User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Twelve medical groups pen letter opposing UHC copay accumulator program
ACR leads outcry against the insurer’s proposed move
Last month, the American College of Rheumatology joined with 11 other medical associations and disease societies asking health insurance giant UnitedHealthcare (UHC) to not proceed with its proposed copay accumulator medical benefit program.
Copay accumulators are policies adopted by insurance companies or their pharmacy benefit managers to exclude patient copayment assistance programs for high-cost drugs, which are promulgated by the drug manufacturers, from being applied to a patient’s annual deductibles or out-of-pocket maximums. The manufacturer’s copay assistance, such as in the form of coupons, is designed to minimize the patient’s out-of-pocket costs. But insurers believe manufacturers will have no pressure to lower the prices of expensive specialty drugs unless patients are unable to afford them. Copay accumulators thus are aimed at giving insurers more leverage in negotiating prices for high-cost drugs.
UHC issued its new copay accumulator protocol for commercial individual and fully insured group plans in early October, effective Jan. 1, 2021, “in order to align employer costs for specialty medications with actual member out of pocket and deductibles,” according to the company’s announcement. In other words, patients will need to pay a higher share of the costs of these medications, said rheumatologist Christopher Phillips, MD, who chairs the Insurance Subcommittee of ACR’s Rheumatologic Care Committee. The annual price of biologic therapies for rheumatologic conditions ranges from $22,000 to $44,000, according to a recent press release from ACR.
The copay accumulator will negate the benefits of manufacturers’ copayment assistance programs for the patient, shifting more of the cost to the patient. With patients being forced to pay a higher share of drug costs for expensive biologic treatments for rheumatoid arthritis, lupus, and other rheumatologic conditions, they’ll stop taking the treatments, Dr. Phillips said.
“In my solo rheumatology practice in Paducah, Kentucky, when I’ve seen this kind of program applied on the pharmacy benefit side, rather than the medical benefit side, almost uniformly patients stop taking the high-cost treatments.” That can lead to disease flares, complications, and permanent disability. The newer rheumatologic drugs can cost $500 to $1,000 per treatment, and in many cases, there’s no generic or lower-cost alternative, he says. “We see policies like this as sacrificing patients to the battle over high drug prices. It’s bad practice, bad for patient outcomes, and nobody – apart from the payer – benefits.”
In ACR’s 2020 Rheumatic Disease Patient Survey, nearly half of 1,109 online survey respondents who had rheumatic diseases reported out-of-pocket costs greater than $1,000 per year for treatment. An IQVIA report from 2016 found that one in four specialty brand prescriptions are abandoned during the deductible phase, three times the rate seen when there is no deductible.
In an Oct. 7 letter to UHC, the 12 groups acknowledged that the drugs targeted by the accumulator policy are expensive. “However, they are also vitally important for our patients.” In addition to the ACR, the organizations involved include the AIDS Institute, American Academy of Dermatology Association, American Academy of Neurology, American College of Gastroenterology, American Gastroenterological Association, American Kidney Fund, Arthritis Foundation, Association for Clinical Oncology, Cancer Support Community, Coalition of State Rheumatology Organizations, and National Multiple Sclerosis Society.
UHC did not reply to questions in time for publication.
First large-scale payer to try copay accumulator program
Under UHC’s proposed policy, providers will be required to use UHC’s portal to report payment information received from drug manufacturer copay assistance programs that are applied to patients’ cost share of these drugs through a complex, 14-step “coupon submission process” involving multiple technology interfaces. “My first oath as a physician is to do no harm to my patient. Many of us are concerned about making these reports, which could harm our patients and undermine the doctor-patient relationship,” Dr. Phillips said.
“If I don’t report, what happens? I don’t think we know the answer to that. Some of us may decide we need to part ways with UHC.” Others may decline to participate in the drug manufacturers’ coupon programs beyond simply informing patients that manufacturer assistance is available.
“We’ve watched these copay accumulator policies for several years,” he said. “Some of them are rather opaque, with names like ‘copay savings programs’ or ‘copay value programs.’ But we had not seen a large-scale payer try to do this until now. Let’s face it: If UHC’s policy goes through, you can count the days until we see it from others.”
The Department of Health & Human Services, in its May 2020 final federal “Notice of Benefit and Payment Parameters for 2021,” indicated that individual states have the responsibility to regulate copay accumulator programs. Five states have banned them or restricted their use for individual and small group health plans. Arizona, Illinois, Virginia, and West Virginia passed such laws in 2019, and Georgia did so earlier this year.
“In next year’s state legislative sessions, we’ll make it a priority to pursue similar laws in other states,” Dr. Phillips said. “I’d encourage rheumatologists to educate their patients on the issues and be active in advocating for them.”
ACR leads outcry against the insurer’s proposed move
ACR leads outcry against the insurer’s proposed move
Last month, the American College of Rheumatology joined with 11 other medical associations and disease societies asking health insurance giant UnitedHealthcare (UHC) to not proceed with its proposed copay accumulator medical benefit program.
Copay accumulators are policies adopted by insurance companies or their pharmacy benefit managers to exclude patient copayment assistance programs for high-cost drugs, which are promulgated by the drug manufacturers, from being applied to a patient’s annual deductibles or out-of-pocket maximums. The manufacturer’s copay assistance, such as in the form of coupons, is designed to minimize the patient’s out-of-pocket costs. But insurers believe manufacturers will have no pressure to lower the prices of expensive specialty drugs unless patients are unable to afford them. Copay accumulators thus are aimed at giving insurers more leverage in negotiating prices for high-cost drugs.
UHC issued its new copay accumulator protocol for commercial individual and fully insured group plans in early October, effective Jan. 1, 2021, “in order to align employer costs for specialty medications with actual member out of pocket and deductibles,” according to the company’s announcement. In other words, patients will need to pay a higher share of the costs of these medications, said rheumatologist Christopher Phillips, MD, who chairs the Insurance Subcommittee of ACR’s Rheumatologic Care Committee. The annual price of biologic therapies for rheumatologic conditions ranges from $22,000 to $44,000, according to a recent press release from ACR.
The copay accumulator will negate the benefits of manufacturers’ copayment assistance programs for the patient, shifting more of the cost to the patient. With patients being forced to pay a higher share of drug costs for expensive biologic treatments for rheumatoid arthritis, lupus, and other rheumatologic conditions, they’ll stop taking the treatments, Dr. Phillips said.
“In my solo rheumatology practice in Paducah, Kentucky, when I’ve seen this kind of program applied on the pharmacy benefit side, rather than the medical benefit side, almost uniformly patients stop taking the high-cost treatments.” That can lead to disease flares, complications, and permanent disability. The newer rheumatologic drugs can cost $500 to $1,000 per treatment, and in many cases, there’s no generic or lower-cost alternative, he says. “We see policies like this as sacrificing patients to the battle over high drug prices. It’s bad practice, bad for patient outcomes, and nobody – apart from the payer – benefits.”
In ACR’s 2020 Rheumatic Disease Patient Survey, nearly half of 1,109 online survey respondents who had rheumatic diseases reported out-of-pocket costs greater than $1,000 per year for treatment. An IQVIA report from 2016 found that one in four specialty brand prescriptions are abandoned during the deductible phase, three times the rate seen when there is no deductible.
In an Oct. 7 letter to UHC, the 12 groups acknowledged that the drugs targeted by the accumulator policy are expensive. “However, they are also vitally important for our patients.” In addition to the ACR, the organizations involved include the AIDS Institute, American Academy of Dermatology Association, American Academy of Neurology, American College of Gastroenterology, American Gastroenterological Association, American Kidney Fund, Arthritis Foundation, Association for Clinical Oncology, Cancer Support Community, Coalition of State Rheumatology Organizations, and National Multiple Sclerosis Society.
UHC did not reply to questions in time for publication.
First large-scale payer to try copay accumulator program
Under UHC’s proposed policy, providers will be required to use UHC’s portal to report payment information received from drug manufacturer copay assistance programs that are applied to patients’ cost share of these drugs through a complex, 14-step “coupon submission process” involving multiple technology interfaces. “My first oath as a physician is to do no harm to my patient. Many of us are concerned about making these reports, which could harm our patients and undermine the doctor-patient relationship,” Dr. Phillips said.
“If I don’t report, what happens? I don’t think we know the answer to that. Some of us may decide we need to part ways with UHC.” Others may decline to participate in the drug manufacturers’ coupon programs beyond simply informing patients that manufacturer assistance is available.
“We’ve watched these copay accumulator policies for several years,” he said. “Some of them are rather opaque, with names like ‘copay savings programs’ or ‘copay value programs.’ But we had not seen a large-scale payer try to do this until now. Let’s face it: If UHC’s policy goes through, you can count the days until we see it from others.”
The Department of Health & Human Services, in its May 2020 final federal “Notice of Benefit and Payment Parameters for 2021,” indicated that individual states have the responsibility to regulate copay accumulator programs. Five states have banned them or restricted their use for individual and small group health plans. Arizona, Illinois, Virginia, and West Virginia passed such laws in 2019, and Georgia did so earlier this year.
“In next year’s state legislative sessions, we’ll make it a priority to pursue similar laws in other states,” Dr. Phillips said. “I’d encourage rheumatologists to educate their patients on the issues and be active in advocating for them.”
Last month, the American College of Rheumatology joined with 11 other medical associations and disease societies asking health insurance giant UnitedHealthcare (UHC) to not proceed with its proposed copay accumulator medical benefit program.
Copay accumulators are policies adopted by insurance companies or their pharmacy benefit managers to exclude patient copayment assistance programs for high-cost drugs, which are promulgated by the drug manufacturers, from being applied to a patient’s annual deductibles or out-of-pocket maximums. The manufacturer’s copay assistance, such as in the form of coupons, is designed to minimize the patient’s out-of-pocket costs. But insurers believe manufacturers will have no pressure to lower the prices of expensive specialty drugs unless patients are unable to afford them. Copay accumulators thus are aimed at giving insurers more leverage in negotiating prices for high-cost drugs.
UHC issued its new copay accumulator protocol for commercial individual and fully insured group plans in early October, effective Jan. 1, 2021, “in order to align employer costs for specialty medications with actual member out of pocket and deductibles,” according to the company’s announcement. In other words, patients will need to pay a higher share of the costs of these medications, said rheumatologist Christopher Phillips, MD, who chairs the Insurance Subcommittee of ACR’s Rheumatologic Care Committee. The annual price of biologic therapies for rheumatologic conditions ranges from $22,000 to $44,000, according to a recent press release from ACR.
The copay accumulator will negate the benefits of manufacturers’ copayment assistance programs for the patient, shifting more of the cost to the patient. With patients being forced to pay a higher share of drug costs for expensive biologic treatments for rheumatoid arthritis, lupus, and other rheumatologic conditions, they’ll stop taking the treatments, Dr. Phillips said.
“In my solo rheumatology practice in Paducah, Kentucky, when I’ve seen this kind of program applied on the pharmacy benefit side, rather than the medical benefit side, almost uniformly patients stop taking the high-cost treatments.” That can lead to disease flares, complications, and permanent disability. The newer rheumatologic drugs can cost $500 to $1,000 per treatment, and in many cases, there’s no generic or lower-cost alternative, he says. “We see policies like this as sacrificing patients to the battle over high drug prices. It’s bad practice, bad for patient outcomes, and nobody – apart from the payer – benefits.”
In ACR’s 2020 Rheumatic Disease Patient Survey, nearly half of 1,109 online survey respondents who had rheumatic diseases reported out-of-pocket costs greater than $1,000 per year for treatment. An IQVIA report from 2016 found that one in four specialty brand prescriptions are abandoned during the deductible phase, three times the rate seen when there is no deductible.
In an Oct. 7 letter to UHC, the 12 groups acknowledged that the drugs targeted by the accumulator policy are expensive. “However, they are also vitally important for our patients.” In addition to the ACR, the organizations involved include the AIDS Institute, American Academy of Dermatology Association, American Academy of Neurology, American College of Gastroenterology, American Gastroenterological Association, American Kidney Fund, Arthritis Foundation, Association for Clinical Oncology, Cancer Support Community, Coalition of State Rheumatology Organizations, and National Multiple Sclerosis Society.
UHC did not reply to questions in time for publication.
First large-scale payer to try copay accumulator program
Under UHC’s proposed policy, providers will be required to use UHC’s portal to report payment information received from drug manufacturer copay assistance programs that are applied to patients’ cost share of these drugs through a complex, 14-step “coupon submission process” involving multiple technology interfaces. “My first oath as a physician is to do no harm to my patient. Many of us are concerned about making these reports, which could harm our patients and undermine the doctor-patient relationship,” Dr. Phillips said.
“If I don’t report, what happens? I don’t think we know the answer to that. Some of us may decide we need to part ways with UHC.” Others may decline to participate in the drug manufacturers’ coupon programs beyond simply informing patients that manufacturer assistance is available.
“We’ve watched these copay accumulator policies for several years,” he said. “Some of them are rather opaque, with names like ‘copay savings programs’ or ‘copay value programs.’ But we had not seen a large-scale payer try to do this until now. Let’s face it: If UHC’s policy goes through, you can count the days until we see it from others.”
The Department of Health & Human Services, in its May 2020 final federal “Notice of Benefit and Payment Parameters for 2021,” indicated that individual states have the responsibility to regulate copay accumulator programs. Five states have banned them or restricted their use for individual and small group health plans. Arizona, Illinois, Virginia, and West Virginia passed such laws in 2019, and Georgia did so earlier this year.
“In next year’s state legislative sessions, we’ll make it a priority to pursue similar laws in other states,” Dr. Phillips said. “I’d encourage rheumatologists to educate their patients on the issues and be active in advocating for them.”
Survey finds European dermatologists unhappy with pandemic teledermatology experience
intensely, according to the findings of a survey presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“The results of our survey clearly show 7 out of 10 participating dermatologists declared that they were not happy with teledermatology, and most of them declared that they were not at all happy,” according to Mariano Suppa, MD, PhD, of the department of dermatology and venereology, Free University of Brussels.
