User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Beyond ‘selfies’: An epidemic of acquired narcissism
Narcissism has an evil reputation. But is it justified? A modicum of narcissism is actually healthy. It can bolster self-confidence, assertiveness, and success in business and in the sociobiology of mating. Perhaps that’s why narcissism as a trait has a survival value from an evolutionary perspective.
Taking an excessive number of “selfies” with a smartphone is probably the most common and relatively benign form of mild narcissism (and not in DSM-5, yet). Narcissistic personality disorder (NPD), with a prevalence of 1%, is on the extreme end of the narcissism continuum. It has become tainted with such an intensely negative halo that it has become a despised trait, an insult, and even a vile epithet, like a 4-letter word. But as psychiatrists and other mental health professionals, we clinically relate to patients with NPD as being afflicted with a serious neuropsychiatric disorder, not as despicable individuals. Many people outside the mental health profession abhor persons with NPD because of their gargantuan hubris, insufferable selfishness, self-aggrandizement, emotional abuse of others, and irremediable vanity. Narcissistic personality disorder deprives its sufferers of the prosocial capacity for empathy, which leads them to belittle others or treat competent individuals with disdain, never as equals. They also seem to be incapable of experiencing shame as they inflate their self-importance and megalomania at the expense of those they degrade. They cannot tolerate any success by others because it threatens to overshadow their own exaggerated achievements. They can be mercilessly harsh towards their underlings. They are incapable of fostering warm, long-term loving relationships, where bidirectional respect is essential. Their lives often are replete with brief, broken-up relationships because they emotionally, physically, or sexually abuse their intimate partners.
Primary NPD has been shown in twin studies to be highly genetic, and more strongly heritable than 17 other personality dimensions.1 It is also resistant to any effective psychotherapeutic, pharmacologic, or somatic treatments. This is particularly relevant given the proclivity of individuals with NPD to experience a crushing disappointment, commonly known as “narcissistic injury,” following a real or imagined failure. This could lead to a painful depression or an outburst of “narcissistic rage” directed at anyone perceived as undermining them, and may even lead to violent behavior.2
Apart from heritable narcissism, there is also another form of narcissism that can develop in some individuals following life events. That hazardous condition, known as “acquired narcissism,” is most often associated with achieving the coveted status of an exalted celebrity. At risk for this acquired personality affliction are famous actors, singers, movie directors, TV anchors, or politicians (although some politicians are natural-born narcissists, driven to seek the powers of public office), and less frequently physicians (perhaps because the practice of medicine is not done in front of spectators) or scientists (because research, no matter how momentous, rarely procures the glamour or public adulation of the entertainment industry). The ardent fans of those “celebs” shower them with such intense attention and adulation that it malignantly transforms previously “normal” individuals into narcissists who start believing they are indeed “very special” and superior to the rest of us mortals (especially as their earning power balloons into the millions after growing up with humble social or economic roots).
Social media has become a catalyst for acquired narcissism, with millions of followers on Twitter, Facebook, or YouTube. Cable TV also caters to politicians, some of whom morph into narcissists, intoxicated with their newfound eminence and stature among their partisan followers, and become genuinely convinced that they have supreme power or influence over the masses. They get carried away with their own exaggerated self-importance as oracles of the “truth,” regardless of how extreme their views may be. Celebrity, politics, social media, and cable TV have converged into a combustible mix, a crucible for acquired narcissism.
An interesting feature of acquired narcissism is “collective narcissism,” in which celebrities coalesce to consolidate their imagined superhuman attributes that go beyond the technical skills of their professions such as acting, singing, sports, or politics. Thus, entertainers or star athletes believe they can enunciate radical statements about contemporary social, political, or environmental issues (at both ends of the debate) as though their artistic success renders them wise arbiters of the truth. What complicates matters is their delirious fans, who revere and mimic whatever their idols say (and their fashion or their tattoos), which further intensifies the grandiosity and megalomania of acquired narcissism. Celebrity triggers mindless idolatry, fueling the narcissism of individuals who are blessed (or cursed?) with runaway personal success. Neuroscientists should conduct research into how the brain is neurobiologically altered by fame, but there are many more urgent questions that demand their attention. It would be important to know if it is reversible or enduring, even as fame inevitably dims.
Continue to: The pursuit of wealth and fame...
The pursuit of wealth and fame is widely prevalent and can be healthy if it is not all-consuming. But if achieved beyond the aspirer’s wildest dreams, he/she may reach an inflection point conducive to a pathologic degree of acquired narcissism. That’s what the French refer to as “les risques du métier” (ie, occupational hazard). I recall reading about celebrities who became enraged when a policeman “dared” to stop their car for some driving violation, confronting the officer with “Do you know who I am?” That question may be a clinical biomarker of acquired narcissism.
Interestingly, several years ago, when the American Psychiatry Association last revised the DSM—sometimes referred to as the “bible” of psychiatric nosology—it came close to dropping NPD from its listed disorders, but then reverted and kept it as one of the 275 diagnostic categories included in DSM-5.3 Had the NPD diagnosis been discarded, one wonders if the mythical god of narcissism would have suffered a transcendental “narcissistic injury”…
1. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831
2. Malmquist CP. Homicide: a psychiatric perspective. Washington, DC: American Psychiatric Publishing, Inc.; 2006:181-182.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
Narcissism has an evil reputation. But is it justified? A modicum of narcissism is actually healthy. It can bolster self-confidence, assertiveness, and success in business and in the sociobiology of mating. Perhaps that’s why narcissism as a trait has a survival value from an evolutionary perspective.
Taking an excessive number of “selfies” with a smartphone is probably the most common and relatively benign form of mild narcissism (and not in DSM-5, yet). Narcissistic personality disorder (NPD), with a prevalence of 1%, is on the extreme end of the narcissism continuum. It has become tainted with such an intensely negative halo that it has become a despised trait, an insult, and even a vile epithet, like a 4-letter word. But as psychiatrists and other mental health professionals, we clinically relate to patients with NPD as being afflicted with a serious neuropsychiatric disorder, not as despicable individuals. Many people outside the mental health profession abhor persons with NPD because of their gargantuan hubris, insufferable selfishness, self-aggrandizement, emotional abuse of others, and irremediable vanity. Narcissistic personality disorder deprives its sufferers of the prosocial capacity for empathy, which leads them to belittle others or treat competent individuals with disdain, never as equals. They also seem to be incapable of experiencing shame as they inflate their self-importance and megalomania at the expense of those they degrade. They cannot tolerate any success by others because it threatens to overshadow their own exaggerated achievements. They can be mercilessly harsh towards their underlings. They are incapable of fostering warm, long-term loving relationships, where bidirectional respect is essential. Their lives often are replete with brief, broken-up relationships because they emotionally, physically, or sexually abuse their intimate partners.
Primary NPD has been shown in twin studies to be highly genetic, and more strongly heritable than 17 other personality dimensions.1 It is also resistant to any effective psychotherapeutic, pharmacologic, or somatic treatments. This is particularly relevant given the proclivity of individuals with NPD to experience a crushing disappointment, commonly known as “narcissistic injury,” following a real or imagined failure. This could lead to a painful depression or an outburst of “narcissistic rage” directed at anyone perceived as undermining them, and may even lead to violent behavior.2
Apart from heritable narcissism, there is also another form of narcissism that can develop in some individuals following life events. That hazardous condition, known as “acquired narcissism,” is most often associated with achieving the coveted status of an exalted celebrity. At risk for this acquired personality affliction are famous actors, singers, movie directors, TV anchors, or politicians (although some politicians are natural-born narcissists, driven to seek the powers of public office), and less frequently physicians (perhaps because the practice of medicine is not done in front of spectators) or scientists (because research, no matter how momentous, rarely procures the glamour or public adulation of the entertainment industry). The ardent fans of those “celebs” shower them with such intense attention and adulation that it malignantly transforms previously “normal” individuals into narcissists who start believing they are indeed “very special” and superior to the rest of us mortals (especially as their earning power balloons into the millions after growing up with humble social or economic roots).
Social media has become a catalyst for acquired narcissism, with millions of followers on Twitter, Facebook, or YouTube. Cable TV also caters to politicians, some of whom morph into narcissists, intoxicated with their newfound eminence and stature among their partisan followers, and become genuinely convinced that they have supreme power or influence over the masses. They get carried away with their own exaggerated self-importance as oracles of the “truth,” regardless of how extreme their views may be. Celebrity, politics, social media, and cable TV have converged into a combustible mix, a crucible for acquired narcissism.
An interesting feature of acquired narcissism is “collective narcissism,” in which celebrities coalesce to consolidate their imagined superhuman attributes that go beyond the technical skills of their professions such as acting, singing, sports, or politics. Thus, entertainers or star athletes believe they can enunciate radical statements about contemporary social, political, or environmental issues (at both ends of the debate) as though their artistic success renders them wise arbiters of the truth. What complicates matters is their delirious fans, who revere and mimic whatever their idols say (and their fashion or their tattoos), which further intensifies the grandiosity and megalomania of acquired narcissism. Celebrity triggers mindless idolatry, fueling the narcissism of individuals who are blessed (or cursed?) with runaway personal success. Neuroscientists should conduct research into how the brain is neurobiologically altered by fame, but there are many more urgent questions that demand their attention. It would be important to know if it is reversible or enduring, even as fame inevitably dims.
Continue to: The pursuit of wealth and fame...
The pursuit of wealth and fame is widely prevalent and can be healthy if it is not all-consuming. But if achieved beyond the aspirer’s wildest dreams, he/she may reach an inflection point conducive to a pathologic degree of acquired narcissism. That’s what the French refer to as “les risques du métier” (ie, occupational hazard). I recall reading about celebrities who became enraged when a policeman “dared” to stop their car for some driving violation, confronting the officer with “Do you know who I am?” That question may be a clinical biomarker of acquired narcissism.
Interestingly, several years ago, when the American Psychiatry Association last revised the DSM—sometimes referred to as the “bible” of psychiatric nosology—it came close to dropping NPD from its listed disorders, but then reverted and kept it as one of the 275 diagnostic categories included in DSM-5.3 Had the NPD diagnosis been discarded, one wonders if the mythical god of narcissism would have suffered a transcendental “narcissistic injury”…
Narcissism has an evil reputation. But is it justified? A modicum of narcissism is actually healthy. It can bolster self-confidence, assertiveness, and success in business and in the sociobiology of mating. Perhaps that’s why narcissism as a trait has a survival value from an evolutionary perspective.
Taking an excessive number of “selfies” with a smartphone is probably the most common and relatively benign form of mild narcissism (and not in DSM-5, yet). Narcissistic personality disorder (NPD), with a prevalence of 1%, is on the extreme end of the narcissism continuum. It has become tainted with such an intensely negative halo that it has become a despised trait, an insult, and even a vile epithet, like a 4-letter word. But as psychiatrists and other mental health professionals, we clinically relate to patients with NPD as being afflicted with a serious neuropsychiatric disorder, not as despicable individuals. Many people outside the mental health profession abhor persons with NPD because of their gargantuan hubris, insufferable selfishness, self-aggrandizement, emotional abuse of others, and irremediable vanity. Narcissistic personality disorder deprives its sufferers of the prosocial capacity for empathy, which leads them to belittle others or treat competent individuals with disdain, never as equals. They also seem to be incapable of experiencing shame as they inflate their self-importance and megalomania at the expense of those they degrade. They cannot tolerate any success by others because it threatens to overshadow their own exaggerated achievements. They can be mercilessly harsh towards their underlings. They are incapable of fostering warm, long-term loving relationships, where bidirectional respect is essential. Their lives often are replete with brief, broken-up relationships because they emotionally, physically, or sexually abuse their intimate partners.
Primary NPD has been shown in twin studies to be highly genetic, and more strongly heritable than 17 other personality dimensions.1 It is also resistant to any effective psychotherapeutic, pharmacologic, or somatic treatments. This is particularly relevant given the proclivity of individuals with NPD to experience a crushing disappointment, commonly known as “narcissistic injury,” following a real or imagined failure. This could lead to a painful depression or an outburst of “narcissistic rage” directed at anyone perceived as undermining them, and may even lead to violent behavior.2
Apart from heritable narcissism, there is also another form of narcissism that can develop in some individuals following life events. That hazardous condition, known as “acquired narcissism,” is most often associated with achieving the coveted status of an exalted celebrity. At risk for this acquired personality affliction are famous actors, singers, movie directors, TV anchors, or politicians (although some politicians are natural-born narcissists, driven to seek the powers of public office), and less frequently physicians (perhaps because the practice of medicine is not done in front of spectators) or scientists (because research, no matter how momentous, rarely procures the glamour or public adulation of the entertainment industry). The ardent fans of those “celebs” shower them with such intense attention and adulation that it malignantly transforms previously “normal” individuals into narcissists who start believing they are indeed “very special” and superior to the rest of us mortals (especially as their earning power balloons into the millions after growing up with humble social or economic roots).
Social media has become a catalyst for acquired narcissism, with millions of followers on Twitter, Facebook, or YouTube. Cable TV also caters to politicians, some of whom morph into narcissists, intoxicated with their newfound eminence and stature among their partisan followers, and become genuinely convinced that they have supreme power or influence over the masses. They get carried away with their own exaggerated self-importance as oracles of the “truth,” regardless of how extreme their views may be. Celebrity, politics, social media, and cable TV have converged into a combustible mix, a crucible for acquired narcissism.
An interesting feature of acquired narcissism is “collective narcissism,” in which celebrities coalesce to consolidate their imagined superhuman attributes that go beyond the technical skills of their professions such as acting, singing, sports, or politics. Thus, entertainers or star athletes believe they can enunciate radical statements about contemporary social, political, or environmental issues (at both ends of the debate) as though their artistic success renders them wise arbiters of the truth. What complicates matters is their delirious fans, who revere and mimic whatever their idols say (and their fashion or their tattoos), which further intensifies the grandiosity and megalomania of acquired narcissism. Celebrity triggers mindless idolatry, fueling the narcissism of individuals who are blessed (or cursed?) with runaway personal success. Neuroscientists should conduct research into how the brain is neurobiologically altered by fame, but there are many more urgent questions that demand their attention. It would be important to know if it is reversible or enduring, even as fame inevitably dims.
Continue to: The pursuit of wealth and fame...
The pursuit of wealth and fame is widely prevalent and can be healthy if it is not all-consuming. But if achieved beyond the aspirer’s wildest dreams, he/she may reach an inflection point conducive to a pathologic degree of acquired narcissism. That’s what the French refer to as “les risques du métier” (ie, occupational hazard). I recall reading about celebrities who became enraged when a policeman “dared” to stop their car for some driving violation, confronting the officer with “Do you know who I am?” That question may be a clinical biomarker of acquired narcissism.
Interestingly, several years ago, when the American Psychiatry Association last revised the DSM—sometimes referred to as the “bible” of psychiatric nosology—it came close to dropping NPD from its listed disorders, but then reverted and kept it as one of the 275 diagnostic categories included in DSM-5.3 Had the NPD diagnosis been discarded, one wonders if the mythical god of narcissism would have suffered a transcendental “narcissistic injury”…
1. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831
2. Malmquist CP. Homicide: a psychiatric perspective. Washington, DC: American Psychiatric Publishing, Inc.; 2006:181-182.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
1. Livesley WJ, Jang KL, Jackson DN, et al. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826-1831
2. Malmquist CP. Homicide: a psychiatric perspective. Washington, DC: American Psychiatric Publishing, Inc.; 2006:181-182.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
Backlash against using rating scales
I strongly disagree with the editorial by Ahmed A. Aboraya, MD, DrPH, and Henry A. Nasrallah, MD, (“It’s time to implement measurement-based care in psychiatric practice,” From the Editor,
We do not have much more to lose before it’s a checklist, vital signs, and a script. I now refer to our profession as “McMedicine.” If you don’t have what is on the menu, you cannot get served. Diseases are rarely treated, symptoms are treated. This is not the profession of medicine. We are not fixing much; we are mostly providing consumers for pharmaceutical companies.
Few psychiatric disorders have been subjected to more measurement than depression. Quite a while ago, someone tried to compare depression scales. They correlated scale scores with the results of evaluations by board-certified psychiatrists. The best scale was a single question: “Are you depressed?” This had been included as a control. Can you do better?
Furthermore, the “paper and numbers” people can’t wait to get an “objective” wrench to tighten the screws and apply the principles of the industrial revolution to squeeze more money out of the system. They will find some way to turn patients into standardized products.
John L. Schenkel, MD
Retired psychiatrist
Peru, NY
With the use of an electronic medical record, what should be a simple 1-page note is transformed into a 5-page note of details. Doctors no longer attend to their patients but rather to their computers. Has this raised consciousness—the most important metric, according to Dr. David Hawkins? I doubt it.
In the words of my great professor, Dr. James Gustafson, I will continue to start my interview with what concerns the patient. Most of the time, they implicitly know.
Our focus should instead be on bringing down the cost of health care. This is what angers our patients most, and yet we do not make it a priority.
Psychiatrist
Glenbeigh Hospital
Rock Creek, Ohio
Signature Health
Ashtabula, Ohio
Behavioral Wellness Group
Mentor, Ohio
Continue to: The authors respond
The authors respond
We appreciate Drs. Schenkel’s and Primc’s comments on our editorial regarding measurement-based care (MBC). However, MBC will not increase the workload of psychiatrists; rather, it will streamline the evaluation of patients and measure the severity of their symptoms or adverse effects as well as the degree of their improvement. The proper use of scales with the appropriate patient populations may actually help clinicians to reduce the extensive amount of details that go into medical records.
The following quote, an excerpt from another article we wrote on MBC,1 speaks to Dr. Primc’s concerns:
“…measures in psychiatry could be considered the equivalent of a thermometer and a stethoscope to a physician.
Ahmed A. Aboraya, MD, DrPH
Assistant Professor
Department of Behavioral Medicine and Psychiatry
Chief of Psychiatry
Sharpe Hospital West Virginia University
Weston, West Virginia
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
Reference
1. Aboraya A, Nasrallah HA, Elswick DE, et al. Measurement-based care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
I strongly disagree with the editorial by Ahmed A. Aboraya, MD, DrPH, and Henry A. Nasrallah, MD, (“It’s time to implement measurement-based care in psychiatric practice,” From the Editor,
We do not have much more to lose before it’s a checklist, vital signs, and a script. I now refer to our profession as “McMedicine.” If you don’t have what is on the menu, you cannot get served. Diseases are rarely treated, symptoms are treated. This is not the profession of medicine. We are not fixing much; we are mostly providing consumers for pharmaceutical companies.
Few psychiatric disorders have been subjected to more measurement than depression. Quite a while ago, someone tried to compare depression scales. They correlated scale scores with the results of evaluations by board-certified psychiatrists. The best scale was a single question: “Are you depressed?” This had been included as a control. Can you do better?
Furthermore, the “paper and numbers” people can’t wait to get an “objective” wrench to tighten the screws and apply the principles of the industrial revolution to squeeze more money out of the system. They will find some way to turn patients into standardized products.
John L. Schenkel, MD
Retired psychiatrist
Peru, NY
With the use of an electronic medical record, what should be a simple 1-page note is transformed into a 5-page note of details. Doctors no longer attend to their patients but rather to their computers. Has this raised consciousness—the most important metric, according to Dr. David Hawkins? I doubt it.
In the words of my great professor, Dr. James Gustafson, I will continue to start my interview with what concerns the patient. Most of the time, they implicitly know.
Our focus should instead be on bringing down the cost of health care. This is what angers our patients most, and yet we do not make it a priority.
Psychiatrist
Glenbeigh Hospital
Rock Creek, Ohio
Signature Health
Ashtabula, Ohio
Behavioral Wellness Group
Mentor, Ohio
Continue to: The authors respond
The authors respond
We appreciate Drs. Schenkel’s and Primc’s comments on our editorial regarding measurement-based care (MBC). However, MBC will not increase the workload of psychiatrists; rather, it will streamline the evaluation of patients and measure the severity of their symptoms or adverse effects as well as the degree of their improvement. The proper use of scales with the appropriate patient populations may actually help clinicians to reduce the extensive amount of details that go into medical records.
The following quote, an excerpt from another article we wrote on MBC,1 speaks to Dr. Primc’s concerns:
“…measures in psychiatry could be considered the equivalent of a thermometer and a stethoscope to a physician.
Ahmed A. Aboraya, MD, DrPH
Assistant Professor
Department of Behavioral Medicine and Psychiatry
Chief of Psychiatry
Sharpe Hospital West Virginia University
Weston, West Virginia
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
Reference
1. Aboraya A, Nasrallah HA, Elswick DE, et al. Measurement-based care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
I strongly disagree with the editorial by Ahmed A. Aboraya, MD, DrPH, and Henry A. Nasrallah, MD, (“It’s time to implement measurement-based care in psychiatric practice,” From the Editor,
We do not have much more to lose before it’s a checklist, vital signs, and a script. I now refer to our profession as “McMedicine.” If you don’t have what is on the menu, you cannot get served. Diseases are rarely treated, symptoms are treated. This is not the profession of medicine. We are not fixing much; we are mostly providing consumers for pharmaceutical companies.
Few psychiatric disorders have been subjected to more measurement than depression. Quite a while ago, someone tried to compare depression scales. They correlated scale scores with the results of evaluations by board-certified psychiatrists. The best scale was a single question: “Are you depressed?” This had been included as a control. Can you do better?
Furthermore, the “paper and numbers” people can’t wait to get an “objective” wrench to tighten the screws and apply the principles of the industrial revolution to squeeze more money out of the system. They will find some way to turn patients into standardized products.
John L. Schenkel, MD
Retired psychiatrist
Peru, NY
With the use of an electronic medical record, what should be a simple 1-page note is transformed into a 5-page note of details. Doctors no longer attend to their patients but rather to their computers. Has this raised consciousness—the most important metric, according to Dr. David Hawkins? I doubt it.
In the words of my great professor, Dr. James Gustafson, I will continue to start my interview with what concerns the patient. Most of the time, they implicitly know.
Our focus should instead be on bringing down the cost of health care. This is what angers our patients most, and yet we do not make it a priority.
Psychiatrist
Glenbeigh Hospital
Rock Creek, Ohio
Signature Health
Ashtabula, Ohio
Behavioral Wellness Group
Mentor, Ohio
Continue to: The authors respond
The authors respond
We appreciate Drs. Schenkel’s and Primc’s comments on our editorial regarding measurement-based care (MBC). However, MBC will not increase the workload of psychiatrists; rather, it will streamline the evaluation of patients and measure the severity of their symptoms or adverse effects as well as the degree of their improvement. The proper use of scales with the appropriate patient populations may actually help clinicians to reduce the extensive amount of details that go into medical records.
The following quote, an excerpt from another article we wrote on MBC,1 speaks to Dr. Primc’s concerns:
“…measures in psychiatry could be considered the equivalent of a thermometer and a stethoscope to a physician.
