User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Study suggests “alarming” diabetes med discontinuation
ORLANDO – Most patients with type 2 diabetes mellitus stop taking their medication within a year, and nearly one-third stop within the first 3 months, a retrospective analysis of claims data for more than 324,000 patients suggests.
The findings in this population of commercially insured adults are startling and highlight a need for interventions to improve treatment persistence, according to Lisa Latts, MD, deputy chief health officer for IBM Watson Health in Cambridge, Mass.
Dr. Latts and her colleagues reviewed medication claims data for 324,136 patients with at least one diagnosis for type 2 diabetes mellitus and one outpatient pharmacy claim for a type 2 diabetes medication after at least 12 prior months without such a claim.
Of those patients, 58% discontinued treatment within 12 months, 31% discontinued within the first 3 months, and 44% discontinued within 6 months, Dr. Latts reported at the annual scientific sessions of the American Diabetes Association.
“Less than half of those patients who discontinued had a restart within the following year. So what we’re seeing here is a huge percentage of individuals who had a diagnosis of type 2 diabetes, were prescribed a medication, and then did not continue the medication,” she said.
Of those who discontinued within the 12-month follow-up, 27% restarted therapy within 60 days and 39% restarted therapy anytime during the 12-month follow-up (mean treatment gap, 107 days). Of those who discontinued by 3 months, 45% restarted within a year (mean treatment gap, 112 days), and of those who discontinued by 6 months, 44% restarted within a year (mean treatment gap, 119 days).
Patients included in the review, which was a collaborative effort of IBM Watson Health and the ADA, had at least 12 months of continuous enrollment in the Truven Health MarketScan Commercial and Medicare Supplemental Databases between 2013 and 2017, before and after the therapy initiation date.
They had a mean age of 55 years, with 28% aged 45-54 years and 35% aged 55-64 years. About 46% were women, and all had at least one diagnosis for type 2 diabetes mellitus during the study period and at least one outpatient pharmacy claim for a type 2 diabetes medication that was prescribed to be taken for at least 30 days.
“As you would expect, far and away the majority [68%] were given metformin,” Dr. Latts said.
Other prescribed treatments after the initial diagnosis included sulfonylureas (7%), insulin (6%), dipeptidyl peptidase–4 inhibitors (6%), sodium-glucose cotransporter 2 inhibitors (1.5%), and a variety of combination treatments – typically metformin plus sulfonylureas (5%).
This study provides real-world evidence that a majority of patients with type 2 diabetes discontinue their treatment within 1 year – an important finding given that medication persistence is imperative for successful treatment, Dr. Latts said. She noted that prior research has shown treatment discontinuation of prescribed medication within the first year is common for a number of chronic disease treatments and is associated with poor clinical outcomes.
“If you treat diabetes, this is alarming,” she said. “These are people who should be on a diabetes med, their doctor probably thinks they’re on a diabetes med, and they’re not taking it.”
The findings are limited by factors associated with the use of administrative claims data, such as possible coding inaccuracies and missed cases in which patients paid out of pocket for medications through low-cost pharmacy offers, as well as by the 12-month window used for the study. She added that the findings may not be generalizable to uninsured or Medicare patients.
Nevertheless, the findings are concerning and may reflect misunderstandings among patients about the need to refill prescriptions after the initial supply runs out, or may relate to side effects that patients don’t report to their physicians, Sherita Golden, MD, of Johns Hopkins University, Baltimore, said during a question-and-answer period following Dr. Latts’ presentation.
“I do treat patients with diabetes so I am very alarmed,” she said, adding that there is a need to improve communication between patients and physicians about treatment and side effects.
Dr. Latts reported relationships with Medtronic, Novo Nordisk, and Sanofi. Her coauthors from IBM Watson and the ADA all reported having no financial disclosures.
SOURCE: Latts L et al. ADA 2018, Abstract 135-OR.
ORLANDO – Most patients with type 2 diabetes mellitus stop taking their medication within a year, and nearly one-third stop within the first 3 months, a retrospective analysis of claims data for more than 324,000 patients suggests.
The findings in this population of commercially insured adults are startling and highlight a need for interventions to improve treatment persistence, according to Lisa Latts, MD, deputy chief health officer for IBM Watson Health in Cambridge, Mass.
Dr. Latts and her colleagues reviewed medication claims data for 324,136 patients with at least one diagnosis for type 2 diabetes mellitus and one outpatient pharmacy claim for a type 2 diabetes medication after at least 12 prior months without such a claim.
Of those patients, 58% discontinued treatment within 12 months, 31% discontinued within the first 3 months, and 44% discontinued within 6 months, Dr. Latts reported at the annual scientific sessions of the American Diabetes Association.
“Less than half of those patients who discontinued had a restart within the following year. So what we’re seeing here is a huge percentage of individuals who had a diagnosis of type 2 diabetes, were prescribed a medication, and then did not continue the medication,” she said.
Of those who discontinued within the 12-month follow-up, 27% restarted therapy within 60 days and 39% restarted therapy anytime during the 12-month follow-up (mean treatment gap, 107 days). Of those who discontinued by 3 months, 45% restarted within a year (mean treatment gap, 112 days), and of those who discontinued by 6 months, 44% restarted within a year (mean treatment gap, 119 days).
Patients included in the review, which was a collaborative effort of IBM Watson Health and the ADA, had at least 12 months of continuous enrollment in the Truven Health MarketScan Commercial and Medicare Supplemental Databases between 2013 and 2017, before and after the therapy initiation date.
They had a mean age of 55 years, with 28% aged 45-54 years and 35% aged 55-64 years. About 46% were women, and all had at least one diagnosis for type 2 diabetes mellitus during the study period and at least one outpatient pharmacy claim for a type 2 diabetes medication that was prescribed to be taken for at least 30 days.
“As you would expect, far and away the majority [68%] were given metformin,” Dr. Latts said.
Other prescribed treatments after the initial diagnosis included sulfonylureas (7%), insulin (6%), dipeptidyl peptidase–4 inhibitors (6%), sodium-glucose cotransporter 2 inhibitors (1.5%), and a variety of combination treatments – typically metformin plus sulfonylureas (5%).
This study provides real-world evidence that a majority of patients with type 2 diabetes discontinue their treatment within 1 year – an important finding given that medication persistence is imperative for successful treatment, Dr. Latts said. She noted that prior research has shown treatment discontinuation of prescribed medication within the first year is common for a number of chronic disease treatments and is associated with poor clinical outcomes.
“If you treat diabetes, this is alarming,” she said. “These are people who should be on a diabetes med, their doctor probably thinks they’re on a diabetes med, and they’re not taking it.”
The findings are limited by factors associated with the use of administrative claims data, such as possible coding inaccuracies and missed cases in which patients paid out of pocket for medications through low-cost pharmacy offers, as well as by the 12-month window used for the study. She added that the findings may not be generalizable to uninsured or Medicare patients.
Nevertheless, the findings are concerning and may reflect misunderstandings among patients about the need to refill prescriptions after the initial supply runs out, or may relate to side effects that patients don’t report to their physicians, Sherita Golden, MD, of Johns Hopkins University, Baltimore, said during a question-and-answer period following Dr. Latts’ presentation.
“I do treat patients with diabetes so I am very alarmed,” she said, adding that there is a need to improve communication between patients and physicians about treatment and side effects.
Dr. Latts reported relationships with Medtronic, Novo Nordisk, and Sanofi. Her coauthors from IBM Watson and the ADA all reported having no financial disclosures.
SOURCE: Latts L et al. ADA 2018, Abstract 135-OR.
ORLANDO – Most patients with type 2 diabetes mellitus stop taking their medication within a year, and nearly one-third stop within the first 3 months, a retrospective analysis of claims data for more than 324,000 patients suggests.
The findings in this population of commercially insured adults are startling and highlight a need for interventions to improve treatment persistence, according to Lisa Latts, MD, deputy chief health officer for IBM Watson Health in Cambridge, Mass.
Dr. Latts and her colleagues reviewed medication claims data for 324,136 patients with at least one diagnosis for type 2 diabetes mellitus and one outpatient pharmacy claim for a type 2 diabetes medication after at least 12 prior months without such a claim.
Of those patients, 58% discontinued treatment within 12 months, 31% discontinued within the first 3 months, and 44% discontinued within 6 months, Dr. Latts reported at the annual scientific sessions of the American Diabetes Association.
“Less than half of those patients who discontinued had a restart within the following year. So what we’re seeing here is a huge percentage of individuals who had a diagnosis of type 2 diabetes, were prescribed a medication, and then did not continue the medication,” she said.
Of those who discontinued within the 12-month follow-up, 27% restarted therapy within 60 days and 39% restarted therapy anytime during the 12-month follow-up (mean treatment gap, 107 days). Of those who discontinued by 3 months, 45% restarted within a year (mean treatment gap, 112 days), and of those who discontinued by 6 months, 44% restarted within a year (mean treatment gap, 119 days).
Patients included in the review, which was a collaborative effort of IBM Watson Health and the ADA, had at least 12 months of continuous enrollment in the Truven Health MarketScan Commercial and Medicare Supplemental Databases between 2013 and 2017, before and after the therapy initiation date.
They had a mean age of 55 years, with 28% aged 45-54 years and 35% aged 55-64 years. About 46% were women, and all had at least one diagnosis for type 2 diabetes mellitus during the study period and at least one outpatient pharmacy claim for a type 2 diabetes medication that was prescribed to be taken for at least 30 days.
“As you would expect, far and away the majority [68%] were given metformin,” Dr. Latts said.
Other prescribed treatments after the initial diagnosis included sulfonylureas (7%), insulin (6%), dipeptidyl peptidase–4 inhibitors (6%), sodium-glucose cotransporter 2 inhibitors (1.5%), and a variety of combination treatments – typically metformin plus sulfonylureas (5%).
This study provides real-world evidence that a majority of patients with type 2 diabetes discontinue their treatment within 1 year – an important finding given that medication persistence is imperative for successful treatment, Dr. Latts said. She noted that prior research has shown treatment discontinuation of prescribed medication within the first year is common for a number of chronic disease treatments and is associated with poor clinical outcomes.
“If you treat diabetes, this is alarming,” she said. “These are people who should be on a diabetes med, their doctor probably thinks they’re on a diabetes med, and they’re not taking it.”
The findings are limited by factors associated with the use of administrative claims data, such as possible coding inaccuracies and missed cases in which patients paid out of pocket for medications through low-cost pharmacy offers, as well as by the 12-month window used for the study. She added that the findings may not be generalizable to uninsured or Medicare patients.
Nevertheless, the findings are concerning and may reflect misunderstandings among patients about the need to refill prescriptions after the initial supply runs out, or may relate to side effects that patients don’t report to their physicians, Sherita Golden, MD, of Johns Hopkins University, Baltimore, said during a question-and-answer period following Dr. Latts’ presentation.
“I do treat patients with diabetes so I am very alarmed,” she said, adding that there is a need to improve communication between patients and physicians about treatment and side effects.
Dr. Latts reported relationships with Medtronic, Novo Nordisk, and Sanofi. Her coauthors from IBM Watson and the ADA all reported having no financial disclosures.
SOURCE: Latts L et al. ADA 2018, Abstract 135-OR.
REPORTING FROM ADA 2018
Key clinical point:
Major finding: In total, 58% of patients discontinued treatment within 12 months, and just 39% of those patients restarted within 1 year.
Study details: An analysis of claims data for more than 324,000 patients.
Disclosures: Dr. Latts reported relationships with Medtronic, Novo Nordisk, and Sanofi. Her coauthors from IBM Watson and the American Diabetes Association all reported having no financial disclosures.
Source: Latts L et al. ADA 2018, Abstract 135-OR.
Statins aren’t preventive in elderly unless they have diabetes
Any benefit of statins for primary prevention in older adult populations may depend on whether or not type 2 diabetes is present, results of a retrospective cohort study suggest.
Statins had no protective effect overall in the study, which included adults older than 74 years who had no clinically recognized atherosclerotic cardiovascular disease (ASCVD).
In older patients with diabetes, statins were associated with reductions in CVD incidence and all-cause mortality. However, this benefit was substantially reduced in patients 85 years and older, and completely absent in those over 90, study authors said in the BMJ.
“These results do not support the widespread use of statins in old and very old populations, but they do support treatment in those with type 2 diabetes younger than 85 years,” said Rafael Ramos, MD, of the University of Girona, Spain, and his coauthors.
While meta-analyses support statins as primary prevention of CVD in individuals 65 years or older, evidence is lacking on those older than 74 years, according to the investigators.
Accordingly, they conducted the present retrospective cohort study based on data from a Spanish primary care database that included patient records for more than 6 million people. They looked specifically for individuals aged 75 years or older with no history of ASCVD who had at least one visit between July 2006 and December 2007.
They found 46,864 people meeting those criteria, of whom 7,502 (16.0%) had started statin treatment and 7,880 (16.8%) had type 2 diabetes.
With a median follow-up of 7.7 years, statin use had no benefit in reducing ASCVD incidence or all-cause mortality for the entire study population, statistical analyses showed.
In participants with diabetes, however, statins did appear protective, at least in the patients aged 75-84 years, with hazard ratios of 0.76 (95% confidence interval, 0.65-0.89) for CVD and 0.84 (95% CI, 0.75-0.94) for all-cause mortality, Dr. Ramos and his colleagues reported.
The 1-year number needed to treat in this 75-84 age group was 164 for atherosclerotic CVD, and 306 for all-cause mortality, they added.
By contrast, the hazard ratios for patients 85 years and older were 0.82 (95% CI, 0.53-1.26) for atherosclerotic CVD, and 1.05 (95% CI, 0.86-1.28) for all-cause mortality, the investigators reported.
The observed reductions in CVD in individuals with diabetes lost statistical significance at age 85 years when investigators looked at hazard ratios for each year of age. Similarly, reductions in all-cause mortality began to lose statistical significance at age 82 years and “definitively disappeared” in those aged 88 years or older, they said.
The project was supported by grants from the Ministerio de Salud, Spain’s Ministry of Science and Innovation through the Carlos III Health Institute and other entities.
Dr. Ramos and his coauthors declared no support in the previous 3 years from any organization related to, or that might have an interest in, the submitted work. They also declared no other relationships or activities that could appear to have influenced the work.
SOURCE: Ramos R et al. BMJ 2018 Sep 5;362:k3359.
Until better evidence is available, patient preference remains the guiding principle in deciding whether to prescribe statins in older adults, according to authors of an accompanying editorial.
“These observational findings are exploratory, however, and should be tested further in randomized trials to rule out any confounding and to study the effect of statins on CVD death, which were not recorded in the database used for this study,” editorial authors Aidan Ryan, MD, Simon Heath, MD, and Paul Cook, MD, wrote in the BMJ.
The ongoing Australian randomized STAREE trial (Statins for Reducing Events in the Elderly) is evaluating primary prevention with atorvastatin 40 mg versus placebo in adults older than 70 years, the authors noted.
While results are awaited, the decision of whether or not to prescribe statins in older adults may depend on their treatment goals, the authors added.
For example, in patients who cite longevity as a treatment goal, the current evidence remains limited that statins as primary prevention could help.
“A patient preference for reduction in myocardial infarction or stroke, however, might help to tilt the balance in favor of statin prescription but the absolute risk reduction, number needed to treat to prevent a CVD event in older patients remains uncertain,” the authors wrote.
Dr. Ryan is an academic clinical fellow and Dr. Cook is a consultant in chemical pathology and metabolic medicine, both at University Hospital Southampton, England. Dr. Heath is in group practice in Warwickshire, England. The authors declared no competing interests.
Until better evidence is available, patient preference remains the guiding principle in deciding whether to prescribe statins in older adults, according to authors of an accompanying editorial.
“These observational findings are exploratory, however, and should be tested further in randomized trials to rule out any confounding and to study the effect of statins on CVD death, which were not recorded in the database used for this study,” editorial authors Aidan Ryan, MD, Simon Heath, MD, and Paul Cook, MD, wrote in the BMJ.
The ongoing Australian randomized STAREE trial (Statins for Reducing Events in the Elderly) is evaluating primary prevention with atorvastatin 40 mg versus placebo in adults older than 70 years, the authors noted.
While results are awaited, the decision of whether or not to prescribe statins in older adults may depend on their treatment goals, the authors added.
For example, in patients who cite longevity as a treatment goal, the current evidence remains limited that statins as primary prevention could help.
“A patient preference for reduction in myocardial infarction or stroke, however, might help to tilt the balance in favor of statin prescription but the absolute risk reduction, number needed to treat to prevent a CVD event in older patients remains uncertain,” the authors wrote.
Dr. Ryan is an academic clinical fellow and Dr. Cook is a consultant in chemical pathology and metabolic medicine, both at University Hospital Southampton, England. Dr. Heath is in group practice in Warwickshire, England. The authors declared no competing interests.
Until better evidence is available, patient preference remains the guiding principle in deciding whether to prescribe statins in older adults, according to authors of an accompanying editorial.
“These observational findings are exploratory, however, and should be tested further in randomized trials to rule out any confounding and to study the effect of statins on CVD death, which were not recorded in the database used for this study,” editorial authors Aidan Ryan, MD, Simon Heath, MD, and Paul Cook, MD, wrote in the BMJ.
The ongoing Australian randomized STAREE trial (Statins for Reducing Events in the Elderly) is evaluating primary prevention with atorvastatin 40 mg versus placebo in adults older than 70 years, the authors noted.
While results are awaited, the decision of whether or not to prescribe statins in older adults may depend on their treatment goals, the authors added.
For example, in patients who cite longevity as a treatment goal, the current evidence remains limited that statins as primary prevention could help.
“A patient preference for reduction in myocardial infarction or stroke, however, might help to tilt the balance in favor of statin prescription but the absolute risk reduction, number needed to treat to prevent a CVD event in older patients remains uncertain,” the authors wrote.
Dr. Ryan is an academic clinical fellow and Dr. Cook is a consultant in chemical pathology and metabolic medicine, both at University Hospital Southampton, England. Dr. Heath is in group practice in Warwickshire, England. The authors declared no competing interests.
Any benefit of statins for primary prevention in older adult populations may depend on whether or not type 2 diabetes is present, results of a retrospective cohort study suggest.
Statins had no protective effect overall in the study, which included adults older than 74 years who had no clinically recognized atherosclerotic cardiovascular disease (ASCVD).
In older patients with diabetes, statins were associated with reductions in CVD incidence and all-cause mortality. However, this benefit was substantially reduced in patients 85 years and older, and completely absent in those over 90, study authors said in the BMJ.
“These results do not support the widespread use of statins in old and very old populations, but they do support treatment in those with type 2 diabetes younger than 85 years,” said Rafael Ramos, MD, of the University of Girona, Spain, and his coauthors.
While meta-analyses support statins as primary prevention of CVD in individuals 65 years or older, evidence is lacking on those older than 74 years, according to the investigators.
Accordingly, they conducted the present retrospective cohort study based on data from a Spanish primary care database that included patient records for more than 6 million people. They looked specifically for individuals aged 75 years or older with no history of ASCVD who had at least one visit between July 2006 and December 2007.
They found 46,864 people meeting those criteria, of whom 7,502 (16.0%) had started statin treatment and 7,880 (16.8%) had type 2 diabetes.
With a median follow-up of 7.7 years, statin use had no benefit in reducing ASCVD incidence or all-cause mortality for the entire study population, statistical analyses showed.
In participants with diabetes, however, statins did appear protective, at least in the patients aged 75-84 years, with hazard ratios of 0.76 (95% confidence interval, 0.65-0.89) for CVD and 0.84 (95% CI, 0.75-0.94) for all-cause mortality, Dr. Ramos and his colleagues reported.
The 1-year number needed to treat in this 75-84 age group was 164 for atherosclerotic CVD, and 306 for all-cause mortality, they added.
By contrast, the hazard ratios for patients 85 years and older were 0.82 (95% CI, 0.53-1.26) for atherosclerotic CVD, and 1.05 (95% CI, 0.86-1.28) for all-cause mortality, the investigators reported.
The observed reductions in CVD in individuals with diabetes lost statistical significance at age 85 years when investigators looked at hazard ratios for each year of age. Similarly, reductions in all-cause mortality began to lose statistical significance at age 82 years and “definitively disappeared” in those aged 88 years or older, they said.
The project was supported by grants from the Ministerio de Salud, Spain’s Ministry of Science and Innovation through the Carlos III Health Institute and other entities.
Dr. Ramos and his coauthors declared no support in the previous 3 years from any organization related to, or that might have an interest in, the submitted work. They also declared no other relationships or activities that could appear to have influenced the work.
SOURCE: Ramos R et al. BMJ 2018 Sep 5;362:k3359.
Any benefit of statins for primary prevention in older adult populations may depend on whether or not type 2 diabetes is present, results of a retrospective cohort study suggest.
Statins had no protective effect overall in the study, which included adults older than 74 years who had no clinically recognized atherosclerotic cardiovascular disease (ASCVD).
In older patients with diabetes, statins were associated with reductions in CVD incidence and all-cause mortality. However, this benefit was substantially reduced in patients 85 years and older, and completely absent in those over 90, study authors said in the BMJ.
“These results do not support the widespread use of statins in old and very old populations, but they do support treatment in those with type 2 diabetes younger than 85 years,” said Rafael Ramos, MD, of the University of Girona, Spain, and his coauthors.
While meta-analyses support statins as primary prevention of CVD in individuals 65 years or older, evidence is lacking on those older than 74 years, according to the investigators.
Accordingly, they conducted the present retrospective cohort study based on data from a Spanish primary care database that included patient records for more than 6 million people. They looked specifically for individuals aged 75 years or older with no history of ASCVD who had at least one visit between July 2006 and December 2007.
They found 46,864 people meeting those criteria, of whom 7,502 (16.0%) had started statin treatment and 7,880 (16.8%) had type 2 diabetes.
With a median follow-up of 7.7 years, statin use had no benefit in reducing ASCVD incidence or all-cause mortality for the entire study population, statistical analyses showed.
In participants with diabetes, however, statins did appear protective, at least in the patients aged 75-84 years, with hazard ratios of 0.76 (95% confidence interval, 0.65-0.89) for CVD and 0.84 (95% CI, 0.75-0.94) for all-cause mortality, Dr. Ramos and his colleagues reported.
The 1-year number needed to treat in this 75-84 age group was 164 for atherosclerotic CVD, and 306 for all-cause mortality, they added.
By contrast, the hazard ratios for patients 85 years and older were 0.82 (95% CI, 0.53-1.26) for atherosclerotic CVD, and 1.05 (95% CI, 0.86-1.28) for all-cause mortality, the investigators reported.
The observed reductions in CVD in individuals with diabetes lost statistical significance at age 85 years when investigators looked at hazard ratios for each year of age. Similarly, reductions in all-cause mortality began to lose statistical significance at age 82 years and “definitively disappeared” in those aged 88 years or older, they said.
The project was supported by grants from the Ministerio de Salud, Spain’s Ministry of Science and Innovation through the Carlos III Health Institute and other entities.
Dr. Ramos and his coauthors declared no support in the previous 3 years from any organization related to, or that might have an interest in, the submitted work. They also declared no other relationships or activities that could appear to have influenced the work.
SOURCE: Ramos R et al. BMJ 2018 Sep 5;362:k3359.
FROM THE BMJ
Key clinical point: Any benefit of statins for primary prevention in older adult populations may depend on whether or not type 2 diabetes is present.