“It was very interesting: it was not just about the lack of a good quality of consultation, but was also related to some extent to a lack of respect from some patients, and also a lack of empathy. The majority of survey respondents felt [attacked] by their own patients because they were proposing teledermatology. So, yes, we were forced to go to teledermatology, and I think we will be again to some extent, but clearly we’re not happy about it,” he elaborated in response to a question from session chair Brigitte Dreno, MD, professor of dermatology and vice dean of the faculty of medicine at the University of Nantes (France).
The survey, conducted by the EADV communication committee, assessed the pandemic’s impact on European dermatologists’ professional practices and personal lives through 30 brief questions, with space at the end for additional open-ended comments. In the comments section, many dermatologists vented about their income loss, the disorganized response to round one of the pandemic, and most of all about teledermatology. Common complaints were that teledermatology required a huge consumption of energy and constituted a major intrusion upon the physicians’ personal lives. And then there was the common theme of unkind treatment by some patients.
The survey was sent twice in June 2020 to more than 4,800 EADV members. It was completed by 490 dermatologists from 39 countries. Dr. Suppa attributed the low response rate to physician weariness of the topic due to saturation news media coverage of the pandemic.
Sixty-nine percent of responding dermatologists were women. Fifty-two percent of participants were over age 50, 81% lived in a city, and 53% worked in a university or public hospital or clinic. Twelve percent lived alone.
Impact on professional practice
Many European dermatologists were on the front lines in dealing with the first wave of COVID-19. Twenty-eight percent worked in a COVID-19 unit. Forty-eight percent of dermatologists performed COVID-19 tests, and those who didn’t either had no patient contact or couldn’t get test kits. Thirty-five percent of dermatologists saw patients who presented with skin signs of COVID-19. Four percent of survey respondents became infected.
Seventy percent rescheduled or canceled all or most patient appointments. Clinical care was prioritized: during the peak of the pandemic, 76% of dermatologists saw only urgent cases – mostly potentially serious rashes – and dermato-oncology patients. Seventy-six percent of dermatologists performed teledermatology, although by June 60% of respondents reported seeing at least three-quarters of their patients face-to-face.
Twenty-three percent of dermatologists reported having lost most or all of their income during March through June, and another 26% lost about half.
Impact on dermatologists’ personal lives
About half of survey respondents reported feeling stressed, and a similar percentage checked the box marked ‘anxiety.’ Nine percent reported depressive symptoms, 15% mentioned feeling anger, 17% uselessness, and 2% admitted suicidal ideation. But 30% of dermatologists reported experiencing no negative psychological effects whatsoever stemming from the pandemic.
Sixteen percent of dermatologists reported drinking more alcohol during sequestration.
But respondents cited positive effects as well: a renewed appreciation of the importance of time, and enjoyment of the additional time spent with family and alone. Many dermatologists relished the opportunity to spend more time cooking, reading literature, doing research, listening to or playing music, and practicing yoga or meditation. And dermatologists took solace and pride in being members of the vital medical community.
Dr. Dreno asked if the survey revealed evidence of underdiagnosis and undertreatment of dermatologic diseases during the pandemic. Dr. Suppa replied that the survey didn’t address that issue, but it’s his personal opinion that this was no doubt the case. Roughly one-quarter of dermatologists canceled all appointments, and when dermatology clinics became filled beginning in June, he and his colleagues saw a number of cases of delayed-diagnosis advanced skin cancer.
“I think that the diseases that were really penalized were the chronic inflammatory diseases, such as psoriasis, hidradenitis suppurativa, and also atopic dermatitis. We were doing a lot of telephone consultations for those patients at that time, and we saw in June that for those particular patients there was an unmet need in the pandemic because some of them really needed to have been seen. I think this is a lesson we should learn for the second wave that we’re unfortunately facing right now: We need to adopt restrictive measures to avoid spreading the pandemic, yes, for sure, but we need to keep in mind that there is not just COVID-19, but also other important diseases,” Dr. Suppa said.
A second EADV survey will be performed during the fall/winter wave of the pandemic.
Dr. Suppa reported having no financial conflicts regarding the EADV-funded survey.
SOURCE: Suppa M. EADV 2020. Presentation D3T03.4D
intensely, according to the findings of a survey presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“The results of our survey clearly show 7 out of 10 participating dermatologists declared that they were not happy with teledermatology, and most of them declared that they were not at all happy,” according to Mariano Suppa, MD, PhD, of the department of dermatology and venereology, Free University of Brussels.
“It was very interesting: it was not just about the lack of a good quality of consultation, but was also related to some extent to a lack of respect from some patients, and also a lack of empathy. The majority of survey respondents felt [attacked] by their own patients because they were proposing teledermatology. So, yes, we were forced to go to teledermatology, and I think we will be again to some extent, but clearly we’re not happy about it,” he elaborated in response to a question from session chair Brigitte Dreno, MD, professor of dermatology and vice dean of the faculty of medicine at the University of Nantes (France).
The survey, conducted by the EADV communication committee, assessed the pandemic’s impact on European dermatologists’ professional practices and personal lives through 30 brief questions, with space at the end for additional open-ended comments. In the comments section, many dermatologists vented about their income loss, the disorganized response to round one of the pandemic, and most of all about teledermatology. Common complaints were that teledermatology required a huge consumption of energy and constituted a major intrusion upon the physicians’ personal lives. And then there was the common theme of unkind treatment by some patients.
The survey was sent twice in June 2020 to more than 4,800 EADV members. It was completed by 490 dermatologists from 39 countries. Dr. Suppa attributed the low response rate to physician weariness of the topic due to saturation news media coverage of the pandemic.
Sixty-nine percent of responding dermatologists were women. Fifty-two percent of participants were over age 50, 81% lived in a city, and 53% worked in a university or public hospital or clinic. Twelve percent lived alone.
Impact on professional practice
Many European dermatologists were on the front lines in dealing with the first wave of COVID-19. Twenty-eight percent worked in a COVID-19 unit. Forty-eight percent of dermatologists performed COVID-19 tests, and those who didn’t either had no patient contact or couldn’t get test kits. Thirty-five percent of dermatologists saw patients who presented with skin signs of COVID-19. Four percent of survey respondents became infected.
Seventy percent rescheduled or canceled all or most patient appointments. Clinical care was prioritized: during the peak of the pandemic, 76% of dermatologists saw only urgent cases – mostly potentially serious rashes – and dermato-oncology patients. Seventy-six percent of dermatologists performed teledermatology, although by June 60% of respondents reported seeing at least three-quarters of their patients face-to-face.
Twenty-three percent of dermatologists reported having lost most or all of their income during March through June, and another 26% lost about half.
Impact on dermatologists’ personal lives
About half of survey respondents reported feeling stressed, and a similar percentage checked the box marked ‘anxiety.’ Nine percent reported depressive symptoms, 15% mentioned feeling anger, 17% uselessness, and 2% admitted suicidal ideation. But 30% of dermatologists reported experiencing no negative psychological effects whatsoever stemming from the pandemic.
Sixteen percent of dermatologists reported drinking more alcohol during sequestration.
But respondents cited positive effects as well: a renewed appreciation of the importance of time, and enjoyment of the additional time spent with family and alone. Many dermatologists relished the opportunity to spend more time cooking, reading literature, doing research, listening to or playing music, and practicing yoga or meditation. And dermatologists took solace and pride in being members of the vital medical community.
Dr. Dreno asked if the survey revealed evidence of underdiagnosis and undertreatment of dermatologic diseases during the pandemic. Dr. Suppa replied that the survey didn’t address that issue, but it’s his personal opinion that this was no doubt the case. Roughly one-quarter of dermatologists canceled all appointments, and when dermatology clinics became filled beginning in June, he and his colleagues saw a number of cases of delayed-diagnosis advanced skin cancer.
“I think that the diseases that were really penalized were the chronic inflammatory diseases, such as psoriasis, hidradenitis suppurativa, and also atopic dermatitis. We were doing a lot of telephone consultations for those patients at that time, and we saw in June that for those particular patients there was an unmet need in the pandemic because some of them really needed to have been seen. I think this is a lesson we should learn for the second wave that we’re unfortunately facing right now: We need to adopt restrictive measures to avoid spreading the pandemic, yes, for sure, but we need to keep in mind that there is not just COVID-19, but also other important diseases,” Dr. Suppa said.
A second EADV survey will be performed during the fall/winter wave of the pandemic.
Dr. Suppa reported having no financial conflicts regarding the EADV-funded survey.
SOURCE: Suppa M. EADV 2020. Presentation D3T03.4D
intensely, according to the findings of a survey presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“The results of our survey clearly show 7 out of 10 participating dermatologists declared that they were not happy with teledermatology, and most of them declared that they were not at all happy,” according to Mariano Suppa, MD, PhD, of the department of dermatology and venereology, Free University of Brussels.
“It was very interesting: it was not just about the lack of a good quality of consultation, but was also related to some extent to a lack of respect from some patients, and also a lack of empathy. The majority of survey respondents felt [attacked] by their own patients because they were proposing teledermatology. So, yes, we were forced to go to teledermatology, and I think we will be again to some extent, but clearly we’re not happy about it,” he elaborated in response to a question from session chair Brigitte Dreno, MD, professor of dermatology and vice dean of the faculty of medicine at the University of Nantes (France).
The survey, conducted by the EADV communication committee, assessed the pandemic’s impact on European dermatologists’ professional practices and personal lives through 30 brief questions, with space at the end for additional open-ended comments. In the comments section, many dermatologists vented about their income loss, the disorganized response to round one of the pandemic, and most of all about teledermatology. Common complaints were that teledermatology required a huge consumption of energy and constituted a major intrusion upon the physicians’ personal lives. And then there was the common theme of unkind treatment by some patients.
The survey was sent twice in June 2020 to more than 4,800 EADV members. It was completed by 490 dermatologists from 39 countries. Dr. Suppa attributed the low response rate to physician weariness of the topic due to saturation news media coverage of the pandemic.
Sixty-nine percent of responding dermatologists were women. Fifty-two percent of participants were over age 50, 81% lived in a city, and 53% worked in a university or public hospital or clinic. Twelve percent lived alone.
Impact on professional practice
Many European dermatologists were on the front lines in dealing with the first wave of COVID-19. Twenty-eight percent worked in a COVID-19 unit. Forty-eight percent of dermatologists performed COVID-19 tests, and those who didn’t either had no patient contact or couldn’t get test kits. Thirty-five percent of dermatologists saw patients who presented with skin signs of COVID-19. Four percent of survey respondents became infected.
Seventy percent rescheduled or canceled all or most patient appointments. Clinical care was prioritized: during the peak of the pandemic, 76% of dermatologists saw only urgent cases – mostly potentially serious rashes – and dermato-oncology patients. Seventy-six percent of dermatologists performed teledermatology, although by June 60% of respondents reported seeing at least three-quarters of their patients face-to-face.
Twenty-three percent of dermatologists reported having lost most or all of their income during March through June, and another 26% lost about half.
Impact on dermatologists’ personal lives
About half of survey respondents reported feeling stressed, and a similar percentage checked the box marked ‘anxiety.’ Nine percent reported depressive symptoms, 15% mentioned feeling anger, 17% uselessness, and 2% admitted suicidal ideation. But 30% of dermatologists reported experiencing no negative psychological effects whatsoever stemming from the pandemic.
Sixteen percent of dermatologists reported drinking more alcohol during sequestration.
But respondents cited positive effects as well: a renewed appreciation of the importance of time, and enjoyment of the additional time spent with family and alone. Many dermatologists relished the opportunity to spend more time cooking, reading literature, doing research, listening to or playing music, and practicing yoga or meditation. And dermatologists took solace and pride in being members of the vital medical community.
Dr. Dreno asked if the survey revealed evidence of underdiagnosis and undertreatment of dermatologic diseases during the pandemic. Dr. Suppa replied that the survey didn’t address that issue, but it’s his personal opinion that this was no doubt the case. Roughly one-quarter of dermatologists canceled all appointments, and when dermatology clinics became filled beginning in June, he and his colleagues saw a number of cases of delayed-diagnosis advanced skin cancer.
“I think that the diseases that were really penalized were the chronic inflammatory diseases, such as psoriasis, hidradenitis suppurativa, and also atopic dermatitis. We were doing a lot of telephone consultations for those patients at that time, and we saw in June that for those particular patients there was an unmet need in the pandemic because some of them really needed to have been seen. I think this is a lesson we should learn for the second wave that we’re unfortunately facing right now: We need to adopt restrictive measures to avoid spreading the pandemic, yes, for sure, but we need to keep in mind that there is not just COVID-19, but also other important diseases,” Dr. Suppa said.
A second EADV survey will be performed during the fall/winter wave of the pandemic.
Dr. Suppa reported having no financial conflicts regarding the EADV-funded survey.
SOURCE: Suppa M. EADV 2020. Presentation D3T03.4D
FROM THE EADV CONGRESS
COVID-19: U.S. sets new weekly high in children
the American Academy of Pediatrics announced Nov. 2.
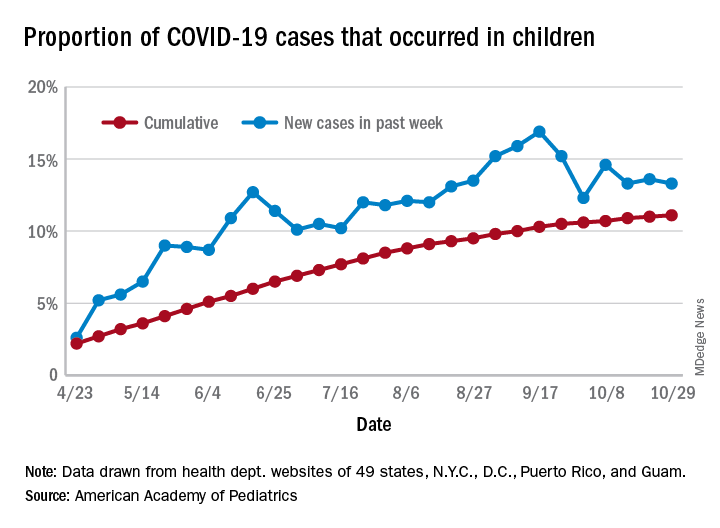
For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.
the American Academy of Pediatrics announced Nov. 2.

