User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Digital mental health training acceptable to boarding teens
ANAHEIM, CALIF. – A modular digital intervention to teach mental health skills to youth awaiting transfer to psychiatric care appeared feasible to implement and acceptable to teens and their parents, according to a study presented at the American Academy of Pediatrics National Conference.
“This program has the potential to teach evidence-based mental health skills to youth during boarding, providing a head start on recovery prior to psychiatric hospitalization,” study coauthor Samantha House, DO, MPH, section chief of pediatric hospital medicine at Dartmouth Hitchcock Medical Center, Lebanon, N.H., told attendees.
Mental health boarding has become increasingly common as psychiatric care resources have been stretched by a crisis in pediatric mental health that began even before the COVID pandemic. Since youth often don’t receive evidence-based therapies while boarding, Dr. House and her coauthor, JoAnna K. Leyenaar, MD, PhD, MPH, developed a pilot program called I-CARE, which stands for Improving Care, Accelerating Recovery and Education.
I-CARE is a digital health intervention that combines videos on a tablet with workbook exercises that teach mental health skills. The seven modules include an introduction and one each on schedule-making, safety planning, psychoeducation, behavioral activation, relaxation skills, and mindfulness skills. Licensed nursing assistants who have received a 6-hour training from a clinical psychologist administer the program and provide safety supervision during boarding.
“I-CARE was designed to be largely self-directed, supported by ‘coaches’ who are not mental health professionals,” Dr. Leyenaar, vice chair of research in the department of pediatrics and an associate professor of pediatrics at Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. With this model, the program requires minimal additional resources beyond the tablets and workbooks, and is designed for implementation in settings with few or no mental health professionals, she said.
Cora Breuner, MD, MPH, a professor of pediatrics at the University of Washington, Seattle, and an attending physician at Seattle Children’s Hospital, was not involved in the study but was excited to see it.
“I think it’s a really good idea, and I like that it’s being studied,” Dr. Breuner said in an interview. She said the health care and public health system has let down an entire population who data had shown were experiencing mental health problems.
“We knew before the pandemic that behavioral health issues were creeping up slowly with anxiety, depression, suicidal ideation, and, of course, substance use disorders and eating disorders, and not a lot was being done about it,” Dr. Breuner said, and the pandemic exacerbated those issues. ”I don’t know why no one realized that this was going to be the downstream effect of having no socialization for kids for 18 months and limited resources for those who we need desperately to provide care for,” especially BIPOC [Black, Indigenous, and people of color] kids and underresourced kids.
That sentiment is exactly what inspired the creation of the program, according to Dr. Leyenaar.
The I-CARE program was implemented at Dartmouth Hitchcock Medical Center in November 2021 for adolescents aged 12-17 who were boarding because of suicidality or self-harm. The program and study excluded youth with psychosis and other cognitive or behavioral conditions that didn’t fit with the skills taught by the module training.
The researchers qualitatively evaluated the I-CARE program in youth who were offered at least two I-CARE modules and with parents present during boarding.
Twenty-four youth, with a median age of 14, were offered the I-CARE program between November 2021 and April 2022 while boarding for a median 8 days. Most of the patients were female (79%), and a third were transgender or gender diverse. Most were White (83%), and about two-thirds had Medicaid (62.5%). The most common diagnoses among the participants were major depressive disorder (71%) and generalized anxiety disorder (46%). Others included PTSD (29%), restrictive eating disorder (21%), and bipolar disorder (12.5%).
All offered the program completed the first module, and 79% participated in additional modules. The main reason for discontinuation was transfer to another facility, but a few youth either refused to engage with the program or felt they knew the material well enough that they weren’t benefiting from it.
The evaluation involved 16 youth, seven parents, and 17 clinicians. On a Likert scale, the composite score for the program’s appropriateness – suitability, applicability, and meeting needs – was an average 3.7, with a higher rating from clinicians (4.3) and caregivers (3.5) than youth (2.8).
“Some youth felt the intervention was better suited for a younger audience or those with less familiarity with mental health skills, but they acknowledged that the intervention would be helpful and appropriate for others,” Dr. House, who is also an assistant professor of pediatrics at Geisel School of Medicine, said.
Youth rated the acceptability of the program more highly (3.6) and all three groups found it easy to use, with an average feasibility score of 4 across the board. The program’s acceptability received an average score of 4 from parents and clinicians.
”Teens seem to particularly value the psychoeducation module that explains the relationship between thoughts and feelings, as well as the opportunity to develop a personalized safety plan,” Dr. Leyenaar said.
Among the challenges expressed by the participating teens were that the loud sounds and beeping in the hospital made it difficult to practice mindfulness and that they often had to wait for staff to be available to do I-CARE.
“I feel like not many people have been trained yet,” one teen said, “so to have more nurses available to do I-CARE would be helpful.”
Another participant found the coaches helpful. “Sometimes they were my nurse, sometimes they were someone I never met before. … and also, they were all really, really nice,” the teen said.
Another teen regarded the material as “really surface-level mental health stuff” that they thought “could be helpful to other people who are here for the first time.” But others found the content more beneficial.
“The videos were helpful. … I was worried that they weren’t going to be very informative, but they did make sense to me,” one participant said. “They weren’t overcomplicating things. … They weren’t saying anything I didn’t understand, so that was good.”
The researchers next plan to conduct a multisite study to determine the program’s effectiveness in improving health outcomes and reducing suicidal ideation. Dr. House and Dr. Leyenaar are looking at ways to refine the program.
”We may narrow the age range for participants, with an upper age limit of 16, since some older teens said that the modules were best suited for a younger audience,” Dr. Leyenaar said. “We are also discussing how to best support youth who are readmitted to our hospital and have participated in I-CARE previously.”
Dr. Breuner said she would be interested to see, in future studies of the program, whether it reduced the likelihood of inpatient psychiatric stay, the length of psychiatric stay after admission, or the risk of readmission. She also wondered if the program might be offered in languages other than English, whether a version might be specifically designed for BIPOC youth, and whether the researchers had considered offering the intervention to caregivers as well.
The modules are teaching the kids but should they also be teaching the parents? Dr. Breuner wondered. A lot of times, she said, the parents are bringing these kids in because they don’t know what to do and can’t deal with them anymore. Offering modules on the same skills to caregivers would also enable the caregivers to reinforce and reteach the skills to their children, especially if the youth struggled to really take in what the modules were trying to teach.
Dr. Leyenaar said she expects buy-in for a program like this would be high at other institutions, but it’s premature to scale it up until they’ve conducted at least another clinical trial on its effectiveness. The biggest potential barrier to buy-in that Dr. Breuner perceived would be cost.
“It’s always difficult when it costs money” since the hospital needs to train the clinicians who provide the care, Dr. Breuner said, but it’s possible those costs could be offset if the program reduces the risk of readmission or return to the emergency department.
While the overall risk of harms from the intervention are low, Dr. Breuner said it is important to be conscious that the intervention may not necessarily be appropriate for all youth.
“There’s always risk when there’s a trauma background, and you have to be very careful, especially with mindfulness training,” Dr. Breuner said. For those with a history of abuse or other adverse childhood experiences “for someone to get into a very calm, still place can actually be counterproductive.”
Dr. Breuner especially appreciated that the researchers involved the youth and caregivers in the evaluation process. “That the parents expressed positive attitudes is really incredible,” she said.
Dr. House, Dr. Leyenaar, and Dr. Breuner had no disclosures. No external funding was noted for the study.
ANAHEIM, CALIF. – A modular digital intervention to teach mental health skills to youth awaiting transfer to psychiatric care appeared feasible to implement and acceptable to teens and their parents, according to a study presented at the American Academy of Pediatrics National Conference.
“This program has the potential to teach evidence-based mental health skills to youth during boarding, providing a head start on recovery prior to psychiatric hospitalization,” study coauthor Samantha House, DO, MPH, section chief of pediatric hospital medicine at Dartmouth Hitchcock Medical Center, Lebanon, N.H., told attendees.
Mental health boarding has become increasingly common as psychiatric care resources have been stretched by a crisis in pediatric mental health that began even before the COVID pandemic. Since youth often don’t receive evidence-based therapies while boarding, Dr. House and her coauthor, JoAnna K. Leyenaar, MD, PhD, MPH, developed a pilot program called I-CARE, which stands for Improving Care, Accelerating Recovery and Education.
I-CARE is a digital health intervention that combines videos on a tablet with workbook exercises that teach mental health skills. The seven modules include an introduction and one each on schedule-making, safety planning, psychoeducation, behavioral activation, relaxation skills, and mindfulness skills. Licensed nursing assistants who have received a 6-hour training from a clinical psychologist administer the program and provide safety supervision during boarding.
“I-CARE was designed to be largely self-directed, supported by ‘coaches’ who are not mental health professionals,” Dr. Leyenaar, vice chair of research in the department of pediatrics and an associate professor of pediatrics at Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. With this model, the program requires minimal additional resources beyond the tablets and workbooks, and is designed for implementation in settings with few or no mental health professionals, she said.
Cora Breuner, MD, MPH, a professor of pediatrics at the University of Washington, Seattle, and an attending physician at Seattle Children’s Hospital, was not involved in the study but was excited to see it.
“I think it’s a really good idea, and I like that it’s being studied,” Dr. Breuner said in an interview. She said the health care and public health system has let down an entire population who data had shown were experiencing mental health problems.
“We knew before the pandemic that behavioral health issues were creeping up slowly with anxiety, depression, suicidal ideation, and, of course, substance use disorders and eating disorders, and not a lot was being done about it,” Dr. Breuner said, and the pandemic exacerbated those issues. ”I don’t know why no one realized that this was going to be the downstream effect of having no socialization for kids for 18 months and limited resources for those who we need desperately to provide care for,” especially BIPOC [Black, Indigenous, and people of color] kids and underresourced kids.
That sentiment is exactly what inspired the creation of the program, according to Dr. Leyenaar.
The I-CARE program was implemented at Dartmouth Hitchcock Medical Center in November 2021 for adolescents aged 12-17 who were boarding because of suicidality or self-harm. The program and study excluded youth with psychosis and other cognitive or behavioral conditions that didn’t fit with the skills taught by the module training.
The researchers qualitatively evaluated the I-CARE program in youth who were offered at least two I-CARE modules and with parents present during boarding.
Twenty-four youth, with a median age of 14, were offered the I-CARE program between November 2021 and April 2022 while boarding for a median 8 days. Most of the patients were female (79%), and a third were transgender or gender diverse. Most were White (83%), and about two-thirds had Medicaid (62.5%). The most common diagnoses among the participants were major depressive disorder (71%) and generalized anxiety disorder (46%). Others included PTSD (29%), restrictive eating disorder (21%), and bipolar disorder (12.5%).
All offered the program completed the first module, and 79% participated in additional modules. The main reason for discontinuation was transfer to another facility, but a few youth either refused to engage with the program or felt they knew the material well enough that they weren’t benefiting from it.
The evaluation involved 16 youth, seven parents, and 17 clinicians. On a Likert scale, the composite score for the program’s appropriateness – suitability, applicability, and meeting needs – was an average 3.7, with a higher rating from clinicians (4.3) and caregivers (3.5) than youth (2.8).
“Some youth felt the intervention was better suited for a younger audience or those with less familiarity with mental health skills, but they acknowledged that the intervention would be helpful and appropriate for others,” Dr. House, who is also an assistant professor of pediatrics at Geisel School of Medicine, said.
Youth rated the acceptability of the program more highly (3.6) and all three groups found it easy to use, with an average feasibility score of 4 across the board. The program’s acceptability received an average score of 4 from parents and clinicians.
”Teens seem to particularly value the psychoeducation module that explains the relationship between thoughts and feelings, as well as the opportunity to develop a personalized safety plan,” Dr. Leyenaar said.
Among the challenges expressed by the participating teens were that the loud sounds and beeping in the hospital made it difficult to practice mindfulness and that they often had to wait for staff to be available to do I-CARE.
“I feel like not many people have been trained yet,” one teen said, “so to have more nurses available to do I-CARE would be helpful.”
Another participant found the coaches helpful. “Sometimes they were my nurse, sometimes they were someone I never met before. … and also, they were all really, really nice,” the teen said.
Another teen regarded the material as “really surface-level mental health stuff” that they thought “could be helpful to other people who are here for the first time.” But others found the content more beneficial.
“The videos were helpful. … I was worried that they weren’t going to be very informative, but they did make sense to me,” one participant said. “They weren’t overcomplicating things. … They weren’t saying anything I didn’t understand, so that was good.”
The researchers next plan to conduct a multisite study to determine the program’s effectiveness in improving health outcomes and reducing suicidal ideation. Dr. House and Dr. Leyenaar are looking at ways to refine the program.
”We may narrow the age range for participants, with an upper age limit of 16, since some older teens said that the modules were best suited for a younger audience,” Dr. Leyenaar said. “We are also discussing how to best support youth who are readmitted to our hospital and have participated in I-CARE previously.”
Dr. Breuner said she would be interested to see, in future studies of the program, whether it reduced the likelihood of inpatient psychiatric stay, the length of psychiatric stay after admission, or the risk of readmission. She also wondered if the program might be offered in languages other than English, whether a version might be specifically designed for BIPOC youth, and whether the researchers had considered offering the intervention to caregivers as well.
The modules are teaching the kids but should they also be teaching the parents? Dr. Breuner wondered. A lot of times, she said, the parents are bringing these kids in because they don’t know what to do and can’t deal with them anymore. Offering modules on the same skills to caregivers would also enable the caregivers to reinforce and reteach the skills to their children, especially if the youth struggled to really take in what the modules were trying to teach.
Dr. Leyenaar said she expects buy-in for a program like this would be high at other institutions, but it’s premature to scale it up until they’ve conducted at least another clinical trial on its effectiveness. The biggest potential barrier to buy-in that Dr. Breuner perceived would be cost.
“It’s always difficult when it costs money” since the hospital needs to train the clinicians who provide the care, Dr. Breuner said, but it’s possible those costs could be offset if the program reduces the risk of readmission or return to the emergency department.
While the overall risk of harms from the intervention are low, Dr. Breuner said it is important to be conscious that the intervention may not necessarily be appropriate for all youth.
“There’s always risk when there’s a trauma background, and you have to be very careful, especially with mindfulness training,” Dr. Breuner said. For those with a history of abuse or other adverse childhood experiences “for someone to get into a very calm, still place can actually be counterproductive.”
Dr. Breuner especially appreciated that the researchers involved the youth and caregivers in the evaluation process. “That the parents expressed positive attitudes is really incredible,” she said.
Dr. House, Dr. Leyenaar, and Dr. Breuner had no disclosures. No external funding was noted for the study.
ANAHEIM, CALIF. – A modular digital intervention to teach mental health skills to youth awaiting transfer to psychiatric care appeared feasible to implement and acceptable to teens and their parents, according to a study presented at the American Academy of Pediatrics National Conference.
“This program has the potential to teach evidence-based mental health skills to youth during boarding, providing a head start on recovery prior to psychiatric hospitalization,” study coauthor Samantha House, DO, MPH, section chief of pediatric hospital medicine at Dartmouth Hitchcock Medical Center, Lebanon, N.H., told attendees.
Mental health boarding has become increasingly common as psychiatric care resources have been stretched by a crisis in pediatric mental health that began even before the COVID pandemic. Since youth often don’t receive evidence-based therapies while boarding, Dr. House and her coauthor, JoAnna K. Leyenaar, MD, PhD, MPH, developed a pilot program called I-CARE, which stands for Improving Care, Accelerating Recovery and Education.
I-CARE is a digital health intervention that combines videos on a tablet with workbook exercises that teach mental health skills. The seven modules include an introduction and one each on schedule-making, safety planning, psychoeducation, behavioral activation, relaxation skills, and mindfulness skills. Licensed nursing assistants who have received a 6-hour training from a clinical psychologist administer the program and provide safety supervision during boarding.
“I-CARE was designed to be largely self-directed, supported by ‘coaches’ who are not mental health professionals,” Dr. Leyenaar, vice chair of research in the department of pediatrics and an associate professor of pediatrics at Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. With this model, the program requires minimal additional resources beyond the tablets and workbooks, and is designed for implementation in settings with few or no mental health professionals, she said.
Cora Breuner, MD, MPH, a professor of pediatrics at the University of Washington, Seattle, and an attending physician at Seattle Children’s Hospital, was not involved in the study but was excited to see it.
“I think it’s a really good idea, and I like that it’s being studied,” Dr. Breuner said in an interview. She said the health care and public health system has let down an entire population who data had shown were experiencing mental health problems.
“We knew before the pandemic that behavioral health issues were creeping up slowly with anxiety, depression, suicidal ideation, and, of course, substance use disorders and eating disorders, and not a lot was being done about it,” Dr. Breuner said, and the pandemic exacerbated those issues. ”I don’t know why no one realized that this was going to be the downstream effect of having no socialization for kids for 18 months and limited resources for those who we need desperately to provide care for,” especially BIPOC [Black, Indigenous, and people of color] kids and underresourced kids.
