User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Treatment of delirium: A review of 3 studies
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
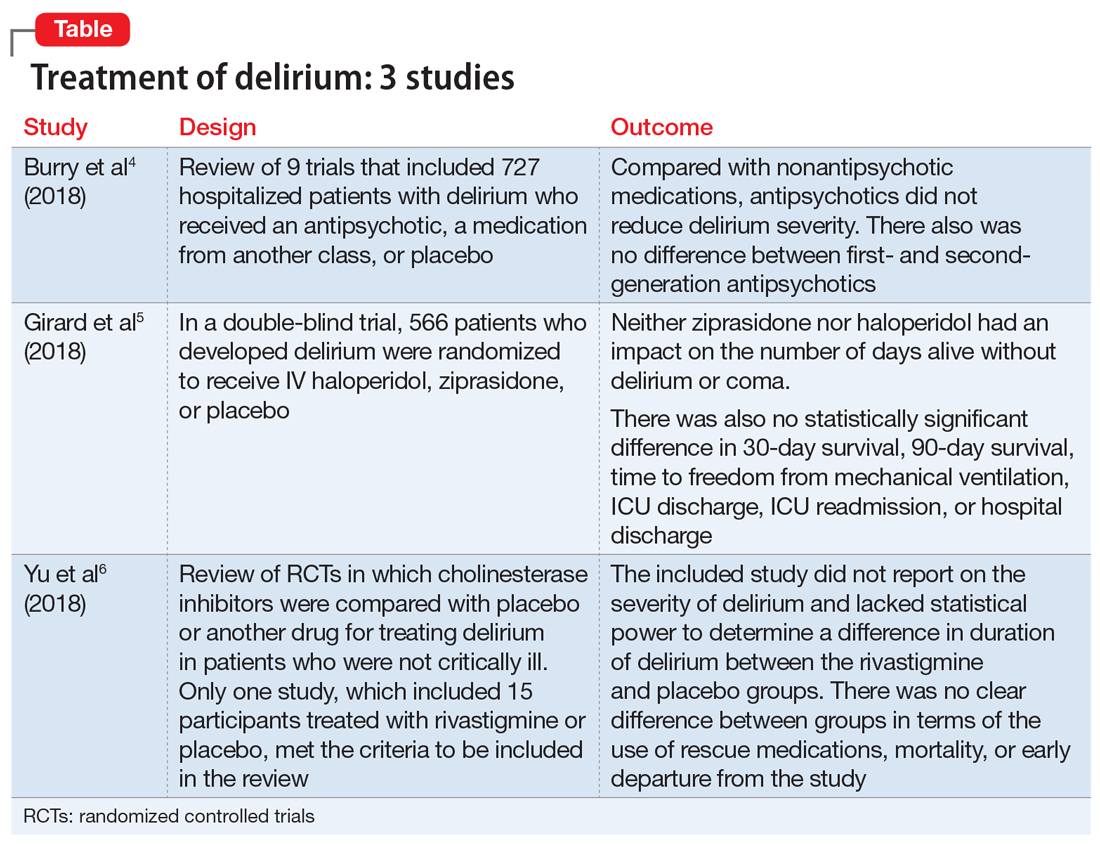
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6

