User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Dupilumab May Reduce Exacerbations in COPD, Type 2 Inflammation
Dupilumab significantly reduced exacerbations and improved lung function in adults with uncontrolled chronic obstructive pulmonary disease (COPD) and type 2 inflammation, based on data from more than 900 individuals.
Data from a phase 3 trial known as NOTUS were presented at the American Thoracic Society’s international conference and published simultaneously in The New England Journal of Medicine.
Dupilumab, a fully human monoclonal antibody, works by inhibiting the signaling of the interleukin 4 (IL-4) and IL-13 pathways and is approved for many conditions characterized by type 2 inflammation, wrote Surya P. Bhatt, MD, of The University of Alabama at Birmingham, and colleagues in the NEJM study.
“Last year, we showed in the BOREAS trial that dupilumab was very effective in lowering exacerbation frequency in patients with COPD who continued to have frequent exacerbations despite being on maximal inhaled therapy,” Dr. Bhatt said in an interview.
12 Months of COPD, Triple Inhaler Therapy
In the NOTUS study, the researchers randomized 470 adults with uncontrolled COPD and type 2 inflammation (defined as a blood eosinophil count of ≥ 300 cells/µL) to 300-mg subcutaneous dupilumab and 465 to a placebo every 2 weeks. Patients were enrolled between July 2020 and May 2023.
The study population included adults aged 40-85 years with physician-diagnosed COPD for at least 12 months who had received background triple inhaler therapy (an inhaled glucocorticoid agent plus long-acting muscarinic antagonist [LAMA]–long-acting beta-agonist [LABA] or LAMA-LABA alone) for at least 3 months and at a stable dose for at least 1 month. All participants were current or former smokers with a smoking history of at least 10 pack-years.
The primary endpoint was a reduction in the annualized rate of moderate or severe COPD exacerbations at 52 weeks.
Patients in the dupilumab group also saw a significantly greater improvement in lung function compared with individuals in the placebo group based on prebronchodilator forced expiratory volume in 1 second from baseline to 12 weeks (least squares mean change of 139 mL vs 57 mL). This improvement was sustained at 52 weeks (least squares mean change of 115 mL vs 54 mL).
Improvement in respiratory symptom severity based on the St. George’s Respiratory Questionnaire was another secondary endpoint, and changes in total score were greater in the dupilumab group than in the placebo group (least squares mean change of 9.8 vs 6.4).
Safety outcomes were similar between the dupilumab and placebo groups, with approximately 66% of patients in each group reporting adverse events during the 52-week study period. Serious adverse events occurred in 13% and 15.9% of dupilumab and placebo patients, respectively, and adverse events resulting in death occurred in 2.6% and 1.5%, respectively. The most common adverse events were COVID-19, which occurred in 9.4% and 8.2% of the dupilumab and placebo patients, respectively, followed by headache, COPD, and nasopharyngitis. Major adverse cardiovascular events occurred in three patients in the dupilumab group and seven patients in the placebo group.
The findings were limited by several factors including the reduced sample size for 52-week endpoints because of the earlier analysis and the primarily White study population, the researchers noted. The study was conducted in part during the COVID-19 pandemic period, which contributed to healthcare disruptions and behavior changes that decreased exposure to viral respiratory infections, they wrote in their discussion. However, the results were strengthened by the large numbers and international population without other major pulmonary diseases, such as asthma, and the 34% reduction in exacerbations with dupilumab vs placebo is clinically significant, they said.
Data May Drive US Food and Drug Administration (FDA) Approval
In the BOREAS trial, dupilumab also improved lung function and quality of life, with no notable safety concerns. “As with any trial evaluating the efficacy and safety of a medication, it is important to confirm the findings in a replicative study,” said Dr. Bhatt. “With NOTUS, we confirmed the findings of BOREAS,” and the researchers were reassured by the substantial reduction in exacerbation frequency and the replication of key secondary outcomes, he said.
With the NOTUS study, “two randomized trials have now shown near identical reductions in exacerbation frequency in a difficult-to-treat population of patients with COPD with type 2 inflammation and frequent exacerbations,” as well as a significant and meaningful improvement in lung function, Dr. Bhatt said in an interview. “We hope these trials pave for the way for regulatory body approval of dupilumab for clinical use,” he said. Looking ahead, more studies are needed to test the potential disease modification effects of dupilumab in patients with COPD, he added.
Potential Change in Patient Management
Approximately 20%-40% of patients with COPD have type 2 inflammation with elevated blood eosinophil count, and this subset of patients has an increased risk for exacerbations, with worsening lung function and quality of life, Dharani K. Narendra, MD, of Baylor College of Medicine, Houston, said in an interview.
Prior phase 3 studies have shown that dupilumab, a blocker of IL-4 and IL-13 pathways, could effectively reduce exacerbations and improve lung function in these patients, and the NOTUS study aimed to confirm the findings in a larger, more diverse population, said Dr. Narendra, who was not involved in the study.
The NOTUS study represents a paradigm shift in the management of COPD patients with type 2 inflammation, said Narendra. “This study validates the previous BOREAS trial and has shown that dupilumab reduces exacerbations, improves lung function and quality of life, and potentially slows disease progression,” she said.
If approved, potential barriers to the use of dupilumab in practice include cost and insurance coverage, education and dissemination of study findings, and limited data on side effects, said Dr. Narendra.
“While the NOTUS study provides valuable insights into the efficacy and safety of dupilumab over 52 weeks, longer-term studies are needed to understand the sustained benefits and risks of continued treatment,” Dr. Narendra told this news organization. “Studies comparing dupilumab with other biological agents and newer COPD treatments could provide insights into its relative efficacy and position in treatment protocols,” she said.
In addition, further research into dupilumab’s underlying mechanisms could provide deeper insights into the pathophysiology of type 2 inflammation in COPD and inform the development of new treatments, Dr. Narendra said. “These steps will help integrate dupilumab more effectively into clinical practice and optimize its use for COPD patients with type 2 inflammation,” she noted.
Dupilumab is undergoing Priority Review by the FDA as an add-on maintenance therapy for adults with uncontrolled COPD and type 2 inflammation, with a target action date of June 27, 2024, according to a company press release.
The study was funded by Sanofi and Regeneron Pharmaceuticals. Dr. Narendra had no financial conflicts to disclose but serves on the Editorial Advisory Board of CHEST Physician.
A version of this article appeared on Medscape.com.
Dupilumab significantly reduced exacerbations and improved lung function in adults with uncontrolled chronic obstructive pulmonary disease (COPD) and type 2 inflammation, based on data from more than 900 individuals.
Data from a phase 3 trial known as NOTUS were presented at the American Thoracic Society’s international conference and published simultaneously in The New England Journal of Medicine.
Dupilumab, a fully human monoclonal antibody, works by inhibiting the signaling of the interleukin 4 (IL-4) and IL-13 pathways and is approved for many conditions characterized by type 2 inflammation, wrote Surya P. Bhatt, MD, of The University of Alabama at Birmingham, and colleagues in the NEJM study.
“Last year, we showed in the BOREAS trial that dupilumab was very effective in lowering exacerbation frequency in patients with COPD who continued to have frequent exacerbations despite being on maximal inhaled therapy,” Dr. Bhatt said in an interview.
12 Months of COPD, Triple Inhaler Therapy
In the NOTUS study, the researchers randomized 470 adults with uncontrolled COPD and type 2 inflammation (defined as a blood eosinophil count of ≥ 300 cells/µL) to 300-mg subcutaneous dupilumab and 465 to a placebo every 2 weeks. Patients were enrolled between July 2020 and May 2023.
The study population included adults aged 40-85 years with physician-diagnosed COPD for at least 12 months who had received background triple inhaler therapy (an inhaled glucocorticoid agent plus long-acting muscarinic antagonist [LAMA]–long-acting beta-agonist [LABA] or LAMA-LABA alone) for at least 3 months and at a stable dose for at least 1 month. All participants were current or former smokers with a smoking history of at least 10 pack-years.
The primary endpoint was a reduction in the annualized rate of moderate or severe COPD exacerbations at 52 weeks.
Patients in the dupilumab group also saw a significantly greater improvement in lung function compared with individuals in the placebo group based on prebronchodilator forced expiratory volume in 1 second from baseline to 12 weeks (least squares mean change of 139 mL vs 57 mL). This improvement was sustained at 52 weeks (least squares mean change of 115 mL vs 54 mL).
Improvement in respiratory symptom severity based on the St. George’s Respiratory Questionnaire was another secondary endpoint, and changes in total score were greater in the dupilumab group than in the placebo group (least squares mean change of 9.8 vs 6.4).
Safety outcomes were similar between the dupilumab and placebo groups, with approximately 66% of patients in each group reporting adverse events during the 52-week study period. Serious adverse events occurred in 13% and 15.9% of dupilumab and placebo patients, respectively, and adverse events resulting in death occurred in 2.6% and 1.5%, respectively. The most common adverse events were COVID-19, which occurred in 9.4% and 8.2% of the dupilumab and placebo patients, respectively, followed by headache, COPD, and nasopharyngitis. Major adverse cardiovascular events occurred in three patients in the dupilumab group and seven patients in the placebo group.
The findings were limited by several factors including the reduced sample size for 52-week endpoints because of the earlier analysis and the primarily White study population, the researchers noted. The study was conducted in part during the COVID-19 pandemic period, which contributed to healthcare disruptions and behavior changes that decreased exposure to viral respiratory infections, they wrote in their discussion. However, the results were strengthened by the large numbers and international population without other major pulmonary diseases, such as asthma, and the 34% reduction in exacerbations with dupilumab vs placebo is clinically significant, they said.
Data May Drive US Food and Drug Administration (FDA) Approval
In the BOREAS trial, dupilumab also improved lung function and quality of life, with no notable safety concerns. “As with any trial evaluating the efficacy and safety of a medication, it is important to confirm the findings in a replicative study,” said Dr. Bhatt. “With NOTUS, we confirmed the findings of BOREAS,” and the researchers were reassured by the substantial reduction in exacerbation frequency and the replication of key secondary outcomes, he said.
With the NOTUS study, “two randomized trials have now shown near identical reductions in exacerbation frequency in a difficult-to-treat population of patients with COPD with type 2 inflammation and frequent exacerbations,” as well as a significant and meaningful improvement in lung function, Dr. Bhatt said in an interview. “We hope these trials pave for the way for regulatory body approval of dupilumab for clinical use,” he said. Looking ahead, more studies are needed to test the potential disease modification effects of dupilumab in patients with COPD, he added.
Potential Change in Patient Management
Approximately 20%-40% of patients with COPD have type 2 inflammation with elevated blood eosinophil count, and this subset of patients has an increased risk for exacerbations, with worsening lung function and quality of life, Dharani K. Narendra, MD, of Baylor College of Medicine, Houston, said in an interview.
Prior phase 3 studies have shown that dupilumab, a blocker of IL-4 and IL-13 pathways, could effectively reduce exacerbations and improve lung function in these patients, and the NOTUS study aimed to confirm the findings in a larger, more diverse population, said Dr. Narendra, who was not involved in the study.
The NOTUS study represents a paradigm shift in the management of COPD patients with type 2 inflammation, said Narendra. “This study validates the previous BOREAS trial and has shown that dupilumab reduces exacerbations, improves lung function and quality of life, and potentially slows disease progression,” she said.
If approved, potential barriers to the use of dupilumab in practice include cost and insurance coverage, education and dissemination of study findings, and limited data on side effects, said Dr. Narendra.
“While the NOTUS study provides valuable insights into the efficacy and safety of dupilumab over 52 weeks, longer-term studies are needed to understand the sustained benefits and risks of continued treatment,” Dr. Narendra told this news organization. “Studies comparing dupilumab with other biological agents and newer COPD treatments could provide insights into its relative efficacy and position in treatment protocols,” she said.
In addition, further research into dupilumab’s underlying mechanisms could provide deeper insights into the pathophysiology of type 2 inflammation in COPD and inform the development of new treatments, Dr. Narendra said. “These steps will help integrate dupilumab more effectively into clinical practice and optimize its use for COPD patients with type 2 inflammation,” she noted.
Dupilumab is undergoing Priority Review by the FDA as an add-on maintenance therapy for adults with uncontrolled COPD and type 2 inflammation, with a target action date of June 27, 2024, according to a company press release.
The study was funded by Sanofi and Regeneron Pharmaceuticals. Dr. Narendra had no financial conflicts to disclose but serves on the Editorial Advisory Board of CHEST Physician.
A version of this article appeared on Medscape.com.
Dupilumab significantly reduced exacerbations and improved lung function in adults with uncontrolled chronic obstructive pulmonary disease (COPD) and type 2 inflammation, based on data from more than 900 individuals.
Data from a phase 3 trial known as NOTUS were presented at the American Thoracic Society’s international conference and published simultaneously in The New England Journal of Medicine.
Dupilumab, a fully human monoclonal antibody, works by inhibiting the signaling of the interleukin 4 (IL-4) and IL-13 pathways and is approved for many conditions characterized by type 2 inflammation, wrote Surya P. Bhatt, MD, of The University of Alabama at Birmingham, and colleagues in the NEJM study.
“Last year, we showed in the BOREAS trial that dupilumab was very effective in lowering exacerbation frequency in patients with COPD who continued to have frequent exacerbations despite being on maximal inhaled therapy,” Dr. Bhatt said in an interview.
12 Months of COPD, Triple Inhaler Therapy
In the NOTUS study, the researchers randomized 470 adults with uncontrolled COPD and type 2 inflammation (defined as a blood eosinophil count of ≥ 300 cells/µL) to 300-mg subcutaneous dupilumab and 465 to a placebo every 2 weeks. Patients were enrolled between July 2020 and May 2023.
The study population included adults aged 40-85 years with physician-diagnosed COPD for at least 12 months who had received background triple inhaler therapy (an inhaled glucocorticoid agent plus long-acting muscarinic antagonist [LAMA]–long-acting beta-agonist [LABA] or LAMA-LABA alone) for at least 3 months and at a stable dose for at least 1 month. All participants were current or former smokers with a smoking history of at least 10 pack-years.
The primary endpoint was a reduction in the annualized rate of moderate or severe COPD exacerbations at 52 weeks.
Patients in the dupilumab group also saw a significantly greater improvement in lung function compared with individuals in the placebo group based on prebronchodilator forced expiratory volume in 1 second from baseline to 12 weeks (least squares mean change of 139 mL vs 57 mL). This improvement was sustained at 52 weeks (least squares mean change of 115 mL vs 54 mL).
Improvement in respiratory symptom severity based on the St. George’s Respiratory Questionnaire was another secondary endpoint, and changes in total score were greater in the dupilumab group than in the placebo group (least squares mean change of 9.8 vs 6.4).
Safety outcomes were similar between the dupilumab and placebo groups, with approximately 66% of patients in each group reporting adverse events during the 52-week study period. Serious adverse events occurred in 13% and 15.9% of dupilumab and placebo patients, respectively, and adverse events resulting in death occurred in 2.6% and 1.5%, respectively. The most common adverse events were COVID-19, which occurred in 9.4% and 8.2% of the dupilumab and placebo patients, respectively, followed by headache, COPD, and nasopharyngitis. Major adverse cardiovascular events occurred in three patients in the dupilumab group and seven patients in the placebo group.
The findings were limited by several factors including the reduced sample size for 52-week endpoints because of the earlier analysis and the primarily White study population, the researchers noted. The study was conducted in part during the COVID-19 pandemic period, which contributed to healthcare disruptions and behavior changes that decreased exposure to viral respiratory infections, they wrote in their discussion. However, the results were strengthened by the large numbers and international population without other major pulmonary diseases, such as asthma, and the 34% reduction in exacerbations with dupilumab vs placebo is clinically significant, they said.
Data May Drive US Food and Drug Administration (FDA) Approval
In the BOREAS trial, dupilumab also improved lung function and quality of life, with no notable safety concerns. “As with any trial evaluating the efficacy and safety of a medication, it is important to confirm the findings in a replicative study,” said Dr. Bhatt. “With NOTUS, we confirmed the findings of BOREAS,” and the researchers were reassured by the substantial reduction in exacerbation frequency and the replication of key secondary outcomes, he said.
With the NOTUS study, “two randomized trials have now shown near identical reductions in exacerbation frequency in a difficult-to-treat population of patients with COPD with type 2 inflammation and frequent exacerbations,” as well as a significant and meaningful improvement in lung function, Dr. Bhatt said in an interview. “We hope these trials pave for the way for regulatory body approval of dupilumab for clinical use,” he said. Looking ahead, more studies are needed to test the potential disease modification effects of dupilumab in patients with COPD, he added.
Potential Change in Patient Management
Approximately 20%-40% of patients with COPD have type 2 inflammation with elevated blood eosinophil count, and this subset of patients has an increased risk for exacerbations, with worsening lung function and quality of life, Dharani K. Narendra, MD, of Baylor College of Medicine, Houston, said in an interview.
Prior phase 3 studies have shown that dupilumab, a blocker of IL-4 and IL-13 pathways, could effectively reduce exacerbations and improve lung function in these patients, and the NOTUS study aimed to confirm the findings in a larger, more diverse population, said Dr. Narendra, who was not involved in the study.
The NOTUS study represents a paradigm shift in the management of COPD patients with type 2 inflammation, said Narendra. “This study validates the previous BOREAS trial and has shown that dupilumab reduces exacerbations, improves lung function and quality of life, and potentially slows disease progression,” she said.
If approved, potential barriers to the use of dupilumab in practice include cost and insurance coverage, education and dissemination of study findings, and limited data on side effects, said Dr. Narendra.
“While the NOTUS study provides valuable insights into the efficacy and safety of dupilumab over 52 weeks, longer-term studies are needed to understand the sustained benefits and risks of continued treatment,” Dr. Narendra told this news organization. “Studies comparing dupilumab with other biological agents and newer COPD treatments could provide insights into its relative efficacy and position in treatment protocols,” she said.
In addition, further research into dupilumab’s underlying mechanisms could provide deeper insights into the pathophysiology of type 2 inflammation in COPD and inform the development of new treatments, Dr. Narendra said. “These steps will help integrate dupilumab more effectively into clinical practice and optimize its use for COPD patients with type 2 inflammation,” she noted.
Dupilumab is undergoing Priority Review by the FDA as an add-on maintenance therapy for adults with uncontrolled COPD and type 2 inflammation, with a target action date of June 27, 2024, according to a company press release.
The study was funded by Sanofi and Regeneron Pharmaceuticals. Dr. Narendra had no financial conflicts to disclose but serves on the Editorial Advisory Board of CHEST Physician.
A version of this article appeared on Medscape.com.
FROM ATS 2024
Understudied Patients With COPD Benefit From BLVR
BLVR has shown promising results in previous studies for carefully selected patients with COPD, said Michael J. Nicholson, DO, of Temple University Hospital, Philadelphia. However, those with AATD have often been excluded from large BLVR trials, so data on its effectiveness in this population are limited, he said.
“The distinct pathophysiology of AATD poses challenges in extrapolating findings from trials involving COPD patients without AATD,” Dr. Nicholson noted. “Variations in affected lung lobes and disease progression are major differences between the AATD and non-AATD populations; we sought to examine if BLVR could provide significant, sustained benefit to AATD patients despite their differences from the typical COPD cohort,” he said.
Patients With COPD and AATD
In a study presented at the American Thoracic Society (ATS) 2024 International Conference, Dr. Nicholson and colleagues reviewed data from 238 adults with COPD including 14 with AATD who underwent BLVR at a single center between August 2018 and December 2022. Pulmonary function test data were collected at baseline and at a median of 7 months post-BLVR. The mean age of patients with AATD was 61.5 years, and 79% were men.
The primary outcome was the percentage of patients with forced expiratory volume per second (FEV1) improvement greater than 15%. Half of the patients with AATD achieved this outcome, with a median improvement in FEV1 of 110 mL and a significant difference in pre- and post-BLVR FEV1 volume based on a Wilcoxon signed rank test (W = 11.5; P < .05).
Patients with AATD also showed significant improvement in several secondary outcomes including BODE index, residual volume (RV), total lung capacity (TLC), RV/TLC ratio, and inspiratory capacity/RV ratio between pre- and post-BLVR.
“The sustained improvements seen at 7 months post-BLVR in patients with lower lobe disease were unexpected and promising,” Dr. Nicholson said in an interview. “In contrast to the National Emphysema Treatment Trial (NETT), which found lung volume reduction surgery ineffective for lower lobe disease, our study revealed significant improvements in lower lobe disease following BLVR,” he said. The sustained improvements up to 7 months post-BLVR are encouraging, given clinical concerns that the ongoing destruction of lung tissue in AATD could cause initial BLVR improvements to regress, he added.
Overall, the results suggest that BLVR is an effective therapy for appropriately selected patients with AATD and COPD, and that significant improvement in lung function can be achieved regardless of the affected lobe, Dr. Nicholson said.
“The primary obstacles to widespread BLVR implementation include the scarcity of equipment, as well as insufficient education and training for pulmonologists outside of major academic institutions,” Dr. Nicholson told this news organization. “Successful outcomes in BLVR require clinicians to have a deep understanding of patient selection criteria, extensive training in BLVR techniques, and access to the necessary technology within their facilities,” he said. However, BLVR has been integrated into pulmonary and interventional pulmonary fellowships nationwide, which paves the way for a new generation of pulmonologists to expand the use of the procedure, he said.
Looking ahead, prospective examination of BLVR vs the current standard of care in patients with AATD would provide invaluable data, Dr. Nicholson said. Since the presentation of the study at the meeting, additional patient data have been added to the analysis and increased the power of the findings, he said. “We intend to extend our assessment of pulmonary function testing beyond 7 months post-BLVR to evaluate the persistence of improvements in the long term,” he added.
Study Confirms Benefits for Wider Patient Population
“Lung volume reduction is an important intervention in patients with severe emphysema,” said David M. Mannino, MD, of the University of Kentucky, Lexington, Kentucky, in an interview. Most emphysema is in the upper lobes, but it tends to occur more in the lower lobes in patients with AATD, said Dr. Mannino, who was not involved in the study.
The findings were not especially surprising, but they were reassuring, Dr. Mannino told this news organization. “We know this intervention works in those with severe emphysema,” and it was helpful to confirm similar success in patients with AATD, he said.
The implications for practice are that BLVR is both a safe and an effective intervention for patients with lower or upper lobe emphysema, although longer-term follow-up studies are needed, he said.
The study received no outside funding. Dr. Nicholson and Dr. Mannino had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
BLVR has shown promising results in previous studies for carefully selected patients with COPD, said Michael J. Nicholson, DO, of Temple University Hospital, Philadelphia. However, those with AATD have often been excluded from large BLVR trials, so data on its effectiveness in this population are limited, he said.
“The distinct pathophysiology of AATD poses challenges in extrapolating findings from trials involving COPD patients without AATD,” Dr. Nicholson noted. “Variations in affected lung lobes and disease progression are major differences between the AATD and non-AATD populations; we sought to examine if BLVR could provide significant, sustained benefit to AATD patients despite their differences from the typical COPD cohort,” he said.
Patients With COPD and AATD
In a study presented at the American Thoracic Society (ATS) 2024 International Conference, Dr. Nicholson and colleagues reviewed data from 238 adults with COPD including 14 with AATD who underwent BLVR at a single center between August 2018 and December 2022. Pulmonary function test data were collected at baseline and at a median of 7 months post-BLVR. The mean age of patients with AATD was 61.5 years, and 79% were men.
The primary outcome was the percentage of patients with forced expiratory volume per second (FEV1) improvement greater than 15%. Half of the patients with AATD achieved this outcome, with a median improvement in FEV1 of 110 mL and a significant difference in pre- and post-BLVR FEV1 volume based on a Wilcoxon signed rank test (W = 11.5; P < .05).
Patients with AATD also showed significant improvement in several secondary outcomes including BODE index, residual volume (RV), total lung capacity (TLC), RV/TLC ratio, and inspiratory capacity/RV ratio between pre- and post-BLVR.
“The sustained improvements seen at 7 months post-BLVR in patients with lower lobe disease were unexpected and promising,” Dr. Nicholson said in an interview. “In contrast to the National Emphysema Treatment Trial (NETT), which found lung volume reduction surgery ineffective for lower lobe disease, our study revealed significant improvements in lower lobe disease following BLVR,” he said. The sustained improvements up to 7 months post-BLVR are encouraging, given clinical concerns that the ongoing destruction of lung tissue in AATD could cause initial BLVR improvements to regress, he added.
Overall, the results suggest that BLVR is an effective therapy for appropriately selected patients with AATD and COPD, and that significant improvement in lung function can be achieved regardless of the affected lobe, Dr. Nicholson said.
“The primary obstacles to widespread BLVR implementation include the scarcity of equipment, as well as insufficient education and training for pulmonologists outside of major academic institutions,” Dr. Nicholson told this news organization. “Successful outcomes in BLVR require clinicians to have a deep understanding of patient selection criteria, extensive training in BLVR techniques, and access to the necessary technology within their facilities,” he said. However, BLVR has been integrated into pulmonary and interventional pulmonary fellowships nationwide, which paves the way for a new generation of pulmonologists to expand the use of the procedure, he said.
Looking ahead, prospective examination of BLVR vs the current standard of care in patients with AATD would provide invaluable data, Dr. Nicholson said. Since the presentation of the study at the meeting, additional patient data have been added to the analysis and increased the power of the findings, he said. “We intend to extend our assessment of pulmonary function testing beyond 7 months post-BLVR to evaluate the persistence of improvements in the long term,” he added.
Study Confirms Benefits for Wider Patient Population
“Lung volume reduction is an important intervention in patients with severe emphysema,” said David M. Mannino, MD, of the University of Kentucky, Lexington, Kentucky, in an interview. Most emphysema is in the upper lobes, but it tends to occur more in the lower lobes in patients with AATD, said Dr. Mannino, who was not involved in the study.
The findings were not especially surprising, but they were reassuring, Dr. Mannino told this news organization. “We know this intervention works in those with severe emphysema,” and it was helpful to confirm similar success in patients with AATD, he said.
The implications for practice are that BLVR is both a safe and an effective intervention for patients with lower or upper lobe emphysema, although longer-term follow-up studies are needed, he said.
The study received no outside funding. Dr. Nicholson and Dr. Mannino had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
BLVR has shown promising results in previous studies for carefully selected patients with COPD, said Michael J. Nicholson, DO, of Temple University Hospital, Philadelphia. However, those with AATD have often been excluded from large BLVR trials, so data on its effectiveness in this population are limited, he said.
“The distinct pathophysiology of AATD poses challenges in extrapolating findings from trials involving COPD patients without AATD,” Dr. Nicholson noted. “Variations in affected lung lobes and disease progression are major differences between the AATD and non-AATD populations; we sought to examine if BLVR could provide significant, sustained benefit to AATD patients despite their differences from the typical COPD cohort,” he said.
Patients With COPD and AATD
In a study presented at the American Thoracic Society (ATS) 2024 International Conference, Dr. Nicholson and colleagues reviewed data from 238 adults with COPD including 14 with AATD who underwent BLVR at a single center between August 2018 and December 2022. Pulmonary function test data were collected at baseline and at a median of 7 months post-BLVR. The mean age of patients with AATD was 61.5 years, and 79% were men.
The primary outcome was the percentage of patients with forced expiratory volume per second (FEV1) improvement greater than 15%. Half of the patients with AATD achieved this outcome, with a median improvement in FEV1 of 110 mL and a significant difference in pre- and post-BLVR FEV1 volume based on a Wilcoxon signed rank test (W = 11.5; P < .05).
Patients with AATD also showed significant improvement in several secondary outcomes including BODE index, residual volume (RV), total lung capacity (TLC), RV/TLC ratio, and inspiratory capacity/RV ratio between pre- and post-BLVR.
“The sustained improvements seen at 7 months post-BLVR in patients with lower lobe disease were unexpected and promising,” Dr. Nicholson said in an interview. “In contrast to the National Emphysema Treatment Trial (NETT), which found lung volume reduction surgery ineffective for lower lobe disease, our study revealed significant improvements in lower lobe disease following BLVR,” he said. The sustained improvements up to 7 months post-BLVR are encouraging, given clinical concerns that the ongoing destruction of lung tissue in AATD could cause initial BLVR improvements to regress, he added.
Overall, the results suggest that BLVR is an effective therapy for appropriately selected patients with AATD and COPD, and that significant improvement in lung function can be achieved regardless of the affected lobe, Dr. Nicholson said.
“The primary obstacles to widespread BLVR implementation include the scarcity of equipment, as well as insufficient education and training for pulmonologists outside of major academic institutions,” Dr. Nicholson told this news organization. “Successful outcomes in BLVR require clinicians to have a deep understanding of patient selection criteria, extensive training in BLVR techniques, and access to the necessary technology within their facilities,” he said. However, BLVR has been integrated into pulmonary and interventional pulmonary fellowships nationwide, which paves the way for a new generation of pulmonologists to expand the use of the procedure, he said.
Looking ahead, prospective examination of BLVR vs the current standard of care in patients with AATD would provide invaluable data, Dr. Nicholson said. Since the presentation of the study at the meeting, additional patient data have been added to the analysis and increased the power of the findings, he said. “We intend to extend our assessment of pulmonary function testing beyond 7 months post-BLVR to evaluate the persistence of improvements in the long term,” he added.
Study Confirms Benefits for Wider Patient Population
“Lung volume reduction is an important intervention in patients with severe emphysema,” said David M. Mannino, MD, of the University of Kentucky, Lexington, Kentucky, in an interview. Most emphysema is in the upper lobes, but it tends to occur more in the lower lobes in patients with AATD, said Dr. Mannino, who was not involved in the study.
The findings were not especially surprising, but they were reassuring, Dr. Mannino told this news organization. “We know this intervention works in those with severe emphysema,” and it was helpful to confirm similar success in patients with AATD, he said.
The implications for practice are that BLVR is both a safe and an effective intervention for patients with lower or upper lobe emphysema, although longer-term follow-up studies are needed, he said.
The study received no outside funding. Dr. Nicholson and Dr. Mannino had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Educational Tool Reduces Unnecessary Inhaler Use in ILD
Use of an electronic tool contributed to the deprescribing of unnecessary inhalers in patients with interstitial lung disease (ILD), based on data from nearly 200 individuals.
Patients with ILD often have symptoms that overlap with those of obstructive airways diseases, Stephanie Nevison, MD, of the University of Toronto, and colleagues wrote in a study presented at the American Thoracic Society’s international conference.
, they noted.
“Our aim was twofold: To quantify the extent of inappropriate inhaler use in patients with ILD and to discontinue them where appropriate,” the researchers wrote.
“We hypothesized that inappropriate inhaler use in ILD is common and that an electronic initiative would improve deprescribing rates,” they said.
The researchers conducted a quality improvement project in an ILD clinic at a single center. They reviewed 5 months of baseline data for 191 patients with ILD to assess baseline frequency of inappropriate inhaler use, defined as one or more of the following criteria: Reported asthma history, smoking history of > 15 pack/years, emphysema on chest CT, patient-reported benefits from therapy, airflow obstruction, or bronchodilator response on spirometry.
A total of 48 patients (25.1%) were on inhalers, and 15 (7.8%) had no indication for them (9% of new referrals and 7% of follow-up patients). The most-prescribed inhalers for patients with no indication were corticosteroids (10 patients), short-acting beta-agonists (8 patients), and long-acting beta-agonists (7 patients).
None of the patients on inhalers received counseling about discontinuing their use. The results of the baseline assessment were shared with clinicians along with education about reducing unnecessary inhaler use in the form of a prompt linked to electronic medical records to discuss deprescription of unnecessary inhalers.
