User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Women are not being warned that anesthetic may reduce birth pill efficacy
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
FROM EUROANAESTHESIA
Abbott baby formula plant in Michigan reopens
The Abbott baby formula factory in Sturgis, Mich., has reopened, a move that could ease the nationwide baby formula shortage.
“Abbott is restarting infant formula production at its Sturgis, Mich., facility today after meeting initial requirements agreed to with the U.S. Food and Drug Administration as part of the consent decree entered into on May 16,” according to a company statement issued June 4.
“Abbott is starting production of EleCare and other specialty and metabolic formulas, with initial EleCare product release to consumers beginning on or about June 20. We’re also working hard to fulfill the steps necessary to restart production of Similac and other formulas and will do so as soon as we can.”
The FDA began investigating when at least four infants became ill with Cronobacter sakazakii bacteria after consuming infant formula produced in the Sturgis plant. Two infants died. Several Abbott baby formula products were recalled and the Sturgis plant was shut down for months.
Abbott said an investigation found no evidence to link the formulas to the infant illnesses, though bacteria was found in parts of the factory that didn’t have contact with formula.
The FDA entered into a consent decree with Abbott in mid-May that allowed the plant to reopen if the company took corrective actions, including the implementation of a sanitation plan and an environmental monitoring plan and employee training programs. Abbott must also retain an independent expert to monitor operations.
The Abbott shutdown, along with supply chain problems, contributed to a nationwide shortage of formula. Reuters, citing the data firm Datasembly, reported that about 73% of baby products were out of stock nationwide as of May 22.
The shortage is so severe that the federal government authorized the importing of formula from overseas.
“We understand the urgent need for formula and our top priority is getting high-quality, safe formula into the hands of families across America. We will ramp production as quickly as we can while meeting all requirements,” the Abbott statement said.
A version of this article first appeared on Webmd.com.
The Abbott baby formula factory in Sturgis, Mich., has reopened, a move that could ease the nationwide baby formula shortage.
“Abbott is restarting infant formula production at its Sturgis, Mich., facility today after meeting initial requirements agreed to with the U.S. Food and Drug Administration as part of the consent decree entered into on May 16,” according to a company statement issued June 4.
“Abbott is starting production of EleCare and other specialty and metabolic formulas, with initial EleCare product release to consumers beginning on or about June 20. We’re also working hard to fulfill the steps necessary to restart production of Similac and other formulas and will do so as soon as we can.”
The FDA began investigating when at least four infants became ill with Cronobacter sakazakii bacteria after consuming infant formula produced in the Sturgis plant. Two infants died. Several Abbott baby formula products were recalled and the Sturgis plant was shut down for months.
Abbott said an investigation found no evidence to link the formulas to the infant illnesses, though bacteria was found in parts of the factory that didn’t have contact with formula.
The FDA entered into a consent decree with Abbott in mid-May that allowed the plant to reopen if the company took corrective actions, including the implementation of a sanitation plan and an environmental monitoring plan and employee training programs. Abbott must also retain an independent expert to monitor operations.
The Abbott shutdown, along with supply chain problems, contributed to a nationwide shortage of formula. Reuters, citing the data firm Datasembly, reported that about 73% of baby products were out of stock nationwide as of May 22.
The shortage is so severe that the federal government authorized the importing of formula from overseas.
“We understand the urgent need for formula and our top priority is getting high-quality, safe formula into the hands of families across America. We will ramp production as quickly as we can while meeting all requirements,” the Abbott statement said.
A version of this article first appeared on Webmd.com.
The Abbott baby formula factory in Sturgis, Mich., has reopened, a move that could ease the nationwide baby formula shortage.
“Abbott is restarting infant formula production at its Sturgis, Mich., facility today after meeting initial requirements agreed to with the U.S. Food and Drug Administration as part of the consent decree entered into on May 16,” according to a company statement issued June 4.
“Abbott is starting production of EleCare and other specialty and metabolic formulas, with initial EleCare product release to consumers beginning on or about June 20. We’re also working hard to fulfill the steps necessary to restart production of Similac and other formulas and will do so as soon as we can.”
The FDA began investigating when at least four infants became ill with Cronobacter sakazakii bacteria after consuming infant formula produced in the Sturgis plant. Two infants died. Several Abbott baby formula products were recalled and the Sturgis plant was shut down for months.
Abbott said an investigation found no evidence to link the formulas to the infant illnesses, though bacteria was found in parts of the factory that didn’t have contact with formula.
The FDA entered into a consent decree with Abbott in mid-May that allowed the plant to reopen if the company took corrective actions, including the implementation of a sanitation plan and an environmental monitoring plan and employee training programs. Abbott must also retain an independent expert to monitor operations.
The Abbott shutdown, along with supply chain problems, contributed to a nationwide shortage of formula. Reuters, citing the data firm Datasembly, reported that about 73% of baby products were out of stock nationwide as of May 22.
The shortage is so severe that the federal government authorized the importing of formula from overseas.
“We understand the urgent need for formula and our top priority is getting high-quality, safe formula into the hands of families across America. We will ramp production as quickly as we can while meeting all requirements,” the Abbott statement said.
A version of this article first appeared on Webmd.com.
Immunosuppressed rheumatic patients not at high risk of breakthrough COVID-19
COPENHAGEN – Most patients with immune-mediated inflammatory diseases (IMID) should not be considered at high risk for severe COVID-19 breakthrough infections, but those on anti-CD20 therapy are the exception, data from a large prospective, cohort study show.
“Overall, the data are reassuring, with conventional risk factors, such as age, and comorbidities seeming to be more important regarding risk of severe COVID-19 breakthrough infections than rheumatic disease or immunosuppressant medication,” said Laura Boekel, MD, from Amsterdam UMC, who presented the study at the annual European Congress of Rheumatology.
But, she added, there was an exception for anti-CD20 therapy. “This is especially relevant for patients with conventional risk factors that might accumulate, and rheumatologists might want to consider alternative treatment options if possible. It is important to inform patients about the risks of anti-CD20.”
Another study, presented during the same session at the congress by Rebecca Hasseli, MD, from the University of Giessen (Germany) saw no deaths and no COVID-19 related complications in a cohort of triple-vaccinated patients with inflammatory rheumatic diseases, despite a higher median age and a higher rate of comorbidities compared to double-vaccinated and unvaccinated cohorts.
Ingrid Jyssum, MD, from Diakonhjemmet Hospital, Oslo, who presented results of the Nor-vaC study investigating the impact of different DMARDs on the immunogenicity of a third COVID-19 vaccine dose, welcomed the research by Dr. Boekel and Dr. Hasseli.
“The findings of Hasseli are interesting in the light of our data on serological response after the third dose, with a lack of breakthrough infections after three doses corresponding well to the robust antibody response that we found in our cohort,” she remarked. “This is very reassuring for our patients. Our own work together with the findings of Hasseli and Boekel demonstrate that additional vaccine doses are important to keep this population well protected against severe COVID-19 infections.”
The Nor-vaC study was conducted with a cohort of 1,100 patients with inflammatory joint and bowel diseases. “These patients had attenuated antibody responses after two vaccine doses; however, we found that a third vaccine dose brought the humoral response in patients up to the antibody levels that healthy controls had after two doses,” said Dr. Jyssum. “In addition, we found that the decline in antibodies after the third dose was less than the decline seen after the second dose. Importantly, the third dose was safe in our patients, with no new safety issues.”
Breakthrough infections and immunosuppressants
“Like the rest of the world, we were wondering if our patients were at increased risk of COVID-19, and if the immunosuppressants used by these patients influenced their risk,” said Dr. Boekel.
The researchers compared both the incidence and severity of COVID-19 breakthrough infections with the SARS-CoV-2 Delta variant in a population of fully vaccinated IMID patients taking immunosuppressants and controls (IMID patients not taking immunosuppressants and healthy controls).
Two large ongoing, prospective, multicenter cohort studies provided pooled data collected between February and December 2021 using digital questionnaires, standardized electronic case record forms, and medical files.
Finger-prick tests were used to collect blood samples that were analyzed after vaccination against SARS-CoV-2 for anti–receptor-binding domain (RBD) antibodies, and antinucleocapsid antibodies to identify asymptomatic breakthrough infections. Any associations between antibodies and the incidence of breakthrough infections were generated, and results were adjusted for sex, cardiovascular disease, chronic pulmonary disease, obesity, and vaccine type.
The analysis included 3,207 IMID patients taking immunosuppressants, and 1,810 controls (985 IMID patients not on immunosuppressants and 825 healthy controls).
Initially, Dr. Boekel and her colleagues looked at incidence of infections and hospitalizations prior to vaccination, and then after vaccination, which was the main aim of the study.
Prior to vaccination, hospitalization risk for COVID-19 was somewhat higher for IMID patients overall compared with controls, reported Dr. Boekel. “But those treated with anti-CD20 therapy, demonstrated much greater risk for severe disease.”
After the SARS-CoV-2 vaccination campaign began, the researchers then looked at how immunosuppressants influenced humoral response to SARS-CoV-2 vaccination.
“Anti-CD20 therapy showed the greatest impact on humoral immune response after SARS-CoV-2 vaccination,” said Dr. Boekel. Other immunosuppressant drugs had variable effects on humoral and cellular immunity.
Once they had established that immunosuppressant drugs impaired immune responses to SARS-CoV-2 vaccination, the researchers wanted to determine if this affected clinical outcomes. Blood samples taken 28 days after the second vaccination enabled Dr. Boekel and her colleagues to see if antibody production was associated with breakthrough infections.
Breakthrough infections were seen in 5% of patients on immunosuppressants, 5% of patients not on immunosuppressants, and 4% of healthy controls. Also, asymptomatic COVID-19 breakthrough cases were comparable between IMID patients taking immunosuppressants and controls, at 10% in each group.
“We saw that the incidence [of getting COVID-19] was comparable between groups, independent of whether they were receiving immunosuppressants or not, or healthy controls. However, if they developed antibodies against the two vaccinations the chance of getting infected was lower,” reported Dr. Boekel.
Hospitalization (severe disease) rates were also comparable between groups. “Patients with rheumatic diseases, even when treated with immunosuppressants were not at increased risk of severe disease from Delta breakthrough infections,” added the researcher. “Cases that were hospitalized were mainly elderly and those with comorbidities, for example cardiovascular disease and cardiopulmonary disease.”
Hospital admissions were 5.4% in patients on immunosuppressants, 5.7% in those not on immunosuppressants, and 6% in health controls.
However, once again, there was one exception, Dr. Boekel stressed. “Patients treated with anti-CD20 therapy were at increased risk of severe disease and hospitalization.”
Omicron variant has a different transmissibility than Delta, so the researchers continued the study looking at the Omicron variant. The data “were mostly reassuring,” said Dr. Boekel. “As expected, hospitalization rates decreased overall, with the exception of patients on anti-CD20 therapy where, despite overall reduced pathogenicity, patients remain at increased risk.”
She said that they were awaiting long-term data so the data reflect only short-term immunity against Omicron. “However, we included many elderly and patients with comorbidities, so this made the analysis very sensitive to detect severe cases,” she added.
Breakthrough infection among double- and triple-vaccinated patients
A lower rate of COVID-19 related complications and deaths were seen in patients who were triple-vaccinated against SARS-CoV-2, than in double-vaccinated or unvaccinated patients, despite the former having more comorbidities and use of rituximab (Rituxan), said Dr. Hasseli.
“These data support the recommendation of booster vaccination to reduce COVID-19-related mortality in patients with inflammatory rheumatic diseases [IRDs],” she said.
“A small number of COVID-19 cases were seen in patients with IRD after vaccinations, and in a few cases, hospitalizations were required. Breakthrough infections were mostly seen in patients on B-cell depletion therapy,” she added.
Dr. Hasseli and her colleagues looked at the characteristics and outcomes of SARS-CoV-2 breakthrough infections among double- and triple-vaccinated patients with IRD.
“We wanted to understand if patients with IRD are protected in the same way as the general population following vaccination, given that these patients receive drugs that might impair the immune response,” she explained.
Data for analysis were drawn from the German COVID-19-IRD registry covering February 2021 and January 2022, and patients who were double- or triple- vaccinated against COVID-19 either 14 days or more prior to a SARS-CoV-2 infection were included. Type of IRD, vaccine, immunomodulation, comorbidities, and outcome of the infection were compared with 737 unvaccinated IRD patients with COVID-19. Those with prior COVID-19 were excluded.
Cases were stratified by vaccinations status: unvaccinated (1,388 patients, median age 57 years); double vaccinated (462, 56 years) and triple vaccinated (301, 53 years). Body mass index was similar across groups (25-26 kg/m2), and time between SARS-CoV-2 infection and last vaccination was 156 days in double-vaccinated patients, and 62 days in triple-vaccinated patients.
Patients had rheumatoid arthritis in 44.7% and 44.4% of unvaccinated and double-vaccinated patients respectively, but fewer triple-vaccinated patients had RA (37.2%). Triple vaccination was seen in 32.2% of patients with spondyloarthritis, 16.6% connective tissue diseases, 5.3% other vasculitis, and 3.3% ANCA-associated vasculitis. Of triple-vaccinated patients, 26.2% were treated with tumor necrosis factor-alpha (TNF-alpha) inhibitors, and 6.3% with rituximab, while 5.3% were not on immunomodulation. At least 25% were treated with glucocorticoids, reported Dr. Hasseli.
“Arterial hypertension and diabetes, that might be risk factors for COVID-19, were less frequently reported in triple-vaccinated patients. More patients in the double-vaccinated group [42.9%] than the triple-vaccinated [23.8%] reported absence of relevant comorbidities,” she said.
COVID-19 related complications were less often reported in double- and triple-vaccinated groups with hospitalizations at 9.5% and 4.3% in double and triple-vaccinated people respectively.
Dr. Boekel and Dr. Hasseli report no relevant conflicts of interest.
COPENHAGEN – Most patients with immune-mediated inflammatory diseases (IMID) should not be considered at high risk for severe COVID-19 breakthrough infections, but those on anti-CD20 therapy are the exception, data from a large prospective, cohort study show.
“Overall, the data are reassuring, with conventional risk factors, such as age, and comorbidities seeming to be more important regarding risk of severe COVID-19 breakthrough infections than rheumatic disease or immunosuppressant medication,” said Laura Boekel, MD, from Amsterdam UMC, who presented the study at the annual European Congress of Rheumatology.
But, she added, there was an exception for anti-CD20 therapy. “This is especially relevant for patients with conventional risk factors that might accumulate, and rheumatologists might want to consider alternative treatment options if possible. It is important to inform patients about the risks of anti-CD20.”
Another study, presented during the same session at the congress by Rebecca Hasseli, MD, from the University of Giessen (Germany) saw no deaths and no COVID-19 related complications in a cohort of triple-vaccinated patients with inflammatory rheumatic diseases, despite a higher median age and a higher rate of comorbidities compared to double-vaccinated and unvaccinated cohorts.
Ingrid Jyssum, MD, from Diakonhjemmet Hospital, Oslo, who presented results of the Nor-vaC study investigating the impact of different DMARDs on the immunogenicity of a third COVID-19 vaccine dose, welcomed the research by Dr. Boekel and Dr. Hasseli.
“The findings of Hasseli are interesting in the light of our data on serological response after the third dose, with a lack of breakthrough infections after three doses corresponding well to the robust antibody response that we found in our cohort,” she remarked. “This is very reassuring for our patients. Our own work together with the findings of Hasseli and Boekel demonstrate that additional vaccine doses are important to keep this population well protected against severe COVID-19 infections.”
The Nor-vaC study was conducted with a cohort of 1,100 patients with inflammatory joint and bowel diseases. “These patients had attenuated antibody responses after two vaccine doses; however, we found that a third vaccine dose brought the humoral response in patients up to the antibody levels that healthy controls had after two doses,” said Dr. Jyssum. “In addition, we found that the decline in antibodies after the third dose was less than the decline seen after the second dose. Importantly, the third dose was safe in our patients, with no new safety issues.”
Breakthrough infections and immunosuppressants
“Like the rest of the world, we were wondering if our patients were at increased risk of COVID-19, and if the immunosuppressants used by these patients influenced their risk,” said Dr. Boekel.
The researchers compared both the incidence and severity of COVID-19 breakthrough infections with the SARS-CoV-2 Delta variant in a population of fully vaccinated IMID patients taking immunosuppressants and controls (IMID patients not taking immunosuppressants and healthy controls).
Two large ongoing, prospective, multicenter cohort studies provided pooled data collected between February and December 2021 using digital questionnaires, standardized electronic case record forms, and medical files.
Finger-prick tests were used to collect blood samples that were analyzed after vaccination against SARS-CoV-2 for anti–receptor-binding domain (RBD) antibodies, and antinucleocapsid antibodies to identify asymptomatic breakthrough infections. Any associations between antibodies and the incidence of breakthrough infections were generated, and results were adjusted for sex, cardiovascular disease, chronic pulmonary disease, obesity, and vaccine type.
The analysis included 3,207 IMID patients taking immunosuppressants, and 1,810 controls (985 IMID patients not on immunosuppressants and 825 healthy controls).
Initially, Dr. Boekel and her colleagues looked at incidence of infections and hospitalizations prior to vaccination, and then after vaccination, which was the main aim of the study.
Prior to vaccination, hospitalization risk for COVID-19 was somewhat higher for IMID patients overall compared with controls, reported Dr. Boekel. “But those treated with anti-CD20 therapy, demonstrated much greater risk for severe disease.”
After the SARS-CoV-2 vaccination campaign began, the researchers then looked at how immunosuppressants influenced humoral response to SARS-CoV-2 vaccination.
“Anti-CD20 therapy showed the greatest impact on humoral immune response after SARS-CoV-2 vaccination,” said Dr. Boekel. Other immunosuppressant drugs had variable effects on humoral and cellular immunity.