1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6

1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Psychosis as a common thread across psychiatric disorders
Ask a psychiatrist to name a psychotic disorder, and the answer will most likely be “schizophrenia.” But if you closely examine the symptom structure of DSM-5 psychiatric disorders, you will note the presence of psychosis in almost all of them.
Fixed false beliefs and impaired reality testing are core features of psychosis. Those are certainly prominent in severe psychoses such as schizophrenia, schizoaffective disorder, or delusional disorder. But psychosis is actually a continuum of varying severity across most psychiatric disorders, although they carry different diagnostic labels. Irrational false beliefs and impaired functioning due to poor reality testing are embedded among many DSM-5 disorders. Hallucinations are less common; they are perceptual aberrations, not thought abnormalities, although they can trigger delusional explanations as to their causation.
Consider the following:
- Bipolar disorder. A large proportion of patients with bipolar disorder manifest delusions, usually grandiose, but often paranoid or referential.
- Major depressive disorder (MDD). Although regarded as a “pure mood disorder,” the core symptoms of MDD—self-deprecation and sense of worthlessness—as well as the poor reality testing of suicidal thoughts (that death is a better option than living) are psychotic false beliefs.
- Anxiety and panic disorder. The central symptom in anxiety and panic attacks is a belief in impending doom and/or death. The fear in anxiety disorders is actually based on a false belief (eg, if I get on the plane, it will crash, and I will die). Thus, technically an irrational/psychotic thought process underpins the terror and fear of anxiety disorders.
- Borderline personality disorder. Frank psychotic symptoms, such as paranoid beliefs, are known to be a component of borderline personality disorder symptoms. Although these symptoms tend to be brief and episodic, they can have a deleterious effect on the person’s coping and relationships.
- Other personality disorders. While many individuals with narcissistic personality disorder are functional, their exaggerated sense of self-importance, entitlement, and self-aggrandizement certainly qualifies as a fixed false belief. Patients with other personality disorders, such as schizotypal and paranoid, are known to harbor false beliefs or magical thinking.
- Body dysmorphic disorder. False beliefs about one’s appearance (such as blemishes or asymmetry) are at the center of this disorder, and it meets the litmus test of a psychosis.
- Anorexia nervosa. This disorder is well known to be characterized by a fixed false belief that one is “fat,” even when the patient’s body borders on being cachectic in appearance according to objective observers.
- Autism. This spectrum of diseases includes false beliefs that drive the ritualistic or odd behaviors.
- Obsessive-compulsive disorder. Although obsessions are usually ego-dystonic, in severe cases, they become ego-syntonic, similar to delusions. On the other hand, compulsions are often driven by a false belief, such as believing that one’s hands are dirty and must be washed incessantly, or that the locks on the door must be rechecked repeatedly because an intruder may break into the house and harm the inhabitants.
- Neurodegenerative syndromes. Neurodegenerative syndromes are neuropsychiatric disorders that very frequently include psychotic symptoms, such as paranoid delusions, delusions of marital infidelity, Capgras syndrome, or folie à deux. These disorders include Alzheimer’s disease, Parkinson’s disease, Lewy body dementia, frontal temporal dementia, metachromatic leukodystrophy, Huntington’s chorea, temporal lobe epilepsy, stroke, xenomelia, reduplicative phenomena, etc. This reflects the common emergence of faulty thinking with disintegration of neural tissue, both gray and white matter.
Continue to: So it should not be...
So it should not be surprising that antipsychotic medications, especially second-generation agents, have been shown to be helpful as monotherapy or adjunctive therapy in practically all the above psychiatric disorders, whether on-label or off-label.
Finally, it should also be noted that a case has been made for the existence of one dimension in all mental disorders manifesting in multiple psychopathologies.1 It is possible that a continuum of delusional thinking is a common thread across many psychiatric disorders due to this putative shared dimension. The milder form of this dimension may also explain the presence of pre-psychotic thinking in a significant proportion of the general population who do not seek psychiatric help.2 Just think of how many people you befriend, socialize with, and regard as perfectly “normal” endorse wild superstitions and astrological predictions, or believe in various conspiracy theories that have no basis in reality.
To comment on this editorial or other topics of interest: [email protected].
1. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
2. van Os J, Linscott RJ, Myin-Germeys I, et al. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179-195.
Ask a psychiatrist to name a psychotic disorder, and the answer will most likely be “schizophrenia.” But if you closely examine the symptom structure of DSM-5 psychiatric disorders, you will note the presence of psychosis in almost all of them.
Fixed false beliefs and impaired reality testing are core features of psychosis. Those are certainly prominent in severe psychoses such as schizophrenia, schizoaffective disorder, or delusional disorder. But psychosis is actually a continuum of varying severity across most psychiatric disorders, although they carry different diagnostic labels. Irrational false beliefs and impaired functioning due to poor reality testing are embedded among many DSM-5 disorders. Hallucinations are less common; they are perceptual aberrations, not thought abnormalities, although they can trigger delusional explanations as to their causation.
Consider the following:
- Bipolar disorder. A large proportion of patients with bipolar disorder manifest delusions, usually grandiose, but often paranoid or referential.
- Major depressive disorder (MDD). Although regarded as a “pure mood disorder,” the core symptoms of MDD—self-deprecation and sense of worthlessness—as well as the poor reality testing of suicidal thoughts (that death is a better option than living) are psychotic false beliefs.
- Anxiety and panic disorder. The central symptom in anxiety and panic attacks is a belief in impending doom and/or death. The fear in anxiety disorders is actually based on a false belief (eg, if I get on the plane, it will crash, and I will die). Thus, technically an irrational/psychotic thought process underpins the terror and fear of anxiety disorders.
- Borderline personality disorder. Frank psychotic symptoms, such as paranoid beliefs, are known to be a component of borderline personality disorder symptoms. Although these symptoms tend to be brief and episodic, they can have a deleterious effect on the person’s coping and relationships.
- Other personality disorders. While many individuals with narcissistic personality disorder are functional, their exaggerated sense of self-importance, entitlement, and self-aggrandizement certainly qualifies as a fixed false belief. Patients with other personality disorders, such as schizotypal and paranoid, are known to harbor false beliefs or magical thinking.
- Body dysmorphic disorder. False beliefs about one’s appearance (such as blemishes or asymmetry) are at the center of this disorder, and it meets the litmus test of a psychosis.
- Anorexia nervosa. This disorder is well known to be characterized by a fixed false belief that one is “fat,” even when the patient’s body borders on being cachectic in appearance according to objective observers.
- Autism. This spectrum of diseases includes false beliefs that drive the ritualistic or odd behaviors.
- Obsessive-compulsive disorder. Although obsessions are usually ego-dystonic, in severe cases, they become ego-syntonic, similar to delusions. On the other hand, compulsions are often driven by a false belief, such as believing that one’s hands are dirty and must be washed incessantly, or that the locks on the door must be rechecked repeatedly because an intruder may break into the house and harm the inhabitants.
- Neurodegenerative syndromes. Neurodegenerative syndromes are neuropsychiatric disorders that very frequently include psychotic symptoms, such as paranoid delusions, delusions of marital infidelity, Capgras syndrome, or folie à deux. These disorders include Alzheimer’s disease, Parkinson’s disease, Lewy body dementia, frontal temporal dementia, metachromatic leukodystrophy, Huntington’s chorea, temporal lobe epilepsy, stroke, xenomelia, reduplicative phenomena, etc. This reflects the common emergence of faulty thinking with disintegration of neural tissue, both gray and white matter.
Continue to: So it should not be...
So it should not be surprising that antipsychotic medications, especially second-generation agents, have been shown to be helpful as monotherapy or adjunctive therapy in practically all the above psychiatric disorders, whether on-label or off-label.
Finally, it should also be noted that a case has been made for the existence of one dimension in all mental disorders manifesting in multiple psychopathologies.1 It is possible that a continuum of delusional thinking is a common thread across many psychiatric disorders due to this putative shared dimension. The milder form of this dimension may also explain the presence of pre-psychotic thinking in a significant proportion of the general population who do not seek psychiatric help.2 Just think of how many people you befriend, socialize with, and regard as perfectly “normal” endorse wild superstitions and astrological predictions, or believe in various conspiracy theories that have no basis in reality.
To comment on this editorial or other topics of interest: [email protected].
Ask a psychiatrist to name a psychotic disorder, and the answer will most likely be “schizophrenia.” But if you closely examine the symptom structure of DSM-5 psychiatric disorders, you will note the presence of psychosis in almost all of them.
Fixed false beliefs and impaired reality testing are core features of psychosis. Those are certainly prominent in severe psychoses such as schizophrenia, schizoaffective disorder, or delusional disorder. But psychosis is actually a continuum of varying severity across most psychiatric disorders, although they carry different diagnostic labels. Irrational false beliefs and impaired functioning due to poor reality testing are embedded among many DSM-5 disorders. Hallucinations are less common; they are perceptual aberrations, not thought abnormalities, although they can trigger delusional explanations as to their causation.
Consider the following:
- Bipolar disorder. A large proportion of patients with bipolar disorder manifest delusions, usually grandiose, but often paranoid or referential.
- Major depressive disorder (MDD). Although regarded as a “pure mood disorder,” the core symptoms of MDD—self-deprecation and sense of worthlessness—as well as the poor reality testing of suicidal thoughts (that death is a better option than living) are psychotic false beliefs.
- Anxiety and panic disorder. The central symptom in anxiety and panic attacks is a belief in impending doom and/or death. The fear in anxiety disorders is actually based on a false belief (eg, if I get on the plane, it will crash, and I will die). Thus, technically an irrational/psychotic thought process underpins the terror and fear of anxiety disorders.
- Borderline personality disorder. Frank psychotic symptoms, such as paranoid beliefs, are known to be a component of borderline personality disorder symptoms. Although these symptoms tend to be brief and episodic, they can have a deleterious effect on the person’s coping and relationships.
- Other personality disorders. While many individuals with narcissistic personality disorder are functional, their exaggerated sense of self-importance, entitlement, and self-aggrandizement certainly qualifies as a fixed false belief. Patients with other personality disorders, such as schizotypal and paranoid, are known to harbor false beliefs or magical thinking.
- Body dysmorphic disorder. False beliefs about one’s appearance (such as blemishes or asymmetry) are at the center of this disorder, and it meets the litmus test of a psychosis.
- Anorexia nervosa. This disorder is well known to be characterized by a fixed false belief that one is “fat,” even when the patient’s body borders on being cachectic in appearance according to objective observers.
- Autism. This spectrum of diseases includes false beliefs that drive the ritualistic or odd behaviors.
- Obsessive-compulsive disorder. Although obsessions are usually ego-dystonic, in severe cases, they become ego-syntonic, similar to delusions. On the other hand, compulsions are often driven by a false belief, such as believing that one’s hands are dirty and must be washed incessantly, or that the locks on the door must be rechecked repeatedly because an intruder may break into the house and harm the inhabitants.
- Neurodegenerative syndromes. Neurodegenerative syndromes are neuropsychiatric disorders that very frequently include psychotic symptoms, such as paranoid delusions, delusions of marital infidelity, Capgras syndrome, or folie à deux. These disorders include Alzheimer’s disease, Parkinson’s disease, Lewy body dementia, frontal temporal dementia, metachromatic leukodystrophy, Huntington’s chorea, temporal lobe epilepsy, stroke, xenomelia, reduplicative phenomena, etc. This reflects the common emergence of faulty thinking with disintegration of neural tissue, both gray and white matter.
Continue to: So it should not be...
So it should not be surprising that antipsychotic medications, especially second-generation agents, have been shown to be helpful as monotherapy or adjunctive therapy in practically all the above psychiatric disorders, whether on-label or off-label.
Finally, it should also be noted that a case has been made for the existence of one dimension in all mental disorders manifesting in multiple psychopathologies.1 It is possible that a continuum of delusional thinking is a common thread across many psychiatric disorders due to this putative shared dimension. The milder form of this dimension may also explain the presence of pre-psychotic thinking in a significant proportion of the general population who do not seek psychiatric help.2 Just think of how many people you befriend, socialize with, and regard as perfectly “normal” endorse wild superstitions and astrological predictions, or believe in various conspiracy theories that have no basis in reality.
To comment on this editorial or other topics of interest: [email protected].
1. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
2. van Os J, Linscott RJ, Myin-Germeys I, et al. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179-195.
1. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
2. van Os J, Linscott RJ, Myin-Germeys I, et al. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179-195.
Career Choices: Academic psychiatry
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, Chief Resident at Nassau University Medical Center, East Meadow, New York, talked with Donald W. Black, MD, Professor of Psychiatry, Department of Psychiatry, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, Iowa. Dr. Black is also Editor-in-Chief of
Dr. Ahmed: What made you choose the academic track, and how did your training lead you towards this path?
Dr. Black: I had always been interested in the idea of working at a medical school, and enjoyed writing and speaking. I was exposed to clinical research as a resident, and that confirmed my interest in academia, because I could envision combining all my interests, along with patient care. I always thought that patients were a major source of ideas for research and writing.
Dr. Ahmed: What are some of the pros and cons of working in academia?
Dr. Black: The pros include being able to influence future physicians through my teaching and writing; being able to pursue important research; and not being isolated from peers. Other advantages are being largely protected from utilization review; having more free time than peers in the private sector, who have difficulty finding coverage; and having defined benefits and a steady salary. I also share call with many peers.
When it comes to the cons, salaries are lower than in the private sector. The cons also include not being my own boss, and sometimes having to bend to the whims of an institution or supervisor.
Continue to: Dr. Ahmed...
Dr. Ahmed: Are you required to conduct research?
Dr. Black: Yes. This is one of the best aspects of my job: being able to make clinical discoveries that I can disseminate through writing and speaking. Over time, this has become increasingly challenging due to the difficulty of obtaining research funding from foundations or the federal government. This has become highly problematic, particularly for clinical researchers, because the National Institutes of Health has clearly been favoring neuroscience.
Dr. Ahmed: What is your typical day like?
Dr. Black: Because of the many hats I wear (or have worn), each day is different from the other. I combine patient care with research, writing, speaking, teaching, and administration. As a tenure-track faculty member, I am expected to write grants, conduct research, and publish. My clinical-track peers primarily provide patient care and teach students and residents.
Dr. Ahmed: What is unique about working in a training institute vs private practice?
Continue to: Dr. Black...
Dr. Black: As an academic psychiatrist, I feel I have the best of both worlds: patient care combined with opportunities my private practice colleagues do not have. Because I have published widely, and have developed a reputation, I am frequently invited to speak at meetings throughout the United States, and sometimes internationally. Travel is a perk of academia, and as someone who loves travel, that is important.
Dr. Ahmed: Where do you see psychiatry going?
Dr. Black: Psychiatry will always be an important specialty because no one else truly cares about patients with psychiatric illnesses. Mental illness will not go away, and society needs highly trained individuals to provide care. There are many “me too” clinicians who now share in caring for patients with psychiatric illnesses, but psychiatrists will always have the most training, and are in a position to provide supervision to others and to direct mental health care teams.
Dr. Ahmed: What advice do you have for residents contemplating a career in academic psychiatry?
Dr. Black: Because most medical schools now have both tenure and clinical tracks, no one needs to feel left out. Those who are interested in scholarly activities will gravitate to the tenure tract, and all that requires in terms of grants and papers, while those who are primarily interested in patient care and teaching will choose the clinical track.
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, Chief Resident at Nassau University Medical Center, East Meadow, New York, talked with Donald W. Black, MD, Professor of Psychiatry, Department of Psychiatry, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, Iowa. Dr. Black is also Editor-in-Chief of
Dr. Ahmed: What made you choose the academic track, and how did your training lead you towards this path?
Dr. Black: I had always been interested in the idea of working at a medical school, and enjoyed writing and speaking. I was exposed to clinical research as a resident, and that confirmed my interest in academia, because I could envision combining all my interests, along with patient care. I always thought that patients were a major source of ideas for research and writing.
Dr. Ahmed: What are some of the pros and cons of working in academia?
Dr. Black: The pros include being able to influence future physicians through my teaching and writing; being able to pursue important research; and not being isolated from peers. Other advantages are being largely protected from utilization review; having more free time than peers in the private sector, who have difficulty finding coverage; and having defined benefits and a steady salary. I also share call with many peers.
When it comes to the cons, salaries are lower than in the private sector. The cons also include not being my own boss, and sometimes having to bend to the whims of an institution or supervisor.
Continue to: Dr. Ahmed...
Dr. Ahmed: Are you required to conduct research?
Dr. Black: Yes. This is one of the best aspects of my job: being able to make clinical discoveries that I can disseminate through writing and speaking. Over time, this has become increasingly challenging due to the difficulty of obtaining research funding from foundations or the federal government. This has become highly problematic, particularly for clinical researchers, because the National Institutes of Health has clearly been favoring neuroscience.
Dr. Ahmed: What is your typical day like?
Dr. Black: Because of the many hats I wear (or have worn), each day is different from the other. I combine patient care with research, writing, speaking, teaching, and administration. As a tenure-track faculty member, I am expected to write grants, conduct research, and publish. My clinical-track peers primarily provide patient care and teach students and residents.
Dr. Ahmed: What is unique about working in a training institute vs private practice?
Continue to: Dr. Black...
Dr. Black: As an academic psychiatrist, I feel I have the best of both worlds: patient care combined with opportunities my private practice colleagues do not have. Because I have published widely, and have developed a reputation, I am frequently invited to speak at meetings throughout the United States, and sometimes internationally. Travel is a perk of academia, and as someone who loves travel, that is important.
Dr. Ahmed: Where do you see psychiatry going?
Dr. Black: Psychiatry will always be an important specialty because no one else truly cares about patients with psychiatric illnesses. Mental illness will not go away, and society needs highly trained individuals to provide care. There are many “me too” clinicians who now share in caring for patients with psychiatric illnesses, but psychiatrists will always have the most training, and are in a position to provide supervision to others and to direct mental health care teams.
Dr. Ahmed: What advice do you have for residents contemplating a career in academic psychiatry?
Dr. Black: Because most medical schools now have both tenure and clinical tracks, no one needs to feel left out. Those who are interested in scholarly activities will gravitate to the tenure tract, and all that requires in terms of grants and papers, while those who are primarily interested in patient care and teaching will choose the clinical track.
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, Chief Resident at Nassau University Medical Center, East Meadow, New York, talked with Donald W. Black, MD, Professor of Psychiatry, Department of Psychiatry, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, Iowa. Dr. Black is also Editor-in-Chief of
Dr. Ahmed: What made you choose the academic track, and how did your training lead you towards this path?
Dr. Black: I had always been interested in the idea of working at a medical school, and enjoyed writing and speaking. I was exposed to clinical research as a resident, and that confirmed my interest in academia, because I could envision combining all my interests, along with patient care. I always thought that patients were a major source of ideas for research and writing.
Dr. Ahmed: What are some of the pros and cons of working in academia?
Dr. Black: The pros include being able to influence future physicians through my teaching and writing; being able to pursue important research; and not being isolated from peers. Other advantages are being largely protected from utilization review; having more free time than peers in the private sector, who have difficulty finding coverage; and having defined benefits and a steady salary. I also share call with many peers.