That sentiment is exactly what inspired the creation of the program, according to Dr. Leyenaar.
The I-CARE program was implemented at Dartmouth Hitchcock Medical Center in November 2021 for adolescents aged 12-17 who were boarding because of suicidality or self-harm. The program and study excluded youth with psychosis and other cognitive or behavioral conditions that didn’t fit with the skills taught by the module training.
The researchers qualitatively evaluated the I-CARE program in youth who were offered at least two I-CARE modules and with parents present during boarding.
Twenty-four youth, with a median age of 14, were offered the I-CARE program between November 2021 and April 2022 while boarding for a median 8 days. Most of the patients were female (79%), and a third were transgender or gender diverse. Most were White (83%), and about two-thirds had Medicaid (62.5%). The most common diagnoses among the participants were major depressive disorder (71%) and generalized anxiety disorder (46%). Others included PTSD (29%), restrictive eating disorder (21%), and bipolar disorder (12.5%).
All offered the program completed the first module, and 79% participated in additional modules. The main reason for discontinuation was transfer to another facility, but a few youth either refused to engage with the program or felt they knew the material well enough that they weren’t benefiting from it.
The evaluation involved 16 youth, seven parents, and 17 clinicians. On a Likert scale, the composite score for the program’s appropriateness – suitability, applicability, and meeting needs – was an average 3.7, with a higher rating from clinicians (4.3) and caregivers (3.5) than youth (2.8).
“Some youth felt the intervention was better suited for a younger audience or those with less familiarity with mental health skills, but they acknowledged that the intervention would be helpful and appropriate for others,” Dr. House, who is also an assistant professor of pediatrics at Geisel School of Medicine, said.
Youth rated the acceptability of the program more highly (3.6) and all three groups found it easy to use, with an average feasibility score of 4 across the board. The program’s acceptability received an average score of 4 from parents and clinicians.
”Teens seem to particularly value the psychoeducation module that explains the relationship between thoughts and feelings, as well as the opportunity to develop a personalized safety plan,” Dr. Leyenaar said.
Among the challenges expressed by the participating teens were that the loud sounds and beeping in the hospital made it difficult to practice mindfulness and that they often had to wait for staff to be available to do I-CARE.
“I feel like not many people have been trained yet,” one teen said, “so to have more nurses available to do I-CARE would be helpful.”
Another participant found the coaches helpful. “Sometimes they were my nurse, sometimes they were someone I never met before. … and also, they were all really, really nice,” the teen said.
Another teen regarded the material as “really surface-level mental health stuff” that they thought “could be helpful to other people who are here for the first time.” But others found the content more beneficial.
“The videos were helpful. … I was worried that they weren’t going to be very informative, but they did make sense to me,” one participant said. “They weren’t overcomplicating things. … They weren’t saying anything I didn’t understand, so that was good.”
The researchers next plan to conduct a multisite study to determine the program’s effectiveness in improving health outcomes and reducing suicidal ideation. Dr. House and Dr. Leyenaar are looking at ways to refine the program.
”We may narrow the age range for participants, with an upper age limit of 16, since some older teens said that the modules were best suited for a younger audience,” Dr. Leyenaar said. “We are also discussing how to best support youth who are readmitted to our hospital and have participated in I-CARE previously.”
Dr. Breuner said she would be interested to see, in future studies of the program, whether it reduced the likelihood of inpatient psychiatric stay, the length of psychiatric stay after admission, or the risk of readmission. She also wondered if the program might be offered in languages other than English, whether a version might be specifically designed for BIPOC youth, and whether the researchers had considered offering the intervention to caregivers as well.
The modules are teaching the kids but should they also be teaching the parents? Dr. Breuner wondered. A lot of times, she said, the parents are bringing these kids in because they don’t know what to do and can’t deal with them anymore. Offering modules on the same skills to caregivers would also enable the caregivers to reinforce and reteach the skills to their children, especially if the youth struggled to really take in what the modules were trying to teach.
Dr. Leyenaar said she expects buy-in for a program like this would be high at other institutions, but it’s premature to scale it up until they’ve conducted at least another clinical trial on its effectiveness. The biggest potential barrier to buy-in that Dr. Breuner perceived would be cost.
“It’s always difficult when it costs money” since the hospital needs to train the clinicians who provide the care, Dr. Breuner said, but it’s possible those costs could be offset if the program reduces the risk of readmission or return to the emergency department.
While the overall risk of harms from the intervention are low, Dr. Breuner said it is important to be conscious that the intervention may not necessarily be appropriate for all youth.
“There’s always risk when there’s a trauma background, and you have to be very careful, especially with mindfulness training,” Dr. Breuner said. For those with a history of abuse or other adverse childhood experiences “for someone to get into a very calm, still place can actually be counterproductive.”
Dr. Breuner especially appreciated that the researchers involved the youth and caregivers in the evaluation process. “That the parents expressed positive attitudes is really incredible,” she said.
Dr. House, Dr. Leyenaar, and Dr. Breuner had no disclosures. No external funding was noted for the study.
AT AAP 2022
63% of long COVID patients are women, study says
according to a new study published in JAMA.
The global study also found that about 6% of people with symptomatic infections had long COVID in 2020 and 2021. The risk for long COVID seemed to be greater among those who needed hospitalization, especially those who needed intensive care.
“Quantifying the number of individuals with long COVID may help policy makers ensure adequate access to services to guide people toward recovery, return to the workplace or school, and restore their mental health and social life,” the researchers wrote.
The study team, which included dozens of researchers across nearly every continent, analyzed data from 54 studies and two databases for more than 1 million patients in 22 countries who had symptomatic COVID infections in 2020 and 2021. They looked at three long COVID symptom types: persistent fatigue with bodily pain or mood swings, ongoing respiratory problems, and cognitive issues. The study included people aged 4-66.
Overall, 6.2% of people reported one of the long COVID symptom types, including 3.7% with ongoing respiratory problems, 3.2% with persistent fatigue and bodily pain or mood swings, and 2.2% with cognitive problems. Among those with long COVID, 38% of people reported more than one symptom cluster.
At 3 months after infection, long COVID symptoms were nearly twice as common in women who were at least 20 years old at 10.6%, compared with men who were at least 20 years old at 5.4%.
Children and teens appeared to have lower risks of long COVID. About 2.8% of patients under age 20 with symptomatic infection developed long-term issues.
The estimated average duration of long COVID symptoms was 9 months among hospitalized patients and 4 months among those who weren’t hospitalized. About 15% of people with long COVID symptoms 3 months after the initial infection continued to have symptoms at 12 months.
The study was largely based on detailed data from ongoing COVID-19 studies in the United States, Austria, the Faroe Islands, Germany, Iran, Italy, the Netherlands, Russia, Sweden, and Switzerland, according to UPI. It was supplemented by published data and research conducted as part of the Global Burden of Diseases, Injuries and Risk Factors Study. The dozens of researchers are referred to as “Global Burden of Disease Long COVID Collaborators.”
The study had limitations, the researchers said, including the assumption that long COVID follows a similar course in all countries. Additional studies may show how long COVID symptoms and severity may vary in different countries and continents.
Ultimately, ongoing studies of large numbers of people with long COVID could help scientists and public health officials understand risk factors and ways to treat the debilitating condition, the study authors wrote, noting that “postinfection fatigue syndrome” has been reported before, namely during the 1918 flu pandemic, after the SARS outbreak in 2003, and after the Ebola epidemic in West Africa in 2014.
“Similar symptoms have been reported after other viral infections, including the Epstein-Barr virus, mononucleosis, and dengue, as well as after nonviral infections such as Q fever, Lyme disease and giardiasis,” they wrote.
Several study investigators reported receiving grants and personal fees from a variety of sources.
A version of this article first appeared on Medscape.com.
according to a new study published in JAMA.
The global study also found that about 6% of people with symptomatic infections had long COVID in 2020 and 2021. The risk for long COVID seemed to be greater among those who needed hospitalization, especially those who needed intensive care.
“Quantifying the number of individuals with long COVID may help policy makers ensure adequate access to services to guide people toward recovery, return to the workplace or school, and restore their mental health and social life,” the researchers wrote.
The study team, which included dozens of researchers across nearly every continent, analyzed data from 54 studies and two databases for more than 1 million patients in 22 countries who had symptomatic COVID infections in 2020 and 2021. They looked at three long COVID symptom types: persistent fatigue with bodily pain or mood swings, ongoing respiratory problems, and cognitive issues. The study included people aged 4-66.
Overall, 6.2% of people reported one of the long COVID symptom types, including 3.7% with ongoing respiratory problems, 3.2% with persistent fatigue and bodily pain or mood swings, and 2.2% with cognitive problems. Among those with long COVID, 38% of people reported more than one symptom cluster.
At 3 months after infection, long COVID symptoms were nearly twice as common in women who were at least 20 years old at 10.6%, compared with men who were at least 20 years old at 5.4%.
Children and teens appeared to have lower risks of long COVID. About 2.8% of patients under age 20 with symptomatic infection developed long-term issues.
The estimated average duration of long COVID symptoms was 9 months among hospitalized patients and 4 months among those who weren’t hospitalized. About 15% of people with long COVID symptoms 3 months after the initial infection continued to have symptoms at 12 months.
The study was largely based on detailed data from ongoing COVID-19 studies in the United States, Austria, the Faroe Islands, Germany, Iran, Italy, the Netherlands, Russia, Sweden, and Switzerland, according to UPI. It was supplemented by published data and research conducted as part of the Global Burden of Diseases, Injuries and Risk Factors Study. The dozens of researchers are referred to as “Global Burden of Disease Long COVID Collaborators.”
The study had limitations, the researchers said, including the assumption that long COVID follows a similar course in all countries. Additional studies may show how long COVID symptoms and severity may vary in different countries and continents.
Ultimately, ongoing studies of large numbers of people with long COVID could help scientists and public health officials understand risk factors and ways to treat the debilitating condition, the study authors wrote, noting that “postinfection fatigue syndrome” has been reported before, namely during the 1918 flu pandemic, after the SARS outbreak in 2003, and after the Ebola epidemic in West Africa in 2014.
“Similar symptoms have been reported after other viral infections, including the Epstein-Barr virus, mononucleosis, and dengue, as well as after nonviral infections such as Q fever, Lyme disease and giardiasis,” they wrote.
Several study investigators reported receiving grants and personal fees from a variety of sources.
A version of this article first appeared on Medscape.com.
according to a new study published in JAMA.
The global study also found that about 6% of people with symptomatic infections had long COVID in 2020 and 2021. The risk for long COVID seemed to be greater among those who needed hospitalization, especially those who needed intensive care.
“Quantifying the number of individuals with long COVID may help policy makers ensure adequate access to services to guide people toward recovery, return to the workplace or school, and restore their mental health and social life,” the researchers wrote.
The study team, which included dozens of researchers across nearly every continent, analyzed data from 54 studies and two databases for more than 1 million patients in 22 countries who had symptomatic COVID infections in 2020 and 2021. They looked at three long COVID symptom types: persistent fatigue with bodily pain or mood swings, ongoing respiratory problems, and cognitive issues. The study included people aged 4-66.
Overall, 6.2% of people reported one of the long COVID symptom types, including 3.7% with ongoing respiratory problems, 3.2% with persistent fatigue and bodily pain or mood swings, and 2.2% with cognitive problems. Among those with long COVID, 38% of people reported more than one symptom cluster.
At 3 months after infection, long COVID symptoms were nearly twice as common in women who were at least 20 years old at 10.6%, compared with men who were at least 20 years old at 5.4%.
Children and teens appeared to have lower risks of long COVID. About 2.8% of patients under age 20 with symptomatic infection developed long-term issues.
The estimated average duration of long COVID symptoms was 9 months among hospitalized patients and 4 months among those who weren’t hospitalized. About 15% of people with long COVID symptoms 3 months after the initial infection continued to have symptoms at 12 months.
The study was largely based on detailed data from ongoing COVID-19 studies in the United States, Austria, the Faroe Islands, Germany, Iran, Italy, the Netherlands, Russia, Sweden, and Switzerland, according to UPI. It was supplemented by published data and research conducted as part of the Global Burden of Diseases, Injuries and Risk Factors Study. The dozens of researchers are referred to as “Global Burden of Disease Long COVID Collaborators.”
The study had limitations, the researchers said, including the assumption that long COVID follows a similar course in all countries. Additional studies may show how long COVID symptoms and severity may vary in different countries and continents.
Ultimately, ongoing studies of large numbers of people with long COVID could help scientists and public health officials understand risk factors and ways to treat the debilitating condition, the study authors wrote, noting that “postinfection fatigue syndrome” has been reported before, namely during the 1918 flu pandemic, after the SARS outbreak in 2003, and after the Ebola epidemic in West Africa in 2014.
“Similar symptoms have been reported after other viral infections, including the Epstein-Barr virus, mononucleosis, and dengue, as well as after nonviral infections such as Q fever, Lyme disease and giardiasis,” they wrote.
Several study investigators reported receiving grants and personal fees from a variety of sources.
A version of this article first appeared on Medscape.com.
FROM JAMA
Older diabetes drugs linked to dementia risk -- one lower, one higher
a new observational study in patients with type 2 diabetes suggests.
The data, obtained from nationwide electronic medical records from the Department of Veterans Affairs, yielded a 22% lower risk of dementia with TZD monotherapy and a 12% elevated risk with sulfonylurea monotherapy, compared with metformin monotherapy. The apparent protective effects of TZDs were greater among individuals with overweight or obesity.
“Our findings provide additional information to aid clinicians’ selection of [glucose-lowering medications] for patients with mild or moderate type 2 diabetes and [who] are at high risk of dementia,” Xin Tang and colleagues wrote in their article, published online in BMJ Open Diabetes Research & Care.
The results “add substantially to the literature concerning the effects of [glucose-lowering medications] on dementia where previous findings have been inconsistent. Studies with a follow-up time of less than 3 years have mainly reported null associations, while studies with longer a follow-up time typically yielded protective findings. With a mean follow-up time of 6.8 years, we had a sufficient duration to detect treatment differences,” the investigators wrote.
“Supplementing [a] sulfonylurea with either metformin or [a] TZD may partially offset its prodementia effects. These findings may help inform medication selection for elderly patients with T2D at high risk of dementia,” they added.
Randomized trials needed to determine cause and effect
Ivan Koychev, PhD, a senior clinical researcher in the department of psychiatry at the University of Oxford (England), told the UK Science Media Centre: “This is a large, well-conducted real-world data study that highlights the importance of checking whether already prescribed medications may be useful for preventing dementia.”
The findings regarding TZDs, also known as glitazones, are in line with existing literature suggesting dementia protection with other drugs prescribed for type 2 diabetes that weren’t examined in the current study, such as newer agents like glucagonlike peptide–1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. Koychev said.
“The main limitations of this study is that following the initial 2-year period the authors were interested in, the participants may have been prescribed one of the other type 2 diabetes drugs [GLP-1 agonists or SGLT2 inhibitors] that have been found to reduce dementia risk, thus potentially making the direct glitazone [TZD] effect more difficult to discern,” Dr. Koychev noted.
And, he pointed out that the study design limits attribution of causality. “It is also important to note that people with type 2 diabetes do run a higher risk of both dementia and cognitive deficits and that these medications are only prescribed in these patients, so all this data is from this patient group rather than the general population.”
James Connell, PhD, head of translational science at Alzheimer’s Research UK, agreed. “While this observational study found that those with type 2 diabetes taking thiazolidinedione had a lower dementia risk than those on the most common medication for type 2 diabetes, it only shows an association between taking the drug and dementia risk and not a causal relationship.
“Double-blind and placebo-controlled clinical trials are needed to see whether the drug [TDZ] could help lower dementia risk in people with and without diabetes. Anyone with any questions about what treatments they are receiving should speak to their doctor,” he told the UK Science Media Centre.
Opposite effects of sulfonylureas, TZDs versus metformin
The study authors analyzed 559,106 VA patients with type 2 diabetes who initiated glucose-lowering medication during 2001-2017 and took it for at least a year. They were aged 60 years or older and did not have dementia at baseline. Most were White (76.8%) and male (96.9%), two-thirds (63.1%) had obesity, and mean hemoglobin A1c was 6.8%.
Overall, 31,125 developed all-cause dementia. The incidence rate was 8.2 cases per 1,000 person-years, ranging from 6.2 cases per 1,000 person-years among those taking metformin monotherapy to 13.4 cases per 1,000 person-years in those taking both sulfonylurea and a TZD.
Compared with metformin monotherapy, the hazard ratio for all-cause dementia for sulfonylurea monotherapy was a significant 1.12. The increased risk was also seen for vascular dementia, with an HR of 1.14.
