User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Experts endorse metabolic surgery for diabetes intervention
Metabolic surgery should be recommended for obese patients with type 2 diabetes mellitus, according to an international consensus statement from 48 clinicians and scholars issued after the Second Diabetes Surgery Summit held as part of the World Congress on Interventional Therapies for Type 2 Diabetes in London in 2015.
Current treatment plans for patients with type 2 diabetes do no include bariatric/metabolic surgery, despite increasing evidence of improved glycemic control and reduced cardiovascular risk factors in surgically treated patients, wrote Dr. Francesco Rubino and colleagues on behalf of members of the Second Diabetes Surgery Summit (DSS-II). The guidelines were endorsed by 45 global medical and scientific societies at the time of publication. The statement was published in a special edition of Diabetes Care (Diabetes Care. 2016;39:861-77. doi:10.2337/dc16-0236).
The guidelines recommend metabolic surgery for patients with type 2 diabetes who have class III obesity (defined as a body mass index of at least 40 kg/m2) or class II obesity (defined as a BMI of 35 kg/m2-39.9 kg/m2). In addition, metabolic surgery should be considered as a option for those patients with type 2 diabetes whose BMI falls within the 30 kg/m2-34.9 kg/m2 range if hyperglycemia remains uncontrolled after treatment attempts with oral or injectable medications. For Asian patients, the BMI thresholds for surgery should be reduced by 2.5 kg/m2, the researchers noted.
The conclusions are based on a review of published evidence on metabolic surgery and type 2 diabetes from January 1, 2005, through September 30, 2015.
The researchers assessed the evidence based on factors including long-term effects of surgery on glycemic control, effectiveness of surgery compared with nonsurgical interventions, comparisons of surgical procedures, and effects of surgery on diabetes complications, cardiovascular risk factors, and mortality. They also considered the short- and long-term safety of different procedures. The recommendations offer guidance on patient selection, pre- and postoperative workups, choice of procedure, and defining goals and success of surgery.
“The success of metabolic surgery needs to be defined in the larger context of comprehensive diabetes care plans,” the researchers noted. “Metabolic surgery should be considered a means to achieve the glycemic control necessary to reduce risk of microvascular complications and CVD.”
The researchers acknowledged that complications from metabolic surgery may require reoperations and rehospitalizations. Other limitations include a lack of evidence in several areas including: cost-effectiveness, optimal nutrition management after surgery, postoperative lifestyle interventions, and long-term effects of surgery, and further research is needed.
However, “there is now sufficient clinical and mechanistic evidence to support inclusion of GI surgery among antidiabetes interventions for people with type 2 diabetes and obesity,” the researchers said. They called for collaboration between clinicians and regulators to recognize the potential value of metabolic surgery for type 2 diabetes and develop appropriate reimbursement plans.
The DSS-II and WCITD 2015 were sponsored by the International Diabetes Society Task Force, King’s College London, King’s College Hospital, Johnson & Johnson, Medtronic, Medimmune, Fractyl, DIAMOND MetaCure, Gore, Novo Nordisk, and NGM Biopharmaceuticals. The researchers reported no relevant conflicts.
“The new guidelines provide much needed guidance for general practitioners, endocrinologists, and diabetes specialists about the use of metabolic surgery in the treatment of obese patients with type 2 diabetes,” wrote Dr. William T. Cefalu and colleagues in an accompanying editorial.
However, “one of the many issues that remains unanswered for metabolic surgery pertains to the exact mechanism of action. Specifically, we now recognize that postoperative improvements in metabolic control occur rapidly and out of proportion to weight loss, yet the physiological and molecular mechanisms underlying these beneficial glycemic effects remain incompletely elucidated,” they wrote. In addition, “before we can fully appreciate the role of metabolic surgery in becoming a readily available, viable option in our treatment algorithm and expand the appropriate candidate pool, we need to fully understand the efficacy, complications, long-term clinical outcomes, and costs. In particular, it will be important to clarify the financial implications to patients, providers, and insurers (both private and government sectors) and to appreciate that these barriers may be hard to overcome in resource poor areas of the world,” they said.
“It is an exciting time for those of us in diabetes research, and the ability to be part of a paradigm change in the understanding, approach, and management of the disease will keep us focused on the next steps to address the larger issue of prevention,” they noted (Diabetes Care. 2016;39:857-60. doi:10.2337/dc16-0686) .
Dr. Cefalu is affiliated with the Pennington Biomedical Research Center at Louisiana State University in Baton Rouge, La. He disclosed financial relationships with multiple companies including AstraZeneca, Janssen, MannKind Corp., Intarcia Therapeutics, and Sanofi.
“The new guidelines provide much needed guidance for general practitioners, endocrinologists, and diabetes specialists about the use of metabolic surgery in the treatment of obese patients with type 2 diabetes,” wrote Dr. William T. Cefalu and colleagues in an accompanying editorial.
However, “one of the many issues that remains unanswered for metabolic surgery pertains to the exact mechanism of action. Specifically, we now recognize that postoperative improvements in metabolic control occur rapidly and out of proportion to weight loss, yet the physiological and molecular mechanisms underlying these beneficial glycemic effects remain incompletely elucidated,” they wrote. In addition, “before we can fully appreciate the role of metabolic surgery in becoming a readily available, viable option in our treatment algorithm and expand the appropriate candidate pool, we need to fully understand the efficacy, complications, long-term clinical outcomes, and costs. In particular, it will be important to clarify the financial implications to patients, providers, and insurers (both private and government sectors) and to appreciate that these barriers may be hard to overcome in resource poor areas of the world,” they said.
“It is an exciting time for those of us in diabetes research, and the ability to be part of a paradigm change in the understanding, approach, and management of the disease will keep us focused on the next steps to address the larger issue of prevention,” they noted (Diabetes Care. 2016;39:857-60. doi:10.2337/dc16-0686) .
Dr. Cefalu is affiliated with the Pennington Biomedical Research Center at Louisiana State University in Baton Rouge, La. He disclosed financial relationships with multiple companies including AstraZeneca, Janssen, MannKind Corp., Intarcia Therapeutics, and Sanofi.
“The new guidelines provide much needed guidance for general practitioners, endocrinologists, and diabetes specialists about the use of metabolic surgery in the treatment of obese patients with type 2 diabetes,” wrote Dr. William T. Cefalu and colleagues in an accompanying editorial.
However, “one of the many issues that remains unanswered for metabolic surgery pertains to the exact mechanism of action. Specifically, we now recognize that postoperative improvements in metabolic control occur rapidly and out of proportion to weight loss, yet the physiological and molecular mechanisms underlying these beneficial glycemic effects remain incompletely elucidated,” they wrote. In addition, “before we can fully appreciate the role of metabolic surgery in becoming a readily available, viable option in our treatment algorithm and expand the appropriate candidate pool, we need to fully understand the efficacy, complications, long-term clinical outcomes, and costs. In particular, it will be important to clarify the financial implications to patients, providers, and insurers (both private and government sectors) and to appreciate that these barriers may be hard to overcome in resource poor areas of the world,” they said.
“It is an exciting time for those of us in diabetes research, and the ability to be part of a paradigm change in the understanding, approach, and management of the disease will keep us focused on the next steps to address the larger issue of prevention,” they noted (Diabetes Care. 2016;39:857-60. doi:10.2337/dc16-0686) .
Dr. Cefalu is affiliated with the Pennington Biomedical Research Center at Louisiana State University in Baton Rouge, La. He disclosed financial relationships with multiple companies including AstraZeneca, Janssen, MannKind Corp., Intarcia Therapeutics, and Sanofi.
Metabolic surgery should be recommended for obese patients with type 2 diabetes mellitus, according to an international consensus statement from 48 clinicians and scholars issued after the Second Diabetes Surgery Summit held as part of the World Congress on Interventional Therapies for Type 2 Diabetes in London in 2015.
Current treatment plans for patients with type 2 diabetes do no include bariatric/metabolic surgery, despite increasing evidence of improved glycemic control and reduced cardiovascular risk factors in surgically treated patients, wrote Dr. Francesco Rubino and colleagues on behalf of members of the Second Diabetes Surgery Summit (DSS-II). The guidelines were endorsed by 45 global medical and scientific societies at the time of publication. The statement was published in a special edition of Diabetes Care (Diabetes Care. 2016;39:861-77. doi:10.2337/dc16-0236).
The guidelines recommend metabolic surgery for patients with type 2 diabetes who have class III obesity (defined as a body mass index of at least 40 kg/m2) or class II obesity (defined as a BMI of 35 kg/m2-39.9 kg/m2). In addition, metabolic surgery should be considered as a option for those patients with type 2 diabetes whose BMI falls within the 30 kg/m2-34.9 kg/m2 range if hyperglycemia remains uncontrolled after treatment attempts with oral or injectable medications. For Asian patients, the BMI thresholds for surgery should be reduced by 2.5 kg/m2, the researchers noted.
The conclusions are based on a review of published evidence on metabolic surgery and type 2 diabetes from January 1, 2005, through September 30, 2015.
The researchers assessed the evidence based on factors including long-term effects of surgery on glycemic control, effectiveness of surgery compared with nonsurgical interventions, comparisons of surgical procedures, and effects of surgery on diabetes complications, cardiovascular risk factors, and mortality. They also considered the short- and long-term safety of different procedures. The recommendations offer guidance on patient selection, pre- and postoperative workups, choice of procedure, and defining goals and success of surgery.
“The success of metabolic surgery needs to be defined in the larger context of comprehensive diabetes care plans,” the researchers noted. “Metabolic surgery should be considered a means to achieve the glycemic control necessary to reduce risk of microvascular complications and CVD.”
The researchers acknowledged that complications from metabolic surgery may require reoperations and rehospitalizations. Other limitations include a lack of evidence in several areas including: cost-effectiveness, optimal nutrition management after surgery, postoperative lifestyle interventions, and long-term effects of surgery, and further research is needed.
However, “there is now sufficient clinical and mechanistic evidence to support inclusion of GI surgery among antidiabetes interventions for people with type 2 diabetes and obesity,” the researchers said. They called for collaboration between clinicians and regulators to recognize the potential value of metabolic surgery for type 2 diabetes and develop appropriate reimbursement plans.
The DSS-II and WCITD 2015 were sponsored by the International Diabetes Society Task Force, King’s College London, King’s College Hospital, Johnson & Johnson, Medtronic, Medimmune, Fractyl, DIAMOND MetaCure, Gore, Novo Nordisk, and NGM Biopharmaceuticals. The researchers reported no relevant conflicts.
Metabolic surgery should be recommended for obese patients with type 2 diabetes mellitus, according to an international consensus statement from 48 clinicians and scholars issued after the Second Diabetes Surgery Summit held as part of the World Congress on Interventional Therapies for Type 2 Diabetes in London in 2015.
Current treatment plans for patients with type 2 diabetes do no include bariatric/metabolic surgery, despite increasing evidence of improved glycemic control and reduced cardiovascular risk factors in surgically treated patients, wrote Dr. Francesco Rubino and colleagues on behalf of members of the Second Diabetes Surgery Summit (DSS-II). The guidelines were endorsed by 45 global medical and scientific societies at the time of publication. The statement was published in a special edition of Diabetes Care (Diabetes Care. 2016;39:861-77. doi:10.2337/dc16-0236).
The guidelines recommend metabolic surgery for patients with type 2 diabetes who have class III obesity (defined as a body mass index of at least 40 kg/m2) or class II obesity (defined as a BMI of 35 kg/m2-39.9 kg/m2). In addition, metabolic surgery should be considered as a option for those patients with type 2 diabetes whose BMI falls within the 30 kg/m2-34.9 kg/m2 range if hyperglycemia remains uncontrolled after treatment attempts with oral or injectable medications. For Asian patients, the BMI thresholds for surgery should be reduced by 2.5 kg/m2, the researchers noted.
The conclusions are based on a review of published evidence on metabolic surgery and type 2 diabetes from January 1, 2005, through September 30, 2015.
The researchers assessed the evidence based on factors including long-term effects of surgery on glycemic control, effectiveness of surgery compared with nonsurgical interventions, comparisons of surgical procedures, and effects of surgery on diabetes complications, cardiovascular risk factors, and mortality. They also considered the short- and long-term safety of different procedures. The recommendations offer guidance on patient selection, pre- and postoperative workups, choice of procedure, and defining goals and success of surgery.
