User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Despite An AI Assist, Imaging Study Shows Disparities in Diagnosing Different Skin Tones
When clinicians in a large-scale study viewed a series of digital images that showed skin diseases across skin tones and were asked to make a diagnosis, the accuracy was 38% among dermatologists and 19% among primary care physicians (PCPs). But when decision support from a deep learning system (DLS) was introduced, diagnostic accuracy increased by 33% among dermatologists and 69% among PCPs, results from a multicenter study showed.
However, the researchers found that across all images, diseases in dark skin (Fitzpatrick skin types 5 and 6) were diagnosed less accurately than diseases in light skin (Fitzpatrick skin types 1-4).
“,” researchers led by Matthew Groh, PhD, of Northwestern University’s Kellogg School of Management, wrote in their study, published online in Nature Medicine.
For the study, 389 board-certified dermatologists and 450 PCPs in 39 countries were presented with 364 images to view spanning 46 skin diseases and asked to submit up to four differential diagnoses. Nearly 80% of the images were of 8 diseases: atopic dermatitis, cutaneous T-cell lymphoma (CTCL), dermatomyositis, lichen planus, Lyme disease, pityriasis rosea, pityriasis rubra pilaris, and secondary syphilis.
Dermatologists and PCPs achieved a diagnostic accuracy of 38% and 19%, respectively, but both groups of clinicians were 4 percentage points less accurate for diagnosis of images of dark skin as compared with light skin. With assistance from DLS decision support, diagnostic accuracy increased by 33% among dermatologists and 69% among primary care physicians. Among dermatologists, DLS support generally increased diagnostic accuracy evenly across skin tones. However, among PCPs, DLS support increased their diagnostic accuracy more in light skin tones than in dark ones.
In the survey component of the study, when the participants were asked, “Do you feel you received sufficient training for diagnosing skin diseases in patients with skin of color (non-white patients)?” 67% of all PCPs and 33% of all dermatologists responded no. “Furthermore, we have found differences in how often BCDs [board-certified dermatologists] and PCPs refer patients with light and dark skin for biopsy,” the authors wrote. “Specifically, for CTCL (a life-threatening disease), we found that both BCDs and PCPs report that they would refer patients for biopsy significantly more often in light skin than dark skin. Moreover, for the common skin diseases atopic dermatitis and pityriasis rosea, we found that BCDs report they would refer patients for biopsy more often in dark skin than light skin, which creates an unnecessary overburden on patients with dark skin.”
In a press release about the study, Dr. Groh emphasized that he and other scientists who investigate human-computer interaction “have to find a way to incorporate underrepresented demographics in our research. That way we will be ready to accurately implement these models in the real world and build AI systems that serve as tools that are designed to avoid the kind of systematic errors we know humans and machines are prone to. Then you can update curricula, you can change norms in different fields and hopefully everyone gets better.”
Ronald Moy, MD, a dermatologist who practices in Beverly Hills, Calif., who was asked to comment on the work, said that the study contributes insights into physician-AI interaction and highlights the need for further training on diagnosing skin diseases in people with darker skin tones. “The strengths of this study include its large sample size of dermatologists and primary care physicians, use of quality-controlled images across skin tones, and thorough evaluation of diagnostic accuracy with and without AI assistance,” said Dr. Moy, who is a past president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, and the American Board of Facial Cosmetic Surgery.
“The study is limited to diagnosis and skin tone estimation based purely on a single image, which does not fully represent a clinical evaluation,” he added. However, “it does provide important benchmark data on diagnostic accuracy disparities across skin tones, but also demonstrates that while AI assistance can improve overall diagnostic accuracy, it may exacerbate disparities for non-specialists.”
Funding for the study was provided by MIT Media Lab consortium members and the Harold Horowitz Student Research Fund. One of the study authors, P. Murali Doraiswamy, MBBS, disclosed that he has received grants, advisory fees, and/or stock from several biotechnology companies outside the scope of this work and that he is a co-inventor on several patents through Duke University. The remaining authors reported having no disclosures. Dr. Moy reported having no disclosures.
When clinicians in a large-scale study viewed a series of digital images that showed skin diseases across skin tones and were asked to make a diagnosis, the accuracy was 38% among dermatologists and 19% among primary care physicians (PCPs). But when decision support from a deep learning system (DLS) was introduced, diagnostic accuracy increased by 33% among dermatologists and 69% among PCPs, results from a multicenter study showed.
However, the researchers found that across all images, diseases in dark skin (Fitzpatrick skin types 5 and 6) were diagnosed less accurately than diseases in light skin (Fitzpatrick skin types 1-4).
“,” researchers led by Matthew Groh, PhD, of Northwestern University’s Kellogg School of Management, wrote in their study, published online in Nature Medicine.
For the study, 389 board-certified dermatologists and 450 PCPs in 39 countries were presented with 364 images to view spanning 46 skin diseases and asked to submit up to four differential diagnoses. Nearly 80% of the images were of 8 diseases: atopic dermatitis, cutaneous T-cell lymphoma (CTCL), dermatomyositis, lichen planus, Lyme disease, pityriasis rosea, pityriasis rubra pilaris, and secondary syphilis.
Dermatologists and PCPs achieved a diagnostic accuracy of 38% and 19%, respectively, but both groups of clinicians were 4 percentage points less accurate for diagnosis of images of dark skin as compared with light skin. With assistance from DLS decision support, diagnostic accuracy increased by 33% among dermatologists and 69% among primary care physicians. Among dermatologists, DLS support generally increased diagnostic accuracy evenly across skin tones. However, among PCPs, DLS support increased their diagnostic accuracy more in light skin tones than in dark ones.
In the survey component of the study, when the participants were asked, “Do you feel you received sufficient training for diagnosing skin diseases in patients with skin of color (non-white patients)?” 67% of all PCPs and 33% of all dermatologists responded no. “Furthermore, we have found differences in how often BCDs [board-certified dermatologists] and PCPs refer patients with light and dark skin for biopsy,” the authors wrote. “Specifically, for CTCL (a life-threatening disease), we found that both BCDs and PCPs report that they would refer patients for biopsy significantly more often in light skin than dark skin. Moreover, for the common skin diseases atopic dermatitis and pityriasis rosea, we found that BCDs report they would refer patients for biopsy more often in dark skin than light skin, which creates an unnecessary overburden on patients with dark skin.”
In a press release about the study, Dr. Groh emphasized that he and other scientists who investigate human-computer interaction “have to find a way to incorporate underrepresented demographics in our research. That way we will be ready to accurately implement these models in the real world and build AI systems that serve as tools that are designed to avoid the kind of systematic errors we know humans and machines are prone to. Then you can update curricula, you can change norms in different fields and hopefully everyone gets better.”
Ronald Moy, MD, a dermatologist who practices in Beverly Hills, Calif., who was asked to comment on the work, said that the study contributes insights into physician-AI interaction and highlights the need for further training on diagnosing skin diseases in people with darker skin tones. “The strengths of this study include its large sample size of dermatologists and primary care physicians, use of quality-controlled images across skin tones, and thorough evaluation of diagnostic accuracy with and without AI assistance,” said Dr. Moy, who is a past president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, and the American Board of Facial Cosmetic Surgery.
“The study is limited to diagnosis and skin tone estimation based purely on a single image, which does not fully represent a clinical evaluation,” he added. However, “it does provide important benchmark data on diagnostic accuracy disparities across skin tones, but also demonstrates that while AI assistance can improve overall diagnostic accuracy, it may exacerbate disparities for non-specialists.”
Funding for the study was provided by MIT Media Lab consortium members and the Harold Horowitz Student Research Fund. One of the study authors, P. Murali Doraiswamy, MBBS, disclosed that he has received grants, advisory fees, and/or stock from several biotechnology companies outside the scope of this work and that he is a co-inventor on several patents through Duke University. The remaining authors reported having no disclosures. Dr. Moy reported having no disclosures.
When clinicians in a large-scale study viewed a series of digital images that showed skin diseases across skin tones and were asked to make a diagnosis, the accuracy was 38% among dermatologists and 19% among primary care physicians (PCPs). But when decision support from a deep learning system (DLS) was introduced, diagnostic accuracy increased by 33% among dermatologists and 69% among PCPs, results from a multicenter study showed.
However, the researchers found that across all images, diseases in dark skin (Fitzpatrick skin types 5 and 6) were diagnosed less accurately than diseases in light skin (Fitzpatrick skin types 1-4).
“,” researchers led by Matthew Groh, PhD, of Northwestern University’s Kellogg School of Management, wrote in their study, published online in Nature Medicine.
For the study, 389 board-certified dermatologists and 450 PCPs in 39 countries were presented with 364 images to view spanning 46 skin diseases and asked to submit up to four differential diagnoses. Nearly 80% of the images were of 8 diseases: atopic dermatitis, cutaneous T-cell lymphoma (CTCL), dermatomyositis, lichen planus, Lyme disease, pityriasis rosea, pityriasis rubra pilaris, and secondary syphilis.
Dermatologists and PCPs achieved a diagnostic accuracy of 38% and 19%, respectively, but both groups of clinicians were 4 percentage points less accurate for diagnosis of images of dark skin as compared with light skin. With assistance from DLS decision support, diagnostic accuracy increased by 33% among dermatologists and 69% among primary care physicians. Among dermatologists, DLS support generally increased diagnostic accuracy evenly across skin tones. However, among PCPs, DLS support increased their diagnostic accuracy more in light skin tones than in dark ones.
In the survey component of the study, when the participants were asked, “Do you feel you received sufficient training for diagnosing skin diseases in patients with skin of color (non-white patients)?” 67% of all PCPs and 33% of all dermatologists responded no. “Furthermore, we have found differences in how often BCDs [board-certified dermatologists] and PCPs refer patients with light and dark skin for biopsy,” the authors wrote. “Specifically, for CTCL (a life-threatening disease), we found that both BCDs and PCPs report that they would refer patients for biopsy significantly more often in light skin than dark skin. Moreover, for the common skin diseases atopic dermatitis and pityriasis rosea, we found that BCDs report they would refer patients for biopsy more often in dark skin than light skin, which creates an unnecessary overburden on patients with dark skin.”
In a press release about the study, Dr. Groh emphasized that he and other scientists who investigate human-computer interaction “have to find a way to incorporate underrepresented demographics in our research. That way we will be ready to accurately implement these models in the real world and build AI systems that serve as tools that are designed to avoid the kind of systematic errors we know humans and machines are prone to. Then you can update curricula, you can change norms in different fields and hopefully everyone gets better.”
Ronald Moy, MD, a dermatologist who practices in Beverly Hills, Calif., who was asked to comment on the work, said that the study contributes insights into physician-AI interaction and highlights the need for further training on diagnosing skin diseases in people with darker skin tones. “The strengths of this study include its large sample size of dermatologists and primary care physicians, use of quality-controlled images across skin tones, and thorough evaluation of diagnostic accuracy with and without AI assistance,” said Dr. Moy, who is a past president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, and the American Board of Facial Cosmetic Surgery.
“The study is limited to diagnosis and skin tone estimation based purely on a single image, which does not fully represent a clinical evaluation,” he added. However, “it does provide important benchmark data on diagnostic accuracy disparities across skin tones, but also demonstrates that while AI assistance can improve overall diagnostic accuracy, it may exacerbate disparities for non-specialists.”
Funding for the study was provided by MIT Media Lab consortium members and the Harold Horowitz Student Research Fund. One of the study authors, P. Murali Doraiswamy, MBBS, disclosed that he has received grants, advisory fees, and/or stock from several biotechnology companies outside the scope of this work and that he is a co-inventor on several patents through Duke University. The remaining authors reported having no disclosures. Dr. Moy reported having no disclosures.
FROM NATURE MEDICINE
Preventing Gout Flares and Hospitalizations Means Targeting These Serum Urate Levels
Clinical efforts to get patients with a history of gout to reach specific target serum urate (SU) levels less than either 5 or 6 mg/dL could prevent the great majority of gout flares and hospitalizations for them, according to a new study that tracked patients for a mean of 8.3 years.
The findings, which appeared February 6 in JAMA, “support the value of target serum urate levels in gout flare prevention in primary care, where most gout patients are treated,” rheumatologist and study coauthor Hyon K. Choi, MD, DrPH, of Harvard Medical School and Massachusetts General Hospital, Boston, told this news organization. However, Dr. Choi noted that “the value of relying on target urate levels is not accepted in primary care practice,” and the author of an accompanying commentary said that the jury is still out about the best strategy to prevent flares.
Gout is caused by monosodium urate crystallization within the joints, which occurs when SU levels exceed the saturation point for uric acid crystallization in the body: approximately 6.8 mg/dL. “Studies have found strongly graded associations between serum urate levels above the saturation point and the risk of developing new cases of gout among individuals without gout at baseline,” Dr. Choi said. “However, associations between serum urate levels and the risk of recurrent flares among preexisting gout patients, which is relevant to clinical gout care practice, has not been established.”
Dr. Choi added that “despite the emphasis in US and European rheumatology guidelines on the use of urate-lowering therapy to treat-to-target serum urate level — eg, under 6 or 5 mg/dL — the proportions of flares associated with such target urate levels remained unknown.”
Study Shows Relationship Between SU Levels and Recurrent Flares
For the study, researchers tracked 3613 patients aged 40-69 with gout in the UK Biobank database from 2006-2010 to 2017 or 2020. The patients, 86% of whom were men, had a mean age of 60 years and about 96% were White.
Among the patients, 1773 new episodes of acute gout occurred in 27% of the patients (16% had one episode, 6% had two episodes, and 5% had at least three episodes). These were treated in primary care or required hospitalizations. The other 73% of patients had no new acute gout episodes.
Overall, 95% of flares occurred in those with baseline SU levels ≥ 6 mg/dL, and 98% occurred in those with levels ≥ 5 mg/dL.
Patients with baseline SU levels < 6.0 mg/dL had an acute gout flare rate of 10.6 per 1000 person-years. In comparison, relative risks for acute gout flares per 1000 person-years were 3.16 at baseline SU levels of 6.0-6.9 mg/dL, 6.20 for 7.0-7.9 mg/dL, 7.70 for 8.0-8.9 mg/dL, 9.80 for 9.0-9.9 mg/dL, and 11.26 for > 10 mg/dL after adjustment for various possible confounders (P < .001).
The researchers identified 64 hospitalizations with gout as the main discharge diagnosis, and 97% occurred in patients with baseline SU levels ≥ 6 mg/dL. All were in patients with baseline SU levels ≥ 5 mg/dL.