In contrast, TZD monotherapy was associated with a significantly lower risk for all-cause dementia (HR, 0.78), as well as for Alzheimer’s disease (HR, 0.89) and vascular dementia (HR, 0.43), compared with metformin monotherapy.
The combination of metformin and TZD also lowered the risk of all-cause dementia, while regimens including sulfonylureas raised the risks for all-cause and vascular dementia.
Most of the results didn’t change significantly when the drug exposure window was extended to 2 years.
Effects more pronounced in those with obesity
The protective 1-year effects of TZD monotherapy and of metformin plus TZD, compared with metformin alone, were more significant among participants aged 75 or younger and with a body mass index above 25 kg/m2, compared with those who were older than 75 years and with normal BMIs, respectively.
On the other hand, the greater risk for dementia incurred with sulfonylureas was further increased among those with higher BMI.
This research was partially funded by grants from the National Human Genome Research Institute, the National Science Foundation, the National Institute of Diabetes and Digestive and Kidney Disease, and the National Heart, Lung, and Blood Institute. Dr. Koychev is chief investigator for a trial, sponsored by Oxford University and funded by Novo Nordisk, testing whether the GLP-1 agonist semaglutide reduces the risk for dementia in aging adults.
A version of this article first appeared on Medscape.com.
a new observational study in patients with type 2 diabetes suggests.
The data, obtained from nationwide electronic medical records from the Department of Veterans Affairs, yielded a 22% lower risk of dementia with TZD monotherapy and a 12% elevated risk with sulfonylurea monotherapy, compared with metformin monotherapy. The apparent protective effects of TZDs were greater among individuals with overweight or obesity.
“Our findings provide additional information to aid clinicians’ selection of [glucose-lowering medications] for patients with mild or moderate type 2 diabetes and [who] are at high risk of dementia,” Xin Tang and colleagues wrote in their article, published online in BMJ Open Diabetes Research & Care.
The results “add substantially to the literature concerning the effects of [glucose-lowering medications] on dementia where previous findings have been inconsistent. Studies with a follow-up time of less than 3 years have mainly reported null associations, while studies with longer a follow-up time typically yielded protective findings. With a mean follow-up time of 6.8 years, we had a sufficient duration to detect treatment differences,” the investigators wrote.
“Supplementing [a] sulfonylurea with either metformin or [a] TZD may partially offset its prodementia effects. These findings may help inform medication selection for elderly patients with T2D at high risk of dementia,” they added.
Randomized trials needed to determine cause and effect
Ivan Koychev, PhD, a senior clinical researcher in the department of psychiatry at the University of Oxford (England), told the UK Science Media Centre: “This is a large, well-conducted real-world data study that highlights the importance of checking whether already prescribed medications may be useful for preventing dementia.”
The findings regarding TZDs, also known as glitazones, are in line with existing literature suggesting dementia protection with other drugs prescribed for type 2 diabetes that weren’t examined in the current study, such as newer agents like glucagonlike peptide–1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. Koychev said.
“The main limitations of this study is that following the initial 2-year period the authors were interested in, the participants may have been prescribed one of the other type 2 diabetes drugs [GLP-1 agonists or SGLT2 inhibitors] that have been found to reduce dementia risk, thus potentially making the direct glitazone [TZD] effect more difficult to discern,” Dr. Koychev noted.
And, he pointed out that the study design limits attribution of causality. “It is also important to note that people with type 2 diabetes do run a higher risk of both dementia and cognitive deficits and that these medications are only prescribed in these patients, so all this data is from this patient group rather than the general population.”
James Connell, PhD, head of translational science at Alzheimer’s Research UK, agreed. “While this observational study found that those with type 2 diabetes taking thiazolidinedione had a lower dementia risk than those on the most common medication for type 2 diabetes, it only shows an association between taking the drug and dementia risk and not a causal relationship.
“Double-blind and placebo-controlled clinical trials are needed to see whether the drug [TDZ] could help lower dementia risk in people with and without diabetes. Anyone with any questions about what treatments they are receiving should speak to their doctor,” he told the UK Science Media Centre.
Opposite effects of sulfonylureas, TZDs versus metformin
The study authors analyzed 559,106 VA patients with type 2 diabetes who initiated glucose-lowering medication during 2001-2017 and took it for at least a year. They were aged 60 years or older and did not have dementia at baseline. Most were White (76.8%) and male (96.9%), two-thirds (63.1%) had obesity, and mean hemoglobin A1c was 6.8%.
Overall, 31,125 developed all-cause dementia. The incidence rate was 8.2 cases per 1,000 person-years, ranging from 6.2 cases per 1,000 person-years among those taking metformin monotherapy to 13.4 cases per 1,000 person-years in those taking both sulfonylurea and a TZD.
Compared with metformin monotherapy, the hazard ratio for all-cause dementia for sulfonylurea monotherapy was a significant 1.12. The increased risk was also seen for vascular dementia, with an HR of 1.14.
In contrast, TZD monotherapy was associated with a significantly lower risk for all-cause dementia (HR, 0.78), as well as for Alzheimer’s disease (HR, 0.89) and vascular dementia (HR, 0.43), compared with metformin monotherapy.
The combination of metformin and TZD also lowered the risk of all-cause dementia, while regimens including sulfonylureas raised the risks for all-cause and vascular dementia.
Most of the results didn’t change significantly when the drug exposure window was extended to 2 years.
Effects more pronounced in those with obesity
The protective 1-year effects of TZD monotherapy and of metformin plus TZD, compared with metformin alone, were more significant among participants aged 75 or younger and with a body mass index above 25 kg/m2, compared with those who were older than 75 years and with normal BMIs, respectively.
On the other hand, the greater risk for dementia incurred with sulfonylureas was further increased among those with higher BMI.
This research was partially funded by grants from the National Human Genome Research Institute, the National Science Foundation, the National Institute of Diabetes and Digestive and Kidney Disease, and the National Heart, Lung, and Blood Institute. Dr. Koychev is chief investigator for a trial, sponsored by Oxford University and funded by Novo Nordisk, testing whether the GLP-1 agonist semaglutide reduces the risk for dementia in aging adults.
A version of this article first appeared on Medscape.com.
a new observational study in patients with type 2 diabetes suggests.
The data, obtained from nationwide electronic medical records from the Department of Veterans Affairs, yielded a 22% lower risk of dementia with TZD monotherapy and a 12% elevated risk with sulfonylurea monotherapy, compared with metformin monotherapy. The apparent protective effects of TZDs were greater among individuals with overweight or obesity.
“Our findings provide additional information to aid clinicians’ selection of [glucose-lowering medications] for patients with mild or moderate type 2 diabetes and [who] are at high risk of dementia,” Xin Tang and colleagues wrote in their article, published online in BMJ Open Diabetes Research & Care.
The results “add substantially to the literature concerning the effects of [glucose-lowering medications] on dementia where previous findings have been inconsistent. Studies with a follow-up time of less than 3 years have mainly reported null associations, while studies with longer a follow-up time typically yielded protective findings. With a mean follow-up time of 6.8 years, we had a sufficient duration to detect treatment differences,” the investigators wrote.
“Supplementing [a] sulfonylurea with either metformin or [a] TZD may partially offset its prodementia effects. These findings may help inform medication selection for elderly patients with T2D at high risk of dementia,” they added.
Randomized trials needed to determine cause and effect
Ivan Koychev, PhD, a senior clinical researcher in the department of psychiatry at the University of Oxford (England), told the UK Science Media Centre: “This is a large, well-conducted real-world data study that highlights the importance of checking whether already prescribed medications may be useful for preventing dementia.”
The findings regarding TZDs, also known as glitazones, are in line with existing literature suggesting dementia protection with other drugs prescribed for type 2 diabetes that weren’t examined in the current study, such as newer agents like glucagonlike peptide–1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. Koychev said.
“The main limitations of this study is that following the initial 2-year period the authors were interested in, the participants may have been prescribed one of the other type 2 diabetes drugs [GLP-1 agonists or SGLT2 inhibitors] that have been found to reduce dementia risk, thus potentially making the direct glitazone [TZD] effect more difficult to discern,” Dr. Koychev noted.
And, he pointed out that the study design limits attribution of causality. “It is also important to note that people with type 2 diabetes do run a higher risk of both dementia and cognitive deficits and that these medications are only prescribed in these patients, so all this data is from this patient group rather than the general population.”
James Connell, PhD, head of translational science at Alzheimer’s Research UK, agreed. “While this observational study found that those with type 2 diabetes taking thiazolidinedione had a lower dementia risk than those on the most common medication for type 2 diabetes, it only shows an association between taking the drug and dementia risk and not a causal relationship.
“Double-blind and placebo-controlled clinical trials are needed to see whether the drug [TDZ] could help lower dementia risk in people with and without diabetes. Anyone with any questions about what treatments they are receiving should speak to their doctor,” he told the UK Science Media Centre.
Opposite effects of sulfonylureas, TZDs versus metformin
The study authors analyzed 559,106 VA patients with type 2 diabetes who initiated glucose-lowering medication during 2001-2017 and took it for at least a year. They were aged 60 years or older and did not have dementia at baseline. Most were White (76.8%) and male (96.9%), two-thirds (63.1%) had obesity, and mean hemoglobin A1c was 6.8%.
Overall, 31,125 developed all-cause dementia. The incidence rate was 8.2 cases per 1,000 person-years, ranging from 6.2 cases per 1,000 person-years among those taking metformin monotherapy to 13.4 cases per 1,000 person-years in those taking both sulfonylurea and a TZD.
Compared with metformin monotherapy, the hazard ratio for all-cause dementia for sulfonylurea monotherapy was a significant 1.12. The increased risk was also seen for vascular dementia, with an HR of 1.14.
In contrast, TZD monotherapy was associated with a significantly lower risk for all-cause dementia (HR, 0.78), as well as for Alzheimer’s disease (HR, 0.89) and vascular dementia (HR, 0.43), compared with metformin monotherapy.
The combination of metformin and TZD also lowered the risk of all-cause dementia, while regimens including sulfonylureas raised the risks for all-cause and vascular dementia.
Most of the results didn’t change significantly when the drug exposure window was extended to 2 years.
Effects more pronounced in those with obesity
The protective 1-year effects of TZD monotherapy and of metformin plus TZD, compared with metformin alone, were more significant among participants aged 75 or younger and with a body mass index above 25 kg/m2, compared with those who were older than 75 years and with normal BMIs, respectively.
On the other hand, the greater risk for dementia incurred with sulfonylureas was further increased among those with higher BMI.
This research was partially funded by grants from the National Human Genome Research Institute, the National Science Foundation, the National Institute of Diabetes and Digestive and Kidney Disease, and the National Heart, Lung, and Blood Institute. Dr. Koychev is chief investigator for a trial, sponsored by Oxford University and funded by Novo Nordisk, testing whether the GLP-1 agonist semaglutide reduces the risk for dementia in aging adults.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN DIABETES RESEARCH & CARE
Epidemic of brain fog? Long COVID’s effects worry experts
Weeks after Jeannie Volpe caught COVID-19 in November 2020, she could no longer do her job running sexual assault support groups in Anniston, Ala., because she kept forgetting the details that survivors had shared with her. “People were telling me they were having to revisit their traumatic memories, which isn’t fair to anybody,” the 47-year-old says.
Ms. Volpe has been diagnosed with long-COVID autonomic dysfunction, which includes severe muscle pain, depression, anxiety, and a loss of thinking skills. Some of her symptoms are more commonly known as brain fog, and they’re among the most frequent problems reported by people who have long-term issues after a bout of COVID-19.
Many experts and medical professionals say they haven’t even begun to scratch the surface of what impact this will have in years to come.
“I’m very worried that we have an epidemic of neurologic dysfunction coming down the pike,” says Pamela Davis, MD, PhD, a research professor at Case Western Reserve University, Cleveland.
In the 2 years Ms. Volpe has been living with long COVID, her executive function – the mental processes that enable people to focus attention, retain information, and multitask – has been so diminished that she had to relearn to drive. One of the various doctors assessing her has suggested speech therapy to help Ms. Volpe relearn how to form words. “I can see the words I want to say in my mind, but I can’t make them come out of my mouth,” she says in a sluggish voice that gives away her condition.
All of those symptoms make it difficult for her to care for herself. Without a job and health insurance, Ms. Volpe says she’s researched assisted suicide in the states that allow it but has ultimately decided she wants to live.
“People tell you things like you should be grateful you survived it, and you should; but you shouldn’t expect somebody to not grieve after losing their autonomy, their career, their finances.”
The findings of researchers studying the brain effects of COVID-19 reinforce what people with long COVID have been dealing with from the start. Their experiences aren’t imaginary; they’re consistent with neurological disorders – including myalgic encephalomyelitis, also known as chronic fatigue syndrome, or ME/CFS – which carry much more weight in the public imagination than the term brain fog, which can often be used dismissively.
Studies have found that COVID-19 is linked to conditions such as strokes; seizures; and mood, memory, and movement disorders.
While there are still a lot of unanswered questions about exactly how COVID-19 affects the brain and what the long-term effects are, there’s enough reason to suggest people should be trying to avoid both infection and reinfection until researchers get more answers.
Worldwide, it’s estimated that COVID-19 has contributed to more than 40 million new cases of neurological disorders, says Ziyad Al-Aly, MD, a clinical epidemiologist and long COVID researcher at Washington University in St. Louis. In his latest study of 14 million medical records of the U.S. Department of Veterans Affairs, the country’s largest integrated health care system, researchers found that regardless of age, gender, race, and lifestyle,
He noted that some of the conditions, such as headaches and mild decline in memory and sharpness, may improve and go away over time. But others that showed up, such as stroke, encephalitis (inflammation of the brain), and Guillain-Barré syndrome (a rare disorder in which the body’s immune system attacks the nerves), often lead to lasting damage. Dr. Al-Aly’s team found that neurological conditions were 7% more likely in those who had COVID-19 than in those who had never been infected.
What’s more, researchers noticed that compared with control groups, the risk of post-COVID thinking problems was more pronounced in people in their 30s, 40s, and 50s – a group that usually would be very unlikely to have these problems. For those over the age of 60, the risks stood out less because at that stage of life, such thinking problems aren’t as rare.
Another study of the veterans system last year showed that COVID-19 survivors were at a 46% higher risk of considering suicide after 1 year.
“We need to be paying attention to this,” says Dr. Al-Aly. “What we’ve seen is really the tip of the iceberg.” He worries that millions of people, including youths, will lose out on employment and education while dealing with long-term disabilities – and the economic and societal implications of such a fallout. “What we will all be left with is the aftermath of sheer devastation in some people’s lives,” he says.
Igor Koralnik, MD, chief of neuro-infectious disease and global neurology at Northwestern University, Chicago, has been running a specialized long COVID clinic. His team published a paper in March 2021 detailing what they saw in their first 100 patients. “About half the population in the study missed at least 10 days of work. This is going to have persistent impact on the workforce,” Dr. Koralnik said in a podcast posted on the Northwestern website. “We have seen that not only [do] patients have symptoms, but they have decreased quality of life.”
For older people and their caregivers, the risk of potential neurodegenerative diseases that the virus has shown to accelerate, such as dementia, is also a big concern. Alzheimer’s is already the fifth leading cause of death for people 65 and older.
In a recent study of more than 6 million people over the age of 65, Dr. Davis and her team at Case Western found the risk of Alzheimer’s in the year after COVID-19 increased by 50%-80%. The chances were especially high for women older than 85.
To date, there are no good treatments for Alzheimer’s, yet total health care costs for long-term care and hospice services for people with dementia topped $300 billion in 2020. That doesn’t even include the related costs to families.
“The downstream effect of having someone with Alzheimer’s being taken care of by a family member can be devastating on everyone,” she says. “Sometimes the caregivers don’t weather that very well.”
When Dr. Davis’s own father got Alzheimer’s at age 86, her mother took care of him until she had a stroke one morning while making breakfast. Dr. Davis attributes the stroke to the stress of caregiving. That left Dr. Davis no choice but to seek housing where both her parents could get care.
Looking at the broader picture, Dr. Davis believes widespread isolation, loneliness, and grief during the pandemic, and the disease of COVID-19 itself, will continue to have a profound impact on psychiatric diagnoses. This in turn could trigger a wave of new substance abuse as a result of unchecked mental health problems.
Still, not all brain experts are jumping to worst-case scenarios, with a lot yet to be understood before sounding the alarm. Joanna Hellmuth, MD, a neurologist and researcher at the University of California, San Francisco, cautions against reading too much into early data, including any assumptions that COVID-19 causes neurodegeneration or irreversible damage in the brain.
Even with before-and-after brain scans by University of Oxford, England, researchers that show structural changes to the brain after infection, she points out that they didn’t actually study the clinical symptoms of the people in the study, so it’s too soon to reach conclusions about associated cognitive problems.
