User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
RAP device being investigated as a way to improve appearance of cellulite
.
“The procedure is relatively painless, without anesthesia and can easily be delegated with physician oversight,” Mathew M. Avram, MD, JD, said during the virtual annual Masters of Aesthetics Symposium. “Side effects have been minimal and transient to date. There is no down time.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples.
“Basically, what you have is a repetition rate and very short rise time that provide microscopic mechanical disruption to the targeted cellular level structures and vacuoles,” Dr. Avram explained. “There’s a high leak pressure and fast repetition rate that exploits the viscoelastic nature of the tissue. You get compressed pulses from electronic filtering and the reflector shape eliminates cavitation, heat, and pain.”
The procedure takes 20-30 minutes to perform and it generates minimal heat and pain, “which is an advantage of the treatment,” he said. “It is completely noninvasive, with no incision whatsoever. No anesthetic is required. There can be physician oversight of delivery, so it is delegable, and there is no recovery time. More study is needed, and we need to stay tuned.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
.
“The procedure is relatively painless, without anesthesia and can easily be delegated with physician oversight,” Mathew M. Avram, MD, JD, said during the virtual annual Masters of Aesthetics Symposium. “Side effects have been minimal and transient to date. There is no down time.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples.
“Basically, what you have is a repetition rate and very short rise time that provide microscopic mechanical disruption to the targeted cellular level structures and vacuoles,” Dr. Avram explained. “There’s a high leak pressure and fast repetition rate that exploits the viscoelastic nature of the tissue. You get compressed pulses from electronic filtering and the reflector shape eliminates cavitation, heat, and pain.”
The procedure takes 20-30 minutes to perform and it generates minimal heat and pain, “which is an advantage of the treatment,” he said. “It is completely noninvasive, with no incision whatsoever. No anesthetic is required. There can be physician oversight of delivery, so it is delegable, and there is no recovery time. More study is needed, and we need to stay tuned.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
.
“The procedure is relatively painless, without anesthesia and can easily be delegated with physician oversight,” Mathew M. Avram, MD, JD, said during the virtual annual Masters of Aesthetics Symposium. “Side effects have been minimal and transient to date. There is no down time.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples.
“Basically, what you have is a repetition rate and very short rise time that provide microscopic mechanical disruption to the targeted cellular level structures and vacuoles,” Dr. Avram explained. “There’s a high leak pressure and fast repetition rate that exploits the viscoelastic nature of the tissue. You get compressed pulses from electronic filtering and the reflector shape eliminates cavitation, heat, and pain.”
The procedure takes 20-30 minutes to perform and it generates minimal heat and pain, “which is an advantage of the treatment,” he said. “It is completely noninvasive, with no incision whatsoever. No anesthetic is required. There can be physician oversight of delivery, so it is delegable, and there is no recovery time. More study is needed, and we need to stay tuned.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
FROM MOA 2020
Tough to tell COVID from smoke inhalation symptoms — And flu season’s coming
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.
COVID-19 outcomes no worse in patients on TNF inhibitors or methotrexate
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Exorcising your ghosts
The COVID-19 pandemic has affected private medical practices on so many levels, not least of which is the loss of employees to illness, fear of illness, early retirement, and other reasons.
If you’re not hip to the slang, “ghosting” is the situation in which an employee disappears without any warning, notice, or explanation. It usually occurs after a candidate accepts a job offer, and you schedule their first day of work. That day dawns, but the new hire never arrives. Less commonly, an employee who has been with you for some time simply stops showing up and cannot be contacted.
Many employers think of ghosting as a relatively new phenomenon, and blame it on the irresponsibility of younger age groups – millennials, in particular. In fact, it has been an issue for many years across all age groups, and employers often share more of the responsibility than they think.
While total prevention is impossible, there are steps you can take as an employer to minimize ghosting in your practice.
- Your hiring process needs to be efficient. If you wait too long to schedule an interview with a promising candidate or to offer them the job, another job offer could lure them away. At the very least, a lengthy process or a lack of transparency may make the applicant apprehensive about accepting a job with you, particularly if other employers are pursuing them.