When it comes to the cons, salaries are lower than in the private sector. The cons also include not being my own boss, and sometimes having to bend to the whims of an institution or supervisor.
Continue to: Dr. Ahmed...
Dr. Ahmed: Are you required to conduct research?
Dr. Black: Yes. This is one of the best aspects of my job: being able to make clinical discoveries that I can disseminate through writing and speaking. Over time, this has become increasingly challenging due to the difficulty of obtaining research funding from foundations or the federal government. This has become highly problematic, particularly for clinical researchers, because the National Institutes of Health has clearly been favoring neuroscience.
Dr. Ahmed: What is your typical day like?
Dr. Black: Because of the many hats I wear (or have worn), each day is different from the other. I combine patient care with research, writing, speaking, teaching, and administration. As a tenure-track faculty member, I am expected to write grants, conduct research, and publish. My clinical-track peers primarily provide patient care and teach students and residents.
Dr. Ahmed: What is unique about working in a training institute vs private practice?
Continue to: Dr. Black...
Dr. Black: As an academic psychiatrist, I feel I have the best of both worlds: patient care combined with opportunities my private practice colleagues do not have. Because I have published widely, and have developed a reputation, I am frequently invited to speak at meetings throughout the United States, and sometimes internationally. Travel is a perk of academia, and as someone who loves travel, that is important.
Dr. Ahmed: Where do you see psychiatry going?
Dr. Black: Psychiatry will always be an important specialty because no one else truly cares about patients with psychiatric illnesses. Mental illness will not go away, and society needs highly trained individuals to provide care. There are many “me too” clinicians who now share in caring for patients with psychiatric illnesses, but psychiatrists will always have the most training, and are in a position to provide supervision to others and to direct mental health care teams.
Dr. Ahmed: What advice do you have for residents contemplating a career in academic psychiatry?
Dr. Black: Because most medical schools now have both tenure and clinical tracks, no one needs to feel left out. Those who are interested in scholarly activities will gravitate to the tenure tract, and all that requires in terms of grants and papers, while those who are primarily interested in patient care and teaching will choose the clinical track.
Factors that change our brains; The APA’s stance on neuroimaging
Factors that change our brains
I greatly enjoyed Dr. Nasrallah’s editorial, “Your patient’s brain is different at every visit” (From the Editor,
In reading this editorial, it is clear that a myriad of factors we consider and address with our patients during each visit underly intricate neurobiologic mechanisms and processes that ever deepen our understanding of the brain. In discussing the changes taking place in our patients, I can’t help but wonder what changes are also occurring in our brains (as Dr. Nasrallah noted). What would be the resulting impact of these changes in our next patient interaction and/or subsequent interaction(s) with the same patient? Looking through the editorial’s bullet points, many (if not all) of the factors contributing to brain changes apply equally and naturally to clinicians as well as patients. In this light, the editorial serves not only as a broad guideline for patient psychoeducation but also as a reminder of wellness and well-being for clinicians.
As a “fresh-out-of-training” psychiatrist, I can definitely work on several of the factors, such as diet and exercise. Trainees and residents can be more susceptible to overlook and befall some of these factors and changes, and may already be basing the clinical advice they give to their patients on these same factors and changes. As a child psychiatrist, I value the importance of modeling healthy behaviors for my patients, and their families and with coworkers or colleagues. In accordance with the impact these factors have on our brains, it’s important to emphasize what we can do to further strengthen rapport and therapeutic value through modeling. I strive to model the desired behaviors, attitudes, and dynamics that are the external, observable manifestation or symptomology of what takes place in my brain. To do so, I understand I need to be mindful in proactively managing the contributing factors, such as those listed in Dr. Nasrallah’s editorial. I imagine patients and their families would easily notice if we are in suboptimal physical and/or mental health that results in us not being prompt, fully engaged, or receptive. I believe that attending to these facets during training falls under the umbrella of professionalism. Being a professional in our field often entails practicing what we preach. So, I’m grateful that what we preach is informed by our field’s exciting research, continued advancements, and expertise that benefits our patients and us professionally and personally.
Philip Yen-Tsun Liu, MD
Child and adolescent psychiatrist
innovaTel Telepsychiatry
San Antonio, Texas
Dr. Nasrallah responds
I would like to thank Dr. Liu for his thoughtful response to my editorial. He seems to be very cognizant of the fact that experiential neuroplasticity and brain tissue remodeling occurs in both the patient and physician. I admire his focus on psychoeducation, wellness, and professionalism. He is right that we as psychiatrists (and nurse practitioners) must be role models for our patients in multiple ways, because it may help enhance clinical outcomes and have a positive impact on their brains.
I would also like to point Dr. Liu to the editorial “The most powerful placebo is not a pill” (From the Editor,
Henry A. Nasrallah, MD
Editor-in-Chief
Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
The APA’s stance on neuroimaging
Can anyone in the modern world argue that the brain is irrelevant to psychiatry? Yet surprisingly, in September 2018, the American Psychiatric Association (APA) officially declared that neuroimaging of the brain has no clinical value in psychiatry.1
Unfortunately, the APA focused almost exclusively on functional magnetic resonance imaging (fMRI) and neglected an extensive library of studies of single-photon emission computed tomography (SPECT) and positron emission tomography (PET). The APA’s position on neuroimaging is as follows1,2:
- A neuroimaging finding must have a sensitivity and specificity (S/sp) of no less than 80%.
- The psychiatric imaging literature does not support using neuroimaging in psychiatric diagnostics or treatment.
- Neuroimaging has not had a significant impact on the diagnosis and treatment of psychiatric disorders.
The APA set unrealistic standards for biomarkers in a field that lacks pathologic markers of specific disease entities.3 Moreover, numerous widely used tests fall below the APA’s unrealistic S/sp cutoff, including the Hamilton Depression Rating Scale,4 Zung Depression Scale,5 the clock drawing test,6 and even the chest X-ray.3 Curiously, numerous replicated SPECT and PET studies were not included in the APA’s analysis.1-3 For example, in a study of 196 veterans, posttraumatic stress disorder was distinguished from traumatic brain injury with an S/sp of 0.92/0.85.7,8 Also, fluorodeoxyglucose (FDG)-PET has an S/sp of 0.84/0.74 in differentiating patients with Alzheimer’s disease from controls, while perfusion SPECT, using multi-detector cameras, has an S/sp of 0.93/0.84.3,9 Moreover, both FDG-PET and SPECT can differentiate other forms of dementia from Alzheimer’s disease, yielding an additional benefit compared to amyloid imaging alone.2,9 As President of the International Society of Applied Neuroimaging, I suggest neuroimaging should not be feared. Neuroimaging does not replace the diagnostician; rather, it aids him/her in a complex case.
Theodore A. Henderson, MD, PhD
President
Neuro-Luminance Brain Health Centers, Inc.
Denver, Colorado
Director
The Synaptic Space
Vice President
The Neuro-Laser Foundation
President
International Society of Applied Neuroimaging
Centennial, Colorado
Disclosure
The author has no ownership in, and receives no remuneration from, any neuroimaging company.
References
1. First MB, Drevets WC, Carter C, et al. Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018:175:
2. First MB, Drevets WC, Carter C, et al. Data supplement for Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018;175(suppl).
3. Henderson TA. Brain SPECT imaging in neuropsychiatric diagnosis and monitoring. EPatient. http://nmpangea.com/2018/10/09/738/. Published 2018. Accessed May 31, 2019.
4. Bagby RM, Ryder AG, Schuller DR, et al. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161(12):2163-2177.
5. Biggs JT, Wylie LT, Ziegler VE. Validity of the Zung Self-rating Depression Scale. Br J Psychiatry. 1978;132:381-385.
6. Seigerschmidt E, Mösch E, Siemen M, et al. The clock drawing test and questionable dementia: reliability and validity. Int J Geriatr Psychiatry. 2002;17(11):1048-1054.
7. Raji CA, Willeumier K, Taylor D, et al. Functional neuroimaging with default mode network regions distinguishes PTSD from TBI in a military veteran population. Brain Imaging Behav. 2015;9(3):527-534.
8. Amen DG, Raji CA, Willeumier K, et al. Functional neuroimaging distinguishes posttraumatic stress disorder from traumatic brain injury in focused and large community datasets. PLoS One. 2015;10(7):e0129659. doi: 10.1371/journal.pone.0129659.
9. Henderson TA. The diagnosis and evaluation of dementia and mild cognitive impairment with emphasis on SPECT perfusion neuroimaging. CNS Spectr. 2012;17(4):176-206.
Factors that change our brains
I greatly enjoyed Dr. Nasrallah’s editorial, “Your patient’s brain is different at every visit” (From the Editor,
In reading this editorial, it is clear that a myriad of factors we consider and address with our patients during each visit underly intricate neurobiologic mechanisms and processes that ever deepen our understanding of the brain. In discussing the changes taking place in our patients, I can’t help but wonder what changes are also occurring in our brains (as Dr. Nasrallah noted). What would be the resulting impact of these changes in our next patient interaction and/or subsequent interaction(s) with the same patient? Looking through the editorial’s bullet points, many (if not all) of the factors contributing to brain changes apply equally and naturally to clinicians as well as patients. In this light, the editorial serves not only as a broad guideline for patient psychoeducation but also as a reminder of wellness and well-being for clinicians.
As a “fresh-out-of-training” psychiatrist, I can definitely work on several of the factors, such as diet and exercise. Trainees and residents can be more susceptible to overlook and befall some of these factors and changes, and may already be basing the clinical advice they give to their patients on these same factors and changes. As a child psychiatrist, I value the importance of modeling healthy behaviors for my patients, and their families and with coworkers or colleagues. In accordance with the impact these factors have on our brains, it’s important to emphasize what we can do to further strengthen rapport and therapeutic value through modeling. I strive to model the desired behaviors, attitudes, and dynamics that are the external, observable manifestation or symptomology of what takes place in my brain. To do so, I understand I need to be mindful in proactively managing the contributing factors, such as those listed in Dr. Nasrallah’s editorial. I imagine patients and their families would easily notice if we are in suboptimal physical and/or mental health that results in us not being prompt, fully engaged, or receptive. I believe that attending to these facets during training falls under the umbrella of professionalism. Being a professional in our field often entails practicing what we preach. So, I’m grateful that what we preach is informed by our field’s exciting research, continued advancements, and expertise that benefits our patients and us professionally and personally.
Philip Yen-Tsun Liu, MD
Child and adolescent psychiatrist
innovaTel Telepsychiatry
San Antonio, Texas
Dr. Nasrallah responds
I would like to thank Dr. Liu for his thoughtful response to my editorial. He seems to be very cognizant of the fact that experiential neuroplasticity and brain tissue remodeling occurs in both the patient and physician. I admire his focus on psychoeducation, wellness, and professionalism. He is right that we as psychiatrists (and nurse practitioners) must be role models for our patients in multiple ways, because it may help enhance clinical outcomes and have a positive impact on their brains.
I would also like to point Dr. Liu to the editorial “The most powerful placebo is not a pill” (From the Editor,
Henry A. Nasrallah, MD
Editor-in-Chief
Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
The APA’s stance on neuroimaging
Can anyone in the modern world argue that the brain is irrelevant to psychiatry? Yet surprisingly, in September 2018, the American Psychiatric Association (APA) officially declared that neuroimaging of the brain has no clinical value in psychiatry.1
Unfortunately, the APA focused almost exclusively on functional magnetic resonance imaging (fMRI) and neglected an extensive library of studies of single-photon emission computed tomography (SPECT) and positron emission tomography (PET). The APA’s position on neuroimaging is as follows1,2:
- A neuroimaging finding must have a sensitivity and specificity (S/sp) of no less than 80%.
- The psychiatric imaging literature does not support using neuroimaging in psychiatric diagnostics or treatment.
- Neuroimaging has not had a significant impact on the diagnosis and treatment of psychiatric disorders.
The APA set unrealistic standards for biomarkers in a field that lacks pathologic markers of specific disease entities.3 Moreover, numerous widely used tests fall below the APA’s unrealistic S/sp cutoff, including the Hamilton Depression Rating Scale,4 Zung Depression Scale,5 the clock drawing test,6 and even the chest X-ray.3 Curiously, numerous replicated SPECT and PET studies were not included in the APA’s analysis.1-3 For example, in a study of 196 veterans, posttraumatic stress disorder was distinguished from traumatic brain injury with an S/sp of 0.92/0.85.7,8 Also, fluorodeoxyglucose (FDG)-PET has an S/sp of 0.84/0.74 in differentiating patients with Alzheimer’s disease from controls, while perfusion SPECT, using multi-detector cameras, has an S/sp of 0.93/0.84.3,9 Moreover, both FDG-PET and SPECT can differentiate other forms of dementia from Alzheimer’s disease, yielding an additional benefit compared to amyloid imaging alone.2,9 As President of the International Society of Applied Neuroimaging, I suggest neuroimaging should not be feared. Neuroimaging does not replace the diagnostician; rather, it aids him/her in a complex case.
Theodore A. Henderson, MD, PhD
President
Neuro-Luminance Brain Health Centers, Inc.
Denver, Colorado
Director
The Synaptic Space
Vice President
The Neuro-Laser Foundation
President
International Society of Applied Neuroimaging
Centennial, Colorado
Disclosure
The author has no ownership in, and receives no remuneration from, any neuroimaging company.
References
1. First MB, Drevets WC, Carter C, et al. Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018:175:
2. First MB, Drevets WC, Carter C, et al. Data supplement for Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018;175(suppl).
3. Henderson TA. Brain SPECT imaging in neuropsychiatric diagnosis and monitoring. EPatient. http://nmpangea.com/2018/10/09/738/. Published 2018. Accessed May 31, 2019.
4. Bagby RM, Ryder AG, Schuller DR, et al. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161(12):2163-2177.
5. Biggs JT, Wylie LT, Ziegler VE. Validity of the Zung Self-rating Depression Scale. Br J Psychiatry. 1978;132:381-385.
6. Seigerschmidt E, Mösch E, Siemen M, et al. The clock drawing test and questionable dementia: reliability and validity. Int J Geriatr Psychiatry. 2002;17(11):1048-1054.
7. Raji CA, Willeumier K, Taylor D, et al. Functional neuroimaging with default mode network regions distinguishes PTSD from TBI in a military veteran population. Brain Imaging Behav. 2015;9(3):527-534.
8. Amen DG, Raji CA, Willeumier K, et al. Functional neuroimaging distinguishes posttraumatic stress disorder from traumatic brain injury in focused and large community datasets. PLoS One. 2015;10(7):e0129659. doi: 10.1371/journal.pone.0129659.
9. Henderson TA. The diagnosis and evaluation of dementia and mild cognitive impairment with emphasis on SPECT perfusion neuroimaging. CNS Spectr. 2012;17(4):176-206.
Factors that change our brains
I greatly enjoyed Dr. Nasrallah’s editorial, “Your patient’s brain is different at every visit” (From the Editor,
In reading this editorial, it is clear that a myriad of factors we consider and address with our patients during each visit underly intricate neurobiologic mechanisms and processes that ever deepen our understanding of the brain. In discussing the changes taking place in our patients, I can’t help but wonder what changes are also occurring in our brains (as Dr. Nasrallah noted). What would be the resulting impact of these changes in our next patient interaction and/or subsequent interaction(s) with the same patient? Looking through the editorial’s bullet points, many (if not all) of the factors contributing to brain changes apply equally and naturally to clinicians as well as patients. In this light, the editorial serves not only as a broad guideline for patient psychoeducation but also as a reminder of wellness and well-being for clinicians.
As a “fresh-out-of-training” psychiatrist, I can definitely work on several of the factors, such as diet and exercise. Trainees and residents can be more susceptible to overlook and befall some of these factors and changes, and may already be basing the clinical advice they give to their patients on these same factors and changes. As a child psychiatrist, I value the importance of modeling healthy behaviors for my patients, and their families and with coworkers or colleagues. In accordance with the impact these factors have on our brains, it’s important to emphasize what we can do to further strengthen rapport and therapeutic value through modeling. I strive to model the desired behaviors, attitudes, and dynamics that are the external, observable manifestation or symptomology of what takes place in my brain. To do so, I understand I need to be mindful in proactively managing the contributing factors, such as those listed in Dr. Nasrallah’s editorial. I imagine patients and their families would easily notice if we are in suboptimal physical and/or mental health that results in us not being prompt, fully engaged, or receptive. I believe that attending to these facets during training falls under the umbrella of professionalism. Being a professional in our field often entails practicing what we preach. So, I’m grateful that what we preach is informed by our field’s exciting research, continued advancements, and expertise that benefits our patients and us professionally and personally.
Philip Yen-Tsun Liu, MD
Child and adolescent psychiatrist
innovaTel Telepsychiatry
San Antonio, Texas
Dr. Nasrallah responds
I would like to thank Dr. Liu for his thoughtful response to my editorial. He seems to be very cognizant of the fact that experiential neuroplasticity and brain tissue remodeling occurs in both the patient and physician. I admire his focus on psychoeducation, wellness, and professionalism. He is right that we as psychiatrists (and nurse practitioners) must be role models for our patients in multiple ways, because it may help enhance clinical outcomes and have a positive impact on their brains.
I would also like to point Dr. Liu to the editorial “The most powerful placebo is not a pill” (From the Editor,
Henry A. Nasrallah, MD
Editor-in-Chief
Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
The APA’s stance on neuroimaging
Can anyone in the modern world argue that the brain is irrelevant to psychiatry? Yet surprisingly, in September 2018, the American Psychiatric Association (APA) officially declared that neuroimaging of the brain has no clinical value in psychiatry.1
Unfortunately, the APA focused almost exclusively on functional magnetic resonance imaging (fMRI) and neglected an extensive library of studies of single-photon emission computed tomography (SPECT) and positron emission tomography (PET). The APA’s position on neuroimaging is as follows1,2:
- A neuroimaging finding must have a sensitivity and specificity (S/sp) of no less than 80%.
- The psychiatric imaging literature does not support using neuroimaging in psychiatric diagnostics or treatment.
- Neuroimaging has not had a significant impact on the diagnosis and treatment of psychiatric disorders.
The APA set unrealistic standards for biomarkers in a field that lacks pathologic markers of specific disease entities.3 Moreover, numerous widely used tests fall below the APA’s unrealistic S/sp cutoff, including the Hamilton Depression Rating Scale,4 Zung Depression Scale,5 the clock drawing test,6 and even the chest X-ray.3 Curiously, numerous replicated SPECT and PET studies were not included in the APA’s analysis.1-3 For example, in a study of 196 veterans, posttraumatic stress disorder was distinguished from traumatic brain injury with an S/sp of 0.92/0.85.7,8 Also, fluorodeoxyglucose (FDG)-PET has an S/sp of 0.84/0.74 in differentiating patients with Alzheimer’s disease from controls, while perfusion SPECT, using multi-detector cameras, has an S/sp of 0.93/0.84.3,9 Moreover, both FDG-PET and SPECT can differentiate other forms of dementia from Alzheimer’s disease, yielding an additional benefit compared to amyloid imaging alone.2,9 As President of the International Society of Applied Neuroimaging, I suggest neuroimaging should not be feared. Neuroimaging does not replace the diagnostician; rather, it aids him/her in a complex case.
Theodore A. Henderson, MD, PhD
President
Neuro-Luminance Brain Health Centers, Inc.
Denver, Colorado
Director
The Synaptic Space
Vice President
The Neuro-Laser Foundation
President
International Society of Applied Neuroimaging
Centennial, Colorado
Disclosure
The author has no ownership in, and receives no remuneration from, any neuroimaging company.
References
1. First MB, Drevets WC, Carter C, et al. Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018:175:
2. First MB, Drevets WC, Carter C, et al. Data supplement for Clinical applications of neuroimaging in psychiatric disorders. Am J Psychiatry. 2018;175(suppl).
3. Henderson TA. Brain SPECT imaging in neuropsychiatric diagnosis and monitoring. EPatient. http://nmpangea.com/2018/10/09/738/. Published 2018. Accessed May 31, 2019.
4. Bagby RM, Ryder AG, Schuller DR, et al. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161(12):2163-2177.
5. Biggs JT, Wylie LT, Ziegler VE. Validity of the Zung Self-rating Depression Scale. Br J Psychiatry. 1978;132:381-385.
6. Seigerschmidt E, Mösch E, Siemen M, et al. The clock drawing test and questionable dementia: reliability and validity. Int J Geriatr Psychiatry. 2002;17(11):1048-1054.
7. Raji CA, Willeumier K, Taylor D, et al. Functional neuroimaging with default mode network regions distinguishes PTSD from TBI in a military veteran population. Brain Imaging Behav. 2015;9(3):527-534.
8. Amen DG, Raji CA, Willeumier K, et al. Functional neuroimaging distinguishes posttraumatic stress disorder from traumatic brain injury in focused and large community datasets. PLoS One. 2015;10(7):e0129659. doi: 10.1371/journal.pone.0129659.
9. Henderson TA. The diagnosis and evaluation of dementia and mild cognitive impairment with emphasis on SPECT perfusion neuroimaging. CNS Spectr. 2012;17(4):176-206.
Nothing to sneeze at: Upper respiratory infections and mood disorders
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.
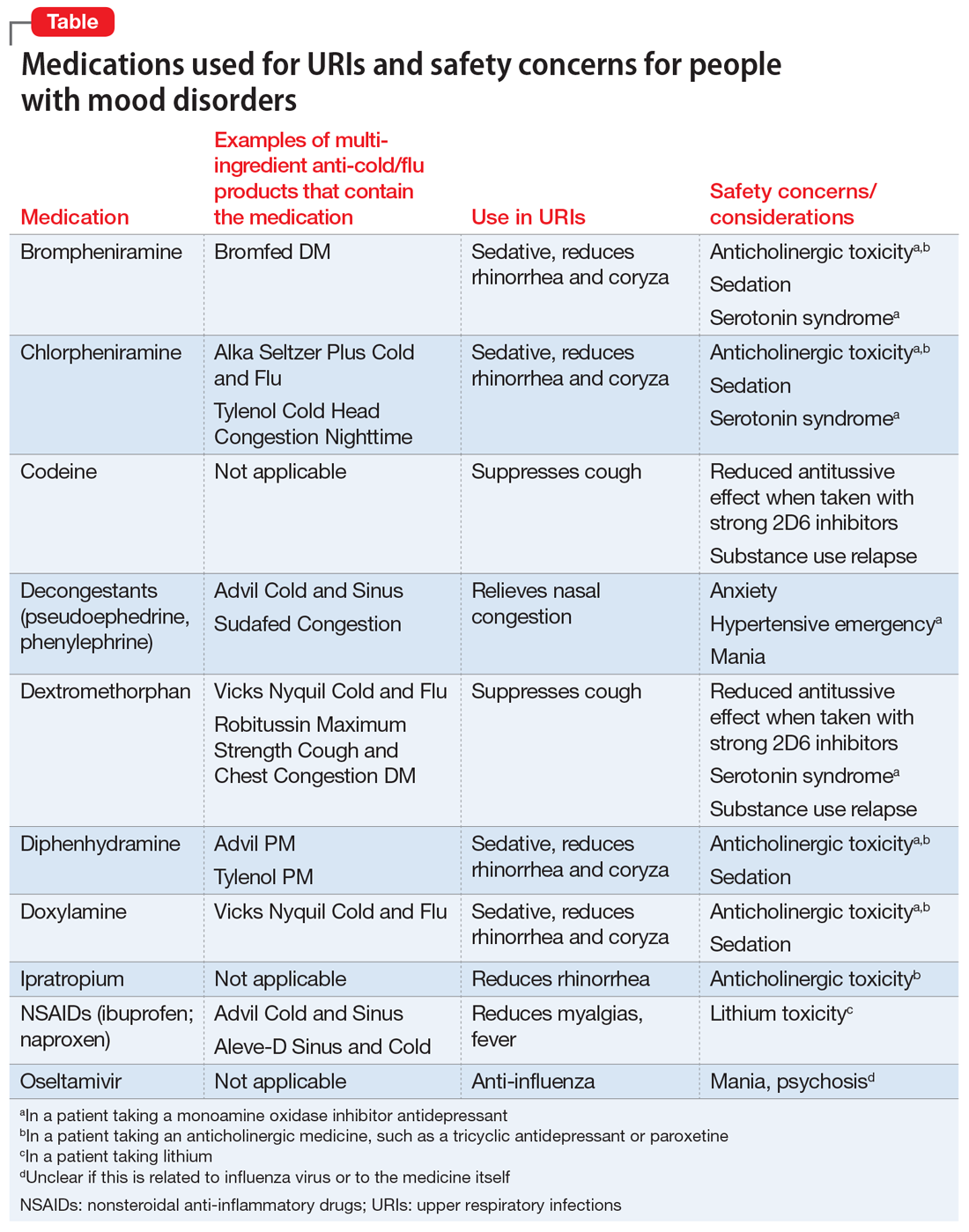
Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.

Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.

Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
Stigma in dementia: It’s time to talk about it
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).

Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.
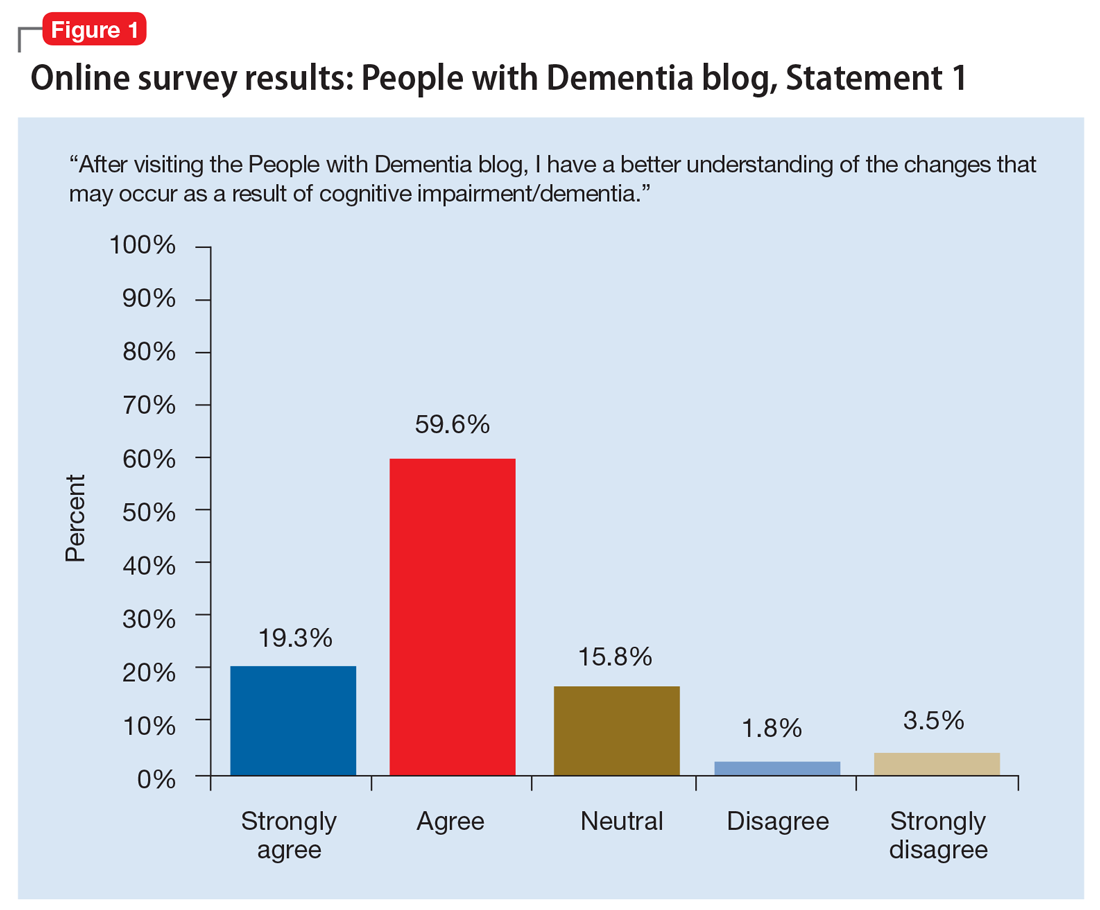
Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.
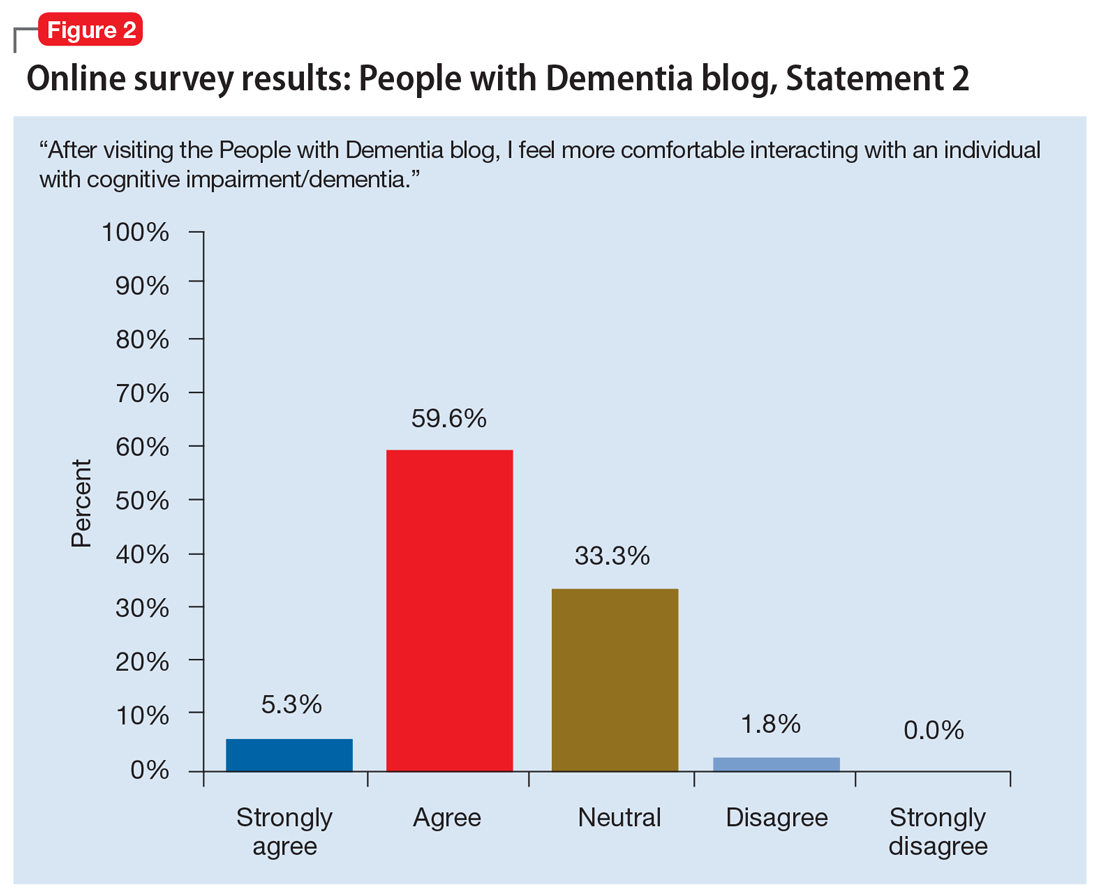
State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a
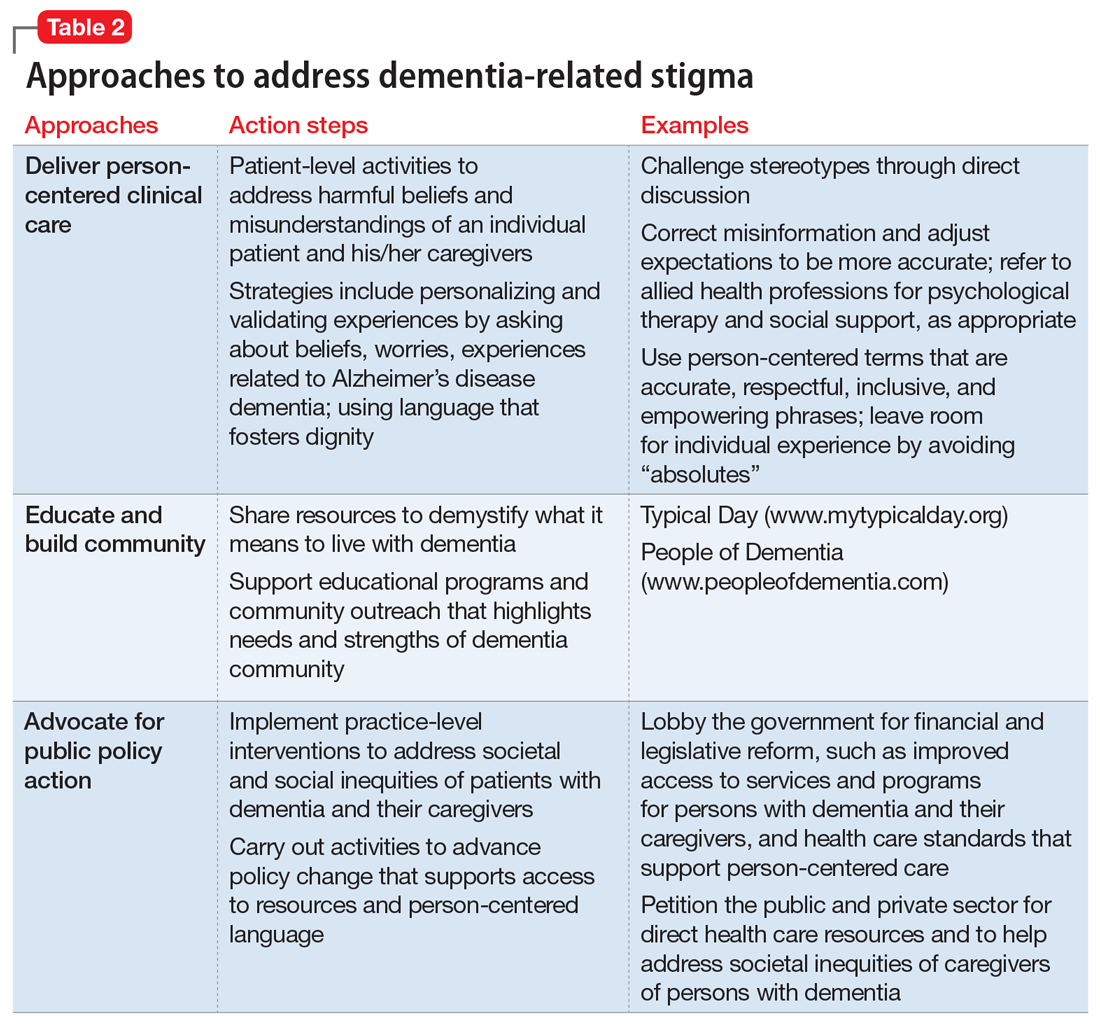
Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).

Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.

Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.

State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a

Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).

Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.

Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.

State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a

Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
Between a rock and a hard place
CASE Irritable and short of breath
Mr. B, age 75, who lives alone, is brought to the emergency department (ED) for evaluation of shortness of breath. Mr. B is normally highly independent, and is able to drive, manage his own finances, attend to activities of daily living, and participate in social functions at church. On the day before he was taken to the ED, his home nurse had come to his home to dispense medications and found Mr. B was irritable, verbally rude, and repeatedly scratching the right side of his head. The nurse was unsure if Mr. B had taken his medications over the weekend. She called for emergency services, but Mr. B refused to go to the ED, and he was able to decline care because he was not in an acute medical emergency (95% oxygen on pulse oximetry).
The next day, when Mr. B’s nurse returned to his home, she found him to be tachypneic and verbigerating the phrase “I don’t know.” She contacted emergency services again, and Mr. B was taken to the ED.
In the ED, Mr. B has tachycardia, tachypnea, increased work of breathing, and diffuse rhonchi. He continues to repeat the phrase “I don’t know” and scratches the right side of his head repeatedly. The ED clinicians consult Psychiatry due to Mr. B’s confusion and because his nurse reports that his presentation is similar to a previous psychiatric hospitalization 9 years earlier.
[polldaddy:10332862]
EVALUATION Complex comorbidities
Mr. B has a lengthy history of schizophrenia, chronic right-sided heart failure secondary to pulmonary hypertension, moderate chronic obstructive pulmonary disease, hypertension, type 2 diabetes mellitus, and prostatic adenocarcinoma after external beam radiation therapy.
His symptoms of schizophrenia had been stable on his long-standing outpatient psychotropic regimen of haloperidol, 5 mg nightly; mirtazapine, 15 mg nightly, for appetite stimulation and insomnia; and trazodone, 100 mg nightly for insomnia. Mr. B has been receiving assertive community treatment (ACT) psychiatric services for schizophrenia; a nurse refills his pill box with his medications weekly. He does not have a history of medication nonadherence, and his nurse did not think he had missed any doses before the weekend.
He has acute changes in depressed mood, perseveration, and a Mini-Mental State Examination (MMSE) score of 26 (missing points for delayed recall and inability to construct a sentence), which indicates a cognitive assessment score on the low end of the normal range for people with at least an eighth grade education.
At the hospital, the psychiatrist diagnoses hypoactive delirium due to Mr. B’s fluctuating attention and disorientation. She also recommends that Mr. B continue his outpatient psychotropic regimen, and adds oral haloperidol, 5 mg, as needed for agitation (his QTc interval is 451 ms; reference range for men <430 ms, borderline prolonged 431 to 450 ms, prolonged >450 ms).
Continue to: An initial laboratory workup...
An initial laboratory workup and electrocardiogram reveal that Mr. B has an elevated troponin level (0.21 ng/mL; reference range <0.04; 0.04 to 0.39 ng/mL is elevated above the 99th percentile of a healthy population), non-ST-elevation myocardial infarction type II, Q waves in lead III, arteriovenous fistula with right axis deviation, acute on chronic kidney failure (creatinine level of 2.1 mg/dL, up from baseline of 1.4 mg/dL; reference range 0.84 to 1.21 mg/dL), elevated brain natriuretic peptide (111 pg/mL; reference range <125 pg/mL), and an elevated lactate level of 5.51 mmol/L (reference range 0.5 to 1 mmol/L). He also has a mixed respiratory alkalosis and metabolic acidosis with increased anion gap, transaminitis (aspartate aminotransferase 149 U/L; reference range 10 to 40 U/L), and elevated alkaline phosphatase (151 IU/L; reference range 44 to 147 IU/L). Urinalysis shows moderate ketones and is negative for nitrite or leukocyte esterase.
A brain CT rules out stroke. A chest X-ray shows subtle left basilar reticular opacity with a follow-up lateral view showing no consolidation and prominent pulmonary vasculature without overt edema.
In the ED, Mr. B is determined to have decision-making capacity and is able to authorize all treatment. Cardiology is also consulted, and Mr. B is admitted to the cardiac intensive care unit (CCU) for cardiogenic shock with close cardiac monitoring.
The Psychiatry and Cardiology teams discuss the risks and benefits of continuing antipsychotics. Due to the imminent risk of harm to Mr. B because of his significant agitation in the ED, which required treatment with one dose of IM haloperidol, 5 mg, and lorazepam, 2 mg, and close monitoring, the teams agree that the benefits of continuing haloperidol outweigh the risks.
On hospital Day 2, Mr. B’s repetitive scratching resolves. He is moved from the CCU to a general medical unit, where he begins to have episodes of mutism and negativism. By hospital Day 6, catatonia is suspected due to a MMSE of 6/30 and a Bush- Francis Catatonia Rating Scale (BFCRS) score of 14 for predominant stereotypy, perseveration, and withdrawal (Table 1). The teams determine that Mr. B lacks decisionmaking capacity due to his inability to rationally manipulate information. His brother is contacted and authorizes all treatment, deferring decision-making to the medical teams caring for Mr. B.
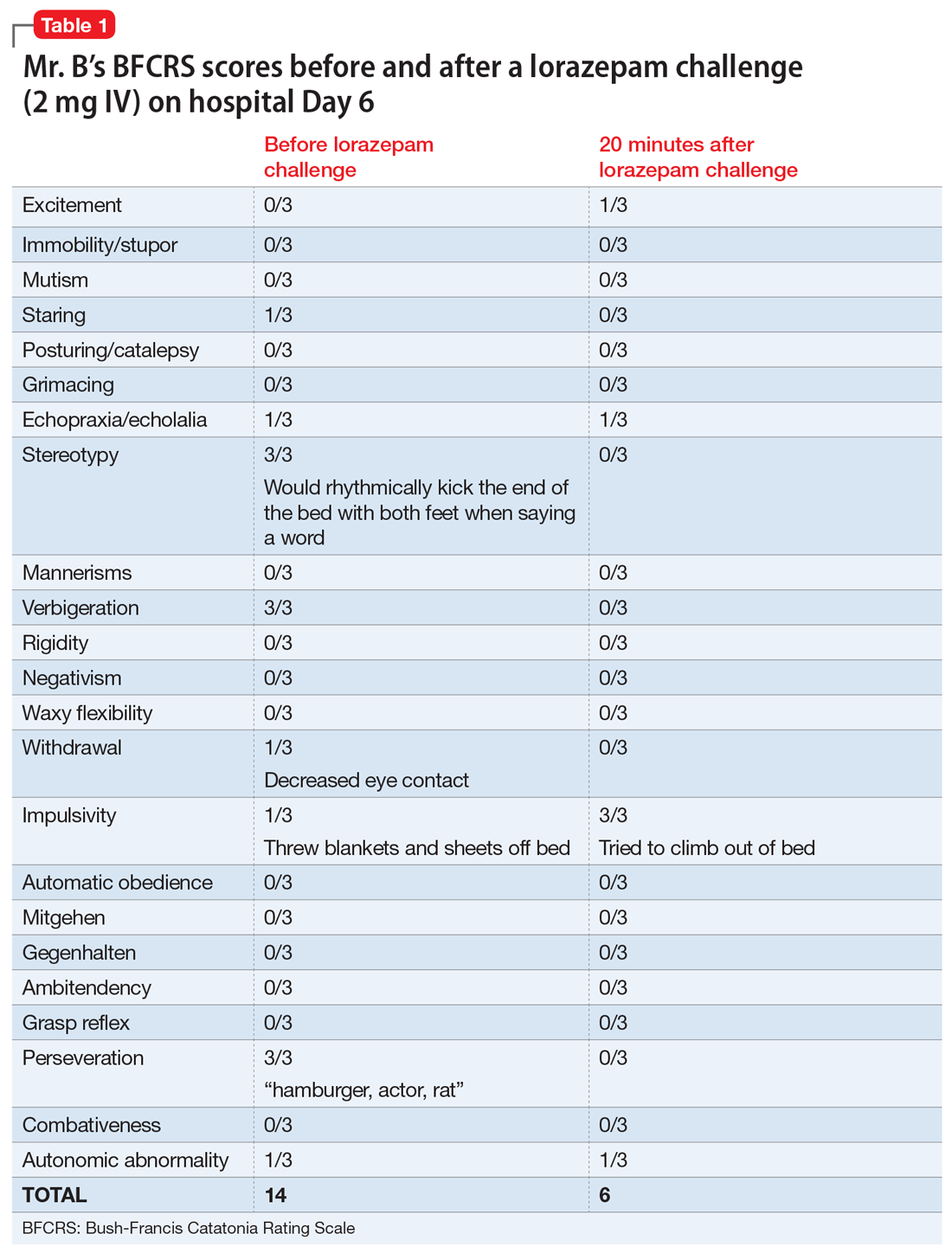
Continue to: Mr. B undergoes an EEG...
Mr. B undergoes an EEG, which rules out nonconvulsive status epilepticus and is consistent with encephalopathy/delirium. Neuroleptic malignant syndrome (NMS) is considered but is less likely because Mr. B had been receiving a stable dose of haloperidol for several years, is afebrile, has stable vital signs, has no muscle rigidity, and no evidence of leukocytosis, creatine kinase elevation, myoglobinuria, hyperkalemia, hyperphosphatemia, thrombocytosis, or hypocalcemia.
Based on these clinical findings, Mr. B is diagnosed with catatonia and delirium.
The authors’ observations
Delirium, characterized by inattention and changes in mental status, is a syndrome due to acute brain dysfunction. It can be subclassified as hyperactive or hypoactive based on the change of activity. Simple catatonia is characterized by changes in behavior, affect, and motor function (with hyper- or hypoactivity). It may arise from gammaaminobutyric acid hypoactivity, dopamine (D2) hypoactivity, and possibly glutamate N-methyl-d-aspartate (NMDA) hyperactivity.1 Malignant catatonia is simple catatonia combined with autonomic instability and hyperthermia, which is a life-threatening condition. The BFCRS is commonly used to assess symptoms.2
Both catatonia and delirium result in significant morbidity and mortality. The 2 conditions share signs and symptoms yet rarely are diagnosed at the same time. DSM-IV, DSM-IV-TR, and DSM-5 state that a diagnosis of catatonia due to another medical condition cannot be made exclusively in the presence of delirium.3,4 DSM-IV and DSM-IV-TR required at least 2 criteria from 5 areas, including motoric immobility, excessive motor activity, extreme negativism or mutism, peculiarities of voluntary movement, and echolalia or echopraxia. Instead of grouping symptoms into clusters, DSM-5 requires 3 criteria of 12 individual symptoms.3,4 A co-occurrence with a medical illness precludes using the DSM-5 “catatonia associated with another mental disorder (catatonia specifier)” with the “unspecified catatonia” diagnosis category.4
However, a growing body of literature suggests that delirium and catatonia can cooccur.5,6 In 2017, Wilson et al6 found that of 136 critically ill patients in the ICU, 43% (58 patients) had only delirium, 3% (4 patients) had only catatonia, 31% (42 patients) had both, and 24% (32 patients) had neither. In patients with both catatonia and delirium, the most common signs of catatonia were autonomic abnormalities (96%), immobility/ stupor (87%), staring (77%), mutism (60%), and posturing (60%).
Continue to: The differential diagnosis...
The differential diagnosis of catatonia is extensive and varied.3,4 The most common psychiatric causes are mood disorders (13% to 31%) and psychotic disorders (7% to 17%).7 Neuromedical etiologies account for 4% to 46% of cases.7 The most common medical and neurologic causes are seizure disorder, acute intermittent porphyria, systemic lupus erythematosus, and drugrelated adverse effects (particularly due to clozapine withdrawal, risperidone, and phencyclidine).7
A workup that includes physical examination, laboratory testing, and neuroimaging can be helpful to identify delirium and catatonia, but there is limited literature to guide identifying coexisting delirium and catatonia other than a blend of physical exam findings of delirium and catatonia. Electroencephalogram may be normal in primary catatonia or may show nonspecific changes in secondary catatonia.8 Additionally, discharges in the frontal lobes and anterior limbic systems with diffuse background slowing and dysrhythmic patterns may be seen.7 Neuroimaging with MRI can help to evaluate catatonia.9 Laboratory testing such as creatine phosphokinase levels can be high in simple catatonia and are often elevated in malignant catatonia.7 Considering the possible co-occurrence of delirium and catatonia is critical to providing good patient care because the 2 conditions are treated differently.
[polldaddy:10332867]
TREATMENT A balancing act
Over the next month, Mr. B alternates between appearing catatonic or delirious. When he appears more catatonic, the dose of lorazepam is increased, which results in increased impulsivity and agitation and leads to multiple interventions from the behavioral emergency response team. At times, the team must use restraints and haloperidol because Mr. B pulls out IV lines and is considered at high risk for falls. When Mr. B appears more delirious and the dose of lorazepam is decreased, he becomes more catatonic.
Following the diagnosis of catatonia on Day 6, oral haloperidol is discontinued to further mitigate Mr. B’s risk of developing NMS. On hospital Day 6, Mr. B improves significantly after a 2-mg IV lorazepam challenge, with a BFCRS score of 6. At this point, he is started on lorazepam, 1 mg IV 3 times a day.
On Day 7, based on the complicated nature of Mr. B’s medical and psychiatric comorbidities, the treatment team considers ECT to minimize medication adverse effects, but Mr. B’s medical condition is too tenuous.
Continue to: On Day 7...
On Day 7, lorazepam is decreased to 0.5 mg/0.5 mg/1 mg IV. On Day 9, it is further decreased to 0.5 mg IV 3 times a day because Mr. B appears to be more delirious. On Day 10, lorazepam is increased to 1 mg IV 3 times a day, and oral haloperidol, 2 mg as needed for agitation, is restarted after multiple nights when Mr. B had behavioral emergencies and was treated with IM haloperidol and lorazepam. On Day 11, lorazepam is decreased and switched from IV formulation to oral, 0.5 mg 3 times a day. On Day 13, oral haloperidol is increased to 2 mg twice a day because of overnight behavioral emergencies requiring treatment with IV haloperidol, 4 mg. On Day 17, oral haloperidol is increased to 2 mg in the morning and 3 mg every night at bedtime because Mr. B has increased morning agitation. On Day 19, oral lorazepam is increased to 1 mg 3 times a day because Mr. B appears more catatonic. On Day 21, oral haloperidol is consolidated to 5 mg every night at bedtime. On Day 31, oral lorazepam is increased to 2 mg/1 mg/1 mg because he appears more catatonic with increased stuttering and mannerisms. On Day 33, oral haloperidol is increased to 6 mg every night at bedtime because Mr. B has morning agitation.
Multiple lorazepam and haloperidol dose adjustments are needed to balance the situation: combating catatonia, addressing delirium, managing schizophrenia symptoms, and improving Mr. B’s cardiac status. Finally, Mr. B is stabilized on oral lorazepam, 2 mg every morning, 1 mg every day at noon, and 1 mg every day at bedtime, and oral haloperidol, 6 mg every day at bedtime. This regimen, Mr. B has a BFCRS score of 1 (Table 2) and returns to his baseline mental status.
The authors’ observations
Delirium and catatonia typically have different treatments. Delirium is routinely treated by addressing the underlying medical and environmental factors, and managing comorbid symptoms such as agitation and disturbing hallucinations by prescribing antipsychotics, restoring the sleep-wake cycle with melatonin, initiating nonpharmacologic behavioral management, and avoiding deliriogenic medications such as benzodiazepines, opioids, and steroids.10 Catatonia is managed by prescribing benzodiazepines (with or without ECT) and by avoiding dopamine antagonists such as antipsychotics and metoclopramide (which may worsen catatonia or precipitate malignant catatonia).
The first-line treatment for catatonia is benzodiazepines, with IV preferred over IM, sublingual, or oral formulations. Electroconvulsive therapy is commonly used with benzodiazepines and is effective in 85% to 90% of patients. For ECT, bitemporal placement and daily treatment with brief pulses are frequently used. It is also effective in 60% of patients who fail to respond to benzodiazepines. Thus, ECT should be considered within the first 48 to 72 hours of benzodiazepine failure.7
Amantadine, a NMDA antagonist, may be a possible treatment for catatonia. A case report published in 1986 described a patient who developed catatonia after the abrupt withdrawal of amantadine during neuroleptic therapy.11 Memantine also may serve as a treatment for catatonia through glutamate antagonism. A review identified 25 cases of patients with catatonia who were treated with amantadine or memantine.12 Oral amantadine was administered at 100 to 400 mg/d in divided doses, with lower doses for patients with diminished renal function.12 Memantine was administered at 5 to 20 mg/d.12 All patients showed improvement after 1 to 7 days of treatment.12 Thus, memantine may be considered for patients with catatonic schizophrenia or comorbid catatonia and delirium. Although memantine was not considered in Mr. B’s case, he would have been a good candidate for treatment with this agent.
Continue to: There are also case reports of...
There are also case reports of aripiprazole being used for catatonia in the context of psychosis or delirium in both adults and adolescents.13-15 Other medications used in case reports for treating catatonia include carbamazepine, valproate, and secondgeneration antipsychotics.7
Because most of the literature on pharmacotherapy for catatonia consists of case reports or small case series, further research on medication management of catatonia and delirium is needed to guide treatment.
OUTCOME Multiple rehospitalizations
On Day 57, Mr. B is discharged to a skilled nursing facility due to significant deconditioning. He is discharged with continued follow-up with his ACT psychiatrist and nurse. Mr. B’s catatonia remains resolved; however, he is unable to be safely managed at the skilled nursing facility.
During the next 7 months, he is readmitted to the ICU for acute on chronic hypoxic respiratory failure 5 times; his rehospitalizations are complicated by delirium due to cardiogenic shock and urosepsis. Mild hyperactive delirium re-emerges after worsening respiratory failure and contributes to falls in the skilled nursing facility.
Six months later, Mr. B continues to receive the initial hospital discharge lorazepam regimen of 2 mg every morning, 1 mg every day at noon, and 1 mg every night at bedtime. The Psychiatry team slowly tapers this to 0.5 mg twice daily.
Continue to: On Day 5...
On Day 5 of Mr. B’s fifth hospital readmission, based on his advance directive, Mr. B’s family implements the do-not-resuscitate and do-not-intubate orders. He is transitioned to comfort measures, and dies on Day 6 with his brother and the hospital chaplain present.
Bottom Line
Delirium and catatonia share signs and symptoms, yet rarely are diagnosed at the same time. Both conditions result in significant morbidity and mortality. An emerging literature supports the concurrence of these 2 syndromes and aids in their diagnosis and treatment. Comorbidity with other medical conditions, common with both delirium and catatonia, substantially complicates treatment; thus, additional research into new treatment approaches is critical.
Related Resources
- Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
- Catatonia Information Center. Penn State University. http://catatonia.org/.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Metoclopramide • Reglan
Mirtazapine • Remeron
Risperidone • Risperdal
Topiramate • Topamax
Trazodone • Desyrel
Valproate • Depacon, Depakene, Depakote
1. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555-577; discussion 578-604.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
6. Wilson JE, Carlson R, Duggan MC. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
7. Fricchione GL, Gross AF, Huffman JC, et al. Chapter 21: Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fricchione GL, Cassem NH, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 6th Ed. Philadelphia, PA: Saunders Elsevier; 2010:273-288.
8. Van der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94-101.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164 (1-3):256-262.
10. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
11. Brown CS, Wittkowsky AK, Bryant SG. Neurolepticinduced catatonia after abrupt withdrawal of amantadine during neuroleptic therapy. Pharmacotherapy. 1986;6(4):193-195.
12. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
13. Huffman JC, Fricchione GL. Catatonia and psychosis in a patient with AIDS: treatment with lorazepam and aripiprazole. J Clin Psychopharmacol. 2005;25(5):508-510.
14. Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517.
15. Voros V, Kovacs A, Herold R, et al. Effectiveness of intramuscular aripiprazole injection in patients with catatonia: report on three cases. Pharmacopsychiatry. 2009;42(6):286-287.
CASE Irritable and short of breath
Mr. B, age 75, who lives alone, is brought to the emergency department (ED) for evaluation of shortness of breath. Mr. B is normally highly independent, and is able to drive, manage his own finances, attend to activities of daily living, and participate in social functions at church. On the day before he was taken to the ED, his home nurse had come to his home to dispense medications and found Mr. B was irritable, verbally rude, and repeatedly scratching the right side of his head. The nurse was unsure if Mr. B had taken his medications over the weekend. She called for emergency services, but Mr. B refused to go to the ED, and he was able to decline care because he was not in an acute medical emergency (95% oxygen on pulse oximetry).
The next day, when Mr. B’s nurse returned to his home, she found him to be tachypneic and verbigerating the phrase “I don’t know.” She contacted emergency services again, and Mr. B was taken to the ED.
In the ED, Mr. B has tachycardia, tachypnea, increased work of breathing, and diffuse rhonchi. He continues to repeat the phrase “I don’t know” and scratches the right side of his head repeatedly. The ED clinicians consult Psychiatry due to Mr. B’s confusion and because his nurse reports that his presentation is similar to a previous psychiatric hospitalization 9 years earlier.
[polldaddy:10332862]
EVALUATION Complex comorbidities
Mr. B has a lengthy history of schizophrenia, chronic right-sided heart failure secondary to pulmonary hypertension, moderate chronic obstructive pulmonary disease, hypertension, type 2 diabetes mellitus, and prostatic adenocarcinoma after external beam radiation therapy.
His symptoms of schizophrenia had been stable on his long-standing outpatient psychotropic regimen of haloperidol, 5 mg nightly; mirtazapine, 15 mg nightly, for appetite stimulation and insomnia; and trazodone, 100 mg nightly for insomnia. Mr. B has been receiving assertive community treatment (ACT) psychiatric services for schizophrenia; a nurse refills his pill box with his medications weekly. He does not have a history of medication nonadherence, and his nurse did not think he had missed any doses before the weekend.
He has acute changes in depressed mood, perseveration, and a Mini-Mental State Examination (MMSE) score of 26 (missing points for delayed recall and inability to construct a sentence), which indicates a cognitive assessment score on the low end of the normal range for people with at least an eighth grade education.
At the hospital, the psychiatrist diagnoses hypoactive delirium due to Mr. B’s fluctuating attention and disorientation. She also recommends that Mr. B continue his outpatient psychotropic regimen, and adds oral haloperidol, 5 mg, as needed for agitation (his QTc interval is 451 ms; reference range for men <430 ms, borderline prolonged 431 to 450 ms, prolonged >450 ms).
Continue to: An initial laboratory workup...
An initial laboratory workup and electrocardiogram reveal that Mr. B has an elevated troponin level (0.21 ng/mL; reference range <0.04; 0.04 to 0.39 ng/mL is elevated above the 99th percentile of a healthy population), non-ST-elevation myocardial infarction type II, Q waves in lead III, arteriovenous fistula with right axis deviation, acute on chronic kidney failure (creatinine level of 2.1 mg/dL, up from baseline of 1.4 mg/dL; reference range 0.84 to 1.21 mg/dL), elevated brain natriuretic peptide (111 pg/mL; reference range <125 pg/mL), and an elevated lactate level of 5.51 mmol/L (reference range 0.5 to 1 mmol/L). He also has a mixed respiratory alkalosis and metabolic acidosis with increased anion gap, transaminitis (aspartate aminotransferase 149 U/L; reference range 10 to 40 U/L), and elevated alkaline phosphatase (151 IU/L; reference range 44 to 147 IU/L). Urinalysis shows moderate ketones and is negative for nitrite or leukocyte esterase.
A brain CT rules out stroke. A chest X-ray shows subtle left basilar reticular opacity with a follow-up lateral view showing no consolidation and prominent pulmonary vasculature without overt edema.
In the ED, Mr. B is determined to have decision-making capacity and is able to authorize all treatment. Cardiology is also consulted, and Mr. B is admitted to the cardiac intensive care unit (CCU) for cardiogenic shock with close cardiac monitoring.
The Psychiatry and Cardiology teams discuss the risks and benefits of continuing antipsychotics. Due to the imminent risk of harm to Mr. B because of his significant agitation in the ED, which required treatment with one dose of IM haloperidol, 5 mg, and lorazepam, 2 mg, and close monitoring, the teams agree that the benefits of continuing haloperidol outweigh the risks.
On hospital Day 2, Mr. B’s repetitive scratching resolves. He is moved from the CCU to a general medical unit, where he begins to have episodes of mutism and negativism. By hospital Day 6, catatonia is suspected due to a MMSE of 6/30 and a Bush- Francis Catatonia Rating Scale (BFCRS) score of 14 for predominant stereotypy, perseveration, and withdrawal (Table 1). The teams determine that Mr. B lacks decisionmaking capacity due to his inability to rationally manipulate information. His brother is contacted and authorizes all treatment, deferring decision-making to the medical teams caring for Mr. B.

