User login
New guideline: Address GTCS frequency to reduce SUDEP risk
BOSTON – Generalized tonic-clonic seizures (GTCS) are a major risk factor for sudden unexpected death in epilepsy (SUDEP), which underscores the importance of advising people with epilepsy about controlling such seizures, according to a new practice guideline from the American Academy of Neurology and the American Epilepsy Society.
Though SUDEP is rare, with an incidence rate of 0.22/1,000 patient-years in children with epilepsy and 1.2/1,000 patient-years in adults with epilepsy, the guideline committee found that people with three or more GTCS per year are 15 times more likely to die suddenly than are those without this seizure type. The risk increases with increasing GTCS frequency. This translates to an absolute risk of up to 18 deaths per 1,000 patient-years for people with epilepsy who have frequent GTCS.
Specifically, the guideline recommends that , she said, adding that the guideline shows that “being free of seizures, particularly tonic-clonic seizures, is strongly associated with a decreased risk.”
Guideline coauthor, Elizabeth Donner, MD, added that, for this reason, the guideline recommends “that health professionals work with people who continue to have, specifically, these kind of seizures to try and reduce them with medications or with epilepsy surgery, actively weighing the risks and benefits of any new approach to seizure management.”
The recommendations are based on moderate (Level B) evidence.
The team also looked at numerous other risk factors for SUDEP and found that the strength of the evidence was too weak to support additional recommendations, said Dr. Donner, director of the comprehensive epilepsy program at The Hospital for Sick Children in Toronto and chair of the American Epilepsy Society SUDEP Task Force.
“More research is now needed to identify other preventable risk factors for SUDEP so that we can focus future studies on finding ways to reduce how often SUDEP occurs,” she added.
While the message regarding the importance of reducing seizure frequency is not new, it is important that this message be reiterated in the context of SUDEP, Dr. Donner said.
“It’s very important for it to be clear that the risk of frequent generalized tonic-clonic seizures – and we’re not talking about really frequent here; we’re talking about significant increased risk of death with only three per year – is not related only to maintaining a driver’s license, maintaining work, or other outcomes like that. It’s actually related to risk of death,” she said, noting that she hopes this is a motivator for pursuing treatments beyond medication when medication isn’t successful for treating seizures.
The guideline, which is endorsed by the International Child Neurology Association, is available online and in print (Neurology. 2017;88:1674–80).
Dr. Harden receives royalties from Wiley and Up-to-Date. Dr. Donner has received research support from the Canadian Institutes of Health Research, Dravet Canada, and SUDEP Aware. Other guideline authors reported numerous disclosures, including many industry sources.
BOSTON – Generalized tonic-clonic seizures (GTCS) are a major risk factor for sudden unexpected death in epilepsy (SUDEP), which underscores the importance of advising people with epilepsy about controlling such seizures, according to a new practice guideline from the American Academy of Neurology and the American Epilepsy Society.
Though SUDEP is rare, with an incidence rate of 0.22/1,000 patient-years in children with epilepsy and 1.2/1,000 patient-years in adults with epilepsy, the guideline committee found that people with three or more GTCS per year are 15 times more likely to die suddenly than are those without this seizure type. The risk increases with increasing GTCS frequency. This translates to an absolute risk of up to 18 deaths per 1,000 patient-years for people with epilepsy who have frequent GTCS.
Specifically, the guideline recommends that , she said, adding that the guideline shows that “being free of seizures, particularly tonic-clonic seizures, is strongly associated with a decreased risk.”
Guideline coauthor, Elizabeth Donner, MD, added that, for this reason, the guideline recommends “that health professionals work with people who continue to have, specifically, these kind of seizures to try and reduce them with medications or with epilepsy surgery, actively weighing the risks and benefits of any new approach to seizure management.”
The recommendations are based on moderate (Level B) evidence.
The team also looked at numerous other risk factors for SUDEP and found that the strength of the evidence was too weak to support additional recommendations, said Dr. Donner, director of the comprehensive epilepsy program at The Hospital for Sick Children in Toronto and chair of the American Epilepsy Society SUDEP Task Force.
“More research is now needed to identify other preventable risk factors for SUDEP so that we can focus future studies on finding ways to reduce how often SUDEP occurs,” she added.
While the message regarding the importance of reducing seizure frequency is not new, it is important that this message be reiterated in the context of SUDEP, Dr. Donner said.
“It’s very important for it to be clear that the risk of frequent generalized tonic-clonic seizures – and we’re not talking about really frequent here; we’re talking about significant increased risk of death with only three per year – is not related only to maintaining a driver’s license, maintaining work, or other outcomes like that. It’s actually related to risk of death,” she said, noting that she hopes this is a motivator for pursuing treatments beyond medication when medication isn’t successful for treating seizures.
The guideline, which is endorsed by the International Child Neurology Association, is available online and in print (Neurology. 2017;88:1674–80).
Dr. Harden receives royalties from Wiley and Up-to-Date. Dr. Donner has received research support from the Canadian Institutes of Health Research, Dravet Canada, and SUDEP Aware. Other guideline authors reported numerous disclosures, including many industry sources.
BOSTON – Generalized tonic-clonic seizures (GTCS) are a major risk factor for sudden unexpected death in epilepsy (SUDEP), which underscores the importance of advising people with epilepsy about controlling such seizures, according to a new practice guideline from the American Academy of Neurology and the American Epilepsy Society.
Though SUDEP is rare, with an incidence rate of 0.22/1,000 patient-years in children with epilepsy and 1.2/1,000 patient-years in adults with epilepsy, the guideline committee found that people with three or more GTCS per year are 15 times more likely to die suddenly than are those without this seizure type. The risk increases with increasing GTCS frequency. This translates to an absolute risk of up to 18 deaths per 1,000 patient-years for people with epilepsy who have frequent GTCS.
Specifically, the guideline recommends that , she said, adding that the guideline shows that “being free of seizures, particularly tonic-clonic seizures, is strongly associated with a decreased risk.”
Guideline coauthor, Elizabeth Donner, MD, added that, for this reason, the guideline recommends “that health professionals work with people who continue to have, specifically, these kind of seizures to try and reduce them with medications or with epilepsy surgery, actively weighing the risks and benefits of any new approach to seizure management.”
The recommendations are based on moderate (Level B) evidence.
The team also looked at numerous other risk factors for SUDEP and found that the strength of the evidence was too weak to support additional recommendations, said Dr. Donner, director of the comprehensive epilepsy program at The Hospital for Sick Children in Toronto and chair of the American Epilepsy Society SUDEP Task Force.
“More research is now needed to identify other preventable risk factors for SUDEP so that we can focus future studies on finding ways to reduce how often SUDEP occurs,” she added.
While the message regarding the importance of reducing seizure frequency is not new, it is important that this message be reiterated in the context of SUDEP, Dr. Donner said.
“It’s very important for it to be clear that the risk of frequent generalized tonic-clonic seizures – and we’re not talking about really frequent here; we’re talking about significant increased risk of death with only three per year – is not related only to maintaining a driver’s license, maintaining work, or other outcomes like that. It’s actually related to risk of death,” she said, noting that she hopes this is a motivator for pursuing treatments beyond medication when medication isn’t successful for treating seizures.
The guideline, which is endorsed by the International Child Neurology Association, is available online and in print (Neurology. 2017;88:1674–80).
Dr. Harden receives royalties from Wiley and Up-to-Date. Dr. Donner has received research support from the Canadian Institutes of Health Research, Dravet Canada, and SUDEP Aware. Other guideline authors reported numerous disclosures, including many industry sources.
AT AAN 2017
Guideline endorses doublet therapy after pancreatic cancer surgery in chemo-naive patients
A recommendation on postop adjuvant chemotherapy has been updated in the Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline, according to an article published online on April 11 in the Journal of Clinical Oncology.
In the absence of medical or surgical contraindications, all patients who have resected pancreatic cancer and did not receive preoperative therapy should be offered 6 months of adjuvant chemotherapy, according to the new recommendation. “The doublet regimen of gemcitabine and capecitabine is preferred in the absence of concerns for toxicity or tolerance; alternatively, monotherapy with gemcitabine or fluorouracil plus folinic acid can be offered. Adjuvant treatment should be initiated within 8 weeks of surgical resection, assuming complete recovery,” Alok A. Khorana, MD, of the Cleveland Clinic, and members of the guideline committee wrote.
The recommendation was based on results of the ESPAC-4 study, a multicenter, international, open-label randomized controlled phase III trial (Lancet. 2017;389:1011-24). ESPAC-4 compared adjuvant combination chemotherapy of gemcitabine and capecitabine with gemcitabine monotherapy in 730 evaluable patients with resected pancreatic ductal adenocarcinoma.
Median overall survival was 28 months (95% confidence interval, 23.5-31.5 months) in the doublet arm and 25.5 months (95% CI, 22.7 to 27.9 months) for gemcitabine alone (hazard ratio, 0.82; 95% CI, 0.68-0.98; P = .032). Grade 3 and 4 adverse events were similar in both arms, although higher rates of hand-foot syndrome and diarrhea occurred in patients randomly assigned to the doublet arm.
The remaining recommendations from the original 2016 ASCO guideline are unchanged.
The revised guideline is available at this link.
[email protected]
On Twitter @maryjodales
A recommendation on postop adjuvant chemotherapy has been updated in the Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline, according to an article published online on April 11 in the Journal of Clinical Oncology.
In the absence of medical or surgical contraindications, all patients who have resected pancreatic cancer and did not receive preoperative therapy should be offered 6 months of adjuvant chemotherapy, according to the new recommendation. “The doublet regimen of gemcitabine and capecitabine is preferred in the absence of concerns for toxicity or tolerance; alternatively, monotherapy with gemcitabine or fluorouracil plus folinic acid can be offered. Adjuvant treatment should be initiated within 8 weeks of surgical resection, assuming complete recovery,” Alok A. Khorana, MD, of the Cleveland Clinic, and members of the guideline committee wrote.
The recommendation was based on results of the ESPAC-4 study, a multicenter, international, open-label randomized controlled phase III trial (Lancet. 2017;389:1011-24). ESPAC-4 compared adjuvant combination chemotherapy of gemcitabine and capecitabine with gemcitabine monotherapy in 730 evaluable patients with resected pancreatic ductal adenocarcinoma.
Median overall survival was 28 months (95% confidence interval, 23.5-31.5 months) in the doublet arm and 25.5 months (95% CI, 22.7 to 27.9 months) for gemcitabine alone (hazard ratio, 0.82; 95% CI, 0.68-0.98; P = .032). Grade 3 and 4 adverse events were similar in both arms, although higher rates of hand-foot syndrome and diarrhea occurred in patients randomly assigned to the doublet arm.
The remaining recommendations from the original 2016 ASCO guideline are unchanged.
The revised guideline is available at this link.
[email protected]
On Twitter @maryjodales
A recommendation on postop adjuvant chemotherapy has been updated in the Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline, according to an article published online on April 11 in the Journal of Clinical Oncology.
In the absence of medical or surgical contraindications, all patients who have resected pancreatic cancer and did not receive preoperative therapy should be offered 6 months of adjuvant chemotherapy, according to the new recommendation. “The doublet regimen of gemcitabine and capecitabine is preferred in the absence of concerns for toxicity or tolerance; alternatively, monotherapy with gemcitabine or fluorouracil plus folinic acid can be offered. Adjuvant treatment should be initiated within 8 weeks of surgical resection, assuming complete recovery,” Alok A. Khorana, MD, of the Cleveland Clinic, and members of the guideline committee wrote.
The recommendation was based on results of the ESPAC-4 study, a multicenter, international, open-label randomized controlled phase III trial (Lancet. 2017;389:1011-24). ESPAC-4 compared adjuvant combination chemotherapy of gemcitabine and capecitabine with gemcitabine monotherapy in 730 evaluable patients with resected pancreatic ductal adenocarcinoma.
Median overall survival was 28 months (95% confidence interval, 23.5-31.5 months) in the doublet arm and 25.5 months (95% CI, 22.7 to 27.9 months) for gemcitabine alone (hazard ratio, 0.82; 95% CI, 0.68-0.98; P = .032). Grade 3 and 4 adverse events were similar in both arms, although higher rates of hand-foot syndrome and diarrhea occurred in patients randomly assigned to the doublet arm.
The remaining recommendations from the original 2016 ASCO guideline are unchanged.
The revised guideline is available at this link.
[email protected]
On Twitter @maryjodales
FROM THE JOURNAL OF CLINICAL ONCOLOGY
NCCN myelofibrosis guideline: Patient voice is key
ORLANDO – Referral to a specialized center with expertise in the management of myeloproliferative neoplasms is strongly recommended for all patients diagnosed with myelofibrosis, according to a new treatment guideline from the National Comprehensive Cancer Network.
The guideline is the first in a series addressing myeloproliferative neoplasms (MPNs), and it focuses on the diagnostic work-up of MPNs, as well as the treatment of myelofibrosis. The guideline panel, led by panel chair Ruben A. Mesa, MD, is working next on guidelines for the other two “core classic” Philadelphia chromosome–negative MPNs: polycythemia vera, and essential thrombocythemia.
Nearly two-thirds of myelofibrosis patients have intermediate-risk 2 or high-risk disease, and treatment decisions in these patients are complex and require patient input – particularly in candidates for allogeneic hematopoietic stem cell transplantation, he said.
“These diseases can be a little different than other malignant diseases,” Dr. Mesa said, explaining that while there is a clear risk of progression to acute myeloid leukemia, and from polycythemia vera and essential thrombocythemia to myelofibrosis, and while the diseases can be fatal, the burden patients face is not solely related to mortality.
There are implications in terms of health that are independent of that, such as the risk of thrombosis and bleeding, the potential for cytopenia, and severe splenomegaly that results in significant symptoms, he said.
Further, while molecular mutations and their implications for prognosis are a “rapidly moving part of the discussion,” the care of patients with MPNs involves far more than a molecular understanding of the disease.
In fact, the role of molecular changes in these patients is speculative, he said.
While such changes can be assessed and used for patient stratification, their role in myelofibrosis – unlike in other diseases such as chronic myeloid leukemia where the level of change in a target gene is highly relevant and prognostic, is not yet clear.
Thus, a core aspect of the guideline is inclusion of the voice of the patient in individualizing care, he said, noting that many factors should be considered, including how well the patient metabolizes drugs, and the symptom profile, psychosocial circumstances, support structure, and personal beliefs.
“It’s not solely about the tumor,” he stressed.
In fact, the answer to the question of whether a patient can be symptomatic enough to require a specific treatment is “no,” because of the potential for side effects, risk, expense, and other considerations.
“So the voice of the patient is always a key part [of the decision],” he said, noting also that as with all NCCN guidelines, this guideline is a partnership with the treating physician; deciding who is a transplant candidate is a nuanced issue for which the panel provides “discussion and guidance.”
“But clearly, these guidelines are the most useful and helpful in the setting of experienced providers bringing all of their experiences to bear,” he said.
In general, however, the guidelines call for allogeneic hematopoietic stem cell transplantation (HCT) in those with intermediate-risk 2 or high-risk disease who are transplant candidates, and treatment based on assessment of symptom burden (using the MPN–Symptom Assessment Form Total Symptom Score–10 Items) in those who are not HCT candidates. Those with platelets at 50,000 or below should be considered for clinical trial enrollment, and those with platelets above 50,000 should be considered for a clinical trial or treatment with the oral JAK1 and JAK2 inhibitor ruxolitinib, which has been shown to have beneficial effects on both symptoms and survival and which is approved for patients with platelets above 50,000. .
Treated patients should be monitored for response and for signs and symptoms of disease progression every 3-6 months. Treatment should continue in those who respond, as well as in those who do not – as long as there is no disease progression.
Those with progressive disease include patients who are moving toward acute leukemia, and those with overt acute leukemia.
“Here is where the key decision occurs. Are they or are they not a transplant candidate? If they are a candidate, we have a potentially curative track which would include cytoreduction followed by transplant,” Dr. Mesa said.
Cytoreduction can involve hypomethylating agents if the patient doesn’t have excess blast cells or too high a burden of disease.
Acute myeloid leukemia–like induction chemotherapy followed by allogeneic HCT is also an option in these patients.
As for treatment of low-risk myelofibrosis, the guideline states that asymptomatic patients can be observed or enrolled in a clinical trial and monitored for progression every 3-6 months, and that symptomatic patients should receive ruxolitinib or interferons (which are used off label), or be enrolled in a clinical trial. Treatment is important for patients with particularly difficult symptoms, he said, noting that some patients have had pruritus so severe that they have committed suicide. Treatment should continue unless monitoring shows signs of progression to intermediate risk 1, intermediate risk 2/high-risk, or advanced stage disease.
For those with intermediate risk 1 disease, the guideline calls for observation or ruxolitinib in those who are symptomatic, or clinical trial enrollment or allogeneic HCT. Treatment should continue unless monitoring shows disease progression, in which case the appropriate algorithm should be considered.
The guideline also addresses several special circumstances, including the management of anemia in myelofibrosis patients, which can be a difficult issue, he said.
Since the guideline was first published in December, two updates have been incorporated, and Dr. Mesa said that he anticipates regular updates given the rapidly evolving understanding of MPNs and new findings with respect to potential treatment strategies.
He noted that a number of drugs are currently in clinical trials involving patients with myelofibrosis, including the JAK2/FLT3 inhibitor pacritinib, the JAK1/JAK2 inhibitor momelotinib, the active antifibrosing agent PRM-151, and the telomerase inhibitor imetelstat, as well as numerous drug combinations.
Going forward, the guideline panel will be focusing on four different areas of assessment, including new therapies and new genetic therapies, improving transplant outcomes, MPN symptom and quality of life assessment, and nonpharmacologic interventions such as yoga.
“We certainly hope to complement things over time, to look not only at pharmacologic interventions, but others that patients may be able to utilize from a toolkit of resources,” he said.
COMFORT-1 update: ruxolitinib responses durable in myelofibrosis
To date, ruxolitinib is the only Food and Drug Administration–approved drug for the treatment of myelofibrosis.
The randomized controlled phase III COMFORT I and II trials conducted in the United States and Europe, respectively, demonstrated that the oral JAK1/JAK2 inhibitor has a rapid, beneficial impact on both survival and disease-associated enlargement of the spleen and improvement in related symptoms, Dr. Mesa said.
A 5-year update on data from 309 patients in the COMFORT-1 trial, as reported at the annual meeting of the American Society of Clinical Oncology in 2016, confirmed the durability of treatment responses to ruxolitinib in patients initially randomized to receive the drug, he said.
“We were able to demonstrate a continued survival advantage for those individuals receiving ruxolitinib,” he added.
At weeks 24 and 264, the mean spleen volume reduction was 31.6% and 37.6%, respectively, in those originally randomized to ruxolitinib. The median duration of at least 35% spleen volume reduction was 168.3 weeks.