For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.
the American Academy of Pediatrics announced Nov. 2.

For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.
Birch bark derivative gel found effective for EB, in phase 3 study
A . The results come from the largest double-blind, randomized trial performed in this patient population.
More than 41% of EB target wounds that were treated with Oleogel-S10 healed within 45 days, compared with about 29% of target wounds treated with placebo, in the EASE phase 3 trial, conducted at 58 sites in 28 countries.
A group of rare genetic disorders, EB “is described as the worst disease you’ve never heard of,” explained lead investigator Dedee Murrell, MD, director of dermatology, St. George Hospital at the University of New South Wales, Sydney. “It starts in children and is like having burns that heal with scars, and no treatment has been approved for it” by the Food and Drug Administration.
“This is the first large clinical trial with placebo of a topical treatment that’s worked for this terrible disease,” Dr. Murrell said in an interview. She noted that standard EB treatment currently consists of applying nonstick dressings to wounds to protect skin from trauma and infection.
Dr. Murrell, who has focused her work on EB patients since 1990, presented the findings at the virtual annual Congress of the European Academy of Dermatology and Venereology.
The trial enrolled 223 patients (average age, 12 years, but ages ranged to 81 years) with three types of EB, including dystrophic and junctional EB and Kindler syndrome. For each participant, a target wound was selected for use as the primary efficacy endpoint. Those wounds had a partial thickness of between 10 cm2 and 50 cm2 and lasted between 21 days and 9 months. Patients were stratified into groups depending on type of EB and size of target wound.
Participants were randomly assigned to receive either Oleogel-S10 (n = 109) or placebo (n = 114). All applied the blinded-study gel to all their wounds at least every 4 days at the time dressings were changed.
The primary endpoint was the percentage of patients whose target wounds completely closed within 45 days. Key secondary endpoints included time to wound healing and percentage of target wounds that healed within 90 days of treatment; incidence and severity of target wound infection; change in total body wound burden, as measured by the Epidermolysis Bullosa Disease Activity and Scarring Index skin activity subscore; change in itching, as measured by the Itch Man Scale and the Leuven Itch Scale; and adverse events.
Nearly 92% of patients who were treated with Oleogel-S10 completed the double-blind phase of the trial, compared with nearly 87% who received placebo. As noted, the primary endpoint was met, with 41.3% of Oleogel-S10 patients achieving target wound closure within 45 days, compared with 28.9% of the patients who received placebo (P = .013).
But the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of Oleogel-S10 patients achieving wound closure vs. 43.9% of control patients.
Target-wound infection occurred in eight participants, including three who used Oleogel-S10 and five who received placebo; all moderate or severe infections occurred in patients who received placebo. Total wound burden was reduced to a greater extent among Oleogel-S10 patients by day 60, but there was no apparent difference at day 90.
Both treatment groups reported qualitative improvements in itch, with no significant differences between groups. The prevalence of adverse events was also similar between groups (Oleogel-S10, 81.7%; placebo, 80.7%). The most frequently reported adverse events among Oleogel-S10 patients, compared with patients who received placebo, were wound complications, pyrexia, wound infection, pruritus, and anemia; only 4.5% of adverse events were deemed severe.
Dr. Murrell said that, on the basis of the trial results, she expects the FDA to fast-track approval of Oleogel-S10, which contains triterpene extract and sunflower oil.
The gel is “a treatment patients will be able to put under their dressings, added to normal treatment, which will accelerate their wound healing, with no significant increase in any side effects,” she added.
Jemima Mellerio, MD, of St. Thomas’ Hospital in London who sees about 400 EB patients each year, agreed with Dr. Murrell that the results are “very exciting.” Dr. Mellerio was not involved in the study.
“Practicing dermatologists seeing people with EB will have something to offer that appears to speed up wound healing in chronic wounds,” Dr. Mellerio said in an interview. “It’s a positive option rather than just supportive treatment, something that makes a difference to the natural history of wounds.”
She said the trial’s biggest strength was including “such a large cohort of patients.
“It’s extremely difficult to do that kind of study, especially with a placebo-controlled arm and especially in a rare disease,” Dr. Mellerio said. “If you think about the product itself, it’s easy to apply, so it’s not particularly onerous for people to add to their daily regimen of dressings.”
The study was funded by Amryt Pharma. Dr. Murrell is an advisory board member for Amryt Pharma. Dr. Mellerio is a consultant for Amryt Pharma.
A version of this article originally appeared on Medscape.com.
A . The results come from the largest double-blind, randomized trial performed in this patient population.
More than 41% of EB target wounds that were treated with Oleogel-S10 healed within 45 days, compared with about 29% of target wounds treated with placebo, in the EASE phase 3 trial, conducted at 58 sites in 28 countries.
A group of rare genetic disorders, EB “is described as the worst disease you’ve never heard of,” explained lead investigator Dedee Murrell, MD, director of dermatology, St. George Hospital at the University of New South Wales, Sydney. “It starts in children and is like having burns that heal with scars, and no treatment has been approved for it” by the Food and Drug Administration.
“This is the first large clinical trial with placebo of a topical treatment that’s worked for this terrible disease,” Dr. Murrell said in an interview. She noted that standard EB treatment currently consists of applying nonstick dressings to wounds to protect skin from trauma and infection.
Dr. Murrell, who has focused her work on EB patients since 1990, presented the findings at the virtual annual Congress of the European Academy of Dermatology and Venereology.
The trial enrolled 223 patients (average age, 12 years, but ages ranged to 81 years) with three types of EB, including dystrophic and junctional EB and Kindler syndrome. For each participant, a target wound was selected for use as the primary efficacy endpoint. Those wounds had a partial thickness of between 10 cm2 and 50 cm2 and lasted between 21 days and 9 months. Patients were stratified into groups depending on type of EB and size of target wound.
Participants were randomly assigned to receive either Oleogel-S10 (n = 109) or placebo (n = 114). All applied the blinded-study gel to all their wounds at least every 4 days at the time dressings were changed.
The primary endpoint was the percentage of patients whose target wounds completely closed within 45 days. Key secondary endpoints included time to wound healing and percentage of target wounds that healed within 90 days of treatment; incidence and severity of target wound infection; change in total body wound burden, as measured by the Epidermolysis Bullosa Disease Activity and Scarring Index skin activity subscore; change in itching, as measured by the Itch Man Scale and the Leuven Itch Scale; and adverse events.
Nearly 92% of patients who were treated with Oleogel-S10 completed the double-blind phase of the trial, compared with nearly 87% who received placebo. As noted, the primary endpoint was met, with 41.3% of Oleogel-S10 patients achieving target wound closure within 45 days, compared with 28.9% of the patients who received placebo (P = .013).
But the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of Oleogel-S10 patients achieving wound closure vs. 43.9% of control patients.
Target-wound infection occurred in eight participants, including three who used Oleogel-S10 and five who received placebo; all moderate or severe infections occurred in patients who received placebo. Total wound burden was reduced to a greater extent among Oleogel-S10 patients by day 60, but there was no apparent difference at day 90.
Both treatment groups reported qualitative improvements in itch, with no significant differences between groups. The prevalence of adverse events was also similar between groups (Oleogel-S10, 81.7%; placebo, 80.7%). The most frequently reported adverse events among Oleogel-S10 patients, compared with patients who received placebo, were wound complications, pyrexia, wound infection, pruritus, and anemia; only 4.5% of adverse events were deemed severe.
Dr. Murrell said that, on the basis of the trial results, she expects the FDA to fast-track approval of Oleogel-S10, which contains triterpene extract and sunflower oil.
The gel is “a treatment patients will be able to put under their dressings, added to normal treatment, which will accelerate their wound healing, with no significant increase in any side effects,” she added.
Jemima Mellerio, MD, of St. Thomas’ Hospital in London who sees about 400 EB patients each year, agreed with Dr. Murrell that the results are “very exciting.” Dr. Mellerio was not involved in the study.
“Practicing dermatologists seeing people with EB will have something to offer that appears to speed up wound healing in chronic wounds,” Dr. Mellerio said in an interview. “It’s a positive option rather than just supportive treatment, something that makes a difference to the natural history of wounds.”
She said the trial’s biggest strength was including “such a large cohort of patients.
“It’s extremely difficult to do that kind of study, especially with a placebo-controlled arm and especially in a rare disease,” Dr. Mellerio said. “If you think about the product itself, it’s easy to apply, so it’s not particularly onerous for people to add to their daily regimen of dressings.”
The study was funded by Amryt Pharma. Dr. Murrell is an advisory board member for Amryt Pharma. Dr. Mellerio is a consultant for Amryt Pharma.
A version of this article originally appeared on Medscape.com.
A . The results come from the largest double-blind, randomized trial performed in this patient population.
More than 41% of EB target wounds that were treated with Oleogel-S10 healed within 45 days, compared with about 29% of target wounds treated with placebo, in the EASE phase 3 trial, conducted at 58 sites in 28 countries.
A group of rare genetic disorders, EB “is described as the worst disease you’ve never heard of,” explained lead investigator Dedee Murrell, MD, director of dermatology, St. George Hospital at the University of New South Wales, Sydney. “It starts in children and is like having burns that heal with scars, and no treatment has been approved for it” by the Food and Drug Administration.
“This is the first large clinical trial with placebo of a topical treatment that’s worked for this terrible disease,” Dr. Murrell said in an interview. She noted that standard EB treatment currently consists of applying nonstick dressings to wounds to protect skin from trauma and infection.
Dr. Murrell, who has focused her work on EB patients since 1990, presented the findings at the virtual annual Congress of the European Academy of Dermatology and Venereology.
The trial enrolled 223 patients (average age, 12 years, but ages ranged to 81 years) with three types of EB, including dystrophic and junctional EB and Kindler syndrome. For each participant, a target wound was selected for use as the primary efficacy endpoint. Those wounds had a partial thickness of between 10 cm2 and 50 cm2 and lasted between 21 days and 9 months. Patients were stratified into groups depending on type of EB and size of target wound.
Participants were randomly assigned to receive either Oleogel-S10 (n = 109) or placebo (n = 114). All applied the blinded-study gel to all their wounds at least every 4 days at the time dressings were changed.
The primary endpoint was the percentage of patients whose target wounds completely closed within 45 days. Key secondary endpoints included time to wound healing and percentage of target wounds that healed within 90 days of treatment; incidence and severity of target wound infection; change in total body wound burden, as measured by the Epidermolysis Bullosa Disease Activity and Scarring Index skin activity subscore; change in itching, as measured by the Itch Man Scale and the Leuven Itch Scale; and adverse events.
Nearly 92% of patients who were treated with Oleogel-S10 completed the double-blind phase of the trial, compared with nearly 87% who received placebo. As noted, the primary endpoint was met, with 41.3% of Oleogel-S10 patients achieving target wound closure within 45 days, compared with 28.9% of the patients who received placebo (P = .013).
But the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of Oleogel-S10 patients achieving wound closure vs. 43.9% of control patients.
Target-wound infection occurred in eight participants, including three who used Oleogel-S10 and five who received placebo; all moderate or severe infections occurred in patients who received placebo. Total wound burden was reduced to a greater extent among Oleogel-S10 patients by day 60, but there was no apparent difference at day 90.
Both treatment groups reported qualitative improvements in itch, with no significant differences between groups. The prevalence of adverse events was also similar between groups (Oleogel-S10, 81.7%; placebo, 80.7%). The most frequently reported adverse events among Oleogel-S10 patients, compared with patients who received placebo, were wound complications, pyrexia, wound infection, pruritus, and anemia; only 4.5% of adverse events were deemed severe.
Dr. Murrell said that, on the basis of the trial results, she expects the FDA to fast-track approval of Oleogel-S10, which contains triterpene extract and sunflower oil.
The gel is “a treatment patients will be able to put under their dressings, added to normal treatment, which will accelerate their wound healing, with no significant increase in any side effects,” she added.
Jemima Mellerio, MD, of St. Thomas’ Hospital in London who sees about 400 EB patients each year, agreed with Dr. Murrell that the results are “very exciting.” Dr. Mellerio was not involved in the study.
“Practicing dermatologists seeing people with EB will have something to offer that appears to speed up wound healing in chronic wounds,” Dr. Mellerio said in an interview. “It’s a positive option rather than just supportive treatment, something that makes a difference to the natural history of wounds.”
She said the trial’s biggest strength was including “such a large cohort of patients.
“It’s extremely difficult to do that kind of study, especially with a placebo-controlled arm and especially in a rare disease,” Dr. Mellerio said. “If you think about the product itself, it’s easy to apply, so it’s not particularly onerous for people to add to their daily regimen of dressings.”
The study was funded by Amryt Pharma. Dr. Murrell is an advisory board member for Amryt Pharma. Dr. Mellerio is a consultant for Amryt Pharma.
A version of this article originally appeared on Medscape.com.
HF an added risk in COVID-19, regardless of ejection fraction
People with a history of heart failure – no matter the type – face more complications and death than their peers without HF once hospitalized with COVID-19, a new observational study shows.
A history of HF was associated with a near doubling risk of in-hospital mortality and ICU care and more than a tripling risk of mechanical ventilation despite adjustment for 18 factors including race, obesity, diabetes, previous treatment with renin-angiotensin-aldosterone system (RAAS) inhibitors, and severity of illness.
