User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
FDA approves first over-the-counter birth control pill
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
“It’s a huge win for patients who need better access to contraception,” said Dr. Gray, who is also a spokesperson for the American College of Obstetricians and Gynecologists.
Women who want hormonal birth control but live in areas without convenient access to a doctor, women who cannot easily take time off of work to see a doctor and get a prescription filled, and women without insurance are examples of people who will benefit, she said.
The Catholic Medical Association, in contrast, expressed “deep concern and disappointment” after an FDA advisory committee’s unanimous vote on May 11 recommending the drug be available over the counter. In a statement after the vote, the group cited “extensive medical studies demonstrating the risks and adverse effects of hormonal contraceptives,” adding that “the social impact of [full approval] would be dramatic.”
But doctors largely disagreed.
“It is definitely a huge win for reproductive autonomy. I’m glad that the FDA is prioritizing patient safety and well-being over politics,” said Catherine Cansino, MD, MPH, an ob.gyn. and clinical professor in the University of California Davis department of obstetrics and gynecology. She said the FDA approved the over-the-counter version because the medication is safe.
While opponents like the Catholic Medical Association cite safety concerns and believe doctors should screen all women before prescribing hormonal contraception, Dr. Gray disagreed. “There’s a lot of evidence that patients can figure out if a progestin-only pill is right for them and safe for them. Medical professionals don’t have to be the gatekeepers for contraception,” she said.
Pricing unknown
Whether insurance companies will pay for Opill now that it will be available without a prescription remains unknown. For some medications, paying a copay through insurance can be less expensive than buying at a retail price.
“Although pricing issues will be relevant, the FDA’s decision will enhance women’s access to hormonal birth control,” said Andrew M. Kaunitz, MD, a professor and associate chairman in the department of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville.
The drugmaker, Perrigo, based in Ireland, has not yet announced how much the pill will cost. The price tag could affect how widely available this form of birth control is. The drug has been shown to be as much as 93% effective for pregnancy prevention. Perrigo says it plans to make the pill available at low or no cost to some women.
Caveats to consider
There are some women for whom hormonal contraceptives have always carried greater risks. For example, women who have breast cancer or a history of breast cancer should not use hormonal contraceptives, the FDA said in a news release announcing the approval. Women with other types of cancer should check with their doctors first, the agency noted.
Women who smoke, who take some medications to lower blood pressure, or who have migraines should also take caution, Dr. Cansino said. “People with migraines may not be suitable for over-the-counter oral contraceptives. But a simple screening through a provider can identify whether you are truly eligible or not.”
Irregular bleeding, headaches, dizziness, nausea, increased appetite, belly pain, cramps, or bloating are the most common side effects of Opill, the FDA said.
The Opill is a progestin-only birth control pill. Similar pills have been available in the United Kingdiom for about 2 years, often referred to as “mini pills” because they contain a single hormone. In contrast, prescription birth control pills in the United States and elsewhere contain more than one hormone, estrogen and progestin, to prevent pregnancy.
Prescription pill packs for combination contraception often feature a week of placebo pills without an active ingredient. While skipping a placebo pill might not make a difference in pregnancy prevention, Opill is different. Every pill in the packet will contain medication, Gray said. “So it’s important to take the pill the same time every day for it to be most effective.”
Even though this may mean one less visit to your doctor, Dr. Kaunitz hopes women will stay up to date on their other medical checkups. “One of our challenges as providers of care to women will be to encourage them to continue to receive important services, including cancer screening and vaccinations, even while they can initiate and continue hormonal contraception without contact with a provider.”
Just the beginning?
The American Medical Association hopes this approval signals more to come.
“While we applaud this move, the AMA continues to urge the FDA and HHS to consider a variety of oral contraceptive options for over-the-counter use,” the association, which has more than 250,000 doctor members, said in a statement. “It is important patients have options when choosing which type of birth control works best for them,”
The American College of Obstetricians and Gynecologists said the FDA’s decision will help many women. “We are glad that more patients will now be empowered to choose when and where they obtain a safe method of contraception without having to wait for a medical appointment or for a prescription to be filled,” Verda J. Hicks, MD, the group’s president, and Christopher M. Zahn, MD, interim chief executive officer, said in a statement.
“Allowing individuals to access birth control at their local pharmacy or drug store will eliminate some barriers,” they said.
A version of this article first appeared on WebMD.com.
This article was updated 7/13/23.
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
“It’s a huge win for patients who need better access to contraception,” said Dr. Gray, who is also a spokesperson for the American College of Obstetricians and Gynecologists.
Women who want hormonal birth control but live in areas without convenient access to a doctor, women who cannot easily take time off of work to see a doctor and get a prescription filled, and women without insurance are examples of people who will benefit, she said.
The Catholic Medical Association, in contrast, expressed “deep concern and disappointment” after an FDA advisory committee’s unanimous vote on May 11 recommending the drug be available over the counter. In a statement after the vote, the group cited “extensive medical studies demonstrating the risks and adverse effects of hormonal contraceptives,” adding that “the social impact of [full approval] would be dramatic.”
But doctors largely disagreed.
“It is definitely a huge win for reproductive autonomy. I’m glad that the FDA is prioritizing patient safety and well-being over politics,” said Catherine Cansino, MD, MPH, an ob.gyn. and clinical professor in the University of California Davis department of obstetrics and gynecology. She said the FDA approved the over-the-counter version because the medication is safe.
While opponents like the Catholic Medical Association cite safety concerns and believe doctors should screen all women before prescribing hormonal contraception, Dr. Gray disagreed. “There’s a lot of evidence that patients can figure out if a progestin-only pill is right for them and safe for them. Medical professionals don’t have to be the gatekeepers for contraception,” she said.
Pricing unknown
Whether insurance companies will pay for Opill now that it will be available without a prescription remains unknown. For some medications, paying a copay through insurance can be less expensive than buying at a retail price.
“Although pricing issues will be relevant, the FDA’s decision will enhance women’s access to hormonal birth control,” said Andrew M. Kaunitz, MD, a professor and associate chairman in the department of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville.
The drugmaker, Perrigo, based in Ireland, has not yet announced how much the pill will cost. The price tag could affect how widely available this form of birth control is. The drug has been shown to be as much as 93% effective for pregnancy prevention. Perrigo says it plans to make the pill available at low or no cost to some women.
Caveats to consider
There are some women for whom hormonal contraceptives have always carried greater risks. For example, women who have breast cancer or a history of breast cancer should not use hormonal contraceptives, the FDA said in a news release announcing the approval. Women with other types of cancer should check with their doctors first, the agency noted.
Women who smoke, who take some medications to lower blood pressure, or who have migraines should also take caution, Dr. Cansino said. “People with migraines may not be suitable for over-the-counter oral contraceptives. But a simple screening through a provider can identify whether you are truly eligible or not.”
Irregular bleeding, headaches, dizziness, nausea, increased appetite, belly pain, cramps, or bloating are the most common side effects of Opill, the FDA said.
The Opill is a progestin-only birth control pill. Similar pills have been available in the United Kingdiom for about 2 years, often referred to as “mini pills” because they contain a single hormone. In contrast, prescription birth control pills in the United States and elsewhere contain more than one hormone, estrogen and progestin, to prevent pregnancy.
Prescription pill packs for combination contraception often feature a week of placebo pills without an active ingredient. While skipping a placebo pill might not make a difference in pregnancy prevention, Opill is different. Every pill in the packet will contain medication, Gray said. “So it’s important to take the pill the same time every day for it to be most effective.”
Even though this may mean one less visit to your doctor, Dr. Kaunitz hopes women will stay up to date on their other medical checkups. “One of our challenges as providers of care to women will be to encourage them to continue to receive important services, including cancer screening and vaccinations, even while they can initiate and continue hormonal contraception without contact with a provider.”
Just the beginning?
The American Medical Association hopes this approval signals more to come.
“While we applaud this move, the AMA continues to urge the FDA and HHS to consider a variety of oral contraceptive options for over-the-counter use,” the association, which has more than 250,000 doctor members, said in a statement. “It is important patients have options when choosing which type of birth control works best for them,”
The American College of Obstetricians and Gynecologists said the FDA’s decision will help many women. “We are glad that more patients will now be empowered to choose when and where they obtain a safe method of contraception without having to wait for a medical appointment or for a prescription to be filled,” Verda J. Hicks, MD, the group’s president, and Christopher M. Zahn, MD, interim chief executive officer, said in a statement.
“Allowing individuals to access birth control at their local pharmacy or drug store will eliminate some barriers,” they said.
A version of this article first appeared on WebMD.com.
This article was updated 7/13/23.
The Food and Drug Administration’s approval today of the first birth control pill for women to be available without a prescription is being hailed by many as a long-needed development, but there remain questions to be resolved, including how much the drug will cost and how it will be used.
The drug, Opill, is expected to be available early next year, and its maker has yet to reveal a retail price. It is the same birth control pill that has been available by prescription for 50 years. But for the first time, women will be able to buy the contraception at a local pharmacy, other retail locations, or online without having to see a doctor first.
Likely to drive debate
Contraception in the United States is not without controversy. The FDA’s approval spurred reactions both for and against making hormonal birth control for women available without a prescription.
“It’s an exciting time, especially right now when reproductive rights are being curtailed in a lot of states. Giving people an additional option for contraception will change people’s lives,” said Beverly Gray, MD, division director of Women’s Community and Population Health at Duke University Medical Center in Durham, N.C.
“It’s a huge win for patients who need better access to contraception,” said Dr. Gray, who is also a spokesperson for the American College of Obstetricians and Gynecologists.
Women who want hormonal birth control but live in areas without convenient access to a doctor, women who cannot easily take time off of work to see a doctor and get a prescription filled, and women without insurance are examples of people who will benefit, she said.
The Catholic Medical Association, in contrast, expressed “deep concern and disappointment” after an FDA advisory committee’s unanimous vote on May 11 recommending the drug be available over the counter. In a statement after the vote, the group cited “extensive medical studies demonstrating the risks and adverse effects of hormonal contraceptives,” adding that “the social impact of [full approval] would be dramatic.”
But doctors largely disagreed.
“It is definitely a huge win for reproductive autonomy. I’m glad that the FDA is prioritizing patient safety and well-being over politics,” said Catherine Cansino, MD, MPH, an ob.gyn. and clinical professor in the University of California Davis department of obstetrics and gynecology. She said the FDA approved the over-the-counter version because the medication is safe.
While opponents like the Catholic Medical Association cite safety concerns and believe doctors should screen all women before prescribing hormonal contraception, Dr. Gray disagreed. “There’s a lot of evidence that patients can figure out if a progestin-only pill is right for them and safe for them. Medical professionals don’t have to be the gatekeepers for contraception,” she said.
Pricing unknown
Whether insurance companies will pay for Opill now that it will be available without a prescription remains unknown. For some medications, paying a copay through insurance can be less expensive than buying at a retail price.
“Although pricing issues will be relevant, the FDA’s decision will enhance women’s access to hormonal birth control,” said Andrew M. Kaunitz, MD, a professor and associate chairman in the department of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville.
The drugmaker, Perrigo, based in Ireland, has not yet announced how much the pill will cost. The price tag could affect how widely available this form of birth control is. The drug has been shown to be as much as 93% effective for pregnancy prevention. Perrigo says it plans to make the pill available at low or no cost to some women.
Caveats to consider
There are some women for whom hormonal contraceptives have always carried greater risks. For example, women who have breast cancer or a history of breast cancer should not use hormonal contraceptives, the FDA said in a news release announcing the approval. Women with other types of cancer should check with their doctors first, the agency noted.
Women who smoke, who take some medications to lower blood pressure, or who have migraines should also take caution, Dr. Cansino said. “People with migraines may not be suitable for over-the-counter oral contraceptives. But a simple screening through a provider can identify whether you are truly eligible or not.”
Irregular bleeding, headaches, dizziness, nausea, increased appetite, belly pain, cramps, or bloating are the most common side effects of Opill, the FDA said.
The Opill is a progestin-only birth control pill. Similar pills have been available in the United Kingdiom for about 2 years, often referred to as “mini pills” because they contain a single hormone. In contrast, prescription birth control pills in the United States and elsewhere contain more than one hormone, estrogen and progestin, to prevent pregnancy.
Prescription pill packs for combination contraception often feature a week of placebo pills without an active ingredient. While skipping a placebo pill might not make a difference in pregnancy prevention, Opill is different. Every pill in the packet will contain medication, Gray said. “So it’s important to take the pill the same time every day for it to be most effective.”
Even though this may mean one less visit to your doctor, Dr. Kaunitz hopes women will stay up to date on their other medical checkups. “One of our challenges as providers of care to women will be to encourage them to continue to receive important services, including cancer screening and vaccinations, even while they can initiate and continue hormonal contraception without contact with a provider.”
Just the beginning?
The American Medical Association hopes this approval signals more to come.
“While we applaud this move, the AMA continues to urge the FDA and HHS to consider a variety of oral contraceptive options for over-the-counter use,” the association, which has more than 250,000 doctor members, said in a statement. “It is important patients have options when choosing which type of birth control works best for them,”
The American College of Obstetricians and Gynecologists said the FDA’s decision will help many women. “We are glad that more patients will now be empowered to choose when and where they obtain a safe method of contraception without having to wait for a medical appointment or for a prescription to be filled,” Verda J. Hicks, MD, the group’s president, and Christopher M. Zahn, MD, interim chief executive officer, said in a statement.
“Allowing individuals to access birth control at their local pharmacy or drug store will eliminate some barriers,” they said.
A version of this article first appeared on WebMD.com.
This article was updated 7/13/23.
Spirometry predicts mortality in type 2 diabetes
Among adults with type 2 diabetes, the presence of preserved ratio impaired spirometry (PRISm) was significantly associated with increased risk of mortality and both macro- and microvascular complications, as well as increased mortality, based on data from more than 20,000 individuals.
Guochen Li, MD, of the Medical College of Soochow University, Suzhou, China, and colleagues wrote.
“A growing number of studies have demonstrated that impaired lung function and type 2 diabetes could trigger shared pathophysiological injuries, such as microangiopathy and chronic inflammation,” they said, but the potential role of PRISm as an early predictor of adverse outcomes in patients with type 2 diabetes has not been fully examined.
In a study published in the journal Chest, the researchers reviewed data from 20,047 individuals with type 2 diabetes in the UK Biobank, a population-based cohort of adults aged 37-73 years recruited between 2006 and 2010.
The main exposure was lung function based on spirometry. PRISm was defined as predicted forced expiratory volume per second (FEV1) less than 80%, with an FEV1/ forced vital capacity (FVC) ratio of at least 0.70. Individuals with normal spirometry (defined as predicted FEV1 ≥ 80% with an FEV1/FVC ratio ≥ 0.70) served as controls.
The primary outcomes were major complications of type 2 diabetes including macrovascular events (myocardial infarction, unstable angina, coronary heart disease [CHD], ischemic stroke, and any type of stroke), microvascular events (diabetic retinopathy and diabetic kidney disease) and mortality (all-cause, cardiovascular, and respiratory).