Once they had established that immunosuppressant drugs impaired immune responses to SARS-CoV-2 vaccination, the researchers wanted to determine if this affected clinical outcomes. Blood samples taken 28 days after the second vaccination enabled Dr. Boekel and her colleagues to see if antibody production was associated with breakthrough infections.
Breakthrough infections were seen in 5% of patients on immunosuppressants, 5% of patients not on immunosuppressants, and 4% of healthy controls. Also, asymptomatic COVID-19 breakthrough cases were comparable between IMID patients taking immunosuppressants and controls, at 10% in each group.
“We saw that the incidence [of getting COVID-19] was comparable between groups, independent of whether they were receiving immunosuppressants or not, or healthy controls. However, if they developed antibodies against the two vaccinations the chance of getting infected was lower,” reported Dr. Boekel.
Hospitalization (severe disease) rates were also comparable between groups. “Patients with rheumatic diseases, even when treated with immunosuppressants were not at increased risk of severe disease from Delta breakthrough infections,” added the researcher. “Cases that were hospitalized were mainly elderly and those with comorbidities, for example cardiovascular disease and cardiopulmonary disease.”
Hospital admissions were 5.4% in patients on immunosuppressants, 5.7% in those not on immunosuppressants, and 6% in health controls.
However, once again, there was one exception, Dr. Boekel stressed. “Patients treated with anti-CD20 therapy were at increased risk of severe disease and hospitalization.”
Omicron variant has a different transmissibility than Delta, so the researchers continued the study looking at the Omicron variant. The data “were mostly reassuring,” said Dr. Boekel. “As expected, hospitalization rates decreased overall, with the exception of patients on anti-CD20 therapy where, despite overall reduced pathogenicity, patients remain at increased risk.”
She said that they were awaiting long-term data so the data reflect only short-term immunity against Omicron. “However, we included many elderly and patients with comorbidities, so this made the analysis very sensitive to detect severe cases,” she added.
Breakthrough infection among double- and triple-vaccinated patients
A lower rate of COVID-19 related complications and deaths were seen in patients who were triple-vaccinated against SARS-CoV-2, than in double-vaccinated or unvaccinated patients, despite the former having more comorbidities and use of rituximab (Rituxan), said Dr. Hasseli.
“These data support the recommendation of booster vaccination to reduce COVID-19-related mortality in patients with inflammatory rheumatic diseases [IRDs],” she said.
“A small number of COVID-19 cases were seen in patients with IRD after vaccinations, and in a few cases, hospitalizations were required. Breakthrough infections were mostly seen in patients on B-cell depletion therapy,” she added.
Dr. Hasseli and her colleagues looked at the characteristics and outcomes of SARS-CoV-2 breakthrough infections among double- and triple-vaccinated patients with IRD.
“We wanted to understand if patients with IRD are protected in the same way as the general population following vaccination, given that these patients receive drugs that might impair the immune response,” she explained.
Data for analysis were drawn from the German COVID-19-IRD registry covering February 2021 and January 2022, and patients who were double- or triple- vaccinated against COVID-19 either 14 days or more prior to a SARS-CoV-2 infection were included. Type of IRD, vaccine, immunomodulation, comorbidities, and outcome of the infection were compared with 737 unvaccinated IRD patients with COVID-19. Those with prior COVID-19 were excluded.
Cases were stratified by vaccinations status: unvaccinated (1,388 patients, median age 57 years); double vaccinated (462, 56 years) and triple vaccinated (301, 53 years). Body mass index was similar across groups (25-26 kg/m2), and time between SARS-CoV-2 infection and last vaccination was 156 days in double-vaccinated patients, and 62 days in triple-vaccinated patients.
Patients had rheumatoid arthritis in 44.7% and 44.4% of unvaccinated and double-vaccinated patients respectively, but fewer triple-vaccinated patients had RA (37.2%). Triple vaccination was seen in 32.2% of patients with spondyloarthritis, 16.6% connective tissue diseases, 5.3% other vasculitis, and 3.3% ANCA-associated vasculitis. Of triple-vaccinated patients, 26.2% were treated with tumor necrosis factor-alpha (TNF-alpha) inhibitors, and 6.3% with rituximab, while 5.3% were not on immunomodulation. At least 25% were treated with glucocorticoids, reported Dr. Hasseli.
“Arterial hypertension and diabetes, that might be risk factors for COVID-19, were less frequently reported in triple-vaccinated patients. More patients in the double-vaccinated group [42.9%] than the triple-vaccinated [23.8%] reported absence of relevant comorbidities,” she said.
COVID-19 related complications were less often reported in double- and triple-vaccinated groups with hospitalizations at 9.5% and 4.3% in double and triple-vaccinated people respectively.
Dr. Boekel and Dr. Hasseli report no relevant conflicts of interest.
COPENHAGEN – Most patients with immune-mediated inflammatory diseases (IMID) should not be considered at high risk for severe COVID-19 breakthrough infections, but those on anti-CD20 therapy are the exception, data from a large prospective, cohort study show.
“Overall, the data are reassuring, with conventional risk factors, such as age, and comorbidities seeming to be more important regarding risk of severe COVID-19 breakthrough infections than rheumatic disease or immunosuppressant medication,” said Laura Boekel, MD, from Amsterdam UMC, who presented the study at the annual European Congress of Rheumatology.
But, she added, there was an exception for anti-CD20 therapy. “This is especially relevant for patients with conventional risk factors that might accumulate, and rheumatologists might want to consider alternative treatment options if possible. It is important to inform patients about the risks of anti-CD20.”
Another study, presented during the same session at the congress by Rebecca Hasseli, MD, from the University of Giessen (Germany) saw no deaths and no COVID-19 related complications in a cohort of triple-vaccinated patients with inflammatory rheumatic diseases, despite a higher median age and a higher rate of comorbidities compared to double-vaccinated and unvaccinated cohorts.
Ingrid Jyssum, MD, from Diakonhjemmet Hospital, Oslo, who presented results of the Nor-vaC study investigating the impact of different DMARDs on the immunogenicity of a third COVID-19 vaccine dose, welcomed the research by Dr. Boekel and Dr. Hasseli.
“The findings of Hasseli are interesting in the light of our data on serological response after the third dose, with a lack of breakthrough infections after three doses corresponding well to the robust antibody response that we found in our cohort,” she remarked. “This is very reassuring for our patients. Our own work together with the findings of Hasseli and Boekel demonstrate that additional vaccine doses are important to keep this population well protected against severe COVID-19 infections.”
The Nor-vaC study was conducted with a cohort of 1,100 patients with inflammatory joint and bowel diseases. “These patients had attenuated antibody responses after two vaccine doses; however, we found that a third vaccine dose brought the humoral response in patients up to the antibody levels that healthy controls had after two doses,” said Dr. Jyssum. “In addition, we found that the decline in antibodies after the third dose was less than the decline seen after the second dose. Importantly, the third dose was safe in our patients, with no new safety issues.”
Breakthrough infections and immunosuppressants
“Like the rest of the world, we were wondering if our patients were at increased risk of COVID-19, and if the immunosuppressants used by these patients influenced their risk,” said Dr. Boekel.
The researchers compared both the incidence and severity of COVID-19 breakthrough infections with the SARS-CoV-2 Delta variant in a population of fully vaccinated IMID patients taking immunosuppressants and controls (IMID patients not taking immunosuppressants and healthy controls).
Two large ongoing, prospective, multicenter cohort studies provided pooled data collected between February and December 2021 using digital questionnaires, standardized electronic case record forms, and medical files.
Finger-prick tests were used to collect blood samples that were analyzed after vaccination against SARS-CoV-2 for anti–receptor-binding domain (RBD) antibodies, and antinucleocapsid antibodies to identify asymptomatic breakthrough infections. Any associations between antibodies and the incidence of breakthrough infections were generated, and results were adjusted for sex, cardiovascular disease, chronic pulmonary disease, obesity, and vaccine type.
The analysis included 3,207 IMID patients taking immunosuppressants, and 1,810 controls (985 IMID patients not on immunosuppressants and 825 healthy controls).
Initially, Dr. Boekel and her colleagues looked at incidence of infections and hospitalizations prior to vaccination, and then after vaccination, which was the main aim of the study.
Prior to vaccination, hospitalization risk for COVID-19 was somewhat higher for IMID patients overall compared with controls, reported Dr. Boekel. “But those treated with anti-CD20 therapy, demonstrated much greater risk for severe disease.”
After the SARS-CoV-2 vaccination campaign began, the researchers then looked at how immunosuppressants influenced humoral response to SARS-CoV-2 vaccination.
“Anti-CD20 therapy showed the greatest impact on humoral immune response after SARS-CoV-2 vaccination,” said Dr. Boekel. Other immunosuppressant drugs had variable effects on humoral and cellular immunity.
Once they had established that immunosuppressant drugs impaired immune responses to SARS-CoV-2 vaccination, the researchers wanted to determine if this affected clinical outcomes. Blood samples taken 28 days after the second vaccination enabled Dr. Boekel and her colleagues to see if antibody production was associated with breakthrough infections.
Breakthrough infections were seen in 5% of patients on immunosuppressants, 5% of patients not on immunosuppressants, and 4% of healthy controls. Also, asymptomatic COVID-19 breakthrough cases were comparable between IMID patients taking immunosuppressants and controls, at 10% in each group.
“We saw that the incidence [of getting COVID-19] was comparable between groups, independent of whether they were receiving immunosuppressants or not, or healthy controls. However, if they developed antibodies against the two vaccinations the chance of getting infected was lower,” reported Dr. Boekel.
Hospitalization (severe disease) rates were also comparable between groups. “Patients with rheumatic diseases, even when treated with immunosuppressants were not at increased risk of severe disease from Delta breakthrough infections,” added the researcher. “Cases that were hospitalized were mainly elderly and those with comorbidities, for example cardiovascular disease and cardiopulmonary disease.”
Hospital admissions were 5.4% in patients on immunosuppressants, 5.7% in those not on immunosuppressants, and 6% in health controls.
However, once again, there was one exception, Dr. Boekel stressed. “Patients treated with anti-CD20 therapy were at increased risk of severe disease and hospitalization.”
Omicron variant has a different transmissibility than Delta, so the researchers continued the study looking at the Omicron variant. The data “were mostly reassuring,” said Dr. Boekel. “As expected, hospitalization rates decreased overall, with the exception of patients on anti-CD20 therapy where, despite overall reduced pathogenicity, patients remain at increased risk.”
She said that they were awaiting long-term data so the data reflect only short-term immunity against Omicron. “However, we included many elderly and patients with comorbidities, so this made the analysis very sensitive to detect severe cases,” she added.
Breakthrough infection among double- and triple-vaccinated patients
A lower rate of COVID-19 related complications and deaths were seen in patients who were triple-vaccinated against SARS-CoV-2, than in double-vaccinated or unvaccinated patients, despite the former having more comorbidities and use of rituximab (Rituxan), said Dr. Hasseli.
“These data support the recommendation of booster vaccination to reduce COVID-19-related mortality in patients with inflammatory rheumatic diseases [IRDs],” she said.
“A small number of COVID-19 cases were seen in patients with IRD after vaccinations, and in a few cases, hospitalizations were required. Breakthrough infections were mostly seen in patients on B-cell depletion therapy,” she added.
Dr. Hasseli and her colleagues looked at the characteristics and outcomes of SARS-CoV-2 breakthrough infections among double- and triple-vaccinated patients with IRD.
“We wanted to understand if patients with IRD are protected in the same way as the general population following vaccination, given that these patients receive drugs that might impair the immune response,” she explained.
Data for analysis were drawn from the German COVID-19-IRD registry covering February 2021 and January 2022, and patients who were double- or triple- vaccinated against COVID-19 either 14 days or more prior to a SARS-CoV-2 infection were included. Type of IRD, vaccine, immunomodulation, comorbidities, and outcome of the infection were compared with 737 unvaccinated IRD patients with COVID-19. Those with prior COVID-19 were excluded.
Cases were stratified by vaccinations status: unvaccinated (1,388 patients, median age 57 years); double vaccinated (462, 56 years) and triple vaccinated (301, 53 years). Body mass index was similar across groups (25-26 kg/m2), and time between SARS-CoV-2 infection and last vaccination was 156 days in double-vaccinated patients, and 62 days in triple-vaccinated patients.
Patients had rheumatoid arthritis in 44.7% and 44.4% of unvaccinated and double-vaccinated patients respectively, but fewer triple-vaccinated patients had RA (37.2%). Triple vaccination was seen in 32.2% of patients with spondyloarthritis, 16.6% connective tissue diseases, 5.3% other vasculitis, and 3.3% ANCA-associated vasculitis. Of triple-vaccinated patients, 26.2% were treated with tumor necrosis factor-alpha (TNF-alpha) inhibitors, and 6.3% with rituximab, while 5.3% were not on immunomodulation. At least 25% were treated with glucocorticoids, reported Dr. Hasseli.
“Arterial hypertension and diabetes, that might be risk factors for COVID-19, were less frequently reported in triple-vaccinated patients. More patients in the double-vaccinated group [42.9%] than the triple-vaccinated [23.8%] reported absence of relevant comorbidities,” she said.
COVID-19 related complications were less often reported in double- and triple-vaccinated groups with hospitalizations at 9.5% and 4.3% in double and triple-vaccinated people respectively.
Dr. Boekel and Dr. Hasseli report no relevant conflicts of interest.
AT THE EULAR 2022 CONGRESS
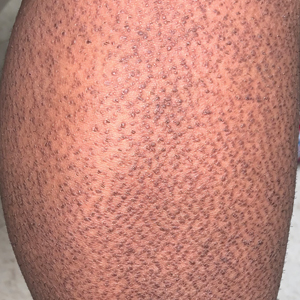
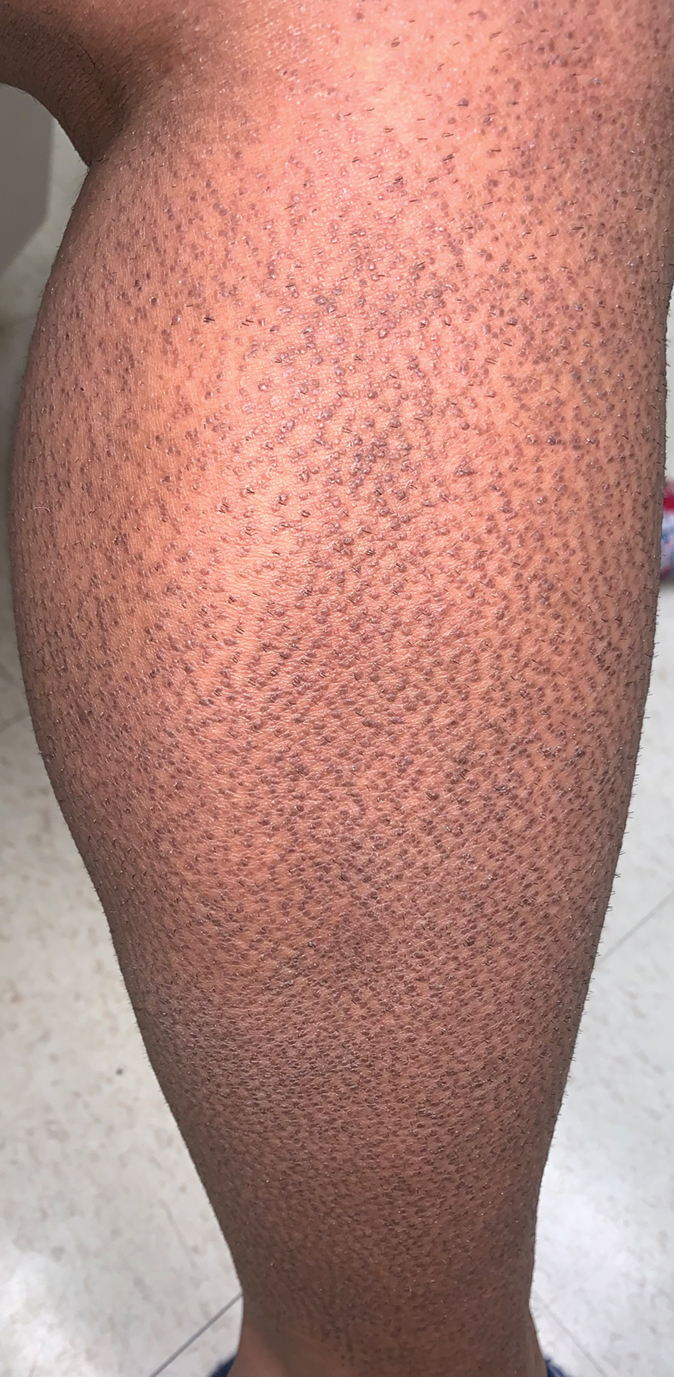
Rippled Macules and Papules on the Legs
The Diagnosis: Cutaneous Amyloidosis
A punch biopsy confirmed the diagnosis of cutaneous amyloidosis, which is characterized by the deposition of amyloid proteins in the skin without systemic involvement. Subtypes of cutaneous amyloidosis include lichenoid, macular, and nodular amyloidosis. A mixed or biphasic amyloidosis can occur when both lichenoid and macular lesions are present.1 Lichenoid and macular amyloidosis generally are characterized by moderate to severe pruritus. Lichenoid amyloidosis favors the shins, calves, ankles, and extensor extremities; macular amyloidosis has a predilection for the interscapular area and less frequently the upper arms, chest, and thighs.2 Atypical variants also have been reported, including amyloidosis cutis dyschromica, poikilodermalike amyloidosis, and bullous amyloidosis, as well as incontinentia pigmenti–like, linear, and nevoid types.3 Macular amyloidosis has been reported to occur in association with progressive systemic sclerosis, primary biliary cirrhosis, systemic lupus erythematosus, paronychia, and multiple endocrine neoplasia type 2.2
Acanthosis nigricans typically presents on the neck and intertriginous areas as velvety hyperpigmented plaques. Confluent and reticulated papillomatosis also appears as slightly elevated papules; however, it occurs in the intermammary region in a reticulated pattern. Ichthyosis vulgaris also may occur on the lower extremities but presents with adherent large scales rather than papules. Keratosis pilaris may present on the proximal lower extremities with smaller, folliculocentric, fleshcolored to pink papules.
