User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Artificial pancreas can improve inpatient glycemic control in type 2 diabetes
NEW ORLEANS – Having people with type 2 diabetes mellitus use an artificial pancreas during hospitalization has the potential to improve control of their glycemia when compared with conventional insulin therapy, based on the results of a small study of inpatients in the United Kingdom.
“This is the first study to show that automated subcutaneous closed-loop insulin delivery without meal-time insulin is feasible and safe in patients with insulin-treated type 2 diabetes in the general wards,” Dr. Hood Thabit of the University of Cambridge (England) reported at the ADA annual scientific sessions. “Closed-loop [delivery] increased time in target, with reduced glucose variability and reduced time spent hyperglycemic without actually increasing time spent hypoglycemic,” he said.
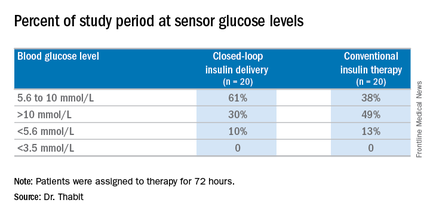
The study involved 40 general ward inpatients evenly assigned to the closed-loop system and conventional insulin therapy for 72 hours.
Dr. Thabit said hyperglycemia in hospital patients is a common problem that’s poorly managed. “There’s an unmet need for an effective and safe glucose control, specifically in the underserved and understudied population of type 2 diabetes in the general wards,” he said. The use of the closed-loop system in inpatients with type 2 diabetes “remains untested until now,” Dr. Thabit added.
The 20 patients randomized to the closed-loop system spent an average of 61% of the whole study period within the sensor glucose target vs. 38% of those on conventional insulin therapy. The closed-loop patients also used comparable insulin daily on average: 62.6 U (±36.3 U) vs. 66.0 U (±39.6 U), Dr. Thabit said.
He noted that those on the closed-loop system did not have to announce meals to the control algorithm, or give any meal-time insulin – “we didn’t want to trouble our nurses with this, due to the increasing workload that health care professionals in the hospital currently face,” he said – and showed “significantly improved” nighttime control of glucose while “simultaneously reducing the risk of nocturnal hypoglycemia”. “The closed loop may potentially be an effective and safe tool to manage hospital inpatient hyperglycemia in this particularly underserved population of patients whilst easing the burden of health care professionals in hospital,” he said.
The cost is not insignificant. The pump and sensor devices together with related consumables can cost up to £6,000 (about $8,600), but he did note the artificial pancreas device itself is reusable. The cost of the automated closed-loop glucose control system can also potentially be offset by the reduced time of health care professionals spent managing inpatient hyperglycemia safely. The study investigators are in the process of planning a larger trial, Dr. Thabit said.
Dr. Thabit had no financial disclosures. Some coauthors disclosed relationships with Novo Nordisk; Medtronic MiniMed; Becton, Dickinson and Co.; Abbott Diabetes Care; Roche Pharmaceuticals; Cell Novo; Animas; Eli Lilly; B. Braun Melsungen; Sanofi-Aventis Deutschland; and Profil Institute for Clinical Research.
NEW ORLEANS – Having people with type 2 diabetes mellitus use an artificial pancreas during hospitalization has the potential to improve control of their glycemia when compared with conventional insulin therapy, based on the results of a small study of inpatients in the United Kingdom.
“This is the first study to show that automated subcutaneous closed-loop insulin delivery without meal-time insulin is feasible and safe in patients with insulin-treated type 2 diabetes in the general wards,” Dr. Hood Thabit of the University of Cambridge (England) reported at the ADA annual scientific sessions. “Closed-loop [delivery] increased time in target, with reduced glucose variability and reduced time spent hyperglycemic without actually increasing time spent hypoglycemic,” he said.
The study involved 40 general ward inpatients evenly assigned to the closed-loop system and conventional insulin therapy for 72 hours.
Dr. Thabit said hyperglycemia in hospital patients is a common problem that’s poorly managed. “There’s an unmet need for an effective and safe glucose control, specifically in the underserved and understudied population of type 2 diabetes in the general wards,” he said. The use of the closed-loop system in inpatients with type 2 diabetes “remains untested until now,” Dr. Thabit added.
The 20 patients randomized to the closed-loop system spent an average of 61% of the whole study period within the sensor glucose target vs. 38% of those on conventional insulin therapy. The closed-loop patients also used comparable insulin daily on average: 62.6 U (±36.3 U) vs. 66.0 U (±39.6 U), Dr. Thabit said.
He noted that those on the closed-loop system did not have to announce meals to the control algorithm, or give any meal-time insulin – “we didn’t want to trouble our nurses with this, due to the increasing workload that health care professionals in the hospital currently face,” he said – and showed “significantly improved” nighttime control of glucose while “simultaneously reducing the risk of nocturnal hypoglycemia”. “The closed loop may potentially be an effective and safe tool to manage hospital inpatient hyperglycemia in this particularly underserved population of patients whilst easing the burden of health care professionals in hospital,” he said.

The cost is not insignificant. The pump and sensor devices together with related consumables can cost up to £6,000 (about $8,600), but he did note the artificial pancreas device itself is reusable. The cost of the automated closed-loop glucose control system can also potentially be offset by the reduced time of health care professionals spent managing inpatient hyperglycemia safely. The study investigators are in the process of planning a larger trial, Dr. Thabit said.
Dr. Thabit had no financial disclosures. Some coauthors disclosed relationships with Novo Nordisk; Medtronic MiniMed; Becton, Dickinson and Co.; Abbott Diabetes Care; Roche Pharmaceuticals; Cell Novo; Animas; Eli Lilly; B. Braun Melsungen; Sanofi-Aventis Deutschland; and Profil Institute for Clinical Research.
NEW ORLEANS – Having people with type 2 diabetes mellitus use an artificial pancreas during hospitalization has the potential to improve control of their glycemia when compared with conventional insulin therapy, based on the results of a small study of inpatients in the United Kingdom.
“This is the first study to show that automated subcutaneous closed-loop insulin delivery without meal-time insulin is feasible and safe in patients with insulin-treated type 2 diabetes in the general wards,” Dr. Hood Thabit of the University of Cambridge (England) reported at the ADA annual scientific sessions. “Closed-loop [delivery] increased time in target, with reduced glucose variability and reduced time spent hyperglycemic without actually increasing time spent hypoglycemic,” he said.
The study involved 40 general ward inpatients evenly assigned to the closed-loop system and conventional insulin therapy for 72 hours.
Dr. Thabit said hyperglycemia in hospital patients is a common problem that’s poorly managed. “There’s an unmet need for an effective and safe glucose control, specifically in the underserved and understudied population of type 2 diabetes in the general wards,” he said. The use of the closed-loop system in inpatients with type 2 diabetes “remains untested until now,” Dr. Thabit added.
The 20 patients randomized to the closed-loop system spent an average of 61% of the whole study period within the sensor glucose target vs. 38% of those on conventional insulin therapy. The closed-loop patients also used comparable insulin daily on average: 62.6 U (±36.3 U) vs. 66.0 U (±39.6 U), Dr. Thabit said.
He noted that those on the closed-loop system did not have to announce meals to the control algorithm, or give any meal-time insulin – “we didn’t want to trouble our nurses with this, due to the increasing workload that health care professionals in the hospital currently face,” he said – and showed “significantly improved” nighttime control of glucose while “simultaneously reducing the risk of nocturnal hypoglycemia”. “The closed loop may potentially be an effective and safe tool to manage hospital inpatient hyperglycemia in this particularly underserved population of patients whilst easing the burden of health care professionals in hospital,” he said.

The cost is not insignificant. The pump and sensor devices together with related consumables can cost up to £6,000 (about $8,600), but he did note the artificial pancreas device itself is reusable. The cost of the automated closed-loop glucose control system can also potentially be offset by the reduced time of health care professionals spent managing inpatient hyperglycemia safely. The study investigators are in the process of planning a larger trial, Dr. Thabit said.
Dr. Thabit had no financial disclosures. Some coauthors disclosed relationships with Novo Nordisk; Medtronic MiniMed; Becton, Dickinson and Co.; Abbott Diabetes Care; Roche Pharmaceuticals; Cell Novo; Animas; Eli Lilly; B. Braun Melsungen; Sanofi-Aventis Deutschland; and Profil Institute for Clinical Research.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point:Hospitalized patients in the general ward with type 2 diabetes mellitus can maintain better glycemic control by using an artificial pancreas than by taking conventional therapy.
Major finding: Patients on the closed-loop system spent an average of 61% of the study period within the sensor glucose target vs. 38% for those on conventional insulin therapy.
Data source: A single-center study of 40 patients randomized to wear the artificial pancreas or take conventional therapy.
Disclosures: Dr. Thabit had no financial disclosures. Some coauthors disclosed relationships with Novo Nordisk; Medtronic MiniMed; Becton, Dickinson and Co.; Abbott Diabetes Care; Roche Pharmaceuticals; Cell Novo; Animas; Eli Lilly; B. Braun Melsungen; Sanofi-Aventis Deutschland; and Profil Institute for Clinical Research.
Pioglitazone safe and effective for steatohepatitis in T2DM
Long-term pioglitazone is not only safe for patients with nonalcoholic steatohepatitis (NASH) and prediabetes or type 2 diabetes but is associated with significant improvements in fatty liver disease outcomes, compared with placebo, research suggests.
Investigators randomized 101 individuals with biopsy-proven NASH and either prediabetes or type 2 diabetes to 18 months of 45 mg/day of pioglitazone or placebo, followed by an 18 month open-label phase of pioglitazone, in addition to a hypocaloric diet, according to a paper published online June 20 in the Annals of Internal Medicine.
Among patients randomized to pioglitazone, 58% achieved at least a two-point reduction in their NASH activity score, without a worsening of fibrosis, compared with only 17% of the placebo group (P less than .001).
The pioglitazone group also showed a significantly greater likelihood that their NASH would resolve, with 51% of the treatment group achieving resolution, compared with 19% of the placebo group (P less than .001).
Pioglitazone treatment was also associated with significantly greater improvements in steatosis, inflammation, and ballooning necrosis, as well as mean histologic scores and fibrosis scores (Ann Intern Med. 2016 Jun 20. doi: 10.7326/M15-1774).
The study also showed that only 12% of the pioglitazone group showed progression of any fibrosis, compared with 28% of the placebo group (P = .039), while pioglitazone was associated with significant 2.5 kg weight gain over placebo.
“Because thiazolidinediones target insulin resistance and adipose tissue dysfunction or inflammation that promotes hepatic ‘lipotoxicity’ in NASH (which is also a prominent feature of T2DM), they may be more helpful for treating steatohepatitis in this population,” wrote Dr. Kenneth Cusi, from the division of endocrinology at the University of Florida, Gainesville, and his coauthors.
However, the authors noted that there have been relatively few studies of pioglitazone and its effects on steatohepatitis in individuals with prediabetes or type 2 diabetes.
“This study also has implications for patients with prediabetes and NASH (about half of our participants) because hepatic steatosis is a risk factor for T2DM, even in nonobese patients,” the authors wrote. They suggested that future studies could explore whether the reversal of hepatic steatosis or nonalcoholic steatohepatitis in patients with prediabetes may help to halt the development of type 2 diabetes.
The study was supported by the Burroughs Wellcome Fund and the American Diabetes Association. One author reported nonfinancial support from Takeda Pharmaceuticals in the form of study medication and placebo, and grants and consultancies from pharmaceutical companies outside the submitted work. No other conflicts of interest were declared.
This study extends previous observations of the therapeutic benefits of pioglitazone in NASH to include patients with impaired glucose regulation, but the use of pioglitazone beyond 18 months did not provide significant additional resolution of fibrosis, although it may retard further progression.