“It’s an important piece of the puzzle, but we don’t know how that fits together with everything else,” says Dr. Hellmuth. “Some of my patients get better. … I haven’t seen a single person get worse since the pandemic started, and so I’m hopeful.”
A version of this article first appeared on WebMD.com.
Weeks after Jeannie Volpe caught COVID-19 in November 2020, she could no longer do her job running sexual assault support groups in Anniston, Ala., because she kept forgetting the details that survivors had shared with her. “People were telling me they were having to revisit their traumatic memories, which isn’t fair to anybody,” the 47-year-old says.
Ms. Volpe has been diagnosed with long-COVID autonomic dysfunction, which includes severe muscle pain, depression, anxiety, and a loss of thinking skills. Some of her symptoms are more commonly known as brain fog, and they’re among the most frequent problems reported by people who have long-term issues after a bout of COVID-19.
Many experts and medical professionals say they haven’t even begun to scratch the surface of what impact this will have in years to come.
“I’m very worried that we have an epidemic of neurologic dysfunction coming down the pike,” says Pamela Davis, MD, PhD, a research professor at Case Western Reserve University, Cleveland.
In the 2 years Ms. Volpe has been living with long COVID, her executive function – the mental processes that enable people to focus attention, retain information, and multitask – has been so diminished that she had to relearn to drive. One of the various doctors assessing her has suggested speech therapy to help Ms. Volpe relearn how to form words. “I can see the words I want to say in my mind, but I can’t make them come out of my mouth,” she says in a sluggish voice that gives away her condition.
All of those symptoms make it difficult for her to care for herself. Without a job and health insurance, Ms. Volpe says she’s researched assisted suicide in the states that allow it but has ultimately decided she wants to live.
“People tell you things like you should be grateful you survived it, and you should; but you shouldn’t expect somebody to not grieve after losing their autonomy, their career, their finances.”
The findings of researchers studying the brain effects of COVID-19 reinforce what people with long COVID have been dealing with from the start. Their experiences aren’t imaginary; they’re consistent with neurological disorders – including myalgic encephalomyelitis, also known as chronic fatigue syndrome, or ME/CFS – which carry much more weight in the public imagination than the term brain fog, which can often be used dismissively.
Studies have found that COVID-19 is linked to conditions such as strokes; seizures; and mood, memory, and movement disorders.
While there are still a lot of unanswered questions about exactly how COVID-19 affects the brain and what the long-term effects are, there’s enough reason to suggest people should be trying to avoid both infection and reinfection until researchers get more answers.
Worldwide, it’s estimated that COVID-19 has contributed to more than 40 million new cases of neurological disorders, says Ziyad Al-Aly, MD, a clinical epidemiologist and long COVID researcher at Washington University in St. Louis. In his latest study of 14 million medical records of the U.S. Department of Veterans Affairs, the country’s largest integrated health care system, researchers found that regardless of age, gender, race, and lifestyle,
He noted that some of the conditions, such as headaches and mild decline in memory and sharpness, may improve and go away over time. But others that showed up, such as stroke, encephalitis (inflammation of the brain), and Guillain-Barré syndrome (a rare disorder in which the body’s immune system attacks the nerves), often lead to lasting damage. Dr. Al-Aly’s team found that neurological conditions were 7% more likely in those who had COVID-19 than in those who had never been infected.
What’s more, researchers noticed that compared with control groups, the risk of post-COVID thinking problems was more pronounced in people in their 30s, 40s, and 50s – a group that usually would be very unlikely to have these problems. For those over the age of 60, the risks stood out less because at that stage of life, such thinking problems aren’t as rare.
Another study of the veterans system last year showed that COVID-19 survivors were at a 46% higher risk of considering suicide after 1 year.
“We need to be paying attention to this,” says Dr. Al-Aly. “What we’ve seen is really the tip of the iceberg.” He worries that millions of people, including youths, will lose out on employment and education while dealing with long-term disabilities – and the economic and societal implications of such a fallout. “What we will all be left with is the aftermath of sheer devastation in some people’s lives,” he says.
Igor Koralnik, MD, chief of neuro-infectious disease and global neurology at Northwestern University, Chicago, has been running a specialized long COVID clinic. His team published a paper in March 2021 detailing what they saw in their first 100 patients. “About half the population in the study missed at least 10 days of work. This is going to have persistent impact on the workforce,” Dr. Koralnik said in a podcast posted on the Northwestern website. “We have seen that not only [do] patients have symptoms, but they have decreased quality of life.”
For older people and their caregivers, the risk of potential neurodegenerative diseases that the virus has shown to accelerate, such as dementia, is also a big concern. Alzheimer’s is already the fifth leading cause of death for people 65 and older.
In a recent study of more than 6 million people over the age of 65, Dr. Davis and her team at Case Western found the risk of Alzheimer’s in the year after COVID-19 increased by 50%-80%. The chances were especially high for women older than 85.
To date, there are no good treatments for Alzheimer’s, yet total health care costs for long-term care and hospice services for people with dementia topped $300 billion in 2020. That doesn’t even include the related costs to families.
“The downstream effect of having someone with Alzheimer’s being taken care of by a family member can be devastating on everyone,” she says. “Sometimes the caregivers don’t weather that very well.”
When Dr. Davis’s own father got Alzheimer’s at age 86, her mother took care of him until she had a stroke one morning while making breakfast. Dr. Davis attributes the stroke to the stress of caregiving. That left Dr. Davis no choice but to seek housing where both her parents could get care.
Looking at the broader picture, Dr. Davis believes widespread isolation, loneliness, and grief during the pandemic, and the disease of COVID-19 itself, will continue to have a profound impact on psychiatric diagnoses. This in turn could trigger a wave of new substance abuse as a result of unchecked mental health problems.
Still, not all brain experts are jumping to worst-case scenarios, with a lot yet to be understood before sounding the alarm. Joanna Hellmuth, MD, a neurologist and researcher at the University of California, San Francisco, cautions against reading too much into early data, including any assumptions that COVID-19 causes neurodegeneration or irreversible damage in the brain.
Even with before-and-after brain scans by University of Oxford, England, researchers that show structural changes to the brain after infection, she points out that they didn’t actually study the clinical symptoms of the people in the study, so it’s too soon to reach conclusions about associated cognitive problems.
“It’s an important piece of the puzzle, but we don’t know how that fits together with everything else,” says Dr. Hellmuth. “Some of my patients get better. … I haven’t seen a single person get worse since the pandemic started, and so I’m hopeful.”
A version of this article first appeared on WebMD.com.
Weeks after Jeannie Volpe caught COVID-19 in November 2020, she could no longer do her job running sexual assault support groups in Anniston, Ala., because she kept forgetting the details that survivors had shared with her. “People were telling me they were having to revisit their traumatic memories, which isn’t fair to anybody,” the 47-year-old says.
Ms. Volpe has been diagnosed with long-COVID autonomic dysfunction, which includes severe muscle pain, depression, anxiety, and a loss of thinking skills. Some of her symptoms are more commonly known as brain fog, and they’re among the most frequent problems reported by people who have long-term issues after a bout of COVID-19.
Many experts and medical professionals say they haven’t even begun to scratch the surface of what impact this will have in years to come.
“I’m very worried that we have an epidemic of neurologic dysfunction coming down the pike,” says Pamela Davis, MD, PhD, a research professor at Case Western Reserve University, Cleveland.
In the 2 years Ms. Volpe has been living with long COVID, her executive function – the mental processes that enable people to focus attention, retain information, and multitask – has been so diminished that she had to relearn to drive. One of the various doctors assessing her has suggested speech therapy to help Ms. Volpe relearn how to form words. “I can see the words I want to say in my mind, but I can’t make them come out of my mouth,” she says in a sluggish voice that gives away her condition.
All of those symptoms make it difficult for her to care for herself. Without a job and health insurance, Ms. Volpe says she’s researched assisted suicide in the states that allow it but has ultimately decided she wants to live.
“People tell you things like you should be grateful you survived it, and you should; but you shouldn’t expect somebody to not grieve after losing their autonomy, their career, their finances.”
The findings of researchers studying the brain effects of COVID-19 reinforce what people with long COVID have been dealing with from the start. Their experiences aren’t imaginary; they’re consistent with neurological disorders – including myalgic encephalomyelitis, also known as chronic fatigue syndrome, or ME/CFS – which carry much more weight in the public imagination than the term brain fog, which can often be used dismissively.
Studies have found that COVID-19 is linked to conditions such as strokes; seizures; and mood, memory, and movement disorders.
While there are still a lot of unanswered questions about exactly how COVID-19 affects the brain and what the long-term effects are, there’s enough reason to suggest people should be trying to avoid both infection and reinfection until researchers get more answers.
Worldwide, it’s estimated that COVID-19 has contributed to more than 40 million new cases of neurological disorders, says Ziyad Al-Aly, MD, a clinical epidemiologist and long COVID researcher at Washington University in St. Louis. In his latest study of 14 million medical records of the U.S. Department of Veterans Affairs, the country’s largest integrated health care system, researchers found that regardless of age, gender, race, and lifestyle,
He noted that some of the conditions, such as headaches and mild decline in memory and sharpness, may improve and go away over time. But others that showed up, such as stroke, encephalitis (inflammation of the brain), and Guillain-Barré syndrome (a rare disorder in which the body’s immune system attacks the nerves), often lead to lasting damage. Dr. Al-Aly’s team found that neurological conditions were 7% more likely in those who had COVID-19 than in those who had never been infected.
What’s more, researchers noticed that compared with control groups, the risk of post-COVID thinking problems was more pronounced in people in their 30s, 40s, and 50s – a group that usually would be very unlikely to have these problems. For those over the age of 60, the risks stood out less because at that stage of life, such thinking problems aren’t as rare.
Another study of the veterans system last year showed that COVID-19 survivors were at a 46% higher risk of considering suicide after 1 year.
“We need to be paying attention to this,” says Dr. Al-Aly. “What we’ve seen is really the tip of the iceberg.” He worries that millions of people, including youths, will lose out on employment and education while dealing with long-term disabilities – and the economic and societal implications of such a fallout. “What we will all be left with is the aftermath of sheer devastation in some people’s lives,” he says.
Igor Koralnik, MD, chief of neuro-infectious disease and global neurology at Northwestern University, Chicago, has been running a specialized long COVID clinic. His team published a paper in March 2021 detailing what they saw in their first 100 patients. “About half the population in the study missed at least 10 days of work. This is going to have persistent impact on the workforce,” Dr. Koralnik said in a podcast posted on the Northwestern website. “We have seen that not only [do] patients have symptoms, but they have decreased quality of life.”
For older people and their caregivers, the risk of potential neurodegenerative diseases that the virus has shown to accelerate, such as dementia, is also a big concern. Alzheimer’s is already the fifth leading cause of death for people 65 and older.
In a recent study of more than 6 million people over the age of 65, Dr. Davis and her team at Case Western found the risk of Alzheimer’s in the year after COVID-19 increased by 50%-80%. The chances were especially high for women older than 85.
To date, there are no good treatments for Alzheimer’s, yet total health care costs for long-term care and hospice services for people with dementia topped $300 billion in 2020. That doesn’t even include the related costs to families.
“The downstream effect of having someone with Alzheimer’s being taken care of by a family member can be devastating on everyone,” she says. “Sometimes the caregivers don’t weather that very well.”
When Dr. Davis’s own father got Alzheimer’s at age 86, her mother took care of him until she had a stroke one morning while making breakfast. Dr. Davis attributes the stroke to the stress of caregiving. That left Dr. Davis no choice but to seek housing where both her parents could get care.
Looking at the broader picture, Dr. Davis believes widespread isolation, loneliness, and grief during the pandemic, and the disease of COVID-19 itself, will continue to have a profound impact on psychiatric diagnoses. This in turn could trigger a wave of new substance abuse as a result of unchecked mental health problems.
Still, not all brain experts are jumping to worst-case scenarios, with a lot yet to be understood before sounding the alarm. Joanna Hellmuth, MD, a neurologist and researcher at the University of California, San Francisco, cautions against reading too much into early data, including any assumptions that COVID-19 causes neurodegeneration or irreversible damage in the brain.
Even with before-and-after brain scans by University of Oxford, England, researchers that show structural changes to the brain after infection, she points out that they didn’t actually study the clinical symptoms of the people in the study, so it’s too soon to reach conclusions about associated cognitive problems.
“It’s an important piece of the puzzle, but we don’t know how that fits together with everything else,” says Dr. Hellmuth. “Some of my patients get better. … I haven’t seen a single person get worse since the pandemic started, and so I’m hopeful.”
A version of this article first appeared on WebMD.com.
USPSTF calls for universal anxiety screening in children 8-18, jury out on suicide screening
For the first time, the task force recommended screening for anxiety in children aged 8-18 years who do not have a diagnosed anxiety disorder and are not showing signs or symptoms of anxiety.
This “B” recommendation reflects “moderate certainty” evidence that screening for anxiety in 8- to 18-year-olds has a moderate net benefit, the task force said.
However, the task force found “insufficient” evidence to weigh the balance of benefits and harms of screening for anxiety in children aged 7 and younger and therefore issued an “I” statement.
The task force also recommended screening for children aged 12-18 years for major depressive disorder (“B” recommendation) but said there is insufficient evidence to weigh the balance of benefits and harms of screening for depression in children aged 11 and younger (“I” statement).
These recommendations are in line with the 2016 recommendations on depression screening from the USPSTF.
“Fortunately, screening older children for anxiety and depression can identify these conditions so children and teens can receive the care that they need,” task force member Martha Kubik, PhD, RN, with George Mason University, Fairfax, Va., said in a statement.
“Unfortunately, there are key evidence gaps related to screening for anxiety and depression in younger children and screening for suicide risk in all youth,” added task force member Lori Pbert, PhD, University of Massachusetts, Worcester.
“We are calling for more research in these critical areas so we can provide health care professionals with evidence-based ways to keep their young patients healthy,” Dr. Pbert said.
Suicide screening
Turning to suicide, the task force says there is not enough evidence to recommend for or against screening for suicide risk in children and adolescents, and therefore issued an “I” statement – in line with the 2014 recommendation statement from the task force.
The task force acknowledged that the American Academy of Pediatrics, the American Foundation for Suicide Prevention, and experts from the National Institute of Mental Health have released a “Blueprint for Youth Suicide Prevention” that recommends universal screening for suicide risk in youth 12 years or older, while children aged 8-11 years should be screened as clinically indicated.
The task force’s final recommendation statements and corresponding evidence summaries on screening children and adolescents for anxiety, depression and suicide were published online Oct. 11, 2022, in JAMA and the USPSTF website.
The final recommendations are consistent with the 2022 draft recommendation statements on these topics.
The task force emphasized that screening is only the first step in helping children and adolescents with anxiety and depression. Youth who screen positive need further evaluation to determine if they have anxiety or depression.
After diagnosis, youth should participate in shared decision-making with their parents and healthcare professional to identify the best treatment or combination of treatments.
Only a first step
In an accompanying editorial, John Walkup, MD, with Ann and Robert H. Lurie Children’s Hospital, Chicago, and coauthors made the point that, for the potential of screening for pediatric anxiety disorders to be fully realized, research focused on the process of screening from evaluation to treatment needs to be a priority.
“Perhaps most critical is developing a smart and sophisticated process of screening aligned with evidence-based treatment strategies that brings added value to routine pediatric medical care and that improves physical and mental health outcomes for children and adolescents,” they wrote.
Members of the USPSTF disclosed no relevant financial relationships. Dr. Walkup reported serving as an unpaid member of the scientific council of the Anxiety and Depression Association of America, receiving royalties for anxiety-related continuing medical education activities from Wolters Kluwer and honoraria for anxiety presentations from the American Academy of Child and Adolescent Psychiatry and the American Academy of Pediatrics.
A version of this article first appeared on Medscape.com.
For the first time, the task force recommended screening for anxiety in children aged 8-18 years who do not have a diagnosed anxiety disorder and are not showing signs or symptoms of anxiety.
This “B” recommendation reflects “moderate certainty” evidence that screening for anxiety in 8- to 18-year-olds has a moderate net benefit, the task force said.
However, the task force found “insufficient” evidence to weigh the balance of benefits and harms of screening for anxiety in children aged 7 and younger and therefore issued an “I” statement.
The task force also recommended screening for children aged 12-18 years for major depressive disorder (“B” recommendation) but said there is insufficient evidence to weigh the balance of benefits and harms of screening for depression in children aged 11 and younger (“I” statement).
These recommendations are in line with the 2016 recommendations on depression screening from the USPSTF.
“Fortunately, screening older children for anxiety and depression can identify these conditions so children and teens can receive the care that they need,” task force member Martha Kubik, PhD, RN, with George Mason University, Fairfax, Va., said in a statement.