Treatment of cutaneous amyloidosis has long been challenging for dermatologists. The primary focus should be treatment of any underlying disease that is causing the pruritus and subsequent manipulation of skin lesions. Topical calcipotriol, phototherapy, oral cyclophosphamide, and Nd:YAG laser have demonstrated beneficial outcomes. IL-31 antibodies may be a potential future treatment.1
1. Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9 2. Rasi A, Khatami A, Javaheri SM. Macular amyloidosis: an assessment of prevalence, sex, and age. Int J Dermatol. 2004;43:898-899. doi:10.1111 /j.1365-4632.2004.01935.x 3. Hamie L, Haddad I, Nasser N, et al. Primary localized cutaneous amyloidosis of keratinocyte origin: an update with emphasis on atypical clinical variants [published online July 21, 2021]. 2021;22:667-680. Am J Clin Dermatol. doi:10.1007/s40257-021-00620-9
The Diagnosis: Cutaneous Amyloidosis
A punch biopsy confirmed the diagnosis of cutaneous amyloidosis, which is characterized by the deposition of amyloid proteins in the skin without systemic involvement. Subtypes of cutaneous amyloidosis include lichenoid, macular, and nodular amyloidosis. A mixed or biphasic amyloidosis can occur when both lichenoid and macular lesions are present.1 Lichenoid and macular amyloidosis generally are characterized by moderate to severe pruritus. Lichenoid amyloidosis favors the shins, calves, ankles, and extensor extremities; macular amyloidosis has a predilection for the interscapular area and less frequently the upper arms, chest, and thighs.2 Atypical variants also have been reported, including amyloidosis cutis dyschromica, poikilodermalike amyloidosis, and bullous amyloidosis, as well as incontinentia pigmenti–like, linear, and nevoid types.3 Macular amyloidosis has been reported to occur in association with progressive systemic sclerosis, primary biliary cirrhosis, systemic lupus erythematosus, paronychia, and multiple endocrine neoplasia type 2.2
Acanthosis nigricans typically presents on the neck and intertriginous areas as velvety hyperpigmented plaques. Confluent and reticulated papillomatosis also appears as slightly elevated papules; however, it occurs in the intermammary region in a reticulated pattern. Ichthyosis vulgaris also may occur on the lower extremities but presents with adherent large scales rather than papules. Keratosis pilaris may present on the proximal lower extremities with smaller, folliculocentric, fleshcolored to pink papules.
Treatment of cutaneous amyloidosis has long been challenging for dermatologists. The primary focus should be treatment of any underlying disease that is causing the pruritus and subsequent manipulation of skin lesions. Topical calcipotriol, phototherapy, oral cyclophosphamide, and Nd:YAG laser have demonstrated beneficial outcomes. IL-31 antibodies may be a potential future treatment.1
The Diagnosis: Cutaneous Amyloidosis
A punch biopsy confirmed the diagnosis of cutaneous amyloidosis, which is characterized by the deposition of amyloid proteins in the skin without systemic involvement. Subtypes of cutaneous amyloidosis include lichenoid, macular, and nodular amyloidosis. A mixed or biphasic amyloidosis can occur when both lichenoid and macular lesions are present.1 Lichenoid and macular amyloidosis generally are characterized by moderate to severe pruritus. Lichenoid amyloidosis favors the shins, calves, ankles, and extensor extremities; macular amyloidosis has a predilection for the interscapular area and less frequently the upper arms, chest, and thighs.2 Atypical variants also have been reported, including amyloidosis cutis dyschromica, poikilodermalike amyloidosis, and bullous amyloidosis, as well as incontinentia pigmenti–like, linear, and nevoid types.3 Macular amyloidosis has been reported to occur in association with progressive systemic sclerosis, primary biliary cirrhosis, systemic lupus erythematosus, paronychia, and multiple endocrine neoplasia type 2.2
Acanthosis nigricans typically presents on the neck and intertriginous areas as velvety hyperpigmented plaques. Confluent and reticulated papillomatosis also appears as slightly elevated papules; however, it occurs in the intermammary region in a reticulated pattern. Ichthyosis vulgaris also may occur on the lower extremities but presents with adherent large scales rather than papules. Keratosis pilaris may present on the proximal lower extremities with smaller, folliculocentric, fleshcolored to pink papules.
Treatment of cutaneous amyloidosis has long been challenging for dermatologists. The primary focus should be treatment of any underlying disease that is causing the pruritus and subsequent manipulation of skin lesions. Topical calcipotriol, phototherapy, oral cyclophosphamide, and Nd:YAG laser have demonstrated beneficial outcomes. IL-31 antibodies may be a potential future treatment.1
1. Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9 2. Rasi A, Khatami A, Javaheri SM. Macular amyloidosis: an assessment of prevalence, sex, and age. Int J Dermatol. 2004;43:898-899. doi:10.1111 /j.1365-4632.2004.01935.x 3. Hamie L, Haddad I, Nasser N, et al. Primary localized cutaneous amyloidosis of keratinocyte origin: an update with emphasis on atypical clinical variants [published online July 21, 2021]. 2021;22:667-680. Am J Clin Dermatol. doi:10.1007/s40257-021-00620-9
1. Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9 2. Rasi A, Khatami A, Javaheri SM. Macular amyloidosis: an assessment of prevalence, sex, and age. Int J Dermatol. 2004;43:898-899. doi:10.1111 /j.1365-4632.2004.01935.x 3. Hamie L, Haddad I, Nasser N, et al. Primary localized cutaneous amyloidosis of keratinocyte origin: an update with emphasis on atypical clinical variants [published online July 21, 2021]. 2021;22:667-680. Am J Clin Dermatol. doi:10.1007/s40257-021-00620-9
A 34-year-old woman presented to our dermatology clinic with an intensely pruritic rash on the legs of 2 years’ duration. The pruritus had waxed and waned in intensity, and the skin lesions were refractory to treatment with low-potency topical steroids. She had no other chronic medical conditions and was not taking any other medications.
Methotrexate enhances pegloticase response in uncontrolled gout
In patients with uncontrolled gout, response rates were increased by 32% when methotrexate was used in conjunction with pegloticase versus pegloticase plus a placebo, it was reported at the annual European Congress of Rheumatology.
In the phase 4 MIRROR trial, 71% of patients who received pretreatment with methotrexate and then the combination of methotrexate and pegloticase achieved uric-acid levels lower than 6 mg/dL for more than 80% of the time during weeks 20-24 of the 52-week study. By comparison, only 39% of those treated with pegloticase plus a placebo achieved this primary endpoint (P < .0001).
“This trial confirms not only improved efficacy but improved safety in patients treated with pegloticase in combination with methotrexate 15 mg orally once weekly,” study investigator John K. Botson, MD, RPh, CCD, said in reporting the trial’s findings.
This is good news for patients, suggested two rheumatologists who were not involved in the study. The combination appears “useful for a select group of gout patients,” observed Christian Ammitzbøll, MD, PhD, from Aarhus University Hospital, Denmark.
“Very promising in refractory gout,” agreed Emre Bilgin, MD, from Ankara, Turkey.
Rationale for using methotrexate
“Oral urate lowering agents are the mainstay of treatment of gout, but there are patients that just don’t respond to oral agents,” said Dr. Botson, a rheumatologist in private practice from Anchorage, Alaska.
“These patients are very difficult to treat,” he added. “They have a lot of physical disabilities, they have high medical comorbidities, and they have a low quality of life. Their treatment options are extremely limited.”
One of the few options they have is pegloticase, a pegylated uric acid specific enzyme sold under the brand name Krystexxa for the past 12 years. It lowers serum uric acid by converting it to allantoin, which is more water soluble and thus is easier to excrete from the body.
However, one of the problems of using the drug is that anti-drug antibodies frequently develop, meaning that discontinuation rates can be as high as 50%, with around a quarter of patients at high risk of experiencing an infusion reaction.
“Methotrexate is a medication we’re very familiar with for other rheumatologic conditions that use biologic medications, and we use this to prevent anti-drug antibodies. So, the MIRROR RCT was a study we performed to examine the pegloticase therapy in combination with methotrexate co therapy,” explained Dr. Botson.
In fact, co-administration of methotrexate and pegloticase was associated with fewer infusion reactions than using pegloticase alone (3% vs. 31%).
Study design and results
A total of 152 patients were included in the trial and were treated with methotrexate at a weekly dose of 15 mg for 2 weeks before being randomized, 2:1, to either continue methotrexate and then receive intravenous pegloticase or receive the latter with a placebo. Pegloticase was given at a dose of 8 mg every 2 weeks. Treatment was for 52 weeks, with the primary endpoint of serum uric acid response tested at 6 months.
The reason for the 2-week run-in period with methotrexate was to check that patients would be able to tolerate it, Dr. Botson explained.
The mean age of patients was around 54 years, the majority (> 84%) were male and were White (69%). The average duration of gout was about 14 years, with over 74% having tophi present at screening and experiencing 10-11 flares in the previous year. Baseline serum uric acid averaged at about 9 mg/dL.
Almost three-quarters of the 100 patients (73%) who were treated with the combination completed treatment to week 24 while the corresponding percentage in the placebo arm (n = 52) was 39%. The main reason for stopping was due to lack of efficacy (27% and 61% of cases, respectively), defined as having serum uric acid levels above 6 mg/dL on two consecutive measurements.
The median time to discontinuation was 69 days for those in the placebo arm; “it was non-estimable” in the methotrexate arm, Dr. Botson reported.
The mean change in serum uric acid through to week 24 was higher in the methotrexate than placebo arm, at a respective 7.66 and 5.23 mg/dL, giving a significant mean difference of 2.43 mg/dL.
There was a “dramatic resolution of tophaceous deposits,” Dr. Botson said. Complete resolution of tophi was seen in 34.6% of methotrexate-treated patients versus 13.8% of pegloticase-placebo–treated patients (P = .043).
One of the most common adverse events associated with pegloticase treatment is gout flare, which occurred in about 70% of participants in both study arms. Overall, the addition of methotrexate did not increase the risk for adverse events in general, and of the two deaths seen in the study – both in methotrexate-treated patients – one was because of a heart attack and another due COVID-19, so they were unrelated to study treatment.
In patients with renal insufficiency
Concern was raised during the discussion, however, on how to handle methotrexate use in patients with renal insufficiency.
“That’s been a debate that we’ve had in this study and others,” said Dr. Botson, acknowledging that “methotrexate is often a concern for the nephrologist that we’re co-treating these patients with.” However, no dose adjustments were needed in the study.
“There are some other studies with other immunomodulators that do suggest that other agents could be used that may be a little less potentially renal toxic, but we didn’t see any toxicity in the patients that we had, even in those that had a reduced [glomerular filtration rate],” he added.
Dr. Botson has received research support from Horizon and Radius Health. He also acknowledged receiving speakers fees from AbbVie, Amgen, Aurinia, ChemoCentryx*, Horizon, Eli Lilly, and Novartis.
Correction, 6/7/22: The name of the company ChemoCentryx was misstated.
In patients with uncontrolled gout, response rates were increased by 32% when methotrexate was used in conjunction with pegloticase versus pegloticase plus a placebo, it was reported at the annual European Congress of Rheumatology.
In the phase 4 MIRROR trial, 71% of patients who received pretreatment with methotrexate and then the combination of methotrexate and pegloticase achieved uric-acid levels lower than 6 mg/dL for more than 80% of the time during weeks 20-24 of the 52-week study. By comparison, only 39% of those treated with pegloticase plus a placebo achieved this primary endpoint (P < .0001).
“This trial confirms not only improved efficacy but improved safety in patients treated with pegloticase in combination with methotrexate 15 mg orally once weekly,” study investigator John K. Botson, MD, RPh, CCD, said in reporting the trial’s findings.
This is good news for patients, suggested two rheumatologists who were not involved in the study. The combination appears “useful for a select group of gout patients,” observed Christian Ammitzbøll, MD, PhD, from Aarhus University Hospital, Denmark.
“Very promising in refractory gout,” agreed Emre Bilgin, MD, from Ankara, Turkey.
Rationale for using methotrexate
“Oral urate lowering agents are the mainstay of treatment of gout, but there are patients that just don’t respond to oral agents,” said Dr. Botson, a rheumatologist in private practice from Anchorage, Alaska.
“These patients are very difficult to treat,” he added. “They have a lot of physical disabilities, they have high medical comorbidities, and they have a low quality of life. Their treatment options are extremely limited.”
One of the few options they have is pegloticase, a pegylated uric acid specific enzyme sold under the brand name Krystexxa for the past 12 years. It lowers serum uric acid by converting it to allantoin, which is more water soluble and thus is easier to excrete from the body.
However, one of the problems of using the drug is that anti-drug antibodies frequently develop, meaning that discontinuation rates can be as high as 50%, with around a quarter of patients at high risk of experiencing an infusion reaction.
“Methotrexate is a medication we’re very familiar with for other rheumatologic conditions that use biologic medications, and we use this to prevent anti-drug antibodies. So, the MIRROR RCT was a study we performed to examine the pegloticase therapy in combination with methotrexate co therapy,” explained Dr. Botson.
In fact, co-administration of methotrexate and pegloticase was associated with fewer infusion reactions than using pegloticase alone (3% vs. 31%).
Study design and results
A total of 152 patients were included in the trial and were treated with methotrexate at a weekly dose of 15 mg for 2 weeks before being randomized, 2:1, to either continue methotrexate and then receive intravenous pegloticase or receive the latter with a placebo. Pegloticase was given at a dose of 8 mg every 2 weeks. Treatment was for 52 weeks, with the primary endpoint of serum uric acid response tested at 6 months.
The reason for the 2-week run-in period with methotrexate was to check that patients would be able to tolerate it, Dr. Botson explained.
The mean age of patients was around 54 years, the majority (> 84%) were male and were White (69%). The average duration of gout was about 14 years, with over 74% having tophi present at screening and experiencing 10-11 flares in the previous year. Baseline serum uric acid averaged at about 9 mg/dL.
Almost three-quarters of the 100 patients (73%) who were treated with the combination completed treatment to week 24 while the corresponding percentage in the placebo arm (n = 52) was 39%. The main reason for stopping was due to lack of efficacy (27% and 61% of cases, respectively), defined as having serum uric acid levels above 6 mg/dL on two consecutive measurements.
The median time to discontinuation was 69 days for those in the placebo arm; “it was non-estimable” in the methotrexate arm, Dr. Botson reported.
The mean change in serum uric acid through to week 24 was higher in the methotrexate than placebo arm, at a respective 7.66 and 5.23 mg/dL, giving a significant mean difference of 2.43 mg/dL.
There was a “dramatic resolution of tophaceous deposits,” Dr. Botson said. Complete resolution of tophi was seen in 34.6% of methotrexate-treated patients versus 13.8% of pegloticase-placebo–treated patients (P = .043).
One of the most common adverse events associated with pegloticase treatment is gout flare, which occurred in about 70% of participants in both study arms. Overall, the addition of methotrexate did not increase the risk for adverse events in general, and of the two deaths seen in the study – both in methotrexate-treated patients – one was because of a heart attack and another due COVID-19, so they were unrelated to study treatment.
In patients with renal insufficiency
Concern was raised during the discussion, however, on how to handle methotrexate use in patients with renal insufficiency.
“That’s been a debate that we’ve had in this study and others,” said Dr. Botson, acknowledging that “methotrexate is often a concern for the nephrologist that we’re co-treating these patients with.” However, no dose adjustments were needed in the study.
“There are some other studies with other immunomodulators that do suggest that other agents could be used that may be a little less potentially renal toxic, but we didn’t see any toxicity in the patients that we had, even in those that had a reduced [glomerular filtration rate],” he added.
Dr. Botson has received research support from Horizon and Radius Health. He also acknowledged receiving speakers fees from AbbVie, Amgen, Aurinia, ChemoCentryx*, Horizon, Eli Lilly, and Novartis.
Correction, 6/7/22: The name of the company ChemoCentryx was misstated.
In patients with uncontrolled gout, response rates were increased by 32% when methotrexate was used in conjunction with pegloticase versus pegloticase plus a placebo, it was reported at the annual European Congress of Rheumatology.
In the phase 4 MIRROR trial, 71% of patients who received pretreatment with methotrexate and then the combination of methotrexate and pegloticase achieved uric-acid levels lower than 6 mg/dL for more than 80% of the time during weeks 20-24 of the 52-week study. By comparison, only 39% of those treated with pegloticase plus a placebo achieved this primary endpoint (P < .0001).
“This trial confirms not only improved efficacy but improved safety in patients treated with pegloticase in combination with methotrexate 15 mg orally once weekly,” study investigator John K. Botson, MD, RPh, CCD, said in reporting the trial’s findings.
This is good news for patients, suggested two rheumatologists who were not involved in the study. The combination appears “useful for a select group of gout patients,” observed Christian Ammitzbøll, MD, PhD, from Aarhus University Hospital, Denmark.
“Very promising in refractory gout,” agreed Emre Bilgin, MD, from Ankara, Turkey.
Rationale for using methotrexate
“Oral urate lowering agents are the mainstay of treatment of gout, but there are patients that just don’t respond to oral agents,” said Dr. Botson, a rheumatologist in private practice from Anchorage, Alaska.