“The success of metabolic surgery needs to be defined in the larger context of comprehensive diabetes care plans,” the researchers noted. “Metabolic surgery should be considered a means to achieve the glycemic control necessary to reduce risk of microvascular complications and CVD.”
The researchers acknowledged that complications from metabolic surgery may require reoperations and rehospitalizations. Other limitations include a lack of evidence in several areas including: cost-effectiveness, optimal nutrition management after surgery, postoperative lifestyle interventions, and long-term effects of surgery, and further research is needed.
However, “there is now sufficient clinical and mechanistic evidence to support inclusion of GI surgery among antidiabetes interventions for people with type 2 diabetes and obesity,” the researchers said. They called for collaboration between clinicians and regulators to recognize the potential value of metabolic surgery for type 2 diabetes and develop appropriate reimbursement plans.
The DSS-II and WCITD 2015 were sponsored by the International Diabetes Society Task Force, King’s College London, King’s College Hospital, Johnson & Johnson, Medtronic, Medimmune, Fractyl, DIAMOND MetaCure, Gore, Novo Nordisk, and NGM Biopharmaceuticals. The researchers reported no relevant conflicts.
FROM DIABETES CARE
Key clinical point: Metabolic surgery should be recommended for obese patients with type 2 diabetes mellitus.
Major finding: Surgery is recommended for patients with type 2 diabetes who have a BMI of 35 kg/m2 or higher and should be considered for patients with a BMI of 30 kg/m2-34.9 kg/m2 with uncontrolled hyperglycemia.
Data source: Published evidence on metabolic surgery and type 2 diabetes identified on Medline between January 1, 2005 and September 30, 2015.
Disclosures: The DSS-II and WCITD 2015 were sponsored by the International Diabetes Society Task Force, King’s College London, King’s College Hospital, Johnson & Johnson, Medtronic, Medimmune, Fractyl, DIAMOND MetaCure, Gore, Novo Nordisk, and NGM Biopharmaceuticals. The researchers reported no relevant conflicts.
Starting with combination diabetes therapy beats initial monotherapy
ORLANDO – Whether to start a patient with newly diagnosed type 2 diabetes mellitus on combination therapy or monotherapy should be based on experimentation and observation rather than expert opinion, according to Dr. Alan Garber, president of the American College of Endocrinology and professor of medicine, biochemistry, and molecular and cellular biology at Baylor College of Medicine in Houston.
Monotherapy for type 2 diabetes with stepwise addition of other antihyperglycemic agents has long been the accepted way to initiate therapy in this population. Beginning in the 1990s, investigators began to compare the efficacy of monotherapy with combination therapy, first with metformin and glyburide alone or together, and then testing metformin in combination with glipizide, rosiglitazone, and sitagliptin, he said.
For metformin and glyburide, each agent alone lowered glycated hemoglobin (HbA1c), compared with placebo, but adding one to the other enhanced lowering. Combining the two drugs had the greatest benefit for higher HbA1c entry levels (e.g., HbA1c strata of 9%-9.9% or 10% or greater vs. less than 8%). At the highest-entry HbA1c levels, half doses of each of metformin and glyburide (250 mg/1.25 mg, respectively) were more efficacious than full doses of each (500 mg/2.5 mg). “This is called drug sparing,” he said.
In a trial of metformin and rosiglitazone, the combination was superior to either alone, producing significantly greater mean reductions in HbA1c and in fasting plasma glucose (FPG) at 32 weeks from their respective baselines, again, with greater reductions for higher-entry HbA1c levels. The combination was also better than either drug alone in the speed of reducing HbA1c or FPG, and in the final attained levels.
The combination of metformin and a sulfonylurea presents a risk of hypoglycemia, but Dr. Garber said the results are “much cleaner” using combinations of metformin with agents such as a thiazolidinedione, a dipeptidyl peptidase-4 inhibitor, or a sodium/glucose cotransporter-2 inhibitor.
Also noteworthy are findings from the EDICT (Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes) trial using insulin-sensitizing and insulin-secreting agents metformin/pioglitazone/exenatide in combination vs. escalating doses of metformin with sequential addition of a sulfonylurea and glargine insulin to treat patients with newly diagnosed type 2 diabetes. Over 2 years, the subjects receiving combination therapy had lower HbA1c, a mean weight loss, compared with weight gain, in the sequential therapy group, and a 7.5-fold lower rate of hypoglycemia, compared with the sequential treatment group (Diabetes Obes Metab. 2015;17:268-75).
Although the agents used in the two treatment strategies were not strictly equivalent, “it’s clear that testing multiple therapeutic mechanisms tends to produce better outcomes than fewer therapeutic mechanisms,” Dr. Garber said. The conclusions are fairly straightforward. “Look for evidence to support what strategies you want to use for your patients’ care.”
Using the Kaiser Permanente database, investigators found that the mean time of having an HbA1c above 8% was 3 years before a second agent was added, and the mean HbA1c was 9%. Many people have ascribed this sort of delay to a problem with the physician. But Dr. Garber said it is more related to patients, who often resist prescriptions for more drugs. So starting with two drugs may produce better efficacy faster as well as overcome the psychological issues of trying to add another one later (Am J Manag Care. 2003;9:213-7).
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, California, raised the possibility of “subtraction therapy, where you start with three agents no matter what, and then if things go well, you subtract. And so you reverse the situation that Alan discussed.” In the patient’s view, “you have a celebration that night instead of a wake,” he said.
Dr. Garber has received honoraria or consulting fees as a member of the advisory boards of Novo Nordisk, Janssen, and Merck. Dr. Einhorn is on the scientific advisory boards of Eli Lilly, Novo Nordisk, Janssen, Boehringer Ingelheim, Sanofi, and Adocia, is a consultant for Halozyme, Glysens, Freedom-Meditech, and Epitracker, and has research funding from Lilly, Novo, Janssen, AstraZeneca, Mannkind, Freedom-Meditech, Merck, Sanofi, and Boehringer Ingelheim.
ORLANDO – Whether to start a patient with newly diagnosed type 2 diabetes mellitus on combination therapy or monotherapy should be based on experimentation and observation rather than expert opinion, according to Dr. Alan Garber, president of the American College of Endocrinology and professor of medicine, biochemistry, and molecular and cellular biology at Baylor College of Medicine in Houston.
Monotherapy for type 2 diabetes with stepwise addition of other antihyperglycemic agents has long been the accepted way to initiate therapy in this population. Beginning in the 1990s, investigators began to compare the efficacy of monotherapy with combination therapy, first with metformin and glyburide alone or together, and then testing metformin in combination with glipizide, rosiglitazone, and sitagliptin, he said.
For metformin and glyburide, each agent alone lowered glycated hemoglobin (HbA1c), compared with placebo, but adding one to the other enhanced lowering. Combining the two drugs had the greatest benefit for higher HbA1c entry levels (e.g., HbA1c strata of 9%-9.9% or 10% or greater vs. less than 8%). At the highest-entry HbA1c levels, half doses of each of metformin and glyburide (250 mg/1.25 mg, respectively) were more efficacious than full doses of each (500 mg/2.5 mg). “This is called drug sparing,” he said.
In a trial of metformin and rosiglitazone, the combination was superior to either alone, producing significantly greater mean reductions in HbA1c and in fasting plasma glucose (FPG) at 32 weeks from their respective baselines, again, with greater reductions for higher-entry HbA1c levels. The combination was also better than either drug alone in the speed of reducing HbA1c or FPG, and in the final attained levels.
The combination of metformin and a sulfonylurea presents a risk of hypoglycemia, but Dr. Garber said the results are “much cleaner” using combinations of metformin with agents such as a thiazolidinedione, a dipeptidyl peptidase-4 inhibitor, or a sodium/glucose cotransporter-2 inhibitor.
Also noteworthy are findings from the EDICT (Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes) trial using insulin-sensitizing and insulin-secreting agents metformin/pioglitazone/exenatide in combination vs. escalating doses of metformin with sequential addition of a sulfonylurea and glargine insulin to treat patients with newly diagnosed type 2 diabetes. Over 2 years, the subjects receiving combination therapy had lower HbA1c, a mean weight loss, compared with weight gain, in the sequential therapy group, and a 7.5-fold lower rate of hypoglycemia, compared with the sequential treatment group (Diabetes Obes Metab. 2015;17:268-75).
Although the agents used in the two treatment strategies were not strictly equivalent, “it’s clear that testing multiple therapeutic mechanisms tends to produce better outcomes than fewer therapeutic mechanisms,” Dr. Garber said. The conclusions are fairly straightforward. “Look for evidence to support what strategies you want to use for your patients’ care.”
Using the Kaiser Permanente database, investigators found that the mean time of having an HbA1c above 8% was 3 years before a second agent was added, and the mean HbA1c was 9%. Many people have ascribed this sort of delay to a problem with the physician. But Dr. Garber said it is more related to patients, who often resist prescriptions for more drugs. So starting with two drugs may produce better efficacy faster as well as overcome the psychological issues of trying to add another one later (Am J Manag Care. 2003;9:213-7).
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, California, raised the possibility of “subtraction therapy, where you start with three agents no matter what, and then if things go well, you subtract. And so you reverse the situation that Alan discussed.” In the patient’s view, “you have a celebration that night instead of a wake,” he said.
Dr. Garber has received honoraria or consulting fees as a member of the advisory boards of Novo Nordisk, Janssen, and Merck. Dr. Einhorn is on the scientific advisory boards of Eli Lilly, Novo Nordisk, Janssen, Boehringer Ingelheim, Sanofi, and Adocia, is a consultant for Halozyme, Glysens, Freedom-Meditech, and Epitracker, and has research funding from Lilly, Novo, Janssen, AstraZeneca, Mannkind, Freedom-Meditech, Merck, Sanofi, and Boehringer Ingelheim.
ORLANDO – Whether to start a patient with newly diagnosed type 2 diabetes mellitus on combination therapy or monotherapy should be based on experimentation and observation rather than expert opinion, according to Dr. Alan Garber, president of the American College of Endocrinology and professor of medicine, biochemistry, and molecular and cellular biology at Baylor College of Medicine in Houston.
Monotherapy for type 2 diabetes with stepwise addition of other antihyperglycemic agents has long been the accepted way to initiate therapy in this population. Beginning in the 1990s, investigators began to compare the efficacy of monotherapy with combination therapy, first with metformin and glyburide alone or together, and then testing metformin in combination with glipizide, rosiglitazone, and sitagliptin, he said.
For metformin and glyburide, each agent alone lowered glycated hemoglobin (HbA1c), compared with placebo, but adding one to the other enhanced lowering. Combining the two drugs had the greatest benefit for higher HbA1c entry levels (e.g., HbA1c strata of 9%-9.9% or 10% or greater vs. less than 8%). At the highest-entry HbA1c levels, half doses of each of metformin and glyburide (250 mg/1.25 mg, respectively) were more efficacious than full doses of each (500 mg/2.5 mg). “This is called drug sparing,” he said.
In a trial of metformin and rosiglitazone, the combination was superior to either alone, producing significantly greater mean reductions in HbA1c and in fasting plasma glucose (FPG) at 32 weeks from their respective baselines, again, with greater reductions for higher-entry HbA1c levels. The combination was also better than either drug alone in the speed of reducing HbA1c or FPG, and in the final attained levels.
The combination of metformin and a sulfonylurea presents a risk of hypoglycemia, but Dr. Garber said the results are “much cleaner” using combinations of metformin with agents such as a thiazolidinedione, a dipeptidyl peptidase-4 inhibitor, or a sodium/glucose cotransporter-2 inhibitor.
Also noteworthy are findings from the EDICT (Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes) trial using insulin-sensitizing and insulin-secreting agents metformin/pioglitazone/exenatide in combination vs. escalating doses of metformin with sequential addition of a sulfonylurea and glargine insulin to treat patients with newly diagnosed type 2 diabetes. Over 2 years, the subjects receiving combination therapy had lower HbA1c, a mean weight loss, compared with weight gain, in the sequential therapy group, and a 7.5-fold lower rate of hypoglycemia, compared with the sequential treatment group (Diabetes Obes Metab. 2015;17:268-75).