Ahmed A. Aboraya, MD, DrPH
Assistant Professor
Department of Behavioral Medicine and Psychiatry
Chief of Psychiatry
Sharpe Hospital West Virginia University
Weston, West Virginia
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
Reference
1. Aboraya A, Nasrallah HA, Elswick DE, et al. Measurement-based care in psychiatry-past, present, and future. Innov Clin Neurosci. 2018;15(11-12):13-26.
Seizure-like episodes, but is it really epilepsy?
CASE Increasingly frequent paroxysmal episodes
Ms. N, age 12, comes to the hospital for evaluation of paroxysmal episodes of pain, weakness, and muscle spasms. A neurologist who evaluated her as an outpatient had recommended a routine electroencephalogram (EEG); after those results were inconclusive, Ms. N’s mother brought her to the hospital for a 24-hour video EEG.
Ms. N has a history of asthma. She has no history of seizures or headache, but her mother has an unspecified seizure disorder that has been stable with antiepileptic medication for many years. Ms. N has no other family history of autoimmune or neurologic disorders.
Ms. N’s episodes began 6 months ago and have progressively increased in frequency from 5 to 12 episodes a day. She says that before she has an episode, she “ feels tingling in her fingers and mouth, and butterflies in her belly,” and then her “whole body clenches up.” She denies experiencing tongue biting, facial or extremity weakness, incontinence, or loss of consciousness during these episodes.
Shortly before her hospitalization, Ms. N had won a scholarship to attend an overnight art camp. Because her episodes were becoming more frequent and their etiology remained unclear, Ms. N and her mother decided it would be unsafe for her to attend, and that she should go to the hospital for evaluation instead.
EVALUATION Tough questions reveal answers
The pediatric team evaluates Ms. N. Her physical exam, laboratory values, and imaging are all within normal limits. Her neurologic exam demonstrates full strength, tone, and sensation in all extremities. All cranial nerves and reflexes are intact. No dysmorphic features or gait abnormalities are noted. All laboratory and imaging tests are normal, including complete blood cell count, electrolytes, calcium, magnesium, phosphorus, glucose, creatine kinase, liver enzymes, urine drug screen, human chorionic gonadotropin (hCG) urine test, and h
After the initial workup, the pediatric team consults the child and adolescent psychiatry team for a complete assessment of Ms. N due to concerns that a psychological component is contributing to her episodes. According to the psychosocial history obtained from Ms. N and her mother, Ms. N had experienced disrupted attachment, trauma, and loss. At age 5, Ms. N was temporarily removed from her mother’s custody after a fight between her mother and brother. At age 9, Ms. N’s stepfather, her primary father figure, died of a brain tumor.
Ms. N also has significant trauma stemming from her relationship with her biological father. Ms. N’s mother reports that her daughter was conceived during nonconsensual sexual intercourse. Ms. N did not have much contact with her biological father until 6 months ago, when he started picking her up at school and taking her to his home for several hours without permission or supervision. Afterwards, Ms. N confided to her mother and a teacher that her father sexually assaulted her during those visits.
Continue to: Ms. N and her mother...
Ms. N and her mother reported the assault to the police and were awaiting legal action.
During the interview with the psychiatry team, Ms. N denies that any thoughts or actions trigger the episodes and reports that she cannot control when they happen. Because she cannot anticipate the episodes, she says she is afraid to leave her house. She does not know why the episodes are happening and feels frustrated that they are getting worse. Ms. N says, “I have been feeling down lately,” but she denies hopelessness, worthlessness, suicidal ideation, homicidal ideation, delusions, or hallucinations.
In the hospital, when the psychiatry team asks Ms. N about her visits with her father, she says that they are “too painful to talk about,” and fears that discussing them will trigger an episode. However, her mother suggests that her daughter’s sexual trauma, as well as ongoing frustrations with the legal system, are influencing her mood; she has had low energy, poor appetite, and is spending more time in bed. Her mother also reports that Ms. N “avoids going out in the sun and spending time with her friends outside. She doesn’t seem to enjoy shopping and art like she used to.” Ms. N told her mother that she was having nightmares about the trauma and “could not stop thinking about some of the bad stuff that happened during the day.”
Ten minutes into the interview, while being questioned about her father, Ms. N experiences a spastic episode. She curls up in bed on her left side, clenches her entire body, and shuts her eyes. Her mother quickly runs to her bedside and counts the seconds until the end of the episode. After 25 seconds, Ms. N awakes with full recollection of the episode. On review of the video EEG during the episode, no ictal patterns are seen.
[polldaddy:10375873]
The authors’ observations
Paroxysmal episodes of weakness, numbness, and muscle spasms in a young female are suggestive of either epilepsy or nonepileptic seizure (NES).1,2 The negative EEG and physical features are inconsistent with epileptiform seizure, and Ms. N’s history and evaluation are suggestive of NES. Nonepileptic seizures are a type of a conversion disorder, or functional neurologic symptom disorder, in which a patient experiences weakness, abnormal movements, or seizure-like episodes that are inconsistent with organic neurologic disease.3 When a diagnosis of conversion disorder is suspected, a clinician must always consider other pathology that can explain the symptoms, such as migraine, vasovagal syncope, or intracranial mass. If a patient has focal neurologic deficits, head imaging should be pursued. Additionally, the clinician must screen for malingering and factitious disorder before establishing a definitive diagnosis. However, conversion disorder is not a diagnosis of exclusion. For example, a negative EEG does not rule out epilepsy, and patients can have both epilepsy and concomitant NES.
Continue to: Although NES is a common...
Although NES is a common type of conversion disorder, it is often difficult to diagnose, manage, and treat. Patients often receive antiepileptic medications but continue to have worsening events that are refractory to treatment. Various clinical features can suggest NES instead of epilepsy. Forced eye closure on video recording is a specific finding suggestive of NES, yet this feature is not sufficient to make the diagnosis.4 A video EEG must be performed to assess for epilepsy. The diagnosis of NES does not exclude the possibility that a patient has epilepsy, as NES can occur in up to 40% of patients with epilepsy.5 A video EEG without ictal patterns before, during, and after an observed episode is diagnostic of NES.6
[polldaddy:10375874]
The authors’ observations
Conversion disorders such as NES are a presentation of neurologic symptoms that cannot be readily accounted for by other conditions and are often associated with antecedent trauma. Multiple factors in Ms. N’s history increase her risk of NES, including loss of multiple loved ones, ongoing legal involvement, and alleged sexual abuse by her father.
Victims of sexual abuse are more likely than the general population to demonstrate symptoms of conversion disorder, especially NES.7,8 The onset of paroxysmal episodes after incestuous abuse in a teenage girl is characteristic of NES. Compared with patients with complex partial epilepsy (CPE), patients with NES are 3 times more likely to report sexual trauma.9,10 Children who report sexual abuse that precedes NES are more likely to have been victimized by a first-degree relative than patients with CPE who report sexual abuse.11 Risk factors for victims developing NES may be related to the severity of adversity, stress sensitivity, and decreased hippocampal volume.12,13
Ms. N endorsed many psychiatric symptoms that accompany her paroxysmal episodes; this is similar to findings in other patients with NES.14 One study found that depression is 3 times more prevalent and PTSD is 8 times more prevalent in patients with NES.12 During the evaluation, Ms. N’s mother said her daughter had low energy, poor appetite, lethargy, and anhedonia for the preceding 5 months, which is consistent with adjustment disorder.3 Her flashbacks, nightmares, difficulty sleeping, and agoraphobia, along with her trouble engaging with the people and activities that used to bring her joy, are symptoms of PTSD. Nonepileptic seizure is often associated with PTSD and can be viewed as an expression of a dissociated subtype.15
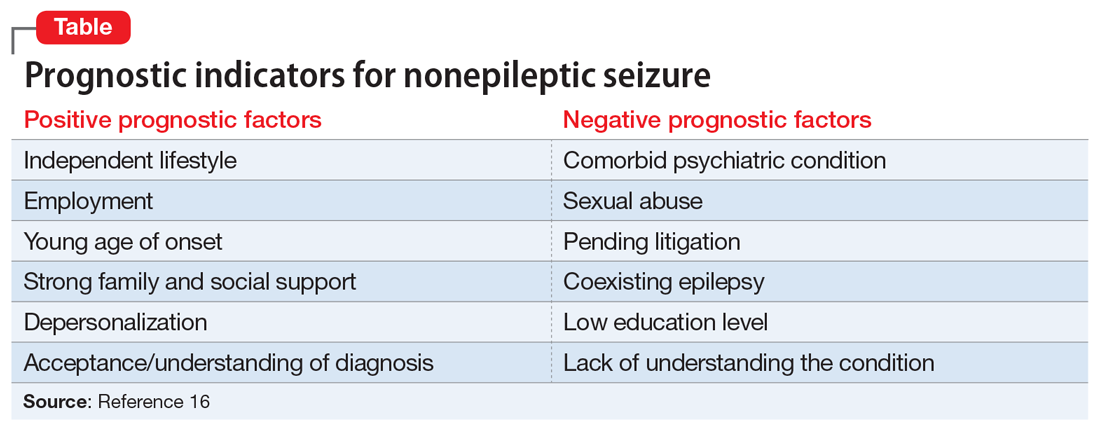
In a literature review, Durrant et al16 isolated prognostic indicators for NES (Table16). This study found that 70% of children and 40% of adults achieve remission from NES. Ms. N’s case has multiple concerning features, such as her comorbid psychiatric conditions, ongoing involvement in a legal case, and sexual trauma; this last factor is associated with the most severe symptoms and worse outcomes.16,17 Despite this somber reality, Ms. N has the support of her mother and is relatively young, which play a vital role in recovery.
Continue to: TREATMENT A strategy for minimizing the episodes
TREATMENT A strategy for minimizing the episodes
Ms. N’s medical workup remains unremarkable throughout the rest of her hospital stay. The psychiatry and pediatric teams discuss their assessments and agree that NES is the most likely diagnosis. The psychiatry team counsels Ms. N and her mother on the diagnosis and etiology of NES.
[polldaddy:10375876]
The authors’ observations
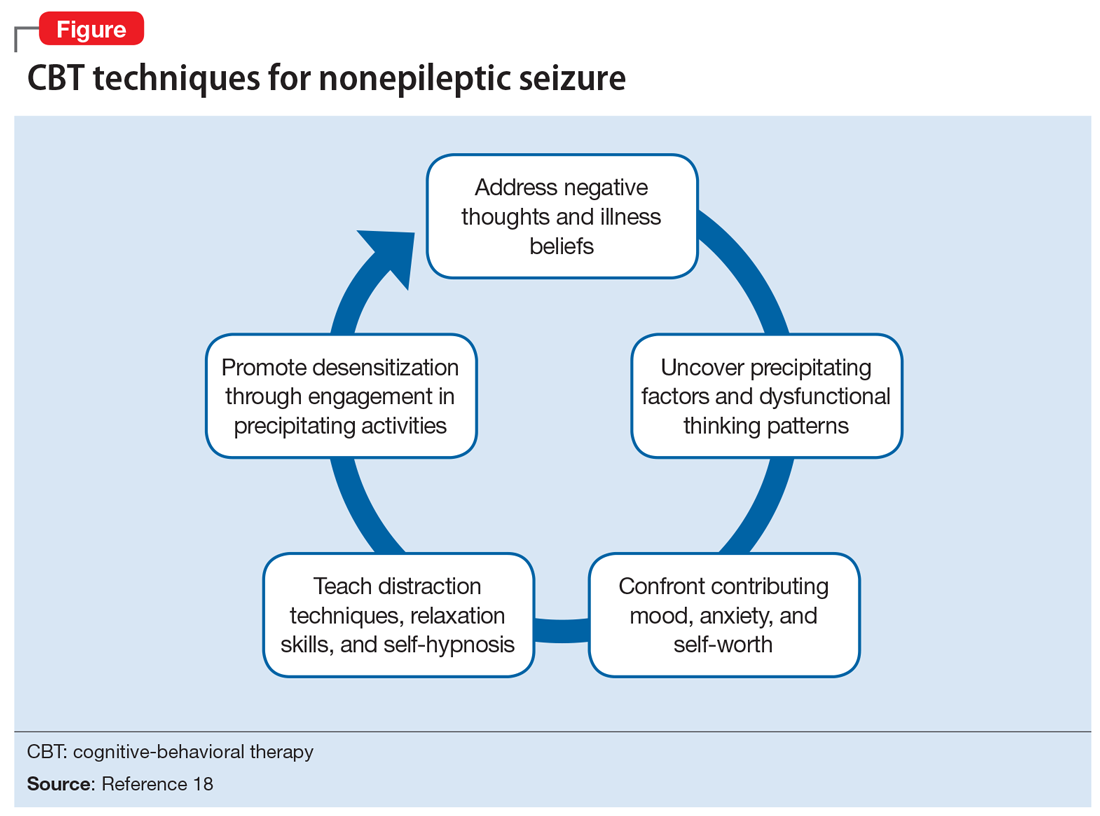
Cognitive-behavioral therapy is currently the treatment of choice for reducing seizure frequency in patients with NES.18,19 The use of CBT was suggested due to the theory that NES represents a dissociative response to trauma. Therapy focuses on changing a patient’s beliefs and perceptions associated with attacks.5 A randomized study of 66 patients with NES compared the use of CBT plus standard medical care with standard medical care alone.18 The standard medical care consisted of supportive treatment, an explanation of NES from a neuropsychiatrist, and supervised withdrawal of antiepileptic drugs. The CBT treatment group was offered weekly hour-long sessions for 12 weeks, accompanied by CBT homework and journaling the frequency and nature of seizure episodes (the CBT techniques are outlined in the Figure18). After 4 months, the CBT treatment group had fewer seizures, and after a 6-month follow-up, they were more likely to be seizure-free. However, in this study, CBT treatment did not improve mood or employment status.
A later investigation looked at using selective serotonin reuptake inhibitors to treat NES in adults.19 This study divided participants into 4 treatment groups: CBT with informed psychotherapy (CBT-ip), CBT-ip plus sertraline, sertraline alone, and treatment as usual. Sertraline was titrated up to a dose of 200 mg/d as tolerated. After 16 weeks of sertraline alone, seizure frequency did not decrease. Although both CBT groups showed a reduction in symptoms of up to 60%, the CBT-ip group reported fewer psychiatric symptoms with better social interactions, quality of life, and global functioning compared with patients treated with CBT-ip plus sertraline. The authors suggested that this may be due to the somatic adverse effects associated with sertraline. This study suggests that CBT without medication is the treatment of choice.
In addition to CBT, studies of psychodynamic psychotherapy for NES have had promising findings.20 Psychodynamic psychotherapy focuses on addressing conscious and unconscious anger, loss, feelings of isolation, and trauma. Through improving emotional processing, insight, coping skills and self-regulation, patients often benefit from an improvement in seizures, psychosocial functioning and health care utilization.
Metin et al21 found that group therapy alongside a family-centered approach elicited a strong and durable reduction in seizures in patients with NES. At enrollment, investigators distributed information on NES to patients and families. Psychoeducation and psychoanalysis with behavior modification techniques were provided in 90-minute weekly group sessions over 3 months. Participants also underwent monthly individualized sessions for standard psychiatric care for 9 months. During the group sessions, operant conditioning techniques were used to prevent secondary gain from seizure-like activity. Families met 4 times for 1 hour each to discuss seizures, receive psychoeducation on a subconscious etiology of NES, and learn behavior modification techniques. All 9 participants who completed group and individual therapy reported a significant and sustained reduction in seizure frequency by at least 50% at 12-month follow-up. Patients also demonstrated improvements in mood, anxiety, and quality of life.
Continue to: A meta-analysis...
A meta-analysis by Carlson and Perry22 that included 13 studies and 228 participants, examined different treatment modalities and their effectiveness for NES. They found that patients who received psychological intervention had a 47% remission rate and 82% improvement in seizure frequency compared with only 14% to 23% of those who did not receive therapy. They postulated that therapy for this illness must be flexible to properly address the socially, psychologically, and functionally heterogenous patient population. Although there are few randomized controlled trials for NES to determine the best evidence-based intervention, there is now consensus that NES has a favorable prognosis when barriers to psychological care are eliminated.
OUTCOME Referral for CBT
The treatment team advises Ms. N to engage in outpatient therapy after discharge from the hospital. Ms. N and her mother agree to the treatment plan, and leave the hospital with a referral for CBT the next day.
Bottom Line
Nonepileptic seizure (NES) is a type of conversion disorder characterized by seizure-like episodes without ictal qualities. Risk factors for NES include concomitant epilepsy, psychiatric disorders, unstable psychosocial situations, and antecedent trauma. Patients with a history of incestuous sexual abuse are most at risk for developing NES. A normal EEG that fully captures a seizure-like episode is diagnostic of NES. Cognitive-behavioral therapy can minimize seizure frequency and intensity.
Related Resources
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-24, 32-35.
- LaFrance WC Jr. Eye-opening behaviors help diagnose nonepileptic seizures. Current Psychiatry. 2006;5(11):121-122, 124, 130.
- LaFrance WC Jr, Kanner AM, Barry JJ. Treating patients with psychological nonepileptic seizures. In: Ettinger AB, Kanner AM, eds. Psychiatric issues in epilepsy: a practical guide to diagnosis and treatment. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2007:461-488.
Drug Brand Name
Sertraline • Zoloft
1. Lesser R. Psychogenic seizures. Neurology. 1996;46(6):1499-1507.
2. Stone J, LaFrance W, Brown R, et al. Conversion disorder: current problems and potential solutions for DSM-5. J Psychosom Res. 2011;71(6):369-376.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Syed T, Arozullah A, Suciu G, et al. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia. 2008;49(5):898-904.
5. Vega-Zelaya L, Alvarez M, Ezquiaga E, et al. Psychogenic non-epileptic seizures in a surgical epilepsy unit: experience and a comprehensive review. Epilepsy Topics. 2014. doi: 10.5772/57439.
6. LaFrance W, Baker G, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach. Epilepsia. 2013;54(11):2005-2018.
7. Roeloes K, Pasman J. Stress, childhood trauma, and cognitive functions in functional neurologic disorders. In: Hallett M, Stone J, Carson A, eds. Handbook of clinical neurology: functional neurologic disorders. 3rd ed. New York, NY: Elsevier; 2017:139-155.
8. Paras M, Murad M, Chen L, et al. Sexual abuse and lifetime diagnosis of somatic disorders. JAMA. 2009;302(5):550.
9. Fiszman A, Alves-Leon SV, Nunes RG, et al. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy Behav. 2004;5(6):818-825.
10. Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev. 2006;26(8):1020-1040.
11. Alper K, Devinsky O, Perrine K, et al. Nonepileptic seizures and childhood sexual and physical abuse. Neurology. 1993;43(10):1950-1953.
12. Plioplys S, Doss J, Siddarth P et al. A multisite controlled study of risk factors in pediatric psychogenic nonepileptic seizures. Epilepsia. 2014;55(11):1739-1747.
13. Andersen S, Tomada A, Vincow E, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292-301.
14. Sar V. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
15. Rosenberg HJ, Rosenberg SD, Williamson PD, et al. A comparative study of trauma and posttraumatic stress disorder prevalence in epilepsy patients and psychogenic nonepileptic seizure patients. Epilepsia. 2000;41(4):447-452.
16. Durrant J, Rickards H, Cavanna A. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res Treat. 2011;2011:1-7.
17. Selkirk M, Duncan R, Oto M, et al. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia. 2008;49(8):1446-1450.
18. Goldstein L, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010;74(24):1986-1994.
19. LaFrance W, Baird G, Barry J, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures. JAMA Psychiatry. 2014;71(9):997.
20. Howlett S, Reuber M. An augmented model of brief psychodynamic interpersonal therapy for patients with nonepileptic seizures. Psychotherapy (Chic). 2009;46(1):125-138.
21. Metin SZ, Ozmen M, Metin B, et al. Treatment with group psychotherapy for chronic psychogenic nonepileptic seizures. Epilepsy Behav. 2013;28(1):91-94.
22. Carlson P, Perry KN. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure. 2017;45:142-150.
CASE Increasingly frequent paroxysmal episodes
Ms. N, age 12, comes to the hospital for evaluation of paroxysmal episodes of pain, weakness, and muscle spasms. A neurologist who evaluated her as an outpatient had recommended a routine electroencephalogram (EEG); after those results were inconclusive, Ms. N’s mother brought her to the hospital for a 24-hour video EEG.
Ms. N has a history of asthma. She has no history of seizures or headache, but her mother has an unspecified seizure disorder that has been stable with antiepileptic medication for many years. Ms. N has no other family history of autoimmune or neurologic disorders.
Ms. N’s episodes began 6 months ago and have progressively increased in frequency from 5 to 12 episodes a day. She says that before she has an episode, she “ feels tingling in her fingers and mouth, and butterflies in her belly,” and then her “whole body clenches up.” She denies experiencing tongue biting, facial or extremity weakness, incontinence, or loss of consciousness during these episodes.
Shortly before her hospitalization, Ms. N had won a scholarship to attend an overnight art camp. Because her episodes were becoming more frequent and their etiology remained unclear, Ms. N and her mother decided it would be unsafe for her to attend, and that she should go to the hospital for evaluation instead.
EVALUATION Tough questions reveal answers
The pediatric team evaluates Ms. N. Her physical exam, laboratory values, and imaging are all within normal limits. Her neurologic exam demonstrates full strength, tone, and sensation in all extremities. All cranial nerves and reflexes are intact. No dysmorphic features or gait abnormalities are noted. All laboratory and imaging tests are normal, including complete blood cell count, electrolytes, calcium, magnesium, phosphorus, glucose, creatine kinase, liver enzymes, urine drug screen, human chorionic gonadotropin (hCG) urine test, and h
After the initial workup, the pediatric team consults the child and adolescent psychiatry team for a complete assessment of Ms. N due to concerns that a psychological component is contributing to her episodes. According to the psychosocial history obtained from Ms. N and her mother, Ms. N had experienced disrupted attachment, trauma, and loss. At age 5, Ms. N was temporarily removed from her mother’s custody after a fight between her mother and brother. At age 9, Ms. N’s stepfather, her primary father figure, died of a brain tumor.
Ms. N also has significant trauma stemming from her relationship with her biological father. Ms. N’s mother reports that her daughter was conceived during nonconsensual sexual intercourse. Ms. N did not have much contact with her biological father until 6 months ago, when he started picking her up at school and taking her to his home for several hours without permission or supervision. Afterwards, Ms. N confided to her mother and a teacher that her father sexually assaulted her during those visits.
Continue to: Ms. N and her mother...
Ms. N and her mother reported the assault to the police and were awaiting legal action.
During the interview with the psychiatry team, Ms. N denies that any thoughts or actions trigger the episodes and reports that she cannot control when they happen. Because she cannot anticipate the episodes, she says she is afraid to leave her house. She does not know why the episodes are happening and feels frustrated that they are getting worse. Ms. N says, “I have been feeling down lately,” but she denies hopelessness, worthlessness, suicidal ideation, homicidal ideation, delusions, or hallucinations.
