User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Heart-protective diet in PURE study allows whole-fat dairy
Most of the protective food categories are in line with standard dietary guidelines for good health, but one that may be heart-protective is not usually included in such recommendations.
The food categories that were found to be protective include fruit, vegetables, nuts, legumes, and fish but also dairy, “mainly whole-fat,” in an analysis based on the international Prospective Urban and Rural Epidemiological (PURE) study and data from five other international trials that encompassed more than 240,000 people.
A healthy diet scoring system was derived from dietary patterns and clinical events observed in the PURE study and was applied to the populations of the other trials. Higher scores, corresponding to greater consumption of the six food categories, tracked with significantly reduced risks for death, myocardial infarction (MI), and stroke.
Reductions in mortality and CV-disease risk that were linked to the higher scores were especially pronounced in lower-income countries in the study published onlinein the European Heart Journal with lead author Andrew Mente, PhD, Population Health Research Institute, McMaster University, Hamilton, Ont.
The study in part refutes the frequent preference for low-fat or no-fat dairy foods over whole-fat dairy in healthy-diet recommendations. But it is consistent with earlier findings from PURE of reduced mortality risk with increased consumption of dietary fat, including saturated fat.
Whereas healthy-diet recommendations tend to emphasize reduced intake of fat, especially saturated fat, the report notes that “there are almost no national or international strategies and policies to increase a number of protective foods,” such as nuts, fish, and dairy.
“Therefore, while the findings from PURE are largely consistent with the nutrition science and modern dietary recommendations to focus on protective foods, the public’s understanding of healthy eating and relevant global policies have not yet caught up to this science,” it states.
“Guidelines and policy actions need to be updated with this newer evidence,” Dr. Mente said in an interview. “For example, the World Health Organization remains mainly focused on reducing certain nutrients, such as fat, saturated fat, added sugar, and salt,” he said. “These recommendations are echoed by government policy actions and industry, as evident by the continued focus on the usual nutrients in food labels of many countries.”
The current findings, Dr. Mente said, “can be used to ensure that the public’s understanding of healthy eating and relevant global policies are able to catch up to the science.”
Healthy diet score
PURE investigators developed their healthy diet score using data from 147,642 people from the general population in 21 countries. The investigators compared self-reported dietary intakes with long-term clinical outcomes.
The scoring system assigned a value of 1 for each of the six health-food categories when individuals’ intake exceeded the entire cohort’s median intake. It assigned a 0 when intake was below the median. The total PURE healthy diet score consisted of the sum of the six values, with higher scores corresponding to a healthier diet. The mean score for cohort was 2.95.
There were 15,707 deaths and 40,764 CV events during a median follow-up of 9.3 years. A score of at least 5 points, compared with 0 or 1 point, was associated with significantly reduced hazard ratios for mortality, MI, and stroke in multivariable analysis:
- Mortality: HR, 0.70 (95% CI, 0.63-0.77; P < .0001).
- Major CV disease: HR, 0.82 (95% CI, 0.75-0.91; P < .0001).
- MI: HR, 0.86 (95% CI, 0.75-0.99; P = .0014).
- Stroke: HR, 0.81 (95% CI, 0.71-0.93; P = .0034).
The healthy diet score’s relationship to clinical outcomes was explored in five other large independent studies, including three prospective trials of patients with CV disease that spanned 50 countries, a case-control study with MI patients in 52 countries, and a case-control study with stroke patients in 33 countries.
In the three prospective trials, higher scores were associated with reduced mortality, CV disease events, and MI:
- Mortality: HR, 0.73 (95% CI, 0.66-0.81).
- Major CV disease: HR, 0.79 (95% CI, 0.72-0.87).
- MI: HR, 0.85 (95% CI, 0.71-0.99).
In the two case-control studies, a higher diet score was associated with reduced odds ratios for first MI and for stroke:
- MI: OR, 0.72 (95% CI, 0.65-0.80).
- Stroke: OR, 0.57 (95% CI, 0.50-0.65).
In an analysis based on the PURE cohort, incorporation of unprocessed red meat or whole grains into the health diet score produced similar results, suggesting that a “modest amount” of meat or whole grains can be part of a healthy diet, the authors contend.
The results were similar in a combined analysis of all the prospective studies. In particular, improvement in diet score by one quintile was associated with significantly reduced risks for the following:
- Mortality: HR, 0.92 (95% CI, 0.90-0.93).
- Major CV disease: HR, 0.94 (95% CI, 0.93-0.95).
- MI: HR, 0.94 (95% CI, 0.92-0.96).
- Stroke: HR, 0.94 (95% CI, 0.89-0.99).
- Death or CV disease: HR, 0.93 (95% CI, 0.92-0.94).
“This strongly indicates that the take-home message for patients is the same as for general populations,” Dr. Mente said. “Eat plenty of fruits, vegetables, nuts, legumes, and a moderate amount of fish and whole-fat dairy to lower risk of CV disease and mortality.”
Dairy foods are not widely consumed in some cultures, he said, “but availability and cost are also factors in determining consumption.” Nonetheless, a high-quality diet can be achieved without including or excluding dairy foods. Context-specific policies and priorities are needed for different populations, “rather than a one-size-fits-all global policy.”
Food labels in many countries mainly focus on “reducing certain nutrients as the end-all, be-all,” Dr. Mente observed. “Our findings can be used as a basis for recommendations regarding what a healthy diet should be globally and then modified for each region based on the specific types of foods that are available and affordable in each region.”
Moreover, he said, “targeted food policies are needed to increase the availability and affordability of healthy foods, especially in lower-income countries where intakes are low.”
Common human biology
The current results from PURE “confirm prior observations from mostly Western nations that low intakes of fruits, vegetables, nuts, legumes, and fish are major risk factors for poor health,” observes Dariush Mozaffarian, MD, DrPH, MPH, Tufts University, Boston, in an accompanying editorial. “This suggests that common human biology, not merely confounding, explains these observed diet–disease relationships, strengthening causal inference on the power of nutrition.”
Moreover, “These findings provide further support that dairy foods, including whole-fat dairy, can be part of a healthy diet,” Dr. Mozaffarian writes. “The new results in PURE, in combination with prior reports, call for a re-evaluation of unrelenting guidelines to avoid whole-fat dairy products.”
Such studies “remind us of the continuing and devastating rise in diet-related chronic diseases globally, and of the power of protective foods to help address these burdens,” the editorial continues. “It is time for national nutrition guidelines, private sector innovations, government tax policy and agricultural incentives, food procurement policies, labeling and other regulatory priorities, and food-based health care interventions to catch up to the science.”
Not automatically superior
“I do not believe guidelines should be changed based on this single study,” contends Howard D. Sesso, ScD, MPH, associate director of the division of preventive medicine at Brigham and Women’s Hospital, Boston, who isn’t part of PURE. “But I welcome the scientific dialog that should come out of any study that challenges what we think we know,” he told this news organization.
“Many other dietary patterns have been identified over the years that also do a great job in predicting disease risk in observational studies,” observed Dr. Sesso. “Is PURE that much better? Maybe, maybe not. But not enough to dismiss other dietary patterns that are already the basis of dietary recommendations in the U.S., Europe, and worldwide.”
The PURE healthy diet score, he said, “appears to work well within the confines of their large pooling of studies around the world, but that doesn’t automatically make it superior to other dietary patterns.” The score “was only modestly, but not greatly, better than existing dietary patterns evaluated.”
Randomized controlled trials are needed, Dr. Sesso said, to “delve into more specific dietary components,” including unprocessed red meat, whole grains, and high-fat dairy foods. And, he said, more observational studies are needed to examine the score’s association with other cardiometabolic outcomes.
The PURE study is funded by the Population Health Research Institute, the Hamilton Health Sciences Research Institute, the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario; with support from Canadian Institutes of Health Research’s Strategy for Patient Oriented Research through the Ontario SPOR Support Unit, as well as the Ontario Ministry of Health and Long-Term Care; and through unrestricted grants from several pharmaceutical companies, with major contributions from AstraZeneca, Sanofi-Aventis, Boehringer Ingelheim, Servier, and GlaxoSmithKline. Additional contributions are from Novartis and King Pharma. Dr. Mente, Dr. Mozaffarian, and Dr. Sesso have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most of the protective food categories are in line with standard dietary guidelines for good health, but one that may be heart-protective is not usually included in such recommendations.
The food categories that were found to be protective include fruit, vegetables, nuts, legumes, and fish but also dairy, “mainly whole-fat,” in an analysis based on the international Prospective Urban and Rural Epidemiological (PURE) study and data from five other international trials that encompassed more than 240,000 people.
A healthy diet scoring system was derived from dietary patterns and clinical events observed in the PURE study and was applied to the populations of the other trials. Higher scores, corresponding to greater consumption of the six food categories, tracked with significantly reduced risks for death, myocardial infarction (MI), and stroke.
Reductions in mortality and CV-disease risk that were linked to the higher scores were especially pronounced in lower-income countries in the study published onlinein the European Heart Journal with lead author Andrew Mente, PhD, Population Health Research Institute, McMaster University, Hamilton, Ont.
The study in part refutes the frequent preference for low-fat or no-fat dairy foods over whole-fat dairy in healthy-diet recommendations. But it is consistent with earlier findings from PURE of reduced mortality risk with increased consumption of dietary fat, including saturated fat.
Whereas healthy-diet recommendations tend to emphasize reduced intake of fat, especially saturated fat, the report notes that “there are almost no national or international strategies and policies to increase a number of protective foods,” such as nuts, fish, and dairy.
“Therefore, while the findings from PURE are largely consistent with the nutrition science and modern dietary recommendations to focus on protective foods, the public’s understanding of healthy eating and relevant global policies have not yet caught up to this science,” it states.
“Guidelines and policy actions need to be updated with this newer evidence,” Dr. Mente said in an interview. “For example, the World Health Organization remains mainly focused on reducing certain nutrients, such as fat, saturated fat, added sugar, and salt,” he said. “These recommendations are echoed by government policy actions and industry, as evident by the continued focus on the usual nutrients in food labels of many countries.”
The current findings, Dr. Mente said, “can be used to ensure that the public’s understanding of healthy eating and relevant global policies are able to catch up to the science.”
Healthy diet score
PURE investigators developed their healthy diet score using data from 147,642 people from the general population in 21 countries. The investigators compared self-reported dietary intakes with long-term clinical outcomes.
The scoring system assigned a value of 1 for each of the six health-food categories when individuals’ intake exceeded the entire cohort’s median intake. It assigned a 0 when intake was below the median. The total PURE healthy diet score consisted of the sum of the six values, with higher scores corresponding to a healthier diet. The mean score for cohort was 2.95.
There were 15,707 deaths and 40,764 CV events during a median follow-up of 9.3 years. A score of at least 5 points, compared with 0 or 1 point, was associated with significantly reduced hazard ratios for mortality, MI, and stroke in multivariable analysis:
- Mortality: HR, 0.70 (95% CI, 0.63-0.77; P < .0001).
- Major CV disease: HR, 0.82 (95% CI, 0.75-0.91; P < .0001).
- MI: HR, 0.86 (95% CI, 0.75-0.99; P = .0014).
- Stroke: HR, 0.81 (95% CI, 0.71-0.93; P = .0034).
The healthy diet score’s relationship to clinical outcomes was explored in five other large independent studies, including three prospective trials of patients with CV disease that spanned 50 countries, a case-control study with MI patients in 52 countries, and a case-control study with stroke patients in 33 countries.
In the three prospective trials, higher scores were associated with reduced mortality, CV disease events, and MI:
- Mortality: HR, 0.73 (95% CI, 0.66-0.81).
- Major CV disease: HR, 0.79 (95% CI, 0.72-0.87).
- MI: HR, 0.85 (95% CI, 0.71-0.99).
In the two case-control studies, a higher diet score was associated with reduced odds ratios for first MI and for stroke:
- MI: OR, 0.72 (95% CI, 0.65-0.80).
- Stroke: OR, 0.57 (95% CI, 0.50-0.65).
In an analysis based on the PURE cohort, incorporation of unprocessed red meat or whole grains into the health diet score produced similar results, suggesting that a “modest amount” of meat or whole grains can be part of a healthy diet, the authors contend.
The results were similar in a combined analysis of all the prospective studies. In particular, improvement in diet score by one quintile was associated with significantly reduced risks for the following:
- Mortality: HR, 0.92 (95% CI, 0.90-0.93).
- Major CV disease: HR, 0.94 (95% CI, 0.93-0.95).
- MI: HR, 0.94 (95% CI, 0.92-0.96).
- Stroke: HR, 0.94 (95% CI, 0.89-0.99).
- Death or CV disease: HR, 0.93 (95% CI, 0.92-0.94).
“This strongly indicates that the take-home message for patients is the same as for general populations,” Dr. Mente said. “Eat plenty of fruits, vegetables, nuts, legumes, and a moderate amount of fish and whole-fat dairy to lower risk of CV disease and mortality.”
Dairy foods are not widely consumed in some cultures, he said, “but availability and cost are also factors in determining consumption.” Nonetheless, a high-quality diet can be achieved without including or excluding dairy foods. Context-specific policies and priorities are needed for different populations, “rather than a one-size-fits-all global policy.”
Food labels in many countries mainly focus on “reducing certain nutrients as the end-all, be-all,” Dr. Mente observed. “Our findings can be used as a basis for recommendations regarding what a healthy diet should be globally and then modified for each region based on the specific types of foods that are available and affordable in each region.”
Moreover, he said, “targeted food policies are needed to increase the availability and affordability of healthy foods, especially in lower-income countries where intakes are low.”
Common human biology
The current results from PURE “confirm prior observations from mostly Western nations that low intakes of fruits, vegetables, nuts, legumes, and fish are major risk factors for poor health,” observes Dariush Mozaffarian, MD, DrPH, MPH, Tufts University, Boston, in an accompanying editorial. “This suggests that common human biology, not merely confounding, explains these observed diet–disease relationships, strengthening causal inference on the power of nutrition.”
Moreover, “These findings provide further support that dairy foods, including whole-fat dairy, can be part of a healthy diet,” Dr. Mozaffarian writes. “The new results in PURE, in combination with prior reports, call for a re-evaluation of unrelenting guidelines to avoid whole-fat dairy products.”
Such studies “remind us of the continuing and devastating rise in diet-related chronic diseases globally, and of the power of protective foods to help address these burdens,” the editorial continues. “It is time for national nutrition guidelines, private sector innovations, government tax policy and agricultural incentives, food procurement policies, labeling and other regulatory priorities, and food-based health care interventions to catch up to the science.”
Not automatically superior
“I do not believe guidelines should be changed based on this single study,” contends Howard D. Sesso, ScD, MPH, associate director of the division of preventive medicine at Brigham and Women’s Hospital, Boston, who isn’t part of PURE. “But I welcome the scientific dialog that should come out of any study that challenges what we think we know,” he told this news organization.
“Many other dietary patterns have been identified over the years that also do a great job in predicting disease risk in observational studies,” observed Dr. Sesso. “Is PURE that much better? Maybe, maybe not. But not enough to dismiss other dietary patterns that are already the basis of dietary recommendations in the U.S., Europe, and worldwide.”
The PURE healthy diet score, he said, “appears to work well within the confines of their large pooling of studies around the world, but that doesn’t automatically make it superior to other dietary patterns.” The score “was only modestly, but not greatly, better than existing dietary patterns evaluated.”
Randomized controlled trials are needed, Dr. Sesso said, to “delve into more specific dietary components,” including unprocessed red meat, whole grains, and high-fat dairy foods. And, he said, more observational studies are needed to examine the score’s association with other cardiometabolic outcomes.
