User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
AI-Powered Clinical Documentation Tool Reduces EHR Time for Clinicians
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Study Reports Safety Data in Children on JAK Inhibitors
TOPLINE:
which also found that acne was the most common skin-related AE in children, and serious AEs were less common.
METHODOLOGY:
- Researchers analyzed 399,649 AEs in 133,216 adult patients and 2883 AEs in 955 pediatric patients (age, < 18 years) from November 2011 to February 2023 using the US Food and Drug Administration Adverse Event Reporting System and the Canada Vigilance Adverse Reaction Online Database.
- AEs were categorized on the basis of the Medical Dictionary for Regulatory Activities system organ class.
- Five JAK inhibitors approved for use in children were included in the study: Baricitinib, upadacitinib, abrocitinib, ruxolitinib, and tofacitinib.
TAKEAWAY:
- The most frequently reported AEs in children were blood and lymphatic system disorders, including neutropenia, thrombocytopenia, and anemia (24%); viral, fungal, and bacterial infections, such as pneumonia and sepsis (17.2%); constitutional symptoms and administrative concerns, including pyrexia and fatigue (15.7%); gastrointestinal disorders, such as vomiting and abdominal pain (13.6%); and respiratory disorders, such as cough and respiratory distress (5.3%).
- In adults, the most common AEs were viral, fungal, and bacterial infections (16.8%); constitutional symptoms and administrative concerns (13.5%); musculoskeletal and connective tissue disorders (7.04%); and gastrointestinal (5.8%) and nervous system (5%) disorders.
- Acne (30.6%), atopic dermatitis (22.2%), and psoriasis (16.7%) were the most common skin and subcutaneous tissue AEs reported in children. Skin and subcutaneous AEs were more common with upadacitinib (21.1%), abrocitinib (9.1%), and tofacitinib (6.3%) in children.
- Serious AEs included in the boxed warning for JAK inhibitors — serious infection, mortality, malignancy, cardiovascular events, and thrombosis — were similar for baricitinib in children (4 of 49 patients, 8.2%) and adults (325 of 3707, 8.8%). For other JAK inhibitors, absolute numbers of these AEs in children were small and rates were lower in children than in adults.
IN PRACTICE:
“This information can support customized treatment and minimize the potential for undesired or intolerable AEs,” the authors wrote.
SOURCE:
This study was led by Sahithi Talasila, BS, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and was published online in Pediatric Dermatology.
LIMITATIONS:
Pharmacovigilance registries did not fully capture the complete range of AEs because of potential reporting bias or recall bias. Additionally, events lacking sufficient objective evidence were underreported, while common AEs associated with JAK inhibitor therapy were overreported.
DISCLOSURES:
No specific funding sources for the study were reported. One author reported being a consultant, one reported serving as a principal investigator in clinical trials, and another reported serving on data and safety monitoring boards of various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which also found that acne was the most common skin-related AE in children, and serious AEs were less common.
METHODOLOGY:
- Researchers analyzed 399,649 AEs in 133,216 adult patients and 2883 AEs in 955 pediatric patients (age, < 18 years) from November 2011 to February 2023 using the US Food and Drug Administration Adverse Event Reporting System and the Canada Vigilance Adverse Reaction Online Database.
- AEs were categorized on the basis of the Medical Dictionary for Regulatory Activities system organ class.
- Five JAK inhibitors approved for use in children were included in the study: Baricitinib, upadacitinib, abrocitinib, ruxolitinib, and tofacitinib.
TAKEAWAY:
- The most frequently reported AEs in children were blood and lymphatic system disorders, including neutropenia, thrombocytopenia, and anemia (24%); viral, fungal, and bacterial infections, such as pneumonia and sepsis (17.2%); constitutional symptoms and administrative concerns, including pyrexia and fatigue (15.7%); gastrointestinal disorders, such as vomiting and abdominal pain (13.6%); and respiratory disorders, such as cough and respiratory distress (5.3%).
- In adults, the most common AEs were viral, fungal, and bacterial infections (16.8%); constitutional symptoms and administrative concerns (13.5%); musculoskeletal and connective tissue disorders (7.04%); and gastrointestinal (5.8%) and nervous system (5%) disorders.
- Acne (30.6%), atopic dermatitis (22.2%), and psoriasis (16.7%) were the most common skin and subcutaneous tissue AEs reported in children. Skin and subcutaneous AEs were more common with upadacitinib (21.1%), abrocitinib (9.1%), and tofacitinib (6.3%) in children.
- Serious AEs included in the boxed warning for JAK inhibitors — serious infection, mortality, malignancy, cardiovascular events, and thrombosis — were similar for baricitinib in children (4 of 49 patients, 8.2%) and adults (325 of 3707, 8.8%). For other JAK inhibitors, absolute numbers of these AEs in children were small and rates were lower in children than in adults.
IN PRACTICE:
“This information can support customized treatment and minimize the potential for undesired or intolerable AEs,” the authors wrote.
SOURCE:
This study was led by Sahithi Talasila, BS, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and was published online in Pediatric Dermatology.
LIMITATIONS:
Pharmacovigilance registries did not fully capture the complete range of AEs because of potential reporting bias or recall bias. Additionally, events lacking sufficient objective evidence were underreported, while common AEs associated with JAK inhibitor therapy were overreported.
DISCLOSURES:
No specific funding sources for the study were reported. One author reported being a consultant, one reported serving as a principal investigator in clinical trials, and another reported serving on data and safety monitoring boards of various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which also found that acne was the most common skin-related AE in children, and serious AEs were less common.
METHODOLOGY:
- Researchers analyzed 399,649 AEs in 133,216 adult patients and 2883 AEs in 955 pediatric patients (age, < 18 years) from November 2011 to February 2023 using the US Food and Drug Administration Adverse Event Reporting System and the Canada Vigilance Adverse Reaction Online Database.
- AEs were categorized on the basis of the Medical Dictionary for Regulatory Activities system organ class.
- Five JAK inhibitors approved for use in children were included in the study: Baricitinib, upadacitinib, abrocitinib, ruxolitinib, and tofacitinib.
TAKEAWAY:
- The most frequently reported AEs in children were blood and lymphatic system disorders, including neutropenia, thrombocytopenia, and anemia (24%); viral, fungal, and bacterial infections, such as pneumonia and sepsis (17.2%); constitutional symptoms and administrative concerns, including pyrexia and fatigue (15.7%); gastrointestinal disorders, such as vomiting and abdominal pain (13.6%); and respiratory disorders, such as cough and respiratory distress (5.3%).
- In adults, the most common AEs were viral, fungal, and bacterial infections (16.8%); constitutional symptoms and administrative concerns (13.5%); musculoskeletal and connective tissue disorders (7.04%); and gastrointestinal (5.8%) and nervous system (5%) disorders.
- Acne (30.6%), atopic dermatitis (22.2%), and psoriasis (16.7%) were the most common skin and subcutaneous tissue AEs reported in children. Skin and subcutaneous AEs were more common with upadacitinib (21.1%), abrocitinib (9.1%), and tofacitinib (6.3%) in children.
- Serious AEs included in the boxed warning for JAK inhibitors — serious infection, mortality, malignancy, cardiovascular events, and thrombosis — were similar for baricitinib in children (4 of 49 patients, 8.2%) and adults (325 of 3707, 8.8%). For other JAK inhibitors, absolute numbers of these AEs in children were small and rates were lower in children than in adults.
IN PRACTICE:
“This information can support customized treatment and minimize the potential for undesired or intolerable AEs,” the authors wrote.
SOURCE:
This study was led by Sahithi Talasila, BS, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and was published online in Pediatric Dermatology.
LIMITATIONS:
Pharmacovigilance registries did not fully capture the complete range of AEs because of potential reporting bias or recall bias. Additionally, events lacking sufficient objective evidence were underreported, while common AEs associated with JAK inhibitor therapy were overreported.
DISCLOSURES:
No specific funding sources for the study were reported. One author reported being a consultant, one reported serving as a principal investigator in clinical trials, and another reported serving on data and safety monitoring boards of various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
FDA OKs Subcutaneous Atezolizumab Formulation for Multiple Cancer Indications
Approved indications include non–small cell lung cancer (NSCLC), SCLC, hepatocellular carcinoma, melanoma, and alveolar soft part sarcoma. Specific indications are available with the full prescribing information at Drugs@FDA.
This is the first programmed death–ligand 1 inhibitor to gain approval for subcutaneous administration.
“This approval represents a significant option to improve the patient experience,” Ann Fish-Steagall, RN, Senior Vice President of Patient Services at the LUNGevity Foundation stated in a Genentech press release.
Subcutaneous atezolizumab and hyaluronidase-tqjs was evaluated in the open-label, randomized IMscin001 trial of 371 adult patients with locally advanced or metastatic NSCLC who were not previously exposed to cancer immunotherapy and who had disease progression following treatment with platinum-based chemotherapy. Patients were randomized 2:1 to receive subcutaneous or IV administration until disease progression or unacceptable toxicity.
Atezolizumab exposure, the primary outcome measure of the study, met the lower limit of geometric mean ratio above the prespecified threshold of 0.8 (cycle 1C trough, 1.05; area under the curve for days 0-21, 0.87).
No notable differences were observed in overall response rate, progression-free survival, or overall survival between the two formulations, according to the FDA approval notice.
The confirmed overall response rate was 9% in the subcutaneous arm and 8% intravenous arm.
Adverse events of any grade occurring in at least 10% of patients were fatigue, musculoskeletal pain, cough, dyspnea, and decreased appetite.
The recommended dose for subcutaneous injection is one 15 mL injection, which contains 1875 mg of atezolizumab and 30,000 units of hyaluronidase.
Injections should be administered in the thigh over approximately 7 minutes every 3 weeks. By contrast, IV administration generally takes 30-60 minutes.
A version of this article first appeared on Medscape.com.
Approved indications include non–small cell lung cancer (NSCLC), SCLC, hepatocellular carcinoma, melanoma, and alveolar soft part sarcoma. Specific indications are available with the full prescribing information at Drugs@FDA.