Major finding: In participants with diabetes who were aged 75-84 years, hazard ratios were 0.76 (95% confidence interval, 0.65-0.89) for CVD and 0.84 (95% CI, 0.75-0.94) for all-cause mortality; participants with diabetes aged 85 years and older had markedly less benefit.
Study details: A retrospective cohort study including 46,864 individuals aged 75 years or older with no history of atherosclerotic CVD.
Disclosures: The project was supported by grants from the Ministerio de Salud and Spain’s Ministry of Science and Innovation through the Carlos III Health Institute, among other entities. Authors declared no support, relationships, or activities related to the submitted work.
Source: Ramos R et al. BMJ 2018;362:k3359.
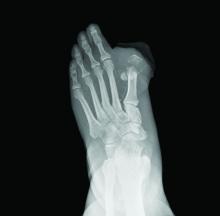
Bone biopsy in suspected osteomyelitis: Culture and histology matter
Diabetic foot ulcers and infections can lead to osteomyelitis, a potentially devastating infection in the bone.
How much of a difference can osteomyelitis make to a patient’s prognosis? A 2014 commentary by Benjamin A. Lipsky, MD, a prominent expert in problems associated with diabetic patients’ feet who’s with the University of Washington, Seattle, hints at the potential toll: “Overall, about 20% of patients with a diabetic foot infection (and over 60% of those with severe infections) have underlying osteomyelitis, which dramatically increases the risk of lower-extremity amputation” (Diabetes Care. 2014 Mar;37[3]:593-5).
Diagnosis of osteomyelitis, which relies on a bone biopsy, is clearly important. But there’s a big gap in diagnostic findings depending on whether doctors request culture or histology results, according to a new study released at the 2018 scientific meeting of the American Diabetes Association (Diabetes. 2018 Jul. doi: 10.2337/db18-110-OR).
In an interview, lead author and podiatrist Peter A. Crisologo, DPM, of University of Texas Southwestern Medical Center, Dallas, explained the study findings and offered guidance for requesting bone biopsies in possible cases of osteomyelitis.
Q: What makes diagnosis and treatment of osteomyelitis unique?
A: In the foot, there’s not a lot of soft tissue between the outside world and your bone. If the wounds on the feet go deep enough, they can spread a bacterial infection to the bone. This changes how foot infections are treated.
If you have a skin infection, it requires 11-12 days of antibiotics. Things start ramping up once you start getting into the bone. You’re talking potential surgery and 6 weeks of antibiotics through IV treatments. This is why it’s really important that you get your diagnosis right.
A lot of people say “I’m going to do the safe thing” and treat a bone infection with an extended course of antibiotics.
That’s not necessarily safe. If you’re overdiagnosing – for example, you identify bacteria that’s just a contaminant – you could put a patient through 6 weeks of IV treatment along with the risks of a PICC (peripherally inserted central catheter ) line infection, complications from IV placement, and complications from the antibiotic.
Also, acute kidney injury develops in at least a third of the patients who undergo 6 weeks of antibiotics. That’s not to mention the cost of the visits and the labs you have to draw. But we don’t want to underdiagnose either. If osteomyelitis is underdiagnosed and then not treated, the infection can smolder and continue to progress and worsen.
Q: Your study looks at the bone biopsy. How does it fit into care of osteomyelitis in the diabetic foot?
A: A bone biopsy is the standard for diagnosis under the guidelines of the Infectious Diseases Society of America/International Working Group on the Diabetic Foot (Clin Infect Dis. 2012;54[12]:e132-73 ).
But beyond that, nobody says anything. Everyone has an operational definition of how a bone biopsy is interpreted, and there’s a need for a consensus on how a bone biopsy can be used to diagnose osteomyelitis.
You’ll get different percentages of your patients diagnosed with osteomyelitis. For example, someone may say the biopsy is only positive if the histology is positive, while another says the histology doesn’t matter if the culture is positive.
Q: Your study looks at histology and culture analyses. What do these reveal?
A: A traditional culture helps you identify the bacteria, as well as guide your treatment when it’s tested against antibiotics.
A traditional histology allows the pathologist to look under a microscope for signs of osteomyelitis: Do they see the right inflammatory cells, white cells, lymphocytes, combinations of cells? Does this look like an acute or chronic osteomyelitis?
Q: Why might it be wise to combine culture and histology analyses?
A: If you have bacteria that’s difficult to culture via traditional methods, it may be a bacteria that doesn’t grow well or easily. If you combine culture with histology, pathologists can look and say, “Your culture was negative but we see these other signs, so we feel this is osteomyelitis.”
Q: Your study examined 35 consecutive patients aged at least 21 years who had moderate or severe infections bone infections in the foot linked with type 1 diabetes (n = 4) or type 2 diabetes (n = 31).
The samples were analyzed via culture, histology, and culture/histology examinations. You also performed genetic sequencing (quantitative polymerase chain reaction targeting 16S rRNA). How does this test fit in to bone biopsies in the clinic?
A: That’s a newer method and not a standard of care treatment for the diabetic foot. This analysis looks at DNA that’s present, bypassing the analysis of difficult-to-grow bacteria.
Q: What did you discover?
A: In this study, histology had the lowest incidence of positively detecting osteomyelitis. (45.7%). The level increases when a culture is taken (68.6% vs. histology; P = .02).
Then it goes up when DNA is used because it’s catching everything (82.9%, P = .001 vs. histology and P = .31 vs. culture).
[The study also found that adding histology to culture or to genetic sequencing did not change positive findings.]
Q: Does the study suggest one approach is better than the others?
A: This paper doesn’t provide enough evidence to use one method over another. The main purpose was to raise the concern that diagnosis can change dramatically depending on how the gold standard of bone biopsy is interpreted.
Q: What were the pros and cons of the genetic sequencing approach?
A: When we use this approach, our positive diagnostic rate significantly increases. But there are also downsides. We don’t know whether the bacteria we see is alive or dead. We just know it was there. So are the patients truly positive? That’s a question we can’t answer.
Genetic sequencing also doesn’t tell us about susceptibilities to antibiotics.
Q: What is the take-home message here for physicians who may order bone biopsies?
A: The thing to do is request both traditional culture and traditional histology.
As far as DNA sequencing, that not something I’d recommend as a standard of care.
Q: Can you comment on cost and insurance coverage for these approaches?
A: As far as I know, genetic sequencing is not covered as it is not standard of care in the diabetic foot and is used mainly for research at this time.
Pathology and culture are standard of care when evaluating for osteomyelitis and should be a covered service. However, a patient should call their insurance company first prior to having the procedure done to see whether it is covered.
Q: What’s next for research in this area?
A: From here, the next step is bigger numbers: Increase the study size and look at this again. Also, we may be able to identify susceptibilities by identifying resistance within the DNA.
Dr. Crisologo and two other study authors report no relevant disclosures. One study author reported various disclosures including research support, consulting, and service on speakers' bureaus.
Diabetic foot ulcers and infections can lead to osteomyelitis, a potentially devastating infection in the bone.
How much of a difference can osteomyelitis make to a patient’s prognosis? A 2014 commentary by Benjamin A. Lipsky, MD, a prominent expert in problems associated with diabetic patients’ feet who’s with the University of Washington, Seattle, hints at the potential toll: “Overall, about 20% of patients with a diabetic foot infection (and over 60% of those with severe infections) have underlying osteomyelitis, which dramatically increases the risk of lower-extremity amputation” (Diabetes Care. 2014 Mar;37[3]:593-5).
Diagnosis of osteomyelitis, which relies on a bone biopsy, is clearly important. But there’s a big gap in diagnostic findings depending on whether doctors request culture or histology results, according to a new study released at the 2018 scientific meeting of the American Diabetes Association (Diabetes. 2018 Jul. doi: 10.2337/db18-110-OR).
In an interview, lead author and podiatrist Peter A. Crisologo, DPM, of University of Texas Southwestern Medical Center, Dallas, explained the study findings and offered guidance for requesting bone biopsies in possible cases of osteomyelitis.
Q: What makes diagnosis and treatment of osteomyelitis unique?
A: In the foot, there’s not a lot of soft tissue between the outside world and your bone. If the wounds on the feet go deep enough, they can spread a bacterial infection to the bone. This changes how foot infections are treated.
If you have a skin infection, it requires 11-12 days of antibiotics. Things start ramping up once you start getting into the bone. You’re talking potential surgery and 6 weeks of antibiotics through IV treatments. This is why it’s really important that you get your diagnosis right.
A lot of people say “I’m going to do the safe thing” and treat a bone infection with an extended course of antibiotics.
That’s not necessarily safe. If you’re overdiagnosing – for example, you identify bacteria that’s just a contaminant – you could put a patient through 6 weeks of IV treatment along with the risks of a PICC (peripherally inserted central catheter ) line infection, complications from IV placement, and complications from the antibiotic.
Also, acute kidney injury develops in at least a third of the patients who undergo 6 weeks of antibiotics. That’s not to mention the cost of the visits and the labs you have to draw. But we don’t want to underdiagnose either. If osteomyelitis is underdiagnosed and then not treated, the infection can smolder and continue to progress and worsen.
Q: Your study looks at the bone biopsy. How does it fit into care of osteomyelitis in the diabetic foot?
A: A bone biopsy is the standard for diagnosis under the guidelines of the Infectious Diseases Society of America/International Working Group on the Diabetic Foot (Clin Infect Dis. 2012;54[12]:e132-73 ).
But beyond that, nobody says anything. Everyone has an operational definition of how a bone biopsy is interpreted, and there’s a need for a consensus on how a bone biopsy can be used to diagnose osteomyelitis.
You’ll get different percentages of your patients diagnosed with osteomyelitis. For example, someone may say the biopsy is only positive if the histology is positive, while another says the histology doesn’t matter if the culture is positive.
Q: Your study looks at histology and culture analyses. What do these reveal?
A: A traditional culture helps you identify the bacteria, as well as guide your treatment when it’s tested against antibiotics.
A traditional histology allows the pathologist to look under a microscope for signs of osteomyelitis: Do they see the right inflammatory cells, white cells, lymphocytes, combinations of cells? Does this look like an acute or chronic osteomyelitis?
Q: Why might it be wise to combine culture and histology analyses?
A: If you have bacteria that’s difficult to culture via traditional methods, it may be a bacteria that doesn’t grow well or easily. If you combine culture with histology, pathologists can look and say, “Your culture was negative but we see these other signs, so we feel this is osteomyelitis.”
Q: Your study examined 35 consecutive patients aged at least 21 years who had moderate or severe infections bone infections in the foot linked with type 1 diabetes (n = 4) or type 2 diabetes (n = 31).
The samples were analyzed via culture, histology, and culture/histology examinations. You also performed genetic sequencing (quantitative polymerase chain reaction targeting 16S rRNA). How does this test fit in to bone biopsies in the clinic?
A: That’s a newer method and not a standard of care treatment for the diabetic foot. This analysis looks at DNA that’s present, bypassing the analysis of difficult-to-grow bacteria.
Q: What did you discover?
A: In this study, histology had the lowest incidence of positively detecting osteomyelitis. (45.7%). The level increases when a culture is taken (68.6% vs. histology; P = .02).
Then it goes up when DNA is used because it’s catching everything (82.9%, P = .001 vs. histology and P = .31 vs. culture).
[The study also found that adding histology to culture or to genetic sequencing did not change positive findings.]
Q: Does the study suggest one approach is better than the others?
A: This paper doesn’t provide enough evidence to use one method over another. The main purpose was to raise the concern that diagnosis can change dramatically depending on how the gold standard of bone biopsy is interpreted.
Q: What were the pros and cons of the genetic sequencing approach?
A: When we use this approach, our positive diagnostic rate significantly increases. But there are also downsides. We don’t know whether the bacteria we see is alive or dead. We just know it was there. So are the patients truly positive? That’s a question we can’t answer.
Genetic sequencing also doesn’t tell us about susceptibilities to antibiotics.
Q: What is the take-home message here for physicians who may order bone biopsies?
A: The thing to do is request both traditional culture and traditional histology.
As far as DNA sequencing, that not something I’d recommend as a standard of care.
Q: Can you comment on cost and insurance coverage for these approaches?
A: As far as I know, genetic sequencing is not covered as it is not standard of care in the diabetic foot and is used mainly for research at this time.
Pathology and culture are standard of care when evaluating for osteomyelitis and should be a covered service. However, a patient should call their insurance company first prior to having the procedure done to see whether it is covered.
Q: What’s next for research in this area?
A: From here, the next step is bigger numbers: Increase the study size and look at this again. Also, we may be able to identify susceptibilities by identifying resistance within the DNA.
Dr. Crisologo and two other study authors report no relevant disclosures. One study author reported various disclosures including research support, consulting, and service on speakers' bureaus.
Diabetic foot ulcers and infections can lead to osteomyelitis, a potentially devastating infection in the bone.
How much of a difference can osteomyelitis make to a patient’s prognosis? A 2014 commentary by Benjamin A. Lipsky, MD, a prominent expert in problems associated with diabetic patients’ feet who’s with the University of Washington, Seattle, hints at the potential toll: “Overall, about 20% of patients with a diabetic foot infection (and over 60% of those with severe infections) have underlying osteomyelitis, which dramatically increases the risk of lower-extremity amputation” (Diabetes Care. 2014 Mar;37[3]:593-5).
Diagnosis of osteomyelitis, which relies on a bone biopsy, is clearly important. But there’s a big gap in diagnostic findings depending on whether doctors request culture or histology results, according to a new study released at the 2018 scientific meeting of the American Diabetes Association (Diabetes. 2018 Jul. doi: 10.2337/db18-110-OR).
In an interview, lead author and podiatrist Peter A. Crisologo, DPM, of University of Texas Southwestern Medical Center, Dallas, explained the study findings and offered guidance for requesting bone biopsies in possible cases of osteomyelitis.
Q: What makes diagnosis and treatment of osteomyelitis unique?
A: In the foot, there’s not a lot of soft tissue between the outside world and your bone. If the wounds on the feet go deep enough, they can spread a bacterial infection to the bone. This changes how foot infections are treated.
If you have a skin infection, it requires 11-12 days of antibiotics. Things start ramping up once you start getting into the bone. You’re talking potential surgery and 6 weeks of antibiotics through IV treatments. This is why it’s really important that you get your diagnosis right.
A lot of people say “I’m going to do the safe thing” and treat a bone infection with an extended course of antibiotics.
That’s not necessarily safe. If you’re overdiagnosing – for example, you identify bacteria that’s just a contaminant – you could put a patient through 6 weeks of IV treatment along with the risks of a PICC (peripherally inserted central catheter ) line infection, complications from IV placement, and complications from the antibiotic.
Also, acute kidney injury develops in at least a third of the patients who undergo 6 weeks of antibiotics. That’s not to mention the cost of the visits and the labs you have to draw. But we don’t want to underdiagnose either. If osteomyelitis is underdiagnosed and then not treated, the infection can smolder and continue to progress and worsen.
Q: Your study looks at the bone biopsy. How does it fit into care of osteomyelitis in the diabetic foot?
A: A bone biopsy is the standard for diagnosis under the guidelines of the Infectious Diseases Society of America/International Working Group on the Diabetic Foot (Clin Infect Dis. 2012;54[12]:e132-73 ).
But beyond that, nobody says anything. Everyone has an operational definition of how a bone biopsy is interpreted, and there’s a need for a consensus on how a bone biopsy can be used to diagnose osteomyelitis.
You’ll get different percentages of your patients diagnosed with osteomyelitis. For example, someone may say the biopsy is only positive if the histology is positive, while another says the histology doesn’t matter if the culture is positive.
Q: Your study looks at histology and culture analyses. What do these reveal?
A: A traditional culture helps you identify the bacteria, as well as guide your treatment when it’s tested against antibiotics.
A traditional histology allows the pathologist to look under a microscope for signs of osteomyelitis: Do they see the right inflammatory cells, white cells, lymphocytes, combinations of cells? Does this look like an acute or chronic osteomyelitis?
Q: Why might it be wise to combine culture and histology analyses?
A: If you have bacteria that’s difficult to culture via traditional methods, it may be a bacteria that doesn’t grow well or easily. If you combine culture with histology, pathologists can look and say, “Your culture was negative but we see these other signs, so we feel this is osteomyelitis.”
Q: Your study examined 35 consecutive patients aged at least 21 years who had moderate or severe infections bone infections in the foot linked with type 1 diabetes (n = 4) or type 2 diabetes (n = 31).
The samples were analyzed via culture, histology, and culture/histology examinations. You also performed genetic sequencing (quantitative polymerase chain reaction targeting 16S rRNA). How does this test fit in to bone biopsies in the clinic?
A: That’s a newer method and not a standard of care treatment for the diabetic foot. This analysis looks at DNA that’s present, bypassing the analysis of difficult-to-grow bacteria.
Q: What did you discover?
A: In this study, histology had the lowest incidence of positively detecting osteomyelitis. (45.7%). The level increases when a culture is taken (68.6% vs. histology; P = .02).
Then it goes up when DNA is used because it’s catching everything (82.9%, P = .001 vs. histology and P = .31 vs. culture).
[The study also found that adding histology to culture or to genetic sequencing did not change positive findings.]
Q: Does the study suggest one approach is better than the others?
A: This paper doesn’t provide enough evidence to use one method over another. The main purpose was to raise the concern that diagnosis can change dramatically depending on how the gold standard of bone biopsy is interpreted.
Q: What were the pros and cons of the genetic sequencing approach?
A: When we use this approach, our positive diagnostic rate significantly increases. But there are also downsides. We don’t know whether the bacteria we see is alive or dead. We just know it was there. So are the patients truly positive? That’s a question we can’t answer.
Genetic sequencing also doesn’t tell us about susceptibilities to antibiotics.
Q: What is the take-home message here for physicians who may order bone biopsies?
A: The thing to do is request both traditional culture and traditional histology.
As far as DNA sequencing, that not something I’d recommend as a standard of care.
Q: Can you comment on cost and insurance coverage for these approaches?
A: As far as I know, genetic sequencing is not covered as it is not standard of care in the diabetic foot and is used mainly for research at this time.
Pathology and culture are standard of care when evaluating for osteomyelitis and should be a covered service. However, a patient should call their insurance company first prior to having the procedure done to see whether it is covered.
Q: What’s next for research in this area?
A: From here, the next step is bigger numbers: Increase the study size and look at this again. Also, we may be able to identify susceptibilities by identifying resistance within the DNA.
Dr. Crisologo and two other study authors report no relevant disclosures. One study author reported various disclosures including research support, consulting, and service on speakers' bureaus.
EXPERT ANALYSIS FROM ADA 2018
Restrictions on EMT glucagon administration should be lifted
ORLANDO – In 42 states, emergency medical technicians aren’t allowed to administer glucagon to patients with severe hypoglycemia, and that’s a problem, according to investigators from the Joslin Diabetes Center, Boston.
Paramedics can administer the drug, but they’re only about a quarter of medical first-responders, so in many parts of the country, ambulance crews show up with only EMTs [emergency medical technicians] on board, and patients have to wait for the ED to get glucagon.
The delay increases the risk of coma, brain damage, and death. Just like in stroke, time is key with severe hypoglycemia. “The minutes really add up,” said lead investigator Nicole Wagner, a one-time researcher at Joslin and now a medical student at Thomas Jefferson Medical College, Philadelphia.
“Increasing the availability of glucagon in the prehospital setting will likely result in reduced cost burden and adverse consequences of severe hypoglycemia. All emergency persons should have access to glucagon along with the training to administer it,” she and her team concluded at the 2018 American Diabetes Association scientific sessions meeting.
The situation is puzzling because glucagon has a good safety profile – the main concern is nausea – and families of diabetics, at least at Joslin, are taught all the time how to mix and inject it.
One of the investigators, a former EMT, ran into the problem when he was working in New York, so the team wanted to find out how widespread it is. They called emergency medical services offices in all 50 states and asked about their glucagon protocols. “We were surprised [by what we found]. We didn’t expect it,” Ms. Wagner said.
In some states, EMTs aren’t even allowed to check blood glucose.
In the eight states that allow EMTs to administer glucagon – Alaska, Montana, Minnesota, Wisconsin, Illinois, Kansas, Virginia, and Rhode Island – it seemed that someone at some point got fired up and lobbied for change. In the other states seemed to have fallen through the cracks. “When we pressed the offices a little bit, we” were told about bureaucratic red tape, “and that maybe it’s something that would be considered down the line,” Ms. Wagner said.
The Joslin team wants to get proactive. Joslin is one of the nation’s leading diabetes institutions, and it has worked on advocacy before. EMT glucagon might be its next campaign. “It’s something we feel should be addressed. We could work with the EMT community to push this through,” she said.
Meanwhile, the glucagon autoinjectors and nasal sprays companies are working on might alleviate the problem. Time will tell.
The team also looked at the 89,263 cases in the National Emergency Management Information System from 2013-2015 in which glucagon was administered; it’s likely the number would have been far higher if EMTs were allowed to give the drug.
Ambulances showed up an average of 15.34 minutes after the first call. Meanwhile, there were 3,944 adverse events with glucagon, mostly nausea.
Less than half of the cases were dispatched correctly as “diabetic problems,” so it’s likely that EMTs who couldn’t give glucagon handled the call.
There was no industry funding for the work. Ms. Wagner had no disclosures.
SOURCE: Wagner NE et al. 2018 American Diabetes Association scientific sessions abstract 387-P
ORLANDO – In 42 states, emergency medical technicians aren’t allowed to administer glucagon to patients with severe hypoglycemia, and that’s a problem, according to investigators from the Joslin Diabetes Center, Boston.
Paramedics can administer the drug, but they’re only about a quarter of medical first-responders, so in many parts of the country, ambulance crews show up with only EMTs [emergency medical technicians] on board, and patients have to wait for the ED to get glucagon.
The delay increases the risk of coma, brain damage, and death. Just like in stroke, time is key with severe hypoglycemia. “The minutes really add up,” said lead investigator Nicole Wagner, a one-time researcher at Joslin and now a medical student at Thomas Jefferson Medical College, Philadelphia.
“Increasing the availability of glucagon in the prehospital setting will likely result in reduced cost burden and adverse consequences of severe hypoglycemia. All emergency persons should have access to glucagon along with the training to administer it,” she and her team concluded at the 2018 American Diabetes Association scientific sessions meeting.
The situation is puzzling because glucagon has a good safety profile – the main concern is nausea – and families of diabetics, at least at Joslin, are taught all the time how to mix and inject it.
One of the investigators, a former EMT, ran into the problem when he was working in New York, so the team wanted to find out how widespread it is. They called emergency medical services offices in all 50 states and asked about their glucagon protocols. “We were surprised [by what we found]. We didn’t expect it,” Ms. Wagner said.
In some states, EMTs aren’t even allowed to check blood glucose.
In the eight states that allow EMTs to administer glucagon – Alaska, Montana, Minnesota, Wisconsin, Illinois, Kansas, Virginia, and Rhode Island – it seemed that someone at some point got fired up and lobbied for change. In the other states seemed to have fallen through the cracks. “When we pressed the offices a little bit, we” were told about bureaucratic red tape, “and that maybe it’s something that would be considered down the line,” Ms. Wagner said.
The Joslin team wants to get proactive. Joslin is one of the nation’s leading diabetes institutions, and it has worked on advocacy before. EMT glucagon might be its next campaign. “It’s something we feel should be addressed. We could work with the EMT community to push this through,” she said.
Meanwhile, the glucagon autoinjectors and nasal sprays companies are working on might alleviate the problem. Time will tell.