“An important feature of this study was that serum urate measurements were obtained from all gout patients at the study baseline, irrespective of clinical needs or flare status,” Dr. Choi said. “Prior studies failed to reveal the truly compelling nature of relations between serum urate levels and recurrent flares among preexisting gout patients.”
As for the cost of SU tests, Dr. Choi said they can run as low as $2. “Portable tests similar to home glucose measurement for diabetes patients are also being adopted by certain gout care practices,” he said.
The findings matter, Dr. Choi said, because SU is not tracked in the “vast majority of gout patients” in primary care. Instead, primary care doctors — as per the guidelines of the American College of Physicians — often adopt an approach that treats symptoms as needed instead of tracking and lowering SU levels, he said. In fact, “95% and 98% of gout flares can be potentially preventable at the population level if serum urate levels < 6 and < 5 mg/dL can be met, respectively, and 100% of hospitalizations for gout could be preventable with serum urate < 5 mg/dL,” he said.
As for limitations, the authors noted that participants in the UK Biobank “typically have a better socioeconomic status and are healthier than the UK general population,” and they added that “these data may underestimate the number of acute gout flares in the cohort.” Also, 55% of the total 502,490 patients in the UK Biobank were excluded owing to lack of primary care data.
Study ‘Offers the Kind of Evidence That We Need’
In an accompanying commentary, University of Alabama at Birmingham rheumatologist Angelo L. Gaffo, MD, MSPH, also noted that the study population was overwhelmingly White, had a low mean SU level (6.9 mg/dL), and had a low level of comorbidities, making the sample “poorly representative of the most commonly described gout populations.”
However, he also noted that there is “growing evidence linking serum urate levels with clinical outcomes,” with a pair of studies — one from 2021 and the other from 2022 — linking reductions in SU to < 6 md/dL to lower flare rates.
Dr. Gaffo told this news organization that although rheumatology guidelines support a treat-to-target strategy, “we haven›t generated a whole lot of important evidence to support it.”
The new study “offers the kind of evidence that we need,” he said, “but this is not going to be the ultimate answer.” That will only come from randomized clinical trials in the works that will pit the treat-to-target approach vs the primary care–favored strategy of titrating treatment until flares are controlled, he said.
Even though evidence is sparse, Dr. Gaffo said he still believes in the treat-to-target strategy: “I believe it is the best way to treat gout.”
What’s next? Researchers hope to understand how to better reach target SU goals in clinical practice, Dr. Choi said. “Involving nurses, pharmacists, or interactive online or app systems — as in other chronic treat-to-target care such as anticoagulation care, blood pressure, or lipid care — is actively being researched.”
He added that “we are trying to find the effective and safe medications and nonpharmacologic measures to reduce the urate burden, which can also simultaneously take care of gout’s frequent cardiovascular-kidney comorbidities.”
The US National Institutes of Health supported the study. Dr. Choi reports receiving grants from Horizon and serving on a board or committee for LG Chem, Shanton, and ANI Pharmaceuticals. Some other authors report an employment and stockholder relationship with Regeneron and support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, and Rheumatology Research Foundation. Dr. Gaffo reports personal fees from PK MED, SOBI/Selecta, Atom, and UpToDate.
A version of this article first appeared on Medscape.com.
Clinical efforts to get patients with a history of gout to reach specific target serum urate (SU) levels less than either 5 or 6 mg/dL could prevent the great majority of gout flares and hospitalizations for them, according to a new study that tracked patients for a mean of 8.3 years.
The findings, which appeared February 6 in JAMA, “support the value of target serum urate levels in gout flare prevention in primary care, where most gout patients are treated,” rheumatologist and study coauthor Hyon K. Choi, MD, DrPH, of Harvard Medical School and Massachusetts General Hospital, Boston, told this news organization. However, Dr. Choi noted that “the value of relying on target urate levels is not accepted in primary care practice,” and the author of an accompanying commentary said that the jury is still out about the best strategy to prevent flares.
Gout is caused by monosodium urate crystallization within the joints, which occurs when SU levels exceed the saturation point for uric acid crystallization in the body: approximately 6.8 mg/dL. “Studies have found strongly graded associations between serum urate levels above the saturation point and the risk of developing new cases of gout among individuals without gout at baseline,” Dr. Choi said. “However, associations between serum urate levels and the risk of recurrent flares among preexisting gout patients, which is relevant to clinical gout care practice, has not been established.”
Dr. Choi added that “despite the emphasis in US and European rheumatology guidelines on the use of urate-lowering therapy to treat-to-target serum urate level — eg, under 6 or 5 mg/dL — the proportions of flares associated with such target urate levels remained unknown.”
Study Shows Relationship Between SU Levels and Recurrent Flares
For the study, researchers tracked 3613 patients aged 40-69 with gout in the UK Biobank database from 2006-2010 to 2017 or 2020. The patients, 86% of whom were men, had a mean age of 60 years and about 96% were White.
Among the patients, 1773 new episodes of acute gout occurred in 27% of the patients (16% had one episode, 6% had two episodes, and 5% had at least three episodes). These were treated in primary care or required hospitalizations. The other 73% of patients had no new acute gout episodes.
Overall, 95% of flares occurred in those with baseline SU levels ≥ 6 mg/dL, and 98% occurred in those with levels ≥ 5 mg/dL.
Patients with baseline SU levels < 6.0 mg/dL had an acute gout flare rate of 10.6 per 1000 person-years. In comparison, relative risks for acute gout flares per 1000 person-years were 3.16 at baseline SU levels of 6.0-6.9 mg/dL, 6.20 for 7.0-7.9 mg/dL, 7.70 for 8.0-8.9 mg/dL, 9.80 for 9.0-9.9 mg/dL, and 11.26 for > 10 mg/dL after adjustment for various possible confounders (P < .001).
The researchers identified 64 hospitalizations with gout as the main discharge diagnosis, and 97% occurred in patients with baseline SU levels ≥ 6 mg/dL. All were in patients with baseline SU levels ≥ 5 mg/dL.
“An important feature of this study was that serum urate measurements were obtained from all gout patients at the study baseline, irrespective of clinical needs or flare status,” Dr. Choi said. “Prior studies failed to reveal the truly compelling nature of relations between serum urate levels and recurrent flares among preexisting gout patients.”
As for the cost of SU tests, Dr. Choi said they can run as low as $2. “Portable tests similar to home glucose measurement for diabetes patients are also being adopted by certain gout care practices,” he said.
The findings matter, Dr. Choi said, because SU is not tracked in the “vast majority of gout patients” in primary care. Instead, primary care doctors — as per the guidelines of the American College of Physicians — often adopt an approach that treats symptoms as needed instead of tracking and lowering SU levels, he said. In fact, “95% and 98% of gout flares can be potentially preventable at the population level if serum urate levels < 6 and < 5 mg/dL can be met, respectively, and 100% of hospitalizations for gout could be preventable with serum urate < 5 mg/dL,” he said.
As for limitations, the authors noted that participants in the UK Biobank “typically have a better socioeconomic status and are healthier than the UK general population,” and they added that “these data may underestimate the number of acute gout flares in the cohort.” Also, 55% of the total 502,490 patients in the UK Biobank were excluded owing to lack of primary care data.
Study ‘Offers the Kind of Evidence That We Need’
In an accompanying commentary, University of Alabama at Birmingham rheumatologist Angelo L. Gaffo, MD, MSPH, also noted that the study population was overwhelmingly White, had a low mean SU level (6.9 mg/dL), and had a low level of comorbidities, making the sample “poorly representative of the most commonly described gout populations.”
However, he also noted that there is “growing evidence linking serum urate levels with clinical outcomes,” with a pair of studies — one from 2021 and the other from 2022 — linking reductions in SU to < 6 md/dL to lower flare rates.
Dr. Gaffo told this news organization that although rheumatology guidelines support a treat-to-target strategy, “we haven›t generated a whole lot of important evidence to support it.”
The new study “offers the kind of evidence that we need,” he said, “but this is not going to be the ultimate answer.” That will only come from randomized clinical trials in the works that will pit the treat-to-target approach vs the primary care–favored strategy of titrating treatment until flares are controlled, he said.
Even though evidence is sparse, Dr. Gaffo said he still believes in the treat-to-target strategy: “I believe it is the best way to treat gout.”
What’s next? Researchers hope to understand how to better reach target SU goals in clinical practice, Dr. Choi said. “Involving nurses, pharmacists, or interactive online or app systems — as in other chronic treat-to-target care such as anticoagulation care, blood pressure, or lipid care — is actively being researched.”
He added that “we are trying to find the effective and safe medications and nonpharmacologic measures to reduce the urate burden, which can also simultaneously take care of gout’s frequent cardiovascular-kidney comorbidities.”
The US National Institutes of Health supported the study. Dr. Choi reports receiving grants from Horizon and serving on a board or committee for LG Chem, Shanton, and ANI Pharmaceuticals. Some other authors report an employment and stockholder relationship with Regeneron and support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, and Rheumatology Research Foundation. Dr. Gaffo reports personal fees from PK MED, SOBI/Selecta, Atom, and UpToDate.
A version of this article first appeared on Medscape.com.
Clinical efforts to get patients with a history of gout to reach specific target serum urate (SU) levels less than either 5 or 6 mg/dL could prevent the great majority of gout flares and hospitalizations for them, according to a new study that tracked patients for a mean of 8.3 years.
The findings, which appeared February 6 in JAMA, “support the value of target serum urate levels in gout flare prevention in primary care, where most gout patients are treated,” rheumatologist and study coauthor Hyon K. Choi, MD, DrPH, of Harvard Medical School and Massachusetts General Hospital, Boston, told this news organization. However, Dr. Choi noted that “the value of relying on target urate levels is not accepted in primary care practice,” and the author of an accompanying commentary said that the jury is still out about the best strategy to prevent flares.
Gout is caused by monosodium urate crystallization within the joints, which occurs when SU levels exceed the saturation point for uric acid crystallization in the body: approximately 6.8 mg/dL. “Studies have found strongly graded associations between serum urate levels above the saturation point and the risk of developing new cases of gout among individuals without gout at baseline,” Dr. Choi said. “However, associations between serum urate levels and the risk of recurrent flares among preexisting gout patients, which is relevant to clinical gout care practice, has not been established.”
Dr. Choi added that “despite the emphasis in US and European rheumatology guidelines on the use of urate-lowering therapy to treat-to-target serum urate level — eg, under 6 or 5 mg/dL — the proportions of flares associated with such target urate levels remained unknown.”
Study Shows Relationship Between SU Levels and Recurrent Flares
For the study, researchers tracked 3613 patients aged 40-69 with gout in the UK Biobank database from 2006-2010 to 2017 or 2020. The patients, 86% of whom were men, had a mean age of 60 years and about 96% were White.
Among the patients, 1773 new episodes of acute gout occurred in 27% of the patients (16% had one episode, 6% had two episodes, and 5% had at least three episodes). These were treated in primary care or required hospitalizations. The other 73% of patients had no new acute gout episodes.
Overall, 95% of flares occurred in those with baseline SU levels ≥ 6 mg/dL, and 98% occurred in those with levels ≥ 5 mg/dL.
Patients with baseline SU levels < 6.0 mg/dL had an acute gout flare rate of 10.6 per 1000 person-years. In comparison, relative risks for acute gout flares per 1000 person-years were 3.16 at baseline SU levels of 6.0-6.9 mg/dL, 6.20 for 7.0-7.9 mg/dL, 7.70 for 8.0-8.9 mg/dL, 9.80 for 9.0-9.9 mg/dL, and 11.26 for > 10 mg/dL after adjustment for various possible confounders (P < .001).
The researchers identified 64 hospitalizations with gout as the main discharge diagnosis, and 97% occurred in patients with baseline SU levels ≥ 6 mg/dL. All were in patients with baseline SU levels ≥ 5 mg/dL.
“An important feature of this study was that serum urate measurements were obtained from all gout patients at the study baseline, irrespective of clinical needs or flare status,” Dr. Choi said. “Prior studies failed to reveal the truly compelling nature of relations between serum urate levels and recurrent flares among preexisting gout patients.”
As for the cost of SU tests, Dr. Choi said they can run as low as $2. “Portable tests similar to home glucose measurement for diabetes patients are also being adopted by certain gout care practices,” he said.
The findings matter, Dr. Choi said, because SU is not tracked in the “vast majority of gout patients” in primary care. Instead, primary care doctors — as per the guidelines of the American College of Physicians — often adopt an approach that treats symptoms as needed instead of tracking and lowering SU levels, he said. In fact, “95% and 98% of gout flares can be potentially preventable at the population level if serum urate levels < 6 and < 5 mg/dL can be met, respectively, and 100% of hospitalizations for gout could be preventable with serum urate < 5 mg/dL,” he said.
As for limitations, the authors noted that participants in the UK Biobank “typically have a better socioeconomic status and are healthier than the UK general population,” and they added that “these data may underestimate the number of acute gout flares in the cohort.” Also, 55% of the total 502,490 patients in the UK Biobank were excluded owing to lack of primary care data.
Study ‘Offers the Kind of Evidence That We Need’
In an accompanying commentary, University of Alabama at Birmingham rheumatologist Angelo L. Gaffo, MD, MSPH, also noted that the study population was overwhelmingly White, had a low mean SU level (6.9 mg/dL), and had a low level of comorbidities, making the sample “poorly representative of the most commonly described gout populations.”
However, he also noted that there is “growing evidence linking serum urate levels with clinical outcomes,” with a pair of studies — one from 2021 and the other from 2022 — linking reductions in SU to < 6 md/dL to lower flare rates.
Dr. Gaffo told this news organization that although rheumatology guidelines support a treat-to-target strategy, “we haven›t generated a whole lot of important evidence to support it.”
The new study “offers the kind of evidence that we need,” he said, “but this is not going to be the ultimate answer.” That will only come from randomized clinical trials in the works that will pit the treat-to-target approach vs the primary care–favored strategy of titrating treatment until flares are controlled, he said.
Even though evidence is sparse, Dr. Gaffo said he still believes in the treat-to-target strategy: “I believe it is the best way to treat gout.”
What’s next? Researchers hope to understand how to better reach target SU goals in clinical practice, Dr. Choi said. “Involving nurses, pharmacists, or interactive online or app systems — as in other chronic treat-to-target care such as anticoagulation care, blood pressure, or lipid care — is actively being researched.”
He added that “we are trying to find the effective and safe medications and nonpharmacologic measures to reduce the urate burden, which can also simultaneously take care of gout’s frequent cardiovascular-kidney comorbidities.”