Although the agents used in the two treatment strategies were not strictly equivalent, “it’s clear that testing multiple therapeutic mechanisms tends to produce better outcomes than fewer therapeutic mechanisms,” Dr. Garber said. The conclusions are fairly straightforward. “Look for evidence to support what strategies you want to use for your patients’ care.”
Using the Kaiser Permanente database, investigators found that the mean time of having an HbA1c above 8% was 3 years before a second agent was added, and the mean HbA1c was 9%. Many people have ascribed this sort of delay to a problem with the physician. But Dr. Garber said it is more related to patients, who often resist prescriptions for more drugs. So starting with two drugs may produce better efficacy faster as well as overcome the psychological issues of trying to add another one later (Am J Manag Care. 2003;9:213-7).
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, California, raised the possibility of “subtraction therapy, where you start with three agents no matter what, and then if things go well, you subtract. And so you reverse the situation that Alan discussed.” In the patient’s view, “you have a celebration that night instead of a wake,” he said.
Dr. Garber has received honoraria or consulting fees as a member of the advisory boards of Novo Nordisk, Janssen, and Merck. Dr. Einhorn is on the scientific advisory boards of Eli Lilly, Novo Nordisk, Janssen, Boehringer Ingelheim, Sanofi, and Adocia, is a consultant for Halozyme, Glysens, Freedom-Meditech, and Epitracker, and has research funding from Lilly, Novo, Janssen, AstraZeneca, Mannkind, Freedom-Meditech, Merck, Sanofi, and Boehringer Ingelheim.
EXPERT ANALYSIS AT AACE 2016
Initial monotherapy safe in type 2 diabetes but must be abandoned quickly if ineffective
ORLANDO – Starting a patient with newly diagnosed type 2 diabetes mellitus on a single agent is a reasonable approach, but leaving them too long on monotherapy that proves ineffective is insupportable by available data, according to Dr. Richard Pratley.
“It’s not so much whether or not you start with a single drug or with two drugs, but it’s the fact that we don’t advance therapy when appropriate,” he said. Allowing hyperglycemia to persist increases health risks. “We can do better with a combination therapy initially, but we also do better just by advancing therapy sequentially better. Nobody has to spend 17 months with an A1c of eight and a half percent or so,” said Dr. Pratley, director of the Florida Hospital Diabetes Institute and an adjunct professor at the Sanford Burnham Medical Research Institute, both in Orlando.
It is reasonable on several grounds to start with a single agent, but “starting” is the operative word. No matter whether one initiates therapy with one drug or a combination, an essential component of any therapy is lifestyle modification, possibly including medically assisted weight loss, he noted during a point/counterpoint session at the annual meeting of the American Association of Clinical Endocrinologists (AACE).
Guidelines from both the American Diabetes Association and the European Association for the Study of Diabetes recommend starting with metformin and then moving on stepwise to add a second drug, then a third as needed, with each step separated by 3 months. If further therapy is needed, then insulin can be added after another 3-6 months. These organizations have taken the position that metformin “remains the optimal drug for monotherapy,” based on low cost, proven safety, weight neutrality, and possible cardiovascular benefits. However, if the hemoglobin A1c is 9% or greater, initial combination therapy with metformin plus another drug may achieve an HbA1c target more quickly than sequential therapy, he said.
In contrast to the ADA/EASD guidelines, the AACE recommends monotherapy with one of several agents only if the entry HbA1c is less than 7.5%, advancing to dual therapy after 1 month if still not at goal. If the entry HbA1c is at or above 7.5% but less than 9.0%, dual therapy including metformin is advised, moving to triple therapy after 1 month if the patient is still not at goal. Any patient with an entry HbA1c above 9% can start with dual or triple therapy if symptoms are absent, but if symptoms are present, insulin is recommended with or without other agents. “Combination therapy is usually required and should involve agents with complementary mechanisms of action,” according to an AACE consensus statement.
With an entry HbA1c between 7.5% and 9%, the decision to use monotherapy or a combination is a “gray area,” Dr. Pratley said. The arguments in favor of combination therapy are that a single agent may have limited efficacy, multiple metabolic abnormalities contribute to hyperglycemia, drugs with complementary mechanisms of action will work better, and getting to an HbA1c goal faster will improve outcomes. With twelve classes of antihyperglycemic agents, however, to treat type 2 diabetes mellitus (2DM), the combination possibilities are almost endless. Even excluding insulin, there are more than 150 possible recommended two-drug combinations.
Based on several studies, Dr. Pratley countered these arguments, saying that one agent is often enough to reach goal when the entry HbA1c is 7.5-9%. Furthermore, evidence is lacking that early control improves outcomes or the durability of response, and there is no comparative efficacy data on initial combination therapies. Initial combinations limit personalizing therapy because of diminished flexibility of dosing, and attribution of side effects to one agent or another may be blurred.
Since diabetes patients may have comorbidities, polypharmacy for 2DM may present more drug interactions and contribute to adverse effects, aside from the risk of severe hypoglycemia from the antidiabetic drugs themselves. Dr. Pratley said that a higher pill burden may decrease adherence, more drugs add to cost, and insurance companies may cover only stepped care.
Comparing drug prices, he said initial monotherapy with metformin for the 1.7 million newly diagnosed 2DM patients in the United States every year could save $1 billion vs. some combination therapies. He said he found metformin free at one supermarket chain pharmacy, while a bag of potting soil was nearly $5 at a store next door, making metformin “cheaper than dirt.”
Citing a retrospective, observational study of more than 7,200 complete courses of treatment for 2DM (Diabetes Care. 2004;27:1535-40), Dr. Pratley said patients had several years of excess glycemic burden before therapy was intensified, which he called “the real problem.” He noted that initial monotherapy works for patients with HbA1c less than 9.0%, with sequential personalized therapy to minimize side effects and cost. “Clinical inertia must be avoided,” he said, so the key to good initial monotherapy is not to delay adding drugs when necessary.
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, Calif., commented in an interview that Dr. Pratley “made some very cogent arguments for minimizing the number of agents used and that the counter argument more recently for multiple-agent therapy has to be looked at in the context of what insurance companies will accept and the patients can tolerate.” He agreed that sequential therapy “is perfectly reasonable” as long as it is done rapidly, meaning waiting no longer than 3 months to add needed therapy. “If you go quickly in sequential therapy, it’s like the equivalent to starting combination from the beginning,” he said.
Dr. Pratley has received research support from Lexcon, Lilly, Merck, Novo Nordisk, sanofi, and Takeda and has been a consultant for AstraZeneca, Boehringer, GlaxoSmithKline, Lilly, Merck, Novo Nordisk, sanofi, and Takeda. All his honoraria are directed toward Florida Hospital, a nonprofit organization, and Dr. Pratley does not receive direct or indirect compensation for these services.
CORRECTION: An earlier version of this article misidentified the top photo of Dr. Daniel Einhorn.
ORLANDO – Starting a patient with newly diagnosed type 2 diabetes mellitus on a single agent is a reasonable approach, but leaving them too long on monotherapy that proves ineffective is insupportable by available data, according to Dr. Richard Pratley.
“It’s not so much whether or not you start with a single drug or with two drugs, but it’s the fact that we don’t advance therapy when appropriate,” he said. Allowing hyperglycemia to persist increases health risks. “We can do better with a combination therapy initially, but we also do better just by advancing therapy sequentially better. Nobody has to spend 17 months with an A1c of eight and a half percent or so,” said Dr. Pratley, director of the Florida Hospital Diabetes Institute and an adjunct professor at the Sanford Burnham Medical Research Institute, both in Orlando.
It is reasonable on several grounds to start with a single agent, but “starting” is the operative word. No matter whether one initiates therapy with one drug or a combination, an essential component of any therapy is lifestyle modification, possibly including medically assisted weight loss, he noted during a point/counterpoint session at the annual meeting of the American Association of Clinical Endocrinologists (AACE).
Guidelines from both the American Diabetes Association and the European Association for the Study of Diabetes recommend starting with metformin and then moving on stepwise to add a second drug, then a third as needed, with each step separated by 3 months. If further therapy is needed, then insulin can be added after another 3-6 months. These organizations have taken the position that metformin “remains the optimal drug for monotherapy,” based on low cost, proven safety, weight neutrality, and possible cardiovascular benefits. However, if the hemoglobin A1c is 9% or greater, initial combination therapy with metformin plus another drug may achieve an HbA1c target more quickly than sequential therapy, he said.
In contrast to the ADA/EASD guidelines, the AACE recommends monotherapy with one of several agents only if the entry HbA1c is less than 7.5%, advancing to dual therapy after 1 month if still not at goal. If the entry HbA1c is at or above 7.5% but less than 9.0%, dual therapy including metformin is advised, moving to triple therapy after 1 month if the patient is still not at goal. Any patient with an entry HbA1c above 9% can start with dual or triple therapy if symptoms are absent, but if symptoms are present, insulin is recommended with or without other agents. “Combination therapy is usually required and should involve agents with complementary mechanisms of action,” according to an AACE consensus statement.
With an entry HbA1c between 7.5% and 9%, the decision to use monotherapy or a combination is a “gray area,” Dr. Pratley said. The arguments in favor of combination therapy are that a single agent may have limited efficacy, multiple metabolic abnormalities contribute to hyperglycemia, drugs with complementary mechanisms of action will work better, and getting to an HbA1c goal faster will improve outcomes. With twelve classes of antihyperglycemic agents, however, to treat type 2 diabetes mellitus (2DM), the combination possibilities are almost endless. Even excluding insulin, there are more than 150 possible recommended two-drug combinations.
Based on several studies, Dr. Pratley countered these arguments, saying that one agent is often enough to reach goal when the entry HbA1c is 7.5-9%. Furthermore, evidence is lacking that early control improves outcomes or the durability of response, and there is no comparative efficacy data on initial combination therapies. Initial combinations limit personalizing therapy because of diminished flexibility of dosing, and attribution of side effects to one agent or another may be blurred.
Since diabetes patients may have comorbidities, polypharmacy for 2DM may present more drug interactions and contribute to adverse effects, aside from the risk of severe hypoglycemia from the antidiabetic drugs themselves. Dr. Pratley said that a higher pill burden may decrease adherence, more drugs add to cost, and insurance companies may cover only stepped care.
Comparing drug prices, he said initial monotherapy with metformin for the 1.7 million newly diagnosed 2DM patients in the United States every year could save $1 billion vs. some combination therapies. He said he found metformin free at one supermarket chain pharmacy, while a bag of potting soil was nearly $5 at a store next door, making metformin “cheaper than dirt.”
Citing a retrospective, observational study of more than 7,200 complete courses of treatment for 2DM (Diabetes Care. 2004;27:1535-40), Dr. Pratley said patients had several years of excess glycemic burden before therapy was intensified, which he called “the real problem.” He noted that initial monotherapy works for patients with HbA1c less than 9.0%, with sequential personalized therapy to minimize side effects and cost. “Clinical inertia must be avoided,” he said, so the key to good initial monotherapy is not to delay adding drugs when necessary.
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, Calif., commented in an interview that Dr. Pratley “made some very cogent arguments for minimizing the number of agents used and that the counter argument more recently for multiple-agent therapy has to be looked at in the context of what insurance companies will accept and the patients can tolerate.” He agreed that sequential therapy “is perfectly reasonable” as long as it is done rapidly, meaning waiting no longer than 3 months to add needed therapy. “If you go quickly in sequential therapy, it’s like the equivalent to starting combination from the beginning,” he said.
Dr. Pratley has received research support from Lexcon, Lilly, Merck, Novo Nordisk, sanofi, and Takeda and has been a consultant for AstraZeneca, Boehringer, GlaxoSmithKline, Lilly, Merck, Novo Nordisk, sanofi, and Takeda. All his honoraria are directed toward Florida Hospital, a nonprofit organization, and Dr. Pratley does not receive direct or indirect compensation for these services.
CORRECTION: An earlier version of this article misidentified the top photo of Dr. Daniel Einhorn.
ORLANDO – Starting a patient with newly diagnosed type 2 diabetes mellitus on a single agent is a reasonable approach, but leaving them too long on monotherapy that proves ineffective is insupportable by available data, according to Dr. Richard Pratley.