Overall survival favored ruxolitinib (hazard ratio, 0.69). Median overall survival in the ruxolitinib group had not yet been reached.
“But we realize our work is not done. The survival curve does not plateau; we are not curing these patients. We’re having meaningful impact, but we have room to continue to improve,” he said.
Also, there is an initial drop in platelet counts that tends to stabilize, but not improve, and there is worsening of anemia (new onset grade 3 or 4 anemia was 25.2% with ruxolitinib, and 26.1% in 111 of 154 patients who crossed over from the placebo group), and although this tends to improve, these are among areas of unmet need, he added.
Further, long-term risks of treatment include cutaneous malignancies (basal cell carcinoma occurred in 7.7% and 9.0% of treatment and crossover patients, respectively), which are difficult to separate from baseline hydroxyurea use, and increased risk of herpes zoster (which occurred in 10.3% and 13.5% of treated and crossover patients).
However, there appears to be no increased risk – and there may be a slight decreased risk – of progression to acute leukemia, Dr. Mesa said.
Dr. Mesa disclosed that he has received consulting fees, honoraria, and/or grant/research support from ARIAD Pharmaceuticals, Celgene, CTI BioPharma, Galena Biopharma, Gilead Sciences, Incyte, Novartis Pharmaceuticals, and Promedior.
A step toward harmonizing treatment
Myelofibrosis is a rare chronic leukemia with a complex biology. Disease heterogeneity poses several challenges in the appropriate selection and timing of treatments in this disorder. The NCCN Practice Guidelines in Myelofibrosis is an important step towards harmonizing clinical practice for treating this disease and improving the care of patients.
Vikas Gupta, MD, FRCP, FRCPath, is Director of The Elizabeth and Tony Comper MPN Program at Princess Margaret Cancer Centre in Toronto and a member of the editorial advisory board of Hematology News.
A step toward harmonizing treatment
Myelofibrosis is a rare chronic leukemia with a complex biology. Disease heterogeneity poses several challenges in the appropriate selection and timing of treatments in this disorder. The NCCN Practice Guidelines in Myelofibrosis is an important step towards harmonizing clinical practice for treating this disease and improving the care of patients.
Vikas Gupta, MD, FRCP, FRCPath, is Director of The Elizabeth and Tony Comper MPN Program at Princess Margaret Cancer Centre in Toronto and a member of the editorial advisory board of Hematology News.
A step toward harmonizing treatment
Myelofibrosis is a rare chronic leukemia with a complex biology. Disease heterogeneity poses several challenges in the appropriate selection and timing of treatments in this disorder. The NCCN Practice Guidelines in Myelofibrosis is an important step towards harmonizing clinical practice for treating this disease and improving the care of patients.
Vikas Gupta, MD, FRCP, FRCPath, is Director of The Elizabeth and Tony Comper MPN Program at Princess Margaret Cancer Centre in Toronto and a member of the editorial advisory board of Hematology News.
ORLANDO – Referral to a specialized center with expertise in the management of myeloproliferative neoplasms is strongly recommended for all patients diagnosed with myelofibrosis, according to a new treatment guideline from the National Comprehensive Cancer Network.
The guideline is the first in a series addressing myeloproliferative neoplasms (MPNs), and it focuses on the diagnostic work-up of MPNs, as well as the treatment of myelofibrosis. The guideline panel, led by panel chair Ruben A. Mesa, MD, is working next on guidelines for the other two “core classic” Philadelphia chromosome–negative MPNs: polycythemia vera, and essential thrombocythemia.
Nearly two-thirds of myelofibrosis patients have intermediate-risk 2 or high-risk disease, and treatment decisions in these patients are complex and require patient input – particularly in candidates for allogeneic hematopoietic stem cell transplantation, he said.
“These diseases can be a little different than other malignant diseases,” Dr. Mesa said, explaining that while there is a clear risk of progression to acute myeloid leukemia, and from polycythemia vera and essential thrombocythemia to myelofibrosis, and while the diseases can be fatal, the burden patients face is not solely related to mortality.
There are implications in terms of health that are independent of that, such as the risk of thrombosis and bleeding, the potential for cytopenia, and severe splenomegaly that results in significant symptoms, he said.
Further, while molecular mutations and their implications for prognosis are a “rapidly moving part of the discussion,” the care of patients with MPNs involves far more than a molecular understanding of the disease.
In fact, the role of molecular changes in these patients is speculative, he said.
While such changes can be assessed and used for patient stratification, their role in myelofibrosis – unlike in other diseases such as chronic myeloid leukemia where the level of change in a target gene is highly relevant and prognostic, is not yet clear.
Thus, a core aspect of the guideline is inclusion of the voice of the patient in individualizing care, he said, noting that many factors should be considered, including how well the patient metabolizes drugs, and the symptom profile, psychosocial circumstances, support structure, and personal beliefs.
“It’s not solely about the tumor,” he stressed.
In fact, the answer to the question of whether a patient can be symptomatic enough to require a specific treatment is “no,” because of the potential for side effects, risk, expense, and other considerations.
“So the voice of the patient is always a key part [of the decision],” he said, noting also that as with all NCCN guidelines, this guideline is a partnership with the treating physician; deciding who is a transplant candidate is a nuanced issue for which the panel provides “discussion and guidance.”
“But clearly, these guidelines are the most useful and helpful in the setting of experienced providers bringing all of their experiences to bear,” he said.
In general, however, the guidelines call for allogeneic hematopoietic stem cell transplantation (HCT) in those with intermediate-risk 2 or high-risk disease who are transplant candidates, and treatment based on assessment of symptom burden (using the MPN–Symptom Assessment Form Total Symptom Score–10 Items) in those who are not HCT candidates. Those with platelets at 50,000 or below should be considered for clinical trial enrollment, and those with platelets above 50,000 should be considered for a clinical trial or treatment with the oral JAK1 and JAK2 inhibitor ruxolitinib, which has been shown to have beneficial effects on both symptoms and survival and which is approved for patients with platelets above 50,000. .
Treated patients should be monitored for response and for signs and symptoms of disease progression every 3-6 months. Treatment should continue in those who respond, as well as in those who do not – as long as there is no disease progression.
Those with progressive disease include patients who are moving toward acute leukemia, and those with overt acute leukemia.
“Here is where the key decision occurs. Are they or are they not a transplant candidate? If they are a candidate, we have a potentially curative track which would include cytoreduction followed by transplant,” Dr. Mesa said.
Cytoreduction can involve hypomethylating agents if the patient doesn’t have excess blast cells or too high a burden of disease.
Acute myeloid leukemia–like induction chemotherapy followed by allogeneic HCT is also an option in these patients.
As for treatment of low-risk myelofibrosis, the guideline states that asymptomatic patients can be observed or enrolled in a clinical trial and monitored for progression every 3-6 months, and that symptomatic patients should receive ruxolitinib or interferons (which are used off label), or be enrolled in a clinical trial. Treatment is important for patients with particularly difficult symptoms, he said, noting that some patients have had pruritus so severe that they have committed suicide. Treatment should continue unless monitoring shows signs of progression to intermediate risk 1, intermediate risk 2/high-risk, or advanced stage disease.
For those with intermediate risk 1 disease, the guideline calls for observation or ruxolitinib in those who are symptomatic, or clinical trial enrollment or allogeneic HCT. Treatment should continue unless monitoring shows disease progression, in which case the appropriate algorithm should be considered.
The guideline also addresses several special circumstances, including the management of anemia in myelofibrosis patients, which can be a difficult issue, he said.
Since the guideline was first published in December, two updates have been incorporated, and Dr. Mesa said that he anticipates regular updates given the rapidly evolving understanding of MPNs and new findings with respect to potential treatment strategies.
He noted that a number of drugs are currently in clinical trials involving patients with myelofibrosis, including the JAK2/FLT3 inhibitor pacritinib, the JAK1/JAK2 inhibitor momelotinib, the active antifibrosing agent PRM-151, and the telomerase inhibitor imetelstat, as well as numerous drug combinations.
Going forward, the guideline panel will be focusing on four different areas of assessment, including new therapies and new genetic therapies, improving transplant outcomes, MPN symptom and quality of life assessment, and nonpharmacologic interventions such as yoga.
“We certainly hope to complement things over time, to look not only at pharmacologic interventions, but others that patients may be able to utilize from a toolkit of resources,” he said.
COMFORT-1 update: ruxolitinib responses durable in myelofibrosis
To date, ruxolitinib is the only Food and Drug Administration–approved drug for the treatment of myelofibrosis.
The randomized controlled phase III COMFORT I and II trials conducted in the United States and Europe, respectively, demonstrated that the oral JAK1/JAK2 inhibitor has a rapid, beneficial impact on both survival and disease-associated enlargement of the spleen and improvement in related symptoms, Dr. Mesa said.
A 5-year update on data from 309 patients in the COMFORT-1 trial, as reported at the annual meeting of the American Society of Clinical Oncology in 2016, confirmed the durability of treatment responses to ruxolitinib in patients initially randomized to receive the drug, he said.
“We were able to demonstrate a continued survival advantage for those individuals receiving ruxolitinib,” he added.
At weeks 24 and 264, the mean spleen volume reduction was 31.6% and 37.6%, respectively, in those originally randomized to ruxolitinib. The median duration of at least 35% spleen volume reduction was 168.3 weeks.
Overall survival favored ruxolitinib (hazard ratio, 0.69). Median overall survival in the ruxolitinib group had not yet been reached.
“But we realize our work is not done. The survival curve does not plateau; we are not curing these patients. We’re having meaningful impact, but we have room to continue to improve,” he said.
Also, there is an initial drop in platelet counts that tends to stabilize, but not improve, and there is worsening of anemia (new onset grade 3 or 4 anemia was 25.2% with ruxolitinib, and 26.1% in 111 of 154 patients who crossed over from the placebo group), and although this tends to improve, these are among areas of unmet need, he added.
Further, long-term risks of treatment include cutaneous malignancies (basal cell carcinoma occurred in 7.7% and 9.0% of treatment and crossover patients, respectively), which are difficult to separate from baseline hydroxyurea use, and increased risk of herpes zoster (which occurred in 10.3% and 13.5% of treated and crossover patients).
However, there appears to be no increased risk – and there may be a slight decreased risk – of progression to acute leukemia, Dr. Mesa said.
Dr. Mesa disclosed that he has received consulting fees, honoraria, and/or grant/research support from ARIAD Pharmaceuticals, Celgene, CTI BioPharma, Galena Biopharma, Gilead Sciences, Incyte, Novartis Pharmaceuticals, and Promedior.
ORLANDO – Referral to a specialized center with expertise in the management of myeloproliferative neoplasms is strongly recommended for all patients diagnosed with myelofibrosis, according to a new treatment guideline from the National Comprehensive Cancer Network.
The guideline is the first in a series addressing myeloproliferative neoplasms (MPNs), and it focuses on the diagnostic work-up of MPNs, as well as the treatment of myelofibrosis. The guideline panel, led by panel chair Ruben A. Mesa, MD, is working next on guidelines for the other two “core classic” Philadelphia chromosome–negative MPNs: polycythemia vera, and essential thrombocythemia.
Nearly two-thirds of myelofibrosis patients have intermediate-risk 2 or high-risk disease, and treatment decisions in these patients are complex and require patient input – particularly in candidates for allogeneic hematopoietic stem cell transplantation, he said.
“These diseases can be a little different than other malignant diseases,” Dr. Mesa said, explaining that while there is a clear risk of progression to acute myeloid leukemia, and from polycythemia vera and essential thrombocythemia to myelofibrosis, and while the diseases can be fatal, the burden patients face is not solely related to mortality.
There are implications in terms of health that are independent of that, such as the risk of thrombosis and bleeding, the potential for cytopenia, and severe splenomegaly that results in significant symptoms, he said.
Further, while molecular mutations and their implications for prognosis are a “rapidly moving part of the discussion,” the care of patients with MPNs involves far more than a molecular understanding of the disease.
In fact, the role of molecular changes in these patients is speculative, he said.
While such changes can be assessed and used for patient stratification, their role in myelofibrosis – unlike in other diseases such as chronic myeloid leukemia where the level of change in a target gene is highly relevant and prognostic, is not yet clear.
Thus, a core aspect of the guideline is inclusion of the voice of the patient in individualizing care, he said, noting that many factors should be considered, including how well the patient metabolizes drugs, and the symptom profile, psychosocial circumstances, support structure, and personal beliefs.
“It’s not solely about the tumor,” he stressed.
In fact, the answer to the question of whether a patient can be symptomatic enough to require a specific treatment is “no,” because of the potential for side effects, risk, expense, and other considerations.
“So the voice of the patient is always a key part [of the decision],” he said, noting also that as with all NCCN guidelines, this guideline is a partnership with the treating physician; deciding who is a transplant candidate is a nuanced issue for which the panel provides “discussion and guidance.”
“But clearly, these guidelines are the most useful and helpful in the setting of experienced providers bringing all of their experiences to bear,” he said.
In general, however, the guidelines call for allogeneic hematopoietic stem cell transplantation (HCT) in those with intermediate-risk 2 or high-risk disease who are transplant candidates, and treatment based on assessment of symptom burden (using the MPN–Symptom Assessment Form Total Symptom Score–10 Items) in those who are not HCT candidates. Those with platelets at 50,000 or below should be considered for clinical trial enrollment, and those with platelets above 50,000 should be considered for a clinical trial or treatment with the oral JAK1 and JAK2 inhibitor ruxolitinib, which has been shown to have beneficial effects on both symptoms and survival and which is approved for patients with platelets above 50,000. .
Treated patients should be monitored for response and for signs and symptoms of disease progression every 3-6 months. Treatment should continue in those who respond, as well as in those who do not – as long as there is no disease progression.
Those with progressive disease include patients who are moving toward acute leukemia, and those with overt acute leukemia.
“Here is where the key decision occurs. Are they or are they not a transplant candidate? If they are a candidate, we have a potentially curative track which would include cytoreduction followed by transplant,” Dr. Mesa said.
Cytoreduction can involve hypomethylating agents if the patient doesn’t have excess blast cells or too high a burden of disease.
Acute myeloid leukemia–like induction chemotherapy followed by allogeneic HCT is also an option in these patients.
As for treatment of low-risk myelofibrosis, the guideline states that asymptomatic patients can be observed or enrolled in a clinical trial and monitored for progression every 3-6 months, and that symptomatic patients should receive ruxolitinib or interferons (which are used off label), or be enrolled in a clinical trial. Treatment is important for patients with particularly difficult symptoms, he said, noting that some patients have had pruritus so severe that they have committed suicide. Treatment should continue unless monitoring shows signs of progression to intermediate risk 1, intermediate risk 2/high-risk, or advanced stage disease.
For those with intermediate risk 1 disease, the guideline calls for observation or ruxolitinib in those who are symptomatic, or clinical trial enrollment or allogeneic HCT. Treatment should continue unless monitoring shows disease progression, in which case the appropriate algorithm should be considered.
The guideline also addresses several special circumstances, including the management of anemia in myelofibrosis patients, which can be a difficult issue, he said.
Since the guideline was first published in December, two updates have been incorporated, and Dr. Mesa said that he anticipates regular updates given the rapidly evolving understanding of MPNs and new findings with respect to potential treatment strategies.
He noted that a number of drugs are currently in clinical trials involving patients with myelofibrosis, including the JAK2/FLT3 inhibitor pacritinib, the JAK1/JAK2 inhibitor momelotinib, the active antifibrosing agent PRM-151, and the telomerase inhibitor imetelstat, as well as numerous drug combinations.
Going forward, the guideline panel will be focusing on four different areas of assessment, including new therapies and new genetic therapies, improving transplant outcomes, MPN symptom and quality of life assessment, and nonpharmacologic interventions such as yoga.
“We certainly hope to complement things over time, to look not only at pharmacologic interventions, but others that patients may be able to utilize from a toolkit of resources,” he said.
COMFORT-1 update: ruxolitinib responses durable in myelofibrosis
To date, ruxolitinib is the only Food and Drug Administration–approved drug for the treatment of myelofibrosis.
The randomized controlled phase III COMFORT I and II trials conducted in the United States and Europe, respectively, demonstrated that the oral JAK1/JAK2 inhibitor has a rapid, beneficial impact on both survival and disease-associated enlargement of the spleen and improvement in related symptoms, Dr. Mesa said.
A 5-year update on data from 309 patients in the COMFORT-1 trial, as reported at the annual meeting of the American Society of Clinical Oncology in 2016, confirmed the durability of treatment responses to ruxolitinib in patients initially randomized to receive the drug, he said.
“We were able to demonstrate a continued survival advantage for those individuals receiving ruxolitinib,” he added.
At weeks 24 and 264, the mean spleen volume reduction was 31.6% and 37.6%, respectively, in those originally randomized to ruxolitinib. The median duration of at least 35% spleen volume reduction was 168.3 weeks.
Overall survival favored ruxolitinib (hazard ratio, 0.69). Median overall survival in the ruxolitinib group had not yet been reached.
“But we realize our work is not done. The survival curve does not plateau; we are not curing these patients. We’re having meaningful impact, but we have room to continue to improve,” he said.
Also, there is an initial drop in platelet counts that tends to stabilize, but not improve, and there is worsening of anemia (new onset grade 3 or 4 anemia was 25.2% with ruxolitinib, and 26.1% in 111 of 154 patients who crossed over from the placebo group), and although this tends to improve, these are among areas of unmet need, he added.
Further, long-term risks of treatment include cutaneous malignancies (basal cell carcinoma occurred in 7.7% and 9.0% of treatment and crossover patients, respectively), which are difficult to separate from baseline hydroxyurea use, and increased risk of herpes zoster (which occurred in 10.3% and 13.5% of treated and crossover patients).
However, there appears to be no increased risk – and there may be a slight decreased risk – of progression to acute leukemia, Dr. Mesa said.
Dr. Mesa disclosed that he has received consulting fees, honoraria, and/or grant/research support from ARIAD Pharmaceuticals, Celgene, CTI BioPharma, Galena Biopharma, Gilead Sciences, Incyte, Novartis Pharmaceuticals, and Promedior.
EXPERT ANALYSIS AT THE NCCN ANNUAL CONFERENCE
Eliminating hepatitis in the United States: A road map
An ambitious new report by the National Academies of Sciences, Engineering, and Medicine lays out a detailed path by which some 90,000 deaths from hepatitis B and C infection could be prevented by 2030.
The National Academies, a group of nongovernmental advisory bodies that includes the former Institute of Medicine, said that “the tools to prevent these deaths” exist – namely vaccination to prevent new hepatitis B infections and antiviral drugs, including new oral medications that can cure chronic hepatitis C infections within months.