This is the first programmed death–ligand 1 inhibitor to gain approval for subcutaneous administration.
“This approval represents a significant option to improve the patient experience,” Ann Fish-Steagall, RN, Senior Vice President of Patient Services at the LUNGevity Foundation stated in a Genentech press release.
Subcutaneous atezolizumab and hyaluronidase-tqjs was evaluated in the open-label, randomized IMscin001 trial of 371 adult patients with locally advanced or metastatic NSCLC who were not previously exposed to cancer immunotherapy and who had disease progression following treatment with platinum-based chemotherapy. Patients were randomized 2:1 to receive subcutaneous or IV administration until disease progression or unacceptable toxicity.
Atezolizumab exposure, the primary outcome measure of the study, met the lower limit of geometric mean ratio above the prespecified threshold of 0.8 (cycle 1C trough, 1.05; area under the curve for days 0-21, 0.87).
No notable differences were observed in overall response rate, progression-free survival, or overall survival between the two formulations, according to the FDA approval notice.
The confirmed overall response rate was 9% in the subcutaneous arm and 8% intravenous arm.
Adverse events of any grade occurring in at least 10% of patients were fatigue, musculoskeletal pain, cough, dyspnea, and decreased appetite.
The recommended dose for subcutaneous injection is one 15 mL injection, which contains 1875 mg of atezolizumab and 30,000 units of hyaluronidase.
Injections should be administered in the thigh over approximately 7 minutes every 3 weeks. By contrast, IV administration generally takes 30-60 minutes.
A version of this article first appeared on Medscape.com.
Approved indications include non–small cell lung cancer (NSCLC), SCLC, hepatocellular carcinoma, melanoma, and alveolar soft part sarcoma. Specific indications are available with the full prescribing information at Drugs@FDA.
This is the first programmed death–ligand 1 inhibitor to gain approval for subcutaneous administration.
“This approval represents a significant option to improve the patient experience,” Ann Fish-Steagall, RN, Senior Vice President of Patient Services at the LUNGevity Foundation stated in a Genentech press release.
Subcutaneous atezolizumab and hyaluronidase-tqjs was evaluated in the open-label, randomized IMscin001 trial of 371 adult patients with locally advanced or metastatic NSCLC who were not previously exposed to cancer immunotherapy and who had disease progression following treatment with platinum-based chemotherapy. Patients were randomized 2:1 to receive subcutaneous or IV administration until disease progression or unacceptable toxicity.
Atezolizumab exposure, the primary outcome measure of the study, met the lower limit of geometric mean ratio above the prespecified threshold of 0.8 (cycle 1C trough, 1.05; area under the curve for days 0-21, 0.87).
No notable differences were observed in overall response rate, progression-free survival, or overall survival between the two formulations, according to the FDA approval notice.
The confirmed overall response rate was 9% in the subcutaneous arm and 8% intravenous arm.
Adverse events of any grade occurring in at least 10% of patients were fatigue, musculoskeletal pain, cough, dyspnea, and decreased appetite.
The recommended dose for subcutaneous injection is one 15 mL injection, which contains 1875 mg of atezolizumab and 30,000 units of hyaluronidase.
Injections should be administered in the thigh over approximately 7 minutes every 3 weeks. By contrast, IV administration generally takes 30-60 minutes.
A version of this article first appeared on Medscape.com.
FDA Approves IL-13 inhibitor for Atopic Dermatitis
The that is not well controlled, despite treatment with topical prescription therapies.
The recommended initial starting dose of lebrikizumab consists of 500 mg (two 250 mg injections) at baseline and week 2, followed by 250 mg every 2 weeks until week 16 or later when adequate clinical response is achieved. Then, maintenance dosing is recommended with one monthly injection (250 mg every 4 weeks). Children aged 12-17 years must weigh at least 88 pounds (40 kg) to be eligible for lebrikizumab treatment.
According to a press release from Lilly, which has been developing lebrikizumab, approval was based on results from the ADvocate 1, ADvocate 2, and ADhere studies, which included over 1000 adults and children aged 12 and older with moderate to severe AD. The primary endpoint for these studies was evaluated at 16 weeks and measured clear or almost clear skin (IGA score of 0 or 1).
According to Lilly, 38% of people in ADvocate 1 and 2 who took lebrikizumab achieved clear or almost-clear skin at 16 weeks, compared with 12% of those in the placebo arm, and 10% experienced these results as early as 4 weeks. Of those treated with lebrikizumab who experienced clear or almost-clear skin at week 16, 77% maintained those results at 1 year on the once-monthly dose. In addition, on average, 43% of those on lebrikizumab experienced relief of itch at 16 weeks, compared with 12% of those on placebo, according to the press release.
The most common side effects of lebrikizumab observed in the clinical trials include eye and eyelid inflammation, such as redness, swelling, and itching; injection-site reactions; and herpes zoster (shingles).
Lebrikizumab was approved in Japan in January 2024, and by the European Commission in 2023.
A version of this article first appeared on Medscape.com.
The that is not well controlled, despite treatment with topical prescription therapies.
The recommended initial starting dose of lebrikizumab consists of 500 mg (two 250 mg injections) at baseline and week 2, followed by 250 mg every 2 weeks until week 16 or later when adequate clinical response is achieved. Then, maintenance dosing is recommended with one monthly injection (250 mg every 4 weeks). Children aged 12-17 years must weigh at least 88 pounds (40 kg) to be eligible for lebrikizumab treatment.
According to a press release from Lilly, which has been developing lebrikizumab, approval was based on results from the ADvocate 1, ADvocate 2, and ADhere studies, which included over 1000 adults and children aged 12 and older with moderate to severe AD. The primary endpoint for these studies was evaluated at 16 weeks and measured clear or almost clear skin (IGA score of 0 or 1).
According to Lilly, 38% of people in ADvocate 1 and 2 who took lebrikizumab achieved clear or almost-clear skin at 16 weeks, compared with 12% of those in the placebo arm, and 10% experienced these results as early as 4 weeks. Of those treated with lebrikizumab who experienced clear or almost-clear skin at week 16, 77% maintained those results at 1 year on the once-monthly dose. In addition, on average, 43% of those on lebrikizumab experienced relief of itch at 16 weeks, compared with 12% of those on placebo, according to the press release.
The most common side effects of lebrikizumab observed in the clinical trials include eye and eyelid inflammation, such as redness, swelling, and itching; injection-site reactions; and herpes zoster (shingles).
Lebrikizumab was approved in Japan in January 2024, and by the European Commission in 2023.
A version of this article first appeared on Medscape.com.
The that is not well controlled, despite treatment with topical prescription therapies.
The recommended initial starting dose of lebrikizumab consists of 500 mg (two 250 mg injections) at baseline and week 2, followed by 250 mg every 2 weeks until week 16 or later when adequate clinical response is achieved. Then, maintenance dosing is recommended with one monthly injection (250 mg every 4 weeks). Children aged 12-17 years must weigh at least 88 pounds (40 kg) to be eligible for lebrikizumab treatment.
According to a press release from Lilly, which has been developing lebrikizumab, approval was based on results from the ADvocate 1, ADvocate 2, and ADhere studies, which included over 1000 adults and children aged 12 and older with moderate to severe AD. The primary endpoint for these studies was evaluated at 16 weeks and measured clear or almost clear skin (IGA score of 0 or 1).
According to Lilly, 38% of people in ADvocate 1 and 2 who took lebrikizumab achieved clear or almost-clear skin at 16 weeks, compared with 12% of those in the placebo arm, and 10% experienced these results as early as 4 weeks. Of those treated with lebrikizumab who experienced clear or almost-clear skin at week 16, 77% maintained those results at 1 year on the once-monthly dose. In addition, on average, 43% of those on lebrikizumab experienced relief of itch at 16 weeks, compared with 12% of those on placebo, according to the press release.
The most common side effects of lebrikizumab observed in the clinical trials include eye and eyelid inflammation, such as redness, swelling, and itching; injection-site reactions; and herpes zoster (shingles).
Lebrikizumab was approved in Japan in January 2024, and by the European Commission in 2023.
A version of this article first appeared on Medscape.com.
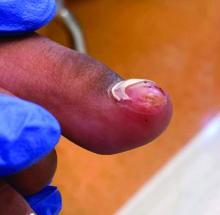
A 14-Year-Old Female Presents With a Growth Under Her Toenail
BY XOCHITL LONGSTAFF, BS; ANGELINA LABIB, MD; AND DAWN EICHENFIELD, MD, PHD
Diagnosis: Subungual bony exostosis
The patient was referred to orthopedics for further evaluation and ultimately underwent excisional surgery. At her most recent follow-up visit with orthopedic surgery, her new nail was observed to be growing well.