Continue to: Mr. B undergoes an EEG...
Mr. B undergoes an EEG, which rules out nonconvulsive status epilepticus and is consistent with encephalopathy/delirium. Neuroleptic malignant syndrome (NMS) is considered but is less likely because Mr. B had been receiving a stable dose of haloperidol for several years, is afebrile, has stable vital signs, has no muscle rigidity, and no evidence of leukocytosis, creatine kinase elevation, myoglobinuria, hyperkalemia, hyperphosphatemia, thrombocytosis, or hypocalcemia.
Based on these clinical findings, Mr. B is diagnosed with catatonia and delirium.
The authors’ observations
Delirium, characterized by inattention and changes in mental status, is a syndrome due to acute brain dysfunction. It can be subclassified as hyperactive or hypoactive based on the change of activity. Simple catatonia is characterized by changes in behavior, affect, and motor function (with hyper- or hypoactivity). It may arise from gammaaminobutyric acid hypoactivity, dopamine (D2) hypoactivity, and possibly glutamate N-methyl-d-aspartate (NMDA) hyperactivity.1 Malignant catatonia is simple catatonia combined with autonomic instability and hyperthermia, which is a life-threatening condition. The BFCRS is commonly used to assess symptoms.2
Both catatonia and delirium result in significant morbidity and mortality. The 2 conditions share signs and symptoms yet rarely are diagnosed at the same time. DSM-IV, DSM-IV-TR, and DSM-5 state that a diagnosis of catatonia due to another medical condition cannot be made exclusively in the presence of delirium.3,4 DSM-IV and DSM-IV-TR required at least 2 criteria from 5 areas, including motoric immobility, excessive motor activity, extreme negativism or mutism, peculiarities of voluntary movement, and echolalia or echopraxia. Instead of grouping symptoms into clusters, DSM-5 requires 3 criteria of 12 individual symptoms.3,4 A co-occurrence with a medical illness precludes using the DSM-5 “catatonia associated with another mental disorder (catatonia specifier)” with the “unspecified catatonia” diagnosis category.4
However, a growing body of literature suggests that delirium and catatonia can cooccur.5,6 In 2017, Wilson et al6 found that of 136 critically ill patients in the ICU, 43% (58 patients) had only delirium, 3% (4 patients) had only catatonia, 31% (42 patients) had both, and 24% (32 patients) had neither. In patients with both catatonia and delirium, the most common signs of catatonia were autonomic abnormalities (96%), immobility/ stupor (87%), staring (77%), mutism (60%), and posturing (60%).
Continue to: The differential diagnosis...
The differential diagnosis of catatonia is extensive and varied.3,4 The most common psychiatric causes are mood disorders (13% to 31%) and psychotic disorders (7% to 17%).7 Neuromedical etiologies account for 4% to 46% of cases.7 The most common medical and neurologic causes are seizure disorder, acute intermittent porphyria, systemic lupus erythematosus, and drugrelated adverse effects (particularly due to clozapine withdrawal, risperidone, and phencyclidine).7
A workup that includes physical examination, laboratory testing, and neuroimaging can be helpful to identify delirium and catatonia, but there is limited literature to guide identifying coexisting delirium and catatonia other than a blend of physical exam findings of delirium and catatonia. Electroencephalogram may be normal in primary catatonia or may show nonspecific changes in secondary catatonia.8 Additionally, discharges in the frontal lobes and anterior limbic systems with diffuse background slowing and dysrhythmic patterns may be seen.7 Neuroimaging with MRI can help to evaluate catatonia.9 Laboratory testing such as creatine phosphokinase levels can be high in simple catatonia and are often elevated in malignant catatonia.7 Considering the possible co-occurrence of delirium and catatonia is critical to providing good patient care because the 2 conditions are treated differently.
[polldaddy:10332867]
TREATMENT A balancing act
Over the next month, Mr. B alternates between appearing catatonic or delirious. When he appears more catatonic, the dose of lorazepam is increased, which results in increased impulsivity and agitation and leads to multiple interventions from the behavioral emergency response team. At times, the team must use restraints and haloperidol because Mr. B pulls out IV lines and is considered at high risk for falls. When Mr. B appears more delirious and the dose of lorazepam is decreased, he becomes more catatonic.
Following the diagnosis of catatonia on Day 6, oral haloperidol is discontinued to further mitigate Mr. B’s risk of developing NMS. On hospital Day 6, Mr. B improves significantly after a 2-mg IV lorazepam challenge, with a BFCRS score of 6. At this point, he is started on lorazepam, 1 mg IV 3 times a day.
On Day 7, based on the complicated nature of Mr. B’s medical and psychiatric comorbidities, the treatment team considers ECT to minimize medication adverse effects, but Mr. B’s medical condition is too tenuous.
Continue to: On Day 7...
On Day 7, lorazepam is decreased to 0.5 mg/0.5 mg/1 mg IV. On Day 9, it is further decreased to 0.5 mg IV 3 times a day because Mr. B appears to be more delirious. On Day 10, lorazepam is increased to 1 mg IV 3 times a day, and oral haloperidol, 2 mg as needed for agitation, is restarted after multiple nights when Mr. B had behavioral emergencies and was treated with IM haloperidol and lorazepam. On Day 11, lorazepam is decreased and switched from IV formulation to oral, 0.5 mg 3 times a day. On Day 13, oral haloperidol is increased to 2 mg twice a day because of overnight behavioral emergencies requiring treatment with IV haloperidol, 4 mg. On Day 17, oral haloperidol is increased to 2 mg in the morning and 3 mg every night at bedtime because Mr. B has increased morning agitation. On Day 19, oral lorazepam is increased to 1 mg 3 times a day because Mr. B appears more catatonic. On Day 21, oral haloperidol is consolidated to 5 mg every night at bedtime. On Day 31, oral lorazepam is increased to 2 mg/1 mg/1 mg because he appears more catatonic with increased stuttering and mannerisms. On Day 33, oral haloperidol is increased to 6 mg every night at bedtime because Mr. B has morning agitation.
Multiple lorazepam and haloperidol dose adjustments are needed to balance the situation: combating catatonia, addressing delirium, managing schizophrenia symptoms, and improving Mr. B’s cardiac status. Finally, Mr. B is stabilized on oral lorazepam, 2 mg every morning, 1 mg every day at noon, and 1 mg every day at bedtime, and oral haloperidol, 6 mg every day at bedtime. This regimen, Mr. B has a BFCRS score of 1 (Table 2) and returns to his baseline mental status.
The authors’ observations
Delirium and catatonia typically have different treatments. Delirium is routinely treated by addressing the underlying medical and environmental factors, and managing comorbid symptoms such as agitation and disturbing hallucinations by prescribing antipsychotics, restoring the sleep-wake cycle with melatonin, initiating nonpharmacologic behavioral management, and avoiding deliriogenic medications such as benzodiazepines, opioids, and steroids.10 Catatonia is managed by prescribing benzodiazepines (with or without ECT) and by avoiding dopamine antagonists such as antipsychotics and metoclopramide (which may worsen catatonia or precipitate malignant catatonia).
The first-line treatment for catatonia is benzodiazepines, with IV preferred over IM, sublingual, or oral formulations. Electroconvulsive therapy is commonly used with benzodiazepines and is effective in 85% to 90% of patients. For ECT, bitemporal placement and daily treatment with brief pulses are frequently used. It is also effective in 60% of patients who fail to respond to benzodiazepines. Thus, ECT should be considered within the first 48 to 72 hours of benzodiazepine failure.7
Amantadine, a NMDA antagonist, may be a possible treatment for catatonia. A case report published in 1986 described a patient who developed catatonia after the abrupt withdrawal of amantadine during neuroleptic therapy.11 Memantine also may serve as a treatment for catatonia through glutamate antagonism. A review identified 25 cases of patients with catatonia who were treated with amantadine or memantine.12 Oral amantadine was administered at 100 to 400 mg/d in divided doses, with lower doses for patients with diminished renal function.12 Memantine was administered at 5 to 20 mg/d.12 All patients showed improvement after 1 to 7 days of treatment.12 Thus, memantine may be considered for patients with catatonic schizophrenia or comorbid catatonia and delirium. Although memantine was not considered in Mr. B’s case, he would have been a good candidate for treatment with this agent.
Continue to: There are also case reports of...
There are also case reports of aripiprazole being used for catatonia in the context of psychosis or delirium in both adults and adolescents.13-15 Other medications used in case reports for treating catatonia include carbamazepine, valproate, and secondgeneration antipsychotics.7
Because most of the literature on pharmacotherapy for catatonia consists of case reports or small case series, further research on medication management of catatonia and delirium is needed to guide treatment.
OUTCOME Multiple rehospitalizations
On Day 57, Mr. B is discharged to a skilled nursing facility due to significant deconditioning. He is discharged with continued follow-up with his ACT psychiatrist and nurse. Mr. B’s catatonia remains resolved; however, he is unable to be safely managed at the skilled nursing facility.
During the next 7 months, he is readmitted to the ICU for acute on chronic hypoxic respiratory failure 5 times; his rehospitalizations are complicated by delirium due to cardiogenic shock and urosepsis. Mild hyperactive delirium re-emerges after worsening respiratory failure and contributes to falls in the skilled nursing facility.
Six months later, Mr. B continues to receive the initial hospital discharge lorazepam regimen of 2 mg every morning, 1 mg every day at noon, and 1 mg every night at bedtime. The Psychiatry team slowly tapers this to 0.5 mg twice daily.
Continue to: On Day 5...
On Day 5 of Mr. B’s fifth hospital readmission, based on his advance directive, Mr. B’s family implements the do-not-resuscitate and do-not-intubate orders. He is transitioned to comfort measures, and dies on Day 6 with his brother and the hospital chaplain present.
Bottom Line
Delirium and catatonia share signs and symptoms, yet rarely are diagnosed at the same time. Both conditions result in significant morbidity and mortality. An emerging literature supports the concurrence of these 2 syndromes and aids in their diagnosis and treatment. Comorbidity with other medical conditions, common with both delirium and catatonia, substantially complicates treatment; thus, additional research into new treatment approaches is critical.
Related Resources
- Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
- Catatonia Information Center. Penn State University. http://catatonia.org/.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Metoclopramide • Reglan
Mirtazapine • Remeron
Risperidone • Risperdal
Topiramate • Topamax
Trazodone • Desyrel
Valproate • Depacon, Depakene, Depakote
CASE Irritable and short of breath
Mr. B, age 75, who lives alone, is brought to the emergency department (ED) for evaluation of shortness of breath. Mr. B is normally highly independent, and is able to drive, manage his own finances, attend to activities of daily living, and participate in social functions at church. On the day before he was taken to the ED, his home nurse had come to his home to dispense medications and found Mr. B was irritable, verbally rude, and repeatedly scratching the right side of his head. The nurse was unsure if Mr. B had taken his medications over the weekend. She called for emergency services, but Mr. B refused to go to the ED, and he was able to decline care because he was not in an acute medical emergency (95% oxygen on pulse oximetry).
The next day, when Mr. B’s nurse returned to his home, she found him to be tachypneic and verbigerating the phrase “I don’t know.” She contacted emergency services again, and Mr. B was taken to the ED.
In the ED, Mr. B has tachycardia, tachypnea, increased work of breathing, and diffuse rhonchi. He continues to repeat the phrase “I don’t know” and scratches the right side of his head repeatedly. The ED clinicians consult Psychiatry due to Mr. B’s confusion and because his nurse reports that his presentation is similar to a previous psychiatric hospitalization 9 years earlier.
[polldaddy:10332862]
EVALUATION Complex comorbidities
Mr. B has a lengthy history of schizophrenia, chronic right-sided heart failure secondary to pulmonary hypertension, moderate chronic obstructive pulmonary disease, hypertension, type 2 diabetes mellitus, and prostatic adenocarcinoma after external beam radiation therapy.
His symptoms of schizophrenia had been stable on his long-standing outpatient psychotropic regimen of haloperidol, 5 mg nightly; mirtazapine, 15 mg nightly, for appetite stimulation and insomnia; and trazodone, 100 mg nightly for insomnia. Mr. B has been receiving assertive community treatment (ACT) psychiatric services for schizophrenia; a nurse refills his pill box with his medications weekly. He does not have a history of medication nonadherence, and his nurse did not think he had missed any doses before the weekend.
He has acute changes in depressed mood, perseveration, and a Mini-Mental State Examination (MMSE) score of 26 (missing points for delayed recall and inability to construct a sentence), which indicates a cognitive assessment score on the low end of the normal range for people with at least an eighth grade education.
At the hospital, the psychiatrist diagnoses hypoactive delirium due to Mr. B’s fluctuating attention and disorientation. She also recommends that Mr. B continue his outpatient psychotropic regimen, and adds oral haloperidol, 5 mg, as needed for agitation (his QTc interval is 451 ms; reference range for men <430 ms, borderline prolonged 431 to 450 ms, prolonged >450 ms).
Continue to: An initial laboratory workup...
An initial laboratory workup and electrocardiogram reveal that Mr. B has an elevated troponin level (0.21 ng/mL; reference range <0.04; 0.04 to 0.39 ng/mL is elevated above the 99th percentile of a healthy population), non-ST-elevation myocardial infarction type II, Q waves in lead III, arteriovenous fistula with right axis deviation, acute on chronic kidney failure (creatinine level of 2.1 mg/dL, up from baseline of 1.4 mg/dL; reference range 0.84 to 1.21 mg/dL), elevated brain natriuretic peptide (111 pg/mL; reference range <125 pg/mL), and an elevated lactate level of 5.51 mmol/L (reference range 0.5 to 1 mmol/L). He also has a mixed respiratory alkalosis and metabolic acidosis with increased anion gap, transaminitis (aspartate aminotransferase 149 U/L; reference range 10 to 40 U/L), and elevated alkaline phosphatase (151 IU/L; reference range 44 to 147 IU/L). Urinalysis shows moderate ketones and is negative for nitrite or leukocyte esterase.
A brain CT rules out stroke. A chest X-ray shows subtle left basilar reticular opacity with a follow-up lateral view showing no consolidation and prominent pulmonary vasculature without overt edema.
In the ED, Mr. B is determined to have decision-making capacity and is able to authorize all treatment. Cardiology is also consulted, and Mr. B is admitted to the cardiac intensive care unit (CCU) for cardiogenic shock with close cardiac monitoring.
The Psychiatry and Cardiology teams discuss the risks and benefits of continuing antipsychotics. Due to the imminent risk of harm to Mr. B because of his significant agitation in the ED, which required treatment with one dose of IM haloperidol, 5 mg, and lorazepam, 2 mg, and close monitoring, the teams agree that the benefits of continuing haloperidol outweigh the risks.
On hospital Day 2, Mr. B’s repetitive scratching resolves. He is moved from the CCU to a general medical unit, where he begins to have episodes of mutism and negativism. By hospital Day 6, catatonia is suspected due to a MMSE of 6/30 and a Bush- Francis Catatonia Rating Scale (BFCRS) score of 14 for predominant stereotypy, perseveration, and withdrawal (Table 1). The teams determine that Mr. B lacks decisionmaking capacity due to his inability to rationally manipulate information. His brother is contacted and authorizes all treatment, deferring decision-making to the medical teams caring for Mr. B.