In the hospital, when the psychiatry team asks Ms. N about her visits with her father, she says that they are “too painful to talk about,” and fears that discussing them will trigger an episode. However, her mother suggests that her daughter’s sexual trauma, as well as ongoing frustrations with the legal system, are influencing her mood; she has had low energy, poor appetite, and is spending more time in bed. Her mother also reports that Ms. N “avoids going out in the sun and spending time with her friends outside. She doesn’t seem to enjoy shopping and art like she used to.” Ms. N told her mother that she was having nightmares about the trauma and “could not stop thinking about some of the bad stuff that happened during the day.”
Ten minutes into the interview, while being questioned about her father, Ms. N experiences a spastic episode. She curls up in bed on her left side, clenches her entire body, and shuts her eyes. Her mother quickly runs to her bedside and counts the seconds until the end of the episode. After 25 seconds, Ms. N awakes with full recollection of the episode. On review of the video EEG during the episode, no ictal patterns are seen.
[polldaddy:10375873]
The authors’ observations
Paroxysmal episodes of weakness, numbness, and muscle spasms in a young female are suggestive of either epilepsy or nonepileptic seizure (NES).1,2 The negative EEG and physical features are inconsistent with epileptiform seizure, and Ms. N’s history and evaluation are suggestive of NES. Nonepileptic seizures are a type of a conversion disorder, or functional neurologic symptom disorder, in which a patient experiences weakness, abnormal movements, or seizure-like episodes that are inconsistent with organic neurologic disease.3 When a diagnosis of conversion disorder is suspected, a clinician must always consider other pathology that can explain the symptoms, such as migraine, vasovagal syncope, or intracranial mass. If a patient has focal neurologic deficits, head imaging should be pursued. Additionally, the clinician must screen for malingering and factitious disorder before establishing a definitive diagnosis. However, conversion disorder is not a diagnosis of exclusion. For example, a negative EEG does not rule out epilepsy, and patients can have both epilepsy and concomitant NES.
Continue to: Although NES is a common...
Although NES is a common type of conversion disorder, it is often difficult to diagnose, manage, and treat. Patients often receive antiepileptic medications but continue to have worsening events that are refractory to treatment. Various clinical features can suggest NES instead of epilepsy. Forced eye closure on video recording is a specific finding suggestive of NES, yet this feature is not sufficient to make the diagnosis.4 A video EEG must be performed to assess for epilepsy. The diagnosis of NES does not exclude the possibility that a patient has epilepsy, as NES can occur in up to 40% of patients with epilepsy.5 A video EEG without ictal patterns before, during, and after an observed episode is diagnostic of NES.6
[polldaddy:10375874]
The authors’ observations
Conversion disorders such as NES are a presentation of neurologic symptoms that cannot be readily accounted for by other conditions and are often associated with antecedent trauma. Multiple factors in Ms. N’s history increase her risk of NES, including loss of multiple loved ones, ongoing legal involvement, and alleged sexual abuse by her father.
Victims of sexual abuse are more likely than the general population to demonstrate symptoms of conversion disorder, especially NES.7,8 The onset of paroxysmal episodes after incestuous abuse in a teenage girl is characteristic of NES. Compared with patients with complex partial epilepsy (CPE), patients with NES are 3 times more likely to report sexual trauma.9,10 Children who report sexual abuse that precedes NES are more likely to have been victimized by a first-degree relative than patients with CPE who report sexual abuse.11 Risk factors for victims developing NES may be related to the severity of adversity, stress sensitivity, and decreased hippocampal volume.12,13
Ms. N endorsed many psychiatric symptoms that accompany her paroxysmal episodes; this is similar to findings in other patients with NES.14 One study found that depression is 3 times more prevalent and PTSD is 8 times more prevalent in patients with NES.12 During the evaluation, Ms. N’s mother said her daughter had low energy, poor appetite, lethargy, and anhedonia for the preceding 5 months, which is consistent with adjustment disorder.3 Her flashbacks, nightmares, difficulty sleeping, and agoraphobia, along with her trouble engaging with the people and activities that used to bring her joy, are symptoms of PTSD. Nonepileptic seizure is often associated with PTSD and can be viewed as an expression of a dissociated subtype.15
In a literature review, Durrant et al16 isolated prognostic indicators for NES (Table16). This study found that 70% of children and 40% of adults achieve remission from NES. Ms. N’s case has multiple concerning features, such as her comorbid psychiatric conditions, ongoing involvement in a legal case, and sexual trauma; this last factor is associated with the most severe symptoms and worse outcomes.16,17 Despite this somber reality, Ms. N has the support of her mother and is relatively young, which play a vital role in recovery.

Continue to: TREATMENT A strategy for minimizing the episodes
TREATMENT A strategy for minimizing the episodes
Ms. N’s medical workup remains unremarkable throughout the rest of her hospital stay. The psychiatry and pediatric teams discuss their assessments and agree that NES is the most likely diagnosis. The psychiatry team counsels Ms. N and her mother on the diagnosis and etiology of NES.
[polldaddy:10375876]
The authors’ observations
Cognitive-behavioral therapy is currently the treatment of choice for reducing seizure frequency in patients with NES.18,19 The use of CBT was suggested due to the theory that NES represents a dissociative response to trauma. Therapy focuses on changing a patient’s beliefs and perceptions associated with attacks.5 A randomized study of 66 patients with NES compared the use of CBT plus standard medical care with standard medical care alone.18 The standard medical care consisted of supportive treatment, an explanation of NES from a neuropsychiatrist, and supervised withdrawal of antiepileptic drugs. The CBT treatment group was offered weekly hour-long sessions for 12 weeks, accompanied by CBT homework and journaling the frequency and nature of seizure episodes (the CBT techniques are outlined in the Figure18). After 4 months, the CBT treatment group had fewer seizures, and after a 6-month follow-up, they were more likely to be seizure-free. However, in this study, CBT treatment did not improve mood or employment status.

A later investigation looked at using selective serotonin reuptake inhibitors to treat NES in adults.19 This study divided participants into 4 treatment groups: CBT with informed psychotherapy (CBT-ip), CBT-ip plus sertraline, sertraline alone, and treatment as usual. Sertraline was titrated up to a dose of 200 mg/d as tolerated. After 16 weeks of sertraline alone, seizure frequency did not decrease. Although both CBT groups showed a reduction in symptoms of up to 60%, the CBT-ip group reported fewer psychiatric symptoms with better social interactions, quality of life, and global functioning compared with patients treated with CBT-ip plus sertraline. The authors suggested that this may be due to the somatic adverse effects associated with sertraline. This study suggests that CBT without medication is the treatment of choice.
In addition to CBT, studies of psychodynamic psychotherapy for NES have had promising findings.20 Psychodynamic psychotherapy focuses on addressing conscious and unconscious anger, loss, feelings of isolation, and trauma. Through improving emotional processing, insight, coping skills and self-regulation, patients often benefit from an improvement in seizures, psychosocial functioning and health care utilization.
Metin et al21 found that group therapy alongside a family-centered approach elicited a strong and durable reduction in seizures in patients with NES. At enrollment, investigators distributed information on NES to patients and families. Psychoeducation and psychoanalysis with behavior modification techniques were provided in 90-minute weekly group sessions over 3 months. Participants also underwent monthly individualized sessions for standard psychiatric care for 9 months. During the group sessions, operant conditioning techniques were used to prevent secondary gain from seizure-like activity. Families met 4 times for 1 hour each to discuss seizures, receive psychoeducation on a subconscious etiology of NES, and learn behavior modification techniques. All 9 participants who completed group and individual therapy reported a significant and sustained reduction in seizure frequency by at least 50% at 12-month follow-up. Patients also demonstrated improvements in mood, anxiety, and quality of life.
Continue to: A meta-analysis...
A meta-analysis by Carlson and Perry22 that included 13 studies and 228 participants, examined different treatment modalities and their effectiveness for NES. They found that patients who received psychological intervention had a 47% remission rate and 82% improvement in seizure frequency compared with only 14% to 23% of those who did not receive therapy. They postulated that therapy for this illness must be flexible to properly address the socially, psychologically, and functionally heterogenous patient population. Although there are few randomized controlled trials for NES to determine the best evidence-based intervention, there is now consensus that NES has a favorable prognosis when barriers to psychological care are eliminated.
OUTCOME Referral for CBT
The treatment team advises Ms. N to engage in outpatient therapy after discharge from the hospital. Ms. N and her mother agree to the treatment plan, and leave the hospital with a referral for CBT the next day.
Bottom Line
Nonepileptic seizure (NES) is a type of conversion disorder characterized by seizure-like episodes without ictal qualities. Risk factors for NES include concomitant epilepsy, psychiatric disorders, unstable psychosocial situations, and antecedent trauma. Patients with a history of incestuous sexual abuse are most at risk for developing NES. A normal EEG that fully captures a seizure-like episode is diagnostic of NES. Cognitive-behavioral therapy can minimize seizure frequency and intensity.
Related Resources
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-24, 32-35.
- LaFrance WC Jr. Eye-opening behaviors help diagnose nonepileptic seizures. Current Psychiatry. 2006;5(11):121-122, 124, 130.
- LaFrance WC Jr, Kanner AM, Barry JJ. Treating patients with psychological nonepileptic seizures. In: Ettinger AB, Kanner AM, eds. Psychiatric issues in epilepsy: a practical guide to diagnosis and treatment. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2007:461-488.
Drug Brand Name
Sertraline • Zoloft
CASE Increasingly frequent paroxysmal episodes
Ms. N, age 12, comes to the hospital for evaluation of paroxysmal episodes of pain, weakness, and muscle spasms. A neurologist who evaluated her as an outpatient had recommended a routine electroencephalogram (EEG); after those results were inconclusive, Ms. N’s mother brought her to the hospital for a 24-hour video EEG.
Ms. N has a history of asthma. She has no history of seizures or headache, but her mother has an unspecified seizure disorder that has been stable with antiepileptic medication for many years. Ms. N has no other family history of autoimmune or neurologic disorders.
Ms. N’s episodes began 6 months ago and have progressively increased in frequency from 5 to 12 episodes a day. She says that before she has an episode, she “ feels tingling in her fingers and mouth, and butterflies in her belly,” and then her “whole body clenches up.” She denies experiencing tongue biting, facial or extremity weakness, incontinence, or loss of consciousness during these episodes.
Shortly before her hospitalization, Ms. N had won a scholarship to attend an overnight art camp. Because her episodes were becoming more frequent and their etiology remained unclear, Ms. N and her mother decided it would be unsafe for her to attend, and that she should go to the hospital for evaluation instead.
EVALUATION Tough questions reveal answers
The pediatric team evaluates Ms. N. Her physical exam, laboratory values, and imaging are all within normal limits. Her neurologic exam demonstrates full strength, tone, and sensation in all extremities. All cranial nerves and reflexes are intact. No dysmorphic features or gait abnormalities are noted. All laboratory and imaging tests are normal, including complete blood cell count, electrolytes, calcium, magnesium, phosphorus, glucose, creatine kinase, liver enzymes, urine drug screen, human chorionic gonadotropin (hCG) urine test, and h
After the initial workup, the pediatric team consults the child and adolescent psychiatry team for a complete assessment of Ms. N due to concerns that a psychological component is contributing to her episodes. According to the psychosocial history obtained from Ms. N and her mother, Ms. N had experienced disrupted attachment, trauma, and loss. At age 5, Ms. N was temporarily removed from her mother’s custody after a fight between her mother and brother. At age 9, Ms. N’s stepfather, her primary father figure, died of a brain tumor.
Ms. N also has significant trauma stemming from her relationship with her biological father. Ms. N’s mother reports that her daughter was conceived during nonconsensual sexual intercourse. Ms. N did not have much contact with her biological father until 6 months ago, when he started picking her up at school and taking her to his home for several hours without permission or supervision. Afterwards, Ms. N confided to her mother and a teacher that her father sexually assaulted her during those visits.
Continue to: Ms. N and her mother...
Ms. N and her mother reported the assault to the police and were awaiting legal action.
During the interview with the psychiatry team, Ms. N denies that any thoughts or actions trigger the episodes and reports that she cannot control when they happen. Because she cannot anticipate the episodes, she says she is afraid to leave her house. She does not know why the episodes are happening and feels frustrated that they are getting worse. Ms. N says, “I have been feeling down lately,” but she denies hopelessness, worthlessness, suicidal ideation, homicidal ideation, delusions, or hallucinations.
In the hospital, when the psychiatry team asks Ms. N about her visits with her father, she says that they are “too painful to talk about,” and fears that discussing them will trigger an episode. However, her mother suggests that her daughter’s sexual trauma, as well as ongoing frustrations with the legal system, are influencing her mood; she has had low energy, poor appetite, and is spending more time in bed. Her mother also reports that Ms. N “avoids going out in the sun and spending time with her friends outside. She doesn’t seem to enjoy shopping and art like she used to.” Ms. N told her mother that she was having nightmares about the trauma and “could not stop thinking about some of the bad stuff that happened during the day.”
Ten minutes into the interview, while being questioned about her father, Ms. N experiences a spastic episode. She curls up in bed on her left side, clenches her entire body, and shuts her eyes. Her mother quickly runs to her bedside and counts the seconds until the end of the episode. After 25 seconds, Ms. N awakes with full recollection of the episode. On review of the video EEG during the episode, no ictal patterns are seen.
[polldaddy:10375873]
The authors’ observations
Paroxysmal episodes of weakness, numbness, and muscle spasms in a young female are suggestive of either epilepsy or nonepileptic seizure (NES).1,2 The negative EEG and physical features are inconsistent with epileptiform seizure, and Ms. N’s history and evaluation are suggestive of NES. Nonepileptic seizures are a type of a conversion disorder, or functional neurologic symptom disorder, in which a patient experiences weakness, abnormal movements, or seizure-like episodes that are inconsistent with organic neurologic disease.3 When a diagnosis of conversion disorder is suspected, a clinician must always consider other pathology that can explain the symptoms, such as migraine, vasovagal syncope, or intracranial mass. If a patient has focal neurologic deficits, head imaging should be pursued. Additionally, the clinician must screen for malingering and factitious disorder before establishing a definitive diagnosis. However, conversion disorder is not a diagnosis of exclusion. For example, a negative EEG does not rule out epilepsy, and patients can have both epilepsy and concomitant NES.
Continue to: Although NES is a common...
Although NES is a common type of conversion disorder, it is often difficult to diagnose, manage, and treat. Patients often receive antiepileptic medications but continue to have worsening events that are refractory to treatment. Various clinical features can suggest NES instead of epilepsy. Forced eye closure on video recording is a specific finding suggestive of NES, yet this feature is not sufficient to make the diagnosis.4 A video EEG must be performed to assess for epilepsy. The diagnosis of NES does not exclude the possibility that a patient has epilepsy, as NES can occur in up to 40% of patients with epilepsy.5 A video EEG without ictal patterns before, during, and after an observed episode is diagnostic of NES.6
[polldaddy:10375874]
The authors’ observations
Conversion disorders such as NES are a presentation of neurologic symptoms that cannot be readily accounted for by other conditions and are often associated with antecedent trauma. Multiple factors in Ms. N’s history increase her risk of NES, including loss of multiple loved ones, ongoing legal involvement, and alleged sexual abuse by her father.
Victims of sexual abuse are more likely than the general population to demonstrate symptoms of conversion disorder, especially NES.7,8 The onset of paroxysmal episodes after incestuous abuse in a teenage girl is characteristic of NES. Compared with patients with complex partial epilepsy (CPE), patients with NES are 3 times more likely to report sexual trauma.9,10 Children who report sexual abuse that precedes NES are more likely to have been victimized by a first-degree relative than patients with CPE who report sexual abuse.11 Risk factors for victims developing NES may be related to the severity of adversity, stress sensitivity, and decreased hippocampal volume.12,13
Ms. N endorsed many psychiatric symptoms that accompany her paroxysmal episodes; this is similar to findings in other patients with NES.14 One study found that depression is 3 times more prevalent and PTSD is 8 times more prevalent in patients with NES.12 During the evaluation, Ms. N’s mother said her daughter had low energy, poor appetite, lethargy, and anhedonia for the preceding 5 months, which is consistent with adjustment disorder.3 Her flashbacks, nightmares, difficulty sleeping, and agoraphobia, along with her trouble engaging with the people and activities that used to bring her joy, are symptoms of PTSD. Nonepileptic seizure is often associated with PTSD and can be viewed as an expression of a dissociated subtype.15
In a literature review, Durrant et al16 isolated prognostic indicators for NES (Table16). This study found that 70% of children and 40% of adults achieve remission from NES. Ms. N’s case has multiple concerning features, such as her comorbid psychiatric conditions, ongoing involvement in a legal case, and sexual trauma; this last factor is associated with the most severe symptoms and worse outcomes.16,17 Despite this somber reality, Ms. N has the support of her mother and is relatively young, which play a vital role in recovery.

Continue to: TREATMENT A strategy for minimizing the episodes
TREATMENT A strategy for minimizing the episodes
Ms. N’s medical workup remains unremarkable throughout the rest of her hospital stay. The psychiatry and pediatric teams discuss their assessments and agree that NES is the most likely diagnosis. The psychiatry team counsels Ms. N and her mother on the diagnosis and etiology of NES.
[polldaddy:10375876]
The authors’ observations
Cognitive-behavioral therapy is currently the treatment of choice for reducing seizure frequency in patients with NES.18,19 The use of CBT was suggested due to the theory that NES represents a dissociative response to trauma. Therapy focuses on changing a patient’s beliefs and perceptions associated with attacks.5 A randomized study of 66 patients with NES compared the use of CBT plus standard medical care with standard medical care alone.18 The standard medical care consisted of supportive treatment, an explanation of NES from a neuropsychiatrist, and supervised withdrawal of antiepileptic drugs. The CBT treatment group was offered weekly hour-long sessions for 12 weeks, accompanied by CBT homework and journaling the frequency and nature of seizure episodes (the CBT techniques are outlined in the Figure18). After 4 months, the CBT treatment group had fewer seizures, and after a 6-month follow-up, they were more likely to be seizure-free. However, in this study, CBT treatment did not improve mood or employment status.

A later investigation looked at using selective serotonin reuptake inhibitors to treat NES in adults.19 This study divided participants into 4 treatment groups: CBT with informed psychotherapy (CBT-ip), CBT-ip plus sertraline, sertraline alone, and treatment as usual. Sertraline was titrated up to a dose of 200 mg/d as tolerated. After 16 weeks of sertraline alone, seizure frequency did not decrease. Although both CBT groups showed a reduction in symptoms of up to 60%, the CBT-ip group reported fewer psychiatric symptoms with better social interactions, quality of life, and global functioning compared with patients treated with CBT-ip plus sertraline. The authors suggested that this may be due to the somatic adverse effects associated with sertraline. This study suggests that CBT without medication is the treatment of choice.
In addition to CBT, studies of psychodynamic psychotherapy for NES have had promising findings.20 Psychodynamic psychotherapy focuses on addressing conscious and unconscious anger, loss, feelings of isolation, and trauma. Through improving emotional processing, insight, coping skills and self-regulation, patients often benefit from an improvement in seizures, psychosocial functioning and health care utilization.
Metin et al21 found that group therapy alongside a family-centered approach elicited a strong and durable reduction in seizures in patients with NES. At enrollment, investigators distributed information on NES to patients and families. Psychoeducation and psychoanalysis with behavior modification techniques were provided in 90-minute weekly group sessions over 3 months. Participants also underwent monthly individualized sessions for standard psychiatric care for 9 months. During the group sessions, operant conditioning techniques were used to prevent secondary gain from seizure-like activity. Families met 4 times for 1 hour each to discuss seizures, receive psychoeducation on a subconscious etiology of NES, and learn behavior modification techniques. All 9 participants who completed group and individual therapy reported a significant and sustained reduction in seizure frequency by at least 50% at 12-month follow-up. Patients also demonstrated improvements in mood, anxiety, and quality of life.
Continue to: A meta-analysis...
A meta-analysis by Carlson and Perry22 that included 13 studies and 228 participants, examined different treatment modalities and their effectiveness for NES. They found that patients who received psychological intervention had a 47% remission rate and 82% improvement in seizure frequency compared with only 14% to 23% of those who did not receive therapy. They postulated that therapy for this illness must be flexible to properly address the socially, psychologically, and functionally heterogenous patient population. Although there are few randomized controlled trials for NES to determine the best evidence-based intervention, there is now consensus that NES has a favorable prognosis when barriers to psychological care are eliminated.
OUTCOME Referral for CBT
The treatment team advises Ms. N to engage in outpatient therapy after discharge from the hospital. Ms. N and her mother agree to the treatment plan, and leave the hospital with a referral for CBT the next day.
Bottom Line
Nonepileptic seizure (NES) is a type of conversion disorder characterized by seizure-like episodes without ictal qualities. Risk factors for NES include concomitant epilepsy, psychiatric disorders, unstable psychosocial situations, and antecedent trauma. Patients with a history of incestuous sexual abuse are most at risk for developing NES. A normal EEG that fully captures a seizure-like episode is diagnostic of NES. Cognitive-behavioral therapy can minimize seizure frequency and intensity.
Related Resources
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-24, 32-35.
- LaFrance WC Jr. Eye-opening behaviors help diagnose nonepileptic seizures. Current Psychiatry. 2006;5(11):121-122, 124, 130.
- LaFrance WC Jr, Kanner AM, Barry JJ. Treating patients with psychological nonepileptic seizures. In: Ettinger AB, Kanner AM, eds. Psychiatric issues in epilepsy: a practical guide to diagnosis and treatment. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2007:461-488.
Drug Brand Name
Sertraline • Zoloft
1. Lesser R. Psychogenic seizures. Neurology. 1996;46(6):1499-1507.
2. Stone J, LaFrance W, Brown R, et al. Conversion disorder: current problems and potential solutions for DSM-5. J Psychosom Res. 2011;71(6):369-376.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Syed T, Arozullah A, Suciu G, et al. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia. 2008;49(5):898-904.
5. Vega-Zelaya L, Alvarez M, Ezquiaga E, et al. Psychogenic non-epileptic seizures in a surgical epilepsy unit: experience and a comprehensive review. Epilepsy Topics. 2014. doi: 10.5772/57439.
6. LaFrance W, Baker G, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach. Epilepsia. 2013;54(11):2005-2018.
7. Roeloes K, Pasman J. Stress, childhood trauma, and cognitive functions in functional neurologic disorders. In: Hallett M, Stone J, Carson A, eds. Handbook of clinical neurology: functional neurologic disorders. 3rd ed. New York, NY: Elsevier; 2017:139-155.
8. Paras M, Murad M, Chen L, et al. Sexual abuse and lifetime diagnosis of somatic disorders. JAMA. 2009;302(5):550.
9. Fiszman A, Alves-Leon SV, Nunes RG, et al. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy Behav. 2004;5(6):818-825.
10. Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev. 2006;26(8):1020-1040.
11. Alper K, Devinsky O, Perrine K, et al. Nonepileptic seizures and childhood sexual and physical abuse. Neurology. 1993;43(10):1950-1953.
12. Plioplys S, Doss J, Siddarth P et al. A multisite controlled study of risk factors in pediatric psychogenic nonepileptic seizures. Epilepsia. 2014;55(11):1739-1747.
13. Andersen S, Tomada A, Vincow E, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292-301.
14. Sar V. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
15. Rosenberg HJ, Rosenberg SD, Williamson PD, et al. A comparative study of trauma and posttraumatic stress disorder prevalence in epilepsy patients and psychogenic nonepileptic seizure patients. Epilepsia. 2000;41(4):447-452.
16. Durrant J, Rickards H, Cavanna A. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res Treat. 2011;2011:1-7.
17. Selkirk M, Duncan R, Oto M, et al. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia. 2008;49(8):1446-1450.
18. Goldstein L, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010;74(24):1986-1994.
19. LaFrance W, Baird G, Barry J, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures. JAMA Psychiatry. 2014;71(9):997.
20. Howlett S, Reuber M. An augmented model of brief psychodynamic interpersonal therapy for patients with nonepileptic seizures. Psychotherapy (Chic). 2009;46(1):125-138.
21. Metin SZ, Ozmen M, Metin B, et al. Treatment with group psychotherapy for chronic psychogenic nonepileptic seizures. Epilepsy Behav. 2013;28(1):91-94.
22. Carlson P, Perry KN. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure. 2017;45:142-150.
1. Lesser R. Psychogenic seizures. Neurology. 1996;46(6):1499-1507.
2. Stone J, LaFrance W, Brown R, et al. Conversion disorder: current problems and potential solutions for DSM-5. J Psychosom Res. 2011;71(6):369-376.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Syed T, Arozullah A, Suciu G, et al. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia. 2008;49(5):898-904.
5. Vega-Zelaya L, Alvarez M, Ezquiaga E, et al. Psychogenic non-epileptic seizures in a surgical epilepsy unit: experience and a comprehensive review. Epilepsy Topics. 2014. doi: 10.5772/57439.
6. LaFrance W, Baker G, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach. Epilepsia. 2013;54(11):2005-2018.
7. Roeloes K, Pasman J. Stress, childhood trauma, and cognitive functions in functional neurologic disorders. In: Hallett M, Stone J, Carson A, eds. Handbook of clinical neurology: functional neurologic disorders. 3rd ed. New York, NY: Elsevier; 2017:139-155.
8. Paras M, Murad M, Chen L, et al. Sexual abuse and lifetime diagnosis of somatic disorders. JAMA. 2009;302(5):550.
9. Fiszman A, Alves-Leon SV, Nunes RG, et al. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy Behav. 2004;5(6):818-825.
10. Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev. 2006;26(8):1020-1040.
11. Alper K, Devinsky O, Perrine K, et al. Nonepileptic seizures and childhood sexual and physical abuse. Neurology. 1993;43(10):1950-1953.
12. Plioplys S, Doss J, Siddarth P et al. A multisite controlled study of risk factors in pediatric psychogenic nonepileptic seizures. Epilepsia. 2014;55(11):1739-1747.
13. Andersen S, Tomada A, Vincow E, et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292-301.
14. Sar V. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161(12):2271-2276.
15. Rosenberg HJ, Rosenberg SD, Williamson PD, et al. A comparative study of trauma and posttraumatic stress disorder prevalence in epilepsy patients and psychogenic nonepileptic seizure patients. Epilepsia. 2000;41(4):447-452.
16. Durrant J, Rickards H, Cavanna A. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res Treat. 2011;2011:1-7.
17. Selkirk M, Duncan R, Oto M, et al. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia. 2008;49(8):1446-1450.
18. Goldstein L, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010;74(24):1986-1994.
19. LaFrance W, Baird G, Barry J, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures. JAMA Psychiatry. 2014;71(9):997.
20. Howlett S, Reuber M. An augmented model of brief psychodynamic interpersonal therapy for patients with nonepileptic seizures. Psychotherapy (Chic). 2009;46(1):125-138.
21. Metin SZ, Ozmen M, Metin B, et al. Treatment with group psychotherapy for chronic psychogenic nonepileptic seizures. Epilepsy Behav. 2013;28(1):91-94.
22. Carlson P, Perry KN. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure. 2017;45:142-150.
How to avoid ‘checklist’ psychiatry
To determine whether a patient meets the criteria for a DSM-5 diagnosis, we rely on objective data, direct observations, and individual biopsychosocial factors as well as our patient’s subjective report of symptoms. However, because the line differentiating normal from abnormal emotional responses can sometimes be blurred, we should be prudent when establishing a diagnosis. Specifically, we need to avoid falling into the trap of “checklist” psychiatry—relegating diagnostic assessments to robotic statements about whether patients meet DSM criteria—because this can lead to making diagnoses too quickly or inaccurately.1 Potential consequences of checklist psychiatry include1,2:
- becoming so “married” to a particular diagnosis that you don’t consider alternative diagnoses
- labeling patients with a diagnosis that many clinicians may view as pejorative (eg, antisocial personality disorder), which might affect their ability to receive future treatment
- developing ineffective treatment plans based on an incorrect diagnosis, including exposing patients to medications that could have serious adverse effects
- performing suicide or violence risk assessments based on inaccurate diagnoses, thereby over- or underestimating the possible risk for an adverse outcome
- leading patients to assume the identity of the inaccurate diagnosis and possibly viewing themselves as dysfunctional or impaired.
When you are uncertain whether your patient has a diagnosable condition, it can be useful to use the terms “no diagnosis” or “diagnosis deferred.” However, many insurance companies will not reimburse without an actual diagnosis. Therefore, the following tips may be helpful in establishing an accurate diagnosis while avoiding checklist psychiatry.1,2
Ask patients about the degree and duration of impairment in functioning. Although impairment in functioning is a criterion of almost all DSM-5 diagnoses, not all endorsed symptoms warrant a diagnosis. Mild symptoms often resolve spontaneously over time without the need for diagnostic labels or interventions.
Make longitudinal observations. Interviewing patients over a long period of time and on multiple occasions can provide data on the consistency of reported symptoms, the presence or absence of behavioral correlates to reported symptomatology, the degree of impairment from the reported symptoms, and the evolution of symptoms.
Collect collateral information. Although we often rely on our patients’ reports of symptoms to establish a diagnosis, this information should not be the sole source. We can obtain a more complete picture if we approach a patient’s family members for their input, including asking about a family history of mental illness or substance use disorders. We can also review prior treatment records and gather observations from clinic or inpatient staff for additional information.
Order laboratory studies. Serum studies and urine toxicology screens provide information that can help form an accurate diagnosis. This information is helpful because certain medical conditions, substance intoxication, and substance withdrawal can mimic psychiatric symptoms.
Continuously re-evaluate your diagnoses. As clinicians, we’d like to provide an accurate diagnosis at the onset of treatment; however, this may not be realistic because the patient’s presentation might change over time. It is paramount that we view diagnoses as evolving, so that we can more readily adjust our approach to treatment, especially when the patient is not benefitting from a well-formulated and comprehensive treatment plan.
Our patients are best served when we take the necessary time to use all resources to conceptualize them as more than a checklist of symptoms.
1. Kontos N, Freudenreich O, Querques J. Thoughtful diagnoses: not ‘checklist’ psychiatry. Current Psychiatry. 2007;6(3):112.
2. Frances A. My 12 best tips on psychiatric diagnosis. Psychiatric Times. http://www.psychiatrictimes.com/dsm-5/my-12-best-tips-psychiatric-diagnosis. Published June 17, 2013. Accessed July 19, 2019.
To determine whether a patient meets the criteria for a DSM-5 diagnosis, we rely on objective data, direct observations, and individual biopsychosocial factors as well as our patient’s subjective report of symptoms. However, because the line differentiating normal from abnormal emotional responses can sometimes be blurred, we should be prudent when establishing a diagnosis. Specifically, we need to avoid falling into the trap of “checklist” psychiatry—relegating diagnostic assessments to robotic statements about whether patients meet DSM criteria—because this can lead to making diagnoses too quickly or inaccurately.1 Potential consequences of checklist psychiatry include1,2:
- becoming so “married” to a particular diagnosis that you don’t consider alternative diagnoses
- labeling patients with a diagnosis that many clinicians may view as pejorative (eg, antisocial personality disorder), which might affect their ability to receive future treatment
- developing ineffective treatment plans based on an incorrect diagnosis, including exposing patients to medications that could have serious adverse effects
- performing suicide or violence risk assessments based on inaccurate diagnoses, thereby over- or underestimating the possible risk for an adverse outcome
- leading patients to assume the identity of the inaccurate diagnosis and possibly viewing themselves as dysfunctional or impaired.
When you are uncertain whether your patient has a diagnosable condition, it can be useful to use the terms “no diagnosis” or “diagnosis deferred.” However, many insurance companies will not reimburse without an actual diagnosis. Therefore, the following tips may be helpful in establishing an accurate diagnosis while avoiding checklist psychiatry.1,2
Ask patients about the degree and duration of impairment in functioning. Although impairment in functioning is a criterion of almost all DSM-5 diagnoses, not all endorsed symptoms warrant a diagnosis. Mild symptoms often resolve spontaneously over time without the need for diagnostic labels or interventions.
Make longitudinal observations. Interviewing patients over a long period of time and on multiple occasions can provide data on the consistency of reported symptoms, the presence or absence of behavioral correlates to reported symptomatology, the degree of impairment from the reported symptoms, and the evolution of symptoms.
Collect collateral information. Although we often rely on our patients’ reports of symptoms to establish a diagnosis, this information should not be the sole source. We can obtain a more complete picture if we approach a patient’s family members for their input, including asking about a family history of mental illness or substance use disorders. We can also review prior treatment records and gather observations from clinic or inpatient staff for additional information.
Order laboratory studies. Serum studies and urine toxicology screens provide information that can help form an accurate diagnosis. This information is helpful because certain medical conditions, substance intoxication, and substance withdrawal can mimic psychiatric symptoms.
Continuously re-evaluate your diagnoses. As clinicians, we’d like to provide an accurate diagnosis at the onset of treatment; however, this may not be realistic because the patient’s presentation might change over time. It is paramount that we view diagnoses as evolving, so that we can more readily adjust our approach to treatment, especially when the patient is not benefitting from a well-formulated and comprehensive treatment plan.
Our patients are best served when we take the necessary time to use all resources to conceptualize them as more than a checklist of symptoms.
To determine whether a patient meets the criteria for a DSM-5 diagnosis, we rely on objective data, direct observations, and individual biopsychosocial factors as well as our patient’s subjective report of symptoms. However, because the line differentiating normal from abnormal emotional responses can sometimes be blurred, we should be prudent when establishing a diagnosis. Specifically, we need to avoid falling into the trap of “checklist” psychiatry—relegating diagnostic assessments to robotic statements about whether patients meet DSM criteria—because this can lead to making diagnoses too quickly or inaccurately.1 Potential consequences of checklist psychiatry include1,2:
- becoming so “married” to a particular diagnosis that you don’t consider alternative diagnoses
- labeling patients with a diagnosis that many clinicians may view as pejorative (eg, antisocial personality disorder), which might affect their ability to receive future treatment
- developing ineffective treatment plans based on an incorrect diagnosis, including exposing patients to medications that could have serious adverse effects
- performing suicide or violence risk assessments based on inaccurate diagnoses, thereby over- or underestimating the possible risk for an adverse outcome
- leading patients to assume the identity of the inaccurate diagnosis and possibly viewing themselves as dysfunctional or impaired.
When you are uncertain whether your patient has a diagnosable condition, it can be useful to use the terms “no diagnosis” or “diagnosis deferred.” However, many insurance companies will not reimburse without an actual diagnosis. Therefore, the following tips may be helpful in establishing an accurate diagnosis while avoiding checklist psychiatry.1,2
Ask patients about the degree and duration of impairment in functioning. Although impairment in functioning is a criterion of almost all DSM-5 diagnoses, not all endorsed symptoms warrant a diagnosis. Mild symptoms often resolve spontaneously over time without the need for diagnostic labels or interventions.
Make longitudinal observations. Interviewing patients over a long period of time and on multiple occasions can provide data on the consistency of reported symptoms, the presence or absence of behavioral correlates to reported symptomatology, the degree of impairment from the reported symptoms, and the evolution of symptoms.
Collect collateral information. Although we often rely on our patients’ reports of symptoms to establish a diagnosis, this information should not be the sole source. We can obtain a more complete picture if we approach a patient’s family members for their input, including asking about a family history of mental illness or substance use disorders. We can also review prior treatment records and gather observations from clinic or inpatient staff for additional information.
Order laboratory studies. Serum studies and urine toxicology screens provide information that can help form an accurate diagnosis. This information is helpful because certain medical conditions, substance intoxication, and substance withdrawal can mimic psychiatric symptoms.
Continuously re-evaluate your diagnoses. As clinicians, we’d like to provide an accurate diagnosis at the onset of treatment; however, this may not be realistic because the patient’s presentation might change over time. It is paramount that we view diagnoses as evolving, so that we can more readily adjust our approach to treatment, especially when the patient is not benefitting from a well-formulated and comprehensive treatment plan.
Our patients are best served when we take the necessary time to use all resources to conceptualize them as more than a checklist of symptoms.
1. Kontos N, Freudenreich O, Querques J. Thoughtful diagnoses: not ‘checklist’ psychiatry. Current Psychiatry. 2007;6(3):112.
2. Frances A. My 12 best tips on psychiatric diagnosis. Psychiatric Times. http://www.psychiatrictimes.com/dsm-5/my-12-best-tips-psychiatric-diagnosis. Published June 17, 2013. Accessed July 19, 2019.
1. Kontos N, Freudenreich O, Querques J. Thoughtful diagnoses: not ‘checklist’ psychiatry. Current Psychiatry. 2007;6(3):112.
2. Frances A. My 12 best tips on psychiatric diagnosis. Psychiatric Times. http://www.psychiatrictimes.com/dsm-5/my-12-best-tips-psychiatric-diagnosis. Published June 17, 2013. Accessed July 19, 2019.
Child trafficking: How to recognize the signs
Child trafficking—a modern-day form of slavery that continues to destroy many lives—often is hidden, even from the clinicians who see its victims. Traffickers typically exploit children for labor or commercial sexual work. The signs and symptoms that suggest a child is being trafficked may be less clear than those of the psychiatric illnesses we usually diagnose and treat. In this article, I summarize characteristics that could be helpful to note when you suspect a child is being trafficked, and offer some resources for helping victims.
How to identify possible victims
Children can be trafficked anywhere. The concept of a child being picked up off a street corner is outdated. Trafficking occurs in cities, suburbs, and rural areas. It happens in hotel rooms, at truck stops, on quiet residential streets, and in expensive homes. The internet has made it easier for traffickers to find victims.
Traffickers typically target youth who are emotionally and physically vulnerable. They often seek out teenagers who are undergoing financial hardships, experiencing family conflict, or have survived natural disasters. Many victims are runaways. In 2016, 1 in 6 child runaways reported to the National Center for Missing and Exploited Children were likely victims of trafficking.1 Of those children, 86% were receiving social services support or living in foster homes.
Traffickers are adept at emotional manipulation, which may explain why a child or adolescent might minimize the abuse during a clinical visit. Traffickers shroud the realities of trafficking with notions of love and inclusion. They use several physical and mental schemes to keep children and adolescents in their grip, such as withholding food, sleep, or medical care. Therefore, we should check for signs and symptoms of chronic medical conditions that have gone untreated, malnutrition, or bruises in various stages of healing.
Connecting risk factors for trafficking to dramatic changes in a young patient’s behavior is challenging. These youth often have dropped out of school, lack consistent family support, and spend their nights in search of a warm place to sleep. Their lives are upended. A child who once was more social may be forced into isolation and make excuses for why she no longer spends time with her friends.
In a study of 106 survivors of domestic sex trafficking, approximately 89% of respondents reported depression during depression. Many respondents reported experiencing anxiety (76.4%), nightmares (73.6%), flashbacks (68%), low self-esteem (81.1%), or feelings of shame or guilt (82.1%).2 Almost 88% of respondents said that they saw a doctor or other clinician while being trafficked, but their clinicians were unable to recognize the signs of trafficking. Part of the challenge is that many children and adolescents are not comfortable discussing their situations with clinicians because they may struggle with shame and guilt. Their traffickers also might have convinced them that they are criminals, not victims. These patients also may have an overwhelming fear of their trafficker, being reported to child welfare authorities, being arrested, being deported, or having their traffickers retaliate against their families. Gaining the trust of a patient who is being trafficked is critical, but not easy, because children may be skeptical of a clinician’s promise of confidentiality.
Some signs of trafficking overlap with the psychiatric presentations with which we are more familiar. These patients may abuse drugs or alcohol as means of escape or because their traffickers force them to use substances.2 They may show symptoms of depression or posttraumatic stress disorder (PTSD) and may be disoriented. Other indicators may be more telling, such as if a child or adolescent describes:
- having no control of their schedules or forms of identification
- having to work excessively long hours, often to pay off an overwhelming debt
- having high security measures installed in their place of residence (such as cameras or barred windows).
Continue to: Also, they may be...
Also, they may be dressed inappropriately for the weather.
We should be concerned when patients’ responses seem coached, if they say they are isolated from their family and community, or if they are submissive or overly timid. In addition, our suspicions should be raised if an accompanying adult guardian insists on sitting in on the appointment or translating for the child. In such instances, we may request that the guardian remain in the waiting area during the appointment so the child will have the opportunity to speak freely.2
How to help a suspected victim
Several local and national organizations help trafficking victims. These organizations provide educational materials and training opportunities for clinicians, as well as direct support for victims. The Homeland Security Blue Campaign advises against confronting a suspected trafficker directly and encourages clinicians to instead report suspected cases to 1-866-347-2423.3
Clinicians can better help children who are trafficked by taking the following 5 steps:
- Learn about the risk factors and signs of child trafficking.
- Post the National Human Trafficking Hotline (1-888-373-7888) in your waiting room.
- Determine if your patient is in danger and needs to be moved to a safe place.
- Connect the patient to social service agencies that can provide financial support and housing assistance so he/she doesn’t feel trapped by financial burdens.
- Work to rebuild their emotional and physical well-being while treating depression, PTSD, substance abuse, or any other mental illness.
1. National Center for Missing and Exploited Childr en. Missing children, state care, and child sex trafficking. http://www.missingkids.com/content/dam/missingkids/pdfs/publications/missingchildrenstatecare.pdf. Accessed June 10, 2019.
2. Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in healthcare facilities. Ann Health Law. 2014;23(1):61-91.
3. Blue Campaign. Identify a victim. US Department of Homeland Security. https://www.dhs.gov/blue-campaign/identify-victim. Accessed June 10, 2019.
Child trafficking—a modern-day form of slavery that continues to destroy many lives—often is hidden, even from the clinicians who see its victims. Traffickers typically exploit children for labor or commercial sexual work. The signs and symptoms that suggest a child is being trafficked may be less clear than those of the psychiatric illnesses we usually diagnose and treat. In this article, I summarize characteristics that could be helpful to note when you suspect a child is being trafficked, and offer some resources for helping victims.
How to identify possible victims
Children can be trafficked anywhere. The concept of a child being picked up off a street corner is outdated. Trafficking occurs in cities, suburbs, and rural areas. It happens in hotel rooms, at truck stops, on quiet residential streets, and in expensive homes. The internet has made it easier for traffickers to find victims.
Traffickers typically target youth who are emotionally and physically vulnerable. They often seek out teenagers who are undergoing financial hardships, experiencing family conflict, or have survived natural disasters. Many victims are runaways. In 2016, 1 in 6 child runaways reported to the National Center for Missing and Exploited Children were likely victims of trafficking.1 Of those children, 86% were receiving social services support or living in foster homes.
Traffickers are adept at emotional manipulation, which may explain why a child or adolescent might minimize the abuse during a clinical visit. Traffickers shroud the realities of trafficking with notions of love and inclusion. They use several physical and mental schemes to keep children and adolescents in their grip, such as withholding food, sleep, or medical care. Therefore, we should check for signs and symptoms of chronic medical conditions that have gone untreated, malnutrition, or bruises in various stages of healing.
Connecting risk factors for trafficking to dramatic changes in a young patient’s behavior is challenging. These youth often have dropped out of school, lack consistent family support, and spend their nights in search of a warm place to sleep. Their lives are upended. A child who once was more social may be forced into isolation and make excuses for why she no longer spends time with her friends.
In a study of 106 survivors of domestic sex trafficking, approximately 89% of respondents reported depression during depression. Many respondents reported experiencing anxiety (76.4%), nightmares (73.6%), flashbacks (68%), low self-esteem (81.1%), or feelings of shame or guilt (82.1%).2 Almost 88% of respondents said that they saw a doctor or other clinician while being trafficked, but their clinicians were unable to recognize the signs of trafficking. Part of the challenge is that many children and adolescents are not comfortable discussing their situations with clinicians because they may struggle with shame and guilt. Their traffickers also might have convinced them that they are criminals, not victims. These patients also may have an overwhelming fear of their trafficker, being reported to child welfare authorities, being arrested, being deported, or having their traffickers retaliate against their families. Gaining the trust of a patient who is being trafficked is critical, but not easy, because children may be skeptical of a clinician’s promise of confidentiality.
Some signs of trafficking overlap with the psychiatric presentations with which we are more familiar. These patients may abuse drugs or alcohol as means of escape or because their traffickers force them to use substances.2 They may show symptoms of depression or posttraumatic stress disorder (PTSD) and may be disoriented. Other indicators may be more telling, such as if a child or adolescent describes:
- having no control of their schedules or forms of identification
- having to work excessively long hours, often to pay off an overwhelming debt
- having high security measures installed in their place of residence (such as cameras or barred windows).
Continue to: Also, they may be...
Also, they may be dressed inappropriately for the weather.
We should be concerned when patients’ responses seem coached, if they say they are isolated from their family and community, or if they are submissive or overly timid. In addition, our suspicions should be raised if an accompanying adult guardian insists on sitting in on the appointment or translating for the child. In such instances, we may request that the guardian remain in the waiting area during the appointment so the child will have the opportunity to speak freely.2
How to help a suspected victim
Several local and national organizations help trafficking victims. These organizations provide educational materials and training opportunities for clinicians, as well as direct support for victims. The Homeland Security Blue Campaign advises against confronting a suspected trafficker directly and encourages clinicians to instead report suspected cases to 1-866-347-2423.3
Clinicians can better help children who are trafficked by taking the following 5 steps:
- Learn about the risk factors and signs of child trafficking.