The authors of the 200-plus-page report, led by Brian Strom, MD, MPH, of Rutgers University in Newark, NJ, calculate that deaths from hepatitis B infection could be halved by 2030 if 90% of patients are diagnosed, if 90% of those diagnosed are connected to care, and if 80% of those for whom treatment is indicated receive it. Treating everyone with chronic hepatitis C would reduce new infections by 90% by 2030, while reducing related deaths by 65%, Dr. Strom and his colleagues estimate.
But the authors also concede that drastic changes to current health policy would be required to reach these goals. These include the adoption of “aggressive testing, diagnosis, treatment, and prevention methods, such as needle exchange.”
They propose that the federal government seek a unique licensing arrangement with one or more manufacturers to bring down the notoriously high cost of direct-acting drugs used in hepatitis C, as a way of raising treatment rates. Currently, fewer than half the patients on Medicaid who are eligible for hepatitis C treatment receive it, and fewer than 1% of prisoners, who have high rates of infection.
Dr. Joseph Lim, director of the viral hepatitis program at Yale University in New Haven. Conn., who was not involved in the National Academies report, called it helpful in the sense that “it casts a spotlight on something that those of us involved in the care of people with viral hepatitis have long known – which is that this is a national and global public health burden that has been under the radar and in the shadow of other important health priorities.”
Both hepatitis B and C increase the risk of liver cancer and are associated with significant morbidity and mortality. Though approximately 4 million people in the United States are estimated to be infected with chronic hepatitis B (1.3 million) or C (2.7 million), these diseases account for less than 1% of the research budget at the National Institutes of Health, the report said. This compares unfavorably to funding for HIV, which affects about 1 million Americans.
As the report states, the tools to radically reduce hepatitis B and C deaths already exist. However, Dr. Lim cautioned in an interview, “the public health infrastructure to address viral hepatitis has been woefully inadequate.” In the United States, he noted, most states receive federal funding for at most a single person in charge of viral hepatitis epidemiology. “The resources currently available are in no way adequate to achieve the very aggressive goals described in the report,” he said.
Even among people with a known diagnosis of hepatitis B or C, only some receive confirmatory testing, Dr. Lim said. And of those with confirmed infections, “only a fraction are linked to care from the diagnosing clinician to a provider with the capacity to assess the state of liver disease and determine whether antiviral therapy is warranted.” Finally, he said, “many patients continue to face barriers to curative therapy due to cost and restrictions by public and private payers.”
Among the recommendations contained in the report is that unrestricted, mass treatment of hepatitis C infections be undertaken – regardless of disease stage. Currently, direct-acting antiviral agents remain costly and are poorly covered, notably by Medicaid. The National Academies advise that the government rectify this by purchasing “a license or assignment to the patent on a direct-acting antiviral drug, and use it only in those market segments where the government pays for treatment and access is now limited, such as Medicaid and prisons.”
Dr. Lim called the licensing proposal “very novel and bold,” but noted that there is no precedent in the United States for diseases such as hepatitis C. “If it could be done it would be an incredible model of government-pharma partnership for the public health good, and have a very significant impact.”
Steven Flamm, MD, chief of the liver transplantation program at Northwestern University in Chicago, who like Dr. Lim was not involved in the creation of the report, said in an interview that it contained innovative ideas and helped underscore the fact that “hepatitis has been given short shrift. The NIH and other agencies do not devote time and energy to this particular medical issue for reasons that are not completely clear.”
But “the problem with these kinds of analyses,” he said, “is that carrying them out is harder than making the recommendations.”
Dr. Flamm echoed Dr. Lim’s concerns about the practicability of implementing some of the recommendations in what he considers a resource-deprived health care environment for viral hepatitis.
“Is elimination possible or can you take a big bite out of it? The answer to that question is yes. We now have agents that can treat chronic viral hepatitis well, which we didn’t have a few years ago.”
Still, he emphasized, having the tools is only one part of the picture. Hepatitis C diagnostic tests have been available since the early 1990s. Yet, Dr. Flamm pointed out, fewer than half of patients have been diagnosed. “If the new CDC screening guidelines gain traction, we will do better than that.”
Dr. Flamm said that he considered the report’s call for a unique government licensing agreement for hepatitis C drugs a tall order. The drugs are already heavily discounted by manufacturers in many cases, he said, yet remain unavailable to those in need of them. In Illinois, Dr. Flamm said, few Medicaid patients with confirmed hepatitis C are given the short-acting antivirals that have revolutionized treatment. “The vast majority have no access to the therapy at all,” he said.
One of the report’s strengths, he said, is in detailing innovative prevention strategies such as delivering and promoting hepatitis B vaccinations to adults through local pharmacies, after the model of influenza vaccinations, and also conducting needle exchanges through pharmacies for intravenous drug users, who are at high risk of contracting both hepatitis B and C.
“Many of these strategies are not very costly,” he said. “The problem is you run into moral platitudes – to eliminate hepatitis, we will have to overcome that,” Dr. Flamm said, something that cannot be taken for granted in the current political environment.
But even if the goals outlined in the report seem ambitious, its authors have done an important service in underscoring the burden of viral hepatitis and laying out how some barriers to prevention, diagnosis, and treatment might be broken, he said.
Viral hepatitis “is a big deal, and it does cost a tremendous amount of money,” he added. “Everybody focuses on the therapeutic cost, but nobody focuses on the costs, direct and indirect, of all the sick people that are out there.”
An ambitious new report by the National Academies of Sciences, Engineering, and Medicine lays out a detailed path by which some 90,000 deaths from hepatitis B and C infection could be prevented by 2030.
The National Academies, a group of nongovernmental advisory bodies that includes the former Institute of Medicine, said that “the tools to prevent these deaths” exist – namely vaccination to prevent new hepatitis B infections and antiviral drugs, including new oral medications that can cure chronic hepatitis C infections within months.
The authors of the 200-plus-page report, led by Brian Strom, MD, MPH, of Rutgers University in Newark, NJ, calculate that deaths from hepatitis B infection could be halved by 2030 if 90% of patients are diagnosed, if 90% of those diagnosed are connected to care, and if 80% of those for whom treatment is indicated receive it. Treating everyone with chronic hepatitis C would reduce new infections by 90% by 2030, while reducing related deaths by 65%, Dr. Strom and his colleagues estimate.
But the authors also concede that drastic changes to current health policy would be required to reach these goals. These include the adoption of “aggressive testing, diagnosis, treatment, and prevention methods, such as needle exchange.”
They propose that the federal government seek a unique licensing arrangement with one or more manufacturers to bring down the notoriously high cost of direct-acting drugs used in hepatitis C, as a way of raising treatment rates. Currently, fewer than half the patients on Medicaid who are eligible for hepatitis C treatment receive it, and fewer than 1% of prisoners, who have high rates of infection.
Dr. Joseph Lim, director of the viral hepatitis program at Yale University in New Haven. Conn., who was not involved in the National Academies report, called it helpful in the sense that “it casts a spotlight on something that those of us involved in the care of people with viral hepatitis have long known – which is that this is a national and global public health burden that has been under the radar and in the shadow of other important health priorities.”
Both hepatitis B and C increase the risk of liver cancer and are associated with significant morbidity and mortality. Though approximately 4 million people in the United States are estimated to be infected with chronic hepatitis B (1.3 million) or C (2.7 million), these diseases account for less than 1% of the research budget at the National Institutes of Health, the report said. This compares unfavorably to funding for HIV, which affects about 1 million Americans.
As the report states, the tools to radically reduce hepatitis B and C deaths already exist. However, Dr. Lim cautioned in an interview, “the public health infrastructure to address viral hepatitis has been woefully inadequate.” In the United States, he noted, most states receive federal funding for at most a single person in charge of viral hepatitis epidemiology. “The resources currently available are in no way adequate to achieve the very aggressive goals described in the report,” he said.
Even among people with a known diagnosis of hepatitis B or C, only some receive confirmatory testing, Dr. Lim said. And of those with confirmed infections, “only a fraction are linked to care from the diagnosing clinician to a provider with the capacity to assess the state of liver disease and determine whether antiviral therapy is warranted.” Finally, he said, “many patients continue to face barriers to curative therapy due to cost and restrictions by public and private payers.”
Among the recommendations contained in the report is that unrestricted, mass treatment of hepatitis C infections be undertaken – regardless of disease stage. Currently, direct-acting antiviral agents remain costly and are poorly covered, notably by Medicaid. The National Academies advise that the government rectify this by purchasing “a license or assignment to the patent on a direct-acting antiviral drug, and use it only in those market segments where the government pays for treatment and access is now limited, such as Medicaid and prisons.”
Dr. Lim called the licensing proposal “very novel and bold,” but noted that there is no precedent in the United States for diseases such as hepatitis C. “If it could be done it would be an incredible model of government-pharma partnership for the public health good, and have a very significant impact.”
Steven Flamm, MD, chief of the liver transplantation program at Northwestern University in Chicago, who like Dr. Lim was not involved in the creation of the report, said in an interview that it contained innovative ideas and helped underscore the fact that “hepatitis has been given short shrift. The NIH and other agencies do not devote time and energy to this particular medical issue for reasons that are not completely clear.”
But “the problem with these kinds of analyses,” he said, “is that carrying them out is harder than making the recommendations.”
Dr. Flamm echoed Dr. Lim’s concerns about the practicability of implementing some of the recommendations in what he considers a resource-deprived health care environment for viral hepatitis.
“Is elimination possible or can you take a big bite out of it? The answer to that question is yes. We now have agents that can treat chronic viral hepatitis well, which we didn’t have a few years ago.”
Still, he emphasized, having the tools is only one part of the picture. Hepatitis C diagnostic tests have been available since the early 1990s. Yet, Dr. Flamm pointed out, fewer than half of patients have been diagnosed. “If the new CDC screening guidelines gain traction, we will do better than that.”
Dr. Flamm said that he considered the report’s call for a unique government licensing agreement for hepatitis C drugs a tall order. The drugs are already heavily discounted by manufacturers in many cases, he said, yet remain unavailable to those in need of them. In Illinois, Dr. Flamm said, few Medicaid patients with confirmed hepatitis C are given the short-acting antivirals that have revolutionized treatment. “The vast majority have no access to the therapy at all,” he said.
One of the report’s strengths, he said, is in detailing innovative prevention strategies such as delivering and promoting hepatitis B vaccinations to adults through local pharmacies, after the model of influenza vaccinations, and also conducting needle exchanges through pharmacies for intravenous drug users, who are at high risk of contracting both hepatitis B and C.
“Many of these strategies are not very costly,” he said. “The problem is you run into moral platitudes – to eliminate hepatitis, we will have to overcome that,” Dr. Flamm said, something that cannot be taken for granted in the current political environment.
But even if the goals outlined in the report seem ambitious, its authors have done an important service in underscoring the burden of viral hepatitis and laying out how some barriers to prevention, diagnosis, and treatment might be broken, he said.
Viral hepatitis “is a big deal, and it does cost a tremendous amount of money,” he added. “Everybody focuses on the therapeutic cost, but nobody focuses on the costs, direct and indirect, of all the sick people that are out there.”
An ambitious new report by the National Academies of Sciences, Engineering, and Medicine lays out a detailed path by which some 90,000 deaths from hepatitis B and C infection could be prevented by 2030.
The National Academies, a group of nongovernmental advisory bodies that includes the former Institute of Medicine, said that “the tools to prevent these deaths” exist – namely vaccination to prevent new hepatitis B infections and antiviral drugs, including new oral medications that can cure chronic hepatitis C infections within months.
The authors of the 200-plus-page report, led by Brian Strom, MD, MPH, of Rutgers University in Newark, NJ, calculate that deaths from hepatitis B infection could be halved by 2030 if 90% of patients are diagnosed, if 90% of those diagnosed are connected to care, and if 80% of those for whom treatment is indicated receive it. Treating everyone with chronic hepatitis C would reduce new infections by 90% by 2030, while reducing related deaths by 65%, Dr. Strom and his colleagues estimate.
But the authors also concede that drastic changes to current health policy would be required to reach these goals. These include the adoption of “aggressive testing, diagnosis, treatment, and prevention methods, such as needle exchange.”
They propose that the federal government seek a unique licensing arrangement with one or more manufacturers to bring down the notoriously high cost of direct-acting drugs used in hepatitis C, as a way of raising treatment rates. Currently, fewer than half the patients on Medicaid who are eligible for hepatitis C treatment receive it, and fewer than 1% of prisoners, who have high rates of infection.
Dr. Joseph Lim, director of the viral hepatitis program at Yale University in New Haven. Conn., who was not involved in the National Academies report, called it helpful in the sense that “it casts a spotlight on something that those of us involved in the care of people with viral hepatitis have long known – which is that this is a national and global public health burden that has been under the radar and in the shadow of other important health priorities.”
Both hepatitis B and C increase the risk of liver cancer and are associated with significant morbidity and mortality. Though approximately 4 million people in the United States are estimated to be infected with chronic hepatitis B (1.3 million) or C (2.7 million), these diseases account for less than 1% of the research budget at the National Institutes of Health, the report said. This compares unfavorably to funding for HIV, which affects about 1 million Americans.
As the report states, the tools to radically reduce hepatitis B and C deaths already exist. However, Dr. Lim cautioned in an interview, “the public health infrastructure to address viral hepatitis has been woefully inadequate.” In the United States, he noted, most states receive federal funding for at most a single person in charge of viral hepatitis epidemiology. “The resources currently available are in no way adequate to achieve the very aggressive goals described in the report,” he said.
Even among people with a known diagnosis of hepatitis B or C, only some receive confirmatory testing, Dr. Lim said. And of those with confirmed infections, “only a fraction are linked to care from the diagnosing clinician to a provider with the capacity to assess the state of liver disease and determine whether antiviral therapy is warranted.” Finally, he said, “many patients continue to face barriers to curative therapy due to cost and restrictions by public and private payers.”
Among the recommendations contained in the report is that unrestricted, mass treatment of hepatitis C infections be undertaken – regardless of disease stage. Currently, direct-acting antiviral agents remain costly and are poorly covered, notably by Medicaid. The National Academies advise that the government rectify this by purchasing “a license or assignment to the patent on a direct-acting antiviral drug, and use it only in those market segments where the government pays for treatment and access is now limited, such as Medicaid and prisons.”
Dr. Lim called the licensing proposal “very novel and bold,” but noted that there is no precedent in the United States for diseases such as hepatitis C. “If it could be done it would be an incredible model of government-pharma partnership for the public health good, and have a very significant impact.”
Steven Flamm, MD, chief of the liver transplantation program at Northwestern University in Chicago, who like Dr. Lim was not involved in the creation of the report, said in an interview that it contained innovative ideas and helped underscore the fact that “hepatitis has been given short shrift. The NIH and other agencies do not devote time and energy to this particular medical issue for reasons that are not completely clear.”
But “the problem with these kinds of analyses,” he said, “is that carrying them out is harder than making the recommendations.”
Dr. Flamm echoed Dr. Lim’s concerns about the practicability of implementing some of the recommendations in what he considers a resource-deprived health care environment for viral hepatitis.
“Is elimination possible or can you take a big bite out of it? The answer to that question is yes. We now have agents that can treat chronic viral hepatitis well, which we didn’t have a few years ago.”
Still, he emphasized, having the tools is only one part of the picture. Hepatitis C diagnostic tests have been available since the early 1990s. Yet, Dr. Flamm pointed out, fewer than half of patients have been diagnosed. “If the new CDC screening guidelines gain traction, we will do better than that.”
Dr. Flamm said that he considered the report’s call for a unique government licensing agreement for hepatitis C drugs a tall order. The drugs are already heavily discounted by manufacturers in many cases, he said, yet remain unavailable to those in need of them. In Illinois, Dr. Flamm said, few Medicaid patients with confirmed hepatitis C are given the short-acting antivirals that have revolutionized treatment. “The vast majority have no access to the therapy at all,” he said.
One of the report’s strengths, he said, is in detailing innovative prevention strategies such as delivering and promoting hepatitis B vaccinations to adults through local pharmacies, after the model of influenza vaccinations, and also conducting needle exchanges through pharmacies for intravenous drug users, who are at high risk of contracting both hepatitis B and C.
“Many of these strategies are not very costly,” he said. “The problem is you run into moral platitudes – to eliminate hepatitis, we will have to overcome that,” Dr. Flamm said, something that cannot be taken for granted in the current political environment.
But even if the goals outlined in the report seem ambitious, its authors have done an important service in underscoring the burden of viral hepatitis and laying out how some barriers to prevention, diagnosis, and treatment might be broken, he said.
Viral hepatitis “is a big deal, and it does cost a tremendous amount of money,” he added. “Everybody focuses on the therapeutic cost, but nobody focuses on the costs, direct and indirect, of all the sick people that are out there.”
FROM THE NATIONAL ACADEMIES OF SCIENCES, ENGINEERING, AND MEDICINE
USPSTF: No recommendation on screening for celiac disease
The current evidence is insufficient for the U.S. Preventive Services Task Force to recommend either for or against routine screening of asymptomatic people for celiac disease, according to a Recommendation Statement published online March 28 in JAMA.
The USPSTF is tasked with making recommendations regarding the effectiveness of specific preventive health care services for patients who have no related signs or symptoms. In this case, the group commissioned a systematic review of the literature on celiac disease from 1946 through June 2016, which became the basis for the evidence report informing their Recommendation Statement, said Kirsten Bibbins-Domingo, PhD, MD, chair of the USPSTF and lead author of the statement, and her associates.
However, only 4 studies out of the 3,036 that were examined addressed the question of screening adequately, and they offered few data of use. According to Roger Chou, MD, lead author of the Evidence Report, and his associates, no trials assessed the screening of asymptomatic children, adolescents, or adults for celiac disease with regard to morbidity, mortality, or quality of life. The evidence also was inadequate concerning targeted screening of at-risk individuals, and there were no studies of the effectiveness of treatment after screen-detected celiac disease was found.
There was some evidence supporting the diagnostic accuracy of the tissue transglutaminase-IgA test to detect celiac disease, but “little or no evidence ... to inform most of the key questions related to benefits and harms of screening for celiac disease in asymptomatic individuals,” said Dr. Chou, of the Pacific Northwest Evidence-Based Practice Center and Oregon Health & Science University, both in Portland, and his associates (JAMA. 2017 Mar 28. doi: 10.1001/jama.2016.10395).
Dr. Bibbins-Domingo noted that the American Academy of Family Physicians also has concluded that the evidence is insufficient to assess the balance of screening’s benefits and harms. In contrast, the American College of Gastroenterology recommends that screening be considered in asymptomatic people who have a first-degree relative with a confirmed diagnosis of celiac disease (JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.1462).