The PURE study is funded by the Population Health Research Institute, the Hamilton Health Sciences Research Institute, the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario; with support from Canadian Institutes of Health Research’s Strategy for Patient Oriented Research through the Ontario SPOR Support Unit, as well as the Ontario Ministry of Health and Long-Term Care; and through unrestricted grants from several pharmaceutical companies, with major contributions from AstraZeneca, Sanofi-Aventis, Boehringer Ingelheim, Servier, and GlaxoSmithKline. Additional contributions are from Novartis and King Pharma. Dr. Mente, Dr. Mozaffarian, and Dr. Sesso have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most of the protective food categories are in line with standard dietary guidelines for good health, but one that may be heart-protective is not usually included in such recommendations.
The food categories that were found to be protective include fruit, vegetables, nuts, legumes, and fish but also dairy, “mainly whole-fat,” in an analysis based on the international Prospective Urban and Rural Epidemiological (PURE) study and data from five other international trials that encompassed more than 240,000 people.
A healthy diet scoring system was derived from dietary patterns and clinical events observed in the PURE study and was applied to the populations of the other trials. Higher scores, corresponding to greater consumption of the six food categories, tracked with significantly reduced risks for death, myocardial infarction (MI), and stroke.
Reductions in mortality and CV-disease risk that were linked to the higher scores were especially pronounced in lower-income countries in the study published onlinein the European Heart Journal with lead author Andrew Mente, PhD, Population Health Research Institute, McMaster University, Hamilton, Ont.
The study in part refutes the frequent preference for low-fat or no-fat dairy foods over whole-fat dairy in healthy-diet recommendations. But it is consistent with earlier findings from PURE of reduced mortality risk with increased consumption of dietary fat, including saturated fat.
Whereas healthy-diet recommendations tend to emphasize reduced intake of fat, especially saturated fat, the report notes that “there are almost no national or international strategies and policies to increase a number of protective foods,” such as nuts, fish, and dairy.
“Therefore, while the findings from PURE are largely consistent with the nutrition science and modern dietary recommendations to focus on protective foods, the public’s understanding of healthy eating and relevant global policies have not yet caught up to this science,” it states.
“Guidelines and policy actions need to be updated with this newer evidence,” Dr. Mente said in an interview. “For example, the World Health Organization remains mainly focused on reducing certain nutrients, such as fat, saturated fat, added sugar, and salt,” he said. “These recommendations are echoed by government policy actions and industry, as evident by the continued focus on the usual nutrients in food labels of many countries.”
The current findings, Dr. Mente said, “can be used to ensure that the public’s understanding of healthy eating and relevant global policies are able to catch up to the science.”
Healthy diet score
PURE investigators developed their healthy diet score using data from 147,642 people from the general population in 21 countries. The investigators compared self-reported dietary intakes with long-term clinical outcomes.
The scoring system assigned a value of 1 for each of the six health-food categories when individuals’ intake exceeded the entire cohort’s median intake. It assigned a 0 when intake was below the median. The total PURE healthy diet score consisted of the sum of the six values, with higher scores corresponding to a healthier diet. The mean score for cohort was 2.95.
There were 15,707 deaths and 40,764 CV events during a median follow-up of 9.3 years. A score of at least 5 points, compared with 0 or 1 point, was associated with significantly reduced hazard ratios for mortality, MI, and stroke in multivariable analysis:
- Mortality: HR, 0.70 (95% CI, 0.63-0.77; P < .0001).
- Major CV disease: HR, 0.82 (95% CI, 0.75-0.91; P < .0001).
- MI: HR, 0.86 (95% CI, 0.75-0.99; P = .0014).
- Stroke: HR, 0.81 (95% CI, 0.71-0.93; P = .0034).
The healthy diet score’s relationship to clinical outcomes was explored in five other large independent studies, including three prospective trials of patients with CV disease that spanned 50 countries, a case-control study with MI patients in 52 countries, and a case-control study with stroke patients in 33 countries.
In the three prospective trials, higher scores were associated with reduced mortality, CV disease events, and MI:
- Mortality: HR, 0.73 (95% CI, 0.66-0.81).
- Major CV disease: HR, 0.79 (95% CI, 0.72-0.87).
- MI: HR, 0.85 (95% CI, 0.71-0.99).
In the two case-control studies, a higher diet score was associated with reduced odds ratios for first MI and for stroke:
- MI: OR, 0.72 (95% CI, 0.65-0.80).
- Stroke: OR, 0.57 (95% CI, 0.50-0.65).
In an analysis based on the PURE cohort, incorporation of unprocessed red meat or whole grains into the health diet score produced similar results, suggesting that a “modest amount” of meat or whole grains can be part of a healthy diet, the authors contend.
The results were similar in a combined analysis of all the prospective studies. In particular, improvement in diet score by one quintile was associated with significantly reduced risks for the following:
- Mortality: HR, 0.92 (95% CI, 0.90-0.93).
- Major CV disease: HR, 0.94 (95% CI, 0.93-0.95).
- MI: HR, 0.94 (95% CI, 0.92-0.96).
- Stroke: HR, 0.94 (95% CI, 0.89-0.99).
- Death or CV disease: HR, 0.93 (95% CI, 0.92-0.94).
“This strongly indicates that the take-home message for patients is the same as for general populations,” Dr. Mente said. “Eat plenty of fruits, vegetables, nuts, legumes, and a moderate amount of fish and whole-fat dairy to lower risk of CV disease and mortality.”
Dairy foods are not widely consumed in some cultures, he said, “but availability and cost are also factors in determining consumption.” Nonetheless, a high-quality diet can be achieved without including or excluding dairy foods. Context-specific policies and priorities are needed for different populations, “rather than a one-size-fits-all global policy.”
Food labels in many countries mainly focus on “reducing certain nutrients as the end-all, be-all,” Dr. Mente observed. “Our findings can be used as a basis for recommendations regarding what a healthy diet should be globally and then modified for each region based on the specific types of foods that are available and affordable in each region.”
Moreover, he said, “targeted food policies are needed to increase the availability and affordability of healthy foods, especially in lower-income countries where intakes are low.”
Common human biology
The current results from PURE “confirm prior observations from mostly Western nations that low intakes of fruits, vegetables, nuts, legumes, and fish are major risk factors for poor health,” observes Dariush Mozaffarian, MD, DrPH, MPH, Tufts University, Boston, in an accompanying editorial. “This suggests that common human biology, not merely confounding, explains these observed diet–disease relationships, strengthening causal inference on the power of nutrition.”
Moreover, “These findings provide further support that dairy foods, including whole-fat dairy, can be part of a healthy diet,” Dr. Mozaffarian writes. “The new results in PURE, in combination with prior reports, call for a re-evaluation of unrelenting guidelines to avoid whole-fat dairy products.”
Such studies “remind us of the continuing and devastating rise in diet-related chronic diseases globally, and of the power of protective foods to help address these burdens,” the editorial continues. “It is time for national nutrition guidelines, private sector innovations, government tax policy and agricultural incentives, food procurement policies, labeling and other regulatory priorities, and food-based health care interventions to catch up to the science.”
Not automatically superior
“I do not believe guidelines should be changed based on this single study,” contends Howard D. Sesso, ScD, MPH, associate director of the division of preventive medicine at Brigham and Women’s Hospital, Boston, who isn’t part of PURE. “But I welcome the scientific dialog that should come out of any study that challenges what we think we know,” he told this news organization.
“Many other dietary patterns have been identified over the years that also do a great job in predicting disease risk in observational studies,” observed Dr. Sesso. “Is PURE that much better? Maybe, maybe not. But not enough to dismiss other dietary patterns that are already the basis of dietary recommendations in the U.S., Europe, and worldwide.”
The PURE healthy diet score, he said, “appears to work well within the confines of their large pooling of studies around the world, but that doesn’t automatically make it superior to other dietary patterns.” The score “was only modestly, but not greatly, better than existing dietary patterns evaluated.”
Randomized controlled trials are needed, Dr. Sesso said, to “delve into more specific dietary components,” including unprocessed red meat, whole grains, and high-fat dairy foods. And, he said, more observational studies are needed to examine the score’s association with other cardiometabolic outcomes.
The PURE study is funded by the Population Health Research Institute, the Hamilton Health Sciences Research Institute, the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario; with support from Canadian Institutes of Health Research’s Strategy for Patient Oriented Research through the Ontario SPOR Support Unit, as well as the Ontario Ministry of Health and Long-Term Care; and through unrestricted grants from several pharmaceutical companies, with major contributions from AstraZeneca, Sanofi-Aventis, Boehringer Ingelheim, Servier, and GlaxoSmithKline. Additional contributions are from Novartis and King Pharma. Dr. Mente, Dr. Mozaffarian, and Dr. Sesso have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN HEART CENTER
FDA expands inclisiran statin-adjunct indication to include primary prevention
Novartis has announced.
The first-in-class small interfering RNA (siRNA) agent was approved in 2021 as an adjunct to statins for patients with clinical cardiovascular disease or heterozygous familial hypercholesterolemia. The indications now include patients taking statins for primary dyslipidemia who have high-risk comorbidities such as diabetes but who do not have a history of cardiovascular events, the company said.
Inclisiran, with a mechanism of action unique among drugs for dyslipidemia, works by “silencing” RNA involved in the synthesis of proprotein convertase subtilisin/kexin type 9. The protein helps regulate the number of LDL cholesterol cell-surface receptors.
Novartis said it has “global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals.”
A version of this article first appeared on Medscape.com.
Novartis has announced.
The first-in-class small interfering RNA (siRNA) agent was approved in 2021 as an adjunct to statins for patients with clinical cardiovascular disease or heterozygous familial hypercholesterolemia. The indications now include patients taking statins for primary dyslipidemia who have high-risk comorbidities such as diabetes but who do not have a history of cardiovascular events, the company said.
Inclisiran, with a mechanism of action unique among drugs for dyslipidemia, works by “silencing” RNA involved in the synthesis of proprotein convertase subtilisin/kexin type 9. The protein helps regulate the number of LDL cholesterol cell-surface receptors.
Novartis said it has “global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals.”
A version of this article first appeared on Medscape.com.
Novartis has announced.
The first-in-class small interfering RNA (siRNA) agent was approved in 2021 as an adjunct to statins for patients with clinical cardiovascular disease or heterozygous familial hypercholesterolemia. The indications now include patients taking statins for primary dyslipidemia who have high-risk comorbidities such as diabetes but who do not have a history of cardiovascular events, the company said.
Inclisiran, with a mechanism of action unique among drugs for dyslipidemia, works by “silencing” RNA involved in the synthesis of proprotein convertase subtilisin/kexin type 9. The protein helps regulate the number of LDL cholesterol cell-surface receptors.
Novartis said it has “global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals.”
A version of this article first appeared on Medscape.com.
The surprising occupations with higher-than-expected ovarian cancer rates
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
Basically, all cancers are caused by a mix of genetic and environmental factors, with some cancers driven more strongly by one or the other. When it comes to ovarian cancer, which kills more than 13,000 women per year in the United States, genetic factors like the BRCA gene mutations are well described.
Other risk factors, like early menarche and nulliparity, are difficult to modify. The only slam-dunk environmental toxin to be linked to ovarian cancer is asbestos. Still, the vast majority of women who develop ovarian cancer do not have a known high-risk gene or asbestos exposure, so other triggers may be out there. How do we find them? The answer may just be good old-fashioned epidemiology.
That’s just what researchers, led by Anita Koushik at the University of Montreal, did in a new study appearing in the journal Occupational and Environmental Medicine.
They identified 497 women in Montreal who had recently been diagnosed with ovarian cancer. They then matched those women to 897 women without ovarian cancer, based on age and address. (This approach would not work well in the United States, as diagnosis of ovarian cancer might depend on access to medical care, which is not universal here. In Canada, however, it’s safer to assume that anyone who could have gotten ovarian cancer in Montreal would have been detected.)
Cases and controls identified, the researchers took a detailed occupational history for each participant: every job they ever worked, and when, and for how long. Each occupation was mapped to a standardized set of industries and, interestingly, to a set of environmental exposures ranging from cosmetic talc to cooking fumes to cotton dust, in what is known as a job-exposure matrix. Of course, they also collected data on other ovarian cancer risk factors.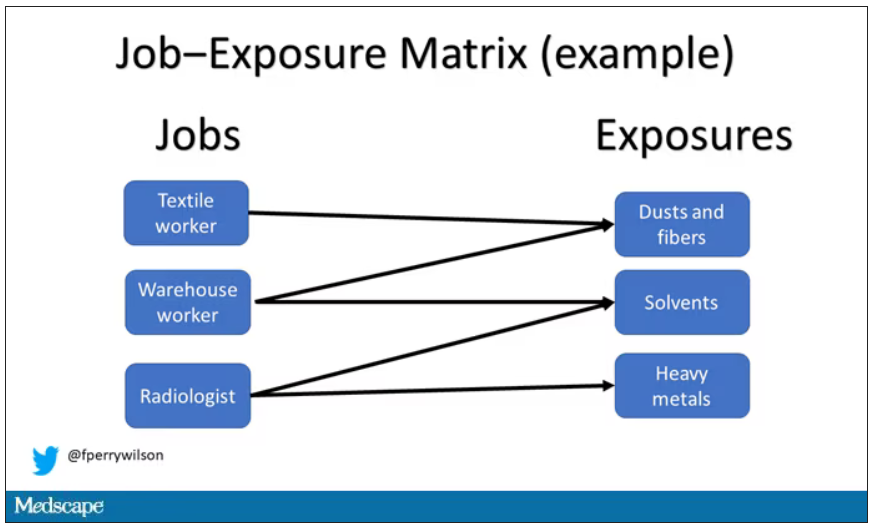
After that, it’s a simple matter of looking at the rate of ovarian cancer by occupation and occupation-associated exposures, accounting for differences in things like pregnancy rates.
A brief aside here. I was at dinner with my wife the other night and telling her about this study, and I asked, “What do you think the occupation with the highest rate of ovarian cancer is?” And without missing a beat, she said: “Hairdressers.” Which blew my mind because of how random that was, but she was also – as usual – 100% correct.
Hairdressers, at least those who had been in the industry for more than 10 years, had a threefold higher risk for ovarian cancer than matched controls who had never been hairdressers.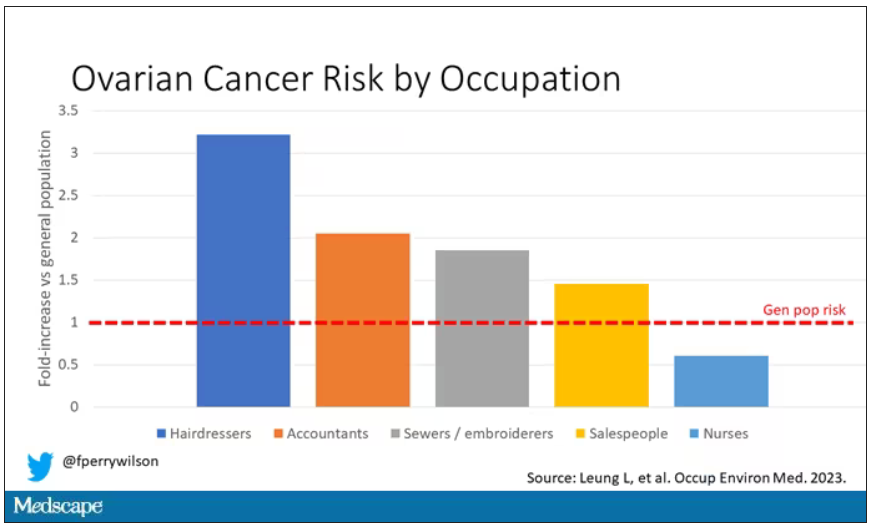
Of course, my wife is a cancer surgeon, so she has a bit of a leg up on me here. Many of you may also know that there is actually a decent body of literature showing higher rates of various cancers among hairdressers, presumably due to the variety of chemicals they are exposed to on a continuous basis.