“These patients are very difficult to treat,” he added. “They have a lot of physical disabilities, they have high medical comorbidities, and they have a low quality of life. Their treatment options are extremely limited.”
One of the few options they have is pegloticase, a pegylated uric acid specific enzyme sold under the brand name Krystexxa for the past 12 years. It lowers serum uric acid by converting it to allantoin, which is more water soluble and thus is easier to excrete from the body.
However, one of the problems of using the drug is that anti-drug antibodies frequently develop, meaning that discontinuation rates can be as high as 50%, with around a quarter of patients at high risk of experiencing an infusion reaction.
“Methotrexate is a medication we’re very familiar with for other rheumatologic conditions that use biologic medications, and we use this to prevent anti-drug antibodies. So, the MIRROR RCT was a study we performed to examine the pegloticase therapy in combination with methotrexate co therapy,” explained Dr. Botson.
In fact, co-administration of methotrexate and pegloticase was associated with fewer infusion reactions than using pegloticase alone (3% vs. 31%).
Study design and results
A total of 152 patients were included in the trial and were treated with methotrexate at a weekly dose of 15 mg for 2 weeks before being randomized, 2:1, to either continue methotrexate and then receive intravenous pegloticase or receive the latter with a placebo. Pegloticase was given at a dose of 8 mg every 2 weeks. Treatment was for 52 weeks, with the primary endpoint of serum uric acid response tested at 6 months.
The reason for the 2-week run-in period with methotrexate was to check that patients would be able to tolerate it, Dr. Botson explained.
The mean age of patients was around 54 years, the majority (> 84%) were male and were White (69%). The average duration of gout was about 14 years, with over 74% having tophi present at screening and experiencing 10-11 flares in the previous year. Baseline serum uric acid averaged at about 9 mg/dL.
Almost three-quarters of the 100 patients (73%) who were treated with the combination completed treatment to week 24 while the corresponding percentage in the placebo arm (n = 52) was 39%. The main reason for stopping was due to lack of efficacy (27% and 61% of cases, respectively), defined as having serum uric acid levels above 6 mg/dL on two consecutive measurements.
The median time to discontinuation was 69 days for those in the placebo arm; “it was non-estimable” in the methotrexate arm, Dr. Botson reported.
The mean change in serum uric acid through to week 24 was higher in the methotrexate than placebo arm, at a respective 7.66 and 5.23 mg/dL, giving a significant mean difference of 2.43 mg/dL.
There was a “dramatic resolution of tophaceous deposits,” Dr. Botson said. Complete resolution of tophi was seen in 34.6% of methotrexate-treated patients versus 13.8% of pegloticase-placebo–treated patients (P = .043).
One of the most common adverse events associated with pegloticase treatment is gout flare, which occurred in about 70% of participants in both study arms. Overall, the addition of methotrexate did not increase the risk for adverse events in general, and of the two deaths seen in the study – both in methotrexate-treated patients – one was because of a heart attack and another due COVID-19, so they were unrelated to study treatment.
In patients with renal insufficiency
Concern was raised during the discussion, however, on how to handle methotrexate use in patients with renal insufficiency.
“That’s been a debate that we’ve had in this study and others,” said Dr. Botson, acknowledging that “methotrexate is often a concern for the nephrologist that we’re co-treating these patients with.” However, no dose adjustments were needed in the study.
“There are some other studies with other immunomodulators that do suggest that other agents could be used that may be a little less potentially renal toxic, but we didn’t see any toxicity in the patients that we had, even in those that had a reduced [glomerular filtration rate],” he added.
Dr. Botson has received research support from Horizon and Radius Health. He also acknowledged receiving speakers fees from AbbVie, Amgen, Aurinia, ChemoCentryx*, Horizon, Eli Lilly, and Novartis.
Correction, 6/7/22: The name of the company ChemoCentryx was misstated.
FROM THE EULAR 2022 CONGRESS
MS and COVID-19: Conflicting signs on risk but some trends are clearer
NATIONAL HARBOR, MD. – While patients with multiple sclerosis (MS) don’t seem to be more likely to be infected with COVID-19, a neurologist told colleagues, the jury is still out over whether they face a higher mortality risk, especially if they take certain disease-modifying therapies (DMTs)
In regard to MS overall, “the data is conflicting, but any increased risk of mortality appears to be slight. And it appears to be chiefly the consequences associated with comorbidities as seen in other populations,” Joseph R. Berger, MD, said at the John F. Kurtzke Memorial Lecture at the annual meeting of the Consortium of Multiple Sclerosis Centers. “If you’re old, if you’re infirm, if you have obesity and cardiovascular disease and underlying pulmonary disease, you’re at risk of dying yourself. It’s not so much the MS,” said Dr. Berger, professor of neurology at the Hospital of the University of Pennsylvania and chief of the multiple sclerosis division at the University of Pennsylvania, Philadelphia.
Dr. Berger had his own COVID-19 story to tell: He couldn’t attend the conference in person because he was quarantining in Portugal since he tested positive. At press time, he was faring well but had reported 4 days of intense back pain.
In regard to MS and COVID-19, Dr. Berger said consistent research suggests that There may be a very small increase in risk of MS relapse in patients with COVID-19, he said, but pseudorelapses are far more common. As for mortality, he highlighted a 2021 pooled analysis of 18 studies with 5,634 patients that suggested they had a crude death rate of 1.97%, standardized lethality ratio of 1.24, and a 24% increased risk of death.
Dr. Berger is skeptical of these findings, however, in light of overall death rate numbers. Early on in the pandemic, the fatality rate in China was estimated at 2.3%.
He said he’s more convinced by a retrospective 2021 German COVID-19 study that compared 551 patients with MS to 156,973 other patients and found lower rates of ICU admission (17.1% in patients with MS vs. 22.7% in those without it), ventilation (9.8% vs. 14.5%), and in‐hospital mortality (11.1% vs. 19.3%).
Meanwhile, a 2021 systematic review found no increase in mortality among 4,310 patients with MS (3% death rate, 20.7% hospitalization), but the death risk was highest among those on no DMTs and those taking anti-CD20 monoclonal antibodies. The COViMS Registry has reported similar findings regarding the anti-CD20 drugs rituximab and ocrelizumab, Dr. Berger noted, and a pooled study of Italian and French data links the monoclonal antibodies to more severe COVID. A 2021 aggregated study also linked the antibodies to increased risk of hospitalization and ICU admission.
“Anti-CD20 monoclonal antibodies appear to increase the risk of hospitalization and perhaps the acquisition of the virus, ICU admission, maybe death,” he said, with rituximab appearing to pose the most risk, followed by ocrelizumab and ofatumumab. “And it appears that the platform [older] therapies may be associated with lesser mortality.”
As for nondrug factors, Dr. Berger said, studies have linked higher risk to age, male sex, and comorbidities.
COVID-19 vaccines are another area of concern, he said. “The recommendation is to administer vaccination prior to the initiation of the anti-CD20s, alemtuzumab, and cladribine, and wait a period of time. Three months is ideal, maybe a little longer, because it appears that the antibody response seems to be best as your CD19 count starts to return.”
Finally, Dr. Berger noted that “passive vaccination” is now available via Evusheld (tixagevimab and cilgavimab) as a preexposure treatment for people with moderate to severe immune compromise who may not mount an effective immune response to COVID-19 vaccination or those who are allergic.
Dr. Berger reported multiple disclosures.
NATIONAL HARBOR, MD. – While patients with multiple sclerosis (MS) don’t seem to be more likely to be infected with COVID-19, a neurologist told colleagues, the jury is still out over whether they face a higher mortality risk, especially if they take certain disease-modifying therapies (DMTs)
In regard to MS overall, “the data is conflicting, but any increased risk of mortality appears to be slight. And it appears to be chiefly the consequences associated with comorbidities as seen in other populations,” Joseph R. Berger, MD, said at the John F. Kurtzke Memorial Lecture at the annual meeting of the Consortium of Multiple Sclerosis Centers. “If you’re old, if you’re infirm, if you have obesity and cardiovascular disease and underlying pulmonary disease, you’re at risk of dying yourself. It’s not so much the MS,” said Dr. Berger, professor of neurology at the Hospital of the University of Pennsylvania and chief of the multiple sclerosis division at the University of Pennsylvania, Philadelphia.
Dr. Berger had his own COVID-19 story to tell: He couldn’t attend the conference in person because he was quarantining in Portugal since he tested positive. At press time, he was faring well but had reported 4 days of intense back pain.
In regard to MS and COVID-19, Dr. Berger said consistent research suggests that There may be a very small increase in risk of MS relapse in patients with COVID-19, he said, but pseudorelapses are far more common. As for mortality, he highlighted a 2021 pooled analysis of 18 studies with 5,634 patients that suggested they had a crude death rate of 1.97%, standardized lethality ratio of 1.24, and a 24% increased risk of death.
Dr. Berger is skeptical of these findings, however, in light of overall death rate numbers. Early on in the pandemic, the fatality rate in China was estimated at 2.3%.
He said he’s more convinced by a retrospective 2021 German COVID-19 study that compared 551 patients with MS to 156,973 other patients and found lower rates of ICU admission (17.1% in patients with MS vs. 22.7% in those without it), ventilation (9.8% vs. 14.5%), and in‐hospital mortality (11.1% vs. 19.3%).
Meanwhile, a 2021 systematic review found no increase in mortality among 4,310 patients with MS (3% death rate, 20.7% hospitalization), but the death risk was highest among those on no DMTs and those taking anti-CD20 monoclonal antibodies. The COViMS Registry has reported similar findings regarding the anti-CD20 drugs rituximab and ocrelizumab, Dr. Berger noted, and a pooled study of Italian and French data links the monoclonal antibodies to more severe COVID. A 2021 aggregated study also linked the antibodies to increased risk of hospitalization and ICU admission.
“Anti-CD20 monoclonal antibodies appear to increase the risk of hospitalization and perhaps the acquisition of the virus, ICU admission, maybe death,” he said, with rituximab appearing to pose the most risk, followed by ocrelizumab and ofatumumab. “And it appears that the platform [older] therapies may be associated with lesser mortality.”
As for nondrug factors, Dr. Berger said, studies have linked higher risk to age, male sex, and comorbidities.
COVID-19 vaccines are another area of concern, he said. “The recommendation is to administer vaccination prior to the initiation of the anti-CD20s, alemtuzumab, and cladribine, and wait a period of time. Three months is ideal, maybe a little longer, because it appears that the antibody response seems to be best as your CD19 count starts to return.”
Finally, Dr. Berger noted that “passive vaccination” is now available via Evusheld (tixagevimab and cilgavimab) as a preexposure treatment for people with moderate to severe immune compromise who may not mount an effective immune response to COVID-19 vaccination or those who are allergic.
Dr. Berger reported multiple disclosures.
NATIONAL HARBOR, MD. – While patients with multiple sclerosis (MS) don’t seem to be more likely to be infected with COVID-19, a neurologist told colleagues, the jury is still out over whether they face a higher mortality risk, especially if they take certain disease-modifying therapies (DMTs)
In regard to MS overall, “the data is conflicting, but any increased risk of mortality appears to be slight. And it appears to be chiefly the consequences associated with comorbidities as seen in other populations,” Joseph R. Berger, MD, said at the John F. Kurtzke Memorial Lecture at the annual meeting of the Consortium of Multiple Sclerosis Centers. “If you’re old, if you’re infirm, if you have obesity and cardiovascular disease and underlying pulmonary disease, you’re at risk of dying yourself. It’s not so much the MS,” said Dr. Berger, professor of neurology at the Hospital of the University of Pennsylvania and chief of the multiple sclerosis division at the University of Pennsylvania, Philadelphia.
Dr. Berger had his own COVID-19 story to tell: He couldn’t attend the conference in person because he was quarantining in Portugal since he tested positive. At press time, he was faring well but had reported 4 days of intense back pain.
In regard to MS and COVID-19, Dr. Berger said consistent research suggests that There may be a very small increase in risk of MS relapse in patients with COVID-19, he said, but pseudorelapses are far more common. As for mortality, he highlighted a 2021 pooled analysis of 18 studies with 5,634 patients that suggested they had a crude death rate of 1.97%, standardized lethality ratio of 1.24, and a 24% increased risk of death.
Dr. Berger is skeptical of these findings, however, in light of overall death rate numbers. Early on in the pandemic, the fatality rate in China was estimated at 2.3%.
He said he’s more convinced by a retrospective 2021 German COVID-19 study that compared 551 patients with MS to 156,973 other patients and found lower rates of ICU admission (17.1% in patients with MS vs. 22.7% in those without it), ventilation (9.8% vs. 14.5%), and in‐hospital mortality (11.1% vs. 19.3%).
Meanwhile, a 2021 systematic review found no increase in mortality among 4,310 patients with MS (3% death rate, 20.7% hospitalization), but the death risk was highest among those on no DMTs and those taking anti-CD20 monoclonal antibodies. The COViMS Registry has reported similar findings regarding the anti-CD20 drugs rituximab and ocrelizumab, Dr. Berger noted, and a pooled study of Italian and French data links the monoclonal antibodies to more severe COVID. A 2021 aggregated study also linked the antibodies to increased risk of hospitalization and ICU admission.
“Anti-CD20 monoclonal antibodies appear to increase the risk of hospitalization and perhaps the acquisition of the virus, ICU admission, maybe death,” he said, with rituximab appearing to pose the most risk, followed by ocrelizumab and ofatumumab. “And it appears that the platform [older] therapies may be associated with lesser mortality.”
As for nondrug factors, Dr. Berger said, studies have linked higher risk to age, male sex, and comorbidities.
COVID-19 vaccines are another area of concern, he said. “The recommendation is to administer vaccination prior to the initiation of the anti-CD20s, alemtuzumab, and cladribine, and wait a period of time. Three months is ideal, maybe a little longer, because it appears that the antibody response seems to be best as your CD19 count starts to return.”
Finally, Dr. Berger noted that “passive vaccination” is now available via Evusheld (tixagevimab and cilgavimab) as a preexposure treatment for people with moderate to severe immune compromise who may not mount an effective immune response to COVID-19 vaccination or those who are allergic.
Dr. Berger reported multiple disclosures.
AT CMSC 2022
Antidiabetes drug costs keep patients away
NEW ORLEANS – , according to findings from two separate studies.
One study looked at the insurance records of more than 70,000 U.S. patients with type 2 diabetes and established cardiovascular disease who were already on metformin. The findings showed that, after adjustment for confounders, the quartile of patients with the highest out-of-pocket cost for an agent from the sodium-glucose cotransporter 2 (SGLT2)–inhibitor class filled a prescription for one of these drugs a significant 21% less often than did patients from the quartile with the lowest personal expense, after adjustment for a variety of potential confounding factors, reported Jing Luo, MD, at the annual scientific sessions of the American Diabetes Association.
A similar analysis run by Dr. Luo and his associates looking at glucagonlike peptide-1 (GLP-1) receptor agonists showed that the quartile of patients who had to pay the most for one of those drugs had an adjusted 12% lower rate of filling a prescription, compared with those with the lowest out-of-pocket expense, a difference that fell just short of significance.
“If we consistently see that high drug costs affect use of highly effective medications in patients with type 2 diabetes and risk factors, it’s quite problematic because it’s not just a matter of money, but it also makes a difference in the patient’s quality of care,” Dr. Luo said in an interview.
Prevention drug lists can help
Consistency turned up in a second report at the same ADA session that retrospectively reviewed data collected during 2004-2017 by a single large U.S. health insurer to identify 3,315 matched pairs of children and adults with diabetes who all had high-deductible health plans for their medical insurance, along with an associated health savings account.
One set of patients in each matched pair began to receive, at some point during follow-up, coverage with a prevention drug list (PDL; also called a formulary) that provided them with a variety of specified agents at no charge. They included oral antidiabetes agents, insulin, antihypertensives, and lipid-lowering drugs. The other half of the matched pairs of patients received no PDL coverage and had copays for their antidiabetes medications.
The findings showed that the rates of out-of-pocket costs for antidiabetes drugs, antidiabetic medications used, and acute diabetes complications all tracked extremely closely between the matched pairs before half of them started to receive their PDL coverage. However, after PDL coverage kicked in, out of pocket costs dropped by 32% for the people with PDL coverage, compared with those who did not receive this coverage. Oral antidiabetes medication use rose modestly, but acute diabetes complications “declined substantially,” with a 14% relative reduction overall in those with PDL coverage, compared with those without, reported J. Franklin Wharam, MBBCh, a professor and health policy researcher at Duke University in Durham, N.C. In the roughly half of the study cohort who fell into a low-income category based on where they lived, the rate of excess acute diabetes complications was 23% higher for those without a PDL, compared with those who had that coverage.
PDL coverage linked with “large reductions in acute, preventable diabetes complications,” concluded Dr. Wharam. “Policy makers and employers should incentivize PDL uptake among low-income patients with diabetes.”
Newer, more effective drugs cost a lot
“The more comorbidities that patients have, the greater is the strength of the evidence for using newer antidiabetes drugs that are more expensive,” but that would mean spending much more on this part of patient care, noted Dr. Luo, an internal medicine physician and researcher at the University of Pittsburgh. “It will cost a lot of money, and I’m not sure what the solution is. It’s a huge conundrum.”
About 30 million Americans have type 2 diabetes. If every one of them went on an SGLT2 inhibitor, or went on an SGLT2 inhibitor plus a GLP-1 receptor agonist, “it would bankrupt the U.S. health care system, so we can’t do that,” commented Sylvio E. Inzucchi, MD, in an interview. “The only thing holding this back is cost. We target these drugs to the patients most apt to benefit from them. If they were generic they would be used much more widely,” noted Dr. Inzucchi, professor and clinical chief of endocrinology at Yale University in New Haven, Conn.