“It’s not so much whether or not you start with a single drug or with two drugs, but it’s the fact that we don’t advance therapy when appropriate,” he said. Allowing hyperglycemia to persist increases health risks. “We can do better with a combination therapy initially, but we also do better just by advancing therapy sequentially better. Nobody has to spend 17 months with an A1c of eight and a half percent or so,” said Dr. Pratley, director of the Florida Hospital Diabetes Institute and an adjunct professor at the Sanford Burnham Medical Research Institute, both in Orlando.
It is reasonable on several grounds to start with a single agent, but “starting” is the operative word. No matter whether one initiates therapy with one drug or a combination, an essential component of any therapy is lifestyle modification, possibly including medically assisted weight loss, he noted during a point/counterpoint session at the annual meeting of the American Association of Clinical Endocrinologists (AACE).
Guidelines from both the American Diabetes Association and the European Association for the Study of Diabetes recommend starting with metformin and then moving on stepwise to add a second drug, then a third as needed, with each step separated by 3 months. If further therapy is needed, then insulin can be added after another 3-6 months. These organizations have taken the position that metformin “remains the optimal drug for monotherapy,” based on low cost, proven safety, weight neutrality, and possible cardiovascular benefits. However, if the hemoglobin A1c is 9% or greater, initial combination therapy with metformin plus another drug may achieve an HbA1c target more quickly than sequential therapy, he said.
In contrast to the ADA/EASD guidelines, the AACE recommends monotherapy with one of several agents only if the entry HbA1c is less than 7.5%, advancing to dual therapy after 1 month if still not at goal. If the entry HbA1c is at or above 7.5% but less than 9.0%, dual therapy including metformin is advised, moving to triple therapy after 1 month if the patient is still not at goal. Any patient with an entry HbA1c above 9% can start with dual or triple therapy if symptoms are absent, but if symptoms are present, insulin is recommended with or without other agents. “Combination therapy is usually required and should involve agents with complementary mechanisms of action,” according to an AACE consensus statement.
With an entry HbA1c between 7.5% and 9%, the decision to use monotherapy or a combination is a “gray area,” Dr. Pratley said. The arguments in favor of combination therapy are that a single agent may have limited efficacy, multiple metabolic abnormalities contribute to hyperglycemia, drugs with complementary mechanisms of action will work better, and getting to an HbA1c goal faster will improve outcomes. With twelve classes of antihyperglycemic agents, however, to treat type 2 diabetes mellitus (2DM), the combination possibilities are almost endless. Even excluding insulin, there are more than 150 possible recommended two-drug combinations.
Based on several studies, Dr. Pratley countered these arguments, saying that one agent is often enough to reach goal when the entry HbA1c is 7.5-9%. Furthermore, evidence is lacking that early control improves outcomes or the durability of response, and there is no comparative efficacy data on initial combination therapies. Initial combinations limit personalizing therapy because of diminished flexibility of dosing, and attribution of side effects to one agent or another may be blurred.
Since diabetes patients may have comorbidities, polypharmacy for 2DM may present more drug interactions and contribute to adverse effects, aside from the risk of severe hypoglycemia from the antidiabetic drugs themselves. Dr. Pratley said that a higher pill burden may decrease adherence, more drugs add to cost, and insurance companies may cover only stepped care.
Comparing drug prices, he said initial monotherapy with metformin for the 1.7 million newly diagnosed 2DM patients in the United States every year could save $1 billion vs. some combination therapies. He said he found metformin free at one supermarket chain pharmacy, while a bag of potting soil was nearly $5 at a store next door, making metformin “cheaper than dirt.”
Citing a retrospective, observational study of more than 7,200 complete courses of treatment for 2DM (Diabetes Care. 2004;27:1535-40), Dr. Pratley said patients had several years of excess glycemic burden before therapy was intensified, which he called “the real problem.” He noted that initial monotherapy works for patients with HbA1c less than 9.0%, with sequential personalized therapy to minimize side effects and cost. “Clinical inertia must be avoided,” he said, so the key to good initial monotherapy is not to delay adding drugs when necessary.
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, Calif., commented in an interview that Dr. Pratley “made some very cogent arguments for minimizing the number of agents used and that the counter argument more recently for multiple-agent therapy has to be looked at in the context of what insurance companies will accept and the patients can tolerate.” He agreed that sequential therapy “is perfectly reasonable” as long as it is done rapidly, meaning waiting no longer than 3 months to add needed therapy. “If you go quickly in sequential therapy, it’s like the equivalent to starting combination from the beginning,” he said.
Dr. Pratley has received research support from Lexcon, Lilly, Merck, Novo Nordisk, sanofi, and Takeda and has been a consultant for AstraZeneca, Boehringer, GlaxoSmithKline, Lilly, Merck, Novo Nordisk, sanofi, and Takeda. All his honoraria are directed toward Florida Hospital, a nonprofit organization, and Dr. Pratley does not receive direct or indirect compensation for these services.
CORRECTION: An earlier version of this article misidentified the top photo of Dr. Daniel Einhorn.
EXPERT ANALYSIS FROM AACE 2016
FDA panel recommends two new combo injectables for diabetes
In back-to back advisory committee hearings, the Food and Drug Administration received recommendations for approval of two new combination medications to treat type 2 diabetes. The two medications each combine long-lasting insulin with a glucagon-like peptide–1 (GLP-1) receptor agonist in a once-daily injectable fixed-dose combination.
On May 24, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) voted unanimously to approve the fixed-dose combination of liraglutide and insulin degludec (individually marketed as Victoza and Tresiba, respectively, by Novo Nordisk). The new combination was referred to as IDegLira in the sponsor’s clinical trials.
The following day, the EMDAC recommended in a 12-2 vote to approve Sanofi-Aventis’ new fixed-dose combination of lixisenatide and insulin glargine (Lantus), also indicated as an add-on to lifestyle management for type 2 diabetes. The sponsor’s proposed name for this combination is iGlarLixi.
For both products, most committee members felt that the combo would most benefit patients who were already on either insulin or a GLP-1 agonist (though the second day’s panel noted that data were lacking on patients transitioning from a GLP-1 agonist to Sanofi-Aventis’ lixisenatide/glargine combo). “I tend to agree with many of the people who have gone before me that the population of patients this should be used in are those that are on one of these two injectable medications already and in particular I think the GLP-1 agonist,” said Dr. Marie Gelato, professor of medicine at Stony Brook (N.Y.) University, who voted in favor of the liraglutide/degludec combination medication.
A concern common to discussion on both days was that some patients with diabetes and a higher body mass might not be able to benefit from these medications, since each one caps insulin dosing.
Other themes common to both days’ deliberations among the largely overlapping panels included the need for fine-tuning the dosing apparatus, labeling, patient interface, and nomenclature for these novel devices. For Dr. Robert Smith, panel chair and professor of endocrinology at Brown University, Providence, R.I., his vote on the second day should be “considered contingent on accomplishing those things adequately.”
Most of the endocrinologists on the committees noted that they personally felt more comfortable beginning a single agent, and probably tended to tinker with patients’ regimens fairly frequently at least during the initial period of diabetes management. However, the committees on both days felt that having the fixed-dose combination agent available might be a particularly useful tool for family practice physicians and those practicing general internal medicine.
Since the proposed lixisenatide/glargine combination would be dispensed as one of two pens, each with a different dose range of lixisenatide, the second day’s panel spent more time in discussion of the potential for confusion or misdosing with two choices.
“It’s incumbent upon the sponsor to make it easier for the doctor. I have confidence that they will work out the delivery system,” said Dr. Peter Wilson, explaining his rationale for voting for approval of the lixisenatide/glargine combo despite some reservations about the device and delivery system, “I think this will be a boon for the patients,” added Dr. Wilson, professor of medicine and public health at Emory University, Atlanta.
Lixisenatide, marketed as Lyxumia in Europe and elsewhere by Sanofi-Aventis, is pending FDA approval, so it received some additional attention during the committee hearing for lixisenatide/glargine. Though it would be the sixth GLP-1 agonist on the U.S. market, committee members did not voice concerns that it would be a “me too” drug; rather, said Dr. Wilson, “It provides yet another choice. ... Choice is very important for physicians and for patients.”
During Sanofi-Aventis’ and the FDA’s presentations, safety data, especially as interpreted by the FDA, seemed to indicate a slightly elevated risk of significant allergic reactions with lixisenatide compared with placebo. However, conceded the FDA’s clinical reviewer Dr. Suchitra Balakrishnan, the postmarketing surveillance program for lixisenatide was “a larger program, which may have contributed to more events occurring.”
Earlier concerns about lixisenatide’s cardiovascular safety have been largely assuaged after publication late last year of results from the ELIXA trial (N Engl J Med. 2015; 373:2247-57) that showed no increased risk – but no benefit – for those with type 2 diabetes taking lixisenatide.
The FDA usually follows the advice of its advisory committees.
On Twitter @karioakes
In back-to back advisory committee hearings, the Food and Drug Administration received recommendations for approval of two new combination medications to treat type 2 diabetes. The two medications each combine long-lasting insulin with a glucagon-like peptide–1 (GLP-1) receptor agonist in a once-daily injectable fixed-dose combination.
On May 24, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) voted unanimously to approve the fixed-dose combination of liraglutide and insulin degludec (individually marketed as Victoza and Tresiba, respectively, by Novo Nordisk). The new combination was referred to as IDegLira in the sponsor’s clinical trials.
The following day, the EMDAC recommended in a 12-2 vote to approve Sanofi-Aventis’ new fixed-dose combination of lixisenatide and insulin glargine (Lantus), also indicated as an add-on to lifestyle management for type 2 diabetes. The sponsor’s proposed name for this combination is iGlarLixi.
For both products, most committee members felt that the combo would most benefit patients who were already on either insulin or a GLP-1 agonist (though the second day’s panel noted that data were lacking on patients transitioning from a GLP-1 agonist to Sanofi-Aventis’ lixisenatide/glargine combo). “I tend to agree with many of the people who have gone before me that the population of patients this should be used in are those that are on one of these two injectable medications already and in particular I think the GLP-1 agonist,” said Dr. Marie Gelato, professor of medicine at Stony Brook (N.Y.) University, who voted in favor of the liraglutide/degludec combination medication.
A concern common to discussion on both days was that some patients with diabetes and a higher body mass might not be able to benefit from these medications, since each one caps insulin dosing.
Other themes common to both days’ deliberations among the largely overlapping panels included the need for fine-tuning the dosing apparatus, labeling, patient interface, and nomenclature for these novel devices. For Dr. Robert Smith, panel chair and professor of endocrinology at Brown University, Providence, R.I., his vote on the second day should be “considered contingent on accomplishing those things adequately.”
Most of the endocrinologists on the committees noted that they personally felt more comfortable beginning a single agent, and probably tended to tinker with patients’ regimens fairly frequently at least during the initial period of diabetes management. However, the committees on both days felt that having the fixed-dose combination agent available might be a particularly useful tool for family practice physicians and those practicing general internal medicine.
Since the proposed lixisenatide/glargine combination would be dispensed as one of two pens, each with a different dose range of lixisenatide, the second day’s panel spent more time in discussion of the potential for confusion or misdosing with two choices.
“It’s incumbent upon the sponsor to make it easier for the doctor. I have confidence that they will work out the delivery system,” said Dr. Peter Wilson, explaining his rationale for voting for approval of the lixisenatide/glargine combo despite some reservations about the device and delivery system, “I think this will be a boon for the patients,” added Dr. Wilson, professor of medicine and public health at Emory University, Atlanta.
Lixisenatide, marketed as Lyxumia in Europe and elsewhere by Sanofi-Aventis, is pending FDA approval, so it received some additional attention during the committee hearing for lixisenatide/glargine. Though it would be the sixth GLP-1 agonist on the U.S. market, committee members did not voice concerns that it would be a “me too” drug; rather, said Dr. Wilson, “It provides yet another choice. ... Choice is very important for physicians and for patients.”
During Sanofi-Aventis’ and the FDA’s presentations, safety data, especially as interpreted by the FDA, seemed to indicate a slightly elevated risk of significant allergic reactions with lixisenatide compared with placebo. However, conceded the FDA’s clinical reviewer Dr. Suchitra Balakrishnan, the postmarketing surveillance program for lixisenatide was “a larger program, which may have contributed to more events occurring.”
