User login
VIDEO: Oral Drugs Replacing Interferon for Hepatitis C
CHICAGO – Two old workhorses in the treatment of hepatitis C are being put out to pasture – interferon and ribavirin.
Newer, less toxic oral regimens can cure more patients with fewer side effects, Dr. Bruce R. Bacon said in this interview at the annual Digestive Disease Week.
Ribavirin is helpful in some patients, but not needed in all. As for interferon: "I used interferon for 30 years," Dr. Bacon said. "I’m glad it’s gone."
Well, almost gone. Some of the oral therapies have yet to be approved, and cost is an issue.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University. He was not involved in the study. He reported financial associations with AbbVie, Gilead Sciences, and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Two old workhorses in the treatment of hepatitis C are being put out to pasture – interferon and ribavirin.
Newer, less toxic oral regimens can cure more patients with fewer side effects, Dr. Bruce R. Bacon said in this interview at the annual Digestive Disease Week.
Ribavirin is helpful in some patients, but not needed in all. As for interferon: "I used interferon for 30 years," Dr. Bacon said. "I’m glad it’s gone."
Well, almost gone. Some of the oral therapies have yet to be approved, and cost is an issue.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University. He was not involved in the study. He reported financial associations with AbbVie, Gilead Sciences, and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Two old workhorses in the treatment of hepatitis C are being put out to pasture – interferon and ribavirin.
Newer, less toxic oral regimens can cure more patients with fewer side effects, Dr. Bruce R. Bacon said in this interview at the annual Digestive Disease Week.
Ribavirin is helpful in some patients, but not needed in all. As for interferon: "I used interferon for 30 years," Dr. Bacon said. "I’m glad it’s gone."
Well, almost gone. Some of the oral therapies have yet to be approved, and cost is an issue.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University. He was not involved in the study. He reported financial associations with AbbVie, Gilead Sciences, and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM DDW 2014
VIDEO: Oral drugs replacing interferon for hepatitis C
CHICAGO – Two old workhorses in the treatment of hepatitis C are being put out to pasture – interferon and ribavirin.
Newer, less toxic oral regimens can cure more patients with fewer side effects, Dr. Bruce R. Bacon said in this interview at the annual Digestive Disease Week.
Ribavirin is helpful in some patients, but not needed in all. As for interferon: "I used interferon for 30 years," Dr. Bacon said. "I’m glad it’s gone."
Well, almost gone. Some of the oral therapies have yet to be approved, and cost is an issue.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University. He was not involved in the study. He reported financial associations with AbbVie, Gilead Sciences, and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
CHICAGO – Two old workhorses in the treatment of hepatitis C are being put out to pasture – interferon and ribavirin.
Newer, less toxic oral regimens can cure more patients with fewer side effects, Dr. Bruce R. Bacon said in this interview at the annual Digestive Disease Week.
Ribavirin is helpful in some patients, but not needed in all. As for interferon: "I used interferon for 30 years," Dr. Bacon said. "I’m glad it’s gone."
Well, almost gone. Some of the oral therapies have yet to be approved, and cost is an issue.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University. He was not involved in the study. He reported financial associations with AbbVie, Gilead Sciences, and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
CHICAGO – Two old workhorses in the treatment of hepatitis C are being put out to pasture – interferon and ribavirin.
Newer, less toxic oral regimens can cure more patients with fewer side effects, Dr. Bruce R. Bacon said in this interview at the annual Digestive Disease Week.
Ribavirin is helpful in some patients, but not needed in all. As for interferon: "I used interferon for 30 years," Dr. Bacon said. "I’m glad it’s gone."
Well, almost gone. Some of the oral therapies have yet to be approved, and cost is an issue.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University. He was not involved in the study. He reported financial associations with AbbVie, Gilead Sciences, and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
EXPERT ANALYSIS FROM DDW 2014
VIDEO: Oral drugs tackle hepatitis C genotypes 2, 3
CHICAGO – The oral drug duo of sofosbuvir and ribavirin produced good cure rates in a study of 419 patients with hepatitis C genotype 2 or 3.
Dr. Bruce R. Bacon, who was not involved in the study, highlighted the regimen’s efficacy even in patients with cirrhosis or who had failed previous therapy. Hear his perspective on treating genotypes 2 and 3 in this interview at the annual Digestive Disease Week.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University.
He reported financial associations with Gilead Sciences, which funded the study, and with AbbVie and Janssen Pharmaceuticals.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The oral drug duo of sofosbuvir and ribavirin produced good cure rates in a study of 419 patients with hepatitis C genotype 2 or 3.
Dr. Bruce R. Bacon, who was not involved in the study, highlighted the regimen’s efficacy even in patients with cirrhosis or who had failed previous therapy. Hear his perspective on treating genotypes 2 and 3 in this interview at the annual Digestive Disease Week.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University.
He reported financial associations with Gilead Sciences, which funded the study, and with AbbVie and Janssen Pharmaceuticals.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The oral drug duo of sofosbuvir and ribavirin produced good cure rates in a study of 419 patients with hepatitis C genotype 2 or 3.