The electronic intervention was applied in 400 of 518 patient encounters, and the researchers reviewed data over another 5-month period. A total of 99 patients were on inhalers, and 3.3% had no indication (5.3% of new referrals and 3.0% of follow-up patients). In the wake of the intervention, “all patients on unnecessary inhalers were counseled on deprescribing, representing a significant increase compared to the preintervention period,” the researchers wrote.
Intervention Shows Potential to Curb Unnecessary Inhaler Use
More research is needed as the findings were limited by the relatively small sample size and use of data from a single center, the researchers noted.
However, the results suggest that electronic reminders are effective for prompting a review of inhaler use, and deprescribing inappropriate inhalers for patients with ILD could reduce the potential for adverse events associated with their use, they concluded.
The current study is important because some patients with ILD may not benefit from inhaler use, David Mannino, MD, of the University of Kentucky, Lexington, said in an interview. In the study, “I was a bit surprised that only 3.3% of patients had no indication for them; this seems rather low,” said Dr. Mannino, who was not involved in the study.
Use of an electronic system that evaluates patients and flags inappropriate therapy is an effective way to decrease overprescribing of medications, Dr. Mannino told this news organization.
As for additional research, application of the tool used in this study to other pulmonary populations could be interesting and potentially useful, he said.
The study received no outside funding. The researchers and Dr. Mannino had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Use of an electronic tool contributed to the deprescribing of unnecessary inhalers in patients with interstitial lung disease (ILD), based on data from nearly 200 individuals.
Patients with ILD often have symptoms that overlap with those of obstructive airways diseases, Stephanie Nevison, MD, of the University of Toronto, and colleagues wrote in a study presented at the American Thoracic Society’s international conference.
, they noted.
“Our aim was twofold: To quantify the extent of inappropriate inhaler use in patients with ILD and to discontinue them where appropriate,” the researchers wrote.
“We hypothesized that inappropriate inhaler use in ILD is common and that an electronic initiative would improve deprescribing rates,” they said.
The researchers conducted a quality improvement project in an ILD clinic at a single center. They reviewed 5 months of baseline data for 191 patients with ILD to assess baseline frequency of inappropriate inhaler use, defined as one or more of the following criteria: Reported asthma history, smoking history of > 15 pack/years, emphysema on chest CT, patient-reported benefits from therapy, airflow obstruction, or bronchodilator response on spirometry.
A total of 48 patients (25.1%) were on inhalers, and 15 (7.8%) had no indication for them (9% of new referrals and 7% of follow-up patients). The most-prescribed inhalers for patients with no indication were corticosteroids (10 patients), short-acting beta-agonists (8 patients), and long-acting beta-agonists (7 patients).
None of the patients on inhalers received counseling about discontinuing their use. The results of the baseline assessment were shared with clinicians along with education about reducing unnecessary inhaler use in the form of a prompt linked to electronic medical records to discuss deprescription of unnecessary inhalers.
The electronic intervention was applied in 400 of 518 patient encounters, and the researchers reviewed data over another 5-month period. A total of 99 patients were on inhalers, and 3.3% had no indication (5.3% of new referrals and 3.0% of follow-up patients). In the wake of the intervention, “all patients on unnecessary inhalers were counseled on deprescribing, representing a significant increase compared to the preintervention period,” the researchers wrote.
Intervention Shows Potential to Curb Unnecessary Inhaler Use
More research is needed as the findings were limited by the relatively small sample size and use of data from a single center, the researchers noted.
However, the results suggest that electronic reminders are effective for prompting a review of inhaler use, and deprescribing inappropriate inhalers for patients with ILD could reduce the potential for adverse events associated with their use, they concluded.
The current study is important because some patients with ILD may not benefit from inhaler use, David Mannino, MD, of the University of Kentucky, Lexington, said in an interview. In the study, “I was a bit surprised that only 3.3% of patients had no indication for them; this seems rather low,” said Dr. Mannino, who was not involved in the study.
Use of an electronic system that evaluates patients and flags inappropriate therapy is an effective way to decrease overprescribing of medications, Dr. Mannino told this news organization.
As for additional research, application of the tool used in this study to other pulmonary populations could be interesting and potentially useful, he said.
The study received no outside funding. The researchers and Dr. Mannino had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Use of an electronic tool contributed to the deprescribing of unnecessary inhalers in patients with interstitial lung disease (ILD), based on data from nearly 200 individuals.
Patients with ILD often have symptoms that overlap with those of obstructive airways diseases, Stephanie Nevison, MD, of the University of Toronto, and colleagues wrote in a study presented at the American Thoracic Society’s international conference.
, they noted.
“Our aim was twofold: To quantify the extent of inappropriate inhaler use in patients with ILD and to discontinue them where appropriate,” the researchers wrote.
“We hypothesized that inappropriate inhaler use in ILD is common and that an electronic initiative would improve deprescribing rates,” they said.
The researchers conducted a quality improvement project in an ILD clinic at a single center. They reviewed 5 months of baseline data for 191 patients with ILD to assess baseline frequency of inappropriate inhaler use, defined as one or more of the following criteria: Reported asthma history, smoking history of > 15 pack/years, emphysema on chest CT, patient-reported benefits from therapy, airflow obstruction, or bronchodilator response on spirometry.
A total of 48 patients (25.1%) were on inhalers, and 15 (7.8%) had no indication for them (9% of new referrals and 7% of follow-up patients). The most-prescribed inhalers for patients with no indication were corticosteroids (10 patients), short-acting beta-agonists (8 patients), and long-acting beta-agonists (7 patients).
None of the patients on inhalers received counseling about discontinuing their use. The results of the baseline assessment were shared with clinicians along with education about reducing unnecessary inhaler use in the form of a prompt linked to electronic medical records to discuss deprescription of unnecessary inhalers.
The electronic intervention was applied in 400 of 518 patient encounters, and the researchers reviewed data over another 5-month period. A total of 99 patients were on inhalers, and 3.3% had no indication (5.3% of new referrals and 3.0% of follow-up patients). In the wake of the intervention, “all patients on unnecessary inhalers were counseled on deprescribing, representing a significant increase compared to the preintervention period,” the researchers wrote.
Intervention Shows Potential to Curb Unnecessary Inhaler Use
More research is needed as the findings were limited by the relatively small sample size and use of data from a single center, the researchers noted.
However, the results suggest that electronic reminders are effective for prompting a review of inhaler use, and deprescribing inappropriate inhalers for patients with ILD could reduce the potential for adverse events associated with their use, they concluded.
The current study is important because some patients with ILD may not benefit from inhaler use, David Mannino, MD, of the University of Kentucky, Lexington, said in an interview. In the study, “I was a bit surprised that only 3.3% of patients had no indication for them; this seems rather low,” said Dr. Mannino, who was not involved in the study.
Use of an electronic system that evaluates patients and flags inappropriate therapy is an effective way to decrease overprescribing of medications, Dr. Mannino told this news organization.
As for additional research, application of the tool used in this study to other pulmonary populations could be interesting and potentially useful, he said.
The study received no outside funding. The researchers and Dr. Mannino had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
FROM ATS 2024
Use of Hypoglossal Nerve Stimulation for Treating OSA in Military Patient Populations
Obstructive sleep apnea (OSA), the repetitive collapse of posterior oropharynx during sleep resulting in hypoxia and/or arousals from sleep, is the most common form of sleep disordered breathing and a common chronic respiratory disorders among middle-aged adults. OSA can lead to significant health problems, such as worsened cardiometabolic disease and cognitive impairment, which can increase morbidity and mortality.1
The gold standard for OSA diagnosis is polysomnography (PSG), although home sleep studies can be performed for select patients. OSA diagnoses are based on the number of times per hour of sleep a patient’s airway narrows or collapses, reducing or stopping airflow, scored as hypopnea or apnea events, respectively. An Apnea-Hypopnea Index (AHI) score of 5 to 14 events/hour is considered mild OSA, 15 to 30 events/hour moderate OSA, and ≥ 30 events/hour severe OSA.2
Treatment commonly includes positive airway pressure (PAP) but more than one-half of patients are not adherent to continuous PAP (CPAP) treatment after about 90 days.3 Efficacy of treatments vary as a function of disease severity and etiology, which—in addition to the classic presentation of obesity with large neck/narrowupper airway—includes craniofacial abnormalities, altered muscle function in the upper airway, pharyngeal neuropathy, and fluid shifts to the neck.
Background
The American Academy of Sleep Medicine (AASM) estimates that 10% to 17% of adults in the United States have OSA.4 Compared with civilians, the military population generally is younger and healthier. Service members have access to regular health care with yearly physical examinations, exercise scheduled into the workday, and mandatory height/weight and fitness standards. Because obesity is a major risk factor for OSA, and the incidence of obesity is relatively low in the military population (estimated at 18.8% in 2021 vs 39.8% among all US adults aged 20 to 39 years), it might be expected that incidence of OSA would be correspondingly low.5,6 However, there is evidence of a rapidly increasing incidence of OSA in military populations. A 2021 study revealed that OSA incidence rates increased from 11 to 333 per 10,000 between 2005 and 2019 across all military branches and demographics, with the highest rate among Army personnel.7 An earlier study revealed a 600% increase in OSA incidence among Army personnel between 2003 and 2011.8
Several factors likely contributed to this increase, including expanding obesity and greater physician awareness and availability of sleep study centers. Rogers and colleagues found that 40% to 50% of incident OSA diagnoses among military personnel occur within 12 months of separation, suggesting that the secondary gains associated with military disability benefits might motivate OSA evaluation.9 It is possible that secondary gain is a factor because an OSA diagnosis can range from a 0% to 100% disability rating, depending on the severity.10 This disability claim is based on evidence that untreated OSA can negatively affect long-term health and mission readiness.8 For example, untreated OSA can lead to hypertension, which contributes to a long list of adverse health and wellness consequences. Most importantly for the military, OSA has been shown to increase daytime sleepiness and reduce cognitive performance.10
The current first-line treatment for OSA is CPAP, which improves symptoms of daytime sleepiness, hypertension management, and daytime alertness.11 Despite its efficacy, nonadherence rates range from 29% to 83%.12-15 Nonadherence factors include lifestyle changes, adverse effects (eg, nasal congestion), and lack of education on proper use.11 Lifestyle changes needed to increase the likelihood of successful therapy, such as regular sleep schedules and proper CPAP cleaning and maintenance, are difficult for military personnel because of the nature of continuous or sustained operations that might require shift work and/or around-the-clock (ie, 24-hour, 7 days a week) task performance. Traveling with CPAP is an added burden for service members deployed to combat operations (ie, added luggage, weight, maintenance). Although alternate treatments such as oral appliances (ie, custom dental devices) are available, they generally are less effective than CPAP.2 Oral appliances could be a reasonable alternative treatment for some patients who cannot manage their OSA with behavioral modifications and are intolerant or unable to effectively use CPAP. This could include patients in the military who are deployed to austere environments.
Surgically implanted hypoglossal nerve stimulator (HGNS) treatment may provide long-term health benefits to service members. After the device is implanted near the hypoglossal nerve, electrical stimulation causes the tongue to move forward, which opens the airway in the anteroposterior dimension. The most important consideration is the mechanism of airway collapse. HGNS is not effective for patients whose OSA events are caused by circumferential collapse of other airway muscles. The cause of airway collapse is ascertained before surgery with drug-induced sleep endoscopy, a procedure that allows visualization of conformational changes in the upper airway during OSA events.
The US Food and Drug Administration (FDA) approved HGNS in 2014. However, it is not considered a first-line treatment for OSA by the AASM. Original candidate criteria for HGNS included an AHI score of 15 to 65 events/hour, age ≥ 18 years, failed CPAP use, body mass index (BMI) < 32, absence of palatal complete concentric collapse, and central apneas comprising < 25% of total events.16 In June 2023, the FDA expanded approval to increase the upper limit of AHI to 100 events/hour and the BMI to < 40.17
HGNS has been reported to be effective in appropriately selected patients with OSA at tertiary care centers with established multidisciplinary sleep surgical programs. These benefits have not been confirmed in larger, community-based settings, where most of these surgeries occur. In community practice, there is significant confusion among patients and clinicians about the optimal pathway for patient selection and clinical follow-up. Many patients view HGNS as a viable alternative to CPAP, but initially do not understand that it requires surgery. Surgical treatments for OSA, such as HGNS, are appealing because they suggest a 1-time intervention that permanently treats the condition, without need for follow-up or equipment resupply. HGNS might be an appealing treatment option because it is less obtrusive than CPAP and requires fewer resources for set-up and maintenance. Also, it does not cause skin irritation (a possible adverse effect of nightly use of a CPAP mask), allows the individual to sleep in a variety of positions, has less impact on social and sex life, and does not require an electric outlet. In the long term, HGNS might be more cost effective because there is no yearly physician follow-up or equipment resupply and/or maintenance.
The military population has specific demands that impact delivery and effectiveness of health care. Among service members with OSA, CPAP treatment can be challenging because of low adherence, required annual follow-up despite frequent moving cycles that pose a challenge for care continuity, and duty limitations for affected service members (ie, the requirement for a waiver to deploy and potential medical separation if symptoms are not adequately controlled). As the incidence of OSA continues to increase among service members, so does the need for OSA treatment options that are efficacious as CPAP but better tolerated and more suitable for use during military operations. The aim of this review is to assess the effectiveness of HGNS and its potential use by the military OSA patient population.
METHODS
To identify eligible studies, we employed PICOS: Population (patients aged ≥ 18 years with a history of OSA), Intervention (HGNS), Comparator (standard of care PAP therapy), Outcome (AHI or Epworth Sleepiness Scale [ESS], and Study (randomized control trial [RCT] or clinical trial). Studies were excluded if they were not written in English or included pediatric populations. The ESS is a subjective rating scale used to determine and quantify a patient’s level of daytime sleepiness, using a 4-point scale for the likelihood of falling asleep totaled across 8 different situations.18 Daytime sleepiness is considered lower normal(0-5 points), higher normal (6-10 points), mild or moderate excessive (11-15 points), and severe excessive (16-24 points).
Literature Search
We conducted a review of PubMed and Scopus for RCTs and controlled trials published from 2013 to 2023 that included the keywords and phrases: obstructive sleep apnea and either hypoglossal nerve stimulation or upper airway stimulation. The final literature search was performed December 8, 2023.
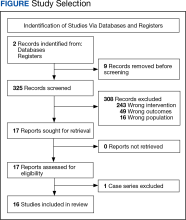
Two authors independently assessed the titles and abstracts of studies identified in the literature search based on the predefined inclusion criteria. If it was not clear whether an article met inclusion criteria based on its title and/or abstract, the 2 review authors assessed the full text of study and resolved any disagreement through consensus. If consensus was not obtained, a third author was consulted. No duplicates were identified. The PRISMA study selection process is presented in the Figure.
Data extraction was performed by 1 independent reviewer. A second author reviewed the extracted data. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third author was consulted. Study data included methods (study design and study objective), participants mean age, inclusion criteria, exclusion criteria, interventions and comparators, and primary study outcomes.
The quality of evidence was assessed using a rating of 1 to 5 based on a modified version of the Oxford Centre for Evidence-based Medicine Levels of Evidence and Grades of Recommendation.19 A rating of 1 indicated a properly powered and conducted RCT, 2 demonstrated a well-designed controlled trial without randomization or prospective comparative cohort trial, 3 designated a case-control study or retrospective cohort study, 4 signified a case series with or without intervention or a cross-sectional study, and 5 denoted an opinion of respected authorities or case reports. Two reviewers independently evaluated the quality of evidence. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third review author was consulted.
RESULTS
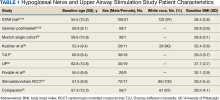
We identified 30 studies; 19 articles did not meet inclusion criteria. The remaining 11 articles were divided into 4 cohorts. Five articles were based on data from the STAR trial, a multicenter study that included adults with moderate-to-severe OSA and inadequate adherence to CPAP.20-24 Four articles used the same patient selection criteria as the STAR trial for a long-term German postmarket study of upper airway stimulation efficacy with OSA.25-28 The third and fourth cohorts each consist of 31 patients with moderate-to-severe OSA with CPAP nonadherence or failure.29,30 The STAR trial included follow-up at 5 years, and the German-postmarket had a follow-up at3 years. The remaining 2 cohorts have 1-year follow-ups.
The Scopus review identified 304 studies; 299 did not meet inclusion criteria and 1 was part of the STAR trial.31 The remaining 4 articles were classified as distinct cohorts. Huntley and colleagues included patients from Thomas Jefferson University (TJU) and University of Pittsburgh (UP) academic medical centers.32 The Pordzik and colleagues cohort received implantation at a tertiary medical center, an RCCT, and a 1:1 comparator trial (Table 1).33-35
STAR Trial
This multicenter, prospective, single-group cohort study was conducted in the US, Germany, Belgium, Netherlands, and France. The STAR trial included 126 patients who were not CPAP therapy adherent. Patients were excluded if they had AHI < 20 or > 50, central sleep apnea > 25% of total AHI, anatomical abnormalities that prevent effective assessment of upper-airway stimulation, complete concentric collapse of the retropalatal airway during drug-induced sleep, neuromuscular disease, hypoglossal-nerve palsy, severe restrictive or obstructive pulmonary disease, moderate-to-severe pulmonary arterial hypertension, severe valvular heart disease, New York Heart Association class III or IV heart failure, recent myocardial infarction or severe cardiac arrhythmias (within the past 6 months), persistent uncontrolled hypertension despite medication use, active psychiatric illness, or coexisting nonrespiratory sleep disorders that would confound functional sleep assessment. Primary outcome measures included the AHI and oxygen desaturation index (ODI) with secondary outcomes using the ESS, the Functional Outcomes of Sleep Questionnaire (FOSQ), and the percentage of sleep time with oxygen saturation < 90%. Of 126 patients who received implantation, 71 underwent an overnight PSG evaluation at 5-year follow-up. Mean (SD) AHI at baseline was reduced with HGNS treatment to from 32.0 (11.8) to 12.4 (16.3). Mean (SD) ESS for 92 participants with 2 measurements declined from 11.6 (5.0) at baseline to 6.9 (4.7) at 5-year follow-up.
The STAR trial included a randomized controlled withdrawal study for 46 patients who had a positive response to therapy to evaluate efficacy and durability of upper airway stimulation. Patients were randomly assigned to therapy maintenance or therapy withdrawal groups for ≥ 1 week. The short-term withdrawal effect was assessed using the original trial outcome measures and indicated that both the withdrawal and maintenance groups showed improvements at 12 months compared with the baseline. However, after the randomized withdrawal, the withdrawal group’s outcome measures deteriorated to baseline levels while the maintenance group showed no change. At 18 months of therapy, outcome measures for both groups were similar to those observed with therapy at 12 months.24 The STAR trial included self-reported outcomes at baseline, 12 months, and 24 months that used ESS to measure daytime sleepiness. These results included subsequent STAR trial reports.20-24,31
The German Postmarket Cohort
This multicenter, prospective, single-arm study used selection criteria that were based on those used in the STAR trial and included patients with moderate-to-severe OSA and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, AHI < 15 or > 65; central apnea index > 25% of total AHI; or complete concentric collapse at the velopharynx during drug-induced sleep. Measured outcomes included AHI, ODI, FOSQ, and ESS. Among the 60 participants, 38 received implantation and a 3-year follow-up. Mean (SD) AHI decreased from 31.2 (13.2) at baseline to 13.1 (14.1) at follow-up, while mean (SD) ESS decreased from 12.8 (5.3) at baseline to 6.0 (3.2) at follow-up.25-28
Munich Cohort
This single-center, prospective clinical trial included patients with AHI > 15 and < 65, central apnea index < 25% of total AHI, and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, anatomical abnormalities that would prevent effective assessment of upper-airway stimulation; all other exclusion criteria matched those used in the STAR trial. Among 31 patients who received implants and completed a 1-year follow-up, mean (SD) AHI decreased from 32.9 (11.2) at baseline to 7.1 (5.9) at follow-up and mean (SD) ESS decreased from 12.6 (5.6) at baseline to 5.9 (5.2) at follow-up.29
Kezirian and Colleagues Cohort
This prospective, single-arm, open-label study was conducted at 4 Australian and 4 US sites. Selection criteria included moderate-to-severe OSA with failure of CPAP, AHI of 20 to 100 with ≥ 15 events/hour occurring in sleep that was non-REM (rapid eye movement) sleep, BMI ≤ 40 (Australia) or ≤ 37 (US), and a predominance of hypopneas (≥ 80% of disordered breathing events during sleep). Patients were excluded if they had earlier upper airway surgery, markedly enlarged tonsils, uncontrolled nasal obstruction, severe retrognathia, > 5% central or mixed apneic events, incompletely treated sleep disorders other than OSA, or a major disorder of the pulmonary, cardiac, renal, or nervous systems. Data were reported for 31 patients whose mean (SD) AHI declined from 45.4 (17.5) at baseline to 25.3 (20.6) at 1-year follow-up and mean (SD) ESS score declined from 12.1 (4.6) at baseline to 7.9 (3.8) 1 year later.30
TJU and UP Cohorts
The TJU and UP cohorts are composed of patients who underwent implantation between May 2014 and August 2016 at 2 academic centers.31,32 Selection criteria was consistent with that used in the STAR trial, and patients completed postoperative titration PSG and outpatient follow-up (48 patients at TJU and 49 at UP). Primary outcomes included AHI, ESS, and O2 nadir. Secondary outcomes consisted of surgical success and percentage of patients tolerating optimal titration setting at follow-up. Postoperative outcomes were assessed during the titration PSG. Time from initial ESS to postoperative PSG at TJU was 1.7 years and at UP was 1.9 years. Time from initial AHI to postoperative PSG at TJU was 90.4 days and 85.2 days at UP. At TJU, mean (SD) AHI and ESS dropped from 35.9 (20.8) and 11.1 (3.8), respectively at baseline to 6.3 (11.5) and 5.8 (3.4), respectively at follow-up. At UP, mean (SD) AHI and ESS fell from 35.3 (15.3) and 10.9 (4.9), respectively at baseline to 6.3 (6.1) and 6.6 (4.5), respectively at follow-up. There were no site-related differences in rates of AHI, ESS, or surgical success.31
Pordzik and Colleagues Cohort
This cohort of 29 patients underwent implantation between February 2020 and June 2022 at a tertiary university medical center with both pre- and postoperative PSG. Selection criteria was consistent with that of the German postmarket cohort. Postoperative PSG was completed a mean (SD) 96.3 (27.0) days after device activation. Mean (SD) AHI dropped from 38.6 (12.7) preoperatively to 24.4 (13.3) postoperatively. Notably, this cohort showed a much lower decrease of postoperative AHI than reported by the STAR trial and UP/TJU cohort.33
Stimulation vs Sham Trial
This multicenter, double-blinded, randomized, crossover trial assessed the effect of HGNS (stim) vs sham stimulation (sham) in 86 patients that completed both phases of the trial. Primary outcomes included AHI and ESS. Secondary outcomes included FOSQ. No carryover effect was found during the crossover phase. The difference between the phases was−15.5 (95% CI, −18.3 to −12.8) for AHI and −3.3 (95% CI, −4.4 to −2.2) for ESS.34
Comparator
The comparator study used propensity score matching to compare outcomes of HGNS and PAP therapy. Primary outcomes included sleepiness, AHI, and effectiveness with outcome measures of AHI and ESS collected at baseline and 12 months postimplantation. The article reported that 126 of 227 patients were matched 1:1. Both groups showed improvement in AHI and ESS. Mean (SD) AHI for the HGNS group at baseline started at 33.9 (15.1) and decreased to 8.1 (6.3). Mean (SD) ESS for the HGNS group at baseline was 15.4 (3.5) and decreased to 7.5 (4.7). In the PAP comparator group, mean (SD) baseline AHI was 36.8 (21.6) and at follow-up was 6.6 (8.0) and mean (SD) ESS was 14.6 (3.9) at baseline and 10.8 (5.6) at follow-up.35
DISCUSSION
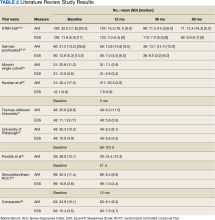
The current clinical data on HGNS suggest that this treatment is effective in adults with moderate-to-severe OSA and effects are sustained at long-term follow-up, as measured by AHI reduction and improvements in sleep related symptoms and quality of life (Table 2). These results have been consistent across several sites.
The STAR trial included a randomized control withdrawal group, for whom HGNS treatment was withdrawn after the 12-month follow-up, and then restored at 18 months.21 This revealed that withdrawal of HGNS treatment resulted in deterioration of both objective and subjective measures of OSA and sleepiness. The beneficial effects of HGNS were restored when treatment was resumed.24 Additionally, the RCCT revealed that therapeutic stimulation via HGNS significantly reduced subjective and objective measures of OSA.34 These studies provide definitive evidence of HGNS efficacy.
Currently, a diagnosis of OSA on PAP is classified as a 50% military disability rating. This rating is based primarily on epidemiologic evidence that untreated OSA is a costly disease that leads to other chronic illnesses that increases health care utilization.9 HGNS requires an initially invasive procedure and higher upfront costs, but it could result in reduced health care use and long-term costs because of improved adherence to treatment—compared with CPAP—that results in better outcomes.
Limitations to OSA Studies
The reviewed studies have several limitations that warrant caution when determining the possible benefits of HGNS treatment. The primary limitation is the lack of active control groups, therefore precluding a direct comparison of the short- and long-term effectiveness of HGNS vs other treatments (eg, CPAP). This is especially problematic because in the reviewed studies HGNS treatment efficacy is reported as a function of the mean—and SD—percent reduction in the AHI, whereas the efficacy of CPAP treatment usually is defined in terms of “adequacy of titration” as suggested by the AASM.36 It has been reported that with CPAP treatment, 50% to 60% of OSA patients achieve AASM-defined optimal improvement of respiratory disturbance index of < 5/hour during a polysomnographic sleep recording of ≥ 15 minutes duration that includes REM sleep in the supine position.37 In most of the reviewed studies, treatment success was more liberally defined as a decrease of AHI by ≥ 50%, regardless of the resulting AHI. It is notable that among the reviewed HGNS studies, the TJU and UP cohorts achieved the best outcome in short-term follow-up of 2 months with a mean (SD) AHI of 6.3 (11.5) and 6.4 (6.1), respectively. Among those cohortsassessed at a 12-month follow-up, the Munich cohort achieved the best outcome with a mean (SD) AHI of 7.1 (5.9).
Although the metrics reported in the reviewed studies are not directly comparable, the reported findings strongly suggest that HGNS generally is less effective than CPAP. How important are these differences? With findings that HGNS “reliably produces clinically meaningful (positive) effects on daytime sleepiness, daytime functioning, and sleep quality,” does it really matter if the outcome metrics for HGNS are a little less positive than those produced by CPAP?38 For individual military OSA patients the answer is yes. This is because in military operational environments—especially during deployment—sleep restriction is nearly ubiquitous, therefore any mild residual deficits in sleep quality and daytime alertness resulting from nominally adequate, but suboptimal OSA treatment, could be exacerbated by sleep restriction, therefore placing the service member and the mission at increased risk.39
Another limitation is the narrow inclusion criteria these studies employed, which limits the generalizability of the findings. Participants in the reviewed clinical trials were selected from a patient population that was mostly middle-aged, White, and obese or overweight. In a Medical Surveillance Monthly Report study, OSA was found to be highest among service members aged > 40 years, male, obese, and Black/non-Hispanic (although it should be noted that more than one-half of enlisted service members aged ≤ 25 years).40,41 Obesity has been noted as a growing concern for the military as the military population is beginning to mirror the civilian population in terms of being overweight or obese despite height and weight standards. HGNS might not be as successful in military populations with different demographics. Moreover, HGNS has been shown to have greater AHI reduction among those with higher BMI.30 Although obese service members have a 6-fold higher 12-year incidence rate of OSA than service members without obesity, this nevertheless suggests that general level of HGNS efficacy might be lower among the military patient population, because obesity is less prevalent in the military than the general population.9
Ethnicity has been found to be a relevant factor, with the highest incidence rate of OSA among non-Hispanic Black males, a demographic that was underrepresented in cohorts included in this review. Further studies will be needed to determine the extent to which findings from HGNS treatment studies are generalizable to the broader OSA patient population.
HGNS Implementation Challenges
Current impediments to widespread use of HGNS as an OSA treatment include no standardized guidance for titration and follow-on care, which varies based on the resources available. Titrating a new device for HGNS requires experienced sleep technicians who have close relationships with device representatives and can troubleshoot problems. Technical expertise, which currently is rare, is required if there are complications after placement or if adjustments to voltage settings are needed over time. In addition, patients may require multiple specialists making it easy to get lost to follow-up after implantation. This is particularly challenging in a transient community, such as the military, because there is no guarantee that a service member will have access to the same specialty care at the next duty station.
Although some evidence suggests that HGNS is a viable alternative treatment for some patients with OSA, the generalizability of these findings to the military patient population is unclear. Specialized facilities and expertise are needed for the surgical procedure and follow-up requirements, which currently constitute significant logistical constraints. As with any implantable device, there is a risk of complications including infection that could result in medical evacuation from a theater of operations. If the device malfunctions or loses effectiveness in a deployed environment, the service member might not have immediate access to medical support, potentially leading to undertreatment of OSA. In future battlefield scenarios in multidomain operations, prolonged, far-forward field care will become the new normal because the military is not expected to have air superiority or the ability to quickly evacuate service members to a higher level of medical care.42
In deployed environments, the potential limitations of HGNS become increasingly risky for the service member and the overall mission. Considering these factors, it will be important to evaluate the practicality of HGNS as a treatment option in military populations. Military-specific challenges associated with HGNS that require further study, include guidance for patient selection outside academic centers, guidance on long-term postsurgical care and device maintenance, duty limitation and military retention considerations, and limitations in training and combat environments. The military medical community needs to conduct its own studies in appropriately selected service members to guide clinical practice.
CONCLUSIONS
HGNS treatment results in improvement of both AHI and ESS scores and could be a deployable treatment option for military patients with OSA. However, HGNS has not been found to be as effective as CPAP, although the current literature is limited by small sample sizes, homogeneous populations that do not reflect the demographics of the military, and mostly short follow-up periods. Future studies should be focused on collecting data on HGNS from demographic groups that are more representative of the military OSA patient population and identifying the subpopulation of patients who derive the greatest benefit from HGNS, so that this treatment can be better individually targeted. Until data on existing military patients is published, it is not possible to fully weigh risks and benefits in this population and generalize civilian guidance to the military.
1. Cumpston E, Chen P. Sleep Apnea Syndrome. PubMed. Updated September 4, 2023. Published January 2024. https://www.ncbi.nlm.nih.gov/books/NBK564431/
2. American Academy of Sleep Medicine. Obstructive sleep apnea. Accessed November 27, 2023. https://aasm.org/resources/factsheets/sleepapnea.pdf
3. Cowen J, Harrison S, Thom L, et al. Use of historical remote monitoring data to determine predictors of CPAP non-compliance in patients with Osa. Sleep Breath. 2023;27(5):1899-1908. doi:10.1007/s11325-023-02806-3
4. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
5. Stiegmann RA, Payne CB, Kiel MA, Stahlman SL. Increased Prevalence of Overweight and Obesity and Incidence of Prediabetes and Type 2 Diabetes During the COVID-19 Pandemic, Active Component Service Members, U.S. Armed Forces, 2018 to 2021. MSMR. 2023;30(1):11-18. Published 2023 Jan 20.