In addition, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition recommends screening at age 3 years for asymptomatic children who have conditions associated with celiac disease, including type 1 diabetes, autoimmune thyroiditis, Down syndrome, Turner syndrome, William’s syndrome, or selective IgA deficiency, said Dr. Bobbins-Domingo, who is also professor of medicine at the University of California, San Francisco.
Copies of the Recommendation Statement, the Evidence Report, the authors’ disclosures, and other materials are available at www.uspreventiveservicestaskforce.org.
Even though the current evidence on the effectiveness of screening for celiac disease is scarce or absent, it remains reasonable for clinicians to have a low threshold for testing patients, especially in high-risk populations such as those with an affected family member or a related autoimmune disorder.
This is because most celiac disease is unrecognized, and patients can present with diverse symptoms rather than the classic triad of abdominal pain, diarrhea, and weight loss.
Rok Seon Choung, MD, and Joseph A. Murray, MD , are in the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn. Dr. Murray reported ties to Alvine Pharmaceuticals, Alba Therapeutics, Celimmune, BioLineRx, and numerous others. Dr. Choung and Dr. Murray made these remarks in an editorial accompanying the USPSTF reports (JAMA. 2017 Mar 28;317:1221-3).
Even though the current evidence on the effectiveness of screening for celiac disease is scarce or absent, it remains reasonable for clinicians to have a low threshold for testing patients, especially in high-risk populations such as those with an affected family member or a related autoimmune disorder.
This is because most celiac disease is unrecognized, and patients can present with diverse symptoms rather than the classic triad of abdominal pain, diarrhea, and weight loss.
Rok Seon Choung, MD, and Joseph A. Murray, MD , are in the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn. Dr. Murray reported ties to Alvine Pharmaceuticals, Alba Therapeutics, Celimmune, BioLineRx, and numerous others. Dr. Choung and Dr. Murray made these remarks in an editorial accompanying the USPSTF reports (JAMA. 2017 Mar 28;317:1221-3).
Even though the current evidence on the effectiveness of screening for celiac disease is scarce or absent, it remains reasonable for clinicians to have a low threshold for testing patients, especially in high-risk populations such as those with an affected family member or a related autoimmune disorder.
This is because most celiac disease is unrecognized, and patients can present with diverse symptoms rather than the classic triad of abdominal pain, diarrhea, and weight loss.
Rok Seon Choung, MD, and Joseph A. Murray, MD , are in the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn. Dr. Murray reported ties to Alvine Pharmaceuticals, Alba Therapeutics, Celimmune, BioLineRx, and numerous others. Dr. Choung and Dr. Murray made these remarks in an editorial accompanying the USPSTF reports (JAMA. 2017 Mar 28;317:1221-3).
The current evidence is insufficient for the U.S. Preventive Services Task Force to recommend either for or against routine screening of asymptomatic people for celiac disease, according to a Recommendation Statement published online March 28 in JAMA.
The USPSTF is tasked with making recommendations regarding the effectiveness of specific preventive health care services for patients who have no related signs or symptoms. In this case, the group commissioned a systematic review of the literature on celiac disease from 1946 through June 2016, which became the basis for the evidence report informing their Recommendation Statement, said Kirsten Bibbins-Domingo, PhD, MD, chair of the USPSTF and lead author of the statement, and her associates.
However, only 4 studies out of the 3,036 that were examined addressed the question of screening adequately, and they offered few data of use. According to Roger Chou, MD, lead author of the Evidence Report, and his associates, no trials assessed the screening of asymptomatic children, adolescents, or adults for celiac disease with regard to morbidity, mortality, or quality of life. The evidence also was inadequate concerning targeted screening of at-risk individuals, and there were no studies of the effectiveness of treatment after screen-detected celiac disease was found.
There was some evidence supporting the diagnostic accuracy of the tissue transglutaminase-IgA test to detect celiac disease, but “little or no evidence ... to inform most of the key questions related to benefits and harms of screening for celiac disease in asymptomatic individuals,” said Dr. Chou, of the Pacific Northwest Evidence-Based Practice Center and Oregon Health & Science University, both in Portland, and his associates (JAMA. 2017 Mar 28. doi: 10.1001/jama.2016.10395).
Dr. Bibbins-Domingo noted that the American Academy of Family Physicians also has concluded that the evidence is insufficient to assess the balance of screening’s benefits and harms. In contrast, the American College of Gastroenterology recommends that screening be considered in asymptomatic people who have a first-degree relative with a confirmed diagnosis of celiac disease (JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.1462).
In addition, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition recommends screening at age 3 years for asymptomatic children who have conditions associated with celiac disease, including type 1 diabetes, autoimmune thyroiditis, Down syndrome, Turner syndrome, William’s syndrome, or selective IgA deficiency, said Dr. Bobbins-Domingo, who is also professor of medicine at the University of California, San Francisco.
Copies of the Recommendation Statement, the Evidence Report, the authors’ disclosures, and other materials are available at www.uspreventiveservicestaskforce.org.
The current evidence is insufficient for the U.S. Preventive Services Task Force to recommend either for or against routine screening of asymptomatic people for celiac disease, according to a Recommendation Statement published online March 28 in JAMA.
The USPSTF is tasked with making recommendations regarding the effectiveness of specific preventive health care services for patients who have no related signs or symptoms. In this case, the group commissioned a systematic review of the literature on celiac disease from 1946 through June 2016, which became the basis for the evidence report informing their Recommendation Statement, said Kirsten Bibbins-Domingo, PhD, MD, chair of the USPSTF and lead author of the statement, and her associates.
However, only 4 studies out of the 3,036 that were examined addressed the question of screening adequately, and they offered few data of use. According to Roger Chou, MD, lead author of the Evidence Report, and his associates, no trials assessed the screening of asymptomatic children, adolescents, or adults for celiac disease with regard to morbidity, mortality, or quality of life. The evidence also was inadequate concerning targeted screening of at-risk individuals, and there were no studies of the effectiveness of treatment after screen-detected celiac disease was found.
There was some evidence supporting the diagnostic accuracy of the tissue transglutaminase-IgA test to detect celiac disease, but “little or no evidence ... to inform most of the key questions related to benefits and harms of screening for celiac disease in asymptomatic individuals,” said Dr. Chou, of the Pacific Northwest Evidence-Based Practice Center and Oregon Health & Science University, both in Portland, and his associates (JAMA. 2017 Mar 28. doi: 10.1001/jama.2016.10395).
Dr. Bibbins-Domingo noted that the American Academy of Family Physicians also has concluded that the evidence is insufficient to assess the balance of screening’s benefits and harms. In contrast, the American College of Gastroenterology recommends that screening be considered in asymptomatic people who have a first-degree relative with a confirmed diagnosis of celiac disease (JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.1462).
In addition, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition recommends screening at age 3 years for asymptomatic children who have conditions associated with celiac disease, including type 1 diabetes, autoimmune thyroiditis, Down syndrome, Turner syndrome, William’s syndrome, or selective IgA deficiency, said Dr. Bobbins-Domingo, who is also professor of medicine at the University of California, San Francisco.
Copies of the Recommendation Statement, the Evidence Report, the authors’ disclosures, and other materials are available at www.uspreventiveservicestaskforce.org.
FROM JAMA
Key clinical point: The current evidence is insufficient for the USPSTF to recommend either for or against routine screening of asymptomatic people for celiac disease.
Major finding: Only 4 studies out of the 3,036 that were examined addressed the question of screening adequately.
Data source: An assessment of the benefits and harms of screening based on a review of four studies.
Disclosures: The USPSTF’s work is supported by the U.S. Agency for Healthcare Research and Quality. The authors’ financial disclosures are available at www.uspreventiveservicestaskforce.org.
Guideline Provides Recommendations for Pharmacologic Treatment of Chronic Insomnia
All patients with chronic insomnia should receive cognitive behavioral therapy as a primary intervention, and clinicians should use a shared decision-making approach when determining whether patients should start pharmacotherapy, according to a new guideline developed by the American Academy of Sleep Medicine (AASM). The guideline, which includes 14 recommendations, was published in the February 15 issue of the Journal of Clinical Sleep Medicine. It is the first guideline from the AASM to provide comprehensive evidence-based analyses of individual drugs commonly used to treat chronic insomnia disorder.
Chronic insomnia is associated with increased work absenteeism and impairment in functional status. Studies have also identified persistent insomnia as a significant risk factor for the development of psychiatric disorders, especially mood disorder. Pharmacologic treatment of insomnia is the most widely used approach to therapy, after treatment of comorbidities. No evidence-based clinical guideline had been published previously, however. The AASM commissioned a task force of four sleep medicine experts to develop the clinical guideline for treatment of chronic insomnia in adults.
The task force conducted a systematic review to identify randomized controlled trials. In addition, the group used the Grading of Recommendations Assessment, Development, and Evaluation process to assess the evidence. Recommendations were then made based on the quality of evidence, the balance of benefits and harms, and patient values and preferences.
Sleep Onset Insomnia Treatment Recommendations
The guideline recommends eszopiclone for the treatment of sleep onset insomnia, based on trials of 2-mg and 3-mg doses of the drug. Mean reduction of sleep latency was 14 minutes greater with eszopiclone than with placebo. Researchers also observed a moderate-to-large improvement in quality of sleep when they compared eszopiclone with placebo.
The experts also recommend ramelteon, based on trials of 8-mg doses of the drug. Mean reduction of sleep latency was nine minutes greater with ramelteon than with placebo. Ramelteon may not improve quality of sleep, however. Clinicians also are advised to use temazepam, based on trials of 15-mg doses. Temazepam reduced sleep latency by 37 minutes, compared with placebo, and provided a small improvement in quality of sleep.
The quality of evidence for the use of triazolam was high, and the drug reduced sleep latency by nine minutes, compared with placebo. The task force determined that the majority of patients would be more likely to use triazolam, compared with no treatment, but many would not use it. Zaleplon was recommended based on trials of 5-mg and 10-mg doses. It reduced sleep latency by 10 minutes, compared with placebo. Finally, zolpidem reduced sleep latency by five to 12 minutes, compared with placebo, and was associated with a moderate improvement in quality of sleep. The quality of evidence was very low, however.
Sleep Maintenance Insomnia Recommendations
The task force found four studies evaluating a 3-mg dose of doxepin and four studies investigating a 6-mg dose in sleep maintenance insomnia. Mean improvement in total sleep time with the drug was 26–32 min longer, compared with placebo. The mean reduction in wake after sleep onset (WASO) associated with doxepin was 22–23 min greater, compared with placebo.
The recommendation for the use of eszopiclone was based on trials of 2-mg and 3-mg doses of the drug. Mean improvement in total sleep time was 28–57 min longer with eszopiclone, compared with placebo. Mean reduction in WASO was 10–14 min greater with eszopiclone, compared with placebo.
Trials of 15-mg doses of temazepam were the basis for the task force’s recommendation of the drug. Mean improvement in total sleep time with temazepam was 99 min longer, compared with placebo. The drug’s effect on WASO was not reported. Compared with placebo, temazepam may provide a small improvement in quality of sleep.
Suvorexant was evaluated in trials of 10-mg, 15–20-mg, and 20-mg doses. Mean improvement in total sleep time was 10 min longer with suvorexant, compared with placebo. Mean reduction in WASO was 16–28 min greater with suvorexant, compared to placebo.
The guideline also recommends zolpidem, based on trials of 10-mg doses. Zolpidem provided a mean improvement in total sleep time that was 29 min longer, compared with placebo. The mean reduction in WASO was 25 min greater with zolpidem, compared with placebo.
Certain Drugs Are Not Recommended
Several drugs were not recommended for treating sleep onset or sleep maintenance insomnia. The task force suggests that clinicians not use diphenhydramine, because the evidence suggests that it is not effective for improving sleep onset or total sleep time. Similarly, evidence suggests that the effects of tiagabine on objective and subjective measures of sleep latency are clinically insignificant, and the drug’s harms may outweigh its benefits. Sleep outcome variables also did not improve with trazodone to a clinically significant degree. The task force also suggested that clinicians not use melatonin, valerian, or L-tryptophan because of the treatments’ lack of clinically significant effects.
All recommendations were classified as weak, due to the task force’s low degree of certainty in the appropriateness of the patient-care strategy. Further study is required to determine the drugs’ efficacy or lack thereof, said the task force. “Clinicians must continue to exercise sound clinical judgment based not only on these recommendations, but also on clinical experience, prior patient response, patient preferences, and potential adverse effects,” the authors concluded.
—Erica Tricarico
Suggested Reading
Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
All patients with chronic insomnia should receive cognitive behavioral therapy as a primary intervention, and clinicians should use a shared decision-making approach when determining whether patients should start pharmacotherapy, according to a new guideline developed by the American Academy of Sleep Medicine (AASM). The guideline, which includes 14 recommendations, was published in the February 15 issue of the Journal of Clinical Sleep Medicine. It is the first guideline from the AASM to provide comprehensive evidence-based analyses of individual drugs commonly used to treat chronic insomnia disorder.
Chronic insomnia is associated with increased work absenteeism and impairment in functional status. Studies have also identified persistent insomnia as a significant risk factor for the development of psychiatric disorders, especially mood disorder. Pharmacologic treatment of insomnia is the most widely used approach to therapy, after treatment of comorbidities. No evidence-based clinical guideline had been published previously, however. The AASM commissioned a task force of four sleep medicine experts to develop the clinical guideline for treatment of chronic insomnia in adults.
The task force conducted a systematic review to identify randomized controlled trials. In addition, the group used the Grading of Recommendations Assessment, Development, and Evaluation process to assess the evidence. Recommendations were then made based on the quality of evidence, the balance of benefits and harms, and patient values and preferences.
Sleep Onset Insomnia Treatment Recommendations
The guideline recommends eszopiclone for the treatment of sleep onset insomnia, based on trials of 2-mg and 3-mg doses of the drug. Mean reduction of sleep latency was 14 minutes greater with eszopiclone than with placebo. Researchers also observed a moderate-to-large improvement in quality of sleep when they compared eszopiclone with placebo.
The experts also recommend ramelteon, based on trials of 8-mg doses of the drug. Mean reduction of sleep latency was nine minutes greater with ramelteon than with placebo. Ramelteon may not improve quality of sleep, however. Clinicians also are advised to use temazepam, based on trials of 15-mg doses. Temazepam reduced sleep latency by 37 minutes, compared with placebo, and provided a small improvement in quality of sleep.
The quality of evidence for the use of triazolam was high, and the drug reduced sleep latency by nine minutes, compared with placebo. The task force determined that the majority of patients would be more likely to use triazolam, compared with no treatment, but many would not use it. Zaleplon was recommended based on trials of 5-mg and 10-mg doses. It reduced sleep latency by 10 minutes, compared with placebo. Finally, zolpidem reduced sleep latency by five to 12 minutes, compared with placebo, and was associated with a moderate improvement in quality of sleep. The quality of evidence was very low, however.
Sleep Maintenance Insomnia Recommendations
The task force found four studies evaluating a 3-mg dose of doxepin and four studies investigating a 6-mg dose in sleep maintenance insomnia. Mean improvement in total sleep time with the drug was 26–32 min longer, compared with placebo. The mean reduction in wake after sleep onset (WASO) associated with doxepin was 22–23 min greater, compared with placebo.
The recommendation for the use of eszopiclone was based on trials of 2-mg and 3-mg doses of the drug. Mean improvement in total sleep time was 28–57 min longer with eszopiclone, compared with placebo. Mean reduction in WASO was 10–14 min greater with eszopiclone, compared with placebo.
Trials of 15-mg doses of temazepam were the basis for the task force’s recommendation of the drug. Mean improvement in total sleep time with temazepam was 99 min longer, compared with placebo. The drug’s effect on WASO was not reported. Compared with placebo, temazepam may provide a small improvement in quality of sleep.
Suvorexant was evaluated in trials of 10-mg, 15–20-mg, and 20-mg doses. Mean improvement in total sleep time was 10 min longer with suvorexant, compared with placebo. Mean reduction in WASO was 16–28 min greater with suvorexant, compared to placebo.
The guideline also recommends zolpidem, based on trials of 10-mg doses. Zolpidem provided a mean improvement in total sleep time that was 29 min longer, compared with placebo. The mean reduction in WASO was 25 min greater with zolpidem, compared with placebo.
Certain Drugs Are Not Recommended
Several drugs were not recommended for treating sleep onset or sleep maintenance insomnia. The task force suggests that clinicians not use diphenhydramine, because the evidence suggests that it is not effective for improving sleep onset or total sleep time. Similarly, evidence suggests that the effects of tiagabine on objective and subjective measures of sleep latency are clinically insignificant, and the drug’s harms may outweigh its benefits. Sleep outcome variables also did not improve with trazodone to a clinically significant degree. The task force also suggested that clinicians not use melatonin, valerian, or L-tryptophan because of the treatments’ lack of clinically significant effects.
All recommendations were classified as weak, due to the task force’s low degree of certainty in the appropriateness of the patient-care strategy. Further study is required to determine the drugs’ efficacy or lack thereof, said the task force. “Clinicians must continue to exercise sound clinical judgment based not only on these recommendations, but also on clinical experience, prior patient response, patient preferences, and potential adverse effects,” the authors concluded.
—Erica Tricarico
Suggested Reading
Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
All patients with chronic insomnia should receive cognitive behavioral therapy as a primary intervention, and clinicians should use a shared decision-making approach when determining whether patients should start pharmacotherapy, according to a new guideline developed by the American Academy of Sleep Medicine (AASM). The guideline, which includes 14 recommendations, was published in the February 15 issue of the Journal of Clinical Sleep Medicine. It is the first guideline from the AASM to provide comprehensive evidence-based analyses of individual drugs commonly used to treat chronic insomnia disorder.
Chronic insomnia is associated with increased work absenteeism and impairment in functional status. Studies have also identified persistent insomnia as a significant risk factor for the development of psychiatric disorders, especially mood disorder. Pharmacologic treatment of insomnia is the most widely used approach to therapy, after treatment of comorbidities. No evidence-based clinical guideline had been published previously, however. The AASM commissioned a task force of four sleep medicine experts to develop the clinical guideline for treatment of chronic insomnia in adults.
The task force conducted a systematic review to identify randomized controlled trials. In addition, the group used the Grading of Recommendations Assessment, Development, and Evaluation process to assess the evidence. Recommendations were then made based on the quality of evidence, the balance of benefits and harms, and patient values and preferences.
Sleep Onset Insomnia Treatment Recommendations
The guideline recommends eszopiclone for the treatment of sleep onset insomnia, based on trials of 2-mg and 3-mg doses of the drug. Mean reduction of sleep latency was 14 minutes greater with eszopiclone than with placebo. Researchers also observed a moderate-to-large improvement in quality of sleep when they compared eszopiclone with placebo.