The team also looked at the 89,263 cases in the National Emergency Management Information System from 2013-2015 in which glucagon was administered; it’s likely the number would have been far higher if EMTs were allowed to give the drug.
Ambulances showed up an average of 15.34 minutes after the first call. Meanwhile, there were 3,944 adverse events with glucagon, mostly nausea.
Less than half of the cases were dispatched correctly as “diabetic problems,” so it’s likely that EMTs who couldn’t give glucagon handled the call.
There was no industry funding for the work. Ms. Wagner had no disclosures.
SOURCE: Wagner NE et al. 2018 American Diabetes Association scientific sessions abstract 387-P
ORLANDO – In 42 states, emergency medical technicians aren’t allowed to administer glucagon to patients with severe hypoglycemia, and that’s a problem, according to investigators from the Joslin Diabetes Center, Boston.
Paramedics can administer the drug, but they’re only about a quarter of medical first-responders, so in many parts of the country, ambulance crews show up with only EMTs [emergency medical technicians] on board, and patients have to wait for the ED to get glucagon.
The delay increases the risk of coma, brain damage, and death. Just like in stroke, time is key with severe hypoglycemia. “The minutes really add up,” said lead investigator Nicole Wagner, a one-time researcher at Joslin and now a medical student at Thomas Jefferson Medical College, Philadelphia.
“Increasing the availability of glucagon in the prehospital setting will likely result in reduced cost burden and adverse consequences of severe hypoglycemia. All emergency persons should have access to glucagon along with the training to administer it,” she and her team concluded at the 2018 American Diabetes Association scientific sessions meeting.
The situation is puzzling because glucagon has a good safety profile – the main concern is nausea – and families of diabetics, at least at Joslin, are taught all the time how to mix and inject it.
One of the investigators, a former EMT, ran into the problem when he was working in New York, so the team wanted to find out how widespread it is. They called emergency medical services offices in all 50 states and asked about their glucagon protocols. “We were surprised [by what we found]. We didn’t expect it,” Ms. Wagner said.
In some states, EMTs aren’t even allowed to check blood glucose.
In the eight states that allow EMTs to administer glucagon – Alaska, Montana, Minnesota, Wisconsin, Illinois, Kansas, Virginia, and Rhode Island – it seemed that someone at some point got fired up and lobbied for change. In the other states seemed to have fallen through the cracks. “When we pressed the offices a little bit, we” were told about bureaucratic red tape, “and that maybe it’s something that would be considered down the line,” Ms. Wagner said.
The Joslin team wants to get proactive. Joslin is one of the nation’s leading diabetes institutions, and it has worked on advocacy before. EMT glucagon might be its next campaign. “It’s something we feel should be addressed. We could work with the EMT community to push this through,” she said.
Meanwhile, the glucagon autoinjectors and nasal sprays companies are working on might alleviate the problem. Time will tell.
The team also looked at the 89,263 cases in the National Emergency Management Information System from 2013-2015 in which glucagon was administered; it’s likely the number would have been far higher if EMTs were allowed to give the drug.
Ambulances showed up an average of 15.34 minutes after the first call. Meanwhile, there were 3,944 adverse events with glucagon, mostly nausea.
Less than half of the cases were dispatched correctly as “diabetic problems,” so it’s likely that EMTs who couldn’t give glucagon handled the call.
There was no industry funding for the work. Ms. Wagner had no disclosures.
SOURCE: Wagner NE et al. 2018 American Diabetes Association scientific sessions abstract 387-P
REPORTING FROM ADA 2018
Key clinical point: States need to allow emergency medical technicians to administer glucagon.
Major finding: Only eight do; elsewhere, delays mean that patients face coma, brain damage, and death.
Study details: Query of emergency medical headquarters in all 50 states
Disclosures: There was no industry funding for the work. The presenter didn’t have any disclosures.
Source: Wagner NE et al. 2018 American Diabetes Association scientific sessions abstract 387-P
Early HbA1c levels may predict gestational diabetes
according to a case-control study drawn from the prospective NICHD Fetal Growth Studies-Singleton cohort.
Women who went on to develop GDM had higher HbA1c levels, and measuring this factor improved prediction when added to traditional GDM risk factors, Stefanie N. Hinkle, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and her associates reported in Scientific Reports.
A previous report showed that GDM-associated fetal overgrowth begins before GDM diagnosis, which suggests a need to identify GDM earlier in pregnancy (Int J Epidemiol. 2018 Feb;47[1]:25–25l).
HbA1c is already used to screen for type 2 diabetes mellitus outside of pregnancy. Previous studies that examined its potential utility for GDM have focused on high-risk patients, examined an HbA1c threshold of 5.7%, used GDM during the first trimester only as the outcome, or had other limitations. There is little research on HbA1c levels and GDM in population-based samples.
Dr. Hinkle and her associates conducted a secondary analysis of a case-control study that involved 2,334 low-risk pregnancies among nonobese women and 468 low-risk pregnancies among obese women (n = 2,802) at 12 U.S. centers. The women were recruited during gestational weeks 8-13 and then followed until the end of the pregnancy. The researchers did a nested GDM case-control study of 107 GDM cases and 214 matched non-GDM controls.
GDM cases had higher HbA1c levels throughout their pregnancies (P less than .03). The researchers found a linear association between HbA1c at enrollment and GDM risk (P = .001). Women with a first trimester HbA1c level of 5.7% had an odds ratio of 2.73 for GDM, compared with women at the median level of 5.2%.
In the adjusted model, for each increment of 0.1% at enrollment, women had an OR of 1.22 for GDM (P less than .001). For every 0.1% difference between HbA1c levels at enrollment and the second visit (24-29 weeks), the OR was 1.21 (P = .04). When the researchers excluded women who were obese, had smoked, had prior GDM, or had a hematologic disorder, the OR per 0.1% increase was similar (OR, 1.23; 95% confidence interval, 1.10-1.38).
A potential optimal cutoff point is 5.1%, which had a sensitivity of 47% (95% CI, 34%-60%) and a specificity of 79% (95% CI, 62%-88%). At 5.7%, which is used as the cutoff for prediabetes in nonpregnant women, the sensitivity was 21% (95% CI, 8%-36%) and the specificity was 95% (95% CI, 91%-99%).
When the model was added to conventional risk factors such as age, race/ethnicity, being overweight or obese before pregnancy, family history of diabetes, previous GDM, and nulliparity, the area under the curve of HbA1c levels at enrollment increased from 0.59 to 0.65.
Robert Atlas, MD, chair of obstetrics and gynecology at Mercy Medical Center, Baltimore, said in an interview, “This is just the first study that needs to be replicated in different patient populations. No one has looked at a continuum of HbA1c and what value above puts you at an increased risk. I think this is a very powerful study that sets the stage for further investigation into how to utilize HbA1c in a better way than we’ve ever looked at it before.”
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural funding and by American Recovery and Reinvestment Act funding. Dr. Hinkle and her associates had no relevant financial disclosures. Dr. Atlas had no relevant financial disclosures.
SOURCE: Hinkle SN et al. Sci Rep. 2018 Aug 16;8(1):12249.
“I don’t think clinically this particular finding can make a change right off the bat. What it shows us is we need to understand, at a deeper level, what mechanistic processes might be going on. What underlying process is going on in the woman that looks apparently normal but has a slightly elevated HbA1c. What are the factors that are making this woman become at (greater) risk?”
Suchi Chandrasekaran, MD, MSCE, is an assistant professor of obstetrics and gynecology in the division of maternal fetal medicine at the University of Washington, Yakima. She had no relevant financial disclosures. She was not associated with the study.
“I don’t think clinically this particular finding can make a change right off the bat. What it shows us is we need to understand, at a deeper level, what mechanistic processes might be going on. What underlying process is going on in the woman that looks apparently normal but has a slightly elevated HbA1c. What are the factors that are making this woman become at (greater) risk?”
Suchi Chandrasekaran, MD, MSCE, is an assistant professor of obstetrics and gynecology in the division of maternal fetal medicine at the University of Washington, Yakima. She had no relevant financial disclosures. She was not associated with the study.
“I don’t think clinically this particular finding can make a change right off the bat. What it shows us is we need to understand, at a deeper level, what mechanistic processes might be going on. What underlying process is going on in the woman that looks apparently normal but has a slightly elevated HbA1c. What are the factors that are making this woman become at (greater) risk?”
Suchi Chandrasekaran, MD, MSCE, is an assistant professor of obstetrics and gynecology in the division of maternal fetal medicine at the University of Washington, Yakima. She had no relevant financial disclosures. She was not associated with the study.
according to a case-control study drawn from the prospective NICHD Fetal Growth Studies-Singleton cohort.
Women who went on to develop GDM had higher HbA1c levels, and measuring this factor improved prediction when added to traditional GDM risk factors, Stefanie N. Hinkle, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and her associates reported in Scientific Reports.
A previous report showed that GDM-associated fetal overgrowth begins before GDM diagnosis, which suggests a need to identify GDM earlier in pregnancy (Int J Epidemiol. 2018 Feb;47[1]:25–25l).
HbA1c is already used to screen for type 2 diabetes mellitus outside of pregnancy. Previous studies that examined its potential utility for GDM have focused on high-risk patients, examined an HbA1c threshold of 5.7%, used GDM during the first trimester only as the outcome, or had other limitations. There is little research on HbA1c levels and GDM in population-based samples.
Dr. Hinkle and her associates conducted a secondary analysis of a case-control study that involved 2,334 low-risk pregnancies among nonobese women and 468 low-risk pregnancies among obese women (n = 2,802) at 12 U.S. centers. The women were recruited during gestational weeks 8-13 and then followed until the end of the pregnancy. The researchers did a nested GDM case-control study of 107 GDM cases and 214 matched non-GDM controls.
GDM cases had higher HbA1c levels throughout their pregnancies (P less than .03). The researchers found a linear association between HbA1c at enrollment and GDM risk (P = .001). Women with a first trimester HbA1c level of 5.7% had an odds ratio of 2.73 for GDM, compared with women at the median level of 5.2%.
In the adjusted model, for each increment of 0.1% at enrollment, women had an OR of 1.22 for GDM (P less than .001). For every 0.1% difference between HbA1c levels at enrollment and the second visit (24-29 weeks), the OR was 1.21 (P = .04). When the researchers excluded women who were obese, had smoked, had prior GDM, or had a hematologic disorder, the OR per 0.1% increase was similar (OR, 1.23; 95% confidence interval, 1.10-1.38).
A potential optimal cutoff point is 5.1%, which had a sensitivity of 47% (95% CI, 34%-60%) and a specificity of 79% (95% CI, 62%-88%). At 5.7%, which is used as the cutoff for prediabetes in nonpregnant women, the sensitivity was 21% (95% CI, 8%-36%) and the specificity was 95% (95% CI, 91%-99%).
When the model was added to conventional risk factors such as age, race/ethnicity, being overweight or obese before pregnancy, family history of diabetes, previous GDM, and nulliparity, the area under the curve of HbA1c levels at enrollment increased from 0.59 to 0.65.
Robert Atlas, MD, chair of obstetrics and gynecology at Mercy Medical Center, Baltimore, said in an interview, “This is just the first study that needs to be replicated in different patient populations. No one has looked at a continuum of HbA1c and what value above puts you at an increased risk. I think this is a very powerful study that sets the stage for further investigation into how to utilize HbA1c in a better way than we’ve ever looked at it before.”
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural funding and by American Recovery and Reinvestment Act funding. Dr. Hinkle and her associates had no relevant financial disclosures. Dr. Atlas had no relevant financial disclosures.
SOURCE: Hinkle SN et al. Sci Rep. 2018 Aug 16;8(1):12249.
according to a case-control study drawn from the prospective NICHD Fetal Growth Studies-Singleton cohort.
Women who went on to develop GDM had higher HbA1c levels, and measuring this factor improved prediction when added to traditional GDM risk factors, Stefanie N. Hinkle, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and her associates reported in Scientific Reports.
A previous report showed that GDM-associated fetal overgrowth begins before GDM diagnosis, which suggests a need to identify GDM earlier in pregnancy (Int J Epidemiol. 2018 Feb;47[1]:25–25l).
HbA1c is already used to screen for type 2 diabetes mellitus outside of pregnancy. Previous studies that examined its potential utility for GDM have focused on high-risk patients, examined an HbA1c threshold of 5.7%, used GDM during the first trimester only as the outcome, or had other limitations. There is little research on HbA1c levels and GDM in population-based samples.
Dr. Hinkle and her associates conducted a secondary analysis of a case-control study that involved 2,334 low-risk pregnancies among nonobese women and 468 low-risk pregnancies among obese women (n = 2,802) at 12 U.S. centers. The women were recruited during gestational weeks 8-13 and then followed until the end of the pregnancy. The researchers did a nested GDM case-control study of 107 GDM cases and 214 matched non-GDM controls.
GDM cases had higher HbA1c levels throughout their pregnancies (P less than .03). The researchers found a linear association between HbA1c at enrollment and GDM risk (P = .001). Women with a first trimester HbA1c level of 5.7% had an odds ratio of 2.73 for GDM, compared with women at the median level of 5.2%.
In the adjusted model, for each increment of 0.1% at enrollment, women had an OR of 1.22 for GDM (P less than .001). For every 0.1% difference between HbA1c levels at enrollment and the second visit (24-29 weeks), the OR was 1.21 (P = .04). When the researchers excluded women who were obese, had smoked, had prior GDM, or had a hematologic disorder, the OR per 0.1% increase was similar (OR, 1.23; 95% confidence interval, 1.10-1.38).
A potential optimal cutoff point is 5.1%, which had a sensitivity of 47% (95% CI, 34%-60%) and a specificity of 79% (95% CI, 62%-88%). At 5.7%, which is used as the cutoff for prediabetes in nonpregnant women, the sensitivity was 21% (95% CI, 8%-36%) and the specificity was 95% (95% CI, 91%-99%).
When the model was added to conventional risk factors such as age, race/ethnicity, being overweight or obese before pregnancy, family history of diabetes, previous GDM, and nulliparity, the area under the curve of HbA1c levels at enrollment increased from 0.59 to 0.65.
Robert Atlas, MD, chair of obstetrics and gynecology at Mercy Medical Center, Baltimore, said in an interview, “This is just the first study that needs to be replicated in different patient populations. No one has looked at a continuum of HbA1c and what value above puts you at an increased risk. I think this is a very powerful study that sets the stage for further investigation into how to utilize HbA1c in a better way than we’ve ever looked at it before.”
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural funding and by American Recovery and Reinvestment Act funding. Dr. Hinkle and her associates had no relevant financial disclosures. Dr. Atlas had no relevant financial disclosures.
SOURCE: Hinkle SN et al. Sci Rep. 2018 Aug 16;8(1):12249.
FROM SCIENTIFIC REPORTS
Key clinical point: First trimester HbA1c levels could improve early diagnosis.
Major finding: HbA1c levels of 5.1% predicted later gestational diabetes with a sensitivity of 47% and a specificity of 79%
Study details: Nested case-control study of 107 gestational diabetes cases and 214 controls.
Disclosures: The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural funding and by the American Recovery and Reinvestment Act funding. .
Source: Hinkle SN et al. Sci Rep. 2018 Aug 16;8(1):12249.
ADA/EASD: Draft consensus statement on managing hyperglycemia in T2DM
ORLANDO – according to a draft consensus report on the management of hyperglycemia in patients with type 2 diabetes mellitus (T2DM).
The report, a project of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD), is currently under review and will be presented in final form Oct. 5 at the EASD annual meeting in Berlin.
The current draft calls, generally, for the initial use of metformin followed by the addition of antihyperglycemic medications based on patient comorbidities and concerns “as we await answers to the many questions that remain,” John Buse, MD, PhD, cochair of the consensus statement writing group, said during a summary of the draft recommendations at the annual scientific sessions of the ADA.
The first step, however, is to assess key patient characteristics; these can include comorbidities, clinical characteristics, issues such as motivation and depression, and cultural and socio-economic context, Deborah J. Wexler, MD, 1 of 10 writing group members, said during the same presentation.
Patients with ASCVD or heart failure
Given new evidence from trials such as EMPA-REG and LEADER showing outcomes benefits with the use of specific antihyperglycemic medications in patients with established atherosclerotic cardiovascular disease (ASCVD), an important early step in the proposed approach is to consider the presence or absence of ASCVD and heart failure, said Dr. Wexler of Massachusetts General Hospital, Boston.
“The presence of cardiovascular disease is a compelling indication for the selection of certain glucose-lowering drugs,” she said.
The draft consensus recommendation in this regard – a new recommendation since the last consensus report in 2015 – differentiates between T2DM patients in whom ASCVD predominates and those in whom heart failure predominates.
“What’s new since 2015 is that we recommend that these comorbidities be considered first and foremost because they do influence the choice of a particular glucose-lowering medication, and the recommendation is that, among patients with type 2 diabetes with established ASCVD, sodium-glucose cotransporter 2 [SGLT2] inhibitors or glucagonlike peptide 1 [GLP-1] receptor agonists with proven cardiovascular benefit are recommended as part of glycemic management,” she said.
However, it is important to note that ASCVD is defined differently across trials, and patients considered in the development of these recommendations are those with much higher cardiovascular risk than the average patient with T2DM, she added.
“It’s also important to keep in mind that each cardiovascular outcomes trial, while large, is but a single experiment ... and we don’t have the benefit of replication,” she said, noting that it is not always clear whether differences in trial findings within a drug class are related to trial design or true differences in individual medications.
“So we try to read into them and interpret these data, but it’s just important to consider that ... and when evidence suggests a hierarchy, we noted that,” she said.
That said, if ASCVD predominates, the recommendation is for treatment with either a GLP-1 receptor agonist with proven cardiovascular benefit (favoring liraglutide over semaglutide and over long-acting exenatide) or an SGLT2 inhibitor with proven cardiovascular benefit if estimated glomerular filtration (eGFR) is adequate (favoring empagliflozin over canagliflozin).
These recommendations are based on the LEADER trial finding of significant improvement in the primary outcome of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke with liraglutide vs. placebo (hazard ratio, 0.87; number needed to treat [NNT], 52 over 3.8 years) and on the EMPA-REG trial finding of significant improvement in the same primary outcome with empagliflozin vs. placebo (HR, 0.86; NNT, 62 over 3.1 years).
Keep in mind that there is no evidence of cardiovascular benefit from these treatments in patients at lower risk and also that the “expensive and complicated” combination of an SGLT2 inhibitor and a GLP-1 receptor agonist has not been tested in cardiovascular outcomes trials and there is no evidence of additional benefit from a cardiovascular perspective with this combination, Dr. Wexler said.
If heart failure predominates, the recommendations call for consideration of an SGLT2 inhibitor as part of the treatment strategy because patients with T2DM are at increased risk for heart failure with reduced or preserved ejection fraction and because significant, consistent reduction in hospitalizations for heart failure were seen in SGLT2 inhibitor trials, writing group member Peter Rossing, MD, explained during the session; he noted, however, that the trials were not designed to adjudicate heart failure and that most patients did not have clinical heart failure at baseline.
In those in whom SGLT2 inhibitors are contraindicated – because of impaired renal function, for example – a GLP-1 receptor agonist with proven cardiovascular benefit is recommended.
“Then we suggest that if you are still not at [hemoglobin A1c] target, you should avoid thiazolidinediones [TZD] because of the risk of fluid overload, and you could then consider, if needed, combining an SGLT2-inhibitor and a GLP-1 receptor agonist ... or you could use a [dipeptidyl peptidase–4 (DPP-4) inhibitor] if you are not on an GLP-1 receptor agonist. And we point out that saxagliptin has unfavorable data on heart failure,” Dr. Rossing of Steno Diabetes Center, Copenhagen, said, noting that basal insulin or sulfonylurea are other alternative options.
In EMPA-REG, hospitalization for heart failure was reduced by 35% with empagliflozin vs. placebo (HR, 0.65; NNT, 71 over 3 years), and similar findings were seen in the CANVAS trial. In LEADER, a nonsignificant 13% reduction was seen in hospitalization for heart failure with liraglutide vs. placebo (HR, 0.87). However, this was a secondary outcome; ongoing studies are addressing heart failure as a primary outcome, Dr. Rossing said.
The report also includes a recommendation that, for patients with chronic kidney disease and high cardiovascular risk, GLP-1 receptor agonists and SGLT2 inhibitors can be used but with dose reductions for some medications – several of which have demonstrated renal benefit and cardiovascular benefits in those populations and can be considered as part of treatment.
Lifestyle management and medication
With respect to lifestyle management and pharmacologic treatment, the proposed recommendations, which are based on several large trials, state that an individualized program of medical nutritional therapy should be offered to all patients and that all overweight and obese patients with diabetes should be advised of the health benefits of weight loss. They also should be encouraged to engage in a program of intensive lifestyle management, which may include food substitution, writing group member Walter Kernan, MD, said at the meeting.
In the DiRECT Trial, the average weight loss was about 10 kg in an intervention group that had complete food replacement for 3 months followed by gradual food reintroduction and ongoing counseling versus about 1 kg in controls, and the diabetes remission rate at 1 year was 46% versus 4%, respectively, said Dr. Kernan of Yale University, New Haven, Conn.
In addition, intentional physical activity is known to improve glycemic control and should be encouraged in all patients with T2DM, he said.
“The foundation of hyperglycemia treatment in type 2 diabetes is, for sure, lifestyle modification,” said group member Geltrude Mingrone, MD, of Catholic University of the Sacred Heart in Rome. “Those patients who are very well motivated and adherent to the [recommendations] can achieve very good results,” she added.
In those in whom lifestyle modification fails to lead to adequate improvement, “a pretty large medication portfolio is available,” she said, adding that the choice of treatment should be based on safety, efficacy, cost, and convenience, factors which are described in the statement.
The hope is that the final consensus statement will make it easier to navigate them, she said.
Finally, bariatric surgery can be considered a very effective salvage therapy, Dr. Mingrone said, noting that only lifestyle modification or bariatric surgery will lead to diabetes remission.
The draft consensus recommendation for bariatric surgery is to consider it in patients with T2DM and a body mass index of 40 kg/m2 or greater (37.5 or greater in those of Asian ancestry), regardless of the level of glycemic control, and in those with BMI of 35-39.9 (32.5-37.4 in those of Asian ancestry) when hyperglycemia is inadequately controlled despite lifestyle and optimal medical therapy.
Decision making and injectable therapies
The statement includes decision-making strategies and algorithms for treatment and addresses issues such as choosing antihyperglycemic medications when weight is a concern (consider an SGLT2 inhibitor or a GLP-1 receptor agonist with good efficacy for weight loss to start – or a combination of both if HbA1c is not on target), when minimizing hypoglycemia is the priority (consider adding an SGLT2 inhibitors, GLP-1 receptor agonist, a TZD, or a DPP-4 inhibitor to metformin therapy to start, followed by reintensification of lifestyle modifications and combination therapies if HbA1c is above target), and when drug costs need to be minimized, as well as when and how to initiate injectable therapies, according to writing group members David D’Alessio, MD, of Duke University, Durham, N.C, and Chantal Mathieu, MD, of Katholieke Universteit Leuven (Belgium).
The draft consensus recommendation regarding the latter is that, in patients who need the greater glucose-lowering effect of an injectable medication, GLP-1 receptor agonists should be considered as the first choice and that, when insulin is the medication of choice on the basis of clinical characteristics, basal insulin is preferred. Additionally, in patients who are unable to maintain glycemic targets on basal insulin in combination with oral medication, intensification with a GLP-1 receptor agonist, SGLT2 inhibitor, or prandial insulin can be considered.
