User login
‘Financial toxicity’ from breast cancer is a worldwide phenomenon
Women across the world face high levels of financial burden from breast cancer, a new systematic review and analysis finds. While the burden of the disease is much higher in less-developed countries, about a third of women in Western nations like the United States say the disease has hurt their financial well-being.
When it comes to financial burden, patients with breast cancer are “a highly vulnerable patient population,” said study coauthor Kavitha Ranganathan, MD, of Brigham and Women’s Hospital, Boston, in an interview. “We need to be both strategic and comprehensive with our approach and use evidence-based methods to come up with these comprehensive solutions,” said Dr. Ranganathan, who noted that she’s hearing more from patients who face monetary hurdles.
The findings were published online in JAMA Network Open.
The researchers believe their analysis is the first to attempt to understand financial toxicity (FT) – excessive financial burden – in breast cancer on a global level. This turned out to be a challenge since there’s no standard way to measure FT.
One approach is to look at financial burden in terms of whether patients are suffering from “catastrophic expenditure,” Dr. Ranganathan said. “That’s what the World Bank and other top health and economic organizations have focused on. It means that the cost of care and – whatever it takes to get care – exceeds 10% of total annual household income.”
Another approach is more subjective and based on patient-reported outcomes, she said: “Are patients having to forgo basic subsistence needs like rent and food?”
For the report, researchers analyzed studies that use both approaches to measure FT from breast cancer. The studies came from high-income countries (n = 24, including 19 from the United States) and middle- and low-income countries (n = 10), and ranged in size from 5 to 2,445 subjects.
The analyzed studies were a range of cross-sectional (n = 26), prospective (n = 7), and retrospective designs (n = 1).
The authors pooled the data from 18 studies and estimated that the rate of patients with FT was 35.3% (14 studies, 27.3%-44.4%) in high-income countries and 78.8% (4 studies, 60.4%-90.0%) in the other countries.
The researchers also conducted a separate pooled analysis of only the U.S. studies (n = 11). It found that 34% (27%-43%) of subjects reported FT. The researchers also conducted a new analysis of Canada-only studies (n = 2) and found that 19% (9%-35%) reported FT.
The researchers weren’t able to provide insight into trends in FT in the United States prior to the period of the studies (2014-2021). But raw numbers suggest the percentage of patients facing financial challenges rose over that time, suggesting a possible increase in burden.
Previous research has suggested that breast cancer poses a higher financial burden than other chronic conditions. “Breast cancer care in particular may be associated with high FT given the need for screening and diagnosis, multidisciplinary care, and longitudinal follow-up,” the researchers write. They add that “notably, gender also affects financial security.”
As for limitations, the researchers report that they only analyzed studies in English, and there was a wide variation in approaches used to analyze FT. The analysis “did not account for different health care systems or control for health care–dedicated gross domestic product,” meaning that there’s no way to know for sure that rates were lower in nations with universal health care.
How could the new findings be useful? “They’re eye-opening for health policymakers. Whenever they see these numbers, they will say, ‘Wow, it is really a problem,’ and they’ll start thinking about solutions,” said study coauthor Rania A. Mekary, PhD, MSc, MSc, of Massachusetts College of Pharmacy and Health Sciences in Boston. “When you give them evidence-based data, then they will take it more seriously.”
The researchers call for interventions in several areas including education about early diagnosis and treatment of breast cancer, expansion of health care coverage, programs to help with nonmedical costs, and better resources for breast cancer care.
In an interview, Mary C. Politi, PhD, of Washington University, St. Louis, said the new report is useful “because it examines financial hardship internationally. Some people wonder whether financial hardship is a U.S. problem because of our health care system, which often relies on insurance and a lot of cost-sharing between insurance and patients. However, financial toxicity is prevalent across countries.”
And, she said, “the study is also useful because it encourages us to measure financial hardship and burden in a more uniform way so we can better compare and pool studies.”
Dr. Politi noted that there are ways to help patients now. “Most hospitals and health centers have staff who can talk to patients about their bills. Sometimes, a payment plan can be set up to space out payments,” she said. “Health care teams can try to consolidate care for patients on the same day to reduce parking expenses or time off for work or child care. Sometimes, changing to less expensive but effective generic medications is an option.”
The study authors received support from the National Cancer Institute, the United Nations Institute for Training and Research, the Global Surgery Foundation, the Harvard Global Health Institute, the Connors Center for Women’s Health and Gender Biology, the Center for Surgery and Public Health, and the National Endowment for Plastic Surgery. Dr. Ranganathan and Dr. Mekary report no disclosures. One coauthor reported a patent (BREAST-Q) and codevelopment of QPROMS, owned by Memorial Sloan Kettering Cancer Center. Another author reports salary support from Blue Cross Blue Shield of Michigan through the collaborative quality initiative known as Michigan Social Health Interventions to Eliminate Disparities. Dr. Politi has no disclosures.
Women across the world face high levels of financial burden from breast cancer, a new systematic review and analysis finds. While the burden of the disease is much higher in less-developed countries, about a third of women in Western nations like the United States say the disease has hurt their financial well-being.
When it comes to financial burden, patients with breast cancer are “a highly vulnerable patient population,” said study coauthor Kavitha Ranganathan, MD, of Brigham and Women’s Hospital, Boston, in an interview. “We need to be both strategic and comprehensive with our approach and use evidence-based methods to come up with these comprehensive solutions,” said Dr. Ranganathan, who noted that she’s hearing more from patients who face monetary hurdles.
The findings were published online in JAMA Network Open.
The researchers believe their analysis is the first to attempt to understand financial toxicity (FT) – excessive financial burden – in breast cancer on a global level. This turned out to be a challenge since there’s no standard way to measure FT.
One approach is to look at financial burden in terms of whether patients are suffering from “catastrophic expenditure,” Dr. Ranganathan said. “That’s what the World Bank and other top health and economic organizations have focused on. It means that the cost of care and – whatever it takes to get care – exceeds 10% of total annual household income.”
Another approach is more subjective and based on patient-reported outcomes, she said: “Are patients having to forgo basic subsistence needs like rent and food?”
For the report, researchers analyzed studies that use both approaches to measure FT from breast cancer. The studies came from high-income countries (n = 24, including 19 from the United States) and middle- and low-income countries (n = 10), and ranged in size from 5 to 2,445 subjects.
The analyzed studies were a range of cross-sectional (n = 26), prospective (n = 7), and retrospective designs (n = 1).
The authors pooled the data from 18 studies and estimated that the rate of patients with FT was 35.3% (14 studies, 27.3%-44.4%) in high-income countries and 78.8% (4 studies, 60.4%-90.0%) in the other countries.
The researchers also conducted a separate pooled analysis of only the U.S. studies (n = 11). It found that 34% (27%-43%) of subjects reported FT. The researchers also conducted a new analysis of Canada-only studies (n = 2) and found that 19% (9%-35%) reported FT.
The researchers weren’t able to provide insight into trends in FT in the United States prior to the period of the studies (2014-2021). But raw numbers suggest the percentage of patients facing financial challenges rose over that time, suggesting a possible increase in burden.
Previous research has suggested that breast cancer poses a higher financial burden than other chronic conditions. “Breast cancer care in particular may be associated with high FT given the need for screening and diagnosis, multidisciplinary care, and longitudinal follow-up,” the researchers write. They add that “notably, gender also affects financial security.”
As for limitations, the researchers report that they only analyzed studies in English, and there was a wide variation in approaches used to analyze FT. The analysis “did not account for different health care systems or control for health care–dedicated gross domestic product,” meaning that there’s no way to know for sure that rates were lower in nations with universal health care.