We believe that physicians should consider adding pioglitazone to their toolboxes when facing patients with NASH and diabetes, but the primary obstacle to the widespread use of pioglitazone remains its safety profile.
Thus, treatment should be considered for patients at the greatest risk (those with NASH and fibrosis) and should be balanced against the common risk for weight gain and the uncommon risks for fracture and heart failure.
Dr. Eduardo Vilar-Gomez is from the University of Seville, Spain, and Dr. Leon A. Adams is from the University of Western Australia, Perth. These comments were made in an accompanying editorial (Ann Intern Med. 2016 Jun 20. doi: 10.7326/M16-1303). No conflicts of interest were declared.
This study extends previous observations of the therapeutic benefits of pioglitazone in NASH to include patients with impaired glucose regulation, but the use of pioglitazone beyond 18 months did not provide significant additional resolution of fibrosis, although it may retard further progression.
We believe that physicians should consider adding pioglitazone to their toolboxes when facing patients with NASH and diabetes, but the primary obstacle to the widespread use of pioglitazone remains its safety profile.
Thus, treatment should be considered for patients at the greatest risk (those with NASH and fibrosis) and should be balanced against the common risk for weight gain and the uncommon risks for fracture and heart failure.
Dr. Eduardo Vilar-Gomez is from the University of Seville, Spain, and Dr. Leon A. Adams is from the University of Western Australia, Perth. These comments were made in an accompanying editorial (Ann Intern Med. 2016 Jun 20. doi: 10.7326/M16-1303). No conflicts of interest were declared.
This study extends previous observations of the therapeutic benefits of pioglitazone in NASH to include patients with impaired glucose regulation, but the use of pioglitazone beyond 18 months did not provide significant additional resolution of fibrosis, although it may retard further progression.
We believe that physicians should consider adding pioglitazone to their toolboxes when facing patients with NASH and diabetes, but the primary obstacle to the widespread use of pioglitazone remains its safety profile.
Thus, treatment should be considered for patients at the greatest risk (those with NASH and fibrosis) and should be balanced against the common risk for weight gain and the uncommon risks for fracture and heart failure.
Dr. Eduardo Vilar-Gomez is from the University of Seville, Spain, and Dr. Leon A. Adams is from the University of Western Australia, Perth. These comments were made in an accompanying editorial (Ann Intern Med. 2016 Jun 20. doi: 10.7326/M16-1303). No conflicts of interest were declared.
Long-term pioglitazone is not only safe for patients with nonalcoholic steatohepatitis (NASH) and prediabetes or type 2 diabetes but is associated with significant improvements in fatty liver disease outcomes, compared with placebo, research suggests.
Investigators randomized 101 individuals with biopsy-proven NASH and either prediabetes or type 2 diabetes to 18 months of 45 mg/day of pioglitazone or placebo, followed by an 18 month open-label phase of pioglitazone, in addition to a hypocaloric diet, according to a paper published online June 20 in the Annals of Internal Medicine.
Among patients randomized to pioglitazone, 58% achieved at least a two-point reduction in their NASH activity score, without a worsening of fibrosis, compared with only 17% of the placebo group (P less than .001).
The pioglitazone group also showed a significantly greater likelihood that their NASH would resolve, with 51% of the treatment group achieving resolution, compared with 19% of the placebo group (P less than .001).
Pioglitazone treatment was also associated with significantly greater improvements in steatosis, inflammation, and ballooning necrosis, as well as mean histologic scores and fibrosis scores (Ann Intern Med. 2016 Jun 20. doi: 10.7326/M15-1774).
The study also showed that only 12% of the pioglitazone group showed progression of any fibrosis, compared with 28% of the placebo group (P = .039), while pioglitazone was associated with significant 2.5 kg weight gain over placebo.
“Because thiazolidinediones target insulin resistance and adipose tissue dysfunction or inflammation that promotes hepatic ‘lipotoxicity’ in NASH (which is also a prominent feature of T2DM), they may be more helpful for treating steatohepatitis in this population,” wrote Dr. Kenneth Cusi, from the division of endocrinology at the University of Florida, Gainesville, and his coauthors.
However, the authors noted that there have been relatively few studies of pioglitazone and its effects on steatohepatitis in individuals with prediabetes or type 2 diabetes.
“This study also has implications for patients with prediabetes and NASH (about half of our participants) because hepatic steatosis is a risk factor for T2DM, even in nonobese patients,” the authors wrote. They suggested that future studies could explore whether the reversal of hepatic steatosis or nonalcoholic steatohepatitis in patients with prediabetes may help to halt the development of type 2 diabetes.
The study was supported by the Burroughs Wellcome Fund and the American Diabetes Association. One author reported nonfinancial support from Takeda Pharmaceuticals in the form of study medication and placebo, and grants and consultancies from pharmaceutical companies outside the submitted work. No other conflicts of interest were declared.
Long-term pioglitazone is not only safe for patients with nonalcoholic steatohepatitis (NASH) and prediabetes or type 2 diabetes but is associated with significant improvements in fatty liver disease outcomes, compared with placebo, research suggests.
Investigators randomized 101 individuals with biopsy-proven NASH and either prediabetes or type 2 diabetes to 18 months of 45 mg/day of pioglitazone or placebo, followed by an 18 month open-label phase of pioglitazone, in addition to a hypocaloric diet, according to a paper published online June 20 in the Annals of Internal Medicine.
Among patients randomized to pioglitazone, 58% achieved at least a two-point reduction in their NASH activity score, without a worsening of fibrosis, compared with only 17% of the placebo group (P less than .001).
The pioglitazone group also showed a significantly greater likelihood that their NASH would resolve, with 51% of the treatment group achieving resolution, compared with 19% of the placebo group (P less than .001).
Pioglitazone treatment was also associated with significantly greater improvements in steatosis, inflammation, and ballooning necrosis, as well as mean histologic scores and fibrosis scores (Ann Intern Med. 2016 Jun 20. doi: 10.7326/M15-1774).
The study also showed that only 12% of the pioglitazone group showed progression of any fibrosis, compared with 28% of the placebo group (P = .039), while pioglitazone was associated with significant 2.5 kg weight gain over placebo.
“Because thiazolidinediones target insulin resistance and adipose tissue dysfunction or inflammation that promotes hepatic ‘lipotoxicity’ in NASH (which is also a prominent feature of T2DM), they may be more helpful for treating steatohepatitis in this population,” wrote Dr. Kenneth Cusi, from the division of endocrinology at the University of Florida, Gainesville, and his coauthors.
However, the authors noted that there have been relatively few studies of pioglitazone and its effects on steatohepatitis in individuals with prediabetes or type 2 diabetes.
“This study also has implications for patients with prediabetes and NASH (about half of our participants) because hepatic steatosis is a risk factor for T2DM, even in nonobese patients,” the authors wrote. They suggested that future studies could explore whether the reversal of hepatic steatosis or nonalcoholic steatohepatitis in patients with prediabetes may help to halt the development of type 2 diabetes.
The study was supported by the Burroughs Wellcome Fund and the American Diabetes Association. One author reported nonfinancial support from Takeda Pharmaceuticals in the form of study medication and placebo, and grants and consultancies from pharmaceutical companies outside the submitted work. No other conflicts of interest were declared.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Long-term pioglitazone is not only safe for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes, but is associated with significant improvements in fatty liver disease outcomes.
Major finding: Among patients randomized to pioglitazone, 58% achieved at least a two-point reduction in their nonalcoholic fatty liver disease activity score, compared with only 17% of the placebo group.
Data source: Randomized placebo-controlled trial of pioglitazone in 101 individuals with biopsy-proven NASH and either prediabetes or type 2 diabetes.
Disclosures: The study was supported by the Burroughs Wellcome Fund and the American Diabetes Association. One author reported nonfinancial support from Takeda Pharmaceuticals in the form of study medication and placebo, and grants and consultancies from pharmaceutical companies outside the submitted work. No other conflicts of interest were declared.
Comprehensive diabetic retinopathy screening challenging
NEW ORLEANS – Fewer than one-third of patients with diabetes being cared for by a public hospital system underwent screening for retinopathy within the past year, judging from the results from a survey of administrative data.
“Diabetic retinopathy is a major cause of vision loss in the United States,” researchers led by Dr. David C. Ziemer wrote in an abstract presented during a poster session at the annual scientific sessions of the American Diabetes Association.
“In 2011, the age-adjusted percentage of adults with diagnosed diabetes reporting visual impairment was 17.6%. This is a pressing issue as the number of Americans with diabetic retinopathy is expected to double from 7.7 million in 2010 to 15.6 million in 2050.”
In an effort to plan for better diabetic retinopathy screening, Dr. Ziemer and his associates analyzed 2014 administrative data from 19,361 patients with diabetes who attended one of several clinics operated by the Atlanta-based Grady Health System. Diabetic retinopathy was considered complete if ophthalmology clinic, optometry, or retinal photograph visit was attended. The researchers also surveyed a convenience sample of 80 patients about their diabetic retinopathy screening in the past year.
The mean age of patients was 57 years, their mean hemoglobin A1c level was 7.8%, 59% were female, and 83% were African-American. Of the 19,361 patients, 5,595 (29%) underwent diabetic retinopathy screening and 13,766 (71%) did not. The unscreened had a mean of 1 clinic visit for diabetes care, compared with a mean of 3.1 for those who underwent screening (P less than .0005). In the analysis of administrative data, Dr. Ziemer, of the division of endocrinology at Emory University, Atlanta, reported that 29% of patients underwent diabetic retinopathy screening in the past year, with variation by care site that ranged from 5% to 66%, and 5,000 had no diabetes continuity care visit.
Factors associated with increased diabetic retinopathy screening were treatment in a diabetes clinic (odds ratio, 2.8), treatment in a primary care clinic (OR, 2.1), and being older (OR, 1.03/year; P less than .001 for all associations), according to a multivariable analysis. Factors associated with decreased diabetic retinopathy screening were Hispanic ethnicity (OR, 0.7) and having a mental health diagnosis (OR, .8; P less than .001 for both associations). The researchers also found that having an in-clinic eye screening doubled the proportion of diabetic retinopathy screenings (48% vs. 22%) and decreased the number of screenings done in an outside clinic (45% vs. 95%).
Of the 80 patients who completed the survey, 68% reported that they underwent diabetic retinopathy screening within the past year, which was in contrast to the 29% reported by administrative data. In addition, 50% of survey respondents who did not undergo diabetic retinopathy screening reported that they received a referral, yet more than 40% failed to honor eye appointments. “The first barrier to address is people who don’t keep appointments,” Dr. Ziemer said in an interview. “Getting people in care is one issue. Having the capacity is another. That’s a real problem.”
The study was supported by the American Diabetes Association. Dr. Ziemer reported having no financial disclosures.
NEW ORLEANS – Fewer than one-third of patients with diabetes being cared for by a public hospital system underwent screening for retinopathy within the past year, judging from the results from a survey of administrative data.
“Diabetic retinopathy is a major cause of vision loss in the United States,” researchers led by Dr. David C. Ziemer wrote in an abstract presented during a poster session at the annual scientific sessions of the American Diabetes Association.
“In 2011, the age-adjusted percentage of adults with diagnosed diabetes reporting visual impairment was 17.6%. This is a pressing issue as the number of Americans with diabetic retinopathy is expected to double from 7.7 million in 2010 to 15.6 million in 2050.”
In an effort to plan for better diabetic retinopathy screening, Dr. Ziemer and his associates analyzed 2014 administrative data from 19,361 patients with diabetes who attended one of several clinics operated by the Atlanta-based Grady Health System. Diabetic retinopathy was considered complete if ophthalmology clinic, optometry, or retinal photograph visit was attended. The researchers also surveyed a convenience sample of 80 patients about their diabetic retinopathy screening in the past year.