This recommendation is based on “overwhelming evidence that GLP-1 receptor agonists give you HbA1c lowering in the same range as basal insulin but can do so without hypoglycemia and with weight loss, in contrast to weight gain with most insulin preparations,” Dr. Mathieu noted.
The bottom line, however, is that “the patient is at the center of everything and ... should become an integral part of the team treating this patient,” Dr. Mathieu said.
Knowledge gaps
In a review of remaining knowledge gaps regarding glycemic control in T2DM, Dr. Buse said that, while the tools available to treat and prevent diabetes are vastly improved, implementation of effective innovation has lagged behind and “requires fundamental changes in health care policy and societal approaches to wellness.”
Additionally, the management of overweight and obesity is clearly inadequate and requires much greater emphasis on lifestyle techniques, behavioral approaches, medication, and surgery, said Dr. Buse, who is the Verne S. Caviness distinguished professor and chief of the division of endocrinology, as well as director of the Diabetes Center, at the University of North Carolina, Chapel Hill.
These and numerous other knowledge gaps (with respect to preserving and enhancing beta-cell function, incorporating personalized medicine, the value of combinations for additive benefit, the identification of biomarkers, the use of early intensive therapy, metabolic surgery decision making, the value of self-monitoring of blood glucose, and the need for better drugs – including those for the primary prevention of cardiovascular disease) need to be addressed and “require additional investment in basic, translational, clinical, and implementation research,” he said.
“More time- and cost-efficient research paradigms to address patient-centered endpoints are needed through regulatory reform and leveraging informatics and coordinated learning health care systems. Additionally, the increasing burden of cardiometabolic disease is an existential threat to society,” he said, stressing that “urgent attention to improve prevention and treatment is of the essence.”
Consensus statement development
The draft consensus statement is the work of group members selected by the ADA and EASD to ensure regional representation (five each from the United States and Europe). The group had two face-to-face meetings, as well as regular teleconferences; the members also conducted a “robust evidence review, which informed the content,” said group cochair Melanie J. Davies, MD, of the University of Leicester (U.K.).
The group reviewed randomized controlled trials, systematic reviews, and meta-analyses published from Jan. 1, 2014, (to capture research that may have been missed during development of the 2015 statement) through Feb. 28, 2018.
The process was based on consensus among members; areas of disagreement were voted on and the group proceeded according to 60% supermajority votes.
The updates were mainly based on research generated over the past 2 years, Dr. Davies said.
The final draft will be submitted for publication to Diabetes Care and Diabetologia.
Dr. Buse reported relationships (research support, stock ownership, and/or advisory roles) with Adocia, AstraZeneca, Boehringer Ingelheim, Dexcom, Elcelyx Therapeutics, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Lexicon Pharmaceuticals, Mellitus Health, Metavention, NovaTarg Therapeutics, Novo Nordisk, PhaseBio Pharmaceuticals, Sanofi, Senseonics, Theracos, and vTv Therapeutics; Dr. Wexler reported having no disclosures; Dr. Rossing reported relationships (consultancy and/or speaking fees, research grants, stock ownership) with AbbVie, Astellas Pharma, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, MDS Medical, Novo Nordisk, and Sanofi; Dr. Kernan reported having no disclosures; Dr. Mingrone is a consultant for Novo Nordisk, Fractyl, and Johnson & Johnson; Dr. D’Alessio reported advisory board membership with and/or research support from Eli Lilly, Intarcia Therapeutics, Merck, and Novo Nordisk; Dr. Mathieu reported relationships (advisory board membership, speaker’s bureau, and/or research support) with Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Hanmi Pharmaceuticals, Intrexon, Janssen Pharmaceuticals, MannKind, Medtronic, Merck Sharp & Dohme (MSD), Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi, and UCB; Dr. Davies reported relationships (advisory panel, consulting, research support, and/or speaker’s bureau) with AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Intarcia Therapeutics, Janssen Pharmaceuticals, MSD, Mitsubishi Tanabi Pharma, Novo Nordisk, Sanofi, and Servier.
ORLANDO – according to a draft consensus report on the management of hyperglycemia in patients with type 2 diabetes mellitus (T2DM).
The report, a project of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD), is currently under review and will be presented in final form Oct. 5 at the EASD annual meeting in Berlin.
The current draft calls, generally, for the initial use of metformin followed by the addition of antihyperglycemic medications based on patient comorbidities and concerns “as we await answers to the many questions that remain,” John Buse, MD, PhD, cochair of the consensus statement writing group, said during a summary of the draft recommendations at the annual scientific sessions of the ADA.
The first step, however, is to assess key patient characteristics; these can include comorbidities, clinical characteristics, issues such as motivation and depression, and cultural and socio-economic context, Deborah J. Wexler, MD, 1 of 10 writing group members, said during the same presentation.
Patients with ASCVD or heart failure
Given new evidence from trials such as EMPA-REG and LEADER showing outcomes benefits with the use of specific antihyperglycemic medications in patients with established atherosclerotic cardiovascular disease (ASCVD), an important early step in the proposed approach is to consider the presence or absence of ASCVD and heart failure, said Dr. Wexler of Massachusetts General Hospital, Boston.
“The presence of cardiovascular disease is a compelling indication for the selection of certain glucose-lowering drugs,” she said.
The draft consensus recommendation in this regard – a new recommendation since the last consensus report in 2015 – differentiates between T2DM patients in whom ASCVD predominates and those in whom heart failure predominates.
“What’s new since 2015 is that we recommend that these comorbidities be considered first and foremost because they do influence the choice of a particular glucose-lowering medication, and the recommendation is that, among patients with type 2 diabetes with established ASCVD, sodium-glucose cotransporter 2 [SGLT2] inhibitors or glucagonlike peptide 1 [GLP-1] receptor agonists with proven cardiovascular benefit are recommended as part of glycemic management,” she said.
However, it is important to note that ASCVD is defined differently across trials, and patients considered in the development of these recommendations are those with much higher cardiovascular risk than the average patient with T2DM, she added.
“It’s also important to keep in mind that each cardiovascular outcomes trial, while large, is but a single experiment ... and we don’t have the benefit of replication,” she said, noting that it is not always clear whether differences in trial findings within a drug class are related to trial design or true differences in individual medications.
“So we try to read into them and interpret these data, but it’s just important to consider that ... and when evidence suggests a hierarchy, we noted that,” she said.
That said, if ASCVD predominates, the recommendation is for treatment with either a GLP-1 receptor agonist with proven cardiovascular benefit (favoring liraglutide over semaglutide and over long-acting exenatide) or an SGLT2 inhibitor with proven cardiovascular benefit if estimated glomerular filtration (eGFR) is adequate (favoring empagliflozin over canagliflozin).
These recommendations are based on the LEADER trial finding of significant improvement in the primary outcome of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke with liraglutide vs. placebo (hazard ratio, 0.87; number needed to treat [NNT], 52 over 3.8 years) and on the EMPA-REG trial finding of significant improvement in the same primary outcome with empagliflozin vs. placebo (HR, 0.86; NNT, 62 over 3.1 years).
Keep in mind that there is no evidence of cardiovascular benefit from these treatments in patients at lower risk and also that the “expensive and complicated” combination of an SGLT2 inhibitor and a GLP-1 receptor agonist has not been tested in cardiovascular outcomes trials and there is no evidence of additional benefit from a cardiovascular perspective with this combination, Dr. Wexler said.
If heart failure predominates, the recommendations call for consideration of an SGLT2 inhibitor as part of the treatment strategy because patients with T2DM are at increased risk for heart failure with reduced or preserved ejection fraction and because significant, consistent reduction in hospitalizations for heart failure were seen in SGLT2 inhibitor trials, writing group member Peter Rossing, MD, explained during the session; he noted, however, that the trials were not designed to adjudicate heart failure and that most patients did not have clinical heart failure at baseline.
In those in whom SGLT2 inhibitors are contraindicated – because of impaired renal function, for example – a GLP-1 receptor agonist with proven cardiovascular benefit is recommended.
“Then we suggest that if you are still not at [hemoglobin A1c] target, you should avoid thiazolidinediones [TZD] because of the risk of fluid overload, and you could then consider, if needed, combining an SGLT2-inhibitor and a GLP-1 receptor agonist ... or you could use a [dipeptidyl peptidase–4 (DPP-4) inhibitor] if you are not on an GLP-1 receptor agonist. And we point out that saxagliptin has unfavorable data on heart failure,” Dr. Rossing of Steno Diabetes Center, Copenhagen, said, noting that basal insulin or sulfonylurea are other alternative options.
In EMPA-REG, hospitalization for heart failure was reduced by 35% with empagliflozin vs. placebo (HR, 0.65; NNT, 71 over 3 years), and similar findings were seen in the CANVAS trial. In LEADER, a nonsignificant 13% reduction was seen in hospitalization for heart failure with liraglutide vs. placebo (HR, 0.87). However, this was a secondary outcome; ongoing studies are addressing heart failure as a primary outcome, Dr. Rossing said.
The report also includes a recommendation that, for patients with chronic kidney disease and high cardiovascular risk, GLP-1 receptor agonists and SGLT2 inhibitors can be used but with dose reductions for some medications – several of which have demonstrated renal benefit and cardiovascular benefits in those populations and can be considered as part of treatment.
Lifestyle management and medication
With respect to lifestyle management and pharmacologic treatment, the proposed recommendations, which are based on several large trials, state that an individualized program of medical nutritional therapy should be offered to all patients and that all overweight and obese patients with diabetes should be advised of the health benefits of weight loss. They also should be encouraged to engage in a program of intensive lifestyle management, which may include food substitution, writing group member Walter Kernan, MD, said at the meeting.
In the DiRECT Trial, the average weight loss was about 10 kg in an intervention group that had complete food replacement for 3 months followed by gradual food reintroduction and ongoing counseling versus about 1 kg in controls, and the diabetes remission rate at 1 year was 46% versus 4%, respectively, said Dr. Kernan of Yale University, New Haven, Conn.
In addition, intentional physical activity is known to improve glycemic control and should be encouraged in all patients with T2DM, he said.
“The foundation of hyperglycemia treatment in type 2 diabetes is, for sure, lifestyle modification,” said group member Geltrude Mingrone, MD, of Catholic University of the Sacred Heart in Rome. “Those patients who are very well motivated and adherent to the [recommendations] can achieve very good results,” she added.
In those in whom lifestyle modification fails to lead to adequate improvement, “a pretty large medication portfolio is available,” she said, adding that the choice of treatment should be based on safety, efficacy, cost, and convenience, factors which are described in the statement.
The hope is that the final consensus statement will make it easier to navigate them, she said.
Finally, bariatric surgery can be considered a very effective salvage therapy, Dr. Mingrone said, noting that only lifestyle modification or bariatric surgery will lead to diabetes remission.
The draft consensus recommendation for bariatric surgery is to consider it in patients with T2DM and a body mass index of 40 kg/m2 or greater (37.5 or greater in those of Asian ancestry), regardless of the level of glycemic control, and in those with BMI of 35-39.9 (32.5-37.4 in those of Asian ancestry) when hyperglycemia is inadequately controlled despite lifestyle and optimal medical therapy.
Decision making and injectable therapies
The statement includes decision-making strategies and algorithms for treatment and addresses issues such as choosing antihyperglycemic medications when weight is a concern (consider an SGLT2 inhibitor or a GLP-1 receptor agonist with good efficacy for weight loss to start – or a combination of both if HbA1c is not on target), when minimizing hypoglycemia is the priority (consider adding an SGLT2 inhibitors, GLP-1 receptor agonist, a TZD, or a DPP-4 inhibitor to metformin therapy to start, followed by reintensification of lifestyle modifications and combination therapies if HbA1c is above target), and when drug costs need to be minimized, as well as when and how to initiate injectable therapies, according to writing group members David D’Alessio, MD, of Duke University, Durham, N.C, and Chantal Mathieu, MD, of Katholieke Universteit Leuven (Belgium).
The draft consensus recommendation regarding the latter is that, in patients who need the greater glucose-lowering effect of an injectable medication, GLP-1 receptor agonists should be considered as the first choice and that, when insulin is the medication of choice on the basis of clinical characteristics, basal insulin is preferred. Additionally, in patients who are unable to maintain glycemic targets on basal insulin in combination with oral medication, intensification with a GLP-1 receptor agonist, SGLT2 inhibitor, or prandial insulin can be considered.
This recommendation is based on “overwhelming evidence that GLP-1 receptor agonists give you HbA1c lowering in the same range as basal insulin but can do so without hypoglycemia and with weight loss, in contrast to weight gain with most insulin preparations,” Dr. Mathieu noted.
The bottom line, however, is that “the patient is at the center of everything and ... should become an integral part of the team treating this patient,” Dr. Mathieu said.
Knowledge gaps
In a review of remaining knowledge gaps regarding glycemic control in T2DM, Dr. Buse said that, while the tools available to treat and prevent diabetes are vastly improved, implementation of effective innovation has lagged behind and “requires fundamental changes in health care policy and societal approaches to wellness.”
Additionally, the management of overweight and obesity is clearly inadequate and requires much greater emphasis on lifestyle techniques, behavioral approaches, medication, and surgery, said Dr. Buse, who is the Verne S. Caviness distinguished professor and chief of the division of endocrinology, as well as director of the Diabetes Center, at the University of North Carolina, Chapel Hill.
These and numerous other knowledge gaps (with respect to preserving and enhancing beta-cell function, incorporating personalized medicine, the value of combinations for additive benefit, the identification of biomarkers, the use of early intensive therapy, metabolic surgery decision making, the value of self-monitoring of blood glucose, and the need for better drugs – including those for the primary prevention of cardiovascular disease) need to be addressed and “require additional investment in basic, translational, clinical, and implementation research,” he said.
“More time- and cost-efficient research paradigms to address patient-centered endpoints are needed through regulatory reform and leveraging informatics and coordinated learning health care systems. Additionally, the increasing burden of cardiometabolic disease is an existential threat to society,” he said, stressing that “urgent attention to improve prevention and treatment is of the essence.”
Consensus statement development
The draft consensus statement is the work of group members selected by the ADA and EASD to ensure regional representation (five each from the United States and Europe). The group had two face-to-face meetings, as well as regular teleconferences; the members also conducted a “robust evidence review, which informed the content,” said group cochair Melanie J. Davies, MD, of the University of Leicester (U.K.).
The group reviewed randomized controlled trials, systematic reviews, and meta-analyses published from Jan. 1, 2014, (to capture research that may have been missed during development of the 2015 statement) through Feb. 28, 2018.
The process was based on consensus among members; areas of disagreement were voted on and the group proceeded according to 60% supermajority votes.
The updates were mainly based on research generated over the past 2 years, Dr. Davies said.
The final draft will be submitted for publication to Diabetes Care and Diabetologia.
Dr. Buse reported relationships (research support, stock ownership, and/or advisory roles) with Adocia, AstraZeneca, Boehringer Ingelheim, Dexcom, Elcelyx Therapeutics, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Lexicon Pharmaceuticals, Mellitus Health, Metavention, NovaTarg Therapeutics, Novo Nordisk, PhaseBio Pharmaceuticals, Sanofi, Senseonics, Theracos, and vTv Therapeutics; Dr. Wexler reported having no disclosures; Dr. Rossing reported relationships (consultancy and/or speaking fees, research grants, stock ownership) with AbbVie, Astellas Pharma, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, MDS Medical, Novo Nordisk, and Sanofi; Dr. Kernan reported having no disclosures; Dr. Mingrone is a consultant for Novo Nordisk, Fractyl, and Johnson & Johnson; Dr. D’Alessio reported advisory board membership with and/or research support from Eli Lilly, Intarcia Therapeutics, Merck, and Novo Nordisk; Dr. Mathieu reported relationships (advisory board membership, speaker’s bureau, and/or research support) with Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Hanmi Pharmaceuticals, Intrexon, Janssen Pharmaceuticals, MannKind, Medtronic, Merck Sharp & Dohme (MSD), Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi, and UCB; Dr. Davies reported relationships (advisory panel, consulting, research support, and/or speaker’s bureau) with AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Intarcia Therapeutics, Janssen Pharmaceuticals, MSD, Mitsubishi Tanabi Pharma, Novo Nordisk, Sanofi, and Servier.
ORLANDO – according to a draft consensus report on the management of hyperglycemia in patients with type 2 diabetes mellitus (T2DM).
The report, a project of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD), is currently under review and will be presented in final form Oct. 5 at the EASD annual meeting in Berlin.
The current draft calls, generally, for the initial use of metformin followed by the addition of antihyperglycemic medications based on patient comorbidities and concerns “as we await answers to the many questions that remain,” John Buse, MD, PhD, cochair of the consensus statement writing group, said during a summary of the draft recommendations at the annual scientific sessions of the ADA.
The first step, however, is to assess key patient characteristics; these can include comorbidities, clinical characteristics, issues such as motivation and depression, and cultural and socio-economic context, Deborah J. Wexler, MD, 1 of 10 writing group members, said during the same presentation.
Patients with ASCVD or heart failure
Given new evidence from trials such as EMPA-REG and LEADER showing outcomes benefits with the use of specific antihyperglycemic medications in patients with established atherosclerotic cardiovascular disease (ASCVD), an important early step in the proposed approach is to consider the presence or absence of ASCVD and heart failure, said Dr. Wexler of Massachusetts General Hospital, Boston.
“The presence of cardiovascular disease is a compelling indication for the selection of certain glucose-lowering drugs,” she said.
The draft consensus recommendation in this regard – a new recommendation since the last consensus report in 2015 – differentiates between T2DM patients in whom ASCVD predominates and those in whom heart failure predominates.
“What’s new since 2015 is that we recommend that these comorbidities be considered first and foremost because they do influence the choice of a particular glucose-lowering medication, and the recommendation is that, among patients with type 2 diabetes with established ASCVD, sodium-glucose cotransporter 2 [SGLT2] inhibitors or glucagonlike peptide 1 [GLP-1] receptor agonists with proven cardiovascular benefit are recommended as part of glycemic management,” she said.
However, it is important to note that ASCVD is defined differently across trials, and patients considered in the development of these recommendations are those with much higher cardiovascular risk than the average patient with T2DM, she added.
“It’s also important to keep in mind that each cardiovascular outcomes trial, while large, is but a single experiment ... and we don’t have the benefit of replication,” she said, noting that it is not always clear whether differences in trial findings within a drug class are related to trial design or true differences in individual medications.
“So we try to read into them and interpret these data, but it’s just important to consider that ... and when evidence suggests a hierarchy, we noted that,” she said.
That said, if ASCVD predominates, the recommendation is for treatment with either a GLP-1 receptor agonist with proven cardiovascular benefit (favoring liraglutide over semaglutide and over long-acting exenatide) or an SGLT2 inhibitor with proven cardiovascular benefit if estimated glomerular filtration (eGFR) is adequate (favoring empagliflozin over canagliflozin).
These recommendations are based on the LEADER trial finding of significant improvement in the primary outcome of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke with liraglutide vs. placebo (hazard ratio, 0.87; number needed to treat [NNT], 52 over 3.8 years) and on the EMPA-REG trial finding of significant improvement in the same primary outcome with empagliflozin vs. placebo (HR, 0.86; NNT, 62 over 3.1 years).
Keep in mind that there is no evidence of cardiovascular benefit from these treatments in patients at lower risk and also that the “expensive and complicated” combination of an SGLT2 inhibitor and a GLP-1 receptor agonist has not been tested in cardiovascular outcomes trials and there is no evidence of additional benefit from a cardiovascular perspective with this combination, Dr. Wexler said.
If heart failure predominates, the recommendations call for consideration of an SGLT2 inhibitor as part of the treatment strategy because patients with T2DM are at increased risk for heart failure with reduced or preserved ejection fraction and because significant, consistent reduction in hospitalizations for heart failure were seen in SGLT2 inhibitor trials, writing group member Peter Rossing, MD, explained during the session; he noted, however, that the trials were not designed to adjudicate heart failure and that most patients did not have clinical heart failure at baseline.
In those in whom SGLT2 inhibitors are contraindicated – because of impaired renal function, for example – a GLP-1 receptor agonist with proven cardiovascular benefit is recommended.
“Then we suggest that if you are still not at [hemoglobin A1c] target, you should avoid thiazolidinediones [TZD] because of the risk of fluid overload, and you could then consider, if needed, combining an SGLT2-inhibitor and a GLP-1 receptor agonist ... or you could use a [dipeptidyl peptidase–4 (DPP-4) inhibitor] if you are not on an GLP-1 receptor agonist. And we point out that saxagliptin has unfavorable data on heart failure,” Dr. Rossing of Steno Diabetes Center, Copenhagen, said, noting that basal insulin or sulfonylurea are other alternative options.
In EMPA-REG, hospitalization for heart failure was reduced by 35% with empagliflozin vs. placebo (HR, 0.65; NNT, 71 over 3 years), and similar findings were seen in the CANVAS trial. In LEADER, a nonsignificant 13% reduction was seen in hospitalization for heart failure with liraglutide vs. placebo (HR, 0.87). However, this was a secondary outcome; ongoing studies are addressing heart failure as a primary outcome, Dr. Rossing said.
The report also includes a recommendation that, for patients with chronic kidney disease and high cardiovascular risk, GLP-1 receptor agonists and SGLT2 inhibitors can be used but with dose reductions for some medications – several of which have demonstrated renal benefit and cardiovascular benefits in those populations and can be considered as part of treatment.
Lifestyle management and medication
With respect to lifestyle management and pharmacologic treatment, the proposed recommendations, which are based on several large trials, state that an individualized program of medical nutritional therapy should be offered to all patients and that all overweight and obese patients with diabetes should be advised of the health benefits of weight loss. They also should be encouraged to engage in a program of intensive lifestyle management, which may include food substitution, writing group member Walter Kernan, MD, said at the meeting.
In the DiRECT Trial, the average weight loss was about 10 kg in an intervention group that had complete food replacement for 3 months followed by gradual food reintroduction and ongoing counseling versus about 1 kg in controls, and the diabetes remission rate at 1 year was 46% versus 4%, respectively, said Dr. Kernan of Yale University, New Haven, Conn.
In addition, intentional physical activity is known to improve glycemic control and should be encouraged in all patients with T2DM, he said.
“The foundation of hyperglycemia treatment in type 2 diabetes is, for sure, lifestyle modification,” said group member Geltrude Mingrone, MD, of Catholic University of the Sacred Heart in Rome. “Those patients who are very well motivated and adherent to the [recommendations] can achieve very good results,” she added.
In those in whom lifestyle modification fails to lead to adequate improvement, “a pretty large medication portfolio is available,” she said, adding that the choice of treatment should be based on safety, efficacy, cost, and convenience, factors which are described in the statement.
The hope is that the final consensus statement will make it easier to navigate them, she said.
Finally, bariatric surgery can be considered a very effective salvage therapy, Dr. Mingrone said, noting that only lifestyle modification or bariatric surgery will lead to diabetes remission.
The draft consensus recommendation for bariatric surgery is to consider it in patients with T2DM and a body mass index of 40 kg/m2 or greater (37.5 or greater in those of Asian ancestry), regardless of the level of glycemic control, and in those with BMI of 35-39.9 (32.5-37.4 in those of Asian ancestry) when hyperglycemia is inadequately controlled despite lifestyle and optimal medical therapy.