How could the new findings be useful? “They’re eye-opening for health policymakers. Whenever they see these numbers, they will say, ‘Wow, it is really a problem,’ and they’ll start thinking about solutions,” said study coauthor Rania A. Mekary, PhD, MSc, MSc, of Massachusetts College of Pharmacy and Health Sciences in Boston. “When you give them evidence-based data, then they will take it more seriously.”
The researchers call for interventions in several areas including education about early diagnosis and treatment of breast cancer, expansion of health care coverage, programs to help with nonmedical costs, and better resources for breast cancer care.
In an interview, Mary C. Politi, PhD, of Washington University, St. Louis, said the new report is useful “because it examines financial hardship internationally. Some people wonder whether financial hardship is a U.S. problem because of our health care system, which often relies on insurance and a lot of cost-sharing between insurance and patients. However, financial toxicity is prevalent across countries.”
And, she said, “the study is also useful because it encourages us to measure financial hardship and burden in a more uniform way so we can better compare and pool studies.”
Dr. Politi noted that there are ways to help patients now. “Most hospitals and health centers have staff who can talk to patients about their bills. Sometimes, a payment plan can be set up to space out payments,” she said. “Health care teams can try to consolidate care for patients on the same day to reduce parking expenses or time off for work or child care. Sometimes, changing to less expensive but effective generic medications is an option.”
The study authors received support from the National Cancer Institute, the United Nations Institute for Training and Research, the Global Surgery Foundation, the Harvard Global Health Institute, the Connors Center for Women’s Health and Gender Biology, the Center for Surgery and Public Health, and the National Endowment for Plastic Surgery. Dr. Ranganathan and Dr. Mekary report no disclosures. One coauthor reported a patent (BREAST-Q) and codevelopment of QPROMS, owned by Memorial Sloan Kettering Cancer Center. Another author reports salary support from Blue Cross Blue Shield of Michigan through the collaborative quality initiative known as Michigan Social Health Interventions to Eliminate Disparities. Dr. Politi has no disclosures.
Women across the world face high levels of financial burden from breast cancer, a new systematic review and analysis finds. While the burden of the disease is much higher in less-developed countries, about a third of women in Western nations like the United States say the disease has hurt their financial well-being.
When it comes to financial burden, patients with breast cancer are “a highly vulnerable patient population,” said study coauthor Kavitha Ranganathan, MD, of Brigham and Women’s Hospital, Boston, in an interview. “We need to be both strategic and comprehensive with our approach and use evidence-based methods to come up with these comprehensive solutions,” said Dr. Ranganathan, who noted that she’s hearing more from patients who face monetary hurdles.
The findings were published online in JAMA Network Open.
The researchers believe their analysis is the first to attempt to understand financial toxicity (FT) – excessive financial burden – in breast cancer on a global level. This turned out to be a challenge since there’s no standard way to measure FT.
One approach is to look at financial burden in terms of whether patients are suffering from “catastrophic expenditure,” Dr. Ranganathan said. “That’s what the World Bank and other top health and economic organizations have focused on. It means that the cost of care and – whatever it takes to get care – exceeds 10% of total annual household income.”
Another approach is more subjective and based on patient-reported outcomes, she said: “Are patients having to forgo basic subsistence needs like rent and food?”
For the report, researchers analyzed studies that use both approaches to measure FT from breast cancer. The studies came from high-income countries (n = 24, including 19 from the United States) and middle- and low-income countries (n = 10), and ranged in size from 5 to 2,445 subjects.
The analyzed studies were a range of cross-sectional (n = 26), prospective (n = 7), and retrospective designs (n = 1).
The authors pooled the data from 18 studies and estimated that the rate of patients with FT was 35.3% (14 studies, 27.3%-44.4%) in high-income countries and 78.8% (4 studies, 60.4%-90.0%) in the other countries.
The researchers also conducted a separate pooled analysis of only the U.S. studies (n = 11). It found that 34% (27%-43%) of subjects reported FT. The researchers also conducted a new analysis of Canada-only studies (n = 2) and found that 19% (9%-35%) reported FT.
The researchers weren’t able to provide insight into trends in FT in the United States prior to the period of the studies (2014-2021). But raw numbers suggest the percentage of patients facing financial challenges rose over that time, suggesting a possible increase in burden.
Previous research has suggested that breast cancer poses a higher financial burden than other chronic conditions. “Breast cancer care in particular may be associated with high FT given the need for screening and diagnosis, multidisciplinary care, and longitudinal follow-up,” the researchers write. They add that “notably, gender also affects financial security.”
As for limitations, the researchers report that they only analyzed studies in English, and there was a wide variation in approaches used to analyze FT. The analysis “did not account for different health care systems or control for health care–dedicated gross domestic product,” meaning that there’s no way to know for sure that rates were lower in nations with universal health care.
How could the new findings be useful? “They’re eye-opening for health policymakers. Whenever they see these numbers, they will say, ‘Wow, it is really a problem,’ and they’ll start thinking about solutions,” said study coauthor Rania A. Mekary, PhD, MSc, MSc, of Massachusetts College of Pharmacy and Health Sciences in Boston. “When you give them evidence-based data, then they will take it more seriously.”
The researchers call for interventions in several areas including education about early diagnosis and treatment of breast cancer, expansion of health care coverage, programs to help with nonmedical costs, and better resources for breast cancer care.
In an interview, Mary C. Politi, PhD, of Washington University, St. Louis, said the new report is useful “because it examines financial hardship internationally. Some people wonder whether financial hardship is a U.S. problem because of our health care system, which often relies on insurance and a lot of cost-sharing between insurance and patients. However, financial toxicity is prevalent across countries.”
And, she said, “the study is also useful because it encourages us to measure financial hardship and burden in a more uniform way so we can better compare and pool studies.”
Dr. Politi noted that there are ways to help patients now. “Most hospitals and health centers have staff who can talk to patients about their bills. Sometimes, a payment plan can be set up to space out payments,” she said. “Health care teams can try to consolidate care for patients on the same day to reduce parking expenses or time off for work or child care. Sometimes, changing to less expensive but effective generic medications is an option.”
The study authors received support from the National Cancer Institute, the United Nations Institute for Training and Research, the Global Surgery Foundation, the Harvard Global Health Institute, the Connors Center for Women’s Health and Gender Biology, the Center for Surgery and Public Health, and the National Endowment for Plastic Surgery. Dr. Ranganathan and Dr. Mekary report no disclosures. One coauthor reported a patent (BREAST-Q) and codevelopment of QPROMS, owned by Memorial Sloan Kettering Cancer Center. Another author reports salary support from Blue Cross Blue Shield of Michigan through the collaborative quality initiative known as Michigan Social Health Interventions to Eliminate Disparities. Dr. Politi has no disclosures.
FROM JAMA NETWORK OPEN
Progress in breast cancer screening over the past 50 years: A remarkable story, but still work to do
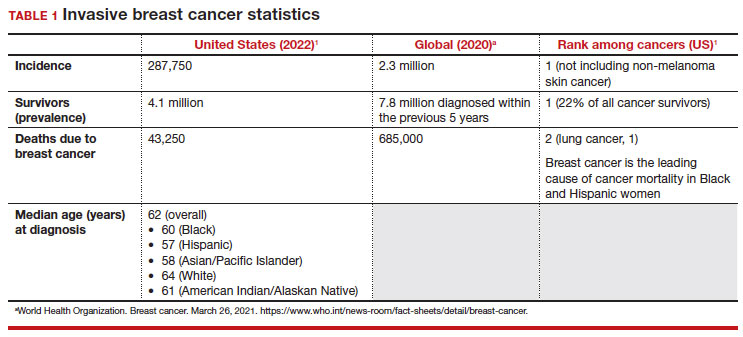
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3
While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
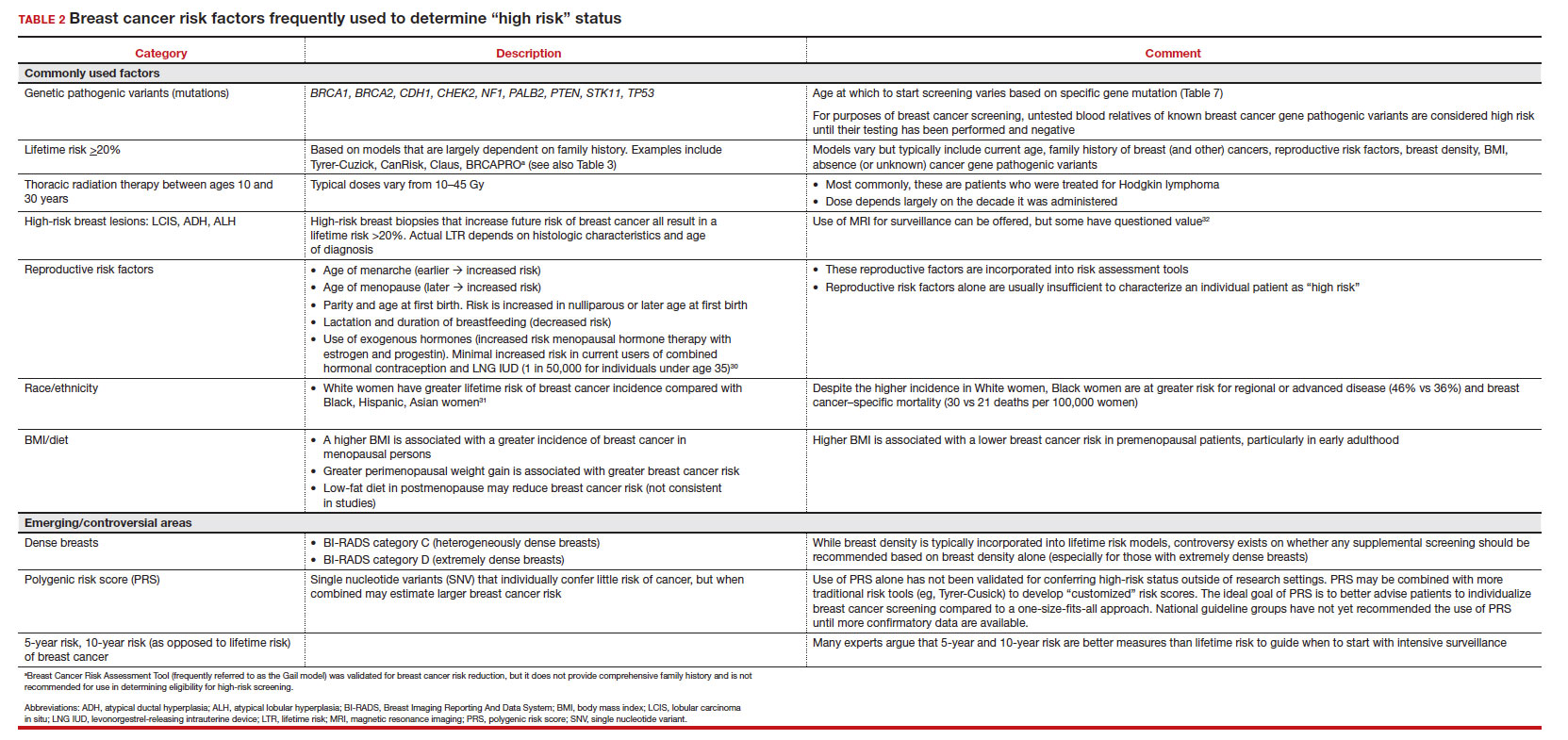
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.
Breast cancer risk assessment
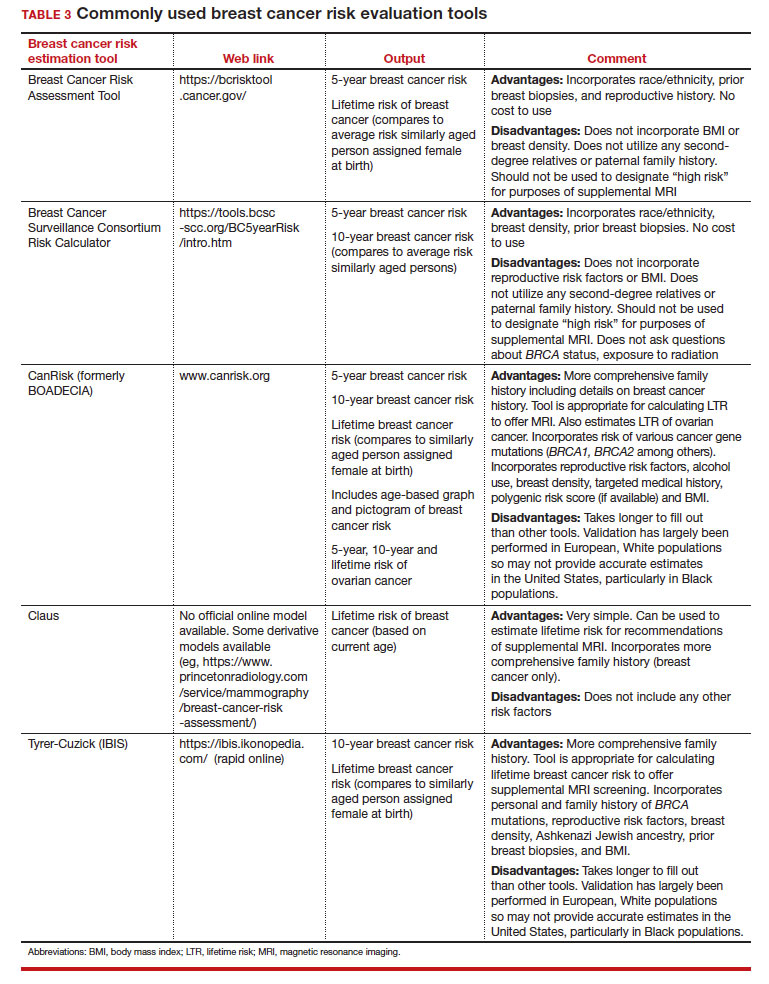
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7
Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10
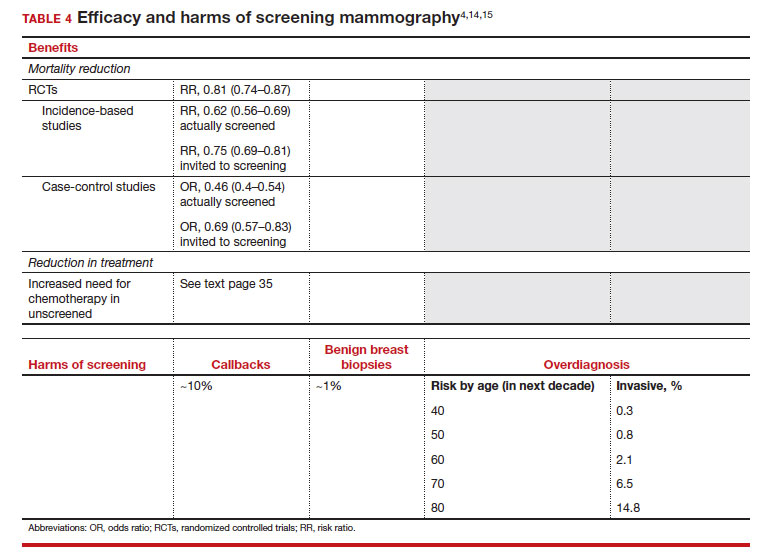
With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).
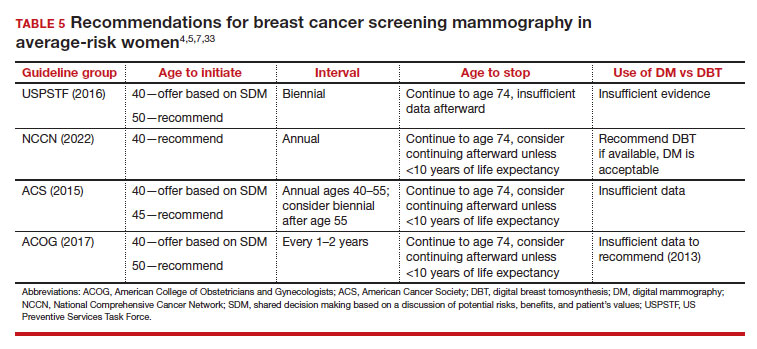
Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.
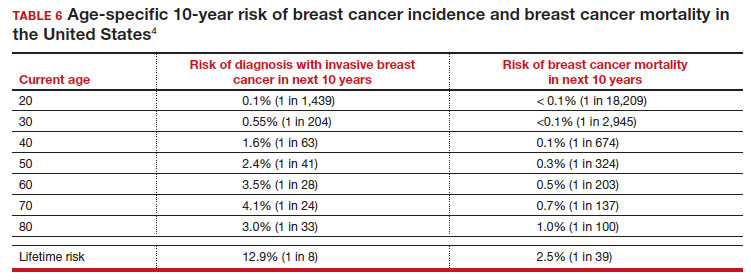
For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.
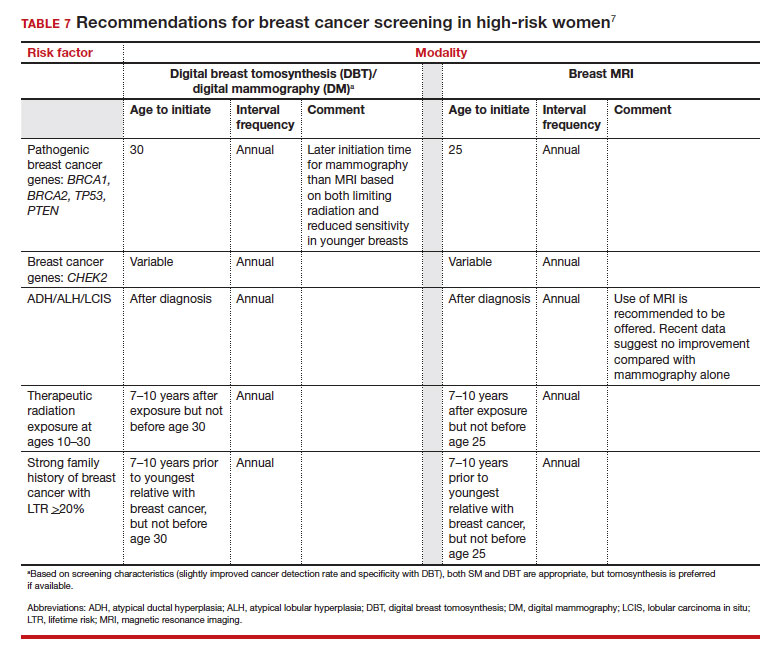
Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
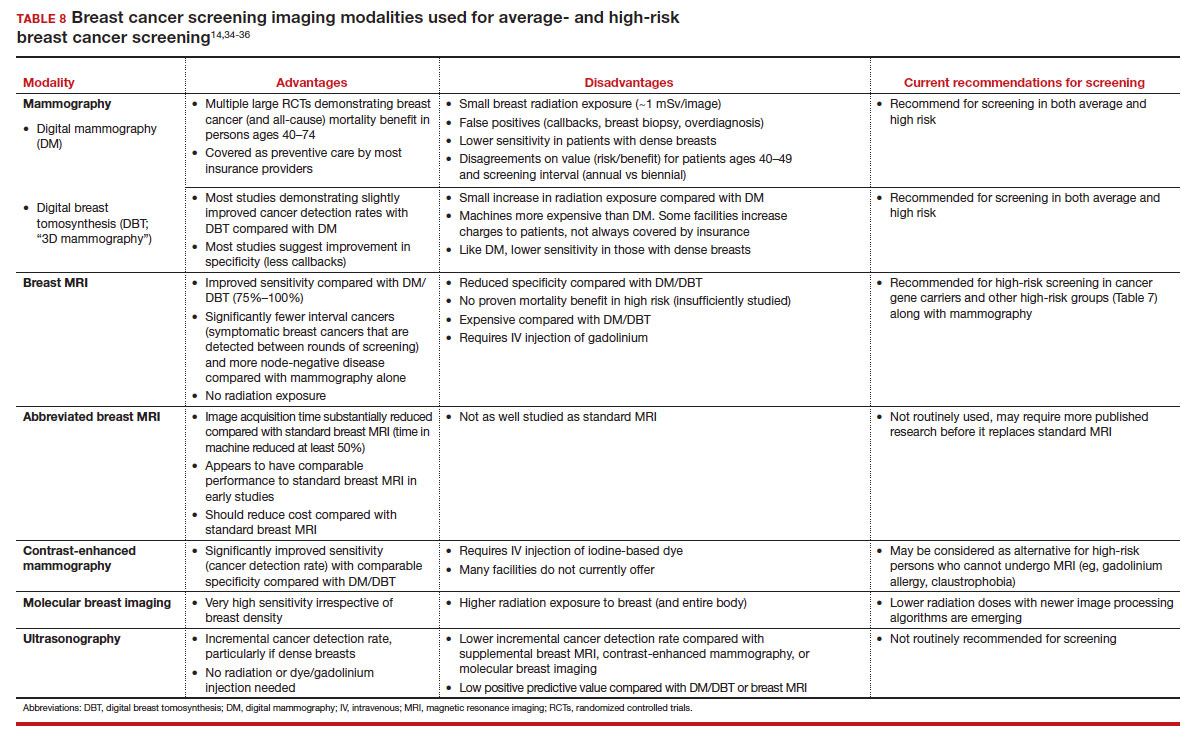
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).
Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3

