User login
Mobile health validation efforts in infancy
Hardly a day goes by anymore without an announcement of a new mobile health app, but there are precious few data to show which apps are useful in clinical or financial terms. Lots of people would like to change that, but how?
Three experts offered ideas in a recent opinion article in the Journal of the American Medical Association, and some partnerships between academia and industry may be laying the groundwork for greater validation of new mobile health (mHealth) tools.
Of the more than 40,000 health, fitness, and medical apps on the market, reviews "have largely focused on personal impressions, rather than evidence-based, unbiased assessments of clinical performance and data security," Adam C. Powell, Ph.D., Dr. Adam B. Landman, and Dr. David W. Bates wrote in their article, "In Search of a Few Good Apps" (JAMA 2014;311:1851-2).
The Office of the National Coordinator for Health Information Technology (ONC) could play a greater role supporting development of mHealth app guidelines and, eventually, commission non-profit or for-profit entities to certify apps, as it does now for electronic health records, they suggested.
Dr. Powell is a Boston-based consultant. Dr. Landman, an emergency medicine specialist, is chief medical information officer for health information innovation and integration at Brigham and Women’s Hospital, Boston. Dr. Bates is chief of general internal medicine and the chief quality officer at Brigham and Women’s. Dr. Landman and Dr. Bates are both at Harvard Medical School in Boston.
The National Institutes of Health mHealth Training Institute is educating an interdisciplinary group of researchers about the potential of mHealth and the need to evaluate these new tools. "If this effort is coupled with increased funding for mHealth research, it may help galvanize a larger body of evidence to inform mHealth app development and certification," the authors wrote.
Meanwhile, a new partnership between the University of California, San Francisco (UCSF), and Samsung Electronics aims to validate and commercialize promising digital tools for health care, including apps, in a Digital Health Innovation Lab. A few other universities recently entered into similar partnerships with industry.
Both UCSF and Samsung are making a "significant investment" to fund the lab but are not ready to release financial details, Dr. Michael Blum said in an interview.
The partnership initially will focus on preventive health, said Dr. Blum, who will direct the lab to be located at UCSF’s Mission Bay campus. "There is no better way to treat disease than by avoiding it in the first place. [Moe than] 70% of our health care dollars are spent on avoidable disease," said Dr. Blum, a cardiologist who has been leading UCSF’s relatively new Center for Digital Health Innovation.
UCSF already has begun testing medical apps in clinical trials, such as a smoking cessation app, and will pursue clinical testing of tools including health sensors, wearable computing, and cloud-based analytics.
"There are many sites designing medical apps but very few are rigorously validated," Dr. Blum said. "We believe that validation is critical to the success of these apps and products. It is important for health care providers to know that they can trust the data and information which will lead to more consistent use and, hopefully, better outcomes for the users."
In Michigan, the William Davidson Foundation in January 2014 awarded $3 million to the Henry Ford Health System to create the William Davidson Center for Entrepreneurs in Digital Health. The center hopes to attract corporate partners and others to create, clinically validate, and commercialize digital health tools and to create a curriculum integrating health care, digital technologies, and entrepreneurship, according to a statement released by the Henry Ford Innovation Institute.
At the University of Colorado, Denver, the Center for Information Technology Innovation recently launched a Digital Health Consortium to bring its business school faculty together with entrepreneurs, health care providers, researchers, educators, and others to develop and clinically validate the next generation of digital health tools. The Center is funded by its members, which include the university and more than 30 Colorado information technology business leaders.
As each of these initiatives and others like them report results from their validation efforts, we’ll bring you the latest news on medical apps. Watch this space.
On Twitter @sherryboschert
Hardly a day goes by anymore without an announcement of a new mobile health app, but there are precious few data to show which apps are useful in clinical or financial terms. Lots of people would like to change that, but how?
Three experts offered ideas in a recent opinion article in the Journal of the American Medical Association, and some partnerships between academia and industry may be laying the groundwork for greater validation of new mobile health (mHealth) tools.
Of the more than 40,000 health, fitness, and medical apps on the market, reviews "have largely focused on personal impressions, rather than evidence-based, unbiased assessments of clinical performance and data security," Adam C. Powell, Ph.D., Dr. Adam B. Landman, and Dr. David W. Bates wrote in their article, "In Search of a Few Good Apps" (JAMA 2014;311:1851-2).
The Office of the National Coordinator for Health Information Technology (ONC) could play a greater role supporting development of mHealth app guidelines and, eventually, commission non-profit or for-profit entities to certify apps, as it does now for electronic health records, they suggested.
Dr. Powell is a Boston-based consultant. Dr. Landman, an emergency medicine specialist, is chief medical information officer for health information innovation and integration at Brigham and Women’s Hospital, Boston. Dr. Bates is chief of general internal medicine and the chief quality officer at Brigham and Women’s. Dr. Landman and Dr. Bates are both at Harvard Medical School in Boston.
The National Institutes of Health mHealth Training Institute is educating an interdisciplinary group of researchers about the potential of mHealth and the need to evaluate these new tools. "If this effort is coupled with increased funding for mHealth research, it may help galvanize a larger body of evidence to inform mHealth app development and certification," the authors wrote.
Meanwhile, a new partnership between the University of California, San Francisco (UCSF), and Samsung Electronics aims to validate and commercialize promising digital tools for health care, including apps, in a Digital Health Innovation Lab. A few other universities recently entered into similar partnerships with industry.
Both UCSF and Samsung are making a "significant investment" to fund the lab but are not ready to release financial details, Dr. Michael Blum said in an interview.
The partnership initially will focus on preventive health, said Dr. Blum, who will direct the lab to be located at UCSF’s Mission Bay campus. "There is no better way to treat disease than by avoiding it in the first place. [Moe than] 70% of our health care dollars are spent on avoidable disease," said Dr. Blum, a cardiologist who has been leading UCSF’s relatively new Center for Digital Health Innovation.
UCSF already has begun testing medical apps in clinical trials, such as a smoking cessation app, and will pursue clinical testing of tools including health sensors, wearable computing, and cloud-based analytics.
"There are many sites designing medical apps but very few are rigorously validated," Dr. Blum said. "We believe that validation is critical to the success of these apps and products. It is important for health care providers to know that they can trust the data and information which will lead to more consistent use and, hopefully, better outcomes for the users."
In Michigan, the William Davidson Foundation in January 2014 awarded $3 million to the Henry Ford Health System to create the William Davidson Center for Entrepreneurs in Digital Health. The center hopes to attract corporate partners and others to create, clinically validate, and commercialize digital health tools and to create a curriculum integrating health care, digital technologies, and entrepreneurship, according to a statement released by the Henry Ford Innovation Institute.
At the University of Colorado, Denver, the Center for Information Technology Innovation recently launched a Digital Health Consortium to bring its business school faculty together with entrepreneurs, health care providers, researchers, educators, and others to develop and clinically validate the next generation of digital health tools. The Center is funded by its members, which include the university and more than 30 Colorado information technology business leaders.
As each of these initiatives and others like them report results from their validation efforts, we’ll bring you the latest news on medical apps. Watch this space.
On Twitter @sherryboschert
Hardly a day goes by anymore without an announcement of a new mobile health app, but there are precious few data to show which apps are useful in clinical or financial terms. Lots of people would like to change that, but how?
Three experts offered ideas in a recent opinion article in the Journal of the American Medical Association, and some partnerships between academia and industry may be laying the groundwork for greater validation of new mobile health (mHealth) tools.
Of the more than 40,000 health, fitness, and medical apps on the market, reviews "have largely focused on personal impressions, rather than evidence-based, unbiased assessments of clinical performance and data security," Adam C. Powell, Ph.D., Dr. Adam B. Landman, and Dr. David W. Bates wrote in their article, "In Search of a Few Good Apps" (JAMA 2014;311:1851-2).
The Office of the National Coordinator for Health Information Technology (ONC) could play a greater role supporting development of mHealth app guidelines and, eventually, commission non-profit or for-profit entities to certify apps, as it does now for electronic health records, they suggested.
Dr. Powell is a Boston-based consultant. Dr. Landman, an emergency medicine specialist, is chief medical information officer for health information innovation and integration at Brigham and Women’s Hospital, Boston. Dr. Bates is chief of general internal medicine and the chief quality officer at Brigham and Women’s. Dr. Landman and Dr. Bates are both at Harvard Medical School in Boston.
The National Institutes of Health mHealth Training Institute is educating an interdisciplinary group of researchers about the potential of mHealth and the need to evaluate these new tools. "If this effort is coupled with increased funding for mHealth research, it may help galvanize a larger body of evidence to inform mHealth app development and certification," the authors wrote.
Meanwhile, a new partnership between the University of California, San Francisco (UCSF), and Samsung Electronics aims to validate and commercialize promising digital tools for health care, including apps, in a Digital Health Innovation Lab. A few other universities recently entered into similar partnerships with industry.
Both UCSF and Samsung are making a "significant investment" to fund the lab but are not ready to release financial details, Dr. Michael Blum said in an interview.
The partnership initially will focus on preventive health, said Dr. Blum, who will direct the lab to be located at UCSF’s Mission Bay campus. "There is no better way to treat disease than by avoiding it in the first place. [Moe than] 70% of our health care dollars are spent on avoidable disease," said Dr. Blum, a cardiologist who has been leading UCSF’s relatively new Center for Digital Health Innovation.
UCSF already has begun testing medical apps in clinical trials, such as a smoking cessation app, and will pursue clinical testing of tools including health sensors, wearable computing, and cloud-based analytics.
"There are many sites designing medical apps but very few are rigorously validated," Dr. Blum said. "We believe that validation is critical to the success of these apps and products. It is important for health care providers to know that they can trust the data and information which will lead to more consistent use and, hopefully, better outcomes for the users."
In Michigan, the William Davidson Foundation in January 2014 awarded $3 million to the Henry Ford Health System to create the William Davidson Center for Entrepreneurs in Digital Health. The center hopes to attract corporate partners and others to create, clinically validate, and commercialize digital health tools and to create a curriculum integrating health care, digital technologies, and entrepreneurship, according to a statement released by the Henry Ford Innovation Institute.
At the University of Colorado, Denver, the Center for Information Technology Innovation recently launched a Digital Health Consortium to bring its business school faculty together with entrepreneurs, health care providers, researchers, educators, and others to develop and clinically validate the next generation of digital health tools. The Center is funded by its members, which include the university and more than 30 Colorado information technology business leaders.
As each of these initiatives and others like them report results from their validation efforts, we’ll bring you the latest news on medical apps. Watch this space.