6. Adult obesity facts. Centers for Disease Control and Prevention. Updated May 17, 2022. Accessed November 27, 2023. https://www.cdc.gov/obesity/data/adult.html
7. Moore BA, Tison LM, Palacios JG, Peterson AL, Mysliwiec V. Incidence of insomnia and obstructive sleep apnea in active duty United States military service members. Sleep. 2021;44(7):zsab024. doi:10.1093/sleep/zsab024
8. Caldwell JA, Knapik JJ, Shing TL, Kardouni JR, Lieberman HR. The association of insomnia and sleep apnea with deployment and combat exposure in the entire population of US army soldiers from 1997 to 2011: a retrospective cohort investigation. Sleep. 2019;42(8):zsz112. doi:10.1093/sleep/zsz112
9. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
10. Veterans Affairs 38 C.F.R. § 4.97-13, Code 6847.
11. Shapiro GK, Shapiro CM. Factors that influence CPAP adherence: an overview. Sleep Breath. 2010;14(4):323-335. doi:10.1007/s11325-010-0391-y
12. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178. doi:10.1513/pats.200708-119mg
13. Sin DD, Mayers I, Man GCW, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121(2):430-435. doi:10.1378/chest.121.2.430
14. Nowak C, Bourgin P, Portier F, Genty E, Escourrou P, Bobin S. Obstruction nasale et compliance à la ventilation nasale à pression positive [Nasal obstruction and compliance to nasal positive airway pressure]. Ann Otolaryngol Chir Cervicofac. 2003;120(3):161-166.
15. Brin YS, Reuveni H, Greenberg S, Tal A, Tarasiuk A. Determinants affecting initiation of continuous positive airway pressure treatment. Isr Med Assoc J. 2005;7(1):13-18.
16. Suurna MV, Jacobowitz O, Chang J, et al. Improving outcomes of hypoglossal nerve stimulation therapy: current practice, future directions, and research gaps. Proceedings of the 2019 International Sleep Surgery Society Research Forum. J Clin Sleep Med. 2021;17(12):2477-2487. doi:10.5664/jcsm.9542
17. Inspire Medical Systems, Inc. Announces FDA approval for apnea hypopnea index indication expansion and increased body mass index labeling. Inspire Medical Systems, Inc. Accessed July 14, 2023. https://investors.inspiresleep.com/investors/press-releases/press-release-details/2023/Inspire-Medical-Systems-Inc.-Announces-FDA-Approval-for-Apnea-Hypopnea-Index-Indication-Expansion-and-Increased-Body-Mass-Index-Labeling/default.aspx
18. Lapin BR, Bena JF, Walia HK, Moul DE. The Epworth Sleepiness Scale: Validation of one-dimensional factor structure in a large clinical sample. J Clin Sleep Med. 2018;14(08):1293-1301. Published 2018 Aug 15. doi:10.5664/jcsm.7258
19. The Centre for Evidence-Based Medicine. November 25, 2020. http://www.cebm.net/index.aspx?o=5653
20. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
21. Strollo PJ Jr, Gillespie MB, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38(10):1593-1598. Published 2015 Oct 1. doi:10.5665/sleep.5054
22. Woodson BT, Soose RJ, Gillespie MB, et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-188. doi:10.1177/0194599815616618
23. Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159(1):194-202. doi:10.1177/0194599818762383
24. Woodson BT, Gillespie MB, Soose RJ, et al. Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg. 2014;151(5):880-887. doi:10.1177/0194599814544445
25. Heiser C, Maurer JT, Hofauer B, Sommer JU, Seitz A, Steffen A. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter German postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-384. doi:10.1177/0194599816683378
26. Steffen A, Sommer JU, Hofauer B, Maurer JT, Hasselbacher K, Heiser C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. Laryngoscope. 2018;128(2):509-515. doi:10.1002/lary.26688
27. Steffen A, Sommer UJ, Maurer JT, Abrams N, Hofauer B, Heiser C. Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea. Sleep Breath. 2020;24(3):979-984. doi:10.1007/s11325-019-01933-028.
28. Hasselbacher K, Hofauer B, Maurer JT, Heiser C, Steffen A, Sommer JU. Patient-reported outcome: results of the multicenter German post-market study. Eur Arch Otorhinolaryngol. 2018;275(7):1913-1919. doi:10.1007/s00405-018-5017-129.
29. Heiser C, Knopf A, Bas M, Gahleitner C, Hofauer B. Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience. Eur Arch Otorhinolaryngol. 2017;274(3):1727-1734. doi:10.1007/s00405-016-4297-6
30. Kezirian EJ, Goding GS Jr, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23(1):77-83. doi:10.1111/jsr.12079
31. Soose RJ, Woodson BT, Gillespie MB, et al. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12(1):43-48. doi:10.5664/jcsm.5390
32. Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med. 2017;13(9):1075-1079. Published 2017 Sep 15. doi:10.5664/jcsm.6726

33. Pordzik J, Seifen C, Ludwig K, et al. Short-term outcome of unilateral inspiration-coupled hypoglossal nerve stimulation in patients with obstructive sleep apnea. Int J Environ Res Public Health. 2022;19(24):16443. Published 2022 Dec 8. doi:10.3390/ijerph192416443
34. Heiser C, Steffen A, Hofauer B, et al. Effect of upper airway stimulation in patients with obstructive sleep apnea (EFFECT): a randomized controlled crossover trial. J Clin Med. 2021;10(13):2880. Published 2021 Jun 29. doi:10.3390/jcm1013288035.
35. Heiser C, Steffen A, Strollo PJ Jr, Giaie-Miniet C, Vanderveken OM, Hofauer B. Hypoglossal nerve stimulation versus positive airway pressure therapy for obstructive sleep apnea. Sleep Breath. 2023;27(2):693-701. doi:10.1007/s11325-022-02663-6
36. Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157-171.
37. Freedman N, Johnson K. Positive airway pressure treatment for obstructive sleep apnea. In: Kryger MH, Roth T, Goldstein CA, Dement WC, eds. Principles and Practice of Sleep Medicine. Elsevier; 2022:1260-1283.
38. Braun M, Stoerzel M, Wollny M, Schoebel C, Ulrich Sommer J, Heiser C. Patient-reported outcomes with hypoglossal nerve stimulation for treatment of obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2023;280(10):4627-4639. doi:10.1007/s00405-023-08062-1
39. Luxton DD, Greenburg D, Ryan J, Niven A, Wheeler G, Mysliwiec V. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34(9):1189-1195. doi:10.5665/SLEEP.1236
40. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
41. Office of the Deputy Assistant Secretary of Defense for Military Community and Family Policy. 2017 Demographics: Profile of the Military Community. US Dept of Defense;2017. Accessed April 4, 2024. http://download.militaryonesource.mil/12038/MOS/Reports/2017-demographics-report.pdf
42. Remondelli MH, Remick KN, Shackelford SA, et al. Casualty care implications of large-scale combat operations. J Trauma Acute Care Surg. 2023;95(2S Suppl 1): S180-S184. doi:10.1097/TA.0000000000004063
Obstructive sleep apnea (OSA), the repetitive collapse of posterior oropharynx during sleep resulting in hypoxia and/or arousals from sleep, is the most common form of sleep disordered breathing and a common chronic respiratory disorders among middle-aged adults. OSA can lead to significant health problems, such as worsened cardiometabolic disease and cognitive impairment, which can increase morbidity and mortality.1
The gold standard for OSA diagnosis is polysomnography (PSG), although home sleep studies can be performed for select patients. OSA diagnoses are based on the number of times per hour of sleep a patient’s airway narrows or collapses, reducing or stopping airflow, scored as hypopnea or apnea events, respectively. An Apnea-Hypopnea Index (AHI) score of 5 to 14 events/hour is considered mild OSA, 15 to 30 events/hour moderate OSA, and ≥ 30 events/hour severe OSA.2
Treatment commonly includes positive airway pressure (PAP) but more than one-half of patients are not adherent to continuous PAP (CPAP) treatment after about 90 days.3 Efficacy of treatments vary as a function of disease severity and etiology, which—in addition to the classic presentation of obesity with large neck/narrowupper airway—includes craniofacial abnormalities, altered muscle function in the upper airway, pharyngeal neuropathy, and fluid shifts to the neck.
Background
The American Academy of Sleep Medicine (AASM) estimates that 10% to 17% of adults in the United States have OSA.4 Compared with civilians, the military population generally is younger and healthier. Service members have access to regular health care with yearly physical examinations, exercise scheduled into the workday, and mandatory height/weight and fitness standards. Because obesity is a major risk factor for OSA, and the incidence of obesity is relatively low in the military population (estimated at 18.8% in 2021 vs 39.8% among all US adults aged 20 to 39 years), it might be expected that incidence of OSA would be correspondingly low.5,6 However, there is evidence of a rapidly increasing incidence of OSA in military populations. A 2021 study revealed that OSA incidence rates increased from 11 to 333 per 10,000 between 2005 and 2019 across all military branches and demographics, with the highest rate among Army personnel.7 An earlier study revealed a 600% increase in OSA incidence among Army personnel between 2003 and 2011.8
Several factors likely contributed to this increase, including expanding obesity and greater physician awareness and availability of sleep study centers. Rogers and colleagues found that 40% to 50% of incident OSA diagnoses among military personnel occur within 12 months of separation, suggesting that the secondary gains associated with military disability benefits might motivate OSA evaluation.9 It is possible that secondary gain is a factor because an OSA diagnosis can range from a 0% to 100% disability rating, depending on the severity.10 This disability claim is based on evidence that untreated OSA can negatively affect long-term health and mission readiness.8 For example, untreated OSA can lead to hypertension, which contributes to a long list of adverse health and wellness consequences. Most importantly for the military, OSA has been shown to increase daytime sleepiness and reduce cognitive performance.10
The current first-line treatment for OSA is CPAP, which improves symptoms of daytime sleepiness, hypertension management, and daytime alertness.11 Despite its efficacy, nonadherence rates range from 29% to 83%.12-15 Nonadherence factors include lifestyle changes, adverse effects (eg, nasal congestion), and lack of education on proper use.11 Lifestyle changes needed to increase the likelihood of successful therapy, such as regular sleep schedules and proper CPAP cleaning and maintenance, are difficult for military personnel because of the nature of continuous or sustained operations that might require shift work and/or around-the-clock (ie, 24-hour, 7 days a week) task performance. Traveling with CPAP is an added burden for service members deployed to combat operations (ie, added luggage, weight, maintenance). Although alternate treatments such as oral appliances (ie, custom dental devices) are available, they generally are less effective than CPAP.2 Oral appliances could be a reasonable alternative treatment for some patients who cannot manage their OSA with behavioral modifications and are intolerant or unable to effectively use CPAP. This could include patients in the military who are deployed to austere environments.
Surgically implanted hypoglossal nerve stimulator (HGNS) treatment may provide long-term health benefits to service members. After the device is implanted near the hypoglossal nerve, electrical stimulation causes the tongue to move forward, which opens the airway in the anteroposterior dimension. The most important consideration is the mechanism of airway collapse. HGNS is not effective for patients whose OSA events are caused by circumferential collapse of other airway muscles. The cause of airway collapse is ascertained before surgery with drug-induced sleep endoscopy, a procedure that allows visualization of conformational changes in the upper airway during OSA events.
The US Food and Drug Administration (FDA) approved HGNS in 2014. However, it is not considered a first-line treatment for OSA by the AASM. Original candidate criteria for HGNS included an AHI score of 15 to 65 events/hour, age ≥ 18 years, failed CPAP use, body mass index (BMI) < 32, absence of palatal complete concentric collapse, and central apneas comprising < 25% of total events.16 In June 2023, the FDA expanded approval to increase the upper limit of AHI to 100 events/hour and the BMI to < 40.17
HGNS has been reported to be effective in appropriately selected patients with OSA at tertiary care centers with established multidisciplinary sleep surgical programs. These benefits have not been confirmed in larger, community-based settings, where most of these surgeries occur. In community practice, there is significant confusion among patients and clinicians about the optimal pathway for patient selection and clinical follow-up. Many patients view HGNS as a viable alternative to CPAP, but initially do not understand that it requires surgery. Surgical treatments for OSA, such as HGNS, are appealing because they suggest a 1-time intervention that permanently treats the condition, without need for follow-up or equipment resupply. HGNS might be an appealing treatment option because it is less obtrusive than CPAP and requires fewer resources for set-up and maintenance. Also, it does not cause skin irritation (a possible adverse effect of nightly use of a CPAP mask), allows the individual to sleep in a variety of positions, has less impact on social and sex life, and does not require an electric outlet. In the long term, HGNS might be more cost effective because there is no yearly physician follow-up or equipment resupply and/or maintenance.
The military population has specific demands that impact delivery and effectiveness of health care. Among service members with OSA, CPAP treatment can be challenging because of low adherence, required annual follow-up despite frequent moving cycles that pose a challenge for care continuity, and duty limitations for affected service members (ie, the requirement for a waiver to deploy and potential medical separation if symptoms are not adequately controlled). As the incidence of OSA continues to increase among service members, so does the need for OSA treatment options that are efficacious as CPAP but better tolerated and more suitable for use during military operations. The aim of this review is to assess the effectiveness of HGNS and its potential use by the military OSA patient population.
METHODS
To identify eligible studies, we employed PICOS: Population (patients aged ≥ 18 years with a history of OSA), Intervention (HGNS), Comparator (standard of care PAP therapy), Outcome (AHI or Epworth Sleepiness Scale [ESS], and Study (randomized control trial [RCT] or clinical trial). Studies were excluded if they were not written in English or included pediatric populations. The ESS is a subjective rating scale used to determine and quantify a patient’s level of daytime sleepiness, using a 4-point scale for the likelihood of falling asleep totaled across 8 different situations.18 Daytime sleepiness is considered lower normal(0-5 points), higher normal (6-10 points), mild or moderate excessive (11-15 points), and severe excessive (16-24 points).
Literature Search
We conducted a review of PubMed and Scopus for RCTs and controlled trials published from 2013 to 2023 that included the keywords and phrases: obstructive sleep apnea and either hypoglossal nerve stimulation or upper airway stimulation. The final literature search was performed December 8, 2023.
Two authors independently assessed the titles and abstracts of studies identified in the literature search based on the predefined inclusion criteria. If it was not clear whether an article met inclusion criteria based on its title and/or abstract, the 2 review authors assessed the full text of study and resolved any disagreement through consensus. If consensus was not obtained, a third author was consulted. No duplicates were identified. The PRISMA study selection process is presented in the Figure.
Data extraction was performed by 1 independent reviewer. A second author reviewed the extracted data. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third author was consulted. Study data included methods (study design and study objective), participants mean age, inclusion criteria, exclusion criteria, interventions and comparators, and primary study outcomes.
The quality of evidence was assessed using a rating of 1 to 5 based on a modified version of the Oxford Centre for Evidence-based Medicine Levels of Evidence and Grades of Recommendation.19 A rating of 1 indicated a properly powered and conducted RCT, 2 demonstrated a well-designed controlled trial without randomization or prospective comparative cohort trial, 3 designated a case-control study or retrospective cohort study, 4 signified a case series with or without intervention or a cross-sectional study, and 5 denoted an opinion of respected authorities or case reports. Two reviewers independently evaluated the quality of evidence. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third review author was consulted.
RESULTS
We identified 30 studies; 19 articles did not meet inclusion criteria. The remaining 11 articles were divided into 4 cohorts. Five articles were based on data from the STAR trial, a multicenter study that included adults with moderate-to-severe OSA and inadequate adherence to CPAP.20-24 Four articles used the same patient selection criteria as the STAR trial for a long-term German postmarket study of upper airway stimulation efficacy with OSA.25-28 The third and fourth cohorts each consist of 31 patients with moderate-to-severe OSA with CPAP nonadherence or failure.29,30 The STAR trial included follow-up at 5 years, and the German-postmarket had a follow-up at3 years. The remaining 2 cohorts have 1-year follow-ups.
The Scopus review identified 304 studies; 299 did not meet inclusion criteria and 1 was part of the STAR trial.31 The remaining 4 articles were classified as distinct cohorts. Huntley and colleagues included patients from Thomas Jefferson University (TJU) and University of Pittsburgh (UP) academic medical centers.32 The Pordzik and colleagues cohort received implantation at a tertiary medical center, an RCCT, and a 1:1 comparator trial (Table 1).33-35
STAR Trial
This multicenter, prospective, single-group cohort study was conducted in the US, Germany, Belgium, Netherlands, and France. The STAR trial included 126 patients who were not CPAP therapy adherent. Patients were excluded if they had AHI < 20 or > 50, central sleep apnea > 25% of total AHI, anatomical abnormalities that prevent effective assessment of upper-airway stimulation, complete concentric collapse of the retropalatal airway during drug-induced sleep, neuromuscular disease, hypoglossal-nerve palsy, severe restrictive or obstructive pulmonary disease, moderate-to-severe pulmonary arterial hypertension, severe valvular heart disease, New York Heart Association class III or IV heart failure, recent myocardial infarction or severe cardiac arrhythmias (within the past 6 months), persistent uncontrolled hypertension despite medication use, active psychiatric illness, or coexisting nonrespiratory sleep disorders that would confound functional sleep assessment. Primary outcome measures included the AHI and oxygen desaturation index (ODI) with secondary outcomes using the ESS, the Functional Outcomes of Sleep Questionnaire (FOSQ), and the percentage of sleep time with oxygen saturation < 90%. Of 126 patients who received implantation, 71 underwent an overnight PSG evaluation at 5-year follow-up. Mean (SD) AHI at baseline was reduced with HGNS treatment to from 32.0 (11.8) to 12.4 (16.3). Mean (SD) ESS for 92 participants with 2 measurements declined from 11.6 (5.0) at baseline to 6.9 (4.7) at 5-year follow-up.
The STAR trial included a randomized controlled withdrawal study for 46 patients who had a positive response to therapy to evaluate efficacy and durability of upper airway stimulation. Patients were randomly assigned to therapy maintenance or therapy withdrawal groups for ≥ 1 week. The short-term withdrawal effect was assessed using the original trial outcome measures and indicated that both the withdrawal and maintenance groups showed improvements at 12 months compared with the baseline. However, after the randomized withdrawal, the withdrawal group’s outcome measures deteriorated to baseline levels while the maintenance group showed no change. At 18 months of therapy, outcome measures for both groups were similar to those observed with therapy at 12 months.24 The STAR trial included self-reported outcomes at baseline, 12 months, and 24 months that used ESS to measure daytime sleepiness. These results included subsequent STAR trial reports.20-24,31
The German Postmarket Cohort
This multicenter, prospective, single-arm study used selection criteria that were based on those used in the STAR trial and included patients with moderate-to-severe OSA and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, AHI < 15 or > 65; central apnea index > 25% of total AHI; or complete concentric collapse at the velopharynx during drug-induced sleep. Measured outcomes included AHI, ODI, FOSQ, and ESS. Among the 60 participants, 38 received implantation and a 3-year follow-up. Mean (SD) AHI decreased from 31.2 (13.2) at baseline to 13.1 (14.1) at follow-up, while mean (SD) ESS decreased from 12.8 (5.3) at baseline to 6.0 (3.2) at follow-up.25-28
Munich Cohort
This single-center, prospective clinical trial included patients with AHI > 15 and < 65, central apnea index < 25% of total AHI, and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, anatomical abnormalities that would prevent effective assessment of upper-airway stimulation; all other exclusion criteria matched those used in the STAR trial. Among 31 patients who received implants and completed a 1-year follow-up, mean (SD) AHI decreased from 32.9 (11.2) at baseline to 7.1 (5.9) at follow-up and mean (SD) ESS decreased from 12.6 (5.6) at baseline to 5.9 (5.2) at follow-up.29
Kezirian and Colleagues Cohort
This prospective, single-arm, open-label study was conducted at 4 Australian and 4 US sites. Selection criteria included moderate-to-severe OSA with failure of CPAP, AHI of 20 to 100 with ≥ 15 events/hour occurring in sleep that was non-REM (rapid eye movement) sleep, BMI ≤ 40 (Australia) or ≤ 37 (US), and a predominance of hypopneas (≥ 80% of disordered breathing events during sleep). Patients were excluded if they had earlier upper airway surgery, markedly enlarged tonsils, uncontrolled nasal obstruction, severe retrognathia, > 5% central or mixed apneic events, incompletely treated sleep disorders other than OSA, or a major disorder of the pulmonary, cardiac, renal, or nervous systems. Data were reported for 31 patients whose mean (SD) AHI declined from 45.4 (17.5) at baseline to 25.3 (20.6) at 1-year follow-up and mean (SD) ESS score declined from 12.1 (4.6) at baseline to 7.9 (3.8) 1 year later.30
TJU and UP Cohorts
The TJU and UP cohorts are composed of patients who underwent implantation between May 2014 and August 2016 at 2 academic centers.31,32 Selection criteria was consistent with that used in the STAR trial, and patients completed postoperative titration PSG and outpatient follow-up (48 patients at TJU and 49 at UP). Primary outcomes included AHI, ESS, and O2 nadir. Secondary outcomes consisted of surgical success and percentage of patients tolerating optimal titration setting at follow-up. Postoperative outcomes were assessed during the titration PSG. Time from initial ESS to postoperative PSG at TJU was 1.7 years and at UP was 1.9 years. Time from initial AHI to postoperative PSG at TJU was 90.4 days and 85.2 days at UP. At TJU, mean (SD) AHI and ESS dropped from 35.9 (20.8) and 11.1 (3.8), respectively at baseline to 6.3 (11.5) and 5.8 (3.4), respectively at follow-up. At UP, mean (SD) AHI and ESS fell from 35.3 (15.3) and 10.9 (4.9), respectively at baseline to 6.3 (6.1) and 6.6 (4.5), respectively at follow-up. There were no site-related differences in rates of AHI, ESS, or surgical success.31
Pordzik and Colleagues Cohort
This cohort of 29 patients underwent implantation between February 2020 and June 2022 at a tertiary university medical center with both pre- and postoperative PSG. Selection criteria was consistent with that of the German postmarket cohort. Postoperative PSG was completed a mean (SD) 96.3 (27.0) days after device activation. Mean (SD) AHI dropped from 38.6 (12.7) preoperatively to 24.4 (13.3) postoperatively. Notably, this cohort showed a much lower decrease of postoperative AHI than reported by the STAR trial and UP/TJU cohort.33
Stimulation vs Sham Trial
This multicenter, double-blinded, randomized, crossover trial assessed the effect of HGNS (stim) vs sham stimulation (sham) in 86 patients that completed both phases of the trial. Primary outcomes included AHI and ESS. Secondary outcomes included FOSQ. No carryover effect was found during the crossover phase. The difference between the phases was−15.5 (95% CI, −18.3 to −12.8) for AHI and −3.3 (95% CI, −4.4 to −2.2) for ESS.34
Comparator
The comparator study used propensity score matching to compare outcomes of HGNS and PAP therapy. Primary outcomes included sleepiness, AHI, and effectiveness with outcome measures of AHI and ESS collected at baseline and 12 months postimplantation. The article reported that 126 of 227 patients were matched 1:1. Both groups showed improvement in AHI and ESS. Mean (SD) AHI for the HGNS group at baseline started at 33.9 (15.1) and decreased to 8.1 (6.3). Mean (SD) ESS for the HGNS group at baseline was 15.4 (3.5) and decreased to 7.5 (4.7). In the PAP comparator group, mean (SD) baseline AHI was 36.8 (21.6) and at follow-up was 6.6 (8.0) and mean (SD) ESS was 14.6 (3.9) at baseline and 10.8 (5.6) at follow-up.35
DISCUSSION
The current clinical data on HGNS suggest that this treatment is effective in adults with moderate-to-severe OSA and effects are sustained at long-term follow-up, as measured by AHI reduction and improvements in sleep related symptoms and quality of life (Table 2). These results have been consistent across several sites.
The STAR trial included a randomized control withdrawal group, for whom HGNS treatment was withdrawn after the 12-month follow-up, and then restored at 18 months.21 This revealed that withdrawal of HGNS treatment resulted in deterioration of both objective and subjective measures of OSA and sleepiness. The beneficial effects of HGNS were restored when treatment was resumed.24 Additionally, the RCCT revealed that therapeutic stimulation via HGNS significantly reduced subjective and objective measures of OSA.34 These studies provide definitive evidence of HGNS efficacy.
Currently, a diagnosis of OSA on PAP is classified as a 50% military disability rating. This rating is based primarily on epidemiologic evidence that untreated OSA is a costly disease that leads to other chronic illnesses that increases health care utilization.9 HGNS requires an initially invasive procedure and higher upfront costs, but it could result in reduced health care use and long-term costs because of improved adherence to treatment—compared with CPAP—that results in better outcomes.
Limitations to OSA Studies
The reviewed studies have several limitations that warrant caution when determining the possible benefits of HGNS treatment. The primary limitation is the lack of active control groups, therefore precluding a direct comparison of the short- and long-term effectiveness of HGNS vs other treatments (eg, CPAP). This is especially problematic because in the reviewed studies HGNS treatment efficacy is reported as a function of the mean—and SD—percent reduction in the AHI, whereas the efficacy of CPAP treatment usually is defined in terms of “adequacy of titration” as suggested by the AASM.36 It has been reported that with CPAP treatment, 50% to 60% of OSA patients achieve AASM-defined optimal improvement of respiratory disturbance index of < 5/hour during a polysomnographic sleep recording of ≥ 15 minutes duration that includes REM sleep in the supine position.37 In most of the reviewed studies, treatment success was more liberally defined as a decrease of AHI by ≥ 50%, regardless of the resulting AHI. It is notable that among the reviewed HGNS studies, the TJU and UP cohorts achieved the best outcome in short-term follow-up of 2 months with a mean (SD) AHI of 6.3 (11.5) and 6.4 (6.1), respectively. Among those cohortsassessed at a 12-month follow-up, the Munich cohort achieved the best outcome with a mean (SD) AHI of 7.1 (5.9).
Although the metrics reported in the reviewed studies are not directly comparable, the reported findings strongly suggest that HGNS generally is less effective than CPAP. How important are these differences? With findings that HGNS “reliably produces clinically meaningful (positive) effects on daytime sleepiness, daytime functioning, and sleep quality,” does it really matter if the outcome metrics for HGNS are a little less positive than those produced by CPAP?38 For individual military OSA patients the answer is yes. This is because in military operational environments—especially during deployment—sleep restriction is nearly ubiquitous, therefore any mild residual deficits in sleep quality and daytime alertness resulting from nominally adequate, but suboptimal OSA treatment, could be exacerbated by sleep restriction, therefore placing the service member and the mission at increased risk.39
Another limitation is the narrow inclusion criteria these studies employed, which limits the generalizability of the findings. Participants in the reviewed clinical trials were selected from a patient population that was mostly middle-aged, White, and obese or overweight. In a Medical Surveillance Monthly Report study, OSA was found to be highest among service members aged > 40 years, male, obese, and Black/non-Hispanic (although it should be noted that more than one-half of enlisted service members aged ≤ 25 years).40,41 Obesity has been noted as a growing concern for the military as the military population is beginning to mirror the civilian population in terms of being overweight or obese despite height and weight standards. HGNS might not be as successful in military populations with different demographics. Moreover, HGNS has been shown to have greater AHI reduction among those with higher BMI.30 Although obese service members have a 6-fold higher 12-year incidence rate of OSA than service members without obesity, this nevertheless suggests that general level of HGNS efficacy might be lower among the military patient population, because obesity is less prevalent in the military than the general population.9
Ethnicity has been found to be a relevant factor, with the highest incidence rate of OSA among non-Hispanic Black males, a demographic that was underrepresented in cohorts included in this review. Further studies will be needed to determine the extent to which findings from HGNS treatment studies are generalizable to the broader OSA patient population.
HGNS Implementation Challenges
Current impediments to widespread use of HGNS as an OSA treatment include no standardized guidance for titration and follow-on care, which varies based on the resources available. Titrating a new device for HGNS requires experienced sleep technicians who have close relationships with device representatives and can troubleshoot problems. Technical expertise, which currently is rare, is required if there are complications after placement or if adjustments to voltage settings are needed over time. In addition, patients may require multiple specialists making it easy to get lost to follow-up after implantation. This is particularly challenging in a transient community, such as the military, because there is no guarantee that a service member will have access to the same specialty care at the next duty station.
Although some evidence suggests that HGNS is a viable alternative treatment for some patients with OSA, the generalizability of these findings to the military patient population is unclear. Specialized facilities and expertise are needed for the surgical procedure and follow-up requirements, which currently constitute significant logistical constraints. As with any implantable device, there is a risk of complications including infection that could result in medical evacuation from a theater of operations. If the device malfunctions or loses effectiveness in a deployed environment, the service member might not have immediate access to medical support, potentially leading to undertreatment of OSA. In future battlefield scenarios in multidomain operations, prolonged, far-forward field care will become the new normal because the military is not expected to have air superiority or the ability to quickly evacuate service members to a higher level of medical care.42
In deployed environments, the potential limitations of HGNS become increasingly risky for the service member and the overall mission. Considering these factors, it will be important to evaluate the practicality of HGNS as a treatment option in military populations. Military-specific challenges associated with HGNS that require further study, include guidance for patient selection outside academic centers, guidance on long-term postsurgical care and device maintenance, duty limitation and military retention considerations, and limitations in training and combat environments. The military medical community needs to conduct its own studies in appropriately selected service members to guide clinical practice.
CONCLUSIONS
HGNS treatment results in improvement of both AHI and ESS scores and could be a deployable treatment option for military patients with OSA. However, HGNS has not been found to be as effective as CPAP, although the current literature is limited by small sample sizes, homogeneous populations that do not reflect the demographics of the military, and mostly short follow-up periods. Future studies should be focused on collecting data on HGNS from demographic groups that are more representative of the military OSA patient population and identifying the subpopulation of patients who derive the greatest benefit from HGNS, so that this treatment can be better individually targeted. Until data on existing military patients is published, it is not possible to fully weigh risks and benefits in this population and generalize civilian guidance to the military.
Obstructive sleep apnea (OSA), the repetitive collapse of posterior oropharynx during sleep resulting in hypoxia and/or arousals from sleep, is the most common form of sleep disordered breathing and a common chronic respiratory disorders among middle-aged adults. OSA can lead to significant health problems, such as worsened cardiometabolic disease and cognitive impairment, which can increase morbidity and mortality.1
The gold standard for OSA diagnosis is polysomnography (PSG), although home sleep studies can be performed for select patients. OSA diagnoses are based on the number of times per hour of sleep a patient’s airway narrows or collapses, reducing or stopping airflow, scored as hypopnea or apnea events, respectively. An Apnea-Hypopnea Index (AHI) score of 5 to 14 events/hour is considered mild OSA, 15 to 30 events/hour moderate OSA, and ≥ 30 events/hour severe OSA.2
Treatment commonly includes positive airway pressure (PAP) but more than one-half of patients are not adherent to continuous PAP (CPAP) treatment after about 90 days.3 Efficacy of treatments vary as a function of disease severity and etiology, which—in addition to the classic presentation of obesity with large neck/narrowupper airway—includes craniofacial abnormalities, altered muscle function in the upper airway, pharyngeal neuropathy, and fluid shifts to the neck.