Decision making and injectable therapies
The statement includes decision-making strategies and algorithms for treatment and addresses issues such as choosing antihyperglycemic medications when weight is a concern (consider an SGLT2 inhibitor or a GLP-1 receptor agonist with good efficacy for weight loss to start – or a combination of both if HbA1c is not on target), when minimizing hypoglycemia is the priority (consider adding an SGLT2 inhibitors, GLP-1 receptor agonist, a TZD, or a DPP-4 inhibitor to metformin therapy to start, followed by reintensification of lifestyle modifications and combination therapies if HbA1c is above target), and when drug costs need to be minimized, as well as when and how to initiate injectable therapies, according to writing group members David D’Alessio, MD, of Duke University, Durham, N.C, and Chantal Mathieu, MD, of Katholieke Universteit Leuven (Belgium).
The draft consensus recommendation regarding the latter is that, in patients who need the greater glucose-lowering effect of an injectable medication, GLP-1 receptor agonists should be considered as the first choice and that, when insulin is the medication of choice on the basis of clinical characteristics, basal insulin is preferred. Additionally, in patients who are unable to maintain glycemic targets on basal insulin in combination with oral medication, intensification with a GLP-1 receptor agonist, SGLT2 inhibitor, or prandial insulin can be considered.
This recommendation is based on “overwhelming evidence that GLP-1 receptor agonists give you HbA1c lowering in the same range as basal insulin but can do so without hypoglycemia and with weight loss, in contrast to weight gain with most insulin preparations,” Dr. Mathieu noted.
The bottom line, however, is that “the patient is at the center of everything and ... should become an integral part of the team treating this patient,” Dr. Mathieu said.
Knowledge gaps
In a review of remaining knowledge gaps regarding glycemic control in T2DM, Dr. Buse said that, while the tools available to treat and prevent diabetes are vastly improved, implementation of effective innovation has lagged behind and “requires fundamental changes in health care policy and societal approaches to wellness.”
Additionally, the management of overweight and obesity is clearly inadequate and requires much greater emphasis on lifestyle techniques, behavioral approaches, medication, and surgery, said Dr. Buse, who is the Verne S. Caviness distinguished professor and chief of the division of endocrinology, as well as director of the Diabetes Center, at the University of North Carolina, Chapel Hill.
These and numerous other knowledge gaps (with respect to preserving and enhancing beta-cell function, incorporating personalized medicine, the value of combinations for additive benefit, the identification of biomarkers, the use of early intensive therapy, metabolic surgery decision making, the value of self-monitoring of blood glucose, and the need for better drugs – including those for the primary prevention of cardiovascular disease) need to be addressed and “require additional investment in basic, translational, clinical, and implementation research,” he said.
“More time- and cost-efficient research paradigms to address patient-centered endpoints are needed through regulatory reform and leveraging informatics and coordinated learning health care systems. Additionally, the increasing burden of cardiometabolic disease is an existential threat to society,” he said, stressing that “urgent attention to improve prevention and treatment is of the essence.”
Consensus statement development
The draft consensus statement is the work of group members selected by the ADA and EASD to ensure regional representation (five each from the United States and Europe). The group had two face-to-face meetings, as well as regular teleconferences; the members also conducted a “robust evidence review, which informed the content,” said group cochair Melanie J. Davies, MD, of the University of Leicester (U.K.).
The group reviewed randomized controlled trials, systematic reviews, and meta-analyses published from Jan. 1, 2014, (to capture research that may have been missed during development of the 2015 statement) through Feb. 28, 2018.
The process was based on consensus among members; areas of disagreement were voted on and the group proceeded according to 60% supermajority votes.
The updates were mainly based on research generated over the past 2 years, Dr. Davies said.
The final draft will be submitted for publication to Diabetes Care and Diabetologia.
Dr. Buse reported relationships (research support, stock ownership, and/or advisory roles) with Adocia, AstraZeneca, Boehringer Ingelheim, Dexcom, Elcelyx Therapeutics, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Lexicon Pharmaceuticals, Mellitus Health, Metavention, NovaTarg Therapeutics, Novo Nordisk, PhaseBio Pharmaceuticals, Sanofi, Senseonics, Theracos, and vTv Therapeutics; Dr. Wexler reported having no disclosures; Dr. Rossing reported relationships (consultancy and/or speaking fees, research grants, stock ownership) with AbbVie, Astellas Pharma, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, MDS Medical, Novo Nordisk, and Sanofi; Dr. Kernan reported having no disclosures; Dr. Mingrone is a consultant for Novo Nordisk, Fractyl, and Johnson & Johnson; Dr. D’Alessio reported advisory board membership with and/or research support from Eli Lilly, Intarcia Therapeutics, Merck, and Novo Nordisk; Dr. Mathieu reported relationships (advisory board membership, speaker’s bureau, and/or research support) with Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Hanmi Pharmaceuticals, Intrexon, Janssen Pharmaceuticals, MannKind, Medtronic, Merck Sharp & Dohme (MSD), Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi, and UCB; Dr. Davies reported relationships (advisory panel, consulting, research support, and/or speaker’s bureau) with AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Intarcia Therapeutics, Janssen Pharmaceuticals, MSD, Mitsubishi Tanabi Pharma, Novo Nordisk, Sanofi, and Servier.
EXPERT ANALYSIS FROM ADA 2018
The VADT at 15 years: No legacy effect of intensive glucose control in T2DM
ORLANDO – , according to final results from the VADT follow-up study (VADT-F).
Participants in the randomized, controlled VADT, which compared the effects of intensive versus standard glucose control in more than 1,700 patients with type 2 diabetes mellitus (T2DM), did not experience a significant improvement in the primary cardiovascular disease (CVD) outcome – a composite of myocardial infarction, stroke, cardiovascular death, new congestive heart failure, cardiovascular surgery or inoperable coronary artery disease, and ischemic amputation – after a median of 5.6 years of active treatment (hazard ratio, 0.88; P = .14). Nor did they experience significant improvement in secondary cardiovascular outcomes, including cardiovascular death and death from any cause (HRs, 1.32 and 1.07, respectively), or in a renal composite outcome (HR, 0.85), according to the findings published in 2009 (N Engl J Med. 2009 Jan 8;360[2]:129-39).
This was despite a rapid and statistically significant separation of hemoglobin A1c (HbA1c) levels between the treatment groups, Peter Reaven, MD, noted during a presentation of the final follow-up data at the annual scientific sessions of the American Diabetes Association.
Approximately 6 months after the start of the VADT, median HbA1c levels decreased from more than 9% in both groups to 6.9% and 8.4% in intensive and standard treatment groups, respectively (a median separation of 1.5%), said Dr. Reaven, director of the diabetes research program at the Phoenix VA Health Care System and a professor of clinical medicine at the University of Arizona in Phoenix.
“This was maintained throughout the study period,” he said. “All other risk factors during this period of time were equal between the two treatment groups.”
10-year outcomes
However, 10-year interim data from VADT-F, published in the New England Journal of Medicine (2015 Jun 4;372[23]:2197-206), showed a delayed benefit in these outcomes among those in the intensive control group: The incidence of the primary CVD composite outcome was reduced by 17% (HR, 0.83; P = .04) in favor of the intensive therapy at that time, Dr. Reaven said.
The incidence of the renal composite outcome, which included estimated glomerular filtration rate less than 54 mL/min per 1.73m2, sustained macroalbuminuria, and end-stage renal disease, was reduced by 32% (HR, 0.68; P = .008), said Nicholas Emanuele, MD, who presented the VADT-F renal and microvascular outcomes at the ADA meeting.
At that 10-year follow-up, HbA1c levels in the intensive and standard treatment groups had nearly equalized (although they remained slightly better in the intensive treatment group), and eventually, the levels stabilized at about 8.2% in both groups through the end of the 15-year follow-up, the investigators said.
“So it was still lower by nearly 1.2 hemoglobin percent units, compared to baseline values nearly 15 years earlier, and despite ending the study in very good control, after we released these patients to the primary care providers for their diabetes care, there was a substantial rise in HbA1c levels over time ... illustrating the difficulty of controlling HbA1c values to this level in this advanced diabetes population,” Dr. Reaven said.
15-year outcomes
At the final 15-year follow up, with the HbA1c levels similar in the groups, nearly all benefits seen at 10 years were lost. Event rates for the CVD primary composite outcome were 51.8 and 47.3 per 1,000 patient-years in the intensive care and standard care groups, respectively (HR, 0.91; P = .23), and event rates for the renal composite outcome were 88 and 85 per 1,000 patient-years (HR, 0.90; P = .55).
Similarly, no differences were seen at 15 years in the secondary VADT-F outcomes of any major diabetes outcome, (HR, 0.90; P = .16), cardiovascular death (HR, 0.94; P = .61), or death from any cause (HR 1.02; P = .81), and no differences were seen in the individual components of the composite outcomes, the investigators said.
The same was true for other outcomes, including hospitalizations and health-related quality of life, Dr. Reaven said.
Ocular events studied in the VADT-F included cataract extraction, laster photocoagulation, vitrectomy, and intravitreal injections, with the latter three constituting a retinal event composite for which there was a difference of “very borderline significance (HR, 0.84; P = .053),” said Dr. Emanuele of Hines (Ill.) VA Hospital and Loyola University of Chicago.
There was no difference between groups for cataract extraction. (HR, 1.16; P = .30) or in participants’ self reported vision at 15 years, he added.
Additional analyses showed that there were no treatment interactions for results based on baseline differences in diabetes duration, prior CV events, or risk scores.
In essence, there was no evidence of a legacy effect, Dr. Reaven said, noting that the findings are “relatively consistent” with those from other recent glucose-lowering trials, including ACCORDION and ADVANCE-ON, which also showed no legacy benefits of intensive glucose lowering.
Dr. Emanuele also concluded that no prolonged legacy effect was apparent for renal and other microvascular outcomes.
The lack of a legacy effect at 15 years, however, shouldn’t discount the benefits seen at the 10-year follow-up because there are other ways to look at “legacy,” Hertzel C. Gerstein, MD, said during an independent “clinical perspective” commentary on the VADT and VADT-F findings.
“Another way to define ‘legacy’ is what happens after the active clinical trial ends, and if you think of it that way, there is a legacy,” said Dr. Gerstein, a professor and Population Health Institute chair in diabetes research at McMaster University and Hamilton Health Sciences, Ontario, Canada.
That is, the intensive glycemic control led to significant improvements at 10-year follow-up. While he acknowledged “that’s just semantics,” he stressed that a number of important lessons have been learned from the VADT and VADT-F – not the least of which relate to mediation analyses that showed the benefit seen at 10 years can be explained, at least statistically, by the differences in HbA1c levels achieved during those intervening 10 years of follow-up.
For example, the 10-year cardiovascular outcome hazard ratios changed from 0.83 with a P value of .04 to 0.86 with a P value of .12 (after controlling for time-varying HbA1c levels) and to 0.94 with a P value of .53 (after controlling for time-varying cumulative mean HbA1c), he said, noting that similar findings have been reported from prior trials.
The VADT design
The VADT was designed to evaluate whether an intensive glycemic control regimen could reduce the incidence of major cardiovascular events compared with standard care in patients with T2DM; secondary objectives included differences in additional cardiovascular, renal, and other outcomes.
Subjects, who were enrolled from 20 VA medical centers beginning in December 2000, were aged 41 years or older (mean of about 60 years) and had failed to respond to a maximum dose of at least one oral agent and/or daily insulin. Patients were excluded if they had HbA1c less than 7.5%, had had a cardiovascular event in the previous 6 months, had advanced congestive heart failure, had severe angina, had a life expectancy of less than 7 years, had a body mass index over 40 kg/m2, had serum creatinine less than 1.6 mg/dL, or had an alanine transaminase level greater than 3 times the upper limit of normal, according to Wyndy L. Wiitala, PhD, of the VA Center for Clinical Management Research in Ann Arbor, Michigan.
A total of 818 patients in the standard care group and 837 in the intensive treatment group completed the study with up to 7.5 years of total follow-up (median, 5.6 years). The groups were similar in age; both were mostly male, which is expected for a VA population; and the average HbA1c level was 9.4% in both groups. Other clinical measures, including lipids, blood pressure, and estimated cardiovascular risk were also similar between the two groups.
“The VADT was designed so that the only planned difference between the treatment groups was the level of glycemic control,” Dr. Wiitala said.
All patients with a BMI of 27 kg/m2 or greater were started on metformin plus rosiglitazone, and those with a BMI less than 27 kg/m2 were started on glimepiride plus rosiglitazone. Those in the intensive therapy arm were started on maximal doses, and those in the standard therapy arm were started on half the maximal doses. Insulin was added for patients in the intensive-therapy group who did not achieve HbA1c below 6%, as well as for those in the standard-therapy group with a level of less than 9%.
Any subsequent medication changes were determined according to protocol guidelines and local assessment, and investigators were allowed to use any approved drug at their discretion.
“The use of medications between the two groups was similar, with differences in dose and insulin intensity only,” Dr. Wiitala said, adding that all other aspects of treatment, including blood pressure control, lipid control, aspirin therapy, diet, and nutrition, were “nearly identical” in the two groups.
The VADT-F design
The negative findings from the VADT raised “a number of questions, which really provided the rationale for the VADT follow-up study,” Dr. Reaven said.
“Would the post-VADT follow-up reveal an emerging cardiovascular benefit? This was particularly relevant as there was an indication that the group differences were increasing toward the end of the study, and benefits in cardiovascular outcomes, as we know, take a fair amount of time,” he said, adding that since the glucose separation seen in the treatment groups was greater than that seen in other recent studies involving patients with advanced T2DM and remained that way for an extended period of time, the follow-up study provided an excellent opportunity to examine whether there was a legacy or other effects.
The VADT-F continued to follow the VADT patients after the intervention ended in 2008; at that time, patients returned to normal care with no further intervention by the research team, Dr. Wiitala said, noting that participants were followed using national data sources, annual mail surveys, and targeted chart reviews.
The 10-year interim analysis was reported in 2015, and the 15-year final analysis, which is currently under review, represents the longest follow-up of patients with advanced T2DM with high risk for cardiovascular disease, she said.
Clinical perspective and future directions
“These results suggest that there are modest long-term cardiovascular disease benefits of therapies directed toward bringing glucose control to near-normal range in high-risk type 2 diabetes and that substantial and continuous glucose separation may be required to maintain these improvements,” Dr. Reaven concluded, adding that “recent studies demonstrating cardiovascular benefit with diabetes agents that only achieve modest improvements in glycemic control highlight the importance of also considering nonglycemic approaches to reducing cardiovascular disease events and mortality in these patients.”
Similarly, Dr. Emanuele concluded that there is a delayed beneficial effect of intensive glycemic control on kidney outcomes but that the effect dissipates as glycemic separation wanes.
However, in his commentary at the meeting, Dr. Gerstein stressed that the findings add value; in addition to showing, via mediation analyses, that HbA1c levels statistically explain the differences seen between the intensive and standard therapy arms at 10 years, the VADT and VADT-F findings also underscore the veracity of the ADA’s recommended target of HbA1c less than 7%, albeit “with all sorts of caveats.”
“But one point to make is that clinical trials do not tell you how to treat the patient in front of you. [They] just tell you what works on average for the average patient. ... You have to take the information you get from randomized trials and put it into your brain as a doctor and treat the patient,” he said.
He and several colleagues further explained this concept in a recent editorial (Diabetes Care. 2018 Jun;41[6]:1121-4) penned in response to new guidance statements published by the American College of Physicians advocating for relaxation of HbA1c control goals in patients with T2DM.
“The ACP proposal may encourage a step backward at a time when accumulating evidence from randomized, controlled trials calls for movement forward in the treatment of diabetes,” they wrote in the editorial entitled “A1c targets should be personalized to maximize benefits while limiting risks.”
Findings from those trials, including the VADT and VADT-F, continue to increase diabetes insights and inform care, and while there is not yet a statin-like “prescribe-and-go” treatment for diabetes, the findings represent a step in the right direction, Dr Gerstein said.
“All you have to do is look at all the clinical trials that are happening. We’re going to get there. ... This is not the end of the end, this is the beginning of the next phase,” he said.
The VADT and VADT-F were funded by the VA Cooperative Studies Program, the ADA, and the National Institutes of Health/National Eye Institute. Medication and additional support were provided by Aventis, GlaxoSmithKline, and Novo Nordisk Pharmaceuticals, which provided funding and supplies, and by Abbott Laboratory, Amylin, Eli Lily, Kos, Roche, and the University of Chicago, which also provided supplies. Dr. Reaven is an advisory panel member for Sanofi and has received research support from AstraZeneca and Novo Nordisk. Dr. Gerstein has received grants or other research support, honoraria, and/or consulting fees from Abbott, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Novo Nordisk, and Sanofi. Dr. Wiitala and Dr. Emanuele reported having no disclosures.
ORLANDO – , according to final results from the VADT follow-up study (VADT-F).
Participants in the randomized, controlled VADT, which compared the effects of intensive versus standard glucose control in more than 1,700 patients with type 2 diabetes mellitus (T2DM), did not experience a significant improvement in the primary cardiovascular disease (CVD) outcome – a composite of myocardial infarction, stroke, cardiovascular death, new congestive heart failure, cardiovascular surgery or inoperable coronary artery disease, and ischemic amputation – after a median of 5.6 years of active treatment (hazard ratio, 0.88; P = .14). Nor did they experience significant improvement in secondary cardiovascular outcomes, including cardiovascular death and death from any cause (HRs, 1.32 and 1.07, respectively), or in a renal composite outcome (HR, 0.85), according to the findings published in 2009 (N Engl J Med. 2009 Jan 8;360[2]:129-39).
This was despite a rapid and statistically significant separation of hemoglobin A1c (HbA1c) levels between the treatment groups, Peter Reaven, MD, noted during a presentation of the final follow-up data at the annual scientific sessions of the American Diabetes Association.
Approximately 6 months after the start of the VADT, median HbA1c levels decreased from more than 9% in both groups to 6.9% and 8.4% in intensive and standard treatment groups, respectively (a median separation of 1.5%), said Dr. Reaven, director of the diabetes research program at the Phoenix VA Health Care System and a professor of clinical medicine at the University of Arizona in Phoenix.
“This was maintained throughout the study period,” he said. “All other risk factors during this period of time were equal between the two treatment groups.”
10-year outcomes
However, 10-year interim data from VADT-F, published in the New England Journal of Medicine (2015 Jun 4;372[23]:2197-206), showed a delayed benefit in these outcomes among those in the intensive control group: The incidence of the primary CVD composite outcome was reduced by 17% (HR, 0.83; P = .04) in favor of the intensive therapy at that time, Dr. Reaven said.
The incidence of the renal composite outcome, which included estimated glomerular filtration rate less than 54 mL/min per 1.73m2, sustained macroalbuminuria, and end-stage renal disease, was reduced by 32% (HR, 0.68; P = .008), said Nicholas Emanuele, MD, who presented the VADT-F renal and microvascular outcomes at the ADA meeting.
At that 10-year follow-up, HbA1c levels in the intensive and standard treatment groups had nearly equalized (although they remained slightly better in the intensive treatment group), and eventually, the levels stabilized at about 8.2% in both groups through the end of the 15-year follow-up, the investigators said.
“So it was still lower by nearly 1.2 hemoglobin percent units, compared to baseline values nearly 15 years earlier, and despite ending the study in very good control, after we released these patients to the primary care providers for their diabetes care, there was a substantial rise in HbA1c levels over time ... illustrating the difficulty of controlling HbA1c values to this level in this advanced diabetes population,” Dr. Reaven said.
15-year outcomes
At the final 15-year follow up, with the HbA1c levels similar in the groups, nearly all benefits seen at 10 years were lost. Event rates for the CVD primary composite outcome were 51.8 and 47.3 per 1,000 patient-years in the intensive care and standard care groups, respectively (HR, 0.91; P = .23), and event rates for the renal composite outcome were 88 and 85 per 1,000 patient-years (HR, 0.90; P = .55).
Similarly, no differences were seen at 15 years in the secondary VADT-F outcomes of any major diabetes outcome, (HR, 0.90; P = .16), cardiovascular death (HR, 0.94; P = .61), or death from any cause (HR 1.02; P = .81), and no differences were seen in the individual components of the composite outcomes, the investigators said.
The same was true for other outcomes, including hospitalizations and health-related quality of life, Dr. Reaven said.
Ocular events studied in the VADT-F included cataract extraction, laster photocoagulation, vitrectomy, and intravitreal injections, with the latter three constituting a retinal event composite for which there was a difference of “very borderline significance (HR, 0.84; P = .053),” said Dr. Emanuele of Hines (Ill.) VA Hospital and Loyola University of Chicago.
There was no difference between groups for cataract extraction. (HR, 1.16; P = .30) or in participants’ self reported vision at 15 years, he added.
Additional analyses showed that there were no treatment interactions for results based on baseline differences in diabetes duration, prior CV events, or risk scores.
In essence, there was no evidence of a legacy effect, Dr. Reaven said, noting that the findings are “relatively consistent” with those from other recent glucose-lowering trials, including ACCORDION and ADVANCE-ON, which also showed no legacy benefits of intensive glucose lowering.
Dr. Emanuele also concluded that no prolonged legacy effect was apparent for renal and other microvascular outcomes.
The lack of a legacy effect at 15 years, however, shouldn’t discount the benefits seen at the 10-year follow-up because there are other ways to look at “legacy,” Hertzel C. Gerstein, MD, said during an independent “clinical perspective” commentary on the VADT and VADT-F findings.
“Another way to define ‘legacy’ is what happens after the active clinical trial ends, and if you think of it that way, there is a legacy,” said Dr. Gerstein, a professor and Population Health Institute chair in diabetes research at McMaster University and Hamilton Health Sciences, Ontario, Canada.
That is, the intensive glycemic control led to significant improvements at 10-year follow-up. While he acknowledged “that’s just semantics,” he stressed that a number of important lessons have been learned from the VADT and VADT-F – not the least of which relate to mediation analyses that showed the benefit seen at 10 years can be explained, at least statistically, by the differences in HbA1c levels achieved during those intervening 10 years of follow-up.
For example, the 10-year cardiovascular outcome hazard ratios changed from 0.83 with a P value of .04 to 0.86 with a P value of .12 (after controlling for time-varying HbA1c levels) and to 0.94 with a P value of .53 (after controlling for time-varying cumulative mean HbA1c), he said, noting that similar findings have been reported from prior trials.
The VADT design
The VADT was designed to evaluate whether an intensive glycemic control regimen could reduce the incidence of major cardiovascular events compared with standard care in patients with T2DM; secondary objectives included differences in additional cardiovascular, renal, and other outcomes.
Subjects, who were enrolled from 20 VA medical centers beginning in December 2000, were aged 41 years or older (mean of about 60 years) and had failed to respond to a maximum dose of at least one oral agent and/or daily insulin. Patients were excluded if they had HbA1c less than 7.5%, had had a cardiovascular event in the previous 6 months, had advanced congestive heart failure, had severe angina, had a life expectancy of less than 7 years, had a body mass index over 40 kg/m2, had serum creatinine less than 1.6 mg/dL, or had an alanine transaminase level greater than 3 times the upper limit of normal, according to Wyndy L. Wiitala, PhD, of the VA Center for Clinical Management Research in Ann Arbor, Michigan.
