User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
My journey with mental illness
I am a retired advanced practice psychiatric nurse who has lived and worked on “both sides of the door.” This wording is paraphrased from psychologist and therapist Lauren Slater, PhD, who wrote about a time she went to McLean Hospital in Belmont, Massachusetts, as a therapist after staying there as a patient years earlier: “And now I am standing on the other—the wrong, I mean the right side of the door and I ring the buzzer.”1 Here I tell my story of the physical and emotional effects of my mental illness and treatment.
Onset of bipolar disorder. My bipolar illness started with a bout of depression in 1963 at age 13, which resulted in a low-key summer of often staying inside. I received no medication, and no one sent me for evaluation. In the fall, I went back to school and finished the year without incident. I continued as a quiet, shy kid through high school in the late 1960s. In my senior year, I decided to take an overload of difficult courses and run on the varsity cross-country team. The amount and intensity of these activities were too much. This resulted in my first manic episode, which started during a weekend visit to a college I hoped to attend. I became excitable, grandiose, and had delusions. A day later, I returned home, and my parents had me admitted to a psychiatric hospital, where I remained for 3 months.
At first, my diagnosis was unclear, and initially no one considered what at the time was called manic depression. At that point, I was unaware of my extensive family psychiatric history. My pharmacologic treatment consisted of chlorpromazine, trifluoperazine, and procyclidine. I returned home just before Christmas and barely finished my senior year of high school. A good college accepted me. But during the orientation, I was asked to leave because I experienced a second manic episode. After 4 more psychiatric hospitalizations, I finally stabilized.
During one of my hospitalizations, I had the good fortune to be interviewed by Dr. Thomas Detre. During this interview, I talked expansively about Don Quixote, Aldonza, and Sancho Panza. Dr. Detre diagnosed me with manic depression, and suggested that I see Dr. Christiaan van der Velde, who was researching lithium carbonate.2 In 1970, I was hospitalized at Norwich State Hospital in Preston, Connecticut and was started on lithium, even though it had not yet been FDA-approved. I responded well to lithium monotherapy.
An extensive family history. Having bipolar disorder was not something I would discuss with others because I felt ashamed. I commonly hid my medication during college, especially from my roommates or other friends. By then, I had learned a little about my family’s psychiatric history, but I knew few specifics. Over time, I became aware of a dense familial cluster of affective illness going back several generations. My maternal grandmother was hospitalized for depression in 1921 after her husband suddenly died during her fourth pregnancy. She became bereft and suicidal because she had no one to support her 4 children. During my grandmother’s hospitalization, her sister and sister’s husband took care of her children. My grandmother remained hospitalized until she died in 1943. At that time, no medications were available to treat her illness. Over the next 2 generations, 2 of her 4 children and 6 of her 12 grandchildren (including me) developed bipolar disorder.
A career and family. In 1970, I started to work as a nursing assistant, then as a nursing technician for 1.5 years in a specialty hospital in New England. In 1973, I began nursing school at a junior college. I received my RN in 1975, a BS in nursing in 1979, and an MS in psychiatric nursing in 1982. I worked steadily as a psychiatric nurse in both inpatient and outpatient settings from 1975 until I retired in 2019.
In the early 1980s, I married my first wife and had 2 wonderful children. During our courtship in 1981 and 1982, I became hypomanic, which perhaps made me more outgoing and sociable. In 1985, after my father required open heart surgery, I had a manic episode that lasted 1 week. Over the next 20 years, although I was not happy with my marriage, I remained euthymic and productive at work. My marriage ended in 2012.
Continue to: By the end of 2012...
By the end of 2012, I had been taking lithium continuously for 42 years. My laboratory tests showed peak lithium levels between 0.6 and 1.2 mmol/L. I remained otherwise healthy, as demonstrated by annual physical exams and laboratory test results. In 2015, I developed an increase in my blood pressure and my primary care physician (PCP) prescribed oral lisinopril, initially 10 mg/d, and later 10 mg twice daily. My blood pressure improved and ranged from 120/74 to 130/82 mm Hg.
Hyperparathyroidism. By 2016, my psychiatrist, PCP, and nephrologist all urged me to consider parathyroid surgery.3-5 Hypercalcemia and hyperparathyroidism caused the most worry. Laboratory tests indicated calcium 11.2 mg/dL, parathyroid hormone (PTH) 88 pg/mL, estimated glomerular filtration rate (eGFR) 59 mL/min, and thyroid-stimulating hormone (TSH) 0.78 mIU/L. Electrocardiographysometimes showed a slight QT elongation. A right bundle branch block, which was first noted in 2015, continued. Due to my elevated calcium levels, I eliminated most calcium from my diet. My psychiatrist began to speak more strongly of parathyroid surgery. I then consulted a senior endocrinologist and a senior nephrologist, who each recommended parathyroid surgery.
I remarried in July 2016, and we moved to a different area of the country. My second wife became a stabilizing force for me. My new PCP, however, found elevated high-density lipoproteins during a routine physical examination, and started me on simvastatin, 10 mg/d. My calcium and PTH levels continued to be elevated. My PCP, nephrologist, therapist, and wife urged me to proceed with the parathyroidectomy. After a short period of watchful waiting and a second consultation with a nephrologist, I agreed to schedule a subtotal parathyroidectomy.
Surgery. In spring 2017, I began preparation for parathyroidectomy. At the time, my lithium carbonate dose was 600 mg/d, alternating with 900 mg/d. My peak level of lithium was 0.6 mmol/L. Lisinopril is synergistic, which allowed me to take a smaller effective dose of lithium.
My parathyroid surgery occurred on June 28, 2017 at Norman Parathyroid Center in Tampa, Florida.6 The surgeon recorded my parathyroid glands as 136, 602, and 348 units using a measure developed at Norman Parathyroid Center. No reading was given for my fourth parathyroid gland, which they did not remove. Following the surgery, I resumed my previous functions, including employment as a visiting nurse. I initially took calcium supplements after surgery, and my lithium dose was reduced to 300 mg orally, twice daily, which I have continued. I have remained euthymic. On August 3, 2017 my laboratory workup showed an eGFR of 64 mL/min, calcium 10.0 mg/dL, and PTH 17 pg/mL. Vitamin D25 OH 33, glucose, BUN/Cr, electrolytes, complete blood count, and albumin were all within normal limits. Repeat bloodwork on September 19, 2017 showed Ca++ 10.1 mg/dL and PTH 18 pg/mL. Nine months after the surgery, I showed an incredibly positive physical and mental response, which has continued to this day.
Continue to: Clinical implications
Clinical implications. This is a single case study. However, it is important for clinicians treating patients with lithium carbonate to regularly order laboratory testing, including for lithium levels, PTH, and calcium, to detect early signs of complications from treatment, including hyperparathyroidism and hypercalcemia.7 These levels could be obtained every 6 months. If a patient’s PTH levels are >70 pg/mL and calcium levels are >11.0 mg/dL, it would be prudent to refer him/her for further medical evaluation. Additionally, it would be helpful to counsel the patient about considering alternative medication and adjunct mental health treatment. At some future point, it could be useful for the clinician and his/her patient to explore the idea of parathyroid surgery.
In addition to chronic lithium use, other causes of hyperparathyroidism include an adenoma on a gland, hyperplasia of ≥2 parathyroid glands, a malignant tumor, severe calcium deficiency, severe vitamin D deficiency, chronic renal failure, and (rarely) an inherited gene that causes hyperparathyroidism.
How I’m doing today. Currently, I am euthymic and in a happy marriage. My laboratory workup in May 2020 included glucose 107 mg/dL, Ca++ 9.5 mg/dL, eGFR 61 mL/min, PTH 32 pg/mL, lithium 0.3 mmol/L (300 mg twice daily), and TSH 1.79 mIU/L. A comprehensive metabolic panel, complete blood count, and lipid panel were all within normal limits.
I am fortunate to continue having excellent care provided by my PCP, nephrologist, urologist, and psychiatric APRN. Together with these wonderful professionals, I have been able to maintain my physical and mental health.
Acknowledgment: I gratefully acknowledge the help and skills of Robin Scharak and Gary Blake for providing some of the editing on this article.
Bill Greenberg MS, RN, APRN
Delray Beach, Florida
1. Slater L. Welcome to my country. New York, NY: Random House; 1996:187.
2. Van der Velde CD. Effectiveness of lithium in the treatment of manic-depressive illness. Am J Psychiatry. 1970;127(3):345-351.
3. Norman Parathyroid Center. Parathyroid glands, high calcium and hyperparathyroidism. www.parathyroid.com. Updated October 21, 2020. Accessed November 11, 2020.
4. Meehan AD, Udumyan R, Kardell M, et al. Lithium-associated hypercalcemia: pathophysiology, prevalence, management. World J Surg. 2018;42(2):415-424.
5. Lally J, Lee B, McDonald C. Prevalence of hypercalcaemia in patients on maintenance lithium therapy monitored in primary care. Ir Med J. 2013;106(1):15-17.
6. Norman Parathyroid Center. Parathyroid surgery: minimally invasive 4-gland parathyroid surgery video. (4-Gland MIRP Parathyroid Operation). https://www.parathyroid.com/parathyroid-surgery.htm. Updated October 1, 2020. Accessed November 5, 2020.
7. MEDSAFE. Hyperparathyroidism and hypercalcaemia with lithium treatment. New Zealand Medicines and Medical Devices Safety Authority. 2014;35(3):37-38.
I am a retired advanced practice psychiatric nurse who has lived and worked on “both sides of the door.” This wording is paraphrased from psychologist and therapist Lauren Slater, PhD, who wrote about a time she went to McLean Hospital in Belmont, Massachusetts, as a therapist after staying there as a patient years earlier: “And now I am standing on the other—the wrong, I mean the right side of the door and I ring the buzzer.”1 Here I tell my story of the physical and emotional effects of my mental illness and treatment.
Onset of bipolar disorder. My bipolar illness started with a bout of depression in 1963 at age 13, which resulted in a low-key summer of often staying inside. I received no medication, and no one sent me for evaluation. In the fall, I went back to school and finished the year without incident. I continued as a quiet, shy kid through high school in the late 1960s. In my senior year, I decided to take an overload of difficult courses and run on the varsity cross-country team. The amount and intensity of these activities were too much. This resulted in my first manic episode, which started during a weekend visit to a college I hoped to attend. I became excitable, grandiose, and had delusions. A day later, I returned home, and my parents had me admitted to a psychiatric hospital, where I remained for 3 months.
At first, my diagnosis was unclear, and initially no one considered what at the time was called manic depression. At that point, I was unaware of my extensive family psychiatric history. My pharmacologic treatment consisted of chlorpromazine, trifluoperazine, and procyclidine. I returned home just before Christmas and barely finished my senior year of high school. A good college accepted me. But during the orientation, I was asked to leave because I experienced a second manic episode. After 4 more psychiatric hospitalizations, I finally stabilized.
During one of my hospitalizations, I had the good fortune to be interviewed by Dr. Thomas Detre. During this interview, I talked expansively about Don Quixote, Aldonza, and Sancho Panza. Dr. Detre diagnosed me with manic depression, and suggested that I see Dr. Christiaan van der Velde, who was researching lithium carbonate.2 In 1970, I was hospitalized at Norwich State Hospital in Preston, Connecticut and was started on lithium, even though it had not yet been FDA-approved. I responded well to lithium monotherapy.
An extensive family history. Having bipolar disorder was not something I would discuss with others because I felt ashamed. I commonly hid my medication during college, especially from my roommates or other friends. By then, I had learned a little about my family’s psychiatric history, but I knew few specifics. Over time, I became aware of a dense familial cluster of affective illness going back several generations. My maternal grandmother was hospitalized for depression in 1921 after her husband suddenly died during her fourth pregnancy. She became bereft and suicidal because she had no one to support her 4 children. During my grandmother’s hospitalization, her sister and sister’s husband took care of her children. My grandmother remained hospitalized until she died in 1943. At that time, no medications were available to treat her illness. Over the next 2 generations, 2 of her 4 children and 6 of her 12 grandchildren (including me) developed bipolar disorder.
A career and family. In 1970, I started to work as a nursing assistant, then as a nursing technician for 1.5 years in a specialty hospital in New England. In 1973, I began nursing school at a junior college. I received my RN in 1975, a BS in nursing in 1979, and an MS in psychiatric nursing in 1982. I worked steadily as a psychiatric nurse in both inpatient and outpatient settings from 1975 until I retired in 2019.
In the early 1980s, I married my first wife and had 2 wonderful children. During our courtship in 1981 and 1982, I became hypomanic, which perhaps made me more outgoing and sociable. In 1985, after my father required open heart surgery, I had a manic episode that lasted 1 week. Over the next 20 years, although I was not happy with my marriage, I remained euthymic and productive at work. My marriage ended in 2012.
Continue to: By the end of 2012...
By the end of 2012, I had been taking lithium continuously for 42 years. My laboratory tests showed peak lithium levels between 0.6 and 1.2 mmol/L. I remained otherwise healthy, as demonstrated by annual physical exams and laboratory test results. In 2015, I developed an increase in my blood pressure and my primary care physician (PCP) prescribed oral lisinopril, initially 10 mg/d, and later 10 mg twice daily. My blood pressure improved and ranged from 120/74 to 130/82 mm Hg.
Hyperparathyroidism. By 2016, my psychiatrist, PCP, and nephrologist all urged me to consider parathyroid surgery.3-5 Hypercalcemia and hyperparathyroidism caused the most worry. Laboratory tests indicated calcium 11.2 mg/dL, parathyroid hormone (PTH) 88 pg/mL, estimated glomerular filtration rate (eGFR) 59 mL/min, and thyroid-stimulating hormone (TSH) 0.78 mIU/L. Electrocardiographysometimes showed a slight QT elongation. A right bundle branch block, which was first noted in 2015, continued. Due to my elevated calcium levels, I eliminated most calcium from my diet. My psychiatrist began to speak more strongly of parathyroid surgery. I then consulted a senior endocrinologist and a senior nephrologist, who each recommended parathyroid surgery.
I remarried in July 2016, and we moved to a different area of the country. My second wife became a stabilizing force for me. My new PCP, however, found elevated high-density lipoproteins during a routine physical examination, and started me on simvastatin, 10 mg/d. My calcium and PTH levels continued to be elevated. My PCP, nephrologist, therapist, and wife urged me to proceed with the parathyroidectomy. After a short period of watchful waiting and a second consultation with a nephrologist, I agreed to schedule a subtotal parathyroidectomy.
Surgery. In spring 2017, I began preparation for parathyroidectomy. At the time, my lithium carbonate dose was 600 mg/d, alternating with 900 mg/d. My peak level of lithium was 0.6 mmol/L. Lisinopril is synergistic, which allowed me to take a smaller effective dose of lithium.
My parathyroid surgery occurred on June 28, 2017 at Norman Parathyroid Center in Tampa, Florida.6 The surgeon recorded my parathyroid glands as 136, 602, and 348 units using a measure developed at Norman Parathyroid Center. No reading was given for my fourth parathyroid gland, which they did not remove. Following the surgery, I resumed my previous functions, including employment as a visiting nurse. I initially took calcium supplements after surgery, and my lithium dose was reduced to 300 mg orally, twice daily, which I have continued. I have remained euthymic. On August 3, 2017 my laboratory workup showed an eGFR of 64 mL/min, calcium 10.0 mg/dL, and PTH 17 pg/mL. Vitamin D25 OH 33, glucose, BUN/Cr, electrolytes, complete blood count, and albumin were all within normal limits. Repeat bloodwork on September 19, 2017 showed Ca++ 10.1 mg/dL and PTH 18 pg/mL. Nine months after the surgery, I showed an incredibly positive physical and mental response, which has continued to this day.
Continue to: Clinical implications
Clinical implications. This is a single case study. However, it is important for clinicians treating patients with lithium carbonate to regularly order laboratory testing, including for lithium levels, PTH, and calcium, to detect early signs of complications from treatment, including hyperparathyroidism and hypercalcemia.7 These levels could be obtained every 6 months. If a patient’s PTH levels are >70 pg/mL and calcium levels are >11.0 mg/dL, it would be prudent to refer him/her for further medical evaluation. Additionally, it would be helpful to counsel the patient about considering alternative medication and adjunct mental health treatment. At some future point, it could be useful for the clinician and his/her patient to explore the idea of parathyroid surgery.
In addition to chronic lithium use, other causes of hyperparathyroidism include an adenoma on a gland, hyperplasia of ≥2 parathyroid glands, a malignant tumor, severe calcium deficiency, severe vitamin D deficiency, chronic renal failure, and (rarely) an inherited gene that causes hyperparathyroidism.
How I’m doing today. Currently, I am euthymic and in a happy marriage. My laboratory workup in May 2020 included glucose 107 mg/dL, Ca++ 9.5 mg/dL, eGFR 61 mL/min, PTH 32 pg/mL, lithium 0.3 mmol/L (300 mg twice daily), and TSH 1.79 mIU/L. A comprehensive metabolic panel, complete blood count, and lipid panel were all within normal limits.
I am fortunate to continue having excellent care provided by my PCP, nephrologist, urologist, and psychiatric APRN. Together with these wonderful professionals, I have been able to maintain my physical and mental health.
Acknowledgment: I gratefully acknowledge the help and skills of Robin Scharak and Gary Blake for providing some of the editing on this article.
Bill Greenberg MS, RN, APRN
Delray Beach, Florida
I am a retired advanced practice psychiatric nurse who has lived and worked on “both sides of the door.” This wording is paraphrased from psychologist and therapist Lauren Slater, PhD, who wrote about a time she went to McLean Hospital in Belmont, Massachusetts, as a therapist after staying there as a patient years earlier: “And now I am standing on the other—the wrong, I mean the right side of the door and I ring the buzzer.”1 Here I tell my story of the physical and emotional effects of my mental illness and treatment.
Onset of bipolar disorder. My bipolar illness started with a bout of depression in 1963 at age 13, which resulted in a low-key summer of often staying inside. I received no medication, and no one sent me for evaluation. In the fall, I went back to school and finished the year without incident. I continued as a quiet, shy kid through high school in the late 1960s. In my senior year, I decided to take an overload of difficult courses and run on the varsity cross-country team. The amount and intensity of these activities were too much. This resulted in my first manic episode, which started during a weekend visit to a college I hoped to attend. I became excitable, grandiose, and had delusions. A day later, I returned home, and my parents had me admitted to a psychiatric hospital, where I remained for 3 months.
At first, my diagnosis was unclear, and initially no one considered what at the time was called manic depression. At that point, I was unaware of my extensive family psychiatric history. My pharmacologic treatment consisted of chlorpromazine, trifluoperazine, and procyclidine. I returned home just before Christmas and barely finished my senior year of high school. A good college accepted me. But during the orientation, I was asked to leave because I experienced a second manic episode. After 4 more psychiatric hospitalizations, I finally stabilized.
During one of my hospitalizations, I had the good fortune to be interviewed by Dr. Thomas Detre. During this interview, I talked expansively about Don Quixote, Aldonza, and Sancho Panza. Dr. Detre diagnosed me with manic depression, and suggested that I see Dr. Christiaan van der Velde, who was researching lithium carbonate.2 In 1970, I was hospitalized at Norwich State Hospital in Preston, Connecticut and was started on lithium, even though it had not yet been FDA-approved. I responded well to lithium monotherapy.
An extensive family history. Having bipolar disorder was not something I would discuss with others because I felt ashamed. I commonly hid my medication during college, especially from my roommates or other friends. By then, I had learned a little about my family’s psychiatric history, but I knew few specifics. Over time, I became aware of a dense familial cluster of affective illness going back several generations. My maternal grandmother was hospitalized for depression in 1921 after her husband suddenly died during her fourth pregnancy. She became bereft and suicidal because she had no one to support her 4 children. During my grandmother’s hospitalization, her sister and sister’s husband took care of her children. My grandmother remained hospitalized until she died in 1943. At that time, no medications were available to treat her illness. Over the next 2 generations, 2 of her 4 children and 6 of her 12 grandchildren (including me) developed bipolar disorder.
A career and family. In 1970, I started to work as a nursing assistant, then as a nursing technician for 1.5 years in a specialty hospital in New England. In 1973, I began nursing school at a junior college. I received my RN in 1975, a BS in nursing in 1979, and an MS in psychiatric nursing in 1982. I worked steadily as a psychiatric nurse in both inpatient and outpatient settings from 1975 until I retired in 2019.
In the early 1980s, I married my first wife and had 2 wonderful children. During our courtship in 1981 and 1982, I became hypomanic, which perhaps made me more outgoing and sociable. In 1985, after my father required open heart surgery, I had a manic episode that lasted 1 week. Over the next 20 years, although I was not happy with my marriage, I remained euthymic and productive at work. My marriage ended in 2012.
Continue to: By the end of 2012...
By the end of 2012, I had been taking lithium continuously for 42 years. My laboratory tests showed peak lithium levels between 0.6 and 1.2 mmol/L. I remained otherwise healthy, as demonstrated by annual physical exams and laboratory test results. In 2015, I developed an increase in my blood pressure and my primary care physician (PCP) prescribed oral lisinopril, initially 10 mg/d, and later 10 mg twice daily. My blood pressure improved and ranged from 120/74 to 130/82 mm Hg.
Hyperparathyroidism. By 2016, my psychiatrist, PCP, and nephrologist all urged me to consider parathyroid surgery.3-5 Hypercalcemia and hyperparathyroidism caused the most worry. Laboratory tests indicated calcium 11.2 mg/dL, parathyroid hormone (PTH) 88 pg/mL, estimated glomerular filtration rate (eGFR) 59 mL/min, and thyroid-stimulating hormone (TSH) 0.78 mIU/L. Electrocardiographysometimes showed a slight QT elongation. A right bundle branch block, which was first noted in 2015, continued. Due to my elevated calcium levels, I eliminated most calcium from my diet. My psychiatrist began to speak more strongly of parathyroid surgery. I then consulted a senior endocrinologist and a senior nephrologist, who each recommended parathyroid surgery.
