User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
VIDEO: HOPE-3 trial supports broader role for statins in primary prevention
CHICAGO – The success in the HOPE-3 trial of a two-pronged strategy of a statin plus moderate-dose antihypertensive therapy has expanded the boundaries of primary preventive pharmacotherapy to incorporate many intermediate-cardiovascular-risk patients without cardiovascular disease.
In the Heart Outcomes Evaluation (HOPE)-3 trial, the combination of rosuvastatin (Crestor) at 10 mg/day plus antihypertensive therapy with 16 mg/day of candesartan and 12.5 mg/day of hydrochlorothiazide reduced cardiovascular events in intermediate-risk patients with hypertension, regardless of their baseline LDL-cholesterol and inflammatory biomarker levels. In nonhypertensive study participants, however, blood pressure-lowering therapy provided no added benefit beyond that achieved with the statin alone, which yielded a 25% relative risk reduction in cardiovascular events compared with placebo, Dr. Salim Yusuf reported at the annual meeting of the American College of Cardiology.
The HOPE-3 trial was the first formal study of the polypill concept of fixed-dose, low-dose combination therapy as a public health tool for primary prevention in an intermediate-risk population without cardiovascular disease. It’s a simple, low-cost, safe, pragmatic preventive strategy that doesn’t require baseline laboratory measurements, dose titration visits, or frequent safety monitoring, he noted.
“The original concept of the polypill was to give it to everyone over age 55. We found in HOPE-3 that the polypill concept is not valid for everybody. It demonstrated benefit in hypertensives but not in nonhypertensives, where a statin alone was beneficial,” said Dr. Yusuf, professor of medicine and executive director of the Population Health Research Institute at McMaster University in Hamilton, Ont.
“The clinical implication is that statins should be used much more widely than they currently are. Most guidelines for hypertension don’t say to give a statin. But HOPE-3 is saying hypertensives will benefit. About half of the 40% reduction in the risk of cardiovascular events we saw with combination therapy in patients in the highest third for baseline systolic blood pressure – that is, above 143.5 mm Hg, with a mean of 154 mm Hg – was due to the rosuvastatin and half to the antihypertensive therapy. Our study suggests you can essentially double the benefit of lowering blood pressure in hypertensives if you also lower cholesterol simultaneously,” he noted. Dr. Yusuf discussed the findings in a video interview.
The double-blind trial included a diverse population of 12,705 men age 55 or older and women age 65 or older in 21 countries. All were at intermediate cardiovascular risk by conventional stratification methods; none had a history of cardiovascular disease. They were randomized to rosuvastatin or placebo, dual antihypertensive therapy or placebo, or to all three medications or double placebo. The combined-therapy group experienced a 33.7 mg/dL greater drop in LDL-cholesterol and a 6.2 mm Hg bigger reduction in systolic blood pressure than in patients on dual placebo.
During a median followup of 5.6 years, the composite rate of cardiovascular death, nonfatal MI, or nonfatal stroke was 3.6% in the combined-therapy group and 5.0% with dual placebo, for a relative risk reduction of 29%. The number needed to treat for 5.6 years in order to prevent one event of the composite outcome was 72. However, the separation in the event rate curves for the two groups grew larger over time. With an additional planned followup of 3-5 years in HOPE-3, it’s likely the benefits will become even greater, according to Dr. Yusuf.
Combination therapy proved safe. Muscle aches and weakness as well as lightheadedness were more common in the combined treatment group than with dual placebo. However, permanent discontinuation rates were similar in the two groups.
HOPE-3 coinvestigator Dr. Eva Lonn presented the comparison between patients randomized to antihypertensive therapy without rosuvastatin or to placebo. In a prespecified subgroup analysis, active treatment resulted in a significant 27% reduction in the risk of the composite outcome compared with placebo in patients in the top one-third of baseline systolic blood pressure, no benefit in those with a systolic blood pressure of 131.6-143.5 mm Hg, and a suggestion of possible harm in patients whose systolic pressure at enrollment was 131.5 mm Hg or less.
Thus, the study helps define the minimum blood pressure at which antihypertensive therapy becomes beneficial, noted Dr. Lonn, professor of cardiology at McMaster University.
Dr. Yusuf said enthusiasm for the polypill concept as a means of reducing the growing global burden of cardiovascular disease remains strong among many experts. There is broad interest in a polypill for secondary prevention that will include aspirin, a beta blocker, and an ACE inhibitor of angiotensin receptor blocker as a low-cost means of improving medication adherence. And several large clinical trials of the polypill concept for primary prevention are underway, including a randomized trial conducted by Dr. Yusuf and coworkers in which several thousand high-risk subjects without baseline cardiovascular disease will receive a combination of a statin plus not two but three antihypertensive drugs.
Discussant Dr. Donald M. Lloyd-Jones commented, “What strikes me about HOPE-3 is that this is a population at risk, but not at particularly high risk.” And yet these patients benefited from statin therapy regardless of their baseline LDL, noted Dr. Lloyd-Jones, chair of the department of preventive medicine at Northwestern University, Chicago, and an architect of the risk-based approach to statin use that’s a centerpiece of the current ACC/AHA guidelines on atherosclerotic cardiovascular risk reduction.
In an interview, Dr. Sidney C. Smith, Jr., said the HOPE-3 data are “worthy of consideration” as experts meet at ACC 16 to begin updating guidelines for the treatment of hypertension. In particular, the key findings that moderate-risk hypertensive patients benefited from a statin regardless of their baseline LDL and initiation of blood pressure-lowering therapy was beneficial for patients with a systolic blood pressure in the 140s but not in those with a systolic pressure in the 120s and 130s could be practice changing, according to Dr. Smith, professor of medicine at the University of North Carolina, Chapel Hill.
Drs. Yusuf and Lonn reported receiving institutional research grants from the Canadian Institutes of Health Research and AstraZeneca, which funded the trial.
Simultaneous with the presentation of the HOPE-3 results at ACC 16 in Chicago, the study on cholesterol lowering and the study on blood pressure and cholesterol lowering led by Dr. Yusef as well as the study on blood pressure lowering led by Dr. Lonn were published online at NEJM.org.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The success in the HOPE-3 trial of a two-pronged strategy of a statin plus moderate-dose antihypertensive therapy has expanded the boundaries of primary preventive pharmacotherapy to incorporate many intermediate-cardiovascular-risk patients without cardiovascular disease.
In the Heart Outcomes Evaluation (HOPE)-3 trial, the combination of rosuvastatin (Crestor) at 10 mg/day plus antihypertensive therapy with 16 mg/day of candesartan and 12.5 mg/day of hydrochlorothiazide reduced cardiovascular events in intermediate-risk patients with hypertension, regardless of their baseline LDL-cholesterol and inflammatory biomarker levels. In nonhypertensive study participants, however, blood pressure-lowering therapy provided no added benefit beyond that achieved with the statin alone, which yielded a 25% relative risk reduction in cardiovascular events compared with placebo, Dr. Salim Yusuf reported at the annual meeting of the American College of Cardiology.
The HOPE-3 trial was the first formal study of the polypill concept of fixed-dose, low-dose combination therapy as a public health tool for primary prevention in an intermediate-risk population without cardiovascular disease. It’s a simple, low-cost, safe, pragmatic preventive strategy that doesn’t require baseline laboratory measurements, dose titration visits, or frequent safety monitoring, he noted.
“The original concept of the polypill was to give it to everyone over age 55. We found in HOPE-3 that the polypill concept is not valid for everybody. It demonstrated benefit in hypertensives but not in nonhypertensives, where a statin alone was beneficial,” said Dr. Yusuf, professor of medicine and executive director of the Population Health Research Institute at McMaster University in Hamilton, Ont.
“The clinical implication is that statins should be used much more widely than they currently are. Most guidelines for hypertension don’t say to give a statin. But HOPE-3 is saying hypertensives will benefit. About half of the 40% reduction in the risk of cardiovascular events we saw with combination therapy in patients in the highest third for baseline systolic blood pressure – that is, above 143.5 mm Hg, with a mean of 154 mm Hg – was due to the rosuvastatin and half to the antihypertensive therapy. Our study suggests you can essentially double the benefit of lowering blood pressure in hypertensives if you also lower cholesterol simultaneously,” he noted. Dr. Yusuf discussed the findings in a video interview.
The double-blind trial included a diverse population of 12,705 men age 55 or older and women age 65 or older in 21 countries. All were at intermediate cardiovascular risk by conventional stratification methods; none had a history of cardiovascular disease. They were randomized to rosuvastatin or placebo, dual antihypertensive therapy or placebo, or to all three medications or double placebo. The combined-therapy group experienced a 33.7 mg/dL greater drop in LDL-cholesterol and a 6.2 mm Hg bigger reduction in systolic blood pressure than in patients on dual placebo.
During a median followup of 5.6 years, the composite rate of cardiovascular death, nonfatal MI, or nonfatal stroke was 3.6% in the combined-therapy group and 5.0% with dual placebo, for a relative risk reduction of 29%. The number needed to treat for 5.6 years in order to prevent one event of the composite outcome was 72. However, the separation in the event rate curves for the two groups grew larger over time. With an additional planned followup of 3-5 years in HOPE-3, it’s likely the benefits will become even greater, according to Dr. Yusuf.
Combination therapy proved safe. Muscle aches and weakness as well as lightheadedness were more common in the combined treatment group than with dual placebo. However, permanent discontinuation rates were similar in the two groups.
HOPE-3 coinvestigator Dr. Eva Lonn presented the comparison between patients randomized to antihypertensive therapy without rosuvastatin or to placebo. In a prespecified subgroup analysis, active treatment resulted in a significant 27% reduction in the risk of the composite outcome compared with placebo in patients in the top one-third of baseline systolic blood pressure, no benefit in those with a systolic blood pressure of 131.6-143.5 mm Hg, and a suggestion of possible harm in patients whose systolic pressure at enrollment was 131.5 mm Hg or less.
Thus, the study helps define the minimum blood pressure at which antihypertensive therapy becomes beneficial, noted Dr. Lonn, professor of cardiology at McMaster University.
Dr. Yusuf said enthusiasm for the polypill concept as a means of reducing the growing global burden of cardiovascular disease remains strong among many experts. There is broad interest in a polypill for secondary prevention that will include aspirin, a beta blocker, and an ACE inhibitor of angiotensin receptor blocker as a low-cost means of improving medication adherence. And several large clinical trials of the polypill concept for primary prevention are underway, including a randomized trial conducted by Dr. Yusuf and coworkers in which several thousand high-risk subjects without baseline cardiovascular disease will receive a combination of a statin plus not two but three antihypertensive drugs.
Discussant Dr. Donald M. Lloyd-Jones commented, “What strikes me about HOPE-3 is that this is a population at risk, but not at particularly high risk.” And yet these patients benefited from statin therapy regardless of their baseline LDL, noted Dr. Lloyd-Jones, chair of the department of preventive medicine at Northwestern University, Chicago, and an architect of the risk-based approach to statin use that’s a centerpiece of the current ACC/AHA guidelines on atherosclerotic cardiovascular risk reduction.
In an interview, Dr. Sidney C. Smith, Jr., said the HOPE-3 data are “worthy of consideration” as experts meet at ACC 16 to begin updating guidelines for the treatment of hypertension. In particular, the key findings that moderate-risk hypertensive patients benefited from a statin regardless of their baseline LDL and initiation of blood pressure-lowering therapy was beneficial for patients with a systolic blood pressure in the 140s but not in those with a systolic pressure in the 120s and 130s could be practice changing, according to Dr. Smith, professor of medicine at the University of North Carolina, Chapel Hill.
Drs. Yusuf and Lonn reported receiving institutional research grants from the Canadian Institutes of Health Research and AstraZeneca, which funded the trial.
Simultaneous with the presentation of the HOPE-3 results at ACC 16 in Chicago, the study on cholesterol lowering and the study on blood pressure and cholesterol lowering led by Dr. Yusef as well as the study on blood pressure lowering led by Dr. Lonn were published online at NEJM.org.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The success in the HOPE-3 trial of a two-pronged strategy of a statin plus moderate-dose antihypertensive therapy has expanded the boundaries of primary preventive pharmacotherapy to incorporate many intermediate-cardiovascular-risk patients without cardiovascular disease.
In the Heart Outcomes Evaluation (HOPE)-3 trial, the combination of rosuvastatin (Crestor) at 10 mg/day plus antihypertensive therapy with 16 mg/day of candesartan and 12.5 mg/day of hydrochlorothiazide reduced cardiovascular events in intermediate-risk patients with hypertension, regardless of their baseline LDL-cholesterol and inflammatory biomarker levels. In nonhypertensive study participants, however, blood pressure-lowering therapy provided no added benefit beyond that achieved with the statin alone, which yielded a 25% relative risk reduction in cardiovascular events compared with placebo, Dr. Salim Yusuf reported at the annual meeting of the American College of Cardiology.