On Twitter @sherryboschert
Brazilian patients favor noninvasive body contouring technique
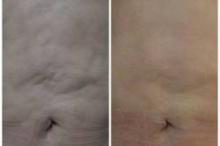
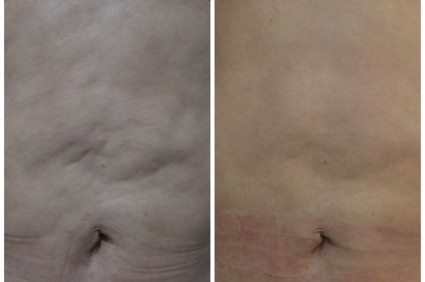
PHOENIX – Patient satisfaction was 100% after treatment with a device combining multipolar radiofrequency and magnetic pulse fields for body contouring, based on data from a series of 260 adults in Brazil.
A total of 60% of patients reported "excellent" satisfaction and 40% reported "good" satisfaction when surveyed shortly after the treatment, Dr. Rafael Nunes said at the annual meeting of the American Society for Laser Medicine and Surgery.
Patients underwent up to six weekly treatments for laxity, wrinkles, or lipodystrophy on the abdomen, flanks, and/or leg areas using the Venus Freeze (MP)2system. The device heats collagen fibers in the skin to produce skin tightening. "The goal is not to reduce volume, but to reshape," said Dr. Nunes, a plastic surgeon in Rio de Janeiro, Brazil.
It’s important to recognize that "if the patient stops the treatments or changes diet habits, probably the results would be totally lost," he said. "Body contouring is challenging."
However, patients who don’t want more invasive body shaping procedures, such as implants, hyaluronic acid, or liposuction, may be pleased with the simplicity of radiofrequency and magnetic pulse, he noted. "The question in our country is, what do patients want? They don’t want to go to the hospital, they don’t like invasive procedures or recovery periods."
The patients in this study had a mean age of 43 years and a mean weight of 66 kg. Ninety percent were female.
After pretreatment with glycerine solution, the 15-minute treatments aimed for a temperature of 41-42° C and uniform erythema on the surface of a 20-cm x 20-cm area.
Side effects immediately after treatment included bruising in 2% of patients, local heat in 78%, edema in 54%, and erythema in 56%. There were no burns. No side effects were seen 5 days later, or at a 3-month follow-up visit.
"In this small study, it [the combination regimen] was a safe and effective treatment for body contouring with no pain, no down time, and high subject satisfaction," Dr. Nunes said
Dr. Nunes reported having no disclosures.
On Twitter @sherryboschert
PHOENIX – Patient satisfaction was 100% after treatment with a device combining multipolar radiofrequency and magnetic pulse fields for body contouring, based on data from a series of 260 adults in Brazil.
A total of 60% of patients reported "excellent" satisfaction and 40% reported "good" satisfaction when surveyed shortly after the treatment, Dr. Rafael Nunes said at the annual meeting of the American Society for Laser Medicine and Surgery.
Patients underwent up to six weekly treatments for laxity, wrinkles, or lipodystrophy on the abdomen, flanks, and/or leg areas using the Venus Freeze (MP)2system. The device heats collagen fibers in the skin to produce skin tightening. "The goal is not to reduce volume, but to reshape," said Dr. Nunes, a plastic surgeon in Rio de Janeiro, Brazil.
It’s important to recognize that "if the patient stops the treatments or changes diet habits, probably the results would be totally lost," he said. "Body contouring is challenging."
However, patients who don’t want more invasive body shaping procedures, such as implants, hyaluronic acid, or liposuction, may be pleased with the simplicity of radiofrequency and magnetic pulse, he noted. "The question in our country is, what do patients want? They don’t want to go to the hospital, they don’t like invasive procedures or recovery periods."
The patients in this study had a mean age of 43 years and a mean weight of 66 kg. Ninety percent were female.
After pretreatment with glycerine solution, the 15-minute treatments aimed for a temperature of 41-42° C and uniform erythema on the surface of a 20-cm x 20-cm area.
Side effects immediately after treatment included bruising in 2% of patients, local heat in 78%, edema in 54%, and erythema in 56%. There were no burns. No side effects were seen 5 days later, or at a 3-month follow-up visit.
"In this small study, it [the combination regimen] was a safe and effective treatment for body contouring with no pain, no down time, and high subject satisfaction," Dr. Nunes said
Dr. Nunes reported having no disclosures.
On Twitter @sherryboschert
PHOENIX – Patient satisfaction was 100% after treatment with a device combining multipolar radiofrequency and magnetic pulse fields for body contouring, based on data from a series of 260 adults in Brazil.
A total of 60% of patients reported "excellent" satisfaction and 40% reported "good" satisfaction when surveyed shortly after the treatment, Dr. Rafael Nunes said at the annual meeting of the American Society for Laser Medicine and Surgery.
Patients underwent up to six weekly treatments for laxity, wrinkles, or lipodystrophy on the abdomen, flanks, and/or leg areas using the Venus Freeze (MP)2system. The device heats collagen fibers in the skin to produce skin tightening. "The goal is not to reduce volume, but to reshape," said Dr. Nunes, a plastic surgeon in Rio de Janeiro, Brazil.
It’s important to recognize that "if the patient stops the treatments or changes diet habits, probably the results would be totally lost," he said. "Body contouring is challenging."
However, patients who don’t want more invasive body shaping procedures, such as implants, hyaluronic acid, or liposuction, may be pleased with the simplicity of radiofrequency and magnetic pulse, he noted. "The question in our country is, what do patients want? They don’t want to go to the hospital, they don’t like invasive procedures or recovery periods."
The patients in this study had a mean age of 43 years and a mean weight of 66 kg. Ninety percent were female.
After pretreatment with glycerine solution, the 15-minute treatments aimed for a temperature of 41-42° C and uniform erythema on the surface of a 20-cm x 20-cm area.
Side effects immediately after treatment included bruising in 2% of patients, local heat in 78%, edema in 54%, and erythema in 56%. There were no burns. No side effects were seen 5 days later, or at a 3-month follow-up visit.
"In this small study, it [the combination regimen] was a safe and effective treatment for body contouring with no pain, no down time, and high subject satisfaction," Dr. Nunes said
Dr. Nunes reported having no disclosures.
On Twitter @sherryboschert
AT LASER 2014
Key clinical point: The findings highlight the importance of understanding patients’ preferences while managing their expectations.
Major finding: Satisfaction after treatment was "excellent" in 60% of patients and "good" in 40%.
Data source: A case series of body contouring using radiofrequency and magnetic pulse in 260 patients at five Brazilian centers.
Disclosures: Dr. Nunes reported having no disclosures.
Peanut Reactivity After Immunotherapy
SAN DIEGO – Waiting 3 months after successful oral immunotherapy for peanut allergy before exposure to peanuts significantly increased the odds of reactivity returning in a prospective study of 20 children.
All 20 patients became desensitized to peanuts while on daily oral immunotherapy and successfully passed double-blind, placebo-controlled food challenges at the end of immunotherapy. All 16 patients who then avoided peanuts for 1 month passed a second food challenge, but only 1 of 4 patients who avoided peanuts for 3 months after immunotherapy passed a second one.
"If you wait long enough, the desensitization effect may well wear off," Dr. Brian P. Vickery said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He and his associates looked at levels of basophil activation and conducted skin prick tests to peanut to get a better sense of why this was happening. Basophil activation to peanut antigen and anti-IgE stimulation increased significantly between the times of the first and second food challenges in the patients who avoided peanuts for 3 months but not in those who avoided the nuts for only 1 month.
The basophil activation levels had been similar between groups at the end of immunotherapy, suggesting that desensitization succeeded in all patients, but the length of peanut avoidance affected basophil responses, leading to revival of clinical reactivity.
Skin prick test results returned to baseline levels in three of the four patients who avoided peanuts for 3 months after the end of immunotherapy.
The amount of time on oral immunotherapy did not seem to be a key factor in the likelihood of sustained suppression of allergic disease, reported Dr. Vickery of the department of pediatrics at the University of North Carolina at Chapel Hill. Patients who waited 1 month before exposure to peanut had been on approximately 20-50 months of oral immunotherapy, and patients who waited 3 months before exposure had been on approximately 60 months of immunotherapy.
The lead author of the study was Michael D. Kulis Jr., Ph.D., also of the university.
Prolonged avoidance of peanut after peanut oral immunotherapy appears to be detrimental and may reverse the effects of the treatment, Dr. A. Wesley Burks said at a press briefing.
The findings may be applicable to other food allergies, but "we don’t know that because there have not been enough studies that long with other foods," said Dr. Burks, a coinvestigator in the study and chairman of the department of pediatrics at the university. "I wouldn’t anticipate that it would be different."
The average age of children in the study was 6 years.
This exploratory study was not controlled and was limited by its small size. The Peanut Oral Immunotherapy in Children (IMPACT) study is underway and should answer the question of how long the clinical effects of oral immunotherapy last, Dr. Vickery said. The study is being sponsored by the Immune Tolerance Network and the National Institute of Allergy and Infectious Diseases.
Dr. Vickery, Dr. Kulis, and Dr. Burks reported having no financial disclosures. The National Institutes of Health funded the study.
On Twitter @sherryboschert
SAN DIEGO – Waiting 3 months after successful oral immunotherapy for peanut allergy before exposure to peanuts significantly increased the odds of reactivity returning in a prospective study of 20 children.
All 20 patients became desensitized to peanuts while on daily oral immunotherapy and successfully passed double-blind, placebo-controlled food challenges at the end of immunotherapy. All 16 patients who then avoided peanuts for 1 month passed a second food challenge, but only 1 of 4 patients who avoided peanuts for 3 months after immunotherapy passed a second one.
"If you wait long enough, the desensitization effect may well wear off," Dr. Brian P. Vickery said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He and his associates looked at levels of basophil activation and conducted skin prick tests to peanut to get a better sense of why this was happening. Basophil activation to peanut antigen and anti-IgE stimulation increased significantly between the times of the first and second food challenges in the patients who avoided peanuts for 3 months but not in those who avoided the nuts for only 1 month.
The basophil activation levels had been similar between groups at the end of immunotherapy, suggesting that desensitization succeeded in all patients, but the length of peanut avoidance affected basophil responses, leading to revival of clinical reactivity.
Skin prick test results returned to baseline levels in three of the four patients who avoided peanuts for 3 months after the end of immunotherapy.
The amount of time on oral immunotherapy did not seem to be a key factor in the likelihood of sustained suppression of allergic disease, reported Dr. Vickery of the department of pediatrics at the University of North Carolina at Chapel Hill. Patients who waited 1 month before exposure to peanut had been on approximately 20-50 months of oral immunotherapy, and patients who waited 3 months before exposure had been on approximately 60 months of immunotherapy.
