User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
COVID-19 in children: Latest weekly increase is largest yet
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
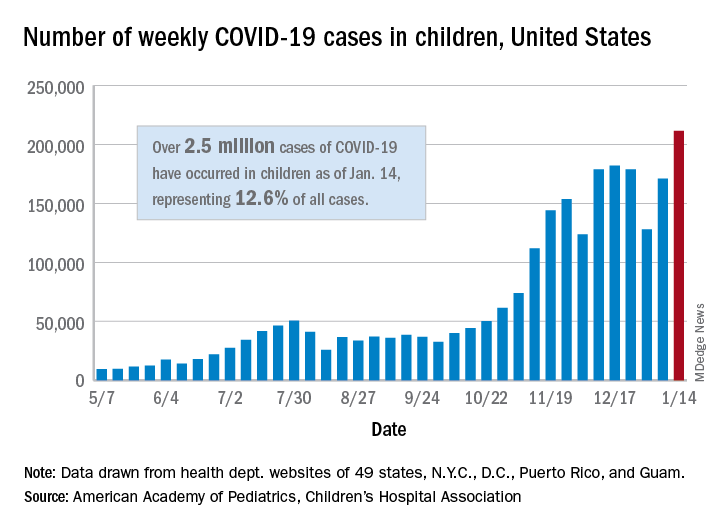
There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
Age at menarche signals potential cardiovascular health risk
“Increases in age at menarche are significantly associated with increases in cardiovascular health among women,” reported Yi Zheng, MPH, and colleagues at the University of Florida, Gainesville.
Mr. Zheng and colleagues conducted a cross-sectional analysis of 20,447 women aged 18 or older using data from a nationally representative sample of the 1999-2016 National Health and Nutrition Examinations Survey (NHANES). In all, 2,292 (11.2%) were determined to have ideal cardiovascular health (CVH).
Early menarche was confirmed to be related to increases in body mass index and greater incidence of type 2 diabetes, consistent with earlier studies, the authors confirmed. Those with nonideal CVH were more likely to have reported early menarche; those with ideal CVH were not only younger, but they also had college or graduate level education or above and higher poverty income ratio. Those with ideal CVH were also less likely to be to be of non-Hispanic Black heritage or to have been previously married.
BMI may be the missing link between early menarche and CVH
Unlike previous studies, the researchers found no significant link between early menarche and blood pressure, total cholesterol, smoking, physical activity, or diet using fully adjusted model data, leading them to conclude that “the associations between early menarche and CVH might be mainly driven by its associations with BMI.”
Mr. Zheng and colleagues suggested that future studies should evaluate the causal relationships between age at menarche and BMI and whether genetic factors and childhood lifestyle predispose women to early menarche and obesity.
“Our findings further highlighted that age at menarche may be used to identify high-risk population[s] and to guide targeted preventions to maintain and improve CVH,” the authors noted. Although they cited several strengths and limitations of the study, they emphasized that the wide use of Life’s Simple 7 factors (blood pressure, total cholesterol, glucose levels, smoking, BMI, physical activity, and diet) to measure CVH should “only be regarded as a surrogate construct, and future efforts are needed to better characterize CVH,” they cautioned.
The findings offer an opportunity to more closely track CVH in racial and ethnic groups
In a separate editorial, Ewa M. Gross-Sawicka, MD, PhD, and Eiran Z. Gorodeski, MD, MPH, both of the Harrington Heart and Vascular Institute, Cleveland, observed: “That the authors found African American women had the lowest overall CVH scores, even after adjusting for differences, highlights the importance of beginning cardiovascular health education earlier, especially for those in certain racial and ethnic groups.”
Dr. Gross-Sawicka and Dr. Gorodeski also raised several key questions that warrant further research: “1) Why do women who experience late menarche have improved cardiovascular health while those who experience early menarche have reduced cardiovascular health? 2) Why do the ‘beneficial’ effects of late menarche on CVH last 10 years longer than the ‘detrimental’ effects of early menarche? 3) Since both early and late menarche are associated with increased risk of cardiovascular disease, are women who experience menarche at an older age more cognizant of the cardiovascular risks compared with younger women and adjust their CVH accordingly?”
A key point also worth further consideration: “It is unclear whether age at menarche is directly associated with CVH, or if this relationship is mediated by the association of age at menarche and BMI and/or hyperglycemia,” said Dr. Gross-Sawicka and Dr. Gorodeski.
In an interview, Jan Shifren, MD, director, Midlife Women’s Health Center, Massachusetts General Hospital, Boston, noted, “The principal finding is that early menarche is associated with worse cardiovascular health, which may reflect the adverse impact of obesity and glucose intolerance on CVH, as obesity also is a risk factor for early menarche. The association between early menarche and worse CVH was significant only in women aged 25-34 years, but not in older women, possibly as other risk factors become more important as women age. One of the most concerning findings in this study ... is that only 11% had ideal CVH based on a combination of behavioral and health factors. As cardiovascular disease is the leading cause of death for women, we must do a better job of optimizing [their] cardiovascular health. Clinicians need to focus on optimizing cardiovascular health for all of their midlife patients, whether or not they experienced early menarche!”
Mr. Zheng and colleagues, as well as Dr. Shifren and Dr. Grodeski, had no conflicts of interest to report. Dr. Gross-Sawicka has received funding from Abbott and Novartis.
“Increases in age at menarche are significantly associated with increases in cardiovascular health among women,” reported Yi Zheng, MPH, and colleagues at the University of Florida, Gainesville.
Mr. Zheng and colleagues conducted a cross-sectional analysis of 20,447 women aged 18 or older using data from a nationally representative sample of the 1999-2016 National Health and Nutrition Examinations Survey (NHANES). In all, 2,292 (11.2%) were determined to have ideal cardiovascular health (CVH).
Early menarche was confirmed to be related to increases in body mass index and greater incidence of type 2 diabetes, consistent with earlier studies, the authors confirmed. Those with nonideal CVH were more likely to have reported early menarche; those with ideal CVH were not only younger, but they also had college or graduate level education or above and higher poverty income ratio. Those with ideal CVH were also less likely to be to be of non-Hispanic Black heritage or to have been previously married.
BMI may be the missing link between early menarche and CVH
Unlike previous studies, the researchers found no significant link between early menarche and blood pressure, total cholesterol, smoking, physical activity, or diet using fully adjusted model data, leading them to conclude that “the associations between early menarche and CVH might be mainly driven by its associations with BMI.”
Mr. Zheng and colleagues suggested that future studies should evaluate the causal relationships between age at menarche and BMI and whether genetic factors and childhood lifestyle predispose women to early menarche and obesity.
“Our findings further highlighted that age at menarche may be used to identify high-risk population[s] and to guide targeted preventions to maintain and improve CVH,” the authors noted. Although they cited several strengths and limitations of the study, they emphasized that the wide use of Life’s Simple 7 factors (blood pressure, total cholesterol, glucose levels, smoking, BMI, physical activity, and diet) to measure CVH should “only be regarded as a surrogate construct, and future efforts are needed to better characterize CVH,” they cautioned.
The findings offer an opportunity to more closely track CVH in racial and ethnic groups
In a separate editorial, Ewa M. Gross-Sawicka, MD, PhD, and Eiran Z. Gorodeski, MD, MPH, both of the Harrington Heart and Vascular Institute, Cleveland, observed: “That the authors found African American women had the lowest overall CVH scores, even after adjusting for differences, highlights the importance of beginning cardiovascular health education earlier, especially for those in certain racial and ethnic groups.”
Dr. Gross-Sawicka and Dr. Gorodeski also raised several key questions that warrant further research: “1) Why do women who experience late menarche have improved cardiovascular health while those who experience early menarche have reduced cardiovascular health? 2) Why do the ‘beneficial’ effects of late menarche on CVH last 10 years longer than the ‘detrimental’ effects of early menarche? 3) Since both early and late menarche are associated with increased risk of cardiovascular disease, are women who experience menarche at an older age more cognizant of the cardiovascular risks compared with younger women and adjust their CVH accordingly?”
A key point also worth further consideration: “It is unclear whether age at menarche is directly associated with CVH, or if this relationship is mediated by the association of age at menarche and BMI and/or hyperglycemia,” said Dr. Gross-Sawicka and Dr. Gorodeski.
In an interview, Jan Shifren, MD, director, Midlife Women’s Health Center, Massachusetts General Hospital, Boston, noted, “The principal finding is that early menarche is associated with worse cardiovascular health, which may reflect the adverse impact of obesity and glucose intolerance on CVH, as obesity also is a risk factor for early menarche. The association between early menarche and worse CVH was significant only in women aged 25-34 years, but not in older women, possibly as other risk factors become more important as women age. One of the most concerning findings in this study ... is that only 11% had ideal CVH based on a combination of behavioral and health factors. As cardiovascular disease is the leading cause of death for women, we must do a better job of optimizing [their] cardiovascular health. Clinicians need to focus on optimizing cardiovascular health for all of their midlife patients, whether or not they experienced early menarche!”
Mr. Zheng and colleagues, as well as Dr. Shifren and Dr. Grodeski, had no conflicts of interest to report. Dr. Gross-Sawicka has received funding from Abbott and Novartis.
“Increases in age at menarche are significantly associated with increases in cardiovascular health among women,” reported Yi Zheng, MPH, and colleagues at the University of Florida, Gainesville.
Mr. Zheng and colleagues conducted a cross-sectional analysis of 20,447 women aged 18 or older using data from a nationally representative sample of the 1999-2016 National Health and Nutrition Examinations Survey (NHANES). In all, 2,292 (11.2%) were determined to have ideal cardiovascular health (CVH).
Early menarche was confirmed to be related to increases in body mass index and greater incidence of type 2 diabetes, consistent with earlier studies, the authors confirmed. Those with nonideal CVH were more likely to have reported early menarche; those with ideal CVH were not only younger, but they also had college or graduate level education or above and higher poverty income ratio. Those with ideal CVH were also less likely to be to be of non-Hispanic Black heritage or to have been previously married.
BMI may be the missing link between early menarche and CVH
Unlike previous studies, the researchers found no significant link between early menarche and blood pressure, total cholesterol, smoking, physical activity, or diet using fully adjusted model data, leading them to conclude that “the associations between early menarche and CVH might be mainly driven by its associations with BMI.”
Mr. Zheng and colleagues suggested that future studies should evaluate the causal relationships between age at menarche and BMI and whether genetic factors and childhood lifestyle predispose women to early menarche and obesity.
“Our findings further highlighted that age at menarche may be used to identify high-risk population[s] and to guide targeted preventions to maintain and improve CVH,” the authors noted. Although they cited several strengths and limitations of the study, they emphasized that the wide use of Life’s Simple 7 factors (blood pressure, total cholesterol, glucose levels, smoking, BMI, physical activity, and diet) to measure CVH should “only be regarded as a surrogate construct, and future efforts are needed to better characterize CVH,” they cautioned.
The findings offer an opportunity to more closely track CVH in racial and ethnic groups
In a separate editorial, Ewa M. Gross-Sawicka, MD, PhD, and Eiran Z. Gorodeski, MD, MPH, both of the Harrington Heart and Vascular Institute, Cleveland, observed: “That the authors found African American women had the lowest overall CVH scores, even after adjusting for differences, highlights the importance of beginning cardiovascular health education earlier, especially for those in certain racial and ethnic groups.”
Dr. Gross-Sawicka and Dr. Gorodeski also raised several key questions that warrant further research: “1) Why do women who experience late menarche have improved cardiovascular health while those who experience early menarche have reduced cardiovascular health? 2) Why do the ‘beneficial’ effects of late menarche on CVH last 10 years longer than the ‘detrimental’ effects of early menarche? 3) Since both early and late menarche are associated with increased risk of cardiovascular disease, are women who experience menarche at an older age more cognizant of the cardiovascular risks compared with younger women and adjust their CVH accordingly?”
A key point also worth further consideration: “It is unclear whether age at menarche is directly associated with CVH, or if this relationship is mediated by the association of age at menarche and BMI and/or hyperglycemia,” said Dr. Gross-Sawicka and Dr. Gorodeski.
In an interview, Jan Shifren, MD, director, Midlife Women’s Health Center, Massachusetts General Hospital, Boston, noted, “The principal finding is that early menarche is associated with worse cardiovascular health, which may reflect the adverse impact of obesity and glucose intolerance on CVH, as obesity also is a risk factor for early menarche. The association between early menarche and worse CVH was significant only in women aged 25-34 years, but not in older women, possibly as other risk factors become more important as women age. One of the most concerning findings in this study ... is that only 11% had ideal CVH based on a combination of behavioral and health factors. As cardiovascular disease is the leading cause of death for women, we must do a better job of optimizing [their] cardiovascular health. Clinicians need to focus on optimizing cardiovascular health for all of their midlife patients, whether or not they experienced early menarche!”
Mr. Zheng and colleagues, as well as Dr. Shifren and Dr. Grodeski, had no conflicts of interest to report. Dr. Gross-Sawicka has received funding from Abbott and Novartis.
FROM THE JOURNAL OF THE NORTH AMERICAN MENOPAUSE SOCIETY
Childhood growth hormones raise risk for adult cardiovascular events
Childhood treatment with recombinant human growth hormone was associated with a significantly increased risk of cardiovascular events, based on data from more than 3,000 individuals.
“Both excess levels of growth hormone and [growth hormone deficiency] have been associated with increased cardiovascular morbidity and mortality,” but data on long-term cardiovascular morbidity in individuals treated with growth hormone in childhood are lacking, wrote Anders Tinblad, MD, of Karolinska Institutet, Stockholm, and colleagues.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 3,408 Swedish patients treated as children with recombinant human growth hormone (rhGH) between Jan. 1, 1985, and Dec. 31, 2010, and compared each with 15 matched controls (a total of 50,036 controls). The patients were treated for one of three conditions: isolated growth hormone deficiency (GHD), small for gestational age (SGA), and idiopathic short stature (ISS).
Data on cardiovascular outcomes were collected from health care and population-based registers and analyzed between Jan. 1, 1985, and Dec. 31, 2014. The average age of the participants at the study’s end was 25.1 years.
In all, 1,809 cardiovascular disease events were recorded over a median follow-up period of 14.9 years, for an incidence rate of 25.6 events per 10,000 person-years in patients and 22.6 events per 10,000 person-years in controls.
When separated by sex, the incidence was higher in female patients compared with controls (31.2 vs. 23.4 events per 10,000 person-years, respectively, but similar in male patients vs. controls (23.3 vs. 22.3 events per 10,000 person-years). “Differences in estrogen levels or responsiveness to rhGH treatment have previously been hypothesized as possible explanations, but the underlying mechanism for this sex difference still remains unclear and merits further investigation,” the researchers wrote.
Overall, the highest adjusted hazard ratios occurred in subgroups of patients with the longest treatment duration (HR 2.08) and highest cumulative dose of growth hormone (HR 2.05), but no association was noted between highest daily hormone dose and cardiovascular event risk. Hazard ratios were higher across all three treatment subgroups of SGA, GHD, and ISS compared with controls (HR 1.97, 1.66, and 1.55, respectively).
“The association between childhood rhGH treatment and CVD events was also seen when assessing only severe CVD outcomes, but with even lower absolute risks,” the researchers noted.
The study findings were limited by several factors including the potential for confounding by treatment indication and the lack of long-term follow-up data given the relatively young age of the study population, the researchers said. The results were strengthened by the large sample size and showed that the absolute risk for overall and severe cardiovascular disease in children treated with growth hormones was low, “which could be reassuring to individual patients,” they added. However, “At the group level, and perhaps especially for female patients and those treated for SGA indication, further close monitoring and future studies of CVD safety are warranted,” they concluded.
Safety and ethical concerns persist
Although the study authors cite limited conclusions on causality and low absolute risk, several issues persist that prompt ongoing analysis of pediatric growth hormone use, namely “worrisome indirect evidence, challenges and limitations in the direct evidence, and the changing world of growth hormone treatment,” Adda Grimberg, MD, of the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
“Although evidence asserts that neither growth hormone nor insulinlike growth factor I is carcinogenic, the basic science and oncology literatures are rife with reports showing that they can make aberrant cells more aggressive,” and such indirect evidence supports the need for more direct evidence of possible harm from growth hormone treatment, Dr. Grimberg wrote. Most current safety data on growth hormone come from postmarketing surveillance studies, but these studies do not include controls or data on outcomes after discontinuation of treatment, she noted.
The current study, while able to follow patients across the lifespan, cannot indicate “whether the small but increased risk of cardiovascular disease found in this study was caused by the pediatric growth hormone treatment that identified the participants, by the conditions being treated, by other potential confounder(s) not captured by the study’s methods, or by a combination of the above,” said Dr. Grimberg.
In addition, “the move from replacement of GHD to pharmacologic height augmentation in children who already make sufficient growth hormone had the potential to change the safety profile of treatment,” she said.