While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.

Breast cancer risk assessment
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7

Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10

With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).

Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.

For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.

Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).

Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3

While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.

Breast cancer risk assessment
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7

Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10

With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).

Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.

For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.

Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).

Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
Omit radiation in older women with low-risk, ER+ breast cancer
say researchers reporting 10-year outcomes from the large phase 3 trial known as PRIME II.
“Our trial provides robust evidence indicating that irradiation can be safely omitted in women 65 years of age or older who have grade 1 or 2 ER-high cancers treated by breast-conserving therapy, provided that they receive 5 years of adjuvant endocrine therapy,” concluded investigators led by Ian Kunkler, MB, a clinical oncology professor at the University of Edinburgh.
The trial randomly assigned 1,326 women who had undergone a lumpectomy to either whole-breast irradiation or no radiation on a background of tamoxifen.
The incidence of local recurrence was lower with radiation (0.9% vs. 9.5%), but there was no significant difference in distant metastases or breast cancer–specific or overall survival.
The findings will “help clinicians guide older patients on whether this particular aspect of early breast cancer treatment can be omitted,” Dr. Kunkler said in a press release. Radiation carries risks of heart and lung damage, and these results show that skipping it does not increase the odds of dying from breast cancer.
The new study was published in the New England Journal of Medicine.
“Any doubt that radiotherapy cannot be omitted in women” who meet the criteria “can be put to rest,” commented breast radiation oncologists Alice Ho, MD, of Duke University in Durham, N.C., and Jennifer Bellon, MD, of Harvard Medical School, Boston, in an accompanying editorial.
Clinical guidelines already support omitting radiation therapy in older women with low-risk tumors treated with lumpectomy and endocrine therapy, but the move has been controversial owing to a lack of long-term data, and use of radiation for such women remains common in the United States, the investigators explain.
The “highly anticipated” results for 10-year outcomes from this trial should help address that issue, as well as “the long-standing problem of overtreatment in older women with low-risk breast cancer,” the editorialists comment.
Study details
PRIME II was conducted from 2003 to 2009 mainly in the United Kingdom. Participants were aged 65 years or older and had T1 or T2 ER-positive tumors no larger than 3 cm and were without nodal involvement.
Following lumpectomies with clear margins, the women underwent endocrine therapy; the investigators recommended tamoxifen at 20 mg/day for 5 years.
Women who were randomly assigned to radiation also received 40-50 Gy of whole-breast irradiation in 20-25 fractions over 3-5 weeks.
At 10 years, 1.6% of women in the no-radiation arm had distant metastases as their first recurrence vs. 3% of women who underwent radiation.
Ten-year breast cancer–specific survival was 97.9% with radiation and 97.4% with no radiation. Ten-year overall survival was 80.7% in the radiotherapy arm vs. 80.8% in the no-radiotherapy group.
In addition, the recurrence rate was lower after radiation. The investigators suggest that lower adherence to endocrine therapy and lower levels of ER positivity increased the risk of local recurrence among women who didn’t receive radiation.
Almost 10% of the women who did not receive radiation had local recurrences by 10 years, but the investigators note that if tumors do recur locally, women still have the option of a second lumpectomy, and if they so choose, they can then receive radiation, so local recurrence “does not necessarily mean loss of the breast.”
PRIME II was funded by the Scottish Government’s chief scientist office and the Breast Cancer Institute at Western General Hospital, Edinburgh. Dr. Kunkler reported no conflicts of interest. A coauthor has acted as a speaker, adviser, and/or researcher for many companies, including Hoffmann-La Roche, Exact Sciences, and Eli Lilly. Dr. Ho reported grants from and/or being a consultant for GlaxoSmithKline, Roche, Merck, and others. Dr. Bellon reported ties to Varian Medical Systems and Veracyte.
A version of this article originally appeared on Medscape.com.
say researchers reporting 10-year outcomes from the large phase 3 trial known as PRIME II.
“Our trial provides robust evidence indicating that irradiation can be safely omitted in women 65 years of age or older who have grade 1 or 2 ER-high cancers treated by breast-conserving therapy, provided that they receive 5 years of adjuvant endocrine therapy,” concluded investigators led by Ian Kunkler, MB, a clinical oncology professor at the University of Edinburgh.
The trial randomly assigned 1,326 women who had undergone a lumpectomy to either whole-breast irradiation or no radiation on a background of tamoxifen.
The incidence of local recurrence was lower with radiation (0.9% vs. 9.5%), but there was no significant difference in distant metastases or breast cancer–specific or overall survival.
The findings will “help clinicians guide older patients on whether this particular aspect of early breast cancer treatment can be omitted,” Dr. Kunkler said in a press release. Radiation carries risks of heart and lung damage, and these results show that skipping it does not increase the odds of dying from breast cancer.
The new study was published in the New England Journal of Medicine.
“Any doubt that radiotherapy cannot be omitted in women” who meet the criteria “can be put to rest,” commented breast radiation oncologists Alice Ho, MD, of Duke University in Durham, N.C., and Jennifer Bellon, MD, of Harvard Medical School, Boston, in an accompanying editorial.
Clinical guidelines already support omitting radiation therapy in older women with low-risk tumors treated with lumpectomy and endocrine therapy, but the move has been controversial owing to a lack of long-term data, and use of radiation for such women remains common in the United States, the investigators explain.
The “highly anticipated” results for 10-year outcomes from this trial should help address that issue, as well as “the long-standing problem of overtreatment in older women with low-risk breast cancer,” the editorialists comment.
Study details
PRIME II was conducted from 2003 to 2009 mainly in the United Kingdom. Participants were aged 65 years or older and had T1 or T2 ER-positive tumors no larger than 3 cm and were without nodal involvement.
Following lumpectomies with clear margins, the women underwent endocrine therapy; the investigators recommended tamoxifen at 20 mg/day for 5 years.
Women who were randomly assigned to radiation also received 40-50 Gy of whole-breast irradiation in 20-25 fractions over 3-5 weeks.
At 10 years, 1.6% of women in the no-radiation arm had distant metastases as their first recurrence vs. 3% of women who underwent radiation.
Ten-year breast cancer–specific survival was 97.9% with radiation and 97.4% with no radiation. Ten-year overall survival was 80.7% in the radiotherapy arm vs. 80.8% in the no-radiotherapy group.
In addition, the recurrence rate was lower after radiation. The investigators suggest that lower adherence to endocrine therapy and lower levels of ER positivity increased the risk of local recurrence among women who didn’t receive radiation.
Almost 10% of the women who did not receive radiation had local recurrences by 10 years, but the investigators note that if tumors do recur locally, women still have the option of a second lumpectomy, and if they so choose, they can then receive radiation, so local recurrence “does not necessarily mean loss of the breast.”
PRIME II was funded by the Scottish Government’s chief scientist office and the Breast Cancer Institute at Western General Hospital, Edinburgh. Dr. Kunkler reported no conflicts of interest. A coauthor has acted as a speaker, adviser, and/or researcher for many companies, including Hoffmann-La Roche, Exact Sciences, and Eli Lilly. Dr. Ho reported grants from and/or being a consultant for GlaxoSmithKline, Roche, Merck, and others. Dr. Bellon reported ties to Varian Medical Systems and Veracyte.
A version of this article originally appeared on Medscape.com.
say researchers reporting 10-year outcomes from the large phase 3 trial known as PRIME II.
“Our trial provides robust evidence indicating that irradiation can be safely omitted in women 65 years of age or older who have grade 1 or 2 ER-high cancers treated by breast-conserving therapy, provided that they receive 5 years of adjuvant endocrine therapy,” concluded investigators led by Ian Kunkler, MB, a clinical oncology professor at the University of Edinburgh.
The trial randomly assigned 1,326 women who had undergone a lumpectomy to either whole-breast irradiation or no radiation on a background of tamoxifen.
The incidence of local recurrence was lower with radiation (0.9% vs. 9.5%), but there was no significant difference in distant metastases or breast cancer–specific or overall survival.
The findings will “help clinicians guide older patients on whether this particular aspect of early breast cancer treatment can be omitted,” Dr. Kunkler said in a press release. Radiation carries risks of heart and lung damage, and these results show that skipping it does not increase the odds of dying from breast cancer.
The new study was published in the New England Journal of Medicine.
“Any doubt that radiotherapy cannot be omitted in women” who meet the criteria “can be put to rest,” commented breast radiation oncologists Alice Ho, MD, of Duke University in Durham, N.C., and Jennifer Bellon, MD, of Harvard Medical School, Boston, in an accompanying editorial.
Clinical guidelines already support omitting radiation therapy in older women with low-risk tumors treated with lumpectomy and endocrine therapy, but the move has been controversial owing to a lack of long-term data, and use of radiation for such women remains common in the United States, the investigators explain.
The “highly anticipated” results for 10-year outcomes from this trial should help address that issue, as well as “the long-standing problem of overtreatment in older women with low-risk breast cancer,” the editorialists comment.
Study details
PRIME II was conducted from 2003 to 2009 mainly in the United Kingdom. Participants were aged 65 years or older and had T1 or T2 ER-positive tumors no larger than 3 cm and were without nodal involvement.
Following lumpectomies with clear margins, the women underwent endocrine therapy; the investigators recommended tamoxifen at 20 mg/day for 5 years.
Women who were randomly assigned to radiation also received 40-50 Gy of whole-breast irradiation in 20-25 fractions over 3-5 weeks.
At 10 years, 1.6% of women in the no-radiation arm had distant metastases as their first recurrence vs. 3% of women who underwent radiation.
Ten-year breast cancer–specific survival was 97.9% with radiation and 97.4% with no radiation. Ten-year overall survival was 80.7% in the radiotherapy arm vs. 80.8% in the no-radiotherapy group.
In addition, the recurrence rate was lower after radiation. The investigators suggest that lower adherence to endocrine therapy and lower levels of ER positivity increased the risk of local recurrence among women who didn’t receive radiation.
Almost 10% of the women who did not receive radiation had local recurrences by 10 years, but the investigators note that if tumors do recur locally, women still have the option of a second lumpectomy, and if they so choose, they can then receive radiation, so local recurrence “does not necessarily mean loss of the breast.”
PRIME II was funded by the Scottish Government’s chief scientist office and the Breast Cancer Institute at Western General Hospital, Edinburgh. Dr. Kunkler reported no conflicts of interest. A coauthor has acted as a speaker, adviser, and/or researcher for many companies, including Hoffmann-La Roche, Exact Sciences, and Eli Lilly. Dr. Ho reported grants from and/or being a consultant for GlaxoSmithKline, Roche, Merck, and others. Dr. Bellon reported ties to Varian Medical Systems and Veracyte.
A version of this article originally appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Breast cancer exacts high financial toll worldwide
but in low- and middle-income countries as well, a meta-analysis found.
Although the rate of financial toxicity was much higher in low- and middle-income countries – affecting 79% of patients – more than 35% of patients in high-income countries also incurred financial hardship, the study team found.
The findings highlight the need for policies to offset the burden of direct and indirect costs for breast cancer care and improve the financial health of vulnerable patients, said the study authors, led by Kavitha Ranganathan, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston.
The study was published online in JAMA Network Open.
The most expensive malignancy?
Patients with breast cancer may be particularly burdened by costs of care, with one study showing substantially higher out-of-pocket costs for patients with breast cancer than colorectal, lung, and prostate cancer combined.
A Lancet Oncology Commission report revealed that breast cancer was the most expensive cancer in the United States in 2010, accounting for $16.5 billion, or 13% of all cancer-related spending. A separate analysis found that individual direct medical costs of breast cancer care can reach $100,000.
In high-income countries, the financial burden of breast cancer care may be the result of novel and costly cancer therapeutics and interventions, overuse of services, increased willingness to pay, and varying insurance coverage. In low- and middle-income countries, women may experience delayed diagnosis because of limited access to screening and high-quality diagnostic services, leading to more later-stage diagnoses requiring more extensive treatments. Lower baseline income, limited insurance coverage, and greater distance to treatment centers may also be factors.
“Establishing the global extent of financial toxicity and comparing the economic burden of disease in different populations is imperative to help policy makers prioritize funding of breast cancer care infrastructure,” Dr. Ranganathan and colleagues write.
In their meta-analysis of 18 studies – 14 from high-income countries and 4 from low – published from 2008 to 2021, the authors found that the definition of financial toxicity varied widely across studies.
For example, some used specific numerical criteria for defining financial toxicity, such as medical cost exceeding 40% of household capacity to pay or potential income or out-of-pocket costs exceeding 30% of annual household income.
Others used patient-reported outcome measures instruments evaluating subjective statements of financial difficulty, such as an affirmative answer to having financial difficulty or trouble paying medical bills, or paying more for medical care than is affordable.
In other studies, financial toxicity was defined according to a patient’s report of specific, objective financial consequences of care, including losing income or a job; having to borrow money or go into debt; having trouble paying for food, rent, or transportation; or having to forgo any type of medical care because of cost.
In their analysis, the pooled rate of financial toxicity among patients with breast cancer was 35.3% in high-income countries and 78.8% in low/middle-income countries, both demonstrating high heterogeneity or variability (P for heterogeneity < .001). In contrast, typical financial toxicity rates across all health conditions in low-income countries ranged from 6% to 12%, the investigators noted.
One study assessing quality of life measures in Egypt found that 47.5% of patients were food insecure, 66% needed financial assistance, 34% used savings to pay for treatment, and 41.2% lacked savings altogether.
Burden reduction
Given the high rates of financial toxicity associated with breast cancer, what strategies might reduce this cost burden?
When exploring potential factors associated with financial toxicity, the researchers found no clear association between financial toxicity and race, employment status, and age, and could draw no firm conclusions about the impact of comorbidities and urban vs. rural place of residence. In addition, cancer stage and treatments were “extremely” heterogeneous across studies and the authors found no clear association between either factor and financial toxicity.
