User login
AAN Guideline Assesses fMRI for Presurgical Evaluation of Patients With Epilepsy
When preparing for epilepsy surgery, neurologists may consider using functional MRI (fMRI) instead of the intracarotid amobarbital procedure (IAP) to map language and memory functions in the brain, according to a practice guideline developed by the American Academy of Neurology (AAN). The current evidence is weak, however, and clinicians should advise patients carefully about the benefits and risks of IAP (also known as the Wada test) and fMRI, according to the guideline, which was published online ahead of print January 11 in Neurology.
IAP, an invasive technique, is the current standard for presurgical evaluation in epilepsy. fMRI is noninvasive and also considered safe. Neither of the two methods has been standardized. “Because fMRI is becoming more widely available, we wanted to see how it compares to the Wada test,” said lead author Jerzy Szaflarski, MD, PhD, Professor of Neurology at the University of Alabama at Birmingham. “While the risks associated with the Wada test are rare, they can be serious, including stroke and injury to the carotid artery.”
The AAN formed an 11-member panel to draft the guidelines. Two panelists independently selected 37 possibly relevant articles. Studies with fewer than 15 cases, case reports, meta-analyses, and editorials were excluded. Two panelists rated each article according to the AAN’s diagnostic and prognostic classification of evidence, and the panel ultimately developed consensus recommendations.
Data Support fMRI for Certain Situations
Class II data indicated that fMRI possibly provides language lateralization information concordant with that of IAP in 87% of people with medial temporal lobe epilepsy and in 81% of patients with extratemporal epilepsy. Current comparative data for temporal tumors or lateral temporal cases are insufficient to draw conclusions. Thus, fMRI may be considered as an option for lateralizing language functions in place of IAP in patients with medial temporal lobe epilepsy (Level C), temporal epilepsy in general (Level C), or extratemporal epilepsy (Level C), said the authors. The evidence is unclear for patients with temporal neocortical epilepsy or temporal tumors (Level U).
One Class II study and one Class III study suggested that fMRI is possibly effective in aiding the prediction of postsurgical language deficits in patients undergoing presurgical evaluation for possible temporal lobectomy. The authors recommended that fMRI may be considered for predicting postsurgical language outcomes after anterior temporal lobe resection for the control of temporal lobe epilepsy (Level C).
Class II evidence suggests that in patients with medial temporal lobe epilepsy, fMRI is comparable with IAP in its ability to lateralize memory functions and may be used for this purpose. The authors recommended that fMRI may be considered as an option to lateralize memory functions in place of IAP in patients with medial temporal lobe epilepsy (Level C).
Nine Class II studies with different methods together suggested that fMRI leftward activation asymmetry during encoding of verbal material, regardless of whether measured in the medial temporal lobe or in the language network, probably predicts verbal memory decline after left medial temporal lobe surgery. The authors therefore recommended that presurgical fMRI of verbal memory or of language encoding should be considered as an option to predict verbal memory outcome in patients with epilepsy who are undergoing evaluation for left medial temporal lobe surgery (Level B).
A Class II study indicated that fMRI activation asymmetry during nonverbal (ie, scene and face recognition) memory tasks possibly predicts nonverbal memory decline after medial temporal lobe surgery. The authors recommended that presurgical fMRI using nonverbal memory encoding may be considered as a means to predict visuospatial memory outcomes in patients with epilepsy who are undergoing evaluation for temporal lobe surgery (Level C).
Based on data from one Class II study and one Class III study, the authors recommended that presurgical fMRI may be used instead of the IAP for language lateralization in patients with epilepsy who are undergoing evaluation for brain surgery (Level C). “However, when fMRI is used for this purpose, task design, data analysis methods, and epilepsy type should be considered,” they added.
In addition, based on nine Class II studies, the authors recommended that fMRI of language and verbal memory lateralization may be an alternative to IAP memory testing for prediction of verbal memory outcome in medial temporal lobe epilepsy (Level C). The authors note that fMRI is not yet established as an alternative to the IAP for prediction of global amnesia in patients who have undergone anterior temporal lobe surgery.
More and Larger Studies Are Needed
“The imperfect concordance between fMRI and IAP language lateralization leaves open the question of which test is more accurate in discordant cases,” said the authors. Although the IAP is the reference standard, it is subject to limitations resulting from individual variation in arterial anatomy, variable effects of anesthesia, the rate of amobarbital injection, variability in patient cooperation, and variation in testing methods.
Like the IAP, cognitive fMRI “is a complex diagnostic procedure that requires both advanced technical expertise in imaging and expert interaction with patients to elicit adequate levels of task performance, select a set of activation tasks appropriate to the patient’s ability and the clinical aims of the study, instruct the patient on the tasks, administer the tasks during scanning, and evaluate and provide corrective feedback on task performance during the scanning session,” said the authors.
Global amnesia may result from bilateral medial temporal lobe damage, and some neurologists depend on the IAP to evaluate a patient’s risk for this outcome. “Global amnesia is rare after unilateral temporal lobe surgery, however, and occurs mainly when there is preexisting contralateral medial temporal lobe dysfunction,” said the authors. “One possible approach, therefore, is to reserve use of the IAP memory test for those patients at greatest risk for global amnesia, that is, patients undergoing unilateral anterior temporal lobe resection who have structural or functional evidence of damage to the contralateral medial temporal lobe.”
“Larger studies need to be conducted to increase the quality of available evidence,” Dr. Szaflarski concluded.
—Erik Greb
Suggested Reading
Szaflarski JP, Gloss D, Binder JR, et al. Practice guideline summary: Use of fMRI in the presurgical evaluation of patients with epilepsy—Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017 Jan 11 [Epub ahead of print].
When preparing for epilepsy surgery, neurologists may consider using functional MRI (fMRI) instead of the intracarotid amobarbital procedure (IAP) to map language and memory functions in the brain, according to a practice guideline developed by the American Academy of Neurology (AAN). The current evidence is weak, however, and clinicians should advise patients carefully about the benefits and risks of IAP (also known as the Wada test) and fMRI, according to the guideline, which was published online ahead of print January 11 in Neurology.
IAP, an invasive technique, is the current standard for presurgical evaluation in epilepsy. fMRI is noninvasive and also considered safe. Neither of the two methods has been standardized. “Because fMRI is becoming more widely available, we wanted to see how it compares to the Wada test,” said lead author Jerzy Szaflarski, MD, PhD, Professor of Neurology at the University of Alabama at Birmingham. “While the risks associated with the Wada test are rare, they can be serious, including stroke and injury to the carotid artery.”
The AAN formed an 11-member panel to draft the guidelines. Two panelists independently selected 37 possibly relevant articles. Studies with fewer than 15 cases, case reports, meta-analyses, and editorials were excluded. Two panelists rated each article according to the AAN’s diagnostic and prognostic classification of evidence, and the panel ultimately developed consensus recommendations.
Data Support fMRI for Certain Situations
Class II data indicated that fMRI possibly provides language lateralization information concordant with that of IAP in 87% of people with medial temporal lobe epilepsy and in 81% of patients with extratemporal epilepsy. Current comparative data for temporal tumors or lateral temporal cases are insufficient to draw conclusions. Thus, fMRI may be considered as an option for lateralizing language functions in place of IAP in patients with medial temporal lobe epilepsy (Level C), temporal epilepsy in general (Level C), or extratemporal epilepsy (Level C), said the authors. The evidence is unclear for patients with temporal neocortical epilepsy or temporal tumors (Level U).
One Class II study and one Class III study suggested that fMRI is possibly effective in aiding the prediction of postsurgical language deficits in patients undergoing presurgical evaluation for possible temporal lobectomy. The authors recommended that fMRI may be considered for predicting postsurgical language outcomes after anterior temporal lobe resection for the control of temporal lobe epilepsy (Level C).
Class II evidence suggests that in patients with medial temporal lobe epilepsy, fMRI is comparable with IAP in its ability to lateralize memory functions and may be used for this purpose. The authors recommended that fMRI may be considered as an option to lateralize memory functions in place of IAP in patients with medial temporal lobe epilepsy (Level C).
Nine Class II studies with different methods together suggested that fMRI leftward activation asymmetry during encoding of verbal material, regardless of whether measured in the medial temporal lobe or in the language network, probably predicts verbal memory decline after left medial temporal lobe surgery. The authors therefore recommended that presurgical fMRI of verbal memory or of language encoding should be considered as an option to predict verbal memory outcome in patients with epilepsy who are undergoing evaluation for left medial temporal lobe surgery (Level B).
A Class II study indicated that fMRI activation asymmetry during nonverbal (ie, scene and face recognition) memory tasks possibly predicts nonverbal memory decline after medial temporal lobe surgery. The authors recommended that presurgical fMRI using nonverbal memory encoding may be considered as a means to predict visuospatial memory outcomes in patients with epilepsy who are undergoing evaluation for temporal lobe surgery (Level C).
Based on data from one Class II study and one Class III study, the authors recommended that presurgical fMRI may be used instead of the IAP for language lateralization in patients with epilepsy who are undergoing evaluation for brain surgery (Level C). “However, when fMRI is used for this purpose, task design, data analysis methods, and epilepsy type should be considered,” they added.
In addition, based on nine Class II studies, the authors recommended that fMRI of language and verbal memory lateralization may be an alternative to IAP memory testing for prediction of verbal memory outcome in medial temporal lobe epilepsy (Level C). The authors note that fMRI is not yet established as an alternative to the IAP for prediction of global amnesia in patients who have undergone anterior temporal lobe surgery.
More and Larger Studies Are Needed
“The imperfect concordance between fMRI and IAP language lateralization leaves open the question of which test is more accurate in discordant cases,” said the authors. Although the IAP is the reference standard, it is subject to limitations resulting from individual variation in arterial anatomy, variable effects of anesthesia, the rate of amobarbital injection, variability in patient cooperation, and variation in testing methods.
Like the IAP, cognitive fMRI “is a complex diagnostic procedure that requires both advanced technical expertise in imaging and expert interaction with patients to elicit adequate levels of task performance, select a set of activation tasks appropriate to the patient’s ability and the clinical aims of the study, instruct the patient on the tasks, administer the tasks during scanning, and evaluate and provide corrective feedback on task performance during the scanning session,” said the authors.
Global amnesia may result from bilateral medial temporal lobe damage, and some neurologists depend on the IAP to evaluate a patient’s risk for this outcome. “Global amnesia is rare after unilateral temporal lobe surgery, however, and occurs mainly when there is preexisting contralateral medial temporal lobe dysfunction,” said the authors. “One possible approach, therefore, is to reserve use of the IAP memory test for those patients at greatest risk for global amnesia, that is, patients undergoing unilateral anterior temporal lobe resection who have structural or functional evidence of damage to the contralateral medial temporal lobe.”
“Larger studies need to be conducted to increase the quality of available evidence,” Dr. Szaflarski concluded.
—Erik Greb
Suggested Reading
Szaflarski JP, Gloss D, Binder JR, et al. Practice guideline summary: Use of fMRI in the presurgical evaluation of patients with epilepsy—Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017 Jan 11 [Epub ahead of print].
When preparing for epilepsy surgery, neurologists may consider using functional MRI (fMRI) instead of the intracarotid amobarbital procedure (IAP) to map language and memory functions in the brain, according to a practice guideline developed by the American Academy of Neurology (AAN). The current evidence is weak, however, and clinicians should advise patients carefully about the benefits and risks of IAP (also known as the Wada test) and fMRI, according to the guideline, which was published online ahead of print January 11 in Neurology.
IAP, an invasive technique, is the current standard for presurgical evaluation in epilepsy. fMRI is noninvasive and also considered safe. Neither of the two methods has been standardized. “Because fMRI is becoming more widely available, we wanted to see how it compares to the Wada test,” said lead author Jerzy Szaflarski, MD, PhD, Professor of Neurology at the University of Alabama at Birmingham. “While the risks associated with the Wada test are rare, they can be serious, including stroke and injury to the carotid artery.”
The AAN formed an 11-member panel to draft the guidelines. Two panelists independently selected 37 possibly relevant articles. Studies with fewer than 15 cases, case reports, meta-analyses, and editorials were excluded. Two panelists rated each article according to the AAN’s diagnostic and prognostic classification of evidence, and the panel ultimately developed consensus recommendations.
Data Support fMRI for Certain Situations
Class II data indicated that fMRI possibly provides language lateralization information concordant with that of IAP in 87% of people with medial temporal lobe epilepsy and in 81% of patients with extratemporal epilepsy. Current comparative data for temporal tumors or lateral temporal cases are insufficient to draw conclusions. Thus, fMRI may be considered as an option for lateralizing language functions in place of IAP in patients with medial temporal lobe epilepsy (Level C), temporal epilepsy in general (Level C), or extratemporal epilepsy (Level C), said the authors. The evidence is unclear for patients with temporal neocortical epilepsy or temporal tumors (Level U).
One Class II study and one Class III study suggested that fMRI is possibly effective in aiding the prediction of postsurgical language deficits in patients undergoing presurgical evaluation for possible temporal lobectomy. The authors recommended that fMRI may be considered for predicting postsurgical language outcomes after anterior temporal lobe resection for the control of temporal lobe epilepsy (Level C).
Class II evidence suggests that in patients with medial temporal lobe epilepsy, fMRI is comparable with IAP in its ability to lateralize memory functions and may be used for this purpose. The authors recommended that fMRI may be considered as an option to lateralize memory functions in place of IAP in patients with medial temporal lobe epilepsy (Level C).
Nine Class II studies with different methods together suggested that fMRI leftward activation asymmetry during encoding of verbal material, regardless of whether measured in the medial temporal lobe or in the language network, probably predicts verbal memory decline after left medial temporal lobe surgery. The authors therefore recommended that presurgical fMRI of verbal memory or of language encoding should be considered as an option to predict verbal memory outcome in patients with epilepsy who are undergoing evaluation for left medial temporal lobe surgery (Level B).
A Class II study indicated that fMRI activation asymmetry during nonverbal (ie, scene and face recognition) memory tasks possibly predicts nonverbal memory decline after medial temporal lobe surgery. The authors recommended that presurgical fMRI using nonverbal memory encoding may be considered as a means to predict visuospatial memory outcomes in patients with epilepsy who are undergoing evaluation for temporal lobe surgery (Level C).
Based on data from one Class II study and one Class III study, the authors recommended that presurgical fMRI may be used instead of the IAP for language lateralization in patients with epilepsy who are undergoing evaluation for brain surgery (Level C). “However, when fMRI is used for this purpose, task design, data analysis methods, and epilepsy type should be considered,” they added.
In addition, based on nine Class II studies, the authors recommended that fMRI of language and verbal memory lateralization may be an alternative to IAP memory testing for prediction of verbal memory outcome in medial temporal lobe epilepsy (Level C). The authors note that fMRI is not yet established as an alternative to the IAP for prediction of global amnesia in patients who have undergone anterior temporal lobe surgery.
More and Larger Studies Are Needed
“The imperfect concordance between fMRI and IAP language lateralization leaves open the question of which test is more accurate in discordant cases,” said the authors. Although the IAP is the reference standard, it is subject to limitations resulting from individual variation in arterial anatomy, variable effects of anesthesia, the rate of amobarbital injection, variability in patient cooperation, and variation in testing methods.
Like the IAP, cognitive fMRI “is a complex diagnostic procedure that requires both advanced technical expertise in imaging and expert interaction with patients to elicit adequate levels of task performance, select a set of activation tasks appropriate to the patient’s ability and the clinical aims of the study, instruct the patient on the tasks, administer the tasks during scanning, and evaluate and provide corrective feedback on task performance during the scanning session,” said the authors.
Global amnesia may result from bilateral medial temporal lobe damage, and some neurologists depend on the IAP to evaluate a patient’s risk for this outcome. “Global amnesia is rare after unilateral temporal lobe surgery, however, and occurs mainly when there is preexisting contralateral medial temporal lobe dysfunction,” said the authors. “One possible approach, therefore, is to reserve use of the IAP memory test for those patients at greatest risk for global amnesia, that is, patients undergoing unilateral anterior temporal lobe resection who have structural or functional evidence of damage to the contralateral medial temporal lobe.”