The No. 2 highest-risk profession on the list? Accountants, with about a twofold higher risk. That one is more of a puzzler. It could be a false positive; after all, there were multiple occupations checked and random error might give a few hits that are meaningless. But there are certainly some occupational factors unique to accountants that might bear further investigation – maybe exposure to volatile organic compounds from office printers, or just a particularly sedentary office environment.
In terms of specific exposures, there were high risks seen with mononuclear aromatic hydrocarbons, bleaches, ethanol, and fluorocarbons, among others, but we have to be a bit more careful here. These exposures were not directly measured. Rather, based on the job category a woman described, the exposures were imputed based on the job-exposure matrix. As such, the correlations between the job and the particular exposure are really quite high, making it essentially impossible to tease out whether it is, for example, being a hairdresser, or being exposed to fluorocarbons as a hairdresser, or being exposed to something else as a hairdresser, that is the problem.
This is how these types of studies work; they tend to raise more questions than they answer. But in a world where a cancer diagnosis can seem to come completely out of the blue, they provide the starting point that someday may lead to a more definitive culprit agent or group of agents. Until then, it might be wise for hairdressers to make sure their workplace is well ventilated.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
Basically, all cancers are caused by a mix of genetic and environmental factors, with some cancers driven more strongly by one or the other. When it comes to ovarian cancer, which kills more than 13,000 women per year in the United States, genetic factors like the BRCA gene mutations are well described.
Other risk factors, like early menarche and nulliparity, are difficult to modify. The only slam-dunk environmental toxin to be linked to ovarian cancer is asbestos. Still, the vast majority of women who develop ovarian cancer do not have a known high-risk gene or asbestos exposure, so other triggers may be out there. How do we find them? The answer may just be good old-fashioned epidemiology.
That’s just what researchers, led by Anita Koushik at the University of Montreal, did in a new study appearing in the journal Occupational and Environmental Medicine.
They identified 497 women in Montreal who had recently been diagnosed with ovarian cancer. They then matched those women to 897 women without ovarian cancer, based on age and address. (This approach would not work well in the United States, as diagnosis of ovarian cancer might depend on access to medical care, which is not universal here. In Canada, however, it’s safer to assume that anyone who could have gotten ovarian cancer in Montreal would have been detected.)
Cases and controls identified, the researchers took a detailed occupational history for each participant: every job they ever worked, and when, and for how long. Each occupation was mapped to a standardized set of industries and, interestingly, to a set of environmental exposures ranging from cosmetic talc to cooking fumes to cotton dust, in what is known as a job-exposure matrix. Of course, they also collected data on other ovarian cancer risk factors.
After that, it’s a simple matter of looking at the rate of ovarian cancer by occupation and occupation-associated exposures, accounting for differences in things like pregnancy rates.
A brief aside here. I was at dinner with my wife the other night and telling her about this study, and I asked, “What do you think the occupation with the highest rate of ovarian cancer is?” And without missing a beat, she said: “Hairdressers.” Which blew my mind because of how random that was, but she was also – as usual – 100% correct.
Hairdressers, at least those who had been in the industry for more than 10 years, had a threefold higher risk for ovarian cancer than matched controls who had never been hairdressers.
Of course, my wife is a cancer surgeon, so she has a bit of a leg up on me here. Many of you may also know that there is actually a decent body of literature showing higher rates of various cancers among hairdressers, presumably due to the variety of chemicals they are exposed to on a continuous basis.
The No. 2 highest-risk profession on the list? Accountants, with about a twofold higher risk. That one is more of a puzzler. It could be a false positive; after all, there were multiple occupations checked and random error might give a few hits that are meaningless. But there are certainly some occupational factors unique to accountants that might bear further investigation – maybe exposure to volatile organic compounds from office printers, or just a particularly sedentary office environment.
In terms of specific exposures, there were high risks seen with mononuclear aromatic hydrocarbons, bleaches, ethanol, and fluorocarbons, among others, but we have to be a bit more careful here. These exposures were not directly measured. Rather, based on the job category a woman described, the exposures were imputed based on the job-exposure matrix. As such, the correlations between the job and the particular exposure are really quite high, making it essentially impossible to tease out whether it is, for example, being a hairdresser, or being exposed to fluorocarbons as a hairdresser, or being exposed to something else as a hairdresser, that is the problem.
This is how these types of studies work; they tend to raise more questions than they answer. But in a world where a cancer diagnosis can seem to come completely out of the blue, they provide the starting point that someday may lead to a more definitive culprit agent or group of agents. Until then, it might be wise for hairdressers to make sure their workplace is well ventilated.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
Basically, all cancers are caused by a mix of genetic and environmental factors, with some cancers driven more strongly by one or the other. When it comes to ovarian cancer, which kills more than 13,000 women per year in the United States, genetic factors like the BRCA gene mutations are well described.
Other risk factors, like early menarche and nulliparity, are difficult to modify. The only slam-dunk environmental toxin to be linked to ovarian cancer is asbestos. Still, the vast majority of women who develop ovarian cancer do not have a known high-risk gene or asbestos exposure, so other triggers may be out there. How do we find them? The answer may just be good old-fashioned epidemiology.
That’s just what researchers, led by Anita Koushik at the University of Montreal, did in a new study appearing in the journal Occupational and Environmental Medicine.
They identified 497 women in Montreal who had recently been diagnosed with ovarian cancer. They then matched those women to 897 women without ovarian cancer, based on age and address. (This approach would not work well in the United States, as diagnosis of ovarian cancer might depend on access to medical care, which is not universal here. In Canada, however, it’s safer to assume that anyone who could have gotten ovarian cancer in Montreal would have been detected.)
Cases and controls identified, the researchers took a detailed occupational history for each participant: every job they ever worked, and when, and for how long. Each occupation was mapped to a standardized set of industries and, interestingly, to a set of environmental exposures ranging from cosmetic talc to cooking fumes to cotton dust, in what is known as a job-exposure matrix. Of course, they also collected data on other ovarian cancer risk factors.
After that, it’s a simple matter of looking at the rate of ovarian cancer by occupation and occupation-associated exposures, accounting for differences in things like pregnancy rates.
A brief aside here. I was at dinner with my wife the other night and telling her about this study, and I asked, “What do you think the occupation with the highest rate of ovarian cancer is?” And without missing a beat, she said: “Hairdressers.” Which blew my mind because of how random that was, but she was also – as usual – 100% correct.
Hairdressers, at least those who had been in the industry for more than 10 years, had a threefold higher risk for ovarian cancer than matched controls who had never been hairdressers.
Of course, my wife is a cancer surgeon, so she has a bit of a leg up on me here. Many of you may also know that there is actually a decent body of literature showing higher rates of various cancers among hairdressers, presumably due to the variety of chemicals they are exposed to on a continuous basis.
The No. 2 highest-risk profession on the list? Accountants, with about a twofold higher risk. That one is more of a puzzler. It could be a false positive; after all, there were multiple occupations checked and random error might give a few hits that are meaningless. But there are certainly some occupational factors unique to accountants that might bear further investigation – maybe exposure to volatile organic compounds from office printers, or just a particularly sedentary office environment.
In terms of specific exposures, there were high risks seen with mononuclear aromatic hydrocarbons, bleaches, ethanol, and fluorocarbons, among others, but we have to be a bit more careful here. These exposures were not directly measured. Rather, based on the job category a woman described, the exposures were imputed based on the job-exposure matrix. As such, the correlations between the job and the particular exposure are really quite high, making it essentially impossible to tease out whether it is, for example, being a hairdresser, or being exposed to fluorocarbons as a hairdresser, or being exposed to something else as a hairdresser, that is the problem.
This is how these types of studies work; they tend to raise more questions than they answer. But in a world where a cancer diagnosis can seem to come completely out of the blue, they provide the starting point that someday may lead to a more definitive culprit agent or group of agents. Until then, it might be wise for hairdressers to make sure their workplace is well ventilated.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
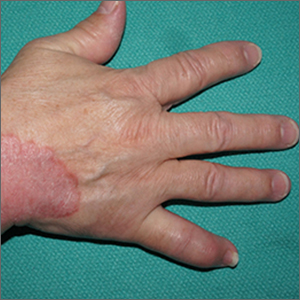
Focal plaques and finger swelling
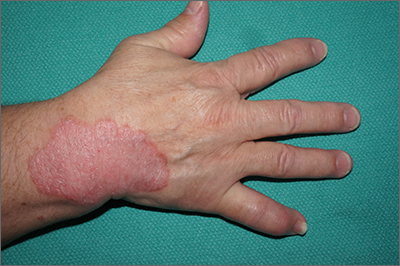
Well-demarcated symmetrical scaly plaques and dactylitis are consistent with psoriasis and psoriatic arthritis (PsA). Even in the absence of significant skin disease, a patient like this should be evaluated by Rheumatology for initiation of disease-modifying antirheumatic drugs (DMARDs).
Psoriatic arthritis manifests as a peripheral arthritis affecting the small joints of the wrists and hands, pain at the insertion of tendons and ligaments (enthesitis), or as axial arthritis. This variable presentation and the lack of specific serological marker can make diagnosis challenging. Associated symptoms beyond the musculoskeletal system include uveitis, inflammatory bowel disease, and cutaneous psoriasis.1 In contrast to osteoarthritis, PsA symptoms are often worse in the morning and improve over the course of the day. Patients with a history of psoriasis on the skin have about a 10% chance of developing PsA, with increased rates in patients who have more widespread plaques and patients with psoriasis at a young age.2 Although not pathognomonic for PsA, pitting of the fingernails may reflect episodic enthesitis in the extensor tendons of the fingers.3 Radiographs of the hands in severe cases may demonstrate narrowing of the proximal portion of the distal or proximal interphalangeal joints with a cup-like concavity of the distal half of the joint.
Conventional DMARDs (such as methotrexate and azathioprine) and biologic DMARDs (including TNF-alpha inhibitors, IL-17 inhibitors, IL-23 inhibitors) are first-line treatments and can stop or slow the progression of disease but will not reverse existing damage. For this reason, it is important to promptly start DMARD therapy after the diagnosis has been established.4
This patient was initiated on adalimumab 40 mg subcutaneously every other week. Her pain improved after 2 months of therapy and her skin plaques almost entirely resolved at 6 months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol. 2020;214:108390. doi: 10.1016/j.clim.2020.108390
2. Ogdie A, Gelfand JM. Clinical risk factors for the development of psoriatic arthritis among patients with psoriasis: a review of available evidence. Curr Rheumatol Rep. 2015;17:64. doi: 10.1007/s11926-015-0540-1
3. Elliott A, Pendleton A, Wright G, et al. The relationship between the nail and systemic enthesitis in psoriatic arthritis. Rheumatol Adv Pract. 2021;5:rkab088. doi: 10.1093/rap/rkab088
4. Coates LC, Soriano ER, Corp N, et al. GRAPPA treatment recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465-479. doi: 10.1038/s41584-022-00798-0

Well-demarcated symmetrical scaly plaques and dactylitis are consistent with psoriasis and psoriatic arthritis (PsA). Even in the absence of significant skin disease, a patient like this should be evaluated by Rheumatology for initiation of disease-modifying antirheumatic drugs (DMARDs).
Psoriatic arthritis manifests as a peripheral arthritis affecting the small joints of the wrists and hands, pain at the insertion of tendons and ligaments (enthesitis), or as axial arthritis. This variable presentation and the lack of specific serological marker can make diagnosis challenging. Associated symptoms beyond the musculoskeletal system include uveitis, inflammatory bowel disease, and cutaneous psoriasis.1 In contrast to osteoarthritis, PsA symptoms are often worse in the morning and improve over the course of the day. Patients with a history of psoriasis on the skin have about a 10% chance of developing PsA, with increased rates in patients who have more widespread plaques and patients with psoriasis at a young age.2 Although not pathognomonic for PsA, pitting of the fingernails may reflect episodic enthesitis in the extensor tendons of the fingers.3 Radiographs of the hands in severe cases may demonstrate narrowing of the proximal portion of the distal or proximal interphalangeal joints with a cup-like concavity of the distal half of the joint.
Conventional DMARDs (such as methotrexate and azathioprine) and biologic DMARDs (including TNF-alpha inhibitors, IL-17 inhibitors, IL-23 inhibitors) are first-line treatments and can stop or slow the progression of disease but will not reverse existing damage. For this reason, it is important to promptly start DMARD therapy after the diagnosis has been established.4
This patient was initiated on adalimumab 40 mg subcutaneously every other week. Her pain improved after 2 months of therapy and her skin plaques almost entirely resolved at 6 months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Well-demarcated symmetrical scaly plaques and dactylitis are consistent with psoriasis and psoriatic arthritis (PsA). Even in the absence of significant skin disease, a patient like this should be evaluated by Rheumatology for initiation of disease-modifying antirheumatic drugs (DMARDs).
Psoriatic arthritis manifests as a peripheral arthritis affecting the small joints of the wrists and hands, pain at the insertion of tendons and ligaments (enthesitis), or as axial arthritis. This variable presentation and the lack of specific serological marker can make diagnosis challenging. Associated symptoms beyond the musculoskeletal system include uveitis, inflammatory bowel disease, and cutaneous psoriasis.1 In contrast to osteoarthritis, PsA symptoms are often worse in the morning and improve over the course of the day. Patients with a history of psoriasis on the skin have about a 10% chance of developing PsA, with increased rates in patients who have more widespread plaques and patients with psoriasis at a young age.2 Although not pathognomonic for PsA, pitting of the fingernails may reflect episodic enthesitis in the extensor tendons of the fingers.3 Radiographs of the hands in severe cases may demonstrate narrowing of the proximal portion of the distal or proximal interphalangeal joints with a cup-like concavity of the distal half of the joint.
Conventional DMARDs (such as methotrexate and azathioprine) and biologic DMARDs (including TNF-alpha inhibitors, IL-17 inhibitors, IL-23 inhibitors) are first-line treatments and can stop or slow the progression of disease but will not reverse existing damage. For this reason, it is important to promptly start DMARD therapy after the diagnosis has been established.4
This patient was initiated on adalimumab 40 mg subcutaneously every other week. Her pain improved after 2 months of therapy and her skin plaques almost entirely resolved at 6 months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol. 2020;214:108390. doi: 10.1016/j.clim.2020.108390
2. Ogdie A, Gelfand JM. Clinical risk factors for the development of psoriatic arthritis among patients with psoriasis: a review of available evidence. Curr Rheumatol Rep. 2015;17:64. doi: 10.1007/s11926-015-0540-1
3. Elliott A, Pendleton A, Wright G, et al. The relationship between the nail and systemic enthesitis in psoriatic arthritis. Rheumatol Adv Pract. 2021;5:rkab088. doi: 10.1093/rap/rkab088
4. Coates LC, Soriano ER, Corp N, et al. GRAPPA treatment recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465-479. doi: 10.1038/s41584-022-00798-0
1. Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol. 2020;214:108390. doi: 10.1016/j.clim.2020.108390
2. Ogdie A, Gelfand JM. Clinical risk factors for the development of psoriatic arthritis among patients with psoriasis: a review of available evidence. Curr Rheumatol Rep. 2015;17:64. doi: 10.1007/s11926-015-0540-1
3. Elliott A, Pendleton A, Wright G, et al. The relationship between the nail and systemic enthesitis in psoriatic arthritis. Rheumatol Adv Pract. 2021;5:rkab088. doi: 10.1093/rap/rkab088
4. Coates LC, Soriano ER, Corp N, et al. GRAPPA treatment recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465-479. doi: 10.1038/s41584-022-00798-0
Long COVID patients turn to doctors for help with disability claims
As millions of Americans face another year of long COVID, some are finding they are unable to return to work or cannot work as they did before they got sick and are turning to doctors for help with documenting their disability.