The experts also recommend ramelteon, based on trials of 8-mg doses of the drug. Mean reduction of sleep latency was nine minutes greater with ramelteon than with placebo. Ramelteon may not improve quality of sleep, however. Clinicians also are advised to use temazepam, based on trials of 15-mg doses. Temazepam reduced sleep latency by 37 minutes, compared with placebo, and provided a small improvement in quality of sleep.
The quality of evidence for the use of triazolam was high, and the drug reduced sleep latency by nine minutes, compared with placebo. The task force determined that the majority of patients would be more likely to use triazolam, compared with no treatment, but many would not use it. Zaleplon was recommended based on trials of 5-mg and 10-mg doses. It reduced sleep latency by 10 minutes, compared with placebo. Finally, zolpidem reduced sleep latency by five to 12 minutes, compared with placebo, and was associated with a moderate improvement in quality of sleep. The quality of evidence was very low, however.
Sleep Maintenance Insomnia Recommendations
The task force found four studies evaluating a 3-mg dose of doxepin and four studies investigating a 6-mg dose in sleep maintenance insomnia. Mean improvement in total sleep time with the drug was 26–32 min longer, compared with placebo. The mean reduction in wake after sleep onset (WASO) associated with doxepin was 22–23 min greater, compared with placebo.
The recommendation for the use of eszopiclone was based on trials of 2-mg and 3-mg doses of the drug. Mean improvement in total sleep time was 28–57 min longer with eszopiclone, compared with placebo. Mean reduction in WASO was 10–14 min greater with eszopiclone, compared with placebo.
Trials of 15-mg doses of temazepam were the basis for the task force’s recommendation of the drug. Mean improvement in total sleep time with temazepam was 99 min longer, compared with placebo. The drug’s effect on WASO was not reported. Compared with placebo, temazepam may provide a small improvement in quality of sleep.
Suvorexant was evaluated in trials of 10-mg, 15–20-mg, and 20-mg doses. Mean improvement in total sleep time was 10 min longer with suvorexant, compared with placebo. Mean reduction in WASO was 16–28 min greater with suvorexant, compared to placebo.
The guideline also recommends zolpidem, based on trials of 10-mg doses. Zolpidem provided a mean improvement in total sleep time that was 29 min longer, compared with placebo. The mean reduction in WASO was 25 min greater with zolpidem, compared with placebo.
Certain Drugs Are Not Recommended
Several drugs were not recommended for treating sleep onset or sleep maintenance insomnia. The task force suggests that clinicians not use diphenhydramine, because the evidence suggests that it is not effective for improving sleep onset or total sleep time. Similarly, evidence suggests that the effects of tiagabine on objective and subjective measures of sleep latency are clinically insignificant, and the drug’s harms may outweigh its benefits. Sleep outcome variables also did not improve with trazodone to a clinically significant degree. The task force also suggested that clinicians not use melatonin, valerian, or L-tryptophan because of the treatments’ lack of clinically significant effects.
All recommendations were classified as weak, due to the task force’s low degree of certainty in the appropriateness of the patient-care strategy. Further study is required to determine the drugs’ efficacy or lack thereof, said the task force. “Clinicians must continue to exercise sound clinical judgment based not only on these recommendations, but also on clinical experience, prior patient response, patient preferences, and potential adverse effects,” the authors concluded.
—Erica Tricarico
Suggested Reading
Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
USPSTF affirms optional pelvic screening
Current evidence fails to support or reject routine screening pelvic exams for asymptomatic, low-risk, nonpregnant adult women, the U.S. Preventive Services Task Force concluded after reviewing the evidence on the accuracy, benefits, and potential harms.
The USPSTF issued an inconclusive “I” statement that was published online March 7 (JAMA. 2017;317[9]:947-53).
Researchers found no data comparing the impact of no screening versus screening pelvic examinations on patient health outcomes including reducing all-cause mortality, reducing cancer-specific and disease-specific morbidity and mortality, and improving quality of life.
“No direct evidence was identified for overall benefits and harms of the pelvic examination as a one-time or periodic screening test,” Janelle M. Guirguis-Blake, MD, of the University of Washington, Tacoma, and colleagues wrote in the accompanying evidence report (JAMA. 2017;317[9]:954-66). The review comprised nine studies: one addressing the harms of screening and eight addressing both harms and accuracy.
Although screening pelvic exams may identify serious conditions as well as benign ones, the potential remains for false-positive and false-negative results that might lead to invasive surgery and unnecessary testing and procedures, the researchers noted. However, the recommendations do not apply to certain conditions for which screening is already recommended, including cervical cancer (via Pap smear), gonorrhea, and chlamydia.
The recommendations are primarily a call for more research rather than a clear guide for clinicians, according to the USPSTF. The research gaps include studies on the physical and psychological harms of pelvic screening for asymptomatic women in primary care; the ability of screening to detect conditions beyond ovarian cancer, genital herpes, bacterial vaginosis, and trichomoniasis; and the impact of screening on a variety of health outcomes, including quality of life.
Given the inadequate evidence to recommend for or against screening, the USPSTF cited the recommendations of other organizations. Both the American College of Physicians and the American Academy of Family Physicians recommend against performing screening pelvic exams in asymptomatic, nonpregnant adult women. The American College of Obstetricians and Gynecologists recommends annual pelvic exams for women 21 years and older but acknowledges a lack of evidence and has said it should be a shared decision between the patient and clinician.
The USPSTF members reported having no relevant financial conflicts.
The USPSTF task force finding of insufficient evidence to support or refute screening pelvic exams conflicts with the views of other organizations, George F. Sawaya, MD, wrote in an editorial (JAMA 2017 Mar 7. doi: 10.1001/jamainternmed.2017.0271).
The American College of Physicians currently recommends against routine screening in asymptomatic, nonpregnant women, while the American College of Obstetricians and Gynecologists recommends in favor of an annual pelvic exam “based on expert opinion” despite the lack of evidence, he said.
“The USPSTF believes that in the setting of an ‘I’ statement, clinicians should be forthright with patients about the uncertainty concerning the balance of benefits and harms,” Dr. Sawaya wrote.
“But perhaps the conversation should focus on the uncertainty among the three professional groups,” he added. “Women should know the facts: that all three groups agree there is no scientific evidence that these examinations are beneficial; that there is evidence of harms including ‘false alarms,’ further testing, and even unnecessary surgery; and that one group strongly recommends against screening examinations, believing them to be more harmful than beneficial,” he said.
The USPSTF recommendation is not a surprise, Colleen McNicholas, DO, MSCI, and Jeffrey F. Peipert, MD, PhD, noted in a second editorial (JAMA 2017;317[9]:910-11). “Despite lack of rigorous research, many would argue that the periodic examination provides opportunity for counseling and trust building between the patient and physician and thus should be universally implemented,” they wrote. However, many women express fear and anxiety before the exam and discomfort, pain, or embarrassment during the exam. “To ignore this aspect when comparing individual parts of the examination seems insensitive and inappropriate,” they added.
“Women, as patients, should be involved in the decision regarding whether to perform a pelvic examination, and clinicians should not require that the patient undergo this procedure to obtain screening, counseling, and age-appropriate health services,” they concluded.
Dr. Sawaya is affiliated with the University of California, San Francisco. He reported having no financial conflicts. Dr. Peipert is affiliated with Indiana University School of Medicine, Indianapolis, and disclosed receiving grants from Teva Pharmaceuticals, Bayer Healthcare Pharmaceuticals, and Merck, as well as serving on the advisory boards of Perrigo and Teva. Dr. McNicholas is affiliated with Washington University, St. Louis, and reported having no financial conflicts.
The USPSTF task force finding of insufficient evidence to support or refute screening pelvic exams conflicts with the views of other organizations, George F. Sawaya, MD, wrote in an editorial (JAMA 2017 Mar 7. doi: 10.1001/jamainternmed.2017.0271).
The American College of Physicians currently recommends against routine screening in asymptomatic, nonpregnant women, while the American College of Obstetricians and Gynecologists recommends in favor of an annual pelvic exam “based on expert opinion” despite the lack of evidence, he said.
“The USPSTF believes that in the setting of an ‘I’ statement, clinicians should be forthright with patients about the uncertainty concerning the balance of benefits and harms,” Dr. Sawaya wrote.
“But perhaps the conversation should focus on the uncertainty among the three professional groups,” he added. “Women should know the facts: that all three groups agree there is no scientific evidence that these examinations are beneficial; that there is evidence of harms including ‘false alarms,’ further testing, and even unnecessary surgery; and that one group strongly recommends against screening examinations, believing them to be more harmful than beneficial,” he said.
The USPSTF recommendation is not a surprise, Colleen McNicholas, DO, MSCI, and Jeffrey F. Peipert, MD, PhD, noted in a second editorial (JAMA 2017;317[9]:910-11). “Despite lack of rigorous research, many would argue that the periodic examination provides opportunity for counseling and trust building between the patient and physician and thus should be universally implemented,” they wrote. However, many women express fear and anxiety before the exam and discomfort, pain, or embarrassment during the exam. “To ignore this aspect when comparing individual parts of the examination seems insensitive and inappropriate,” they added.
“Women, as patients, should be involved in the decision regarding whether to perform a pelvic examination, and clinicians should not require that the patient undergo this procedure to obtain screening, counseling, and age-appropriate health services,” they concluded.
Dr. Sawaya is affiliated with the University of California, San Francisco. He reported having no financial conflicts. Dr. Peipert is affiliated with Indiana University School of Medicine, Indianapolis, and disclosed receiving grants from Teva Pharmaceuticals, Bayer Healthcare Pharmaceuticals, and Merck, as well as serving on the advisory boards of Perrigo and Teva. Dr. McNicholas is affiliated with Washington University, St. Louis, and reported having no financial conflicts.
The USPSTF task force finding of insufficient evidence to support or refute screening pelvic exams conflicts with the views of other organizations, George F. Sawaya, MD, wrote in an editorial (JAMA 2017 Mar 7. doi: 10.1001/jamainternmed.2017.0271).
The American College of Physicians currently recommends against routine screening in asymptomatic, nonpregnant women, while the American College of Obstetricians and Gynecologists recommends in favor of an annual pelvic exam “based on expert opinion” despite the lack of evidence, he said.
“The USPSTF believes that in the setting of an ‘I’ statement, clinicians should be forthright with patients about the uncertainty concerning the balance of benefits and harms,” Dr. Sawaya wrote.
“But perhaps the conversation should focus on the uncertainty among the three professional groups,” he added. “Women should know the facts: that all three groups agree there is no scientific evidence that these examinations are beneficial; that there is evidence of harms including ‘false alarms,’ further testing, and even unnecessary surgery; and that one group strongly recommends against screening examinations, believing them to be more harmful than beneficial,” he said.
The USPSTF recommendation is not a surprise, Colleen McNicholas, DO, MSCI, and Jeffrey F. Peipert, MD, PhD, noted in a second editorial (JAMA 2017;317[9]:910-11). “Despite lack of rigorous research, many would argue that the periodic examination provides opportunity for counseling and trust building between the patient and physician and thus should be universally implemented,” they wrote. However, many women express fear and anxiety before the exam and discomfort, pain, or embarrassment during the exam. “To ignore this aspect when comparing individual parts of the examination seems insensitive and inappropriate,” they added.
“Women, as patients, should be involved in the decision regarding whether to perform a pelvic examination, and clinicians should not require that the patient undergo this procedure to obtain screening, counseling, and age-appropriate health services,” they concluded.
Dr. Sawaya is affiliated with the University of California, San Francisco. He reported having no financial conflicts. Dr. Peipert is affiliated with Indiana University School of Medicine, Indianapolis, and disclosed receiving grants from Teva Pharmaceuticals, Bayer Healthcare Pharmaceuticals, and Merck, as well as serving on the advisory boards of Perrigo and Teva. Dr. McNicholas is affiliated with Washington University, St. Louis, and reported having no financial conflicts.
Current evidence fails to support or reject routine screening pelvic exams for asymptomatic, low-risk, nonpregnant adult women, the U.S. Preventive Services Task Force concluded after reviewing the evidence on the accuracy, benefits, and potential harms.
The USPSTF issued an inconclusive “I” statement that was published online March 7 (JAMA. 2017;317[9]:947-53).
Researchers found no data comparing the impact of no screening versus screening pelvic examinations on patient health outcomes including reducing all-cause mortality, reducing cancer-specific and disease-specific morbidity and mortality, and improving quality of life.
“No direct evidence was identified for overall benefits and harms of the pelvic examination as a one-time or periodic screening test,” Janelle M. Guirguis-Blake, MD, of the University of Washington, Tacoma, and colleagues wrote in the accompanying evidence report (JAMA. 2017;317[9]:954-66). The review comprised nine studies: one addressing the harms of screening and eight addressing both harms and accuracy.
Although screening pelvic exams may identify serious conditions as well as benign ones, the potential remains for false-positive and false-negative results that might lead to invasive surgery and unnecessary testing and procedures, the researchers noted. However, the recommendations do not apply to certain conditions for which screening is already recommended, including cervical cancer (via Pap smear), gonorrhea, and chlamydia.
The recommendations are primarily a call for more research rather than a clear guide for clinicians, according to the USPSTF. The research gaps include studies on the physical and psychological harms of pelvic screening for asymptomatic women in primary care; the ability of screening to detect conditions beyond ovarian cancer, genital herpes, bacterial vaginosis, and trichomoniasis; and the impact of screening on a variety of health outcomes, including quality of life.
Given the inadequate evidence to recommend for or against screening, the USPSTF cited the recommendations of other organizations. Both the American College of Physicians and the American Academy of Family Physicians recommend against performing screening pelvic exams in asymptomatic, nonpregnant adult women. The American College of Obstetricians and Gynecologists recommends annual pelvic exams for women 21 years and older but acknowledges a lack of evidence and has said it should be a shared decision between the patient and clinician.
The USPSTF members reported having no relevant financial conflicts.
Current evidence fails to support or reject routine screening pelvic exams for asymptomatic, low-risk, nonpregnant adult women, the U.S. Preventive Services Task Force concluded after reviewing the evidence on the accuracy, benefits, and potential harms.
The USPSTF issued an inconclusive “I” statement that was published online March 7 (JAMA. 2017;317[9]:947-53).
Researchers found no data comparing the impact of no screening versus screening pelvic examinations on patient health outcomes including reducing all-cause mortality, reducing cancer-specific and disease-specific morbidity and mortality, and improving quality of life.
“No direct evidence was identified for overall benefits and harms of the pelvic examination as a one-time or periodic screening test,” Janelle M. Guirguis-Blake, MD, of the University of Washington, Tacoma, and colleagues wrote in the accompanying evidence report (JAMA. 2017;317[9]:954-66). The review comprised nine studies: one addressing the harms of screening and eight addressing both harms and accuracy.
Although screening pelvic exams may identify serious conditions as well as benign ones, the potential remains for false-positive and false-negative results that might lead to invasive surgery and unnecessary testing and procedures, the researchers noted. However, the recommendations do not apply to certain conditions for which screening is already recommended, including cervical cancer (via Pap smear), gonorrhea, and chlamydia.
The recommendations are primarily a call for more research rather than a clear guide for clinicians, according to the USPSTF. The research gaps include studies on the physical and psychological harms of pelvic screening for asymptomatic women in primary care; the ability of screening to detect conditions beyond ovarian cancer, genital herpes, bacterial vaginosis, and trichomoniasis; and the impact of screening on a variety of health outcomes, including quality of life.
Given the inadequate evidence to recommend for or against screening, the USPSTF cited the recommendations of other organizations. Both the American College of Physicians and the American Academy of Family Physicians recommend against performing screening pelvic exams in asymptomatic, nonpregnant adult women. The American College of Obstetricians and Gynecologists recommends annual pelvic exams for women 21 years and older but acknowledges a lack of evidence and has said it should be a shared decision between the patient and clinician.
The USPSTF members reported having no relevant financial conflicts.
FROM JAMA
ACIP vaccine update, 2017
The Advisory Committee on Immunization Practices (ACIP) met 3 times in 2016 and introduced or revised recommendations on influenza, meningococcal, human papillomavirus (HPV), cholera, and hepatitis B vaccines. This Practice Alert highlights the most important new recommendations, except those for influenza vaccines, which were described in a previous Practice Alert.1 (See the summary of how this year’s flu season compares to last year’s.)
SIDEBAR
PRACTICE ALERT UPDATE
How this year's flu season compares to last yearThe 2016-2017 influenza season has been relatively mild, with activity nationwide picking up in late January and continuing to increase in February. As of February 16, 90% of the infections typed were type A, and most of those cases (more than 90%) were H3N1. Not surprisingly, the age group most heavily affected has been the elderly.
The hospitalization rate among those ≥65 years as of early February was 113.5/100,000, which is about half the rate of the same week during the 2014-2015 flu season. The hospitalization rate among those ages 50 to 64 years was 23.5/100,000—about 40% lower than the rate during the same week last flu season. At press time, 20 pediatric deaths had occurred, which is less than one-quarter of the number that occurred during the same time last year, and resistance to oseltamivir had not yet been detected in any isolates.
Source: Centers for Disease Control and Prevention. Situation update: summary of weekly FluView report. Available at: https://www.cdc.gov/flu/weekly/summary.htm. Accessed February 16, 2017.
Meningococcal vaccine: Now recommended for HIV-positive patients
Meningococcal conjugate vaccine (serogroups A, C, W, and Y) is recommended for all adolescents ages 11 to 12 as a single dose with a booster at age 16.2 It is also recommended for adults and for children (starting at age 2 months) who have high-risk conditions such as functional or anatomic asplenia or complement deficiencies. Others at high risk include microbiologists routinely exposed to isolates of Neisseria meningitidis and those traveling to areas of high meningococcal incidence. ACIP recently added human immunodeficiency virus (HIV) infection to the list of high-risk conditions.3
Two meningococcal conjugate vaccines are available in the United States: Menactra, (Sanofi Pasteur), licensed for use in individuals ages 9 months to 55 years; and Menveo (GlaxoSmithKline), licensed for use in individuals ages 2 months to 55 years. Menveo is the preferred vaccine for children younger than 2 years infected with HIV. However, if Menactra is used, give it at least 4 weeks after completing all pneumococcal conjugate vaccine doses and either before or concomitantly with diphtheria and tetanus toxoid and acellular pertussis vaccine (DTaP). All individuals who are HIV positive should receive a multi-dose primary series and booster doses. The number of primary doses and timing of boosters depends on the product used and the ages of those vaccinated (TABLE3).
