User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Cutaneous Manifestations and Clinical Disparities in Patients Without Housing
More than half a million individuals are without housing (NWH) on any given night in the United States, as estimated by the US Department of Housing and Urban Development. 1 Lack of hygiene, increased risk of infection and infestation due to living conditions, and barriers to health care put these individuals at increased risk for disease. 2 Skin disease, including fungal infection and acne, are within the top 10 most prevalent diseases worldwide and can cause major psychologic impairment, yet dermatologic concerns and clinical outcomes in NWH patients have not been well characterized. 2-5 Further, because this vulnerable demographic tends to be underinsured, they frequently present to the emergency department (ED) for management of disease. 1,6 Survey of common concerns in NWH patients is of utility to consulting dermatologists and nondermatologist providers in the ED, who can familiarize themselves with management of diseases they are more likely to encounter. Few studies examine dermatologic conditions in the ED, and a thorough literature review indicates none have included homelessness as a variable. 6,7 Additionally, comparison with a matched control group of patients with housing (WH) is limited. 5,8 We present one of the largest comparisons of cutaneous disease in NWH vs WH patients in a single hospital system to elucidate the types of cutaneous disease that motivate patients to seek care, the location of skin disease, and differences in clinical care.
Methods
A retrospective medical record review of patients seen for an inclusive list of dermatologic diagnoses in the ED or while admitted at University Medical Center New Orleans, Louisiana (UMC), between January 1, 2018, and April 21, 2020, was conducted. This study was qualified as exempt from the institutional review board by Louisiana State University because it proposed zero risk to the patients and remained completely anonymous. Eight hundred forty-two total medical records were reviewed (NWH, 421; WH, 421)(Table 1). Patients with housing were matched based on self-identified race and ethnicity, sex, and age. Disease categories were constructed based on fundamental pathophysiology adapted from Dermatology9: infectious, noninfectious inflammatory, neoplasm, trauma and wounds, drug-related eruptions, vascular, pruritic, pigmented, bullous, neuropsychiatric, and other. Other included unspecified eruptions as well as miscellaneous lesions such as calluses. The current chief concern, anatomic location, and configuration were recorded, as well as biopsied lesions and outpatient referrals or inpatient consultations to dermatology or other specialties, including wound care, infectious disease, podiatry, and surgery. χ2 analysis was used to analyze significance of cutaneous categories, body location, and referrals. Groups smaller than 5 defaulted to the Fisher exact test.

Results
The total diagnoses (including both chief concerns and secondary diagnoses) are shown in Table 2. Chief concerns were more frequently cutaneous or dermatologic for WH (NWH, 209; WH, 307; P<.001). In both groups, cutaneous infectious etiologies were more likely to be a patient’s presenting chief concern (58% NWH, P=.002; 42% WH, P<.001). Noninfectious inflammatory etiologies and pigmented lesions were more likely to be secondary diagnoses with an unrelated noncutaneous concern; noninfectious inflammatory etiologies were only 16% of the total cutaneous chief concerns (11% NWH, P=.04; 20% WH, P=.03), and no pigmented lesions were chief concerns.
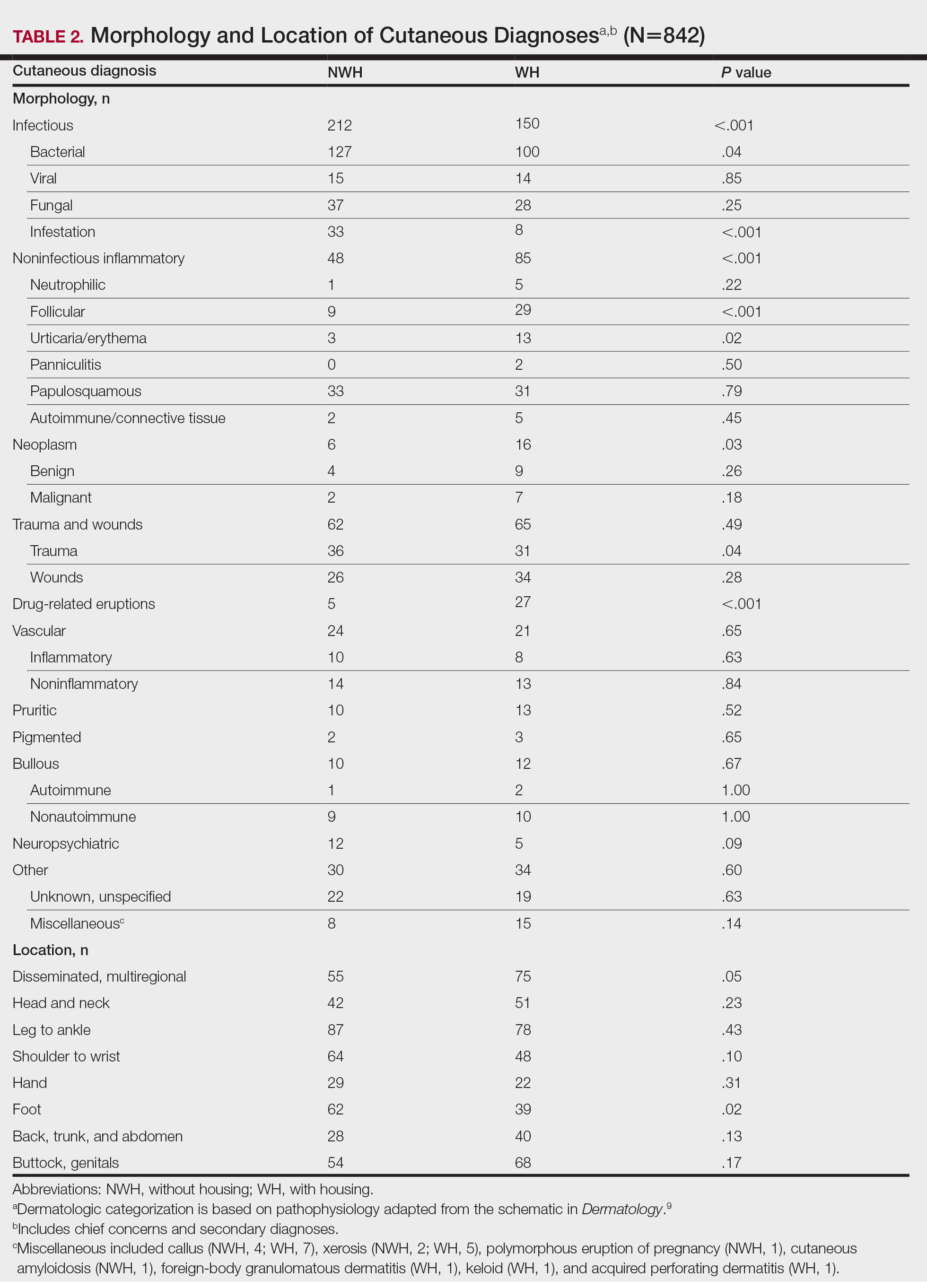
Infection was the most common chief concern, though NWH patients presented with significantly more infectious concerns (NWH, 212; WH, 150; P<.001), particularly infestations (NWH, 33; WH, 8; P<.001) and bacterial etiologies (NWH, 127; WH, 100; P=.04). The majority of bacterial etiologies were either an abscess or cellulitis (NWH, 106; WH, 83), though infected chronic wounds were categorized as bacterial infection when treated definitively as such (eg, in the case of sacral ulcers causing osteomyelitis)(NWH, 21; WH, 17). Of note, infectious etiology was associated with intravenous drug use (IVDU) in both NWH and WH patients. Of 184 NWH who reported IVDU, 127 had an infectious diagnosis (P<.001). Similarly, 43 of 56 total WH patients who reported IVDU had an infectious diagnosis (P<.001). Infestation (within the infectious category) included scabies (NWH, 20; WH, 3) and insect or arthropod bites (NWH, 12; WH, 5). Two NWH patients also presented with swelling of the lower extremities and were subsequently diagnosed with maggot infestations. Fungal and viral etiologies were not significantly increased in either group; however, NWH did have a higher incidence of tinea pedis (NWH, 14; WH, 4; P=.03).
More neoplasms (NWH, 6; WH, 16; P=.03), noninfectious inflammatory eruptions (NWH, 48; WH, 85; P<.001), and cutaneous drug eruptions (NWH, 5; WH, 27; P<.001) were reported in WH patients. There was no significant difference in benign vs malignant neoplastic processes between groups. More noninfectious inflammatory eruptions in WH were specifically driven by a markedly increased incidence of follicular (NWH, 9; WH, 29; P<.001) and urticarial/erythematous (NWH, 3; WH, 13; P=.02) lesions. Follicular etiologies included acne (NWH, 1; WH, 6; P=.12), folliculitis (NWH, 5; WH, 2; P=.45), hidradenitis suppurativa (NWH, 2; WH, 11; P=.02), and pilonidal and sebaceous cysts (NWH, 1; WH, 10; P=.01). Allergic urticaria dominated the urticarial/erythematous category (NWH, 3; WH, 11; P=.06), though there were 2 WH presentations of diffuse erythema and skin peeling.
Another substantial proportion of cutaneous etiologies were due to trauma or chronic wounds. Significantly more traumatic injuries presented in NWH patients vs WH patients (36 vs 31; P=.04). Trauma included human or dog bites (NWH, 5; WH, 4), sunburns (NWH, 3; WH, 0), other burns (NWH, 11; WH, 13), abrasions and lacerations (NWH, 16; WH, 3; P=.004), and foreign bodies (NWH, 1; WH, 1). Wounds consisted of chronic wounds such as those due to diabetes mellitus (foot ulcers) or immobility (sacral ulcers); numbers were similar between groups.
Looking at location, NWH patients had more pathology on the feet (NWH, 62; WH, 39; P=.02), whereas WH patients had more disseminated multiregional concerns (NWH, 55; WH, 75; P=.05). No one body location was notably more likely to warrant a chief concern.
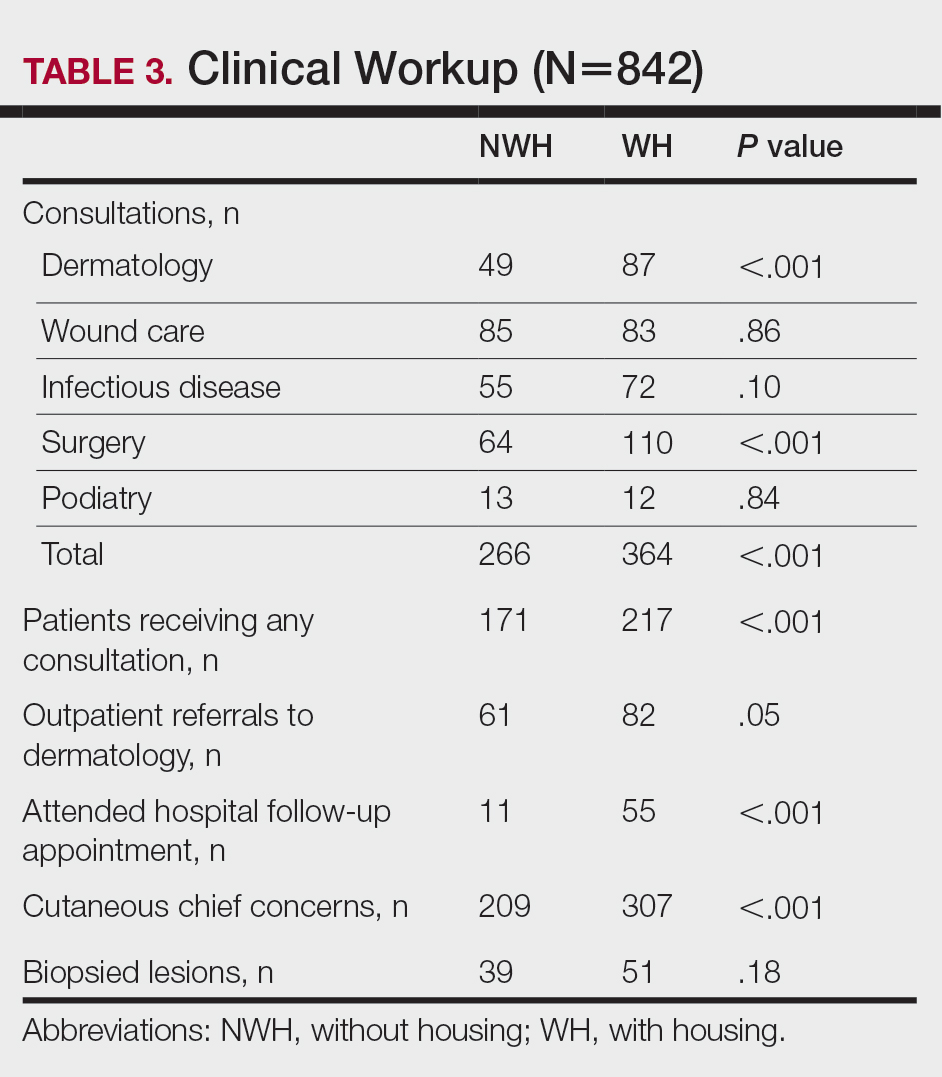
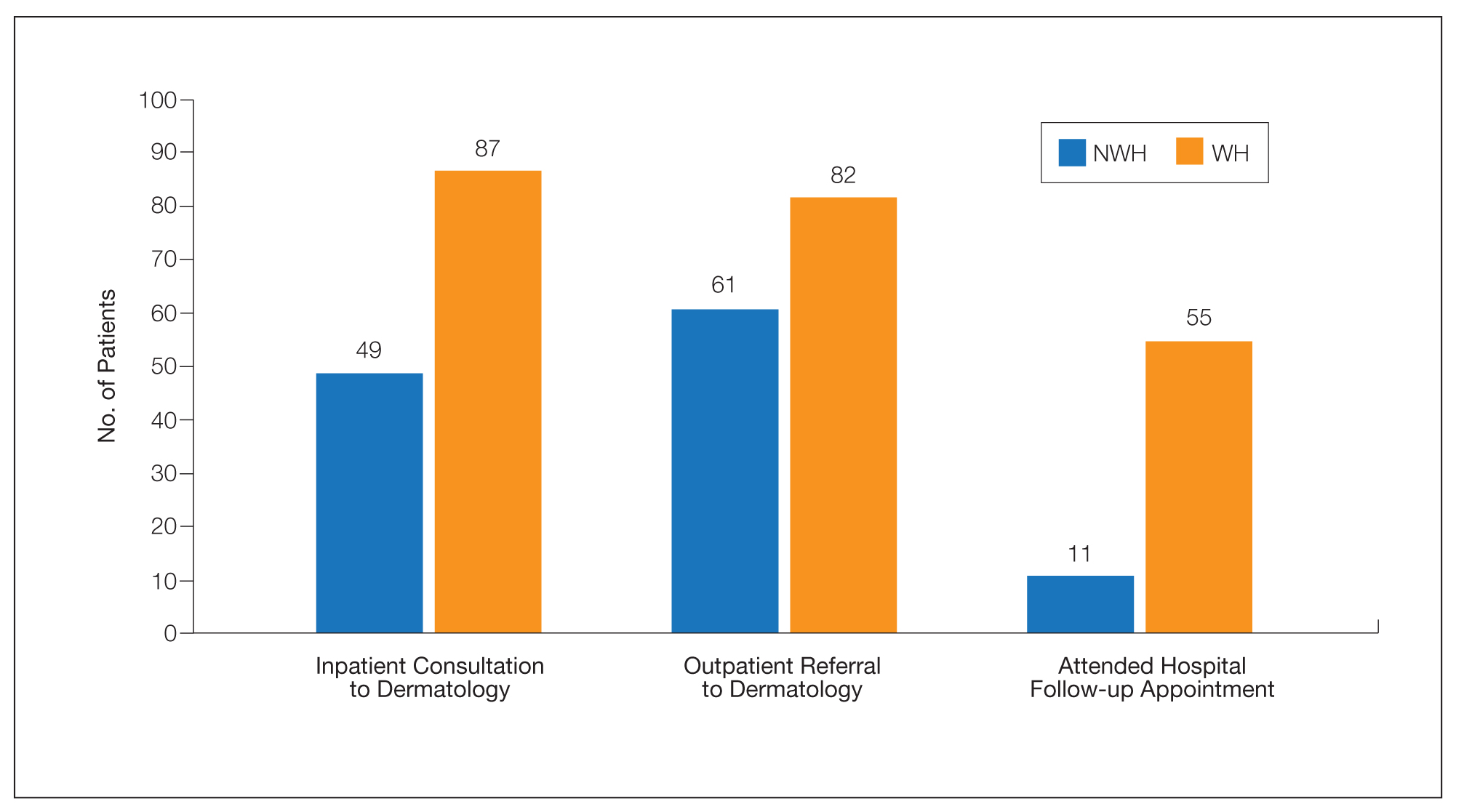
For clinical outcomes, more WH patients received a consultation of any kind (NWH, 171; WH, 217; P<.001), consultation to dermatology (NWH, 49; WH, 87; P<.001), and consultation to surgery (NWH, 64; WH, 110; P<.001)(Table 3 and Figure). More outpatient referrals to dermatology were made for WH patients (NWH, 61; WH, 82; P=.05). Notably, NWH patients presented for 80% fewer hospital follow-up appointments (NWH, 11; WH, 55; P<.001). It is essential to note that these findings were not affected by self-reported race or ethnicity. Results remained significant when broken into cohorts consisting of patients with and without skin of color.
Comment
Cutaneous Concerns in NWH Patients—Although cutaneous disease has been reported to disproportionately affect NWH patients,10 in our cohort, NWH patients had fewer cutaneous chief concerns than WH patients. However, without comparing with all patients entering the ED at UMC, we cannot make a statement on this claim. We do present a few reasons why NWH patients do not have more cutaneous concerns. First, they may wait to present with cutaneous disease until it becomes more severe (eg, until chronic wounds have progressed to infections). Second, as discussed in depth by Hollestein and Nijsten,3 dermatologic disease may be a major contributor to the overall count of disability-adjusted life years but may play a minor role in individual disability. Therefore, skin disease often is considered less important on an individual basis, despite substantial psychosocial burden, leading to further stigmatization of this vulnerable population and discouraged care-seeking behavior, particularly for noninfectious inflammatory eruptions, which were notably more present in WH individuals. Third, fewer dermatologic lesions were reported on NWH patients, which may explain why all 3 WH pigmented lesions were diagnosed after presentation with a noncutaneous concern (eg, headache, anemia, nausea).
Infectious Cutaneous Diagnoses—The increased presentation of infectious etiologies, especially bacterial, is linked to the increased numbers of IVDUs reported in NWH individuals as well as increased exposure and decreased access to basic hygienic supplies. Intravenous drug use acted as an effect modifier of infectious etiology diagnoses, playing a major role in both NWH and WH cohorts. Although Black and Hispanic individuals as well as individuals with low socioeconomic status have increased proportions of skin cancer, there are inadequate data on the prevalence in NWH individuals.4 We found no increase in malignant dermatologic processes in NWH individuals; however, this may be secondary to inadequate screening with a total body skin examination.
Clinical Workup of NWH Patients—Because most NWH individuals present to the ED to receive care, their care compared with WH patients should be considered. In this cohort, WH patients received a less extensive clinical workup. They received almost half as many dermatologic consultations and fewer outpatient referrals to dermatology. Major communication barriers may affect NWH presentation to follow-up, which was drastically lower than WH individuals, as scheduling typically occurs well after discharge from the ED or inpatient unit. We suggest a few alterations to improve dermatologic care for NWH individuals:
• Consider inpatient consultation for serious dermatologic conditions—even if chronic—to improve disease control, considering that many barriers inhibit follow-up in clinic.
• Involve outreach teams, such as the Assertive Community Treatment teams, that assist individuals by delivering medicine for psychiatric disorders, conducting total-body skin examinations, assisting with wound care, providing basic skin barrier creams or medicaments, and carrying information regarding outpatient follow-up.
• Educate ED providers on the most common skin concerns, especially those that fall within the noninfectious inflammatory category, such as hidradenitis suppurativa, which could easily be misdiagnosed as an abscess.
Future Directions—Owing to limitations of a retrospective cohort study, we present several opportunities for further research on this vulnerable population. The severity of disease, especially infectious etiologies, should be graded to determine if NWH patients truly present later in the disease course. The duration and quality of housing for NWH patients could be categorized based on living conditions (eg, on the street vs in a shelter). Although the findings of our NWH cohort presenting to the ED at UMC provide helpful insight into dermatologic disease, these findings may be disparate from those conducted at other locations in the United States. University Medical Center provides care to mostly subsidized insurance plans in a racially diverse community. Improved outcomes for the NWH individuals living in New Orleans start with obtaining a greater understanding of their diseases and where disparities exist that can be bridged with better care.
Acknowledgment—The dataset generated during this study and used for analysis is not publicly available to protect public health information but is available from the corresponding author on reasonable request.
- Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. 2014;384:1529-1540. doi:10.1016/S0140-6736(14)61132-6
- Contag C, Lowenstein SE, Jain S, et al. Survey of symptomatic dermatologic disease in homeless patients at a shelter-based clinic. Our Dermatol Online. 2017;8:133-137. doi:10.7241/ourd.20172.37
- Hollestein LM, Nijsten T. An insight into the global burden of skin diseases. J Invest Dermatol. 2014;134:1499-1501. doi:10.1038/jid.2013.513
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Mackelprang JL, Graves JM, Rivara FP. Homeless in America: injuries treated in US emergency departments, 2007-2011. Int J Inj Contr Saf Promot. 2014;21:289-297. doi:10.1038/jid.2014.371
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313. doi:10.1016/j.jaad.2014.03.013
- Stratigos AJ, Stern R, Gonzalez E, et al. Prevalence of skin disease in a cohort of shelter-based homeless men. J Am Acad Dermatol. 1999;41:197-202. doi:10.1016/S0190-9622(99)70048-4
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012.
- Badiaga S, Menard A, Tissot Dupont H, et al. Prevalence of skin infections in sheltered homeless. Eur J Dermatol. 2005;15:382-386.
More than half a million individuals are without housing (NWH) on any given night in the United States, as estimated by the US Department of Housing and Urban Development. 1 Lack of hygiene, increased risk of infection and infestation due to living conditions, and barriers to health care put these individuals at increased risk for disease. 2 Skin disease, including fungal infection and acne, are within the top 10 most prevalent diseases worldwide and can cause major psychologic impairment, yet dermatologic concerns and clinical outcomes in NWH patients have not been well characterized. 2-5 Further, because this vulnerable demographic tends to be underinsured, they frequently present to the emergency department (ED) for management of disease. 1,6 Survey of common concerns in NWH patients is of utility to consulting dermatologists and nondermatologist providers in the ED, who can familiarize themselves with management of diseases they are more likely to encounter. Few studies examine dermatologic conditions in the ED, and a thorough literature review indicates none have included homelessness as a variable. 6,7 Additionally, comparison with a matched control group of patients with housing (WH) is limited. 5,8 We present one of the largest comparisons of cutaneous disease in NWH vs WH patients in a single hospital system to elucidate the types of cutaneous disease that motivate patients to seek care, the location of skin disease, and differences in clinical care.
Methods
A retrospective medical record review of patients seen for an inclusive list of dermatologic diagnoses in the ED or while admitted at University Medical Center New Orleans, Louisiana (UMC), between January 1, 2018, and April 21, 2020, was conducted. This study was qualified as exempt from the institutional review board by Louisiana State University because it proposed zero risk to the patients and remained completely anonymous. Eight hundred forty-two total medical records were reviewed (NWH, 421; WH, 421)(Table 1). Patients with housing were matched based on self-identified race and ethnicity, sex, and age. Disease categories were constructed based on fundamental pathophysiology adapted from Dermatology9: infectious, noninfectious inflammatory, neoplasm, trauma and wounds, drug-related eruptions, vascular, pruritic, pigmented, bullous, neuropsychiatric, and other. Other included unspecified eruptions as well as miscellaneous lesions such as calluses. The current chief concern, anatomic location, and configuration were recorded, as well as biopsied lesions and outpatient referrals or inpatient consultations to dermatology or other specialties, including wound care, infectious disease, podiatry, and surgery. χ2 analysis was used to analyze significance of cutaneous categories, body location, and referrals. Groups smaller than 5 defaulted to the Fisher exact test.