I remarried in July 2016, and we moved to a different area of the country. My second wife became a stabilizing force for me. My new PCP, however, found elevated high-density lipoproteins during a routine physical examination, and started me on simvastatin, 10 mg/d. My calcium and PTH levels continued to be elevated. My PCP, nephrologist, therapist, and wife urged me to proceed with the parathyroidectomy. After a short period of watchful waiting and a second consultation with a nephrologist, I agreed to schedule a subtotal parathyroidectomy.
Surgery. In spring 2017, I began preparation for parathyroidectomy. At the time, my lithium carbonate dose was 600 mg/d, alternating with 900 mg/d. My peak level of lithium was 0.6 mmol/L. Lisinopril is synergistic, which allowed me to take a smaller effective dose of lithium.
My parathyroid surgery occurred on June 28, 2017 at Norman Parathyroid Center in Tampa, Florida.6 The surgeon recorded my parathyroid glands as 136, 602, and 348 units using a measure developed at Norman Parathyroid Center. No reading was given for my fourth parathyroid gland, which they did not remove. Following the surgery, I resumed my previous functions, including employment as a visiting nurse. I initially took calcium supplements after surgery, and my lithium dose was reduced to 300 mg orally, twice daily, which I have continued. I have remained euthymic. On August 3, 2017 my laboratory workup showed an eGFR of 64 mL/min, calcium 10.0 mg/dL, and PTH 17 pg/mL. Vitamin D25 OH 33, glucose, BUN/Cr, electrolytes, complete blood count, and albumin were all within normal limits. Repeat bloodwork on September 19, 2017 showed Ca++ 10.1 mg/dL and PTH 18 pg/mL. Nine months after the surgery, I showed an incredibly positive physical and mental response, which has continued to this day.
Continue to: Clinical implications
Clinical implications. This is a single case study. However, it is important for clinicians treating patients with lithium carbonate to regularly order laboratory testing, including for lithium levels, PTH, and calcium, to detect early signs of complications from treatment, including hyperparathyroidism and hypercalcemia.7 These levels could be obtained every 6 months. If a patient’s PTH levels are >70 pg/mL and calcium levels are >11.0 mg/dL, it would be prudent to refer him/her for further medical evaluation. Additionally, it would be helpful to counsel the patient about considering alternative medication and adjunct mental health treatment. At some future point, it could be useful for the clinician and his/her patient to explore the idea of parathyroid surgery.
In addition to chronic lithium use, other causes of hyperparathyroidism include an adenoma on a gland, hyperplasia of ≥2 parathyroid glands, a malignant tumor, severe calcium deficiency, severe vitamin D deficiency, chronic renal failure, and (rarely) an inherited gene that causes hyperparathyroidism.
How I’m doing today. Currently, I am euthymic and in a happy marriage. My laboratory workup in May 2020 included glucose 107 mg/dL, Ca++ 9.5 mg/dL, eGFR 61 mL/min, PTH 32 pg/mL, lithium 0.3 mmol/L (300 mg twice daily), and TSH 1.79 mIU/L. A comprehensive metabolic panel, complete blood count, and lipid panel were all within normal limits.
I am fortunate to continue having excellent care provided by my PCP, nephrologist, urologist, and psychiatric APRN. Together with these wonderful professionals, I have been able to maintain my physical and mental health.
Acknowledgment: I gratefully acknowledge the help and skills of Robin Scharak and Gary Blake for providing some of the editing on this article.
Bill Greenberg MS, RN, APRN
Delray Beach, Florida
1. Slater L. Welcome to my country. New York, NY: Random House; 1996:187.
2. Van der Velde CD. Effectiveness of lithium in the treatment of manic-depressive illness. Am J Psychiatry. 1970;127(3):345-351.
3. Norman Parathyroid Center. Parathyroid glands, high calcium and hyperparathyroidism. www.parathyroid.com. Updated October 21, 2020. Accessed November 11, 2020.
4. Meehan AD, Udumyan R, Kardell M, et al. Lithium-associated hypercalcemia: pathophysiology, prevalence, management. World J Surg. 2018;42(2):415-424.
5. Lally J, Lee B, McDonald C. Prevalence of hypercalcaemia in patients on maintenance lithium therapy monitored in primary care. Ir Med J. 2013;106(1):15-17.
6. Norman Parathyroid Center. Parathyroid surgery: minimally invasive 4-gland parathyroid surgery video. (4-Gland MIRP Parathyroid Operation). https://www.parathyroid.com/parathyroid-surgery.htm. Updated October 1, 2020. Accessed November 5, 2020.
7. MEDSAFE. Hyperparathyroidism and hypercalcaemia with lithium treatment. New Zealand Medicines and Medical Devices Safety Authority. 2014;35(3):37-38.
1. Slater L. Welcome to my country. New York, NY: Random House; 1996:187.
2. Van der Velde CD. Effectiveness of lithium in the treatment of manic-depressive illness. Am J Psychiatry. 1970;127(3):345-351.
3. Norman Parathyroid Center. Parathyroid glands, high calcium and hyperparathyroidism. www.parathyroid.com. Updated October 21, 2020. Accessed November 11, 2020.
4. Meehan AD, Udumyan R, Kardell M, et al. Lithium-associated hypercalcemia: pathophysiology, prevalence, management. World J Surg. 2018;42(2):415-424.
5. Lally J, Lee B, McDonald C. Prevalence of hypercalcaemia in patients on maintenance lithium therapy monitored in primary care. Ir Med J. 2013;106(1):15-17.
6. Norman Parathyroid Center. Parathyroid surgery: minimally invasive 4-gland parathyroid surgery video. (4-Gland MIRP Parathyroid Operation). https://www.parathyroid.com/parathyroid-surgery.htm. Updated October 1, 2020. Accessed November 5, 2020.
7. MEDSAFE. Hyperparathyroidism and hypercalcaemia with lithium treatment. New Zealand Medicines and Medical Devices Safety Authority. 2014;35(3):37-38.
Death anxiety among psychiatry trainees during COVID-19
The coronavirus disease 2019 (COVID-19) pandemic
The far-reaching effects of death anxiety
Postgraduate psychiatry training may expose one to stressful situations with adverse psychologic consequences.1 Furthermore, when caring for patients, psychiatry trainees frequently need to face issues of death and dying in the form of suicide risk assessments, grief and bereavement processes, near-death experiences, posttraumatic stress disorder, and psycho-oncology rotations. Because these interactions are incredibly personal, the emotions they provoke inevitably affect every interaction, theoretical discussion, diagnostic work-up, and treatment plan.
How each of us experiences death anxiety is unique. For some, it could be a fear of nonexistence, ultimate loss, disruption of the flow of life, worry about leaving loved ones behind, or fear of pain or loneliness in dying. Some might fear an untimely or violent death and subsequent judgment and retributions. The literature suggests that fear of death may be at the root of various mental health problems and, if left unaddressed, may adversely impact long-term treatment outcomes.2 Despite this, many standard treatment approaches typically do not target death anxiety, which potentially contributes to a “revolving door” of mental health problems.3
American existential psychiatrist Irvin Yalom, MD, cautioned psychiatrists not to “scratch where it does not itch.”4 Yet death, according to Dr. Yalom, does itch. Violent death is that caused by human intent or negligence, and is characterized by feeling helpless and terrorized at the time of dying. It may occur as an acute incident that denies the dying individual and his/her family members the time and space to prepare for the death.5 For survivors, accommodating the mental, emotional, psychological, and spiritual effects of violent death is a complex process that rarely has a conclusion. It often is accompanied by survivors’ guilt, which is replayed in the form of flashbacks and nightmares.6 With this understanding, I view COVID-19 deaths as violent deaths.
Pay close attention to countertransference
As much as we influence our patients and their families, we also are profoundly influenced by them. We need to pay attention to any feelings our clinical encounters generate within us, and to carefully use these feelings in our clinical judgment, and not just make causal inferences. For instance, if a clinician thinks that a patient with suicidal ideation would be better off dead, these feelings are a reliable indicator that the patient is, indeed, at a high risk of completing suicide.7 It is our ethical and moral responsibility towards our patients to listen to our countertransference responses. The aim is to identify countertransference and use it to inform us, not to rule us. By taking an active role in managing our emotional responses in the face of loss, we can harness the spirit of resilience. This is not always as easy as it seems. We need our peers, experienced clinicians, and supervisors to help us explore our feelings, resistances, and countertransference reactions.
Strategies to combat burnout
Psychiatric trainees must be encouraged to establish and maintain rigorous plans of self-care to prevent compassion fatigue and burnout. Most importantly, training programs must diversify residents’ clinical exposure by providing activities that promote mental health promotion activities, scholarly endeavors, and peer support groups. This will help trainees to restore meaning and purpose in life beyond.
1. Coverdale J, Balon R, Beresin EV, et al. What are some stressful adversities in psychiatry residency training, and how should they be managed professionally? Acad Psychiatry. 2019;43(2):145-150.
2. Russac RJ, Gatliff C, Reece M, et al. Death anxiety across the adult years: an examination of age and gender effects. Death Stud. 2007;31(6):549-561.
3. Lisa I, Menzies RG, Menzies RE. Death anxiety and its role in psychopathology: reviewing the status of a transdiagnostic construct. Clinical Psychology Review. 2014;34(7):580-593.
4. Yalom ID. Staring at the sun: being at peace with your own mortality. London, UK: Piatkus; 2011.
5. Rynearson EK, Johnson TA, Correa F. The horror and helplessness of violent death. In: Katz RS, Johnson TA (eds). When professionals weep: emotional and countertransference responses in palliative and end-of-life care. Abingdon, UK: Routledge; 2016:91-103.
6. Breggin PR. Guilt, shame, and anxiety: understanding and overcoming negative emotions. Buffalo, NY: Prometheus Books; 2014.
7. Katz RS, Johnson TA, (eds). When professionals weep: Emotional and countertransference responses in palliative and end-of-life care. Abingdon, UK: Routledge; 2016.
The coronavirus disease 2019 (COVID-19) pandemic
The far-reaching effects of death anxiety
Postgraduate psychiatry training may expose one to stressful situations with adverse psychologic consequences.1 Furthermore, when caring for patients, psychiatry trainees frequently need to face issues of death and dying in the form of suicide risk assessments, grief and bereavement processes, near-death experiences, posttraumatic stress disorder, and psycho-oncology rotations. Because these interactions are incredibly personal, the emotions they provoke inevitably affect every interaction, theoretical discussion, diagnostic work-up, and treatment plan.
How each of us experiences death anxiety is unique. For some, it could be a fear of nonexistence, ultimate loss, disruption of the flow of life, worry about leaving loved ones behind, or fear of pain or loneliness in dying. Some might fear an untimely or violent death and subsequent judgment and retributions. The literature suggests that fear of death may be at the root of various mental health problems and, if left unaddressed, may adversely impact long-term treatment outcomes.2 Despite this, many standard treatment approaches typically do not target death anxiety, which potentially contributes to a “revolving door” of mental health problems.3
American existential psychiatrist Irvin Yalom, MD, cautioned psychiatrists not to “scratch where it does not itch.”4 Yet death, according to Dr. Yalom, does itch. Violent death is that caused by human intent or negligence, and is characterized by feeling helpless and terrorized at the time of dying. It may occur as an acute incident that denies the dying individual and his/her family members the time and space to prepare for the death.5 For survivors, accommodating the mental, emotional, psychological, and spiritual effects of violent death is a complex process that rarely has a conclusion. It often is accompanied by survivors’ guilt, which is replayed in the form of flashbacks and nightmares.6 With this understanding, I view COVID-19 deaths as violent deaths.
Pay close attention to countertransference
As much as we influence our patients and their families, we also are profoundly influenced by them. We need to pay attention to any feelings our clinical encounters generate within us, and to carefully use these feelings in our clinical judgment, and not just make causal inferences. For instance, if a clinician thinks that a patient with suicidal ideation would be better off dead, these feelings are a reliable indicator that the patient is, indeed, at a high risk of completing suicide.7 It is our ethical and moral responsibility towards our patients to listen to our countertransference responses. The aim is to identify countertransference and use it to inform us, not to rule us. By taking an active role in managing our emotional responses in the face of loss, we can harness the spirit of resilience. This is not always as easy as it seems. We need our peers, experienced clinicians, and supervisors to help us explore our feelings, resistances, and countertransference reactions.
Strategies to combat burnout
Psychiatric trainees must be encouraged to establish and maintain rigorous plans of self-care to prevent compassion fatigue and burnout. Most importantly, training programs must diversify residents’ clinical exposure by providing activities that promote mental health promotion activities, scholarly endeavors, and peer support groups. This will help trainees to restore meaning and purpose in life beyond.
The coronavirus disease 2019 (COVID-19) pandemic
The far-reaching effects of death anxiety
Postgraduate psychiatry training may expose one to stressful situations with adverse psychologic consequences.1 Furthermore, when caring for patients, psychiatry trainees frequently need to face issues of death and dying in the form of suicide risk assessments, grief and bereavement processes, near-death experiences, posttraumatic stress disorder, and psycho-oncology rotations. Because these interactions are incredibly personal, the emotions they provoke inevitably affect every interaction, theoretical discussion, diagnostic work-up, and treatment plan.
How each of us experiences death anxiety is unique. For some, it could be a fear of nonexistence, ultimate loss, disruption of the flow of life, worry about leaving loved ones behind, or fear of pain or loneliness in dying. Some might fear an untimely or violent death and subsequent judgment and retributions. The literature suggests that fear of death may be at the root of various mental health problems and, if left unaddressed, may adversely impact long-term treatment outcomes.2 Despite this, many standard treatment approaches typically do not target death anxiety, which potentially contributes to a “revolving door” of mental health problems.3
American existential psychiatrist Irvin Yalom, MD, cautioned psychiatrists not to “scratch where it does not itch.”4 Yet death, according to Dr. Yalom, does itch. Violent death is that caused by human intent or negligence, and is characterized by feeling helpless and terrorized at the time of dying. It may occur as an acute incident that denies the dying individual and his/her family members the time and space to prepare for the death.5 For survivors, accommodating the mental, emotional, psychological, and spiritual effects of violent death is a complex process that rarely has a conclusion. It often is accompanied by survivors’ guilt, which is replayed in the form of flashbacks and nightmares.6 With this understanding, I view COVID-19 deaths as violent deaths.
Pay close attention to countertransference
As much as we influence our patients and their families, we also are profoundly influenced by them. We need to pay attention to any feelings our clinical encounters generate within us, and to carefully use these feelings in our clinical judgment, and not just make causal inferences. For instance, if a clinician thinks that a patient with suicidal ideation would be better off dead, these feelings are a reliable indicator that the patient is, indeed, at a high risk of completing suicide.7 It is our ethical and moral responsibility towards our patients to listen to our countertransference responses. The aim is to identify countertransference and use it to inform us, not to rule us. By taking an active role in managing our emotional responses in the face of loss, we can harness the spirit of resilience. This is not always as easy as it seems. We need our peers, experienced clinicians, and supervisors to help us explore our feelings, resistances, and countertransference reactions.
Strategies to combat burnout
Psychiatric trainees must be encouraged to establish and maintain rigorous plans of self-care to prevent compassion fatigue and burnout. Most importantly, training programs must diversify residents’ clinical exposure by providing activities that promote mental health promotion activities, scholarly endeavors, and peer support groups. This will help trainees to restore meaning and purpose in life beyond.
1. Coverdale J, Balon R, Beresin EV, et al. What are some stressful adversities in psychiatry residency training, and how should they be managed professionally? Acad Psychiatry. 2019;43(2):145-150.
2. Russac RJ, Gatliff C, Reece M, et al. Death anxiety across the adult years: an examination of age and gender effects. Death Stud. 2007;31(6):549-561.
3. Lisa I, Menzies RG, Menzies RE. Death anxiety and its role in psychopathology: reviewing the status of a transdiagnostic construct. Clinical Psychology Review. 2014;34(7):580-593.
4. Yalom ID. Staring at the sun: being at peace with your own mortality. London, UK: Piatkus; 2011.
5. Rynearson EK, Johnson TA, Correa F. The horror and helplessness of violent death. In: Katz RS, Johnson TA (eds). When professionals weep: emotional and countertransference responses in palliative and end-of-life care. Abingdon, UK: Routledge; 2016:91-103.
6. Breggin PR. Guilt, shame, and anxiety: understanding and overcoming negative emotions. Buffalo, NY: Prometheus Books; 2014.
7. Katz RS, Johnson TA, (eds). When professionals weep: Emotional and countertransference responses in palliative and end-of-life care. Abingdon, UK: Routledge; 2016.
1. Coverdale J, Balon R, Beresin EV, et al. What are some stressful adversities in psychiatry residency training, and how should they be managed professionally? Acad Psychiatry. 2019;43(2):145-150.
2. Russac RJ, Gatliff C, Reece M, et al. Death anxiety across the adult years: an examination of age and gender effects. Death Stud. 2007;31(6):549-561.
3. Lisa I, Menzies RG, Menzies RE. Death anxiety and its role in psychopathology: reviewing the status of a transdiagnostic construct. Clinical Psychology Review. 2014;34(7):580-593.
4. Yalom ID. Staring at the sun: being at peace with your own mortality. London, UK: Piatkus; 2011.
5. Rynearson EK, Johnson TA, Correa F. The horror and helplessness of violent death. In: Katz RS, Johnson TA (eds). When professionals weep: emotional and countertransference responses in palliative and end-of-life care. Abingdon, UK: Routledge; 2016:91-103.
6. Breggin PR. Guilt, shame, and anxiety: understanding and overcoming negative emotions. Buffalo, NY: Prometheus Books; 2014.
7. Katz RS, Johnson TA, (eds). When professionals weep: Emotional and countertransference responses in palliative and end-of-life care. Abingdon, UK: Routledge; 2016.
P3SONG: Evaluation for autism spectrum disorder
Autism spectrum disorder (ASD) is characterized by impairments in communication and social interactions, along with repetitive and perseverant behaviors.1 It has a prevalence of 0.75% to 1.1% among the general population.1 The presentation of ASD can vary, and patients may have a wide range of comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), neurologic disorders, and genetic disorders.1 Therefore, a comprehensive evaluation needs to include a multidisciplinary assessment by clinicians from several specialties, including primary care, psychiatry, psychology, and neurology. Here I offer psychiatrists 3 Ps and the mnemonic SONG to describe a multidisciplinary approach to assessing a patient with suspected or confirmed ASD.
Primary care evaluation of patients with ASD is important for the diagnosis and treatment of any co-existing medical conditions. Primary care physicians are often the source of referrals to psychiatry, although the reason for the referral may not always be suspicion of autism. In my clinical practice, almost all referrals from primary care involve a chief complaint of anger or behavioral problems, or even obsessive-compulsive behaviors.
Psychiatric evaluation should include obtaining a detailed history of the patient’s conception, birth, development, and social life, and his/her family history of genetic conditions. In my practice, ADHD and elimination disorders are common comorbidities in patients with ASD. Consider communicating with daycare staff or teachers and auxiliary staff, such as guidance counselors, because doing so can help elucidate the diagnosis. Also, ask adult family members, preferably a parent, for collateral information to help establish an accurate diagnosis in your adult patients.
Psychological evaluation should include testing to rule out intellectual disability and learning disorders, which are common in patients with ASD.2 Tests commonly used for evaluation of ASD include the Autism Diagnostic Observation Schedule (ADOS), Childhood Autism Rating Scale (CARS), and Autism Diagnostic Interview-Revised (ADI-R).
Speech evaluation. Deficits in language and communication are commonly observed in patients with ASD, especially in younger patients.3 A study of the relationship between early language skills (age of first word production) and later functioning in children with ASD indicated that earlier age of first word acquisition was associated with higher cognitive ability and adaptive skills when measured later in childhood.3 Therefore, timely intervention following speech evaluation can result in favorable outcomes.
Occupational evaluation. Approximately 69% to 93% of children and adults with ASD exhibit sensory symptoms (hyperresponsive, hyporesponsive, and sensory-seeking behaviors).4 Patients with sensory symptoms often experience limitations in multiple areas of their life. Early intervention by an occupational therapist can help improve long-term outcomes.4
Neurologic evaluation is important because ASD is a neurodevelopmental disorder. Patients with ASD often have comorbid seizure disorders.1 The estimated prevalence of epilepsy in these patients ranges from 2.7% to 44.4%.1 A baseline EEG and neuroimaging can help improve your understanding of the relationship between ASD and seizure disorders, and guide treatment.
Genetic testing. Between 10% to 15% of individuals with ASD have a medical condition, such as cytogenetic or single-gene disorder, that causes ASD.5 Fragile X syndrome, tuberous sclerosis, and Prader-Willi syndrome are a few common examples of genetic disorders associated with ASD.5 Autism spectrum disorder has also been known to have a strong genetic basis with high probability of heritability in families.5 Genetic testing can help to detect any underlying genetic disorders in your patients as well as their family members. Chromosomal microarray analysis has become more accessible due to improved insurance coverage, and is convenient to perform by collection of a buccal mucosa sample in the office setting.
1. Strasser L, Downes M, Kung J, et al. Prevalence and risk factors for autism spectrum disorder in epilepsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2018;60(1):19-29.
2. Schwatrz CE, Neri G. Autism and intellectual disability: two sides of the same coin. Am J Med Genet Part C Semin Med Genet. 2012;160C(2):89-89.
3. Mayo J, Chlebowski C, Fein DA, et al. Age of first words predicts cognitive ability and adaptive skills in children with ASD. J Autism Dev Disord. 2013;43(2):253-264.
4. McCormick C, Hepburn S, Young GS, et al. Sensory symptoms in children with autism spectrum disorder, other developmental disorders and typical development: a longitudinal study. Autism. 2016;20(5):572-579.
5. Balasubramanian B, Bhatt CV, Goyel NA. Genetic studies in children with intellectual disability and autistic spectrum of disorders. Indian J Hum Genet. 2009;15(3):103-107.
Autism spectrum disorder (ASD) is characterized by impairments in communication and social interactions, along with repetitive and perseverant behaviors.1 It has a prevalence of 0.75% to 1.1% among the general population.1 The presentation of ASD can vary, and patients may have a wide range of comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), neurologic disorders, and genetic disorders.1 Therefore, a comprehensive evaluation needs to include a multidisciplinary assessment by clinicians from several specialties, including primary care, psychiatry, psychology, and neurology. Here I offer psychiatrists 3 Ps and the mnemonic SONG to describe a multidisciplinary approach to assessing a patient with suspected or confirmed ASD.