- Keep applicants in the loop. Follow up with every candidate; let them know where they are in your hiring process. Applicants who have no clue whether they have a shot at the job are going to start looking elsewhere. And make sure they know the job description and starting salary from the outset.
- Talk to new hires before their first day. Contact them personally to see if they have any questions or concerns, and let them know that you’re looking forward to their arrival.
- Once they start, make them feel welcome. An employee’s first few days on the job set the tone for the rest of the employment relationship. During this time, clearly communicate what the employee can expect from you and what you expect from them. Take time to discuss key issues, such as work schedules, timekeeping practices, how performance is measured, and dress codes. Introduce them to coworkers, and get them started shadowing more experienced staff members.
- Take a hard look at your supervision and your supervisors. Business people like to say that employees don’t quit their job, they quit their boss. If an employee quits – with or without notice – it may very well be because of a poor working relationship with you or the supervisor. To be effective, you and your supervisors need to be diligent in setting goals, managing performance, and applying workplace rules and policies. Numerous third-party companies provide training and guidance in these areas when needed.
- Recognize and reward. As I’ve written many times, positive feedback is a simple, low-cost way to improve employee retention. It demonstrates that you value an employee’s contributions and sets an excellent example for other employees. Effective recognition can come from anyone – including patients – and should be given openly. (Another old adage: “Praise publicly, criticize privately.”) It never hurts to catch an employee doing something right and acknowledge it.
- Don’t jump to conclusions. If a new hire or employee is absent without notice, don’t just assume you’ve been ghosted. There may be extenuating circumstances, such as an emergency or illness. In some states, an employee’s absence is protected under a law where the employee may not be required to provide advance notice, and taking adverse action could violate these laws. Establish procedures for attempting to contact absent employees, and make sure you’re complying with all applicable leave laws before taking any action.
If an employee does abandon their job, think before you act. Comply with all applicable laws. Act consistently with how you’ve handled similar situations in the past. Your attorney should be involved, especially if the decision involves termination. Notify the employee in writing. As with all employment decisions, keep adequate documentation in case the decision is ever challenged, or you need it to support future disciplinary decisions.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. He has no disclosures related to this column. Write to him at [email protected].
The COVID-19 pandemic has affected private medical practices on so many levels, not least of which is the loss of employees to illness, fear of illness, early retirement, and other reasons.
If you’re not hip to the slang, “ghosting” is the situation in which an employee disappears without any warning, notice, or explanation. It usually occurs after a candidate accepts a job offer, and you schedule their first day of work. That day dawns, but the new hire never arrives. Less commonly, an employee who has been with you for some time simply stops showing up and cannot be contacted.
Many employers think of ghosting as a relatively new phenomenon, and blame it on the irresponsibility of younger age groups – millennials, in particular. In fact, it has been an issue for many years across all age groups, and employers often share more of the responsibility than they think.
While total prevention is impossible, there are steps you can take as an employer to minimize ghosting in your practice.
- Your hiring process needs to be efficient. If you wait too long to schedule an interview with a promising candidate or to offer them the job, another job offer could lure them away. At the very least, a lengthy process or a lack of transparency may make the applicant apprehensive about accepting a job with you, particularly if other employers are pursuing them.
- Keep applicants in the loop. Follow up with every candidate; let them know where they are in your hiring process. Applicants who have no clue whether they have a shot at the job are going to start looking elsewhere. And make sure they know the job description and starting salary from the outset.
- Talk to new hires before their first day. Contact them personally to see if they have any questions or concerns, and let them know that you’re looking forward to their arrival.
- Once they start, make them feel welcome. An employee’s first few days on the job set the tone for the rest of the employment relationship. During this time, clearly communicate what the employee can expect from you and what you expect from them. Take time to discuss key issues, such as work schedules, timekeeping practices, how performance is measured, and dress codes. Introduce them to coworkers, and get them started shadowing more experienced staff members.
- Take a hard look at your supervision and your supervisors. Business people like to say that employees don’t quit their job, they quit their boss. If an employee quits – with or without notice – it may very well be because of a poor working relationship with you or the supervisor. To be effective, you and your supervisors need to be diligent in setting goals, managing performance, and applying workplace rules and policies. Numerous third-party companies provide training and guidance in these areas when needed.