The US National Institutes of Health supported the study. Dr. Choi reports receiving grants from Horizon and serving on a board or committee for LG Chem, Shanton, and ANI Pharmaceuticals. Some other authors report an employment and stockholder relationship with Regeneron and support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, and Rheumatology Research Foundation. Dr. Gaffo reports personal fees from PK MED, SOBI/Selecta, Atom, and UpToDate.
A version of this article first appeared on Medscape.com.
FROM JAMA
Higher HDL Tied to Prediabetes Reversion — Up to a Point
TOPLINE:
Higher high-density lipoprotein cholesterol (HDL-C) levels show a positive association with prediabetes reversal to normoglycemia in Chinese adults, but only up to a certain threshold.
METHODOLOGY:
- Researchers examined the correlation between HDL-C levels and the reversion of people with prediabetes to normoglycemia in a secondary analysis of data from a population-based cohort study.
- The analysis included 15,420 Chinese patients with prediabetes who underwent health screening between 2010 and 2016 (mean age, 51 ± 13 years; 5414 (35%) women).
- The outcome measure, reversion to normoglycemia, was determined by no self-reported diabetic event and fasting plasma glucose < 5.6 mmol/L at follow-up.
- They categorized the adults into four groups on the basis of HDL-C quartiles.
- They used multiple statistical models to investigate the association between HDL-C levels and reversion from prediabetes, assess the linearity of the association, and account for independent variables and confounding factors.
TAKEAWAY:
- After a median follow-up of nearly 3 years, 6627 (43%) of patients with prediabetes had a reversion to normoglycemia.
- The groups with higher HDL-C levels had a higher likelihood of prediabetes reversal to normoglycemia (adjusted hazard ratio [HR], 1.90; P < .001).
- They found a nonlinear association and threshold effect: The probability of reversal from prediabetes to normoglycemia stabilized rather than continued to increase at an inflection point (1.54 mmol/L in men, 1.62 mmol/L in women).
- A significant positive correlation with reversal to normoglycemia was observed below the HDL-C threshold (men: HR, 2.78; 95% CI, 2.37-3.26; women: HR, 2.22; 95% CI, 1.80-2.73).
IN PRACTICE:
“Keeping HDL-C levels near the inflection point in patients with prediabetes may greatly increase the likelihood of reversion from prediabetes to normoglycemia,” the authors wrote.
SOURCE:
The study, with lead author Zihe Mo, Department of Physical Examination, Dongguan Tungwah Hospital, Dongguan, China, was published online in Scientific Reports.
LIMITATIONS:
The study included individuals of Chinese descent, necessitating more studies into the HDL-C and normoglycemia relationship across diverse genetic backgrounds. The study relied solely on fasting plasma glucose measurements and was unable to capture the entirety of prediabetes complexity. As a secondary analysis of previously published data, the study faces limitations in managing unmeasured variables not initially included in the dataset. The observational study cannot determine a causal relationship between HDL-C and reversion from prediabetes to normoglycemia.
DISCLOSURES:
The study was supported by the Natural Science Funding of China. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher high-density lipoprotein cholesterol (HDL-C) levels show a positive association with prediabetes reversal to normoglycemia in Chinese adults, but only up to a certain threshold.
METHODOLOGY:
- Researchers examined the correlation between HDL-C levels and the reversion of people with prediabetes to normoglycemia in a secondary analysis of data from a population-based cohort study.
- The analysis included 15,420 Chinese patients with prediabetes who underwent health screening between 2010 and 2016 (mean age, 51 ± 13 years; 5414 (35%) women).
- The outcome measure, reversion to normoglycemia, was determined by no self-reported diabetic event and fasting plasma glucose < 5.6 mmol/L at follow-up.
- They categorized the adults into four groups on the basis of HDL-C quartiles.
- They used multiple statistical models to investigate the association between HDL-C levels and reversion from prediabetes, assess the linearity of the association, and account for independent variables and confounding factors.
TAKEAWAY:
- After a median follow-up of nearly 3 years, 6627 (43%) of patients with prediabetes had a reversion to normoglycemia.
- The groups with higher HDL-C levels had a higher likelihood of prediabetes reversal to normoglycemia (adjusted hazard ratio [HR], 1.90; P < .001).
- They found a nonlinear association and threshold effect: The probability of reversal from prediabetes to normoglycemia stabilized rather than continued to increase at an inflection point (1.54 mmol/L in men, 1.62 mmol/L in women).
- A significant positive correlation with reversal to normoglycemia was observed below the HDL-C threshold (men: HR, 2.78; 95% CI, 2.37-3.26; women: HR, 2.22; 95% CI, 1.80-2.73).
IN PRACTICE:
“Keeping HDL-C levels near the inflection point in patients with prediabetes may greatly increase the likelihood of reversion from prediabetes to normoglycemia,” the authors wrote.
SOURCE:
The study, with lead author Zihe Mo, Department of Physical Examination, Dongguan Tungwah Hospital, Dongguan, China, was published online in Scientific Reports.
LIMITATIONS:
The study included individuals of Chinese descent, necessitating more studies into the HDL-C and normoglycemia relationship across diverse genetic backgrounds. The study relied solely on fasting plasma glucose measurements and was unable to capture the entirety of prediabetes complexity. As a secondary analysis of previously published data, the study faces limitations in managing unmeasured variables not initially included in the dataset. The observational study cannot determine a causal relationship between HDL-C and reversion from prediabetes to normoglycemia.
DISCLOSURES:
The study was supported by the Natural Science Funding of China. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher high-density lipoprotein cholesterol (HDL-C) levels show a positive association with prediabetes reversal to normoglycemia in Chinese adults, but only up to a certain threshold.
METHODOLOGY:
- Researchers examined the correlation between HDL-C levels and the reversion of people with prediabetes to normoglycemia in a secondary analysis of data from a population-based cohort study.
- The analysis included 15,420 Chinese patients with prediabetes who underwent health screening between 2010 and 2016 (mean age, 51 ± 13 years; 5414 (35%) women).
- The outcome measure, reversion to normoglycemia, was determined by no self-reported diabetic event and fasting plasma glucose < 5.6 mmol/L at follow-up.
- They categorized the adults into four groups on the basis of HDL-C quartiles.
- They used multiple statistical models to investigate the association between HDL-C levels and reversion from prediabetes, assess the linearity of the association, and account for independent variables and confounding factors.
TAKEAWAY:
- After a median follow-up of nearly 3 years, 6627 (43%) of patients with prediabetes had a reversion to normoglycemia.
- The groups with higher HDL-C levels had a higher likelihood of prediabetes reversal to normoglycemia (adjusted hazard ratio [HR], 1.90; P < .001).
- They found a nonlinear association and threshold effect: The probability of reversal from prediabetes to normoglycemia stabilized rather than continued to increase at an inflection point (1.54 mmol/L in men, 1.62 mmol/L in women).
- A significant positive correlation with reversal to normoglycemia was observed below the HDL-C threshold (men: HR, 2.78; 95% CI, 2.37-3.26; women: HR, 2.22; 95% CI, 1.80-2.73).
IN PRACTICE:
“Keeping HDL-C levels near the inflection point in patients with prediabetes may greatly increase the likelihood of reversion from prediabetes to normoglycemia,” the authors wrote.
SOURCE:
The study, with lead author Zihe Mo, Department of Physical Examination, Dongguan Tungwah Hospital, Dongguan, China, was published online in Scientific Reports.
LIMITATIONS:
The study included individuals of Chinese descent, necessitating more studies into the HDL-C and normoglycemia relationship across diverse genetic backgrounds. The study relied solely on fasting plasma glucose measurements and was unable to capture the entirety of prediabetes complexity. As a secondary analysis of previously published data, the study faces limitations in managing unmeasured variables not initially included in the dataset. The observational study cannot determine a causal relationship between HDL-C and reversion from prediabetes to normoglycemia.
DISCLOSURES:
The study was supported by the Natural Science Funding of China. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
10 Reasons to Refer Your Patient to an Endocrinologist
The blockbuster drugs of the century have arrived: glucagon-like peptide 1 receptor agonists (GLP-1 RAs). These drugs were developed to control blood sugar but have gained immense popularity for weight loss. Patients are clamoring for the drugs, and physicians are inundated with patient inquiries.
As doctors in primary care and other specialties are discovering, the GLP-1 RA drugs add another layer of complexity to the long-term management of a chronic disease. Managing diabetes and obesity requires a multidisciplinary team and a multispecialty treatment approach.
That’s why it’s more important than ever to know when and why to refer patients to an endocrinologist, who can offer unparalleled expertise as part of a multidisciplinary treatment approach.
Here are 10 reasons to refer your patients with diabetes to an endocrinologist.
1. To help make an optimal medication choice. Endocrinologists navigate diabetes management by considering individualized glycemic, cardiorenal, and weight goals as per guidelines, incorporating knowledge of medication side effects, simplifying regimens for adherence, and addressing practical factors like access and cost. Optimal medication selection is crucial, as a recent study found that nearly two thirds of patients altered their treatment by discontinuing their medication, switching their medication, or changing the dose of their medication within 12 months. Whether diabetes is controlled or uncontrolled, patients should consult an endocrinologist due to the potential complexity of cases, including late autoimmune onset of diabetes; medication-induced diabetes; and factors such as age, fragility, and chronic illnesses.
2. To facilitate medication approvals, alternatives, and authorizations. Attaining medication approval for patients entails a nuanced understanding and resources. Through experience and careful consideration, endocrinologists develop insights into potential barriers, especially in cases where approval for specific medications necessitates prior failures with multiple GLP-1 RAs or antihyperglycemic agents. This expertise positions them to advocate effectively for alternative options, often involving the meticulous process of prior authorizations. Certain endocrinology practices may augment this endeavor by offering dedicated resources, such as a specialized prior authorization team.
3. To deal with diabetes complications. Endocrinologists can help address emerging issues in GLP-1 RA drugs such as retinopathy, gastroparesis, and mental health effects. They can also help manage coexisting conditions, such as addressing thyroid nodules before considering the use of GLP-1 RAs. Recognizing the interconnected nature of diabetes and its influence on diverse body systems, endocrinologists ensure a thorough and integrated management strategy for their patients.
4. To titrate other glucose-lowering agents. Patients with diabetes are often on combination therapy. Endocrinologists adeptly adjust and titrate these treatments to optimize glucose control while minimizing side effects like hypoglycemia. Beyond insulin, their expertise encompasses various glucose-lowering agents. Notably, patients who use GLP-1 RAs in combination with medications such as insulin secretagogues (eg, sulfonylurea) and insulin face an elevated risk for hypoglycemia, including severe cases, necessitating careful titration to mitigate these effects.
5. To integrate advances in diabetes technology. Endocrinologists stay abreast of technological advancements in diabetes care, incorporating innovations in monitoring and treatment strategies such as continuous glucose monitors and insulin pumps. This ensures that patients benefit from the latest technologies for more precise management of their condition.
6. To ensure a comprehensive care team. Endocrinologists engage in collaborative efforts with a multidisciplinary team composed of professionals like nurses, diabetes educators, and nutritionists. These experts may be situated within endocrinology offices or accessible through a well-established referral network. Together, the team delivers thorough counseling on medication use and effectively addresses essential lifestyle factors, ensuring a comprehensive approach to diabetes management.
7. To counsel on side effects and management. Ensuring adherence and persistence with medication therapy poses considerable challenges. One study noted discontinuation rates for non-insulin diabetes medications of about 38%, with a higher 50% rate for GLP-1 RA drugs. The study didn›t provide specific reasons for discontinuation, but discontinuation was lower when medications were prescribed by an endocrinologist. Endocrinologists can provide valuable guidance on potential medication side effects and their management. This proactive approach not only fosters patient understanding but also empowers individuals to promptly address side effects, significantly enhancing treatment adherence and overall effectiveness.
8. To work around drug shortages. Given their frequent involvement in prescribing and obtaining medications for patients, endocrinologists adeptly utilize community relationships to navigate medication shortages. Their awareness of drug availability provides patients with a strategic advantage in overcoming supply challenges.
9. To determine dosing equivalents. In situations where supply-chain shortages persist, a thorough understanding of alternative options and dosing equivalents becomes paramount for ensuring uninterrupted care.
To provide follow-up. Endocrinologists prioritize regular follow-ups, providing patients with dedicated time slots for 10. ongoing monitoring and adjustments to their treatment plans. This commitment to follow-up care contributes to sustained, optimal outcomes in diabetes management.
Navigating the intricate healthcare landscape requires a delicate balance between primary care proficiency and specialist expertise, with endocrinologists playing a pivotal role in diabetes management. Our collaborative strength lies in acknowledging challenges and resource limitations, especially a physician’s familiarity with the latest diabetes medications.
Dr. Jaisinghani has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from Novo Nordisk.
A version of this article appeared on Medscape.com.
The blockbuster drugs of the century have arrived: glucagon-like peptide 1 receptor agonists (GLP-1 RAs). These drugs were developed to control blood sugar but have gained immense popularity for weight loss. Patients are clamoring for the drugs, and physicians are inundated with patient inquiries.
As doctors in primary care and other specialties are discovering, the GLP-1 RA drugs add another layer of complexity to the long-term management of a chronic disease. Managing diabetes and obesity requires a multidisciplinary team and a multispecialty treatment approach.
That’s why it’s more important than ever to know when and why to refer patients to an endocrinologist, who can offer unparalleled expertise as part of a multidisciplinary treatment approach.
Here are 10 reasons to refer your patients with diabetes to an endocrinologist.
1. To help make an optimal medication choice. Endocrinologists navigate diabetes management by considering individualized glycemic, cardiorenal, and weight goals as per guidelines, incorporating knowledge of medication side effects, simplifying regimens for adherence, and addressing practical factors like access and cost. Optimal medication selection is crucial, as a recent study found that nearly two thirds of patients altered their treatment by discontinuing their medication, switching their medication, or changing the dose of their medication within 12 months. Whether diabetes is controlled or uncontrolled, patients should consult an endocrinologist due to the potential complexity of cases, including late autoimmune onset of diabetes; medication-induced diabetes; and factors such as age, fragility, and chronic illnesses.
2. To facilitate medication approvals, alternatives, and authorizations. Attaining medication approval for patients entails a nuanced understanding and resources. Through experience and careful consideration, endocrinologists develop insights into potential barriers, especially in cases where approval for specific medications necessitates prior failures with multiple GLP-1 RAs or antihyperglycemic agents. This expertise positions them to advocate effectively for alternative options, often involving the meticulous process of prior authorizations. Certain endocrinology practices may augment this endeavor by offering dedicated resources, such as a specialized prior authorization team.