“Larger studies need to be conducted to increase the quality of available evidence,” Dr. Szaflarski concluded.
—Erik Greb
Suggested Reading
Szaflarski JP, Gloss D, Binder JR, et al. Practice guideline summary: Use of fMRI in the presurgical evaluation of patients with epilepsy—Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017 Jan 11 [Epub ahead of print].
Complex congenital heart conditions call for complex care in pregnancy
A new scientific statement from the American Heart Association (AHA) brings together recommendations for management of pregnancy for women with serious congenital heart disease. The 38-page document addresses a wide range of complex congenital heart conditions, presenting a newly unified set of recommendations for care that ranges from preconception counseling, through pregnancy, labor, and delivery, to the postpartum period.
Caring for women with complex congenital heart lesions is becoming more commonplace, as more infants undergo successful repairs of previously-unsurvivable cardiac anomalies. “More moms with congenital heart disease are showing up pregnant, having survived the tumultuous peripartum and neonatal period, and are now facing a new set of risks in pregnancy,” Michael Foley, MD, chair of the department of obstetrics and gynecology at the University of Arizona, Phoenix, said in an interview.
Joseph Kay, MD, a cardiologist and professor of medicine and pediatrics at the University of Colorado, Aurora, said that one big benefit of the new scientific statement is having a single reference point for care of these patients. “The scientific statement brings all of the information about caring for these patients together into one document. This will be a very valuable resource for trainees to get a sense of what’s important; it also represents a platform for new programs to understand the scope of services needed,” said Dr. Kay in an interview.
The document provides a thorough review of the physiologic changes of pregnancy and the intrapartum and postpartum periods, noting that the heterogeneity of congenital heart disease means that women who have different lesions carry different risks in pregnancy.
Examples of lesions presenting intermediate risk include most arrhythmias (category II), hypertrophic cardiomyopathy, and a repaired coarctation (both category II-III). The most severe lesions carry a contraindication for pregnancy; the WHO guidelines suggest discussing termination should women with a category IV lesion become pregnant. Severe mitral stenosis, severe symptomatic aortic stenosis, and severe systemic ventricular dysfunction all place women into category IV.
Beginning with pregnancy risk category III, the WHO guidelines recommend intensive cardiac and obstetric monitoring throughout pregnancy, childbirth, and the puerperium. Several maternal-fetal medicine specialists interviewed all agreed that an interdisciplinary team is a must for good obstetric care in this population.
How interdisciplinary care plays out can depend on geography and facility-dependent resources. Dr. Kay said that his facility is the referral site for pregnant women with complex congenital lesions in an area that spans the Canadian and the Mexican borders from north to south, and ranges from parts of Kansas to eastern Montana from east to west. Still, Dr. Kay said that even for patients with lower-risk lesions, “We will see patients at least once, at approximately the midpoint of pregnancy, and again during the third trimester if possible.” The specifics of care depend on “the nature of the lesion and the complexity of the disease,” said Dr. Kay.
In his facility, said Dr. Kay, telemetry is available for all of the labor and delivery unit beds. This means that the mother and infant can usually stay together and receive postpartum nursing and lactation care from a skilled staff.
In no circumstances should ob.gyns. go it alone, said Dr. Foley. “The conversation with the ob.gyn. needs to be about comanaging these patients, at the very least. Even the most learned maternal-fetal medicine specialist needs to be working with a cardiologist and an anesthesiologist to create a delivery plan that includes pain management, fluid management, and consideration for intrapartum hemodynamic monitoring,” he said.
And the team needs to be in place long before delivery, Dr. Foley pointed out. “In many hospitals, the care delivery gap may be the inability to have this consistent proactive approach. You can’t expect the best outcomes when you have to hurriedly assemble an unfamiliar ad hoc team when a woman with congenital heart disease presents in labor. Despite their best intentions, inconsistent team members may not have the knowledge and experience to provide the safest care for these patients,” he said.
Though an individualized labor and delivery plan is a must, and a multispecialty team should be assembled, maternal congenital heart disease doesn’t necessarily consign a woman to cesarean delivery. “Most women can and should have a vaginal delivery. It’s safer for them. If a natural delivery may increase risk of issues, we may consider a facilitated second stage of labor with epidural anesthesia and forceps- or vacuum-assisted delivery,” said Dr. Kay.
It’s important to understand the nuances of an individual patient’s health and risk status, said Dr. Norton. “A simplified view is often bad. It’s not the case that ‘it’s always better to deliver’ or ‘it’s always better to have a cesarean delivery.’”
Especially for women who need anticoagulation or who may have lesions that put them at great risk should pregnancy occur, preconception counseling is a vital part of their care, and guidance in the scientific statement can help specialists avoid the complications that can occur in the absence of evidence-based treatment. Said Dr. Kay, “I have seen an unfortunate case or two of patients whose anticoagulation was stopped or changed, contrary to guidelines, and who suffered strokes. I hope more people will see this document.”
Ms. Canobbio echoed the sentiment: “You don’t want to have to backpedal once a young woman presents with a pregnancy. Appropriate contraceptive counseling needs to be part of the conversation.”
One key concept underscored in the scientific statement is that elevated risk persists into the postpartum period. “Following delivery, the mother is still at risk for an extended period of time. The greatest risk for mortality in these patients is post delivery, when a large volume of blood is expelled from the uterus back into the maternal circulation,” said Ms. Canobbio. “These women need close follow-up; we can’t say they are home free until several weeks to 2 months after delivery. The need for vigilance and surveillance continues.”
Since the scientific statement is not a new set of guidelines, but rather a compilation of currently existing reference documents, the authors noted that management differences may exist in some cases, but did not assign greater value to one practice than another. “We addressed that there are differences between the European and the American guidelines. For example, with regard to anticoagulation, both would agree to use Lovenox [enoxaparin], but the difference is whether it should be used for the entire pregnancy or for parts of the pregnancy,” said Ms. Canobbio.
Looking forward, more women with complex congenital heart disease will bear children, but their future is not certain. Said Ms. Canobbio: “The data are growing that if the patient is clinically stable at the time of pregnancy, it’s likely we can get them through safely. What’s not yet known is whether the burden of pregnancy in a woman who is otherwise healthy will shorten her lifespan. However, early data are promising, and it’s looking like these women can fare well.”
Topics covered in the scientific statement include:
- Defining which patients are at increased risk in pregnancy.
- Physiological adaptations of pregnancy, the puerperium, and the postpartum period, with an emphasis on hemodynamic changes.
- Assessment and evaluation in the preconception and early prenatal periods.
- Pregnancy management, including appropriate testing.
- Medications in pregnancy, including a table of common cardiac drugs and their pregnancy categories and lactation risks.
- Breakdown of suggested prenatal care by trimester.
- Intrapartum care, including indications for fluid management, ECG and hemodynamic monitoring, and management of the second stage of delivery.
- Postpartum care, with attention to the very rapid increase in blood volume and concomitant leap in stroke volume and cardiac output.
- Considerations when choosing contraceptive method.
- Cardiac complications seen in pregnancy, including arrhythmias, managing mechanical valves and anticoagulation, heart failure, and cyanosis.
- Indications for and risks associated with interventional therapies during pregnancy.
- Detailed discussion of management of pregnancy for women with specific lesions.
None of the members of the writing committee for the scientific statement had relevant disclosures. Dr. Foley and Dr. Kay reported no disclosures. Dr. Norton reported that she has received research funding from Natera and Ultragenyx.
[email protected]
On Twitter @karioakes
A new scientific statement from the American Heart Association (AHA) brings together recommendations for management of pregnancy for women with serious congenital heart disease. The 38-page document addresses a wide range of complex congenital heart conditions, presenting a newly unified set of recommendations for care that ranges from preconception counseling, through pregnancy, labor, and delivery, to the postpartum period.
Caring for women with complex congenital heart lesions is becoming more commonplace, as more infants undergo successful repairs of previously-unsurvivable cardiac anomalies. “More moms with congenital heart disease are showing up pregnant, having survived the tumultuous peripartum and neonatal period, and are now facing a new set of risks in pregnancy,” Michael Foley, MD, chair of the department of obstetrics and gynecology at the University of Arizona, Phoenix, said in an interview.
Joseph Kay, MD, a cardiologist and professor of medicine and pediatrics at the University of Colorado, Aurora, said that one big benefit of the new scientific statement is having a single reference point for care of these patients. “The scientific statement brings all of the information about caring for these patients together into one document. This will be a very valuable resource for trainees to get a sense of what’s important; it also represents a platform for new programs to understand the scope of services needed,” said Dr. Kay in an interview.
The document provides a thorough review of the physiologic changes of pregnancy and the intrapartum and postpartum periods, noting that the heterogeneity of congenital heart disease means that women who have different lesions carry different risks in pregnancy.
Examples of lesions presenting intermediate risk include most arrhythmias (category II), hypertrophic cardiomyopathy, and a repaired coarctation (both category II-III). The most severe lesions carry a contraindication for pregnancy; the WHO guidelines suggest discussing termination should women with a category IV lesion become pregnant. Severe mitral stenosis, severe symptomatic aortic stenosis, and severe systemic ventricular dysfunction all place women into category IV.
Beginning with pregnancy risk category III, the WHO guidelines recommend intensive cardiac and obstetric monitoring throughout pregnancy, childbirth, and the puerperium. Several maternal-fetal medicine specialists interviewed all agreed that an interdisciplinary team is a must for good obstetric care in this population.
How interdisciplinary care plays out can depend on geography and facility-dependent resources. Dr. Kay said that his facility is the referral site for pregnant women with complex congenital lesions in an area that spans the Canadian and the Mexican borders from north to south, and ranges from parts of Kansas to eastern Montana from east to west. Still, Dr. Kay said that even for patients with lower-risk lesions, “We will see patients at least once, at approximately the midpoint of pregnancy, and again during the third trimester if possible.” The specifics of care depend on “the nature of the lesion and the complexity of the disease,” said Dr. Kay.
In his facility, said Dr. Kay, telemetry is available for all of the labor and delivery unit beds. This means that the mother and infant can usually stay together and receive postpartum nursing and lactation care from a skilled staff.
In no circumstances should ob.gyns. go it alone, said Dr. Foley. “The conversation with the ob.gyn. needs to be about comanaging these patients, at the very least. Even the most learned maternal-fetal medicine specialist needs to be working with a cardiologist and an anesthesiologist to create a delivery plan that includes pain management, fluid management, and consideration for intrapartum hemodynamic monitoring,” he said.
And the team needs to be in place long before delivery, Dr. Foley pointed out. “In many hospitals, the care delivery gap may be the inability to have this consistent proactive approach. You can’t expect the best outcomes when you have to hurriedly assemble an unfamiliar ad hoc team when a woman with congenital heart disease presents in labor. Despite their best intentions, inconsistent team members may not have the knowledge and experience to provide the safest care for these patients,” he said.
Though an individualized labor and delivery plan is a must, and a multispecialty team should be assembled, maternal congenital heart disease doesn’t necessarily consign a woman to cesarean delivery. “Most women can and should have a vaginal delivery. It’s safer for them. If a natural delivery may increase risk of issues, we may consider a facilitated second stage of labor with epidural anesthesia and forceps- or vacuum-assisted delivery,” said Dr. Kay.
It’s important to understand the nuances of an individual patient’s health and risk status, said Dr. Norton. “A simplified view is often bad. It’s not the case that ‘it’s always better to deliver’ or ‘it’s always better to have a cesarean delivery.’”
Especially for women who need anticoagulation or who may have lesions that put them at great risk should pregnancy occur, preconception counseling is a vital part of their care, and guidance in the scientific statement can help specialists avoid the complications that can occur in the absence of evidence-based treatment. Said Dr. Kay, “I have seen an unfortunate case or two of patients whose anticoagulation was stopped or changed, contrary to guidelines, and who suffered strokes. I hope more people will see this document.”
Ms. Canobbio echoed the sentiment: “You don’t want to have to backpedal once a young woman presents with a pregnancy. Appropriate contraceptive counseling needs to be part of the conversation.”
One key concept underscored in the scientific statement is that elevated risk persists into the postpartum period. “Following delivery, the mother is still at risk for an extended period of time. The greatest risk for mortality in these patients is post delivery, when a large volume of blood is expelled from the uterus back into the maternal circulation,” said Ms. Canobbio. “These women need close follow-up; we can’t say they are home free until several weeks to 2 months after delivery. The need for vigilance and surveillance continues.”
Since the scientific statement is not a new set of guidelines, but rather a compilation of currently existing reference documents, the authors noted that management differences may exist in some cases, but did not assign greater value to one practice than another. “We addressed that there are differences between the European and the American guidelines. For example, with regard to anticoagulation, both would agree to use Lovenox [enoxaparin], but the difference is whether it should be used for the entire pregnancy or for parts of the pregnancy,” said Ms. Canobbio.
Looking forward, more women with complex congenital heart disease will bear children, but their future is not certain. Said Ms. Canobbio: “The data are growing that if the patient is clinically stable at the time of pregnancy, it’s likely we can get them through safely. What’s not yet known is whether the burden of pregnancy in a woman who is otherwise healthy will shorten her lifespan. However, early data are promising, and it’s looking like these women can fare well.”
Topics covered in the scientific statement include:
- Defining which patients are at increased risk in pregnancy.
- Physiological adaptations of pregnancy, the puerperium, and the postpartum period, with an emphasis on hemodynamic changes.
- Assessment and evaluation in the preconception and early prenatal periods.
- Pregnancy management, including appropriate testing.
- Medications in pregnancy, including a table of common cardiac drugs and their pregnancy categories and lactation risks.
- Breakdown of suggested prenatal care by trimester.
- Intrapartum care, including indications for fluid management, ECG and hemodynamic monitoring, and management of the second stage of delivery.
- Postpartum care, with attention to the very rapid increase in blood volume and concomitant leap in stroke volume and cardiac output.
- Considerations when choosing contraceptive method.
- Cardiac complications seen in pregnancy, including arrhythmias, managing mechanical valves and anticoagulation, heart failure, and cyanosis.
- Indications for and risks associated with interventional therapies during pregnancy.
- Detailed discussion of management of pregnancy for women with specific lesions.
None of the members of the writing committee for the scientific statement had relevant disclosures. Dr. Foley and Dr. Kay reported no disclosures. Dr. Norton reported that she has received research funding from Natera and Ultragenyx.
[email protected]
On Twitter @karioakes
A new scientific statement from the American Heart Association (AHA) brings together recommendations for management of pregnancy for women with serious congenital heart disease. The 38-page document addresses a wide range of complex congenital heart conditions, presenting a newly unified set of recommendations for care that ranges from preconception counseling, through pregnancy, labor, and delivery, to the postpartum period.
Caring for women with complex congenital heart lesions is becoming more commonplace, as more infants undergo successful repairs of previously-unsurvivable cardiac anomalies. “More moms with congenital heart disease are showing up pregnant, having survived the tumultuous peripartum and neonatal period, and are now facing a new set of risks in pregnancy,” Michael Foley, MD, chair of the department of obstetrics and gynecology at the University of Arizona, Phoenix, said in an interview.
Joseph Kay, MD, a cardiologist and professor of medicine and pediatrics at the University of Colorado, Aurora, said that one big benefit of the new scientific statement is having a single reference point for care of these patients. “The scientific statement brings all of the information about caring for these patients together into one document. This will be a very valuable resource for trainees to get a sense of what’s important; it also represents a platform for new programs to understand the scope of services needed,” said Dr. Kay in an interview.
The document provides a thorough review of the physiologic changes of pregnancy and the intrapartum and postpartum periods, noting that the heterogeneity of congenital heart disease means that women who have different lesions carry different risks in pregnancy.
