User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Call to action on obesity amid COVID-19 pandemic
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Potential COVID-19 variant surge looms over U.S.
Another coronavirus surge may be on the way in the United States as daily COVID-19 cases continue to plateau around 60,000, states begin to lift restrictions, and people embark on spring break trips this week, according to CNN.
Outbreaks will likely stem from the B.1.1.7 variant, which was first identified in the United Kingdom, and gain momentum during the next 6-14 weeks.
“Four weeks ago, the B.1.1.7 variant made up about 1%-4% of the virus that we were seeing in communities across the country. Today it’s up to 30%-40%,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told NBC’s Meet the Press on March 7.
Dr. Osterholm compared the current situation with the “eye of the hurricane,” where the skies appear clear but more storms are on the way. Across Europe, 27 countries are seeing significant B.1.1.7 case increases, and 10 are getting hit hard, he said.
“What we’ve seen in Europe, when we hit that 50% mark, you see cases surge,” he said. “So right now, we do have to keep America as safe as we can from this virus by not letting up on any of the public health measures we’ve taken.”
In January, the CDC warned that B.1.1.7 variant cases would increase in 2021 and become the dominant variant in the country by this month. The United States has now reported more than 3,000 cases across 46 states, according to the latest CDC tally updated on March 7. More than 600 cases have been found in Florida, followed by more than 400 in Michigan.
The CDC has said the tally doesn’t represent the total number of B.1.1.7 cases in the United States, only the ones that have been identified by analyzing samples through genomic sequencing.
“Where it has hit in the U.K. and now elsewhere in Europe, it has been catastrophic,” Celine Gounder, MD, an infectious disease specialist with New York University Langone Health, told CNN on March 7.
The variant is more transmissible than the original novel coronavirus, and the cases in the United States are “increasing exponentially,” she said.
“It has driven up rates of hospitalizations and deaths and it’s very difficult to control,” Dr. Gounder said.
Vaccination numbers aren’t yet high enough to stop the predicted surge, she added. The United States has shipped more than 116 million vaccine doses, according to the latest CDC update on March 7. Nearly 59 million people have received at least one dose, and 30.6 million people have received two vaccine doses. About 9% of the U.S. population has been fully vaccinated.
States shouldn’t ease restrictions until the vaccination numbers are much higher and daily COVID-19 cases fall below 10,000 – and maybe “considerably less than that,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told CNN on March 4.
Several states have already begun to lift COVID-19 safety protocols, with Texas and Mississippi removing mask mandates last week. Businesses in Texas will be able to reopen at full capacity on March 10. For now, public health officials are urging Americans to continue to wear masks, avoid crowds, and follow social distancing guidelines as vaccines roll out across the country.
“This is sort of like we’ve been running this really long marathon, and we’re 100 yards from the finish line and we sit down and we give up,” Dr. Gounder told CNN on Sunday. ‘We’re almost there, we just need to give ourselves a bit more time to get a larger proportion of the population covered with vaccines.”
A version of this article first appeared on WebMD.com.
Another coronavirus surge may be on the way in the United States as daily COVID-19 cases continue to plateau around 60,000, states begin to lift restrictions, and people embark on spring break trips this week, according to CNN.
Outbreaks will likely stem from the B.1.1.7 variant, which was first identified in the United Kingdom, and gain momentum during the next 6-14 weeks.
“Four weeks ago, the B.1.1.7 variant made up about 1%-4% of the virus that we were seeing in communities across the country. Today it’s up to 30%-40%,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told NBC’s Meet the Press on March 7.
Dr. Osterholm compared the current situation with the “eye of the hurricane,” where the skies appear clear but more storms are on the way. Across Europe, 27 countries are seeing significant B.1.1.7 case increases, and 10 are getting hit hard, he said.
“What we’ve seen in Europe, when we hit that 50% mark, you see cases surge,” he said. “So right now, we do have to keep America as safe as we can from this virus by not letting up on any of the public health measures we’ve taken.”
In January, the CDC warned that B.1.1.7 variant cases would increase in 2021 and become the dominant variant in the country by this month. The United States has now reported more than 3,000 cases across 46 states, according to the latest CDC tally updated on March 7. More than 600 cases have been found in Florida, followed by more than 400 in Michigan.
The CDC has said the tally doesn’t represent the total number of B.1.1.7 cases in the United States, only the ones that have been identified by analyzing samples through genomic sequencing.
“Where it has hit in the U.K. and now elsewhere in Europe, it has been catastrophic,” Celine Gounder, MD, an infectious disease specialist with New York University Langone Health, told CNN on March 7.
The variant is more transmissible than the original novel coronavirus, and the cases in the United States are “increasing exponentially,” she said.
“It has driven up rates of hospitalizations and deaths and it’s very difficult to control,” Dr. Gounder said.
Vaccination numbers aren’t yet high enough to stop the predicted surge, she added. The United States has shipped more than 116 million vaccine doses, according to the latest CDC update on March 7. Nearly 59 million people have received at least one dose, and 30.6 million people have received two vaccine doses. About 9% of the U.S. population has been fully vaccinated.
States shouldn’t ease restrictions until the vaccination numbers are much higher and daily COVID-19 cases fall below 10,000 – and maybe “considerably less than that,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told CNN on March 4.
Several states have already begun to lift COVID-19 safety protocols, with Texas and Mississippi removing mask mandates last week. Businesses in Texas will be able to reopen at full capacity on March 10. For now, public health officials are urging Americans to continue to wear masks, avoid crowds, and follow social distancing guidelines as vaccines roll out across the country.
“This is sort of like we’ve been running this really long marathon, and we’re 100 yards from the finish line and we sit down and we give up,” Dr. Gounder told CNN on Sunday. ‘We’re almost there, we just need to give ourselves a bit more time to get a larger proportion of the population covered with vaccines.”
A version of this article first appeared on WebMD.com.
Another coronavirus surge may be on the way in the United States as daily COVID-19 cases continue to plateau around 60,000, states begin to lift restrictions, and people embark on spring break trips this week, according to CNN.
Outbreaks will likely stem from the B.1.1.7 variant, which was first identified in the United Kingdom, and gain momentum during the next 6-14 weeks.
“Four weeks ago, the B.1.1.7 variant made up about 1%-4% of the virus that we were seeing in communities across the country. Today it’s up to 30%-40%,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told NBC’s Meet the Press on March 7.
Dr. Osterholm compared the current situation with the “eye of the hurricane,” where the skies appear clear but more storms are on the way. Across Europe, 27 countries are seeing significant B.1.1.7 case increases, and 10 are getting hit hard, he said.
“What we’ve seen in Europe, when we hit that 50% mark, you see cases surge,” he said. “So right now, we do have to keep America as safe as we can from this virus by not letting up on any of the public health measures we’ve taken.”
In January, the CDC warned that B.1.1.7 variant cases would increase in 2021 and become the dominant variant in the country by this month. The United States has now reported more than 3,000 cases across 46 states, according to the latest CDC tally updated on March 7. More than 600 cases have been found in Florida, followed by more than 400 in Michigan.
The CDC has said the tally doesn’t represent the total number of B.1.1.7 cases in the United States, only the ones that have been identified by analyzing samples through genomic sequencing.
“Where it has hit in the U.K. and now elsewhere in Europe, it has been catastrophic,” Celine Gounder, MD, an infectious disease specialist with New York University Langone Health, told CNN on March 7.
The variant is more transmissible than the original novel coronavirus, and the cases in the United States are “increasing exponentially,” she said.
“It has driven up rates of hospitalizations and deaths and it’s very difficult to control,” Dr. Gounder said.
Vaccination numbers aren’t yet high enough to stop the predicted surge, she added. The United States has shipped more than 116 million vaccine doses, according to the latest CDC update on March 7. Nearly 59 million people have received at least one dose, and 30.6 million people have received two vaccine doses. About 9% of the U.S. population has been fully vaccinated.
States shouldn’t ease restrictions until the vaccination numbers are much higher and daily COVID-19 cases fall below 10,000 – and maybe “considerably less than that,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told CNN on March 4.
Several states have already begun to lift COVID-19 safety protocols, with Texas and Mississippi removing mask mandates last week. Businesses in Texas will be able to reopen at full capacity on March 10. For now, public health officials are urging Americans to continue to wear masks, avoid crowds, and follow social distancing guidelines as vaccines roll out across the country.
“This is sort of like we’ve been running this really long marathon, and we’re 100 yards from the finish line and we sit down and we give up,” Dr. Gounder told CNN on Sunday. ‘We’re almost there, we just need to give ourselves a bit more time to get a larger proportion of the population covered with vaccines.”
A version of this article first appeared on WebMD.com.
DOACs offered after heart valve surgery despite absence of data
Direct oral anticoagulants (DOACs) are used in about 1% of patients undergoing surgical mechanical aortic and mitral valve replacement, but in up to 6% of surgical bioprosthetic valve replacements, according to registry data presented at CRT 2021.
In an analysis of the Society of Thoracic Surgery (STS) registry during 2014-2017, DOAC use increased steadily among those undergoing surgical bioprosthetic valve replacement, reaching a number that is potentially clinically significant, according to Ankur Kalra, MD, an interventional cardiologist at Akron General Hospital who has an academic appointment at the Cleveland Clinic.
There was no increase in the use of DOACs observed among patients undergoing mechanical valve replacement, “but even if the number is 1%, they should probably not be used at all until we accrue more data,” Dr. Kalra said.
DOACs discouraged in patients with mechanical or bioprosthetic valves
In Food and Drug Administration labeling, DOACs are contraindicated or not recommended. This can be traced to the randomized RE-ALIGN trial, which was stopped prematurely due to evidence of harm from a DOAC, according to Dr. Kalra.
In RE-ALIGN, which enrolled patients undergoing mechanical aortic or mitral valve replacement, dabigatran was associated not only with more bleeding events than warfarin, but also more thromboembolic events.
There are no randomized data comparing the factor Xa inhibitors rivaroxaban or apixaban to warfarin in heart valve surgery, but Dr. Kalra noted cautionary language is found in the labeling of both, “perhaps due to the RE-ALIGN data.”
Registry shows trends in prescribing
In the STS registry data, 193 (1.1%) of the 18,142 patients undergoing mechanical aortic valve surgery, 139 (1.0%) of the 13,942 patients undergoing mechanical mitral valve surgery, 5,625 (4.7%) of the 116,203 patients undergoing aortic bioprosthetic aortic valve surgery, and 2,180 (5.9%) of the 39,243 patients undergoing bioprosthetic mitral valve surgery were on a DOAC at discharge.
Among those receiving a mechanical value and placed on a DOAC, about two-thirds were on a factor Xa inhibitor rather than dabigatran. For those receiving a bioprosthetic value, the proportion was greater than 80%. Dr. Kalra speculated that the RE-ALIGN trial might be the reason factor Xa inhibitors were favored.
In both types of valves, whether mechanical or bioprosthetic, more comorbidities predicted a greater likelihood of receiving a DOAC rather than warfarin. For those receiving mechanical values, the comorbidities with a significant association with greater DOAC use included hypertension (P = .003), dyslipidemia (P = .02), arrhythmia (P < .001), and peripheral arterial disease (P < 0.001).
The same factors were significant for predicting increased likelihood of a DOAC following bioprosthetic valve replacement, but there were additional factors, including atrial fibrillation independent of other types of arrhythmias (P < .001), a factor not significant for mechanical valves, as well as diabetes (P < .001), cerebrovascular disease (P < .001), dialysis (P < .001), and endocarditis (P < .001).
“This is probably intuitive, but patients who were on a factor Xa inhibitor before their valve replacement were also more likely to be discharged on a factor Xa inhibitor,” Dr. Kalra said at the virtual meeting, sponsored by MedStar Heart & Vascular Institute.
The year-to-year increase in DOAC use among those undergoing bioprosthetic valve replacement over the study period, which was a significant trend, was not observed among those undergoing mechanical valve replacement. Rather, the 1% proportion remained stable over the study period.
“We wanted to look at outcomes, but we found that the STS database, which only includes data out to 30 days, is not structured for this type of analysis,” Dr. Kalra said. He was also concerned about the limitations of a comparison in which 1% of the sample was being compared to 99%.
Expert: One percent is ‘very small number’
David J. Cohen, MD, commented on the 1% figure, which was so low that a moderator questioned whether it could be due mostly to coding errors.
“This is a very, very small number so at some level it is reassuring that it is so low in the mechanical valves,” Dr. Cohen said. However, he was more circumspect about the larger number in bioprosthetic valves.
“I have always thought it was a bit strange there was a warning against using them in bioprosthetic valves, especially in the aortic position,” he said.
“The trials that established the benefits of DOACs were all in nonvalvular atrial fibrillation, but this did not mean non–aortic stenosis; it meant non–mitral valvular. There have been articles written about how that has been misinterpreted,” said Dr. Cohen, director of clinical and outcomes research at the Cardiovascular Research Foundation and director of academic affairs at St. Francis Hospital, Roslyn, N.Y.
For his part, Dr. Kalra reported that he does not consider DOACs in patients who have undergone a surgical mechanical valve replacement. For bioprosthetic valves, he “prefers” warfarin over DOACs.
Overall, the evidence from the registry led Dr. Kalra to suggest that physicians should continue to “exercise caution” in using DOACs instead of warfarin after any surgical valve replacement “until randomized clinical trials provide sufficient evidence” to make a judgment about relative efficacy and safety.
Results of the study were published online as a research letter in Jama Network Open after Dr. Kalra’s presentation. Dr. Kalra and Dr. Cohen report no potential conflicts of interest.
Direct oral anticoagulants (DOACs) are used in about 1% of patients undergoing surgical mechanical aortic and mitral valve replacement, but in up to 6% of surgical bioprosthetic valve replacements, according to registry data presented at CRT 2021.
In an analysis of the Society of Thoracic Surgery (STS) registry during 2014-2017, DOAC use increased steadily among those undergoing surgical bioprosthetic valve replacement, reaching a number that is potentially clinically significant, according to Ankur Kalra, MD, an interventional cardiologist at Akron General Hospital who has an academic appointment at the Cleveland Clinic.
There was no increase in the use of DOACs observed among patients undergoing mechanical valve replacement, “but even if the number is 1%, they should probably not be used at all until we accrue more data,” Dr. Kalra said.
DOACs discouraged in patients with mechanical or bioprosthetic valves
In Food and Drug Administration labeling, DOACs are contraindicated or not recommended. This can be traced to the randomized RE-ALIGN trial, which was stopped prematurely due to evidence of harm from a DOAC, according to Dr. Kalra.
In RE-ALIGN, which enrolled patients undergoing mechanical aortic or mitral valve replacement, dabigatran was associated not only with more bleeding events than warfarin, but also more thromboembolic events.
There are no randomized data comparing the factor Xa inhibitors rivaroxaban or apixaban to warfarin in heart valve surgery, but Dr. Kalra noted cautionary language is found in the labeling of both, “perhaps due to the RE-ALIGN data.”
Registry shows trends in prescribing
In the STS registry data, 193 (1.1%) of the 18,142 patients undergoing mechanical aortic valve surgery, 139 (1.0%) of the 13,942 patients undergoing mechanical mitral valve surgery, 5,625 (4.7%) of the 116,203 patients undergoing aortic bioprosthetic aortic valve surgery, and 2,180 (5.9%) of the 39,243 patients undergoing bioprosthetic mitral valve surgery were on a DOAC at discharge.
Among those receiving a mechanical value and placed on a DOAC, about two-thirds were on a factor Xa inhibitor rather than dabigatran. For those receiving a bioprosthetic value, the proportion was greater than 80%. Dr. Kalra speculated that the RE-ALIGN trial might be the reason factor Xa inhibitors were favored.