But the authors noted that the highest-priority patients are typically those who have low education, have low socioeconomic status, lack health insurance, and live in low-resource areas.
To reduce financial toxicity and improve outcomes among patients with breast cancer, the study team recommended four potential strategies:
- Use targeted educational campaigns to raise awareness about the signs and symptoms of breast cancer and the importance of early diagnosis and treatment.
- Expand health care coverage to minimize direct medical out-of-pocket costs.
- Develop programs to assist with direct nonmedical and indirect costs, such as transportation to and lodging near treatment centers and childcare.
- Improve screening, referral, and treatment infrastructure for breast cancer care.
The researchers also noted that their data highlight the value of universal health care coverage as a policy strategy, with evidence of lower financial toxicity rates in countries with universal health coverage.
Support for the study was provided in part by the National Cancer Institute, United Nations Institute for Training and Research and the Global Surgery Foundation, Harvard Global Health Institute, Connors Center for Women’s Health and Gender Biology, the Center for Surgery and Public Health, and the National Endowment for Plastic Surgery. Dr. Ranganathan reports no relevant financial relationships. Several coauthors have disclosures; the full list can be found with the original article.
A version of this article originally appeared on Medscape.com.
but in low- and middle-income countries as well, a meta-analysis found.
Although the rate of financial toxicity was much higher in low- and middle-income countries – affecting 79% of patients – more than 35% of patients in high-income countries also incurred financial hardship, the study team found.
The findings highlight the need for policies to offset the burden of direct and indirect costs for breast cancer care and improve the financial health of vulnerable patients, said the study authors, led by Kavitha Ranganathan, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston.
The study was published online in JAMA Network Open.
The most expensive malignancy?
Patients with breast cancer may be particularly burdened by costs of care, with one study showing substantially higher out-of-pocket costs for patients with breast cancer than colorectal, lung, and prostate cancer combined.
A Lancet Oncology Commission report revealed that breast cancer was the most expensive cancer in the United States in 2010, accounting for $16.5 billion, or 13% of all cancer-related spending. A separate analysis found that individual direct medical costs of breast cancer care can reach $100,000.
In high-income countries, the financial burden of breast cancer care may be the result of novel and costly cancer therapeutics and interventions, overuse of services, increased willingness to pay, and varying insurance coverage. In low- and middle-income countries, women may experience delayed diagnosis because of limited access to screening and high-quality diagnostic services, leading to more later-stage diagnoses requiring more extensive treatments. Lower baseline income, limited insurance coverage, and greater distance to treatment centers may also be factors.
“Establishing the global extent of financial toxicity and comparing the economic burden of disease in different populations is imperative to help policy makers prioritize funding of breast cancer care infrastructure,” Dr. Ranganathan and colleagues write.
In their meta-analysis of 18 studies – 14 from high-income countries and 4 from low – published from 2008 to 2021, the authors found that the definition of financial toxicity varied widely across studies.
For example, some used specific numerical criteria for defining financial toxicity, such as medical cost exceeding 40% of household capacity to pay or potential income or out-of-pocket costs exceeding 30% of annual household income.
Others used patient-reported outcome measures instruments evaluating subjective statements of financial difficulty, such as an affirmative answer to having financial difficulty or trouble paying medical bills, or paying more for medical care than is affordable.
In other studies, financial toxicity was defined according to a patient’s report of specific, objective financial consequences of care, including losing income or a job; having to borrow money or go into debt; having trouble paying for food, rent, or transportation; or having to forgo any type of medical care because of cost.
In their analysis, the pooled rate of financial toxicity among patients with breast cancer was 35.3% in high-income countries and 78.8% in low/middle-income countries, both demonstrating high heterogeneity or variability (P for heterogeneity < .001). In contrast, typical financial toxicity rates across all health conditions in low-income countries ranged from 6% to 12%, the investigators noted.
One study assessing quality of life measures in Egypt found that 47.5% of patients were food insecure, 66% needed financial assistance, 34% used savings to pay for treatment, and 41.2% lacked savings altogether.
Burden reduction
Given the high rates of financial toxicity associated with breast cancer, what strategies might reduce this cost burden?
When exploring potential factors associated with financial toxicity, the researchers found no clear association between financial toxicity and race, employment status, and age, and could draw no firm conclusions about the impact of comorbidities and urban vs. rural place of residence. In addition, cancer stage and treatments were “extremely” heterogeneous across studies and the authors found no clear association between either factor and financial toxicity.
But the authors noted that the highest-priority patients are typically those who have low education, have low socioeconomic status, lack health insurance, and live in low-resource areas.
To reduce financial toxicity and improve outcomes among patients with breast cancer, the study team recommended four potential strategies:
- Use targeted educational campaigns to raise awareness about the signs and symptoms of breast cancer and the importance of early diagnosis and treatment.
- Expand health care coverage to minimize direct medical out-of-pocket costs.
- Develop programs to assist with direct nonmedical and indirect costs, such as transportation to and lodging near treatment centers and childcare.
- Improve screening, referral, and treatment infrastructure for breast cancer care.
The researchers also noted that their data highlight the value of universal health care coverage as a policy strategy, with evidence of lower financial toxicity rates in countries with universal health coverage.
Support for the study was provided in part by the National Cancer Institute, United Nations Institute for Training and Research and the Global Surgery Foundation, Harvard Global Health Institute, Connors Center for Women’s Health and Gender Biology, the Center for Surgery and Public Health, and the National Endowment for Plastic Surgery. Dr. Ranganathan reports no relevant financial relationships. Several coauthors have disclosures; the full list can be found with the original article.
A version of this article originally appeared on Medscape.com.
but in low- and middle-income countries as well, a meta-analysis found.
Although the rate of financial toxicity was much higher in low- and middle-income countries – affecting 79% of patients – more than 35% of patients in high-income countries also incurred financial hardship, the study team found.
The findings highlight the need for policies to offset the burden of direct and indirect costs for breast cancer care and improve the financial health of vulnerable patients, said the study authors, led by Kavitha Ranganathan, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston.
The study was published online in JAMA Network Open.
The most expensive malignancy?
Patients with breast cancer may be particularly burdened by costs of care, with one study showing substantially higher out-of-pocket costs for patients with breast cancer than colorectal, lung, and prostate cancer combined.
A Lancet Oncology Commission report revealed that breast cancer was the most expensive cancer in the United States in 2010, accounting for $16.5 billion, or 13% of all cancer-related spending. A separate analysis found that individual direct medical costs of breast cancer care can reach $100,000.
In high-income countries, the financial burden of breast cancer care may be the result of novel and costly cancer therapeutics and interventions, overuse of services, increased willingness to pay, and varying insurance coverage. In low- and middle-income countries, women may experience delayed diagnosis because of limited access to screening and high-quality diagnostic services, leading to more later-stage diagnoses requiring more extensive treatments. Lower baseline income, limited insurance coverage, and greater distance to treatment centers may also be factors.
“Establishing the global extent of financial toxicity and comparing the economic burden of disease in different populations is imperative to help policy makers prioritize funding of breast cancer care infrastructure,” Dr. Ranganathan and colleagues write.
In their meta-analysis of 18 studies – 14 from high-income countries and 4 from low – published from 2008 to 2021, the authors found that the definition of financial toxicity varied widely across studies.
For example, some used specific numerical criteria for defining financial toxicity, such as medical cost exceeding 40% of household capacity to pay or potential income or out-of-pocket costs exceeding 30% of annual household income.
Others used patient-reported outcome measures instruments evaluating subjective statements of financial difficulty, such as an affirmative answer to having financial difficulty or trouble paying medical bills, or paying more for medical care than is affordable.
In other studies, financial toxicity was defined according to a patient’s report of specific, objective financial consequences of care, including losing income or a job; having to borrow money or go into debt; having trouble paying for food, rent, or transportation; or having to forgo any type of medical care because of cost.
In their analysis, the pooled rate of financial toxicity among patients with breast cancer was 35.3% in high-income countries and 78.8% in low/middle-income countries, both demonstrating high heterogeneity or variability (P for heterogeneity < .001). In contrast, typical financial toxicity rates across all health conditions in low-income countries ranged from 6% to 12%, the investigators noted.
One study assessing quality of life measures in Egypt found that 47.5% of patients were food insecure, 66% needed financial assistance, 34% used savings to pay for treatment, and 41.2% lacked savings altogether.
Burden reduction
Given the high rates of financial toxicity associated with breast cancer, what strategies might reduce this cost burden?
When exploring potential factors associated with financial toxicity, the researchers found no clear association between financial toxicity and race, employment status, and age, and could draw no firm conclusions about the impact of comorbidities and urban vs. rural place of residence. In addition, cancer stage and treatments were “extremely” heterogeneous across studies and the authors found no clear association between either factor and financial toxicity.
But the authors noted that the highest-priority patients are typically those who have low education, have low socioeconomic status, lack health insurance, and live in low-resource areas.
To reduce financial toxicity and improve outcomes among patients with breast cancer, the study team recommended four potential strategies:
- Use targeted educational campaigns to raise awareness about the signs and symptoms of breast cancer and the importance of early diagnosis and treatment.
- Expand health care coverage to minimize direct medical out-of-pocket costs.
- Develop programs to assist with direct nonmedical and indirect costs, such as transportation to and lodging near treatment centers and childcare.
- Improve screening, referral, and treatment infrastructure for breast cancer care.
The researchers also noted that their data highlight the value of universal health care coverage as a policy strategy, with evidence of lower financial toxicity rates in countries with universal health coverage.
Support for the study was provided in part by the National Cancer Institute, United Nations Institute for Training and Research and the Global Surgery Foundation, Harvard Global Health Institute, Connors Center for Women’s Health and Gender Biology, the Center for Surgery and Public Health, and the National Endowment for Plastic Surgery. Dr. Ranganathan reports no relevant financial relationships. Several coauthors have disclosures; the full list can be found with the original article.
A version of this article originally appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Genomic clues to poor outcomes in young breast cancer patients
and offer clues about molecular targets for future trials.
Compared with older women with early stage HR-positive breast cancer, women under 40 years of age had significantly higher frequencies of certain mutations, such as GATA3, as well as genomic features associated with a poor prognosis. Notably, the researchers found that women with such poor prognostic features vs. those with none had a significantly worse 8-year distant recurrence-free interval and overall survival.
“We have demonstrated age-related differences in genomic profiles with enrichment of genomic features associated with poor prognosis in these younger premenopausal women compared with older premenopausal and postmenopausal women,” the authors wrote in the study, published in the Annals of Oncology. Importantly, the genomic features highlight “the potential for age-focused treatment strategies.”
Charis Eng, MD, PhD, of the Cleveland Clinic Genomic Medicine Institute, Ohio, noted that the findings are promising but need further validation.
“With time and the appropriate clinical trials in place, I envision that these findings will enable the personalized genomics-driven management of these cancers – not only treatment, but also toward prevention,” said Dr. Eng, who was not involved in the study.
Young premenopausal women, particularly those with HR-positive, luminal breast cancer, are known to have significantly higher recurrence rates and worse survival, compared with older women, but the reasons have remained unclear.
Although previous studies have identified key gene expression signatures linked to worse outcomes in younger patients with breast cancer, there are limited data on this younger patient population, especially by breast cancer subtype. Given that breast cancer treatment strategies are often similar across age groups, such evidence gaps could represent missed opportunities for developing more targeted treatment strategies for this high-risk population of young women.
To further investigate the cancer-specific genetic profiles in younger women, Sherene Loi, MD, PhD, of the Peter MacCallum Cancer Centre, University of Melbourne, and colleagues turned to data from the pivotal, multicenter Suppression of Ovarian Function Trial (SOFT).
Using next-generation sequencing, Dr. Loi and colleagues evaluated HR-positive, HER2-negative tumors among a subset of 1,276 premenopausal women who were diagnosed with early stage breast cancer. The study employed deep-targeted sequencing for most patients (n = 1,258) as well as whole-exome sequencing in a matched case-control subsample of young women with a median age of 38 years (n = 82).
Compared with women aged 40 and older, those under 40 years of age (n = 359) had significantly higher frequencies of mutations in GATA3 (19% vs. 16%) and copy number-amplifications (47% vs. 26%).
Younger women also had significantly higher features suggestive of homologous recombination deficiency (27% vs. 21% in older women), and a higher proportion of PIK3CA mutations with concurrent copy number-amplifications (23% vs. 11%, respectively), all considered to be poor prognostic features.
In addition, younger women had significantly lower frequencies of certain mutations, including PIK3CA (32% vs. 47%), CDH1 (3% vs. 9%), and MAP3K1 (7% vs. 12%), compared with older women.
Overall, 46% of women had poor prognostic features. These poor prognostic features were observed in 72% of patients under age 35, compared with 54% aged 35-39, and 40% of those 40 and over.
Compared with women without those features, women with poor prognostic features had a lower 8-year distant recurrence-free interval of 84% vs. 94% (hazard ratio, 1.85), and worse 8-year overall survival of 88% vs. 96%, respectively (HR, 2.20). Notably, younger women under age 40 had the poorest outcomes, with an 8-year distant recurrence-free interval rate of 74% vs. 85% in older women, and an 8-year overall survival of 80% vs. 93%, respectively.