Continue to: Mr. B undergoes an EEG...
Mr. B undergoes an EEG, which rules out nonconvulsive status epilepticus and is consistent with encephalopathy/delirium. Neuroleptic malignant syndrome (NMS) is considered but is less likely because Mr. B had been receiving a stable dose of haloperidol for several years, is afebrile, has stable vital signs, has no muscle rigidity, and no evidence of leukocytosis, creatine kinase elevation, myoglobinuria, hyperkalemia, hyperphosphatemia, thrombocytosis, or hypocalcemia.
Based on these clinical findings, Mr. B is diagnosed with catatonia and delirium.
The authors’ observations
Delirium, characterized by inattention and changes in mental status, is a syndrome due to acute brain dysfunction. It can be subclassified as hyperactive or hypoactive based on the change of activity. Simple catatonia is characterized by changes in behavior, affect, and motor function (with hyper- or hypoactivity). It may arise from gammaaminobutyric acid hypoactivity, dopamine (D2) hypoactivity, and possibly glutamate N-methyl-d-aspartate (NMDA) hyperactivity.1 Malignant catatonia is simple catatonia combined with autonomic instability and hyperthermia, which is a life-threatening condition. The BFCRS is commonly used to assess symptoms.2
Both catatonia and delirium result in significant morbidity and mortality. The 2 conditions share signs and symptoms yet rarely are diagnosed at the same time. DSM-IV, DSM-IV-TR, and DSM-5 state that a diagnosis of catatonia due to another medical condition cannot be made exclusively in the presence of delirium.3,4 DSM-IV and DSM-IV-TR required at least 2 criteria from 5 areas, including motoric immobility, excessive motor activity, extreme negativism or mutism, peculiarities of voluntary movement, and echolalia or echopraxia. Instead of grouping symptoms into clusters, DSM-5 requires 3 criteria of 12 individual symptoms.3,4 A co-occurrence with a medical illness precludes using the DSM-5 “catatonia associated with another mental disorder (catatonia specifier)” with the “unspecified catatonia” diagnosis category.4
However, a growing body of literature suggests that delirium and catatonia can cooccur.5,6 In 2017, Wilson et al6 found that of 136 critically ill patients in the ICU, 43% (58 patients) had only delirium, 3% (4 patients) had only catatonia, 31% (42 patients) had both, and 24% (32 patients) had neither. In patients with both catatonia and delirium, the most common signs of catatonia were autonomic abnormalities (96%), immobility/ stupor (87%), staring (77%), mutism (60%), and posturing (60%).
Continue to: The differential diagnosis...
The differential diagnosis of catatonia is extensive and varied.3,4 The most common psychiatric causes are mood disorders (13% to 31%) and psychotic disorders (7% to 17%).7 Neuromedical etiologies account for 4% to 46% of cases.7 The most common medical and neurologic causes are seizure disorder, acute intermittent porphyria, systemic lupus erythematosus, and drugrelated adverse effects (particularly due to clozapine withdrawal, risperidone, and phencyclidine).7
A workup that includes physical examination, laboratory testing, and neuroimaging can be helpful to identify delirium and catatonia, but there is limited literature to guide identifying coexisting delirium and catatonia other than a blend of physical exam findings of delirium and catatonia. Electroencephalogram may be normal in primary catatonia or may show nonspecific changes in secondary catatonia.8 Additionally, discharges in the frontal lobes and anterior limbic systems with diffuse background slowing and dysrhythmic patterns may be seen.7 Neuroimaging with MRI can help to evaluate catatonia.9 Laboratory testing such as creatine phosphokinase levels can be high in simple catatonia and are often elevated in malignant catatonia.7 Considering the possible co-occurrence of delirium and catatonia is critical to providing good patient care because the 2 conditions are treated differently.
[polldaddy:10332867]
TREATMENT A balancing act
Over the next month, Mr. B alternates between appearing catatonic or delirious. When he appears more catatonic, the dose of lorazepam is increased, which results in increased impulsivity and agitation and leads to multiple interventions from the behavioral emergency response team. At times, the team must use restraints and haloperidol because Mr. B pulls out IV lines and is considered at high risk for falls. When Mr. B appears more delirious and the dose of lorazepam is decreased, he becomes more catatonic.
Following the diagnosis of catatonia on Day 6, oral haloperidol is discontinued to further mitigate Mr. B’s risk of developing NMS. On hospital Day 6, Mr. B improves significantly after a 2-mg IV lorazepam challenge, with a BFCRS score of 6. At this point, he is started on lorazepam, 1 mg IV 3 times a day.
On Day 7, based on the complicated nature of Mr. B’s medical and psychiatric comorbidities, the treatment team considers ECT to minimize medication adverse effects, but Mr. B’s medical condition is too tenuous.
Continue to: On Day 7...
On Day 7, lorazepam is decreased to 0.5 mg/0.5 mg/1 mg IV. On Day 9, it is further decreased to 0.5 mg IV 3 times a day because Mr. B appears to be more delirious. On Day 10, lorazepam is increased to 1 mg IV 3 times a day, and oral haloperidol, 2 mg as needed for agitation, is restarted after multiple nights when Mr. B had behavioral emergencies and was treated with IM haloperidol and lorazepam. On Day 11, lorazepam is decreased and switched from IV formulation to oral, 0.5 mg 3 times a day. On Day 13, oral haloperidol is increased to 2 mg twice a day because of overnight behavioral emergencies requiring treatment with IV haloperidol, 4 mg. On Day 17, oral haloperidol is increased to 2 mg in the morning and 3 mg every night at bedtime because Mr. B has increased morning agitation. On Day 19, oral lorazepam is increased to 1 mg 3 times a day because Mr. B appears more catatonic. On Day 21, oral haloperidol is consolidated to 5 mg every night at bedtime. On Day 31, oral lorazepam is increased to 2 mg/1 mg/1 mg because he appears more catatonic with increased stuttering and mannerisms. On Day 33, oral haloperidol is increased to 6 mg every night at bedtime because Mr. B has morning agitation.
Multiple lorazepam and haloperidol dose adjustments are needed to balance the situation: combating catatonia, addressing delirium, managing schizophrenia symptoms, and improving Mr. B’s cardiac status. Finally, Mr. B is stabilized on oral lorazepam, 2 mg every morning, 1 mg every day at noon, and 1 mg every day at bedtime, and oral haloperidol, 6 mg every day at bedtime. This regimen, Mr. B has a BFCRS score of 1 (Table 2) and returns to his baseline mental status.
The authors’ observations
Delirium and catatonia typically have different treatments. Delirium is routinely treated by addressing the underlying medical and environmental factors, and managing comorbid symptoms such as agitation and disturbing hallucinations by prescribing antipsychotics, restoring the sleep-wake cycle with melatonin, initiating nonpharmacologic behavioral management, and avoiding deliriogenic medications such as benzodiazepines, opioids, and steroids.10 Catatonia is managed by prescribing benzodiazepines (with or without ECT) and by avoiding dopamine antagonists such as antipsychotics and metoclopramide (which may worsen catatonia or precipitate malignant catatonia).
The first-line treatment for catatonia is benzodiazepines, with IV preferred over IM, sublingual, or oral formulations. Electroconvulsive therapy is commonly used with benzodiazepines and is effective in 85% to 90% of patients. For ECT, bitemporal placement and daily treatment with brief pulses are frequently used. It is also effective in 60% of patients who fail to respond to benzodiazepines. Thus, ECT should be considered within the first 48 to 72 hours of benzodiazepine failure.7
Amantadine, a NMDA antagonist, may be a possible treatment for catatonia. A case report published in 1986 described a patient who developed catatonia after the abrupt withdrawal of amantadine during neuroleptic therapy.11 Memantine also may serve as a treatment for catatonia through glutamate antagonism. A review identified 25 cases of patients with catatonia who were treated with amantadine or memantine.12 Oral amantadine was administered at 100 to 400 mg/d in divided doses, with lower doses for patients with diminished renal function.12 Memantine was administered at 5 to 20 mg/d.12 All patients showed improvement after 1 to 7 days of treatment.12 Thus, memantine may be considered for patients with catatonic schizophrenia or comorbid catatonia and delirium. Although memantine was not considered in Mr. B’s case, he would have been a good candidate for treatment with this agent.
Continue to: There are also case reports of...
There are also case reports of aripiprazole being used for catatonia in the context of psychosis or delirium in both adults and adolescents.13-15 Other medications used in case reports for treating catatonia include carbamazepine, valproate, and secondgeneration antipsychotics.7
Because most of the literature on pharmacotherapy for catatonia consists of case reports or small case series, further research on medication management of catatonia and delirium is needed to guide treatment.
OUTCOME Multiple rehospitalizations
On Day 57, Mr. B is discharged to a skilled nursing facility due to significant deconditioning. He is discharged with continued follow-up with his ACT psychiatrist and nurse. Mr. B’s catatonia remains resolved; however, he is unable to be safely managed at the skilled nursing facility.
During the next 7 months, he is readmitted to the ICU for acute on chronic hypoxic respiratory failure 5 times; his rehospitalizations are complicated by delirium due to cardiogenic shock and urosepsis. Mild hyperactive delirium re-emerges after worsening respiratory failure and contributes to falls in the skilled nursing facility.
Six months later, Mr. B continues to receive the initial hospital discharge lorazepam regimen of 2 mg every morning, 1 mg every day at noon, and 1 mg every night at bedtime. The Psychiatry team slowly tapers this to 0.5 mg twice daily.
Continue to: On Day 5...
On Day 5 of Mr. B’s fifth hospital readmission, based on his advance directive, Mr. B’s family implements the do-not-resuscitate and do-not-intubate orders. He is transitioned to comfort measures, and dies on Day 6 with his brother and the hospital chaplain present.
Bottom Line
Delirium and catatonia share signs and symptoms, yet rarely are diagnosed at the same time. Both conditions result in significant morbidity and mortality. An emerging literature supports the concurrence of these 2 syndromes and aids in their diagnosis and treatment. Comorbidity with other medical conditions, common with both delirium and catatonia, substantially complicates treatment; thus, additional research into new treatment approaches is critical.
Related Resources
- Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
- Catatonia Information Center. Penn State University. http://catatonia.org/.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Metoclopramide • Reglan
Mirtazapine • Remeron
Risperidone • Risperdal
Topiramate • Topamax
Trazodone • Desyrel
Valproate • Depacon, Depakene, Depakote
1. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555-577; discussion 578-604.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
6. Wilson JE, Carlson R, Duggan MC. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
7. Fricchione GL, Gross AF, Huffman JC, et al. Chapter 21: Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fricchione GL, Cassem NH, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 6th Ed. Philadelphia, PA: Saunders Elsevier; 2010:273-288.
8. Van der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94-101.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164 (1-3):256-262.
10. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
11. Brown CS, Wittkowsky AK, Bryant SG. Neurolepticinduced catatonia after abrupt withdrawal of amantadine during neuroleptic therapy. Pharmacotherapy. 1986;6(4):193-195.
12. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
13. Huffman JC, Fricchione GL. Catatonia and psychosis in a patient with AIDS: treatment with lorazepam and aripiprazole. J Clin Psychopharmacol. 2005;25(5):508-510.
14. Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517.
15. Voros V, Kovacs A, Herold R, et al. Effectiveness of intramuscular aripiprazole injection in patients with catatonia: report on three cases. Pharmacopsychiatry. 2009;42(6):286-287.
1. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555-577; discussion 578-604.
2. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
3. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
6. Wilson JE, Carlson R, Duggan MC. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837-1844.
7. Fricchione GL, Gross AF, Huffman JC, et al. Chapter 21: Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fricchione GL, Cassem NH, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 6th Ed. Philadelphia, PA: Saunders Elsevier; 2010:273-288.
8. Van der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147(1):94-101.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164 (1-3):256-262.
10. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
11. Brown CS, Wittkowsky AK, Bryant SG. Neurolepticinduced catatonia after abrupt withdrawal of amantadine during neuroleptic therapy. Pharmacotherapy. 1986;6(4):193-195.
12. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
13. Huffman JC, Fricchione GL. Catatonia and psychosis in a patient with AIDS: treatment with lorazepam and aripiprazole. J Clin Psychopharmacol. 2005;25(5):508-510.
14. Roberto AJ, Pinnaka S, Mohan A, et al. Adolescent catatonia successfully treated with lorazepam and aripiprazole. Case Rep Psychiatry. 2014;2014:309517.
15. Voros V, Kovacs A, Herold R, et al. Effectiveness of intramuscular aripiprazole injection in patients with catatonia: report on three cases. Pharmacopsychiatry. 2009;42(6):286-287.
Psychiatry and neurology, more
Dr. Nasrallah’s “Psychiatry and neurology: Sister neuroscience specialties with different approaches to the brain” (From the Editor,
In mathematics, chaos theory deals with the impossible complexity of simplicity. From primitive initial states, self-interacting systems give rise to short-term predictability, but an unpredictable long-term. Classically, this is illustrated as a hurricane born from the flapping of a butterfly’s wings. Neurology has found great clinical utility in understanding butterfly wings. However, psychiatry forsakes simplicity for complexity: it dives into the emergent systems that arise from self-interacting neurons, asking us to stand within the eye of the hurricane and understand it in its entirety. Psychiatry asks us to transcend the traditional medical focus of discrete physiological mechanisms, and ask—from the standpoint of biologic, social, and spiritual well-being—how can we calm the hurricane?
Psychiatry once had a widely-encompassing understanding of its remit: to appreciate the multifaceted experience of the human life and grant succor to the fractured or anguished soul. In such times, psychiatry was a popular destination for seniors graduating in the United States. Annually, 7% to 10% of US graduates chose psychiatry as a career, and continued to do so until the late 1970s.1 In the 1970s, the reductive understanding of the mind increased in prominence, and the role of psychiatry transitioned to one similar to that of other medical specialties: putting patients in boxes, and chronically titrating their medications. The interest of graduating seniors waned alongside the scope of our interest: in 1977, only 4.4% of US graduates pursued psychiatry.2 In 2019, 4.06% of graduating senior applications were to the field of psychiatry.3 (This is not meant to undervalue the quality of international medical graduates, but to focus on local trends in cultural values.)
Psychiatry offers diagnostic and therapeutic avenues that are traditionally undervalued in other fields of medicine. Nephrosis may not care if a patient feels that his or her life is spiritually satisfying and their actions meaningful. However, a patient’s anguish at his reduced functional status does not care for whether his albumin level is normalized—he requires that his suffering be recognized, and that we make an earnest effort to cloak “the shameful nakedness of pain.”4
Psychiatry also makes unique demands of, and offers benefits to, the practitioner. Neurologists complete their residencies feeling that their clinical acumen has increased: “I can formulate a thorough differential now.” Psychiatry asks us not only to cultivate technical proficiency, but also wisdom. The prolonged reflection on the quality and nature of human experience, and the need to guide such patients in a manner far wider and more meaningful in scope than their serotonin pathways, offers the opportunity to emerge from residency a more mindful and grateful human being.
Ultimately, the loss of this sense of scope has not been a failure of medical education. It has been a surrender of the current generation of psychiatry attendings. We have ceded responsibility for the social and spiritual care of our patients to other fields, or to no one at all. If we give up on understanding the hurricane, how can we be surprised that students prefer to chase butterflies?
James Steinberg, MPH, OMS-IV
New York Institute of Technology
College of Osteopathic Medicine
Old Westbury, New York
Robert Barris, MD
Director
Inpatient Psychiatric Services
Nassau University Medical Center
East Meadow, New York
References
1. Sierles FS, Taylor MA. Decline of U.S. medical student career choice of psychiatry and what to do about it. Am J Psychiatry. 1995;152(10):1416-1426.
2. Results and data: main residency match. NRMP data. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2013/08/resultsanddata1984.pdf. Published May 1984. Accessed May 8, 2019.
3. Advanced Data Tables. The Match 2019. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2019/03/Advance-Data-Tables-2019_WWW.pdf. Published March 2019. Accessed May 8, 2019.
4. Kipling R. Doctors. In: Kipling: poems (Everyman’s Library Pocket Poets Series). New York, NY: Random House. 2007:234.
Dr. Nasrallah responds
Thank you, Mr. Steinberg and Dr. Barris, for your comments about my editorial. I genuinely enjoyed the eloquence of your letter. In computers, which we all own and use, hardware is indispensable because it enables us to exploit the software, but the richness of the software is far more interesting than the hardware for the creative productivity of humans. So what you say is correct: T
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Continue to: Perspectives on motherhood and psychiatry
Perspectives on motherhood and psychiatry
I very much enjoyed Drs. Helen M. Farrell’s and Katherine A. Kosman’s recent article “Motherhood and the working psychiatrist” (Psychiatry 2.0,
Christina Ford, MD
Private psychiatric practice
Los Angeles, California
I doubt that anyone—male or female—would argue against the points made by Drs. Farrell and Kosman’s “Motherhood and the working psychiatrist,” which emphasized the need for breaking down the barriers that continue to exist for female physicians who choose to balance their careers with motherhood. As a female psychiatrist who has known since high school that I would choose to remain child-free, I would like to add a different perspective to this discussion and possibly help represent the 20% of women, age 40 to 44, with an MD or PhD who are also child-free.1
While Drs. Farrell and Kosman referenced many assumptions made about working physician mothers, I have not been able to move through medical school, residency, and my career without battling certain assumptions as well. Although every mother is a woman, logic dictates that the converse—every woman is a mother—is certainly not true. However, when interviewing for residency, I was paired specifically with a female attending who had children, and I was told that I could ask her questions about how to balance work-life and raising a family, despite the fact that I did not say or indicate that I had any interest in having such a conversation. There is also the assumption (sometimes more explicit than others) that those of us without children are missing out on something—that we are not included in the “having it all” category. However, in my mind, “having it all” means having the choice to remain child-free, to focus more intensely on my career, to travel when I want, and to own a white couch—without feeling the social obligation to fulfill a role in which I really have no interest.
Cherishing that ability to focus more on my career, however, does not imply that I am boundlessly able and willing to take extra calls, work holidays, or cover for all my colleagues with children (which is also a common assumption). And while I may not be a caregiver to children, that should not detract from the devotion and time I want to spend helping my parents, relatives, and friends.
The article also made the case that facilities, medical schools, and residency programs need to implement policies and procedures that guide the development of accommodations, such as flexible scheduling and lactation rooms, to meet the needs of trainees and physicians without having to jump through hoops or rely on colleagues for coverage and other assistance. Having been in situations where such policies and procedures were not in place, I can affirm that the absence of such guidelines leads not only parents but also child-free physicians to feeling unnecessarily stressed. There was no clear coverage in place when fellow classmates in my residency program went on maternity leave. Essentially, everyone else was expected to step up and take on the additional caseloads, leading the pregnant classmates to try to time things around rotations where there were lighter demands or more residents assigned—not a simple task by any means.
Post-residency, there have been continued challenges. At one point, I was working in a clinic with 2 other female psychiatrists, one of whom was making plans to take maternity leave. During a meeting with our supervisors, the other physician and I were told that we were taking on the third doctor’s patients (without any extension of our own hours or reimbursement) while she was on leave. In addition to disgruntlement over the extra work being sprung on us, I pointed out that this would, in effect, make the third physician’s role obsolete. If 2 of us were able to do the work of 3, what would be the point in keeping her position when she returned? I was assured that this wouldn’t be the case. We dealt with the weeks of covering additional patients, and when she returned from leave, she was asked to shift some of her hours to a different (and, in my opinion, less desirable) clinic.
So, yes, it is incumbent upon facilities and training programs to take responsibility and to remove the barriers that make the jobs of female physicians with children even more challenging than they need to be. This can benefit not only those physicians and their children, but also their colleagues and, ultimately, the patients, who often bear the brunt of stressed, burnt-out physicians and disorganized programs. While I am not going to take a stance on whether it truly takes a village to raise a child, I certainly do not think that it should take a village to organize maternity leave and lactation rooms.
Jessica L. Langenhan, MD, MBA, CHCQM
Medical DirectorBeacon Health Options
Cypress, California
Reference
1. Livingston G. Childlessness. Pew Research Center. https://www.pewsocialtrends.org/2015/05/07/childlessness/. Published May 7, 2015. Accessed May 9, 2019.
Dr. Nasrallah’s “Psychiatry and neurology: Sister neuroscience specialties with different approaches to the brain” (From the Editor,
In mathematics, chaos theory deals with the impossible complexity of simplicity. From primitive initial states, self-interacting systems give rise to short-term predictability, but an unpredictable long-term. Classically, this is illustrated as a hurricane born from the flapping of a butterfly’s wings. Neurology has found great clinical utility in understanding butterfly wings. However, psychiatry forsakes simplicity for complexity: it dives into the emergent systems that arise from self-interacting neurons, asking us to stand within the eye of the hurricane and understand it in its entirety. Psychiatry asks us to transcend the traditional medical focus of discrete physiological mechanisms, and ask—from the standpoint of biologic, social, and spiritual well-being—how can we calm the hurricane?
Psychiatry once had a widely-encompassing understanding of its remit: to appreciate the multifaceted experience of the human life and grant succor to the fractured or anguished soul. In such times, psychiatry was a popular destination for seniors graduating in the United States. Annually, 7% to 10% of US graduates chose psychiatry as a career, and continued to do so until the late 1970s.1 In the 1970s, the reductive understanding of the mind increased in prominence, and the role of psychiatry transitioned to one similar to that of other medical specialties: putting patients in boxes, and chronically titrating their medications. The interest of graduating seniors waned alongside the scope of our interest: in 1977, only 4.4% of US graduates pursued psychiatry.2 In 2019, 4.06% of graduating senior applications were to the field of psychiatry.3 (This is not meant to undervalue the quality of international medical graduates, but to focus on local trends in cultural values.)
Psychiatry offers diagnostic and therapeutic avenues that are traditionally undervalued in other fields of medicine. Nephrosis may not care if a patient feels that his or her life is spiritually satisfying and their actions meaningful. However, a patient’s anguish at his reduced functional status does not care for whether his albumin level is normalized—he requires that his suffering be recognized, and that we make an earnest effort to cloak “the shameful nakedness of pain.”4