“Unfortunately, there are key evidence gaps related to screening for anxiety and depression in younger children and screening for suicide risk in all youth,” added task force member Lori Pbert, PhD, University of Massachusetts, Worcester.
“We are calling for more research in these critical areas so we can provide health care professionals with evidence-based ways to keep their young patients healthy,” Dr. Pbert said.
Suicide screening
Turning to suicide, the task force says there is not enough evidence to recommend for or against screening for suicide risk in children and adolescents, and therefore issued an “I” statement – in line with the 2014 recommendation statement from the task force.
The task force acknowledged that the American Academy of Pediatrics, the American Foundation for Suicide Prevention, and experts from the National Institute of Mental Health have released a “Blueprint for Youth Suicide Prevention” that recommends universal screening for suicide risk in youth 12 years or older, while children aged 8-11 years should be screened as clinically indicated.
The task force’s final recommendation statements and corresponding evidence summaries on screening children and adolescents for anxiety, depression and suicide were published online Oct. 11, 2022, in JAMA and the USPSTF website.
The final recommendations are consistent with the 2022 draft recommendation statements on these topics.
The task force emphasized that screening is only the first step in helping children and adolescents with anxiety and depression. Youth who screen positive need further evaluation to determine if they have anxiety or depression.
After diagnosis, youth should participate in shared decision-making with their parents and healthcare professional to identify the best treatment or combination of treatments.
Only a first step
In an accompanying editorial, John Walkup, MD, with Ann and Robert H. Lurie Children’s Hospital, Chicago, and coauthors made the point that, for the potential of screening for pediatric anxiety disorders to be fully realized, research focused on the process of screening from evaluation to treatment needs to be a priority.
“Perhaps most critical is developing a smart and sophisticated process of screening aligned with evidence-based treatment strategies that brings added value to routine pediatric medical care and that improves physical and mental health outcomes for children and adolescents,” they wrote.
Members of the USPSTF disclosed no relevant financial relationships. Dr. Walkup reported serving as an unpaid member of the scientific council of the Anxiety and Depression Association of America, receiving royalties for anxiety-related continuing medical education activities from Wolters Kluwer and honoraria for anxiety presentations from the American Academy of Child and Adolescent Psychiatry and the American Academy of Pediatrics.
A version of this article first appeared on Medscape.com.
For the first time, the task force recommended screening for anxiety in children aged 8-18 years who do not have a diagnosed anxiety disorder and are not showing signs or symptoms of anxiety.
This “B” recommendation reflects “moderate certainty” evidence that screening for anxiety in 8- to 18-year-olds has a moderate net benefit, the task force said.
However, the task force found “insufficient” evidence to weigh the balance of benefits and harms of screening for anxiety in children aged 7 and younger and therefore issued an “I” statement.
The task force also recommended screening for children aged 12-18 years for major depressive disorder (“B” recommendation) but said there is insufficient evidence to weigh the balance of benefits and harms of screening for depression in children aged 11 and younger (“I” statement).
These recommendations are in line with the 2016 recommendations on depression screening from the USPSTF.
“Fortunately, screening older children for anxiety and depression can identify these conditions so children and teens can receive the care that they need,” task force member Martha Kubik, PhD, RN, with George Mason University, Fairfax, Va., said in a statement.
“Unfortunately, there are key evidence gaps related to screening for anxiety and depression in younger children and screening for suicide risk in all youth,” added task force member Lori Pbert, PhD, University of Massachusetts, Worcester.
“We are calling for more research in these critical areas so we can provide health care professionals with evidence-based ways to keep their young patients healthy,” Dr. Pbert said.
Suicide screening
Turning to suicide, the task force says there is not enough evidence to recommend for or against screening for suicide risk in children and adolescents, and therefore issued an “I” statement – in line with the 2014 recommendation statement from the task force.
The task force acknowledged that the American Academy of Pediatrics, the American Foundation for Suicide Prevention, and experts from the National Institute of Mental Health have released a “Blueprint for Youth Suicide Prevention” that recommends universal screening for suicide risk in youth 12 years or older, while children aged 8-11 years should be screened as clinically indicated.
The task force’s final recommendation statements and corresponding evidence summaries on screening children and adolescents for anxiety, depression and suicide were published online Oct. 11, 2022, in JAMA and the USPSTF website.
The final recommendations are consistent with the 2022 draft recommendation statements on these topics.
The task force emphasized that screening is only the first step in helping children and adolescents with anxiety and depression. Youth who screen positive need further evaluation to determine if they have anxiety or depression.
After diagnosis, youth should participate in shared decision-making with their parents and healthcare professional to identify the best treatment or combination of treatments.
Only a first step
In an accompanying editorial, John Walkup, MD, with Ann and Robert H. Lurie Children’s Hospital, Chicago, and coauthors made the point that, for the potential of screening for pediatric anxiety disorders to be fully realized, research focused on the process of screening from evaluation to treatment needs to be a priority.
“Perhaps most critical is developing a smart and sophisticated process of screening aligned with evidence-based treatment strategies that brings added value to routine pediatric medical care and that improves physical and mental health outcomes for children and adolescents,” they wrote.
Members of the USPSTF disclosed no relevant financial relationships. Dr. Walkup reported serving as an unpaid member of the scientific council of the Anxiety and Depression Association of America, receiving royalties for anxiety-related continuing medical education activities from Wolters Kluwer and honoraria for anxiety presentations from the American Academy of Child and Adolescent Psychiatry and the American Academy of Pediatrics.
A version of this article first appeared on Medscape.com.
FROM JAMA
The truth about the ‘happy hormone’: Why we shouldn’t mess with dopamine
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Weighted blankets promote melatonin release, may improve sleep
, compared with a lighter blanket of only about 2.4% of body weight.
This suggests that weighted blankets may help promote sleep in patients suffering from insomnia, according to the results from the small, in-laboratory crossover study.
“Melatonin is produced by the pineal gland and plays an essential role in sleep timing,” lead author Elisa Meth, PhD student, Uppsala University, Sweden, and colleagues observe.
“Using a weighted blanket increased melatonin concentration in saliva by about 30%,” Ms. Meth added in a statement.
“Future studies should investigate whether the stimulatory effect on melatonin secretion remains when using a weighted blanket over more extended periods,” the researchers observe, and caution that “it is also unclear whether the observed increase in melatonin is therapeutically relevant.”
The study was published online in the Journal of Sleep Research.
Weighted blankets are commercially available at least in some countries in Scandinavia and Germany, as examples, and in general, they are sold for therapeutic purposes. And at least one study found that weighted blankets were an effective and safe intervention for insomnia in patients with major depressive disorder, bipolar disorder, generalized anxiety disorder, and attention deficit hyperactivity disorder and led to improvements in daytime symptoms and levels of activity.
Study done in healthy volunteers
The study involved a total of 26 healthy volunteers, 15 men and 11 women, none of whom had any sleep issues. “The day before the first testing session, the participants visited the laboratory for an adaptation night,” the authors observe. There were two experimental test nights, one in which the weighted blanket was used and the second during which the lighter blanket was used.
On the test nights, lights were dimmed between 9 PM and 11 PM and participants used a weighted blanket covering the extremities, abdomen, and chest 1 hour before and during 8 hours of sleep. As the authors explain, the filling of the weighted blanket consisted of honed glass pearls, combined with polyester wadding, which corresponded to 12.2% of participants’ body weight.
“Saliva was collected every 20 minutes between 22:00 and 23:00,” Ms. Meth and colleagues note. Participants’ subjective sleepiness was also assessed every 20 minutes using the Karolinska Sleepiness Scale both before the hour that lights were turned off and the next morning.
“Sleep duration in each experimental night was recorded with the OURA ring,” investigators explain.
The OURA ring is a commercial multisensor wearable device that measures physiological variables indicative of sleep. Investigators focused on total sleep duration as the primary outcome measure.
On average, salivary melatonin concentrations rose by about 5.8 pg/mL between 10 PM and 11 PM (P < .001), but the average increase in salivary melatonin concentrations was greater under weighted blanket conditions at 6.6 pg/mL, compared with 5.0 pg/mL during the lighter blanket session (P = .011).
Oxytocin in turn rose by about 315 pg/mL initially, but this rise was only transient, and over time, no significant difference in oxytocin levels was observed between the two blanket conditions. There were also no differences in cortisol levels or the activity of the sympathetic nervous system between the weighted and light blanket sessions.
Importantly, as well, no significant differences were seen in the level of sleepiness between participants when either blanket was used nor was there a significant difference in total sleep duration.
“Our study cannot identify the underlying mechanism for the observed stimulatory effects of the weighted blanket on melatonin,” the investigators caution.
However, one explanation could be that the pressure exerted by the weighted blanket activates cutaneous sensory afferent nerves, carrying information to the brain. The region where the sensory information is delivered stimulates oxytocinergic neurons that can promote calm and well-being and decrease fear, stress, and pain. In addition, these neurons also connect to the pineal gland to influence the release of melatonin, the authors explain.
Melatonin often viewed in the wrong context
Senior author Christian Benedict, PhD, associate professor of pharmacology, Uppsala University, Sweden, explained that some people think of melatonin in the wrong context.
In point of fact, “it’s not a sleep-promoting hormone. It prepares your body and brain for the biological night ... [and] sleep coincides with the biological night, but it’s not like you take melatonin and you have a very nice uninterrupted slumber – this is not true,” he told this news organization.
He also noted that certain groups respond to melatonin better than others. For example, children with attention deficit hyperactivity disorder may have some benefit from melatonin supplements, as may the elderly who can no longer produce sufficient amounts of melatonin and for whom supplements may help promote the timing of sleep.
However, the bottom line is that, even in those who do respond to melatonin supplements, they likely do so through a placebo effect that meta-analyses have shown plays a powerful role in promoting sleep.
Dr. Benedict also stressed that just because the body makes melatonin, itself, does not mean that melatonin supplements are necessarily “safe.”
“We know melatonin has some impact on puberty – it may delay the onset of puberty – and we know that it can also impair blood glucose, so when people are eating and have a lot of melatonin on board, the melatonin will tell the pancreas to turn off insulin production, which can give rise to hyperglycemia,” he said.
However, Dr. Benedict cautioned that weighted blankets don’t come cheap. A quick Google search brings up examples that cost upwards of $350. “MDs can say try one if you can afford these blankets, but perhaps people can use several less costly blankets,” he said. “But I definitely think if there are cheap options, why not?” he concluded.
Dr. Benedict has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, compared with a lighter blanket of only about 2.4% of body weight.
This suggests that weighted blankets may help promote sleep in patients suffering from insomnia, according to the results from the small, in-laboratory crossover study.
“Melatonin is produced by the pineal gland and plays an essential role in sleep timing,” lead author Elisa Meth, PhD student, Uppsala University, Sweden, and colleagues observe.
“Using a weighted blanket increased melatonin concentration in saliva by about 30%,” Ms. Meth added in a statement.
“Future studies should investigate whether the stimulatory effect on melatonin secretion remains when using a weighted blanket over more extended periods,” the researchers observe, and caution that “it is also unclear whether the observed increase in melatonin is therapeutically relevant.”
The study was published online in the Journal of Sleep Research.
Weighted blankets are commercially available at least in some countries in Scandinavia and Germany, as examples, and in general, they are sold for therapeutic purposes. And at least one study found that weighted blankets were an effective and safe intervention for insomnia in patients with major depressive disorder, bipolar disorder, generalized anxiety disorder, and attention deficit hyperactivity disorder and led to improvements in daytime symptoms and levels of activity.
Study done in healthy volunteers
The study involved a total of 26 healthy volunteers, 15 men and 11 women, none of whom had any sleep issues. “The day before the first testing session, the participants visited the laboratory for an adaptation night,” the authors observe. There were two experimental test nights, one in which the weighted blanket was used and the second during which the lighter blanket was used.
On the test nights, lights were dimmed between 9 PM and 11 PM and participants used a weighted blanket covering the extremities, abdomen, and chest 1 hour before and during 8 hours of sleep. As the authors explain, the filling of the weighted blanket consisted of honed glass pearls, combined with polyester wadding, which corresponded to 12.2% of participants’ body weight.
“Saliva was collected every 20 minutes between 22:00 and 23:00,” Ms. Meth and colleagues note. Participants’ subjective sleepiness was also assessed every 20 minutes using the Karolinska Sleepiness Scale both before the hour that lights were turned off and the next morning.
“Sleep duration in each experimental night was recorded with the OURA ring,” investigators explain.
The OURA ring is a commercial multisensor wearable device that measures physiological variables indicative of sleep. Investigators focused on total sleep duration as the primary outcome measure.
On average, salivary melatonin concentrations rose by about 5.8 pg/mL between 10 PM and 11 PM (P < .001), but the average increase in salivary melatonin concentrations was greater under weighted blanket conditions at 6.6 pg/mL, compared with 5.0 pg/mL during the lighter blanket session (P = .011).
Oxytocin in turn rose by about 315 pg/mL initially, but this rise was only transient, and over time, no significant difference in oxytocin levels was observed between the two blanket conditions. There were also no differences in cortisol levels or the activity of the sympathetic nervous system between the weighted and light blanket sessions.
Importantly, as well, no significant differences were seen in the level of sleepiness between participants when either blanket was used nor was there a significant difference in total sleep duration.
“Our study cannot identify the underlying mechanism for the observed stimulatory effects of the weighted blanket on melatonin,” the investigators caution.
However, one explanation could be that the pressure exerted by the weighted blanket activates cutaneous sensory afferent nerves, carrying information to the brain. The region where the sensory information is delivered stimulates oxytocinergic neurons that can promote calm and well-being and decrease fear, stress, and pain. In addition, these neurons also connect to the pineal gland to influence the release of melatonin, the authors explain.
Melatonin often viewed in the wrong context
Senior author Christian Benedict, PhD, associate professor of pharmacology, Uppsala University, Sweden, explained that some people think of melatonin in the wrong context.
In point of fact, “it’s not a sleep-promoting hormone. It prepares your body and brain for the biological night ... [and] sleep coincides with the biological night, but it’s not like you take melatonin and you have a very nice uninterrupted slumber – this is not true,” he told this news organization.
He also noted that certain groups respond to melatonin better than others. For example, children with attention deficit hyperactivity disorder may have some benefit from melatonin supplements, as may the elderly who can no longer produce sufficient amounts of melatonin and for whom supplements may help promote the timing of sleep.
However, the bottom line is that, even in those who do respond to melatonin supplements, they likely do so through a placebo effect that meta-analyses have shown plays a powerful role in promoting sleep.
Dr. Benedict also stressed that just because the body makes melatonin, itself, does not mean that melatonin supplements are necessarily “safe.”
“We know melatonin has some impact on puberty – it may delay the onset of puberty – and we know that it can also impair blood glucose, so when people are eating and have a lot of melatonin on board, the melatonin will tell the pancreas to turn off insulin production, which can give rise to hyperglycemia,” he said.
However, Dr. Benedict cautioned that weighted blankets don’t come cheap. A quick Google search brings up examples that cost upwards of $350. “MDs can say try one if you can afford these blankets, but perhaps people can use several less costly blankets,” he said. “But I definitely think if there are cheap options, why not?” he concluded.
Dr. Benedict has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, compared with a lighter blanket of only about 2.4% of body weight.
This suggests that weighted blankets may help promote sleep in patients suffering from insomnia, according to the results from the small, in-laboratory crossover study.
“Melatonin is produced by the pineal gland and plays an essential role in sleep timing,” lead author Elisa Meth, PhD student, Uppsala University, Sweden, and colleagues observe.
“Using a weighted blanket increased melatonin concentration in saliva by about 30%,” Ms. Meth added in a statement.
“Future studies should investigate whether the stimulatory effect on melatonin secretion remains when using a weighted blanket over more extended periods,” the researchers observe, and caution that “it is also unclear whether the observed increase in melatonin is therapeutically relevant.”
The study was published online in the Journal of Sleep Research.
Weighted blankets are commercially available at least in some countries in Scandinavia and Germany, as examples, and in general, they are sold for therapeutic purposes. And at least one study found that weighted blankets were an effective and safe intervention for insomnia in patients with major depressive disorder, bipolar disorder, generalized anxiety disorder, and attention deficit hyperactivity disorder and led to improvements in daytime symptoms and levels of activity.
Study done in healthy volunteers
The study involved a total of 26 healthy volunteers, 15 men and 11 women, none of whom had any sleep issues. “The day before the first testing session, the participants visited the laboratory for an adaptation night,” the authors observe. There were two experimental test nights, one in which the weighted blanket was used and the second during which the lighter blanket was used.