The mean age of patients was 57 years, their mean hemoglobin A1c level was 7.8%, 59% were female, and 83% were African-American. Of the 19,361 patients, 5,595 (29%) underwent diabetic retinopathy screening and 13,766 (71%) did not. The unscreened had a mean of 1 clinic visit for diabetes care, compared with a mean of 3.1 for those who underwent screening (P less than .0005). In the analysis of administrative data, Dr. Ziemer, of the division of endocrinology at Emory University, Atlanta, reported that 29% of patients underwent diabetic retinopathy screening in the past year, with variation by care site that ranged from 5% to 66%, and 5,000 had no diabetes continuity care visit.
Factors associated with increased diabetic retinopathy screening were treatment in a diabetes clinic (odds ratio, 2.8), treatment in a primary care clinic (OR, 2.1), and being older (OR, 1.03/year; P less than .001 for all associations), according to a multivariable analysis. Factors associated with decreased diabetic retinopathy screening were Hispanic ethnicity (OR, 0.7) and having a mental health diagnosis (OR, .8; P less than .001 for both associations). The researchers also found that having an in-clinic eye screening doubled the proportion of diabetic retinopathy screenings (48% vs. 22%) and decreased the number of screenings done in an outside clinic (45% vs. 95%).
Of the 80 patients who completed the survey, 68% reported that they underwent diabetic retinopathy screening within the past year, which was in contrast to the 29% reported by administrative data. In addition, 50% of survey respondents who did not undergo diabetic retinopathy screening reported that they received a referral, yet more than 40% failed to honor eye appointments. “The first barrier to address is people who don’t keep appointments,” Dr. Ziemer said in an interview. “Getting people in care is one issue. Having the capacity is another. That’s a real problem.”
The study was supported by the American Diabetes Association. Dr. Ziemer reported having no financial disclosures.
NEW ORLEANS – Fewer than one-third of patients with diabetes being cared for by a public hospital system underwent screening for retinopathy within the past year, judging from the results from a survey of administrative data.
“Diabetic retinopathy is a major cause of vision loss in the United States,” researchers led by Dr. David C. Ziemer wrote in an abstract presented during a poster session at the annual scientific sessions of the American Diabetes Association.
“In 2011, the age-adjusted percentage of adults with diagnosed diabetes reporting visual impairment was 17.6%. This is a pressing issue as the number of Americans with diabetic retinopathy is expected to double from 7.7 million in 2010 to 15.6 million in 2050.”
In an effort to plan for better diabetic retinopathy screening, Dr. Ziemer and his associates analyzed 2014 administrative data from 19,361 patients with diabetes who attended one of several clinics operated by the Atlanta-based Grady Health System. Diabetic retinopathy was considered complete if ophthalmology clinic, optometry, or retinal photograph visit was attended. The researchers also surveyed a convenience sample of 80 patients about their diabetic retinopathy screening in the past year.
The mean age of patients was 57 years, their mean hemoglobin A1c level was 7.8%, 59% were female, and 83% were African-American. Of the 19,361 patients, 5,595 (29%) underwent diabetic retinopathy screening and 13,766 (71%) did not. The unscreened had a mean of 1 clinic visit for diabetes care, compared with a mean of 3.1 for those who underwent screening (P less than .0005). In the analysis of administrative data, Dr. Ziemer, of the division of endocrinology at Emory University, Atlanta, reported that 29% of patients underwent diabetic retinopathy screening in the past year, with variation by care site that ranged from 5% to 66%, and 5,000 had no diabetes continuity care visit.
Factors associated with increased diabetic retinopathy screening were treatment in a diabetes clinic (odds ratio, 2.8), treatment in a primary care clinic (OR, 2.1), and being older (OR, 1.03/year; P less than .001 for all associations), according to a multivariable analysis. Factors associated with decreased diabetic retinopathy screening were Hispanic ethnicity (OR, 0.7) and having a mental health diagnosis (OR, .8; P less than .001 for both associations). The researchers also found that having an in-clinic eye screening doubled the proportion of diabetic retinopathy screenings (48% vs. 22%) and decreased the number of screenings done in an outside clinic (45% vs. 95%).
Of the 80 patients who completed the survey, 68% reported that they underwent diabetic retinopathy screening within the past year, which was in contrast to the 29% reported by administrative data. In addition, 50% of survey respondents who did not undergo diabetic retinopathy screening reported that they received a referral, yet more than 40% failed to honor eye appointments. “The first barrier to address is people who don’t keep appointments,” Dr. Ziemer said in an interview. “Getting people in care is one issue. Having the capacity is another. That’s a real problem.”
The study was supported by the American Diabetes Association. Dr. Ziemer reported having no financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Some 71% of diabetes patients did not undergo screening for diabetic retinopathy.
Major finding: Only 29% of patients underwent diabetic retinopathy screening in the past year, with variation by care site that ranged from 5% to 66%.
Data source: An analysis of administrative data from 19,361 patients with diabetes who attended one of several clinics operated by the Atlanta-based Grady Health System in 2014.
Disclosures: The study was supported by the American Diabetes Association. Dr. Ziemer reported having no financial disclosures.
Phentermine-topiramate shows best chance of weight loss at 1 year
The combination weight-loss drug phentermine plus topiramate is associated with the highest odds of individuals being able to lose 5% of their body weight within 1 year, according to a meta-analysis comparing outcomes and adverse events for orlistat, lorcaserin, naltrexone-bupropion, phentermine-topiramate, and liraglutide.
Researchers analyzed 28 randomized placebo- or active-controlled clinical trials involving a total of 29,018 participants and found those who took phentermine-topiramate had a ninefold greater likelihood of achieving a 5% weight loss by 1 year than did those on placebo, according to a paper published in the June 14 issue of JAMA.
Liraglutide showed the second-highest odds of achieving a 5% weight loss at 1 year (odds ratio, 5.54), followed by naltrexone-bupropion (OR, 3.96), lorcaserin (OR, 3.10), and orlistat (OR, 2.70).
Nearly one-quarter of individuals on placebo achieved at least a 5% weight loss by 1 year, compared with three-quarters of individuals taking phentermine-topiramate, 63% of those taking liraglutide, 55% taking naltrexone-bupropion, 49% taking lorcaserin, and 44% taking orlistat (JAMA 2016;315:2424-34. doi: 10.1001/jama.2016.7602).
Of those on placebo, only 9% achieved at least a 10% weight loss at 1 year, compared with 54% of patients taking phentermine-topiramate, 34% of patients on liraglutide, 30% of patients on naltrexone-bupropion, 25% of those taking lorcaserin, and 20% of those taking orlistat.
Phentermine-topiramate was also associated with the greatest weight loss, compared with placebo, with patients losing a mean of 8.8 kg vs. 5.2 kg with liraglutide, 5 kg with naltrexone-bupropion, 3.2 kg with lorcaserin, and 2.6 kg with orlistat.
While all active drugs were associated with a higher rate of discontinuation because of adverse events than was seen with placebo, liraglutide was associated with the greatest risk of discontinuation, compared with placebo, followed by naltrexone-bupropion, phentermine-topiramate, orlistat, and then lorcaserin.
Dr. Rohan Khera of the department of internal medicine at the University of Iowa, Iowa City, and coauthors wrote that pharmacologic treatment decisions should consider coexisting medical conditions that might influence for or against a particular choice for weight loss.
“For example, liraglutide may be a more appropriate agent in people with diabetes because of its glucose-lowering effects,” they wrote. “Conversely, naltrexone-bupropion in patients with chronic opiate or alcohol dependence may be associated with neuropsychiatric complications.
“Ultimately, given the differences in safety, efficacy, and response to therapy, the ideal approach to weight loss should be highly individualized, identifying appropriate candidates for pharmacotherapy, behavioral interventions, and surgical interventions.”
Two study authors were supported by a grant from the National Library of Medicine or the National Institute of Diabetes and Digestive and Kidney Diseases. One author reported receiving funding, participating on advisory committees, and serving as a consultant with a range of pharmaceutical manufacturers, as well as being a cofounder of Liponexus. Another author reported research support from NovoNordisk for research on liraglutide. No other disclosures were reported.
The combination weight-loss drug phentermine plus topiramate is associated with the highest odds of individuals being able to lose 5% of their body weight within 1 year, according to a meta-analysis comparing outcomes and adverse events for orlistat, lorcaserin, naltrexone-bupropion, phentermine-topiramate, and liraglutide.
Researchers analyzed 28 randomized placebo- or active-controlled clinical trials involving a total of 29,018 participants and found those who took phentermine-topiramate had a ninefold greater likelihood of achieving a 5% weight loss by 1 year than did those on placebo, according to a paper published in the June 14 issue of JAMA.
Liraglutide showed the second-highest odds of achieving a 5% weight loss at 1 year (odds ratio, 5.54), followed by naltrexone-bupropion (OR, 3.96), lorcaserin (OR, 3.10), and orlistat (OR, 2.70).
Nearly one-quarter of individuals on placebo achieved at least a 5% weight loss by 1 year, compared with three-quarters of individuals taking phentermine-topiramate, 63% of those taking liraglutide, 55% taking naltrexone-bupropion, 49% taking lorcaserin, and 44% taking orlistat (JAMA 2016;315:2424-34. doi: 10.1001/jama.2016.7602).
Of those on placebo, only 9% achieved at least a 10% weight loss at 1 year, compared with 54% of patients taking phentermine-topiramate, 34% of patients on liraglutide, 30% of patients on naltrexone-bupropion, 25% of those taking lorcaserin, and 20% of those taking orlistat.
Phentermine-topiramate was also associated with the greatest weight loss, compared with placebo, with patients losing a mean of 8.8 kg vs. 5.2 kg with liraglutide, 5 kg with naltrexone-bupropion, 3.2 kg with lorcaserin, and 2.6 kg with orlistat.
While all active drugs were associated with a higher rate of discontinuation because of adverse events than was seen with placebo, liraglutide was associated with the greatest risk of discontinuation, compared with placebo, followed by naltrexone-bupropion, phentermine-topiramate, orlistat, and then lorcaserin.
Dr. Rohan Khera of the department of internal medicine at the University of Iowa, Iowa City, and coauthors wrote that pharmacologic treatment decisions should consider coexisting medical conditions that might influence for or against a particular choice for weight loss.
“For example, liraglutide may be a more appropriate agent in people with diabetes because of its glucose-lowering effects,” they wrote. “Conversely, naltrexone-bupropion in patients with chronic opiate or alcohol dependence may be associated with neuropsychiatric complications.
“Ultimately, given the differences in safety, efficacy, and response to therapy, the ideal approach to weight loss should be highly individualized, identifying appropriate candidates for pharmacotherapy, behavioral interventions, and surgical interventions.”
Two study authors were supported by a grant from the National Library of Medicine or the National Institute of Diabetes and Digestive and Kidney Diseases. One author reported receiving funding, participating on advisory committees, and serving as a consultant with a range of pharmaceutical manufacturers, as well as being a cofounder of Liponexus. Another author reported research support from NovoNordisk for research on liraglutide. No other disclosures were reported.
The combination weight-loss drug phentermine plus topiramate is associated with the highest odds of individuals being able to lose 5% of their body weight within 1 year, according to a meta-analysis comparing outcomes and adverse events for orlistat, lorcaserin, naltrexone-bupropion, phentermine-topiramate, and liraglutide.
Researchers analyzed 28 randomized placebo- or active-controlled clinical trials involving a total of 29,018 participants and found those who took phentermine-topiramate had a ninefold greater likelihood of achieving a 5% weight loss by 1 year than did those on placebo, according to a paper published in the June 14 issue of JAMA.