How might these results inform potential therapeutics?
Drugs targeting the homologous recombination deficiency pathway are well established, and up to 36% of very young patients in the study showed genomic features of homologous recombination deficiency, the authors noted.
In addition, Dr. Eng explained, there are other Food and Drug Administration–approved treatments that can target the copy number amplified, PIK3CA-mutated tumors, including therapies that target PIK3CA itself, or proteins downstream of it. However, use of such therapies would need “to be tested experimentally, especially since pathway inhibition sometimes may result in rebound signaling to promote tumor growth,” Dr. Eng said.
An important caveat is that patients with germline BRCA1 or BRCA2 mutations may be underrepresented in the SOFT clinical trial, as the trial excluded patients who already had bilateral oophorectomy or planned to within 5 years, the authors noted.
Nevertheless, Dr. Loi said that the study is important because “there are no other datasets as large or with this long follow-up for very young women with breast cancer.”
Furthermore, “the SOFT clinical trial was practice-changing, so using the tumor samples associated with this study is more impactful than smaller cohorts with no outcome data or institutional retrospective cohorts,” she said.
Dr. Eng agreed that the study’s size is an important attribute, allowing the authors to “identify differences that would have been missed in a smaller and more heterogeneous series.”
She added that future research should also include ancestry and racial diversity.
“While young women have higher occurrences of aggressive breast cancers, mortality is twice as likely in young Black women, compared to young White women,” Dr. Eng said.
The study received funding from a Susan G. Komen for the Cure Promise Grant, the National Health and Research Council of Australia, the Breast Cancer Research Foundation, and the National Breast Cancer Foundation of Australia, and support from the family of Judy Eisman in Australia. Dr. Loi and Dr. Eng report no relevant financial disclosures.
A version of this article originally appeared on Medscape.com.
and offer clues about molecular targets for future trials.
Compared with older women with early stage HR-positive breast cancer, women under 40 years of age had significantly higher frequencies of certain mutations, such as GATA3, as well as genomic features associated with a poor prognosis. Notably, the researchers found that women with such poor prognostic features vs. those with none had a significantly worse 8-year distant recurrence-free interval and overall survival.
“We have demonstrated age-related differences in genomic profiles with enrichment of genomic features associated with poor prognosis in these younger premenopausal women compared with older premenopausal and postmenopausal women,” the authors wrote in the study, published in the Annals of Oncology. Importantly, the genomic features highlight “the potential for age-focused treatment strategies.”
Charis Eng, MD, PhD, of the Cleveland Clinic Genomic Medicine Institute, Ohio, noted that the findings are promising but need further validation.
“With time and the appropriate clinical trials in place, I envision that these findings will enable the personalized genomics-driven management of these cancers – not only treatment, but also toward prevention,” said Dr. Eng, who was not involved in the study.
Young premenopausal women, particularly those with HR-positive, luminal breast cancer, are known to have significantly higher recurrence rates and worse survival, compared with older women, but the reasons have remained unclear.
Although previous studies have identified key gene expression signatures linked to worse outcomes in younger patients with breast cancer, there are limited data on this younger patient population, especially by breast cancer subtype. Given that breast cancer treatment strategies are often similar across age groups, such evidence gaps could represent missed opportunities for developing more targeted treatment strategies for this high-risk population of young women.
To further investigate the cancer-specific genetic profiles in younger women, Sherene Loi, MD, PhD, of the Peter MacCallum Cancer Centre, University of Melbourne, and colleagues turned to data from the pivotal, multicenter Suppression of Ovarian Function Trial (SOFT).
Using next-generation sequencing, Dr. Loi and colleagues evaluated HR-positive, HER2-negative tumors among a subset of 1,276 premenopausal women who were diagnosed with early stage breast cancer. The study employed deep-targeted sequencing for most patients (n = 1,258) as well as whole-exome sequencing in a matched case-control subsample of young women with a median age of 38 years (n = 82).
Compared with women aged 40 and older, those under 40 years of age (n = 359) had significantly higher frequencies of mutations in GATA3 (19% vs. 16%) and copy number-amplifications (47% vs. 26%).
Younger women also had significantly higher features suggestive of homologous recombination deficiency (27% vs. 21% in older women), and a higher proportion of PIK3CA mutations with concurrent copy number-amplifications (23% vs. 11%, respectively), all considered to be poor prognostic features.
In addition, younger women had significantly lower frequencies of certain mutations, including PIK3CA (32% vs. 47%), CDH1 (3% vs. 9%), and MAP3K1 (7% vs. 12%), compared with older women.
Overall, 46% of women had poor prognostic features. These poor prognostic features were observed in 72% of patients under age 35, compared with 54% aged 35-39, and 40% of those 40 and over.
Compared with women without those features, women with poor prognostic features had a lower 8-year distant recurrence-free interval of 84% vs. 94% (hazard ratio, 1.85), and worse 8-year overall survival of 88% vs. 96%, respectively (HR, 2.20). Notably, younger women under age 40 had the poorest outcomes, with an 8-year distant recurrence-free interval rate of 74% vs. 85% in older women, and an 8-year overall survival of 80% vs. 93%, respectively.
How might these results inform potential therapeutics?
Drugs targeting the homologous recombination deficiency pathway are well established, and up to 36% of very young patients in the study showed genomic features of homologous recombination deficiency, the authors noted.
In addition, Dr. Eng explained, there are other Food and Drug Administration–approved treatments that can target the copy number amplified, PIK3CA-mutated tumors, including therapies that target PIK3CA itself, or proteins downstream of it. However, use of such therapies would need “to be tested experimentally, especially since pathway inhibition sometimes may result in rebound signaling to promote tumor growth,” Dr. Eng said.
An important caveat is that patients with germline BRCA1 or BRCA2 mutations may be underrepresented in the SOFT clinical trial, as the trial excluded patients who already had bilateral oophorectomy or planned to within 5 years, the authors noted.
Nevertheless, Dr. Loi said that the study is important because “there are no other datasets as large or with this long follow-up for very young women with breast cancer.”
Furthermore, “the SOFT clinical trial was practice-changing, so using the tumor samples associated with this study is more impactful than smaller cohorts with no outcome data or institutional retrospective cohorts,” she said.
Dr. Eng agreed that the study’s size is an important attribute, allowing the authors to “identify differences that would have been missed in a smaller and more heterogeneous series.”
She added that future research should also include ancestry and racial diversity.
“While young women have higher occurrences of aggressive breast cancers, mortality is twice as likely in young Black women, compared to young White women,” Dr. Eng said.
The study received funding from a Susan G. Komen for the Cure Promise Grant, the National Health and Research Council of Australia, the Breast Cancer Research Foundation, and the National Breast Cancer Foundation of Australia, and support from the family of Judy Eisman in Australia. Dr. Loi and Dr. Eng report no relevant financial disclosures.
A version of this article originally appeared on Medscape.com.
and offer clues about molecular targets for future trials.
Compared with older women with early stage HR-positive breast cancer, women under 40 years of age had significantly higher frequencies of certain mutations, such as GATA3, as well as genomic features associated with a poor prognosis. Notably, the researchers found that women with such poor prognostic features vs. those with none had a significantly worse 8-year distant recurrence-free interval and overall survival.
“We have demonstrated age-related differences in genomic profiles with enrichment of genomic features associated with poor prognosis in these younger premenopausal women compared with older premenopausal and postmenopausal women,” the authors wrote in the study, published in the Annals of Oncology. Importantly, the genomic features highlight “the potential for age-focused treatment strategies.”
Charis Eng, MD, PhD, of the Cleveland Clinic Genomic Medicine Institute, Ohio, noted that the findings are promising but need further validation.
“With time and the appropriate clinical trials in place, I envision that these findings will enable the personalized genomics-driven management of these cancers – not only treatment, but also toward prevention,” said Dr. Eng, who was not involved in the study.
Young premenopausal women, particularly those with HR-positive, luminal breast cancer, are known to have significantly higher recurrence rates and worse survival, compared with older women, but the reasons have remained unclear.
Although previous studies have identified key gene expression signatures linked to worse outcomes in younger patients with breast cancer, there are limited data on this younger patient population, especially by breast cancer subtype. Given that breast cancer treatment strategies are often similar across age groups, such evidence gaps could represent missed opportunities for developing more targeted treatment strategies for this high-risk population of young women.
To further investigate the cancer-specific genetic profiles in younger women, Sherene Loi, MD, PhD, of the Peter MacCallum Cancer Centre, University of Melbourne, and colleagues turned to data from the pivotal, multicenter Suppression of Ovarian Function Trial (SOFT).
Using next-generation sequencing, Dr. Loi and colleagues evaluated HR-positive, HER2-negative tumors among a subset of 1,276 premenopausal women who were diagnosed with early stage breast cancer. The study employed deep-targeted sequencing for most patients (n = 1,258) as well as whole-exome sequencing in a matched case-control subsample of young women with a median age of 38 years (n = 82).
Compared with women aged 40 and older, those under 40 years of age (n = 359) had significantly higher frequencies of mutations in GATA3 (19% vs. 16%) and copy number-amplifications (47% vs. 26%).
Younger women also had significantly higher features suggestive of homologous recombination deficiency (27% vs. 21% in older women), and a higher proportion of PIK3CA mutations with concurrent copy number-amplifications (23% vs. 11%, respectively), all considered to be poor prognostic features.
In addition, younger women had significantly lower frequencies of certain mutations, including PIK3CA (32% vs. 47%), CDH1 (3% vs. 9%), and MAP3K1 (7% vs. 12%), compared with older women.
Overall, 46% of women had poor prognostic features. These poor prognostic features were observed in 72% of patients under age 35, compared with 54% aged 35-39, and 40% of those 40 and over.
Compared with women without those features, women with poor prognostic features had a lower 8-year distant recurrence-free interval of 84% vs. 94% (hazard ratio, 1.85), and worse 8-year overall survival of 88% vs. 96%, respectively (HR, 2.20). Notably, younger women under age 40 had the poorest outcomes, with an 8-year distant recurrence-free interval rate of 74% vs. 85% in older women, and an 8-year overall survival of 80% vs. 93%, respectively.
How might these results inform potential therapeutics?
Drugs targeting the homologous recombination deficiency pathway are well established, and up to 36% of very young patients in the study showed genomic features of homologous recombination deficiency, the authors noted.
In addition, Dr. Eng explained, there are other Food and Drug Administration–approved treatments that can target the copy number amplified, PIK3CA-mutated tumors, including therapies that target PIK3CA itself, or proteins downstream of it. However, use of such therapies would need “to be tested experimentally, especially since pathway inhibition sometimes may result in rebound signaling to promote tumor growth,” Dr. Eng said.
An important caveat is that patients with germline BRCA1 or BRCA2 mutations may be underrepresented in the SOFT clinical trial, as the trial excluded patients who already had bilateral oophorectomy or planned to within 5 years, the authors noted.
Nevertheless, Dr. Loi said that the study is important because “there are no other datasets as large or with this long follow-up for very young women with breast cancer.”
Furthermore, “the SOFT clinical trial was practice-changing, so using the tumor samples associated with this study is more impactful than smaller cohorts with no outcome data or institutional retrospective cohorts,” she said.
Dr. Eng agreed that the study’s size is an important attribute, allowing the authors to “identify differences that would have been missed in a smaller and more heterogeneous series.”
She added that future research should also include ancestry and racial diversity.
“While young women have higher occurrences of aggressive breast cancers, mortality is twice as likely in young Black women, compared to young White women,” Dr. Eng said.
The study received funding from a Susan G. Komen for the Cure Promise Grant, the National Health and Research Council of Australia, the Breast Cancer Research Foundation, and the National Breast Cancer Foundation of Australia, and support from the family of Judy Eisman in Australia. Dr. Loi and Dr. Eng report no relevant financial disclosures.
A version of this article originally appeared on Medscape.com.
FROM ANNALS OF ONCOLOGY
‘Valid option’ for partial breast irradiation in breast cancer
The study covered in this summary was published on researchsquare.com as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
- According to numerous guidelines, partial breast irradiation after lumpectomy is a sound approach for early-stage breast cancer, but there is a lack of consensus about treatment schedules.
- The investigators suggest that 30 Gy in five daily fractions is a “valid option” for these patients in a field that lacks consensus.
Study design
- The team reviewed 381 women with early breast cancer treated with this approach (30 Gy in five daily fractions) at their center from 2013 to 2022.
- Half of patients had left-sided tumors, 94.5% had invasive ductal carcinomas, 96.6% had grade 1 or grade 2 disease, and tumors were luminal like in 99.2% of patients.
- Following lumpectomy, women underwent partial breast irradiation to the tumor bed plus 15 mm of isometric expansion beyond it.
- Follow-up was a median of 28 months.
Key results
- Seven patients (2%) had a local recurrence, of which two were in the treatment field.
- Three-year local control, disease-free survival, and overall survival were high (97.5%, 95.7%, and 96.9%, respectively).
- Nearly 90% of patients and 97% of physicians reported good or excellent cosmesis.
- Ten patients (2.9%) had grade 2 late toxicities, including edema, asthenia, and fibrosis; there were no grade 3 or higher adverse events.
- Five patients (1.5%) had late cardiac major events, four of whom were treated on the right breast; three patients (0.9%) had late pulmonary fibrosis.
- The safety and efficacy outcomes are in line with previous reports, including those that used different dosage and/or fractionation schedules.
Limitations
- The study was retrospective, with a relatively short follow-up.
- Quality of life was not assessed.
- There was no objective baseline measure of cosmesis against which to compare cosmetic results.
Disclosures
- There was no funding for the study, and the investigators didn’t have any conflicts of interest to report.
This is a summary of a preprint research study, “One-Week External Beam Partial Breast Irradiation: Survival and Toxicity Outcomes,” led by Riccardo Ray Colciago from the Fondazione IRCCS Istituto Nazionale dei Tumori, Milan. The study has not been peer reviewed. The full text can be found at researchsquare.com.
A version of this article first appeared on Medscape.com.
The study covered in this summary was published on researchsquare.com as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