On the test nights, lights were dimmed between 9 PM and 11 PM and participants used a weighted blanket covering the extremities, abdomen, and chest 1 hour before and during 8 hours of sleep. As the authors explain, the filling of the weighted blanket consisted of honed glass pearls, combined with polyester wadding, which corresponded to 12.2% of participants’ body weight.
“Saliva was collected every 20 minutes between 22:00 and 23:00,” Ms. Meth and colleagues note. Participants’ subjective sleepiness was also assessed every 20 minutes using the Karolinska Sleepiness Scale both before the hour that lights were turned off and the next morning.
“Sleep duration in each experimental night was recorded with the OURA ring,” investigators explain.
The OURA ring is a commercial multisensor wearable device that measures physiological variables indicative of sleep. Investigators focused on total sleep duration as the primary outcome measure.
On average, salivary melatonin concentrations rose by about 5.8 pg/mL between 10 PM and 11 PM (P < .001), but the average increase in salivary melatonin concentrations was greater under weighted blanket conditions at 6.6 pg/mL, compared with 5.0 pg/mL during the lighter blanket session (P = .011).
Oxytocin in turn rose by about 315 pg/mL initially, but this rise was only transient, and over time, no significant difference in oxytocin levels was observed between the two blanket conditions. There were also no differences in cortisol levels or the activity of the sympathetic nervous system between the weighted and light blanket sessions.
Importantly, as well, no significant differences were seen in the level of sleepiness between participants when either blanket was used nor was there a significant difference in total sleep duration.
“Our study cannot identify the underlying mechanism for the observed stimulatory effects of the weighted blanket on melatonin,” the investigators caution.
However, one explanation could be that the pressure exerted by the weighted blanket activates cutaneous sensory afferent nerves, carrying information to the brain. The region where the sensory information is delivered stimulates oxytocinergic neurons that can promote calm and well-being and decrease fear, stress, and pain. In addition, these neurons also connect to the pineal gland to influence the release of melatonin, the authors explain.
Melatonin often viewed in the wrong context
Senior author Christian Benedict, PhD, associate professor of pharmacology, Uppsala University, Sweden, explained that some people think of melatonin in the wrong context.
In point of fact, “it’s not a sleep-promoting hormone. It prepares your body and brain for the biological night ... [and] sleep coincides with the biological night, but it’s not like you take melatonin and you have a very nice uninterrupted slumber – this is not true,” he told this news organization.
He also noted that certain groups respond to melatonin better than others. For example, children with attention deficit hyperactivity disorder may have some benefit from melatonin supplements, as may the elderly who can no longer produce sufficient amounts of melatonin and for whom supplements may help promote the timing of sleep.
However, the bottom line is that, even in those who do respond to melatonin supplements, they likely do so through a placebo effect that meta-analyses have shown plays a powerful role in promoting sleep.
Dr. Benedict also stressed that just because the body makes melatonin, itself, does not mean that melatonin supplements are necessarily “safe.”
“We know melatonin has some impact on puberty – it may delay the onset of puberty – and we know that it can also impair blood glucose, so when people are eating and have a lot of melatonin on board, the melatonin will tell the pancreas to turn off insulin production, which can give rise to hyperglycemia,” he said.
However, Dr. Benedict cautioned that weighted blankets don’t come cheap. A quick Google search brings up examples that cost upwards of $350. “MDs can say try one if you can afford these blankets, but perhaps people can use several less costly blankets,” he said. “But I definitely think if there are cheap options, why not?” he concluded.
Dr. Benedict has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF SLEEP RESEARCH
Youth killed by guns in U.S. equals classroom a day
according to the American Academy of Pediatrics.
Preventing firearm-related injuries and deaths in children and youth “demands a public safety approach like regulation of motor vehicles,” the group said.
The organization on Oct. 8 released an updated policy statement and technical report about gun violence and children at its 2022 annual meeting in Anaheim, Calif. The reports were published in the journal Pediatrics, and the authors plan to discuss them during the conference.
“Each day, 28 U.S. children and teens – the equivalent of a high school classroom – die from gun violence, making it the No. 1 killer of youth through age 24,” the AAP said in a statement about the reports. “The national death rate is significantly higher than all other high-income countries combined, largely due to an alarming increase in suicides and homicides that do not make national headlines.”
Firearms have become the leading cause of death among children in the United States.
In 2020, guns caused 10,197 deaths of Americans younger than 24, according to the Society for Adolescent Health and Medicine.
In 2015, more than 7,200 American youth were killed by firearms. That same year in 28 other high-income countries – which combined would have had a population twice that of the United States – just 685 youth were killed by firearms, according to the AAP.
Separately at the AAP conference, physicians are presenting new research about gun violence and children. And on Oct. 10, a pediatrician who was at Uvalde Memorial Hospital in Texas after the deadly school shooting in May is scheduled to address attendees. The doctor, Roy Guerrero, MD, testified on Capitol Hill to advocate for gun control after the shooting at Robb Elementary School, which killed 19 children and two adults.
“This is not a simple problem, and it cannot be fixed with a simple solution,” Lois K. Lee, MD, MPH, said in the AAP news release. Dr. Lee chairs the AAP Council on Injury, Violence, and Poison Prevention that wrote the new reports. “Pediatricians as a start can offer families guidance and education on more safely storing guns. AAP also calls for supporting legislation that, much like the common-sense requirements for obtaining a driver’s license, would improve gun ownership safety.”
Many deaths occur at home
The rate of homicide from firearms in U.S. youth, especially those aged 15-24 years, increased by 14% during the past decade, and the rate of suicide from firearms increased by 39%, according to the AAP.
Homicides account for 58% of youth firearm deaths, whereas suicides account for 37%. Another 2% of youth firearm deaths are unintentional, and 1% result from law enforcement actions, the group said.
Among children 12 years old and younger, about 85% of firearm deaths occur at home. Teen firearm deaths are about as likely to occur at home (39%) as on the street or sidewalk (38%), according to research based on 2014 data.
“School shootings represent a relatively new phenomenon over the last half-century, and the United States has the highest rate of school shootings in the world,” the AAP technical report noted. Between 1966 and 2008, according to the group, 44 such shootings occurred in the United States, or an average of about one per year. Fast forward a few years and the violence became dramatically worse: Between 2013 and 2015, officials counted 154 school shootings – or about one per week.
Still, school shootings are responsible for less than 1% of all firearm deaths among children 17 years or younger in the United States. While school shootings “receive a tremendous amount of attention,” the report stated, other child firearm deaths may be less likely to make national headlines.
“Many firearm tragedies escape public attention because they occur in a home, sometimes in a child’s own home or at a friend’s house, or their neighbor’s or grandparent’s residence,” Eric W. Fleegler, MD, MPH, Boston Children’s Hospital, a co-author of the new reports, said in a statement from AAP. “Research tells us that families tend to underestimate how children will behave when they encounter a gun and miscalculate the risks. Suicide risks are also a huge concern, especially in families where teens are struggling with their mental health.”
AAP-recommended actions include:
- Mental health screenings and safe gun storage education provided by clinicians as part of routine patient visits
- Increased funding for violence intervention programs in hospital and community settings
- Regulation of firearms like other consumer products, with national requirements that address training, licensing, insurance coverage, registration of individuals purchasing firearms, and safe storage
- The use of technology that allows only authorized users to pull the trigger
- Universal background checks that use federal databases and information from local police before all gun purchases
- Extreme risk protection order laws, or “red flag laws,” that prohibit individuals at risk for harming themselves or others from purchasing or owning a firearm
- More funding for firearm injury and prevention research.
A noticeable increase in the ED
Irma Ugalde, MD, associate professor and director of pediatric emergency medicine research at McGovern Medical School at UTHealth Houston, noticed that firearm-related injuries in children at her hospital were more common during the COVID-19 pandemic, even as pediatric emergency department visits decreased overall.
She and her colleagues studied the trends and reported their findings at the AAP meeting.
“We saw a drop in pediatric admissions overall,” Dr. Ugalde said in a statement about the study. “But what was really noticeable was that trauma was still very prevalent – in fact probably more so – and we were seeing more firearm injuries.”
The researchers found that firearm injuries in children rose from 88 cases in 2019 to 118 in 2020. The number of incidents remained elevated in 2021, with 115 cases.
In addition, the researchers found an initial increase in injuries occurring at home where the shooter was a known family member or friend, and in cases involving firearms that were not properly stored.
By comparison, pediatric ED visits overall decreased by 34.2% from 2019 to 2020, and by 11.8% from 2019 to 2021.
The increase in firearm injuries coincided with an increase in gun sales in the United States, the researchers noted.
“National and statewide initiatives to mitigate the risk of firearm-related injury and death are necessary,” Dr. Ugalde’s group said. “We recommend that health care workers remain vigilant about screening for potential risk factors and safe storage of firearms.”
Accidental injuries
Daniel D. Guzman, MD, with Cook Children’s Health Care Center, Fort Worth, Tex., conducted a study focused on unintentional firearm injuries in children. Dr. Guzman’s group analyzed data from 204 patients younger than age 19 seen at Cook Children’s from January 2015 to June 2021.
Dr. Guzman and his colleagues examined outcomes for injuries caused by powder guns – shotguns, rifles, and handguns – and air-power guns that shoot BBs and pellets.
The researchers found that 29% of the unintentional firearm injuries occurred with powder guns and 71% with air-power weapons, often BB guns.
“It is important that all firearms, powdered and air-powered, be stored safely in a lock box or safe,” Dr. Guzman said in a statement. To that end, Cook Children’s has developed a program called Aim for Safety to teach children and parents about the dangers of unsupervised play with BB guns and pellet guns, as well as the importance of storing all firearms unloaded and in a locked safe.
A version of this article first appeared on Medscape.com.
according to the American Academy of Pediatrics.
Preventing firearm-related injuries and deaths in children and youth “demands a public safety approach like regulation of motor vehicles,” the group said.
The organization on Oct. 8 released an updated policy statement and technical report about gun violence and children at its 2022 annual meeting in Anaheim, Calif. The reports were published in the journal Pediatrics, and the authors plan to discuss them during the conference.
“Each day, 28 U.S. children and teens – the equivalent of a high school classroom – die from gun violence, making it the No. 1 killer of youth through age 24,” the AAP said in a statement about the reports. “The national death rate is significantly higher than all other high-income countries combined, largely due to an alarming increase in suicides and homicides that do not make national headlines.”
Firearms have become the leading cause of death among children in the United States.
In 2020, guns caused 10,197 deaths of Americans younger than 24, according to the Society for Adolescent Health and Medicine.
In 2015, more than 7,200 American youth were killed by firearms. That same year in 28 other high-income countries – which combined would have had a population twice that of the United States – just 685 youth were killed by firearms, according to the AAP.
Separately at the AAP conference, physicians are presenting new research about gun violence and children. And on Oct. 10, a pediatrician who was at Uvalde Memorial Hospital in Texas after the deadly school shooting in May is scheduled to address attendees. The doctor, Roy Guerrero, MD, testified on Capitol Hill to advocate for gun control after the shooting at Robb Elementary School, which killed 19 children and two adults.
“This is not a simple problem, and it cannot be fixed with a simple solution,” Lois K. Lee, MD, MPH, said in the AAP news release. Dr. Lee chairs the AAP Council on Injury, Violence, and Poison Prevention that wrote the new reports. “Pediatricians as a start can offer families guidance and education on more safely storing guns. AAP also calls for supporting legislation that, much like the common-sense requirements for obtaining a driver’s license, would improve gun ownership safety.”
Many deaths occur at home
The rate of homicide from firearms in U.S. youth, especially those aged 15-24 years, increased by 14% during the past decade, and the rate of suicide from firearms increased by 39%, according to the AAP.
Homicides account for 58% of youth firearm deaths, whereas suicides account for 37%. Another 2% of youth firearm deaths are unintentional, and 1% result from law enforcement actions, the group said.
Among children 12 years old and younger, about 85% of firearm deaths occur at home. Teen firearm deaths are about as likely to occur at home (39%) as on the street or sidewalk (38%), according to research based on 2014 data.
“School shootings represent a relatively new phenomenon over the last half-century, and the United States has the highest rate of school shootings in the world,” the AAP technical report noted. Between 1966 and 2008, according to the group, 44 such shootings occurred in the United States, or an average of about one per year. Fast forward a few years and the violence became dramatically worse: Between 2013 and 2015, officials counted 154 school shootings – or about one per week.
Still, school shootings are responsible for less than 1% of all firearm deaths among children 17 years or younger in the United States. While school shootings “receive a tremendous amount of attention,” the report stated, other child firearm deaths may be less likely to make national headlines.
“Many firearm tragedies escape public attention because they occur in a home, sometimes in a child’s own home or at a friend’s house, or their neighbor’s or grandparent’s residence,” Eric W. Fleegler, MD, MPH, Boston Children’s Hospital, a co-author of the new reports, said in a statement from AAP. “Research tells us that families tend to underestimate how children will behave when they encounter a gun and miscalculate the risks. Suicide risks are also a huge concern, especially in families where teens are struggling with their mental health.”
AAP-recommended actions include:
- Mental health screenings and safe gun storage education provided by clinicians as part of routine patient visits
- Increased funding for violence intervention programs in hospital and community settings
- Regulation of firearms like other consumer products, with national requirements that address training, licensing, insurance coverage, registration of individuals purchasing firearms, and safe storage
- The use of technology that allows only authorized users to pull the trigger
- Universal background checks that use federal databases and information from local police before all gun purchases
- Extreme risk protection order laws, or “red flag laws,” that prohibit individuals at risk for harming themselves or others from purchasing or owning a firearm
- More funding for firearm injury and prevention research.
A noticeable increase in the ED
Irma Ugalde, MD, associate professor and director of pediatric emergency medicine research at McGovern Medical School at UTHealth Houston, noticed that firearm-related injuries in children at her hospital were more common during the COVID-19 pandemic, even as pediatric emergency department visits decreased overall.
She and her colleagues studied the trends and reported their findings at the AAP meeting.
“We saw a drop in pediatric admissions overall,” Dr. Ugalde said in a statement about the study. “But what was really noticeable was that trauma was still very prevalent – in fact probably more so – and we were seeing more firearm injuries.”
The researchers found that firearm injuries in children rose from 88 cases in 2019 to 118 in 2020. The number of incidents remained elevated in 2021, with 115 cases.
In addition, the researchers found an initial increase in injuries occurring at home where the shooter was a known family member or friend, and in cases involving firearms that were not properly stored.
By comparison, pediatric ED visits overall decreased by 34.2% from 2019 to 2020, and by 11.8% from 2019 to 2021.
The increase in firearm injuries coincided with an increase in gun sales in the United States, the researchers noted.
“National and statewide initiatives to mitigate the risk of firearm-related injury and death are necessary,” Dr. Ugalde’s group said. “We recommend that health care workers remain vigilant about screening for potential risk factors and safe storage of firearms.”
Accidental injuries
Daniel D. Guzman, MD, with Cook Children’s Health Care Center, Fort Worth, Tex., conducted a study focused on unintentional firearm injuries in children. Dr. Guzman’s group analyzed data from 204 patients younger than age 19 seen at Cook Children’s from January 2015 to June 2021.
Dr. Guzman and his colleagues examined outcomes for injuries caused by powder guns – shotguns, rifles, and handguns – and air-power guns that shoot BBs and pellets.
The researchers found that 29% of the unintentional firearm injuries occurred with powder guns and 71% with air-power weapons, often BB guns.
“It is important that all firearms, powdered and air-powered, be stored safely in a lock box or safe,” Dr. Guzman said in a statement. To that end, Cook Children’s has developed a program called Aim for Safety to teach children and parents about the dangers of unsupervised play with BB guns and pellet guns, as well as the importance of storing all firearms unloaded and in a locked safe.
A version of this article first appeared on Medscape.com.
according to the American Academy of Pediatrics.
Preventing firearm-related injuries and deaths in children and youth “demands a public safety approach like regulation of motor vehicles,” the group said.
The organization on Oct. 8 released an updated policy statement and technical report about gun violence and children at its 2022 annual meeting in Anaheim, Calif. The reports were published in the journal Pediatrics, and the authors plan to discuss them during the conference.
“Each day, 28 U.S. children and teens – the equivalent of a high school classroom – die from gun violence, making it the No. 1 killer of youth through age 24,” the AAP said in a statement about the reports. “The national death rate is significantly higher than all other high-income countries combined, largely due to an alarming increase in suicides and homicides that do not make national headlines.”
Firearms have become the leading cause of death among children in the United States.
In 2020, guns caused 10,197 deaths of Americans younger than 24, according to the Society for Adolescent Health and Medicine.
In 2015, more than 7,200 American youth were killed by firearms. That same year in 28 other high-income countries – which combined would have had a population twice that of the United States – just 685 youth were killed by firearms, according to the AAP.