3. To deal with diabetes complications. Endocrinologists can help address emerging issues in GLP-1 RA drugs such as retinopathy, gastroparesis, and mental health effects. They can also help manage coexisting conditions, such as addressing thyroid nodules before considering the use of GLP-1 RAs. Recognizing the interconnected nature of diabetes and its influence on diverse body systems, endocrinologists ensure a thorough and integrated management strategy for their patients.
4. To titrate other glucose-lowering agents. Patients with diabetes are often on combination therapy. Endocrinologists adeptly adjust and titrate these treatments to optimize glucose control while minimizing side effects like hypoglycemia. Beyond insulin, their expertise encompasses various glucose-lowering agents. Notably, patients who use GLP-1 RAs in combination with medications such as insulin secretagogues (eg, sulfonylurea) and insulin face an elevated risk for hypoglycemia, including severe cases, necessitating careful titration to mitigate these effects.
5. To integrate advances in diabetes technology. Endocrinologists stay abreast of technological advancements in diabetes care, incorporating innovations in monitoring and treatment strategies such as continuous glucose monitors and insulin pumps. This ensures that patients benefit from the latest technologies for more precise management of their condition.
6. To ensure a comprehensive care team. Endocrinologists engage in collaborative efforts with a multidisciplinary team composed of professionals like nurses, diabetes educators, and nutritionists. These experts may be situated within endocrinology offices or accessible through a well-established referral network. Together, the team delivers thorough counseling on medication use and effectively addresses essential lifestyle factors, ensuring a comprehensive approach to diabetes management.
7. To counsel on side effects and management. Ensuring adherence and persistence with medication therapy poses considerable challenges. One study noted discontinuation rates for non-insulin diabetes medications of about 38%, with a higher 50% rate for GLP-1 RA drugs. The study didn›t provide specific reasons for discontinuation, but discontinuation was lower when medications were prescribed by an endocrinologist. Endocrinologists can provide valuable guidance on potential medication side effects and their management. This proactive approach not only fosters patient understanding but also empowers individuals to promptly address side effects, significantly enhancing treatment adherence and overall effectiveness.
8. To work around drug shortages. Given their frequent involvement in prescribing and obtaining medications for patients, endocrinologists adeptly utilize community relationships to navigate medication shortages. Their awareness of drug availability provides patients with a strategic advantage in overcoming supply challenges.
9. To determine dosing equivalents. In situations where supply-chain shortages persist, a thorough understanding of alternative options and dosing equivalents becomes paramount for ensuring uninterrupted care.
To provide follow-up. Endocrinologists prioritize regular follow-ups, providing patients with dedicated time slots for 10. ongoing monitoring and adjustments to their treatment plans. This commitment to follow-up care contributes to sustained, optimal outcomes in diabetes management.
Navigating the intricate healthcare landscape requires a delicate balance between primary care proficiency and specialist expertise, with endocrinologists playing a pivotal role in diabetes management. Our collaborative strength lies in acknowledging challenges and resource limitations, especially a physician’s familiarity with the latest diabetes medications.
Dr. Jaisinghani has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from Novo Nordisk.
A version of this article appeared on Medscape.com.
The blockbuster drugs of the century have arrived: glucagon-like peptide 1 receptor agonists (GLP-1 RAs). These drugs were developed to control blood sugar but have gained immense popularity for weight loss. Patients are clamoring for the drugs, and physicians are inundated with patient inquiries.
As doctors in primary care and other specialties are discovering, the GLP-1 RA drugs add another layer of complexity to the long-term management of a chronic disease. Managing diabetes and obesity requires a multidisciplinary team and a multispecialty treatment approach.
That’s why it’s more important than ever to know when and why to refer patients to an endocrinologist, who can offer unparalleled expertise as part of a multidisciplinary treatment approach.
Here are 10 reasons to refer your patients with diabetes to an endocrinologist.
1. To help make an optimal medication choice. Endocrinologists navigate diabetes management by considering individualized glycemic, cardiorenal, and weight goals as per guidelines, incorporating knowledge of medication side effects, simplifying regimens for adherence, and addressing practical factors like access and cost. Optimal medication selection is crucial, as a recent study found that nearly two thirds of patients altered their treatment by discontinuing their medication, switching their medication, or changing the dose of their medication within 12 months. Whether diabetes is controlled or uncontrolled, patients should consult an endocrinologist due to the potential complexity of cases, including late autoimmune onset of diabetes; medication-induced diabetes; and factors such as age, fragility, and chronic illnesses.
2. To facilitate medication approvals, alternatives, and authorizations. Attaining medication approval for patients entails a nuanced understanding and resources. Through experience and careful consideration, endocrinologists develop insights into potential barriers, especially in cases where approval for specific medications necessitates prior failures with multiple GLP-1 RAs or antihyperglycemic agents. This expertise positions them to advocate effectively for alternative options, often involving the meticulous process of prior authorizations. Certain endocrinology practices may augment this endeavor by offering dedicated resources, such as a specialized prior authorization team.
3. To deal with diabetes complications. Endocrinologists can help address emerging issues in GLP-1 RA drugs such as retinopathy, gastroparesis, and mental health effects. They can also help manage coexisting conditions, such as addressing thyroid nodules before considering the use of GLP-1 RAs. Recognizing the interconnected nature of diabetes and its influence on diverse body systems, endocrinologists ensure a thorough and integrated management strategy for their patients.
4. To titrate other glucose-lowering agents. Patients with diabetes are often on combination therapy. Endocrinologists adeptly adjust and titrate these treatments to optimize glucose control while minimizing side effects like hypoglycemia. Beyond insulin, their expertise encompasses various glucose-lowering agents. Notably, patients who use GLP-1 RAs in combination with medications such as insulin secretagogues (eg, sulfonylurea) and insulin face an elevated risk for hypoglycemia, including severe cases, necessitating careful titration to mitigate these effects.
5. To integrate advances in diabetes technology. Endocrinologists stay abreast of technological advancements in diabetes care, incorporating innovations in monitoring and treatment strategies such as continuous glucose monitors and insulin pumps. This ensures that patients benefit from the latest technologies for more precise management of their condition.
6. To ensure a comprehensive care team. Endocrinologists engage in collaborative efforts with a multidisciplinary team composed of professionals like nurses, diabetes educators, and nutritionists. These experts may be situated within endocrinology offices or accessible through a well-established referral network. Together, the team delivers thorough counseling on medication use and effectively addresses essential lifestyle factors, ensuring a comprehensive approach to diabetes management.
7. To counsel on side effects and management. Ensuring adherence and persistence with medication therapy poses considerable challenges. One study noted discontinuation rates for non-insulin diabetes medications of about 38%, with a higher 50% rate for GLP-1 RA drugs. The study didn›t provide specific reasons for discontinuation, but discontinuation was lower when medications were prescribed by an endocrinologist. Endocrinologists can provide valuable guidance on potential medication side effects and their management. This proactive approach not only fosters patient understanding but also empowers individuals to promptly address side effects, significantly enhancing treatment adherence and overall effectiveness.
8. To work around drug shortages. Given their frequent involvement in prescribing and obtaining medications for patients, endocrinologists adeptly utilize community relationships to navigate medication shortages. Their awareness of drug availability provides patients with a strategic advantage in overcoming supply challenges.
9. To determine dosing equivalents. In situations where supply-chain shortages persist, a thorough understanding of alternative options and dosing equivalents becomes paramount for ensuring uninterrupted care.
To provide follow-up. Endocrinologists prioritize regular follow-ups, providing patients with dedicated time slots for 10. ongoing monitoring and adjustments to their treatment plans. This commitment to follow-up care contributes to sustained, optimal outcomes in diabetes management.
Navigating the intricate healthcare landscape requires a delicate balance between primary care proficiency and specialist expertise, with endocrinologists playing a pivotal role in diabetes management. Our collaborative strength lies in acknowledging challenges and resource limitations, especially a physician’s familiarity with the latest diabetes medications.
Dr. Jaisinghani has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from Novo Nordisk.
A version of this article appeared on Medscape.com.
A Neurotoxin, an Antidepressant, and More Emerging Options for Treating Rosacea
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
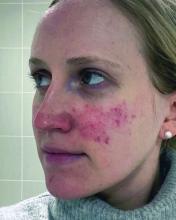
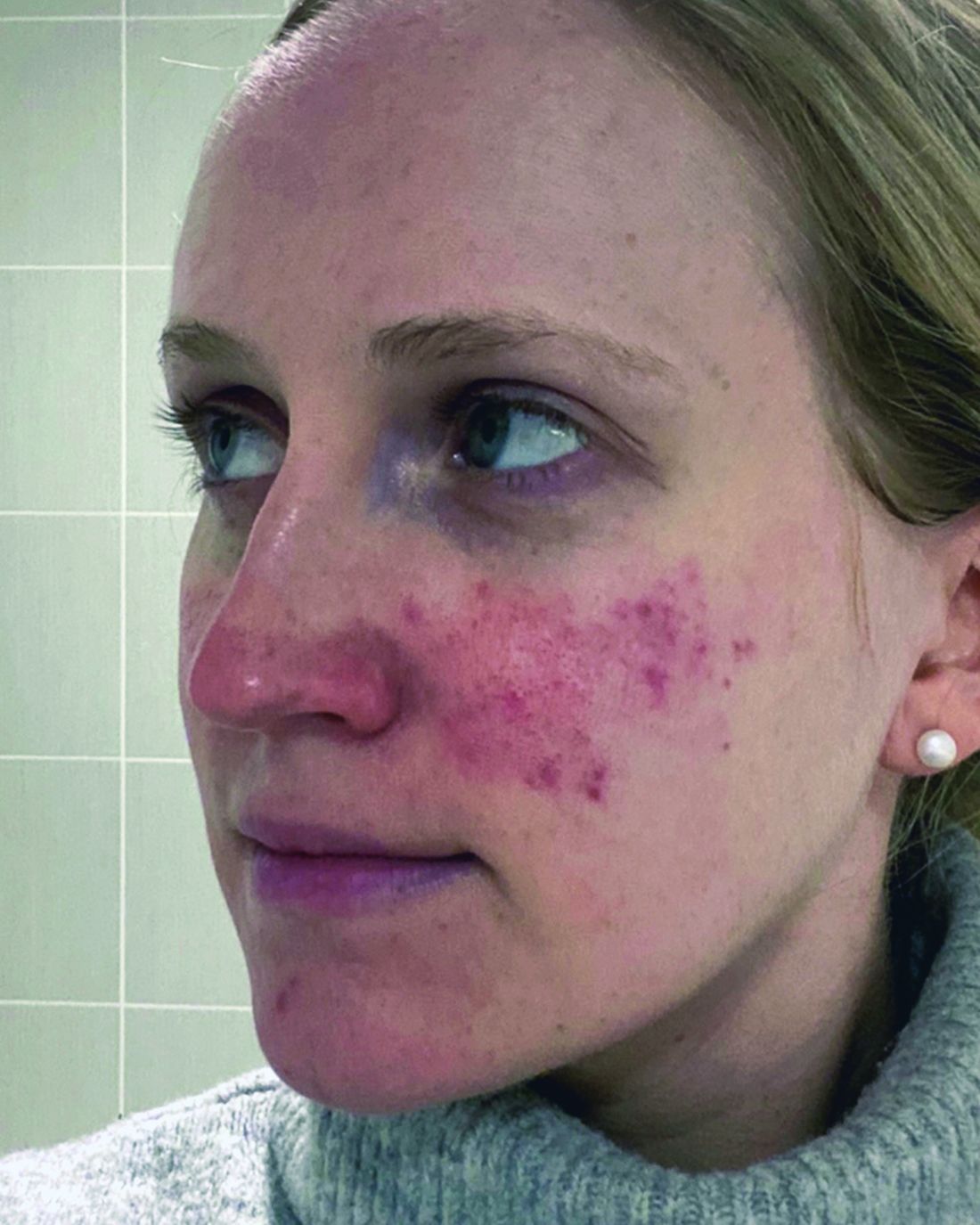
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
FROM ODAC 2024
RNA Vaccines: Risk for Heavy Menstrual Bleeding Clarified
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Disparities Seen in Weight Loss Drug Prescriptions, Fills
Socioeconomic factors and insurance type greatly influence the odds of a person with obesity receiving a prescription for a weight loss medication and subsequently filling it, new research finds.
The results come from a retrospective study of Florida and Ohio electronic health records of more than 50,000 adults with a body mass index (BMI) of ≥ 30 kg/m2 who sought care for obesity from 2015 through June 2023.
The fill rate increased to 26% in 2022-2023 after the newer glucagon-like peptide 1 (GLP-1) agonists became available, but the identified disparities persisted throughout, study author Hamlet Gasoyan, PhD, told this news organization. “Things are changing, but this study provides a very good picture of who’s getting prescriptions and the implications for policy decisions.”
Dr. Gasoyan, of the Center for Value-Based Care Research at the Cleveland Clinic, Cleveland, Ohio, noted that Medicare doesn’t currently cover antiobesity medications nor do most Medicaid programs (neither Florida’s nor Ohio’s do), but there is now at least one bill in Congress to change that. “Medicare and other government payers are currently facing important policy decisions about antiobesity medication coverage. I think they should consider how their policies could impact existing inequalities in obesity care.”
Another noteworthy finding, Dr. Gasoyan said, is that “despite all the recent hype, the real data shows these medications are underutilized and probably will remain so.”
Asked to comment, David B. Sarwer, PhD, Director of the Center for Obesity Research and Education at Temple University, Philadelphia, Pennsylvania, told this news organization, “there’s a tremendous amount of enthusiasm in the obesity treatment community that these newer medications have the potential to be game-changers. I think what this study shows us, as does other work from this group and others, is that we still have some significant issues around access to care and long-term engagement with these medications that we need to address for them to realize their full potential.”
Dr. Sarwer acknowledged, as did Dr. Gasoyan, that the study timing is a limitation and more data will need to be collected prospectively with the new incretin drugs. As of now, though, “These medications are very expensive. While there are some insurance plans that are offering payment for them, many are not. Until we wrestle that to the ground there are always going to be questions about whether these medications are getting to the people who need them the most. I think one of the highlights of this paper is it reminds us that obesity is a disease that differentially impacts persons from underserved groups.”
Moreover, Dr. Sarwer noted, “In this day and age, many physicians don’t have a lot of time to spend with individual patients. Conversations around weight can be challenging and often very emotional for patients. I’m not sure we’ve trained physicians how to have productive, targeted conversations that lead to effective use of a weight-loss intervention. Maybe in some ways that’s what we’re seeing here.”