Liraglutide showed the second-highest odds of achieving a 5% weight loss at 1 year (odds ratio, 5.54), followed by naltrexone-bupropion (OR, 3.96), lorcaserin (OR, 3.10), and orlistat (OR, 2.70).
Nearly one-quarter of individuals on placebo achieved at least a 5% weight loss by 1 year, compared with three-quarters of individuals taking phentermine-topiramate, 63% of those taking liraglutide, 55% taking naltrexone-bupropion, 49% taking lorcaserin, and 44% taking orlistat (JAMA 2016;315:2424-34. doi: 10.1001/jama.2016.7602).
Of those on placebo, only 9% achieved at least a 10% weight loss at 1 year, compared with 54% of patients taking phentermine-topiramate, 34% of patients on liraglutide, 30% of patients on naltrexone-bupropion, 25% of those taking lorcaserin, and 20% of those taking orlistat.
Phentermine-topiramate was also associated with the greatest weight loss, compared with placebo, with patients losing a mean of 8.8 kg vs. 5.2 kg with liraglutide, 5 kg with naltrexone-bupropion, 3.2 kg with lorcaserin, and 2.6 kg with orlistat.
While all active drugs were associated with a higher rate of discontinuation because of adverse events than was seen with placebo, liraglutide was associated with the greatest risk of discontinuation, compared with placebo, followed by naltrexone-bupropion, phentermine-topiramate, orlistat, and then lorcaserin.
Dr. Rohan Khera of the department of internal medicine at the University of Iowa, Iowa City, and coauthors wrote that pharmacologic treatment decisions should consider coexisting medical conditions that might influence for or against a particular choice for weight loss.
“For example, liraglutide may be a more appropriate agent in people with diabetes because of its glucose-lowering effects,” they wrote. “Conversely, naltrexone-bupropion in patients with chronic opiate or alcohol dependence may be associated with neuropsychiatric complications.
“Ultimately, given the differences in safety, efficacy, and response to therapy, the ideal approach to weight loss should be highly individualized, identifying appropriate candidates for pharmacotherapy, behavioral interventions, and surgical interventions.”
Two study authors were supported by a grant from the National Library of Medicine or the National Institute of Diabetes and Digestive and Kidney Diseases. One author reported receiving funding, participating on advisory committees, and serving as a consultant with a range of pharmaceutical manufacturers, as well as being a cofounder of Liponexus. Another author reported research support from NovoNordisk for research on liraglutide. No other disclosures were reported.
FROM JAMA
Key clinical point: The combination weight-loss drug phentermine plus topiramate is associated with the highest odds of individuals being able to lose 5% of their body weight by 1 year, compared with four other weight-loss drugs.
Major finding: Patients taking phentermine-topiramate had a ninefold greater likelihood of achieving a 5% weight loss by 1 year than did those on placebo.
Data source: A systematic review and meta-analysis of 28 randomized placebo- or active-controlled clinical trials involving a total of 29,018 participants.
Disclosures: Two study authors were supported by a grant from the National Library of Medicine or the National Institute of Diabetes and Digestive and Kidney Diseases. One author reported receiving funding, participating on advisory committees, and serving as a consultant with a range of pharmaceutical manufacturers, as well as being a cofounder of Liponexus. Another author reported research support from NovoNordisk for research on liraglutide. No other disclosures were reported.
Hydroxychloroquine, abatacept linked with reduced type 2 diabetes
LONDON – U.S. rheumatoid arthritis patients had a significantly reduced incidence of type 2 diabetes when on treatment with either hydroxychloroquine or abatacept in an analysis of more than 13,000 patients enrolled in a U.S. national registry during 2000-2015.
The same analysis also showed statistically significant increases in the risk for new-onset type 2 diabetes in rheumatoid arthritis (RA) patients treated with a glucocorticoid or a statin, Kaleb Michaud, Ph.D., reported in a poster at the European Congress of Rheumatology.
“Our findings can inform clinicians about determining appropriate treatment decisions in rheumatoid arthritis patients at increased risk for developing type 2 diabetes,” said Dr. Michaud, an epidemiologist at the University of Nebraska in Omaha and also codirector of the National Data Bank for Rheumatic Diseases in Wichita, Kan., the source of the data used in his study.
Hydroxychloroquine, a relatively inexpensive drug that’s often used in RA patients in combination with other drugs, might be a good agent to consider adding to the therapeutic mix of RA patients at risk for developing type 2 diabetes, he said in an interview. Although the implications of this finding for the use of abatacept (Orencia) are less clear, it is a signal of a novel benefit from the drug that merits further study, Dr. Michaud said.
The findings also suggest that, when rheumatoid arthritis patients receive statin treatment, they are candidates for more intensive monitoring of diabetes onset, he added.
His analysis focused on the 13,669 RA patients who participated in the National Data Bank for Rheumatic Diseases for at least a year during 2000-2015 and had not been diagnosed as having diabetes of any type at the time they entered the registry. During a median 4.6 years of follow-up, 1,139 patients either self-reported receiving a new diagnosis of type 2 diabetes or began treatment with a hypoglycemic medication. Patients averaged about 59 years old at the time they entered the registry, and about 80% were women.
Dr. Michaud and his associates assessed the incidence rate of type 2 diabetes according to six categories of RA treatment: methotrexate monotherapy, which they used as the reference group; any treatment with abatacept with or without methotrexate; any treatment with hydroxychloroquine; any treatment with a glucocorticoid; treatment with any other disease-modifying antirheumatic drug (DMARD) with methotrexate; and treatment with any other DMARD without methotrexate or no DMARD treatment. A seventh treatment category was treatment with a statin.
A series of time-varying Cox proportional hazard models that adjusted for sociodemographic variables, comorbidities, body mass index, and measures of RA severity showed that, when compared with methotrexate monotherapy, patients treated with abatacept had a statistically significant 48% reduced rate of developing diabetes, and those treated with hydroxychloroquine had a statistically significant 33% reduced diabetes incidence, they reported.
In contrast, compared with methotrexate monotherapy, treatment with a glucocorticoid linked with a statistically significant 31% increased rate of type 2 diabetes, and treatment with a statin linked with a statistically significant 56% increased rate of diabetes. Other forms of DMARD treatment, including tumor necrosis factor inhibitors and other biologic DMARDs, had no statistically significant link with diabetes development.
Dr. Michaud also reported additional analyses that further defined the associations between hydroxychloroquine treatment and reduced diabetes incidence: The reduction in diabetes incidence became statistically significant in patients only when they had received the drug for at least 2 years. The link with reduced diabetes incidence seemed dose dependent, with a stronger protective effect in patients who received at least 400 mg/day. Also, the strength of the protection waned in patients who had discontinued hydroxychloroquine for at least 6 months, and the protective effect completely disappeared once patients were off hydroxychloroquine for at least 1 year. Finally, the link between hydroxychloroquine use and reduced diabetes incidence also was statistically significant in patients who concurrently received a glucocorticoid, but a significant protective association disappeared in patients who received a statin as well as hydroxychloroquine.
Dr. Michaud had no disclosures. The study received no commercial support.
On Twitter @mitchelzoler
LONDON – U.S. rheumatoid arthritis patients had a significantly reduced incidence of type 2 diabetes when on treatment with either hydroxychloroquine or abatacept in an analysis of more than 13,000 patients enrolled in a U.S. national registry during 2000-2015.
The same analysis also showed statistically significant increases in the risk for new-onset type 2 diabetes in rheumatoid arthritis (RA) patients treated with a glucocorticoid or a statin, Kaleb Michaud, Ph.D., reported in a poster at the European Congress of Rheumatology.
“Our findings can inform clinicians about determining appropriate treatment decisions in rheumatoid arthritis patients at increased risk for developing type 2 diabetes,” said Dr. Michaud, an epidemiologist at the University of Nebraska in Omaha and also codirector of the National Data Bank for Rheumatic Diseases in Wichita, Kan., the source of the data used in his study.
Hydroxychloroquine, a relatively inexpensive drug that’s often used in RA patients in combination with other drugs, might be a good agent to consider adding to the therapeutic mix of RA patients at risk for developing type 2 diabetes, he said in an interview. Although the implications of this finding for the use of abatacept (Orencia) are less clear, it is a signal of a novel benefit from the drug that merits further study, Dr. Michaud said.
The findings also suggest that, when rheumatoid arthritis patients receive statin treatment, they are candidates for more intensive monitoring of diabetes onset, he added.
His analysis focused on the 13,669 RA patients who participated in the National Data Bank for Rheumatic Diseases for at least a year during 2000-2015 and had not been diagnosed as having diabetes of any type at the time they entered the registry. During a median 4.6 years of follow-up, 1,139 patients either self-reported receiving a new diagnosis of type 2 diabetes or began treatment with a hypoglycemic medication. Patients averaged about 59 years old at the time they entered the registry, and about 80% were women.
Dr. Michaud and his associates assessed the incidence rate of type 2 diabetes according to six categories of RA treatment: methotrexate monotherapy, which they used as the reference group; any treatment with abatacept with or without methotrexate; any treatment with hydroxychloroquine; any treatment with a glucocorticoid; treatment with any other disease-modifying antirheumatic drug (DMARD) with methotrexate; and treatment with any other DMARD without methotrexate or no DMARD treatment. A seventh treatment category was treatment with a statin.
A series of time-varying Cox proportional hazard models that adjusted for sociodemographic variables, comorbidities, body mass index, and measures of RA severity showed that, when compared with methotrexate monotherapy, patients treated with abatacept had a statistically significant 48% reduced rate of developing diabetes, and those treated with hydroxychloroquine had a statistically significant 33% reduced diabetes incidence, they reported.
In contrast, compared with methotrexate monotherapy, treatment with a glucocorticoid linked with a statistically significant 31% increased rate of type 2 diabetes, and treatment with a statin linked with a statistically significant 56% increased rate of diabetes. Other forms of DMARD treatment, including tumor necrosis factor inhibitors and other biologic DMARDs, had no statistically significant link with diabetes development.
Dr. Michaud also reported additional analyses that further defined the associations between hydroxychloroquine treatment and reduced diabetes incidence: The reduction in diabetes incidence became statistically significant in patients only when they had received the drug for at least 2 years. The link with reduced diabetes incidence seemed dose dependent, with a stronger protective effect in patients who received at least 400 mg/day. Also, the strength of the protection waned in patients who had discontinued hydroxychloroquine for at least 6 months, and the protective effect completely disappeared once patients were off hydroxychloroquine for at least 1 year. Finally, the link between hydroxychloroquine use and reduced diabetes incidence also was statistically significant in patients who concurrently received a glucocorticoid, but a significant protective association disappeared in patients who received a statin as well as hydroxychloroquine.
Dr. Michaud had no disclosures. The study received no commercial support.
On Twitter @mitchelzoler
LONDON – U.S. rheumatoid arthritis patients had a significantly reduced incidence of type 2 diabetes when on treatment with either hydroxychloroquine or abatacept in an analysis of more than 13,000 patients enrolled in a U.S. national registry during 2000-2015.
The same analysis also showed statistically significant increases in the risk for new-onset type 2 diabetes in rheumatoid arthritis (RA) patients treated with a glucocorticoid or a statin, Kaleb Michaud, Ph.D., reported in a poster at the European Congress of Rheumatology.
“Our findings can inform clinicians about determining appropriate treatment decisions in rheumatoid arthritis patients at increased risk for developing type 2 diabetes,” said Dr. Michaud, an epidemiologist at the University of Nebraska in Omaha and also codirector of the National Data Bank for Rheumatic Diseases in Wichita, Kan., the source of the data used in his study.