Separately at the AAP conference, physicians are presenting new research about gun violence and children. And on Oct. 10, a pediatrician who was at Uvalde Memorial Hospital in Texas after the deadly school shooting in May is scheduled to address attendees. The doctor, Roy Guerrero, MD, testified on Capitol Hill to advocate for gun control after the shooting at Robb Elementary School, which killed 19 children and two adults.
“This is not a simple problem, and it cannot be fixed with a simple solution,” Lois K. Lee, MD, MPH, said in the AAP news release. Dr. Lee chairs the AAP Council on Injury, Violence, and Poison Prevention that wrote the new reports. “Pediatricians as a start can offer families guidance and education on more safely storing guns. AAP also calls for supporting legislation that, much like the common-sense requirements for obtaining a driver’s license, would improve gun ownership safety.”
Many deaths occur at home
The rate of homicide from firearms in U.S. youth, especially those aged 15-24 years, increased by 14% during the past decade, and the rate of suicide from firearms increased by 39%, according to the AAP.
Homicides account for 58% of youth firearm deaths, whereas suicides account for 37%. Another 2% of youth firearm deaths are unintentional, and 1% result from law enforcement actions, the group said.
Among children 12 years old and younger, about 85% of firearm deaths occur at home. Teen firearm deaths are about as likely to occur at home (39%) as on the street or sidewalk (38%), according to research based on 2014 data.
“School shootings represent a relatively new phenomenon over the last half-century, and the United States has the highest rate of school shootings in the world,” the AAP technical report noted. Between 1966 and 2008, according to the group, 44 such shootings occurred in the United States, or an average of about one per year. Fast forward a few years and the violence became dramatically worse: Between 2013 and 2015, officials counted 154 school shootings – or about one per week.
Still, school shootings are responsible for less than 1% of all firearm deaths among children 17 years or younger in the United States. While school shootings “receive a tremendous amount of attention,” the report stated, other child firearm deaths may be less likely to make national headlines.
“Many firearm tragedies escape public attention because they occur in a home, sometimes in a child’s own home or at a friend’s house, or their neighbor’s or grandparent’s residence,” Eric W. Fleegler, MD, MPH, Boston Children’s Hospital, a co-author of the new reports, said in a statement from AAP. “Research tells us that families tend to underestimate how children will behave when they encounter a gun and miscalculate the risks. Suicide risks are also a huge concern, especially in families where teens are struggling with their mental health.”
AAP-recommended actions include:
- Mental health screenings and safe gun storage education provided by clinicians as part of routine patient visits
- Increased funding for violence intervention programs in hospital and community settings
- Regulation of firearms like other consumer products, with national requirements that address training, licensing, insurance coverage, registration of individuals purchasing firearms, and safe storage
- The use of technology that allows only authorized users to pull the trigger
- Universal background checks that use federal databases and information from local police before all gun purchases
- Extreme risk protection order laws, or “red flag laws,” that prohibit individuals at risk for harming themselves or others from purchasing or owning a firearm
- More funding for firearm injury and prevention research.
A noticeable increase in the ED
Irma Ugalde, MD, associate professor and director of pediatric emergency medicine research at McGovern Medical School at UTHealth Houston, noticed that firearm-related injuries in children at her hospital were more common during the COVID-19 pandemic, even as pediatric emergency department visits decreased overall.
She and her colleagues studied the trends and reported their findings at the AAP meeting.
“We saw a drop in pediatric admissions overall,” Dr. Ugalde said in a statement about the study. “But what was really noticeable was that trauma was still very prevalent – in fact probably more so – and we were seeing more firearm injuries.”
The researchers found that firearm injuries in children rose from 88 cases in 2019 to 118 in 2020. The number of incidents remained elevated in 2021, with 115 cases.
In addition, the researchers found an initial increase in injuries occurring at home where the shooter was a known family member or friend, and in cases involving firearms that were not properly stored.
By comparison, pediatric ED visits overall decreased by 34.2% from 2019 to 2020, and by 11.8% from 2019 to 2021.
The increase in firearm injuries coincided with an increase in gun sales in the United States, the researchers noted.
“National and statewide initiatives to mitigate the risk of firearm-related injury and death are necessary,” Dr. Ugalde’s group said. “We recommend that health care workers remain vigilant about screening for potential risk factors and safe storage of firearms.”
Accidental injuries
Daniel D. Guzman, MD, with Cook Children’s Health Care Center, Fort Worth, Tex., conducted a study focused on unintentional firearm injuries in children. Dr. Guzman’s group analyzed data from 204 patients younger than age 19 seen at Cook Children’s from January 2015 to June 2021.
Dr. Guzman and his colleagues examined outcomes for injuries caused by powder guns – shotguns, rifles, and handguns – and air-power guns that shoot BBs and pellets.
The researchers found that 29% of the unintentional firearm injuries occurred with powder guns and 71% with air-power weapons, often BB guns.
“It is important that all firearms, powdered and air-powered, be stored safely in a lock box or safe,” Dr. Guzman said in a statement. To that end, Cook Children’s has developed a program called Aim for Safety to teach children and parents about the dangers of unsupervised play with BB guns and pellet guns, as well as the importance of storing all firearms unloaded and in a locked safe.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
Gut microbiota disruption a driver of aggression in schizophrenia?
However, at least one expert expressed concerns over the study’s conclusions.
Results from a study of 50 inpatients with schizophrenia showed significantly higher pro-inflammation, pro-oxidation, and leaky gut biomarkers in those with aggression vs. their peers who did not display aggression.
In addition, those with aggression showed less alpha diversity and evenness of the fecal bacterial community, lower levels of several beneficial gut bacteria, and higher levels of the fecal genera Prevotella.
Six short-chain fatty acids (SCFAs) and six neurotransmitters were also lower in the aggression vs. no-aggression groups.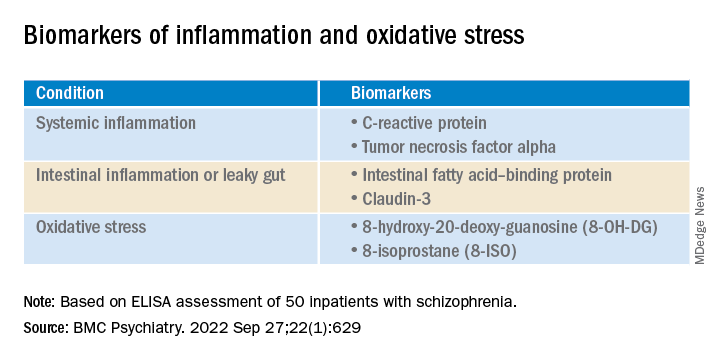
“The present study was the first to compare the state of inflammation, oxidation, intestinal microbiota, and metabolites” in inpatients with schizophrenia and aggression, compared with those who did not show aggression, write the investigators, led by Hongxin Deng, department of psychiatry, Zhumadian (China) Psychiatric Hospital.
“Results indicate pro-inflammation, pro-oxidation and leaky gut phenotypes relating to enteric dysbacteriosis and microbial SCFAs feature the aggression in [individuals with schizophrenia], which provides clues for future microbial-based or anti-inflammatory/oxidative therapies on aggression,” they add.
The findings were published online in BMC Psychiatry.
Unknown pathogenesis
Although emerging evidence suggests that schizophrenia “may augment the propensity for aggression incidence about fourfold to sevenfold,” the pathogenesis of aggression “remains largely unknown,” the investigators note.
The same researchers previously found an association between the systemic pro-inflammation response and the onset or severity of aggression in schizophrenia, “possibly caused by leaky gut-induced bacterial translocation.”
The researchers suggest that peripheral cytokines “could cross the blood-brain barrier, thus precipitating changes in mood and behavior through hypothalamic-pituitary-adrenal axis.”
However, they note that the pro-inflammation phenotype is “often a synergistic effect of multiple causes.” Of these, chronic pro-oxidative stress has been shown to contribute to aggression onset in intermittent explosive disorder, but this association has rarely been confirmed in patients with schizophrenia.
In addition, increasing evidence points to enteric dysbacteriosis and dysbiosis of intestinal flora metabolites, including SCFAs or neurotransmitters, as potentially “integral parts of psychiatric disorders’ pathophysiology” by changing the state of both oxidative stress and inflammation.
The investigators hypothesized that the systemic pro-inflammation phenotype in aggression-affected schizophrenia cases “involves alterations to gut microbiota and its metabolites, leaky gut, and oxidative stress.” However, the profiles of these variables and their interrelationships have been “poorly investigated” in inpatients with schizophrenia and aggression.
To fill this gap, they assessed adult psychiatric inpatients with schizophrenia and aggressive behaviors and inpatients with schizophrenia but no aggressive behavior within 1 week before admission (n = 25 per group; mean age, 33.52 years, and 32.88 years, respectively; 68% and 64% women, respectively).
They collected stool samples from each patient and used enzyme-linked immunoassay (ELISA) to detect fecal calprotectin protein, an indicator of intestinal inflammation. They also collected fasting peripheral blood samples, using ELISA to detect several biomarkers.
The researchers also used the Modified Overt Aggression Scale (MOAS) to characterize aggressive behaviors and the Positive and Negative Syndrome Scale to characterize psychiatric symptoms.
‘Vital role’
Significantly higher biomarkers for systemic pro-inflammation, pro-oxidation and leaky gut were found in the aggression vs the no-aggression group (all P < .05).
After controlling for potential confounders, the researchers also found positive associations between MOAS scores and biomarkers, both serum and fecal.
There were also positive associations between serum 8-hydroxy-20-deoxy-guanosine (8-OH-DG) or 8-isoprostane (8-ISO) and systemic inflammatory biomarkers (all R > 0; P < .05).
In addition, the alpha diversity and evenness of the fecal bacterial community were lower in the aggression vs. no aggression groups.
When the researchers compared the relative abundance of the top 15 genera composition of intestinal microflora in the two groups, Bacteroides, Faecalibacterium, Blautia, Bifidobacterium, Collinsella, and Eubacterium coprostanoligenes were “remarkably reduced” in the group with aggression, whereas the abundance of fecal genera Prevotella was significantly increased (all corrected P < .001).
In the patients who had schizophrenia with aggression, levels of six SCFAs and six neurotransmitters were much lower than in the patients with schizophrenia but no aggression (all P < .05).
Inpatients with schizophrenia and aggression “had dramatically increased serum level of 8-OH-DG (nucleic acid oxidation biomarker) and 8-ISO (lipid oxidation biomarker) than those without, and further correlation analysis also showed positive correlativity between pro-oxidation and systemic pro-inflammation response or aggression severity,” the investigators write.
The findings “collectively suggest the cocontributory role of systemic pro-inflammation and pro-oxidation in the development of aggression” in schizophrenia, they add. “Gut dysbacteriosis with leaky gut seems to play a vital role in the pathophysiology.”
Correlation vs. causality
Commenting for this article, Emeran Mayer, MD, distinguished research professor of medicine at the G. Oppenheimer Center for Neurobiology of Stress and Resilience and UCLA Brain Gut Microbiome Center, Los Angeles, said that “at first glance, it is interesting that the behavioral trait of aggression but not the diagnosis of schizophrenia showed the differences in markers of systemic inflammation, increased gut permeability, and microbiome parameters.”
However, like many such descriptive studies, the research is flawed by comparing two patient groups and concluding causality between the biomarkers and the behavior traits, added Dr. Mayer, who was not involved with the study.
The study’s shortcomings include its small sample size as well as several confounding factors – particularly diet, sleep, exercise, and stress and anxiety levels – that were not considered, he said. The study also lacked a control group with high levels of aggression but without schizophrenia.
“The observed changes in intestinal permeability, unscientifically referred to as ‘leaky gut,’ as well as the gut microbiome differences, could be secondary to chronically increased sympathetic nervous system activation in the high aggression group,” Dr. Mayer said. “This is an interesting hypothesis which should be discussed and should have been addressed in this study.”
The differences in gut microbial composition and SCFA production “could be secondary to differences in plant-based diet components,” Dr. Mayer speculated, wondering how well dietary intake was controlled.
“Overall, it is an interesting descriptive study, which unfortunately does not contribute significantly to a better understanding of the role of the brain-gut microbiome system in schizophrenic patients,” he said.
The study was funded by a grant from China Postdoctoral Science Foundation. The investigators have reported no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com.
However, at least one expert expressed concerns over the study’s conclusions.
Results from a study of 50 inpatients with schizophrenia showed significantly higher pro-inflammation, pro-oxidation, and leaky gut biomarkers in those with aggression vs. their peers who did not display aggression.
In addition, those with aggression showed less alpha diversity and evenness of the fecal bacterial community, lower levels of several beneficial gut bacteria, and higher levels of the fecal genera Prevotella.
Six short-chain fatty acids (SCFAs) and six neurotransmitters were also lower in the aggression vs. no-aggression groups.
“The present study was the first to compare the state of inflammation, oxidation, intestinal microbiota, and metabolites” in inpatients with schizophrenia and aggression, compared with those who did not show aggression, write the investigators, led by Hongxin Deng, department of psychiatry, Zhumadian (China) Psychiatric Hospital.
“Results indicate pro-inflammation, pro-oxidation and leaky gut phenotypes relating to enteric dysbacteriosis and microbial SCFAs feature the aggression in [individuals with schizophrenia], which provides clues for future microbial-based or anti-inflammatory/oxidative therapies on aggression,” they add.
The findings were published online in BMC Psychiatry.
Unknown pathogenesis
Although emerging evidence suggests that schizophrenia “may augment the propensity for aggression incidence about fourfold to sevenfold,” the pathogenesis of aggression “remains largely unknown,” the investigators note.
The same researchers previously found an association between the systemic pro-inflammation response and the onset or severity of aggression in schizophrenia, “possibly caused by leaky gut-induced bacterial translocation.”
The researchers suggest that peripheral cytokines “could cross the blood-brain barrier, thus precipitating changes in mood and behavior through hypothalamic-pituitary-adrenal axis.”
However, they note that the pro-inflammation phenotype is “often a synergistic effect of multiple causes.” Of these, chronic pro-oxidative stress has been shown to contribute to aggression onset in intermittent explosive disorder, but this association has rarely been confirmed in patients with schizophrenia.
In addition, increasing evidence points to enteric dysbacteriosis and dysbiosis of intestinal flora metabolites, including SCFAs or neurotransmitters, as potentially “integral parts of psychiatric disorders’ pathophysiology” by changing the state of both oxidative stress and inflammation.
The investigators hypothesized that the systemic pro-inflammation phenotype in aggression-affected schizophrenia cases “involves alterations to gut microbiota and its metabolites, leaky gut, and oxidative stress.” However, the profiles of these variables and their interrelationships have been “poorly investigated” in inpatients with schizophrenia and aggression.
To fill this gap, they assessed adult psychiatric inpatients with schizophrenia and aggressive behaviors and inpatients with schizophrenia but no aggressive behavior within 1 week before admission (n = 25 per group; mean age, 33.52 years, and 32.88 years, respectively; 68% and 64% women, respectively).
They collected stool samples from each patient and used enzyme-linked immunoassay (ELISA) to detect fecal calprotectin protein, an indicator of intestinal inflammation. They also collected fasting peripheral blood samples, using ELISA to detect several biomarkers.
The researchers also used the Modified Overt Aggression Scale (MOAS) to characterize aggressive behaviors and the Positive and Negative Syndrome Scale to characterize psychiatric symptoms.
‘Vital role’
Significantly higher biomarkers for systemic pro-inflammation, pro-oxidation and leaky gut were found in the aggression vs the no-aggression group (all P < .05).
After controlling for potential confounders, the researchers also found positive associations between MOAS scores and biomarkers, both serum and fecal.
There were also positive associations between serum 8-hydroxy-20-deoxy-guanosine (8-OH-DG) or 8-isoprostane (8-ISO) and systemic inflammatory biomarkers (all R > 0; P < .05).
In addition, the alpha diversity and evenness of the fecal bacterial community were lower in the aggression vs. no aggression groups.
When the researchers compared the relative abundance of the top 15 genera composition of intestinal microflora in the two groups, Bacteroides, Faecalibacterium, Blautia, Bifidobacterium, Collinsella, and Eubacterium coprostanoligenes were “remarkably reduced” in the group with aggression, whereas the abundance of fecal genera Prevotella was significantly increased (all corrected P < .001).
In the patients who had schizophrenia with aggression, levels of six SCFAs and six neurotransmitters were much lower than in the patients with schizophrenia but no aggression (all P < .05).
Inpatients with schizophrenia and aggression “had dramatically increased serum level of 8-OH-DG (nucleic acid oxidation biomarker) and 8-ISO (lipid oxidation biomarker) than those without, and further correlation analysis also showed positive correlativity between pro-oxidation and systemic pro-inflammation response or aggression severity,” the investigators write.
The findings “collectively suggest the cocontributory role of systemic pro-inflammation and pro-oxidation in the development of aggression” in schizophrenia, they add. “Gut dysbacteriosis with leaky gut seems to play a vital role in the pathophysiology.”
Correlation vs. causality
Commenting for this article, Emeran Mayer, MD, distinguished research professor of medicine at the G. Oppenheimer Center for Neurobiology of Stress and Resilience and UCLA Brain Gut Microbiome Center, Los Angeles, said that “at first glance, it is interesting that the behavioral trait of aggression but not the diagnosis of schizophrenia showed the differences in markers of systemic inflammation, increased gut permeability, and microbiome parameters.”
However, like many such descriptive studies, the research is flawed by comparing two patient groups and concluding causality between the biomarkers and the behavior traits, added Dr. Mayer, who was not involved with the study.
The study’s shortcomings include its small sample size as well as several confounding factors – particularly diet, sleep, exercise, and stress and anxiety levels – that were not considered, he said. The study also lacked a control group with high levels of aggression but without schizophrenia.
“The observed changes in intestinal permeability, unscientifically referred to as ‘leaky gut,’ as well as the gut microbiome differences, could be secondary to chronically increased sympathetic nervous system activation in the high aggression group,” Dr. Mayer said. “This is an interesting hypothesis which should be discussed and should have been addressed in this study.”
The differences in gut microbial composition and SCFA production “could be secondary to differences in plant-based diet components,” Dr. Mayer speculated, wondering how well dietary intake was controlled.
“Overall, it is an interesting descriptive study, which unfortunately does not contribute significantly to a better understanding of the role of the brain-gut microbiome system in schizophrenic patients,” he said.
The study was funded by a grant from China Postdoctoral Science Foundation. The investigators have reported no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com.
However, at least one expert expressed concerns over the study’s conclusions.
Results from a study of 50 inpatients with schizophrenia showed significantly higher pro-inflammation, pro-oxidation, and leaky gut biomarkers in those with aggression vs. their peers who did not display aggression.
In addition, those with aggression showed less alpha diversity and evenness of the fecal bacterial community, lower levels of several beneficial gut bacteria, and higher levels of the fecal genera Prevotella.
Six short-chain fatty acids (SCFAs) and six neurotransmitters were also lower in the aggression vs. no-aggression groups.
“The present study was the first to compare the state of inflammation, oxidation, intestinal microbiota, and metabolites” in inpatients with schizophrenia and aggression, compared with those who did not show aggression, write the investigators, led by Hongxin Deng, department of psychiatry, Zhumadian (China) Psychiatric Hospital.
“Results indicate pro-inflammation, pro-oxidation and leaky gut phenotypes relating to enteric dysbacteriosis and microbial SCFAs feature the aggression in [individuals with schizophrenia], which provides clues for future microbial-based or anti-inflammatory/oxidative therapies on aggression,” they add.
The findings were published online in BMC Psychiatry.
Unknown pathogenesis
Although emerging evidence suggests that schizophrenia “may augment the propensity for aggression incidence about fourfold to sevenfold,” the pathogenesis of aggression “remains largely unknown,” the investigators note.
The same researchers previously found an association between the systemic pro-inflammation response and the onset or severity of aggression in schizophrenia, “possibly caused by leaky gut-induced bacterial translocation.”
The researchers suggest that peripheral cytokines “could cross the blood-brain barrier, thus precipitating changes in mood and behavior through hypothalamic-pituitary-adrenal axis.”
However, they note that the pro-inflammation phenotype is “often a synergistic effect of multiple causes.” Of these, chronic pro-oxidative stress has been shown to contribute to aggression onset in intermittent explosive disorder, but this association has rarely been confirmed in patients with schizophrenia.
In addition, increasing evidence points to enteric dysbacteriosis and dysbiosis of intestinal flora metabolites, including SCFAs or neurotransmitters, as potentially “integral parts of psychiatric disorders’ pathophysiology” by changing the state of both oxidative stress and inflammation.
The investigators hypothesized that the systemic pro-inflammation phenotype in aggression-affected schizophrenia cases “involves alterations to gut microbiota and its metabolites, leaky gut, and oxidative stress.” However, the profiles of these variables and their interrelationships have been “poorly investigated” in inpatients with schizophrenia and aggression.
To fill this gap, they assessed adult psychiatric inpatients with schizophrenia and aggressive behaviors and inpatients with schizophrenia but no aggressive behavior within 1 week before admission (n = 25 per group; mean age, 33.52 years, and 32.88 years, respectively; 68% and 64% women, respectively).
They collected stool samples from each patient and used enzyme-linked immunoassay (ELISA) to detect fecal calprotectin protein, an indicator of intestinal inflammation. They also collected fasting peripheral blood samples, using ELISA to detect several biomarkers.
The researchers also used the Modified Overt Aggression Scale (MOAS) to characterize aggressive behaviors and the Positive and Negative Syndrome Scale to characterize psychiatric symptoms.
‘Vital role’
Significantly higher biomarkers for systemic pro-inflammation, pro-oxidation and leaky gut were found in the aggression vs the no-aggression group (all P < .05).
After controlling for potential confounders, the researchers also found positive associations between MOAS scores and biomarkers, both serum and fecal.
There were also positive associations between serum 8-hydroxy-20-deoxy-guanosine (8-OH-DG) or 8-isoprostane (8-ISO) and systemic inflammatory biomarkers (all R > 0; P < .05).
In addition, the alpha diversity and evenness of the fecal bacterial community were lower in the aggression vs. no aggression groups.
When the researchers compared the relative abundance of the top 15 genera composition of intestinal microflora in the two groups, Bacteroides, Faecalibacterium, Blautia, Bifidobacterium, Collinsella, and Eubacterium coprostanoligenes were “remarkably reduced” in the group with aggression, whereas the abundance of fecal genera Prevotella was significantly increased (all corrected P < .001).
In the patients who had schizophrenia with aggression, levels of six SCFAs and six neurotransmitters were much lower than in the patients with schizophrenia but no aggression (all P < .05).
Inpatients with schizophrenia and aggression “had dramatically increased serum level of 8-OH-DG (nucleic acid oxidation biomarker) and 8-ISO (lipid oxidation biomarker) than those without, and further correlation analysis also showed positive correlativity between pro-oxidation and systemic pro-inflammation response or aggression severity,” the investigators write.
The findings “collectively suggest the cocontributory role of systemic pro-inflammation and pro-oxidation in the development of aggression” in schizophrenia, they add. “Gut dysbacteriosis with leaky gut seems to play a vital role in the pathophysiology.”
Correlation vs. causality
Commenting for this article, Emeran Mayer, MD, distinguished research professor of medicine at the G. Oppenheimer Center for Neurobiology of Stress and Resilience and UCLA Brain Gut Microbiome Center, Los Angeles, said that “at first glance, it is interesting that the behavioral trait of aggression but not the diagnosis of schizophrenia showed the differences in markers of systemic inflammation, increased gut permeability, and microbiome parameters.”
However, like many such descriptive studies, the research is flawed by comparing two patient groups and concluding causality between the biomarkers and the behavior traits, added Dr. Mayer, who was not involved with the study.
The study’s shortcomings include its small sample size as well as several confounding factors – particularly diet, sleep, exercise, and stress and anxiety levels – that were not considered, he said. The study also lacked a control group with high levels of aggression but without schizophrenia.
“The observed changes in intestinal permeability, unscientifically referred to as ‘leaky gut,’ as well as the gut microbiome differences, could be secondary to chronically increased sympathetic nervous system activation in the high aggression group,” Dr. Mayer said. “This is an interesting hypothesis which should be discussed and should have been addressed in this study.”
The differences in gut microbial composition and SCFA production “could be secondary to differences in plant-based diet components,” Dr. Mayer speculated, wondering how well dietary intake was controlled.
“Overall, it is an interesting descriptive study, which unfortunately does not contribute significantly to a better understanding of the role of the brain-gut microbiome system in schizophrenic patients,” he said.
The study was funded by a grant from China Postdoctoral Science Foundation. The investigators have reported no relevant financial relationships. Dr. Mayer is a scientific advisory board member of Danone, Axial Therapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
A version of this article first appeared on Medscape.com.
FROM BMC PSYCHIATRY
New ICD-10-CM codes a ‘big switch-over’ for neurocognitive disorders
Revised ICD-10-CM codes for neurocognitive disorders are now in effect, the American Psychiatric Association has announced
The coding changes for major and mild neurocognitive disorders represent “the most consequential” coding changes for DSM-5 disorders since the Oct. 1, 2015, changeover from ICD-9-CM to ICD-10-CM,” Michael First, MD, professor of clinical psychiatry at Columbia University, in New York, wrote in a statement published in Psychiatric News.
The updated codes for neurocognitive disorders are “much more specific and indicate all the different types of behavioral problems that could occur with dementia,” First, who served as editor of the DSM-5-TR, added in an interview.
This year, coding changes that affect psychiatry are largely confined to major and mild neurocognitive disorders, but they represent “a big switch-over,” Dr. First said.
What’s new
The first three characters that make up the ICD-10-CM code for major neurocognitive disorder depend on the type of etiologic medical condition and are unchanged:
- F01 for major neurocognitive disorder caused by vascular disease
- F02 for major neurocognitive disorder caused by other medical conditions in which the specific etiologic medical condition is indicated by also listing the ICD-10-CM code for the medical condition
- F03 for major neurocognitive disorder when the medical etiology is unknown
However, DSM-5-TR diagnostic criteria for major neurocognitive disorder include severity specifiers (mild, moderate, severe), but there is no provision for indicating this “clinically important” information in the current ICD-10-CM code for major neurocognitive disorder, Dr. First explained.
The 2022 coding changes for major neurocognitive disorder include the provision of a fourth character code to indicate the severity of the major neurocognitive disorder – “A” indicates mild (difficulties with instrumental activities of daily living, such as housework and managing money); “B,” moderate (difficulties with basic activities of daily living, such as feeding and dressing); and “C,” severe (fully dependent) impairment.
The coding changes for major neurocognitive disorder also now include fifth and sixth characters to indicate the presence of an accompanying behavioral or psychological disturbance, such as agitation, psychotic disturbance, mood symptoms, and anxiety.
The update, which went into effect Oct. 1, also adds to ICD-10-CM two new mental disorder codes, F06.71 and F06.70 for mild neurocognitive disorder caused by a medical condition with or without a behavioral disturbance, respectively.
The coding changes affecting psychiatry are outlined in the APA’s 2022 DSM-5-TR Update: Supplement to the Diagnostic and Statistical Manual of Mental Disorders and DSM-5-TR Neurocognitive Disorders Supplement.
Annual event
Every Oct. 1, ICD-10-CM codes for all of medicine are updated, with new codes being added and others revised or deleted. Only a small fraction of the 68,000 codes is affected. Last year, 159 new codes were added, 25 codes were deleted, and 27 existing codes were revised.
All HIPAA-compliant health care entities are required to use the most up-to-date ICD-10-CM codes.
“I think there’s a grace period where you can still use the old codes, but there will be a point where if you use the old code, it’ll get rejected because it won’t be considered a valid code,” said Dr. First.
A version of this article first appeared on Medscape.com.
Revised ICD-10-CM codes for neurocognitive disorders are now in effect, the American Psychiatric Association has announced
The coding changes for major and mild neurocognitive disorders represent “the most consequential” coding changes for DSM-5 disorders since the Oct. 1, 2015, changeover from ICD-9-CM to ICD-10-CM,” Michael First, MD, professor of clinical psychiatry at Columbia University, in New York, wrote in a statement published in Psychiatric News.
The updated codes for neurocognitive disorders are “much more specific and indicate all the different types of behavioral problems that could occur with dementia,” First, who served as editor of the DSM-5-TR, added in an interview.
This year, coding changes that affect psychiatry are largely confined to major and mild neurocognitive disorders, but they represent “a big switch-over,” Dr. First said.
What’s new
The first three characters that make up the ICD-10-CM code for major neurocognitive disorder depend on the type of etiologic medical condition and are unchanged:
- F01 for major neurocognitive disorder caused by vascular disease
- F02 for major neurocognitive disorder caused by other medical conditions in which the specific etiologic medical condition is indicated by also listing the ICD-10-CM code for the medical condition
- F03 for major neurocognitive disorder when the medical etiology is unknown
However, DSM-5-TR diagnostic criteria for major neurocognitive disorder include severity specifiers (mild, moderate, severe), but there is no provision for indicating this “clinically important” information in the current ICD-10-CM code for major neurocognitive disorder, Dr. First explained.
The 2022 coding changes for major neurocognitive disorder include the provision of a fourth character code to indicate the severity of the major neurocognitive disorder – “A” indicates mild (difficulties with instrumental activities of daily living, such as housework and managing money); “B,” moderate (difficulties with basic activities of daily living, such as feeding and dressing); and “C,” severe (fully dependent) impairment.
The coding changes for major neurocognitive disorder also now include fifth and sixth characters to indicate the presence of an accompanying behavioral or psychological disturbance, such as agitation, psychotic disturbance, mood symptoms, and anxiety.
The update, which went into effect Oct. 1, also adds to ICD-10-CM two new mental disorder codes, F06.71 and F06.70 for mild neurocognitive disorder caused by a medical condition with or without a behavioral disturbance, respectively.
The coding changes affecting psychiatry are outlined in the APA’s 2022 DSM-5-TR Update: Supplement to the Diagnostic and Statistical Manual of Mental Disorders and DSM-5-TR Neurocognitive Disorders Supplement.
Annual event
Every Oct. 1, ICD-10-CM codes for all of medicine are updated, with new codes being added and others revised or deleted. Only a small fraction of the 68,000 codes is affected. Last year, 159 new codes were added, 25 codes were deleted, and 27 existing codes were revised.
All HIPAA-compliant health care entities are required to use the most up-to-date ICD-10-CM codes.
“I think there’s a grace period where you can still use the old codes, but there will be a point where if you use the old code, it’ll get rejected because it won’t be considered a valid code,” said Dr. First.
A version of this article first appeared on Medscape.com.
Revised ICD-10-CM codes for neurocognitive disorders are now in effect, the American Psychiatric Association has announced
The coding changes for major and mild neurocognitive disorders represent “the most consequential” coding changes for DSM-5 disorders since the Oct. 1, 2015, changeover from ICD-9-CM to ICD-10-CM,” Michael First, MD, professor of clinical psychiatry at Columbia University, in New York, wrote in a statement published in Psychiatric News.
The updated codes for neurocognitive disorders are “much more specific and indicate all the different types of behavioral problems that could occur with dementia,” First, who served as editor of the DSM-5-TR, added in an interview.
This year, coding changes that affect psychiatry are largely confined to major and mild neurocognitive disorders, but they represent “a big switch-over,” Dr. First said.
What’s new
The first three characters that make up the ICD-10-CM code for major neurocognitive disorder depend on the type of etiologic medical condition and are unchanged:
- F01 for major neurocognitive disorder caused by vascular disease
- F02 for major neurocognitive disorder caused by other medical conditions in which the specific etiologic medical condition is indicated by also listing the ICD-10-CM code for the medical condition
- F03 for major neurocognitive disorder when the medical etiology is unknown
However, DSM-5-TR diagnostic criteria for major neurocognitive disorder include severity specifiers (mild, moderate, severe), but there is no provision for indicating this “clinically important” information in the current ICD-10-CM code for major neurocognitive disorder, Dr. First explained.
The 2022 coding changes for major neurocognitive disorder include the provision of a fourth character code to indicate the severity of the major neurocognitive disorder – “A” indicates mild (difficulties with instrumental activities of daily living, such as housework and managing money); “B,” moderate (difficulties with basic activities of daily living, such as feeding and dressing); and “C,” severe (fully dependent) impairment.
The coding changes for major neurocognitive disorder also now include fifth and sixth characters to indicate the presence of an accompanying behavioral or psychological disturbance, such as agitation, psychotic disturbance, mood symptoms, and anxiety.
The update, which went into effect Oct. 1, also adds to ICD-10-CM two new mental disorder codes, F06.71 and F06.70 for mild neurocognitive disorder caused by a medical condition with or without a behavioral disturbance, respectively.
The coding changes affecting psychiatry are outlined in the APA’s 2022 DSM-5-TR Update: Supplement to the Diagnostic and Statistical Manual of Mental Disorders and DSM-5-TR Neurocognitive Disorders Supplement.
Annual event
Every Oct. 1, ICD-10-CM codes for all of medicine are updated, with new codes being added and others revised or deleted. Only a small fraction of the 68,000 codes is affected. Last year, 159 new codes were added, 25 codes were deleted, and 27 existing codes were revised.
All HIPAA-compliant health care entities are required to use the most up-to-date ICD-10-CM codes.
“I think there’s a grace period where you can still use the old codes, but there will be a point where if you use the old code, it’ll get rejected because it won’t be considered a valid code,” said Dr. First.
A version of this article first appeared on Medscape.com.