Primary care evaluation of patients with ASD is important for the diagnosis and treatment of any co-existing medical conditions. Primary care physicians are often the source of referrals to psychiatry, although the reason for the referral may not always be suspicion of autism. In my clinical practice, almost all referrals from primary care involve a chief complaint of anger or behavioral problems, or even obsessive-compulsive behaviors.
Psychiatric evaluation should include obtaining a detailed history of the patient’s conception, birth, development, and social life, and his/her family history of genetic conditions. In my practice, ADHD and elimination disorders are common comorbidities in patients with ASD. Consider communicating with daycare staff or teachers and auxiliary staff, such as guidance counselors, because doing so can help elucidate the diagnosis. Also, ask adult family members, preferably a parent, for collateral information to help establish an accurate diagnosis in your adult patients.
Psychological evaluation should include testing to rule out intellectual disability and learning disorders, which are common in patients with ASD.2 Tests commonly used for evaluation of ASD include the Autism Diagnostic Observation Schedule (ADOS), Childhood Autism Rating Scale (CARS), and Autism Diagnostic Interview-Revised (ADI-R).
Speech evaluation. Deficits in language and communication are commonly observed in patients with ASD, especially in younger patients.3 A study of the relationship between early language skills (age of first word production) and later functioning in children with ASD indicated that earlier age of first word acquisition was associated with higher cognitive ability and adaptive skills when measured later in childhood.3 Therefore, timely intervention following speech evaluation can result in favorable outcomes.
Occupational evaluation. Approximately 69% to 93% of children and adults with ASD exhibit sensory symptoms (hyperresponsive, hyporesponsive, and sensory-seeking behaviors).4 Patients with sensory symptoms often experience limitations in multiple areas of their life. Early intervention by an occupational therapist can help improve long-term outcomes.4
Neurologic evaluation is important because ASD is a neurodevelopmental disorder. Patients with ASD often have comorbid seizure disorders.1 The estimated prevalence of epilepsy in these patients ranges from 2.7% to 44.4%.1 A baseline EEG and neuroimaging can help improve your understanding of the relationship between ASD and seizure disorders, and guide treatment.
Genetic testing. Between 10% to 15% of individuals with ASD have a medical condition, such as cytogenetic or single-gene disorder, that causes ASD.5 Fragile X syndrome, tuberous sclerosis, and Prader-Willi syndrome are a few common examples of genetic disorders associated with ASD.5 Autism spectrum disorder has also been known to have a strong genetic basis with high probability of heritability in families.5 Genetic testing can help to detect any underlying genetic disorders in your patients as well as their family members. Chromosomal microarray analysis has become more accessible due to improved insurance coverage, and is convenient to perform by collection of a buccal mucosa sample in the office setting.
Autism spectrum disorder (ASD) is characterized by impairments in communication and social interactions, along with repetitive and perseverant behaviors.1 It has a prevalence of 0.75% to 1.1% among the general population.1 The presentation of ASD can vary, and patients may have a wide range of comorbidities, such as attention-deficit/hyperactivity disorder (ADHD), neurologic disorders, and genetic disorders.1 Therefore, a comprehensive evaluation needs to include a multidisciplinary assessment by clinicians from several specialties, including primary care, psychiatry, psychology, and neurology. Here I offer psychiatrists 3 Ps and the mnemonic SONG to describe a multidisciplinary approach to assessing a patient with suspected or confirmed ASD.
Primary care evaluation of patients with ASD is important for the diagnosis and treatment of any co-existing medical conditions. Primary care physicians are often the source of referrals to psychiatry, although the reason for the referral may not always be suspicion of autism. In my clinical practice, almost all referrals from primary care involve a chief complaint of anger or behavioral problems, or even obsessive-compulsive behaviors.
Psychiatric evaluation should include obtaining a detailed history of the patient’s conception, birth, development, and social life, and his/her family history of genetic conditions. In my practice, ADHD and elimination disorders are common comorbidities in patients with ASD. Consider communicating with daycare staff or teachers and auxiliary staff, such as guidance counselors, because doing so can help elucidate the diagnosis. Also, ask adult family members, preferably a parent, for collateral information to help establish an accurate diagnosis in your adult patients.
Psychological evaluation should include testing to rule out intellectual disability and learning disorders, which are common in patients with ASD.2 Tests commonly used for evaluation of ASD include the Autism Diagnostic Observation Schedule (ADOS), Childhood Autism Rating Scale (CARS), and Autism Diagnostic Interview-Revised (ADI-R).
Speech evaluation. Deficits in language and communication are commonly observed in patients with ASD, especially in younger patients.3 A study of the relationship between early language skills (age of first word production) and later functioning in children with ASD indicated that earlier age of first word acquisition was associated with higher cognitive ability and adaptive skills when measured later in childhood.3 Therefore, timely intervention following speech evaluation can result in favorable outcomes.
Occupational evaluation. Approximately 69% to 93% of children and adults with ASD exhibit sensory symptoms (hyperresponsive, hyporesponsive, and sensory-seeking behaviors).4 Patients with sensory symptoms often experience limitations in multiple areas of their life. Early intervention by an occupational therapist can help improve long-term outcomes.4
Neurologic evaluation is important because ASD is a neurodevelopmental disorder. Patients with ASD often have comorbid seizure disorders.1 The estimated prevalence of epilepsy in these patients ranges from 2.7% to 44.4%.1 A baseline EEG and neuroimaging can help improve your understanding of the relationship between ASD and seizure disorders, and guide treatment.
Genetic testing. Between 10% to 15% of individuals with ASD have a medical condition, such as cytogenetic or single-gene disorder, that causes ASD.5 Fragile X syndrome, tuberous sclerosis, and Prader-Willi syndrome are a few common examples of genetic disorders associated with ASD.5 Autism spectrum disorder has also been known to have a strong genetic basis with high probability of heritability in families.5 Genetic testing can help to detect any underlying genetic disorders in your patients as well as their family members. Chromosomal microarray analysis has become more accessible due to improved insurance coverage, and is convenient to perform by collection of a buccal mucosa sample in the office setting.
1. Strasser L, Downes M, Kung J, et al. Prevalence and risk factors for autism spectrum disorder in epilepsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2018;60(1):19-29.
2. Schwatrz CE, Neri G. Autism and intellectual disability: two sides of the same coin. Am J Med Genet Part C Semin Med Genet. 2012;160C(2):89-89.
3. Mayo J, Chlebowski C, Fein DA, et al. Age of first words predicts cognitive ability and adaptive skills in children with ASD. J Autism Dev Disord. 2013;43(2):253-264.
4. McCormick C, Hepburn S, Young GS, et al. Sensory symptoms in children with autism spectrum disorder, other developmental disorders and typical development: a longitudinal study. Autism. 2016;20(5):572-579.
5. Balasubramanian B, Bhatt CV, Goyel NA. Genetic studies in children with intellectual disability and autistic spectrum of disorders. Indian J Hum Genet. 2009;15(3):103-107.
1. Strasser L, Downes M, Kung J, et al. Prevalence and risk factors for autism spectrum disorder in epilepsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2018;60(1):19-29.
2. Schwatrz CE, Neri G. Autism and intellectual disability: two sides of the same coin. Am J Med Genet Part C Semin Med Genet. 2012;160C(2):89-89.
3. Mayo J, Chlebowski C, Fein DA, et al. Age of first words predicts cognitive ability and adaptive skills in children with ASD. J Autism Dev Disord. 2013;43(2):253-264.
4. McCormick C, Hepburn S, Young GS, et al. Sensory symptoms in children with autism spectrum disorder, other developmental disorders and typical development: a longitudinal study. Autism. 2016;20(5):572-579.
5. Balasubramanian B, Bhatt CV, Goyel NA. Genetic studies in children with intellectual disability and autistic spectrum of disorders. Indian J Hum Genet. 2009;15(3):103-107.
How to handle negative online reviews
Online reviews have become a popular method for patients to rate their psychiatrists. Patients’ online reviews can help other patients make more informed decisions about pursuing treatment, offer us valuable feedback on our performance, and help improve standards of care.1 However, during the course of our careers, we may receive negative online reviews. These reviews may range from mild dissatisfaction to abusive comments, and they could have adverse personal and professional consequences.2 For example, online discussions might make current patients question your practices or consider ending their treatment with you.2 Also, potential patients might decide to not inquire about your services.2 Here I offer suggestions for approaching negative online reviews, and point out some potential pitfalls of responding to them.
Remain professional. You might become upset or frazzled after reading online criticisms about your performance, particularly if the information is erroneous or deceptive. As much as you would like to immediately respond, a public tit-for-tat could prolong or fuel a conflict, or make you come across as angry.2
There may be occasions, however, when it would be appropriate to respond. If you choose to respond to a negative online review, you need to have a methodical plan. Avoid reacting in a knee-jerk manner because this is usually unproductive. In addition, ensure that your response is professional and polite, because an intemperate response could undermine the public’s confidence in our profession.2
Maintain patient confidentiality. Although patients are free to post anything they desire, psychiatrists must maintain confidentiality. The Health Insurance Portability and Accountability Act (HIPAA) applies to online reviews, which prevents us from disclosing information about patients without their permission, including even acknowledging that someone is our patient.3 Your patients’ disclosures are not permission to disclose their health information. Potential patients might avoid us or existing patients may end their treatment with us if they believe their personal information could be disclosed online without their consent. To avoid such concerns, reply to online reviews with generic comments about your practice’s general policies without violating confidentiality. Also, to avoid violating HIPAA rules, you may want to contact your malpractice carrier or your facility’s legal department before replying.1
Invite patients to discuss their grievances. If your patients identify themselves in a review, or if you are able to identify them, consider inviting them to discuss their concerns with you (over the phone, face-to-face, or via video conferencing). During such conversations, thank the patient for their review, and do not ask them to delete it.2 Focus on addressing their concerns and resolving any problems they experienced during treatment; doing so can help improve your practice. This approach also might lead a patient to remove their negative review or to write a review that lets other patients know that you are listening to them.
Even if you choose not to invite your patients to air their concerns, do not entirely dismiss negative reviews. Instead, try to step back from your emotions and take an objective look at such reviews so you can determine what steps to take to improve your practices. Improving your communication with patients could decrease the likelihood that they will write negative reviews in the first place.
Take action on fake reviews. If a negative review is fake (not written by one of your patients) or blatantly untrue, contact the web site administrator and provide evidence to support having the review deleted, especially if it violates the site’s terms of service.1 However, this approach may not be fruitful. Web sites can be manipulated, and many do not require users to authenticate that they are actual patients.1 Although most web sites would not want their reputation damaged by users posting fake reviews, more dramatic reviews could help lead to increased traffic, which lowers an administrator’s incentive to remove negative reviews.1
Continue to: Consider legal repercussions
Consider legal repercussions. Stay up-to-date with online reviews about you by conducting internet searches once every 3 months.1 Consider notifying your malpractice carrier or facility’s legal department if a review suggests a patient or family might initiate legal action against you or the facility.1 You might consider pursuing legal action if an online review is defamatory, but such claims often are difficult to prove in court.1 Even if you win, such a case could later be repeatedly mentioned in articles and journals, thus creating a permanent record of the negative review in the literature.1
Enlist help with your online image. If financially feasible, hire a professional service to help improve your online image or assist in responding to negative reviews.1 Build your profile on review web sites to help frame your online image, and include information that mentions the pertinent steps you are taking to address any legitimate concerns your patients raise in their reviews. Encourage your patients to post reviews because that could produce a more equitable sample and paint a more accurate picture of your practice.
Lobby professional medical organizations to take action to protect psychiatrists from negative online reviews by creating legislation that holds web sites accountable for their content.1
Stay positive. Unfounded or not, negative online reviews are an inevitable part of a psychiatrist’s professional life.2 One negative review (or even several) is not going to destroy your reputation or career. Do not feel alone if you receive a negative review. Seek advice from colleagues who have received negative reviews; in addition to offering advice, they can also provide a listening ear.2
1. Kendall L, Botello T. Internet sabotage: negative online reviews of psychiatrists. Psychiatr Ann. 2016;46(12):715-716, 718-719.
2. Rimmer A. A patient has complained about me online. What should I do? BMJ. 2019;366:I5705. doi: 10.1136/bmj.I5705.
3. Health Insurance Portability and Accountability Act of 1996 (HIPAA), S 1028, 104th Cong, Public Law No. 104-191, 110 Stat. 1936 (1996). https://www.govinfo.gov/content/pkg/PLAW-104publ191/pdf/PLAW-104publ191.pdf. Accessed Novermber 16, 2020.
Online reviews have become a popular method for patients to rate their psychiatrists. Patients’ online reviews can help other patients make more informed decisions about pursuing treatment, offer us valuable feedback on our performance, and help improve standards of care.1 However, during the course of our careers, we may receive negative online reviews. These reviews may range from mild dissatisfaction to abusive comments, and they could have adverse personal and professional consequences.2 For example, online discussions might make current patients question your practices or consider ending their treatment with you.2 Also, potential patients might decide to not inquire about your services.2 Here I offer suggestions for approaching negative online reviews, and point out some potential pitfalls of responding to them.
Remain professional. You might become upset or frazzled after reading online criticisms about your performance, particularly if the information is erroneous or deceptive. As much as you would like to immediately respond, a public tit-for-tat could prolong or fuel a conflict, or make you come across as angry.2
There may be occasions, however, when it would be appropriate to respond. If you choose to respond to a negative online review, you need to have a methodical plan. Avoid reacting in a knee-jerk manner because this is usually unproductive. In addition, ensure that your response is professional and polite, because an intemperate response could undermine the public’s confidence in our profession.2
Maintain patient confidentiality. Although patients are free to post anything they desire, psychiatrists must maintain confidentiality. The Health Insurance Portability and Accountability Act (HIPAA) applies to online reviews, which prevents us from disclosing information about patients without their permission, including even acknowledging that someone is our patient.3 Your patients’ disclosures are not permission to disclose their health information. Potential patients might avoid us or existing patients may end their treatment with us if they believe their personal information could be disclosed online without their consent. To avoid such concerns, reply to online reviews with generic comments about your practice’s general policies without violating confidentiality. Also, to avoid violating HIPAA rules, you may want to contact your malpractice carrier or your facility’s legal department before replying.1
Invite patients to discuss their grievances. If your patients identify themselves in a review, or if you are able to identify them, consider inviting them to discuss their concerns with you (over the phone, face-to-face, or via video conferencing). During such conversations, thank the patient for their review, and do not ask them to delete it.2 Focus on addressing their concerns and resolving any problems they experienced during treatment; doing so can help improve your practice. This approach also might lead a patient to remove their negative review or to write a review that lets other patients know that you are listening to them.
Even if you choose not to invite your patients to air their concerns, do not entirely dismiss negative reviews. Instead, try to step back from your emotions and take an objective look at such reviews so you can determine what steps to take to improve your practices. Improving your communication with patients could decrease the likelihood that they will write negative reviews in the first place.
Take action on fake reviews. If a negative review is fake (not written by one of your patients) or blatantly untrue, contact the web site administrator and provide evidence to support having the review deleted, especially if it violates the site’s terms of service.1 However, this approach may not be fruitful. Web sites can be manipulated, and many do not require users to authenticate that they are actual patients.1 Although most web sites would not want their reputation damaged by users posting fake reviews, more dramatic reviews could help lead to increased traffic, which lowers an administrator’s incentive to remove negative reviews.1
Continue to: Consider legal repercussions
Consider legal repercussions. Stay up-to-date with online reviews about you by conducting internet searches once every 3 months.1 Consider notifying your malpractice carrier or facility’s legal department if a review suggests a patient or family might initiate legal action against you or the facility.1 You might consider pursuing legal action if an online review is defamatory, but such claims often are difficult to prove in court.1 Even if you win, such a case could later be repeatedly mentioned in articles and journals, thus creating a permanent record of the negative review in the literature.1
Enlist help with your online image. If financially feasible, hire a professional service to help improve your online image or assist in responding to negative reviews.1 Build your profile on review web sites to help frame your online image, and include information that mentions the pertinent steps you are taking to address any legitimate concerns your patients raise in their reviews. Encourage your patients to post reviews because that could produce a more equitable sample and paint a more accurate picture of your practice.
Lobby professional medical organizations to take action to protect psychiatrists from negative online reviews by creating legislation that holds web sites accountable for their content.1
Stay positive. Unfounded or not, negative online reviews are an inevitable part of a psychiatrist’s professional life.2 One negative review (or even several) is not going to destroy your reputation or career. Do not feel alone if you receive a negative review. Seek advice from colleagues who have received negative reviews; in addition to offering advice, they can also provide a listening ear.2
Online reviews have become a popular method for patients to rate their psychiatrists. Patients’ online reviews can help other patients make more informed decisions about pursuing treatment, offer us valuable feedback on our performance, and help improve standards of care.1 However, during the course of our careers, we may receive negative online reviews. These reviews may range from mild dissatisfaction to abusive comments, and they could have adverse personal and professional consequences.2 For example, online discussions might make current patients question your practices or consider ending their treatment with you.2 Also, potential patients might decide to not inquire about your services.2 Here I offer suggestions for approaching negative online reviews, and point out some potential pitfalls of responding to them.
Remain professional. You might become upset or frazzled after reading online criticisms about your performance, particularly if the information is erroneous or deceptive. As much as you would like to immediately respond, a public tit-for-tat could prolong or fuel a conflict, or make you come across as angry.2
There may be occasions, however, when it would be appropriate to respond. If you choose to respond to a negative online review, you need to have a methodical plan. Avoid reacting in a knee-jerk manner because this is usually unproductive. In addition, ensure that your response is professional and polite, because an intemperate response could undermine the public’s confidence in our profession.2
Maintain patient confidentiality. Although patients are free to post anything they desire, psychiatrists must maintain confidentiality. The Health Insurance Portability and Accountability Act (HIPAA) applies to online reviews, which prevents us from disclosing information about patients without their permission, including even acknowledging that someone is our patient.3 Your patients’ disclosures are not permission to disclose their health information. Potential patients might avoid us or existing patients may end their treatment with us if they believe their personal information could be disclosed online without their consent. To avoid such concerns, reply to online reviews with generic comments about your practice’s general policies without violating confidentiality. Also, to avoid violating HIPAA rules, you may want to contact your malpractice carrier or your facility’s legal department before replying.1
Invite patients to discuss their grievances. If your patients identify themselves in a review, or if you are able to identify them, consider inviting them to discuss their concerns with you (over the phone, face-to-face, or via video conferencing). During such conversations, thank the patient for their review, and do not ask them to delete it.2 Focus on addressing their concerns and resolving any problems they experienced during treatment; doing so can help improve your practice. This approach also might lead a patient to remove their negative review or to write a review that lets other patients know that you are listening to them.
Even if you choose not to invite your patients to air their concerns, do not entirely dismiss negative reviews. Instead, try to step back from your emotions and take an objective look at such reviews so you can determine what steps to take to improve your practices. Improving your communication with patients could decrease the likelihood that they will write negative reviews in the first place.
Take action on fake reviews. If a negative review is fake (not written by one of your patients) or blatantly untrue, contact the web site administrator and provide evidence to support having the review deleted, especially if it violates the site’s terms of service.1 However, this approach may not be fruitful. Web sites can be manipulated, and many do not require users to authenticate that they are actual patients.1 Although most web sites would not want their reputation damaged by users posting fake reviews, more dramatic reviews could help lead to increased traffic, which lowers an administrator’s incentive to remove negative reviews.1
Continue to: Consider legal repercussions
Consider legal repercussions. Stay up-to-date with online reviews about you by conducting internet searches once every 3 months.1 Consider notifying your malpractice carrier or facility’s legal department if a review suggests a patient or family might initiate legal action against you or the facility.1 You might consider pursuing legal action if an online review is defamatory, but such claims often are difficult to prove in court.1 Even if you win, such a case could later be repeatedly mentioned in articles and journals, thus creating a permanent record of the negative review in the literature.1
Enlist help with your online image. If financially feasible, hire a professional service to help improve your online image or assist in responding to negative reviews.1 Build your profile on review web sites to help frame your online image, and include information that mentions the pertinent steps you are taking to address any legitimate concerns your patients raise in their reviews. Encourage your patients to post reviews because that could produce a more equitable sample and paint a more accurate picture of your practice.
Lobby professional medical organizations to take action to protect psychiatrists from negative online reviews by creating legislation that holds web sites accountable for their content.1
Stay positive. Unfounded or not, negative online reviews are an inevitable part of a psychiatrist’s professional life.2 One negative review (or even several) is not going to destroy your reputation or career. Do not feel alone if you receive a negative review. Seek advice from colleagues who have received negative reviews; in addition to offering advice, they can also provide a listening ear.2
1. Kendall L, Botello T. Internet sabotage: negative online reviews of psychiatrists. Psychiatr Ann. 2016;46(12):715-716, 718-719.
2. Rimmer A. A patient has complained about me online. What should I do? BMJ. 2019;366:I5705. doi: 10.1136/bmj.I5705.
3. Health Insurance Portability and Accountability Act of 1996 (HIPAA), S 1028, 104th Cong, Public Law No. 104-191, 110 Stat. 1936 (1996). https://www.govinfo.gov/content/pkg/PLAW-104publ191/pdf/PLAW-104publ191.pdf. Accessed Novermber 16, 2020.
1. Kendall L, Botello T. Internet sabotage: negative online reviews of psychiatrists. Psychiatr Ann. 2016;46(12):715-716, 718-719.
2. Rimmer A. A patient has complained about me online. What should I do? BMJ. 2019;366:I5705. doi: 10.1136/bmj.I5705.
3. Health Insurance Portability and Accountability Act of 1996 (HIPAA), S 1028, 104th Cong, Public Law No. 104-191, 110 Stat. 1936 (1996). https://www.govinfo.gov/content/pkg/PLAW-104publ191/pdf/PLAW-104publ191.pdf. Accessed Novermber 16, 2020.