“Parents of patients in pediatric primary care practices and of patients seeking growth-related care in a subspecialty endocrine clinic rated treatment characteristics (i.e., proven efficacy and safety) as the factor most having a big or extreme effect on their growth-related medical decision-making,” Dr. Grimberg said. “The centrality of treatment safety to patient-family decision-making underscores the importance of continued scrutiny of growth hormone safety as the treatment and its recipients continue to evolve,” she concluded.
The study was supported by the Swedish Research Council, the Stockholm City Council, the Karolinska Institute, the Society for Child Care, Sahlgrenska University Hospital, and the Stockholm County Council’s combined clinical residency and PhD training program. Lead author Dr. Tidblad disclosed funding from the Society for Child Care and Stockholm County Council during the conduct of the study and personal fees from Pfizer. Dr. Grimberg disclosed serving as a member of the steering committee for the Pfizer International Growth Study Database, and as a consultant for the Pediatric Endocrine Society GH Deficiency Knowledge Center, sponsored by Sandoz AG.
Childhood treatment with recombinant human growth hormone was associated with a significantly increased risk of cardiovascular events, based on data from more than 3,000 individuals.
“Both excess levels of growth hormone and [growth hormone deficiency] have been associated with increased cardiovascular morbidity and mortality,” but data on long-term cardiovascular morbidity in individuals treated with growth hormone in childhood are lacking, wrote Anders Tinblad, MD, of Karolinska Institutet, Stockholm, and colleagues.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 3,408 Swedish patients treated as children with recombinant human growth hormone (rhGH) between Jan. 1, 1985, and Dec. 31, 2010, and compared each with 15 matched controls (a total of 50,036 controls). The patients were treated for one of three conditions: isolated growth hormone deficiency (GHD), small for gestational age (SGA), and idiopathic short stature (ISS).
Data on cardiovascular outcomes were collected from health care and population-based registers and analyzed between Jan. 1, 1985, and Dec. 31, 2014. The average age of the participants at the study’s end was 25.1 years.
In all, 1,809 cardiovascular disease events were recorded over a median follow-up period of 14.9 years, for an incidence rate of 25.6 events per 10,000 person-years in patients and 22.6 events per 10,000 person-years in controls.
When separated by sex, the incidence was higher in female patients compared with controls (31.2 vs. 23.4 events per 10,000 person-years, respectively, but similar in male patients vs. controls (23.3 vs. 22.3 events per 10,000 person-years). “Differences in estrogen levels or responsiveness to rhGH treatment have previously been hypothesized as possible explanations, but the underlying mechanism for this sex difference still remains unclear and merits further investigation,” the researchers wrote.
Overall, the highest adjusted hazard ratios occurred in subgroups of patients with the longest treatment duration (HR 2.08) and highest cumulative dose of growth hormone (HR 2.05), but no association was noted between highest daily hormone dose and cardiovascular event risk. Hazard ratios were higher across all three treatment subgroups of SGA, GHD, and ISS compared with controls (HR 1.97, 1.66, and 1.55, respectively).
“The association between childhood rhGH treatment and CVD events was also seen when assessing only severe CVD outcomes, but with even lower absolute risks,” the researchers noted.
The study findings were limited by several factors including the potential for confounding by treatment indication and the lack of long-term follow-up data given the relatively young age of the study population, the researchers said. The results were strengthened by the large sample size and showed that the absolute risk for overall and severe cardiovascular disease in children treated with growth hormones was low, “which could be reassuring to individual patients,” they added. However, “At the group level, and perhaps especially for female patients and those treated for SGA indication, further close monitoring and future studies of CVD safety are warranted,” they concluded.
Safety and ethical concerns persist
Although the study authors cite limited conclusions on causality and low absolute risk, several issues persist that prompt ongoing analysis of pediatric growth hormone use, namely “worrisome indirect evidence, challenges and limitations in the direct evidence, and the changing world of growth hormone treatment,” Adda Grimberg, MD, of the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
“Although evidence asserts that neither growth hormone nor insulinlike growth factor I is carcinogenic, the basic science and oncology literatures are rife with reports showing that they can make aberrant cells more aggressive,” and such indirect evidence supports the need for more direct evidence of possible harm from growth hormone treatment, Dr. Grimberg wrote. Most current safety data on growth hormone come from postmarketing surveillance studies, but these studies do not include controls or data on outcomes after discontinuation of treatment, she noted.
The current study, while able to follow patients across the lifespan, cannot indicate “whether the small but increased risk of cardiovascular disease found in this study was caused by the pediatric growth hormone treatment that identified the participants, by the conditions being treated, by other potential confounder(s) not captured by the study’s methods, or by a combination of the above,” said Dr. Grimberg.
In addition, “the move from replacement of GHD to pharmacologic height augmentation in children who already make sufficient growth hormone had the potential to change the safety profile of treatment,” she said.
“Parents of patients in pediatric primary care practices and of patients seeking growth-related care in a subspecialty endocrine clinic rated treatment characteristics (i.e., proven efficacy and safety) as the factor most having a big or extreme effect on their growth-related medical decision-making,” Dr. Grimberg said. “The centrality of treatment safety to patient-family decision-making underscores the importance of continued scrutiny of growth hormone safety as the treatment and its recipients continue to evolve,” she concluded.
The study was supported by the Swedish Research Council, the Stockholm City Council, the Karolinska Institute, the Society for Child Care, Sahlgrenska University Hospital, and the Stockholm County Council’s combined clinical residency and PhD training program. Lead author Dr. Tidblad disclosed funding from the Society for Child Care and Stockholm County Council during the conduct of the study and personal fees from Pfizer. Dr. Grimberg disclosed serving as a member of the steering committee for the Pfizer International Growth Study Database, and as a consultant for the Pediatric Endocrine Society GH Deficiency Knowledge Center, sponsored by Sandoz AG.
Childhood treatment with recombinant human growth hormone was associated with a significantly increased risk of cardiovascular events, based on data from more than 3,000 individuals.
“Both excess levels of growth hormone and [growth hormone deficiency] have been associated with increased cardiovascular morbidity and mortality,” but data on long-term cardiovascular morbidity in individuals treated with growth hormone in childhood are lacking, wrote Anders Tinblad, MD, of Karolinska Institutet, Stockholm, and colleagues.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 3,408 Swedish patients treated as children with recombinant human growth hormone (rhGH) between Jan. 1, 1985, and Dec. 31, 2010, and compared each with 15 matched controls (a total of 50,036 controls). The patients were treated for one of three conditions: isolated growth hormone deficiency (GHD), small for gestational age (SGA), and idiopathic short stature (ISS).
Data on cardiovascular outcomes were collected from health care and population-based registers and analyzed between Jan. 1, 1985, and Dec. 31, 2014. The average age of the participants at the study’s end was 25.1 years.
In all, 1,809 cardiovascular disease events were recorded over a median follow-up period of 14.9 years, for an incidence rate of 25.6 events per 10,000 person-years in patients and 22.6 events per 10,000 person-years in controls.
When separated by sex, the incidence was higher in female patients compared with controls (31.2 vs. 23.4 events per 10,000 person-years, respectively, but similar in male patients vs. controls (23.3 vs. 22.3 events per 10,000 person-years). “Differences in estrogen levels or responsiveness to rhGH treatment have previously been hypothesized as possible explanations, but the underlying mechanism for this sex difference still remains unclear and merits further investigation,” the researchers wrote.
Overall, the highest adjusted hazard ratios occurred in subgroups of patients with the longest treatment duration (HR 2.08) and highest cumulative dose of growth hormone (HR 2.05), but no association was noted between highest daily hormone dose and cardiovascular event risk. Hazard ratios were higher across all three treatment subgroups of SGA, GHD, and ISS compared with controls (HR 1.97, 1.66, and 1.55, respectively).
“The association between childhood rhGH treatment and CVD events was also seen when assessing only severe CVD outcomes, but with even lower absolute risks,” the researchers noted.
The study findings were limited by several factors including the potential for confounding by treatment indication and the lack of long-term follow-up data given the relatively young age of the study population, the researchers said. The results were strengthened by the large sample size and showed that the absolute risk for overall and severe cardiovascular disease in children treated with growth hormones was low, “which could be reassuring to individual patients,” they added. However, “At the group level, and perhaps especially for female patients and those treated for SGA indication, further close monitoring and future studies of CVD safety are warranted,” they concluded.
Safety and ethical concerns persist
Although the study authors cite limited conclusions on causality and low absolute risk, several issues persist that prompt ongoing analysis of pediatric growth hormone use, namely “worrisome indirect evidence, challenges and limitations in the direct evidence, and the changing world of growth hormone treatment,” Adda Grimberg, MD, of the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
“Although evidence asserts that neither growth hormone nor insulinlike growth factor I is carcinogenic, the basic science and oncology literatures are rife with reports showing that they can make aberrant cells more aggressive,” and such indirect evidence supports the need for more direct evidence of possible harm from growth hormone treatment, Dr. Grimberg wrote. Most current safety data on growth hormone come from postmarketing surveillance studies, but these studies do not include controls or data on outcomes after discontinuation of treatment, she noted.
The current study, while able to follow patients across the lifespan, cannot indicate “whether the small but increased risk of cardiovascular disease found in this study was caused by the pediatric growth hormone treatment that identified the participants, by the conditions being treated, by other potential confounder(s) not captured by the study’s methods, or by a combination of the above,” said Dr. Grimberg.
In addition, “the move from replacement of GHD to pharmacologic height augmentation in children who already make sufficient growth hormone had the potential to change the safety profile of treatment,” she said.
“Parents of patients in pediatric primary care practices and of patients seeking growth-related care in a subspecialty endocrine clinic rated treatment characteristics (i.e., proven efficacy and safety) as the factor most having a big or extreme effect on their growth-related medical decision-making,” Dr. Grimberg said. “The centrality of treatment safety to patient-family decision-making underscores the importance of continued scrutiny of growth hormone safety as the treatment and its recipients continue to evolve,” she concluded.
The study was supported by the Swedish Research Council, the Stockholm City Council, the Karolinska Institute, the Society for Child Care, Sahlgrenska University Hospital, and the Stockholm County Council’s combined clinical residency and PhD training program. Lead author Dr. Tidblad disclosed funding from the Society for Child Care and Stockholm County Council during the conduct of the study and personal fees from Pfizer. Dr. Grimberg disclosed serving as a member of the steering committee for the Pfizer International Growth Study Database, and as a consultant for the Pediatric Endocrine Society GH Deficiency Knowledge Center, sponsored by Sandoz AG.
FROM JAMA PEDIATRICS
Biomarker HF risk score envisioned as SGLT2 inhibitor lodestar in diabetes
A scoring system that predicts risk for new heart failure over 5 years that is based solely on a few familiar, readily available biomarkers could potentially help steer patients with diabetes or even prediabetes toward HF-preventive therapies, researchers proposed based on a new study.
They foresee the risk-stratification tool, based on data pooled from three major community-based cohort studies but not independently validated, as a way to select patients with diabetes and prediabetes for treatment with SGLT2 inhibitors.
Several members of that drug class, conceived as antidiabetic agents, have been shown to help in prevention or treatment of HF in patients with diabetes and those without diabetes but at increased cardiovascular (CV) risk. Yet their uptake in practice has been lagging, the group noted.
Most HF benefits in the SGLT2 inhibitor trials “were seen in patients who have established cardiovascular disease – basically a history of heart attack or stroke,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“So we wanted to see how we can identify high-risk patients without a history of cardiovascular disease using these biomarkers, as an approach to targeting SGLT2 inhibitors, which are fairly expensive therapies,” he said. Without such risk stratification, “you end up treating so many more patients to get very modest returns.”
The group developed a scoring system based on four biomarkers that are “easily measured with inexpensive tests,” Dr. Pandey said: high-sensitivity-assay cardiac troponin T (hs-cTnT) and C-reactive protein (hs-CRP) levels, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) levels, and electrocardiography for evidence of left-ventricular hypertrophy (ECG-LVH).
The derivation cohort consisted of participants in the Atherosclerosis Risk in Communities RIC, Dallas Heart Study, and Multi-Ethnic Study of Atherosclerosis Multi-Ethnic Study of Atherosclerosis epidemiologic studies who were free of coronary heart disease, stroke, or HF for whom there were sufficient data on CV risk factors and the four biomarkers. None were taking SGLT2 inhibitors at enrollment in their respective studies, the researchers noted.
Members of the pooled cohorts who had diabetes or prediabetes were assigned 1 point for each abnormal biomarker. The 5-year risk for incident HF went up continuously along with the score in people with diabetes and in those with prediabetes, the latter defined as a fasting plasma glucose level from 100 mg/dL to less than 126 mg/dL.
For those with a score of 1, compared with 0, for example, the risk for HF went up 82% with diabetes and 40% with prediabetes. But for those with a score of 3 or 4, the risk went up more than four and a half times with diabetes and more than three and a half times for those with prediabetes. Risk increases were independent of other likely HF risk factors and consistently significant.
The analysis was published Jan. 6 in JACC: Heart Failure.
The biomarker score should be especially useful in patients considered at low to intermediate risk, based on clinical characteristics, as a means to identify residual HF risk and, potentially, select candidates for SGLT2-inhibitor therapy, Dr. Pandey said.
“The other purpose of the study was to broaden the scope of heart failure prevention in dysglycemia by looking also at prediabetes, not just diabetes,” he said. There isn’t much high-quality evidence supporting SGLT2-inhibitor therapy in prediabetes, but it follows that the drugs may be helpful in prediabetes because they are protective in patients with and without diabetes.
“Our work suggests that prediabetes patients who have elevated biomarkers are at a higher risk of heart failure,” Dr. Pandey said, suggesting that the HF risk score could potentially help select their drug therapy as well.
The current study seems “to provide a proof of concept that one can use circulating biomarkers to more precisely identify patients in whom therapies might be expected to exert greatest benefit,” which is especially important for potentially expensive agents like the SGLT2 inhibitors, James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Importantly in the analysis, a greater number of biomarker abnormalities not only corresponded to rising levels of risk, the risk increases were “dramatic,” and therefore so was the supposed potential benefit of SGLT2-inhibitor therapy, said Dr. Januzzi, who isn’t a coauthor but was an editor for its publication in JACC: Heart Failure.
The uptake of SGLT2 inhibitors for heart failure in practice has been less rapid than hoped, he observed, so if “this hypothetical construct holds up” for the drug class, “it might actually help kick-start focusing on who might optimally receive the drugs.”
Elevated levels of hs-cTnT, hs-CRP, and NT-proBNP, as well as presence of ECG-LVH, were each independently associated with a significantly increased 5-year risk for HF in unadjusted and adjusted analyses of the 6,799 people in the pooled cohort, 33.2% of whom had diabetes and 66.8% of whom had prediabetes, the group writes.
The scoring system would require validation in other cohorts before it could be used, Dr. Pandey observed; once there is “robust validation,” it might be applied first to patients with dysglycemia at intermediate CV risk by standard clinical measures.
Certainly the HF risk-stratification scoring system requires validation in other studies, Dr. Januzzi agreed. But it is intuitively appealing, and the study’s results are consistent with “data that we’re submitting for publication imminently” based on the CANVAS CV-outcomes trial of the SGLT2 inhibitor canagliflozin (Invokana) in patients with diabetes.
Dr. Pandey disclosed receiving support from the Gilead Sciences Research Scholar Program and serving on an advisory board of Roche Diagnostics. Dr. Januzzi disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on end-point committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda.
A version of this article first appeared on Medscape.com.
A scoring system that predicts risk for new heart failure over 5 years that is based solely on a few familiar, readily available biomarkers could potentially help steer patients with diabetes or even prediabetes toward HF-preventive therapies, researchers proposed based on a new study.
They foresee the risk-stratification tool, based on data pooled from three major community-based cohort studies but not independently validated, as a way to select patients with diabetes and prediabetes for treatment with SGLT2 inhibitors.
Several members of that drug class, conceived as antidiabetic agents, have been shown to help in prevention or treatment of HF in patients with diabetes and those without diabetes but at increased cardiovascular (CV) risk. Yet their uptake in practice has been lagging, the group noted.
Most HF benefits in the SGLT2 inhibitor trials “were seen in patients who have established cardiovascular disease – basically a history of heart attack or stroke,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“So we wanted to see how we can identify high-risk patients without a history of cardiovascular disease using these biomarkers, as an approach to targeting SGLT2 inhibitors, which are fairly expensive therapies,” he said. Without such risk stratification, “you end up treating so many more patients to get very modest returns.”
The group developed a scoring system based on four biomarkers that are “easily measured with inexpensive tests,” Dr. Pandey said: high-sensitivity-assay cardiac troponin T (hs-cTnT) and C-reactive protein (hs-CRP) levels, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) levels, and electrocardiography for evidence of left-ventricular hypertrophy (ECG-LVH).
