iPad,” says Dr. Manson. “We also hope to have it incorporated into electronic health records, where it could be used for clinical decision-making within the record.”
The app is not intended to replace clinical judgment, she adds. “I think clinicians are really familiar with the concept that, when you’re using an app, clinical judgment remains paramount. The app is not going to replace the clinician’s own discernment of what is going on with the patient.”
For detailed information, see an article on the app in the journal Menopause, available at http://www.menopause.org/docs/default-source/professional/our-new-paper.pdf
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
A new mobile app for iPhone and iPad enables both clinicians and patients to make decisions about menopausal therapies for moderate to severe hot flashes, night sweats, and/or genitourinary symptoms. The app also aids in assessing the patient’s risk of cardiovascular disease, breast cancer, and fracture.

|

|

|
The MenoPro app, developed in association with the North American Menopause Society (NAMS), is available free of charge from Apple. The app is designed to aid in the assessment and management of bothersome menopausal symptoms in women aged 45 and older.
Designed for both clinician and patient
A novel feature of the app is its two modes—one for the clinician and another for the patient. The clinician mode enables risk assessment and decision-making to determine whether hormonal therapy might be indicated and to determine the formulation and dosage of the therapy selected. It also features assessment of the patient’s 10-year risk of cardiovascular disease, her risk of breast cancer using the Gail model, and her fracture risk using the FRAX tool. When hormonal therapies are not appropriate, the app steers the clinician to nonhormonal options.
The patient can make use of the app to learn about her different treatment options, including lifestyle modifications. The app guides her through a self-assessment to gauge how far along she is in the menopausal transition, the severity of her symptoms, and her interest in hormonal or nonhormonal therapy. The app begins by recommending lifestyle changes and behavioral factors that can reduce menopausal symptoms. After a 3-month trial of these modifications, the patient is prompted to visit her health-care provider if further relief is needed.
Only FDA-approved drugs are recommended
“The app is completely up to date in terms of information about the newest medications that have been approved by the US Food and Drug Administration,” says JoAnn E. Manson, MD, DrPH, current chair of the NAMS Scientific Program and a past president of NAMS. Dr. Manson is Chief of the Division of Preventive Medicine at Brigham and Women’s Hospital in Boston. She also is Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health at Harvard Medical School.
“The app focuses on FDA-approved medications, including off-label use of medications that may be commonly prescribed in practice to treat hot flashes, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs),” she says.
“I think another big advantage is that very often clinicians who are managing patients during the menopausal transition or in early menopause may not be thinking that much about cardiovascular risk or even know how to evaluate it or make use of a 10-year risk score. So the app really helps them to become very familiar with the evaluation of cardiovascular risk, breast cancer risk, and fracture risk, and provides them with the resources to make use of the information.”
An algorithm is available within the app
The app is based on an algorithm that can be accessed within the app by choosing the “About” button. Another feature: the clinician can email a summary of the patient’s assessment directly to her, along with links to resources on a variety of relevant topics.
“In the future, there is a plan to have the app available for other mobile phones and tablet devices in addition to the iPhone and iPad,” says Dr. Manson. “We also hope to have it incorporated into electronic health records, where it could be used for clinical decision-making within the record.”
The app is not intended to replace clinical judgment, she adds. “I think clinicians are really familiar with the concept that, when you’re using an app, clinical judgment remains paramount. The app is not going to replace the clinician’s own discernment of what is going on with the patient.”
For detailed information, see an article on the app in the journal Menopause, available at http://www.menopause.org/docs/default-source/professional/our-new-paper.pdf
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
First large study on the risk of cancer spread using power morcellation pinpoints risk of uterine malignancy
Among women who undergo minimally invasive hysterectomy with electric power morcellation, the rate of uterine cancer is 27 cases per 10,000 women at the time of the procedure, according to a recent study published in the Journal of the American Medical Association.1 That figure translates into approximately one case of undetected uterine cancer in every 368 women undergoing hysterectomy. Earlier this year the US Food and Drug Administration (FDA) estimated the prevalence of uterine sarcoma at one case in every 352 women.2
Leading up to publication of this study in late July, there had been concern and considerable discussion—including a 2-day hearing convened by the FDA— about whether power morcellation may result in the spread of undetected malignancies and, if so, how often that may occur.
Although power morcellators have been available commercially for two decades, accurate estimates of the prevalence of malignancy at the time of power morcellation have been lacking.
Jason D. Wright, MD, and colleagues from Columbia University used the Perspective database, a large insurance database, to investigate the prevalence of underlying cancer in women who underwent uterine morcellation during minimally invasive hysterectomy from 2006 to 2012. This database is an “all-payer” database that includes more than 500 hospitals in the United States, many of them urban teaching centers.
The cohort included 232,882 women who underwent minimally invasive hysterectomy, including 36,470 (15.7%) who had uterine morcellation during the procedure. Among women who underwent morcellation, 99 cases of uterine cancer were identified, a prevalence of 27 cases per 10,000 women (95% confidence interval [CI], 22–32).