- According to numerous guidelines, partial breast irradiation after lumpectomy is a sound approach for early-stage breast cancer, but there is a lack of consensus about treatment schedules.
- The investigators suggest that 30 Gy in five daily fractions is a “valid option” for these patients in a field that lacks consensus.
Study design
- The team reviewed 381 women with early breast cancer treated with this approach (30 Gy in five daily fractions) at their center from 2013 to 2022.
- Half of patients had left-sided tumors, 94.5% had invasive ductal carcinomas, 96.6% had grade 1 or grade 2 disease, and tumors were luminal like in 99.2% of patients.
- Following lumpectomy, women underwent partial breast irradiation to the tumor bed plus 15 mm of isometric expansion beyond it.
- Follow-up was a median of 28 months.
Key results
- Seven patients (2%) had a local recurrence, of which two were in the treatment field.
- Three-year local control, disease-free survival, and overall survival were high (97.5%, 95.7%, and 96.9%, respectively).
- Nearly 90% of patients and 97% of physicians reported good or excellent cosmesis.
- Ten patients (2.9%) had grade 2 late toxicities, including edema, asthenia, and fibrosis; there were no grade 3 or higher adverse events.
- Five patients (1.5%) had late cardiac major events, four of whom were treated on the right breast; three patients (0.9%) had late pulmonary fibrosis.
- The safety and efficacy outcomes are in line with previous reports, including those that used different dosage and/or fractionation schedules.
Limitations
- The study was retrospective, with a relatively short follow-up.
- Quality of life was not assessed.
- There was no objective baseline measure of cosmesis against which to compare cosmetic results.
Disclosures
- There was no funding for the study, and the investigators didn’t have any conflicts of interest to report.
This is a summary of a preprint research study, “One-Week External Beam Partial Breast Irradiation: Survival and Toxicity Outcomes,” led by Riccardo Ray Colciago from the Fondazione IRCCS Istituto Nazionale dei Tumori, Milan. The study has not been peer reviewed. The full text can be found at researchsquare.com.
A version of this article first appeared on Medscape.com.
The study covered in this summary was published on researchsquare.com as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
- According to numerous guidelines, partial breast irradiation after lumpectomy is a sound approach for early-stage breast cancer, but there is a lack of consensus about treatment schedules.
- The investigators suggest that 30 Gy in five daily fractions is a “valid option” for these patients in a field that lacks consensus.
Study design
- The team reviewed 381 women with early breast cancer treated with this approach (30 Gy in five daily fractions) at their center from 2013 to 2022.
- Half of patients had left-sided tumors, 94.5% had invasive ductal carcinomas, 96.6% had grade 1 or grade 2 disease, and tumors were luminal like in 99.2% of patients.
- Following lumpectomy, women underwent partial breast irradiation to the tumor bed plus 15 mm of isometric expansion beyond it.
- Follow-up was a median of 28 months.
Key results
- Seven patients (2%) had a local recurrence, of which two were in the treatment field.
- Three-year local control, disease-free survival, and overall survival were high (97.5%, 95.7%, and 96.9%, respectively).
- Nearly 90% of patients and 97% of physicians reported good or excellent cosmesis.
- Ten patients (2.9%) had grade 2 late toxicities, including edema, asthenia, and fibrosis; there were no grade 3 or higher adverse events.
- Five patients (1.5%) had late cardiac major events, four of whom were treated on the right breast; three patients (0.9%) had late pulmonary fibrosis.
- The safety and efficacy outcomes are in line with previous reports, including those that used different dosage and/or fractionation schedules.
Limitations
- The study was retrospective, with a relatively short follow-up.
- Quality of life was not assessed.
- There was no objective baseline measure of cosmesis against which to compare cosmetic results.
Disclosures
- There was no funding for the study, and the investigators didn’t have any conflicts of interest to report.
This is a summary of a preprint research study, “One-Week External Beam Partial Breast Irradiation: Survival and Toxicity Outcomes,” led by Riccardo Ray Colciago from the Fondazione IRCCS Istituto Nazionale dei Tumori, Milan. The study has not been peer reviewed. The full text can be found at researchsquare.com.
A version of this article first appeared on Medscape.com.
Radiotherapy for early breast cancer: Sharp cutoff at age 70
say researchers reporting new data showing a sharp cut-off at age 70.
“In our study, one of the most significant variables in determining whether breast cancer patients who are close their 70th birthday are recommended standard-of-care radiation or de-escalated treatment is whether they show up a few months before or a few months after that 70th birthday,” commented study author Wesley J. Talcott, MD, of the department of therapeutic radiology at the Yale School of Medicine, New Haven, Conn.
The results show a trend in which radiation therapy is 50% less likely to be prescribed for patients age 70 and older with early-stage breast cancer, even when controlling for population size, patient demographics, and disease specific variables.
This suggests that oncologists are weighing the variable of age too heavily when deciding on adjuvant treatments, the authors suggest.
“In certain circumstances, breast cancer oncology providers are treating age like a binary categorical variable when selecting patients for treatments or diagnostic procedures, rather than the continuous variable that it is,” Dr. Talcott commented.
The study was published online in the International Journal of Radiation Oncology: Biology, Physics.
Approached for comment, Casey Chollet-Lipscomb, MD, radiation oncologist with Tennessee Oncology, Nashville, who was not associated with the study, agreed with its main finding.
“The study helps emphasize the importance of individualized care,” she said. “Increasing age is the most common risk factor for breast cancer, but breast cancer is an incredibly diverse disease. While you can observe trends based on age, every patient is unique, and they can’t be lumped into one bucket and prescribed treatment based on a strict age cutoff.”
The retrospective study included two cohorts of women identified in the National Cancer Data Base (2004-2017) all of whom underwent lumpectomy for early-stage breast cancer. All patients had “strong indications” for adjuvant treatment.
Patients in cohort 1 (n = 160,990) included women with estrogen-receptor negative cancer, tumor size greater than 3 cm, who were determined to be “appropriate” for radiation therapy.
Patients in cohort 2 (n = 394,946) had hormone-receptor positive cancer, tumor size greater than 5 mm, and were considered to be “appropriate” candidates for endocrine therapy.
Multivariable analysis was performed to control for comorbidity burden (measured by the Charlson-Deyo Comorbidity Index), race and ethnicity, insurance status, academic versus non-academic treatment center, median annual income of a patient’s area of residence, distance from the site of treatment, and pathology variables including number of lymph nodes sampled, histologic grade, and genomic risk score.
In cohort 1, radiation was recommended for 90%-92% of patients between the ages of 50-69; this dropped to 81% for those aged 70.
After MVA, it was determined that age difference was an independent predictor for adjuvant radiation recommendation only at age 70 versus 69 (odds ratio, 0.47; 95% confidence interval 0.39-0.57, P < .001).
For cohort 2, year-over-year age difference predicted endocrine therapy recommendation only at the juncture between age 70 versus 69 (OR, 0.86, 95% CI 0.74-0.99, P = .001).
“Our results don’t say that we should be increasing the amount of treatment for patients over the age [of] 70 or decreasing that patient treatment for patients younger than age 70. What we believe is that we need to be assessing physiologic age of our patients when treating patients,” Dr. Talcott said.
“We would do this by looking at not just how many years a patient has been on this Earth but also what their current health status is, how many good quality-of-life years they might have after treatment or without it, and what the patient wants in terms of burden of treatment. This is a much more valuable way to approach the allocation of treatments than using age alone,” he added.
Both Dr. Talcott and Dr. Chollet-Lipscomb agreed that a limitation of the study was a lack of data on how physicians decided on a specific treatment in each individual case, but they agree that even without this information the results were “significant.”
Dr. Chollet-Lipscomb also highlighted the factors other than age she would use to determine the best adjuvant treatment for a patient with early stage breast cancer, including the individual features of the tumor, how aggressive it looks under the microscope, what the receptor status is, and a patient’s overall performance status and comorbidities.
Dr. Talcott and Dr. Chollet-Lipscomb report no relevant financial relationships. The authors had no acknowledgement of research support for this study.
A version of this article first appeared on Medscape.com.
say researchers reporting new data showing a sharp cut-off at age 70.
“In our study, one of the most significant variables in determining whether breast cancer patients who are close their 70th birthday are recommended standard-of-care radiation or de-escalated treatment is whether they show up a few months before or a few months after that 70th birthday,” commented study author Wesley J. Talcott, MD, of the department of therapeutic radiology at the Yale School of Medicine, New Haven, Conn.
The results show a trend in which radiation therapy is 50% less likely to be prescribed for patients age 70 and older with early-stage breast cancer, even when controlling for population size, patient demographics, and disease specific variables.
This suggests that oncologists are weighing the variable of age too heavily when deciding on adjuvant treatments, the authors suggest.
“In certain circumstances, breast cancer oncology providers are treating age like a binary categorical variable when selecting patients for treatments or diagnostic procedures, rather than the continuous variable that it is,” Dr. Talcott commented.
The study was published online in the International Journal of Radiation Oncology: Biology, Physics.
Approached for comment, Casey Chollet-Lipscomb, MD, radiation oncologist with Tennessee Oncology, Nashville, who was not associated with the study, agreed with its main finding.
“The study helps emphasize the importance of individualized care,” she said. “Increasing age is the most common risk factor for breast cancer, but breast cancer is an incredibly diverse disease. While you can observe trends based on age, every patient is unique, and they can’t be lumped into one bucket and prescribed treatment based on a strict age cutoff.”
The retrospective study included two cohorts of women identified in the National Cancer Data Base (2004-2017) all of whom underwent lumpectomy for early-stage breast cancer. All patients had “strong indications” for adjuvant treatment.
Patients in cohort 1 (n = 160,990) included women with estrogen-receptor negative cancer, tumor size greater than 3 cm, who were determined to be “appropriate” for radiation therapy.
Patients in cohort 2 (n = 394,946) had hormone-receptor positive cancer, tumor size greater than 5 mm, and were considered to be “appropriate” candidates for endocrine therapy.
Multivariable analysis was performed to control for comorbidity burden (measured by the Charlson-Deyo Comorbidity Index), race and ethnicity, insurance status, academic versus non-academic treatment center, median annual income of a patient’s area of residence, distance from the site of treatment, and pathology variables including number of lymph nodes sampled, histologic grade, and genomic risk score.
In cohort 1, radiation was recommended for 90%-92% of patients between the ages of 50-69; this dropped to 81% for those aged 70.
After MVA, it was determined that age difference was an independent predictor for adjuvant radiation recommendation only at age 70 versus 69 (odds ratio, 0.47; 95% confidence interval 0.39-0.57, P < .001).
For cohort 2, year-over-year age difference predicted endocrine therapy recommendation only at the juncture between age 70 versus 69 (OR, 0.86, 95% CI 0.74-0.99, P = .001).
“Our results don’t say that we should be increasing the amount of treatment for patients over the age [of] 70 or decreasing that patient treatment for patients younger than age 70. What we believe is that we need to be assessing physiologic age of our patients when treating patients,” Dr. Talcott said.
“We would do this by looking at not just how many years a patient has been on this Earth but also what their current health status is, how many good quality-of-life years they might have after treatment or without it, and what the patient wants in terms of burden of treatment. This is a much more valuable way to approach the allocation of treatments than using age alone,” he added.
Both Dr. Talcott and Dr. Chollet-Lipscomb agreed that a limitation of the study was a lack of data on how physicians decided on a specific treatment in each individual case, but they agree that even without this information the results were “significant.”
Dr. Chollet-Lipscomb also highlighted the factors other than age she would use to determine the best adjuvant treatment for a patient with early stage breast cancer, including the individual features of the tumor, how aggressive it looks under the microscope, what the receptor status is, and a patient’s overall performance status and comorbidities.
Dr. Talcott and Dr. Chollet-Lipscomb report no relevant financial relationships. The authors had no acknowledgement of research support for this study.
A version of this article first appeared on Medscape.com.
say researchers reporting new data showing a sharp cut-off at age 70.
“In our study, one of the most significant variables in determining whether breast cancer patients who are close their 70th birthday are recommended standard-of-care radiation or de-escalated treatment is whether they show up a few months before or a few months after that 70th birthday,” commented study author Wesley J. Talcott, MD, of the department of therapeutic radiology at the Yale School of Medicine, New Haven, Conn.
The results show a trend in which radiation therapy is 50% less likely to be prescribed for patients age 70 and older with early-stage breast cancer, even when controlling for population size, patient demographics, and disease specific variables.
This suggests that oncologists are weighing the variable of age too heavily when deciding on adjuvant treatments, the authors suggest.
“In certain circumstances, breast cancer oncology providers are treating age like a binary categorical variable when selecting patients for treatments or diagnostic procedures, rather than the continuous variable that it is,” Dr. Talcott commented.
The study was published online in the International Journal of Radiation Oncology: Biology, Physics.
Approached for comment, Casey Chollet-Lipscomb, MD, radiation oncologist with Tennessee Oncology, Nashville, who was not associated with the study, agreed with its main finding.
“The study helps emphasize the importance of individualized care,” she said. “Increasing age is the most common risk factor for breast cancer, but breast cancer is an incredibly diverse disease. While you can observe trends based on age, every patient is unique, and they can’t be lumped into one bucket and prescribed treatment based on a strict age cutoff.”
The retrospective study included two cohorts of women identified in the National Cancer Data Base (2004-2017) all of whom underwent lumpectomy for early-stage breast cancer. All patients had “strong indications” for adjuvant treatment.
Patients in cohort 1 (n = 160,990) included women with estrogen-receptor negative cancer, tumor size greater than 3 cm, who were determined to be “appropriate” for radiation therapy.
Patients in cohort 2 (n = 394,946) had hormone-receptor positive cancer, tumor size greater than 5 mm, and were considered to be “appropriate” candidates for endocrine therapy.
Multivariable analysis was performed to control for comorbidity burden (measured by the Charlson-Deyo Comorbidity Index), race and ethnicity, insurance status, academic versus non-academic treatment center, median annual income of a patient’s area of residence, distance from the site of treatment, and pathology variables including number of lymph nodes sampled, histologic grade, and genomic risk score.
In cohort 1, radiation was recommended for 90%-92% of patients between the ages of 50-69; this dropped to 81% for those aged 70.