The lead author of the study was Michael D. Kulis Jr., Ph.D., also of the university.
Prolonged avoidance of peanut after peanut oral immunotherapy appears to be detrimental and may reverse the effects of the treatment, Dr. A. Wesley Burks said at a press briefing.
The findings may be applicable to other food allergies, but "we don’t know that because there have not been enough studies that long with other foods," said Dr. Burks, a coinvestigator in the study and chairman of the department of pediatrics at the university. "I wouldn’t anticipate that it would be different."
The average age of children in the study was 6 years.
This exploratory study was not controlled and was limited by its small size. The Peanut Oral Immunotherapy in Children (IMPACT) study is underway and should answer the question of how long the clinical effects of oral immunotherapy last, Dr. Vickery said. The study is being sponsored by the Immune Tolerance Network and the National Institute of Allergy and Infectious Diseases.
Dr. Vickery, Dr. Kulis, and Dr. Burks reported having no financial disclosures. The National Institutes of Health funded the study.
On Twitter @sherryboschert
SAN DIEGO – Waiting 3 months after successful oral immunotherapy for peanut allergy before exposure to peanuts significantly increased the odds of reactivity returning in a prospective study of 20 children.
All 20 patients became desensitized to peanuts while on daily oral immunotherapy and successfully passed double-blind, placebo-controlled food challenges at the end of immunotherapy. All 16 patients who then avoided peanuts for 1 month passed a second food challenge, but only 1 of 4 patients who avoided peanuts for 3 months after immunotherapy passed a second one.
"If you wait long enough, the desensitization effect may well wear off," Dr. Brian P. Vickery said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He and his associates looked at levels of basophil activation and conducted skin prick tests to peanut to get a better sense of why this was happening. Basophil activation to peanut antigen and anti-IgE stimulation increased significantly between the times of the first and second food challenges in the patients who avoided peanuts for 3 months but not in those who avoided the nuts for only 1 month.
The basophil activation levels had been similar between groups at the end of immunotherapy, suggesting that desensitization succeeded in all patients, but the length of peanut avoidance affected basophil responses, leading to revival of clinical reactivity.
Skin prick test results returned to baseline levels in three of the four patients who avoided peanuts for 3 months after the end of immunotherapy.
The amount of time on oral immunotherapy did not seem to be a key factor in the likelihood of sustained suppression of allergic disease, reported Dr. Vickery of the department of pediatrics at the University of North Carolina at Chapel Hill. Patients who waited 1 month before exposure to peanut had been on approximately 20-50 months of oral immunotherapy, and patients who waited 3 months before exposure had been on approximately 60 months of immunotherapy.
The lead author of the study was Michael D. Kulis Jr., Ph.D., also of the university.
Prolonged avoidance of peanut after peanut oral immunotherapy appears to be detrimental and may reverse the effects of the treatment, Dr. A. Wesley Burks said at a press briefing.
The findings may be applicable to other food allergies, but "we don’t know that because there have not been enough studies that long with other foods," said Dr. Burks, a coinvestigator in the study and chairman of the department of pediatrics at the university. "I wouldn’t anticipate that it would be different."
The average age of children in the study was 6 years.
This exploratory study was not controlled and was limited by its small size. The Peanut Oral Immunotherapy in Children (IMPACT) study is underway and should answer the question of how long the clinical effects of oral immunotherapy last, Dr. Vickery said. The study is being sponsored by the Immune Tolerance Network and the National Institute of Allergy and Infectious Diseases.
Dr. Vickery, Dr. Kulis, and Dr. Burks reported having no financial disclosures. The National Institutes of Health funded the study.
On Twitter @sherryboschert
AT 2014 AAAAI ANNUAL MEETING
Peanut reactivity returned if exposure delayed after immunotherapy
SAN DIEGO – Waiting 3 months after successful oral immunotherapy for peanut allergy before exposure to peanuts significantly increased the odds of reactivity returning in a prospective study of 20 children.
All 20 patients became desensitized to peanuts while on daily oral immunotherapy and successfully passed double-blind, placebo-controlled food challenges at the end of immunotherapy. All 16 patients who then avoided peanuts for 1 month passed a second food challenge, but only 1 of 4 patients who avoided peanuts for 3 months after immunotherapy passed a second one.
"If you wait long enough, the desensitization effect may well wear off," Dr. Brian P. Vickery said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He and his associates looked at levels of basophil activation and conducted skin prick tests to peanut to get a better sense of why this was happening. Basophil activation to peanut antigen and anti-IgE stimulation increased significantly between the times of the first and second food challenges in the patients who avoided peanuts for 3 months but not in those who avoided the nuts for only 1 month.
The basophil activation levels had been similar between groups at the end of immunotherapy, suggesting that desensitization succeeded in all patients, but the length of peanut avoidance affected basophil responses, leading to revival of clinical reactivity.
Skin prick test results returned to baseline levels in three of the four patients who avoided peanuts for 3 months after the end of immunotherapy.
The amount of time on oral immunotherapy did not seem to be a key factor in the likelihood of sustained suppression of allergic disease, reported Dr. Vickery of the department of pediatrics at the University of North Carolina at Chapel Hill. Patients who waited 1 month before exposure to peanut had been on approximately 20-50 months of oral immunotherapy, and patients who waited 3 months before exposure had been on approximately 60 months of immunotherapy.
The lead author of the study was Michael D. Kulis Jr., Ph.D., also of the university.
Prolonged avoidance of peanut after peanut oral immunotherapy appears to be detrimental and may reverse the effects of the treatment, Dr. A. Wesley Burks said at a press briefing.
The findings may be applicable to other food allergies, but "we don’t know that because there have not been enough studies that long with other foods," said Dr. Burks, a coinvestigator in the study and chairman of the department of pediatrics at the university. "I wouldn’t anticipate that it would be different."
The average age of children in the study was 6 years.
This exploratory study was not controlled and was limited by its small size. The Peanut Oral Immunotherapy in Children (IMPACT) study is underway and should answer the question of how long the clinical effects of oral immunotherapy last, Dr. Vickery said. The study is being sponsored by the Immune Tolerance Network and the National Institute of Allergy and Infectious Diseases.
Dr. Vickery, Dr. Kulis, and Dr. Burks reported having no financial disclosures. The National Institutes of Health funded the study.
On Twitter @sherryboschert
SAN DIEGO – Waiting 3 months after successful oral immunotherapy for peanut allergy before exposure to peanuts significantly increased the odds of reactivity returning in a prospective study of 20 children.
All 20 patients became desensitized to peanuts while on daily oral immunotherapy and successfully passed double-blind, placebo-controlled food challenges at the end of immunotherapy. All 16 patients who then avoided peanuts for 1 month passed a second food challenge, but only 1 of 4 patients who avoided peanuts for 3 months after immunotherapy passed a second one.
"If you wait long enough, the desensitization effect may well wear off," Dr. Brian P. Vickery said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He and his associates looked at levels of basophil activation and conducted skin prick tests to peanut to get a better sense of why this was happening. Basophil activation to peanut antigen and anti-IgE stimulation increased significantly between the times of the first and second food challenges in the patients who avoided peanuts for 3 months but not in those who avoided the nuts for only 1 month.
The basophil activation levels had been similar between groups at the end of immunotherapy, suggesting that desensitization succeeded in all patients, but the length of peanut avoidance affected basophil responses, leading to revival of clinical reactivity.
Skin prick test results returned to baseline levels in three of the four patients who avoided peanuts for 3 months after the end of immunotherapy.
The amount of time on oral immunotherapy did not seem to be a key factor in the likelihood of sustained suppression of allergic disease, reported Dr. Vickery of the department of pediatrics at the University of North Carolina at Chapel Hill. Patients who waited 1 month before exposure to peanut had been on approximately 20-50 months of oral immunotherapy, and patients who waited 3 months before exposure had been on approximately 60 months of immunotherapy.
The lead author of the study was Michael D. Kulis Jr., Ph.D., also of the university.
Prolonged avoidance of peanut after peanut oral immunotherapy appears to be detrimental and may reverse the effects of the treatment, Dr. A. Wesley Burks said at a press briefing.
The findings may be applicable to other food allergies, but "we don’t know that because there have not been enough studies that long with other foods," said Dr. Burks, a coinvestigator in the study and chairman of the department of pediatrics at the university. "I wouldn’t anticipate that it would be different."
The average age of children in the study was 6 years.
This exploratory study was not controlled and was limited by its small size. The Peanut Oral Immunotherapy in Children (IMPACT) study is underway and should answer the question of how long the clinical effects of oral immunotherapy last, Dr. Vickery said. The study is being sponsored by the Immune Tolerance Network and the National Institute of Allergy and Infectious Diseases.
Dr. Vickery, Dr. Kulis, and Dr. Burks reported having no financial disclosures. The National Institutes of Health funded the study.
On Twitter @sherryboschert
SAN DIEGO – Waiting 3 months after successful oral immunotherapy for peanut allergy before exposure to peanuts significantly increased the odds of reactivity returning in a prospective study of 20 children.
All 20 patients became desensitized to peanuts while on daily oral immunotherapy and successfully passed double-blind, placebo-controlled food challenges at the end of immunotherapy. All 16 patients who then avoided peanuts for 1 month passed a second food challenge, but only 1 of 4 patients who avoided peanuts for 3 months after immunotherapy passed a second one.
"If you wait long enough, the desensitization effect may well wear off," Dr. Brian P. Vickery said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He and his associates looked at levels of basophil activation and conducted skin prick tests to peanut to get a better sense of why this was happening. Basophil activation to peanut antigen and anti-IgE stimulation increased significantly between the times of the first and second food challenges in the patients who avoided peanuts for 3 months but not in those who avoided the nuts for only 1 month.
The basophil activation levels had been similar between groups at the end of immunotherapy, suggesting that desensitization succeeded in all patients, but the length of peanut avoidance affected basophil responses, leading to revival of clinical reactivity.
Skin prick test results returned to baseline levels in three of the four patients who avoided peanuts for 3 months after the end of immunotherapy.
The amount of time on oral immunotherapy did not seem to be a key factor in the likelihood of sustained suppression of allergic disease, reported Dr. Vickery of the department of pediatrics at the University of North Carolina at Chapel Hill. Patients who waited 1 month before exposure to peanut had been on approximately 20-50 months of oral immunotherapy, and patients who waited 3 months before exposure had been on approximately 60 months of immunotherapy.
The lead author of the study was Michael D. Kulis Jr., Ph.D., also of the university.