The derivation cohort consisted of participants in the Atherosclerosis Risk in Communities RIC, Dallas Heart Study, and Multi-Ethnic Study of Atherosclerosis Multi-Ethnic Study of Atherosclerosis epidemiologic studies who were free of coronary heart disease, stroke, or HF for whom there were sufficient data on CV risk factors and the four biomarkers. None were taking SGLT2 inhibitors at enrollment in their respective studies, the researchers noted.
Members of the pooled cohorts who had diabetes or prediabetes were assigned 1 point for each abnormal biomarker. The 5-year risk for incident HF went up continuously along with the score in people with diabetes and in those with prediabetes, the latter defined as a fasting plasma glucose level from 100 mg/dL to less than 126 mg/dL.
For those with a score of 1, compared with 0, for example, the risk for HF went up 82% with diabetes and 40% with prediabetes. But for those with a score of 3 or 4, the risk went up more than four and a half times with diabetes and more than three and a half times for those with prediabetes. Risk increases were independent of other likely HF risk factors and consistently significant.
The analysis was published Jan. 6 in JACC: Heart Failure.
The biomarker score should be especially useful in patients considered at low to intermediate risk, based on clinical characteristics, as a means to identify residual HF risk and, potentially, select candidates for SGLT2-inhibitor therapy, Dr. Pandey said.
“The other purpose of the study was to broaden the scope of heart failure prevention in dysglycemia by looking also at prediabetes, not just diabetes,” he said. There isn’t much high-quality evidence supporting SGLT2-inhibitor therapy in prediabetes, but it follows that the drugs may be helpful in prediabetes because they are protective in patients with and without diabetes.
“Our work suggests that prediabetes patients who have elevated biomarkers are at a higher risk of heart failure,” Dr. Pandey said, suggesting that the HF risk score could potentially help select their drug therapy as well.
The current study seems “to provide a proof of concept that one can use circulating biomarkers to more precisely identify patients in whom therapies might be expected to exert greatest benefit,” which is especially important for potentially expensive agents like the SGLT2 inhibitors, James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Importantly in the analysis, a greater number of biomarker abnormalities not only corresponded to rising levels of risk, the risk increases were “dramatic,” and therefore so was the supposed potential benefit of SGLT2-inhibitor therapy, said Dr. Januzzi, who isn’t a coauthor but was an editor for its publication in JACC: Heart Failure.
The uptake of SGLT2 inhibitors for heart failure in practice has been less rapid than hoped, he observed, so if “this hypothetical construct holds up” for the drug class, “it might actually help kick-start focusing on who might optimally receive the drugs.”
Elevated levels of hs-cTnT, hs-CRP, and NT-proBNP, as well as presence of ECG-LVH, were each independently associated with a significantly increased 5-year risk for HF in unadjusted and adjusted analyses of the 6,799 people in the pooled cohort, 33.2% of whom had diabetes and 66.8% of whom had prediabetes, the group writes.
The scoring system would require validation in other cohorts before it could be used, Dr. Pandey observed; once there is “robust validation,” it might be applied first to patients with dysglycemia at intermediate CV risk by standard clinical measures.
Certainly the HF risk-stratification scoring system requires validation in other studies, Dr. Januzzi agreed. But it is intuitively appealing, and the study’s results are consistent with “data that we’re submitting for publication imminently” based on the CANVAS CV-outcomes trial of the SGLT2 inhibitor canagliflozin (Invokana) in patients with diabetes.
Dr. Pandey disclosed receiving support from the Gilead Sciences Research Scholar Program and serving on an advisory board of Roche Diagnostics. Dr. Januzzi disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on end-point committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda.
A version of this article first appeared on Medscape.com.
A scoring system that predicts risk for new heart failure over 5 years that is based solely on a few familiar, readily available biomarkers could potentially help steer patients with diabetes or even prediabetes toward HF-preventive therapies, researchers proposed based on a new study.
They foresee the risk-stratification tool, based on data pooled from three major community-based cohort studies but not independently validated, as a way to select patients with diabetes and prediabetes for treatment with SGLT2 inhibitors.
Several members of that drug class, conceived as antidiabetic agents, have been shown to help in prevention or treatment of HF in patients with diabetes and those without diabetes but at increased cardiovascular (CV) risk. Yet their uptake in practice has been lagging, the group noted.
Most HF benefits in the SGLT2 inhibitor trials “were seen in patients who have established cardiovascular disease – basically a history of heart attack or stroke,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“So we wanted to see how we can identify high-risk patients without a history of cardiovascular disease using these biomarkers, as an approach to targeting SGLT2 inhibitors, which are fairly expensive therapies,” he said. Without such risk stratification, “you end up treating so many more patients to get very modest returns.”
The group developed a scoring system based on four biomarkers that are “easily measured with inexpensive tests,” Dr. Pandey said: high-sensitivity-assay cardiac troponin T (hs-cTnT) and C-reactive protein (hs-CRP) levels, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) levels, and electrocardiography for evidence of left-ventricular hypertrophy (ECG-LVH).
The derivation cohort consisted of participants in the Atherosclerosis Risk in Communities RIC, Dallas Heart Study, and Multi-Ethnic Study of Atherosclerosis Multi-Ethnic Study of Atherosclerosis epidemiologic studies who were free of coronary heart disease, stroke, or HF for whom there were sufficient data on CV risk factors and the four biomarkers. None were taking SGLT2 inhibitors at enrollment in their respective studies, the researchers noted.
Members of the pooled cohorts who had diabetes or prediabetes were assigned 1 point for each abnormal biomarker. The 5-year risk for incident HF went up continuously along with the score in people with diabetes and in those with prediabetes, the latter defined as a fasting plasma glucose level from 100 mg/dL to less than 126 mg/dL.
For those with a score of 1, compared with 0, for example, the risk for HF went up 82% with diabetes and 40% with prediabetes. But for those with a score of 3 or 4, the risk went up more than four and a half times with diabetes and more than three and a half times for those with prediabetes. Risk increases were independent of other likely HF risk factors and consistently significant.
The analysis was published Jan. 6 in JACC: Heart Failure.
The biomarker score should be especially useful in patients considered at low to intermediate risk, based on clinical characteristics, as a means to identify residual HF risk and, potentially, select candidates for SGLT2-inhibitor therapy, Dr. Pandey said.
“The other purpose of the study was to broaden the scope of heart failure prevention in dysglycemia by looking also at prediabetes, not just diabetes,” he said. There isn’t much high-quality evidence supporting SGLT2-inhibitor therapy in prediabetes, but it follows that the drugs may be helpful in prediabetes because they are protective in patients with and without diabetes.
“Our work suggests that prediabetes patients who have elevated biomarkers are at a higher risk of heart failure,” Dr. Pandey said, suggesting that the HF risk score could potentially help select their drug therapy as well.
The current study seems “to provide a proof of concept that one can use circulating biomarkers to more precisely identify patients in whom therapies might be expected to exert greatest benefit,” which is especially important for potentially expensive agents like the SGLT2 inhibitors, James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Importantly in the analysis, a greater number of biomarker abnormalities not only corresponded to rising levels of risk, the risk increases were “dramatic,” and therefore so was the supposed potential benefit of SGLT2-inhibitor therapy, said Dr. Januzzi, who isn’t a coauthor but was an editor for its publication in JACC: Heart Failure.
The uptake of SGLT2 inhibitors for heart failure in practice has been less rapid than hoped, he observed, so if “this hypothetical construct holds up” for the drug class, “it might actually help kick-start focusing on who might optimally receive the drugs.”
Elevated levels of hs-cTnT, hs-CRP, and NT-proBNP, as well as presence of ECG-LVH, were each independently associated with a significantly increased 5-year risk for HF in unadjusted and adjusted analyses of the 6,799 people in the pooled cohort, 33.2% of whom had diabetes and 66.8% of whom had prediabetes, the group writes.
The scoring system would require validation in other cohorts before it could be used, Dr. Pandey observed; once there is “robust validation,” it might be applied first to patients with dysglycemia at intermediate CV risk by standard clinical measures.
Certainly the HF risk-stratification scoring system requires validation in other studies, Dr. Januzzi agreed. But it is intuitively appealing, and the study’s results are consistent with “data that we’re submitting for publication imminently” based on the CANVAS CV-outcomes trial of the SGLT2 inhibitor canagliflozin (Invokana) in patients with diabetes.
Dr. Pandey disclosed receiving support from the Gilead Sciences Research Scholar Program and serving on an advisory board of Roche Diagnostics. Dr. Januzzi disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on end-point committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda.
A version of this article first appeared on Medscape.com.
Moderna needs more kids for COVID vaccine trials
according to the company CEO and a federal official.
The Moderna vaccine was authorized for use in December and is now being given to people 18 and over. But children would receive lower doses, so new clinical trials must be done, Moderna CEO Stephane Bancel said at the JPMorgan virtual Health Care Conference on Monday.
Clinical trials on children 11 and younger “will take much longer, because we have to age deescalate and start at a lower dose. So we should not anticipate clinical data in 2021, but more in 2022,” Ms. Bancel said, according to Business Insider.
Moderna’s clinical trials for 12- to 17-year-olds started 4 weeks ago, but the company is having trouble getting enough participants, said Moncef Slaoui, PhD, the scientific head of Operation Warp Speed, the U.S. government’s vaccine effort. That could delay Food and Drug Administration approval, he said.
“It’s really very important for all of us, for all the population in America, to realize that we can’t have that indication unless adolescents aged 12-18 decide to participate,” Dr. Slaoui said, according to USA Today.
He said the adolescent trials are getting only about 800 volunteers a month, but need at least 3,000 volunteers to complete the study, USA Today reported. Parents interested in having their child participate can check eligibility and sign at this website.
The Pfizer/BioNTech vaccine won authorization for use in 16- to 17-year-olds as well as adults.
The coronavirus doesn’t appear to have as serious complications for children as for adults.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the American Association of Pediatrics says. “However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
The association says 179 children had died of COVID-related reasons in 43 states and New York City as of Dec. 31, 2020. That’s about 0.06% of total COVID deaths, it says.
But children do get sick. As of Jan. 7, 2021, nearly 2.3 million children had tested positive for COVID-19 since the start of the pandemic, the association says.
A version of this article first appeared on WebMD.com.
according to the company CEO and a federal official.
The Moderna vaccine was authorized for use in December and is now being given to people 18 and over. But children would receive lower doses, so new clinical trials must be done, Moderna CEO Stephane Bancel said at the JPMorgan virtual Health Care Conference on Monday.
Clinical trials on children 11 and younger “will take much longer, because we have to age deescalate and start at a lower dose. So we should not anticipate clinical data in 2021, but more in 2022,” Ms. Bancel said, according to Business Insider.
Moderna’s clinical trials for 12- to 17-year-olds started 4 weeks ago, but the company is having trouble getting enough participants, said Moncef Slaoui, PhD, the scientific head of Operation Warp Speed, the U.S. government’s vaccine effort. That could delay Food and Drug Administration approval, he said.
“It’s really very important for all of us, for all the population in America, to realize that we can’t have that indication unless adolescents aged 12-18 decide to participate,” Dr. Slaoui said, according to USA Today.
He said the adolescent trials are getting only about 800 volunteers a month, but need at least 3,000 volunteers to complete the study, USA Today reported. Parents interested in having their child participate can check eligibility and sign at this website.
The Pfizer/BioNTech vaccine won authorization for use in 16- to 17-year-olds as well as adults.
The coronavirus doesn’t appear to have as serious complications for children as for adults.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the American Association of Pediatrics says. “However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
The association says 179 children had died of COVID-related reasons in 43 states and New York City as of Dec. 31, 2020. That’s about 0.06% of total COVID deaths, it says.
But children do get sick. As of Jan. 7, 2021, nearly 2.3 million children had tested positive for COVID-19 since the start of the pandemic, the association says.
A version of this article first appeared on WebMD.com.
according to the company CEO and a federal official.
The Moderna vaccine was authorized for use in December and is now being given to people 18 and over. But children would receive lower doses, so new clinical trials must be done, Moderna CEO Stephane Bancel said at the JPMorgan virtual Health Care Conference on Monday.
Clinical trials on children 11 and younger “will take much longer, because we have to age deescalate and start at a lower dose. So we should not anticipate clinical data in 2021, but more in 2022,” Ms. Bancel said, according to Business Insider.
Moderna’s clinical trials for 12- to 17-year-olds started 4 weeks ago, but the company is having trouble getting enough participants, said Moncef Slaoui, PhD, the scientific head of Operation Warp Speed, the U.S. government’s vaccine effort. That could delay Food and Drug Administration approval, he said.
“It’s really very important for all of us, for all the population in America, to realize that we can’t have that indication unless adolescents aged 12-18 decide to participate,” Dr. Slaoui said, according to USA Today.
He said the adolescent trials are getting only about 800 volunteers a month, but need at least 3,000 volunteers to complete the study, USA Today reported. Parents interested in having their child participate can check eligibility and sign at this website.
The Pfizer/BioNTech vaccine won authorization for use in 16- to 17-year-olds as well as adults.
The coronavirus doesn’t appear to have as serious complications for children as for adults.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the American Association of Pediatrics says. “However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
The association says 179 children had died of COVID-related reasons in 43 states and New York City as of Dec. 31, 2020. That’s about 0.06% of total COVID deaths, it says.
But children do get sick. As of Jan. 7, 2021, nearly 2.3 million children had tested positive for COVID-19 since the start of the pandemic, the association says.
A version of this article first appeared on WebMD.com.
Could an osteoporosis drug reduce need for hip revision surgery?
A single injection of denosumab (Prolia, Amgen), frequently used to treat osteoporosis, may reduce the need for revision surgery in patients with symptomatic osteolysis following total hip arthroplasty, a new proof-of-concept study suggests.
Aseptic loosening is the result of wear-induced osteolysis caused by the prosthetic hip and is a major contributor to the need for revision surgery in many parts of the world.
“The only established treatment for prosthesis-related osteolysis after joint replacement is revision surgery, which carries substantially greater morbidity and mortality than primary joint replacement,” Mohit M. Mahatma, MRes, of the University of Sheffield, England, and colleagues wrote in their article, published online Jan. 11 in The Lancet Rheumatology.
As well as an increased risk of infection and other complications, revision surgery is much more costly than a first-time operation, they added.
“The results of this proof-of-concept clinical trial indicate that denosumab is effective at reducing bone resorption activity within osteolytic lesion tissue and is well tolerated within the limitations of the single dose used here,” they concluded.
Commenting on the findings, Antonia Chen, MD, associate professor of orthopedic surgery, Harvard Medical School, Boston, emphasized that further studies are needed to assess the effectiveness of this strategy to reduce the need for hip revision surgery.
Nevertheless, “osteolysis is still unfortunately a problem we do have to deal with and we do not have any other way to prevent it,” she said in an interview. “So it’s a good start ... although further studies are definitely needed,” Dr. Chen added.
In an accompanying editorial, Hannu Aro, MD, Turku University Hospital in Finland, agreed: “Without a doubt, the trial is a breakthrough, but it represents only the first step in the development of pharmacological therapy aiming to slow, prevent, or even reverse the process of wear-induced periprosthetic osteolysis.”
Small single-center study
The phase 2, single-center, randomized, controlled trial involved 22 patients who had previously undergone hip replacement surgery at Sheffield Teaching Hospitals and were scheduled for revision surgery due to symptomatic osteolysis. They were randomized to a single subcutaneous injection of denosumab at a dose of 60 mg, or placebo, on their second hospital visit.
“The primary outcome was the between-group difference in the number of osteoclasts per mm of osteolytic membrane at the osteolytic membrane-bone interface at week 8,” the authors noted.
At this time point, there were 83% fewer osteoclasts at the interface in the denosumab group compared with placebo, at a median of 0.05 per mm in the treatment group compared with 0.30 per mm in the placebo group (P = .011).
Secondary histological outcomes were also significantly improved in favor of the denosumab group compared with placebo.
Potential to prevent half of all hip revision surgeries?
Patients who received denosumab also demonstrated an acute fall in serum and urinary markers of bone resorption following administration of the drug, reaching a nadir at week 4, which was maintained until revision surgery at week 8.
In contrast, “no change in these markers was observed in the placebo group [P < .0003 for all biomarkers],” the investigators noted. Rates of adverse events were comparable in both treatment groups.
As the authors explained, osteolysis occurs following joint replacement surgery when particles of plastic wear off from the prosthesis, triggering an immune reaction that attacks the bone around the implant, causing the joint to loosen.
“It is very clear from our bone biopsies and bone imaging that the [denosumab] injection stops the bone absorbing the microplastic particles from the replacement joint and therefore could prevent the bone from being eaten away and the need for revision surgery,” senior author Mark Wilkinson, MBChB, PhD, honorary consultant orthopedic surgeon, Sheffield Teaching Hospitals, said in a press release from his institution.
“This study is a significant breakthrough as we’ve demonstrated that there is a drug, already available and successful in the treatment of osteoporosis, that has the potential to prevent up to half of all revised replacement surgeries which are caused by osteolysis,” he added.