Subungual exostosis, also known as Dupuytren’s exostosis, is a benign osteocartilaginous tumor that classically presents as a bony growth at the dorsal aspect of the distal phalanx of the great toe, near the nail bed. The pathogenesis remains unclear, but suggested etiologies include prior trauma, infection, and hereditary abnormalities.1
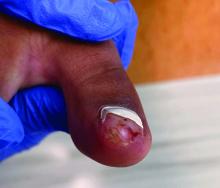
Clinically, lesions can be painful and may be associated with skin ulceration. The location at the dorsal distal great toe is a key distinguishing feature. Physical exam reveals a firm, fixed nodule with a hyperkeratotic smooth surface.2
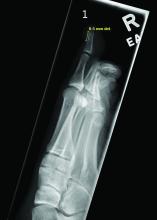
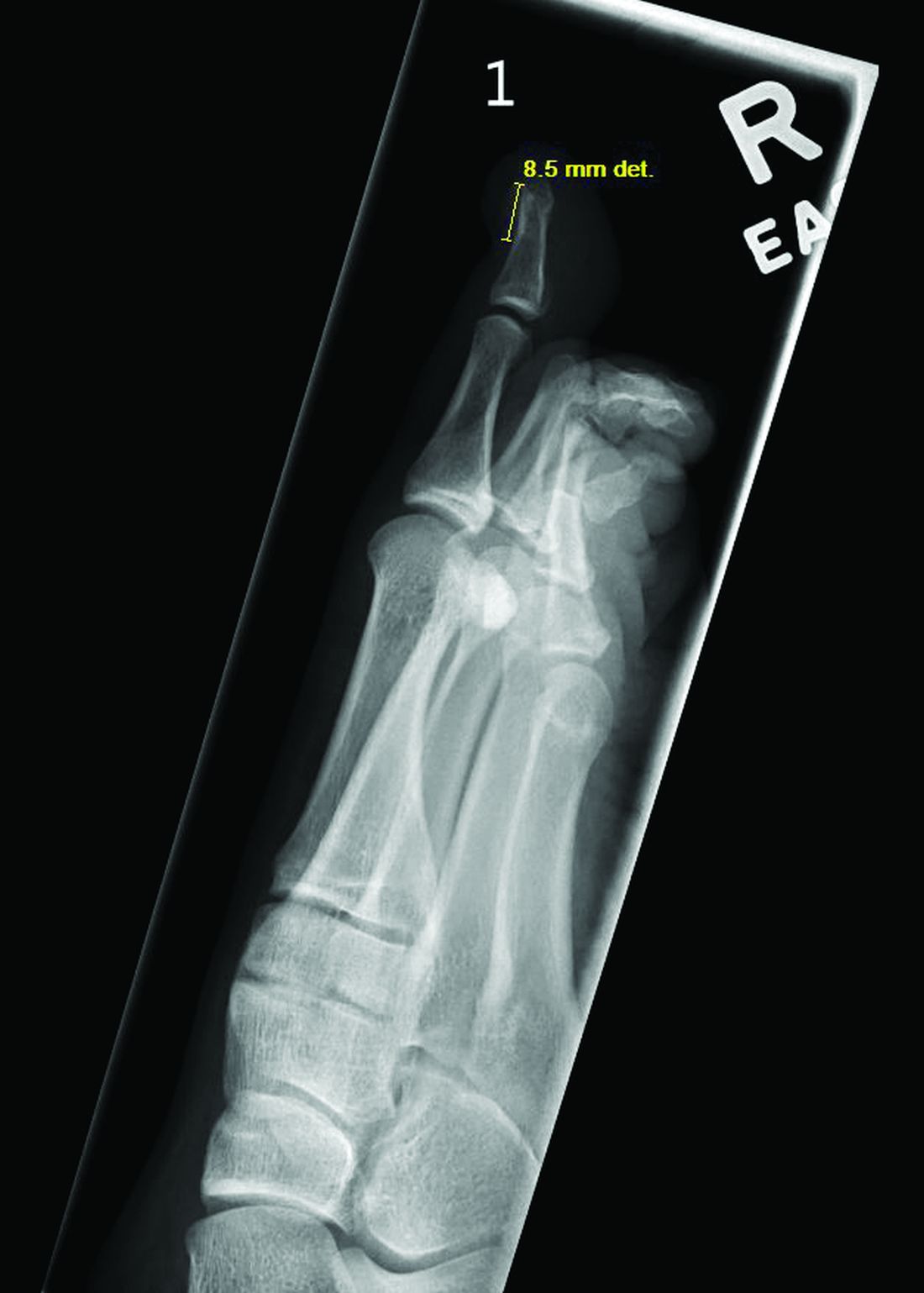
Radiographic evaluation, particularly with a lateral view, is often diagnostic. The classic radiographic finding in subungual exostosis is an osseous structure connected to the distal phalanx, with a hazy periphery representing a fibrocartilage cap.
Treatment involves complete marginal excision. The complications from surgical excision are minimal, with the most common being recurrence.3 However, the recurrence rate is also generally low, around 4%.1
Ms. Longstaff is currently completing a research year as a Pediatric Clinical Research Fellow at University of California San Diego (UCSD) Rady Children’s Hospital prior to finishing her final year at the David Geffen School of Medicine at the University of California, Los Angeles. Dr. Labib is the Post-Doctoral Pediatric Clinical Research Fellow at UCSD Rady Children’s Hospital. Dr. Eichenfield is a dermatologist at Rady Children’s Hospital–San Diego and assistant clinical professor at UCSD.
References
1. Alabdullrahman LW et al. Osteochondroma. In: StatPearls [Internet]. 2024 Feb 26. https://www.ncbi.nlm.nih.gov/books/NBK544296/#.
2. DaCambra MP et al. Clin Orthop Relat Res. 2014 Apr;472(4):1251-9. doi: 10.1007/s11999-013-3345-4.
3. Womack ME et al. J Am Acad Orthop Surg Glob Res Rev. 2022 Mar 22;6(3):e21.00239. doi: 10.5435/JAAOSGlobal-D-21-00239.
BY XOCHITL LONGSTAFF, BS; ANGELINA LABIB, MD; AND DAWN EICHENFIELD, MD, PHD
Diagnosis: Subungual bony exostosis
The patient was referred to orthopedics for further evaluation and ultimately underwent excisional surgery. At her most recent follow-up visit with orthopedic surgery, her new nail was observed to be growing well.
Subungual exostosis, also known as Dupuytren’s exostosis, is a benign osteocartilaginous tumor that classically presents as a bony growth at the dorsal aspect of the distal phalanx of the great toe, near the nail bed. The pathogenesis remains unclear, but suggested etiologies include prior trauma, infection, and hereditary abnormalities.1
Clinically, lesions can be painful and may be associated with skin ulceration. The location at the dorsal distal great toe is a key distinguishing feature. Physical exam reveals a firm, fixed nodule with a hyperkeratotic smooth surface.2
Radiographic evaluation, particularly with a lateral view, is often diagnostic. The classic radiographic finding in subungual exostosis is an osseous structure connected to the distal phalanx, with a hazy periphery representing a fibrocartilage cap.
Treatment involves complete marginal excision. The complications from surgical excision are minimal, with the most common being recurrence.3 However, the recurrence rate is also generally low, around 4%.1
Ms. Longstaff is currently completing a research year as a Pediatric Clinical Research Fellow at University of California San Diego (UCSD) Rady Children’s Hospital prior to finishing her final year at the David Geffen School of Medicine at the University of California, Los Angeles. Dr. Labib is the Post-Doctoral Pediatric Clinical Research Fellow at UCSD Rady Children’s Hospital. Dr. Eichenfield is a dermatologist at Rady Children’s Hospital–San Diego and assistant clinical professor at UCSD.
References
1. Alabdullrahman LW et al. Osteochondroma. In: StatPearls [Internet]. 2024 Feb 26. https://www.ncbi.nlm.nih.gov/books/NBK544296/#.
2. DaCambra MP et al. Clin Orthop Relat Res. 2014 Apr;472(4):1251-9. doi: 10.1007/s11999-013-3345-4.
3. Womack ME et al. J Am Acad Orthop Surg Glob Res Rev. 2022 Mar 22;6(3):e21.00239. doi: 10.5435/JAAOSGlobal-D-21-00239.
BY XOCHITL LONGSTAFF, BS; ANGELINA LABIB, MD; AND DAWN EICHENFIELD, MD, PHD
Diagnosis: Subungual bony exostosis
The patient was referred to orthopedics for further evaluation and ultimately underwent excisional surgery. At her most recent follow-up visit with orthopedic surgery, her new nail was observed to be growing well.
Subungual exostosis, also known as Dupuytren’s exostosis, is a benign osteocartilaginous tumor that classically presents as a bony growth at the dorsal aspect of the distal phalanx of the great toe, near the nail bed. The pathogenesis remains unclear, but suggested etiologies include prior trauma, infection, and hereditary abnormalities.1
Clinically, lesions can be painful and may be associated with skin ulceration. The location at the dorsal distal great toe is a key distinguishing feature. Physical exam reveals a firm, fixed nodule with a hyperkeratotic smooth surface.2
Radiographic evaluation, particularly with a lateral view, is often diagnostic. The classic radiographic finding in subungual exostosis is an osseous structure connected to the distal phalanx, with a hazy periphery representing a fibrocartilage cap.
Treatment involves complete marginal excision. The complications from surgical excision are minimal, with the most common being recurrence.3 However, the recurrence rate is also generally low, around 4%.1
Ms. Longstaff is currently completing a research year as a Pediatric Clinical Research Fellow at University of California San Diego (UCSD) Rady Children’s Hospital prior to finishing her final year at the David Geffen School of Medicine at the University of California, Los Angeles. Dr. Labib is the Post-Doctoral Pediatric Clinical Research Fellow at UCSD Rady Children’s Hospital. Dr. Eichenfield is a dermatologist at Rady Children’s Hospital–San Diego and assistant clinical professor at UCSD.
References
1. Alabdullrahman LW et al. Osteochondroma. In: StatPearls [Internet]. 2024 Feb 26. https://www.ncbi.nlm.nih.gov/books/NBK544296/#.
2. DaCambra MP et al. Clin Orthop Relat Res. 2014 Apr;472(4):1251-9. doi: 10.1007/s11999-013-3345-4.
3. Womack ME et al. J Am Acad Orthop Surg Glob Res Rev. 2022 Mar 22;6(3):e21.00239. doi: 10.5435/JAAOSGlobal-D-21-00239.
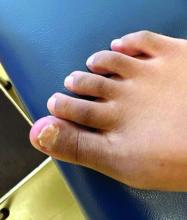
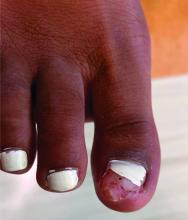
A 14-year-old healthy female presents with a painful nodule under her great toenail. The nodule had been present for 2 months and there was no preceding trauma. Three days prior to presentation, her nail cracked and bled after bumping her toe. The toe is painful to palpation. Given the associated pain, the patient visited urgent care and was prescribed cephalexin and acetaminophen.
Physical examination reveals a skin-colored subungual nodule with hypertrophic tissue originating from the nail bed of the right great toe, but no thickening of the nail plate (Figures 1-3).