Results
The total diagnoses (including both chief concerns and secondary diagnoses) are shown in Table 2. Chief concerns were more frequently cutaneous or dermatologic for WH (NWH, 209; WH, 307; P<.001). In both groups, cutaneous infectious etiologies were more likely to be a patient’s presenting chief concern (58% NWH, P=.002; 42% WH, P<.001). Noninfectious inflammatory etiologies and pigmented lesions were more likely to be secondary diagnoses with an unrelated noncutaneous concern; noninfectious inflammatory etiologies were only 16% of the total cutaneous chief concerns (11% NWH, P=.04; 20% WH, P=.03), and no pigmented lesions were chief concerns.

Infection was the most common chief concern, though NWH patients presented with significantly more infectious concerns (NWH, 212; WH, 150; P<.001), particularly infestations (NWH, 33; WH, 8; P<.001) and bacterial etiologies (NWH, 127; WH, 100; P=.04). The majority of bacterial etiologies were either an abscess or cellulitis (NWH, 106; WH, 83), though infected chronic wounds were categorized as bacterial infection when treated definitively as such (eg, in the case of sacral ulcers causing osteomyelitis)(NWH, 21; WH, 17). Of note, infectious etiology was associated with intravenous drug use (IVDU) in both NWH and WH patients. Of 184 NWH who reported IVDU, 127 had an infectious diagnosis (P<.001). Similarly, 43 of 56 total WH patients who reported IVDU had an infectious diagnosis (P<.001). Infestation (within the infectious category) included scabies (NWH, 20; WH, 3) and insect or arthropod bites (NWH, 12; WH, 5). Two NWH patients also presented with swelling of the lower extremities and were subsequently diagnosed with maggot infestations. Fungal and viral etiologies were not significantly increased in either group; however, NWH did have a higher incidence of tinea pedis (NWH, 14; WH, 4; P=.03).
More neoplasms (NWH, 6; WH, 16; P=.03), noninfectious inflammatory eruptions (NWH, 48; WH, 85; P<.001), and cutaneous drug eruptions (NWH, 5; WH, 27; P<.001) were reported in WH patients. There was no significant difference in benign vs malignant neoplastic processes between groups. More noninfectious inflammatory eruptions in WH were specifically driven by a markedly increased incidence of follicular (NWH, 9; WH, 29; P<.001) and urticarial/erythematous (NWH, 3; WH, 13; P=.02) lesions. Follicular etiologies included acne (NWH, 1; WH, 6; P=.12), folliculitis (NWH, 5; WH, 2; P=.45), hidradenitis suppurativa (NWH, 2; WH, 11; P=.02), and pilonidal and sebaceous cysts (NWH, 1; WH, 10; P=.01). Allergic urticaria dominated the urticarial/erythematous category (NWH, 3; WH, 11; P=.06), though there were 2 WH presentations of diffuse erythema and skin peeling.
Another substantial proportion of cutaneous etiologies were due to trauma or chronic wounds. Significantly more traumatic injuries presented in NWH patients vs WH patients (36 vs 31; P=.04). Trauma included human or dog bites (NWH, 5; WH, 4), sunburns (NWH, 3; WH, 0), other burns (NWH, 11; WH, 13), abrasions and lacerations (NWH, 16; WH, 3; P=.004), and foreign bodies (NWH, 1; WH, 1). Wounds consisted of chronic wounds such as those due to diabetes mellitus (foot ulcers) or immobility (sacral ulcers); numbers were similar between groups.
Looking at location, NWH patients had more pathology on the feet (NWH, 62; WH, 39; P=.02), whereas WH patients had more disseminated multiregional concerns (NWH, 55; WH, 75; P=.05). No one body location was notably more likely to warrant a chief concern.
For clinical outcomes, more WH patients received a consultation of any kind (NWH, 171; WH, 217; P<.001), consultation to dermatology (NWH, 49; WH, 87; P<.001), and consultation to surgery (NWH, 64; WH, 110; P<.001)(Table 3 and Figure). More outpatient referrals to dermatology were made for WH patients (NWH, 61; WH, 82; P=.05). Notably, NWH patients presented for 80% fewer hospital follow-up appointments (NWH, 11; WH, 55; P<.001). It is essential to note that these findings were not affected by self-reported race or ethnicity. Results remained significant when broken into cohorts consisting of patients with and without skin of color.

Comment
Cutaneous Concerns in NWH Patients—Although cutaneous disease has been reported to disproportionately affect NWH patients,10 in our cohort, NWH patients had fewer cutaneous chief concerns than WH patients. However, without comparing with all patients entering the ED at UMC, we cannot make a statement on this claim. We do present a few reasons why NWH patients do not have more cutaneous concerns. First, they may wait to present with cutaneous disease until it becomes more severe (eg, until chronic wounds have progressed to infections). Second, as discussed in depth by Hollestein and Nijsten,3 dermatologic disease may be a major contributor to the overall count of disability-adjusted life years but may play a minor role in individual disability. Therefore, skin disease often is considered less important on an individual basis, despite substantial psychosocial burden, leading to further stigmatization of this vulnerable population and discouraged care-seeking behavior, particularly for noninfectious inflammatory eruptions, which were notably more present in WH individuals. Third, fewer dermatologic lesions were reported on NWH patients, which may explain why all 3 WH pigmented lesions were diagnosed after presentation with a noncutaneous concern (eg, headache, anemia, nausea).

Infectious Cutaneous Diagnoses—The increased presentation of infectious etiologies, especially bacterial, is linked to the increased numbers of IVDUs reported in NWH individuals as well as increased exposure and decreased access to basic hygienic supplies. Intravenous drug use acted as an effect modifier of infectious etiology diagnoses, playing a major role in both NWH and WH cohorts. Although Black and Hispanic individuals as well as individuals with low socioeconomic status have increased proportions of skin cancer, there are inadequate data on the prevalence in NWH individuals.4 We found no increase in malignant dermatologic processes in NWH individuals; however, this may be secondary to inadequate screening with a total body skin examination.
Clinical Workup of NWH Patients—Because most NWH individuals present to the ED to receive care, their care compared with WH patients should be considered. In this cohort, WH patients received a less extensive clinical workup. They received almost half as many dermatologic consultations and fewer outpatient referrals to dermatology. Major communication barriers may affect NWH presentation to follow-up, which was drastically lower than WH individuals, as scheduling typically occurs well after discharge from the ED or inpatient unit. We suggest a few alterations to improve dermatologic care for NWH individuals:
• Consider inpatient consultation for serious dermatologic conditions—even if chronic—to improve disease control, considering that many barriers inhibit follow-up in clinic.
• Involve outreach teams, such as the Assertive Community Treatment teams, that assist individuals by delivering medicine for psychiatric disorders, conducting total-body skin examinations, assisting with wound care, providing basic skin barrier creams or medicaments, and carrying information regarding outpatient follow-up.
• Educate ED providers on the most common skin concerns, especially those that fall within the noninfectious inflammatory category, such as hidradenitis suppurativa, which could easily be misdiagnosed as an abscess.
Future Directions—Owing to limitations of a retrospective cohort study, we present several opportunities for further research on this vulnerable population. The severity of disease, especially infectious etiologies, should be graded to determine if NWH patients truly present later in the disease course. The duration and quality of housing for NWH patients could be categorized based on living conditions (eg, on the street vs in a shelter). Although the findings of our NWH cohort presenting to the ED at UMC provide helpful insight into dermatologic disease, these findings may be disparate from those conducted at other locations in the United States. University Medical Center provides care to mostly subsidized insurance plans in a racially diverse community. Improved outcomes for the NWH individuals living in New Orleans start with obtaining a greater understanding of their diseases and where disparities exist that can be bridged with better care.
Acknowledgment—The dataset generated during this study and used for analysis is not publicly available to protect public health information but is available from the corresponding author on reasonable request.
More than half a million individuals are without housing (NWH) on any given night in the United States, as estimated by the US Department of Housing and Urban Development. 1 Lack of hygiene, increased risk of infection and infestation due to living conditions, and barriers to health care put these individuals at increased risk for disease. 2 Skin disease, including fungal infection and acne, are within the top 10 most prevalent diseases worldwide and can cause major psychologic impairment, yet dermatologic concerns and clinical outcomes in NWH patients have not been well characterized. 2-5 Further, because this vulnerable demographic tends to be underinsured, they frequently present to the emergency department (ED) for management of disease. 1,6 Survey of common concerns in NWH patients is of utility to consulting dermatologists and nondermatologist providers in the ED, who can familiarize themselves with management of diseases they are more likely to encounter. Few studies examine dermatologic conditions in the ED, and a thorough literature review indicates none have included homelessness as a variable. 6,7 Additionally, comparison with a matched control group of patients with housing (WH) is limited. 5,8 We present one of the largest comparisons of cutaneous disease in NWH vs WH patients in a single hospital system to elucidate the types of cutaneous disease that motivate patients to seek care, the location of skin disease, and differences in clinical care.
Methods
A retrospective medical record review of patients seen for an inclusive list of dermatologic diagnoses in the ED or while admitted at University Medical Center New Orleans, Louisiana (UMC), between January 1, 2018, and April 21, 2020, was conducted. This study was qualified as exempt from the institutional review board by Louisiana State University because it proposed zero risk to the patients and remained completely anonymous. Eight hundred forty-two total medical records were reviewed (NWH, 421; WH, 421)(Table 1). Patients with housing were matched based on self-identified race and ethnicity, sex, and age. Disease categories were constructed based on fundamental pathophysiology adapted from Dermatology9: infectious, noninfectious inflammatory, neoplasm, trauma and wounds, drug-related eruptions, vascular, pruritic, pigmented, bullous, neuropsychiatric, and other. Other included unspecified eruptions as well as miscellaneous lesions such as calluses. The current chief concern, anatomic location, and configuration were recorded, as well as biopsied lesions and outpatient referrals or inpatient consultations to dermatology or other specialties, including wound care, infectious disease, podiatry, and surgery. χ2 analysis was used to analyze significance of cutaneous categories, body location, and referrals. Groups smaller than 5 defaulted to the Fisher exact test.

Results
The total diagnoses (including both chief concerns and secondary diagnoses) are shown in Table 2. Chief concerns were more frequently cutaneous or dermatologic for WH (NWH, 209; WH, 307; P<.001). In both groups, cutaneous infectious etiologies were more likely to be a patient’s presenting chief concern (58% NWH, P=.002; 42% WH, P<.001). Noninfectious inflammatory etiologies and pigmented lesions were more likely to be secondary diagnoses with an unrelated noncutaneous concern; noninfectious inflammatory etiologies were only 16% of the total cutaneous chief concerns (11% NWH, P=.04; 20% WH, P=.03), and no pigmented lesions were chief concerns.

Infection was the most common chief concern, though NWH patients presented with significantly more infectious concerns (NWH, 212; WH, 150; P<.001), particularly infestations (NWH, 33; WH, 8; P<.001) and bacterial etiologies (NWH, 127; WH, 100; P=.04). The majority of bacterial etiologies were either an abscess or cellulitis (NWH, 106; WH, 83), though infected chronic wounds were categorized as bacterial infection when treated definitively as such (eg, in the case of sacral ulcers causing osteomyelitis)(NWH, 21; WH, 17). Of note, infectious etiology was associated with intravenous drug use (IVDU) in both NWH and WH patients. Of 184 NWH who reported IVDU, 127 had an infectious diagnosis (P<.001). Similarly, 43 of 56 total WH patients who reported IVDU had an infectious diagnosis (P<.001). Infestation (within the infectious category) included scabies (NWH, 20; WH, 3) and insect or arthropod bites (NWH, 12; WH, 5). Two NWH patients also presented with swelling of the lower extremities and were subsequently diagnosed with maggot infestations. Fungal and viral etiologies were not significantly increased in either group; however, NWH did have a higher incidence of tinea pedis (NWH, 14; WH, 4; P=.03).
More neoplasms (NWH, 6; WH, 16; P=.03), noninfectious inflammatory eruptions (NWH, 48; WH, 85; P<.001), and cutaneous drug eruptions (NWH, 5; WH, 27; P<.001) were reported in WH patients. There was no significant difference in benign vs malignant neoplastic processes between groups. More noninfectious inflammatory eruptions in WH were specifically driven by a markedly increased incidence of follicular (NWH, 9; WH, 29; P<.001) and urticarial/erythematous (NWH, 3; WH, 13; P=.02) lesions. Follicular etiologies included acne (NWH, 1; WH, 6; P=.12), folliculitis (NWH, 5; WH, 2; P=.45), hidradenitis suppurativa (NWH, 2; WH, 11; P=.02), and pilonidal and sebaceous cysts (NWH, 1; WH, 10; P=.01). Allergic urticaria dominated the urticarial/erythematous category (NWH, 3; WH, 11; P=.06), though there were 2 WH presentations of diffuse erythema and skin peeling.
Another substantial proportion of cutaneous etiologies were due to trauma or chronic wounds. Significantly more traumatic injuries presented in NWH patients vs WH patients (36 vs 31; P=.04). Trauma included human or dog bites (NWH, 5; WH, 4), sunburns (NWH, 3; WH, 0), other burns (NWH, 11; WH, 13), abrasions and lacerations (NWH, 16; WH, 3; P=.004), and foreign bodies (NWH, 1; WH, 1). Wounds consisted of chronic wounds such as those due to diabetes mellitus (foot ulcers) or immobility (sacral ulcers); numbers were similar between groups.
Looking at location, NWH patients had more pathology on the feet (NWH, 62; WH, 39; P=.02), whereas WH patients had more disseminated multiregional concerns (NWH, 55; WH, 75; P=.05). No one body location was notably more likely to warrant a chief concern.
For clinical outcomes, more WH patients received a consultation of any kind (NWH, 171; WH, 217; P<.001), consultation to dermatology (NWH, 49; WH, 87; P<.001), and consultation to surgery (NWH, 64; WH, 110; P<.001)(Table 3 and Figure). More outpatient referrals to dermatology were made for WH patients (NWH, 61; WH, 82; P=.05). Notably, NWH patients presented for 80% fewer hospital follow-up appointments (NWH, 11; WH, 55; P<.001). It is essential to note that these findings were not affected by self-reported race or ethnicity. Results remained significant when broken into cohorts consisting of patients with and without skin of color.

Comment
Cutaneous Concerns in NWH Patients—Although cutaneous disease has been reported to disproportionately affect NWH patients,10 in our cohort, NWH patients had fewer cutaneous chief concerns than WH patients. However, without comparing with all patients entering the ED at UMC, we cannot make a statement on this claim. We do present a few reasons why NWH patients do not have more cutaneous concerns. First, they may wait to present with cutaneous disease until it becomes more severe (eg, until chronic wounds have progressed to infections). Second, as discussed in depth by Hollestein and Nijsten,3 dermatologic disease may be a major contributor to the overall count of disability-adjusted life years but may play a minor role in individual disability. Therefore, skin disease often is considered less important on an individual basis, despite substantial psychosocial burden, leading to further stigmatization of this vulnerable population and discouraged care-seeking behavior, particularly for noninfectious inflammatory eruptions, which were notably more present in WH individuals. Third, fewer dermatologic lesions were reported on NWH patients, which may explain why all 3 WH pigmented lesions were diagnosed after presentation with a noncutaneous concern (eg, headache, anemia, nausea).