Dr. Wilkinson and coauthors said their results justify the need for future trials targeting earlier-stage disease to further test the use of denosumab to prevent or reduce the need for revision surgery.
In 2018, aseptic loosening accounted for over half of all revision procedures, as reported to the National Joint Registry in England and Wales.
Older polyethylene prostheses are the main culprit
Commenting further on the study, Dr. Chen noted that osteolysis still plagues orthopedic surgeons because the original polyethylene prostheses were not very good. A better prosthesis developed at Massachusetts General Hospital is made up of highly crossed-link polyethylene and still wears over time but to a much lesser extent than the older polyethylene prostheses.
Metal and ceramic prostheses also can induce osteolysis, but again to a much lesser extent than the older polyethylene implants.
“Any particle can technically cause osteolysis but plastic produces the most particles,” Dr. Chen explained. Although hip revision rates in the United States are low to begin with, aseptic loosening is still one of the main reasons that patients need to undergo revision surgery, she observed.
“A lot of patients are still living with the old plastic [implants] so there is still a need for something like this,” she stressed.
However, many questions about this potential new strategy remain to be answered, including when best to initiate treatment and how to manage patients at risk for osteolysis 20-30 years after they have received their original implant.
In his editorial, Dr. Aro said that serious adverse consequences often become evident 10-20 years after patients have undergone the original hip replacement procedures, when they are potentially less physically fit than they were at the time of the operation and thus less able to withstand the rigors of a difficult revision surgery.
“In this context, the concept of nonsurgical pharmacological treatment of periprosthetic osteolysis ... brings a new hope for the ever-increasing population of patients with total hip arthroplasty to avoid revision surgery,” Dr. Aro suggested.
However, Dr. Aro cautioned that reduction of bone turnover by antiresorptive agents such as denosumab has been associated with the development of atypical femoral fractures.
The study was funded by Amgen. Dr. Wilkinson has reported receiving a grant from Amgen. Dr. Chen has reported serving as a consultant for Striker and b-One Ortho. Dr. Aro has reported receiving a grant to his institution from Amgen Finland and the Academy of Finland. He has also served as a member of an advisory scientific board for Amgen Finland.
A version of this article first appeared on Medscape.com.
A single injection of denosumab (Prolia, Amgen), frequently used to treat osteoporosis, may reduce the need for revision surgery in patients with symptomatic osteolysis following total hip arthroplasty, a new proof-of-concept study suggests.
Aseptic loosening is the result of wear-induced osteolysis caused by the prosthetic hip and is a major contributor to the need for revision surgery in many parts of the world.
“The only established treatment for prosthesis-related osteolysis after joint replacement is revision surgery, which carries substantially greater morbidity and mortality than primary joint replacement,” Mohit M. Mahatma, MRes, of the University of Sheffield, England, and colleagues wrote in their article, published online Jan. 11 in The Lancet Rheumatology.
As well as an increased risk of infection and other complications, revision surgery is much more costly than a first-time operation, they added.
“The results of this proof-of-concept clinical trial indicate that denosumab is effective at reducing bone resorption activity within osteolytic lesion tissue and is well tolerated within the limitations of the single dose used here,” they concluded.
Commenting on the findings, Antonia Chen, MD, associate professor of orthopedic surgery, Harvard Medical School, Boston, emphasized that further studies are needed to assess the effectiveness of this strategy to reduce the need for hip revision surgery.
Nevertheless, “osteolysis is still unfortunately a problem we do have to deal with and we do not have any other way to prevent it,” she said in an interview. “So it’s a good start ... although further studies are definitely needed,” Dr. Chen added.
In an accompanying editorial, Hannu Aro, MD, Turku University Hospital in Finland, agreed: “Without a doubt, the trial is a breakthrough, but it represents only the first step in the development of pharmacological therapy aiming to slow, prevent, or even reverse the process of wear-induced periprosthetic osteolysis.”
Small single-center study
The phase 2, single-center, randomized, controlled trial involved 22 patients who had previously undergone hip replacement surgery at Sheffield Teaching Hospitals and were scheduled for revision surgery due to symptomatic osteolysis. They were randomized to a single subcutaneous injection of denosumab at a dose of 60 mg, or placebo, on their second hospital visit.
“The primary outcome was the between-group difference in the number of osteoclasts per mm of osteolytic membrane at the osteolytic membrane-bone interface at week 8,” the authors noted.
At this time point, there were 83% fewer osteoclasts at the interface in the denosumab group compared with placebo, at a median of 0.05 per mm in the treatment group compared with 0.30 per mm in the placebo group (P = .011).
Secondary histological outcomes were also significantly improved in favor of the denosumab group compared with placebo.
Potential to prevent half of all hip revision surgeries?
Patients who received denosumab also demonstrated an acute fall in serum and urinary markers of bone resorption following administration of the drug, reaching a nadir at week 4, which was maintained until revision surgery at week 8.
In contrast, “no change in these markers was observed in the placebo group [P < .0003 for all biomarkers],” the investigators noted. Rates of adverse events were comparable in both treatment groups.
As the authors explained, osteolysis occurs following joint replacement surgery when particles of plastic wear off from the prosthesis, triggering an immune reaction that attacks the bone around the implant, causing the joint to loosen.
“It is very clear from our bone biopsies and bone imaging that the [denosumab] injection stops the bone absorbing the microplastic particles from the replacement joint and therefore could prevent the bone from being eaten away and the need for revision surgery,” senior author Mark Wilkinson, MBChB, PhD, honorary consultant orthopedic surgeon, Sheffield Teaching Hospitals, said in a press release from his institution.
“This study is a significant breakthrough as we’ve demonstrated that there is a drug, already available and successful in the treatment of osteoporosis, that has the potential to prevent up to half of all revised replacement surgeries which are caused by osteolysis,” he added.
Dr. Wilkinson and coauthors said their results justify the need for future trials targeting earlier-stage disease to further test the use of denosumab to prevent or reduce the need for revision surgery.
In 2018, aseptic loosening accounted for over half of all revision procedures, as reported to the National Joint Registry in England and Wales.
Older polyethylene prostheses are the main culprit
Commenting further on the study, Dr. Chen noted that osteolysis still plagues orthopedic surgeons because the original polyethylene prostheses were not very good. A better prosthesis developed at Massachusetts General Hospital is made up of highly crossed-link polyethylene and still wears over time but to a much lesser extent than the older polyethylene prostheses.
Metal and ceramic prostheses also can induce osteolysis, but again to a much lesser extent than the older polyethylene implants.
“Any particle can technically cause osteolysis but plastic produces the most particles,” Dr. Chen explained. Although hip revision rates in the United States are low to begin with, aseptic loosening is still one of the main reasons that patients need to undergo revision surgery, she observed.
“A lot of patients are still living with the old plastic [implants] so there is still a need for something like this,” she stressed.
However, many questions about this potential new strategy remain to be answered, including when best to initiate treatment and how to manage patients at risk for osteolysis 20-30 years after they have received their original implant.
In his editorial, Dr. Aro said that serious adverse consequences often become evident 10-20 years after patients have undergone the original hip replacement procedures, when they are potentially less physically fit than they were at the time of the operation and thus less able to withstand the rigors of a difficult revision surgery.
“In this context, the concept of nonsurgical pharmacological treatment of periprosthetic osteolysis ... brings a new hope for the ever-increasing population of patients with total hip arthroplasty to avoid revision surgery,” Dr. Aro suggested.
However, Dr. Aro cautioned that reduction of bone turnover by antiresorptive agents such as denosumab has been associated with the development of atypical femoral fractures.
The study was funded by Amgen. Dr. Wilkinson has reported receiving a grant from Amgen. Dr. Chen has reported serving as a consultant for Striker and b-One Ortho. Dr. Aro has reported receiving a grant to his institution from Amgen Finland and the Academy of Finland. He has also served as a member of an advisory scientific board for Amgen Finland.
A version of this article first appeared on Medscape.com.
A single injection of denosumab (Prolia, Amgen), frequently used to treat osteoporosis, may reduce the need for revision surgery in patients with symptomatic osteolysis following total hip arthroplasty, a new proof-of-concept study suggests.
Aseptic loosening is the result of wear-induced osteolysis caused by the prosthetic hip and is a major contributor to the need for revision surgery in many parts of the world.
“The only established treatment for prosthesis-related osteolysis after joint replacement is revision surgery, which carries substantially greater morbidity and mortality than primary joint replacement,” Mohit M. Mahatma, MRes, of the University of Sheffield, England, and colleagues wrote in their article, published online Jan. 11 in The Lancet Rheumatology.
As well as an increased risk of infection and other complications, revision surgery is much more costly than a first-time operation, they added.
“The results of this proof-of-concept clinical trial indicate that denosumab is effective at reducing bone resorption activity within osteolytic lesion tissue and is well tolerated within the limitations of the single dose used here,” they concluded.
Commenting on the findings, Antonia Chen, MD, associate professor of orthopedic surgery, Harvard Medical School, Boston, emphasized that further studies are needed to assess the effectiveness of this strategy to reduce the need for hip revision surgery.
Nevertheless, “osteolysis is still unfortunately a problem we do have to deal with and we do not have any other way to prevent it,” she said in an interview. “So it’s a good start ... although further studies are definitely needed,” Dr. Chen added.
In an accompanying editorial, Hannu Aro, MD, Turku University Hospital in Finland, agreed: “Without a doubt, the trial is a breakthrough, but it represents only the first step in the development of pharmacological therapy aiming to slow, prevent, or even reverse the process of wear-induced periprosthetic osteolysis.”
Small single-center study
The phase 2, single-center, randomized, controlled trial involved 22 patients who had previously undergone hip replacement surgery at Sheffield Teaching Hospitals and were scheduled for revision surgery due to symptomatic osteolysis. They were randomized to a single subcutaneous injection of denosumab at a dose of 60 mg, or placebo, on their second hospital visit.
“The primary outcome was the between-group difference in the number of osteoclasts per mm of osteolytic membrane at the osteolytic membrane-bone interface at week 8,” the authors noted.
At this time point, there were 83% fewer osteoclasts at the interface in the denosumab group compared with placebo, at a median of 0.05 per mm in the treatment group compared with 0.30 per mm in the placebo group (P = .011).
Secondary histological outcomes were also significantly improved in favor of the denosumab group compared with placebo.
Potential to prevent half of all hip revision surgeries?
Patients who received denosumab also demonstrated an acute fall in serum and urinary markers of bone resorption following administration of the drug, reaching a nadir at week 4, which was maintained until revision surgery at week 8.
In contrast, “no change in these markers was observed in the placebo group [P < .0003 for all biomarkers],” the investigators noted. Rates of adverse events were comparable in both treatment groups.
As the authors explained, osteolysis occurs following joint replacement surgery when particles of plastic wear off from the prosthesis, triggering an immune reaction that attacks the bone around the implant, causing the joint to loosen.
“It is very clear from our bone biopsies and bone imaging that the [denosumab] injection stops the bone absorbing the microplastic particles from the replacement joint and therefore could prevent the bone from being eaten away and the need for revision surgery,” senior author Mark Wilkinson, MBChB, PhD, honorary consultant orthopedic surgeon, Sheffield Teaching Hospitals, said in a press release from his institution.
“This study is a significant breakthrough as we’ve demonstrated that there is a drug, already available and successful in the treatment of osteoporosis, that has the potential to prevent up to half of all revised replacement surgeries which are caused by osteolysis,” he added.
Dr. Wilkinson and coauthors said their results justify the need for future trials targeting earlier-stage disease to further test the use of denosumab to prevent or reduce the need for revision surgery.
In 2018, aseptic loosening accounted for over half of all revision procedures, as reported to the National Joint Registry in England and Wales.
Older polyethylene prostheses are the main culprit
Commenting further on the study, Dr. Chen noted that osteolysis still plagues orthopedic surgeons because the original polyethylene prostheses were not very good. A better prosthesis developed at Massachusetts General Hospital is made up of highly crossed-link polyethylene and still wears over time but to a much lesser extent than the older polyethylene prostheses.
Metal and ceramic prostheses also can induce osteolysis, but again to a much lesser extent than the older polyethylene implants.
“Any particle can technically cause osteolysis but plastic produces the most particles,” Dr. Chen explained. Although hip revision rates in the United States are low to begin with, aseptic loosening is still one of the main reasons that patients need to undergo revision surgery, she observed.
“A lot of patients are still living with the old plastic [implants] so there is still a need for something like this,” she stressed.
However, many questions about this potential new strategy remain to be answered, including when best to initiate treatment and how to manage patients at risk for osteolysis 20-30 years after they have received their original implant.
In his editorial, Dr. Aro said that serious adverse consequences often become evident 10-20 years after patients have undergone the original hip replacement procedures, when they are potentially less physically fit than they were at the time of the operation and thus less able to withstand the rigors of a difficult revision surgery.
“In this context, the concept of nonsurgical pharmacological treatment of periprosthetic osteolysis ... brings a new hope for the ever-increasing population of patients with total hip arthroplasty to avoid revision surgery,” Dr. Aro suggested.
However, Dr. Aro cautioned that reduction of bone turnover by antiresorptive agents such as denosumab has been associated with the development of atypical femoral fractures.
The study was funded by Amgen. Dr. Wilkinson has reported receiving a grant from Amgen. Dr. Chen has reported serving as a consultant for Striker and b-One Ortho. Dr. Aro has reported receiving a grant to his institution from Amgen Finland and the Academy of Finland. He has also served as a member of an advisory scientific board for Amgen Finland.
A version of this article first appeared on Medscape.com.
Normal FLIP findings usually ruled out esophageal motility disorders
Most patients with normal findings on functional luminal imaging probe (FLIP) showed no clinical evidence of a major esophageal motor disorder, even when their high-resolution manometry (HRM) test results were abnormal, according to the results of a single-center retrospective cohort study.
Among 111 study participants with normal FLIP findings, 79% also showed no evidence of a major esophageal motor disorder on esophageal high-resolution manometry (HRM), wrote Alexandra J. Baumann, DO, of Northwestern University, Chicago, and associates. “Among the remaining 21% with apparent disagreement with HRM, [those] with normal FLIP panometry carried overall clinical impressions of not having a major esophageal motor disorder and subsequently were treated conservatively without the need for surgical interventions,” they reported. For patients with normal upper endoscopy and normal FLIP panometry, “the initial clinical management strategy could be directed toward addressing gastroesophageal reflux or a functional syndrome,” they wrote in Clinical Gastroenterology and Hepatology.
FLIP uses high-resolution impedance planimetry to evaluate esophageal lumen parameters, distensibility, and contractility in response to distension. Although HRM is standard for evaluating esophageal motility, false negatives and positives can result from challenges with interpreting outflow obstructions and normal lower-esophageal sphincter relaxation pressures among patients with clinical achalasia.
Hence, the researchers evaluated correlations between FLIP and HRM in 111 patients with esophageal symptoms and nonobstructive endoscopy findings who were evaluated at the Esophageal Center of Northwestern University between 2012 and 2019. Gastroenterologists performed additional studies, such as barium esophagrams, at their discretion. By study design, all patients had normal FLIP results, defined as an esophagogastric junction distensibility index above 3.0 mm2 per mm Hg and a normal contractile response (that is, normal repetitive retrograde contractions and a repetitive antegrade contraction pattern that met the Rule-of-6s). Three clinicians evaluated and reached consensus on each FLIP study. Esophageal HRM data were interpreted based on the Chicago classification system (version 3.0).
Patients with normal FLIP panometry findings “did not have a clinical impression of a major esophageal motor disorder,” the researchers reported. In all, 23 (21%) patients with normal FLIP results had discrepant (abnormal) HRM findings, most of which were false positives or equivocal.
For example, among 20 patients whose HRM suggested an esophagogastric junction outflow obstruction, 17 showed normal bolus transit on supine swallows and 16 showed normalization of integrated relaxation pressure after adjunctive maneuvers. Similarly, among 10 patients who underwent a barium esophagram, 8 showed normal emptying, 1 showed a temporary delay but no retention, and 1 had an incomplete study. “The overall clinical impression was not of an achalasia variant in any of these 20 patients with [esophagogastric junction outflow obstruction] on HRM, and thus none underwent botulinum toxin injection, pneumatic dilation, or lower-esophageal sphincter myotomy at our center,” the researchers wrote. Among 17 patients who were available for clinical follow-up, 4 underwent empiric dilation, of whom none had mucosal disruption. One patient was diagnosed with dysphagia lusoria based on cross-sectional imaging, while the rest were managed conservatively.
Similarly, among 10 patients with at least 50% ineffective swallows on HRM, 5 showed normal barium emptying and 9 were managed conservatively (the remaining patient underwent cricopharyngeal dilation for concurrent oropharyngeal dysphagia). The strong correlation between HRM and esophagrams in this study indicates that“[n]ormal findings from FLIP panometry can be used to exclude esophageal motility disorders at the time of endoscopy, possibly reducing the need for high-resolution manometry evaluation of some patients,” the investigators concluded. “However, further longitudinal studies are needed to support this approach.”