A total of 818 patients in the standard care group and 837 in the intensive treatment group completed the study with up to 7.5 years of total follow-up (median, 5.6 years). The groups were similar in age; both were mostly male, which is expected for a VA population; and the average HbA1c level was 9.4% in both groups. Other clinical measures, including lipids, blood pressure, and estimated cardiovascular risk were also similar between the two groups.
“The VADT was designed so that the only planned difference between the treatment groups was the level of glycemic control,” Dr. Wiitala said.
All patients with a BMI of 27 kg/m2 or greater were started on metformin plus rosiglitazone, and those with a BMI less than 27 kg/m2 were started on glimepiride plus rosiglitazone. Those in the intensive therapy arm were started on maximal doses, and those in the standard therapy arm were started on half the maximal doses. Insulin was added for patients in the intensive-therapy group who did not achieve HbA1c below 6%, as well as for those in the standard-therapy group with a level of less than 9%.
Any subsequent medication changes were determined according to protocol guidelines and local assessment, and investigators were allowed to use any approved drug at their discretion.
“The use of medications between the two groups was similar, with differences in dose and insulin intensity only,” Dr. Wiitala said, adding that all other aspects of treatment, including blood pressure control, lipid control, aspirin therapy, diet, and nutrition, were “nearly identical” in the two groups.
The VADT-F design
The negative findings from the VADT raised “a number of questions, which really provided the rationale for the VADT follow-up study,” Dr. Reaven said.
“Would the post-VADT follow-up reveal an emerging cardiovascular benefit? This was particularly relevant as there was an indication that the group differences were increasing toward the end of the study, and benefits in cardiovascular outcomes, as we know, take a fair amount of time,” he said, adding that since the glucose separation seen in the treatment groups was greater than that seen in other recent studies involving patients with advanced T2DM and remained that way for an extended period of time, the follow-up study provided an excellent opportunity to examine whether there was a legacy or other effects.
The VADT-F continued to follow the VADT patients after the intervention ended in 2008; at that time, patients returned to normal care with no further intervention by the research team, Dr. Wiitala said, noting that participants were followed using national data sources, annual mail surveys, and targeted chart reviews.
The 10-year interim analysis was reported in 2015, and the 15-year final analysis, which is currently under review, represents the longest follow-up of patients with advanced T2DM with high risk for cardiovascular disease, she said.
Clinical perspective and future directions
“These results suggest that there are modest long-term cardiovascular disease benefits of therapies directed toward bringing glucose control to near-normal range in high-risk type 2 diabetes and that substantial and continuous glucose separation may be required to maintain these improvements,” Dr. Reaven concluded, adding that “recent studies demonstrating cardiovascular benefit with diabetes agents that only achieve modest improvements in glycemic control highlight the importance of also considering nonglycemic approaches to reducing cardiovascular disease events and mortality in these patients.”
Similarly, Dr. Emanuele concluded that there is a delayed beneficial effect of intensive glycemic control on kidney outcomes but that the effect dissipates as glycemic separation wanes.
However, in his commentary at the meeting, Dr. Gerstein stressed that the findings add value; in addition to showing, via mediation analyses, that HbA1c levels statistically explain the differences seen between the intensive and standard therapy arms at 10 years, the VADT and VADT-F findings also underscore the veracity of the ADA’s recommended target of HbA1c less than 7%, albeit “with all sorts of caveats.”
“But one point to make is that clinical trials do not tell you how to treat the patient in front of you. [They] just tell you what works on average for the average patient. ... You have to take the information you get from randomized trials and put it into your brain as a doctor and treat the patient,” he said.
He and several colleagues further explained this concept in a recent editorial (Diabetes Care. 2018 Jun;41[6]:1121-4) penned in response to new guidance statements published by the American College of Physicians advocating for relaxation of HbA1c control goals in patients with T2DM.
“The ACP proposal may encourage a step backward at a time when accumulating evidence from randomized, controlled trials calls for movement forward in the treatment of diabetes,” they wrote in the editorial entitled “A1c targets should be personalized to maximize benefits while limiting risks.”
Findings from those trials, including the VADT and VADT-F, continue to increase diabetes insights and inform care, and while there is not yet a statin-like “prescribe-and-go” treatment for diabetes, the findings represent a step in the right direction, Dr Gerstein said.
“All you have to do is look at all the clinical trials that are happening. We’re going to get there. ... This is not the end of the end, this is the beginning of the next phase,” he said.
The VADT and VADT-F were funded by the VA Cooperative Studies Program, the ADA, and the National Institutes of Health/National Eye Institute. Medication and additional support were provided by Aventis, GlaxoSmithKline, and Novo Nordisk Pharmaceuticals, which provided funding and supplies, and by Abbott Laboratory, Amylin, Eli Lily, Kos, Roche, and the University of Chicago, which also provided supplies. Dr. Reaven is an advisory panel member for Sanofi and has received research support from AstraZeneca and Novo Nordisk. Dr. Gerstein has received grants or other research support, honoraria, and/or consulting fees from Abbott, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Novo Nordisk, and Sanofi. Dr. Wiitala and Dr. Emanuele reported having no disclosures.
ORLANDO – , according to final results from the VADT follow-up study (VADT-F).
Participants in the randomized, controlled VADT, which compared the effects of intensive versus standard glucose control in more than 1,700 patients with type 2 diabetes mellitus (T2DM), did not experience a significant improvement in the primary cardiovascular disease (CVD) outcome – a composite of myocardial infarction, stroke, cardiovascular death, new congestive heart failure, cardiovascular surgery or inoperable coronary artery disease, and ischemic amputation – after a median of 5.6 years of active treatment (hazard ratio, 0.88; P = .14). Nor did they experience significant improvement in secondary cardiovascular outcomes, including cardiovascular death and death from any cause (HRs, 1.32 and 1.07, respectively), or in a renal composite outcome (HR, 0.85), according to the findings published in 2009 (N Engl J Med. 2009 Jan 8;360[2]:129-39).
This was despite a rapid and statistically significant separation of hemoglobin A1c (HbA1c) levels between the treatment groups, Peter Reaven, MD, noted during a presentation of the final follow-up data at the annual scientific sessions of the American Diabetes Association.
Approximately 6 months after the start of the VADT, median HbA1c levels decreased from more than 9% in both groups to 6.9% and 8.4% in intensive and standard treatment groups, respectively (a median separation of 1.5%), said Dr. Reaven, director of the diabetes research program at the Phoenix VA Health Care System and a professor of clinical medicine at the University of Arizona in Phoenix.
“This was maintained throughout the study period,” he said. “All other risk factors during this period of time were equal between the two treatment groups.”
10-year outcomes
However, 10-year interim data from VADT-F, published in the New England Journal of Medicine (2015 Jun 4;372[23]:2197-206), showed a delayed benefit in these outcomes among those in the intensive control group: The incidence of the primary CVD composite outcome was reduced by 17% (HR, 0.83; P = .04) in favor of the intensive therapy at that time, Dr. Reaven said.
The incidence of the renal composite outcome, which included estimated glomerular filtration rate less than 54 mL/min per 1.73m2, sustained macroalbuminuria, and end-stage renal disease, was reduced by 32% (HR, 0.68; P = .008), said Nicholas Emanuele, MD, who presented the VADT-F renal and microvascular outcomes at the ADA meeting.
At that 10-year follow-up, HbA1c levels in the intensive and standard treatment groups had nearly equalized (although they remained slightly better in the intensive treatment group), and eventually, the levels stabilized at about 8.2% in both groups through the end of the 15-year follow-up, the investigators said.
“So it was still lower by nearly 1.2 hemoglobin percent units, compared to baseline values nearly 15 years earlier, and despite ending the study in very good control, after we released these patients to the primary care providers for their diabetes care, there was a substantial rise in HbA1c levels over time ... illustrating the difficulty of controlling HbA1c values to this level in this advanced diabetes population,” Dr. Reaven said.
15-year outcomes
At the final 15-year follow up, with the HbA1c levels similar in the groups, nearly all benefits seen at 10 years were lost. Event rates for the CVD primary composite outcome were 51.8 and 47.3 per 1,000 patient-years in the intensive care and standard care groups, respectively (HR, 0.91; P = .23), and event rates for the renal composite outcome were 88 and 85 per 1,000 patient-years (HR, 0.90; P = .55).
Similarly, no differences were seen at 15 years in the secondary VADT-F outcomes of any major diabetes outcome, (HR, 0.90; P = .16), cardiovascular death (HR, 0.94; P = .61), or death from any cause (HR 1.02; P = .81), and no differences were seen in the individual components of the composite outcomes, the investigators said.
The same was true for other outcomes, including hospitalizations and health-related quality of life, Dr. Reaven said.
Ocular events studied in the VADT-F included cataract extraction, laster photocoagulation, vitrectomy, and intravitreal injections, with the latter three constituting a retinal event composite for which there was a difference of “very borderline significance (HR, 0.84; P = .053),” said Dr. Emanuele of Hines (Ill.) VA Hospital and Loyola University of Chicago.
There was no difference between groups for cataract extraction. (HR, 1.16; P = .30) or in participants’ self reported vision at 15 years, he added.
Additional analyses showed that there were no treatment interactions for results based on baseline differences in diabetes duration, prior CV events, or risk scores.
In essence, there was no evidence of a legacy effect, Dr. Reaven said, noting that the findings are “relatively consistent” with those from other recent glucose-lowering trials, including ACCORDION and ADVANCE-ON, which also showed no legacy benefits of intensive glucose lowering.
Dr. Emanuele also concluded that no prolonged legacy effect was apparent for renal and other microvascular outcomes.
The lack of a legacy effect at 15 years, however, shouldn’t discount the benefits seen at the 10-year follow-up because there are other ways to look at “legacy,” Hertzel C. Gerstein, MD, said during an independent “clinical perspective” commentary on the VADT and VADT-F findings.
“Another way to define ‘legacy’ is what happens after the active clinical trial ends, and if you think of it that way, there is a legacy,” said Dr. Gerstein, a professor and Population Health Institute chair in diabetes research at McMaster University and Hamilton Health Sciences, Ontario, Canada.
That is, the intensive glycemic control led to significant improvements at 10-year follow-up. While he acknowledged “that’s just semantics,” he stressed that a number of important lessons have been learned from the VADT and VADT-F – not the least of which relate to mediation analyses that showed the benefit seen at 10 years can be explained, at least statistically, by the differences in HbA1c levels achieved during those intervening 10 years of follow-up.
For example, the 10-year cardiovascular outcome hazard ratios changed from 0.83 with a P value of .04 to 0.86 with a P value of .12 (after controlling for time-varying HbA1c levels) and to 0.94 with a P value of .53 (after controlling for time-varying cumulative mean HbA1c), he said, noting that similar findings have been reported from prior trials.
The VADT design
The VADT was designed to evaluate whether an intensive glycemic control regimen could reduce the incidence of major cardiovascular events compared with standard care in patients with T2DM; secondary objectives included differences in additional cardiovascular, renal, and other outcomes.
Subjects, who were enrolled from 20 VA medical centers beginning in December 2000, were aged 41 years or older (mean of about 60 years) and had failed to respond to a maximum dose of at least one oral agent and/or daily insulin. Patients were excluded if they had HbA1c less than 7.5%, had had a cardiovascular event in the previous 6 months, had advanced congestive heart failure, had severe angina, had a life expectancy of less than 7 years, had a body mass index over 40 kg/m2, had serum creatinine less than 1.6 mg/dL, or had an alanine transaminase level greater than 3 times the upper limit of normal, according to Wyndy L. Wiitala, PhD, of the VA Center for Clinical Management Research in Ann Arbor, Michigan.
A total of 818 patients in the standard care group and 837 in the intensive treatment group completed the study with up to 7.5 years of total follow-up (median, 5.6 years). The groups were similar in age; both were mostly male, which is expected for a VA population; and the average HbA1c level was 9.4% in both groups. Other clinical measures, including lipids, blood pressure, and estimated cardiovascular risk were also similar between the two groups.
“The VADT was designed so that the only planned difference between the treatment groups was the level of glycemic control,” Dr. Wiitala said.
All patients with a BMI of 27 kg/m2 or greater were started on metformin plus rosiglitazone, and those with a BMI less than 27 kg/m2 were started on glimepiride plus rosiglitazone. Those in the intensive therapy arm were started on maximal doses, and those in the standard therapy arm were started on half the maximal doses. Insulin was added for patients in the intensive-therapy group who did not achieve HbA1c below 6%, as well as for those in the standard-therapy group with a level of less than 9%.
Any subsequent medication changes were determined according to protocol guidelines and local assessment, and investigators were allowed to use any approved drug at their discretion.
“The use of medications between the two groups was similar, with differences in dose and insulin intensity only,” Dr. Wiitala said, adding that all other aspects of treatment, including blood pressure control, lipid control, aspirin therapy, diet, and nutrition, were “nearly identical” in the two groups.
The VADT-F design
The negative findings from the VADT raised “a number of questions, which really provided the rationale for the VADT follow-up study,” Dr. Reaven said.
“Would the post-VADT follow-up reveal an emerging cardiovascular benefit? This was particularly relevant as there was an indication that the group differences were increasing toward the end of the study, and benefits in cardiovascular outcomes, as we know, take a fair amount of time,” he said, adding that since the glucose separation seen in the treatment groups was greater than that seen in other recent studies involving patients with advanced T2DM and remained that way for an extended period of time, the follow-up study provided an excellent opportunity to examine whether there was a legacy or other effects.
The VADT-F continued to follow the VADT patients after the intervention ended in 2008; at that time, patients returned to normal care with no further intervention by the research team, Dr. Wiitala said, noting that participants were followed using national data sources, annual mail surveys, and targeted chart reviews.
The 10-year interim analysis was reported in 2015, and the 15-year final analysis, which is currently under review, represents the longest follow-up of patients with advanced T2DM with high risk for cardiovascular disease, she said.
Clinical perspective and future directions
“These results suggest that there are modest long-term cardiovascular disease benefits of therapies directed toward bringing glucose control to near-normal range in high-risk type 2 diabetes and that substantial and continuous glucose separation may be required to maintain these improvements,” Dr. Reaven concluded, adding that “recent studies demonstrating cardiovascular benefit with diabetes agents that only achieve modest improvements in glycemic control highlight the importance of also considering nonglycemic approaches to reducing cardiovascular disease events and mortality in these patients.”
Similarly, Dr. Emanuele concluded that there is a delayed beneficial effect of intensive glycemic control on kidney outcomes but that the effect dissipates as glycemic separation wanes.
However, in his commentary at the meeting, Dr. Gerstein stressed that the findings add value; in addition to showing, via mediation analyses, that HbA1c levels statistically explain the differences seen between the intensive and standard therapy arms at 10 years, the VADT and VADT-F findings also underscore the veracity of the ADA’s recommended target of HbA1c less than 7%, albeit “with all sorts of caveats.”
“But one point to make is that clinical trials do not tell you how to treat the patient in front of you. [They] just tell you what works on average for the average patient. ... You have to take the information you get from randomized trials and put it into your brain as a doctor and treat the patient,” he said.
He and several colleagues further explained this concept in a recent editorial (Diabetes Care. 2018 Jun;41[6]:1121-4) penned in response to new guidance statements published by the American College of Physicians advocating for relaxation of HbA1c control goals in patients with T2DM.
“The ACP proposal may encourage a step backward at a time when accumulating evidence from randomized, controlled trials calls for movement forward in the treatment of diabetes,” they wrote in the editorial entitled “A1c targets should be personalized to maximize benefits while limiting risks.”
Findings from those trials, including the VADT and VADT-F, continue to increase diabetes insights and inform care, and while there is not yet a statin-like “prescribe-and-go” treatment for diabetes, the findings represent a step in the right direction, Dr Gerstein said.
“All you have to do is look at all the clinical trials that are happening. We’re going to get there. ... This is not the end of the end, this is the beginning of the next phase,” he said.
The VADT and VADT-F were funded by the VA Cooperative Studies Program, the ADA, and the National Institutes of Health/National Eye Institute. Medication and additional support were provided by Aventis, GlaxoSmithKline, and Novo Nordisk Pharmaceuticals, which provided funding and supplies, and by Abbott Laboratory, Amylin, Eli Lily, Kos, Roche, and the University of Chicago, which also provided supplies. Dr. Reaven is an advisory panel member for Sanofi and has received research support from AstraZeneca and Novo Nordisk. Dr. Gerstein has received grants or other research support, honoraria, and/or consulting fees from Abbott, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Novo Nordisk, and Sanofi. Dr. Wiitala and Dr. Emanuele reported having no disclosures.
EXPERT ANALYSIS FROM ADA 2018
Studies support cardiovascular risk management in T2DM
Taking steps to manage cardiovascular risk can help improve long-term outcomes for type 2 diabetes mellitus, according to two new studies published Aug. 16 in the New England Journal of Medicine.
The first study, which examined weight gain after smoking cessation, found that despite a temporary increase in T2DM risk, post-cessation weight gain did not diminish the long-term benefits of reduced cardiovascular and all-cause mortality.
The analysis included three cohort studies: the Nurses’ Health Study (NHS), Nurses’ Health Study II (NHS II), and the Health Professionals Follow-Up Study (HPFS), with follow-up questionnaires every 2 years. After exclusions, a total of 162,807 patients were included in the diabetes analysis and 170,723 in the mortality analysis, reported Yang Hu of the department of nutrition at Harvard T.H. Chan School of Public Health, Boston, and his coauthors (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1803626).
In each follow-up cycle, participants who reported being smokers in the previous cycle but “past” smokers in the current cycle were identified. Quitters were defined as either transient quitters (past smokers in the current cycle but current smokers in previous and next cycles), recent quitters (2-6 consecutive years since smoking cessation), and long-term quitters (6 or more consecutive years since cessation). Weight change was observed for the first 6 years after quitting.
Overall, 12,384 cases of T2DM were confirmed. Diabetes risk was higher for recent quitters than for current smokers (hazard ratio, 1.22; 95% confidence interval, 1.12-1.32); this risk peaked 5-7 years after quitting and then gradually decreased. In analysis of patients with the longest follow-up time, diabetes risk dropped after 30 years of cessation to that of participants who had never smoked, the authors reported.
Compared with current smokers, hazard ratios for T2DM in recent quitters were 1.08 (95% CI, 0.93-1.26) for those without weight gain, 1.15 (95% CI, 0.99-1.33) for those with weight gain of 0.1-5.0 kg, 1.36 (95% CI, 1.16-1.58) for those with weight gain of 5.1-10 kg, and 1.59 (95% CI, 1.36-1.85) in those with weight gain of more than 10 kg.
In the mortality analysis, 23,867 deaths occurred, of which 5,492 were due to cardiovascular disease. Compared with current smokers, hazard ratios for death from cardiovascular disease in recent quitters were 0.69 (95% CI, 0.54-0.88) in those without weight gain; 0.47 (95% CI, 0.35-0.63) in those with weight gain of 0.1-5 kg; 0.25 (95% CI, 0.15-0.42) in those with weight gain of 5.1-10 kg; 0.33 (95% CI, 0.18-0.60) in those with weight gain of more than 10 kg; and 0.50 (95% CI, 0.46-0.55) for longer term quitters. The corresponding hazard ratios for all-cause deaths in the same weight gain groups were 0.81 (95% CI, 0.73-0.90); 0.52 (95% CI, 0.46-0.59); 0.46 (95% CI, 0.38-0.55); 0.50 (95% CI, 0.40-0.63); and 0.57 (95% CI, 0.54-0.59).
The findings suggest that weight gain after quitting smoking “did not attenuate the apparent benefits of smoking cessation on reducing cardiovascular mortality or extending longevity,” the authors said. “However, preventing excessive weight gain may maximize the health benefits of smoking cessation through reducing the short-term risk of diabetes and further lowering the long-term risk of death.”
The second study, which included 271,174 patients with T2DM from the Swedish National Diabetes Register and 1,355,870 controls, examined five risk factors: elevated glycated hemoglobin level, elevated low-density lipoprotein cholesterol level, albuminuria, smoking, and elevated blood pressure.
All-cause mortality, myocardial infarction, stroke, and hospitalization for heart failure were evaluated. The risk of each outcome among patients with T2DM was estimated according to the number of risk-factor variables within guideline-recommended target ranges, compared with matched controls, wrote Aidin Rawshani, MD, of the department of molecular and clinical medicine at the University of Gothenburg (Sweden), and his coauthors (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1800256).
Among the T2DM patients, the excess risk of outcomes was reduced with each risk factor variable within the recommended target range. A total of 37,825 patients with T2DM (13.9%) and 137,520 controls (10.1%) died during the study period.
Among T2DM patients with all variables within target range, the hazard ratio was 1.06 for all-cause death (95% CI, 1.00-1.12); 0.84 for acute myocardial infarction (95% CI, 0.75-0.93); and 0.95 for stroke (95% CI, 0.84-1.07). Smoking was the strongest predictor of death, followed by physical activity, marital status, glycated hemoglobin level, and use of statins.
The study results “indicate that having all five risk-factor variables within the target ranges could theoretically eliminate the excess risk of acute myocardial infarction,” Dr. Rawshani and his colleagues wrote. “Patients with type 2 diabetes who had five risk-factor variables within target ranges appeared to have little or no excess risks of death, myocardial infarction, and stroke as compared with the general population.”
Dr. Hu and his coauthors did not report any disclosures. Dr. Rawshani’s coauthors disclosed relationships with numerous companies including Amgen, Astra Zeneca, and Boehringer Ingelheim.
SOURCE: Hu Y et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1803626. Rawshani A et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1800256.
“Given the high prevalence of obesity and the related increase in type 2 diabetes, prevention of cardiovascular complications is essential,” Steven A. Schroeder, MD, wrote in an editorial published along with the studies (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMe1809004).
The findings reported by Hu and colleagues demonstrate that “the cardiovascular and overall mortality benefits of stopping smoking far outweigh the risks of acquiring type 2 diabetes,” he wrote.
The results reported by Rawshani and coauthors “provide clear support for active management of risk factors” because of the fact that patients with risk factor variables within recommended range had little or no excess risk of death or cardiovascular events.
The results of these two studies “provide support for control of cardiovascular risk factors in patients with diabetes, as well as reassurance that the benefits of smoking cessation outweigh the risks of obesity-associated diabetes,” he concluded.
Dr. Schroeder is on the faculty of the department of medicine, University of California, San Francisco. He had no financial conflicts of interest to disclose.
“Given the high prevalence of obesity and the related increase in type 2 diabetes, prevention of cardiovascular complications is essential,” Steven A. Schroeder, MD, wrote in an editorial published along with the studies (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMe1809004).
The findings reported by Hu and colleagues demonstrate that “the cardiovascular and overall mortality benefits of stopping smoking far outweigh the risks of acquiring type 2 diabetes,” he wrote.
The results reported by Rawshani and coauthors “provide clear support for active management of risk factors” because of the fact that patients with risk factor variables within recommended range had little or no excess risk of death or cardiovascular events.
The results of these two studies “provide support for control of cardiovascular risk factors in patients with diabetes, as well as reassurance that the benefits of smoking cessation outweigh the risks of obesity-associated diabetes,” he concluded.
Dr. Schroeder is on the faculty of the department of medicine, University of California, San Francisco. He had no financial conflicts of interest to disclose.
“Given the high prevalence of obesity and the related increase in type 2 diabetes, prevention of cardiovascular complications is essential,” Steven A. Schroeder, MD, wrote in an editorial published along with the studies (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMe1809004).
The findings reported by Hu and colleagues demonstrate that “the cardiovascular and overall mortality benefits of stopping smoking far outweigh the risks of acquiring type 2 diabetes,” he wrote.