Adverse outcomes were high regardless of whether patients had HF with a preserved, mid-range, or reduced left ventricular ejection fraction (HFpEF/HFmrEF/HFrEF).
“That for me was the real zinger,” senior author Anuradha Lala, MD, said in an interview . “Because as clinicians, oftentimes, and wrongly so, we think this person has preserved ejection fraction, so they’re not needing my heart failure expertise as much as someone with heart failure with reduced ejection fraction.”
In the peak of the pandemic, that may have meant triaging patients with HFpEF to a regular floor, whereas those with HFrEF were seen by the specialist team.
“What this alerted me to is to take heart failure as a diagnosis very seriously, regardless of ejection fraction, and that is very much in line with all of the emerging data about heart failure with preserved ejection fraction,” said Dr. Lala, from the Icahn School of Medicine at Mount Sinai, New York.
“Now when I see patients in the clinic, I incorporate part of our visit to talking about what they are doing to prevent COVID, which I really wasn’t doing before. It was like ‘Oh yeah, what crazy times we’re dealing with’ and then addressing their heart failure as I normally would,” she said. “But now, interwoven into every visit is: Are you wearing a mask, what’s your social distancing policy, who are you living with at home, has anyone at home or who you’ve interacted with been sick? I’m asking those questions just as a knee-jerk reaction for these patients because I know the repercussions. We have to keep in mind these are observational studies, so I can’t prove causality but these are observations that are, nonetheless, quite robust.”
Although cardiovascular disease, including HF, is recognized as a risk factor for worse outcomes in COVID-19 patients, data are sparse on the clinical course and prognosis of patients with preexisting HF.
“I would have expected that there would have been a gradation of risk from the people with very low ejection fractions up into the normal range, but here it didn’t seem to matter at all. So that’s an important point that bad outcomes were independent of ejection fraction,” commented Lee Goldberg, MD, professor of medicine and chief of advanced heart failure and cardiac transplant at the University of Pennsylvania, Philadelphia.
The study also validated that there is no association between use of RAAS inhibitors and bad outcomes in patients with COVID-19, he said.
Although this has been demonstrated in several studies, concerns were raised early in the pandemic that ACE inhibitors and angiotensin receptor blockers could facilitate infection with SARS-CoV-2 and increase the risk of severe or lethal COVID-19.
“For most clinicians that question has been put to bed, but we’re still getting patients that will ask during office visits ‘Is it safe for me to stay on?’ They still have that doubt [about] ‘Are we doing the right thing?’ ” Dr. Goldberg said.
“We can reassure them now. A lot of us are able to say there’s nothing to that, we’re very clear about this, stay on the meds. If anything, there’s data that suggest actually it may be better to be on an ACE inhibitor; that the hospitalizations were shorter and the outcomes were a little bit better.”
For the current study, published online Oct. 28 in the Journal of the American College of Cardiology, the investigators analyzed 6,439 patients admitted for COVID-19 at one of five Mount Sinai Health System hospitals in New York between Feb. 27 and June 26. Their mean age was 65.3 years, 45% were women, and one-third were treated with RAAS inhibitors before admission.
Using ICD-9/10 codes and individual chart review, HF was identified in 422 patients (6.6%), of which 250 patients had HFpEF (≥50%), 44 had HFmrEF (41%-49%), and 128 had HFrEF (≤40%).
Patients with HFpEF were older, more frequently women with a higher body mass index and history of lung disease than patients with HFrEF, whereas those with HFmrEF fell in between.
The HFpEF group was also treated with hydroxychloroquine or macrolides and noninvasive ventilation more frequently than the other two groups, whereas antiplatelet and neurohormonal therapies were more common in the HFrEF group.
Patients with a history of HF had significantly longer hospital stays than those without HF (8 days vs. 6 days), increased need for intubation (22.8% vs. 11.9%) and ICU care (23.2% vs. 16.6%), and worse in-hospital mortality (40% vs. 24.9%).
After multivariable regression adjustment, HF persisted as an independent risk factor for ICU care (odds ratio, 1.71; 95% CI, 1.25-2.34), intubation and mechanical ventilation (OR, 3.64; 95% CI, 2.56-5.16), and in-hospital mortality (OR, 1.88; 95% CI, 1.27-2.78).
“I knew to expect higher rates of adverse outcomes but I didn’t expect it to be nearly a twofold increase,” Dr. Lala said. “I thought that was pretty powerful.”
No significant differences were seen across LVEF categories in length of stay, need for ICU care, intubation and mechanical ventilation, acute kidney injury, shock, thromboembolic events, arrhythmias, or 30-day readmission rates.
However, cardiogenic shock (7.8% vs. 2.3% vs. 2%) and HF-related causes for 30-day readmissions (47.1% vs. 0% vs. 8.6%) were significantly higher in patients with HFrEF than in those with HFmrEF or HFpEF.
Also, mortality was lower in those with HFmrEF (22.7%) than with HFrEF (38.3%) and HFpEF (44%). The group was small but the “results suggested that patients with HFmrEF could have a better prognosis, because they can represent a distinct and more favorable HF phenotype,” the authors wrote.
The statistical testing didn’t show much difference and the patient numbers were very small, noted Dr. Goldberg. “So they might be overreaching a little bit there.”
“To me, the take-home message is that just having the phenotype of heart failure, regardless of EF, is associated with bad outcomes and we need to be vigilant on two fronts,” he said. “We really need to be doing prevention in the folks with heart failure because if they get COVID their outcomes are not going to be as good. Second, as clinicians, if we see a patient presenting with COVID who has a history of heart failure we may want to be much more vigilant with that individual than we might otherwise be. So I think there’s something to be said for kind of risk-stratifying people in that way.”
Dr. Goldberg pointed out that the study had many “amazing strengths,” including a large, racially diverse population, direct chart review to identify HF patients, and capturing a patient’s specific HF phenotype.
Weaknesses are that it was a single-center study, so the biases of how these patients were treated are not easily controlled for, he said. “We also don’t know when the hospital system was very strained as they were making some decisions: Were the older patients who had advanced heart and lung disease ultimately less aggressively treated because they felt they wouldn’t survive?”
Dr. Lala has received personal fees from Zoll, outside the submitted work. Dr. Goldberg reported research funding with Respicardia and consulting fees from Abbott.
This article first appeared on Medscape.com.
People with a history of heart failure – no matter the type – face more complications and death than their peers without HF once hospitalized with COVID-19, a new observational study shows.
A history of HF was associated with a near doubling risk of in-hospital mortality and ICU care and more than a tripling risk of mechanical ventilation despite adjustment for 18 factors including race, obesity, diabetes, previous treatment with renin-angiotensin-aldosterone system (RAAS) inhibitors, and severity of illness.
Adverse outcomes were high regardless of whether patients had HF with a preserved, mid-range, or reduced left ventricular ejection fraction (HFpEF/HFmrEF/HFrEF).
“That for me was the real zinger,” senior author Anuradha Lala, MD, said in an interview . “Because as clinicians, oftentimes, and wrongly so, we think this person has preserved ejection fraction, so they’re not needing my heart failure expertise as much as someone with heart failure with reduced ejection fraction.”
In the peak of the pandemic, that may have meant triaging patients with HFpEF to a regular floor, whereas those with HFrEF were seen by the specialist team.
“What this alerted me to is to take heart failure as a diagnosis very seriously, regardless of ejection fraction, and that is very much in line with all of the emerging data about heart failure with preserved ejection fraction,” said Dr. Lala, from the Icahn School of Medicine at Mount Sinai, New York.
“Now when I see patients in the clinic, I incorporate part of our visit to talking about what they are doing to prevent COVID, which I really wasn’t doing before. It was like ‘Oh yeah, what crazy times we’re dealing with’ and then addressing their heart failure as I normally would,” she said. “But now, interwoven into every visit is: Are you wearing a mask, what’s your social distancing policy, who are you living with at home, has anyone at home or who you’ve interacted with been sick? I’m asking those questions just as a knee-jerk reaction for these patients because I know the repercussions. We have to keep in mind these are observational studies, so I can’t prove causality but these are observations that are, nonetheless, quite robust.”
Although cardiovascular disease, including HF, is recognized as a risk factor for worse outcomes in COVID-19 patients, data are sparse on the clinical course and prognosis of patients with preexisting HF.
“I would have expected that there would have been a gradation of risk from the people with very low ejection fractions up into the normal range, but here it didn’t seem to matter at all. So that’s an important point that bad outcomes were independent of ejection fraction,” commented Lee Goldberg, MD, professor of medicine and chief of advanced heart failure and cardiac transplant at the University of Pennsylvania, Philadelphia.
The study also validated that there is no association between use of RAAS inhibitors and bad outcomes in patients with COVID-19, he said.
Although this has been demonstrated in several studies, concerns were raised early in the pandemic that ACE inhibitors and angiotensin receptor blockers could facilitate infection with SARS-CoV-2 and increase the risk of severe or lethal COVID-19.
“For most clinicians that question has been put to bed, but we’re still getting patients that will ask during office visits ‘Is it safe for me to stay on?’ They still have that doubt [about] ‘Are we doing the right thing?’ ” Dr. Goldberg said.
“We can reassure them now. A lot of us are able to say there’s nothing to that, we’re very clear about this, stay on the meds. If anything, there’s data that suggest actually it may be better to be on an ACE inhibitor; that the hospitalizations were shorter and the outcomes were a little bit better.”
For the current study, published online Oct. 28 in the Journal of the American College of Cardiology, the investigators analyzed 6,439 patients admitted for COVID-19 at one of five Mount Sinai Health System hospitals in New York between Feb. 27 and June 26. Their mean age was 65.3 years, 45% were women, and one-third were treated with RAAS inhibitors before admission.
Using ICD-9/10 codes and individual chart review, HF was identified in 422 patients (6.6%), of which 250 patients had HFpEF (≥50%), 44 had HFmrEF (41%-49%), and 128 had HFrEF (≤40%).
Patients with HFpEF were older, more frequently women with a higher body mass index and history of lung disease than patients with HFrEF, whereas those with HFmrEF fell in between.
The HFpEF group was also treated with hydroxychloroquine or macrolides and noninvasive ventilation more frequently than the other two groups, whereas antiplatelet and neurohormonal therapies were more common in the HFrEF group.
Patients with a history of HF had significantly longer hospital stays than those without HF (8 days vs. 6 days), increased need for intubation (22.8% vs. 11.9%) and ICU care (23.2% vs. 16.6%), and worse in-hospital mortality (40% vs. 24.9%).
After multivariable regression adjustment, HF persisted as an independent risk factor for ICU care (odds ratio, 1.71; 95% CI, 1.25-2.34), intubation and mechanical ventilation (OR, 3.64; 95% CI, 2.56-5.16), and in-hospital mortality (OR, 1.88; 95% CI, 1.27-2.78).
“I knew to expect higher rates of adverse outcomes but I didn’t expect it to be nearly a twofold increase,” Dr. Lala said. “I thought that was pretty powerful.”
No significant differences were seen across LVEF categories in length of stay, need for ICU care, intubation and mechanical ventilation, acute kidney injury, shock, thromboembolic events, arrhythmias, or 30-day readmission rates.
However, cardiogenic shock (7.8% vs. 2.3% vs. 2%) and HF-related causes for 30-day readmissions (47.1% vs. 0% vs. 8.6%) were significantly higher in patients with HFrEF than in those with HFmrEF or HFpEF.
Also, mortality was lower in those with HFmrEF (22.7%) than with HFrEF (38.3%) and HFpEF (44%). The group was small but the “results suggested that patients with HFmrEF could have a better prognosis, because they can represent a distinct and more favorable HF phenotype,” the authors wrote.
The statistical testing didn’t show much difference and the patient numbers were very small, noted Dr. Goldberg. “So they might be overreaching a little bit there.”
“To me, the take-home message is that just having the phenotype of heart failure, regardless of EF, is associated with bad outcomes and we need to be vigilant on two fronts,” he said. “We really need to be doing prevention in the folks with heart failure because if they get COVID their outcomes are not going to be as good. Second, as clinicians, if we see a patient presenting with COVID who has a history of heart failure we may want to be much more vigilant with that individual than we might otherwise be. So I think there’s something to be said for kind of risk-stratifying people in that way.”
Dr. Goldberg pointed out that the study had many “amazing strengths,” including a large, racially diverse population, direct chart review to identify HF patients, and capturing a patient’s specific HF phenotype.
Weaknesses are that it was a single-center study, so the biases of how these patients were treated are not easily controlled for, he said. “We also don’t know when the hospital system was very strained as they were making some decisions: Were the older patients who had advanced heart and lung disease ultimately less aggressively treated because they felt they wouldn’t survive?”
Dr. Lala has received personal fees from Zoll, outside the submitted work. Dr. Goldberg reported research funding with Respicardia and consulting fees from Abbott.
This article first appeared on Medscape.com.
People with a history of heart failure – no matter the type – face more complications and death than their peers without HF once hospitalized with COVID-19, a new observational study shows.
A history of HF was associated with a near doubling risk of in-hospital mortality and ICU care and more than a tripling risk of mechanical ventilation despite adjustment for 18 factors including race, obesity, diabetes, previous treatment with renin-angiotensin-aldosterone system (RAAS) inhibitors, and severity of illness.
Adverse outcomes were high regardless of whether patients had HF with a preserved, mid-range, or reduced left ventricular ejection fraction (HFpEF/HFmrEF/HFrEF).
“That for me was the real zinger,” senior author Anuradha Lala, MD, said in an interview . “Because as clinicians, oftentimes, and wrongly so, we think this person has preserved ejection fraction, so they’re not needing my heart failure expertise as much as someone with heart failure with reduced ejection fraction.”
In the peak of the pandemic, that may have meant triaging patients with HFpEF to a regular floor, whereas those with HFrEF were seen by the specialist team.