Examples of lesions presenting intermediate risk include most arrhythmias (category II), hypertrophic cardiomyopathy, and a repaired coarctation (both category II-III). The most severe lesions carry a contraindication for pregnancy; the WHO guidelines suggest discussing termination should women with a category IV lesion become pregnant. Severe mitral stenosis, severe symptomatic aortic stenosis, and severe systemic ventricular dysfunction all place women into category IV.
Beginning with pregnancy risk category III, the WHO guidelines recommend intensive cardiac and obstetric monitoring throughout pregnancy, childbirth, and the puerperium. Several maternal-fetal medicine specialists interviewed all agreed that an interdisciplinary team is a must for good obstetric care in this population.
How interdisciplinary care plays out can depend on geography and facility-dependent resources. Dr. Kay said that his facility is the referral site for pregnant women with complex congenital lesions in an area that spans the Canadian and the Mexican borders from north to south, and ranges from parts of Kansas to eastern Montana from east to west. Still, Dr. Kay said that even for patients with lower-risk lesions, “We will see patients at least once, at approximately the midpoint of pregnancy, and again during the third trimester if possible.” The specifics of care depend on “the nature of the lesion and the complexity of the disease,” said Dr. Kay.
In his facility, said Dr. Kay, telemetry is available for all of the labor and delivery unit beds. This means that the mother and infant can usually stay together and receive postpartum nursing and lactation care from a skilled staff.
In no circumstances should ob.gyns. go it alone, said Dr. Foley. “The conversation with the ob.gyn. needs to be about comanaging these patients, at the very least. Even the most learned maternal-fetal medicine specialist needs to be working with a cardiologist and an anesthesiologist to create a delivery plan that includes pain management, fluid management, and consideration for intrapartum hemodynamic monitoring,” he said.
And the team needs to be in place long before delivery, Dr. Foley pointed out. “In many hospitals, the care delivery gap may be the inability to have this consistent proactive approach. You can’t expect the best outcomes when you have to hurriedly assemble an unfamiliar ad hoc team when a woman with congenital heart disease presents in labor. Despite their best intentions, inconsistent team members may not have the knowledge and experience to provide the safest care for these patients,” he said.
Though an individualized labor and delivery plan is a must, and a multispecialty team should be assembled, maternal congenital heart disease doesn’t necessarily consign a woman to cesarean delivery. “Most women can and should have a vaginal delivery. It’s safer for them. If a natural delivery may increase risk of issues, we may consider a facilitated second stage of labor with epidural anesthesia and forceps- or vacuum-assisted delivery,” said Dr. Kay.
It’s important to understand the nuances of an individual patient’s health and risk status, said Dr. Norton. “A simplified view is often bad. It’s not the case that ‘it’s always better to deliver’ or ‘it’s always better to have a cesarean delivery.’”
Especially for women who need anticoagulation or who may have lesions that put them at great risk should pregnancy occur, preconception counseling is a vital part of their care, and guidance in the scientific statement can help specialists avoid the complications that can occur in the absence of evidence-based treatment. Said Dr. Kay, “I have seen an unfortunate case or two of patients whose anticoagulation was stopped or changed, contrary to guidelines, and who suffered strokes. I hope more people will see this document.”
Ms. Canobbio echoed the sentiment: “You don’t want to have to backpedal once a young woman presents with a pregnancy. Appropriate contraceptive counseling needs to be part of the conversation.”
One key concept underscored in the scientific statement is that elevated risk persists into the postpartum period. “Following delivery, the mother is still at risk for an extended period of time. The greatest risk for mortality in these patients is post delivery, when a large volume of blood is expelled from the uterus back into the maternal circulation,” said Ms. Canobbio. “These women need close follow-up; we can’t say they are home free until several weeks to 2 months after delivery. The need for vigilance and surveillance continues.”
Since the scientific statement is not a new set of guidelines, but rather a compilation of currently existing reference documents, the authors noted that management differences may exist in some cases, but did not assign greater value to one practice than another. “We addressed that there are differences between the European and the American guidelines. For example, with regard to anticoagulation, both would agree to use Lovenox [enoxaparin], but the difference is whether it should be used for the entire pregnancy or for parts of the pregnancy,” said Ms. Canobbio.
Looking forward, more women with complex congenital heart disease will bear children, but their future is not certain. Said Ms. Canobbio: “The data are growing that if the patient is clinically stable at the time of pregnancy, it’s likely we can get them through safely. What’s not yet known is whether the burden of pregnancy in a woman who is otherwise healthy will shorten her lifespan. However, early data are promising, and it’s looking like these women can fare well.”
Topics covered in the scientific statement include:
- Defining which patients are at increased risk in pregnancy.
- Physiological adaptations of pregnancy, the puerperium, and the postpartum period, with an emphasis on hemodynamic changes.
- Assessment and evaluation in the preconception and early prenatal periods.
- Pregnancy management, including appropriate testing.
- Medications in pregnancy, including a table of common cardiac drugs and their pregnancy categories and lactation risks.
- Breakdown of suggested prenatal care by trimester.
- Intrapartum care, including indications for fluid management, ECG and hemodynamic monitoring, and management of the second stage of delivery.
- Postpartum care, with attention to the very rapid increase in blood volume and concomitant leap in stroke volume and cardiac output.
- Considerations when choosing contraceptive method.
- Cardiac complications seen in pregnancy, including arrhythmias, managing mechanical valves and anticoagulation, heart failure, and cyanosis.
- Indications for and risks associated with interventional therapies during pregnancy.
- Detailed discussion of management of pregnancy for women with specific lesions.
None of the members of the writing committee for the scientific statement had relevant disclosures. Dr. Foley and Dr. Kay reported no disclosures. Dr. Norton reported that she has received research funding from Natera and Ultragenyx.
[email protected]
On Twitter @karioakes
AGA Clinical Practice Update: Treatment for severe alcohol hepatitis challenging
Acute alcoholic hepatitis carries a high risk of mortality, yet only a minority of patients admitted to the hospital with the condition receive appropriate treatment, said the authors of an expert review.
Writing in the January 2017 issue of Clinical Gastroenterology and Hepatology, Mack C. Mitchell Jr., MD, of the University of Texas Southwestern Medical Center, Dallas, and Craig J. McClain, MD, of the University of Louisville (Ky.), described the challenges associated with treating acute alcoholic hepatitis and its consequences.
Acute alcohol hepatitis develops in heavy drinkers and presents with rapid onset of malaise, anorexia, tender hepatomegaly, and features of the systemic inflammatory response syndrome. Patients with alcoholic hepatitis also are at high risk of nutritional deficiency, infection, acute kidney injury, and multiorgan failure.
The two most widely used therapies are glucocorticoids – generally considered the standard of care for severe alcoholic hepatitis – and the phosphodiesterase inhibitor pentoxifylline (Clin Gastroenterol Hepatol. 2017. doi: 10.1016/j.cgh.2016.08.047).
“Although in its most severe form AH has a high short-term mortality rate if untreated, in 2011, only 28% of more than 1,600 patients admitted to U.S. hospitals were treated with glucocorticoids and 17% with pentoxifylline (PTX), suggesting a lack of widespread confidence in the two most frequently used therapies for AH,” the authors wrote.
Both drugs work by addressing the underlying inflammation that plays a key role in liver injury, but the evidence for both is mixed: A 2008 Cochrane systematic review of 15 trials concluded there was no benefit from glucocorticoids, largely because of substantial variability in bias across the trials, while two meta-analyses of pentoxifylline trials concluded that there were no differences in short-term mortality between those who received it and those who did not.
Some patients are unsuitable for glucocorticoids and others may develop resistance. There is also the possibility that, while glucocorticoids may improve short-term survival, the associated increase in infection risk removes that advantage at 90 days and 1 year after diagnosis. These infections, in turn, often precede the development of acute kidney injury and multiorgan failure.
The authors, however, did suggest that the approach of very high, short-term bursts of glucocorticoids to induce “immune paralysis” – an approach taken for lupus nephritis – might be considered.
They stressed that abstinence was the cornerstone of treatment for acute alcoholic hepatitis, with studies showing that patients with alcoholic hepatitis who resume heavy drinking have significantly worse outcomes than those who don’t.
“Although abstinence is important at all stages, it is particularly important to emphasize abstinence beyond 90 days when many patients are regaining normal functioning,” Dr. Mitchell and Dr. McClain wrote.
Infection, kidney injury, and malnutrition are all significant concerns in patients with acute alcoholic hepatitis.
With respect to infection, the authors said considerable suspicion is required to pick up bacterial and fungal infections, as patients may not always have a fever and an elevated white blood cell count is an unreliable indicator. Infection also can lead to acute kidney injury.
Malnutrition is not only common in patients with alcohol hepatitis, but it has a significant negative impact on recovery. All patients should be encouraged to meet nutritional goals as early as possible, but just how to achieve this is controversial, the authors stressed.
For example, one study suggested that enteral nutrition was as good as glucocorticoids in reducing 28-day mortality, while another found enteral nutrition via nasogastric tube – in addition to glucocorticoids – was no better than glucocorticoids alone. “Whether [nasogastric] tubes should be used to provide enteral nutrition is a subject of controversy,” the authors wrote. “Normal- to high-protein diets are safe and do not increase the risk of encephalopathy in patients with AH.”
No conflicts of interest were declared.
Acute alcoholic hepatitis carries a high risk of mortality, yet only a minority of patients admitted to the hospital with the condition receive appropriate treatment, said the authors of an expert review.
Writing in the January 2017 issue of Clinical Gastroenterology and Hepatology, Mack C. Mitchell Jr., MD, of the University of Texas Southwestern Medical Center, Dallas, and Craig J. McClain, MD, of the University of Louisville (Ky.), described the challenges associated with treating acute alcoholic hepatitis and its consequences.
Acute alcohol hepatitis develops in heavy drinkers and presents with rapid onset of malaise, anorexia, tender hepatomegaly, and features of the systemic inflammatory response syndrome. Patients with alcoholic hepatitis also are at high risk of nutritional deficiency, infection, acute kidney injury, and multiorgan failure.
The two most widely used therapies are glucocorticoids – generally considered the standard of care for severe alcoholic hepatitis – and the phosphodiesterase inhibitor pentoxifylline (Clin Gastroenterol Hepatol. 2017. doi: 10.1016/j.cgh.2016.08.047).
“Although in its most severe form AH has a high short-term mortality rate if untreated, in 2011, only 28% of more than 1,600 patients admitted to U.S. hospitals were treated with glucocorticoids and 17% with pentoxifylline (PTX), suggesting a lack of widespread confidence in the two most frequently used therapies for AH,” the authors wrote.
Both drugs work by addressing the underlying inflammation that plays a key role in liver injury, but the evidence for both is mixed: A 2008 Cochrane systematic review of 15 trials concluded there was no benefit from glucocorticoids, largely because of substantial variability in bias across the trials, while two meta-analyses of pentoxifylline trials concluded that there were no differences in short-term mortality between those who received it and those who did not.
Some patients are unsuitable for glucocorticoids and others may develop resistance. There is also the possibility that, while glucocorticoids may improve short-term survival, the associated increase in infection risk removes that advantage at 90 days and 1 year after diagnosis. These infections, in turn, often precede the development of acute kidney injury and multiorgan failure.
The authors, however, did suggest that the approach of very high, short-term bursts of glucocorticoids to induce “immune paralysis” – an approach taken for lupus nephritis – might be considered.
They stressed that abstinence was the cornerstone of treatment for acute alcoholic hepatitis, with studies showing that patients with alcoholic hepatitis who resume heavy drinking have significantly worse outcomes than those who don’t.
“Although abstinence is important at all stages, it is particularly important to emphasize abstinence beyond 90 days when many patients are regaining normal functioning,” Dr. Mitchell and Dr. McClain wrote.
Infection, kidney injury, and malnutrition are all significant concerns in patients with acute alcoholic hepatitis.
With respect to infection, the authors said considerable suspicion is required to pick up bacterial and fungal infections, as patients may not always have a fever and an elevated white blood cell count is an unreliable indicator. Infection also can lead to acute kidney injury.
Malnutrition is not only common in patients with alcohol hepatitis, but it has a significant negative impact on recovery. All patients should be encouraged to meet nutritional goals as early as possible, but just how to achieve this is controversial, the authors stressed.
For example, one study suggested that enteral nutrition was as good as glucocorticoids in reducing 28-day mortality, while another found enteral nutrition via nasogastric tube – in addition to glucocorticoids – was no better than glucocorticoids alone. “Whether [nasogastric] tubes should be used to provide enteral nutrition is a subject of controversy,” the authors wrote. “Normal- to high-protein diets are safe and do not increase the risk of encephalopathy in patients with AH.”
No conflicts of interest were declared.
Acute alcoholic hepatitis carries a high risk of mortality, yet only a minority of patients admitted to the hospital with the condition receive appropriate treatment, said the authors of an expert review.
Writing in the January 2017 issue of Clinical Gastroenterology and Hepatology, Mack C. Mitchell Jr., MD, of the University of Texas Southwestern Medical Center, Dallas, and Craig J. McClain, MD, of the University of Louisville (Ky.), described the challenges associated with treating acute alcoholic hepatitis and its consequences.
Acute alcohol hepatitis develops in heavy drinkers and presents with rapid onset of malaise, anorexia, tender hepatomegaly, and features of the systemic inflammatory response syndrome. Patients with alcoholic hepatitis also are at high risk of nutritional deficiency, infection, acute kidney injury, and multiorgan failure.
The two most widely used therapies are glucocorticoids – generally considered the standard of care for severe alcoholic hepatitis – and the phosphodiesterase inhibitor pentoxifylline (Clin Gastroenterol Hepatol. 2017. doi: 10.1016/j.cgh.2016.08.047).
“Although in its most severe form AH has a high short-term mortality rate if untreated, in 2011, only 28% of more than 1,600 patients admitted to U.S. hospitals were treated with glucocorticoids and 17% with pentoxifylline (PTX), suggesting a lack of widespread confidence in the two most frequently used therapies for AH,” the authors wrote.
Both drugs work by addressing the underlying inflammation that plays a key role in liver injury, but the evidence for both is mixed: A 2008 Cochrane systematic review of 15 trials concluded there was no benefit from glucocorticoids, largely because of substantial variability in bias across the trials, while two meta-analyses of pentoxifylline trials concluded that there were no differences in short-term mortality between those who received it and those who did not.
Some patients are unsuitable for glucocorticoids and others may develop resistance. There is also the possibility that, while glucocorticoids may improve short-term survival, the associated increase in infection risk removes that advantage at 90 days and 1 year after diagnosis. These infections, in turn, often precede the development of acute kidney injury and multiorgan failure.
The authors, however, did suggest that the approach of very high, short-term bursts of glucocorticoids to induce “immune paralysis” – an approach taken for lupus nephritis – might be considered.
They stressed that abstinence was the cornerstone of treatment for acute alcoholic hepatitis, with studies showing that patients with alcoholic hepatitis who resume heavy drinking have significantly worse outcomes than those who don’t.
“Although abstinence is important at all stages, it is particularly important to emphasize abstinence beyond 90 days when many patients are regaining normal functioning,” Dr. Mitchell and Dr. McClain wrote.
Infection, kidney injury, and malnutrition are all significant concerns in patients with acute alcoholic hepatitis.
With respect to infection, the authors said considerable suspicion is required to pick up bacterial and fungal infections, as patients may not always have a fever and an elevated white blood cell count is an unreliable indicator. Infection also can lead to acute kidney injury.
Malnutrition is not only common in patients with alcohol hepatitis, but it has a significant negative impact on recovery. All patients should be encouraged to meet nutritional goals as early as possible, but just how to achieve this is controversial, the authors stressed.
For example, one study suggested that enteral nutrition was as good as glucocorticoids in reducing 28-day mortality, while another found enteral nutrition via nasogastric tube – in addition to glucocorticoids – was no better than glucocorticoids alone. “Whether [nasogastric] tubes should be used to provide enteral nutrition is a subject of controversy,” the authors wrote. “Normal- to high-protein diets are safe and do not increase the risk of encephalopathy in patients with AH.”