In both types of valves, whether mechanical or bioprosthetic, more comorbidities predicted a greater likelihood of receiving a DOAC rather than warfarin. For those receiving mechanical values, the comorbidities with a significant association with greater DOAC use included hypertension (P = .003), dyslipidemia (P = .02), arrhythmia (P < .001), and peripheral arterial disease (P < 0.001).
The same factors were significant for predicting increased likelihood of a DOAC following bioprosthetic valve replacement, but there were additional factors, including atrial fibrillation independent of other types of arrhythmias (P < .001), a factor not significant for mechanical valves, as well as diabetes (P < .001), cerebrovascular disease (P < .001), dialysis (P < .001), and endocarditis (P < .001).
“This is probably intuitive, but patients who were on a factor Xa inhibitor before their valve replacement were also more likely to be discharged on a factor Xa inhibitor,” Dr. Kalra said at the virtual meeting, sponsored by MedStar Heart & Vascular Institute.
The year-to-year increase in DOAC use among those undergoing bioprosthetic valve replacement over the study period, which was a significant trend, was not observed among those undergoing mechanical valve replacement. Rather, the 1% proportion remained stable over the study period.
“We wanted to look at outcomes, but we found that the STS database, which only includes data out to 30 days, is not structured for this type of analysis,” Dr. Kalra said. He was also concerned about the limitations of a comparison in which 1% of the sample was being compared to 99%.
Expert: One percent is ‘very small number’
David J. Cohen, MD, commented on the 1% figure, which was so low that a moderator questioned whether it could be due mostly to coding errors.
“This is a very, very small number so at some level it is reassuring that it is so low in the mechanical valves,” Dr. Cohen said. However, he was more circumspect about the larger number in bioprosthetic valves.
“I have always thought it was a bit strange there was a warning against using them in bioprosthetic valves, especially in the aortic position,” he said.
“The trials that established the benefits of DOACs were all in nonvalvular atrial fibrillation, but this did not mean non–aortic stenosis; it meant non–mitral valvular. There have been articles written about how that has been misinterpreted,” said Dr. Cohen, director of clinical and outcomes research at the Cardiovascular Research Foundation and director of academic affairs at St. Francis Hospital, Roslyn, N.Y.
For his part, Dr. Kalra reported that he does not consider DOACs in patients who have undergone a surgical mechanical valve replacement. For bioprosthetic valves, he “prefers” warfarin over DOACs.
Overall, the evidence from the registry led Dr. Kalra to suggest that physicians should continue to “exercise caution” in using DOACs instead of warfarin after any surgical valve replacement “until randomized clinical trials provide sufficient evidence” to make a judgment about relative efficacy and safety.
Results of the study were published online as a research letter in Jama Network Open after Dr. Kalra’s presentation. Dr. Kalra and Dr. Cohen report no potential conflicts of interest.
Direct oral anticoagulants (DOACs) are used in about 1% of patients undergoing surgical mechanical aortic and mitral valve replacement, but in up to 6% of surgical bioprosthetic valve replacements, according to registry data presented at CRT 2021.
In an analysis of the Society of Thoracic Surgery (STS) registry during 2014-2017, DOAC use increased steadily among those undergoing surgical bioprosthetic valve replacement, reaching a number that is potentially clinically significant, according to Ankur Kalra, MD, an interventional cardiologist at Akron General Hospital who has an academic appointment at the Cleveland Clinic.
There was no increase in the use of DOACs observed among patients undergoing mechanical valve replacement, “but even if the number is 1%, they should probably not be used at all until we accrue more data,” Dr. Kalra said.
DOACs discouraged in patients with mechanical or bioprosthetic valves
In Food and Drug Administration labeling, DOACs are contraindicated or not recommended. This can be traced to the randomized RE-ALIGN trial, which was stopped prematurely due to evidence of harm from a DOAC, according to Dr. Kalra.
In RE-ALIGN, which enrolled patients undergoing mechanical aortic or mitral valve replacement, dabigatran was associated not only with more bleeding events than warfarin, but also more thromboembolic events.
There are no randomized data comparing the factor Xa inhibitors rivaroxaban or apixaban to warfarin in heart valve surgery, but Dr. Kalra noted cautionary language is found in the labeling of both, “perhaps due to the RE-ALIGN data.”
Registry shows trends in prescribing
In the STS registry data, 193 (1.1%) of the 18,142 patients undergoing mechanical aortic valve surgery, 139 (1.0%) of the 13,942 patients undergoing mechanical mitral valve surgery, 5,625 (4.7%) of the 116,203 patients undergoing aortic bioprosthetic aortic valve surgery, and 2,180 (5.9%) of the 39,243 patients undergoing bioprosthetic mitral valve surgery were on a DOAC at discharge.
Among those receiving a mechanical value and placed on a DOAC, about two-thirds were on a factor Xa inhibitor rather than dabigatran. For those receiving a bioprosthetic value, the proportion was greater than 80%. Dr. Kalra speculated that the RE-ALIGN trial might be the reason factor Xa inhibitors were favored.
In both types of valves, whether mechanical or bioprosthetic, more comorbidities predicted a greater likelihood of receiving a DOAC rather than warfarin. For those receiving mechanical values, the comorbidities with a significant association with greater DOAC use included hypertension (P = .003), dyslipidemia (P = .02), arrhythmia (P < .001), and peripheral arterial disease (P < 0.001).
The same factors were significant for predicting increased likelihood of a DOAC following bioprosthetic valve replacement, but there were additional factors, including atrial fibrillation independent of other types of arrhythmias (P < .001), a factor not significant for mechanical valves, as well as diabetes (P < .001), cerebrovascular disease (P < .001), dialysis (P < .001), and endocarditis (P < .001).
“This is probably intuitive, but patients who were on a factor Xa inhibitor before their valve replacement were also more likely to be discharged on a factor Xa inhibitor,” Dr. Kalra said at the virtual meeting, sponsored by MedStar Heart & Vascular Institute.
The year-to-year increase in DOAC use among those undergoing bioprosthetic valve replacement over the study period, which was a significant trend, was not observed among those undergoing mechanical valve replacement. Rather, the 1% proportion remained stable over the study period.
“We wanted to look at outcomes, but we found that the STS database, which only includes data out to 30 days, is not structured for this type of analysis,” Dr. Kalra said. He was also concerned about the limitations of a comparison in which 1% of the sample was being compared to 99%.
Expert: One percent is ‘very small number’
David J. Cohen, MD, commented on the 1% figure, which was so low that a moderator questioned whether it could be due mostly to coding errors.
“This is a very, very small number so at some level it is reassuring that it is so low in the mechanical valves,” Dr. Cohen said. However, he was more circumspect about the larger number in bioprosthetic valves.
“I have always thought it was a bit strange there was a warning against using them in bioprosthetic valves, especially in the aortic position,” he said.
“The trials that established the benefits of DOACs were all in nonvalvular atrial fibrillation, but this did not mean non–aortic stenosis; it meant non–mitral valvular. There have been articles written about how that has been misinterpreted,” said Dr. Cohen, director of clinical and outcomes research at the Cardiovascular Research Foundation and director of academic affairs at St. Francis Hospital, Roslyn, N.Y.
For his part, Dr. Kalra reported that he does not consider DOACs in patients who have undergone a surgical mechanical valve replacement. For bioprosthetic valves, he “prefers” warfarin over DOACs.
Overall, the evidence from the registry led Dr. Kalra to suggest that physicians should continue to “exercise caution” in using DOACs instead of warfarin after any surgical valve replacement “until randomized clinical trials provide sufficient evidence” to make a judgment about relative efficacy and safety.
Results of the study were published online as a research letter in Jama Network Open after Dr. Kalra’s presentation. Dr. Kalra and Dr. Cohen report no potential conflicts of interest.
FROM CRT 2021
Five-day course of oral antiviral appears to stop SARS-CoV-2 in its tracks
A single pill of the investigational drug molnupiravir taken twice a day for 5 days eliminated SARS-CoV-2 from the nasopharynx of 49 participants.
That led Carlos del Rio, MD, distinguished professor of medicine at Emory University, Atlanta, to suggest a future in which a drug like molnupiravir could be taken in the first few days of symptoms to prevent severe disease, similar to Tamiflu for influenza.
“I think it’s critically important,” he said of the data. Emory University was involved in the trial of molnupiravir but Dr. del Rio was not part of that team. “This drug offers the first antiviral oral drug that then could be used in an outpatient setting.”
Still, Dr. del Rio said it’s too soon to call this particular drug the breakthrough clinicians need to keep people out of the ICU. “It has the potential to be practice changing; it’s not practice changing at the moment.”
Wendy Painter, MD, of Ridgeback Biotherapeutics, who presented the data at the Conference on Retroviruses and Opportunistic Infections, agreed. While the data are promising, “We will need to see if people get better from actual illness” to assess the real value of the drug in clinical care.
“That’s a phase 3 objective we’ll need to prove,” she said in an interview.
Phase 2/3 efficacy and safety studies of the drug are now underway in hospitalized and nonhospitalized patients.
In a brief prerecorded presentation of the data, Dr. Painter laid out what researchers know so far: Preclinical studies suggest that molnupiravir is effective against a number of viruses, including coronaviruses and specifically SARS-CoV-2. It prevents a virus from replicating by inducing viral error catastrophe (Proc Natl Acad Sci U S A. 2002 Oct 15;99[21]:13374-6) – essentially overloading the virus with replication and mutation until the virus burns itself out and can’t produce replicable copies.
In this phase 2a, randomized, double-blind, controlled trial, researchers recruited 202 adults who were treated at an outpatient clinic with fever or other symptoms of a respiratory virus and confirmed SARS-CoV-2 infection by day 4. Participants were randomly assigned to three different groups: 200 mg of molnupiravir, 400 mg, or 800 mg. The 200-mg arm was matched 1:1 with a placebo-controlled group, and the other two groups had three participants in the active group for every one control.
Participants took the pills twice daily for 5 days, and then were followed for a total of 28 days to monitor for complications or adverse events. At days 3, 5, 7, 14, and 28, researchers also took nasopharyngeal swabs for polymerase chain reaction tests, to sequence the virus, and to grow cultures of SARS-CoV-2 to see if the virus that’s present is actually capable of infecting others.
Notably, the pills do not have to be refrigerated at any point in the process, alleviating the cold-chain challenges that have plagued vaccines.
“There’s an urgent need for an easily produced, transported, stored, and administered antiviral drug against SARS-CoV-2,” Dr. Painter said.
Of the 202 people recruited, 182 had swabs that could be evaluated, of which 78 showed infection at baseline. The results are based on labs of those 78 participants.
By day 3, 28% of patients in the placebo arm had SARS-CoV-2 in their nasopharynx, compared with 20.4% of patients receiving any dose of molnupiravir. But by day 5, none of the participants receiving the active drug had evidence of SARS-CoV-2 in their nasopharynx. In comparison, 24% of people in the placebo arm still had detectable virus.
Halfway through the treatment course, differences in the presence of infectious virus were already evident. By day 3 of the 5-day course, 36.4% of participants in the 200-mg group had detectable virus in the nasopharynx, compared with 21% in the 400-mg group and just 12.5% in the 800-mg group. And although the reduction in SARS-CoV-2 was noticeable in the 200-mg and the 400-mg arms, it was only statistically significant in the 800-mg arm.
In contrast, by the end of the 5 days in the placebo groups, infectious virus varied from 18.2% in the 200-mg placebo group to 30% in the 800-mg group. This points out the variability of the disease course of SARS-CoV-2.
“You just don’t know” which infections will lead to serious disease, Dr. Painter said in an interview. “And don’t you wish we did?”
Seven participants discontinued treatment, though only four experienced adverse events. Three of those discontinued the trial because of adverse events. The study is still blinded, so it’s unclear what those events were, but Dr. Painter said that they were not thought to be related to the study drug.
The bottom line, said Dr. Painter, was that people treated with molnupiravir had starkly different outcomes in lab measures during the study.
“An average of 10 days after symptom onset, 24% of placebo patients remained culture positive” for SARS-CoV-2 – meaning there wasn’t just virus in the nasopharynx, but it was capable of replicating, Dr. Painter said. “In contrast, no infectious virus could be recovered at study day 5 in any molnupiravir-treated patients.”
A version of this article first appeared on Medscape.com.
A single pill of the investigational drug molnupiravir taken twice a day for 5 days eliminated SARS-CoV-2 from the nasopharynx of 49 participants.
That led Carlos del Rio, MD, distinguished professor of medicine at Emory University, Atlanta, to suggest a future in which a drug like molnupiravir could be taken in the first few days of symptoms to prevent severe disease, similar to Tamiflu for influenza.
“I think it’s critically important,” he said of the data. Emory University was involved in the trial of molnupiravir but Dr. del Rio was not part of that team. “This drug offers the first antiviral oral drug that then could be used in an outpatient setting.”
Still, Dr. del Rio said it’s too soon to call this particular drug the breakthrough clinicians need to keep people out of the ICU. “It has the potential to be practice changing; it’s not practice changing at the moment.”
Wendy Painter, MD, of Ridgeback Biotherapeutics, who presented the data at the Conference on Retroviruses and Opportunistic Infections, agreed. While the data are promising, “We will need to see if people get better from actual illness” to assess the real value of the drug in clinical care.
“That’s a phase 3 objective we’ll need to prove,” she said in an interview.
Phase 2/3 efficacy and safety studies of the drug are now underway in hospitalized and nonhospitalized patients.
In a brief prerecorded presentation of the data, Dr. Painter laid out what researchers know so far: Preclinical studies suggest that molnupiravir is effective against a number of viruses, including coronaviruses and specifically SARS-CoV-2. It prevents a virus from replicating by inducing viral error catastrophe (Proc Natl Acad Sci U S A. 2002 Oct 15;99[21]:13374-6) – essentially overloading the virus with replication and mutation until the virus burns itself out and can’t produce replicable copies.
In this phase 2a, randomized, double-blind, controlled trial, researchers recruited 202 adults who were treated at an outpatient clinic with fever or other symptoms of a respiratory virus and confirmed SARS-CoV-2 infection by day 4. Participants were randomly assigned to three different groups: 200 mg of molnupiravir, 400 mg, or 800 mg. The 200-mg arm was matched 1:1 with a placebo-controlled group, and the other two groups had three participants in the active group for every one control.
Participants took the pills twice daily for 5 days, and then were followed for a total of 28 days to monitor for complications or adverse events. At days 3, 5, 7, 14, and 28, researchers also took nasopharyngeal swabs for polymerase chain reaction tests, to sequence the virus, and to grow cultures of SARS-CoV-2 to see if the virus that’s present is actually capable of infecting others.
Notably, the pills do not have to be refrigerated at any point in the process, alleviating the cold-chain challenges that have plagued vaccines.
“There’s an urgent need for an easily produced, transported, stored, and administered antiviral drug against SARS-CoV-2,” Dr. Painter said.
Of the 202 people recruited, 182 had swabs that could be evaluated, of which 78 showed infection at baseline. The results are based on labs of those 78 participants.
By day 3, 28% of patients in the placebo arm had SARS-CoV-2 in their nasopharynx, compared with 20.4% of patients receiving any dose of molnupiravir. But by day 5, none of the participants receiving the active drug had evidence of SARS-CoV-2 in their nasopharynx. In comparison, 24% of people in the placebo arm still had detectable virus.
Halfway through the treatment course, differences in the presence of infectious virus were already evident. By day 3 of the 5-day course, 36.4% of participants in the 200-mg group had detectable virus in the nasopharynx, compared with 21% in the 400-mg group and just 12.5% in the 800-mg group. And although the reduction in SARS-CoV-2 was noticeable in the 200-mg and the 400-mg arms, it was only statistically significant in the 800-mg arm.