Prolonged avoidance of peanut after peanut oral immunotherapy appears to be detrimental and may reverse the effects of the treatment, Dr. A. Wesley Burks said at a press briefing.
The findings may be applicable to other food allergies, but "we don’t know that because there have not been enough studies that long with other foods," said Dr. Burks, a coinvestigator in the study and chairman of the department of pediatrics at the university. "I wouldn’t anticipate that it would be different."
The average age of children in the study was 6 years.
This exploratory study was not controlled and was limited by its small size. The Peanut Oral Immunotherapy in Children (IMPACT) study is underway and should answer the question of how long the clinical effects of oral immunotherapy last, Dr. Vickery said. The study is being sponsored by the Immune Tolerance Network and the National Institute of Allergy and Infectious Diseases.
Dr. Vickery, Dr. Kulis, and Dr. Burks reported having no financial disclosures. The National Institutes of Health funded the study.
On Twitter @sherryboschert
AT 2014 AAAAI ANNUAL MEETING
Major finding: Reactivity to peanuts returned in none of the 16 patients who avoided peanuts for 1 month after oral immunotherapy and in three of four patients who avoided peanuts for 3 months.
Data source: A prospective study of 20 children who underwent food challenges after oral immunotherapy treatment for peanut allergy.
Disclosures: Dr. Vickery, Dr. Kulis, and Dr. Burks reported having no financial disclosures.
Mindfulness improved irritable bowel for a year
CHICAGO – An 8-week course in mindfulness-based stress reduction reduced the severity of irritable bowel syndrome symptoms 6 and 12 months later, compared with 8 weeks of participation in a control group, follow-up on 68 women found.
Scores for overall irritable bowel syndrome (IBS) severity on the IBS Severity Scale (IBS-SS) were similar between groups at baseline (284 in the intervention group and 288 in the control group) but had improved significantly more in the mindfulness training group at 6 months (scores decreased 151 and 108 points, respectively) and at 12 months (scores decreased 115 vs. 26 points, respectively) compared with baseline.
The investigators originally reported significant benefits from the mindfulness course, compared with the control group immediately after the group sessions and at 3 months of follow-up in the prospective, randomized, controlled trial involving 75 patients (Am. J. Gastroenterol. 2011;106:1678-88). The current follow-up to 6 and 12 months shows lasting symptomatic improvements from mindfulness training, Olafur S. Palsson, Psy.D., and his associates reported at the annual Digestive Disease Week.
Among the 68 patients who completed 1 year of follow-up in the current analysis, the 33 who got mindfulness training also showed significantly greater improvements in secondary outcomes, compared with the 35 patients in the support group, said Dr. Palsson, a professor of medicine at the University of North Carolina, Chapel Hill.
Scores on the IBS Quality of Life Instrument were similar between groups at baseline (65 in the mindfulness group and 67 in the control group) but improved significantly more in the mindfulness group by 12 months (by 15 vs. 3, respectively).
Scores for gut-focused anxiety on the Visceral Sensitivity Index – which were not significantly different between groups at baseline or immediately after the group sessions – improved significantly more in the mindfulness group than in the control group by 3 months and the gains remained significantly greater at 6 months (by 12 vs. 2, respectively) and at 12 months (by 9 vs. –1, respectively).
"To our knowledge, these follow-up findings demonstrate some of the longest-duration therapeutic effects of mindfulness training ever reported in a clinical trial," he said.
Both interventions consisted of eight weekly sessions and a half-day retreat. The control group attended a conventional support group. The mindfulness course was based on the Mindfulness-Based Stress Reduction Program of Jon Kabat-Zinn, Ph.D., and Saki F. Santorelli, Ed.D., both of the University of Massachusetts, Worcester.
The longitudinal study controlled for the effects of race and income (less than or at least $40,000/year). The results suggest that the impact of mindfulness training on bowel symptom severity and gut-focused anxiety are well maintained and that improvements in health-related quality of life develop gradually over many months after the training, Dr. Palsson said. General psychological well-being did not change significantly based on the training, he added.
Scores for mindfulness on the Five-Facet Mindfulness Questionnaire were higher at every follow-up in the mindfulness group, compared with the control group, but the differences were not statistically significant. Mindfulness scores peaked in the mindfulness group at around 6 months and were attenuated at 12 months.
Patients ranged in age from 19 to 71 years, with a mean age of 43 years. Most patients were white, and women who were minorities or had lower incomes were more likely to drop out of the trial over time.
The National Center for Complementary and Alternative Medicine funded the study. Dr. Palsson and his coinvestigators reported financial associations with Takeda Pharmaceuticals, Ono Pharmaceuticals, Ironwood Pharmaceuticals, Entera Health, and/or the Rome Foundation.
On Twitter @sherryboschert
Mindfulness, an ancient Buddhist meditative practice, seeks to maintain awareness of the present moment by reducing attachment to thoughts or feelings about the past or future (for example, worry). Associated with reduced suffering in chronic pain and depression (JAMA 2008;300:1350-2), mindfulness may potentially operate through top-down modulation of thalamocortical alpha-rhythms, facilitating more efficient filtering of sensory information in the brain (Front. Hum. Neurosci. 2013;7:12). In IBS, mindfulness may "uncouple" the sensory experiences of abdominal pain (for example, visceral hypersensitivity) from its associated negative evaluative and emotional reactions (for example, catastrophizing, fear, avoidance). Mindfulness practice has been successfully incorporated into cognitive therapy for a host of psychological conditions (Br. J. Psych. 2012;200:359-60).
Mindfulness-based stress reduction (MBSR), a stand-alone therapy (not just a skill) developed in 1979 by Dr. Jon Kabat-Zinn (Gen. Hosp. Psych. 1982;4:33-47), has advantages over other therapies - it is a standardized, eight-session program that can be administered in groups to a heterogeneous patient population by a wide range of medical providers. MBSR features the skill of mindfulness but also incorporates yoga, acceptance, and stress management. In addition to intensive coursework and a weekend retreat, patients engage in home practice 45 minutes a day. In this study, MBSR may have been less feasible or acceptable to women of lower socioeconomic status or in certain ethnic/racial minority groups.
The long-term success of MBSR on IBS symptoms suggested that the acquisition of mindfulness skills and their incorporation into everyday life may not always alleviate symptoms immediately - in other words, we should not abandon its practice too soon. As mindfulness improved, so did symptoms. Despite limitations, these results suggest we could focus research on increasing adherence to the lifelong practice of mindfulness, include mindfulness as a skill in other IBS therapies, and increase its acceptability to a broader population of patients.
Dr. Laurie Keefer, AGAF, is with the departments of psychiatry and behavioral sciences at Northwestern University, Chicago, and director of the center for psychosocial research in GI, and director of clinical research, division of gastroenterology and hepatology. She has no financial disclosures.
Mindfulness, an ancient Buddhist meditative practice, seeks to maintain awareness of the present moment by reducing attachment to thoughts or feelings about the past or future (for example, worry). Associated with reduced suffering in chronic pain and depression (JAMA 2008;300:1350-2), mindfulness may potentially operate through top-down modulation of thalamocortical alpha-rhythms, facilitating more efficient filtering of sensory information in the brain (Front. Hum. Neurosci. 2013;7:12). In IBS, mindfulness may "uncouple" the sensory experiences of abdominal pain (for example, visceral hypersensitivity) from its associated negative evaluative and emotional reactions (for example, catastrophizing, fear, avoidance). Mindfulness practice has been successfully incorporated into cognitive therapy for a host of psychological conditions (Br. J. Psych. 2012;200:359-60).
Mindfulness-based stress reduction (MBSR), a stand-alone therapy (not just a skill) developed in 1979 by Dr. Jon Kabat-Zinn (Gen. Hosp. Psych. 1982;4:33-47), has advantages over other therapies - it is a standardized, eight-session program that can be administered in groups to a heterogeneous patient population by a wide range of medical providers. MBSR features the skill of mindfulness but also incorporates yoga, acceptance, and stress management. In addition to intensive coursework and a weekend retreat, patients engage in home practice 45 minutes a day. In this study, MBSR may have been less feasible or acceptable to women of lower socioeconomic status or in certain ethnic/racial minority groups.
The long-term success of MBSR on IBS symptoms suggested that the acquisition of mindfulness skills and their incorporation into everyday life may not always alleviate symptoms immediately - in other words, we should not abandon its practice too soon. As mindfulness improved, so did symptoms. Despite limitations, these results suggest we could focus research on increasing adherence to the lifelong practice of mindfulness, include mindfulness as a skill in other IBS therapies, and increase its acceptability to a broader population of patients.
Dr. Laurie Keefer, AGAF, is with the departments of psychiatry and behavioral sciences at Northwestern University, Chicago, and director of the center for psychosocial research in GI, and director of clinical research, division of gastroenterology and hepatology. She has no financial disclosures.
Mindfulness, an ancient Buddhist meditative practice, seeks to maintain awareness of the present moment by reducing attachment to thoughts or feelings about the past or future (for example, worry). Associated with reduced suffering in chronic pain and depression (JAMA 2008;300:1350-2), mindfulness may potentially operate through top-down modulation of thalamocortical alpha-rhythms, facilitating more efficient filtering of sensory information in the brain (Front. Hum. Neurosci. 2013;7:12). In IBS, mindfulness may "uncouple" the sensory experiences of abdominal pain (for example, visceral hypersensitivity) from its associated negative evaluative and emotional reactions (for example, catastrophizing, fear, avoidance). Mindfulness practice has been successfully incorporated into cognitive therapy for a host of psychological conditions (Br. J. Psych. 2012;200:359-60).
Mindfulness-based stress reduction (MBSR), a stand-alone therapy (not just a skill) developed in 1979 by Dr. Jon Kabat-Zinn (Gen. Hosp. Psych. 1982;4:33-47), has advantages over other therapies - it is a standardized, eight-session program that can be administered in groups to a heterogeneous patient population by a wide range of medical providers. MBSR features the skill of mindfulness but also incorporates yoga, acceptance, and stress management. In addition to intensive coursework and a weekend retreat, patients engage in home practice 45 minutes a day. In this study, MBSR may have been less feasible or acceptable to women of lower socioeconomic status or in certain ethnic/racial minority groups.
The long-term success of MBSR on IBS symptoms suggested that the acquisition of mindfulness skills and their incorporation into everyday life may not always alleviate symptoms immediately - in other words, we should not abandon its practice too soon. As mindfulness improved, so did symptoms. Despite limitations, these results suggest we could focus research on increasing adherence to the lifelong practice of mindfulness, include mindfulness as a skill in other IBS therapies, and increase its acceptability to a broader population of patients.