2020 Peer Reviewers
Aparna Atluru, MD, MBA
Stanford Medicine
Sandra Barker, PhD
Virginia Commonwealth University
Kamal Bhatia, MD
MedStar Georgetown University Hospital
Charles F. Caley, PharmD
Western New England University Pharmacy Practice
Khushminder Chahal, MD
Homewood Health Centre
Craig Chepke, MD, FAPA
University of North Carolina at Chapel Hill School of Medicine
O. Greg Deardorff, PharmD, BCPP
Fulton State Hospital
Parikshit Deshmukh, MD, FAPA, FASAM
Oxford, FL
David Dunner, MD
Center for Anxiety and Depression
Lee Flowers, MD, MPH
Aspire Locums LLC
Melissa D. Grady, PhD, MSW
Catholic University, National Catholic School of Social Services
Staci Gruber, PhD
McLean Hospital
Vikas Gupta, MD, MPH
South Carolina Department of Mental Health
Susan Hatters-Friedman, MD
Case Western Reserve University
Robert Hendren, DO
University of California, San Francisco
Veeraraghavan Iyer, MD
Rutgers New Jersey Medical School
Abigail Kay, MD
Thomas Jefferson University
Rebecca Klisz-Hulbert, MD
Wayne State University
Gaurav Kulkarni, MD
Compass Health Network Psychiatry
Jill Levenson, PhD
Barry University
Steven B. Lippmann, MD
University of Louisville
Muhammad Hassan Majeed, MD
Lehigh Valley Health Network
David N. Neubauer, MD
Johns Hopkins University
John Onate, MD
UC Davis Health
Joel Paris, MD
McGill University
Brett Parmenter, PhD
VA Puget Sound Health Care System American Lake Division, Mental Health Clinic
Andrew Penn, RN, MS, NP
University of California, San Francisco
Fady Rachid, MD
Private Practice Geneva, Switzerland
Eduardo Rueda Vasquez, MD
Williamsport, PA
Marsal Sanches, MD, PhD, FAPA
University of Texas John P. and Kathrine G. McGovern Medical School
Matthew A. Schreiber, MD, PhD
Puget Sound VA Health Care System University of Washington School of Medicine
Mary Seeman, MD
University of Toronto
Ravi Shankar, MD
University of Missouri
Ashish Sharma, MD
University of Nebraska Medical Center
James Shore, MD, MPH/MSPH
University of Colorado Denver
Tawny Smith, PharmD, BCPP
University of Texas at Austin
Renee Sorrentino, MD
Massachusetts General Hospital
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Justin Strickland, PhD
Johns Hopkins University
Yilang Tang, MD, PhD
Emory University
Robyn Thom, MD
Massachusetts General Hospital
Katherine Unverferth, MD
University of California, Los Angeles
Amy M. VandenBerg, PharmD, BCPP
University of Michigan
Shikha Verma, MD
Rogers Behavioral Health
Roopma Wadhwa, MD, MHA
South Carolina Department of Mental Health
Patricia Westmoreland, MD
Eating Recovery Center
Glen Xiong, MD
University of California at Davis
Aparna Atluru, MD, MBA
Stanford Medicine
Sandra Barker, PhD
Virginia Commonwealth University
Kamal Bhatia, MD
MedStar Georgetown University Hospital
Charles F. Caley, PharmD
Western New England University Pharmacy Practice
Khushminder Chahal, MD
Homewood Health Centre
Craig Chepke, MD, FAPA
University of North Carolina at Chapel Hill School of Medicine
O. Greg Deardorff, PharmD, BCPP
Fulton State Hospital
Parikshit Deshmukh, MD, FAPA, FASAM
Oxford, FL
David Dunner, MD
Center for Anxiety and Depression
Lee Flowers, MD, MPH
Aspire Locums LLC
Melissa D. Grady, PhD, MSW
Catholic University, National Catholic School of Social Services
Staci Gruber, PhD
McLean Hospital
Vikas Gupta, MD, MPH
South Carolina Department of Mental Health
Susan Hatters-Friedman, MD
Case Western Reserve University
Robert Hendren, DO
University of California, San Francisco
Veeraraghavan Iyer, MD
Rutgers New Jersey Medical School
Abigail Kay, MD
Thomas Jefferson University
Rebecca Klisz-Hulbert, MD
Wayne State University
Gaurav Kulkarni, MD
Compass Health Network Psychiatry
Jill Levenson, PhD
Barry University
Steven B. Lippmann, MD
University of Louisville
Muhammad Hassan Majeed, MD
Lehigh Valley Health Network
David N. Neubauer, MD
Johns Hopkins University
John Onate, MD
UC Davis Health
Joel Paris, MD
McGill University
Brett Parmenter, PhD
VA Puget Sound Health Care System American Lake Division, Mental Health Clinic
Andrew Penn, RN, MS, NP
University of California, San Francisco
Fady Rachid, MD
Private Practice Geneva, Switzerland
Eduardo Rueda Vasquez, MD
Williamsport, PA
Marsal Sanches, MD, PhD, FAPA
University of Texas John P. and Kathrine G. McGovern Medical School
Matthew A. Schreiber, MD, PhD
Puget Sound VA Health Care System University of Washington School of Medicine
Mary Seeman, MD
University of Toronto
Ravi Shankar, MD
University of Missouri
Ashish Sharma, MD
University of Nebraska Medical Center
James Shore, MD, MPH/MSPH
University of Colorado Denver
Tawny Smith, PharmD, BCPP
University of Texas at Austin
Renee Sorrentino, MD
Massachusetts General Hospital
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Justin Strickland, PhD
Johns Hopkins University
Yilang Tang, MD, PhD
Emory University
Robyn Thom, MD
Massachusetts General Hospital
Katherine Unverferth, MD
University of California, Los Angeles
Amy M. VandenBerg, PharmD, BCPP
University of Michigan
Shikha Verma, MD
Rogers Behavioral Health
Roopma Wadhwa, MD, MHA
South Carolina Department of Mental Health
Patricia Westmoreland, MD
Eating Recovery Center
Glen Xiong, MD
University of California at Davis
Aparna Atluru, MD, MBA
Stanford Medicine
Sandra Barker, PhD
Virginia Commonwealth University
Kamal Bhatia, MD
MedStar Georgetown University Hospital
Charles F. Caley, PharmD
Western New England University Pharmacy Practice
Khushminder Chahal, MD
Homewood Health Centre
Craig Chepke, MD, FAPA
University of North Carolina at Chapel Hill School of Medicine
O. Greg Deardorff, PharmD, BCPP
Fulton State Hospital
Parikshit Deshmukh, MD, FAPA, FASAM
Oxford, FL
David Dunner, MD
Center for Anxiety and Depression
Lee Flowers, MD, MPH
Aspire Locums LLC
Melissa D. Grady, PhD, MSW
Catholic University, National Catholic School of Social Services
Staci Gruber, PhD
McLean Hospital
Vikas Gupta, MD, MPH
South Carolina Department of Mental Health
Susan Hatters-Friedman, MD
Case Western Reserve University
Robert Hendren, DO
University of California, San Francisco
Veeraraghavan Iyer, MD
Rutgers New Jersey Medical School
Abigail Kay, MD
Thomas Jefferson University
Rebecca Klisz-Hulbert, MD
Wayne State University
Gaurav Kulkarni, MD
Compass Health Network Psychiatry
Jill Levenson, PhD
Barry University
Steven B. Lippmann, MD
University of Louisville
Muhammad Hassan Majeed, MD
Lehigh Valley Health Network
David N. Neubauer, MD
Johns Hopkins University
John Onate, MD
UC Davis Health
Joel Paris, MD
McGill University
Brett Parmenter, PhD
VA Puget Sound Health Care System American Lake Division, Mental Health Clinic
Andrew Penn, RN, MS, NP
University of California, San Francisco
Fady Rachid, MD
Private Practice Geneva, Switzerland
Eduardo Rueda Vasquez, MD
Williamsport, PA
Marsal Sanches, MD, PhD, FAPA
University of Texas John P. and Kathrine G. McGovern Medical School
Matthew A. Schreiber, MD, PhD
Puget Sound VA Health Care System University of Washington School of Medicine
Mary Seeman, MD
University of Toronto
Ravi Shankar, MD
University of Missouri
Ashish Sharma, MD
University of Nebraska Medical Center
James Shore, MD, MPH/MSPH
University of Colorado Denver
Tawny Smith, PharmD, BCPP
University of Texas at Austin
Renee Sorrentino, MD
Massachusetts General Hospital
Cornel Stanciu, MD
Dartmouth’s Geisel School of Medicine
Justin Strickland, PhD
Johns Hopkins University
Yilang Tang, MD, PhD
Emory University
Robyn Thom, MD
Massachusetts General Hospital
Katherine Unverferth, MD
University of California, Los Angeles
Amy M. VandenBerg, PharmD, BCPP
University of Michigan
Shikha Verma, MD
Rogers Behavioral Health
Roopma Wadhwa, MD, MHA
South Carolina Department of Mental Health
Patricia Westmoreland, MD
Eating Recovery Center
Glen Xiong, MD
University of California at Davis
The COPD patient who couldn’t stop worrying
CASE A passive wish to die
Ms. M, age 76, has a history of major depressive disorder, unspecified anxiety disorder, and severe chronic obstructive pulmonary disease (COPD), for which she requires supplemental oxygen. She is admitted to a psychiatric hospital after several months of increased dysphoria, rumination, anhedonia, and a passive wish to die. She also has a decreased appetite and has lost 10 lb, experiences frequent daily episodes of shortness of breath and associated racing thoughts, and has a rapid heart rate.
HISTORY Past medication trials
In addition to COPD, Ms. M’s medical history includes hypertension. Past psychotropic medication trials used to treat her depression and anxiety have included aripiprazole, 5 mg/d; duloxetine, 60 mg/d; fluoxetine, 40 mg/d; mirtazapine, 30 mg nightly; buspirone, 10 mg twice daily; and clonazepam, 0.5 mg twice daily. She has no history of psychotherapy, and because of her uncontrolled anxiety and depression, she has never completed a pulmonary rehabilitation program.
Her current medications include salmeterol, 50 mcg inhaled twice daily, for COPD; amlodipine, 10 mg/d, for hypertension; buspirone, 10 mg twice daily, for anxiety; and duloxetine, 60 mg/d, for depression.
EXAMINATION No evidence of dementia
On examination, Ms. M is alert and oriented to person, place, date, and situation. Overall, she has mild difficulty with attention and short-term recall, which appears to be due to poor effort; intact long-term memory; and is able to abstract appropriately. There is no evidence of dementia.
A mental status exam reveals a frail, elderly woman with fair-to-poor hygiene, cooperative behavior, slowed motor activity, slowed speech with low volume, low mood, and depressed affect with constricted range. Her thought process is linear, her thought content includes passive death wishes, and she does not have hallucinations.
Bitemporal electroconvulsive therapy (ECT), 1.0 ms pulse width at 1.5 times Ms. M’s seizure threshold 3 times weekly, is initiated to treat her depression, with seizure duration averaging 45 seconds for each session. She receives a total of 8 treatments over the course of admission. Buspirone, 10 mg twice daily, is stopped shortly after admission, but she continues to receive duloxetine, 60 mg/d. Ms. M continues to have shortness of breath, palpitations, fearful ruminations about the future, and difficulty falling asleep.
[polldaddy:10673878]
The authors’ observations
The treatment team explores other options, such as benzodiazepines, psychotherapy modalities, and mindfulness exercises, to treat Ms. M’s anxiety and comorbid COPD. Lorazepam, 0.5 mg twice daily, was chosen to treat her acute anxiety. Due to Ms. M’s need for supplemental oxygen, the treatment team attempted to mitigate the risk of using a benzodiazepine by limiting its use to the minimum effective dose. The teams also looked for alternative therapies.
Continue to: Evalution of anxiety...
Evaluation of anxiety and depression in a patient with COPD is complicated by a high degree of symptom overlap. Patients with COPD may experience anxiety symptoms such as shortness of breath, rapid heart rate, numbness/tingling, and racing thoughts, and/or depressive symptoms such as decreased energy, impaired sleep, and impaired concentration. It can therefore be difficult to discern if a symptom is attributable to the physical diagnosis, the psychiatric diagnosis, or a combination of both. Catastrophic thinking about mild physical symptoms is common in patients with COPD. This can lead to hyperventilation and hypocapnia (manifested by lightheadedness, dizziness, paresthesia, and altered consciousness), with a reciprocally escalating cascade of anxiety and somatic symptoms.1
First-line therapy for anxiety disorders with comorbid COPD is CBT and other nonpharmacologic interventions.2,3
Although there is little evidence that traditional pharmacologic treatments (eg, antidepressants, benzodiazepines) have a statistically significant effect on anxiety and depression in COPD, studies have found that they have some clinical benefit.3 Risks, however, limit the utility of certain agents. Sedative-hypnotics potentially decrease respiratory drive and, particularly in older patients, antidepressants’ sedating effects can increase the risk of falls3 leading to increased morbidity, hospitalization, and mortality.
TREATMENT Mindfulness techniques and meditation
Ms. M’s symptoms show no improvement with the addition of lorazepam, 0.5 mg twice daily. A clinician teaches Ms. M mindfulness techniques, and she begins a trial of daily, individual, guided meditation using a meditation app. Respiratory therapists also instruct her on controlled breathing techniques such as pursed-lips breathing, diaphragmatic breathing, and deep breathing. They also encourage Ms. M to participate in the daily exercise group while on the unit.
[polldaddy:10673881]
The authors’ observations
Research indicates that low doses of opioids are safe and effective for refractory breathlessness in patients with severe COPD(those with an arterial partial pressure of oxygen ≤55 mm Hg or arterial oxygen saturation ≤88%).6,7
Continue to: The current opioid crisis...
The current opioid crisis prompts additional caution in prescribing, especially when considering using short-acting, immediate-release opioids such as morphine, which have a greater potential for abuse and dependence.
Many patients with COPD in the end-of-life phase and in severe pain or discomfort due to the advanced stages of their illness receive opioids as part of palliative care. Patients with COPD whose medical care is predominantly palliative may benefit greatly from being prescribed opioids. Most patients with COPD who find relief from low-dose opioids usually have 6 to 12 months to live, and low-dose opioids may help them obtain the best possible quality of life.
Choosing opioids as a treatment involves the risk of physiologic dependence and opioid use disorder. For Ms. M, the potential benefits were thought to outweigh such risks.
OUTCOME Breathlessness improves, anxiety decreases
Ms. M’s lorazepam is discontinued, and immediate-release morphine is prescribed at a low dose of 1 mg/d on an as-needed basis for anxiety with good effect. Ms. M’s breathlessness improves, leading to an overall decrease in anxiety. She does not experience sedation, confusion, or adverse respiratory effects.
Ms. M’s anxiety and depression improve over the course of the hospitalization with this regimen. On hospital Day 25, she is discharged with a plan to continue duloxetine, 60 mg/d, ECT twice weekly, and low-dose morphine, 1 mg/d, as needed for anxiety. She is referred for pulmonary rehabilitation and CBT to maintain remission.
[polldaddy:10673882]
Continue to: The authors' observations
The authors’ observations
Ms. M’s case highlights several challenges associated with treating psychiatric illness in a patient with a chronic medical illness. The relationship between COPD, anxiety, and depression is complex, and is associated with reduced quality of life, increasing severity of pulmonary disease, increased dyspnea, a sense of loss and inability to cope, and decreased self-efficacy and adherence to treatment.9-11
Bottom Line
When traditional antidepressant and anxiolytic therapies have not sufficiently helped, consider low-dose, once-daily opioids to address refractory breathlessness in a patient with COPD with comorbid anxiety and depression. This treatment can lead patients to participate in rehabilitation therapies and improve their quality of life.
Related Resources
- Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Buspirone • Buspar
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Hydromorphone • Dilaudid
Levodopa • Sinemet
Lorazepam • Ativan
Mirtazapine • Remeron
Morphine • MS Contin
Naloxone • Narcan
Oxycodone • Oxycontin
Salmeterol • Serevent Diskus
1. Harnett D. The difficult-to-treat psychiatric patient with comorbid medical illness. In: Dewan M, Pies R, eds. The difficult-to-treat psychiatric patient. Washington, DC: American Psychiatric Association Publishing; 2001:325-357.
2. Panagioti M, Scott C, Blakemore A, et al. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
3. Cafarella P, Effing T, Usmani ZA, et al. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627-638.
4. Heslop-Marshall K, Baker C, Carrick-Sen D, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res. 2018;4:00094-2018. doi: 10.1183/23120541.00094-2018.
5. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84(8):1154-1157.
6. Abernethy A, Currow D, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523-528.
7. Janowiak P, Krajnik M, Podolec Z, et al. Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial. BMC Pulm Med. 2017;17:186.
8. Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64(10):910-915.
9. Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
10. Carmen Valenza M, Valenza-Peña G, Torres-Sánchez I, et al. Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: a randomized clinical trial. Respir Care. 2014;59(2):209-215.
11. Pollok J, van Agteren J, Esterman A, et al. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;3:CD012347. doi: 10.1002/14651858.CD012347.pub2.
12. Roberts N, Kidd L, Kirkwood K, et al. A systematic review of the content and delivery of education in pulmonary rehabilitation programmes. Respiratory Medicine. 2018;145:161-181.
13. Pumar M, Gray C, Walsh J, et al. Anxiety and depression-important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
14. Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
15. Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
CASE A passive wish to die
Ms. M, age 76, has a history of major depressive disorder, unspecified anxiety disorder, and severe chronic obstructive pulmonary disease (COPD), for which she requires supplemental oxygen. She is admitted to a psychiatric hospital after several months of increased dysphoria, rumination, anhedonia, and a passive wish to die. She also has a decreased appetite and has lost 10 lb, experiences frequent daily episodes of shortness of breath and associated racing thoughts, and has a rapid heart rate.
HISTORY Past medication trials
In addition to COPD, Ms. M’s medical history includes hypertension. Past psychotropic medication trials used to treat her depression and anxiety have included aripiprazole, 5 mg/d; duloxetine, 60 mg/d; fluoxetine, 40 mg/d; mirtazapine, 30 mg nightly; buspirone, 10 mg twice daily; and clonazepam, 0.5 mg twice daily. She has no history of psychotherapy, and because of her uncontrolled anxiety and depression, she has never completed a pulmonary rehabilitation program.
Her current medications include salmeterol, 50 mcg inhaled twice daily, for COPD; amlodipine, 10 mg/d, for hypertension; buspirone, 10 mg twice daily, for anxiety; and duloxetine, 60 mg/d, for depression.
EXAMINATION No evidence of dementia
On examination, Ms. M is alert and oriented to person, place, date, and situation. Overall, she has mild difficulty with attention and short-term recall, which appears to be due to poor effort; intact long-term memory; and is able to abstract appropriately. There is no evidence of dementia.
A mental status exam reveals a frail, elderly woman with fair-to-poor hygiene, cooperative behavior, slowed motor activity, slowed speech with low volume, low mood, and depressed affect with constricted range. Her thought process is linear, her thought content includes passive death wishes, and she does not have hallucinations.
Bitemporal electroconvulsive therapy (ECT), 1.0 ms pulse width at 1.5 times Ms. M’s seizure threshold 3 times weekly, is initiated to treat her depression, with seizure duration averaging 45 seconds for each session. She receives a total of 8 treatments over the course of admission. Buspirone, 10 mg twice daily, is stopped shortly after admission, but she continues to receive duloxetine, 60 mg/d. Ms. M continues to have shortness of breath, palpitations, fearful ruminations about the future, and difficulty falling asleep.
[polldaddy:10673878]
The authors’ observations
The treatment team explores other options, such as benzodiazepines, psychotherapy modalities, and mindfulness exercises, to treat Ms. M’s anxiety and comorbid COPD. Lorazepam, 0.5 mg twice daily, was chosen to treat her acute anxiety. Due to Ms. M’s need for supplemental oxygen, the treatment team attempted to mitigate the risk of using a benzodiazepine by limiting its use to the minimum effective dose. The teams also looked for alternative therapies.
Continue to: Evalution of anxiety...
Evaluation of anxiety and depression in a patient with COPD is complicated by a high degree of symptom overlap. Patients with COPD may experience anxiety symptoms such as shortness of breath, rapid heart rate, numbness/tingling, and racing thoughts, and/or depressive symptoms such as decreased energy, impaired sleep, and impaired concentration. It can therefore be difficult to discern if a symptom is attributable to the physical diagnosis, the psychiatric diagnosis, or a combination of both. Catastrophic thinking about mild physical symptoms is common in patients with COPD. This can lead to hyperventilation and hypocapnia (manifested by lightheadedness, dizziness, paresthesia, and altered consciousness), with a reciprocally escalating cascade of anxiety and somatic symptoms.1
First-line therapy for anxiety disorders with comorbid COPD is CBT and other nonpharmacologic interventions.2,3
Although there is little evidence that traditional pharmacologic treatments (eg, antidepressants, benzodiazepines) have a statistically significant effect on anxiety and depression in COPD, studies have found that they have some clinical benefit.3 Risks, however, limit the utility of certain agents. Sedative-hypnotics potentially decrease respiratory drive and, particularly in older patients, antidepressants’ sedating effects can increase the risk of falls3 leading to increased morbidity, hospitalization, and mortality.
TREATMENT Mindfulness techniques and meditation
Ms. M’s symptoms show no improvement with the addition of lorazepam, 0.5 mg twice daily. A clinician teaches Ms. M mindfulness techniques, and she begins a trial of daily, individual, guided meditation using a meditation app. Respiratory therapists also instruct her on controlled breathing techniques such as pursed-lips breathing, diaphragmatic breathing, and deep breathing. They also encourage Ms. M to participate in the daily exercise group while on the unit.
[polldaddy:10673881]
The authors’ observations
Research indicates that low doses of opioids are safe and effective for refractory breathlessness in patients with severe COPD(those with an arterial partial pressure of oxygen ≤55 mm Hg or arterial oxygen saturation ≤88%).6,7
Continue to: The current opioid crisis...
The current opioid crisis prompts additional caution in prescribing, especially when considering using short-acting, immediate-release opioids such as morphine, which have a greater potential for abuse and dependence.