- Post the National Human Trafficking Hotline (1-888-373-7888) in your waiting room.
- Determine if your patient is in danger and needs to be moved to a safe place.
- Connect the patient to social service agencies that can provide financial support and housing assistance so he/she doesn’t feel trapped by financial burdens.
- Work to rebuild their emotional and physical well-being while treating depression, PTSD, substance abuse, or any other mental illness.
Child trafficking—a modern-day form of slavery that continues to destroy many lives—often is hidden, even from the clinicians who see its victims. Traffickers typically exploit children for labor or commercial sexual work. The signs and symptoms that suggest a child is being trafficked may be less clear than those of the psychiatric illnesses we usually diagnose and treat. In this article, I summarize characteristics that could be helpful to note when you suspect a child is being trafficked, and offer some resources for helping victims.
How to identify possible victims
Children can be trafficked anywhere. The concept of a child being picked up off a street corner is outdated. Trafficking occurs in cities, suburbs, and rural areas. It happens in hotel rooms, at truck stops, on quiet residential streets, and in expensive homes. The internet has made it easier for traffickers to find victims.
Traffickers typically target youth who are emotionally and physically vulnerable. They often seek out teenagers who are undergoing financial hardships, experiencing family conflict, or have survived natural disasters. Many victims are runaways. In 2016, 1 in 6 child runaways reported to the National Center for Missing and Exploited Children were likely victims of trafficking.1 Of those children, 86% were receiving social services support or living in foster homes.
Traffickers are adept at emotional manipulation, which may explain why a child or adolescent might minimize the abuse during a clinical visit. Traffickers shroud the realities of trafficking with notions of love and inclusion. They use several physical and mental schemes to keep children and adolescents in their grip, such as withholding food, sleep, or medical care. Therefore, we should check for signs and symptoms of chronic medical conditions that have gone untreated, malnutrition, or bruises in various stages of healing.
Connecting risk factors for trafficking to dramatic changes in a young patient’s behavior is challenging. These youth often have dropped out of school, lack consistent family support, and spend their nights in search of a warm place to sleep. Their lives are upended. A child who once was more social may be forced into isolation and make excuses for why she no longer spends time with her friends.
In a study of 106 survivors of domestic sex trafficking, approximately 89% of respondents reported depression during depression. Many respondents reported experiencing anxiety (76.4%), nightmares (73.6%), flashbacks (68%), low self-esteem (81.1%), or feelings of shame or guilt (82.1%).2 Almost 88% of respondents said that they saw a doctor or other clinician while being trafficked, but their clinicians were unable to recognize the signs of trafficking. Part of the challenge is that many children and adolescents are not comfortable discussing their situations with clinicians because they may struggle with shame and guilt. Their traffickers also might have convinced them that they are criminals, not victims. These patients also may have an overwhelming fear of their trafficker, being reported to child welfare authorities, being arrested, being deported, or having their traffickers retaliate against their families. Gaining the trust of a patient who is being trafficked is critical, but not easy, because children may be skeptical of a clinician’s promise of confidentiality.
Some signs of trafficking overlap with the psychiatric presentations with which we are more familiar. These patients may abuse drugs or alcohol as means of escape or because their traffickers force them to use substances.2 They may show symptoms of depression or posttraumatic stress disorder (PTSD) and may be disoriented. Other indicators may be more telling, such as if a child or adolescent describes:
- having no control of their schedules or forms of identification
- having to work excessively long hours, often to pay off an overwhelming debt
- having high security measures installed in their place of residence (such as cameras or barred windows).
Continue to: Also, they may be...
Also, they may be dressed inappropriately for the weather.
We should be concerned when patients’ responses seem coached, if they say they are isolated from their family and community, or if they are submissive or overly timid. In addition, our suspicions should be raised if an accompanying adult guardian insists on sitting in on the appointment or translating for the child. In such instances, we may request that the guardian remain in the waiting area during the appointment so the child will have the opportunity to speak freely.2
How to help a suspected victim
Several local and national organizations help trafficking victims. These organizations provide educational materials and training opportunities for clinicians, as well as direct support for victims. The Homeland Security Blue Campaign advises against confronting a suspected trafficker directly and encourages clinicians to instead report suspected cases to 1-866-347-2423.3
Clinicians can better help children who are trafficked by taking the following 5 steps:
- Learn about the risk factors and signs of child trafficking.
- Post the National Human Trafficking Hotline (1-888-373-7888) in your waiting room.
- Determine if your patient is in danger and needs to be moved to a safe place.
- Connect the patient to social service agencies that can provide financial support and housing assistance so he/she doesn’t feel trapped by financial burdens.
- Work to rebuild their emotional and physical well-being while treating depression, PTSD, substance abuse, or any other mental illness.
1. National Center for Missing and Exploited Childr en. Missing children, state care, and child sex trafficking. http://www.missingkids.com/content/dam/missingkids/pdfs/publications/missingchildrenstatecare.pdf. Accessed June 10, 2019.
2. Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in healthcare facilities. Ann Health Law. 2014;23(1):61-91.
3. Blue Campaign. Identify a victim. US Department of Homeland Security. https://www.dhs.gov/blue-campaign/identify-victim. Accessed June 10, 2019.
1. National Center for Missing and Exploited Childr en. Missing children, state care, and child sex trafficking. http://www.missingkids.com/content/dam/missingkids/pdfs/publications/missingchildrenstatecare.pdf. Accessed June 10, 2019.
2. Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in healthcare facilities. Ann Health Law. 2014;23(1):61-91.
3. Blue Campaign. Identify a victim. US Department of Homeland Security. https://www.dhs.gov/blue-campaign/identify-victim. Accessed June 10, 2019.
Hypersomnolence: Unraveling the causes
Establishing a diagnosis of hypersomnia—recurrent episodes of excessive daytime sleepiness (EDS) or prolonged nighttime sleep—requires a stepwise assessment. We describe a complex case of an older adult who presented with multiple potential causes of hypersomnolence.
CASE REPORT
Persistent daytime sleepiness
Mr. W, age 63, is a veteran with a medical history significant for severe obstructive sleep apnea (OSA), insomnia, restless leg syndrome, hypertension, and major depressive disorder. He reported long-standing EDS that was causing functional and social impairment. Mr. W’s EDS persisted despite the use of continuous positive airway pressure (CPAP) therapy. A download of his CPAP compliance summary revealed both optimal CPAP adherence (>7-hour usage for 95%) and control of OSA (Apnea Hypopnea Index <5). His Epworth Sleepiness Scale (ESS) score remained at 20 out of 24. Another clinician had previously prescribed modafinil to treat Mr. W’s EDS, which was presumed to be related to sleep apnea. At the time of assessment, Mr. W was taking modafinil, 200 mg every morning, without significant relief of his daytime somnolence. Laboratory results revealed normal liver function tests, electrolytes, and hormonal levels, and a urine toxicology was negative. Mr. W said he constantly rubbed his legs to ease his bilateral leg movement. He reported both sensory and motor components, and relief with movement and absence of sensations in the morning.1 Gabapentin was initiated and titrated to a therapeutic dose to stabilize these symptoms.
Further contemplation led the treating clinician to investigate sleep deprivation or insomnia as potential causes of Mr. W’s daytime somnolence. Mr. W also reported occasional insomnia symptoms. To probe for the culprit of daytime sleepiness, actigraphy wrist monitoring was performed and showed no persistent insomnia or circadian rhythm disturbances.2 Medication reconciliation revealed Mr. W was taking 2 medications (fluoxetine and modafinil) that made him alert, but because he took these in the morning, it was unlikely that they were affecting his sleep. Upon review of his sleep habits, Mr. W’s naps were rare and unrefreshing during the day and he was not drinking excessive amounts of caffeinated beverages.
The diagnostic uncertainty led the treating clinician to order a polysomnography sleep study (PSG) with Multiple Sleep Latency Test (MSLT), which revealed a mean sleep latency of 4.1 minutes with no rapid eye movement (REM) periods during his PSG nor next-day napping.3 The PSG showed sleep fragmentation with a sleep efficiency of 90%. The results indicated residual sleepiness secondary to OSA.
Next, the clinician prescribed dextroamphetamine, 25 mg/d, which lowered Mr. W’s ESS score by 2 points (18 out of 24). The clinician presumed that if the stimulant worked, the diagnosis would more likely fit the criteria for residual sleepiness from OSA, rather than idiopathic hypersomnia (IH). Due to a lack of efficacy and adverse effects, the patient was tapered off this medication.
Mr. W reported that he experienced sleepiness during his service in the military at age 23. He also said he did not feel refreshed if he napped during the day.
To address the hypersomnia, he was prescribed off-label sodium oxybate. Sodium oxybate was efficacious and well tolerated; it was slowly titrated up to 9 g/d. After taking sodium oxybate for 2 months, Mr. W’s ESS score diminished to 6. Currently, he reports no functional impairment. A repeat actigraphy showed minimal sleep fragmentation and a strong normal circadian rhythm.
Continue to: Identifying hypersomnia
Identifying hypersomnia
Idiopathic hypersomnia should be considered when a patient’s excessive sleep or EDS are not better explained by another sleep disorder, other medical or psychiatric disorders, or the use of illicit drugs or medications.4 Idiopathic hypersomnia is characterized by EDS that occurs in the absence of cataplexy and is accompanied by no more than 1 sleep-onset REM (SOREM) period on an MSLT and the preceding PSG combined. The differential diagnosis includes narcolepsy, sleep apnea, and
In IH, evidence of hypersomnia must be demonstrated by an MSLT showing a mean sleep latency of <8 minutes or by PSG or wrist actigraphy showing a total 24-hour sleep time of >660 minutes.4 A prolonged and severe form of sleep inertia, consisting of prolonged difficulty waking up with repeated returns to sleep, irritability, automatic behavior, and confusion, often occurs in IH but is not pathognomonic.4
Naps are long—often 60 minutes—and described as unrefreshing by 46% to 78% of patients.4 Sleep efficiency on polysomnography is usually high (mean 90% to 94%). Self-reported total sleep time is longer than in controls and is >10 hours in at least 30% of patients.4 Unfortunately, symptoms and certain objective findings of IH are not unique to the disorder and are considered ubiquitous.
For Mr. W, a diagnosis of narcolepsy was unlikely due to his MSLT results. Patients with narcolepsy have cataplexy (REM dissociation) and/or at least 2 SOREM periods on MLST, or at least 1 SOREM period on MLST in conjunction with a SOREM on the preceding PSG,4 which Mr. W did not exhibit. Patients with narcolepsy typically take refreshing naps lasting 15 to 30 minutes. Although not unique to narcolepsy, common findings include hypnagogic hallucinations and sleep paralysis. Patients with narcolepsy typically do not have sleep inertia but, when seemingly awake, have lapses in vigilance sometimes in combination with automatic behavior, such as writing gibberish or interrupting a conversation with a completely different topic. Another characteristic PSG finding is moderate to severe sleep fragmentation, which may be due to associated periodic limb movements or instability in sleep/wake transitions.5 Mr. W had no history of traumatic brain injury that would suggest hypersomnolence secondary to a brain injury.
Among medical conditions, OSA is the predominant cause of EDS, but this, too, was unlikely for Mr. W because the CPAP therapy reports indicated excellent chronic use and effect. His apnea/hypopnea index was low, and the lowest oxygen saturation recorded on his pre-MSLT PSG using CPAP was 93%. Subjectively, Mr. W reported no choking, gasping, or snoring while receiving CPAP therapy.
Continue to: Restless leg syndrome...
Restless leg syndrome was excluded because after receiving gabapentin, both Mr. W and his wife reported improvement in his leg movements.
Although patients with mood disorders such as depression have normal MSLT results, Mr. W reported no excessive time lying in bed awake, which patients with depression often describe as fatigue and sleepiness. In addition, Mr. W’s score on the Clinically Useful Depression Outcome Scale indicated he was not depressed.
Mr. W’s clinician prescribed off-label sodium oxybate to address his EDS. Its potential benefit in this case may be related to its activity on gamma-aminobutyric acid (GABAB) receptors and its effects in prolonging slow-wave sleep, which has restorative properties. This treatment’s effectiveness in this patient was surprising and without precedent. Because the causes of IH often are not precisely defined, we do not recommend administering a trial of this medication without stepwise exclusion of other causes of sleepiness as demonstrated in Pagel’s algorithm “Diagnosis and Management of Conditions That Cause Excessive Daytime Sleepiness,”6 available at www.aafp.org/afp/2009/0301/p391.html.
1. Kallweit U, Siccoli MM, Poryazova R, et al. Excessive daytime sleepiness in idiopathic restless legs syndrome: characteristics and evolution under dopaminergic treatment. Eur Neurol. 2009;62(3):176-179.
2. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514-1527.
3. Carskadon MA. Guidelines for the Multiple Sleep Latency Test (MSLT): a standard measure of sleepiness. Sleep. 1986;9(4):519-524.
4. American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
5. Bahammam A. Periodic leg movements in narcolepsy patients: impact on sleep architecture. Acta Neurol Scand. 2007;115(5):351-355.
6. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
Establishing a diagnosis of hypersomnia—recurrent episodes of excessive daytime sleepiness (EDS) or prolonged nighttime sleep—requires a stepwise assessment. We describe a complex case of an older adult who presented with multiple potential causes of hypersomnolence.
CASE REPORT
Persistent daytime sleepiness
Mr. W, age 63, is a veteran with a medical history significant for severe obstructive sleep apnea (OSA), insomnia, restless leg syndrome, hypertension, and major depressive disorder. He reported long-standing EDS that was causing functional and social impairment. Mr. W’s EDS persisted despite the use of continuous positive airway pressure (CPAP) therapy. A download of his CPAP compliance summary revealed both optimal CPAP adherence (>7-hour usage for 95%) and control of OSA (Apnea Hypopnea Index <5). His Epworth Sleepiness Scale (ESS) score remained at 20 out of 24. Another clinician had previously prescribed modafinil to treat Mr. W’s EDS, which was presumed to be related to sleep apnea. At the time of assessment, Mr. W was taking modafinil, 200 mg every morning, without significant relief of his daytime somnolence. Laboratory results revealed normal liver function tests, electrolytes, and hormonal levels, and a urine toxicology was negative. Mr. W said he constantly rubbed his legs to ease his bilateral leg movement. He reported both sensory and motor components, and relief with movement and absence of sensations in the morning.1 Gabapentin was initiated and titrated to a therapeutic dose to stabilize these symptoms.
Further contemplation led the treating clinician to investigate sleep deprivation or insomnia as potential causes of Mr. W’s daytime somnolence. Mr. W also reported occasional insomnia symptoms. To probe for the culprit of daytime sleepiness, actigraphy wrist monitoring was performed and showed no persistent insomnia or circadian rhythm disturbances.2 Medication reconciliation revealed Mr. W was taking 2 medications (fluoxetine and modafinil) that made him alert, but because he took these in the morning, it was unlikely that they were affecting his sleep. Upon review of his sleep habits, Mr. W’s naps were rare and unrefreshing during the day and he was not drinking excessive amounts of caffeinated beverages.
The diagnostic uncertainty led the treating clinician to order a polysomnography sleep study (PSG) with Multiple Sleep Latency Test (MSLT), which revealed a mean sleep latency of 4.1 minutes with no rapid eye movement (REM) periods during his PSG nor next-day napping.3 The PSG showed sleep fragmentation with a sleep efficiency of 90%. The results indicated residual sleepiness secondary to OSA.
Next, the clinician prescribed dextroamphetamine, 25 mg/d, which lowered Mr. W’s ESS score by 2 points (18 out of 24). The clinician presumed that if the stimulant worked, the diagnosis would more likely fit the criteria for residual sleepiness from OSA, rather than idiopathic hypersomnia (IH). Due to a lack of efficacy and adverse effects, the patient was tapered off this medication.
Mr. W reported that he experienced sleepiness during his service in the military at age 23. He also said he did not feel refreshed if he napped during the day.
To address the hypersomnia, he was prescribed off-label sodium oxybate. Sodium oxybate was efficacious and well tolerated; it was slowly titrated up to 9 g/d. After taking sodium oxybate for 2 months, Mr. W’s ESS score diminished to 6. Currently, he reports no functional impairment. A repeat actigraphy showed minimal sleep fragmentation and a strong normal circadian rhythm.
Continue to: Identifying hypersomnia
Identifying hypersomnia
Idiopathic hypersomnia should be considered when a patient’s excessive sleep or EDS are not better explained by another sleep disorder, other medical or psychiatric disorders, or the use of illicit drugs or medications.4 Idiopathic hypersomnia is characterized by EDS that occurs in the absence of cataplexy and is accompanied by no more than 1 sleep-onset REM (SOREM) period on an MSLT and the preceding PSG combined. The differential diagnosis includes narcolepsy, sleep apnea, and
In IH, evidence of hypersomnia must be demonstrated by an MSLT showing a mean sleep latency of <8 minutes or by PSG or wrist actigraphy showing a total 24-hour sleep time of >660 minutes.4 A prolonged and severe form of sleep inertia, consisting of prolonged difficulty waking up with repeated returns to sleep, irritability, automatic behavior, and confusion, often occurs in IH but is not pathognomonic.4
Naps are long—often 60 minutes—and described as unrefreshing by 46% to 78% of patients.4 Sleep efficiency on polysomnography is usually high (mean 90% to 94%). Self-reported total sleep time is longer than in controls and is >10 hours in at least 30% of patients.4 Unfortunately, symptoms and certain objective findings of IH are not unique to the disorder and are considered ubiquitous.
For Mr. W, a diagnosis of narcolepsy was unlikely due to his MSLT results. Patients with narcolepsy have cataplexy (REM dissociation) and/or at least 2 SOREM periods on MLST, or at least 1 SOREM period on MLST in conjunction with a SOREM on the preceding PSG,4 which Mr. W did not exhibit. Patients with narcolepsy typically take refreshing naps lasting 15 to 30 minutes. Although not unique to narcolepsy, common findings include hypnagogic hallucinations and sleep paralysis. Patients with narcolepsy typically do not have sleep inertia but, when seemingly awake, have lapses in vigilance sometimes in combination with automatic behavior, such as writing gibberish or interrupting a conversation with a completely different topic. Another characteristic PSG finding is moderate to severe sleep fragmentation, which may be due to associated periodic limb movements or instability in sleep/wake transitions.5 Mr. W had no history of traumatic brain injury that would suggest hypersomnolence secondary to a brain injury.
Among medical conditions, OSA is the predominant cause of EDS, but this, too, was unlikely for Mr. W because the CPAP therapy reports indicated excellent chronic use and effect. His apnea/hypopnea index was low, and the lowest oxygen saturation recorded on his pre-MSLT PSG using CPAP was 93%. Subjectively, Mr. W reported no choking, gasping, or snoring while receiving CPAP therapy.
Continue to: Restless leg syndrome...
Restless leg syndrome was excluded because after receiving gabapentin, both Mr. W and his wife reported improvement in his leg movements.
Although patients with mood disorders such as depression have normal MSLT results, Mr. W reported no excessive time lying in bed awake, which patients with depression often describe as fatigue and sleepiness. In addition, Mr. W’s score on the Clinically Useful Depression Outcome Scale indicated he was not depressed.
Mr. W’s clinician prescribed off-label sodium oxybate to address his EDS. Its potential benefit in this case may be related to its activity on gamma-aminobutyric acid (GABAB) receptors and its effects in prolonging slow-wave sleep, which has restorative properties. This treatment’s effectiveness in this patient was surprising and without precedent. Because the causes of IH often are not precisely defined, we do not recommend administering a trial of this medication without stepwise exclusion of other causes of sleepiness as demonstrated in Pagel’s algorithm “Diagnosis and Management of Conditions That Cause Excessive Daytime Sleepiness,”6 available at www.aafp.org/afp/2009/0301/p391.html.
Establishing a diagnosis of hypersomnia—recurrent episodes of excessive daytime sleepiness (EDS) or prolonged nighttime sleep—requires a stepwise assessment. We describe a complex case of an older adult who presented with multiple potential causes of hypersomnolence.
CASE REPORT
Persistent daytime sleepiness
Mr. W, age 63, is a veteran with a medical history significant for severe obstructive sleep apnea (OSA), insomnia, restless leg syndrome, hypertension, and major depressive disorder. He reported long-standing EDS that was causing functional and social impairment. Mr. W’s EDS persisted despite the use of continuous positive airway pressure (CPAP) therapy. A download of his CPAP compliance summary revealed both optimal CPAP adherence (>7-hour usage for 95%) and control of OSA (Apnea Hypopnea Index <5). His Epworth Sleepiness Scale (ESS) score remained at 20 out of 24. Another clinician had previously prescribed modafinil to treat Mr. W’s EDS, which was presumed to be related to sleep apnea. At the time of assessment, Mr. W was taking modafinil, 200 mg every morning, without significant relief of his daytime somnolence. Laboratory results revealed normal liver function tests, electrolytes, and hormonal levels, and a urine toxicology was negative. Mr. W said he constantly rubbed his legs to ease his bilateral leg movement. He reported both sensory and motor components, and relief with movement and absence of sensations in the morning.1 Gabapentin was initiated and titrated to a therapeutic dose to stabilize these symptoms.
Further contemplation led the treating clinician to investigate sleep deprivation or insomnia as potential causes of Mr. W’s daytime somnolence. Mr. W also reported occasional insomnia symptoms. To probe for the culprit of daytime sleepiness, actigraphy wrist monitoring was performed and showed no persistent insomnia or circadian rhythm disturbances.2 Medication reconciliation revealed Mr. W was taking 2 medications (fluoxetine and modafinil) that made him alert, but because he took these in the morning, it was unlikely that they were affecting his sleep. Upon review of his sleep habits, Mr. W’s naps were rare and unrefreshing during the day and he was not drinking excessive amounts of caffeinated beverages.
The diagnostic uncertainty led the treating clinician to order a polysomnography sleep study (PSG) with Multiple Sleep Latency Test (MSLT), which revealed a mean sleep latency of 4.1 minutes with no rapid eye movement (REM) periods during his PSG nor next-day napping.3 The PSG showed sleep fragmentation with a sleep efficiency of 90%. The results indicated residual sleepiness secondary to OSA.
Next, the clinician prescribed dextroamphetamine, 25 mg/d, which lowered Mr. W’s ESS score by 2 points (18 out of 24). The clinician presumed that if the stimulant worked, the diagnosis would more likely fit the criteria for residual sleepiness from OSA, rather than idiopathic hypersomnia (IH). Due to a lack of efficacy and adverse effects, the patient was tapered off this medication.
Mr. W reported that he experienced sleepiness during his service in the military at age 23. He also said he did not feel refreshed if he napped during the day.
To address the hypersomnia, he was prescribed off-label sodium oxybate. Sodium oxybate was efficacious and well tolerated; it was slowly titrated up to 9 g/d. After taking sodium oxybate for 2 months, Mr. W’s ESS score diminished to 6. Currently, he reports no functional impairment. A repeat actigraphy showed minimal sleep fragmentation and a strong normal circadian rhythm.