For those who can return to work, a doctor’s diagnosis of long COVID is key to gaining access to workplace accommodations, such as working flex hours or remotely. For those who cannot work, a note from the doctor is the first step to collecting disability payments.
With no definitive blood tests or scans for long COVID that could confirm a diagnosis, some say doctors may feel uncomfortable in this role, which puts them in a tough spot, said Wes Ely, MD, MPH, codirector of the critical illness, brain dysfunction and survivorship center at Vanderbilt University, Nashville, Tenn.
Doctors typically are not taught to deal with vagueness in diagnostics.
“Long COVID falls straight into the gray zone,” he said. There are no tests and a long list of common symptoms. “It makes a lot of doctors feel super insecure,” he said.
Now, patients and their advocates are calling for doctors to be more open-minded about how they assess those with long COVID and other chronic illnesses. Although their disability may not be visible, many with long COVID struggle to function. If they need help, they say, they need a doctor to confirm their limitations – test results or no test results.
Better documentation of patient-reported symptoms would go a long way, according to a perspective published in The New England Journal of Medicine.
“There’s a long history of people with disabilities being forced to ask doctors to legitimize their symptoms,” said study author Zackary Berger, MD, PhD, Johns Hopkins University, Baltimore, Md. Dr. Berger believes doctors should learn to listen more closely to patients, turn their narratives into patient notes, and use the new International Classification of Diseases 10 (ICD-10) code, a worldwide system for identifying and generating data on diseases, when they diagnose long COVID. He also thinks doctors should become advocates for their patients.
The Americans With Disabilities Act allows employers to request medical proof of disability, “and thereby assigns physicians the gate-keeping role of determining patients’ eligibility for reasonable accommodations,” according to the analysis. Those accommodations may mean a handicapped parking space or extra days working remotely.
Without a definitive diagnostic test, long COVID joins fibromyalgia and ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome), which lack biomarkers or imaging tests to support a diagnosis, they write.
“These diagnoses are therefore contentious, and government agencies, employers, and many physicians do not accept these conditions as real,” they write.
Physicians make a good faith effort in trying to understand long COVID, but both doctors and the courts like to see evidence, said Michael Ashley Stein, JD, PHD, director of the Harvard Law School Project on Disability. Dr. Stein and others say that doctors should listen closely to their patients’ descriptions of their symptoms.
“In the absence of agreed-upon biomarkers, doctors need to listen to their patients and look for other [indications] and other consistent evidence of conditions, and then work from there rather than dismiss the existence of these conditions,” he said.
Dr. Ely said he and others were taught in medical school that if it doesn’t come up on a diagnostic test, there’s no problem. “I am absolutely complicit,” he said. “I’m part of the community that did that for so many years.”
Dr. Ely agreed that the demand for clinical test results does not work for long COVID and chronic diseases such as ME/CFS. People come in with complaints and they get a typical medical workup with labs, he said, and the labs look normal on paper.
“And [the doctor is] thinking: ‘I don’t know what is wrong with this person and there’s nothing on paper I can treat. I don’t know if I even believe in long COVID.’ ”
At the same time, patients might need support from a doctor to get accommodations at work under the ADA, such as flexible hours. Or doctors’ notes may be required if a patient is trying to collect private disability insurance, workers compensation, or federal disability payments through Social Security.
The U.S. Centers for Disease Control and Prevention guidelines on diagnosing long COVID, updated last December, point out that normal laboratory or imaging findings do not rule out long COVID.
In addition, 12 key symptoms of long COVID were identified in May by scientists working with the RECOVER Initiative, the federal government’s long COVID research program. These symptoms include fatigue, brain fog, dizziness, gastrointestinal symptoms, loss of or change in smell or taste, chest pain, and abnormal movements.
Still, patients with long COVID seeking help also face the “disability con,” a term coined by the second author of the NEJM article, Doron Dorfman, a professor at Seton Hall Law School in Newark, N.J.
“Nowadays, when people think disability, they immediately think fraud,” he said.
Prof. Dorfman thinks the perception that many people are faking disabilities to gain an unfair advantage is the biggest barrier for anyone seeking help. The disability system is “preventing people who deserve legal rights from actually obtaining them,” he said.
He urged doctors to believe their patients. One way is to try to “translate the person’s narrative into medical language.”
His coauthor Dr. Berger did not agree with the argument that doctors cannot diagnose without tests.
“Any clinician knows that lab tests are not everything,” he said. “There are conditions that don’t have specific biomarkers that we diagnose all the time.” He cited acquired pneumonia and urinary tract infections as examples.
Benefits lawyers have taken note of the complexities for people with long COVID who seek help through the ADA and federal disability program.
One law firm noted: “The government safety net is not designed to help an emerging disease with no clear diagnosis or treatment plans. Insurance carriers are denying claims, and long-term disability benefits are being denied.”
About 16 million working-age Americans have long COVID, according to an update of a 2022 report by the Brookings Institute. Up to 4 million of these people are out of work because of the condition, the study found. The research is based on newly collected U.S. Census Bureau data that show 24% of those with long COVID report “significant activity limitations.”
Dr. Ely said he sees progress in this area. Many of these issues have come up at the committee convened by the National Academy of Science to look at the working definition of long COVID. NAS, a Washington research group, held a public meeting on their findings on June 22.
A version of this article first appeared on Medscape.com.
As millions of Americans face another year of long COVID, some are finding they are unable to return to work or cannot work as they did before they got sick and are turning to doctors for help with documenting their disability.
For those who can return to work, a doctor’s diagnosis of long COVID is key to gaining access to workplace accommodations, such as working flex hours or remotely. For those who cannot work, a note from the doctor is the first step to collecting disability payments.
With no definitive blood tests or scans for long COVID that could confirm a diagnosis, some say doctors may feel uncomfortable in this role, which puts them in a tough spot, said Wes Ely, MD, MPH, codirector of the critical illness, brain dysfunction and survivorship center at Vanderbilt University, Nashville, Tenn.
Doctors typically are not taught to deal with vagueness in diagnostics.
“Long COVID falls straight into the gray zone,” he said. There are no tests and a long list of common symptoms. “It makes a lot of doctors feel super insecure,” he said.
Now, patients and their advocates are calling for doctors to be more open-minded about how they assess those with long COVID and other chronic illnesses. Although their disability may not be visible, many with long COVID struggle to function. If they need help, they say, they need a doctor to confirm their limitations – test results or no test results.
Better documentation of patient-reported symptoms would go a long way, according to a perspective published in The New England Journal of Medicine.
“There’s a long history of people with disabilities being forced to ask doctors to legitimize their symptoms,” said study author Zackary Berger, MD, PhD, Johns Hopkins University, Baltimore, Md. Dr. Berger believes doctors should learn to listen more closely to patients, turn their narratives into patient notes, and use the new International Classification of Diseases 10 (ICD-10) code, a worldwide system for identifying and generating data on diseases, when they diagnose long COVID. He also thinks doctors should become advocates for their patients.
The Americans With Disabilities Act allows employers to request medical proof of disability, “and thereby assigns physicians the gate-keeping role of determining patients’ eligibility for reasonable accommodations,” according to the analysis. Those accommodations may mean a handicapped parking space or extra days working remotely.
Without a definitive diagnostic test, long COVID joins fibromyalgia and ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome), which lack biomarkers or imaging tests to support a diagnosis, they write.
“These diagnoses are therefore contentious, and government agencies, employers, and many physicians do not accept these conditions as real,” they write.
Physicians make a good faith effort in trying to understand long COVID, but both doctors and the courts like to see evidence, said Michael Ashley Stein, JD, PHD, director of the Harvard Law School Project on Disability. Dr. Stein and others say that doctors should listen closely to their patients’ descriptions of their symptoms.
“In the absence of agreed-upon biomarkers, doctors need to listen to their patients and look for other [indications] and other consistent evidence of conditions, and then work from there rather than dismiss the existence of these conditions,” he said.
Dr. Ely said he and others were taught in medical school that if it doesn’t come up on a diagnostic test, there’s no problem. “I am absolutely complicit,” he said. “I’m part of the community that did that for so many years.”
Dr. Ely agreed that the demand for clinical test results does not work for long COVID and chronic diseases such as ME/CFS. People come in with complaints and they get a typical medical workup with labs, he said, and the labs look normal on paper.
“And [the doctor is] thinking: ‘I don’t know what is wrong with this person and there’s nothing on paper I can treat. I don’t know if I even believe in long COVID.’ ”
At the same time, patients might need support from a doctor to get accommodations at work under the ADA, such as flexible hours. Or doctors’ notes may be required if a patient is trying to collect private disability insurance, workers compensation, or federal disability payments through Social Security.
The U.S. Centers for Disease Control and Prevention guidelines on diagnosing long COVID, updated last December, point out that normal laboratory or imaging findings do not rule out long COVID.
In addition, 12 key symptoms of long COVID were identified in May by scientists working with the RECOVER Initiative, the federal government’s long COVID research program. These symptoms include fatigue, brain fog, dizziness, gastrointestinal symptoms, loss of or change in smell or taste, chest pain, and abnormal movements.
Still, patients with long COVID seeking help also face the “disability con,” a term coined by the second author of the NEJM article, Doron Dorfman, a professor at Seton Hall Law School in Newark, N.J.
“Nowadays, when people think disability, they immediately think fraud,” he said.
Prof. Dorfman thinks the perception that many people are faking disabilities to gain an unfair advantage is the biggest barrier for anyone seeking help. The disability system is “preventing people who deserve legal rights from actually obtaining them,” he said.
He urged doctors to believe their patients. One way is to try to “translate the person’s narrative into medical language.”
His coauthor Dr. Berger did not agree with the argument that doctors cannot diagnose without tests.
“Any clinician knows that lab tests are not everything,” he said. “There are conditions that don’t have specific biomarkers that we diagnose all the time.” He cited acquired pneumonia and urinary tract infections as examples.
Benefits lawyers have taken note of the complexities for people with long COVID who seek help through the ADA and federal disability program.
One law firm noted: “The government safety net is not designed to help an emerging disease with no clear diagnosis or treatment plans. Insurance carriers are denying claims, and long-term disability benefits are being denied.”
About 16 million working-age Americans have long COVID, according to an update of a 2022 report by the Brookings Institute. Up to 4 million of these people are out of work because of the condition, the study found. The research is based on newly collected U.S. Census Bureau data that show 24% of those with long COVID report “significant activity limitations.”
Dr. Ely said he sees progress in this area. Many of these issues have come up at the committee convened by the National Academy of Science to look at the working definition of long COVID. NAS, a Washington research group, held a public meeting on their findings on June 22.
A version of this article first appeared on Medscape.com.
As millions of Americans face another year of long COVID, some are finding they are unable to return to work or cannot work as they did before they got sick and are turning to doctors for help with documenting their disability.
For those who can return to work, a doctor’s diagnosis of long COVID is key to gaining access to workplace accommodations, such as working flex hours or remotely. For those who cannot work, a note from the doctor is the first step to collecting disability payments.
With no definitive blood tests or scans for long COVID that could confirm a diagnosis, some say doctors may feel uncomfortable in this role, which puts them in a tough spot, said Wes Ely, MD, MPH, codirector of the critical illness, brain dysfunction and survivorship center at Vanderbilt University, Nashville, Tenn.
Doctors typically are not taught to deal with vagueness in diagnostics.
“Long COVID falls straight into the gray zone,” he said. There are no tests and a long list of common symptoms. “It makes a lot of doctors feel super insecure,” he said.
Now, patients and their advocates are calling for doctors to be more open-minded about how they assess those with long COVID and other chronic illnesses. Although their disability may not be visible, many with long COVID struggle to function. If they need help, they say, they need a doctor to confirm their limitations – test results or no test results.
Better documentation of patient-reported symptoms would go a long way, according to a perspective published in The New England Journal of Medicine.
“There’s a long history of people with disabilities being forced to ask doctors to legitimize their symptoms,” said study author Zackary Berger, MD, PhD, Johns Hopkins University, Baltimore, Md. Dr. Berger believes doctors should learn to listen more closely to patients, turn their narratives into patient notes, and use the new International Classification of Diseases 10 (ICD-10) code, a worldwide system for identifying and generating data on diseases, when they diagnose long COVID. He also thinks doctors should become advocates for their patients.
The Americans With Disabilities Act allows employers to request medical proof of disability, “and thereby assigns physicians the gate-keeping role of determining patients’ eligibility for reasonable accommodations,” according to the analysis. Those accommodations may mean a handicapped parking space or extra days working remotely.
Without a definitive diagnostic test, long COVID joins fibromyalgia and ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome), which lack biomarkers or imaging tests to support a diagnosis, they write.
“These diagnoses are therefore contentious, and government agencies, employers, and many physicians do not accept these conditions as real,” they write.
Physicians make a good faith effort in trying to understand long COVID, but both doctors and the courts like to see evidence, said Michael Ashley Stein, JD, PHD, director of the Harvard Law School Project on Disability. Dr. Stein and others say that doctors should listen closely to their patients’ descriptions of their symptoms.
“In the absence of agreed-upon biomarkers, doctors need to listen to their patients and look for other [indications] and other consistent evidence of conditions, and then work from there rather than dismiss the existence of these conditions,” he said.
Dr. Ely said he and others were taught in medical school that if it doesn’t come up on a diagnostic test, there’s no problem. “I am absolutely complicit,” he said. “I’m part of the community that did that for so many years.”
Dr. Ely agreed that the demand for clinical test results does not work for long COVID and chronic diseases such as ME/CFS. People come in with complaints and they get a typical medical workup with labs, he said, and the labs look normal on paper.
“And [the doctor is] thinking: ‘I don’t know what is wrong with this person and there’s nothing on paper I can treat. I don’t know if I even believe in long COVID.’ ”
At the same time, patients might need support from a doctor to get accommodations at work under the ADA, such as flexible hours. Or doctors’ notes may be required if a patient is trying to collect private disability insurance, workers compensation, or federal disability payments through Social Security.
The U.S. Centers for Disease Control and Prevention guidelines on diagnosing long COVID, updated last December, point out that normal laboratory or imaging findings do not rule out long COVID.
In addition, 12 key symptoms of long COVID were identified in May by scientists working with the RECOVER Initiative, the federal government’s long COVID research program. These symptoms include fatigue, brain fog, dizziness, gastrointestinal symptoms, loss of or change in smell or taste, chest pain, and abnormal movements.
Still, patients with long COVID seeking help also face the “disability con,” a term coined by the second author of the NEJM article, Doron Dorfman, a professor at Seton Hall Law School in Newark, N.J.
“Nowadays, when people think disability, they immediately think fraud,” he said.
Prof. Dorfman thinks the perception that many people are faking disabilities to gain an unfair advantage is the biggest barrier for anyone seeking help. The disability system is “preventing people who deserve legal rights from actually obtaining them,” he said.
He urged doctors to believe their patients. One way is to try to “translate the person’s narrative into medical language.”
His coauthor Dr. Berger did not agree with the argument that doctors cannot diagnose without tests.
“Any clinician knows that lab tests are not everything,” he said. “There are conditions that don’t have specific biomarkers that we diagnose all the time.” He cited acquired pneumonia and urinary tract infections as examples.
Benefits lawyers have taken note of the complexities for people with long COVID who seek help through the ADA and federal disability program.
One law firm noted: “The government safety net is not designed to help an emerging disease with no clear diagnosis or treatment plans. Insurance carriers are denying claims, and long-term disability benefits are being denied.”