The study run by Dr. Luo and his associates retrospectively reviewed data from 72,743 U.S. adults included in the Optum Clinformatics database during December 2017–December 2019. All included patients had type 2 diabetes, received metformin monotherapy, and had established atherosclerotic cardiovascular disease. They averaged 72 years of age, 56% were men, and 88% were on a Medicare Advantage plan, while the remainder had commercial insurance. Their average hemoglobin A1c level was 6.8%.
People in the quartile with the lowest copays spent an average of about $20/month for either an SGLT2 inhibitor or a GLP-1 receptor agonist. Those in the quartile with the highest copays spent roughly $100/month for agents from each of these two classes. The analysis followed patients for a median of 914 days.
In addition to finding disparate rates of drug use between these two quartiles, the analysis also showed that higher copays linked with longer times to initially fill prescriptions for these drugs. But while those with higher copays took longer to start both classes than did those with the smallest copays, even those with the lowest out-of-pocket costs averaged about a year to initiate treatment.
Dr. Luo attributed this delay to other factors besides costs to patients, such as clinicians prescribing other classes of second-line oral antidiabetes agents, clinical inertia, and lack of awareness by clinicians of the special benefits of SGLT2 inhibitors and GLP-1 receptor antagonists for patients with type 2 diabetes and cardiovascular disease.
“A lot of clinical and social factors drive medication use,” not just out-of-pocket cost, he explained.
Dr. Luo is a consultant to Alosa Health. Dr. Wharam had no disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk.
NEW ORLEANS – , according to findings from two separate studies.
One study looked at the insurance records of more than 70,000 U.S. patients with type 2 diabetes and established cardiovascular disease who were already on metformin. The findings showed that, after adjustment for confounders, the quartile of patients with the highest out-of-pocket cost for an agent from the sodium-glucose cotransporter 2 (SGLT2)–inhibitor class filled a prescription for one of these drugs a significant 21% less often than did patients from the quartile with the lowest personal expense, after adjustment for a variety of potential confounding factors, reported Jing Luo, MD, at the annual scientific sessions of the American Diabetes Association.
A similar analysis run by Dr. Luo and his associates looking at glucagonlike peptide-1 (GLP-1) receptor agonists showed that the quartile of patients who had to pay the most for one of those drugs had an adjusted 12% lower rate of filling a prescription, compared with those with the lowest out-of-pocket expense, a difference that fell just short of significance.
“If we consistently see that high drug costs affect use of highly effective medications in patients with type 2 diabetes and risk factors, it’s quite problematic because it’s not just a matter of money, but it also makes a difference in the patient’s quality of care,” Dr. Luo said in an interview.
Prevention drug lists can help
Consistency turned up in a second report at the same ADA session that retrospectively reviewed data collected during 2004-2017 by a single large U.S. health insurer to identify 3,315 matched pairs of children and adults with diabetes who all had high-deductible health plans for their medical insurance, along with an associated health savings account.
One set of patients in each matched pair began to receive, at some point during follow-up, coverage with a prevention drug list (PDL; also called a formulary) that provided them with a variety of specified agents at no charge. They included oral antidiabetes agents, insulin, antihypertensives, and lipid-lowering drugs. The other half of the matched pairs of patients received no PDL coverage and had copays for their antidiabetes medications.
The findings showed that the rates of out-of-pocket costs for antidiabetes drugs, antidiabetic medications used, and acute diabetes complications all tracked extremely closely between the matched pairs before half of them started to receive their PDL coverage. However, after PDL coverage kicked in, out of pocket costs dropped by 32% for the people with PDL coverage, compared with those who did not receive this coverage. Oral antidiabetes medication use rose modestly, but acute diabetes complications “declined substantially,” with a 14% relative reduction overall in those with PDL coverage, compared with those without, reported J. Franklin Wharam, MBBCh, a professor and health policy researcher at Duke University in Durham, N.C. In the roughly half of the study cohort who fell into a low-income category based on where they lived, the rate of excess acute diabetes complications was 23% higher for those without a PDL, compared with those who had that coverage.
PDL coverage linked with “large reductions in acute, preventable diabetes complications,” concluded Dr. Wharam. “Policy makers and employers should incentivize PDL uptake among low-income patients with diabetes.”
Newer, more effective drugs cost a lot
“The more comorbidities that patients have, the greater is the strength of the evidence for using newer antidiabetes drugs that are more expensive,” but that would mean spending much more on this part of patient care, noted Dr. Luo, an internal medicine physician and researcher at the University of Pittsburgh. “It will cost a lot of money, and I’m not sure what the solution is. It’s a huge conundrum.”
About 30 million Americans have type 2 diabetes. If every one of them went on an SGLT2 inhibitor, or went on an SGLT2 inhibitor plus a GLP-1 receptor agonist, “it would bankrupt the U.S. health care system, so we can’t do that,” commented Sylvio E. Inzucchi, MD, in an interview. “The only thing holding this back is cost. We target these drugs to the patients most apt to benefit from them. If they were generic they would be used much more widely,” noted Dr. Inzucchi, professor and clinical chief of endocrinology at Yale University in New Haven, Conn.
The study run by Dr. Luo and his associates retrospectively reviewed data from 72,743 U.S. adults included in the Optum Clinformatics database during December 2017–December 2019. All included patients had type 2 diabetes, received metformin monotherapy, and had established atherosclerotic cardiovascular disease. They averaged 72 years of age, 56% were men, and 88% were on a Medicare Advantage plan, while the remainder had commercial insurance. Their average hemoglobin A1c level was 6.8%.
People in the quartile with the lowest copays spent an average of about $20/month for either an SGLT2 inhibitor or a GLP-1 receptor agonist. Those in the quartile with the highest copays spent roughly $100/month for agents from each of these two classes. The analysis followed patients for a median of 914 days.
In addition to finding disparate rates of drug use between these two quartiles, the analysis also showed that higher copays linked with longer times to initially fill prescriptions for these drugs. But while those with higher copays took longer to start both classes than did those with the smallest copays, even those with the lowest out-of-pocket costs averaged about a year to initiate treatment.
Dr. Luo attributed this delay to other factors besides costs to patients, such as clinicians prescribing other classes of second-line oral antidiabetes agents, clinical inertia, and lack of awareness by clinicians of the special benefits of SGLT2 inhibitors and GLP-1 receptor antagonists for patients with type 2 diabetes and cardiovascular disease.
“A lot of clinical and social factors drive medication use,” not just out-of-pocket cost, he explained.
Dr. Luo is a consultant to Alosa Health. Dr. Wharam had no disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk.
NEW ORLEANS – , according to findings from two separate studies.
One study looked at the insurance records of more than 70,000 U.S. patients with type 2 diabetes and established cardiovascular disease who were already on metformin. The findings showed that, after adjustment for confounders, the quartile of patients with the highest out-of-pocket cost for an agent from the sodium-glucose cotransporter 2 (SGLT2)–inhibitor class filled a prescription for one of these drugs a significant 21% less often than did patients from the quartile with the lowest personal expense, after adjustment for a variety of potential confounding factors, reported Jing Luo, MD, at the annual scientific sessions of the American Diabetes Association.
A similar analysis run by Dr. Luo and his associates looking at glucagonlike peptide-1 (GLP-1) receptor agonists showed that the quartile of patients who had to pay the most for one of those drugs had an adjusted 12% lower rate of filling a prescription, compared with those with the lowest out-of-pocket expense, a difference that fell just short of significance.
“If we consistently see that high drug costs affect use of highly effective medications in patients with type 2 diabetes and risk factors, it’s quite problematic because it’s not just a matter of money, but it also makes a difference in the patient’s quality of care,” Dr. Luo said in an interview.
Prevention drug lists can help
Consistency turned up in a second report at the same ADA session that retrospectively reviewed data collected during 2004-2017 by a single large U.S. health insurer to identify 3,315 matched pairs of children and adults with diabetes who all had high-deductible health plans for their medical insurance, along with an associated health savings account.
One set of patients in each matched pair began to receive, at some point during follow-up, coverage with a prevention drug list (PDL; also called a formulary) that provided them with a variety of specified agents at no charge. They included oral antidiabetes agents, insulin, antihypertensives, and lipid-lowering drugs. The other half of the matched pairs of patients received no PDL coverage and had copays for their antidiabetes medications.
The findings showed that the rates of out-of-pocket costs for antidiabetes drugs, antidiabetic medications used, and acute diabetes complications all tracked extremely closely between the matched pairs before half of them started to receive their PDL coverage. However, after PDL coverage kicked in, out of pocket costs dropped by 32% for the people with PDL coverage, compared with those who did not receive this coverage. Oral antidiabetes medication use rose modestly, but acute diabetes complications “declined substantially,” with a 14% relative reduction overall in those with PDL coverage, compared with those without, reported J. Franklin Wharam, MBBCh, a professor and health policy researcher at Duke University in Durham, N.C. In the roughly half of the study cohort who fell into a low-income category based on where they lived, the rate of excess acute diabetes complications was 23% higher for those without a PDL, compared with those who had that coverage.
PDL coverage linked with “large reductions in acute, preventable diabetes complications,” concluded Dr. Wharam. “Policy makers and employers should incentivize PDL uptake among low-income patients with diabetes.”
Newer, more effective drugs cost a lot
“The more comorbidities that patients have, the greater is the strength of the evidence for using newer antidiabetes drugs that are more expensive,” but that would mean spending much more on this part of patient care, noted Dr. Luo, an internal medicine physician and researcher at the University of Pittsburgh. “It will cost a lot of money, and I’m not sure what the solution is. It’s a huge conundrum.”
About 30 million Americans have type 2 diabetes. If every one of them went on an SGLT2 inhibitor, or went on an SGLT2 inhibitor plus a GLP-1 receptor agonist, “it would bankrupt the U.S. health care system, so we can’t do that,” commented Sylvio E. Inzucchi, MD, in an interview. “The only thing holding this back is cost. We target these drugs to the patients most apt to benefit from them. If they were generic they would be used much more widely,” noted Dr. Inzucchi, professor and clinical chief of endocrinology at Yale University in New Haven, Conn.
The study run by Dr. Luo and his associates retrospectively reviewed data from 72,743 U.S. adults included in the Optum Clinformatics database during December 2017–December 2019. All included patients had type 2 diabetes, received metformin monotherapy, and had established atherosclerotic cardiovascular disease. They averaged 72 years of age, 56% were men, and 88% were on a Medicare Advantage plan, while the remainder had commercial insurance. Their average hemoglobin A1c level was 6.8%.
People in the quartile with the lowest copays spent an average of about $20/month for either an SGLT2 inhibitor or a GLP-1 receptor agonist. Those in the quartile with the highest copays spent roughly $100/month for agents from each of these two classes. The analysis followed patients for a median of 914 days.
In addition to finding disparate rates of drug use between these two quartiles, the analysis also showed that higher copays linked with longer times to initially fill prescriptions for these drugs. But while those with higher copays took longer to start both classes than did those with the smallest copays, even those with the lowest out-of-pocket costs averaged about a year to initiate treatment.
Dr. Luo attributed this delay to other factors besides costs to patients, such as clinicians prescribing other classes of second-line oral antidiabetes agents, clinical inertia, and lack of awareness by clinicians of the special benefits of SGLT2 inhibitors and GLP-1 receptor antagonists for patients with type 2 diabetes and cardiovascular disease.
“A lot of clinical and social factors drive medication use,” not just out-of-pocket cost, he explained.
Dr. Luo is a consultant to Alosa Health. Dr. Wharam had no disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk.
AT ADA 2022
Obesity in adolescence raises risk for adult type 1 diabetes
NEW ORLEANS – Obesity in adolescence is linked to an increased risk for type 1 diabetes onset in adulthood, new research suggests.
These new data, from Israeli military recruits followed for over a decade, suggest that obesity may be playing a causal role in type 1 as well as type 2 diabetes.
The incidence of type 1 diabetes has been increasing by about 2%-3% annually over recent decades, but the reasons aren’t clear. The study is the first to examine the role of obesity in adolescence and type 1 diabetes in young adulthood, and also the first to examine the question of using antibody status as part of the criteria for a type 1 diagnosis.
The findings were reported at the annual scientific sessions of the American Diabetes Association by Gilad Twig, MD, PhD, professor of medicine at Sheba Medical Center, Tel HaShomer, Israel. “For people who might have a high risk for developing type 1 diabetes, these results emphasize the importance of maintaining a normal weight,” he said in an interview. He noted that, although this recommendation applies to everyone, “here it’s becoming more precise for the population – more individualized in the sense that this might specifically help you.”
Naveed Sattar, PhD, professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow, said in an interview that carrying too much weight “will make the pancreas have to work harder to make insulin to keep the sugar normal. So, if you’re stressing the system and the pancreas is already likely to fail, it will fail faster.”
Clinically, Dr. Sattar said, “Lifestyle does matter to the risk of developing type 1 diabetes. The weighting may be different [from type 2]. The major factor in type 1 is still the genetics, but if you have a family history of type 1 and your genetic potential is greater, you will minimize your risk by staying leaner.”
Study highlights that type 1 is not always ‘juvenile’
In addition to countering the long-held belief that type 1 diabetes is primarily a condition of thin individuals and unrelated to obesity, the data also reinforce the emerging recognition that type 1 diabetes isn’t always “juvenile” and in fact often arises in adulthood.
“About half of all cases of type 1 diabetes develop after age 18. By reputation, people think it’s a disease of children. But it’s begun to grow so that now 50% of cases occur after late adolescence,” noted Dr. Twig.
Dr. Sattar pointed to a UK Biobank study showing that nearly half of all cases of type 1 diabetes arise after age 30 years. “You absolutely can get type 1 in adulthood. It’s not rare.”
Direct correlation seen in otherwise healthy young people
The retrospective nationwide cohort study included 1,426,362 17-year-olds (834,050 male and 592,312 female) who underwent medical evaluation prior to military conscription starting in January 1996 and followed them through 2016. At baseline, none had a history of dysglycemia.
The data were linked with information about adult-onset type 1 diabetes in the Israeli National Diabetes Registry. In all, 777 incident type 1 diabetes cases were recorded over the study period, with a rate of 4.9 cases per 100,000 person-years.
Over a median follow-up of 11.2 years, there was a graded incidence of type 1 diabetes across BMI groups from underweight to obesity, from 3.6 to 8.4 cases per 100,000 person-years.
After adjustment for sex, birth year, age at study entry, education, and cognitive performance with BMI 5th-49th percentiles as the reference, the hazard ratios were 1.05 for the 50th-74th BMI percentiles, 1.41 for 75th-84th, 1.54 for those who were overweight, and 2.05 for those with obesity.
Every 5-unit increment in BMI corresponded to a 35% greater incidence of type 1 diabetes (adjusted hazard ratio 1.35) and every one increment was associated with a 35% greater risk (1.25), both values significant.
Sensitivity analyses resulted in similar findings for those with no other chronic health conditions at baseline. The results also didn’t change in a separate analysis of 574,720 subjects in whom autoantibody data were available to confirm the type 1 diabetes diagnosis.
Hypotheses for mechanisms
The mechanism for the association isn’t clear, but in a simultaneously published article in Diabetologia, Dr. Twig and colleagues outline several hypotheses. One relates to the growing evidence of a link between various autoimmune conditions, which point to the possibility of elevated adipokines and cytokines in obesity diminishing self-tolerance by promoting proinflammatory processes.
The authors cite data from the TrialNet Pathway to Prevention study of relatives of people with type 1 diabetes in which participants who were overweight and obese had an increased risk of islet autoantibody expression. However, not all data have supported this finding.
“Obesity is related to several other autoimmune conditions, so it’s not a complete surprise it might be related to another,” Dr. Twig noted.
Other possibilities include vitamin D deficiency, a high-fat diet, and alterations in gut microbiota.
And then there’s the “accelerator hypothesis,” suggesting that both type 1 and type 2 diabetes result from insulin resistance and genetic background that affect the rate of beta cell loss and the disease phenotype. Dr. Sattar said that the accelerator hypotheses “makes complete sense to me. Because the population is so obese, we’re seeing it more now whereas we might not have seen it 40 years ago when the BMI differentials were far less in society.”
Dr. Twig has no disclosures. Dr. Sattar has consulted for or received lecture fees from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceutical, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi, and received grant support from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics through his institution.
NEW ORLEANS – Obesity in adolescence is linked to an increased risk for type 1 diabetes onset in adulthood, new research suggests.
These new data, from Israeli military recruits followed for over a decade, suggest that obesity may be playing a causal role in type 1 as well as type 2 diabetes.
The incidence of type 1 diabetes has been increasing by about 2%-3% annually over recent decades, but the reasons aren’t clear. The study is the first to examine the role of obesity in adolescence and type 1 diabetes in young adulthood, and also the first to examine the question of using antibody status as part of the criteria for a type 1 diagnosis.
The findings were reported at the annual scientific sessions of the American Diabetes Association by Gilad Twig, MD, PhD, professor of medicine at Sheba Medical Center, Tel HaShomer, Israel. “For people who might have a high risk for developing type 1 diabetes, these results emphasize the importance of maintaining a normal weight,” he said in an interview. He noted that, although this recommendation applies to everyone, “here it’s becoming more precise for the population – more individualized in the sense that this might specifically help you.”
Naveed Sattar, PhD, professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow, said in an interview that carrying too much weight “will make the pancreas have to work harder to make insulin to keep the sugar normal. So, if you’re stressing the system and the pancreas is already likely to fail, it will fail faster.”
Clinically, Dr. Sattar said, “Lifestyle does matter to the risk of developing type 1 diabetes. The weighting may be different [from type 2]. The major factor in type 1 is still the genetics, but if you have a family history of type 1 and your genetic potential is greater, you will minimize your risk by staying leaner.”