No conflicts of interest were declared.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
ADA: Empagliflozin and liraglutide reduce type 2 CV death
to reduce the risk of CV death, according to the American Diabetes Association 2017 Standards of Medical Care.
ADA updates it standards annually based on new information and research; like its predecessors, the 2017 guidance is comprehensive, addressing mental, social, and other challenges faced by patients with diabetes, along with clinical care (Diabetes Care. 2017 Jan;40(Suppl 1):S4-S5).
The 2017 guidance contains a great deal of new information. At 135 pages, there are 22 more pages than in 2016. “They did a really nice job. This guide is useful for anyone helping patients with diabetes,” including diabetologists, dietitians, educators, psychologists, and social workers, Richard Hellman, MD, a clinical endocrinologist in North Kansas City, Mo., said in an interview.
The empagliflozin and liraglutide recommendation applies to any patient with type 2 diabetes who has a history of stroke, heart attack, acute coronary syndrome, angina, or peripheral arterial disease. Data from recent trials have shown use of the drugs modestly reduces cardiovascular mortality in this population.
It’s unclear if the benefits are drug specific or group effects. “We anxiously await the results of several ongoing cardiovascular outcomes trials” to find out, said Helena Rodbard, MD, a clinical endocrinologist in Rockville, Md., who also commented on the new standards.
Basal insulin plus a GLP-1 receptor agonist, like liraglutide, are also now recommended for insulin-dependent type 2 disease. “This combination gives rise to a markedly reduced risk of hypoglycemia compared with basal insulin ... basal bolus insulin, or premixed insulins,” according to the ADA.
The newer drugs and insulins are expensive. To help doctors and patients negotiate the price hurdle, ADA added tables on how much the various options cost per month. It was a good move; “the cost of care is going up so fast” in diabetes “that many patients can no longer afford” what’s prescribed. “It’s a major problem,” said Dr. Hellman, clinical professor at the University of Missouri–Kansas City.
The ADA also set a blood glucose level of 54 mg/dL to trigger aggressive hypoglycemia treatment. “There has been confusion over when to treat aggressively. It was a good choice to land on 54 mg/dL” a safe, conservative number a bit higher than others have suggested, Dr. Hellman said.
Meanwhile, the group lowered its metabolic surgery cut point – the ADA has stopped using the term “bariatric surgery” – to type 2 patients with a body mass index of 30 kg/m2 when medications don’t work. The group also set a new hypertension treatment target of 120-160/80-105 mm Hg in pregnancy, and said that insulin is the treatment of choice for gestational diabetes, given concerns about metformin crossing the placenta and glyburide in cord blood.
The ADA expanded its list of diabetes comorbidities to include autoimmune disease, HIV, anxiety, depression, and disordered eating. In addition, doctors should ask patients how well they sleep – since sleep problems affect glycemic control – and should intervene when there’s a problem, according to the guidance.
The group updated its combination injection algorithm for type 2 diabetes “to reflect studies demonstrating the noninferiority of basal insulin plus” liraglutide and its class members “versus basal insulin plus rapid-acting insulin” or two daily injections of premixed insulin. The ADA added a section on the role of newly available biosimilar insulins, as well, and clarified that either basal insulin or basal plus bolus correctional insulin can be used to treat noncritical inpatients, but noted that “sole use of sliding scale insulin in the inpatient hospital setting is strongly discouraged.”
People on long-term metformin should have their vitamin B12 checked periodically, because of new evidence about the risk of B12 deficiency, the group said, and “due to the risk of malformations associated with unplanned pregnancies and poor metabolic control.” The group added “a new recommendation ... encouraging preconception counseling starting at puberty for all girls of childbearing potential.”
“Even though most of this information should be well known to practitioners treating patients, [it’s] a worthwhile read for everyone who treats people with diabetes,” Dr. Rodbard said.
The majority of the people on the ADA’s update committee had no disclosures, but a few reported ties to various companies, including Novo Nordisk, the maker of liraglutide, and Boehringer Ingelheim and Lilly, the companies that developed and/or marketed empagliflozin. Dr. Hellman had no conflicts. Dr. Rodbard is an adviser or researcher for AstraZeneca, Lilly, Janssen, Merck, Novo Nordisk, Sanofi, and Regeneron.
to reduce the risk of CV death, according to the American Diabetes Association 2017 Standards of Medical Care.
ADA updates it standards annually based on new information and research; like its predecessors, the 2017 guidance is comprehensive, addressing mental, social, and other challenges faced by patients with diabetes, along with clinical care (Diabetes Care. 2017 Jan;40(Suppl 1):S4-S5).
The 2017 guidance contains a great deal of new information. At 135 pages, there are 22 more pages than in 2016. “They did a really nice job. This guide is useful for anyone helping patients with diabetes,” including diabetologists, dietitians, educators, psychologists, and social workers, Richard Hellman, MD, a clinical endocrinologist in North Kansas City, Mo., said in an interview.
The empagliflozin and liraglutide recommendation applies to any patient with type 2 diabetes who has a history of stroke, heart attack, acute coronary syndrome, angina, or peripheral arterial disease. Data from recent trials have shown use of the drugs modestly reduces cardiovascular mortality in this population.
It’s unclear if the benefits are drug specific or group effects. “We anxiously await the results of several ongoing cardiovascular outcomes trials” to find out, said Helena Rodbard, MD, a clinical endocrinologist in Rockville, Md., who also commented on the new standards.
Basal insulin plus a GLP-1 receptor agonist, like liraglutide, are also now recommended for insulin-dependent type 2 disease. “This combination gives rise to a markedly reduced risk of hypoglycemia compared with basal insulin ... basal bolus insulin, or premixed insulins,” according to the ADA.
The newer drugs and insulins are expensive. To help doctors and patients negotiate the price hurdle, ADA added tables on how much the various options cost per month. It was a good move; “the cost of care is going up so fast” in diabetes “that many patients can no longer afford” what’s prescribed. “It’s a major problem,” said Dr. Hellman, clinical professor at the University of Missouri–Kansas City.
The ADA also set a blood glucose level of 54 mg/dL to trigger aggressive hypoglycemia treatment. “There has been confusion over when to treat aggressively. It was a good choice to land on 54 mg/dL” a safe, conservative number a bit higher than others have suggested, Dr. Hellman said.
Meanwhile, the group lowered its metabolic surgery cut point – the ADA has stopped using the term “bariatric surgery” – to type 2 patients with a body mass index of 30 kg/m2 when medications don’t work. The group also set a new hypertension treatment target of 120-160/80-105 mm Hg in pregnancy, and said that insulin is the treatment of choice for gestational diabetes, given concerns about metformin crossing the placenta and glyburide in cord blood.
The ADA expanded its list of diabetes comorbidities to include autoimmune disease, HIV, anxiety, depression, and disordered eating. In addition, doctors should ask patients how well they sleep – since sleep problems affect glycemic control – and should intervene when there’s a problem, according to the guidance.
The group updated its combination injection algorithm for type 2 diabetes “to reflect studies demonstrating the noninferiority of basal insulin plus” liraglutide and its class members “versus basal insulin plus rapid-acting insulin” or two daily injections of premixed insulin. The ADA added a section on the role of newly available biosimilar insulins, as well, and clarified that either basal insulin or basal plus bolus correctional insulin can be used to treat noncritical inpatients, but noted that “sole use of sliding scale insulin in the inpatient hospital setting is strongly discouraged.”
People on long-term metformin should have their vitamin B12 checked periodically, because of new evidence about the risk of B12 deficiency, the group said, and “due to the risk of malformations associated with unplanned pregnancies and poor metabolic control.” The group added “a new recommendation ... encouraging preconception counseling starting at puberty for all girls of childbearing potential.”
“Even though most of this information should be well known to practitioners treating patients, [it’s] a worthwhile read for everyone who treats people with diabetes,” Dr. Rodbard said.
The majority of the people on the ADA’s update committee had no disclosures, but a few reported ties to various companies, including Novo Nordisk, the maker of liraglutide, and Boehringer Ingelheim and Lilly, the companies that developed and/or marketed empagliflozin. Dr. Hellman had no conflicts. Dr. Rodbard is an adviser or researcher for AstraZeneca, Lilly, Janssen, Merck, Novo Nordisk, Sanofi, and Regeneron.
to reduce the risk of CV death, according to the American Diabetes Association 2017 Standards of Medical Care.
ADA updates it standards annually based on new information and research; like its predecessors, the 2017 guidance is comprehensive, addressing mental, social, and other challenges faced by patients with diabetes, along with clinical care (Diabetes Care. 2017 Jan;40(Suppl 1):S4-S5).
The 2017 guidance contains a great deal of new information. At 135 pages, there are 22 more pages than in 2016. “They did a really nice job. This guide is useful for anyone helping patients with diabetes,” including diabetologists, dietitians, educators, psychologists, and social workers, Richard Hellman, MD, a clinical endocrinologist in North Kansas City, Mo., said in an interview.
The empagliflozin and liraglutide recommendation applies to any patient with type 2 diabetes who has a history of stroke, heart attack, acute coronary syndrome, angina, or peripheral arterial disease. Data from recent trials have shown use of the drugs modestly reduces cardiovascular mortality in this population.
It’s unclear if the benefits are drug specific or group effects. “We anxiously await the results of several ongoing cardiovascular outcomes trials” to find out, said Helena Rodbard, MD, a clinical endocrinologist in Rockville, Md., who also commented on the new standards.
Basal insulin plus a GLP-1 receptor agonist, like liraglutide, are also now recommended for insulin-dependent type 2 disease. “This combination gives rise to a markedly reduced risk of hypoglycemia compared with basal insulin ... basal bolus insulin, or premixed insulins,” according to the ADA.
The newer drugs and insulins are expensive. To help doctors and patients negotiate the price hurdle, ADA added tables on how much the various options cost per month. It was a good move; “the cost of care is going up so fast” in diabetes “that many patients can no longer afford” what’s prescribed. “It’s a major problem,” said Dr. Hellman, clinical professor at the University of Missouri–Kansas City.
The ADA also set a blood glucose level of 54 mg/dL to trigger aggressive hypoglycemia treatment. “There has been confusion over when to treat aggressively. It was a good choice to land on 54 mg/dL” a safe, conservative number a bit higher than others have suggested, Dr. Hellman said.
Meanwhile, the group lowered its metabolic surgery cut point – the ADA has stopped using the term “bariatric surgery” – to type 2 patients with a body mass index of 30 kg/m2 when medications don’t work. The group also set a new hypertension treatment target of 120-160/80-105 mm Hg in pregnancy, and said that insulin is the treatment of choice for gestational diabetes, given concerns about metformin crossing the placenta and glyburide in cord blood.
The ADA expanded its list of diabetes comorbidities to include autoimmune disease, HIV, anxiety, depression, and disordered eating. In addition, doctors should ask patients how well they sleep – since sleep problems affect glycemic control – and should intervene when there’s a problem, according to the guidance.
The group updated its combination injection algorithm for type 2 diabetes “to reflect studies demonstrating the noninferiority of basal insulin plus” liraglutide and its class members “versus basal insulin plus rapid-acting insulin” or two daily injections of premixed insulin. The ADA added a section on the role of newly available biosimilar insulins, as well, and clarified that either basal insulin or basal plus bolus correctional insulin can be used to treat noncritical inpatients, but noted that “sole use of sliding scale insulin in the inpatient hospital setting is strongly discouraged.”
People on long-term metformin should have their vitamin B12 checked periodically, because of new evidence about the risk of B12 deficiency, the group said, and “due to the risk of malformations associated with unplanned pregnancies and poor metabolic control.” The group added “a new recommendation ... encouraging preconception counseling starting at puberty for all girls of childbearing potential.”
“Even though most of this information should be well known to practitioners treating patients, [it’s] a worthwhile read for everyone who treats people with diabetes,” Dr. Rodbard said.
The majority of the people on the ADA’s update committee had no disclosures, but a few reported ties to various companies, including Novo Nordisk, the maker of liraglutide, and Boehringer Ingelheim and Lilly, the companies that developed and/or marketed empagliflozin. Dr. Hellman had no conflicts. Dr. Rodbard is an adviser or researcher for AstraZeneca, Lilly, Janssen, Merck, Novo Nordisk, Sanofi, and Regeneron.
Coronary revascularization appropriate use criteria updated
For ST segment–elevation myocardial infarction (STEMI) patients presenting between 12 and 24 hours from symptom onset but with no signs of clinical instability, coronary revascularization “may be appropriate,” according to a new report. At the same time, for STEMI patients initially treated with fibrinolysis, revascularization was rated as “appropriate therapy” in the setting of suspected failed fibrinolytic therapy or in stable and asymptomatic patients from 3 to 24 hours after fibrinolysis.
Those are two conclusions contained in a revision of the appropriate use criteria (AUC) for coronary revascularization published on Dec. 21 (J Am Coll Cardiol. doi: 10.1016/j.jacc.2016.10.034).
“This update provides a reassessment of clinical scenarios that the writing group felt to be affected by significant changes in the medical literature or gaps from prior criteria,” Manesh R. Patel, MD, chief of the division of cardiology and codirector of the Duke Heart Center at Duke University, Durham, N.C., and chair of the seven-member writing committee for the document, said in a prepared statement. “The primary objective of the appropriate use criteria is to provide a framework for the assessment of practice patterns that will hopefully improve physician decision making and ultimately lead to better patient outcomes.”
The 22-page document contains 17 clinical scenarios that were scored by a separate committee of 17 experts to indicate whether revascularization in patients with acute coronary syndromes is appropriate, may be appropriate, or is rarely appropriate for the clinical scenario presented. Step-by-step flow charts are included to help use the criteria. “Since publication of the 2012 AUC document (J Am Coll Cardiol. 2012;59:857-81), new guidelines for [STEMI] and non–ST segment elevation myocardial infarction (NSTEMI)/unstable angina have been published with additional focused updates of the [stable ischemic heart disease] guideline and a combined focused update of the percutaneous coronary intervention (PCI) and STEMI guideline,” the writing committee noted. “New clinical trials have been published extending the knowledge and evidence around coronary revascularization, including trials that challenge earlier recommendations about the timing of nonculprit vessel PCI in the setting of STEMI. Additional studies related to coronary artery bypass graft surgery, medical therapy, and diagnostic technologies such as fractional flow reserve (FFR) have emerged as well as analyses from the National Cardiovascular Data Registry (NCDR) on the existing AUC that provide insights into practice patterns, clinical scenarios, and patient features not previously addressed.”
Conclusions in the document include those for nonculprit artery revascularization during the index hospitalization after primary PCI or fibrinolysis. This was rated as “appropriate and reasonable” for patients with one or more severe stenoses and spontaneous or easily provoked ischemia or for asymptomatic patients with ischemic findings on noninvasive testing. Meanwhile, in the presence of an intermediate-severity nonculprit artery stenosis, revascularization was rated as “appropriate therapy” in cases where the fractional flow reserve is at or below 0.80. For patients who are stable and asymptomatic after primary PCI, revascularization was rated as “may be appropriate” for one or more severe stenoses even in the absence of further testing.
The only “rarely appropriate” rating in patients with acute coronary syndromes occurred for asymptomatic patients with intermediate-severity nonculprit artery stenoses in the absence of any additional testing to demonstrate the functional significance of the stenosis.
“As in prior versions of the AUC, these revascularization ratings should be used to reinforce existing management strategies and identify patient populations that need more information to identify the most effective treatments,” the authors concluded. Dr. Patel reported having no financial disclosures.
For ST segment–elevation myocardial infarction (STEMI) patients presenting between 12 and 24 hours from symptom onset but with no signs of clinical instability, coronary revascularization “may be appropriate,” according to a new report. At the same time, for STEMI patients initially treated with fibrinolysis, revascularization was rated as “appropriate therapy” in the setting of suspected failed fibrinolytic therapy or in stable and asymptomatic patients from 3 to 24 hours after fibrinolysis.