Dr. Bruce R. Bacon, who was not involved in the study, highlighted the regimen’s efficacy even in patients with cirrhosis or who had failed previous therapy. Hear his perspective on treating genotypes 2 and 3 in this interview at the annual Digestive Disease Week.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University.
He reported financial associations with Gilead Sciences, which funded the study, and with AbbVie and Janssen Pharmaceuticals.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT DDW 2014
VIDEO: ‘Three D’ for hepatitis C called ‘revolutionary’
CHICAGO – An experimental triple-drug regimen with or without ribavirin cured more than 90% of 724 previously untreated patients with hepatitis C genotype 1 who did not have cirrhosis.
And it was done without interferon.
Dr. Bruce R. Bacon called this oral regimen "revolutionary" in an interview during the annual Digestive Disease Week. Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at St. Louis University. He was not involved in the study.
Hear his thoughts on treatment with ABT-450 with ritonavir, ombitasvir, and dasabuvir (known as the "three D" regimen) with or without ribavirin.
Dr. Bacon reported financial associations with AbbVie, which is developing the new drugs; Gilead Sciences; and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
CHICAGO – An experimental triple-drug regimen with or without ribavirin cured more than 90% of 724 previously untreated patients with hepatitis C genotype 1 who did not have cirrhosis.
And it was done without interferon.
Dr. Bruce R. Bacon called this oral regimen "revolutionary" in an interview during the annual Digestive Disease Week. Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at St. Louis University. He was not involved in the study.
Hear his thoughts on treatment with ABT-450 with ritonavir, ombitasvir, and dasabuvir (known as the "three D" regimen) with or without ribavirin.
Dr. Bacon reported financial associations with AbbVie, which is developing the new drugs; Gilead Sciences; and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
CHICAGO – An experimental triple-drug regimen with or without ribavirin cured more than 90% of 724 previously untreated patients with hepatitis C genotype 1 who did not have cirrhosis.
And it was done without interferon.
Dr. Bruce R. Bacon called this oral regimen "revolutionary" in an interview during the annual Digestive Disease Week. Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at St. Louis University. He was not involved in the study.
Hear his thoughts on treatment with ABT-450 with ritonavir, ombitasvir, and dasabuvir (known as the "three D" regimen) with or without ribavirin.
Dr. Bacon reported financial associations with AbbVie, which is developing the new drugs; Gilead Sciences; and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
AT DDW 2014
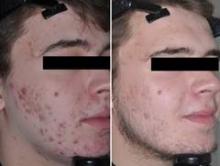
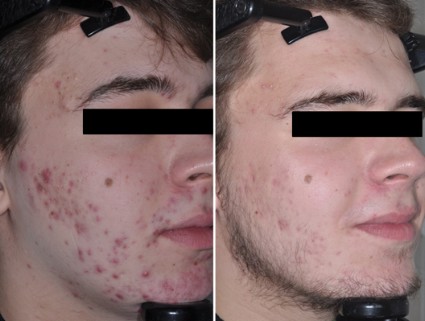
Gold-coated microparticles aid acne treatment
PHOENIX – Application of gold-coated microparticles to the skin may enhance laser photothermolysis of sebaceous glands to treat acne, according to three small, preliminary studies.
Inadequate contrast in sebaceous glands has limited the effectiveness of selective photothermolysis to treat acne. Specially engineered 0.150-mcm microparticles of inert gold coating a silica core were designed for surface plasmon resonance at near-infrared wavelengths to optimize contrast and high absorption of light in the sebaceous glands during laser treatment.
In a randomized, controlled crossover study, 48 patients with acne vulgaris received either immediate or delayed treatment for 12 weeks, after which the delayed-treatment group also began receiving the treatment. Immediate treatment included three treatments at 2-week intervals. The treatment consisted of a microparticle suspension gently massaged into facial skin for about 8 minutes, superficial skin cleansing, and pulsed irradiation with two passes of an 800-nm laser. Patients in the delayed-treatment group used an over-the-counter face wash (2% salicylic acid) twice daily.
The mean number of inflammatory lesions decreased by 34% at 12 weeks after treatment started, compared with a 16% reduction in the control group, a statistically significant difference, Dilip Paithankar, Ph.D., reported at the annual meeting of the American Society for Laser Medicine and Surgery.
At 28 weeks after the crossover to treatment for all subjects (40 weeks after the start of the study), the mean number of inflammatory lesions decreased by 61%, reported Dr. Paithankar, chief technology officer at Sebacia, Duluth, Ga., which is developing the gold-coated microparticles treatment.
In another randomized, sham-controlled study, 49 patients with acne vulgaris underwent three laser photothermolysis treatments at weekly intervals using either the gold-coated microparticles or a control vehicle without the particles. Mean inflammatory lesion counts decreased significantly more in the treatment group than in the control group at 8, 12, and 16 weeks of follow-up. In the treatment group, mean inflammatory lesion counts were 44% lower at 8 weeks, 49% lower at 12 weeks, and 53% lower at 16 weeks, compared with baseline. In the control group, mean inflammatory lesion counts were 14% lower, 22% lower, and 31% lower at those time points, respectively, compared with baseline.