The HOPE-3 trial was the first formal study of the polypill concept of fixed-dose, low-dose combination therapy as a public health tool for primary prevention in an intermediate-risk population without cardiovascular disease. It’s a simple, low-cost, safe, pragmatic preventive strategy that doesn’t require baseline laboratory measurements, dose titration visits, or frequent safety monitoring, he noted.
“The original concept of the polypill was to give it to everyone over age 55. We found in HOPE-3 that the polypill concept is not valid for everybody. It demonstrated benefit in hypertensives but not in nonhypertensives, where a statin alone was beneficial,” said Dr. Yusuf, professor of medicine and executive director of the Population Health Research Institute at McMaster University in Hamilton, Ont.
“The clinical implication is that statins should be used much more widely than they currently are. Most guidelines for hypertension don’t say to give a statin. But HOPE-3 is saying hypertensives will benefit. About half of the 40% reduction in the risk of cardiovascular events we saw with combination therapy in patients in the highest third for baseline systolic blood pressure – that is, above 143.5 mm Hg, with a mean of 154 mm Hg – was due to the rosuvastatin and half to the antihypertensive therapy. Our study suggests you can essentially double the benefit of lowering blood pressure in hypertensives if you also lower cholesterol simultaneously,” he noted. Dr. Yusuf discussed the findings in a video interview.
The double-blind trial included a diverse population of 12,705 men age 55 or older and women age 65 or older in 21 countries. All were at intermediate cardiovascular risk by conventional stratification methods; none had a history of cardiovascular disease. They were randomized to rosuvastatin or placebo, dual antihypertensive therapy or placebo, or to all three medications or double placebo. The combined-therapy group experienced a 33.7 mg/dL greater drop in LDL-cholesterol and a 6.2 mm Hg bigger reduction in systolic blood pressure than in patients on dual placebo.
During a median followup of 5.6 years, the composite rate of cardiovascular death, nonfatal MI, or nonfatal stroke was 3.6% in the combined-therapy group and 5.0% with dual placebo, for a relative risk reduction of 29%. The number needed to treat for 5.6 years in order to prevent one event of the composite outcome was 72. However, the separation in the event rate curves for the two groups grew larger over time. With an additional planned followup of 3-5 years in HOPE-3, it’s likely the benefits will become even greater, according to Dr. Yusuf.
Combination therapy proved safe. Muscle aches and weakness as well as lightheadedness were more common in the combined treatment group than with dual placebo. However, permanent discontinuation rates were similar in the two groups.
HOPE-3 coinvestigator Dr. Eva Lonn presented the comparison between patients randomized to antihypertensive therapy without rosuvastatin or to placebo. In a prespecified subgroup analysis, active treatment resulted in a significant 27% reduction in the risk of the composite outcome compared with placebo in patients in the top one-third of baseline systolic blood pressure, no benefit in those with a systolic blood pressure of 131.6-143.5 mm Hg, and a suggestion of possible harm in patients whose systolic pressure at enrollment was 131.5 mm Hg or less.
Thus, the study helps define the minimum blood pressure at which antihypertensive therapy becomes beneficial, noted Dr. Lonn, professor of cardiology at McMaster University.
Dr. Yusuf said enthusiasm for the polypill concept as a means of reducing the growing global burden of cardiovascular disease remains strong among many experts. There is broad interest in a polypill for secondary prevention that will include aspirin, a beta blocker, and an ACE inhibitor of angiotensin receptor blocker as a low-cost means of improving medication adherence. And several large clinical trials of the polypill concept for primary prevention are underway, including a randomized trial conducted by Dr. Yusuf and coworkers in which several thousand high-risk subjects without baseline cardiovascular disease will receive a combination of a statin plus not two but three antihypertensive drugs.
Discussant Dr. Donald M. Lloyd-Jones commented, “What strikes me about HOPE-3 is that this is a population at risk, but not at particularly high risk.” And yet these patients benefited from statin therapy regardless of their baseline LDL, noted Dr. Lloyd-Jones, chair of the department of preventive medicine at Northwestern University, Chicago, and an architect of the risk-based approach to statin use that’s a centerpiece of the current ACC/AHA guidelines on atherosclerotic cardiovascular risk reduction.
In an interview, Dr. Sidney C. Smith, Jr., said the HOPE-3 data are “worthy of consideration” as experts meet at ACC 16 to begin updating guidelines for the treatment of hypertension. In particular, the key findings that moderate-risk hypertensive patients benefited from a statin regardless of their baseline LDL and initiation of blood pressure-lowering therapy was beneficial for patients with a systolic blood pressure in the 140s but not in those with a systolic pressure in the 120s and 130s could be practice changing, according to Dr. Smith, professor of medicine at the University of North Carolina, Chapel Hill.
Drs. Yusuf and Lonn reported receiving institutional research grants from the Canadian Institutes of Health Research and AstraZeneca, which funded the trial.
Simultaneous with the presentation of the HOPE-3 results at ACC 16 in Chicago, the study on cholesterol lowering and the study on blood pressure and cholesterol lowering led by Dr. Yusef as well as the study on blood pressure lowering led by Dr. Lonn were published online at NEJM.org.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 16
Key clinical point: Statin therapy should be used much more widely.
Major finding: The number of moderate-cardiovascular-risk persons needed to treat with a combination of a statin and two antihypertensive drugs for 5.6 years in order to prevent one cardiovascular death, nonfatal MI, or nonfatal stroke was 72.
Data source: The HOPE-3 study was a double-blind, randomized trial including 12,705 men and women at intermediate cardiovascular risk but without cardiovascular disease at enrollment.
Disclosures: The study presenters reported receiving institutional research grants from the Canadian Institutes of Health Research and AstraZeneca, which funded the trial.
VIDEO: Liraglutide acts on GLP-1 receptors to lessen desire for high-fat foods
BOSTON – Two related studies of brain structure and the mechanism of the analog of glucagon-like peptide (GLP) hormone liraglutide indicate that the drug works to decrease reward-related activation of brain sites linked to desire for unhealthy foods in patients with type 2 diabetes.
“Our finding suggests that liraglutide may make people more attentive to what they are eating, particularly high-calories or high-fat foods,” said study co-investigator Olivia Farr, PhD, of Beth Israel Deaconess Hospital and Harvard Medical School, Boston.
Liraglutide, which has been approved for weight management for obese patients and those with type 2 diabetes, is known to promote weight loss, but the mechanism by which this occurs has not been fully understood. The investigators undertook two studies, one to examine human brains to identify GLP-1 receptors and the other to examine the impact liraglutide administration may have on neural responses to food cues in patients with type 2 diabetes.
Immunohistochemical examination of 22 human brain samples identified GLP-1 receptors in the hypothalamus, medulla oblongata, and parietal cortex. GLP-1 receptors have previously only been identified in animals. The findings support the role of the receptors in weight loss in patients on liraglutide.
The researchers then performed a second randomized, placebo-controlled, double-blind, cross-over study involving 18 adult patients with type 2 diabetes. The subjects received, in random order, injections of placebo or liraglutide. Liraglutide was titrated to 0.6 mg at visit 1, 1.2 mg at visit 2, and 1.8 mg at visit 3, which were a week apart, with the highest dose maintained in the 3 days between visits 3 and 4. The total period was 17 days. Visit 4 was an overnight stay followed by functional magnetic resonance imaging (fMRI). Then, after a 3-week washout period, the participants received the other treatment on the same schedule, with another fMRI scan.
During the fMRI, participants viewed images of different foods that had been determined in pre-trial testing to be generally perceived as desirable (typically cakes, pastries, fried food, and fast food) and undesirable (typically leafy greens, fruits, vegetables, and other low-calorie food). In addition, non-food images were shown to verify that the brain activation was driven by the food images. The regions of the brain that became active during inspection of the images were determined.
Liraglutide decreased activation of the parietal cortex in response to the highly desirable food images. Additionally, activation in the insula and putamen was reduced; these regions are involved in the brain’s reward system. Increased perception of hunger and appetite by the participants when they viewed images of desirable foods correlated with increased activation of GLP-1 receptors in the parietal and visual cortices during liraglutide treatment. In participants experiencing nausea, decreased brain activation in the cingulate cortex was apparent. Hypothalamus-related activity was not evident.
“This decreased activation means that individuals on liraglutide find highly desirable foods less attention-grabbing and less rewarding than they typically would without liraglutide,” said Dr. Farr.
The researchers suggested that liraglutide could be suited for weight loss in those who opt for high-fat food as a means of pleasure. Further, the data point to a central mechanism contributing to or underlying effects of liraglutide on metabolism/weight loss.
The Harvard researchers are seeking to confirm the findings in a larger study using the 3-mg dose of liraglutide that has been approved for obesity. In addition, they will explore whether the brain response to liraglutide is a general phenomenon or whether individuals differ.
Dr. Farr had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – Two related studies of brain structure and the mechanism of the analog of glucagon-like peptide (GLP) hormone liraglutide indicate that the drug works to decrease reward-related activation of brain sites linked to desire for unhealthy foods in patients with type 2 diabetes.
“Our finding suggests that liraglutide may make people more attentive to what they are eating, particularly high-calories or high-fat foods,” said study co-investigator Olivia Farr, PhD, of Beth Israel Deaconess Hospital and Harvard Medical School, Boston.
Liraglutide, which has been approved for weight management for obese patients and those with type 2 diabetes, is known to promote weight loss, but the mechanism by which this occurs has not been fully understood. The investigators undertook two studies, one to examine human brains to identify GLP-1 receptors and the other to examine the impact liraglutide administration may have on neural responses to food cues in patients with type 2 diabetes.
Immunohistochemical examination of 22 human brain samples identified GLP-1 receptors in the hypothalamus, medulla oblongata, and parietal cortex. GLP-1 receptors have previously only been identified in animals. The findings support the role of the receptors in weight loss in patients on liraglutide.
The researchers then performed a second randomized, placebo-controlled, double-blind, cross-over study involving 18 adult patients with type 2 diabetes. The subjects received, in random order, injections of placebo or liraglutide. Liraglutide was titrated to 0.6 mg at visit 1, 1.2 mg at visit 2, and 1.8 mg at visit 3, which were a week apart, with the highest dose maintained in the 3 days between visits 3 and 4. The total period was 17 days. Visit 4 was an overnight stay followed by functional magnetic resonance imaging (fMRI). Then, after a 3-week washout period, the participants received the other treatment on the same schedule, with another fMRI scan.
During the fMRI, participants viewed images of different foods that had been determined in pre-trial testing to be generally perceived as desirable (typically cakes, pastries, fried food, and fast food) and undesirable (typically leafy greens, fruits, vegetables, and other low-calorie food). In addition, non-food images were shown to verify that the brain activation was driven by the food images. The regions of the brain that became active during inspection of the images were determined.
Liraglutide decreased activation of the parietal cortex in response to the highly desirable food images. Additionally, activation in the insula and putamen was reduced; these regions are involved in the brain’s reward system. Increased perception of hunger and appetite by the participants when they viewed images of desirable foods correlated with increased activation of GLP-1 receptors in the parietal and visual cortices during liraglutide treatment. In participants experiencing nausea, decreased brain activation in the cingulate cortex was apparent. Hypothalamus-related activity was not evident.
“This decreased activation means that individuals on liraglutide find highly desirable foods less attention-grabbing and less rewarding than they typically would without liraglutide,” said Dr. Farr.
The researchers suggested that liraglutide could be suited for weight loss in those who opt for high-fat food as a means of pleasure. Further, the data point to a central mechanism contributing to or underlying effects of liraglutide on metabolism/weight loss.
The Harvard researchers are seeking to confirm the findings in a larger study using the 3-mg dose of liraglutide that has been approved for obesity. In addition, they will explore whether the brain response to liraglutide is a general phenomenon or whether individuals differ.
Dr. Farr had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – Two related studies of brain structure and the mechanism of the analog of glucagon-like peptide (GLP) hormone liraglutide indicate that the drug works to decrease reward-related activation of brain sites linked to desire for unhealthy foods in patients with type 2 diabetes.
“Our finding suggests that liraglutide may make people more attentive to what they are eating, particularly high-calories or high-fat foods,” said study co-investigator Olivia Farr, PhD, of Beth Israel Deaconess Hospital and Harvard Medical School, Boston.
Liraglutide, which has been approved for weight management for obese patients and those with type 2 diabetes, is known to promote weight loss, but the mechanism by which this occurs has not been fully understood. The investigators undertook two studies, one to examine human brains to identify GLP-1 receptors and the other to examine the impact liraglutide administration may have on neural responses to food cues in patients with type 2 diabetes.