Disparities Seen in Both Prescriptions and Fills
The 50,678 study subjects all not only met BMI criteria (≥ 30 kg/m2) but also attended at least one weight management program (n = 48,711) and/or received a weight-loss medication prescription (n = 4047). “We know BMI isn’t a perfect measure of obesity, so we specifically looked at where the patient or provider had identified excess weight as an issue and wanted to do something about it…You would expect that in this group the use of antiobesity medications would be high, but it wasn’t, unfortunately,” Dr. Gasoyan commented.
Participants had a mean BMI of 38 kg/m2 and mean age 50 years. Slightly more than half (54%) were women, 66% were White individuals, 24% Black individuals, and 5.3% Hispanic individuals. A majority (56%) had private insurance, and 41% had diabetes. Mean follow-up time was 4.7 years.
The main measures were prescriptions for naltrexone-bupropion, orlistat, phentermine-topiramate, 3.0 mg liraglutide, 2.4 mg semaglutide, and a fill for one of those during the study follow-up.
Overall, 8.0% had a new anti-obesity medication prescription, and of those, 55% had at least one documented fill of the prescription. Among the fills, 39% were for naltrexone-buproprion, 29% for phentermine-topiramate, 19% for semaglutide, 11% for liraglutide, and 1.2% for orlistat.
In the multivariable model, receipt of an antiobesity medication prescription was significantly less likely among Black patients (adjusted odds ratio, 0.68), Hispanic individuals (0.72), and those from other racial or ethnic backgrounds (0.70) than among White patients. Men had lower odds than women (0.38).
Compared with privately insured patients, significantly lower odds of receiving prescriptions were seen in those with Medicaid (0.44), traditional Medicare (0.35), Medicare Advantage (0.36), and self-paying (0.65) and other insurance types (0.53). Those in the highest quartile of economic disadvantage also had lower antiobesity medication prescription odds (0.81).
Also associated with lower prescription odds were younger age, higher age-adjusted Charlson comorbidity score, presence of diabetes diagnosis, and a history of myocardial infarction or heart failure.
Factors associated with lower odds of filling antiobesity medication prescriptions included Hispanic ethnicity vs White ethnicity (0.51) but not Black race. Compared with private insurance, lower odds of filling the prescriptions were seen among those with Medicaid (0.41), traditional Medicare (0.38), and Medicare Advantage (0.37).
Over the study period, compared with naltrexone-buproprion, phentermine-topiramate had higher odds of being filled (1.27), while liraglutide (0.61) and orlistat (0.11) had lower odds, and semaglutide didn’t differ significantly (0.90).
Older age, female sex, and the presence of diabetes diagnosis were associated with higher odds of prescription fills, while deprivation quartile, history of myocardial infarction, history of heart failure, and age-adjusted Charlson comorbidity score were not significantly associated with medication fill.
Dr. Gasoyan told this news organization, “This study is unique in that we were able to look at patterns of use and barriers at several stages…We just recently published another study where we found patients weren’t often taking these medications long-term. So, patients are facing challenges on receiving obesity pharmacotherapy at several stages. …Hopefully these data will highlight the issues and inform future decisions. We see clear areas where we could obviously do better.”
Dr. Gasoyan had no disclosures. Dr. Sarwer received grant funding from the National Institutes of Health and declared having consulting relationships with NovoNordisk and Twenty30 Health.
A version of this article appeared on Medscape.com.
Socioeconomic factors and insurance type greatly influence the odds of a person with obesity receiving a prescription for a weight loss medication and subsequently filling it, new research finds.
The results come from a retrospective study of Florida and Ohio electronic health records of more than 50,000 adults with a body mass index (BMI) of ≥ 30 kg/m2 who sought care for obesity from 2015 through June 2023.
The fill rate increased to 26% in 2022-2023 after the newer glucagon-like peptide 1 (GLP-1) agonists became available, but the identified disparities persisted throughout, study author Hamlet Gasoyan, PhD, told this news organization. “Things are changing, but this study provides a very good picture of who’s getting prescriptions and the implications for policy decisions.”
Dr. Gasoyan, of the Center for Value-Based Care Research at the Cleveland Clinic, Cleveland, Ohio, noted that Medicare doesn’t currently cover antiobesity medications nor do most Medicaid programs (neither Florida’s nor Ohio’s do), but there is now at least one bill in Congress to change that. “Medicare and other government payers are currently facing important policy decisions about antiobesity medication coverage. I think they should consider how their policies could impact existing inequalities in obesity care.”
Another noteworthy finding, Dr. Gasoyan said, is that “despite all the recent hype, the real data shows these medications are underutilized and probably will remain so.”
Asked to comment, David B. Sarwer, PhD, Director of the Center for Obesity Research and Education at Temple University, Philadelphia, Pennsylvania, told this news organization, “there’s a tremendous amount of enthusiasm in the obesity treatment community that these newer medications have the potential to be game-changers. I think what this study shows us, as does other work from this group and others, is that we still have some significant issues around access to care and long-term engagement with these medications that we need to address for them to realize their full potential.”
Dr. Sarwer acknowledged, as did Dr. Gasoyan, that the study timing is a limitation and more data will need to be collected prospectively with the new incretin drugs. As of now, though, “These medications are very expensive. While there are some insurance plans that are offering payment for them, many are not. Until we wrestle that to the ground there are always going to be questions about whether these medications are getting to the people who need them the most. I think one of the highlights of this paper is it reminds us that obesity is a disease that differentially impacts persons from underserved groups.”
Moreover, Dr. Sarwer noted, “In this day and age, many physicians don’t have a lot of time to spend with individual patients. Conversations around weight can be challenging and often very emotional for patients. I’m not sure we’ve trained physicians how to have productive, targeted conversations that lead to effective use of a weight-loss intervention. Maybe in some ways that’s what we’re seeing here.”
Disparities Seen in Both Prescriptions and Fills
The 50,678 study subjects all not only met BMI criteria (≥ 30 kg/m2) but also attended at least one weight management program (n = 48,711) and/or received a weight-loss medication prescription (n = 4047). “We know BMI isn’t a perfect measure of obesity, so we specifically looked at where the patient or provider had identified excess weight as an issue and wanted to do something about it…You would expect that in this group the use of antiobesity medications would be high, but it wasn’t, unfortunately,” Dr. Gasoyan commented.
Participants had a mean BMI of 38 kg/m2 and mean age 50 years. Slightly more than half (54%) were women, 66% were White individuals, 24% Black individuals, and 5.3% Hispanic individuals. A majority (56%) had private insurance, and 41% had diabetes. Mean follow-up time was 4.7 years.
The main measures were prescriptions for naltrexone-bupropion, orlistat, phentermine-topiramate, 3.0 mg liraglutide, 2.4 mg semaglutide, and a fill for one of those during the study follow-up.
Overall, 8.0% had a new anti-obesity medication prescription, and of those, 55% had at least one documented fill of the prescription. Among the fills, 39% were for naltrexone-buproprion, 29% for phentermine-topiramate, 19% for semaglutide, 11% for liraglutide, and 1.2% for orlistat.
In the multivariable model, receipt of an antiobesity medication prescription was significantly less likely among Black patients (adjusted odds ratio, 0.68), Hispanic individuals (0.72), and those from other racial or ethnic backgrounds (0.70) than among White patients. Men had lower odds than women (0.38).
Compared with privately insured patients, significantly lower odds of receiving prescriptions were seen in those with Medicaid (0.44), traditional Medicare (0.35), Medicare Advantage (0.36), and self-paying (0.65) and other insurance types (0.53). Those in the highest quartile of economic disadvantage also had lower antiobesity medication prescription odds (0.81).
Also associated with lower prescription odds were younger age, higher age-adjusted Charlson comorbidity score, presence of diabetes diagnosis, and a history of myocardial infarction or heart failure.
Factors associated with lower odds of filling antiobesity medication prescriptions included Hispanic ethnicity vs White ethnicity (0.51) but not Black race. Compared with private insurance, lower odds of filling the prescriptions were seen among those with Medicaid (0.41), traditional Medicare (0.38), and Medicare Advantage (0.37).
Over the study period, compared with naltrexone-buproprion, phentermine-topiramate had higher odds of being filled (1.27), while liraglutide (0.61) and orlistat (0.11) had lower odds, and semaglutide didn’t differ significantly (0.90).
Older age, female sex, and the presence of diabetes diagnosis were associated with higher odds of prescription fills, while deprivation quartile, history of myocardial infarction, history of heart failure, and age-adjusted Charlson comorbidity score were not significantly associated with medication fill.
Dr. Gasoyan told this news organization, “This study is unique in that we were able to look at patterns of use and barriers at several stages…We just recently published another study where we found patients weren’t often taking these medications long-term. So, patients are facing challenges on receiving obesity pharmacotherapy at several stages. …Hopefully these data will highlight the issues and inform future decisions. We see clear areas where we could obviously do better.”
Dr. Gasoyan had no disclosures. Dr. Sarwer received grant funding from the National Institutes of Health and declared having consulting relationships with NovoNordisk and Twenty30 Health.
A version of this article appeared on Medscape.com.
Socioeconomic factors and insurance type greatly influence the odds of a person with obesity receiving a prescription for a weight loss medication and subsequently filling it, new research finds.
The results come from a retrospective study of Florida and Ohio electronic health records of more than 50,000 adults with a body mass index (BMI) of ≥ 30 kg/m2 who sought care for obesity from 2015 through June 2023.
The fill rate increased to 26% in 2022-2023 after the newer glucagon-like peptide 1 (GLP-1) agonists became available, but the identified disparities persisted throughout, study author Hamlet Gasoyan, PhD, told this news organization. “Things are changing, but this study provides a very good picture of who’s getting prescriptions and the implications for policy decisions.”
Dr. Gasoyan, of the Center for Value-Based Care Research at the Cleveland Clinic, Cleveland, Ohio, noted that Medicare doesn’t currently cover antiobesity medications nor do most Medicaid programs (neither Florida’s nor Ohio’s do), but there is now at least one bill in Congress to change that. “Medicare and other government payers are currently facing important policy decisions about antiobesity medication coverage. I think they should consider how their policies could impact existing inequalities in obesity care.”
Another noteworthy finding, Dr. Gasoyan said, is that “despite all the recent hype, the real data shows these medications are underutilized and probably will remain so.”
Asked to comment, David B. Sarwer, PhD, Director of the Center for Obesity Research and Education at Temple University, Philadelphia, Pennsylvania, told this news organization, “there’s a tremendous amount of enthusiasm in the obesity treatment community that these newer medications have the potential to be game-changers. I think what this study shows us, as does other work from this group and others, is that we still have some significant issues around access to care and long-term engagement with these medications that we need to address for them to realize their full potential.”
Dr. Sarwer acknowledged, as did Dr. Gasoyan, that the study timing is a limitation and more data will need to be collected prospectively with the new incretin drugs. As of now, though, “These medications are very expensive. While there are some insurance plans that are offering payment for them, many are not. Until we wrestle that to the ground there are always going to be questions about whether these medications are getting to the people who need them the most. I think one of the highlights of this paper is it reminds us that obesity is a disease that differentially impacts persons from underserved groups.”
Moreover, Dr. Sarwer noted, “In this day and age, many physicians don’t have a lot of time to spend with individual patients. Conversations around weight can be challenging and often very emotional for patients. I’m not sure we’ve trained physicians how to have productive, targeted conversations that lead to effective use of a weight-loss intervention. Maybe in some ways that’s what we’re seeing here.”
Disparities Seen in Both Prescriptions and Fills
The 50,678 study subjects all not only met BMI criteria (≥ 30 kg/m2) but also attended at least one weight management program (n = 48,711) and/or received a weight-loss medication prescription (n = 4047). “We know BMI isn’t a perfect measure of obesity, so we specifically looked at where the patient or provider had identified excess weight as an issue and wanted to do something about it…You would expect that in this group the use of antiobesity medications would be high, but it wasn’t, unfortunately,” Dr. Gasoyan commented.
Participants had a mean BMI of 38 kg/m2 and mean age 50 years. Slightly more than half (54%) were women, 66% were White individuals, 24% Black individuals, and 5.3% Hispanic individuals. A majority (56%) had private insurance, and 41% had diabetes. Mean follow-up time was 4.7 years.
The main measures were prescriptions for naltrexone-bupropion, orlistat, phentermine-topiramate, 3.0 mg liraglutide, 2.4 mg semaglutide, and a fill for one of those during the study follow-up.
Overall, 8.0% had a new anti-obesity medication prescription, and of those, 55% had at least one documented fill of the prescription. Among the fills, 39% were for naltrexone-buproprion, 29% for phentermine-topiramate, 19% for semaglutide, 11% for liraglutide, and 1.2% for orlistat.
In the multivariable model, receipt of an antiobesity medication prescription was significantly less likely among Black patients (adjusted odds ratio, 0.68), Hispanic individuals (0.72), and those from other racial or ethnic backgrounds (0.70) than among White patients. Men had lower odds than women (0.38).
Compared with privately insured patients, significantly lower odds of receiving prescriptions were seen in those with Medicaid (0.44), traditional Medicare (0.35), Medicare Advantage (0.36), and self-paying (0.65) and other insurance types (0.53). Those in the highest quartile of economic disadvantage also had lower antiobesity medication prescription odds (0.81).
Also associated with lower prescription odds were younger age, higher age-adjusted Charlson comorbidity score, presence of diabetes diagnosis, and a history of myocardial infarction or heart failure.
Factors associated with lower odds of filling antiobesity medication prescriptions included Hispanic ethnicity vs White ethnicity (0.51) but not Black race. Compared with private insurance, lower odds of filling the prescriptions were seen among those with Medicaid (0.41), traditional Medicare (0.38), and Medicare Advantage (0.37).
Over the study period, compared with naltrexone-buproprion, phentermine-topiramate had higher odds of being filled (1.27), while liraglutide (0.61) and orlistat (0.11) had lower odds, and semaglutide didn’t differ significantly (0.90).
Older age, female sex, and the presence of diabetes diagnosis were associated with higher odds of prescription fills, while deprivation quartile, history of myocardial infarction, history of heart failure, and age-adjusted Charlson comorbidity score were not significantly associated with medication fill.
Dr. Gasoyan told this news organization, “This study is unique in that we were able to look at patterns of use and barriers at several stages…We just recently published another study where we found patients weren’t often taking these medications long-term. So, patients are facing challenges on receiving obesity pharmacotherapy at several stages. …Hopefully these data will highlight the issues and inform future decisions. We see clear areas where we could obviously do better.”
Dr. Gasoyan had no disclosures. Dr. Sarwer received grant funding from the National Institutes of Health and declared having consulting relationships with NovoNordisk and Twenty30 Health.
A version of this article appeared on Medscape.com.