About 16 million working-age Americans have long COVID, according to an update of a 2022 report by the Brookings Institute. Up to 4 million of these people are out of work because of the condition, the study found. The research is based on newly collected U.S. Census Bureau data that show 24% of those with long COVID report “significant activity limitations.”
Dr. Ely said he sees progress in this area. Many of these issues have come up at the committee convened by the National Academy of Science to look at the working definition of long COVID. NAS, a Washington research group, held a public meeting on their findings on June 22.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
‘Body size is not a choice’ and deserves legal protections
Legislators in New York City recently approved a bill specifically prohibiting weight- and height-based discrimination, on par with existing protections for gender, race, sexual orientation, and other personal identities. Other U.S. cities, as well as New York state, are considering similar moves.
Weight-based discrimination in the United States has increased by an estimated 66% over the past decade, putting it on par with the prevalence of racial discrimination. More than 40% of adult Americans and 18% of children report experiencing weight discrimination in employment, school, and/or health care settings – as well as within interpersonal relationships – demonstrating a clear need to have legal protections in place.
For obesity advocates in Canada, the news from New York triggered a moment of reflection to consider how our own advocacy efforts have fared over the years, or not. Just like in the United States, body size and obesity (and appearance in general) are not specifically protected grounds under human rights legislation in Canada (for example, the Canadian Human Rights Act), unlike race, gender, sexual orientation, and religion.
Case law is uneven across the Canadian provinces when it comes to determining whether obesity is even a disease and/or a disability. And despite broad support for anti–weight discrimination policies in Canada (Front Public Health. 2023 Apr 17;11:1060794; Milbank Q. 2015 Dec;93[4]:691-731), years of advocacy at the national and provincial levels have not led to any legislative changes (Ramos Salas Obes Rev. 2017 Nov;18[11]:1323-35; Can J Diabetes. 2015 Apr. doi: 10.1016/j.jcjd.2015.01.009). A 2017 private members bill seeking to add protection for body size to Manitoba’s human rights code was defeated, with many members of the legislature citing enforcement difficulties as the reason for voting down the proposition.
Some obesity advocates have argued that people living with obesity can be protected under the grounds of disability in the Canadian Human Rights Act. To be protected, however, individuals must demonstrate that there is actual or perceived disability relating to their weight or size; yet, many people living with obesity and those who have a higher weight don’t perceive themselves as having a disability.
In our view, the disparate viewpoints on the worthiness of considering body size a human rights issue could be resolved, at least partially, by wider understanding and adoption of the relatively new clinical definition of obesity. This definition holds that obesity is not about size; an obesity diagnosis can be made only when objective clinical investigations identify that excess or abnormal adiposity (fat tissue) impairs health.
While obesity advocates use the clinical definition of obesity, weight and body size proponents disagree that obesity is a chronic disease, and in fact believe that treating it as such can be stigmatizing. In a sense, this can sometimes be true, as not all people with larger bodies have obesity per the new definition but risk being identified as “unhealthy” in the clinical world. Bias, it turns out, can be a two-way street.
Regardless of the advocacy strategy used, it’s clear that specific anti–weight discrimination laws are needed in Canada. One in four Canadian adults report experiencing discrimination in their day-to-day life, with race, gender, age, and weight being the most commonly reported forms. To refuse to protect them against some, but not all, forms of discrimination is itself unjust, and is surely rooted in the age-old misinformed concept that excess weight is the result of laziness, poor food choices, and lack of physical activity, among other moral failings.
Including body size in human rights codes may provide a mechanism to seek legal remedy from discriminatory acts, but it will do little to address rampant weight bias, in the same way that race-based legal protections don’t eradicate racism. And it’s not just the legal community that fails to understand that weight is, by and large, a product of our environment and our genes. Weight bias and stigma are well documented in media, workplaces, the home, and in health care systems.
The solution, in our minds, is meaningful education across all these domains, reinforcing that weight is not a behavior, just as health is not a size. If we truly understand and embrace these concepts, then as a society we may someday recognize that body size is not a choice, just like race, sexual orientation, gender identity, and other individual characteristics. And if it’s not a choice, if it’s not a behavior, then it deserves the same protections.
At the same time, people with obesity deserve to seek evidence-based treatment, just as those at higher weights who experience no weight or adiposity-related health issues deserve not to be identified as having a disease simply because of their size.
If we all follow the science, we might yet turn a common understanding into more equitable outcomes for all.
Dr. Ramos Salas and Mr. Hussey are research consultants for Replica Communications, Hamilton, Ont. She disclosed ties with the Canadian Institutes of Health Research, European Association for the Study of Obesity, Novo Nordisk, Obesity Canada, The Obesity Society, World Obesity, and the World Health Organization. Mr. Hussey disclosed ties with the European Association for the Study of Obesity, Novo Nordisk, Obesity Canada, and the World Health Organization (Nutrition and Food Safety).
A version of this article originally appeared on Medscape.com.
Legislators in New York City recently approved a bill specifically prohibiting weight- and height-based discrimination, on par with existing protections for gender, race, sexual orientation, and other personal identities. Other U.S. cities, as well as New York state, are considering similar moves.
Weight-based discrimination in the United States has increased by an estimated 66% over the past decade, putting it on par with the prevalence of racial discrimination. More than 40% of adult Americans and 18% of children report experiencing weight discrimination in employment, school, and/or health care settings – as well as within interpersonal relationships – demonstrating a clear need to have legal protections in place.
For obesity advocates in Canada, the news from New York triggered a moment of reflection to consider how our own advocacy efforts have fared over the years, or not. Just like in the United States, body size and obesity (and appearance in general) are not specifically protected grounds under human rights legislation in Canada (for example, the Canadian Human Rights Act), unlike race, gender, sexual orientation, and religion.
Case law is uneven across the Canadian provinces when it comes to determining whether obesity is even a disease and/or a disability. And despite broad support for anti–weight discrimination policies in Canada (Front Public Health. 2023 Apr 17;11:1060794; Milbank Q. 2015 Dec;93[4]:691-731), years of advocacy at the national and provincial levels have not led to any legislative changes (Ramos Salas Obes Rev. 2017 Nov;18[11]:1323-35; Can J Diabetes. 2015 Apr. doi: 10.1016/j.jcjd.2015.01.009). A 2017 private members bill seeking to add protection for body size to Manitoba’s human rights code was defeated, with many members of the legislature citing enforcement difficulties as the reason for voting down the proposition.
Some obesity advocates have argued that people living with obesity can be protected under the grounds of disability in the Canadian Human Rights Act. To be protected, however, individuals must demonstrate that there is actual or perceived disability relating to their weight or size; yet, many people living with obesity and those who have a higher weight don’t perceive themselves as having a disability.
In our view, the disparate viewpoints on the worthiness of considering body size a human rights issue could be resolved, at least partially, by wider understanding and adoption of the relatively new clinical definition of obesity. This definition holds that obesity is not about size; an obesity diagnosis can be made only when objective clinical investigations identify that excess or abnormal adiposity (fat tissue) impairs health.
While obesity advocates use the clinical definition of obesity, weight and body size proponents disagree that obesity is a chronic disease, and in fact believe that treating it as such can be stigmatizing. In a sense, this can sometimes be true, as not all people with larger bodies have obesity per the new definition but risk being identified as “unhealthy” in the clinical world. Bias, it turns out, can be a two-way street.
Regardless of the advocacy strategy used, it’s clear that specific anti–weight discrimination laws are needed in Canada. One in four Canadian adults report experiencing discrimination in their day-to-day life, with race, gender, age, and weight being the most commonly reported forms. To refuse to protect them against some, but not all, forms of discrimination is itself unjust, and is surely rooted in the age-old misinformed concept that excess weight is the result of laziness, poor food choices, and lack of physical activity, among other moral failings.
Including body size in human rights codes may provide a mechanism to seek legal remedy from discriminatory acts, but it will do little to address rampant weight bias, in the same way that race-based legal protections don’t eradicate racism. And it’s not just the legal community that fails to understand that weight is, by and large, a product of our environment and our genes. Weight bias and stigma are well documented in media, workplaces, the home, and in health care systems.
The solution, in our minds, is meaningful education across all these domains, reinforcing that weight is not a behavior, just as health is not a size. If we truly understand and embrace these concepts, then as a society we may someday recognize that body size is not a choice, just like race, sexual orientation, gender identity, and other individual characteristics. And if it’s not a choice, if it’s not a behavior, then it deserves the same protections.
At the same time, people with obesity deserve to seek evidence-based treatment, just as those at higher weights who experience no weight or adiposity-related health issues deserve not to be identified as having a disease simply because of their size.
If we all follow the science, we might yet turn a common understanding into more equitable outcomes for all.
Dr. Ramos Salas and Mr. Hussey are research consultants for Replica Communications, Hamilton, Ont. She disclosed ties with the Canadian Institutes of Health Research, European Association for the Study of Obesity, Novo Nordisk, Obesity Canada, The Obesity Society, World Obesity, and the World Health Organization. Mr. Hussey disclosed ties with the European Association for the Study of Obesity, Novo Nordisk, Obesity Canada, and the World Health Organization (Nutrition and Food Safety).
A version of this article originally appeared on Medscape.com.
Legislators in New York City recently approved a bill specifically prohibiting weight- and height-based discrimination, on par with existing protections for gender, race, sexual orientation, and other personal identities. Other U.S. cities, as well as New York state, are considering similar moves.
Weight-based discrimination in the United States has increased by an estimated 66% over the past decade, putting it on par with the prevalence of racial discrimination. More than 40% of adult Americans and 18% of children report experiencing weight discrimination in employment, school, and/or health care settings – as well as within interpersonal relationships – demonstrating a clear need to have legal protections in place.
For obesity advocates in Canada, the news from New York triggered a moment of reflection to consider how our own advocacy efforts have fared over the years, or not. Just like in the United States, body size and obesity (and appearance in general) are not specifically protected grounds under human rights legislation in Canada (for example, the Canadian Human Rights Act), unlike race, gender, sexual orientation, and religion.
Case law is uneven across the Canadian provinces when it comes to determining whether obesity is even a disease and/or a disability. And despite broad support for anti–weight discrimination policies in Canada (Front Public Health. 2023 Apr 17;11:1060794; Milbank Q. 2015 Dec;93[4]:691-731), years of advocacy at the national and provincial levels have not led to any legislative changes (Ramos Salas Obes Rev. 2017 Nov;18[11]:1323-35; Can J Diabetes. 2015 Apr. doi: 10.1016/j.jcjd.2015.01.009). A 2017 private members bill seeking to add protection for body size to Manitoba’s human rights code was defeated, with many members of the legislature citing enforcement difficulties as the reason for voting down the proposition.
Some obesity advocates have argued that people living with obesity can be protected under the grounds of disability in the Canadian Human Rights Act. To be protected, however, individuals must demonstrate that there is actual or perceived disability relating to their weight or size; yet, many people living with obesity and those who have a higher weight don’t perceive themselves as having a disability.
In our view, the disparate viewpoints on the worthiness of considering body size a human rights issue could be resolved, at least partially, by wider understanding and adoption of the relatively new clinical definition of obesity. This definition holds that obesity is not about size; an obesity diagnosis can be made only when objective clinical investigations identify that excess or abnormal adiposity (fat tissue) impairs health.
While obesity advocates use the clinical definition of obesity, weight and body size proponents disagree that obesity is a chronic disease, and in fact believe that treating it as such can be stigmatizing. In a sense, this can sometimes be true, as not all people with larger bodies have obesity per the new definition but risk being identified as “unhealthy” in the clinical world. Bias, it turns out, can be a two-way street.
Regardless of the advocacy strategy used, it’s clear that specific anti–weight discrimination laws are needed in Canada. One in four Canadian adults report experiencing discrimination in their day-to-day life, with race, gender, age, and weight being the most commonly reported forms. To refuse to protect them against some, but not all, forms of discrimination is itself unjust, and is surely rooted in the age-old misinformed concept that excess weight is the result of laziness, poor food choices, and lack of physical activity, among other moral failings.
Including body size in human rights codes may provide a mechanism to seek legal remedy from discriminatory acts, but it will do little to address rampant weight bias, in the same way that race-based legal protections don’t eradicate racism. And it’s not just the legal community that fails to understand that weight is, by and large, a product of our environment and our genes. Weight bias and stigma are well documented in media, workplaces, the home, and in health care systems.
The solution, in our minds, is meaningful education across all these domains, reinforcing that weight is not a behavior, just as health is not a size. If we truly understand and embrace these concepts, then as a society we may someday recognize that body size is not a choice, just like race, sexual orientation, gender identity, and other individual characteristics. And if it’s not a choice, if it’s not a behavior, then it deserves the same protections.
At the same time, people with obesity deserve to seek evidence-based treatment, just as those at higher weights who experience no weight or adiposity-related health issues deserve not to be identified as having a disease simply because of their size.
If we all follow the science, we might yet turn a common understanding into more equitable outcomes for all.
Dr. Ramos Salas and Mr. Hussey are research consultants for Replica Communications, Hamilton, Ont. She disclosed ties with the Canadian Institutes of Health Research, European Association for the Study of Obesity, Novo Nordisk, Obesity Canada, The Obesity Society, World Obesity, and the World Health Organization. Mr. Hussey disclosed ties with the European Association for the Study of Obesity, Novo Nordisk, Obesity Canada, and the World Health Organization (Nutrition and Food Safety).
A version of this article originally appeared on Medscape.com.
AMA supports APRN oversight by both medical and nursing boards
In a move that raises the stakes in doctors’ ongoing scope-creep battle against nonphysician providers, the American Medical Association’s legislative body voted recently to change its policy on the supervision of advanced practice registered nurses (APRNs). AMA’s House of Delegates called for state medical boards to regulate APRNs in addition to nursing boards.
The AMA has long claimed that nonphysician providers, such as nurse practitioners (NPs) and physician assistants (PAs), need greater oversight because expanded scope of practice for advanced practice practitioners threatens patient safety and undermines the physician-led team model.
APRNs have been touted as a solution to expand access to care and reduce disparities, especially in rural and underserved communities, and they have been promoted by organizations such as the National Academy of Medicine. But the AMA disputes that scope expansions are necessary to increase access to care.
The organization that represents the nation’s physicians said in a prepared statement that it opposes scope expansions because removing doctors from the care team results in higher costs to the patient and lower quality care.
Several nursing organizations swiftly criticized the policy recommendation, including the American Nurses Association, the American Association of Nurse Practitioners, and the National Council of State Boards of Nursing.
The policy shift would create more administrative burdens for APRNs and generate “a downstream effect that only hurts patients,” particularly those in underserved communities without timely access to care, ANA president Jennifer Mensik Kennedy, PhD, MBA, RN, NEA-BC, told this news organization.
“The licensing and regulation of APRNs have never required the oversight of state medical boards,” she said, adding that it should remain the obligation of nursing regulatory bodies.
Jon Fanning, MS, CAE, CNED, chief executive officer of the AANP, called the AMA proposal “flawed.”
“The restrictive involvement of the board of medicine directly contributes to health care access challenges, resulting in continued low health care rankings, geographic disparities in care, and unnecessary regulatory cost in these states,” he said in a press release.
Still, the AMA has vowed to #StopScopeCreep. Securing stricter practice guidelines was a central theme of the association’s recent annual meeting and a goal of its plan to strengthen the physician workforce. The organization invests heavily in advocacy and education efforts to defeat state bills seeking to extend APRN authority. To that end, the AMA Scope of Practice Partnership, a coalition of over 100 medical associations, has awarded members $3.5 million in grants to combat scope-expansion legislation.
The AMA and the American College of Radiology recently partnered to create advocacy materials, including handouts encouraging patients to ask questions such as: “Will a physician be reviewing my chart, lab results, x-rays, and other tests?”