Dr. Laurie Keefer, AGAF, is with the departments of psychiatry and behavioral sciences at Northwestern University, Chicago, and director of the center for psychosocial research in GI, and director of clinical research, division of gastroenterology and hepatology. She has no financial disclosures.
CHICAGO – An 8-week course in mindfulness-based stress reduction reduced the severity of irritable bowel syndrome symptoms 6 and 12 months later, compared with 8 weeks of participation in a control group, follow-up on 68 women found.
Scores for overall irritable bowel syndrome (IBS) severity on the IBS Severity Scale (IBS-SS) were similar between groups at baseline (284 in the intervention group and 288 in the control group) but had improved significantly more in the mindfulness training group at 6 months (scores decreased 151 and 108 points, respectively) and at 12 months (scores decreased 115 vs. 26 points, respectively) compared with baseline.
The investigators originally reported significant benefits from the mindfulness course, compared with the control group immediately after the group sessions and at 3 months of follow-up in the prospective, randomized, controlled trial involving 75 patients (Am. J. Gastroenterol. 2011;106:1678-88). The current follow-up to 6 and 12 months shows lasting symptomatic improvements from mindfulness training, Olafur S. Palsson, Psy.D., and his associates reported at the annual Digestive Disease Week.
Among the 68 patients who completed 1 year of follow-up in the current analysis, the 33 who got mindfulness training also showed significantly greater improvements in secondary outcomes, compared with the 35 patients in the support group, said Dr. Palsson, a professor of medicine at the University of North Carolina, Chapel Hill.
Scores on the IBS Quality of Life Instrument were similar between groups at baseline (65 in the mindfulness group and 67 in the control group) but improved significantly more in the mindfulness group by 12 months (by 15 vs. 3, respectively).
Scores for gut-focused anxiety on the Visceral Sensitivity Index – which were not significantly different between groups at baseline or immediately after the group sessions – improved significantly more in the mindfulness group than in the control group by 3 months and the gains remained significantly greater at 6 months (by 12 vs. 2, respectively) and at 12 months (by 9 vs. –1, respectively).
"To our knowledge, these follow-up findings demonstrate some of the longest-duration therapeutic effects of mindfulness training ever reported in a clinical trial," he said.
Both interventions consisted of eight weekly sessions and a half-day retreat. The control group attended a conventional support group. The mindfulness course was based on the Mindfulness-Based Stress Reduction Program of Jon Kabat-Zinn, Ph.D., and Saki F. Santorelli, Ed.D., both of the University of Massachusetts, Worcester.
The longitudinal study controlled for the effects of race and income (less than or at least $40,000/year). The results suggest that the impact of mindfulness training on bowel symptom severity and gut-focused anxiety are well maintained and that improvements in health-related quality of life develop gradually over many months after the training, Dr. Palsson said. General psychological well-being did not change significantly based on the training, he added.
Scores for mindfulness on the Five-Facet Mindfulness Questionnaire were higher at every follow-up in the mindfulness group, compared with the control group, but the differences were not statistically significant. Mindfulness scores peaked in the mindfulness group at around 6 months and were attenuated at 12 months.
Patients ranged in age from 19 to 71 years, with a mean age of 43 years. Most patients were white, and women who were minorities or had lower incomes were more likely to drop out of the trial over time.
The National Center for Complementary and Alternative Medicine funded the study. Dr. Palsson and his coinvestigators reported financial associations with Takeda Pharmaceuticals, Ono Pharmaceuticals, Ironwood Pharmaceuticals, Entera Health, and/or the Rome Foundation.
On Twitter @sherryboschert
CHICAGO – An 8-week course in mindfulness-based stress reduction reduced the severity of irritable bowel syndrome symptoms 6 and 12 months later, compared with 8 weeks of participation in a control group, follow-up on 68 women found.
Scores for overall irritable bowel syndrome (IBS) severity on the IBS Severity Scale (IBS-SS) were similar between groups at baseline (284 in the intervention group and 288 in the control group) but had improved significantly more in the mindfulness training group at 6 months (scores decreased 151 and 108 points, respectively) and at 12 months (scores decreased 115 vs. 26 points, respectively) compared with baseline.
The investigators originally reported significant benefits from the mindfulness course, compared with the control group immediately after the group sessions and at 3 months of follow-up in the prospective, randomized, controlled trial involving 75 patients (Am. J. Gastroenterol. 2011;106:1678-88). The current follow-up to 6 and 12 months shows lasting symptomatic improvements from mindfulness training, Olafur S. Palsson, Psy.D., and his associates reported at the annual Digestive Disease Week.
Among the 68 patients who completed 1 year of follow-up in the current analysis, the 33 who got mindfulness training also showed significantly greater improvements in secondary outcomes, compared with the 35 patients in the support group, said Dr. Palsson, a professor of medicine at the University of North Carolina, Chapel Hill.
Scores on the IBS Quality of Life Instrument were similar between groups at baseline (65 in the mindfulness group and 67 in the control group) but improved significantly more in the mindfulness group by 12 months (by 15 vs. 3, respectively).
Scores for gut-focused anxiety on the Visceral Sensitivity Index – which were not significantly different between groups at baseline or immediately after the group sessions – improved significantly more in the mindfulness group than in the control group by 3 months and the gains remained significantly greater at 6 months (by 12 vs. 2, respectively) and at 12 months (by 9 vs. –1, respectively).
"To our knowledge, these follow-up findings demonstrate some of the longest-duration therapeutic effects of mindfulness training ever reported in a clinical trial," he said.
Both interventions consisted of eight weekly sessions and a half-day retreat. The control group attended a conventional support group. The mindfulness course was based on the Mindfulness-Based Stress Reduction Program of Jon Kabat-Zinn, Ph.D., and Saki F. Santorelli, Ed.D., both of the University of Massachusetts, Worcester.
The longitudinal study controlled for the effects of race and income (less than or at least $40,000/year). The results suggest that the impact of mindfulness training on bowel symptom severity and gut-focused anxiety are well maintained and that improvements in health-related quality of life develop gradually over many months after the training, Dr. Palsson said. General psychological well-being did not change significantly based on the training, he added.
Scores for mindfulness on the Five-Facet Mindfulness Questionnaire were higher at every follow-up in the mindfulness group, compared with the control group, but the differences were not statistically significant. Mindfulness scores peaked in the mindfulness group at around 6 months and were attenuated at 12 months.
Patients ranged in age from 19 to 71 years, with a mean age of 43 years. Most patients were white, and women who were minorities or had lower incomes were more likely to drop out of the trial over time.
The National Center for Complementary and Alternative Medicine funded the study. Dr. Palsson and his coinvestigators reported financial associations with Takeda Pharmaceuticals, Ono Pharmaceuticals, Ironwood Pharmaceuticals, Entera Health, and/or the Rome Foundation.
On Twitter @sherryboschert
AT DDW 2014
Major finding: IBS Severity Scale scores improved in the mindfulness group by 151 points at 6 months and by 115 points at 12 months, compared with baseline, significantly greater improvements than changes in the control group of 108 and 26 points at 6 and 12 months.
Data source: Longitudinal follow-up on a randomized, controlled trial of an 8-week, mindfulness-based stress-reduction course compared with a control group in 68 women with IBS.
Disclosures: The National Center for Complementary and Alternative Medicine funded the study. Dr. Palsson and his coinvestigators reported financial associations with Takeda Pharmaceuticals, Ono Pharmaceuticals, Ironwood Pharmaceuticals, Entera Health, and/or the Rome Foundation.
Food allergy overdiagnosed with IgE tests
SAN DIEGO – Unwarranted food allergy testing in 67% of 274 patients who underwent testing identified a new food allergen in only 4 patients, in a retrospective study.
The review of charts on patients referred to a tertiary food allergy center from September 2011 to December 2012 found 274 children with results from a standard panel of food-specific IgE tests obtained prior to referral. Only 33% of cases warranted evaluation for food allergy according to criteria set by the National Institute of Allergy and Infectious Diseases, according to Dr. Maryam Saifi and her associates.
In the 90 patients who underwent food allergy testing appropriately, testing identified a previously unknown allergen in 38 patients (42%) and found no new allergen in 52 (58%). When food allergy testing was not warranted, however, testing identified a previously unknown allergen in only 4 of 184 patients (5%) and no new allergen in 180 patients (95%), Dr. Saifi reported in a poster presentation at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The most common reason for conducting food-specific IgE panel testing in patients who did not meet criteria for testing was allergic rhinitis, followed by mild atopic dermatitis, urticaria, GI complaints, rash (not otherwise specified), angioedema without urticaria, and cough.
Test results and recommendations from primary care providers led 126 of the 274 patients to alter their diets (46%), yet only 54 food-avoiding patients had a history warranting evaluation for food allergy (20% of the whole cohort), reported Dr. Saifi, a pediatrician at the University of Texas Southwestern Medical Center, Dallas.
Diet-altering patients were avoiding foods such as milk, eggs, peanuts, tree nuts, soy, wheat, fish, shellfish, sesame seed, corn, chocolate, or beef. After being seen at the referral clinic for a history, repeat testing, and observed challenges when necessary, patients were able to reintroduce an average of two foods per person, most commonly among milk, eggs, peanuts, soy, and wheat. All patients who had been avoiding corn and chocolate were able to reintroduce those foods.
Serum allergy testing should be used judiciously and only when indicated by history and physical exam, Dr. Saifi concluded. IgE panels for food allergy seem to have little utility as screening tests and often lead to misdiagnosis of food allergy, resulting in inappropriate food avoidance that could cause nutritional deficiencies, increased anxiety, and lower quality of life, she said.
The study excluded patients diagnosed with eosinophilic esophagitis and patients whose records lacked results from food-specific IgE tests prior to referral.
The investigators’ financial disclosures were not available.
On Twitter @sherryboschert
SAN DIEGO – Unwarranted food allergy testing in 67% of 274 patients who underwent testing identified a new food allergen in only 4 patients, in a retrospective study.
The review of charts on patients referred to a tertiary food allergy center from September 2011 to December 2012 found 274 children with results from a standard panel of food-specific IgE tests obtained prior to referral. Only 33% of cases warranted evaluation for food allergy according to criteria set by the National Institute of Allergy and Infectious Diseases, according to Dr. Maryam Saifi and her associates.
In the 90 patients who underwent food allergy testing appropriately, testing identified a previously unknown allergen in 38 patients (42%) and found no new allergen in 52 (58%). When food allergy testing was not warranted, however, testing identified a previously unknown allergen in only 4 of 184 patients (5%) and no new allergen in 180 patients (95%), Dr. Saifi reported in a poster presentation at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The most common reason for conducting food-specific IgE panel testing in patients who did not meet criteria for testing was allergic rhinitis, followed by mild atopic dermatitis, urticaria, GI complaints, rash (not otherwise specified), angioedema without urticaria, and cough.