The work was supported by the Public Health Service and the American College of Gastroenterology. Dr. Baumann reported having no conflicts of interest. Four coinvestigators disclosed relevant ties to Crospon, Given Imaging, Ironwood, Medtronic, Sandhill Scientific, Torax, and other companies..
SOURCE: Baumann AJ et al. Clin Gastroenterol Hepatol 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.040.
Endoscopy is often the first step in the evaluation of dysphagia and other esophageal symptoms such as chest pain. When endoscopy is negative for a cause of these esophageal symptoms and biopsies rule out eosinophilic esophagitis, an esophageal motility disorder should be excluded, and high-resolution esophageal manometry is considered the standard method for this purpose.
Functional lumen imaging probe (FLIP) panometry offers the opportunity to evaluate esophageal motor function during sedated endoscopy, and it can be easily added to the endoscopic procedure if there are no findings to explain esophageal symptoms. The prospect of establishing the presence of normal esophageal motility and ruling out a major motility disorder during endoscopy is very attractive because it would increase diagnostic efficiency while also obviating the need for an additional and potentially uncomfortable study for the patient. This study by Buamann and colleagues explores the yield of normal FLIP panometry to predict the presence of normal esophageal motility and rule out a major motility disorder. Their study showed that manometry was negative for a major motility disorder in 88 of 111 (79%) patients with normal FLIP panometry. Manometry revealed a major motility disorder in 23 patients with normal FLIP topography, mainly because of esophagogastric junction outflow obstruction (EGJOO) seen in 20 patients, along with absent contractility in 2, and distal esophageal spasm in 1. The EGJOO was for the most part not confirmed by adjunctive swallows on manometry or by esophagram, and aggressive therapies were not needed, indicating likely falsely positive EGJOO diagnosed by manometry. These are very encouraging results. If the findings are confirmed in larger prospective studies, it would be reasonable to consider modifying our paradigm for the evaluation of esophageal symptoms, and FLIP panometry could be considered as a screening tool to rule out a clinically significant major motility disorders during the initial endoscopic evaluation for esophageal symptoms.
Marcelo F. Vela, MD, MSCR, AGAF, is professor of medicine, director of Esophageal Disorders, and program director of Esophageal Fellowship in the division of gastroenterology and hepatology at Mayo Clinic Arizona in Scottsdale. He reports being a consultant for Medtronic and receiving research support from Diversatek.
Endoscopy is often the first step in the evaluation of dysphagia and other esophageal symptoms such as chest pain. When endoscopy is negative for a cause of these esophageal symptoms and biopsies rule out eosinophilic esophagitis, an esophageal motility disorder should be excluded, and high-resolution esophageal manometry is considered the standard method for this purpose.
Functional lumen imaging probe (FLIP) panometry offers the opportunity to evaluate esophageal motor function during sedated endoscopy, and it can be easily added to the endoscopic procedure if there are no findings to explain esophageal symptoms. The prospect of establishing the presence of normal esophageal motility and ruling out a major motility disorder during endoscopy is very attractive because it would increase diagnostic efficiency while also obviating the need for an additional and potentially uncomfortable study for the patient. This study by Buamann and colleagues explores the yield of normal FLIP panometry to predict the presence of normal esophageal motility and rule out a major motility disorder. Their study showed that manometry was negative for a major motility disorder in 88 of 111 (79%) patients with normal FLIP panometry. Manometry revealed a major motility disorder in 23 patients with normal FLIP topography, mainly because of esophagogastric junction outflow obstruction (EGJOO) seen in 20 patients, along with absent contractility in 2, and distal esophageal spasm in 1. The EGJOO was for the most part not confirmed by adjunctive swallows on manometry or by esophagram, and aggressive therapies were not needed, indicating likely falsely positive EGJOO diagnosed by manometry. These are very encouraging results. If the findings are confirmed in larger prospective studies, it would be reasonable to consider modifying our paradigm for the evaluation of esophageal symptoms, and FLIP panometry could be considered as a screening tool to rule out a clinically significant major motility disorders during the initial endoscopic evaluation for esophageal symptoms.
Marcelo F. Vela, MD, MSCR, AGAF, is professor of medicine, director of Esophageal Disorders, and program director of Esophageal Fellowship in the division of gastroenterology and hepatology at Mayo Clinic Arizona in Scottsdale. He reports being a consultant for Medtronic and receiving research support from Diversatek.
Endoscopy is often the first step in the evaluation of dysphagia and other esophageal symptoms such as chest pain. When endoscopy is negative for a cause of these esophageal symptoms and biopsies rule out eosinophilic esophagitis, an esophageal motility disorder should be excluded, and high-resolution esophageal manometry is considered the standard method for this purpose.
Functional lumen imaging probe (FLIP) panometry offers the opportunity to evaluate esophageal motor function during sedated endoscopy, and it can be easily added to the endoscopic procedure if there are no findings to explain esophageal symptoms. The prospect of establishing the presence of normal esophageal motility and ruling out a major motility disorder during endoscopy is very attractive because it would increase diagnostic efficiency while also obviating the need for an additional and potentially uncomfortable study for the patient. This study by Buamann and colleagues explores the yield of normal FLIP panometry to predict the presence of normal esophageal motility and rule out a major motility disorder. Their study showed that manometry was negative for a major motility disorder in 88 of 111 (79%) patients with normal FLIP panometry. Manometry revealed a major motility disorder in 23 patients with normal FLIP topography, mainly because of esophagogastric junction outflow obstruction (EGJOO) seen in 20 patients, along with absent contractility in 2, and distal esophageal spasm in 1. The EGJOO was for the most part not confirmed by adjunctive swallows on manometry or by esophagram, and aggressive therapies were not needed, indicating likely falsely positive EGJOO diagnosed by manometry. These are very encouraging results. If the findings are confirmed in larger prospective studies, it would be reasonable to consider modifying our paradigm for the evaluation of esophageal symptoms, and FLIP panometry could be considered as a screening tool to rule out a clinically significant major motility disorders during the initial endoscopic evaluation for esophageal symptoms.
Marcelo F. Vela, MD, MSCR, AGAF, is professor of medicine, director of Esophageal Disorders, and program director of Esophageal Fellowship in the division of gastroenterology and hepatology at Mayo Clinic Arizona in Scottsdale. He reports being a consultant for Medtronic and receiving research support from Diversatek.
Most patients with normal findings on functional luminal imaging probe (FLIP) showed no clinical evidence of a major esophageal motor disorder, even when their high-resolution manometry (HRM) test results were abnormal, according to the results of a single-center retrospective cohort study.
Among 111 study participants with normal FLIP findings, 79% also showed no evidence of a major esophageal motor disorder on esophageal high-resolution manometry (HRM), wrote Alexandra J. Baumann, DO, of Northwestern University, Chicago, and associates. “Among the remaining 21% with apparent disagreement with HRM, [those] with normal FLIP panometry carried overall clinical impressions of not having a major esophageal motor disorder and subsequently were treated conservatively without the need for surgical interventions,” they reported. For patients with normal upper endoscopy and normal FLIP panometry, “the initial clinical management strategy could be directed toward addressing gastroesophageal reflux or a functional syndrome,” they wrote in Clinical Gastroenterology and Hepatology.
FLIP uses high-resolution impedance planimetry to evaluate esophageal lumen parameters, distensibility, and contractility in response to distension. Although HRM is standard for evaluating esophageal motility, false negatives and positives can result from challenges with interpreting outflow obstructions and normal lower-esophageal sphincter relaxation pressures among patients with clinical achalasia.
Hence, the researchers evaluated correlations between FLIP and HRM in 111 patients with esophageal symptoms and nonobstructive endoscopy findings who were evaluated at the Esophageal Center of Northwestern University between 2012 and 2019. Gastroenterologists performed additional studies, such as barium esophagrams, at their discretion. By study design, all patients had normal FLIP results, defined as an esophagogastric junction distensibility index above 3.0 mm2 per mm Hg and a normal contractile response (that is, normal repetitive retrograde contractions and a repetitive antegrade contraction pattern that met the Rule-of-6s). Three clinicians evaluated and reached consensus on each FLIP study. Esophageal HRM data were interpreted based on the Chicago classification system (version 3.0).
Patients with normal FLIP panometry findings “did not have a clinical impression of a major esophageal motor disorder,” the researchers reported. In all, 23 (21%) patients with normal FLIP results had discrepant (abnormal) HRM findings, most of which were false positives or equivocal.
For example, among 20 patients whose HRM suggested an esophagogastric junction outflow obstruction, 17 showed normal bolus transit on supine swallows and 16 showed normalization of integrated relaxation pressure after adjunctive maneuvers. Similarly, among 10 patients who underwent a barium esophagram, 8 showed normal emptying, 1 showed a temporary delay but no retention, and 1 had an incomplete study. “The overall clinical impression was not of an achalasia variant in any of these 20 patients with [esophagogastric junction outflow obstruction] on HRM, and thus none underwent botulinum toxin injection, pneumatic dilation, or lower-esophageal sphincter myotomy at our center,” the researchers wrote. Among 17 patients who were available for clinical follow-up, 4 underwent empiric dilation, of whom none had mucosal disruption. One patient was diagnosed with dysphagia lusoria based on cross-sectional imaging, while the rest were managed conservatively.
Similarly, among 10 patients with at least 50% ineffective swallows on HRM, 5 showed normal barium emptying and 9 were managed conservatively (the remaining patient underwent cricopharyngeal dilation for concurrent oropharyngeal dysphagia). The strong correlation between HRM and esophagrams in this study indicates that“[n]ormal findings from FLIP panometry can be used to exclude esophageal motility disorders at the time of endoscopy, possibly reducing the need for high-resolution manometry evaluation of some patients,” the investigators concluded. “However, further longitudinal studies are needed to support this approach.”
The work was supported by the Public Health Service and the American College of Gastroenterology. Dr. Baumann reported having no conflicts of interest. Four coinvestigators disclosed relevant ties to Crospon, Given Imaging, Ironwood, Medtronic, Sandhill Scientific, Torax, and other companies..
SOURCE: Baumann AJ et al. Clin Gastroenterol Hepatol 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.040.
Most patients with normal findings on functional luminal imaging probe (FLIP) showed no clinical evidence of a major esophageal motor disorder, even when their high-resolution manometry (HRM) test results were abnormal, according to the results of a single-center retrospective cohort study.
Among 111 study participants with normal FLIP findings, 79% also showed no evidence of a major esophageal motor disorder on esophageal high-resolution manometry (HRM), wrote Alexandra J. Baumann, DO, of Northwestern University, Chicago, and associates. “Among the remaining 21% with apparent disagreement with HRM, [those] with normal FLIP panometry carried overall clinical impressions of not having a major esophageal motor disorder and subsequently were treated conservatively without the need for surgical interventions,” they reported. For patients with normal upper endoscopy and normal FLIP panometry, “the initial clinical management strategy could be directed toward addressing gastroesophageal reflux or a functional syndrome,” they wrote in Clinical Gastroenterology and Hepatology.
FLIP uses high-resolution impedance planimetry to evaluate esophageal lumen parameters, distensibility, and contractility in response to distension. Although HRM is standard for evaluating esophageal motility, false negatives and positives can result from challenges with interpreting outflow obstructions and normal lower-esophageal sphincter relaxation pressures among patients with clinical achalasia.
Hence, the researchers evaluated correlations between FLIP and HRM in 111 patients with esophageal symptoms and nonobstructive endoscopy findings who were evaluated at the Esophageal Center of Northwestern University between 2012 and 2019. Gastroenterologists performed additional studies, such as barium esophagrams, at their discretion. By study design, all patients had normal FLIP results, defined as an esophagogastric junction distensibility index above 3.0 mm2 per mm Hg and a normal contractile response (that is, normal repetitive retrograde contractions and a repetitive antegrade contraction pattern that met the Rule-of-6s). Three clinicians evaluated and reached consensus on each FLIP study. Esophageal HRM data were interpreted based on the Chicago classification system (version 3.0).
Patients with normal FLIP panometry findings “did not have a clinical impression of a major esophageal motor disorder,” the researchers reported. In all, 23 (21%) patients with normal FLIP results had discrepant (abnormal) HRM findings, most of which were false positives or equivocal.
For example, among 20 patients whose HRM suggested an esophagogastric junction outflow obstruction, 17 showed normal bolus transit on supine swallows and 16 showed normalization of integrated relaxation pressure after adjunctive maneuvers. Similarly, among 10 patients who underwent a barium esophagram, 8 showed normal emptying, 1 showed a temporary delay but no retention, and 1 had an incomplete study. “The overall clinical impression was not of an achalasia variant in any of these 20 patients with [esophagogastric junction outflow obstruction] on HRM, and thus none underwent botulinum toxin injection, pneumatic dilation, or lower-esophageal sphincter myotomy at our center,” the researchers wrote. Among 17 patients who were available for clinical follow-up, 4 underwent empiric dilation, of whom none had mucosal disruption. One patient was diagnosed with dysphagia lusoria based on cross-sectional imaging, while the rest were managed conservatively.
Similarly, among 10 patients with at least 50% ineffective swallows on HRM, 5 showed normal barium emptying and 9 were managed conservatively (the remaining patient underwent cricopharyngeal dilation for concurrent oropharyngeal dysphagia). The strong correlation between HRM and esophagrams in this study indicates that“[n]ormal findings from FLIP panometry can be used to exclude esophageal motility disorders at the time of endoscopy, possibly reducing the need for high-resolution manometry evaluation of some patients,” the investigators concluded. “However, further longitudinal studies are needed to support this approach.”
The work was supported by the Public Health Service and the American College of Gastroenterology. Dr. Baumann reported having no conflicts of interest. Four coinvestigators disclosed relevant ties to Crospon, Given Imaging, Ironwood, Medtronic, Sandhill Scientific, Torax, and other companies..
SOURCE: Baumann AJ et al. Clin Gastroenterol Hepatol 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.040.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Invasive bacterial infections uncommon in afebrile infants with diagnosed AOM
Outpatient management of most afebrile infants with acute otitis media who haven’t been tested for invasive bacterial infection may be reasonable given the low occurrence of adverse events, said Son H. McLaren, MD, MS, of Columbia University, New York, and colleagues.
Dr. McLaren and associates conducted an international cross-sectional study at 33 emergency departments participating in the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics (AAP): 29 in the United States, 2 in Canada and 2 in Spain.
The researchers sought first to assess prevalence of invasive bacterial infections and adverse events tied to acute otitis media (AOM) in infants 90 days and younger. Those who were clinically diagnosed with AOM and presented without fever between January 2007 and December 2017 were included in the study. The presence of fever, they explained, “is a primary driver for more expanded testing and/or empirical treatment of invasive bacterial infection (IBI). Secondarily, they sought to characterize patterns of diagnostic testing and the factors associated with it specifically in this patient population.
Of 5,270 patients screened, 1,637 met study criteria. Included patients were a median age of 68 days. A total of 1,459 (89.1%) met AAP diagnostic criteria for AOM. The remaining 178 patients were examined and found to have more than one of these criteria: 113 had opacification of tympanic membrane, 57 had dull tympanic membrane, 25 had decreased visualization of middle ear structures, 9 had middle ear effusion, 8 had visible tympanic membrane perforation and 5 had decreased tympanic membrane mobility with insufflation. None of the 278 infants with blood cultures had bacteremia, nor were they diagnosed with bacterial meningitis. Two of 645 (0.3%) infants experienced adverse events, as evidenced with 30-day follow-up or history of hospitalization.
Dr. McLaren and colleagues observed that despite a low prevalence of IBI and AOM-associated adverse events, more than one-fifth of patients were prescribed diagnostic testing for IBI and subsequently hospitalized, a practice that appeared more common with younger patients.
Significant testing and hospitalizations persisted despite low prevalence of IBIs
Although diagnostic testing and hospitalizations differed by site, they were, in fact, “substantial in contrast to the low prevalence of IBIs and adverse events,” the researchers noted. “Our data may be used to help guide clinical management of afebrile infants with clinician-diagnosed AOM, who are not included in the current AAP AOM practice guideline,” the authors said. They speculated that this practice may be due, in part, to young-age risk of IBI and the concern for IBI in this population based on febrile infant population data and a general hesitance to begin antibiotics without first evaluating for IBI. They also cited a low prevalence ranging from 0.8% to 2.5% as evidence for low risk of IBI in afebrile infants with AOM.
Also of note, given that roughly three-fourths of infants included in the study were reported to have symptoms of upper respiratory infection that can lead to viral AOM, including these infants who could have a lower likelihood of IBI than those with known bacterial AOM, may have led the researchers to underestimate IBI prevalence. Because existing data do not allow for clear distinction of viral from bacterial AOM without tympanocentesis, and because more than 85% of older patients with clinically diagnosed AOM also have observed bacterial otopathogens, the authors clarify that “it is understandable why clinicians would manage infants with AOM conservatively, regardless of the presence of concurrent viral illnesses.” They also acknowledged that one major challenge in working with infants believed to have AOM is ensuring that it is actually present since it is so hard to diagnose.