Continue to: Identifying hypersomnia
Identifying hypersomnia
Idiopathic hypersomnia should be considered when a patient’s excessive sleep or EDS are not better explained by another sleep disorder, other medical or psychiatric disorders, or the use of illicit drugs or medications.4 Idiopathic hypersomnia is characterized by EDS that occurs in the absence of cataplexy and is accompanied by no more than 1 sleep-onset REM (SOREM) period on an MSLT and the preceding PSG combined. The differential diagnosis includes narcolepsy, sleep apnea, and
In IH, evidence of hypersomnia must be demonstrated by an MSLT showing a mean sleep latency of <8 minutes or by PSG or wrist actigraphy showing a total 24-hour sleep time of >660 minutes.4 A prolonged and severe form of sleep inertia, consisting of prolonged difficulty waking up with repeated returns to sleep, irritability, automatic behavior, and confusion, often occurs in IH but is not pathognomonic.4
Naps are long—often 60 minutes—and described as unrefreshing by 46% to 78% of patients.4 Sleep efficiency on polysomnography is usually high (mean 90% to 94%). Self-reported total sleep time is longer than in controls and is >10 hours in at least 30% of patients.4 Unfortunately, symptoms and certain objective findings of IH are not unique to the disorder and are considered ubiquitous.
For Mr. W, a diagnosis of narcolepsy was unlikely due to his MSLT results. Patients with narcolepsy have cataplexy (REM dissociation) and/or at least 2 SOREM periods on MLST, or at least 1 SOREM period on MLST in conjunction with a SOREM on the preceding PSG,4 which Mr. W did not exhibit. Patients with narcolepsy typically take refreshing naps lasting 15 to 30 minutes. Although not unique to narcolepsy, common findings include hypnagogic hallucinations and sleep paralysis. Patients with narcolepsy typically do not have sleep inertia but, when seemingly awake, have lapses in vigilance sometimes in combination with automatic behavior, such as writing gibberish or interrupting a conversation with a completely different topic. Another characteristic PSG finding is moderate to severe sleep fragmentation, which may be due to associated periodic limb movements or instability in sleep/wake transitions.5 Mr. W had no history of traumatic brain injury that would suggest hypersomnolence secondary to a brain injury.
Among medical conditions, OSA is the predominant cause of EDS, but this, too, was unlikely for Mr. W because the CPAP therapy reports indicated excellent chronic use and effect. His apnea/hypopnea index was low, and the lowest oxygen saturation recorded on his pre-MSLT PSG using CPAP was 93%. Subjectively, Mr. W reported no choking, gasping, or snoring while receiving CPAP therapy.
Continue to: Restless leg syndrome...
Restless leg syndrome was excluded because after receiving gabapentin, both Mr. W and his wife reported improvement in his leg movements.
Although patients with mood disorders such as depression have normal MSLT results, Mr. W reported no excessive time lying in bed awake, which patients with depression often describe as fatigue and sleepiness. In addition, Mr. W’s score on the Clinically Useful Depression Outcome Scale indicated he was not depressed.
Mr. W’s clinician prescribed off-label sodium oxybate to address his EDS. Its potential benefit in this case may be related to its activity on gamma-aminobutyric acid (GABAB) receptors and its effects in prolonging slow-wave sleep, which has restorative properties. This treatment’s effectiveness in this patient was surprising and without precedent. Because the causes of IH often are not precisely defined, we do not recommend administering a trial of this medication without stepwise exclusion of other causes of sleepiness as demonstrated in Pagel’s algorithm “Diagnosis and Management of Conditions That Cause Excessive Daytime Sleepiness,”6 available at www.aafp.org/afp/2009/0301/p391.html.
1. Kallweit U, Siccoli MM, Poryazova R, et al. Excessive daytime sleepiness in idiopathic restless legs syndrome: characteristics and evolution under dopaminergic treatment. Eur Neurol. 2009;62(3):176-179.
2. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514-1527.
3. Carskadon MA. Guidelines for the Multiple Sleep Latency Test (MSLT): a standard measure of sleepiness. Sleep. 1986;9(4):519-524.
4. American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
5. Bahammam A. Periodic leg movements in narcolepsy patients: impact on sleep architecture. Acta Neurol Scand. 2007;115(5):351-355.
6. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
1. Kallweit U, Siccoli MM, Poryazova R, et al. Excessive daytime sleepiness in idiopathic restless legs syndrome: characteristics and evolution under dopaminergic treatment. Eur Neurol. 2009;62(3):176-179.
2. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514-1527.
3. Carskadon MA. Guidelines for the Multiple Sleep Latency Test (MSLT): a standard measure of sleepiness. Sleep. 1986;9(4):519-524.
4. American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
5. Bahammam A. Periodic leg movements in narcolepsy patients: impact on sleep architecture. Acta Neurol Scand. 2007;115(5):351-355.
6. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
Polypharmacy: When might it make sense?
Polypharmacy is often defined as the simultaneous prescription of multiple medications (usually ≥5) to a single patient for a single condition or multiple conditions.1 Patients with psychiatric illnesses may easily be prescribed multiple psychotropic medications regardless of how many other medications they may already take for nonpsychiatric comorbidities. According to 2011-2014 Centers for Disease Control and Prevention data, 11.9% of the US population used ≥5 medications in the past 30 days.2 Risks of polypharmacy include higher rates of adverse effects as well as treatment noncompliance.3
There are, however, many patients for whom a combination of psychotropic agents can be beneficial. It is important to carefully assess your patient’s regimen, and to document the rationale for prescribing multiple medications. Here I describe some factors that can help you to determine whether a multi-medication regimen might be warranted for your patient.
Accepted medication pairings. This describes a medication combination that has been recognized as generally safe and may provide more benefits than either single agent alone. Examples of clinically accepted medication combinations include4,5:
- a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) plus bupropion
- an SSRI or SNRI plus mirtazapine
- ziprasidone as an adjunct to valproate or lithium for treating bipolar disorder
- aripiprazole as an adjunctive treatment for major depressive disorder (MDD).
Comorbid diagnoses. Each of a patient’s psychiatric comorbidities may require a different medication to address specific symptoms.3 Psychiatric comorbidities that might be appropriate for multiple medications include attention-deficit/hyperactivity disorder and bipolar disorder, MDD and generalized anxiety disorder, and a mood disorder and a substance use disorder.
Treatment resistance. The patient has demonstrated poor or no response to prior trials with simpler medication regimens, and/or there is a history of decompensation or hospitalization when medications were pared down.
Severe acute symptoms. The patient has been experiencing acute symptoms that do not respond to one medication class. For example, a patient with bipolar disorder who has acute mania and psychosis may require significant doses of both a mood stabilizer and an antipsychotic.
Amelioration of adverse effects. One medication may be prescribed to address the adverse effects of other medications. For example, propranolol may be added to address akathisia from aripiprazole or tremors from lithium. In these cases, it is important to determine if the medication that’s causing adverse effects continues to provide benefits, in order to justify continuing it as well as adding a new agent.3
Continue to: After reviewing...
After reviewing your patient’s medication regimen, if one of these scenarios does not clearly exist, consider a “deprescribing” approach—reducing or stopping medications—to address unnecessary and potentially detrimental polypharmacy. For more information on dep
1. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230.
Polypharmacy is often defined as the simultaneous prescription of multiple medications (usually ≥5) to a single patient for a single condition or multiple conditions.1 Patients with psychiatric illnesses may easily be prescribed multiple psychotropic medications regardless of how many other medications they may already take for nonpsychiatric comorbidities. According to 2011-2014 Centers for Disease Control and Prevention data, 11.9% of the US population used ≥5 medications in the past 30 days.2 Risks of polypharmacy include higher rates of adverse effects as well as treatment noncompliance.3
There are, however, many patients for whom a combination of psychotropic agents can be beneficial. It is important to carefully assess your patient’s regimen, and to document the rationale for prescribing multiple medications. Here I describe some factors that can help you to determine whether a multi-medication regimen might be warranted for your patient.
Accepted medication pairings. This describes a medication combination that has been recognized as generally safe and may provide more benefits than either single agent alone. Examples of clinically accepted medication combinations include4,5:
- a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) plus bupropion
- an SSRI or SNRI plus mirtazapine
- ziprasidone as an adjunct to valproate or lithium for treating bipolar disorder
- aripiprazole as an adjunctive treatment for major depressive disorder (MDD).
Comorbid diagnoses. Each of a patient’s psychiatric comorbidities may require a different medication to address specific symptoms.3 Psychiatric comorbidities that might be appropriate for multiple medications include attention-deficit/hyperactivity disorder and bipolar disorder, MDD and generalized anxiety disorder, and a mood disorder and a substance use disorder.
Treatment resistance. The patient has demonstrated poor or no response to prior trials with simpler medication regimens, and/or there is a history of decompensation or hospitalization when medications were pared down.
Severe acute symptoms. The patient has been experiencing acute symptoms that do not respond to one medication class. For example, a patient with bipolar disorder who has acute mania and psychosis may require significant doses of both a mood stabilizer and an antipsychotic.
Amelioration of adverse effects. One medication may be prescribed to address the adverse effects of other medications. For example, propranolol may be added to address akathisia from aripiprazole or tremors from lithium. In these cases, it is important to determine if the medication that’s causing adverse effects continues to provide benefits, in order to justify continuing it as well as adding a new agent.3
Continue to: After reviewing...
After reviewing your patient’s medication regimen, if one of these scenarios does not clearly exist, consider a “deprescribing” approach—reducing or stopping medications—to address unnecessary and potentially detrimental polypharmacy. For more information on dep
Polypharmacy is often defined as the simultaneous prescription of multiple medications (usually ≥5) to a single patient for a single condition or multiple conditions.1 Patients with psychiatric illnesses may easily be prescribed multiple psychotropic medications regardless of how many other medications they may already take for nonpsychiatric comorbidities. According to 2011-2014 Centers for Disease Control and Prevention data, 11.9% of the US population used ≥5 medications in the past 30 days.2 Risks of polypharmacy include higher rates of adverse effects as well as treatment noncompliance.3
There are, however, many patients for whom a combination of psychotropic agents can be beneficial. It is important to carefully assess your patient’s regimen, and to document the rationale for prescribing multiple medications. Here I describe some factors that can help you to determine whether a multi-medication regimen might be warranted for your patient.
Accepted medication pairings. This describes a medication combination that has been recognized as generally safe and may provide more benefits than either single agent alone. Examples of clinically accepted medication combinations include4,5:
- a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) plus bupropion
- an SSRI or SNRI plus mirtazapine
- ziprasidone as an adjunct to valproate or lithium for treating bipolar disorder
- aripiprazole as an adjunctive treatment for major depressive disorder (MDD).
Comorbid diagnoses. Each of a patient’s psychiatric comorbidities may require a different medication to address specific symptoms.3 Psychiatric comorbidities that might be appropriate for multiple medications include attention-deficit/hyperactivity disorder and bipolar disorder, MDD and generalized anxiety disorder, and a mood disorder and a substance use disorder.
Treatment resistance. The patient has demonstrated poor or no response to prior trials with simpler medication regimens, and/or there is a history of decompensation or hospitalization when medications were pared down.
Severe acute symptoms. The patient has been experiencing acute symptoms that do not respond to one medication class. For example, a patient with bipolar disorder who has acute mania and psychosis may require significant doses of both a mood stabilizer and an antipsychotic.
Amelioration of adverse effects. One medication may be prescribed to address the adverse effects of other medications. For example, propranolol may be added to address akathisia from aripiprazole or tremors from lithium. In these cases, it is important to determine if the medication that’s causing adverse effects continues to provide benefits, in order to justify continuing it as well as adding a new agent.3
Continue to: After reviewing...
After reviewing your patient’s medication regimen, if one of these scenarios does not clearly exist, consider a “deprescribing” approach—reducing or stopping medications—to address unnecessary and potentially detrimental polypharmacy. For more information on dep
1. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230.
1. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230.
The jealous insomniac
CASE Anxious and jealous
Mrs. H, age 28, presents to the emergency department (ED) with pressured speech, emotional lability, loose associations, and echolalia. On physical examination, Mrs. H is noted to have hand tremors. Mrs. H says she has not slept for the past 5 days and is experiencing anxiety and heart palpitations.
She also says that for the past 2 years she has believed that her husband is having an affair with her best friend. However, her current presentation—which she attributes to the alleged affair—began a week before she came to the ED. According to her husband, Mrs. H was “perfectly fine until a week ago” and her symptoms “appeared out of nowhere.” He reports that this has never happened before.
Mrs. H is admitted to the psychiatry unit. The nursing team reports that on the first night, Mrs. H was “running and screaming on the unit, out of control,” and was “tearful, manicky, and dysphoric.”
Mrs. H has no significant medical or psychiatric history. Her family history is significant for hyperthyroidism in her mother and maternal grandmother. Mrs. H says she smokes cigarettes (1 pack/d) but denies alcohol or illicit drug use.
EVALUATION A telling thyroid panel
Mrs. H undergoes laboratory testing, including a complete blood count, comprehensive metabolic panel, and thyroid panel due to her family history of thyroid-related disorders. The thyroid panel shows the presence of the thyroid-stimulating hormone (TSH) receptor antibody; a low TSH level; elevated triiodothyronine (T3) and thyroxine (T4) levels, with T3 > T4; elevated thyroid peroxidase (TPO) antibody; and elevated thyroglobulin antibody (Table 1). A scan shows the thyroid gland to be normal/top-normal size and is read by radiology to be indicative of a resolving thyroiditis vs Graves’ disease. An electrocardiogram indicates a heart rate of 139 beats per minute.
[polldaddy:10352133]
The authors’ observations
Mrs. H fits the presentation of psychosis secondary to Graves’ disease. However, our differential consisted of thyroiditis, brief psychotic disorder, delusional disorder (jealous type), and bipolar mania.
Brief psychotic disorder, bipolar mania, and delusional disorder were better explained by Graves’ disease, and Mrs. H’s jealous delusion resulted in functional impairment, which eliminated delusional disorder. Her family history of hyperthyroidism, as well as her sex and history of tobacco use, supported the diagnosis of Graves’ disease. Although Mrs. H did not experience goiter, ophthalmopathy, or dermopathy, which are common signs and symptoms of Graves’ disease (Table 2), she did present with irritability, insomnia, tachycardia, and a hand tremor. Her psychiatric symptoms included anxiety, emotional lability and, most importantly, psychosis. Her laboratory results included the presence of the TSH-receptor antibody, a low TSH level, and elevated T3 and T4 levels (T3>T4), confirming the diagnosis of early-onset Graves’ disease.
Continue to: Graves' disease
Graves’ disease
Graves’ disease is the most common cause of hyperthyroidism, representing approximately 50% to 80% of cases.1 Graves’ disease occurs most often in women, smokers, and those with a personal or family history of autoimmune disease; although patients of any age may be affected, the peak incidence occurs between age 40 and 60.1
Graves’ disease results from the production of immunoglobulin G (IgG) antibodies that activate the TSH receptor on the surface of thyroid follicular cells.1 The presence of the TSH-receptor antibody, in addition to a low TSH and elevated T3 and T4 levels (T3>T4), are common laboratory findings in patients with this disease. A thyroid scan will also show increased radiotracer accumulation.
Patients with Graves’ disease, as well as those with hyperthyroidism, tend to report weight loss, increased appetite, heat intolerance, irritability, insomnia, and palpitations. In addition to the above symptoms, the identifying signs and symptoms of Graves’ disease include a goiter, ophthalmopathy, and dermopathy (Table 2). Rarely, patients with Graves’ disease can present with psychosis, which is often complicated by thyrotoxicosis.2
[polldaddy:10352135]
TREATMENT Antipsychotic and a beta blocker
Based on her signs, symptoms, and laboratory findings, Mrs. H receives risperidone, 1 mg twice daily, for psychosis, and atenolol, 25 mg twice daily, for heart palpitations. Over 4 days, her symptoms decrease; she experiences more linear thought and decreased flight-of-ideas, and becomes unsure about the truth of her husband’s alleged affair. Her impulsive behaviors and severe mood lability cease. Her tachycardia remains controlled with atenolol.
The authors’ observations
Rapid initiation of treatment is important when managing patients with Graves’ disease, because untreated patients have a higher risk of psychiatric illness, cardiac disease, arrhythmia, and sudden cardiac death.1 Patients with Graves’ disease typically are treated with thionamides, radioactive iodine, and/or surgery. When a patient presents with psychosis as a result of thyrotoxicosis, treatment focuses on improving the thyrotoxicosis through anti-thyroid medications and beta blockers (Table 33). Psychotropic medications, such as antipsychotics, are not indicated for primary treatment, but are given to patients who have severe psychosis until symptoms have resolved.3 For Mrs. H, the severity of her psychosis necessitated risperidone in addition to atenolol.
OUTCOME Continuous medical management; no ablation
Mrs. H is discharged with immediate outpatient follow-up with an endocrinology team to discuss the best long-term management of her thyroiditis. Mrs. H opts for continuous medical management (as opposed to ablation) and is administered methimazole, 15 mg/d, to treat Graves’ disease.
The authors’ observations
This case provides useful information regarding recognizing psychosis as the initial sign of Graves’ disease. Although Graves’ disease represents 50% to 80% of cases of hyperthyroidism,1 psychosis as the first clinical presentation of this disease is extremely rare. Several case reports, however, have described this phenomenon,2,3 and further studies would be helpful to determine its true prevalence.
Continue to: Bottom Line
Bottom Line
Although extremely rare, psychosis as the initial clinical presentation of Graves’ disease can occur. The early diagnosis of Graves’ disease is critical to prevent cardiovascular implications and death.
Related Resources
- Abraham P, Acharya S. Current and emerging treatment options for Graves’ hyperthyroidism. Ther Clin Risk Manag. 2010;6:29-40.
- Bunevicius R, Prange AJ Jr. Psychiatric manifestations of Graves’ hyperthyroidism: pathophysiology and treatment options. CNS Drugs. 2006;20(11):897-909.
- Ginsberg J. Diagnosis and management of Graves’ disease. CMAJ. 2003;168(5):575-585.
Drug Brand Names
Atenolol • Tenormin
Methimazole • Tapazole
Risperidone • Risperdal
1. Girgis C, Champion B, Wall J. Current concepts in Graves’ disease. Ther Adv Endocrinol Metab. 2011;2(3):135-144.
2. Urias-Uribe L, Valdez-Solis E, González-Milán C, et al. Psychosis crisis associated with thyrotoxicosis due to Graves’ disease. Case Rep Psychiatry. 2017;2017:6803682. doi: 10.1155/2017/6803682.
3. Ugwu ET, Maluze J, Onyebueke GC. Graves’ thyrotoxicosis presenting as schizophreniform psychosis: a case report and literature review. Int J Endocrinol Metab. 2017;15(1):e41977. doi: 10.5812/ijem.41977.
CASE Anxious and jealous
Mrs. H, age 28, presents to the emergency department (ED) with pressured speech, emotional lability, loose associations, and echolalia. On physical examination, Mrs. H is noted to have hand tremors. Mrs. H says she has not slept for the past 5 days and is experiencing anxiety and heart palpitations.
She also says that for the past 2 years she has believed that her husband is having an affair with her best friend. However, her current presentation—which she attributes to the alleged affair—began a week before she came to the ED. According to her husband, Mrs. H was “perfectly fine until a week ago” and her symptoms “appeared out of nowhere.” He reports that this has never happened before.
Mrs. H is admitted to the psychiatry unit. The nursing team reports that on the first night, Mrs. H was “running and screaming on the unit, out of control,” and was “tearful, manicky, and dysphoric.”
Mrs. H has no significant medical or psychiatric history. Her family history is significant for hyperthyroidism in her mother and maternal grandmother. Mrs. H says she smokes cigarettes (1 pack/d) but denies alcohol or illicit drug use.

EVALUATION A telling thyroid panel
Mrs. H undergoes laboratory testing, including a complete blood count, comprehensive metabolic panel, and thyroid panel due to her family history of thyroid-related disorders. The thyroid panel shows the presence of the thyroid-stimulating hormone (TSH) receptor antibody; a low TSH level; elevated triiodothyronine (T3) and thyroxine (T4) levels, with T3 > T4; elevated thyroid peroxidase (TPO) antibody; and elevated thyroglobulin antibody (Table 1). A scan shows the thyroid gland to be normal/top-normal size and is read by radiology to be indicative of a resolving thyroiditis vs Graves’ disease. An electrocardiogram indicates a heart rate of 139 beats per minute.
[polldaddy:10352133]
The authors’ observations
Mrs. H fits the presentation of psychosis secondary to Graves’ disease. However, our differential consisted of thyroiditis, brief psychotic disorder, delusional disorder (jealous type), and bipolar mania.
Brief psychotic disorder, bipolar mania, and delusional disorder were better explained by Graves’ disease, and Mrs. H’s jealous delusion resulted in functional impairment, which eliminated delusional disorder. Her family history of hyperthyroidism, as well as her sex and history of tobacco use, supported the diagnosis of Graves’ disease. Although Mrs. H did not experience goiter, ophthalmopathy, or dermopathy, which are common signs and symptoms of Graves’ disease (Table 2), she did present with irritability, insomnia, tachycardia, and a hand tremor. Her psychiatric symptoms included anxiety, emotional lability and, most importantly, psychosis. Her laboratory results included the presence of the TSH-receptor antibody, a low TSH level, and elevated T3 and T4 levels (T3>T4), confirming the diagnosis of early-onset Graves’ disease.
Continue to: Graves' disease
Graves’ disease
Graves’ disease is the most common cause of hyperthyroidism, representing approximately 50% to 80% of cases.1 Graves’ disease occurs most often in women, smokers, and those with a personal or family history of autoimmune disease; although patients of any age may be affected, the peak incidence occurs between age 40 and 60.1
Graves’ disease results from the production of immunoglobulin G (IgG) antibodies that activate the TSH receptor on the surface of thyroid follicular cells.1 The presence of the TSH-receptor antibody, in addition to a low TSH and elevated T3 and T4 levels (T3>T4), are common laboratory findings in patients with this disease. A thyroid scan will also show increased radiotracer accumulation.
Patients with Graves’ disease, as well as those with hyperthyroidism, tend to report weight loss, increased appetite, heat intolerance, irritability, insomnia, and palpitations. In addition to the above symptoms, the identifying signs and symptoms of Graves’ disease include a goiter, ophthalmopathy, and dermopathy (Table 2). Rarely, patients with Graves’ disease can present with psychosis, which is often complicated by thyrotoxicosis.2
[polldaddy:10352135]
TREATMENT Antipsychotic and a beta blocker
Based on her signs, symptoms, and laboratory findings, Mrs. H receives risperidone, 1 mg twice daily, for psychosis, and atenolol, 25 mg twice daily, for heart palpitations. Over 4 days, her symptoms decrease; she experiences more linear thought and decreased flight-of-ideas, and becomes unsure about the truth of her husband’s alleged affair. Her impulsive behaviors and severe mood lability cease. Her tachycardia remains controlled with atenolol.