In contrast, by the end of the 5 days in the placebo groups, infectious virus varied from 18.2% in the 200-mg placebo group to 30% in the 800-mg group. This points out the variability of the disease course of SARS-CoV-2.
“You just don’t know” which infections will lead to serious disease, Dr. Painter said in an interview. “And don’t you wish we did?”
Seven participants discontinued treatment, though only four experienced adverse events. Three of those discontinued the trial because of adverse events. The study is still blinded, so it’s unclear what those events were, but Dr. Painter said that they were not thought to be related to the study drug.
The bottom line, said Dr. Painter, was that people treated with molnupiravir had starkly different outcomes in lab measures during the study.
“An average of 10 days after symptom onset, 24% of placebo patients remained culture positive” for SARS-CoV-2 – meaning there wasn’t just virus in the nasopharynx, but it was capable of replicating, Dr. Painter said. “In contrast, no infectious virus could be recovered at study day 5 in any molnupiravir-treated patients.”
A version of this article first appeared on Medscape.com.
A single pill of the investigational drug molnupiravir taken twice a day for 5 days eliminated SARS-CoV-2 from the nasopharynx of 49 participants.
That led Carlos del Rio, MD, distinguished professor of medicine at Emory University, Atlanta, to suggest a future in which a drug like molnupiravir could be taken in the first few days of symptoms to prevent severe disease, similar to Tamiflu for influenza.
“I think it’s critically important,” he said of the data. Emory University was involved in the trial of molnupiravir but Dr. del Rio was not part of that team. “This drug offers the first antiviral oral drug that then could be used in an outpatient setting.”
Still, Dr. del Rio said it’s too soon to call this particular drug the breakthrough clinicians need to keep people out of the ICU. “It has the potential to be practice changing; it’s not practice changing at the moment.”
Wendy Painter, MD, of Ridgeback Biotherapeutics, who presented the data at the Conference on Retroviruses and Opportunistic Infections, agreed. While the data are promising, “We will need to see if people get better from actual illness” to assess the real value of the drug in clinical care.
“That’s a phase 3 objective we’ll need to prove,” she said in an interview.
Phase 2/3 efficacy and safety studies of the drug are now underway in hospitalized and nonhospitalized patients.
In a brief prerecorded presentation of the data, Dr. Painter laid out what researchers know so far: Preclinical studies suggest that molnupiravir is effective against a number of viruses, including coronaviruses and specifically SARS-CoV-2. It prevents a virus from replicating by inducing viral error catastrophe (Proc Natl Acad Sci U S A. 2002 Oct 15;99[21]:13374-6) – essentially overloading the virus with replication and mutation until the virus burns itself out and can’t produce replicable copies.
In this phase 2a, randomized, double-blind, controlled trial, researchers recruited 202 adults who were treated at an outpatient clinic with fever or other symptoms of a respiratory virus and confirmed SARS-CoV-2 infection by day 4. Participants were randomly assigned to three different groups: 200 mg of molnupiravir, 400 mg, or 800 mg. The 200-mg arm was matched 1:1 with a placebo-controlled group, and the other two groups had three participants in the active group for every one control.
Participants took the pills twice daily for 5 days, and then were followed for a total of 28 days to monitor for complications or adverse events. At days 3, 5, 7, 14, and 28, researchers also took nasopharyngeal swabs for polymerase chain reaction tests, to sequence the virus, and to grow cultures of SARS-CoV-2 to see if the virus that’s present is actually capable of infecting others.
Notably, the pills do not have to be refrigerated at any point in the process, alleviating the cold-chain challenges that have plagued vaccines.
“There’s an urgent need for an easily produced, transported, stored, and administered antiviral drug against SARS-CoV-2,” Dr. Painter said.
Of the 202 people recruited, 182 had swabs that could be evaluated, of which 78 showed infection at baseline. The results are based on labs of those 78 participants.
By day 3, 28% of patients in the placebo arm had SARS-CoV-2 in their nasopharynx, compared with 20.4% of patients receiving any dose of molnupiravir. But by day 5, none of the participants receiving the active drug had evidence of SARS-CoV-2 in their nasopharynx. In comparison, 24% of people in the placebo arm still had detectable virus.
Halfway through the treatment course, differences in the presence of infectious virus were already evident. By day 3 of the 5-day course, 36.4% of participants in the 200-mg group had detectable virus in the nasopharynx, compared with 21% in the 400-mg group and just 12.5% in the 800-mg group. And although the reduction in SARS-CoV-2 was noticeable in the 200-mg and the 400-mg arms, it was only statistically significant in the 800-mg arm.
In contrast, by the end of the 5 days in the placebo groups, infectious virus varied from 18.2% in the 200-mg placebo group to 30% in the 800-mg group. This points out the variability of the disease course of SARS-CoV-2.
“You just don’t know” which infections will lead to serious disease, Dr. Painter said in an interview. “And don’t you wish we did?”
Seven participants discontinued treatment, though only four experienced adverse events. Three of those discontinued the trial because of adverse events. The study is still blinded, so it’s unclear what those events were, but Dr. Painter said that they were not thought to be related to the study drug.
The bottom line, said Dr. Painter, was that people treated with molnupiravir had starkly different outcomes in lab measures during the study.
“An average of 10 days after symptom onset, 24% of placebo patients remained culture positive” for SARS-CoV-2 – meaning there wasn’t just virus in the nasopharynx, but it was capable of replicating, Dr. Painter said. “In contrast, no infectious virus could be recovered at study day 5 in any molnupiravir-treated patients.”
A version of this article first appeared on Medscape.com.
CDC: Vaccinated people can gather indoors without masks
People who are fully vaccinated against COVID-19 can safely gather unmasked and inside with nonvulnerable people who are not yet immunized, according to long-awaited guidance released by the CDC.
“Today’s action represents an important first step. It is not our final destination,” CDC Director Rochelle Walensky, MD, said March 8 at a White House briefing. “As more people get vaccinated, levels of COVID-19 infection decline in communities, and as our understanding of COVID immunity improves, we look forward to updating these recommendations to the public.”
According to the new guidance, people who are at least 2 weeks out from their last dose can:
- Visit with other fully vaccinated people indoors without wearing masks or physical distancing.
- Visit with unvaccinated people from a single household who are at low risk for severe COVID-19 disease indoors without wearing masks or physical distancing
- Avoid quarantine and testing following exposure to someone if they remain asymptomatic.
However, there are still restrictions that will remain until further data are collected. Those who are fully vaccinated must still:
- Wear masks and physically distance in public settings and around people at high risk for severe disease.
- Wear masks and physically distance when visiting unvaccinated people from more than one household.
- Avoid medium- and large-sized gatherings.
- Avoid travel.
People considered at high risk for severe disease include older adults and those with cancer, chronic kidney disease, COPD, Down syndrome, heart disease, heart failure, a weakened immune system, obesity, sickle cell disease, and type 2 diabetes. The category also includes pregnant women and smokers.
“In public spaces, fully vaccinated people should continue to follow guidance to protect themselves and others, including wearing a well-fitted mask, physical distancing (at least 6 feet), avoiding crowds, avoiding poorly ventilated spaces, covering coughs and sneezes, washing hands often, and following any applicable workplace or school guidance,” the guidance says. “Fully vaccinated people should still watch for symptoms of COVID-19, especially following an exposure to someone with suspected or confirmed COVID-19.”
Respecting travel restrictions is still crucial, Dr. Walensky said, given past surges and variants that have emerged after periods of increased travel.
"We would like to give the opportunity for vaccinated grandparents to visit children and grandchildren who are healthy and local,” Dr. Walensky said.
But, she said, “It’s important to realize as we’re working through this that over 90% of the population is not yet vaccinated.”
For now, there are not enough data on transmission rates from those who are vaccinated to the rest of the public. However, Anthony Fauci, MD, said at a briefing last month that preliminary data are “pointing in a very favorable direction.”
Studies from Spain and Israel published last month showed the amount of viral load – or the amount of the COVID-19 virus in someone’s body – is significantly lower if someone gets infected after they’ve been vaccinated, compared with people who get infected and didn’t have the vaccine. Lower viral load means much lower chances of passing the virus to someone else, Dr. Fauci said.
“The science of COVID-19 is complex,” Dr. Walensky said, “and our understanding of it continues to evolve.”
A version of this article first appeared on WebMD.com.
People who are fully vaccinated against COVID-19 can safely gather unmasked and inside with nonvulnerable people who are not yet immunized, according to long-awaited guidance released by the CDC.
“Today’s action represents an important first step. It is not our final destination,” CDC Director Rochelle Walensky, MD, said March 8 at a White House briefing. “As more people get vaccinated, levels of COVID-19 infection decline in communities, and as our understanding of COVID immunity improves, we look forward to updating these recommendations to the public.”
According to the new guidance, people who are at least 2 weeks out from their last dose can:
- Visit with other fully vaccinated people indoors without wearing masks or physical distancing.
- Visit with unvaccinated people from a single household who are at low risk for severe COVID-19 disease indoors without wearing masks or physical distancing
- Avoid quarantine and testing following exposure to someone if they remain asymptomatic.
However, there are still restrictions that will remain until further data are collected. Those who are fully vaccinated must still:
- Wear masks and physically distance in public settings and around people at high risk for severe disease.
- Wear masks and physically distance when visiting unvaccinated people from more than one household.
- Avoid medium- and large-sized gatherings.
- Avoid travel.
People considered at high risk for severe disease include older adults and those with cancer, chronic kidney disease, COPD, Down syndrome, heart disease, heart failure, a weakened immune system, obesity, sickle cell disease, and type 2 diabetes. The category also includes pregnant women and smokers.
“In public spaces, fully vaccinated people should continue to follow guidance to protect themselves and others, including wearing a well-fitted mask, physical distancing (at least 6 feet), avoiding crowds, avoiding poorly ventilated spaces, covering coughs and sneezes, washing hands often, and following any applicable workplace or school guidance,” the guidance says. “Fully vaccinated people should still watch for symptoms of COVID-19, especially following an exposure to someone with suspected or confirmed COVID-19.”
Respecting travel restrictions is still crucial, Dr. Walensky said, given past surges and variants that have emerged after periods of increased travel.
"We would like to give the opportunity for vaccinated grandparents to visit children and grandchildren who are healthy and local,” Dr. Walensky said.
But, she said, “It’s important to realize as we’re working through this that over 90% of the population is not yet vaccinated.”
For now, there are not enough data on transmission rates from those who are vaccinated to the rest of the public. However, Anthony Fauci, MD, said at a briefing last month that preliminary data are “pointing in a very favorable direction.”
Studies from Spain and Israel published last month showed the amount of viral load – or the amount of the COVID-19 virus in someone’s body – is significantly lower if someone gets infected after they’ve been vaccinated, compared with people who get infected and didn’t have the vaccine. Lower viral load means much lower chances of passing the virus to someone else, Dr. Fauci said.
“The science of COVID-19 is complex,” Dr. Walensky said, “and our understanding of it continues to evolve.”
A version of this article first appeared on WebMD.com.
People who are fully vaccinated against COVID-19 can safely gather unmasked and inside with nonvulnerable people who are not yet immunized, according to long-awaited guidance released by the CDC.
“Today’s action represents an important first step. It is not our final destination,” CDC Director Rochelle Walensky, MD, said March 8 at a White House briefing. “As more people get vaccinated, levels of COVID-19 infection decline in communities, and as our understanding of COVID immunity improves, we look forward to updating these recommendations to the public.”
According to the new guidance, people who are at least 2 weeks out from their last dose can:
- Visit with other fully vaccinated people indoors without wearing masks or physical distancing.
- Visit with unvaccinated people from a single household who are at low risk for severe COVID-19 disease indoors without wearing masks or physical distancing
- Avoid quarantine and testing following exposure to someone if they remain asymptomatic.
However, there are still restrictions that will remain until further data are collected. Those who are fully vaccinated must still:
- Wear masks and physically distance in public settings and around people at high risk for severe disease.
- Wear masks and physically distance when visiting unvaccinated people from more than one household.
- Avoid medium- and large-sized gatherings.
- Avoid travel.
People considered at high risk for severe disease include older adults and those with cancer, chronic kidney disease, COPD, Down syndrome, heart disease, heart failure, a weakened immune system, obesity, sickle cell disease, and type 2 diabetes. The category also includes pregnant women and smokers.
“In public spaces, fully vaccinated people should continue to follow guidance to protect themselves and others, including wearing a well-fitted mask, physical distancing (at least 6 feet), avoiding crowds, avoiding poorly ventilated spaces, covering coughs and sneezes, washing hands often, and following any applicable workplace or school guidance,” the guidance says. “Fully vaccinated people should still watch for symptoms of COVID-19, especially following an exposure to someone with suspected or confirmed COVID-19.”
Respecting travel restrictions is still crucial, Dr. Walensky said, given past surges and variants that have emerged after periods of increased travel.
"We would like to give the opportunity for vaccinated grandparents to visit children and grandchildren who are healthy and local,” Dr. Walensky said.
But, she said, “It’s important to realize as we’re working through this that over 90% of the population is not yet vaccinated.”
For now, there are not enough data on transmission rates from those who are vaccinated to the rest of the public. However, Anthony Fauci, MD, said at a briefing last month that preliminary data are “pointing in a very favorable direction.”
Studies from Spain and Israel published last month showed the amount of viral load – or the amount of the COVID-19 virus in someone’s body – is significantly lower if someone gets infected after they’ve been vaccinated, compared with people who get infected and didn’t have the vaccine. Lower viral load means much lower chances of passing the virus to someone else, Dr. Fauci said.
“The science of COVID-19 is complex,” Dr. Walensky said, “and our understanding of it continues to evolve.”
A version of this article first appeared on WebMD.com.
How to make resident mental health care stigma free
Sarah Sofka, MD, FACP, noticed a pattern. As program director for the internal medicine (IM) residency at West Virginia University, Morgantown, she was informed when residents were sent to counseling because they were affected by burnout, depression, or anxiety. When trainees returned from these visits, many told her the same thing: They wished they had sought help sooner.
IM residents and their families had access to free counseling at WVU, but few used the resource, says Dr. Sofka. “So, we thought, let’s just schedule all of our residents for a therapy visit so they can go and see what it’s like,” she said. “This will hopefully decrease the stigma for seeking mental health care. If everybody’s going, it’s not a big deal.”
In July 2015, Dr. Sofka and her colleagues launched a universal well-being assessment program for the IM residents at WVU. The program leaders automatically scheduled first- and second-year residents for a visit to the faculty staff assistance program counselors. The visits were not mandatory, and residents could choose not to go; but if they did go, they received the entire day of their visit off from work.
Five and a half years after launching their program, Dr. Sofka and her colleagues conducted one of the first studies of the efficacy of an opt-out approach for resident mental wellness. They found that , suggesting that residents were seeking help proactively after having to at least consider it.
Opt-out counseling is a recent concept in residency programs – one that’s attracting interest from training programs across the country. Brown University, Providence, R.I.; the University of Colorado at Denver, Aurora; University of Pennsylvania, Philadelphia; and the University of California, San Francisco have at least one residency program that uses the approach.
Lisa Meeks, PhD, an assistant professor of family medicine at Michigan Medicine, in Ann Arbor, and other experts also believe opt-out counseling could decrease stigma and help normalize seeking care for mental health problems in the medical community while lowering the barriers for trainees who need help.
No time, no access, plenty of stigma
Burnout and mental health are known to be major concerns for health care workers, especially trainees. College graduates starting medical education have lower rates of burnout and depression, compared with demographically matched peers; however, once they’ve started training, medical students, residents, and fellows are more likely to be burned out and exhibit symptoms of depression. The ongoing COVID-19 pandemic is further fraying the well-being of overworked and traumatized health care professionals, and experts predict a mental health crisis will follow the viral crisis.