GLP-1s for Obesity: Your Questions Answered
The arrival of GLP-1 receptor agonists has revolutionized treatment options for people with obesity and medical practice.
This news organization recently hosted a panel of experts across specialties — including endocrinology, gastroenterology, and obesity medicine — to discuss these potentially life-changing medications and to answer questions from the audience.
Because of the flood of queries from our audience, we asked our panelists to address some of the questions that didn’t make the recording. Their answers are below.
Beverly Tchang, MD, endocrinologist, Weill Cornell Medicine, New York City
Audience member: Can you initiate glucagon-like peptide-1 agonists (GLP-1 RAs) as a primary drug in a patient with obesity and newly diagnosed type 2 diabetes?
BT: We often prescribe GLP-1 RAs to individuals with type 2 diabetes as a first-line medication. Guidelines from the American Diabetes Association are really emphasizing a patient-centered approach, and metformin may not be the best first-line medication anymore.
Audience member:
BT: GLP-1 RAs do not need to be renally dosed, but I still recommend conferring with the patient’s nephrologist because the glomerular filtration rate might decrease in the setting of dehydration. Because GLP1s suppress the thirst, not just appetite, patients can go all day without drinking water and not feel thirsty.
Michael Camilleri, MD, gastroenterologist, Mayo Clinic, Rochester, Minnesota
Audience member: Should GLP-1 RAs be held for 1 week or 4 weeks prior to surgery to reduce the patient’s risk for aspiration? And is tapering required?
MC: For a patient taking liraglutide, I would hold the drug for 1 week prior to surgery. For patients taking other GLP-1 RAs, including extended exenatide, I advise holding for between 2 and 3 weeks before the procedure. It’s also important to make sure the patient’s diabetes is well-controlled with other medications — not GLP-1 RAs — during this period.
After surgery, you can restart GLP-1 RA therapy once there is recovery of oral food intake and normal bowel function.
Audience member: Is treatment with GLP-1 RAs appropriate for a patient with a family history of colon cancer but an otherwise unremarkable medical and family history?
MC: I have not seen a contraindication to receiving GLP-1 RAs based on a family history of colorectal cancer or other malignancies. An analysis of the French national healthcare insurance system database has suggested 1-3 years use of GLP-1 RAs (exenatide, liraglutide, and dulaglutide) may be linked with increased occurrence of thyroid cancer. Data from 37 randomized controlled trials and 19 real-world studies having 16,839 patients in placebo control group, 16,550 patients in active control group, and 13,330 patients in real-world studies were analyzed in a 2023 systematic review and meta-analysis. Compared to placebo or active control treatments, occurrence of pancreatic cancer, thyroid cancer, and all neoplasms — benign, malignant, and otherwise unspecified — were similar in the semaglutide group.
Toshi Iroku-Malize, MD, MPH, MBA, FAAFP, family physician, Zucker School of Medicine, Hempstead, New York
Audience member: What do you do about elevated liver functions after starting treatment with GLP-1 RAs, and what do you do when a patient has reached their weight loss goal?
TI-M: I recommend monitoring the liver function tests, evaluating for underlying causes, such as viral hepatitis, alcohol-related damage, or problems with other medications, and consulting a gastroenterologist or liver specialist if necessary. It’s also important to discuss the risk-benefit of continuing on the GP-1 RA for that particular patient.
Audience member: What effects will GLP-1 RAs have on sleep-disordered breathing/obstructive sleep apnea (OSA)? Are you aware of any ongoing trials addressing this subject?
TI-M: GLP-1 RAs may have beneficial effects on sleep-disordered breathing and OSA through weight loss, which can lead to a reduction in excess adipose tissue, and improvements in metabolic parameters. In terms of studies, a 2023 paper addressed this question, but more research is needed.
Audience member: Is it within a psychiatric provider’s scope of practice to prescribe GLP-1 agents for the reduction of weight gain associated with psychiatric medications?
TI-M: Obesity medicine is an interdisciplinary process. Numerous medications prescribed for mental health can contribute to obesity, and psychiatrists can play a role in collaborating with a patient’s primary care provider and/or obesity medicine specialist to determine which medications can be adjusted or replaced. It is important to remember that obesity management is not just about medications. It requires managing nutrition and activity in addition to behavioral health issues and social determinants of health. If the clinician has had the training to manage these pillars and is comfortable managing this chronic illness — similar to diabetes, hypertension, and other conditions — then this is a possibility. Otherwise, team-based care is appropriate.
Holly Lofton, MD, obesity medicine, NYU Langone Health, New York City
Audience member: Can we safely use them on patients who have had bariatric surgery and regularly develop dumping syndrome?
HL: These medications can be used after bariatric surgery in patients who meet the criteria for pharmacologic treatment. If a patient is having postoperative symptoms of dumping syndrome or excessive gastrointestinal losses from vomiting or diarrhea, dietary adjustments and other methods of managing the dumping syndrome in gastric bypass patients should be initiated before considering GLP-1 RAs because these patients do not have a functioning pylorus in their alimentary tract and these drugs are not indicated to treat dumping syndrome. The first-line approach typically involves reducing the patient’s intake of simple carbohydrates but can also include medications or surgical intervention when appropriate.
Audience member: Would teaching a patient to fast intermittently while they’re on GLP-1 RAs help them preserve weight loss if they choose to wean off the medication?
HL: Personally, I feel it is best to use the titration period and the time in which the patient is actively losing weight when on GLP-1 RAs. These are the best periods to help develop an individualized treatment plan, one that includes nutrition, activity, behavior modification, and resistance training. The patient’s lifestyle plan will likely change based on their environment and other factors. Intermittent fasting can be a part of such a plan. There is no consensus as to exactly which eating pattern will help patients maintain weight once they lose the physiologic benefit of the weight loss medications. However, studies have been published that demonstrate an average weight regain of 66% or greater when patients go from taking the maximum dose of a GLP-1 RA to taking none at all. Thus, patients should still be followed closely for weight regain when they discontinue a GLP-1 RA.
A version of this article appeared on Medscape.com.
The arrival of GLP-1 receptor agonists has revolutionized treatment options for people with obesity and medical practice.
This news organization recently hosted a panel of experts across specialties — including endocrinology, gastroenterology, and obesity medicine — to discuss these potentially life-changing medications and to answer questions from the audience.
Because of the flood of queries from our audience, we asked our panelists to address some of the questions that didn’t make the recording. Their answers are below.
Beverly Tchang, MD, endocrinologist, Weill Cornell Medicine, New York City
Audience member: Can you initiate glucagon-like peptide-1 agonists (GLP-1 RAs) as a primary drug in a patient with obesity and newly diagnosed type 2 diabetes?
BT: We often prescribe GLP-1 RAs to individuals with type 2 diabetes as a first-line medication. Guidelines from the American Diabetes Association are really emphasizing a patient-centered approach, and metformin may not be the best first-line medication anymore.
Audience member:
BT: GLP-1 RAs do not need to be renally dosed, but I still recommend conferring with the patient’s nephrologist because the glomerular filtration rate might decrease in the setting of dehydration. Because GLP1s suppress the thirst, not just appetite, patients can go all day without drinking water and not feel thirsty.
Michael Camilleri, MD, gastroenterologist, Mayo Clinic, Rochester, Minnesota
Audience member: Should GLP-1 RAs be held for 1 week or 4 weeks prior to surgery to reduce the patient’s risk for aspiration? And is tapering required?
MC: For a patient taking liraglutide, I would hold the drug for 1 week prior to surgery. For patients taking other GLP-1 RAs, including extended exenatide, I advise holding for between 2 and 3 weeks before the procedure. It’s also important to make sure the patient’s diabetes is well-controlled with other medications — not GLP-1 RAs — during this period.
After surgery, you can restart GLP-1 RA therapy once there is recovery of oral food intake and normal bowel function.
Audience member: Is treatment with GLP-1 RAs appropriate for a patient with a family history of colon cancer but an otherwise unremarkable medical and family history?
MC: I have not seen a contraindication to receiving GLP-1 RAs based on a family history of colorectal cancer or other malignancies. An analysis of the French national healthcare insurance system database has suggested 1-3 years use of GLP-1 RAs (exenatide, liraglutide, and dulaglutide) may be linked with increased occurrence of thyroid cancer. Data from 37 randomized controlled trials and 19 real-world studies having 16,839 patients in placebo control group, 16,550 patients in active control group, and 13,330 patients in real-world studies were analyzed in a 2023 systematic review and meta-analysis. Compared to placebo or active control treatments, occurrence of pancreatic cancer, thyroid cancer, and all neoplasms — benign, malignant, and otherwise unspecified — were similar in the semaglutide group.
Toshi Iroku-Malize, MD, MPH, MBA, FAAFP, family physician, Zucker School of Medicine, Hempstead, New York
Audience member: What do you do about elevated liver functions after starting treatment with GLP-1 RAs, and what do you do when a patient has reached their weight loss goal?
TI-M: I recommend monitoring the liver function tests, evaluating for underlying causes, such as viral hepatitis, alcohol-related damage, or problems with other medications, and consulting a gastroenterologist or liver specialist if necessary. It’s also important to discuss the risk-benefit of continuing on the GP-1 RA for that particular patient.
Audience member: What effects will GLP-1 RAs have on sleep-disordered breathing/obstructive sleep apnea (OSA)? Are you aware of any ongoing trials addressing this subject?
TI-M: GLP-1 RAs may have beneficial effects on sleep-disordered breathing and OSA through weight loss, which can lead to a reduction in excess adipose tissue, and improvements in metabolic parameters. In terms of studies, a 2023 paper addressed this question, but more research is needed.
Audience member: Is it within a psychiatric provider’s scope of practice to prescribe GLP-1 agents for the reduction of weight gain associated with psychiatric medications?
TI-M: Obesity medicine is an interdisciplinary process. Numerous medications prescribed for mental health can contribute to obesity, and psychiatrists can play a role in collaborating with a patient’s primary care provider and/or obesity medicine specialist to determine which medications can be adjusted or replaced. It is important to remember that obesity management is not just about medications. It requires managing nutrition and activity in addition to behavioral health issues and social determinants of health. If the clinician has had the training to manage these pillars and is comfortable managing this chronic illness — similar to diabetes, hypertension, and other conditions — then this is a possibility. Otherwise, team-based care is appropriate.
Holly Lofton, MD, obesity medicine, NYU Langone Health, New York City
Audience member: Can we safely use them on patients who have had bariatric surgery and regularly develop dumping syndrome?
HL: These medications can be used after bariatric surgery in patients who meet the criteria for pharmacologic treatment. If a patient is having postoperative symptoms of dumping syndrome or excessive gastrointestinal losses from vomiting or diarrhea, dietary adjustments and other methods of managing the dumping syndrome in gastric bypass patients should be initiated before considering GLP-1 RAs because these patients do not have a functioning pylorus in their alimentary tract and these drugs are not indicated to treat dumping syndrome. The first-line approach typically involves reducing the patient’s intake of simple carbohydrates but can also include medications or surgical intervention when appropriate.
Audience member: Would teaching a patient to fast intermittently while they’re on GLP-1 RAs help them preserve weight loss if they choose to wean off the medication?
HL: Personally, I feel it is best to use the titration period and the time in which the patient is actively losing weight when on GLP-1 RAs. These are the best periods to help develop an individualized treatment plan, one that includes nutrition, activity, behavior modification, and resistance training. The patient’s lifestyle plan will likely change based on their environment and other factors. Intermittent fasting can be a part of such a plan. There is no consensus as to exactly which eating pattern will help patients maintain weight once they lose the physiologic benefit of the weight loss medications. However, studies have been published that demonstrate an average weight regain of 66% or greater when patients go from taking the maximum dose of a GLP-1 RA to taking none at all. Thus, patients should still be followed closely for weight regain when they discontinue a GLP-1 RA.
A version of this article appeared on Medscape.com.
The arrival of GLP-1 receptor agonists has revolutionized treatment options for people with obesity and medical practice.
This news organization recently hosted a panel of experts across specialties — including endocrinology, gastroenterology, and obesity medicine — to discuss these potentially life-changing medications and to answer questions from the audience.
Because of the flood of queries from our audience, we asked our panelists to address some of the questions that didn’t make the recording. Their answers are below.
Beverly Tchang, MD, endocrinologist, Weill Cornell Medicine, New York City
Audience member: Can you initiate glucagon-like peptide-1 agonists (GLP-1 RAs) as a primary drug in a patient with obesity and newly diagnosed type 2 diabetes?
BT: We often prescribe GLP-1 RAs to individuals with type 2 diabetes as a first-line medication. Guidelines from the American Diabetes Association are really emphasizing a patient-centered approach, and metformin may not be the best first-line medication anymore.
Audience member:
BT: GLP-1 RAs do not need to be renally dosed, but I still recommend conferring with the patient’s nephrologist because the glomerular filtration rate might decrease in the setting of dehydration. Because GLP1s suppress the thirst, not just appetite, patients can go all day without drinking water and not feel thirsty.
Michael Camilleri, MD, gastroenterologist, Mayo Clinic, Rochester, Minnesota
Audience member: Should GLP-1 RAs be held for 1 week or 4 weeks prior to surgery to reduce the patient’s risk for aspiration? And is tapering required?
MC: For a patient taking liraglutide, I would hold the drug for 1 week prior to surgery. For patients taking other GLP-1 RAs, including extended exenatide, I advise holding for between 2 and 3 weeks before the procedure. It’s also important to make sure the patient’s diabetes is well-controlled with other medications — not GLP-1 RAs — during this period.
After surgery, you can restart GLP-1 RA therapy once there is recovery of oral food intake and normal bowel function.
Audience member: Is treatment with GLP-1 RAs appropriate for a patient with a family history of colon cancer but an otherwise unremarkable medical and family history?
MC: I have not seen a contraindication to receiving GLP-1 RAs based on a family history of colorectal cancer or other malignancies. An analysis of the French national healthcare insurance system database has suggested 1-3 years use of GLP-1 RAs (exenatide, liraglutide, and dulaglutide) may be linked with increased occurrence of thyroid cancer. Data from 37 randomized controlled trials and 19 real-world studies having 16,839 patients in placebo control group, 16,550 patients in active control group, and 13,330 patients in real-world studies were analyzed in a 2023 systematic review and meta-analysis. Compared to placebo or active control treatments, occurrence of pancreatic cancer, thyroid cancer, and all neoplasms — benign, malignant, and otherwise unspecified — were similar in the semaglutide group.