Dr. McLaren and colleagues cited several study limitations: 1) completeness and accuracy of data couldn’t be ensured because of the retrospective study design; 2) because not all infants were tested for IBI, its prevalence may have been underestimated; 3) infants whose discharge codes did not include AOM may have been missed, although all infants with positive blood or cerebrospinal fluid cultures were screened for missed AOM diagnosis; and 4) it is important to consider that any issues associated with testing and hospitalization that were identified may have been the result of management decisions driven by factors that cannot be captured retrospectively or by a diagnosis of AOM.
The findings are not generalizable to infants aged younger than 28 days
Finally, the authors cautioned that because the number of infants younger than 28 days was quite small, and it is therefore infinitely more challenging to diagnose AOM for these patients, results of the study should be applied to infants older than 28 days and are not generalizable to febrile infants.
“This report will not resolve the significant challenge faced by clinicians in treating infants aged [younger than] 28 days who have the highest risk of occult bacteremia and systemic spread of a focal bacterial infection,” Joseph Ravera, MD, and M.W. Stevens, MD, of the University of Vermont, Burlington, noted in an accompanying editorial. Previous studies have identified this age group “to be at the highest risk for systemic bacterial involvement and the most difficult to risk stratify on the basis of physical examination findings and initial laboratory results,” they noted. That the subjects aged younger than 28 days in this study had nearly a 50% admission rate illustrates the clinical uncertainty pediatric emergency medicine providers are challenged with, they added. Just 100 (6%) of the 1,637 patients in the study sample were in this age category, which makes it difficult, given the lack of sufficient data, to generalize findings to the youngest infants.
“Despite a paucity of young infants and limitations inherent to the design, this study does contribute to the literature with a robust retrospective data set of afebrile infants between 1 and 3 months of age with an ED diagnosis of AOM ... It certainly provides a base of support for carefully designed prospective studies in which researchers aim to determine the best care for AOM in children under 6 months of age,” reflected Dr. Ravera and Dr. Stevens.
In a separate interview, Karalyn Kinsella, MD, private practice, Cheshire, Conn. noted, “What is confusing is the absence of documented symptoms for infants presenting to the emergency department, as the symptoms they presented with would influence our concern for IBI. Diagnosing AOM in infants under 90 days old is extremely uncommon as an outpatient pediatrician. Although the finding of AOM in an afebrile infant is very rare in the outpatient setting, this study assures us the risk of IBI is almost nonexistent. Therefore, further workup is unnecessary unless providers have clinical suspicions to the contrary.”
Dr. McLaren and colleagues as well as Dr. Ravera, Dr. Stevens, and Dr. Kinsella, had no conflicts of interest and no relevant financial disclosures.
Outpatient management of most afebrile infants with acute otitis media who haven’t been tested for invasive bacterial infection may be reasonable given the low occurrence of adverse events, said Son H. McLaren, MD, MS, of Columbia University, New York, and colleagues.
Dr. McLaren and associates conducted an international cross-sectional study at 33 emergency departments participating in the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics (AAP): 29 in the United States, 2 in Canada and 2 in Spain.
The researchers sought first to assess prevalence of invasive bacterial infections and adverse events tied to acute otitis media (AOM) in infants 90 days and younger. Those who were clinically diagnosed with AOM and presented without fever between January 2007 and December 2017 were included in the study. The presence of fever, they explained, “is a primary driver for more expanded testing and/or empirical treatment of invasive bacterial infection (IBI). Secondarily, they sought to characterize patterns of diagnostic testing and the factors associated with it specifically in this patient population.
Of 5,270 patients screened, 1,637 met study criteria. Included patients were a median age of 68 days. A total of 1,459 (89.1%) met AAP diagnostic criteria for AOM. The remaining 178 patients were examined and found to have more than one of these criteria: 113 had opacification of tympanic membrane, 57 had dull tympanic membrane, 25 had decreased visualization of middle ear structures, 9 had middle ear effusion, 8 had visible tympanic membrane perforation and 5 had decreased tympanic membrane mobility with insufflation. None of the 278 infants with blood cultures had bacteremia, nor were they diagnosed with bacterial meningitis. Two of 645 (0.3%) infants experienced adverse events, as evidenced with 30-day follow-up or history of hospitalization.
Dr. McLaren and colleagues observed that despite a low prevalence of IBI and AOM-associated adverse events, more than one-fifth of patients were prescribed diagnostic testing for IBI and subsequently hospitalized, a practice that appeared more common with younger patients.
Significant testing and hospitalizations persisted despite low prevalence of IBIs
Although diagnostic testing and hospitalizations differed by site, they were, in fact, “substantial in contrast to the low prevalence of IBIs and adverse events,” the researchers noted. “Our data may be used to help guide clinical management of afebrile infants with clinician-diagnosed AOM, who are not included in the current AAP AOM practice guideline,” the authors said. They speculated that this practice may be due, in part, to young-age risk of IBI and the concern for IBI in this population based on febrile infant population data and a general hesitance to begin antibiotics without first evaluating for IBI. They also cited a low prevalence ranging from 0.8% to 2.5% as evidence for low risk of IBI in afebrile infants with AOM.
Also of note, given that roughly three-fourths of infants included in the study were reported to have symptoms of upper respiratory infection that can lead to viral AOM, including these infants who could have a lower likelihood of IBI than those with known bacterial AOM, may have led the researchers to underestimate IBI prevalence. Because existing data do not allow for clear distinction of viral from bacterial AOM without tympanocentesis, and because more than 85% of older patients with clinically diagnosed AOM also have observed bacterial otopathogens, the authors clarify that “it is understandable why clinicians would manage infants with AOM conservatively, regardless of the presence of concurrent viral illnesses.” They also acknowledged that one major challenge in working with infants believed to have AOM is ensuring that it is actually present since it is so hard to diagnose.
Dr. McLaren and colleagues cited several study limitations: 1) completeness and accuracy of data couldn’t be ensured because of the retrospective study design; 2) because not all infants were tested for IBI, its prevalence may have been underestimated; 3) infants whose discharge codes did not include AOM may have been missed, although all infants with positive blood or cerebrospinal fluid cultures were screened for missed AOM diagnosis; and 4) it is important to consider that any issues associated with testing and hospitalization that were identified may have been the result of management decisions driven by factors that cannot be captured retrospectively or by a diagnosis of AOM.
The findings are not generalizable to infants aged younger than 28 days
Finally, the authors cautioned that because the number of infants younger than 28 days was quite small, and it is therefore infinitely more challenging to diagnose AOM for these patients, results of the study should be applied to infants older than 28 days and are not generalizable to febrile infants.
“This report will not resolve the significant challenge faced by clinicians in treating infants aged [younger than] 28 days who have the highest risk of occult bacteremia and systemic spread of a focal bacterial infection,” Joseph Ravera, MD, and M.W. Stevens, MD, of the University of Vermont, Burlington, noted in an accompanying editorial. Previous studies have identified this age group “to be at the highest risk for systemic bacterial involvement and the most difficult to risk stratify on the basis of physical examination findings and initial laboratory results,” they noted. That the subjects aged younger than 28 days in this study had nearly a 50% admission rate illustrates the clinical uncertainty pediatric emergency medicine providers are challenged with, they added. Just 100 (6%) of the 1,637 patients in the study sample were in this age category, which makes it difficult, given the lack of sufficient data, to generalize findings to the youngest infants.
“Despite a paucity of young infants and limitations inherent to the design, this study does contribute to the literature with a robust retrospective data set of afebrile infants between 1 and 3 months of age with an ED diagnosis of AOM ... It certainly provides a base of support for carefully designed prospective studies in which researchers aim to determine the best care for AOM in children under 6 months of age,” reflected Dr. Ravera and Dr. Stevens.
In a separate interview, Karalyn Kinsella, MD, private practice, Cheshire, Conn. noted, “What is confusing is the absence of documented symptoms for infants presenting to the emergency department, as the symptoms they presented with would influence our concern for IBI. Diagnosing AOM in infants under 90 days old is extremely uncommon as an outpatient pediatrician. Although the finding of AOM in an afebrile infant is very rare in the outpatient setting, this study assures us the risk of IBI is almost nonexistent. Therefore, further workup is unnecessary unless providers have clinical suspicions to the contrary.”
Dr. McLaren and colleagues as well as Dr. Ravera, Dr. Stevens, and Dr. Kinsella, had no conflicts of interest and no relevant financial disclosures.
Outpatient management of most afebrile infants with acute otitis media who haven’t been tested for invasive bacterial infection may be reasonable given the low occurrence of adverse events, said Son H. McLaren, MD, MS, of Columbia University, New York, and colleagues.
Dr. McLaren and associates conducted an international cross-sectional study at 33 emergency departments participating in the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics (AAP): 29 in the United States, 2 in Canada and 2 in Spain.
The researchers sought first to assess prevalence of invasive bacterial infections and adverse events tied to acute otitis media (AOM) in infants 90 days and younger. Those who were clinically diagnosed with AOM and presented without fever between January 2007 and December 2017 were included in the study. The presence of fever, they explained, “is a primary driver for more expanded testing and/or empirical treatment of invasive bacterial infection (IBI). Secondarily, they sought to characterize patterns of diagnostic testing and the factors associated with it specifically in this patient population.
Of 5,270 patients screened, 1,637 met study criteria. Included patients were a median age of 68 days. A total of 1,459 (89.1%) met AAP diagnostic criteria for AOM. The remaining 178 patients were examined and found to have more than one of these criteria: 113 had opacification of tympanic membrane, 57 had dull tympanic membrane, 25 had decreased visualization of middle ear structures, 9 had middle ear effusion, 8 had visible tympanic membrane perforation and 5 had decreased tympanic membrane mobility with insufflation. None of the 278 infants with blood cultures had bacteremia, nor were they diagnosed with bacterial meningitis. Two of 645 (0.3%) infants experienced adverse events, as evidenced with 30-day follow-up or history of hospitalization.
Dr. McLaren and colleagues observed that despite a low prevalence of IBI and AOM-associated adverse events, more than one-fifth of patients were prescribed diagnostic testing for IBI and subsequently hospitalized, a practice that appeared more common with younger patients.
Significant testing and hospitalizations persisted despite low prevalence of IBIs
Although diagnostic testing and hospitalizations differed by site, they were, in fact, “substantial in contrast to the low prevalence of IBIs and adverse events,” the researchers noted. “Our data may be used to help guide clinical management of afebrile infants with clinician-diagnosed AOM, who are not included in the current AAP AOM practice guideline,” the authors said. They speculated that this practice may be due, in part, to young-age risk of IBI and the concern for IBI in this population based on febrile infant population data and a general hesitance to begin antibiotics without first evaluating for IBI. They also cited a low prevalence ranging from 0.8% to 2.5% as evidence for low risk of IBI in afebrile infants with AOM.
Also of note, given that roughly three-fourths of infants included in the study were reported to have symptoms of upper respiratory infection that can lead to viral AOM, including these infants who could have a lower likelihood of IBI than those with known bacterial AOM, may have led the researchers to underestimate IBI prevalence. Because existing data do not allow for clear distinction of viral from bacterial AOM without tympanocentesis, and because more than 85% of older patients with clinically diagnosed AOM also have observed bacterial otopathogens, the authors clarify that “it is understandable why clinicians would manage infants with AOM conservatively, regardless of the presence of concurrent viral illnesses.” They also acknowledged that one major challenge in working with infants believed to have AOM is ensuring that it is actually present since it is so hard to diagnose.
Dr. McLaren and colleagues cited several study limitations: 1) completeness and accuracy of data couldn’t be ensured because of the retrospective study design; 2) because not all infants were tested for IBI, its prevalence may have been underestimated; 3) infants whose discharge codes did not include AOM may have been missed, although all infants with positive blood or cerebrospinal fluid cultures were screened for missed AOM diagnosis; and 4) it is important to consider that any issues associated with testing and hospitalization that were identified may have been the result of management decisions driven by factors that cannot be captured retrospectively or by a diagnosis of AOM.
The findings are not generalizable to infants aged younger than 28 days
Finally, the authors cautioned that because the number of infants younger than 28 days was quite small, and it is therefore infinitely more challenging to diagnose AOM for these patients, results of the study should be applied to infants older than 28 days and are not generalizable to febrile infants.
“This report will not resolve the significant challenge faced by clinicians in treating infants aged [younger than] 28 days who have the highest risk of occult bacteremia and systemic spread of a focal bacterial infection,” Joseph Ravera, MD, and M.W. Stevens, MD, of the University of Vermont, Burlington, noted in an accompanying editorial. Previous studies have identified this age group “to be at the highest risk for systemic bacterial involvement and the most difficult to risk stratify on the basis of physical examination findings and initial laboratory results,” they noted. That the subjects aged younger than 28 days in this study had nearly a 50% admission rate illustrates the clinical uncertainty pediatric emergency medicine providers are challenged with, they added. Just 100 (6%) of the 1,637 patients in the study sample were in this age category, which makes it difficult, given the lack of sufficient data, to generalize findings to the youngest infants.
“Despite a paucity of young infants and limitations inherent to the design, this study does contribute to the literature with a robust retrospective data set of afebrile infants between 1 and 3 months of age with an ED diagnosis of AOM ... It certainly provides a base of support for carefully designed prospective studies in which researchers aim to determine the best care for AOM in children under 6 months of age,” reflected Dr. Ravera and Dr. Stevens.
In a separate interview, Karalyn Kinsella, MD, private practice, Cheshire, Conn. noted, “What is confusing is the absence of documented symptoms for infants presenting to the emergency department, as the symptoms they presented with would influence our concern for IBI. Diagnosing AOM in infants under 90 days old is extremely uncommon as an outpatient pediatrician. Although the finding of AOM in an afebrile infant is very rare in the outpatient setting, this study assures us the risk of IBI is almost nonexistent. Therefore, further workup is unnecessary unless providers have clinical suspicions to the contrary.”
Dr. McLaren and colleagues as well as Dr. Ravera, Dr. Stevens, and Dr. Kinsella, had no conflicts of interest and no relevant financial disclosures.
FROM PEDIATRICS
Asthma-COPD overlap: Patients have high disease burden
Patients with asthma–chronic obstructive pulmonary disease overlap (ACO) experienced a higher burden of disease than patients with either asthma or COPD alone, a recent study has found.
Approximately 20% of chronic obstructive airway disease cases are ACO, but data on these patients are limited, as they are often excluded from clinical trials, wrote Sarah A. Hiles, MD, of the University of Newcastle (Australia) and colleagues.
“Comparing the burden of eosinophilic ACO, eosinophilic severe asthma, and eosinophilic COPD may also help contextualize findings from phenotype-targeted treatments in different diagnostic groups, such as the limited success of anti-IL [interleukin]–5 monoclonal antibodies as therapy in eosinophilic COPD,” they said.
In a cross-sectional, observational study published in Respirology the researchers recruited patients aged 18 years and older with a confirmed diagnosis of COPD only (153) severe asthma only (64), or ACO (106). Patients were assessed for demographic and clinical factors including health-related quality of life, past-year exacerbation, and other indicators of disease burden. In addition, patients were identified as having eosinophilic airway disease based on a blood eosinophil count of at least 0.3x109/L.
Overall, eosinophilic airway disease was present in 41% of the patients; 55%, 44%, and 29% for those with ACO, severe asthma, and COPD, respectively. Reports of poor health-related quality of life and past-year exacerbations were similar for eosinophilic patients across all three conditions.
However, patients with eosinophilic ACO experienced significantly more past-year exacerbations, notably those requiring oral corticosteroids, compared with patients with asthma alone. In addition, the cumulative number of past-year exacerbations in patient with eosinophilic disease was 164 in those with ACO, compared with severe asthma alone (44) and COPD alone (59).
Patients with ACO also had significantly higher disease burden based on the St. George’s Respiratory Questionnaire (SGRQ), which assessed functional limitation. “For 100 patients, the cumulative SGRQ score attributable to eosinophilic airways disease in ACO was 2,872.8, which was higher than in severe asthma (1,942.5) or COPD (1,638.1),” the researchers said.
The study was limited by several factors including the cross-sectional design and use of a single measurement to classify eosinophilia, the researchers noted. “The non-eosinophilic group likely included a mix of patients with treated eosinophilia and patients without eosinophilia, regardless of treatment, which is a limitation to consider when interpreting the disease burden estimates in this group,” they added.
However, the results add to the understanding of blood eosinophils in airway disease and the study “supports eosinophilia as a phenotype that spans across disease labels of severe asthma and COPD, and their overlap,” they concluded.
The study was supported by AstraZeneca; lead author Dr. Hiles received a salary through a grant from AstraZeneca to the University of Newcastle while conducting the study. Other coauthors disclosed relationships with companies including AstraZeneca, GlaxoSmithKline, Menarini, and Novartis.