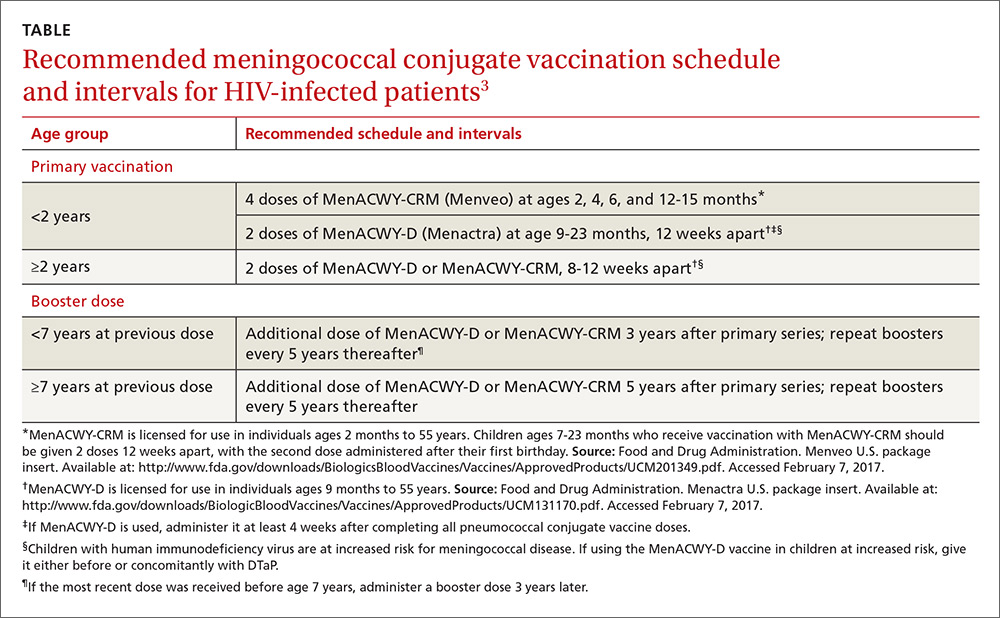
Although neither meningococcal conjugate vaccine product is licensed for use in individuals 56 years or older, ACIP recommends using one of the products for HIV-infected individuals in this age group because the only meningococcal vaccine licensed for use in adults 56 or older, meningococcal polysaccharide vaccine (MPSV4, Menomune, Sanofi Pasteur), has not been studied in patients with HIV infection.
Serogroup B. Two vaccine products provide short-term protection against meningococcal serogroup B: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). In 2015, ACIP made a “B” recommendation for the use of these vaccines in individuals 16 to 23 years of age, with the preferred age range being 16 to 18.4 A “B” recommendation means that while ACIP does not advise routine use of the vaccines in this age group, the vaccines can be administered to those who desire them. ACIP has recommended routine use of these products only for individuals 10 years and older who are at high risk for meningococcal disease.5
Trumenba was approved as a 3-dose vaccine, administered at 0, 2, and 6 months. Bexsero requires 2 doses given at least one month apart. At its October 2016 meeting, ACIP approved a 2-dose Trumenba schedule, at 0 and 6 months, when administered to those not at risk for meningococcal disease.6 However, during an outbreak, and for those at high risk for meningococcal disease, adhere to the original 3-dose schedule.
HPV vaccine: Now a 2-dose schedule for younger patients
The only HPV vaccine available in the United States is the 9-valent HPV vaccine (9vHPV), Gardasil 9. It is approved for both males and females ages 9 to 26 years. ACIP recommends it for both sexes at ages 11 or 12, and advises catch-up doses for men through age 21 and women through age 26. It also recommends vaccination through age 26 for men who have sex with men and men
The HPV vaccine is approved for a 3-dose schedule at 0, 1 to 2, and 6 months. At its October 2016 meeting, ACIP approved a 2-dose schedule (0, 6-12 months) for those starting the vaccine before their 15th birthday.7 Those starting the vaccine after their 15th birthday, and individuals at any age with an immune-compromising condition, should receive 3 doses. It is hoped that a 2-dose schedule will help to increase the uptake of this safe, effective, and underused vaccine.
Cholera: A new vaccine is available
In June 2016, the FDA approved a live, attenuated, single-dose, oral vaccine (Vaxchora, PaxVax, Inc.) for the prevention of cholera in adults ages 18 to 64 years. It is the only cholera vaccine approved in the United States.
Cholera occurs at low rates among travelers to areas where the disease is endemic. The key to prevention is food and water precautions, and thus the vaccine is not recommended for most travelers—only for those who are at increased risk of exposure to cholera or who have a medical condition that predisposes them to a poor response to medical care if cholera is contracted.8 Risk increases with long-term or frequent travel to endemic areas where safe food and water is not always available. Examples of compromising medical conditions include a blood type O, low gastric acidity, and heart or kidney disease.
Duration of the vaccine’s effectiveness is unknown, given a lack of data beyond 6 months. No recommendation for revaccination has been made, and this issue will be assessed as more data are collected. Other unknowns about the vaccine include its effectiveness among immune-suppressed individuals and pregnant women, as well as for those who live in cholera endemic areas or were previously vaccinated with another cholera vaccine.
Hepatitis B: Vaccinate newborns sooner
The incidence of hepatitis B virus (HBV) infection has declined by more than 90% since the introduction of a vaccine in 1982.9
Current recommendations for the prevention of HBV include:9
- Screen all pregnant women for hepatitis B surface antigen (HBsAg), and use HBIG and hepatitis B vaccines within 12 hours of birth for all newborns whose mothers are HBsAg positive or have an unknown HBsAg status.
- Administer the 3-dose hepatitis B vaccine to all other infants.
- Routinely vaccinate previously unvaccinated children and adolescents.
- Routinely vaccinate adults who are non-immune and at risk for HBV infection.
At its October 2016 meeting, ACIP adopted a comprehensive update of all HBV prevention recommendations. (This will be the subject of a future Practice Alert.) Included was a revision of a previously permissive recommendation that allowed the first dose of hepatitis B vaccine for newborns to be given within 2 months of hospital discharge. The new recommendation9 states that newborns of mothers known to be HBsAg negative should be vaccinated within 24 hours (if weight is ≥2000 g) or at age one month or at hospital discharge (if weight is <2000 g).
The first dose should be given within 12 hours of birth to all newborns whose mothers are HBsAg positive or have an unknown HBsAg status.9
Immunization schedules
Every year ACIP updates the adult and child immunization schedules to incorporate the changes from the previous year. These can be found on the ACIP Web site at https://www.cdc.gov/vaccines/schedules/hcp/index.html. This Web site remains the most authoritative and accurate source of information on vaccines and immunizations for both professionals and the public.
1. Campos-Outcalt D. Need-to-know information for the 2016-2017 flu season. J Fam Pract. 2016;65:613-617.
2. Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62:1-28.
3. MacNeil JR, Rubin LG, Patton M, et al. Recommendations for use of meningococcal conjugate vaccines in HIV-infected persons— Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1189-1194.
4. MacNeil JR, Rubin LG, Folaranmi T, et al. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1171-1176.
5. Folaranmi T, Rubin L, Martin SW, et al. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608-612.
6. MacNeil J. Considerations for Use of 2- and 3-Dose Schedules of MenB-FHbp (Trumenba). Presentation at: Advisory Committee on Immunization Practices; October 19, 2016; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-10/meningococcal-05-macneil.pdf. Accessed February 6, 2017.
7. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
8. Wong KW. Cholera vaccine update and proposed recommendations. Presentation at: Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/cholera-02-wong.pdf. Accessed January 27, 2017.
9. Schillie S. Revised ACIP Hepatitis B (HepB) vaccine recommendations. Presentation at: Advisory Committee on Immunization Practices; October 19, 2016. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-10/hepatitis-02-schillie-october-2016.pdf. Accessed January 27, 2017.
10.
11. Ko SC, Fan L, Smith EA, et al. Estimated annual perinatal hepatitis B virus infections in the United States, 2000-2009. J Pediatric Infect Dis Soc. 2016;5:114-121.
12. Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. MMWR Morb Mortal Wkly Rep. 2006;55:1-25.
13. Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099-1102.
The Advisory Committee on Immunization Practices (ACIP) met 3 times in 2016 and introduced or revised recommendations on influenza, meningococcal, human papillomavirus (HPV), cholera, and hepatitis B vaccines. This Practice Alert highlights the most important new recommendations, except those for influenza vaccines, which were described in a previous Practice Alert.1 (See the summary of how this year’s flu season compares to last year’s.)
SIDEBAR
PRACTICE ALERT UPDATE
How this year's flu season compares to last yearThe 2016-2017 influenza season has been relatively mild, with activity nationwide picking up in late January and continuing to increase in February. As of February 16, 90% of the infections typed were type A, and most of those cases (more than 90%) were H3N1. Not surprisingly, the age group most heavily affected has been the elderly.
The hospitalization rate among those ≥65 years as of early February was 113.5/100,000, which is about half the rate of the same week during the 2014-2015 flu season. The hospitalization rate among those ages 50 to 64 years was 23.5/100,000—about 40% lower than the rate during the same week last flu season. At press time, 20 pediatric deaths had occurred, which is less than one-quarter of the number that occurred during the same time last year, and resistance to oseltamivir had not yet been detected in any isolates.
Source: Centers for Disease Control and Prevention. Situation update: summary of weekly FluView report. Available at: https://www.cdc.gov/flu/weekly/summary.htm. Accessed February 16, 2017.
Meningococcal vaccine: Now recommended for HIV-positive patients
Meningococcal conjugate vaccine (serogroups A, C, W, and Y) is recommended for all adolescents ages 11 to 12 as a single dose with a booster at age 16.2 It is also recommended for adults and for children (starting at age 2 months) who have high-risk conditions such as functional or anatomic asplenia or complement deficiencies. Others at high risk include microbiologists routinely exposed to isolates of Neisseria meningitidis and those traveling to areas of high meningococcal incidence. ACIP recently added human immunodeficiency virus (HIV) infection to the list of high-risk conditions.3
Two meningococcal conjugate vaccines are available in the United States: Menactra, (Sanofi Pasteur), licensed for use in individuals ages 9 months to 55 years; and Menveo (GlaxoSmithKline), licensed for use in individuals ages 2 months to 55 years. Menveo is the preferred vaccine for children younger than 2 years infected with HIV. However, if Menactra is used, give it at least 4 weeks after completing all pneumococcal conjugate vaccine doses and either before or concomitantly with diphtheria and tetanus toxoid and acellular pertussis vaccine (DTaP). All individuals who are HIV positive should receive a multi-dose primary series and booster doses. The number of primary doses and timing of boosters depends on the product used and the ages of those vaccinated (TABLE3).

Although neither meningococcal conjugate vaccine product is licensed for use in individuals 56 years or older, ACIP recommends using one of the products for HIV-infected individuals in this age group because the only meningococcal vaccine licensed for use in adults 56 or older, meningococcal polysaccharide vaccine (MPSV4, Menomune, Sanofi Pasteur), has not been studied in patients with HIV infection.
Serogroup B. Two vaccine products provide short-term protection against meningococcal serogroup B: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). In 2015, ACIP made a “B” recommendation for the use of these vaccines in individuals 16 to 23 years of age, with the preferred age range being 16 to 18.4 A “B” recommendation means that while ACIP does not advise routine use of the vaccines in this age group, the vaccines can be administered to those who desire them. ACIP has recommended routine use of these products only for individuals 10 years and older who are at high risk for meningococcal disease.5
Trumenba was approved as a 3-dose vaccine, administered at 0, 2, and 6 months. Bexsero requires 2 doses given at least one month apart. At its October 2016 meeting, ACIP approved a 2-dose Trumenba schedule, at 0 and 6 months, when administered to those not at risk for meningococcal disease.6 However, during an outbreak, and for those at high risk for meningococcal disease, adhere to the original 3-dose schedule.
HPV vaccine: Now a 2-dose schedule for younger patients
The only HPV vaccine available in the United States is the 9-valent HPV vaccine (9vHPV), Gardasil 9. It is approved for both males and females ages 9 to 26 years. ACIP recommends it for both sexes at ages 11 or 12, and advises catch-up doses for men through age 21 and women through age 26. It also recommends vaccination through age 26 for men who have sex with men and men
The HPV vaccine is approved for a 3-dose schedule at 0, 1 to 2, and 6 months. At its October 2016 meeting, ACIP approved a 2-dose schedule (0, 6-12 months) for those starting the vaccine before their 15th birthday.7 Those starting the vaccine after their 15th birthday, and individuals at any age with an immune-compromising condition, should receive 3 doses. It is hoped that a 2-dose schedule will help to increase the uptake of this safe, effective, and underused vaccine.
Cholera: A new vaccine is available
In June 2016, the FDA approved a live, attenuated, single-dose, oral vaccine (Vaxchora, PaxVax, Inc.) for the prevention of cholera in adults ages 18 to 64 years. It is the only cholera vaccine approved in the United States.
Cholera occurs at low rates among travelers to areas where the disease is endemic. The key to prevention is food and water precautions, and thus the vaccine is not recommended for most travelers—only for those who are at increased risk of exposure to cholera or who have a medical condition that predisposes them to a poor response to medical care if cholera is contracted.8 Risk increases with long-term or frequent travel to endemic areas where safe food and water is not always available. Examples of compromising medical conditions include a blood type O, low gastric acidity, and heart or kidney disease.
Duration of the vaccine’s effectiveness is unknown, given a lack of data beyond 6 months. No recommendation for revaccination has been made, and this issue will be assessed as more data are collected. Other unknowns about the vaccine include its effectiveness among immune-suppressed individuals and pregnant women, as well as for those who live in cholera endemic areas or were previously vaccinated with another cholera vaccine.
Hepatitis B: Vaccinate newborns sooner
The incidence of hepatitis B virus (HBV) infection has declined by more than 90% since the introduction of a vaccine in 1982.9
Current recommendations for the prevention of HBV include:9
- Screen all pregnant women for hepatitis B surface antigen (HBsAg), and use HBIG and hepatitis B vaccines within 12 hours of birth for all newborns whose mothers are HBsAg positive or have an unknown HBsAg status.
- Administer the 3-dose hepatitis B vaccine to all other infants.
- Routinely vaccinate previously unvaccinated children and adolescents.
- Routinely vaccinate adults who are non-immune and at risk for HBV infection.
At its October 2016 meeting, ACIP adopted a comprehensive update of all HBV prevention recommendations. (This will be the subject of a future Practice Alert.) Included was a revision of a previously permissive recommendation that allowed the first dose of hepatitis B vaccine for newborns to be given within 2 months of hospital discharge. The new recommendation9 states that newborns of mothers known to be HBsAg negative should be vaccinated within 24 hours (if weight is ≥2000 g) or at age one month or at hospital discharge (if weight is <2000 g).
The first dose should be given within 12 hours of birth to all newborns whose mothers are HBsAg positive or have an unknown HBsAg status.9
Immunization schedules
Every year ACIP updates the adult and child immunization schedules to incorporate the changes from the previous year. These can be found on the ACIP Web site at https://www.cdc.gov/vaccines/schedules/hcp/index.html. This Web site remains the most authoritative and accurate source of information on vaccines and immunizations for both professionals and the public.
The Advisory Committee on Immunization Practices (ACIP) met 3 times in 2016 and introduced or revised recommendations on influenza, meningococcal, human papillomavirus (HPV), cholera, and hepatitis B vaccines. This Practice Alert highlights the most important new recommendations, except those for influenza vaccines, which were described in a previous Practice Alert.1 (See the summary of how this year’s flu season compares to last year’s.)
SIDEBAR
PRACTICE ALERT UPDATE
How this year's flu season compares to last yearThe 2016-2017 influenza season has been relatively mild, with activity nationwide picking up in late January and continuing to increase in February. As of February 16, 90% of the infections typed were type A, and most of those cases (more than 90%) were H3N1. Not surprisingly, the age group most heavily affected has been the elderly.
The hospitalization rate among those ≥65 years as of early February was 113.5/100,000, which is about half the rate of the same week during the 2014-2015 flu season. The hospitalization rate among those ages 50 to 64 years was 23.5/100,000—about 40% lower than the rate during the same week last flu season. At press time, 20 pediatric deaths had occurred, which is less than one-quarter of the number that occurred during the same time last year, and resistance to oseltamivir had not yet been detected in any isolates.
Source: Centers for Disease Control and Prevention. Situation update: summary of weekly FluView report. Available at: https://www.cdc.gov/flu/weekly/summary.htm. Accessed February 16, 2017.
Meningococcal vaccine: Now recommended for HIV-positive patients
Meningococcal conjugate vaccine (serogroups A, C, W, and Y) is recommended for all adolescents ages 11 to 12 as a single dose with a booster at age 16.2 It is also recommended for adults and for children (starting at age 2 months) who have high-risk conditions such as functional or anatomic asplenia or complement deficiencies. Others at high risk include microbiologists routinely exposed to isolates of Neisseria meningitidis and those traveling to areas of high meningococcal incidence. ACIP recently added human immunodeficiency virus (HIV) infection to the list of high-risk conditions.3
Two meningococcal conjugate vaccines are available in the United States: Menactra, (Sanofi Pasteur), licensed for use in individuals ages 9 months to 55 years; and Menveo (GlaxoSmithKline), licensed for use in individuals ages 2 months to 55 years. Menveo is the preferred vaccine for children younger than 2 years infected with HIV. However, if Menactra is used, give it at least 4 weeks after completing all pneumococcal conjugate vaccine doses and either before or concomitantly with diphtheria and tetanus toxoid and acellular pertussis vaccine (DTaP). All individuals who are HIV positive should receive a multi-dose primary series and booster doses. The number of primary doses and timing of boosters depends on the product used and the ages of those vaccinated (TABLE3).

Although neither meningococcal conjugate vaccine product is licensed for use in individuals 56 years or older, ACIP recommends using one of the products for HIV-infected individuals in this age group because the only meningococcal vaccine licensed for use in adults 56 or older, meningococcal polysaccharide vaccine (MPSV4, Menomune, Sanofi Pasteur), has not been studied in patients with HIV infection.
Serogroup B. Two vaccine products provide short-term protection against meningococcal serogroup B: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). In 2015, ACIP made a “B” recommendation for the use of these vaccines in individuals 16 to 23 years of age, with the preferred age range being 16 to 18.4 A “B” recommendation means that while ACIP does not advise routine use of the vaccines in this age group, the vaccines can be administered to those who desire them. ACIP has recommended routine use of these products only for individuals 10 years and older who are at high risk for meningococcal disease.5
Trumenba was approved as a 3-dose vaccine, administered at 0, 2, and 6 months. Bexsero requires 2 doses given at least one month apart. At its October 2016 meeting, ACIP approved a 2-dose Trumenba schedule, at 0 and 6 months, when administered to those not at risk for meningococcal disease.6 However, during an outbreak, and for those at high risk for meningococcal disease, adhere to the original 3-dose schedule.