Earlier concerns about lixisenatide’s cardiovascular safety have been largely assuaged after publication late last year of results from the ELIXA trial (N Engl J Med. 2015; 373:2247-57) that showed no increased risk – but no benefit – for those with type 2 diabetes taking lixisenatide.
The FDA usually follows the advice of its advisory committees.
On Twitter @karioakes
In back-to back advisory committee hearings, the Food and Drug Administration received recommendations for approval of two new combination medications to treat type 2 diabetes. The two medications each combine long-lasting insulin with a glucagon-like peptide–1 (GLP-1) receptor agonist in a once-daily injectable fixed-dose combination.
On May 24, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) voted unanimously to approve the fixed-dose combination of liraglutide and insulin degludec (individually marketed as Victoza and Tresiba, respectively, by Novo Nordisk). The new combination was referred to as IDegLira in the sponsor’s clinical trials.
The following day, the EMDAC recommended in a 12-2 vote to approve Sanofi-Aventis’ new fixed-dose combination of lixisenatide and insulin glargine (Lantus), also indicated as an add-on to lifestyle management for type 2 diabetes. The sponsor’s proposed name for this combination is iGlarLixi.
For both products, most committee members felt that the combo would most benefit patients who were already on either insulin or a GLP-1 agonist (though the second day’s panel noted that data were lacking on patients transitioning from a GLP-1 agonist to Sanofi-Aventis’ lixisenatide/glargine combo). “I tend to agree with many of the people who have gone before me that the population of patients this should be used in are those that are on one of these two injectable medications already and in particular I think the GLP-1 agonist,” said Dr. Marie Gelato, professor of medicine at Stony Brook (N.Y.) University, who voted in favor of the liraglutide/degludec combination medication.
A concern common to discussion on both days was that some patients with diabetes and a higher body mass might not be able to benefit from these medications, since each one caps insulin dosing.
Other themes common to both days’ deliberations among the largely overlapping panels included the need for fine-tuning the dosing apparatus, labeling, patient interface, and nomenclature for these novel devices. For Dr. Robert Smith, panel chair and professor of endocrinology at Brown University, Providence, R.I., his vote on the second day should be “considered contingent on accomplishing those things adequately.”
Most of the endocrinologists on the committees noted that they personally felt more comfortable beginning a single agent, and probably tended to tinker with patients’ regimens fairly frequently at least during the initial period of diabetes management. However, the committees on both days felt that having the fixed-dose combination agent available might be a particularly useful tool for family practice physicians and those practicing general internal medicine.
Since the proposed lixisenatide/glargine combination would be dispensed as one of two pens, each with a different dose range of lixisenatide, the second day’s panel spent more time in discussion of the potential for confusion or misdosing with two choices.
“It’s incumbent upon the sponsor to make it easier for the doctor. I have confidence that they will work out the delivery system,” said Dr. Peter Wilson, explaining his rationale for voting for approval of the lixisenatide/glargine combo despite some reservations about the device and delivery system, “I think this will be a boon for the patients,” added Dr. Wilson, professor of medicine and public health at Emory University, Atlanta.
Lixisenatide, marketed as Lyxumia in Europe and elsewhere by Sanofi-Aventis, is pending FDA approval, so it received some additional attention during the committee hearing for lixisenatide/glargine. Though it would be the sixth GLP-1 agonist on the U.S. market, committee members did not voice concerns that it would be a “me too” drug; rather, said Dr. Wilson, “It provides yet another choice. ... Choice is very important for physicians and for patients.”
During Sanofi-Aventis’ and the FDA’s presentations, safety data, especially as interpreted by the FDA, seemed to indicate a slightly elevated risk of significant allergic reactions with lixisenatide compared with placebo. However, conceded the FDA’s clinical reviewer Dr. Suchitra Balakrishnan, the postmarketing surveillance program for lixisenatide was “a larger program, which may have contributed to more events occurring.”
Earlier concerns about lixisenatide’s cardiovascular safety have been largely assuaged after publication late last year of results from the ELIXA trial (N Engl J Med. 2015; 373:2247-57) that showed no increased risk – but no benefit – for those with type 2 diabetes taking lixisenatide.
The FDA usually follows the advice of its advisory committees.
On Twitter @karioakes
FROM AN FDA ADVISORY COMMITTEE MEETING
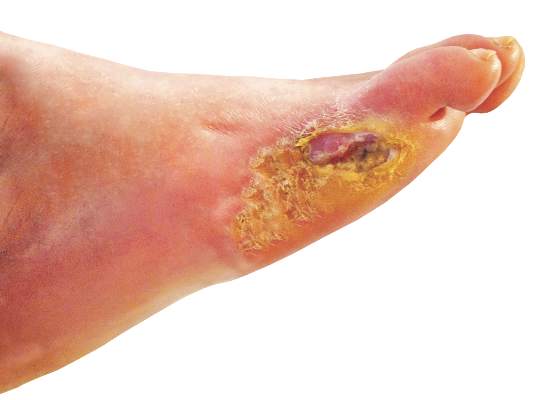
Diabetic foot ulcers linked to cognitive decline
Among patients with type 2 diabetes, those with foot ulcers show cognitive impairment across all domains when compared with those without, according to a report published in Diabetes Care.
In what they described as one of the first studies to examine cognitive function in people with diabetic foot ulcers, researchers found that these patients “remember less, have decreased ability to concentrate, and more difficulty with learning, less inhibition, slower cognitive and psychomotor responses, and less verbal fluency” than patients with diabetes that does not include foot involvement.
Although this study had a cross-sectional design that precluded drawing conclusions about causality, an analysis that estimated the participants’ premorbid and postmorbid cognitive abilities suggested that people with diabetic foot ulcers had experienced a recent significant cognitive decline, while those without foot ulcers had not, said Rachel Natovich, Ph.D., of the department of public health, Ben-Gurion University of the Negev, Be’er Sheva (Israel) and the Endocrinology Institute, Sheba Medical Center, Ramat Gan (Israel) and her associates. These findings indicate that patients with diabetic foot ulcers – the very patients who face the greatest self-treatment challenges – are the ones who have the weakest cognitive resources to do so, they noted.
The investigators examined this issue after noting that recent consensus guidelines require patients with diabetic foot ulcers to take on even more self-management than is already required for the diabetes. This demands “applying complex cognitive abilities in learning, understanding, and remembering new information; planning and initiating self-care practices; adopting behavioral changes that involve psychomotor abilities; and maintaining these behaviors while controlling and repressing impulses.” So Dr. Natovich and her associates assessed whether the cognitive profile of patients who have diabetic foot problems differs from that of patients who don’t, using a case-control study design.
The 194 study participants were aged 45-75 years. The 99 subjects who had at least one diabetic foot ulcer (cases) were matched for age and duration of diabetes with 95 subjects who did not (controls). All underwent a comprehensive battery of neuropsychological tests assessing general intelligence, short- and long-term memory, attention and concentration, psychomotor efficiency, reaction time, executive function, nonverbal IQ, visual-motor speed, coordination, capacity for learning, verbal production, semantic memory, and language. All were also assessed for depression via the Patient Health Questionnaire.
After scores were standardized according to the expected performance by age and education level, patients with diabetic foot ulcers showed significantly lower scores in all the domains tested, compared with the patients without foot ulcers. This difference persisted after the data were adjusted to account for possible confounding factors such as smoking status, hemoglobin A1c level, presence or absence of depressive symptoms, and presence or absence of macrovascular disease (Diab Care. 2016 May. doi:10.2337/dc15-2838).
The estimated premorbid cognitive function was similar between the two study groups, but current cognitive function declined significantly in the patients with foot ulcers while remaining relatively constant in the patients without foot ulcers. Prospective studies are needed to explore the timing of cognitive decline and the possibility of causation, Dr. Natovich and her associates said.
The study results “highlight the importance of focusing on cognitive functioning, a less-studied area in diabetic foot research,” they added.
“We feel that it is important to screen the cognitive status of these patients regularly and to take cognitive abilities into consideration in treatment-planning recommendations and follow-up.”
Among patients with type 2 diabetes, those with foot ulcers show cognitive impairment across all domains when compared with those without, according to a report published in Diabetes Care.
In what they described as one of the first studies to examine cognitive function in people with diabetic foot ulcers, researchers found that these patients “remember less, have decreased ability to concentrate, and more difficulty with learning, less inhibition, slower cognitive and psychomotor responses, and less verbal fluency” than patients with diabetes that does not include foot involvement.
Although this study had a cross-sectional design that precluded drawing conclusions about causality, an analysis that estimated the participants’ premorbid and postmorbid cognitive abilities suggested that people with diabetic foot ulcers had experienced a recent significant cognitive decline, while those without foot ulcers had not, said Rachel Natovich, Ph.D., of the department of public health, Ben-Gurion University of the Negev, Be’er Sheva (Israel) and the Endocrinology Institute, Sheba Medical Center, Ramat Gan (Israel) and her associates. These findings indicate that patients with diabetic foot ulcers – the very patients who face the greatest self-treatment challenges – are the ones who have the weakest cognitive resources to do so, they noted.
The investigators examined this issue after noting that recent consensus guidelines require patients with diabetic foot ulcers to take on even more self-management than is already required for the diabetes. This demands “applying complex cognitive abilities in learning, understanding, and remembering new information; planning and initiating self-care practices; adopting behavioral changes that involve psychomotor abilities; and maintaining these behaviors while controlling and repressing impulses.” So Dr. Natovich and her associates assessed whether the cognitive profile of patients who have diabetic foot problems differs from that of patients who don’t, using a case-control study design.
The 194 study participants were aged 45-75 years. The 99 subjects who had at least one diabetic foot ulcer (cases) were matched for age and duration of diabetes with 95 subjects who did not (controls). All underwent a comprehensive battery of neuropsychological tests assessing general intelligence, short- and long-term memory, attention and concentration, psychomotor efficiency, reaction time, executive function, nonverbal IQ, visual-motor speed, coordination, capacity for learning, verbal production, semantic memory, and language. All were also assessed for depression via the Patient Health Questionnaire.
After scores were standardized according to the expected performance by age and education level, patients with diabetic foot ulcers showed significantly lower scores in all the domains tested, compared with the patients without foot ulcers. This difference persisted after the data were adjusted to account for possible confounding factors such as smoking status, hemoglobin A1c level, presence or absence of depressive symptoms, and presence or absence of macrovascular disease (Diab Care. 2016 May. doi:10.2337/dc15-2838).
The estimated premorbid cognitive function was similar between the two study groups, but current cognitive function declined significantly in the patients with foot ulcers while remaining relatively constant in the patients without foot ulcers. Prospective studies are needed to explore the timing of cognitive decline and the possibility of causation, Dr. Natovich and her associates said.
The study results “highlight the importance of focusing on cognitive functioning, a less-studied area in diabetic foot research,” they added.
“We feel that it is important to screen the cognitive status of these patients regularly and to take cognitive abilities into consideration in treatment-planning recommendations and follow-up.”
Among patients with type 2 diabetes, those with foot ulcers show cognitive impairment across all domains when compared with those without, according to a report published in Diabetes Care.
In what they described as one of the first studies to examine cognitive function in people with diabetic foot ulcers, researchers found that these patients “remember less, have decreased ability to concentrate, and more difficulty with learning, less inhibition, slower cognitive and psychomotor responses, and less verbal fluency” than patients with diabetes that does not include foot involvement.
Although this study had a cross-sectional design that precluded drawing conclusions about causality, an analysis that estimated the participants’ premorbid and postmorbid cognitive abilities suggested that people with diabetic foot ulcers had experienced a recent significant cognitive decline, while those without foot ulcers had not, said Rachel Natovich, Ph.D., of the department of public health, Ben-Gurion University of the Negev, Be’er Sheva (Israel) and the Endocrinology Institute, Sheba Medical Center, Ramat Gan (Israel) and her associates. These findings indicate that patients with diabetic foot ulcers – the very patients who face the greatest self-treatment challenges – are the ones who have the weakest cognitive resources to do so, they noted.
The investigators examined this issue after noting that recent consensus guidelines require patients with diabetic foot ulcers to take on even more self-management than is already required for the diabetes. This demands “applying complex cognitive abilities in learning, understanding, and remembering new information; planning and initiating self-care practices; adopting behavioral changes that involve psychomotor abilities; and maintaining these behaviors while controlling and repressing impulses.” So Dr. Natovich and her associates assessed whether the cognitive profile of patients who have diabetic foot problems differs from that of patients who don’t, using a case-control study design.