The Accreditation Council for Graduate Medical Education recently mandated that programs offer wellness services to trainees. Yet this doesn’t mean they are always used; well-known barriers stand between residents, medical students, and physicians and their receiving effective mental health treatment.
Two of the most obvious are access and time, given the grueling and often inflexible schedules of most trainees, says Jessica Gold, MD, a psychiatrist at Washington University, St. Louis, who specializes in treating medical professionals. Dr. Gold also points out that, to be done correctly, these programs require institutional support and investment – resources that aren’t always adequate.
“A lack of transparency and clear messaging around what is available, who provides the services, and how to access these services can be a major barrier,” says Erene Stergiopoulos, MD, a second-year psychiatry resident at the University of Toronto. In addition, there can be considerable lag between when a resident realizes they need help and when they manage to find a provider and schedule an appointment, says Dr. Meeks.
Even when these logistical barriers are overcome, trainees and physicians have to contend with the persistent stigma associated with mental health treatment in the culture of medicine, says Dr. Gold. A recent survey by the American College of Emergency Physicians found that 73% of surveyed physicians feel there is stigma in their workplace about seeking mental health treatment. Many state medical licensing boards still require physicians to disclose mental health treatment, which discourages many trainees and providers from seeking proactive care, says Mary Moffit, PhD, associate professor of psychiatry and director of the resident and faculty wellness program at Oregon Health & Science University, Portland.
How the opt-out approach works
“The idea is by making it opt-out, you really normalize it,” says Maneesh Batra, MD, MPH, associate director of the University of Washington, Seattle, Children’s Hospital residency program. Similar approaches have proven effective at shaping human behavior in other health care settings, including boosting testing rates for HIV and increasing immunization rates for childhood vaccines, Dr. Batra says.
In general, opt-out programs acknowledge that people are busy and won’t take that extra step or click that extra button if they don’t have to, says Oana Tomescu, MD, PhD, associate professor of clinical medicine and pediatrics at the University of Pennsylvania, Philadelphia.
In 2018, Dr. Sofka and her colleagues at WVU conducted a survey that showed that a majority of residents thought favorably of their opt-out program and said they would return to counseling for follow-up care. In their most recent study, published in the Journal of Graduate Medical Education in 2021, Dr. Sofka and her colleagues found that residents did just that – only 8 of 239 opted out of universally scheduled visits. Resident-initiated visits increased significantly from zero during the 2014-2015 academic year to 23 in 2018-2019. Between those periods, program-mandated visits decreased significantly from 12 to 3.
The initiative has succeeded in creating a culture of openness and caring at WVU, says 2nd-year internal medicine resident Nistha Modi, MD. “It sets the tone for the program – we talk about mental health openly,” says Dr. Modi.
Crucially, the counselors work out of a different building than the hospital where Dr. Modi and her fellow residents work and use a separate electronic medical record system to protect resident privacy. This is hugely important for medical trainees, note Dr. Tomescu, Dr. Gold, and many other experts. The therapists understand residency and medical education, and there is no limit to the number of visits a resident or fellow can make with the program counselors, says Dr. Modi.
Opt-out programs offer a counterbalance to many negative tendencies in residency, says Dr. Meeks. “We’ve normalized so many things that are not healthy and productive. ... We need to counterbalance that with normalizing help seeking. And it’s really difficult to normalize something that’s not part of a system.”
Costs, concerns, and systematic support
Providing unlimited, free counseling for trainees can be very beneficial, but it requires adequate funding and personnel resources. Offering unlimited access means that an institution has to follow through in making this degree of care available while also ensuring that the system doesn’t get overwhelmed or is unable to accommodate very sick individuals, says Dr. Gold.
Another concern that experts like Dr. Batra, Dr. Moffit, and Dr. Gold share is that residents who go to their scheduled appointments may not completely buy into the experience because it wasn’t their idea in the first place. Participation alone doesn’t necessarily indicate full acceptance. Program personnel don’t intend for these appointments to be thought of as mandatory, yet residents may still experience them that way. Several leading resident well-being programs instead emphasize outreach to trainees, institutional support, and accessible mental health resources that are – and feel – entirely voluntary.
“If I tell someone that they have to do something, it’s very different than if they arrive at that conclusion for themselves,” says Dr. Batra. “That’s how life works.”
When it comes to cost, a recent study published in Academic Medicine provides encouraging data. At the University of Colorado, an opt-out pilot program for IM and pediatrics interns during the 2017-2018 academic year cost just $940 total, equal to $11.75 per intern. As in West Virginia, the program in Colorado covered the cost of the visit, interns were provided a half day off (whether they attended their appointment or not), and the visits and surveys were entirely optional and confidential. During the 1-year pilot program, 29% of 80 interns attended the scheduled appointment, 56% opted out in advance, and 15% didn’t show up. The majority of interns who were surveyed (85%), however, thought the program should continue and that it had a positive effect on their wellness even if they didn’t attend their appointment.
In West Virginia, program costs are higher. The program has $20,000 in annual funding to cover the opt-out program and unlimited counseling visits for residents and fellows. With that funding, Dr. Sofka and her colleagues were also able to expand the program slightly last year to schedule all the critical care faculty for counseling visits. Cost is a barrier to expanding these services to the entire institution, which Dr. Sofka says she hopes to do one day.
Research in this area is still preliminary. The WVU and Colorado studies provide some of the first evidence in support of an opt-out approach. Eventually, it would be beneficial for multicenter studies and longitudinal research to track the effects of such programs over time, say Dr. Sofka and Ajay Major, MD, MBA, one of the study’s coauthors and a hematology/oncology fellow at the University of Chicago.
Whether a program goes with an opt-out approach or not, the systematic supports – protecting resident privacy, providing flexible scheduling, and more – are crucial.
As Dr. Tomescu notes, wellness shouldn’t be just something trainees have to do. “The key with really working on burnout at a huge level is for all programs and schools to recognize that it’s a shared responsibility.”
“I felt very fortunate that I was able to get some help throughout residency,” says Dr. Modi. “About how to be a better daughter. How to be content with things I have in life. How to be happy, and grateful. With the kind of job we have, I think we sometimes forget to be grateful.”
A version of this article first appeared on Medscape.com.
Sarah Sofka, MD, FACP, noticed a pattern. As program director for the internal medicine (IM) residency at West Virginia University, Morgantown, she was informed when residents were sent to counseling because they were affected by burnout, depression, or anxiety. When trainees returned from these visits, many told her the same thing: They wished they had sought help sooner.
IM residents and their families had access to free counseling at WVU, but few used the resource, says Dr. Sofka. “So, we thought, let’s just schedule all of our residents for a therapy visit so they can go and see what it’s like,” she said. “This will hopefully decrease the stigma for seeking mental health care. If everybody’s going, it’s not a big deal.”
In July 2015, Dr. Sofka and her colleagues launched a universal well-being assessment program for the IM residents at WVU. The program leaders automatically scheduled first- and second-year residents for a visit to the faculty staff assistance program counselors. The visits were not mandatory, and residents could choose not to go; but if they did go, they received the entire day of their visit off from work.
Five and a half years after launching their program, Dr. Sofka and her colleagues conducted one of the first studies of the efficacy of an opt-out approach for resident mental wellness. They found that , suggesting that residents were seeking help proactively after having to at least consider it.
Opt-out counseling is a recent concept in residency programs – one that’s attracting interest from training programs across the country. Brown University, Providence, R.I.; the University of Colorado at Denver, Aurora; University of Pennsylvania, Philadelphia; and the University of California, San Francisco have at least one residency program that uses the approach.
Lisa Meeks, PhD, an assistant professor of family medicine at Michigan Medicine, in Ann Arbor, and other experts also believe opt-out counseling could decrease stigma and help normalize seeking care for mental health problems in the medical community while lowering the barriers for trainees who need help.
No time, no access, plenty of stigma
Burnout and mental health are known to be major concerns for health care workers, especially trainees. College graduates starting medical education have lower rates of burnout and depression, compared with demographically matched peers; however, once they’ve started training, medical students, residents, and fellows are more likely to be burned out and exhibit symptoms of depression. The ongoing COVID-19 pandemic is further fraying the well-being of overworked and traumatized health care professionals, and experts predict a mental health crisis will follow the viral crisis.
The Accreditation Council for Graduate Medical Education recently mandated that programs offer wellness services to trainees. Yet this doesn’t mean they are always used; well-known barriers stand between residents, medical students, and physicians and their receiving effective mental health treatment.
Two of the most obvious are access and time, given the grueling and often inflexible schedules of most trainees, says Jessica Gold, MD, a psychiatrist at Washington University, St. Louis, who specializes in treating medical professionals. Dr. Gold also points out that, to be done correctly, these programs require institutional support and investment – resources that aren’t always adequate.
“A lack of transparency and clear messaging around what is available, who provides the services, and how to access these services can be a major barrier,” says Erene Stergiopoulos, MD, a second-year psychiatry resident at the University of Toronto. In addition, there can be considerable lag between when a resident realizes they need help and when they manage to find a provider and schedule an appointment, says Dr. Meeks.
Even when these logistical barriers are overcome, trainees and physicians have to contend with the persistent stigma associated with mental health treatment in the culture of medicine, says Dr. Gold. A recent survey by the American College of Emergency Physicians found that 73% of surveyed physicians feel there is stigma in their workplace about seeking mental health treatment. Many state medical licensing boards still require physicians to disclose mental health treatment, which discourages many trainees and providers from seeking proactive care, says Mary Moffit, PhD, associate professor of psychiatry and director of the resident and faculty wellness program at Oregon Health & Science University, Portland.
How the opt-out approach works
“The idea is by making it opt-out, you really normalize it,” says Maneesh Batra, MD, MPH, associate director of the University of Washington, Seattle, Children’s Hospital residency program. Similar approaches have proven effective at shaping human behavior in other health care settings, including boosting testing rates for HIV and increasing immunization rates for childhood vaccines, Dr. Batra says.
In general, opt-out programs acknowledge that people are busy and won’t take that extra step or click that extra button if they don’t have to, says Oana Tomescu, MD, PhD, associate professor of clinical medicine and pediatrics at the University of Pennsylvania, Philadelphia.
In 2018, Dr. Sofka and her colleagues at WVU conducted a survey that showed that a majority of residents thought favorably of their opt-out program and said they would return to counseling for follow-up care. In their most recent study, published in the Journal of Graduate Medical Education in 2021, Dr. Sofka and her colleagues found that residents did just that – only 8 of 239 opted out of universally scheduled visits. Resident-initiated visits increased significantly from zero during the 2014-2015 academic year to 23 in 2018-2019. Between those periods, program-mandated visits decreased significantly from 12 to 3.
The initiative has succeeded in creating a culture of openness and caring at WVU, says 2nd-year internal medicine resident Nistha Modi, MD. “It sets the tone for the program – we talk about mental health openly,” says Dr. Modi.
Crucially, the counselors work out of a different building than the hospital where Dr. Modi and her fellow residents work and use a separate electronic medical record system to protect resident privacy. This is hugely important for medical trainees, note Dr. Tomescu, Dr. Gold, and many other experts. The therapists understand residency and medical education, and there is no limit to the number of visits a resident or fellow can make with the program counselors, says Dr. Modi.
Opt-out programs offer a counterbalance to many negative tendencies in residency, says Dr. Meeks. “We’ve normalized so many things that are not healthy and productive. ... We need to counterbalance that with normalizing help seeking. And it’s really difficult to normalize something that’s not part of a system.”
Costs, concerns, and systematic support
Providing unlimited, free counseling for trainees can be very beneficial, but it requires adequate funding and personnel resources. Offering unlimited access means that an institution has to follow through in making this degree of care available while also ensuring that the system doesn’t get overwhelmed or is unable to accommodate very sick individuals, says Dr. Gold.
Another concern that experts like Dr. Batra, Dr. Moffit, and Dr. Gold share is that residents who go to their scheduled appointments may not completely buy into the experience because it wasn’t their idea in the first place. Participation alone doesn’t necessarily indicate full acceptance. Program personnel don’t intend for these appointments to be thought of as mandatory, yet residents may still experience them that way. Several leading resident well-being programs instead emphasize outreach to trainees, institutional support, and accessible mental health resources that are – and feel – entirely voluntary.
“If I tell someone that they have to do something, it’s very different than if they arrive at that conclusion for themselves,” says Dr. Batra. “That’s how life works.”
When it comes to cost, a recent study published in Academic Medicine provides encouraging data. At the University of Colorado, an opt-out pilot program for IM and pediatrics interns during the 2017-2018 academic year cost just $940 total, equal to $11.75 per intern. As in West Virginia, the program in Colorado covered the cost of the visit, interns were provided a half day off (whether they attended their appointment or not), and the visits and surveys were entirely optional and confidential. During the 1-year pilot program, 29% of 80 interns attended the scheduled appointment, 56% opted out in advance, and 15% didn’t show up. The majority of interns who were surveyed (85%), however, thought the program should continue and that it had a positive effect on their wellness even if they didn’t attend their appointment.
In West Virginia, program costs are higher. The program has $20,000 in annual funding to cover the opt-out program and unlimited counseling visits for residents and fellows. With that funding, Dr. Sofka and her colleagues were also able to expand the program slightly last year to schedule all the critical care faculty for counseling visits. Cost is a barrier to expanding these services to the entire institution, which Dr. Sofka says she hopes to do one day.
Research in this area is still preliminary. The WVU and Colorado studies provide some of the first evidence in support of an opt-out approach. Eventually, it would be beneficial for multicenter studies and longitudinal research to track the effects of such programs over time, say Dr. Sofka and Ajay Major, MD, MBA, one of the study’s coauthors and a hematology/oncology fellow at the University of Chicago.
Whether a program goes with an opt-out approach or not, the systematic supports – protecting resident privacy, providing flexible scheduling, and more – are crucial.
As Dr. Tomescu notes, wellness shouldn’t be just something trainees have to do. “The key with really working on burnout at a huge level is for all programs and schools to recognize that it’s a shared responsibility.”
“I felt very fortunate that I was able to get some help throughout residency,” says Dr. Modi. “About how to be a better daughter. How to be content with things I have in life. How to be happy, and grateful. With the kind of job we have, I think we sometimes forget to be grateful.”
A version of this article first appeared on Medscape.com.
Sarah Sofka, MD, FACP, noticed a pattern. As program director for the internal medicine (IM) residency at West Virginia University, Morgantown, she was informed when residents were sent to counseling because they were affected by burnout, depression, or anxiety. When trainees returned from these visits, many told her the same thing: They wished they had sought help sooner.
IM residents and their families had access to free counseling at WVU, but few used the resource, says Dr. Sofka. “So, we thought, let’s just schedule all of our residents for a therapy visit so they can go and see what it’s like,” she said. “This will hopefully decrease the stigma for seeking mental health care. If everybody’s going, it’s not a big deal.”
In July 2015, Dr. Sofka and her colleagues launched a universal well-being assessment program for the IM residents at WVU. The program leaders automatically scheduled first- and second-year residents for a visit to the faculty staff assistance program counselors. The visits were not mandatory, and residents could choose not to go; but if they did go, they received the entire day of their visit off from work.
Five and a half years after launching their program, Dr. Sofka and her colleagues conducted one of the first studies of the efficacy of an opt-out approach for resident mental wellness. They found that , suggesting that residents were seeking help proactively after having to at least consider it.
Opt-out counseling is a recent concept in residency programs – one that’s attracting interest from training programs across the country. Brown University, Providence, R.I.; the University of Colorado at Denver, Aurora; University of Pennsylvania, Philadelphia; and the University of California, San Francisco have at least one residency program that uses the approach.