Inflammatory lesions were clear or almost clear by the end of the study in 24% in the treatment group and in none of the control patients.
Data from a separate histologic study suggest that using ultrasound to assist delivery of the gold-coated microparticles may improve selective destruction from laser treatment, Dr. Girish Munavalli reported at the meeting. Ultrasound has been used in other settings to enhance delivery of small molecules through the stratum corneum.
The study used an ultrasound horn vibrating in a microparticle suspension placed in an enclosure above preauricular skin in 37 subjects, followed by 800-nm laser treatment. Biopsies taken within 15 minutes showed localized thermal injury to infundibuli and sebaceous glands with no collateral damage to surrounding tissue or to the epidermis, reported Dr. Munavalli, a dermatologist in group practice in Charlotte, N.C. The extent of thermal damage correlated with the duration of the ultrasound.
The level of damage could be expected to improve acne, but that needs confirmation in clinical trials, he said.
In each of the studies, the treatments were well tolerated, with minimal side effects including mild to moderate pain (using no anesthetic) and short-lived erythema and edema.
Dr. Paithankar works for Sebacia. Dr. Munavalli reported having no disclosures; some of his coinvestigators work for Sebacia.
On Twitter @sherryboschert
PHOENIX – Application of gold-coated microparticles to the skin may enhance laser photothermolysis of sebaceous glands to treat acne, according to three small, preliminary studies.
Inadequate contrast in sebaceous glands has limited the effectiveness of selective photothermolysis to treat acne. Specially engineered 0.150-mcm microparticles of inert gold coating a silica core were designed for surface plasmon resonance at near-infrared wavelengths to optimize contrast and high absorption of light in the sebaceous glands during laser treatment.
In a randomized, controlled crossover study, 48 patients with acne vulgaris received either immediate or delayed treatment for 12 weeks, after which the delayed-treatment group also began receiving the treatment. Immediate treatment included three treatments at 2-week intervals. The treatment consisted of a microparticle suspension gently massaged into facial skin for about 8 minutes, superficial skin cleansing, and pulsed irradiation with two passes of an 800-nm laser. Patients in the delayed-treatment group used an over-the-counter face wash (2% salicylic acid) twice daily.
The mean number of inflammatory lesions decreased by 34% at 12 weeks after treatment started, compared with a 16% reduction in the control group, a statistically significant difference, Dilip Paithankar, Ph.D., reported at the annual meeting of the American Society for Laser Medicine and Surgery.
At 28 weeks after the crossover to treatment for all subjects (40 weeks after the start of the study), the mean number of inflammatory lesions decreased by 61%, reported Dr. Paithankar, chief technology officer at Sebacia, Duluth, Ga., which is developing the gold-coated microparticles treatment.
In another randomized, sham-controlled study, 49 patients with acne vulgaris underwent three laser photothermolysis treatments at weekly intervals using either the gold-coated microparticles or a control vehicle without the particles. Mean inflammatory lesion counts decreased significantly more in the treatment group than in the control group at 8, 12, and 16 weeks of follow-up. In the treatment group, mean inflammatory lesion counts were 44% lower at 8 weeks, 49% lower at 12 weeks, and 53% lower at 16 weeks, compared with baseline. In the control group, mean inflammatory lesion counts were 14% lower, 22% lower, and 31% lower at those time points, respectively, compared with baseline.
Inflammatory lesions were clear or almost clear by the end of the study in 24% in the treatment group and in none of the control patients.
Data from a separate histologic study suggest that using ultrasound to assist delivery of the gold-coated microparticles may improve selective destruction from laser treatment, Dr. Girish Munavalli reported at the meeting. Ultrasound has been used in other settings to enhance delivery of small molecules through the stratum corneum.
The study used an ultrasound horn vibrating in a microparticle suspension placed in an enclosure above preauricular skin in 37 subjects, followed by 800-nm laser treatment. Biopsies taken within 15 minutes showed localized thermal injury to infundibuli and sebaceous glands with no collateral damage to surrounding tissue or to the epidermis, reported Dr. Munavalli, a dermatologist in group practice in Charlotte, N.C. The extent of thermal damage correlated with the duration of the ultrasound.
The level of damage could be expected to improve acne, but that needs confirmation in clinical trials, he said.
In each of the studies, the treatments were well tolerated, with minimal side effects including mild to moderate pain (using no anesthetic) and short-lived erythema and edema.
Dr. Paithankar works for Sebacia. Dr. Munavalli reported having no disclosures; some of his coinvestigators work for Sebacia.
On Twitter @sherryboschert
PHOENIX – Application of gold-coated microparticles to the skin may enhance laser photothermolysis of sebaceous glands to treat acne, according to three small, preliminary studies.
Inadequate contrast in sebaceous glands has limited the effectiveness of selective photothermolysis to treat acne. Specially engineered 0.150-mcm microparticles of inert gold coating a silica core were designed for surface plasmon resonance at near-infrared wavelengths to optimize contrast and high absorption of light in the sebaceous glands during laser treatment.