- Recognize and reward. As I’ve written many times, positive feedback is a simple, low-cost way to improve employee retention. It demonstrates that you value an employee’s contributions and sets an excellent example for other employees. Effective recognition can come from anyone – including patients – and should be given openly. (Another old adage: “Praise publicly, criticize privately.”) It never hurts to catch an employee doing something right and acknowledge it.
- Don’t jump to conclusions. If a new hire or employee is absent without notice, don’t just assume you’ve been ghosted. There may be extenuating circumstances, such as an emergency or illness. In some states, an employee’s absence is protected under a law where the employee may not be required to provide advance notice, and taking adverse action could violate these laws. Establish procedures for attempting to contact absent employees, and make sure you’re complying with all applicable leave laws before taking any action.
If an employee does abandon their job, think before you act. Comply with all applicable laws. Act consistently with how you’ve handled similar situations in the past. Your attorney should be involved, especially if the decision involves termination. Notify the employee in writing. As with all employment decisions, keep adequate documentation in case the decision is ever challenged, or you need it to support future disciplinary decisions.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. He has no disclosures related to this column. Write to him at [email protected].
The COVID-19 pandemic has affected private medical practices on so many levels, not least of which is the loss of employees to illness, fear of illness, early retirement, and other reasons.
If you’re not hip to the slang, “ghosting” is the situation in which an employee disappears without any warning, notice, or explanation. It usually occurs after a candidate accepts a job offer, and you schedule their first day of work. That day dawns, but the new hire never arrives. Less commonly, an employee who has been with you for some time simply stops showing up and cannot be contacted.
Many employers think of ghosting as a relatively new phenomenon, and blame it on the irresponsibility of younger age groups – millennials, in particular. In fact, it has been an issue for many years across all age groups, and employers often share more of the responsibility than they think.
While total prevention is impossible, there are steps you can take as an employer to minimize ghosting in your practice.
- Your hiring process needs to be efficient. If you wait too long to schedule an interview with a promising candidate or to offer them the job, another job offer could lure them away. At the very least, a lengthy process or a lack of transparency may make the applicant apprehensive about accepting a job with you, particularly if other employers are pursuing them.
- Keep applicants in the loop. Follow up with every candidate; let them know where they are in your hiring process. Applicants who have no clue whether they have a shot at the job are going to start looking elsewhere. And make sure they know the job description and starting salary from the outset.
- Talk to new hires before their first day. Contact them personally to see if they have any questions or concerns, and let them know that you’re looking forward to their arrival.
- Once they start, make them feel welcome. An employee’s first few days on the job set the tone for the rest of the employment relationship. During this time, clearly communicate what the employee can expect from you and what you expect from them. Take time to discuss key issues, such as work schedules, timekeeping practices, how performance is measured, and dress codes. Introduce them to coworkers, and get them started shadowing more experienced staff members.
- Take a hard look at your supervision and your supervisors. Business people like to say that employees don’t quit their job, they quit their boss. If an employee quits – with or without notice – it may very well be because of a poor working relationship with you or the supervisor. To be effective, you and your supervisors need to be diligent in setting goals, managing performance, and applying workplace rules and policies. Numerous third-party companies provide training and guidance in these areas when needed.
- Recognize and reward. As I’ve written many times, positive feedback is a simple, low-cost way to improve employee retention. It demonstrates that you value an employee’s contributions and sets an excellent example for other employees. Effective recognition can come from anyone – including patients – and should be given openly. (Another old adage: “Praise publicly, criticize privately.”) It never hurts to catch an employee doing something right and acknowledge it.
- Don’t jump to conclusions. If a new hire or employee is absent without notice, don’t just assume you’ve been ghosted. There may be extenuating circumstances, such as an emergency or illness. In some states, an employee’s absence is protected under a law where the employee may not be required to provide advance notice, and taking adverse action could violate these laws. Establish procedures for attempting to contact absent employees, and make sure you’re complying with all applicable leave laws before taking any action.