Immunohistochemical examination of 22 human brain samples identified GLP-1 receptors in the hypothalamus, medulla oblongata, and parietal cortex. GLP-1 receptors have previously only been identified in animals. The findings support the role of the receptors in weight loss in patients on liraglutide.
The researchers then performed a second randomized, placebo-controlled, double-blind, cross-over study involving 18 adult patients with type 2 diabetes. The subjects received, in random order, injections of placebo or liraglutide. Liraglutide was titrated to 0.6 mg at visit 1, 1.2 mg at visit 2, and 1.8 mg at visit 3, which were a week apart, with the highest dose maintained in the 3 days between visits 3 and 4. The total period was 17 days. Visit 4 was an overnight stay followed by functional magnetic resonance imaging (fMRI). Then, after a 3-week washout period, the participants received the other treatment on the same schedule, with another fMRI scan.
During the fMRI, participants viewed images of different foods that had been determined in pre-trial testing to be generally perceived as desirable (typically cakes, pastries, fried food, and fast food) and undesirable (typically leafy greens, fruits, vegetables, and other low-calorie food). In addition, non-food images were shown to verify that the brain activation was driven by the food images. The regions of the brain that became active during inspection of the images were determined.
Liraglutide decreased activation of the parietal cortex in response to the highly desirable food images. Additionally, activation in the insula and putamen was reduced; these regions are involved in the brain’s reward system. Increased perception of hunger and appetite by the participants when they viewed images of desirable foods correlated with increased activation of GLP-1 receptors in the parietal and visual cortices during liraglutide treatment. In participants experiencing nausea, decreased brain activation in the cingulate cortex was apparent. Hypothalamus-related activity was not evident.
“This decreased activation means that individuals on liraglutide find highly desirable foods less attention-grabbing and less rewarding than they typically would without liraglutide,” said Dr. Farr.
The researchers suggested that liraglutide could be suited for weight loss in those who opt for high-fat food as a means of pleasure. Further, the data point to a central mechanism contributing to or underlying effects of liraglutide on metabolism/weight loss.
The Harvard researchers are seeking to confirm the findings in a larger study using the 3-mg dose of liraglutide that has been approved for obesity. In addition, they will explore whether the brain response to liraglutide is a general phenomenon or whether individuals differ.
Dr. Farr had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: HOPE-3 trial expands scope of primary cardiovascular prevention
CHICAGO – The HOPE-3 trial has brought the primary prevention of cardiovascular disease front-and-center as a hot topic of discussion at the annual meeting of the American College of Cardiology.
The large, randomized trial provides new evidence of a significant reduction in cardiovascular events in an intermediate-risk population that hasn’t previously been the focus of risk reduction via pharmacotherapy.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The double-blind study randomized nearly 13,000 intermediate-risk men and women with no baseline cardiovascular disease to lipid-lowering with rosuvastatin (Crestor) at 10 mg/day or placebo, dual-antihypertensive therapy with candesartan plus chlorothiazide or placebo regardless of baseline blood pressure, or all three drugs or placebo. After a median of 5.6 years, the combined-therapy group had a 29% reduction in the composite of cardiovascular death or nonfatal MI or stroke compared with placebo-treated controls. Impressively, the benefit was similar regardless of baseline LDL-cholesterol level; however, only subjects with hypertension benefited from the dual-antihypertensive therapy.
In this interview, Dr. B. Hadley Wilson of the Sanger Clinic in Charlotte, N.C., explains why he considers HOPE-3 to be a giant step forward in preventive cardiolo
CHICAGO – The HOPE-3 trial has brought the primary prevention of cardiovascular disease front-and-center as a hot topic of discussion at the annual meeting of the American College of Cardiology.
The large, randomized trial provides new evidence of a significant reduction in cardiovascular events in an intermediate-risk population that hasn’t previously been the focus of risk reduction via pharmacotherapy.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The double-blind study randomized nearly 13,000 intermediate-risk men and women with no baseline cardiovascular disease to lipid-lowering with rosuvastatin (Crestor) at 10 mg/day or placebo, dual-antihypertensive therapy with candesartan plus chlorothiazide or placebo regardless of baseline blood pressure, or all three drugs or placebo. After a median of 5.6 years, the combined-therapy group had a 29% reduction in the composite of cardiovascular death or nonfatal MI or stroke compared with placebo-treated controls. Impressively, the benefit was similar regardless of baseline LDL-cholesterol level; however, only subjects with hypertension benefited from the dual-antihypertensive therapy.
In this interview, Dr. B. Hadley Wilson of the Sanger Clinic in Charlotte, N.C., explains why he considers HOPE-3 to be a giant step forward in preventive cardiolo
CHICAGO – The HOPE-3 trial has brought the primary prevention of cardiovascular disease front-and-center as a hot topic of discussion at the annual meeting of the American College of Cardiology.
The large, randomized trial provides new evidence of a significant reduction in cardiovascular events in an intermediate-risk population that hasn’t previously been the focus of risk reduction via pharmacotherapy.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The double-blind study randomized nearly 13,000 intermediate-risk men and women with no baseline cardiovascular disease to lipid-lowering with rosuvastatin (Crestor) at 10 mg/day or placebo, dual-antihypertensive therapy with candesartan plus chlorothiazide or placebo regardless of baseline blood pressure, or all three drugs or placebo. After a median of 5.6 years, the combined-therapy group had a 29% reduction in the composite of cardiovascular death or nonfatal MI or stroke compared with placebo-treated controls. Impressively, the benefit was similar regardless of baseline LDL-cholesterol level; however, only subjects with hypertension benefited from the dual-antihypertensive therapy.
In this interview, Dr. B. Hadley Wilson of the Sanger Clinic in Charlotte, N.C., explains why he considers HOPE-3 to be a giant step forward in preventive cardiolo
AT ACC 16
Prepsychosis links with elevated metabolic syndrome
MADRID – Untreated people at high risk for developing psychosis also showed an increased prevalence of certain components of metabolic syndrome in data collected from 163 German study participants, a finding that gives new insight into the well-documented but poorly delineated link between schizophrenia and metabolic syndrome.
“The findings point out that a high risk for schizophrenia implies a certain risk for patients to develop metabolic syndrome independent of treatment effects,” said Dr. Joachim Cordes, a psychiatrist at the LVR Clinic of the Heinrich-Heine University in Düsseldorf, Germany. He assumed that genetic factors underlie the shared risk some people face for both developing schizophrenia and metabolic syndrome. “I think there is a direct connection between schizophrenia and metabolic syndrome, an inherent factor like a genetic factor,” Dr. Cordes said in an interview. This understanding should influence how patients with newly diagnosed schizophrenia or those at risk for psychosis are managed, he added.
Dr. Cordes’s report was one of several at the meeting sponsored by the European Psychiatric Association that examined different facets of the complex links that tie schizophrenia to metabolic syndrome, an association that already had lots of evidence, including a recent meta-analysis (Schizophr Bull. 2013 March;39[2]:306-18).
He used data collected on 163 people enrolled in the PREVENT study and at high risk for a first psychotic episode. Run at nine German centers, PREVENT primarily tested very early intervention with drug and behavioral therapy to improve outcomes. Dr. Cordes took data collected from these prepsychosis, high-risk patients to assess their prevalence of metabolic syndrome and of the various individual features that define metabolic syndrome, using a definition published by the American Heart Association and the U.S. National Heart, Lung, and Blood Institute (Circulation. 2005 Oct 18;112[17]:2735-52). He compared these metabolic syndrome rates with the general German population, using data from 35,869 randomly selected German adults in more than 1,500 German primary care practices, the German Metabolic and Cardiovascular Risk Project (GEMCAS).
The findings showed a 9.2% prevalence of metabolic syndrome in the prepsychosis group and a 7.4% rate among the general adult population, Dr. Cordes reported. Among men in the prepsychosis group, the metabolic syndrome definers with the largest increments in prevalence were low HDL, in 21% of the prepsychosis people and in 12% of the general population, and elevated blood glucose in 11%, compared with 6%. Among women, the metabolic syndrome definers with the greatest between-group differences were elevated waist circumference, in 30% of those with prepsychosis, compared with 17% in the general population, and low HDL in 19%, compared with 14%.
This apparently inherent link between a tendency toward psychosis and schizophrenia and a tendency to develop features of metabolic syndrome suggests that patients with newly diagnosed schizophrenia need a preventive approach to weight management, Dr. Cordes said. He also suggested prescribing antipsychotic medications that pose the lowest risk for causing further metabolic derangements in patients.
A second report at the meeting came from an assessment of cognitive function and its relationship to metabolic syndrome in 54 women diagnosed with schizophrenia and on stable treatment. The schizophrenia patients with metabolic syndrome, nearly half of the total group, performed significantly worse than those without metabolic syndrome in tests of verbal memory, executive function, and attention and processing speed, findings that support an increased incidence of selective cognitive impairment in patients with schizophrenia and metabolic syndrome, said Dr. Adela C. Botis, a psychiatrist and researcher at the University of Medicine and Pharmacy in Cluj-Napoca, Romania.
Dr. Botis and her associates studied 54 women diagnosed with schizophrenia who had remitted symptoms for at least 6 months on stable antipsychotic treatment. Using the metabolic syndrome definition of the International Diabetes Federation 25 (46%) had metabolic syndrome, and the other 29 (54%) did not. These numbers document the high prevalence of metabolic syndrome in schizophrenia patients.
A multivariate analysis identified demographic and metabolic factors that significantly linked with decrements in several cognitive domains. Economic status and living situation linked with deficits in verbal memory; elevated systolic blood pressure significantly linked with worsened attention and processing speed; high body mass index linked with loss of motor speed; and less education significantly linked with all these increments as well as four other domains,
A third report used a post-hoc analysis of data from two separate trials to show that treatment with a relatively new antipsychotic drug, lurasidone (Latuda), produced less metabolic syndrome, compared with risperidone or extended-release quetiapine (Seroquel XR), said Dr. Andrei Pikalov, head of global medical affairs at Sunovion Pharmaceuticals, the company that markets Latuda. Lurasidone received approval for treating schizophrenia in 2010.
He took data from two studies designed to assess lurasidone’s efficacy for treating adults with schizophrenia for 12 months, compared with either risperidone in a study with 621 patients, or with quetiapine XR in a study with 292 patients. He applied the same metabolic syndrome definition used by Dr. Cordes to clinical measurements taken at baseline and after 12 months on treatment.
The results showed that treatment with lurasidone produced less than half the rate of new metabolic syndrome cases, compared with risperidone, a statistically significant difference, and less than two-thirds the rate of quetiapine XR, a difference that did not reach statistical significance.
Dr. Cordes said he has been a speaker for Servier. Dr. Botis had no disclosures. Dr. Pikalov is an employee of Sunovion, which markets lurasidone (Latuda).
On Twitter @mitchelzoler
MADRID – Untreated people at high risk for developing psychosis also showed an increased prevalence of certain components of metabolic syndrome in data collected from 163 German study participants, a finding that gives new insight into the well-documented but poorly delineated link between schizophrenia and metabolic syndrome.
“The findings point out that a high risk for schizophrenia implies a certain risk for patients to develop metabolic syndrome independent of treatment effects,” said Dr. Joachim Cordes, a psychiatrist at the LVR Clinic of the Heinrich-Heine University in Düsseldorf, Germany. He assumed that genetic factors underlie the shared risk some people face for both developing schizophrenia and metabolic syndrome. “I think there is a direct connection between schizophrenia and metabolic syndrome, an inherent factor like a genetic factor,” Dr. Cordes said in an interview. This understanding should influence how patients with newly diagnosed schizophrenia or those at risk for psychosis are managed, he added.
Dr. Cordes’s report was one of several at the meeting sponsored by the European Psychiatric Association that examined different facets of the complex links that tie schizophrenia to metabolic syndrome, an association that already had lots of evidence, including a recent meta-analysis (Schizophr Bull. 2013 March;39[2]:306-18).
He used data collected on 163 people enrolled in the PREVENT study and at high risk for a first psychotic episode. Run at nine German centers, PREVENT primarily tested very early intervention with drug and behavioral therapy to improve outcomes. Dr. Cordes took data collected from these prepsychosis, high-risk patients to assess their prevalence of metabolic syndrome and of the various individual features that define metabolic syndrome, using a definition published by the American Heart Association and the U.S. National Heart, Lung, and Blood Institute (Circulation. 2005 Oct 18;112[17]:2735-52). He compared these metabolic syndrome rates with the general German population, using data from 35,869 randomly selected German adults in more than 1,500 German primary care practices, the German Metabolic and Cardiovascular Risk Project (GEMCAS).
The findings showed a 9.2% prevalence of metabolic syndrome in the prepsychosis group and a 7.4% rate among the general adult population, Dr. Cordes reported. Among men in the prepsychosis group, the metabolic syndrome definers with the largest increments in prevalence were low HDL, in 21% of the prepsychosis people and in 12% of the general population, and elevated blood glucose in 11%, compared with 6%. Among women, the metabolic syndrome definers with the greatest between-group differences were elevated waist circumference, in 30% of those with prepsychosis, compared with 17% in the general population, and low HDL in 19%, compared with 14%.