Hydroxychloroquine, a relatively inexpensive drug that’s often used in RA patients in combination with other drugs, might be a good agent to consider adding to the therapeutic mix of RA patients at risk for developing type 2 diabetes, he said in an interview. Although the implications of this finding for the use of abatacept (Orencia) are less clear, it is a signal of a novel benefit from the drug that merits further study, Dr. Michaud said.
The findings also suggest that, when rheumatoid arthritis patients receive statin treatment, they are candidates for more intensive monitoring of diabetes onset, he added.
His analysis focused on the 13,669 RA patients who participated in the National Data Bank for Rheumatic Diseases for at least a year during 2000-2015 and had not been diagnosed as having diabetes of any type at the time they entered the registry. During a median 4.6 years of follow-up, 1,139 patients either self-reported receiving a new diagnosis of type 2 diabetes or began treatment with a hypoglycemic medication. Patients averaged about 59 years old at the time they entered the registry, and about 80% were women.
Dr. Michaud and his associates assessed the incidence rate of type 2 diabetes according to six categories of RA treatment: methotrexate monotherapy, which they used as the reference group; any treatment with abatacept with or without methotrexate; any treatment with hydroxychloroquine; any treatment with a glucocorticoid; treatment with any other disease-modifying antirheumatic drug (DMARD) with methotrexate; and treatment with any other DMARD without methotrexate or no DMARD treatment. A seventh treatment category was treatment with a statin.
A series of time-varying Cox proportional hazard models that adjusted for sociodemographic variables, comorbidities, body mass index, and measures of RA severity showed that, when compared with methotrexate monotherapy, patients treated with abatacept had a statistically significant 48% reduced rate of developing diabetes, and those treated with hydroxychloroquine had a statistically significant 33% reduced diabetes incidence, they reported.
In contrast, compared with methotrexate monotherapy, treatment with a glucocorticoid linked with a statistically significant 31% increased rate of type 2 diabetes, and treatment with a statin linked with a statistically significant 56% increased rate of diabetes. Other forms of DMARD treatment, including tumor necrosis factor inhibitors and other biologic DMARDs, had no statistically significant link with diabetes development.
Dr. Michaud also reported additional analyses that further defined the associations between hydroxychloroquine treatment and reduced diabetes incidence: The reduction in diabetes incidence became statistically significant in patients only when they had received the drug for at least 2 years. The link with reduced diabetes incidence seemed dose dependent, with a stronger protective effect in patients who received at least 400 mg/day. Also, the strength of the protection waned in patients who had discontinued hydroxychloroquine for at least 6 months, and the protective effect completely disappeared once patients were off hydroxychloroquine for at least 1 year. Finally, the link between hydroxychloroquine use and reduced diabetes incidence also was statistically significant in patients who concurrently received a glucocorticoid, but a significant protective association disappeared in patients who received a statin as well as hydroxychloroquine.
Dr. Michaud had no disclosures. The study received no commercial support.
On Twitter @mitchelzoler
AT THE EULAR 2016 CONGRESS
Key clinical point: U.S. rheumatoid arthritis patients on hydroxychloroquine or abatacept had a significantly reduced incidence of type 2 diabetes, compared with patients on methotrexate monotherapy.
Major finding: Hydroxychloroquine cut type 2 diabetes incidence by 33% and abatacept cut it by 48%, compared with patients on methotrexate monotherapy.
Data source: Analysis of data collected from 13,669 U.S. rheumatoid arthritis patients enrolled in a registry during 2000-2015.
Disclosures: Dr. Michaud had no disclosures. The study received no commercial support.
Bevacizumab offers best value of anti-VEGF drugs to treat DME
Despite recent data showing that aflibercept is the most effective anti–vascular endothelial growth factor (anti-VEGF) treatment available for patients with diabetic macular edema, bevacizumab is still by far the best drug option available from a cost-effectiveness perspective, according to a post hoc analysis.
Dr. Joshua D. Stein of the University of Michigan, Ann Arbor, and his coauthors analyzed the anti-VEGF drugs prescribed to 624 diabetic macular edema (DME) patients – 209 taking aflibercept, 207 taking bevacizumab, and 208 taking ranibizumab – enrolled in the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial for incremental cost-effectiveness ratios (ICERs).
“On the basis of 2015 wholesale acquisition costs, aflibercept (2 mg) costs $1,850, ranibizumab (0.3mg) costs $1,170, and bevacizumab repackaged at compounding pharmacies into syringes for ophthalmologic use containing 1.25 mg of bevacizumab costs approximately $60 per dose,” Dr. Stein and his coauthors wrote (JAMA Ophthalmol. 2016 June 9. doi: 10.1001/jamaophthalmol.2016.1669). “Considering that these medicines may be given 9 to 11 times in the first year of treatment and, on average, 17 times during 5 years, total costs can be substantial.”
Data from the randomized clinical trial were used to calculate projected benefit, costs, and cost-effectiveness of aflibercept and ranibizumab compared with bevacizumab as baseline. In addition, the investigators also determined both ICERs and Quality Life-Adjusted Years (QALY) for each drug over periods of 1 and 10 years. Results indicated that for 1 year of treatment, the ICER of aflibercept was $1.11 million per QALY and for ranibizumab, $1.73 million per QALY. Over the course of 10 years, aflibercept would come out to $349,000 per QALY, while ranibizumab would be $603,000 per QALY. In an analysis of a subgroup with highly reduced eyesight due to DME, the 10-year ICER of aflibercept would be $287,000 per QALY and for ranibizumab, $817,000, according to Dr. Stein and his associates.
Over a 1-year period, bevacizumab would cost $4,100, compared to $26,100 for aflibercept and $18,600 for ranibizumab. Over a 10-year period, those costs would jump up to $102,500 for aflibercept and $79,400 for ranibizumab, while bevacizumab would cost $39,800. Overall, the costs of aflibercept and ranibizumab would have to decrease by 69% and 80%, respectively, for the costs to become competitive with bevacizumab.
“Aflibercept (2.0 mg) and ranibizumab (0.3 mg) are not cost-effective relative to bevacizumab for treatment of DME unless their prices decrease substantially [and] in contexts where bevacizumab is unavailable for DME treatment, aflibercept is not cost-effective relative to ranibizumab,” the authors concluded, adding that bevacizumab makes the most sense as a primary anti-VEGF treatment because it allows for the greatest overall value.
The National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the U.S. Department of Health and Human Services funded the study. Dr. Stein did not report any relevant financial disclosures; however, other coauthors reported potentially relevant disclosures.
Despite recent data showing that aflibercept is the most effective anti–vascular endothelial growth factor (anti-VEGF) treatment available for patients with diabetic macular edema, bevacizumab is still by far the best drug option available from a cost-effectiveness perspective, according to a post hoc analysis.
Dr. Joshua D. Stein of the University of Michigan, Ann Arbor, and his coauthors analyzed the anti-VEGF drugs prescribed to 624 diabetic macular edema (DME) patients – 209 taking aflibercept, 207 taking bevacizumab, and 208 taking ranibizumab – enrolled in the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial for incremental cost-effectiveness ratios (ICERs).
“On the basis of 2015 wholesale acquisition costs, aflibercept (2 mg) costs $1,850, ranibizumab (0.3mg) costs $1,170, and bevacizumab repackaged at compounding pharmacies into syringes for ophthalmologic use containing 1.25 mg of bevacizumab costs approximately $60 per dose,” Dr. Stein and his coauthors wrote (JAMA Ophthalmol. 2016 June 9. doi: 10.1001/jamaophthalmol.2016.1669). “Considering that these medicines may be given 9 to 11 times in the first year of treatment and, on average, 17 times during 5 years, total costs can be substantial.”
Data from the randomized clinical trial were used to calculate projected benefit, costs, and cost-effectiveness of aflibercept and ranibizumab compared with bevacizumab as baseline. In addition, the investigators also determined both ICERs and Quality Life-Adjusted Years (QALY) for each drug over periods of 1 and 10 years. Results indicated that for 1 year of treatment, the ICER of aflibercept was $1.11 million per QALY and for ranibizumab, $1.73 million per QALY. Over the course of 10 years, aflibercept would come out to $349,000 per QALY, while ranibizumab would be $603,000 per QALY. In an analysis of a subgroup with highly reduced eyesight due to DME, the 10-year ICER of aflibercept would be $287,000 per QALY and for ranibizumab, $817,000, according to Dr. Stein and his associates.
Over a 1-year period, bevacizumab would cost $4,100, compared to $26,100 for aflibercept and $18,600 for ranibizumab. Over a 10-year period, those costs would jump up to $102,500 for aflibercept and $79,400 for ranibizumab, while bevacizumab would cost $39,800. Overall, the costs of aflibercept and ranibizumab would have to decrease by 69% and 80%, respectively, for the costs to become competitive with bevacizumab.
“Aflibercept (2.0 mg) and ranibizumab (0.3 mg) are not cost-effective relative to bevacizumab for treatment of DME unless their prices decrease substantially [and] in contexts where bevacizumab is unavailable for DME treatment, aflibercept is not cost-effective relative to ranibizumab,” the authors concluded, adding that bevacizumab makes the most sense as a primary anti-VEGF treatment because it allows for the greatest overall value.
The National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the U.S. Department of Health and Human Services funded the study. Dr. Stein did not report any relevant financial disclosures; however, other coauthors reported potentially relevant disclosures.
Despite recent data showing that aflibercept is the most effective anti–vascular endothelial growth factor (anti-VEGF) treatment available for patients with diabetic macular edema, bevacizumab is still by far the best drug option available from a cost-effectiveness perspective, according to a post hoc analysis.
Dr. Joshua D. Stein of the University of Michigan, Ann Arbor, and his coauthors analyzed the anti-VEGF drugs prescribed to 624 diabetic macular edema (DME) patients – 209 taking aflibercept, 207 taking bevacizumab, and 208 taking ranibizumab – enrolled in the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial for incremental cost-effectiveness ratios (ICERs).
“On the basis of 2015 wholesale acquisition costs, aflibercept (2 mg) costs $1,850, ranibizumab (0.3mg) costs $1,170, and bevacizumab repackaged at compounding pharmacies into syringes for ophthalmologic use containing 1.25 mg of bevacizumab costs approximately $60 per dose,” Dr. Stein and his coauthors wrote (JAMA Ophthalmol. 2016 June 9. doi: 10.1001/jamaophthalmol.2016.1669). “Considering that these medicines may be given 9 to 11 times in the first year of treatment and, on average, 17 times during 5 years, total costs can be substantial.”
Data from the randomized clinical trial were used to calculate projected benefit, costs, and cost-effectiveness of aflibercept and ranibizumab compared with bevacizumab as baseline. In addition, the investigators also determined both ICERs and Quality Life-Adjusted Years (QALY) for each drug over periods of 1 and 10 years. Results indicated that for 1 year of treatment, the ICER of aflibercept was $1.11 million per QALY and for ranibizumab, $1.73 million per QALY. Over the course of 10 years, aflibercept would come out to $349,000 per QALY, while ranibizumab would be $603,000 per QALY. In an analysis of a subgroup with highly reduced eyesight due to DME, the 10-year ICER of aflibercept would be $287,000 per QALY and for ranibizumab, $817,000, according to Dr. Stein and his associates.
Over a 1-year period, bevacizumab would cost $4,100, compared to $26,100 for aflibercept and $18,600 for ranibizumab. Over a 10-year period, those costs would jump up to $102,500 for aflibercept and $79,400 for ranibizumab, while bevacizumab would cost $39,800. Overall, the costs of aflibercept and ranibizumab would have to decrease by 69% and 80%, respectively, for the costs to become competitive with bevacizumab.