If an employee does abandon their job, think before you act. Comply with all applicable laws. Act consistently with how you’ve handled similar situations in the past. Your attorney should be involved, especially if the decision involves termination. Notify the employee in writing. As with all employment decisions, keep adequate documentation in case the decision is ever challenged, or you need it to support future disciplinary decisions.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. He has no disclosures related to this column. Write to him at [email protected].
Physician income drops, burnout spikes globally in pandemic
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
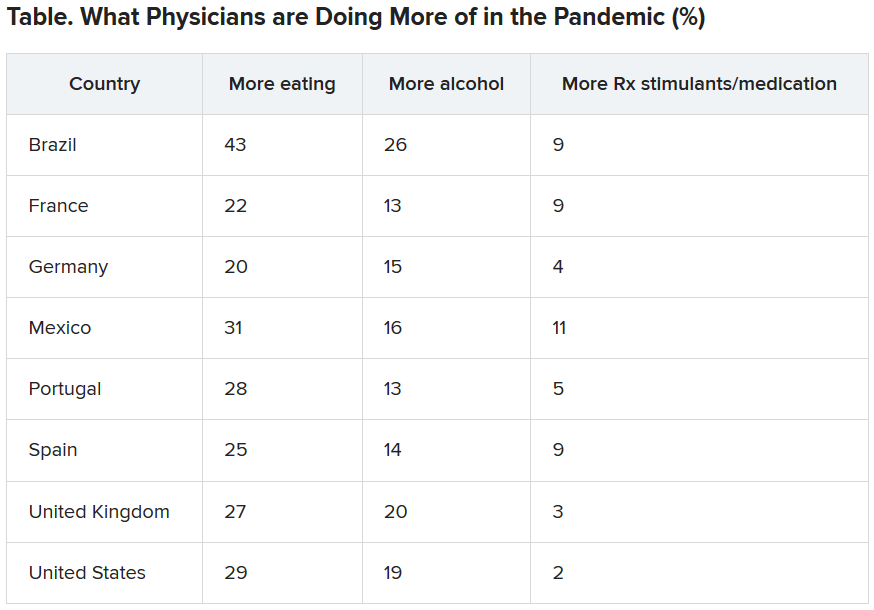
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
Infectious COVID-19 can persist in gut for weeks
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.
Worry over family, friends the main driver of COVID-19 stress
Individuals are more worried about family members becoming ill with COVID-19 or about unknowingly transmitting the disease to family members than they are about contracting it themselves, results of a new survey show.
Investigators surveyed over 3,000 adults, using an online questionnaire. Of the respondents, about 20% were health care workers, and most were living in locations with active stay-at-home orders at the time of the survey.
Close to half of participants were worried about family members contracting the virus, one third were worried about unknowingly infecting others, and 20% were worried about contracting the virus themselves.
“We were a little surprised to see that people were more concerned about others than about themselves, specifically worrying about whether a family member would contract COVID-19 and whether they might unintentionally infect others,” lead author Ran Barzilay, MD, PhD, child and adolescent psychiatrist at the Children’s Hospital of Philadelphia (CHOP), told Medscape Medical News.
The study was published online August 20 in Translational Psychiatry.
Interactive platform
“The pandemic has provided a unique opportunity to study resilience in healthcare professionals and others,” said Barzilay, assistant professor at the Lifespan Brain Institute, a collaboration between CHOP and the University of Pennsylvania, under the directorship of Raquel Gur, MD, PhD.
“After the pandemic broke out in March, we launched a website in early April where we surveyed people for levels of resilience, mental health, and well-being during the outbreak,” he added.
Survey participants then shared it with their contacts.
“To date, over 7000 people have completed it – mostly from the US but also from Israel,” Barzilay said.
The survey was anonymous, but participants could choose to have follow-up contact. The survey included an interactive 21-item resilience questionnaire and an assessment of COVID-19-related items related to worries concerning the following: contracting, dying from, or currently having the illness; having a family member contract the illness; unknowingly infecting others; and experiencing significant financial burden.
A total of 1350 participants took a second survey on anxiety and depression that utilized the Generalized Anxiety Disorder–7 and the Patient Health Questionnaire–2.
“What makes the survey unique is that it’s not just a means of collecting data but also an interactive platform that gives participants immediate personalized feedback, based on their responses to the resilience and well-being surveys, with practical tips and recommendations for stress management and ways of boosting resilience,” Barzilay said.