Those are two conclusions contained in a revision of the appropriate use criteria (AUC) for coronary revascularization published on Dec. 21 (J Am Coll Cardiol. doi: 10.1016/j.jacc.2016.10.034).
“This update provides a reassessment of clinical scenarios that the writing group felt to be affected by significant changes in the medical literature or gaps from prior criteria,” Manesh R. Patel, MD, chief of the division of cardiology and codirector of the Duke Heart Center at Duke University, Durham, N.C., and chair of the seven-member writing committee for the document, said in a prepared statement. “The primary objective of the appropriate use criteria is to provide a framework for the assessment of practice patterns that will hopefully improve physician decision making and ultimately lead to better patient outcomes.”
The 22-page document contains 17 clinical scenarios that were scored by a separate committee of 17 experts to indicate whether revascularization in patients with acute coronary syndromes is appropriate, may be appropriate, or is rarely appropriate for the clinical scenario presented. Step-by-step flow charts are included to help use the criteria. “Since publication of the 2012 AUC document (J Am Coll Cardiol. 2012;59:857-81), new guidelines for [STEMI] and non–ST segment elevation myocardial infarction (NSTEMI)/unstable angina have been published with additional focused updates of the [stable ischemic heart disease] guideline and a combined focused update of the percutaneous coronary intervention (PCI) and STEMI guideline,” the writing committee noted. “New clinical trials have been published extending the knowledge and evidence around coronary revascularization, including trials that challenge earlier recommendations about the timing of nonculprit vessel PCI in the setting of STEMI. Additional studies related to coronary artery bypass graft surgery, medical therapy, and diagnostic technologies such as fractional flow reserve (FFR) have emerged as well as analyses from the National Cardiovascular Data Registry (NCDR) on the existing AUC that provide insights into practice patterns, clinical scenarios, and patient features not previously addressed.”
Conclusions in the document include those for nonculprit artery revascularization during the index hospitalization after primary PCI or fibrinolysis. This was rated as “appropriate and reasonable” for patients with one or more severe stenoses and spontaneous or easily provoked ischemia or for asymptomatic patients with ischemic findings on noninvasive testing. Meanwhile, in the presence of an intermediate-severity nonculprit artery stenosis, revascularization was rated as “appropriate therapy” in cases where the fractional flow reserve is at or below 0.80. For patients who are stable and asymptomatic after primary PCI, revascularization was rated as “may be appropriate” for one or more severe stenoses even in the absence of further testing.
The only “rarely appropriate” rating in patients with acute coronary syndromes occurred for asymptomatic patients with intermediate-severity nonculprit artery stenoses in the absence of any additional testing to demonstrate the functional significance of the stenosis.
“As in prior versions of the AUC, these revascularization ratings should be used to reinforce existing management strategies and identify patient populations that need more information to identify the most effective treatments,” the authors concluded. Dr. Patel reported having no financial disclosures.
For ST segment–elevation myocardial infarction (STEMI) patients presenting between 12 and 24 hours from symptom onset but with no signs of clinical instability, coronary revascularization “may be appropriate,” according to a new report. At the same time, for STEMI patients initially treated with fibrinolysis, revascularization was rated as “appropriate therapy” in the setting of suspected failed fibrinolytic therapy or in stable and asymptomatic patients from 3 to 24 hours after fibrinolysis.
Those are two conclusions contained in a revision of the appropriate use criteria (AUC) for coronary revascularization published on Dec. 21 (J Am Coll Cardiol. doi: 10.1016/j.jacc.2016.10.034).
“This update provides a reassessment of clinical scenarios that the writing group felt to be affected by significant changes in the medical literature or gaps from prior criteria,” Manesh R. Patel, MD, chief of the division of cardiology and codirector of the Duke Heart Center at Duke University, Durham, N.C., and chair of the seven-member writing committee for the document, said in a prepared statement. “The primary objective of the appropriate use criteria is to provide a framework for the assessment of practice patterns that will hopefully improve physician decision making and ultimately lead to better patient outcomes.”
The 22-page document contains 17 clinical scenarios that were scored by a separate committee of 17 experts to indicate whether revascularization in patients with acute coronary syndromes is appropriate, may be appropriate, or is rarely appropriate for the clinical scenario presented. Step-by-step flow charts are included to help use the criteria. “Since publication of the 2012 AUC document (J Am Coll Cardiol. 2012;59:857-81), new guidelines for [STEMI] and non–ST segment elevation myocardial infarction (NSTEMI)/unstable angina have been published with additional focused updates of the [stable ischemic heart disease] guideline and a combined focused update of the percutaneous coronary intervention (PCI) and STEMI guideline,” the writing committee noted. “New clinical trials have been published extending the knowledge and evidence around coronary revascularization, including trials that challenge earlier recommendations about the timing of nonculprit vessel PCI in the setting of STEMI. Additional studies related to coronary artery bypass graft surgery, medical therapy, and diagnostic technologies such as fractional flow reserve (FFR) have emerged as well as analyses from the National Cardiovascular Data Registry (NCDR) on the existing AUC that provide insights into practice patterns, clinical scenarios, and patient features not previously addressed.”
Conclusions in the document include those for nonculprit artery revascularization during the index hospitalization after primary PCI or fibrinolysis. This was rated as “appropriate and reasonable” for patients with one or more severe stenoses and spontaneous or easily provoked ischemia or for asymptomatic patients with ischemic findings on noninvasive testing. Meanwhile, in the presence of an intermediate-severity nonculprit artery stenosis, revascularization was rated as “appropriate therapy” in cases where the fractional flow reserve is at or below 0.80. For patients who are stable and asymptomatic after primary PCI, revascularization was rated as “may be appropriate” for one or more severe stenoses even in the absence of further testing.
The only “rarely appropriate” rating in patients with acute coronary syndromes occurred for asymptomatic patients with intermediate-severity nonculprit artery stenoses in the absence of any additional testing to demonstrate the functional significance of the stenosis.
“As in prior versions of the AUC, these revascularization ratings should be used to reinforce existing management strategies and identify patient populations that need more information to identify the most effective treatments,” the authors concluded. Dr. Patel reported having no financial disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
ASCO and AHA: Maintain high suspicion for cardiac dysfunction
Maintain a high suspicion for cardiac dysfunction and a low threshold for cardiac assessment with any patients who are survivors of adult cancers and may have received cardiotoxic therapy, a new guideline suggests.
The guideline, released by the American Society of Clinical Oncology and endorsed by the American Heart Association, is intended to assist primary care physicians, oncologists, cardiologists, and any members of multidisciplinary cancer care teams in preventing and monitoring systolic cardiac dysfunction, which is “typically detected as low left ventricular ejection fraction,” said Saro H. Armenian, DO, and his associates on the expert panel that drafted the guidelines.
To develop the guidelines, the panel conducted a systematic review of 8 metaanalyses, 12 randomized clinical trials, 49 cohort studies, 32 before-and-after studies, and 3 cross-sectional studies published in 1999-2016. They addressed five key questions: Which cancer survivors are at increased risk for developing cardiac dysfunction? Which preventive strategies minimize that risk before cancer therapy is initiated? Which preventive strategies minimize that risk during administration of potentially cardiotoxic cancer therapies? Which cardiac monitoring approaches are preferred during cancer therapies? And which cardiac monitoring approaches are preferred after cancer therapy is completed?
Regarding the fifth question, the guideline advises clinicians to regularly assess and manage cardiovascular risk factors such as smoking, hypertension, diabetes, dyslipidemia, and obesity in survivors of adult cancers, as well as to complete careful histories and physical examinations regularly. Any signs or symptoms that raise the suspicion of cardiac dysfunction should prompt an ECG (or cardiac MRI or multigated acquisition if an ECG isn’t available or technically feasible), assays of serum cardiac biomarkers, and, depending on the findings of these assessments, referral to a cardiologist.
“Patients also need to be advised that cardiac dysfunction can be a progressive disorder and may initially be asymptomatic; therefore, early and late warning signs and symptoms should be discussed,” said Dr. Armenian, a pediatric hematologist/oncologist and director of outcomes research in the department of population sciences at City of Hope in Duarte, Calif., and his associates.
The guideline also includes a special section concerning health disparities. “Patients with cancer who are members of racial or ethnic minorities suffer disproportionately from comorbidities, experience more obstacles to receiving care, are more likely to be uninsured, and are at greater risk of receiving care of poor quality than other Americans. [They] may have a substantially higher burden of cardiovascular complications during and after cancer treatment, in part because of inequities in the management of cardiovascular risk factors,” the guidelines state (J Clin Oncol. 2016 Dec 5 [doi:10.1200/JCO.2016.70.5400]).
A copy of the guideline and further information, including a data supplement, a methodology supplement, slide sets, and clinical tools and resources, are available at www.asco.org/cardiac-guidelineThis guideline was supported by the American Society of Clinical Oncology. Dr. Armenian reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Maintain a high suspicion for cardiac dysfunction and a low threshold for cardiac assessment with any patients who are survivors of adult cancers and may have received cardiotoxic therapy, a new guideline suggests.
The guideline, released by the American Society of Clinical Oncology and endorsed by the American Heart Association, is intended to assist primary care physicians, oncologists, cardiologists, and any members of multidisciplinary cancer care teams in preventing and monitoring systolic cardiac dysfunction, which is “typically detected as low left ventricular ejection fraction,” said Saro H. Armenian, DO, and his associates on the expert panel that drafted the guidelines.
To develop the guidelines, the panel conducted a systematic review of 8 metaanalyses, 12 randomized clinical trials, 49 cohort studies, 32 before-and-after studies, and 3 cross-sectional studies published in 1999-2016. They addressed five key questions: Which cancer survivors are at increased risk for developing cardiac dysfunction? Which preventive strategies minimize that risk before cancer therapy is initiated? Which preventive strategies minimize that risk during administration of potentially cardiotoxic cancer therapies? Which cardiac monitoring approaches are preferred during cancer therapies? And which cardiac monitoring approaches are preferred after cancer therapy is completed?
Regarding the fifth question, the guideline advises clinicians to regularly assess and manage cardiovascular risk factors such as smoking, hypertension, diabetes, dyslipidemia, and obesity in survivors of adult cancers, as well as to complete careful histories and physical examinations regularly. Any signs or symptoms that raise the suspicion of cardiac dysfunction should prompt an ECG (or cardiac MRI or multigated acquisition if an ECG isn’t available or technically feasible), assays of serum cardiac biomarkers, and, depending on the findings of these assessments, referral to a cardiologist.
“Patients also need to be advised that cardiac dysfunction can be a progressive disorder and may initially be asymptomatic; therefore, early and late warning signs and symptoms should be discussed,” said Dr. Armenian, a pediatric hematologist/oncologist and director of outcomes research in the department of population sciences at City of Hope in Duarte, Calif., and his associates.
The guideline also includes a special section concerning health disparities. “Patients with cancer who are members of racial or ethnic minorities suffer disproportionately from comorbidities, experience more obstacles to receiving care, are more likely to be uninsured, and are at greater risk of receiving care of poor quality than other Americans. [They] may have a substantially higher burden of cardiovascular complications during and after cancer treatment, in part because of inequities in the management of cardiovascular risk factors,” the guidelines state (J Clin Oncol. 2016 Dec 5 [doi:10.1200/JCO.2016.70.5400]).
A copy of the guideline and further information, including a data supplement, a methodology supplement, slide sets, and clinical tools and resources, are available at www.asco.org/cardiac-guidelineThis guideline was supported by the American Society of Clinical Oncology. Dr. Armenian reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Maintain a high suspicion for cardiac dysfunction and a low threshold for cardiac assessment with any patients who are survivors of adult cancers and may have received cardiotoxic therapy, a new guideline suggests.
The guideline, released by the American Society of Clinical Oncology and endorsed by the American Heart Association, is intended to assist primary care physicians, oncologists, cardiologists, and any members of multidisciplinary cancer care teams in preventing and monitoring systolic cardiac dysfunction, which is “typically detected as low left ventricular ejection fraction,” said Saro H. Armenian, DO, and his associates on the expert panel that drafted the guidelines.
To develop the guidelines, the panel conducted a systematic review of 8 metaanalyses, 12 randomized clinical trials, 49 cohort studies, 32 before-and-after studies, and 3 cross-sectional studies published in 1999-2016. They addressed five key questions: Which cancer survivors are at increased risk for developing cardiac dysfunction? Which preventive strategies minimize that risk before cancer therapy is initiated? Which preventive strategies minimize that risk during administration of potentially cardiotoxic cancer therapies? Which cardiac monitoring approaches are preferred during cancer therapies? And which cardiac monitoring approaches are preferred after cancer therapy is completed?
Regarding the fifth question, the guideline advises clinicians to regularly assess and manage cardiovascular risk factors such as smoking, hypertension, diabetes, dyslipidemia, and obesity in survivors of adult cancers, as well as to complete careful histories and physical examinations regularly. Any signs or symptoms that raise the suspicion of cardiac dysfunction should prompt an ECG (or cardiac MRI or multigated acquisition if an ECG isn’t available or technically feasible), assays of serum cardiac biomarkers, and, depending on the findings of these assessments, referral to a cardiologist.
“Patients also need to be advised that cardiac dysfunction can be a progressive disorder and may initially be asymptomatic; therefore, early and late warning signs and symptoms should be discussed,” said Dr. Armenian, a pediatric hematologist/oncologist and director of outcomes research in the department of population sciences at City of Hope in Duarte, Calif., and his associates.
The guideline also includes a special section concerning health disparities. “Patients with cancer who are members of racial or ethnic minorities suffer disproportionately from comorbidities, experience more obstacles to receiving care, are more likely to be uninsured, and are at greater risk of receiving care of poor quality than other Americans. [They] may have a substantially higher burden of cardiovascular complications during and after cancer treatment, in part because of inequities in the management of cardiovascular risk factors,” the guidelines state (J Clin Oncol. 2016 Dec 5 [doi:10.1200/JCO.2016.70.5400]).
A copy of the guideline and further information, including a data supplement, a methodology supplement, slide sets, and clinical tools and resources, are available at www.asco.org/cardiac-guidelineThis guideline was supported by the American Society of Clinical Oncology. Dr. Armenian reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
New guidelines provide standardized hypoglycemia values for clinical evaluation
, joining with the European Association for the Study of Diabetes to specify that a level of less than 3 mmol/L (54 mg/dL) should be considered “clinically important hypoglycemia.”
“A single glucose level should be agreed to that has serious clinical and health-economic consequences,” the ADA and EASD stated in the new guidelines. “This would enable the diabetes and regulatory communities to compare the effectiveness of interventions in reducing hypoglycemia, be they pharmacological, technological, or educational. It would also permit the use of meta-analysis as a statistical tool to increase power when comparing interventions.”
An international, multidisciplinary group – the International Hypoglycemia Study Group – was formed to create distinct definitions of the various levels of severity that hypoglycemia can have. The new guidelines contain three levels, which should be used by clinicians to determine what amounts of blood glucose are significant enough to be clinically reported.
“Currently, there is no uniform agreement to what constitutes reportable hypoglycemia in clinical trials,” she said. “In some studies, it is defined as a blood glucose level of less than 70 mg/dL, whereas in others it is defined as a blood glucose level less than 54 mg/dL.”
The guidelines define first-level hypoglycemia as any glucose level of 3.9 mmol/L (70 mg/dL) or less. This is not considered low enough to be reported on a consistent basis in clinical studies; however, that determination must ultimately be made by the investigators, as the parameters for what is significant often vary from study to study.
The second level is the 3 mmol/L (54 mg/dL), which now is deemed to be a clinically significant level of hypoglycemia. Because it is “sufficiently low to indicate serious, clinically important hypoglycemia,” it should be reported as part of any clinical studies. Finally, the third level, less than 2.8 mmol/L (50 mg/dL), indicates severe hypoglycemia and is classified as any individual with “severe cognitive impairment requiring external assistance for recovery,” according to the guidelines (Diabetes Care. 2016 Dec 1. doi: 10.2337/dc16-2215).