The 194 study participants were aged 45-75 years. The 99 subjects who had at least one diabetic foot ulcer (cases) were matched for age and duration of diabetes with 95 subjects who did not (controls). All underwent a comprehensive battery of neuropsychological tests assessing general intelligence, short- and long-term memory, attention and concentration, psychomotor efficiency, reaction time, executive function, nonverbal IQ, visual-motor speed, coordination, capacity for learning, verbal production, semantic memory, and language. All were also assessed for depression via the Patient Health Questionnaire.
After scores were standardized according to the expected performance by age and education level, patients with diabetic foot ulcers showed significantly lower scores in all the domains tested, compared with the patients without foot ulcers. This difference persisted after the data were adjusted to account for possible confounding factors such as smoking status, hemoglobin A1c level, presence or absence of depressive symptoms, and presence or absence of macrovascular disease (Diab Care. 2016 May. doi:10.2337/dc15-2838).
The estimated premorbid cognitive function was similar between the two study groups, but current cognitive function declined significantly in the patients with foot ulcers while remaining relatively constant in the patients without foot ulcers. Prospective studies are needed to explore the timing of cognitive decline and the possibility of causation, Dr. Natovich and her associates said.
The study results “highlight the importance of focusing on cognitive functioning, a less-studied area in diabetic foot research,” they added.
“We feel that it is important to screen the cognitive status of these patients regularly and to take cognitive abilities into consideration in treatment-planning recommendations and follow-up.”
FROM DIABETES CARE
Key clinical point: Among patients with type 2 diabetes, those with foot ulcers show cognitive impairment, compared with those who don’t have foot ulcers.
Major finding: Ninety-nine patients with at least one diabetic foot showed significantly lower scores in all cognitive domains tested compared with the 95 patients without foot ulcers.
Data source: A cross-sectional case-control study comparing cognitive performance between 99 patients with and 95 patients without diabetic foot ulcers.
Disclosures: No sponsor of this study was identified. Dr. Natovich and her associates reported having no relevant disclosures.
FDA alert: Canagliflozin use may be associated with toe, foot amputations
Interim safety results from an ongoing clinical trial found an increase in leg and foot amputations, mostly affecting the toes, in patients treated with the diabetes medicine canagliflozin, according to an FDA Drug Safety Communication on May 18, 2016.
The agency currently is investigating the safety issue but has yet to determine if taking canagliflozin is associated with an increased risk of leg and foot amputations. A sodium-glucose cotransporter 2 inhibitor, canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013.
“Patients should not stop or change their diabetes medicines without first talking to their health care professional,” the communication states. “Doing so can lead to uncontrolled blood sugar levels that can be harmful. Over time, this can cause serious problems, including blindness, nerve and kidney damage, and heart disease. Patients taking canagliflozin should notify their health care professionals right away if they notice any new pain or tenderness, sores or ulcers, or infections in their legs or feet.”
The agency advises health care professionals to follow the recommendations in the canagliflozin drug labels and to monitor patients for the signs and symptoms described above.
Upon its approval, the FDA required five postmarketing studies for canagliflozin: a cardiovascular outcomes trial; an enhanced pharmacovigilance program to monitor for malignancies, serious cases of pancreatitis, severe hypersensitivity reactions, photosensitivity reactions, liver abnormalities, and adverse pregnancy outcomes; a bone safety study; and two pediatric studies under the Pediatric Research Equity Act (PREA), including a pharmacokinetic and pharmacodynamic study and a safety and efficacy study. In late 2015, investigators determined that the risk of bone fracture is increased with canagliflozin treatment.
Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
Interim safety results from an ongoing clinical trial found an increase in leg and foot amputations, mostly affecting the toes, in patients treated with the diabetes medicine canagliflozin, according to an FDA Drug Safety Communication on May 18, 2016.
The agency currently is investigating the safety issue but has yet to determine if taking canagliflozin is associated with an increased risk of leg and foot amputations. A sodium-glucose cotransporter 2 inhibitor, canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013.
“Patients should not stop or change their diabetes medicines without first talking to their health care professional,” the communication states. “Doing so can lead to uncontrolled blood sugar levels that can be harmful. Over time, this can cause serious problems, including blindness, nerve and kidney damage, and heart disease. Patients taking canagliflozin should notify their health care professionals right away if they notice any new pain or tenderness, sores or ulcers, or infections in their legs or feet.”
The agency advises health care professionals to follow the recommendations in the canagliflozin drug labels and to monitor patients for the signs and symptoms described above.
Upon its approval, the FDA required five postmarketing studies for canagliflozin: a cardiovascular outcomes trial; an enhanced pharmacovigilance program to monitor for malignancies, serious cases of pancreatitis, severe hypersensitivity reactions, photosensitivity reactions, liver abnormalities, and adverse pregnancy outcomes; a bone safety study; and two pediatric studies under the Pediatric Research Equity Act (PREA), including a pharmacokinetic and pharmacodynamic study and a safety and efficacy study. In late 2015, investigators determined that the risk of bone fracture is increased with canagliflozin treatment.
Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
Interim safety results from an ongoing clinical trial found an increase in leg and foot amputations, mostly affecting the toes, in patients treated with the diabetes medicine canagliflozin, according to an FDA Drug Safety Communication on May 18, 2016.
The agency currently is investigating the safety issue but has yet to determine if taking canagliflozin is associated with an increased risk of leg and foot amputations. A sodium-glucose cotransporter 2 inhibitor, canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013.
“Patients should not stop or change their diabetes medicines without first talking to their health care professional,” the communication states. “Doing so can lead to uncontrolled blood sugar levels that can be harmful. Over time, this can cause serious problems, including blindness, nerve and kidney damage, and heart disease. Patients taking canagliflozin should notify their health care professionals right away if they notice any new pain or tenderness, sores or ulcers, or infections in their legs or feet.”
The agency advises health care professionals to follow the recommendations in the canagliflozin drug labels and to monitor patients for the signs and symptoms described above.
Upon its approval, the FDA required five postmarketing studies for canagliflozin: a cardiovascular outcomes trial; an enhanced pharmacovigilance program to monitor for malignancies, serious cases of pancreatitis, severe hypersensitivity reactions, photosensitivity reactions, liver abnormalities, and adverse pregnancy outcomes; a bone safety study; and two pediatric studies under the Pediatric Research Equity Act (PREA), including a pharmacokinetic and pharmacodynamic study and a safety and efficacy study. In late 2015, investigators determined that the risk of bone fracture is increased with canagliflozin treatment.
Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
Some improvements seen in neurocognition post-bariatric surgery
ATLANTA – Some patients experienced improvement in at least one neurocognitive domain up to 3 years after having bariatric surgery, a small, systematic review has shown.
The most significant improvements were reported in memory, with nine studies showing some statistically significant improvement in a post-bariatric surgery cohort. Four studies showed statistically significant improvement in attention and executive function, and two did so in language.
Dr. Gurneet S. Thiara, a psychiatry resident at the University of Toronto, presented the findings during a scientific session at this year’s annual meeting of the American Psychiatric Association.
Because the studies that form the basis of the analysis did not follow a standard pre-surgery neurocognitive assessment, the actual scope of bariatric surgery’s impact on neurocognition is hard to determine. This shortcoming provides evidence that instituting a standardized method of psychiatric assessment pre-bariatric surgery could help clinicians better anticipate overall neurocognitive outcomes, he said.
“It’s hard to pinpoint the one domain that affects [this cohort] most,” said Dr. Thiara.
One study included in the analysis showed no neurocognitive improvement, although Dr. Thiara noted this was possibly due to the under- or non-reporting of negative outcomes by researchers who conducted studies that might have met his inclusion criteria.
Dr. Thiara and his colleagues were not able to draw conclusions as to which patients would be affected in which domains and by what mechanism of action. Their analysis did suggest possible relationships between gastric bypass and changes in metabolism, levels of leptin and ghrelin, vascular function, hypoperfusion in the brain, and even shifts in the gut microbiome.
Dr. Thiara sought studies with bariatric surgery patients whose neurocognitive and psychological outcomes were followed anywhere from one to three years post-surgery. After analyzing 422 studies published between January 1990 and August 2015, only ten studies, with patient sample sizes ranging from 10 to 156, met the criteria.
The study was not intended to determine a relationship between neurocognitive outcomes and type of bypass surgery performed, but Dr. Thiara said the majority of the procedures analyzed tended to be Roux-en-Y rather than the gastric bypass sleeve.
On Twitter @whitneymcknight
ATLANTA – Some patients experienced improvement in at least one neurocognitive domain up to 3 years after having bariatric surgery, a small, systematic review has shown.
The most significant improvements were reported in memory, with nine studies showing some statistically significant improvement in a post-bariatric surgery cohort. Four studies showed statistically significant improvement in attention and executive function, and two did so in language.
Dr. Gurneet S. Thiara, a psychiatry resident at the University of Toronto, presented the findings during a scientific session at this year’s annual meeting of the American Psychiatric Association.
Because the studies that form the basis of the analysis did not follow a standard pre-surgery neurocognitive assessment, the actual scope of bariatric surgery’s impact on neurocognition is hard to determine. This shortcoming provides evidence that instituting a standardized method of psychiatric assessment pre-bariatric surgery could help clinicians better anticipate overall neurocognitive outcomes, he said.
“It’s hard to pinpoint the one domain that affects [this cohort] most,” said Dr. Thiara.
One study included in the analysis showed no neurocognitive improvement, although Dr. Thiara noted this was possibly due to the under- or non-reporting of negative outcomes by researchers who conducted studies that might have met his inclusion criteria.
Dr. Thiara and his colleagues were not able to draw conclusions as to which patients would be affected in which domains and by what mechanism of action. Their analysis did suggest possible relationships between gastric bypass and changes in metabolism, levels of leptin and ghrelin, vascular function, hypoperfusion in the brain, and even shifts in the gut microbiome.
Dr. Thiara sought studies with bariatric surgery patients whose neurocognitive and psychological outcomes were followed anywhere from one to three years post-surgery. After analyzing 422 studies published between January 1990 and August 2015, only ten studies, with patient sample sizes ranging from 10 to 156, met the criteria.
The study was not intended to determine a relationship between neurocognitive outcomes and type of bypass surgery performed, but Dr. Thiara said the majority of the procedures analyzed tended to be Roux-en-Y rather than the gastric bypass sleeve.
On Twitter @whitneymcknight
ATLANTA – Some patients experienced improvement in at least one neurocognitive domain up to 3 years after having bariatric surgery, a small, systematic review has shown.
The most significant improvements were reported in memory, with nine studies showing some statistically significant improvement in a post-bariatric surgery cohort. Four studies showed statistically significant improvement in attention and executive function, and two did so in language.
Dr. Gurneet S. Thiara, a psychiatry resident at the University of Toronto, presented the findings during a scientific session at this year’s annual meeting of the American Psychiatric Association.
Because the studies that form the basis of the analysis did not follow a standard pre-surgery neurocognitive assessment, the actual scope of bariatric surgery’s impact on neurocognition is hard to determine. This shortcoming provides evidence that instituting a standardized method of psychiatric assessment pre-bariatric surgery could help clinicians better anticipate overall neurocognitive outcomes, he said.
“It’s hard to pinpoint the one domain that affects [this cohort] most,” said Dr. Thiara.
One study included in the analysis showed no neurocognitive improvement, although Dr. Thiara noted this was possibly due to the under- or non-reporting of negative outcomes by researchers who conducted studies that might have met his inclusion criteria.
Dr. Thiara and his colleagues were not able to draw conclusions as to which patients would be affected in which domains and by what mechanism of action. Their analysis did suggest possible relationships between gastric bypass and changes in metabolism, levels of leptin and ghrelin, vascular function, hypoperfusion in the brain, and even shifts in the gut microbiome.
Dr. Thiara sought studies with bariatric surgery patients whose neurocognitive and psychological outcomes were followed anywhere from one to three years post-surgery. After analyzing 422 studies published between January 1990 and August 2015, only ten studies, with patient sample sizes ranging from 10 to 156, met the criteria.