“What this alerted me to is to take heart failure as a diagnosis very seriously, regardless of ejection fraction, and that is very much in line with all of the emerging data about heart failure with preserved ejection fraction,” said Dr. Lala, from the Icahn School of Medicine at Mount Sinai, New York.
“Now when I see patients in the clinic, I incorporate part of our visit to talking about what they are doing to prevent COVID, which I really wasn’t doing before. It was like ‘Oh yeah, what crazy times we’re dealing with’ and then addressing their heart failure as I normally would,” she said. “But now, interwoven into every visit is: Are you wearing a mask, what’s your social distancing policy, who are you living with at home, has anyone at home or who you’ve interacted with been sick? I’m asking those questions just as a knee-jerk reaction for these patients because I know the repercussions. We have to keep in mind these are observational studies, so I can’t prove causality but these are observations that are, nonetheless, quite robust.”
Although cardiovascular disease, including HF, is recognized as a risk factor for worse outcomes in COVID-19 patients, data are sparse on the clinical course and prognosis of patients with preexisting HF.
“I would have expected that there would have been a gradation of risk from the people with very low ejection fractions up into the normal range, but here it didn’t seem to matter at all. So that’s an important point that bad outcomes were independent of ejection fraction,” commented Lee Goldberg, MD, professor of medicine and chief of advanced heart failure and cardiac transplant at the University of Pennsylvania, Philadelphia.
The study also validated that there is no association between use of RAAS inhibitors and bad outcomes in patients with COVID-19, he said.
Although this has been demonstrated in several studies, concerns were raised early in the pandemic that ACE inhibitors and angiotensin receptor blockers could facilitate infection with SARS-CoV-2 and increase the risk of severe or lethal COVID-19.
“For most clinicians that question has been put to bed, but we’re still getting patients that will ask during office visits ‘Is it safe for me to stay on?’ They still have that doubt [about] ‘Are we doing the right thing?’ ” Dr. Goldberg said.
“We can reassure them now. A lot of us are able to say there’s nothing to that, we’re very clear about this, stay on the meds. If anything, there’s data that suggest actually it may be better to be on an ACE inhibitor; that the hospitalizations were shorter and the outcomes were a little bit better.”
For the current study, published online Oct. 28 in the Journal of the American College of Cardiology, the investigators analyzed 6,439 patients admitted for COVID-19 at one of five Mount Sinai Health System hospitals in New York between Feb. 27 and June 26. Their mean age was 65.3 years, 45% were women, and one-third were treated with RAAS inhibitors before admission.
Using ICD-9/10 codes and individual chart review, HF was identified in 422 patients (6.6%), of which 250 patients had HFpEF (≥50%), 44 had HFmrEF (41%-49%), and 128 had HFrEF (≤40%).
Patients with HFpEF were older, more frequently women with a higher body mass index and history of lung disease than patients with HFrEF, whereas those with HFmrEF fell in between.
The HFpEF group was also treated with hydroxychloroquine or macrolides and noninvasive ventilation more frequently than the other two groups, whereas antiplatelet and neurohormonal therapies were more common in the HFrEF group.
Patients with a history of HF had significantly longer hospital stays than those without HF (8 days vs. 6 days), increased need for intubation (22.8% vs. 11.9%) and ICU care (23.2% vs. 16.6%), and worse in-hospital mortality (40% vs. 24.9%).
After multivariable regression adjustment, HF persisted as an independent risk factor for ICU care (odds ratio, 1.71; 95% CI, 1.25-2.34), intubation and mechanical ventilation (OR, 3.64; 95% CI, 2.56-5.16), and in-hospital mortality (OR, 1.88; 95% CI, 1.27-2.78).
“I knew to expect higher rates of adverse outcomes but I didn’t expect it to be nearly a twofold increase,” Dr. Lala said. “I thought that was pretty powerful.”
No significant differences were seen across LVEF categories in length of stay, need for ICU care, intubation and mechanical ventilation, acute kidney injury, shock, thromboembolic events, arrhythmias, or 30-day readmission rates.
However, cardiogenic shock (7.8% vs. 2.3% vs. 2%) and HF-related causes for 30-day readmissions (47.1% vs. 0% vs. 8.6%) were significantly higher in patients with HFrEF than in those with HFmrEF or HFpEF.
Also, mortality was lower in those with HFmrEF (22.7%) than with HFrEF (38.3%) and HFpEF (44%). The group was small but the “results suggested that patients with HFmrEF could have a better prognosis, because they can represent a distinct and more favorable HF phenotype,” the authors wrote.
The statistical testing didn’t show much difference and the patient numbers were very small, noted Dr. Goldberg. “So they might be overreaching a little bit there.”
“To me, the take-home message is that just having the phenotype of heart failure, regardless of EF, is associated with bad outcomes and we need to be vigilant on two fronts,” he said. “We really need to be doing prevention in the folks with heart failure because if they get COVID their outcomes are not going to be as good. Second, as clinicians, if we see a patient presenting with COVID who has a history of heart failure we may want to be much more vigilant with that individual than we might otherwise be. So I think there’s something to be said for kind of risk-stratifying people in that way.”
Dr. Goldberg pointed out that the study had many “amazing strengths,” including a large, racially diverse population, direct chart review to identify HF patients, and capturing a patient’s specific HF phenotype.
Weaknesses are that it was a single-center study, so the biases of how these patients were treated are not easily controlled for, he said. “We also don’t know when the hospital system was very strained as they were making some decisions: Were the older patients who had advanced heart and lung disease ultimately less aggressively treated because they felt they wouldn’t survive?”
Dr. Lala has received personal fees from Zoll, outside the submitted work. Dr. Goldberg reported research funding with Respicardia and consulting fees from Abbott.
This article first appeared on Medscape.com.
Biologics may protect psoriasis patients against severe COVID-19
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
FROM THE EADV CONGRESS
Mirikizumab beats placebo, secukinumab for psoriasis
The investigational monoclonal antibody according to new long-term OASIS-2 trial data.
Both doses of mirikizumab in the international, double-blind trial achieved improvements in Psoriasis Area and Severity Index (PASI) scores in larger numbers of participants at week 52 than secukinumab (Cosentyx), with low adverse event rates.
If approved, mirikizumab, which binds the p19 subunit of IL-23, would join three other IL-23 drugs already marketed in the United States for moderate to severe psoriasis, said OASIS-2 lead investigator Kim A. Papp, MD, PhD, founder and president of Probity Medical Research in Waterloo, Ont.
But Dr. Papp feels larger studies “will be necessary to put these data into perspective,” he said during a presentation at the virtual annual European Academy of Dermatology and Venereology Congress.
“Probably the most important takeaway here is that we may have another option to choose from,” Dr. Papp said in an interview. “People tend to think we have an adequate stable of treatment options, and I would argue we do not.”
“There are variations over time that occur in terms of an individual’s biological response, and the consequence is that nothing we have works for everyone, and nothing we have works forever,” he added. Psoriasis biologics “are increasingly competent, compared to medications we had even 5 or 10 years ago ... but they still don’t satisfy all our needs, so we do need to keep replenishing our stock.”
The multicenter trial included 1,465 patients who were randomly split into four groups. Subcutaneously, one group received 250 mg of mirikizumab every 4 weeks, and then 250 mg of the drug every 8 weeks starting at week 16. Another group received 250 mg of mirikizumab every 4 weeks and then 125 mg every 8 weeks starting at week 16.
The third group received 300 mg of secukinumab weekly for 4 weeks and then every 4 weeks starting at week 4. The last group received placebo every 4 weeks, and then 250 mg of mirikizumab every 4 weeks from week 16 to 32 and every 8 weeks thereafter.
Primary endpoints measured the percentage of patients achieving a static Physician’s Global Assessment (sPGA) of 0 or 1, with an improvement of at least 2 points from baseline; and the proportion of patients with PASI 90 at week 16, compared with placebo.
Major secondary endpoints were PASI 75 and PASI 100, compared with placebo at week 16; an sPGA of 0 or 1 and PASI 90 noninferiority, compared with secukinumab at week 16; and sPGA of 0 or 1, PASI 90, and PASI 100 superiority, compared with secukinumab at week 52.
More than 91% of participants completed all 52 weeks in the trial. Mirikizumab met primary endpoints compared with placebo and major secondary endpoints vs secukinumab at week 16 (P < .001). PASI 90 and sPGA (0,1) response rates far exceeded placebo for both 250 mg mirikizumab (74.4% and 79.7%, respectively) and secukinumab (72.8% and 76.3%, respectively).
At week 52, major secondary endpoints for both mirikizumab doses were superior to secukinumab (all P < .001). PASI 90 was achieved by 81.4% of 125 mg and 82.4% of 250 mg mirikizumab patients versus 69.4% of secukinumab patients; sPGA (0,1) by 83.1% of 125 mg and 83.3% of 250 mg mirikizumab patients versus 68.5% of secukinumab patients; and PASI 100 by 53.9% of 125 mg and 58.8% of 250 mg mirikizumab patients versus 42.9% of secukinumab patients.
Treatment-associated adverse effects were similar across all treatment groups and study periods. The most common were nasopharyngitis, upper respiratory tract infection, headache, back pain, and arthralgia. But serious adverse effects were minimal, Dr. Papp said. One death occurred in a mirikizumab patient from acute MI, which was deemed unrelated to the study drug.
Myrto Georgia Trakatelli, MD, PhD, from Aristotle University of Thessaloniki (Greece), said the results indicate that dermatologists “should not be afraid to use” mirikizumab long term if it is approved by the Food and Drug Administration.
“Sometimes patients use many treatments for a long time and all of a sudden, they stop working,” Dr. Trakatelli said in an interview. “A new biologic is always welcome because we do see patients not responding to other treatment.”
But Dr. Trakatelli said “a point that troubled me in the study” was that mirikizumab was compared with an IL-17 inhibitor “instead of a molecule targeting IL-23, such as guselkumab [Tremfya], for example.”
“I would have liked to see a head-to-head comparison with a molecule that blocks the same target,” said Dr. Trakatelli, chair of the EADV education committee.
Dr. Papp countered that “there are various reasons for running comparator studies.” Secukinumab, he said, “was the market leader and was widely used, so it makes sense that one is going to compare against a product as the market lead.”
“Not to say there won’t be future studies” in which mirikizumab is compared “head to head with IL-23s,” Dr. Papp added.
But larger patient numbers and longer treatment times are still needed with mirikizumab “to characterize the level of response, duration of response, and any adverse event profiles,” Dr. Papp stressed.
“One study does not a drug make,” he said. “It’s just exciting that we still have things to offer. This is an important example, and of course opportunity, for patients.”
The trial was funded by Lilly. Dr. Papp disclosed financial relationships with AbbVie, Amgen, Astellas, Valeant, Baxalta, Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Coherus, Dermira, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Lilly, Medimmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Stiefel, Sun Pharma, Takeda, and UCB. Dr. Trakatelli is a speaker for Novartis.
A version of this article originally appeared on Medscape.com.
The investigational monoclonal antibody according to new long-term OASIS-2 trial data.
Both doses of mirikizumab in the international, double-blind trial achieved improvements in Psoriasis Area and Severity Index (PASI) scores in larger numbers of participants at week 52 than secukinumab (Cosentyx), with low adverse event rates.
If approved, mirikizumab, which binds the p19 subunit of IL-23, would join three other IL-23 drugs already marketed in the United States for moderate to severe psoriasis, said OASIS-2 lead investigator Kim A. Papp, MD, PhD, founder and president of Probity Medical Research in Waterloo, Ont.
But Dr. Papp feels larger studies “will be necessary to put these data into perspective,” he said during a presentation at the virtual annual European Academy of Dermatology and Venereology Congress.
“Probably the most important takeaway here is that we may have another option to choose from,” Dr. Papp said in an interview. “People tend to think we have an adequate stable of treatment options, and I would argue we do not.”
“There are variations over time that occur in terms of an individual’s biological response, and the consequence is that nothing we have works for everyone, and nothing we have works forever,” he added. Psoriasis biologics “are increasingly competent, compared to medications we had even 5 or 10 years ago ... but they still don’t satisfy all our needs, so we do need to keep replenishing our stock.”
The multicenter trial included 1,465 patients who were randomly split into four groups. Subcutaneously, one group received 250 mg of mirikizumab every 4 weeks, and then 250 mg of the drug every 8 weeks starting at week 16. Another group received 250 mg of mirikizumab every 4 weeks and then 125 mg every 8 weeks starting at week 16.
The third group received 300 mg of secukinumab weekly for 4 weeks and then every 4 weeks starting at week 4. The last group received placebo every 4 weeks, and then 250 mg of mirikizumab every 4 weeks from week 16 to 32 and every 8 weeks thereafter.
Primary endpoints measured the percentage of patients achieving a static Physician’s Global Assessment (sPGA) of 0 or 1, with an improvement of at least 2 points from baseline; and the proportion of patients with PASI 90 at week 16, compared with placebo.
Major secondary endpoints were PASI 75 and PASI 100, compared with placebo at week 16; an sPGA of 0 or 1 and PASI 90 noninferiority, compared with secukinumab at week 16; and sPGA of 0 or 1, PASI 90, and PASI 100 superiority, compared with secukinumab at week 52.
More than 91% of participants completed all 52 weeks in the trial. Mirikizumab met primary endpoints compared with placebo and major secondary endpoints vs secukinumab at week 16 (P < .001). PASI 90 and sPGA (0,1) response rates far exceeded placebo for both 250 mg mirikizumab (74.4% and 79.7%, respectively) and secukinumab (72.8% and 76.3%, respectively).
At week 52, major secondary endpoints for both mirikizumab doses were superior to secukinumab (all P < .001). PASI 90 was achieved by 81.4% of 125 mg and 82.4% of 250 mg mirikizumab patients versus 69.4% of secukinumab patients; sPGA (0,1) by 83.1% of 125 mg and 83.3% of 250 mg mirikizumab patients versus 68.5% of secukinumab patients; and PASI 100 by 53.9% of 125 mg and 58.8% of 250 mg mirikizumab patients versus 42.9% of secukinumab patients.