Are Pharmacy Deserts Worsening Health Disparities?
TOPLINE:
Pharmacy closures in the United States are creating “pharmacy deserts,” disproportionately affecting socially vulnerable communities. High social vulnerability and low primary care practitioner (PCP) density are linked to increased pharmacy desert density.
METHODOLOGY:
- Data through 2020 on communities located 10 or more miles from the nearest retail pharmacy were sourced from TelePharm Map.
- Counties were stratified as having a high pharmacy desert density if the number of pharmacy deserts per 1000 inhabitants was in the 80th percentile or higher.
- Social vulnerability index and healthcare practitioner data were obtained from the Agency for Toxic Substances and Disease Registry and the Area Health Resources Files.
- PCP density was calculated as the number of PCPs per 10,000 inhabitants.
- A total of 3143 counties were analyzed, with 1447 (46%) having at least one pharmacy desert.
TAKEAWAY:
- Counties with a high pharmacy desert density had a higher social vulnerability index than those with a low pharmacy desert density (P = .006).
- Areas with a high pharmacy desert density had lower median PCP density than those with low or no pharmacy desert density (P < .001).
- High social vulnerability index (odds ratio [OR], 1.35; 95% CI, 1.07-1.70; P = .01) and low PCP density (OR, 2.27; 95% CI, 1.80-2.86; P < .001) were associated with a higher likelihood for a county to have a high pharmacy desert density.
- Pharmacy closures are leaving more individuals without easy access to medications, with disproportionate consequences for certain communities.
IN PRACTICE:
“As high pharmacy desert density counties also have a lower PCP density, patients residing in these regions face increased barriers to accessing primary healthcare needs,” wrote the authors of the study.
SOURCE:
The study was led by Giovanni Catalano, MD, Muhammad Muntazir Mehdi Khan, MBBS, and Timothy M. Pawlik, MD, PhD, MPH, MTS, MBA, Department of Surgery, The Ohio State University Wexner Medical Center in Columbus, Ohio. It was published online in JAMA Network Open.
LIMITATIONS:
The cross-sectional design of the study limited the ability to draw causal inferences. The study relied on public county-level data, which may not have captured all relevant variables. The use of the social vulnerability index and PCP density as proxies did not fully represent the complexity of pharmacy access issues. The study’s findings were not generalizable to regions outside the United States.
DISCLOSURES:
No relevant conflicts of interest were disclosed by the authors. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Pharmacy closures in the United States are creating “pharmacy deserts,” disproportionately affecting socially vulnerable communities. High social vulnerability and low primary care practitioner (PCP) density are linked to increased pharmacy desert density.
METHODOLOGY:
- Data through 2020 on communities located 10 or more miles from the nearest retail pharmacy were sourced from TelePharm Map.
- Counties were stratified as having a high pharmacy desert density if the number of pharmacy deserts per 1000 inhabitants was in the 80th percentile or higher.
- Social vulnerability index and healthcare practitioner data were obtained from the Agency for Toxic Substances and Disease Registry and the Area Health Resources Files.
- PCP density was calculated as the number of PCPs per 10,000 inhabitants.
- A total of 3143 counties were analyzed, with 1447 (46%) having at least one pharmacy desert.
TAKEAWAY:
- Counties with a high pharmacy desert density had a higher social vulnerability index than those with a low pharmacy desert density (P = .006).
- Areas with a high pharmacy desert density had lower median PCP density than those with low or no pharmacy desert density (P < .001).
- High social vulnerability index (odds ratio [OR], 1.35; 95% CI, 1.07-1.70; P = .01) and low PCP density (OR, 2.27; 95% CI, 1.80-2.86; P < .001) were associated with a higher likelihood for a county to have a high pharmacy desert density.
- Pharmacy closures are leaving more individuals without easy access to medications, with disproportionate consequences for certain communities.
IN PRACTICE:
“As high pharmacy desert density counties also have a lower PCP density, patients residing in these regions face increased barriers to accessing primary healthcare needs,” wrote the authors of the study.
SOURCE:
The study was led by Giovanni Catalano, MD, Muhammad Muntazir Mehdi Khan, MBBS, and Timothy M. Pawlik, MD, PhD, MPH, MTS, MBA, Department of Surgery, The Ohio State University Wexner Medical Center in Columbus, Ohio. It was published online in JAMA Network Open.
LIMITATIONS:
The cross-sectional design of the study limited the ability to draw causal inferences. The study relied on public county-level data, which may not have captured all relevant variables. The use of the social vulnerability index and PCP density as proxies did not fully represent the complexity of pharmacy access issues. The study’s findings were not generalizable to regions outside the United States.
DISCLOSURES:
No relevant conflicts of interest were disclosed by the authors. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Pharmacy closures in the United States are creating “pharmacy deserts,” disproportionately affecting socially vulnerable communities. High social vulnerability and low primary care practitioner (PCP) density are linked to increased pharmacy desert density.
METHODOLOGY:
- Data through 2020 on communities located 10 or more miles from the nearest retail pharmacy were sourced from TelePharm Map.
- Counties were stratified as having a high pharmacy desert density if the number of pharmacy deserts per 1000 inhabitants was in the 80th percentile or higher.
- Social vulnerability index and healthcare practitioner data were obtained from the Agency for Toxic Substances and Disease Registry and the Area Health Resources Files.
- PCP density was calculated as the number of PCPs per 10,000 inhabitants.
- A total of 3143 counties were analyzed, with 1447 (46%) having at least one pharmacy desert.
TAKEAWAY:
- Counties with a high pharmacy desert density had a higher social vulnerability index than those with a low pharmacy desert density (P = .006).
- Areas with a high pharmacy desert density had lower median PCP density than those with low or no pharmacy desert density (P < .001).
- High social vulnerability index (odds ratio [OR], 1.35; 95% CI, 1.07-1.70; P = .01) and low PCP density (OR, 2.27; 95% CI, 1.80-2.86; P < .001) were associated with a higher likelihood for a county to have a high pharmacy desert density.
- Pharmacy closures are leaving more individuals without easy access to medications, with disproportionate consequences for certain communities.
IN PRACTICE:
“As high pharmacy desert density counties also have a lower PCP density, patients residing in these regions face increased barriers to accessing primary healthcare needs,” wrote the authors of the study.
SOURCE:
The study was led by Giovanni Catalano, MD, Muhammad Muntazir Mehdi Khan, MBBS, and Timothy M. Pawlik, MD, PhD, MPH, MTS, MBA, Department of Surgery, The Ohio State University Wexner Medical Center in Columbus, Ohio. It was published online in JAMA Network Open.
LIMITATIONS:
The cross-sectional design of the study limited the ability to draw causal inferences. The study relied on public county-level data, which may not have captured all relevant variables. The use of the social vulnerability index and PCP density as proxies did not fully represent the complexity of pharmacy access issues. The study’s findings were not generalizable to regions outside the United States.
DISCLOSURES:
No relevant conflicts of interest were disclosed by the authors. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
UVA Defends Medical School Dean, Hospital CEO After Docs Call for Their Removal
The University of Virginia (UVA) is defending the CEO of its health system and its medical school dean in the wake of a very public call for their removal.
At least 128 members of the University of Virginia faculty who are employed by both the medical school and the UVA Physicians Group wrote to the UVA Board of Visitors and its peer-elected faculty leaders, expressing no confidence in K. Craig Kent, MD, CEO of UVA Health and executive vice president for health affairs, and Melina Kibbe, MD, dean of the medical school and chief health affairs officer.
Dr. Kibbe, a vascular surgeon and researcher, is also the editor in chief of JAMA Surgery.
“We call for the immediate removal of Craig Kent and Melina Kibbe,” wrote the physicians.
The letter alleged that patient safety was compromised because doctors, nurses, and other staff were pressured to abstain from reporting safety concerns and that physicians had been hired “despite concerns regarding integrity and quality.” Those who raised safety concerns faced “explicit and implicit threats and retaliation,” including delays and denials of promotion and tenure, said the letter.
The September 5 letter did not include signatures. The authors said that names were being protected, but that they would share the names with a limited audience.
UVA President Jim Ryan took issue with the notion that the signees were anonymous. He said in his own letter to medical school faculty that some of the accusations were about matters that had already been addressed or that were being worked on. As far as allegations that he was not previously aware of, “we will do our best to investigate,” he said.
The faculty who signed the letter “have besmirched the reputations of not just Melina and Craig,” wrote Mr. Ryan. “They have unfairly — and I trust unwittingly — cast a shadow over the great work of the entire health system and medical school.”
The authors claimed that reports about bullying and harassment of trainees had been “suppressed, minimized, and subsequently altered.”
And they said that spending on leadership was prioritized over addressing clinical and technical staff shortages. Whistleblowers who reported fraud were not protected, and clinicians were pressured to modify patient records to “obfuscate adverse outcomes and boost productivity metrics,” they wrote.
The 128 members of the UVA Physicians Group who signed the letter represent about 10% of the 1400 medical school faculty members.
It is not the first time that Dr. Kent has been given a vote of no confidence. In 2017, when he was the dean of the College of Medicine at the Ohio State University, Dr. Kent was accused in a “no confidence” letter from 25 physicians and faculty of helping to undermine the school’s mission and taking actions that led to resignations and early retirements of many staff, the Columbus Dispatch reported.
William G. Crutchfield Jr., a member of the UVA Health System Board, defended Dr. Kent and Dr. Kibbe in a lengthy statement shared with this news organization. He said that UVA Health’s four hospitals had received “A” ratings for safety, and that the system has a 5.1% turnover rate compared with a national average of 8.3%.
Dr. Kent and Dr. Kibbe have recruited faculty from top academic medical centers, Mr. Crutchfield wrote.
“If our work environment were so toxic, these people would not have joined our faculty,” he wrote.
Mr. Crutchfield credited Dr. Kent and Dr. Kibbe with crafting a new 10-year strategic plan and for hiring a chief strategy officer to lead the plan — a move that replaced “expensive outside consultants.”