The study was not intended to determine a relationship between neurocognitive outcomes and type of bypass surgery performed, but Dr. Thiara said the majority of the procedures analyzed tended to be Roux-en-Y rather than the gastric bypass sleeve.
On Twitter @whitneymcknight
AT APA 2016
Key clinical point: Neurocognitive testing in patients before bariatric surgery could be a useful tool for tracking overall psychosocial outcomes.
Major finding: Improvements in neurocognitive function were found across several domains in some patients in the years after bariatric surgery.
Data source: Systematic review of neurocognitive outcomes in post-bariatric surgery patients followed for at least 1 year in 10 studies of between 10 and 156 patients.
Disclosures: Dr. Thiara had no relevant disclosures. This study was sponsored in part by the Toronto Western Hospital Bariatric Psychosocial Surgery Program, part of the University Health Network, Toronto, Ont.
Sharp blood pressure rise spikes stroke risk
Individuals whose blood pressure rose sharply over time had a significantly increased risk of stroke and death from nonstroke causes, compared with other blood pressure trajectories in a study of more than 6,000 adults published online May 9 in Hypertension.
The current association of blood pressure with stroke does not account for variations in blood pressure trajectories over the long term, wrote Dr. M. Arfan Ikram of Erasmus University, Rotterdam, the Netherlands, and his colleagues.
The researchers reviewed data from 6,745 adults aged 55-106 years participating in the population-based Rotterdam Study, and identified four blood pressure trajectories over 5 decades. Class 1 included individuals whose blood pressure increased from 120 to 160 mm Hg; class 2 increased from 120 to 200 mm Hg; class 3 included those with moderate midlife blood pressure averaging 140 mm Hg; class 4 included those with a high midlife blood pressure averaging 160 mm Hg.
After controlling for confounding variables, class 3 had the highest overall risk of stroke, but the lowest risk of dying from a nonstroke event. Class 2 and class 4 individuals were at the greatest risk of stroke and of dying from nonstroke disease before 80 years of age. Class 1 individuals (with a normal baseline blood pressure and gradual increase) were least likely to suffer a stroke or die from a nonstroke event (Hypertension. 2016 May 9).
Although the study was limited by its homogenous nature (small geographical region, mostly white population), the findings show the importance of regular blood pressure measurement, the researchers noted.
“Identifying the patterns described in our study is an important step, since they evoke new causal and treatment questions that can motivate future studies to explore the etiologic significance and predictive value of associations,” they said. The researchers had no financial conflicts to disclose.
Individuals whose blood pressure rose sharply over time had a significantly increased risk of stroke and death from nonstroke causes, compared with other blood pressure trajectories in a study of more than 6,000 adults published online May 9 in Hypertension.
The current association of blood pressure with stroke does not account for variations in blood pressure trajectories over the long term, wrote Dr. M. Arfan Ikram of Erasmus University, Rotterdam, the Netherlands, and his colleagues.
The researchers reviewed data from 6,745 adults aged 55-106 years participating in the population-based Rotterdam Study, and identified four blood pressure trajectories over 5 decades. Class 1 included individuals whose blood pressure increased from 120 to 160 mm Hg; class 2 increased from 120 to 200 mm Hg; class 3 included those with moderate midlife blood pressure averaging 140 mm Hg; class 4 included those with a high midlife blood pressure averaging 160 mm Hg.
After controlling for confounding variables, class 3 had the highest overall risk of stroke, but the lowest risk of dying from a nonstroke event. Class 2 and class 4 individuals were at the greatest risk of stroke and of dying from nonstroke disease before 80 years of age. Class 1 individuals (with a normal baseline blood pressure and gradual increase) were least likely to suffer a stroke or die from a nonstroke event (Hypertension. 2016 May 9).
Although the study was limited by its homogenous nature (small geographical region, mostly white population), the findings show the importance of regular blood pressure measurement, the researchers noted.
“Identifying the patterns described in our study is an important step, since they evoke new causal and treatment questions that can motivate future studies to explore the etiologic significance and predictive value of associations,” they said. The researchers had no financial conflicts to disclose.
Individuals whose blood pressure rose sharply over time had a significantly increased risk of stroke and death from nonstroke causes, compared with other blood pressure trajectories in a study of more than 6,000 adults published online May 9 in Hypertension.
The current association of blood pressure with stroke does not account for variations in blood pressure trajectories over the long term, wrote Dr. M. Arfan Ikram of Erasmus University, Rotterdam, the Netherlands, and his colleagues.
The researchers reviewed data from 6,745 adults aged 55-106 years participating in the population-based Rotterdam Study, and identified four blood pressure trajectories over 5 decades. Class 1 included individuals whose blood pressure increased from 120 to 160 mm Hg; class 2 increased from 120 to 200 mm Hg; class 3 included those with moderate midlife blood pressure averaging 140 mm Hg; class 4 included those with a high midlife blood pressure averaging 160 mm Hg.
After controlling for confounding variables, class 3 had the highest overall risk of stroke, but the lowest risk of dying from a nonstroke event. Class 2 and class 4 individuals were at the greatest risk of stroke and of dying from nonstroke disease before 80 years of age. Class 1 individuals (with a normal baseline blood pressure and gradual increase) were least likely to suffer a stroke or die from a nonstroke event (Hypertension. 2016 May 9).
Although the study was limited by its homogenous nature (small geographical region, mostly white population), the findings show the importance of regular blood pressure measurement, the researchers noted.
“Identifying the patterns described in our study is an important step, since they evoke new causal and treatment questions that can motivate future studies to explore the etiologic significance and predictive value of associations,” they said. The researchers had no financial conflicts to disclose.
FROM HYPERTENSION
Key clinical point: Blood pressure trajectories can help develop prevention strategies.
Major finding: The risk of stroke was significantly higher in classes 2-4 (4.7%-13.6%) vs. 0.7% for class 1.
Data source: A review of 6,745 community-dwelling adults aged 55-106 years participating in a population-based study (the Rotterdam Study).
Disclosures: The researchers had no financial conflicts to disclose.
Study of twins quantifies diabetes, obesity link with psoriasis
The statistically significant association between psoriasis and both obesity and type 2 diabetes comes down to genetics, according to a large population-based study of twins.
Across the entire cohort of 33,588 Danish twins, researchers found the prevalence of psoriasis was 53% higher in individuals with type 2 diabetes, while the prevalence of psoriasis was 81% higher in individuals with a body mass index of at least 35, after adjustment for confounders such as sex, age, and smoking (JAMA Dermatol. 2016 April 27. doi:10.1001/jamadermatol.2015.6262).
Among the 449 twin pairs discordant for psoriasis, the risk for obesity was more than double in the twin with psoriasis compared to the unaffected twin, although this was significant only among dizygotic pairs, not monozygotic pairs.
The twin analysis also found that the risk of type 2 diabetes was the same between twins with and without psoriasis.
“Increased plasma levels of tumor necrosis factor, tumor necrosis factor receptors, and interleukin 6, which have important roles in the pathogenesis of psoriasis, have been found to be linked with obesity,” wrote Dr. Ann Sophie Lønnberg of the department of dermato-allergology, Gentofte Hospital, University of Copenhagen, and her associates.
“The association between type 2 diabetes mellitus and psoriasis also might be owing to increased tumor necrosis factor production from psoriatic inflammation and low-grade obesity inflammation, because it contributes to insulin resistance,” they wrote.
While obesity and type 2 diabetes are known comorbidities of psoriasis, Dr. Lønnberg and colleagues said this was the first study, to their knowledge, to explore the contribution of genetic and environmental factors to this interaction, and they suggested that future studies could look for genes and epigenetic factors that might underlie these associations.
Their analysis showed the genetic correlation between psoriasis and type 2 diabetes was 0.13, while the environmental correlation was 0.10. Similarly, the genetic correlation between psoriasis and BMI was 0.12, while environmental correlation was –0.05.
No conflicts of interest were declared.
Given the association of psoriasis – particularly more severe disease – and increases in BMI, most of our patients would be recommended for diabetes screening based on standard recommendations.

|
Dr. Joel M. Gelfand |
Therefore, dermatologists have the opportunity to educate patients with psoriasis and initiate appropriate screenings (or refer them to primary care physicians), which can result in better health outcomes through evidence-based interventions.
Dr. Joel M. Gelfand is from the department of dermatology and Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania Perelman School of Medicine. He made this comments in an accompanying editorial (JAMA Dermatol. 2016 April 27. doi:10.1001/jamadermatol.2016.0670). Dr. Gelfand declared consultancies for Abbvie, AstraZeneca, Celgene, Coherus, Eli Lilly, Janssen Biologics (formerly Centocor), Sanofi, Merck, Novartis, Endo, Valeant, and Pfizer; research grant support from Abbvie, Amgen, Eli Lilly, Janssen, Novartis, Regeneron, and Pfizer (to the trustees of the University of Pennsylvania); and having received payment for continuing medical education work related to psoriasis. He is is a co–patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
Given the association of psoriasis – particularly more severe disease – and increases in BMI, most of our patients would be recommended for diabetes screening based on standard recommendations.

|
Dr. Joel M. Gelfand |
Therefore, dermatologists have the opportunity to educate patients with psoriasis and initiate appropriate screenings (or refer them to primary care physicians), which can result in better health outcomes through evidence-based interventions.
Dr. Joel M. Gelfand is from the department of dermatology and Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania Perelman School of Medicine. He made this comments in an accompanying editorial (JAMA Dermatol. 2016 April 27. doi:10.1001/jamadermatol.2016.0670). Dr. Gelfand declared consultancies for Abbvie, AstraZeneca, Celgene, Coherus, Eli Lilly, Janssen Biologics (formerly Centocor), Sanofi, Merck, Novartis, Endo, Valeant, and Pfizer; research grant support from Abbvie, Amgen, Eli Lilly, Janssen, Novartis, Regeneron, and Pfizer (to the trustees of the University of Pennsylvania); and having received payment for continuing medical education work related to psoriasis. He is is a co–patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
Given the association of psoriasis – particularly more severe disease – and increases in BMI, most of our patients would be recommended for diabetes screening based on standard recommendations.

|
Dr. Joel M. Gelfand |
Therefore, dermatologists have the opportunity to educate patients with psoriasis and initiate appropriate screenings (or refer them to primary care physicians), which can result in better health outcomes through evidence-based interventions.
Dr. Joel M. Gelfand is from the department of dermatology and Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania Perelman School of Medicine. He made this comments in an accompanying editorial (JAMA Dermatol. 2016 April 27. doi:10.1001/jamadermatol.2016.0670). Dr. Gelfand declared consultancies for Abbvie, AstraZeneca, Celgene, Coherus, Eli Lilly, Janssen Biologics (formerly Centocor), Sanofi, Merck, Novartis, Endo, Valeant, and Pfizer; research grant support from Abbvie, Amgen, Eli Lilly, Janssen, Novartis, Regeneron, and Pfizer (to the trustees of the University of Pennsylvania); and having received payment for continuing medical education work related to psoriasis. He is is a co–patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
The statistically significant association between psoriasis and both obesity and type 2 diabetes comes down to genetics, according to a large population-based study of twins.
Across the entire cohort of 33,588 Danish twins, researchers found the prevalence of psoriasis was 53% higher in individuals with type 2 diabetes, while the prevalence of psoriasis was 81% higher in individuals with a body mass index of at least 35, after adjustment for confounders such as sex, age, and smoking (JAMA Dermatol. 2016 April 27. doi:10.1001/jamadermatol.2015.6262).
Among the 449 twin pairs discordant for psoriasis, the risk for obesity was more than double in the twin with psoriasis compared to the unaffected twin, although this was significant only among dizygotic pairs, not monozygotic pairs.
The twin analysis also found that the risk of type 2 diabetes was the same between twins with and without psoriasis.
“Increased plasma levels of tumor necrosis factor, tumor necrosis factor receptors, and interleukin 6, which have important roles in the pathogenesis of psoriasis, have been found to be linked with obesity,” wrote Dr. Ann Sophie Lønnberg of the department of dermato-allergology, Gentofte Hospital, University of Copenhagen, and her associates.
“The association between type 2 diabetes mellitus and psoriasis also might be owing to increased tumor necrosis factor production from psoriatic inflammation and low-grade obesity inflammation, because it contributes to insulin resistance,” they wrote.