After MVA, it was determined that age difference was an independent predictor for adjuvant radiation recommendation only at age 70 versus 69 (odds ratio, 0.47; 95% confidence interval 0.39-0.57, P < .001).
For cohort 2, year-over-year age difference predicted endocrine therapy recommendation only at the juncture between age 70 versus 69 (OR, 0.86, 95% CI 0.74-0.99, P = .001).
“Our results don’t say that we should be increasing the amount of treatment for patients over the age [of] 70 or decreasing that patient treatment for patients younger than age 70. What we believe is that we need to be assessing physiologic age of our patients when treating patients,” Dr. Talcott said.
“We would do this by looking at not just how many years a patient has been on this Earth but also what their current health status is, how many good quality-of-life years they might have after treatment or without it, and what the patient wants in terms of burden of treatment. This is a much more valuable way to approach the allocation of treatments than using age alone,” he added.
Both Dr. Talcott and Dr. Chollet-Lipscomb agreed that a limitation of the study was a lack of data on how physicians decided on a specific treatment in each individual case, but they agree that even without this information the results were “significant.”
Dr. Chollet-Lipscomb also highlighted the factors other than age she would use to determine the best adjuvant treatment for a patient with early stage breast cancer, including the individual features of the tumor, how aggressive it looks under the microscope, what the receptor status is, and a patient’s overall performance status and comorbidities.
Dr. Talcott and Dr. Chollet-Lipscomb report no relevant financial relationships. The authors had no acknowledgement of research support for this study.
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF RADIATION ONCOLOGY: BIOLOGY, PHYSICS
Use of diagnostic mammograms is inconsistent, survey finds
Existing guidelines offer little help, according to Pavani Chalasani, MD, MPH, who presented the study at the San Antonio Breast Cancer Symposium. “They just say [do an] annual mammogram, but they don’t say, ‘Do we need to do screening? Do we need to do breast MRIs?’”
Her personal experience also reflected a general confusion. “I asked my colleagues and got different answers from seven colleagues,” said Dr. Chalasani, who is an oncologist at the University of Arizona Cancer Center, Tucson.
She noted that diagnostic mammograms are generally similar to screening mammograms, but the radiologist is viewing the images in real time and can take additional views as needed while the patient is still present. “That is the biggest difference,” said Dr. Chalasani. No studies have been conducted to determine which method produces better results.
To get a snapshot of current practice, she and her colleagues developed a survey, which the American Society of Clinical Oncology sent to 1,000 randomly selected members between Oct. 19 and Nov. 22, 2021. 244 individuals responded; 93.5% were physicians, and half identified as female. A total of 174 respondents were medical oncologists, 31 were radiation oncologists, and 20 were surgical oncologists. The imbalance among respondents is a limitation of the study. That “may or may not be reflective of our real-time practices (among surgeons), but we do think that since a lot of times patients are seen by medical oncologists, there could be overlap,” said Dr. Chalasani.
About 50% of respondents said that they use breast MRI in the diagnosis of 25% or fewer patients. Approximately 64% of respondents said they used diagnostic mammograms versus about 31% who used imaging mammograms at first imaging. About 53% said they ordered mammograms within the first 6 months after treatment.
38% of those who ordered diagnostic mammograms for surveillance used it for 3-5 years, while 29% continued it for 5 years or more. One-quarter employed additional imaging during follow-up, most commonly breast ultrasound. About 65% said they had no stop date for screening mammograms, as long as the patient remained healthy. The choice of screening or diagnostic mammography was about 50:50, though about 55% said they use screening mammography for patients 80 years of age or older.
Dr. Chalasani pointed out that both screening and diagnostic mammograms provide similar imaging quality. Screening mammograms are completely covered by insurance, while diagnostic mammograms typically require a copay. “We’re doing this [diagnostic mammography] with no guidelines, but there is this out of pocket cost, without knowing if it’s the right thing to do,” she said.
National Comprehensive Cancer Network guidelines indicate that diagnostic mammograms can be conducted for 5 years after a ductal carcinoma in situ diagnosis, but it doesn’t provide guidance for invasive cancers. Some past studies suggested that doing diagnostic mammograms for 3 years may increase diagnosis, but it isn’t clear if any such advantage would actually result in a clinical difference, according to Dr. Chalasani. “With the treatments we have, we still might cure [the cancer]. So what endpoints are we looking for? Are we changing care to add on toxicity to the patient, and stress to the patient and also for the health care system?”
She hopes that physicians will look at the results and understand that diagnostic mammograms, while they intuitively feel superior, are not supported by guidelines, and patients must incur an extra cost.
Her team also plans to conduct cost-effectiveness analysis of diagnostic mammograms.
Dr. Chalasani has no relevant financial disclosures.
Existing guidelines offer little help, according to Pavani Chalasani, MD, MPH, who presented the study at the San Antonio Breast Cancer Symposium. “They just say [do an] annual mammogram, but they don’t say, ‘Do we need to do screening? Do we need to do breast MRIs?’”
Her personal experience also reflected a general confusion. “I asked my colleagues and got different answers from seven colleagues,” said Dr. Chalasani, who is an oncologist at the University of Arizona Cancer Center, Tucson.
She noted that diagnostic mammograms are generally similar to screening mammograms, but the radiologist is viewing the images in real time and can take additional views as needed while the patient is still present. “That is the biggest difference,” said Dr. Chalasani. No studies have been conducted to determine which method produces better results.
To get a snapshot of current practice, she and her colleagues developed a survey, which the American Society of Clinical Oncology sent to 1,000 randomly selected members between Oct. 19 and Nov. 22, 2021. 244 individuals responded; 93.5% were physicians, and half identified as female. A total of 174 respondents were medical oncologists, 31 were radiation oncologists, and 20 were surgical oncologists. The imbalance among respondents is a limitation of the study. That “may or may not be reflective of our real-time practices (among surgeons), but we do think that since a lot of times patients are seen by medical oncologists, there could be overlap,” said Dr. Chalasani.
About 50% of respondents said that they use breast MRI in the diagnosis of 25% or fewer patients. Approximately 64% of respondents said they used diagnostic mammograms versus about 31% who used imaging mammograms at first imaging. About 53% said they ordered mammograms within the first 6 months after treatment.
38% of those who ordered diagnostic mammograms for surveillance used it for 3-5 years, while 29% continued it for 5 years or more. One-quarter employed additional imaging during follow-up, most commonly breast ultrasound. About 65% said they had no stop date for screening mammograms, as long as the patient remained healthy. The choice of screening or diagnostic mammography was about 50:50, though about 55% said they use screening mammography for patients 80 years of age or older.
Dr. Chalasani pointed out that both screening and diagnostic mammograms provide similar imaging quality. Screening mammograms are completely covered by insurance, while diagnostic mammograms typically require a copay. “We’re doing this [diagnostic mammography] with no guidelines, but there is this out of pocket cost, without knowing if it’s the right thing to do,” she said.
National Comprehensive Cancer Network guidelines indicate that diagnostic mammograms can be conducted for 5 years after a ductal carcinoma in situ diagnosis, but it doesn’t provide guidance for invasive cancers. Some past studies suggested that doing diagnostic mammograms for 3 years may increase diagnosis, but it isn’t clear if any such advantage would actually result in a clinical difference, according to Dr. Chalasani. “With the treatments we have, we still might cure [the cancer]. So what endpoints are we looking for? Are we changing care to add on toxicity to the patient, and stress to the patient and also for the health care system?”
She hopes that physicians will look at the results and understand that diagnostic mammograms, while they intuitively feel superior, are not supported by guidelines, and patients must incur an extra cost.
Her team also plans to conduct cost-effectiveness analysis of diagnostic mammograms.
Dr. Chalasani has no relevant financial disclosures.
Existing guidelines offer little help, according to Pavani Chalasani, MD, MPH, who presented the study at the San Antonio Breast Cancer Symposium. “They just say [do an] annual mammogram, but they don’t say, ‘Do we need to do screening? Do we need to do breast MRIs?’”
Her personal experience also reflected a general confusion. “I asked my colleagues and got different answers from seven colleagues,” said Dr. Chalasani, who is an oncologist at the University of Arizona Cancer Center, Tucson.
She noted that diagnostic mammograms are generally similar to screening mammograms, but the radiologist is viewing the images in real time and can take additional views as needed while the patient is still present. “That is the biggest difference,” said Dr. Chalasani. No studies have been conducted to determine which method produces better results.
To get a snapshot of current practice, she and her colleagues developed a survey, which the American Society of Clinical Oncology sent to 1,000 randomly selected members between Oct. 19 and Nov. 22, 2021. 244 individuals responded; 93.5% were physicians, and half identified as female. A total of 174 respondents were medical oncologists, 31 were radiation oncologists, and 20 were surgical oncologists. The imbalance among respondents is a limitation of the study. That “may or may not be reflective of our real-time practices (among surgeons), but we do think that since a lot of times patients are seen by medical oncologists, there could be overlap,” said Dr. Chalasani.
About 50% of respondents said that they use breast MRI in the diagnosis of 25% or fewer patients. Approximately 64% of respondents said they used diagnostic mammograms versus about 31% who used imaging mammograms at first imaging. About 53% said they ordered mammograms within the first 6 months after treatment.
38% of those who ordered diagnostic mammograms for surveillance used it for 3-5 years, while 29% continued it for 5 years or more. One-quarter employed additional imaging during follow-up, most commonly breast ultrasound. About 65% said they had no stop date for screening mammograms, as long as the patient remained healthy. The choice of screening or diagnostic mammography was about 50:50, though about 55% said they use screening mammography for patients 80 years of age or older.
Dr. Chalasani pointed out that both screening and diagnostic mammograms provide similar imaging quality. Screening mammograms are completely covered by insurance, while diagnostic mammograms typically require a copay. “We’re doing this [diagnostic mammography] with no guidelines, but there is this out of pocket cost, without knowing if it’s the right thing to do,” she said.
National Comprehensive Cancer Network guidelines indicate that diagnostic mammograms can be conducted for 5 years after a ductal carcinoma in situ diagnosis, but it doesn’t provide guidance for invasive cancers. Some past studies suggested that doing diagnostic mammograms for 3 years may increase diagnosis, but it isn’t clear if any such advantage would actually result in a clinical difference, according to Dr. Chalasani. “With the treatments we have, we still might cure [the cancer]. So what endpoints are we looking for? Are we changing care to add on toxicity to the patient, and stress to the patient and also for the health care system?”
She hopes that physicians will look at the results and understand that diagnostic mammograms, while they intuitively feel superior, are not supported by guidelines, and patients must incur an extra cost.
Her team also plans to conduct cost-effectiveness analysis of diagnostic mammograms.
Dr. Chalasani has no relevant financial disclosures.
FROM SABCS 2022
FDA OKs sacituzumab govitecan for HR+ metastatic breast cancer
for patients with unresectable, locally advanced or metastatic hormone receptor (HR)–positive, HER2-negative breast cancer after endocrine-based therapy and at least two additional systemic therapies for metastatic disease.
Label expansion for the Trop-2–directed antibody-drug conjugate was based on the TROPICS-02 trial, which randomized 543 adults 1:1 to either sacituzumab govitecan 10 mg/kg IV on days 1 and 8 of a 21-day cycle or single agent chemotherapy, most often eribulin but also vinorelbine, gemcitabine, or capecitabine.
Median progression free survival was 5.5 months with sacituzumab govitecan versus 4 months with single agent chemotherapy (hazard ratio, 0.66; P = .0003). Median overall survival was 14.4 months in the sacituzumab govitecan group versus 11.2 months with chemotherapy (HR, 0.79), according to an FDA press release announcing the approval.
In a Gilead press release, Hope Rugo, MD, a breast cancer specialist at the University of California, San Francisco, and principal investigator for TROPICS-02, said the approval “is significant for the breast cancer community. We have had limited options to offer patients after endocrine-based therapy and chemotherapy, and to see a clinically meaningful survival benefit of more than 3 months with a quality-of-life benefit for these women is exceptional.”
The most common adverse events associated with sacituzumab govitecan in the trial, occurring in a quarter or more of participants, were decreased leukocyte count, decreased neutrophil count, decreased hemoglobin, decreased lymphocyte count, diarrhea, fatigue, nausea, alopecia, glucose elevation, constipation, and decreased albumin.
Labeling for the agent carries a boxedwarning of severe or life-threatening neutropenia and severe diarrhea.
The recommended dose is the trial dose: 10 mg/kg IV on days 1 and 8 of 21-day cycles until disease progression or unacceptable toxicity.
Sacituzumab govitecan was previously approved for unresectable, locally advanced or metastatic triple-negative breast cancer after two or more prior systemic therapies and locally advanced or metastatic urothelial cancer after platinum-based chemotherapy and either a PD-1 or PD-L1 inhibitor.
A version of this article first appeared on Medscape.com.
for patients with unresectable, locally advanced or metastatic hormone receptor (HR)–positive, HER2-negative breast cancer after endocrine-based therapy and at least two additional systemic therapies for metastatic disease.
Label expansion for the Trop-2–directed antibody-drug conjugate was based on the TROPICS-02 trial, which randomized 543 adults 1:1 to either sacituzumab govitecan 10 mg/kg IV on days 1 and 8 of a 21-day cycle or single agent chemotherapy, most often eribulin but also vinorelbine, gemcitabine, or capecitabine.
Median progression free survival was 5.5 months with sacituzumab govitecan versus 4 months with single agent chemotherapy (hazard ratio, 0.66; P = .0003). Median overall survival was 14.4 months in the sacituzumab govitecan group versus 11.2 months with chemotherapy (HR, 0.79), according to an FDA press release announcing the approval.
In a Gilead press release, Hope Rugo, MD, a breast cancer specialist at the University of California, San Francisco, and principal investigator for TROPICS-02, said the approval “is significant for the breast cancer community. We have had limited options to offer patients after endocrine-based therapy and chemotherapy, and to see a clinically meaningful survival benefit of more than 3 months with a quality-of-life benefit for these women is exceptional.”
The most common adverse events associated with sacituzumab govitecan in the trial, occurring in a quarter or more of participants, were decreased leukocyte count, decreased neutrophil count, decreased hemoglobin, decreased lymphocyte count, diarrhea, fatigue, nausea, alopecia, glucose elevation, constipation, and decreased albumin.
Labeling for the agent carries a boxedwarning of severe or life-threatening neutropenia and severe diarrhea.
The recommended dose is the trial dose: 10 mg/kg IV on days 1 and 8 of 21-day cycles until disease progression or unacceptable toxicity.