This apparently inherent link between a tendency toward psychosis and schizophrenia and a tendency to develop features of metabolic syndrome suggests that patients with newly diagnosed schizophrenia need a preventive approach to weight management, Dr. Cordes said. He also suggested prescribing antipsychotic medications that pose the lowest risk for causing further metabolic derangements in patients.
A second report at the meeting came from an assessment of cognitive function and its relationship to metabolic syndrome in 54 women diagnosed with schizophrenia and on stable treatment. The schizophrenia patients with metabolic syndrome, nearly half of the total group, performed significantly worse than those without metabolic syndrome in tests of verbal memory, executive function, and attention and processing speed, findings that support an increased incidence of selective cognitive impairment in patients with schizophrenia and metabolic syndrome, said Dr. Adela C. Botis, a psychiatrist and researcher at the University of Medicine and Pharmacy in Cluj-Napoca, Romania.
Dr. Botis and her associates studied 54 women diagnosed with schizophrenia who had remitted symptoms for at least 6 months on stable antipsychotic treatment. Using the metabolic syndrome definition of the International Diabetes Federation 25 (46%) had metabolic syndrome, and the other 29 (54%) did not. These numbers document the high prevalence of metabolic syndrome in schizophrenia patients.
A multivariate analysis identified demographic and metabolic factors that significantly linked with decrements in several cognitive domains. Economic status and living situation linked with deficits in verbal memory; elevated systolic blood pressure significantly linked with worsened attention and processing speed; high body mass index linked with loss of motor speed; and less education significantly linked with all these increments as well as four other domains,
A third report used a post-hoc analysis of data from two separate trials to show that treatment with a relatively new antipsychotic drug, lurasidone (Latuda), produced less metabolic syndrome, compared with risperidone or extended-release quetiapine (Seroquel XR), said Dr. Andrei Pikalov, head of global medical affairs at Sunovion Pharmaceuticals, the company that markets Latuda. Lurasidone received approval for treating schizophrenia in 2010.
He took data from two studies designed to assess lurasidone’s efficacy for treating adults with schizophrenia for 12 months, compared with either risperidone in a study with 621 patients, or with quetiapine XR in a study with 292 patients. He applied the same metabolic syndrome definition used by Dr. Cordes to clinical measurements taken at baseline and after 12 months on treatment.
The results showed that treatment with lurasidone produced less than half the rate of new metabolic syndrome cases, compared with risperidone, a statistically significant difference, and less than two-thirds the rate of quetiapine XR, a difference that did not reach statistical significance.
Dr. Cordes said he has been a speaker for Servier. Dr. Botis had no disclosures. Dr. Pikalov is an employee of Sunovion, which markets lurasidone (Latuda).
On Twitter @mitchelzoler
MADRID – Untreated people at high risk for developing psychosis also showed an increased prevalence of certain components of metabolic syndrome in data collected from 163 German study participants, a finding that gives new insight into the well-documented but poorly delineated link between schizophrenia and metabolic syndrome.
“The findings point out that a high risk for schizophrenia implies a certain risk for patients to develop metabolic syndrome independent of treatment effects,” said Dr. Joachim Cordes, a psychiatrist at the LVR Clinic of the Heinrich-Heine University in Düsseldorf, Germany. He assumed that genetic factors underlie the shared risk some people face for both developing schizophrenia and metabolic syndrome. “I think there is a direct connection between schizophrenia and metabolic syndrome, an inherent factor like a genetic factor,” Dr. Cordes said in an interview. This understanding should influence how patients with newly diagnosed schizophrenia or those at risk for psychosis are managed, he added.
Dr. Cordes’s report was one of several at the meeting sponsored by the European Psychiatric Association that examined different facets of the complex links that tie schizophrenia to metabolic syndrome, an association that already had lots of evidence, including a recent meta-analysis (Schizophr Bull. 2013 March;39[2]:306-18).
He used data collected on 163 people enrolled in the PREVENT study and at high risk for a first psychotic episode. Run at nine German centers, PREVENT primarily tested very early intervention with drug and behavioral therapy to improve outcomes. Dr. Cordes took data collected from these prepsychosis, high-risk patients to assess their prevalence of metabolic syndrome and of the various individual features that define metabolic syndrome, using a definition published by the American Heart Association and the U.S. National Heart, Lung, and Blood Institute (Circulation. 2005 Oct 18;112[17]:2735-52). He compared these metabolic syndrome rates with the general German population, using data from 35,869 randomly selected German adults in more than 1,500 German primary care practices, the German Metabolic and Cardiovascular Risk Project (GEMCAS).
The findings showed a 9.2% prevalence of metabolic syndrome in the prepsychosis group and a 7.4% rate among the general adult population, Dr. Cordes reported. Among men in the prepsychosis group, the metabolic syndrome definers with the largest increments in prevalence were low HDL, in 21% of the prepsychosis people and in 12% of the general population, and elevated blood glucose in 11%, compared with 6%. Among women, the metabolic syndrome definers with the greatest between-group differences were elevated waist circumference, in 30% of those with prepsychosis, compared with 17% in the general population, and low HDL in 19%, compared with 14%.
This apparently inherent link between a tendency toward psychosis and schizophrenia and a tendency to develop features of metabolic syndrome suggests that patients with newly diagnosed schizophrenia need a preventive approach to weight management, Dr. Cordes said. He also suggested prescribing antipsychotic medications that pose the lowest risk for causing further metabolic derangements in patients.
A second report at the meeting came from an assessment of cognitive function and its relationship to metabolic syndrome in 54 women diagnosed with schizophrenia and on stable treatment. The schizophrenia patients with metabolic syndrome, nearly half of the total group, performed significantly worse than those without metabolic syndrome in tests of verbal memory, executive function, and attention and processing speed, findings that support an increased incidence of selective cognitive impairment in patients with schizophrenia and metabolic syndrome, said Dr. Adela C. Botis, a psychiatrist and researcher at the University of Medicine and Pharmacy in Cluj-Napoca, Romania.
Dr. Botis and her associates studied 54 women diagnosed with schizophrenia who had remitted symptoms for at least 6 months on stable antipsychotic treatment. Using the metabolic syndrome definition of the International Diabetes Federation 25 (46%) had metabolic syndrome, and the other 29 (54%) did not. These numbers document the high prevalence of metabolic syndrome in schizophrenia patients.
A multivariate analysis identified demographic and metabolic factors that significantly linked with decrements in several cognitive domains. Economic status and living situation linked with deficits in verbal memory; elevated systolic blood pressure significantly linked with worsened attention and processing speed; high body mass index linked with loss of motor speed; and less education significantly linked with all these increments as well as four other domains,
A third report used a post-hoc analysis of data from two separate trials to show that treatment with a relatively new antipsychotic drug, lurasidone (Latuda), produced less metabolic syndrome, compared with risperidone or extended-release quetiapine (Seroquel XR), said Dr. Andrei Pikalov, head of global medical affairs at Sunovion Pharmaceuticals, the company that markets Latuda. Lurasidone received approval for treating schizophrenia in 2010.
He took data from two studies designed to assess lurasidone’s efficacy for treating adults with schizophrenia for 12 months, compared with either risperidone in a study with 621 patients, or with quetiapine XR in a study with 292 patients. He applied the same metabolic syndrome definition used by Dr. Cordes to clinical measurements taken at baseline and after 12 months on treatment.
The results showed that treatment with lurasidone produced less than half the rate of new metabolic syndrome cases, compared with risperidone, a statistically significant difference, and less than two-thirds the rate of quetiapine XR, a difference that did not reach statistical significance.
Dr. Cordes said he has been a speaker for Servier. Dr. Botis had no disclosures. Dr. Pikalov is an employee of Sunovion, which markets lurasidone (Latuda).
On Twitter @mitchelzoler
AT THE EUROPEAN CONGRESS OF PSYCHIATRY
Key clinical point: People at high risk for a first psychosis episode had an increased prevalence of certain metabolic syndrome components, compared with the general population.
Major finding: Metabolic syndrome was prevalent in 9.2% of the prepsychosis group and in 7.4% of the general population.
Data source: Post-hoc analysis of data from 163 German people with prepsychosis.
Disclosures: Dr. Cordes said he has been a speaker for Servier. Dr. Botis had no disclosures. Dr. Pikalov is an employee of Sunovion, which markets lurasidone (Latuda).
Women with suspected CAD classified as lower risk than men
Women with suspected coronary artery disease had similar symptoms and more heart disease risk factors, compared with men, but were assessed as lower risk by their providers and on all standard risk scores, according to a secondary analysis of the PROMISE trial.
The results “highlight the need for sex-specific approaches to coronary artery disease evaluation and testing,” said Kshipra Hemal at Duke Clinical Research Institute in Durham, N.C., and her associates. The findings will be presented April 3 at the annual meeting of the American College of Cardiology and were published online March 23 in the Journal of the American College of Cardiology: Cardiovascular Imaging.
The PROMISE (Prospective Multicenter Imaging Study for the Evaluation of Chest Pain) trial is one of the largest contemporary trials of symptomatic, nonacute suspected CAD. The study included 10,003 stable outpatients, nearly half of whom were women. The researchers calculated the 2008 Framingham score, 2013 Atherosclerotic Cardiovascular Disease score, 1979 Diamond and Forrester score, modified 2011 Diamond and Forrester score, and 2012 combined Diamond-Forrester and Coronary Artery Surgery Study scores for all patients. Patients also were randomly assigned to either anatomical testing with CT angiography or to functional testing with exercise electrocardiogram, stress nuclear imaging, or stress echocardiogram (J Am Coll Cardiol Img. 2016 Mar 23. doi: 10.1016/j.jcmg.2016.02.001).
Women in the study were an average of 3 years older than the men and were significantly more likely to be hypertensive (67% vs. 63%), dyslipidemic (69% vs. 66%), and to have a family history of premature CAD (35% vs. 29%; P less than .01 for all comparisons), the researchers reported. Nonetheless, all five risk scores characterized women as lower risk than men (P less than .001 for mean differences). Moreover, before testing, providers characterized 41% of women having a low (less than 30%) likelihood of CAD, compared with 34% of men (P less than .001).
Women were more likely than men to be referred for stress echocardiography or nuclear stress test, but only 9.7% had a positive noninvasive test, compared with 15% of men (P less than .001), the researchers also reported. “A number of characteristics predicted positive test results, and many characteristics were similar between the sexes,” they added. “However, in multivariable models, key predictors of test positivity were few and varied by sex.” Body mass index and Framingham risk score predicted a positive test for women, while both the Framingham and modified Diamond-Forrester risk scores predicted a positive test for men.
Chest pain was the most common primary symptom reported by nearly three-quarters of women and men and was described as “crushing/pressure/squeezing/tightness” 53% and 46% of the time, respectively (P less than .001). Dyspnea was the second most frequent primary symptom at 15% for both sexes. Women were more likely than men to describe back pain, neck or jaw pain, or palpitations, but only 0.6% to 2.7% of patients ranked these among their main symptoms.
“Further studies are warranted to examine the underlying pathophysiology and implications for clinical care of the sex-based clinical differences observed along the entire diagnostic pathway of suspected CAD, including risk factor burden, presenting symptoms, and testing results,” the researchers concluded.
The PROMISE study was funded by the National Heart, Lung, and Blood Institute. Ms. Hemal had no disclosures. Senior author Dr. Pamela S. Douglas disclosed grant support from HeartFlow and having served on a data and safety monitoring board for General Electric Healthcare. Two of the other 15 coinvestigators also disclosed relationships with industry; the rest had no disclosures.
Despite symptomatic presentation, greater family history of premature coronary artery disease, and higher risk factor burden, including older age and greater prevalence of hypertension and dyslipidemia, the women in PROMISE were more likely to be characterized as low risk based on standard cardiovascular risk assessment scores and thus, not surprisingly, also were considered to be at lower risk by their providers. These findings add credence to the ongoing concerns that women are preferentially likely to receive less intensive management of CAD than their male counterparts.
The 2014 American Heart Association Consensus Statement on noninvasive diagnostic testing in women with suspected ischemic heart disease highlighted the development of novel diagnostic tools that have an expanded role in the evaluation of symptomatic female patients to detect not only focal epicardial coronary stenosis, but also nonobstructive atherosclerosis as well as the identification of ischemia resulting from microvascular dysfunction. Such methods using advanced imaging are making steady progress in the understanding of microvascular disease and its consequences.