Study highlights that type 1 is not always ‘juvenile’
In addition to countering the long-held belief that type 1 diabetes is primarily a condition of thin individuals and unrelated to obesity, the data also reinforce the emerging recognition that type 1 diabetes isn’t always “juvenile” and in fact often arises in adulthood.
“About half of all cases of type 1 diabetes develop after age 18. By reputation, people think it’s a disease of children. But it’s begun to grow so that now 50% of cases occur after late adolescence,” noted Dr. Twig.
Dr. Sattar pointed to a UK Biobank study showing that nearly half of all cases of type 1 diabetes arise after age 30 years. “You absolutely can get type 1 in adulthood. It’s not rare.”
Direct correlation seen in otherwise healthy young people
The retrospective nationwide cohort study included 1,426,362 17-year-olds (834,050 male and 592,312 female) who underwent medical evaluation prior to military conscription starting in January 1996 and followed them through 2016. At baseline, none had a history of dysglycemia.
The data were linked with information about adult-onset type 1 diabetes in the Israeli National Diabetes Registry. In all, 777 incident type 1 diabetes cases were recorded over the study period, with a rate of 4.9 cases per 100,000 person-years.
Over a median follow-up of 11.2 years, there was a graded incidence of type 1 diabetes across BMI groups from underweight to obesity, from 3.6 to 8.4 cases per 100,000 person-years.
After adjustment for sex, birth year, age at study entry, education, and cognitive performance with BMI 5th-49th percentiles as the reference, the hazard ratios were 1.05 for the 50th-74th BMI percentiles, 1.41 for 75th-84th, 1.54 for those who were overweight, and 2.05 for those with obesity.
Every 5-unit increment in BMI corresponded to a 35% greater incidence of type 1 diabetes (adjusted hazard ratio 1.35) and every one increment was associated with a 35% greater risk (1.25), both values significant.
Sensitivity analyses resulted in similar findings for those with no other chronic health conditions at baseline. The results also didn’t change in a separate analysis of 574,720 subjects in whom autoantibody data were available to confirm the type 1 diabetes diagnosis.
Hypotheses for mechanisms
The mechanism for the association isn’t clear, but in a simultaneously published article in Diabetologia, Dr. Twig and colleagues outline several hypotheses. One relates to the growing evidence of a link between various autoimmune conditions, which point to the possibility of elevated adipokines and cytokines in obesity diminishing self-tolerance by promoting proinflammatory processes.
The authors cite data from the TrialNet Pathway to Prevention study of relatives of people with type 1 diabetes in which participants who were overweight and obese had an increased risk of islet autoantibody expression. However, not all data have supported this finding.
“Obesity is related to several other autoimmune conditions, so it’s not a complete surprise it might be related to another,” Dr. Twig noted.
Other possibilities include vitamin D deficiency, a high-fat diet, and alterations in gut microbiota.
And then there’s the “accelerator hypothesis,” suggesting that both type 1 and type 2 diabetes result from insulin resistance and genetic background that affect the rate of beta cell loss and the disease phenotype. Dr. Sattar said that the accelerator hypotheses “makes complete sense to me. Because the population is so obese, we’re seeing it more now whereas we might not have seen it 40 years ago when the BMI differentials were far less in society.”
Dr. Twig has no disclosures. Dr. Sattar has consulted for or received lecture fees from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceutical, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi, and received grant support from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics through his institution.
NEW ORLEANS – Obesity in adolescence is linked to an increased risk for type 1 diabetes onset in adulthood, new research suggests.
These new data, from Israeli military recruits followed for over a decade, suggest that obesity may be playing a causal role in type 1 as well as type 2 diabetes.
The incidence of type 1 diabetes has been increasing by about 2%-3% annually over recent decades, but the reasons aren’t clear. The study is the first to examine the role of obesity in adolescence and type 1 diabetes in young adulthood, and also the first to examine the question of using antibody status as part of the criteria for a type 1 diagnosis.
The findings were reported at the annual scientific sessions of the American Diabetes Association by Gilad Twig, MD, PhD, professor of medicine at Sheba Medical Center, Tel HaShomer, Israel. “For people who might have a high risk for developing type 1 diabetes, these results emphasize the importance of maintaining a normal weight,” he said in an interview. He noted that, although this recommendation applies to everyone, “here it’s becoming more precise for the population – more individualized in the sense that this might specifically help you.”
Naveed Sattar, PhD, professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow, said in an interview that carrying too much weight “will make the pancreas have to work harder to make insulin to keep the sugar normal. So, if you’re stressing the system and the pancreas is already likely to fail, it will fail faster.”
Clinically, Dr. Sattar said, “Lifestyle does matter to the risk of developing type 1 diabetes. The weighting may be different [from type 2]. The major factor in type 1 is still the genetics, but if you have a family history of type 1 and your genetic potential is greater, you will minimize your risk by staying leaner.”
Study highlights that type 1 is not always ‘juvenile’
In addition to countering the long-held belief that type 1 diabetes is primarily a condition of thin individuals and unrelated to obesity, the data also reinforce the emerging recognition that type 1 diabetes isn’t always “juvenile” and in fact often arises in adulthood.
“About half of all cases of type 1 diabetes develop after age 18. By reputation, people think it’s a disease of children. But it’s begun to grow so that now 50% of cases occur after late adolescence,” noted Dr. Twig.
Dr. Sattar pointed to a UK Biobank study showing that nearly half of all cases of type 1 diabetes arise after age 30 years. “You absolutely can get type 1 in adulthood. It’s not rare.”
Direct correlation seen in otherwise healthy young people
The retrospective nationwide cohort study included 1,426,362 17-year-olds (834,050 male and 592,312 female) who underwent medical evaluation prior to military conscription starting in January 1996 and followed them through 2016. At baseline, none had a history of dysglycemia.
The data were linked with information about adult-onset type 1 diabetes in the Israeli National Diabetes Registry. In all, 777 incident type 1 diabetes cases were recorded over the study period, with a rate of 4.9 cases per 100,000 person-years.
Over a median follow-up of 11.2 years, there was a graded incidence of type 1 diabetes across BMI groups from underweight to obesity, from 3.6 to 8.4 cases per 100,000 person-years.
After adjustment for sex, birth year, age at study entry, education, and cognitive performance with BMI 5th-49th percentiles as the reference, the hazard ratios were 1.05 for the 50th-74th BMI percentiles, 1.41 for 75th-84th, 1.54 for those who were overweight, and 2.05 for those with obesity.
Every 5-unit increment in BMI corresponded to a 35% greater incidence of type 1 diabetes (adjusted hazard ratio 1.35) and every one increment was associated with a 35% greater risk (1.25), both values significant.
Sensitivity analyses resulted in similar findings for those with no other chronic health conditions at baseline. The results also didn’t change in a separate analysis of 574,720 subjects in whom autoantibody data were available to confirm the type 1 diabetes diagnosis.
Hypotheses for mechanisms
The mechanism for the association isn’t clear, but in a simultaneously published article in Diabetologia, Dr. Twig and colleagues outline several hypotheses. One relates to the growing evidence of a link between various autoimmune conditions, which point to the possibility of elevated adipokines and cytokines in obesity diminishing self-tolerance by promoting proinflammatory processes.
The authors cite data from the TrialNet Pathway to Prevention study of relatives of people with type 1 diabetes in which participants who were overweight and obese had an increased risk of islet autoantibody expression. However, not all data have supported this finding.
“Obesity is related to several other autoimmune conditions, so it’s not a complete surprise it might be related to another,” Dr. Twig noted.
Other possibilities include vitamin D deficiency, a high-fat diet, and alterations in gut microbiota.
And then there’s the “accelerator hypothesis,” suggesting that both type 1 and type 2 diabetes result from insulin resistance and genetic background that affect the rate of beta cell loss and the disease phenotype. Dr. Sattar said that the accelerator hypotheses “makes complete sense to me. Because the population is so obese, we’re seeing it more now whereas we might not have seen it 40 years ago when the BMI differentials were far less in society.”
Dr. Twig has no disclosures. Dr. Sattar has consulted for or received lecture fees from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceutical, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi, and received grant support from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics through his institution.
AT ADA 2022
Weekly dulaglutide promising in youth with type 2 diabetes
Another glucagonlike peptide-1 (GLP1) agonist, dulaglutide (Trulicity, Lilly), is poised to be a new option for glycemic control in youth aged 10-18 years with type 2 diabetes, given as a weekly injection, based on the AWARD-PEDS clinical trial.
The U.S. Food and Drug Administration has already approved daily injection liraglutide (Victoza, Novo Nordisk) in 2019 and weekly exenatide (Bydureon/Bydureon BCise, AstraZeneca) in 2021 for glycemic control in young patients with type 2 diabetes, both of which are also GLP-1 agonists.
AWARD-PEDS showed that youth with type 2 diabetes and obesity treated with or without metformin or basal insulin who received weekly injections of 0.75 mg or 1.5 mg of dulaglutide had lower hemoglobin A1c at 26 weeks than patients who received placebo.
Eli Lilly is now submitting these trial results to the FDA for this indication.
Dulaglutide was cleared for use in adults with type 2 diabetes in the United States in 2014 and was additionally approved for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes at high risk of such events in 2020.
The most common adverse symptoms were gastrointestinal, and the safety profile was consistent with that in adults. However, the drug had no effect on body mass index.
The study was simultaneously published in the New England Journal of Medicine and presented as a late-breaking poster at the annual scientific sessions of the American Diabetes Association in New Orleans.
Might dulaglutide target pathophysiologic impairments in youth?
Dulaglutide would “offer a new treatment that targets the pathophysiologic impairments of type 2 diabetes in youth,” Silva A. Arslanian, MD, lead investigator, told this news organization.
Exenatide is also given as a weekly injection but is associated with a smaller decrease in A1c and does not improve fasting glucose concentrations, plus it requires more steps compared with the dulaglutide single-use pen, said Dr. Arslanian, who is scientific director at the Center for Pediatric Research in Obesity & Metabolism, UPMC Children’s Hospital of Pittsburgh.
“Liraglutide is a daily injection, and I believe most patients, particularly adolescents, would prefer a weekly injection,” she added.
Invited to comment, Elvira Isganaitis, MD, MPH, said “the significance of this paper lies in the fact that options for treating type 2 diabetes in children are currently much more limited than in adults – which is a major problem given recent studies that show that type 2 diabetes in youth is much more aggressive and more likely to cause complications early in the disease course.”
Dr. Isganaitis was not involved with the trial but is an investigator for the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study.
“With supply chain shortages and health insurance coverage issues that are common in the U.S., it would be helpful to have more than one FDA-approved option for a weekly GLP-1 receptor agonist in children [and] access to other classes of medications,” added Dr. Isganaitis, a pediatric endocrinologist at the Joslin Diabetes Center, Boston.
Phase 3 trials of sodium-glucose cotransporter 2 (SGLT2) inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors in youth with type 2 diabetes are also ongoing, Dr. Arslanian noted, “but as always, recruitment is slow with adolescents.”
“I am not optimistic that DPP4 inhibitors will have a place in the treatment of youth with type 2 diabetes,” she said. A recent study showed the addition of sitagliptin to metformin in youth with type 2 diabetes did not provide durable improvement in glycemic control.
Potentially promising therapy
In their published article, Dr. Arslanian and colleagues write that “considering the progressive increase in [A1c] over time that was observed in the TODAY trial, with 34% of youths having [an A1c] of at least 10% after up to 15 years of follow-up, we believe that the effects of dulaglutide therapy appear to be potentially promising.”
The TODAY trial showed that more than 50% of youth with type 2 diabetes taking metformin failed to maintain glycemic control within a median of 11.5 months, Dr. Arslanian elaborated, and over time their A1c escalated while their beta-cell function deteriorated rapidly, and complications progressed quickly.
“Therefore,” she noted, “considering that dulaglutide and the GLP-1 receptor agonist class of drugs improve A1c, improve beta-cell function, suppress glucagon concentrations, and improve insulin sensitivity, dulaglutide would provide a promising new treatment option for youth with type 2 diabetes.”
Phase 3 superiority trial
The AWARD-PEDS trial included 154 youth with type 2 diabetes and a BMI greater than the 85th percentile for their age and sex at 46 centers in nine countries. Researchers randomized participants 1:1:1 to the two doses of dulaglutide or placebo for 26 weeks, followed by a 26-week open-label study (during which the placebo group received 0.75 mg dulaglutide) and a 4-week safety extension.
Participants were a mean age of 14.5 years and had a mean BMI of 34 kg/m2.
In each of the dulaglutide groups, roughly 66% of patients were female and 58% were White, 18% were Black, and about 57% were Hispanic. They had a mean weight of 91 kg (200 lb) and a mean A1c of about 8%; 62% were taking metformin only, 27% were taking metformin plus basal insulin, 3% were taking basal insulin only, and 10% were on diet and exercise only.
At 26 weeks, mean A1c increased by 0.6% in the placebo group but decreased by 0.6% in the 0.75-mg dulaglutide group and by 0.9% in the 1.5-mg dulaglutide group (P < .001 for both comparisons versus placebo).
Also at 26 weeks, more participants in the pooled dulaglutide groups than in the placebo group had an A1c <7.0% (51% vs. 14%; P < .001).
Fasting glucose concentration increased in the placebo group (+17.1 mg/dL ) and decreased in the pooled dulaglutide groups (–18.9 mg/dL; P < .001).
There were no group differences in BMI or adiposity-related parameters even at 52 weeks.
“I believe adolescents may be somewhat resistant to the weight-reducing effects of GLP-1 agonists in diabetes trials (liraglutide and exenatide youth type 2 diabetes trials showed the same thing) and they may need higher doses,” Dr. Arslanian speculated.
“Only future studies will be able to address this issue,” she concluded.
The study was funded by Eli Lilly. Dr. Arslanian has disclosed being a consultant for Eli Lilly, Novo Nordisk, and Rhythm Pharmaceuticals; participating in data safety monitoring for AstraZeneca and Eli Lilly trials; and receiving institutional research funding from Eli Lilly and Novo Nordisk. Dr. Isganaitis has disclosed receiving research funding (paid to her institution) from Dexcom and AstraZeneca.
A version of this article first appeared on Medscape.com.
Another glucagonlike peptide-1 (GLP1) agonist, dulaglutide (Trulicity, Lilly), is poised to be a new option for glycemic control in youth aged 10-18 years with type 2 diabetes, given as a weekly injection, based on the AWARD-PEDS clinical trial.
The U.S. Food and Drug Administration has already approved daily injection liraglutide (Victoza, Novo Nordisk) in 2019 and weekly exenatide (Bydureon/Bydureon BCise, AstraZeneca) in 2021 for glycemic control in young patients with type 2 diabetes, both of which are also GLP-1 agonists.
AWARD-PEDS showed that youth with type 2 diabetes and obesity treated with or without metformin or basal insulin who received weekly injections of 0.75 mg or 1.5 mg of dulaglutide had lower hemoglobin A1c at 26 weeks than patients who received placebo.
Eli Lilly is now submitting these trial results to the FDA for this indication.
Dulaglutide was cleared for use in adults with type 2 diabetes in the United States in 2014 and was additionally approved for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes at high risk of such events in 2020.
The most common adverse symptoms were gastrointestinal, and the safety profile was consistent with that in adults. However, the drug had no effect on body mass index.
The study was simultaneously published in the New England Journal of Medicine and presented as a late-breaking poster at the annual scientific sessions of the American Diabetes Association in New Orleans.
Might dulaglutide target pathophysiologic impairments in youth?
Dulaglutide would “offer a new treatment that targets the pathophysiologic impairments of type 2 diabetes in youth,” Silva A. Arslanian, MD, lead investigator, told this news organization.
Exenatide is also given as a weekly injection but is associated with a smaller decrease in A1c and does not improve fasting glucose concentrations, plus it requires more steps compared with the dulaglutide single-use pen, said Dr. Arslanian, who is scientific director at the Center for Pediatric Research in Obesity & Metabolism, UPMC Children’s Hospital of Pittsburgh.
“Liraglutide is a daily injection, and I believe most patients, particularly adolescents, would prefer a weekly injection,” she added.
Invited to comment, Elvira Isganaitis, MD, MPH, said “the significance of this paper lies in the fact that options for treating type 2 diabetes in children are currently much more limited than in adults – which is a major problem given recent studies that show that type 2 diabetes in youth is much more aggressive and more likely to cause complications early in the disease course.”
Dr. Isganaitis was not involved with the trial but is an investigator for the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study.
“With supply chain shortages and health insurance coverage issues that are common in the U.S., it would be helpful to have more than one FDA-approved option for a weekly GLP-1 receptor agonist in children [and] access to other classes of medications,” added Dr. Isganaitis, a pediatric endocrinologist at the Joslin Diabetes Center, Boston.
Phase 3 trials of sodium-glucose cotransporter 2 (SGLT2) inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors in youth with type 2 diabetes are also ongoing, Dr. Arslanian noted, “but as always, recruitment is slow with adolescents.”
“I am not optimistic that DPP4 inhibitors will have a place in the treatment of youth with type 2 diabetes,” she said. A recent study showed the addition of sitagliptin to metformin in youth with type 2 diabetes did not provide durable improvement in glycemic control.
Potentially promising therapy
In their published article, Dr. Arslanian and colleagues write that “considering the progressive increase in [A1c] over time that was observed in the TODAY trial, with 34% of youths having [an A1c] of at least 10% after up to 15 years of follow-up, we believe that the effects of dulaglutide therapy appear to be potentially promising.”
The TODAY trial showed that more than 50% of youth with type 2 diabetes taking metformin failed to maintain glycemic control within a median of 11.5 months, Dr. Arslanian elaborated, and over time their A1c escalated while their beta-cell function deteriorated rapidly, and complications progressed quickly.