Lisa Meeks, PhD, an assistant professor of family medicine at Michigan Medicine, in Ann Arbor, and other experts also believe opt-out counseling could decrease stigma and help normalize seeking care for mental health problems in the medical community while lowering the barriers for trainees who need help.
No time, no access, plenty of stigma
Burnout and mental health are known to be major concerns for health care workers, especially trainees. College graduates starting medical education have lower rates of burnout and depression, compared with demographically matched peers; however, once they’ve started training, medical students, residents, and fellows are more likely to be burned out and exhibit symptoms of depression. The ongoing COVID-19 pandemic is further fraying the well-being of overworked and traumatized health care professionals, and experts predict a mental health crisis will follow the viral crisis.
The Accreditation Council for Graduate Medical Education recently mandated that programs offer wellness services to trainees. Yet this doesn’t mean they are always used; well-known barriers stand between residents, medical students, and physicians and their receiving effective mental health treatment.
Two of the most obvious are access and time, given the grueling and often inflexible schedules of most trainees, says Jessica Gold, MD, a psychiatrist at Washington University, St. Louis, who specializes in treating medical professionals. Dr. Gold also points out that, to be done correctly, these programs require institutional support and investment – resources that aren’t always adequate.
“A lack of transparency and clear messaging around what is available, who provides the services, and how to access these services can be a major barrier,” says Erene Stergiopoulos, MD, a second-year psychiatry resident at the University of Toronto. In addition, there can be considerable lag between when a resident realizes they need help and when they manage to find a provider and schedule an appointment, says Dr. Meeks.
Even when these logistical barriers are overcome, trainees and physicians have to contend with the persistent stigma associated with mental health treatment in the culture of medicine, says Dr. Gold. A recent survey by the American College of Emergency Physicians found that 73% of surveyed physicians feel there is stigma in their workplace about seeking mental health treatment. Many state medical licensing boards still require physicians to disclose mental health treatment, which discourages many trainees and providers from seeking proactive care, says Mary Moffit, PhD, associate professor of psychiatry and director of the resident and faculty wellness program at Oregon Health & Science University, Portland.
How the opt-out approach works
“The idea is by making it opt-out, you really normalize it,” says Maneesh Batra, MD, MPH, associate director of the University of Washington, Seattle, Children’s Hospital residency program. Similar approaches have proven effective at shaping human behavior in other health care settings, including boosting testing rates for HIV and increasing immunization rates for childhood vaccines, Dr. Batra says.
In general, opt-out programs acknowledge that people are busy and won’t take that extra step or click that extra button if they don’t have to, says Oana Tomescu, MD, PhD, associate professor of clinical medicine and pediatrics at the University of Pennsylvania, Philadelphia.
In 2018, Dr. Sofka and her colleagues at WVU conducted a survey that showed that a majority of residents thought favorably of their opt-out program and said they would return to counseling for follow-up care. In their most recent study, published in the Journal of Graduate Medical Education in 2021, Dr. Sofka and her colleagues found that residents did just that – only 8 of 239 opted out of universally scheduled visits. Resident-initiated visits increased significantly from zero during the 2014-2015 academic year to 23 in 2018-2019. Between those periods, program-mandated visits decreased significantly from 12 to 3.
The initiative has succeeded in creating a culture of openness and caring at WVU, says 2nd-year internal medicine resident Nistha Modi, MD. “It sets the tone for the program – we talk about mental health openly,” says Dr. Modi.
Crucially, the counselors work out of a different building than the hospital where Dr. Modi and her fellow residents work and use a separate electronic medical record system to protect resident privacy. This is hugely important for medical trainees, note Dr. Tomescu, Dr. Gold, and many other experts. The therapists understand residency and medical education, and there is no limit to the number of visits a resident or fellow can make with the program counselors, says Dr. Modi.
Opt-out programs offer a counterbalance to many negative tendencies in residency, says Dr. Meeks. “We’ve normalized so many things that are not healthy and productive. ... We need to counterbalance that with normalizing help seeking. And it’s really difficult to normalize something that’s not part of a system.”
Costs, concerns, and systematic support
Providing unlimited, free counseling for trainees can be very beneficial, but it requires adequate funding and personnel resources. Offering unlimited access means that an institution has to follow through in making this degree of care available while also ensuring that the system doesn’t get overwhelmed or is unable to accommodate very sick individuals, says Dr. Gold.
Another concern that experts like Dr. Batra, Dr. Moffit, and Dr. Gold share is that residents who go to their scheduled appointments may not completely buy into the experience because it wasn’t their idea in the first place. Participation alone doesn’t necessarily indicate full acceptance. Program personnel don’t intend for these appointments to be thought of as mandatory, yet residents may still experience them that way. Several leading resident well-being programs instead emphasize outreach to trainees, institutional support, and accessible mental health resources that are – and feel – entirely voluntary.
“If I tell someone that they have to do something, it’s very different than if they arrive at that conclusion for themselves,” says Dr. Batra. “That’s how life works.”
When it comes to cost, a recent study published in Academic Medicine provides encouraging data. At the University of Colorado, an opt-out pilot program for IM and pediatrics interns during the 2017-2018 academic year cost just $940 total, equal to $11.75 per intern. As in West Virginia, the program in Colorado covered the cost of the visit, interns were provided a half day off (whether they attended their appointment or not), and the visits and surveys were entirely optional and confidential. During the 1-year pilot program, 29% of 80 interns attended the scheduled appointment, 56% opted out in advance, and 15% didn’t show up. The majority of interns who were surveyed (85%), however, thought the program should continue and that it had a positive effect on their wellness even if they didn’t attend their appointment.
In West Virginia, program costs are higher. The program has $20,000 in annual funding to cover the opt-out program and unlimited counseling visits for residents and fellows. With that funding, Dr. Sofka and her colleagues were also able to expand the program slightly last year to schedule all the critical care faculty for counseling visits. Cost is a barrier to expanding these services to the entire institution, which Dr. Sofka says she hopes to do one day.
Research in this area is still preliminary. The WVU and Colorado studies provide some of the first evidence in support of an opt-out approach. Eventually, it would be beneficial for multicenter studies and longitudinal research to track the effects of such programs over time, say Dr. Sofka and Ajay Major, MD, MBA, one of the study’s coauthors and a hematology/oncology fellow at the University of Chicago.
Whether a program goes with an opt-out approach or not, the systematic supports – protecting resident privacy, providing flexible scheduling, and more – are crucial.
As Dr. Tomescu notes, wellness shouldn’t be just something trainees have to do. “The key with really working on burnout at a huge level is for all programs and schools to recognize that it’s a shared responsibility.”
“I felt very fortunate that I was able to get some help throughout residency,” says Dr. Modi. “About how to be a better daughter. How to be content with things I have in life. How to be happy, and grateful. With the kind of job we have, I think we sometimes forget to be grateful.”
A version of this article first appeared on Medscape.com.
RECOVERY trial of COVID-19 treatments stops colchicine arm
On the advice of its independent data monitoring committee (DMC), the RECOVERY trial has stopped recruitment to the colchicine arm for lack of efficacy in patients hospitalized with COVID-19.
“The DMC saw no convincing evidence that further recruitment would provide conclusive proof of worthwhile mortality benefit either overall or in any prespecified subgroup,” the British investigators announced on March 5.
“The RECOVERY trial has already identified two anti-inflammatory drugs – dexamethasone and tocilizumab – that improve the chances of survival for patients with severe COVID-19. So, it is disappointing that colchicine, which is widely used to treat gout and other inflammatory conditions, has no effect in these patients,” cochief investigator Martin Landray, MBChB, PhD, said in a statement.
“We do large, randomized trials to establish whether a drug that seems promising in theory has real benefits for patients in practice. Unfortunately, colchicine is not one of those,” said Dr. Landry, University of Oxford (England).
The RECOVERY trial is evaluating a range of potential treatments for COVID-19 at 180 hospitals in the United Kingdom, Indonesia, and Nepal, and was designed with the expectation that drugs would be added or dropped as the evidence changes. Since November 2020, the trial has included an arm comparing colchicine with usual care alone.
As part of a routine meeting March 4, the DMC reviewed data from a preliminary analysis based on 2,178 deaths among 11,162 patients, 94% of whom were being treated with a corticosteroid such as dexamethasone.
The results showed no significant difference in the primary endpoint of 28-day mortality in patients randomized to colchicine versus usual care alone (20% vs. 19%; risk ratio, 1.02; 95% confidence interval, 0.94-1.11; P = .63).
Follow-up is ongoing and final results will be published as soon as possible, the investigators said. Thus far, there has been no convincing evidence of an effect of colchicine on clinical outcomes in hospitalized COVID-19 patients.
Recruitment will continue to all other treatment arms – aspirin, baricitinib, Regeneron’s antibody cocktail, and, in select hospitals, dimethyl fumarate – the investigators said.
Cochief investigator Peter Hornby, MD, PhD, also from the University of Oxford, noted that this has been the largest trial ever of colchicine. “Whilst we are disappointed that the overall result is negative, it is still important information for the future care of patients in the U.K. and worldwide.”
A version of this article first appeared on Medscape.com.
On the advice of its independent data monitoring committee (DMC), the RECOVERY trial has stopped recruitment to the colchicine arm for lack of efficacy in patients hospitalized with COVID-19.
“The DMC saw no convincing evidence that further recruitment would provide conclusive proof of worthwhile mortality benefit either overall or in any prespecified subgroup,” the British investigators announced on March 5.
“The RECOVERY trial has already identified two anti-inflammatory drugs – dexamethasone and tocilizumab – that improve the chances of survival for patients with severe COVID-19. So, it is disappointing that colchicine, which is widely used to treat gout and other inflammatory conditions, has no effect in these patients,” cochief investigator Martin Landray, MBChB, PhD, said in a statement.
“We do large, randomized trials to establish whether a drug that seems promising in theory has real benefits for patients in practice. Unfortunately, colchicine is not one of those,” said Dr. Landry, University of Oxford (England).
The RECOVERY trial is evaluating a range of potential treatments for COVID-19 at 180 hospitals in the United Kingdom, Indonesia, and Nepal, and was designed with the expectation that drugs would be added or dropped as the evidence changes. Since November 2020, the trial has included an arm comparing colchicine with usual care alone.
As part of a routine meeting March 4, the DMC reviewed data from a preliminary analysis based on 2,178 deaths among 11,162 patients, 94% of whom were being treated with a corticosteroid such as dexamethasone.
The results showed no significant difference in the primary endpoint of 28-day mortality in patients randomized to colchicine versus usual care alone (20% vs. 19%; risk ratio, 1.02; 95% confidence interval, 0.94-1.11; P = .63).
Follow-up is ongoing and final results will be published as soon as possible, the investigators said. Thus far, there has been no convincing evidence of an effect of colchicine on clinical outcomes in hospitalized COVID-19 patients.
Recruitment will continue to all other treatment arms – aspirin, baricitinib, Regeneron’s antibody cocktail, and, in select hospitals, dimethyl fumarate – the investigators said.
Cochief investigator Peter Hornby, MD, PhD, also from the University of Oxford, noted that this has been the largest trial ever of colchicine. “Whilst we are disappointed that the overall result is negative, it is still important information for the future care of patients in the U.K. and worldwide.”
A version of this article first appeared on Medscape.com.
On the advice of its independent data monitoring committee (DMC), the RECOVERY trial has stopped recruitment to the colchicine arm for lack of efficacy in patients hospitalized with COVID-19.
“The DMC saw no convincing evidence that further recruitment would provide conclusive proof of worthwhile mortality benefit either overall or in any prespecified subgroup,” the British investigators announced on March 5.
“The RECOVERY trial has already identified two anti-inflammatory drugs – dexamethasone and tocilizumab – that improve the chances of survival for patients with severe COVID-19. So, it is disappointing that colchicine, which is widely used to treat gout and other inflammatory conditions, has no effect in these patients,” cochief investigator Martin Landray, MBChB, PhD, said in a statement.
“We do large, randomized trials to establish whether a drug that seems promising in theory has real benefits for patients in practice. Unfortunately, colchicine is not one of those,” said Dr. Landry, University of Oxford (England).
The RECOVERY trial is evaluating a range of potential treatments for COVID-19 at 180 hospitals in the United Kingdom, Indonesia, and Nepal, and was designed with the expectation that drugs would be added or dropped as the evidence changes. Since November 2020, the trial has included an arm comparing colchicine with usual care alone.
As part of a routine meeting March 4, the DMC reviewed data from a preliminary analysis based on 2,178 deaths among 11,162 patients, 94% of whom were being treated with a corticosteroid such as dexamethasone.
The results showed no significant difference in the primary endpoint of 28-day mortality in patients randomized to colchicine versus usual care alone (20% vs. 19%; risk ratio, 1.02; 95% confidence interval, 0.94-1.11; P = .63).
Follow-up is ongoing and final results will be published as soon as possible, the investigators said. Thus far, there has been no convincing evidence of an effect of colchicine on clinical outcomes in hospitalized COVID-19 patients.
Recruitment will continue to all other treatment arms – aspirin, baricitinib, Regeneron’s antibody cocktail, and, in select hospitals, dimethyl fumarate – the investigators said.
Cochief investigator Peter Hornby, MD, PhD, also from the University of Oxford, noted that this has been the largest trial ever of colchicine. “Whilst we are disappointed that the overall result is negative, it is still important information for the future care of patients in the U.K. and worldwide.”
A version of this article first appeared on Medscape.com.
MIS-C follow-up proves challenging across pediatric hospitals
The discovery of any novel disease or condition means a steep learning curve as physicians must develop protocols for diagnosis, management, and follow-up on the fly in the midst of admitting and treating patients. Medical society task forces and committees often release interim guidance during the learning process, but each institution ultimately has to determine what works for them based on their resources, clinical experience, and patient population.
But when the novel condition demands the involvement of multiple different specialties, the challenge of management grows even more complex – as does follow-up after patients are discharged. Such has been the story with multisystem inflammatory syndrome in children (MIS-C), a complication of COVID-19 that shares some features with Kawasaki disease.
The similarities to Kawasaki provided physicians a place to start in developing appropriate treatment regimens and involved a similar interdisciplinary team from, at the least, cardiology and rheumatology, plus infectious disease since MIS-C results from COVID-19.
“It literally has it in the name – multisystem essentially hints that there are multiple specialties involved, multiple hands in the pot trying to manage the kids, and so each specialty has their own kind of unique role in the patient’s care even on the outpatient side,” said Samina S. Bhumbra, MD, an infectious disease pediatrician at Riley Hospital for Children and assistant professor of clinical pediatrics at Indiana University in Indianapolis. “This isn’t a disease that falls under one specialty.”
By July, the American College of Rheumatology had issued interim clinical guidance for management that most children’s hospitals have followed or slightly adapted. But ACR guidelines could not address how each institution should handle outpatient follow-up visits, especially since those visits required, again, at least cardiology and rheumatology if not infectious disease or other specialties as well.
“When their kids are admitted to the hospital, to be told at discharge you have to be followed up by all these specialists is a lot to handle,” Dr. Bhumbra said. But just as it’s difficult for parents to deal with the need to see several different doctors after discharge, it can be difficult at some institutions for physicians to design a follow-up schedule that can accommodate families, especially families who live far from the hospital in the first place.
“Some of our follow-up is disjointed because all of our clinics had never been on the same day just because of staff availability,” Dr. Bhumbra said. “But it can be a 2- to 3-hour drive for some of our patients, depending on how far they’re coming.”
Many of them can’t make that drive more than once in the same month, much less the same week.
“If you have multiple visits, it makes it more likely that they’re not showing up,” said Ryan M. Serrano, MD, a pediatric cardiologist at Riley and assistant professor of pediatrics at Indiana University. Riley used telehealth when possible, especially if families could get labs done near home. But pediatric echocardiograms require technicians who have experience with children, so families need to come to the hospital.