Test results and recommendations from primary care providers led 126 of the 274 patients to alter their diets (46%), yet only 54 food-avoiding patients had a history warranting evaluation for food allergy (20% of the whole cohort), reported Dr. Saifi, a pediatrician at the University of Texas Southwestern Medical Center, Dallas.
Diet-altering patients were avoiding foods such as milk, eggs, peanuts, tree nuts, soy, wheat, fish, shellfish, sesame seed, corn, chocolate, or beef. After being seen at the referral clinic for a history, repeat testing, and observed challenges when necessary, patients were able to reintroduce an average of two foods per person, most commonly among milk, eggs, peanuts, soy, and wheat. All patients who had been avoiding corn and chocolate were able to reintroduce those foods.
Serum allergy testing should be used judiciously and only when indicated by history and physical exam, Dr. Saifi concluded. IgE panels for food allergy seem to have little utility as screening tests and often lead to misdiagnosis of food allergy, resulting in inappropriate food avoidance that could cause nutritional deficiencies, increased anxiety, and lower quality of life, she said.
The study excluded patients diagnosed with eosinophilic esophagitis and patients whose records lacked results from food-specific IgE tests prior to referral.
The investigators’ financial disclosures were not available.
On Twitter @sherryboschert
SAN DIEGO – Unwarranted food allergy testing in 67% of 274 patients who underwent testing identified a new food allergen in only 4 patients, in a retrospective study.
The review of charts on patients referred to a tertiary food allergy center from September 2011 to December 2012 found 274 children with results from a standard panel of food-specific IgE tests obtained prior to referral. Only 33% of cases warranted evaluation for food allergy according to criteria set by the National Institute of Allergy and Infectious Diseases, according to Dr. Maryam Saifi and her associates.
In the 90 patients who underwent food allergy testing appropriately, testing identified a previously unknown allergen in 38 patients (42%) and found no new allergen in 52 (58%). When food allergy testing was not warranted, however, testing identified a previously unknown allergen in only 4 of 184 patients (5%) and no new allergen in 180 patients (95%), Dr. Saifi reported in a poster presentation at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The most common reason for conducting food-specific IgE panel testing in patients who did not meet criteria for testing was allergic rhinitis, followed by mild atopic dermatitis, urticaria, GI complaints, rash (not otherwise specified), angioedema without urticaria, and cough.
Test results and recommendations from primary care providers led 126 of the 274 patients to alter their diets (46%), yet only 54 food-avoiding patients had a history warranting evaluation for food allergy (20% of the whole cohort), reported Dr. Saifi, a pediatrician at the University of Texas Southwestern Medical Center, Dallas.
Diet-altering patients were avoiding foods such as milk, eggs, peanuts, tree nuts, soy, wheat, fish, shellfish, sesame seed, corn, chocolate, or beef. After being seen at the referral clinic for a history, repeat testing, and observed challenges when necessary, patients were able to reintroduce an average of two foods per person, most commonly among milk, eggs, peanuts, soy, and wheat. All patients who had been avoiding corn and chocolate were able to reintroduce those foods.
Serum allergy testing should be used judiciously and only when indicated by history and physical exam, Dr. Saifi concluded. IgE panels for food allergy seem to have little utility as screening tests and often lead to misdiagnosis of food allergy, resulting in inappropriate food avoidance that could cause nutritional deficiencies, increased anxiety, and lower quality of life, she said.
The study excluded patients diagnosed with eosinophilic esophagitis and patients whose records lacked results from food-specific IgE tests prior to referral.
The investigators’ financial disclosures were not available.
On Twitter @sherryboschert
AT THE 2014 AAAAI ANNUAL MEETING
Major finding: Food allergy testing identified a new allergen in 5% of 184 patients in whom testing was not warranted and in 42% of 90 patients in whom testing was warranted.
Data source: Retrospective study of 274 patients referred to a tertiary allergy center who had results from a panel of food-specific IgE tests before referral.
Disclosures: The investigators’ financial disclosures were not available.
Know the urban myths that compromise allergy care
SAN DIEGO – There’s no such thing as a hypoallergenic dog. Blood tests for sale on the Internet won’t identify a child’s allergies. And parents don’t have to wait until a child is 1, 2 or 3 years of age to introduce dietary milk, eggs, or nuts.
These are some of the facts that physicians need to know in order to counter common myths about allergy, Dr. David R. Stukus said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Patients aren’t the only ones who need enlightening. Too many physicians still believe some of the myths listed below, said Dr. Stukus of the department of pediatrics at Nationwide Children’s Hospital and Ohio State University, Columbus:
• Hypoallergenic pets: "There’s a lot of false advertising" by companies marketing supposedly hypoallergenic pets, some selling cats for $7,000-$28,000 or dogs for $16,000, he said. While some of these animals have been bred to produce fewer major allergens from their saliva, sebaceous glands, or perianal glands, they still produce minor allergens that cause clinical symptoms in sensitized people.
• Blood testing: Allergen-specific serum IgE testing is not a reliable screen for allergy, and often leads to misinterpretation and false-positive results – which in turn lead to diagnostic confusion and unnecessarily eliminating foods from a diet. Patients can purchase kits on the Internet for $49.95 that purport to test blood for 10 food, animal, environmental, and inhalant allergens, the results of which are sent back to patients for them to interpret or to ask their doctors to interpret.
Other blood-test kits selling for $450 on the Internet claim to test for IgG antibodies toward foods and additives, even thought IgG antibodies indicate exposure to products, not allergy, and may be a marker for food tolerance, not intolerance, Dr. Stukus said. A physician in the audience said that some allergists in his community are doing these IgG antibody tests, "so we have to watch ourselves, too," he said.
• No milk, eggs, or nuts for babies: Changes in recommendations over the years have contributed to the myth that highly allergenic food such as milk, eggs, or nuts should be avoided by infants until ages 1, 2, or 3 years. The most current recommendations from the American Academy of Pediatrics say that there’s no evidence to support avoiding highly allergenic foods past 4-6 months of age (Pediatrics 2008;121:183-91).
Some evidence is emerging from recent trials that early introduction of highly allergenic foods may promote tolerance, but "if they have a sibling with a peanut allergy, it makes sense to do IgE testing before peanut introduction," he said.
• Artificial dye: Despite controversy around artificial food coloring since the 1950s and around food additives in the 1970s, there is no scientific evidence to support a link between exposure to artificial dye or coloring and IgE-mediated allergic reactions, and there are no skin test extracts or serum-specific IgE tests to test for artificial dye allergy. On the contrary, many studies have found no associations.
There is some evidence, however, that an additive-free diet may improve symptoms of attention-deficit/hyperactivity disorder in a small subset of children (Clin. Pediatr. 2011;50:279-93) And rare cases of anaphylaxis have been reported in reaction to carmine, a natural red coloring derived from dried insects that is commonly used in cosmetics, but not in reaction to artificial dye.
• Egg in vaccines: Dr. Stukus handled a recent consultation in which "they were refusing to give MMR vaccine to someone with egg allergy. So, there is still a lot of confusion over this," he said.
MMR vaccine is safe for anyone with a history of egg allergy, with no testing or allergy referral required, he said. Influenza vaccine also can be given safely to egg-allergic patients, dozens of trials and guidelines conclude, with some differences in recommendations, he said. The Joint Council of Allergy, Asthma, and Immunology says there’s no need for a waiting period or referral to an allergy specialist, while the Centers for Disease Control and Prevention and the American Academy of Pediatrics recommend 30 minutes of observation for egg-allergic patients who receive influenza vaccine and referral to an allergist if there’s a history of anaphylaxis to egg. Egg-free influenza vaccine is a relatively new alternative.
Vaccine for yellow fever or rabies is contraindicated in patients with allergy to egg, but there are tests and procedures that may allow these vaccinations in a graded manner in some patients. Egg-free alternatives to rabies vaccine also are an alternative. Gelatin in both of these vaccines can cause allergic reactions, so evaluate gelatin-hypersensitive patients before vaccinating.
• Shellfish, iodine, and radiocontrast media: Surveys suggest that a majority of radiologists and cardiologists routinely ask patients about shellfish allergy before administering iodinated contrast media, even though iodine is not an allergen, Dr. Stukus said. This myth seems to have originated from a 1975 study in which patients with any kind of reported allergy were twice as likely to react to contrast media (Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1975;124:145-52). Reports of seafood allergy in 15% of patients were associated with reaction to contrast media, but so were reported egg, milk, or chocolate allergy, each in 15% of patients. "Have any of you ever asked patients if they have a chocolate allergy before irradiating them?" Dr. Stukus asked.
Reactions to radiocontrast media with high osmolality agents are common, however, affecting 5%-12% of patients, with elevated risk in patients with atopy. Premedication regimens for patients with a previous reaction to radiocontrast media can lower the risk to less than 1%.
• Skin testing: The idea that skin testing is unreliable until 2, 3, or 5 years of age is sheer myth, but an ongoing one. "I had one of my colleagues say this to me 2 weeks ago," Dr. Stukus said. Skin testing is reliable at any age and can accurately assess for the presence of specific IgE, he said.
• Penicillin allergy: Adverse reactions to antibiotics are very common, but true allergic reactions are uncommon. Approximately 10% of people in general say they are allergic to penicillin, but fewer than 10% of those will have a positive skin test or symptoms if challenged. "If patients get labeled allergic" to penicillin "on their chart, that follows them forever" and makes them more likely to use less-effective, more-toxic, costlier antibiotic alternatives, Dr. Stukus said. "We can improve their lives by proving they don’t have it and taking this label off their chart."
• Gluten: Eating gluten "is currently being blamed for the ails of humanity," largely driven by companies with products to sell – so be prepared to talk about this with patients with self-diagnosed gluten allergy, Dr. Stukus said. IgE-mediated hypersensitivity reactions can occur toward wheat, rye, or barley, but not to gluten. Celiac disease is an autoimmune condition (not IgE-mediated hypersensitivity) that improves with a gluten-free diet. IgE-mediated hypersensitivity to gluten is very uncommon, but patients more commonly report having "gluten sensitivity" and GI symptoms after eating foods with gluten. That’s a poorly defined condition that’s hard to prove. A double-blind, placebo-controlled challenge is the only available method of diagnosing gluten sensitivity.