“Aflibercept (2.0 mg) and ranibizumab (0.3 mg) are not cost-effective relative to bevacizumab for treatment of DME unless their prices decrease substantially [and] in contexts where bevacizumab is unavailable for DME treatment, aflibercept is not cost-effective relative to ranibizumab,” the authors concluded, adding that bevacizumab makes the most sense as a primary anti-VEGF treatment because it allows for the greatest overall value.
The National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the U.S. Department of Health and Human Services funded the study. Dr. Stein did not report any relevant financial disclosures; however, other coauthors reported potentially relevant disclosures.
FROM JAMA OPHTHALMOLOGY
Key clinical point: Bevacizumab remains the best option, in terms of price and value, over aflibercept and ranibizumab for treatment of diabetic macular edema.
Major finding: Based on incremental cost-effectiveness ratios (ICERs) over 1- and 10-year periods, the costs of aflibercept and ranibizumab would have to decrease by 69% and 80%, respectively, to match the cost of bevacizumab over the same time periods.
Data source: Post hoc analysis of patients enrolled in the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial at 1-year follow-up.
Disclosures: The National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the U.S. Department of Health and Human Services funded the study. Dr. Stein did not report any relevant financial disclosures.
Postpartum glycemic screening rates are low for women with GDM history
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Glycemic screening rates for women who had gestational diabetes mellitus are generally low and vary based on race, geography, and medication treatment in pregnancy.
Major finding: More than three-quarters of women with a history of gestational diabetes mellitus did not receive a glycemic screen in their first year postpartum.
Data sources: A retrospective analysis of medical records from women enrolled in a large, U.S. commercial health plan from 2000-2012.
Disclosures: The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
Adolescent obesity rose slightly, again
Nearly one in five young people in the United States are obese, and proportionally more adolescents have been obese during every time period measured since 1994, according to an analysis published online June 7 in JAMA.
But the most recent data suggest only a “small” rise in adolescent obesity since 2011, and stable rates among children during this time period, said Cynthia L. Ogden, Ph.D., of the National Center for Health Statistics at the Centers for Disease Control and Prevention.
Few studies of obesity in young people have teased out rates by age, according to Dr. Ogden and her associates. Using National Health and Nutrition Examination Survey data, they calculated rates of obesity and extreme obesity among 40,780 children and adolescents aged 2-19 years for the periods 1988-1994 through 2013-2014. They defined obesity as a body mass index (BMI) at or above the sex-specific 95th percentile on the CDC BMI-for-age growth charts, and they defined extreme obesity as a BMI at least 120% of the sex-specific 95th percentile on the charts (JAMA. 2016 Jun 7. doi: 10.1001/jama.2016.6361).
Based on these definitions, 17% of children and adolescents were obese between 2011 and 2014, while 6% were extremely obese, the investigators reported. Furthermore, 21% of adolescents were obese in 2013-2014, compared with 17% during 2003-2004 and 11% during 1988-1994.
Rates for 6- to -11-year-olds have remained fairly stable in the high teens for more than a decade, while rates among 2- to 5-year-olds peaked in 2003-2004 at nearly 14% before dropping to about 9% during 2013-2014. The prevalence of obesity varied little by sex, but diverged substantially by race and ethnicity. For example, in 2011-2014, 23% of Hispanics and about 23% of black children were obese, and 9% and 12% were extremely obese, respectively the researchers reported. Rates for the same ages of non-Hispanic Asian children were 9% and 2%, respectively, and those for non-Hispanic whites were 20% and 7%, respectively.
“Body mass index is an imperfect measure of body fat and health risk,” the investigators cautioned. “There are racial and ethnic differences in body fat at the same BMI level. Among children and adolescents, the definition of obesity is statistical. Children and adolescents are compared with a group of U.S. children in the 1960s to early 1990s, so the prevalence of obesity is dependent on the characteristics of the age-specific population during that period. In addition, among young children, small changes in weight can lead to relatively large changes in BMI percentile”
The researchers reported no funding sources and had no disclosures.
Numerous foundations, industries, professional societies, and governmental agencies have provided hundreds of millions of dollars in funding to support basic science research in obesity, clinical trials, and observational studies, development of new drugs and devices, and hospital and community programs to help stem the tide of the obesity epidemic. In addition, communities, schools, places of worship, and professional societies have become active in attempting to counteract obesity – emphasizing exercise, better dietary choices, and nutritional labeling of foods.
Although it is impossible to know what the extent of the obesity epidemic would have been without these efforts, [these data] certainly do not suggest much success. Perhaps new incentives are needed to encourage the food industry to work with families and the medical community to prevent obesity. The stakes for the health of people in the United States are high, and creative solutions are needed.
Dr. Jody W. Zylke is deputy editor of JAMA. Dr. Howard Bauchner is editor in chief of JAMA. These comments are excerpted from their accompanying editorial (JAMA. 2016 Jun. doi: 10.1001/jama.2016.6190).
Numerous foundations, industries, professional societies, and governmental agencies have provided hundreds of millions of dollars in funding to support basic science research in obesity, clinical trials, and observational studies, development of new drugs and devices, and hospital and community programs to help stem the tide of the obesity epidemic. In addition, communities, schools, places of worship, and professional societies have become active in attempting to counteract obesity – emphasizing exercise, better dietary choices, and nutritional labeling of foods.
Although it is impossible to know what the extent of the obesity epidemic would have been without these efforts, [these data] certainly do not suggest much success. Perhaps new incentives are needed to encourage the food industry to work with families and the medical community to prevent obesity. The stakes for the health of people in the United States are high, and creative solutions are needed.
Dr. Jody W. Zylke is deputy editor of JAMA. Dr. Howard Bauchner is editor in chief of JAMA. These comments are excerpted from their accompanying editorial (JAMA. 2016 Jun. doi: 10.1001/jama.2016.6190).
Numerous foundations, industries, professional societies, and governmental agencies have provided hundreds of millions of dollars in funding to support basic science research in obesity, clinical trials, and observational studies, development of new drugs and devices, and hospital and community programs to help stem the tide of the obesity epidemic. In addition, communities, schools, places of worship, and professional societies have become active in attempting to counteract obesity – emphasizing exercise, better dietary choices, and nutritional labeling of foods.
Although it is impossible to know what the extent of the obesity epidemic would have been without these efforts, [these data] certainly do not suggest much success. Perhaps new incentives are needed to encourage the food industry to work with families and the medical community to prevent obesity. The stakes for the health of people in the United States are high, and creative solutions are needed.
Dr. Jody W. Zylke is deputy editor of JAMA. Dr. Howard Bauchner is editor in chief of JAMA. These comments are excerpted from their accompanying editorial (JAMA. 2016 Jun. doi: 10.1001/jama.2016.6190).
Nearly one in five young people in the United States are obese, and proportionally more adolescents have been obese during every time period measured since 1994, according to an analysis published online June 7 in JAMA.
But the most recent data suggest only a “small” rise in adolescent obesity since 2011, and stable rates among children during this time period, said Cynthia L. Ogden, Ph.D., of the National Center for Health Statistics at the Centers for Disease Control and Prevention.
Few studies of obesity in young people have teased out rates by age, according to Dr. Ogden and her associates. Using National Health and Nutrition Examination Survey data, they calculated rates of obesity and extreme obesity among 40,780 children and adolescents aged 2-19 years for the periods 1988-1994 through 2013-2014. They defined obesity as a body mass index (BMI) at or above the sex-specific 95th percentile on the CDC BMI-for-age growth charts, and they defined extreme obesity as a BMI at least 120% of the sex-specific 95th percentile on the charts (JAMA. 2016 Jun 7. doi: 10.1001/jama.2016.6361).
Based on these definitions, 17% of children and adolescents were obese between 2011 and 2014, while 6% were extremely obese, the investigators reported. Furthermore, 21% of adolescents were obese in 2013-2014, compared with 17% during 2003-2004 and 11% during 1988-1994.
Rates for 6- to -11-year-olds have remained fairly stable in the high teens for more than a decade, while rates among 2- to 5-year-olds peaked in 2003-2004 at nearly 14% before dropping to about 9% during 2013-2014. The prevalence of obesity varied little by sex, but diverged substantially by race and ethnicity. For example, in 2011-2014, 23% of Hispanics and about 23% of black children were obese, and 9% and 12% were extremely obese, respectively the researchers reported. Rates for the same ages of non-Hispanic Asian children were 9% and 2%, respectively, and those for non-Hispanic whites were 20% and 7%, respectively.
“Body mass index is an imperfect measure of body fat and health risk,” the investigators cautioned. “There are racial and ethnic differences in body fat at the same BMI level. Among children and adolescents, the definition of obesity is statistical. Children and adolescents are compared with a group of U.S. children in the 1960s to early 1990s, so the prevalence of obesity is dependent on the characteristics of the age-specific population during that period. In addition, among young children, small changes in weight can lead to relatively large changes in BMI percentile”
The researchers reported no funding sources and had no disclosures.
Nearly one in five young people in the United States are obese, and proportionally more adolescents have been obese during every time period measured since 1994, according to an analysis published online June 7 in JAMA.
But the most recent data suggest only a “small” rise in adolescent obesity since 2011, and stable rates among children during this time period, said Cynthia L. Ogden, Ph.D., of the National Center for Health Statistics at the Centers for Disease Control and Prevention.
Few studies of obesity in young people have teased out rates by age, according to Dr. Ogden and her associates. Using National Health and Nutrition Examination Survey data, they calculated rates of obesity and extreme obesity among 40,780 children and adolescents aged 2-19 years for the periods 1988-1994 through 2013-2014. They defined obesity as a body mass index (BMI) at or above the sex-specific 95th percentile on the CDC BMI-for-age growth charts, and they defined extreme obesity as a BMI at least 120% of the sex-specific 95th percentile on the charts (JAMA. 2016 Jun 7. doi: 10.1001/jama.2016.6361).
Based on these definitions, 17% of children and adolescents were obese between 2011 and 2014, while 6% were extremely obese, the investigators reported. Furthermore, 21% of adolescents were obese in 2013-2014, compared with 17% during 2003-2004 and 11% during 1988-1994.
Rates for 6- to -11-year-olds have remained fairly stable in the high teens for more than a decade, while rates among 2- to 5-year-olds peaked in 2003-2004 at nearly 14% before dropping to about 9% during 2013-2014. The prevalence of obesity varied little by sex, but diverged substantially by race and ethnicity. For example, in 2011-2014, 23% of Hispanics and about 23% of black children were obese, and 9% and 12% were extremely obese, respectively the researchers reported. Rates for the same ages of non-Hispanic Asian children were 9% and 2%, respectively, and those for non-Hispanic whites were 20% and 7%, respectively.
“Body mass index is an imperfect measure of body fat and health risk,” the investigators cautioned. “There are racial and ethnic differences in body fat at the same BMI level. Among children and adolescents, the definition of obesity is statistical. Children and adolescents are compared with a group of U.S. children in the 1960s to early 1990s, so the prevalence of obesity is dependent on the characteristics of the age-specific population during that period. In addition, among young children, small changes in weight can lead to relatively large changes in BMI percentile”
The researchers reported no funding sources and had no disclosures.
FROM JAMA
Key clinical point:Nearly one in five children and adolescents are obese, and rates of adolescent obesity have risen during every time period measured since 1994.
Major finding: About 17% of children and adolescents in the United States were obese between 2011 and 2014 (95% confidence interval, 15.5%-18.6%). Nearly 21% of adolescents were obese in 2013-2014, compared with 17% during 2003-2004 and 10% during 1988-1994.
Data source: An analysis of the body mass indexes of 40,780 individuals aged 2-19 years from the National Health and Nutrition Examination Survey.
Disclosures: The researchers reported no funding sources and had no disclosures.