“Therefore,” she noted, “considering that dulaglutide and the GLP-1 receptor agonist class of drugs improve A1c, improve beta-cell function, suppress glucagon concentrations, and improve insulin sensitivity, dulaglutide would provide a promising new treatment option for youth with type 2 diabetes.”
Phase 3 superiority trial
The AWARD-PEDS trial included 154 youth with type 2 diabetes and a BMI greater than the 85th percentile for their age and sex at 46 centers in nine countries. Researchers randomized participants 1:1:1 to the two doses of dulaglutide or placebo for 26 weeks, followed by a 26-week open-label study (during which the placebo group received 0.75 mg dulaglutide) and a 4-week safety extension.
Participants were a mean age of 14.5 years and had a mean BMI of 34 kg/m2.
In each of the dulaglutide groups, roughly 66% of patients were female and 58% were White, 18% were Black, and about 57% were Hispanic. They had a mean weight of 91 kg (200 lb) and a mean A1c of about 8%; 62% were taking metformin only, 27% were taking metformin plus basal insulin, 3% were taking basal insulin only, and 10% were on diet and exercise only.
At 26 weeks, mean A1c increased by 0.6% in the placebo group but decreased by 0.6% in the 0.75-mg dulaglutide group and by 0.9% in the 1.5-mg dulaglutide group (P < .001 for both comparisons versus placebo).
Also at 26 weeks, more participants in the pooled dulaglutide groups than in the placebo group had an A1c <7.0% (51% vs. 14%; P < .001).
Fasting glucose concentration increased in the placebo group (+17.1 mg/dL ) and decreased in the pooled dulaglutide groups (–18.9 mg/dL; P < .001).
There were no group differences in BMI or adiposity-related parameters even at 52 weeks.
“I believe adolescents may be somewhat resistant to the weight-reducing effects of GLP-1 agonists in diabetes trials (liraglutide and exenatide youth type 2 diabetes trials showed the same thing) and they may need higher doses,” Dr. Arslanian speculated.
“Only future studies will be able to address this issue,” she concluded.
The study was funded by Eli Lilly. Dr. Arslanian has disclosed being a consultant for Eli Lilly, Novo Nordisk, and Rhythm Pharmaceuticals; participating in data safety monitoring for AstraZeneca and Eli Lilly trials; and receiving institutional research funding from Eli Lilly and Novo Nordisk. Dr. Isganaitis has disclosed receiving research funding (paid to her institution) from Dexcom and AstraZeneca.
A version of this article first appeared on Medscape.com.
Another glucagonlike peptide-1 (GLP1) agonist, dulaglutide (Trulicity, Lilly), is poised to be a new option for glycemic control in youth aged 10-18 years with type 2 diabetes, given as a weekly injection, based on the AWARD-PEDS clinical trial.
The U.S. Food and Drug Administration has already approved daily injection liraglutide (Victoza, Novo Nordisk) in 2019 and weekly exenatide (Bydureon/Bydureon BCise, AstraZeneca) in 2021 for glycemic control in young patients with type 2 diabetes, both of which are also GLP-1 agonists.
AWARD-PEDS showed that youth with type 2 diabetes and obesity treated with or without metformin or basal insulin who received weekly injections of 0.75 mg or 1.5 mg of dulaglutide had lower hemoglobin A1c at 26 weeks than patients who received placebo.
Eli Lilly is now submitting these trial results to the FDA for this indication.
Dulaglutide was cleared for use in adults with type 2 diabetes in the United States in 2014 and was additionally approved for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes at high risk of such events in 2020.
The most common adverse symptoms were gastrointestinal, and the safety profile was consistent with that in adults. However, the drug had no effect on body mass index.
The study was simultaneously published in the New England Journal of Medicine and presented as a late-breaking poster at the annual scientific sessions of the American Diabetes Association in New Orleans.
Might dulaglutide target pathophysiologic impairments in youth?
Dulaglutide would “offer a new treatment that targets the pathophysiologic impairments of type 2 diabetes in youth,” Silva A. Arslanian, MD, lead investigator, told this news organization.
Exenatide is also given as a weekly injection but is associated with a smaller decrease in A1c and does not improve fasting glucose concentrations, plus it requires more steps compared with the dulaglutide single-use pen, said Dr. Arslanian, who is scientific director at the Center for Pediatric Research in Obesity & Metabolism, UPMC Children’s Hospital of Pittsburgh.
“Liraglutide is a daily injection, and I believe most patients, particularly adolescents, would prefer a weekly injection,” she added.
Invited to comment, Elvira Isganaitis, MD, MPH, said “the significance of this paper lies in the fact that options for treating type 2 diabetes in children are currently much more limited than in adults – which is a major problem given recent studies that show that type 2 diabetes in youth is much more aggressive and more likely to cause complications early in the disease course.”
Dr. Isganaitis was not involved with the trial but is an investigator for the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study.
“With supply chain shortages and health insurance coverage issues that are common in the U.S., it would be helpful to have more than one FDA-approved option for a weekly GLP-1 receptor agonist in children [and] access to other classes of medications,” added Dr. Isganaitis, a pediatric endocrinologist at the Joslin Diabetes Center, Boston.
Phase 3 trials of sodium-glucose cotransporter 2 (SGLT2) inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors in youth with type 2 diabetes are also ongoing, Dr. Arslanian noted, “but as always, recruitment is slow with adolescents.”
“I am not optimistic that DPP4 inhibitors will have a place in the treatment of youth with type 2 diabetes,” she said. A recent study showed the addition of sitagliptin to metformin in youth with type 2 diabetes did not provide durable improvement in glycemic control.
Potentially promising therapy
In their published article, Dr. Arslanian and colleagues write that “considering the progressive increase in [A1c] over time that was observed in the TODAY trial, with 34% of youths having [an A1c] of at least 10% after up to 15 years of follow-up, we believe that the effects of dulaglutide therapy appear to be potentially promising.”
The TODAY trial showed that more than 50% of youth with type 2 diabetes taking metformin failed to maintain glycemic control within a median of 11.5 months, Dr. Arslanian elaborated, and over time their A1c escalated while their beta-cell function deteriorated rapidly, and complications progressed quickly.
“Therefore,” she noted, “considering that dulaglutide and the GLP-1 receptor agonist class of drugs improve A1c, improve beta-cell function, suppress glucagon concentrations, and improve insulin sensitivity, dulaglutide would provide a promising new treatment option for youth with type 2 diabetes.”
Phase 3 superiority trial
The AWARD-PEDS trial included 154 youth with type 2 diabetes and a BMI greater than the 85th percentile for their age and sex at 46 centers in nine countries. Researchers randomized participants 1:1:1 to the two doses of dulaglutide or placebo for 26 weeks, followed by a 26-week open-label study (during which the placebo group received 0.75 mg dulaglutide) and a 4-week safety extension.
Participants were a mean age of 14.5 years and had a mean BMI of 34 kg/m2.
In each of the dulaglutide groups, roughly 66% of patients were female and 58% were White, 18% were Black, and about 57% were Hispanic. They had a mean weight of 91 kg (200 lb) and a mean A1c of about 8%; 62% were taking metformin only, 27% were taking metformin plus basal insulin, 3% were taking basal insulin only, and 10% were on diet and exercise only.
At 26 weeks, mean A1c increased by 0.6% in the placebo group but decreased by 0.6% in the 0.75-mg dulaglutide group and by 0.9% in the 1.5-mg dulaglutide group (P < .001 for both comparisons versus placebo).
Also at 26 weeks, more participants in the pooled dulaglutide groups than in the placebo group had an A1c <7.0% (51% vs. 14%; P < .001).
Fasting glucose concentration increased in the placebo group (+17.1 mg/dL ) and decreased in the pooled dulaglutide groups (–18.9 mg/dL; P < .001).
There were no group differences in BMI or adiposity-related parameters even at 52 weeks.
“I believe adolescents may be somewhat resistant to the weight-reducing effects of GLP-1 agonists in diabetes trials (liraglutide and exenatide youth type 2 diabetes trials showed the same thing) and they may need higher doses,” Dr. Arslanian speculated.
“Only future studies will be able to address this issue,” she concluded.
The study was funded by Eli Lilly. Dr. Arslanian has disclosed being a consultant for Eli Lilly, Novo Nordisk, and Rhythm Pharmaceuticals; participating in data safety monitoring for AstraZeneca and Eli Lilly trials; and receiving institutional research funding from Eli Lilly and Novo Nordisk. Dr. Isganaitis has disclosed receiving research funding (paid to her institution) from Dexcom and AstraZeneca.
A version of this article first appeared on Medscape.com.
FROM ADA 2022
Tirzepatide powers ‘unprecedented’ weight loss in SURMOUNT-1
NEW ORLEANS – Treatment of people with obesity but no diabetes with the dual–incretin agonist tirzepatide safely produced “unprecedented” levels of weight loss in the vast majority of patients in SURMOUNT-1, a placebo-controlled trial with more than 2,500 people with obesity or overweight plus at least one weight-related complication.
Although the pivotal trial did not directly compare weekly subcutaneous injection with the twincretin tirzepatide (at 5 mg, 10 mg, or 15 mg) with either bariatric surgery or what has been the reigning champ of weight-loss agents, a 2.4-mg/week injection of semaglutide (Wegovy), the new findings are impressive because they eclipsed semaglutide’s past performance in at least three important ways, said Ania M. Jastreboff, MD, PhD, SURMOUNT-1’s lead investigator, at the annual scientific sessions of the American Diabetes Association.
First, the highest-tested dosage of tirzepatide, 15 mg/week, for 72 weeks, produced a 5% or greater loss in baseline weight in 91%-96% of patients, an effect “not previously seen” in any prior phase 3 trial of a weight-loss agent, noted Dr. Jastreboff, an endocrinologist and director of Weight Management & Obesity Prevention at Yale University in New Haven, Conn.
Second, the average level of weight loss among the 630 people who received 15 mg/week was 22.5% in the on-treatment analysis, and 20.9% in the intention-to-treat analysis, again a magnitude of effect never before seen with any other medical intervention.
And in an exploratory analysis, 40% of people who received the highest-tested tirzepatide dose of 15 mg/week had at least a 25% loss in baseline weight in the on-treatment analysis, another example of unprecedented weight-loss achievement, said Dr. Jastreboff.
Looking at the data another way, the average baseline weight of those in the trial was 104 kg (230 lb) at the start, and the average weight loss was between 35 and 52 lbs by 72 weeks on treatment, Dr. Jastreboff said in a press conference.
She noted, however, that not everyone will respond to tirzepatide, “but if you do respond to this medicine, you will feel full earlier, you won’t want to go back for seconds, and you may eat smaller amounts more often.”
Such weight-loss agents will need to be taken chronically, in the same way that medications are for hypertension or dyslipidemia, Dr. Jastreboff stressed. “If you stop the antiobesity medication then the body fat mass set point will go back up so this necessitates long-term treatment.”
A new era: Weight loss ‘in the range of bariatric surgery’
Tirzepatide, developed by Lilly, has recently been approved in the United States for the treatment of type 2 diabetes, under the brand name Mounjaro.
SURMOUNT-1 was designed to examine the effect of the agent in overweight/obesity, and the company will be filing for the additional indication of weight loss in the future. Top-line results of SURMOUNT-1 generated much excitement when Lilly reported them back in April, including a story in The New York Times.
Semaglutide, a Novo Nordisk drug, is approved in the United States for type 2 diabetes (as Ozempic at doses of either 1 mg or 2 mg per week) and also for weight loss, as Wegovy, at the higher dose of 2.4 mg per week. When Wegovy was given the green light by the Food and Drug Administration a year ago, it too was hailed as a “game changer” for obesity.
The weight-loss results seen in SURMOUNT-1 “put tirzepatide squarely in the range of weight loss achieved with bariatric surgery,” concluded Louis J. Aronne, MD, a coinvestigator on the trial, professor at Weill-Cornell Medicine in New York, and director of the Center for Weight Management and Metabolic Clinical Research of Weill-Cornell.
The results are “amazing,” and propel the weight-loss field into “a new era of obesity treatment,” commented Lee M. Kaplan, MD, who was not involved in the study and served as designated discussant for the trial.
Despite the lack of direct comparison, the findings indicate that “tirzepatide causes more weight loss than semaglutide,” and it provides “an opportunity to meet or exceed” the weight-loss effects of bariatric surgery, added Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
Simultaneously with Dr. Jastreboff’s report at the meeting, the results were published online in The New England Journal of Medicine.
An accompanying editorial agrees with Dr. Kaplan: “It is remarkable that the magnitude of weight loss with tirzepatide was similar to that with gastric bypass, which raises the potential for alternative medical approaches to the treatment of obesity.”
“The tides are shifting, and there are now more options for people with obesity to lose weight,” write Clifford J. Rosen, MD, of Tufts University, Boston, and Julie R. Ingelfinger, MD, of Harvard University and Massachusetts General Hospital, Boston.
Dual incretin agonism ‘enhances activity,’ says expert
Tirzepatide is the first agent on the U.S. market from a novel class of dual-incretin agonists, with a molecular structure engineered to activate both the glucagonlike protein-1 (GLP-1) receptor and the glucose-dependent insulinotropic polypeptide (GIP), the two predominant incretins in the human gut. This combined activity has led to the twincretin nickname for tirzepatide.
Semaglutide is a single-incretin agonist, with its activity focused exclusively on the GLP-1 receptor.
Dr. Aronne tied the apparently superior efficacy of tirzepatide relative to semaglutide directly to the added incretin activity of tirzepatide. “The dual approach enhances efficacy,” he proposed during his presentation at the meeting.
The impressive efficacy and reassuring safety profile reported from SURMOUNT-1 opens the door to a new approach to treating obesity, which in the past has often taken a back seat to treatments for dyslipidemia, hypertension, and diabetes.
“Now that we can treat obesity safely and effectively, it makes sense to treat obesity first,” Dr. Aronne recommended.
Dr. Jastreboff agreed: “Perhaps we can prevent diabetes by treating obesity head-on,” she remarked.
Weight-loss agents gain U.S. traction
There have been concerns about patient access to these newer weight-loss drugs in the United States, given that the retail cost of semaglutide for obesity exceeds $1,000/month, but Dr. Aronne reported data that painted a more optimistic picture.
His numbers showed that during the first months that semaglutide was on the U.S. market as a weight-loss agent, the number of U.S. prescriptions written for branded antiobesity medications roughly doubled, a spike that seemed mostly driven by the introduction and growing use of semaglutide.
With tirzepatide, every prespecified cardiometabolic parameter assessed in the trial showed clinically meaningful improvements, reported Dr. Jastreboff, including an average 17% reduction in waist circumference in patients on either of the highest two dosages, a 34% average drop in total fat mass, an average 0.5–percentage point cut in baseline hemoglobin A1c at the highest two dosages, substantial cuts in fasting plasma glucose and fasting insulin levels, an average 28% drop in triglyceride levels, and an average systolic blood pressure reduction of about 8 mm Hg that occurred within 24 weeks on treatment.
“I think that insurers will sign up” for tirzepatide coverage based on benefits like this, Dr. Aronne predicted.
SURMOUNT-1 randomized 2,539 patients with obesity or with overweight plus at least one weight-related complication at any of 119 sites in nine countries. They had a body mass index of 30 kg/m2 or more, or 27 kg/m2 or more and at least one weight-related complication, excluding diabetes. They were randomized in a 1:1:1:1 ratio to receive once-weekly, subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks, including a 20-week dose-escalation period.
The study’s two primary endpoints were the average percentage change in body weight from entry to 72 weeks, and the percentage of participants reaching at least a 5% reduction in their baseline body weight by 72 weeks.
The most common adverse events with tirzepatide were gastrointestinal, and most were mild to moderate in severity, occurring primarily during dose escalation. Adverse events caused treatment discontinuation in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg tirzepatide doses and placebo, respectively
The trial ran from December 2019 to April 2022, so during the peak of the COVID-19 pandemic, which Dr. Jastreboff described as an “amazing feat.”
Jamy Ard, MD, who chaired the SURMOUNT-1 session quipped, after hearing the results, “Wow; that’s exciting. If you’re not excited by the results, you’d better check your pulse.”
Dr. Ard is a professor at Wake Forest University, Winston-Salem, N.C., and codirector of the Wake Forest Baptist Health Weight Management Center in Winston-Salem.
SURMOUNT-1 was sponsored by Eli Lilly, the company that markets tirzepatide (Mounjaro). Dr. Jastreboff has been an advisor or consultant to Eli Lilly, as well as to Boehringer Ingelheim, Intellihealth, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, Scholar Rock, and Weight Watchers, and she has received research funding from Eli Lilly and Novo Nordisk. Dr. Aronne has been a consultant or advisor to, speaker on behalf of, or received research funding from Eli Lilly as well as from Altimmune, Amgen, Allurion, Intellihealth, Janssen, Novo Nordisk, Pfizer, and United Health group; he has an ownership interest in ERX, Gelesis, and Intellihealth; and he serves on the board of ERX, Jamieson Wellness, and Intellihealth. Dr. Kaplan has been a consultant to Eli Lilly, as well as to Amgen, Boehringer Ingelheim, Gelesis, Gilead, Novo Nordisk, Optum Health, Pfizer, Rhythm Pharmaceuticals, the Obesity and Nutrition Institute, and Xeno Biosciences. Dr. Ard has been a consultant to Eli Lilly, as well as to Nestle Health Sciences and Novo Nordisk, and he has received research funding from Boehringer Ingelheim, Epitomee, Medical, and United Health Group.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Treatment of people with obesity but no diabetes with the dual–incretin agonist tirzepatide safely produced “unprecedented” levels of weight loss in the vast majority of patients in SURMOUNT-1, a placebo-controlled trial with more than 2,500 people with obesity or overweight plus at least one weight-related complication.