HPV vaccine: Now a 2-dose schedule for younger patients
The only HPV vaccine available in the United States is the 9-valent HPV vaccine (9vHPV), Gardasil 9. It is approved for both males and females ages 9 to 26 years. ACIP recommends it for both sexes at ages 11 or 12, and advises catch-up doses for men through age 21 and women through age 26. It also recommends vaccination through age 26 for men who have sex with men and men
The HPV vaccine is approved for a 3-dose schedule at 0, 1 to 2, and 6 months. At its October 2016 meeting, ACIP approved a 2-dose schedule (0, 6-12 months) for those starting the vaccine before their 15th birthday.7 Those starting the vaccine after their 15th birthday, and individuals at any age with an immune-compromising condition, should receive 3 doses. It is hoped that a 2-dose schedule will help to increase the uptake of this safe, effective, and underused vaccine.
Cholera: A new vaccine is available
In June 2016, the FDA approved a live, attenuated, single-dose, oral vaccine (Vaxchora, PaxVax, Inc.) for the prevention of cholera in adults ages 18 to 64 years. It is the only cholera vaccine approved in the United States.
Cholera occurs at low rates among travelers to areas where the disease is endemic. The key to prevention is food and water precautions, and thus the vaccine is not recommended for most travelers—only for those who are at increased risk of exposure to cholera or who have a medical condition that predisposes them to a poor response to medical care if cholera is contracted.8 Risk increases with long-term or frequent travel to endemic areas where safe food and water is not always available. Examples of compromising medical conditions include a blood type O, low gastric acidity, and heart or kidney disease.
Duration of the vaccine’s effectiveness is unknown, given a lack of data beyond 6 months. No recommendation for revaccination has been made, and this issue will be assessed as more data are collected. Other unknowns about the vaccine include its effectiveness among immune-suppressed individuals and pregnant women, as well as for those who live in cholera endemic areas or were previously vaccinated with another cholera vaccine.
Hepatitis B: Vaccinate newborns sooner
The incidence of hepatitis B virus (HBV) infection has declined by more than 90% since the introduction of a vaccine in 1982.9
Current recommendations for the prevention of HBV include:9
- Screen all pregnant women for hepatitis B surface antigen (HBsAg), and use HBIG and hepatitis B vaccines within 12 hours of birth for all newborns whose mothers are HBsAg positive or have an unknown HBsAg status.
- Administer the 3-dose hepatitis B vaccine to all other infants.
- Routinely vaccinate previously unvaccinated children and adolescents.
- Routinely vaccinate adults who are non-immune and at risk for HBV infection.
At its October 2016 meeting, ACIP adopted a comprehensive update of all HBV prevention recommendations. (This will be the subject of a future Practice Alert.) Included was a revision of a previously permissive recommendation that allowed the first dose of hepatitis B vaccine for newborns to be given within 2 months of hospital discharge. The new recommendation9 states that newborns of mothers known to be HBsAg negative should be vaccinated within 24 hours (if weight is ≥2000 g) or at age one month or at hospital discharge (if weight is <2000 g).
The first dose should be given within 12 hours of birth to all newborns whose mothers are HBsAg positive or have an unknown HBsAg status.9
Immunization schedules
Every year ACIP updates the adult and child immunization schedules to incorporate the changes from the previous year. These can be found on the ACIP Web site at https://www.cdc.gov/vaccines/schedules/hcp/index.html. This Web site remains the most authoritative and accurate source of information on vaccines and immunizations for both professionals and the public.
1. Campos-Outcalt D. Need-to-know information for the 2016-2017 flu season. J Fam Pract. 2016;65:613-617.
2. Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62:1-28.
3. MacNeil JR, Rubin LG, Patton M, et al. Recommendations for use of meningococcal conjugate vaccines in HIV-infected persons— Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1189-1194.
4. MacNeil JR, Rubin LG, Folaranmi T, et al. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1171-1176.
5. Folaranmi T, Rubin L, Martin SW, et al. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608-612.
6. MacNeil J. Considerations for Use of 2- and 3-Dose Schedules of MenB-FHbp (Trumenba). Presentation at: Advisory Committee on Immunization Practices; October 19, 2016; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-10/meningococcal-05-macneil.pdf. Accessed February 6, 2017.
7. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
8. Wong KW. Cholera vaccine update and proposed recommendations. Presentation at: Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/cholera-02-wong.pdf. Accessed January 27, 2017.
9. Schillie S. Revised ACIP Hepatitis B (HepB) vaccine recommendations. Presentation at: Advisory Committee on Immunization Practices; October 19, 2016. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-10/hepatitis-02-schillie-october-2016.pdf. Accessed January 27, 2017.
10.
11. Ko SC, Fan L, Smith EA, et al. Estimated annual perinatal hepatitis B virus infections in the United States, 2000-2009. J Pediatric Infect Dis Soc. 2016;5:114-121.
12. Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. MMWR Morb Mortal Wkly Rep. 2006;55:1-25.
13. Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099-1102.
1. Campos-Outcalt D. Need-to-know information for the 2016-2017 flu season. J Fam Pract. 2016;65:613-617.
2. Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62:1-28.
3. MacNeil JR, Rubin LG, Patton M, et al. Recommendations for use of meningococcal conjugate vaccines in HIV-infected persons— Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1189-1194.
4. MacNeil JR, Rubin LG, Folaranmi T, et al. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1171-1176.
5. Folaranmi T, Rubin L, Martin SW, et al. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608-612.
6. MacNeil J. Considerations for Use of 2- and 3-Dose Schedules of MenB-FHbp (Trumenba). Presentation at: Advisory Committee on Immunization Practices; October 19, 2016; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-10/meningococcal-05-macneil.pdf. Accessed February 6, 2017.
7. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
8. Wong KW. Cholera vaccine update and proposed recommendations. Presentation at: Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/cholera-02-wong.pdf. Accessed January 27, 2017.
9. Schillie S. Revised ACIP Hepatitis B (HepB) vaccine recommendations. Presentation at: Advisory Committee on Immunization Practices; October 19, 2016. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-10/hepatitis-02-schillie-october-2016.pdf. Accessed January 27, 2017.
10.
11. Ko SC, Fan L, Smith EA, et al. Estimated annual perinatal hepatitis B virus infections in the United States, 2000-2009. J Pediatric Infect Dis Soc. 2016;5:114-121.
12. Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. MMWR Morb Mortal Wkly Rep. 2006;55:1-25.
13. Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099-1102.
Guidelines tackle long-term screening, management of myeloma
New guidelines recommend proactively screening for the late-term effects of both myeloma itself and the multiple therapies many patients receive.
“We are entering a watershed period in which patients are expecting to live in excess of 5 to 10 years after a diagnosis of myeloma, and issues of survivorship are becoming increasingly important,” wrote John A. Snowden, MD, of Sheffield (England) Teaching Hospitals NHS Foundation Trust and his associates on behalf of the UK Myeloma Forum and the British Society for Haematology.
Late effects of myeloma and therapies “constitute a unique syndrome,” the guideline authors emphasized. “Survivorship in myeloma therefore requires specialized screening, coordinated management and multidisciplinary care” (Br J Haematol. 2017 Jan 20. doi: 10.1111/bjh.14514).
Patients with myeloma should not receive live attenuated vaccines, the guidelines noted. Inactivated vaccinations should be timed to periods of minimal disease and after treatment recovery. The authors recommended influenza and varicella-zoster vaccines for both patients and household contacts. For patients, they also recommended Haemophilus influenzae type b vaccine and conjugate pneumococcal vaccine, followed by polysaccharide PPV23 at least 2 months later. They also suggested revaccination after HSCT.
About half of myeloma patients have renal impairment and should undergo routine tests of serum calcium, parathyroid hormone, and vitamin D, the authors stated. Moderate to severe renal impairment, renal-related hyperparathyroidism, and nephrotic syndrome merit specialty referrals, they added. They also advised carefully managing diabetes and hypertension to delay end-stage kidney disease, modifying doses of lenalidomide and bisphosphonate doses in renally impaired patients, avoiding nephrotoxic drugs when possible, and considering erythropoiesis-stimulating agents and iron supplementation for anemia.
Endocrine disorders are also common in myeloma, and the authors recommended annual screening for hypothyroidism, hypogonadism in males, and menopausal symptoms in younger females, especially after HSCT. They emphasized annual measurements of weight, height, body mass index, waist circumference, strength and frailty, blood pressure, HbA1c, and serum lipids, with referral to primary care when needed. For bone loss, they emphasized weight-bearing exercise, bisphosphonates, dietary changes, and calcium and vitamin D supplementation. They recommended specialist input on hormone therapy, if indicated.
Spinal cord or nerve-root compression often accompanies myeloma, and long-term survivors also may have peripheral neuropathy secondary to chemotherapy and other drug treatments, the guidelines noted. They recommended testing thyroid function and vitamin B12 levels, reducing or eliminating neurotoxic agents, offering gabapentin or pregabalin for symptom control, and referring patients to pain specialists and neurologists for peripheral neuropathy beyond grade I. They also advised annual ophthalmic screening because even intermittent high-dose corticosteroid therapy can lead to cataracts.
Cardiopulmonary abnormalities affect about half of myeloma patients and deserve heightened attention, the authors stressed. They recommended routinely screening cardiovascular risk factors, testing natriuretic peptide annually, and performing electrocardiograms, echocardiography, and pulmonary function tests in at-risk patients. They also advised diet, weight control, smoking cessation, physical activity, and specialist referral for patients with established cardiovascular or pulmonary disease.
Bisphosphonates can cause osteonecrosis of the jaw, and chemotherapy and other therapies can cause oral dryness. The guidelines emphasized – in addition to monitoring for these adverse outcomes – the importance of oral hygiene, artificial saliva rinses, annual dental exams, and specialty evaluations for nonhealing lesions.
Novel myeloma therapies often cause diarrhea, but chronic diarrhea should be evaluated by a gastroenterologist to rule out malignancies, underlying bowel disease, AL amyloidosis, and bile acid malabsorption, the authors stressed. They also recommended annual assessments of liver function tests, drug and alcohol history, and vitamin D, B12, folate, and ferritin levels. Nutritionists should provide input if patients are losing weight, they added.
Myeloma confers at least eight times the risk of myelodysplastic syndrome and acute myeloid leukemia, compared with the general population, the guidelines noted. Second primary malignancies can result from long-term exposure to lenalidomide and to such alkylating agents as melphalan. They advised considering myelodysplastic syndrome and acute myeloid leukemia in patients with persistent or worsening cytopenias, investigating symptoms that could indicate other malignancies, participating in cancer screening registries, and developing formal surveillance for second primary malignancies.
Additional recommendations included baseline geriatric assessment in elderly and frail patients; holistic assessments at the start of each line of treatment to pinpoint needs and concerns and to plan support services; and regular assessments of mood, anxiety, and cognitive status, with referrals for therapy, psychiatry, and support groups as needed. The authors also stressed the role of routine holistic needs-assessments to detect and track both pain and fatigue. Therapy should always include prehabilitation and rehabilitation, and clinicians should recommend ongoing regular physical activity and a healthy lifestyle, they emphasized.
To develop the guidelines, the experts searched Medline and the Cochrane databases for literature published between 2006 and March 31, 2016. They based key recommendations on evidence from randomized, controlled trials. When those data were not available, they resorted to other studies and to consensus expert opinion. The recommendations take cost-effectiveness into account, but are not based on formal health economic assessments, the experts noted.
Myeloma UK paid for an independent medical writer to help search the literature and draft the manuscript. Dr. Snowden also disclosed support from Sheffield Hospitals Charity.
New guidelines recommend proactively screening for the late-term effects of both myeloma itself and the multiple therapies many patients receive.
“We are entering a watershed period in which patients are expecting to live in excess of 5 to 10 years after a diagnosis of myeloma, and issues of survivorship are becoming increasingly important,” wrote John A. Snowden, MD, of Sheffield (England) Teaching Hospitals NHS Foundation Trust and his associates on behalf of the UK Myeloma Forum and the British Society for Haematology.
Late effects of myeloma and therapies “constitute a unique syndrome,” the guideline authors emphasized. “Survivorship in myeloma therefore requires specialized screening, coordinated management and multidisciplinary care” (Br J Haematol. 2017 Jan 20. doi: 10.1111/bjh.14514).
Patients with myeloma should not receive live attenuated vaccines, the guidelines noted. Inactivated vaccinations should be timed to periods of minimal disease and after treatment recovery. The authors recommended influenza and varicella-zoster vaccines for both patients and household contacts. For patients, they also recommended Haemophilus influenzae type b vaccine and conjugate pneumococcal vaccine, followed by polysaccharide PPV23 at least 2 months later. They also suggested revaccination after HSCT.
About half of myeloma patients have renal impairment and should undergo routine tests of serum calcium, parathyroid hormone, and vitamin D, the authors stated. Moderate to severe renal impairment, renal-related hyperparathyroidism, and nephrotic syndrome merit specialty referrals, they added. They also advised carefully managing diabetes and hypertension to delay end-stage kidney disease, modifying doses of lenalidomide and bisphosphonate doses in renally impaired patients, avoiding nephrotoxic drugs when possible, and considering erythropoiesis-stimulating agents and iron supplementation for anemia.
Endocrine disorders are also common in myeloma, and the authors recommended annual screening for hypothyroidism, hypogonadism in males, and menopausal symptoms in younger females, especially after HSCT. They emphasized annual measurements of weight, height, body mass index, waist circumference, strength and frailty, blood pressure, HbA1c, and serum lipids, with referral to primary care when needed. For bone loss, they emphasized weight-bearing exercise, bisphosphonates, dietary changes, and calcium and vitamin D supplementation. They recommended specialist input on hormone therapy, if indicated.
Spinal cord or nerve-root compression often accompanies myeloma, and long-term survivors also may have peripheral neuropathy secondary to chemotherapy and other drug treatments, the guidelines noted. They recommended testing thyroid function and vitamin B12 levels, reducing or eliminating neurotoxic agents, offering gabapentin or pregabalin for symptom control, and referring patients to pain specialists and neurologists for peripheral neuropathy beyond grade I. They also advised annual ophthalmic screening because even intermittent high-dose corticosteroid therapy can lead to cataracts.
Cardiopulmonary abnormalities affect about half of myeloma patients and deserve heightened attention, the authors stressed. They recommended routinely screening cardiovascular risk factors, testing natriuretic peptide annually, and performing electrocardiograms, echocardiography, and pulmonary function tests in at-risk patients. They also advised diet, weight control, smoking cessation, physical activity, and specialist referral for patients with established cardiovascular or pulmonary disease.
Bisphosphonates can cause osteonecrosis of the jaw, and chemotherapy and other therapies can cause oral dryness. The guidelines emphasized – in addition to monitoring for these adverse outcomes – the importance of oral hygiene, artificial saliva rinses, annual dental exams, and specialty evaluations for nonhealing lesions.
Novel myeloma therapies often cause diarrhea, but chronic diarrhea should be evaluated by a gastroenterologist to rule out malignancies, underlying bowel disease, AL amyloidosis, and bile acid malabsorption, the authors stressed. They also recommended annual assessments of liver function tests, drug and alcohol history, and vitamin D, B12, folate, and ferritin levels. Nutritionists should provide input if patients are losing weight, they added.
Myeloma confers at least eight times the risk of myelodysplastic syndrome and acute myeloid leukemia, compared with the general population, the guidelines noted. Second primary malignancies can result from long-term exposure to lenalidomide and to such alkylating agents as melphalan. They advised considering myelodysplastic syndrome and acute myeloid leukemia in patients with persistent or worsening cytopenias, investigating symptoms that could indicate other malignancies, participating in cancer screening registries, and developing formal surveillance for second primary malignancies.
Additional recommendations included baseline geriatric assessment in elderly and frail patients; holistic assessments at the start of each line of treatment to pinpoint needs and concerns and to plan support services; and regular assessments of mood, anxiety, and cognitive status, with referrals for therapy, psychiatry, and support groups as needed. The authors also stressed the role of routine holistic needs-assessments to detect and track both pain and fatigue. Therapy should always include prehabilitation and rehabilitation, and clinicians should recommend ongoing regular physical activity and a healthy lifestyle, they emphasized.
To develop the guidelines, the experts searched Medline and the Cochrane databases for literature published between 2006 and March 31, 2016. They based key recommendations on evidence from randomized, controlled trials. When those data were not available, they resorted to other studies and to consensus expert opinion. The recommendations take cost-effectiveness into account, but are not based on formal health economic assessments, the experts noted.
Myeloma UK paid for an independent medical writer to help search the literature and draft the manuscript. Dr. Snowden also disclosed support from Sheffield Hospitals Charity.
New guidelines recommend proactively screening for the late-term effects of both myeloma itself and the multiple therapies many patients receive.
“We are entering a watershed period in which patients are expecting to live in excess of 5 to 10 years after a diagnosis of myeloma, and issues of survivorship are becoming increasingly important,” wrote John A. Snowden, MD, of Sheffield (England) Teaching Hospitals NHS Foundation Trust and his associates on behalf of the UK Myeloma Forum and the British Society for Haematology.
Late effects of myeloma and therapies “constitute a unique syndrome,” the guideline authors emphasized. “Survivorship in myeloma therefore requires specialized screening, coordinated management and multidisciplinary care” (Br J Haematol. 2017 Jan 20. doi: 10.1111/bjh.14514).
Patients with myeloma should not receive live attenuated vaccines, the guidelines noted. Inactivated vaccinations should be timed to periods of minimal disease and after treatment recovery. The authors recommended influenza and varicella-zoster vaccines for both patients and household contacts. For patients, they also recommended Haemophilus influenzae type b vaccine and conjugate pneumococcal vaccine, followed by polysaccharide PPV23 at least 2 months later. They also suggested revaccination after HSCT.
About half of myeloma patients have renal impairment and should undergo routine tests of serum calcium, parathyroid hormone, and vitamin D, the authors stated. Moderate to severe renal impairment, renal-related hyperparathyroidism, and nephrotic syndrome merit specialty referrals, they added. They also advised carefully managing diabetes and hypertension to delay end-stage kidney disease, modifying doses of lenalidomide and bisphosphonate doses in renally impaired patients, avoiding nephrotoxic drugs when possible, and considering erythropoiesis-stimulating agents and iron supplementation for anemia.
Endocrine disorders are also common in myeloma, and the authors recommended annual screening for hypothyroidism, hypogonadism in males, and menopausal symptoms in younger females, especially after HSCT. They emphasized annual measurements of weight, height, body mass index, waist circumference, strength and frailty, blood pressure, HbA1c, and serum lipids, with referral to primary care when needed. For bone loss, they emphasized weight-bearing exercise, bisphosphonates, dietary changes, and calcium and vitamin D supplementation. They recommended specialist input on hormone therapy, if indicated.