Patients with asthma–chronic obstructive pulmonary disease overlap (ACO) experienced a higher burden of disease than patients with either asthma or COPD alone, a recent study has found.
Approximately 20% of chronic obstructive airway disease cases are ACO, but data on these patients are limited, as they are often excluded from clinical trials, wrote Sarah A. Hiles, MD, of the University of Newcastle (Australia) and colleagues.
“Comparing the burden of eosinophilic ACO, eosinophilic severe asthma, and eosinophilic COPD may also help contextualize findings from phenotype-targeted treatments in different diagnostic groups, such as the limited success of anti-IL [interleukin]–5 monoclonal antibodies as therapy in eosinophilic COPD,” they said.
In a cross-sectional, observational study published in Respirology the researchers recruited patients aged 18 years and older with a confirmed diagnosis of COPD only (153) severe asthma only (64), or ACO (106). Patients were assessed for demographic and clinical factors including health-related quality of life, past-year exacerbation, and other indicators of disease burden. In addition, patients were identified as having eosinophilic airway disease based on a blood eosinophil count of at least 0.3x109/L.
Overall, eosinophilic airway disease was present in 41% of the patients; 55%, 44%, and 29% for those with ACO, severe asthma, and COPD, respectively. Reports of poor health-related quality of life and past-year exacerbations were similar for eosinophilic patients across all three conditions.
However, patients with eosinophilic ACO experienced significantly more past-year exacerbations, notably those requiring oral corticosteroids, compared with patients with asthma alone. In addition, the cumulative number of past-year exacerbations in patient with eosinophilic disease was 164 in those with ACO, compared with severe asthma alone (44) and COPD alone (59).
Patients with ACO also had significantly higher disease burden based on the St. George’s Respiratory Questionnaire (SGRQ), which assessed functional limitation. “For 100 patients, the cumulative SGRQ score attributable to eosinophilic airways disease in ACO was 2,872.8, which was higher than in severe asthma (1,942.5) or COPD (1,638.1),” the researchers said.
The study was limited by several factors including the cross-sectional design and use of a single measurement to classify eosinophilia, the researchers noted. “The non-eosinophilic group likely included a mix of patients with treated eosinophilia and patients without eosinophilia, regardless of treatment, which is a limitation to consider when interpreting the disease burden estimates in this group,” they added.
However, the results add to the understanding of blood eosinophils in airway disease and the study “supports eosinophilia as a phenotype that spans across disease labels of severe asthma and COPD, and their overlap,” they concluded.
The study was supported by AstraZeneca; lead author Dr. Hiles received a salary through a grant from AstraZeneca to the University of Newcastle while conducting the study. Other coauthors disclosed relationships with companies including AstraZeneca, GlaxoSmithKline, Menarini, and Novartis.
Patients with asthma–chronic obstructive pulmonary disease overlap (ACO) experienced a higher burden of disease than patients with either asthma or COPD alone, a recent study has found.
Approximately 20% of chronic obstructive airway disease cases are ACO, but data on these patients are limited, as they are often excluded from clinical trials, wrote Sarah A. Hiles, MD, of the University of Newcastle (Australia) and colleagues.
“Comparing the burden of eosinophilic ACO, eosinophilic severe asthma, and eosinophilic COPD may also help contextualize findings from phenotype-targeted treatments in different diagnostic groups, such as the limited success of anti-IL [interleukin]–5 monoclonal antibodies as therapy in eosinophilic COPD,” they said.
In a cross-sectional, observational study published in Respirology the researchers recruited patients aged 18 years and older with a confirmed diagnosis of COPD only (153) severe asthma only (64), or ACO (106). Patients were assessed for demographic and clinical factors including health-related quality of life, past-year exacerbation, and other indicators of disease burden. In addition, patients were identified as having eosinophilic airway disease based on a blood eosinophil count of at least 0.3x109/L.
Overall, eosinophilic airway disease was present in 41% of the patients; 55%, 44%, and 29% for those with ACO, severe asthma, and COPD, respectively. Reports of poor health-related quality of life and past-year exacerbations were similar for eosinophilic patients across all three conditions.
However, patients with eosinophilic ACO experienced significantly more past-year exacerbations, notably those requiring oral corticosteroids, compared with patients with asthma alone. In addition, the cumulative number of past-year exacerbations in patient with eosinophilic disease was 164 in those with ACO, compared with severe asthma alone (44) and COPD alone (59).
Patients with ACO also had significantly higher disease burden based on the St. George’s Respiratory Questionnaire (SGRQ), which assessed functional limitation. “For 100 patients, the cumulative SGRQ score attributable to eosinophilic airways disease in ACO was 2,872.8, which was higher than in severe asthma (1,942.5) or COPD (1,638.1),” the researchers said.
The study was limited by several factors including the cross-sectional design and use of a single measurement to classify eosinophilia, the researchers noted. “The non-eosinophilic group likely included a mix of patients with treated eosinophilia and patients without eosinophilia, regardless of treatment, which is a limitation to consider when interpreting the disease burden estimates in this group,” they added.
However, the results add to the understanding of blood eosinophils in airway disease and the study “supports eosinophilia as a phenotype that spans across disease labels of severe asthma and COPD, and their overlap,” they concluded.
The study was supported by AstraZeneca; lead author Dr. Hiles received a salary through a grant from AstraZeneca to the University of Newcastle while conducting the study. Other coauthors disclosed relationships with companies including AstraZeneca, GlaxoSmithKline, Menarini, and Novartis.
FROM RESPIROLOGY
To fast or not? The new dieting dilemma
Cardiologist Ethan J. Weiss, MD, followed an intermittent-fasting diet for 7 years. He lost about 3.6 kg (8 lb) and began recommending the approach to friends and patients who wanted to lose weight.
“I liked the way the diet was so simple,” said Dr. Weiss, an associate professor at the Cardiovascular Research Institute, University of California, San Francisco. But he also felt “it was too good to be true because you can eat what you want as long as it’s within a narrow window.”
So when, last year, he conducted a randomized, controlled trial, TREAT, testing such an approach – eating during just 8 hours a day, fasting for the remaining 16 hours – versus an eating plan of three meals a day without restrictions, he was somewhat dismayed to find the group of people who fasted didn’t lose any more weight than the other group.
The approach used in this study is known as time-restricted eating. It involves designating periods of time within the day when people can consume whatever they want; they then “fast” at times outside those eating windows. Other methods include alternate-day fasting, or the well-known 5:2 diet. In the latter, people eat a “normal” amount of around 2,000 calories per day on 5 days of the week, but for the other 2 days, they restrict caloric intake to 500 calories per day.
Intermittent fasting is an umbrella term encompassing all of these different approaches.
Dr. Weiss’s work builds on more than a decade of research into this type of eating plan by scientists, including Krista Varady, PhD, professor of nutrition at the University of Illinois at Chicago, who presented an overview of her own studies last fall at the virtual annual meeting of the European Association for the Study of Diabetes.
Although much of the work has suggested that the shorter duration of eating period in this type of diet leads to lower calorie intake and weight loss while avoiding the need for the tedious calorie-counting of conventional diets, Dr. Weiss’s data – published last year – throws a spanner in the works and now complicates the evidence base.
A promise of simplicity: ‘All you have to do is watch the clock’
Dr. Varady said she, too, is intrigued by the simplicity of intermittent-fasting diets.
In 2018, Dr. Varady and colleagues tested the weight-loss efficacy of 12 weeks of time-restricted feeding in a pilot study of 23 people with obesity.
Participants were permitted an 8-hour eating window (10 a.m. to 6 p.m.) followed by water-only fasting of 16 hours (6 p.m. to 10 a.m.) the next day (sometimes referred to as the 16:8 diet). Researchers measured weight loss and fat mass, as well as metabolic parameters, and compared the active group with 23 matched-control participants who ate freely.
There were no restrictions on type or quantity of food consumed by the control group during the 8-hour period, but individuals in the time-restricted feeding group consumed around 350 calories less than the comparator group.
Dr. Varady thinks this is most likely because of the fact that people normally eat during a 14-hour window and time-restricted feeding cuts that down by 6 hours.
“One of the most beautiful things about time-restricted feeding is that it doesn’t require calorie monitoring,” she explained. “People get burnt out with having to constantly monitor calories. All you have to do is watch the clock.”
Adherence was quite high, she reported, although most people skipped 1 day, often a Saturday, likely because of social engagements.
Weight loss in the time-restricted feeding group was mild to moderate. After 3 months, mean body weight decreased by 2.6%, or approximately 3 kg (7-8 lb), relative to those who ate freely, but this was a significant difference (P < .05).
But the researchers observed little change in metabolic disease risk factors between the groups.
In the time-restricted feeding group, systolic blood pressure dropped from 128 mm Hg to 121 mm Hg over the 12-week period, which was significant relative to the control group (P < .05) but there were no significant changes in fasting glucose, fasting lipids, fasting insulin, or insulin resistance relative to the comparator group.
In contrast to Dr. Varady’s findings, Dr. Weiss’s randomized TREAT trial, which used a similar 16:8 period of time-restricted versus unrestricted eating in 116 individuals with overweight or obesity, did not find greater weight loss in the group restricted to eating within the 8-hour window.
As previously reported by this news organization, those who fasted for 16 hours of each day (n = 59) did lose some weight, compared with the control group (n = 57) over 12 weeks, but the difference in weight loss between the groups was not significant (−0.26 kg; P = .63).
And there were no significant differences in any of the secondary outcomes of fat mass, fasting insulin, fasting glucose, hemoglobin A1c levels, estimated energy intake, total energy expenditure, and resting energy expenditure between the time-restricted eating and regular feeding groups.
“I don’t claim time-restricted eating is dead,” Dr. Weiss said, “but the hope that you can eat for a limited time each day and solve metabolic disease is not there.”
Does the length of the eating window matter?
Following her pilot study of an 8-hour eating window, Dr. Varady conducted further research with 4- or 6-hour eating windows to see if even shorter periods would precipitate greater weight loss, ideally a clinically significant loss of 5% of body weight.
She ran a 2-month randomized, controlled study in people with obesity, published in 2020, which was the first to examine both a 4-hour (3 p.m. to 7 p.m.; n = 19) or 6-hour (1 p.m to 7 p.m.; n = 20) eating window versus a diet without any food restrictions as a control (n = 19) (Cell Metab. 2020;32:366-78.e3).
Dr. Varady explained that they decided to shift the eating window to later in the day for this trial (in contrast to the earlier 8-hour study) to allow people to eat dinner at a sociable time, and thereby hopefully reduce dropouts from the study.
“Unlike with alternate-day fasting, most people find time-restricted feeding easy to incorporate into their lifestyles,” she remarked.
Both the 4- and 6-hour eating window groups experienced a mean 3.2% body weight loss, compared with controls, and this correlated with a 550-calorie reduction in their daily consumption, compared with their baseline calorie intake.
In terms of other outcomes – and in contrast to the 8-hour window study which showed very little changed other than a minor decrease in blood pressure – researchers saw some changes in metabolic risk factors with the 4- and 6-hour eating windows, Dr. Varady reported.
Compared with the control group, fasting insulin decreased in both time-restricted feeding groups by a mean of 15% (P < .05). Insulin resistance also decreased by 25% in the 4-hour group and by 15% in the 6-hour group, compared with the control group. Fasting glucose did not change in either group, however.
The researchers did not observe any effect on blood pressure or plasma lipids in the 4- or 6-hour eating window groups, compared with controls. However, measures of oxidative stress and inflammation decreased in both groups versus controls by approximately 35% (P < .05).
“These findings suggest that this form of severe time-restricted feeding is achievable and can help adults with obesity lose weight, without having to count calories,” Dr. Varady and colleagues conclude.
Is intermittent fasting better for weight loss than calorie restriction?
Ultimately, if weight loss is the primary goal, many want to know how time-restricted feeding compares with conventional daily calorie restriction.
Back in 2017, Dr. Varady published a year-long randomized, controlled study that compared alternate-day fasting with a calorie-restriction diet and a conventional/usual diet among 100 participants with obesity who were otherwise healthy.
Participants on the alternate-day fasting plan (n = 34) consumed 500 calories on fasting days for the first 6 months for weight loss (approximately 25% of energy needs) followed by 125% of energy needs on alternating “feast days”. For an additional 6 months, they ate 1,000 calories on fasting days – aimed at weight maintenance.
Those following the calorie-restriction diet (n = 35) reduced energy intake by 25% (approximately 500 kcal) for the first 6 months for weight loss, followed by enough calories sufficient for weight maintenance (so no further loss nor gain).
However, the study showed alternate-day fasting did not produce better weight loss than conventional calorie counting.
“Over the first 6 months [during the weight-loss period] both groups lost an average of 6% body weight. After 12 months it crept back to 5% weight loss,” reported Dr. Varady.
“Realistically, if the study continued for 2 or 3 years, they probably would have regained much of their weight,” she admitted.
Dr. Varady suspects it might be better for the alternate-day fasting participants to continue eating only 500 calories on their fast day during the weight-loss maintenance period rather than increasing calorie intake during this phase.
Heart rate and blood pressure did not change in either group, while triglycerides decreased in the alternate-day fasting group, and LDL cholesterol decreased in the calorie-restriction group.
Glucose level decreased in the calorie-restriction group but not the alternate-day fasting group, and insulin and HOMA-IR were unaffected in both groups, reported Dr. Varady, noting that these findings were in healthy people with obesity.
In people with obesity and insulin resistance – evaluated as a subgroup in a separate study by Dr. Varady of alternate-day fasting versus daily calorie restriction published in 2019 – she noted that when insulin levels and HOMA-IR were measured, there was a greater reduction in both variables in the fasting group, compared with the calorie-restriction group.
“For people at risk of diabetes, maybe fasting produces more potent effects on glycemic control?” she ventured.
Who fares best with which fasting diets?
Summing up, Dr. Varady provided some practical pointers regarding who she feels is best suited to intermittent fasting and who should avoid it.
Those who binge eat, shift-workers, and frequent snackers do not do well with fasting, she said.
The first 10 days of intermittent fasting are rough, she pointed out, with the most common complaint being headaches.
“Eventually, people do feel an energy boost on fast days, and they say they concentrate better and have lots of energy. People won’t feel lethargic. Also, eating protein on fast days has been shown to keep hunger at bay.”
She cautiously concluded that weight loss with “alternate-day fasting” is quicker than some other methods, at 4.5-7 kg (10-15 lb) in 3 months, but is harder to follow and requires some calorie counting.
“In comparison, with time-restricted feeding, for which there have been very few ... studies to date, weight loss is slower at 2-4.5 kg (5-10 lb) in 3 months, but it is easier to follow and tolerable because you don’t need to count calories.”
Dr. Weiss has reported no relevant financial relationships. Dr. Varady has reported receiving author fees from Hachette for her book, “Every Other Day Diet.” (New York: Hachette, 2013)
A version of this article first appeared on Medscape.com.
Cardiologist Ethan J. Weiss, MD, followed an intermittent-fasting diet for 7 years. He lost about 3.6 kg (8 lb) and began recommending the approach to friends and patients who wanted to lose weight.
“I liked the way the diet was so simple,” said Dr. Weiss, an associate professor at the Cardiovascular Research Institute, University of California, San Francisco. But he also felt “it was too good to be true because you can eat what you want as long as it’s within a narrow window.”
So when, last year, he conducted a randomized, controlled trial, TREAT, testing such an approach – eating during just 8 hours a day, fasting for the remaining 16 hours – versus an eating plan of three meals a day without restrictions, he was somewhat dismayed to find the group of people who fasted didn’t lose any more weight than the other group.
The approach used in this study is known as time-restricted eating. It involves designating periods of time within the day when people can consume whatever they want; they then “fast” at times outside those eating windows. Other methods include alternate-day fasting, or the well-known 5:2 diet. In the latter, people eat a “normal” amount of around 2,000 calories per day on 5 days of the week, but for the other 2 days, they restrict caloric intake to 500 calories per day.
Intermittent fasting is an umbrella term encompassing all of these different approaches.
Dr. Weiss’s work builds on more than a decade of research into this type of eating plan by scientists, including Krista Varady, PhD, professor of nutrition at the University of Illinois at Chicago, who presented an overview of her own studies last fall at the virtual annual meeting of the European Association for the Study of Diabetes.
Although much of the work has suggested that the shorter duration of eating period in this type of diet leads to lower calorie intake and weight loss while avoiding the need for the tedious calorie-counting of conventional diets, Dr. Weiss’s data – published last year – throws a spanner in the works and now complicates the evidence base.
A promise of simplicity: ‘All you have to do is watch the clock’
Dr. Varady said she, too, is intrigued by the simplicity of intermittent-fasting diets.
In 2018, Dr. Varady and colleagues tested the weight-loss efficacy of 12 weeks of time-restricted feeding in a pilot study of 23 people with obesity.