In a randomized, controlled crossover study, 48 patients with acne vulgaris received either immediate or delayed treatment for 12 weeks, after which the delayed-treatment group also began receiving the treatment. Immediate treatment included three treatments at 2-week intervals. The treatment consisted of a microparticle suspension gently massaged into facial skin for about 8 minutes, superficial skin cleansing, and pulsed irradiation with two passes of an 800-nm laser. Patients in the delayed-treatment group used an over-the-counter face wash (2% salicylic acid) twice daily.
The mean number of inflammatory lesions decreased by 34% at 12 weeks after treatment started, compared with a 16% reduction in the control group, a statistically significant difference, Dilip Paithankar, Ph.D., reported at the annual meeting of the American Society for Laser Medicine and Surgery.
At 28 weeks after the crossover to treatment for all subjects (40 weeks after the start of the study), the mean number of inflammatory lesions decreased by 61%, reported Dr. Paithankar, chief technology officer at Sebacia, Duluth, Ga., which is developing the gold-coated microparticles treatment.
In another randomized, sham-controlled study, 49 patients with acne vulgaris underwent three laser photothermolysis treatments at weekly intervals using either the gold-coated microparticles or a control vehicle without the particles. Mean inflammatory lesion counts decreased significantly more in the treatment group than in the control group at 8, 12, and 16 weeks of follow-up. In the treatment group, mean inflammatory lesion counts were 44% lower at 8 weeks, 49% lower at 12 weeks, and 53% lower at 16 weeks, compared with baseline. In the control group, mean inflammatory lesion counts were 14% lower, 22% lower, and 31% lower at those time points, respectively, compared with baseline.
Inflammatory lesions were clear or almost clear by the end of the study in 24% in the treatment group and in none of the control patients.
Data from a separate histologic study suggest that using ultrasound to assist delivery of the gold-coated microparticles may improve selective destruction from laser treatment, Dr. Girish Munavalli reported at the meeting. Ultrasound has been used in other settings to enhance delivery of small molecules through the stratum corneum.
The study used an ultrasound horn vibrating in a microparticle suspension placed in an enclosure above preauricular skin in 37 subjects, followed by 800-nm laser treatment. Biopsies taken within 15 minutes showed localized thermal injury to infundibuli and sebaceous glands with no collateral damage to surrounding tissue or to the epidermis, reported Dr. Munavalli, a dermatologist in group practice in Charlotte, N.C. The extent of thermal damage correlated with the duration of the ultrasound.
The level of damage could be expected to improve acne, but that needs confirmation in clinical trials, he said.
In each of the studies, the treatments were well tolerated, with minimal side effects including mild to moderate pain (using no anesthetic) and short-lived erythema and edema.
Dr. Paithankar works for Sebacia. Dr. Munavalli reported having no disclosures; some of his coinvestigators work for Sebacia.
On Twitter @sherryboschert
AT LASER 2014
Key clinical point: Inadequate contrast has limited the use of laser targeting of the sebaceous glands; specially designed gold-coated microparticles are designed to improve contrast and promote light absorption.
Major finding: Laser treatment of acne vulgaris preceded by application of gold-coated microparticles decreased mean inflammatory lesion counts by 34% and 49% at 12 weeks in two separate studies, compared with reductions of 16% and 22% in control groups.
Data source: Two randomized, controlled trials of 48 patients and 49 patients, respectively, with acne vulgaris, and a separate histologic study of 37 subjects.
Disclosures: Dr. Paithankar works for Sebacia, which developed the gold-coated microparticles treatment. Dr. Munavalli reported having no disclosures; some of his coinvestigators work for Sebacia.
VIDEO: New directions in bariatric surgery quality measures
CHICAGO – Bariatric surgery has made great strides in safety and efficacy – so many, in fact, that the field is ready to focus on a new range of quality goals, explained Dr. John Morton.
In an interview at the annual Digestive Disease Week, Dr. Morton, chief of bariatric surgery at Stanford (Calif.) University, discussed how new quality targets, such as 30-day readmissions, could improve bariatric surgery outcomes and boost patient satisfaction.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Bariatric surgery has made great strides in safety and efficacy – so many, in fact, that the field is ready to focus on a new range of quality goals, explained Dr. John Morton.
In an interview at the annual Digestive Disease Week, Dr. Morton, chief of bariatric surgery at Stanford (Calif.) University, discussed how new quality targets, such as 30-day readmissions, could improve bariatric surgery outcomes and boost patient satisfaction.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Bariatric surgery has made great strides in safety and efficacy – so many, in fact, that the field is ready to focus on a new range of quality goals, explained Dr. John Morton.
In an interview at the annual Digestive Disease Week, Dr. Morton, chief of bariatric surgery at Stanford (Calif.) University, discussed how new quality targets, such as 30-day readmissions, could improve bariatric surgery outcomes and boost patient satisfaction.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At DDW 2014
Interferon-free Regimen Improves Response in HCV
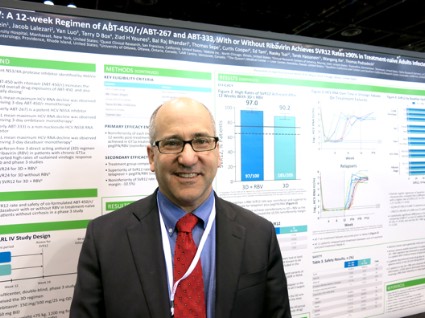
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
AT DDW 2014
Interferon-free regimen improves response in HCV
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
AT DDW 2014
Major finding: For patients with genotype 1a infection, SVR occurred in 97% with ribavirin and 90.2% without ribavirin. For patients with genotype 1b infection, rates were 99.5% and 99%, respectively.