Spinal cord or nerve-root compression often accompanies myeloma, and long-term survivors also may have peripheral neuropathy secondary to chemotherapy and other drug treatments, the guidelines noted. They recommended testing thyroid function and vitamin B12 levels, reducing or eliminating neurotoxic agents, offering gabapentin or pregabalin for symptom control, and referring patients to pain specialists and neurologists for peripheral neuropathy beyond grade I. They also advised annual ophthalmic screening because even intermittent high-dose corticosteroid therapy can lead to cataracts.
Cardiopulmonary abnormalities affect about half of myeloma patients and deserve heightened attention, the authors stressed. They recommended routinely screening cardiovascular risk factors, testing natriuretic peptide annually, and performing electrocardiograms, echocardiography, and pulmonary function tests in at-risk patients. They also advised diet, weight control, smoking cessation, physical activity, and specialist referral for patients with established cardiovascular or pulmonary disease.
Bisphosphonates can cause osteonecrosis of the jaw, and chemotherapy and other therapies can cause oral dryness. The guidelines emphasized – in addition to monitoring for these adverse outcomes – the importance of oral hygiene, artificial saliva rinses, annual dental exams, and specialty evaluations for nonhealing lesions.
Novel myeloma therapies often cause diarrhea, but chronic diarrhea should be evaluated by a gastroenterologist to rule out malignancies, underlying bowel disease, AL amyloidosis, and bile acid malabsorption, the authors stressed. They also recommended annual assessments of liver function tests, drug and alcohol history, and vitamin D, B12, folate, and ferritin levels. Nutritionists should provide input if patients are losing weight, they added.
Myeloma confers at least eight times the risk of myelodysplastic syndrome and acute myeloid leukemia, compared with the general population, the guidelines noted. Second primary malignancies can result from long-term exposure to lenalidomide and to such alkylating agents as melphalan. They advised considering myelodysplastic syndrome and acute myeloid leukemia in patients with persistent or worsening cytopenias, investigating symptoms that could indicate other malignancies, participating in cancer screening registries, and developing formal surveillance for second primary malignancies.
Additional recommendations included baseline geriatric assessment in elderly and frail patients; holistic assessments at the start of each line of treatment to pinpoint needs and concerns and to plan support services; and regular assessments of mood, anxiety, and cognitive status, with referrals for therapy, psychiatry, and support groups as needed. The authors also stressed the role of routine holistic needs-assessments to detect and track both pain and fatigue. Therapy should always include prehabilitation and rehabilitation, and clinicians should recommend ongoing regular physical activity and a healthy lifestyle, they emphasized.
To develop the guidelines, the experts searched Medline and the Cochrane databases for literature published between 2006 and March 31, 2016. They based key recommendations on evidence from randomized, controlled trials. When those data were not available, they resorted to other studies and to consensus expert opinion. The recommendations take cost-effectiveness into account, but are not based on formal health economic assessments, the experts noted.
Myeloma UK paid for an independent medical writer to help search the literature and draft the manuscript. Dr. Snowden also disclosed support from Sheffield Hospitals Charity.
FROM BRITISH JOURNAL OF HAEMATOLOGY
Guideline: Prioritize nondrug therapies for low back pain
Clinicians and patients should prioritize nonpharmacologic therapies for low back pain of any duration, according to an updated guideline from the American College of Physicians.
For acute and subacute low back pain, first-line choices include heat, massage, acupuncture, and spinal manipulation, Amir Qaseem, MD, PhD, and his associates wrote in the Annals of Internal Medicine. Patients with chronic low back pain have many nondrug options, ranging from exercise and tai chi to mindfulness-based stress reduction and spinal manipulation, the authors add (Ann Intern Med. 2017 Feb 14. doi: 10.7326/M16-2367).
The updated therapeutic recommendations focus on clinical presentations. They define acute low back pain as lasting less than 4 weeks, subacute low back pain as lasting 4-12 weeks, and chronic low back pain as lasting more than 12 weeks. For acute and subacute low back pain, low to moderate quality evidence supports the efficacy of acupuncture, massage, spinal manipulation, superficial heat, lumbar supports, and low-level laser therapy, the guideline authors conclude.
They recommend considering nonsteroidal anti-inflammatory drugs or skeletal muscle relaxants for patients who want medications for acute or subacute low back pain. There is moderate-quality evidence that NSAIDs confer a small analgesic benefit, compared with placebo, but their renal and gastrointestinal risks call for careful patient selection and use of the lowest possible doses and treatment durations, the authors emphasized.
Likewise, moderate-quality evidence supports the use of skeletal muscle relaxants for short-term pain relief, but patients should know that these drugs can lead to sedation and other adverse effects on the central nervous system, they stated.
Acetaminophen is no longer recommended for low back pain, having failed to shorten time to recovery, compared with placebo, in a large, multicenter, randomized trial (Lancet. 2014 Nov 1;384[9954]:1586-96).
Likewise, short-term oral or intramuscular corticosteroids have been found ineffective for acute low back pain, while benzodiazepines are ineffective for radiculopathy, the experts noted.
“Evidence was insufficient to determine effectiveness of antidepressants, benzodiazepines, antiseizure medications, or opioids, versus placebo, in patients with acute or subacute low back pain,” they added.
The guideline authors also noted insufficient evidence for many nondrug therapies for acute and subacute low back pain, including transcutaneous electrical nerve stimulation, electrical muscle stimulation, inferential therapy, short-wave diathermy, traction, superficial cold, motor control exercise, Pilates, tai chi, yoga, psychological therapies, multidisciplinary rehabilitation, ultrasound, and taping.
For chronic low back pain, the guideline strongly recommends starting with nondrug therapies, including exercise, multidisciplinary rehabilitation, acupuncture, mindfulness-based stress reduction, tai chi, yoga, motor control exercise, progressive relaxation, electromyography biofeedback, low-level laser therapy, operant therapy, cognitive behavioral therapy, or spinal manipulation.
Despite low-quality evidence for these modalities, “fewer harms are associated with these types of therapies than with pharmacologic options,” the authors wrote.
If nonpharmacologic interventions fail to improve chronic low back pain, the experts recommended NSAIDs in the first line, followed by second-line therapy with tramadol or duloxetine (Cymbalta). Recent evidence suggests that NSAIDs are less effective for low back pain than previously thought, while the trials that reported a modest analgesic benefit of duloxetine over placebo were industry funded, the authors note.
Opioids should only be considered for chronic low back pain that fails both nondrug and nonopioid therapies, “and only if the potential benefits outweigh the risks for individual patients, and after a discussion of known risks and realistic benefits,” the guideline authors emphasized.
This update does not cover topical therapies or epidural injections. Epidural steroid injections decreased pain associated with radiculopathy in the short term but did not confer long-term benefits, according to a recent separate review (Ann Intern Med. 2015 Sep 1;163[5]:373-81).
The Agency for Healthcare Research and Quality funded the work. One coauthor disclosed personal fees from Takeda Pharmaceuticals outside the submitted work, and membership in the American College of Physicians Clinical Guidelines Committee and the American College of Rheumatology Quality of Care Committee. The other authors had no conflicts. Two members of the ACP Clinical Guidelines Committee disclosed ties to Healthwise and UpToDate outside the submitted work.
Despite the considerable effort invested in these systematic reviews and in providing clinicians with rational recommendations for care, doubts exist as to whether simply publishing this work will be sufficient to drive guideline-concordant care.
Systematic reviews and recommendations from governmental organizations and professional societies are not new and predate large increases in diagnostic and therapeutic services.
For example, the lack of evidence supporting opiates for low back pain did not prevent their dramatic increase in use. Moreover, these updated reviews and recommendations do not focus on diagnostic tests, such as magnetic resonance imaging, and invasive therapies, such as injections and surgery, which are major drivers of health care spending for low back pain.
If clinicians and their professional societies cannot demonstrate that their recommendations are improving the delivery of high-value services, what are the alternatives?
Likely what is needed is an “all of the above” approach: more pragmatic trials to evaluate proven therapies and their combinations in real-world settings; efforts to reduce the use of low-value services, such as payer coverage policies based on guideline recommendations; patient engagement through shared decision making; and pressure on insurers to cover nonpharmacologic, noninvasive therapies that have shown benefit.
Steven J. Atlas, MD, MPH, is at Massachusetts General Hospital in Boston. He disclosed royalties from UpToDate and personal fees from Healthwise. These comments are from his editorial (Ann Intern Med. 2017 Feb 14. doi: 10.7326/M17-0923).
Despite the considerable effort invested in these systematic reviews and in providing clinicians with rational recommendations for care, doubts exist as to whether simply publishing this work will be sufficient to drive guideline-concordant care.
Systematic reviews and recommendations from governmental organizations and professional societies are not new and predate large increases in diagnostic and therapeutic services.
For example, the lack of evidence supporting opiates for low back pain did not prevent their dramatic increase in use. Moreover, these updated reviews and recommendations do not focus on diagnostic tests, such as magnetic resonance imaging, and invasive therapies, such as injections and surgery, which are major drivers of health care spending for low back pain.
If clinicians and their professional societies cannot demonstrate that their recommendations are improving the delivery of high-value services, what are the alternatives?
Likely what is needed is an “all of the above” approach: more pragmatic trials to evaluate proven therapies and their combinations in real-world settings; efforts to reduce the use of low-value services, such as payer coverage policies based on guideline recommendations; patient engagement through shared decision making; and pressure on insurers to cover nonpharmacologic, noninvasive therapies that have shown benefit.
Steven J. Atlas, MD, MPH, is at Massachusetts General Hospital in Boston. He disclosed royalties from UpToDate and personal fees from Healthwise. These comments are from his editorial (Ann Intern Med. 2017 Feb 14. doi: 10.7326/M17-0923).
Despite the considerable effort invested in these systematic reviews and in providing clinicians with rational recommendations for care, doubts exist as to whether simply publishing this work will be sufficient to drive guideline-concordant care.
Systematic reviews and recommendations from governmental organizations and professional societies are not new and predate large increases in diagnostic and therapeutic services.
For example, the lack of evidence supporting opiates for low back pain did not prevent their dramatic increase in use. Moreover, these updated reviews and recommendations do not focus on diagnostic tests, such as magnetic resonance imaging, and invasive therapies, such as injections and surgery, which are major drivers of health care spending for low back pain.
If clinicians and their professional societies cannot demonstrate that their recommendations are improving the delivery of high-value services, what are the alternatives?
Likely what is needed is an “all of the above” approach: more pragmatic trials to evaluate proven therapies and their combinations in real-world settings; efforts to reduce the use of low-value services, such as payer coverage policies based on guideline recommendations; patient engagement through shared decision making; and pressure on insurers to cover nonpharmacologic, noninvasive therapies that have shown benefit.
Steven J. Atlas, MD, MPH, is at Massachusetts General Hospital in Boston. He disclosed royalties from UpToDate and personal fees from Healthwise. These comments are from his editorial (Ann Intern Med. 2017 Feb 14. doi: 10.7326/M17-0923).
Clinicians and patients should prioritize nonpharmacologic therapies for low back pain of any duration, according to an updated guideline from the American College of Physicians.
For acute and subacute low back pain, first-line choices include heat, massage, acupuncture, and spinal manipulation, Amir Qaseem, MD, PhD, and his associates wrote in the Annals of Internal Medicine. Patients with chronic low back pain have many nondrug options, ranging from exercise and tai chi to mindfulness-based stress reduction and spinal manipulation, the authors add (Ann Intern Med. 2017 Feb 14. doi: 10.7326/M16-2367).
The updated therapeutic recommendations focus on clinical presentations. They define acute low back pain as lasting less than 4 weeks, subacute low back pain as lasting 4-12 weeks, and chronic low back pain as lasting more than 12 weeks. For acute and subacute low back pain, low to moderate quality evidence supports the efficacy of acupuncture, massage, spinal manipulation, superficial heat, lumbar supports, and low-level laser therapy, the guideline authors conclude.
They recommend considering nonsteroidal anti-inflammatory drugs or skeletal muscle relaxants for patients who want medications for acute or subacute low back pain. There is moderate-quality evidence that NSAIDs confer a small analgesic benefit, compared with placebo, but their renal and gastrointestinal risks call for careful patient selection and use of the lowest possible doses and treatment durations, the authors emphasized.
Likewise, moderate-quality evidence supports the use of skeletal muscle relaxants for short-term pain relief, but patients should know that these drugs can lead to sedation and other adverse effects on the central nervous system, they stated.
Acetaminophen is no longer recommended for low back pain, having failed to shorten time to recovery, compared with placebo, in a large, multicenter, randomized trial (Lancet. 2014 Nov 1;384[9954]:1586-96).
Likewise, short-term oral or intramuscular corticosteroids have been found ineffective for acute low back pain, while benzodiazepines are ineffective for radiculopathy, the experts noted.
“Evidence was insufficient to determine effectiveness of antidepressants, benzodiazepines, antiseizure medications, or opioids, versus placebo, in patients with acute or subacute low back pain,” they added.
The guideline authors also noted insufficient evidence for many nondrug therapies for acute and subacute low back pain, including transcutaneous electrical nerve stimulation, electrical muscle stimulation, inferential therapy, short-wave diathermy, traction, superficial cold, motor control exercise, Pilates, tai chi, yoga, psychological therapies, multidisciplinary rehabilitation, ultrasound, and taping.
For chronic low back pain, the guideline strongly recommends starting with nondrug therapies, including exercise, multidisciplinary rehabilitation, acupuncture, mindfulness-based stress reduction, tai chi, yoga, motor control exercise, progressive relaxation, electromyography biofeedback, low-level laser therapy, operant therapy, cognitive behavioral therapy, or spinal manipulation.
Despite low-quality evidence for these modalities, “fewer harms are associated with these types of therapies than with pharmacologic options,” the authors wrote.
If nonpharmacologic interventions fail to improve chronic low back pain, the experts recommended NSAIDs in the first line, followed by second-line therapy with tramadol or duloxetine (Cymbalta). Recent evidence suggests that NSAIDs are less effective for low back pain than previously thought, while the trials that reported a modest analgesic benefit of duloxetine over placebo were industry funded, the authors note.
Opioids should only be considered for chronic low back pain that fails both nondrug and nonopioid therapies, “and only if the potential benefits outweigh the risks for individual patients, and after a discussion of known risks and realistic benefits,” the guideline authors emphasized.
This update does not cover topical therapies or epidural injections. Epidural steroid injections decreased pain associated with radiculopathy in the short term but did not confer long-term benefits, according to a recent separate review (Ann Intern Med. 2015 Sep 1;163[5]:373-81).
The Agency for Healthcare Research and Quality funded the work. One coauthor disclosed personal fees from Takeda Pharmaceuticals outside the submitted work, and membership in the American College of Physicians Clinical Guidelines Committee and the American College of Rheumatology Quality of Care Committee. The other authors had no conflicts. Two members of the ACP Clinical Guidelines Committee disclosed ties to Healthwise and UpToDate outside the submitted work.
Clinicians and patients should prioritize nonpharmacologic therapies for low back pain of any duration, according to an updated guideline from the American College of Physicians.
For acute and subacute low back pain, first-line choices include heat, massage, acupuncture, and spinal manipulation, Amir Qaseem, MD, PhD, and his associates wrote in the Annals of Internal Medicine. Patients with chronic low back pain have many nondrug options, ranging from exercise and tai chi to mindfulness-based stress reduction and spinal manipulation, the authors add (Ann Intern Med. 2017 Feb 14. doi: 10.7326/M16-2367).
The updated therapeutic recommendations focus on clinical presentations. They define acute low back pain as lasting less than 4 weeks, subacute low back pain as lasting 4-12 weeks, and chronic low back pain as lasting more than 12 weeks. For acute and subacute low back pain, low to moderate quality evidence supports the efficacy of acupuncture, massage, spinal manipulation, superficial heat, lumbar supports, and low-level laser therapy, the guideline authors conclude.
They recommend considering nonsteroidal anti-inflammatory drugs or skeletal muscle relaxants for patients who want medications for acute or subacute low back pain. There is moderate-quality evidence that NSAIDs confer a small analgesic benefit, compared with placebo, but their renal and gastrointestinal risks call for careful patient selection and use of the lowest possible doses and treatment durations, the authors emphasized.
Likewise, moderate-quality evidence supports the use of skeletal muscle relaxants for short-term pain relief, but patients should know that these drugs can lead to sedation and other adverse effects on the central nervous system, they stated.
Acetaminophen is no longer recommended for low back pain, having failed to shorten time to recovery, compared with placebo, in a large, multicenter, randomized trial (Lancet. 2014 Nov 1;384[9954]:1586-96).
Likewise, short-term oral or intramuscular corticosteroids have been found ineffective for acute low back pain, while benzodiazepines are ineffective for radiculopathy, the experts noted.
“Evidence was insufficient to determine effectiveness of antidepressants, benzodiazepines, antiseizure medications, or opioids, versus placebo, in patients with acute or subacute low back pain,” they added.
The guideline authors also noted insufficient evidence for many nondrug therapies for acute and subacute low back pain, including transcutaneous electrical nerve stimulation, electrical muscle stimulation, inferential therapy, short-wave diathermy, traction, superficial cold, motor control exercise, Pilates, tai chi, yoga, psychological therapies, multidisciplinary rehabilitation, ultrasound, and taping.
For chronic low back pain, the guideline strongly recommends starting with nondrug therapies, including exercise, multidisciplinary rehabilitation, acupuncture, mindfulness-based stress reduction, tai chi, yoga, motor control exercise, progressive relaxation, electromyography biofeedback, low-level laser therapy, operant therapy, cognitive behavioral therapy, or spinal manipulation.
Despite low-quality evidence for these modalities, “fewer harms are associated with these types of therapies than with pharmacologic options,” the authors wrote.
If nonpharmacologic interventions fail to improve chronic low back pain, the experts recommended NSAIDs in the first line, followed by second-line therapy with tramadol or duloxetine (Cymbalta). Recent evidence suggests that NSAIDs are less effective for low back pain than previously thought, while the trials that reported a modest analgesic benefit of duloxetine over placebo were industry funded, the authors note.
Opioids should only be considered for chronic low back pain that fails both nondrug and nonopioid therapies, “and only if the potential benefits outweigh the risks for individual patients, and after a discussion of known risks and realistic benefits,” the guideline authors emphasized.
This update does not cover topical therapies or epidural injections. Epidural steroid injections decreased pain associated with radiculopathy in the short term but did not confer long-term benefits, according to a recent separate review (Ann Intern Med. 2015 Sep 1;163[5]:373-81).
The Agency for Healthcare Research and Quality funded the work. One coauthor disclosed personal fees from Takeda Pharmaceuticals outside the submitted work, and membership in the American College of Physicians Clinical Guidelines Committee and the American College of Rheumatology Quality of Care Committee. The other authors had no conflicts. Two members of the ACP Clinical Guidelines Committee disclosed ties to Healthwise and UpToDate outside the submitted work.
FROM ANNALS OF INTERNAL MEDICINE