With a new standard of hypoglycemic values that are deemed clinically significant, the ADA and EASD hope that comparing different insulins, medications, technologies, and educational interventions will now become easier and more standardized, leading to better care worldwide.
Although there is general agreement as to where severe hypoglycemia really begins, the newly defined glucose levels are “a step in the right direction,” according to Dr. Rodbard.
The International Hypoglycaemia Study Group developed these guidelines through a grant from Novo Nordisk, awarded to the Six Degrees Academy of Toronto. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, Merck, Sharp & Dohme, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic U.K. Dr. Rodbard did not report any financial disclosures.
, joining with the European Association for the Study of Diabetes to specify that a level of less than 3 mmol/L (54 mg/dL) should be considered “clinically important hypoglycemia.”
“A single glucose level should be agreed to that has serious clinical and health-economic consequences,” the ADA and EASD stated in the new guidelines. “This would enable the diabetes and regulatory communities to compare the effectiveness of interventions in reducing hypoglycemia, be they pharmacological, technological, or educational. It would also permit the use of meta-analysis as a statistical tool to increase power when comparing interventions.”
An international, multidisciplinary group – the International Hypoglycemia Study Group – was formed to create distinct definitions of the various levels of severity that hypoglycemia can have. The new guidelines contain three levels, which should be used by clinicians to determine what amounts of blood glucose are significant enough to be clinically reported.
“Currently, there is no uniform agreement to what constitutes reportable hypoglycemia in clinical trials,” she said. “In some studies, it is defined as a blood glucose level of less than 70 mg/dL, whereas in others it is defined as a blood glucose level less than 54 mg/dL.”
The guidelines define first-level hypoglycemia as any glucose level of 3.9 mmol/L (70 mg/dL) or less. This is not considered low enough to be reported on a consistent basis in clinical studies; however, that determination must ultimately be made by the investigators, as the parameters for what is significant often vary from study to study.
The second level is the 3 mmol/L (54 mg/dL), which now is deemed to be a clinically significant level of hypoglycemia. Because it is “sufficiently low to indicate serious, clinically important hypoglycemia,” it should be reported as part of any clinical studies. Finally, the third level, less than 2.8 mmol/L (50 mg/dL), indicates severe hypoglycemia and is classified as any individual with “severe cognitive impairment requiring external assistance for recovery,” according to the guidelines (Diabetes Care. 2016 Dec 1. doi: 10.2337/dc16-2215).
With a new standard of hypoglycemic values that are deemed clinically significant, the ADA and EASD hope that comparing different insulins, medications, technologies, and educational interventions will now become easier and more standardized, leading to better care worldwide.
Although there is general agreement as to where severe hypoglycemia really begins, the newly defined glucose levels are “a step in the right direction,” according to Dr. Rodbard.
The International Hypoglycaemia Study Group developed these guidelines through a grant from Novo Nordisk, awarded to the Six Degrees Academy of Toronto. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, Merck, Sharp & Dohme, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic U.K. Dr. Rodbard did not report any financial disclosures.
, joining with the European Association for the Study of Diabetes to specify that a level of less than 3 mmol/L (54 mg/dL) should be considered “clinically important hypoglycemia.”
“A single glucose level should be agreed to that has serious clinical and health-economic consequences,” the ADA and EASD stated in the new guidelines. “This would enable the diabetes and regulatory communities to compare the effectiveness of interventions in reducing hypoglycemia, be they pharmacological, technological, or educational. It would also permit the use of meta-analysis as a statistical tool to increase power when comparing interventions.”
An international, multidisciplinary group – the International Hypoglycemia Study Group – was formed to create distinct definitions of the various levels of severity that hypoglycemia can have. The new guidelines contain three levels, which should be used by clinicians to determine what amounts of blood glucose are significant enough to be clinically reported.
“Currently, there is no uniform agreement to what constitutes reportable hypoglycemia in clinical trials,” she said. “In some studies, it is defined as a blood glucose level of less than 70 mg/dL, whereas in others it is defined as a blood glucose level less than 54 mg/dL.”
The guidelines define first-level hypoglycemia as any glucose level of 3.9 mmol/L (70 mg/dL) or less. This is not considered low enough to be reported on a consistent basis in clinical studies; however, that determination must ultimately be made by the investigators, as the parameters for what is significant often vary from study to study.
The second level is the 3 mmol/L (54 mg/dL), which now is deemed to be a clinically significant level of hypoglycemia. Because it is “sufficiently low to indicate serious, clinically important hypoglycemia,” it should be reported as part of any clinical studies. Finally, the third level, less than 2.8 mmol/L (50 mg/dL), indicates severe hypoglycemia and is classified as any individual with “severe cognitive impairment requiring external assistance for recovery,” according to the guidelines (Diabetes Care. 2016 Dec 1. doi: 10.2337/dc16-2215).
With a new standard of hypoglycemic values that are deemed clinically significant, the ADA and EASD hope that comparing different insulins, medications, technologies, and educational interventions will now become easier and more standardized, leading to better care worldwide.
Although there is general agreement as to where severe hypoglycemia really begins, the newly defined glucose levels are “a step in the right direction,” according to Dr. Rodbard.
The International Hypoglycaemia Study Group developed these guidelines through a grant from Novo Nordisk, awarded to the Six Degrees Academy of Toronto. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, Merck, Sharp & Dohme, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic U.K. Dr. Rodbard did not report any financial disclosures.
Opioids for chronic pain: The CDC’s 12 recommendations
Earlier this year, the Centers for Disease Control and Prevention (CDC) published a clinical practice guideline aimed at decreasing opioid use in the treatment of chronic pain.1 It developed this guideline in response to the increasing problem of opioid abuse and opioid-related mortality in the United States.
The CDC notes that an estimated 1.9 million people abused or were dependent on prescription opioid pain medication in 2013.1 Between 1999 and 2014, more than 165,000 people in the United States died from an overdose of opioid pain medication, with that rate increasing markedly in the past decade.1 In 2011, an estimated 420,000 emergency department visits were related to the abuse of narcotic pain relievers.2
While the problem of increasing opioid-related abuse and deaths has been apparent for some time, effective interventions have been elusive. Evidence remains sparse on the benefits and harms of long-term opioid therapy for chronic pain, except for those at the end of life. Evidence has been insufficient to determine long-term benefits of opioid therapy vs no opioid therapy, although the potential for harms from high doses of opioids are documented. There is not much evidence comparing nonpharmacologic and non-opioid pharmacologic treatments with long-term opioid therapy.
This lack of an evidence base is reflected in the CDC guideline. Of the guideline’s 12 recommendations, not one has high-level supporting evidence and only one has even moderate-level evidence behind it. Four recommendations are supported by low-level evidence, and 7 by very-low-level evidence. Yet 11 of the 12 are given an A recommendation, meaning that the guideline panel feels that most patients should receive this course of action.
Methodology used to create the guideline
The guideline committee used a modified GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) to develop the guideline. It is the same system the Advisory Committee on Immunization Practices adopted to assess and make recommendations on vaccines.3 The system’s classification of levels of evidence and recommendation categories are described in FIGURE 1.1
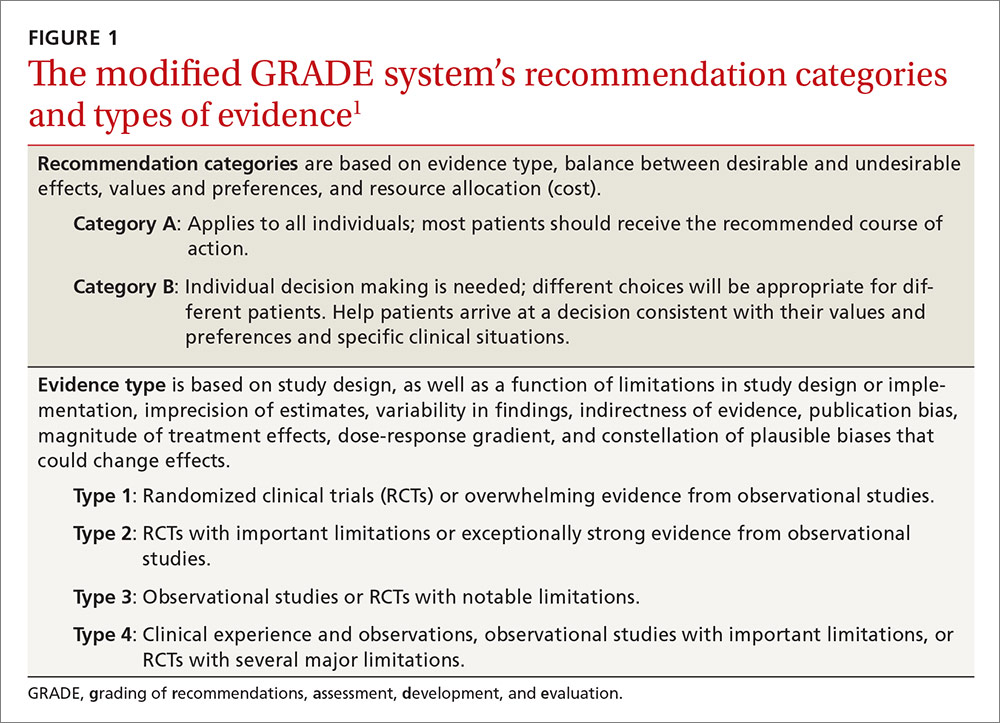
The committee started by assessing evidence with a report on the long-term effectiveness of opioids for chronic pain, produced by the Agency for Health Care Research and Quality in 2014;4 it then augmented that report by performing an updated search for new evidence published since the report came out.5 The committee then conducted a “contextual evidence review”6 on the following 4 areas:
- the effectiveness of nonpharmacologic (cognitive behavioral therapy, exercise therapy, interventional treatments, multimodal pain treatment) and non-opioid pharmacologic treatments (acetaminophen, nonsteroidal anti-inflammatory drugs, antidepressants, anticonvulsants)
- the benefits and harms of opioid therapy
- clinician and patient values and preferences related to opioids and medication risks, benefits, and use
- resource allocation, including costs and economic analyses.
The guideline wording indicates that, for this contextual analysis, the committee used a rapid systematic review methodology, in part because of time constraints given the imperative to produce a guideline to address a pressing problem, and because of a recognition that evidence on the questions would be scant and not of high quality.1 The 12 recommendations are categorized under 3 main headings.
Determining when to initiate or continue opioids for chronic pain
1. Nonpharmacologic therapy and non-opioid pharmacologic therapy are preferred for chronic pain. Consider opioid therapy only if you anticipate that benefits for both pain and function will outweigh risks to the patient. If opioids are used, combine them as appropriate with nonpharmacologic therapy and non-opioid pharmacologic therapy. (Recommendation category: A; evidence type: 3)
2. Before starting opioid therapy for chronic pain, establish treatment goals with the patient, including realistic goals for pain and function, and consider how therapy will be discontinued if the benefits do not outweigh the risks. Continue opioid therapy only if there is clinically meaningful improvement in pain and function that outweighs risks to patient safety. (Recommendation category: A; evidence type: 4)
3. Before starting opioid therapy, and periodically during its course, discuss with patients known risks and realistic benefits of opioid therapy and patient and clinician responsibilities for managing therapy. (Recommendation category: A; evidence type: 3)
Opioid selection, dosage, duration, follow-up, and discontinuation
4. When starting opioid therapy for chronic pain, prescribe immediate-release opioids instead of extended-release/long-acting (ER/LA) agents. (Recommendation category: A; evidence type: 4)
5. When starting opioids, prescribe the lowest effective dosage. Use caution when prescribing opioids at any dosage; carefully reassess the evidence for individual benefits and risks when increasing the dosage to ≥50 morphine milligram equivalents (MME)/d; and avoid increasing the dosage to ≥90 MME/d (or carefully justify such a decision, if made). (Recommendation category: A; evidence type: 3)
6. Long-term opioid use often begins with treatment of acute pain. When opioids are used for acute pain, prescribe the lowest effective dose of immediate-release opioids at a quantity no greater than is needed for the expected duration of pain severe enough to require opioids. Three days or less will often be sufficient; more than 7 days will rarely be needed. (Recommendation category: A; evidence type: 4)
7. In monitoring opioid therapy for chronic pain, reevaluate benefits and harms with patients within one to 4 weeks of starting opioid therapy or escalating the dose. Also, evaluate the benefits and harms of continued therapy with patients every 3 months or more frequently. If the benefits of continued opioid therapy do not outweigh the harms, optimize other therapies and work with patients to taper opioids to lower dosages or to taper and discontinue them. (Recommendation category: A; evidence type: 4)
Assessing risk and addressing harms of opioid use
8. Before starting opioid therapy, and periodically during its continuation, evaluate risk factors for opioid-related harms. Incorporate strategies into the management plan to mitigate risk; consider offering naloxone when factors are present that increase the risk for opioid overdose—eg, a history of overdose, history of substance use disorder, higher opioid dosages (≥50 MME/d), or concurrent benzodiazepine use. (Recommendation category: A; evidence type: 4)
9. Review the patient’s history of controlled substance prescriptions. Use data from the state prescription drug monitoring program (PDMP) to determine whether the patient is receiving opioid dosages or dangerous combinations that put him or her at high risk for overdose. (State Web sites are available at http://www.pdmpassist.org/content/state-pdmp-websites.) Review PDMP data when starting opioid therapy for chronic pain and periodically during its continuation, at least every 3 months and with each new prescription. (Recommendation category: A; evidence type: 4)
10. Before prescribing opioids for chronic pain, use urine drug testing to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs, and consider urine drug testing at least annually. (Recommendation category: B; evidence type: 4)
11. Avoid prescribing opioid pain medication and benzodiazepines concurrently whenever possible. (Recommendation category: A; evidence type: 3)
12. For patients with opioid use disorder, offer or arrange for evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies). (Recommendation category: A; evidence type: 2)
Aids for guideline implementation
The CDC has produced materials to assist physicians in implementing this guideline, including checklists for prescribing or continuing opioids. The checklist for initiation of opioids is reproduced in FIGURE 2.7
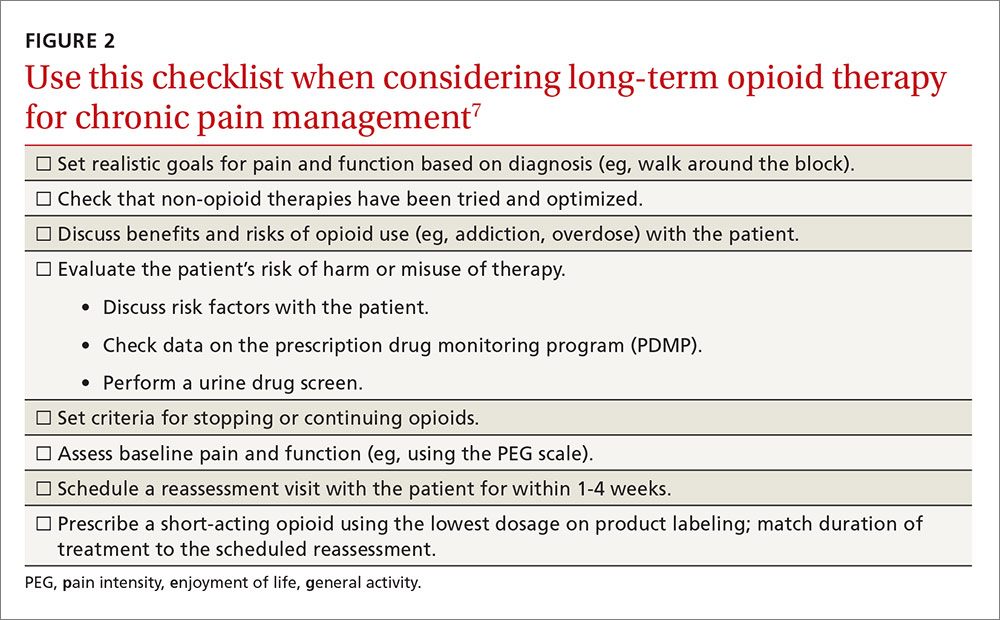
The CDC is addressing a severe public health problem and doing so by using contemporary evidence-based methodology and guideline development processes. The lack of high-quality evidence on the topic and the use of a less-than-optimal evidence review process for some key questions may hamper this effort. However, given the prominence of the CDC, this clinical guideline will likely be considered the standard of care for family physicians.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1–49. Available at: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed October 17, 20
2. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. 20
3. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
4. Chou R, Deyo R, Devine B, et al. The effectiveness and risks of long-term opioid treatment of chronic pain. Evidence Report/Technology Assessment No. 218. AHRQ Publication No. 14-E005-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/557/1971/chronic-pain-opioid-treatment-report-141205.pdf. Accessed October 17, 2016.