Data source: Two randomized, phase III trials of previously untreated patients with hepatitis C and no cirrhosis who were treated with 12 weeks of ABT-450/r, ombitasvir, and dasabuvir with or without ribavirin.
Disclosures: AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
Sofosbuvir-ribavirin treats hepatitis C virus genotypes 2, 3
CHICAGO – Oral sofosbuvir and ribavirin given for 12 weeks produced a sustained virologic response in 93% of 250 patients with hepatitis C virus genotype 2 and in 85% of patients with genotype 3 infection who were treated for 24 weeks.
The 77-center European study defined a sustained virologic response as a hepatitis C virus RNA level of less than 25 IU/mL, Dr. Stefan Zeuzem and his associates reported. More than half of the patients (58%) had undergone prior therapy with interferon-based regimens, with no response in 30%. Cirrhosis was present in 21% of patients.
The study began as a phase III trial randomizing patients to receive 12 weeks of once-daily oral sofosbuvir 400 mg and twice-daily oral ribavirin (administered according to body weight), or matching placebo. The investigators turned the trial into a descriptive study after a previous study reported that patients with genotype 3 infection might benefit from more than 12 weeks of sofosbuvir therapy (N. Engl. J. Med. 2013;368:1867-77).
Dr. Zeuzem and his associates unblinded the treatment arms, dropped placebo treatment, and extended treatment for patients with genotype 3 infection to 24 weeks. The findings were published online in the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi: 1056/NEJMoa1316145]).
Among patients with genotype 3 infection, a sustained virologic response developed in 68% of those with cirrhosis and 91% without cirrhosis, reported Dr. Zeuzem, who is professor of medicine and chief of the department of medicine I at Johann Wolfgang Goethe University Medical Center, Frankfurt, Germany.
Headache, fatigue, and pruritus were the most common adverse events. Approximately 1% of patients in both the genotype 3 and 4 groups discontinued treatment due to side effects.
Treatment history and the presence of cirrhosis affected response rates in patients with genotype 3 infection. In previously untreated patients, a sustained virologic response was seen in 92% of those with cirrhosis and in 95% without cirrhosis. Response rates were lower in previously treated patients with cirrhosis (62%) and without cirrhosis (87%). The reasons for these differences are unknown.
Four factors were independently associated with a sustained virologic response in patients with genotype 3 infection, a multivariate regression analysis found. A sustained response was four times as likely in those with a baseline hepatitis C virus RNA level of less than 6 log10 IU and three times as likely in women, patients without cirrhosis, or those under age 50, compared with patients without those characteristics. These predictive factors must be validated in further studies, Dr. Zeuzem said.
Three patients with genotype 3 infection had a virologic relapse. The response rate and relapse rate for patients with genotype 3 who were treated for 24 weeks were "substantially" better than rates reported in previous studies using the same regimen for 12 or 16 weeks, the investigators said. Extending the duration of treatment did not significantly increase adverse events or discontinuation of therapy.
The findings suggest that sofosbuvir-ribavirin can be an alternative therapy for patients with hepatitis C virus genotype 2 or 3 who have contraindications to peginterferon-based treatment, Dr. Zeuzem said.
Gilead Sciences, which markets sofosbuvir, funded the study. Dr. Zeuzem reported financial associations with Gilead and multiple other pharmaceutical companies, as did many of his coinvestigators.
On Twitter @sherryboschert
CHICAGO – Oral sofosbuvir and ribavirin given for 12 weeks produced a sustained virologic response in 93% of 250 patients with hepatitis C virus genotype 2 and in 85% of patients with genotype 3 infection who were treated for 24 weeks.
The 77-center European study defined a sustained virologic response as a hepatitis C virus RNA level of less than 25 IU/mL, Dr. Stefan Zeuzem and his associates reported. More than half of the patients (58%) had undergone prior therapy with interferon-based regimens, with no response in 30%. Cirrhosis was present in 21% of patients.
The study began as a phase III trial randomizing patients to receive 12 weeks of once-daily oral sofosbuvir 400 mg and twice-daily oral ribavirin (administered according to body weight), or matching placebo. The investigators turned the trial into a descriptive study after a previous study reported that patients with genotype 3 infection might benefit from more than 12 weeks of sofosbuvir therapy (N. Engl. J. Med. 2013;368:1867-77).
Dr. Zeuzem and his associates unblinded the treatment arms, dropped placebo treatment, and extended treatment for patients with genotype 3 infection to 24 weeks. The findings were published online in the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi: 1056/NEJMoa1316145]).