The policy recommendation comes as concerns mount over the potential for significant physician shortages, fueled partly by older physicians’ retirements and doctors reducing hours or exiting the workforce due to pandemic fatigue and burnout.
While practice regulations vary by state, a new federal bill could change that by broadening the authority of APRNs under Medicare and Medicaid guidelines. Introduced in the U.S. House of Representatives in April and supported by the ANA, the Improving Care and Access to Nurses Act would allow APRNs to perform more procedures, including cardiac and pulmonary rehabilitation and certification of terminal illness for hospice, according to an ANA press release.
In the meantime, several state legislatures are considering bills that would expand APRN scope of practice. Utah is the latest to join a growing list of states – about half now – offering full practice authority to NPs.
Other states offer a reduced scope of practice for APRNs, typically requiring a collaborative agreement with a supervising physician. The remaining states enforce tighter regulations and physician oversight.
A recent Medscape survey found that most physicians report having a good rapport with NPs but many have mixed feelings about giving them expanded practice roles, with one-third saying it would harm patient care. Feelings were only slightly more favorable toward PAs. However, about 75% of patients were either neutral or supportive of independent practice for NPs and PAs.
A version of this article first appeared on Medscape.com.
In a move that raises the stakes in doctors’ ongoing scope-creep battle against nonphysician providers, the American Medical Association’s legislative body voted recently to change its policy on the supervision of advanced practice registered nurses (APRNs). AMA’s House of Delegates called for state medical boards to regulate APRNs in addition to nursing boards.
The AMA has long claimed that nonphysician providers, such as nurse practitioners (NPs) and physician assistants (PAs), need greater oversight because expanded scope of practice for advanced practice practitioners threatens patient safety and undermines the physician-led team model.
APRNs have been touted as a solution to expand access to care and reduce disparities, especially in rural and underserved communities, and they have been promoted by organizations such as the National Academy of Medicine. But the AMA disputes that scope expansions are necessary to increase access to care.
The organization that represents the nation’s physicians said in a prepared statement that it opposes scope expansions because removing doctors from the care team results in higher costs to the patient and lower quality care.
Several nursing organizations swiftly criticized the policy recommendation, including the American Nurses Association, the American Association of Nurse Practitioners, and the National Council of State Boards of Nursing.
The policy shift would create more administrative burdens for APRNs and generate “a downstream effect that only hurts patients,” particularly those in underserved communities without timely access to care, ANA president Jennifer Mensik Kennedy, PhD, MBA, RN, NEA-BC, told this news organization.
“The licensing and regulation of APRNs have never required the oversight of state medical boards,” she said, adding that it should remain the obligation of nursing regulatory bodies.
Jon Fanning, MS, CAE, CNED, chief executive officer of the AANP, called the AMA proposal “flawed.”
“The restrictive involvement of the board of medicine directly contributes to health care access challenges, resulting in continued low health care rankings, geographic disparities in care, and unnecessary regulatory cost in these states,” he said in a press release.
Still, the AMA has vowed to #StopScopeCreep. Securing stricter practice guidelines was a central theme of the association’s recent annual meeting and a goal of its plan to strengthen the physician workforce. The organization invests heavily in advocacy and education efforts to defeat state bills seeking to extend APRN authority. To that end, the AMA Scope of Practice Partnership, a coalition of over 100 medical associations, has awarded members $3.5 million in grants to combat scope-expansion legislation.
The AMA and the American College of Radiology recently partnered to create advocacy materials, including handouts encouraging patients to ask questions such as: “Will a physician be reviewing my chart, lab results, x-rays, and other tests?”
The policy recommendation comes as concerns mount over the potential for significant physician shortages, fueled partly by older physicians’ retirements and doctors reducing hours or exiting the workforce due to pandemic fatigue and burnout.
While practice regulations vary by state, a new federal bill could change that by broadening the authority of APRNs under Medicare and Medicaid guidelines. Introduced in the U.S. House of Representatives in April and supported by the ANA, the Improving Care and Access to Nurses Act would allow APRNs to perform more procedures, including cardiac and pulmonary rehabilitation and certification of terminal illness for hospice, according to an ANA press release.
In the meantime, several state legislatures are considering bills that would expand APRN scope of practice. Utah is the latest to join a growing list of states – about half now – offering full practice authority to NPs.
Other states offer a reduced scope of practice for APRNs, typically requiring a collaborative agreement with a supervising physician. The remaining states enforce tighter regulations and physician oversight.
A recent Medscape survey found that most physicians report having a good rapport with NPs but many have mixed feelings about giving them expanded practice roles, with one-third saying it would harm patient care. Feelings were only slightly more favorable toward PAs. However, about 75% of patients were either neutral or supportive of independent practice for NPs and PAs.
A version of this article first appeared on Medscape.com.
In a move that raises the stakes in doctors’ ongoing scope-creep battle against nonphysician providers, the American Medical Association’s legislative body voted recently to change its policy on the supervision of advanced practice registered nurses (APRNs). AMA’s House of Delegates called for state medical boards to regulate APRNs in addition to nursing boards.
The AMA has long claimed that nonphysician providers, such as nurse practitioners (NPs) and physician assistants (PAs), need greater oversight because expanded scope of practice for advanced practice practitioners threatens patient safety and undermines the physician-led team model.
APRNs have been touted as a solution to expand access to care and reduce disparities, especially in rural and underserved communities, and they have been promoted by organizations such as the National Academy of Medicine. But the AMA disputes that scope expansions are necessary to increase access to care.
The organization that represents the nation’s physicians said in a prepared statement that it opposes scope expansions because removing doctors from the care team results in higher costs to the patient and lower quality care.
Several nursing organizations swiftly criticized the policy recommendation, including the American Nurses Association, the American Association of Nurse Practitioners, and the National Council of State Boards of Nursing.
The policy shift would create more administrative burdens for APRNs and generate “a downstream effect that only hurts patients,” particularly those in underserved communities without timely access to care, ANA president Jennifer Mensik Kennedy, PhD, MBA, RN, NEA-BC, told this news organization.
“The licensing and regulation of APRNs have never required the oversight of state medical boards,” she said, adding that it should remain the obligation of nursing regulatory bodies.
Jon Fanning, MS, CAE, CNED, chief executive officer of the AANP, called the AMA proposal “flawed.”
“The restrictive involvement of the board of medicine directly contributes to health care access challenges, resulting in continued low health care rankings, geographic disparities in care, and unnecessary regulatory cost in these states,” he said in a press release.
Still, the AMA has vowed to #StopScopeCreep. Securing stricter practice guidelines was a central theme of the association’s recent annual meeting and a goal of its plan to strengthen the physician workforce. The organization invests heavily in advocacy and education efforts to defeat state bills seeking to extend APRN authority. To that end, the AMA Scope of Practice Partnership, a coalition of over 100 medical associations, has awarded members $3.5 million in grants to combat scope-expansion legislation.
The AMA and the American College of Radiology recently partnered to create advocacy materials, including handouts encouraging patients to ask questions such as: “Will a physician be reviewing my chart, lab results, x-rays, and other tests?”
The policy recommendation comes as concerns mount over the potential for significant physician shortages, fueled partly by older physicians’ retirements and doctors reducing hours or exiting the workforce due to pandemic fatigue and burnout.
While practice regulations vary by state, a new federal bill could change that by broadening the authority of APRNs under Medicare and Medicaid guidelines. Introduced in the U.S. House of Representatives in April and supported by the ANA, the Improving Care and Access to Nurses Act would allow APRNs to perform more procedures, including cardiac and pulmonary rehabilitation and certification of terminal illness for hospice, according to an ANA press release.
In the meantime, several state legislatures are considering bills that would expand APRN scope of practice. Utah is the latest to join a growing list of states – about half now – offering full practice authority to NPs.
Other states offer a reduced scope of practice for APRNs, typically requiring a collaborative agreement with a supervising physician. The remaining states enforce tighter regulations and physician oversight.
A recent Medscape survey found that most physicians report having a good rapport with NPs but many have mixed feelings about giving them expanded practice roles, with one-third saying it would harm patient care. Feelings were only slightly more favorable toward PAs. However, about 75% of patients were either neutral or supportive of independent practice for NPs and PAs.
A version of this article first appeared on Medscape.com.
Nearly one in five in U.S. still hadn’t gotten COVID by end of 2022
, according to a new estimate.
The findings came from an analysis of blood donations. The Centers for Disease Control and Prevention analyzed donor blood from 143,000 people every 3 months during 2022, looking for the presence of COVID antibodies that meant a person had previously been infected with the virus. The prevalence of antibodies from previous infections steadily rose throughout the year. Antibodies from prior infection were found in 49% of donors as of Feb. 15, 2022, 59% of donors as of May 15, 2022, 70% of donors as of Aug. 15, 2022, and 78% of donors as of Nov. 15, 2022.
Donor blood also was analyzed for the presence of antibodies known to come from COVID vaccination. When the vaccine-induced and infection-induced antibody data were combined, the CDC estimated that 97% of people had antibodies as of the end of the 2022.
In the report, CDC authors explained that while the presence of antibodies is related to protection from infection and to less severe disease, the level of antibodies that a person has can vary. The authors said that no standards have yet been set that show a minimum level of antibodies needed to provide protection.
As of July 3, more than 1.1 million people had died in the United States from COVID-19, according to CDC data. Deaths for the first half of 2023 are down dramatically, compared with the first 3 years of the pandemic, with just 41,538 death certificates this year listing the virus as an underlying or contributing cause. About two in three COVID deaths this year occurred in a hospital or nursing home, and 89% of people who died from the virus this year have been age 65 or older.
A version of this article first appeared on WebMD.com.
, according to a new estimate.
The findings came from an analysis of blood donations. The Centers for Disease Control and Prevention analyzed donor blood from 143,000 people every 3 months during 2022, looking for the presence of COVID antibodies that meant a person had previously been infected with the virus. The prevalence of antibodies from previous infections steadily rose throughout the year. Antibodies from prior infection were found in 49% of donors as of Feb. 15, 2022, 59% of donors as of May 15, 2022, 70% of donors as of Aug. 15, 2022, and 78% of donors as of Nov. 15, 2022.
Donor blood also was analyzed for the presence of antibodies known to come from COVID vaccination. When the vaccine-induced and infection-induced antibody data were combined, the CDC estimated that 97% of people had antibodies as of the end of the 2022.
In the report, CDC authors explained that while the presence of antibodies is related to protection from infection and to less severe disease, the level of antibodies that a person has can vary. The authors said that no standards have yet been set that show a minimum level of antibodies needed to provide protection.
As of July 3, more than 1.1 million people had died in the United States from COVID-19, according to CDC data. Deaths for the first half of 2023 are down dramatically, compared with the first 3 years of the pandemic, with just 41,538 death certificates this year listing the virus as an underlying or contributing cause. About two in three COVID deaths this year occurred in a hospital or nursing home, and 89% of people who died from the virus this year have been age 65 or older.
A version of this article first appeared on WebMD.com.
, according to a new estimate.
The findings came from an analysis of blood donations. The Centers for Disease Control and Prevention analyzed donor blood from 143,000 people every 3 months during 2022, looking for the presence of COVID antibodies that meant a person had previously been infected with the virus. The prevalence of antibodies from previous infections steadily rose throughout the year. Antibodies from prior infection were found in 49% of donors as of Feb. 15, 2022, 59% of donors as of May 15, 2022, 70% of donors as of Aug. 15, 2022, and 78% of donors as of Nov. 15, 2022.
Donor blood also was analyzed for the presence of antibodies known to come from COVID vaccination. When the vaccine-induced and infection-induced antibody data were combined, the CDC estimated that 97% of people had antibodies as of the end of the 2022.
In the report, CDC authors explained that while the presence of antibodies is related to protection from infection and to less severe disease, the level of antibodies that a person has can vary. The authors said that no standards have yet been set that show a minimum level of antibodies needed to provide protection.
As of July 3, more than 1.1 million people had died in the United States from COVID-19, according to CDC data. Deaths for the first half of 2023 are down dramatically, compared with the first 3 years of the pandemic, with just 41,538 death certificates this year listing the virus as an underlying or contributing cause. About two in three COVID deaths this year occurred in a hospital or nursing home, and 89% of people who died from the virus this year have been age 65 or older.
A version of this article first appeared on WebMD.com.
Treating obesity: Will new drugs end the crisis?
This is the second in a three-part series on the obesity crisis. Part one tackles a complicated question – why does the obesity rate keep rising despite our efforts to stop it? – and can be found here. Part three shows how doctors and patients can make treatment better and can be found here.
In the mid-1980s, Louis J. Aronne, MD, strolled into a lab at Rockefeller University in New York where a colleague was breeding mice. “I will never forget what he showed me,” said Dr. Aronne, now the director of obesity research and treatment at Weill Cornell Medicine in New York. “He had a cage with 10 mice, one severely obese and the others normal weight. He took blood from one of the thin mice and gave it to the fat mouse.”
When Dr. Aronne returned 3 days later, that obese mouse had turned thin.
It was proof of something Dr. Aronne already suspected: Obesity had biological causes and wasn’t just a failure of willpower.
Years later, in 1994, that research led to the discovery of leptin, a hormone released from fat cells that’s involved in the regulation of body weight. It was a watershed moment in obesity research.
Since then, Dr. Aronne and others have worked to build the clinical field of obesity medicine, attempting to shift the public and medical view of obesity from a purely behavioral issue to a disease worthy of medical treatment.
All the while, the U.S. obesity rate soared.
Now, another watershed moment: We finally have highly effective obesity drugs. The hype is real, and so are the weight loss results.
“I’ve been saying for 30 years that when we find treatments that really work, people aren’t going to believe the results,” Dr. Aronne said. “It took longer than I expected, but it’s gratifying now to see.”
The big question
The emerging class of obesity medications known as GLP-1 agonists is indeed a game changer. The weight loss drug semaglutide (Ozempic, Wegovy) showed groundbreaking results, and studies suggest a parade of even more impressive drugs is on the way.
Yes, the drugs offer new hope to millions with obesity complications. But to truly turn the tide on our 42% obesity rate, much more work remains to be done, researchers said, including answering a big question:
How do these weight loss drugs work?
“We have new blockbuster drugs, and we don’t even know why they reduce body weight,” said Samuel Klein, MD, professor of medicine and nutritional science at Washington University in St. Louis. “It was by accident that this was discovered.”
Oops, we created a weight loss drug
Developed to treat diabetes, the GLP-1 drugs’ weight loss effects were a surprise. Now that those effects are confirmed, pharmaceutical companies and researchers are racing to figure out how these drugs work.
In the 1960s, scientists discovered the incretin effect – when you eat glucose (sugar), your body makes more insulin than it does if glucose is given intravenously. Glucose passes through the GI tract and the gut releases hormones that stimulate insulin secretion. It’s “essentially a feed-forward signal to your pancreas to tell it, ‘By the way, you need to be ready because there’s a bunch of glucose coming,’ ” said Randy Seeley, MD, director of the Michigan Nutrition Obesity Research Center, funded by the National Institutes of Health.
One of these hormones – or “incretins” – is GLP-1. In experiments, people with type 2 diabetes who were hooked up to GLP-1 saw their blood sugar go down.
“That led to the idea that if we could take this native hormone and make it last longer, we’d have a therapy for type 2 diabetes,” said Dr. Seeley. Thanks to a GLP-1-like compound in the saliva of the Gila monster, that idea became reality in the 2000s.
Along the way, a surprising side finding came to light: In early trials, diabetes patients on these drugs dropped weight.
Both Ozempic and Wegovy – brand names for semaglutide – are once-weekly injections (pill forms to treat obesity are on the way), but the latter is a higher dose.