We agree with the PROMISE investigators that focused sex-specific diagnostic strategies are needed to reduce the cardiovascular mortality and morbidity in women. With emerging data on the full pathophysiologic spectrum of ischemic heart disease in women, diagnostic algorithms must include functional and anatomic cardiac tests as well as physiologic assessments of endothelial and microvascular function, for accurately establishing the diagnosis and prognosis of women with suspected IHD.
Dr. Jennifer H. Mieres is with Hofstra University, Hempstead, N.Y. Dr. Robert O. Bonow is with Northwestern University, Chicago. They had no disclosures. These comments are from their editorial (J Am Coll Cardiol Img. 2016 Mar 23. doi: 10.1016/j.jcmg.2016.02.0089).
Despite symptomatic presentation, greater family history of premature coronary artery disease, and higher risk factor burden, including older age and greater prevalence of hypertension and dyslipidemia, the women in PROMISE were more likely to be characterized as low risk based on standard cardiovascular risk assessment scores and thus, not surprisingly, also were considered to be at lower risk by their providers. These findings add credence to the ongoing concerns that women are preferentially likely to receive less intensive management of CAD than their male counterparts.
The 2014 American Heart Association Consensus Statement on noninvasive diagnostic testing in women with suspected ischemic heart disease highlighted the development of novel diagnostic tools that have an expanded role in the evaluation of symptomatic female patients to detect not only focal epicardial coronary stenosis, but also nonobstructive atherosclerosis as well as the identification of ischemia resulting from microvascular dysfunction. Such methods using advanced imaging are making steady progress in the understanding of microvascular disease and its consequences.
We agree with the PROMISE investigators that focused sex-specific diagnostic strategies are needed to reduce the cardiovascular mortality and morbidity in women. With emerging data on the full pathophysiologic spectrum of ischemic heart disease in women, diagnostic algorithms must include functional and anatomic cardiac tests as well as physiologic assessments of endothelial and microvascular function, for accurately establishing the diagnosis and prognosis of women with suspected IHD.
Dr. Jennifer H. Mieres is with Hofstra University, Hempstead, N.Y. Dr. Robert O. Bonow is with Northwestern University, Chicago. They had no disclosures. These comments are from their editorial (J Am Coll Cardiol Img. 2016 Mar 23. doi: 10.1016/j.jcmg.2016.02.0089).
Despite symptomatic presentation, greater family history of premature coronary artery disease, and higher risk factor burden, including older age and greater prevalence of hypertension and dyslipidemia, the women in PROMISE were more likely to be characterized as low risk based on standard cardiovascular risk assessment scores and thus, not surprisingly, also were considered to be at lower risk by their providers. These findings add credence to the ongoing concerns that women are preferentially likely to receive less intensive management of CAD than their male counterparts.
The 2014 American Heart Association Consensus Statement on noninvasive diagnostic testing in women with suspected ischemic heart disease highlighted the development of novel diagnostic tools that have an expanded role in the evaluation of symptomatic female patients to detect not only focal epicardial coronary stenosis, but also nonobstructive atherosclerosis as well as the identification of ischemia resulting from microvascular dysfunction. Such methods using advanced imaging are making steady progress in the understanding of microvascular disease and its consequences.
We agree with the PROMISE investigators that focused sex-specific diagnostic strategies are needed to reduce the cardiovascular mortality and morbidity in women. With emerging data on the full pathophysiologic spectrum of ischemic heart disease in women, diagnostic algorithms must include functional and anatomic cardiac tests as well as physiologic assessments of endothelial and microvascular function, for accurately establishing the diagnosis and prognosis of women with suspected IHD.
Dr. Jennifer H. Mieres is with Hofstra University, Hempstead, N.Y. Dr. Robert O. Bonow is with Northwestern University, Chicago. They had no disclosures. These comments are from their editorial (J Am Coll Cardiol Img. 2016 Mar 23. doi: 10.1016/j.jcmg.2016.02.0089).
Women with suspected coronary artery disease had similar symptoms and more heart disease risk factors, compared with men, but were assessed as lower risk by their providers and on all standard risk scores, according to a secondary analysis of the PROMISE trial.
The results “highlight the need for sex-specific approaches to coronary artery disease evaluation and testing,” said Kshipra Hemal at Duke Clinical Research Institute in Durham, N.C., and her associates. The findings will be presented April 3 at the annual meeting of the American College of Cardiology and were published online March 23 in the Journal of the American College of Cardiology: Cardiovascular Imaging.
The PROMISE (Prospective Multicenter Imaging Study for the Evaluation of Chest Pain) trial is one of the largest contemporary trials of symptomatic, nonacute suspected CAD. The study included 10,003 stable outpatients, nearly half of whom were women. The researchers calculated the 2008 Framingham score, 2013 Atherosclerotic Cardiovascular Disease score, 1979 Diamond and Forrester score, modified 2011 Diamond and Forrester score, and 2012 combined Diamond-Forrester and Coronary Artery Surgery Study scores for all patients. Patients also were randomly assigned to either anatomical testing with CT angiography or to functional testing with exercise electrocardiogram, stress nuclear imaging, or stress echocardiogram (J Am Coll Cardiol Img. 2016 Mar 23. doi: 10.1016/j.jcmg.2016.02.001).
Women in the study were an average of 3 years older than the men and were significantly more likely to be hypertensive (67% vs. 63%), dyslipidemic (69% vs. 66%), and to have a family history of premature CAD (35% vs. 29%; P less than .01 for all comparisons), the researchers reported. Nonetheless, all five risk scores characterized women as lower risk than men (P less than .001 for mean differences). Moreover, before testing, providers characterized 41% of women having a low (less than 30%) likelihood of CAD, compared with 34% of men (P less than .001).
Women were more likely than men to be referred for stress echocardiography or nuclear stress test, but only 9.7% had a positive noninvasive test, compared with 15% of men (P less than .001), the researchers also reported. “A number of characteristics predicted positive test results, and many characteristics were similar between the sexes,” they added. “However, in multivariable models, key predictors of test positivity were few and varied by sex.” Body mass index and Framingham risk score predicted a positive test for women, while both the Framingham and modified Diamond-Forrester risk scores predicted a positive test for men.
Chest pain was the most common primary symptom reported by nearly three-quarters of women and men and was described as “crushing/pressure/squeezing/tightness” 53% and 46% of the time, respectively (P less than .001). Dyspnea was the second most frequent primary symptom at 15% for both sexes. Women were more likely than men to describe back pain, neck or jaw pain, or palpitations, but only 0.6% to 2.7% of patients ranked these among their main symptoms.
“Further studies are warranted to examine the underlying pathophysiology and implications for clinical care of the sex-based clinical differences observed along the entire diagnostic pathway of suspected CAD, including risk factor burden, presenting symptoms, and testing results,” the researchers concluded.
The PROMISE study was funded by the National Heart, Lung, and Blood Institute. Ms. Hemal had no disclosures. Senior author Dr. Pamela S. Douglas disclosed grant support from HeartFlow and having served on a data and safety monitoring board for General Electric Healthcare. Two of the other 15 coinvestigators also disclosed relationships with industry; the rest had no disclosures.
Women with suspected coronary artery disease had similar symptoms and more heart disease risk factors, compared with men, but were assessed as lower risk by their providers and on all standard risk scores, according to a secondary analysis of the PROMISE trial.
The results “highlight the need for sex-specific approaches to coronary artery disease evaluation and testing,” said Kshipra Hemal at Duke Clinical Research Institute in Durham, N.C., and her associates. The findings will be presented April 3 at the annual meeting of the American College of Cardiology and were published online March 23 in the Journal of the American College of Cardiology: Cardiovascular Imaging.
The PROMISE (Prospective Multicenter Imaging Study for the Evaluation of Chest Pain) trial is one of the largest contemporary trials of symptomatic, nonacute suspected CAD. The study included 10,003 stable outpatients, nearly half of whom were women. The researchers calculated the 2008 Framingham score, 2013 Atherosclerotic Cardiovascular Disease score, 1979 Diamond and Forrester score, modified 2011 Diamond and Forrester score, and 2012 combined Diamond-Forrester and Coronary Artery Surgery Study scores for all patients. Patients also were randomly assigned to either anatomical testing with CT angiography or to functional testing with exercise electrocardiogram, stress nuclear imaging, or stress echocardiogram (J Am Coll Cardiol Img. 2016 Mar 23. doi: 10.1016/j.jcmg.2016.02.001).
Women in the study were an average of 3 years older than the men and were significantly more likely to be hypertensive (67% vs. 63%), dyslipidemic (69% vs. 66%), and to have a family history of premature CAD (35% vs. 29%; P less than .01 for all comparisons), the researchers reported. Nonetheless, all five risk scores characterized women as lower risk than men (P less than .001 for mean differences). Moreover, before testing, providers characterized 41% of women having a low (less than 30%) likelihood of CAD, compared with 34% of men (P less than .001).
Women were more likely than men to be referred for stress echocardiography or nuclear stress test, but only 9.7% had a positive noninvasive test, compared with 15% of men (P less than .001), the researchers also reported. “A number of characteristics predicted positive test results, and many characteristics were similar between the sexes,” they added. “However, in multivariable models, key predictors of test positivity were few and varied by sex.” Body mass index and Framingham risk score predicted a positive test for women, while both the Framingham and modified Diamond-Forrester risk scores predicted a positive test for men.
Chest pain was the most common primary symptom reported by nearly three-quarters of women and men and was described as “crushing/pressure/squeezing/tightness” 53% and 46% of the time, respectively (P less than .001). Dyspnea was the second most frequent primary symptom at 15% for both sexes. Women were more likely than men to describe back pain, neck or jaw pain, or palpitations, but only 0.6% to 2.7% of patients ranked these among their main symptoms.
“Further studies are warranted to examine the underlying pathophysiology and implications for clinical care of the sex-based clinical differences observed along the entire diagnostic pathway of suspected CAD, including risk factor burden, presenting symptoms, and testing results,” the researchers concluded.
The PROMISE study was funded by the National Heart, Lung, and Blood Institute. Ms. Hemal had no disclosures. Senior author Dr. Pamela S. Douglas disclosed grant support from HeartFlow and having served on a data and safety monitoring board for General Electric Healthcare. Two of the other 15 coinvestigators also disclosed relationships with industry; the rest had no disclosures.
FROM ACC 16
Key clinical point: Women with suspected coronary artery disease had similar symptoms and more risk factors for coronary artery disease, compared with men, but were classified as lower risk on risk scores and by providers.
Major finding: All risk scores assessed women as being at lower risk than men. Providers characterized 41% of pretest women and 34% of men as low risk (P less than .001).
Data source: A prospective, multicenter, randomized trial of 10,003 symptomatic outpatients with suspected coronary artery disease.
Disclosures: The PROMISE study was funded by the National Heart, Lung, and Blood Institute. Dr. Hemal had no disclosures. Senior author Dr. Pamela Douglas disclosed grant support from HeartFlow and having served on a data and safety monitoring board for General Electric Healthcare. Two of the other 15 coinvestigators also disclosed relationships with industry; the rest had no disclosures.
Large cohort study confirms link between pioglitazone, bladder cancer
Pioglitazone was linked to a 63% increase in bladder cancer risk, compared with other noninsulin antidiabetic drugs in a large population-based cohort study.
Furthermore, incidence rose the longer patients took pioglitazone and the higher their dose, according to Dr. Marco Tuccori at Jewish General Hospital in Montreal, and his associates. “Rosiglitazone was not associated with an increased risk of bladder cancer in any analysis, suggesting the risk is drug-specific and not a class effect,” they wrote online March 30 in BMJ.
Both pioglitazone and rosiglitazone are thiazolidinediones that help treat insulin resistance in patients with type 2 diabetes. In 2005, Proactive trial investigators reported that patients were more likely to develop bladder cancer on pioglitazone, compared with placebo. But later observational studies reported mixed associations between pioglitazone and bladder cancer, perhaps because of biases and other methodological shortcomings, the researchers said (BMJ. 2016. doi: 10.1136/bmj.i1541).
Dr. Tuccori and his colleagues studied 145,806 patients with newly diagnosed type 2 diabetes, defined as a first-ever prescription for thiazolidinediones, metformin, sulfonylureas, prandial glucose regulators, acarbose, dipeptidyl peptidase-4 inhibitors, glucagonlike peptide agonists, or sodium-glucose cotransporter-2 inhibitors. The investigators excluded patients with any history of bladder cancer or less than a year of follow-up, and set a lag period of 1 year to help control for bladder cancer cases that were undiagnosed at study entry. The study included data for 2000-2013 from a national primary care registry that correlated well with the national cancer registry, the investigators said.