Children’s hospitals have therefore had to adapt scheduling strategies or develop pediatric specialty clinics to coordinate across the multiple departments and accommodate a complex follow-up regimen that is still evolving as physicians learn more about MIS-C.
Determining a follow-up regimen
Even before determining how to coordinate appointments, hospitals had to decide what follow-up itself should be.
“How long do we follow these patients and how often do we follow them?” said Melissa S. Oliver, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University.
“We’re seeing that a lot of our patients rapidly respond when they get appropriate therapy, but we don’t know about long-term outcomes yet. We’re all still learning.”
At Children’s Hospital of Philadelphia, infectious disease follows up 4-6 weeks post discharge. The cardiology division came up with a follow-up plan that has evolved over time, said Matthew Elias, MD, an attending cardiologist at CHOP’s Cardiac Center and clinical assistant professor of pediatrics at the University of Pennsylvania, Philadelphia.
Patients get an EKG and echocardiogram at 2 weeks and, if their condition is stable, 6 weeks after discharge. After that, it depends on the patient’s clinical situation. Patients with moderately diminished left ventricular systolic function are recommended to get an MRI scan 3 months after discharge and, if old enough, exercise stress tests. Otherwise, they are seen at 6 months, but that appointment is optional for those whose prior echos have consistently been normal.
Other institutions, including Riley, are following a similar schedule of 2-week, 6-week, and 6-month postdischarge follow-ups, and most plan to do a 1-year follow-up as well, although that 1-year mark hasn’t arrived yet for most. Most do rheumatology labs at the 2-week appointment and use that to determine steroids management and whether labs are needed at the 6-week appointment. If labs have normalized, they aren’t done at 6 months. Small variations in follow-up management exist across institutions, but all are remaining open to changes. Riley, for example, is considering MRI screening for ongoing cardiac inflammation at 6 months to a year for all patients, Dr. Serrano said.
The dedicated clinic model
The two challenges Riley needed to address were the lack of a clear consensus on what MIS-C follow-up should look like and the need for continuity of care, Dr. Serrano said.
Regular discussion in departmental meetings at Riley “progressed from how do we take care of them and what treatments do we give them to how do we follow them and manage them in outpatient,” Dr. Oliver said. In the inpatient setting, they had an interdisciplinary team, but how could they maintain that for outpatients without overwhelming the families?
“I think the main challenge is for the families to identify who is leading the care for them,” said Martha M. Rodriguez, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University. That sometimes led to families picking which follow-up appointments they would attend and which they would skip if they could not make them all – and sometimes they skipped the more important ones. “They would go to the appointment with me and then miss the cardiology appointments and the echocardiogram, which was more important to follow any abnormalities in the heart,” Dr. Rodriguez said.
After trying to coordinate separate follow-up appointments for months, Riley ultimately decided to form a dedicated clinic for MIS-C follow-up – a “one-stop shop” single appointment at each follow-up, Dr. Bhumbra said, that covers labs, EKG, echocardiogram, and any other necessary tests.
“Our goal with the clinic is to make life easier for the families and to be able to coordinate the appointments,” Dr. Rodriguez said. “They will be able to see the three of us, and it would be easier for us to communicate with each other about their plan.”
The clinic began Feb. 11 and occurs twice a month. Though it’s just begun, Dr. Oliver said the first clinic went well, and it’s helping them figure out the role each specialty needs to play in follow-up care.
“For us with rheumatology, after lab values have returned to normal and they’re off steroids, sometimes we think there isn’t much more we can contribute to,” she said. And then there are the patients who didn’t see any rheumatologists while inpatients.
“That’s what we’re trying to figure out as well,” Dr. Oliver said. “Should we be seeing every single kid regardless of whether we were involved in their inpatient [stay] or only seeing the ones we’ve seen?” She expects the coming months will help them work that out.
Texas Children’s Hospital in Houston also uses a dedicated clinic, but they set it up before the first MIS-C patient came through the doors, said Sara Kristen Sexson Tejtel, MD, a pediatric cardiologist at Texas Children’s. The hospital already has other types of multidisciplinary clinics, and they anticipated the challenge of getting families to come to too many appointments in a short period of time.
“Getting someone to come back once is hard enough,” Dr. Sexson Tejtel said. “Getting them to come back twice is impossible.”
Infectious disease is less involved at Texas Children’s, so it’s primarily Dr. Sexson Tejtel and her rheumatologist colleague who see the patients. They hold the clinic once a week, twice if needed.
“It does make the appointment a little longer, but I think the patients appreciate that everything can be addressed with that one visit,” Dr. Sexson Tejtel said. “Being in the hospital as long as some of these kids are is so hard, so making any of that easy as possible is so helpful.” A single appointment also allows the doctors to work together on what labs are needed so that children don’t need multiple labs drawn.
At the appointment, she and the rheumatologist enter the patient’s room and take the patient’s history together.
“It’s nice because it makes the family not to have to repeat things and tell the same story over and over,” she said. “Sometimes I ask questions that then the rheumatologist jumps off of, and then sometimes he’ll ask questions, and I’ll think, ‘Ooh, I’ll ask more questions about that.’ ”
In fact, this team approach at all clinics has made her a more thoughtful, well-rounded physician, she said.
“I have learned so much going to all of my multidisciplinary clinics, and I think I’m able to better care for my patients because I’m not just thinking about it from a cardiac perspective,” she said. “It takes some work, but it’s not hard and I think it is beneficial both for the patient and for the physician. This team approach is definitely where we’re trying to live right now.”
Separate but coordinated appointments
A dedicated clinic isn’t the answer for all institutions, however. At Children’s Hospital of Philadelphia, the size of the networks and all its satellites made a one-stop shop impractical.
“We talked about a consolidated clinic early on, when MIS-C was first emerging and all our groups were collaborating and coming up with our inpatient and outpatient care pathways,” said Sanjeev K. Swami, MD, an infectious disease pediatrician at CHOP and associate professor of clinical pediatrics at the University of Pennsylvania. But timing varies on when each specialist wants to see the families return, and existing clinic schedules and locations varied too much.
So CHOP coordinates appointments individually for each patient, depending on where the patient lives and sometimes stacking them on the same day when possible. Sometimes infectious disease or rheumatology use telehealth, and CHOP, like the other hospitals, prioritizes cardiology, especially for the patients who had cardiac abnormalities in the hospital, Dr. Swami said.
“All three of our groups try to be as flexible as possible. We’ve had a really good collaboration between our groups,” he said, and spreading out follow-up allows specialists to ask about concerns raised at previous appointments, ensuring stronger continuity of care.
“We can make sure things are getting followed up on,” Dr. Swami said. “I think that has been beneficial to make sure things aren’t falling through the cracks.”
CHOP cardiologist Dr. Elias said that ongoing communication, among providers and with families, has been absolutely crucial.
“Everyone’s been talking so frequently about our MIS-C patients while inpatient that by the time they’re an outpatient, it seems to work smoothly, where families are hearing similar items but with a different flair, one from infectious, one from rheumatology, and one from cardiology,” he said.
Children’s Mercy in Kansas City, Mo., also has multiple satellite clinics and follows a model similar to that of CHOP. They discussed having a dedicated multidisciplinary team for each MIS-C patient, but even the logistics of that were difficult, said Emily J. Fox, MD, a rheumatologist and assistant professor of pediatrics at the University of Missouri-Kansas City.
Instead, Children’s Mercy tries to coordinate follow-up appointments to be on the same day and often use telehealth for the rheumatology appointments. Families that live closer to the hospital’s location in Joplin, Mo., go in for their cardiology appointment there, and then Dr. Fox conducts a telehealth appointment with the help of nurses in Joplin.
“We really do try hard, especially since these kids are in the hospital for a long time, to make the coordination as easy as possible,” Dr. Fox said. “This was all was very new, especially in the beginning, but I think at least our group is getting a little bit more comfortable in managing these patients.”
Looking ahead
The biggest question that still looms is what happens to these children, if anything, down the line.
“What was unique about this was this was a new disease we were all learning about together with no baseline,” Dr. Swami said. “None of us had ever seen this condition before.”
So far, the prognosis for the vast majority of children is good. “Most of these kids survive, most of them are doing well, and they almost all recover,” Dr. Serrano said. Labs tend to normalize by 6 weeks post discharge, if not much earlier, and not much cardiac involvement is showing up at later follow-ups. But not even a year has passed, so there’s plenty to learn. “We don’t know if there’s long-term risk. I would not be surprised if 20 years down the road we’re finding out things about this that we had no idea” about, Dr. Serrano said. “Everybody wants answers, and nobody has any, and the answers we have may end up being wrong. That’s how it goes when you’re dealing with something you’ve never seen.”
Research underway will ideally begin providing those answers soon. CHOP is a participating site in an NIH-NHLBI–sponsored study, called COVID MUSIC, that is tracking long-term outcomes for MIS-C at 30 centers across the United States and Canada for 5 years.
“That will really definitely be helpful in answering some of the questions about long-term outcomes,” Dr. Elias said. “We hope this is going to be a transient issue and that patients won’t have any long-term manifestations, but we don’t know that yet.”
Meanwhile, one benefit that has come out of the pandemic is strong collaboration, Dr. Bhumbra said.
“The biggest thing we’re all eagerly waiting and hoping for is standard guidelines on how best to follow-up on these kids, but I know that’s a ways away,” Dr. Bhumbra said. So for now, each institution is doing what it can to develop protocols that they feel best serve the patients’ needs, such as Riley’s new dedicated MIS-C clinic. “It takes a village to take care of these kids, and MIS-C has proven that having a clinic with all three specialties at one clinic is going to be great for the families.”
Dr. Fox serves on a committee for Pfizer unrelated to MIS-C. No other doctors interviewed for this story had relevant conflicts of interest to disclose.
The discovery of any novel disease or condition means a steep learning curve as physicians must develop protocols for diagnosis, management, and follow-up on the fly in the midst of admitting and treating patients. Medical society task forces and committees often release interim guidance during the learning process, but each institution ultimately has to determine what works for them based on their resources, clinical experience, and patient population.
But when the novel condition demands the involvement of multiple different specialties, the challenge of management grows even more complex – as does follow-up after patients are discharged. Such has been the story with multisystem inflammatory syndrome in children (MIS-C), a complication of COVID-19 that shares some features with Kawasaki disease.
The similarities to Kawasaki provided physicians a place to start in developing appropriate treatment regimens and involved a similar interdisciplinary team from, at the least, cardiology and rheumatology, plus infectious disease since MIS-C results from COVID-19.
“It literally has it in the name – multisystem essentially hints that there are multiple specialties involved, multiple hands in the pot trying to manage the kids, and so each specialty has their own kind of unique role in the patient’s care even on the outpatient side,” said Samina S. Bhumbra, MD, an infectious disease pediatrician at Riley Hospital for Children and assistant professor of clinical pediatrics at Indiana University in Indianapolis. “This isn’t a disease that falls under one specialty.”
By July, the American College of Rheumatology had issued interim clinical guidance for management that most children’s hospitals have followed or slightly adapted. But ACR guidelines could not address how each institution should handle outpatient follow-up visits, especially since those visits required, again, at least cardiology and rheumatology if not infectious disease or other specialties as well.
“When their kids are admitted to the hospital, to be told at discharge you have to be followed up by all these specialists is a lot to handle,” Dr. Bhumbra said. But just as it’s difficult for parents to deal with the need to see several different doctors after discharge, it can be difficult at some institutions for physicians to design a follow-up schedule that can accommodate families, especially families who live far from the hospital in the first place.
“Some of our follow-up is disjointed because all of our clinics had never been on the same day just because of staff availability,” Dr. Bhumbra said. “But it can be a 2- to 3-hour drive for some of our patients, depending on how far they’re coming.”
Many of them can’t make that drive more than once in the same month, much less the same week.
“If you have multiple visits, it makes it more likely that they’re not showing up,” said Ryan M. Serrano, MD, a pediatric cardiologist at Riley and assistant professor of pediatrics at Indiana University. Riley used telehealth when possible, especially if families could get labs done near home. But pediatric echocardiograms require technicians who have experience with children, so families need to come to the hospital.
Children’s hospitals have therefore had to adapt scheduling strategies or develop pediatric specialty clinics to coordinate across the multiple departments and accommodate a complex follow-up regimen that is still evolving as physicians learn more about MIS-C.
Determining a follow-up regimen
Even before determining how to coordinate appointments, hospitals had to decide what follow-up itself should be.
“How long do we follow these patients and how often do we follow them?” said Melissa S. Oliver, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University.
“We’re seeing that a lot of our patients rapidly respond when they get appropriate therapy, but we don’t know about long-term outcomes yet. We’re all still learning.”
At Children’s Hospital of Philadelphia, infectious disease follows up 4-6 weeks post discharge. The cardiology division came up with a follow-up plan that has evolved over time, said Matthew Elias, MD, an attending cardiologist at CHOP’s Cardiac Center and clinical assistant professor of pediatrics at the University of Pennsylvania, Philadelphia.
Patients get an EKG and echocardiogram at 2 weeks and, if their condition is stable, 6 weeks after discharge. After that, it depends on the patient’s clinical situation. Patients with moderately diminished left ventricular systolic function are recommended to get an MRI scan 3 months after discharge and, if old enough, exercise stress tests. Otherwise, they are seen at 6 months, but that appointment is optional for those whose prior echos have consistently been normal.
Other institutions, including Riley, are following a similar schedule of 2-week, 6-week, and 6-month postdischarge follow-ups, and most plan to do a 1-year follow-up as well, although that 1-year mark hasn’t arrived yet for most. Most do rheumatology labs at the 2-week appointment and use that to determine steroids management and whether labs are needed at the 6-week appointment. If labs have normalized, they aren’t done at 6 months. Small variations in follow-up management exist across institutions, but all are remaining open to changes. Riley, for example, is considering MRI screening for ongoing cardiac inflammation at 6 months to a year for all patients, Dr. Serrano said.
The dedicated clinic model
The two challenges Riley needed to address were the lack of a clear consensus on what MIS-C follow-up should look like and the need for continuity of care, Dr. Serrano said.
Regular discussion in departmental meetings at Riley “progressed from how do we take care of them and what treatments do we give them to how do we follow them and manage them in outpatient,” Dr. Oliver said. In the inpatient setting, they had an interdisciplinary team, but how could they maintain that for outpatients without overwhelming the families?
“I think the main challenge is for the families to identify who is leading the care for them,” said Martha M. Rodriguez, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University. That sometimes led to families picking which follow-up appointments they would attend and which they would skip if they could not make them all – and sometimes they skipped the more important ones. “They would go to the appointment with me and then miss the cardiology appointments and the echocardiogram, which was more important to follow any abnormalities in the heart,” Dr. Rodriguez said.
After trying to coordinate separate follow-up appointments for months, Riley ultimately decided to form a dedicated clinic for MIS-C follow-up – a “one-stop shop” single appointment at each follow-up, Dr. Bhumbra said, that covers labs, EKG, echocardiogram, and any other necessary tests.
“Our goal with the clinic is to make life easier for the families and to be able to coordinate the appointments,” Dr. Rodriguez said. “They will be able to see the three of us, and it would be easier for us to communicate with each other about their plan.”
The clinic began Feb. 11 and occurs twice a month. Though it’s just begun, Dr. Oliver said the first clinic went well, and it’s helping them figure out the role each specialty needs to play in follow-up care.
“For us with rheumatology, after lab values have returned to normal and they’re off steroids, sometimes we think there isn’t much more we can contribute to,” she said. And then there are the patients who didn’t see any rheumatologists while inpatients.