Treatment-associated adverse effects were similar across all treatment groups and study periods. The most common were nasopharyngitis, upper respiratory tract infection, headache, back pain, and arthralgia. But serious adverse effects were minimal, Dr. Papp said. One death occurred in a mirikizumab patient from acute MI, which was deemed unrelated to the study drug.
Myrto Georgia Trakatelli, MD, PhD, from Aristotle University of Thessaloniki (Greece), said the results indicate that dermatologists “should not be afraid to use” mirikizumab long term if it is approved by the Food and Drug Administration.
“Sometimes patients use many treatments for a long time and all of a sudden, they stop working,” Dr. Trakatelli said in an interview. “A new biologic is always welcome because we do see patients not responding to other treatment.”
But Dr. Trakatelli said “a point that troubled me in the study” was that mirikizumab was compared with an IL-17 inhibitor “instead of a molecule targeting IL-23, such as guselkumab [Tremfya], for example.”
“I would have liked to see a head-to-head comparison with a molecule that blocks the same target,” said Dr. Trakatelli, chair of the EADV education committee.
Dr. Papp countered that “there are various reasons for running comparator studies.” Secukinumab, he said, “was the market leader and was widely used, so it makes sense that one is going to compare against a product as the market lead.”
“Not to say there won’t be future studies” in which mirikizumab is compared “head to head with IL-23s,” Dr. Papp added.
But larger patient numbers and longer treatment times are still needed with mirikizumab “to characterize the level of response, duration of response, and any adverse event profiles,” Dr. Papp stressed.
“One study does not a drug make,” he said. “It’s just exciting that we still have things to offer. This is an important example, and of course opportunity, for patients.”
The trial was funded by Lilly. Dr. Papp disclosed financial relationships with AbbVie, Amgen, Astellas, Valeant, Baxalta, Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Coherus, Dermira, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Lilly, Medimmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Stiefel, Sun Pharma, Takeda, and UCB. Dr. Trakatelli is a speaker for Novartis.
A version of this article originally appeared on Medscape.com.
The investigational monoclonal antibody according to new long-term OASIS-2 trial data.
Both doses of mirikizumab in the international, double-blind trial achieved improvements in Psoriasis Area and Severity Index (PASI) scores in larger numbers of participants at week 52 than secukinumab (Cosentyx), with low adverse event rates.
If approved, mirikizumab, which binds the p19 subunit of IL-23, would join three other IL-23 drugs already marketed in the United States for moderate to severe psoriasis, said OASIS-2 lead investigator Kim A. Papp, MD, PhD, founder and president of Probity Medical Research in Waterloo, Ont.
But Dr. Papp feels larger studies “will be necessary to put these data into perspective,” he said during a presentation at the virtual annual European Academy of Dermatology and Venereology Congress.
“Probably the most important takeaway here is that we may have another option to choose from,” Dr. Papp said in an interview. “People tend to think we have an adequate stable of treatment options, and I would argue we do not.”
“There are variations over time that occur in terms of an individual’s biological response, and the consequence is that nothing we have works for everyone, and nothing we have works forever,” he added. Psoriasis biologics “are increasingly competent, compared to medications we had even 5 or 10 years ago ... but they still don’t satisfy all our needs, so we do need to keep replenishing our stock.”
The multicenter trial included 1,465 patients who were randomly split into four groups. Subcutaneously, one group received 250 mg of mirikizumab every 4 weeks, and then 250 mg of the drug every 8 weeks starting at week 16. Another group received 250 mg of mirikizumab every 4 weeks and then 125 mg every 8 weeks starting at week 16.
The third group received 300 mg of secukinumab weekly for 4 weeks and then every 4 weeks starting at week 4. The last group received placebo every 4 weeks, and then 250 mg of mirikizumab every 4 weeks from week 16 to 32 and every 8 weeks thereafter.
Primary endpoints measured the percentage of patients achieving a static Physician’s Global Assessment (sPGA) of 0 or 1, with an improvement of at least 2 points from baseline; and the proportion of patients with PASI 90 at week 16, compared with placebo.
Major secondary endpoints were PASI 75 and PASI 100, compared with placebo at week 16; an sPGA of 0 or 1 and PASI 90 noninferiority, compared with secukinumab at week 16; and sPGA of 0 or 1, PASI 90, and PASI 100 superiority, compared with secukinumab at week 52.
More than 91% of participants completed all 52 weeks in the trial. Mirikizumab met primary endpoints compared with placebo and major secondary endpoints vs secukinumab at week 16 (P < .001). PASI 90 and sPGA (0,1) response rates far exceeded placebo for both 250 mg mirikizumab (74.4% and 79.7%, respectively) and secukinumab (72.8% and 76.3%, respectively).
At week 52, major secondary endpoints for both mirikizumab doses were superior to secukinumab (all P < .001). PASI 90 was achieved by 81.4% of 125 mg and 82.4% of 250 mg mirikizumab patients versus 69.4% of secukinumab patients; sPGA (0,1) by 83.1% of 125 mg and 83.3% of 250 mg mirikizumab patients versus 68.5% of secukinumab patients; and PASI 100 by 53.9% of 125 mg and 58.8% of 250 mg mirikizumab patients versus 42.9% of secukinumab patients.
Treatment-associated adverse effects were similar across all treatment groups and study periods. The most common were nasopharyngitis, upper respiratory tract infection, headache, back pain, and arthralgia. But serious adverse effects were minimal, Dr. Papp said. One death occurred in a mirikizumab patient from acute MI, which was deemed unrelated to the study drug.
Myrto Georgia Trakatelli, MD, PhD, from Aristotle University of Thessaloniki (Greece), said the results indicate that dermatologists “should not be afraid to use” mirikizumab long term if it is approved by the Food and Drug Administration.
“Sometimes patients use many treatments for a long time and all of a sudden, they stop working,” Dr. Trakatelli said in an interview. “A new biologic is always welcome because we do see patients not responding to other treatment.”
But Dr. Trakatelli said “a point that troubled me in the study” was that mirikizumab was compared with an IL-17 inhibitor “instead of a molecule targeting IL-23, such as guselkumab [Tremfya], for example.”
“I would have liked to see a head-to-head comparison with a molecule that blocks the same target,” said Dr. Trakatelli, chair of the EADV education committee.
Dr. Papp countered that “there are various reasons for running comparator studies.” Secukinumab, he said, “was the market leader and was widely used, so it makes sense that one is going to compare against a product as the market lead.”
“Not to say there won’t be future studies” in which mirikizumab is compared “head to head with IL-23s,” Dr. Papp added.
But larger patient numbers and longer treatment times are still needed with mirikizumab “to characterize the level of response, duration of response, and any adverse event profiles,” Dr. Papp stressed.
“One study does not a drug make,” he said. “It’s just exciting that we still have things to offer. This is an important example, and of course opportunity, for patients.”
The trial was funded by Lilly. Dr. Papp disclosed financial relationships with AbbVie, Amgen, Astellas, Valeant, Baxalta, Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Coherus, Dermira, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Lilly, Medimmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Stiefel, Sun Pharma, Takeda, and UCB. Dr. Trakatelli is a speaker for Novartis.
A version of this article originally appeared on Medscape.com.
FROM THE EADV CONGRESS
Health sector has spent $464 million on lobbying in 2020
, according to the Center for Responsive Politics.
PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
Hand eczema: Pan-JAK inhibitor delgocitinib shows dose-dependent response in phase 2b trial
a new international phase 2b research suggests.
An investigational pan–Janus kinase inhibitor that blocks all four members of the JAK family, twice-daily delgocitinib doses of 8 mg/g and 20 mg/g demonstrated the highest efficacy in adults with mild to severe chronic hand eczema. By week 16, nearly 40% of patients receiving either dose were clear or almost clear of symptoms.
“By mode of action, we think delgocitinib is more selective in the way of acting,” said lead investigator Margitta Worm, MD, PhD, of the department of dermatology, venereology, and allergology at Charité University Hospital in Berlin, during a presentation of the results at the virtual annual congress of the European Academy of Dermatology and Venereology.
“We do know that JAKs play an important role in chronic inflammation and interfering with the JAK pathway can have anti-inflammatory effects,” Dr. Worm said in an interview. “Whenever it’s possible to use a molecule topically or locally, it’s advantageous for patients because it’s only acting where you apply it and there are no systemic side effects.”
Defined as lasting more than 3 months or relapsing twice or more within a year, chronic hand eczema is a particularly problematic form of atopic dermatitis because “we need our hands every day for almost every activity, so having eczema on your hands has a huge impact on quality of life,” Dr. Worm said.
Many people whose hands are integral to their occupations also have trouble working because of the disorder, she explained. But current topical treatments are limited to emollients, corticosteroids, and calcineurin inhibitors.
“Topical corticosteroids are efficacious, but can cause skin atrophy,” she said. “Their long-term side-effect profile limits their use.”
The number of patients in each treatment group was too small to focus on different subtypes of chronic hand eczema, “but this is something that will probably be looked at in the future,” Dr. Worm said. “At the moment it’s nice to see a dose-dependent clinical efficacy and good tolerability, and now we have to wait for phase 3 data in the future.”
Dr. Worm and colleagues aimed to establish the dose-response relationship of twice-daily applications of delgocitinib cream in doses of 1, 3, 8, and 20 mg/g and a delgocitinib cream vehicle for 16 weeks. The 258 participants (61% women; average age, 46 years) were randomly assigned in equal groups to each dose of delgocitinib cream or the vehicle cream twice daily at centers in Denmark, Germany, and the United States.
The primary endpoint for the double-blind, 26-center trial was the proportion of patients who achieved an Investigator’s Global Assessment score of 0 (“clear”) or 1 (“almost clear”), with a 2-point or higher improvement from baseline over the study period. A key secondary endpoint was a change in the Hand Eczema Severity Index (HECSI) from baseline to week 16.
At week 16, a statistically significant dose response was established for both primary and secondary endpoints (P < .025). More patients in the delgocitinib 8-mg/g and 20-mg/g groups met the primary endpoint (36.5% and 37.7%, respectively) than patients in the 1-mg/g and 3-mg/g groups (21.2% and 7.8%, respectively) and vehicle group (8%, P = .0004).
This primary skin clearance effect at week 16 was demonstrated from week 4 in the 8-mg/g group and week 6 in the 20-mg/g group. But all active doses achieved a statistically significant greater jump in HECSI from baseline to week 16 than the vehicle cream (P < .05).
“The strength of the trial is that there were different concentrations of the substance used,” Dr. Worm said. “When you look to the results, you can demonstrate a dose-dependent clinical efficacy. This is of great value to really compare the efficacy of single doses.”
Most adverse events reported were not considered treatment related and were mild or moderate. The most frequently reported side effects were nasopharyngitis, eczema, and headache.
Commenting on the results, Asli Bilgic, MD, from Akdeniz University in Antalya, Turkey, who was not involved with the study, said that phase 3 studies of delgocitinib should probe further into the effects of the 8-mg/g dosage in this patient group since it appears to show similar efficacy and safety to 20 mg/g.
It’s important for research to focus on hand eczema “because it’s a very common disease, and treatment options are really sparse,” Dr. Bilgic said in an interview.
“Especially in the COVID era, many health care professionals, along with cleaning, catering, and mechanical jobs” are essential workers affected by the condition, she said. “It affects people’s self-esteem and their ability to do their job.”
The study was funded by LEO Pharma. Dr. Worm received lecture honoraria from LEO Pharma. Dr. Bilgic disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a new international phase 2b research suggests.
An investigational pan–Janus kinase inhibitor that blocks all four members of the JAK family, twice-daily delgocitinib doses of 8 mg/g and 20 mg/g demonstrated the highest efficacy in adults with mild to severe chronic hand eczema. By week 16, nearly 40% of patients receiving either dose were clear or almost clear of symptoms.
“By mode of action, we think delgocitinib is more selective in the way of acting,” said lead investigator Margitta Worm, MD, PhD, of the department of dermatology, venereology, and allergology at Charité University Hospital in Berlin, during a presentation of the results at the virtual annual congress of the European Academy of Dermatology and Venereology.
“We do know that JAKs play an important role in chronic inflammation and interfering with the JAK pathway can have anti-inflammatory effects,” Dr. Worm said in an interview. “Whenever it’s possible to use a molecule topically or locally, it’s advantageous for patients because it’s only acting where you apply it and there are no systemic side effects.”
Defined as lasting more than 3 months or relapsing twice or more within a year, chronic hand eczema is a particularly problematic form of atopic dermatitis because “we need our hands every day for almost every activity, so having eczema on your hands has a huge impact on quality of life,” Dr. Worm said.
Many people whose hands are integral to their occupations also have trouble working because of the disorder, she explained. But current topical treatments are limited to emollients, corticosteroids, and calcineurin inhibitors.
“Topical corticosteroids are efficacious, but can cause skin atrophy,” she said. “Their long-term side-effect profile limits their use.”
The number of patients in each treatment group was too small to focus on different subtypes of chronic hand eczema, “but this is something that will probably be looked at in the future,” Dr. Worm said. “At the moment it’s nice to see a dose-dependent clinical efficacy and good tolerability, and now we have to wait for phase 3 data in the future.”
Dr. Worm and colleagues aimed to establish the dose-response relationship of twice-daily applications of delgocitinib cream in doses of 1, 3, 8, and 20 mg/g and a delgocitinib cream vehicle for 16 weeks. The 258 participants (61% women; average age, 46 years) were randomly assigned in equal groups to each dose of delgocitinib cream or the vehicle cream twice daily at centers in Denmark, Germany, and the United States.
The primary endpoint for the double-blind, 26-center trial was the proportion of patients who achieved an Investigator’s Global Assessment score of 0 (“clear”) or 1 (“almost clear”), with a 2-point or higher improvement from baseline over the study period. A key secondary endpoint was a change in the Hand Eczema Severity Index (HECSI) from baseline to week 16.