Background
The American Academy of Sleep Medicine (AASM) estimates that 10% to 17% of adults in the United States have OSA.4 Compared with civilians, the military population generally is younger and healthier. Service members have access to regular health care with yearly physical examinations, exercise scheduled into the workday, and mandatory height/weight and fitness standards. Because obesity is a major risk factor for OSA, and the incidence of obesity is relatively low in the military population (estimated at 18.8% in 2021 vs 39.8% among all US adults aged 20 to 39 years), it might be expected that incidence of OSA would be correspondingly low.5,6 However, there is evidence of a rapidly increasing incidence of OSA in military populations. A 2021 study revealed that OSA incidence rates increased from 11 to 333 per 10,000 between 2005 and 2019 across all military branches and demographics, with the highest rate among Army personnel.7 An earlier study revealed a 600% increase in OSA incidence among Army personnel between 2003 and 2011.8
Several factors likely contributed to this increase, including expanding obesity and greater physician awareness and availability of sleep study centers. Rogers and colleagues found that 40% to 50% of incident OSA diagnoses among military personnel occur within 12 months of separation, suggesting that the secondary gains associated with military disability benefits might motivate OSA evaluation.9 It is possible that secondary gain is a factor because an OSA diagnosis can range from a 0% to 100% disability rating, depending on the severity.10 This disability claim is based on evidence that untreated OSA can negatively affect long-term health and mission readiness.8 For example, untreated OSA can lead to hypertension, which contributes to a long list of adverse health and wellness consequences. Most importantly for the military, OSA has been shown to increase daytime sleepiness and reduce cognitive performance.10
The current first-line treatment for OSA is CPAP, which improves symptoms of daytime sleepiness, hypertension management, and daytime alertness.11 Despite its efficacy, nonadherence rates range from 29% to 83%.12-15 Nonadherence factors include lifestyle changes, adverse effects (eg, nasal congestion), and lack of education on proper use.11 Lifestyle changes needed to increase the likelihood of successful therapy, such as regular sleep schedules and proper CPAP cleaning and maintenance, are difficult for military personnel because of the nature of continuous or sustained operations that might require shift work and/or around-the-clock (ie, 24-hour, 7 days a week) task performance. Traveling with CPAP is an added burden for service members deployed to combat operations (ie, added luggage, weight, maintenance). Although alternate treatments such as oral appliances (ie, custom dental devices) are available, they generally are less effective than CPAP.2 Oral appliances could be a reasonable alternative treatment for some patients who cannot manage their OSA with behavioral modifications and are intolerant or unable to effectively use CPAP. This could include patients in the military who are deployed to austere environments.
Surgically implanted hypoglossal nerve stimulator (HGNS) treatment may provide long-term health benefits to service members. After the device is implanted near the hypoglossal nerve, electrical stimulation causes the tongue to move forward, which opens the airway in the anteroposterior dimension. The most important consideration is the mechanism of airway collapse. HGNS is not effective for patients whose OSA events are caused by circumferential collapse of other airway muscles. The cause of airway collapse is ascertained before surgery with drug-induced sleep endoscopy, a procedure that allows visualization of conformational changes in the upper airway during OSA events.
The US Food and Drug Administration (FDA) approved HGNS in 2014. However, it is not considered a first-line treatment for OSA by the AASM. Original candidate criteria for HGNS included an AHI score of 15 to 65 events/hour, age ≥ 18 years, failed CPAP use, body mass index (BMI) < 32, absence of palatal complete concentric collapse, and central apneas comprising < 25% of total events.16 In June 2023, the FDA expanded approval to increase the upper limit of AHI to 100 events/hour and the BMI to < 40.17
HGNS has been reported to be effective in appropriately selected patients with OSA at tertiary care centers with established multidisciplinary sleep surgical programs. These benefits have not been confirmed in larger, community-based settings, where most of these surgeries occur. In community practice, there is significant confusion among patients and clinicians about the optimal pathway for patient selection and clinical follow-up. Many patients view HGNS as a viable alternative to CPAP, but initially do not understand that it requires surgery. Surgical treatments for OSA, such as HGNS, are appealing because they suggest a 1-time intervention that permanently treats the condition, without need for follow-up or equipment resupply. HGNS might be an appealing treatment option because it is less obtrusive than CPAP and requires fewer resources for set-up and maintenance. Also, it does not cause skin irritation (a possible adverse effect of nightly use of a CPAP mask), allows the individual to sleep in a variety of positions, has less impact on social and sex life, and does not require an electric outlet. In the long term, HGNS might be more cost effective because there is no yearly physician follow-up or equipment resupply and/or maintenance.
The military population has specific demands that impact delivery and effectiveness of health care. Among service members with OSA, CPAP treatment can be challenging because of low adherence, required annual follow-up despite frequent moving cycles that pose a challenge for care continuity, and duty limitations for affected service members (ie, the requirement for a waiver to deploy and potential medical separation if symptoms are not adequately controlled). As the incidence of OSA continues to increase among service members, so does the need for OSA treatment options that are efficacious as CPAP but better tolerated and more suitable for use during military operations. The aim of this review is to assess the effectiveness of HGNS and its potential use by the military OSA patient population.
METHODS
To identify eligible studies, we employed PICOS: Population (patients aged ≥ 18 years with a history of OSA), Intervention (HGNS), Comparator (standard of care PAP therapy), Outcome (AHI or Epworth Sleepiness Scale [ESS], and Study (randomized control trial [RCT] or clinical trial). Studies were excluded if they were not written in English or included pediatric populations. The ESS is a subjective rating scale used to determine and quantify a patient’s level of daytime sleepiness, using a 4-point scale for the likelihood of falling asleep totaled across 8 different situations.18 Daytime sleepiness is considered lower normal(0-5 points), higher normal (6-10 points), mild or moderate excessive (11-15 points), and severe excessive (16-24 points).
Literature Search
We conducted a review of PubMed and Scopus for RCTs and controlled trials published from 2013 to 2023 that included the keywords and phrases: obstructive sleep apnea and either hypoglossal nerve stimulation or upper airway stimulation. The final literature search was performed December 8, 2023.
Two authors independently assessed the titles and abstracts of studies identified in the literature search based on the predefined inclusion criteria. If it was not clear whether an article met inclusion criteria based on its title and/or abstract, the 2 review authors assessed the full text of study and resolved any disagreement through consensus. If consensus was not obtained, a third author was consulted. No duplicates were identified. The PRISMA study selection process is presented in the Figure.
Data extraction was performed by 1 independent reviewer. A second author reviewed the extracted data. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third author was consulted. Study data included methods (study design and study objective), participants mean age, inclusion criteria, exclusion criteria, interventions and comparators, and primary study outcomes.
The quality of evidence was assessed using a rating of 1 to 5 based on a modified version of the Oxford Centre for Evidence-based Medicine Levels of Evidence and Grades of Recommendation.19 A rating of 1 indicated a properly powered and conducted RCT, 2 demonstrated a well-designed controlled trial without randomization or prospective comparative cohort trial, 3 designated a case-control study or retrospective cohort study, 4 signified a case series with or without intervention or a cross-sectional study, and 5 denoted an opinion of respected authorities or case reports. Two reviewers independently evaluated the quality of evidence. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third review author was consulted.
RESULTS
We identified 30 studies; 19 articles did not meet inclusion criteria. The remaining 11 articles were divided into 4 cohorts. Five articles were based on data from the STAR trial, a multicenter study that included adults with moderate-to-severe OSA and inadequate adherence to CPAP.20-24 Four articles used the same patient selection criteria as the STAR trial for a long-term German postmarket study of upper airway stimulation efficacy with OSA.25-28 The third and fourth cohorts each consist of 31 patients with moderate-to-severe OSA with CPAP nonadherence or failure.29,30 The STAR trial included follow-up at 5 years, and the German-postmarket had a follow-up at3 years. The remaining 2 cohorts have 1-year follow-ups.
The Scopus review identified 304 studies; 299 did not meet inclusion criteria and 1 was part of the STAR trial.31 The remaining 4 articles were classified as distinct cohorts. Huntley and colleagues included patients from Thomas Jefferson University (TJU) and University of Pittsburgh (UP) academic medical centers.32 The Pordzik and colleagues cohort received implantation at a tertiary medical center, an RCCT, and a 1:1 comparator trial (Table 1).33-35
STAR Trial
This multicenter, prospective, single-group cohort study was conducted in the US, Germany, Belgium, Netherlands, and France. The STAR trial included 126 patients who were not CPAP therapy adherent. Patients were excluded if they had AHI < 20 or > 50, central sleep apnea > 25% of total AHI, anatomical abnormalities that prevent effective assessment of upper-airway stimulation, complete concentric collapse of the retropalatal airway during drug-induced sleep, neuromuscular disease, hypoglossal-nerve palsy, severe restrictive or obstructive pulmonary disease, moderate-to-severe pulmonary arterial hypertension, severe valvular heart disease, New York Heart Association class III or IV heart failure, recent myocardial infarction or severe cardiac arrhythmias (within the past 6 months), persistent uncontrolled hypertension despite medication use, active psychiatric illness, or coexisting nonrespiratory sleep disorders that would confound functional sleep assessment. Primary outcome measures included the AHI and oxygen desaturation index (ODI) with secondary outcomes using the ESS, the Functional Outcomes of Sleep Questionnaire (FOSQ), and the percentage of sleep time with oxygen saturation < 90%. Of 126 patients who received implantation, 71 underwent an overnight PSG evaluation at 5-year follow-up. Mean (SD) AHI at baseline was reduced with HGNS treatment to from 32.0 (11.8) to 12.4 (16.3). Mean (SD) ESS for 92 participants with 2 measurements declined from 11.6 (5.0) at baseline to 6.9 (4.7) at 5-year follow-up.
The STAR trial included a randomized controlled withdrawal study for 46 patients who had a positive response to therapy to evaluate efficacy and durability of upper airway stimulation. Patients were randomly assigned to therapy maintenance or therapy withdrawal groups for ≥ 1 week. The short-term withdrawal effect was assessed using the original trial outcome measures and indicated that both the withdrawal and maintenance groups showed improvements at 12 months compared with the baseline. However, after the randomized withdrawal, the withdrawal group’s outcome measures deteriorated to baseline levels while the maintenance group showed no change. At 18 months of therapy, outcome measures for both groups were similar to those observed with therapy at 12 months.24 The STAR trial included self-reported outcomes at baseline, 12 months, and 24 months that used ESS to measure daytime sleepiness. These results included subsequent STAR trial reports.20-24,31
The German Postmarket Cohort
This multicenter, prospective, single-arm study used selection criteria that were based on those used in the STAR trial and included patients with moderate-to-severe OSA and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, AHI < 15 or > 65; central apnea index > 25% of total AHI; or complete concentric collapse at the velopharynx during drug-induced sleep. Measured outcomes included AHI, ODI, FOSQ, and ESS. Among the 60 participants, 38 received implantation and a 3-year follow-up. Mean (SD) AHI decreased from 31.2 (13.2) at baseline to 13.1 (14.1) at follow-up, while mean (SD) ESS decreased from 12.8 (5.3) at baseline to 6.0 (3.2) at follow-up.25-28
Munich Cohort
This single-center, prospective clinical trial included patients with AHI > 15 and < 65, central apnea index < 25% of total AHI, and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, anatomical abnormalities that would prevent effective assessment of upper-airway stimulation; all other exclusion criteria matched those used in the STAR trial. Among 31 patients who received implants and completed a 1-year follow-up, mean (SD) AHI decreased from 32.9 (11.2) at baseline to 7.1 (5.9) at follow-up and mean (SD) ESS decreased from 12.6 (5.6) at baseline to 5.9 (5.2) at follow-up.29
Kezirian and Colleagues Cohort
This prospective, single-arm, open-label study was conducted at 4 Australian and 4 US sites. Selection criteria included moderate-to-severe OSA with failure of CPAP, AHI of 20 to 100 with ≥ 15 events/hour occurring in sleep that was non-REM (rapid eye movement) sleep, BMI ≤ 40 (Australia) or ≤ 37 (US), and a predominance of hypopneas (≥ 80% of disordered breathing events during sleep). Patients were excluded if they had earlier upper airway surgery, markedly enlarged tonsils, uncontrolled nasal obstruction, severe retrognathia, > 5% central or mixed apneic events, incompletely treated sleep disorders other than OSA, or a major disorder of the pulmonary, cardiac, renal, or nervous systems. Data were reported for 31 patients whose mean (SD) AHI declined from 45.4 (17.5) at baseline to 25.3 (20.6) at 1-year follow-up and mean (SD) ESS score declined from 12.1 (4.6) at baseline to 7.9 (3.8) 1 year later.30
TJU and UP Cohorts
The TJU and UP cohorts are composed of patients who underwent implantation between May 2014 and August 2016 at 2 academic centers.31,32 Selection criteria was consistent with that used in the STAR trial, and patients completed postoperative titration PSG and outpatient follow-up (48 patients at TJU and 49 at UP). Primary outcomes included AHI, ESS, and O2 nadir. Secondary outcomes consisted of surgical success and percentage of patients tolerating optimal titration setting at follow-up. Postoperative outcomes were assessed during the titration PSG. Time from initial ESS to postoperative PSG at TJU was 1.7 years and at UP was 1.9 years. Time from initial AHI to postoperative PSG at TJU was 90.4 days and 85.2 days at UP. At TJU, mean (SD) AHI and ESS dropped from 35.9 (20.8) and 11.1 (3.8), respectively at baseline to 6.3 (11.5) and 5.8 (3.4), respectively at follow-up. At UP, mean (SD) AHI and ESS fell from 35.3 (15.3) and 10.9 (4.9), respectively at baseline to 6.3 (6.1) and 6.6 (4.5), respectively at follow-up. There were no site-related differences in rates of AHI, ESS, or surgical success.31
Pordzik and Colleagues Cohort
This cohort of 29 patients underwent implantation between February 2020 and June 2022 at a tertiary university medical center with both pre- and postoperative PSG. Selection criteria was consistent with that of the German postmarket cohort. Postoperative PSG was completed a mean (SD) 96.3 (27.0) days after device activation. Mean (SD) AHI dropped from 38.6 (12.7) preoperatively to 24.4 (13.3) postoperatively. Notably, this cohort showed a much lower decrease of postoperative AHI than reported by the STAR trial and UP/TJU cohort.33
Stimulation vs Sham Trial
This multicenter, double-blinded, randomized, crossover trial assessed the effect of HGNS (stim) vs sham stimulation (sham) in 86 patients that completed both phases of the trial. Primary outcomes included AHI and ESS. Secondary outcomes included FOSQ. No carryover effect was found during the crossover phase. The difference between the phases was−15.5 (95% CI, −18.3 to −12.8) for AHI and −3.3 (95% CI, −4.4 to −2.2) for ESS.34
Comparator
The comparator study used propensity score matching to compare outcomes of HGNS and PAP therapy. Primary outcomes included sleepiness, AHI, and effectiveness with outcome measures of AHI and ESS collected at baseline and 12 months postimplantation. The article reported that 126 of 227 patients were matched 1:1. Both groups showed improvement in AHI and ESS. Mean (SD) AHI for the HGNS group at baseline started at 33.9 (15.1) and decreased to 8.1 (6.3). Mean (SD) ESS for the HGNS group at baseline was 15.4 (3.5) and decreased to 7.5 (4.7). In the PAP comparator group, mean (SD) baseline AHI was 36.8 (21.6) and at follow-up was 6.6 (8.0) and mean (SD) ESS was 14.6 (3.9) at baseline and 10.8 (5.6) at follow-up.35
DISCUSSION
The current clinical data on HGNS suggest that this treatment is effective in adults with moderate-to-severe OSA and effects are sustained at long-term follow-up, as measured by AHI reduction and improvements in sleep related symptoms and quality of life (Table 2). These results have been consistent across several sites.
The STAR trial included a randomized control withdrawal group, for whom HGNS treatment was withdrawn after the 12-month follow-up, and then restored at 18 months.21 This revealed that withdrawal of HGNS treatment resulted in deterioration of both objective and subjective measures of OSA and sleepiness. The beneficial effects of HGNS were restored when treatment was resumed.24 Additionally, the RCCT revealed that therapeutic stimulation via HGNS significantly reduced subjective and objective measures of OSA.34 These studies provide definitive evidence of HGNS efficacy.
Currently, a diagnosis of OSA on PAP is classified as a 50% military disability rating. This rating is based primarily on epidemiologic evidence that untreated OSA is a costly disease that leads to other chronic illnesses that increases health care utilization.9 HGNS requires an initially invasive procedure and higher upfront costs, but it could result in reduced health care use and long-term costs because of improved adherence to treatment—compared with CPAP—that results in better outcomes.
Limitations to OSA Studies
The reviewed studies have several limitations that warrant caution when determining the possible benefits of HGNS treatment. The primary limitation is the lack of active control groups, therefore precluding a direct comparison of the short- and long-term effectiveness of HGNS vs other treatments (eg, CPAP). This is especially problematic because in the reviewed studies HGNS treatment efficacy is reported as a function of the mean—and SD—percent reduction in the AHI, whereas the efficacy of CPAP treatment usually is defined in terms of “adequacy of titration” as suggested by the AASM.36 It has been reported that with CPAP treatment, 50% to 60% of OSA patients achieve AASM-defined optimal improvement of respiratory disturbance index of < 5/hour during a polysomnographic sleep recording of ≥ 15 minutes duration that includes REM sleep in the supine position.37 In most of the reviewed studies, treatment success was more liberally defined as a decrease of AHI by ≥ 50%, regardless of the resulting AHI. It is notable that among the reviewed HGNS studies, the TJU and UP cohorts achieved the best outcome in short-term follow-up of 2 months with a mean (SD) AHI of 6.3 (11.5) and 6.4 (6.1), respectively. Among those cohortsassessed at a 12-month follow-up, the Munich cohort achieved the best outcome with a mean (SD) AHI of 7.1 (5.9).
Although the metrics reported in the reviewed studies are not directly comparable, the reported findings strongly suggest that HGNS generally is less effective than CPAP. How important are these differences? With findings that HGNS “reliably produces clinically meaningful (positive) effects on daytime sleepiness, daytime functioning, and sleep quality,” does it really matter if the outcome metrics for HGNS are a little less positive than those produced by CPAP?38 For individual military OSA patients the answer is yes. This is because in military operational environments—especially during deployment—sleep restriction is nearly ubiquitous, therefore any mild residual deficits in sleep quality and daytime alertness resulting from nominally adequate, but suboptimal OSA treatment, could be exacerbated by sleep restriction, therefore placing the service member and the mission at increased risk.39
Another limitation is the narrow inclusion criteria these studies employed, which limits the generalizability of the findings. Participants in the reviewed clinical trials were selected from a patient population that was mostly middle-aged, White, and obese or overweight. In a Medical Surveillance Monthly Report study, OSA was found to be highest among service members aged > 40 years, male, obese, and Black/non-Hispanic (although it should be noted that more than one-half of enlisted service members aged ≤ 25 years).40,41 Obesity has been noted as a growing concern for the military as the military population is beginning to mirror the civilian population in terms of being overweight or obese despite height and weight standards. HGNS might not be as successful in military populations with different demographics. Moreover, HGNS has been shown to have greater AHI reduction among those with higher BMI.30 Although obese service members have a 6-fold higher 12-year incidence rate of OSA than service members without obesity, this nevertheless suggests that general level of HGNS efficacy might be lower among the military patient population, because obesity is less prevalent in the military than the general population.9
Ethnicity has been found to be a relevant factor, with the highest incidence rate of OSA among non-Hispanic Black males, a demographic that was underrepresented in cohorts included in this review. Further studies will be needed to determine the extent to which findings from HGNS treatment studies are generalizable to the broader OSA patient population.
HGNS Implementation Challenges
Current impediments to widespread use of HGNS as an OSA treatment include no standardized guidance for titration and follow-on care, which varies based on the resources available. Titrating a new device for HGNS requires experienced sleep technicians who have close relationships with device representatives and can troubleshoot problems. Technical expertise, which currently is rare, is required if there are complications after placement or if adjustments to voltage settings are needed over time. In addition, patients may require multiple specialists making it easy to get lost to follow-up after implantation. This is particularly challenging in a transient community, such as the military, because there is no guarantee that a service member will have access to the same specialty care at the next duty station.
Although some evidence suggests that HGNS is a viable alternative treatment for some patients with OSA, the generalizability of these findings to the military patient population is unclear. Specialized facilities and expertise are needed for the surgical procedure and follow-up requirements, which currently constitute significant logistical constraints. As with any implantable device, there is a risk of complications including infection that could result in medical evacuation from a theater of operations. If the device malfunctions or loses effectiveness in a deployed environment, the service member might not have immediate access to medical support, potentially leading to undertreatment of OSA. In future battlefield scenarios in multidomain operations, prolonged, far-forward field care will become the new normal because the military is not expected to have air superiority or the ability to quickly evacuate service members to a higher level of medical care.42
In deployed environments, the potential limitations of HGNS become increasingly risky for the service member and the overall mission. Considering these factors, it will be important to evaluate the practicality of HGNS as a treatment option in military populations. Military-specific challenges associated with HGNS that require further study, include guidance for patient selection outside academic centers, guidance on long-term postsurgical care and device maintenance, duty limitation and military retention considerations, and limitations in training and combat environments. The military medical community needs to conduct its own studies in appropriately selected service members to guide clinical practice.
CONCLUSIONS
HGNS treatment results in improvement of both AHI and ESS scores and could be a deployable treatment option for military patients with OSA. However, HGNS has not been found to be as effective as CPAP, although the current literature is limited by small sample sizes, homogeneous populations that do not reflect the demographics of the military, and mostly short follow-up periods. Future studies should be focused on collecting data on HGNS from demographic groups that are more representative of the military OSA patient population and identifying the subpopulation of patients who derive the greatest benefit from HGNS, so that this treatment can be better individually targeted. Until data on existing military patients is published, it is not possible to fully weigh risks and benefits in this population and generalize civilian guidance to the military.
1. Cumpston E, Chen P. Sleep Apnea Syndrome. PubMed. Updated September 4, 2023. Published January 2024. https://www.ncbi.nlm.nih.gov/books/NBK564431/
2. American Academy of Sleep Medicine. Obstructive sleep apnea. Accessed November 27, 2023. https://aasm.org/resources/factsheets/sleepapnea.pdf
3. Cowen J, Harrison S, Thom L, et al. Use of historical remote monitoring data to determine predictors of CPAP non-compliance in patients with Osa. Sleep Breath. 2023;27(5):1899-1908. doi:10.1007/s11325-023-02806-3
4. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
5. Stiegmann RA, Payne CB, Kiel MA, Stahlman SL. Increased Prevalence of Overweight and Obesity and Incidence of Prediabetes and Type 2 Diabetes During the COVID-19 Pandemic, Active Component Service Members, U.S. Armed Forces, 2018 to 2021. MSMR. 2023;30(1):11-18. Published 2023 Jan 20.
6. Adult obesity facts. Centers for Disease Control and Prevention. Updated May 17, 2022. Accessed November 27, 2023. https://www.cdc.gov/obesity/data/adult.html
7. Moore BA, Tison LM, Palacios JG, Peterson AL, Mysliwiec V. Incidence of insomnia and obstructive sleep apnea in active duty United States military service members. Sleep. 2021;44(7):zsab024. doi:10.1093/sleep/zsab024
8. Caldwell JA, Knapik JJ, Shing TL, Kardouni JR, Lieberman HR. The association of insomnia and sleep apnea with deployment and combat exposure in the entire population of US army soldiers from 1997 to 2011: a retrospective cohort investigation. Sleep. 2019;42(8):zsz112. doi:10.1093/sleep/zsz112
9. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
10. Veterans Affairs 38 C.F.R. § 4.97-13, Code 6847.
11. Shapiro GK, Shapiro CM. Factors that influence CPAP adherence: an overview. Sleep Breath. 2010;14(4):323-335. doi:10.1007/s11325-010-0391-y
12. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178. doi:10.1513/pats.200708-119mg
13. Sin DD, Mayers I, Man GCW, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121(2):430-435. doi:10.1378/chest.121.2.430
14. Nowak C, Bourgin P, Portier F, Genty E, Escourrou P, Bobin S. Obstruction nasale et compliance à la ventilation nasale à pression positive [Nasal obstruction and compliance to nasal positive airway pressure]. Ann Otolaryngol Chir Cervicofac. 2003;120(3):161-166.
15. Brin YS, Reuveni H, Greenberg S, Tal A, Tarasiuk A. Determinants affecting initiation of continuous positive airway pressure treatment. Isr Med Assoc J. 2005;7(1):13-18.
16. Suurna MV, Jacobowitz O, Chang J, et al. Improving outcomes of hypoglossal nerve stimulation therapy: current practice, future directions, and research gaps. Proceedings of the 2019 International Sleep Surgery Society Research Forum. J Clin Sleep Med. 2021;17(12):2477-2487. doi:10.5664/jcsm.9542
17. Inspire Medical Systems, Inc. Announces FDA approval for apnea hypopnea index indication expansion and increased body mass index labeling. Inspire Medical Systems, Inc. Accessed July 14, 2023. https://investors.inspiresleep.com/investors/press-releases/press-release-details/2023/Inspire-Medical-Systems-Inc.-Announces-FDA-Approval-for-Apnea-Hypopnea-Index-Indication-Expansion-and-Increased-Body-Mass-Index-Labeling/default.aspx
18. Lapin BR, Bena JF, Walia HK, Moul DE. The Epworth Sleepiness Scale: Validation of one-dimensional factor structure in a large clinical sample. J Clin Sleep Med. 2018;14(08):1293-1301. Published 2018 Aug 15. doi:10.5664/jcsm.7258
19. The Centre for Evidence-Based Medicine. November 25, 2020. http://www.cebm.net/index.aspx?o=5653
20. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
21. Strollo PJ Jr, Gillespie MB, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38(10):1593-1598. Published 2015 Oct 1. doi:10.5665/sleep.5054
22. Woodson BT, Soose RJ, Gillespie MB, et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-188. doi:10.1177/0194599815616618
23. Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159(1):194-202. doi:10.1177/0194599818762383
24. Woodson BT, Gillespie MB, Soose RJ, et al. Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg. 2014;151(5):880-887. doi:10.1177/0194599814544445
25. Heiser C, Maurer JT, Hofauer B, Sommer JU, Seitz A, Steffen A. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter German postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-384. doi:10.1177/0194599816683378
26. Steffen A, Sommer JU, Hofauer B, Maurer JT, Hasselbacher K, Heiser C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. Laryngoscope. 2018;128(2):509-515. doi:10.1002/lary.26688
27. Steffen A, Sommer UJ, Maurer JT, Abrams N, Hofauer B, Heiser C. Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea. Sleep Breath. 2020;24(3):979-984. doi:10.1007/s11325-019-01933-028.
28. Hasselbacher K, Hofauer B, Maurer JT, Heiser C, Steffen A, Sommer JU. Patient-reported outcome: results of the multicenter German post-market study. Eur Arch Otorhinolaryngol. 2018;275(7):1913-1919. doi:10.1007/s00405-018-5017-129.
29. Heiser C, Knopf A, Bas M, Gahleitner C, Hofauer B. Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience. Eur Arch Otorhinolaryngol. 2017;274(3):1727-1734. doi:10.1007/s00405-016-4297-6
30. Kezirian EJ, Goding GS Jr, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23(1):77-83. doi:10.1111/jsr.12079
31. Soose RJ, Woodson BT, Gillespie MB, et al. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12(1):43-48. doi:10.5664/jcsm.5390
32. Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med. 2017;13(9):1075-1079. Published 2017 Sep 15. doi:10.5664/jcsm.6726

33. Pordzik J, Seifen C, Ludwig K, et al. Short-term outcome of unilateral inspiration-coupled hypoglossal nerve stimulation in patients with obstructive sleep apnea. Int J Environ Res Public Health. 2022;19(24):16443. Published 2022 Dec 8. doi:10.3390/ijerph192416443
34. Heiser C, Steffen A, Hofauer B, et al. Effect of upper airway stimulation in patients with obstructive sleep apnea (EFFECT): a randomized controlled crossover trial. J Clin Med. 2021;10(13):2880. Published 2021 Jun 29. doi:10.3390/jcm1013288035.
35. Heiser C, Steffen A, Strollo PJ Jr, Giaie-Miniet C, Vanderveken OM, Hofauer B. Hypoglossal nerve stimulation versus positive airway pressure therapy for obstructive sleep apnea. Sleep Breath. 2023;27(2):693-701. doi:10.1007/s11325-022-02663-6
36. Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157-171.
37. Freedman N, Johnson K. Positive airway pressure treatment for obstructive sleep apnea. In: Kryger MH, Roth T, Goldstein CA, Dement WC, eds. Principles and Practice of Sleep Medicine. Elsevier; 2022:1260-1283.
38. Braun M, Stoerzel M, Wollny M, Schoebel C, Ulrich Sommer J, Heiser C. Patient-reported outcomes with hypoglossal nerve stimulation for treatment of obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2023;280(10):4627-4639. doi:10.1007/s00405-023-08062-1
39. Luxton DD, Greenburg D, Ryan J, Niven A, Wheeler G, Mysliwiec V. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34(9):1189-1195. doi:10.5665/SLEEP.1236
40. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
41. Office of the Deputy Assistant Secretary of Defense for Military Community and Family Policy. 2017 Demographics: Profile of the Military Community. US Dept of Defense;2017. Accessed April 4, 2024. http://download.militaryonesource.mil/12038/MOS/Reports/2017-demographics-report.pdf
42. Remondelli MH, Remick KN, Shackelford SA, et al. Casualty care implications of large-scale combat operations. J Trauma Acute Care Surg. 2023;95(2S Suppl 1): S180-S184. doi:10.1097/TA.0000000000004063
1. Cumpston E, Chen P. Sleep Apnea Syndrome. PubMed. Updated September 4, 2023. Published January 2024. https://www.ncbi.nlm.nih.gov/books/NBK564431/
2. American Academy of Sleep Medicine. Obstructive sleep apnea. Accessed November 27, 2023. https://aasm.org/resources/factsheets/sleepapnea.pdf
3. Cowen J, Harrison S, Thom L, et al. Use of historical remote monitoring data to determine predictors of CPAP non-compliance in patients with Osa. Sleep Breath. 2023;27(5):1899-1908. doi:10.1007/s11325-023-02806-3
4. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
5. Stiegmann RA, Payne CB, Kiel MA, Stahlman SL. Increased Prevalence of Overweight and Obesity and Incidence of Prediabetes and Type 2 Diabetes During the COVID-19 Pandemic, Active Component Service Members, U.S. Armed Forces, 2018 to 2021. MSMR. 2023;30(1):11-18. Published 2023 Jan 20.
6. Adult obesity facts. Centers for Disease Control and Prevention. Updated May 17, 2022. Accessed November 27, 2023. https://www.cdc.gov/obesity/data/adult.html
7. Moore BA, Tison LM, Palacios JG, Peterson AL, Mysliwiec V. Incidence of insomnia and obstructive sleep apnea in active duty United States military service members. Sleep. 2021;44(7):zsab024. doi:10.1093/sleep/zsab024
8. Caldwell JA, Knapik JJ, Shing TL, Kardouni JR, Lieberman HR. The association of insomnia and sleep apnea with deployment and combat exposure in the entire population of US army soldiers from 1997 to 2011: a retrospective cohort investigation. Sleep. 2019;42(8):zsz112. doi:10.1093/sleep/zsz112
9. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
10. Veterans Affairs 38 C.F.R. § 4.97-13, Code 6847.
11. Shapiro GK, Shapiro CM. Factors that influence CPAP adherence: an overview. Sleep Breath. 2010;14(4):323-335. doi:10.1007/s11325-010-0391-y
12. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178. doi:10.1513/pats.200708-119mg
13. Sin DD, Mayers I, Man GCW, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121(2):430-435. doi:10.1378/chest.121.2.430
14. Nowak C, Bourgin P, Portier F, Genty E, Escourrou P, Bobin S. Obstruction nasale et compliance à la ventilation nasale à pression positive [Nasal obstruction and compliance to nasal positive airway pressure]. Ann Otolaryngol Chir Cervicofac. 2003;120(3):161-166.