The authors’ observations
Rapid initiation of treatment is important when managing patients with Graves’ disease, because untreated patients have a higher risk of psychiatric illness, cardiac disease, arrhythmia, and sudden cardiac death.1 Patients with Graves’ disease typically are treated with thionamides, radioactive iodine, and/or surgery. When a patient presents with psychosis as a result of thyrotoxicosis, treatment focuses on improving the thyrotoxicosis through anti-thyroid medications and beta blockers (Table 33). Psychotropic medications, such as antipsychotics, are not indicated for primary treatment, but are given to patients who have severe psychosis until symptoms have resolved.3 For Mrs. H, the severity of her psychosis necessitated risperidone in addition to atenolol.
OUTCOME Continuous medical management; no ablation
Mrs. H is discharged with immediate outpatient follow-up with an endocrinology team to discuss the best long-term management of her thyroiditis. Mrs. H opts for continuous medical management (as opposed to ablation) and is administered methimazole, 15 mg/d, to treat Graves’ disease.
The authors’ observations
This case provides useful information regarding recognizing psychosis as the initial sign of Graves’ disease. Although Graves’ disease represents 50% to 80% of cases of hyperthyroidism,1 psychosis as the first clinical presentation of this disease is extremely rare. Several case reports, however, have described this phenomenon,2,3 and further studies would be helpful to determine its true prevalence.
Continue to: Bottom Line
Bottom Line
Although extremely rare, psychosis as the initial clinical presentation of Graves’ disease can occur. The early diagnosis of Graves’ disease is critical to prevent cardiovascular implications and death.
Related Resources
- Abraham P, Acharya S. Current and emerging treatment options for Graves’ hyperthyroidism. Ther Clin Risk Manag. 2010;6:29-40.
- Bunevicius R, Prange AJ Jr. Psychiatric manifestations of Graves’ hyperthyroidism: pathophysiology and treatment options. CNS Drugs. 2006;20(11):897-909.
- Ginsberg J. Diagnosis and management of Graves’ disease. CMAJ. 2003;168(5):575-585.
Drug Brand Names
Atenolol • Tenormin
Methimazole • Tapazole
Risperidone • Risperdal
CASE Anxious and jealous
Mrs. H, age 28, presents to the emergency department (ED) with pressured speech, emotional lability, loose associations, and echolalia. On physical examination, Mrs. H is noted to have hand tremors. Mrs. H says she has not slept for the past 5 days and is experiencing anxiety and heart palpitations.
She also says that for the past 2 years she has believed that her husband is having an affair with her best friend. However, her current presentation—which she attributes to the alleged affair—began a week before she came to the ED. According to her husband, Mrs. H was “perfectly fine until a week ago” and her symptoms “appeared out of nowhere.” He reports that this has never happened before.
Mrs. H is admitted to the psychiatry unit. The nursing team reports that on the first night, Mrs. H was “running and screaming on the unit, out of control,” and was “tearful, manicky, and dysphoric.”
Mrs. H has no significant medical or psychiatric history. Her family history is significant for hyperthyroidism in her mother and maternal grandmother. Mrs. H says she smokes cigarettes (1 pack/d) but denies alcohol or illicit drug use.

EVALUATION A telling thyroid panel
Mrs. H undergoes laboratory testing, including a complete blood count, comprehensive metabolic panel, and thyroid panel due to her family history of thyroid-related disorders. The thyroid panel shows the presence of the thyroid-stimulating hormone (TSH) receptor antibody; a low TSH level; elevated triiodothyronine (T3) and thyroxine (T4) levels, with T3 > T4; elevated thyroid peroxidase (TPO) antibody; and elevated thyroglobulin antibody (Table 1). A scan shows the thyroid gland to be normal/top-normal size and is read by radiology to be indicative of a resolving thyroiditis vs Graves’ disease. An electrocardiogram indicates a heart rate of 139 beats per minute.
[polldaddy:10352133]
The authors’ observations
Mrs. H fits the presentation of psychosis secondary to Graves’ disease. However, our differential consisted of thyroiditis, brief psychotic disorder, delusional disorder (jealous type), and bipolar mania.
Brief psychotic disorder, bipolar mania, and delusional disorder were better explained by Graves’ disease, and Mrs. H’s jealous delusion resulted in functional impairment, which eliminated delusional disorder. Her family history of hyperthyroidism, as well as her sex and history of tobacco use, supported the diagnosis of Graves’ disease. Although Mrs. H did not experience goiter, ophthalmopathy, or dermopathy, which are common signs and symptoms of Graves’ disease (Table 2), she did present with irritability, insomnia, tachycardia, and a hand tremor. Her psychiatric symptoms included anxiety, emotional lability and, most importantly, psychosis. Her laboratory results included the presence of the TSH-receptor antibody, a low TSH level, and elevated T3 and T4 levels (T3>T4), confirming the diagnosis of early-onset Graves’ disease.
Continue to: Graves' disease
Graves’ disease
Graves’ disease is the most common cause of hyperthyroidism, representing approximately 50% to 80% of cases.1 Graves’ disease occurs most often in women, smokers, and those with a personal or family history of autoimmune disease; although patients of any age may be affected, the peak incidence occurs between age 40 and 60.1
Graves’ disease results from the production of immunoglobulin G (IgG) antibodies that activate the TSH receptor on the surface of thyroid follicular cells.1 The presence of the TSH-receptor antibody, in addition to a low TSH and elevated T3 and T4 levels (T3>T4), are common laboratory findings in patients with this disease. A thyroid scan will also show increased radiotracer accumulation.
Patients with Graves’ disease, as well as those with hyperthyroidism, tend to report weight loss, increased appetite, heat intolerance, irritability, insomnia, and palpitations. In addition to the above symptoms, the identifying signs and symptoms of Graves’ disease include a goiter, ophthalmopathy, and dermopathy (Table 2). Rarely, patients with Graves’ disease can present with psychosis, which is often complicated by thyrotoxicosis.2
[polldaddy:10352135]
TREATMENT Antipsychotic and a beta blocker
Based on her signs, symptoms, and laboratory findings, Mrs. H receives risperidone, 1 mg twice daily, for psychosis, and atenolol, 25 mg twice daily, for heart palpitations. Over 4 days, her symptoms decrease; she experiences more linear thought and decreased flight-of-ideas, and becomes unsure about the truth of her husband’s alleged affair. Her impulsive behaviors and severe mood lability cease. Her tachycardia remains controlled with atenolol.
The authors’ observations
Rapid initiation of treatment is important when managing patients with Graves’ disease, because untreated patients have a higher risk of psychiatric illness, cardiac disease, arrhythmia, and sudden cardiac death.1 Patients with Graves’ disease typically are treated with thionamides, radioactive iodine, and/or surgery. When a patient presents with psychosis as a result of thyrotoxicosis, treatment focuses on improving the thyrotoxicosis through anti-thyroid medications and beta blockers (Table 33). Psychotropic medications, such as antipsychotics, are not indicated for primary treatment, but are given to patients who have severe psychosis until symptoms have resolved.3 For Mrs. H, the severity of her psychosis necessitated risperidone in addition to atenolol.
OUTCOME Continuous medical management; no ablation
Mrs. H is discharged with immediate outpatient follow-up with an endocrinology team to discuss the best long-term management of her thyroiditis. Mrs. H opts for continuous medical management (as opposed to ablation) and is administered methimazole, 15 mg/d, to treat Graves’ disease.
The authors’ observations
This case provides useful information regarding recognizing psychosis as the initial sign of Graves’ disease. Although Graves’ disease represents 50% to 80% of cases of hyperthyroidism,1 psychosis as the first clinical presentation of this disease is extremely rare. Several case reports, however, have described this phenomenon,2,3 and further studies would be helpful to determine its true prevalence.
Continue to: Bottom Line
Bottom Line
Although extremely rare, psychosis as the initial clinical presentation of Graves’ disease can occur. The early diagnosis of Graves’ disease is critical to prevent cardiovascular implications and death.
Related Resources
- Abraham P, Acharya S. Current and emerging treatment options for Graves’ hyperthyroidism. Ther Clin Risk Manag. 2010;6:29-40.
- Bunevicius R, Prange AJ Jr. Psychiatric manifestations of Graves’ hyperthyroidism: pathophysiology and treatment options. CNS Drugs. 2006;20(11):897-909.
- Ginsberg J. Diagnosis and management of Graves’ disease. CMAJ. 2003;168(5):575-585.
Drug Brand Names
Atenolol • Tenormin
Methimazole • Tapazole
Risperidone • Risperdal
1. Girgis C, Champion B, Wall J. Current concepts in Graves’ disease. Ther Adv Endocrinol Metab. 2011;2(3):135-144.
2. Urias-Uribe L, Valdez-Solis E, González-Milán C, et al. Psychosis crisis associated with thyrotoxicosis due to Graves’ disease. Case Rep Psychiatry. 2017;2017:6803682. doi: 10.1155/2017/6803682.
3. Ugwu ET, Maluze J, Onyebueke GC. Graves’ thyrotoxicosis presenting as schizophreniform psychosis: a case report and literature review. Int J Endocrinol Metab. 2017;15(1):e41977. doi: 10.5812/ijem.41977.
1. Girgis C, Champion B, Wall J. Current concepts in Graves’ disease. Ther Adv Endocrinol Metab. 2011;2(3):135-144.
2. Urias-Uribe L, Valdez-Solis E, González-Milán C, et al. Psychosis crisis associated with thyrotoxicosis due to Graves’ disease. Case Rep Psychiatry. 2017;2017:6803682. doi: 10.1155/2017/6803682.
3. Ugwu ET, Maluze J, Onyebueke GC. Graves’ thyrotoxicosis presenting as schizophreniform psychosis: a case report and literature review. Int J Endocrinol Metab. 2017;15(1):e41977. doi: 10.5812/ijem.41977.
Serotonin syndrome: How to keep your patients safe
Mr. S, age 55, comes to your clinic as a walk-in for management of major depressive disorder, insomnia, and migraines. He also has tobacco use disorder and hypertension. Several days ago, Mr. S had visited the clinic because he was continuing to experience depressive symptoms, so his sertraline was increased from 100 to 200 mg/d. His current medication regimen includes sertraline 200 mg/d, trazodone 100 mg/d, lisinopril 10 mg/d, and sumatriptan, 100 mg as needed for migraine. He says last week he used 4 or 5 doses of sumatriptan because he experienced several migraines. Mr. S also reports occasionally taking 2 tablets of trazodone instead of 1 on nights that he has trouble falling asleep.
Today, Mr. S presents with a low-grade fever, diarrhea, internal restlessness, and a racing heartbeat that started shortly after his last visit. During physical examination, he exhibits slow, continuous lateral eye movements. His vital signs are markedly elevated: blood pressure, 175/85 mm Hg; heart rate, 110 beats per minute; and temperature, 39°C (102.2°F). Based on his presentation, the treatment team decides to send Mr. S to urgent care for closer monitoring.
Serotonin syndrome is a drug-induced syndrome caused by overstimulation of serotonin receptors. The syndrome is characterized by a classic clinical triad consisting of mental status changes, autonomic hyperactivity, and neuromuscular abnormalities. The clinical presentation is highly variable, and the severity ranges from mild to life-threatening.1-3 The incidence and prevalence of serotonin syndrome has not been well defined.3 Serotonin syndrome may be underreported because mild cases are often overlooked due to nonspecific symptoms. In addition, lack of physician awareness of drug–drug interactions, signs and symptoms, and differential diagnoses may result in underdiagnosis or misdiagnosis.1-3
What causes it?
Serotonin syndrome is usually a consequence of a drug–drug interaction between 2 or more serotonergic agents.4 Serotonin syndrome may result following medication misuse, overdose, initiation of a serotonergic agent, or increase in the dose of a currently prescribed serotonergic agent.3,4 In addition to medication classes and specific agents, Table 12-5 lists the drug mechanisms associated with serotonin syndrome:
- inhibition of serotonin reuptake
- inhibition of serotonin metabolism
- increased serotonin synthesis
- agonism of the serotonin receptor.
The amount of serotonergic activity most likely to cause serotonin syndrome is unclear.4
Pathophysiology. Serotonin, also known as 5-hydroxytryptamine (5-HT), is a metabolite of the amino acid tryptophan. This neurotransmitter is located in both the CNS and the periphery. Regulation of the serotonergic system begins in the presynaptic neurons with decarboxylation and hydroxylation of tryptophan resulting in serotonin synthesis. Once serotonin is produced, it is released into the synaptic cleft, where it binds to serotonin receptors.1,4,5 After receptor binding, serotonin reuptake occurs in the presynaptic neurons, where it can be metabolized by the monoamine oxidase enzyme. Finally, the metabolites are excreted in the urine. Serotonin syndrome results when this regulatory system is disrupted due to hyperstimulation of the postsynaptic serotonin receptors, mainly via agonism of the 5-HT2A and 5-HT1A receptors.1,4,5
Continue to: A nonspecific presentation
A nonspecific presentation
Unfortunately, many of the symptoms of serotonin syndrome are nonspecific, and the severity varies among patients.2,3 The onset of symptoms usually occurs within 6 to 8 hours after ingestion of a serotonergic agent.5 It is important to immediately recognize the symptoms (Table 22-5) and formulate a differential diagnosis because sudden progression of symptoms is common and may lead to life-threatening circumstances.1,3

In mild cases of serotonin syndrome, patients may have a low-grade fever or be afebrile. Hyperthermia tends to be present in moderate and severe cases, with temperatures >41°C (105.8°F) during life-threatening cases. Diaphoresis and tachycardia may be present regardless of severity. Additional autonomic irregularities include hypertension, tachypnea, nausea, vomiting, diarrhea, and hyperactive bowel sounds. In terms of neuromuscular abnormalities, hyperreflexia is a primary concern, as well as myoclonus. As the severity progresses to life-threatening, the clonus may convert from inducible to spontaneous and slow, continuous lateral eye movements may be present. Additional neuromuscular symptoms include tremor, akathisia, and muscle rigidity.1,3-5
Common mental status changes during mild cases include restlessness and anxiety. Abnormal mentation during moderate cases may present as increased hypervigilance and agitation, and this may advance to delirium or coma in severe cases. As the severity intensifies, the risk of developing additional physiological complications also increases. Rhabdomyolysis may occur due to muscle damage and myoglobinuria secondary to hyperreflexia, myoclonus, hypertonicity, and muscle rigidity. Muscle breakdown may then progress to further complications, such as renal failure. In rare instances, serotonin syndrome can result in seizures or death.1,3-5
Medication history tips off the diagnosis
The first step in diagnosing serotonin syndrome is to conduct a thorough review of the patient’s medication history, specifically taking into account any recent exposure to serotonergic agents.3,5 It is important to ask about prescription medications as well as over-the-counter products, herbal supplements, and illicit substances.1,4 When reviewing the medication history, investigate whether there may have been a recent change in therapy with serotonergic agents. Also, determine when the patient’s symptoms began in relation to exposure to serotonergic agents.4
After the medication review, conduct a thorough physical and neurologic examination to identify current symptoms and severity.1,3 No specific laboratory test is available to definitively confirm the diagnosis of serotonin syndrome.1,4 Monitoring of serum serotonin is not recommended because the levels do not correlate with symptom severity.3 The recommended diagnostic tool is the Hunter Serotonin Toxicity Criteria (Figure1,3).3,4 Historically, the Sternbach’s Diagnostic Criteria for serotonin syndrome were used for diagnosis; however, the Hunter Serotonin Toxicity Criteria are more sensitive (96% vs 75%) and more specific (97% vs 84%) than the Sternbach’s Diagnostic Criteria for serotonin syndrome.1,3-5
Continue to: In addition to using the proper diagnostic tool...
In addition to using the proper diagnostic tool, conduct a differential diagnosis to rule out other drug-induced syndromes, such as anticholinergic toxidrome, neuroleptic malignant syndrome, or malignant hyperthermia.1,3,5 Autonomic instability, including hypertension, tachycardia, tachypnea, and hyperthermia, may be present in all of the aforementioned drug-induced syndromes.1 As a result, the clinician must monitor for other symptoms that may differentiate the disease states to establish a clear diagnosis.
Discontinue agents, offer supportive care
There are no official published guidelines for managing serotonin syndrome.5 Regardless of the severity of a patient’s presentation, all serotonergic agents should be discontinued immediately. In addition, supportive care should be initiated for symptom management. Intravenous fluid replacement is recommended for hydration and to treat hyperthermia. External cooling may also be warranted to reduce body temperatures. Vital signs should be stabilized with appropriate pharmacotherapy.1,3-5
Benzodiazepines are considered a mainstay for relief of agitation during serotonin syndrome of any severity. In life-threatening cases—which are characterized by hyperthermia >41°C (105.8°F)—sedation, paralysis, and intubation may be necessary to maintain the airway, breathing, and circulation.1,3-5 Because treatment of hyperthermia requires elimination of hyperreflexia, paralysis is recommended.1 Nondepolarizing neuromuscular blocking agents, such as vecuronium, are preferred over depolarizing agents due to their decreased potential for rhabdomyolysis.1,3
Cyproheptadine, a histamine-1 receptor antagonist and a 5-HT2A receptor antagonist, is recommended for off-label treatment of serotonin syndrome to help decrease the intensity of symptoms. This should be initiated as a single dose of 12 mg followed by 2 mg every 2 hours until symptoms improve.1,3,5 After stabilization, a maintenance dose of 8 mg every 6 hours is recommended. Doses should not exceed the maximum recommended dose of 0.5 mg/kg/d.1,3,6 The most common adverse reactions associated with cyproheptadine are sedation and anticholinergic adverse effects.1,4,6
Antipsychotics, such as olanzapine and chlorpromazine, have been considered treatment alternatives due to their associated 5-HT2A receptor antagonism. However, there is limited data supporting such use.1,4 Antipsychotics should be used with caution because neuroleptic malignant syndrome may be mistaken for serotonin syndrome. Use of antipyretics is not recommended for treating fever and hyperthermia because the increase in body temperature is secondary to excessive muscle activity rather than dysfunction of the hypothalamic temperature set point.1,3,5 Physical restraints are also not recommended because their use may provoke further hyperthermia and increase the risk of rhabdomyolysis.3,5
Continue to: Ultimately, the duration of treatment...
Ultimately, the duration of treatment will be influenced by the pharmacokinetics of the serotonergic agents that induced the serotonin syndrome. Following resolution, retrial of the offending serotonergic agents should be carefully assessed. A retrial should only be considered after an adequate washout period has been observed, and clinicians should consider utilizing lower doses.2,5
Take steps for prevention
Patients at highest risk of developing serotonin syndrome are those who have multiple comorbidities that result in treatment with multiple serotonergic agents.3 Clinicians and patients alike need to be educated about the signs and symptoms of serotonin syndrome to promote early recognition. Also consider modifying your prescribing practices to minimize the use of multiple serotonergic agents. When switching between serotonergic agents, institute safe washout periods. Encourage patients to adhere to their prescribed medication regimens. Using electronic ordering systems can help detect drug–drug interactions.1,3 Prophylaxis with cyproheptadine may be considered in high-risk patients; however, no clinical trials have been conducted to evaluate using cyproheptadine to prevent serotonin syndrome.7
CASE CONTINUED
Upon further assessment in urgent care, Mr. S is found to have muscle rigidity in addition to ocular clonus and a temperature >38°C (100.4°F). Because Mr. S’s symptoms coincide with a recent increase of sertraline and increased use of both trazodone and sumatriptan, he meets Hunter Serotonin Toxicity Criteria. Therefore, his symptoms are likely related to excessive increase in serotonergic activity. Mr. S is admitted to the hospital for closer monitoring, and his sertraline, trazodone, and sumatriptan are held. He receives IV fluids for several days as well as cyproheptadine, 8 mg every 6 hours after stabilization, until his symptoms resolve. On Day 4, Mr. S no longer experiences diarrhea and internal restlessness. His vital signs return to normal, and as a result of symptom resolution, he is discharged from the hospital. The treatment team discusses changing his medication regimen to avoid multiple serotonergic agents. Mr. S is switched from sertraline to bupropion XL, 150 mg/d. Sumatriptan, 100 mg/d as needed, is continued for acute migraine treatment. Trazodone is discontinued and replaced with melatonin, 3 mg/d. The team also counsels Mr. S on the importance of proper adherence to his medication regimen. He is advised to return to the clinic in 2 weeks for reassessment of safety and efficacy.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Almotriptan • Axert
Buprenorphine • Subutex
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cyproheptadine • Periactin
Eletriptan • Relpax
Frovatriptan • Frova
Granisetron • Kytril
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Methadone • Dolophine, Methadose
Metoclopramide • Reglan
Mirtazapine • Remeron
Naratriptan • Amerge
Olanzapine • Zyprexa
Ondansetron • Zofran
Rizatriptan • Maxalt
Sertraline • Zoloft
Sumatriptan • Imitrex tablets
Tapentadol • Nucynta
Tramadol • Conzip
Trazodone • Desyrel, Oleptro
Valproic acid • Depakene, Depakote
Vecuronium • Norcuron
Zolmitriptan • Zomig
1. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
2. Beakley BD, Kaye AM, Kaye AD. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician. 2015;18(4):395-400.
3. Wang RZ, Vashistha V, Kaur S, et al. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817.
4. Bartlett D. Drug-induced serotonin syndrome. Crit Care Nurse. 2017;37(1):49-54.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. Cyproheptadine hydrochloride tablets [package insert]. Hayward, CA: Impax Generics; 2017.
7. Deardorff OG, Khan T, Kulkarni G, et al. Serotonin syndrome: prophylactic treatment with cyproheptadine. Prim Care Companion CNS Disord. 2016;18(4). doi: 10.4088/PCC.16br01966.
Mr. S, age 55, comes to your clinic as a walk-in for management of major depressive disorder, insomnia, and migraines. He also has tobacco use disorder and hypertension. Several days ago, Mr. S had visited the clinic because he was continuing to experience depressive symptoms, so his sertraline was increased from 100 to 200 mg/d. His current medication regimen includes sertraline 200 mg/d, trazodone 100 mg/d, lisinopril 10 mg/d, and sumatriptan, 100 mg as needed for migraine. He says last week he used 4 or 5 doses of sumatriptan because he experienced several migraines. Mr. S also reports occasionally taking 2 tablets of trazodone instead of 1 on nights that he has trouble falling asleep.
Today, Mr. S presents with a low-grade fever, diarrhea, internal restlessness, and a racing heartbeat that started shortly after his last visit. During physical examination, he exhibits slow, continuous lateral eye movements. His vital signs are markedly elevated: blood pressure, 175/85 mm Hg; heart rate, 110 beats per minute; and temperature, 39°C (102.2°F). Based on his presentation, the treatment team decides to send Mr. S to urgent care for closer monitoring.