Participants were permitted an 8-hour eating window (10 a.m. to 6 p.m.) followed by water-only fasting of 16 hours (6 p.m. to 10 a.m.) the next day (sometimes referred to as the 16:8 diet). Researchers measured weight loss and fat mass, as well as metabolic parameters, and compared the active group with 23 matched-control participants who ate freely.
There were no restrictions on type or quantity of food consumed by the control group during the 8-hour period, but individuals in the time-restricted feeding group consumed around 350 calories less than the comparator group.
Dr. Varady thinks this is most likely because of the fact that people normally eat during a 14-hour window and time-restricted feeding cuts that down by 6 hours.
“One of the most beautiful things about time-restricted feeding is that it doesn’t require calorie monitoring,” she explained. “People get burnt out with having to constantly monitor calories. All you have to do is watch the clock.”
Adherence was quite high, she reported, although most people skipped 1 day, often a Saturday, likely because of social engagements.
Weight loss in the time-restricted feeding group was mild to moderate. After 3 months, mean body weight decreased by 2.6%, or approximately 3 kg (7-8 lb), relative to those who ate freely, but this was a significant difference (P < .05).
But the researchers observed little change in metabolic disease risk factors between the groups.
In the time-restricted feeding group, systolic blood pressure dropped from 128 mm Hg to 121 mm Hg over the 12-week period, which was significant relative to the control group (P < .05) but there were no significant changes in fasting glucose, fasting lipids, fasting insulin, or insulin resistance relative to the comparator group.
In contrast to Dr. Varady’s findings, Dr. Weiss’s randomized TREAT trial, which used a similar 16:8 period of time-restricted versus unrestricted eating in 116 individuals with overweight or obesity, did not find greater weight loss in the group restricted to eating within the 8-hour window.
As previously reported by this news organization, those who fasted for 16 hours of each day (n = 59) did lose some weight, compared with the control group (n = 57) over 12 weeks, but the difference in weight loss between the groups was not significant (−0.26 kg; P = .63).
And there were no significant differences in any of the secondary outcomes of fat mass, fasting insulin, fasting glucose, hemoglobin A1c levels, estimated energy intake, total energy expenditure, and resting energy expenditure between the time-restricted eating and regular feeding groups.
“I don’t claim time-restricted eating is dead,” Dr. Weiss said, “but the hope that you can eat for a limited time each day and solve metabolic disease is not there.”
Does the length of the eating window matter?
Following her pilot study of an 8-hour eating window, Dr. Varady conducted further research with 4- or 6-hour eating windows to see if even shorter periods would precipitate greater weight loss, ideally a clinically significant loss of 5% of body weight.
She ran a 2-month randomized, controlled study in people with obesity, published in 2020, which was the first to examine both a 4-hour (3 p.m. to 7 p.m.; n = 19) or 6-hour (1 p.m to 7 p.m.; n = 20) eating window versus a diet without any food restrictions as a control (n = 19) (Cell Metab. 2020;32:366-78.e3).
Dr. Varady explained that they decided to shift the eating window to later in the day for this trial (in contrast to the earlier 8-hour study) to allow people to eat dinner at a sociable time, and thereby hopefully reduce dropouts from the study.
“Unlike with alternate-day fasting, most people find time-restricted feeding easy to incorporate into their lifestyles,” she remarked.
Both the 4- and 6-hour eating window groups experienced a mean 3.2% body weight loss, compared with controls, and this correlated with a 550-calorie reduction in their daily consumption, compared with their baseline calorie intake.
In terms of other outcomes – and in contrast to the 8-hour window study which showed very little changed other than a minor decrease in blood pressure – researchers saw some changes in metabolic risk factors with the 4- and 6-hour eating windows, Dr. Varady reported.
Compared with the control group, fasting insulin decreased in both time-restricted feeding groups by a mean of 15% (P < .05). Insulin resistance also decreased by 25% in the 4-hour group and by 15% in the 6-hour group, compared with the control group. Fasting glucose did not change in either group, however.
The researchers did not observe any effect on blood pressure or plasma lipids in the 4- or 6-hour eating window groups, compared with controls. However, measures of oxidative stress and inflammation decreased in both groups versus controls by approximately 35% (P < .05).
“These findings suggest that this form of severe time-restricted feeding is achievable and can help adults with obesity lose weight, without having to count calories,” Dr. Varady and colleagues conclude.
Is intermittent fasting better for weight loss than calorie restriction?
Ultimately, if weight loss is the primary goal, many want to know how time-restricted feeding compares with conventional daily calorie restriction.
Back in 2017, Dr. Varady published a year-long randomized, controlled study that compared alternate-day fasting with a calorie-restriction diet and a conventional/usual diet among 100 participants with obesity who were otherwise healthy.
Participants on the alternate-day fasting plan (n = 34) consumed 500 calories on fasting days for the first 6 months for weight loss (approximately 25% of energy needs) followed by 125% of energy needs on alternating “feast days”. For an additional 6 months, they ate 1,000 calories on fasting days – aimed at weight maintenance.
Those following the calorie-restriction diet (n = 35) reduced energy intake by 25% (approximately 500 kcal) for the first 6 months for weight loss, followed by enough calories sufficient for weight maintenance (so no further loss nor gain).
However, the study showed alternate-day fasting did not produce better weight loss than conventional calorie counting.
“Over the first 6 months [during the weight-loss period] both groups lost an average of 6% body weight. After 12 months it crept back to 5% weight loss,” reported Dr. Varady.
“Realistically, if the study continued for 2 or 3 years, they probably would have regained much of their weight,” she admitted.
Dr. Varady suspects it might be better for the alternate-day fasting participants to continue eating only 500 calories on their fast day during the weight-loss maintenance period rather than increasing calorie intake during this phase.
Heart rate and blood pressure did not change in either group, while triglycerides decreased in the alternate-day fasting group, and LDL cholesterol decreased in the calorie-restriction group.
Glucose level decreased in the calorie-restriction group but not the alternate-day fasting group, and insulin and HOMA-IR were unaffected in both groups, reported Dr. Varady, noting that these findings were in healthy people with obesity.
In people with obesity and insulin resistance – evaluated as a subgroup in a separate study by Dr. Varady of alternate-day fasting versus daily calorie restriction published in 2019 – she noted that when insulin levels and HOMA-IR were measured, there was a greater reduction in both variables in the fasting group, compared with the calorie-restriction group.
“For people at risk of diabetes, maybe fasting produces more potent effects on glycemic control?” she ventured.
Who fares best with which fasting diets?
Summing up, Dr. Varady provided some practical pointers regarding who she feels is best suited to intermittent fasting and who should avoid it.
Those who binge eat, shift-workers, and frequent snackers do not do well with fasting, she said.
The first 10 days of intermittent fasting are rough, she pointed out, with the most common complaint being headaches.
“Eventually, people do feel an energy boost on fast days, and they say they concentrate better and have lots of energy. People won’t feel lethargic. Also, eating protein on fast days has been shown to keep hunger at bay.”
She cautiously concluded that weight loss with “alternate-day fasting” is quicker than some other methods, at 4.5-7 kg (10-15 lb) in 3 months, but is harder to follow and requires some calorie counting.
“In comparison, with time-restricted feeding, for which there have been very few ... studies to date, weight loss is slower at 2-4.5 kg (5-10 lb) in 3 months, but it is easier to follow and tolerable because you don’t need to count calories.”
Dr. Weiss has reported no relevant financial relationships. Dr. Varady has reported receiving author fees from Hachette for her book, “Every Other Day Diet.” (New York: Hachette, 2013)
A version of this article first appeared on Medscape.com.
Cardiologist Ethan J. Weiss, MD, followed an intermittent-fasting diet for 7 years. He lost about 3.6 kg (8 lb) and began recommending the approach to friends and patients who wanted to lose weight.
“I liked the way the diet was so simple,” said Dr. Weiss, an associate professor at the Cardiovascular Research Institute, University of California, San Francisco. But he also felt “it was too good to be true because you can eat what you want as long as it’s within a narrow window.”
So when, last year, he conducted a randomized, controlled trial, TREAT, testing such an approach – eating during just 8 hours a day, fasting for the remaining 16 hours – versus an eating plan of three meals a day without restrictions, he was somewhat dismayed to find the group of people who fasted didn’t lose any more weight than the other group.
The approach used in this study is known as time-restricted eating. It involves designating periods of time within the day when people can consume whatever they want; they then “fast” at times outside those eating windows. Other methods include alternate-day fasting, or the well-known 5:2 diet. In the latter, people eat a “normal” amount of around 2,000 calories per day on 5 days of the week, but for the other 2 days, they restrict caloric intake to 500 calories per day.
Intermittent fasting is an umbrella term encompassing all of these different approaches.
Dr. Weiss’s work builds on more than a decade of research into this type of eating plan by scientists, including Krista Varady, PhD, professor of nutrition at the University of Illinois at Chicago, who presented an overview of her own studies last fall at the virtual annual meeting of the European Association for the Study of Diabetes.
Although much of the work has suggested that the shorter duration of eating period in this type of diet leads to lower calorie intake and weight loss while avoiding the need for the tedious calorie-counting of conventional diets, Dr. Weiss’s data – published last year – throws a spanner in the works and now complicates the evidence base.
A promise of simplicity: ‘All you have to do is watch the clock’
Dr. Varady said she, too, is intrigued by the simplicity of intermittent-fasting diets.
In 2018, Dr. Varady and colleagues tested the weight-loss efficacy of 12 weeks of time-restricted feeding in a pilot study of 23 people with obesity.
Participants were permitted an 8-hour eating window (10 a.m. to 6 p.m.) followed by water-only fasting of 16 hours (6 p.m. to 10 a.m.) the next day (sometimes referred to as the 16:8 diet). Researchers measured weight loss and fat mass, as well as metabolic parameters, and compared the active group with 23 matched-control participants who ate freely.
There were no restrictions on type or quantity of food consumed by the control group during the 8-hour period, but individuals in the time-restricted feeding group consumed around 350 calories less than the comparator group.
Dr. Varady thinks this is most likely because of the fact that people normally eat during a 14-hour window and time-restricted feeding cuts that down by 6 hours.
“One of the most beautiful things about time-restricted feeding is that it doesn’t require calorie monitoring,” she explained. “People get burnt out with having to constantly monitor calories. All you have to do is watch the clock.”
Adherence was quite high, she reported, although most people skipped 1 day, often a Saturday, likely because of social engagements.
Weight loss in the time-restricted feeding group was mild to moderate. After 3 months, mean body weight decreased by 2.6%, or approximately 3 kg (7-8 lb), relative to those who ate freely, but this was a significant difference (P < .05).
But the researchers observed little change in metabolic disease risk factors between the groups.
In the time-restricted feeding group, systolic blood pressure dropped from 128 mm Hg to 121 mm Hg over the 12-week period, which was significant relative to the control group (P < .05) but there were no significant changes in fasting glucose, fasting lipids, fasting insulin, or insulin resistance relative to the comparator group.
In contrast to Dr. Varady’s findings, Dr. Weiss’s randomized TREAT trial, which used a similar 16:8 period of time-restricted versus unrestricted eating in 116 individuals with overweight or obesity, did not find greater weight loss in the group restricted to eating within the 8-hour window.
As previously reported by this news organization, those who fasted for 16 hours of each day (n = 59) did lose some weight, compared with the control group (n = 57) over 12 weeks, but the difference in weight loss between the groups was not significant (−0.26 kg; P = .63).
And there were no significant differences in any of the secondary outcomes of fat mass, fasting insulin, fasting glucose, hemoglobin A1c levels, estimated energy intake, total energy expenditure, and resting energy expenditure between the time-restricted eating and regular feeding groups.
“I don’t claim time-restricted eating is dead,” Dr. Weiss said, “but the hope that you can eat for a limited time each day and solve metabolic disease is not there.”
Does the length of the eating window matter?
Following her pilot study of an 8-hour eating window, Dr. Varady conducted further research with 4- or 6-hour eating windows to see if even shorter periods would precipitate greater weight loss, ideally a clinically significant loss of 5% of body weight.
She ran a 2-month randomized, controlled study in people with obesity, published in 2020, which was the first to examine both a 4-hour (3 p.m. to 7 p.m.; n = 19) or 6-hour (1 p.m to 7 p.m.; n = 20) eating window versus a diet without any food restrictions as a control (n = 19) (Cell Metab. 2020;32:366-78.e3).
Dr. Varady explained that they decided to shift the eating window to later in the day for this trial (in contrast to the earlier 8-hour study) to allow people to eat dinner at a sociable time, and thereby hopefully reduce dropouts from the study.
“Unlike with alternate-day fasting, most people find time-restricted feeding easy to incorporate into their lifestyles,” she remarked.
Both the 4- and 6-hour eating window groups experienced a mean 3.2% body weight loss, compared with controls, and this correlated with a 550-calorie reduction in their daily consumption, compared with their baseline calorie intake.
In terms of other outcomes – and in contrast to the 8-hour window study which showed very little changed other than a minor decrease in blood pressure – researchers saw some changes in metabolic risk factors with the 4- and 6-hour eating windows, Dr. Varady reported.
Compared with the control group, fasting insulin decreased in both time-restricted feeding groups by a mean of 15% (P < .05). Insulin resistance also decreased by 25% in the 4-hour group and by 15% in the 6-hour group, compared with the control group. Fasting glucose did not change in either group, however.
The researchers did not observe any effect on blood pressure or plasma lipids in the 4- or 6-hour eating window groups, compared with controls. However, measures of oxidative stress and inflammation decreased in both groups versus controls by approximately 35% (P < .05).
“These findings suggest that this form of severe time-restricted feeding is achievable and can help adults with obesity lose weight, without having to count calories,” Dr. Varady and colleagues conclude.
Is intermittent fasting better for weight loss than calorie restriction?
Ultimately, if weight loss is the primary goal, many want to know how time-restricted feeding compares with conventional daily calorie restriction.
Back in 2017, Dr. Varady published a year-long randomized, controlled study that compared alternate-day fasting with a calorie-restriction diet and a conventional/usual diet among 100 participants with obesity who were otherwise healthy.
Participants on the alternate-day fasting plan (n = 34) consumed 500 calories on fasting days for the first 6 months for weight loss (approximately 25% of energy needs) followed by 125% of energy needs on alternating “feast days”. For an additional 6 months, they ate 1,000 calories on fasting days – aimed at weight maintenance.
Those following the calorie-restriction diet (n = 35) reduced energy intake by 25% (approximately 500 kcal) for the first 6 months for weight loss, followed by enough calories sufficient for weight maintenance (so no further loss nor gain).
However, the study showed alternate-day fasting did not produce better weight loss than conventional calorie counting.
“Over the first 6 months [during the weight-loss period] both groups lost an average of 6% body weight. After 12 months it crept back to 5% weight loss,” reported Dr. Varady.
“Realistically, if the study continued for 2 or 3 years, they probably would have regained much of their weight,” she admitted.
Dr. Varady suspects it might be better for the alternate-day fasting participants to continue eating only 500 calories on their fast day during the weight-loss maintenance period rather than increasing calorie intake during this phase.
Heart rate and blood pressure did not change in either group, while triglycerides decreased in the alternate-day fasting group, and LDL cholesterol decreased in the calorie-restriction group.
Glucose level decreased in the calorie-restriction group but not the alternate-day fasting group, and insulin and HOMA-IR were unaffected in both groups, reported Dr. Varady, noting that these findings were in healthy people with obesity.
In people with obesity and insulin resistance – evaluated as a subgroup in a separate study by Dr. Varady of alternate-day fasting versus daily calorie restriction published in 2019 – she noted that when insulin levels and HOMA-IR were measured, there was a greater reduction in both variables in the fasting group, compared with the calorie-restriction group.
“For people at risk of diabetes, maybe fasting produces more potent effects on glycemic control?” she ventured.
Who fares best with which fasting diets?
Summing up, Dr. Varady provided some practical pointers regarding who she feels is best suited to intermittent fasting and who should avoid it.
Those who binge eat, shift-workers, and frequent snackers do not do well with fasting, she said.
The first 10 days of intermittent fasting are rough, she pointed out, with the most common complaint being headaches.
“Eventually, people do feel an energy boost on fast days, and they say they concentrate better and have lots of energy. People won’t feel lethargic. Also, eating protein on fast days has been shown to keep hunger at bay.”
She cautiously concluded that weight loss with “alternate-day fasting” is quicker than some other methods, at 4.5-7 kg (10-15 lb) in 3 months, but is harder to follow and requires some calorie counting.
“In comparison, with time-restricted feeding, for which there have been very few ... studies to date, weight loss is slower at 2-4.5 kg (5-10 lb) in 3 months, but it is easier to follow and tolerable because you don’t need to count calories.”
Dr. Weiss has reported no relevant financial relationships. Dr. Varady has reported receiving author fees from Hachette for her book, “Every Other Day Diet.” (New York: Hachette, 2013)
A version of this article first appeared on Medscape.com.