The results reported by Rawshani and coauthors “provide clear support for active management of risk factors” because of the fact that patients with risk factor variables within recommended range had little or no excess risk of death or cardiovascular events.
The results of these two studies “provide support for control of cardiovascular risk factors in patients with diabetes, as well as reassurance that the benefits of smoking cessation outweigh the risks of obesity-associated diabetes,” he concluded.
Dr. Schroeder is on the faculty of the department of medicine, University of California, San Francisco. He had no financial conflicts of interest to disclose.
Taking steps to manage cardiovascular risk can help improve long-term outcomes for type 2 diabetes mellitus, according to two new studies published Aug. 16 in the New England Journal of Medicine.
The first study, which examined weight gain after smoking cessation, found that despite a temporary increase in T2DM risk, post-cessation weight gain did not diminish the long-term benefits of reduced cardiovascular and all-cause mortality.
The analysis included three cohort studies: the Nurses’ Health Study (NHS), Nurses’ Health Study II (NHS II), and the Health Professionals Follow-Up Study (HPFS), with follow-up questionnaires every 2 years. After exclusions, a total of 162,807 patients were included in the diabetes analysis and 170,723 in the mortality analysis, reported Yang Hu of the department of nutrition at Harvard T.H. Chan School of Public Health, Boston, and his coauthors (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1803626).
In each follow-up cycle, participants who reported being smokers in the previous cycle but “past” smokers in the current cycle were identified. Quitters were defined as either transient quitters (past smokers in the current cycle but current smokers in previous and next cycles), recent quitters (2-6 consecutive years since smoking cessation), and long-term quitters (6 or more consecutive years since cessation). Weight change was observed for the first 6 years after quitting.
Overall, 12,384 cases of T2DM were confirmed. Diabetes risk was higher for recent quitters than for current smokers (hazard ratio, 1.22; 95% confidence interval, 1.12-1.32); this risk peaked 5-7 years after quitting and then gradually decreased. In analysis of patients with the longest follow-up time, diabetes risk dropped after 30 years of cessation to that of participants who had never smoked, the authors reported.
Compared with current smokers, hazard ratios for T2DM in recent quitters were 1.08 (95% CI, 0.93-1.26) for those without weight gain, 1.15 (95% CI, 0.99-1.33) for those with weight gain of 0.1-5.0 kg, 1.36 (95% CI, 1.16-1.58) for those with weight gain of 5.1-10 kg, and 1.59 (95% CI, 1.36-1.85) in those with weight gain of more than 10 kg.
In the mortality analysis, 23,867 deaths occurred, of which 5,492 were due to cardiovascular disease. Compared with current smokers, hazard ratios for death from cardiovascular disease in recent quitters were 0.69 (95% CI, 0.54-0.88) in those without weight gain; 0.47 (95% CI, 0.35-0.63) in those with weight gain of 0.1-5 kg; 0.25 (95% CI, 0.15-0.42) in those with weight gain of 5.1-10 kg; 0.33 (95% CI, 0.18-0.60) in those with weight gain of more than 10 kg; and 0.50 (95% CI, 0.46-0.55) for longer term quitters. The corresponding hazard ratios for all-cause deaths in the same weight gain groups were 0.81 (95% CI, 0.73-0.90); 0.52 (95% CI, 0.46-0.59); 0.46 (95% CI, 0.38-0.55); 0.50 (95% CI, 0.40-0.63); and 0.57 (95% CI, 0.54-0.59).
The findings suggest that weight gain after quitting smoking “did not attenuate the apparent benefits of smoking cessation on reducing cardiovascular mortality or extending longevity,” the authors said. “However, preventing excessive weight gain may maximize the health benefits of smoking cessation through reducing the short-term risk of diabetes and further lowering the long-term risk of death.”
The second study, which included 271,174 patients with T2DM from the Swedish National Diabetes Register and 1,355,870 controls, examined five risk factors: elevated glycated hemoglobin level, elevated low-density lipoprotein cholesterol level, albuminuria, smoking, and elevated blood pressure.
All-cause mortality, myocardial infarction, stroke, and hospitalization for heart failure were evaluated. The risk of each outcome among patients with T2DM was estimated according to the number of risk-factor variables within guideline-recommended target ranges, compared with matched controls, wrote Aidin Rawshani, MD, of the department of molecular and clinical medicine at the University of Gothenburg (Sweden), and his coauthors (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1800256).
Among the T2DM patients, the excess risk of outcomes was reduced with each risk factor variable within the recommended target range. A total of 37,825 patients with T2DM (13.9%) and 137,520 controls (10.1%) died during the study period.
Among T2DM patients with all variables within target range, the hazard ratio was 1.06 for all-cause death (95% CI, 1.00-1.12); 0.84 for acute myocardial infarction (95% CI, 0.75-0.93); and 0.95 for stroke (95% CI, 0.84-1.07). Smoking was the strongest predictor of death, followed by physical activity, marital status, glycated hemoglobin level, and use of statins.
The study results “indicate that having all five risk-factor variables within the target ranges could theoretically eliminate the excess risk of acute myocardial infarction,” Dr. Rawshani and his colleagues wrote. “Patients with type 2 diabetes who had five risk-factor variables within target ranges appeared to have little or no excess risks of death, myocardial infarction, and stroke as compared with the general population.”
Dr. Hu and his coauthors did not report any disclosures. Dr. Rawshani’s coauthors disclosed relationships with numerous companies including Amgen, Astra Zeneca, and Boehringer Ingelheim.
SOURCE: Hu Y et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1803626. Rawshani A et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1800256.
Taking steps to manage cardiovascular risk can help improve long-term outcomes for type 2 diabetes mellitus, according to two new studies published Aug. 16 in the New England Journal of Medicine.
The first study, which examined weight gain after smoking cessation, found that despite a temporary increase in T2DM risk, post-cessation weight gain did not diminish the long-term benefits of reduced cardiovascular and all-cause mortality.
The analysis included three cohort studies: the Nurses’ Health Study (NHS), Nurses’ Health Study II (NHS II), and the Health Professionals Follow-Up Study (HPFS), with follow-up questionnaires every 2 years. After exclusions, a total of 162,807 patients were included in the diabetes analysis and 170,723 in the mortality analysis, reported Yang Hu of the department of nutrition at Harvard T.H. Chan School of Public Health, Boston, and his coauthors (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1803626).
In each follow-up cycle, participants who reported being smokers in the previous cycle but “past” smokers in the current cycle were identified. Quitters were defined as either transient quitters (past smokers in the current cycle but current smokers in previous and next cycles), recent quitters (2-6 consecutive years since smoking cessation), and long-term quitters (6 or more consecutive years since cessation). Weight change was observed for the first 6 years after quitting.
Overall, 12,384 cases of T2DM were confirmed. Diabetes risk was higher for recent quitters than for current smokers (hazard ratio, 1.22; 95% confidence interval, 1.12-1.32); this risk peaked 5-7 years after quitting and then gradually decreased. In analysis of patients with the longest follow-up time, diabetes risk dropped after 30 years of cessation to that of participants who had never smoked, the authors reported.
Compared with current smokers, hazard ratios for T2DM in recent quitters were 1.08 (95% CI, 0.93-1.26) for those without weight gain, 1.15 (95% CI, 0.99-1.33) for those with weight gain of 0.1-5.0 kg, 1.36 (95% CI, 1.16-1.58) for those with weight gain of 5.1-10 kg, and 1.59 (95% CI, 1.36-1.85) in those with weight gain of more than 10 kg.
In the mortality analysis, 23,867 deaths occurred, of which 5,492 were due to cardiovascular disease. Compared with current smokers, hazard ratios for death from cardiovascular disease in recent quitters were 0.69 (95% CI, 0.54-0.88) in those without weight gain; 0.47 (95% CI, 0.35-0.63) in those with weight gain of 0.1-5 kg; 0.25 (95% CI, 0.15-0.42) in those with weight gain of 5.1-10 kg; 0.33 (95% CI, 0.18-0.60) in those with weight gain of more than 10 kg; and 0.50 (95% CI, 0.46-0.55) for longer term quitters. The corresponding hazard ratios for all-cause deaths in the same weight gain groups were 0.81 (95% CI, 0.73-0.90); 0.52 (95% CI, 0.46-0.59); 0.46 (95% CI, 0.38-0.55); 0.50 (95% CI, 0.40-0.63); and 0.57 (95% CI, 0.54-0.59).
The findings suggest that weight gain after quitting smoking “did not attenuate the apparent benefits of smoking cessation on reducing cardiovascular mortality or extending longevity,” the authors said. “However, preventing excessive weight gain may maximize the health benefits of smoking cessation through reducing the short-term risk of diabetes and further lowering the long-term risk of death.”
The second study, which included 271,174 patients with T2DM from the Swedish National Diabetes Register and 1,355,870 controls, examined five risk factors: elevated glycated hemoglobin level, elevated low-density lipoprotein cholesterol level, albuminuria, smoking, and elevated blood pressure.
All-cause mortality, myocardial infarction, stroke, and hospitalization for heart failure were evaluated. The risk of each outcome among patients with T2DM was estimated according to the number of risk-factor variables within guideline-recommended target ranges, compared with matched controls, wrote Aidin Rawshani, MD, of the department of molecular and clinical medicine at the University of Gothenburg (Sweden), and his coauthors (N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1800256).
Among the T2DM patients, the excess risk of outcomes was reduced with each risk factor variable within the recommended target range. A total of 37,825 patients with T2DM (13.9%) and 137,520 controls (10.1%) died during the study period.
Among T2DM patients with all variables within target range, the hazard ratio was 1.06 for all-cause death (95% CI, 1.00-1.12); 0.84 for acute myocardial infarction (95% CI, 0.75-0.93); and 0.95 for stroke (95% CI, 0.84-1.07). Smoking was the strongest predictor of death, followed by physical activity, marital status, glycated hemoglobin level, and use of statins.
The study results “indicate that having all five risk-factor variables within the target ranges could theoretically eliminate the excess risk of acute myocardial infarction,” Dr. Rawshani and his colleagues wrote. “Patients with type 2 diabetes who had five risk-factor variables within target ranges appeared to have little or no excess risks of death, myocardial infarction, and stroke as compared with the general population.”
Dr. Hu and his coauthors did not report any disclosures. Dr. Rawshani’s coauthors disclosed relationships with numerous companies including Amgen, Astra Zeneca, and Boehringer Ingelheim.
SOURCE: Hu Y et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1803626. Rawshani A et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1800256.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Taking steps to manage cardiovascular risk can help improve the long-term outlook for type 2 diabetes mellitus.
Major finding: Despite a temporary increase in type 2 diabetes mellitus risk, post–smoking cessation weight gain did not diminish the long-term benefits of reduced cardiovascular and all-cause mortality. In patients with type 2 diabetes, the excess risk of adverse outcomes was reduced with each risk factor variable within the recommended target range.
Study details: An analysis of three cohort studies and a separate analysis of 271,174 patients with T2DM from the Swedish National Diabetes Register and 1,355,870 controls.
Disclosures: Dr. Hu and coauthors did not report any disclosures. Dr. Rawshani’s coauthors disclosed relationships with numerous companies including Amgen, Astra Zeneca, and Boehringer Ingelheim.
Source: Hu Y et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1803626. Rawshani A et al. N Engl J Med. 2018 Aug 16. doi: 10.1056/NEJMoa1800256.
ADA underscores distinctions in youth, adult T1DM
Management of type 1 diabetes mellitus in children should include careful consideration of the unique features and challenges that differentiate it from T1DM in adults, according to a new position statement released by the American Diabetes Association.
The statement, published Aug. 10 in Diabetes Care, includes guidance on diagnosis, staging, screening, monitoring, treatment, nutrition, physical activity, and transition from pediatric to adult care.
With regard to diagnosis and staging, the recommendations emphasize the importance of distinguishing between T1DM, type 2 diabetes mellitus, and monogenic diabetes. It also asserts that a pediatric endocrinologist should be consulted before making a diagnosis when “isolated glycosuria or hyperglycemia is discovered in the setting of acute illness and in the absence of classic symptoms,” wrote Jane L. Chiang, MD, of McKinsey & Company and chief medical officer at Diasome Pharmaceuticals in Palo Alto, Calif., and coauthors.
The guidance also describes the three stages of type 1 diabetes development. Stage 1 is presymptomatic and features the presence of beta-cell autoimmunity. Stage 2, also presymptomatic, includes the presence of beta-cell autoimmunity with dysglycemia. Symptomatic disease from insulin deficiency begins in stage 3, and may include hyperglycemia, polyuria, polydipsia, weight loss, polyphagia, fatigue, and blurred vision. Perineal candidiasis is common in girls, and about one-third of cases present with diabetic ketoacidosis (DKA).
In patients with hyperglycemia symptoms, blood glucose, not hemoglobin A1c, should be used to diagnose acute onset of disease. Delays in diagnosis and insulin replacement therapy should be avoided and a definitive diagnosis made quickly, the authors added.
Because the current method of using HbA1c to diagnose diabetes was based on studies limited to adults, there is still debate over whether to use HbA1c to diagnose T1DM in children and adolescents, Dr. Chiang and colleagues noted. Additionally, physicians must take care to distinguish between diabetes types because of increased numbers of overweight children with T1DM, as well as frequent misdiagnosis of monogenic diabetes as T1DM.
The position statement emphasizes the importance of insulin therapy as treatment for children with T1DM and recommends that most patients should be treated with either multiple injections of prandial and basal insulin, or with continuous subcutaneous insulin infusion. HbA1c should be measured at 3-month intervals to assess glycemic control, with a target HbA1c of less than 7.5%, the authors said. Also covered are recommendations for blood glucose monitoring, blood and urine ketone monitoring, and continuous glucose monitoring.
The importance of integrating an exercise and nutrition plan is also highlighted in the guidance. In addition to monitoring carbohydrate and caloric intake with the help of a dietitian, 60 minutes of moderate to vigorous activity daily are recommended as an exercise goal. Steps should also be taken to prevent hypoglycemia during and after exercise, the authors added.
Measures must also be taken to anticipate and address the unique behavioral and social challenges that accompany diabetes management in developing adolescents, the authors said. Social and family issues, peer relationships, and disordered eating should all be considered, and, starting at age 12 years, patients should be allowed time to speak in confidentiality with their health care provider, Dr. Chiang and colleagues said.
Additionally, as adolescents assert increased independence and autonomy, independent disease management should be facilitated, and issues such as depression and risky behaviors discussed.
The guidelines also discuss the importance of following the Centers for Disease Control and Prevention immunization schedule, and monitoring growth and weight gain. Patients with T1DM and their caregivers should also be sufficiently educated on comorbidities such as diabetic ketoacidosis, hypoglycemia, retinopathy, dyslipidemia, autoimmune diseases, and other complications.
Supportive environments such as diabetes camps, as well as technological advances, may be effective tools in encouraging diabetes self-management. Though there is no “optimal transition age” for the shift from pediatric to adult care, ADA recommends that providers begin transition preparation in the early adolescent years, and provide counseling on diabetes self-management.
“An ineffective transition from pediatric to adult diabetes care may contribute to fragmentation of health care and increased risk for adverse outcomes,” the authors said. “An individualized approach to transition timing is recommended, prioritizing the developmental needs and preferences of the patient.”
The authors reported relationships with Diasome Pharmaceuticals and numerous other companies.
SOURCE: Chiang J et al. Diabetes Care. 2018 Jul. doi: 10.2337/dci18-0023.
Management of type 1 diabetes mellitus in children should include careful consideration of the unique features and challenges that differentiate it from T1DM in adults, according to a new position statement released by the American Diabetes Association.
The statement, published Aug. 10 in Diabetes Care, includes guidance on diagnosis, staging, screening, monitoring, treatment, nutrition, physical activity, and transition from pediatric to adult care.
With regard to diagnosis and staging, the recommendations emphasize the importance of distinguishing between T1DM, type 2 diabetes mellitus, and monogenic diabetes. It also asserts that a pediatric endocrinologist should be consulted before making a diagnosis when “isolated glycosuria or hyperglycemia is discovered in the setting of acute illness and in the absence of classic symptoms,” wrote Jane L. Chiang, MD, of McKinsey & Company and chief medical officer at Diasome Pharmaceuticals in Palo Alto, Calif., and coauthors.
The guidance also describes the three stages of type 1 diabetes development. Stage 1 is presymptomatic and features the presence of beta-cell autoimmunity. Stage 2, also presymptomatic, includes the presence of beta-cell autoimmunity with dysglycemia. Symptomatic disease from insulin deficiency begins in stage 3, and may include hyperglycemia, polyuria, polydipsia, weight loss, polyphagia, fatigue, and blurred vision. Perineal candidiasis is common in girls, and about one-third of cases present with diabetic ketoacidosis (DKA).
In patients with hyperglycemia symptoms, blood glucose, not hemoglobin A1c, should be used to diagnose acute onset of disease. Delays in diagnosis and insulin replacement therapy should be avoided and a definitive diagnosis made quickly, the authors added.
Because the current method of using HbA1c to diagnose diabetes was based on studies limited to adults, there is still debate over whether to use HbA1c to diagnose T1DM in children and adolescents, Dr. Chiang and colleagues noted. Additionally, physicians must take care to distinguish between diabetes types because of increased numbers of overweight children with T1DM, as well as frequent misdiagnosis of monogenic diabetes as T1DM.
The position statement emphasizes the importance of insulin therapy as treatment for children with T1DM and recommends that most patients should be treated with either multiple injections of prandial and basal insulin, or with continuous subcutaneous insulin infusion. HbA1c should be measured at 3-month intervals to assess glycemic control, with a target HbA1c of less than 7.5%, the authors said. Also covered are recommendations for blood glucose monitoring, blood and urine ketone monitoring, and continuous glucose monitoring.
The importance of integrating an exercise and nutrition plan is also highlighted in the guidance. In addition to monitoring carbohydrate and caloric intake with the help of a dietitian, 60 minutes of moderate to vigorous activity daily are recommended as an exercise goal. Steps should also be taken to prevent hypoglycemia during and after exercise, the authors added.
Measures must also be taken to anticipate and address the unique behavioral and social challenges that accompany diabetes management in developing adolescents, the authors said. Social and family issues, peer relationships, and disordered eating should all be considered, and, starting at age 12 years, patients should be allowed time to speak in confidentiality with their health care provider, Dr. Chiang and colleagues said.
Additionally, as adolescents assert increased independence and autonomy, independent disease management should be facilitated, and issues such as depression and risky behaviors discussed.
The guidelines also discuss the importance of following the Centers for Disease Control and Prevention immunization schedule, and monitoring growth and weight gain. Patients with T1DM and their caregivers should also be sufficiently educated on comorbidities such as diabetic ketoacidosis, hypoglycemia, retinopathy, dyslipidemia, autoimmune diseases, and other complications.
Supportive environments such as diabetes camps, as well as technological advances, may be effective tools in encouraging diabetes self-management. Though there is no “optimal transition age” for the shift from pediatric to adult care, ADA recommends that providers begin transition preparation in the early adolescent years, and provide counseling on diabetes self-management.
“An ineffective transition from pediatric to adult diabetes care may contribute to fragmentation of health care and increased risk for adverse outcomes,” the authors said. “An individualized approach to transition timing is recommended, prioritizing the developmental needs and preferences of the patient.”
The authors reported relationships with Diasome Pharmaceuticals and numerous other companies.
SOURCE: Chiang J et al. Diabetes Care. 2018 Jul. doi: 10.2337/dci18-0023.
Management of type 1 diabetes mellitus in children should include careful consideration of the unique features and challenges that differentiate it from T1DM in adults, according to a new position statement released by the American Diabetes Association.
The statement, published Aug. 10 in Diabetes Care, includes guidance on diagnosis, staging, screening, monitoring, treatment, nutrition, physical activity, and transition from pediatric to adult care.
With regard to diagnosis and staging, the recommendations emphasize the importance of distinguishing between T1DM, type 2 diabetes mellitus, and monogenic diabetes. It also asserts that a pediatric endocrinologist should be consulted before making a diagnosis when “isolated glycosuria or hyperglycemia is discovered in the setting of acute illness and in the absence of classic symptoms,” wrote Jane L. Chiang, MD, of McKinsey & Company and chief medical officer at Diasome Pharmaceuticals in Palo Alto, Calif., and coauthors.
The guidance also describes the three stages of type 1 diabetes development. Stage 1 is presymptomatic and features the presence of beta-cell autoimmunity. Stage 2, also presymptomatic, includes the presence of beta-cell autoimmunity with dysglycemia. Symptomatic disease from insulin deficiency begins in stage 3, and may include hyperglycemia, polyuria, polydipsia, weight loss, polyphagia, fatigue, and blurred vision. Perineal candidiasis is common in girls, and about one-third of cases present with diabetic ketoacidosis (DKA).
In patients with hyperglycemia symptoms, blood glucose, not hemoglobin A1c, should be used to diagnose acute onset of disease. Delays in diagnosis and insulin replacement therapy should be avoided and a definitive diagnosis made quickly, the authors added.
Because the current method of using HbA1c to diagnose diabetes was based on studies limited to adults, there is still debate over whether to use HbA1c to diagnose T1DM in children and adolescents, Dr. Chiang and colleagues noted. Additionally, physicians must take care to distinguish between diabetes types because of increased numbers of overweight children with T1DM, as well as frequent misdiagnosis of monogenic diabetes as T1DM.
The position statement emphasizes the importance of insulin therapy as treatment for children with T1DM and recommends that most patients should be treated with either multiple injections of prandial and basal insulin, or with continuous subcutaneous insulin infusion. HbA1c should be measured at 3-month intervals to assess glycemic control, with a target HbA1c of less than 7.5%, the authors said. Also covered are recommendations for blood glucose monitoring, blood and urine ketone monitoring, and continuous glucose monitoring.
The importance of integrating an exercise and nutrition plan is also highlighted in the guidance. In addition to monitoring carbohydrate and caloric intake with the help of a dietitian, 60 minutes of moderate to vigorous activity daily are recommended as an exercise goal. Steps should also be taken to prevent hypoglycemia during and after exercise, the authors added.
Measures must also be taken to anticipate and address the unique behavioral and social challenges that accompany diabetes management in developing adolescents, the authors said. Social and family issues, peer relationships, and disordered eating should all be considered, and, starting at age 12 years, patients should be allowed time to speak in confidentiality with their health care provider, Dr. Chiang and colleagues said.
Additionally, as adolescents assert increased independence and autonomy, independent disease management should be facilitated, and issues such as depression and risky behaviors discussed.
The guidelines also discuss the importance of following the Centers for Disease Control and Prevention immunization schedule, and monitoring growth and weight gain. Patients with T1DM and their caregivers should also be sufficiently educated on comorbidities such as diabetic ketoacidosis, hypoglycemia, retinopathy, dyslipidemia, autoimmune diseases, and other complications.
Supportive environments such as diabetes camps, as well as technological advances, may be effective tools in encouraging diabetes self-management. Though there is no “optimal transition age” for the shift from pediatric to adult care, ADA recommends that providers begin transition preparation in the early adolescent years, and provide counseling on diabetes self-management.
“An ineffective transition from pediatric to adult diabetes care may contribute to fragmentation of health care and increased risk for adverse outcomes,” the authors said. “An individualized approach to transition timing is recommended, prioritizing the developmental needs and preferences of the patient.”
The authors reported relationships with Diasome Pharmaceuticals and numerous other companies.