Obesity continues to trend up among women over the past decade
Four in 10 women in the United States are obese, 1 in 10 women has a body mass index above 40 kg/m2, and significantly more women are obese than a decade ago, according to a large study published June 7 in JAMA.
In contrast, obesity rates among men in the United States have remained stable since 2005, said Dr. Katherine Flegal of the National Center for Health Statistics. “Other studies are needed to determine the reasons for these trends,” she and her associates wrote.
Between 1980 and 2000, obesity rates in the United States rose significantly among both men and women. Between 2000 and 2004, rates rose significantly for men, but not women. Rates then leveled off for both sexes through 2012. To further explore these trends, Dr. Flegal and her associates calculated the prevalence of obesity (BMI greater than 30 kg/m2) and class 3 obesity (BMI greater than 40 kg/m2) for 2,638 men and 2,817 women aged 20 and up during 2013-2014, the most recently available 2-year data period from the National Health and Nutrition Examination Survey (NHANES). The researchers also examined trends in obesity since 2005, based on NHANES data from 21,013 adults (JAMA. 2016 Jun 7. doi: 10.1001/jama.2016.6458).
About 38% of adults in the United States were obese during 2013-2014 (95% confidence interval, 36%-40%), including about 40% of women and 35% of men, the researchers found. A total of 7.7% of adults had a BMI of at least 40, including 5.5 % of men and 9.9% of women.
During the decade from 2005 through 2014, the prevalence of obesity among women rose significantly from 35.6% to 41.1% (P = .004), even after the investigators adjusted for age, race and Hispanic origin, smoking status, and education. Among men, the adjusted prevalence of obesity remained about 35% during this time period. Likewise, the adjusted prevalence of class 3 obesity (BMI of at least 40) rose significantly for women (P = .01), but not for men.
Black women also were significantly more likely to be obese or severely obese, compared with non-Hispanic white women in the study, the investigators found. Among men, current smokers were less likely to be obese than never smokers, and women with education beyond high school were less likely to be obese than women who had not finished high school.
The investigators reported no funding sources and had no disclosures.
Much research and attention have been directed toward the treatment of obesity, but the development of new drugs and procedures will not solve the problem. Perhaps genetics will unlock some of the mysteries of obesity, but this will take time, and more immediate solutions are needed. The emphasis has to be on prevention, despite evidence that school- and community-based prevention programs and education campaigns by local governments and professional societies have not been highly successful.
The obesity epidemic in the United States is now 3 decades old, and huge investments have been made in research, clinical care, and development of various programs to counteract obesity. However, few data suggest the epidemic is diminishing. Perhaps it is time for an entirely different approach, one that emphasizes collaboration with the food and restaurant industries that are in part responsible for putting food on our dinner tables.
Dr. Jody W. Zylke is deputy editor of JAMA. Dr. Howard Bauchner is editor in chief of JAMA. These comments are from their accompanying editorial (JAMA. 2016 Jun 7. doi: 10.1001/jama.2016.6190).
Much research and attention have been directed toward the treatment of obesity, but the development of new drugs and procedures will not solve the problem. Perhaps genetics will unlock some of the mysteries of obesity, but this will take time, and more immediate solutions are needed. The emphasis has to be on prevention, despite evidence that school- and community-based prevention programs and education campaigns by local governments and professional societies have not been highly successful.
The obesity epidemic in the United States is now 3 decades old, and huge investments have been made in research, clinical care, and development of various programs to counteract obesity. However, few data suggest the epidemic is diminishing. Perhaps it is time for an entirely different approach, one that emphasizes collaboration with the food and restaurant industries that are in part responsible for putting food on our dinner tables.
Dr. Jody W. Zylke is deputy editor of JAMA. Dr. Howard Bauchner is editor in chief of JAMA. These comments are from their accompanying editorial (JAMA. 2016 Jun 7. doi: 10.1001/jama.2016.6190).
Much research and attention have been directed toward the treatment of obesity, but the development of new drugs and procedures will not solve the problem. Perhaps genetics will unlock some of the mysteries of obesity, but this will take time, and more immediate solutions are needed. The emphasis has to be on prevention, despite evidence that school- and community-based prevention programs and education campaigns by local governments and professional societies have not been highly successful.
The obesity epidemic in the United States is now 3 decades old, and huge investments have been made in research, clinical care, and development of various programs to counteract obesity. However, few data suggest the epidemic is diminishing. Perhaps it is time for an entirely different approach, one that emphasizes collaboration with the food and restaurant industries that are in part responsible for putting food on our dinner tables.
Dr. Jody W. Zylke is deputy editor of JAMA. Dr. Howard Bauchner is editor in chief of JAMA. These comments are from their accompanying editorial (JAMA. 2016 Jun 7. doi: 10.1001/jama.2016.6190).
Four in 10 women in the United States are obese, 1 in 10 women has a body mass index above 40 kg/m2, and significantly more women are obese than a decade ago, according to a large study published June 7 in JAMA.
In contrast, obesity rates among men in the United States have remained stable since 2005, said Dr. Katherine Flegal of the National Center for Health Statistics. “Other studies are needed to determine the reasons for these trends,” she and her associates wrote.
Between 1980 and 2000, obesity rates in the United States rose significantly among both men and women. Between 2000 and 2004, rates rose significantly for men, but not women. Rates then leveled off for both sexes through 2012. To further explore these trends, Dr. Flegal and her associates calculated the prevalence of obesity (BMI greater than 30 kg/m2) and class 3 obesity (BMI greater than 40 kg/m2) for 2,638 men and 2,817 women aged 20 and up during 2013-2014, the most recently available 2-year data period from the National Health and Nutrition Examination Survey (NHANES). The researchers also examined trends in obesity since 2005, based on NHANES data from 21,013 adults (JAMA. 2016 Jun 7. doi: 10.1001/jama.2016.6458).
About 38% of adults in the United States were obese during 2013-2014 (95% confidence interval, 36%-40%), including about 40% of women and 35% of men, the researchers found. A total of 7.7% of adults had a BMI of at least 40, including 5.5 % of men and 9.9% of women.
During the decade from 2005 through 2014, the prevalence of obesity among women rose significantly from 35.6% to 41.1% (P = .004), even after the investigators adjusted for age, race and Hispanic origin, smoking status, and education. Among men, the adjusted prevalence of obesity remained about 35% during this time period. Likewise, the adjusted prevalence of class 3 obesity (BMI of at least 40) rose significantly for women (P = .01), but not for men.
Black women also were significantly more likely to be obese or severely obese, compared with non-Hispanic white women in the study, the investigators found. Among men, current smokers were less likely to be obese than never smokers, and women with education beyond high school were less likely to be obese than women who had not finished high school.
The investigators reported no funding sources and had no disclosures.
Four in 10 women in the United States are obese, 1 in 10 women has a body mass index above 40 kg/m2, and significantly more women are obese than a decade ago, according to a large study published June 7 in JAMA.
In contrast, obesity rates among men in the United States have remained stable since 2005, said Dr. Katherine Flegal of the National Center for Health Statistics. “Other studies are needed to determine the reasons for these trends,” she and her associates wrote.
Between 1980 and 2000, obesity rates in the United States rose significantly among both men and women. Between 2000 and 2004, rates rose significantly for men, but not women. Rates then leveled off for both sexes through 2012. To further explore these trends, Dr. Flegal and her associates calculated the prevalence of obesity (BMI greater than 30 kg/m2) and class 3 obesity (BMI greater than 40 kg/m2) for 2,638 men and 2,817 women aged 20 and up during 2013-2014, the most recently available 2-year data period from the National Health and Nutrition Examination Survey (NHANES). The researchers also examined trends in obesity since 2005, based on NHANES data from 21,013 adults (JAMA. 2016 Jun 7. doi: 10.1001/jama.2016.6458).
About 38% of adults in the United States were obese during 2013-2014 (95% confidence interval, 36%-40%), including about 40% of women and 35% of men, the researchers found. A total of 7.7% of adults had a BMI of at least 40, including 5.5 % of men and 9.9% of women.
During the decade from 2005 through 2014, the prevalence of obesity among women rose significantly from 35.6% to 41.1% (P = .004), even after the investigators adjusted for age, race and Hispanic origin, smoking status, and education. Among men, the adjusted prevalence of obesity remained about 35% during this time period. Likewise, the adjusted prevalence of class 3 obesity (BMI of at least 40) rose significantly for women (P = .01), but not for men.
Black women also were significantly more likely to be obese or severely obese, compared with non-Hispanic white women in the study, the investigators found. Among men, current smokers were less likely to be obese than never smokers, and women with education beyond high school were less likely to be obese than women who had not finished high school.
The investigators reported no funding sources and had no disclosures.
FROM JAMA
Key clinical point: Significantly more women in the United States are obese now than a decade ago, while obesity rates among men have leveled off.
Major finding: During 2005 through 2014, the adjusted prevalence of obesity among women rose from 35.6% to 41.1% (P = .004).
Data source: An analysis of the body mass index for 26,468 adults from the National Health and Nutrition Examination Survey.
Disclosures: The researchers reported no funding sources and had no disclosures.
Experts endorse metabolic surgery for diabetes intervention
Metabolic surgery should be recommended for obese patients with type 2 diabetes mellitus, according to an international consensus statement from 48 clinicians and scholars issued after the Second Diabetes Surgery Summit held as part of the World Congress on Interventional Therapies for Type 2 Diabetes in London in 2015.
Current treatment plans for patients with type 2 diabetes do no include bariatric/metabolic surgery, despite increasing evidence of improved glycemic control and reduced cardiovascular risk factors in surgically treated patients, wrote Dr. Francesco Rubino and colleagues on behalf of members of the Second Diabetes Surgery Summit (DSS-II). The guidelines were endorsed by 45 global medical and scientific societies at the time of publication. The statement was published in a special edition of Diabetes Care (Diabetes Care. 2016;39:861-77. doi:10.2337/dc16-0236).
The guidelines recommend metabolic surgery for patients with type 2 diabetes who have class III obesity (defined as a body mass index of at least 40 kg/m2) or class II obesity (defined as a BMI of 35 kg/m2-39.9 kg/m2). In addition, metabolic surgery should be considered as a option for those patients with type 2 diabetes whose BMI falls within the 30 kg/m2-34.9 kg/m2 range if hyperglycemia remains uncontrolled after treatment attempts with oral or injectable medications. For Asian patients, the BMI thresholds for surgery should be reduced by 2.5 kg/m2, the researchers noted.
The conclusions are based on a review of published evidence on metabolic surgery and type 2 diabetes from January 1, 2005, through September 30, 2015.
The researchers assessed the evidence based on factors including long-term effects of surgery on glycemic control, effectiveness of surgery compared with nonsurgical interventions, comparisons of surgical procedures, and effects of surgery on diabetes complications, cardiovascular risk factors, and mortality. They also considered the short- and long-term safety of different procedures. The recommendations offer guidance on patient selection, pre- and postoperative workups, choice of procedure, and defining goals and success of surgery.
“The success of metabolic surgery needs to be defined in the larger context of comprehensive diabetes care plans,” the researchers noted. “Metabolic surgery should be considered a means to achieve the glycemic control necessary to reduce risk of microvascular complications and CVD.”
The researchers acknowledged that complications from metabolic surgery may require reoperations and rehospitalizations. Other limitations include a lack of evidence in several areas including: cost-effectiveness, optimal nutrition management after surgery, postoperative lifestyle interventions, and long-term effects of surgery, and further research is needed.
However, “there is now sufficient clinical and mechanistic evidence to support inclusion of GI surgery among antidiabetes interventions for people with type 2 diabetes and obesity,” the researchers said. They called for collaboration between clinicians and regulators to recognize the potential value of metabolic surgery for type 2 diabetes and develop appropriate reimbursement plans.