In all, 622 patients were diagnosed with bladder cancer (90 cases per 100,000 person-years), with a median of 4.4 years between study entry and cancer diagnosis (interquartile range, 2.5-6.5 years). Pioglitazone was associated with 121 cases of bladder cancer for every 100,000 person-years, compared with only 89 cases per 100,000 person-years for other antidiabetic drugs (hazard ratio, 1.6; 95% confidence interval, 1.2-2.2). Patients were no more likely to develop bladder cancer on rosiglitazone than other antidiabetic drugs (86.2 and 88.9 cases per 100,000 person-years of exposure, respectively; HR, 1.1; 95% CI, 0.8-1.5). Furthermore, pioglitazone was linked to nearly a 50% greater risk of bladder cancer, compared with rosiglitazone in a head-to-head comparison.
Not only did the link between pioglitazone and bladder cancer hold up in nine sensitivity analyses, but the hazard ratio increased to 1.73 when the researchers increased the required lag period to 2 years. Pharmacologic differences between pioglitazone and rosiglitazone might explain their distinct risk profiles in terms of bladder cancer, the investigators noted. Rosiglitazone is selective for peroxisome proliferator-activated receptor (PPAR) gamma, while pioglitazone has dual PPAR alpha/gamma activity. Only the latter increased expression of carcinogenic bladder biomarkers in rat models, they noted. This mechanism needs more exploration, they added.
The Canadian Institutes of Health Research funded the study. The researchers had no disclosures.
The results of such a large study are troubling Although it represents a retroactive observational assessment of the risk of bladder cancer from pioglitazone rather than a prospective randomized trial, the large number of patients gives this study strength. Yet, it is at odds, as the authors themselves point out, with other published studies like the Kaiser Permanente Northern California study. That study used the same cohort with follow-up extended to 10 years, and the use of pioglitazone was no longer significantly associated with an increased risk of bladder cancer. It is important to note that, although the authors show an increased risk for bladder cancer, the numbers are very small. I wish the authors would balance the risk in their study with potential benefit in the same cohort. They did cite the recently published Insulin Resistance Intervention after Stroke (IRIS) trial, which randomized 3,895 people. After a similar median follow-up of 4.8 years (4.7 years in the United Kingdom study), pioglitazone was associated with a decreased risk of a composite endpoint of stroke or myocardial infarction. In the IRIS study, there was an increased number of bladder cancer events in the pioglitazone group: 12 vs. 8 in the placebo group. In IRIS we see a clear benefit-risk ratio. By prescribing pioglitazone, there is significant cardiovascular risk improvement, while the number of cancer cases was quite small. In summary, continued prescribing of pioglitazone seems reasonable, and although the controversy has not been resolved, it seems that benefit-risk ratio favors prescribing pioglitazone. It makes sense, however, to exercise caution and follow the recommendations of the Food and Drug Administration in patients with a high risk for bladder cancer.
Dr. Yehuda Handelsman is medical director and principal investigator, Metabolic Institute of America, Tarzana, Calif., and immediate past president, American College of Endocrinology.
The results of such a large study are troubling Although it represents a retroactive observational assessment of the risk of bladder cancer from pioglitazone rather than a prospective randomized trial, the large number of patients gives this study strength. Yet, it is at odds, as the authors themselves point out, with other published studies like the Kaiser Permanente Northern California study. That study used the same cohort with follow-up extended to 10 years, and the use of pioglitazone was no longer significantly associated with an increased risk of bladder cancer. It is important to note that, although the authors show an increased risk for bladder cancer, the numbers are very small. I wish the authors would balance the risk in their study with potential benefit in the same cohort. They did cite the recently published Insulin Resistance Intervention after Stroke (IRIS) trial, which randomized 3,895 people. After a similar median follow-up of 4.8 years (4.7 years in the United Kingdom study), pioglitazone was associated with a decreased risk of a composite endpoint of stroke or myocardial infarction. In the IRIS study, there was an increased number of bladder cancer events in the pioglitazone group: 12 vs. 8 in the placebo group. In IRIS we see a clear benefit-risk ratio. By prescribing pioglitazone, there is significant cardiovascular risk improvement, while the number of cancer cases was quite small. In summary, continued prescribing of pioglitazone seems reasonable, and although the controversy has not been resolved, it seems that benefit-risk ratio favors prescribing pioglitazone. It makes sense, however, to exercise caution and follow the recommendations of the Food and Drug Administration in patients with a high risk for bladder cancer.
Dr. Yehuda Handelsman is medical director and principal investigator, Metabolic Institute of America, Tarzana, Calif., and immediate past president, American College of Endocrinology.
The results of such a large study are troubling Although it represents a retroactive observational assessment of the risk of bladder cancer from pioglitazone rather than a prospective randomized trial, the large number of patients gives this study strength. Yet, it is at odds, as the authors themselves point out, with other published studies like the Kaiser Permanente Northern California study. That study used the same cohort with follow-up extended to 10 years, and the use of pioglitazone was no longer significantly associated with an increased risk of bladder cancer. It is important to note that, although the authors show an increased risk for bladder cancer, the numbers are very small. I wish the authors would balance the risk in their study with potential benefit in the same cohort. They did cite the recently published Insulin Resistance Intervention after Stroke (IRIS) trial, which randomized 3,895 people. After a similar median follow-up of 4.8 years (4.7 years in the United Kingdom study), pioglitazone was associated with a decreased risk of a composite endpoint of stroke or myocardial infarction. In the IRIS study, there was an increased number of bladder cancer events in the pioglitazone group: 12 vs. 8 in the placebo group. In IRIS we see a clear benefit-risk ratio. By prescribing pioglitazone, there is significant cardiovascular risk improvement, while the number of cancer cases was quite small. In summary, continued prescribing of pioglitazone seems reasonable, and although the controversy has not been resolved, it seems that benefit-risk ratio favors prescribing pioglitazone. It makes sense, however, to exercise caution and follow the recommendations of the Food and Drug Administration in patients with a high risk for bladder cancer.
Dr. Yehuda Handelsman is medical director and principal investigator, Metabolic Institute of America, Tarzana, Calif., and immediate past president, American College of Endocrinology.
Pioglitazone was linked to a 63% increase in bladder cancer risk, compared with other noninsulin antidiabetic drugs in a large population-based cohort study.
Furthermore, incidence rose the longer patients took pioglitazone and the higher their dose, according to Dr. Marco Tuccori at Jewish General Hospital in Montreal, and his associates. “Rosiglitazone was not associated with an increased risk of bladder cancer in any analysis, suggesting the risk is drug-specific and not a class effect,” they wrote online March 30 in BMJ.
Both pioglitazone and rosiglitazone are thiazolidinediones that help treat insulin resistance in patients with type 2 diabetes. In 2005, Proactive trial investigators reported that patients were more likely to develop bladder cancer on pioglitazone, compared with placebo. But later observational studies reported mixed associations between pioglitazone and bladder cancer, perhaps because of biases and other methodological shortcomings, the researchers said (BMJ. 2016. doi: 10.1136/bmj.i1541).
Dr. Tuccori and his colleagues studied 145,806 patients with newly diagnosed type 2 diabetes, defined as a first-ever prescription for thiazolidinediones, metformin, sulfonylureas, prandial glucose regulators, acarbose, dipeptidyl peptidase-4 inhibitors, glucagonlike peptide agonists, or sodium-glucose cotransporter-2 inhibitors. The investigators excluded patients with any history of bladder cancer or less than a year of follow-up, and set a lag period of 1 year to help control for bladder cancer cases that were undiagnosed at study entry. The study included data for 2000-2013 from a national primary care registry that correlated well with the national cancer registry, the investigators said.
In all, 622 patients were diagnosed with bladder cancer (90 cases per 100,000 person-years), with a median of 4.4 years between study entry and cancer diagnosis (interquartile range, 2.5-6.5 years). Pioglitazone was associated with 121 cases of bladder cancer for every 100,000 person-years, compared with only 89 cases per 100,000 person-years for other antidiabetic drugs (hazard ratio, 1.6; 95% confidence interval, 1.2-2.2). Patients were no more likely to develop bladder cancer on rosiglitazone than other antidiabetic drugs (86.2 and 88.9 cases per 100,000 person-years of exposure, respectively; HR, 1.1; 95% CI, 0.8-1.5). Furthermore, pioglitazone was linked to nearly a 50% greater risk of bladder cancer, compared with rosiglitazone in a head-to-head comparison.
Not only did the link between pioglitazone and bladder cancer hold up in nine sensitivity analyses, but the hazard ratio increased to 1.73 when the researchers increased the required lag period to 2 years. Pharmacologic differences between pioglitazone and rosiglitazone might explain their distinct risk profiles in terms of bladder cancer, the investigators noted. Rosiglitazone is selective for peroxisome proliferator-activated receptor (PPAR) gamma, while pioglitazone has dual PPAR alpha/gamma activity. Only the latter increased expression of carcinogenic bladder biomarkers in rat models, they noted. This mechanism needs more exploration, they added.
The Canadian Institutes of Health Research funded the study. The researchers had no disclosures.
Pioglitazone was linked to a 63% increase in bladder cancer risk, compared with other noninsulin antidiabetic drugs in a large population-based cohort study.
Furthermore, incidence rose the longer patients took pioglitazone and the higher their dose, according to Dr. Marco Tuccori at Jewish General Hospital in Montreal, and his associates. “Rosiglitazone was not associated with an increased risk of bladder cancer in any analysis, suggesting the risk is drug-specific and not a class effect,” they wrote online March 30 in BMJ.
Both pioglitazone and rosiglitazone are thiazolidinediones that help treat insulin resistance in patients with type 2 diabetes. In 2005, Proactive trial investigators reported that patients were more likely to develop bladder cancer on pioglitazone, compared with placebo. But later observational studies reported mixed associations between pioglitazone and bladder cancer, perhaps because of biases and other methodological shortcomings, the researchers said (BMJ. 2016. doi: 10.1136/bmj.i1541).
Dr. Tuccori and his colleagues studied 145,806 patients with newly diagnosed type 2 diabetes, defined as a first-ever prescription for thiazolidinediones, metformin, sulfonylureas, prandial glucose regulators, acarbose, dipeptidyl peptidase-4 inhibitors, glucagonlike peptide agonists, or sodium-glucose cotransporter-2 inhibitors. The investigators excluded patients with any history of bladder cancer or less than a year of follow-up, and set a lag period of 1 year to help control for bladder cancer cases that were undiagnosed at study entry. The study included data for 2000-2013 from a national primary care registry that correlated well with the national cancer registry, the investigators said.
In all, 622 patients were diagnosed with bladder cancer (90 cases per 100,000 person-years), with a median of 4.4 years between study entry and cancer diagnosis (interquartile range, 2.5-6.5 years). Pioglitazone was associated with 121 cases of bladder cancer for every 100,000 person-years, compared with only 89 cases per 100,000 person-years for other antidiabetic drugs (hazard ratio, 1.6; 95% confidence interval, 1.2-2.2). Patients were no more likely to develop bladder cancer on rosiglitazone than other antidiabetic drugs (86.2 and 88.9 cases per 100,000 person-years of exposure, respectively; HR, 1.1; 95% CI, 0.8-1.5). Furthermore, pioglitazone was linked to nearly a 50% greater risk of bladder cancer, compared with rosiglitazone in a head-to-head comparison.
Not only did the link between pioglitazone and bladder cancer hold up in nine sensitivity analyses, but the hazard ratio increased to 1.73 when the researchers increased the required lag period to 2 years. Pharmacologic differences between pioglitazone and rosiglitazone might explain their distinct risk profiles in terms of bladder cancer, the investigators noted. Rosiglitazone is selective for peroxisome proliferator-activated receptor (PPAR) gamma, while pioglitazone has dual PPAR alpha/gamma activity. Only the latter increased expression of carcinogenic bladder biomarkers in rat models, they noted. This mechanism needs more exploration, they added.
The Canadian Institutes of Health Research funded the study. The researchers had no disclosures.
FROM BMJ
Key clinical point: Pioglitazone exposure increased the risk of bladder cancer by 63%, compared with other antidiabetic drugs in a large observational study.
Major finding: Pioglitazone was associated with 121 cases of bladder cancer for every 100,000 person-years, compared with 89 cases for every 100,000 person-years for other drugs (HR, 1.63; 95% CI, 1.22-2.19).
Data source: A population-based cohort study of 145,806 patients newly treated with antidiabetic drugs between 2000 and 2013.
Disclosures: The Canadian Institutes of Health Research funded the study. The researchers had no disclosures.
Incretin-based diabetes drugs don’t raise heart failure risk
Incretin-based antidiabetic drugs didn’t raise the risk of hospitalization for heart failure in an international observational study involving 1.5 million patients reported online March 24 in the New England Journal of Medicine.
The safety of dipeptidyl peptidase 4 (DPP-4) inhibitors such as sitagliptin, saxagliptin, and linagliptin, and of glucagon-like peptide–1 (GLP-1) analogues such as exenatide and liraglutide is controversial. Some clinical trials have reported these agents raise the risk of heart failure (HF) while others have found no increase in risk, but all of the studies are underpowered to settle the question, said Kristian B. Filion, Ph.D., of McGill University and the Center for Clinical Epidemiology, Lady Davis Research Institute, Jewish General Hospital, and his associates.
They examined this issue by analyzing data from several large cohorts of diabetes patients treated in routine clinical practice in the United States, Canada, and England. Their study population comprised 1,499,650 adults who began taking noninsulin antidiabetic drugs at or after the date that incretin-based agents entered the market. “With 3.2 million person-years of observations, we had the statistical power to robustly assess this important drug safety issue,” the investigators said.
Patients taking DPP-4 inhibitors and GLP-1 analogues were compared with those taking non–incretin-based drugs such as biguanides, sulfonylureas, thiazolidinediones, alpha-glucosidase inhibitors, meglitinides, and sodium-glucose cotransporter-2 inhibitors. A total of 29,741 patients were hospitalized for HF, for an overall rate of 9.2 events per 1,000 person-years.
Incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs (hazard ratio, 0.82) among the roughly 1.4 million patients who had no history of HF at baseline. Individually, neither DPP-4 inhibitors (HR, 0.84) nor GLP-1 analogues (HR, 0.95) were associated with an increased risk of hospitalization for HF. These findings remained consistent through several subgroup and sensitivity analyses that categorized the data according to duration of exposure, presence or absence of a history of MI, and duration of diabetes, Dr. Filion and his associates said (N Engl J Med. 2016 Mar 24. doi: 10.1056/NEJMoa1506115).
Similarly, incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs among the approximately 80,000 patients who had a history of HF at baseline (HR, 0.86).
This study was supported by the Canadian Institutes of Health Research and the Quebec Foundation for Health Research. Dr. Filion reported having no relevant financial disclosures; some of his associates reported ties to numerous industry sources.
Incretin-based antidiabetic drugs didn’t raise the risk of hospitalization for heart failure in an international observational study involving 1.5 million patients reported online March 24 in the New England Journal of Medicine.
The safety of dipeptidyl peptidase 4 (DPP-4) inhibitors such as sitagliptin, saxagliptin, and linagliptin, and of glucagon-like peptide–1 (GLP-1) analogues such as exenatide and liraglutide is controversial. Some clinical trials have reported these agents raise the risk of heart failure (HF) while others have found no increase in risk, but all of the studies are underpowered to settle the question, said Kristian B. Filion, Ph.D., of McGill University and the Center for Clinical Epidemiology, Lady Davis Research Institute, Jewish General Hospital, and his associates.
They examined this issue by analyzing data from several large cohorts of diabetes patients treated in routine clinical practice in the United States, Canada, and England. Their study population comprised 1,499,650 adults who began taking noninsulin antidiabetic drugs at or after the date that incretin-based agents entered the market. “With 3.2 million person-years of observations, we had the statistical power to robustly assess this important drug safety issue,” the investigators said.
Patients taking DPP-4 inhibitors and GLP-1 analogues were compared with those taking non–incretin-based drugs such as biguanides, sulfonylureas, thiazolidinediones, alpha-glucosidase inhibitors, meglitinides, and sodium-glucose cotransporter-2 inhibitors. A total of 29,741 patients were hospitalized for HF, for an overall rate of 9.2 events per 1,000 person-years.
Incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs (hazard ratio, 0.82) among the roughly 1.4 million patients who had no history of HF at baseline. Individually, neither DPP-4 inhibitors (HR, 0.84) nor GLP-1 analogues (HR, 0.95) were associated with an increased risk of hospitalization for HF. These findings remained consistent through several subgroup and sensitivity analyses that categorized the data according to duration of exposure, presence or absence of a history of MI, and duration of diabetes, Dr. Filion and his associates said (N Engl J Med. 2016 Mar 24. doi: 10.1056/NEJMoa1506115).
Similarly, incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs among the approximately 80,000 patients who had a history of HF at baseline (HR, 0.86).
This study was supported by the Canadian Institutes of Health Research and the Quebec Foundation for Health Research. Dr. Filion reported having no relevant financial disclosures; some of his associates reported ties to numerous industry sources.
Incretin-based antidiabetic drugs didn’t raise the risk of hospitalization for heart failure in an international observational study involving 1.5 million patients reported online March 24 in the New England Journal of Medicine.
The safety of dipeptidyl peptidase 4 (DPP-4) inhibitors such as sitagliptin, saxagliptin, and linagliptin, and of glucagon-like peptide–1 (GLP-1) analogues such as exenatide and liraglutide is controversial. Some clinical trials have reported these agents raise the risk of heart failure (HF) while others have found no increase in risk, but all of the studies are underpowered to settle the question, said Kristian B. Filion, Ph.D., of McGill University and the Center for Clinical Epidemiology, Lady Davis Research Institute, Jewish General Hospital, and his associates.
They examined this issue by analyzing data from several large cohorts of diabetes patients treated in routine clinical practice in the United States, Canada, and England. Their study population comprised 1,499,650 adults who began taking noninsulin antidiabetic drugs at or after the date that incretin-based agents entered the market. “With 3.2 million person-years of observations, we had the statistical power to robustly assess this important drug safety issue,” the investigators said.
Patients taking DPP-4 inhibitors and GLP-1 analogues were compared with those taking non–incretin-based drugs such as biguanides, sulfonylureas, thiazolidinediones, alpha-glucosidase inhibitors, meglitinides, and sodium-glucose cotransporter-2 inhibitors. A total of 29,741 patients were hospitalized for HF, for an overall rate of 9.2 events per 1,000 person-years.
Incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs (hazard ratio, 0.82) among the roughly 1.4 million patients who had no history of HF at baseline. Individually, neither DPP-4 inhibitors (HR, 0.84) nor GLP-1 analogues (HR, 0.95) were associated with an increased risk of hospitalization for HF. These findings remained consistent through several subgroup and sensitivity analyses that categorized the data according to duration of exposure, presence or absence of a history of MI, and duration of diabetes, Dr. Filion and his associates said (N Engl J Med. 2016 Mar 24. doi: 10.1056/NEJMoa1506115).
Similarly, incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs among the approximately 80,000 patients who had a history of HF at baseline (HR, 0.86).
This study was supported by the Canadian Institutes of Health Research and the Quebec Foundation for Health Research. Dr. Filion reported having no relevant financial disclosures; some of his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Incretin-based antidiabetic drugs did not raise the risk of hospitalization for heart failure in a large international observational study.
Major finding: Neither DPP-4 inhibitors (HR, 0.84) nor GLP-1 analogues (HR, 0.95) were associated with an increased risk of hospitalization for HF, compared with non–incretin-based antidiabetic drugs.
Data source: A retrospective international observational cohort study involving roughly 1.5 million diabetes patients, of whom 29,741 were hospitalized for HF.
Disclosures: This study was supported by the Canadian Institutes of Health Research and the Quebec Foundation for Health Research. Dr. Filion reported having no relevant financial disclosures; some of his associates reported ties to numerous industry sources.
VIDEO: Lifestyle intervention blocks antipsychotic-associated weight gain
MADRID – The weight gain most patients have when first starting treatment with antipsychotic medications is not inevitable.
A 12-week intervention program designed to promote exercise and a healthy diet largely blunted a big weight gain by 16 adolescents and young adults starting treatment after an initial diagnosis of psychosis in a controlled study at a single Australian center. Later follow-up further showed that a majority of participants in the program remained mostly free of excess weight 2 years after the life-style intervention, Dr. Philip B. Ward said at the meeting sponsored by the European Psychiatric Association.
Implementing this type of intervention is very important because antipsychotic-induced weight gain launches young psychiatric patients into a middle age that often includes metabolic syndrome, type 2 diabetes, and a significantly increased risk for cardiovascular disease events, said Dr. Ward, a psychiatrist at the University of New South Wales in Sydney.
He reported results from a pilot program, Keeping the Body in Mind, run at one of the university’s community clinics that gave newly-diagnosed psychiatric patients aged 15-25 years regular instruction in diet, food shopping, and cooking and in an exercise class that included individualized coaching over the course of 12 weeks. At the end of the program, average weight gain relative to baseline weight was 1.8 kg among 16 patients in the intervention group and 7.8 kg among 12 patients who received standard care at a different community clinic. (Early Interv Psychiatry. 2015. doi: 10.1111/eip.12230).
Expressed another way, 2 of the 16 participating patients (13%) had a clinically significant (at least 7%) weight gain during the 12-week program, compared with 9 of the 12 (75%) of patients with a significant weight gain in the control group.
To assess the durability of this effect, Dr. Ward and his associates did follow-up 2 years later on 12 of the participants, who showed an average 1.3-kg weight gain after 2 years, compared with their weight at the time they entered the program, Dr Ward reported. Based on this success, the university’s psychiatric clinics are now expanding the program to make it available to all patients starting treatment on antipsychotic medications, about 80 patients a year, Dr. Ward said in an interview.
Although Dr. Ward stressed the importance of lifestyle intervention, he noted that the antipsychotic drug selected for treatment can also affect the magnitude of acute weight gain. Two drugs that seem to pose some of the lowest weight-gain risks are aripiprazole and ziprasidone.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
MADRID – The weight gain most patients have when first starting treatment with antipsychotic medications is not inevitable.
A 12-week intervention program designed to promote exercise and a healthy diet largely blunted a big weight gain by 16 adolescents and young adults starting treatment after an initial diagnosis of psychosis in a controlled study at a single Australian center. Later follow-up further showed that a majority of participants in the program remained mostly free of excess weight 2 years after the life-style intervention, Dr. Philip B. Ward said at the meeting sponsored by the European Psychiatric Association.
Implementing this type of intervention is very important because antipsychotic-induced weight gain launches young psychiatric patients into a middle age that often includes metabolic syndrome, type 2 diabetes, and a significantly increased risk for cardiovascular disease events, said Dr. Ward, a psychiatrist at the University of New South Wales in Sydney.
He reported results from a pilot program, Keeping the Body in Mind, run at one of the university’s community clinics that gave newly-diagnosed psychiatric patients aged 15-25 years regular instruction in diet, food shopping, and cooking and in an exercise class that included individualized coaching over the course of 12 weeks. At the end of the program, average weight gain relative to baseline weight was 1.8 kg among 16 patients in the intervention group and 7.8 kg among 12 patients who received standard care at a different community clinic. (Early Interv Psychiatry. 2015. doi: 10.1111/eip.12230).
Expressed another way, 2 of the 16 participating patients (13%) had a clinically significant (at least 7%) weight gain during the 12-week program, compared with 9 of the 12 (75%) of patients with a significant weight gain in the control group.
To assess the durability of this effect, Dr. Ward and his associates did follow-up 2 years later on 12 of the participants, who showed an average 1.3-kg weight gain after 2 years, compared with their weight at the time they entered the program, Dr Ward reported. Based on this success, the university’s psychiatric clinics are now expanding the program to make it available to all patients starting treatment on antipsychotic medications, about 80 patients a year, Dr. Ward said in an interview.
Although Dr. Ward stressed the importance of lifestyle intervention, he noted that the antipsychotic drug selected for treatment can also affect the magnitude of acute weight gain. Two drugs that seem to pose some of the lowest weight-gain risks are aripiprazole and ziprasidone.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
MADRID – The weight gain most patients have when first starting treatment with antipsychotic medications is not inevitable.
A 12-week intervention program designed to promote exercise and a healthy diet largely blunted a big weight gain by 16 adolescents and young adults starting treatment after an initial diagnosis of psychosis in a controlled study at a single Australian center. Later follow-up further showed that a majority of participants in the program remained mostly free of excess weight 2 years after the life-style intervention, Dr. Philip B. Ward said at the meeting sponsored by the European Psychiatric Association.
Implementing this type of intervention is very important because antipsychotic-induced weight gain launches young psychiatric patients into a middle age that often includes metabolic syndrome, type 2 diabetes, and a significantly increased risk for cardiovascular disease events, said Dr. Ward, a psychiatrist at the University of New South Wales in Sydney.
He reported results from a pilot program, Keeping the Body in Mind, run at one of the university’s community clinics that gave newly-diagnosed psychiatric patients aged 15-25 years regular instruction in diet, food shopping, and cooking and in an exercise class that included individualized coaching over the course of 12 weeks. At the end of the program, average weight gain relative to baseline weight was 1.8 kg among 16 patients in the intervention group and 7.8 kg among 12 patients who received standard care at a different community clinic. (Early Interv Psychiatry. 2015. doi: 10.1111/eip.12230).
Expressed another way, 2 of the 16 participating patients (13%) had a clinically significant (at least 7%) weight gain during the 12-week program, compared with 9 of the 12 (75%) of patients with a significant weight gain in the control group.
To assess the durability of this effect, Dr. Ward and his associates did follow-up 2 years later on 12 of the participants, who showed an average 1.3-kg weight gain after 2 years, compared with their weight at the time they entered the program, Dr Ward reported. Based on this success, the university’s psychiatric clinics are now expanding the program to make it available to all patients starting treatment on antipsychotic medications, about 80 patients a year, Dr. Ward said in an interview.
Although Dr. Ward stressed the importance of lifestyle intervention, he noted that the antipsychotic drug selected for treatment can also affect the magnitude of acute weight gain. Two drugs that seem to pose some of the lowest weight-gain risks are aripiprazole and ziprasidone.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT THE EUROPEAN CONGRESS OF PSYCHIATRY
Key clinical point: Most adolescents and young adults who participated in a lifestyle intervention when they began receiving an antipsychotic medication avoided acute weight gain.
Major finding: At 2-year follow-up, the average weight gain by program participants was 1.3 kg above baseline weight.
Data source: Sixteen adolescent and young-adult patients newly diagnosed with psychosis treated at one Australian center.
Disclosures: Dr. Ward had no disclosures.
Diabetes hospitalizations down, but disparities persist
The overall adult hospitalization rate for uncontrolled diabetes without complications dropped by 38% from 2001 to 2012, but considerable racial and ethnic disparities remained, according to the Agency for Healthcare Research and Quality (AHRQ).
That 38% represents a decrease from 27.9 per 100,000 U.S. adults in 2001 to 17.3 per 100,000 in 2012. The admission rate among blacks dropped by a slightly higher 40% over that period, but it started at a much-higher 88.3 per 100,000 adults before going down to 53.1 per 100,000 – still three times higher than the overall rate. The rate for Hispanics fell 42%, but it also was much higher than the overall rate to begin with: 46 per 100,000. The 2012 rate for Hispanic adults was 26.7 per 100,000, about 54% higher than the overall admission rate, the AHRQ reported.
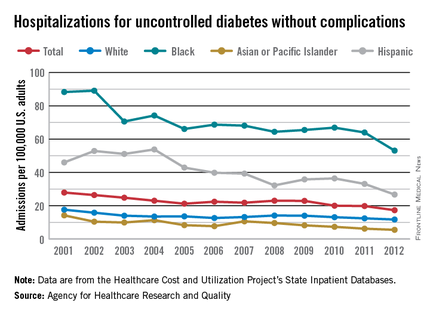
Much closer to reaching the 2008 achievable benchmark of 5 admissions per 100,000 adults were Asians or Pacific Islanders, who went from a rate of 14.2 per 100,000 to 5.5 per 100,000, and whites, who went from 17.6 per 100,000 to 11.7 between 2001 and 2012, according to data from the AHRQ Healthcare Cost and Utilization Project’s State Inpatient Databases.
The report noted that, “at the current rate, the [2008 achievable] benchmark could not be met for the total population for approximately 17 years.” Asians or Pacific Islanders could meet that goal in 2 years, the AHRQ added, compared with 10 years for Hispanics, 20 years for blacks, and 22 years for whites.
The overall adult hospitalization rate for uncontrolled diabetes without complications dropped by 38% from 2001 to 2012, but considerable racial and ethnic disparities remained, according to the Agency for Healthcare Research and Quality (AHRQ).
That 38% represents a decrease from 27.9 per 100,000 U.S. adults in 2001 to 17.3 per 100,000 in 2012. The admission rate among blacks dropped by a slightly higher 40% over that period, but it started at a much-higher 88.3 per 100,000 adults before going down to 53.1 per 100,000 – still three times higher than the overall rate. The rate for Hispanics fell 42%, but it also was much higher than the overall rate to begin with: 46 per 100,000. The 2012 rate for Hispanic adults was 26.7 per 100,000, about 54% higher than the overall admission rate, the AHRQ reported.

Much closer to reaching the 2008 achievable benchmark of 5 admissions per 100,000 adults were Asians or Pacific Islanders, who went from a rate of 14.2 per 100,000 to 5.5 per 100,000, and whites, who went from 17.6 per 100,000 to 11.7 between 2001 and 2012, according to data from the AHRQ Healthcare Cost and Utilization Project’s State Inpatient Databases.
The report noted that, “at the current rate, the [2008 achievable] benchmark could not be met for the total population for approximately 17 years.” Asians or Pacific Islanders could meet that goal in 2 years, the AHRQ added, compared with 10 years for Hispanics, 20 years for blacks, and 22 years for whites.
The overall adult hospitalization rate for uncontrolled diabetes without complications dropped by 38% from 2001 to 2012, but considerable racial and ethnic disparities remained, according to the Agency for Healthcare Research and Quality (AHRQ).
That 38% represents a decrease from 27.9 per 100,000 U.S. adults in 2001 to 17.3 per 100,000 in 2012. The admission rate among blacks dropped by a slightly higher 40% over that period, but it started at a much-higher 88.3 per 100,000 adults before going down to 53.1 per 100,000 – still three times higher than the overall rate. The rate for Hispanics fell 42%, but it also was much higher than the overall rate to begin with: 46 per 100,000. The 2012 rate for Hispanic adults was 26.7 per 100,000, about 54% higher than the overall admission rate, the AHRQ reported.

Much closer to reaching the 2008 achievable benchmark of 5 admissions per 100,000 adults were Asians or Pacific Islanders, who went from a rate of 14.2 per 100,000 to 5.5 per 100,000, and whites, who went from 17.6 per 100,000 to 11.7 between 2001 and 2012, according to data from the AHRQ Healthcare Cost and Utilization Project’s State Inpatient Databases.
The report noted that, “at the current rate, the [2008 achievable] benchmark could not be met for the total population for approximately 17 years.” Asians or Pacific Islanders could meet that goal in 2 years, the AHRQ added, compared with 10 years for Hispanics, 20 years for blacks, and 22 years for whites.
Low BMI, high body fat both raise mortality risk
Both a low body mass index and a high percentage of body fat are independently associated with an increased risk of all-cause mortality in middle-aged and older adults, according to an observational study reported online in the Annals of Internal Medicine.
The finding that these somewhat contradictory measures are both associated with higher mortality helps account for the so-called obesity paradox – the fact that mortality is lower in overweight, mildly obese, and moderately obese persons than in those who are underweight or of low-normal weight, said Dr. Raj Padwal of the University of Alberta, Edmonton, and his associates.
The investigators assessed the relationships among body mass index, body fat, and mortality by analyzing data from a population-based cohort study of 54,420 men and women aged 40 and older. These study participants underwent assessment of body composition as part of a study of bone mineral density, then were followed for a median of 4-7 years. BMI and body fat percentage were categorized into quintiles; men and women were analyzed separately.
Among women (mean age, 63.5 years), 2% were underweight, 38% were of normal weight, 34% were overweight, 17% had class I obesity, and 8% had class II or III obesity. The mean percentage of body fat was 32%. There were 4,965 deaths during follow-up. Among men (mean age, 65.5 years), 1% were underweight, 29% were of normal weight, 45% were overweight, 18% had class I obesity, and 6% had class II or III obesity. The mean percentage of body fat was 30%. There were 984 deaths during follow-up.
Higher BMI correlated with decreased mortality in both sexes. Among women, death rates declined from 18.6/1,000 person-years in the lowest BMI quintile to 13.9/1,000 person-years in the highest BMI quintile. Among men, death rates declined from 51.5/1,000 person-years in the lowest BMI quintile to 32.7/1,000 person-years in the highest BMI quintile.
In contrast, higher body fat percentage correlated with increased mortality. Among women, a high percentage of body fat was associated with significantly higher mortality (hazard ratio, 1.19), as it was among men (HR, 1.59). “Our results suggest that BMI may be an inappropriate surrogate for adiposity, and this limitation may explain the presence of the obesity paradox in many studies,” Dr. Padwal and his associates reported (Ann Intern Med. 2016 March 8. doi: 10.7326/M15-1181).
This study was limited in that the study population was predominantly female and white, and it may have included more “health-seeking” and lower-weight individuals than there are in the general population, the investigators added.
Both a low body mass index and a high percentage of body fat are independently associated with an increased risk of all-cause mortality in middle-aged and older adults, according to an observational study reported online in the Annals of Internal Medicine.
The finding that these somewhat contradictory measures are both associated with higher mortality helps account for the so-called obesity paradox – the fact that mortality is lower in overweight, mildly obese, and moderately obese persons than in those who are underweight or of low-normal weight, said Dr. Raj Padwal of the University of Alberta, Edmonton, and his associates.
The investigators assessed the relationships among body mass index, body fat, and mortality by analyzing data from a population-based cohort study of 54,420 men and women aged 40 and older. These study participants underwent assessment of body composition as part of a study of bone mineral density, then were followed for a median of 4-7 years. BMI and body fat percentage were categorized into quintiles; men and women were analyzed separately.
Among women (mean age, 63.5 years), 2% were underweight, 38% were of normal weight, 34% were overweight, 17% had class I obesity, and 8% had class II or III obesity. The mean percentage of body fat was 32%. There were 4,965 deaths during follow-up. Among men (mean age, 65.5 years), 1% were underweight, 29% were of normal weight, 45% were overweight, 18% had class I obesity, and 6% had class II or III obesity. The mean percentage of body fat was 30%. There were 984 deaths during follow-up.
Higher BMI correlated with decreased mortality in both sexes. Among women, death rates declined from 18.6/1,000 person-years in the lowest BMI quintile to 13.9/1,000 person-years in the highest BMI quintile. Among men, death rates declined from 51.5/1,000 person-years in the lowest BMI quintile to 32.7/1,000 person-years in the highest BMI quintile.
In contrast, higher body fat percentage correlated with increased mortality. Among women, a high percentage of body fat was associated with significantly higher mortality (hazard ratio, 1.19), as it was among men (HR, 1.59). “Our results suggest that BMI may be an inappropriate surrogate for adiposity, and this limitation may explain the presence of the obesity paradox in many studies,” Dr. Padwal and his associates reported (Ann Intern Med. 2016 March 8. doi: 10.7326/M15-1181).
This study was limited in that the study population was predominantly female and white, and it may have included more “health-seeking” and lower-weight individuals than there are in the general population, the investigators added.
Both a low body mass index and a high percentage of body fat are independently associated with an increased risk of all-cause mortality in middle-aged and older adults, according to an observational study reported online in the Annals of Internal Medicine.
The finding that these somewhat contradictory measures are both associated with higher mortality helps account for the so-called obesity paradox – the fact that mortality is lower in overweight, mildly obese, and moderately obese persons than in those who are underweight or of low-normal weight, said Dr. Raj Padwal of the University of Alberta, Edmonton, and his associates.
The investigators assessed the relationships among body mass index, body fat, and mortality by analyzing data from a population-based cohort study of 54,420 men and women aged 40 and older. These study participants underwent assessment of body composition as part of a study of bone mineral density, then were followed for a median of 4-7 years. BMI and body fat percentage were categorized into quintiles; men and women were analyzed separately.
Among women (mean age, 63.5 years), 2% were underweight, 38% were of normal weight, 34% were overweight, 17% had class I obesity, and 8% had class II or III obesity. The mean percentage of body fat was 32%. There were 4,965 deaths during follow-up. Among men (mean age, 65.5 years), 1% were underweight, 29% were of normal weight, 45% were overweight, 18% had class I obesity, and 6% had class II or III obesity. The mean percentage of body fat was 30%. There were 984 deaths during follow-up.
Higher BMI correlated with decreased mortality in both sexes. Among women, death rates declined from 18.6/1,000 person-years in the lowest BMI quintile to 13.9/1,000 person-years in the highest BMI quintile. Among men, death rates declined from 51.5/1,000 person-years in the lowest BMI quintile to 32.7/1,000 person-years in the highest BMI quintile.
In contrast, higher body fat percentage correlated with increased mortality. Among women, a high percentage of body fat was associated with significantly higher mortality (hazard ratio, 1.19), as it was among men (HR, 1.59). “Our results suggest that BMI may be an inappropriate surrogate for adiposity, and this limitation may explain the presence of the obesity paradox in many studies,” Dr. Padwal and his associates reported (Ann Intern Med. 2016 March 8. doi: 10.7326/M15-1181).
This study was limited in that the study population was predominantly female and white, and it may have included more “health-seeking” and lower-weight individuals than there are in the general population, the investigators added.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Both a low BMI and a high percentage of body fat are independently associated with an increased risk of all-cause mortality in middle-aged and older adults.
Major finding: Among women, a high percentage of body fat was associated with significantly higher mortality (HR, 1.19), as it was among men (HR, 1.59).
Data source: A secondary analysis of data from a population-based cohort study involving 54,420 Canadian men and women followed for a median of 4-7 years.
Disclosures: No sponsor or funding source was identified for this study. Dr. Padwal reported having no relevant financial disclosures; one of his associates reported ties to Genzyme, Amgen, Eli Lilly, and Novartis.