Among patients with genotype 3 infection, a sustained virologic response developed in 68% of those with cirrhosis and 91% without cirrhosis, reported Dr. Zeuzem, who is professor of medicine and chief of the department of medicine I at Johann Wolfgang Goethe University Medical Center, Frankfurt, Germany.
Headache, fatigue, and pruritus were the most common adverse events. Approximately 1% of patients in both the genotype 3 and 4 groups discontinued treatment due to side effects.
Treatment history and the presence of cirrhosis affected response rates in patients with genotype 3 infection. In previously untreated patients, a sustained virologic response was seen in 92% of those with cirrhosis and in 95% without cirrhosis. Response rates were lower in previously treated patients with cirrhosis (62%) and without cirrhosis (87%). The reasons for these differences are unknown.
Four factors were independently associated with a sustained virologic response in patients with genotype 3 infection, a multivariate regression analysis found. A sustained response was four times as likely in those with a baseline hepatitis C virus RNA level of less than 6 log10 IU and three times as likely in women, patients without cirrhosis, or those under age 50, compared with patients without those characteristics. These predictive factors must be validated in further studies, Dr. Zeuzem said.
Three patients with genotype 3 infection had a virologic relapse. The response rate and relapse rate for patients with genotype 3 who were treated for 24 weeks were "substantially" better than rates reported in previous studies using the same regimen for 12 or 16 weeks, the investigators said. Extending the duration of treatment did not significantly increase adverse events or discontinuation of therapy.
The findings suggest that sofosbuvir-ribavirin can be an alternative therapy for patients with hepatitis C virus genotype 2 or 3 who have contraindications to peginterferon-based treatment, Dr. Zeuzem said.
Gilead Sciences, which markets sofosbuvir, funded the study. Dr. Zeuzem reported financial associations with Gilead and multiple other pharmaceutical companies, as did many of his coinvestigators.
On Twitter @sherryboschert
CHICAGO – Oral sofosbuvir and ribavirin given for 12 weeks produced a sustained virologic response in 93% of 250 patients with hepatitis C virus genotype 2 and in 85% of patients with genotype 3 infection who were treated for 24 weeks.
The 77-center European study defined a sustained virologic response as a hepatitis C virus RNA level of less than 25 IU/mL, Dr. Stefan Zeuzem and his associates reported. More than half of the patients (58%) had undergone prior therapy with interferon-based regimens, with no response in 30%. Cirrhosis was present in 21% of patients.
The study began as a phase III trial randomizing patients to receive 12 weeks of once-daily oral sofosbuvir 400 mg and twice-daily oral ribavirin (administered according to body weight), or matching placebo. The investigators turned the trial into a descriptive study after a previous study reported that patients with genotype 3 infection might benefit from more than 12 weeks of sofosbuvir therapy (N. Engl. J. Med. 2013;368:1867-77).
Dr. Zeuzem and his associates unblinded the treatment arms, dropped placebo treatment, and extended treatment for patients with genotype 3 infection to 24 weeks. The findings were published online in the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi: 1056/NEJMoa1316145]).
Among patients with genotype 3 infection, a sustained virologic response developed in 68% of those with cirrhosis and 91% without cirrhosis, reported Dr. Zeuzem, who is professor of medicine and chief of the department of medicine I at Johann Wolfgang Goethe University Medical Center, Frankfurt, Germany.
Headache, fatigue, and pruritus were the most common adverse events. Approximately 1% of patients in both the genotype 3 and 4 groups discontinued treatment due to side effects.
Treatment history and the presence of cirrhosis affected response rates in patients with genotype 3 infection. In previously untreated patients, a sustained virologic response was seen in 92% of those with cirrhosis and in 95% without cirrhosis. Response rates were lower in previously treated patients with cirrhosis (62%) and without cirrhosis (87%). The reasons for these differences are unknown.
Four factors were independently associated with a sustained virologic response in patients with genotype 3 infection, a multivariate regression analysis found. A sustained response was four times as likely in those with a baseline hepatitis C virus RNA level of less than 6 log10 IU and three times as likely in women, patients without cirrhosis, or those under age 50, compared with patients without those characteristics. These predictive factors must be validated in further studies, Dr. Zeuzem said.
Three patients with genotype 3 infection had a virologic relapse. The response rate and relapse rate for patients with genotype 3 who were treated for 24 weeks were "substantially" better than rates reported in previous studies using the same regimen for 12 or 16 weeks, the investigators said. Extending the duration of treatment did not significantly increase adverse events or discontinuation of therapy.
The findings suggest that sofosbuvir-ribavirin can be an alternative therapy for patients with hepatitis C virus genotype 2 or 3 who have contraindications to peginterferon-based treatment, Dr. Zeuzem said.
Gilead Sciences, which markets sofosbuvir, funded the study. Dr. Zeuzem reported financial associations with Gilead and multiple other pharmaceutical companies, as did many of his coinvestigators.
On Twitter @sherryboschert
AT DDW 2014
Major finding: Sustained virologic response occurred in 93% after 12 weeks of treatment for genotype 2 and in 85% after 24 weeks of therapy for genotype 3.