Infectious Cutaneous Diagnoses—The increased presentation of infectious etiologies, especially bacterial, is linked to the increased numbers of IVDUs reported in NWH individuals as well as increased exposure and decreased access to basic hygienic supplies. Intravenous drug use acted as an effect modifier of infectious etiology diagnoses, playing a major role in both NWH and WH cohorts. Although Black and Hispanic individuals as well as individuals with low socioeconomic status have increased proportions of skin cancer, there are inadequate data on the prevalence in NWH individuals.4 We found no increase in malignant dermatologic processes in NWH individuals; however, this may be secondary to inadequate screening with a total body skin examination.
Clinical Workup of NWH Patients—Because most NWH individuals present to the ED to receive care, their care compared with WH patients should be considered. In this cohort, WH patients received a less extensive clinical workup. They received almost half as many dermatologic consultations and fewer outpatient referrals to dermatology. Major communication barriers may affect NWH presentation to follow-up, which was drastically lower than WH individuals, as scheduling typically occurs well after discharge from the ED or inpatient unit. We suggest a few alterations to improve dermatologic care for NWH individuals:
• Consider inpatient consultation for serious dermatologic conditions—even if chronic—to improve disease control, considering that many barriers inhibit follow-up in clinic.
• Involve outreach teams, such as the Assertive Community Treatment teams, that assist individuals by delivering medicine for psychiatric disorders, conducting total-body skin examinations, assisting with wound care, providing basic skin barrier creams or medicaments, and carrying information regarding outpatient follow-up.
• Educate ED providers on the most common skin concerns, especially those that fall within the noninfectious inflammatory category, such as hidradenitis suppurativa, which could easily be misdiagnosed as an abscess.
Future Directions—Owing to limitations of a retrospective cohort study, we present several opportunities for further research on this vulnerable population. The severity of disease, especially infectious etiologies, should be graded to determine if NWH patients truly present later in the disease course. The duration and quality of housing for NWH patients could be categorized based on living conditions (eg, on the street vs in a shelter). Although the findings of our NWH cohort presenting to the ED at UMC provide helpful insight into dermatologic disease, these findings may be disparate from those conducted at other locations in the United States. University Medical Center provides care to mostly subsidized insurance plans in a racially diverse community. Improved outcomes for the NWH individuals living in New Orleans start with obtaining a greater understanding of their diseases and where disparities exist that can be bridged with better care.
Acknowledgment—The dataset generated during this study and used for analysis is not publicly available to protect public health information but is available from the corresponding author on reasonable request.
- Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. 2014;384:1529-1540. doi:10.1016/S0140-6736(14)61132-6
- Contag C, Lowenstein SE, Jain S, et al. Survey of symptomatic dermatologic disease in homeless patients at a shelter-based clinic. Our Dermatol Online. 2017;8:133-137. doi:10.7241/ourd.20172.37
- Hollestein LM, Nijsten T. An insight into the global burden of skin diseases. J Invest Dermatol. 2014;134:1499-1501. doi:10.1038/jid.2013.513
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Mackelprang JL, Graves JM, Rivara FP. Homeless in America: injuries treated in US emergency departments, 2007-2011. Int J Inj Contr Saf Promot. 2014;21:289-297. doi:10.1038/jid.2014.371
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313. doi:10.1016/j.jaad.2014.03.013
- Stratigos AJ, Stern R, Gonzalez E, et al. Prevalence of skin disease in a cohort of shelter-based homeless men. J Am Acad Dermatol. 1999;41:197-202. doi:10.1016/S0190-9622(99)70048-4
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012.
- Badiaga S, Menard A, Tissot Dupont H, et al. Prevalence of skin infections in sheltered homeless. Eur J Dermatol. 2005;15:382-386.
- Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. 2014;384:1529-1540. doi:10.1016/S0140-6736(14)61132-6
- Contag C, Lowenstein SE, Jain S, et al. Survey of symptomatic dermatologic disease in homeless patients at a shelter-based clinic. Our Dermatol Online. 2017;8:133-137. doi:10.7241/ourd.20172.37
- Hollestein LM, Nijsten T. An insight into the global burden of skin diseases. J Invest Dermatol. 2014;134:1499-1501. doi:10.1038/jid.2013.513
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Mackelprang JL, Graves JM, Rivara FP. Homeless in America: injuries treated in US emergency departments, 2007-2011. Int J Inj Contr Saf Promot. 2014;21:289-297. doi:10.1038/jid.2014.371
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313. doi:10.1016/j.jaad.2014.03.013
- Stratigos AJ, Stern R, Gonzalez E, et al. Prevalence of skin disease in a cohort of shelter-based homeless men. J Am Acad Dermatol. 1999;41:197-202. doi:10.1016/S0190-9622(99)70048-4
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012.
- Badiaga S, Menard A, Tissot Dupont H, et al. Prevalence of skin infections in sheltered homeless. Eur J Dermatol. 2005;15:382-386.
Practice Points
- Dermatologic disease in patients without housing (NWH) is characterized by more infectious concerns and fewer follicular and urticarial noninfectious inflammatory eruptions compared with matched controls of those with housing.
- Patients with housing more frequently presented with cutaneous chief concerns and received more consultations while in the hospital.
- This study uncovered notable pathological and clinical differences in treating dermatologic conditions in NWH patients.
Acyclovir-Resistant Cutaneous Herpes Simplex Virus in DOCK8 Deficiency
Dedicator of cytokinesis 8 (DOCK8 ) deficiency is the major cause of autosomal-recessive hyper-IgEsyndrome. 1 Characteristic clinical features including eosinophilia, eczema, and recurrent Staphylococcus aureus cutaneous and respiratory tract infections are common in DOCK8 deficiency, similar to the autosomal-dominant form of hyper-IgE syndrome that is due to defi c iency of signal transducer and activation of transcription 3 (STAT-3 ). 1 In addition, patients with DOCK8 deficiency are particularly susceptible to asthma; food allergies; lymphomas; and severe cutaneous viral infections, including herpes simplex virus (HSV), molluscum contagiosum, varicella-zoster virus, and human papillomavirus. Since the discovery of the DOCK8 gene in 2009, various studies have sought to elucidate the mechanistic contribution of DOCK8 to the dermatologic immune environment. 2 Although cutaneous viral infections such as those caused by HSV typically are short lived and self-limiting in immunocompetent hosts, they have proven to be severe and recalcitrant in the setting of DOCK8 deficiency. 1 Herein, we report the case of a 32-month-old girl with homozygous DOCK8 deficiency who developed acyclovir-resistant cutaneous HSV.
Case Report
A 32-month-old girl presented with an approximately 2-cm linear erosion along the left posterior auricular sulcus at month 9 of a hospital stay for recurrent infections. Her medical history was notable for multiple upper respiratory tract infections, diffuse eczema, and food allergies. She had presented to an outside hospital at 14 months of age with herpetic gingivostomatitis and eczema herpeticum that was successfully treated with acyclovir. She was readmitted at 20 months of age due to Pneumocystis jiroveci pneumonia, pancytopenia, and disseminated histoplasmosis. Prophylactic oral acyclovir (20 mg/kg twice daily) was started, given her history of HSV infection. Because of recurrent infections, she underwent an immunodeficiency workup. Whole exome sequencing analysis revealed a homozygous deletion c.(528+1_529−1)_(1516+1_1517−1)del in DOCK8 gene–affecting exons 5 to 13. The patient was transferred to our hospital for continued care and as a potential candidate for bone marrow transplant following resolution of the disseminated histoplasmosis infection.
During her hospitalization at the current presentation, she was noted to have a 2-cm linear erosion along the left posterior auricular sulcus. Initial wound care with bacitracin ointment was applied to the area while specimens were obtained and empiric oral acyclovir therapy was initiated (20 mg/kg 4 times daily [QID]), given a clinical impression consistent with cutaneous HSV infection despite acyclovir prophylaxis. Direct immunofluorescence and viral cultures were positive for HSV-1, while bacterial cultures grew methicillin-susceptible S aureus. Cephalexin and mupirocin ointment were started, and acyclovir was continued. After 2 weeks of therapy, there was no visible change in the wound; cultures were repeated, again showing the wound contained HSV. Bacterial cultures this time grew Pseudomonas putida, and the antibiotic regimen was transitioned to cefepime.
After no response to the continued course of therapeutic acyclovir, HSV cultures were sent to the Centers for Disease Control and Prevention for resistance testing, and biopsy of the lesion was performed by the otolaryngology service to rule out malignancy and potential alternative diagnoses. Histopathology showed only reactive inflammation without visible microorganisms on tissue HSV-1/HSV-2 immunostain; however, tissue viral culture was positive for HSV-1. The patient was transitioned back to acyclovir (intravenous [IV] 20 mg/kg QID) with the addition of empiric foscarnet (IV 40 mg/kg 3 times daily) given the worsening appearance of the lesion. The HSV acyclovir resistance test results from the Centers for Disease Control and Prevention returned soon after and were positive for resistance (median infectious dose, 3.29 µg/L [reference interval, sensitive <2.00 µg/L; resistant >1.90 µg/L]). The patient completed a 21-day course of combination foscarnet and acyclovir therapy, during which time the lesion showed notable improvement and healing. The patient was continued on prophylactic acyclovir (IV 20 mg/kg QID). Unfortunately, the patient eventually died due to complications related to pneumonia.
Comment
Infection in Patients With DOCK8 Deficiency—The gene DOCK8 has emerged as playing a central role in both innate and adaptive immunity, as it is expressed primarily in immune cells and serves as a mediator of numerous processes, including immune synapse formation, cell signaling and trafficking, antibody and cytokine production, and lymphocyte memory.3 Cells that are critical for combating cutaneous viral infections, including skin-resident memory T cells and natural killer cells, are defective, which leads to a severely immunocompromised state in DOCK8-deficient patients with a particular susceptibility to infectious and inflammatory dermatologic disease.4
Herpes simplex virus infection commonly is seen in DOCK8 deficiency, with retrospective analysis of a DOCK8-deficient cohort revealing HSV infection in approximately 38% of patients.5 Prophylactic acyclovir is essential for DOCK8-deficient individuals with a history of HSV infection given the tendency of the virus to reactivate.6 However, despite prophylaxis, our patient developed an HSV-positive posterior auricular erosion that continued to progress even after increase of the acyclovir dose. Acyclovir resistance testing of the HSV isolated from the wound was positive, confirming the clinical suspicion of the presence of acyclovir-resistant HSV infection.
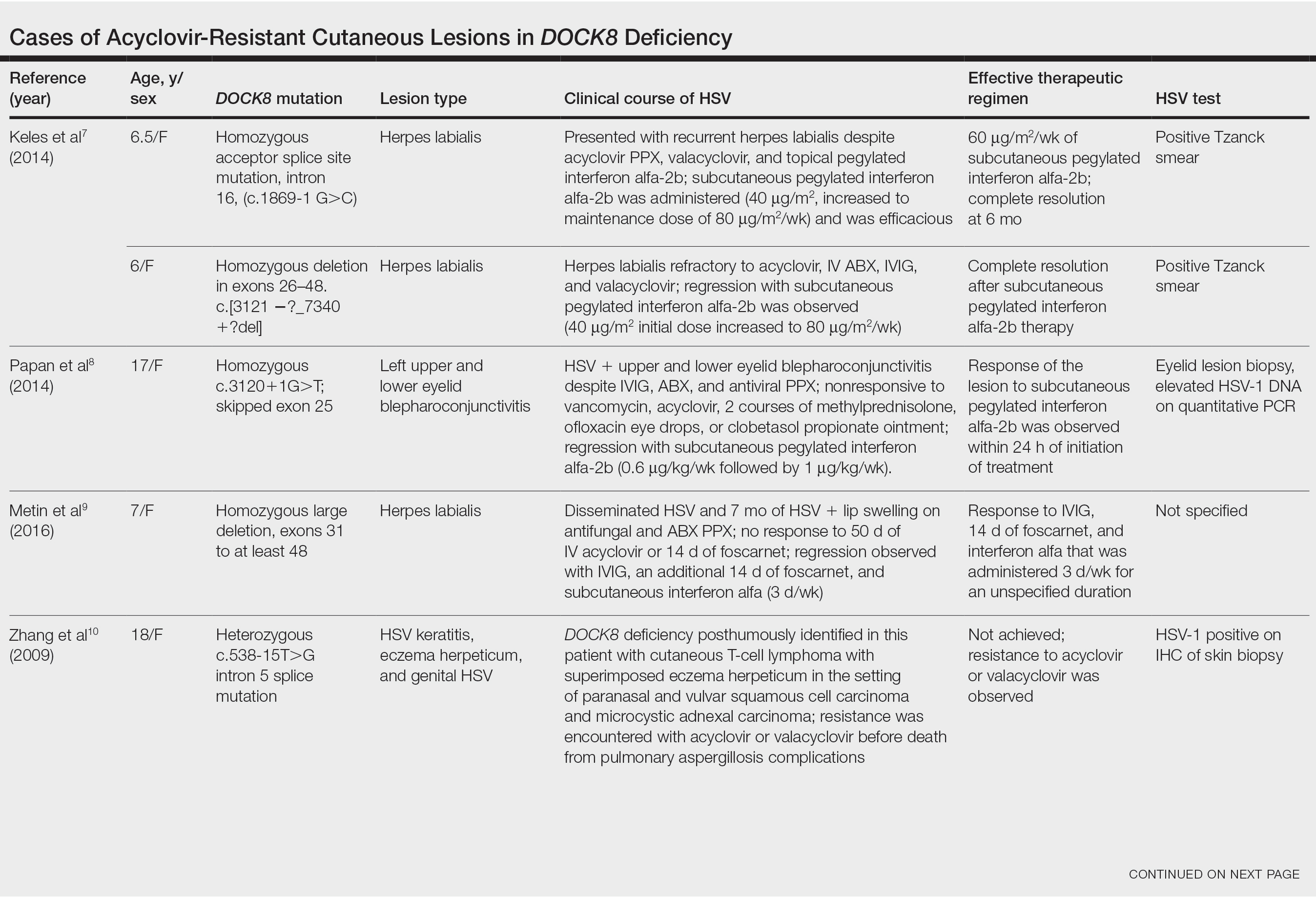
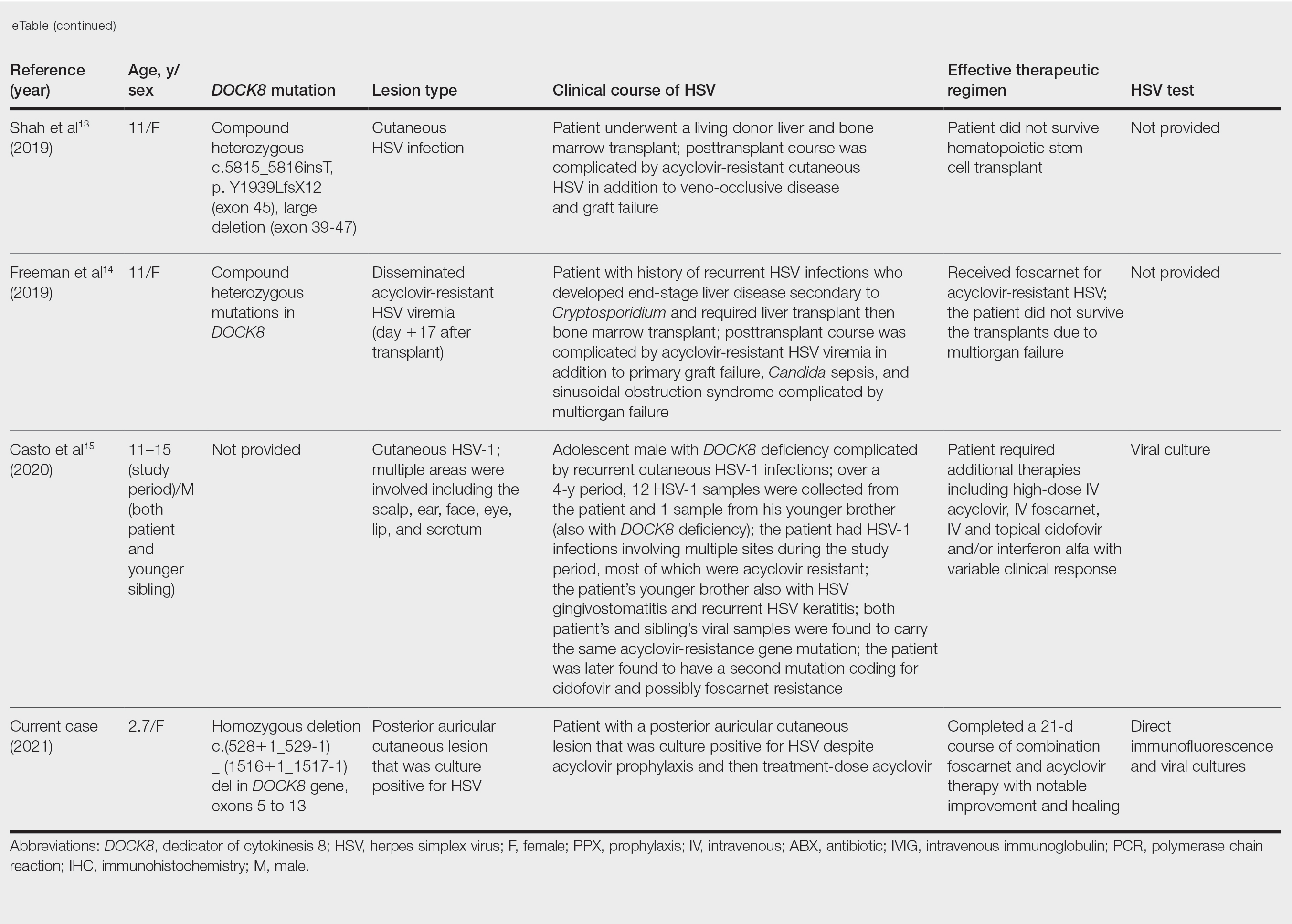
Acyclovir-Resistant HSV—Acyclovir-resistant HSV in immunosuppressed individuals was first noted in 1982, and most cases since then have occurred in the setting of AIDS and in organ transplant recipients.6 Few reports of acyclovir-resistant HSV in DOCK8 deficiency exist, and to our knowledge, our patient is the youngest DOCK8-deficient individual to be documented with acyclovir-resistant HSV infection.1,7-15 We identified relevant cases from the PubMed and EMBASE databases using the search terms DOCK8 deficiency and acyclovir and DOCK8 deficiency and herpes. The eTable lists other reported cases of acyclovir-resistant HSV in DOCK8-deficient patients. The majority of cases involved school-aged females. Lesion types varied and included herpes labialis, eczema herpeticum, and blepharoconjunctivitis. Escalation of therapy and resolution of the lesion was seen in some cases with administration of subcutaneous pegylated interferon alfa-2b.
Treatment Alternatives—Acyclovir competitively inhibits viral DNA polymerase by incorporating into elongating viral DNA strands and halting chain synthesis. Acyclovir requires triphosphorylation for activation, and viral thymidine kinase is responsible for the first phosphorylation event. Ninety-five percent of cases of acyclovir resistance are secondary to mutations in viral thymidine kinase. Foscarnet also inhibits viral DNA polymerase but does so directly without the need to be phosphorylated first.6 For this reason, foscarnet often is the drug of choice in the treatment of acyclovir-resistant HSV, as evidenced in our patient. However, foscarnet-resistant HSV strains may develop from mutations in the DNA polymerase gene.
Cidofovir is a nucleotide analogue that requires phosphorylation by host, as opposed to viral, kinases for antiviral activity. Intravenous and topical formulations of cidofovir have proven effective in the treatment of acyclovir- and foscarnet-resistant HSV lesions.6 Cidofovir also can be applied intralesionally, a method that provides targeted therapy and minimizes cidofovir-associated nephrotoxicity.12 Reports of systemic interferon alfa therapy for acyclovir-resistant HSV also exist. A study found IFN-⍺ production by peripheral blood mononuclear cells in DOCK8-deficient individuals to be significantly reduced relative to controls (P<.05).7 There has been complete resolution of acyclovir-resistant HSV lesions with subcutaneous pegylated interferon alfa-2b injections in several DOCK8-deficient patients.7-9
The need for escalating therapy in DOCK8-deficient individuals with acyclovir-resistant HSV infection underscores the essential role of DOCK8 in dermatologic immunity. Our case demonstrates that a high degree of suspicion for cutaneous HSV infection should be adopted in DOCK8-deficient patients of any age, regardless of acyclovir prophylaxis. Viral culture in addition to bacterial cultures should be performed early in patients with cutaneous erosions, and the threshold for HSV resistance testing should be low to minimize morbidity associated with these infections. Early resistance testing in our case could have prevented prolongation of infection and likely eliminated the need for a biopsy.
Conclusion
DOCK8 deficiency presents a unique challenge to dermatologists and other health care providers given the susceptibility of affected individuals to developing a reservoir of severe and potentially resistant viral cutaneous infections. Prophylactic acyclovir may not be sufficient for HSV suppression, even in the youngest of patients, and suspicion for resistance should be high to avoid delays in adequate treatment.
- Chu EY, Freeman AF, Jing H, et al. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol. 2012;148:79-84. doi:10.1001/archdermatol.2011.262
- Aydin SE, Kilic SS, Aytekin C, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options—a review of 136 patients. J Clin Immunol. 2015;35:189-198. doi:10.1007/s10875-014-0126-0
- Kearney CJ, Randall KL, Oliaro J. DOCK8 regulates signal transduction events to control immunity. Cell Mol Immunol. 2017;14:406-411. doi:10.1038/cmi.2017.9
- Zhang Q, Dove CG, Hor JL, et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med. 2014;211:2549-2566. doi:10.1084/jem.20141307
- Engelhardt KR, Gertz EM, Keles S, et al. The extended clinical phenotype of 64 patients with DOCK8 deficiency. J Allergy Clin Immunol. 2015;136:402-412. doi:10.1016/j.jaci.2014.12.1945
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320. doi:10.1016/S0733-8635(02)00093-1
- Keles S, Jabara HH, Reisli I, et al. Plasmacytoid dendritic cell depletion in DOCK8 deficiency: rescue of severe herpetic infections with interferon alpha-2b therapy. J Allergy Clin Immunol. 2014;133:1753-1755.e3. doi:10.1016/j.jaci.2014.03.032
- Papan C, Hagl B, Heinz V, et al Beneficial IFN-α treatment of tumorous herpes simplex blepharoconjunctivitis in dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2014;133:1456-1458. doi:10.1016/j.jaci.2014.02.008
- Metin A, Kanik-Yuksek S, Ozkaya-Parlakay A, et al. Giant herpes labialis in a child with DOCK8-deficient hyper-IgE syndrome. Pediatr Neonatol. 2016;57:79-80. doi:10.1016/j.pedneo.2015.04.011
- Zhang Q, Davis JC, Lamborn IT, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046-2055. doi:10.1056/NEJMoa0905506
- Lei JY, Wang Y, Jaffe ES, et al. Microcystic adnexal carcinoma associated with primary immunodeficiency, recurrent diffuse herpes simplex virus infection, and cutaneous T-cell lymphoma. Am J Dermatopathol. 2000;22:524-529. doi:10.1097/00000372-200012000-00008
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Shah NN, Freeman AF, Hickstein DD. Addendum to: haploidentical related donor hematopoietic stem cell transplantation for DOCK8 deficiency using post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2019;25:E65-E67. doi:10.1016/j.bbmt.2018.11.014
- Freeman AF, Yazigi N, Shah NN, et al. Tandem orthotopic living donor liver transplantation followed by same donor haploidentical hematopoietic stem cell transplantation for DOCK8 deficiency. Transplantation. 2019;103:2144-2149. doi:10.1097/TP.0000000000002649
- Casto AM, Stout SC, Selvarangan R, et al. Evaluation of genotypic antiviral resistance testing as an alternative to phenotypic testing in a patient with DOCK8 deficiency and severe HSV-1 disease. J Infect Dis. 2020;221:2035-2042. doi:10.1093/infdis/jiaa020
Dedicator of cytokinesis 8 (DOCK8 ) deficiency is the major cause of autosomal-recessive hyper-IgEsyndrome. 1 Characteristic clinical features including eosinophilia, eczema, and recurrent Staphylococcus aureus cutaneous and respiratory tract infections are common in DOCK8 deficiency, similar to the autosomal-dominant form of hyper-IgE syndrome that is due to defi c iency of signal transducer and activation of transcription 3 (STAT-3 ). 1 In addition, patients with DOCK8 deficiency are particularly susceptible to asthma; food allergies; lymphomas; and severe cutaneous viral infections, including herpes simplex virus (HSV), molluscum contagiosum, varicella-zoster virus, and human papillomavirus. Since the discovery of the DOCK8 gene in 2009, various studies have sought to elucidate the mechanistic contribution of DOCK8 to the dermatologic immune environment. 2 Although cutaneous viral infections such as those caused by HSV typically are short lived and self-limiting in immunocompetent hosts, they have proven to be severe and recalcitrant in the setting of DOCK8 deficiency. 1 Herein, we report the case of a 32-month-old girl with homozygous DOCK8 deficiency who developed acyclovir-resistant cutaneous HSV.
Case Report
A 32-month-old girl presented with an approximately 2-cm linear erosion along the left posterior auricular sulcus at month 9 of a hospital stay for recurrent infections. Her medical history was notable for multiple upper respiratory tract infections, diffuse eczema, and food allergies. She had presented to an outside hospital at 14 months of age with herpetic gingivostomatitis and eczema herpeticum that was successfully treated with acyclovir. She was readmitted at 20 months of age due to Pneumocystis jiroveci pneumonia, pancytopenia, and disseminated histoplasmosis. Prophylactic oral acyclovir (20 mg/kg twice daily) was started, given her history of HSV infection. Because of recurrent infections, she underwent an immunodeficiency workup. Whole exome sequencing analysis revealed a homozygous deletion c.(528+1_529−1)_(1516+1_1517−1)del in DOCK8 gene–affecting exons 5 to 13. The patient was transferred to our hospital for continued care and as a potential candidate for bone marrow transplant following resolution of the disseminated histoplasmosis infection.
During her hospitalization at the current presentation, she was noted to have a 2-cm linear erosion along the left posterior auricular sulcus. Initial wound care with bacitracin ointment was applied to the area while specimens were obtained and empiric oral acyclovir therapy was initiated (20 mg/kg 4 times daily [QID]), given a clinical impression consistent with cutaneous HSV infection despite acyclovir prophylaxis. Direct immunofluorescence and viral cultures were positive for HSV-1, while bacterial cultures grew methicillin-susceptible S aureus. Cephalexin and mupirocin ointment were started, and acyclovir was continued. After 2 weeks of therapy, there was no visible change in the wound; cultures were repeated, again showing the wound contained HSV. Bacterial cultures this time grew Pseudomonas putida, and the antibiotic regimen was transitioned to cefepime.
After no response to the continued course of therapeutic acyclovir, HSV cultures were sent to the Centers for Disease Control and Prevention for resistance testing, and biopsy of the lesion was performed by the otolaryngology service to rule out malignancy and potential alternative diagnoses. Histopathology showed only reactive inflammation without visible microorganisms on tissue HSV-1/HSV-2 immunostain; however, tissue viral culture was positive for HSV-1. The patient was transitioned back to acyclovir (intravenous [IV] 20 mg/kg QID) with the addition of empiric foscarnet (IV 40 mg/kg 3 times daily) given the worsening appearance of the lesion. The HSV acyclovir resistance test results from the Centers for Disease Control and Prevention returned soon after and were positive for resistance (median infectious dose, 3.29 µg/L [reference interval, sensitive <2.00 µg/L; resistant >1.90 µg/L]). The patient completed a 21-day course of combination foscarnet and acyclovir therapy, during which time the lesion showed notable improvement and healing. The patient was continued on prophylactic acyclovir (IV 20 mg/kg QID). Unfortunately, the patient eventually died due to complications related to pneumonia.
Comment
Infection in Patients With DOCK8 Deficiency—The gene DOCK8 has emerged as playing a central role in both innate and adaptive immunity, as it is expressed primarily in immune cells and serves as a mediator of numerous processes, including immune synapse formation, cell signaling and trafficking, antibody and cytokine production, and lymphocyte memory.3 Cells that are critical for combating cutaneous viral infections, including skin-resident memory T cells and natural killer cells, are defective, which leads to a severely immunocompromised state in DOCK8-deficient patients with a particular susceptibility to infectious and inflammatory dermatologic disease.4
Herpes simplex virus infection commonly is seen in DOCK8 deficiency, with retrospective analysis of a DOCK8-deficient cohort revealing HSV infection in approximately 38% of patients.5 Prophylactic acyclovir is essential for DOCK8-deficient individuals with a history of HSV infection given the tendency of the virus to reactivate.6 However, despite prophylaxis, our patient developed an HSV-positive posterior auricular erosion that continued to progress even after increase of the acyclovir dose. Acyclovir resistance testing of the HSV isolated from the wound was positive, confirming the clinical suspicion of the presence of acyclovir-resistant HSV infection.