“That’s what we’re trying to figure out as well,” Dr. Oliver said. “Should we be seeing every single kid regardless of whether we were involved in their inpatient [stay] or only seeing the ones we’ve seen?” She expects the coming months will help them work that out.
Texas Children’s Hospital in Houston also uses a dedicated clinic, but they set it up before the first MIS-C patient came through the doors, said Sara Kristen Sexson Tejtel, MD, a pediatric cardiologist at Texas Children’s. The hospital already has other types of multidisciplinary clinics, and they anticipated the challenge of getting families to come to too many appointments in a short period of time.
“Getting someone to come back once is hard enough,” Dr. Sexson Tejtel said. “Getting them to come back twice is impossible.”
Infectious disease is less involved at Texas Children’s, so it’s primarily Dr. Sexson Tejtel and her rheumatologist colleague who see the patients. They hold the clinic once a week, twice if needed.
“It does make the appointment a little longer, but I think the patients appreciate that everything can be addressed with that one visit,” Dr. Sexson Tejtel said. “Being in the hospital as long as some of these kids are is so hard, so making any of that easy as possible is so helpful.” A single appointment also allows the doctors to work together on what labs are needed so that children don’t need multiple labs drawn.
At the appointment, she and the rheumatologist enter the patient’s room and take the patient’s history together.
“It’s nice because it makes the family not to have to repeat things and tell the same story over and over,” she said. “Sometimes I ask questions that then the rheumatologist jumps off of, and then sometimes he’ll ask questions, and I’ll think, ‘Ooh, I’ll ask more questions about that.’ ”
In fact, this team approach at all clinics has made her a more thoughtful, well-rounded physician, she said.
“I have learned so much going to all of my multidisciplinary clinics, and I think I’m able to better care for my patients because I’m not just thinking about it from a cardiac perspective,” she said. “It takes some work, but it’s not hard and I think it is beneficial both for the patient and for the physician. This team approach is definitely where we’re trying to live right now.”
Separate but coordinated appointments
A dedicated clinic isn’t the answer for all institutions, however. At Children’s Hospital of Philadelphia, the size of the networks and all its satellites made a one-stop shop impractical.
“We talked about a consolidated clinic early on, when MIS-C was first emerging and all our groups were collaborating and coming up with our inpatient and outpatient care pathways,” said Sanjeev K. Swami, MD, an infectious disease pediatrician at CHOP and associate professor of clinical pediatrics at the University of Pennsylvania. But timing varies on when each specialist wants to see the families return, and existing clinic schedules and locations varied too much.
So CHOP coordinates appointments individually for each patient, depending on where the patient lives and sometimes stacking them on the same day when possible. Sometimes infectious disease or rheumatology use telehealth, and CHOP, like the other hospitals, prioritizes cardiology, especially for the patients who had cardiac abnormalities in the hospital, Dr. Swami said.
“All three of our groups try to be as flexible as possible. We’ve had a really good collaboration between our groups,” he said, and spreading out follow-up allows specialists to ask about concerns raised at previous appointments, ensuring stronger continuity of care.
“We can make sure things are getting followed up on,” Dr. Swami said. “I think that has been beneficial to make sure things aren’t falling through the cracks.”
CHOP cardiologist Dr. Elias said that ongoing communication, among providers and with families, has been absolutely crucial.
“Everyone’s been talking so frequently about our MIS-C patients while inpatient that by the time they’re an outpatient, it seems to work smoothly, where families are hearing similar items but with a different flair, one from infectious, one from rheumatology, and one from cardiology,” he said.
Children’s Mercy in Kansas City, Mo., also has multiple satellite clinics and follows a model similar to that of CHOP. They discussed having a dedicated multidisciplinary team for each MIS-C patient, but even the logistics of that were difficult, said Emily J. Fox, MD, a rheumatologist and assistant professor of pediatrics at the University of Missouri-Kansas City.
Instead, Children’s Mercy tries to coordinate follow-up appointments to be on the same day and often use telehealth for the rheumatology appointments. Families that live closer to the hospital’s location in Joplin, Mo., go in for their cardiology appointment there, and then Dr. Fox conducts a telehealth appointment with the help of nurses in Joplin.
“We really do try hard, especially since these kids are in the hospital for a long time, to make the coordination as easy as possible,” Dr. Fox said. “This was all was very new, especially in the beginning, but I think at least our group is getting a little bit more comfortable in managing these patients.”
Looking ahead
The biggest question that still looms is what happens to these children, if anything, down the line.
“What was unique about this was this was a new disease we were all learning about together with no baseline,” Dr. Swami said. “None of us had ever seen this condition before.”
So far, the prognosis for the vast majority of children is good. “Most of these kids survive, most of them are doing well, and they almost all recover,” Dr. Serrano said. Labs tend to normalize by 6 weeks post discharge, if not much earlier, and not much cardiac involvement is showing up at later follow-ups. But not even a year has passed, so there’s plenty to learn. “We don’t know if there’s long-term risk. I would not be surprised if 20 years down the road we’re finding out things about this that we had no idea” about, Dr. Serrano said. “Everybody wants answers, and nobody has any, and the answers we have may end up being wrong. That’s how it goes when you’re dealing with something you’ve never seen.”
Research underway will ideally begin providing those answers soon. CHOP is a participating site in an NIH-NHLBI–sponsored study, called COVID MUSIC, that is tracking long-term outcomes for MIS-C at 30 centers across the United States and Canada for 5 years.
“That will really definitely be helpful in answering some of the questions about long-term outcomes,” Dr. Elias said. “We hope this is going to be a transient issue and that patients won’t have any long-term manifestations, but we don’t know that yet.”
Meanwhile, one benefit that has come out of the pandemic is strong collaboration, Dr. Bhumbra said.
“The biggest thing we’re all eagerly waiting and hoping for is standard guidelines on how best to follow-up on these kids, but I know that’s a ways away,” Dr. Bhumbra said. So for now, each institution is doing what it can to develop protocols that they feel best serve the patients’ needs, such as Riley’s new dedicated MIS-C clinic. “It takes a village to take care of these kids, and MIS-C has proven that having a clinic with all three specialties at one clinic is going to be great for the families.”
Dr. Fox serves on a committee for Pfizer unrelated to MIS-C. No other doctors interviewed for this story had relevant conflicts of interest to disclose.
The discovery of any novel disease or condition means a steep learning curve as physicians must develop protocols for diagnosis, management, and follow-up on the fly in the midst of admitting and treating patients. Medical society task forces and committees often release interim guidance during the learning process, but each institution ultimately has to determine what works for them based on their resources, clinical experience, and patient population.
But when the novel condition demands the involvement of multiple different specialties, the challenge of management grows even more complex – as does follow-up after patients are discharged. Such has been the story with multisystem inflammatory syndrome in children (MIS-C), a complication of COVID-19 that shares some features with Kawasaki disease.
The similarities to Kawasaki provided physicians a place to start in developing appropriate treatment regimens and involved a similar interdisciplinary team from, at the least, cardiology and rheumatology, plus infectious disease since MIS-C results from COVID-19.
“It literally has it in the name – multisystem essentially hints that there are multiple specialties involved, multiple hands in the pot trying to manage the kids, and so each specialty has their own kind of unique role in the patient’s care even on the outpatient side,” said Samina S. Bhumbra, MD, an infectious disease pediatrician at Riley Hospital for Children and assistant professor of clinical pediatrics at Indiana University in Indianapolis. “This isn’t a disease that falls under one specialty.”
By July, the American College of Rheumatology had issued interim clinical guidance for management that most children’s hospitals have followed or slightly adapted. But ACR guidelines could not address how each institution should handle outpatient follow-up visits, especially since those visits required, again, at least cardiology and rheumatology if not infectious disease or other specialties as well.
“When their kids are admitted to the hospital, to be told at discharge you have to be followed up by all these specialists is a lot to handle,” Dr. Bhumbra said. But just as it’s difficult for parents to deal with the need to see several different doctors after discharge, it can be difficult at some institutions for physicians to design a follow-up schedule that can accommodate families, especially families who live far from the hospital in the first place.
“Some of our follow-up is disjointed because all of our clinics had never been on the same day just because of staff availability,” Dr. Bhumbra said. “But it can be a 2- to 3-hour drive for some of our patients, depending on how far they’re coming.”
Many of them can’t make that drive more than once in the same month, much less the same week.
“If you have multiple visits, it makes it more likely that they’re not showing up,” said Ryan M. Serrano, MD, a pediatric cardiologist at Riley and assistant professor of pediatrics at Indiana University. Riley used telehealth when possible, especially if families could get labs done near home. But pediatric echocardiograms require technicians who have experience with children, so families need to come to the hospital.
Children’s hospitals have therefore had to adapt scheduling strategies or develop pediatric specialty clinics to coordinate across the multiple departments and accommodate a complex follow-up regimen that is still evolving as physicians learn more about MIS-C.
Determining a follow-up regimen
Even before determining how to coordinate appointments, hospitals had to decide what follow-up itself should be.
“How long do we follow these patients and how often do we follow them?” said Melissa S. Oliver, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University.
“We’re seeing that a lot of our patients rapidly respond when they get appropriate therapy, but we don’t know about long-term outcomes yet. We’re all still learning.”
At Children’s Hospital of Philadelphia, infectious disease follows up 4-6 weeks post discharge. The cardiology division came up with a follow-up plan that has evolved over time, said Matthew Elias, MD, an attending cardiologist at CHOP’s Cardiac Center and clinical assistant professor of pediatrics at the University of Pennsylvania, Philadelphia.
Patients get an EKG and echocardiogram at 2 weeks and, if their condition is stable, 6 weeks after discharge. After that, it depends on the patient’s clinical situation. Patients with moderately diminished left ventricular systolic function are recommended to get an MRI scan 3 months after discharge and, if old enough, exercise stress tests. Otherwise, they are seen at 6 months, but that appointment is optional for those whose prior echos have consistently been normal.
Other institutions, including Riley, are following a similar schedule of 2-week, 6-week, and 6-month postdischarge follow-ups, and most plan to do a 1-year follow-up as well, although that 1-year mark hasn’t arrived yet for most. Most do rheumatology labs at the 2-week appointment and use that to determine steroids management and whether labs are needed at the 6-week appointment. If labs have normalized, they aren’t done at 6 months. Small variations in follow-up management exist across institutions, but all are remaining open to changes. Riley, for example, is considering MRI screening for ongoing cardiac inflammation at 6 months to a year for all patients, Dr. Serrano said.
The dedicated clinic model
The two challenges Riley needed to address were the lack of a clear consensus on what MIS-C follow-up should look like and the need for continuity of care, Dr. Serrano said.
Regular discussion in departmental meetings at Riley “progressed from how do we take care of them and what treatments do we give them to how do we follow them and manage them in outpatient,” Dr. Oliver said. In the inpatient setting, they had an interdisciplinary team, but how could they maintain that for outpatients without overwhelming the families?
“I think the main challenge is for the families to identify who is leading the care for them,” said Martha M. Rodriguez, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University. That sometimes led to families picking which follow-up appointments they would attend and which they would skip if they could not make them all – and sometimes they skipped the more important ones. “They would go to the appointment with me and then miss the cardiology appointments and the echocardiogram, which was more important to follow any abnormalities in the heart,” Dr. Rodriguez said.
After trying to coordinate separate follow-up appointments for months, Riley ultimately decided to form a dedicated clinic for MIS-C follow-up – a “one-stop shop” single appointment at each follow-up, Dr. Bhumbra said, that covers labs, EKG, echocardiogram, and any other necessary tests.
“Our goal with the clinic is to make life easier for the families and to be able to coordinate the appointments,” Dr. Rodriguez said. “They will be able to see the three of us, and it would be easier for us to communicate with each other about their plan.”
The clinic began Feb. 11 and occurs twice a month. Though it’s just begun, Dr. Oliver said the first clinic went well, and it’s helping them figure out the role each specialty needs to play in follow-up care.
“For us with rheumatology, after lab values have returned to normal and they’re off steroids, sometimes we think there isn’t much more we can contribute to,” she said. And then there are the patients who didn’t see any rheumatologists while inpatients.
“That’s what we’re trying to figure out as well,” Dr. Oliver said. “Should we be seeing every single kid regardless of whether we were involved in their inpatient [stay] or only seeing the ones we’ve seen?” She expects the coming months will help them work that out.
Texas Children’s Hospital in Houston also uses a dedicated clinic, but they set it up before the first MIS-C patient came through the doors, said Sara Kristen Sexson Tejtel, MD, a pediatric cardiologist at Texas Children’s. The hospital already has other types of multidisciplinary clinics, and they anticipated the challenge of getting families to come to too many appointments in a short period of time.
“Getting someone to come back once is hard enough,” Dr. Sexson Tejtel said. “Getting them to come back twice is impossible.”
Infectious disease is less involved at Texas Children’s, so it’s primarily Dr. Sexson Tejtel and her rheumatologist colleague who see the patients. They hold the clinic once a week, twice if needed.
“It does make the appointment a little longer, but I think the patients appreciate that everything can be addressed with that one visit,” Dr. Sexson Tejtel said. “Being in the hospital as long as some of these kids are is so hard, so making any of that easy as possible is so helpful.” A single appointment also allows the doctors to work together on what labs are needed so that children don’t need multiple labs drawn.
At the appointment, she and the rheumatologist enter the patient’s room and take the patient’s history together.
“It’s nice because it makes the family not to have to repeat things and tell the same story over and over,” she said. “Sometimes I ask questions that then the rheumatologist jumps off of, and then sometimes he’ll ask questions, and I’ll think, ‘Ooh, I’ll ask more questions about that.’ ”
In fact, this team approach at all clinics has made her a more thoughtful, well-rounded physician, she said.
“I have learned so much going to all of my multidisciplinary clinics, and I think I’m able to better care for my patients because I’m not just thinking about it from a cardiac perspective,” she said. “It takes some work, but it’s not hard and I think it is beneficial both for the patient and for the physician. This team approach is definitely where we’re trying to live right now.”
Separate but coordinated appointments
A dedicated clinic isn’t the answer for all institutions, however. At Children’s Hospital of Philadelphia, the size of the networks and all its satellites made a one-stop shop impractical.
“We talked about a consolidated clinic early on, when MIS-C was first emerging and all our groups were collaborating and coming up with our inpatient and outpatient care pathways,” said Sanjeev K. Swami, MD, an infectious disease pediatrician at CHOP and associate professor of clinical pediatrics at the University of Pennsylvania. But timing varies on when each specialist wants to see the families return, and existing clinic schedules and locations varied too much.
So CHOP coordinates appointments individually for each patient, depending on where the patient lives and sometimes stacking them on the same day when possible. Sometimes infectious disease or rheumatology use telehealth, and CHOP, like the other hospitals, prioritizes cardiology, especially for the patients who had cardiac abnormalities in the hospital, Dr. Swami said.
“All three of our groups try to be as flexible as possible. We’ve had a really good collaboration between our groups,” he said, and spreading out follow-up allows specialists to ask about concerns raised at previous appointments, ensuring stronger continuity of care.
“We can make sure things are getting followed up on,” Dr. Swami said. “I think that has been beneficial to make sure things aren’t falling through the cracks.”
CHOP cardiologist Dr. Elias said that ongoing communication, among providers and with families, has been absolutely crucial.
“Everyone’s been talking so frequently about our MIS-C patients while inpatient that by the time they’re an outpatient, it seems to work smoothly, where families are hearing similar items but with a different flair, one from infectious, one from rheumatology, and one from cardiology,” he said.
Children’s Mercy in Kansas City, Mo., also has multiple satellite clinics and follows a model similar to that of CHOP. They discussed having a dedicated multidisciplinary team for each MIS-C patient, but even the logistics of that were difficult, said Emily J. Fox, MD, a rheumatologist and assistant professor of pediatrics at the University of Missouri-Kansas City.