“That dose results in about 40% of patients in the clinical trials achieving a 20% weight loss. We’ve just had nothing like that in terms of efficacy before,” said Dr. Seeley, who has worked with some of the drug companies (including Novo Nordisk, the maker of Ozempic and Wegovy, and Eli Lilly, maker of Mounjaro) that market the GLP-1s.
By contrast, semaglutide’s once-a-day predecessor liraglutide (Saxenda, made by Novo Nordisk) can lead to about 10% weight loss.
“And one of the ironies is, we don’t really know why,” Dr. Seeley said. “We don’t know why semaglutide is a better molecule for weight loss than liraglutide.”
Initially, scientists believed that the drugs, in addition to telling the pancreas to secrete more insulin, were also signaling the brain that you’re full. “Turns out that’s not really the way it works,” Dr. Seeley said. “GLP-1 made from your gut probably doesn’t get into your brain very much. But you make GLP-1 in your brain as well.”
For weight loss, it’s the brain’s GLP-1 system, not the gut’s, that the drugs are thought to hijack. But exactly which parts of the brain they affect and how is unknown. “That’s something lots of people are working on, including our own lab,” Dr. Seeley said. (Another surprise: The drugs may have potential as an anti-addiction treatment.)
The diabetes medication tirzepatide (Mounjaro), expected to be approved for weight loss as early as this year, is also a weekly injection, but it has a unique feature: It starts a response not just for GLP-1 but also for another incretin called GIP. Turns out, two is better than one: Trial participants on tirzepatide lost up to 22.5% of their body weight.
More of these hybrid drugs are on the way, Dr. Seeley said. In mid-stage clinical trials, the drug retatrutide, which targets three hormones, led to 24% weight loss. “The idea is the more bullets we can load into the gun, the more we can push the biology into a place where it’s easier to lose weight.”
Shifting from prevention to damage control
Less invasive and more scalable than surgery (only 1% of the eligible population gets bariatric surgery), the drugs offer doctors a safe, effective way to treat many patients with obesity. That’s cause for excitement, but concerns remain because they are expensive, costing about $800 to $1,300 per month out of pocket. Many health insurers, including Medicare, do not cover them for weight loss.
“You have this significant advance in obesity treatment, but very few will be able to access it,” said Gary Foster, PhD, adjunct professor of psychology in psychiatry at the University of Pennsylvania, Philadelphia, and chief scientific officer at WW (formerly Weight Watchers).
There is a push, including a proposed bill, to get Medicare to cover obesity medication. But given the expense of the drugs, the health economics do not support that move, according to an editorial in the New England Journal of Medicine. If Medicare were to cover obesity meds, the budget impact would likely be huge, potentially driving up premiums. If other payers followed suit, the impact could be felt across the U.S. health care system.
Other drawbacks include side effects – including nausea, diarrhea, stomach pain, and vomiting – that can be so bad that some patients can’t tolerate them.
And critically, the drugs do not deal with the root cause of the problem, said Robert Lustig, MD, an endocrinologist and pediatrician at the University of California, San Francisco, who has suggested that excess insulin is driving obesity. “No one has the disease that these drugs are treating. No one has GLP-1 deficiency. They’re bypassing the problem. They’re band-aiding the problem.”
Because the drugs work by mimicking starvation – they appear to curb hunger, so you eat less – people on them lose not just fat but also healthy lean mass, Dr. Lustig said.
Concerns about pancreatitis did not really bear out in postmarketing reports. (The drugs are still not recommended in people with pancreatitis or multiple endocrine neoplasia.) But predicting longer-term outcomes can be difficult, noted Dr. Lustig.
Then there are philosophical questions, said James Hill, PhD, director of the Nutrition Obesity Research Center at the University of Alabama at Birmingham.. “If you’re continuing to not exercise and eat not healthy foods and take a medication, is that success? Have we won when people are at a lower weight but not doing a healthy behavior?”
‘We can’t treat our way out of this’
The fact is, ending the obesity epidemic is a tall order, even for drugs as impressive as these.
“We can’t treat our way out of this,” said Jamy Ard, MD, codirector of Wake Forest Baptist Health Weight Management Center in Winston-Salem, N.C. “The treatments we have now are great, and there will be more coming. But we do need to figure out the prevention side of things.”
Dr. Seeley agreed but added that we can’t diet-and-exercise our way out either.
“There’s no switch to be flipped,” Dr. Seeley said. “If you told me we shouldn’t spend all this money on these drugs, we should spend it on prevention – great! What would we do?”
And prevention efforts won’t help the millions already living with health problems from obesity, Dr. Aronne said.
“Getting people to stop smoking prevents lung cancer. But stopping smoking doesn’t treat lung cancer,” Dr. Aronne said. “Once the physical changes occur in the lung that cause a tumor to grow, it’s too late. You have to think of obesity the same way.”
Dr. Seeley pointed out that “fearmongering” around the drugs highlights our lingering bias that obesity is a lifestyle issue that should not be medically treated.
“People say, ‘When you stop taking it, you’re going to gain the weight back,’ ” Dr. Seeley said. “There’s truth to that, but when you stop taking your hypertension medication, your blood pressure goes up. We don’t think of that as a [reason] for why you shouldn’t take your blood pressure medication. But that gets trumpeted into all these conversations about whether people [with obesity] should be treated at all.”
Like obesity, blood pressure was once thought to be a behavioral problem, Dr. Aronne said. But blood pressure meds prevent heart attacks and strokes. And it’s likely obesity meds will do the same.
One 55-year-old patient on the road to kidney failure lost weight on obesity medications, including semaglutide, Dr. Aronne said. Now, 6 years later, his kidney function is back to normal. “Normally, we think of kidney disease as irreversible,” Dr. Aronne said.
In that respect, these drugs should save money in the long run by virtue of heading off those health care costs, said Dr. Seeley, who imagines a future where obesity is not gone but better managed, like high blood pressure is now.
In the end, the drugs are another step toward what Dr. Aronne and many others have always pushed for: Treating obesity as a disease.
A version of this article originally appeared on WebMD.com.
This is the second in a three-part series on the obesity crisis. Part one tackles a complicated question – why does the obesity rate keep rising despite our efforts to stop it? – and can be found here. Part three shows how doctors and patients can make treatment better and can be found here.
In the mid-1980s, Louis J. Aronne, MD, strolled into a lab at Rockefeller University in New York where a colleague was breeding mice. “I will never forget what he showed me,” said Dr. Aronne, now the director of obesity research and treatment at Weill Cornell Medicine in New York. “He had a cage with 10 mice, one severely obese and the others normal weight. He took blood from one of the thin mice and gave it to the fat mouse.”
When Dr. Aronne returned 3 days later, that obese mouse had turned thin.
It was proof of something Dr. Aronne already suspected: Obesity had biological causes and wasn’t just a failure of willpower.
Years later, in 1994, that research led to the discovery of leptin, a hormone released from fat cells that’s involved in the regulation of body weight. It was a watershed moment in obesity research.
Since then, Dr. Aronne and others have worked to build the clinical field of obesity medicine, attempting to shift the public and medical view of obesity from a purely behavioral issue to a disease worthy of medical treatment.
All the while, the U.S. obesity rate soared.
Now, another watershed moment: We finally have highly effective obesity drugs. The hype is real, and so are the weight loss results.
“I’ve been saying for 30 years that when we find treatments that really work, people aren’t going to believe the results,” Dr. Aronne said. “It took longer than I expected, but it’s gratifying now to see.”
The big question
The emerging class of obesity medications known as GLP-1 agonists is indeed a game changer. The weight loss drug semaglutide (Ozempic, Wegovy) showed groundbreaking results, and studies suggest a parade of even more impressive drugs is on the way.
Yes, the drugs offer new hope to millions with obesity complications. But to truly turn the tide on our 42% obesity rate, much more work remains to be done, researchers said, including answering a big question:
How do these weight loss drugs work?
“We have new blockbuster drugs, and we don’t even know why they reduce body weight,” said Samuel Klein, MD, professor of medicine and nutritional science at Washington University in St. Louis. “It was by accident that this was discovered.”
Oops, we created a weight loss drug
Developed to treat diabetes, the GLP-1 drugs’ weight loss effects were a surprise. Now that those effects are confirmed, pharmaceutical companies and researchers are racing to figure out how these drugs work.
In the 1960s, scientists discovered the incretin effect – when you eat glucose (sugar), your body makes more insulin than it does if glucose is given intravenously. Glucose passes through the GI tract and the gut releases hormones that stimulate insulin secretion. It’s “essentially a feed-forward signal to your pancreas to tell it, ‘By the way, you need to be ready because there’s a bunch of glucose coming,’ ” said Randy Seeley, MD, director of the Michigan Nutrition Obesity Research Center, funded by the National Institutes of Health.
One of these hormones – or “incretins” – is GLP-1. In experiments, people with type 2 diabetes who were hooked up to GLP-1 saw their blood sugar go down.
“That led to the idea that if we could take this native hormone and make it last longer, we’d have a therapy for type 2 diabetes,” said Dr. Seeley. Thanks to a GLP-1-like compound in the saliva of the Gila monster, that idea became reality in the 2000s.
Along the way, a surprising side finding came to light: In early trials, diabetes patients on these drugs dropped weight.
Both Ozempic and Wegovy – brand names for semaglutide – are once-weekly injections (pill forms to treat obesity are on the way), but the latter is a higher dose.
“That dose results in about 40% of patients in the clinical trials achieving a 20% weight loss. We’ve just had nothing like that in terms of efficacy before,” said Dr. Seeley, who has worked with some of the drug companies (including Novo Nordisk, the maker of Ozempic and Wegovy, and Eli Lilly, maker of Mounjaro) that market the GLP-1s.
By contrast, semaglutide’s once-a-day predecessor liraglutide (Saxenda, made by Novo Nordisk) can lead to about 10% weight loss.
“And one of the ironies is, we don’t really know why,” Dr. Seeley said. “We don’t know why semaglutide is a better molecule for weight loss than liraglutide.”
Initially, scientists believed that the drugs, in addition to telling the pancreas to secrete more insulin, were also signaling the brain that you’re full. “Turns out that’s not really the way it works,” Dr. Seeley said. “GLP-1 made from your gut probably doesn’t get into your brain very much. But you make GLP-1 in your brain as well.”
For weight loss, it’s the brain’s GLP-1 system, not the gut’s, that the drugs are thought to hijack. But exactly which parts of the brain they affect and how is unknown. “That’s something lots of people are working on, including our own lab,” Dr. Seeley said. (Another surprise: The drugs may have potential as an anti-addiction treatment.)
The diabetes medication tirzepatide (Mounjaro), expected to be approved for weight loss as early as this year, is also a weekly injection, but it has a unique feature: It starts a response not just for GLP-1 but also for another incretin called GIP. Turns out, two is better than one: Trial participants on tirzepatide lost up to 22.5% of their body weight.
More of these hybrid drugs are on the way, Dr. Seeley said. In mid-stage clinical trials, the drug retatrutide, which targets three hormones, led to 24% weight loss. “The idea is the more bullets we can load into the gun, the more we can push the biology into a place where it’s easier to lose weight.”
Shifting from prevention to damage control
Less invasive and more scalable than surgery (only 1% of the eligible population gets bariatric surgery), the drugs offer doctors a safe, effective way to treat many patients with obesity. That’s cause for excitement, but concerns remain because they are expensive, costing about $800 to $1,300 per month out of pocket. Many health insurers, including Medicare, do not cover them for weight loss.
“You have this significant advance in obesity treatment, but very few will be able to access it,” said Gary Foster, PhD, adjunct professor of psychology in psychiatry at the University of Pennsylvania, Philadelphia, and chief scientific officer at WW (formerly Weight Watchers).
There is a push, including a proposed bill, to get Medicare to cover obesity medication. But given the expense of the drugs, the health economics do not support that move, according to an editorial in the New England Journal of Medicine. If Medicare were to cover obesity meds, the budget impact would likely be huge, potentially driving up premiums. If other payers followed suit, the impact could be felt across the U.S. health care system.
Other drawbacks include side effects – including nausea, diarrhea, stomach pain, and vomiting – that can be so bad that some patients can’t tolerate them.
And critically, the drugs do not deal with the root cause of the problem, said Robert Lustig, MD, an endocrinologist and pediatrician at the University of California, San Francisco, who has suggested that excess insulin is driving obesity. “No one has the disease that these drugs are treating. No one has GLP-1 deficiency. They’re bypassing the problem. They’re band-aiding the problem.”
Because the drugs work by mimicking starvation – they appear to curb hunger, so you eat less – people on them lose not just fat but also healthy lean mass, Dr. Lustig said.
Concerns about pancreatitis did not really bear out in postmarketing reports. (The drugs are still not recommended in people with pancreatitis or multiple endocrine neoplasia.) But predicting longer-term outcomes can be difficult, noted Dr. Lustig.
Then there are philosophical questions, said James Hill, PhD, director of the Nutrition Obesity Research Center at the University of Alabama at Birmingham.. “If you’re continuing to not exercise and eat not healthy foods and take a medication, is that success? Have we won when people are at a lower weight but not doing a healthy behavior?”
‘We can’t treat our way out of this’
The fact is, ending the obesity epidemic is a tall order, even for drugs as impressive as these.
“We can’t treat our way out of this,” said Jamy Ard, MD, codirector of Wake Forest Baptist Health Weight Management Center in Winston-Salem, N.C. “The treatments we have now are great, and there will be more coming. But we do need to figure out the prevention side of things.”
Dr. Seeley agreed but added that we can’t diet-and-exercise our way out either.
“There’s no switch to be flipped,” Dr. Seeley said. “If you told me we shouldn’t spend all this money on these drugs, we should spend it on prevention – great! What would we do?”
And prevention efforts won’t help the millions already living with health problems from obesity, Dr. Aronne said.
“Getting people to stop smoking prevents lung cancer. But stopping smoking doesn’t treat lung cancer,” Dr. Aronne said. “Once the physical changes occur in the lung that cause a tumor to grow, it’s too late. You have to think of obesity the same way.”
Dr. Seeley pointed out that “fearmongering” around the drugs highlights our lingering bias that obesity is a lifestyle issue that should not be medically treated.
“People say, ‘When you stop taking it, you’re going to gain the weight back,’ ” Dr. Seeley said. “There’s truth to that, but when you stop taking your hypertension medication, your blood pressure goes up. We don’t think of that as a [reason] for why you shouldn’t take your blood pressure medication. But that gets trumpeted into all these conversations about whether people [with obesity] should be treated at all.”
Like obesity, blood pressure was once thought to be a behavioral problem, Dr. Aronne said. But blood pressure meds prevent heart attacks and strokes. And it’s likely obesity meds will do the same.
One 55-year-old patient on the road to kidney failure lost weight on obesity medications, including semaglutide, Dr. Aronne said. Now, 6 years later, his kidney function is back to normal. “Normally, we think of kidney disease as irreversible,” Dr. Aronne said.
In that respect, these drugs should save money in the long run by virtue of heading off those health care costs, said Dr. Seeley, who imagines a future where obesity is not gone but better managed, like high blood pressure is now.
In the end, the drugs are another step toward what Dr. Aronne and many others have always pushed for: Treating obesity as a disease.
A version of this article originally appeared on WebMD.com.
This is the second in a three-part series on the obesity crisis. Part one tackles a complicated question – why does the obesity rate keep rising despite our efforts to stop it? – and can be found here. Part three shows how doctors and patients can make treatment better and can be found here.
In the mid-1980s, Louis J. Aronne, MD, strolled into a lab at Rockefeller University in New York where a colleague was breeding mice. “I will never forget what he showed me,” said Dr. Aronne, now the director of obesity research and treatment at Weill Cornell Medicine in New York. “He had a cage with 10 mice, one severely obese and the others normal weight. He took blood from one of the thin mice and gave it to the fat mouse.”