Acyclovir-Resistant HSV—Acyclovir-resistant HSV in immunosuppressed individuals was first noted in 1982, and most cases since then have occurred in the setting of AIDS and in organ transplant recipients.6 Few reports of acyclovir-resistant HSV in DOCK8 deficiency exist, and to our knowledge, our patient is the youngest DOCK8-deficient individual to be documented with acyclovir-resistant HSV infection.1,7-15 We identified relevant cases from the PubMed and EMBASE databases using the search terms DOCK8 deficiency and acyclovir and DOCK8 deficiency and herpes. The eTable lists other reported cases of acyclovir-resistant HSV in DOCK8-deficient patients. The majority of cases involved school-aged females. Lesion types varied and included herpes labialis, eczema herpeticum, and blepharoconjunctivitis. Escalation of therapy and resolution of the lesion was seen in some cases with administration of subcutaneous pegylated interferon alfa-2b.

Treatment Alternatives—Acyclovir competitively inhibits viral DNA polymerase by incorporating into elongating viral DNA strands and halting chain synthesis. Acyclovir requires triphosphorylation for activation, and viral thymidine kinase is responsible for the first phosphorylation event. Ninety-five percent of cases of acyclovir resistance are secondary to mutations in viral thymidine kinase. Foscarnet also inhibits viral DNA polymerase but does so directly without the need to be phosphorylated first.6 For this reason, foscarnet often is the drug of choice in the treatment of acyclovir-resistant HSV, as evidenced in our patient. However, foscarnet-resistant HSV strains may develop from mutations in the DNA polymerase gene.
Cidofovir is a nucleotide analogue that requires phosphorylation by host, as opposed to viral, kinases for antiviral activity. Intravenous and topical formulations of cidofovir have proven effective in the treatment of acyclovir- and foscarnet-resistant HSV lesions.6 Cidofovir also can be applied intralesionally, a method that provides targeted therapy and minimizes cidofovir-associated nephrotoxicity.12 Reports of systemic interferon alfa therapy for acyclovir-resistant HSV also exist. A study found IFN-⍺ production by peripheral blood mononuclear cells in DOCK8-deficient individuals to be significantly reduced relative to controls (P<.05).7 There has been complete resolution of acyclovir-resistant HSV lesions with subcutaneous pegylated interferon alfa-2b injections in several DOCK8-deficient patients.7-9
The need for escalating therapy in DOCK8-deficient individuals with acyclovir-resistant HSV infection underscores the essential role of DOCK8 in dermatologic immunity. Our case demonstrates that a high degree of suspicion for cutaneous HSV infection should be adopted in DOCK8-deficient patients of any age, regardless of acyclovir prophylaxis. Viral culture in addition to bacterial cultures should be performed early in patients with cutaneous erosions, and the threshold for HSV resistance testing should be low to minimize morbidity associated with these infections. Early resistance testing in our case could have prevented prolongation of infection and likely eliminated the need for a biopsy.
Conclusion
DOCK8 deficiency presents a unique challenge to dermatologists and other health care providers given the susceptibility of affected individuals to developing a reservoir of severe and potentially resistant viral cutaneous infections. Prophylactic acyclovir may not be sufficient for HSV suppression, even in the youngest of patients, and suspicion for resistance should be high to avoid delays in adequate treatment.
Dedicator of cytokinesis 8 (DOCK8 ) deficiency is the major cause of autosomal-recessive hyper-IgEsyndrome. 1 Characteristic clinical features including eosinophilia, eczema, and recurrent Staphylococcus aureus cutaneous and respiratory tract infections are common in DOCK8 deficiency, similar to the autosomal-dominant form of hyper-IgE syndrome that is due to defi c iency of signal transducer and activation of transcription 3 (STAT-3 ). 1 In addition, patients with DOCK8 deficiency are particularly susceptible to asthma; food allergies; lymphomas; and severe cutaneous viral infections, including herpes simplex virus (HSV), molluscum contagiosum, varicella-zoster virus, and human papillomavirus. Since the discovery of the DOCK8 gene in 2009, various studies have sought to elucidate the mechanistic contribution of DOCK8 to the dermatologic immune environment. 2 Although cutaneous viral infections such as those caused by HSV typically are short lived and self-limiting in immunocompetent hosts, they have proven to be severe and recalcitrant in the setting of DOCK8 deficiency. 1 Herein, we report the case of a 32-month-old girl with homozygous DOCK8 deficiency who developed acyclovir-resistant cutaneous HSV.
Case Report
A 32-month-old girl presented with an approximately 2-cm linear erosion along the left posterior auricular sulcus at month 9 of a hospital stay for recurrent infections. Her medical history was notable for multiple upper respiratory tract infections, diffuse eczema, and food allergies. She had presented to an outside hospital at 14 months of age with herpetic gingivostomatitis and eczema herpeticum that was successfully treated with acyclovir. She was readmitted at 20 months of age due to Pneumocystis jiroveci pneumonia, pancytopenia, and disseminated histoplasmosis. Prophylactic oral acyclovir (20 mg/kg twice daily) was started, given her history of HSV infection. Because of recurrent infections, she underwent an immunodeficiency workup. Whole exome sequencing analysis revealed a homozygous deletion c.(528+1_529−1)_(1516+1_1517−1)del in DOCK8 gene–affecting exons 5 to 13. The patient was transferred to our hospital for continued care and as a potential candidate for bone marrow transplant following resolution of the disseminated histoplasmosis infection.
During her hospitalization at the current presentation, she was noted to have a 2-cm linear erosion along the left posterior auricular sulcus. Initial wound care with bacitracin ointment was applied to the area while specimens were obtained and empiric oral acyclovir therapy was initiated (20 mg/kg 4 times daily [QID]), given a clinical impression consistent with cutaneous HSV infection despite acyclovir prophylaxis. Direct immunofluorescence and viral cultures were positive for HSV-1, while bacterial cultures grew methicillin-susceptible S aureus. Cephalexin and mupirocin ointment were started, and acyclovir was continued. After 2 weeks of therapy, there was no visible change in the wound; cultures were repeated, again showing the wound contained HSV. Bacterial cultures this time grew Pseudomonas putida, and the antibiotic regimen was transitioned to cefepime.
After no response to the continued course of therapeutic acyclovir, HSV cultures were sent to the Centers for Disease Control and Prevention for resistance testing, and biopsy of the lesion was performed by the otolaryngology service to rule out malignancy and potential alternative diagnoses. Histopathology showed only reactive inflammation without visible microorganisms on tissue HSV-1/HSV-2 immunostain; however, tissue viral culture was positive for HSV-1. The patient was transitioned back to acyclovir (intravenous [IV] 20 mg/kg QID) with the addition of empiric foscarnet (IV 40 mg/kg 3 times daily) given the worsening appearance of the lesion. The HSV acyclovir resistance test results from the Centers for Disease Control and Prevention returned soon after and were positive for resistance (median infectious dose, 3.29 µg/L [reference interval, sensitive <2.00 µg/L; resistant >1.90 µg/L]). The patient completed a 21-day course of combination foscarnet and acyclovir therapy, during which time the lesion showed notable improvement and healing. The patient was continued on prophylactic acyclovir (IV 20 mg/kg QID). Unfortunately, the patient eventually died due to complications related to pneumonia.
Comment
Infection in Patients With DOCK8 Deficiency—The gene DOCK8 has emerged as playing a central role in both innate and adaptive immunity, as it is expressed primarily in immune cells and serves as a mediator of numerous processes, including immune synapse formation, cell signaling and trafficking, antibody and cytokine production, and lymphocyte memory.3 Cells that are critical for combating cutaneous viral infections, including skin-resident memory T cells and natural killer cells, are defective, which leads to a severely immunocompromised state in DOCK8-deficient patients with a particular susceptibility to infectious and inflammatory dermatologic disease.4
Herpes simplex virus infection commonly is seen in DOCK8 deficiency, with retrospective analysis of a DOCK8-deficient cohort revealing HSV infection in approximately 38% of patients.5 Prophylactic acyclovir is essential for DOCK8-deficient individuals with a history of HSV infection given the tendency of the virus to reactivate.6 However, despite prophylaxis, our patient developed an HSV-positive posterior auricular erosion that continued to progress even after increase of the acyclovir dose. Acyclovir resistance testing of the HSV isolated from the wound was positive, confirming the clinical suspicion of the presence of acyclovir-resistant HSV infection.

Acyclovir-Resistant HSV—Acyclovir-resistant HSV in immunosuppressed individuals was first noted in 1982, and most cases since then have occurred in the setting of AIDS and in organ transplant recipients.6 Few reports of acyclovir-resistant HSV in DOCK8 deficiency exist, and to our knowledge, our patient is the youngest DOCK8-deficient individual to be documented with acyclovir-resistant HSV infection.1,7-15 We identified relevant cases from the PubMed and EMBASE databases using the search terms DOCK8 deficiency and acyclovir and DOCK8 deficiency and herpes. The eTable lists other reported cases of acyclovir-resistant HSV in DOCK8-deficient patients. The majority of cases involved school-aged females. Lesion types varied and included herpes labialis, eczema herpeticum, and blepharoconjunctivitis. Escalation of therapy and resolution of the lesion was seen in some cases with administration of subcutaneous pegylated interferon alfa-2b.