Mr. Ryan said in his letter that his inbox “is overflowing with testimonials from some of the 1200-plus faculty who did not sign the letter, who attest that the health system today — under Melina and Craig’s leadership — is in the best shape it has ever been in, and that they have addressed changes that have needed to be made for more than two decades.”
A request to see some of these positive testimonials was not answered by press time.
Mr. Crutchfield, like Mr. Ryan, said that the letter writers were doing more harm than good.
“If a small cabal of people hiding behind anonymity can force outstanding leaders out of UVA, it will make it extremely difficult to recruit outstanding new physicians, nurses, technicians, and administrators,” he wrote.
A version of this article first appeared on Medscape.com.
The University of Virginia (UVA) is defending the CEO of its health system and its medical school dean in the wake of a very public call for their removal.
At least 128 members of the University of Virginia faculty who are employed by both the medical school and the UVA Physicians Group wrote to the UVA Board of Visitors and its peer-elected faculty leaders, expressing no confidence in K. Craig Kent, MD, CEO of UVA Health and executive vice president for health affairs, and Melina Kibbe, MD, dean of the medical school and chief health affairs officer.
Dr. Kibbe, a vascular surgeon and researcher, is also the editor in chief of JAMA Surgery.
“We call for the immediate removal of Craig Kent and Melina Kibbe,” wrote the physicians.
The letter alleged that patient safety was compromised because doctors, nurses, and other staff were pressured to abstain from reporting safety concerns and that physicians had been hired “despite concerns regarding integrity and quality.” Those who raised safety concerns faced “explicit and implicit threats and retaliation,” including delays and denials of promotion and tenure, said the letter.
The September 5 letter did not include signatures. The authors said that names were being protected, but that they would share the names with a limited audience.
UVA President Jim Ryan took issue with the notion that the signees were anonymous. He said in his own letter to medical school faculty that some of the accusations were about matters that had already been addressed or that were being worked on. As far as allegations that he was not previously aware of, “we will do our best to investigate,” he said.
The faculty who signed the letter “have besmirched the reputations of not just Melina and Craig,” wrote Mr. Ryan. “They have unfairly — and I trust unwittingly — cast a shadow over the great work of the entire health system and medical school.”
The authors claimed that reports about bullying and harassment of trainees had been “suppressed, minimized, and subsequently altered.”
And they said that spending on leadership was prioritized over addressing clinical and technical staff shortages. Whistleblowers who reported fraud were not protected, and clinicians were pressured to modify patient records to “obfuscate adverse outcomes and boost productivity metrics,” they wrote.
The 128 members of the UVA Physicians Group who signed the letter represent about 10% of the 1400 medical school faculty members.
It is not the first time that Dr. Kent has been given a vote of no confidence. In 2017, when he was the dean of the College of Medicine at the Ohio State University, Dr. Kent was accused in a “no confidence” letter from 25 physicians and faculty of helping to undermine the school’s mission and taking actions that led to resignations and early retirements of many staff, the Columbus Dispatch reported.
William G. Crutchfield Jr., a member of the UVA Health System Board, defended Dr. Kent and Dr. Kibbe in a lengthy statement shared with this news organization. He said that UVA Health’s four hospitals had received “A” ratings for safety, and that the system has a 5.1% turnover rate compared with a national average of 8.3%.
Dr. Kent and Dr. Kibbe have recruited faculty from top academic medical centers, Mr. Crutchfield wrote.
“If our work environment were so toxic, these people would not have joined our faculty,” he wrote.
Mr. Crutchfield credited Dr. Kent and Dr. Kibbe with crafting a new 10-year strategic plan and for hiring a chief strategy officer to lead the plan — a move that replaced “expensive outside consultants.”
Mr. Ryan said in his letter that his inbox “is overflowing with testimonials from some of the 1200-plus faculty who did not sign the letter, who attest that the health system today — under Melina and Craig’s leadership — is in the best shape it has ever been in, and that they have addressed changes that have needed to be made for more than two decades.”
A request to see some of these positive testimonials was not answered by press time.
Mr. Crutchfield, like Mr. Ryan, said that the letter writers were doing more harm than good.
“If a small cabal of people hiding behind anonymity can force outstanding leaders out of UVA, it will make it extremely difficult to recruit outstanding new physicians, nurses, technicians, and administrators,” he wrote.
A version of this article first appeared on Medscape.com.
The University of Virginia (UVA) is defending the CEO of its health system and its medical school dean in the wake of a very public call for their removal.
At least 128 members of the University of Virginia faculty who are employed by both the medical school and the UVA Physicians Group wrote to the UVA Board of Visitors and its peer-elected faculty leaders, expressing no confidence in K. Craig Kent, MD, CEO of UVA Health and executive vice president for health affairs, and Melina Kibbe, MD, dean of the medical school and chief health affairs officer.
Dr. Kibbe, a vascular surgeon and researcher, is also the editor in chief of JAMA Surgery.
“We call for the immediate removal of Craig Kent and Melina Kibbe,” wrote the physicians.
The letter alleged that patient safety was compromised because doctors, nurses, and other staff were pressured to abstain from reporting safety concerns and that physicians had been hired “despite concerns regarding integrity and quality.” Those who raised safety concerns faced “explicit and implicit threats and retaliation,” including delays and denials of promotion and tenure, said the letter.
The September 5 letter did not include signatures. The authors said that names were being protected, but that they would share the names with a limited audience.
UVA President Jim Ryan took issue with the notion that the signees were anonymous. He said in his own letter to medical school faculty that some of the accusations were about matters that had already been addressed or that were being worked on. As far as allegations that he was not previously aware of, “we will do our best to investigate,” he said.
The faculty who signed the letter “have besmirched the reputations of not just Melina and Craig,” wrote Mr. Ryan. “They have unfairly — and I trust unwittingly — cast a shadow over the great work of the entire health system and medical school.”
The authors claimed that reports about bullying and harassment of trainees had been “suppressed, minimized, and subsequently altered.”
And they said that spending on leadership was prioritized over addressing clinical and technical staff shortages. Whistleblowers who reported fraud were not protected, and clinicians were pressured to modify patient records to “obfuscate adverse outcomes and boost productivity metrics,” they wrote.
The 128 members of the UVA Physicians Group who signed the letter represent about 10% of the 1400 medical school faculty members.
It is not the first time that Dr. Kent has been given a vote of no confidence. In 2017, when he was the dean of the College of Medicine at the Ohio State University, Dr. Kent was accused in a “no confidence” letter from 25 physicians and faculty of helping to undermine the school’s mission and taking actions that led to resignations and early retirements of many staff, the Columbus Dispatch reported.
William G. Crutchfield Jr., a member of the UVA Health System Board, defended Dr. Kent and Dr. Kibbe in a lengthy statement shared with this news organization. He said that UVA Health’s four hospitals had received “A” ratings for safety, and that the system has a 5.1% turnover rate compared with a national average of 8.3%.
Dr. Kent and Dr. Kibbe have recruited faculty from top academic medical centers, Mr. Crutchfield wrote.
“If our work environment were so toxic, these people would not have joined our faculty,” he wrote.
Mr. Crutchfield credited Dr. Kent and Dr. Kibbe with crafting a new 10-year strategic plan and for hiring a chief strategy officer to lead the plan — a move that replaced “expensive outside consultants.”
Mr. Ryan said in his letter that his inbox “is overflowing with testimonials from some of the 1200-plus faculty who did not sign the letter, who attest that the health system today — under Melina and Craig’s leadership — is in the best shape it has ever been in, and that they have addressed changes that have needed to be made for more than two decades.”
A request to see some of these positive testimonials was not answered by press time.
Mr. Crutchfield, like Mr. Ryan, said that the letter writers were doing more harm than good.
“If a small cabal of people hiding behind anonymity can force outstanding leaders out of UVA, it will make it extremely difficult to recruit outstanding new physicians, nurses, technicians, and administrators,” he wrote.
A version of this article first appeared on Medscape.com.
Current Hydroxychloroquine Use in Lupus May Provide Protection Against Cardiovascular Events
TOPLINE:
Current use of hydroxychloroquine is associated with a lower risk for myocardial infarction (MI), stroke, and other thromboembolic events in patients with systemic lupus erythematosus (SLE). This protective effect diminishes after discontinuation of hydroxychloroquine treatment.
METHODOLOGY:
- Researchers used a nested case-control design to evaluate the association between exposure to hydroxychloroquine and the risk for cardiovascular events in patients with SLE.
- They included 52,883 adults with SLE (mean age, 44.23 years; 86.6% women) identified from the National System of Health Databases, which includes 99% of the French population.
- Among these, 1981 individuals with composite cardiovascular conditions were matched with 16,892 control individuals without cardiovascular conditions.
- Patients were categorized on the basis of hydroxychloroquine exposure into current users (last exposure within 90 days before a cardiovascular event), remote users (91-365 days before), and nonusers (no exposure within 365 days).
- The study outcomes included a composite of cardiovascular events, including MI, stroke (including transient ischemic attack), and other thromboembolic events such as phlebitis, thrombophlebitis, venous thrombosis, venous thromboembolism, and pulmonary embolism.
TAKEAWAY:
- Current hydroxychloroquine users had lower odds of experiencing a composite cardiovascular outcome than nonusers (adjusted odds ratio [aOR], 0.63; 95% CI, 0.57-0.70).
- The odds of MI (aOR, 0.72; 95% CI, 0.60-0.87), stroke (aOR, 0.71; 95% CI, 0.61-0.83), and other thromboembolic events (aOR, 0.58; 95% CI, 0.48-0.69) were also lower among current users than among nonusers.
- No significant association was found for remote hydroxychloroquine exposure and the risk for composite cardiovascular events, MI, stroke, and other thromboembolic events.
IN PRACTICE:
“These findings support the protective association of hydroxychloroquine against CV [cardiovascular] events and underscore the importance of continuous hydroxychloroquine therapy for patients diagnosed with SLE,” the authors wrote.
SOURCE:
The study was led by Lamiae Grimaldi-Bensouda, PharmD, PhD, Department of Pharmacology, Hospital Group Paris-Saclay, Assistance Publique-Hôpitaux de Paris, France. It was published online on August 30, 2024, in JAMA Network Open.
LIMITATIONS:
The observational nature of the study may have introduced confounding. Current hydroxychloroquine users were younger than nonusers, with an average age difference of almost 5 years. Current hydroxychloroquine users had a twofold longer duration of onset of SLE and had a higher prevalence of chronic kidney disease compared with nonusers.
DISCLOSURES:
This study was funded by the Banque pour l’Investissement, Deeptech. Some authors declared having financial ties with various institutions and companies outside of the current study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Current use of hydroxychloroquine is associated with a lower risk for myocardial infarction (MI), stroke, and other thromboembolic events in patients with systemic lupus erythematosus (SLE). This protective effect diminishes after discontinuation of hydroxychloroquine treatment.
METHODOLOGY:
- Researchers used a nested case-control design to evaluate the association between exposure to hydroxychloroquine and the risk for cardiovascular events in patients with SLE.
- They included 52,883 adults with SLE (mean age, 44.23 years; 86.6% women) identified from the National System of Health Databases, which includes 99% of the French population.
- Among these, 1981 individuals with composite cardiovascular conditions were matched with 16,892 control individuals without cardiovascular conditions.
- Patients were categorized on the basis of hydroxychloroquine exposure into current users (last exposure within 90 days before a cardiovascular event), remote users (91-365 days before), and nonusers (no exposure within 365 days).
- The study outcomes included a composite of cardiovascular events, including MI, stroke (including transient ischemic attack), and other thromboembolic events such as phlebitis, thrombophlebitis, venous thrombosis, venous thromboembolism, and pulmonary embolism.
TAKEAWAY:
- Current hydroxychloroquine users had lower odds of experiencing a composite cardiovascular outcome than nonusers (adjusted odds ratio [aOR], 0.63; 95% CI, 0.57-0.70).
- The odds of MI (aOR, 0.72; 95% CI, 0.60-0.87), stroke (aOR, 0.71; 95% CI, 0.61-0.83), and other thromboembolic events (aOR, 0.58; 95% CI, 0.48-0.69) were also lower among current users than among nonusers.
- No significant association was found for remote hydroxychloroquine exposure and the risk for composite cardiovascular events, MI, stroke, and other thromboembolic events.
IN PRACTICE:
“These findings support the protective association of hydroxychloroquine against CV [cardiovascular] events and underscore the importance of continuous hydroxychloroquine therapy for patients diagnosed with SLE,” the authors wrote.
SOURCE:
The study was led by Lamiae Grimaldi-Bensouda, PharmD, PhD, Department of Pharmacology, Hospital Group Paris-Saclay, Assistance Publique-Hôpitaux de Paris, France. It was published online on August 30, 2024, in JAMA Network Open.
LIMITATIONS:
The observational nature of the study may have introduced confounding. Current hydroxychloroquine users were younger than nonusers, with an average age difference of almost 5 years. Current hydroxychloroquine users had a twofold longer duration of onset of SLE and had a higher prevalence of chronic kidney disease compared with nonusers.
DISCLOSURES:
This study was funded by the Banque pour l’Investissement, Deeptech. Some authors declared having financial ties with various institutions and companies outside of the current study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Current use of hydroxychloroquine is associated with a lower risk for myocardial infarction (MI), stroke, and other thromboembolic events in patients with systemic lupus erythematosus (SLE). This protective effect diminishes after discontinuation of hydroxychloroquine treatment.
METHODOLOGY:
- Researchers used a nested case-control design to evaluate the association between exposure to hydroxychloroquine and the risk for cardiovascular events in patients with SLE.
- They included 52,883 adults with SLE (mean age, 44.23 years; 86.6% women) identified from the National System of Health Databases, which includes 99% of the French population.
- Among these, 1981 individuals with composite cardiovascular conditions were matched with 16,892 control individuals without cardiovascular conditions.
- Patients were categorized on the basis of hydroxychloroquine exposure into current users (last exposure within 90 days before a cardiovascular event), remote users (91-365 days before), and nonusers (no exposure within 365 days).
- The study outcomes included a composite of cardiovascular events, including MI, stroke (including transient ischemic attack), and other thromboembolic events such as phlebitis, thrombophlebitis, venous thrombosis, venous thromboembolism, and pulmonary embolism.
TAKEAWAY:
- Current hydroxychloroquine users had lower odds of experiencing a composite cardiovascular outcome than nonusers (adjusted odds ratio [aOR], 0.63; 95% CI, 0.57-0.70).
- The odds of MI (aOR, 0.72; 95% CI, 0.60-0.87), stroke (aOR, 0.71; 95% CI, 0.61-0.83), and other thromboembolic events (aOR, 0.58; 95% CI, 0.48-0.69) were also lower among current users than among nonusers.
- No significant association was found for remote hydroxychloroquine exposure and the risk for composite cardiovascular events, MI, stroke, and other thromboembolic events.
IN PRACTICE:
“These findings support the protective association of hydroxychloroquine against CV [cardiovascular] events and underscore the importance of continuous hydroxychloroquine therapy for patients diagnosed with SLE,” the authors wrote.
SOURCE:
The study was led by Lamiae Grimaldi-Bensouda, PharmD, PhD, Department of Pharmacology, Hospital Group Paris-Saclay, Assistance Publique-Hôpitaux de Paris, France. It was published online on August 30, 2024, in JAMA Network Open.
LIMITATIONS:
The observational nature of the study may have introduced confounding. Current hydroxychloroquine users were younger than nonusers, with an average age difference of almost 5 years. Current hydroxychloroquine users had a twofold longer duration of onset of SLE and had a higher prevalence of chronic kidney disease compared with nonusers.
DISCLOSURES:
This study was funded by the Banque pour l’Investissement, Deeptech. Some authors declared having financial ties with various institutions and companies outside of the current study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Do Cannabis Users Need More Anesthesia During Surgery?
TOPLINE:
However, the clinical relevance of this difference remains unclear.
METHODOLOGY:
- To assess if cannabis use leads to higher doses of inhalational anesthesia during surgery, the researchers conducted a retrospective cohort study comparing the average intraoperative minimum alveolar concentrations of volatile anesthetics (isoflurane and sevoflurane) between older adults who used cannabis products and those who did not.
- The researchers reviewed electronic health records of 22,476 patients aged 65 years or older who underwent surgery at the University of Florida Health System between 2018 and 2020.
- Overall, 268 patients who reported using cannabis within 60 days of surgery (median age, 69 years; 35% women) were matched to 1072 nonusers.
- The median duration of anesthesia was 175 minutes.
- The primary outcome was the intraoperative time-weighted average of isoflurane or sevoflurane minimum alveolar concentration equivalents.
TAKEAWAY:
- Cannabis users had significantly higher average minimum alveolar concentrations of isoflurane or sevoflurane than nonusers (mean, 0.58 vs 0.54; mean difference, 0.04; P = .021).
- The findings were confirmed in a sensitivity analysis that revealed higher mean average minimum alveolar concentrations of anesthesia in cannabis users than in nonusers (0.57 vs 0.53; P = .029).
- Although the 0.04 difference in minimum alveolar concentration between cannabis users and nonusers was statistically significant, its clinical importance is unclear.
IN PRACTICE:
“While recent guidelines underscore the importance of universal screening for cannabinoids before surgery, caution is paramount to prevent clinical bias leading to the administration of unnecessary higher doses of inhalational anesthesia, especially as robust evidence supporting such practices remains lacking,” the authors of the study wrote.
SOURCE:
This study was led by Ruba Sajdeya, MD, PhD, of the Department of Epidemiology at the University of Florida, Gainesville, and was published online in August 2024 in Anesthesiology.
LIMITATIONS:
This study lacked access to prescription or dispensed medications, including opioids, which may have introduced residual confounding. Potential underdocumentation of cannabis use in medical records could have led to exposure misclassification. The causality between cannabis usage and increased anesthetic dosing could not be established due to the observational nature of this study.
DISCLOSURES:
This study was supported by the National Institute on Aging, the National Institutes of Health, and in part by the University of Florida Clinical and Translational Science Institute. Some authors declared receiving research support, consulting fees, and honoraria and having other ties with pharmaceutical companies and various other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
However, the clinical relevance of this difference remains unclear.
METHODOLOGY:
- To assess if cannabis use leads to higher doses of inhalational anesthesia during surgery, the researchers conducted a retrospective cohort study comparing the average intraoperative minimum alveolar concentrations of volatile anesthetics (isoflurane and sevoflurane) between older adults who used cannabis products and those who did not.
- The researchers reviewed electronic health records of 22,476 patients aged 65 years or older who underwent surgery at the University of Florida Health System between 2018 and 2020.
- Overall, 268 patients who reported using cannabis within 60 days of surgery (median age, 69 years; 35% women) were matched to 1072 nonusers.
- The median duration of anesthesia was 175 minutes.
- The primary outcome was the intraoperative time-weighted average of isoflurane or sevoflurane minimum alveolar concentration equivalents.
TAKEAWAY:
- Cannabis users had significantly higher average minimum alveolar concentrations of isoflurane or sevoflurane than nonusers (mean, 0.58 vs 0.54; mean difference, 0.04; P = .021).
- The findings were confirmed in a sensitivity analysis that revealed higher mean average minimum alveolar concentrations of anesthesia in cannabis users than in nonusers (0.57 vs 0.53; P = .029).
- Although the 0.04 difference in minimum alveolar concentration between cannabis users and nonusers was statistically significant, its clinical importance is unclear.
IN PRACTICE:
“While recent guidelines underscore the importance of universal screening for cannabinoids before surgery, caution is paramount to prevent clinical bias leading to the administration of unnecessary higher doses of inhalational anesthesia, especially as robust evidence supporting such practices remains lacking,” the authors of the study wrote.
SOURCE:
This study was led by Ruba Sajdeya, MD, PhD, of the Department of Epidemiology at the University of Florida, Gainesville, and was published online in August 2024 in Anesthesiology.
LIMITATIONS:
This study lacked access to prescription or dispensed medications, including opioids, which may have introduced residual confounding. Potential underdocumentation of cannabis use in medical records could have led to exposure misclassification. The causality between cannabis usage and increased anesthetic dosing could not be established due to the observational nature of this study.
DISCLOSURES:
This study was supported by the National Institute on Aging, the National Institutes of Health, and in part by the University of Florida Clinical and Translational Science Institute. Some authors declared receiving research support, consulting fees, and honoraria and having other ties with pharmaceutical companies and various other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
However, the clinical relevance of this difference remains unclear.
METHODOLOGY:
- To assess if cannabis use leads to higher doses of inhalational anesthesia during surgery, the researchers conducted a retrospective cohort study comparing the average intraoperative minimum alveolar concentrations of volatile anesthetics (isoflurane and sevoflurane) between older adults who used cannabis products and those who did not.
- The researchers reviewed electronic health records of 22,476 patients aged 65 years or older who underwent surgery at the University of Florida Health System between 2018 and 2020.
- Overall, 268 patients who reported using cannabis within 60 days of surgery (median age, 69 years; 35% women) were matched to 1072 nonusers.
- The median duration of anesthesia was 175 minutes.
- The primary outcome was the intraoperative time-weighted average of isoflurane or sevoflurane minimum alveolar concentration equivalents.
TAKEAWAY:
- Cannabis users had significantly higher average minimum alveolar concentrations of isoflurane or sevoflurane than nonusers (mean, 0.58 vs 0.54; mean difference, 0.04; P = .021).
- The findings were confirmed in a sensitivity analysis that revealed higher mean average minimum alveolar concentrations of anesthesia in cannabis users than in nonusers (0.57 vs 0.53; P = .029).
- Although the 0.04 difference in minimum alveolar concentration between cannabis users and nonusers was statistically significant, its clinical importance is unclear.
IN PRACTICE:
“While recent guidelines underscore the importance of universal screening for cannabinoids before surgery, caution is paramount to prevent clinical bias leading to the administration of unnecessary higher doses of inhalational anesthesia, especially as robust evidence supporting such practices remains lacking,” the authors of the study wrote.
SOURCE:
This study was led by Ruba Sajdeya, MD, PhD, of the Department of Epidemiology at the University of Florida, Gainesville, and was published online in August 2024 in Anesthesiology.
LIMITATIONS:
This study lacked access to prescription or dispensed medications, including opioids, which may have introduced residual confounding. Potential underdocumentation of cannabis use in medical records could have led to exposure misclassification. The causality between cannabis usage and increased anesthetic dosing could not be established due to the observational nature of this study.
DISCLOSURES:
This study was supported by the National Institute on Aging, the National Institutes of Health, and in part by the University of Florida Clinical and Translational Science Institute. Some authors declared receiving research support, consulting fees, and honoraria and having other ties with pharmaceutical companies and various other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Why More Doctors Are Joining Unions
With huge shifts over the past decade in the way doctors are employed — half of all doctors now work for a health system or large medical group — the idea of unionizing is not only being explored but gaining traction within the profession. In fact, 8% of the physician workforce (or 70,000 physicians) belong to a union, according to statistics gathered in 2022.
Exact numbers are hard to come by, and, interestingly, although the American Medical Association (AMA) “ supports the right of physicians to engage in collective bargaining,” the organization doesn’t track union membership among physicians, according to an AMA spokesperson.
Forming a Union
One challenge is that forming a union is not only time-consuming but also difficult, owing to several barriers. For starters, the laws dictating unionization differ by state, and the rules governing unionization vary if a hospital is public or private. If there’s enough momentum from doctors leading unionization efforts, approval from hospital leaders is required before an official election can be requested from the National Labor Relations Board.
That said, for doctors who are in a union — the two most popular are the Union of American Physicians and Dentists and the Doctors Council branch of the Service Employees International Union (SEIU)—the benefits are immense, especially because union members can focus on what matters, such as providing the best patient care possible.
, reported WBUR in Boston.
Belonging Matters
“When you build a relationship with your patients, it’s special, and that connection isn’t replaceable,” said Nicholas VenOsdel, MD, a pediatrician at Allina Health Primary Care in Hastings, Minnesota, and a union member of the Doctors Council. “However, a lot of us have felt like that hasn’t been respected as the climate of healthcare has changed so fast.”
In fact, autonomy over how much time doctors spend with patients is driving a lot of interest in unionization.
“We don’t necessarily have that autonomy now,” said Amber Higgins, MD, an emergency physician and an obstetrician at ChristianaCare, a hospital network in Newark, Delaware, and a member of the Doctors Council. “There are so many other demands, whether it’s billing, patient documentation, or other demands from the employer, and all of that takes time away from patient care.”
Another primary driver of physician unionization is the physician burnout epidemic. Physicians collectively complain that they spend more time on electronic health record documentation and bureaucratic administration. Yet if unions can improve these working conditions, the benefit to physicians and their patients would be a welcome change.
Union members are bullish and believe that having a cohesive voice will make a difference.
“We need to use our collective voices to get back to focusing on patient care instead of staring at a computer screen for 80% of the day,” Dr. Higgins told this news organization. “So much of medicine involves getting to the correct diagnosis, listening to patients, observing them, and building a relationship with them. We need time to build that.”
With corporate consolidation and a profit-driven mandate by healthcare systems, doctors are increasingly frustrated and feel that their voices haven’t been heard enough when it comes to issues like workplace safety, working hours, and benefits, said Stuart Bussey, MD, JD, a family practice physician and president of the Union of American Physicians and Dentists in Sacramento, California.
However, he adds that urging doctors to join together to fight for a better working environment hasn’t been easy.
“Doctors are individualists, and they don’t know how to work in packs like hospital administrators do,” said Dr. Bussey. “They’re hard to organize, but I want them to understand that unless they join hands, sign petitions, and speak as one voice, they’re going to lose out on an amazing opportunity.”
Overcoming Misperceptions About Unions
One barrier to doctors getting involved is the sentiment that unions might do the opposite of what’s intended — that is, they might further reduce a doctor’s autonomy and work flexibility. Or there may be a perception that the drive to join a union is predicated on making more money.
Though he’s now in a union, Dr. VenOsdel, who has been in a hospital-based practice for 7 years, admits that he initially felt very differently about unions than he does today.
“Even though I have family members in healthcare unions, I had a neutral to even slightly negative view of unions,” said Dr. VenOsdel. “It took me working directly with the Minnesota Nurses Association and the Doctors Council to learn the other side of the story.”
Armed with more information, he began lobbying for stricter rules about how his state’s large healthcare systems were closing hospitals and ending much-needed community services.
“I remember standing at the Capitol in Minnesota and telling one of the members that I once felt negatively about unions,” he added. “I realized then that I only knew what employers were telling me via such things as emails about strikes — that information was all being shared from the employers’ perspective.”
The other misperception is that unions only exist to argue against management, including against colleagues who are also part of the management structure, said Dr. Higgins.
“Some doctors perceive being in a union as ‘how can those same leaders also be in a union,’” she said. She feels that they currently don’t have leadership representing them that can help with such things as restructuring their support teams or getting them help with certain tasks. “That’s another way unions can help.”
Social Justice Plays a Role
For Dr. VenOsdel, being part of a union has helped him return to what he calls the “art” of medicine.
“Philosophically, the union gave me an option for change in what felt like a hopeless situation,” he said. “It wasn’t just that I was tossing the keys to someone else and saying, ‘I can’t fix this.’ Instead, we’re taking the reins back and fixing things ourselves.”
Bussey argues that as the uneven balance between administrators and providers in many healthcare organizations grows, the time to consider forming a union is now.
“We’re in a $4 trillion medical industrial revolution,” he said. “Administrators and bureaucrats are multiplying 30-fold times vs providers, and most of that $4 trillion supports things that don’t contribute to the doctor-patient relationship.”
Furthermore, union proponents say that where a one-on-one relationship between doctor and patient once existed, that has now been “triangulated” to include administrators.
“We’ve lost power in every way,” Dr. Bussey said. “We have the degrees, the liability, and the knowledge — we should have more power to make our workplaces safer and better.”
Ultimately, for some unionized doctors, the very holding of a union card is rooted in supporting social justice issues.
“When doctors realize how powerful a tool a union can be for social justice and change, this will alter perceptions of unions within our profession,” Dr. VenOsdel said. “Our union helps give us a voice to stand up for other staff who aren’t unionized and, most importantly, to stand up for the patients who need us.”
A version of this article first appeared on Medscape.com.
With huge shifts over the past decade in the way doctors are employed — half of all doctors now work for a health system or large medical group — the idea of unionizing is not only being explored but gaining traction within the profession. In fact, 8% of the physician workforce (or 70,000 physicians) belong to a union, according to statistics gathered in 2022.
Exact numbers are hard to come by, and, interestingly, although the American Medical Association (AMA) “ supports the right of physicians to engage in collective bargaining,” the organization doesn’t track union membership among physicians, according to an AMA spokesperson.
Forming a Union
One challenge is that forming a union is not only time-consuming but also difficult, owing to several barriers. For starters, the laws dictating unionization differ by state, and the rules governing unionization vary if a hospital is public or private. If there’s enough momentum from doctors leading unionization efforts, approval from hospital leaders is required before an official election can be requested from the National Labor Relations Board.
That said, for doctors who are in a union — the two most popular are the Union of American Physicians and Dentists and the Doctors Council branch of the Service Employees International Union (SEIU)—the benefits are immense, especially because union members can focus on what matters, such as providing the best patient care possible.
, reported WBUR in Boston.
Belonging Matters
“When you build a relationship with your patients, it’s special, and that connection isn’t replaceable,” said Nicholas VenOsdel, MD, a pediatrician at Allina Health Primary Care in Hastings, Minnesota, and a union member of the Doctors Council. “However, a lot of us have felt like that hasn’t been respected as the climate of healthcare has changed so fast.”