Many patients with COPD in the end-of-life phase and in severe pain or discomfort due to the advanced stages of their illness receive opioids as part of palliative care. Patients with COPD whose medical care is predominantly palliative may benefit greatly from being prescribed opioids. Most patients with COPD who find relief from low-dose opioids usually have 6 to 12 months to live, and low-dose opioids may help them obtain the best possible quality of life.
Choosing opioids as a treatment involves the risk of physiologic dependence and opioid use disorder. For Ms. M, the potential benefits were thought to outweigh such risks.
OUTCOME Breathlessness improves, anxiety decreases
Ms. M’s lorazepam is discontinued, and immediate-release morphine is prescribed at a low dose of 1 mg/d on an as-needed basis for anxiety with good effect. Ms. M’s breathlessness improves, leading to an overall decrease in anxiety. She does not experience sedation, confusion, or adverse respiratory effects.
Ms. M’s anxiety and depression improve over the course of the hospitalization with this regimen. On hospital Day 25, she is discharged with a plan to continue duloxetine, 60 mg/d, ECT twice weekly, and low-dose morphine, 1 mg/d, as needed for anxiety. She is referred for pulmonary rehabilitation and CBT to maintain remission.
[polldaddy:10673882]
Continue to: The authors' observations
The authors’ observations
Ms. M’s case highlights several challenges associated with treating psychiatric illness in a patient with a chronic medical illness. The relationship between COPD, anxiety, and depression is complex, and is associated with reduced quality of life, increasing severity of pulmonary disease, increased dyspnea, a sense of loss and inability to cope, and decreased self-efficacy and adherence to treatment.9-11
Bottom Line
When traditional antidepressant and anxiolytic therapies have not sufficiently helped, consider low-dose, once-daily opioids to address refractory breathlessness in a patient with COPD with comorbid anxiety and depression. This treatment can lead patients to participate in rehabilitation therapies and improve their quality of life.
Related Resources
- Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Buspirone • Buspar
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Hydromorphone • Dilaudid
Levodopa • Sinemet
Lorazepam • Ativan
Mirtazapine • Remeron
Morphine • MS Contin
Naloxone • Narcan
Oxycodone • Oxycontin
Salmeterol • Serevent Diskus
CASE A passive wish to die
Ms. M, age 76, has a history of major depressive disorder, unspecified anxiety disorder, and severe chronic obstructive pulmonary disease (COPD), for which she requires supplemental oxygen. She is admitted to a psychiatric hospital after several months of increased dysphoria, rumination, anhedonia, and a passive wish to die. She also has a decreased appetite and has lost 10 lb, experiences frequent daily episodes of shortness of breath and associated racing thoughts, and has a rapid heart rate.
HISTORY Past medication trials
In addition to COPD, Ms. M’s medical history includes hypertension. Past psychotropic medication trials used to treat her depression and anxiety have included aripiprazole, 5 mg/d; duloxetine, 60 mg/d; fluoxetine, 40 mg/d; mirtazapine, 30 mg nightly; buspirone, 10 mg twice daily; and clonazepam, 0.5 mg twice daily. She has no history of psychotherapy, and because of her uncontrolled anxiety and depression, she has never completed a pulmonary rehabilitation program.
Her current medications include salmeterol, 50 mcg inhaled twice daily, for COPD; amlodipine, 10 mg/d, for hypertension; buspirone, 10 mg twice daily, for anxiety; and duloxetine, 60 mg/d, for depression.
EXAMINATION No evidence of dementia
On examination, Ms. M is alert and oriented to person, place, date, and situation. Overall, she has mild difficulty with attention and short-term recall, which appears to be due to poor effort; intact long-term memory; and is able to abstract appropriately. There is no evidence of dementia.
A mental status exam reveals a frail, elderly woman with fair-to-poor hygiene, cooperative behavior, slowed motor activity, slowed speech with low volume, low mood, and depressed affect with constricted range. Her thought process is linear, her thought content includes passive death wishes, and she does not have hallucinations.
Bitemporal electroconvulsive therapy (ECT), 1.0 ms pulse width at 1.5 times Ms. M’s seizure threshold 3 times weekly, is initiated to treat her depression, with seizure duration averaging 45 seconds for each session. She receives a total of 8 treatments over the course of admission. Buspirone, 10 mg twice daily, is stopped shortly after admission, but she continues to receive duloxetine, 60 mg/d. Ms. M continues to have shortness of breath, palpitations, fearful ruminations about the future, and difficulty falling asleep.
[polldaddy:10673878]
The authors’ observations
The treatment team explores other options, such as benzodiazepines, psychotherapy modalities, and mindfulness exercises, to treat Ms. M’s anxiety and comorbid COPD. Lorazepam, 0.5 mg twice daily, was chosen to treat her acute anxiety. Due to Ms. M’s need for supplemental oxygen, the treatment team attempted to mitigate the risk of using a benzodiazepine by limiting its use to the minimum effective dose. The teams also looked for alternative therapies.
Continue to: Evalution of anxiety...
Evaluation of anxiety and depression in a patient with COPD is complicated by a high degree of symptom overlap. Patients with COPD may experience anxiety symptoms such as shortness of breath, rapid heart rate, numbness/tingling, and racing thoughts, and/or depressive symptoms such as decreased energy, impaired sleep, and impaired concentration. It can therefore be difficult to discern if a symptom is attributable to the physical diagnosis, the psychiatric diagnosis, or a combination of both. Catastrophic thinking about mild physical symptoms is common in patients with COPD. This can lead to hyperventilation and hypocapnia (manifested by lightheadedness, dizziness, paresthesia, and altered consciousness), with a reciprocally escalating cascade of anxiety and somatic symptoms.1
First-line therapy for anxiety disorders with comorbid COPD is CBT and other nonpharmacologic interventions.2,3
Although there is little evidence that traditional pharmacologic treatments (eg, antidepressants, benzodiazepines) have a statistically significant effect on anxiety and depression in COPD, studies have found that they have some clinical benefit.3 Risks, however, limit the utility of certain agents. Sedative-hypnotics potentially decrease respiratory drive and, particularly in older patients, antidepressants’ sedating effects can increase the risk of falls3 leading to increased morbidity, hospitalization, and mortality.
TREATMENT Mindfulness techniques and meditation
Ms. M’s symptoms show no improvement with the addition of lorazepam, 0.5 mg twice daily. A clinician teaches Ms. M mindfulness techniques, and she begins a trial of daily, individual, guided meditation using a meditation app. Respiratory therapists also instruct her on controlled breathing techniques such as pursed-lips breathing, diaphragmatic breathing, and deep breathing. They also encourage Ms. M to participate in the daily exercise group while on the unit.
[polldaddy:10673881]
The authors’ observations
Research indicates that low doses of opioids are safe and effective for refractory breathlessness in patients with severe COPD(those with an arterial partial pressure of oxygen ≤55 mm Hg or arterial oxygen saturation ≤88%).6,7
Continue to: The current opioid crisis...
The current opioid crisis prompts additional caution in prescribing, especially when considering using short-acting, immediate-release opioids such as morphine, which have a greater potential for abuse and dependence.
Many patients with COPD in the end-of-life phase and in severe pain or discomfort due to the advanced stages of their illness receive opioids as part of palliative care. Patients with COPD whose medical care is predominantly palliative may benefit greatly from being prescribed opioids. Most patients with COPD who find relief from low-dose opioids usually have 6 to 12 months to live, and low-dose opioids may help them obtain the best possible quality of life.
Choosing opioids as a treatment involves the risk of physiologic dependence and opioid use disorder. For Ms. M, the potential benefits were thought to outweigh such risks.
OUTCOME Breathlessness improves, anxiety decreases
Ms. M’s lorazepam is discontinued, and immediate-release morphine is prescribed at a low dose of 1 mg/d on an as-needed basis for anxiety with good effect. Ms. M’s breathlessness improves, leading to an overall decrease in anxiety. She does not experience sedation, confusion, or adverse respiratory effects.
Ms. M’s anxiety and depression improve over the course of the hospitalization with this regimen. On hospital Day 25, she is discharged with a plan to continue duloxetine, 60 mg/d, ECT twice weekly, and low-dose morphine, 1 mg/d, as needed for anxiety. She is referred for pulmonary rehabilitation and CBT to maintain remission.
[polldaddy:10673882]
Continue to: The authors' observations
The authors’ observations
Ms. M’s case highlights several challenges associated with treating psychiatric illness in a patient with a chronic medical illness. The relationship between COPD, anxiety, and depression is complex, and is associated with reduced quality of life, increasing severity of pulmonary disease, increased dyspnea, a sense of loss and inability to cope, and decreased self-efficacy and adherence to treatment.9-11
Bottom Line
When traditional antidepressant and anxiolytic therapies have not sufficiently helped, consider low-dose, once-daily opioids to address refractory breathlessness in a patient with COPD with comorbid anxiety and depression. This treatment can lead patients to participate in rehabilitation therapies and improve their quality of life.
Related Resources
- Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
- Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Buspirone • Buspar
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Hydromorphone • Dilaudid
Levodopa • Sinemet
Lorazepam • Ativan
Mirtazapine • Remeron
Morphine • MS Contin
Naloxone • Narcan
Oxycodone • Oxycontin
Salmeterol • Serevent Diskus
1. Harnett D. The difficult-to-treat psychiatric patient with comorbid medical illness. In: Dewan M, Pies R, eds. The difficult-to-treat psychiatric patient. Washington, DC: American Psychiatric Association Publishing; 2001:325-357.
2. Panagioti M, Scott C, Blakemore A, et al. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
3. Cafarella P, Effing T, Usmani ZA, et al. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627-638.
4. Heslop-Marshall K, Baker C, Carrick-Sen D, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res. 2018;4:00094-2018. doi: 10.1183/23120541.00094-2018.
5. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84(8):1154-1157.
6. Abernethy A, Currow D, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523-528.
7. Janowiak P, Krajnik M, Podolec Z, et al. Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial. BMC Pulm Med. 2017;17:186.
8. Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64(10):910-915.
9. Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
10. Carmen Valenza M, Valenza-Peña G, Torres-Sánchez I, et al. Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: a randomized clinical trial. Respir Care. 2014;59(2):209-215.
11. Pollok J, van Agteren J, Esterman A, et al. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;3:CD012347. doi: 10.1002/14651858.CD012347.pub2.
12. Roberts N, Kidd L, Kirkwood K, et al. A systematic review of the content and delivery of education in pulmonary rehabilitation programmes. Respiratory Medicine. 2018;145:161-181.
13. Pumar M, Gray C, Walsh J, et al. Anxiety and depression-important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
14. Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
15. Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
1. Harnett D. The difficult-to-treat psychiatric patient with comorbid medical illness. In: Dewan M, Pies R, eds. The difficult-to-treat psychiatric patient. Washington, DC: American Psychiatric Association Publishing; 2001:325-357.
2. Panagioti M, Scott C, Blakemore A, et al. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
3. Cafarella P, Effing T, Usmani ZA, et al. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627-638.
4. Heslop-Marshall K, Baker C, Carrick-Sen D, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res. 2018;4:00094-2018. doi: 10.1183/23120541.00094-2018.
5. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84(8):1154-1157.
6. Abernethy A, Currow D, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523-528.
7. Janowiak P, Krajnik M, Podolec Z, et al. Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial. BMC Pulm Med. 2017;17:186.
8. Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64(10):910-915.
9. Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
10. Carmen Valenza M, Valenza-Peña G, Torres-Sánchez I, et al. Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: a randomized clinical trial. Respir Care. 2014;59(2):209-215.
11. Pollok J, van Agteren J, Esterman A, et al. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;3:CD012347. doi: 10.1002/14651858.CD012347.pub2.
12. Roberts N, Kidd L, Kirkwood K, et al. A systematic review of the content and delivery of education in pulmonary rehabilitation programmes. Respiratory Medicine. 2018;145:161-181.
13. Pumar M, Gray C, Walsh J, et al. Anxiety and depression-important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
14. Alexopoulos G, Kiosses D, Sirey J, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. Am J Geriatr Psychiatry. 2014;22(11):1316-1324.
15. Jackson D, Banerjee S, Sirey J, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on quality of life. Am J Geriatr Psychiatry. 2018;27(5):502-511.
Virtual residency/fellowship interviews: The good, the bad, and the future
As a psychiatry resident in the age of coronavirus disease 2019 (COVID-19), many of my educational experiences have undergone adjustments. Now, as I interview for a fellowship, I see firsthand that recruitment activities have not been spared from shifting paradigms levied by the pandemic. To adhere to social distancing guidelines and limit trainee interpersonal exposure, the American Association of Directors of Psychiatric Residency Training recommended that all psychiatry residency interviews be conducted virtually for 2020/2021.1 Trainees and programs alike are embarking on a new frontier of virtual interviews, and it is important that we evaluate the advantages and disadvantages of this approach. Because uncertainty abounds regarding when a sense of normalcy might eventually return to psychiatry residency and fellowship recruitment activities, I also provide recommendations to interviewers and interviewees who may navigate virtual recruitment in the future.
Advantages of virtual interviews
An immediately significant advantage of virtual interviews is the lack of travel, which for some applicants can be cost-prohibitive. The costs of airfare, rental vehicles, and lodging in multiple cities can add up, sometimes requiring students to budget interview travel into already-high student loans. In some cases, applicants may have limited days to interview, which makes the flexibility afforded by the lack of travel advantageous. Furthermore, navigating new locations can add to preexisting interview stress. Without travel, applicants can consider a broader set of programs and accept more interviews.
Another advantage is that virtual interviews allow interviewees latitude to shift the interview’s “frame.” Rather than sitting in an interviewer’s office, interviewees can choose a more comfortable environment for themselves, imparting a “home-field advantage” that may put them at ease. During my fellowship interviews, controlling the room temperature, using a familiar chair, and wearing comfortable shoes helped to reduce the anxiety inherent to interviewing.
Disadvantages of virtual interviews
Any new or unfamiliar experience can impart challenges. For example, applicants and interviewers must adjust to and observe different sets of etiquette during virtual interviews. These include muting microphones to avoid talking over each other, maintaining consistent eye contact, avoiding multitasking, and following up to avoid miscommunication.
Another potential problem is that virtual interviews can dampen an applicant’s ability to appreciate a program’s culture. Observing informal interactions between trainees and faculty is often as important as the formal interviews in ascertaining which programs have a supportive culture. Because my virtual fellowship interviews have generally been limited to formal one-on-one interviews, assessing program culture has become more challenging. Conversely, programs may find it difficult to grasp an applicant’s temperament and interaction style.
Virtual interviewing, while undeniably convenient in many regards, may fall prey to its own convenience. There can be disparities in the quantity, duration, and frequency of interviews. For me, the number of and time allotted for interviews has varied widely, ranging from 2.5 to 8 hours. The amount of allotted break time has also differed, with some programs providing longer breaks between interviews (30 to 60 minutes) and others offering shorter (5 to 10 minutes) or no breaks. Minimal breaks may fatigue applicants, while longer breaks may seem like wasted time. While virtual interviews may require no physical travel between offices, breaks are a necessity that should be implemented thoughtfully.
Finally, a troublesome challenge I encountered surprisingly often was unreliable internet service and other technical difficulties. Several times, my interviewers’ (or my) screen froze or shut off due to connectivity issues. This is an obstacle unique to virtual interviews that requires both a backup plan as well as patience and calm to navigate during an already taxing situation.
Continue to: What's next?
What’s next?
As applicants and programs adjust to the realities of virtual interviewing, this is likely an unfamiliar experience for all. While the benefits and shortcomings must be considered together, I, along with many of my peers,2 continue to prefer traditional in-person interviews. As the ongoing COVID-19 pandemic makes in-person interviews difficult, applicants and programs must embrace the experience of virtual interviews. However, a good understanding of the advantages and disadvantages are instrumental in preempting prospective challenges. Based on my recent experiences with virtual fellowship interviews, I have created some recommendations for applicants and psychiatry programs participating in virtual recruitment (Table).

After the COVID-19 pandemic subsides, it is conceivable that the advantages of virtual interviewing may justify its continued use. For example, applicants may be able to apply to geographically diverse programs without travel expenses. Currently, there is a paucity of evidence regarding virtual interviews specifically in psychiatry training programs, but the experiences of both applicants and psychiatry programs during this atypical time will allow us to improve the process going forward, and evaluate its utility well after COVID-19 recedes.
1. Arbuckle M, Kerlek A, Kovach J, et al. Consensus statement from the Association of Directors of Medical Student Education in Psychiatry (ADMSEP) and the American Association of Directors of Psychiatric Residency Training (AADPRT) on the 2020-21 Residency Application Cycle. https://www.aadprt.org/application/files/8816/0017/8240/admsep_aadprt_statement_9-14-20_Rev.pdf. Published May 18, 2020. Accessed November 20, 2020.
2. Seifi A, Mirahmadizadeh A, Eslami V. Perception of medical students and residents about virtual interviews for residency applications in the United States. PLoS ONE. 2020;15(8):e0238239. doi: 10.1371/journal.pone.0238239.
As a psychiatry resident in the age of coronavirus disease 2019 (COVID-19), many of my educational experiences have undergone adjustments. Now, as I interview for a fellowship, I see firsthand that recruitment activities have not been spared from shifting paradigms levied by the pandemic. To adhere to social distancing guidelines and limit trainee interpersonal exposure, the American Association of Directors of Psychiatric Residency Training recommended that all psychiatry residency interviews be conducted virtually for 2020/2021.1 Trainees and programs alike are embarking on a new frontier of virtual interviews, and it is important that we evaluate the advantages and disadvantages of this approach. Because uncertainty abounds regarding when a sense of normalcy might eventually return to psychiatry residency and fellowship recruitment activities, I also provide recommendations to interviewers and interviewees who may navigate virtual recruitment in the future.
Advantages of virtual interviews
An immediately significant advantage of virtual interviews is the lack of travel, which for some applicants can be cost-prohibitive. The costs of airfare, rental vehicles, and lodging in multiple cities can add up, sometimes requiring students to budget interview travel into already-high student loans. In some cases, applicants may have limited days to interview, which makes the flexibility afforded by the lack of travel advantageous. Furthermore, navigating new locations can add to preexisting interview stress. Without travel, applicants can consider a broader set of programs and accept more interviews.
Another advantage is that virtual interviews allow interviewees latitude to shift the interview’s “frame.” Rather than sitting in an interviewer’s office, interviewees can choose a more comfortable environment for themselves, imparting a “home-field advantage” that may put them at ease. During my fellowship interviews, controlling the room temperature, using a familiar chair, and wearing comfortable shoes helped to reduce the anxiety inherent to interviewing.
Disadvantages of virtual interviews
Any new or unfamiliar experience can impart challenges. For example, applicants and interviewers must adjust to and observe different sets of etiquette during virtual interviews. These include muting microphones to avoid talking over each other, maintaining consistent eye contact, avoiding multitasking, and following up to avoid miscommunication.
Another potential problem is that virtual interviews can dampen an applicant’s ability to appreciate a program’s culture. Observing informal interactions between trainees and faculty is often as important as the formal interviews in ascertaining which programs have a supportive culture. Because my virtual fellowship interviews have generally been limited to formal one-on-one interviews, assessing program culture has become more challenging. Conversely, programs may find it difficult to grasp an applicant’s temperament and interaction style.
Virtual interviewing, while undeniably convenient in many regards, may fall prey to its own convenience. There can be disparities in the quantity, duration, and frequency of interviews. For me, the number of and time allotted for interviews has varied widely, ranging from 2.5 to 8 hours. The amount of allotted break time has also differed, with some programs providing longer breaks between interviews (30 to 60 minutes) and others offering shorter (5 to 10 minutes) or no breaks. Minimal breaks may fatigue applicants, while longer breaks may seem like wasted time. While virtual interviews may require no physical travel between offices, breaks are a necessity that should be implemented thoughtfully.
Finally, a troublesome challenge I encountered surprisingly often was unreliable internet service and other technical difficulties. Several times, my interviewers’ (or my) screen froze or shut off due to connectivity issues. This is an obstacle unique to virtual interviews that requires both a backup plan as well as patience and calm to navigate during an already taxing situation.
Continue to: What's next?
What’s next?
As applicants and programs adjust to the realities of virtual interviewing, this is likely an unfamiliar experience for all. While the benefits and shortcomings must be considered together, I, along with many of my peers,2 continue to prefer traditional in-person interviews. As the ongoing COVID-19 pandemic makes in-person interviews difficult, applicants and programs must embrace the experience of virtual interviews. However, a good understanding of the advantages and disadvantages are instrumental in preempting prospective challenges. Based on my recent experiences with virtual fellowship interviews, I have created some recommendations for applicants and psychiatry programs participating in virtual recruitment (Table).

After the COVID-19 pandemic subsides, it is conceivable that the advantages of virtual interviewing may justify its continued use. For example, applicants may be able to apply to geographically diverse programs without travel expenses. Currently, there is a paucity of evidence regarding virtual interviews specifically in psychiatry training programs, but the experiences of both applicants and psychiatry programs during this atypical time will allow us to improve the process going forward, and evaluate its utility well after COVID-19 recedes.
As a psychiatry resident in the age of coronavirus disease 2019 (COVID-19), many of my educational experiences have undergone adjustments. Now, as I interview for a fellowship, I see firsthand that recruitment activities have not been spared from shifting paradigms levied by the pandemic. To adhere to social distancing guidelines and limit trainee interpersonal exposure, the American Association of Directors of Psychiatric Residency Training recommended that all psychiatry residency interviews be conducted virtually for 2020/2021.1 Trainees and programs alike are embarking on a new frontier of virtual interviews, and it is important that we evaluate the advantages and disadvantages of this approach. Because uncertainty abounds regarding when a sense of normalcy might eventually return to psychiatry residency and fellowship recruitment activities, I also provide recommendations to interviewers and interviewees who may navigate virtual recruitment in the future.