Treatment Alternatives—Acyclovir competitively inhibits viral DNA polymerase by incorporating into elongating viral DNA strands and halting chain synthesis. Acyclovir requires triphosphorylation for activation, and viral thymidine kinase is responsible for the first phosphorylation event. Ninety-five percent of cases of acyclovir resistance are secondary to mutations in viral thymidine kinase. Foscarnet also inhibits viral DNA polymerase but does so directly without the need to be phosphorylated first.6 For this reason, foscarnet often is the drug of choice in the treatment of acyclovir-resistant HSV, as evidenced in our patient. However, foscarnet-resistant HSV strains may develop from mutations in the DNA polymerase gene.
Cidofovir is a nucleotide analogue that requires phosphorylation by host, as opposed to viral, kinases for antiviral activity. Intravenous and topical formulations of cidofovir have proven effective in the treatment of acyclovir- and foscarnet-resistant HSV lesions.6 Cidofovir also can be applied intralesionally, a method that provides targeted therapy and minimizes cidofovir-associated nephrotoxicity.12 Reports of systemic interferon alfa therapy for acyclovir-resistant HSV also exist. A study found IFN-⍺ production by peripheral blood mononuclear cells in DOCK8-deficient individuals to be significantly reduced relative to controls (P<.05).7 There has been complete resolution of acyclovir-resistant HSV lesions with subcutaneous pegylated interferon alfa-2b injections in several DOCK8-deficient patients.7-9
The need for escalating therapy in DOCK8-deficient individuals with acyclovir-resistant HSV infection underscores the essential role of DOCK8 in dermatologic immunity. Our case demonstrates that a high degree of suspicion for cutaneous HSV infection should be adopted in DOCK8-deficient patients of any age, regardless of acyclovir prophylaxis. Viral culture in addition to bacterial cultures should be performed early in patients with cutaneous erosions, and the threshold for HSV resistance testing should be low to minimize morbidity associated with these infections. Early resistance testing in our case could have prevented prolongation of infection and likely eliminated the need for a biopsy.
Conclusion
DOCK8 deficiency presents a unique challenge to dermatologists and other health care providers given the susceptibility of affected individuals to developing a reservoir of severe and potentially resistant viral cutaneous infections. Prophylactic acyclovir may not be sufficient for HSV suppression, even in the youngest of patients, and suspicion for resistance should be high to avoid delays in adequate treatment.
- Chu EY, Freeman AF, Jing H, et al. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol. 2012;148:79-84. doi:10.1001/archdermatol.2011.262
- Aydin SE, Kilic SS, Aytekin C, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options—a review of 136 patients. J Clin Immunol. 2015;35:189-198. doi:10.1007/s10875-014-0126-0
- Kearney CJ, Randall KL, Oliaro J. DOCK8 regulates signal transduction events to control immunity. Cell Mol Immunol. 2017;14:406-411. doi:10.1038/cmi.2017.9
- Zhang Q, Dove CG, Hor JL, et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med. 2014;211:2549-2566. doi:10.1084/jem.20141307
- Engelhardt KR, Gertz EM, Keles S, et al. The extended clinical phenotype of 64 patients with DOCK8 deficiency. J Allergy Clin Immunol. 2015;136:402-412. doi:10.1016/j.jaci.2014.12.1945
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320. doi:10.1016/S0733-8635(02)00093-1
- Keles S, Jabara HH, Reisli I, et al. Plasmacytoid dendritic cell depletion in DOCK8 deficiency: rescue of severe herpetic infections with interferon alpha-2b therapy. J Allergy Clin Immunol. 2014;133:1753-1755.e3. doi:10.1016/j.jaci.2014.03.032
- Papan C, Hagl B, Heinz V, et al Beneficial IFN-α treatment of tumorous herpes simplex blepharoconjunctivitis in dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2014;133:1456-1458. doi:10.1016/j.jaci.2014.02.008
- Metin A, Kanik-Yuksek S, Ozkaya-Parlakay A, et al. Giant herpes labialis in a child with DOCK8-deficient hyper-IgE syndrome. Pediatr Neonatol. 2016;57:79-80. doi:10.1016/j.pedneo.2015.04.011
- Zhang Q, Davis JC, Lamborn IT, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046-2055. doi:10.1056/NEJMoa0905506
- Lei JY, Wang Y, Jaffe ES, et al. Microcystic adnexal carcinoma associated with primary immunodeficiency, recurrent diffuse herpes simplex virus infection, and cutaneous T-cell lymphoma. Am J Dermatopathol. 2000;22:524-529. doi:10.1097/00000372-200012000-00008
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Shah NN, Freeman AF, Hickstein DD. Addendum to: haploidentical related donor hematopoietic stem cell transplantation for DOCK8 deficiency using post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2019;25:E65-E67. doi:10.1016/j.bbmt.2018.11.014
- Freeman AF, Yazigi N, Shah NN, et al. Tandem orthotopic living donor liver transplantation followed by same donor haploidentical hematopoietic stem cell transplantation for DOCK8 deficiency. Transplantation. 2019;103:2144-2149. doi:10.1097/TP.0000000000002649
- Casto AM, Stout SC, Selvarangan R, et al. Evaluation of genotypic antiviral resistance testing as an alternative to phenotypic testing in a patient with DOCK8 deficiency and severe HSV-1 disease. J Infect Dis. 2020;221:2035-2042. doi:10.1093/infdis/jiaa020
- Chu EY, Freeman AF, Jing H, et al. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol. 2012;148:79-84. doi:10.1001/archdermatol.2011.262
- Aydin SE, Kilic SS, Aytekin C, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options—a review of 136 patients. J Clin Immunol. 2015;35:189-198. doi:10.1007/s10875-014-0126-0
- Kearney CJ, Randall KL, Oliaro J. DOCK8 regulates signal transduction events to control immunity. Cell Mol Immunol. 2017;14:406-411. doi:10.1038/cmi.2017.9
- Zhang Q, Dove CG, Hor JL, et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med. 2014;211:2549-2566. doi:10.1084/jem.20141307
- Engelhardt KR, Gertz EM, Keles S, et al. The extended clinical phenotype of 64 patients with DOCK8 deficiency. J Allergy Clin Immunol. 2015;136:402-412. doi:10.1016/j.jaci.2014.12.1945
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320. doi:10.1016/S0733-8635(02)00093-1
- Keles S, Jabara HH, Reisli I, et al. Plasmacytoid dendritic cell depletion in DOCK8 deficiency: rescue of severe herpetic infections with interferon alpha-2b therapy. J Allergy Clin Immunol. 2014;133:1753-1755.e3. doi:10.1016/j.jaci.2014.03.032
- Papan C, Hagl B, Heinz V, et al Beneficial IFN-α treatment of tumorous herpes simplex blepharoconjunctivitis in dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2014;133:1456-1458. doi:10.1016/j.jaci.2014.02.008
- Metin A, Kanik-Yuksek S, Ozkaya-Parlakay A, et al. Giant herpes labialis in a child with DOCK8-deficient hyper-IgE syndrome. Pediatr Neonatol. 2016;57:79-80. doi:10.1016/j.pedneo.2015.04.011
- Zhang Q, Davis JC, Lamborn IT, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046-2055. doi:10.1056/NEJMoa0905506
- Lei JY, Wang Y, Jaffe ES, et al. Microcystic adnexal carcinoma associated with primary immunodeficiency, recurrent diffuse herpes simplex virus infection, and cutaneous T-cell lymphoma. Am J Dermatopathol. 2000;22:524-529. doi:10.1097/00000372-200012000-00008
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Shah NN, Freeman AF, Hickstein DD. Addendum to: haploidentical related donor hematopoietic stem cell transplantation for DOCK8 deficiency using post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2019;25:E65-E67. doi:10.1016/j.bbmt.2018.11.014
- Freeman AF, Yazigi N, Shah NN, et al. Tandem orthotopic living donor liver transplantation followed by same donor haploidentical hematopoietic stem cell transplantation for DOCK8 deficiency. Transplantation. 2019;103:2144-2149. doi:10.1097/TP.0000000000002649
- Casto AM, Stout SC, Selvarangan R, et al. Evaluation of genotypic antiviral resistance testing as an alternative to phenotypic testing in a patient with DOCK8 deficiency and severe HSV-1 disease. J Infect Dis. 2020;221:2035-2042. doi:10.1093/infdis/jiaa020
Practice Points
- Patients with dedicator of cytokinesis 8 ( DOCK 8 ) deficiency are susceptible to development of severe recalcitrant viral cutaneous infections, including herpes simplex virus (HSV).
- Dermatologists should be aware that prophylactic acyclovir may not be sufficient for HSV suppression in the setting of severe immunodeficiency.
- Acyclovir-resistant cutaneous HSV lesions require escalation of therapy, which may include addition of foscarnet, cidofovir, or subcutaneous pegylated interferon alfa-2b to the therapeutic regimen.
- Viral culture should be performed on suspicious lesions in DOCK 8 -deficient patients despite acyclovir prophylaxis, and the threshold for HSV resistance testing should be low.
Merck seeks FDA authorization for antiviral COVID-19 pill
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
PDT for actinic keratoses continues to be refined
.

“We have conventional PDT, daylight PDT; we can combine with a range of topicals, and we can combine a range of different physical treatment procedures in order to provide better and individualized treatment regimens for our patients,” Merete Haedersdal, MD, PhD, DMSc, professor of dermatology at the University of Copenhagen, said during a course on laser and aesthetic skin therapy.
In Europe, PDT consists of a three-step procedure: curettage of AKs, application of photosensitizer on the skin (typically methyl aminolevulinate, versus aminolevulinic acid, used more often in the United States), and illumination with red light (versus blue light, used more often in the United States), which causes a photochemical reaction.
“It’s a photochemical-reaction concept with which we can achieve up to 90% cure rates of AKs at 3 months,” Dr. Haedersdal said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
PDT is also used in Europe for select patients with Bowen’s disease (yielding a 90% cure rate at 3 months, 70% at 2 years); superficial basal cell carcinoma (yielding a 90% cure rate at 3 months, 75% at 5 years), and nodular BCC (yielding a 90% cure rate at 3 months, 75% at 5 years.
“With conventional PDT, whether it’s blue light, red light, MAL, or ALA, we have beautiful cosmesis, but we also have a challenge, which is pain,” she said. This is behind the motivation to look at other ways to provide PDT.
Daylight PDT, which was pioneered by Dr. Haedersdal’s mentor, Hans Christian Wulf, MD, DMSc, PharmD, professor of dermatology at the University of Copenhagen, provides 80%-90% clearance of thin AKs, lower clearance of thick AKs – and is a nearly pain-free procedure because of continuous photoactivation of protoporphyrin IX, with a Visual Analog Scale in the range of 1-3. “Globally, thousands of patients have been treated [with daylight PDT], which is backed up in the literature with more than 150 publications,” she said.
According to Dr. Haedersdal, MAL cream with daylight activation for treatment of AK was approved in Colombia and Mexico in 2013; in Australia, Brazil, and Costa Rica in 2014; and in Chile, Europe, and New Zealand in 2015. “I do hope that one day you will have daylight PDT approved in the United States,” she said.
The suggested protocol for daylight PDT starts by applying a sunscreen with an organic filter. After about 15 minutes, the lesion or lesions are prepared, and MAL is applied with no occlusion. Patients should start their exposure to daylight within 30 minutes of application, remaining outdoors for 2 hours of continuous exposure, either at a dedicated space located on the ground of the hospital or clinic or at their home. After 2 hours, patients wipe off the remaining cream and are advised to stay indoors for the rest of the day.
“Ideal candidates are those who have large skin areas that can be easily exposed to sunlight,” such as the scalp and lower legs, said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston. “If patients are treated in areas covered by clothing, it can be greasy and sticky with the cream. In these cases, you can cover the area with Tegaderm, which allows for 99% light transmission. Daylight can shine through and the Tegaderm can be removed after the procedure.”
On rainy days between April 1 and October 1 in Copenhagen, she said, daylight PDT is provided in a greenhouse in the hospital garden, with a heater and blankets for patients when the temperature falls below 10° C.
The amount of light required for a treatment effect is 5,000-10,000 lux, and the number of lux on a sunny day in Denmark is about 100,000, she said. “That means that all year round in countries south of latitude 45 degrees N, patients can be treated with daylight PDT.”
To intensify the treatment efficacy of conventional PDT and daylight PDT, especially in those with severely photodamaged skin, combining treatment with a physical pretreatment technique such as curettage, ablative fractional laser, microdermabrasion, microneedling, and nonablative fractional laser is another strategy. A small randomized controlled trial found that ablative fractional laser treatment extended notable relative effectiveness, compared with other physical enhancement techniques.
Dr. Haedersdal and colleagues published a study that compared pretreatment with ablative fractional laser and microdermabrasion pads before daylight PDT for AKs in field-cancerized skin. They found that with a single treatment, combination therapy with ablative fractional laser before daylight PDT led to significantly greater efficacious AK clearance and skin rejuvenation, compared with treatment with microdermabrasion.
“We don’t know why this is, but we believe it may be due to the fact that with the laser, we have a photothermal response, which in combination with the photochemical response from the photodynamic therapy induces a synergistic effect,” she said.
A range of topical treatments can also be given in combination with PDT. In a meta-analysis of 10 randomized controlled trials, German researchers evaluated the efficacy of PDT combined with imiquimod cream, 5-fluorouracil cream, tazarotene gel, and calcipotriol ointment. They concluded that the combination of PDT with another topical drug intervention improves AK clearance rates, compared with monotherapy.
More recently, the same authors summarized the current knowledge on the efficacy and safety of local combination therapies for the treatment of patients with AK in a review article, which Dr. Haedersdal said provides a nice overview of this topic.
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
.

“We have conventional PDT, daylight PDT; we can combine with a range of topicals, and we can combine a range of different physical treatment procedures in order to provide better and individualized treatment regimens for our patients,” Merete Haedersdal, MD, PhD, DMSc, professor of dermatology at the University of Copenhagen, said during a course on laser and aesthetic skin therapy.
In Europe, PDT consists of a three-step procedure: curettage of AKs, application of photosensitizer on the skin (typically methyl aminolevulinate, versus aminolevulinic acid, used more often in the United States), and illumination with red light (versus blue light, used more often in the United States), which causes a photochemical reaction.
“It’s a photochemical-reaction concept with which we can achieve up to 90% cure rates of AKs at 3 months,” Dr. Haedersdal said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
PDT is also used in Europe for select patients with Bowen’s disease (yielding a 90% cure rate at 3 months, 70% at 2 years); superficial basal cell carcinoma (yielding a 90% cure rate at 3 months, 75% at 5 years), and nodular BCC (yielding a 90% cure rate at 3 months, 75% at 5 years.
“With conventional PDT, whether it’s blue light, red light, MAL, or ALA, we have beautiful cosmesis, but we also have a challenge, which is pain,” she said. This is behind the motivation to look at other ways to provide PDT.
Daylight PDT, which was pioneered by Dr. Haedersdal’s mentor, Hans Christian Wulf, MD, DMSc, PharmD, professor of dermatology at the University of Copenhagen, provides 80%-90% clearance of thin AKs, lower clearance of thick AKs – and is a nearly pain-free procedure because of continuous photoactivation of protoporphyrin IX, with a Visual Analog Scale in the range of 1-3. “Globally, thousands of patients have been treated [with daylight PDT], which is backed up in the literature with more than 150 publications,” she said.
According to Dr. Haedersdal, MAL cream with daylight activation for treatment of AK was approved in Colombia and Mexico in 2013; in Australia, Brazil, and Costa Rica in 2014; and in Chile, Europe, and New Zealand in 2015. “I do hope that one day you will have daylight PDT approved in the United States,” she said.
The suggested protocol for daylight PDT starts by applying a sunscreen with an organic filter. After about 15 minutes, the lesion or lesions are prepared, and MAL is applied with no occlusion. Patients should start their exposure to daylight within 30 minutes of application, remaining outdoors for 2 hours of continuous exposure, either at a dedicated space located on the ground of the hospital or clinic or at their home. After 2 hours, patients wipe off the remaining cream and are advised to stay indoors for the rest of the day.
“Ideal candidates are those who have large skin areas that can be easily exposed to sunlight,” such as the scalp and lower legs, said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston. “If patients are treated in areas covered by clothing, it can be greasy and sticky with the cream. In these cases, you can cover the area with Tegaderm, which allows for 99% light transmission. Daylight can shine through and the Tegaderm can be removed after the procedure.”
On rainy days between April 1 and October 1 in Copenhagen, she said, daylight PDT is provided in a greenhouse in the hospital garden, with a heater and blankets for patients when the temperature falls below 10° C.
The amount of light required for a treatment effect is 5,000-10,000 lux, and the number of lux on a sunny day in Denmark is about 100,000, she said. “That means that all year round in countries south of latitude 45 degrees N, patients can be treated with daylight PDT.”
To intensify the treatment efficacy of conventional PDT and daylight PDT, especially in those with severely photodamaged skin, combining treatment with a physical pretreatment technique such as curettage, ablative fractional laser, microdermabrasion, microneedling, and nonablative fractional laser is another strategy. A small randomized controlled trial found that ablative fractional laser treatment extended notable relative effectiveness, compared with other physical enhancement techniques.
Dr. Haedersdal and colleagues published a study that compared pretreatment with ablative fractional laser and microdermabrasion pads before daylight PDT for AKs in field-cancerized skin. They found that with a single treatment, combination therapy with ablative fractional laser before daylight PDT led to significantly greater efficacious AK clearance and skin rejuvenation, compared with treatment with microdermabrasion.
“We don’t know why this is, but we believe it may be due to the fact that with the laser, we have a photothermal response, which in combination with the photochemical response from the photodynamic therapy induces a synergistic effect,” she said.
A range of topical treatments can also be given in combination with PDT. In a meta-analysis of 10 randomized controlled trials, German researchers evaluated the efficacy of PDT combined with imiquimod cream, 5-fluorouracil cream, tazarotene gel, and calcipotriol ointment. They concluded that the combination of PDT with another topical drug intervention improves AK clearance rates, compared with monotherapy.
More recently, the same authors summarized the current knowledge on the efficacy and safety of local combination therapies for the treatment of patients with AK in a review article, which Dr. Haedersdal said provides a nice overview of this topic.
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
.