At week 16, a statistically significant dose response was established for both primary and secondary endpoints (P < .025). More patients in the delgocitinib 8-mg/g and 20-mg/g groups met the primary endpoint (36.5% and 37.7%, respectively) than patients in the 1-mg/g and 3-mg/g groups (21.2% and 7.8%, respectively) and vehicle group (8%, P = .0004).
This primary skin clearance effect at week 16 was demonstrated from week 4 in the 8-mg/g group and week 6 in the 20-mg/g group. But all active doses achieved a statistically significant greater jump in HECSI from baseline to week 16 than the vehicle cream (P < .05).
“The strength of the trial is that there were different concentrations of the substance used,” Dr. Worm said. “When you look to the results, you can demonstrate a dose-dependent clinical efficacy. This is of great value to really compare the efficacy of single doses.”
Most adverse events reported were not considered treatment related and were mild or moderate. The most frequently reported side effects were nasopharyngitis, eczema, and headache.
Commenting on the results, Asli Bilgic, MD, from Akdeniz University in Antalya, Turkey, who was not involved with the study, said that phase 3 studies of delgocitinib should probe further into the effects of the 8-mg/g dosage in this patient group since it appears to show similar efficacy and safety to 20 mg/g.
It’s important for research to focus on hand eczema “because it’s a very common disease, and treatment options are really sparse,” Dr. Bilgic said in an interview.
“Especially in the COVID era, many health care professionals, along with cleaning, catering, and mechanical jobs” are essential workers affected by the condition, she said. “It affects people’s self-esteem and their ability to do their job.”
The study was funded by LEO Pharma. Dr. Worm received lecture honoraria from LEO Pharma. Dr. Bilgic disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a new international phase 2b research suggests.
An investigational pan–Janus kinase inhibitor that blocks all four members of the JAK family, twice-daily delgocitinib doses of 8 mg/g and 20 mg/g demonstrated the highest efficacy in adults with mild to severe chronic hand eczema. By week 16, nearly 40% of patients receiving either dose were clear or almost clear of symptoms.
“By mode of action, we think delgocitinib is more selective in the way of acting,” said lead investigator Margitta Worm, MD, PhD, of the department of dermatology, venereology, and allergology at Charité University Hospital in Berlin, during a presentation of the results at the virtual annual congress of the European Academy of Dermatology and Venereology.
“We do know that JAKs play an important role in chronic inflammation and interfering with the JAK pathway can have anti-inflammatory effects,” Dr. Worm said in an interview. “Whenever it’s possible to use a molecule topically or locally, it’s advantageous for patients because it’s only acting where you apply it and there are no systemic side effects.”
Defined as lasting more than 3 months or relapsing twice or more within a year, chronic hand eczema is a particularly problematic form of atopic dermatitis because “we need our hands every day for almost every activity, so having eczema on your hands has a huge impact on quality of life,” Dr. Worm said.
Many people whose hands are integral to their occupations also have trouble working because of the disorder, she explained. But current topical treatments are limited to emollients, corticosteroids, and calcineurin inhibitors.
“Topical corticosteroids are efficacious, but can cause skin atrophy,” she said. “Their long-term side-effect profile limits their use.”
The number of patients in each treatment group was too small to focus on different subtypes of chronic hand eczema, “but this is something that will probably be looked at in the future,” Dr. Worm said. “At the moment it’s nice to see a dose-dependent clinical efficacy and good tolerability, and now we have to wait for phase 3 data in the future.”
Dr. Worm and colleagues aimed to establish the dose-response relationship of twice-daily applications of delgocitinib cream in doses of 1, 3, 8, and 20 mg/g and a delgocitinib cream vehicle for 16 weeks. The 258 participants (61% women; average age, 46 years) were randomly assigned in equal groups to each dose of delgocitinib cream or the vehicle cream twice daily at centers in Denmark, Germany, and the United States.
The primary endpoint for the double-blind, 26-center trial was the proportion of patients who achieved an Investigator’s Global Assessment score of 0 (“clear”) or 1 (“almost clear”), with a 2-point or higher improvement from baseline over the study period. A key secondary endpoint was a change in the Hand Eczema Severity Index (HECSI) from baseline to week 16.
At week 16, a statistically significant dose response was established for both primary and secondary endpoints (P < .025). More patients in the delgocitinib 8-mg/g and 20-mg/g groups met the primary endpoint (36.5% and 37.7%, respectively) than patients in the 1-mg/g and 3-mg/g groups (21.2% and 7.8%, respectively) and vehicle group (8%, P = .0004).
This primary skin clearance effect at week 16 was demonstrated from week 4 in the 8-mg/g group and week 6 in the 20-mg/g group. But all active doses achieved a statistically significant greater jump in HECSI from baseline to week 16 than the vehicle cream (P < .05).
“The strength of the trial is that there were different concentrations of the substance used,” Dr. Worm said. “When you look to the results, you can demonstrate a dose-dependent clinical efficacy. This is of great value to really compare the efficacy of single doses.”
Most adverse events reported were not considered treatment related and were mild or moderate. The most frequently reported side effects were nasopharyngitis, eczema, and headache.
Commenting on the results, Asli Bilgic, MD, from Akdeniz University in Antalya, Turkey, who was not involved with the study, said that phase 3 studies of delgocitinib should probe further into the effects of the 8-mg/g dosage in this patient group since it appears to show similar efficacy and safety to 20 mg/g.
It’s important for research to focus on hand eczema “because it’s a very common disease, and treatment options are really sparse,” Dr. Bilgic said in an interview.
“Especially in the COVID era, many health care professionals, along with cleaning, catering, and mechanical jobs” are essential workers affected by the condition, she said. “It affects people’s self-esteem and their ability to do their job.”
The study was funded by LEO Pharma. Dr. Worm received lecture honoraria from LEO Pharma. Dr. Bilgic disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE EADV CONGRESS
Med student’s cardiac crisis a COVID-era medical mystery
Within minutes of her arrival at Community North Hospital in Indianapolis, Ramya Yeleti’s vital signs plummeted; her pulse was at 45 beats per minute and her ejection fraction was hovering near 10%. “I definitely thought there was a chance I would close my eyes and never open them again, but I only had a few seconds to process that,” she recalled. Then everything went black. Ramya fell unconscious as shock pads were positioned and a swarm of clinicians prepared to insert an Impella heart pump through a catheter into her aorta.
The third-year medical student and aspiring psychiatrist had been doing in-person neurology rotations in July when she began to experience fever and uncontrolled vomiting. Her initial thought was that she must have caught the flu from a patient.
After all, Ramya, along with her father Ram Yeleti, MD, mother Indira, and twin sister Divya, had all weathered COVID-19 in previous months and later tested positive for SARS-CoV-2 antibodies. The only family member who had been spared was her younger brother Rohith.
Indira suffered a severe case, requiring ICU care for 2 days but no ventilator; the others experienced mostly mild symptoms. Ramya — who was studying for her third-year board exams after classes at Marian University College of Osteopathic Medicine in Indianapolis went virtual in March — was left with lingering fatigue; however, her cough and muscle aches abated and her sense of taste and smell returned. When she started rotations, she thought her life was getting back to normal.
Ramya’s flu symptoms did not improve. A university-mandated rapid COVID test came back negative, but 2 more days of vomiting started to worry both her and her father, who is a cardiologist and chief physician executive at Community Health Network in Indianapolis. After Ramya felt some chest pain, she asked her father to listen to her heart. All sounded normal, and Ram prescribed ondansetron for her nausea.
But the antiemetic didn’t work, and by the next morning both father and daughter were convinced that they needed to head to the emergency department.
“I wanted to double-check if I was missing something about her being dehydrated,” Ram told Medscape Medical News. “Several things can cause protracted nausea, like hepatitis, appendicitis, or another infection. I feel terribly guilty I didn’t realize she had a heart condition.”
A surprising turn for the worst
Ramya’s subtle symptoms quickly gave way to the dramatic cardiac crisis that unfolded just after her arrival at Community North. “Her EKG looked absolutely horrendous, like a 75-year-old having a heart attack,” Ram said.
As a cardiologist, he knew his daughter’s situation was growing dire when he heard physicians shouting that the Impella wasn’t working and she needed extracorporeal membrane oxygenation (ECMO).
“At that point, I didn’t think she’d survive,” her father recalled. “We had 10 physicians in the room who worked on her for 5 hours to get her stabilized.”
“It was especially traumatic because, obviously, I knew exactly what was happening,” he added. “You can’t sugarcoat anything.”
After being connected to the heart–lung equipment, Ramya was transferred to IU Health Methodist Hospital, also in Indianapolis, where she was tested again for COVID-19. Unlike the rapid test administered just days earlier, the PCR assay came back positive.
“I knew she had acute myocarditis, but coronavirus never crossed my mind,” said Ram.
“As we were dealing with her heart, we were also dealing with this challenge: she was coming back positive for COVID-19 again,” said Roopa Rao, MD, the heart failure transplant cardiologist at IU Health who treated Ramya.
“We weren’t sure whether we were dealing with an active infection or dead virus” from her previous infection, Rao said, “so we started treating her like she had active COVID-19 and gave her remdesivir, convalescent plasma, and steroids, which was the protocol in our hospital.”
A biopsy of Ramya’s heart tissue, along with blood tests, indicated a past parvovirus infection. It’s possible that Ramya’s previous coronavirus infection made her susceptible to heart damage from a newer parvovirus infection, said Rao. Either virus, or both together, could have been responsible for the calamity.
Although it was unheard of during Ramya’s cardiac crisis in early August, evolving evidence now raises the possibility that she is one of a handful of people in the world to be reinfected with SARS-CoV-2. Also emerging are cases of COVID-related myocarditis and other extreme heart complications, particularly in young people.
“At the time, it wasn’t really clear if people could have another infection so quickly,” Rao told Medscape Medical News. “It is possible she is one of these rare individuals to have COVID-19 twice. I’m hoping at some point we will have some clarity.”
“I would favor a coinfection as probably the triggering factor for her sickness,” she said. “It may take some time, but like any other disease — and it doesn’t look like COVID will go away magically — I hope we’ll have some answers down the road.”
Another wrinkle
The next 48 hours brought astonishing news: Ramya’s heart function had rebounded to nearly normal, and her ejection fraction increased to about 45%. Heart transplantation wouldn’t be necessary, although Rao stood poised to follow through if ECMO only sustained, rather than improved, Ramya’s prognosis.
“Ramya was so sick that if she didn’t recover, the only option would be a heart transplant,” said Rao. “But we wanted to do everything to keep that heart.”
After steroid and COVID treatment, Ramya’s heart started to come back. “It didn’t make sense to me,” said Rao. “I don’t know what helped. If we hadn’t done ECMO, her heart probably wouldn’t have recovered, so I would say we have to support these patients and give them time for the heart to recover, even to the point of ECMO.”
Despite the good news, Ramya’s survival still hung in the balance. When she was disconnected from ECMO, clinicians discovered that the Impella device had caused a rare complication, damaging her mitral valve. The valve could be repaired surgically, but both Rao and Ram felt great trepidation at the prospect of cardiopulmonary bypass during the open-heart procedure.
“They would need to stop her heart and restart it, and I was concerned it would not restart,” Ram explained. “I didn’t like the idea of open-heart surgery, but my biggest fear was she was not going to survive it because of a really fresh, sick heart.”
The cardiologists’ fears did, in fact, come to pass: it took an hour to coax Ramya’s heart back at the end of surgery. But, just as the surgeon was preparing to reconnect Ramya to ECMO in desperation, “her heart recovered again,” Rao reported.
“Some things you never forget in life,” she said. “I can’t describe how everyone in the OR felt, all taking care of her. I told Ramya, ‘you are a fighter’.”
New strength
Six days would pass before Ramya woke up and learned of the astounding series of events that saved her. She knew “something was really wrong” because of the incision at the center of her chest, but learning she’d been on ECMO and the heart transplant list drove home how close to death she’d actually come.
“Most people don’t get off ECMO; they die on it,” she said. “And the chances of dying on the heart transplant list are very high. It was very strange to me that this was my story all of a sudden, when a week and a half earlier I was on rotation.”
Ongoing physical therapy over the past 3 months has transformed Ramya from a state of profound physical weakness to a place of relative strength. The now-fourth-year med student is turning 26 in November and is hungry to restart in-person rotations. Her downtime has been filled in part with researching myocarditis and collaborating with Rao on her own case study for journal publication.
But the mental trauma from her experience has girded her in ways she knows will make her stronger personally and professionally in the years ahead.
“It’s still very hard. I’m still recovering,” she acknowledged. “I described it to my therapist as an invisible wound on my brain.”
“When I came out of the hospital, I still had ECMO wounds, deep gashes on my legs that affected how fast and how long I could walk,” she said. “I felt like the same thing was going on my brain — a huge cut no one could see.”
Her intention to specialize in psychiatry has become more pressing now that Ramya has realized the impact of trauma on mental health.
“My body failing me was awful, but I could handle it,” she said. “Losing any part of my mind would have been way worse. I want to take care of that in my patients.”
This article first appeared on Medscape.com.
Within minutes of her arrival at Community North Hospital in Indianapolis, Ramya Yeleti’s vital signs plummeted; her pulse was at 45 beats per minute and her ejection fraction was hovering near 10%. “I definitely thought there was a chance I would close my eyes and never open them again, but I only had a few seconds to process that,” she recalled. Then everything went black. Ramya fell unconscious as shock pads were positioned and a swarm of clinicians prepared to insert an Impella heart pump through a catheter into her aorta.
The third-year medical student and aspiring psychiatrist had been doing in-person neurology rotations in July when she began to experience fever and uncontrolled vomiting. Her initial thought was that she must have caught the flu from a patient.