Tend and befriend
Ten days into the survey, data were available on 3,042 participants (64% women, 54% with advanced education, 20.5% health care providers), who ranged in age from 18 to 70 years (mean [SD], 38.9 [11.9] years).
After accounting for covariates, the researchers found that participants reported more distress about family members contracting COVID-19 and about unknowingly infecting others than about getting COVID-19 themselves (48.5% and 36% vs. 19.9%, respectively; P < .0005).
Increased COVID-19-related worries were associated with 22% higher anxiety and 16.1% higher depression scores; women had higher scores than men on both.
Each 1-SD increase in the composite score of COVID-19 worries was associated with over twice the increased probability of generalized anxiety and depression (odds ratio, 2.23; 95% confidence interval, 1.88-2.65; and OR, 1.67; 95% CI, 1.41-1.98, respectively; for both, P < .001).
On the other hand, for every 1-SD increase in the resilience score, there was a 64.9% decrease in the possibility of screening positive for generalized anxiety disorder and a 69.3% decrease in the possibility of screening positive for depression (for both, P < .0001).
Compared to participants from Israel, US participants were “more stressed” about contracting, dying from, and currently having COVID-19 themselves. Overall, Israeli participants scored higher than US participants on the resilience scale.
Rates of anxiety and depression did not differ significantly between healthcare providers and others. Health care providers worried more about contracting COVID-19 themselves and worried less about finances after COVID-19.
The authors propose that survey participants were more worried about others than about themselves because of “prosocial behavior under stress” and “tend-and-befriend,” whereby, “in response to threat, humans tend to protect their close ones (tending) and seek out their social group for mutual defense (befriending).”
This type of altruistic behavior has been “described in acute situations throughout history” and has been “linked to mechanisms of resilience for overcoming adversity,” the authors indicate.
Demographic biases
Commenting on the findings for Medscape Medical News, Golnaz Tabibnia, PhD, a neuroscientist at the University of California, Irvine, who was not involved in the research, suggested that although higher resilience scores were associated with lower COVID-related worries, it is possible, “as the authors suggest, that having more resilience resources makes you less worried, but the causality could go the other direction as well, and less worry/rumination may lead to more resilience.”
Also commenting on the study for Medscape Medical News, Christiaan Vinkers, MD, PhD, a psychiatrist at the Amsterdam University Medical Center, Amsterdam, the Netherlands, said it was noteworthy that healthcare providers reported similar levels of mood and anxiety symptoms, compared to others.
“This is encouraging, as it suggests adequate resilience levels in professionals who work in the front lines of the COVID-19 pandemic,” he said.
Resilience occurs not only at the individual level but also at the community level, which may help explain the striking differences in COVID-19-related worries and anxiety between participants from the United States and Israel, Vinkers added.
E. Alison Holman, PhD, professor, Sue and Bill Gross School of Nursing, University of California, Irvine, noted that respondents were predominantly white, female, and had relatively high incomes, “suggesting strong demographic biases in those who chose to participate.”
Holman, who was not involved with the study, told Medscape Medical News that the “findings do not address the real impact of COVID-19 on the hardest-hit communities in America – poor, Black, and Latinx communities, where a large proportion of essential workers live.”
Barzilay acknowledged that, “unfortunately, because of the way the study was circulated, it did not reach minorities, which is one of the things we want to improve.”
The study is ongoing and has been translated into Spanish, French, and Hebrew. The team plans to collect data on diverse populations.
The study was supported by grants from the National Institute of Mental Health, the Lifespan Brain Institute of Children’s Hospital of Philadelphia, Penn Medicine, the University of Pennsylvania, and in part by the Zuckerman STEM Leadership Program. Barzilay serves on the scientific board and reports stock ownership in Taliaz Health. The other authors, Golnaz, Vinkers, and Holman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Individuals are more worried about family members becoming ill with COVID-19 or about unknowingly transmitting the disease to family members than they are about contracting it themselves, results of a new survey show.