SOURCE: Chiang J et al. Diabetes Care. 2018 Jul. doi: 10.2337/dci18-0023.
FROM DIABETES CARE
Key clinical point: Management of type 1 diabetes in children and adolescents should take into account the unique challenges of disease management in that age group, and facilitate an effective transition to adult care.
Major finding: The position statement emphasizes the importance of insulin therapy as treatment for children with T1DM and the importance of integrating an exercise and nutrition plan.
Study details: An analysis of numerous diabetes studies and clinical trials.
Disclosures: The authors reported relationships with Diasome Pharmaceuticals and numerous other companies.
Source: Chiang J et al. Diabetes Care. 2018 Jul. doi: 10.2337/dci18-0023.
What do you call a koala who is too sweet for its own good? Diabetic
SAN DIEGO – The 14-pound patient with the deep-pile complexion was lethargic, kept drinking a lot of water, and had a glucose level in the range of 600-700 mg/dL. He was nearly comatose by the time medical staff transferred him to a specialized facility.
The diagnosis: Diabetes. The treatment: Insulin. But multiple daily skin pricks were quite a challenge for Quincy the koala. After all, he requires up to 22 hours of shut-eye each day.
What to do? The veterinary staff at the San Diego Zoo turned to the experts – an endocrinologist and a manufacturer of continuous glucose monitors. Now, Quincy has his own CGM, and a medical team that is tracking his glucose levels in real time on their smartphones.
In fact, Athena Philis-Tsimikas, MD, of the Scripps Whittier Diabetes Institute, pulls out her phone and checks on him at least a couple times a day. She also gets alerts if his blood sugar drops too quickly.
“He is definitely another one of my patients,” she said in an interview. But he’s the only one who lives in trees and enjoys a nice eucalyptus smoothie.
Humans are hardly the only mammals who get diabetes
Veterinarians are quite familiar with diabetes. A wide variety of mammals from pigs and apes to horses and dolphins can develop an equivalent of the human condition. Dogs may be prescribed daily insulin shots, and cats even develop peripheral neuropathy and retinopathy like humans with diabetes.
So it’s not entirely surprising that a team at the Los Angeles Zoo diagnosed Quincy, a 3-year-old Queensland koala, with diabetes.
Quincy’s glucose levels should have been around 80-130 mg/dL, similar to the ideal levels in humans, said San Diego Zoo senior veterinarian Cora Singleton, DVM, in an interview. But tests prompted by his symptoms showed his levels were high, she said, and they stayed that way. According to her, that suggested he wasn’t just having a one-time elevation that animals can experience when they’re stressed.
Unfortunately, there are only a few scattered reports of diabetes in koalas, and “there’s not anything documented about treating a koala over a long term,” Dr. Singleton said. “We’re in uncharted territory here.”
So the Los Angeles Zoo sent Quincy down the California coast for more specialized treatment. The San Diego Zoo’s veterinary staff took in Quincy and treated him with glucose tests and insulin shots, Dr. Singleton said. “But we were looking a way for to get more information with less disturbance to Quincy.”
Someone mentioned the idea of a sensor. “We thought, ‘What a great idea,’” Dr. Singleton said. “It would be a way for us to get a lot of information and find out how his highs and lows are related.”
That’s when the team turned to local endocrinologist Dr. Tsimikas for a helping hand.
The key to koala calming: Eucalyptus smoothies
“They did reach out to us and asked what kind of sensors might be available. We connected them to Dexcom,” a CGM company that’s based in San Diego, Dr. Tsimikas said. “We knew the newest one was coming along and suggested they place that on him as a starting point.”
On June 1, a zoo team attached a Dexcom G6 Continuous Glucose Monitoring System to the koala’s side.
“He’s doing very well. He tolerates the CGM superbly,” Dr. Singleton said. And Quincy doesn’t react when sensors are applied, she said, although it helps that he gets to enjoy a eucalyptus smoothie during the procedure. “Put that in a big syringe, and he’ll volunteer for most anything,” she said.
Obesity can trigger diabetes in mammals other than humans. Could eucalyptus overindulgence explain Quincy’s case of diabetes? Nope.
According to Dr. Tsimikas, the ingredients of the eucalyptus smoothie are just pureed eucalyptus leaves that “go down fast and easy.” These naturally have a nice mix of carbohydrates, fat, and protein to better manage the koala’s sugars and other nutritional needs. If he is dropping his blood sugar values fast, there is another dextrose drink they give him in small amounts, which contains 5-10 g carbohydrates. This is enough to help bring his glucose values back up. It is similar to the treatment recommendations provided to humans with diabetes where they are told to take 15 g of carbohydrates such as honey, hard candies, or juice to prevent a severe hypoglycemic episode.
Dr. Singleton noted that Quincy appears to have the koala equivalent is type 1 diabetes mellitus (T1DM).
Dr. Tsimikas noted “We are not finding the typical antibodies that we find in human T1DM. Quincy is showing low insulin levels, which is why it more closely resembles T1DM. We will be doing further analysis and comparisons with nondiabetic koalas in the future to see if it can be better differentiated.
While he appears to have type 1 diabetes, it’s not clear why he developed it, Dr. Singleton said.
While Quincy is only 3 years old, he’s a full-fledged adult in koala terms. Koalas typically live up to their mid-teens, she said.
This speechless patient still manages to communicate
The San Diego Zoo’s veterinary staff is monitoring Quincy and trying to understand how his glucose levels and daily insulin shots affect him. His tiny size has ruled out use of an insulin pump: Although the insulin pumps have been getting smaller and lighter, they are still too large to attach to our tiny friend. Especially since he would need both the CGM device and the pump, there is not a lot of surface area on his body for attachment of all the devices, according to Dr. Tsimikas.
Since Quincy is so tiny, insulin doses must be minuscule to avoid sending him into hypoglycemia, Dr. Tsimikas said. She said the koala’s medical team is planning to try using a NovoPen Echo injector with a half-unit of insulin.
Dr. Singleton noted that for now, “he’s maintaining his body weight, and he has days when he feels spunky. Sometimes, when he knows it’s breakfast time, and he hears his caretakers coming up the doorway with his breakfast, he’ll be very active on his perch.”
But he has sluggish days, too, when he’ll try to sleep in. Dr. Singleton keeps an eye out for grogginess and signs of weakness and hypoglycemia or hyperglycemia like “a little wobble in his step.”
“The biggest thing I’ve learned from Quincy is the value of his particular nonverbal cues,” she said. “I’m starting to understand when he feels like his sugars are a little high or a little low. I imagine that doctors and parents have the same challenges with little patients, along with figuring out how you communicate that this is supposed to help them.”
Dr. Tsimikas agreed, noting that she sees similarities between Quincy and patients who are hospitalized and can’t easily communicate. Now, “we can track the folks who are on the CGM and intervene earlier than before,” said Dr. Tsimikas, who’s part of a clinical trial team testing CGM devices in two hospitals. “It’s almost like having another vital sign.
“It is only when we have all the data on all the other factors that can influence blood sugar, such as eating patterns, insulin dose and timing, and activity level that we can more accurately adjust the medical interventions.” This requires collaboration between all the groups involved in Quincy’s care. In koalas, the collaboration is with the veterinarian, koala zookeepers, dietitian, and the technology monitoring team. Whereas, for humans, we need parents, care providers, diabetes educators, dietitians, and physicians.
It’s not clear if Quincy will need his CGM for the rest of his life. If he’s stable on a specific insulin dose, Dr. Tsimikas said, he may not need it. But it sounds like eucalyptus smoothies will always be a vital part of his regimen.
In the name of thoroughness, take note that Quincy is not the first diabetic zoo animal whose care involved physicians from Scripps. “We have had several other consultations for animals with diabetes. Nearly 25 years ago, a roller-skating chimpanzee with diabetes was brought to the Scripps Whittier Institute labs for evaluation and treatment recommendations. A few years later, one of our medical directors, Alberto Hayek, MD, advised on the care of Lune, a diabetic baboon at the San Diego Zoo, for insulin management. This time we are making house calls to the zoo to treat Quincy in his home environment. Each animal experience offers opportunities to expand our knowledge about diabetes care and exchange approaches that we might not otherwise be aware of. This has been fun and rewarding. I am looking forward to seeing further outcomes from our interactions with Quincy,” according to Dr. Tsimikas.
Dr. Tsimikas reports that her center conducts research with Dexcom and Novo Nordisk. Dr. Singleton reports no relevant disclosures.
SAN DIEGO – The 14-pound patient with the deep-pile complexion was lethargic, kept drinking a lot of water, and had a glucose level in the range of 600-700 mg/dL. He was nearly comatose by the time medical staff transferred him to a specialized facility.
The diagnosis: Diabetes. The treatment: Insulin. But multiple daily skin pricks were quite a challenge for Quincy the koala. After all, he requires up to 22 hours of shut-eye each day.
What to do? The veterinary staff at the San Diego Zoo turned to the experts – an endocrinologist and a manufacturer of continuous glucose monitors. Now, Quincy has his own CGM, and a medical team that is tracking his glucose levels in real time on their smartphones.
In fact, Athena Philis-Tsimikas, MD, of the Scripps Whittier Diabetes Institute, pulls out her phone and checks on him at least a couple times a day. She also gets alerts if his blood sugar drops too quickly.
“He is definitely another one of my patients,” she said in an interview. But he’s the only one who lives in trees and enjoys a nice eucalyptus smoothie.
Humans are hardly the only mammals who get diabetes
Veterinarians are quite familiar with diabetes. A wide variety of mammals from pigs and apes to horses and dolphins can develop an equivalent of the human condition. Dogs may be prescribed daily insulin shots, and cats even develop peripheral neuropathy and retinopathy like humans with diabetes.
So it’s not entirely surprising that a team at the Los Angeles Zoo diagnosed Quincy, a 3-year-old Queensland koala, with diabetes.
Quincy’s glucose levels should have been around 80-130 mg/dL, similar to the ideal levels in humans, said San Diego Zoo senior veterinarian Cora Singleton, DVM, in an interview. But tests prompted by his symptoms showed his levels were high, she said, and they stayed that way. According to her, that suggested he wasn’t just having a one-time elevation that animals can experience when they’re stressed.
Unfortunately, there are only a few scattered reports of diabetes in koalas, and “there’s not anything documented about treating a koala over a long term,” Dr. Singleton said. “We’re in uncharted territory here.”
So the Los Angeles Zoo sent Quincy down the California coast for more specialized treatment. The San Diego Zoo’s veterinary staff took in Quincy and treated him with glucose tests and insulin shots, Dr. Singleton said. “But we were looking a way for to get more information with less disturbance to Quincy.”
Someone mentioned the idea of a sensor. “We thought, ‘What a great idea,’” Dr. Singleton said. “It would be a way for us to get a lot of information and find out how his highs and lows are related.”
That’s when the team turned to local endocrinologist Dr. Tsimikas for a helping hand.
The key to koala calming: Eucalyptus smoothies
“They did reach out to us and asked what kind of sensors might be available. We connected them to Dexcom,” a CGM company that’s based in San Diego, Dr. Tsimikas said. “We knew the newest one was coming along and suggested they place that on him as a starting point.”
On June 1, a zoo team attached a Dexcom G6 Continuous Glucose Monitoring System to the koala’s side.
“He’s doing very well. He tolerates the CGM superbly,” Dr. Singleton said. And Quincy doesn’t react when sensors are applied, she said, although it helps that he gets to enjoy a eucalyptus smoothie during the procedure. “Put that in a big syringe, and he’ll volunteer for most anything,” she said.
Obesity can trigger diabetes in mammals other than humans. Could eucalyptus overindulgence explain Quincy’s case of diabetes? Nope.
According to Dr. Tsimikas, the ingredients of the eucalyptus smoothie are just pureed eucalyptus leaves that “go down fast and easy.” These naturally have a nice mix of carbohydrates, fat, and protein to better manage the koala’s sugars and other nutritional needs. If he is dropping his blood sugar values fast, there is another dextrose drink they give him in small amounts, which contains 5-10 g carbohydrates. This is enough to help bring his glucose values back up. It is similar to the treatment recommendations provided to humans with diabetes where they are told to take 15 g of carbohydrates such as honey, hard candies, or juice to prevent a severe hypoglycemic episode.
Dr. Singleton noted that Quincy appears to have the koala equivalent is type 1 diabetes mellitus (T1DM).
Dr. Tsimikas noted “We are not finding the typical antibodies that we find in human T1DM. Quincy is showing low insulin levels, which is why it more closely resembles T1DM. We will be doing further analysis and comparisons with nondiabetic koalas in the future to see if it can be better differentiated.
While he appears to have type 1 diabetes, it’s not clear why he developed it, Dr. Singleton said.
While Quincy is only 3 years old, he’s a full-fledged adult in koala terms. Koalas typically live up to their mid-teens, she said.
This speechless patient still manages to communicate
The San Diego Zoo’s veterinary staff is monitoring Quincy and trying to understand how his glucose levels and daily insulin shots affect him. His tiny size has ruled out use of an insulin pump: Although the insulin pumps have been getting smaller and lighter, they are still too large to attach to our tiny friend. Especially since he would need both the CGM device and the pump, there is not a lot of surface area on his body for attachment of all the devices, according to Dr. Tsimikas.
Since Quincy is so tiny, insulin doses must be minuscule to avoid sending him into hypoglycemia, Dr. Tsimikas said. She said the koala’s medical team is planning to try using a NovoPen Echo injector with a half-unit of insulin.
Dr. Singleton noted that for now, “he’s maintaining his body weight, and he has days when he feels spunky. Sometimes, when he knows it’s breakfast time, and he hears his caretakers coming up the doorway with his breakfast, he’ll be very active on his perch.”
But he has sluggish days, too, when he’ll try to sleep in. Dr. Singleton keeps an eye out for grogginess and signs of weakness and hypoglycemia or hyperglycemia like “a little wobble in his step.”
“The biggest thing I’ve learned from Quincy is the value of his particular nonverbal cues,” she said. “I’m starting to understand when he feels like his sugars are a little high or a little low. I imagine that doctors and parents have the same challenges with little patients, along with figuring out how you communicate that this is supposed to help them.”
Dr. Tsimikas agreed, noting that she sees similarities between Quincy and patients who are hospitalized and can’t easily communicate. Now, “we can track the folks who are on the CGM and intervene earlier than before,” said Dr. Tsimikas, who’s part of a clinical trial team testing CGM devices in two hospitals. “It’s almost like having another vital sign.
“It is only when we have all the data on all the other factors that can influence blood sugar, such as eating patterns, insulin dose and timing, and activity level that we can more accurately adjust the medical interventions.” This requires collaboration between all the groups involved in Quincy’s care. In koalas, the collaboration is with the veterinarian, koala zookeepers, dietitian, and the technology monitoring team. Whereas, for humans, we need parents, care providers, diabetes educators, dietitians, and physicians.
It’s not clear if Quincy will need his CGM for the rest of his life. If he’s stable on a specific insulin dose, Dr. Tsimikas said, he may not need it. But it sounds like eucalyptus smoothies will always be a vital part of his regimen.
In the name of thoroughness, take note that Quincy is not the first diabetic zoo animal whose care involved physicians from Scripps. “We have had several other consultations for animals with diabetes. Nearly 25 years ago, a roller-skating chimpanzee with diabetes was brought to the Scripps Whittier Institute labs for evaluation and treatment recommendations. A few years later, one of our medical directors, Alberto Hayek, MD, advised on the care of Lune, a diabetic baboon at the San Diego Zoo, for insulin management. This time we are making house calls to the zoo to treat Quincy in his home environment. Each animal experience offers opportunities to expand our knowledge about diabetes care and exchange approaches that we might not otherwise be aware of. This has been fun and rewarding. I am looking forward to seeing further outcomes from our interactions with Quincy,” according to Dr. Tsimikas.
Dr. Tsimikas reports that her center conducts research with Dexcom and Novo Nordisk. Dr. Singleton reports no relevant disclosures.
SAN DIEGO – The 14-pound patient with the deep-pile complexion was lethargic, kept drinking a lot of water, and had a glucose level in the range of 600-700 mg/dL. He was nearly comatose by the time medical staff transferred him to a specialized facility.
The diagnosis: Diabetes. The treatment: Insulin. But multiple daily skin pricks were quite a challenge for Quincy the koala. After all, he requires up to 22 hours of shut-eye each day.
What to do? The veterinary staff at the San Diego Zoo turned to the experts – an endocrinologist and a manufacturer of continuous glucose monitors. Now, Quincy has his own CGM, and a medical team that is tracking his glucose levels in real time on their smartphones.
In fact, Athena Philis-Tsimikas, MD, of the Scripps Whittier Diabetes Institute, pulls out her phone and checks on him at least a couple times a day. She also gets alerts if his blood sugar drops too quickly.
“He is definitely another one of my patients,” she said in an interview. But he’s the only one who lives in trees and enjoys a nice eucalyptus smoothie.
Humans are hardly the only mammals who get diabetes
Veterinarians are quite familiar with diabetes. A wide variety of mammals from pigs and apes to horses and dolphins can develop an equivalent of the human condition. Dogs may be prescribed daily insulin shots, and cats even develop peripheral neuropathy and retinopathy like humans with diabetes.
So it’s not entirely surprising that a team at the Los Angeles Zoo diagnosed Quincy, a 3-year-old Queensland koala, with diabetes.
Quincy’s glucose levels should have been around 80-130 mg/dL, similar to the ideal levels in humans, said San Diego Zoo senior veterinarian Cora Singleton, DVM, in an interview. But tests prompted by his symptoms showed his levels were high, she said, and they stayed that way. According to her, that suggested he wasn’t just having a one-time elevation that animals can experience when they’re stressed.
Unfortunately, there are only a few scattered reports of diabetes in koalas, and “there’s not anything documented about treating a koala over a long term,” Dr. Singleton said. “We’re in uncharted territory here.”
So the Los Angeles Zoo sent Quincy down the California coast for more specialized treatment. The San Diego Zoo’s veterinary staff took in Quincy and treated him with glucose tests and insulin shots, Dr. Singleton said. “But we were looking a way for to get more information with less disturbance to Quincy.”
Someone mentioned the idea of a sensor. “We thought, ‘What a great idea,’” Dr. Singleton said. “It would be a way for us to get a lot of information and find out how his highs and lows are related.”
That’s when the team turned to local endocrinologist Dr. Tsimikas for a helping hand.
The key to koala calming: Eucalyptus smoothies
“They did reach out to us and asked what kind of sensors might be available. We connected them to Dexcom,” a CGM company that’s based in San Diego, Dr. Tsimikas said. “We knew the newest one was coming along and suggested they place that on him as a starting point.”
On June 1, a zoo team attached a Dexcom G6 Continuous Glucose Monitoring System to the koala’s side.
“He’s doing very well. He tolerates the CGM superbly,” Dr. Singleton said. And Quincy doesn’t react when sensors are applied, she said, although it helps that he gets to enjoy a eucalyptus smoothie during the procedure. “Put that in a big syringe, and he’ll volunteer for most anything,” she said.
Obesity can trigger diabetes in mammals other than humans. Could eucalyptus overindulgence explain Quincy’s case of diabetes? Nope.
According to Dr. Tsimikas, the ingredients of the eucalyptus smoothie are just pureed eucalyptus leaves that “go down fast and easy.” These naturally have a nice mix of carbohydrates, fat, and protein to better manage the koala’s sugars and other nutritional needs. If he is dropping his blood sugar values fast, there is another dextrose drink they give him in small amounts, which contains 5-10 g carbohydrates. This is enough to help bring his glucose values back up. It is similar to the treatment recommendations provided to humans with diabetes where they are told to take 15 g of carbohydrates such as honey, hard candies, or juice to prevent a severe hypoglycemic episode.
Dr. Singleton noted that Quincy appears to have the koala equivalent is type 1 diabetes mellitus (T1DM).
Dr. Tsimikas noted “We are not finding the typical antibodies that we find in human T1DM. Quincy is showing low insulin levels, which is why it more closely resembles T1DM. We will be doing further analysis and comparisons with nondiabetic koalas in the future to see if it can be better differentiated.
While he appears to have type 1 diabetes, it’s not clear why he developed it, Dr. Singleton said.
While Quincy is only 3 years old, he’s a full-fledged adult in koala terms. Koalas typically live up to their mid-teens, she said.
This speechless patient still manages to communicate
The San Diego Zoo’s veterinary staff is monitoring Quincy and trying to understand how his glucose levels and daily insulin shots affect him. His tiny size has ruled out use of an insulin pump: Although the insulin pumps have been getting smaller and lighter, they are still too large to attach to our tiny friend. Especially since he would need both the CGM device and the pump, there is not a lot of surface area on his body for attachment of all the devices, according to Dr. Tsimikas.
Since Quincy is so tiny, insulin doses must be minuscule to avoid sending him into hypoglycemia, Dr. Tsimikas said. She said the koala’s medical team is planning to try using a NovoPen Echo injector with a half-unit of insulin.
Dr. Singleton noted that for now, “he’s maintaining his body weight, and he has days when he feels spunky. Sometimes, when he knows it’s breakfast time, and he hears his caretakers coming up the doorway with his breakfast, he’ll be very active on his perch.”
But he has sluggish days, too, when he’ll try to sleep in. Dr. Singleton keeps an eye out for grogginess and signs of weakness and hypoglycemia or hyperglycemia like “a little wobble in his step.”
“The biggest thing I’ve learned from Quincy is the value of his particular nonverbal cues,” she said. “I’m starting to understand when he feels like his sugars are a little high or a little low. I imagine that doctors and parents have the same challenges with little patients, along with figuring out how you communicate that this is supposed to help them.”
Dr. Tsimikas agreed, noting that she sees similarities between Quincy and patients who are hospitalized and can’t easily communicate. Now, “we can track the folks who are on the CGM and intervene earlier than before,” said Dr. Tsimikas, who’s part of a clinical trial team testing CGM devices in two hospitals. “It’s almost like having another vital sign.
“It is only when we have all the data on all the other factors that can influence blood sugar, such as eating patterns, insulin dose and timing, and activity level that we can more accurately adjust the medical interventions.” This requires collaboration between all the groups involved in Quincy’s care. In koalas, the collaboration is with the veterinarian, koala zookeepers, dietitian, and the technology monitoring team. Whereas, for humans, we need parents, care providers, diabetes educators, dietitians, and physicians.
It’s not clear if Quincy will need his CGM for the rest of his life. If he’s stable on a specific insulin dose, Dr. Tsimikas said, he may not need it. But it sounds like eucalyptus smoothies will always be a vital part of his regimen.
In the name of thoroughness, take note that Quincy is not the first diabetic zoo animal whose care involved physicians from Scripps. “We have had several other consultations for animals with diabetes. Nearly 25 years ago, a roller-skating chimpanzee with diabetes was brought to the Scripps Whittier Institute labs for evaluation and treatment recommendations. A few years later, one of our medical directors, Alberto Hayek, MD, advised on the care of Lune, a diabetic baboon at the San Diego Zoo, for insulin management. This time we are making house calls to the zoo to treat Quincy in his home environment. Each animal experience offers opportunities to expand our knowledge about diabetes care and exchange approaches that we might not otherwise be aware of. This has been fun and rewarding. I am looking forward to seeing further outcomes from our interactions with Quincy,” according to Dr. Tsimikas.
Dr. Tsimikas reports that her center conducts research with Dexcom and Novo Nordisk. Dr. Singleton reports no relevant disclosures.