After all, Ramya, along with her father Ram Yeleti, MD, mother Indira, and twin sister Divya, had all weathered COVID-19 in previous months and later tested positive for SARS-CoV-2 antibodies. The only family member who had been spared was her younger brother Rohith.
Indira suffered a severe case, requiring ICU care for 2 days but no ventilator; the others experienced mostly mild symptoms. Ramya — who was studying for her third-year board exams after classes at Marian University College of Osteopathic Medicine in Indianapolis went virtual in March — was left with lingering fatigue; however, her cough and muscle aches abated and her sense of taste and smell returned. When she started rotations, she thought her life was getting back to normal.
Ramya’s flu symptoms did not improve. A university-mandated rapid COVID test came back negative, but 2 more days of vomiting started to worry both her and her father, who is a cardiologist and chief physician executive at Community Health Network in Indianapolis. After Ramya felt some chest pain, she asked her father to listen to her heart. All sounded normal, and Ram prescribed ondansetron for her nausea.
But the antiemetic didn’t work, and by the next morning both father and daughter were convinced that they needed to head to the emergency department.
“I wanted to double-check if I was missing something about her being dehydrated,” Ram told Medscape Medical News. “Several things can cause protracted nausea, like hepatitis, appendicitis, or another infection. I feel terribly guilty I didn’t realize she had a heart condition.”
A surprising turn for the worst
Ramya’s subtle symptoms quickly gave way to the dramatic cardiac crisis that unfolded just after her arrival at Community North. “Her EKG looked absolutely horrendous, like a 75-year-old having a heart attack,” Ram said.
As a cardiologist, he knew his daughter’s situation was growing dire when he heard physicians shouting that the Impella wasn’t working and she needed extracorporeal membrane oxygenation (ECMO).
“At that point, I didn’t think she’d survive,” her father recalled. “We had 10 physicians in the room who worked on her for 5 hours to get her stabilized.”
“It was especially traumatic because, obviously, I knew exactly what was happening,” he added. “You can’t sugarcoat anything.”
After being connected to the heart–lung equipment, Ramya was transferred to IU Health Methodist Hospital, also in Indianapolis, where she was tested again for COVID-19. Unlike the rapid test administered just days earlier, the PCR assay came back positive.
“I knew she had acute myocarditis, but coronavirus never crossed my mind,” said Ram.
“As we were dealing with her heart, we were also dealing with this challenge: she was coming back positive for COVID-19 again,” said Roopa Rao, MD, the heart failure transplant cardiologist at IU Health who treated Ramya.
“We weren’t sure whether we were dealing with an active infection or dead virus” from her previous infection, Rao said, “so we started treating her like she had active COVID-19 and gave her remdesivir, convalescent plasma, and steroids, which was the protocol in our hospital.”
A biopsy of Ramya’s heart tissue, along with blood tests, indicated a past parvovirus infection. It’s possible that Ramya’s previous coronavirus infection made her susceptible to heart damage from a newer parvovirus infection, said Rao. Either virus, or both together, could have been responsible for the calamity.
Although it was unheard of during Ramya’s cardiac crisis in early August, evolving evidence now raises the possibility that she is one of a handful of people in the world to be reinfected with SARS-CoV-2. Also emerging are cases of COVID-related myocarditis and other extreme heart complications, particularly in young people.
“At the time, it wasn’t really clear if people could have another infection so quickly,” Rao told Medscape Medical News. “It is possible she is one of these rare individuals to have COVID-19 twice. I’m hoping at some point we will have some clarity.”
“I would favor a coinfection as probably the triggering factor for her sickness,” she said. “It may take some time, but like any other disease — and it doesn’t look like COVID will go away magically — I hope we’ll have some answers down the road.”
Another wrinkle
The next 48 hours brought astonishing news: Ramya’s heart function had rebounded to nearly normal, and her ejection fraction increased to about 45%. Heart transplantation wouldn’t be necessary, although Rao stood poised to follow through if ECMO only sustained, rather than improved, Ramya’s prognosis.
“Ramya was so sick that if she didn’t recover, the only option would be a heart transplant,” said Rao. “But we wanted to do everything to keep that heart.”
After steroid and COVID treatment, Ramya’s heart started to come back. “It didn’t make sense to me,” said Rao. “I don’t know what helped. If we hadn’t done ECMO, her heart probably wouldn’t have recovered, so I would say we have to support these patients and give them time for the heart to recover, even to the point of ECMO.”
Despite the good news, Ramya’s survival still hung in the balance. When she was disconnected from ECMO, clinicians discovered that the Impella device had caused a rare complication, damaging her mitral valve. The valve could be repaired surgically, but both Rao and Ram felt great trepidation at the prospect of cardiopulmonary bypass during the open-heart procedure.
“They would need to stop her heart and restart it, and I was concerned it would not restart,” Ram explained. “I didn’t like the idea of open-heart surgery, but my biggest fear was she was not going to survive it because of a really fresh, sick heart.”
The cardiologists’ fears did, in fact, come to pass: it took an hour to coax Ramya’s heart back at the end of surgery. But, just as the surgeon was preparing to reconnect Ramya to ECMO in desperation, “her heart recovered again,” Rao reported.
“Some things you never forget in life,” she said. “I can’t describe how everyone in the OR felt, all taking care of her. I told Ramya, ‘you are a fighter’.”
New strength
Six days would pass before Ramya woke up and learned of the astounding series of events that saved her. She knew “something was really wrong” because of the incision at the center of her chest, but learning she’d been on ECMO and the heart transplant list drove home how close to death she’d actually come.
“Most people don’t get off ECMO; they die on it,” she said. “And the chances of dying on the heart transplant list are very high. It was very strange to me that this was my story all of a sudden, when a week and a half earlier I was on rotation.”
Ongoing physical therapy over the past 3 months has transformed Ramya from a state of profound physical weakness to a place of relative strength. The now-fourth-year med student is turning 26 in November and is hungry to restart in-person rotations. Her downtime has been filled in part with researching myocarditis and collaborating with Rao on her own case study for journal publication.
But the mental trauma from her experience has girded her in ways she knows will make her stronger personally and professionally in the years ahead.
“It’s still very hard. I’m still recovering,” she acknowledged. “I described it to my therapist as an invisible wound on my brain.”
“When I came out of the hospital, I still had ECMO wounds, deep gashes on my legs that affected how fast and how long I could walk,” she said. “I felt like the same thing was going on my brain — a huge cut no one could see.”
Her intention to specialize in psychiatry has become more pressing now that Ramya has realized the impact of trauma on mental health.
“My body failing me was awful, but I could handle it,” she said. “Losing any part of my mind would have been way worse. I want to take care of that in my patients.”
This article first appeared on Medscape.com.
Within minutes of her arrival at Community North Hospital in Indianapolis, Ramya Yeleti’s vital signs plummeted; her pulse was at 45 beats per minute and her ejection fraction was hovering near 10%. “I definitely thought there was a chance I would close my eyes and never open them again, but I only had a few seconds to process that,” she recalled. Then everything went black. Ramya fell unconscious as shock pads were positioned and a swarm of clinicians prepared to insert an Impella heart pump through a catheter into her aorta.
The third-year medical student and aspiring psychiatrist had been doing in-person neurology rotations in July when she began to experience fever and uncontrolled vomiting. Her initial thought was that she must have caught the flu from a patient.
After all, Ramya, along with her father Ram Yeleti, MD, mother Indira, and twin sister Divya, had all weathered COVID-19 in previous months and later tested positive for SARS-CoV-2 antibodies. The only family member who had been spared was her younger brother Rohith.
Indira suffered a severe case, requiring ICU care for 2 days but no ventilator; the others experienced mostly mild symptoms. Ramya — who was studying for her third-year board exams after classes at Marian University College of Osteopathic Medicine in Indianapolis went virtual in March — was left with lingering fatigue; however, her cough and muscle aches abated and her sense of taste and smell returned. When she started rotations, she thought her life was getting back to normal.
Ramya’s flu symptoms did not improve. A university-mandated rapid COVID test came back negative, but 2 more days of vomiting started to worry both her and her father, who is a cardiologist and chief physician executive at Community Health Network in Indianapolis. After Ramya felt some chest pain, she asked her father to listen to her heart. All sounded normal, and Ram prescribed ondansetron for her nausea.
But the antiemetic didn’t work, and by the next morning both father and daughter were convinced that they needed to head to the emergency department.
“I wanted to double-check if I was missing something about her being dehydrated,” Ram told Medscape Medical News. “Several things can cause protracted nausea, like hepatitis, appendicitis, or another infection. I feel terribly guilty I didn’t realize she had a heart condition.”
A surprising turn for the worst
Ramya’s subtle symptoms quickly gave way to the dramatic cardiac crisis that unfolded just after her arrival at Community North. “Her EKG looked absolutely horrendous, like a 75-year-old having a heart attack,” Ram said.
As a cardiologist, he knew his daughter’s situation was growing dire when he heard physicians shouting that the Impella wasn’t working and she needed extracorporeal membrane oxygenation (ECMO).
“At that point, I didn’t think she’d survive,” her father recalled. “We had 10 physicians in the room who worked on her for 5 hours to get her stabilized.”
“It was especially traumatic because, obviously, I knew exactly what was happening,” he added. “You can’t sugarcoat anything.”
After being connected to the heart–lung equipment, Ramya was transferred to IU Health Methodist Hospital, also in Indianapolis, where she was tested again for COVID-19. Unlike the rapid test administered just days earlier, the PCR assay came back positive.
“I knew she had acute myocarditis, but coronavirus never crossed my mind,” said Ram.
“As we were dealing with her heart, we were also dealing with this challenge: she was coming back positive for COVID-19 again,” said Roopa Rao, MD, the heart failure transplant cardiologist at IU Health who treated Ramya.
“We weren’t sure whether we were dealing with an active infection or dead virus” from her previous infection, Rao said, “so we started treating her like she had active COVID-19 and gave her remdesivir, convalescent plasma, and steroids, which was the protocol in our hospital.”
A biopsy of Ramya’s heart tissue, along with blood tests, indicated a past parvovirus infection. It’s possible that Ramya’s previous coronavirus infection made her susceptible to heart damage from a newer parvovirus infection, said Rao. Either virus, or both together, could have been responsible for the calamity.
Although it was unheard of during Ramya’s cardiac crisis in early August, evolving evidence now raises the possibility that she is one of a handful of people in the world to be reinfected with SARS-CoV-2. Also emerging are cases of COVID-related myocarditis and other extreme heart complications, particularly in young people.
“At the time, it wasn’t really clear if people could have another infection so quickly,” Rao told Medscape Medical News. “It is possible she is one of these rare individuals to have COVID-19 twice. I’m hoping at some point we will have some clarity.”
“I would favor a coinfection as probably the triggering factor for her sickness,” she said. “It may take some time, but like any other disease — and it doesn’t look like COVID will go away magically — I hope we’ll have some answers down the road.”
Another wrinkle
The next 48 hours brought astonishing news: Ramya’s heart function had rebounded to nearly normal, and her ejection fraction increased to about 45%. Heart transplantation wouldn’t be necessary, although Rao stood poised to follow through if ECMO only sustained, rather than improved, Ramya’s prognosis.
“Ramya was so sick that if she didn’t recover, the only option would be a heart transplant,” said Rao. “But we wanted to do everything to keep that heart.”
After steroid and COVID treatment, Ramya’s heart started to come back. “It didn’t make sense to me,” said Rao. “I don’t know what helped. If we hadn’t done ECMO, her heart probably wouldn’t have recovered, so I would say we have to support these patients and give them time for the heart to recover, even to the point of ECMO.”
Despite the good news, Ramya’s survival still hung in the balance. When she was disconnected from ECMO, clinicians discovered that the Impella device had caused a rare complication, damaging her mitral valve. The valve could be repaired surgically, but both Rao and Ram felt great trepidation at the prospect of cardiopulmonary bypass during the open-heart procedure.
“They would need to stop her heart and restart it, and I was concerned it would not restart,” Ram explained. “I didn’t like the idea of open-heart surgery, but my biggest fear was she was not going to survive it because of a really fresh, sick heart.”
The cardiologists’ fears did, in fact, come to pass: it took an hour to coax Ramya’s heart back at the end of surgery. But, just as the surgeon was preparing to reconnect Ramya to ECMO in desperation, “her heart recovered again,” Rao reported.
“Some things you never forget in life,” she said. “I can’t describe how everyone in the OR felt, all taking care of her. I told Ramya, ‘you are a fighter’.”
New strength
Six days would pass before Ramya woke up and learned of the astounding series of events that saved her. She knew “something was really wrong” because of the incision at the center of her chest, but learning she’d been on ECMO and the heart transplant list drove home how close to death she’d actually come.
“Most people don’t get off ECMO; they die on it,” she said. “And the chances of dying on the heart transplant list are very high. It was very strange to me that this was my story all of a sudden, when a week and a half earlier I was on rotation.”
Ongoing physical therapy over the past 3 months has transformed Ramya from a state of profound physical weakness to a place of relative strength. The now-fourth-year med student is turning 26 in November and is hungry to restart in-person rotations. Her downtime has been filled in part with researching myocarditis and collaborating with Rao on her own case study for journal publication.
But the mental trauma from her experience has girded her in ways she knows will make her stronger personally and professionally in the years ahead.
“It’s still very hard. I’m still recovering,” she acknowledged. “I described it to my therapist as an invisible wound on my brain.”
“When I came out of the hospital, I still had ECMO wounds, deep gashes on my legs that affected how fast and how long I could walk,” she said. “I felt like the same thing was going on my brain — a huge cut no one could see.”
Her intention to specialize in psychiatry has become more pressing now that Ramya has realized the impact of trauma on mental health.
“My body failing me was awful, but I could handle it,” she said. “Losing any part of my mind would have been way worse. I want to take care of that in my patients.”
This article first appeared on Medscape.com.