15. Brin YS, Reuveni H, Greenberg S, Tal A, Tarasiuk A. Determinants affecting initiation of continuous positive airway pressure treatment. Isr Med Assoc J. 2005;7(1):13-18.
16. Suurna MV, Jacobowitz O, Chang J, et al. Improving outcomes of hypoglossal nerve stimulation therapy: current practice, future directions, and research gaps. Proceedings of the 2019 International Sleep Surgery Society Research Forum. J Clin Sleep Med. 2021;17(12):2477-2487. doi:10.5664/jcsm.9542
17. Inspire Medical Systems, Inc. Announces FDA approval for apnea hypopnea index indication expansion and increased body mass index labeling. Inspire Medical Systems, Inc. Accessed July 14, 2023. https://investors.inspiresleep.com/investors/press-releases/press-release-details/2023/Inspire-Medical-Systems-Inc.-Announces-FDA-Approval-for-Apnea-Hypopnea-Index-Indication-Expansion-and-Increased-Body-Mass-Index-Labeling/default.aspx
18. Lapin BR, Bena JF, Walia HK, Moul DE. The Epworth Sleepiness Scale: Validation of one-dimensional factor structure in a large clinical sample. J Clin Sleep Med. 2018;14(08):1293-1301. Published 2018 Aug 15. doi:10.5664/jcsm.7258
19. The Centre for Evidence-Based Medicine. November 25, 2020. http://www.cebm.net/index.aspx?o=5653
20. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
21. Strollo PJ Jr, Gillespie MB, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38(10):1593-1598. Published 2015 Oct 1. doi:10.5665/sleep.5054
22. Woodson BT, Soose RJ, Gillespie MB, et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-188. doi:10.1177/0194599815616618
23. Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159(1):194-202. doi:10.1177/0194599818762383
24. Woodson BT, Gillespie MB, Soose RJ, et al. Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg. 2014;151(5):880-887. doi:10.1177/0194599814544445
25. Heiser C, Maurer JT, Hofauer B, Sommer JU, Seitz A, Steffen A. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter German postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-384. doi:10.1177/0194599816683378
26. Steffen A, Sommer JU, Hofauer B, Maurer JT, Hasselbacher K, Heiser C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. Laryngoscope. 2018;128(2):509-515. doi:10.1002/lary.26688
27. Steffen A, Sommer UJ, Maurer JT, Abrams N, Hofauer B, Heiser C. Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea. Sleep Breath. 2020;24(3):979-984. doi:10.1007/s11325-019-01933-028.
28. Hasselbacher K, Hofauer B, Maurer JT, Heiser C, Steffen A, Sommer JU. Patient-reported outcome: results of the multicenter German post-market study. Eur Arch Otorhinolaryngol. 2018;275(7):1913-1919. doi:10.1007/s00405-018-5017-129.
29. Heiser C, Knopf A, Bas M, Gahleitner C, Hofauer B. Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience. Eur Arch Otorhinolaryngol. 2017;274(3):1727-1734. doi:10.1007/s00405-016-4297-6
30. Kezirian EJ, Goding GS Jr, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23(1):77-83. doi:10.1111/jsr.12079
31. Soose RJ, Woodson BT, Gillespie MB, et al. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12(1):43-48. doi:10.5664/jcsm.5390
32. Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med. 2017;13(9):1075-1079. Published 2017 Sep 15. doi:10.5664/jcsm.6726

33. Pordzik J, Seifen C, Ludwig K, et al. Short-term outcome of unilateral inspiration-coupled hypoglossal nerve stimulation in patients with obstructive sleep apnea. Int J Environ Res Public Health. 2022;19(24):16443. Published 2022 Dec 8. doi:10.3390/ijerph192416443
34. Heiser C, Steffen A, Hofauer B, et al. Effect of upper airway stimulation in patients with obstructive sleep apnea (EFFECT): a randomized controlled crossover trial. J Clin Med. 2021;10(13):2880. Published 2021 Jun 29. doi:10.3390/jcm1013288035.
35. Heiser C, Steffen A, Strollo PJ Jr, Giaie-Miniet C, Vanderveken OM, Hofauer B. Hypoglossal nerve stimulation versus positive airway pressure therapy for obstructive sleep apnea. Sleep Breath. 2023;27(2):693-701. doi:10.1007/s11325-022-02663-6
36. Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157-171.
37. Freedman N, Johnson K. Positive airway pressure treatment for obstructive sleep apnea. In: Kryger MH, Roth T, Goldstein CA, Dement WC, eds. Principles and Practice of Sleep Medicine. Elsevier; 2022:1260-1283.
38. Braun M, Stoerzel M, Wollny M, Schoebel C, Ulrich Sommer J, Heiser C. Patient-reported outcomes with hypoglossal nerve stimulation for treatment of obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2023;280(10):4627-4639. doi:10.1007/s00405-023-08062-1
39. Luxton DD, Greenburg D, Ryan J, Niven A, Wheeler G, Mysliwiec V. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34(9):1189-1195. doi:10.5665/SLEEP.1236
40. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
41. Office of the Deputy Assistant Secretary of Defense for Military Community and Family Policy. 2017 Demographics: Profile of the Military Community. US Dept of Defense;2017. Accessed April 4, 2024. http://download.militaryonesource.mil/12038/MOS/Reports/2017-demographics-report.pdf
42. Remondelli MH, Remick KN, Shackelford SA, et al. Casualty care implications of large-scale combat operations. J Trauma Acute Care Surg. 2023;95(2S Suppl 1): S180-S184. doi:10.1097/TA.0000000000004063
New Drug Offers Hope for CPAP-Free Nights for Sleep Apnea
Roughly 30 million to 40 million people in the United States, and nearly a billion people worldwide, have sleep apnea. Because they are cumbersome and often uncomfortable, many sleep apnea patients don’t use their continuous positive airway pressure (CPAP) machine.
“In my patients, I’d say a quarter of them don’t get compliant on the machine and require other treatments,” said David Kuhlmann, MD, medical director of sleep medicine at Bothwell Regional Health Center in Sedalia, MO. That’s often because they “just don’t want to wear a mask at night.”
For Dr. Kuhlmann, who’s also a spokesperson for the American Academy of Sleep Medicine, no other treatment can replace something that continually supplies air throughout the night.
But that may be changing.
New Pill Making Waves in Sleep Apnea
That’s what researchers at Apnimed hope. Apnimed is a company that’s developed a new oral drug for sleep apnea — currently called AD109. AD109 combines the drugs aroxybutynin and atomoxetine.
Aroxybutynin is used to treat symptoms of an overactive bladder, while atomoxetine is used to treat attention deficit hyperactivity disorder.
“The drug is unique in the sense that, currently, there’s no approved drug for the treatment of sleep apnea,” said Douglas Kirsch, MD, medical director of sleep medicine at Atrium Health in Charlotte, NC. “AD109 keeps the airway from collapsing during the night. And that function is through a combination of drugs, which, in theory, both help keep the airway a little bit more open, but also helps keep people asleep.”
AD109 is currently in phase 3 trials, but results are already out for phase 2.
The conclusion of those phase 2 studies?
“AD109 showed clinically meaningful improvement in [sleep apnea], suggesting that further development of the compound is warranted.” That’s taken straight from the study’s published data.
And onto phase 3 clinical trials the drug goes. But there’s something to consider when looking at these results.
Evaluating AD109’s Results
One promising result out of the phase 2 trials was the lack of major side effects in people who took the drug.
“What you are kind of hoping for from a phase 2 trial, both from a set safety perspective and an efficacy perspective, is that it did change the level of sleep apnea when compared to placebo,” said Dr. Kirsch, who’s also a former president of the American Academy of Sleep Medicine.
For phase 2 trials, patients were separated into groups after they were tested to see how severe their sleep apnea was, using the apnea-hypopnea index (AHI).
Dr. Kuhlmann said there are two big things they noticed: The apnea-hypopnea index dropped in patients given two different doses of the drug. Those in the group that took the lower dosage actually saw “clinically significant improvement in fatigue.”
For those with an index score of 10-15 (mild), 77% had their scores lowered to below 10.
But only 42% with a score of 15-30 (moderate) were able to get below 10. And only 7% of those with a score of over 30 were able to get all the way down to 10 or below.
Regarding some of the index score drops, Dr. Kuhlmann said, “If you drop from an AHI of 20-10, that’s still OSA [obstructive sleep apnea] if you have diabetes, high blood pressure, depression, daytime sleepiness, or insomnia.”
Phase 3 should include a broader range of people. “Phase 2 provides a proof of concept…phase 3 is a little bit broader…you can open the use of the drug to more people,” said Dr. Kirsch.
A Suspicious Omission
Significantly, the AD109 phase 2 trial also seemed not to include a crucial thing when sleep experts look at how well treatments work: Oxygen saturation.
“Often, when you review a sleep study with a patient, you’ll talk about both AHI and minimum oxygen saturation,” Dr. Kirsch said.
Dr. Kuhlmann was skeptical of this omission. Instead of reporting the minimum oxygen saturation, Apnimed used something called “hypoxic burden,” he said.
“They didn’t give us oxygen saturation information at all. But there’s a big difference between somebody who has a minimum oxygen saturation of 89% and went from an AHI of 20 to 12…which sounds great…but had minimum oxygen saturation stay the same after.”
In explaining the importance of hypoxic burden, Dr. Kirsch said, “If 99% of a sleep study was at 90% and above, but there was one dip at 80%, that’s not the same as spending 45 minutes below 88%. What you really want to talk about is how much or how long does that oxygen get low?”
What Therapies Must Consider for the Future
Until phase 3 data is out, it’s not possible to say for sure where AD109 can work alone for people across the spectrum of severity.
“Like any form of data, there are going to be targeted populations that may do better…with any drug, you’re unlikely to fix everything…Until we see that phase 3 data…you really can’t say for sure,” Dr. Kirsch said.
“It seems AD109 treats more of a milder spectrum than maybe the ones who would get the most benefit,” Dr. Kuhlmann said.
But he said AD109 may still work well for a number of people. It’s just important to understand that a pill can’t be compared to positive airway pressure.
Dr. Kuhlmann said he’d like to see a medication — including AD109 — that could measure up as well to oral appliances or anything that treats mild to moderate cases and “have some clinical scales associated with it that are positive.”
Besides AD109, Dr. Kirsch said, “I think we are potentially on the precipice of having some drugs that may help with sleep apnea in the coming years.”
Big Need for Progress
The American Academy of Sleep Medicine estimates up to 80% of people with obstructive sleep apnea — the most common form — remain undiagnosed.
Cigarette smoking, high alcohol intake, drugs, or neurological disorders are common risk factors. But most importantly, it’s anything that decreases muscle tone around the upper airway — like obesity — or changes in structural features that narrow the airway.
Dr. Kuhlmann stressed the importance of weight issues linked to sleep apnea. “It’s a very common condition, especially as people are getting older and heavier…you have loss of muscle tone to your entire body, including the upper airway muscles.”
SOURCES:
- David Kuhlmann, MD, spokesperson, American Academy of Sleep Medicine; medical director of sleep medicine, Bothwell Regional Health Center, Sedalia, MO.
- Apnimed: “Parallel Arm Trial of AD109 and Placebo With Patients With OSA (LunAIRo),” “Parallel-Arm Study to Compare AD109 to Placebo With Patients With OSA (SynAIRgy Study).”
- Douglas Kirsch, MD, former president, American Academy of Sleep Medicine; medical director of sleep medicine, Atrium Health, Charlotte, NC.
- American Academy of Sleep Medicine: “Rising Prevalence of Sleep Apnea in US Threatens Public Health.”
- National Council on Aging: “Sleep Apnea Statistics and Facts You Should Know.”
This article originally appeared on WebMD.
Roughly 30 million to 40 million people in the United States, and nearly a billion people worldwide, have sleep apnea. Because they are cumbersome and often uncomfortable, many sleep apnea patients don’t use their continuous positive airway pressure (CPAP) machine.
“In my patients, I’d say a quarter of them don’t get compliant on the machine and require other treatments,” said David Kuhlmann, MD, medical director of sleep medicine at Bothwell Regional Health Center in Sedalia, MO. That’s often because they “just don’t want to wear a mask at night.”
For Dr. Kuhlmann, who’s also a spokesperson for the American Academy of Sleep Medicine, no other treatment can replace something that continually supplies air throughout the night.
But that may be changing.
New Pill Making Waves in Sleep Apnea
That’s what researchers at Apnimed hope. Apnimed is a company that’s developed a new oral drug for sleep apnea — currently called AD109. AD109 combines the drugs aroxybutynin and atomoxetine.
Aroxybutynin is used to treat symptoms of an overactive bladder, while atomoxetine is used to treat attention deficit hyperactivity disorder.
“The drug is unique in the sense that, currently, there’s no approved drug for the treatment of sleep apnea,” said Douglas Kirsch, MD, medical director of sleep medicine at Atrium Health in Charlotte, NC. “AD109 keeps the airway from collapsing during the night. And that function is through a combination of drugs, which, in theory, both help keep the airway a little bit more open, but also helps keep people asleep.”
AD109 is currently in phase 3 trials, but results are already out for phase 2.
The conclusion of those phase 2 studies?
“AD109 showed clinically meaningful improvement in [sleep apnea], suggesting that further development of the compound is warranted.” That’s taken straight from the study’s published data.
And onto phase 3 clinical trials the drug goes. But there’s something to consider when looking at these results.
Evaluating AD109’s Results
One promising result out of the phase 2 trials was the lack of major side effects in people who took the drug.
“What you are kind of hoping for from a phase 2 trial, both from a set safety perspective and an efficacy perspective, is that it did change the level of sleep apnea when compared to placebo,” said Dr. Kirsch, who’s also a former president of the American Academy of Sleep Medicine.
For phase 2 trials, patients were separated into groups after they were tested to see how severe their sleep apnea was, using the apnea-hypopnea index (AHI).
Dr. Kuhlmann said there are two big things they noticed: The apnea-hypopnea index dropped in patients given two different doses of the drug. Those in the group that took the lower dosage actually saw “clinically significant improvement in fatigue.”
For those with an index score of 10-15 (mild), 77% had their scores lowered to below 10.
But only 42% with a score of 15-30 (moderate) were able to get below 10. And only 7% of those with a score of over 30 were able to get all the way down to 10 or below.
Regarding some of the index score drops, Dr. Kuhlmann said, “If you drop from an AHI of 20-10, that’s still OSA [obstructive sleep apnea] if you have diabetes, high blood pressure, depression, daytime sleepiness, or insomnia.”
Phase 3 should include a broader range of people. “Phase 2 provides a proof of concept…phase 3 is a little bit broader…you can open the use of the drug to more people,” said Dr. Kirsch.
A Suspicious Omission
Significantly, the AD109 phase 2 trial also seemed not to include a crucial thing when sleep experts look at how well treatments work: Oxygen saturation.
“Often, when you review a sleep study with a patient, you’ll talk about both AHI and minimum oxygen saturation,” Dr. Kirsch said.
Dr. Kuhlmann was skeptical of this omission. Instead of reporting the minimum oxygen saturation, Apnimed used something called “hypoxic burden,” he said.
“They didn’t give us oxygen saturation information at all. But there’s a big difference between somebody who has a minimum oxygen saturation of 89% and went from an AHI of 20 to 12…which sounds great…but had minimum oxygen saturation stay the same after.”
In explaining the importance of hypoxic burden, Dr. Kirsch said, “If 99% of a sleep study was at 90% and above, but there was one dip at 80%, that’s not the same as spending 45 minutes below 88%. What you really want to talk about is how much or how long does that oxygen get low?”
What Therapies Must Consider for the Future
Until phase 3 data is out, it’s not possible to say for sure where AD109 can work alone for people across the spectrum of severity.
“Like any form of data, there are going to be targeted populations that may do better…with any drug, you’re unlikely to fix everything…Until we see that phase 3 data…you really can’t say for sure,” Dr. Kirsch said.
“It seems AD109 treats more of a milder spectrum than maybe the ones who would get the most benefit,” Dr. Kuhlmann said.
But he said AD109 may still work well for a number of people. It’s just important to understand that a pill can’t be compared to positive airway pressure.
Dr. Kuhlmann said he’d like to see a medication — including AD109 — that could measure up as well to oral appliances or anything that treats mild to moderate cases and “have some clinical scales associated with it that are positive.”
Besides AD109, Dr. Kirsch said, “I think we are potentially on the precipice of having some drugs that may help with sleep apnea in the coming years.”
Big Need for Progress
The American Academy of Sleep Medicine estimates up to 80% of people with obstructive sleep apnea — the most common form — remain undiagnosed.
Cigarette smoking, high alcohol intake, drugs, or neurological disorders are common risk factors. But most importantly, it’s anything that decreases muscle tone around the upper airway — like obesity — or changes in structural features that narrow the airway.
Dr. Kuhlmann stressed the importance of weight issues linked to sleep apnea. “It’s a very common condition, especially as people are getting older and heavier…you have loss of muscle tone to your entire body, including the upper airway muscles.”
SOURCES:
- David Kuhlmann, MD, spokesperson, American Academy of Sleep Medicine; medical director of sleep medicine, Bothwell Regional Health Center, Sedalia, MO.
- Apnimed: “Parallel Arm Trial of AD109 and Placebo With Patients With OSA (LunAIRo),” “Parallel-Arm Study to Compare AD109 to Placebo With Patients With OSA (SynAIRgy Study).”
- Douglas Kirsch, MD, former president, American Academy of Sleep Medicine; medical director of sleep medicine, Atrium Health, Charlotte, NC.
- American Academy of Sleep Medicine: “Rising Prevalence of Sleep Apnea in US Threatens Public Health.”
- National Council on Aging: “Sleep Apnea Statistics and Facts You Should Know.”
This article originally appeared on WebMD.
Roughly 30 million to 40 million people in the United States, and nearly a billion people worldwide, have sleep apnea. Because they are cumbersome and often uncomfortable, many sleep apnea patients don’t use their continuous positive airway pressure (CPAP) machine.
“In my patients, I’d say a quarter of them don’t get compliant on the machine and require other treatments,” said David Kuhlmann, MD, medical director of sleep medicine at Bothwell Regional Health Center in Sedalia, MO. That’s often because they “just don’t want to wear a mask at night.”
For Dr. Kuhlmann, who’s also a spokesperson for the American Academy of Sleep Medicine, no other treatment can replace something that continually supplies air throughout the night.
But that may be changing.
New Pill Making Waves in Sleep Apnea
That’s what researchers at Apnimed hope. Apnimed is a company that’s developed a new oral drug for sleep apnea — currently called AD109. AD109 combines the drugs aroxybutynin and atomoxetine.
Aroxybutynin is used to treat symptoms of an overactive bladder, while atomoxetine is used to treat attention deficit hyperactivity disorder.
“The drug is unique in the sense that, currently, there’s no approved drug for the treatment of sleep apnea,” said Douglas Kirsch, MD, medical director of sleep medicine at Atrium Health in Charlotte, NC. “AD109 keeps the airway from collapsing during the night. And that function is through a combination of drugs, which, in theory, both help keep the airway a little bit more open, but also helps keep people asleep.”
AD109 is currently in phase 3 trials, but results are already out for phase 2.
The conclusion of those phase 2 studies?
“AD109 showed clinically meaningful improvement in [sleep apnea], suggesting that further development of the compound is warranted.” That’s taken straight from the study’s published data.
And onto phase 3 clinical trials the drug goes. But there’s something to consider when looking at these results.
Evaluating AD109’s Results
One promising result out of the phase 2 trials was the lack of major side effects in people who took the drug.
“What you are kind of hoping for from a phase 2 trial, both from a set safety perspective and an efficacy perspective, is that it did change the level of sleep apnea when compared to placebo,” said Dr. Kirsch, who’s also a former president of the American Academy of Sleep Medicine.
For phase 2 trials, patients were separated into groups after they were tested to see how severe their sleep apnea was, using the apnea-hypopnea index (AHI).
Dr. Kuhlmann said there are two big things they noticed: The apnea-hypopnea index dropped in patients given two different doses of the drug. Those in the group that took the lower dosage actually saw “clinically significant improvement in fatigue.”
For those with an index score of 10-15 (mild), 77% had their scores lowered to below 10.
But only 42% with a score of 15-30 (moderate) were able to get below 10. And only 7% of those with a score of over 30 were able to get all the way down to 10 or below.
Regarding some of the index score drops, Dr. Kuhlmann said, “If you drop from an AHI of 20-10, that’s still OSA [obstructive sleep apnea] if you have diabetes, high blood pressure, depression, daytime sleepiness, or insomnia.”
Phase 3 should include a broader range of people. “Phase 2 provides a proof of concept…phase 3 is a little bit broader…you can open the use of the drug to more people,” said Dr. Kirsch.
A Suspicious Omission
Significantly, the AD109 phase 2 trial also seemed not to include a crucial thing when sleep experts look at how well treatments work: Oxygen saturation.
“Often, when you review a sleep study with a patient, you’ll talk about both AHI and minimum oxygen saturation,” Dr. Kirsch said.
Dr. Kuhlmann was skeptical of this omission. Instead of reporting the minimum oxygen saturation, Apnimed used something called “hypoxic burden,” he said.
“They didn’t give us oxygen saturation information at all. But there’s a big difference between somebody who has a minimum oxygen saturation of 89% and went from an AHI of 20 to 12…which sounds great…but had minimum oxygen saturation stay the same after.”
In explaining the importance of hypoxic burden, Dr. Kirsch said, “If 99% of a sleep study was at 90% and above, but there was one dip at 80%, that’s not the same as spending 45 minutes below 88%. What you really want to talk about is how much or how long does that oxygen get low?”
What Therapies Must Consider for the Future
Until phase 3 data is out, it’s not possible to say for sure where AD109 can work alone for people across the spectrum of severity.
“Like any form of data, there are going to be targeted populations that may do better…with any drug, you’re unlikely to fix everything…Until we see that phase 3 data…you really can’t say for sure,” Dr. Kirsch said.
“It seems AD109 treats more of a milder spectrum than maybe the ones who would get the most benefit,” Dr. Kuhlmann said.
But he said AD109 may still work well for a number of people. It’s just important to understand that a pill can’t be compared to positive airway pressure.
Dr. Kuhlmann said he’d like to see a medication — including AD109 — that could measure up as well to oral appliances or anything that treats mild to moderate cases and “have some clinical scales associated with it that are positive.”
Besides AD109, Dr. Kirsch said, “I think we are potentially on the precipice of having some drugs that may help with sleep apnea in the coming years.”
Big Need for Progress
The American Academy of Sleep Medicine estimates up to 80% of people with obstructive sleep apnea — the most common form — remain undiagnosed.
Cigarette smoking, high alcohol intake, drugs, or neurological disorders are common risk factors. But most importantly, it’s anything that decreases muscle tone around the upper airway — like obesity — or changes in structural features that narrow the airway.
Dr. Kuhlmann stressed the importance of weight issues linked to sleep apnea. “It’s a very common condition, especially as people are getting older and heavier…you have loss of muscle tone to your entire body, including the upper airway muscles.”
SOURCES:
- David Kuhlmann, MD, spokesperson, American Academy of Sleep Medicine; medical director of sleep medicine, Bothwell Regional Health Center, Sedalia, MO.
- Apnimed: “Parallel Arm Trial of AD109 and Placebo With Patients With OSA (LunAIRo),” “Parallel-Arm Study to Compare AD109 to Placebo With Patients With OSA (SynAIRgy Study).”
- Douglas Kirsch, MD, former president, American Academy of Sleep Medicine; medical director of sleep medicine, Atrium Health, Charlotte, NC.
- American Academy of Sleep Medicine: “Rising Prevalence of Sleep Apnea in US Threatens Public Health.”
- National Council on Aging: “Sleep Apnea Statistics and Facts You Should Know.”
This article originally appeared on WebMD.
Gene Tests Could Predict if a Drug Will Work for a Patient
What if there were tests that could tell you whether the following drugs were a good match for your patients: Antidepressants, statins, painkillers, anticlotting medicines, chemotherapy agents, HIV treatments, organ transplant antirejection drugs, proton pump inhibitors for heartburn, and more?
That’s quite a list. And that’s pharmacogenetics, testing patients for genetic differences that affect how well a given drug will work for them and what kind of side effects to expect.
“About 9 out of 10 people will have a genetic difference in their DNA that can impact how they respond to common medications,” said Emily J. Cicali, PharmD, a clinical associate at the University of Florida College of Pharmacy, Gainesville.
Dr. Cicali is the clinical director of UF Health’s MyRx, a virtual program that gives Florida and New Jersey residents access to pharmacogenetic (PGx) tests plus expert interpretation by the health system’s pharmacists. Genetic factors are thought to contribute to about 25% or more of inappropriate drug responses or adverse events, said Kristin Wiisanen, PharmD, dean of the College of Pharmacy at Rosalind Franklin University of Medicine and Science in North Chicago.
Dr. Cicali said.
Through a cheek swab or blood sample, the MyRx program — and a growing number of health system programs, doctors’ offices, and home tests available across the United States — gives consumers a window on inherited gene variants that can affect how their body activates, metabolizes, and clears away medications from a long list of widely used drugs.
Why PGx Tests Can Have a Big Impact
These tests work by looking for genes that control drug metabolism.
“You have several different drug-metabolizing enzymes in your liver,” Dr. Cicali explained. “Pharmacogenetic tests look for gene variants that encode for these enzymes. If you’re an ultrarapid metabolizer, you have more of the enzymes that metabolize certain drugs, and there could be a risk the drug won’t work well because it doesn’t stay in the body long enough. On the other end of the spectrum, poor metabolizers have low levels of enzymes that affect certain drugs, so the drugs hang around longer and cause side effects.”
While pharmacogenetics is still considered an emerging science, it’s becoming more mainstream as test prices drop, insurance coverage expands, and an explosion of new research boosts understanding of gene-drug interactions, Dr. Wiisanen said.
Politicians are trying to extend its reach, too. The Right Drug Dose Now Act of 2024, introduced in Congress in late March, aims to accelerate the use of PGx by boosting public awareness and by inserting PGx test results into consumers’ electronic health records. (Though a similar bill died in a US House subcommittee in 2023.)
“The use of pharmacogenetic data to guide prescribing is growing rapidly,” Dr. Wiisanen said. “It’s becoming a routine part of drug therapy for many medications.”
What the Research Shows
When researchers sequenced the DNA of more than 10,000 Mayo Clinic patients, they made a discovery that might surprise many Americans: Gene variants that affect the effectiveness and safety of widely used drugs are not rare glitches. More than 99% of study participants had at least one. And 79% had three or more.
The Mayo-Baylor RIGHT 10K Study — one of the largest PGx studies ever conducted in the United States — looked at 77 gene variants, most involved with drug metabolism in the liver. Researchers focused closely on 13 with extensively studied, gene-based prescribing recommendations for 21 drugs including antidepressants, statins, pain killers, anticlotting medications for heart conditions, HIV treatments, chemotherapy agents, and antirejection drugs for organ transplants.
When researchers added participants’ genetic data to their electronic health records, they also sent semi-urgent alerts, which are alerts with the potential for severe harm, to the clinicians of 61 study volunteers. Over half changed patients’ drugs or doses.
The changes made a difference. One participant taking the pain drug tramadol turned out to be a poor metabolizer and was having dizzy spells because blood levels of the drug stayed high for long periods. Stopping tramadol stopped the dizziness. A participant taking escitalopram plus bupropion for major depression found out that the combo was likely ineffective because they metabolized escitalopram rapidly. A switch to a higher dose of bupropion alone put their depression into full remission.
“So many factors play into how you respond to medications,” said Mayo Clinic pharmacogenomics pharmacist Jessica Wright, PharmD, BCACP, one of the study authors. “Genetics is one of those pieces. Pharmacogenetic testing can reveal things that clinicians may not have been aware of or could help explain a patient’s exaggerated side effect.”
Pharmacogenetics is also called pharmacogenomics. The terms are often used interchangeably, even among PGx pharmacists, though the first refers to how individual genes influence drug response and the second to the effects of multiple genes, said Kelly E. Caudle, PharmD, PhD, an associate member of the Department of Pharmacy and Pharmaceutical Sciences at St. Jude Children’s Research Hospital in Memphis, Tennessee. Dr. Caudle is also co-principal investigator and director of the National Institutes of Health (NIH)-funded Clinical Pharmacogenetics Implementation Consortium (CPIC). The group creates, publishes, and posts evidence-based clinical practice guidelines for drugs with well-researched PGx influences.
By any name, PGx may help explain, predict, and sidestep unpredictable responses to a variety of drugs:
- In a 2023 multicenter study of 6944 people from seven European countries in The Lancet, those given customized drug treatments based on a 12-gene PGx panel had 30% fewer side effects than those who didn’t get this personalized prescribing. People in the study were being treated for cancer, heart disease, and mental health issues, among other conditions.
- In a 2023 from China’s Tongji University, Shanghai, of 650 survivors of strokes and transient ischemic attacks, those whose antiplatelet drugs (such as clopidogrel) were customized based on PGx testing had a lower risk for stroke and other vascular events in the next 90 days. The study was published in Frontiers in Pharmacology.
- In a University of Pennsylvania of 1944 adults with major depression, published in the Journal of the American Medical Association, those whose antidepressants were guided by PGx test results were 28% more likely to go into remission during the first 24 weeks of treatment than those in a control group. But by 24 weeks, equal numbers were in remission. A 2023 Chinese of 11 depression studies, published in BMC Psychiatry, came to a similar conclusion: PGx-guided antidepressant prescriptions may help people feel better quicker, perhaps by avoiding some of the usual trial-and-error of different depression drugs.
PGx checks are already strongly recommended or considered routine before some medications are prescribed. These include abacavir (Ziagen), an antiviral treatment for HIV that can have severe side effects in people with one gene variant.
The US Food and Drug Administration (FDA) recommends genetic testing for people with colon cancer before starting the drug irinotecan (Camptosar), which can cause severe diarrhea and raise infection risk in people with a gene variant that slows the drug’s elimination from the body.
Genetic testing is also recommended by the FDA for people with acute lymphoblastic leukemia before receiving the chemotherapy drug mercaptopurine (Purinethol) because a gene variant that affects drug processing can trigger serious side effects and raise the risk for infection at standard dosages.
“One of the key benefits of pharmacogenomic testing is in preventing adverse drug reactions,” Dr. Wiisanen said. “Testing of the thiopurine methyltransferase enzyme to guide dosing with 6-mercaptopurine or azathioprine can help prevent myelosuppression, a serious adverse drug reaction caused by lower production of blood cells in bone marrow.”
When, Why, and How to Test
“A family doctor should consider a PGx test if a patient is planning on taking a medication for which there is a CPIC guideline with a dosing recommendation,” said Teri Klein, PhD, professor of biomedical data science at Stanford University in California, and principal investigator at PharmGKB, an online resource funded by the NIH that provides information for healthcare practitioners, researchers, and consumers about PGx. Affiliated with CPIC, it’s based at Stanford University.
You might also consider it for patients already on a drug who are “not responding or experiencing side effects,” Dr. Caudle said.
Here’s how four PGx experts suggest consumers and physicians approach this option.