Serotonin syndrome is a drug-induced syndrome caused by overstimulation of serotonin receptors. The syndrome is characterized by a classic clinical triad consisting of mental status changes, autonomic hyperactivity, and neuromuscular abnormalities. The clinical presentation is highly variable, and the severity ranges from mild to life-threatening.1-3 The incidence and prevalence of serotonin syndrome has not been well defined.3 Serotonin syndrome may be underreported because mild cases are often overlooked due to nonspecific symptoms. In addition, lack of physician awareness of drug–drug interactions, signs and symptoms, and differential diagnoses may result in underdiagnosis or misdiagnosis.1-3
What causes it?
Serotonin syndrome is usually a consequence of a drug–drug interaction between 2 or more serotonergic agents.4 Serotonin syndrome may result following medication misuse, overdose, initiation of a serotonergic agent, or increase in the dose of a currently prescribed serotonergic agent.3,4 In addition to medication classes and specific agents, Table 12-5 lists the drug mechanisms associated with serotonin syndrome:
- inhibition of serotonin reuptake
- inhibition of serotonin metabolism
- increased serotonin synthesis
- agonism of the serotonin receptor.

The amount of serotonergic activity most likely to cause serotonin syndrome is unclear.4
Pathophysiology. Serotonin, also known as 5-hydroxytryptamine (5-HT), is a metabolite of the amino acid tryptophan. This neurotransmitter is located in both the CNS and the periphery. Regulation of the serotonergic system begins in the presynaptic neurons with decarboxylation and hydroxylation of tryptophan resulting in serotonin synthesis. Once serotonin is produced, it is released into the synaptic cleft, where it binds to serotonin receptors.1,4,5 After receptor binding, serotonin reuptake occurs in the presynaptic neurons, where it can be metabolized by the monoamine oxidase enzyme. Finally, the metabolites are excreted in the urine. Serotonin syndrome results when this regulatory system is disrupted due to hyperstimulation of the postsynaptic serotonin receptors, mainly via agonism of the 5-HT2A and 5-HT1A receptors.1,4,5
Continue to: A nonspecific presentation
A nonspecific presentation
Unfortunately, many of the symptoms of serotonin syndrome are nonspecific, and the severity varies among patients.2,3 The onset of symptoms usually occurs within 6 to 8 hours after ingestion of a serotonergic agent.5 It is important to immediately recognize the symptoms (Table 22-5) and formulate a differential diagnosis because sudden progression of symptoms is common and may lead to life-threatening circumstances.1,3

In mild cases of serotonin syndrome, patients may have a low-grade fever or be afebrile. Hyperthermia tends to be present in moderate and severe cases, with temperatures >41°C (105.8°F) during life-threatening cases. Diaphoresis and tachycardia may be present regardless of severity. Additional autonomic irregularities include hypertension, tachypnea, nausea, vomiting, diarrhea, and hyperactive bowel sounds. In terms of neuromuscular abnormalities, hyperreflexia is a primary concern, as well as myoclonus. As the severity progresses to life-threatening, the clonus may convert from inducible to spontaneous and slow, continuous lateral eye movements may be present. Additional neuromuscular symptoms include tremor, akathisia, and muscle rigidity.1,3-5
Common mental status changes during mild cases include restlessness and anxiety. Abnormal mentation during moderate cases may present as increased hypervigilance and agitation, and this may advance to delirium or coma in severe cases. As the severity intensifies, the risk of developing additional physiological complications also increases. Rhabdomyolysis may occur due to muscle damage and myoglobinuria secondary to hyperreflexia, myoclonus, hypertonicity, and muscle rigidity. Muscle breakdown may then progress to further complications, such as renal failure. In rare instances, serotonin syndrome can result in seizures or death.1,3-5
Medication history tips off the diagnosis
The first step in diagnosing serotonin syndrome is to conduct a thorough review of the patient’s medication history, specifically taking into account any recent exposure to serotonergic agents.3,5 It is important to ask about prescription medications as well as over-the-counter products, herbal supplements, and illicit substances.1,4 When reviewing the medication history, investigate whether there may have been a recent change in therapy with serotonergic agents. Also, determine when the patient’s symptoms began in relation to exposure to serotonergic agents.4
After the medication review, conduct a thorough physical and neurologic examination to identify current symptoms and severity.1,3 No specific laboratory test is available to definitively confirm the diagnosis of serotonin syndrome.1,4 Monitoring of serum serotonin is not recommended because the levels do not correlate with symptom severity.3 The recommended diagnostic tool is the Hunter Serotonin Toxicity Criteria (Figure1,3).3,4 Historically, the Sternbach’s Diagnostic Criteria for serotonin syndrome were used for diagnosis; however, the Hunter Serotonin Toxicity Criteria are more sensitive (96% vs 75%) and more specific (97% vs 84%) than the Sternbach’s Diagnostic Criteria for serotonin syndrome.1,3-5
Continue to: In addition to using the proper diagnostic tool...
In addition to using the proper diagnostic tool, conduct a differential diagnosis to rule out other drug-induced syndromes, such as anticholinergic toxidrome, neuroleptic malignant syndrome, or malignant hyperthermia.1,3,5 Autonomic instability, including hypertension, tachycardia, tachypnea, and hyperthermia, may be present in all of the aforementioned drug-induced syndromes.1 As a result, the clinician must monitor for other symptoms that may differentiate the disease states to establish a clear diagnosis.
Discontinue agents, offer supportive care
There are no official published guidelines for managing serotonin syndrome.5 Regardless of the severity of a patient’s presentation, all serotonergic agents should be discontinued immediately. In addition, supportive care should be initiated for symptom management. Intravenous fluid replacement is recommended for hydration and to treat hyperthermia. External cooling may also be warranted to reduce body temperatures. Vital signs should be stabilized with appropriate pharmacotherapy.1,3-5
Benzodiazepines are considered a mainstay for relief of agitation during serotonin syndrome of any severity. In life-threatening cases—which are characterized by hyperthermia >41°C (105.8°F)—sedation, paralysis, and intubation may be necessary to maintain the airway, breathing, and circulation.1,3-5 Because treatment of hyperthermia requires elimination of hyperreflexia, paralysis is recommended.1 Nondepolarizing neuromuscular blocking agents, such as vecuronium, are preferred over depolarizing agents due to their decreased potential for rhabdomyolysis.1,3
Cyproheptadine, a histamine-1 receptor antagonist and a 5-HT2A receptor antagonist, is recommended for off-label treatment of serotonin syndrome to help decrease the intensity of symptoms. This should be initiated as a single dose of 12 mg followed by 2 mg every 2 hours until symptoms improve.1,3,5 After stabilization, a maintenance dose of 8 mg every 6 hours is recommended. Doses should not exceed the maximum recommended dose of 0.5 mg/kg/d.1,3,6 The most common adverse reactions associated with cyproheptadine are sedation and anticholinergic adverse effects.1,4,6
Antipsychotics, such as olanzapine and chlorpromazine, have been considered treatment alternatives due to their associated 5-HT2A receptor antagonism. However, there is limited data supporting such use.1,4 Antipsychotics should be used with caution because neuroleptic malignant syndrome may be mistaken for serotonin syndrome. Use of antipyretics is not recommended for treating fever and hyperthermia because the increase in body temperature is secondary to excessive muscle activity rather than dysfunction of the hypothalamic temperature set point.1,3,5 Physical restraints are also not recommended because their use may provoke further hyperthermia and increase the risk of rhabdomyolysis.3,5
Continue to: Ultimately, the duration of treatment...
Ultimately, the duration of treatment will be influenced by the pharmacokinetics of the serotonergic agents that induced the serotonin syndrome. Following resolution, retrial of the offending serotonergic agents should be carefully assessed. A retrial should only be considered after an adequate washout period has been observed, and clinicians should consider utilizing lower doses.2,5
Take steps for prevention
Patients at highest risk of developing serotonin syndrome are those who have multiple comorbidities that result in treatment with multiple serotonergic agents.3 Clinicians and patients alike need to be educated about the signs and symptoms of serotonin syndrome to promote early recognition. Also consider modifying your prescribing practices to minimize the use of multiple serotonergic agents. When switching between serotonergic agents, institute safe washout periods. Encourage patients to adhere to their prescribed medication regimens. Using electronic ordering systems can help detect drug–drug interactions.1,3 Prophylaxis with cyproheptadine may be considered in high-risk patients; however, no clinical trials have been conducted to evaluate using cyproheptadine to prevent serotonin syndrome.7
CASE CONTINUED
Upon further assessment in urgent care, Mr. S is found to have muscle rigidity in addition to ocular clonus and a temperature >38°C (100.4°F). Because Mr. S’s symptoms coincide with a recent increase of sertraline and increased use of both trazodone and sumatriptan, he meets Hunter Serotonin Toxicity Criteria. Therefore, his symptoms are likely related to excessive increase in serotonergic activity. Mr. S is admitted to the hospital for closer monitoring, and his sertraline, trazodone, and sumatriptan are held. He receives IV fluids for several days as well as cyproheptadine, 8 mg every 6 hours after stabilization, until his symptoms resolve. On Day 4, Mr. S no longer experiences diarrhea and internal restlessness. His vital signs return to normal, and as a result of symptom resolution, he is discharged from the hospital. The treatment team discusses changing his medication regimen to avoid multiple serotonergic agents. Mr. S is switched from sertraline to bupropion XL, 150 mg/d. Sumatriptan, 100 mg/d as needed, is continued for acute migraine treatment. Trazodone is discontinued and replaced with melatonin, 3 mg/d. The team also counsels Mr. S on the importance of proper adherence to his medication regimen. He is advised to return to the clinic in 2 weeks for reassessment of safety and efficacy.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Almotriptan • Axert
Buprenorphine • Subutex
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cyproheptadine • Periactin
Eletriptan • Relpax
Frovatriptan • Frova
Granisetron • Kytril
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Methadone • Dolophine, Methadose
Metoclopramide • Reglan
Mirtazapine • Remeron
Naratriptan • Amerge
Olanzapine • Zyprexa
Ondansetron • Zofran
Rizatriptan • Maxalt
Sertraline • Zoloft
Sumatriptan • Imitrex tablets
Tapentadol • Nucynta
Tramadol • Conzip
Trazodone • Desyrel, Oleptro
Valproic acid • Depakene, Depakote
Vecuronium • Norcuron
Zolmitriptan • Zomig
Mr. S, age 55, comes to your clinic as a walk-in for management of major depressive disorder, insomnia, and migraines. He also has tobacco use disorder and hypertension. Several days ago, Mr. S had visited the clinic because he was continuing to experience depressive symptoms, so his sertraline was increased from 100 to 200 mg/d. His current medication regimen includes sertraline 200 mg/d, trazodone 100 mg/d, lisinopril 10 mg/d, and sumatriptan, 100 mg as needed for migraine. He says last week he used 4 or 5 doses of sumatriptan because he experienced several migraines. Mr. S also reports occasionally taking 2 tablets of trazodone instead of 1 on nights that he has trouble falling asleep.
Today, Mr. S presents with a low-grade fever, diarrhea, internal restlessness, and a racing heartbeat that started shortly after his last visit. During physical examination, he exhibits slow, continuous lateral eye movements. His vital signs are markedly elevated: blood pressure, 175/85 mm Hg; heart rate, 110 beats per minute; and temperature, 39°C (102.2°F). Based on his presentation, the treatment team decides to send Mr. S to urgent care for closer monitoring.
Serotonin syndrome is a drug-induced syndrome caused by overstimulation of serotonin receptors. The syndrome is characterized by a classic clinical triad consisting of mental status changes, autonomic hyperactivity, and neuromuscular abnormalities. The clinical presentation is highly variable, and the severity ranges from mild to life-threatening.1-3 The incidence and prevalence of serotonin syndrome has not been well defined.3 Serotonin syndrome may be underreported because mild cases are often overlooked due to nonspecific symptoms. In addition, lack of physician awareness of drug–drug interactions, signs and symptoms, and differential diagnoses may result in underdiagnosis or misdiagnosis.1-3
What causes it?
Serotonin syndrome is usually a consequence of a drug–drug interaction between 2 or more serotonergic agents.4 Serotonin syndrome may result following medication misuse, overdose, initiation of a serotonergic agent, or increase in the dose of a currently prescribed serotonergic agent.3,4 In addition to medication classes and specific agents, Table 12-5 lists the drug mechanisms associated with serotonin syndrome:
- inhibition of serotonin reuptake
- inhibition of serotonin metabolism
- increased serotonin synthesis
- agonism of the serotonin receptor.

The amount of serotonergic activity most likely to cause serotonin syndrome is unclear.4
Pathophysiology. Serotonin, also known as 5-hydroxytryptamine (5-HT), is a metabolite of the amino acid tryptophan. This neurotransmitter is located in both the CNS and the periphery. Regulation of the serotonergic system begins in the presynaptic neurons with decarboxylation and hydroxylation of tryptophan resulting in serotonin synthesis. Once serotonin is produced, it is released into the synaptic cleft, where it binds to serotonin receptors.1,4,5 After receptor binding, serotonin reuptake occurs in the presynaptic neurons, where it can be metabolized by the monoamine oxidase enzyme. Finally, the metabolites are excreted in the urine. Serotonin syndrome results when this regulatory system is disrupted due to hyperstimulation of the postsynaptic serotonin receptors, mainly via agonism of the 5-HT2A and 5-HT1A receptors.1,4,5
Continue to: A nonspecific presentation
A nonspecific presentation
Unfortunately, many of the symptoms of serotonin syndrome are nonspecific, and the severity varies among patients.2,3 The onset of symptoms usually occurs within 6 to 8 hours after ingestion of a serotonergic agent.5 It is important to immediately recognize the symptoms (Table 22-5) and formulate a differential diagnosis because sudden progression of symptoms is common and may lead to life-threatening circumstances.1,3

In mild cases of serotonin syndrome, patients may have a low-grade fever or be afebrile. Hyperthermia tends to be present in moderate and severe cases, with temperatures >41°C (105.8°F) during life-threatening cases. Diaphoresis and tachycardia may be present regardless of severity. Additional autonomic irregularities include hypertension, tachypnea, nausea, vomiting, diarrhea, and hyperactive bowel sounds. In terms of neuromuscular abnormalities, hyperreflexia is a primary concern, as well as myoclonus. As the severity progresses to life-threatening, the clonus may convert from inducible to spontaneous and slow, continuous lateral eye movements may be present. Additional neuromuscular symptoms include tremor, akathisia, and muscle rigidity.1,3-5
Common mental status changes during mild cases include restlessness and anxiety. Abnormal mentation during moderate cases may present as increased hypervigilance and agitation, and this may advance to delirium or coma in severe cases. As the severity intensifies, the risk of developing additional physiological complications also increases. Rhabdomyolysis may occur due to muscle damage and myoglobinuria secondary to hyperreflexia, myoclonus, hypertonicity, and muscle rigidity. Muscle breakdown may then progress to further complications, such as renal failure. In rare instances, serotonin syndrome can result in seizures or death.1,3-5
Medication history tips off the diagnosis
The first step in diagnosing serotonin syndrome is to conduct a thorough review of the patient’s medication history, specifically taking into account any recent exposure to serotonergic agents.3,5 It is important to ask about prescription medications as well as over-the-counter products, herbal supplements, and illicit substances.1,4 When reviewing the medication history, investigate whether there may have been a recent change in therapy with serotonergic agents. Also, determine when the patient’s symptoms began in relation to exposure to serotonergic agents.4
After the medication review, conduct a thorough physical and neurologic examination to identify current symptoms and severity.1,3 No specific laboratory test is available to definitively confirm the diagnosis of serotonin syndrome.1,4 Monitoring of serum serotonin is not recommended because the levels do not correlate with symptom severity.3 The recommended diagnostic tool is the Hunter Serotonin Toxicity Criteria (Figure1,3).3,4 Historically, the Sternbach’s Diagnostic Criteria for serotonin syndrome were used for diagnosis; however, the Hunter Serotonin Toxicity Criteria are more sensitive (96% vs 75%) and more specific (97% vs 84%) than the Sternbach’s Diagnostic Criteria for serotonin syndrome.1,3-5
Continue to: In addition to using the proper diagnostic tool...
In addition to using the proper diagnostic tool, conduct a differential diagnosis to rule out other drug-induced syndromes, such as anticholinergic toxidrome, neuroleptic malignant syndrome, or malignant hyperthermia.1,3,5 Autonomic instability, including hypertension, tachycardia, tachypnea, and hyperthermia, may be present in all of the aforementioned drug-induced syndromes.1 As a result, the clinician must monitor for other symptoms that may differentiate the disease states to establish a clear diagnosis.
Discontinue agents, offer supportive care
There are no official published guidelines for managing serotonin syndrome.5 Regardless of the severity of a patient’s presentation, all serotonergic agents should be discontinued immediately. In addition, supportive care should be initiated for symptom management. Intravenous fluid replacement is recommended for hydration and to treat hyperthermia. External cooling may also be warranted to reduce body temperatures. Vital signs should be stabilized with appropriate pharmacotherapy.1,3-5
Benzodiazepines are considered a mainstay for relief of agitation during serotonin syndrome of any severity. In life-threatening cases—which are characterized by hyperthermia >41°C (105.8°F)—sedation, paralysis, and intubation may be necessary to maintain the airway, breathing, and circulation.1,3-5 Because treatment of hyperthermia requires elimination of hyperreflexia, paralysis is recommended.1 Nondepolarizing neuromuscular blocking agents, such as vecuronium, are preferred over depolarizing agents due to their decreased potential for rhabdomyolysis.1,3
Cyproheptadine, a histamine-1 receptor antagonist and a 5-HT2A receptor antagonist, is recommended for off-label treatment of serotonin syndrome to help decrease the intensity of symptoms. This should be initiated as a single dose of 12 mg followed by 2 mg every 2 hours until symptoms improve.1,3,5 After stabilization, a maintenance dose of 8 mg every 6 hours is recommended. Doses should not exceed the maximum recommended dose of 0.5 mg/kg/d.1,3,6 The most common adverse reactions associated with cyproheptadine are sedation and anticholinergic adverse effects.1,4,6
Antipsychotics, such as olanzapine and chlorpromazine, have been considered treatment alternatives due to their associated 5-HT2A receptor antagonism. However, there is limited data supporting such use.1,4 Antipsychotics should be used with caution because neuroleptic malignant syndrome may be mistaken for serotonin syndrome. Use of antipyretics is not recommended for treating fever and hyperthermia because the increase in body temperature is secondary to excessive muscle activity rather than dysfunction of the hypothalamic temperature set point.1,3,5 Physical restraints are also not recommended because their use may provoke further hyperthermia and increase the risk of rhabdomyolysis.3,5
Continue to: Ultimately, the duration of treatment...
Ultimately, the duration of treatment will be influenced by the pharmacokinetics of the serotonergic agents that induced the serotonin syndrome. Following resolution, retrial of the offending serotonergic agents should be carefully assessed. A retrial should only be considered after an adequate washout period has been observed, and clinicians should consider utilizing lower doses.2,5
Take steps for prevention
Patients at highest risk of developing serotonin syndrome are those who have multiple comorbidities that result in treatment with multiple serotonergic agents.3 Clinicians and patients alike need to be educated about the signs and symptoms of serotonin syndrome to promote early recognition. Also consider modifying your prescribing practices to minimize the use of multiple serotonergic agents. When switching between serotonergic agents, institute safe washout periods. Encourage patients to adhere to their prescribed medication regimens. Using electronic ordering systems can help detect drug–drug interactions.1,3 Prophylaxis with cyproheptadine may be considered in high-risk patients; however, no clinical trials have been conducted to evaluate using cyproheptadine to prevent serotonin syndrome.7
CASE CONTINUED
Upon further assessment in urgent care, Mr. S is found to have muscle rigidity in addition to ocular clonus and a temperature >38°C (100.4°F). Because Mr. S’s symptoms coincide with a recent increase of sertraline and increased use of both trazodone and sumatriptan, he meets Hunter Serotonin Toxicity Criteria. Therefore, his symptoms are likely related to excessive increase in serotonergic activity. Mr. S is admitted to the hospital for closer monitoring, and his sertraline, trazodone, and sumatriptan are held. He receives IV fluids for several days as well as cyproheptadine, 8 mg every 6 hours after stabilization, until his symptoms resolve. On Day 4, Mr. S no longer experiences diarrhea and internal restlessness. His vital signs return to normal, and as a result of symptom resolution, he is discharged from the hospital. The treatment team discusses changing his medication regimen to avoid multiple serotonergic agents. Mr. S is switched from sertraline to bupropion XL, 150 mg/d. Sumatriptan, 100 mg/d as needed, is continued for acute migraine treatment. Trazodone is discontinued and replaced with melatonin, 3 mg/d. The team also counsels Mr. S on the importance of proper adherence to his medication regimen. He is advised to return to the clinic in 2 weeks for reassessment of safety and efficacy.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Almotriptan • Axert
Buprenorphine • Subutex
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cyproheptadine • Periactin
Eletriptan • Relpax
Frovatriptan • Frova
Granisetron • Kytril
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Methadone • Dolophine, Methadose
Metoclopramide • Reglan
Mirtazapine • Remeron
Naratriptan • Amerge
Olanzapine • Zyprexa
Ondansetron • Zofran
Rizatriptan • Maxalt
Sertraline • Zoloft
Sumatriptan • Imitrex tablets
Tapentadol • Nucynta
Tramadol • Conzip
Trazodone • Desyrel, Oleptro
Valproic acid • Depakene, Depakote
Vecuronium • Norcuron
Zolmitriptan • Zomig
1. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
2. Beakley BD, Kaye AM, Kaye AD. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician. 2015;18(4):395-400.
3. Wang RZ, Vashistha V, Kaur S, et al. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817.
4. Bartlett D. Drug-induced serotonin syndrome. Crit Care Nurse. 2017;37(1):49-54.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. Cyproheptadine hydrochloride tablets [package insert]. Hayward, CA: Impax Generics; 2017.
7. Deardorff OG, Khan T, Kulkarni G, et al. Serotonin syndrome: prophylactic treatment with cyproheptadine. Prim Care Companion CNS Disord. 2016;18(4). doi: 10.4088/PCC.16br01966.
1. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
2. Beakley BD, Kaye AM, Kaye AD. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician. 2015;18(4):395-400.
3. Wang RZ, Vashistha V, Kaur S, et al. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817.
4. Bartlett D. Drug-induced serotonin syndrome. Crit Care Nurse. 2017;37(1):49-54.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. Cyproheptadine hydrochloride tablets [package insert]. Hayward, CA: Impax Generics; 2017.
7. Deardorff OG, Khan T, Kulkarni G, et al. Serotonin syndrome: prophylactic treatment with cyproheptadine. Prim Care Companion CNS Disord. 2016;18(4). doi: 10.4088/PCC.16br01966.