Advantages of virtual interviews
An immediately significant advantage of virtual interviews is the lack of travel, which for some applicants can be cost-prohibitive. The costs of airfare, rental vehicles, and lodging in multiple cities can add up, sometimes requiring students to budget interview travel into already-high student loans. In some cases, applicants may have limited days to interview, which makes the flexibility afforded by the lack of travel advantageous. Furthermore, navigating new locations can add to preexisting interview stress. Without travel, applicants can consider a broader set of programs and accept more interviews.
Another advantage is that virtual interviews allow interviewees latitude to shift the interview’s “frame.” Rather than sitting in an interviewer’s office, interviewees can choose a more comfortable environment for themselves, imparting a “home-field advantage” that may put them at ease. During my fellowship interviews, controlling the room temperature, using a familiar chair, and wearing comfortable shoes helped to reduce the anxiety inherent to interviewing.
Disadvantages of virtual interviews
Any new or unfamiliar experience can impart challenges. For example, applicants and interviewers must adjust to and observe different sets of etiquette during virtual interviews. These include muting microphones to avoid talking over each other, maintaining consistent eye contact, avoiding multitasking, and following up to avoid miscommunication.
Another potential problem is that virtual interviews can dampen an applicant’s ability to appreciate a program’s culture. Observing informal interactions between trainees and faculty is often as important as the formal interviews in ascertaining which programs have a supportive culture. Because my virtual fellowship interviews have generally been limited to formal one-on-one interviews, assessing program culture has become more challenging. Conversely, programs may find it difficult to grasp an applicant’s temperament and interaction style.
Virtual interviewing, while undeniably convenient in many regards, may fall prey to its own convenience. There can be disparities in the quantity, duration, and frequency of interviews. For me, the number of and time allotted for interviews has varied widely, ranging from 2.5 to 8 hours. The amount of allotted break time has also differed, with some programs providing longer breaks between interviews (30 to 60 minutes) and others offering shorter (5 to 10 minutes) or no breaks. Minimal breaks may fatigue applicants, while longer breaks may seem like wasted time. While virtual interviews may require no physical travel between offices, breaks are a necessity that should be implemented thoughtfully.
Finally, a troublesome challenge I encountered surprisingly often was unreliable internet service and other technical difficulties. Several times, my interviewers’ (or my) screen froze or shut off due to connectivity issues. This is an obstacle unique to virtual interviews that requires both a backup plan as well as patience and calm to navigate during an already taxing situation.
Continue to: What's next?
What’s next?
As applicants and programs adjust to the realities of virtual interviewing, this is likely an unfamiliar experience for all. While the benefits and shortcomings must be considered together, I, along with many of my peers,2 continue to prefer traditional in-person interviews. As the ongoing COVID-19 pandemic makes in-person interviews difficult, applicants and programs must embrace the experience of virtual interviews. However, a good understanding of the advantages and disadvantages are instrumental in preempting prospective challenges. Based on my recent experiences with virtual fellowship interviews, I have created some recommendations for applicants and psychiatry programs participating in virtual recruitment (Table).

After the COVID-19 pandemic subsides, it is conceivable that the advantages of virtual interviewing may justify its continued use. For example, applicants may be able to apply to geographically diverse programs without travel expenses. Currently, there is a paucity of evidence regarding virtual interviews specifically in psychiatry training programs, but the experiences of both applicants and psychiatry programs during this atypical time will allow us to improve the process going forward, and evaluate its utility well after COVID-19 recedes.
1. Arbuckle M, Kerlek A, Kovach J, et al. Consensus statement from the Association of Directors of Medical Student Education in Psychiatry (ADMSEP) and the American Association of Directors of Psychiatric Residency Training (AADPRT) on the 2020-21 Residency Application Cycle. https://www.aadprt.org/application/files/8816/0017/8240/admsep_aadprt_statement_9-14-20_Rev.pdf. Published May 18, 2020. Accessed November 20, 2020.
2. Seifi A, Mirahmadizadeh A, Eslami V. Perception of medical students and residents about virtual interviews for residency applications in the United States. PLoS ONE. 2020;15(8):e0238239. doi: 10.1371/journal.pone.0238239.
1. Arbuckle M, Kerlek A, Kovach J, et al. Consensus statement from the Association of Directors of Medical Student Education in Psychiatry (ADMSEP) and the American Association of Directors of Psychiatric Residency Training (AADPRT) on the 2020-21 Residency Application Cycle. https://www.aadprt.org/application/files/8816/0017/8240/admsep_aadprt_statement_9-14-20_Rev.pdf. Published May 18, 2020. Accessed November 20, 2020.
2. Seifi A, Mirahmadizadeh A, Eslami V. Perception of medical students and residents about virtual interviews for residency applications in the United States. PLoS ONE. 2020;15(8):e0238239. doi: 10.1371/journal.pone.0238239.
COVID-19’s religious strain: Differentiating spirituality from pathology
As the world grapples with the coronavirus disease 2019 (COVID-19) pandemic, the search for answers, comfort, how to cope, and how to make sense of it all has become paramount. People commonly turn to their faith in times of crisis, but this recent global public health emergency is unlike many have ever seen or could have imagined.1 What happens when the well-intentioned journey for spiritual insight intersects with psychiatric symptomatology? Where does the line between these phenomena get crossed? As a psychiatric resident and person who was raised in the Pentecostal faith, I have observed faith and psychopathology come to a head in the last 6 months. COVID-19 has dealt a religious strain of undocumented cases; I hope to shed light on the topic by sharing my experience of navigating the assessment and treatment plan of patients with psychiatric symptoms whose spiritual beliefs are a cornerstone of life.
Piety, or pathology?
The following approaches have helped me to identify what is driven by faith vs what is psychopathology:
While taking the patient history. Obtaining a history from a patient who professes to have strong spiritual beliefs and presents with psychiatric symptoms is similar to a standard patient interview, but pay special attention to how the patient came to the emergency department. Was there a family member, friend, or emergency medical services present at that time? During the interview, patients often appear “normal,” which may lead a clinician to question the reason for the consult, yet considering the recent events preceding the presentation will be a good place to start gathering the appropriate information for investigation.
Next, compare the patient’s recent daily functioning with his/her baseline. If this information comes solely from the patient, it may be skewed, so try to retrieve information from a collateral source. If the patient was accompanied by someone, request permission from the patient to speak with him/her. It may also be best in some instances to speak with the collateral source out of earshot of the patient. Be aware that collateral information that comes from just one source also could be biased, so search for additional contacts to help acquire a comprehensive representation of the circumstances.
Information about a patient could come from a faith leader because people often rely on their faith leaders when they are ill, in need of support, or in crisis.2 Faith leaders may have valuable information and insight into the patient and the history of the patient’s illness. In addition, diverse sources of collateral reports may be helpful because specific spiritual views and practices can vary even within one family or congregation. What may be an abnormal practice to some followers may be normal for others.3 When approaching these situations with parishioners, it is essential to maintain confidentiality.
While performing the clinical examination. As with any psychiatric diagnosis, other causative factors (metabolic and organic) need to be ruled out. Also, assess for the use of mood-altering substances. The patient may express offense or resistance to such questions, but maintain a matter-of-fact approach and explain that assessment for substance use is a routine part of the clinical examination. Approximately 18% of people in the United States with psychiatric disorders have a comorbid substance use disorder.4 However, keep in mind that a patient who refuses substance use screening is not necessarily hiding something. The road to being thorough may lead to strained rapport with the patient, and this risk must be balanced with providing the best care. As in any other clinical situation, seek evidence to both verify and clarify information without being deterred by a patient’s vocalization of spiritual tenants.
Learn about your patients’ beliefs
Do not feel defeated if you find these interviews difficult. Religion and symptomatology can overlap and fluctuate within the same faith group, which can make these types of assessments complex.5 In an effort to understand the patient more clearly, be sensitive to their spiritual practices and receptive to learning about unfamiliar spiritual beliefs. Be transparent about not knowing a specific belief or practice, and exhibit humility. Most patients are open to sharing their religious/spiritual views with a clinician who is sincere about wanting insight. Understanding the value of spiritual care is an important skill that many medical practitioners often lack.6 This understanding is especially critical when patients express worries related to the COVID-19 pandemic and how they are coping.
Continue to: Integrate your patient's spiritual requests
Integrate your patient’s spiritual requests
If you are comfortable with certain practices that do not compromise your values or beliefs or put a patient at risk, try to integrate your patients’ spiritual request(s) in their care. For a patient who serves a higher power, admitting to a problem (eg, fears related to COVID-19) or seeking professional help for symptoms (eg, anxiety, depression) may imply spiritual doubt. Patients may believe that seeking professional assistance means they are questioning the omnipotence of their deity to prevent or heal a condition. While spiritual distress can stimulate changes in behavior, it may not be pathological.
To avoid misdiagnosis, refer to the description “V62.89 (Z65.8) Religious or Spiritual Problem” in the DSM-5.7 If you find that it is a discord in faith that is affecting the patient’s presentation, and that this has not caused a psychiatric disorder, document this appropriately and provide the necessary resources to continue supporting the patient holistically.
1. Dein S, Loewenthal K, Lewis CA, et al. COVID-19, mental health and religion: an agenda for future research. Mental Health, Religion & Culture. 2020;23(1):1-9.
2. American Psychiatric Association Foundation. Mental health: a guide for faith leaders. Arlington, VA: American Psychiatric Association Foundation; 2018.
3. Johnson CV, Friedman HL. Enlightened or delusional? Differentiating religious, spiritual, and transpersonal experiences from psychopathology. Journal of Humanistic Psychology. 2008;48(4):505-527.
4. Han B, Compton WM, Blanco C, et al. Prevalence, treatment, and unmet treatment needs of US adults with mental health and substance use disorders. Health Aff (Millwood). 2017;36(10):1739-1747.
5. Menezes Jr A, Moreira-Almeida A. Differential diagnosis between spiritual experiences and mental disorders of religious content. Rev Psiq Clín. 2009;36(2):75-82.
6. Best M, Butow P, Olver I. Doctors discussing religion and spirituality: a systematic literature review. Palliat Med. 2016;30(4):327-337.
7. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:725.
As the world grapples with the coronavirus disease 2019 (COVID-19) pandemic, the search for answers, comfort, how to cope, and how to make sense of it all has become paramount. People commonly turn to their faith in times of crisis, but this recent global public health emergency is unlike many have ever seen or could have imagined.1 What happens when the well-intentioned journey for spiritual insight intersects with psychiatric symptomatology? Where does the line between these phenomena get crossed? As a psychiatric resident and person who was raised in the Pentecostal faith, I have observed faith and psychopathology come to a head in the last 6 months. COVID-19 has dealt a religious strain of undocumented cases; I hope to shed light on the topic by sharing my experience of navigating the assessment and treatment plan of patients with psychiatric symptoms whose spiritual beliefs are a cornerstone of life.
Piety, or pathology?
The following approaches have helped me to identify what is driven by faith vs what is psychopathology:
While taking the patient history. Obtaining a history from a patient who professes to have strong spiritual beliefs and presents with psychiatric symptoms is similar to a standard patient interview, but pay special attention to how the patient came to the emergency department. Was there a family member, friend, or emergency medical services present at that time? During the interview, patients often appear “normal,” which may lead a clinician to question the reason for the consult, yet considering the recent events preceding the presentation will be a good place to start gathering the appropriate information for investigation.
Next, compare the patient’s recent daily functioning with his/her baseline. If this information comes solely from the patient, it may be skewed, so try to retrieve information from a collateral source. If the patient was accompanied by someone, request permission from the patient to speak with him/her. It may also be best in some instances to speak with the collateral source out of earshot of the patient. Be aware that collateral information that comes from just one source also could be biased, so search for additional contacts to help acquire a comprehensive representation of the circumstances.
Information about a patient could come from a faith leader because people often rely on their faith leaders when they are ill, in need of support, or in crisis.2 Faith leaders may have valuable information and insight into the patient and the history of the patient’s illness. In addition, diverse sources of collateral reports may be helpful because specific spiritual views and practices can vary even within one family or congregation. What may be an abnormal practice to some followers may be normal for others.3 When approaching these situations with parishioners, it is essential to maintain confidentiality.
While performing the clinical examination. As with any psychiatric diagnosis, other causative factors (metabolic and organic) need to be ruled out. Also, assess for the use of mood-altering substances. The patient may express offense or resistance to such questions, but maintain a matter-of-fact approach and explain that assessment for substance use is a routine part of the clinical examination. Approximately 18% of people in the United States with psychiatric disorders have a comorbid substance use disorder.4 However, keep in mind that a patient who refuses substance use screening is not necessarily hiding something. The road to being thorough may lead to strained rapport with the patient, and this risk must be balanced with providing the best care. As in any other clinical situation, seek evidence to both verify and clarify information without being deterred by a patient’s vocalization of spiritual tenants.
Learn about your patients’ beliefs
Do not feel defeated if you find these interviews difficult. Religion and symptomatology can overlap and fluctuate within the same faith group, which can make these types of assessments complex.5 In an effort to understand the patient more clearly, be sensitive to their spiritual practices and receptive to learning about unfamiliar spiritual beliefs. Be transparent about not knowing a specific belief or practice, and exhibit humility. Most patients are open to sharing their religious/spiritual views with a clinician who is sincere about wanting insight. Understanding the value of spiritual care is an important skill that many medical practitioners often lack.6 This understanding is especially critical when patients express worries related to the COVID-19 pandemic and how they are coping.
Continue to: Integrate your patient's spiritual requests
Integrate your patient’s spiritual requests
If you are comfortable with certain practices that do not compromise your values or beliefs or put a patient at risk, try to integrate your patients’ spiritual request(s) in their care. For a patient who serves a higher power, admitting to a problem (eg, fears related to COVID-19) or seeking professional help for symptoms (eg, anxiety, depression) may imply spiritual doubt. Patients may believe that seeking professional assistance means they are questioning the omnipotence of their deity to prevent or heal a condition. While spiritual distress can stimulate changes in behavior, it may not be pathological.
To avoid misdiagnosis, refer to the description “V62.89 (Z65.8) Religious or Spiritual Problem” in the DSM-5.7 If you find that it is a discord in faith that is affecting the patient’s presentation, and that this has not caused a psychiatric disorder, document this appropriately and provide the necessary resources to continue supporting the patient holistically.
As the world grapples with the coronavirus disease 2019 (COVID-19) pandemic, the search for answers, comfort, how to cope, and how to make sense of it all has become paramount. People commonly turn to their faith in times of crisis, but this recent global public health emergency is unlike many have ever seen or could have imagined.1 What happens when the well-intentioned journey for spiritual insight intersects with psychiatric symptomatology? Where does the line between these phenomena get crossed? As a psychiatric resident and person who was raised in the Pentecostal faith, I have observed faith and psychopathology come to a head in the last 6 months. COVID-19 has dealt a religious strain of undocumented cases; I hope to shed light on the topic by sharing my experience of navigating the assessment and treatment plan of patients with psychiatric symptoms whose spiritual beliefs are a cornerstone of life.
Piety, or pathology?
The following approaches have helped me to identify what is driven by faith vs what is psychopathology:
While taking the patient history. Obtaining a history from a patient who professes to have strong spiritual beliefs and presents with psychiatric symptoms is similar to a standard patient interview, but pay special attention to how the patient came to the emergency department. Was there a family member, friend, or emergency medical services present at that time? During the interview, patients often appear “normal,” which may lead a clinician to question the reason for the consult, yet considering the recent events preceding the presentation will be a good place to start gathering the appropriate information for investigation.
Next, compare the patient’s recent daily functioning with his/her baseline. If this information comes solely from the patient, it may be skewed, so try to retrieve information from a collateral source. If the patient was accompanied by someone, request permission from the patient to speak with him/her. It may also be best in some instances to speak with the collateral source out of earshot of the patient. Be aware that collateral information that comes from just one source also could be biased, so search for additional contacts to help acquire a comprehensive representation of the circumstances.
Information about a patient could come from a faith leader because people often rely on their faith leaders when they are ill, in need of support, or in crisis.2 Faith leaders may have valuable information and insight into the patient and the history of the patient’s illness. In addition, diverse sources of collateral reports may be helpful because specific spiritual views and practices can vary even within one family or congregation. What may be an abnormal practice to some followers may be normal for others.3 When approaching these situations with parishioners, it is essential to maintain confidentiality.
While performing the clinical examination. As with any psychiatric diagnosis, other causative factors (metabolic and organic) need to be ruled out. Also, assess for the use of mood-altering substances. The patient may express offense or resistance to such questions, but maintain a matter-of-fact approach and explain that assessment for substance use is a routine part of the clinical examination. Approximately 18% of people in the United States with psychiatric disorders have a comorbid substance use disorder.4 However, keep in mind that a patient who refuses substance use screening is not necessarily hiding something. The road to being thorough may lead to strained rapport with the patient, and this risk must be balanced with providing the best care. As in any other clinical situation, seek evidence to both verify and clarify information without being deterred by a patient’s vocalization of spiritual tenants.
Learn about your patients’ beliefs
Do not feel defeated if you find these interviews difficult. Religion and symptomatology can overlap and fluctuate within the same faith group, which can make these types of assessments complex.5 In an effort to understand the patient more clearly, be sensitive to their spiritual practices and receptive to learning about unfamiliar spiritual beliefs. Be transparent about not knowing a specific belief or practice, and exhibit humility. Most patients are open to sharing their religious/spiritual views with a clinician who is sincere about wanting insight. Understanding the value of spiritual care is an important skill that many medical practitioners often lack.6 This understanding is especially critical when patients express worries related to the COVID-19 pandemic and how they are coping.
Continue to: Integrate your patient's spiritual requests
Integrate your patient’s spiritual requests
If you are comfortable with certain practices that do not compromise your values or beliefs or put a patient at risk, try to integrate your patients’ spiritual request(s) in their care. For a patient who serves a higher power, admitting to a problem (eg, fears related to COVID-19) or seeking professional help for symptoms (eg, anxiety, depression) may imply spiritual doubt. Patients may believe that seeking professional assistance means they are questioning the omnipotence of their deity to prevent or heal a condition. While spiritual distress can stimulate changes in behavior, it may not be pathological.
To avoid misdiagnosis, refer to the description “V62.89 (Z65.8) Religious or Spiritual Problem” in the DSM-5.7 If you find that it is a discord in faith that is affecting the patient’s presentation, and that this has not caused a psychiatric disorder, document this appropriately and provide the necessary resources to continue supporting the patient holistically.
1. Dein S, Loewenthal K, Lewis CA, et al. COVID-19, mental health and religion: an agenda for future research. Mental Health, Religion & Culture. 2020;23(1):1-9.
2. American Psychiatric Association Foundation. Mental health: a guide for faith leaders. Arlington, VA: American Psychiatric Association Foundation; 2018.
3. Johnson CV, Friedman HL. Enlightened or delusional? Differentiating religious, spiritual, and transpersonal experiences from psychopathology. Journal of Humanistic Psychology. 2008;48(4):505-527.
4. Han B, Compton WM, Blanco C, et al. Prevalence, treatment, and unmet treatment needs of US adults with mental health and substance use disorders. Health Aff (Millwood). 2017;36(10):1739-1747.
5. Menezes Jr A, Moreira-Almeida A. Differential diagnosis between spiritual experiences and mental disorders of religious content. Rev Psiq Clín. 2009;36(2):75-82.
6. Best M, Butow P, Olver I. Doctors discussing religion and spirituality: a systematic literature review. Palliat Med. 2016;30(4):327-337.
7. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:725.
1. Dein S, Loewenthal K, Lewis CA, et al. COVID-19, mental health and religion: an agenda for future research. Mental Health, Religion & Culture. 2020;23(1):1-9.
2. American Psychiatric Association Foundation. Mental health: a guide for faith leaders. Arlington, VA: American Psychiatric Association Foundation; 2018.
3. Johnson CV, Friedman HL. Enlightened or delusional? Differentiating religious, spiritual, and transpersonal experiences from psychopathology. Journal of Humanistic Psychology. 2008;48(4):505-527.
4. Han B, Compton WM, Blanco C, et al. Prevalence, treatment, and unmet treatment needs of US adults with mental health and substance use disorders. Health Aff (Millwood). 2017;36(10):1739-1747.
5. Menezes Jr A, Moreira-Almeida A. Differential diagnosis between spiritual experiences and mental disorders of religious content. Rev Psiq Clín. 2009;36(2):75-82.
6. Best M, Butow P, Olver I. Doctors discussing religion and spirituality: a systematic literature review. Palliat Med. 2016;30(4):327-337.
7. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:725.
Caring for outpatients during COVID-19: 4 themes
As a result of the coronavirus disease 2019 (COVID-19) pandemic, the content of outpatient psychotherapy and psychopharmacology sessions has seen significant change, with many patients focusing on how the pandemic has altered their daily lives and emotional well-being. Most patients were suddenly limited in both the amount of time they spent, and in their interactions with people, outside of their homes. Additionally, employment-related stressors such as working from home and the potential loss of a job and/or income added to pandemic stress.1 Patients simultaneously processed their experiences of the COVID-19 pandemic while often striving to adapt to new virtual modes of mental health care delivery via phone or video conferencing.
The clinic staff at our large, multidisciplinary, urban outpatient mental health practice conducts weekly case consultation meetings. In meetings held during the early stages of the COVID-19 pandemic, we noted 4 dominant clinical themes emerging across our patients’ experiences:
- isolation
- uncertainty
- household stress
- grief.
These themes occurred across many diagnostic categories, suggesting they reflect a dramatic shift brought on by the pandemic. Our group compared clinical experiences from the beginning of the pandemic through the end of May 2020. For this article, we considered several patients who expressed these 4 themes and created a “composite patient.” In the following sections, we describe the typical presentation of, and recommended interventions for, a composite patient for each of these 4 themes.
Isolation
Mr. J, a 60-year-old, single, African American man diagnosed with bipolar disorder with psychotic features, lives alone in an apartment in a densely populated area. Before COVID-19, he had been attending a day treatment program. His daily walks for coffee and cigarettes provided the scaffolding to his emotional stability and gave him a sense of belonging to a world outside of his home. Mr. J also had been able to engage in informal social activities in the common areas of his apartment complex.