Toshi Iroku-Malize, MD, MPH, MBA, FAAFP, family physician, Zucker School of Medicine, Hempstead, New York
Audience member: What do you do about elevated liver functions after starting treatment with GLP-1 RAs, and what do you do when a patient has reached their weight loss goal?
TI-M: I recommend monitoring the liver function tests, evaluating for underlying causes, such as viral hepatitis, alcohol-related damage, or problems with other medications, and consulting a gastroenterologist or liver specialist if necessary. It’s also important to discuss the risk-benefit of continuing on the GP-1 RA for that particular patient.
Audience member: What effects will GLP-1 RAs have on sleep-disordered breathing/obstructive sleep apnea (OSA)? Are you aware of any ongoing trials addressing this subject?
TI-M: GLP-1 RAs may have beneficial effects on sleep-disordered breathing and OSA through weight loss, which can lead to a reduction in excess adipose tissue, and improvements in metabolic parameters. In terms of studies, a 2023 paper addressed this question, but more research is needed.
Audience member: Is it within a psychiatric provider’s scope of practice to prescribe GLP-1 agents for the reduction of weight gain associated with psychiatric medications?
TI-M: Obesity medicine is an interdisciplinary process. Numerous medications prescribed for mental health can contribute to obesity, and psychiatrists can play a role in collaborating with a patient’s primary care provider and/or obesity medicine specialist to determine which medications can be adjusted or replaced. It is important to remember that obesity management is not just about medications. It requires managing nutrition and activity in addition to behavioral health issues and social determinants of health. If the clinician has had the training to manage these pillars and is comfortable managing this chronic illness — similar to diabetes, hypertension, and other conditions — then this is a possibility. Otherwise, team-based care is appropriate.
Holly Lofton, MD, obesity medicine, NYU Langone Health, New York City
Audience member: Can we safely use them on patients who have had bariatric surgery and regularly develop dumping syndrome?
HL: These medications can be used after bariatric surgery in patients who meet the criteria for pharmacologic treatment. If a patient is having postoperative symptoms of dumping syndrome or excessive gastrointestinal losses from vomiting or diarrhea, dietary adjustments and other methods of managing the dumping syndrome in gastric bypass patients should be initiated before considering GLP-1 RAs because these patients do not have a functioning pylorus in their alimentary tract and these drugs are not indicated to treat dumping syndrome. The first-line approach typically involves reducing the patient’s intake of simple carbohydrates but can also include medications or surgical intervention when appropriate.
Audience member: Would teaching a patient to fast intermittently while they’re on GLP-1 RAs help them preserve weight loss if they choose to wean off the medication?
HL: Personally, I feel it is best to use the titration period and the time in which the patient is actively losing weight when on GLP-1 RAs. These are the best periods to help develop an individualized treatment plan, one that includes nutrition, activity, behavior modification, and resistance training. The patient’s lifestyle plan will likely change based on their environment and other factors. Intermittent fasting can be a part of such a plan. There is no consensus as to exactly which eating pattern will help patients maintain weight once they lose the physiologic benefit of the weight loss medications. However, studies have been published that demonstrate an average weight regain of 66% or greater when patients go from taking the maximum dose of a GLP-1 RA to taking none at all. Thus, patients should still be followed closely for weight regain when they discontinue a GLP-1 RA.
A version of this article appeared on Medscape.com.
Is Your Patient With PCOS at Risk for Suicide?
Women with polycystic ovary syndrome (PCOS) may be as much as eight times more likely to attempt suicide than are those without the disorder, according to a new study published in the Annals of Internal Medicine on February 5.
The results point to the importance of mental health screening for all patients who may have syndrome, the researchers concluded.
“If we can know such conditions earlier in our clinical practice, we may reduce the subsequence risk and bad consequences,” said Mu-Hong Chen, MD, PhD, an attending psychiatrist at the Department of Psychiatry at Taipei Veterans General Hospital in Taiwan, a coauthor of the study.
PCOS affects as many as 15% of reproductive-age women in the United States, or approximately six million people. The condition is associated with an increased risk for metabolic disorders, like diabetes and metabolic syndrome, and cardiovascular problems, like hypertension and stroke. The disorder is associated with infertility, weight gain, hirsutism, and skin changes. Evidence also shows that these changes can lead to poorer self-image and mental health conditions like depression and anxiety.
Dr. Chen and his coauthors compared the records of nearly 19,000 women between ages 12 and 64 years who had a PCOS diagnosis with a matched control group of 189,600 women and girls without PCOS using data from 1997 to 2012 in the Taiwan National Health Insurance Research Database. Cohorts were matched by age, income, urbanization level, and mental health conditions.
Older women with PCOS had slightly lower risk compared with younger women, but the risk was higher compared with older women without PCOS. Studies in other countries have shown similar results.
Adolescents with PCOS had more than five times the risk for attempted suicide than did the control group (hazard ratio [HR], 5.38; 95% CI, 3.93-7.3). Those between ages 20 and 40 years had more than nine times the risk for attempted suicide (HR, 9.15; 95% CI, 8.03-10.42), and those older than 40 years had the lowest risk (HR, 3.75; 95% CI, 2.23-6.28).
The number of women with PCOS in the study was likely underreported, and those who were included likely had more serious cases, according to Ricardo Azziz, MD, MPH, MBA, professor in the Department of Obstetrics & Gynecology and the Department of Medicine at the University of Alabama at Birmingham.
The findings, “speak to the fact that women with PCOS do have a greater incidence of mental health disorders and do require clinicians and patients themselves and their families to be aware of these risks,” said Dr. Azziz, former CEO of the American Society for Reproductive Medicine.
Clinicians should ask their patients with PCOS about suicide risk and mental health, according to Dr. Azziz.
“It’s not infrequent that those of us in clinical practice see patients who are significantly depressed, and we need to ask the right questions,” he said.
Though he was only aware of a few patients with PCOS who have attempted suicide, he said that clinicians should be prepared to refer these patients to another professional who can address mental health concerns if they express any signs of distress.
“Simply asking and inviting patients to speak about this will allow physicians to identify patients who may need to be referred,” Dr. Azziz said.
The study was funded by grants from the Taipei Veterans General Hospital, Yen Tjing Ling Medical Foundation, and the Ministry of Science and Technology of Taiwan.
The study authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Women with polycystic ovary syndrome (PCOS) may be as much as eight times more likely to attempt suicide than are those without the disorder, according to a new study published in the Annals of Internal Medicine on February 5.
The results point to the importance of mental health screening for all patients who may have syndrome, the researchers concluded.
“If we can know such conditions earlier in our clinical practice, we may reduce the subsequence risk and bad consequences,” said Mu-Hong Chen, MD, PhD, an attending psychiatrist at the Department of Psychiatry at Taipei Veterans General Hospital in Taiwan, a coauthor of the study.
PCOS affects as many as 15% of reproductive-age women in the United States, or approximately six million people. The condition is associated with an increased risk for metabolic disorders, like diabetes and metabolic syndrome, and cardiovascular problems, like hypertension and stroke. The disorder is associated with infertility, weight gain, hirsutism, and skin changes. Evidence also shows that these changes can lead to poorer self-image and mental health conditions like depression and anxiety.
Dr. Chen and his coauthors compared the records of nearly 19,000 women between ages 12 and 64 years who had a PCOS diagnosis with a matched control group of 189,600 women and girls without PCOS using data from 1997 to 2012 in the Taiwan National Health Insurance Research Database. Cohorts were matched by age, income, urbanization level, and mental health conditions.
Older women with PCOS had slightly lower risk compared with younger women, but the risk was higher compared with older women without PCOS. Studies in other countries have shown similar results.
Adolescents with PCOS had more than five times the risk for attempted suicide than did the control group (hazard ratio [HR], 5.38; 95% CI, 3.93-7.3). Those between ages 20 and 40 years had more than nine times the risk for attempted suicide (HR, 9.15; 95% CI, 8.03-10.42), and those older than 40 years had the lowest risk (HR, 3.75; 95% CI, 2.23-6.28).
The number of women with PCOS in the study was likely underreported, and those who were included likely had more serious cases, according to Ricardo Azziz, MD, MPH, MBA, professor in the Department of Obstetrics & Gynecology and the Department of Medicine at the University of Alabama at Birmingham.
The findings, “speak to the fact that women with PCOS do have a greater incidence of mental health disorders and do require clinicians and patients themselves and their families to be aware of these risks,” said Dr. Azziz, former CEO of the American Society for Reproductive Medicine.
Clinicians should ask their patients with PCOS about suicide risk and mental health, according to Dr. Azziz.
“It’s not infrequent that those of us in clinical practice see patients who are significantly depressed, and we need to ask the right questions,” he said.
Though he was only aware of a few patients with PCOS who have attempted suicide, he said that clinicians should be prepared to refer these patients to another professional who can address mental health concerns if they express any signs of distress.
“Simply asking and inviting patients to speak about this will allow physicians to identify patients who may need to be referred,” Dr. Azziz said.
The study was funded by grants from the Taipei Veterans General Hospital, Yen Tjing Ling Medical Foundation, and the Ministry of Science and Technology of Taiwan.
The study authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Women with polycystic ovary syndrome (PCOS) may be as much as eight times more likely to attempt suicide than are those without the disorder, according to a new study published in the Annals of Internal Medicine on February 5.
The results point to the importance of mental health screening for all patients who may have syndrome, the researchers concluded.
“If we can know such conditions earlier in our clinical practice, we may reduce the subsequence risk and bad consequences,” said Mu-Hong Chen, MD, PhD, an attending psychiatrist at the Department of Psychiatry at Taipei Veterans General Hospital in Taiwan, a coauthor of the study.
PCOS affects as many as 15% of reproductive-age women in the United States, or approximately six million people. The condition is associated with an increased risk for metabolic disorders, like diabetes and metabolic syndrome, and cardiovascular problems, like hypertension and stroke. The disorder is associated with infertility, weight gain, hirsutism, and skin changes. Evidence also shows that these changes can lead to poorer self-image and mental health conditions like depression and anxiety.
Dr. Chen and his coauthors compared the records of nearly 19,000 women between ages 12 and 64 years who had a PCOS diagnosis with a matched control group of 189,600 women and girls without PCOS using data from 1997 to 2012 in the Taiwan National Health Insurance Research Database. Cohorts were matched by age, income, urbanization level, and mental health conditions.
Older women with PCOS had slightly lower risk compared with younger women, but the risk was higher compared with older women without PCOS. Studies in other countries have shown similar results.
Adolescents with PCOS had more than five times the risk for attempted suicide than did the control group (hazard ratio [HR], 5.38; 95% CI, 3.93-7.3). Those between ages 20 and 40 years had more than nine times the risk for attempted suicide (HR, 9.15; 95% CI, 8.03-10.42), and those older than 40 years had the lowest risk (HR, 3.75; 95% CI, 2.23-6.28).
The number of women with PCOS in the study was likely underreported, and those who were included likely had more serious cases, according to Ricardo Azziz, MD, MPH, MBA, professor in the Department of Obstetrics & Gynecology and the Department of Medicine at the University of Alabama at Birmingham.
The findings, “speak to the fact that women with PCOS do have a greater incidence of mental health disorders and do require clinicians and patients themselves and their families to be aware of these risks,” said Dr. Azziz, former CEO of the American Society for Reproductive Medicine.
Clinicians should ask their patients with PCOS about suicide risk and mental health, according to Dr. Azziz.
“It’s not infrequent that those of us in clinical practice see patients who are significantly depressed, and we need to ask the right questions,” he said.
Though he was only aware of a few patients with PCOS who have attempted suicide, he said that clinicians should be prepared to refer these patients to another professional who can address mental health concerns if they express any signs of distress.
“Simply asking and inviting patients to speak about this will allow physicians to identify patients who may need to be referred,” Dr. Azziz said.
The study was funded by grants from the Taipei Veterans General Hospital, Yen Tjing Ling Medical Foundation, and the Ministry of Science and Technology of Taiwan.
The study authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
New Findings on Vitamin D, Omega-3 Supplements for Preventing Autoimmune Diseases
Two years after the end of a randomized trial that showed a benefit of daily vitamin D and omega-3 fatty acid (n-3 FA) supplementation for reducing risk for autoimmune diseases, the salubrious effects of daily vitamin D appear to have waned after the supplement was discontinued, while the protection from n-3 lived on for at least 2 additional years.
As previously reported, the randomized VITAL, which was designed primarily to study the effects of vitamin D and n-3 supplementation on incident cancer and cardiovascular disease, also showed that 5 years of vitamin D supplementation was associated with a 22% reduction in risk for confirmed autoimmune diseases, and 5 years of n-3 FA supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases.
Now, investigators Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston, Massachusetts, and colleagues reported that among 21,592 participants in VITAL who agreed to be followed for an additional 2 years after discontinuation, the protection against autoimmune diseases from daily vitamin D (cholecalciferol; 2000 IU/d) was no longer statistically significant, but the benefits of daily marine n-3 FAs (1 g/d as a fish-oil capsule containing 460 mg of eicosapentaenoic acid and 380 mg of docosahexaenoic acid) remained significant.
“VITAL observational extension results suggest that vitamin D supplementation should be given on a continuous basis for long-term prevention of [autoimmune diseases]. The beneficial effects of n-3 fatty acids, however, may be prolonged for at least 2 years after discontinuation,” they wrote in an article published in Arthritis & Rheumatology.
Dr. Costenbader told this news organization that the results of the observational extension study suggest that the benefits of vitamin D “wear off more quickly, and it should be continued for a longer period of time or indefinitely, rather than only for 5 years.”
In addition to the disparity in the duration of the protective effect, the investigators also saw differences in the effects across different autoimmune diseases.
“The protective effect of vitamin D seemed strongest for psoriasis, while for omega-3 fatty acids, the protective effects were strongest for rheumatoid arthritis and inflammatory bowel disease,” she said.
Mixed Effects
In an interview with this news organization, Janet Funk, MD, MS, vice chair of research in the Department of Medicine and professor in the School of Nutritional Science and Wellness at the University of Arizona, Tucson, Arizona, who was not involved in the study, saidthat the results suggest that while each supplement may offer protection against autoimmune diseases, the effects are inconsistent and may not apply to all patients.
“I think the VITAL extension results suggest that either supplement (or both together) may have benefits in reducing risk of autoimmune diseases, including possible persistent effects posttreatment, but that these effects are nuanced (ie, only in normal weight post-vitamin D treatment) and possibly not uniform across all autoimmune diseases (including possible adverse effects for some — eg, inverse association between prior omega-3 and psoriasis and tendency for increased autoimmune thyroid disease for vitamin D), although the study was not powered sufficiently to draw disease-specific conclusions,” she said.