Find a Test
More than a dozen PGx tests are on the market — some only a provider can order, others a consumer can order after a review by their provider or by a provider from the testing company. Some of the tests (using saliva) may be administered at home, while blood tests are done in a doctor’s office or laboratory. Companies that offer the tests include ARUP Laboratories, Genomind, Labcorp, Mayo Clinic Laboratories, Myriad Neuroscience, Precision Sciences Inc., Tempus, and OneOme, but there are many others online. (Keep in mind that many laboratories offer “lab-developed tests” — created for use in a single laboratory — but these can be harder to verify. “The FDA regulates pharmacogenomic testing in laboratories,” Dr. Wiisanen said, “but many of the regulatory parameters are still being defined.”)
Because PGx is so new, there is no official list of recommended tests. So you’ll have to do a little homework. You can check that the laboratory is accredited by searching for it in the NIH Genetic Testing Laboratory Registry database. Beyond that, you’ll have to consult other evidence-based resources to confirm that the drug you’re interested in has research-backed data about specific gene variants (alleles) that affect metabolism as well as research-based clinical guidelines for using PGx results to make prescribing decisions.
The CPIC’s guidelines include dosing and alternate drug recommendations for more than 100 antidepressants, chemotherapy drugs, the antiplatelet and anticlotting drugs clopidogrel and warfarin, local anesthetics, antivirals and antibacterials, pain killers and anti-inflammatory drugs, and some cholesterol-lowering statins such as lovastatin and fluvastatin.
For help figuring out if a test looks for the right gene variants, Dr. Caudle and Dr. Wright recommended checking with the Association for Molecular Pathology’s website. The group published a brief list of best practices for pharmacogenomic testing in 2019. And it keeps a list of gene variants (alleles) that should be included in tests. Clinical guidelines from the CPIC and other groups, available on PharmGKB’s website, also list gene variants that affect the metabolism of the drug.
Consider Cost
The price tag for a test is typically several hundred dollars — but it can run as high as $1000-$2500. And health insurance doesn’t always pick up the tab.
In a 2023 University of Florida study of more than 1000 insurance claims for PGx testing, the number reimbursed varied from 72% for a pain diagnosis to 52% for cardiology to 46% for psychiatry.
Medicare covers some PGx testing when a consumer and their providers meet certain criteria, including whether a drug being considered has a significant gene-drug interaction. California’s Medi-Cal health insurance program covers PGx as do Medicaid programs in some states, including Arkansas and Rhode Island. You can find state-by-state coverage information on the Genetics Policy Hub’s website.
Understand the Results
As more insurers cover PGx, Dr. Klein and Dr. Wiisanen say the field will grow and more providers will use it to inform prescribing. But some health systems aren’t waiting.
In addition to UF Health’s MyRx, PGx is part of personalized medicine programs at the University of Pennsylvania in Philadelphia, Endeavor Health in Chicago, the Mayo Clinic, the University of California, San Francisco, Sanford Health in Sioux Falls, South Dakota, and St. Jude Children’s Research Hospital in Memphis, Tennessee.
Beyond testing, they offer a very useful service: A consult with a pharmacogenetics pharmacist to review the results and explain what they mean for a consumer’s current and future medications.
Physicians and curious consumers can also consult CPIC’s guidelines, which give recommendations about how to interpret the results of a PGx test, said Dr. Klein, a co-principal investigator at CPIC. CPIC has a grading system for both the evidence that supports the recommendation (high, moderate, or weak) and the recommendation itself (strong, moderate, or optional).
Currently, labeling for 456 prescription drugs sold in the United States includes some type of PGx information, according to the FDA’s Table of Pharmacogenomic Biomarkers in Drug Labeling and an annotated guide from PharmGKB.
Just 108 drug labels currently tell doctors and patients what to do with the information — such as requiring or suggesting testing or offering prescribing recommendations, according to PharmGKB. In contrast, PharmGKB’s online resources include evidence-based clinical guidelines for 201 drugs from CPIC and from professional PGx societies in the Netherlands, Canada, France, and elsewhere.
Consumers and physicians can also look for a pharmacist with pharmacogenetics training in their area or through a nearby medical center to learn more, Dr. Wright suggested. And while consumers can test without working with their own physician, the experts advise against it. Don’t stop or change the dose of medications you already take on your own, they say . And do work with your primary care practitioner or specialist to get tested and understand how the results fit into the bigger picture of how your body responds to your medications.
A version of this article appeared on Medscape.com.
What if there were tests that could tell you whether the following drugs were a good match for your patients: Antidepressants, statins, painkillers, anticlotting medicines, chemotherapy agents, HIV treatments, organ transplant antirejection drugs, proton pump inhibitors for heartburn, and more?
That’s quite a list. And that’s pharmacogenetics, testing patients for genetic differences that affect how well a given drug will work for them and what kind of side effects to expect.
“About 9 out of 10 people will have a genetic difference in their DNA that can impact how they respond to common medications,” said Emily J. Cicali, PharmD, a clinical associate at the University of Florida College of Pharmacy, Gainesville.
Dr. Cicali is the clinical director of UF Health’s MyRx, a virtual program that gives Florida and New Jersey residents access to pharmacogenetic (PGx) tests plus expert interpretation by the health system’s pharmacists. Genetic factors are thought to contribute to about 25% or more of inappropriate drug responses or adverse events, said Kristin Wiisanen, PharmD, dean of the College of Pharmacy at Rosalind Franklin University of Medicine and Science in North Chicago.
Dr. Cicali said.
Through a cheek swab or blood sample, the MyRx program — and a growing number of health system programs, doctors’ offices, and home tests available across the United States — gives consumers a window on inherited gene variants that can affect how their body activates, metabolizes, and clears away medications from a long list of widely used drugs.
Why PGx Tests Can Have a Big Impact
These tests work by looking for genes that control drug metabolism.
“You have several different drug-metabolizing enzymes in your liver,” Dr. Cicali explained. “Pharmacogenetic tests look for gene variants that encode for these enzymes. If you’re an ultrarapid metabolizer, you have more of the enzymes that metabolize certain drugs, and there could be a risk the drug won’t work well because it doesn’t stay in the body long enough. On the other end of the spectrum, poor metabolizers have low levels of enzymes that affect certain drugs, so the drugs hang around longer and cause side effects.”
While pharmacogenetics is still considered an emerging science, it’s becoming more mainstream as test prices drop, insurance coverage expands, and an explosion of new research boosts understanding of gene-drug interactions, Dr. Wiisanen said.
Politicians are trying to extend its reach, too. The Right Drug Dose Now Act of 2024, introduced in Congress in late March, aims to accelerate the use of PGx by boosting public awareness and by inserting PGx test results into consumers’ electronic health records. (Though a similar bill died in a US House subcommittee in 2023.)
“The use of pharmacogenetic data to guide prescribing is growing rapidly,” Dr. Wiisanen said. “It’s becoming a routine part of drug therapy for many medications.”
What the Research Shows
When researchers sequenced the DNA of more than 10,000 Mayo Clinic patients, they made a discovery that might surprise many Americans: Gene variants that affect the effectiveness and safety of widely used drugs are not rare glitches. More than 99% of study participants had at least one. And 79% had three or more.
The Mayo-Baylor RIGHT 10K Study — one of the largest PGx studies ever conducted in the United States — looked at 77 gene variants, most involved with drug metabolism in the liver. Researchers focused closely on 13 with extensively studied, gene-based prescribing recommendations for 21 drugs including antidepressants, statins, pain killers, anticlotting medications for heart conditions, HIV treatments, chemotherapy agents, and antirejection drugs for organ transplants.
When researchers added participants’ genetic data to their electronic health records, they also sent semi-urgent alerts, which are alerts with the potential for severe harm, to the clinicians of 61 study volunteers. Over half changed patients’ drugs or doses.
The changes made a difference. One participant taking the pain drug tramadol turned out to be a poor metabolizer and was having dizzy spells because blood levels of the drug stayed high for long periods. Stopping tramadol stopped the dizziness. A participant taking escitalopram plus bupropion for major depression found out that the combo was likely ineffective because they metabolized escitalopram rapidly. A switch to a higher dose of bupropion alone put their depression into full remission.
“So many factors play into how you respond to medications,” said Mayo Clinic pharmacogenomics pharmacist Jessica Wright, PharmD, BCACP, one of the study authors. “Genetics is one of those pieces. Pharmacogenetic testing can reveal things that clinicians may not have been aware of or could help explain a patient’s exaggerated side effect.”
Pharmacogenetics is also called pharmacogenomics. The terms are often used interchangeably, even among PGx pharmacists, though the first refers to how individual genes influence drug response and the second to the effects of multiple genes, said Kelly E. Caudle, PharmD, PhD, an associate member of the Department of Pharmacy and Pharmaceutical Sciences at St. Jude Children’s Research Hospital in Memphis, Tennessee. Dr. Caudle is also co-principal investigator and director of the National Institutes of Health (NIH)-funded Clinical Pharmacogenetics Implementation Consortium (CPIC). The group creates, publishes, and posts evidence-based clinical practice guidelines for drugs with well-researched PGx influences.
By any name, PGx may help explain, predict, and sidestep unpredictable responses to a variety of drugs:
- In a 2023 multicenter study of 6944 people from seven European countries in The Lancet, those given customized drug treatments based on a 12-gene PGx panel had 30% fewer side effects than those who didn’t get this personalized prescribing. People in the study were being treated for cancer, heart disease, and mental health issues, among other conditions.
- In a 2023 from China’s Tongji University, Shanghai, of 650 survivors of strokes and transient ischemic attacks, those whose antiplatelet drugs (such as clopidogrel) were customized based on PGx testing had a lower risk for stroke and other vascular events in the next 90 days. The study was published in Frontiers in Pharmacology.
- In a University of Pennsylvania of 1944 adults with major depression, published in the Journal of the American Medical Association, those whose antidepressants were guided by PGx test results were 28% more likely to go into remission during the first 24 weeks of treatment than those in a control group. But by 24 weeks, equal numbers were in remission. A 2023 Chinese of 11 depression studies, published in BMC Psychiatry, came to a similar conclusion: PGx-guided antidepressant prescriptions may help people feel better quicker, perhaps by avoiding some of the usual trial-and-error of different depression drugs.
PGx checks are already strongly recommended or considered routine before some medications are prescribed. These include abacavir (Ziagen), an antiviral treatment for HIV that can have severe side effects in people with one gene variant.
The US Food and Drug Administration (FDA) recommends genetic testing for people with colon cancer before starting the drug irinotecan (Camptosar), which can cause severe diarrhea and raise infection risk in people with a gene variant that slows the drug’s elimination from the body.
Genetic testing is also recommended by the FDA for people with acute lymphoblastic leukemia before receiving the chemotherapy drug mercaptopurine (Purinethol) because a gene variant that affects drug processing can trigger serious side effects and raise the risk for infection at standard dosages.
“One of the key benefits of pharmacogenomic testing is in preventing adverse drug reactions,” Dr. Wiisanen said. “Testing of the thiopurine methyltransferase enzyme to guide dosing with 6-mercaptopurine or azathioprine can help prevent myelosuppression, a serious adverse drug reaction caused by lower production of blood cells in bone marrow.”
When, Why, and How to Test
“A family doctor should consider a PGx test if a patient is planning on taking a medication for which there is a CPIC guideline with a dosing recommendation,” said Teri Klein, PhD, professor of biomedical data science at Stanford University in California, and principal investigator at PharmGKB, an online resource funded by the NIH that provides information for healthcare practitioners, researchers, and consumers about PGx. Affiliated with CPIC, it’s based at Stanford University.
You might also consider it for patients already on a drug who are “not responding or experiencing side effects,” Dr. Caudle said.
Here’s how four PGx experts suggest consumers and physicians approach this option.
Find a Test
More than a dozen PGx tests are on the market — some only a provider can order, others a consumer can order after a review by their provider or by a provider from the testing company. Some of the tests (using saliva) may be administered at home, while blood tests are done in a doctor’s office or laboratory. Companies that offer the tests include ARUP Laboratories, Genomind, Labcorp, Mayo Clinic Laboratories, Myriad Neuroscience, Precision Sciences Inc., Tempus, and OneOme, but there are many others online. (Keep in mind that many laboratories offer “lab-developed tests” — created for use in a single laboratory — but these can be harder to verify. “The FDA regulates pharmacogenomic testing in laboratories,” Dr. Wiisanen said, “but many of the regulatory parameters are still being defined.”)
Because PGx is so new, there is no official list of recommended tests. So you’ll have to do a little homework. You can check that the laboratory is accredited by searching for it in the NIH Genetic Testing Laboratory Registry database. Beyond that, you’ll have to consult other evidence-based resources to confirm that the drug you’re interested in has research-backed data about specific gene variants (alleles) that affect metabolism as well as research-based clinical guidelines for using PGx results to make prescribing decisions.
The CPIC’s guidelines include dosing and alternate drug recommendations for more than 100 antidepressants, chemotherapy drugs, the antiplatelet and anticlotting drugs clopidogrel and warfarin, local anesthetics, antivirals and antibacterials, pain killers and anti-inflammatory drugs, and some cholesterol-lowering statins such as lovastatin and fluvastatin.
For help figuring out if a test looks for the right gene variants, Dr. Caudle and Dr. Wright recommended checking with the Association for Molecular Pathology’s website. The group published a brief list of best practices for pharmacogenomic testing in 2019. And it keeps a list of gene variants (alleles) that should be included in tests. Clinical guidelines from the CPIC and other groups, available on PharmGKB’s website, also list gene variants that affect the metabolism of the drug.
Consider Cost
The price tag for a test is typically several hundred dollars — but it can run as high as $1000-$2500. And health insurance doesn’t always pick up the tab.
In a 2023 University of Florida study of more than 1000 insurance claims for PGx testing, the number reimbursed varied from 72% for a pain diagnosis to 52% for cardiology to 46% for psychiatry.
Medicare covers some PGx testing when a consumer and their providers meet certain criteria, including whether a drug being considered has a significant gene-drug interaction. California’s Medi-Cal health insurance program covers PGx as do Medicaid programs in some states, including Arkansas and Rhode Island. You can find state-by-state coverage information on the Genetics Policy Hub’s website.
Understand the Results
As more insurers cover PGx, Dr. Klein and Dr. Wiisanen say the field will grow and more providers will use it to inform prescribing. But some health systems aren’t waiting.
In addition to UF Health’s MyRx, PGx is part of personalized medicine programs at the University of Pennsylvania in Philadelphia, Endeavor Health in Chicago, the Mayo Clinic, the University of California, San Francisco, Sanford Health in Sioux Falls, South Dakota, and St. Jude Children’s Research Hospital in Memphis, Tennessee.
Beyond testing, they offer a very useful service: A consult with a pharmacogenetics pharmacist to review the results and explain what they mean for a consumer’s current and future medications.
Physicians and curious consumers can also consult CPIC’s guidelines, which give recommendations about how to interpret the results of a PGx test, said Dr. Klein, a co-principal investigator at CPIC. CPIC has a grading system for both the evidence that supports the recommendation (high, moderate, or weak) and the recommendation itself (strong, moderate, or optional).
Currently, labeling for 456 prescription drugs sold in the United States includes some type of PGx information, according to the FDA’s Table of Pharmacogenomic Biomarkers in Drug Labeling and an annotated guide from PharmGKB.
Just 108 drug labels currently tell doctors and patients what to do with the information — such as requiring or suggesting testing or offering prescribing recommendations, according to PharmGKB. In contrast, PharmGKB’s online resources include evidence-based clinical guidelines for 201 drugs from CPIC and from professional PGx societies in the Netherlands, Canada, France, and elsewhere.
Consumers and physicians can also look for a pharmacist with pharmacogenetics training in their area or through a nearby medical center to learn more, Dr. Wright suggested. And while consumers can test without working with their own physician, the experts advise against it. Don’t stop or change the dose of medications you already take on your own, they say . And do work with your primary care practitioner or specialist to get tested and understand how the results fit into the bigger picture of how your body responds to your medications.
A version of this article appeared on Medscape.com.
What if there were tests that could tell you whether the following drugs were a good match for your patients: Antidepressants, statins, painkillers, anticlotting medicines, chemotherapy agents, HIV treatments, organ transplant antirejection drugs, proton pump inhibitors for heartburn, and more?
That’s quite a list. And that’s pharmacogenetics, testing patients for genetic differences that affect how well a given drug will work for them and what kind of side effects to expect.
“About 9 out of 10 people will have a genetic difference in their DNA that can impact how they respond to common medications,” said Emily J. Cicali, PharmD, a clinical associate at the University of Florida College of Pharmacy, Gainesville.
Dr. Cicali is the clinical director of UF Health’s MyRx, a virtual program that gives Florida and New Jersey residents access to pharmacogenetic (PGx) tests plus expert interpretation by the health system’s pharmacists. Genetic factors are thought to contribute to about 25% or more of inappropriate drug responses or adverse events, said Kristin Wiisanen, PharmD, dean of the College of Pharmacy at Rosalind Franklin University of Medicine and Science in North Chicago.
Dr. Cicali said.
Through a cheek swab or blood sample, the MyRx program — and a growing number of health system programs, doctors’ offices, and home tests available across the United States — gives consumers a window on inherited gene variants that can affect how their body activates, metabolizes, and clears away medications from a long list of widely used drugs.
Why PGx Tests Can Have a Big Impact
These tests work by looking for genes that control drug metabolism.
“You have several different drug-metabolizing enzymes in your liver,” Dr. Cicali explained. “Pharmacogenetic tests look for gene variants that encode for these enzymes. If you’re an ultrarapid metabolizer, you have more of the enzymes that metabolize certain drugs, and there could be a risk the drug won’t work well because it doesn’t stay in the body long enough. On the other end of the spectrum, poor metabolizers have low levels of enzymes that affect certain drugs, so the drugs hang around longer and cause side effects.”
While pharmacogenetics is still considered an emerging science, it’s becoming more mainstream as test prices drop, insurance coverage expands, and an explosion of new research boosts understanding of gene-drug interactions, Dr. Wiisanen said.
Politicians are trying to extend its reach, too. The Right Drug Dose Now Act of 2024, introduced in Congress in late March, aims to accelerate the use of PGx by boosting public awareness and by inserting PGx test results into consumers’ electronic health records. (Though a similar bill died in a US House subcommittee in 2023.)
“The use of pharmacogenetic data to guide prescribing is growing rapidly,” Dr. Wiisanen said. “It’s becoming a routine part of drug therapy for many medications.”
What the Research Shows
When researchers sequenced the DNA of more than 10,000 Mayo Clinic patients, they made a discovery that might surprise many Americans: Gene variants that affect the effectiveness and safety of widely used drugs are not rare glitches. More than 99% of study participants had at least one. And 79% had three or more.
The Mayo-Baylor RIGHT 10K Study — one of the largest PGx studies ever conducted in the United States — looked at 77 gene variants, most involved with drug metabolism in the liver. Researchers focused closely on 13 with extensively studied, gene-based prescribing recommendations for 21 drugs including antidepressants, statins, pain killers, anticlotting medications for heart conditions, HIV treatments, chemotherapy agents, and antirejection drugs for organ transplants.
When researchers added participants’ genetic data to their electronic health records, they also sent semi-urgent alerts, which are alerts with the potential for severe harm, to the clinicians of 61 study volunteers. Over half changed patients’ drugs or doses.
The changes made a difference. One participant taking the pain drug tramadol turned out to be a poor metabolizer and was having dizzy spells because blood levels of the drug stayed high for long periods. Stopping tramadol stopped the dizziness. A participant taking escitalopram plus bupropion for major depression found out that the combo was likely ineffective because they metabolized escitalopram rapidly. A switch to a higher dose of bupropion alone put their depression into full remission.
“So many factors play into how you respond to medications,” said Mayo Clinic pharmacogenomics pharmacist Jessica Wright, PharmD, BCACP, one of the study authors. “Genetics is one of those pieces. Pharmacogenetic testing can reveal things that clinicians may not have been aware of or could help explain a patient’s exaggerated side effect.”
Pharmacogenetics is also called pharmacogenomics. The terms are often used interchangeably, even among PGx pharmacists, though the first refers to how individual genes influence drug response and the second to the effects of multiple genes, said Kelly E. Caudle, PharmD, PhD, an associate member of the Department of Pharmacy and Pharmaceutical Sciences at St. Jude Children’s Research Hospital in Memphis, Tennessee. Dr. Caudle is also co-principal investigator and director of the National Institutes of Health (NIH)-funded Clinical Pharmacogenetics Implementation Consortium (CPIC). The group creates, publishes, and posts evidence-based clinical practice guidelines for drugs with well-researched PGx influences.
By any name, PGx may help explain, predict, and sidestep unpredictable responses to a variety of drugs:
- In a 2023 multicenter study of 6944 people from seven European countries in The Lancet, those given customized drug treatments based on a 12-gene PGx panel had 30% fewer side effects than those who didn’t get this personalized prescribing. People in the study were being treated for cancer, heart disease, and mental health issues, among other conditions.
- In a 2023 from China’s Tongji University, Shanghai, of 650 survivors of strokes and transient ischemic attacks, those whose antiplatelet drugs (such as clopidogrel) were customized based on PGx testing had a lower risk for stroke and other vascular events in the next 90 days. The study was published in Frontiers in Pharmacology.
- In a University of Pennsylvania of 1944 adults with major depression, published in the Journal of the American Medical Association, those whose antidepressants were guided by PGx test results were 28% more likely to go into remission during the first 24 weeks of treatment than those in a control group. But by 24 weeks, equal numbers were in remission. A 2023 Chinese of 11 depression studies, published in BMC Psychiatry, came to a similar conclusion: PGx-guided antidepressant prescriptions may help people feel better quicker, perhaps by avoiding some of the usual trial-and-error of different depression drugs.
PGx checks are already strongly recommended or considered routine before some medications are prescribed. These include abacavir (Ziagen), an antiviral treatment for HIV that can have severe side effects in people with one gene variant.
The US Food and Drug Administration (FDA) recommends genetic testing for people with colon cancer before starting the drug irinotecan (Camptosar), which can cause severe diarrhea and raise infection risk in people with a gene variant that slows the drug’s elimination from the body.
Genetic testing is also recommended by the FDA for people with acute lymphoblastic leukemia before receiving the chemotherapy drug mercaptopurine (Purinethol) because a gene variant that affects drug processing can trigger serious side effects and raise the risk for infection at standard dosages.
“One of the key benefits of pharmacogenomic testing is in preventing adverse drug reactions,” Dr. Wiisanen said. “Testing of the thiopurine methyltransferase enzyme to guide dosing with 6-mercaptopurine or azathioprine can help prevent myelosuppression, a serious adverse drug reaction caused by lower production of blood cells in bone marrow.”
When, Why, and How to Test
“A family doctor should consider a PGx test if a patient is planning on taking a medication for which there is a CPIC guideline with a dosing recommendation,” said Teri Klein, PhD, professor of biomedical data science at Stanford University in California, and principal investigator at PharmGKB, an online resource funded by the NIH that provides information for healthcare practitioners, researchers, and consumers about PGx. Affiliated with CPIC, it’s based at Stanford University.
You might also consider it for patients already on a drug who are “not responding or experiencing side effects,” Dr. Caudle said.
Here’s how four PGx experts suggest consumers and physicians approach this option.
Find a Test
More than a dozen PGx tests are on the market — some only a provider can order, others a consumer can order after a review by their provider or by a provider from the testing company. Some of the tests (using saliva) may be administered at home, while blood tests are done in a doctor’s office or laboratory. Companies that offer the tests include ARUP Laboratories, Genomind, Labcorp, Mayo Clinic Laboratories, Myriad Neuroscience, Precision Sciences Inc., Tempus, and OneOme, but there are many others online. (Keep in mind that many laboratories offer “lab-developed tests” — created for use in a single laboratory — but these can be harder to verify. “The FDA regulates pharmacogenomic testing in laboratories,” Dr. Wiisanen said, “but many of the regulatory parameters are still being defined.”)
Because PGx is so new, there is no official list of recommended tests. So you’ll have to do a little homework. You can check that the laboratory is accredited by searching for it in the NIH Genetic Testing Laboratory Registry database. Beyond that, you’ll have to consult other evidence-based resources to confirm that the drug you’re interested in has research-backed data about specific gene variants (alleles) that affect metabolism as well as research-based clinical guidelines for using PGx results to make prescribing decisions.
The CPIC’s guidelines include dosing and alternate drug recommendations for more than 100 antidepressants, chemotherapy drugs, the antiplatelet and anticlotting drugs clopidogrel and warfarin, local anesthetics, antivirals and antibacterials, pain killers and anti-inflammatory drugs, and some cholesterol-lowering statins such as lovastatin and fluvastatin.
For help figuring out if a test looks for the right gene variants, Dr. Caudle and Dr. Wright recommended checking with the Association for Molecular Pathology’s website. The group published a brief list of best practices for pharmacogenomic testing in 2019. And it keeps a list of gene variants (alleles) that should be included in tests. Clinical guidelines from the CPIC and other groups, available on PharmGKB’s website, also list gene variants that affect the metabolism of the drug.
Consider Cost
The price tag for a test is typically several hundred dollars — but it can run as high as $1000-$2500. And health insurance doesn’t always pick up the tab.
In a 2023 University of Florida study of more than 1000 insurance claims for PGx testing, the number reimbursed varied from 72% for a pain diagnosis to 52% for cardiology to 46% for psychiatry.
Medicare covers some PGx testing when a consumer and their providers meet certain criteria, including whether a drug being considered has a significant gene-drug interaction. California’s Medi-Cal health insurance program covers PGx as do Medicaid programs in some states, including Arkansas and Rhode Island. You can find state-by-state coverage information on the Genetics Policy Hub’s website.
Understand the Results
As more insurers cover PGx, Dr. Klein and Dr. Wiisanen say the field will grow and more providers will use it to inform prescribing. But some health systems aren’t waiting.
In addition to UF Health’s MyRx, PGx is part of personalized medicine programs at the University of Pennsylvania in Philadelphia, Endeavor Health in Chicago, the Mayo Clinic, the University of California, San Francisco, Sanford Health in Sioux Falls, South Dakota, and St. Jude Children’s Research Hospital in Memphis, Tennessee.
Beyond testing, they offer a very useful service: A consult with a pharmacogenetics pharmacist to review the results and explain what they mean for a consumer’s current and future medications.
Physicians and curious consumers can also consult CPIC’s guidelines, which give recommendations about how to interpret the results of a PGx test, said Dr. Klein, a co-principal investigator at CPIC. CPIC has a grading system for both the evidence that supports the recommendation (high, moderate, or weak) and the recommendation itself (strong, moderate, or optional).
Currently, labeling for 456 prescription drugs sold in the United States includes some type of PGx information, according to the FDA’s Table of Pharmacogenomic Biomarkers in Drug Labeling and an annotated guide from PharmGKB.
Just 108 drug labels currently tell doctors and patients what to do with the information — such as requiring or suggesting testing or offering prescribing recommendations, according to PharmGKB. In contrast, PharmGKB’s online resources include evidence-based clinical guidelines for 201 drugs from CPIC and from professional PGx societies in the Netherlands, Canada, France, and elsewhere.
Consumers and physicians can also look for a pharmacist with pharmacogenetics training in their area or through a nearby medical center to learn more, Dr. Wright suggested. And while consumers can test without working with their own physician, the experts advise against it. Don’t stop or change the dose of medications you already take on your own, they say . And do work with your primary care practitioner or specialist to get tested and understand how the results fit into the bigger picture of how your body responds to your medications.
A version of this article appeared on Medscape.com.
The ASCO Annual Meeting Starts This Week
From its origins in 1964, ASCO’s annual event has grown to become the world’s largest clinical oncology meeting, drawing attendees from across the globe.
More than 7000 abstracts were submitted for this year’s meeting a new record — and over 5000 were selected for presentation.
This year’s chair of the Annual Meeting Education Committee, Thomas William LeBlanc, MD, told us he has been attending the meeting since his training days more than a decade ago.
The event is “just incredibly empowering and energizing,” Dr. LeBlanc said, with opportunities to catch up with old colleagues and meet new ones, learn how far oncology has come and where it’s headed, and hear clinical pearls to take back the clinic.
This year’s theme, selected by ASCO President Lynn M. Schuchter, MD, is “The Art and Science of Cancer Care: From Comfort to Cure.”
Dr. LeBlanc, a blood cancer specialist at Duke University, Durham, North Carolina, said the theme has been woven throughout the abstract and educational sessions. Most sessions will have at least one presentation related to how we support people — not only “when we cure them but also when we can’t cure them,” he said.
Topics will include patient well-being, comfort measures, and survivorship. And for the first time the plenary session will include a palliative care abstract that addresses whether or not palliative care can be delivered effectively through telemedicine. The session is on Sunday, June 2.
Other potentially practice changing plenary abstracts tackle immunotherapy combinations for resectable melanoma, perioperative chemotherapy vs neoadjuvant chemoradiation for esophageal cancer, and osimertinib after definitive chemoradiotherapy for unresectable non–small cell lung cancer.
ASCO is piloting a slightly different format for research presentations this year. Instead of starting with context and background, speakers have been asked to present study results upfront as well as repeat them at the end of the talk. The reason behind the tweak is that engagement and retention tend to be better when results are presented upfront, instead of just at the end of a talk.
A popular session — ASCO Voices — has also been given a more central position in the conference: Friday, May 31. In this session, speakers will give short presentations about their personal experiences as providers, researchers, or patients.
ASCO Voices is a relatively recent addition to the meeting that has grown and gotten better. The talks are usually “very powerful narratives” that remind clinicians about “the importance of what they’re doing each day,” Dr. LeBlanc said.
Snippets of the talks will be played while people wait for sessions to begin at the meeting, so attendees who miss the Friday talks can still hear them.
In terms of educational sessions, Dr. LeBlanc highlighted two that might be of general interest to practicing oncologists: A joint ASCO/American Association for Cancer Research session entitled “Drugging the ‘Undruggable’ Target: Successes, Challenges, and the Road Ahead,” on Sunday morning and “Common Sense Oncology: Equity, Value, and Outcomes That Matter” on Monday morning.
As a blood cancer specialist, he said he is particularly interested in the topline results from the ASC4FIRST trial of asciminib, a newer kinase inhibitor, in newly diagnosed chronic myeloid leukemia, presented on Friday.
As in past years, this news organization will be on hand providing coverage with a dedicated team of reporters, editors, and videographers. Stop by our exhibit hall booth — number 26030 — to learn about the tools we offer to support your practice.
A version of this article appeared on Medscape.com .
From its origins in 1964, ASCO’s annual event has grown to become the world’s largest clinical oncology meeting, drawing attendees from across the globe.
More than 7000 abstracts were submitted for this year’s meeting a new record — and over 5000 were selected for presentation.
This year’s chair of the Annual Meeting Education Committee, Thomas William LeBlanc, MD, told us he has been attending the meeting since his training days more than a decade ago.
The event is “just incredibly empowering and energizing,” Dr. LeBlanc said, with opportunities to catch up with old colleagues and meet new ones, learn how far oncology has come and where it’s headed, and hear clinical pearls to take back the clinic.
This year’s theme, selected by ASCO President Lynn M. Schuchter, MD, is “The Art and Science of Cancer Care: From Comfort to Cure.”
Dr. LeBlanc, a blood cancer specialist at Duke University, Durham, North Carolina, said the theme has been woven throughout the abstract and educational sessions. Most sessions will have at least one presentation related to how we support people — not only “when we cure them but also when we can’t cure them,” he said.