Sacituzumab govitecan was previously approved for unresectable, locally advanced or metastatic triple-negative breast cancer after two or more prior systemic therapies and locally advanced or metastatic urothelial cancer after platinum-based chemotherapy and either a PD-1 or PD-L1 inhibitor.
A version of this article first appeared on Medscape.com.
for patients with unresectable, locally advanced or metastatic hormone receptor (HR)–positive, HER2-negative breast cancer after endocrine-based therapy and at least two additional systemic therapies for metastatic disease.
Label expansion for the Trop-2–directed antibody-drug conjugate was based on the TROPICS-02 trial, which randomized 543 adults 1:1 to either sacituzumab govitecan 10 mg/kg IV on days 1 and 8 of a 21-day cycle or single agent chemotherapy, most often eribulin but also vinorelbine, gemcitabine, or capecitabine.
Median progression free survival was 5.5 months with sacituzumab govitecan versus 4 months with single agent chemotherapy (hazard ratio, 0.66; P = .0003). Median overall survival was 14.4 months in the sacituzumab govitecan group versus 11.2 months with chemotherapy (HR, 0.79), according to an FDA press release announcing the approval.
In a Gilead press release, Hope Rugo, MD, a breast cancer specialist at the University of California, San Francisco, and principal investigator for TROPICS-02, said the approval “is significant for the breast cancer community. We have had limited options to offer patients after endocrine-based therapy and chemotherapy, and to see a clinically meaningful survival benefit of more than 3 months with a quality-of-life benefit for these women is exceptional.”
The most common adverse events associated with sacituzumab govitecan in the trial, occurring in a quarter or more of participants, were decreased leukocyte count, decreased neutrophil count, decreased hemoglobin, decreased lymphocyte count, diarrhea, fatigue, nausea, alopecia, glucose elevation, constipation, and decreased albumin.
Labeling for the agent carries a boxedwarning of severe or life-threatening neutropenia and severe diarrhea.
The recommended dose is the trial dose: 10 mg/kg IV on days 1 and 8 of 21-day cycles until disease progression or unacceptable toxicity.
Sacituzumab govitecan was previously approved for unresectable, locally advanced or metastatic triple-negative breast cancer after two or more prior systemic therapies and locally advanced or metastatic urothelial cancer after platinum-based chemotherapy and either a PD-1 or PD-L1 inhibitor.
A version of this article first appeared on Medscape.com.
Race and geography tied to breast cancer care delays
suggesting the need to target high-risk geographic regions and patient groups to ensure timely care, new research suggests.
Among nearly 33,000 women from North Carolina with stage I-III breast cancer, Black patients were nearly twice as likely has non-Black patients to experience treatment delays of more than 60 days, researchers found.
“Our findings suggest that treatment delays are alarmingly common in patients at high risk for breast cancer death, including young Black women and patients with stage III disease,” the authors note in their article, which was published online in Cancer.
Research shows that breast cancer treatment delays of 30-60 days can lower survival, and Black patients face a “disproportionate risk of treatment delays across the breast cancer care delivery spectrum,” the authors explain.
However, studies exploring whether or how racial disparities in treatment delays relate to geography are more limited.
In the current analysis, researchers amassed a retrospective cohort of all patients with stage I-III breast cancer between 2004 and 2015 in the North Carolina Central Cancer Registry and explored the risk of treatment delay by race and geographic subregion.
The cohort included 32,626 women, 6,190 (19.0%) of whom were Black. Counties were divided into the nine Area Health Education Center regions for North Carolina.
Compared with non‐Black patients, Black patients were more likely to have stage III disease (15.2% vs. 9.3%), hormone receptor–negative tumors (29.3% vs. 15.6%), Medicaid insurance (46.7% vs. 14.9%), and to live within 5 miles of their treatment site (30.6% vs. 25.2%).
Overall, Black patients were almost two times more likely to experience a treatment delay of more than 60 days (15% vs. 8%).
On average, about one in seven Black women experienced a lengthy delay, but the risk varied depending on geographic location. Patients living in certain regions of the state were more likely to experience delays; those in the highest-risk region were about twice as likely to experience a delay as those in the lowest-risk region (relative risk, 2.1 among Black patients; and RR, 1.9 among non-Black patients).
The magnitude of the racial gap in treatment delay varied by region – from 0% to 9.4%. But overall, of patients who experienced treatment delays, a significantly greater proportion were Black patients in every region except region 2, where only 2.7% (93 of 3,362) of patients were Black.
Notably, two regions with the greatest disparities in treatment delay, as well as the highest absolute risk of treatment delay for Black patients, surround large cities.
“These delays weren’t explained by the patients’ distance from cancer treatment facilities, their specific stage of cancer or type of treatment, or what insurance they had,” lead author Katherine Reeder-Hayes, MD, with the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, said in a news release.
Instead, Dr. Reeder-Hayes said, the findings suggest that the structure of local health care systems, rather than patient characteristics, may better explain why some patients experience treatment delays.
In other words, “if cancer care teams in certain areas say, ‘Oh, it’s particularly hard to treat breast cancer in our area because people are poor or have really advanced stages of cancer when they come in,’ our research does not bear out that explanation,” Dr. Reeder-Hayes said in email to this news organization.
This study “highlights the persistent disparities in treatment delays Black women encounter, which often lead to worse outcomes,” said Kathie-Ann Joseph, MD, MPH, who was not involved in the research.
“Interestingly, the authors could not attribute these delays in treatment to patient-level factors,” said Dr. Joseph, a breast cancer surgeon at NYU Langone Perlmutter Cancer Center, New York. But the authors “did find substantial geographic variation, which suggests the need to address structural barriers contributing to treatment delays in Black women.”
Sara P. Cate, MD, who was not involved with the research, also noted that the study highlights a known issue – “that racial minorities have longer delays in cancer treatment.” And notably, she said, the findings reveal that this disparity persists in areas where access to care is better and more robust.
“The nuances of the delays to care are multifactorial,” said Dr. Cate, a breast cancer surgeon and director of the Breast Surgery Quality Program at Mount Sinai in New York. “We need to do better with this population, and it is a multilevel solution of financial assistance, social work, and patient navigation.”
The study was supported in part by grants from the Susan G. Komen Foundation and the NC State Employees’ Credit Union. Dr. Reeder-Hayes, Dr. Cate, and Dr. Joseph have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggesting the need to target high-risk geographic regions and patient groups to ensure timely care, new research suggests.
Among nearly 33,000 women from North Carolina with stage I-III breast cancer, Black patients were nearly twice as likely has non-Black patients to experience treatment delays of more than 60 days, researchers found.
“Our findings suggest that treatment delays are alarmingly common in patients at high risk for breast cancer death, including young Black women and patients with stage III disease,” the authors note in their article, which was published online in Cancer.
Research shows that breast cancer treatment delays of 30-60 days can lower survival, and Black patients face a “disproportionate risk of treatment delays across the breast cancer care delivery spectrum,” the authors explain.
However, studies exploring whether or how racial disparities in treatment delays relate to geography are more limited.
In the current analysis, researchers amassed a retrospective cohort of all patients with stage I-III breast cancer between 2004 and 2015 in the North Carolina Central Cancer Registry and explored the risk of treatment delay by race and geographic subregion.
The cohort included 32,626 women, 6,190 (19.0%) of whom were Black. Counties were divided into the nine Area Health Education Center regions for North Carolina.
Compared with non‐Black patients, Black patients were more likely to have stage III disease (15.2% vs. 9.3%), hormone receptor–negative tumors (29.3% vs. 15.6%), Medicaid insurance (46.7% vs. 14.9%), and to live within 5 miles of their treatment site (30.6% vs. 25.2%).
Overall, Black patients were almost two times more likely to experience a treatment delay of more than 60 days (15% vs. 8%).
On average, about one in seven Black women experienced a lengthy delay, but the risk varied depending on geographic location. Patients living in certain regions of the state were more likely to experience delays; those in the highest-risk region were about twice as likely to experience a delay as those in the lowest-risk region (relative risk, 2.1 among Black patients; and RR, 1.9 among non-Black patients).
The magnitude of the racial gap in treatment delay varied by region – from 0% to 9.4%. But overall, of patients who experienced treatment delays, a significantly greater proportion were Black patients in every region except region 2, where only 2.7% (93 of 3,362) of patients were Black.
Notably, two regions with the greatest disparities in treatment delay, as well as the highest absolute risk of treatment delay for Black patients, surround large cities.
“These delays weren’t explained by the patients’ distance from cancer treatment facilities, their specific stage of cancer or type of treatment, or what insurance they had,” lead author Katherine Reeder-Hayes, MD, with the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, said in a news release.
Instead, Dr. Reeder-Hayes said, the findings suggest that the structure of local health care systems, rather than patient characteristics, may better explain why some patients experience treatment delays.
In other words, “if cancer care teams in certain areas say, ‘Oh, it’s particularly hard to treat breast cancer in our area because people are poor or have really advanced stages of cancer when they come in,’ our research does not bear out that explanation,” Dr. Reeder-Hayes said in email to this news organization.
This study “highlights the persistent disparities in treatment delays Black women encounter, which often lead to worse outcomes,” said Kathie-Ann Joseph, MD, MPH, who was not involved in the research.
“Interestingly, the authors could not attribute these delays in treatment to patient-level factors,” said Dr. Joseph, a breast cancer surgeon at NYU Langone Perlmutter Cancer Center, New York. But the authors “did find substantial geographic variation, which suggests the need to address structural barriers contributing to treatment delays in Black women.”
Sara P. Cate, MD, who was not involved with the research, also noted that the study highlights a known issue – “that racial minorities have longer delays in cancer treatment.” And notably, she said, the findings reveal that this disparity persists in areas where access to care is better and more robust.
“The nuances of the delays to care are multifactorial,” said Dr. Cate, a breast cancer surgeon and director of the Breast Surgery Quality Program at Mount Sinai in New York. “We need to do better with this population, and it is a multilevel solution of financial assistance, social work, and patient navigation.”
The study was supported in part by grants from the Susan G. Komen Foundation and the NC State Employees’ Credit Union. Dr. Reeder-Hayes, Dr. Cate, and Dr. Joseph have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggesting the need to target high-risk geographic regions and patient groups to ensure timely care, new research suggests.
Among nearly 33,000 women from North Carolina with stage I-III breast cancer, Black patients were nearly twice as likely has non-Black patients to experience treatment delays of more than 60 days, researchers found.
“Our findings suggest that treatment delays are alarmingly common in patients at high risk for breast cancer death, including young Black women and patients with stage III disease,” the authors note in their article, which was published online in Cancer.
Research shows that breast cancer treatment delays of 30-60 days can lower survival, and Black patients face a “disproportionate risk of treatment delays across the breast cancer care delivery spectrum,” the authors explain.
However, studies exploring whether or how racial disparities in treatment delays relate to geography are more limited.
In the current analysis, researchers amassed a retrospective cohort of all patients with stage I-III breast cancer between 2004 and 2015 in the North Carolina Central Cancer Registry and explored the risk of treatment delay by race and geographic subregion.
The cohort included 32,626 women, 6,190 (19.0%) of whom were Black. Counties were divided into the nine Area Health Education Center regions for North Carolina.
Compared with non‐Black patients, Black patients were more likely to have stage III disease (15.2% vs. 9.3%), hormone receptor–negative tumors (29.3% vs. 15.6%), Medicaid insurance (46.7% vs. 14.9%), and to live within 5 miles of their treatment site (30.6% vs. 25.2%).
Overall, Black patients were almost two times more likely to experience a treatment delay of more than 60 days (15% vs. 8%).
On average, about one in seven Black women experienced a lengthy delay, but the risk varied depending on geographic location. Patients living in certain regions of the state were more likely to experience delays; those in the highest-risk region were about twice as likely to experience a delay as those in the lowest-risk region (relative risk, 2.1 among Black patients; and RR, 1.9 among non-Black patients).
The magnitude of the racial gap in treatment delay varied by region – from 0% to 9.4%. But overall, of patients who experienced treatment delays, a significantly greater proportion were Black patients in every region except region 2, where only 2.7% (93 of 3,362) of patients were Black.
Notably, two regions with the greatest disparities in treatment delay, as well as the highest absolute risk of treatment delay for Black patients, surround large cities.
“These delays weren’t explained by the patients’ distance from cancer treatment facilities, their specific stage of cancer or type of treatment, or what insurance they had,” lead author Katherine Reeder-Hayes, MD, with the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, said in a news release.
Instead, Dr. Reeder-Hayes said, the findings suggest that the structure of local health care systems, rather than patient characteristics, may better explain why some patients experience treatment delays.
In other words, “if cancer care teams in certain areas say, ‘Oh, it’s particularly hard to treat breast cancer in our area because people are poor or have really advanced stages of cancer when they come in,’ our research does not bear out that explanation,” Dr. Reeder-Hayes said in email to this news organization.
This study “highlights the persistent disparities in treatment delays Black women encounter, which often lead to worse outcomes,” said Kathie-Ann Joseph, MD, MPH, who was not involved in the research.
“Interestingly, the authors could not attribute these delays in treatment to patient-level factors,” said Dr. Joseph, a breast cancer surgeon at NYU Langone Perlmutter Cancer Center, New York. But the authors “did find substantial geographic variation, which suggests the need to address structural barriers contributing to treatment delays in Black women.”
Sara P. Cate, MD, who was not involved with the research, also noted that the study highlights a known issue – “that racial minorities have longer delays in cancer treatment.” And notably, she said, the findings reveal that this disparity persists in areas where access to care is better and more robust.
“The nuances of the delays to care are multifactorial,” said Dr. Cate, a breast cancer surgeon and director of the Breast Surgery Quality Program at Mount Sinai in New York. “We need to do better with this population, and it is a multilevel solution of financial assistance, social work, and patient navigation.”
The study was supported in part by grants from the Susan G. Komen Foundation and the NC State Employees’ Credit Union. Dr. Reeder-Hayes, Dr. Cate, and Dr. Joseph have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CANCER