Investigators surveyed over 3,000 adults, using an online questionnaire. Of the respondents, about 20% were health care workers, and most were living in locations with active stay-at-home orders at the time of the survey.
Close to half of participants were worried about family members contracting the virus, one third were worried about unknowingly infecting others, and 20% were worried about contracting the virus themselves.
“We were a little surprised to see that people were more concerned about others than about themselves, specifically worrying about whether a family member would contract COVID-19 and whether they might unintentionally infect others,” lead author Ran Barzilay, MD, PhD, child and adolescent psychiatrist at the Children’s Hospital of Philadelphia (CHOP), told Medscape Medical News.
The study was published online August 20 in Translational Psychiatry.
Interactive platform
“The pandemic has provided a unique opportunity to study resilience in healthcare professionals and others,” said Barzilay, assistant professor at the Lifespan Brain Institute, a collaboration between CHOP and the University of Pennsylvania, under the directorship of Raquel Gur, MD, PhD.
“After the pandemic broke out in March, we launched a website in early April where we surveyed people for levels of resilience, mental health, and well-being during the outbreak,” he added.
Survey participants then shared it with their contacts.
“To date, over 7000 people have completed it – mostly from the US but also from Israel,” Barzilay said.
The survey was anonymous, but participants could choose to have follow-up contact. The survey included an interactive 21-item resilience questionnaire and an assessment of COVID-19-related items related to worries concerning the following: contracting, dying from, or currently having the illness; having a family member contract the illness; unknowingly infecting others; and experiencing significant financial burden.
A total of 1350 participants took a second survey on anxiety and depression that utilized the Generalized Anxiety Disorder–7 and the Patient Health Questionnaire–2.
“What makes the survey unique is that it’s not just a means of collecting data but also an interactive platform that gives participants immediate personalized feedback, based on their responses to the resilience and well-being surveys, with practical tips and recommendations for stress management and ways of boosting resilience,” Barzilay said.
Tend and befriend
Ten days into the survey, data were available on 3,042 participants (64% women, 54% with advanced education, 20.5% health care providers), who ranged in age from 18 to 70 years (mean [SD], 38.9 [11.9] years).
After accounting for covariates, the researchers found that participants reported more distress about family members contracting COVID-19 and about unknowingly infecting others than about getting COVID-19 themselves (48.5% and 36% vs. 19.9%, respectively; P < .0005).
Increased COVID-19-related worries were associated with 22% higher anxiety and 16.1% higher depression scores; women had higher scores than men on both.
Each 1-SD increase in the composite score of COVID-19 worries was associated with over twice the increased probability of generalized anxiety and depression (odds ratio, 2.23; 95% confidence interval, 1.88-2.65; and OR, 1.67; 95% CI, 1.41-1.98, respectively; for both, P < .001).
On the other hand, for every 1-SD increase in the resilience score, there was a 64.9% decrease in the possibility of screening positive for generalized anxiety disorder and a 69.3% decrease in the possibility of screening positive for depression (for both, P < .0001).
Compared to participants from Israel, US participants were “more stressed” about contracting, dying from, and currently having COVID-19 themselves. Overall, Israeli participants scored higher than US participants on the resilience scale.
Rates of anxiety and depression did not differ significantly between healthcare providers and others. Health care providers worried more about contracting COVID-19 themselves and worried less about finances after COVID-19.
The authors propose that survey participants were more worried about others than about themselves because of “prosocial behavior under stress” and “tend-and-befriend,” whereby, “in response to threat, humans tend to protect their close ones (tending) and seek out their social group for mutual defense (befriending).”
This type of altruistic behavior has been “described in acute situations throughout history” and has been “linked to mechanisms of resilience for overcoming adversity,” the authors indicate.
Demographic biases
Commenting on the findings for Medscape Medical News, Golnaz Tabibnia, PhD, a neuroscientist at the University of California, Irvine, who was not involved in the research, suggested that although higher resilience scores were associated with lower COVID-related worries, it is possible, “as the authors suggest, that having more resilience resources makes you less worried, but the causality could go the other direction as well, and less worry/rumination may lead to more resilience.”