In an editorial accompanying the study, rheumatologist Joel M. Kremer, MD, of Albany Medical College and the Corrona Research Foundation in Delray Beach, Florida, wrote that “[T]he studies by Dr. Costenbader, et al. have shed new light on the possibility that dietary supplements of n-3 FA [fatty acid] may prevent the onset of [autoimmune disease]. The sustained benefits they describe for as long as 2 years after the supplements are discontinued are consistent with the chronicity of FA species in cellular plasma membranes where they serve as substrates for a diverse array of salient metabolic and inflammatory pathways.”
VITAL Then
To test whether vitamin D or marine-derived long-chain n-3 FA supplementation could protect against autoimmune disease over time, Dr. Costenbader and colleagues piggybacked an ancillary study onto the VITAL trial, which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older and 13,085 women aged 55 and older. The study had a 2 × 2 factorial design, with patients randomly assigned to vitamin D 2000 IU/d or placebo and then further randomized to either 1 g/d n-3 FAs or placebo in both the vitamin D and placebo primary randomization arms.
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with a hazard ratio (HR) of 0.68 (P = .02) for incident autoimmune disease, n-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03). However, when probable incident autoimmune disease cases were included, the effect of n-3 became significant, with an HR of 0.82.
VITAL Now
In the current analysis, Dr. Costenbader and colleagues reported observational data on 21,592 VITAL participants, a sample representing 83.5% of those who were initially randomized, and 87.9% of those who were alive and could be contacted at the end of the study.
As in the initial trial, the investigators used annual questionnaires to assess incident autoimmune diseases during the randomized follow-up. Participants were asked about new-onset, doctor-diagnosed rheumatoid arthritis, polymyalgia rheumatica, psoriasis, autoimmune thyroid disease, and inflammatory bowel disease. Participants could also write in any other new autoimmune disease diagnoses.
There were 236 new cases of confirmed autoimmune disease that occurred since the initial publication of the trial results, as well as 65 probable cases identified during the median 5.3 years of the randomized portion, and 42 probable cases diagnosed during the 2-year observational phase.
The investigators found that after the 2-year observation period, 255 participants initially randomized to receive vitamin D had a newly developed confirmed autoimmune disease, compared with 259 of those initially randomized to a vitamin D placebo. This translated into a nonsignificant HR of 0.98.
Adding probable autoimmune cases to the confirmed cases made little difference, resulting in a nonsignificant adjusted HR of 0.95.
In contrast, there were 234 confirmed autoimmune disease cases among patients initially assigned to n-3, compared with 280 among patients randomized to the n-3 placebo, translating into a statistically significant HR of 0.83 for new-onset autoimmune disease with n-3.
Dr. Costenbader and colleagues acknowledged that the study was limited by the use of doses intended to prevent cancer or cardiovascular disease and that higher doses intended for high-risk or nutritionally deficient populations might reveal larger effects of supplementation. In addition, they noted the difficulty of identifying the timing and onset of incident disease, and that the small number of cases that occurred during the 2-year observational period precluded detailed analyses of individual autoimmune diseases.
The study was funded by grants from the National Institutes of Health. Dr. Costenbader, Dr. Funk, and Dr. Kremer reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Two years after the end of a randomized trial that showed a benefit of daily vitamin D and omega-3 fatty acid (n-3 FA) supplementation for reducing risk for autoimmune diseases, the salubrious effects of daily vitamin D appear to have waned after the supplement was discontinued, while the protection from n-3 lived on for at least 2 additional years.
As previously reported, the randomized VITAL, which was designed primarily to study the effects of vitamin D and n-3 supplementation on incident cancer and cardiovascular disease, also showed that 5 years of vitamin D supplementation was associated with a 22% reduction in risk for confirmed autoimmune diseases, and 5 years of n-3 FA supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases.
Now, investigators Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston, Massachusetts, and colleagues reported that among 21,592 participants in VITAL who agreed to be followed for an additional 2 years after discontinuation, the protection against autoimmune diseases from daily vitamin D (cholecalciferol; 2000 IU/d) was no longer statistically significant, but the benefits of daily marine n-3 FAs (1 g/d as a fish-oil capsule containing 460 mg of eicosapentaenoic acid and 380 mg of docosahexaenoic acid) remained significant.
“VITAL observational extension results suggest that vitamin D supplementation should be given on a continuous basis for long-term prevention of [autoimmune diseases]. The beneficial effects of n-3 fatty acids, however, may be prolonged for at least 2 years after discontinuation,” they wrote in an article published in Arthritis & Rheumatology.
Dr. Costenbader told this news organization that the results of the observational extension study suggest that the benefits of vitamin D “wear off more quickly, and it should be continued for a longer period of time or indefinitely, rather than only for 5 years.”
In addition to the disparity in the duration of the protective effect, the investigators also saw differences in the effects across different autoimmune diseases.
“The protective effect of vitamin D seemed strongest for psoriasis, while for omega-3 fatty acids, the protective effects were strongest for rheumatoid arthritis and inflammatory bowel disease,” she said.
Mixed Effects
In an interview with this news organization, Janet Funk, MD, MS, vice chair of research in the Department of Medicine and professor in the School of Nutritional Science and Wellness at the University of Arizona, Tucson, Arizona, who was not involved in the study, saidthat the results suggest that while each supplement may offer protection against autoimmune diseases, the effects are inconsistent and may not apply to all patients.
“I think the VITAL extension results suggest that either supplement (or both together) may have benefits in reducing risk of autoimmune diseases, including possible persistent effects posttreatment, but that these effects are nuanced (ie, only in normal weight post-vitamin D treatment) and possibly not uniform across all autoimmune diseases (including possible adverse effects for some — eg, inverse association between prior omega-3 and psoriasis and tendency for increased autoimmune thyroid disease for vitamin D), although the study was not powered sufficiently to draw disease-specific conclusions,” she said.
In an editorial accompanying the study, rheumatologist Joel M. Kremer, MD, of Albany Medical College and the Corrona Research Foundation in Delray Beach, Florida, wrote that “[T]he studies by Dr. Costenbader, et al. have shed new light on the possibility that dietary supplements of n-3 FA [fatty acid] may prevent the onset of [autoimmune disease]. The sustained benefits they describe for as long as 2 years after the supplements are discontinued are consistent with the chronicity of FA species in cellular plasma membranes where they serve as substrates for a diverse array of salient metabolic and inflammatory pathways.”
VITAL Then
To test whether vitamin D or marine-derived long-chain n-3 FA supplementation could protect against autoimmune disease over time, Dr. Costenbader and colleagues piggybacked an ancillary study onto the VITAL trial, which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older and 13,085 women aged 55 and older. The study had a 2 × 2 factorial design, with patients randomly assigned to vitamin D 2000 IU/d or placebo and then further randomized to either 1 g/d n-3 FAs or placebo in both the vitamin D and placebo primary randomization arms.
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with a hazard ratio (HR) of 0.68 (P = .02) for incident autoimmune disease, n-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03). However, when probable incident autoimmune disease cases were included, the effect of n-3 became significant, with an HR of 0.82.
VITAL Now
In the current analysis, Dr. Costenbader and colleagues reported observational data on 21,592 VITAL participants, a sample representing 83.5% of those who were initially randomized, and 87.9% of those who were alive and could be contacted at the end of the study.
As in the initial trial, the investigators used annual questionnaires to assess incident autoimmune diseases during the randomized follow-up. Participants were asked about new-onset, doctor-diagnosed rheumatoid arthritis, polymyalgia rheumatica, psoriasis, autoimmune thyroid disease, and inflammatory bowel disease. Participants could also write in any other new autoimmune disease diagnoses.
There were 236 new cases of confirmed autoimmune disease that occurred since the initial publication of the trial results, as well as 65 probable cases identified during the median 5.3 years of the randomized portion, and 42 probable cases diagnosed during the 2-year observational phase.
The investigators found that after the 2-year observation period, 255 participants initially randomized to receive vitamin D had a newly developed confirmed autoimmune disease, compared with 259 of those initially randomized to a vitamin D placebo. This translated into a nonsignificant HR of 0.98.
Adding probable autoimmune cases to the confirmed cases made little difference, resulting in a nonsignificant adjusted HR of 0.95.
In contrast, there were 234 confirmed autoimmune disease cases among patients initially assigned to n-3, compared with 280 among patients randomized to the n-3 placebo, translating into a statistically significant HR of 0.83 for new-onset autoimmune disease with n-3.
Dr. Costenbader and colleagues acknowledged that the study was limited by the use of doses intended to prevent cancer or cardiovascular disease and that higher doses intended for high-risk or nutritionally deficient populations might reveal larger effects of supplementation. In addition, they noted the difficulty of identifying the timing and onset of incident disease, and that the small number of cases that occurred during the 2-year observational period precluded detailed analyses of individual autoimmune diseases.
The study was funded by grants from the National Institutes of Health. Dr. Costenbader, Dr. Funk, and Dr. Kremer reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Two years after the end of a randomized trial that showed a benefit of daily vitamin D and omega-3 fatty acid (n-3 FA) supplementation for reducing risk for autoimmune diseases, the salubrious effects of daily vitamin D appear to have waned after the supplement was discontinued, while the protection from n-3 lived on for at least 2 additional years.
As previously reported, the randomized VITAL, which was designed primarily to study the effects of vitamin D and n-3 supplementation on incident cancer and cardiovascular disease, also showed that 5 years of vitamin D supplementation was associated with a 22% reduction in risk for confirmed autoimmune diseases, and 5 years of n-3 FA supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases.
Now, investigators Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston, Massachusetts, and colleagues reported that among 21,592 participants in VITAL who agreed to be followed for an additional 2 years after discontinuation, the protection against autoimmune diseases from daily vitamin D (cholecalciferol; 2000 IU/d) was no longer statistically significant, but the benefits of daily marine n-3 FAs (1 g/d as a fish-oil capsule containing 460 mg of eicosapentaenoic acid and 380 mg of docosahexaenoic acid) remained significant.
“VITAL observational extension results suggest that vitamin D supplementation should be given on a continuous basis for long-term prevention of [autoimmune diseases]. The beneficial effects of n-3 fatty acids, however, may be prolonged for at least 2 years after discontinuation,” they wrote in an article published in Arthritis & Rheumatology.
Dr. Costenbader told this news organization that the results of the observational extension study suggest that the benefits of vitamin D “wear off more quickly, and it should be continued for a longer period of time or indefinitely, rather than only for 5 years.”
In addition to the disparity in the duration of the protective effect, the investigators also saw differences in the effects across different autoimmune diseases.
“The protective effect of vitamin D seemed strongest for psoriasis, while for omega-3 fatty acids, the protective effects were strongest for rheumatoid arthritis and inflammatory bowel disease,” she said.
Mixed Effects
In an interview with this news organization, Janet Funk, MD, MS, vice chair of research in the Department of Medicine and professor in the School of Nutritional Science and Wellness at the University of Arizona, Tucson, Arizona, who was not involved in the study, saidthat the results suggest that while each supplement may offer protection against autoimmune diseases, the effects are inconsistent and may not apply to all patients.
“I think the VITAL extension results suggest that either supplement (or both together) may have benefits in reducing risk of autoimmune diseases, including possible persistent effects posttreatment, but that these effects are nuanced (ie, only in normal weight post-vitamin D treatment) and possibly not uniform across all autoimmune diseases (including possible adverse effects for some — eg, inverse association between prior omega-3 and psoriasis and tendency for increased autoimmune thyroid disease for vitamin D), although the study was not powered sufficiently to draw disease-specific conclusions,” she said.
In an editorial accompanying the study, rheumatologist Joel M. Kremer, MD, of Albany Medical College and the Corrona Research Foundation in Delray Beach, Florida, wrote that “[T]he studies by Dr. Costenbader, et al. have shed new light on the possibility that dietary supplements of n-3 FA [fatty acid] may prevent the onset of [autoimmune disease]. The sustained benefits they describe for as long as 2 years after the supplements are discontinued are consistent with the chronicity of FA species in cellular plasma membranes where they serve as substrates for a diverse array of salient metabolic and inflammatory pathways.”
VITAL Then
To test whether vitamin D or marine-derived long-chain n-3 FA supplementation could protect against autoimmune disease over time, Dr. Costenbader and colleagues piggybacked an ancillary study onto the VITAL trial, which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older and 13,085 women aged 55 and older. The study had a 2 × 2 factorial design, with patients randomly assigned to vitamin D 2000 IU/d or placebo and then further randomized to either 1 g/d n-3 FAs or placebo in both the vitamin D and placebo primary randomization arms.
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with a hazard ratio (HR) of 0.68 (P = .02) for incident autoimmune disease, n-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03). However, when probable incident autoimmune disease cases were included, the effect of n-3 became significant, with an HR of 0.82.
VITAL Now
In the current analysis, Dr. Costenbader and colleagues reported observational data on 21,592 VITAL participants, a sample representing 83.5% of those who were initially randomized, and 87.9% of those who were alive and could be contacted at the end of the study.
As in the initial trial, the investigators used annual questionnaires to assess incident autoimmune diseases during the randomized follow-up. Participants were asked about new-onset, doctor-diagnosed rheumatoid arthritis, polymyalgia rheumatica, psoriasis, autoimmune thyroid disease, and inflammatory bowel disease. Participants could also write in any other new autoimmune disease diagnoses.
There were 236 new cases of confirmed autoimmune disease that occurred since the initial publication of the trial results, as well as 65 probable cases identified during the median 5.3 years of the randomized portion, and 42 probable cases diagnosed during the 2-year observational phase.
The investigators found that after the 2-year observation period, 255 participants initially randomized to receive vitamin D had a newly developed confirmed autoimmune disease, compared with 259 of those initially randomized to a vitamin D placebo. This translated into a nonsignificant HR of 0.98.
Adding probable autoimmune cases to the confirmed cases made little difference, resulting in a nonsignificant adjusted HR of 0.95.
In contrast, there were 234 confirmed autoimmune disease cases among patients initially assigned to n-3, compared with 280 among patients randomized to the n-3 placebo, translating into a statistically significant HR of 0.83 for new-onset autoimmune disease with n-3.
Dr. Costenbader and colleagues acknowledged that the study was limited by the use of doses intended to prevent cancer or cardiovascular disease and that higher doses intended for high-risk or nutritionally deficient populations might reveal larger effects of supplementation. In addition, they noted the difficulty of identifying the timing and onset of incident disease, and that the small number of cases that occurred during the 2-year observational period precluded detailed analyses of individual autoimmune diseases.
The study was funded by grants from the National Institutes of Health. Dr. Costenbader, Dr. Funk, and Dr. Kremer reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY