User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
No LIGHT shed on CV safety of naltrexone-bupropion
The cardiovascular risks of the weight-loss combination drug naltrexone-bupropion are still uncertain, because the FDA-mandated safety trial, LIGHT, was terminated prematurely due to the sponsoring drug company’s breach of confidentiality. Other irregularities also were discovered, according to a report published online March 8 in JAMA.
Orexigen violated its agreement with FDA not to disclose the findings of an interim analysis performed after only 25% of expected CV events accrued. These findings appeared highly favorable for the drug, prompting the sponsor to publicly, and prematurely, claim naltrexone-bupropion reduced CV risk by 41% independently of its weight-loss effect. This favorable outcome was later found to be “driven almost exclusively by a 12-to-1 imbalance in death favoring naltrexone-bupropion among patients who were no longer taking the study drug,” said Dr. Steven E. Nissen of the Cleveland Clinic Center for Cardiovascular Research and his associates.
They now report the results of a second interim analysis of LIGHT (Cardiovascular Outcomes Study of Naltrexone SR/Bupropion SR in Overweight and Obese Subjects With Cardiovascular Risk Factors) performed after 50% of expected CV events accrued, as well as those of a final analysis performed when the trial was halted and 64% of expected CV events accrued. These findings are much less favorable, showing no significant difference between naltrexone-bupropion and placebo in the rate of major adverse cardiovascular events (MACE). In addition, extremely poor adherence to both active treatment and placebo over time, together with the complex statistical issues involved, call into question any interpretation of the results and preclude “any reliable conclusions about the long-term safety of naltrexone-bupropion,” the investigators said.
“The cardiovascular safety of this treatment remains uncertain and will require evaluation in a new, adequately powered outcome trial.” The results of such a study, should it be undertaken, wouldn’t be available for at least 3-4 years, they noted.
In their report, the investigators described in detail why the FDA required a CV safety study even though the agency had already approved naltrexone-bupropion as a weight-loss treatment.
The study was a phase IIIb randomized, double-blind, placebo-controlled trial involving 8,910 overweight/obese patients aged 45 or older (mean age, 61 years) who were at risk of adverse CV outcomes due to preexisting CV disease; exercise-induced angina; an unfavorable ankle/brachial index; stenosis of coronary, carotid, or lower extremity arteries; type 2 diabetes; hypertension; dyslipidemia; or smoking. These participants received daily tablets of either 32 mg naltrexone plus 360 mg bupropion or a matching placebo and were to be followed for 4 years at 266 U.S. medical centers.
The analysis performed after 50% of expected CV events accrued showed that MACE – the primary safety outcome – occurred in 2.0% of the naltrexone-bupropion group and 2.3% of the placebo group, a nonsignificant difference. Differences between the two study groups also were nonsignificant for the individual components of the primary outcome, including cardiovascular death (0.4% vs. 0.8%), nonfatal stroke (0.5% vs. 0.4%), and nonfatal MI (1.2% vs. 1.2%). These results were confirmed in a sensitivity analysis based on the final data collection, when 64% of expected CV events accrued: MACE occurred in 2.7% of the naltrexone-bupropion group and 2.8% of the placebo group, another nonsignificant difference.
The active treatment also was no better than placebo regarding all secondary outcomes, including all-cause mortality, CV hospitalization, and coronary revascularization, Dr. Nissen and his associates said (JAMA. 2016 Mar 8. doi: 10.1001/jama.2016.1558). Treatment adherence was low. At 16 weeks, only 64% of the naltrexone-bupropion group and 72% of the placebo group were still taking the study treatment. By 2 years, only 27% of the naltrexone-bupropion group and 17% of the placebo group were. The mean duration of treatment was only 18.4 weeks for naltrexone-bupropion and 16.3 weeks for placebo. Most treatment discontinuations were due to a failure to lose weight, but a substantial proportion occurred because of treatment-related increases in blood pressure or heart rate.
The active treatment’s ability to reduce body weight was deemed “modest.” When the trial was halted, the mean decrease in weight was 3.9 kg with naltrexone-bupropion, representing a 3.6% reduction in total weight, and was 1.2 kg with placebo, representing a 1.1% reduction.
Adverse effects developed in 28.1% of patients taking naltrexone-bupropion, which was a significantly greater proportion than the 8.7% rate in the placebo group. The most common adverse events leading to discontinuation of the study drug affected the gastrointestinal system (nausea, constipation, vomiting), the CNS (tremor, dizziness, headache), and mental/emotional problems (insomnia, anxiety, hallucinations, depression).
The sponsor of this trial, Orexigen Therapeutics, disregarded the multiple harms caused by breaches of confidentiality, treated the academic investigators unfairly, ignored the trial’s data monitoring committee, and defied the FDA. Their statements about the naltrexone-bupropion’s cardiovascular efficacy were highly misleading, and there were obvious signs that the supporting data were not reliable. For example, nearly all of the CV mortality in the first interim analysis occurred well after participants had stopped taking their medication.
Yet the drug company was not sanctioned, and the naltrexone-bupropion retains FDA approval. At a minimum, the FDA should withhold approval until a viable replacement study is conducted, or it should require Risk Evaluation and Mitigation Strategies to counter the misinformation disseminated by the study sponsor, or it should restrict use of the drug outright.
Clinicians should be aware of these research improprieties and should know that the purported weight-loss benefit of naltrexone-bupropion is only 2.7 kg more than that achieved with placebo. How does this modest benefit balance against an unknown cardiovascular risk? In addition, the drug’s lack of efficacy clearly contributed to the very poor adherence rate in this trial: At 1 year, only 37.5% were still taking naltrexone-bupropion and 26.3% were still taking placebo.
Dr. Joshua M. Sharfstein is in the department of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore. Dr. Bruce M. Psaty is in the Cardiovascular Health Research Unit and the departments of medicine, epidemiology, and health services at the University of Washington, Seattle. They reported that this work was supported by the National Heart, Lung, and Blood Institute. Dr. Sharfstein reported serving as principal deputy commissioner of the FDA in 2009-2011. Dr. Psaty reported serving on the data monitoring committee of a clinical trial funded by Zoll LifeCor, on the steering committee of the Yale Open Data Access Project funded by Johnson & Johnson, and on the FDA Science Board. Dr. Sharfstein and Dr. Psaty made these remarks in an editorial accompanying Dr. Nissen’s report (JAMA. 2016;315:984-6).
The sponsor of this trial, Orexigen Therapeutics, disregarded the multiple harms caused by breaches of confidentiality, treated the academic investigators unfairly, ignored the trial’s data monitoring committee, and defied the FDA. Their statements about the naltrexone-bupropion’s cardiovascular efficacy were highly misleading, and there were obvious signs that the supporting data were not reliable. For example, nearly all of the CV mortality in the first interim analysis occurred well after participants had stopped taking their medication.
Yet the drug company was not sanctioned, and the naltrexone-bupropion retains FDA approval. At a minimum, the FDA should withhold approval until a viable replacement study is conducted, or it should require Risk Evaluation and Mitigation Strategies to counter the misinformation disseminated by the study sponsor, or it should restrict use of the drug outright.
Clinicians should be aware of these research improprieties and should know that the purported weight-loss benefit of naltrexone-bupropion is only 2.7 kg more than that achieved with placebo. How does this modest benefit balance against an unknown cardiovascular risk? In addition, the drug’s lack of efficacy clearly contributed to the very poor adherence rate in this trial: At 1 year, only 37.5% were still taking naltrexone-bupropion and 26.3% were still taking placebo.
Dr. Joshua M. Sharfstein is in the department of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore. Dr. Bruce M. Psaty is in the Cardiovascular Health Research Unit and the departments of medicine, epidemiology, and health services at the University of Washington, Seattle. They reported that this work was supported by the National Heart, Lung, and Blood Institute. Dr. Sharfstein reported serving as principal deputy commissioner of the FDA in 2009-2011. Dr. Psaty reported serving on the data monitoring committee of a clinical trial funded by Zoll LifeCor, on the steering committee of the Yale Open Data Access Project funded by Johnson & Johnson, and on the FDA Science Board. Dr. Sharfstein and Dr. Psaty made these remarks in an editorial accompanying Dr. Nissen’s report (JAMA. 2016;315:984-6).
The sponsor of this trial, Orexigen Therapeutics, disregarded the multiple harms caused by breaches of confidentiality, treated the academic investigators unfairly, ignored the trial’s data monitoring committee, and defied the FDA. Their statements about the naltrexone-bupropion’s cardiovascular efficacy were highly misleading, and there were obvious signs that the supporting data were not reliable. For example, nearly all of the CV mortality in the first interim analysis occurred well after participants had stopped taking their medication.
Yet the drug company was not sanctioned, and the naltrexone-bupropion retains FDA approval. At a minimum, the FDA should withhold approval until a viable replacement study is conducted, or it should require Risk Evaluation and Mitigation Strategies to counter the misinformation disseminated by the study sponsor, or it should restrict use of the drug outright.
Clinicians should be aware of these research improprieties and should know that the purported weight-loss benefit of naltrexone-bupropion is only 2.7 kg more than that achieved with placebo. How does this modest benefit balance against an unknown cardiovascular risk? In addition, the drug’s lack of efficacy clearly contributed to the very poor adherence rate in this trial: At 1 year, only 37.5% were still taking naltrexone-bupropion and 26.3% were still taking placebo.
Dr. Joshua M. Sharfstein is in the department of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore. Dr. Bruce M. Psaty is in the Cardiovascular Health Research Unit and the departments of medicine, epidemiology, and health services at the University of Washington, Seattle. They reported that this work was supported by the National Heart, Lung, and Blood Institute. Dr. Sharfstein reported serving as principal deputy commissioner of the FDA in 2009-2011. Dr. Psaty reported serving on the data monitoring committee of a clinical trial funded by Zoll LifeCor, on the steering committee of the Yale Open Data Access Project funded by Johnson & Johnson, and on the FDA Science Board. Dr. Sharfstein and Dr. Psaty made these remarks in an editorial accompanying Dr. Nissen’s report (JAMA. 2016;315:984-6).
The cardiovascular risks of the weight-loss combination drug naltrexone-bupropion are still uncertain, because the FDA-mandated safety trial, LIGHT, was terminated prematurely due to the sponsoring drug company’s breach of confidentiality. Other irregularities also were discovered, according to a report published online March 8 in JAMA.
Orexigen violated its agreement with FDA not to disclose the findings of an interim analysis performed after only 25% of expected CV events accrued. These findings appeared highly favorable for the drug, prompting the sponsor to publicly, and prematurely, claim naltrexone-bupropion reduced CV risk by 41% independently of its weight-loss effect. This favorable outcome was later found to be “driven almost exclusively by a 12-to-1 imbalance in death favoring naltrexone-bupropion among patients who were no longer taking the study drug,” said Dr. Steven E. Nissen of the Cleveland Clinic Center for Cardiovascular Research and his associates.
They now report the results of a second interim analysis of LIGHT (Cardiovascular Outcomes Study of Naltrexone SR/Bupropion SR in Overweight and Obese Subjects With Cardiovascular Risk Factors) performed after 50% of expected CV events accrued, as well as those of a final analysis performed when the trial was halted and 64% of expected CV events accrued. These findings are much less favorable, showing no significant difference between naltrexone-bupropion and placebo in the rate of major adverse cardiovascular events (MACE). In addition, extremely poor adherence to both active treatment and placebo over time, together with the complex statistical issues involved, call into question any interpretation of the results and preclude “any reliable conclusions about the long-term safety of naltrexone-bupropion,” the investigators said.
“The cardiovascular safety of this treatment remains uncertain and will require evaluation in a new, adequately powered outcome trial.” The results of such a study, should it be undertaken, wouldn’t be available for at least 3-4 years, they noted.
In their report, the investigators described in detail why the FDA required a CV safety study even though the agency had already approved naltrexone-bupropion as a weight-loss treatment.
The study was a phase IIIb randomized, double-blind, placebo-controlled trial involving 8,910 overweight/obese patients aged 45 or older (mean age, 61 years) who were at risk of adverse CV outcomes due to preexisting CV disease; exercise-induced angina; an unfavorable ankle/brachial index; stenosis of coronary, carotid, or lower extremity arteries; type 2 diabetes; hypertension; dyslipidemia; or smoking. These participants received daily tablets of either 32 mg naltrexone plus 360 mg bupropion or a matching placebo and were to be followed for 4 years at 266 U.S. medical centers.
The analysis performed after 50% of expected CV events accrued showed that MACE – the primary safety outcome – occurred in 2.0% of the naltrexone-bupropion group and 2.3% of the placebo group, a nonsignificant difference. Differences between the two study groups also were nonsignificant for the individual components of the primary outcome, including cardiovascular death (0.4% vs. 0.8%), nonfatal stroke (0.5% vs. 0.4%), and nonfatal MI (1.2% vs. 1.2%). These results were confirmed in a sensitivity analysis based on the final data collection, when 64% of expected CV events accrued: MACE occurred in 2.7% of the naltrexone-bupropion group and 2.8% of the placebo group, another nonsignificant difference.
The active treatment also was no better than placebo regarding all secondary outcomes, including all-cause mortality, CV hospitalization, and coronary revascularization, Dr. Nissen and his associates said (JAMA. 2016 Mar 8. doi: 10.1001/jama.2016.1558). Treatment adherence was low. At 16 weeks, only 64% of the naltrexone-bupropion group and 72% of the placebo group were still taking the study treatment. By 2 years, only 27% of the naltrexone-bupropion group and 17% of the placebo group were. The mean duration of treatment was only 18.4 weeks for naltrexone-bupropion and 16.3 weeks for placebo. Most treatment discontinuations were due to a failure to lose weight, but a substantial proportion occurred because of treatment-related increases in blood pressure or heart rate.
The active treatment’s ability to reduce body weight was deemed “modest.” When the trial was halted, the mean decrease in weight was 3.9 kg with naltrexone-bupropion, representing a 3.6% reduction in total weight, and was 1.2 kg with placebo, representing a 1.1% reduction.
Adverse effects developed in 28.1% of patients taking naltrexone-bupropion, which was a significantly greater proportion than the 8.7% rate in the placebo group. The most common adverse events leading to discontinuation of the study drug affected the gastrointestinal system (nausea, constipation, vomiting), the CNS (tremor, dizziness, headache), and mental/emotional problems (insomnia, anxiety, hallucinations, depression).
The cardiovascular risks of the weight-loss combination drug naltrexone-bupropion are still uncertain, because the FDA-mandated safety trial, LIGHT, was terminated prematurely due to the sponsoring drug company’s breach of confidentiality. Other irregularities also were discovered, according to a report published online March 8 in JAMA.
Orexigen violated its agreement with FDA not to disclose the findings of an interim analysis performed after only 25% of expected CV events accrued. These findings appeared highly favorable for the drug, prompting the sponsor to publicly, and prematurely, claim naltrexone-bupropion reduced CV risk by 41% independently of its weight-loss effect. This favorable outcome was later found to be “driven almost exclusively by a 12-to-1 imbalance in death favoring naltrexone-bupropion among patients who were no longer taking the study drug,” said Dr. Steven E. Nissen of the Cleveland Clinic Center for Cardiovascular Research and his associates.
They now report the results of a second interim analysis of LIGHT (Cardiovascular Outcomes Study of Naltrexone SR/Bupropion SR in Overweight and Obese Subjects With Cardiovascular Risk Factors) performed after 50% of expected CV events accrued, as well as those of a final analysis performed when the trial was halted and 64% of expected CV events accrued. These findings are much less favorable, showing no significant difference between naltrexone-bupropion and placebo in the rate of major adverse cardiovascular events (MACE). In addition, extremely poor adherence to both active treatment and placebo over time, together with the complex statistical issues involved, call into question any interpretation of the results and preclude “any reliable conclusions about the long-term safety of naltrexone-bupropion,” the investigators said.
“The cardiovascular safety of this treatment remains uncertain and will require evaluation in a new, adequately powered outcome trial.” The results of such a study, should it be undertaken, wouldn’t be available for at least 3-4 years, they noted.
In their report, the investigators described in detail why the FDA required a CV safety study even though the agency had already approved naltrexone-bupropion as a weight-loss treatment.
The study was a phase IIIb randomized, double-blind, placebo-controlled trial involving 8,910 overweight/obese patients aged 45 or older (mean age, 61 years) who were at risk of adverse CV outcomes due to preexisting CV disease; exercise-induced angina; an unfavorable ankle/brachial index; stenosis of coronary, carotid, or lower extremity arteries; type 2 diabetes; hypertension; dyslipidemia; or smoking. These participants received daily tablets of either 32 mg naltrexone plus 360 mg bupropion or a matching placebo and were to be followed for 4 years at 266 U.S. medical centers.
The analysis performed after 50% of expected CV events accrued showed that MACE – the primary safety outcome – occurred in 2.0% of the naltrexone-bupropion group and 2.3% of the placebo group, a nonsignificant difference. Differences between the two study groups also were nonsignificant for the individual components of the primary outcome, including cardiovascular death (0.4% vs. 0.8%), nonfatal stroke (0.5% vs. 0.4%), and nonfatal MI (1.2% vs. 1.2%). These results were confirmed in a sensitivity analysis based on the final data collection, when 64% of expected CV events accrued: MACE occurred in 2.7% of the naltrexone-bupropion group and 2.8% of the placebo group, another nonsignificant difference.
The active treatment also was no better than placebo regarding all secondary outcomes, including all-cause mortality, CV hospitalization, and coronary revascularization, Dr. Nissen and his associates said (JAMA. 2016 Mar 8. doi: 10.1001/jama.2016.1558). Treatment adherence was low. At 16 weeks, only 64% of the naltrexone-bupropion group and 72% of the placebo group were still taking the study treatment. By 2 years, only 27% of the naltrexone-bupropion group and 17% of the placebo group were. The mean duration of treatment was only 18.4 weeks for naltrexone-bupropion and 16.3 weeks for placebo. Most treatment discontinuations were due to a failure to lose weight, but a substantial proportion occurred because of treatment-related increases in blood pressure or heart rate.
The active treatment’s ability to reduce body weight was deemed “modest.” When the trial was halted, the mean decrease in weight was 3.9 kg with naltrexone-bupropion, representing a 3.6% reduction in total weight, and was 1.2 kg with placebo, representing a 1.1% reduction.
Adverse effects developed in 28.1% of patients taking naltrexone-bupropion, which was a significantly greater proportion than the 8.7% rate in the placebo group. The most common adverse events leading to discontinuation of the study drug affected the gastrointestinal system (nausea, constipation, vomiting), the CNS (tremor, dizziness, headache), and mental/emotional problems (insomnia, anxiety, hallucinations, depression).
FROM JAMA
Key clinical point: The cardiovascular risks of the weight-loss combination drug naltrexone-bupropion are still uncertain.
Major finding: The primary safety outcome, major adverse cardiovascular events, occurred in 2.0% of the naltrexone-bupropion group and 2.3% of the placebo group, a nonsignificant difference.
Data source: LIGHT, a multicenter randomized placebo-controlled double-blind noninferiority trial involving 8,910 overweight/obese patients.
Disclosures: This trial was sponsored by Orexigen Therapeutics and Takeda Pharmaceuticals. Dr. Nissen reported receiving grants from The Medicines Company, Amgen, Pfizer, AstraZeneca, Esperion Therapeutics, Eli Lilly, and Cerenis, and consulting for numerous drug companies that pay his fees directly to charities. His associates reported ties to numerous industry sources.
Poor physical fitness upped diabetes risk regardless of weight
Young, out-of-shape men were about three times more likely than physically fit men to develop type 2 diabetes later in life, even if their body weight was normal, reported the authors of a large registry study.
“These findings suggest that interventions to improve aerobic and muscle fitness levels early in life could help reduce risk for type 2 diabetes mellitus in adulthood,” Dr. Casey Crump, at the Icahn School of Medicine at Mount Sinai, New York, and his associates wrote in a study published online March 7 in Annals of Internal Medicine.
Future longitudinal studies of physical fitness could help identify “windows of susceptibility” and the best preventive measures, the researchers added.
A sedentary lifestyle is known to increase the risk of type 2 diabetes, but less is known about how physical fitness affects risk.
To explore the question, the researchers identified 1,534,425 men without baseline diabetes who underwent military conscription physical examinations between 1969 and 1997. They tracked the men until up to 62 years of age by analyzing both the Swedish Hospital Registry and the Swedish Outpatient Registry (Ann Intern Med. 2016 Mar 8; doi: 10.7326/M15-2002).
In all, 34,008 men developed type 2 diabetes over 39.4 million years of follow-up, the investigators said. Both low cardiorespiratory fitness and low muscle strength independently increased the risk for type 2 diabetes, regardless of whether the men had a high or normal body weight.
Moreover, the combination of low cardiorespiratory fitness and poor muscular fitness increased type 2 diabetes risk threefold (adjusted hazard ratio, 3.07; 95% confidence interval, 2.88 to 3.27; P less than .001), with a positive additive interaction (P less than .001).
Accounting for smoking lowered the associations between poor baseline fitness and type 2 diabetes by about 9%; but they remained significant (P less than .001), suggesting that unmeasured confounding “had little influence on our main findings,” the investigators said. If the associations are causal, then aerobic conditioning programs targeting men with low muscle strength might have the greatest public health impact, they added.
The U.S. National Institutes of Health, the Swedish Research Council, and Region Skåne/Lund University funded the study. The researchers had no disclosures.
Given the global increase in type 2 diabetes prevalence, there is a pressing need for intervention strategies that reduce the progression of the disease as well as the risk for related complications and premature death. The study by Dr. Crump and his colleagues fills an important research gap by demonstrating a strong inverse association between physical fitness at a young age and long-term risk for type 2 diabetes, independent of weight status. The enhancement of cardiorespiratory and muscular fitness through habitual physical activity in all persons should be recommended as a frontline therapy to address the public health burden of type 2 diabetes.
Peter T. Katzmarzyk, Ph.D., is at Pennington Biomedical Research Center in Baton Rouge, La. He had no disclosures. These comments were taken from his accompanying editorial (Ann Intern Med. 2016 Mar 8; doi: 10.7326/M16-0269).
Given the global increase in type 2 diabetes prevalence, there is a pressing need for intervention strategies that reduce the progression of the disease as well as the risk for related complications and premature death. The study by Dr. Crump and his colleagues fills an important research gap by demonstrating a strong inverse association between physical fitness at a young age and long-term risk for type 2 diabetes, independent of weight status. The enhancement of cardiorespiratory and muscular fitness through habitual physical activity in all persons should be recommended as a frontline therapy to address the public health burden of type 2 diabetes.
Peter T. Katzmarzyk, Ph.D., is at Pennington Biomedical Research Center in Baton Rouge, La. He had no disclosures. These comments were taken from his accompanying editorial (Ann Intern Med. 2016 Mar 8; doi: 10.7326/M16-0269).
Given the global increase in type 2 diabetes prevalence, there is a pressing need for intervention strategies that reduce the progression of the disease as well as the risk for related complications and premature death. The study by Dr. Crump and his colleagues fills an important research gap by demonstrating a strong inverse association between physical fitness at a young age and long-term risk for type 2 diabetes, independent of weight status. The enhancement of cardiorespiratory and muscular fitness through habitual physical activity in all persons should be recommended as a frontline therapy to address the public health burden of type 2 diabetes.
Peter T. Katzmarzyk, Ph.D., is at Pennington Biomedical Research Center in Baton Rouge, La. He had no disclosures. These comments were taken from his accompanying editorial (Ann Intern Med. 2016 Mar 8; doi: 10.7326/M16-0269).
Young, out-of-shape men were about three times more likely than physically fit men to develop type 2 diabetes later in life, even if their body weight was normal, reported the authors of a large registry study.
“These findings suggest that interventions to improve aerobic and muscle fitness levels early in life could help reduce risk for type 2 diabetes mellitus in adulthood,” Dr. Casey Crump, at the Icahn School of Medicine at Mount Sinai, New York, and his associates wrote in a study published online March 7 in Annals of Internal Medicine.
Future longitudinal studies of physical fitness could help identify “windows of susceptibility” and the best preventive measures, the researchers added.
A sedentary lifestyle is known to increase the risk of type 2 diabetes, but less is known about how physical fitness affects risk.
To explore the question, the researchers identified 1,534,425 men without baseline diabetes who underwent military conscription physical examinations between 1969 and 1997. They tracked the men until up to 62 years of age by analyzing both the Swedish Hospital Registry and the Swedish Outpatient Registry (Ann Intern Med. 2016 Mar 8; doi: 10.7326/M15-2002).
In all, 34,008 men developed type 2 diabetes over 39.4 million years of follow-up, the investigators said. Both low cardiorespiratory fitness and low muscle strength independently increased the risk for type 2 diabetes, regardless of whether the men had a high or normal body weight.
Moreover, the combination of low cardiorespiratory fitness and poor muscular fitness increased type 2 diabetes risk threefold (adjusted hazard ratio, 3.07; 95% confidence interval, 2.88 to 3.27; P less than .001), with a positive additive interaction (P less than .001).
Accounting for smoking lowered the associations between poor baseline fitness and type 2 diabetes by about 9%; but they remained significant (P less than .001), suggesting that unmeasured confounding “had little influence on our main findings,” the investigators said. If the associations are causal, then aerobic conditioning programs targeting men with low muscle strength might have the greatest public health impact, they added.
The U.S. National Institutes of Health, the Swedish Research Council, and Region Skåne/Lund University funded the study. The researchers had no disclosures.
Young, out-of-shape men were about three times more likely than physically fit men to develop type 2 diabetes later in life, even if their body weight was normal, reported the authors of a large registry study.
“These findings suggest that interventions to improve aerobic and muscle fitness levels early in life could help reduce risk for type 2 diabetes mellitus in adulthood,” Dr. Casey Crump, at the Icahn School of Medicine at Mount Sinai, New York, and his associates wrote in a study published online March 7 in Annals of Internal Medicine.
Future longitudinal studies of physical fitness could help identify “windows of susceptibility” and the best preventive measures, the researchers added.
A sedentary lifestyle is known to increase the risk of type 2 diabetes, but less is known about how physical fitness affects risk.
To explore the question, the researchers identified 1,534,425 men without baseline diabetes who underwent military conscription physical examinations between 1969 and 1997. They tracked the men until up to 62 years of age by analyzing both the Swedish Hospital Registry and the Swedish Outpatient Registry (Ann Intern Med. 2016 Mar 8; doi: 10.7326/M15-2002).
In all, 34,008 men developed type 2 diabetes over 39.4 million years of follow-up, the investigators said. Both low cardiorespiratory fitness and low muscle strength independently increased the risk for type 2 diabetes, regardless of whether the men had a high or normal body weight.
Moreover, the combination of low cardiorespiratory fitness and poor muscular fitness increased type 2 diabetes risk threefold (adjusted hazard ratio, 3.07; 95% confidence interval, 2.88 to 3.27; P less than .001), with a positive additive interaction (P less than .001).
Accounting for smoking lowered the associations between poor baseline fitness and type 2 diabetes by about 9%; but they remained significant (P less than .001), suggesting that unmeasured confounding “had little influence on our main findings,” the investigators said. If the associations are causal, then aerobic conditioning programs targeting men with low muscle strength might have the greatest public health impact, they added.
The U.S. National Institutes of Health, the Swedish Research Council, and Region Skåne/Lund University funded the study. The researchers had no disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: In young men, both low cardiorespiratory fitness and low muscle strength independently increased the risk for type 2 diabetes mellitus, regardless of body weight.
Major finding: Low cardiorespiratory fitness combined with poor muscular fitness increased T2DM risk threefold (adjusted hazard ratio, 3.07; P less than .001).
Data source: A registry study of 1,534,425 Swedish men without baseline diabetes mellitus who underwent military conscription physical examinations between 1969 and 1997.
Disclosures: The U.S. National Institutes of Health, the Swedish Research Council, and Region Skåne/Lund University funded the study. The researchers had no disclosures.
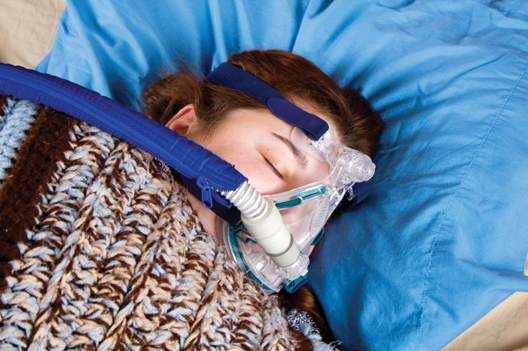
In newly diagnosed hypertension with OSA, adding CPAP augmented the benefits of losartan
In patients with new-onset hypertension and obstructive sleep apnea, continuous positive airway pressure (CPAP) therapy plus antihypertensive treatment with losartan led to reductions in systolic blood pressure beyond those achieved with losartan alone, a two-phase study found.
“Adding CPAP treatment to losartan may reduce blood pressure in a clinically relevant way if the patients are compliant with the device,” said Dr. Erik Thunström of the Sahlgrenska Academy at the University of Gothenburg, Sweden, and his associates.
In their open-label study, 89 men and women with new-onset untreated hypertension – 54 of whom were found to have obstructive sleep apnea (OSA) through a home sleep study and 35 of whom were determined to not have OSA – were treated for 6 weeks with losartan, 50 mg daily. Ambulatory 24-hour blood pressure monitoring was performed before and after treatment.
The patients with OSA were then randomized to receive 6 weeks of nightly add-on CPAP therapy or to continue losartan alone. Ambulatory 24-hour blood pressure monitoring was performed again.
Losartan alone reduced blood pressure in patients with hypertension and concomitant OSA, but the effect was smaller than that seen in patients without OSA. Statistically significant differences were seen in the mean net reduction in morning systolic blood pressure and morning mean arterial pressure. Overall, losartan appeared to be less effective at night and during the early morning hours in patients with OSA, the researchers reported.
After 6 weeks of losartan alone, a blood pressure less than 130/80 mm Hg was achieved by 12.5% of the patients with OSA and by 29% of the patients without OSA.
After 6 weeks of add-on CPAP therapy, 25% of patients with OSA achieved blood pressures less than 130/80 mm Hg. The differences in blood pressures for the OSA patients receiving CPAP plus losartan and those receiving losartan alone were 4.4 mm Hg for 24-hour systolic blood pressure, 1.9 mm Hg for diastolic, and 2.5 mm Hg for mean arterial pressure.
The most “robust” blood pressure changes were seen in the patients who used CPAP therapy for more than 4 hours every night, reducing the mean 24-hour systolic blood pressure by 6.5 mm Hg, the diastolic pressure by 3.8 mm Hg, and the mean arterial blood pressure by 4.6 mm Hg, the researchers reported (Am J Respir Crit Care Med. 2016 Feb.;193:310-20). “Adding CPAP to treatment with losartan reduced the mean 24-hour systolic blood pressure by 6.5 mm Hg in the subgroup of patients with OSA who were adherent with CPAP,” they wrote.
Patients included in the study all had a body mass index of 35 kg/m2; those with OSA had slightly higher BMIs that did not differ significantly from those without OSA.
That CPAP seems to have additive blood pressure–lowering effect when used concomitantly with losartan “favors the idea that it contributes to a further down-regulation of RAAS [renin-angiotensin-aldosterone system] activity in new-onset hypertension and OSA,” the authors wrote.
RAAS activity is often changed in hypertension, and in animal studies it has been shown to be up-regulated by intermittent hypoxia. Angiotensin II receptor antagonists are thus viewed as a good choice in the treatment of patients with OSA and new-onset hypertension, they wrote.
Treating OSA may make hypertension easier to address pharmacologically. The effect of CPAP on blood pressure is relatively small when all patients are considered but is more substantial and clinically important for those who use CPAP for more than 4 hours per night.
Can treatment of OSA effectively reduce blood pressure in an otherwise asymptomatic hypertensive patient with OSA? I believe the study would suggest that the answer remains “maybe.”
Most of the patients in the study would require a higher dose of losartan or an additional antihypertensive drug, even while using CPAP, to get to target blood pressures. Getting patients to use CPAP is a difficult task, as is adherence with any long-term pharmacologic management.
All in all, however, CPAP could contribute to blood pressure control while also improving quality of life and possibly reducing the risk for cardiovascular disease.
Dr. David P. White is with Harvard Medical School in Boston. His comments are excerpted from an accompanying editorial (Am J Respir Crit Care Med. 2016 Feb;193:238-9).
Treating OSA may make hypertension easier to address pharmacologically. The effect of CPAP on blood pressure is relatively small when all patients are considered but is more substantial and clinically important for those who use CPAP for more than 4 hours per night.
Can treatment of OSA effectively reduce blood pressure in an otherwise asymptomatic hypertensive patient with OSA? I believe the study would suggest that the answer remains “maybe.”
Most of the patients in the study would require a higher dose of losartan or an additional antihypertensive drug, even while using CPAP, to get to target blood pressures. Getting patients to use CPAP is a difficult task, as is adherence with any long-term pharmacologic management.
All in all, however, CPAP could contribute to blood pressure control while also improving quality of life and possibly reducing the risk for cardiovascular disease.
Dr. David P. White is with Harvard Medical School in Boston. His comments are excerpted from an accompanying editorial (Am J Respir Crit Care Med. 2016 Feb;193:238-9).
Treating OSA may make hypertension easier to address pharmacologically. The effect of CPAP on blood pressure is relatively small when all patients are considered but is more substantial and clinically important for those who use CPAP for more than 4 hours per night.
Can treatment of OSA effectively reduce blood pressure in an otherwise asymptomatic hypertensive patient with OSA? I believe the study would suggest that the answer remains “maybe.”
Most of the patients in the study would require a higher dose of losartan or an additional antihypertensive drug, even while using CPAP, to get to target blood pressures. Getting patients to use CPAP is a difficult task, as is adherence with any long-term pharmacologic management.
All in all, however, CPAP could contribute to blood pressure control while also improving quality of life and possibly reducing the risk for cardiovascular disease.
Dr. David P. White is with Harvard Medical School in Boston. His comments are excerpted from an accompanying editorial (Am J Respir Crit Care Med. 2016 Feb;193:238-9).
In patients with new-onset hypertension and obstructive sleep apnea, continuous positive airway pressure (CPAP) therapy plus antihypertensive treatment with losartan led to reductions in systolic blood pressure beyond those achieved with losartan alone, a two-phase study found.
“Adding CPAP treatment to losartan may reduce blood pressure in a clinically relevant way if the patients are compliant with the device,” said Dr. Erik Thunström of the Sahlgrenska Academy at the University of Gothenburg, Sweden, and his associates.
In their open-label study, 89 men and women with new-onset untreated hypertension – 54 of whom were found to have obstructive sleep apnea (OSA) through a home sleep study and 35 of whom were determined to not have OSA – were treated for 6 weeks with losartan, 50 mg daily. Ambulatory 24-hour blood pressure monitoring was performed before and after treatment.
The patients with OSA were then randomized to receive 6 weeks of nightly add-on CPAP therapy or to continue losartan alone. Ambulatory 24-hour blood pressure monitoring was performed again.
Losartan alone reduced blood pressure in patients with hypertension and concomitant OSA, but the effect was smaller than that seen in patients without OSA. Statistically significant differences were seen in the mean net reduction in morning systolic blood pressure and morning mean arterial pressure. Overall, losartan appeared to be less effective at night and during the early morning hours in patients with OSA, the researchers reported.
After 6 weeks of losartan alone, a blood pressure less than 130/80 mm Hg was achieved by 12.5% of the patients with OSA and by 29% of the patients without OSA.
After 6 weeks of add-on CPAP therapy, 25% of patients with OSA achieved blood pressures less than 130/80 mm Hg. The differences in blood pressures for the OSA patients receiving CPAP plus losartan and those receiving losartan alone were 4.4 mm Hg for 24-hour systolic blood pressure, 1.9 mm Hg for diastolic, and 2.5 mm Hg for mean arterial pressure.
The most “robust” blood pressure changes were seen in the patients who used CPAP therapy for more than 4 hours every night, reducing the mean 24-hour systolic blood pressure by 6.5 mm Hg, the diastolic pressure by 3.8 mm Hg, and the mean arterial blood pressure by 4.6 mm Hg, the researchers reported (Am J Respir Crit Care Med. 2016 Feb.;193:310-20). “Adding CPAP to treatment with losartan reduced the mean 24-hour systolic blood pressure by 6.5 mm Hg in the subgroup of patients with OSA who were adherent with CPAP,” they wrote.
Patients included in the study all had a body mass index of 35 kg/m2; those with OSA had slightly higher BMIs that did not differ significantly from those without OSA.
That CPAP seems to have additive blood pressure–lowering effect when used concomitantly with losartan “favors the idea that it contributes to a further down-regulation of RAAS [renin-angiotensin-aldosterone system] activity in new-onset hypertension and OSA,” the authors wrote.
RAAS activity is often changed in hypertension, and in animal studies it has been shown to be up-regulated by intermittent hypoxia. Angiotensin II receptor antagonists are thus viewed as a good choice in the treatment of patients with OSA and new-onset hypertension, they wrote.
In patients with new-onset hypertension and obstructive sleep apnea, continuous positive airway pressure (CPAP) therapy plus antihypertensive treatment with losartan led to reductions in systolic blood pressure beyond those achieved with losartan alone, a two-phase study found.
“Adding CPAP treatment to losartan may reduce blood pressure in a clinically relevant way if the patients are compliant with the device,” said Dr. Erik Thunström of the Sahlgrenska Academy at the University of Gothenburg, Sweden, and his associates.
In their open-label study, 89 men and women with new-onset untreated hypertension – 54 of whom were found to have obstructive sleep apnea (OSA) through a home sleep study and 35 of whom were determined to not have OSA – were treated for 6 weeks with losartan, 50 mg daily. Ambulatory 24-hour blood pressure monitoring was performed before and after treatment.
The patients with OSA were then randomized to receive 6 weeks of nightly add-on CPAP therapy or to continue losartan alone. Ambulatory 24-hour blood pressure monitoring was performed again.
Losartan alone reduced blood pressure in patients with hypertension and concomitant OSA, but the effect was smaller than that seen in patients without OSA. Statistically significant differences were seen in the mean net reduction in morning systolic blood pressure and morning mean arterial pressure. Overall, losartan appeared to be less effective at night and during the early morning hours in patients with OSA, the researchers reported.
After 6 weeks of losartan alone, a blood pressure less than 130/80 mm Hg was achieved by 12.5% of the patients with OSA and by 29% of the patients without OSA.
After 6 weeks of add-on CPAP therapy, 25% of patients with OSA achieved blood pressures less than 130/80 mm Hg. The differences in blood pressures for the OSA patients receiving CPAP plus losartan and those receiving losartan alone were 4.4 mm Hg for 24-hour systolic blood pressure, 1.9 mm Hg for diastolic, and 2.5 mm Hg for mean arterial pressure.
The most “robust” blood pressure changes were seen in the patients who used CPAP therapy for more than 4 hours every night, reducing the mean 24-hour systolic blood pressure by 6.5 mm Hg, the diastolic pressure by 3.8 mm Hg, and the mean arterial blood pressure by 4.6 mm Hg, the researchers reported (Am J Respir Crit Care Med. 2016 Feb.;193:310-20). “Adding CPAP to treatment with losartan reduced the mean 24-hour systolic blood pressure by 6.5 mm Hg in the subgroup of patients with OSA who were adherent with CPAP,” they wrote.
Patients included in the study all had a body mass index of 35 kg/m2; those with OSA had slightly higher BMIs that did not differ significantly from those without OSA.
That CPAP seems to have additive blood pressure–lowering effect when used concomitantly with losartan “favors the idea that it contributes to a further down-regulation of RAAS [renin-angiotensin-aldosterone system] activity in new-onset hypertension and OSA,” the authors wrote.
RAAS activity is often changed in hypertension, and in animal studies it has been shown to be up-regulated by intermittent hypoxia. Angiotensin II receptor antagonists are thus viewed as a good choice in the treatment of patients with OSA and new-onset hypertension, they wrote.
FROM AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Key clinical point: In patients with new-onset hypertension and obstructive sleep apnea, adding continuous positive airway pressure may reduce blood pressure levels further than achieved with losartan alone.
Major finding: In adherent patients, CPAP reduced the mean 24-hour systolic blood pressure by an additional 6.5 mm Hg as compared to the levels seen in patients on losartan alone.
Data source: A study of 89 men and women with new-onset untreated hypertension who were treated with losartan for 6 weeks and tested for OSA. In a second 6-week study, patients found to have OSA were randomized to receive CPAP or no CPAP.
Disclosures: The researchers had no relevant financial disclosures.
Degludec/liraglutide noninferior to uptitrated glargine
For adults whose type 2 diabetes is not controlled by glargine plus metformin, switching from glargine to combination degludec/liraglutide lowered hemoglobin A1c levels to a noninferior degree, compared with continued uptitrating of glargine, according to a report published online March 1 in JAMA.
In DUAL V, an international, randomized, phase III clinical trial sponsored and conducted by Novo Nordisk, maker of degludec/liraglutide, 557 patients (mean age, 59 years) on a regimen of glargine plus metformin were randomly assigned to switch from glargine to combination degludec/liraglutide or to uptitrate their glargine dose to a target HbA1c level, said Dr. Ildiko Lingvay of the departments of internal medicine and clinical sciences, University of Texas, Dallas, and her associates.
After 26 weeks, HbA1c levels decreased to 6.6% with the study intervention and to 7.1% with the increased glargine. This nonsignificant difference in the primary efficacy endpoint demonstrates the noninferiority of degludec/liraglutide. Combination degludec/liraglutide also was associated with a 1.4-kg reduction in mean body weight to 86.9 kg, while increasing glargine was associated with a 1.8-kg increase in body weight to 89.1 kg.
In addition, hypoglycemic episodes occurred in 28.4% of the degludec/liraglutide group, significantly fewer than the 49.1% of the glargine group who experienced hypoglycemic episodes. The respective rates of hypoglycemic episodes were 2.23 vs. 5.05 per patient-year of exposure to the drugs.
Patients taking degludec/liraglutide reported improved scores on measures of physical health, physical functioning, bodily pain, and the impact of diabetes treatment on daily living, while those taking glargine reported worsening of these scores. Patients in the intervention group also reported higher satisfaction with treatment than did those in the control group, Dr. Lingvay and her associates said (JAMA. 2016 Mar 1. doi: 10.101/jama.2016.1252). The rate of adverse events was 343.3 per 100 person-years of exposure for degludec/liraglutide and 286.4 per 100 person-years for glargine. The respective rates of serious adverse events were 3.9 and 6.7 per 100 person-years of exposure.
“As a once-daily, single injection that is effective, is associated with weight loss, and [carries] a low risk of hypoglycemia, degludec/liraglutide may overcome many of the barriers to treatment intensification in patients treated with basal insulin,” the investigators said.
DUAL V was sponsored by Novo Nordisk, maker of degludec/liraglutide, which also participated in the study design and conduct; the collection, analysis, and interpretation of the data; and the writing of the report. Dr. Lingvay reported ties to Novo Nordisk, Pfizer/Merck, GI Dynamics, AstraZeneca, Boehringer-Ingelheim, Janssen, and Sanofi; her associates reported ties to numerous industry sources.
For adults whose type 2 diabetes is not controlled by glargine plus metformin, switching from glargine to combination degludec/liraglutide lowered hemoglobin A1c levels to a noninferior degree, compared with continued uptitrating of glargine, according to a report published online March 1 in JAMA.
In DUAL V, an international, randomized, phase III clinical trial sponsored and conducted by Novo Nordisk, maker of degludec/liraglutide, 557 patients (mean age, 59 years) on a regimen of glargine plus metformin were randomly assigned to switch from glargine to combination degludec/liraglutide or to uptitrate their glargine dose to a target HbA1c level, said Dr. Ildiko Lingvay of the departments of internal medicine and clinical sciences, University of Texas, Dallas, and her associates.
After 26 weeks, HbA1c levels decreased to 6.6% with the study intervention and to 7.1% with the increased glargine. This nonsignificant difference in the primary efficacy endpoint demonstrates the noninferiority of degludec/liraglutide. Combination degludec/liraglutide also was associated with a 1.4-kg reduction in mean body weight to 86.9 kg, while increasing glargine was associated with a 1.8-kg increase in body weight to 89.1 kg.
In addition, hypoglycemic episodes occurred in 28.4% of the degludec/liraglutide group, significantly fewer than the 49.1% of the glargine group who experienced hypoglycemic episodes. The respective rates of hypoglycemic episodes were 2.23 vs. 5.05 per patient-year of exposure to the drugs.
Patients taking degludec/liraglutide reported improved scores on measures of physical health, physical functioning, bodily pain, and the impact of diabetes treatment on daily living, while those taking glargine reported worsening of these scores. Patients in the intervention group also reported higher satisfaction with treatment than did those in the control group, Dr. Lingvay and her associates said (JAMA. 2016 Mar 1. doi: 10.101/jama.2016.1252). The rate of adverse events was 343.3 per 100 person-years of exposure for degludec/liraglutide and 286.4 per 100 person-years for glargine. The respective rates of serious adverse events were 3.9 and 6.7 per 100 person-years of exposure.
“As a once-daily, single injection that is effective, is associated with weight loss, and [carries] a low risk of hypoglycemia, degludec/liraglutide may overcome many of the barriers to treatment intensification in patients treated with basal insulin,” the investigators said.
DUAL V was sponsored by Novo Nordisk, maker of degludec/liraglutide, which also participated in the study design and conduct; the collection, analysis, and interpretation of the data; and the writing of the report. Dr. Lingvay reported ties to Novo Nordisk, Pfizer/Merck, GI Dynamics, AstraZeneca, Boehringer-Ingelheim, Janssen, and Sanofi; her associates reported ties to numerous industry sources.
For adults whose type 2 diabetes is not controlled by glargine plus metformin, switching from glargine to combination degludec/liraglutide lowered hemoglobin A1c levels to a noninferior degree, compared with continued uptitrating of glargine, according to a report published online March 1 in JAMA.
In DUAL V, an international, randomized, phase III clinical trial sponsored and conducted by Novo Nordisk, maker of degludec/liraglutide, 557 patients (mean age, 59 years) on a regimen of glargine plus metformin were randomly assigned to switch from glargine to combination degludec/liraglutide or to uptitrate their glargine dose to a target HbA1c level, said Dr. Ildiko Lingvay of the departments of internal medicine and clinical sciences, University of Texas, Dallas, and her associates.
After 26 weeks, HbA1c levels decreased to 6.6% with the study intervention and to 7.1% with the increased glargine. This nonsignificant difference in the primary efficacy endpoint demonstrates the noninferiority of degludec/liraglutide. Combination degludec/liraglutide also was associated with a 1.4-kg reduction in mean body weight to 86.9 kg, while increasing glargine was associated with a 1.8-kg increase in body weight to 89.1 kg.
In addition, hypoglycemic episodes occurred in 28.4% of the degludec/liraglutide group, significantly fewer than the 49.1% of the glargine group who experienced hypoglycemic episodes. The respective rates of hypoglycemic episodes were 2.23 vs. 5.05 per patient-year of exposure to the drugs.
Patients taking degludec/liraglutide reported improved scores on measures of physical health, physical functioning, bodily pain, and the impact of diabetes treatment on daily living, while those taking glargine reported worsening of these scores. Patients in the intervention group also reported higher satisfaction with treatment than did those in the control group, Dr. Lingvay and her associates said (JAMA. 2016 Mar 1. doi: 10.101/jama.2016.1252). The rate of adverse events was 343.3 per 100 person-years of exposure for degludec/liraglutide and 286.4 per 100 person-years for glargine. The respective rates of serious adverse events were 3.9 and 6.7 per 100 person-years of exposure.
“As a once-daily, single injection that is effective, is associated with weight loss, and [carries] a low risk of hypoglycemia, degludec/liraglutide may overcome many of the barriers to treatment intensification in patients treated with basal insulin,” the investigators said.
DUAL V was sponsored by Novo Nordisk, maker of degludec/liraglutide, which also participated in the study design and conduct; the collection, analysis, and interpretation of the data; and the writing of the report. Dr. Lingvay reported ties to Novo Nordisk, Pfizer/Merck, GI Dynamics, AstraZeneca, Boehringer-Ingelheim, Janssen, and Sanofi; her associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Switching to combination degludec/liraglutide produced noninferior reductions in HbA1c levels in adults with uncontrolled type 2 diabetes, compared with continued uptitrating of glargine.
Major finding: After 26 weeks, mean HbA1c levels decreased to 6.6% with degludec/liraglutide and to 7.1% with increased glargine, a nonsignificant difference.
Data source: DUAL V, an international, randomized, phase III noninferiority trial in 557 adults with uncontrolled type 2 diabetes treated for 26 weeks.
Disclosures: DUAL V was sponsored by Novo Nordisk, maker of degludec/liraglutide, which also participated in the study design and conduct; the collection, analysis, and interpretation of the data; and the writing of the report. Dr. Lingvay reported ties to Novo Nordisk, Pfizer/Merck, GI Dynamics, AstraZeneca, Boehringer-Ingelheim, Janssen, and Sanofi; her associates reported ties to numerous industry sources.
Could PCSK9 inhibitors be cost effective for all comers?
WASHINGTON - Treating all of the estimated 23 million individuals in the United States who would qualify for PCSK9 inhibitor therapy on the basis of current Food and Drug Administration indications and insurance company reimbursement criteria may be cost effective, according to data extrapolated from a tertiary center lipid clinic.
That is, if the two approved PCSK9 inhibitors are shown to reduce cardiovascular events in outcome studies.
With the current annual cost of PCSK9 therapy estimated to be roughly $14,000 per patient, the total bill for treating all candidates is an estimated $320 billion per year, but this huge sum may be offset by savings in medical care and lost productivity, Dr. Marloe Prince said at Cardiovascular Research Technologies 2016.
“Cost is a real issue for PCSK9 inhibitors,” reported Dr. Prince, an internal medicine resident at Jewish Hospital in Cincinnati. “Our aim was to determine first what percentage of patients would quality for PCSK9 inhibitor therapy and then to perform a cost efficacy analysis based on these estimates.”
The two PCSK9 inhibitors approved for lowering LDL cholesterol, evolocumab and alirocumab, are priced similarly. Both reduce LDL cholesterol by about 60% in most patients. Several published trials have demonstrated that PCSK9 inhibitors, which are administered by injection at intervals ranging from 2 weeks to 1 month, can bring a substantial proportion of high-risk patients to guideline recommended goals when maximally tolerated statins fail to do so.
In this study, the first step was to determine what proportion of patients referred to a lipid clinic for inadequate control of LDL cholesterol are eligible for PCSK9 inhibitors on the basis of current FDA indications and commercial insurance criteria. These indications include LDL cholesterol levels above guideline recommended targets in patients on maximally tolerated statins with heterozygous (HeFH) or homozygous (HoFH) familial hypercholesterolemia as well as those who have had a previous cardiovascular event.
Of 734 consecutive patients referred to the lipid clinic who had been managed for at least 2 months on maximally tolerated statins, 220 (30%) were eligible for a PCSK9 prescription on the basis of both the current FDA indication and commercial insurance reimbursement criteria. Applying this 30% figure to previously published estimates of patients not at goal on maximally tolerated statins, Dr. Prince and his coinvestigators estimated that there are 23 million Americans with hypercholesterolemia who would similarly qualify for a PCSK9 inhibitor.
The cost of supplying all 23 million individuals with a PCSK9 inhibitor is roughly $322 billion annually, but Dr. Prince cited data indicating that the estimated costs for treating cardiovascular events and stroke per year is also about $320 billion. Outcome trials with PCSK9 inhibitors have not yet been completed, but the estimate of the risk reduction based on preliminary data is 50%.
“If the preliminary findings are correct, PCSK9 inhibitors would be expected to provide direct savings of $160 billion,” Dr. Prince said. These savings are less surprising when current average costs for a single percutaneous coronary intervention are at $70,000, Dr. Prince said at the meeting, sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“In addition, indirect saving from avoiding loss of productivity and even death is reasonably estimated to be in the range of $170 billion,” said Dr. Prince, citing data that would support these figures.
While he acknowledged the limitations of extrapolating data from a single center for these cost estimates, Dr. Prince suggested this is a useful exercise for considering the role of these agents, which provide an unprecedented opportunity to bring high-risk patients to guideline-recommended LDL-C goals. Of the weaknesses of the study, however, the most important may be the absence of level 1 evidence that the cardiovascular risk reductions provided by PCSK9 inhibitors will be commensurate with their potent lipid lowering. Data from the ongoing outcome trials are more than a year away.
WASHINGTON - Treating all of the estimated 23 million individuals in the United States who would qualify for PCSK9 inhibitor therapy on the basis of current Food and Drug Administration indications and insurance company reimbursement criteria may be cost effective, according to data extrapolated from a tertiary center lipid clinic.
That is, if the two approved PCSK9 inhibitors are shown to reduce cardiovascular events in outcome studies.
With the current annual cost of PCSK9 therapy estimated to be roughly $14,000 per patient, the total bill for treating all candidates is an estimated $320 billion per year, but this huge sum may be offset by savings in medical care and lost productivity, Dr. Marloe Prince said at Cardiovascular Research Technologies 2016.
“Cost is a real issue for PCSK9 inhibitors,” reported Dr. Prince, an internal medicine resident at Jewish Hospital in Cincinnati. “Our aim was to determine first what percentage of patients would quality for PCSK9 inhibitor therapy and then to perform a cost efficacy analysis based on these estimates.”
The two PCSK9 inhibitors approved for lowering LDL cholesterol, evolocumab and alirocumab, are priced similarly. Both reduce LDL cholesterol by about 60% in most patients. Several published trials have demonstrated that PCSK9 inhibitors, which are administered by injection at intervals ranging from 2 weeks to 1 month, can bring a substantial proportion of high-risk patients to guideline recommended goals when maximally tolerated statins fail to do so.
In this study, the first step was to determine what proportion of patients referred to a lipid clinic for inadequate control of LDL cholesterol are eligible for PCSK9 inhibitors on the basis of current FDA indications and commercial insurance criteria. These indications include LDL cholesterol levels above guideline recommended targets in patients on maximally tolerated statins with heterozygous (HeFH) or homozygous (HoFH) familial hypercholesterolemia as well as those who have had a previous cardiovascular event.
Of 734 consecutive patients referred to the lipid clinic who had been managed for at least 2 months on maximally tolerated statins, 220 (30%) were eligible for a PCSK9 prescription on the basis of both the current FDA indication and commercial insurance reimbursement criteria. Applying this 30% figure to previously published estimates of patients not at goal on maximally tolerated statins, Dr. Prince and his coinvestigators estimated that there are 23 million Americans with hypercholesterolemia who would similarly qualify for a PCSK9 inhibitor.
The cost of supplying all 23 million individuals with a PCSK9 inhibitor is roughly $322 billion annually, but Dr. Prince cited data indicating that the estimated costs for treating cardiovascular events and stroke per year is also about $320 billion. Outcome trials with PCSK9 inhibitors have not yet been completed, but the estimate of the risk reduction based on preliminary data is 50%.
“If the preliminary findings are correct, PCSK9 inhibitors would be expected to provide direct savings of $160 billion,” Dr. Prince said. These savings are less surprising when current average costs for a single percutaneous coronary intervention are at $70,000, Dr. Prince said at the meeting, sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“In addition, indirect saving from avoiding loss of productivity and even death is reasonably estimated to be in the range of $170 billion,” said Dr. Prince, citing data that would support these figures.
While he acknowledged the limitations of extrapolating data from a single center for these cost estimates, Dr. Prince suggested this is a useful exercise for considering the role of these agents, which provide an unprecedented opportunity to bring high-risk patients to guideline-recommended LDL-C goals. Of the weaknesses of the study, however, the most important may be the absence of level 1 evidence that the cardiovascular risk reductions provided by PCSK9 inhibitors will be commensurate with their potent lipid lowering. Data from the ongoing outcome trials are more than a year away.
WASHINGTON - Treating all of the estimated 23 million individuals in the United States who would qualify for PCSK9 inhibitor therapy on the basis of current Food and Drug Administration indications and insurance company reimbursement criteria may be cost effective, according to data extrapolated from a tertiary center lipid clinic.
That is, if the two approved PCSK9 inhibitors are shown to reduce cardiovascular events in outcome studies.
With the current annual cost of PCSK9 therapy estimated to be roughly $14,000 per patient, the total bill for treating all candidates is an estimated $320 billion per year, but this huge sum may be offset by savings in medical care and lost productivity, Dr. Marloe Prince said at Cardiovascular Research Technologies 2016.
“Cost is a real issue for PCSK9 inhibitors,” reported Dr. Prince, an internal medicine resident at Jewish Hospital in Cincinnati. “Our aim was to determine first what percentage of patients would quality for PCSK9 inhibitor therapy and then to perform a cost efficacy analysis based on these estimates.”
The two PCSK9 inhibitors approved for lowering LDL cholesterol, evolocumab and alirocumab, are priced similarly. Both reduce LDL cholesterol by about 60% in most patients. Several published trials have demonstrated that PCSK9 inhibitors, which are administered by injection at intervals ranging from 2 weeks to 1 month, can bring a substantial proportion of high-risk patients to guideline recommended goals when maximally tolerated statins fail to do so.
In this study, the first step was to determine what proportion of patients referred to a lipid clinic for inadequate control of LDL cholesterol are eligible for PCSK9 inhibitors on the basis of current FDA indications and commercial insurance criteria. These indications include LDL cholesterol levels above guideline recommended targets in patients on maximally tolerated statins with heterozygous (HeFH) or homozygous (HoFH) familial hypercholesterolemia as well as those who have had a previous cardiovascular event.
Of 734 consecutive patients referred to the lipid clinic who had been managed for at least 2 months on maximally tolerated statins, 220 (30%) were eligible for a PCSK9 prescription on the basis of both the current FDA indication and commercial insurance reimbursement criteria. Applying this 30% figure to previously published estimates of patients not at goal on maximally tolerated statins, Dr. Prince and his coinvestigators estimated that there are 23 million Americans with hypercholesterolemia who would similarly qualify for a PCSK9 inhibitor.
The cost of supplying all 23 million individuals with a PCSK9 inhibitor is roughly $322 billion annually, but Dr. Prince cited data indicating that the estimated costs for treating cardiovascular events and stroke per year is also about $320 billion. Outcome trials with PCSK9 inhibitors have not yet been completed, but the estimate of the risk reduction based on preliminary data is 50%.
“If the preliminary findings are correct, PCSK9 inhibitors would be expected to provide direct savings of $160 billion,” Dr. Prince said. These savings are less surprising when current average costs for a single percutaneous coronary intervention are at $70,000, Dr. Prince said at the meeting, sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“In addition, indirect saving from avoiding loss of productivity and even death is reasonably estimated to be in the range of $170 billion,” said Dr. Prince, citing data that would support these figures.
While he acknowledged the limitations of extrapolating data from a single center for these cost estimates, Dr. Prince suggested this is a useful exercise for considering the role of these agents, which provide an unprecedented opportunity to bring high-risk patients to guideline-recommended LDL-C goals. Of the weaknesses of the study, however, the most important may be the absence of level 1 evidence that the cardiovascular risk reductions provided by PCSK9 inhibitors will be commensurate with their potent lipid lowering. Data from the ongoing outcome trials are more than a year away.
AT CARDIOVASCULAR RESEARCH TECHNOLOGIES 2016
Key clinical point: If cardiovascular protection with PCSK9 inhibitors meets expectations, treating all 23 million Americans who may be indicated for this treatment is likely to be cost effective.
Major finding: The estimated annual drug costs of $320 billion would be offset by an estimated $160 billion in direct and $170 billion in indirect cost savings.
Data source: Extrapolation from single-center experience.
Disclosures: Dr. Prince reports no financial relationships relevant to this study.
Among hospitalized patients with diabetes, 25% have undiagnosed diabetic retinopathy
The prevalence of undiagnosed diabetic retinopathy was 25% and that of sight-threatening diabetic retinopathy was 19% of an inpatient population of patients with diabetes, compared with the general population; researchers identified several barriers to ophthalmic care.
Diabetic retinopathy and sight-threatening diabetic retinopathy are estimated at a prevalence of 28.5% and 4.4%, respectively. In contrast, there is little research in to the prevalence of undiagnosed diabetic retinopathy or sight-threatening diabetic retinopathy in higher risk inpatients.
Dr. Jessica Kovarik, who at the time of this research was with the UPMC Eye Center at the University of Pittsburgh, and her associates sought to identify the prevalence of undiagnosed diabetic retinopathy among inpatients with established diabetes as well as barriers to diabetic retinopathy examinations and treatment.
They conducted a cross-sectional analysis of diabetic patients admitted to an urban teaching hospital in Pittsburgh. Digital funduscopic images were obtained to determine the presence and severity of diabetic retinopathy and macular edema. Questionnaires assessed barriers to ophthalmic examinations and demographics (BMJ Open Diab Res Care. 2016;4:e000164 [doi: 10.1136/bmjdrc-2015-000164]).
In total, 113 patients were eligible and 5 were excluded from analysis of diabetic retinopathy prevalence due to an inability to take images or poor-quality images.
Among the patients, 61 were women, 83 were white, and 34 were aged 50-60 years. Most had health insurance (89%) and an ophthalmologist (64%), and most understood that diabetic retinopathy affects vision (91%). Further, patients reported a history of type 2 diabetes (96%), hypertension (85%), hyperlipidemia (68%), renal disease (25%), peripheral vascular disease (55%), and coronary artery disease (52%).
Among those who had not had a dilated funduscopic examination within a year, barriers to screening examination included transportation issues, physical disability, too many appointments or being too sick, cost, lack of time or priority, or no visual impairment. Forty percent reported having an eye examination within the year and 5% reported never having an eye examination.
The investigators identified 7 patients with clinically significant macular edema (6%), 13 with proliferative diabetic retinopathy (12%), and 1 with severe (1%), 14 with moderate (13%), and 16 with mild nonproliferative diabetic retinopathy (15%). Overall, 44% of the patients had diabetic retinopathy, with 25% previously undiagnosed. Further, sight-threatening diabetic retinopathy was found in 19%, with 3.7% previously undiagnosed.
Finally, after multivariable analysis, a longer duration of diabetes (odds ratio, 1.08 per year; 95% confidence interval, 1.014-1.147; P =.017) and renal disease (OR, 3.86; 95% CI, 1.22-12.27; P =.022) was associated with diabetic retinopathy. Further, of the 17 patients admitted with osteomyelitis or a nonhealing diabetic ulcer, 15 (88.2%) had diabetic retinopathy.
“Curiously, most inpatients in our population (91%) are aware of the ocular complications of diabetes, and many (64%) do have ophthalmologists (more than any other subspecialty listed), yet only a minority (40%) of patients are getting the recommended standard of care screening examinations. Barriers that are unique to this high-risk population may explain this disparity,” the authors wrote.
The study was funded by the National Institutes of Health, Eye and Ear Foundation of Pittsburgh, Clinical and Translational Science Institute, the University of Pittsburgh, and a grant from Research to Prevent Blindness. One of the researchers, Dr. Jann Johnston, reported speaking for Medtronic, Lilly, and Sanofi.
The prevalence of undiagnosed diabetic retinopathy was 25% and that of sight-threatening diabetic retinopathy was 19% of an inpatient population of patients with diabetes, compared with the general population; researchers identified several barriers to ophthalmic care.
Diabetic retinopathy and sight-threatening diabetic retinopathy are estimated at a prevalence of 28.5% and 4.4%, respectively. In contrast, there is little research in to the prevalence of undiagnosed diabetic retinopathy or sight-threatening diabetic retinopathy in higher risk inpatients.
Dr. Jessica Kovarik, who at the time of this research was with the UPMC Eye Center at the University of Pittsburgh, and her associates sought to identify the prevalence of undiagnosed diabetic retinopathy among inpatients with established diabetes as well as barriers to diabetic retinopathy examinations and treatment.
They conducted a cross-sectional analysis of diabetic patients admitted to an urban teaching hospital in Pittsburgh. Digital funduscopic images were obtained to determine the presence and severity of diabetic retinopathy and macular edema. Questionnaires assessed barriers to ophthalmic examinations and demographics (BMJ Open Diab Res Care. 2016;4:e000164 [doi: 10.1136/bmjdrc-2015-000164]).
In total, 113 patients were eligible and 5 were excluded from analysis of diabetic retinopathy prevalence due to an inability to take images or poor-quality images.
Among the patients, 61 were women, 83 were white, and 34 were aged 50-60 years. Most had health insurance (89%) and an ophthalmologist (64%), and most understood that diabetic retinopathy affects vision (91%). Further, patients reported a history of type 2 diabetes (96%), hypertension (85%), hyperlipidemia (68%), renal disease (25%), peripheral vascular disease (55%), and coronary artery disease (52%).
Among those who had not had a dilated funduscopic examination within a year, barriers to screening examination included transportation issues, physical disability, too many appointments or being too sick, cost, lack of time or priority, or no visual impairment. Forty percent reported having an eye examination within the year and 5% reported never having an eye examination.
The investigators identified 7 patients with clinically significant macular edema (6%), 13 with proliferative diabetic retinopathy (12%), and 1 with severe (1%), 14 with moderate (13%), and 16 with mild nonproliferative diabetic retinopathy (15%). Overall, 44% of the patients had diabetic retinopathy, with 25% previously undiagnosed. Further, sight-threatening diabetic retinopathy was found in 19%, with 3.7% previously undiagnosed.
Finally, after multivariable analysis, a longer duration of diabetes (odds ratio, 1.08 per year; 95% confidence interval, 1.014-1.147; P =.017) and renal disease (OR, 3.86; 95% CI, 1.22-12.27; P =.022) was associated with diabetic retinopathy. Further, of the 17 patients admitted with osteomyelitis or a nonhealing diabetic ulcer, 15 (88.2%) had diabetic retinopathy.
“Curiously, most inpatients in our population (91%) are aware of the ocular complications of diabetes, and many (64%) do have ophthalmologists (more than any other subspecialty listed), yet only a minority (40%) of patients are getting the recommended standard of care screening examinations. Barriers that are unique to this high-risk population may explain this disparity,” the authors wrote.
The study was funded by the National Institutes of Health, Eye and Ear Foundation of Pittsburgh, Clinical and Translational Science Institute, the University of Pittsburgh, and a grant from Research to Prevent Blindness. One of the researchers, Dr. Jann Johnston, reported speaking for Medtronic, Lilly, and Sanofi.
The prevalence of undiagnosed diabetic retinopathy was 25% and that of sight-threatening diabetic retinopathy was 19% of an inpatient population of patients with diabetes, compared with the general population; researchers identified several barriers to ophthalmic care.
Diabetic retinopathy and sight-threatening diabetic retinopathy are estimated at a prevalence of 28.5% and 4.4%, respectively. In contrast, there is little research in to the prevalence of undiagnosed diabetic retinopathy or sight-threatening diabetic retinopathy in higher risk inpatients.
Dr. Jessica Kovarik, who at the time of this research was with the UPMC Eye Center at the University of Pittsburgh, and her associates sought to identify the prevalence of undiagnosed diabetic retinopathy among inpatients with established diabetes as well as barriers to diabetic retinopathy examinations and treatment.
They conducted a cross-sectional analysis of diabetic patients admitted to an urban teaching hospital in Pittsburgh. Digital funduscopic images were obtained to determine the presence and severity of diabetic retinopathy and macular edema. Questionnaires assessed barriers to ophthalmic examinations and demographics (BMJ Open Diab Res Care. 2016;4:e000164 [doi: 10.1136/bmjdrc-2015-000164]).
In total, 113 patients were eligible and 5 were excluded from analysis of diabetic retinopathy prevalence due to an inability to take images or poor-quality images.
Among the patients, 61 were women, 83 were white, and 34 were aged 50-60 years. Most had health insurance (89%) and an ophthalmologist (64%), and most understood that diabetic retinopathy affects vision (91%). Further, patients reported a history of type 2 diabetes (96%), hypertension (85%), hyperlipidemia (68%), renal disease (25%), peripheral vascular disease (55%), and coronary artery disease (52%).
Among those who had not had a dilated funduscopic examination within a year, barriers to screening examination included transportation issues, physical disability, too many appointments or being too sick, cost, lack of time or priority, or no visual impairment. Forty percent reported having an eye examination within the year and 5% reported never having an eye examination.
The investigators identified 7 patients with clinically significant macular edema (6%), 13 with proliferative diabetic retinopathy (12%), and 1 with severe (1%), 14 with moderate (13%), and 16 with mild nonproliferative diabetic retinopathy (15%). Overall, 44% of the patients had diabetic retinopathy, with 25% previously undiagnosed. Further, sight-threatening diabetic retinopathy was found in 19%, with 3.7% previously undiagnosed.
Finally, after multivariable analysis, a longer duration of diabetes (odds ratio, 1.08 per year; 95% confidence interval, 1.014-1.147; P =.017) and renal disease (OR, 3.86; 95% CI, 1.22-12.27; P =.022) was associated with diabetic retinopathy. Further, of the 17 patients admitted with osteomyelitis or a nonhealing diabetic ulcer, 15 (88.2%) had diabetic retinopathy.
“Curiously, most inpatients in our population (91%) are aware of the ocular complications of diabetes, and many (64%) do have ophthalmologists (more than any other subspecialty listed), yet only a minority (40%) of patients are getting the recommended standard of care screening examinations. Barriers that are unique to this high-risk population may explain this disparity,” the authors wrote.
The study was funded by the National Institutes of Health, Eye and Ear Foundation of Pittsburgh, Clinical and Translational Science Institute, the University of Pittsburgh, and a grant from Research to Prevent Blindness. One of the researchers, Dr. Jann Johnston, reported speaking for Medtronic, Lilly, and Sanofi.
FROM BMJ DIABETES RESEARCH AND CARE
Key clinical point: Among hospitalized patients with diabetes, 25% had undiagnosed diabetic retinopathy and 19% had undiagnosed sight-threatening diabetic retinopathy; it was most common in patients with a nonhealing ulcer and/or a history of renal disease at admission.
Major finding: While many patients knew they were at risk for retinopathy, they faced significant barriers to receiving ophthalmic care, including lack of transportation and physical disability.
Data source: A cross-sectional analysis of 113 consecutive patients with diabetes who were admitted to an urban teaching hospital with digital funduscopic images to determine the presence and severity of diabetic retinopathy and questionnaires to assess barriers to care.
Disclosures: The study was funded by the National Institutes of Health, Eye and Ear Foundation of Pittsburgh, Clinical and Translational Science Institute, the University of Pittsburgh, and a grant from Research to Prevent Blindness. Dr. Jann Johnston reported speaking for Lilly, Medtronic, and Sanofi.
Pediatric BMI increases linked to rises in blood pressure, hypertension risk
Children’s and adolescents’ risk of blood pressure increases and hypertension rose as their body mass index increased, even over a short period of a few years, according to a recent study.
“Obesity, especially severe obesity, at a young age confers an increased risk of early onset of cardiometabolic diseases such as hypertension,” wrote Emily D. Parker, Ph.D., of the HealthPartners Institute for Education and Research in Minneapolis, and her associates online (Pediatrics. 2016 Feb 19. doi: 10.1542/peds.2015-1662). “The significant adverse effect of weight gain and obesity early in life, and over a short period of time, emphasizes the importance of developing early and effective clinical and public health strategies directed at the primary prevention of overweight and obesity.”
The researchers retrospectively analyzed the health care records of 100,606 children and adolescents, aged 3-17 years, who received care from HealthPartners Medical Group in Minnesota, Kaiser Permanente Colorado, or Kaiser Permanente Northern California. All the patients had not been hypertensive within the 6 months before baseline measurements and had at least three primary care visits with blood pressure measurements between January 2007 and December 2011.
At baseline, 16% of the patients were overweight, 2% were obese, and 4% were severely obese. The majority (92%) were below the 90th percentile for their systolic blood pressure at baseline, while 4% were prehypertensive and 4% were hypertensive (at or above 95th percentile). Over a median 3.1 years of follow-up per person, 0.3% of the patients became hypertensive, translating to an incidence rate of 0.15 new cases per year.
After accounting for demographics, baseline blood pressure percentiles, year, and site, both children (aged 3-11) and adolescents with obesity were about twice as likely as children and adolescents with low healthy weights to develop hypertension (hazard ratio, 2.02 and HR, 2.20, respectively). Children and adolescents with severe obesity had more than a four times greater risk of developing hypertension (HR, 4.42 and HR, 4.46, respectively), compared with those with a low healthy weight. These were significant differences. No association appeared between those with low-normal weights at baseline and either high-normal or overweight categories during follow-up.
Forty percent of the children and 24% of the adolescents dropped from severely obese to obese during follow-up, and 45% of the children and 55% of the adolescents who were obese at baseline remained so throughout follow-up. Among children overweight at baseline, 19% became obese, 0.7% became severely obese, and 44% became a healthy weight. Among initially overweight adolescents, 13% became obese, 0.1% became severely obese, and 34% became a healthy weight.
“There was a strong association between change in BMI [body mass index] category and change in blood pressure across BMI categories in both age groups and genders,” Dr. Parker and her associates wrote. “In girls and boys 3-11 years old, both systolic blood pressure and diastolic blood pressure percentiles increased significantly when BMI increased from normal to either overweight or obese and when it increased from overweight to obese.” Similar but greater changes were seen among the adolescents, particularly among girls aged 12-17 years.
Correspondingly, children and teens who dropped from a higher to a lower BMI category had statistically significant drops in both systolic and diastolic blood pressure.
Risk of hypertension tripled for those with obesity at baseline who remained obese through follow-up (HR, 3.71 for children; HR, 3.64 for teens).
The study was funded by the National Heart, Lung, and Blood Institute. Most of the investigators had no relevant financial disclosures. Dr. Joan C. Lo has received previous research funding from Sanofi unrelated to this study.
Children’s and adolescents’ risk of blood pressure increases and hypertension rose as their body mass index increased, even over a short period of a few years, according to a recent study.
“Obesity, especially severe obesity, at a young age confers an increased risk of early onset of cardiometabolic diseases such as hypertension,” wrote Emily D. Parker, Ph.D., of the HealthPartners Institute for Education and Research in Minneapolis, and her associates online (Pediatrics. 2016 Feb 19. doi: 10.1542/peds.2015-1662). “The significant adverse effect of weight gain and obesity early in life, and over a short period of time, emphasizes the importance of developing early and effective clinical and public health strategies directed at the primary prevention of overweight and obesity.”
The researchers retrospectively analyzed the health care records of 100,606 children and adolescents, aged 3-17 years, who received care from HealthPartners Medical Group in Minnesota, Kaiser Permanente Colorado, or Kaiser Permanente Northern California. All the patients had not been hypertensive within the 6 months before baseline measurements and had at least three primary care visits with blood pressure measurements between January 2007 and December 2011.
At baseline, 16% of the patients were overweight, 2% were obese, and 4% were severely obese. The majority (92%) were below the 90th percentile for their systolic blood pressure at baseline, while 4% were prehypertensive and 4% were hypertensive (at or above 95th percentile). Over a median 3.1 years of follow-up per person, 0.3% of the patients became hypertensive, translating to an incidence rate of 0.15 new cases per year.
After accounting for demographics, baseline blood pressure percentiles, year, and site, both children (aged 3-11) and adolescents with obesity were about twice as likely as children and adolescents with low healthy weights to develop hypertension (hazard ratio, 2.02 and HR, 2.20, respectively). Children and adolescents with severe obesity had more than a four times greater risk of developing hypertension (HR, 4.42 and HR, 4.46, respectively), compared with those with a low healthy weight. These were significant differences. No association appeared between those with low-normal weights at baseline and either high-normal or overweight categories during follow-up.
Forty percent of the children and 24% of the adolescents dropped from severely obese to obese during follow-up, and 45% of the children and 55% of the adolescents who were obese at baseline remained so throughout follow-up. Among children overweight at baseline, 19% became obese, 0.7% became severely obese, and 44% became a healthy weight. Among initially overweight adolescents, 13% became obese, 0.1% became severely obese, and 34% became a healthy weight.
“There was a strong association between change in BMI [body mass index] category and change in blood pressure across BMI categories in both age groups and genders,” Dr. Parker and her associates wrote. “In girls and boys 3-11 years old, both systolic blood pressure and diastolic blood pressure percentiles increased significantly when BMI increased from normal to either overweight or obese and when it increased from overweight to obese.” Similar but greater changes were seen among the adolescents, particularly among girls aged 12-17 years.
Correspondingly, children and teens who dropped from a higher to a lower BMI category had statistically significant drops in both systolic and diastolic blood pressure.
Risk of hypertension tripled for those with obesity at baseline who remained obese through follow-up (HR, 3.71 for children; HR, 3.64 for teens).
The study was funded by the National Heart, Lung, and Blood Institute. Most of the investigators had no relevant financial disclosures. Dr. Joan C. Lo has received previous research funding from Sanofi unrelated to this study.
Children’s and adolescents’ risk of blood pressure increases and hypertension rose as their body mass index increased, even over a short period of a few years, according to a recent study.
“Obesity, especially severe obesity, at a young age confers an increased risk of early onset of cardiometabolic diseases such as hypertension,” wrote Emily D. Parker, Ph.D., of the HealthPartners Institute for Education and Research in Minneapolis, and her associates online (Pediatrics. 2016 Feb 19. doi: 10.1542/peds.2015-1662). “The significant adverse effect of weight gain and obesity early in life, and over a short period of time, emphasizes the importance of developing early and effective clinical and public health strategies directed at the primary prevention of overweight and obesity.”
The researchers retrospectively analyzed the health care records of 100,606 children and adolescents, aged 3-17 years, who received care from HealthPartners Medical Group in Minnesota, Kaiser Permanente Colorado, or Kaiser Permanente Northern California. All the patients had not been hypertensive within the 6 months before baseline measurements and had at least three primary care visits with blood pressure measurements between January 2007 and December 2011.
At baseline, 16% of the patients were overweight, 2% were obese, and 4% were severely obese. The majority (92%) were below the 90th percentile for their systolic blood pressure at baseline, while 4% were prehypertensive and 4% were hypertensive (at or above 95th percentile). Over a median 3.1 years of follow-up per person, 0.3% of the patients became hypertensive, translating to an incidence rate of 0.15 new cases per year.
After accounting for demographics, baseline blood pressure percentiles, year, and site, both children (aged 3-11) and adolescents with obesity were about twice as likely as children and adolescents with low healthy weights to develop hypertension (hazard ratio, 2.02 and HR, 2.20, respectively). Children and adolescents with severe obesity had more than a four times greater risk of developing hypertension (HR, 4.42 and HR, 4.46, respectively), compared with those with a low healthy weight. These were significant differences. No association appeared between those with low-normal weights at baseline and either high-normal or overweight categories during follow-up.
Forty percent of the children and 24% of the adolescents dropped from severely obese to obese during follow-up, and 45% of the children and 55% of the adolescents who were obese at baseline remained so throughout follow-up. Among children overweight at baseline, 19% became obese, 0.7% became severely obese, and 44% became a healthy weight. Among initially overweight adolescents, 13% became obese, 0.1% became severely obese, and 34% became a healthy weight.
“There was a strong association between change in BMI [body mass index] category and change in blood pressure across BMI categories in both age groups and genders,” Dr. Parker and her associates wrote. “In girls and boys 3-11 years old, both systolic blood pressure and diastolic blood pressure percentiles increased significantly when BMI increased from normal to either overweight or obese and when it increased from overweight to obese.” Similar but greater changes were seen among the adolescents, particularly among girls aged 12-17 years.
Correspondingly, children and teens who dropped from a higher to a lower BMI category had statistically significant drops in both systolic and diastolic blood pressure.
Risk of hypertension tripled for those with obesity at baseline who remained obese through follow-up (HR, 3.71 for children; HR, 3.64 for teens).
The study was funded by the National Heart, Lung, and Blood Institute. Most of the investigators had no relevant financial disclosures. Dr. Joan C. Lo has received previous research funding from Sanofi unrelated to this study.
FROM PEDIATRICS
Key clinical point: The risk of blood pressure increases and even hypertension rises with increasing BMI among youth aged 3-17 years.
Major finding: Incident hypertension risk doubled for children and adolescents with obesity (HR, 2.02 and HR, 2.20, respectively) and quadrupled for those with severe obesity (HR, 4.42 and HR, 4.46, respectively).
Data source: The findings are based on a retrospective cohort study of 100,606 individuals, aged 3-17 years, from one of three U.S. health systems who were tracked over a median 3.1 years.
Disclosures: The study was funded by the National Heart, Lung, and Blood Institute. Dr. Lo has received previous research funding from Sanofi.
Diabetes duration, depression linked in elderly men
A longer duration of diabetes is associated with a greater risk of depression in men aged 70-89, according to Dr. Osvaldo P. Almeida and associates.
In their sample of 5,462 elderly men, 932 had diabetes, and 976 had current or past depression. Of those with diabetes, 215 had current or past depression. The odds ratio of diabetic men ever being depressed was 1.49, and the OR of current depression was 1.94.
The association between depression and diabetes duration was J shaped, with ORs of 1.92 for those with less than 10 years of diabetes history, 1.56 for those with 10-19.9 years of diabetes, 2.49 for those with 20-29.9 years of diabetes, and 3.13 for those with more than 30 years of diabetes.
Frailty was a very significant predictor of depression in diabetic men, but it accounted for about 15% of the association between diabetes and depression, the investigators noted.
“The severity of comorbidity may also play a role, and this could explain why the association between diabetes and depression becomes more obvious during the later stages of illness. Sufficiently powered prospective studies with prolonged follow-up, limited attrition, and robust measures of comorbidity should provide greater certainty about the true nature of these associations,” the investigators concluded.
Find the study in Maturitas (doi: 10.1016/j.maturitas.2016.01.003).
A longer duration of diabetes is associated with a greater risk of depression in men aged 70-89, according to Dr. Osvaldo P. Almeida and associates.
In their sample of 5,462 elderly men, 932 had diabetes, and 976 had current or past depression. Of those with diabetes, 215 had current or past depression. The odds ratio of diabetic men ever being depressed was 1.49, and the OR of current depression was 1.94.
The association between depression and diabetes duration was J shaped, with ORs of 1.92 for those with less than 10 years of diabetes history, 1.56 for those with 10-19.9 years of diabetes, 2.49 for those with 20-29.9 years of diabetes, and 3.13 for those with more than 30 years of diabetes.
Frailty was a very significant predictor of depression in diabetic men, but it accounted for about 15% of the association between diabetes and depression, the investigators noted.
“The severity of comorbidity may also play a role, and this could explain why the association between diabetes and depression becomes more obvious during the later stages of illness. Sufficiently powered prospective studies with prolonged follow-up, limited attrition, and robust measures of comorbidity should provide greater certainty about the true nature of these associations,” the investigators concluded.
Find the study in Maturitas (doi: 10.1016/j.maturitas.2016.01.003).
A longer duration of diabetes is associated with a greater risk of depression in men aged 70-89, according to Dr. Osvaldo P. Almeida and associates.
In their sample of 5,462 elderly men, 932 had diabetes, and 976 had current or past depression. Of those with diabetes, 215 had current or past depression. The odds ratio of diabetic men ever being depressed was 1.49, and the OR of current depression was 1.94.
The association between depression and diabetes duration was J shaped, with ORs of 1.92 for those with less than 10 years of diabetes history, 1.56 for those with 10-19.9 years of diabetes, 2.49 for those with 20-29.9 years of diabetes, and 3.13 for those with more than 30 years of diabetes.
Frailty was a very significant predictor of depression in diabetic men, but it accounted for about 15% of the association between diabetes and depression, the investigators noted.
“The severity of comorbidity may also play a role, and this could explain why the association between diabetes and depression becomes more obvious during the later stages of illness. Sufficiently powered prospective studies with prolonged follow-up, limited attrition, and robust measures of comorbidity should provide greater certainty about the true nature of these associations,” the investigators concluded.
Find the study in Maturitas (doi: 10.1016/j.maturitas.2016.01.003).
FROM MATURITAS
Primary care endures in heart failure management
Heart failure management has become increasingly complex over the past couple of decades, with new drugs and drug combinations, new uses for potentially life-saving implanted devices, and a more sophisticated appreciation of the ways that various comorbidities complicate a heart failure patient’s clinical status. These expanded dimensions of heart failure care resulted in the establishment in 2008 of a new secondary subspecialty, Advanced Heart Failure and Transplant Cardiology, aimed at training and certifying physicians in all the nuances of complex heart failure diagnostics and care.
But as the 2009 manifesto announcing this new heart failure subspecialty detailed, care for the vast majority of U.S. patients with heart failure remains in the hands of internal medicine primary care physicians (PCPs) and general cardiologists (J Am Coll Cardiol. 2009 Mar 10;53[10]:834-6). To some extent this is a manpower issue. The estimated number of Americans living with heart failure exceeds 5 million, a figure that dwarfs the very modest number of U.S. physicians and clinicians who are certified or self-identified heart failure specialists.
As of today, fewer than 1,000 U.S. physicians have received formal certification as heart failure subspecialists through the examination administered in 2010, 2012, and 2014, said Michele Blair, chief executive officer of the Heart Failure Society of America. A more liberal definition of a heart failure specialist might include the roughly 3,000 unique physicians (mostly cardiologists, but also some hospitalists and emergency physicians) who have recently attended an annual meeting of the HFSA, as well as the roughly 2,300 physician assistants and nurse practitioners who have shown a heart failure interest by coming to a recent HFSA meeting. But even these expanded estimates calculate out to about 1 clinician with a special interest in heart failure for each 1,000 heart failure patients, not a very reassuring ratio.
The burgeoning numbers of heart failure patients, compared with the relative scarcity of both heart failure experts and general cardiologists, raises issues of how primary-care internists best share this management responsibility. Recent interviews with several heart failure subspecialists and primary care internists provide some insight into how this division of labor is now playing out in routine U.S. practice. What often occurs is that primary care internists take exclusive responsibility for caring for heart failure patients until they feel they are getting in over their heads, at which time they’ll consult with a cardiology colleague or refer the patient to a cardiologist. That moment of recognition by the generalist – that the demands and complexity of the case exceed their comfort level – varies widely, with some PCPs referring patients as soon as heart failure symptoms appear while others stay comfortable as the primary care giver even as a patient’s disease deteriorates to a more advanced stage.
Heart failure specialists highlighted their reliance on PCPs to take an ongoing, active role even for patients with significantly advanced heart failure, as generalists are well suited to coordinating the multispecialty care that such patients usually require, with attention to their need for lifestyle modifications as well as management of their diabetes, sleep apnea, chronic obstructive pulmonary disease, renal failure, and other comorbidities.
As Dr. Michael K. Ong, a primary care internist at the University of California, Los Angeles, said in an interview, his heart failure specialist colleague manages patients’ heart failure; “I manage [or refer] everything else not directly related to the heart failure.”
The most successful U.S. care models seem to be some variation on a team-care approach, in which physicians collaborate with pharmacists, nurses, rehabilitation specialists, and social workers as well as specialists, a team that would include and perhaps be led by either a primary care internist, a cardiologist, or a heart failure specialist but would also broadly include physicians able to deal with all the morbidity facets of heart failure. It’s a model that remains unavailable in many U.S. settings or is just starting to emerge, as fee-for-service coverage of patients gets replaced by population-management models that better accommodate the upfront financial demands of coordinated team care. It makes financial sense a few years down the road when improved patient outcomes result in cost savings.
Primary care and patients with symptomatic heart failure
The heart failure definitions and staging system established in 2001 by a guidelines panel of the American College of Cardiology and American Heart Association defined stage A heart failure as starting before a patient exhibits any heart failure symptoms (the classic ones include dyspnea, rales, and peripheral edema). The panel designated symptomatic heart failure patients as stage C. Patients without heart failure symptoms but with one or more risk factors (such as hypertension, diabetes, obesity, and cardiovascular disease) plus structural heart disease (such as cardiomyopathy or other forms of heart remodeling) were designated stage B. The panel said that people at stage A had one or more risk factors but no structural heart changes and no heart failure symptoms.
Although stage-A heart failure patients are clearly the types of people most often seen and cared for by PCPs, many of these physicians, as well as many heart failure specialists, don’t consider patients who have only hypertension or only diabetes or only obesity as yet having heart failure. That paradox deserves more discussion, but the best way to begin talking about PCPs and heart failure patients is when patients are symptomatic and have what everyone would agree is heart failure.
Even though the ACC/AHA staging system places stage C patients well down the heart failure road, stage C is usually when patients are first diagnosed with heart failure. Although the diagnosis is often first made by a hospitalist or emergency-department physician when severe and sudden-onset heart failure symptoms drive the patient to a hospital, or the diagnosis originates with a cardiologist or heart failure specialist when the patient’s presentation and differential diagnosis isn’t straightforward, most commonly the diagnosis starts with a PCP in an office encounter with a patient who is symptomatic but not acutely ill.
“Patients with shortness of breath or other forms of effort intolerance most often seek care from PCPs. The differential diagnosis of dyspnea is long and complex. Recognition that a patient with dyspnea may have HF is crucial” for timely management and treatment, said Dr. Mary Norine Walsh, medical director of Heart Failure and Cardiac Transplantation at St. Vincent Heart Center in Indianapolis.
At the Mayo Clinic in Rochester, Minn., “most of the heart failure diagnoses are done by PCPs, usually first identified at stage C when a patient comes in with symptoms. Stage B heart failure is usually only identified as an incidental finding when echocardiography is done for some other reason,” said Dr. Paul M. McKie, a heart failure cardiologist who works closely with the primary-care staff at Mayo as an embedded consultant cardiologist.
According to Dr. Mariell L. Jessup, a heart failure physician and professor at the University of Pennsylvania in Philadelphia, a key to PCPs promptly identifying patients with recent-onset, stage C heart failure is to keep the disease as well as its prominent risk factors at the top of their differential-diagnosis list for at-risk patients. “Heart failure is a common disorder,” Dr. Jessup said, and must be considered for patients with shortness of breath. “The leading causes of heart failure are hypertension, obesity, and diabetes. So keep heart failure in mind, especially for patients with one or more of these risk factors.”
Although PCPs might order an echocardiography examination or a lab test like measurement of brain natriuretic protein (BNP) to help nail down the diagnosis, they often leave reading the echocardiography results to a cardiologist colleague. “When a PCP orders an echo it’s automatically read by a cardiologist, and then we get the cardiologist’s report. I don’t read echos myself,” said Dr. Rebecca J. Cunningham, an internal medicine PCP at Brigham and Women’s Hospital in Boston who frequently sees patients with heart failure as medical director of the hospital’s Integrated Care Management Program. “I had one PCP colleague who undertook additional training to learn to read echos himself, but that’s unusual.”
Dr. Mary Ann Bauman, an internal medicine PCP and medical director for Women’s Health and Community Relations at INTEGRIS Health in Oklahoma City, noted a similar division of labor. “If a patient has shortness of breath, maybe some edema, and I hear a few rales, but is totally functional, I always order an echo but I don’t read it. I refer the echo to a cardiologist who then sends me a report,” Dr. Bauman said in an interview. “If I think the patient may have heart failure I’ll also order a BNP or NT-proBNP test. If I suspect heart failure and the BNP is high, it’s a red flag. BNP is another tool for getting the diagnosis right.”
The next step seems much more variable. Some PCPs retain primary control of heart failure management for many of their patients, especially when stage C patients remain stable and functional on simple, straightforward treatment and particularly when they have heart failure with preserved ejection fraction (HFpEF), usually defined as a left ventricular ejection fraction that is at least 40%-45%. Consultation or referral to a cardiologist or heart-failure physician seems much more common for patients with frequent decompensations and hospitalizations or patients with heart failure with reduced ejection fraction (HFrEF). But the main thread reported by both PCPs and cardiologists is that it all depends and varies for each patient and for each PCP depending on what patient responsibilities a PCP feels comfortable taking on.
Dr. Bauman sits at one end of the spectrum: “If it looks like a patient has heart failure, I refer them right away; I don’t wait for decompensation to occur. I want to be sure that there are no nuances in the patient that need something before I recognize it. Most of my PCP partners do the same. You don’t know what it is you don’t know. For me, it’s better to refer the patient right away so the patient has a cardiologist who already knows them who can be called if they start to decompensate.”
Dr. Bauman cited the increasing complexity of heart failure management as the main driver of her current approach, which she contrasted to how she dealt with heart failure patients 20 years ago. “It’s become so complicated that, as a PCP, I don’t feel that I can keep up” with the optimal ways to manage every heart failure patient. “I might not give my heart failure patients the best care they could receive.” The aspects of care that Dr. Bauman said she can provide to heart failure patients she has referred include “dealing with lifestyle changes, making sure patients are taking their medications and getting to their appointments, adjusting their heart-failure medication dosages as needed once they start on the drugs, and seeing that their diabetes and hypertension are well controlled. That is the role of the PCP. But when it comes to deciding which HF medications to use, that’s when I like to have a cardiologist involved.”
But the PCPs at Mayo Clinic often take a different tack, said Dr. McKie. “If the patient is a simple case of heart failure with no red flags and the patient is doing relatively well on treatment with simple diuretic treatment, then initiation of heart failure medications and ongoing management is often directed by the PCP with some cardiology backup as needed,” he said. But Dr. McKie conceded that a spectrum of PCP approaches exists at Mayo as well. “A lot depends on the patient and on the specific provider. Some patients we never get calls about; their PCPs are excellent at managing diuretics and uptitrating beta-blockers and ACE inhibitors. We may only get called if the patient decompensates, But other PCPs are very uncomfortable and they request that we get involved as soon as the diagnosis of stage C heart failure is made. So there is a wide range.” Dr. McKie noted that he thinks it is appropriate for himself or one of his cardiology colleagues to get more active when the HFrEF patient’s ejection fraction drops below 40% and certainly below 35%. That’s because at this stage, patients also need treatment with an aldosterone receptor antagonist such as spironolactone, and they undergo consideration for receiving an implantable cardioverter defibrillator or a cardiac resynchronization therapy device.
“There is nothing magic about heart failure management; it is very well proscribed by guidelines. Nothing precludes a PCP from taking ownership” of heart failure patients, said Dr. Akshay S. Desai, a heart failure cardiologist at Brigham and Women’s Hospital. “I think there is some fear among PCPs that they intrude” by managing heart failure patients. But for patients with structural heart disease or even left ventricular dysfunction, “PCPs should feel empowered to start standard heart failure treatments, including ACE inhibitors and beta-blockers, especially because half of heart failure patients have HFpEF, and PCPs often don’t refer HFpEF patients to cardiologists. It’s the patients with left ventricular dysfunction who end up in heart failure clinics,” Dr. Desai said.
On the other hand, Dr. Desai cautioned PCPs against waiting too long to bring more complex, sicker, and harder-to-manage patients to the attention of a heart failure specialist.
“What we worry about are late referrals, when patients are profoundly decompensated,” he said. “By the time they show up [at a heart failure clinic or emergency department] they have end-organ dysfunction,” which makes them much harder to treat and maybe irreversible. “Recognizing heart failure early is the key, and early referral is an obligation” when a heart failure patient is deteriorating or becomes too complex for a PCP to properly manage, Dr. Desai advised.
But even when heart failure patients develop more severe disease, with significantly depressed left ventricular function or frequent decompensations, PCPs continue to play a valuable role in coordinating the wide range of treatments patients need for their various comorbidities.
“Once a cardiologist or heart failure physician is involved there is still a role for PCPs” said Dr. Monica R. Shah, deputy chief of the Heart Failure and Arrhythmia Branch of the National Heart, Lung, and Blood Institute in Bethesda, Md. “Heart failure patients are complex, it’s not just one organ system that’s affected, and you need a partnership between cardiologists and PCPs to coordinate all of a patient’s care. A heart failure physician needs to work with a PCP to be sure that the patient’s health is optimal. Collaboration between cardiologists and PCPs is key to ensure that optimal care is effectively delivered to patients,” Dr. Shah said in an interview.
“Keeping the PCP at the center of the care team is critical, especially with the multiple comorbidities that HF patients can have, including chronic obstructive pulmonary disease, diabetes, renal failure, sleep apnea, atrial fibrillation, and degenerative joint disease. Before you know it you have a half-dozen subspecialists involved in care and it can become uncoordinated. Keeping the PCP at the center of the team and providing the PCP with support from specialists as needed is critical,” said Dr. McKie.
Even for the most severe heart failure patients, PCPs can still play an important role by providing palliative care and dealing with end-of-life issues, specialists said.
Primary care and heart failure’s antecedents
The other, obvious time in heart failure’s severity spectrum for PCPs to take a very active role is with presymptomatic, stage A patients. Perhaps the only controversial element of this is whether such patients really have a form of heart failure and whether is it important to conceptualize heart failure this way.
The notion of stage A heart failure dates back to the 2001 edition of heart failure diagnosis and management recommendations issued by a panel organized by the ACC and AHA (J Am Coll Cardiol. 2001 Dec;38[7]:2101-13). The 2001 writing committee members said that they “decided to take a new approach to the classification of heart failure that emphasized both the evolution and progression of the disease.” They defined stage A patients as presymptomatic and without structural heart disease but with “conditions strongly associated with the development of heart failure,” specifically systemic hypertension, coronary artery disease, diabetes, a history of cardiotoxic drug therapy or alcohol abuse, a history of rheumatic fever, or a family history of cardiomyopathy.
When the ACC and AHA panel members next updated the heart failure recommendations in 2005, they seemed to take a rhetorical step back, saying that stage A and B “are clearly not heart failure but are an attempt to help healthcare providers identify patients early who are at risk for developing heart failure. Stage A and B patients are best defined as those with risk factors that clearly predispose toward the development of HF.” (J Am Coll Cardiol. 2005 Sept. 46[6]:1116-43) In 2005, the panel also streamlined the list of risk factors that identify stage A heart failure patients: hypertension, atherosclerotic disease, diabetes, obesity, metabolic syndrome, patients who have taken cardiotoxins, or patients with a family history of cardiomyopathy. The 2009 recommendation update left this definition of stage A heart failure unchanged, but in 2013 the most recent update devoted less attention to explaining the significance of the stage-A heart failure, although it clearly highlighted the importance of controlling hypertension, diabetes, and obesity as ways to prevent patients from developing symptomatic heart failure (J Am Coll Cardiol. 2013 Oct 15;62[16]:e147-e239).
The subtle, official tweaking of the stage A (and B) heart failure concept during 2001-2013, as well establishment of stage A in the first place, seems to have left both PCPs and heart failure specialists unsure on exactly how to think about presymptomatic people with one or more of the prominent heart failure risk factors of hypertension, diabetes, and obesity. While they uniformly agree that identifying these risk factors and then treating them according to contemporary guidelines is hugely important for stopping or deferring the onset of heart failure, and they also agree that this aspect of patient care is clearly a core responsibility for PCPs, many also say that they don’t think of presymptomatic patients as having heart failure of any type despite the stage A designation on the books.
One exception is St. Vincent’s Dr. Walsh. “I think the writers of the 2001 heart failure guidelines had an inspired approach. Identifying patients with hypertension, diabetes, coronary artery disease, etc., as patients with heart failure has helped drive home the point that treatment and control of these diseases is crucial,” she said in an interview. “But I am not sure all physicians have adopted the concept. “Uncontrolled hypertension is prevalent, and not viewed by all as resulting in heart failure down the road. Diabetes and hypertension are very important risk factors for the development of heart failure in women,” she added. “I’m especially diligent in ensuring that women with one or both of these diseases get treated aggressively.”
Highlighting specifically the fundamental role that uncontrolled hypertension plays in causing heart failure, the University of Pennsylvania’s Dr. Jessup estimated that controlling hypertension throughout the U.S. population could probably cut heart failure incidence in half.
Others draw a sharper contrast between the risk factor stage and the symptomatic stages of heart failure, though they all agree on the importance of risk factor management by PCPs. “Hypertension does not mean that a patient has heart failure; it means they have a risk factor for heart failure and the patient is in the prevention stage,” said the NHLBI’s Dr. Shah. ”The most important role for PCPs is to identify the risk factors and prevent development of [symptomatic] heart failure. This is where PCPs are critically important because patients present to them at the early stages.”
Dr. Bauman, the PCP with INTEGRIS in Oklahoma City, generally doesn’t conflate risk factors with stage A heart failure. “I look at every patient with hypertension or diabetes as a person at risk for cardiovascular disease. I push them to get their blood pressure and glycemia under control. But I don’t think of them as stage A heart failure patients. I think of them as patients at risk for heart failure, but also at risk for atrial fibrillation, MI, and stroke. I think about their risk, but I don’t label them in my mind as having stage A heart failure. I think that this is a patient at risk for cardiovascular disease and that I must do what I should to manage their risk factors.”
“I don’t personally think about patients having stage A heart failure,” agreed Dr. Cunningham, a PCP at Brigham and Women’s Hospital. “When I see patients with hypertension, I counsel them about what matters to them so that they will take their medications, because if they currently feel fine they may not understand the long-term risk they face. So I invest time in making the patient understand why their hypertension is important and the risks it poses, so that in the long-run they won’t have a stroke or MI or develop heart failure. But I don’t think that the stage A definition has changed my approach; I already think of hypertension as a precursor to a variety of bad downstream consequences. I don’t think of someone as a heart failure patient just because they have hypertension, and I don’t think that every patient with hypertension will develop heart failure.” Speaking of her colleagues, Dr. Cunningham added, “I don’t have a sense that the stages of heart failure have made much of an impact on how other PCPs talk with patients or plan their care.”
“The heart failure staging system is useful from the standpoint of emphasizing that the disease begins with primordial risk and progresses through a period of structural injury during which patients may not be symptomatic,” summed up Dr. Desai. “But practically, most of us confront the diagnosis of heart failure when patients become symptomatic and reach stage C.”
Can an intensified approach better slow stage A progression?
One of the inherent limitations right now in referring to patients as having stage A heart failure is that it adds little to how heart failure risk factors are managed. A patient with hypertension undergoing appropriate care will receive treatment to lower blood pressure to recommended goal levels. The antihypertensive treatment remains the same regardless of whether the patient is considered to have only hypertension or whether the treating physician also thinks of the patient as having stage A heart failure. The same applies to patients diagnosed with diabetes; their hyperglycemia-controlling treatment remains unchanged whether or not their physician labels them as stage A heart failure patients.
But what if an evidence-based way existed to not only identify patients with hypertension or diabetes, but to identify within those patients the subset who faced a particularly increased risk for developing heart failure? And what if an evidence-based intervention existed that could be added to standard blood pressure–lowering or hyperglycemia-controlling interventions and had proved to slow or stop progression of patients to heart failure?
Preliminary evidence that screening for stage A heart failure patients can successfully identify a subset at elevated risk for developing symptomatic heart failure and that intensified risk-factor control helped mitigate this risk appeared in two reports published in 2013. But both studies were relatively small, they ran in Europe, and neither has undergone replication in a U.S. study in the 2.5 years since their publication.
The larger study, STOP-HF (St. Vincent’s Screening to Prevent Heart Failure), included patients at 39 primary care practices in Ireland, a study organized by researchers at St. Vincent’s University Hospital in Dublin. They enrolled people without symptoms of heart failure who were at least 41 years old and had at least one of these risk factors: hypertension, hypercholesterolemia, obesity, vascular disease, diabetes, an arrhythmia, or valvular disease: In short, primarily stage A heart failure patients.
The researchers then tested 1,374 of these people for their baseline blood level of BNP and randomized them into two intervention arms. For those randomized to the active arm, the PCPs for these people received an unblinded report of the BNP results, and those with a level of 50 pg/mL or higher underwent further assessment by screening echocardiography and intensified risk-factor control, including risk-factor coaching by a nurse. Those randomized to this arm who had a lower BNP level at baseline underwent annual follow-up BNP screening, and if their level reached the 50 pg/ML threshold they switched to the more intensified protocol. Those randomized to the control arm received a more standard program of risk-factor modification and their BNP levels were never unblinded.
After an average follow-up of 4.2 years, people in the active intervention arm of STOP-HF had a 5% cumulative incidence of left ventricular dysfunction or heart failure, while those in the control arm had a 9% rate, a 45% relative risk reduction from the active intervention that was statistically significant for the study’s primary endpoint (JAMA. 2013 July 3;310[1]:66-74).
The second study, PONTIAC (NT-proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients Without a History of Cardiac Disease), ran in Austria and Germany and involved 300 patients who had type 2 diabetes and were free from cardiac disease at baseline. At baseline, all people considered for the study underwent a screening measure of their blood level of NT-proBNP (a physiologic precursor to BNP) and those with a level above 125 pg/mL were randomized to either a usual-care group or an arm that underwent more intensified up-titration treatment with a renin-angiotensin system antagonist drug and with a beta-blocker. The primary endpoint was the incidence of hospitalization or death due to cardiac disease after 2 years, which was a relative 65% lower in the intensified intervention group, a statistically significant difference (J Am Coll Cardiol. 2013 Oct 8;62[15]:1365-72).
Both studies focused on people with common risk factors seen in primary care practices and used BNP or a BNP-like blood marker to identify people with an elevated risk for developing heart failure or other cardiac disease, and both studies showed that application of a more aggressive risk-factor intervention program resulted in a significant reduction in heart failure or heart failure–related outcomes after 2-4 years. Both studies appeared to offer models for improving risk-factor management by PCPs for people with stage A heart failure, but at the end of 2015 neither model had undergone U.S. testing.
“The STOP-HF and PONTIAC studies were proofs of concept for using biomarkers to gain a better sense of cardiac health,” said Dr. Tariq Ahmad, a heart failure physician at Yale University in New Haven, Conn., who is interested in developing biomarkers for guiding heart failure management. “Metrics like blood pressure and heart rate are relatively crude measures of cardiac health. We need to see in a large trial if we can use these more objective measures of cardiac health to decide how to treat patients,” In addition to BNP and NT-proBNP, Dr. Ahmad cited ST2 and galectin-3 as other promising biomarkers in the blood that may better gauge a person’s risk for developing heart failure and the need for intensified risk-factor control. The current inability of PCPs to better risk stratify people who meet the stage A heart failure definition so that those at highest risk could undergo more intensified interventions constitutes a missed opportunity for heart failure prevention, he said.
“The STOP-HF trial is really important and desperately needs replication,” said Dr. Margaret M. Redfield, professor of medicine and a heart failure physician at Mayo Clinic in Rochester, Minn.
She, and her Mayo associates, including Dr. McKie, are planning to launch a research protocol this year to finally test a STOP-HF type of program in a U.S. setting. They are planning to measure NT-proBNP levels in patients with stage A heart failure and then randomize some to an intervention arm with intensified risk reduction treatments.
“The problem with stage A today is, if we apply it according to the ACC and AHA definition, it would include quite a large number of patients, and not all of them – in fact a minority – would go on to develop symptomatic heart failure,” said Dr. McKie. “How you can further risk stratify the stage A population with simple testing is an issue for ongoing research,” he said. “The STOP-HF and PONTIAC strategies need more testing. Both studies were done in Europe, and we haven’t studied this approach in the U.S. Their approach makes sense and is appealing but it needs more testing.”
The economic barrier to intensified stage-A management
Even if a U.S. based study could replicate the STOP-HF results and provide an evidence base for improved prevention of symptomatic heart failure by interventions instituted by PCPs, it’s not clear whether the U.S. health care system as it currently is structured provides a framework that is able to invest in intensified upfront management of risk factors to achieve a reduced incidence of symptomatic heart failure several years later.
“One of the interesting aspects of STOP-HF was its use of a nurse-based intervention. We don’t have the resources for that in our practices right now,” noted Dr. Cunningham, the PCP at Brigham and Women’s Hospital who is medical director of the hospital’s Integrated Care Management Program for medically complex patients. While that program uses nurse care coordinators to pull together the disparate elements of care for heart failure patients and others with more severe, chronic illnesses, the program currently serves only patients with advanced disease, not presymptomatic patients who face a potentially elevated risk for bad outcomes that would happen many years in the future.
“This speaks to the need for more population-based preventive management, which PCPs are trying to start to do, but currently we are nowhere near fulfilling that potential,” said Dr. Cunningham. The barrier is having clinical resources for help in managing lower-risk patients, to make sure they receive all the interventions they should. We’re now trying to start using care teams for patients with diabetes or other conditions. The biggest gap is that we don’t have the resources; we don’t have enough nurses on our staff to intervene” for all the patients who could potentially benefit. “Right now, we can only afford to use nurses for selected, high-risk patients.” The challenge is to have a care model that allows a lot of upfront costs to generate savings over a long-term time horizon, he said. “It’s very important for improving population health, but it’s hard to make it happen in our current health care system.”
Dr. Ahmad noted the enormous downside of a health system that is not proactive and often waits for heart failure patients to declare themselves with severe illness.
“The majority of heart failure patients I see drifted through the health care system” without recognition of their accumulating morbidity. “By the time they show heart failure symptoms, their disease is pretty advanced and we have real difficulty managing it. A lot of patients do not have their heart failure managed until they fall off the edge and their condition is much less modifiable. If we could identify these patients sooner, it would help both them and the health care system. It would be great to have objective measures that could help PCPs identify early abnormal patients who need more aggressive management. In much of U.S. practice, heart failure management is more specialty driven. It might be different in closed systems, but in many heart failure practices there is no PCP coordination. The health care system is not set up to allow PCPs to take care of these issues.”
Dr. Bauman said she sees some reason for optimism in looming reimbursement changes, where population management might help drive a shift toward more team care for heart failure and a focus on earlier identification of patients at risk and intervention at early stages of their disease.
“As we move toward population management it becomes more obvious that you need a team approach to managing heart failure, involving not just physicians but also pharmacists, nurses, social workers, and care coordinators. In my system, INTEGRIS, the whole-team management approach is beginning to happen. It’s new to primary care to apply a large team of clinicians; it takes a lot of resources. Being able to afford a team was a problem when we were paid by fee-for-service, it wasn’t practical. Population management will make it possible.”
Dr. Desai has been a consultant to Novartis, Merck, St. Jude, and Relypsa and has received research funding from Novartis and AtCor Medical. Dr. Redfield has been a consultant to Merck and Eli Lilly. Dr. Ahmad has been a consultant to Roche. Dr. Ong, Dr. Walsh, Dr. Jessup, Dr. McKie, Dr. Bauman, Dr. Shah, and Dr. Cunningham had no disclosures.
On Twitter @mitchelzoler
Heart failure management has become increasingly complex over the past couple of decades, with new drugs and drug combinations, new uses for potentially life-saving implanted devices, and a more sophisticated appreciation of the ways that various comorbidities complicate a heart failure patient’s clinical status. These expanded dimensions of heart failure care resulted in the establishment in 2008 of a new secondary subspecialty, Advanced Heart Failure and Transplant Cardiology, aimed at training and certifying physicians in all the nuances of complex heart failure diagnostics and care.
But as the 2009 manifesto announcing this new heart failure subspecialty detailed, care for the vast majority of U.S. patients with heart failure remains in the hands of internal medicine primary care physicians (PCPs) and general cardiologists (J Am Coll Cardiol. 2009 Mar 10;53[10]:834-6). To some extent this is a manpower issue. The estimated number of Americans living with heart failure exceeds 5 million, a figure that dwarfs the very modest number of U.S. physicians and clinicians who are certified or self-identified heart failure specialists.
As of today, fewer than 1,000 U.S. physicians have received formal certification as heart failure subspecialists through the examination administered in 2010, 2012, and 2014, said Michele Blair, chief executive officer of the Heart Failure Society of America. A more liberal definition of a heart failure specialist might include the roughly 3,000 unique physicians (mostly cardiologists, but also some hospitalists and emergency physicians) who have recently attended an annual meeting of the HFSA, as well as the roughly 2,300 physician assistants and nurse practitioners who have shown a heart failure interest by coming to a recent HFSA meeting. But even these expanded estimates calculate out to about 1 clinician with a special interest in heart failure for each 1,000 heart failure patients, not a very reassuring ratio.
The burgeoning numbers of heart failure patients, compared with the relative scarcity of both heart failure experts and general cardiologists, raises issues of how primary-care internists best share this management responsibility. Recent interviews with several heart failure subspecialists and primary care internists provide some insight into how this division of labor is now playing out in routine U.S. practice. What often occurs is that primary care internists take exclusive responsibility for caring for heart failure patients until they feel they are getting in over their heads, at which time they’ll consult with a cardiology colleague or refer the patient to a cardiologist. That moment of recognition by the generalist – that the demands and complexity of the case exceed their comfort level – varies widely, with some PCPs referring patients as soon as heart failure symptoms appear while others stay comfortable as the primary care giver even as a patient’s disease deteriorates to a more advanced stage.
Heart failure specialists highlighted their reliance on PCPs to take an ongoing, active role even for patients with significantly advanced heart failure, as generalists are well suited to coordinating the multispecialty care that such patients usually require, with attention to their need for lifestyle modifications as well as management of their diabetes, sleep apnea, chronic obstructive pulmonary disease, renal failure, and other comorbidities.
As Dr. Michael K. Ong, a primary care internist at the University of California, Los Angeles, said in an interview, his heart failure specialist colleague manages patients’ heart failure; “I manage [or refer] everything else not directly related to the heart failure.”
The most successful U.S. care models seem to be some variation on a team-care approach, in which physicians collaborate with pharmacists, nurses, rehabilitation specialists, and social workers as well as specialists, a team that would include and perhaps be led by either a primary care internist, a cardiologist, or a heart failure specialist but would also broadly include physicians able to deal with all the morbidity facets of heart failure. It’s a model that remains unavailable in many U.S. settings or is just starting to emerge, as fee-for-service coverage of patients gets replaced by population-management models that better accommodate the upfront financial demands of coordinated team care. It makes financial sense a few years down the road when improved patient outcomes result in cost savings.
Primary care and patients with symptomatic heart failure
The heart failure definitions and staging system established in 2001 by a guidelines panel of the American College of Cardiology and American Heart Association defined stage A heart failure as starting before a patient exhibits any heart failure symptoms (the classic ones include dyspnea, rales, and peripheral edema). The panel designated symptomatic heart failure patients as stage C. Patients without heart failure symptoms but with one or more risk factors (such as hypertension, diabetes, obesity, and cardiovascular disease) plus structural heart disease (such as cardiomyopathy or other forms of heart remodeling) were designated stage B. The panel said that people at stage A had one or more risk factors but no structural heart changes and no heart failure symptoms.
Although stage-A heart failure patients are clearly the types of people most often seen and cared for by PCPs, many of these physicians, as well as many heart failure specialists, don’t consider patients who have only hypertension or only diabetes or only obesity as yet having heart failure. That paradox deserves more discussion, but the best way to begin talking about PCPs and heart failure patients is when patients are symptomatic and have what everyone would agree is heart failure.
Even though the ACC/AHA staging system places stage C patients well down the heart failure road, stage C is usually when patients are first diagnosed with heart failure. Although the diagnosis is often first made by a hospitalist or emergency-department physician when severe and sudden-onset heart failure symptoms drive the patient to a hospital, or the diagnosis originates with a cardiologist or heart failure specialist when the patient’s presentation and differential diagnosis isn’t straightforward, most commonly the diagnosis starts with a PCP in an office encounter with a patient who is symptomatic but not acutely ill.
“Patients with shortness of breath or other forms of effort intolerance most often seek care from PCPs. The differential diagnosis of dyspnea is long and complex. Recognition that a patient with dyspnea may have HF is crucial” for timely management and treatment, said Dr. Mary Norine Walsh, medical director of Heart Failure and Cardiac Transplantation at St. Vincent Heart Center in Indianapolis.
At the Mayo Clinic in Rochester, Minn., “most of the heart failure diagnoses are done by PCPs, usually first identified at stage C when a patient comes in with symptoms. Stage B heart failure is usually only identified as an incidental finding when echocardiography is done for some other reason,” said Dr. Paul M. McKie, a heart failure cardiologist who works closely with the primary-care staff at Mayo as an embedded consultant cardiologist.
According to Dr. Mariell L. Jessup, a heart failure physician and professor at the University of Pennsylvania in Philadelphia, a key to PCPs promptly identifying patients with recent-onset, stage C heart failure is to keep the disease as well as its prominent risk factors at the top of their differential-diagnosis list for at-risk patients. “Heart failure is a common disorder,” Dr. Jessup said, and must be considered for patients with shortness of breath. “The leading causes of heart failure are hypertension, obesity, and diabetes. So keep heart failure in mind, especially for patients with one or more of these risk factors.”
Although PCPs might order an echocardiography examination or a lab test like measurement of brain natriuretic protein (BNP) to help nail down the diagnosis, they often leave reading the echocardiography results to a cardiologist colleague. “When a PCP orders an echo it’s automatically read by a cardiologist, and then we get the cardiologist’s report. I don’t read echos myself,” said Dr. Rebecca J. Cunningham, an internal medicine PCP at Brigham and Women’s Hospital in Boston who frequently sees patients with heart failure as medical director of the hospital’s Integrated Care Management Program. “I had one PCP colleague who undertook additional training to learn to read echos himself, but that’s unusual.”
Dr. Mary Ann Bauman, an internal medicine PCP and medical director for Women’s Health and Community Relations at INTEGRIS Health in Oklahoma City, noted a similar division of labor. “If a patient has shortness of breath, maybe some edema, and I hear a few rales, but is totally functional, I always order an echo but I don’t read it. I refer the echo to a cardiologist who then sends me a report,” Dr. Bauman said in an interview. “If I think the patient may have heart failure I’ll also order a BNP or NT-proBNP test. If I suspect heart failure and the BNP is high, it’s a red flag. BNP is another tool for getting the diagnosis right.”
The next step seems much more variable. Some PCPs retain primary control of heart failure management for many of their patients, especially when stage C patients remain stable and functional on simple, straightforward treatment and particularly when they have heart failure with preserved ejection fraction (HFpEF), usually defined as a left ventricular ejection fraction that is at least 40%-45%. Consultation or referral to a cardiologist or heart-failure physician seems much more common for patients with frequent decompensations and hospitalizations or patients with heart failure with reduced ejection fraction (HFrEF). But the main thread reported by both PCPs and cardiologists is that it all depends and varies for each patient and for each PCP depending on what patient responsibilities a PCP feels comfortable taking on.
Dr. Bauman sits at one end of the spectrum: “If it looks like a patient has heart failure, I refer them right away; I don’t wait for decompensation to occur. I want to be sure that there are no nuances in the patient that need something before I recognize it. Most of my PCP partners do the same. You don’t know what it is you don’t know. For me, it’s better to refer the patient right away so the patient has a cardiologist who already knows them who can be called if they start to decompensate.”
Dr. Bauman cited the increasing complexity of heart failure management as the main driver of her current approach, which she contrasted to how she dealt with heart failure patients 20 years ago. “It’s become so complicated that, as a PCP, I don’t feel that I can keep up” with the optimal ways to manage every heart failure patient. “I might not give my heart failure patients the best care they could receive.” The aspects of care that Dr. Bauman said she can provide to heart failure patients she has referred include “dealing with lifestyle changes, making sure patients are taking their medications and getting to their appointments, adjusting their heart-failure medication dosages as needed once they start on the drugs, and seeing that their diabetes and hypertension are well controlled. That is the role of the PCP. But when it comes to deciding which HF medications to use, that’s when I like to have a cardiologist involved.”
But the PCPs at Mayo Clinic often take a different tack, said Dr. McKie. “If the patient is a simple case of heart failure with no red flags and the patient is doing relatively well on treatment with simple diuretic treatment, then initiation of heart failure medications and ongoing management is often directed by the PCP with some cardiology backup as needed,” he said. But Dr. McKie conceded that a spectrum of PCP approaches exists at Mayo as well. “A lot depends on the patient and on the specific provider. Some patients we never get calls about; their PCPs are excellent at managing diuretics and uptitrating beta-blockers and ACE inhibitors. We may only get called if the patient decompensates, But other PCPs are very uncomfortable and they request that we get involved as soon as the diagnosis of stage C heart failure is made. So there is a wide range.” Dr. McKie noted that he thinks it is appropriate for himself or one of his cardiology colleagues to get more active when the HFrEF patient’s ejection fraction drops below 40% and certainly below 35%. That’s because at this stage, patients also need treatment with an aldosterone receptor antagonist such as spironolactone, and they undergo consideration for receiving an implantable cardioverter defibrillator or a cardiac resynchronization therapy device.
“There is nothing magic about heart failure management; it is very well proscribed by guidelines. Nothing precludes a PCP from taking ownership” of heart failure patients, said Dr. Akshay S. Desai, a heart failure cardiologist at Brigham and Women’s Hospital. “I think there is some fear among PCPs that they intrude” by managing heart failure patients. But for patients with structural heart disease or even left ventricular dysfunction, “PCPs should feel empowered to start standard heart failure treatments, including ACE inhibitors and beta-blockers, especially because half of heart failure patients have HFpEF, and PCPs often don’t refer HFpEF patients to cardiologists. It’s the patients with left ventricular dysfunction who end up in heart failure clinics,” Dr. Desai said.
On the other hand, Dr. Desai cautioned PCPs against waiting too long to bring more complex, sicker, and harder-to-manage patients to the attention of a heart failure specialist.
“What we worry about are late referrals, when patients are profoundly decompensated,” he said. “By the time they show up [at a heart failure clinic or emergency department] they have end-organ dysfunction,” which makes them much harder to treat and maybe irreversible. “Recognizing heart failure early is the key, and early referral is an obligation” when a heart failure patient is deteriorating or becomes too complex for a PCP to properly manage, Dr. Desai advised.
But even when heart failure patients develop more severe disease, with significantly depressed left ventricular function or frequent decompensations, PCPs continue to play a valuable role in coordinating the wide range of treatments patients need for their various comorbidities.
“Once a cardiologist or heart failure physician is involved there is still a role for PCPs” said Dr. Monica R. Shah, deputy chief of the Heart Failure and Arrhythmia Branch of the National Heart, Lung, and Blood Institute in Bethesda, Md. “Heart failure patients are complex, it’s not just one organ system that’s affected, and you need a partnership between cardiologists and PCPs to coordinate all of a patient’s care. A heart failure physician needs to work with a PCP to be sure that the patient’s health is optimal. Collaboration between cardiologists and PCPs is key to ensure that optimal care is effectively delivered to patients,” Dr. Shah said in an interview.
“Keeping the PCP at the center of the care team is critical, especially with the multiple comorbidities that HF patients can have, including chronic obstructive pulmonary disease, diabetes, renal failure, sleep apnea, atrial fibrillation, and degenerative joint disease. Before you know it you have a half-dozen subspecialists involved in care and it can become uncoordinated. Keeping the PCP at the center of the team and providing the PCP with support from specialists as needed is critical,” said Dr. McKie.
Even for the most severe heart failure patients, PCPs can still play an important role by providing palliative care and dealing with end-of-life issues, specialists said.
Primary care and heart failure’s antecedents
The other, obvious time in heart failure’s severity spectrum for PCPs to take a very active role is with presymptomatic, stage A patients. Perhaps the only controversial element of this is whether such patients really have a form of heart failure and whether is it important to conceptualize heart failure this way.
The notion of stage A heart failure dates back to the 2001 edition of heart failure diagnosis and management recommendations issued by a panel organized by the ACC and AHA (J Am Coll Cardiol. 2001 Dec;38[7]:2101-13). The 2001 writing committee members said that they “decided to take a new approach to the classification of heart failure that emphasized both the evolution and progression of the disease.” They defined stage A patients as presymptomatic and without structural heart disease but with “conditions strongly associated with the development of heart failure,” specifically systemic hypertension, coronary artery disease, diabetes, a history of cardiotoxic drug therapy or alcohol abuse, a history of rheumatic fever, or a family history of cardiomyopathy.
When the ACC and AHA panel members next updated the heart failure recommendations in 2005, they seemed to take a rhetorical step back, saying that stage A and B “are clearly not heart failure but are an attempt to help healthcare providers identify patients early who are at risk for developing heart failure. Stage A and B patients are best defined as those with risk factors that clearly predispose toward the development of HF.” (J Am Coll Cardiol. 2005 Sept. 46[6]:1116-43) In 2005, the panel also streamlined the list of risk factors that identify stage A heart failure patients: hypertension, atherosclerotic disease, diabetes, obesity, metabolic syndrome, patients who have taken cardiotoxins, or patients with a family history of cardiomyopathy. The 2009 recommendation update left this definition of stage A heart failure unchanged, but in 2013 the most recent update devoted less attention to explaining the significance of the stage-A heart failure, although it clearly highlighted the importance of controlling hypertension, diabetes, and obesity as ways to prevent patients from developing symptomatic heart failure (J Am Coll Cardiol. 2013 Oct 15;62[16]:e147-e239).
The subtle, official tweaking of the stage A (and B) heart failure concept during 2001-2013, as well establishment of stage A in the first place, seems to have left both PCPs and heart failure specialists unsure on exactly how to think about presymptomatic people with one or more of the prominent heart failure risk factors of hypertension, diabetes, and obesity. While they uniformly agree that identifying these risk factors and then treating them according to contemporary guidelines is hugely important for stopping or deferring the onset of heart failure, and they also agree that this aspect of patient care is clearly a core responsibility for PCPs, many also say that they don’t think of presymptomatic patients as having heart failure of any type despite the stage A designation on the books.
One exception is St. Vincent’s Dr. Walsh. “I think the writers of the 2001 heart failure guidelines had an inspired approach. Identifying patients with hypertension, diabetes, coronary artery disease, etc., as patients with heart failure has helped drive home the point that treatment and control of these diseases is crucial,” she said in an interview. “But I am not sure all physicians have adopted the concept. “Uncontrolled hypertension is prevalent, and not viewed by all as resulting in heart failure down the road. Diabetes and hypertension are very important risk factors for the development of heart failure in women,” she added. “I’m especially diligent in ensuring that women with one or both of these diseases get treated aggressively.”
Highlighting specifically the fundamental role that uncontrolled hypertension plays in causing heart failure, the University of Pennsylvania’s Dr. Jessup estimated that controlling hypertension throughout the U.S. population could probably cut heart failure incidence in half.
Others draw a sharper contrast between the risk factor stage and the symptomatic stages of heart failure, though they all agree on the importance of risk factor management by PCPs. “Hypertension does not mean that a patient has heart failure; it means they have a risk factor for heart failure and the patient is in the prevention stage,” said the NHLBI’s Dr. Shah. ”The most important role for PCPs is to identify the risk factors and prevent development of [symptomatic] heart failure. This is where PCPs are critically important because patients present to them at the early stages.”
Dr. Bauman, the PCP with INTEGRIS in Oklahoma City, generally doesn’t conflate risk factors with stage A heart failure. “I look at every patient with hypertension or diabetes as a person at risk for cardiovascular disease. I push them to get their blood pressure and glycemia under control. But I don’t think of them as stage A heart failure patients. I think of them as patients at risk for heart failure, but also at risk for atrial fibrillation, MI, and stroke. I think about their risk, but I don’t label them in my mind as having stage A heart failure. I think that this is a patient at risk for cardiovascular disease and that I must do what I should to manage their risk factors.”
“I don’t personally think about patients having stage A heart failure,” agreed Dr. Cunningham, a PCP at Brigham and Women’s Hospital. “When I see patients with hypertension, I counsel them about what matters to them so that they will take their medications, because if they currently feel fine they may not understand the long-term risk they face. So I invest time in making the patient understand why their hypertension is important and the risks it poses, so that in the long-run they won’t have a stroke or MI or develop heart failure. But I don’t think that the stage A definition has changed my approach; I already think of hypertension as a precursor to a variety of bad downstream consequences. I don’t think of someone as a heart failure patient just because they have hypertension, and I don’t think that every patient with hypertension will develop heart failure.” Speaking of her colleagues, Dr. Cunningham added, “I don’t have a sense that the stages of heart failure have made much of an impact on how other PCPs talk with patients or plan their care.”
“The heart failure staging system is useful from the standpoint of emphasizing that the disease begins with primordial risk and progresses through a period of structural injury during which patients may not be symptomatic,” summed up Dr. Desai. “But practically, most of us confront the diagnosis of heart failure when patients become symptomatic and reach stage C.”
Can an intensified approach better slow stage A progression?
One of the inherent limitations right now in referring to patients as having stage A heart failure is that it adds little to how heart failure risk factors are managed. A patient with hypertension undergoing appropriate care will receive treatment to lower blood pressure to recommended goal levels. The antihypertensive treatment remains the same regardless of whether the patient is considered to have only hypertension or whether the treating physician also thinks of the patient as having stage A heart failure. The same applies to patients diagnosed with diabetes; their hyperglycemia-controlling treatment remains unchanged whether or not their physician labels them as stage A heart failure patients.
But what if an evidence-based way existed to not only identify patients with hypertension or diabetes, but to identify within those patients the subset who faced a particularly increased risk for developing heart failure? And what if an evidence-based intervention existed that could be added to standard blood pressure–lowering or hyperglycemia-controlling interventions and had proved to slow or stop progression of patients to heart failure?
Preliminary evidence that screening for stage A heart failure patients can successfully identify a subset at elevated risk for developing symptomatic heart failure and that intensified risk-factor control helped mitigate this risk appeared in two reports published in 2013. But both studies were relatively small, they ran in Europe, and neither has undergone replication in a U.S. study in the 2.5 years since their publication.
The larger study, STOP-HF (St. Vincent’s Screening to Prevent Heart Failure), included patients at 39 primary care practices in Ireland, a study organized by researchers at St. Vincent’s University Hospital in Dublin. They enrolled people without symptoms of heart failure who were at least 41 years old and had at least one of these risk factors: hypertension, hypercholesterolemia, obesity, vascular disease, diabetes, an arrhythmia, or valvular disease: In short, primarily stage A heart failure patients.
The researchers then tested 1,374 of these people for their baseline blood level of BNP and randomized them into two intervention arms. For those randomized to the active arm, the PCPs for these people received an unblinded report of the BNP results, and those with a level of 50 pg/mL or higher underwent further assessment by screening echocardiography and intensified risk-factor control, including risk-factor coaching by a nurse. Those randomized to this arm who had a lower BNP level at baseline underwent annual follow-up BNP screening, and if their level reached the 50 pg/ML threshold they switched to the more intensified protocol. Those randomized to the control arm received a more standard program of risk-factor modification and their BNP levels were never unblinded.
After an average follow-up of 4.2 years, people in the active intervention arm of STOP-HF had a 5% cumulative incidence of left ventricular dysfunction or heart failure, while those in the control arm had a 9% rate, a 45% relative risk reduction from the active intervention that was statistically significant for the study’s primary endpoint (JAMA. 2013 July 3;310[1]:66-74).
The second study, PONTIAC (NT-proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients Without a History of Cardiac Disease), ran in Austria and Germany and involved 300 patients who had type 2 diabetes and were free from cardiac disease at baseline. At baseline, all people considered for the study underwent a screening measure of their blood level of NT-proBNP (a physiologic precursor to BNP) and those with a level above 125 pg/mL were randomized to either a usual-care group or an arm that underwent more intensified up-titration treatment with a renin-angiotensin system antagonist drug and with a beta-blocker. The primary endpoint was the incidence of hospitalization or death due to cardiac disease after 2 years, which was a relative 65% lower in the intensified intervention group, a statistically significant difference (J Am Coll Cardiol. 2013 Oct 8;62[15]:1365-72).
Both studies focused on people with common risk factors seen in primary care practices and used BNP or a BNP-like blood marker to identify people with an elevated risk for developing heart failure or other cardiac disease, and both studies showed that application of a more aggressive risk-factor intervention program resulted in a significant reduction in heart failure or heart failure–related outcomes after 2-4 years. Both studies appeared to offer models for improving risk-factor management by PCPs for people with stage A heart failure, but at the end of 2015 neither model had undergone U.S. testing.
“The STOP-HF and PONTIAC studies were proofs of concept for using biomarkers to gain a better sense of cardiac health,” said Dr. Tariq Ahmad, a heart failure physician at Yale University in New Haven, Conn., who is interested in developing biomarkers for guiding heart failure management. “Metrics like blood pressure and heart rate are relatively crude measures of cardiac health. We need to see in a large trial if we can use these more objective measures of cardiac health to decide how to treat patients,” In addition to BNP and NT-proBNP, Dr. Ahmad cited ST2 and galectin-3 as other promising biomarkers in the blood that may better gauge a person’s risk for developing heart failure and the need for intensified risk-factor control. The current inability of PCPs to better risk stratify people who meet the stage A heart failure definition so that those at highest risk could undergo more intensified interventions constitutes a missed opportunity for heart failure prevention, he said.
“The STOP-HF trial is really important and desperately needs replication,” said Dr. Margaret M. Redfield, professor of medicine and a heart failure physician at Mayo Clinic in Rochester, Minn.
She, and her Mayo associates, including Dr. McKie, are planning to launch a research protocol this year to finally test a STOP-HF type of program in a U.S. setting. They are planning to measure NT-proBNP levels in patients with stage A heart failure and then randomize some to an intervention arm with intensified risk reduction treatments.
“The problem with stage A today is, if we apply it according to the ACC and AHA definition, it would include quite a large number of patients, and not all of them – in fact a minority – would go on to develop symptomatic heart failure,” said Dr. McKie. “How you can further risk stratify the stage A population with simple testing is an issue for ongoing research,” he said. “The STOP-HF and PONTIAC strategies need more testing. Both studies were done in Europe, and we haven’t studied this approach in the U.S. Their approach makes sense and is appealing but it needs more testing.”
The economic barrier to intensified stage-A management
Even if a U.S. based study could replicate the STOP-HF results and provide an evidence base for improved prevention of symptomatic heart failure by interventions instituted by PCPs, it’s not clear whether the U.S. health care system as it currently is structured provides a framework that is able to invest in intensified upfront management of risk factors to achieve a reduced incidence of symptomatic heart failure several years later.
“One of the interesting aspects of STOP-HF was its use of a nurse-based intervention. We don’t have the resources for that in our practices right now,” noted Dr. Cunningham, the PCP at Brigham and Women’s Hospital who is medical director of the hospital’s Integrated Care Management Program for medically complex patients. While that program uses nurse care coordinators to pull together the disparate elements of care for heart failure patients and others with more severe, chronic illnesses, the program currently serves only patients with advanced disease, not presymptomatic patients who face a potentially elevated risk for bad outcomes that would happen many years in the future.
“This speaks to the need for more population-based preventive management, which PCPs are trying to start to do, but currently we are nowhere near fulfilling that potential,” said Dr. Cunningham. The barrier is having clinical resources for help in managing lower-risk patients, to make sure they receive all the interventions they should. We’re now trying to start using care teams for patients with diabetes or other conditions. The biggest gap is that we don’t have the resources; we don’t have enough nurses on our staff to intervene” for all the patients who could potentially benefit. “Right now, we can only afford to use nurses for selected, high-risk patients.” The challenge is to have a care model that allows a lot of upfront costs to generate savings over a long-term time horizon, he said. “It’s very important for improving population health, but it’s hard to make it happen in our current health care system.”
Dr. Ahmad noted the enormous downside of a health system that is not proactive and often waits for heart failure patients to declare themselves with severe illness.
“The majority of heart failure patients I see drifted through the health care system” without recognition of their accumulating morbidity. “By the time they show heart failure symptoms, their disease is pretty advanced and we have real difficulty managing it. A lot of patients do not have their heart failure managed until they fall off the edge and their condition is much less modifiable. If we could identify these patients sooner, it would help both them and the health care system. It would be great to have objective measures that could help PCPs identify early abnormal patients who need more aggressive management. In much of U.S. practice, heart failure management is more specialty driven. It might be different in closed systems, but in many heart failure practices there is no PCP coordination. The health care system is not set up to allow PCPs to take care of these issues.”
Dr. Bauman said she sees some reason for optimism in looming reimbursement changes, where population management might help drive a shift toward more team care for heart failure and a focus on earlier identification of patients at risk and intervention at early stages of their disease.
“As we move toward population management it becomes more obvious that you need a team approach to managing heart failure, involving not just physicians but also pharmacists, nurses, social workers, and care coordinators. In my system, INTEGRIS, the whole-team management approach is beginning to happen. It’s new to primary care to apply a large team of clinicians; it takes a lot of resources. Being able to afford a team was a problem when we were paid by fee-for-service, it wasn’t practical. Population management will make it possible.”
Dr. Desai has been a consultant to Novartis, Merck, St. Jude, and Relypsa and has received research funding from Novartis and AtCor Medical. Dr. Redfield has been a consultant to Merck and Eli Lilly. Dr. Ahmad has been a consultant to Roche. Dr. Ong, Dr. Walsh, Dr. Jessup, Dr. McKie, Dr. Bauman, Dr. Shah, and Dr. Cunningham had no disclosures.
On Twitter @mitchelzoler
Heart failure management has become increasingly complex over the past couple of decades, with new drugs and drug combinations, new uses for potentially life-saving implanted devices, and a more sophisticated appreciation of the ways that various comorbidities complicate a heart failure patient’s clinical status. These expanded dimensions of heart failure care resulted in the establishment in 2008 of a new secondary subspecialty, Advanced Heart Failure and Transplant Cardiology, aimed at training and certifying physicians in all the nuances of complex heart failure diagnostics and care.
But as the 2009 manifesto announcing this new heart failure subspecialty detailed, care for the vast majority of U.S. patients with heart failure remains in the hands of internal medicine primary care physicians (PCPs) and general cardiologists (J Am Coll Cardiol. 2009 Mar 10;53[10]:834-6). To some extent this is a manpower issue. The estimated number of Americans living with heart failure exceeds 5 million, a figure that dwarfs the very modest number of U.S. physicians and clinicians who are certified or self-identified heart failure specialists.
As of today, fewer than 1,000 U.S. physicians have received formal certification as heart failure subspecialists through the examination administered in 2010, 2012, and 2014, said Michele Blair, chief executive officer of the Heart Failure Society of America. A more liberal definition of a heart failure specialist might include the roughly 3,000 unique physicians (mostly cardiologists, but also some hospitalists and emergency physicians) who have recently attended an annual meeting of the HFSA, as well as the roughly 2,300 physician assistants and nurse practitioners who have shown a heart failure interest by coming to a recent HFSA meeting. But even these expanded estimates calculate out to about 1 clinician with a special interest in heart failure for each 1,000 heart failure patients, not a very reassuring ratio.
The burgeoning numbers of heart failure patients, compared with the relative scarcity of both heart failure experts and general cardiologists, raises issues of how primary-care internists best share this management responsibility. Recent interviews with several heart failure subspecialists and primary care internists provide some insight into how this division of labor is now playing out in routine U.S. practice. What often occurs is that primary care internists take exclusive responsibility for caring for heart failure patients until they feel they are getting in over their heads, at which time they’ll consult with a cardiology colleague or refer the patient to a cardiologist. That moment of recognition by the generalist – that the demands and complexity of the case exceed their comfort level – varies widely, with some PCPs referring patients as soon as heart failure symptoms appear while others stay comfortable as the primary care giver even as a patient’s disease deteriorates to a more advanced stage.
Heart failure specialists highlighted their reliance on PCPs to take an ongoing, active role even for patients with significantly advanced heart failure, as generalists are well suited to coordinating the multispecialty care that such patients usually require, with attention to their need for lifestyle modifications as well as management of their diabetes, sleep apnea, chronic obstructive pulmonary disease, renal failure, and other comorbidities.
As Dr. Michael K. Ong, a primary care internist at the University of California, Los Angeles, said in an interview, his heart failure specialist colleague manages patients’ heart failure; “I manage [or refer] everything else not directly related to the heart failure.”
The most successful U.S. care models seem to be some variation on a team-care approach, in which physicians collaborate with pharmacists, nurses, rehabilitation specialists, and social workers as well as specialists, a team that would include and perhaps be led by either a primary care internist, a cardiologist, or a heart failure specialist but would also broadly include physicians able to deal with all the morbidity facets of heart failure. It’s a model that remains unavailable in many U.S. settings or is just starting to emerge, as fee-for-service coverage of patients gets replaced by population-management models that better accommodate the upfront financial demands of coordinated team care. It makes financial sense a few years down the road when improved patient outcomes result in cost savings.
Primary care and patients with symptomatic heart failure
The heart failure definitions and staging system established in 2001 by a guidelines panel of the American College of Cardiology and American Heart Association defined stage A heart failure as starting before a patient exhibits any heart failure symptoms (the classic ones include dyspnea, rales, and peripheral edema). The panel designated symptomatic heart failure patients as stage C. Patients without heart failure symptoms but with one or more risk factors (such as hypertension, diabetes, obesity, and cardiovascular disease) plus structural heart disease (such as cardiomyopathy or other forms of heart remodeling) were designated stage B. The panel said that people at stage A had one or more risk factors but no structural heart changes and no heart failure symptoms.
Although stage-A heart failure patients are clearly the types of people most often seen and cared for by PCPs, many of these physicians, as well as many heart failure specialists, don’t consider patients who have only hypertension or only diabetes or only obesity as yet having heart failure. That paradox deserves more discussion, but the best way to begin talking about PCPs and heart failure patients is when patients are symptomatic and have what everyone would agree is heart failure.
Even though the ACC/AHA staging system places stage C patients well down the heart failure road, stage C is usually when patients are first diagnosed with heart failure. Although the diagnosis is often first made by a hospitalist or emergency-department physician when severe and sudden-onset heart failure symptoms drive the patient to a hospital, or the diagnosis originates with a cardiologist or heart failure specialist when the patient’s presentation and differential diagnosis isn’t straightforward, most commonly the diagnosis starts with a PCP in an office encounter with a patient who is symptomatic but not acutely ill.
“Patients with shortness of breath or other forms of effort intolerance most often seek care from PCPs. The differential diagnosis of dyspnea is long and complex. Recognition that a patient with dyspnea may have HF is crucial” for timely management and treatment, said Dr. Mary Norine Walsh, medical director of Heart Failure and Cardiac Transplantation at St. Vincent Heart Center in Indianapolis.
At the Mayo Clinic in Rochester, Minn., “most of the heart failure diagnoses are done by PCPs, usually first identified at stage C when a patient comes in with symptoms. Stage B heart failure is usually only identified as an incidental finding when echocardiography is done for some other reason,” said Dr. Paul M. McKie, a heart failure cardiologist who works closely with the primary-care staff at Mayo as an embedded consultant cardiologist.
According to Dr. Mariell L. Jessup, a heart failure physician and professor at the University of Pennsylvania in Philadelphia, a key to PCPs promptly identifying patients with recent-onset, stage C heart failure is to keep the disease as well as its prominent risk factors at the top of their differential-diagnosis list for at-risk patients. “Heart failure is a common disorder,” Dr. Jessup said, and must be considered for patients with shortness of breath. “The leading causes of heart failure are hypertension, obesity, and diabetes. So keep heart failure in mind, especially for patients with one or more of these risk factors.”
Although PCPs might order an echocardiography examination or a lab test like measurement of brain natriuretic protein (BNP) to help nail down the diagnosis, they often leave reading the echocardiography results to a cardiologist colleague. “When a PCP orders an echo it’s automatically read by a cardiologist, and then we get the cardiologist’s report. I don’t read echos myself,” said Dr. Rebecca J. Cunningham, an internal medicine PCP at Brigham and Women’s Hospital in Boston who frequently sees patients with heart failure as medical director of the hospital’s Integrated Care Management Program. “I had one PCP colleague who undertook additional training to learn to read echos himself, but that’s unusual.”
Dr. Mary Ann Bauman, an internal medicine PCP and medical director for Women’s Health and Community Relations at INTEGRIS Health in Oklahoma City, noted a similar division of labor. “If a patient has shortness of breath, maybe some edema, and I hear a few rales, but is totally functional, I always order an echo but I don’t read it. I refer the echo to a cardiologist who then sends me a report,” Dr. Bauman said in an interview. “If I think the patient may have heart failure I’ll also order a BNP or NT-proBNP test. If I suspect heart failure and the BNP is high, it’s a red flag. BNP is another tool for getting the diagnosis right.”
The next step seems much more variable. Some PCPs retain primary control of heart failure management for many of their patients, especially when stage C patients remain stable and functional on simple, straightforward treatment and particularly when they have heart failure with preserved ejection fraction (HFpEF), usually defined as a left ventricular ejection fraction that is at least 40%-45%. Consultation or referral to a cardiologist or heart-failure physician seems much more common for patients with frequent decompensations and hospitalizations or patients with heart failure with reduced ejection fraction (HFrEF). But the main thread reported by both PCPs and cardiologists is that it all depends and varies for each patient and for each PCP depending on what patient responsibilities a PCP feels comfortable taking on.
Dr. Bauman sits at one end of the spectrum: “If it looks like a patient has heart failure, I refer them right away; I don’t wait for decompensation to occur. I want to be sure that there are no nuances in the patient that need something before I recognize it. Most of my PCP partners do the same. You don’t know what it is you don’t know. For me, it’s better to refer the patient right away so the patient has a cardiologist who already knows them who can be called if they start to decompensate.”
Dr. Bauman cited the increasing complexity of heart failure management as the main driver of her current approach, which she contrasted to how she dealt with heart failure patients 20 years ago. “It’s become so complicated that, as a PCP, I don’t feel that I can keep up” with the optimal ways to manage every heart failure patient. “I might not give my heart failure patients the best care they could receive.” The aspects of care that Dr. Bauman said she can provide to heart failure patients she has referred include “dealing with lifestyle changes, making sure patients are taking their medications and getting to their appointments, adjusting their heart-failure medication dosages as needed once they start on the drugs, and seeing that their diabetes and hypertension are well controlled. That is the role of the PCP. But when it comes to deciding which HF medications to use, that’s when I like to have a cardiologist involved.”
But the PCPs at Mayo Clinic often take a different tack, said Dr. McKie. “If the patient is a simple case of heart failure with no red flags and the patient is doing relatively well on treatment with simple diuretic treatment, then initiation of heart failure medications and ongoing management is often directed by the PCP with some cardiology backup as needed,” he said. But Dr. McKie conceded that a spectrum of PCP approaches exists at Mayo as well. “A lot depends on the patient and on the specific provider. Some patients we never get calls about; their PCPs are excellent at managing diuretics and uptitrating beta-blockers and ACE inhibitors. We may only get called if the patient decompensates, But other PCPs are very uncomfortable and they request that we get involved as soon as the diagnosis of stage C heart failure is made. So there is a wide range.” Dr. McKie noted that he thinks it is appropriate for himself or one of his cardiology colleagues to get more active when the HFrEF patient’s ejection fraction drops below 40% and certainly below 35%. That’s because at this stage, patients also need treatment with an aldosterone receptor antagonist such as spironolactone, and they undergo consideration for receiving an implantable cardioverter defibrillator or a cardiac resynchronization therapy device.
“There is nothing magic about heart failure management; it is very well proscribed by guidelines. Nothing precludes a PCP from taking ownership” of heart failure patients, said Dr. Akshay S. Desai, a heart failure cardiologist at Brigham and Women’s Hospital. “I think there is some fear among PCPs that they intrude” by managing heart failure patients. But for patients with structural heart disease or even left ventricular dysfunction, “PCPs should feel empowered to start standard heart failure treatments, including ACE inhibitors and beta-blockers, especially because half of heart failure patients have HFpEF, and PCPs often don’t refer HFpEF patients to cardiologists. It’s the patients with left ventricular dysfunction who end up in heart failure clinics,” Dr. Desai said.
On the other hand, Dr. Desai cautioned PCPs against waiting too long to bring more complex, sicker, and harder-to-manage patients to the attention of a heart failure specialist.
“What we worry about are late referrals, when patients are profoundly decompensated,” he said. “By the time they show up [at a heart failure clinic or emergency department] they have end-organ dysfunction,” which makes them much harder to treat and maybe irreversible. “Recognizing heart failure early is the key, and early referral is an obligation” when a heart failure patient is deteriorating or becomes too complex for a PCP to properly manage, Dr. Desai advised.
But even when heart failure patients develop more severe disease, with significantly depressed left ventricular function or frequent decompensations, PCPs continue to play a valuable role in coordinating the wide range of treatments patients need for their various comorbidities.
“Once a cardiologist or heart failure physician is involved there is still a role for PCPs” said Dr. Monica R. Shah, deputy chief of the Heart Failure and Arrhythmia Branch of the National Heart, Lung, and Blood Institute in Bethesda, Md. “Heart failure patients are complex, it’s not just one organ system that’s affected, and you need a partnership between cardiologists and PCPs to coordinate all of a patient’s care. A heart failure physician needs to work with a PCP to be sure that the patient’s health is optimal. Collaboration between cardiologists and PCPs is key to ensure that optimal care is effectively delivered to patients,” Dr. Shah said in an interview.
“Keeping the PCP at the center of the care team is critical, especially with the multiple comorbidities that HF patients can have, including chronic obstructive pulmonary disease, diabetes, renal failure, sleep apnea, atrial fibrillation, and degenerative joint disease. Before you know it you have a half-dozen subspecialists involved in care and it can become uncoordinated. Keeping the PCP at the center of the team and providing the PCP with support from specialists as needed is critical,” said Dr. McKie.
Even for the most severe heart failure patients, PCPs can still play an important role by providing palliative care and dealing with end-of-life issues, specialists said.
Primary care and heart failure’s antecedents
The other, obvious time in heart failure’s severity spectrum for PCPs to take a very active role is with presymptomatic, stage A patients. Perhaps the only controversial element of this is whether such patients really have a form of heart failure and whether is it important to conceptualize heart failure this way.
The notion of stage A heart failure dates back to the 2001 edition of heart failure diagnosis and management recommendations issued by a panel organized by the ACC and AHA (J Am Coll Cardiol. 2001 Dec;38[7]:2101-13). The 2001 writing committee members said that they “decided to take a new approach to the classification of heart failure that emphasized both the evolution and progression of the disease.” They defined stage A patients as presymptomatic and without structural heart disease but with “conditions strongly associated with the development of heart failure,” specifically systemic hypertension, coronary artery disease, diabetes, a history of cardiotoxic drug therapy or alcohol abuse, a history of rheumatic fever, or a family history of cardiomyopathy.
When the ACC and AHA panel members next updated the heart failure recommendations in 2005, they seemed to take a rhetorical step back, saying that stage A and B “are clearly not heart failure but are an attempt to help healthcare providers identify patients early who are at risk for developing heart failure. Stage A and B patients are best defined as those with risk factors that clearly predispose toward the development of HF.” (J Am Coll Cardiol. 2005 Sept. 46[6]:1116-43) In 2005, the panel also streamlined the list of risk factors that identify stage A heart failure patients: hypertension, atherosclerotic disease, diabetes, obesity, metabolic syndrome, patients who have taken cardiotoxins, or patients with a family history of cardiomyopathy. The 2009 recommendation update left this definition of stage A heart failure unchanged, but in 2013 the most recent update devoted less attention to explaining the significance of the stage-A heart failure, although it clearly highlighted the importance of controlling hypertension, diabetes, and obesity as ways to prevent patients from developing symptomatic heart failure (J Am Coll Cardiol. 2013 Oct 15;62[16]:e147-e239).
The subtle, official tweaking of the stage A (and B) heart failure concept during 2001-2013, as well establishment of stage A in the first place, seems to have left both PCPs and heart failure specialists unsure on exactly how to think about presymptomatic people with one or more of the prominent heart failure risk factors of hypertension, diabetes, and obesity. While they uniformly agree that identifying these risk factors and then treating them according to contemporary guidelines is hugely important for stopping or deferring the onset of heart failure, and they also agree that this aspect of patient care is clearly a core responsibility for PCPs, many also say that they don’t think of presymptomatic patients as having heart failure of any type despite the stage A designation on the books.
One exception is St. Vincent’s Dr. Walsh. “I think the writers of the 2001 heart failure guidelines had an inspired approach. Identifying patients with hypertension, diabetes, coronary artery disease, etc., as patients with heart failure has helped drive home the point that treatment and control of these diseases is crucial,” she said in an interview. “But I am not sure all physicians have adopted the concept. “Uncontrolled hypertension is prevalent, and not viewed by all as resulting in heart failure down the road. Diabetes and hypertension are very important risk factors for the development of heart failure in women,” she added. “I’m especially diligent in ensuring that women with one or both of these diseases get treated aggressively.”
Highlighting specifically the fundamental role that uncontrolled hypertension plays in causing heart failure, the University of Pennsylvania’s Dr. Jessup estimated that controlling hypertension throughout the U.S. population could probably cut heart failure incidence in half.
Others draw a sharper contrast between the risk factor stage and the symptomatic stages of heart failure, though they all agree on the importance of risk factor management by PCPs. “Hypertension does not mean that a patient has heart failure; it means they have a risk factor for heart failure and the patient is in the prevention stage,” said the NHLBI’s Dr. Shah. ”The most important role for PCPs is to identify the risk factors and prevent development of [symptomatic] heart failure. This is where PCPs are critically important because patients present to them at the early stages.”
Dr. Bauman, the PCP with INTEGRIS in Oklahoma City, generally doesn’t conflate risk factors with stage A heart failure. “I look at every patient with hypertension or diabetes as a person at risk for cardiovascular disease. I push them to get their blood pressure and glycemia under control. But I don’t think of them as stage A heart failure patients. I think of them as patients at risk for heart failure, but also at risk for atrial fibrillation, MI, and stroke. I think about their risk, but I don’t label them in my mind as having stage A heart failure. I think that this is a patient at risk for cardiovascular disease and that I must do what I should to manage their risk factors.”
“I don’t personally think about patients having stage A heart failure,” agreed Dr. Cunningham, a PCP at Brigham and Women’s Hospital. “When I see patients with hypertension, I counsel them about what matters to them so that they will take their medications, because if they currently feel fine they may not understand the long-term risk they face. So I invest time in making the patient understand why their hypertension is important and the risks it poses, so that in the long-run they won’t have a stroke or MI or develop heart failure. But I don’t think that the stage A definition has changed my approach; I already think of hypertension as a precursor to a variety of bad downstream consequences. I don’t think of someone as a heart failure patient just because they have hypertension, and I don’t think that every patient with hypertension will develop heart failure.” Speaking of her colleagues, Dr. Cunningham added, “I don’t have a sense that the stages of heart failure have made much of an impact on how other PCPs talk with patients or plan their care.”
“The heart failure staging system is useful from the standpoint of emphasizing that the disease begins with primordial risk and progresses through a period of structural injury during which patients may not be symptomatic,” summed up Dr. Desai. “But practically, most of us confront the diagnosis of heart failure when patients become symptomatic and reach stage C.”
Can an intensified approach better slow stage A progression?
One of the inherent limitations right now in referring to patients as having stage A heart failure is that it adds little to how heart failure risk factors are managed. A patient with hypertension undergoing appropriate care will receive treatment to lower blood pressure to recommended goal levels. The antihypertensive treatment remains the same regardless of whether the patient is considered to have only hypertension or whether the treating physician also thinks of the patient as having stage A heart failure. The same applies to patients diagnosed with diabetes; their hyperglycemia-controlling treatment remains unchanged whether or not their physician labels them as stage A heart failure patients.
But what if an evidence-based way existed to not only identify patients with hypertension or diabetes, but to identify within those patients the subset who faced a particularly increased risk for developing heart failure? And what if an evidence-based intervention existed that could be added to standard blood pressure–lowering or hyperglycemia-controlling interventions and had proved to slow or stop progression of patients to heart failure?
Preliminary evidence that screening for stage A heart failure patients can successfully identify a subset at elevated risk for developing symptomatic heart failure and that intensified risk-factor control helped mitigate this risk appeared in two reports published in 2013. But both studies were relatively small, they ran in Europe, and neither has undergone replication in a U.S. study in the 2.5 years since their publication.
The larger study, STOP-HF (St. Vincent’s Screening to Prevent Heart Failure), included patients at 39 primary care practices in Ireland, a study organized by researchers at St. Vincent’s University Hospital in Dublin. They enrolled people without symptoms of heart failure who were at least 41 years old and had at least one of these risk factors: hypertension, hypercholesterolemia, obesity, vascular disease, diabetes, an arrhythmia, or valvular disease: In short, primarily stage A heart failure patients.
The researchers then tested 1,374 of these people for their baseline blood level of BNP and randomized them into two intervention arms. For those randomized to the active arm, the PCPs for these people received an unblinded report of the BNP results, and those with a level of 50 pg/mL or higher underwent further assessment by screening echocardiography and intensified risk-factor control, including risk-factor coaching by a nurse. Those randomized to this arm who had a lower BNP level at baseline underwent annual follow-up BNP screening, and if their level reached the 50 pg/ML threshold they switched to the more intensified protocol. Those randomized to the control arm received a more standard program of risk-factor modification and their BNP levels were never unblinded.
After an average follow-up of 4.2 years, people in the active intervention arm of STOP-HF had a 5% cumulative incidence of left ventricular dysfunction or heart failure, while those in the control arm had a 9% rate, a 45% relative risk reduction from the active intervention that was statistically significant for the study’s primary endpoint (JAMA. 2013 July 3;310[1]:66-74).
The second study, PONTIAC (NT-proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients Without a History of Cardiac Disease), ran in Austria and Germany and involved 300 patients who had type 2 diabetes and were free from cardiac disease at baseline. At baseline, all people considered for the study underwent a screening measure of their blood level of NT-proBNP (a physiologic precursor to BNP) and those with a level above 125 pg/mL were randomized to either a usual-care group or an arm that underwent more intensified up-titration treatment with a renin-angiotensin system antagonist drug and with a beta-blocker. The primary endpoint was the incidence of hospitalization or death due to cardiac disease after 2 years, which was a relative 65% lower in the intensified intervention group, a statistically significant difference (J Am Coll Cardiol. 2013 Oct 8;62[15]:1365-72).
Both studies focused on people with common risk factors seen in primary care practices and used BNP or a BNP-like blood marker to identify people with an elevated risk for developing heart failure or other cardiac disease, and both studies showed that application of a more aggressive risk-factor intervention program resulted in a significant reduction in heart failure or heart failure–related outcomes after 2-4 years. Both studies appeared to offer models for improving risk-factor management by PCPs for people with stage A heart failure, but at the end of 2015 neither model had undergone U.S. testing.
“The STOP-HF and PONTIAC studies were proofs of concept for using biomarkers to gain a better sense of cardiac health,” said Dr. Tariq Ahmad, a heart failure physician at Yale University in New Haven, Conn., who is interested in developing biomarkers for guiding heart failure management. “Metrics like blood pressure and heart rate are relatively crude measures of cardiac health. We need to see in a large trial if we can use these more objective measures of cardiac health to decide how to treat patients,” In addition to BNP and NT-proBNP, Dr. Ahmad cited ST2 and galectin-3 as other promising biomarkers in the blood that may better gauge a person’s risk for developing heart failure and the need for intensified risk-factor control. The current inability of PCPs to better risk stratify people who meet the stage A heart failure definition so that those at highest risk could undergo more intensified interventions constitutes a missed opportunity for heart failure prevention, he said.
“The STOP-HF trial is really important and desperately needs replication,” said Dr. Margaret M. Redfield, professor of medicine and a heart failure physician at Mayo Clinic in Rochester, Minn.
She, and her Mayo associates, including Dr. McKie, are planning to launch a research protocol this year to finally test a STOP-HF type of program in a U.S. setting. They are planning to measure NT-proBNP levels in patients with stage A heart failure and then randomize some to an intervention arm with intensified risk reduction treatments.
“The problem with stage A today is, if we apply it according to the ACC and AHA definition, it would include quite a large number of patients, and not all of them – in fact a minority – would go on to develop symptomatic heart failure,” said Dr. McKie. “How you can further risk stratify the stage A population with simple testing is an issue for ongoing research,” he said. “The STOP-HF and PONTIAC strategies need more testing. Both studies were done in Europe, and we haven’t studied this approach in the U.S. Their approach makes sense and is appealing but it needs more testing.”
The economic barrier to intensified stage-A management
Even if a U.S. based study could replicate the STOP-HF results and provide an evidence base for improved prevention of symptomatic heart failure by interventions instituted by PCPs, it’s not clear whether the U.S. health care system as it currently is structured provides a framework that is able to invest in intensified upfront management of risk factors to achieve a reduced incidence of symptomatic heart failure several years later.
“One of the interesting aspects of STOP-HF was its use of a nurse-based intervention. We don’t have the resources for that in our practices right now,” noted Dr. Cunningham, the PCP at Brigham and Women’s Hospital who is medical director of the hospital’s Integrated Care Management Program for medically complex patients. While that program uses nurse care coordinators to pull together the disparate elements of care for heart failure patients and others with more severe, chronic illnesses, the program currently serves only patients with advanced disease, not presymptomatic patients who face a potentially elevated risk for bad outcomes that would happen many years in the future.
“This speaks to the need for more population-based preventive management, which PCPs are trying to start to do, but currently we are nowhere near fulfilling that potential,” said Dr. Cunningham. The barrier is having clinical resources for help in managing lower-risk patients, to make sure they receive all the interventions they should. We’re now trying to start using care teams for patients with diabetes or other conditions. The biggest gap is that we don’t have the resources; we don’t have enough nurses on our staff to intervene” for all the patients who could potentially benefit. “Right now, we can only afford to use nurses for selected, high-risk patients.” The challenge is to have a care model that allows a lot of upfront costs to generate savings over a long-term time horizon, he said. “It’s very important for improving population health, but it’s hard to make it happen in our current health care system.”
Dr. Ahmad noted the enormous downside of a health system that is not proactive and often waits for heart failure patients to declare themselves with severe illness.
“The majority of heart failure patients I see drifted through the health care system” without recognition of their accumulating morbidity. “By the time they show heart failure symptoms, their disease is pretty advanced and we have real difficulty managing it. A lot of patients do not have their heart failure managed until they fall off the edge and their condition is much less modifiable. If we could identify these patients sooner, it would help both them and the health care system. It would be great to have objective measures that could help PCPs identify early abnormal patients who need more aggressive management. In much of U.S. practice, heart failure management is more specialty driven. It might be different in closed systems, but in many heart failure practices there is no PCP coordination. The health care system is not set up to allow PCPs to take care of these issues.”
Dr. Bauman said she sees some reason for optimism in looming reimbursement changes, where population management might help drive a shift toward more team care for heart failure and a focus on earlier identification of patients at risk and intervention at early stages of their disease.
“As we move toward population management it becomes more obvious that you need a team approach to managing heart failure, involving not just physicians but also pharmacists, nurses, social workers, and care coordinators. In my system, INTEGRIS, the whole-team management approach is beginning to happen. It’s new to primary care to apply a large team of clinicians; it takes a lot of resources. Being able to afford a team was a problem when we were paid by fee-for-service, it wasn’t practical. Population management will make it possible.”
Dr. Desai has been a consultant to Novartis, Merck, St. Jude, and Relypsa and has received research funding from Novartis and AtCor Medical. Dr. Redfield has been a consultant to Merck and Eli Lilly. Dr. Ahmad has been a consultant to Roche. Dr. Ong, Dr. Walsh, Dr. Jessup, Dr. McKie, Dr. Bauman, Dr. Shah, and Dr. Cunningham had no disclosures.
On Twitter @mitchelzoler
Chlorthalidone controls blood pressure longer than HCTZ
Low-dose chlorthalidone is significantly better at reducing blood pressure over a 24-hour period than is the most commonly prescribed formulation of hydrochlorothiazide for essential hypertension, findings from a randomized, controlled trial published Jan. 25 suggest.
Standard HCTZ at 12.5 mg was seen reducing blood pressure during daytime or office hours, resulting in undetected or masked hypertension during nighttime and early-morning hours, investigators found.
However, both 6.25 mg chlorthalidone and an extended-release preparation of 12.5 mg HCTZ were shown to be effective at sustaining a smooth, 24-hour control as measured by ambulatory blood pressure monitoring.
For their research, Dr. Anil K. Pareek of Ipca Laboratories in Mumbai, India, and his colleagues randomized 54 patients aged 65 years and younger with stage 1 hypertension and no comorbidities to chlorthalidone, 6.25 mg (16 patients); HCTZ 12.5 mg (18); or HCTZ-controlled release 12.5 mg (20) for 12 weeks.
For the cohort as a whole, patients’ mean in-office blood pressure was 149/93 mm Hg. At both 4 and 12 weeks, all three study arms saw significant reductions in office BP (P less than .01).
However, only patients treated with chlorthalidone or HCTZ-CR saw statistically significant reductions in ambulatory diastolic and systolic blood pressure at both time points, while the standard HCTZ group did not see statistically significant changes. At 12 weeks, patients treated with chlorthalidone or HCTZ-CR achieved reductions of 11.1/7.8 mm Hg, and 10.3/8.2 mm Hg, respectively, while those in the standard HCTZ arm saw drops of 6.0/4.2 mm Hg (J Am Coll Cardiol. 2016 Jan 25;67:379-89).
Also at 12 weeks, the chlorthalidone and HCTZ-CR groups had mean nocturnal systolic pressure decreases of 10.2 and 12.7 mm/Hg, respectively, while those receiving standard HCTZ saw nighttime SBP reduced by mean 4.9 mm Hg.
Low potassium was seen in 10% (n = 2) of patients taking HCTZ-CR, 5.6% of patients on standard HCTZ, and 6.3% (n = 1) of patients on chlorthalidone.
Dr. Pareek and his colleagues noted that chlorthalidone possesses a distinct pharmacokinetic profile from HCTZ, with a higher potency that allows it to be used as monotherapy in smaller doses and has “a wider volume of distribution, with partitioning into red blood cells” that may be responsible for its longer duration of action.
Dr. Pareek and his colleagues wrote that previous studies have shown an overestimation of the response to standard HCTZ, based on office blood pressure measurements alone.
“Assessing the antihypertensive efficacy of HCTZ by [office] BP measurements only is deceptive and prone to lull physicians and patients into a false sense of security,” the researchers wrote. “With HCTZ therapy, sustained hypertension merely will be converted into masked hypertension.”
Low-dose chlorthalidone can be used as monotherapy in treating essential hypertension, Dr. Pareek and his colleagues concluded, whereas standard low-dose HCTZ should no longer be considered an acceptable option.
The investigators noted as a limitation of their study its small size.
Ipca Laboratories, a manufacturer of chlorthalidone, sponsored the study, whose lead author and two coauthors are also employees of Ipca.
Despite the overwhelming cardiovascular outcome data and established 24-hour efficacy of chlorthalidone in hypertension, prescription patterns are unchanged, with HCTZ remaining the diuretic of choice due to concerns regarding hypokalemia and greater likelihood of new-onset diabetes associated with chlorthalidone. Ambulatory blood pressure monitoring data have shown that chlorthalidone has antihypertensive effects beyond 24 hours, whereas HCTZ has antihypertensive effects for roughly 12-14 hours.
It could be argued that the superiority of chlorthalidone in cardiovascular outcome trials is not attributable to its potency, but rather its duration of action, given its extended effects on nocturnal blood pressure. As such, controlled-release HCTZ was also able to achieve comparable antihypertensive effects in terms of blood pressure reduction and duration of effect. These long-acting preparations are crucial to blood pressure control during the early morning, when one is most vulnerable to CV events.
Although this trial may appear to reflect a lower rate of side effects, specifically the absence of hyponatremia, the trial duration was short, the study was underpowered to detect this outcome, and those at highest risk, that is, people older than 65 years of age, were excluded. Only one-third of those screened were enrolled, suggesting that just a fraction of the patients seen on a daily basis would fit the focused study criteria, namely those without comorbidities and younger than 65 years. Additionally, because the study was conducted in a Southeast Asian country, it remains unclear whether the results can be extrapolated to those on Western diets or of other ethnicities.
Dr. Hillel Z. Sternlicht and Dr. George Bakris of the ASH Comprehensive Hypertension Center, University of Chicago Medicine, made these comments in an accompanying editorial (J Am Coll Cardiol. 2016 Jan 25. doi: 10.1016/j.jacc.2015.11.025). Dr. Sternlicht has reported that he has no relationships relevant to this paper. Dr. Bakris disclosed financial relationships with AbbVie, Janssen, AstraZeneca, Bayer, Takeda, NxStage, and Daiichi-Sankyo.
Despite the overwhelming cardiovascular outcome data and established 24-hour efficacy of chlorthalidone in hypertension, prescription patterns are unchanged, with HCTZ remaining the diuretic of choice due to concerns regarding hypokalemia and greater likelihood of new-onset diabetes associated with chlorthalidone. Ambulatory blood pressure monitoring data have shown that chlorthalidone has antihypertensive effects beyond 24 hours, whereas HCTZ has antihypertensive effects for roughly 12-14 hours.
It could be argued that the superiority of chlorthalidone in cardiovascular outcome trials is not attributable to its potency, but rather its duration of action, given its extended effects on nocturnal blood pressure. As such, controlled-release HCTZ was also able to achieve comparable antihypertensive effects in terms of blood pressure reduction and duration of effect. These long-acting preparations are crucial to blood pressure control during the early morning, when one is most vulnerable to CV events.
Although this trial may appear to reflect a lower rate of side effects, specifically the absence of hyponatremia, the trial duration was short, the study was underpowered to detect this outcome, and those at highest risk, that is, people older than 65 years of age, were excluded. Only one-third of those screened were enrolled, suggesting that just a fraction of the patients seen on a daily basis would fit the focused study criteria, namely those without comorbidities and younger than 65 years. Additionally, because the study was conducted in a Southeast Asian country, it remains unclear whether the results can be extrapolated to those on Western diets or of other ethnicities.
Dr. Hillel Z. Sternlicht and Dr. George Bakris of the ASH Comprehensive Hypertension Center, University of Chicago Medicine, made these comments in an accompanying editorial (J Am Coll Cardiol. 2016 Jan 25. doi: 10.1016/j.jacc.2015.11.025). Dr. Sternlicht has reported that he has no relationships relevant to this paper. Dr. Bakris disclosed financial relationships with AbbVie, Janssen, AstraZeneca, Bayer, Takeda, NxStage, and Daiichi-Sankyo.
Despite the overwhelming cardiovascular outcome data and established 24-hour efficacy of chlorthalidone in hypertension, prescription patterns are unchanged, with HCTZ remaining the diuretic of choice due to concerns regarding hypokalemia and greater likelihood of new-onset diabetes associated with chlorthalidone. Ambulatory blood pressure monitoring data have shown that chlorthalidone has antihypertensive effects beyond 24 hours, whereas HCTZ has antihypertensive effects for roughly 12-14 hours.
It could be argued that the superiority of chlorthalidone in cardiovascular outcome trials is not attributable to its potency, but rather its duration of action, given its extended effects on nocturnal blood pressure. As such, controlled-release HCTZ was also able to achieve comparable antihypertensive effects in terms of blood pressure reduction and duration of effect. These long-acting preparations are crucial to blood pressure control during the early morning, when one is most vulnerable to CV events.
Although this trial may appear to reflect a lower rate of side effects, specifically the absence of hyponatremia, the trial duration was short, the study was underpowered to detect this outcome, and those at highest risk, that is, people older than 65 years of age, were excluded. Only one-third of those screened were enrolled, suggesting that just a fraction of the patients seen on a daily basis would fit the focused study criteria, namely those without comorbidities and younger than 65 years. Additionally, because the study was conducted in a Southeast Asian country, it remains unclear whether the results can be extrapolated to those on Western diets or of other ethnicities.
Dr. Hillel Z. Sternlicht and Dr. George Bakris of the ASH Comprehensive Hypertension Center, University of Chicago Medicine, made these comments in an accompanying editorial (J Am Coll Cardiol. 2016 Jan 25. doi: 10.1016/j.jacc.2015.11.025). Dr. Sternlicht has reported that he has no relationships relevant to this paper. Dr. Bakris disclosed financial relationships with AbbVie, Janssen, AstraZeneca, Bayer, Takeda, NxStage, and Daiichi-Sankyo.
Low-dose chlorthalidone is significantly better at reducing blood pressure over a 24-hour period than is the most commonly prescribed formulation of hydrochlorothiazide for essential hypertension, findings from a randomized, controlled trial published Jan. 25 suggest.
Standard HCTZ at 12.5 mg was seen reducing blood pressure during daytime or office hours, resulting in undetected or masked hypertension during nighttime and early-morning hours, investigators found.
However, both 6.25 mg chlorthalidone and an extended-release preparation of 12.5 mg HCTZ were shown to be effective at sustaining a smooth, 24-hour control as measured by ambulatory blood pressure monitoring.
For their research, Dr. Anil K. Pareek of Ipca Laboratories in Mumbai, India, and his colleagues randomized 54 patients aged 65 years and younger with stage 1 hypertension and no comorbidities to chlorthalidone, 6.25 mg (16 patients); HCTZ 12.5 mg (18); or HCTZ-controlled release 12.5 mg (20) for 12 weeks.
For the cohort as a whole, patients’ mean in-office blood pressure was 149/93 mm Hg. At both 4 and 12 weeks, all three study arms saw significant reductions in office BP (P less than .01).
However, only patients treated with chlorthalidone or HCTZ-CR saw statistically significant reductions in ambulatory diastolic and systolic blood pressure at both time points, while the standard HCTZ group did not see statistically significant changes. At 12 weeks, patients treated with chlorthalidone or HCTZ-CR achieved reductions of 11.1/7.8 mm Hg, and 10.3/8.2 mm Hg, respectively, while those in the standard HCTZ arm saw drops of 6.0/4.2 mm Hg (J Am Coll Cardiol. 2016 Jan 25;67:379-89).
Also at 12 weeks, the chlorthalidone and HCTZ-CR groups had mean nocturnal systolic pressure decreases of 10.2 and 12.7 mm/Hg, respectively, while those receiving standard HCTZ saw nighttime SBP reduced by mean 4.9 mm Hg.
Low potassium was seen in 10% (n = 2) of patients taking HCTZ-CR, 5.6% of patients on standard HCTZ, and 6.3% (n = 1) of patients on chlorthalidone.
Dr. Pareek and his colleagues noted that chlorthalidone possesses a distinct pharmacokinetic profile from HCTZ, with a higher potency that allows it to be used as monotherapy in smaller doses and has “a wider volume of distribution, with partitioning into red blood cells” that may be responsible for its longer duration of action.
Dr. Pareek and his colleagues wrote that previous studies have shown an overestimation of the response to standard HCTZ, based on office blood pressure measurements alone.
“Assessing the antihypertensive efficacy of HCTZ by [office] BP measurements only is deceptive and prone to lull physicians and patients into a false sense of security,” the researchers wrote. “With HCTZ therapy, sustained hypertension merely will be converted into masked hypertension.”
Low-dose chlorthalidone can be used as monotherapy in treating essential hypertension, Dr. Pareek and his colleagues concluded, whereas standard low-dose HCTZ should no longer be considered an acceptable option.
The investigators noted as a limitation of their study its small size.
Ipca Laboratories, a manufacturer of chlorthalidone, sponsored the study, whose lead author and two coauthors are also employees of Ipca.
Low-dose chlorthalidone is significantly better at reducing blood pressure over a 24-hour period than is the most commonly prescribed formulation of hydrochlorothiazide for essential hypertension, findings from a randomized, controlled trial published Jan. 25 suggest.
Standard HCTZ at 12.5 mg was seen reducing blood pressure during daytime or office hours, resulting in undetected or masked hypertension during nighttime and early-morning hours, investigators found.
However, both 6.25 mg chlorthalidone and an extended-release preparation of 12.5 mg HCTZ were shown to be effective at sustaining a smooth, 24-hour control as measured by ambulatory blood pressure monitoring.
For their research, Dr. Anil K. Pareek of Ipca Laboratories in Mumbai, India, and his colleagues randomized 54 patients aged 65 years and younger with stage 1 hypertension and no comorbidities to chlorthalidone, 6.25 mg (16 patients); HCTZ 12.5 mg (18); or HCTZ-controlled release 12.5 mg (20) for 12 weeks.
For the cohort as a whole, patients’ mean in-office blood pressure was 149/93 mm Hg. At both 4 and 12 weeks, all three study arms saw significant reductions in office BP (P less than .01).
However, only patients treated with chlorthalidone or HCTZ-CR saw statistically significant reductions in ambulatory diastolic and systolic blood pressure at both time points, while the standard HCTZ group did not see statistically significant changes. At 12 weeks, patients treated with chlorthalidone or HCTZ-CR achieved reductions of 11.1/7.8 mm Hg, and 10.3/8.2 mm Hg, respectively, while those in the standard HCTZ arm saw drops of 6.0/4.2 mm Hg (J Am Coll Cardiol. 2016 Jan 25;67:379-89).
Also at 12 weeks, the chlorthalidone and HCTZ-CR groups had mean nocturnal systolic pressure decreases of 10.2 and 12.7 mm/Hg, respectively, while those receiving standard HCTZ saw nighttime SBP reduced by mean 4.9 mm Hg.
Low potassium was seen in 10% (n = 2) of patients taking HCTZ-CR, 5.6% of patients on standard HCTZ, and 6.3% (n = 1) of patients on chlorthalidone.
Dr. Pareek and his colleagues noted that chlorthalidone possesses a distinct pharmacokinetic profile from HCTZ, with a higher potency that allows it to be used as monotherapy in smaller doses and has “a wider volume of distribution, with partitioning into red blood cells” that may be responsible for its longer duration of action.
Dr. Pareek and his colleagues wrote that previous studies have shown an overestimation of the response to standard HCTZ, based on office blood pressure measurements alone.
“Assessing the antihypertensive efficacy of HCTZ by [office] BP measurements only is deceptive and prone to lull physicians and patients into a false sense of security,” the researchers wrote. “With HCTZ therapy, sustained hypertension merely will be converted into masked hypertension.”
Low-dose chlorthalidone can be used as monotherapy in treating essential hypertension, Dr. Pareek and his colleagues concluded, whereas standard low-dose HCTZ should no longer be considered an acceptable option.
The investigators noted as a limitation of their study its small size.
Ipca Laboratories, a manufacturer of chlorthalidone, sponsored the study, whose lead author and two coauthors are also employees of Ipca.
FROM JACC
Key clinical point: Standard HCTZ 12.5 mg did not significantly lower blood pressure over 24 hours, while 6.25 mg chlorthalidone and 12.5 mg controlled-release HCTZ did.
Major finding: At 12 weeks, patients treated with chlorthalidone or HCTZ-CR achieved reductions of 11.1/7.8 mm Hg and 10.3/8.2 mm Hg (P less than .01 for both), respectively, while those in the standard HCTZ arm saw drops of 6.0/4.2 mm Hg.
Data source: A randomized, double-blind, comparative study enrolling 54 patients with stage 1 hypertension. Patients were randomized to receive chlorthalidone 6.25 mg, HCTZ-CR 12.5 mg, or conventional HCTZ 12.5 mg and were followed up at 4 and 12 weeks with ambulatory and in-office BP monitoring.
Disclosures: The study was sponsored by a manufacturer of chlorthalidone, with lead author and two coauthors who are company employees.