In fact, autonomy over how much time doctors spend with patients is driving a lot of interest in unionization.
“We don’t necessarily have that autonomy now,” said Amber Higgins, MD, an emergency physician and an obstetrician at ChristianaCare, a hospital network in Newark, Delaware, and a member of the Doctors Council. “There are so many other demands, whether it’s billing, patient documentation, or other demands from the employer, and all of that takes time away from patient care.”
Another primary driver of physician unionization is the physician burnout epidemic. Physicians collectively complain that they spend more time on electronic health record documentation and bureaucratic administration. Yet if unions can improve these working conditions, the benefit to physicians and their patients would be a welcome change.
Union members are bullish and believe that having a cohesive voice will make a difference.
“We need to use our collective voices to get back to focusing on patient care instead of staring at a computer screen for 80% of the day,” Dr. Higgins told this news organization. “So much of medicine involves getting to the correct diagnosis, listening to patients, observing them, and building a relationship with them. We need time to build that.”
With corporate consolidation and a profit-driven mandate by healthcare systems, doctors are increasingly frustrated and feel that their voices haven’t been heard enough when it comes to issues like workplace safety, working hours, and benefits, said Stuart Bussey, MD, JD, a family practice physician and president of the Union of American Physicians and Dentists in Sacramento, California.
However, he adds that urging doctors to join together to fight for a better working environment hasn’t been easy.
“Doctors are individualists, and they don’t know how to work in packs like hospital administrators do,” said Dr. Bussey. “They’re hard to organize, but I want them to understand that unless they join hands, sign petitions, and speak as one voice, they’re going to lose out on an amazing opportunity.”
Overcoming Misperceptions About Unions
One barrier to doctors getting involved is the sentiment that unions might do the opposite of what’s intended — that is, they might further reduce a doctor’s autonomy and work flexibility. Or there may be a perception that the drive to join a union is predicated on making more money.
Though he’s now in a union, Dr. VenOsdel, who has been in a hospital-based practice for 7 years, admits that he initially felt very differently about unions than he does today.
“Even though I have family members in healthcare unions, I had a neutral to even slightly negative view of unions,” said Dr. VenOsdel. “It took me working directly with the Minnesota Nurses Association and the Doctors Council to learn the other side of the story.”
Armed with more information, he began lobbying for stricter rules about how his state’s large healthcare systems were closing hospitals and ending much-needed community services.
“I remember standing at the Capitol in Minnesota and telling one of the members that I once felt negatively about unions,” he added. “I realized then that I only knew what employers were telling me via such things as emails about strikes — that information was all being shared from the employers’ perspective.”
The other misperception is that unions only exist to argue against management, including against colleagues who are also part of the management structure, said Dr. Higgins.
“Some doctors perceive being in a union as ‘how can those same leaders also be in a union,’” she said. She feels that they currently don’t have leadership representing them that can help with such things as restructuring their support teams or getting them help with certain tasks. “That’s another way unions can help.”
Social Justice Plays a Role
For Dr. VenOsdel, being part of a union has helped him return to what he calls the “art” of medicine.
“Philosophically, the union gave me an option for change in what felt like a hopeless situation,” he said. “It wasn’t just that I was tossing the keys to someone else and saying, ‘I can’t fix this.’ Instead, we’re taking the reins back and fixing things ourselves.”
Bussey argues that as the uneven balance between administrators and providers in many healthcare organizations grows, the time to consider forming a union is now.
“We’re in a $4 trillion medical industrial revolution,” he said. “Administrators and bureaucrats are multiplying 30-fold times vs providers, and most of that $4 trillion supports things that don’t contribute to the doctor-patient relationship.”
Furthermore, union proponents say that where a one-on-one relationship between doctor and patient once existed, that has now been “triangulated” to include administrators.
“We’ve lost power in every way,” Dr. Bussey said. “We have the degrees, the liability, and the knowledge — we should have more power to make our workplaces safer and better.”
Ultimately, for some unionized doctors, the very holding of a union card is rooted in supporting social justice issues.
“When doctors realize how powerful a tool a union can be for social justice and change, this will alter perceptions of unions within our profession,” Dr. VenOsdel said. “Our union helps give us a voice to stand up for other staff who aren’t unionized and, most importantly, to stand up for the patients who need us.”
A version of this article first appeared on Medscape.com.
With huge shifts over the past decade in the way doctors are employed — half of all doctors now work for a health system or large medical group — the idea of unionizing is not only being explored but gaining traction within the profession. In fact, 8% of the physician workforce (or 70,000 physicians) belong to a union, according to statistics gathered in 2022.
Exact numbers are hard to come by, and, interestingly, although the American Medical Association (AMA) “ supports the right of physicians to engage in collective bargaining,” the organization doesn’t track union membership among physicians, according to an AMA spokesperson.
Forming a Union
One challenge is that forming a union is not only time-consuming but also difficult, owing to several barriers. For starters, the laws dictating unionization differ by state, and the rules governing unionization vary if a hospital is public or private. If there’s enough momentum from doctors leading unionization efforts, approval from hospital leaders is required before an official election can be requested from the National Labor Relations Board.
That said, for doctors who are in a union — the two most popular are the Union of American Physicians and Dentists and the Doctors Council branch of the Service Employees International Union (SEIU)—the benefits are immense, especially because union members can focus on what matters, such as providing the best patient care possible.
, reported WBUR in Boston.
Belonging Matters
“When you build a relationship with your patients, it’s special, and that connection isn’t replaceable,” said Nicholas VenOsdel, MD, a pediatrician at Allina Health Primary Care in Hastings, Minnesota, and a union member of the Doctors Council. “However, a lot of us have felt like that hasn’t been respected as the climate of healthcare has changed so fast.”
In fact, autonomy over how much time doctors spend with patients is driving a lot of interest in unionization.
“We don’t necessarily have that autonomy now,” said Amber Higgins, MD, an emergency physician and an obstetrician at ChristianaCare, a hospital network in Newark, Delaware, and a member of the Doctors Council. “There are so many other demands, whether it’s billing, patient documentation, or other demands from the employer, and all of that takes time away from patient care.”
Another primary driver of physician unionization is the physician burnout epidemic. Physicians collectively complain that they spend more time on electronic health record documentation and bureaucratic administration. Yet if unions can improve these working conditions, the benefit to physicians and their patients would be a welcome change.
Union members are bullish and believe that having a cohesive voice will make a difference.
“We need to use our collective voices to get back to focusing on patient care instead of staring at a computer screen for 80% of the day,” Dr. Higgins told this news organization. “So much of medicine involves getting to the correct diagnosis, listening to patients, observing them, and building a relationship with them. We need time to build that.”
With corporate consolidation and a profit-driven mandate by healthcare systems, doctors are increasingly frustrated and feel that their voices haven’t been heard enough when it comes to issues like workplace safety, working hours, and benefits, said Stuart Bussey, MD, JD, a family practice physician and president of the Union of American Physicians and Dentists in Sacramento, California.
However, he adds that urging doctors to join together to fight for a better working environment hasn’t been easy.
“Doctors are individualists, and they don’t know how to work in packs like hospital administrators do,” said Dr. Bussey. “They’re hard to organize, but I want them to understand that unless they join hands, sign petitions, and speak as one voice, they’re going to lose out on an amazing opportunity.”
Overcoming Misperceptions About Unions
One barrier to doctors getting involved is the sentiment that unions might do the opposite of what’s intended — that is, they might further reduce a doctor’s autonomy and work flexibility. Or there may be a perception that the drive to join a union is predicated on making more money.
Though he’s now in a union, Dr. VenOsdel, who has been in a hospital-based practice for 7 years, admits that he initially felt very differently about unions than he does today.
“Even though I have family members in healthcare unions, I had a neutral to even slightly negative view of unions,” said Dr. VenOsdel. “It took me working directly with the Minnesota Nurses Association and the Doctors Council to learn the other side of the story.”
Armed with more information, he began lobbying for stricter rules about how his state’s large healthcare systems were closing hospitals and ending much-needed community services.
“I remember standing at the Capitol in Minnesota and telling one of the members that I once felt negatively about unions,” he added. “I realized then that I only knew what employers were telling me via such things as emails about strikes — that information was all being shared from the employers’ perspective.”
The other misperception is that unions only exist to argue against management, including against colleagues who are also part of the management structure, said Dr. Higgins.
“Some doctors perceive being in a union as ‘how can those same leaders also be in a union,’” she said. She feels that they currently don’t have leadership representing them that can help with such things as restructuring their support teams or getting them help with certain tasks. “That’s another way unions can help.”
Social Justice Plays a Role
For Dr. VenOsdel, being part of a union has helped him return to what he calls the “art” of medicine.
“Philosophically, the union gave me an option for change in what felt like a hopeless situation,” he said. “It wasn’t just that I was tossing the keys to someone else and saying, ‘I can’t fix this.’ Instead, we’re taking the reins back and fixing things ourselves.”
Bussey argues that as the uneven balance between administrators and providers in many healthcare organizations grows, the time to consider forming a union is now.
“We’re in a $4 trillion medical industrial revolution,” he said. “Administrators and bureaucrats are multiplying 30-fold times vs providers, and most of that $4 trillion supports things that don’t contribute to the doctor-patient relationship.”
Furthermore, union proponents say that where a one-on-one relationship between doctor and patient once existed, that has now been “triangulated” to include administrators.
“We’ve lost power in every way,” Dr. Bussey said. “We have the degrees, the liability, and the knowledge — we should have more power to make our workplaces safer and better.”
Ultimately, for some unionized doctors, the very holding of a union card is rooted in supporting social justice issues.
“When doctors realize how powerful a tool a union can be for social justice and change, this will alter perceptions of unions within our profession,” Dr. VenOsdel said. “Our union helps give us a voice to stand up for other staff who aren’t unionized and, most importantly, to stand up for the patients who need us.”
A version of this article first appeared on Medscape.com.