• Mold: Mold is everywhere and can cause real disease in susceptible persons, but mycotoxins rarely cause disease unless ingested in large quantities. Most health problems attributed to mold exposure are exaggerated, with no scientific basis or supportive evidence, Dr. Stukus said. But "hysteria" around mold has been a boon to some lawyers and companies that sell air purifiers and other detoxification equipment. Know your approach to identifying mold allergy, and be straightforward with patients, he advised.
Dr. Stukus reported having no financial disclosures.
On Twitter @sherryboschert
SAN DIEGO – There’s no such thing as a hypoallergenic dog. Blood tests for sale on the Internet won’t identify a child’s allergies. And parents don’t have to wait until a child is 1, 2 or 3 years of age to introduce dietary milk, eggs, or nuts.
These are some of the facts that physicians need to know in order to counter common myths about allergy, Dr. David R. Stukus said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Patients aren’t the only ones who need enlightening. Too many physicians still believe some of the myths listed below, said Dr. Stukus of the department of pediatrics at Nationwide Children’s Hospital and Ohio State University, Columbus:
• Hypoallergenic pets: "There’s a lot of false advertising" by companies marketing supposedly hypoallergenic pets, some selling cats for $7,000-$28,000 or dogs for $16,000, he said. While some of these animals have been bred to produce fewer major allergens from their saliva, sebaceous glands, or perianal glands, they still produce minor allergens that cause clinical symptoms in sensitized people.
• Blood testing: Allergen-specific serum IgE testing is not a reliable screen for allergy, and often leads to misinterpretation and false-positive results – which in turn lead to diagnostic confusion and unnecessarily eliminating foods from a diet. Patients can purchase kits on the Internet for $49.95 that purport to test blood for 10 food, animal, environmental, and inhalant allergens, the results of which are sent back to patients for them to interpret or to ask their doctors to interpret.
Other blood-test kits selling for $450 on the Internet claim to test for IgG antibodies toward foods and additives, even thought IgG antibodies indicate exposure to products, not allergy, and may be a marker for food tolerance, not intolerance, Dr. Stukus said. A physician in the audience said that some allergists in his community are doing these IgG antibody tests, "so we have to watch ourselves, too," he said.
• No milk, eggs, or nuts for babies: Changes in recommendations over the years have contributed to the myth that highly allergenic food such as milk, eggs, or nuts should be avoided by infants until ages 1, 2, or 3 years. The most current recommendations from the American Academy of Pediatrics say that there’s no evidence to support avoiding highly allergenic foods past 4-6 months of age (Pediatrics 2008;121:183-91).
Some evidence is emerging from recent trials that early introduction of highly allergenic foods may promote tolerance, but "if they have a sibling with a peanut allergy, it makes sense to do IgE testing before peanut introduction," he said.
• Artificial dye: Despite controversy around artificial food coloring since the 1950s and around food additives in the 1970s, there is no scientific evidence to support a link between exposure to artificial dye or coloring and IgE-mediated allergic reactions, and there are no skin test extracts or serum-specific IgE tests to test for artificial dye allergy. On the contrary, many studies have found no associations.
There is some evidence, however, that an additive-free diet may improve symptoms of attention-deficit/hyperactivity disorder in a small subset of children (Clin. Pediatr. 2011;50:279-93) And rare cases of anaphylaxis have been reported in reaction to carmine, a natural red coloring derived from dried insects that is commonly used in cosmetics, but not in reaction to artificial dye.
• Egg in vaccines: Dr. Stukus handled a recent consultation in which "they were refusing to give MMR vaccine to someone with egg allergy. So, there is still a lot of confusion over this," he said.
MMR vaccine is safe for anyone with a history of egg allergy, with no testing or allergy referral required, he said. Influenza vaccine also can be given safely to egg-allergic patients, dozens of trials and guidelines conclude, with some differences in recommendations, he said. The Joint Council of Allergy, Asthma, and Immunology says there’s no need for a waiting period or referral to an allergy specialist, while the Centers for Disease Control and Prevention and the American Academy of Pediatrics recommend 30 minutes of observation for egg-allergic patients who receive influenza vaccine and referral to an allergist if there’s a history of anaphylaxis to egg. Egg-free influenza vaccine is a relatively new alternative.
Vaccine for yellow fever or rabies is contraindicated in patients with allergy to egg, but there are tests and procedures that may allow these vaccinations in a graded manner in some patients. Egg-free alternatives to rabies vaccine also are an alternative. Gelatin in both of these vaccines can cause allergic reactions, so evaluate gelatin-hypersensitive patients before vaccinating.
• Shellfish, iodine, and radiocontrast media: Surveys suggest that a majority of radiologists and cardiologists routinely ask patients about shellfish allergy before administering iodinated contrast media, even though iodine is not an allergen, Dr. Stukus said. This myth seems to have originated from a 1975 study in which patients with any kind of reported allergy were twice as likely to react to contrast media (Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1975;124:145-52). Reports of seafood allergy in 15% of patients were associated with reaction to contrast media, but so were reported egg, milk, or chocolate allergy, each in 15% of patients. "Have any of you ever asked patients if they have a chocolate allergy before irradiating them?" Dr. Stukus asked.
Reactions to radiocontrast media with high osmolality agents are common, however, affecting 5%-12% of patients, with elevated risk in patients with atopy. Premedication regimens for patients with a previous reaction to radiocontrast media can lower the risk to less than 1%.
• Skin testing: The idea that skin testing is unreliable until 2, 3, or 5 years of age is sheer myth, but an ongoing one. "I had one of my colleagues say this to me 2 weeks ago," Dr. Stukus said. Skin testing is reliable at any age and can accurately assess for the presence of specific IgE, he said.
• Penicillin allergy: Adverse reactions to antibiotics are very common, but true allergic reactions are uncommon. Approximately 10% of people in general say they are allergic to penicillin, but fewer than 10% of those will have a positive skin test or symptoms if challenged. "If patients get labeled allergic" to penicillin "on their chart, that follows them forever" and makes them more likely to use less-effective, more-toxic, costlier antibiotic alternatives, Dr. Stukus said. "We can improve their lives by proving they don’t have it and taking this label off their chart."
• Gluten: Eating gluten "is currently being blamed for the ails of humanity," largely driven by companies with products to sell – so be prepared to talk about this with patients with self-diagnosed gluten allergy, Dr. Stukus said. IgE-mediated hypersensitivity reactions can occur toward wheat, rye, or barley, but not to gluten. Celiac disease is an autoimmune condition (not IgE-mediated hypersensitivity) that improves with a gluten-free diet. IgE-mediated hypersensitivity to gluten is very uncommon, but patients more commonly report having "gluten sensitivity" and GI symptoms after eating foods with gluten. That’s a poorly defined condition that’s hard to prove. A double-blind, placebo-controlled challenge is the only available method of diagnosing gluten sensitivity.
• Mold: Mold is everywhere and can cause real disease in susceptible persons, but mycotoxins rarely cause disease unless ingested in large quantities. Most health problems attributed to mold exposure are exaggerated, with no scientific basis or supportive evidence, Dr. Stukus said. But "hysteria" around mold has been a boon to some lawyers and companies that sell air purifiers and other detoxification equipment. Know your approach to identifying mold allergy, and be straightforward with patients, he advised.
Dr. Stukus reported having no financial disclosures.
On Twitter @sherryboschert
SAN DIEGO – There’s no such thing as a hypoallergenic dog. Blood tests for sale on the Internet won’t identify a child’s allergies. And parents don’t have to wait until a child is 1, 2 or 3 years of age to introduce dietary milk, eggs, or nuts.
These are some of the facts that physicians need to know in order to counter common myths about allergy, Dr. David R. Stukus said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Patients aren’t the only ones who need enlightening. Too many physicians still believe some of the myths listed below, said Dr. Stukus of the department of pediatrics at Nationwide Children’s Hospital and Ohio State University, Columbus:
• Hypoallergenic pets: "There’s a lot of false advertising" by companies marketing supposedly hypoallergenic pets, some selling cats for $7,000-$28,000 or dogs for $16,000, he said. While some of these animals have been bred to produce fewer major allergens from their saliva, sebaceous glands, or perianal glands, they still produce minor allergens that cause clinical symptoms in sensitized people.
• Blood testing: Allergen-specific serum IgE testing is not a reliable screen for allergy, and often leads to misinterpretation and false-positive results – which in turn lead to diagnostic confusion and unnecessarily eliminating foods from a diet. Patients can purchase kits on the Internet for $49.95 that purport to test blood for 10 food, animal, environmental, and inhalant allergens, the results of which are sent back to patients for them to interpret or to ask their doctors to interpret.
Other blood-test kits selling for $450 on the Internet claim to test for IgG antibodies toward foods and additives, even thought IgG antibodies indicate exposure to products, not allergy, and may be a marker for food tolerance, not intolerance, Dr. Stukus said. A physician in the audience said that some allergists in his community are doing these IgG antibody tests, "so we have to watch ourselves, too," he said.
• No milk, eggs, or nuts for babies: Changes in recommendations over the years have contributed to the myth that highly allergenic food such as milk, eggs, or nuts should be avoided by infants until ages 1, 2, or 3 years. The most current recommendations from the American Academy of Pediatrics say that there’s no evidence to support avoiding highly allergenic foods past 4-6 months of age (Pediatrics 2008;121:183-91).
Some evidence is emerging from recent trials that early introduction of highly allergenic foods may promote tolerance, but "if they have a sibling with a peanut allergy, it makes sense to do IgE testing before peanut introduction," he said.
• Artificial dye: Despite controversy around artificial food coloring since the 1950s and around food additives in the 1970s, there is no scientific evidence to support a link between exposure to artificial dye or coloring and IgE-mediated allergic reactions, and there are no skin test extracts or serum-specific IgE tests to test for artificial dye allergy. On the contrary, many studies have found no associations.
There is some evidence, however, that an additive-free diet may improve symptoms of attention-deficit/hyperactivity disorder in a small subset of children (Clin. Pediatr. 2011;50:279-93) And rare cases of anaphylaxis have been reported in reaction to carmine, a natural red coloring derived from dried insects that is commonly used in cosmetics, but not in reaction to artificial dye.
• Egg in vaccines: Dr. Stukus handled a recent consultation in which "they were refusing to give MMR vaccine to someone with egg allergy. So, there is still a lot of confusion over this," he said.
MMR vaccine is safe for anyone with a history of egg allergy, with no testing or allergy referral required, he said. Influenza vaccine also can be given safely to egg-allergic patients, dozens of trials and guidelines conclude, with some differences in recommendations, he said. The Joint Council of Allergy, Asthma, and Immunology says there’s no need for a waiting period or referral to an allergy specialist, while the Centers for Disease Control and Prevention and the American Academy of Pediatrics recommend 30 minutes of observation for egg-allergic patients who receive influenza vaccine and referral to an allergist if there’s a history of anaphylaxis to egg. Egg-free influenza vaccine is a relatively new alternative.