5. Centers for Disease Control and Prevention. Clinical evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38026. Accessed October 17, 2016.
6. Centers for Disease Control and Prevention. Contextual evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain – United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38027. Accessed October 17, 2016.
7. Centers for Disease Control and Prevention. Checklist for prescribing opioids for chronic pain. Available at: https://stacks.cdc.gov/view/cdc/38025. Accessed October 17, 2016.
Earlier this year, the Centers for Disease Control and Prevention (CDC) published a clinical practice guideline aimed at decreasing opioid use in the treatment of chronic pain.1 It developed this guideline in response to the increasing problem of opioid abuse and opioid-related mortality in the United States.
The CDC notes that an estimated 1.9 million people abused or were dependent on prescription opioid pain medication in 2013.1 Between 1999 and 2014, more than 165,000 people in the United States died from an overdose of opioid pain medication, with that rate increasing markedly in the past decade.1 In 2011, an estimated 420,000 emergency department visits were related to the abuse of narcotic pain relievers.2
While the problem of increasing opioid-related abuse and deaths has been apparent for some time, effective interventions have been elusive. Evidence remains sparse on the benefits and harms of long-term opioid therapy for chronic pain, except for those at the end of life. Evidence has been insufficient to determine long-term benefits of opioid therapy vs no opioid therapy, although the potential for harms from high doses of opioids are documented. There is not much evidence comparing nonpharmacologic and non-opioid pharmacologic treatments with long-term opioid therapy.
This lack of an evidence base is reflected in the CDC guideline. Of the guideline’s 12 recommendations, not one has high-level supporting evidence and only one has even moderate-level evidence behind it. Four recommendations are supported by low-level evidence, and 7 by very-low-level evidence. Yet 11 of the 12 are given an A recommendation, meaning that the guideline panel feels that most patients should receive this course of action.
Methodology used to create the guideline
The guideline committee used a modified GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) to develop the guideline. It is the same system the Advisory Committee on Immunization Practices adopted to assess and make recommendations on vaccines.3 The system’s classification of levels of evidence and recommendation categories are described in FIGURE 1.1

The committee started by assessing evidence with a report on the long-term effectiveness of opioids for chronic pain, produced by the Agency for Health Care Research and Quality in 2014;4 it then augmented that report by performing an updated search for new evidence published since the report came out.5 The committee then conducted a “contextual evidence review”6 on the following 4 areas:
- the effectiveness of nonpharmacologic (cognitive behavioral therapy, exercise therapy, interventional treatments, multimodal pain treatment) and non-opioid pharmacologic treatments (acetaminophen, nonsteroidal anti-inflammatory drugs, antidepressants, anticonvulsants)
- the benefits and harms of opioid therapy
- clinician and patient values and preferences related to opioids and medication risks, benefits, and use
- resource allocation, including costs and economic analyses.
The guideline wording indicates that, for this contextual analysis, the committee used a rapid systematic review methodology, in part because of time constraints given the imperative to produce a guideline to address a pressing problem, and because of a recognition that evidence on the questions would be scant and not of high quality.1 The 12 recommendations are categorized under 3 main headings.
Determining when to initiate or continue opioids for chronic pain
1. Nonpharmacologic therapy and non-opioid pharmacologic therapy are preferred for chronic pain. Consider opioid therapy only if you anticipate that benefits for both pain and function will outweigh risks to the patient. If opioids are used, combine them as appropriate with nonpharmacologic therapy and non-opioid pharmacologic therapy. (Recommendation category: A; evidence type: 3)
2. Before starting opioid therapy for chronic pain, establish treatment goals with the patient, including realistic goals for pain and function, and consider how therapy will be discontinued if the benefits do not outweigh the risks. Continue opioid therapy only if there is clinically meaningful improvement in pain and function that outweighs risks to patient safety. (Recommendation category: A; evidence type: 4)
3. Before starting opioid therapy, and periodically during its course, discuss with patients known risks and realistic benefits of opioid therapy and patient and clinician responsibilities for managing therapy. (Recommendation category: A; evidence type: 3)
Opioid selection, dosage, duration, follow-up, and discontinuation
4. When starting opioid therapy for chronic pain, prescribe immediate-release opioids instead of extended-release/long-acting (ER/LA) agents. (Recommendation category: A; evidence type: 4)
5. When starting opioids, prescribe the lowest effective dosage. Use caution when prescribing opioids at any dosage; carefully reassess the evidence for individual benefits and risks when increasing the dosage to ≥50 morphine milligram equivalents (MME)/d; and avoid increasing the dosage to ≥90 MME/d (or carefully justify such a decision, if made). (Recommendation category: A; evidence type: 3)
6. Long-term opioid use often begins with treatment of acute pain. When opioids are used for acute pain, prescribe the lowest effective dose of immediate-release opioids at a quantity no greater than is needed for the expected duration of pain severe enough to require opioids. Three days or less will often be sufficient; more than 7 days will rarely be needed. (Recommendation category: A; evidence type: 4)
7. In monitoring opioid therapy for chronic pain, reevaluate benefits and harms with patients within one to 4 weeks of starting opioid therapy or escalating the dose. Also, evaluate the benefits and harms of continued therapy with patients every 3 months or more frequently. If the benefits of continued opioid therapy do not outweigh the harms, optimize other therapies and work with patients to taper opioids to lower dosages or to taper and discontinue them. (Recommendation category: A; evidence type: 4)
Assessing risk and addressing harms of opioid use
8. Before starting opioid therapy, and periodically during its continuation, evaluate risk factors for opioid-related harms. Incorporate strategies into the management plan to mitigate risk; consider offering naloxone when factors are present that increase the risk for opioid overdose—eg, a history of overdose, history of substance use disorder, higher opioid dosages (≥50 MME/d), or concurrent benzodiazepine use. (Recommendation category: A; evidence type: 4)
9. Review the patient’s history of controlled substance prescriptions. Use data from the state prescription drug monitoring program (PDMP) to determine whether the patient is receiving opioid dosages or dangerous combinations that put him or her at high risk for overdose. (State Web sites are available at http://www.pdmpassist.org/content/state-pdmp-websites.) Review PDMP data when starting opioid therapy for chronic pain and periodically during its continuation, at least every 3 months and with each new prescription. (Recommendation category: A; evidence type: 4)
10. Before prescribing opioids for chronic pain, use urine drug testing to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs, and consider urine drug testing at least annually. (Recommendation category: B; evidence type: 4)
11. Avoid prescribing opioid pain medication and benzodiazepines concurrently whenever possible. (Recommendation category: A; evidence type: 3)
12. For patients with opioid use disorder, offer or arrange for evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies). (Recommendation category: A; evidence type: 2)
Aids for guideline implementation
The CDC has produced materials to assist physicians in implementing this guideline, including checklists for prescribing or continuing opioids. The checklist for initiation of opioids is reproduced in FIGURE 2.7

The CDC is addressing a severe public health problem and doing so by using contemporary evidence-based methodology and guideline development processes. The lack of high-quality evidence on the topic and the use of a less-than-optimal evidence review process for some key questions may hamper this effort. However, given the prominence of the CDC, this clinical guideline will likely be considered the standard of care for family physicians.
Earlier this year, the Centers for Disease Control and Prevention (CDC) published a clinical practice guideline aimed at decreasing opioid use in the treatment of chronic pain.1 It developed this guideline in response to the increasing problem of opioid abuse and opioid-related mortality in the United States.
The CDC notes that an estimated 1.9 million people abused or were dependent on prescription opioid pain medication in 2013.1 Between 1999 and 2014, more than 165,000 people in the United States died from an overdose of opioid pain medication, with that rate increasing markedly in the past decade.1 In 2011, an estimated 420,000 emergency department visits were related to the abuse of narcotic pain relievers.2
While the problem of increasing opioid-related abuse and deaths has been apparent for some time, effective interventions have been elusive. Evidence remains sparse on the benefits and harms of long-term opioid therapy for chronic pain, except for those at the end of life. Evidence has been insufficient to determine long-term benefits of opioid therapy vs no opioid therapy, although the potential for harms from high doses of opioids are documented. There is not much evidence comparing nonpharmacologic and non-opioid pharmacologic treatments with long-term opioid therapy.
This lack of an evidence base is reflected in the CDC guideline. Of the guideline’s 12 recommendations, not one has high-level supporting evidence and only one has even moderate-level evidence behind it. Four recommendations are supported by low-level evidence, and 7 by very-low-level evidence. Yet 11 of the 12 are given an A recommendation, meaning that the guideline panel feels that most patients should receive this course of action.
Methodology used to create the guideline
The guideline committee used a modified GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) to develop the guideline. It is the same system the Advisory Committee on Immunization Practices adopted to assess and make recommendations on vaccines.3 The system’s classification of levels of evidence and recommendation categories are described in FIGURE 1.1

The committee started by assessing evidence with a report on the long-term effectiveness of opioids for chronic pain, produced by the Agency for Health Care Research and Quality in 2014;4 it then augmented that report by performing an updated search for new evidence published since the report came out.5 The committee then conducted a “contextual evidence review”6 on the following 4 areas:
- the effectiveness of nonpharmacologic (cognitive behavioral therapy, exercise therapy, interventional treatments, multimodal pain treatment) and non-opioid pharmacologic treatments (acetaminophen, nonsteroidal anti-inflammatory drugs, antidepressants, anticonvulsants)
- the benefits and harms of opioid therapy
- clinician and patient values and preferences related to opioids and medication risks, benefits, and use
- resource allocation, including costs and economic analyses.
The guideline wording indicates that, for this contextual analysis, the committee used a rapid systematic review methodology, in part because of time constraints given the imperative to produce a guideline to address a pressing problem, and because of a recognition that evidence on the questions would be scant and not of high quality.1 The 12 recommendations are categorized under 3 main headings.
Determining when to initiate or continue opioids for chronic pain
1. Nonpharmacologic therapy and non-opioid pharmacologic therapy are preferred for chronic pain. Consider opioid therapy only if you anticipate that benefits for both pain and function will outweigh risks to the patient. If opioids are used, combine them as appropriate with nonpharmacologic therapy and non-opioid pharmacologic therapy. (Recommendation category: A; evidence type: 3)
2. Before starting opioid therapy for chronic pain, establish treatment goals with the patient, including realistic goals for pain and function, and consider how therapy will be discontinued if the benefits do not outweigh the risks. Continue opioid therapy only if there is clinically meaningful improvement in pain and function that outweighs risks to patient safety. (Recommendation category: A; evidence type: 4)
3. Before starting opioid therapy, and periodically during its course, discuss with patients known risks and realistic benefits of opioid therapy and patient and clinician responsibilities for managing therapy. (Recommendation category: A; evidence type: 3)
Opioid selection, dosage, duration, follow-up, and discontinuation
4. When starting opioid therapy for chronic pain, prescribe immediate-release opioids instead of extended-release/long-acting (ER/LA) agents. (Recommendation category: A; evidence type: 4)
5. When starting opioids, prescribe the lowest effective dosage. Use caution when prescribing opioids at any dosage; carefully reassess the evidence for individual benefits and risks when increasing the dosage to ≥50 morphine milligram equivalents (MME)/d; and avoid increasing the dosage to ≥90 MME/d (or carefully justify such a decision, if made). (Recommendation category: A; evidence type: 3)
6. Long-term opioid use often begins with treatment of acute pain. When opioids are used for acute pain, prescribe the lowest effective dose of immediate-release opioids at a quantity no greater than is needed for the expected duration of pain severe enough to require opioids. Three days or less will often be sufficient; more than 7 days will rarely be needed. (Recommendation category: A; evidence type: 4)
7. In monitoring opioid therapy for chronic pain, reevaluate benefits and harms with patients within one to 4 weeks of starting opioid therapy or escalating the dose. Also, evaluate the benefits and harms of continued therapy with patients every 3 months or more frequently. If the benefits of continued opioid therapy do not outweigh the harms, optimize other therapies and work with patients to taper opioids to lower dosages or to taper and discontinue them. (Recommendation category: A; evidence type: 4)
Assessing risk and addressing harms of opioid use
8. Before starting opioid therapy, and periodically during its continuation, evaluate risk factors for opioid-related harms. Incorporate strategies into the management plan to mitigate risk; consider offering naloxone when factors are present that increase the risk for opioid overdose—eg, a history of overdose, history of substance use disorder, higher opioid dosages (≥50 MME/d), or concurrent benzodiazepine use. (Recommendation category: A; evidence type: 4)
9. Review the patient’s history of controlled substance prescriptions. Use data from the state prescription drug monitoring program (PDMP) to determine whether the patient is receiving opioid dosages or dangerous combinations that put him or her at high risk for overdose. (State Web sites are available at http://www.pdmpassist.org/content/state-pdmp-websites.) Review PDMP data when starting opioid therapy for chronic pain and periodically during its continuation, at least every 3 months and with each new prescription. (Recommendation category: A; evidence type: 4)
10. Before prescribing opioids for chronic pain, use urine drug testing to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs, and consider urine drug testing at least annually. (Recommendation category: B; evidence type: 4)
11. Avoid prescribing opioid pain medication and benzodiazepines concurrently whenever possible. (Recommendation category: A; evidence type: 3)
12. For patients with opioid use disorder, offer or arrange for evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies). (Recommendation category: A; evidence type: 2)
Aids for guideline implementation
The CDC has produced materials to assist physicians in implementing this guideline, including checklists for prescribing or continuing opioids. The checklist for initiation of opioids is reproduced in FIGURE 2.7

The CDC is addressing a severe public health problem and doing so by using contemporary evidence-based methodology and guideline development processes. The lack of high-quality evidence on the topic and the use of a less-than-optimal evidence review process for some key questions may hamper this effort. However, given the prominence of the CDC, this clinical guideline will likely be considered the standard of care for family physicians.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1–49. Available at: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed October 17, 20
2. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. 20
3. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
4. Chou R, Deyo R, Devine B, et al. The effectiveness and risks of long-term opioid treatment of chronic pain. Evidence Report/Technology Assessment No. 218. AHRQ Publication No. 14-E005-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/557/1971/chronic-pain-opioid-treatment-report-141205.pdf. Accessed October 17, 2016.
5. Centers for Disease Control and Prevention. Clinical evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38026. Accessed October 17, 2016.
6. Centers for Disease Control and Prevention. Contextual evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain – United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38027. Accessed October 17, 2016.
7. Centers for Disease Control and Prevention. Checklist for prescribing opioids for chronic pain. Available at: https://stacks.cdc.gov/view/cdc/38025. Accessed October 17, 2016.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1–49. Available at: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed October 17, 20
2. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. 20
3. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
4. Chou R, Deyo R, Devine B, et al. The effectiveness and risks of long-term opioid treatment of chronic pain. Evidence Report/Technology Assessment No. 218. AHRQ Publication No. 14-E005-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/557/1971/chronic-pain-opioid-treatment-report-141205.pdf. Accessed October 17, 2016.
5. Centers for Disease Control and Prevention. Clinical evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38026. Accessed October 17, 2016.