Psychiatry also makes unique demands of, and offers benefits to, the practitioner. Neurologists complete their residencies feeling that their clinical acumen has increased: “I can formulate a thorough differential now.” Psychiatry asks us not only to cultivate technical proficiency, but also wisdom. The prolonged reflection on the quality and nature of human experience, and the need to guide such patients in a manner far wider and more meaningful in scope than their serotonin pathways, offers the opportunity to emerge from residency a more mindful and grateful human being.
Ultimately, the loss of this sense of scope has not been a failure of medical education. It has been a surrender of the current generation of psychiatry attendings. We have ceded responsibility for the social and spiritual care of our patients to other fields, or to no one at all. If we give up on understanding the hurricane, how can we be surprised that students prefer to chase butterflies?
James Steinberg, MPH, OMS-IV
New York Institute of Technology
College of Osteopathic Medicine
Old Westbury, New York
Robert Barris, MD
Director
Inpatient Psychiatric Services
Nassau University Medical Center
East Meadow, New York
References
1. Sierles FS, Taylor MA. Decline of U.S. medical student career choice of psychiatry and what to do about it. Am J Psychiatry. 1995;152(10):1416-1426.
2. Results and data: main residency match. NRMP data. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2013/08/resultsanddata1984.pdf. Published May 1984. Accessed May 8, 2019.
3. Advanced Data Tables. The Match 2019. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2019/03/Advance-Data-Tables-2019_WWW.pdf. Published March 2019. Accessed May 8, 2019.
4. Kipling R. Doctors. In: Kipling: poems (Everyman’s Library Pocket Poets Series). New York, NY: Random House. 2007:234.
Dr. Nasrallah responds
Thank you, Mr. Steinberg and Dr. Barris, for your comments about my editorial. I genuinely enjoyed the eloquence of your letter. In computers, which we all own and use, hardware is indispensable because it enables us to exploit the software, but the richness of the software is far more interesting than the hardware for the creative productivity of humans. So what you say is correct: T
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Continue to: Perspectives on motherhood and psychiatry
Perspectives on motherhood and psychiatry
I very much enjoyed Drs. Helen M. Farrell’s and Katherine A. Kosman’s recent article “Motherhood and the working psychiatrist” (Psychiatry 2.0,
Christina Ford, MD
Private psychiatric practice
Los Angeles, California
I doubt that anyone—male or female—would argue against the points made by Drs. Farrell and Kosman’s “Motherhood and the working psychiatrist,” which emphasized the need for breaking down the barriers that continue to exist for female physicians who choose to balance their careers with motherhood. As a female psychiatrist who has known since high school that I would choose to remain child-free, I would like to add a different perspective to this discussion and possibly help represent the 20% of women, age 40 to 44, with an MD or PhD who are also child-free.1
While Drs. Farrell and Kosman referenced many assumptions made about working physician mothers, I have not been able to move through medical school, residency, and my career without battling certain assumptions as well. Although every mother is a woman, logic dictates that the converse—every woman is a mother—is certainly not true. However, when interviewing for residency, I was paired specifically with a female attending who had children, and I was told that I could ask her questions about how to balance work-life and raising a family, despite the fact that I did not say or indicate that I had any interest in having such a conversation. There is also the assumption (sometimes more explicit than others) that those of us without children are missing out on something—that we are not included in the “having it all” category. However, in my mind, “having it all” means having the choice to remain child-free, to focus more intensely on my career, to travel when I want, and to own a white couch—without feeling the social obligation to fulfill a role in which I really have no interest.
Cherishing that ability to focus more on my career, however, does not imply that I am boundlessly able and willing to take extra calls, work holidays, or cover for all my colleagues with children (which is also a common assumption). And while I may not be a caregiver to children, that should not detract from the devotion and time I want to spend helping my parents, relatives, and friends.
The article also made the case that facilities, medical schools, and residency programs need to implement policies and procedures that guide the development of accommodations, such as flexible scheduling and lactation rooms, to meet the needs of trainees and physicians without having to jump through hoops or rely on colleagues for coverage and other assistance. Having been in situations where such policies and procedures were not in place, I can affirm that the absence of such guidelines leads not only parents but also child-free physicians to feeling unnecessarily stressed. There was no clear coverage in place when fellow classmates in my residency program went on maternity leave. Essentially, everyone else was expected to step up and take on the additional caseloads, leading the pregnant classmates to try to time things around rotations where there were lighter demands or more residents assigned—not a simple task by any means.
Post-residency, there have been continued challenges. At one point, I was working in a clinic with 2 other female psychiatrists, one of whom was making plans to take maternity leave. During a meeting with our supervisors, the other physician and I were told that we were taking on the third doctor’s patients (without any extension of our own hours or reimbursement) while she was on leave. In addition to disgruntlement over the extra work being sprung on us, I pointed out that this would, in effect, make the third physician’s role obsolete. If 2 of us were able to do the work of 3, what would be the point in keeping her position when she returned? I was assured that this wouldn’t be the case. We dealt with the weeks of covering additional patients, and when she returned from leave, she was asked to shift some of her hours to a different (and, in my opinion, less desirable) clinic.
So, yes, it is incumbent upon facilities and training programs to take responsibility and to remove the barriers that make the jobs of female physicians with children even more challenging than they need to be. This can benefit not only those physicians and their children, but also their colleagues and, ultimately, the patients, who often bear the brunt of stressed, burnt-out physicians and disorganized programs. While I am not going to take a stance on whether it truly takes a village to raise a child, I certainly do not think that it should take a village to organize maternity leave and lactation rooms.
Jessica L. Langenhan, MD, MBA, CHCQM
Medical DirectorBeacon Health Options
Cypress, California
Reference
1. Livingston G. Childlessness. Pew Research Center. https://www.pewsocialtrends.org/2015/05/07/childlessness/. Published May 7, 2015. Accessed May 9, 2019.
Dr. Nasrallah’s “Psychiatry and neurology: Sister neuroscience specialties with different approaches to the brain” (From the Editor,
In mathematics, chaos theory deals with the impossible complexity of simplicity. From primitive initial states, self-interacting systems give rise to short-term predictability, but an unpredictable long-term. Classically, this is illustrated as a hurricane born from the flapping of a butterfly’s wings. Neurology has found great clinical utility in understanding butterfly wings. However, psychiatry forsakes simplicity for complexity: it dives into the emergent systems that arise from self-interacting neurons, asking us to stand within the eye of the hurricane and understand it in its entirety. Psychiatry asks us to transcend the traditional medical focus of discrete physiological mechanisms, and ask—from the standpoint of biologic, social, and spiritual well-being—how can we calm the hurricane?
Psychiatry once had a widely-encompassing understanding of its remit: to appreciate the multifaceted experience of the human life and grant succor to the fractured or anguished soul. In such times, psychiatry was a popular destination for seniors graduating in the United States. Annually, 7% to 10% of US graduates chose psychiatry as a career, and continued to do so until the late 1970s.1 In the 1970s, the reductive understanding of the mind increased in prominence, and the role of psychiatry transitioned to one similar to that of other medical specialties: putting patients in boxes, and chronically titrating their medications. The interest of graduating seniors waned alongside the scope of our interest: in 1977, only 4.4% of US graduates pursued psychiatry.2 In 2019, 4.06% of graduating senior applications were to the field of psychiatry.3 (This is not meant to undervalue the quality of international medical graduates, but to focus on local trends in cultural values.)
Psychiatry offers diagnostic and therapeutic avenues that are traditionally undervalued in other fields of medicine. Nephrosis may not care if a patient feels that his or her life is spiritually satisfying and their actions meaningful. However, a patient’s anguish at his reduced functional status does not care for whether his albumin level is normalized—he requires that his suffering be recognized, and that we make an earnest effort to cloak “the shameful nakedness of pain.”4
Psychiatry also makes unique demands of, and offers benefits to, the practitioner. Neurologists complete their residencies feeling that their clinical acumen has increased: “I can formulate a thorough differential now.” Psychiatry asks us not only to cultivate technical proficiency, but also wisdom. The prolonged reflection on the quality and nature of human experience, and the need to guide such patients in a manner far wider and more meaningful in scope than their serotonin pathways, offers the opportunity to emerge from residency a more mindful and grateful human being.
Ultimately, the loss of this sense of scope has not been a failure of medical education. It has been a surrender of the current generation of psychiatry attendings. We have ceded responsibility for the social and spiritual care of our patients to other fields, or to no one at all. If we give up on understanding the hurricane, how can we be surprised that students prefer to chase butterflies?
James Steinberg, MPH, OMS-IV
New York Institute of Technology
College of Osteopathic Medicine
Old Westbury, New York
Robert Barris, MD
Director
Inpatient Psychiatric Services
Nassau University Medical Center
East Meadow, New York
References
1. Sierles FS, Taylor MA. Decline of U.S. medical student career choice of psychiatry and what to do about it. Am J Psychiatry. 1995;152(10):1416-1426.
2. Results and data: main residency match. NRMP data. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2013/08/resultsanddata1984.pdf. Published May 1984. Accessed May 8, 2019.
3. Advanced Data Tables. The Match 2019. The National Resident Matching Program. https://mk0nrmpcikgb8jxyd19h.kinstacdn.com/wp-content/uploads/2019/03/Advance-Data-Tables-2019_WWW.pdf. Published March 2019. Accessed May 8, 2019.
4. Kipling R. Doctors. In: Kipling: poems (Everyman’s Library Pocket Poets Series). New York, NY: Random House. 2007:234.
Dr. Nasrallah responds
Thank you, Mr. Steinberg and Dr. Barris, for your comments about my editorial. I genuinely enjoyed the eloquence of your letter. In computers, which we all own and use, hardware is indispensable because it enables us to exploit the software, but the richness of the software is far more interesting than the hardware for the creative productivity of humans. So what you say is correct: T
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Continue to: Perspectives on motherhood and psychiatry
Perspectives on motherhood and psychiatry
I very much enjoyed Drs. Helen M. Farrell’s and Katherine A. Kosman’s recent article “Motherhood and the working psychiatrist” (Psychiatry 2.0,
Christina Ford, MD
Private psychiatric practice
Los Angeles, California
I doubt that anyone—male or female—would argue against the points made by Drs. Farrell and Kosman’s “Motherhood and the working psychiatrist,” which emphasized the need for breaking down the barriers that continue to exist for female physicians who choose to balance their careers with motherhood. As a female psychiatrist who has known since high school that I would choose to remain child-free, I would like to add a different perspective to this discussion and possibly help represent the 20% of women, age 40 to 44, with an MD or PhD who are also child-free.1
While Drs. Farrell and Kosman referenced many assumptions made about working physician mothers, I have not been able to move through medical school, residency, and my career without battling certain assumptions as well. Although every mother is a woman, logic dictates that the converse—every woman is a mother—is certainly not true. However, when interviewing for residency, I was paired specifically with a female attending who had children, and I was told that I could ask her questions about how to balance work-life and raising a family, despite the fact that I did not say or indicate that I had any interest in having such a conversation. There is also the assumption (sometimes more explicit than others) that those of us without children are missing out on something—that we are not included in the “having it all” category. However, in my mind, “having it all” means having the choice to remain child-free, to focus more intensely on my career, to travel when I want, and to own a white couch—without feeling the social obligation to fulfill a role in which I really have no interest.
Cherishing that ability to focus more on my career, however, does not imply that I am boundlessly able and willing to take extra calls, work holidays, or cover for all my colleagues with children (which is also a common assumption). And while I may not be a caregiver to children, that should not detract from the devotion and time I want to spend helping my parents, relatives, and friends.
The article also made the case that facilities, medical schools, and residency programs need to implement policies and procedures that guide the development of accommodations, such as flexible scheduling and lactation rooms, to meet the needs of trainees and physicians without having to jump through hoops or rely on colleagues for coverage and other assistance. Having been in situations where such policies and procedures were not in place, I can affirm that the absence of such guidelines leads not only parents but also child-free physicians to feeling unnecessarily stressed. There was no clear coverage in place when fellow classmates in my residency program went on maternity leave. Essentially, everyone else was expected to step up and take on the additional caseloads, leading the pregnant classmates to try to time things around rotations where there were lighter demands or more residents assigned—not a simple task by any means.
Post-residency, there have been continued challenges. At one point, I was working in a clinic with 2 other female psychiatrists, one of whom was making plans to take maternity leave. During a meeting with our supervisors, the other physician and I were told that we were taking on the third doctor’s patients (without any extension of our own hours or reimbursement) while she was on leave. In addition to disgruntlement over the extra work being sprung on us, I pointed out that this would, in effect, make the third physician’s role obsolete. If 2 of us were able to do the work of 3, what would be the point in keeping her position when she returned? I was assured that this wouldn’t be the case. We dealt with the weeks of covering additional patients, and when she returned from leave, she was asked to shift some of her hours to a different (and, in my opinion, less desirable) clinic.
So, yes, it is incumbent upon facilities and training programs to take responsibility and to remove the barriers that make the jobs of female physicians with children even more challenging than they need to be. This can benefit not only those physicians and their children, but also their colleagues and, ultimately, the patients, who often bear the brunt of stressed, burnt-out physicians and disorganized programs. While I am not going to take a stance on whether it truly takes a village to raise a child, I certainly do not think that it should take a village to organize maternity leave and lactation rooms.
Jessica L. Langenhan, MD, MBA, CHCQM
Medical DirectorBeacon Health Options
Cypress, California
Reference
1. Livingston G. Childlessness. Pew Research Center. https://www.pewsocialtrends.org/2015/05/07/childlessness/. Published May 7, 2015. Accessed May 9, 2019.
SPIRITT: What does ‘spirituality’ mean?
Both patients and clinicians alike have shown increasing interest in spirituality as a component of physical and mental well-being.1 However, there’s no clear consensus on what spirituality actually means. The Merriam-Webster dictionary defines it “affecting the spirit, relating to sacred matters, concerned with religious issues.”2 Spirituality is sometimes defined in broadly secular terms, such as the feeling of “being part of something greater than ourselves,” or in connection to ideas rooted in a specific belief system, such as “aligning oneself with the Will of God.”
I prefer to think of the word “spiritual” as encompassing multiple practices and beliefs that have the common goal of helping us deepen our capacity for self-awareness, joy, compassion, love, freedom, justice, and mutual cooperation, not only for our own benefit, but also to create a better world. To help clinicians better understand what the term spirituality implies, whether for themselves or for their patients, I offer the acronym SPIRITT to describe core components of varied spiritual perspectives, beliefs, and practices.
Sacred. Considering certain aspects of life, time, or place as non-ordinary and worthy of reverence and awe.
Presence. Cultivating an inner presence that is open, accepting, compassionate, and loving toward others. During a spiritual experience, some may feel embraced in this way by a presence outside of themselves, such as an encounter with a spiritual teacher or an experience of feeling held lovingly by a transcendent power.
Interconnection. Understanding that we are not separate entities but are interconnected beings existing in interdependent unity, starting with our families and extending out universally. According to this perspective, harming anything or anyone is doing harm to ourself.
Rest. Taking a Sabbath or unplugging. Dedicating time each week for resting your mind and body. Spending quality time with family. Decreasing excessive stimulation and loosening the grip of consumerism.
Introspection. Looking inwardly. Eastern traditions emphasize deepening self-awareness through mindful meditation practices, while Western traditions include taking a personal inventory through self-examination or confessional practices.
Continue to: Traditions
Traditions. Studying sacred texts, participating in communal prayer, meditating, or engaging in rituals. This requires sorting through outmoded beliefs and ways of thinking while updating beliefs that are compatible with our lived experiences.
Transcendence. Experiencing moments, whether through nature, music, dance, ritual, prayer, art, etc., in which the narrow sense of being a separate self fades away and there is a deeper sense of a larger connection and belonging that is transpersonal, timeless, and expansive.
The components of SPIRITT have helped me to think about and pursue the physical, emotional, and social benefits of adopting a spiritual practice for my well-being as well as for the benefit of my patients.
1. Koenig HG. Religion, spirituality, and health: a review and update. Adv Mind Body Med. 2015;29(3):19-26.
2. Spiritual. Miriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/spiritual. Accessed May 9, 2019.
Both patients and clinicians alike have shown increasing interest in spirituality as a component of physical and mental well-being.1 However, there’s no clear consensus on what spirituality actually means. The Merriam-Webster dictionary defines it “affecting the spirit, relating to sacred matters, concerned with religious issues.”2 Spirituality is sometimes defined in broadly secular terms, such as the feeling of “being part of something greater than ourselves,” or in connection to ideas rooted in a specific belief system, such as “aligning oneself with the Will of God.”
I prefer to think of the word “spiritual” as encompassing multiple practices and beliefs that have the common goal of helping us deepen our capacity for self-awareness, joy, compassion, love, freedom, justice, and mutual cooperation, not only for our own benefit, but also to create a better world. To help clinicians better understand what the term spirituality implies, whether for themselves or for their patients, I offer the acronym SPIRITT to describe core components of varied spiritual perspectives, beliefs, and practices.
Sacred. Considering certain aspects of life, time, or place as non-ordinary and worthy of reverence and awe.
Presence. Cultivating an inner presence that is open, accepting, compassionate, and loving toward others. During a spiritual experience, some may feel embraced in this way by a presence outside of themselves, such as an encounter with a spiritual teacher or an experience of feeling held lovingly by a transcendent power.
Interconnection. Understanding that we are not separate entities but are interconnected beings existing in interdependent unity, starting with our families and extending out universally. According to this perspective, harming anything or anyone is doing harm to ourself.
Rest. Taking a Sabbath or unplugging. Dedicating time each week for resting your mind and body. Spending quality time with family. Decreasing excessive stimulation and loosening the grip of consumerism.
Introspection. Looking inwardly. Eastern traditions emphasize deepening self-awareness through mindful meditation practices, while Western traditions include taking a personal inventory through self-examination or confessional practices.
Continue to: Traditions
Traditions. Studying sacred texts, participating in communal prayer, meditating, or engaging in rituals. This requires sorting through outmoded beliefs and ways of thinking while updating beliefs that are compatible with our lived experiences.
Transcendence. Experiencing moments, whether through nature, music, dance, ritual, prayer, art, etc., in which the narrow sense of being a separate self fades away and there is a deeper sense of a larger connection and belonging that is transpersonal, timeless, and expansive.
The components of SPIRITT have helped me to think about and pursue the physical, emotional, and social benefits of adopting a spiritual practice for my well-being as well as for the benefit of my patients.
Both patients and clinicians alike have shown increasing interest in spirituality as a component of physical and mental well-being.1 However, there’s no clear consensus on what spirituality actually means. The Merriam-Webster dictionary defines it “affecting the spirit, relating to sacred matters, concerned with religious issues.”2 Spirituality is sometimes defined in broadly secular terms, such as the feeling of “being part of something greater than ourselves,” or in connection to ideas rooted in a specific belief system, such as “aligning oneself with the Will of God.”
I prefer to think of the word “spiritual” as encompassing multiple practices and beliefs that have the common goal of helping us deepen our capacity for self-awareness, joy, compassion, love, freedom, justice, and mutual cooperation, not only for our own benefit, but also to create a better world. To help clinicians better understand what the term spirituality implies, whether for themselves or for their patients, I offer the acronym SPIRITT to describe core components of varied spiritual perspectives, beliefs, and practices.
Sacred. Considering certain aspects of life, time, or place as non-ordinary and worthy of reverence and awe.
Presence. Cultivating an inner presence that is open, accepting, compassionate, and loving toward others. During a spiritual experience, some may feel embraced in this way by a presence outside of themselves, such as an encounter with a spiritual teacher or an experience of feeling held lovingly by a transcendent power.
Interconnection. Understanding that we are not separate entities but are interconnected beings existing in interdependent unity, starting with our families and extending out universally. According to this perspective, harming anything or anyone is doing harm to ourself.
Rest. Taking a Sabbath or unplugging. Dedicating time each week for resting your mind and body. Spending quality time with family. Decreasing excessive stimulation and loosening the grip of consumerism.
Introspection. Looking inwardly. Eastern traditions emphasize deepening self-awareness through mindful meditation practices, while Western traditions include taking a personal inventory through self-examination or confessional practices.
Continue to: Traditions
Traditions. Studying sacred texts, participating in communal prayer, meditating, or engaging in rituals. This requires sorting through outmoded beliefs and ways of thinking while updating beliefs that are compatible with our lived experiences.
Transcendence. Experiencing moments, whether through nature, music, dance, ritual, prayer, art, etc., in which the narrow sense of being a separate self fades away and there is a deeper sense of a larger connection and belonging that is transpersonal, timeless, and expansive.
The components of SPIRITT have helped me to think about and pursue the physical, emotional, and social benefits of adopting a spiritual practice for my well-being as well as for the benefit of my patients.
1. Koenig HG. Religion, spirituality, and health: a review and update. Adv Mind Body Med. 2015;29(3):19-26.
2. Spiritual. Miriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/spiritual. Accessed May 9, 2019.
1. Koenig HG. Religion, spirituality, and health: a review and update. Adv Mind Body Med. 2015;29(3):19-26.
2. Spiritual. Miriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/spiritual. Accessed May 9, 2019.