Topics will include patient well-being, comfort measures, and survivorship. And for the first time the plenary session will include a palliative care abstract that addresses whether or not palliative care can be delivered effectively through telemedicine. The session is on Sunday, June 2.
Other potentially practice changing plenary abstracts tackle immunotherapy combinations for resectable melanoma, perioperative chemotherapy vs neoadjuvant chemoradiation for esophageal cancer, and osimertinib after definitive chemoradiotherapy for unresectable non–small cell lung cancer.
ASCO is piloting a slightly different format for research presentations this year. Instead of starting with context and background, speakers have been asked to present study results upfront as well as repeat them at the end of the talk. The reason behind the tweak is that engagement and retention tend to be better when results are presented upfront, instead of just at the end of a talk.
A popular session — ASCO Voices — has also been given a more central position in the conference: Friday, May 31. In this session, speakers will give short presentations about their personal experiences as providers, researchers, or patients.
ASCO Voices is a relatively recent addition to the meeting that has grown and gotten better. The talks are usually “very powerful narratives” that remind clinicians about “the importance of what they’re doing each day,” Dr. LeBlanc said.
Snippets of the talks will be played while people wait for sessions to begin at the meeting, so attendees who miss the Friday talks can still hear them.
In terms of educational sessions, Dr. LeBlanc highlighted two that might be of general interest to practicing oncologists: A joint ASCO/American Association for Cancer Research session entitled “Drugging the ‘Undruggable’ Target: Successes, Challenges, and the Road Ahead,” on Sunday morning and “Common Sense Oncology: Equity, Value, and Outcomes That Matter” on Monday morning.
As a blood cancer specialist, he said he is particularly interested in the topline results from the ASC4FIRST trial of asciminib, a newer kinase inhibitor, in newly diagnosed chronic myeloid leukemia, presented on Friday.
As in past years, this news organization will be on hand providing coverage with a dedicated team of reporters, editors, and videographers. Stop by our exhibit hall booth — number 26030 — to learn about the tools we offer to support your practice.
A version of this article appeared on Medscape.com .
From its origins in 1964, ASCO’s annual event has grown to become the world’s largest clinical oncology meeting, drawing attendees from across the globe.
More than 7000 abstracts were submitted for this year’s meeting a new record — and over 5000 were selected for presentation.
This year’s chair of the Annual Meeting Education Committee, Thomas William LeBlanc, MD, told us he has been attending the meeting since his training days more than a decade ago.
The event is “just incredibly empowering and energizing,” Dr. LeBlanc said, with opportunities to catch up with old colleagues and meet new ones, learn how far oncology has come and where it’s headed, and hear clinical pearls to take back the clinic.
This year’s theme, selected by ASCO President Lynn M. Schuchter, MD, is “The Art and Science of Cancer Care: From Comfort to Cure.”
Dr. LeBlanc, a blood cancer specialist at Duke University, Durham, North Carolina, said the theme has been woven throughout the abstract and educational sessions. Most sessions will have at least one presentation related to how we support people — not only “when we cure them but also when we can’t cure them,” he said.
Topics will include patient well-being, comfort measures, and survivorship. And for the first time the plenary session will include a palliative care abstract that addresses whether or not palliative care can be delivered effectively through telemedicine. The session is on Sunday, June 2.
Other potentially practice changing plenary abstracts tackle immunotherapy combinations for resectable melanoma, perioperative chemotherapy vs neoadjuvant chemoradiation for esophageal cancer, and osimertinib after definitive chemoradiotherapy for unresectable non–small cell lung cancer.
ASCO is piloting a slightly different format for research presentations this year. Instead of starting with context and background, speakers have been asked to present study results upfront as well as repeat them at the end of the talk. The reason behind the tweak is that engagement and retention tend to be better when results are presented upfront, instead of just at the end of a talk.
A popular session — ASCO Voices — has also been given a more central position in the conference: Friday, May 31. In this session, speakers will give short presentations about their personal experiences as providers, researchers, or patients.
ASCO Voices is a relatively recent addition to the meeting that has grown and gotten better. The talks are usually “very powerful narratives” that remind clinicians about “the importance of what they’re doing each day,” Dr. LeBlanc said.
Snippets of the talks will be played while people wait for sessions to begin at the meeting, so attendees who miss the Friday talks can still hear them.
In terms of educational sessions, Dr. LeBlanc highlighted two that might be of general interest to practicing oncologists: A joint ASCO/American Association for Cancer Research session entitled “Drugging the ‘Undruggable’ Target: Successes, Challenges, and the Road Ahead,” on Sunday morning and “Common Sense Oncology: Equity, Value, and Outcomes That Matter” on Monday morning.
As a blood cancer specialist, he said he is particularly interested in the topline results from the ASC4FIRST trial of asciminib, a newer kinase inhibitor, in newly diagnosed chronic myeloid leukemia, presented on Friday.
As in past years, this news organization will be on hand providing coverage with a dedicated team of reporters, editors, and videographers. Stop by our exhibit hall booth — number 26030 — to learn about the tools we offer to support your practice.
A version of this article appeared on Medscape.com .
Could a Fungal Infection Cause a Future Pandemic?
The principle of resilience and survival is crucial for medically significant fungi. These microorganisms are far from creating the postapocalyptic scenario depicted in TV series like The Last of Us, and much work is necessary to learn more about them. Accurate statistics on fungal infections, accompanied by clinical histories, simple laboratory tests, new antifungals, and a necessary One Health approach are lacking.
The entomopathogenic fungus Ophiocordyceps unilateralis was made notorious by the TV series, but for now, it only manages to control the brains of some ants at will. Luckily, there are no signs that fungi affecting humans are inclined to create zombies.
What is clear is that the world belongs to the kingdom of fungi and that fungi are everywhere. There are already close to 150,000 described species, but millions remain to be discovered. They abound in decomposing organic matter, soil, or animal excrement, including that of bats and pigeons. Some fungi have even managed to find a home in hospitals. Lastly, we must not forget those that establish themselves in the human microbiome.
Given such diversity, it is legitimate to ask whether any of them could be capable of generating new pandemics. Could the forgotten Cryptococcus neoformans, Aspergillus fumigatus, or Histoplasma species, among others, trigger new health emergencies on the scale of the one generated by SARS-CoV-2?
We cannot forget that a coronavirus has already confirmed that reality can surpass fiction. However, Edith Sánchez Paredes, a biologist, doctor in biomedical sciences, and specialist in medical mycology, provided a reassuring response to Medscape Spanish Edition on this point.
“That would be very difficult to see because the way fungal infections are acquired is not from person to person, in most cases,” said Dr. Sánchez Paredes, from the Mycology Unit of the Faculty of Medicine at the National Autonomous University of Mexico.
Close to 300 species have already been classified as pathogenic in humans. Although the numbers are not precise and are increasing, it is estimated that around 1,500,000 people worldwide die each year of systemic fungal infections.
“However, it is important to emphasize that establishment of an infection depends not only on the causal agent. A crucial factor is the host, in this case, the human. Generally, these types of infections will develop in individuals with some deficiency in their immune system. The more deficient the immune response, the more likely a fungal infection may occur,” stated Dr. Sánchez Paredes.
The possibility of a pandemic like the one experienced with SARS-CoV-2 in the short term is remote, but the threat posed by fungal infections persists.
In 2022, the World Health Organization (WHO) defined a priority list of pathogenic fungi, with the aim of guiding actions to control them. It is mentioned there that invasive fungal diseases are on the rise worldwide, particularly in immunocompromised populations.
“Despite the growing concern, fungal infections receive very little attention and resources, leading to a paucity of quality data on fungal disease distribution and antifungal resistance patterns. Consequently, it is impossible to estimate their exact burden,” as stated in the document.
In line with this, an article published in Mycoses in 2022 concluded that fungal infections are neglected diseases in Latin America. Among other difficulties, deficiencies in access to tests such as polymerase chain reaction or serum detection of beta-1,3-D-glucan have been reported there.
In terms of treatments, most countries encounter problems with access to liposomal amphotericin B and new azoles, such as posaconazole and isavuconazole.
“Unfortunately, in Latin America, we suffer from a poor infrastructure for diagnosing fungal infections; likewise, we have limited access to antifungals available in the global market. What’s more, we lack reliable data on the epidemiology of fungal infections in the region, so many times governments are unaware of the true extent of the problem,” said Rogelio de Jesús Treviño Rangel, PhD, a medical microbiologist and expert in clinical mycology, professor, and researcher at the Faculty of Medicine of the Autonomous University of Nuevo León in Mexico.
Need for More Medical Mycology Training
Dr. Fernando Messina is a medical mycologist with the Mycology Unit of the Francisco Javier Muñiz Infectious Diseases Hospital in Buenos Aires, Argentina. He has noted an increase in the number of cases of cryptococcosis, histoplasmosis, and aspergillosis in his daily practice.
“Particularly, pulmonary aspergillosis is steadily increasing. This is because many patients have structural lung alterations that favor the appearance of this mycosis. This is related to the increase in cases of tuberculosis and the rise in life expectancy of patients with chronic obstructive pulmonary disease or other pulmonary or systemic diseases,” Dr. Messina stated.
For Dr. Messina, the main obstacle in current clinical practice is the low level of awareness among nonspecialist physicians regarding the presence of systemic fungal infections, and because these infections are more common than realized, it is vital to consider fungal etiology before starting empirical antibiotic therapy.
“Health professionals usually do not think about mycoses because mycology occupies a very small space in medical education at universities. As the Venezuelan mycologist Gioconda Cunto de San Blas once said, ‘Mycology is the Cinderella of microbiology.’ To change this, we need to give more space to mycoses in undergraduate and postgraduate studies,” Dr. Messina asserted.
He added, “The main challenge is to train professionals with an emphasis on the clinical interpretation of cases. Current medicine has a strong trend toward molecular biology and the use of rapid diagnostic methods, without considering the clinical symptoms or the patient’s history. Determinations are very useful, but it is necessary to interpret the results.”
Dr. Messina sees it as unlikely in the short term for a pandemic to be caused by fungi, but if it were to occur, he believes it would happen in healthcare systems in regions that are not prepared in terms of infrastructure. However, as seen in the health emergency resulting from SARS-CoV-2, he thinks the impact would be mitigated by the performance of healthcare professionals.
“In general, we have the ability to adapt to any adverse situation or change — although it is clear that we need more doctors, biochemists, and microbiologists trained in mycology,” emphasized Dr. Messina.
More than 40 interns pass through Muñiz Hospital each year. They are doctors and biochemists from Argentina, other countries in the region, or even Europe, seeking to enhance their training in mycology. Regarding fungal infection laboratory work, the interest lies in learning to use traditional techniques and innovative molecular methods.
“Rapid diagnostic methods, especially the detection of circulating antigens, have marked a change in the prognosis of deep mycosis in immunocompromised hosts. The possibility of screening and monitoring in this group of patients is very important and has a great benefit,” said Gabriela Santiso, PhD, a biochemist and head of the Mycology Unit of the Francisco Javier Muñiz Infectious Diseases Hospital.
According to Dr. Santiso, the current landscape includes the ability to identify genus and species, which can help in understanding resistance to antifungals. Furthermore, conducting sensitivity tests to these drugs, using standardized commercial methods, also provides timely information for treatment.
But Dr. Santiso warns that Latin America is a vast region with great disparity in human and technological resources. Although most countries in the region have networks facilitating access to timely diagnosis, resources are generally more available in major urban centers.
This often clashes with the epidemiology of most fungal infections. “Let’s not forget that many fungal pathologies affect low-income people who have difficulties accessing health centers, which sometimes turns them into chronic diseases that are hard to treat,” Dr. Santiso pointed out.
In mycology laboratories, the biggest cost is incurred by new diagnostic tests, such as those allowing molecular identification. Conventional methods are not usually expensive, but they require time and effort to train human resources to handle them.
Because new methodologies are not always available or easily accessible throughout the region, Dr. Santiso recommended not neglecting traditional mycological techniques. “Molecular methods, rapid diagnostic methods, and conventional mycology techniques are complementary and not mutually exclusive tests. Continuous training and updating are needed in this area,” she emphasized.
Why Are Resistant Fungal Infections Becoming Increasingly Common?
The first barrier for fungi to cause infection in humans is body temperature; most of them cannot withstand 37 °C. However, they also struggle to evade the immune response that is activated when they try to enter the body.
“We are normally exposed to many of these fungi, almost all the time, but if our immune system is adequate, it may not go beyond a mild infection, in most cases subclinical, which will resolve quickly,” Dr. Sánchez Paredes stated.
However, according to Dr. Sánchez Paredes, if the immune response is weak, “the fungus will have no trouble establishing itself in our organs. Some are even part of our microbiota, such as Candida albicans, which in the face of an imbalance or immunocompromise, can lead to serious infections.”
It is clear that the population at risk for immunosuppression has increased. According to the WHO, this is due to the high prevalence of such diseases as tuberculosis, cancer, and HIV infection, among others.
But the WHO also believes that the increase in fungal infections is related to greater population access to critical care units, invasive procedures, chemotherapy, or immunotherapy treatments.
Furthermore, factors related to the fungus itself and the environment play a role. “These organisms have enzymes, proteins, and other molecules that allow them to survive in the environment in which they normally inhabit. When they face a new and stressful one, they must express other molecules that will allow them to survive. All of this helps them evade elements of the immune system, antifungals, and, of course, body temperature,” according to Dr. Sánchez Paredes.
It is possible that climate change is also behind the noticeable increase in fungal infections and that this crisis may have an even greater impact in the future. The temperature of the environment has increased, and fungi will have to adapt to the planet’s temperature, to the point where body temperature may no longer be a significant barrier for them.
Environmental changes would also be responsible for modifications in the distribution of endemic mycoses, and it is believed that fungi will more frequently find new ecological niches, be able to survive in other environments, and alter distribution zones.
This is what is happening between Mexico and the United States with coccidioidomycosis, or valley fever. “We will begin to see cases of some mycoses where they were not normally seen, so we will have to conduct more studies to confirm that the fungus is inhabiting these new areas or is simply appearing in new sites owing to migration and the great mobility of populations,” Dr. Sánchez Paredes said.
Finally, exposure to environmental factors would partly be responsible for the increasing resistance to first-line antifungals observed in these microorganisms. This seems to be the case with A. fumigatus when exposed to azoles used as fungicides in agriculture.
One Health in Fungal Infections
The increasing resistance to antifungals is a clear testament that human, animal, and environmental health are interconnected. This is why a multidisciplinary approach that adopts the perspective of One Health is necessary for its management.
“The use of fungicides in agriculture, structurally similar to the azoles used in clinics, generates resistance in Aspergillus fumigatus found in the environment. These fungi in humans can be associated with infections that do not respond to first-line treatment,” explained Carlos Arturo Álvarez, an infectious diseases physician and professor at the Faculty of Medicine at the National University of Colombia.
According to Dr. Álvarez, the approach to control them should not only focus on the search for diagnostic methods that allow early detection of antifungal resistance or research on new antifungal treatments. He believes that progress must also be made with strategies that allow for the proper use of antifungals in agriculture.
“Unfortunately, the One Health approach is not yet well implemented in the region, and in my view, there is a lack of articulation in the different sectors. That is, there is a need for true coordination between government offices of agriculture, animal and human health, academia, and international organizations. This is not happening yet, and I believe we are in the initial stage of visibility,” Dr. Álvarez opined.
Veterinary public health is another pillar of the aforementioned approach. For various reasons, animals experience a higher frequency of fungal infections. A few carry and transmit true zoonoses that affect human health, but most often, animals act only as sentinels indicating a potential source of transmission.
Carolina Segundo Zaragoza, PhD, has worked in veterinary mycology for 30 years. She currently heads the veterinary mycology laboratory at the Animal Production Teaching, Research, and Extension Center in Altiplano, under the Faculty of Veterinary Medicine and Animal Husbandry at the National Autonomous University of Mexico. Because she has frequent contact with specialists in human mycology, during her professional career she has received several patient consultations, most of which were for cutaneous mycoses.
“They detect some dermatomycosis and realize that the common factor is owning a companion animal or a production animal with which the patient has contact. Both animals and humans present the same type of lesions, and then comes the question: Who infected whom? I remind them that the main source of infection is the soil and that animals should not be blamed in the first instance,” Dr. Segundo Zaragoza clarified.
She is currently collaborating on a research project analyzing the presence of Coccidioides immitis in the soil. This pathogen is responsible for coccidioidomycosis in dogs and humans, and she sees with satisfaction how these types of initiatives, which include some components of the One Health vision, are becoming more common in Mexico.
“Fortunately, human mycologists are increasingly providing more space for the dissemination of veterinary mycology. So I have had the opportunity to be invited to different forums on medical mycology to present the clinical cases we can have in animals and talk about the research projects we carry out. I have more and more opportunities to conduct joint research with human mycologists and veterinary doctors,” she said.
Dr. Segundo Zaragoza believes that to better implement the One Health vision, standardizing the criteria for detecting, diagnosing, and treating mycoses is necessary. She considers that teamwork will be key to achieving the common goal of safeguarding the well-being and health of humans and animals.
Alarms Sound for Candida auris
The WHO included the yeast Candida auris in its group of pathogens with critical priority, and since 2009, it has raised alarm owing to the ease with which it grows in hospitals. In that setting, C auris is known for its high transmissibility, its ability to cause outbreaks, and the high mortality rate from disseminated infections.
“It has been a concern for the mycological community because it shows resistance to most antifungals used clinically, mainly azoles, but also for causing epidemic outbreaks,” emphasized Dr. Sánchez Paredes.
Its mode of transmission is not very clear, but it has been documented to be present on the skin and persist in hospital materials and furniture. It causes nosocomial infections in critically ill patients, such as those in intensive care, and those with cancer or who have received a transplant.
Risk factors for its development include renal insufficiency, hospital stays of more than 15 days, mechanical ventilation, central lines, use of parenteral nutrition, and presence of sepsis.
As for other mycoses, there are no precise studies reporting global incidence rates, but the trend indicates an increase in the detection of outbreaks in various countries lately — something that began to be visible during the COVID-19 pandemic.
In Mexico, Dr. Treviño Rangel and colleagues from Nuevo León reported the first case of candidemia caused by this agent. It occurred in May 2020 and involved a 58-year-old woman with a history of severe endometriosis and multiple complications in the gastrointestinal tract. The patient’s condition improved favorably thanks to antifungal therapy with caspofungin and liposomal amphotericin B.
However, 3 months after that episode, the group reported an outbreak of C. auris at the same hospital in 12 critically ill patients co-infected with SARS-CoV-2. All were on mechanical ventilation, had peripherally inserted central catheters and urinary catheters, and had a prolonged hospital stay (20-70 days). The mortality in patients with candidemia in this cohort was 83.3%.
Open Ending
As seen in some science fiction series, fungal infections in the region still have an open ending, and Global Action For Fungal Infections (GAFFI) has estimated that with better diagnostics and treatments, deaths caused by fungi could decrease to less than 750,000 per year worldwide.
But if everything continues as is, some aspects of what is to come may resemble the dystopia depicted in The Last of Us. No zombies, but emerging and reemerging fungi in a chaotic distribution, and resistant to all established treatments.
“The risk factors of patients and their immune status, combined with the behavior of mycoses, bring a complicated scenario. But therapeutic failure resulting from multidrug resistance to antifungals could make it catastrophic,” Dr. Sánchez Paredes summarized.
At the moment, there are only four families of drugs capable of counteracting fungal infections — and as mentioned, some are already scarce in Latin America’s hospital pharmacies.
“Historically, fungal infections have been given less importance than those caused by viruses or bacteria. Even in some developed countries, the true extent of morbidity and mortality they present is unknown. This results in less investment in the development of new antifungal molecules because knowledge is lacking about the incidence and prevalence of these diseases,” Dr. Treviño Rangel pointed out.
He added that the main limitation for the development of new drugs is economic. “Unfortunately, not many pharmaceutical companies are willing to invest in the development of new antifungals, and there are no government programs specifically promoting and supporting research into new therapeutic options against these neglected diseases,” he asserted.
Development of vaccines to prevent fungal infections faces the same barriers. Although, according to Dr. Treviño Rangel, the difficulties are compounded by the great similarity between fungal cells and human cells. This makes it possible for harmful cross-reactivity to occur. In addition, because most severe fungal infections occur in individuals with immunosuppression, a vaccine would need to trigger an adequate immune response despite this issue.
Meanwhile, fungi quietly continue to do what they do best: resist and survive. For millions of years, they have mutated and adapted to new environments. Some theories even blame them for the extinction of dinosaurs and the subsequent rise of mammals. They exist on the edge of life and death, decomposing and creating. There is consensus that at the moment, it does not seem feasible for them to generate a pandemic like the one due to SARS-CoV-2, given their transmission mechanism. But who is willing to rule out that this may not happen in the long or medium term?
Dr. Sánchez Paredes, Dr. Treviño Rangel, Dr. Messina, Dr. Santiso, Dr. Álvarez, and Dr. Segundo Zaragoza have declared no relevant financial conflicts of interest.
This story was translated from Medscape Spanish Edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The principle of resilience and survival is crucial for medically significant fungi. These microorganisms are far from creating the postapocalyptic scenario depicted in TV series like The Last of Us, and much work is necessary to learn more about them. Accurate statistics on fungal infections, accompanied by clinical histories, simple laboratory tests, new antifungals, and a necessary One Health approach are lacking.
The entomopathogenic fungus Ophiocordyceps unilateralis was made notorious by the TV series, but for now, it only manages to control the brains of some ants at will. Luckily, there are no signs that fungi affecting humans are inclined to create zombies.
What is clear is that the world belongs to the kingdom of fungi and that fungi are everywhere. There are already close to 150,000 described species, but millions remain to be discovered. They abound in decomposing organic matter, soil, or animal excrement, including that of bats and pigeons. Some fungi have even managed to find a home in hospitals. Lastly, we must not forget those that establish themselves in the human microbiome.
Given such diversity, it is legitimate to ask whether any of them could be capable of generating new pandemics. Could the forgotten Cryptococcus neoformans, Aspergillus fumigatus, or Histoplasma species, among others, trigger new health emergencies on the scale of the one generated by SARS-CoV-2?
We cannot forget that a coronavirus has already confirmed that reality can surpass fiction. However, Edith Sánchez Paredes, a biologist, doctor in biomedical sciences, and specialist in medical mycology, provided a reassuring response to Medscape Spanish Edition on this point.
“That would be very difficult to see because the way fungal infections are acquired is not from person to person, in most cases,” said Dr. Sánchez Paredes, from the Mycology Unit of the Faculty of Medicine at the National Autonomous University of Mexico.
Close to 300 species have already been classified as pathogenic in humans. Although the numbers are not precise and are increasing, it is estimated that around 1,500,000 people worldwide die each year of systemic fungal infections.
“However, it is important to emphasize that establishment of an infection depends not only on the causal agent. A crucial factor is the host, in this case, the human. Generally, these types of infections will develop in individuals with some deficiency in their immune system. The more deficient the immune response, the more likely a fungal infection may occur,” stated Dr. Sánchez Paredes.
The possibility of a pandemic like the one experienced with SARS-CoV-2 in the short term is remote, but the threat posed by fungal infections persists.
In 2022, the World Health Organization (WHO) defined a priority list of pathogenic fungi, with the aim of guiding actions to control them. It is mentioned there that invasive fungal diseases are on the rise worldwide, particularly in immunocompromised populations.
“Despite the growing concern, fungal infections receive very little attention and resources, leading to a paucity of quality data on fungal disease distribution and antifungal resistance patterns. Consequently, it is impossible to estimate their exact burden,” as stated in the document.
In line with this, an article published in Mycoses in 2022 concluded that fungal infections are neglected diseases in Latin America. Among other difficulties, deficiencies in access to tests such as polymerase chain reaction or serum detection of beta-1,3-D-glucan have been reported there.
In terms of treatments, most countries encounter problems with access to liposomal amphotericin B and new azoles, such as posaconazole and isavuconazole.
“Unfortunately, in Latin America, we suffer from a poor infrastructure for diagnosing fungal infections; likewise, we have limited access to antifungals available in the global market. What’s more, we lack reliable data on the epidemiology of fungal infections in the region, so many times governments are unaware of the true extent of the problem,” said Rogelio de Jesús Treviño Rangel, PhD, a medical microbiologist and expert in clinical mycology, professor, and researcher at the Faculty of Medicine of the Autonomous University of Nuevo León in Mexico.
Need for More Medical Mycology Training
Dr. Fernando Messina is a medical mycologist with the Mycology Unit of the Francisco Javier Muñiz Infectious Diseases Hospital in Buenos Aires, Argentina. He has noted an increase in the number of cases of cryptococcosis, histoplasmosis, and aspergillosis in his daily practice.
“Particularly, pulmonary aspergillosis is steadily increasing. This is because many patients have structural lung alterations that favor the appearance of this mycosis. This is related to the increase in cases of tuberculosis and the rise in life expectancy of patients with chronic obstructive pulmonary disease or other pulmonary or systemic diseases,” Dr. Messina stated.
For Dr. Messina, the main obstacle in current clinical practice is the low level of awareness among nonspecialist physicians regarding the presence of systemic fungal infections, and because these infections are more common than realized, it is vital to consider fungal etiology before starting empirical antibiotic therapy.
“Health professionals usually do not think about mycoses because mycology occupies a very small space in medical education at universities. As the Venezuelan mycologist Gioconda Cunto de San Blas once said, ‘Mycology is the Cinderella of microbiology.’ To change this, we need to give more space to mycoses in undergraduate and postgraduate studies,” Dr. Messina asserted.
He added, “The main challenge is to train professionals with an emphasis on the clinical interpretation of cases. Current medicine has a strong trend toward molecular biology and the use of rapid diagnostic methods, without considering the clinical symptoms or the patient’s history. Determinations are very useful, but it is necessary to interpret the results.”
Dr. Messina sees it as unlikely in the short term for a pandemic to be caused by fungi, but if it were to occur, he believes it would happen in healthcare systems in regions that are not prepared in terms of infrastructure. However, as seen in the health emergency resulting from SARS-CoV-2, he thinks the impact would be mitigated by the performance of healthcare professionals.
“In general, we have the ability to adapt to any adverse situation or change — although it is clear that we need more doctors, biochemists, and microbiologists trained in mycology,” emphasized Dr. Messina.
More than 40 interns pass through Muñiz Hospital each year. They are doctors and biochemists from Argentina, other countries in the region, or even Europe, seeking to enhance their training in mycology. Regarding fungal infection laboratory work, the interest lies in learning to use traditional techniques and innovative molecular methods.
“Rapid diagnostic methods, especially the detection of circulating antigens, have marked a change in the prognosis of deep mycosis in immunocompromised hosts. The possibility of screening and monitoring in this group of patients is very important and has a great benefit,” said Gabriela Santiso, PhD, a biochemist and head of the Mycology Unit of the Francisco Javier Muñiz Infectious Diseases Hospital.
According to Dr. Santiso, the current landscape includes the ability to identify genus and species, which can help in understanding resistance to antifungals. Furthermore, conducting sensitivity tests to these drugs, using standardized commercial methods, also provides timely information for treatment.
But Dr. Santiso warns that Latin America is a vast region with great disparity in human and technological resources. Although most countries in the region have networks facilitating access to timely diagnosis, resources are generally more available in major urban centers.
This often clashes with the epidemiology of most fungal infections. “Let’s not forget that many fungal pathologies affect low-income people who have difficulties accessing health centers, which sometimes turns them into chronic diseases that are hard to treat,” Dr. Santiso pointed out.
In mycology laboratories, the biggest cost is incurred by new diagnostic tests, such as those allowing molecular identification. Conventional methods are not usually expensive, but they require time and effort to train human resources to handle them.
Because new methodologies are not always available or easily accessible throughout the region, Dr. Santiso recommended not neglecting traditional mycological techniques. “Molecular methods, rapid diagnostic methods, and conventional mycology techniques are complementary and not mutually exclusive tests. Continuous training and updating are needed in this area,” she emphasized.
Why Are Resistant Fungal Infections Becoming Increasingly Common?
The first barrier for fungi to cause infection in humans is body temperature; most of them cannot withstand 37 °C. However, they also struggle to evade the immune response that is activated when they try to enter the body.
“We are normally exposed to many of these fungi, almost all the time, but if our immune system is adequate, it may not go beyond a mild infection, in most cases subclinical, which will resolve quickly,” Dr. Sánchez Paredes stated.
However, according to Dr. Sánchez Paredes, if the immune response is weak, “the fungus will have no trouble establishing itself in our organs. Some are even part of our microbiota, such as Candida albicans, which in the face of an imbalance or immunocompromise, can lead to serious infections.”
It is clear that the population at risk for immunosuppression has increased. According to the WHO, this is due to the high prevalence of such diseases as tuberculosis, cancer, and HIV infection, among others.
But the WHO also believes that the increase in fungal infections is related to greater population access to critical care units, invasive procedures, chemotherapy, or immunotherapy treatments.
Furthermore, factors related to the fungus itself and the environment play a role. “These organisms have enzymes, proteins, and other molecules that allow them to survive in the environment in which they normally inhabit. When they face a new and stressful one, they must express other molecules that will allow them to survive. All of this helps them evade elements of the immune system, antifungals, and, of course, body temperature,” according to Dr. Sánchez Paredes.
It is possible that climate change is also behind the noticeable increase in fungal infections and that this crisis may have an even greater impact in the future. The temperature of the environment has increased, and fungi will have to adapt to the planet’s temperature, to the point where body temperature may no longer be a significant barrier for them.
Environmental changes would also be responsible for modifications in the distribution of endemic mycoses, and it is believed that fungi will more frequently find new ecological niches, be able to survive in other environments, and alter distribution zones.
This is what is happening between Mexico and the United States with coccidioidomycosis, or valley fever. “We will begin to see cases of some mycoses where they were not normally seen, so we will have to conduct more studies to confirm that the fungus is inhabiting these new areas or is simply appearing in new sites owing to migration and the great mobility of populations,” Dr. Sánchez Paredes said.
Finally, exposure to environmental factors would partly be responsible for the increasing resistance to first-line antifungals observed in these microorganisms. This seems to be the case with A. fumigatus when exposed to azoles used as fungicides in agriculture.
One Health in Fungal Infections
The increasing resistance to antifungals is a clear testament that human, animal, and environmental health are interconnected. This is why a multidisciplinary approach that adopts the perspective of One Health is necessary for its management.
“The use of fungicides in agriculture, structurally similar to the azoles used in clinics, generates resistance in Aspergillus fumigatus found in the environment. These fungi in humans can be associated with infections that do not respond to first-line treatment,” explained Carlos Arturo Álvarez, an infectious diseases physician and professor at the Faculty of Medicine at the National University of Colombia.
According to Dr. Álvarez, the approach to control them should not only focus on the search for diagnostic methods that allow early detection of antifungal resistance or research on new antifungal treatments. He believes that progress must also be made with strategies that allow for the proper use of antifungals in agriculture.
“Unfortunately, the One Health approach is not yet well implemented in the region, and in my view, there is a lack of articulation in the different sectors. That is, there is a need for true coordination between government offices of agriculture, animal and human health, academia, and international organizations. This is not happening yet, and I believe we are in the initial stage of visibility,” Dr. Álvarez opined.
Veterinary public health is another pillar of the aforementioned approach. For various reasons, animals experience a higher frequency of fungal infections. A few carry and transmit true zoonoses that affect human health, but most often, animals act only as sentinels indicating a potential source of transmission.