The start of the COVID-19 pandemic ends his interpersonal interactions, from the passive and superficial conversations he had with strangers in coffee shops to the more intimate engagement with his peers in his treatment program. The common areas of Mr. J’s apartment building are closed, and his routine cigarette breaks with neighbors have become solitary events, with the added stress of having to schedule his use of the building’s designated smoking area. Before COVID-19, Mr. J had been regularly meeting his brother for coffee to talk about the recent death of their father, but these meetings end due to infection concerns by Mr. J and his brother, who cares for their ailing mother who is at high risk for COVID-19 infection.
Mr. J begins to report self-referential ideation when walking in public, citing his inability to see peoples’ facial expressions because they are wearing masks. As a result of the pandemic restrictions, he becomes depressed and develops increased paranoid ideation. Fortunately, Mr. J begins to participate in a virtual partial hospitalization program to address his paranoid ideation through intensive and clinically-based social interactions. He is unfamiliar with the technology used for virtual visits, but is given the necessary technical support. He is also able to begin virtual visits with his brother and mother. Mr. J soon reports his symptoms are reduced and his mood is more stable.
Engaging in interpersonal interactions can have a positive impact on mental health. Social isolation has demonstrated negative effects that are amplified in individuals with psychiatric disorders.2 Interpersonal interactions can provide a shared experience, promote positive feelings of social connection, and aid in the development of social skills.3,4 Among our patients, we have begun to see the effects of isolation manifest as loneliness and demoralization.
Continue to: Interventions
Interventions. Due to restrictions imposed to limit the spread of COVID-19, evidence-based interventions such as meeting a friend for a meal or participating in in-person support groups typically are not options, thus forcing clinicians to accommodate, adapt, and use technology to develop parallel interventions to provide the same therapeutic effects.5,6 These solutions need to be individualized to accommodate each patient’s unique social and clinical situation (Table 1). Engaging through technology can be problematic for patients with psychosis and paranoid ideation, or those with depressive symptoms. Psychopharmacology or therapy visit time has to be dedicated to helping patients become comfortable and confident when using technology to access their clinicians. Patients can use this same technology to establish virtual social connections. Providing patients with accurate, factual information about infection control during clinical visits ultimately supports their mental health. Delivering clinical care during COVID-19 has required creativity and flexibility to optimize available resources and capitalize on patients’ social supports. These strategies help decrease isolation, loneliness, and exacerbation of psychiatric symptoms.
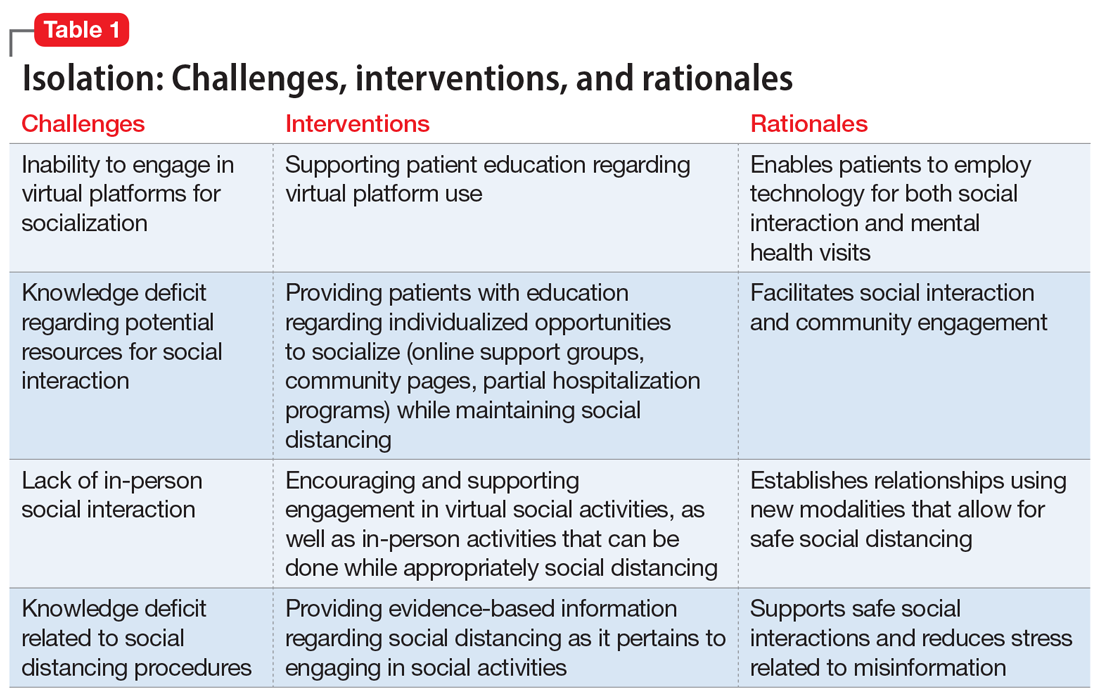
Uncertainty
Ms. L, age 42, has a history of posttraumatic stress disorder and obstructive sleep apnea, for which she uses a continuous airway positive pressure (CPAP) device. She had been working as a part-time nanny when her employer furloughed her early in the COVID-19 pandemic. Her anxiety has gotten worse throughout the quarantine; she fears her unemployment benefits will run out and she will lose her job. Her anxiety manifests as somatic “pit-of-stomach” sensations. Her sleep has been disrupted; she reports more frequent nightmares, and her partner says that Ms. L has had apneic episodes and bruxism. The parameters of Ms. L’s CPAP device need to be adjusted, but a previously scheduled overnight polysomnography test is deemed a nonessential procedure and canceled. Ms. L has been reluctant to go to a food pantry because she is afraid of being exposed to COVID-19. In virtual sessions, Ms. L says she is uncertain if she will be able to pay her rent, buy food, or access medical care, and expresses overriding helplessness.
During COVID-19, anxiety and insomnia are driven by the sudden manifestation of uncertainty regarding being able to work, pay rent or mortgage, buy food and other provisions, or visit family and friends, including those who are hospitalized or live in nursing homes. Additional uncertainties include how long the quarantine will last, who will become ill, and when, or if, life will return to normal. Taken together, these uncertainties impart a pervasive dread to daily experience.
Interventions. Clinicians can facilitate access to services (eg, social services, benefits specialists) and help patients parse out what they should and can address practically, and which challenges are outside of their personal or communal control (Table 2). Patients can be encouraged to identify paralytic rumination and shift their mental focus to engage in constructive projects. They can be advised to limit their intake of media that increases their anxiety and replace it with phone calls or e-mails to family and friends. Scheduled practice of mindfulness meditation and diaphragmatic breathing can help reduce anxiety.7,8 Pharmacotherapeutic interventions should be low-risk to minimize burdening emergency departments saturated with patients who have COVID-19 and serve to reduce symptoms that interfere with behavioral activation. While the research on benzodiazepines and non-benzodiazepine receptor agonists (“Z-drugs” such as zolpidem and eszopiclone) in the setting of obstructive sleep apnea is complex, and there is some evidence that the latter may not exacerbate apnea,9 benzodiazepines and Z-drugs are associated with an array of risks, including tolerance, withdrawal, and traumatic falls, particularly in older adults.10 Sleep hygiene and cognitive-behavioral therapy are first-line therapies for insomnia.11
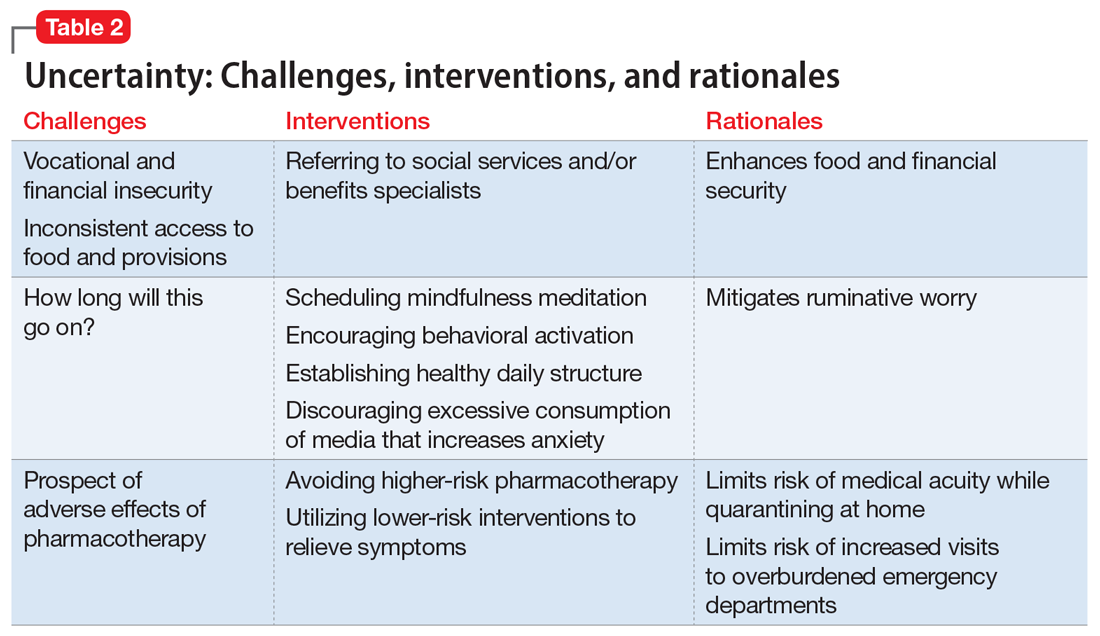
Household stress
Ms. M, a 45-year-old single mother with a history of generalized anxiety disorder, is suddenly thrust into homeschooling her 2 children, ages 10 and 8, while trying to remain productive at her job as a software engineer. She no longer has time for herself, and spends much of her day helping her children with schoolwork or planning activities to keep them engaged rather than arguing with each other. She feels intense pressure, heightened stress, and increased anxiety as she tries to navigate this new daily routine.
Continue to: New household dynamics...
New household dynamics abound when people are suddenly forced into atypical routines. In the context of COVID-19, working parents may be forced to balance the demands of their jobs with homeschooling their children. Couples may find themselves arguing more frequently. Adult children may find themselves needing to care for their ill parents. Limited space, a lack of leisure activities, and uncertainty about the future coalesce to increase conflict and stress. Research suggests that how people cope with a stressor is a more reliable determinant of health and well-being than the stressor itself.12
Interventions. Mental health clinicians can offer several recommendations to help patients cope with increased household stress (Table 3). We can encourage patients to have clear communication with their loved ones regarding new expectations, roles, and their feelings. Demarcating specific areas within living spaces to each person in the household can help each member feel a sense of autonomy, regardless of how small their area may be. Clinicians can help patients learn to take the time as a family to work on establishing new household routines. Telepsychiatry offers clinicians a unique window into patients’ lives and family dynamics, and we can use this perspective to deepen our understanding of the patient’s context and household relationships and help them navigate the situation thrust upon them.

Grief
Following a psychiatric hospitalization for an acute exacerbation of psychosis, Ms. S, age 79, is transferred to a rehabilitation facility, where she contracts COVID-19. Because Ms. S did not have a history of chronic medical illness, her family anticipates a full recovery. Early in the course of Ms. S’s admission, the rehabilitation facility restricts visitations, and her family is unable to see her. Ms. S dies in this facility without her family’s presence and without her family having the opportunity to say goodbye. Ms. S’s psychiatrist offers her family a virtual session to provide support. During the virtual session, the psychiatrist notes signs of complicated bereavement among Ms. S’s family members, including nonacceptance of the death, rumination about the circumstances of the death, and describing life as having no purpose.
The COVID-19 pandemic has complicated the natural process of loss and grief across multiple dimensions. Studies have shown that an inability to say goodbye before death, a lack of social support,13 and a lack of preparation for loss14 are associated with complicated bereavement and depression. Many people are experiencing the loss of loved ones without having a chance to appropriately mourn. Forbidding visits to family members who are hospitalized also prevents the practice of religious and spiritual rituals that typically occur at the end of life. This is worsened by truncated or absent funeral services. Support for those who are grieving may be offered from a distance, if at all. When surviving family members have been with the deceased prior to hospitalization, they may be required to self-quarantine, potentially exacerbating their grief and other symptoms associated with loss.
Interventions. Because social support is a protective factor against complicated grief,14 there are several recommendations for survivors as they work through the process of grief (Table 4). These include preparing families for a potential death; discussing desired spiritual and memorial services15; connecting families to resources such as community grief support programs, counseling/therapy, funeral services, video conferencing, and other communication tools; and planning for additional support for surviving family and friends, both immediately after the death and in the long term. It is also important to provide appropriate counseling and support for surviving family members to focus on their own well-being by exercising, eating nutritious meals, getting enough sleep, and abstaining from alcohol and drugs of abuse.16
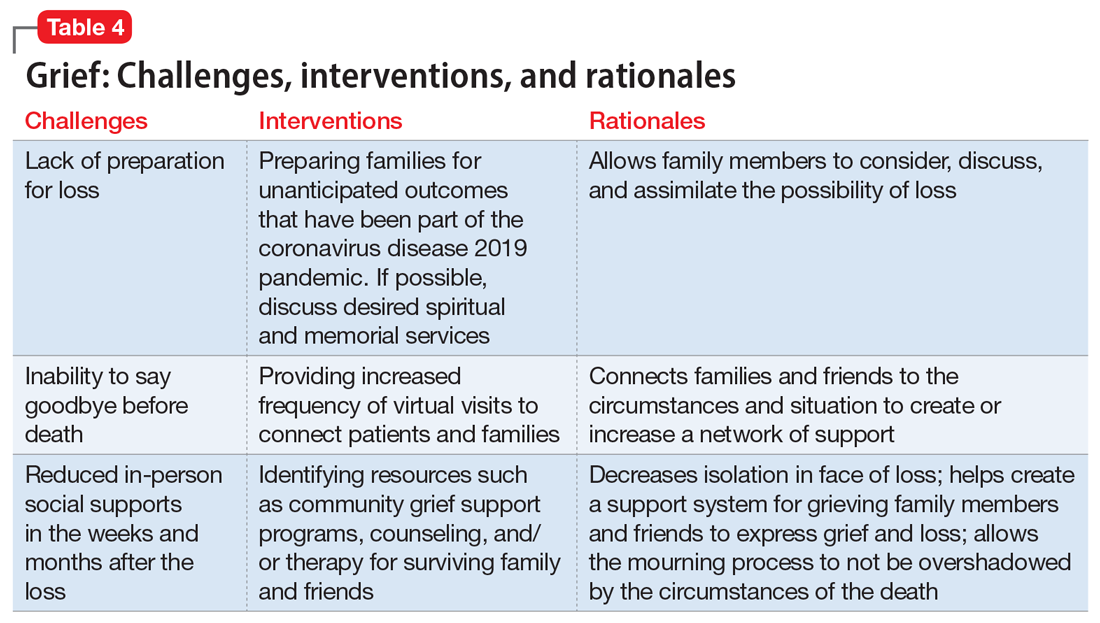
Continue to: An ongoing challenge
An ongoing challenge
Our clinical team recommends further investigation to define additional psychotherapeutic themes arising from the COVID-19 pandemic and provide evidence-based interventions to address these categories, which we expect will increase in clinical salience in the months and years ahead. Close monitoring, follow-up by clinical and research staff, and evidence-based interventions will help address these dominant themes, with the goal of alleviating patient suffering.
Bottom Line
Our team identified 4 dominant clinical themes emerging across our patients’ experiences during the coronavirus disease 2019 pandemic: isolation, uncertainty, household stress, and grief. Clinicians can implement specific interventions to reduce the impact of these themes, which we expect to remain clinically relevant in the upcoming months and years.
Related Resources
- Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-32,39.
- Carr D, Boerner K, Moorman S. Bereavement in the time of coronavirus: unprecedented challenges demand novel interventions. J Aging Soc Policy. 2020;32(4-5):425-431.
Drug Brand Names
Eszopiclone • Lunesta
Zolpidem • Ambien
1. Bloom N. How working from home works out. Stanford Institute for Economic Policy Research Policy Brief. https://siepr.stanford.edu/research/publications/how-working-home-works-out. Published June 2020. Accessed October 28, 2020.
2. Linz SJ, Sturm BA. The phenomenon of social isolation in the severely mentally ill. Perspect Psychiatr Care. 2013;49(4):243-254.
3. Smith KP, Christakis NA. Social networks and health. Annual Review of Sociology. 2008;34(1):405-429.
4. Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51(suppl):S54‐S66.
5. Mann F, Bone JK, Lloyd-Evans B. A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. Soc Psychiatry Psychiatr Epidemiol. 2017;52(6):627-638.
6. Choi M, Kong S, Jung D. Computer and internet interventions for loneliness and depression in older adults: a meta-analysis. Healthc Inform Res. 2012;18(3):191‐198.
7. Chen YF, Huang ZY, Chien CH, et al. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care. 2017;53(4):329-336.
8. Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786‐792.
9. Carberry JC, Grunstein RR, Eckert DJ. The effects of zolpidem in obstructive sleep apnea - an open-label pilot study. Sleep Res. 2019;28(6):e12853. doi: 10.1111/jsr.12853.
10. Markota M, Rummans TA, Bostwick JM, et al. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632-1639.
11. Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician. 2017;96(1):29-35.
12. Dijkstra MT, Homan AC. Engaging in rather than disengaging from stress: effective coping and perceived control. Front Psychol. 2016;7:1415.
13. Romero MM, Ott CH, Kelber ST. Predictors of grief in bereaved family caregivers of person’s with Alzheimer’s disease: a prospective study. Death Stud. 2014;38(6-10):395-403.
14. Lobb EA, Kristjanson LJ, Aoun SM, et al. Predictors of complicated grief: a systematic review of empirical studies. Death Stud. 2010;34(8):673-698.
15. Wallace CL, Wladkowski SP, Gibson A, et al. Grief during the COVID-19 pandemic: considerations for palliative care providers. J Pain Symptom Manage. 2020;60(1):e70-e76. doi: 10.1016/j.jpainsymman.2020.04.012
16. Selman LE, Chao D, Sowden R, et al. Bereavement support on the frontline of COVID-19: recommendations for hospital clinicians. J Pain Symptom Manage. 2020;60(2):e81-e86. doi: 10.1016/j.jpainsymman.2020.04.024
As a result of the coronavirus disease 2019 (COVID-19) pandemic, the content of outpatient psychotherapy and psychopharmacology sessions has seen significant change, with many patients focusing on how the pandemic has altered their daily lives and emotional well-being. Most patients were suddenly limited in both the amount of time they spent, and in their interactions with people, outside of their homes. Additionally, employment-related stressors such as working from home and the potential loss of a job and/or income added to pandemic stress.1 Patients simultaneously processed their experiences of the COVID-19 pandemic while often striving to adapt to new virtual modes of mental health care delivery via phone or video conferencing.
The clinic staff at our large, multidisciplinary, urban outpatient mental health practice conducts weekly case consultation meetings. In meetings held during the early stages of the COVID-19 pandemic, we noted 4 dominant clinical themes emerging across our patients’ experiences:
- isolation
- uncertainty
- household stress
- grief.
These themes occurred across many diagnostic categories, suggesting they reflect a dramatic shift brought on by the pandemic. Our group compared clinical experiences from the beginning of the pandemic through the end of May 2020. For this article, we considered several patients who expressed these 4 themes and created a “composite patient.” In the following sections, we describe the typical presentation of, and recommended interventions for, a composite patient for each of these 4 themes.
Isolation
Mr. J, a 60-year-old, single, African American man diagnosed with bipolar disorder with psychotic features, lives alone in an apartment in a densely populated area. Before COVID-19, he had been attending a day treatment program. His daily walks for coffee and cigarettes provided the scaffolding to his emotional stability and gave him a sense of belonging to a world outside of his home. Mr. J also had been able to engage in informal social activities in the common areas of his apartment complex.
The start of the COVID-19 pandemic ends his interpersonal interactions, from the passive and superficial conversations he had with strangers in coffee shops to the more intimate engagement with his peers in his treatment program. The common areas of Mr. J’s apartment building are closed, and his routine cigarette breaks with neighbors have become solitary events, with the added stress of having to schedule his use of the building’s designated smoking area. Before COVID-19, Mr. J had been regularly meeting his brother for coffee to talk about the recent death of their father, but these meetings end due to infection concerns by Mr. J and his brother, who cares for their ailing mother who is at high risk for COVID-19 infection.
Mr. J begins to report self-referential ideation when walking in public, citing his inability to see peoples’ facial expressions because they are wearing masks. As a result of the pandemic restrictions, he becomes depressed and develops increased paranoid ideation. Fortunately, Mr. J begins to participate in a virtual partial hospitalization program to address his paranoid ideation through intensive and clinically-based social interactions. He is unfamiliar with the technology used for virtual visits, but is given the necessary technical support. He is also able to begin virtual visits with his brother and mother. Mr. J soon reports his symptoms are reduced and his mood is more stable.
Engaging in interpersonal interactions can have a positive impact on mental health. Social isolation has demonstrated negative effects that are amplified in individuals with psychiatric disorders.2 Interpersonal interactions can provide a shared experience, promote positive feelings of social connection, and aid in the development of social skills.3,4 Among our patients, we have begun to see the effects of isolation manifest as loneliness and demoralization.
Continue to: Interventions
Interventions. Due to restrictions imposed to limit the spread of COVID-19, evidence-based interventions such as meeting a friend for a meal or participating in in-person support groups typically are not options, thus forcing clinicians to accommodate, adapt, and use technology to develop parallel interventions to provide the same therapeutic effects.5,6 These solutions need to be individualized to accommodate each patient’s unique social and clinical situation (Table 1). Engaging through technology can be problematic for patients with psychosis and paranoid ideation, or those with depressive symptoms. Psychopharmacology or therapy visit time has to be dedicated to helping patients become comfortable and confident when using technology to access their clinicians. Patients can use this same technology to establish virtual social connections. Providing patients with accurate, factual information about infection control during clinical visits ultimately supports their mental health. Delivering clinical care during COVID-19 has required creativity and flexibility to optimize available resources and capitalize on patients’ social supports. These strategies help decrease isolation, loneliness, and exacerbation of psychiatric symptoms.