6. Centers for Disease Control and Prevention. Contextual evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain – United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38027. Accessed October 17, 2016.
7. Centers for Disease Control and Prevention. Checklist for prescribing opioids for chronic pain. Available at: https://stacks.cdc.gov/view/cdc/38025. Accessed October 17, 2016.
ASTRO guidelines lower age thresholds for APBI
The American Society for Radiation Oncology has issued new guidelines recommending accelerated partial breast irradiation brachytherapy (APBI) as an alternative to whole breast irradiation (WBI) after surgery in early-stage breast cancer patients, and lowering the age range of patients considered suitable for the procedure to people 50 and older, from 60.
With APBI, localized radiation is delivered to the region around the excised tissue, reducing treatment time and sparing healthy tissue. APBI may also be considered for patients 40 and older, according to ASTRO, if they meet all of the pathologic criteria for suitability listed in the guidelines for patients 50 and above.
The guidelines represent the first ASTRO update on APBI since 2009. In addition to expanding the age range for APBI treatment, the guidelines add low-risk ductal carcinoma in situ as an indication. The guidelines also address intraoperative radiation therapy, or IORT, in which patients receive low-energy photon or electron radiation during surgery (Pract Rad Oncol. 2016 Nov. 17 doi: 10.1016/j.prro.2016.09.007).
While IORT is suitable for patients with invasive cancer eligible for APBI, the guidelines say, patients considering this option should be counseled about the risk of recurrence compared with standard treatment, and, with photon IORT, about potential toxicity risk requiring follow-up. Though more than 40 studies were considered by the ASTRO committee, including large randomized trials comparing APBI with WBI, the new recommendations represent “more of a tweak than a revolution,” said Jay Harris, MD, of Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, one of the guideline authors.
Dr. Harris noted in an interview that two important randomized controlled trials comparing APBI and WBI are still underway, with full follow-up results expected in 2-3 years, after which more definitive recommendations can be made. For the intraoperative radiation advice contained in the guidelines, “we had evidence from two trials looking at different approaches,” Dr. Harris said. “One has long-term data using an electron beam in the operating room – this group showed that that approach seems reasonable in patients that we at ASTRO considered suitable in general for APBI. The other approach is low-dose photon radiation, for which we have only short-term follow-up, making us more hesitant to endorse it.” As for the new recommendation sanctioning APBI for ductal carcinoma, “There’s a lot of variation [in protocols] across the country, compared with invasive cancer,” Dr. Harris said. “We’re kind of all over the map with DCIS. This guideline presents another option.”
The guidelines were sponsored by ASTRO; two authors disclosed financial relationships with firms that make radiologic technology.
The American Society for Radiation Oncology has issued new guidelines recommending accelerated partial breast irradiation brachytherapy (APBI) as an alternative to whole breast irradiation (WBI) after surgery in early-stage breast cancer patients, and lowering the age range of patients considered suitable for the procedure to people 50 and older, from 60.
With APBI, localized radiation is delivered to the region around the excised tissue, reducing treatment time and sparing healthy tissue. APBI may also be considered for patients 40 and older, according to ASTRO, if they meet all of the pathologic criteria for suitability listed in the guidelines for patients 50 and above.
The guidelines represent the first ASTRO update on APBI since 2009. In addition to expanding the age range for APBI treatment, the guidelines add low-risk ductal carcinoma in situ as an indication. The guidelines also address intraoperative radiation therapy, or IORT, in which patients receive low-energy photon or electron radiation during surgery (Pract Rad Oncol. 2016 Nov. 17 doi: 10.1016/j.prro.2016.09.007).
While IORT is suitable for patients with invasive cancer eligible for APBI, the guidelines say, patients considering this option should be counseled about the risk of recurrence compared with standard treatment, and, with photon IORT, about potential toxicity risk requiring follow-up. Though more than 40 studies were considered by the ASTRO committee, including large randomized trials comparing APBI with WBI, the new recommendations represent “more of a tweak than a revolution,” said Jay Harris, MD, of Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, one of the guideline authors.
Dr. Harris noted in an interview that two important randomized controlled trials comparing APBI and WBI are still underway, with full follow-up results expected in 2-3 years, after which more definitive recommendations can be made. For the intraoperative radiation advice contained in the guidelines, “we had evidence from two trials looking at different approaches,” Dr. Harris said. “One has long-term data using an electron beam in the operating room – this group showed that that approach seems reasonable in patients that we at ASTRO considered suitable in general for APBI. The other approach is low-dose photon radiation, for which we have only short-term follow-up, making us more hesitant to endorse it.” As for the new recommendation sanctioning APBI for ductal carcinoma, “There’s a lot of variation [in protocols] across the country, compared with invasive cancer,” Dr. Harris said. “We’re kind of all over the map with DCIS. This guideline presents another option.”
The guidelines were sponsored by ASTRO; two authors disclosed financial relationships with firms that make radiologic technology.
The American Society for Radiation Oncology has issued new guidelines recommending accelerated partial breast irradiation brachytherapy (APBI) as an alternative to whole breast irradiation (WBI) after surgery in early-stage breast cancer patients, and lowering the age range of patients considered suitable for the procedure to people 50 and older, from 60.
With APBI, localized radiation is delivered to the region around the excised tissue, reducing treatment time and sparing healthy tissue. APBI may also be considered for patients 40 and older, according to ASTRO, if they meet all of the pathologic criteria for suitability listed in the guidelines for patients 50 and above.
The guidelines represent the first ASTRO update on APBI since 2009. In addition to expanding the age range for APBI treatment, the guidelines add low-risk ductal carcinoma in situ as an indication. The guidelines also address intraoperative radiation therapy, or IORT, in which patients receive low-energy photon or electron radiation during surgery (Pract Rad Oncol. 2016 Nov. 17 doi: 10.1016/j.prro.2016.09.007).
While IORT is suitable for patients with invasive cancer eligible for APBI, the guidelines say, patients considering this option should be counseled about the risk of recurrence compared with standard treatment, and, with photon IORT, about potential toxicity risk requiring follow-up. Though more than 40 studies were considered by the ASTRO committee, including large randomized trials comparing APBI with WBI, the new recommendations represent “more of a tweak than a revolution,” said Jay Harris, MD, of Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, one of the guideline authors.
Dr. Harris noted in an interview that two important randomized controlled trials comparing APBI and WBI are still underway, with full follow-up results expected in 2-3 years, after which more definitive recommendations can be made. For the intraoperative radiation advice contained in the guidelines, “we had evidence from two trials looking at different approaches,” Dr. Harris said. “One has long-term data using an electron beam in the operating room – this group showed that that approach seems reasonable in patients that we at ASTRO considered suitable in general for APBI. The other approach is low-dose photon radiation, for which we have only short-term follow-up, making us more hesitant to endorse it.” As for the new recommendation sanctioning APBI for ductal carcinoma, “There’s a lot of variation [in protocols] across the country, compared with invasive cancer,” Dr. Harris said. “We’re kind of all over the map with DCIS. This guideline presents another option.”
The guidelines were sponsored by ASTRO; two authors disclosed financial relationships with firms that make radiologic technology.
FROM PRACTICAL RADIATION ONCOLOGY
NCCN: Deliver vincristine by mini IV drip bag
Always dilute chemotherapy agent vincristine and administer it by mini IV-drip bag, instead of syringe, urges the National Comprehensive Cancer Network in a new campaign.
The goal of “Just Bag It” is to prevent a rare but uniformly fatal medical error – administering vincristine to the spinal fluid. When syringes are side by side – one with vincristine for IV push, another with a chemotherapeutic agent meant for push into the spinal fluid – it is just too easy to make a mistake. When administered intrathecally, vincristine causes ascending paralysis, neurological defects, and eventually death.
Despite all the warning labels and checks, “this still happens,” Marc Stewart, MD, cochair of the National Comprehensive Cancer Network (NCCN) Best Practices Committee, as well as medical director of the Seattle Cancer Care Alliance and professor of medicine at the University of Washington, said at a press conference.
Mini IV-drip bag administration will make it “virtually impossible. No physician would hook the bag up to a needle in someone’s spine” and even if they did, there wouldn’t be enough pressure in the bag to push vincristine in, he said.
The group has encouraged drip-bag delivery of vincristine for years, but only about half of hospitals have adopted the policy. The mistake happens so rarely – about 125 cases since the 1960s – “that the motivation for change is just not there.” Until somebody like NCCN calls it out in a high-profile campaign, “it’s not high on the radar screen,” Dr. Stewart said. It should be a relatively easy fix because bagging vincristine is not more costly. In general, the cost difference versus syringe “is going to be pennies,” he said.
“We challenge all medical centers, hospitals, and oncology practices around the nation and the world to implement this medication safety policy so this error never occurs again,” NCCN Chief Executive Officer Robert Carlson, MD, said in a press release. A medical oncologist, he witnessed the death of a 21-year-old patient after an intrathecal vincristine injection in 2005.
“Some health care providers may associate the use of an IV bag with a heightened risk of extravasation, but research shows that the risk of extravasation is extremely low (less than 0.05%) regardless of how vincristine is administered,” the press release noted.
Vincristine is widely used in treating patients with leukemia or lymphoma.
The safety of intravenous administration of vincristine has been a long-standing concern for anyone who participates in the management of patients with hematologic malignancies. As we all know, accidental intrathecal administration of vincristine is uniformly fatal.
At many centers, including ours, policies related to intravenous infusion of vesicants via a peripheral line have made the implementation of the safety recommendations difficult. It is not surprising that only 50% of hospitals surveyed by NCCN have fully implemented the mini-bag recommendation given the concern for extravasation. However, the newest ONS guidelines for vesicant administration allow for short-term infusions via a peripheral line. For our center, this support has been instrumental in allowing us to move to a practice with the recommended mini-bags. The NCCN “Just Bag It” campaign will likely help to move institutions such as ours to be in compliance with this important safety initiative.
Donna Capozzi, PharmD, is associate director of ambulatory services in the department of pharmacy at the Hospital of the University of Pennsylvania Perelman Center for Advanced Medicine in Philadelphia. She is on the editorial advisory board of Hematology News, a publication of this news company.
The safety of intravenous administration of vincristine has been a long-standing concern for anyone who participates in the management of patients with hematologic malignancies. As we all know, accidental intrathecal administration of vincristine is uniformly fatal.
At many centers, including ours, policies related to intravenous infusion of vesicants via a peripheral line have made the implementation of the safety recommendations difficult. It is not surprising that only 50% of hospitals surveyed by NCCN have fully implemented the mini-bag recommendation given the concern for extravasation. However, the newest ONS guidelines for vesicant administration allow for short-term infusions via a peripheral line. For our center, this support has been instrumental in allowing us to move to a practice with the recommended mini-bags. The NCCN “Just Bag It” campaign will likely help to move institutions such as ours to be in compliance with this important safety initiative.
Donna Capozzi, PharmD, is associate director of ambulatory services in the department of pharmacy at the Hospital of the University of Pennsylvania Perelman Center for Advanced Medicine in Philadelphia. She is on the editorial advisory board of Hematology News, a publication of this news company.
The safety of intravenous administration of vincristine has been a long-standing concern for anyone who participates in the management of patients with hematologic malignancies. As we all know, accidental intrathecal administration of vincristine is uniformly fatal.
At many centers, including ours, policies related to intravenous infusion of vesicants via a peripheral line have made the implementation of the safety recommendations difficult. It is not surprising that only 50% of hospitals surveyed by NCCN have fully implemented the mini-bag recommendation given the concern for extravasation. However, the newest ONS guidelines for vesicant administration allow for short-term infusions via a peripheral line. For our center, this support has been instrumental in allowing us to move to a practice with the recommended mini-bags. The NCCN “Just Bag It” campaign will likely help to move institutions such as ours to be in compliance with this important safety initiative.
Donna Capozzi, PharmD, is associate director of ambulatory services in the department of pharmacy at the Hospital of the University of Pennsylvania Perelman Center for Advanced Medicine in Philadelphia. She is on the editorial advisory board of Hematology News, a publication of this news company.
Always dilute chemotherapy agent vincristine and administer it by mini IV-drip bag, instead of syringe, urges the National Comprehensive Cancer Network in a new campaign.
The goal of “Just Bag It” is to prevent a rare but uniformly fatal medical error – administering vincristine to the spinal fluid. When syringes are side by side – one with vincristine for IV push, another with a chemotherapeutic agent meant for push into the spinal fluid – it is just too easy to make a mistake. When administered intrathecally, vincristine causes ascending paralysis, neurological defects, and eventually death.
Despite all the warning labels and checks, “this still happens,” Marc Stewart, MD, cochair of the National Comprehensive Cancer Network (NCCN) Best Practices Committee, as well as medical director of the Seattle Cancer Care Alliance and professor of medicine at the University of Washington, said at a press conference.
Mini IV-drip bag administration will make it “virtually impossible. No physician would hook the bag up to a needle in someone’s spine” and even if they did, there wouldn’t be enough pressure in the bag to push vincristine in, he said.
The group has encouraged drip-bag delivery of vincristine for years, but only about half of hospitals have adopted the policy. The mistake happens so rarely – about 125 cases since the 1960s – “that the motivation for change is just not there.” Until somebody like NCCN calls it out in a high-profile campaign, “it’s not high on the radar screen,” Dr. Stewart said. It should be a relatively easy fix because bagging vincristine is not more costly. In general, the cost difference versus syringe “is going to be pennies,” he said.
“We challenge all medical centers, hospitals, and oncology practices around the nation and the world to implement this medication safety policy so this error never occurs again,” NCCN Chief Executive Officer Robert Carlson, MD, said in a press release. A medical oncologist, he witnessed the death of a 21-year-old patient after an intrathecal vincristine injection in 2005.
“Some health care providers may associate the use of an IV bag with a heightened risk of extravasation, but research shows that the risk of extravasation is extremely low (less than 0.05%) regardless of how vincristine is administered,” the press release noted.
Vincristine is widely used in treating patients with leukemia or lymphoma.
Always dilute chemotherapy agent vincristine and administer it by mini IV-drip bag, instead of syringe, urges the National Comprehensive Cancer Network in a new campaign.
The goal of “Just Bag It” is to prevent a rare but uniformly fatal medical error – administering vincristine to the spinal fluid. When syringes are side by side – one with vincristine for IV push, another with a chemotherapeutic agent meant for push into the spinal fluid – it is just too easy to make a mistake. When administered intrathecally, vincristine causes ascending paralysis, neurological defects, and eventually death.
Despite all the warning labels and checks, “this still happens,” Marc Stewart, MD, cochair of the National Comprehensive Cancer Network (NCCN) Best Practices Committee, as well as medical director of the Seattle Cancer Care Alliance and professor of medicine at the University of Washington, said at a press conference.
Mini IV-drip bag administration will make it “virtually impossible. No physician would hook the bag up to a needle in someone’s spine” and even if they did, there wouldn’t be enough pressure in the bag to push vincristine in, he said.
The group has encouraged drip-bag delivery of vincristine for years, but only about half of hospitals have adopted the policy. The mistake happens so rarely – about 125 cases since the 1960s – “that the motivation for change is just not there.” Until somebody like NCCN calls it out in a high-profile campaign, “it’s not high on the radar screen,” Dr. Stewart said. It should be a relatively easy fix because bagging vincristine is not more costly. In general, the cost difference versus syringe “is going to be pennies,” he said.
“We challenge all medical centers, hospitals, and oncology practices around the nation and the world to implement this medication safety policy so this error never occurs again,” NCCN Chief Executive Officer Robert Carlson, MD, said in a press release. A medical oncologist, he witnessed the death of a 21-year-old patient after an intrathecal vincristine injection in 2005.
“Some health care providers may associate the use of an IV bag with a heightened risk of extravasation, but research shows that the risk of extravasation is extremely low (less than 0.05%) regardless of how vincristine is administered,” the press release noted.
Vincristine is widely used in treating patients with leukemia or lymphoma.

