Carolina Segundo Zaragoza, PhD, has worked in veterinary mycology for 30 years. She currently heads the veterinary mycology laboratory at the Animal Production Teaching, Research, and Extension Center in Altiplano, under the Faculty of Veterinary Medicine and Animal Husbandry at the National Autonomous University of Mexico. Because she has frequent contact with specialists in human mycology, during her professional career she has received several patient consultations, most of which were for cutaneous mycoses.
“They detect some dermatomycosis and realize that the common factor is owning a companion animal or a production animal with which the patient has contact. Both animals and humans present the same type of lesions, and then comes the question: Who infected whom? I remind them that the main source of infection is the soil and that animals should not be blamed in the first instance,” Dr. Segundo Zaragoza clarified.
She is currently collaborating on a research project analyzing the presence of Coccidioides immitis in the soil. This pathogen is responsible for coccidioidomycosis in dogs and humans, and she sees with satisfaction how these types of initiatives, which include some components of the One Health vision, are becoming more common in Mexico.
“Fortunately, human mycologists are increasingly providing more space for the dissemination of veterinary mycology. So I have had the opportunity to be invited to different forums on medical mycology to present the clinical cases we can have in animals and talk about the research projects we carry out. I have more and more opportunities to conduct joint research with human mycologists and veterinary doctors,” she said.
Dr. Segundo Zaragoza believes that to better implement the One Health vision, standardizing the criteria for detecting, diagnosing, and treating mycoses is necessary. She considers that teamwork will be key to achieving the common goal of safeguarding the well-being and health of humans and animals.
Alarms Sound for Candida auris
The WHO included the yeast Candida auris in its group of pathogens with critical priority, and since 2009, it has raised alarm owing to the ease with which it grows in hospitals. In that setting, C auris is known for its high transmissibility, its ability to cause outbreaks, and the high mortality rate from disseminated infections.
“It has been a concern for the mycological community because it shows resistance to most antifungals used clinically, mainly azoles, but also for causing epidemic outbreaks,” emphasized Dr. Sánchez Paredes.
Its mode of transmission is not very clear, but it has been documented to be present on the skin and persist in hospital materials and furniture. It causes nosocomial infections in critically ill patients, such as those in intensive care, and those with cancer or who have received a transplant.
Risk factors for its development include renal insufficiency, hospital stays of more than 15 days, mechanical ventilation, central lines, use of parenteral nutrition, and presence of sepsis.
As for other mycoses, there are no precise studies reporting global incidence rates, but the trend indicates an increase in the detection of outbreaks in various countries lately — something that began to be visible during the COVID-19 pandemic.
In Mexico, Dr. Treviño Rangel and colleagues from Nuevo León reported the first case of candidemia caused by this agent. It occurred in May 2020 and involved a 58-year-old woman with a history of severe endometriosis and multiple complications in the gastrointestinal tract. The patient’s condition improved favorably thanks to antifungal therapy with caspofungin and liposomal amphotericin B.
However, 3 months after that episode, the group reported an outbreak of C. auris at the same hospital in 12 critically ill patients co-infected with SARS-CoV-2. All were on mechanical ventilation, had peripherally inserted central catheters and urinary catheters, and had a prolonged hospital stay (20-70 days). The mortality in patients with candidemia in this cohort was 83.3%.
Open Ending
As seen in some science fiction series, fungal infections in the region still have an open ending, and Global Action For Fungal Infections (GAFFI) has estimated that with better diagnostics and treatments, deaths caused by fungi could decrease to less than 750,000 per year worldwide.
But if everything continues as is, some aspects of what is to come may resemble the dystopia depicted in The Last of Us. No zombies, but emerging and reemerging fungi in a chaotic distribution, and resistant to all established treatments.
“The risk factors of patients and their immune status, combined with the behavior of mycoses, bring a complicated scenario. But therapeutic failure resulting from multidrug resistance to antifungals could make it catastrophic,” Dr. Sánchez Paredes summarized.
At the moment, there are only four families of drugs capable of counteracting fungal infections — and as mentioned, some are already scarce in Latin America’s hospital pharmacies.
“Historically, fungal infections have been given less importance than those caused by viruses or bacteria. Even in some developed countries, the true extent of morbidity and mortality they present is unknown. This results in less investment in the development of new antifungal molecules because knowledge is lacking about the incidence and prevalence of these diseases,” Dr. Treviño Rangel pointed out.
He added that the main limitation for the development of new drugs is economic. “Unfortunately, not many pharmaceutical companies are willing to invest in the development of new antifungals, and there are no government programs specifically promoting and supporting research into new therapeutic options against these neglected diseases,” he asserted.
Development of vaccines to prevent fungal infections faces the same barriers. Although, according to Dr. Treviño Rangel, the difficulties are compounded by the great similarity between fungal cells and human cells. This makes it possible for harmful cross-reactivity to occur. In addition, because most severe fungal infections occur in individuals with immunosuppression, a vaccine would need to trigger an adequate immune response despite this issue.
Meanwhile, fungi quietly continue to do what they do best: resist and survive. For millions of years, they have mutated and adapted to new environments. Some theories even blame them for the extinction of dinosaurs and the subsequent rise of mammals. They exist on the edge of life and death, decomposing and creating. There is consensus that at the moment, it does not seem feasible for them to generate a pandemic like the one due to SARS-CoV-2, given their transmission mechanism. But who is willing to rule out that this may not happen in the long or medium term?
Dr. Sánchez Paredes, Dr. Treviño Rangel, Dr. Messina, Dr. Santiso, Dr. Álvarez, and Dr. Segundo Zaragoza have declared no relevant financial conflicts of interest.
This story was translated from Medscape Spanish Edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The principle of resilience and survival is crucial for medically significant fungi. These microorganisms are far from creating the postapocalyptic scenario depicted in TV series like The Last of Us, and much work is necessary to learn more about them. Accurate statistics on fungal infections, accompanied by clinical histories, simple laboratory tests, new antifungals, and a necessary One Health approach are lacking.
The entomopathogenic fungus Ophiocordyceps unilateralis was made notorious by the TV series, but for now, it only manages to control the brains of some ants at will. Luckily, there are no signs that fungi affecting humans are inclined to create zombies.
What is clear is that the world belongs to the kingdom of fungi and that fungi are everywhere. There are already close to 150,000 described species, but millions remain to be discovered. They abound in decomposing organic matter, soil, or animal excrement, including that of bats and pigeons. Some fungi have even managed to find a home in hospitals. Lastly, we must not forget those that establish themselves in the human microbiome.
Given such diversity, it is legitimate to ask whether any of them could be capable of generating new pandemics. Could the forgotten Cryptococcus neoformans, Aspergillus fumigatus, or Histoplasma species, among others, trigger new health emergencies on the scale of the one generated by SARS-CoV-2?
We cannot forget that a coronavirus has already confirmed that reality can surpass fiction. However, Edith Sánchez Paredes, a biologist, doctor in biomedical sciences, and specialist in medical mycology, provided a reassuring response to Medscape Spanish Edition on this point.
“That would be very difficult to see because the way fungal infections are acquired is not from person to person, in most cases,” said Dr. Sánchez Paredes, from the Mycology Unit of the Faculty of Medicine at the National Autonomous University of Mexico.
Close to 300 species have already been classified as pathogenic in humans. Although the numbers are not precise and are increasing, it is estimated that around 1,500,000 people worldwide die each year of systemic fungal infections.
“However, it is important to emphasize that establishment of an infection depends not only on the causal agent. A crucial factor is the host, in this case, the human. Generally, these types of infections will develop in individuals with some deficiency in their immune system. The more deficient the immune response, the more likely a fungal infection may occur,” stated Dr. Sánchez Paredes.
The possibility of a pandemic like the one experienced with SARS-CoV-2 in the short term is remote, but the threat posed by fungal infections persists.
In 2022, the World Health Organization (WHO) defined a priority list of pathogenic fungi, with the aim of guiding actions to control them. It is mentioned there that invasive fungal diseases are on the rise worldwide, particularly in immunocompromised populations.
“Despite the growing concern, fungal infections receive very little attention and resources, leading to a paucity of quality data on fungal disease distribution and antifungal resistance patterns. Consequently, it is impossible to estimate their exact burden,” as stated in the document.
In line with this, an article published in Mycoses in 2022 concluded that fungal infections are neglected diseases in Latin America. Among other difficulties, deficiencies in access to tests such as polymerase chain reaction or serum detection of beta-1,3-D-glucan have been reported there.
In terms of treatments, most countries encounter problems with access to liposomal amphotericin B and new azoles, such as posaconazole and isavuconazole.
“Unfortunately, in Latin America, we suffer from a poor infrastructure for diagnosing fungal infections; likewise, we have limited access to antifungals available in the global market. What’s more, we lack reliable data on the epidemiology of fungal infections in the region, so many times governments are unaware of the true extent of the problem,” said Rogelio de Jesús Treviño Rangel, PhD, a medical microbiologist and expert in clinical mycology, professor, and researcher at the Faculty of Medicine of the Autonomous University of Nuevo León in Mexico.
Need for More Medical Mycology Training
Dr. Fernando Messina is a medical mycologist with the Mycology Unit of the Francisco Javier Muñiz Infectious Diseases Hospital in Buenos Aires, Argentina. He has noted an increase in the number of cases of cryptococcosis, histoplasmosis, and aspergillosis in his daily practice.
“Particularly, pulmonary aspergillosis is steadily increasing. This is because many patients have structural lung alterations that favor the appearance of this mycosis. This is related to the increase in cases of tuberculosis and the rise in life expectancy of patients with chronic obstructive pulmonary disease or other pulmonary or systemic diseases,” Dr. Messina stated.
For Dr. Messina, the main obstacle in current clinical practice is the low level of awareness among nonspecialist physicians regarding the presence of systemic fungal infections, and because these infections are more common than realized, it is vital to consider fungal etiology before starting empirical antibiotic therapy.
“Health professionals usually do not think about mycoses because mycology occupies a very small space in medical education at universities. As the Venezuelan mycologist Gioconda Cunto de San Blas once said, ‘Mycology is the Cinderella of microbiology.’ To change this, we need to give more space to mycoses in undergraduate and postgraduate studies,” Dr. Messina asserted.
He added, “The main challenge is to train professionals with an emphasis on the clinical interpretation of cases. Current medicine has a strong trend toward molecular biology and the use of rapid diagnostic methods, without considering the clinical symptoms or the patient’s history. Determinations are very useful, but it is necessary to interpret the results.”
Dr. Messina sees it as unlikely in the short term for a pandemic to be caused by fungi, but if it were to occur, he believes it would happen in healthcare systems in regions that are not prepared in terms of infrastructure. However, as seen in the health emergency resulting from SARS-CoV-2, he thinks the impact would be mitigated by the performance of healthcare professionals.
“In general, we have the ability to adapt to any adverse situation or change — although it is clear that we need more doctors, biochemists, and microbiologists trained in mycology,” emphasized Dr. Messina.
More than 40 interns pass through Muñiz Hospital each year. They are doctors and biochemists from Argentina, other countries in the region, or even Europe, seeking to enhance their training in mycology. Regarding fungal infection laboratory work, the interest lies in learning to use traditional techniques and innovative molecular methods.
“Rapid diagnostic methods, especially the detection of circulating antigens, have marked a change in the prognosis of deep mycosis in immunocompromised hosts. The possibility of screening and monitoring in this group of patients is very important and has a great benefit,” said Gabriela Santiso, PhD, a biochemist and head of the Mycology Unit of the Francisco Javier Muñiz Infectious Diseases Hospital.
According to Dr. Santiso, the current landscape includes the ability to identify genus and species, which can help in understanding resistance to antifungals. Furthermore, conducting sensitivity tests to these drugs, using standardized commercial methods, also provides timely information for treatment.
But Dr. Santiso warns that Latin America is a vast region with great disparity in human and technological resources. Although most countries in the region have networks facilitating access to timely diagnosis, resources are generally more available in major urban centers.
This often clashes with the epidemiology of most fungal infections. “Let’s not forget that many fungal pathologies affect low-income people who have difficulties accessing health centers, which sometimes turns them into chronic diseases that are hard to treat,” Dr. Santiso pointed out.
In mycology laboratories, the biggest cost is incurred by new diagnostic tests, such as those allowing molecular identification. Conventional methods are not usually expensive, but they require time and effort to train human resources to handle them.
Because new methodologies are not always available or easily accessible throughout the region, Dr. Santiso recommended not neglecting traditional mycological techniques. “Molecular methods, rapid diagnostic methods, and conventional mycology techniques are complementary and not mutually exclusive tests. Continuous training and updating are needed in this area,” she emphasized.
Why Are Resistant Fungal Infections Becoming Increasingly Common?
The first barrier for fungi to cause infection in humans is body temperature; most of them cannot withstand 37 °C. However, they also struggle to evade the immune response that is activated when they try to enter the body.
“We are normally exposed to many of these fungi, almost all the time, but if our immune system is adequate, it may not go beyond a mild infection, in most cases subclinical, which will resolve quickly,” Dr. Sánchez Paredes stated.
However, according to Dr. Sánchez Paredes, if the immune response is weak, “the fungus will have no trouble establishing itself in our organs. Some are even part of our microbiota, such as Candida albicans, which in the face of an imbalance or immunocompromise, can lead to serious infections.”
It is clear that the population at risk for immunosuppression has increased. According to the WHO, this is due to the high prevalence of such diseases as tuberculosis, cancer, and HIV infection, among others.
But the WHO also believes that the increase in fungal infections is related to greater population access to critical care units, invasive procedures, chemotherapy, or immunotherapy treatments.
Furthermore, factors related to the fungus itself and the environment play a role. “These organisms have enzymes, proteins, and other molecules that allow them to survive in the environment in which they normally inhabit. When they face a new and stressful one, they must express other molecules that will allow them to survive. All of this helps them evade elements of the immune system, antifungals, and, of course, body temperature,” according to Dr. Sánchez Paredes.
It is possible that climate change is also behind the noticeable increase in fungal infections and that this crisis may have an even greater impact in the future. The temperature of the environment has increased, and fungi will have to adapt to the planet’s temperature, to the point where body temperature may no longer be a significant barrier for them.
Environmental changes would also be responsible for modifications in the distribution of endemic mycoses, and it is believed that fungi will more frequently find new ecological niches, be able to survive in other environments, and alter distribution zones.
This is what is happening between Mexico and the United States with coccidioidomycosis, or valley fever. “We will begin to see cases of some mycoses where they were not normally seen, so we will have to conduct more studies to confirm that the fungus is inhabiting these new areas or is simply appearing in new sites owing to migration and the great mobility of populations,” Dr. Sánchez Paredes said.
Finally, exposure to environmental factors would partly be responsible for the increasing resistance to first-line antifungals observed in these microorganisms. This seems to be the case with A. fumigatus when exposed to azoles used as fungicides in agriculture.
One Health in Fungal Infections
The increasing resistance to antifungals is a clear testament that human, animal, and environmental health are interconnected. This is why a multidisciplinary approach that adopts the perspective of One Health is necessary for its management.
“The use of fungicides in agriculture, structurally similar to the azoles used in clinics, generates resistance in Aspergillus fumigatus found in the environment. These fungi in humans can be associated with infections that do not respond to first-line treatment,” explained Carlos Arturo Álvarez, an infectious diseases physician and professor at the Faculty of Medicine at the National University of Colombia.
According to Dr. Álvarez, the approach to control them should not only focus on the search for diagnostic methods that allow early detection of antifungal resistance or research on new antifungal treatments. He believes that progress must also be made with strategies that allow for the proper use of antifungals in agriculture.
“Unfortunately, the One Health approach is not yet well implemented in the region, and in my view, there is a lack of articulation in the different sectors. That is, there is a need for true coordination between government offices of agriculture, animal and human health, academia, and international organizations. This is not happening yet, and I believe we are in the initial stage of visibility,” Dr. Álvarez opined.
Veterinary public health is another pillar of the aforementioned approach. For various reasons, animals experience a higher frequency of fungal infections. A few carry and transmit true zoonoses that affect human health, but most often, animals act only as sentinels indicating a potential source of transmission.
Carolina Segundo Zaragoza, PhD, has worked in veterinary mycology for 30 years. She currently heads the veterinary mycology laboratory at the Animal Production Teaching, Research, and Extension Center in Altiplano, under the Faculty of Veterinary Medicine and Animal Husbandry at the National Autonomous University of Mexico. Because she has frequent contact with specialists in human mycology, during her professional career she has received several patient consultations, most of which were for cutaneous mycoses.
“They detect some dermatomycosis and realize that the common factor is owning a companion animal or a production animal with which the patient has contact. Both animals and humans present the same type of lesions, and then comes the question: Who infected whom? I remind them that the main source of infection is the soil and that animals should not be blamed in the first instance,” Dr. Segundo Zaragoza clarified.
She is currently collaborating on a research project analyzing the presence of Coccidioides immitis in the soil. This pathogen is responsible for coccidioidomycosis in dogs and humans, and she sees with satisfaction how these types of initiatives, which include some components of the One Health vision, are becoming more common in Mexico.
“Fortunately, human mycologists are increasingly providing more space for the dissemination of veterinary mycology. So I have had the opportunity to be invited to different forums on medical mycology to present the clinical cases we can have in animals and talk about the research projects we carry out. I have more and more opportunities to conduct joint research with human mycologists and veterinary doctors,” she said.
Dr. Segundo Zaragoza believes that to better implement the One Health vision, standardizing the criteria for detecting, diagnosing, and treating mycoses is necessary. She considers that teamwork will be key to achieving the common goal of safeguarding the well-being and health of humans and animals.
Alarms Sound for Candida auris
The WHO included the yeast Candida auris in its group of pathogens with critical priority, and since 2009, it has raised alarm owing to the ease with which it grows in hospitals. In that setting, C auris is known for its high transmissibility, its ability to cause outbreaks, and the high mortality rate from disseminated infections.
“It has been a concern for the mycological community because it shows resistance to most antifungals used clinically, mainly azoles, but also for causing epidemic outbreaks,” emphasized Dr. Sánchez Paredes.
Its mode of transmission is not very clear, but it has been documented to be present on the skin and persist in hospital materials and furniture. It causes nosocomial infections in critically ill patients, such as those in intensive care, and those with cancer or who have received a transplant.
Risk factors for its development include renal insufficiency, hospital stays of more than 15 days, mechanical ventilation, central lines, use of parenteral nutrition, and presence of sepsis.
As for other mycoses, there are no precise studies reporting global incidence rates, but the trend indicates an increase in the detection of outbreaks in various countries lately — something that began to be visible during the COVID-19 pandemic.
In Mexico, Dr. Treviño Rangel and colleagues from Nuevo León reported the first case of candidemia caused by this agent. It occurred in May 2020 and involved a 58-year-old woman with a history of severe endometriosis and multiple complications in the gastrointestinal tract. The patient’s condition improved favorably thanks to antifungal therapy with caspofungin and liposomal amphotericin B.
However, 3 months after that episode, the group reported an outbreak of C. auris at the same hospital in 12 critically ill patients co-infected with SARS-CoV-2. All were on mechanical ventilation, had peripherally inserted central catheters and urinary catheters, and had a prolonged hospital stay (20-70 days). The mortality in patients with candidemia in this cohort was 83.3%.
Open Ending
As seen in some science fiction series, fungal infections in the region still have an open ending, and Global Action For Fungal Infections (GAFFI) has estimated that with better diagnostics and treatments, deaths caused by fungi could decrease to less than 750,000 per year worldwide.
But if everything continues as is, some aspects of what is to come may resemble the dystopia depicted in The Last of Us. No zombies, but emerging and reemerging fungi in a chaotic distribution, and resistant to all established treatments.
“The risk factors of patients and their immune status, combined with the behavior of mycoses, bring a complicated scenario. But therapeutic failure resulting from multidrug resistance to antifungals could make it catastrophic,” Dr. Sánchez Paredes summarized.
At the moment, there are only four families of drugs capable of counteracting fungal infections — and as mentioned, some are already scarce in Latin America’s hospital pharmacies.
“Historically, fungal infections have been given less importance than those caused by viruses or bacteria. Even in some developed countries, the true extent of morbidity and mortality they present is unknown. This results in less investment in the development of new antifungal molecules because knowledge is lacking about the incidence and prevalence of these diseases,” Dr. Treviño Rangel pointed out.
He added that the main limitation for the development of new drugs is economic. “Unfortunately, not many pharmaceutical companies are willing to invest in the development of new antifungals, and there are no government programs specifically promoting and supporting research into new therapeutic options against these neglected diseases,” he asserted.
Development of vaccines to prevent fungal infections faces the same barriers. Although, according to Dr. Treviño Rangel, the difficulties are compounded by the great similarity between fungal cells and human cells. This makes it possible for harmful cross-reactivity to occur. In addition, because most severe fungal infections occur in individuals with immunosuppression, a vaccine would need to trigger an adequate immune response despite this issue.
Meanwhile, fungi quietly continue to do what they do best: resist and survive. For millions of years, they have mutated and adapted to new environments. Some theories even blame them for the extinction of dinosaurs and the subsequent rise of mammals. They exist on the edge of life and death, decomposing and creating. There is consensus that at the moment, it does not seem feasible for them to generate a pandemic like the one due to SARS-CoV-2, given their transmission mechanism. But who is willing to rule out that this may not happen in the long or medium term?
Dr. Sánchez Paredes, Dr. Treviño Rangel, Dr. Messina, Dr. Santiso, Dr. Álvarez, and Dr. Segundo Zaragoza have declared no relevant financial conflicts of interest.
This story was translated from Medscape Spanish Edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Roche Blood Test for Lp(a) Designated Breakthrough Device
The Tina-quant Lp(a) RxDx assay, developed by Roche in partnership with Amgen, is designed to identify adults with elevated Lp(a) levels who may benefit from lipid-lowering therapies currently in development.
Lp(a) is a type of lipoprotein that is genetically inherited. Elevated levels have been associated with an increased risk for heart disease, stroke, and other blood vessel diseases.
Worldwide, about 1 in 5 people have high Lp(a) levels that are not significantly affected by lifestyle changes, such as diet and exercise. Elevated Lp(a) is particularly prevalent among women and people of African descent.
Lp(a) testing is “an important tool for clinicians, enabling them to make a more accurate assessment of [cardiovascular] risk, and it is expected to become a part of regular diagnostic testing in the coming years,” Roche said in a news release announcing the breakthrough designation for the Lp(a) blood test.
If approved, the Tina-quant Lp(a) RxDx assay will be available on select Roche cobas platforms, the company reported.
Although low-density-lipoprotein (LDL) cholesterol particles are much more abundant than Lp(a) particles and carry the greatest overall risk for heart disease, on a per-particle basis, atherogenic risk associated with Lp(a) is about six times higher than that associated with LDL cholesterol, a recent study showed.
There currently are no approved pharmacologic therapies to lower Lp(a) levels in the United States, but several hopefuls are in development.
One is zerlasiran (Silence Therapeutics), a short interfering RNA (siRNA) agent, or “gene silencing” therapy, which binds to and temporarily blocks the action of the LPA gene, which encodes for apolipoprotein A, a dominant and rate-limiting component in the hepatic synthesis of the Lp(a) particle.
Treatment with zerlasiran produced significant and sustained reductions in Lp(a) concentrations in adults with elevated Lp(a) in the phase 1 APOLLO trial and the phase 2 ALPACAR-360 trial.
Other siRNA agents in development to lower Lp(a) levels include pelacarsen, lepodisiran, olpasiran, and muvalaplin.
A version of this article appeared on Medscape.com.
The Tina-quant Lp(a) RxDx assay, developed by Roche in partnership with Amgen, is designed to identify adults with elevated Lp(a) levels who may benefit from lipid-lowering therapies currently in development.
Lp(a) is a type of lipoprotein that is genetically inherited. Elevated levels have been associated with an increased risk for heart disease, stroke, and other blood vessel diseases.
Worldwide, about 1 in 5 people have high Lp(a) levels that are not significantly affected by lifestyle changes, such as diet and exercise. Elevated Lp(a) is particularly prevalent among women and people of African descent.
Lp(a) testing is “an important tool for clinicians, enabling them to make a more accurate assessment of [cardiovascular] risk, and it is expected to become a part of regular diagnostic testing in the coming years,” Roche said in a news release announcing the breakthrough designation for the Lp(a) blood test.
If approved, the Tina-quant Lp(a) RxDx assay will be available on select Roche cobas platforms, the company reported.
Although low-density-lipoprotein (LDL) cholesterol particles are much more abundant than Lp(a) particles and carry the greatest overall risk for heart disease, on a per-particle basis, atherogenic risk associated with Lp(a) is about six times higher than that associated with LDL cholesterol, a recent study showed.
There currently are no approved pharmacologic therapies to lower Lp(a) levels in the United States, but several hopefuls are in development.
One is zerlasiran (Silence Therapeutics), a short interfering RNA (siRNA) agent, or “gene silencing” therapy, which binds to and temporarily blocks the action of the LPA gene, which encodes for apolipoprotein A, a dominant and rate-limiting component in the hepatic synthesis of the Lp(a) particle.
Treatment with zerlasiran produced significant and sustained reductions in Lp(a) concentrations in adults with elevated Lp(a) in the phase 1 APOLLO trial and the phase 2 ALPACAR-360 trial.
Other siRNA agents in development to lower Lp(a) levels include pelacarsen, lepodisiran, olpasiran, and muvalaplin.
A version of this article appeared on Medscape.com.
The Tina-quant Lp(a) RxDx assay, developed by Roche in partnership with Amgen, is designed to identify adults with elevated Lp(a) levels who may benefit from lipid-lowering therapies currently in development.
Lp(a) is a type of lipoprotein that is genetically inherited. Elevated levels have been associated with an increased risk for heart disease, stroke, and other blood vessel diseases.
Worldwide, about 1 in 5 people have high Lp(a) levels that are not significantly affected by lifestyle changes, such as diet and exercise. Elevated Lp(a) is particularly prevalent among women and people of African descent.
Lp(a) testing is “an important tool for clinicians, enabling them to make a more accurate assessment of [cardiovascular] risk, and it is expected to become a part of regular diagnostic testing in the coming years,” Roche said in a news release announcing the breakthrough designation for the Lp(a) blood test.
If approved, the Tina-quant Lp(a) RxDx assay will be available on select Roche cobas platforms, the company reported.
Although low-density-lipoprotein (LDL) cholesterol particles are much more abundant than Lp(a) particles and carry the greatest overall risk for heart disease, on a per-particle basis, atherogenic risk associated with Lp(a) is about six times higher than that associated with LDL cholesterol, a recent study showed.
There currently are no approved pharmacologic therapies to lower Lp(a) levels in the United States, but several hopefuls are in development.
One is zerlasiran (Silence Therapeutics), a short interfering RNA (siRNA) agent, or “gene silencing” therapy, which binds to and temporarily blocks the action of the LPA gene, which encodes for apolipoprotein A, a dominant and rate-limiting component in the hepatic synthesis of the Lp(a) particle.
Treatment with zerlasiran produced significant and sustained reductions in Lp(a) concentrations in adults with elevated Lp(a) in the phase 1 APOLLO trial and the phase 2 ALPACAR-360 trial.
Other siRNA agents in development to lower Lp(a) levels include pelacarsen, lepodisiran, olpasiran, and muvalaplin.
A version of this article appeared on Medscape.com.
Right heart catheterization practice patterns in pulmonary hypertension in the US
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Pulmonary Vascular Disease Section
While these cutoffs are straightforward, a gap in practical application is evidenced by considerable variability in how PH providers perform and interpret RHC hemodynamic information.
A recent survey of 145 PH providers conducted by CHEST’s Pulmonary Vascular Disease Section shed light on the current RHC practices in the US.2 Regarding the respondents’ characteristics, 85% were in the 30-60 age range, 68% were males, and 71% were pulmonologists.
About half of the providers perform the RHC themselves. Most review the hemodynamic tracings, but up to 21% rely on the final report alone. Regarding PCWP, most (86%) obtain it during end-expiration, but only 42% routinely measure a PCWP saturation for confirmation. When faced with PVR discrepancies between thermodilution and indirect Fick (IFick), up to 30% chose either IFick or didn’t know which one to trust. Nearly 20% repeat the RHC at least annually, and 80% whenever the patient declines.
This study provides the largest reported data on real-world RHC practices by PH physicians in the US. We found significant variability in hemodynamic interpretation. Standardization of RHC performance and hemodynamic evaluation is crucial to ensure appropriate PH management.
– Abubakr A. Bajwa, MBBS, FCCP
Member-at-Large
– Samantha Pettigrew, MD
Fellow-in-Training
– Francisco J. Soto, MD, MS, FCCP
Section Vice Chair
References
1. Simonneau et al. Eur Resp J. 2019;53(1):1801913
2. Soto et al. CHEST. 2023;164(4):Supplement A5832-A5834
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Pulmonary Vascular Disease Section
While these cutoffs are straightforward, a gap in practical application is evidenced by considerable variability in how PH providers perform and interpret RHC hemodynamic information.
A recent survey of 145 PH providers conducted by CHEST’s Pulmonary Vascular Disease Section shed light on the current RHC practices in the US.2 Regarding the respondents’ characteristics, 85% were in the 30-60 age range, 68% were males, and 71% were pulmonologists.
About half of the providers perform the RHC themselves. Most review the hemodynamic tracings, but up to 21% rely on the final report alone. Regarding PCWP, most (86%) obtain it during end-expiration, but only 42% routinely measure a PCWP saturation for confirmation. When faced with PVR discrepancies between thermodilution and indirect Fick (IFick), up to 30% chose either IFick or didn’t know which one to trust. Nearly 20% repeat the RHC at least annually, and 80% whenever the patient declines.
This study provides the largest reported data on real-world RHC practices by PH physicians in the US. We found significant variability in hemodynamic interpretation. Standardization of RHC performance and hemodynamic evaluation is crucial to ensure appropriate PH management.
– Abubakr A. Bajwa, MBBS, FCCP
Member-at-Large
– Samantha Pettigrew, MD
Fellow-in-Training
– Francisco J. Soto, MD, MS, FCCP
Section Vice Chair
References
1. Simonneau et al. Eur Resp J. 2019;53(1):1801913
2. Soto et al. CHEST. 2023;164(4):Supplement A5832-A5834
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Pulmonary Vascular Disease Section
While these cutoffs are straightforward, a gap in practical application is evidenced by considerable variability in how PH providers perform and interpret RHC hemodynamic information.
A recent survey of 145 PH providers conducted by CHEST’s Pulmonary Vascular Disease Section shed light on the current RHC practices in the US.2 Regarding the respondents’ characteristics, 85% were in the 30-60 age range, 68% were males, and 71% were pulmonologists.
About half of the providers perform the RHC themselves. Most review the hemodynamic tracings, but up to 21% rely on the final report alone. Regarding PCWP, most (86%) obtain it during end-expiration, but only 42% routinely measure a PCWP saturation for confirmation. When faced with PVR discrepancies between thermodilution and indirect Fick (IFick), up to 30% chose either IFick or didn’t know which one to trust. Nearly 20% repeat the RHC at least annually, and 80% whenever the patient declines.
This study provides the largest reported data on real-world RHC practices by PH physicians in the US. We found significant variability in hemodynamic interpretation. Standardization of RHC performance and hemodynamic evaluation is crucial to ensure appropriate PH management.
– Abubakr A. Bajwa, MBBS, FCCP
Member-at-Large
– Samantha Pettigrew, MD
Fellow-in-Training
– Francisco J. Soto, MD, MS, FCCP
Section Vice Chair
References
1. Simonneau et al. Eur Resp J. 2019;53(1):1801913
2. Soto et al. CHEST. 2023;164(4):Supplement A5832-A5834