“We have conventional PDT, daylight PDT; we can combine with a range of topicals, and we can combine a range of different physical treatment procedures in order to provide better and individualized treatment regimens for our patients,” Merete Haedersdal, MD, PhD, DMSc, professor of dermatology at the University of Copenhagen, said during a course on laser and aesthetic skin therapy.
In Europe, PDT consists of a three-step procedure: curettage of AKs, application of photosensitizer on the skin (typically methyl aminolevulinate, versus aminolevulinic acid, used more often in the United States), and illumination with red light (versus blue light, used more often in the United States), which causes a photochemical reaction.
“It’s a photochemical-reaction concept with which we can achieve up to 90% cure rates of AKs at 3 months,” Dr. Haedersdal said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
PDT is also used in Europe for select patients with Bowen’s disease (yielding a 90% cure rate at 3 months, 70% at 2 years); superficial basal cell carcinoma (yielding a 90% cure rate at 3 months, 75% at 5 years), and nodular BCC (yielding a 90% cure rate at 3 months, 75% at 5 years.
“With conventional PDT, whether it’s blue light, red light, MAL, or ALA, we have beautiful cosmesis, but we also have a challenge, which is pain,” she said. This is behind the motivation to look at other ways to provide PDT.
Daylight PDT, which was pioneered by Dr. Haedersdal’s mentor, Hans Christian Wulf, MD, DMSc, PharmD, professor of dermatology at the University of Copenhagen, provides 80%-90% clearance of thin AKs, lower clearance of thick AKs – and is a nearly pain-free procedure because of continuous photoactivation of protoporphyrin IX, with a Visual Analog Scale in the range of 1-3. “Globally, thousands of patients have been treated [with daylight PDT], which is backed up in the literature with more than 150 publications,” she said.
According to Dr. Haedersdal, MAL cream with daylight activation for treatment of AK was approved in Colombia and Mexico in 2013; in Australia, Brazil, and Costa Rica in 2014; and in Chile, Europe, and New Zealand in 2015. “I do hope that one day you will have daylight PDT approved in the United States,” she said.
The suggested protocol for daylight PDT starts by applying a sunscreen with an organic filter. After about 15 minutes, the lesion or lesions are prepared, and MAL is applied with no occlusion. Patients should start their exposure to daylight within 30 minutes of application, remaining outdoors for 2 hours of continuous exposure, either at a dedicated space located on the ground of the hospital or clinic or at their home. After 2 hours, patients wipe off the remaining cream and are advised to stay indoors for the rest of the day.
“Ideal candidates are those who have large skin areas that can be easily exposed to sunlight,” such as the scalp and lower legs, said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston. “If patients are treated in areas covered by clothing, it can be greasy and sticky with the cream. In these cases, you can cover the area with Tegaderm, which allows for 99% light transmission. Daylight can shine through and the Tegaderm can be removed after the procedure.”
On rainy days between April 1 and October 1 in Copenhagen, she said, daylight PDT is provided in a greenhouse in the hospital garden, with a heater and blankets for patients when the temperature falls below 10° C.
The amount of light required for a treatment effect is 5,000-10,000 lux, and the number of lux on a sunny day in Denmark is about 100,000, she said. “That means that all year round in countries south of latitude 45 degrees N, patients can be treated with daylight PDT.”
To intensify the treatment efficacy of conventional PDT and daylight PDT, especially in those with severely photodamaged skin, combining treatment with a physical pretreatment technique such as curettage, ablative fractional laser, microdermabrasion, microneedling, and nonablative fractional laser is another strategy. A small randomized controlled trial found that ablative fractional laser treatment extended notable relative effectiveness, compared with other physical enhancement techniques.
Dr. Haedersdal and colleagues published a study that compared pretreatment with ablative fractional laser and microdermabrasion pads before daylight PDT for AKs in field-cancerized skin. They found that with a single treatment, combination therapy with ablative fractional laser before daylight PDT led to significantly greater efficacious AK clearance and skin rejuvenation, compared with treatment with microdermabrasion.
“We don’t know why this is, but we believe it may be due to the fact that with the laser, we have a photothermal response, which in combination with the photochemical response from the photodynamic therapy induces a synergistic effect,” she said.
A range of topical treatments can also be given in combination with PDT. In a meta-analysis of 10 randomized controlled trials, German researchers evaluated the efficacy of PDT combined with imiquimod cream, 5-fluorouracil cream, tazarotene gel, and calcipotriol ointment. They concluded that the combination of PDT with another topical drug intervention improves AK clearance rates, compared with monotherapy.
More recently, the same authors summarized the current knowledge on the efficacy and safety of local combination therapies for the treatment of patients with AK in a review article, which Dr. Haedersdal said provides a nice overview of this topic.
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
HEPA filters may clean SARS-CoV-2 from the air: Study
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
FDA approves avacopan for rare ANCA autoimmune disease
U.S. regulators approved avacopan (Tavneos) for a rare immune disorder after receiving additional information to address concerns raised about the drug that were previously discussed at a public meeting in May.
ChemoCentryx, the drug’s manufacturer, today announced that the U.S. Food and Drug Administration approved the drug as an adjunctive treatment for severe active antineutrophil cytoplasmic autoantibody–associated vasculitis (also known as ANCA-associated vasculitis or ANCA vasculitis).
This systemic disease results from overactivation of the complement system, leading to inflammation and eventual destruction of small blood vessels. This can lead to organ damage and failure, with the kidney as the major target, said the company in a statement.
The avacopan approval was based in large part on the results of the ADVOCATE trial, which were highlighted in a February 2021 editorial in the New England Journal of Medicine , titled “Avacopan – Time to replace glucocorticoids?” But the FDA-approved indication for avacopan is as an adjunctive treatment of adult patients with severe active ANCA-associated vasculitis (granulomatosis with polyangiitis [GPA] and microscopic polyangiitis [MPA]) in combination with standard therapy including glucocorticoids. “Tavneos does not eliminate glucocorticoid use,” the label states.
The ADVOCATE trial was a global, randomized, double-blind, active-controlled, double-dummy phase 3 trial of 330 patients with ANCA-associated vasculitis conducted in 20 countries, ChemoCentryx said. Participants were randomly assigned to receive either rituximab or cyclophosphamide (followed by azathioprine/mycophenolate) and either avacopan or study-supplied oral prednisone.
Subjects in both treatment groups could also receive nonprotocol glucocorticoids as needed. The study met its primary endpoints of disease remission at 26 weeks and sustained remission at 52 weeks, as assessed by the Birmingham Vasculitis Activity Score (BVAS), ChemoCentryx said. Common adverse reactions among study participants included nausea, headache, hypertension, diarrhea, vomiting, rash, fatigue, upper abdominal pain, dizziness, blood creatinine increase, and paresthesia.
In the ChemoCentryx statement, Peter A. Merkel, MD, MPH, a consultant to the company and the chief of rheumatology at the University of Pennsylvania, Philadelphia, called the avacopan clearance a “first-in-a-decade approval of a medicine for ANCA-associated vasculitis.”
“Patients will now have access to a new class of medication that provides beneficial effects for the treatment of ANCA-associated vasculitis,” Dr. Merkel said.
In reviewing the avacopan application, the FDA noted that the medicine is intended to treat “a rare and serious disease associated with high morbidity and increased mortality.”
“It is also a disease with high unmet need for new therapies,” the FDA staff said in a review of the ChemoCentryx application for approval of avacopan, which was posted online ahead of a meeting this past May.
Previous FDA concerns
In that review, FDA staff made public various concerns about the evidence used in seeking approval of the medicine. The FDA staff said there were “substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.”
Members of the FDA’s Arthritis Advisory Committee voted 10-8 on May 6 on a question of whether the risk-benefit profile of avacopan is adequate to support approval. The panel also voted 9-9 on whether the efficacy data support approval of avacopan, and 10-8 that the safety profile of avacopan is adequate to support approval.
ChemoCentryx in July said it filed an amendment to its new drug application (NDA) for avacopan. This appears to have answered regulators’ questions about the drug.
On a call with analysts Friday, ChemoCentryx officials outlined a marketing strategy for avacopan, with efforts focused on reaching influential rheumatologists and nephrologists. The company will set a U.S. wholesale acquisition cost for the drug of about $150,000-$200,000 a patient, in keeping with the range of prices often seen for orphan drugs. ChemoCentryx said it intends to offer financial support programs for the medicine.
ChemoCentryx said avacopan is also approved for the treatment of microscopic polyangiitis and granulomatosis with polyangiitis (the two main forms of ANCA-associated vasculitis) in Japan. The regulatory decision in Europe is expected by the end of this year.
A version of this article first appeared on Medscape.com.
U.S. regulators approved avacopan (Tavneos) for a rare immune disorder after receiving additional information to address concerns raised about the drug that were previously discussed at a public meeting in May.
ChemoCentryx, the drug’s manufacturer, today announced that the U.S. Food and Drug Administration approved the drug as an adjunctive treatment for severe active antineutrophil cytoplasmic autoantibody–associated vasculitis (also known as ANCA-associated vasculitis or ANCA vasculitis).
This systemic disease results from overactivation of the complement system, leading to inflammation and eventual destruction of small blood vessels. This can lead to organ damage and failure, with the kidney as the major target, said the company in a statement.
The avacopan approval was based in large part on the results of the ADVOCATE trial, which were highlighted in a February 2021 editorial in the New England Journal of Medicine , titled “Avacopan – Time to replace glucocorticoids?” But the FDA-approved indication for avacopan is as an adjunctive treatment of adult patients with severe active ANCA-associated vasculitis (granulomatosis with polyangiitis [GPA] and microscopic polyangiitis [MPA]) in combination with standard therapy including glucocorticoids. “Tavneos does not eliminate glucocorticoid use,” the label states.
The ADVOCATE trial was a global, randomized, double-blind, active-controlled, double-dummy phase 3 trial of 330 patients with ANCA-associated vasculitis conducted in 20 countries, ChemoCentryx said. Participants were randomly assigned to receive either rituximab or cyclophosphamide (followed by azathioprine/mycophenolate) and either avacopan or study-supplied oral prednisone.
Subjects in both treatment groups could also receive nonprotocol glucocorticoids as needed. The study met its primary endpoints of disease remission at 26 weeks and sustained remission at 52 weeks, as assessed by the Birmingham Vasculitis Activity Score (BVAS), ChemoCentryx said. Common adverse reactions among study participants included nausea, headache, hypertension, diarrhea, vomiting, rash, fatigue, upper abdominal pain, dizziness, blood creatinine increase, and paresthesia.
In the ChemoCentryx statement, Peter A. Merkel, MD, MPH, a consultant to the company and the chief of rheumatology at the University of Pennsylvania, Philadelphia, called the avacopan clearance a “first-in-a-decade approval of a medicine for ANCA-associated vasculitis.”
“Patients will now have access to a new class of medication that provides beneficial effects for the treatment of ANCA-associated vasculitis,” Dr. Merkel said.
In reviewing the avacopan application, the FDA noted that the medicine is intended to treat “a rare and serious disease associated with high morbidity and increased mortality.”
“It is also a disease with high unmet need for new therapies,” the FDA staff said in a review of the ChemoCentryx application for approval of avacopan, which was posted online ahead of a meeting this past May.
Previous FDA concerns
In that review, FDA staff made public various concerns about the evidence used in seeking approval of the medicine. The FDA staff said there were “substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.”
Members of the FDA’s Arthritis Advisory Committee voted 10-8 on May 6 on a question of whether the risk-benefit profile of avacopan is adequate to support approval. The panel also voted 9-9 on whether the efficacy data support approval of avacopan, and 10-8 that the safety profile of avacopan is adequate to support approval.
ChemoCentryx in July said it filed an amendment to its new drug application (NDA) for avacopan. This appears to have answered regulators’ questions about the drug.
On a call with analysts Friday, ChemoCentryx officials outlined a marketing strategy for avacopan, with efforts focused on reaching influential rheumatologists and nephrologists. The company will set a U.S. wholesale acquisition cost for the drug of about $150,000-$200,000 a patient, in keeping with the range of prices often seen for orphan drugs. ChemoCentryx said it intends to offer financial support programs for the medicine.
ChemoCentryx said avacopan is also approved for the treatment of microscopic polyangiitis and granulomatosis with polyangiitis (the two main forms of ANCA-associated vasculitis) in Japan. The regulatory decision in Europe is expected by the end of this year.
A version of this article first appeared on Medscape.com.
U.S. regulators approved avacopan (Tavneos) for a rare immune disorder after receiving additional information to address concerns raised about the drug that were previously discussed at a public meeting in May.
ChemoCentryx, the drug’s manufacturer, today announced that the U.S. Food and Drug Administration approved the drug as an adjunctive treatment for severe active antineutrophil cytoplasmic autoantibody–associated vasculitis (also known as ANCA-associated vasculitis or ANCA vasculitis).
This systemic disease results from overactivation of the complement system, leading to inflammation and eventual destruction of small blood vessels. This can lead to organ damage and failure, with the kidney as the major target, said the company in a statement.
The avacopan approval was based in large part on the results of the ADVOCATE trial, which were highlighted in a February 2021 editorial in the New England Journal of Medicine , titled “Avacopan – Time to replace glucocorticoids?” But the FDA-approved indication for avacopan is as an adjunctive treatment of adult patients with severe active ANCA-associated vasculitis (granulomatosis with polyangiitis [GPA] and microscopic polyangiitis [MPA]) in combination with standard therapy including glucocorticoids. “Tavneos does not eliminate glucocorticoid use,” the label states.
The ADVOCATE trial was a global, randomized, double-blind, active-controlled, double-dummy phase 3 trial of 330 patients with ANCA-associated vasculitis conducted in 20 countries, ChemoCentryx said. Participants were randomly assigned to receive either rituximab or cyclophosphamide (followed by azathioprine/mycophenolate) and either avacopan or study-supplied oral prednisone.
Subjects in both treatment groups could also receive nonprotocol glucocorticoids as needed. The study met its primary endpoints of disease remission at 26 weeks and sustained remission at 52 weeks, as assessed by the Birmingham Vasculitis Activity Score (BVAS), ChemoCentryx said. Common adverse reactions among study participants included nausea, headache, hypertension, diarrhea, vomiting, rash, fatigue, upper abdominal pain, dizziness, blood creatinine increase, and paresthesia.
In the ChemoCentryx statement, Peter A. Merkel, MD, MPH, a consultant to the company and the chief of rheumatology at the University of Pennsylvania, Philadelphia, called the avacopan clearance a “first-in-a-decade approval of a medicine for ANCA-associated vasculitis.”
“Patients will now have access to a new class of medication that provides beneficial effects for the treatment of ANCA-associated vasculitis,” Dr. Merkel said.
In reviewing the avacopan application, the FDA noted that the medicine is intended to treat “a rare and serious disease associated with high morbidity and increased mortality.”
“It is also a disease with high unmet need for new therapies,” the FDA staff said in a review of the ChemoCentryx application for approval of avacopan, which was posted online ahead of a meeting this past May.
Previous FDA concerns
In that review, FDA staff made public various concerns about the evidence used in seeking approval of the medicine. The FDA staff said there were “substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.”
Members of the FDA’s Arthritis Advisory Committee voted 10-8 on May 6 on a question of whether the risk-benefit profile of avacopan is adequate to support approval. The panel also voted 9-9 on whether the efficacy data support approval of avacopan, and 10-8 that the safety profile of avacopan is adequate to support approval.
ChemoCentryx in July said it filed an amendment to its new drug application (NDA) for avacopan. This appears to have answered regulators’ questions about the drug.
On a call with analysts Friday, ChemoCentryx officials outlined a marketing strategy for avacopan, with efforts focused on reaching influential rheumatologists and nephrologists. The company will set a U.S. wholesale acquisition cost for the drug of about $150,000-$200,000 a patient, in keeping with the range of prices often seen for orphan drugs. ChemoCentryx said it intends to offer financial support programs for the medicine.
ChemoCentryx said avacopan is also approved for the treatment of microscopic polyangiitis and granulomatosis with polyangiitis (the two main forms of ANCA-associated vasculitis) in Japan. The regulatory decision in Europe is expected by the end of this year.
A version of this article first appeared on Medscape.com.
FDA issues warning about use of dermal fillers with needle-free devices
.
Specifically, the warning advises consumers and health care professionals “not to use needle-free devices such as hyaluron pens for injection of hyaluronic acid (HA) or other lip and facial fillers, collectively and commonly referred to as dermal fillers or fillers.”
According to the statement, the agency “is aware of serious injuries and in some cases, permanent harm to the skin, lips, or eyes with the use of needle-free devices for injection of fillers.”
Needle-free devices and lip and facial fillers for use with these devices are being sold directly to consumers online, and are promoted on social media “to increase lip volume, improve the appearance of wrinkles, change the shape of the nose, and other similar procedures,” according to the FDA warning.
The FDA points out that FDA-approved dermal fillers are for prescription use only, and should be administered only by licensed health care professionals using a syringe with a needle or cannula, and advises consumers not to buy or use lip or facial fillers sold directly to the public.
These products may be contaminated with infectious agents or chemicals. Moreover, “needle-free injection devices for aesthetic purposes do not provide enough control over where the injected product is placed,” the statement adds. In addition to infections, other risks include bleeding and bruising, formation of lumps, allergic reactions, blockage of a blood vessel (which can result in necrosis, blindness, or stroke), and transmission of diseases from sharing devices.
The FDA’s recommendations for health care providers include not using any aesthetic fillers with a needle-free device, and not using approved dermal fillers in such devices.
The American Society for Dermatologic Surgery Association (ASDSA) commended the FDA on the safety communication in a statement issued on October 11. In February, the ASDSA issued an alert about children using hyaluron pens to self-inject hyaluronic filler into the epidermal and upper dermal skin layers.
“I am pleased that the FDA has taken notice of this disturbing new trend, especially that of children using these devices on social media,” ASDSA president Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center, at Massachusetts General Hospital, Boston, said in the statement. “The complexity of facial anatomy requires in-depth knowledge and expertise, and patients should always have medical procedures done by a physician who also has knowledge of adverse events,” he added, urging consumers to see a board-certified dermatologist before undergoing any cosmetic procedure.
In response to a query, an FDA spokesperson did not have an estimate of the number of reports of these adverse events.
People who have problems or are concerned about having had a filler injected with a needle-free device should contact a licensed health care provider. Consumers and health care professionals should report adverse events related to injection of fillers with a needle-free device to the FDA’s MedWatch program. In addition to MedWatch, adverse events can also be reported to the Cutaneous Procedures Adverse Events Reporting (CAPER) Registry, established earlier this year by the ASDSA with the department of dermatology at Northwestern University, Chicago.
*This story was updated on October 12.
.
Specifically, the warning advises consumers and health care professionals “not to use needle-free devices such as hyaluron pens for injection of hyaluronic acid (HA) or other lip and facial fillers, collectively and commonly referred to as dermal fillers or fillers.”
According to the statement, the agency “is aware of serious injuries and in some cases, permanent harm to the skin, lips, or eyes with the use of needle-free devices for injection of fillers.”
Needle-free devices and lip and facial fillers for use with these devices are being sold directly to consumers online, and are promoted on social media “to increase lip volume, improve the appearance of wrinkles, change the shape of the nose, and other similar procedures,” according to the FDA warning.
The FDA points out that FDA-approved dermal fillers are for prescription use only, and should be administered only by licensed health care professionals using a syringe with a needle or cannula, and advises consumers not to buy or use lip or facial fillers sold directly to the public.
These products may be contaminated with infectious agents or chemicals. Moreover, “needle-free injection devices for aesthetic purposes do not provide enough control over where the injected product is placed,” the statement adds. In addition to infections, other risks include bleeding and bruising, formation of lumps, allergic reactions, blockage of a blood vessel (which can result in necrosis, blindness, or stroke), and transmission of diseases from sharing devices.
The FDA’s recommendations for health care providers include not using any aesthetic fillers with a needle-free device, and not using approved dermal fillers in such devices.
The American Society for Dermatologic Surgery Association (ASDSA) commended the FDA on the safety communication in a statement issued on October 11. In February, the ASDSA issued an alert about children using hyaluron pens to self-inject hyaluronic filler into the epidermal and upper dermal skin layers.
“I am pleased that the FDA has taken notice of this disturbing new trend, especially that of children using these devices on social media,” ASDSA president Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center, at Massachusetts General Hospital, Boston, said in the statement. “The complexity of facial anatomy requires in-depth knowledge and expertise, and patients should always have medical procedures done by a physician who also has knowledge of adverse events,” he added, urging consumers to see a board-certified dermatologist before undergoing any cosmetic procedure.
In response to a query, an FDA spokesperson did not have an estimate of the number of reports of these adverse events.
People who have problems or are concerned about having had a filler injected with a needle-free device should contact a licensed health care provider. Consumers and health care professionals should report adverse events related to injection of fillers with a needle-free device to the FDA’s MedWatch program. In addition to MedWatch, adverse events can also be reported to the Cutaneous Procedures Adverse Events Reporting (CAPER) Registry, established earlier this year by the ASDSA with the department of dermatology at Northwestern University, Chicago.
*This story was updated on October 12.
.
Specifically, the warning advises consumers and health care professionals “not to use needle-free devices such as hyaluron pens for injection of hyaluronic acid (HA) or other lip and facial fillers, collectively and commonly referred to as dermal fillers or fillers.”
According to the statement, the agency “is aware of serious injuries and in some cases, permanent harm to the skin, lips, or eyes with the use of needle-free devices for injection of fillers.”