Vaccine for yellow fever or rabies is contraindicated in patients with allergy to egg, but there are tests and procedures that may allow these vaccinations in a graded manner in some patients. Egg-free alternatives to rabies vaccine also are an alternative. Gelatin in both of these vaccines can cause allergic reactions, so evaluate gelatin-hypersensitive patients before vaccinating.
• Shellfish, iodine, and radiocontrast media: Surveys suggest that a majority of radiologists and cardiologists routinely ask patients about shellfish allergy before administering iodinated contrast media, even though iodine is not an allergen, Dr. Stukus said. This myth seems to have originated from a 1975 study in which patients with any kind of reported allergy were twice as likely to react to contrast media (Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1975;124:145-52). Reports of seafood allergy in 15% of patients were associated with reaction to contrast media, but so were reported egg, milk, or chocolate allergy, each in 15% of patients. "Have any of you ever asked patients if they have a chocolate allergy before irradiating them?" Dr. Stukus asked.
Reactions to radiocontrast media with high osmolality agents are common, however, affecting 5%-12% of patients, with elevated risk in patients with atopy. Premedication regimens for patients with a previous reaction to radiocontrast media can lower the risk to less than 1%.
• Skin testing: The idea that skin testing is unreliable until 2, 3, or 5 years of age is sheer myth, but an ongoing one. "I had one of my colleagues say this to me 2 weeks ago," Dr. Stukus said. Skin testing is reliable at any age and can accurately assess for the presence of specific IgE, he said.
• Penicillin allergy: Adverse reactions to antibiotics are very common, but true allergic reactions are uncommon. Approximately 10% of people in general say they are allergic to penicillin, but fewer than 10% of those will have a positive skin test or symptoms if challenged. "If patients get labeled allergic" to penicillin "on their chart, that follows them forever" and makes them more likely to use less-effective, more-toxic, costlier antibiotic alternatives, Dr. Stukus said. "We can improve their lives by proving they don’t have it and taking this label off their chart."
• Gluten: Eating gluten "is currently being blamed for the ails of humanity," largely driven by companies with products to sell – so be prepared to talk about this with patients with self-diagnosed gluten allergy, Dr. Stukus said. IgE-mediated hypersensitivity reactions can occur toward wheat, rye, or barley, but not to gluten. Celiac disease is an autoimmune condition (not IgE-mediated hypersensitivity) that improves with a gluten-free diet. IgE-mediated hypersensitivity to gluten is very uncommon, but patients more commonly report having "gluten sensitivity" and GI symptoms after eating foods with gluten. That’s a poorly defined condition that’s hard to prove. A double-blind, placebo-controlled challenge is the only available method of diagnosing gluten sensitivity.
• Mold: Mold is everywhere and can cause real disease in susceptible persons, but mycotoxins rarely cause disease unless ingested in large quantities. Most health problems attributed to mold exposure are exaggerated, with no scientific basis or supportive evidence, Dr. Stukus said. But "hysteria" around mold has been a boon to some lawyers and companies that sell air purifiers and other detoxification equipment. Know your approach to identifying mold allergy, and be straightforward with patients, he advised.
Dr. Stukus reported having no financial disclosures.
On Twitter @sherryboschert
AT 2014 AAAAI ANNUAL MEETING
What Puts C difficile Patients at Greater Readmission Risk?
CHICAGO – More and more elderly people are getting infected with Clostridium difficile, and many get readmitted to the hospital after initial treatment for this potentially deadly diarrheal illness. Why?
A large analysis of Medicare data identified risk factors for readmission due to C difficile in the elderly, Dr. Courtney Collins of the University of Massachusetts, Worcester, and her associates reported at the annual Digestive Disease Week.
The first month after discharge from a C difficile hospitalization appears to be a crucial time to think about preventing reinfection. Dr. Collins spoke with us about her findings, and how they will change her clinical practice and, perhaps, yours.
Dr. Collins reported having no disclosures.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – More and more elderly people are getting infected with Clostridium difficile, and many get readmitted to the hospital after initial treatment for this potentially deadly diarrheal illness. Why?
A large analysis of Medicare data identified risk factors for readmission due to C difficile in the elderly, Dr. Courtney Collins of the University of Massachusetts, Worcester, and her associates reported at the annual Digestive Disease Week.
The first month after discharge from a C difficile hospitalization appears to be a crucial time to think about preventing reinfection. Dr. Collins spoke with us about her findings, and how they will change her clinical practice and, perhaps, yours.
Dr. Collins reported having no disclosures.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – More and more elderly people are getting infected with Clostridium difficile, and many get readmitted to the hospital after initial treatment for this potentially deadly diarrheal illness. Why?
A large analysis of Medicare data identified risk factors for readmission due to C difficile in the elderly, Dr. Courtney Collins of the University of Massachusetts, Worcester, and her associates reported at the annual Digestive Disease Week.
The first month after discharge from a C difficile hospitalization appears to be a crucial time to think about preventing reinfection. Dr. Collins spoke with us about her findings, and how they will change her clinical practice and, perhaps, yours.
Dr. Collins reported having no disclosures.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT DDW 2014
What puts C. diff patients at greater readmission risk?
CHICAGO – More and more elderly people are getting infected with Clostridium difficile, and many get readmitted to the hospital after initial treatment for this potentially deadly diarrheal illness. Why?
A large analysis of Medicare data identified risk factors for readmission due to C. difficile in the elderly, Dr. Courtney Collins of the University of Massachusetts, Worcester, and her associates reported at the annual Digestive Disease Week.
The first month after discharge from a C. difficile hospitalization appears to be a crucial time to think about preventing reinfection. Dr. Collins spoke with us about her findings, and how they will change her clinical practice and, perhaps, yours.
Dr. Collins reported having no disclosures.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – More and more elderly people are getting infected with Clostridium difficile, and many get readmitted to the hospital after initial treatment for this potentially deadly diarrheal illness. Why?
A large analysis of Medicare data identified risk factors for readmission due to C. difficile in the elderly, Dr. Courtney Collins of the University of Massachusetts, Worcester, and her associates reported at the annual Digestive Disease Week.
The first month after discharge from a C. difficile hospitalization appears to be a crucial time to think about preventing reinfection. Dr. Collins spoke with us about her findings, and how they will change her clinical practice and, perhaps, yours.
Dr. Collins reported having no disclosures.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – More and more elderly people are getting infected with Clostridium difficile, and many get readmitted to the hospital after initial treatment for this potentially deadly diarrheal illness. Why?
A large analysis of Medicare data identified risk factors for readmission due to C. difficile in the elderly, Dr. Courtney Collins of the University of Massachusetts, Worcester, and her associates reported at the annual Digestive Disease Week.
The first month after discharge from a C. difficile hospitalization appears to be a crucial time to think about preventing reinfection. Dr. Collins spoke with us about her findings, and how they will change her clinical practice and, perhaps, yours.
Dr. Collins reported having no disclosures.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT DDW 2014
VIDEO: Acupuncture beats propofol for endoscopy analgesia
CHICAGO – Endoscopic ultrasound can be a long, uncomfortable, and even painful procedure. But electroacupuncture starting 45 minutes before the procedure significantly decreased the need for drugs to control pain in a prospective, randomized, double-blind, sham-controlled study of 64 patients in Hong Kong.
Dr. Anthony Y. Teoh, who did the endoscopies, and Dr. Wing Wa Leung, the acupuncturist, showed us how they did it in an interview at the annual Digestive Disease Week. Both are of the department of surgery at Chinese University of Hong Kong.
The 32 patients in the electroacupuncture group took a median of only two hits of patient-controlled analgesia with a mean of 0.22 mg/kg propofol, compared with 10 hits for the 32 patients in the control group with a mean of 0.71 mg/kg propofol. Patients rated their pain significantly lower and their satisfaction with the procedure significantly higher in the electroacupuncture group than in the control group, while it made no difference for the endoscopist, whose satisfaction scores were similar between groups. Fifteen patients with acupuncture said they’d be willing to repeat the procedure, compared with eight control patients.
Does that mean electroacupuncture will be coming to your endoscopy suite? Hear what Dr. Teoh and Dr. Leung have to say.
They reported having no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
CHICAGO – Endoscopic ultrasound can be a long, uncomfortable, and even painful procedure. But electroacupuncture starting 45 minutes before the procedure significantly decreased the need for drugs to control pain in a prospective, randomized, double-blind, sham-controlled study of 64 patients in Hong Kong.
Dr. Anthony Y. Teoh, who did the endoscopies, and Dr. Wing Wa Leung, the acupuncturist, showed us how they did it in an interview at the annual Digestive Disease Week. Both are of the department of surgery at Chinese University of Hong Kong.
The 32 patients in the electroacupuncture group took a median of only two hits of patient-controlled analgesia with a mean of 0.22 mg/kg propofol, compared with 10 hits for the 32 patients in the control group with a mean of 0.71 mg/kg propofol. Patients rated their pain significantly lower and their satisfaction with the procedure significantly higher in the electroacupuncture group than in the control group, while it made no difference for the endoscopist, whose satisfaction scores were similar between groups. Fifteen patients with acupuncture said they’d be willing to repeat the procedure, compared with eight control patients.
Does that mean electroacupuncture will be coming to your endoscopy suite? Hear what Dr. Teoh and Dr. Leung have to say.
They reported having no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
CHICAGO – Endoscopic ultrasound can be a long, uncomfortable, and even painful procedure. But electroacupuncture starting 45 minutes before the procedure significantly decreased the need for drugs to control pain in a prospective, randomized, double-blind, sham-controlled study of 64 patients in Hong Kong.
Dr. Anthony Y. Teoh, who did the endoscopies, and Dr. Wing Wa Leung, the acupuncturist, showed us how they did it in an interview at the annual Digestive Disease Week. Both are of the department of surgery at Chinese University of Hong Kong.
The 32 patients in the electroacupuncture group took a median of only two hits of patient-controlled analgesia with a mean of 0.22 mg/kg propofol, compared with 10 hits for the 32 patients in the control group with a mean of 0.71 mg/kg propofol. Patients rated their pain significantly lower and their satisfaction with the procedure significantly higher in the electroacupuncture group than in the control group, while it made no difference for the endoscopist, whose satisfaction scores were similar between groups. Fifteen patients with acupuncture said they’d be willing to repeat the procedure, compared with eight control patients.
Does that mean electroacupuncture will be coming to your endoscopy suite? Hear what Dr. Teoh and Dr. Leung have to say.
They reported having no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
AT DDW 2014