While obesity and type 2 diabetes are known comorbidities of psoriasis, Dr. Lønnberg and colleagues said this was the first study, to their knowledge, to explore the contribution of genetic and environmental factors to this interaction, and they suggested that future studies could look for genes and epigenetic factors that might underlie these associations.
Their analysis showed the genetic correlation between psoriasis and type 2 diabetes was 0.13, while the environmental correlation was 0.10. Similarly, the genetic correlation between psoriasis and BMI was 0.12, while environmental correlation was –0.05.
No conflicts of interest were declared.
The statistically significant association between psoriasis and both obesity and type 2 diabetes comes down to genetics, according to a large population-based study of twins.
Across the entire cohort of 33,588 Danish twins, researchers found the prevalence of psoriasis was 53% higher in individuals with type 2 diabetes, while the prevalence of psoriasis was 81% higher in individuals with a body mass index of at least 35, after adjustment for confounders such as sex, age, and smoking (JAMA Dermatol. 2016 April 27. doi:10.1001/jamadermatol.2015.6262).
Among the 449 twin pairs discordant for psoriasis, the risk for obesity was more than double in the twin with psoriasis compared to the unaffected twin, although this was significant only among dizygotic pairs, not monozygotic pairs.
The twin analysis also found that the risk of type 2 diabetes was the same between twins with and without psoriasis.
“Increased plasma levels of tumor necrosis factor, tumor necrosis factor receptors, and interleukin 6, which have important roles in the pathogenesis of psoriasis, have been found to be linked with obesity,” wrote Dr. Ann Sophie Lønnberg of the department of dermato-allergology, Gentofte Hospital, University of Copenhagen, and her associates.
“The association between type 2 diabetes mellitus and psoriasis also might be owing to increased tumor necrosis factor production from psoriatic inflammation and low-grade obesity inflammation, because it contributes to insulin resistance,” they wrote.
While obesity and type 2 diabetes are known comorbidities of psoriasis, Dr. Lønnberg and colleagues said this was the first study, to their knowledge, to explore the contribution of genetic and environmental factors to this interaction, and they suggested that future studies could look for genes and epigenetic factors that might underlie these associations.
Their analysis showed the genetic correlation between psoriasis and type 2 diabetes was 0.13, while the environmental correlation was 0.10. Similarly, the genetic correlation between psoriasis and BMI was 0.12, while environmental correlation was –0.05.
No conflicts of interest were declared.
FROM JAMA DERMATOLOGY
Key clinical point: The prevalence of both type 2 diabetes and psoriasis is significantly higher in individuals with psoriasis.
Major finding: The risk of obesity is twofold higher in a twin with psoriasis compared to the co-twin without psoriasis.
Data source: Cross-sectional, population-based study of 33,588 Danish twins, including 449 pairs discordant for psoriasis.
Disclosures: No conflicts of interest were declared.
No rise in serious HF seen in patients taking saxagliptin or sitagliptin
Neither saxagliptin nor sitagliptin, the two oral DPP-4 inhibitors most commonly used as antihyperglycemic medications, raised the risk of hospitalization for heart failure in a large population-based cohort study that analyzed data from a Food and Drug Administration surveillance program.
The report was published online April 25 in Annals of Internal Medicine.
The cardiovascular safety of DPP-4 inhibitors is controversial: Several postmarketing studies have produced conflicting results, particularly with regard to HF risk. “Patients with diabetes have a higher HF risk than those without, so any antihyperglycemic agent that modifies the risk warrants further examination,” said Sengwee Toh, Sc.D., a pharmacoepidemiologist in the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Institute, Boston, and his associates.
They compared rates of HF among demographically and geographically diverse patients who initiated antidiabetic medications during a 7-year period in routine clinical settings. The study population included 78,553 adults who initiated saxagliptin and 298,124 who initiated sitagliptin, who were compared with patients who initiated pioglitazone, second-generation sulfonylureas, or long-acting insulins. Mean follow-up was 7-9 months.
There was no evidence of an increased risk of hospitalization for HF among new users of saxagliptin or sitagliptin. The hazard ratios for developing HF were 0.83 for saxagliptin vs. sitagliptin, 0.63 for saxagliptin vs. pioglitazone, 0.69 for saxagliptin vs. sulfonylureas, and 0.61 for saxagliptin vs. insulin. Similarly, the hazard ratios for developing HF were 0.74 for sitagliptin vs. pioglitazone, 0.86 for sitagliptin vs. sulfonylureas, and 0.71 for sitagliptin vs. insulin.
These results were consistent across sensitivity analyses and subgroup analyses that categorized patients by whether or not they had preexisting cardiovascular disease and whether or not they had a history of prior HF, the investigators said (Ann Intern Med. 2016 April 25. doi:10.7326/M15-2568).
However, this was an observational study with a relatively short follow-up. “Well-designed randomized trials with hospitalization for HF as the main endpoint or observational studies that address the limitations of our study will help provide more definitive evidence on the topic,” Dr. Toh and his associates said.
This study was supported by the FDA. Dr. Toh reported having no relevant financial disclosures; one of his associates reported receiving personal fees from Novartis unrelated to this work.
The findings of Toh et al. allay concerns about a saxagliptin- or sitagliptin-associated risk for heart failure. This risk was similar between the two agents and either comparable to or lower than that in all other comparator groups.
Beyond reassuring clinicians, this study illustrates the value of large, longitudinal databases built from clinical and administrative data, to complement the findings of clinical trials. These investigators were able to draw their conclusions from rich demographic, diagnostic, prescription, and utilization data based in routine real-world practice.
Joseph V. Selby, M.D., is at the Patient-Centered Outcomes Research Institute, Washington. He reported having no relevant financial disclosures. Dr. Selby made these remarks in an editorial accompanying Dr. Toh’s report (Ann. Intern. Med. 2016 April 25. doi:10.7326/M16-0869).
The findings of Toh et al. allay concerns about a saxagliptin- or sitagliptin-associated risk for heart failure. This risk was similar between the two agents and either comparable to or lower than that in all other comparator groups.
Beyond reassuring clinicians, this study illustrates the value of large, longitudinal databases built from clinical and administrative data, to complement the findings of clinical trials. These investigators were able to draw their conclusions from rich demographic, diagnostic, prescription, and utilization data based in routine real-world practice.
Joseph V. Selby, M.D., is at the Patient-Centered Outcomes Research Institute, Washington. He reported having no relevant financial disclosures. Dr. Selby made these remarks in an editorial accompanying Dr. Toh’s report (Ann. Intern. Med. 2016 April 25. doi:10.7326/M16-0869).
The findings of Toh et al. allay concerns about a saxagliptin- or sitagliptin-associated risk for heart failure. This risk was similar between the two agents and either comparable to or lower than that in all other comparator groups.
Beyond reassuring clinicians, this study illustrates the value of large, longitudinal databases built from clinical and administrative data, to complement the findings of clinical trials. These investigators were able to draw their conclusions from rich demographic, diagnostic, prescription, and utilization data based in routine real-world practice.
Joseph V. Selby, M.D., is at the Patient-Centered Outcomes Research Institute, Washington. He reported having no relevant financial disclosures. Dr. Selby made these remarks in an editorial accompanying Dr. Toh’s report (Ann. Intern. Med. 2016 April 25. doi:10.7326/M16-0869).
Neither saxagliptin nor sitagliptin, the two oral DPP-4 inhibitors most commonly used as antihyperglycemic medications, raised the risk of hospitalization for heart failure in a large population-based cohort study that analyzed data from a Food and Drug Administration surveillance program.
The report was published online April 25 in Annals of Internal Medicine.
The cardiovascular safety of DPP-4 inhibitors is controversial: Several postmarketing studies have produced conflicting results, particularly with regard to HF risk. “Patients with diabetes have a higher HF risk than those without, so any antihyperglycemic agent that modifies the risk warrants further examination,” said Sengwee Toh, Sc.D., a pharmacoepidemiologist in the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Institute, Boston, and his associates.
They compared rates of HF among demographically and geographically diverse patients who initiated antidiabetic medications during a 7-year period in routine clinical settings. The study population included 78,553 adults who initiated saxagliptin and 298,124 who initiated sitagliptin, who were compared with patients who initiated pioglitazone, second-generation sulfonylureas, or long-acting insulins. Mean follow-up was 7-9 months.
There was no evidence of an increased risk of hospitalization for HF among new users of saxagliptin or sitagliptin. The hazard ratios for developing HF were 0.83 for saxagliptin vs. sitagliptin, 0.63 for saxagliptin vs. pioglitazone, 0.69 for saxagliptin vs. sulfonylureas, and 0.61 for saxagliptin vs. insulin. Similarly, the hazard ratios for developing HF were 0.74 for sitagliptin vs. pioglitazone, 0.86 for sitagliptin vs. sulfonylureas, and 0.71 for sitagliptin vs. insulin.
These results were consistent across sensitivity analyses and subgroup analyses that categorized patients by whether or not they had preexisting cardiovascular disease and whether or not they had a history of prior HF, the investigators said (Ann Intern Med. 2016 April 25. doi:10.7326/M15-2568).
However, this was an observational study with a relatively short follow-up. “Well-designed randomized trials with hospitalization for HF as the main endpoint or observational studies that address the limitations of our study will help provide more definitive evidence on the topic,” Dr. Toh and his associates said.
This study was supported by the FDA. Dr. Toh reported having no relevant financial disclosures; one of his associates reported receiving personal fees from Novartis unrelated to this work.
Neither saxagliptin nor sitagliptin, the two oral DPP-4 inhibitors most commonly used as antihyperglycemic medications, raised the risk of hospitalization for heart failure in a large population-based cohort study that analyzed data from a Food and Drug Administration surveillance program.
The report was published online April 25 in Annals of Internal Medicine.
The cardiovascular safety of DPP-4 inhibitors is controversial: Several postmarketing studies have produced conflicting results, particularly with regard to HF risk. “Patients with diabetes have a higher HF risk than those without, so any antihyperglycemic agent that modifies the risk warrants further examination,” said Sengwee Toh, Sc.D., a pharmacoepidemiologist in the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Institute, Boston, and his associates.
They compared rates of HF among demographically and geographically diverse patients who initiated antidiabetic medications during a 7-year period in routine clinical settings. The study population included 78,553 adults who initiated saxagliptin and 298,124 who initiated sitagliptin, who were compared with patients who initiated pioglitazone, second-generation sulfonylureas, or long-acting insulins. Mean follow-up was 7-9 months.
There was no evidence of an increased risk of hospitalization for HF among new users of saxagliptin or sitagliptin. The hazard ratios for developing HF were 0.83 for saxagliptin vs. sitagliptin, 0.63 for saxagliptin vs. pioglitazone, 0.69 for saxagliptin vs. sulfonylureas, and 0.61 for saxagliptin vs. insulin. Similarly, the hazard ratios for developing HF were 0.74 for sitagliptin vs. pioglitazone, 0.86 for sitagliptin vs. sulfonylureas, and 0.71 for sitagliptin vs. insulin.
These results were consistent across sensitivity analyses and subgroup analyses that categorized patients by whether or not they had preexisting cardiovascular disease and whether or not they had a history of prior HF, the investigators said (Ann Intern Med. 2016 April 25. doi:10.7326/M15-2568).
However, this was an observational study with a relatively short follow-up. “Well-designed randomized trials with hospitalization for HF as the main endpoint or observational studies that address the limitations of our study will help provide more definitive evidence on the topic,” Dr. Toh and his associates said.
This study was supported by the FDA. Dr. Toh reported having no relevant financial disclosures; one of his associates reported receiving personal fees from Novartis unrelated to this work.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: No increase in the risk of hospitalization for heart failure was found in a large cohort study that used an FDA surveillance program.
Major finding: The hazard ratios for developing HF were 0.83 for saxagliptin vs. sitagliptin, 0.63 for saxagliptin vs. pioglitazone, 0.69 for saxagliptin vs. sulfonylureas, and 0.61 for saxagliptin vs. insulin.
Data source: A population-based retrospective cohort study involving 78,553 new users of saxagliptin and 298,124 of sitagliptin during a 7-year period.
Disclosures: This study was supported by the FDA. Dr. Toh reported having no relevant financial disclosures; one of his associates reported receiving personal fees from Novartis unrelated to this work.