Data source: A descriptive cohort study of 419 patients with hepatitis C genotypes 2 or 3 treated with sofosbuvir and ribavirin.
Disclosures: Gilead Sciences, which markets sofosbuvir, funded the study. Dr. Zeuzem reported financial associations with Gilead and multiple other pharmaceutical companies, as did many of his coinvestigators.
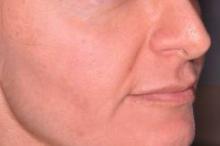
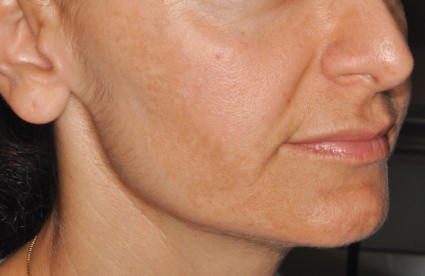
Laser settings tested against melasma
PHOENIX – Treating moderate to severe melasma with a 50-ns, 1,064-nm Q-switched neodynium:YAG laser produced equivalent temporary results with less pain than did a 5-ns, 1,064-nm Q-switched Nd:YAG laser in a split-face study of 10 women.
In the 6-month study, scores on a melasma area and severity index decreased by 16% using the 50-ns pulse width, and by 27% using the 5-ns pulse width at month 4, which was 1 month after the last of three monthly treatments that combined microdermabrasion, laser therapy, and a topical therapy regimen. Changes from both lasers were statistically significant compared with baseline, but not significantly different between laser groups, Dr. Vineet Mishra reported at the annual meeting of the American Society for Laser Medicine and Surgery.
The investigators sought to optimize laser settings for treating melasma to see whether lasers could be used in combination with other melasma therapies, Dr. Mishra said in an interview.
"During the study, everybody improved," Dr. Mishra said.
Melanin (as measured by a reflectance spectrophotometer at each visit) decreased by 20% with the 50-ns pulse width and by 17% with the 5-ns pulse width during the study.
However, pigment relapsed in both treatment areas after the study, to a level not significantly different from that at baseline. This was not unexpected when treating melasma, and was "likely due to sun exposure in sunny San Diego," said Dr. Mishra, director of Mohs surgery and procedural dermatology at the University of Texas Health Science Center, San Antonio.
Patients who completed the study rated pain from the procedure as a mean of 1.2 on the side of the face treated with the 50-ns laser and 2.9 with the 5-ns laser, using a 10-point scale with 0 representing no pain and 10 the worst pain, Dr. Mishra said.
Each treatment session consisted of microdermabrasion of the entire face followed by treatment with the 50-ns laser on one side and treatment with the 5-ns laser on the other side. Two laser passes were applied using a fluence of 1.6 J/cm2 and a 5-mm spot size. For 2 weeks prior to each treatment session and starting 2 days after each laser treatment, patients were asked to follow a daily skin care regimen that consisted of topical 4% hydroquinone, 0.05% tretinoin cream, and sunscreen with a sun protection factor of 50.
"Lasers have exploded on the market" but can be tricky to use against melasma, Dr. Mishra said. Too-high levels of laser energy can cause postinflammatory hyperpigmentation, which can worsen melasma, he noted.
Melasma is a difficult condition to treat, but "combination therapy is optimal," Dr. Mishra added in an interview. Next steps for research should include longer-term studies to determine how patients would fare if they continued with the combination treatment regimen. In addition, using a picosecond laser for melasma "would be a new frontier" to explore in long-term studies, he said.
The study won an award at the meeting.
The study received funding from Syneron and Candela, which market the 50-ns laser, and one of Dr. Mishra’s coinvestigators reported financial associations with Syneron and Candela and with Cynosure, which markets the 5-ns laser.
On Twitter @sherryboschert
PHOENIX – Treating moderate to severe melasma with a 50-ns, 1,064-nm Q-switched neodynium:YAG laser produced equivalent temporary results with less pain than did a 5-ns, 1,064-nm Q-switched Nd:YAG laser in a split-face study of 10 women.
In the 6-month study, scores on a melasma area and severity index decreased by 16% using the 50-ns pulse width, and by 27% using the 5-ns pulse width at month 4, which was 1 month after the last of three monthly treatments that combined microdermabrasion, laser therapy, and a topical therapy regimen. Changes from both lasers were statistically significant compared with baseline, but not significantly different between laser groups, Dr. Vineet Mishra reported at the annual meeting of the American Society for Laser Medicine and Surgery.
The investigators sought to optimize laser settings for treating melasma to see whether lasers could be used in combination with other melasma therapies, Dr. Mishra said in an interview.
"During the study, everybody improved," Dr. Mishra said.
Melanin (as measured by a reflectance spectrophotometer at each visit) decreased by 20% with the 50-ns pulse width and by 17% with the 5-ns pulse width during the study.
However, pigment relapsed in both treatment areas after the study, to a level not significantly different from that at baseline. This was not unexpected when treating melasma, and was "likely due to sun exposure in sunny San Diego," said Dr. Mishra, director of Mohs surgery and procedural dermatology at the University of Texas Health Science Center, San Antonio.