Among women who underwent power morcellation, the prevalence of underlying cancer and endometrial hyperplasia increased with age. For example, compared with women younger than 40 years, the prevalence ratio for uterine malignancy was:
- 4.97 (95% CI, 1.91–12.93) in women aged 50 to 54 years
- 19.37 (95% CI, 7.66–48.95) in those aged 55 to 59 years
- 21.36 (95% CI, 7.22–63.21) in women aged 60 to 64
- 35.97 (95% CI, 14.14–91.53) in women aged 65 or older.
“Prevalence information is the first step in determining the risk of spreading cancer with morcellation,” Wright and colleagues observe. “Patients considering morcellation should be adequately counseled about the prevalence of cancerous and precancerous conditions prior to undergoing the procedure.”
Reference
- Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy using morcellation [published online ahead of print July 22, 2014]. JAMA. doi: 10.1001/jama.2014.9005.
- US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy. FDA Safety Communication. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed July 28, 2014.
Among women who undergo minimally invasive hysterectomy with electric power morcellation, the rate of uterine cancer is 27 cases per 10,000 women at the time of the procedure, according to a recent study published in the Journal of the American Medical Association.1 That figure translates into approximately one case of undetected uterine cancer in every 368 women undergoing hysterectomy. Earlier this year the US Food and Drug Administration (FDA) estimated the prevalence of uterine sarcoma at one case in every 352 women.2
Leading up to publication of this study in late July, there had been concern and considerable discussion—including a 2-day hearing convened by the FDA— about whether power morcellation may result in the spread of undetected malignancies and, if so, how often that may occur.
Although power morcellators have been available commercially for two decades, accurate estimates of the prevalence of malignancy at the time of power morcellation have been lacking.
Jason D. Wright, MD, and colleagues from Columbia University used the Perspective database, a large insurance database, to investigate the prevalence of underlying cancer in women who underwent uterine morcellation during minimally invasive hysterectomy from 2006 to 2012. This database is an “all-payer” database that includes more than 500 hospitals in the United States, many of them urban teaching centers.
The cohort included 232,882 women who underwent minimally invasive hysterectomy, including 36,470 (15.7%) who had uterine morcellation during the procedure. Among women who underwent morcellation, 99 cases of uterine cancer were identified, a prevalence of 27 cases per 10,000 women (95% confidence interval [CI], 22–32).
Among women who underwent power morcellation, the prevalence of underlying cancer and endometrial hyperplasia increased with age. For example, compared with women younger than 40 years, the prevalence ratio for uterine malignancy was:
- 4.97 (95% CI, 1.91–12.93) in women aged 50 to 54 years
- 19.37 (95% CI, 7.66–48.95) in those aged 55 to 59 years
- 21.36 (95% CI, 7.22–63.21) in women aged 60 to 64
- 35.97 (95% CI, 14.14–91.53) in women aged 65 or older.
“Prevalence information is the first step in determining the risk of spreading cancer with morcellation,” Wright and colleagues observe. “Patients considering morcellation should be adequately counseled about the prevalence of cancerous and precancerous conditions prior to undergoing the procedure.”
Among women who undergo minimally invasive hysterectomy with electric power morcellation, the rate of uterine cancer is 27 cases per 10,000 women at the time of the procedure, according to a recent study published in the Journal of the American Medical Association.1 That figure translates into approximately one case of undetected uterine cancer in every 368 women undergoing hysterectomy. Earlier this year the US Food and Drug Administration (FDA) estimated the prevalence of uterine sarcoma at one case in every 352 women.2
Leading up to publication of this study in late July, there had been concern and considerable discussion—including a 2-day hearing convened by the FDA— about whether power morcellation may result in the spread of undetected malignancies and, if so, how often that may occur.
Although power morcellators have been available commercially for two decades, accurate estimates of the prevalence of malignancy at the time of power morcellation have been lacking.
Jason D. Wright, MD, and colleagues from Columbia University used the Perspective database, a large insurance database, to investigate the prevalence of underlying cancer in women who underwent uterine morcellation during minimally invasive hysterectomy from 2006 to 2012. This database is an “all-payer” database that includes more than 500 hospitals in the United States, many of them urban teaching centers.
The cohort included 232,882 women who underwent minimally invasive hysterectomy, including 36,470 (15.7%) who had uterine morcellation during the procedure. Among women who underwent morcellation, 99 cases of uterine cancer were identified, a prevalence of 27 cases per 10,000 women (95% confidence interval [CI], 22–32).
Among women who underwent power morcellation, the prevalence of underlying cancer and endometrial hyperplasia increased with age. For example, compared with women younger than 40 years, the prevalence ratio for uterine malignancy was:
- 4.97 (95% CI, 1.91–12.93) in women aged 50 to 54 years
- 19.37 (95% CI, 7.66–48.95) in those aged 55 to 59 years
- 21.36 (95% CI, 7.22–63.21) in women aged 60 to 64
- 35.97 (95% CI, 14.14–91.53) in women aged 65 or older.
“Prevalence information is the first step in determining the risk of spreading cancer with morcellation,” Wright and colleagues observe. “Patients considering morcellation should be adequately counseled about the prevalence of cancerous and precancerous conditions prior to undergoing the procedure.”
Reference
- Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy using morcellation [published online ahead of print July 22, 2014]. JAMA. doi: 10.1001/jama.2014.9005.
- US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy. FDA Safety Communication. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed July 28, 2014.
Reference
- Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy using morcellation [published online ahead of print July 22, 2014]. JAMA. doi: 10.1001/jama.2014.9005.
- US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy. FDA Safety Communication. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed July 28, 2014.