Although the pivotal trial did not directly compare weekly subcutaneous injection with the twincretin tirzepatide (at 5 mg, 10 mg, or 15 mg) with either bariatric surgery or what has been the reigning champ of weight-loss agents, a 2.4-mg/week injection of semaglutide (Wegovy), the new findings are impressive because they eclipsed semaglutide’s past performance in at least three important ways, said Ania M. Jastreboff, MD, PhD, SURMOUNT-1’s lead investigator, at the annual scientific sessions of the American Diabetes Association.
First, the highest-tested dosage of tirzepatide, 15 mg/week, for 72 weeks, produced a 5% or greater loss in baseline weight in 91%-96% of patients, an effect “not previously seen” in any prior phase 3 trial of a weight-loss agent, noted Dr. Jastreboff, an endocrinologist and director of Weight Management & Obesity Prevention at Yale University in New Haven, Conn.
Second, the average level of weight loss among the 630 people who received 15 mg/week was 22.5% in the on-treatment analysis, and 20.9% in the intention-to-treat analysis, again a magnitude of effect never before seen with any other medical intervention.
And in an exploratory analysis, 40% of people who received the highest-tested tirzepatide dose of 15 mg/week had at least a 25% loss in baseline weight in the on-treatment analysis, another example of unprecedented weight-loss achievement, said Dr. Jastreboff.
Looking at the data another way, the average baseline weight of those in the trial was 104 kg (230 lb) at the start, and the average weight loss was between 35 and 52 lbs by 72 weeks on treatment, Dr. Jastreboff said in a press conference.
She noted, however, that not everyone will respond to tirzepatide, “but if you do respond to this medicine, you will feel full earlier, you won’t want to go back for seconds, and you may eat smaller amounts more often.”
Such weight-loss agents will need to be taken chronically, in the same way that medications are for hypertension or dyslipidemia, Dr. Jastreboff stressed. “If you stop the antiobesity medication then the body fat mass set point will go back up so this necessitates long-term treatment.”
A new era: Weight loss ‘in the range of bariatric surgery’
Tirzepatide, developed by Lilly, has recently been approved in the United States for the treatment of type 2 diabetes, under the brand name Mounjaro.
SURMOUNT-1 was designed to examine the effect of the agent in overweight/obesity, and the company will be filing for the additional indication of weight loss in the future. Top-line results of SURMOUNT-1 generated much excitement when Lilly reported them back in April, including a story in The New York Times.
Semaglutide, a Novo Nordisk drug, is approved in the United States for type 2 diabetes (as Ozempic at doses of either 1 mg or 2 mg per week) and also for weight loss, as Wegovy, at the higher dose of 2.4 mg per week. When Wegovy was given the green light by the Food and Drug Administration a year ago, it too was hailed as a “game changer” for obesity.
The weight-loss results seen in SURMOUNT-1 “put tirzepatide squarely in the range of weight loss achieved with bariatric surgery,” concluded Louis J. Aronne, MD, a coinvestigator on the trial, professor at Weill-Cornell Medicine in New York, and director of the Center for Weight Management and Metabolic Clinical Research of Weill-Cornell.
The results are “amazing,” and propel the weight-loss field into “a new era of obesity treatment,” commented Lee M. Kaplan, MD, who was not involved in the study and served as designated discussant for the trial.
Despite the lack of direct comparison, the findings indicate that “tirzepatide causes more weight loss than semaglutide,” and it provides “an opportunity to meet or exceed” the weight-loss effects of bariatric surgery, added Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
Simultaneously with Dr. Jastreboff’s report at the meeting, the results were published online in The New England Journal of Medicine.
An accompanying editorial agrees with Dr. Kaplan: “It is remarkable that the magnitude of weight loss with tirzepatide was similar to that with gastric bypass, which raises the potential for alternative medical approaches to the treatment of obesity.”
“The tides are shifting, and there are now more options for people with obesity to lose weight,” write Clifford J. Rosen, MD, of Tufts University, Boston, and Julie R. Ingelfinger, MD, of Harvard University and Massachusetts General Hospital, Boston.
Dual incretin agonism ‘enhances activity,’ says expert
Tirzepatide is the first agent on the U.S. market from a novel class of dual-incretin agonists, with a molecular structure engineered to activate both the glucagonlike protein-1 (GLP-1) receptor and the glucose-dependent insulinotropic polypeptide (GIP), the two predominant incretins in the human gut. This combined activity has led to the twincretin nickname for tirzepatide.
Semaglutide is a single-incretin agonist, with its activity focused exclusively on the GLP-1 receptor.
Dr. Aronne tied the apparently superior efficacy of tirzepatide relative to semaglutide directly to the added incretin activity of tirzepatide. “The dual approach enhances efficacy,” he proposed during his presentation at the meeting.
The impressive efficacy and reassuring safety profile reported from SURMOUNT-1 opens the door to a new approach to treating obesity, which in the past has often taken a back seat to treatments for dyslipidemia, hypertension, and diabetes.
“Now that we can treat obesity safely and effectively, it makes sense to treat obesity first,” Dr. Aronne recommended.
Dr. Jastreboff agreed: “Perhaps we can prevent diabetes by treating obesity head-on,” she remarked.
Weight-loss agents gain U.S. traction
There have been concerns about patient access to these newer weight-loss drugs in the United States, given that the retail cost of semaglutide for obesity exceeds $1,000/month, but Dr. Aronne reported data that painted a more optimistic picture.
His numbers showed that during the first months that semaglutide was on the U.S. market as a weight-loss agent, the number of U.S. prescriptions written for branded antiobesity medications roughly doubled, a spike that seemed mostly driven by the introduction and growing use of semaglutide.
With tirzepatide, every prespecified cardiometabolic parameter assessed in the trial showed clinically meaningful improvements, reported Dr. Jastreboff, including an average 17% reduction in waist circumference in patients on either of the highest two dosages, a 34% average drop in total fat mass, an average 0.5–percentage point cut in baseline hemoglobin A1c at the highest two dosages, substantial cuts in fasting plasma glucose and fasting insulin levels, an average 28% drop in triglyceride levels, and an average systolic blood pressure reduction of about 8 mm Hg that occurred within 24 weeks on treatment.
“I think that insurers will sign up” for tirzepatide coverage based on benefits like this, Dr. Aronne predicted.
SURMOUNT-1 randomized 2,539 patients with obesity or with overweight plus at least one weight-related complication at any of 119 sites in nine countries. They had a body mass index of 30 kg/m2 or more, or 27 kg/m2 or more and at least one weight-related complication, excluding diabetes. They were randomized in a 1:1:1:1 ratio to receive once-weekly, subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks, including a 20-week dose-escalation period.
The study’s two primary endpoints were the average percentage change in body weight from entry to 72 weeks, and the percentage of participants reaching at least a 5% reduction in their baseline body weight by 72 weeks.
The most common adverse events with tirzepatide were gastrointestinal, and most were mild to moderate in severity, occurring primarily during dose escalation. Adverse events caused treatment discontinuation in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg tirzepatide doses and placebo, respectively
The trial ran from December 2019 to April 2022, so during the peak of the COVID-19 pandemic, which Dr. Jastreboff described as an “amazing feat.”
Jamy Ard, MD, who chaired the SURMOUNT-1 session quipped, after hearing the results, “Wow; that’s exciting. If you’re not excited by the results, you’d better check your pulse.”
Dr. Ard is a professor at Wake Forest University, Winston-Salem, N.C., and codirector of the Wake Forest Baptist Health Weight Management Center in Winston-Salem.
SURMOUNT-1 was sponsored by Eli Lilly, the company that markets tirzepatide (Mounjaro). Dr. Jastreboff has been an advisor or consultant to Eli Lilly, as well as to Boehringer Ingelheim, Intellihealth, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, Scholar Rock, and Weight Watchers, and she has received research funding from Eli Lilly and Novo Nordisk. Dr. Aronne has been a consultant or advisor to, speaker on behalf of, or received research funding from Eli Lilly as well as from Altimmune, Amgen, Allurion, Intellihealth, Janssen, Novo Nordisk, Pfizer, and United Health group; he has an ownership interest in ERX, Gelesis, and Intellihealth; and he serves on the board of ERX, Jamieson Wellness, and Intellihealth. Dr. Kaplan has been a consultant to Eli Lilly, as well as to Amgen, Boehringer Ingelheim, Gelesis, Gilead, Novo Nordisk, Optum Health, Pfizer, Rhythm Pharmaceuticals, the Obesity and Nutrition Institute, and Xeno Biosciences. Dr. Ard has been a consultant to Eli Lilly, as well as to Nestle Health Sciences and Novo Nordisk, and he has received research funding from Boehringer Ingelheim, Epitomee, Medical, and United Health Group.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Treatment of people with obesity but no diabetes with the dual–incretin agonist tirzepatide safely produced “unprecedented” levels of weight loss in the vast majority of patients in SURMOUNT-1, a placebo-controlled trial with more than 2,500 people with obesity or overweight plus at least one weight-related complication.
Although the pivotal trial did not directly compare weekly subcutaneous injection with the twincretin tirzepatide (at 5 mg, 10 mg, or 15 mg) with either bariatric surgery or what has been the reigning champ of weight-loss agents, a 2.4-mg/week injection of semaglutide (Wegovy), the new findings are impressive because they eclipsed semaglutide’s past performance in at least three important ways, said Ania M. Jastreboff, MD, PhD, SURMOUNT-1’s lead investigator, at the annual scientific sessions of the American Diabetes Association.
First, the highest-tested dosage of tirzepatide, 15 mg/week, for 72 weeks, produced a 5% or greater loss in baseline weight in 91%-96% of patients, an effect “not previously seen” in any prior phase 3 trial of a weight-loss agent, noted Dr. Jastreboff, an endocrinologist and director of Weight Management & Obesity Prevention at Yale University in New Haven, Conn.
Second, the average level of weight loss among the 630 people who received 15 mg/week was 22.5% in the on-treatment analysis, and 20.9% in the intention-to-treat analysis, again a magnitude of effect never before seen with any other medical intervention.
And in an exploratory analysis, 40% of people who received the highest-tested tirzepatide dose of 15 mg/week had at least a 25% loss in baseline weight in the on-treatment analysis, another example of unprecedented weight-loss achievement, said Dr. Jastreboff.
Looking at the data another way, the average baseline weight of those in the trial was 104 kg (230 lb) at the start, and the average weight loss was between 35 and 52 lbs by 72 weeks on treatment, Dr. Jastreboff said in a press conference.
She noted, however, that not everyone will respond to tirzepatide, “but if you do respond to this medicine, you will feel full earlier, you won’t want to go back for seconds, and you may eat smaller amounts more often.”
Such weight-loss agents will need to be taken chronically, in the same way that medications are for hypertension or dyslipidemia, Dr. Jastreboff stressed. “If you stop the antiobesity medication then the body fat mass set point will go back up so this necessitates long-term treatment.”
A new era: Weight loss ‘in the range of bariatric surgery’
Tirzepatide, developed by Lilly, has recently been approved in the United States for the treatment of type 2 diabetes, under the brand name Mounjaro.
SURMOUNT-1 was designed to examine the effect of the agent in overweight/obesity, and the company will be filing for the additional indication of weight loss in the future. Top-line results of SURMOUNT-1 generated much excitement when Lilly reported them back in April, including a story in The New York Times.
Semaglutide, a Novo Nordisk drug, is approved in the United States for type 2 diabetes (as Ozempic at doses of either 1 mg or 2 mg per week) and also for weight loss, as Wegovy, at the higher dose of 2.4 mg per week. When Wegovy was given the green light by the Food and Drug Administration a year ago, it too was hailed as a “game changer” for obesity.
The weight-loss results seen in SURMOUNT-1 “put tirzepatide squarely in the range of weight loss achieved with bariatric surgery,” concluded Louis J. Aronne, MD, a coinvestigator on the trial, professor at Weill-Cornell Medicine in New York, and director of the Center for Weight Management and Metabolic Clinical Research of Weill-Cornell.
The results are “amazing,” and propel the weight-loss field into “a new era of obesity treatment,” commented Lee M. Kaplan, MD, who was not involved in the study and served as designated discussant for the trial.
Despite the lack of direct comparison, the findings indicate that “tirzepatide causes more weight loss than semaglutide,” and it provides “an opportunity to meet or exceed” the weight-loss effects of bariatric surgery, added Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
Simultaneously with Dr. Jastreboff’s report at the meeting, the results were published online in The New England Journal of Medicine.
An accompanying editorial agrees with Dr. Kaplan: “It is remarkable that the magnitude of weight loss with tirzepatide was similar to that with gastric bypass, which raises the potential for alternative medical approaches to the treatment of obesity.”
“The tides are shifting, and there are now more options for people with obesity to lose weight,” write Clifford J. Rosen, MD, of Tufts University, Boston, and Julie R. Ingelfinger, MD, of Harvard University and Massachusetts General Hospital, Boston.
Dual incretin agonism ‘enhances activity,’ says expert
Tirzepatide is the first agent on the U.S. market from a novel class of dual-incretin agonists, with a molecular structure engineered to activate both the glucagonlike protein-1 (GLP-1) receptor and the glucose-dependent insulinotropic polypeptide (GIP), the two predominant incretins in the human gut. This combined activity has led to the twincretin nickname for tirzepatide.
Semaglutide is a single-incretin agonist, with its activity focused exclusively on the GLP-1 receptor.
Dr. Aronne tied the apparently superior efficacy of tirzepatide relative to semaglutide directly to the added incretin activity of tirzepatide. “The dual approach enhances efficacy,” he proposed during his presentation at the meeting.
The impressive efficacy and reassuring safety profile reported from SURMOUNT-1 opens the door to a new approach to treating obesity, which in the past has often taken a back seat to treatments for dyslipidemia, hypertension, and diabetes.
“Now that we can treat obesity safely and effectively, it makes sense to treat obesity first,” Dr. Aronne recommended.
Dr. Jastreboff agreed: “Perhaps we can prevent diabetes by treating obesity head-on,” she remarked.
Weight-loss agents gain U.S. traction
There have been concerns about patient access to these newer weight-loss drugs in the United States, given that the retail cost of semaglutide for obesity exceeds $1,000/month, but Dr. Aronne reported data that painted a more optimistic picture.
His numbers showed that during the first months that semaglutide was on the U.S. market as a weight-loss agent, the number of U.S. prescriptions written for branded antiobesity medications roughly doubled, a spike that seemed mostly driven by the introduction and growing use of semaglutide.
With tirzepatide, every prespecified cardiometabolic parameter assessed in the trial showed clinically meaningful improvements, reported Dr. Jastreboff, including an average 17% reduction in waist circumference in patients on either of the highest two dosages, a 34% average drop in total fat mass, an average 0.5–percentage point cut in baseline hemoglobin A1c at the highest two dosages, substantial cuts in fasting plasma glucose and fasting insulin levels, an average 28% drop in triglyceride levels, and an average systolic blood pressure reduction of about 8 mm Hg that occurred within 24 weeks on treatment.
“I think that insurers will sign up” for tirzepatide coverage based on benefits like this, Dr. Aronne predicted.
SURMOUNT-1 randomized 2,539 patients with obesity or with overweight plus at least one weight-related complication at any of 119 sites in nine countries. They had a body mass index of 30 kg/m2 or more, or 27 kg/m2 or more and at least one weight-related complication, excluding diabetes. They were randomized in a 1:1:1:1 ratio to receive once-weekly, subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks, including a 20-week dose-escalation period.
The study’s two primary endpoints were the average percentage change in body weight from entry to 72 weeks, and the percentage of participants reaching at least a 5% reduction in their baseline body weight by 72 weeks.
The most common adverse events with tirzepatide were gastrointestinal, and most were mild to moderate in severity, occurring primarily during dose escalation. Adverse events caused treatment discontinuation in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg tirzepatide doses and placebo, respectively
The trial ran from December 2019 to April 2022, so during the peak of the COVID-19 pandemic, which Dr. Jastreboff described as an “amazing feat.”
Jamy Ard, MD, who chaired the SURMOUNT-1 session quipped, after hearing the results, “Wow; that’s exciting. If you’re not excited by the results, you’d better check your pulse.”
Dr. Ard is a professor at Wake Forest University, Winston-Salem, N.C., and codirector of the Wake Forest Baptist Health Weight Management Center in Winston-Salem.
SURMOUNT-1 was sponsored by Eli Lilly, the company that markets tirzepatide (Mounjaro). Dr. Jastreboff has been an advisor or consultant to Eli Lilly, as well as to Boehringer Ingelheim, Intellihealth, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, Scholar Rock, and Weight Watchers, and she has received research funding from Eli Lilly and Novo Nordisk. Dr. Aronne has been a consultant or advisor to, speaker on behalf of, or received research funding from Eli Lilly as well as from Altimmune, Amgen, Allurion, Intellihealth, Janssen, Novo Nordisk, Pfizer, and United Health group; he has an ownership interest in ERX, Gelesis, and Intellihealth; and he serves on the board of ERX, Jamieson Wellness, and Intellihealth. Dr. Kaplan has been a consultant to Eli Lilly, as well as to Amgen, Boehringer Ingelheim, Gelesis, Gilead, Novo Nordisk, Optum Health, Pfizer, Rhythm Pharmaceuticals, the Obesity and Nutrition Institute, and Xeno Biosciences. Dr. Ard has been a consultant to Eli Lilly, as well as to Nestle Health Sciences and Novo Nordisk, and he has received research funding from Boehringer Ingelheim, Epitomee, Medical, and United Health Group.
A version of this article first appeared on Medscape.com.
AT ADA 2022