The DSS-II and WCITD 2015 were sponsored by the International Diabetes Society Task Force, King’s College London, King’s College Hospital, Johnson & Johnson, Medtronic, Medimmune, Fractyl, DIAMOND MetaCure, Gore, Novo Nordisk, and NGM Biopharmaceuticals. The researchers reported no relevant conflicts.
“The new guidelines provide much needed guidance for general practitioners, endocrinologists, and diabetes specialists about the use of metabolic surgery in the treatment of obese patients with type 2 diabetes,” wrote Dr. William T. Cefalu and colleagues in an accompanying editorial.
However, “one of the many issues that remains unanswered for metabolic surgery pertains to the exact mechanism of action. Specifically, we now recognize that postoperative improvements in metabolic control occur rapidly and out of proportion to weight loss, yet the physiological and molecular mechanisms underlying these beneficial glycemic effects remain incompletely elucidated,” they wrote. In addition, “before we can fully appreciate the role of metabolic surgery in becoming a readily available, viable option in our treatment algorithm and expand the appropriate candidate pool, we need to fully understand the efficacy, complications, long-term clinical outcomes, and costs. In particular, it will be important to clarify the financial implications to patients, providers, and insurers (both private and government sectors) and to appreciate that these barriers may be hard to overcome in resource poor areas of the world,” they said.
“It is an exciting time for those of us in diabetes research, and the ability to be part of a paradigm change in the understanding, approach, and management of the disease will keep us focused on the next steps to address the larger issue of prevention,” they noted (Diabetes Care. 2016;39:857-60. doi:10.2337/dc16-0686) .
Dr. Cefalu is affiliated with the Pennington Biomedical Research Center at Louisiana State University in Baton Rouge, La. He disclosed financial relationships with multiple companies including AstraZeneca, Janssen, MannKind Corp., Intarcia Therapeutics, and Sanofi.
“The new guidelines provide much needed guidance for general practitioners, endocrinologists, and diabetes specialists about the use of metabolic surgery in the treatment of obese patients with type 2 diabetes,” wrote Dr. William T. Cefalu and colleagues in an accompanying editorial.
However, “one of the many issues that remains unanswered for metabolic surgery pertains to the exact mechanism of action. Specifically, we now recognize that postoperative improvements in metabolic control occur rapidly and out of proportion to weight loss, yet the physiological and molecular mechanisms underlying these beneficial glycemic effects remain incompletely elucidated,” they wrote. In addition, “before we can fully appreciate the role of metabolic surgery in becoming a readily available, viable option in our treatment algorithm and expand the appropriate candidate pool, we need to fully understand the efficacy, complications, long-term clinical outcomes, and costs. In particular, it will be important to clarify the financial implications to patients, providers, and insurers (both private and government sectors) and to appreciate that these barriers may be hard to overcome in resource poor areas of the world,” they said.
“It is an exciting time for those of us in diabetes research, and the ability to be part of a paradigm change in the understanding, approach, and management of the disease will keep us focused on the next steps to address the larger issue of prevention,” they noted (Diabetes Care. 2016;39:857-60. doi:10.2337/dc16-0686) .
Dr. Cefalu is affiliated with the Pennington Biomedical Research Center at Louisiana State University in Baton Rouge, La. He disclosed financial relationships with multiple companies including AstraZeneca, Janssen, MannKind Corp., Intarcia Therapeutics, and Sanofi.
“The new guidelines provide much needed guidance for general practitioners, endocrinologists, and diabetes specialists about the use of metabolic surgery in the treatment of obese patients with type 2 diabetes,” wrote Dr. William T. Cefalu and colleagues in an accompanying editorial.
However, “one of the many issues that remains unanswered for metabolic surgery pertains to the exact mechanism of action. Specifically, we now recognize that postoperative improvements in metabolic control occur rapidly and out of proportion to weight loss, yet the physiological and molecular mechanisms underlying these beneficial glycemic effects remain incompletely elucidated,” they wrote. In addition, “before we can fully appreciate the role of metabolic surgery in becoming a readily available, viable option in our treatment algorithm and expand the appropriate candidate pool, we need to fully understand the efficacy, complications, long-term clinical outcomes, and costs. In particular, it will be important to clarify the financial implications to patients, providers, and insurers (both private and government sectors) and to appreciate that these barriers may be hard to overcome in resource poor areas of the world,” they said.
“It is an exciting time for those of us in diabetes research, and the ability to be part of a paradigm change in the understanding, approach, and management of the disease will keep us focused on the next steps to address the larger issue of prevention,” they noted (Diabetes Care. 2016;39:857-60. doi:10.2337/dc16-0686) .
Dr. Cefalu is affiliated with the Pennington Biomedical Research Center at Louisiana State University in Baton Rouge, La. He disclosed financial relationships with multiple companies including AstraZeneca, Janssen, MannKind Corp., Intarcia Therapeutics, and Sanofi.
Metabolic surgery should be recommended for obese patients with type 2 diabetes mellitus, according to an international consensus statement from 48 clinicians and scholars issued after the Second Diabetes Surgery Summit held as part of the World Congress on Interventional Therapies for Type 2 Diabetes in London in 2015.
Current treatment plans for patients with type 2 diabetes do no include bariatric/metabolic surgery, despite increasing evidence of improved glycemic control and reduced cardiovascular risk factors in surgically treated patients, wrote Dr. Francesco Rubino and colleagues on behalf of members of the Second Diabetes Surgery Summit (DSS-II). The guidelines were endorsed by 45 global medical and scientific societies at the time of publication. The statement was published in a special edition of Diabetes Care (Diabetes Care. 2016;39:861-77. doi:10.2337/dc16-0236).
The guidelines recommend metabolic surgery for patients with type 2 diabetes who have class III obesity (defined as a body mass index of at least 40 kg/m2) or class II obesity (defined as a BMI of 35 kg/m2-39.9 kg/m2). In addition, metabolic surgery should be considered as a option for those patients with type 2 diabetes whose BMI falls within the 30 kg/m2-34.9 kg/m2 range if hyperglycemia remains uncontrolled after treatment attempts with oral or injectable medications. For Asian patients, the BMI thresholds for surgery should be reduced by 2.5 kg/m2, the researchers noted.
The conclusions are based on a review of published evidence on metabolic surgery and type 2 diabetes from January 1, 2005, through September 30, 2015.
The researchers assessed the evidence based on factors including long-term effects of surgery on glycemic control, effectiveness of surgery compared with nonsurgical interventions, comparisons of surgical procedures, and effects of surgery on diabetes complications, cardiovascular risk factors, and mortality. They also considered the short- and long-term safety of different procedures. The recommendations offer guidance on patient selection, pre- and postoperative workups, choice of procedure, and defining goals and success of surgery.
“The success of metabolic surgery needs to be defined in the larger context of comprehensive diabetes care plans,” the researchers noted. “Metabolic surgery should be considered a means to achieve the glycemic control necessary to reduce risk of microvascular complications and CVD.”
The researchers acknowledged that complications from metabolic surgery may require reoperations and rehospitalizations. Other limitations include a lack of evidence in several areas including: cost-effectiveness, optimal nutrition management after surgery, postoperative lifestyle interventions, and long-term effects of surgery, and further research is needed.
However, “there is now sufficient clinical and mechanistic evidence to support inclusion of GI surgery among antidiabetes interventions for people with type 2 diabetes and obesity,” the researchers said. They called for collaboration between clinicians and regulators to recognize the potential value of metabolic surgery for type 2 diabetes and develop appropriate reimbursement plans.
The DSS-II and WCITD 2015 were sponsored by the International Diabetes Society Task Force, King’s College London, King’s College Hospital, Johnson & Johnson, Medtronic, Medimmune, Fractyl, DIAMOND MetaCure, Gore, Novo Nordisk, and NGM Biopharmaceuticals. The researchers reported no relevant conflicts.
Metabolic surgery should be recommended for obese patients with type 2 diabetes mellitus, according to an international consensus statement from 48 clinicians and scholars issued after the Second Diabetes Surgery Summit held as part of the World Congress on Interventional Therapies for Type 2 Diabetes in London in 2015.
Current treatment plans for patients with type 2 diabetes do no include bariatric/metabolic surgery, despite increasing evidence of improved glycemic control and reduced cardiovascular risk factors in surgically treated patients, wrote Dr. Francesco Rubino and colleagues on behalf of members of the Second Diabetes Surgery Summit (DSS-II). The guidelines were endorsed by 45 global medical and scientific societies at the time of publication. The statement was published in a special edition of Diabetes Care (Diabetes Care. 2016;39:861-77. doi:10.2337/dc16-0236).
The guidelines recommend metabolic surgery for patients with type 2 diabetes who have class III obesity (defined as a body mass index of at least 40 kg/m2) or class II obesity (defined as a BMI of 35 kg/m2-39.9 kg/m2). In addition, metabolic surgery should be considered as a option for those patients with type 2 diabetes whose BMI falls within the 30 kg/m2-34.9 kg/m2 range if hyperglycemia remains uncontrolled after treatment attempts with oral or injectable medications. For Asian patients, the BMI thresholds for surgery should be reduced by 2.5 kg/m2, the researchers noted.
The conclusions are based on a review of published evidence on metabolic surgery and type 2 diabetes from January 1, 2005, through September 30, 2015.
The researchers assessed the evidence based on factors including long-term effects of surgery on glycemic control, effectiveness of surgery compared with nonsurgical interventions, comparisons of surgical procedures, and effects of surgery on diabetes complications, cardiovascular risk factors, and mortality. They also considered the short- and long-term safety of different procedures. The recommendations offer guidance on patient selection, pre- and postoperative workups, choice of procedure, and defining goals and success of surgery.
“The success of metabolic surgery needs to be defined in the larger context of comprehensive diabetes care plans,” the researchers noted. “Metabolic surgery should be considered a means to achieve the glycemic control necessary to reduce risk of microvascular complications and CVD.”
The researchers acknowledged that complications from metabolic surgery may require reoperations and rehospitalizations. Other limitations include a lack of evidence in several areas including: cost-effectiveness, optimal nutrition management after surgery, postoperative lifestyle interventions, and long-term effects of surgery, and further research is needed.
However, “there is now sufficient clinical and mechanistic evidence to support inclusion of GI surgery among antidiabetes interventions for people with type 2 diabetes and obesity,” the researchers said. They called for collaboration between clinicians and regulators to recognize the potential value of metabolic surgery for type 2 diabetes and develop appropriate reimbursement plans.
The DSS-II and WCITD 2015 were sponsored by the International Diabetes Society Task Force, King’s College London, King’s College Hospital, Johnson & Johnson, Medtronic, Medimmune, Fractyl, DIAMOND MetaCure, Gore, Novo Nordisk, and NGM Biopharmaceuticals. The researchers reported no relevant conflicts.
FROM DIABETES CARE
Key clinical point: Metabolic surgery should be recommended for obese patients with type 2 diabetes mellitus.
Major finding: Surgery is recommended for patients with type 2 diabetes who have a BMI of 35 kg/m2 or higher and should be considered for patients with a BMI of 30 kg/m2-34.9 kg/m2 with uncontrolled hyperglycemia.
Data source: Published evidence on metabolic surgery and type 2 diabetes identified on Medline between January 1, 2005 and September 30, 2015.
Disclosures: The DSS-II and WCITD 2015 were sponsored by the International Diabetes Society Task Force, King’s College London, King’s College Hospital, Johnson & Johnson, Medtronic, Medimmune, Fractyl, DIAMOND MetaCure, Gore, Novo Nordisk, and NGM Biopharmaceuticals. The researchers reported no relevant conflicts.