Uncertainty
Ms. L, age 42, has a history of posttraumatic stress disorder and obstructive sleep apnea, for which she uses a continuous airway positive pressure (CPAP) device. She had been working as a part-time nanny when her employer furloughed her early in the COVID-19 pandemic. Her anxiety has gotten worse throughout the quarantine; she fears her unemployment benefits will run out and she will lose her job. Her anxiety manifests as somatic “pit-of-stomach” sensations. Her sleep has been disrupted; she reports more frequent nightmares, and her partner says that Ms. L has had apneic episodes and bruxism. The parameters of Ms. L’s CPAP device need to be adjusted, but a previously scheduled overnight polysomnography test is deemed a nonessential procedure and canceled. Ms. L has been reluctant to go to a food pantry because she is afraid of being exposed to COVID-19. In virtual sessions, Ms. L says she is uncertain if she will be able to pay her rent, buy food, or access medical care, and expresses overriding helplessness.
During COVID-19, anxiety and insomnia are driven by the sudden manifestation of uncertainty regarding being able to work, pay rent or mortgage, buy food and other provisions, or visit family and friends, including those who are hospitalized or live in nursing homes. Additional uncertainties include how long the quarantine will last, who will become ill, and when, or if, life will return to normal. Taken together, these uncertainties impart a pervasive dread to daily experience.
Interventions. Clinicians can facilitate access to services (eg, social services, benefits specialists) and help patients parse out what they should and can address practically, and which challenges are outside of their personal or communal control (Table 2). Patients can be encouraged to identify paralytic rumination and shift their mental focus to engage in constructive projects. They can be advised to limit their intake of media that increases their anxiety and replace it with phone calls or e-mails to family and friends. Scheduled practice of mindfulness meditation and diaphragmatic breathing can help reduce anxiety.7,8 Pharmacotherapeutic interventions should be low-risk to minimize burdening emergency departments saturated with patients who have COVID-19 and serve to reduce symptoms that interfere with behavioral activation. While the research on benzodiazepines and non-benzodiazepine receptor agonists (“Z-drugs” such as zolpidem and eszopiclone) in the setting of obstructive sleep apnea is complex, and there is some evidence that the latter may not exacerbate apnea,9 benzodiazepines and Z-drugs are associated with an array of risks, including tolerance, withdrawal, and traumatic falls, particularly in older adults.10 Sleep hygiene and cognitive-behavioral therapy are first-line therapies for insomnia.11

Household stress
Ms. M, a 45-year-old single mother with a history of generalized anxiety disorder, is suddenly thrust into homeschooling her 2 children, ages 10 and 8, while trying to remain productive at her job as a software engineer. She no longer has time for herself, and spends much of her day helping her children with schoolwork or planning activities to keep them engaged rather than arguing with each other. She feels intense pressure, heightened stress, and increased anxiety as she tries to navigate this new daily routine.
Continue to: New household dynamics...
New household dynamics abound when people are suddenly forced into atypical routines. In the context of COVID-19, working parents may be forced to balance the demands of their jobs with homeschooling their children. Couples may find themselves arguing more frequently. Adult children may find themselves needing to care for their ill parents. Limited space, a lack of leisure activities, and uncertainty about the future coalesce to increase conflict and stress. Research suggests that how people cope with a stressor is a more reliable determinant of health and well-being than the stressor itself.12
Interventions. Mental health clinicians can offer several recommendations to help patients cope with increased household stress (Table 3). We can encourage patients to have clear communication with their loved ones regarding new expectations, roles, and their feelings. Demarcating specific areas within living spaces to each person in the household can help each member feel a sense of autonomy, regardless of how small their area may be. Clinicians can help patients learn to take the time as a family to work on establishing new household routines. Telepsychiatry offers clinicians a unique window into patients’ lives and family dynamics, and we can use this perspective to deepen our understanding of the patient’s context and household relationships and help them navigate the situation thrust upon them.

Grief
Following a psychiatric hospitalization for an acute exacerbation of psychosis, Ms. S, age 79, is transferred to a rehabilitation facility, where she contracts COVID-19. Because Ms. S did not have a history of chronic medical illness, her family anticipates a full recovery. Early in the course of Ms. S’s admission, the rehabilitation facility restricts visitations, and her family is unable to see her. Ms. S dies in this facility without her family’s presence and without her family having the opportunity to say goodbye. Ms. S’s psychiatrist offers her family a virtual session to provide support. During the virtual session, the psychiatrist notes signs of complicated bereavement among Ms. S’s family members, including nonacceptance of the death, rumination about the circumstances of the death, and describing life as having no purpose.
The COVID-19 pandemic has complicated the natural process of loss and grief across multiple dimensions. Studies have shown that an inability to say goodbye before death, a lack of social support,13 and a lack of preparation for loss14 are associated with complicated bereavement and depression. Many people are experiencing the loss of loved ones without having a chance to appropriately mourn. Forbidding visits to family members who are hospitalized also prevents the practice of religious and spiritual rituals that typically occur at the end of life. This is worsened by truncated or absent funeral services. Support for those who are grieving may be offered from a distance, if at all. When surviving family members have been with the deceased prior to hospitalization, they may be required to self-quarantine, potentially exacerbating their grief and other symptoms associated with loss.
Interventions. Because social support is a protective factor against complicated grief,14 there are several recommendations for survivors as they work through the process of grief (Table 4). These include preparing families for a potential death; discussing desired spiritual and memorial services15; connecting families to resources such as community grief support programs, counseling/therapy, funeral services, video conferencing, and other communication tools; and planning for additional support for surviving family and friends, both immediately after the death and in the long term. It is also important to provide appropriate counseling and support for surviving family members to focus on their own well-being by exercising, eating nutritious meals, getting enough sleep, and abstaining from alcohol and drugs of abuse.16

Continue to: An ongoing challenge
An ongoing challenge
Our clinical team recommends further investigation to define additional psychotherapeutic themes arising from the COVID-19 pandemic and provide evidence-based interventions to address these categories, which we expect will increase in clinical salience in the months and years ahead. Close monitoring, follow-up by clinical and research staff, and evidence-based interventions will help address these dominant themes, with the goal of alleviating patient suffering.
Bottom Line
Our team identified 4 dominant clinical themes emerging across our patients’ experiences during the coronavirus disease 2019 pandemic: isolation, uncertainty, household stress, and grief. Clinicians can implement specific interventions to reduce the impact of these themes, which we expect to remain clinically relevant in the upcoming months and years.
Related Resources
- Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-32,39.
- Carr D, Boerner K, Moorman S. Bereavement in the time of coronavirus: unprecedented challenges demand novel interventions. J Aging Soc Policy. 2020;32(4-5):425-431.
Drug Brand Names
Eszopiclone • Lunesta
Zolpidem • Ambien
As a result of the coronavirus disease 2019 (COVID-19) pandemic, the content of outpatient psychotherapy and psychopharmacology sessions has seen significant change, with many patients focusing on how the pandemic has altered their daily lives and emotional well-being. Most patients were suddenly limited in both the amount of time they spent, and in their interactions with people, outside of their homes. Additionally, employment-related stressors such as working from home and the potential loss of a job and/or income added to pandemic stress.1 Patients simultaneously processed their experiences of the COVID-19 pandemic while often striving to adapt to new virtual modes of mental health care delivery via phone or video conferencing.
The clinic staff at our large, multidisciplinary, urban outpatient mental health practice conducts weekly case consultation meetings. In meetings held during the early stages of the COVID-19 pandemic, we noted 4 dominant clinical themes emerging across our patients’ experiences:
- isolation
- uncertainty
- household stress
- grief.
These themes occurred across many diagnostic categories, suggesting they reflect a dramatic shift brought on by the pandemic. Our group compared clinical experiences from the beginning of the pandemic through the end of May 2020. For this article, we considered several patients who expressed these 4 themes and created a “composite patient.” In the following sections, we describe the typical presentation of, and recommended interventions for, a composite patient for each of these 4 themes.
Isolation
Mr. J, a 60-year-old, single, African American man diagnosed with bipolar disorder with psychotic features, lives alone in an apartment in a densely populated area. Before COVID-19, he had been attending a day treatment program. His daily walks for coffee and cigarettes provided the scaffolding to his emotional stability and gave him a sense of belonging to a world outside of his home. Mr. J also had been able to engage in informal social activities in the common areas of his apartment complex.
The start of the COVID-19 pandemic ends his interpersonal interactions, from the passive and superficial conversations he had with strangers in coffee shops to the more intimate engagement with his peers in his treatment program. The common areas of Mr. J’s apartment building are closed, and his routine cigarette breaks with neighbors have become solitary events, with the added stress of having to schedule his use of the building’s designated smoking area. Before COVID-19, Mr. J had been regularly meeting his brother for coffee to talk about the recent death of their father, but these meetings end due to infection concerns by Mr. J and his brother, who cares for their ailing mother who is at high risk for COVID-19 infection.
Mr. J begins to report self-referential ideation when walking in public, citing his inability to see peoples’ facial expressions because they are wearing masks. As a result of the pandemic restrictions, he becomes depressed and develops increased paranoid ideation. Fortunately, Mr. J begins to participate in a virtual partial hospitalization program to address his paranoid ideation through intensive and clinically-based social interactions. He is unfamiliar with the technology used for virtual visits, but is given the necessary technical support. He is also able to begin virtual visits with his brother and mother. Mr. J soon reports his symptoms are reduced and his mood is more stable.
Engaging in interpersonal interactions can have a positive impact on mental health. Social isolation has demonstrated negative effects that are amplified in individuals with psychiatric disorders.2 Interpersonal interactions can provide a shared experience, promote positive feelings of social connection, and aid in the development of social skills.3,4 Among our patients, we have begun to see the effects of isolation manifest as loneliness and demoralization.
Continue to: Interventions
Interventions. Due to restrictions imposed to limit the spread of COVID-19, evidence-based interventions such as meeting a friend for a meal or participating in in-person support groups typically are not options, thus forcing clinicians to accommodate, adapt, and use technology to develop parallel interventions to provide the same therapeutic effects.5,6 These solutions need to be individualized to accommodate each patient’s unique social and clinical situation (Table 1). Engaging through technology can be problematic for patients with psychosis and paranoid ideation, or those with depressive symptoms. Psychopharmacology or therapy visit time has to be dedicated to helping patients become comfortable and confident when using technology to access their clinicians. Patients can use this same technology to establish virtual social connections. Providing patients with accurate, factual information about infection control during clinical visits ultimately supports their mental health. Delivering clinical care during COVID-19 has required creativity and flexibility to optimize available resources and capitalize on patients’ social supports. These strategies help decrease isolation, loneliness, and exacerbation of psychiatric symptoms.

Uncertainty
Ms. L, age 42, has a history of posttraumatic stress disorder and obstructive sleep apnea, for which she uses a continuous airway positive pressure (CPAP) device. She had been working as a part-time nanny when her employer furloughed her early in the COVID-19 pandemic. Her anxiety has gotten worse throughout the quarantine; she fears her unemployment benefits will run out and she will lose her job. Her anxiety manifests as somatic “pit-of-stomach” sensations. Her sleep has been disrupted; she reports more frequent nightmares, and her partner says that Ms. L has had apneic episodes and bruxism. The parameters of Ms. L’s CPAP device need to be adjusted, but a previously scheduled overnight polysomnography test is deemed a nonessential procedure and canceled. Ms. L has been reluctant to go to a food pantry because she is afraid of being exposed to COVID-19. In virtual sessions, Ms. L says she is uncertain if she will be able to pay her rent, buy food, or access medical care, and expresses overriding helplessness.
During COVID-19, anxiety and insomnia are driven by the sudden manifestation of uncertainty regarding being able to work, pay rent or mortgage, buy food and other provisions, or visit family and friends, including those who are hospitalized or live in nursing homes. Additional uncertainties include how long the quarantine will last, who will become ill, and when, or if, life will return to normal. Taken together, these uncertainties impart a pervasive dread to daily experience.
Interventions. Clinicians can facilitate access to services (eg, social services, benefits specialists) and help patients parse out what they should and can address practically, and which challenges are outside of their personal or communal control (Table 2). Patients can be encouraged to identify paralytic rumination and shift their mental focus to engage in constructive projects. They can be advised to limit their intake of media that increases their anxiety and replace it with phone calls or e-mails to family and friends. Scheduled practice of mindfulness meditation and diaphragmatic breathing can help reduce anxiety.7,8 Pharmacotherapeutic interventions should be low-risk to minimize burdening emergency departments saturated with patients who have COVID-19 and serve to reduce symptoms that interfere with behavioral activation. While the research on benzodiazepines and non-benzodiazepine receptor agonists (“Z-drugs” such as zolpidem and eszopiclone) in the setting of obstructive sleep apnea is complex, and there is some evidence that the latter may not exacerbate apnea,9 benzodiazepines and Z-drugs are associated with an array of risks, including tolerance, withdrawal, and traumatic falls, particularly in older adults.10 Sleep hygiene and cognitive-behavioral therapy are first-line therapies for insomnia.11

Household stress
Ms. M, a 45-year-old single mother with a history of generalized anxiety disorder, is suddenly thrust into homeschooling her 2 children, ages 10 and 8, while trying to remain productive at her job as a software engineer. She no longer has time for herself, and spends much of her day helping her children with schoolwork or planning activities to keep them engaged rather than arguing with each other. She feels intense pressure, heightened stress, and increased anxiety as she tries to navigate this new daily routine.
Continue to: New household dynamics...
New household dynamics abound when people are suddenly forced into atypical routines. In the context of COVID-19, working parents may be forced to balance the demands of their jobs with homeschooling their children. Couples may find themselves arguing more frequently. Adult children may find themselves needing to care for their ill parents. Limited space, a lack of leisure activities, and uncertainty about the future coalesce to increase conflict and stress. Research suggests that how people cope with a stressor is a more reliable determinant of health and well-being than the stressor itself.12
Interventions. Mental health clinicians can offer several recommendations to help patients cope with increased household stress (Table 3). We can encourage patients to have clear communication with their loved ones regarding new expectations, roles, and their feelings. Demarcating specific areas within living spaces to each person in the household can help each member feel a sense of autonomy, regardless of how small their area may be. Clinicians can help patients learn to take the time as a family to work on establishing new household routines. Telepsychiatry offers clinicians a unique window into patients’ lives and family dynamics, and we can use this perspective to deepen our understanding of the patient’s context and household relationships and help them navigate the situation thrust upon them.

Grief
Following a psychiatric hospitalization for an acute exacerbation of psychosis, Ms. S, age 79, is transferred to a rehabilitation facility, where she contracts COVID-19. Because Ms. S did not have a history of chronic medical illness, her family anticipates a full recovery. Early in the course of Ms. S’s admission, the rehabilitation facility restricts visitations, and her family is unable to see her. Ms. S dies in this facility without her family’s presence and without her family having the opportunity to say goodbye. Ms. S’s psychiatrist offers her family a virtual session to provide support. During the virtual session, the psychiatrist notes signs of complicated bereavement among Ms. S’s family members, including nonacceptance of the death, rumination about the circumstances of the death, and describing life as having no purpose.
The COVID-19 pandemic has complicated the natural process of loss and grief across multiple dimensions. Studies have shown that an inability to say goodbye before death, a lack of social support,13 and a lack of preparation for loss14 are associated with complicated bereavement and depression. Many people are experiencing the loss of loved ones without having a chance to appropriately mourn. Forbidding visits to family members who are hospitalized also prevents the practice of religious and spiritual rituals that typically occur at the end of life. This is worsened by truncated or absent funeral services. Support for those who are grieving may be offered from a distance, if at all. When surviving family members have been with the deceased prior to hospitalization, they may be required to self-quarantine, potentially exacerbating their grief and other symptoms associated with loss.
Interventions. Because social support is a protective factor against complicated grief,14 there are several recommendations for survivors as they work through the process of grief (Table 4). These include preparing families for a potential death; discussing desired spiritual and memorial services15; connecting families to resources such as community grief support programs, counseling/therapy, funeral services, video conferencing, and other communication tools; and planning for additional support for surviving family and friends, both immediately after the death and in the long term. It is also important to provide appropriate counseling and support for surviving family members to focus on their own well-being by exercising, eating nutritious meals, getting enough sleep, and abstaining from alcohol and drugs of abuse.16

Continue to: An ongoing challenge
An ongoing challenge
Our clinical team recommends further investigation to define additional psychotherapeutic themes arising from the COVID-19 pandemic and provide evidence-based interventions to address these categories, which we expect will increase in clinical salience in the months and years ahead. Close monitoring, follow-up by clinical and research staff, and evidence-based interventions will help address these dominant themes, with the goal of alleviating patient suffering.
Bottom Line
Our team identified 4 dominant clinical themes emerging across our patients’ experiences during the coronavirus disease 2019 pandemic: isolation, uncertainty, household stress, and grief. Clinicians can implement specific interventions to reduce the impact of these themes, which we expect to remain clinically relevant in the upcoming months and years.
Related Resources
- Sharma RA, Maheshwari S, Bronsther R. COVID-19 in the era of loneliness. Current Psychiatry. 2020;19(5):31-32,39.
- Carr D, Boerner K, Moorman S. Bereavement in the time of coronavirus: unprecedented challenges demand novel interventions. J Aging Soc Policy. 2020;32(4-5):425-431.
Drug Brand Names
Eszopiclone • Lunesta
Zolpidem • Ambien
1. Bloom N. How working from home works out. Stanford Institute for Economic Policy Research Policy Brief. https://siepr.stanford.edu/research/publications/how-working-home-works-out. Published June 2020. Accessed October 28, 2020.
2. Linz SJ, Sturm BA. The phenomenon of social isolation in the severely mentally ill. Perspect Psychiatr Care. 2013;49(4):243-254.
3. Smith KP, Christakis NA. Social networks and health. Annual Review of Sociology. 2008;34(1):405-429.
4. Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51(suppl):S54‐S66.
5. Mann F, Bone JK, Lloyd-Evans B. A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. Soc Psychiatry Psychiatr Epidemiol. 2017;52(6):627-638.
6. Choi M, Kong S, Jung D. Computer and internet interventions for loneliness and depression in older adults: a meta-analysis. Healthc Inform Res. 2012;18(3):191‐198.
7. Chen YF, Huang ZY, Chien CH, et al. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care. 2017;53(4):329-336.
8. Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786‐792.
9. Carberry JC, Grunstein RR, Eckert DJ. The effects of zolpidem in obstructive sleep apnea - an open-label pilot study. Sleep Res. 2019;28(6):e12853. doi: 10.1111/jsr.12853.
10. Markota M, Rummans TA, Bostwick JM, et al. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632-1639.
11. Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician. 2017;96(1):29-35.
12. Dijkstra MT, Homan AC. Engaging in rather than disengaging from stress: effective coping and perceived control. Front Psychol. 2016;7:1415.
13. Romero MM, Ott CH, Kelber ST. Predictors of grief in bereaved family caregivers of person’s with Alzheimer’s disease: a prospective study. Death Stud. 2014;38(6-10):395-403.
14. Lobb EA, Kristjanson LJ, Aoun SM, et al. Predictors of complicated grief: a systematic review of empirical studies. Death Stud. 2010;34(8):673-698.
15. Wallace CL, Wladkowski SP, Gibson A, et al. Grief during the COVID-19 pandemic: considerations for palliative care providers. J Pain Symptom Manage. 2020;60(1):e70-e76. doi: 10.1016/j.jpainsymman.2020.04.012
16. Selman LE, Chao D, Sowden R, et al. Bereavement support on the frontline of COVID-19: recommendations for hospital clinicians. J Pain Symptom Manage. 2020;60(2):e81-e86. doi: 10.1016/j.jpainsymman.2020.04.024
1. Bloom N. How working from home works out. Stanford Institute for Economic Policy Research Policy Brief. https://siepr.stanford.edu/research/publications/how-working-home-works-out. Published June 2020. Accessed October 28, 2020.
2. Linz SJ, Sturm BA. The phenomenon of social isolation in the severely mentally ill. Perspect Psychiatr Care. 2013;49(4):243-254.
3. Smith KP, Christakis NA. Social networks and health. Annual Review of Sociology. 2008;34(1):405-429.
4. Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51(suppl):S54‐S66.
5. Mann F, Bone JK, Lloyd-Evans B. A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. Soc Psychiatry Psychiatr Epidemiol. 2017;52(6):627-638.
6. Choi M, Kong S, Jung D. Computer and internet interventions for loneliness and depression in older adults: a meta-analysis. Healthc Inform Res. 2012;18(3):191‐198.
7. Chen YF, Huang ZY, Chien CH, et al. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care. 2017;53(4):329-336.
8. Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786‐792.
9. Carberry JC, Grunstein RR, Eckert DJ. The effects of zolpidem in obstructive sleep apnea - an open-label pilot study. Sleep Res. 2019;28(6):e12853. doi: 10.1111/jsr.12853.
10. Markota M, Rummans TA, Bostwick JM, et al. Benzodiazepine use in older adults: dangers, management, and alternative therapies. Mayo Clin Proc. 2016;91(11):1632-1639.
11. Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician. 2017;96(1):29-35.
12. Dijkstra MT, Homan AC. Engaging in rather than disengaging from stress: effective coping and perceived control. Front Psychol. 2016;7:1415.
13. Romero MM, Ott CH, Kelber ST. Predictors of grief in bereaved family caregivers of person’s with Alzheimer’s disease: a prospective study. Death Stud. 2014;38(6-10):395-403.
14. Lobb EA, Kristjanson LJ, Aoun SM, et al. Predictors of complicated grief: a systematic review of empirical studies. Death Stud. 2010;34(8):673-698.
15. Wallace CL, Wladkowski SP, Gibson A, et al. Grief during the COVID-19 pandemic: considerations for palliative care providers. J Pain Symptom Manage. 2020;60(1):e70-e76. doi: 10.1016/j.jpainsymman.2020.04.012
16. Selman LE, Chao D, Sowden R, et al. Bereavement support on the frontline of COVID-19: recommendations for hospital clinicians. J Pain Symptom Manage. 2020;60(2):e81-e86. doi: 10.1016/j.jpainsymman.2020.04.024
Managing metabolic syndrome in patients with schizophrenia
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.
Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.

Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.

Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.