Overall, 16.9% of study participants (3385 patients) had obstructive spirometry and 22.6% (4521 patients) had PRISm. Compared with individuals with normal spirometry, those with PRISm were more likely to be current smokers, obese, and living in economically disadvantaged areas. Individuals with PRISm also were significantly more likely to be long-term patients with diabetes who were taking glucose-lowering or lipid-lowering drugs (P < .001 for all).
The median follow-up for each of the type 2 diabetes complications and mortality was approximately 12 years. Over this time, 5.0% of patients developed incident MI, 1.3% developed unstable angina, 15.6% had CHD, 3.5% had an ischemic stroke, and 4.7% had any type of stroke. As for microvascular events, 7.8% developed diabetic retinopathy and 6.7% developed diabetic kidney disease. A total of 2588 patients died during the study period (15.1%), including 544 from cardiovascular disease and 319 from respiratory disease.
PRISm was significantly associated with increased risk of each of the complications and mortality types. These associations persisted after adjusting for lifestyle and other factors. The fully adjusted hazard ratios for PRISm versus normal spirometry were 1.23 for MI, 1.23 for unstable angina, 1.21 for CHD, 1.38 for ischemic stroke, 1.41 for any type of stroke, 1.31 for diabetic retinopathy, and 1.38 for diabetic kidney disease. Adjusted HRs for mortality were 1.34, 1.60, and 1.56 for all-cause, cardiovascular, and respiratory mortality, respectively.
The researchers also found that adding PRISm to an office-based risk score significantly improved the risk classification and predictive power for type 2 diabetes complications with the exception of unstable angina and mortality. They found little evidence for an association with sex, smoking, or PRISm duration and any mortality types. However, in subgroup analyses by age, sex, and duration of diabetes, PRISm remained associated with increased risk of macrovascular and microvascular complications, as well as mortality.
Potential mechanisms for the association between PRISm and diabetes complications include the role of insulin resistance in the exacerbation of lung damage in patients with type 2 diabetes, the increased rate of supplemental oxygen use among individuals with PRISm, and the increased prevalence of pulmonary artery enlargement in the PRISm subjects, the researchers wrote.
The findings were limited by several factors including the prospective design, the homogeneous population of individuals primarily of British or Irish ancestry, and the exclusion of diabetic neuropathy from the analysis, the researchers noted.
However, the results were strengthened by the large cohort, use of professional spirometry, and relatively long follow-up. “The findings underscore the relevance of PRISm for prognostic classification in type 2 diabetes and its potential for optimizing prevention strategies in this condition,” they concluded.
The study was supported by the National Natural Science Foundation of China, Natural Science Foundation of Jiangsu Province, and the Priority Academic Program Development of Jiangsu Higher Education Institutions. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com
Among adults with type 2 diabetes, the presence of preserved ratio impaired spirometry (PRISm) was significantly associated with increased risk of mortality and both macro- and microvascular complications, as well as increased mortality, based on data from more than 20,000 individuals.
Guochen Li, MD, of the Medical College of Soochow University, Suzhou, China, and colleagues wrote.
“A growing number of studies have demonstrated that impaired lung function and type 2 diabetes could trigger shared pathophysiological injuries, such as microangiopathy and chronic inflammation,” they said, but the potential role of PRISm as an early predictor of adverse outcomes in patients with type 2 diabetes has not been fully examined.
In a study published in the journal Chest, the researchers reviewed data from 20,047 individuals with type 2 diabetes in the UK Biobank, a population-based cohort of adults aged 37-73 years recruited between 2006 and 2010.
The main exposure was lung function based on spirometry. PRISm was defined as predicted forced expiratory volume per second (FEV1) less than 80%, with an FEV1/ forced vital capacity (FVC) ratio of at least 0.70. Individuals with normal spirometry (defined as predicted FEV1 ≥ 80% with an FEV1/FVC ratio ≥ 0.70) served as controls.
The primary outcomes were major complications of type 2 diabetes including macrovascular events (myocardial infarction, unstable angina, coronary heart disease [CHD], ischemic stroke, and any type of stroke), microvascular events (diabetic retinopathy and diabetic kidney disease) and mortality (all-cause, cardiovascular, and respiratory).
Overall, 16.9% of study participants (3385 patients) had obstructive spirometry and 22.6% (4521 patients) had PRISm. Compared with individuals with normal spirometry, those with PRISm were more likely to be current smokers, obese, and living in economically disadvantaged areas. Individuals with PRISm also were significantly more likely to be long-term patients with diabetes who were taking glucose-lowering or lipid-lowering drugs (P < .001 for all).
The median follow-up for each of the type 2 diabetes complications and mortality was approximately 12 years. Over this time, 5.0% of patients developed incident MI, 1.3% developed unstable angina, 15.6% had CHD, 3.5% had an ischemic stroke, and 4.7% had any type of stroke. As for microvascular events, 7.8% developed diabetic retinopathy and 6.7% developed diabetic kidney disease. A total of 2588 patients died during the study period (15.1%), including 544 from cardiovascular disease and 319 from respiratory disease.
PRISm was significantly associated with increased risk of each of the complications and mortality types. These associations persisted after adjusting for lifestyle and other factors. The fully adjusted hazard ratios for PRISm versus normal spirometry were 1.23 for MI, 1.23 for unstable angina, 1.21 for CHD, 1.38 for ischemic stroke, 1.41 for any type of stroke, 1.31 for diabetic retinopathy, and 1.38 for diabetic kidney disease. Adjusted HRs for mortality were 1.34, 1.60, and 1.56 for all-cause, cardiovascular, and respiratory mortality, respectively.
The researchers also found that adding PRISm to an office-based risk score significantly improved the risk classification and predictive power for type 2 diabetes complications with the exception of unstable angina and mortality. They found little evidence for an association with sex, smoking, or PRISm duration and any mortality types. However, in subgroup analyses by age, sex, and duration of diabetes, PRISm remained associated with increased risk of macrovascular and microvascular complications, as well as mortality.
Potential mechanisms for the association between PRISm and diabetes complications include the role of insulin resistance in the exacerbation of lung damage in patients with type 2 diabetes, the increased rate of supplemental oxygen use among individuals with PRISm, and the increased prevalence of pulmonary artery enlargement in the PRISm subjects, the researchers wrote.
The findings were limited by several factors including the prospective design, the homogeneous population of individuals primarily of British or Irish ancestry, and the exclusion of diabetic neuropathy from the analysis, the researchers noted.
However, the results were strengthened by the large cohort, use of professional spirometry, and relatively long follow-up. “The findings underscore the relevance of PRISm for prognostic classification in type 2 diabetes and its potential for optimizing prevention strategies in this condition,” they concluded.
The study was supported by the National Natural Science Foundation of China, Natural Science Foundation of Jiangsu Province, and the Priority Academic Program Development of Jiangsu Higher Education Institutions. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com
Among adults with type 2 diabetes, the presence of preserved ratio impaired spirometry (PRISm) was significantly associated with increased risk of mortality and both macro- and microvascular complications, as well as increased mortality, based on data from more than 20,000 individuals.
Guochen Li, MD, of the Medical College of Soochow University, Suzhou, China, and colleagues wrote.
“A growing number of studies have demonstrated that impaired lung function and type 2 diabetes could trigger shared pathophysiological injuries, such as microangiopathy and chronic inflammation,” they said, but the potential role of PRISm as an early predictor of adverse outcomes in patients with type 2 diabetes has not been fully examined.
In a study published in the journal Chest, the researchers reviewed data from 20,047 individuals with type 2 diabetes in the UK Biobank, a population-based cohort of adults aged 37-73 years recruited between 2006 and 2010.
The main exposure was lung function based on spirometry. PRISm was defined as predicted forced expiratory volume per second (FEV1) less than 80%, with an FEV1/ forced vital capacity (FVC) ratio of at least 0.70. Individuals with normal spirometry (defined as predicted FEV1 ≥ 80% with an FEV1/FVC ratio ≥ 0.70) served as controls.
The primary outcomes were major complications of type 2 diabetes including macrovascular events (myocardial infarction, unstable angina, coronary heart disease [CHD], ischemic stroke, and any type of stroke), microvascular events (diabetic retinopathy and diabetic kidney disease) and mortality (all-cause, cardiovascular, and respiratory).
Overall, 16.9% of study participants (3385 patients) had obstructive spirometry and 22.6% (4521 patients) had PRISm. Compared with individuals with normal spirometry, those with PRISm were more likely to be current smokers, obese, and living in economically disadvantaged areas. Individuals with PRISm also were significantly more likely to be long-term patients with diabetes who were taking glucose-lowering or lipid-lowering drugs (P < .001 for all).
The median follow-up for each of the type 2 diabetes complications and mortality was approximately 12 years. Over this time, 5.0% of patients developed incident MI, 1.3% developed unstable angina, 15.6% had CHD, 3.5% had an ischemic stroke, and 4.7% had any type of stroke. As for microvascular events, 7.8% developed diabetic retinopathy and 6.7% developed diabetic kidney disease. A total of 2588 patients died during the study period (15.1%), including 544 from cardiovascular disease and 319 from respiratory disease.
PRISm was significantly associated with increased risk of each of the complications and mortality types. These associations persisted after adjusting for lifestyle and other factors. The fully adjusted hazard ratios for PRISm versus normal spirometry were 1.23 for MI, 1.23 for unstable angina, 1.21 for CHD, 1.38 for ischemic stroke, 1.41 for any type of stroke, 1.31 for diabetic retinopathy, and 1.38 for diabetic kidney disease. Adjusted HRs for mortality were 1.34, 1.60, and 1.56 for all-cause, cardiovascular, and respiratory mortality, respectively.
The researchers also found that adding PRISm to an office-based risk score significantly improved the risk classification and predictive power for type 2 diabetes complications with the exception of unstable angina and mortality. They found little evidence for an association with sex, smoking, or PRISm duration and any mortality types. However, in subgroup analyses by age, sex, and duration of diabetes, PRISm remained associated with increased risk of macrovascular and microvascular complications, as well as mortality.
Potential mechanisms for the association between PRISm and diabetes complications include the role of insulin resistance in the exacerbation of lung damage in patients with type 2 diabetes, the increased rate of supplemental oxygen use among individuals with PRISm, and the increased prevalence of pulmonary artery enlargement in the PRISm subjects, the researchers wrote.
The findings were limited by several factors including the prospective design, the homogeneous population of individuals primarily of British or Irish ancestry, and the exclusion of diabetic neuropathy from the analysis, the researchers noted.
However, the results were strengthened by the large cohort, use of professional spirometry, and relatively long follow-up. “The findings underscore the relevance of PRISm for prognostic classification in type 2 diabetes and its potential for optimizing prevention strategies in this condition,” they concluded.
The study was supported by the National Natural Science Foundation of China, Natural Science Foundation of Jiangsu Province, and the Priority Academic Program Development of Jiangsu Higher Education Institutions. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com
FROM THE JOURNAL CHEST
Hearing loss tied to more fatigue in middle and older age
Like many stressful chronic conditions, hearing loss appears to foster fatigue, according to an analysis of National Health and Nutrition Examination Study data published in JAMA Otolaryngology – Head & Neck Surgery.
Researchers at Johns Hopkins University, Baltimore, examined NHANES data from 2015 to 2016 and 2017 to 2018, including findings on more than 3,000 participants aged 40 and older. Based on the audiometry subset of NHANES data, hearing loss was associated with a higher frequency of fatigue – even after adjustment for demographics, comorbidities, and lifestyle variables such as smoking, alcohol, and body mass index, in a nationally representative sample of adults in middle and older age.
“We wanted to get away from small clinical data and take a look at the population level to see if hearing loss was related to fatigue and, further perhaps, to cognitive decline,” said coauthor Nicholas S. Reed, AuD, PhD, an assistant professor of epidemiology at Johns Hopkins University, Baltimore, in an interview. “We found people with hearing loss had twice the risk of reporting fatigue nearly every day versus those not reporting fatigue.” This cross-sectional study provides needed population-based evidence from a nationally representative sample, according to Dr. Reed and associates, who have been researching the possible connection between age-related hearing loss, physical activity levels, and cognitive decline.
Study details
The 3,031 age-eligible participants had a mean age of 58 years; 48% were male, and 10% were Black. Some hearing loss was reported by 24%.
They responded to the following question: “Over the last 2 weeks, how often have you been bothered by feeling tired or having little energy?” Response categories were “not at all,” “several days,” “more than half the days,” and “nearly every day.” Those with hearing loss were more likely to report fatigue for more than half the days (relative risk ratio, 2.16; 95% confidence interval, 1.27-3.67) and nearly every day (RRR, 2.05; 95% CI, 1.16-3.65), compared with not having fatigue. Additional adjustment for comorbidities and depressive symptoms showed similar results.
Hearing loss was defined as > 25 decibels hearing level (dB HL) versus normal hearing of ≤ 25 dB HL, and continuously by every 10 dB HL poorer. Each 10-dB HL of audiometric hearing loss was associated with a higher likelihood of reporting fatigue nearly every day (RRR, 1.24; 95% CI,1.04-1.47), but not for more than half the days.
The association tended to be stronger in younger, non-Hispanic White, and female participants, but statistical testing did not support differential associations by age, sex, race, or ethnicity.
While some might intuitively expect hearing loss to cause noticeably more fatigue in middle-aged people who may be straining to hear during hours in the daily workplace or at home, Dr. Reed said older people probably feel more hearing-related fatigue owing to age and comorbidities. “And higher physical activity levels of middle-aged adults can be protective.”
Dr. Reed advised primary care physicians to be sure to ask about fatigue and hearing status during wellness exams and take appropriate steps to diagnose and correct hearing problems. “Make sure hearing is part of the health equation because hearing loss can be part of the culprit. And it’s very possible that hearing loss is also contributing to cognitive decline.”
Dr. Reed’s group will soon release data on a clinical trial on hearing loss and cognitive decline.
The authors called for studies incorporating fatigue assessments in order to clarify how hearing loss might contribute to physical and mental fatigue and how it could be associated with downstream outcomes such as fatigue-related physical impairment. Dr. Reed reported grants from the National Institute on Aging during the conduct of the study and stock compensation from the Neosensory Advisory Board outside of the submitted work. Several coauthors reported academic or government research funding as well as fees and honoraria from various private-sector companies.
Like many stressful chronic conditions, hearing loss appears to foster fatigue, according to an analysis of National Health and Nutrition Examination Study data published in JAMA Otolaryngology – Head & Neck Surgery.
Researchers at Johns Hopkins University, Baltimore, examined NHANES data from 2015 to 2016 and 2017 to 2018, including findings on more than 3,000 participants aged 40 and older. Based on the audiometry subset of NHANES data, hearing loss was associated with a higher frequency of fatigue – even after adjustment for demographics, comorbidities, and lifestyle variables such as smoking, alcohol, and body mass index, in a nationally representative sample of adults in middle and older age.
“We wanted to get away from small clinical data and take a look at the population level to see if hearing loss was related to fatigue and, further perhaps, to cognitive decline,” said coauthor Nicholas S. Reed, AuD, PhD, an assistant professor of epidemiology at Johns Hopkins University, Baltimore, in an interview. “We found people with hearing loss had twice the risk of reporting fatigue nearly every day versus those not reporting fatigue.” This cross-sectional study provides needed population-based evidence from a nationally representative sample, according to Dr. Reed and associates, who have been researching the possible connection between age-related hearing loss, physical activity levels, and cognitive decline.
Study details
The 3,031 age-eligible participants had a mean age of 58 years; 48% were male, and 10% were Black. Some hearing loss was reported by 24%.
They responded to the following question: “Over the last 2 weeks, how often have you been bothered by feeling tired or having little energy?” Response categories were “not at all,” “several days,” “more than half the days,” and “nearly every day.” Those with hearing loss were more likely to report fatigue for more than half the days (relative risk ratio, 2.16; 95% confidence interval, 1.27-3.67) and nearly every day (RRR, 2.05; 95% CI, 1.16-3.65), compared with not having fatigue. Additional adjustment for comorbidities and depressive symptoms showed similar results.
Hearing loss was defined as > 25 decibels hearing level (dB HL) versus normal hearing of ≤ 25 dB HL, and continuously by every 10 dB HL poorer. Each 10-dB HL of audiometric hearing loss was associated with a higher likelihood of reporting fatigue nearly every day (RRR, 1.24; 95% CI,1.04-1.47), but not for more than half the days.
The association tended to be stronger in younger, non-Hispanic White, and female participants, but statistical testing did not support differential associations by age, sex, race, or ethnicity.
While some might intuitively expect hearing loss to cause noticeably more fatigue in middle-aged people who may be straining to hear during hours in the daily workplace or at home, Dr. Reed said older people probably feel more hearing-related fatigue owing to age and comorbidities. “And higher physical activity levels of middle-aged adults can be protective.”
Dr. Reed advised primary care physicians to be sure to ask about fatigue and hearing status during wellness exams and take appropriate steps to diagnose and correct hearing problems. “Make sure hearing is part of the health equation because hearing loss can be part of the culprit. And it’s very possible that hearing loss is also contributing to cognitive decline.”
Dr. Reed’s group will soon release data on a clinical trial on hearing loss and cognitive decline.
The authors called for studies incorporating fatigue assessments in order to clarify how hearing loss might contribute to physical and mental fatigue and how it could be associated with downstream outcomes such as fatigue-related physical impairment. Dr. Reed reported grants from the National Institute on Aging during the conduct of the study and stock compensation from the Neosensory Advisory Board outside of the submitted work. Several coauthors reported academic or government research funding as well as fees and honoraria from various private-sector companies.
Like many stressful chronic conditions, hearing loss appears to foster fatigue, according to an analysis of National Health and Nutrition Examination Study data published in JAMA Otolaryngology – Head & Neck Surgery.
Researchers at Johns Hopkins University, Baltimore, examined NHANES data from 2015 to 2016 and 2017 to 2018, including findings on more than 3,000 participants aged 40 and older. Based on the audiometry subset of NHANES data, hearing loss was associated with a higher frequency of fatigue – even after adjustment for demographics, comorbidities, and lifestyle variables such as smoking, alcohol, and body mass index, in a nationally representative sample of adults in middle and older age.
“We wanted to get away from small clinical data and take a look at the population level to see if hearing loss was related to fatigue and, further perhaps, to cognitive decline,” said coauthor Nicholas S. Reed, AuD, PhD, an assistant professor of epidemiology at Johns Hopkins University, Baltimore, in an interview. “We found people with hearing loss had twice the risk of reporting fatigue nearly every day versus those not reporting fatigue.” This cross-sectional study provides needed population-based evidence from a nationally representative sample, according to Dr. Reed and associates, who have been researching the possible connection between age-related hearing loss, physical activity levels, and cognitive decline.
Study details
The 3,031 age-eligible participants had a mean age of 58 years; 48% were male, and 10% were Black. Some hearing loss was reported by 24%.
They responded to the following question: “Over the last 2 weeks, how often have you been bothered by feeling tired or having little energy?” Response categories were “not at all,” “several days,” “more than half the days,” and “nearly every day.” Those with hearing loss were more likely to report fatigue for more than half the days (relative risk ratio, 2.16; 95% confidence interval, 1.27-3.67) and nearly every day (RRR, 2.05; 95% CI, 1.16-3.65), compared with not having fatigue. Additional adjustment for comorbidities and depressive symptoms showed similar results.
Hearing loss was defined as > 25 decibels hearing level (dB HL) versus normal hearing of ≤ 25 dB HL, and continuously by every 10 dB HL poorer. Each 10-dB HL of audiometric hearing loss was associated with a higher likelihood of reporting fatigue nearly every day (RRR, 1.24; 95% CI,1.04-1.47), but not for more than half the days.
The association tended to be stronger in younger, non-Hispanic White, and female participants, but statistical testing did not support differential associations by age, sex, race, or ethnicity.
While some might intuitively expect hearing loss to cause noticeably more fatigue in middle-aged people who may be straining to hear during hours in the daily workplace or at home, Dr. Reed said older people probably feel more hearing-related fatigue owing to age and comorbidities. “And higher physical activity levels of middle-aged adults can be protective.”
Dr. Reed advised primary care physicians to be sure to ask about fatigue and hearing status during wellness exams and take appropriate steps to diagnose and correct hearing problems. “Make sure hearing is part of the health equation because hearing loss can be part of the culprit. And it’s very possible that hearing loss is also contributing to cognitive decline.”
Dr. Reed’s group will soon release data on a clinical trial on hearing loss and cognitive decline.
The authors called for studies incorporating fatigue assessments in order to clarify how hearing loss might contribute to physical and mental fatigue and how it could be associated with downstream outcomes such as fatigue-related physical impairment. Dr. Reed reported grants from the National Institute on Aging during the conduct of the study and stock compensation from the Neosensory Advisory Board outside of the submitted work. Several coauthors reported academic or government research funding as well as fees and honoraria from various private-sector companies.
FROM JAMA OTOLARYNGOLOGY – HEAD & NECK SURGERY
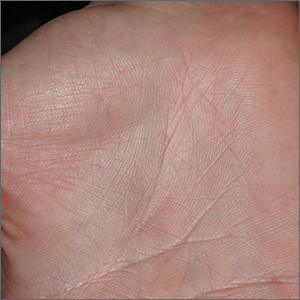
Intensely itchy normal skin
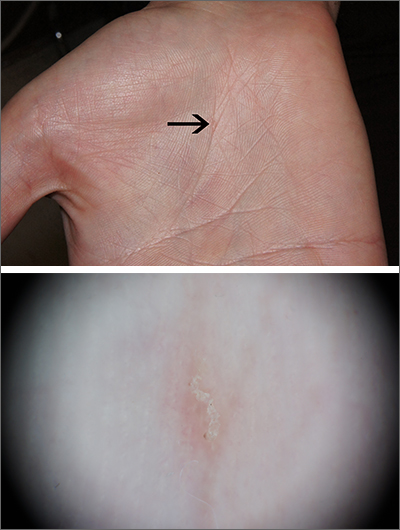
Severe itching should prompt suspicion for scabies and the hands are the highest-yield location. In this patient’s case, there weren’t findings in the web spaces and, in general, skin findings were largely absent; dermoscopy confirmed the diagnosis of scabies.
Sarcoptes scabiei, is a parasitic mite that lives and reproduces in and on human skin and is transmitted by very close contact, either skin-to-skin or by living within a household or institution with shared linens and furnishings. After infection, itching develops within days to weeks from both the physical movement and burrowing of mites within the skin and from the allergic and inflammatory response to mite bodies and their waste.1 Symptoms and infections may persist for years in the absence of treatment.
Sometimes (as in this case), burrows are few and very subtle. More often, there are widespread burrows and excoriated papules over the hands, trunk, extremities, and genitals. A burrowed mite is often adjacent to, but not directly in, an excoriation. Dermoscopy has transformed the ability to diagnose this condition quickly by enabling clinicians to visualize the triangular shape of the head and front legs of a mite (called the “delta sign”). This localization allows easy microscopic confirmation by paring the mite from the skin with a small scalpel blade. (A #11 or #15 blade works very well.)
Topical permethrin 5% cream is highly curative. The cream should be applied from the top of the neck to the tips of the patient’s toes and left on for 8 hours; the process should be repeated a week later. Very close contacts (eg, symptomatic household members or sexual partners) should be treated concurrently. A 60 g tube will treat 1 adult twice. (A 60 g tube of permethrin with a refill, therefore, will treat 2 adults twice.) Oral ivermectin 3 mg dosed at 200 mcg/kg in a single dose repeated in 1 to 2 weeks is an alternative.
Outbreaks in an institutional setting present a significant challenge and require population-based control and often the assistance of infection control specialists or local public health officials. Often this involves weekly treatment with ivermectin for all potentially affected individuals for 3 to 4 weeks and surveillance for follow-up. While there is some resistance to ivermectin, many failures relate more to reinfection from unidentified sources.
This patient received topical permethrin 5% cream dosed as noted above. Itching can be expected to persist for 3 to 4 weeks, so topical triamcinolone 0.1% cream was prescribed as needed for itching on days when permethrin wasn’t applied. At 6 weeks, this patient’s symptoms had resolved.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Richards RN. Scabies: diagnostic and therapeutic update. J Cutan Med Surg. 2021;25:95-101. doi: 10.1177/1203475420960446

Severe itching should prompt suspicion for scabies and the hands are the highest-yield location. In this patient’s case, there weren’t findings in the web spaces and, in general, skin findings were largely absent; dermoscopy confirmed the diagnosis of scabies.
Sarcoptes scabiei, is a parasitic mite that lives and reproduces in and on human skin and is transmitted by very close contact, either skin-to-skin or by living within a household or institution with shared linens and furnishings. After infection, itching develops within days to weeks from both the physical movement and burrowing of mites within the skin and from the allergic and inflammatory response to mite bodies and their waste.1 Symptoms and infections may persist for years in the absence of treatment.
Sometimes (as in this case), burrows are few and very subtle. More often, there are widespread burrows and excoriated papules over the hands, trunk, extremities, and genitals. A burrowed mite is often adjacent to, but not directly in, an excoriation. Dermoscopy has transformed the ability to diagnose this condition quickly by enabling clinicians to visualize the triangular shape of the head and front legs of a mite (called the “delta sign”). This localization allows easy microscopic confirmation by paring the mite from the skin with a small scalpel blade. (A #11 or #15 blade works very well.)
Topical permethrin 5% cream is highly curative. The cream should be applied from the top of the neck to the tips of the patient’s toes and left on for 8 hours; the process should be repeated a week later. Very close contacts (eg, symptomatic household members or sexual partners) should be treated concurrently. A 60 g tube will treat 1 adult twice. (A 60 g tube of permethrin with a refill, therefore, will treat 2 adults twice.) Oral ivermectin 3 mg dosed at 200 mcg/kg in a single dose repeated in 1 to 2 weeks is an alternative.
Outbreaks in an institutional setting present a significant challenge and require population-based control and often the assistance of infection control specialists or local public health officials. Often this involves weekly treatment with ivermectin for all potentially affected individuals for 3 to 4 weeks and surveillance for follow-up. While there is some resistance to ivermectin, many failures relate more to reinfection from unidentified sources.
This patient received topical permethrin 5% cream dosed as noted above. Itching can be expected to persist for 3 to 4 weeks, so topical triamcinolone 0.1% cream was prescribed as needed for itching on days when permethrin wasn’t applied. At 6 weeks, this patient’s symptoms had resolved.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Severe itching should prompt suspicion for scabies and the hands are the highest-yield location. In this patient’s case, there weren’t findings in the web spaces and, in general, skin findings were largely absent; dermoscopy confirmed the diagnosis of scabies.
Sarcoptes scabiei, is a parasitic mite that lives and reproduces in and on human skin and is transmitted by very close contact, either skin-to-skin or by living within a household or institution with shared linens and furnishings. After infection, itching develops within days to weeks from both the physical movement and burrowing of mites within the skin and from the allergic and inflammatory response to mite bodies and their waste.1 Symptoms and infections may persist for years in the absence of treatment.
Sometimes (as in this case), burrows are few and very subtle. More often, there are widespread burrows and excoriated papules over the hands, trunk, extremities, and genitals. A burrowed mite is often adjacent to, but not directly in, an excoriation. Dermoscopy has transformed the ability to diagnose this condition quickly by enabling clinicians to visualize the triangular shape of the head and front legs of a mite (called the “delta sign”). This localization allows easy microscopic confirmation by paring the mite from the skin with a small scalpel blade. (A #11 or #15 blade works very well.)
Topical permethrin 5% cream is highly curative. The cream should be applied from the top of the neck to the tips of the patient’s toes and left on for 8 hours; the process should be repeated a week later. Very close contacts (eg, symptomatic household members or sexual partners) should be treated concurrently. A 60 g tube will treat 1 adult twice. (A 60 g tube of permethrin with a refill, therefore, will treat 2 adults twice.) Oral ivermectin 3 mg dosed at 200 mcg/kg in a single dose repeated in 1 to 2 weeks is an alternative.
Outbreaks in an institutional setting present a significant challenge and require population-based control and often the assistance of infection control specialists or local public health officials. Often this involves weekly treatment with ivermectin for all potentially affected individuals for 3 to 4 weeks and surveillance for follow-up. While there is some resistance to ivermectin, many failures relate more to reinfection from unidentified sources.
This patient received topical permethrin 5% cream dosed as noted above. Itching can be expected to persist for 3 to 4 weeks, so topical triamcinolone 0.1% cream was prescribed as needed for itching on days when permethrin wasn’t applied. At 6 weeks, this patient’s symptoms had resolved.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Richards RN. Scabies: diagnostic and therapeutic update. J Cutan Med Surg. 2021;25:95-101. doi: 10.1177/1203475420960446
1. Richards RN. Scabies: diagnostic and therapeutic update. J Cutan Med Surg. 2021;25:95-101. doi: 10.1177/1203475420960446
Evidence weighed for suicide/self-harm with obesity drugs
Following reports that the European Medicines Agency is looking into instances of suicide or self-harm after patients took the weight loss drugs semaglutide or liraglutide, the manufacturer, Novo Nordisk, issued a statement to this news organization in which it says it “remains confident in the benefit risk profile of the products and remains committed to ensuring patient safety.”
U.S. experts say they haven’t personally seen this adverse effect in any patients except for one isolated case. An increase in suicidal ideation, particularly among younger people, has been reported following bariatric surgery for weight loss.
In the United States, the two drugs – both GLP-1 agonists – already come with a warning about the potential for these adverse effects on the branded versions approved for weight loss, Wegovy and Saxenda. (Years earlier, both drugs, marketed as Ozempic and Victoza, were also approved for treatment of type 2 diabetes.)
Of more than 1,200 reports of adverse reactions with semaglutide, 60 cases of suicidal ideation and 7 suicide attempts have been reported since 2018, according to the Food and Drug Administration’s Adverse Event Reporting System (FAERS) public database. For liraglutide, there were 71 cases of suicidal ideation, 28 suicide attempts, and 25 completed suicides out of more than 35,000 reports of adverse reactions.
The FAERS website cautions users that the data may be duplicated or incomplete, that rates of occurrence cannot be established using the data, that reports have not been verified, and that the existence of a report cannot establish causation.
The EMA is looking into about 150 reports of possible cases of self-injury and suicidal thoughts, according to a press release from the agency.
“It is not yet clear whether the reported cases are linked to the medicines themselves or to the patients’ underlying conditions or other factors,” it says. The medicines are widely used in the European Union, according to the press release.
The review of Ozempic, Saxenda, and Wegovy, which started on July 3, 2023, has been extended to include other GLP-1 receptor agonists, which include dulaglutide, exenatide, and lixisenatide. This review is expected to conclude in November 2023.
In a statement, Novo Nordisk did not directly dispute a potential link between the drugs and suicidal ideation.
“In the U.S., FDA requires medications for chronic weight management that work on the central nervous system, including Wegovy and Saxenda, to carry a warning about suicidal behavior and ideation,” the statement indicates. “This event had been reported in clinical trials with other weight management products.”
It adds: “Novo Nordisk is continuously performing surveillance of the data from ongoing clinical trials and real-world use of its products and collaborates closely with the authorities to ensure patient safety and adequate information to healthcare professionals.”
Important to know the denominator
“What’s important to know is the denominator,” said Holly Lofton, MD, a clinical associate professor of surgery and medicine and the director of the medical weight management program at NYU Langone, New York. “It needs a denominator with the total population on the medication so we can determine if that’s really a significant risk.”
Dr. Lofton described an isolated, anecdotal case of a patient who had no history of depression or mental health problems but developed suicidal thoughts after taking Saxenda for several months. In that case, the 25-year-old was experiencing problems in a personal relationship and with social media.
Two other weight loss specialists contacted by this news organization had not had patients who had experienced suicidal ideation with the drugs. “These are not very common in practice,” Dr. Lofton said in an interview.
The U.S. prescribing information for Saxenda, which contains liraglutide and has been approved as an adjunct to diet and exercise for chronic weight management, recommends monitoring for the emergence of depression and suicidal thoughts. In the clinical trials, 6 of the 3,384 patients who took the drug reported suicidal ideation; none of the 1,941 patients who received placebo did so, according to the FDA.
Similarly, the U.S. prescribing information for Wegovy, which contains semaglutide, recommends monitoring for the emergence of suicidal thoughts or depression, but this recommendation was based on clinical trials of other weight management products. The prescribing information for Ozempic, the brand name for semaglutide for type 2 diabetes, does not include this recommendation.
Is it the weight loss, rather than the meds? Seen with bariatric surgery too
Speculating what the link, if any, might be, Dr. Lofton suggested dopamine release could be playing a role. Small trials in humans as well as animal studies hint at a blunting of dopamine responses to usual triggers – including addictive substances and possibly food – that may also affect mood.
Young people (aged 18-34) who undergo bariatric surgery are at an increased risk of suicide during follow-up compared to their peers who don’t have surgery. And a study found an increase in events involving self-harm after bariatric surgery, especially among patients who already had a mental health disorder.
For a patient who derives comfort from food, not being able to eat in response to a stressful event may lead that patient to act out in more serious ways, according to Dr. Lofton. “That’s why, again, surgical follow-up is so important and their presurgical psychiatric evaluation is so important.”
A version of this article originally appeared on Medscape.com.
Following reports that the European Medicines Agency is looking into instances of suicide or self-harm after patients took the weight loss drugs semaglutide or liraglutide, the manufacturer, Novo Nordisk, issued a statement to this news organization in which it says it “remains confident in the benefit risk profile of the products and remains committed to ensuring patient safety.”
U.S. experts say they haven’t personally seen this adverse effect in any patients except for one isolated case. An increase in suicidal ideation, particularly among younger people, has been reported following bariatric surgery for weight loss.
In the United States, the two drugs – both GLP-1 agonists – already come with a warning about the potential for these adverse effects on the branded versions approved for weight loss, Wegovy and Saxenda. (Years earlier, both drugs, marketed as Ozempic and Victoza, were also approved for treatment of type 2 diabetes.)
Of more than 1,200 reports of adverse reactions with semaglutide, 60 cases of suicidal ideation and 7 suicide attempts have been reported since 2018, according to the Food and Drug Administration’s Adverse Event Reporting System (FAERS) public database. For liraglutide, there were 71 cases of suicidal ideation, 28 suicide attempts, and 25 completed suicides out of more than 35,000 reports of adverse reactions.
The FAERS website cautions users that the data may be duplicated or incomplete, that rates of occurrence cannot be established using the data, that reports have not been verified, and that the existence of a report cannot establish causation.
The EMA is looking into about 150 reports of possible cases of self-injury and suicidal thoughts, according to a press release from the agency.
“It is not yet clear whether the reported cases are linked to the medicines themselves or to the patients’ underlying conditions or other factors,” it says. The medicines are widely used in the European Union, according to the press release.
The review of Ozempic, Saxenda, and Wegovy, which started on July 3, 2023, has been extended to include other GLP-1 receptor agonists, which include dulaglutide, exenatide, and lixisenatide. This review is expected to conclude in November 2023.
In a statement, Novo Nordisk did not directly dispute a potential link between the drugs and suicidal ideation.
“In the U.S., FDA requires medications for chronic weight management that work on the central nervous system, including Wegovy and Saxenda, to carry a warning about suicidal behavior and ideation,” the statement indicates. “This event had been reported in clinical trials with other weight management products.”
It adds: “Novo Nordisk is continuously performing surveillance of the data from ongoing clinical trials and real-world use of its products and collaborates closely with the authorities to ensure patient safety and adequate information to healthcare professionals.”
Important to know the denominator
“What’s important to know is the denominator,” said Holly Lofton, MD, a clinical associate professor of surgery and medicine and the director of the medical weight management program at NYU Langone, New York. “It needs a denominator with the total population on the medication so we can determine if that’s really a significant risk.”
Dr. Lofton described an isolated, anecdotal case of a patient who had no history of depression or mental health problems but developed suicidal thoughts after taking Saxenda for several months. In that case, the 25-year-old was experiencing problems in a personal relationship and with social media.
Two other weight loss specialists contacted by this news organization had not had patients who had experienced suicidal ideation with the drugs. “These are not very common in practice,” Dr. Lofton said in an interview.
The U.S. prescribing information for Saxenda, which contains liraglutide and has been approved as an adjunct to diet and exercise for chronic weight management, recommends monitoring for the emergence of depression and suicidal thoughts. In the clinical trials, 6 of the 3,384 patients who took the drug reported suicidal ideation; none of the 1,941 patients who received placebo did so, according to the FDA.
Similarly, the U.S. prescribing information for Wegovy, which contains semaglutide, recommends monitoring for the emergence of suicidal thoughts or depression, but this recommendation was based on clinical trials of other weight management products. The prescribing information for Ozempic, the brand name for semaglutide for type 2 diabetes, does not include this recommendation.
Is it the weight loss, rather than the meds? Seen with bariatric surgery too
Speculating what the link, if any, might be, Dr. Lofton suggested dopamine release could be playing a role. Small trials in humans as well as animal studies hint at a blunting of dopamine responses to usual triggers – including addictive substances and possibly food – that may also affect mood.
Young people (aged 18-34) who undergo bariatric surgery are at an increased risk of suicide during follow-up compared to their peers who don’t have surgery. And a study found an increase in events involving self-harm after bariatric surgery, especially among patients who already had a mental health disorder.
For a patient who derives comfort from food, not being able to eat in response to a stressful event may lead that patient to act out in more serious ways, according to Dr. Lofton. “That’s why, again, surgical follow-up is so important and their presurgical psychiatric evaluation is so important.”
A version of this article originally appeared on Medscape.com.
Following reports that the European Medicines Agency is looking into instances of suicide or self-harm after patients took the weight loss drugs semaglutide or liraglutide, the manufacturer, Novo Nordisk, issued a statement to this news organization in which it says it “remains confident in the benefit risk profile of the products and remains committed to ensuring patient safety.”
U.S. experts say they haven’t personally seen this adverse effect in any patients except for one isolated case. An increase in suicidal ideation, particularly among younger people, has been reported following bariatric surgery for weight loss.
In the United States, the two drugs – both GLP-1 agonists – already come with a warning about the potential for these adverse effects on the branded versions approved for weight loss, Wegovy and Saxenda. (Years earlier, both drugs, marketed as Ozempic and Victoza, were also approved for treatment of type 2 diabetes.)
Of more than 1,200 reports of adverse reactions with semaglutide, 60 cases of suicidal ideation and 7 suicide attempts have been reported since 2018, according to the Food and Drug Administration’s Adverse Event Reporting System (FAERS) public database. For liraglutide, there were 71 cases of suicidal ideation, 28 suicide attempts, and 25 completed suicides out of more than 35,000 reports of adverse reactions.
The FAERS website cautions users that the data may be duplicated or incomplete, that rates of occurrence cannot be established using the data, that reports have not been verified, and that the existence of a report cannot establish causation.
The EMA is looking into about 150 reports of possible cases of self-injury and suicidal thoughts, according to a press release from the agency.
“It is not yet clear whether the reported cases are linked to the medicines themselves or to the patients’ underlying conditions or other factors,” it says. The medicines are widely used in the European Union, according to the press release.
The review of Ozempic, Saxenda, and Wegovy, which started on July 3, 2023, has been extended to include other GLP-1 receptor agonists, which include dulaglutide, exenatide, and lixisenatide. This review is expected to conclude in November 2023.
In a statement, Novo Nordisk did not directly dispute a potential link between the drugs and suicidal ideation.
“In the U.S., FDA requires medications for chronic weight management that work on the central nervous system, including Wegovy and Saxenda, to carry a warning about suicidal behavior and ideation,” the statement indicates. “This event had been reported in clinical trials with other weight management products.”
It adds: “Novo Nordisk is continuously performing surveillance of the data from ongoing clinical trials and real-world use of its products and collaborates closely with the authorities to ensure patient safety and adequate information to healthcare professionals.”
Important to know the denominator
“What’s important to know is the denominator,” said Holly Lofton, MD, a clinical associate professor of surgery and medicine and the director of the medical weight management program at NYU Langone, New York. “It needs a denominator with the total population on the medication so we can determine if that’s really a significant risk.”
Dr. Lofton described an isolated, anecdotal case of a patient who had no history of depression or mental health problems but developed suicidal thoughts after taking Saxenda for several months. In that case, the 25-year-old was experiencing problems in a personal relationship and with social media.
Two other weight loss specialists contacted by this news organization had not had patients who had experienced suicidal ideation with the drugs. “These are not very common in practice,” Dr. Lofton said in an interview.
The U.S. prescribing information for Saxenda, which contains liraglutide and has been approved as an adjunct to diet and exercise for chronic weight management, recommends monitoring for the emergence of depression and suicidal thoughts. In the clinical trials, 6 of the 3,384 patients who took the drug reported suicidal ideation; none of the 1,941 patients who received placebo did so, according to the FDA.
Similarly, the U.S. prescribing information for Wegovy, which contains semaglutide, recommends monitoring for the emergence of suicidal thoughts or depression, but this recommendation was based on clinical trials of other weight management products. The prescribing information for Ozempic, the brand name for semaglutide for type 2 diabetes, does not include this recommendation.
Is it the weight loss, rather than the meds? Seen with bariatric surgery too
Speculating what the link, if any, might be, Dr. Lofton suggested dopamine release could be playing a role. Small trials in humans as well as animal studies hint at a blunting of dopamine responses to usual triggers – including addictive substances and possibly food – that may also affect mood.
Young people (aged 18-34) who undergo bariatric surgery are at an increased risk of suicide during follow-up compared to their peers who don’t have surgery. And a study found an increase in events involving self-harm after bariatric surgery, especially among patients who already had a mental health disorder.
For a patient who derives comfort from food, not being able to eat in response to a stressful event may lead that patient to act out in more serious ways, according to Dr. Lofton. “That’s why, again, surgical follow-up is so important and their presurgical psychiatric evaluation is so important.”
A version of this article originally appeared on Medscape.com.
Link between low co-pays for new diabetes drugs and patient adherence
Findings from a recent study indicate that the less U.S. patients pay out of pocket for drugs that often have high co-pays, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors or glucagonlike peptide-1 (GLP-1) agonists, the more they adhere to taking these medications.
The study, led by Utibe R. Essien, MD, from University of California, Los Angeles, and Balvindar Singh, MD, PhD, from University of Pittsburgh, was published online in JAMA Cardiology.
Patient data from Clinformatics Data Mart, a health insurance claims database, was analyzed for the study. The information for 90,041 adults from the United States who had commercial and Medicare health insurance, and who started taking a GLP-1 agonist or SGLT2 inhibitor between 2014 and 2020 was reviewed. Participants had type 2 diabetes, heart failure, or both.
The primary outcome showed patients with a lower drug co-pay had significantly higher odds of 12-month adherence to GLP-1 agonists and SGLT2 inhibitors, compared with those with a higher co-pay. These differences persisted after controlling for patient demographic, clinical, and socioeconomic covariates.
After full adjustments were made and after the 12 months, patients with a high co-pay of $50 per month or more were 53% less likely to adhere to an SGLT2 inhibitor and 32% less likely to adhere to a GLP-1 agonist, compared with patients with a co-pay of less than $10 per month for these agents.
“Lowering high out-of-pocket prescription costs may be key to improving adherence to guideline-recommended therapies and advancing overall quality of care in patients with type 2 diabetes and heart failure,” the authors conclude.
The authors acknowledge the study’s limitations, including the inability to exclude residual confounding, uncertain generalizability for those without health insurance or with public insurance and possible misclassifications of type 2 diabetes and heart failure diagnoses or medical comorbidities. Additionally, this study did not have information on patients’ preferences associated with medication use, including specific reasons for poor adherence, and could not assess how co-payments influenced initial prescription receipt or abandonment at the pharmacy, or other factors including possible price inflation.
The study received no commercial funding. One author (not a lead author) is an adviser to several drug companies including ones that market SGLT2 inhibitors or GLP-1 agonists.
Findings from a recent study indicate that the less U.S. patients pay out of pocket for drugs that often have high co-pays, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors or glucagonlike peptide-1 (GLP-1) agonists, the more they adhere to taking these medications.
The study, led by Utibe R. Essien, MD, from University of California, Los Angeles, and Balvindar Singh, MD, PhD, from University of Pittsburgh, was published online in JAMA Cardiology.
Patient data from Clinformatics Data Mart, a health insurance claims database, was analyzed for the study. The information for 90,041 adults from the United States who had commercial and Medicare health insurance, and who started taking a GLP-1 agonist or SGLT2 inhibitor between 2014 and 2020 was reviewed. Participants had type 2 diabetes, heart failure, or both.
The primary outcome showed patients with a lower drug co-pay had significantly higher odds of 12-month adherence to GLP-1 agonists and SGLT2 inhibitors, compared with those with a higher co-pay. These differences persisted after controlling for patient demographic, clinical, and socioeconomic covariates.
After full adjustments were made and after the 12 months, patients with a high co-pay of $50 per month or more were 53% less likely to adhere to an SGLT2 inhibitor and 32% less likely to adhere to a GLP-1 agonist, compared with patients with a co-pay of less than $10 per month for these agents.
“Lowering high out-of-pocket prescription costs may be key to improving adherence to guideline-recommended therapies and advancing overall quality of care in patients with type 2 diabetes and heart failure,” the authors conclude.
The authors acknowledge the study’s limitations, including the inability to exclude residual confounding, uncertain generalizability for those without health insurance or with public insurance and possible misclassifications of type 2 diabetes and heart failure diagnoses or medical comorbidities. Additionally, this study did not have information on patients’ preferences associated with medication use, including specific reasons for poor adherence, and could not assess how co-payments influenced initial prescription receipt or abandonment at the pharmacy, or other factors including possible price inflation.
The study received no commercial funding. One author (not a lead author) is an adviser to several drug companies including ones that market SGLT2 inhibitors or GLP-1 agonists.
Findings from a recent study indicate that the less U.S. patients pay out of pocket for drugs that often have high co-pays, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors or glucagonlike peptide-1 (GLP-1) agonists, the more they adhere to taking these medications.
The study, led by Utibe R. Essien, MD, from University of California, Los Angeles, and Balvindar Singh, MD, PhD, from University of Pittsburgh, was published online in JAMA Cardiology.
Patient data from Clinformatics Data Mart, a health insurance claims database, was analyzed for the study. The information for 90,041 adults from the United States who had commercial and Medicare health insurance, and who started taking a GLP-1 agonist or SGLT2 inhibitor between 2014 and 2020 was reviewed. Participants had type 2 diabetes, heart failure, or both.
The primary outcome showed patients with a lower drug co-pay had significantly higher odds of 12-month adherence to GLP-1 agonists and SGLT2 inhibitors, compared with those with a higher co-pay. These differences persisted after controlling for patient demographic, clinical, and socioeconomic covariates.
After full adjustments were made and after the 12 months, patients with a high co-pay of $50 per month or more were 53% less likely to adhere to an SGLT2 inhibitor and 32% less likely to adhere to a GLP-1 agonist, compared with patients with a co-pay of less than $10 per month for these agents.
“Lowering high out-of-pocket prescription costs may be key to improving adherence to guideline-recommended therapies and advancing overall quality of care in patients with type 2 diabetes and heart failure,” the authors conclude.
The authors acknowledge the study’s limitations, including the inability to exclude residual confounding, uncertain generalizability for those without health insurance or with public insurance and possible misclassifications of type 2 diabetes and heart failure diagnoses or medical comorbidities. Additionally, this study did not have information on patients’ preferences associated with medication use, including specific reasons for poor adherence, and could not assess how co-payments influenced initial prescription receipt or abandonment at the pharmacy, or other factors including possible price inflation.
The study received no commercial funding. One author (not a lead author) is an adviser to several drug companies including ones that market SGLT2 inhibitors or GLP-1 agonists.
FROM JAMA CARDIOLOGY
FDA approves cognitive-behavioral app for adults with type 2 diabetes
A smartphone-based app designed to deliver cognitive-behavioral therapy (CBT) to adults with type 2 diabetes received marketing approval as a class II medical device from the Food and Drug Administration on July 10, becoming the first digital behavioral therapeutic device for people with diabetes to receive this designation for U.S. patients.
Better Therapeutics representatives said that the app, formerly known as BT-001, will be called AspyreRX, with U.S. sales planned to launch in October-December 2023.
The app will be available to patients exclusively by prescription, with a planned 90-day use duration and an option for a second 90-day prescription. A company official said the price per prescription will be about $500-800, although this is not yet finalized. The app is intended for use in concert with the conventional pillars of glycemic control in people with type 2 diabetes: lifestyle modification and treatment with antidiabetes medications.
Senior staff members of Better Therapeutics acknowledged the critical need for an education program, which they will now launch for clinicians, payers, and patients to get across the message of the potential benefit and safety associated with using the CBT app. Their initial marketing will target patients with type 2 diabetes and poorly controlled hemoglobin A1c levels in five to six U.S. regions with high numbers of these patients. The company will also attempt to make the app available through the Department of Veterans Affairs health system and try to secure coverage by Medicare and commercial health-insurance providers.
Approval based on pivotal trial results
The FDA approval focused on data collected in the BT-001 randomized, controlled trial, which included 669 U.S. adults with poorly controlled type 2 diabetes. Results, published in 2022 in Diabetes Care, showed that after 90 days, people using the app had an average incremental reduction in A1c of 0.39 percentage points, compared with control patients who didn’t use the app, the primary endpoint. Use of the app also appeared safe.
Subsequent meeting presentations of study findings showed that A1c-lowering linked with app use was durable during continued use for a total of 180 days, that the effectiveness of the app in helping to lower A1c levels was “dose dependent” relative to the number of lessons a person completed, and that using the app significantly linked with a reduced need for intensified glycemic control through added medications.
Another finding of the extended-use phase of the study was that 81% of patients assigned to the app-using group continued to regularly use the app after 180 days, a level of durable engagement by patients that “exceeded our expectations,” said Diane Gomez-Thinnes, chief commercial officer of Better Therapeutics, during a press conference.
The company plans to tweak the app prior to its launch based on additional analyses of results from the pivotal study to further improve patient engagement and app ease of use. The company is also planning to expand the range of smartphones that can support the app, although about 90%-95% of U.S. smartphones have this capability.
Better Therapeutics is also actively developing and testing other modifications to the basic CBT app to make it usable by people with other cardiometabolic disorders such as hypertension, obesity, and fatty liver disease.
The BT-001 study was funded by Better Therapeutics.
A version of this article first appeared on Medscape.com.
A smartphone-based app designed to deliver cognitive-behavioral therapy (CBT) to adults with type 2 diabetes received marketing approval as a class II medical device from the Food and Drug Administration on July 10, becoming the first digital behavioral therapeutic device for people with diabetes to receive this designation for U.S. patients.
Better Therapeutics representatives said that the app, formerly known as BT-001, will be called AspyreRX, with U.S. sales planned to launch in October-December 2023.
The app will be available to patients exclusively by prescription, with a planned 90-day use duration and an option for a second 90-day prescription. A company official said the price per prescription will be about $500-800, although this is not yet finalized. The app is intended for use in concert with the conventional pillars of glycemic control in people with type 2 diabetes: lifestyle modification and treatment with antidiabetes medications.
Senior staff members of Better Therapeutics acknowledged the critical need for an education program, which they will now launch for clinicians, payers, and patients to get across the message of the potential benefit and safety associated with using the CBT app. Their initial marketing will target patients with type 2 diabetes and poorly controlled hemoglobin A1c levels in five to six U.S. regions with high numbers of these patients. The company will also attempt to make the app available through the Department of Veterans Affairs health system and try to secure coverage by Medicare and commercial health-insurance providers.
Approval based on pivotal trial results
The FDA approval focused on data collected in the BT-001 randomized, controlled trial, which included 669 U.S. adults with poorly controlled type 2 diabetes. Results, published in 2022 in Diabetes Care, showed that after 90 days, people using the app had an average incremental reduction in A1c of 0.39 percentage points, compared with control patients who didn’t use the app, the primary endpoint. Use of the app also appeared safe.
Subsequent meeting presentations of study findings showed that A1c-lowering linked with app use was durable during continued use for a total of 180 days, that the effectiveness of the app in helping to lower A1c levels was “dose dependent” relative to the number of lessons a person completed, and that using the app significantly linked with a reduced need for intensified glycemic control through added medications.
Another finding of the extended-use phase of the study was that 81% of patients assigned to the app-using group continued to regularly use the app after 180 days, a level of durable engagement by patients that “exceeded our expectations,” said Diane Gomez-Thinnes, chief commercial officer of Better Therapeutics, during a press conference.
The company plans to tweak the app prior to its launch based on additional analyses of results from the pivotal study to further improve patient engagement and app ease of use. The company is also planning to expand the range of smartphones that can support the app, although about 90%-95% of U.S. smartphones have this capability.
Better Therapeutics is also actively developing and testing other modifications to the basic CBT app to make it usable by people with other cardiometabolic disorders such as hypertension, obesity, and fatty liver disease.
The BT-001 study was funded by Better Therapeutics.
A version of this article first appeared on Medscape.com.
A smartphone-based app designed to deliver cognitive-behavioral therapy (CBT) to adults with type 2 diabetes received marketing approval as a class II medical device from the Food and Drug Administration on July 10, becoming the first digital behavioral therapeutic device for people with diabetes to receive this designation for U.S. patients.
Better Therapeutics representatives said that the app, formerly known as BT-001, will be called AspyreRX, with U.S. sales planned to launch in October-December 2023.
The app will be available to patients exclusively by prescription, with a planned 90-day use duration and an option for a second 90-day prescription. A company official said the price per prescription will be about $500-800, although this is not yet finalized. The app is intended for use in concert with the conventional pillars of glycemic control in people with type 2 diabetes: lifestyle modification and treatment with antidiabetes medications.
Senior staff members of Better Therapeutics acknowledged the critical need for an education program, which they will now launch for clinicians, payers, and patients to get across the message of the potential benefit and safety associated with using the CBT app. Their initial marketing will target patients with type 2 diabetes and poorly controlled hemoglobin A1c levels in five to six U.S. regions with high numbers of these patients. The company will also attempt to make the app available through the Department of Veterans Affairs health system and try to secure coverage by Medicare and commercial health-insurance providers.
Approval based on pivotal trial results
The FDA approval focused on data collected in the BT-001 randomized, controlled trial, which included 669 U.S. adults with poorly controlled type 2 diabetes. Results, published in 2022 in Diabetes Care, showed that after 90 days, people using the app had an average incremental reduction in A1c of 0.39 percentage points, compared with control patients who didn’t use the app, the primary endpoint. Use of the app also appeared safe.
Subsequent meeting presentations of study findings showed that A1c-lowering linked with app use was durable during continued use for a total of 180 days, that the effectiveness of the app in helping to lower A1c levels was “dose dependent” relative to the number of lessons a person completed, and that using the app significantly linked with a reduced need for intensified glycemic control through added medications.
Another finding of the extended-use phase of the study was that 81% of patients assigned to the app-using group continued to regularly use the app after 180 days, a level of durable engagement by patients that “exceeded our expectations,” said Diane Gomez-Thinnes, chief commercial officer of Better Therapeutics, during a press conference.
The company plans to tweak the app prior to its launch based on additional analyses of results from the pivotal study to further improve patient engagement and app ease of use. The company is also planning to expand the range of smartphones that can support the app, although about 90%-95% of U.S. smartphones have this capability.
Better Therapeutics is also actively developing and testing other modifications to the basic CBT app to make it usable by people with other cardiometabolic disorders such as hypertension, obesity, and fatty liver disease.
The BT-001 study was funded by Better Therapeutics.
A version of this article first appeared on Medscape.com.
Aging and type 1 diabetes: ‘Complete picture’ 40 years on
SAN DIEGO –
New funding for 2022-2027 for the DCCT long-term observational follow-up study, the Epidemiology of Diabetes Interventions and Complications (EDIC) will go toward investigating aspects of type 1 diabetes that are associated with aging and are also common in type 2 diabetes, including cardiovascular disease, fatty liver disease, and sleep apnea.
The original randomized DCCT clinical trial results, published in 1993 in the New England Journal of Medicine, proved that early intensive glycemic control was the key to preventing or slowing the progression of long-term eye, kidney, and nerve complications of type 1 diabetes. Subsequently, EDIC has yielded many more major findings including that early tight glycemic control also reduces cardiovascular risk and prolongs survival in type 1 diabetes.
And although the phenomenon of metabolic memory initially seen in EDIC means that early glycemic control is important, subsequent EDIC data also have suggested that it is never too late to initiate intensive glycemic control, speakers emphasized during a special symposium commemorating 40 years since the start of DCCT, held during the annual scientific sessions of the American Diabetes Association. As with the 30-year DCCT/EDIC commemorative symposium held in 2013, local study participants were in the audience and were acknowledged with long applause.
Together, DCCT and EDIC – both funded by the National Institutes of Health at 27 sites in the United States and Canada – have changed the standard of care for people with type 1 diabetes and continue to inform clinical practice. Prior to the DCCT, between 1930 and 1970, about a third of people with type 1 diabetes developed vision loss and one in five experienced kidney failure and/or myocardial infarction. Stroke and amputation were also common, DCCT/EDIC chair David M. Nathan, MD, said while introducing the symposium.
“All of the advances in care of type 1 diabetes have developed because this study demonstrated that it was important – continuous glucose monitoring (CGM), new insulins, better [insulin] pumps. ... I think the most profound finding is that mortality in our intensively treated cohort is the same as in the general population. That says it all,” Dr. Nathan said in an interview.
And now, “what we still have yet to contribute is what happens to type 1 diabetes as people get older,” added Dr. Nathan, a professor of medicine at Harvard Medical School and director of the Diabetes Center at Massachusetts General Hospital, both in Boston.
‘Something that heretofore none of us could have imagined’
The 1,441 DCCT participants had a mean age of 27 years at baseline in 1983, when they were randomized to intensive insulin therapy or usual care. The 1,375 participants (96%) who continued into EDIC in 1994 were an average of 35 years old at that point, when the usual care group was taught intensive glycemic management and all participants returned to their personal health care teams. The 1,075 participants in EDIC today are an average age of 63 years.
Only 11 participants had died at the start of EDIC, and just 250 (17%) have died as of 2023, said study coordinator cochair Gayle Lorenzi, RN, who is a certified diabetes care and education specialist at the University of California, San Diego.
“DCCT/EDIC because of its longevity represents a unique opportunity to explore aging in long duration of type 1 diabetes, something that heretofore none of us could have imagined, especially for those of you in the audience who started your careers in the 70s and 80s,” Ms. Lorenzi commented.
About 36% of the cohort now has overweight and 40% have obesity, mirroring the general population. And they now have a mean hemoglobin A1c of 7.3%.
According to Barbara H. Braffett, PhD, co–principal investigator at the DCCT/EDIC data coordinating center: “The EDIC study is now shifting its focus during the next 5 years to understand the clinical course of type 1 diabetes in the setting of advancing duration and age, as well as increasing adiposity, which has progressively affected individuals with type 1 diabetes and has potential long-term adverse consequences.”
Dr. Braffett outlined the new study approaches added in 2022-2027. Cardiopulmonary exercise testing, two-dimensional Doppler echocardiography, and carotid-femoral pulse wave velocity will be used to quantify functional and structural changes central to heart failure.
Dr. Nathan commented that, although enough cardiovascular events were available in EDIC by 2006 to demonstrate a significant 58% reduction in the intensive therapy group, “now we can start looking at the aging heart. We have a bunch of great cardiologists working with us who will be guiding us on measuring everything.”
Fatty liver disease in the setting of increasing adiposity will also be investigated using transient elastography (FibroScan) and the Fibrosis-4 index, a quantification of liver enzymes and platelet count.
Dr. Nathan noted that the study participants have had “this kind of funny metabolic milieu in their liver for decades. They don’t make insulin in their pancreas, and therefore, the insulin they get is peripheral and then it goes to their liver. Well, what does that do to them?”
Participants will also complete three symptom questionnaires assessing obstructive sleep apnea, aimed at guiding future sleep studies in those found to be at high risk, Dr. Braffett said.
DCCT/EDIC over 40 years: ‘Incredibly complete picture’
As of 2023, the DCCT/EDIC participants have been studied for longer than 60% of their lifespans and for over 80% of their diabetes duration, Dr. Braffett noted.
During the EDIC 2017-2022 cycle, Dr. Braffett and other speakers summarized, prior EDIC efforts had focused on aspects of cognitive function, physical function, and cheiroarthropathy.
Other DCCT/EDIC studies examined the relationship of A1c and diabetes duration in cardiovascular disease risk, the association of microvascular complications with the risk of cardiovascular disease beyond traditional risk factors, and the risk of severe hypoglycemia over the first 30 years of DCCT/EDIC follow-up.
Moreover, the longitudinal eye and kidney assessments over the 40 years have informed screening guidelines for retinopathy and urinary albumin.
Dr. Nathan said: “Today, the number with horrible complications is very few, but we haven’t erased complications entirely. ... We have this incredibly complete picture of type 1 diabetes that allows us to explore everything. We welcome people to come to us with ideas. That’s the value of this research.”
Dr. Nathan, Ms. Lorenzi, and Dr. Braffett reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO –
New funding for 2022-2027 for the DCCT long-term observational follow-up study, the Epidemiology of Diabetes Interventions and Complications (EDIC) will go toward investigating aspects of type 1 diabetes that are associated with aging and are also common in type 2 diabetes, including cardiovascular disease, fatty liver disease, and sleep apnea.
The original randomized DCCT clinical trial results, published in 1993 in the New England Journal of Medicine, proved that early intensive glycemic control was the key to preventing or slowing the progression of long-term eye, kidney, and nerve complications of type 1 diabetes. Subsequently, EDIC has yielded many more major findings including that early tight glycemic control also reduces cardiovascular risk and prolongs survival in type 1 diabetes.
And although the phenomenon of metabolic memory initially seen in EDIC means that early glycemic control is important, subsequent EDIC data also have suggested that it is never too late to initiate intensive glycemic control, speakers emphasized during a special symposium commemorating 40 years since the start of DCCT, held during the annual scientific sessions of the American Diabetes Association. As with the 30-year DCCT/EDIC commemorative symposium held in 2013, local study participants were in the audience and were acknowledged with long applause.
Together, DCCT and EDIC – both funded by the National Institutes of Health at 27 sites in the United States and Canada – have changed the standard of care for people with type 1 diabetes and continue to inform clinical practice. Prior to the DCCT, between 1930 and 1970, about a third of people with type 1 diabetes developed vision loss and one in five experienced kidney failure and/or myocardial infarction. Stroke and amputation were also common, DCCT/EDIC chair David M. Nathan, MD, said while introducing the symposium.
“All of the advances in care of type 1 diabetes have developed because this study demonstrated that it was important – continuous glucose monitoring (CGM), new insulins, better [insulin] pumps. ... I think the most profound finding is that mortality in our intensively treated cohort is the same as in the general population. That says it all,” Dr. Nathan said in an interview.
And now, “what we still have yet to contribute is what happens to type 1 diabetes as people get older,” added Dr. Nathan, a professor of medicine at Harvard Medical School and director of the Diabetes Center at Massachusetts General Hospital, both in Boston.
‘Something that heretofore none of us could have imagined’
The 1,441 DCCT participants had a mean age of 27 years at baseline in 1983, when they were randomized to intensive insulin therapy or usual care. The 1,375 participants (96%) who continued into EDIC in 1994 were an average of 35 years old at that point, when the usual care group was taught intensive glycemic management and all participants returned to their personal health care teams. The 1,075 participants in EDIC today are an average age of 63 years.
Only 11 participants had died at the start of EDIC, and just 250 (17%) have died as of 2023, said study coordinator cochair Gayle Lorenzi, RN, who is a certified diabetes care and education specialist at the University of California, San Diego.
“DCCT/EDIC because of its longevity represents a unique opportunity to explore aging in long duration of type 1 diabetes, something that heretofore none of us could have imagined, especially for those of you in the audience who started your careers in the 70s and 80s,” Ms. Lorenzi commented.
About 36% of the cohort now has overweight and 40% have obesity, mirroring the general population. And they now have a mean hemoglobin A1c of 7.3%.
According to Barbara H. Braffett, PhD, co–principal investigator at the DCCT/EDIC data coordinating center: “The EDIC study is now shifting its focus during the next 5 years to understand the clinical course of type 1 diabetes in the setting of advancing duration and age, as well as increasing adiposity, which has progressively affected individuals with type 1 diabetes and has potential long-term adverse consequences.”
Dr. Braffett outlined the new study approaches added in 2022-2027. Cardiopulmonary exercise testing, two-dimensional Doppler echocardiography, and carotid-femoral pulse wave velocity will be used to quantify functional and structural changes central to heart failure.
Dr. Nathan commented that, although enough cardiovascular events were available in EDIC by 2006 to demonstrate a significant 58% reduction in the intensive therapy group, “now we can start looking at the aging heart. We have a bunch of great cardiologists working with us who will be guiding us on measuring everything.”
Fatty liver disease in the setting of increasing adiposity will also be investigated using transient elastography (FibroScan) and the Fibrosis-4 index, a quantification of liver enzymes and platelet count.
Dr. Nathan noted that the study participants have had “this kind of funny metabolic milieu in their liver for decades. They don’t make insulin in their pancreas, and therefore, the insulin they get is peripheral and then it goes to their liver. Well, what does that do to them?”
Participants will also complete three symptom questionnaires assessing obstructive sleep apnea, aimed at guiding future sleep studies in those found to be at high risk, Dr. Braffett said.
DCCT/EDIC over 40 years: ‘Incredibly complete picture’
As of 2023, the DCCT/EDIC participants have been studied for longer than 60% of their lifespans and for over 80% of their diabetes duration, Dr. Braffett noted.
During the EDIC 2017-2022 cycle, Dr. Braffett and other speakers summarized, prior EDIC efforts had focused on aspects of cognitive function, physical function, and cheiroarthropathy.
Other DCCT/EDIC studies examined the relationship of A1c and diabetes duration in cardiovascular disease risk, the association of microvascular complications with the risk of cardiovascular disease beyond traditional risk factors, and the risk of severe hypoglycemia over the first 30 years of DCCT/EDIC follow-up.
Moreover, the longitudinal eye and kidney assessments over the 40 years have informed screening guidelines for retinopathy and urinary albumin.
Dr. Nathan said: “Today, the number with horrible complications is very few, but we haven’t erased complications entirely. ... We have this incredibly complete picture of type 1 diabetes that allows us to explore everything. We welcome people to come to us with ideas. That’s the value of this research.”
Dr. Nathan, Ms. Lorenzi, and Dr. Braffett reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO –
New funding for 2022-2027 for the DCCT long-term observational follow-up study, the Epidemiology of Diabetes Interventions and Complications (EDIC) will go toward investigating aspects of type 1 diabetes that are associated with aging and are also common in type 2 diabetes, including cardiovascular disease, fatty liver disease, and sleep apnea.
The original randomized DCCT clinical trial results, published in 1993 in the New England Journal of Medicine, proved that early intensive glycemic control was the key to preventing or slowing the progression of long-term eye, kidney, and nerve complications of type 1 diabetes. Subsequently, EDIC has yielded many more major findings including that early tight glycemic control also reduces cardiovascular risk and prolongs survival in type 1 diabetes.
And although the phenomenon of metabolic memory initially seen in EDIC means that early glycemic control is important, subsequent EDIC data also have suggested that it is never too late to initiate intensive glycemic control, speakers emphasized during a special symposium commemorating 40 years since the start of DCCT, held during the annual scientific sessions of the American Diabetes Association. As with the 30-year DCCT/EDIC commemorative symposium held in 2013, local study participants were in the audience and were acknowledged with long applause.
Together, DCCT and EDIC – both funded by the National Institutes of Health at 27 sites in the United States and Canada – have changed the standard of care for people with type 1 diabetes and continue to inform clinical practice. Prior to the DCCT, between 1930 and 1970, about a third of people with type 1 diabetes developed vision loss and one in five experienced kidney failure and/or myocardial infarction. Stroke and amputation were also common, DCCT/EDIC chair David M. Nathan, MD, said while introducing the symposium.
“All of the advances in care of type 1 diabetes have developed because this study demonstrated that it was important – continuous glucose monitoring (CGM), new insulins, better [insulin] pumps. ... I think the most profound finding is that mortality in our intensively treated cohort is the same as in the general population. That says it all,” Dr. Nathan said in an interview.
And now, “what we still have yet to contribute is what happens to type 1 diabetes as people get older,” added Dr. Nathan, a professor of medicine at Harvard Medical School and director of the Diabetes Center at Massachusetts General Hospital, both in Boston.
‘Something that heretofore none of us could have imagined’
The 1,441 DCCT participants had a mean age of 27 years at baseline in 1983, when they were randomized to intensive insulin therapy or usual care. The 1,375 participants (96%) who continued into EDIC in 1994 were an average of 35 years old at that point, when the usual care group was taught intensive glycemic management and all participants returned to their personal health care teams. The 1,075 participants in EDIC today are an average age of 63 years.
Only 11 participants had died at the start of EDIC, and just 250 (17%) have died as of 2023, said study coordinator cochair Gayle Lorenzi, RN, who is a certified diabetes care and education specialist at the University of California, San Diego.
“DCCT/EDIC because of its longevity represents a unique opportunity to explore aging in long duration of type 1 diabetes, something that heretofore none of us could have imagined, especially for those of you in the audience who started your careers in the 70s and 80s,” Ms. Lorenzi commented.
About 36% of the cohort now has overweight and 40% have obesity, mirroring the general population. And they now have a mean hemoglobin A1c of 7.3%.
According to Barbara H. Braffett, PhD, co–principal investigator at the DCCT/EDIC data coordinating center: “The EDIC study is now shifting its focus during the next 5 years to understand the clinical course of type 1 diabetes in the setting of advancing duration and age, as well as increasing adiposity, which has progressively affected individuals with type 1 diabetes and has potential long-term adverse consequences.”
Dr. Braffett outlined the new study approaches added in 2022-2027. Cardiopulmonary exercise testing, two-dimensional Doppler echocardiography, and carotid-femoral pulse wave velocity will be used to quantify functional and structural changes central to heart failure.
Dr. Nathan commented that, although enough cardiovascular events were available in EDIC by 2006 to demonstrate a significant 58% reduction in the intensive therapy group, “now we can start looking at the aging heart. We have a bunch of great cardiologists working with us who will be guiding us on measuring everything.”
Fatty liver disease in the setting of increasing adiposity will also be investigated using transient elastography (FibroScan) and the Fibrosis-4 index, a quantification of liver enzymes and platelet count.
Dr. Nathan noted that the study participants have had “this kind of funny metabolic milieu in their liver for decades. They don’t make insulin in their pancreas, and therefore, the insulin they get is peripheral and then it goes to their liver. Well, what does that do to them?”
Participants will also complete three symptom questionnaires assessing obstructive sleep apnea, aimed at guiding future sleep studies in those found to be at high risk, Dr. Braffett said.
DCCT/EDIC over 40 years: ‘Incredibly complete picture’
As of 2023, the DCCT/EDIC participants have been studied for longer than 60% of their lifespans and for over 80% of their diabetes duration, Dr. Braffett noted.
During the EDIC 2017-2022 cycle, Dr. Braffett and other speakers summarized, prior EDIC efforts had focused on aspects of cognitive function, physical function, and cheiroarthropathy.
Other DCCT/EDIC studies examined the relationship of A1c and diabetes duration in cardiovascular disease risk, the association of microvascular complications with the risk of cardiovascular disease beyond traditional risk factors, and the risk of severe hypoglycemia over the first 30 years of DCCT/EDIC follow-up.
Moreover, the longitudinal eye and kidney assessments over the 40 years have informed screening guidelines for retinopathy and urinary albumin.
Dr. Nathan said: “Today, the number with horrible complications is very few, but we haven’t erased complications entirely. ... We have this incredibly complete picture of type 1 diabetes that allows us to explore everything. We welcome people to come to us with ideas. That’s the value of this research.”
Dr. Nathan, Ms. Lorenzi, and Dr. Braffett reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADA 2023
Oral IL-23 receptor antagonist for psoriasis promising: Phase 2b study
SINGAPORE – across all doses, compared with placebo, according to results of the FRONTIER 1 trial.
In the 16-week phase 2b study, 255 adults with moderate to severe plaque psoriasis were randomly assigned into six treatment groups: placebo (n = 43), JNJ-2113 25 mg daily (n = 43), 25 mg twice daily (n = 41), 50 mg daily (n = 43), 100 mg daily (n = 43), or 100 mg twice daily (n = 42).
Of those who took the placebo, only 9.3% achieved the study’s primary endpoint of a 75% or greater improvement in the Psoriasis Area and Severity Index (PASI-75) by week 16. This was compared with 78.6% in the group that took the highest dose.
“Additionally, the onset of action was fairly fast: at week 4, more than 20% of patients had achieved PASI 75,” said Robert Bissonnette, MD, CEO of Innovaderm Research in Montreal, who presented the findings during a late-breaker session at the World Congress of Dermatology.
Patients in the remaining groups demonstrated a response that corresponded to dosing level: with 37.2%, 51.2%, 58.1%, and 65.1% achieving PASI-75 in the 25 mg daily, 25 mg twice-daily, 50 mg daily, and 100 mg daily groups, respectively.
“These results are very interesting because in terms of psoriasis treatment, if this is confirmed in phase 3, it would give us an oral alternative that would be selective for IL-23,” said Dr. Bissonnette, referring to the signaling pathway that plays a critical role in the pathogenesis of several immune-mediated inflammatory diseases, including plaque psoriasis.
Although rarely life-threatening, the skin disorder is often intractable to treatment. In recent years, therapies that block IL-23 signaling and downstream inflammatory cytokine production have proven useful. “We have on the market a number of biological agents targeting IL-23 that we use on a regular basis,” said Dr. Bissonnette. “However, there are currently no orally delivered therapies.”
If successful, JNJ-2113 – a first-in-class oral IL-23 antagonist peptide developed by Janssen – could change the treatment paradigm for patients with moderate to severe plaque psoriasis. “When I was first introduced to the concept, I thought it wouldn’t work as it’s a peptide, that it would be digested by the stomach,” he told the audience. “But because of its GI stability and its potency, when you administer it orally, you can detect pharmacological activity.”
A well-tolerated alternative
Participants in the FRONTIER 1 trial were on average about 44 years old and weighed 88.9 kg (195 lb). Most had been living with psoriasis for about 18 years, with a total PASI score of 19.05. In addition, 43.1% had been treated with phototherapy in the past, 22% with biologics, and 78.4% with systemics.
PASI 90 and 100 were among some of the secondary outcomes measured. Similar to the primary outcome of PASI 75, all treatment groups demonstrated a statistically significant dose-response in PASI 90, compared with placebo. For those on the highest dose of JNJ-2113, 59.5% and 40.5% achieved PASI 90 and PASI 100, respectively, by week 16. The corresponding figures for those receiving placebo were 2.3% and 0%.
The safety profile for JNJ-2113 across all doses was similar to that of placebo, with no evidence of a dose-dependent increase in the occurrence of adverse events (AEs). The most frequently reported AEs were COVID-19 and nasopharyngitis. There were three serious AEs (COVID-19, infected cyst, suicide attempt) among those on the active drug, but the investigators assessed that they were not related to the study intervention. No deaths, major adverse cardiac events, or malignancies were reported during the study.
Approached for an independent comment, Marius-Anton Ionescu, MD, PhD, from the University Hospital Saint Louis, Paris, who specializes in psoriasis, told this news organization that the new development with JNJ-2113 “is really promising.”
Treatment for plaque psoriasis has improved to the point where some biologics, such as risankizumab (Skyrizi), only require patients to have “four shots a year,” he says. “This is the future of psoriasis treatment; it might go down to two shots a year” – a regimen that will be easier than taking an oral medication once or twice a day.
“But it’s good to have an oral option because you will always have some patients who say: ‘Shots are not for me, I’m afraid,’ ” he says.
However, Dr. Ionescu noted that if JNJ-2113 were to pass phase 3 trials, it might face stiff competition from the selective tyrosine kinase 2 (TYK2) inhibitor deucravacitinib (Sotyktu), which the U.S. Food and Drug Administration approved for use in adults with moderate to severe plaque psoriasis last September. “It has very good results and is the first oral therapy that is comparable with biologics for plaque psoriasis,” he says.
But Dr. Bissonnette remains hopeful for the future. “I think JNJ-2113 goes way beyond psoriasis because this type of strategy using oral peptide–blocking receptors could be used in other immune-mediated diseases, including atopic dermatitis and other diseases outside of dermatology.” In addition to running a phase 3 study for moderate to severe plaque psoriasis, Janssen is planning to initiate a phase 2b clinical trial of JNJ-2113 in adults with ulcerative colitis.
The study was funded by Janssen. Dr. Bissonnette reports consulting and investigating for Janssen, and being on advisory panels and receiving research funding from multiple other pharmaceutical companies. Dr. Ionescu is an investigator for Psoriasis National Register France Psobioteq (no honoraria), and an investigator and speaker for Uriage cosmetics (honoraria).
A version of this article first appeared on Medscape.com.
SINGAPORE – across all doses, compared with placebo, according to results of the FRONTIER 1 trial.
In the 16-week phase 2b study, 255 adults with moderate to severe plaque psoriasis were randomly assigned into six treatment groups: placebo (n = 43), JNJ-2113 25 mg daily (n = 43), 25 mg twice daily (n = 41), 50 mg daily (n = 43), 100 mg daily (n = 43), or 100 mg twice daily (n = 42).
Of those who took the placebo, only 9.3% achieved the study’s primary endpoint of a 75% or greater improvement in the Psoriasis Area and Severity Index (PASI-75) by week 16. This was compared with 78.6% in the group that took the highest dose.
“Additionally, the onset of action was fairly fast: at week 4, more than 20% of patients had achieved PASI 75,” said Robert Bissonnette, MD, CEO of Innovaderm Research in Montreal, who presented the findings during a late-breaker session at the World Congress of Dermatology.
Patients in the remaining groups demonstrated a response that corresponded to dosing level: with 37.2%, 51.2%, 58.1%, and 65.1% achieving PASI-75 in the 25 mg daily, 25 mg twice-daily, 50 mg daily, and 100 mg daily groups, respectively.
“These results are very interesting because in terms of psoriasis treatment, if this is confirmed in phase 3, it would give us an oral alternative that would be selective for IL-23,” said Dr. Bissonnette, referring to the signaling pathway that plays a critical role in the pathogenesis of several immune-mediated inflammatory diseases, including plaque psoriasis.
Although rarely life-threatening, the skin disorder is often intractable to treatment. In recent years, therapies that block IL-23 signaling and downstream inflammatory cytokine production have proven useful. “We have on the market a number of biological agents targeting IL-23 that we use on a regular basis,” said Dr. Bissonnette. “However, there are currently no orally delivered therapies.”
If successful, JNJ-2113 – a first-in-class oral IL-23 antagonist peptide developed by Janssen – could change the treatment paradigm for patients with moderate to severe plaque psoriasis. “When I was first introduced to the concept, I thought it wouldn’t work as it’s a peptide, that it would be digested by the stomach,” he told the audience. “But because of its GI stability and its potency, when you administer it orally, you can detect pharmacological activity.”
A well-tolerated alternative
Participants in the FRONTIER 1 trial were on average about 44 years old and weighed 88.9 kg (195 lb). Most had been living with psoriasis for about 18 years, with a total PASI score of 19.05. In addition, 43.1% had been treated with phototherapy in the past, 22% with biologics, and 78.4% with systemics.
PASI 90 and 100 were among some of the secondary outcomes measured. Similar to the primary outcome of PASI 75, all treatment groups demonstrated a statistically significant dose-response in PASI 90, compared with placebo. For those on the highest dose of JNJ-2113, 59.5% and 40.5% achieved PASI 90 and PASI 100, respectively, by week 16. The corresponding figures for those receiving placebo were 2.3% and 0%.
The safety profile for JNJ-2113 across all doses was similar to that of placebo, with no evidence of a dose-dependent increase in the occurrence of adverse events (AEs). The most frequently reported AEs were COVID-19 and nasopharyngitis. There were three serious AEs (COVID-19, infected cyst, suicide attempt) among those on the active drug, but the investigators assessed that they were not related to the study intervention. No deaths, major adverse cardiac events, or malignancies were reported during the study.
Approached for an independent comment, Marius-Anton Ionescu, MD, PhD, from the University Hospital Saint Louis, Paris, who specializes in psoriasis, told this news organization that the new development with JNJ-2113 “is really promising.”
Treatment for plaque psoriasis has improved to the point where some biologics, such as risankizumab (Skyrizi), only require patients to have “four shots a year,” he says. “This is the future of psoriasis treatment; it might go down to two shots a year” – a regimen that will be easier than taking an oral medication once or twice a day.
“But it’s good to have an oral option because you will always have some patients who say: ‘Shots are not for me, I’m afraid,’ ” he says.
However, Dr. Ionescu noted that if JNJ-2113 were to pass phase 3 trials, it might face stiff competition from the selective tyrosine kinase 2 (TYK2) inhibitor deucravacitinib (Sotyktu), which the U.S. Food and Drug Administration approved for use in adults with moderate to severe plaque psoriasis last September. “It has very good results and is the first oral therapy that is comparable with biologics for plaque psoriasis,” he says.
But Dr. Bissonnette remains hopeful for the future. “I think JNJ-2113 goes way beyond psoriasis because this type of strategy using oral peptide–blocking receptors could be used in other immune-mediated diseases, including atopic dermatitis and other diseases outside of dermatology.” In addition to running a phase 3 study for moderate to severe plaque psoriasis, Janssen is planning to initiate a phase 2b clinical trial of JNJ-2113 in adults with ulcerative colitis.
The study was funded by Janssen. Dr. Bissonnette reports consulting and investigating for Janssen, and being on advisory panels and receiving research funding from multiple other pharmaceutical companies. Dr. Ionescu is an investigator for Psoriasis National Register France Psobioteq (no honoraria), and an investigator and speaker for Uriage cosmetics (honoraria).
A version of this article first appeared on Medscape.com.
SINGAPORE – across all doses, compared with placebo, according to results of the FRONTIER 1 trial.
In the 16-week phase 2b study, 255 adults with moderate to severe plaque psoriasis were randomly assigned into six treatment groups: placebo (n = 43), JNJ-2113 25 mg daily (n = 43), 25 mg twice daily (n = 41), 50 mg daily (n = 43), 100 mg daily (n = 43), or 100 mg twice daily (n = 42).
Of those who took the placebo, only 9.3% achieved the study’s primary endpoint of a 75% or greater improvement in the Psoriasis Area and Severity Index (PASI-75) by week 16. This was compared with 78.6% in the group that took the highest dose.
“Additionally, the onset of action was fairly fast: at week 4, more than 20% of patients had achieved PASI 75,” said Robert Bissonnette, MD, CEO of Innovaderm Research in Montreal, who presented the findings during a late-breaker session at the World Congress of Dermatology.
Patients in the remaining groups demonstrated a response that corresponded to dosing level: with 37.2%, 51.2%, 58.1%, and 65.1% achieving PASI-75 in the 25 mg daily, 25 mg twice-daily, 50 mg daily, and 100 mg daily groups, respectively.
“These results are very interesting because in terms of psoriasis treatment, if this is confirmed in phase 3, it would give us an oral alternative that would be selective for IL-23,” said Dr. Bissonnette, referring to the signaling pathway that plays a critical role in the pathogenesis of several immune-mediated inflammatory diseases, including plaque psoriasis.
Although rarely life-threatening, the skin disorder is often intractable to treatment. In recent years, therapies that block IL-23 signaling and downstream inflammatory cytokine production have proven useful. “We have on the market a number of biological agents targeting IL-23 that we use on a regular basis,” said Dr. Bissonnette. “However, there are currently no orally delivered therapies.”
If successful, JNJ-2113 – a first-in-class oral IL-23 antagonist peptide developed by Janssen – could change the treatment paradigm for patients with moderate to severe plaque psoriasis. “When I was first introduced to the concept, I thought it wouldn’t work as it’s a peptide, that it would be digested by the stomach,” he told the audience. “But because of its GI stability and its potency, when you administer it orally, you can detect pharmacological activity.”
A well-tolerated alternative
Participants in the FRONTIER 1 trial were on average about 44 years old and weighed 88.9 kg (195 lb). Most had been living with psoriasis for about 18 years, with a total PASI score of 19.05. In addition, 43.1% had been treated with phototherapy in the past, 22% with biologics, and 78.4% with systemics.
PASI 90 and 100 were among some of the secondary outcomes measured. Similar to the primary outcome of PASI 75, all treatment groups demonstrated a statistically significant dose-response in PASI 90, compared with placebo. For those on the highest dose of JNJ-2113, 59.5% and 40.5% achieved PASI 90 and PASI 100, respectively, by week 16. The corresponding figures for those receiving placebo were 2.3% and 0%.
The safety profile for JNJ-2113 across all doses was similar to that of placebo, with no evidence of a dose-dependent increase in the occurrence of adverse events (AEs). The most frequently reported AEs were COVID-19 and nasopharyngitis. There were three serious AEs (COVID-19, infected cyst, suicide attempt) among those on the active drug, but the investigators assessed that they were not related to the study intervention. No deaths, major adverse cardiac events, or malignancies were reported during the study.
Approached for an independent comment, Marius-Anton Ionescu, MD, PhD, from the University Hospital Saint Louis, Paris, who specializes in psoriasis, told this news organization that the new development with JNJ-2113 “is really promising.”
Treatment for plaque psoriasis has improved to the point where some biologics, such as risankizumab (Skyrizi), only require patients to have “four shots a year,” he says. “This is the future of psoriasis treatment; it might go down to two shots a year” – a regimen that will be easier than taking an oral medication once or twice a day.
“But it’s good to have an oral option because you will always have some patients who say: ‘Shots are not for me, I’m afraid,’ ” he says.
However, Dr. Ionescu noted that if JNJ-2113 were to pass phase 3 trials, it might face stiff competition from the selective tyrosine kinase 2 (TYK2) inhibitor deucravacitinib (Sotyktu), which the U.S. Food and Drug Administration approved for use in adults with moderate to severe plaque psoriasis last September. “It has very good results and is the first oral therapy that is comparable with biologics for plaque psoriasis,” he says.
But Dr. Bissonnette remains hopeful for the future. “I think JNJ-2113 goes way beyond psoriasis because this type of strategy using oral peptide–blocking receptors could be used in other immune-mediated diseases, including atopic dermatitis and other diseases outside of dermatology.” In addition to running a phase 3 study for moderate to severe plaque psoriasis, Janssen is planning to initiate a phase 2b clinical trial of JNJ-2113 in adults with ulcerative colitis.
The study was funded by Janssen. Dr. Bissonnette reports consulting and investigating for Janssen, and being on advisory panels and receiving research funding from multiple other pharmaceutical companies. Dr. Ionescu is an investigator for Psoriasis National Register France Psobioteq (no honoraria), and an investigator and speaker for Uriage cosmetics (honoraria).
A version of this article first appeared on Medscape.com.
AT WCD 2023
JAK inhibitors efficacious for atopic dermatitis in Asian patients, study finds
SINGAPORE – conducted in Singapore has found.
“Abrocitinib and upadacitinib surprisingly appeared to have better treatment efficacy compared to baricitinib,” said study lead Yik Weng Yew, MD, PhD, MPH, deputy head of research at Singapore’s National Skin Centre (NSC), who presented the results at the 25th World Congress of Dermatology. “But overall, as a group, I think they show a very good treatment response, as well as a good effect on itch response.”
JAK inhibitors are used to treat a variety of inflammatory diseases including alopecia areata, rheumatoid arthritis, and inflammatory bowel disease. Although treatment for severe eczema was previously limited to topical steroids and oral immunosuppressants, there are now two oral JAK inhibitors – abrocitinib and upadacitinib – approved in 2022 by the Food and Drug Administration for treating AD, which affects up to 2.4% of the global population. (A topical formulation of ruxolitinib, a JAK inhibitor, was approved for AD in 2021.)
The Singapore study is one of the few that have examined the safety and efficacy of JAK inhibitors for treatment of AD in a non-White population.
Chinese population
For the 12-week trial, conducted in 2022, Dr. Yew and associates recruited 35 patients from the NSC. More than half of participants (64%) were men and most (96%) were of Chinese ethnicity. Four of every five patients had previously received systemic agents: 17% had been treated with one systemic agent, 18.9% with two, 15.1% with three, 22.6% with four, and 3.8% with five. The most commonly used agents were cyclosporine (62.3%), methotrexate (47.2%), azathioprine (39.6%), and dupilumab (35.8%).
“The switch in therapy could have been a result of inadequate efficacy or cost reasons because in Singapore patients pay out of pocket for AD treatments,” said Dr. Yew.
Additionally, he offered a caveat on the profile of participants: “Perhaps they were more difficult atopic eczema patients, and therefore, the efficacy [of JAK inhibitors] might be a bit different.”
Clearer skin, less itch
Patients received one of the three study drugs: baricitinib (66%), abrocitinib (21%), and upadacitinib (13%). The distribution was “affected by reimbursement patterns and availability of the drug,” explained Dr. Yew.
They were assessed at weeks 4 and 12. By study end, the proportion of patients who self-reported an improvement in their condition was 100% for upadacitinib, 90% for abrocitinib, and 69% for baricitinib.
Scores on the Investigator Global Assessment (IGA) also improved with treatment. Patients in the baricitinib group saw their mean score fall from 4.0 to 3.0 by week 4, then to 2.0 by week 12. With upadacitinib and abrocitinib, “you can see that there is a nice decrease in IGA responses,” said Dr. Yew, referring to the larger improvement in scores experienced by patients on those two treatments. For patients on upadacitinib, IGA decreased from 3.5 to 2 at 4 weeks, then to 0.5 at 12 weeks, while those taking abrocitinib had their scores drop from 4.0 to 2.0 at 4 weeks, then to 1.0 at 12 weeks.
When it came to itch reduction, the abrocitinib group experienced the biggest reduction, with a median reduction of 5.5 points in itch score. Median reduction in itch score was 4 points for the other two groups. “Oral JAK inhibitors appear to have a good effect on itch response,” said Dr. Yew.
However, the researchers observed no significant reduction in percentage of body surface area affected, the last outcome assessed.
The most commonly reported adverse events were increased creatine kinase levels (11.3% of patients), increased LDL cholesterol levels (9.4%), and herpes zoster (9.4%). Those in the abrocitinib reported a higher number of these adverse events, compared with the other two treatment groups. (There were no herpes zoster cases among those taking baricitinib.)
For herpes zoster, Dr. Yew said “the common recommendation” is to give the inactivated shingles vaccine. “But the problem is that, number one, these patients would have probably failed multiple agents so they probably can’t wait for you to vaccinate before you initiate treatment.”
In addition, people in Singapore have to pay out-of-pocket for the two vaccine doses, “which is probably a month’s worth of medication,” he noted. “So we have a lot of resistance from patients.”
Additionally, Dr. Yew noted that contrary to what has previously been reported in the literature, there were few complaints of acne as a side effect in the Singaporean study population.
Toward greater representation
Dr. Yew pointed out that the study was limited by a few factors: neither the Eczema Area and Severity Index or Scoring of Atopic Dermatitis index data was used, and the study population was small and not representative of the real world.
Still, the new findings contribute to the overall safety and efficacy profile of JAK inhibitors in AD, which has so far been scarce in non-White populations.
“In Western studies, unfortunately, the representation of the population of skin of color or different ethnicities is underrepresented,” said Yousef Binamer, MD, chair of the dermatology department at King Faisal Specialist Hospital, Riyadh, Saudi Arabia, when approached for an independent comment on the results.
“This is now why researchers are looking into specific groups to study them,” which he pointed out, is crucial because “the immunophenotyping of AD is different for each background.”
The incidence and severity of AD tend to be higher in Asian and Middle Eastern populations, for instance, he noted. “It’s very common in Asia, and not so common in very white skin. I did my training in Canada so I see the difference,” said Dr. Binamer. “Asian people tend to be more itchy and have a tendency to scar on pigmentation.” Whereas White people “usually do not have this issue.”
“So I think real-world evidence of JAK inhibitors in the other populations is important,” he said. Studies such as the one conducted in Singapore, as well as the recently reported QUARTZ3 study, which examined the use of the JAK inhibitor ivarmacitinib in 256 Chinese patients with AD, are helping to pave the way.
The study was independently supported. Dr. Yew and Dr. Binamer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SINGAPORE – conducted in Singapore has found.
“Abrocitinib and upadacitinib surprisingly appeared to have better treatment efficacy compared to baricitinib,” said study lead Yik Weng Yew, MD, PhD, MPH, deputy head of research at Singapore’s National Skin Centre (NSC), who presented the results at the 25th World Congress of Dermatology. “But overall, as a group, I think they show a very good treatment response, as well as a good effect on itch response.”
JAK inhibitors are used to treat a variety of inflammatory diseases including alopecia areata, rheumatoid arthritis, and inflammatory bowel disease. Although treatment for severe eczema was previously limited to topical steroids and oral immunosuppressants, there are now two oral JAK inhibitors – abrocitinib and upadacitinib – approved in 2022 by the Food and Drug Administration for treating AD, which affects up to 2.4% of the global population. (A topical formulation of ruxolitinib, a JAK inhibitor, was approved for AD in 2021.)
The Singapore study is one of the few that have examined the safety and efficacy of JAK inhibitors for treatment of AD in a non-White population.
Chinese population
For the 12-week trial, conducted in 2022, Dr. Yew and associates recruited 35 patients from the NSC. More than half of participants (64%) were men and most (96%) were of Chinese ethnicity. Four of every five patients had previously received systemic agents: 17% had been treated with one systemic agent, 18.9% with two, 15.1% with three, 22.6% with four, and 3.8% with five. The most commonly used agents were cyclosporine (62.3%), methotrexate (47.2%), azathioprine (39.6%), and dupilumab (35.8%).
“The switch in therapy could have been a result of inadequate efficacy or cost reasons because in Singapore patients pay out of pocket for AD treatments,” said Dr. Yew.
Additionally, he offered a caveat on the profile of participants: “Perhaps they were more difficult atopic eczema patients, and therefore, the efficacy [of JAK inhibitors] might be a bit different.”
Clearer skin, less itch
Patients received one of the three study drugs: baricitinib (66%), abrocitinib (21%), and upadacitinib (13%). The distribution was “affected by reimbursement patterns and availability of the drug,” explained Dr. Yew.
They were assessed at weeks 4 and 12. By study end, the proportion of patients who self-reported an improvement in their condition was 100% for upadacitinib, 90% for abrocitinib, and 69% for baricitinib.
Scores on the Investigator Global Assessment (IGA) also improved with treatment. Patients in the baricitinib group saw their mean score fall from 4.0 to 3.0 by week 4, then to 2.0 by week 12. With upadacitinib and abrocitinib, “you can see that there is a nice decrease in IGA responses,” said Dr. Yew, referring to the larger improvement in scores experienced by patients on those two treatments. For patients on upadacitinib, IGA decreased from 3.5 to 2 at 4 weeks, then to 0.5 at 12 weeks, while those taking abrocitinib had their scores drop from 4.0 to 2.0 at 4 weeks, then to 1.0 at 12 weeks.
When it came to itch reduction, the abrocitinib group experienced the biggest reduction, with a median reduction of 5.5 points in itch score. Median reduction in itch score was 4 points for the other two groups. “Oral JAK inhibitors appear to have a good effect on itch response,” said Dr. Yew.
However, the researchers observed no significant reduction in percentage of body surface area affected, the last outcome assessed.
The most commonly reported adverse events were increased creatine kinase levels (11.3% of patients), increased LDL cholesterol levels (9.4%), and herpes zoster (9.4%). Those in the abrocitinib reported a higher number of these adverse events, compared with the other two treatment groups. (There were no herpes zoster cases among those taking baricitinib.)
For herpes zoster, Dr. Yew said “the common recommendation” is to give the inactivated shingles vaccine. “But the problem is that, number one, these patients would have probably failed multiple agents so they probably can’t wait for you to vaccinate before you initiate treatment.”
In addition, people in Singapore have to pay out-of-pocket for the two vaccine doses, “which is probably a month’s worth of medication,” he noted. “So we have a lot of resistance from patients.”
Additionally, Dr. Yew noted that contrary to what has previously been reported in the literature, there were few complaints of acne as a side effect in the Singaporean study population.
Toward greater representation
Dr. Yew pointed out that the study was limited by a few factors: neither the Eczema Area and Severity Index or Scoring of Atopic Dermatitis index data was used, and the study population was small and not representative of the real world.
Still, the new findings contribute to the overall safety and efficacy profile of JAK inhibitors in AD, which has so far been scarce in non-White populations.
“In Western studies, unfortunately, the representation of the population of skin of color or different ethnicities is underrepresented,” said Yousef Binamer, MD, chair of the dermatology department at King Faisal Specialist Hospital, Riyadh, Saudi Arabia, when approached for an independent comment on the results.
“This is now why researchers are looking into specific groups to study them,” which he pointed out, is crucial because “the immunophenotyping of AD is different for each background.”
The incidence and severity of AD tend to be higher in Asian and Middle Eastern populations, for instance, he noted. “It’s very common in Asia, and not so common in very white skin. I did my training in Canada so I see the difference,” said Dr. Binamer. “Asian people tend to be more itchy and have a tendency to scar on pigmentation.” Whereas White people “usually do not have this issue.”
“So I think real-world evidence of JAK inhibitors in the other populations is important,” he said. Studies such as the one conducted in Singapore, as well as the recently reported QUARTZ3 study, which examined the use of the JAK inhibitor ivarmacitinib in 256 Chinese patients with AD, are helping to pave the way.
The study was independently supported. Dr. Yew and Dr. Binamer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SINGAPORE – conducted in Singapore has found.
“Abrocitinib and upadacitinib surprisingly appeared to have better treatment efficacy compared to baricitinib,” said study lead Yik Weng Yew, MD, PhD, MPH, deputy head of research at Singapore’s National Skin Centre (NSC), who presented the results at the 25th World Congress of Dermatology. “But overall, as a group, I think they show a very good treatment response, as well as a good effect on itch response.”
JAK inhibitors are used to treat a variety of inflammatory diseases including alopecia areata, rheumatoid arthritis, and inflammatory bowel disease. Although treatment for severe eczema was previously limited to topical steroids and oral immunosuppressants, there are now two oral JAK inhibitors – abrocitinib and upadacitinib – approved in 2022 by the Food and Drug Administration for treating AD, which affects up to 2.4% of the global population. (A topical formulation of ruxolitinib, a JAK inhibitor, was approved for AD in 2021.)
The Singapore study is one of the few that have examined the safety and efficacy of JAK inhibitors for treatment of AD in a non-White population.
Chinese population
For the 12-week trial, conducted in 2022, Dr. Yew and associates recruited 35 patients from the NSC. More than half of participants (64%) were men and most (96%) were of Chinese ethnicity. Four of every five patients had previously received systemic agents: 17% had been treated with one systemic agent, 18.9% with two, 15.1% with three, 22.6% with four, and 3.8% with five. The most commonly used agents were cyclosporine (62.3%), methotrexate (47.2%), azathioprine (39.6%), and dupilumab (35.8%).
“The switch in therapy could have been a result of inadequate efficacy or cost reasons because in Singapore patients pay out of pocket for AD treatments,” said Dr. Yew.
Additionally, he offered a caveat on the profile of participants: “Perhaps they were more difficult atopic eczema patients, and therefore, the efficacy [of JAK inhibitors] might be a bit different.”
Clearer skin, less itch
Patients received one of the three study drugs: baricitinib (66%), abrocitinib (21%), and upadacitinib (13%). The distribution was “affected by reimbursement patterns and availability of the drug,” explained Dr. Yew.
They were assessed at weeks 4 and 12. By study end, the proportion of patients who self-reported an improvement in their condition was 100% for upadacitinib, 90% for abrocitinib, and 69% for baricitinib.
Scores on the Investigator Global Assessment (IGA) also improved with treatment. Patients in the baricitinib group saw their mean score fall from 4.0 to 3.0 by week 4, then to 2.0 by week 12. With upadacitinib and abrocitinib, “you can see that there is a nice decrease in IGA responses,” said Dr. Yew, referring to the larger improvement in scores experienced by patients on those two treatments. For patients on upadacitinib, IGA decreased from 3.5 to 2 at 4 weeks, then to 0.5 at 12 weeks, while those taking abrocitinib had their scores drop from 4.0 to 2.0 at 4 weeks, then to 1.0 at 12 weeks.
When it came to itch reduction, the abrocitinib group experienced the biggest reduction, with a median reduction of 5.5 points in itch score. Median reduction in itch score was 4 points for the other two groups. “Oral JAK inhibitors appear to have a good effect on itch response,” said Dr. Yew.
However, the researchers observed no significant reduction in percentage of body surface area affected, the last outcome assessed.
The most commonly reported adverse events were increased creatine kinase levels (11.3% of patients), increased LDL cholesterol levels (9.4%), and herpes zoster (9.4%). Those in the abrocitinib reported a higher number of these adverse events, compared with the other two treatment groups. (There were no herpes zoster cases among those taking baricitinib.)
For herpes zoster, Dr. Yew said “the common recommendation” is to give the inactivated shingles vaccine. “But the problem is that, number one, these patients would have probably failed multiple agents so they probably can’t wait for you to vaccinate before you initiate treatment.”
In addition, people in Singapore have to pay out-of-pocket for the two vaccine doses, “which is probably a month’s worth of medication,” he noted. “So we have a lot of resistance from patients.”
Additionally, Dr. Yew noted that contrary to what has previously been reported in the literature, there were few complaints of acne as a side effect in the Singaporean study population.
Toward greater representation
Dr. Yew pointed out that the study was limited by a few factors: neither the Eczema Area and Severity Index or Scoring of Atopic Dermatitis index data was used, and the study population was small and not representative of the real world.
Still, the new findings contribute to the overall safety and efficacy profile of JAK inhibitors in AD, which has so far been scarce in non-White populations.
“In Western studies, unfortunately, the representation of the population of skin of color or different ethnicities is underrepresented,” said Yousef Binamer, MD, chair of the dermatology department at King Faisal Specialist Hospital, Riyadh, Saudi Arabia, when approached for an independent comment on the results.
“This is now why researchers are looking into specific groups to study them,” which he pointed out, is crucial because “the immunophenotyping of AD is different for each background.”
The incidence and severity of AD tend to be higher in Asian and Middle Eastern populations, for instance, he noted. “It’s very common in Asia, and not so common in very white skin. I did my training in Canada so I see the difference,” said Dr. Binamer. “Asian people tend to be more itchy and have a tendency to scar on pigmentation.” Whereas White people “usually do not have this issue.”
“So I think real-world evidence of JAK inhibitors in the other populations is important,” he said. Studies such as the one conducted in Singapore, as well as the recently reported QUARTZ3 study, which examined the use of the JAK inhibitor ivarmacitinib in 256 Chinese patients with AD, are helping to pave the way.
The study was independently supported. Dr. Yew and Dr. Binamer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT WCD 2023