Needle-free devices and lip and facial fillers for use with these devices are being sold directly to consumers online, and are promoted on social media “to increase lip volume, improve the appearance of wrinkles, change the shape of the nose, and other similar procedures,” according to the FDA warning.
The FDA points out that FDA-approved dermal fillers are for prescription use only, and should be administered only by licensed health care professionals using a syringe with a needle or cannula, and advises consumers not to buy or use lip or facial fillers sold directly to the public.
These products may be contaminated with infectious agents or chemicals. Moreover, “needle-free injection devices for aesthetic purposes do not provide enough control over where the injected product is placed,” the statement adds. In addition to infections, other risks include bleeding and bruising, formation of lumps, allergic reactions, blockage of a blood vessel (which can result in necrosis, blindness, or stroke), and transmission of diseases from sharing devices.
The FDA’s recommendations for health care providers include not using any aesthetic fillers with a needle-free device, and not using approved dermal fillers in such devices.
The American Society for Dermatologic Surgery Association (ASDSA) commended the FDA on the safety communication in a statement issued on October 11. In February, the ASDSA issued an alert about children using hyaluron pens to self-inject hyaluronic filler into the epidermal and upper dermal skin layers.
“I am pleased that the FDA has taken notice of this disturbing new trend, especially that of children using these devices on social media,” ASDSA president Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center, at Massachusetts General Hospital, Boston, said in the statement. “The complexity of facial anatomy requires in-depth knowledge and expertise, and patients should always have medical procedures done by a physician who also has knowledge of adverse events,” he added, urging consumers to see a board-certified dermatologist before undergoing any cosmetic procedure.
In response to a query, an FDA spokesperson did not have an estimate of the number of reports of these adverse events.
People who have problems or are concerned about having had a filler injected with a needle-free device should contact a licensed health care provider. Consumers and health care professionals should report adverse events related to injection of fillers with a needle-free device to the FDA’s MedWatch program. In addition to MedWatch, adverse events can also be reported to the Cutaneous Procedures Adverse Events Reporting (CAPER) Registry, established earlier this year by the ASDSA with the department of dermatology at Northwestern University, Chicago.
*This story was updated on October 12.
Benzene prompts recalls of spray antifungals and sunscreens
Bayer has voluntarily recalled batches of its Lotrimin and Tinactin products because of benzene detected in some samples, according to an Oct. 1 company announcement, available on the Food and Drug Administration website. “It is important to note that Bayer’s decision to voluntarily recall these products is a precautionary measure and that the levels detected are not expected to cause adverse health consequences in consumers,” the announcement said.
Benzene is classified as a human carcinogen present in the environment from both natural sources and human activity, and it has been shown to cause cancer with long-term exposure.
The products included in the recall – all in aerosol spray cans – are unexpired Lotrimin and Tinactin sprays with lot numbers starting with TN, CV, or NAA that were distributed to consumer venues between September 2018 and September 2021. The over-the-counter products are Lotrimin Anti-Fungal Athlete’s Foot Powder Spray, Lotrimin Anti-Fungal Jock Itch (AFJI) Athlete’s Foot Powder Spray, Lotrimin Anti-Fungal (AF) Athlete’s Foot Deodorant Powder Spray, Lotrimin AF Athlete’s Foot Liquid Spray, Lotrimin AF Athlete’s Foot Daily Prevention Deodorant Powder Spray, Tinactin Jock Itch (JI) Powder Spray, Tinactin Athlete’s Foot Deodorant Powder Spray, Tinactin Athlete’s Foot Powder Spray, and Tinactin Athlete’s Foot Liquid Spray.
Bayer has received no reports of adverse events related to the recall. The company also reported no concerns with its antifungal creams or other products.
In addition, Coppertone has issued a voluntary recall of specific lots of five spray sunscreen products because of the presence of benzene, according to a Sept. 30th company announcement, also posted on the FDA website. The recall includes Pure&Simple spray for babies, children, and adults; Coppertone Sport Mineral Spray; and Travel-sized Coppertone Sport spray. The specific lots were manufactured between January and June 2021, and are listed on the company announcement.
“Daily exposure to benzene at the levels detected in these affected Coppertone aerosol sunscreen spray products would not be expected to cause adverse health consequences based on generally accepted exposure modeling by numerous regulatory agencies,” according to the announcement. Coppertone has received no reports of adverse events related to the recall.
In the announcement, Coppertone advised consumers to discontinue use of the impacted products, dispose of the aerosol cans properly, and contact their physician or health care provider if they experience any problems related to the sunscreen sprays.
In May 2021, online pharmacy Valisure, which routinely tests their medications, petitioned the FDA to recall specific sunscreens after detecting high benzene levels in several brands and batches of sunscreen products. The FDA evaluated the petition, but the agency itself did not issue any recalls of sunscreens.
Clinicians are advised to report any adverse events to the FDA’s MedWatch Adverse Event Reporting program either online or by regular mail or fax using this form.
Bayer has voluntarily recalled batches of its Lotrimin and Tinactin products because of benzene detected in some samples, according to an Oct. 1 company announcement, available on the Food and Drug Administration website. “It is important to note that Bayer’s decision to voluntarily recall these products is a precautionary measure and that the levels detected are not expected to cause adverse health consequences in consumers,” the announcement said.
Benzene is classified as a human carcinogen present in the environment from both natural sources and human activity, and it has been shown to cause cancer with long-term exposure.
The products included in the recall – all in aerosol spray cans – are unexpired Lotrimin and Tinactin sprays with lot numbers starting with TN, CV, or NAA that were distributed to consumer venues between September 2018 and September 2021. The over-the-counter products are Lotrimin Anti-Fungal Athlete’s Foot Powder Spray, Lotrimin Anti-Fungal Jock Itch (AFJI) Athlete’s Foot Powder Spray, Lotrimin Anti-Fungal (AF) Athlete’s Foot Deodorant Powder Spray, Lotrimin AF Athlete’s Foot Liquid Spray, Lotrimin AF Athlete’s Foot Daily Prevention Deodorant Powder Spray, Tinactin Jock Itch (JI) Powder Spray, Tinactin Athlete’s Foot Deodorant Powder Spray, Tinactin Athlete’s Foot Powder Spray, and Tinactin Athlete’s Foot Liquid Spray.
Bayer has received no reports of adverse events related to the recall. The company also reported no concerns with its antifungal creams or other products.
In addition, Coppertone has issued a voluntary recall of specific lots of five spray sunscreen products because of the presence of benzene, according to a Sept. 30th company announcement, also posted on the FDA website. The recall includes Pure&Simple spray for babies, children, and adults; Coppertone Sport Mineral Spray; and Travel-sized Coppertone Sport spray. The specific lots were manufactured between January and June 2021, and are listed on the company announcement.
“Daily exposure to benzene at the levels detected in these affected Coppertone aerosol sunscreen spray products would not be expected to cause adverse health consequences based on generally accepted exposure modeling by numerous regulatory agencies,” according to the announcement. Coppertone has received no reports of adverse events related to the recall.
In the announcement, Coppertone advised consumers to discontinue use of the impacted products, dispose of the aerosol cans properly, and contact their physician or health care provider if they experience any problems related to the sunscreen sprays.
In May 2021, online pharmacy Valisure, which routinely tests their medications, petitioned the FDA to recall specific sunscreens after detecting high benzene levels in several brands and batches of sunscreen products. The FDA evaluated the petition, but the agency itself did not issue any recalls of sunscreens.
Clinicians are advised to report any adverse events to the FDA’s MedWatch Adverse Event Reporting program either online or by regular mail or fax using this form.
Bayer has voluntarily recalled batches of its Lotrimin and Tinactin products because of benzene detected in some samples, according to an Oct. 1 company announcement, available on the Food and Drug Administration website. “It is important to note that Bayer’s decision to voluntarily recall these products is a precautionary measure and that the levels detected are not expected to cause adverse health consequences in consumers,” the announcement said.
Benzene is classified as a human carcinogen present in the environment from both natural sources and human activity, and it has been shown to cause cancer with long-term exposure.
The products included in the recall – all in aerosol spray cans – are unexpired Lotrimin and Tinactin sprays with lot numbers starting with TN, CV, or NAA that were distributed to consumer venues between September 2018 and September 2021. The over-the-counter products are Lotrimin Anti-Fungal Athlete’s Foot Powder Spray, Lotrimin Anti-Fungal Jock Itch (AFJI) Athlete’s Foot Powder Spray, Lotrimin Anti-Fungal (AF) Athlete’s Foot Deodorant Powder Spray, Lotrimin AF Athlete’s Foot Liquid Spray, Lotrimin AF Athlete’s Foot Daily Prevention Deodorant Powder Spray, Tinactin Jock Itch (JI) Powder Spray, Tinactin Athlete’s Foot Deodorant Powder Spray, Tinactin Athlete’s Foot Powder Spray, and Tinactin Athlete’s Foot Liquid Spray.
Bayer has received no reports of adverse events related to the recall. The company also reported no concerns with its antifungal creams or other products.
In addition, Coppertone has issued a voluntary recall of specific lots of five spray sunscreen products because of the presence of benzene, according to a Sept. 30th company announcement, also posted on the FDA website. The recall includes Pure&Simple spray for babies, children, and adults; Coppertone Sport Mineral Spray; and Travel-sized Coppertone Sport spray. The specific lots were manufactured between January and June 2021, and are listed on the company announcement.
“Daily exposure to benzene at the levels detected in these affected Coppertone aerosol sunscreen spray products would not be expected to cause adverse health consequences based on generally accepted exposure modeling by numerous regulatory agencies,” according to the announcement. Coppertone has received no reports of adverse events related to the recall.
In the announcement, Coppertone advised consumers to discontinue use of the impacted products, dispose of the aerosol cans properly, and contact their physician or health care provider if they experience any problems related to the sunscreen sprays.
In May 2021, online pharmacy Valisure, which routinely tests their medications, petitioned the FDA to recall specific sunscreens after detecting high benzene levels in several brands and batches of sunscreen products. The FDA evaluated the petition, but the agency itself did not issue any recalls of sunscreens.
Clinicians are advised to report any adverse events to the FDA’s MedWatch Adverse Event Reporting program either online or by regular mail or fax using this form.
Major insurers running billions of dollars behind on payments to hospitals and doctors
Anthem Blue Cross, the country’s second-biggest health insurance company, is behind on billions of dollars in payments owed to hospitals and doctors because of onerous new reimbursement rules, computer problems and mishandled claims, say hospital officials in multiple states.
Anthem, like other big insurers, is using the COVID-19 crisis as cover to institute “egregious” policies that harm patients and pinch hospital finances, said Molly Smith, group vice president at the American Hospital Association. “There’s this sense of ‘Everyone’s distracted. We can get this through.’ ”
Hospitals are also dealing with a spike in retroactive claims denials by UnitedHealthcare, the biggest health insurer, for ED care, the AHA said.
Hospitals say it is hurting their finances as many cope with COVID surges – even after the industry has received tens of billions of dollars in emergency assistance from the federal government.
“We recognize there have been some challenges” to prompt payments caused by claims-processing changes and “a new set of dynamics” amid the pandemic, Anthem spokesperson Colin Manning said in an email. “We apologize for any delays or inconvenience this may have caused.”
Virginia law requires insurers to pay claims within 40 days. In a Sept. 24 letter to state insurance regulators, VCU Health, a system that operates a large teaching hospital in Richmond associated with Virginia Commonwealth University, said Anthem owes it $385 million. More than 40% of the claims are more than 90 days old, VCU said.
For all Virginia hospitals, Anthem’s late, unpaid claims amount to “hundreds of millions of dollars,” the Virginia Hospital and Healthcare Association said in a June 23 letter to state regulators.
Nationwide, the payment delays “are creating an untenable situation,” the American Hospital Association said in a Sept. 9 letter to Anthem CEO Gail Boudreaux. “Patients are facing greater hurdles to accessing care; clinicians are burning out on unnecessary administrative tasks; and the system is straining to finance the personnel and supplies” needed to fight Covid.
Complaints about Anthem extend “from sea to shining sea, from New Hampshire to California,” AHA CEO Rick Pollack told KHN.
Substantial payment delays can be seen on Anthem’s books. On June 30, 2019, before the pandemic, 43% of the insurer’s medical bills for that quarter were unpaid, according to regulatory filings. Two years later that figure had risen to 53% – a difference of $2.5 billion.
Anthem profits were $4.6 billion in 2020 and $3.5 billion in the first half of 2021.
Alexis Thurber, who lives near Seattle, was insured by Anthem when she got an $18,192 hospital bill in May for radiation therapy that doctors said was essential to treat her breast cancer.
The treatments were “experimental” and “not medically necessary,” Anthem said, according to Ms. Thurber. She spent much of the summer trying to get the insurer to pay up – placing two dozen phone calls, spending hours on hold, sending multiple emails and enduring unmeasurable stress and worry. It finally covered the claim months later.
“It’s so egregious. It’s a game they’re playing,” said Ms. Thurber, 51, whose cancer was diagnosed in November. “Trying to get true help was impossible.”
Privacy rules prevent Anthem from commenting on Ms. Thurber’s case, said Anthem spokesperson Colin Manning.
When insurers fail to promptly pay medical bills, patients are left in the lurch. They might first get a notice saying payment is pending or denied. A hospital might bill them for treatment they thought would be covered. Hospitals and doctors often sue patients whose insurance didn’t pay up.
Hospitals point to a variety of Anthem practices contributing to payment delays or denials, including new layers of document requirements, prior-authorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers. “This requires providers to literally leave the patient[’s] bedside to get on the phone with Anthem,” AHA said in its letter.
Anthem often hinders coverage for outpatient surgery, specialty pharmacy and other services in health systems listed as in network, amounting to a “bait and switch” on Anthem members, AHA officials said.
“Demanding that patients be treated outside of the hospital setting, against the advice of the patient’s in-network treating physician, appears to be motivated by a desire to drive up Empire’s profits,” the Greater New York Hospital Association wrote in an April letter to Empire Blue Cross, which is owned by Anthem.
Anthem officials pushed back in a recent letter to the AHA, saying the insurer’s changing rules are intended partly to control excessive prices charged by hospitals for specialty drugs and nonemergency surgery, screening and diagnostic procedures.
Severe problems with Anthem’s new claims management system surfaced months ago and “persist without meaningful improvement,” AHA said in its letter.
Claims have gotten lost in Anthem’s computers, and in some cases VCU Health has had to print medical records and mail them to get paid, VCU said in its letter. The cash slowdown imposes “an unmanageable disruption that threatens to undermine our financial footing,” VCU said.
United denied $31,557 in claims for Emily Long’s care after she was struck in June by a motorcycle in New York City. She needed surgery to repair a fractured cheekbone. United said there was a lack of documentation for “medical necessity” – an “incredibly aggravating” response on top of the distress of the accident, Ms. Long said.
The Brooklyn hospital that treated Ms. Long was “paid appropriately under her plan and within the required time frame,” said United spokesperson Maria Gordon Shydlo. “The facility has the right to appeal the decision.”
United’s unpaid claims came to 54% as of June 30, about the same level as 2 years previously.
When Erin Conlisk initially had trouble gaining approval for a piece of medical equipment for her elderly father this summer, United employees told her the insurer’s entire prior-authorization database had gone down for weeks, said Ms. Conlisk, who lives in California.
“There was a brief issue with our prior-authorization process in mid-July, which was resolved quickly,” Gordon Shydlo said.
When asked by Wall Street analysts about the payment backups, Anthem executives said it partly reflects their decision to increase financial reserves amid the health crisis.
“Really a ton of uncertainty associated with this environment,” John Gallina, the company’s chief financial officer, said on a conference call in July. “We’ve tried to be extremely prudent and conservative in our approach.”
During the pandemic, hospitals have benefited from two extraordinary cash infusions. They and other medical providers have received more than $100 billion through the CARES Act of 2020 and the American Rescue Plan of 2021. Last year United, Anthem and other insurers accelerated billions in hospital reimbursements.
The federal payments enriched many of the biggest, wealthiest systems while poorer hospitals serving low-income patients and rural areas struggled.
Those are the systems most hurt now by insurer payment delays, hospital officials said. Federal relief funds “have been a lifeline, but they don’t make people whole in terms of the losses from increased expenses and lost revenue as a result of the COVID experience,” Mr. Pollack said.
Several health systems declined to comment about claims payment delays or didn’t respond to a reporter’s queries. Among individual hospitals “there is a deep fear of talking on the record about your largest business partner,” AHA’s Ms. Smith said.
Alexis Thurber worried she might have to pay her $18,192 radiation bill herself, and she’s not confident her Anthem policy will do a better job next time of covering the cost of her care.
“It makes me not want to go to the doctor anymore,” she said. “I’m scared to get another mammogram because you can’t rely on it.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Anthem Blue Cross, the country’s second-biggest health insurance company, is behind on billions of dollars in payments owed to hospitals and doctors because of onerous new reimbursement rules, computer problems and mishandled claims, say hospital officials in multiple states.
Anthem, like other big insurers, is using the COVID-19 crisis as cover to institute “egregious” policies that harm patients and pinch hospital finances, said Molly Smith, group vice president at the American Hospital Association. “There’s this sense of ‘Everyone’s distracted. We can get this through.’ ”
Hospitals are also dealing with a spike in retroactive claims denials by UnitedHealthcare, the biggest health insurer, for ED care, the AHA said.
Hospitals say it is hurting their finances as many cope with COVID surges – even after the industry has received tens of billions of dollars in emergency assistance from the federal government.
“We recognize there have been some challenges” to prompt payments caused by claims-processing changes and “a new set of dynamics” amid the pandemic, Anthem spokesperson Colin Manning said in an email. “We apologize for any delays or inconvenience this may have caused.”
Virginia law requires insurers to pay claims within 40 days. In a Sept. 24 letter to state insurance regulators, VCU Health, a system that operates a large teaching hospital in Richmond associated with Virginia Commonwealth University, said Anthem owes it $385 million. More than 40% of the claims are more than 90 days old, VCU said.
For all Virginia hospitals, Anthem’s late, unpaid claims amount to “hundreds of millions of dollars,” the Virginia Hospital and Healthcare Association said in a June 23 letter to state regulators.
Nationwide, the payment delays “are creating an untenable situation,” the American Hospital Association said in a Sept. 9 letter to Anthem CEO Gail Boudreaux. “Patients are facing greater hurdles to accessing care; clinicians are burning out on unnecessary administrative tasks; and the system is straining to finance the personnel and supplies” needed to fight Covid.
Complaints about Anthem extend “from sea to shining sea, from New Hampshire to California,” AHA CEO Rick Pollack told KHN.
Substantial payment delays can be seen on Anthem’s books. On June 30, 2019, before the pandemic, 43% of the insurer’s medical bills for that quarter were unpaid, according to regulatory filings. Two years later that figure had risen to 53% – a difference of $2.5 billion.
Anthem profits were $4.6 billion in 2020 and $3.5 billion in the first half of 2021.
Alexis Thurber, who lives near Seattle, was insured by Anthem when she got an $18,192 hospital bill in May for radiation therapy that doctors said was essential to treat her breast cancer.
The treatments were “experimental” and “not medically necessary,” Anthem said, according to Ms. Thurber. She spent much of the summer trying to get the insurer to pay up – placing two dozen phone calls, spending hours on hold, sending multiple emails and enduring unmeasurable stress and worry. It finally covered the claim months later.
“It’s so egregious. It’s a game they’re playing,” said Ms. Thurber, 51, whose cancer was diagnosed in November. “Trying to get true help was impossible.”
Privacy rules prevent Anthem from commenting on Ms. Thurber’s case, said Anthem spokesperson Colin Manning.
When insurers fail to promptly pay medical bills, patients are left in the lurch. They might first get a notice saying payment is pending or denied. A hospital might bill them for treatment they thought would be covered. Hospitals and doctors often sue patients whose insurance didn’t pay up.
Hospitals point to a variety of Anthem practices contributing to payment delays or denials, including new layers of document requirements, prior-authorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers. “This requires providers to literally leave the patient[’s] bedside to get on the phone with Anthem,” AHA said in its letter.
Anthem often hinders coverage for outpatient surgery, specialty pharmacy and other services in health systems listed as in network, amounting to a “bait and switch” on Anthem members, AHA officials said.
“Demanding that patients be treated outside of the hospital setting, against the advice of the patient’s in-network treating physician, appears to be motivated by a desire to drive up Empire’s profits,” the Greater New York Hospital Association wrote in an April letter to Empire Blue Cross, which is owned by Anthem.
Anthem officials pushed back in a recent letter to the AHA, saying the insurer’s changing rules are intended partly to control excessive prices charged by hospitals for specialty drugs and nonemergency surgery, screening and diagnostic procedures.
Severe problems with Anthem’s new claims management system surfaced months ago and “persist without meaningful improvement,” AHA said in its letter.
Claims have gotten lost in Anthem’s computers, and in some cases VCU Health has had to print medical records and mail them to get paid, VCU said in its letter. The cash slowdown imposes “an unmanageable disruption that threatens to undermine our financial footing,” VCU said.
United denied $31,557 in claims for Emily Long’s care after she was struck in June by a motorcycle in New York City. She needed surgery to repair a fractured cheekbone. United said there was a lack of documentation for “medical necessity” – an “incredibly aggravating” response on top of the distress of the accident, Ms. Long said.
The Brooklyn hospital that treated Ms. Long was “paid appropriately under her plan and within the required time frame,” said United spokesperson Maria Gordon Shydlo. “The facility has the right to appeal the decision.”
United’s unpaid claims came to 54% as of June 30, about the same level as 2 years previously.
When Erin Conlisk initially had trouble gaining approval for a piece of medical equipment for her elderly father this summer, United employees told her the insurer’s entire prior-authorization database had gone down for weeks, said Ms. Conlisk, who lives in California.
“There was a brief issue with our prior-authorization process in mid-July, which was resolved quickly,” Gordon Shydlo said.