Patients who completed the study rated pain from the procedure as a mean of 1.2 on the side of the face treated with the 50-ns laser and 2.9 with the 5-ns laser, using a 10-point scale with 0 representing no pain and 10 the worst pain, Dr. Mishra said.
Each treatment session consisted of microdermabrasion of the entire face followed by treatment with the 50-ns laser on one side and treatment with the 5-ns laser on the other side. Two laser passes were applied using a fluence of 1.6 J/cm2 and a 5-mm spot size. For 2 weeks prior to each treatment session and starting 2 days after each laser treatment, patients were asked to follow a daily skin care regimen that consisted of topical 4% hydroquinone, 0.05% tretinoin cream, and sunscreen with a sun protection factor of 50.
"Lasers have exploded on the market" but can be tricky to use against melasma, Dr. Mishra said. Too-high levels of laser energy can cause postinflammatory hyperpigmentation, which can worsen melasma, he noted.
Melasma is a difficult condition to treat, but "combination therapy is optimal," Dr. Mishra added in an interview. Next steps for research should include longer-term studies to determine how patients would fare if they continued with the combination treatment regimen. In addition, using a picosecond laser for melasma "would be a new frontier" to explore in long-term studies, he said.
The study won an award at the meeting.
The study received funding from Syneron and Candela, which market the 50-ns laser, and one of Dr. Mishra’s coinvestigators reported financial associations with Syneron and Candela and with Cynosure, which markets the 5-ns laser.
On Twitter @sherryboschert
PHOENIX – Treating moderate to severe melasma with a 50-ns, 1,064-nm Q-switched neodynium:YAG laser produced equivalent temporary results with less pain than did a 5-ns, 1,064-nm Q-switched Nd:YAG laser in a split-face study of 10 women.
In the 6-month study, scores on a melasma area and severity index decreased by 16% using the 50-ns pulse width, and by 27% using the 5-ns pulse width at month 4, which was 1 month after the last of three monthly treatments that combined microdermabrasion, laser therapy, and a topical therapy regimen. Changes from both lasers were statistically significant compared with baseline, but not significantly different between laser groups, Dr. Vineet Mishra reported at the annual meeting of the American Society for Laser Medicine and Surgery.
The investigators sought to optimize laser settings for treating melasma to see whether lasers could be used in combination with other melasma therapies, Dr. Mishra said in an interview.
"During the study, everybody improved," Dr. Mishra said.
Melanin (as measured by a reflectance spectrophotometer at each visit) decreased by 20% with the 50-ns pulse width and by 17% with the 5-ns pulse width during the study.
However, pigment relapsed in both treatment areas after the study, to a level not significantly different from that at baseline. This was not unexpected when treating melasma, and was "likely due to sun exposure in sunny San Diego," said Dr. Mishra, director of Mohs surgery and procedural dermatology at the University of Texas Health Science Center, San Antonio.
Patients who completed the study rated pain from the procedure as a mean of 1.2 on the side of the face treated with the 50-ns laser and 2.9 with the 5-ns laser, using a 10-point scale with 0 representing no pain and 10 the worst pain, Dr. Mishra said.
Each treatment session consisted of microdermabrasion of the entire face followed by treatment with the 50-ns laser on one side and treatment with the 5-ns laser on the other side. Two laser passes were applied using a fluence of 1.6 J/cm2 and a 5-mm spot size. For 2 weeks prior to each treatment session and starting 2 days after each laser treatment, patients were asked to follow a daily skin care regimen that consisted of topical 4% hydroquinone, 0.05% tretinoin cream, and sunscreen with a sun protection factor of 50.
"Lasers have exploded on the market" but can be tricky to use against melasma, Dr. Mishra said. Too-high levels of laser energy can cause postinflammatory hyperpigmentation, which can worsen melasma, he noted.
Melasma is a difficult condition to treat, but "combination therapy is optimal," Dr. Mishra added in an interview. Next steps for research should include longer-term studies to determine how patients would fare if they continued with the combination treatment regimen. In addition, using a picosecond laser for melasma "would be a new frontier" to explore in long-term studies, he said.
The study won an award at the meeting.
The study received funding from Syneron and Candela, which market the 50-ns laser, and one of Dr. Mishra’s coinvestigators reported financial associations with Syneron and Candela and with Cynosure, which markets the 5-ns laser.
On Twitter @sherryboschert
AT LASER 2014
Key clinical point: Finding the optimal settings for lasers in patients with melasma could help improve the condition as part of a combination treatment regimen.
Major finding: Both the 50-ns and 5-ns pulse widths significantly decreased melasma area and severity index scores temporarily, but mean pain scores were significantly lower with the 50-ns laser (1.2) than with the 5-ns laser (2.9).
Data source: A prospective, randomized split-face comparison of 50-ns and 5-ns 1,064-nm Q-switched Nd:YAG laser therapy, plus dermabrasion and topical therapy for melasma.
Disclosures: The study received funding from Syneron and Candela, which market the 50-ns laser, and one of Dr. Mishra’s coinvestigators reported financial associations with Syneron and Candela and with Cynosure, which markets the 5-ns laser.