Instead, Children’s Mercy tries to coordinate follow-up appointments to be on the same day and often use telehealth for the rheumatology appointments. Families that live closer to the hospital’s location in Joplin, Mo., go in for their cardiology appointment there, and then Dr. Fox conducts a telehealth appointment with the help of nurses in Joplin.
“We really do try hard, especially since these kids are in the hospital for a long time, to make the coordination as easy as possible,” Dr. Fox said. “This was all was very new, especially in the beginning, but I think at least our group is getting a little bit more comfortable in managing these patients.”
Looking ahead
The biggest question that still looms is what happens to these children, if anything, down the line.
“What was unique about this was this was a new disease we were all learning about together with no baseline,” Dr. Swami said. “None of us had ever seen this condition before.”
So far, the prognosis for the vast majority of children is good. “Most of these kids survive, most of them are doing well, and they almost all recover,” Dr. Serrano said. Labs tend to normalize by 6 weeks post discharge, if not much earlier, and not much cardiac involvement is showing up at later follow-ups. But not even a year has passed, so there’s plenty to learn. “We don’t know if there’s long-term risk. I would not be surprised if 20 years down the road we’re finding out things about this that we had no idea” about, Dr. Serrano said. “Everybody wants answers, and nobody has any, and the answers we have may end up being wrong. That’s how it goes when you’re dealing with something you’ve never seen.”
Research underway will ideally begin providing those answers soon. CHOP is a participating site in an NIH-NHLBI–sponsored study, called COVID MUSIC, that is tracking long-term outcomes for MIS-C at 30 centers across the United States and Canada for 5 years.
“That will really definitely be helpful in answering some of the questions about long-term outcomes,” Dr. Elias said. “We hope this is going to be a transient issue and that patients won’t have any long-term manifestations, but we don’t know that yet.”
Meanwhile, one benefit that has come out of the pandemic is strong collaboration, Dr. Bhumbra said.
“The biggest thing we’re all eagerly waiting and hoping for is standard guidelines on how best to follow-up on these kids, but I know that’s a ways away,” Dr. Bhumbra said. So for now, each institution is doing what it can to develop protocols that they feel best serve the patients’ needs, such as Riley’s new dedicated MIS-C clinic. “It takes a village to take care of these kids, and MIS-C has proven that having a clinic with all three specialties at one clinic is going to be great for the families.”
Dr. Fox serves on a committee for Pfizer unrelated to MIS-C. No other doctors interviewed for this story had relevant conflicts of interest to disclose.
Heart failure redefined with new classifications, staging
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.
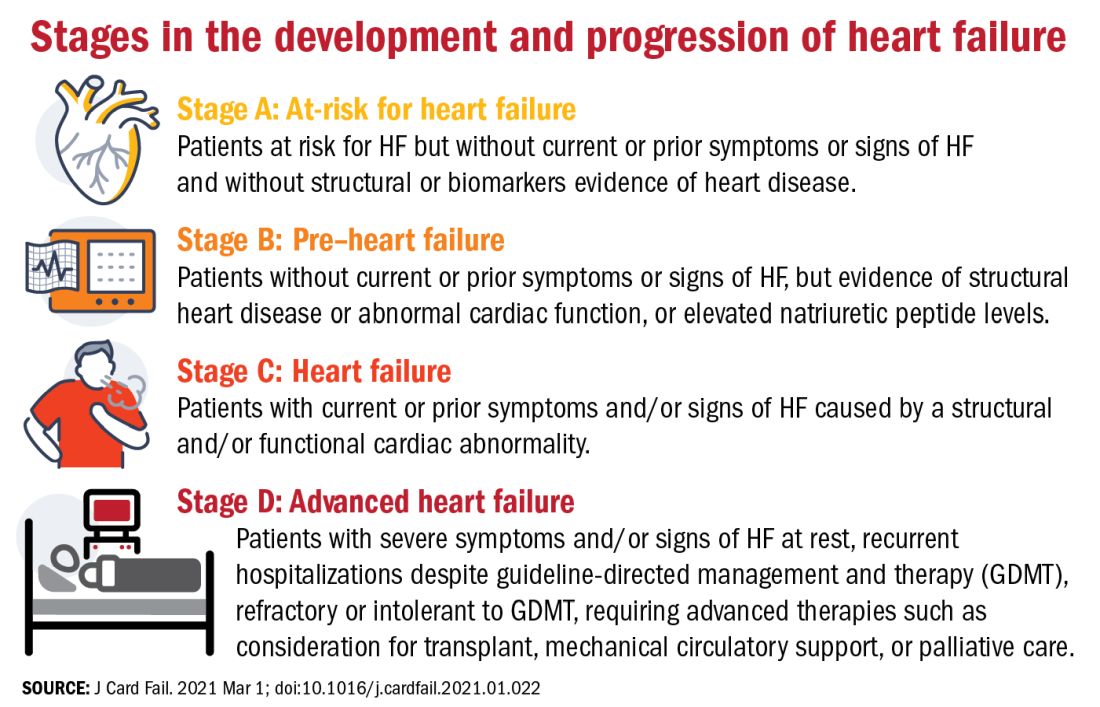
One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.

One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.

One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
FROM THE JOURNAL OF CARDIAC FAILURE
Dining restrictions, mask mandates tied to less illness, death, CDC reaffirms
The numbers are in to back up two policies designed to restrict the spread of the COVID-19 pandemic.
Researchers at the Centers for Disease Control and Prevention) found that when states lifted restrictions on dining on premises at restaurants, rates of daily COVID-19 cases jumped 41-100 days later. COVID-19-related deaths also increased significantly after 60 days.
On the other hand, the same report demonstrates that state mask mandates slowed the spread of SARS-CoV-2 within a few weeks.
The study was published online March 5 in the CDC Morbidity and Mortality Weekly Report.
The investigators did not distinguish between outdoor and indoor restaurant dining. But they did compare COVID-19 case and death rates before and after most states banned restaurants from serving patrons on-premises in March and April 2020.
They found, for example, that COVID-19 daily cases increased by 0.9% at 41-60 days after on-premise dining was permitted. Similarly, rates jumped by 1.2% at 61-80 days, and 1.1% at 81-100 days after the restaurant restrictions were lifted.
The differences were statistically significant, with P values of .02, <.01, and .04, respectively.
COVID-19–related death rates did not increase significantly at first – but did jump 2.2% between 61 and 80 days after the return of on-premises dining, for example. Deaths also increased by 3% at 81-100 days.
Both these differences were statistically significant (P < .01).
This is not the first report where the CDC announced reservations about in-person dining. In September 2020, CDC investigators implicated the inability to wear a mask while eating and drinking as likely contributing to the heightened risk.
Masks make a difference
The CDC report also provided more evidence to back mask-wearing policies for public spaces. Between March 1 and Dec. 31, 2020, 74% of U.S. counties issued mask mandates.
Investigators found that these policies had a more immediate effect, reducing daily COVID-19 cases by 0.5% in the first 20 days. Mask mandates likewise were linked to daily cases dropping 1.1% between 21 and 40 days, 1.5% between 41 and 60 days, 1.7% between 61 and 80 days, and 1.8% between 81 and 100 days.
These decreases in daily COVID-19 cases were statistically significant (P < .01) compared with a reference period before March 1, 2020.
The CDC also linked mask mandates to lower mortality. For example, these state policies were associated with 0.7% fewer deaths at 1-20 days post implementation. The effect increased thereafter – 1.0% drop at 21-40 days, 1.4% decrease at 41-60 days, 1.6% drop between 61 and 80 days, and 1.9% fewer deaths between 81 and 100 days.
The decrease in deaths was statistically significant at 1-20 days after the mask mandate (P = .03), as well as during the other periods (each P < .01) compared with the reference period.
CDC Director Rochelle Walensky, MD, reacted to the new findings at a White House press briefing. She cited how increases in COVID-19 cases and death rates “slowed significantly within 20 days of putting mask mandates into place. This is why I’m asking you to double down on prevention measures.
“We have seen this movie before,” Dr. Walensky added. “When prevention measures like mask-wearing mandates are lifted, cases go up.”
Recently, multiple states have announced plans to roll back restrictions related to the pandemic, including mask mandates, which prompted warnings from some public health officials.
These are not the first CDC data to show that mask mandates make a difference.
In February 2021, for example, the agency pointed out that state-wide mask mandates reduced COVID-19 hospitalizations by 5.5% among adults 18-64 years old within 3 weeks of implementation.
Restrictions regarding on-premises restaurant dining and implementation of state-wide mask mandates are two tactics within a more comprehensive CDC strategy to reduce the spread of SARS-CoV-2. The researchers note that “such efforts are increasingly important given the emergence of highly transmissible SARS-CoV-2 variants in the United States.”
The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The numbers are in to back up two policies designed to restrict the spread of the COVID-19 pandemic.
Researchers at the Centers for Disease Control and Prevention) found that when states lifted restrictions on dining on premises at restaurants, rates of daily COVID-19 cases jumped 41-100 days later. COVID-19-related deaths also increased significantly after 60 days.
On the other hand, the same report demonstrates that state mask mandates slowed the spread of SARS-CoV-2 within a few weeks.
The study was published online March 5 in the CDC Morbidity and Mortality Weekly Report.
The investigators did not distinguish between outdoor and indoor restaurant dining. But they did compare COVID-19 case and death rates before and after most states banned restaurants from serving patrons on-premises in March and April 2020.
They found, for example, that COVID-19 daily cases increased by 0.9% at 41-60 days after on-premise dining was permitted. Similarly, rates jumped by 1.2% at 61-80 days, and 1.1% at 81-100 days after the restaurant restrictions were lifted.
The differences were statistically significant, with P values of .02, <.01, and .04, respectively.
COVID-19–related death rates did not increase significantly at first – but did jump 2.2% between 61 and 80 days after the return of on-premises dining, for example. Deaths also increased by 3% at 81-100 days.
Both these differences were statistically significant (P < .01).
This is not the first report where the CDC announced reservations about in-person dining. In September 2020, CDC investigators implicated the inability to wear a mask while eating and drinking as likely contributing to the heightened risk.
Masks make a difference
The CDC report also provided more evidence to back mask-wearing policies for public spaces. Between March 1 and Dec. 31, 2020, 74% of U.S. counties issued mask mandates.
Investigators found that these policies had a more immediate effect, reducing daily COVID-19 cases by 0.5% in the first 20 days. Mask mandates likewise were linked to daily cases dropping 1.1% between 21 and 40 days, 1.5% between 41 and 60 days, 1.7% between 61 and 80 days, and 1.8% between 81 and 100 days.
These decreases in daily COVID-19 cases were statistically significant (P < .01) compared with a reference period before March 1, 2020.
The CDC also linked mask mandates to lower mortality. For example, these state policies were associated with 0.7% fewer deaths at 1-20 days post implementation. The effect increased thereafter – 1.0% drop at 21-40 days, 1.4% decrease at 41-60 days, 1.6% drop between 61 and 80 days, and 1.9% fewer deaths between 81 and 100 days.
The decrease in deaths was statistically significant at 1-20 days after the mask mandate (P = .03), as well as during the other periods (each P < .01) compared with the reference period.
CDC Director Rochelle Walensky, MD, reacted to the new findings at a White House press briefing. She cited how increases in COVID-19 cases and death rates “slowed significantly within 20 days of putting mask mandates into place. This is why I’m asking you to double down on prevention measures.
“We have seen this movie before,” Dr. Walensky added. “When prevention measures like mask-wearing mandates are lifted, cases go up.”
Recently, multiple states have announced plans to roll back restrictions related to the pandemic, including mask mandates, which prompted warnings from some public health officials.
These are not the first CDC data to show that mask mandates make a difference.
In February 2021, for example, the agency pointed out that state-wide mask mandates reduced COVID-19 hospitalizations by 5.5% among adults 18-64 years old within 3 weeks of implementation.
Restrictions regarding on-premises restaurant dining and implementation of state-wide mask mandates are two tactics within a more comprehensive CDC strategy to reduce the spread of SARS-CoV-2. The researchers note that “such efforts are increasingly important given the emergence of highly transmissible SARS-CoV-2 variants in the United States.”
The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The numbers are in to back up two policies designed to restrict the spread of the COVID-19 pandemic.
Researchers at the Centers for Disease Control and Prevention) found that when states lifted restrictions on dining on premises at restaurants, rates of daily COVID-19 cases jumped 41-100 days later. COVID-19-related deaths also increased significantly after 60 days.
On the other hand, the same report demonstrates that state mask mandates slowed the spread of SARS-CoV-2 within a few weeks.
The study was published online March 5 in the CDC Morbidity and Mortality Weekly Report.
The investigators did not distinguish between outdoor and indoor restaurant dining. But they did compare COVID-19 case and death rates before and after most states banned restaurants from serving patrons on-premises in March and April 2020.
They found, for example, that COVID-19 daily cases increased by 0.9% at 41-60 days after on-premise dining was permitted. Similarly, rates jumped by 1.2% at 61-80 days, and 1.1% at 81-100 days after the restaurant restrictions were lifted.
The differences were statistically significant, with P values of .02, <.01, and .04, respectively.
COVID-19–related death rates did not increase significantly at first – but did jump 2.2% between 61 and 80 days after the return of on-premises dining, for example. Deaths also increased by 3% at 81-100 days.
Both these differences were statistically significant (P < .01).
This is not the first report where the CDC announced reservations about in-person dining. In September 2020, CDC investigators implicated the inability to wear a mask while eating and drinking as likely contributing to the heightened risk.
Masks make a difference
The CDC report also provided more evidence to back mask-wearing policies for public spaces. Between March 1 and Dec. 31, 2020, 74% of U.S. counties issued mask mandates.
Investigators found that these policies had a more immediate effect, reducing daily COVID-19 cases by 0.5% in the first 20 days. Mask mandates likewise were linked to daily cases dropping 1.1% between 21 and 40 days, 1.5% between 41 and 60 days, 1.7% between 61 and 80 days, and 1.8% between 81 and 100 days.
These decreases in daily COVID-19 cases were statistically significant (P < .01) compared with a reference period before March 1, 2020.
The CDC also linked mask mandates to lower mortality. For example, these state policies were associated with 0.7% fewer deaths at 1-20 days post implementation. The effect increased thereafter – 1.0% drop at 21-40 days, 1.4% decrease at 41-60 days, 1.6% drop between 61 and 80 days, and 1.9% fewer deaths between 81 and 100 days.
The decrease in deaths was statistically significant at 1-20 days after the mask mandate (P = .03), as well as during the other periods (each P < .01) compared with the reference period.
CDC Director Rochelle Walensky, MD, reacted to the new findings at a White House press briefing. She cited how increases in COVID-19 cases and death rates “slowed significantly within 20 days of putting mask mandates into place. This is why I’m asking you to double down on prevention measures.
“We have seen this movie before,” Dr. Walensky added. “When prevention measures like mask-wearing mandates are lifted, cases go up.”
Recently, multiple states have announced plans to roll back restrictions related to the pandemic, including mask mandates, which prompted warnings from some public health officials.
These are not the first CDC data to show that mask mandates make a difference.
In February 2021, for example, the agency pointed out that state-wide mask mandates reduced COVID-19 hospitalizations by 5.5% among adults 18-64 years old within 3 weeks of implementation.
Restrictions regarding on-premises restaurant dining and implementation of state-wide mask mandates are two tactics within a more comprehensive CDC strategy to reduce the spread of SARS-CoV-2. The researchers note that “such efforts are increasingly important given the emergence of highly transmissible SARS-CoV-2 variants in the United States.”
The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.




