When asked by Wall Street analysts about the payment backups, Anthem executives said it partly reflects their decision to increase financial reserves amid the health crisis.
“Really a ton of uncertainty associated with this environment,” John Gallina, the company’s chief financial officer, said on a conference call in July. “We’ve tried to be extremely prudent and conservative in our approach.”
During the pandemic, hospitals have benefited from two extraordinary cash infusions. They and other medical providers have received more than $100 billion through the CARES Act of 2020 and the American Rescue Plan of 2021. Last year United, Anthem and other insurers accelerated billions in hospital reimbursements.
The federal payments enriched many of the biggest, wealthiest systems while poorer hospitals serving low-income patients and rural areas struggled.
Those are the systems most hurt now by insurer payment delays, hospital officials said. Federal relief funds “have been a lifeline, but they don’t make people whole in terms of the losses from increased expenses and lost revenue as a result of the COVID experience,” Mr. Pollack said.
Several health systems declined to comment about claims payment delays or didn’t respond to a reporter’s queries. Among individual hospitals “there is a deep fear of talking on the record about your largest business partner,” AHA’s Ms. Smith said.
Alexis Thurber worried she might have to pay her $18,192 radiation bill herself, and she’s not confident her Anthem policy will do a better job next time of covering the cost of her care.
“It makes me not want to go to the doctor anymore,” she said. “I’m scared to get another mammogram because you can’t rely on it.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Anthem Blue Cross, the country’s second-biggest health insurance company, is behind on billions of dollars in payments owed to hospitals and doctors because of onerous new reimbursement rules, computer problems and mishandled claims, say hospital officials in multiple states.
Anthem, like other big insurers, is using the COVID-19 crisis as cover to institute “egregious” policies that harm patients and pinch hospital finances, said Molly Smith, group vice president at the American Hospital Association. “There’s this sense of ‘Everyone’s distracted. We can get this through.’ ”
Hospitals are also dealing with a spike in retroactive claims denials by UnitedHealthcare, the biggest health insurer, for ED care, the AHA said.
Hospitals say it is hurting their finances as many cope with COVID surges – even after the industry has received tens of billions of dollars in emergency assistance from the federal government.
“We recognize there have been some challenges” to prompt payments caused by claims-processing changes and “a new set of dynamics” amid the pandemic, Anthem spokesperson Colin Manning said in an email. “We apologize for any delays or inconvenience this may have caused.”
Virginia law requires insurers to pay claims within 40 days. In a Sept. 24 letter to state insurance regulators, VCU Health, a system that operates a large teaching hospital in Richmond associated with Virginia Commonwealth University, said Anthem owes it $385 million. More than 40% of the claims are more than 90 days old, VCU said.
For all Virginia hospitals, Anthem’s late, unpaid claims amount to “hundreds of millions of dollars,” the Virginia Hospital and Healthcare Association said in a June 23 letter to state regulators.
Nationwide, the payment delays “are creating an untenable situation,” the American Hospital Association said in a Sept. 9 letter to Anthem CEO Gail Boudreaux. “Patients are facing greater hurdles to accessing care; clinicians are burning out on unnecessary administrative tasks; and the system is straining to finance the personnel and supplies” needed to fight Covid.
Complaints about Anthem extend “from sea to shining sea, from New Hampshire to California,” AHA CEO Rick Pollack told KHN.
Substantial payment delays can be seen on Anthem’s books. On June 30, 2019, before the pandemic, 43% of the insurer’s medical bills for that quarter were unpaid, according to regulatory filings. Two years later that figure had risen to 53% – a difference of $2.5 billion.
Anthem profits were $4.6 billion in 2020 and $3.5 billion in the first half of 2021.
Alexis Thurber, who lives near Seattle, was insured by Anthem when she got an $18,192 hospital bill in May for radiation therapy that doctors said was essential to treat her breast cancer.
The treatments were “experimental” and “not medically necessary,” Anthem said, according to Ms. Thurber. She spent much of the summer trying to get the insurer to pay up – placing two dozen phone calls, spending hours on hold, sending multiple emails and enduring unmeasurable stress and worry. It finally covered the claim months later.
“It’s so egregious. It’s a game they’re playing,” said Ms. Thurber, 51, whose cancer was diagnosed in November. “Trying to get true help was impossible.”
Privacy rules prevent Anthem from commenting on Ms. Thurber’s case, said Anthem spokesperson Colin Manning.
When insurers fail to promptly pay medical bills, patients are left in the lurch. They might first get a notice saying payment is pending or denied. A hospital might bill them for treatment they thought would be covered. Hospitals and doctors often sue patients whose insurance didn’t pay up.
Hospitals point to a variety of Anthem practices contributing to payment delays or denials, including new layers of document requirements, prior-authorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers. “This requires providers to literally leave the patient[’s] bedside to get on the phone with Anthem,” AHA said in its letter.
Anthem often hinders coverage for outpatient surgery, specialty pharmacy and other services in health systems listed as in network, amounting to a “bait and switch” on Anthem members, AHA officials said.
“Demanding that patients be treated outside of the hospital setting, against the advice of the patient’s in-network treating physician, appears to be motivated by a desire to drive up Empire’s profits,” the Greater New York Hospital Association wrote in an April letter to Empire Blue Cross, which is owned by Anthem.
Anthem officials pushed back in a recent letter to the AHA, saying the insurer’s changing rules are intended partly to control excessive prices charged by hospitals for specialty drugs and nonemergency surgery, screening and diagnostic procedures.
Severe problems with Anthem’s new claims management system surfaced months ago and “persist without meaningful improvement,” AHA said in its letter.
Claims have gotten lost in Anthem’s computers, and in some cases VCU Health has had to print medical records and mail them to get paid, VCU said in its letter. The cash slowdown imposes “an unmanageable disruption that threatens to undermine our financial footing,” VCU said.
United denied $31,557 in claims for Emily Long’s care after she was struck in June by a motorcycle in New York City. She needed surgery to repair a fractured cheekbone. United said there was a lack of documentation for “medical necessity” – an “incredibly aggravating” response on top of the distress of the accident, Ms. Long said.
The Brooklyn hospital that treated Ms. Long was “paid appropriately under her plan and within the required time frame,” said United spokesperson Maria Gordon Shydlo. “The facility has the right to appeal the decision.”
United’s unpaid claims came to 54% as of June 30, about the same level as 2 years previously.
When Erin Conlisk initially had trouble gaining approval for a piece of medical equipment for her elderly father this summer, United employees told her the insurer’s entire prior-authorization database had gone down for weeks, said Ms. Conlisk, who lives in California.
“There was a brief issue with our prior-authorization process in mid-July, which was resolved quickly,” Gordon Shydlo said.
When asked by Wall Street analysts about the payment backups, Anthem executives said it partly reflects their decision to increase financial reserves amid the health crisis.
“Really a ton of uncertainty associated with this environment,” John Gallina, the company’s chief financial officer, said on a conference call in July. “We’ve tried to be extremely prudent and conservative in our approach.”
During the pandemic, hospitals have benefited from two extraordinary cash infusions. They and other medical providers have received more than $100 billion through the CARES Act of 2020 and the American Rescue Plan of 2021. Last year United, Anthem and other insurers accelerated billions in hospital reimbursements.
The federal payments enriched many of the biggest, wealthiest systems while poorer hospitals serving low-income patients and rural areas struggled.
Those are the systems most hurt now by insurer payment delays, hospital officials said. Federal relief funds “have been a lifeline, but they don’t make people whole in terms of the losses from increased expenses and lost revenue as a result of the COVID experience,” Mr. Pollack said.
Several health systems declined to comment about claims payment delays or didn’t respond to a reporter’s queries. Among individual hospitals “there is a deep fear of talking on the record about your largest business partner,” AHA’s Ms. Smith said.
Alexis Thurber worried she might have to pay her $18,192 radiation bill herself, and she’s not confident her Anthem policy will do a better job next time of covering the cost of her care.
“It makes me not want to go to the doctor anymore,” she said. “I’m scared to get another mammogram because you can’t rely on it.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
In atopic dermatitis trial, abrocitinib offers faster itch relief than dupilumab
), in a multicenter randomized trial presented as a late breaker at the annual meeting of the European Academy of Dermatology and Venereology.
The earlier onset of action with the JAK inhibitor was achieved even though most patients in both arms were on topical corticosteroids, a design element that “is clinically relevant” for a practical comparison of these two agents, according to Kristian Reich, MD, PhD, Center for Translational Research in Inflammatory Skin Diseases, University Medical Center, Hamburg-Eppendorf, Germany.
The goal of this phase 3b trial, called JADE DARE, was to compare relative safety and efficacy of these strategies over the early course of treatment, he said.
Over 700 patients randomized
JADE DARE enrolled 727 patients over age 18 years who previously had an inadequate response to conventional topical therapies. All had moderate to severe AD defined by criteria such as body surface area greater than or equal to 10% and Eczema Area Severity Index (EASI) greater than or equal to 16. They were randomly assigned to 200 mg oral abrocitinib once daily or 300 mg subcutaneous dupilumab (after a loading dose of 600 mg) every 2 weeks. A double-dummy design preserved blinding.
The coprimary endpoints were at least a 4-point improvement in pruritus as measured with the Peak Pruritus Numerical Rating Scale (PP-NRS) score at week 2 and at least a 90% improvement in the EASI (EASI 90) at week 4.
The primary endpoint for pruritus at 2 weeks was reached by nearly twice as many patients randomly assigned to abrocitinib (46.2% vs. 25.5%; P < .001). The proportion of those meeting the EASI 90 endpoint at week 4 was also superior on abrocitinib (28.5% vs. 14.6%; P < .001)
Advantage for pruritus control dissipates
For the pruritus endpoint, the advantage of abrocitinib slowly diminished over time after the peak difference observed at 2 weeks. Although the advantage at week 4 (58.1% vs. 40.8%) and week 8 (65.8% vs. 52.7%) remained sizable, there were very small differences thereafter. However, Dr. Reich pointed out that the percentages continued to favor abrocitinib at least numerically through the 26 weeks of follow-up completed so far.
The pattern of response on EASI 90 was not the same. After demonstrating superiority at the 4-week timepoint, the advantage of abrocitinib persisted. When compared at week 16, which was a secondary endpoint of the JADE DARE trial, the advantage of abrocitinib remained significant (54.3% vs. 41.9%; P < .001). The advantage of abrocitinib narrowed but remained numerically superior at 26 weeks (54.6% vs. 47.6%).
Based on the data collected to date, “abrocitinib is clearly superior early on,” Dr. Reich said. Moreover, he reiterated that topical corticosteroids were allowed as background therapy in both arms.
“It is difficult to show an advantage for one active therapy over the other in patients on background corticosteroids,” Dr. Reich maintained.
Both drugs are well tolerated
The drugs were similarly well tolerated. Serious adverse events were uncommon in either arm. The rate of study dropouts due to an adverse event potentially related to treatment assignment was 3% in each group.
Nausea (19% vs. 2%), acne (13.5% vs. 2%), and headache (13% vs. 7.5%) were all more common in patients randomly assigned to abrocitinib. Conjunctivitis was more common in the group randomly assigned to dupilumab (10% vs. 2%).
The two deaths that occurred during this study were in the abrocitinib arm, but one was the result of COVID-19 infection and the other was a cardiovascular event in a patient with risk factors. Neither was considered to be treatment-related.
Abrocitinib’s relative selectivity for the JAK1 inhibitor is a potential differentiator from other currently available JAK inhibitors, although direct comparisons of these therapies for clinical activity in AD as well as most other diseases remains limited.
The relatively rapid relief of pruritus with the JAK inhibitor relative to the monoclonal antibody in the JADE DARE trial is likely to be perceived as clinically significant by patients with AD, according to Sonja Ständer, MD, professor of dermatology and neurodermatology at the University Hospital Münster, Germany.
“One of the highest needs of patients with atopic dermatitis is a rapid and profound relief of itch,” Dr. Ständer, who wrote a review article on AD earlier this year, said in an interview.
Although several current therapies are effective against pruritus, Dr. Ständer believes that the higher proportion of patients achieving itch control at 2 weeks on abrocitinib “will attract the attention of affected patients.”
However, she added that patients need to take both benefits and risks into account, indicating that clinical utility cannot be judged on a single outcome. In selecting one drug over the others, she advised “a balanced use of therapies.”
Abrocitinib was first approved in the United Kingdom in early September, followed by Japan last Thursday, for the treatment of moderate to severe AD in patients ages 12 and older. It is under review elsewhere, including in the United States and the European Union for AD.
In September, the FDA approved the first JAK inhibitor for treating AD – a topical JAK inhibitor, ruxolitinib.
Dr. Reich reports financial relationships with 20 pharmaceutical companies, including Pfizer, which provided funding for the JADE DARE trial. Dr. Ständer reports financial relationships with Beiersdorf AG, Galderma, Kliniska, Lilly, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
), in a multicenter randomized trial presented as a late breaker at the annual meeting of the European Academy of Dermatology and Venereology.
The earlier onset of action with the JAK inhibitor was achieved even though most patients in both arms were on topical corticosteroids, a design element that “is clinically relevant” for a practical comparison of these two agents, according to Kristian Reich, MD, PhD, Center for Translational Research in Inflammatory Skin Diseases, University Medical Center, Hamburg-Eppendorf, Germany.
The goal of this phase 3b trial, called JADE DARE, was to compare relative safety and efficacy of these strategies over the early course of treatment, he said.
Over 700 patients randomized
JADE DARE enrolled 727 patients over age 18 years who previously had an inadequate response to conventional topical therapies. All had moderate to severe AD defined by criteria such as body surface area greater than or equal to 10% and Eczema Area Severity Index (EASI) greater than or equal to 16. They were randomly assigned to 200 mg oral abrocitinib once daily or 300 mg subcutaneous dupilumab (after a loading dose of 600 mg) every 2 weeks. A double-dummy design preserved blinding.
The coprimary endpoints were at least a 4-point improvement in pruritus as measured with the Peak Pruritus Numerical Rating Scale (PP-NRS) score at week 2 and at least a 90% improvement in the EASI (EASI 90) at week 4.
The primary endpoint for pruritus at 2 weeks was reached by nearly twice as many patients randomly assigned to abrocitinib (46.2% vs. 25.5%; P < .001). The proportion of those meeting the EASI 90 endpoint at week 4 was also superior on abrocitinib (28.5% vs. 14.6%; P < .001)
Advantage for pruritus control dissipates
For the pruritus endpoint, the advantage of abrocitinib slowly diminished over time after the peak difference observed at 2 weeks. Although the advantage at week 4 (58.1% vs. 40.8%) and week 8 (65.8% vs. 52.7%) remained sizable, there were very small differences thereafter. However, Dr. Reich pointed out that the percentages continued to favor abrocitinib at least numerically through the 26 weeks of follow-up completed so far.
The pattern of response on EASI 90 was not the same. After demonstrating superiority at the 4-week timepoint, the advantage of abrocitinib persisted. When compared at week 16, which was a secondary endpoint of the JADE DARE trial, the advantage of abrocitinib remained significant (54.3% vs. 41.9%; P < .001). The advantage of abrocitinib narrowed but remained numerically superior at 26 weeks (54.6% vs. 47.6%).
Based on the data collected to date, “abrocitinib is clearly superior early on,” Dr. Reich said. Moreover, he reiterated that topical corticosteroids were allowed as background therapy in both arms.
“It is difficult to show an advantage for one active therapy over the other in patients on background corticosteroids,” Dr. Reich maintained.
Both drugs are well tolerated
The drugs were similarly well tolerated. Serious adverse events were uncommon in either arm. The rate of study dropouts due to an adverse event potentially related to treatment assignment was 3% in each group.
Nausea (19% vs. 2%), acne (13.5% vs. 2%), and headache (13% vs. 7.5%) were all more common in patients randomly assigned to abrocitinib. Conjunctivitis was more common in the group randomly assigned to dupilumab (10% vs. 2%).
The two deaths that occurred during this study were in the abrocitinib arm, but one was the result of COVID-19 infection and the other was a cardiovascular event in a patient with risk factors. Neither was considered to be treatment-related.
Abrocitinib’s relative selectivity for the JAK1 inhibitor is a potential differentiator from other currently available JAK inhibitors, although direct comparisons of these therapies for clinical activity in AD as well as most other diseases remains limited.
The relatively rapid relief of pruritus with the JAK inhibitor relative to the monoclonal antibody in the JADE DARE trial is likely to be perceived as clinically significant by patients with AD, according to Sonja Ständer, MD, professor of dermatology and neurodermatology at the University Hospital Münster, Germany.
“One of the highest needs of patients with atopic dermatitis is a rapid and profound relief of itch,” Dr. Ständer, who wrote a review article on AD earlier this year, said in an interview.
Although several current therapies are effective against pruritus, Dr. Ständer believes that the higher proportion of patients achieving itch control at 2 weeks on abrocitinib “will attract the attention of affected patients.”
However, she added that patients need to take both benefits and risks into account, indicating that clinical utility cannot be judged on a single outcome. In selecting one drug over the others, she advised “a balanced use of therapies.”
Abrocitinib was first approved in the United Kingdom in early September, followed by Japan last Thursday, for the treatment of moderate to severe AD in patients ages 12 and older. It is under review elsewhere, including in the United States and the European Union for AD.
In September, the FDA approved the first JAK inhibitor for treating AD – a topical JAK inhibitor, ruxolitinib.
Dr. Reich reports financial relationships with 20 pharmaceutical companies, including Pfizer, which provided funding for the JADE DARE trial. Dr. Ständer reports financial relationships with Beiersdorf AG, Galderma, Kliniska, Lilly, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
), in a multicenter randomized trial presented as a late breaker at the annual meeting of the European Academy of Dermatology and Venereology.
The earlier onset of action with the JAK inhibitor was achieved even though most patients in both arms were on topical corticosteroids, a design element that “is clinically relevant” for a practical comparison of these two agents, according to Kristian Reich, MD, PhD, Center for Translational Research in Inflammatory Skin Diseases, University Medical Center, Hamburg-Eppendorf, Germany.
The goal of this phase 3b trial, called JADE DARE, was to compare relative safety and efficacy of these strategies over the early course of treatment, he said.
Over 700 patients randomized
JADE DARE enrolled 727 patients over age 18 years who previously had an inadequate response to conventional topical therapies. All had moderate to severe AD defined by criteria such as body surface area greater than or equal to 10% and Eczema Area Severity Index (EASI) greater than or equal to 16. They were randomly assigned to 200 mg oral abrocitinib once daily or 300 mg subcutaneous dupilumab (after a loading dose of 600 mg) every 2 weeks. A double-dummy design preserved blinding.
The coprimary endpoints were at least a 4-point improvement in pruritus as measured with the Peak Pruritus Numerical Rating Scale (PP-NRS) score at week 2 and at least a 90% improvement in the EASI (EASI 90) at week 4.
The primary endpoint for pruritus at 2 weeks was reached by nearly twice as many patients randomly assigned to abrocitinib (46.2% vs. 25.5%; P < .001). The proportion of those meeting the EASI 90 endpoint at week 4 was also superior on abrocitinib (28.5% vs. 14.6%; P < .001)
Advantage for pruritus control dissipates
For the pruritus endpoint, the advantage of abrocitinib slowly diminished over time after the peak difference observed at 2 weeks. Although the advantage at week 4 (58.1% vs. 40.8%) and week 8 (65.8% vs. 52.7%) remained sizable, there were very small differences thereafter. However, Dr. Reich pointed out that the percentages continued to favor abrocitinib at least numerically through the 26 weeks of follow-up completed so far.
The pattern of response on EASI 90 was not the same. After demonstrating superiority at the 4-week timepoint, the advantage of abrocitinib persisted. When compared at week 16, which was a secondary endpoint of the JADE DARE trial, the advantage of abrocitinib remained significant (54.3% vs. 41.9%; P < .001). The advantage of abrocitinib narrowed but remained numerically superior at 26 weeks (54.6% vs. 47.6%).
Based on the data collected to date, “abrocitinib is clearly superior early on,” Dr. Reich said. Moreover, he reiterated that topical corticosteroids were allowed as background therapy in both arms.
“It is difficult to show an advantage for one active therapy over the other in patients on background corticosteroids,” Dr. Reich maintained.
Both drugs are well tolerated
The drugs were similarly well tolerated. Serious adverse events were uncommon in either arm. The rate of study dropouts due to an adverse event potentially related to treatment assignment was 3% in each group.
Nausea (19% vs. 2%), acne (13.5% vs. 2%), and headache (13% vs. 7.5%) were all more common in patients randomly assigned to abrocitinib. Conjunctivitis was more common in the group randomly assigned to dupilumab (10% vs. 2%).
The two deaths that occurred during this study were in the abrocitinib arm, but one was the result of COVID-19 infection and the other was a cardiovascular event in a patient with risk factors. Neither was considered to be treatment-related.
Abrocitinib’s relative selectivity for the JAK1 inhibitor is a potential differentiator from other currently available JAK inhibitors, although direct comparisons of these therapies for clinical activity in AD as well as most other diseases remains limited.
The relatively rapid relief of pruritus with the JAK inhibitor relative to the monoclonal antibody in the JADE DARE trial is likely to be perceived as clinically significant by patients with AD, according to Sonja Ständer, MD, professor of dermatology and neurodermatology at the University Hospital Münster, Germany.
“One of the highest needs of patients with atopic dermatitis is a rapid and profound relief of itch,” Dr. Ständer, who wrote a review article on AD earlier this year, said in an interview.
Although several current therapies are effective against pruritus, Dr. Ständer believes that the higher proportion of patients achieving itch control at 2 weeks on abrocitinib “will attract the attention of affected patients.”
However, she added that patients need to take both benefits and risks into account, indicating that clinical utility cannot be judged on a single outcome. In selecting one drug over the others, she advised “a balanced use of therapies.”
Abrocitinib was first approved in the United Kingdom in early September, followed by Japan last Thursday, for the treatment of moderate to severe AD in patients ages 12 and older. It is under review elsewhere, including in the United States and the European Union for AD.
In September, the FDA approved the first JAK inhibitor for treating AD – a topical JAK inhibitor, ruxolitinib.
Dr. Reich reports financial relationships with 20 pharmaceutical companies, including Pfizer, which provided funding for the JADE DARE trial. Dr. Ständer reports financial relationships with Beiersdorf AG, Galderma, Kliniska, Lilly, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.