When Dr. Aronne returned 3 days later, that obese mouse had turned thin.
It was proof of something Dr. Aronne already suspected: Obesity had biological causes and wasn’t just a failure of willpower.
Years later, in 1994, that research led to the discovery of leptin, a hormone released from fat cells that’s involved in the regulation of body weight. It was a watershed moment in obesity research.
Since then, Dr. Aronne and others have worked to build the clinical field of obesity medicine, attempting to shift the public and medical view of obesity from a purely behavioral issue to a disease worthy of medical treatment.
All the while, the U.S. obesity rate soared.
Now, another watershed moment: We finally have highly effective obesity drugs. The hype is real, and so are the weight loss results.
“I’ve been saying for 30 years that when we find treatments that really work, people aren’t going to believe the results,” Dr. Aronne said. “It took longer than I expected, but it’s gratifying now to see.”
The big question
The emerging class of obesity medications known as GLP-1 agonists is indeed a game changer. The weight loss drug semaglutide (Ozempic, Wegovy) showed groundbreaking results, and studies suggest a parade of even more impressive drugs is on the way.
Yes, the drugs offer new hope to millions with obesity complications. But to truly turn the tide on our 42% obesity rate, much more work remains to be done, researchers said, including answering a big question:
How do these weight loss drugs work?
“We have new blockbuster drugs, and we don’t even know why they reduce body weight,” said Samuel Klein, MD, professor of medicine and nutritional science at Washington University in St. Louis. “It was by accident that this was discovered.”
Oops, we created a weight loss drug
Developed to treat diabetes, the GLP-1 drugs’ weight loss effects were a surprise. Now that those effects are confirmed, pharmaceutical companies and researchers are racing to figure out how these drugs work.
In the 1960s, scientists discovered the incretin effect – when you eat glucose (sugar), your body makes more insulin than it does if glucose is given intravenously. Glucose passes through the GI tract and the gut releases hormones that stimulate insulin secretion. It’s “essentially a feed-forward signal to your pancreas to tell it, ‘By the way, you need to be ready because there’s a bunch of glucose coming,’ ” said Randy Seeley, MD, director of the Michigan Nutrition Obesity Research Center, funded by the National Institutes of Health.
One of these hormones – or “incretins” – is GLP-1. In experiments, people with type 2 diabetes who were hooked up to GLP-1 saw their blood sugar go down.
“That led to the idea that if we could take this native hormone and make it last longer, we’d have a therapy for type 2 diabetes,” said Dr. Seeley. Thanks to a GLP-1-like compound in the saliva of the Gila monster, that idea became reality in the 2000s.
Along the way, a surprising side finding came to light: In early trials, diabetes patients on these drugs dropped weight.
Both Ozempic and Wegovy – brand names for semaglutide – are once-weekly injections (pill forms to treat obesity are on the way), but the latter is a higher dose.
“That dose results in about 40% of patients in the clinical trials achieving a 20% weight loss. We’ve just had nothing like that in terms of efficacy before,” said Dr. Seeley, who has worked with some of the drug companies (including Novo Nordisk, the maker of Ozempic and Wegovy, and Eli Lilly, maker of Mounjaro) that market the GLP-1s.
By contrast, semaglutide’s once-a-day predecessor liraglutide (Saxenda, made by Novo Nordisk) can lead to about 10% weight loss.
“And one of the ironies is, we don’t really know why,” Dr. Seeley said. “We don’t know why semaglutide is a better molecule for weight loss than liraglutide.”
Initially, scientists believed that the drugs, in addition to telling the pancreas to secrete more insulin, were also signaling the brain that you’re full. “Turns out that’s not really the way it works,” Dr. Seeley said. “GLP-1 made from your gut probably doesn’t get into your brain very much. But you make GLP-1 in your brain as well.”
For weight loss, it’s the brain’s GLP-1 system, not the gut’s, that the drugs are thought to hijack. But exactly which parts of the brain they affect and how is unknown. “That’s something lots of people are working on, including our own lab,” Dr. Seeley said. (Another surprise: The drugs may have potential as an anti-addiction treatment.)
The diabetes medication tirzepatide (Mounjaro), expected to be approved for weight loss as early as this year, is also a weekly injection, but it has a unique feature: It starts a response not just for GLP-1 but also for another incretin called GIP. Turns out, two is better than one: Trial participants on tirzepatide lost up to 22.5% of their body weight.
More of these hybrid drugs are on the way, Dr. Seeley said. In mid-stage clinical trials, the drug retatrutide, which targets three hormones, led to 24% weight loss. “The idea is the more bullets we can load into the gun, the more we can push the biology into a place where it’s easier to lose weight.”
Shifting from prevention to damage control
Less invasive and more scalable than surgery (only 1% of the eligible population gets bariatric surgery), the drugs offer doctors a safe, effective way to treat many patients with obesity. That’s cause for excitement, but concerns remain because they are expensive, costing about $800 to $1,300 per month out of pocket. Many health insurers, including Medicare, do not cover them for weight loss.
“You have this significant advance in obesity treatment, but very few will be able to access it,” said Gary Foster, PhD, adjunct professor of psychology in psychiatry at the University of Pennsylvania, Philadelphia, and chief scientific officer at WW (formerly Weight Watchers).
There is a push, including a proposed bill, to get Medicare to cover obesity medication. But given the expense of the drugs, the health economics do not support that move, according to an editorial in the New England Journal of Medicine. If Medicare were to cover obesity meds, the budget impact would likely be huge, potentially driving up premiums. If other payers followed suit, the impact could be felt across the U.S. health care system.
Other drawbacks include side effects – including nausea, diarrhea, stomach pain, and vomiting – that can be so bad that some patients can’t tolerate them.
And critically, the drugs do not deal with the root cause of the problem, said Robert Lustig, MD, an endocrinologist and pediatrician at the University of California, San Francisco, who has suggested that excess insulin is driving obesity. “No one has the disease that these drugs are treating. No one has GLP-1 deficiency. They’re bypassing the problem. They’re band-aiding the problem.”
Because the drugs work by mimicking starvation – they appear to curb hunger, so you eat less – people on them lose not just fat but also healthy lean mass, Dr. Lustig said.
Concerns about pancreatitis did not really bear out in postmarketing reports. (The drugs are still not recommended in people with pancreatitis or multiple endocrine neoplasia.) But predicting longer-term outcomes can be difficult, noted Dr. Lustig.
Then there are philosophical questions, said James Hill, PhD, director of the Nutrition Obesity Research Center at the University of Alabama at Birmingham.. “If you’re continuing to not exercise and eat not healthy foods and take a medication, is that success? Have we won when people are at a lower weight but not doing a healthy behavior?”
‘We can’t treat our way out of this’
The fact is, ending the obesity epidemic is a tall order, even for drugs as impressive as these.
“We can’t treat our way out of this,” said Jamy Ard, MD, codirector of Wake Forest Baptist Health Weight Management Center in Winston-Salem, N.C. “The treatments we have now are great, and there will be more coming. But we do need to figure out the prevention side of things.”
Dr. Seeley agreed but added that we can’t diet-and-exercise our way out either.
“There’s no switch to be flipped,” Dr. Seeley said. “If you told me we shouldn’t spend all this money on these drugs, we should spend it on prevention – great! What would we do?”
And prevention efforts won’t help the millions already living with health problems from obesity, Dr. Aronne said.
“Getting people to stop smoking prevents lung cancer. But stopping smoking doesn’t treat lung cancer,” Dr. Aronne said. “Once the physical changes occur in the lung that cause a tumor to grow, it’s too late. You have to think of obesity the same way.”
Dr. Seeley pointed out that “fearmongering” around the drugs highlights our lingering bias that obesity is a lifestyle issue that should not be medically treated.
“People say, ‘When you stop taking it, you’re going to gain the weight back,’ ” Dr. Seeley said. “There’s truth to that, but when you stop taking your hypertension medication, your blood pressure goes up. We don’t think of that as a [reason] for why you shouldn’t take your blood pressure medication. But that gets trumpeted into all these conversations about whether people [with obesity] should be treated at all.”
Like obesity, blood pressure was once thought to be a behavioral problem, Dr. Aronne said. But blood pressure meds prevent heart attacks and strokes. And it’s likely obesity meds will do the same.
One 55-year-old patient on the road to kidney failure lost weight on obesity medications, including semaglutide, Dr. Aronne said. Now, 6 years later, his kidney function is back to normal. “Normally, we think of kidney disease as irreversible,” Dr. Aronne said.
In that respect, these drugs should save money in the long run by virtue of heading off those health care costs, said Dr. Seeley, who imagines a future where obesity is not gone but better managed, like high blood pressure is now.
In the end, the drugs are another step toward what Dr. Aronne and many others have always pushed for: Treating obesity as a disease.
A version of this article originally appeared on WebMD.com.
Higher alcohol consumption linked to early-onset CRC
TOPLINE:
Higher levels of alcohol consumption appear to increase an individual’s risk of early-onset colorectal cancer (CRC), particularly distal colon and rectal cancers, according to a population-based study from South Korea.
METHODOLOGY:
- The investigators retrospectively compared average daily alcohol consumption with early-onset CRC risk among nearly 5.7 million adults younger than 50 years, using data from the Korean National Health Insurance Service.
- Alcohol consumption levels were defined as nondrinker, light (< 10 g/day or < 0.7 U.S. drinks/day), moderate (10-30 g/day for men, 10-20 g/day for women), and heavy (≥ 30 g/day or ≥ 2.1 drinks/day for men, ≥ 20 g/day or ≥ 1.4 drinks/day for women).
- The primary outcome was incidence of early-onset CRC diagnosed before age 50. Models were adjusted for age, sex, smoking status, exercise, and income, as well as for comorbidities.
TAKEAWAY:
- Overall, 8,314 incident early-onset CRC cases occurred during the mean follow-up period of 7.4 years.
- Compared with light drinking, moderate and heavy drinking were associated with a significantly elevated risk of early-onset CRC (adjusted hazard ratio, 1.09 and 1.20, respectively); by sex, significant associations were found only among men.
- Among men, heavy drinking vs. light drinking was associated with a 26% increased risk of distal colon cancer, a 17% higher risk of rectal cancer, and a 29% higher risk of unspecified colon cancer (but not proximal colon cancer).
- Among women, moderate drinking was associated with a 47% increased risk of distal colon cancer. Among nondrinkers, there was a 14% reduced risk of rectal cancer, compared with light drinkers.
IN PRACTICE:
“This population-based study provides evidence that higher levels of alcohol consumption may increase the risk of early-onset CRC,” the investigators concluded. “[E]ffective interventions are required to discourage alcohol consumption among young people and to tailor CRC screening approaches for high-risk individuals.”
SOURCE:
The study was led by researchers at Seoul National University, South Korea. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Study limitations include self-reported alcohol consumption. Data were missing for a higher number of male participants and younger participants, and there was a potential problem related to multiple comparisons and confounders. Only Korean individuals were included in the study, so larger studies involving various races are needed.
DISCLOSURES:
Funding was provided by grants from the Korea Health Technology R&D Project and the Ministry of Health and Welfare, Republic of Korea. No potential conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
Higher levels of alcohol consumption appear to increase an individual’s risk of early-onset colorectal cancer (CRC), particularly distal colon and rectal cancers, according to a population-based study from South Korea.
METHODOLOGY:
- The investigators retrospectively compared average daily alcohol consumption with early-onset CRC risk among nearly 5.7 million adults younger than 50 years, using data from the Korean National Health Insurance Service.
- Alcohol consumption levels were defined as nondrinker, light (< 10 g/day or < 0.7 U.S. drinks/day), moderate (10-30 g/day for men, 10-20 g/day for women), and heavy (≥ 30 g/day or ≥ 2.1 drinks/day for men, ≥ 20 g/day or ≥ 1.4 drinks/day for women).
- The primary outcome was incidence of early-onset CRC diagnosed before age 50. Models were adjusted for age, sex, smoking status, exercise, and income, as well as for comorbidities.
TAKEAWAY:
- Overall, 8,314 incident early-onset CRC cases occurred during the mean follow-up period of 7.4 years.
- Compared with light drinking, moderate and heavy drinking were associated with a significantly elevated risk of early-onset CRC (adjusted hazard ratio, 1.09 and 1.20, respectively); by sex, significant associations were found only among men.
- Among men, heavy drinking vs. light drinking was associated with a 26% increased risk of distal colon cancer, a 17% higher risk of rectal cancer, and a 29% higher risk of unspecified colon cancer (but not proximal colon cancer).
- Among women, moderate drinking was associated with a 47% increased risk of distal colon cancer. Among nondrinkers, there was a 14% reduced risk of rectal cancer, compared with light drinkers.
IN PRACTICE:
“This population-based study provides evidence that higher levels of alcohol consumption may increase the risk of early-onset CRC,” the investigators concluded. “[E]ffective interventions are required to discourage alcohol consumption among young people and to tailor CRC screening approaches for high-risk individuals.”
SOURCE:
The study was led by researchers at Seoul National University, South Korea. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Study limitations include self-reported alcohol consumption. Data were missing for a higher number of male participants and younger participants, and there was a potential problem related to multiple comparisons and confounders. Only Korean individuals were included in the study, so larger studies involving various races are needed.
DISCLOSURES:
Funding was provided by grants from the Korea Health Technology R&D Project and the Ministry of Health and Welfare, Republic of Korea. No potential conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
Higher levels of alcohol consumption appear to increase an individual’s risk of early-onset colorectal cancer (CRC), particularly distal colon and rectal cancers, according to a population-based study from South Korea.
METHODOLOGY:
- The investigators retrospectively compared average daily alcohol consumption with early-onset CRC risk among nearly 5.7 million adults younger than 50 years, using data from the Korean National Health Insurance Service.
- Alcohol consumption levels were defined as nondrinker, light (< 10 g/day or < 0.7 U.S. drinks/day), moderate (10-30 g/day for men, 10-20 g/day for women), and heavy (≥ 30 g/day or ≥ 2.1 drinks/day for men, ≥ 20 g/day or ≥ 1.4 drinks/day for women).
- The primary outcome was incidence of early-onset CRC diagnosed before age 50. Models were adjusted for age, sex, smoking status, exercise, and income, as well as for comorbidities.
TAKEAWAY:
- Overall, 8,314 incident early-onset CRC cases occurred during the mean follow-up period of 7.4 years.
- Compared with light drinking, moderate and heavy drinking were associated with a significantly elevated risk of early-onset CRC (adjusted hazard ratio, 1.09 and 1.20, respectively); by sex, significant associations were found only among men.
- Among men, heavy drinking vs. light drinking was associated with a 26% increased risk of distal colon cancer, a 17% higher risk of rectal cancer, and a 29% higher risk of unspecified colon cancer (but not proximal colon cancer).
- Among women, moderate drinking was associated with a 47% increased risk of distal colon cancer. Among nondrinkers, there was a 14% reduced risk of rectal cancer, compared with light drinkers.
IN PRACTICE:
“This population-based study provides evidence that higher levels of alcohol consumption may increase the risk of early-onset CRC,” the investigators concluded. “[E]ffective interventions are required to discourage alcohol consumption among young people and to tailor CRC screening approaches for high-risk individuals.”
SOURCE:
The study was led by researchers at Seoul National University, South Korea. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Study limitations include self-reported alcohol consumption. Data were missing for a higher number of male participants and younger participants, and there was a potential problem related to multiple comparisons and confounders. Only Korean individuals were included in the study, so larger studies involving various races are needed.
DISCLOSURES:
Funding was provided by grants from the Korea Health Technology R&D Project and the Ministry of Health and Welfare, Republic of Korea. No potential conflicts of interest were reported.
A version of this article first appeared on Medscape.com.