Also commenting on the study for Medscape Medical News, Christiaan Vinkers, MD, PhD, a psychiatrist at the Amsterdam University Medical Center, Amsterdam, the Netherlands, said it was noteworthy that healthcare providers reported similar levels of mood and anxiety symptoms, compared to others.
“This is encouraging, as it suggests adequate resilience levels in professionals who work in the front lines of the COVID-19 pandemic,” he said.
Resilience occurs not only at the individual level but also at the community level, which may help explain the striking differences in COVID-19-related worries and anxiety between participants from the United States and Israel, Vinkers added.
E. Alison Holman, PhD, professor, Sue and Bill Gross School of Nursing, University of California, Irvine, noted that respondents were predominantly white, female, and had relatively high incomes, “suggesting strong demographic biases in those who chose to participate.”
Holman, who was not involved with the study, told Medscape Medical News that the “findings do not address the real impact of COVID-19 on the hardest-hit communities in America – poor, Black, and Latinx communities, where a large proportion of essential workers live.”
Barzilay acknowledged that, “unfortunately, because of the way the study was circulated, it did not reach minorities, which is one of the things we want to improve.”
The study is ongoing and has been translated into Spanish, French, and Hebrew. The team plans to collect data on diverse populations.
The study was supported by grants from the National Institute of Mental Health, the Lifespan Brain Institute of Children’s Hospital of Philadelphia, Penn Medicine, the University of Pennsylvania, and in part by the Zuckerman STEM Leadership Program. Barzilay serves on the scientific board and reports stock ownership in Taliaz Health. The other authors, Golnaz, Vinkers, and Holman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Individuals are more worried about family members becoming ill with COVID-19 or about unknowingly transmitting the disease to family members than they are about contracting it themselves, results of a new survey show.
Investigators surveyed over 3,000 adults, using an online questionnaire. Of the respondents, about 20% were health care workers, and most were living in locations with active stay-at-home orders at the time of the survey.
Close to half of participants were worried about family members contracting the virus, one third were worried about unknowingly infecting others, and 20% were worried about contracting the virus themselves.
“We were a little surprised to see that people were more concerned about others than about themselves, specifically worrying about whether a family member would contract COVID-19 and whether they might unintentionally infect others,” lead author Ran Barzilay, MD, PhD, child and adolescent psychiatrist at the Children’s Hospital of Philadelphia (CHOP), told Medscape Medical News.
The study was published online August 20 in Translational Psychiatry.
Interactive platform
“The pandemic has provided a unique opportunity to study resilience in healthcare professionals and others,” said Barzilay, assistant professor at the Lifespan Brain Institute, a collaboration between CHOP and the University of Pennsylvania, under the directorship of Raquel Gur, MD, PhD.
“After the pandemic broke out in March, we launched a website in early April where we surveyed people for levels of resilience, mental health, and well-being during the outbreak,” he added.
Survey participants then shared it with their contacts.
“To date, over 7000 people have completed it – mostly from the US but also from Israel,” Barzilay said.
The survey was anonymous, but participants could choose to have follow-up contact. The survey included an interactive 21-item resilience questionnaire and an assessment of COVID-19-related items related to worries concerning the following: contracting, dying from, or currently having the illness; having a family member contract the illness; unknowingly infecting others; and experiencing significant financial burden.
A total of 1350 participants took a second survey on anxiety and depression that utilized the Generalized Anxiety Disorder–7 and the Patient Health Questionnaire–2.
“What makes the survey unique is that it’s not just a means of collecting data but also an interactive platform that gives participants immediate personalized feedback, based on their responses to the resilience and well-being surveys, with practical tips and recommendations for stress management and ways of boosting resilience,” Barzilay said.
Tend and befriend
Ten days into the survey, data were available on 3,042 participants (64% women, 54% with advanced education, 20.5% health care providers), who ranged in age from 18 to 70 years (mean [SD], 38.9 [11.9] years).
After accounting for covariates, the researchers found that participants reported more distress about family members contracting COVID-19 and about unknowingly infecting others than about getting COVID-19 themselves (48.5% and 36% vs. 19.9%, respectively; P < .0005).
Increased COVID-19-related worries were associated with 22% higher anxiety and 16.1% higher depression scores; women had higher scores than men on both.
Each 1-SD increase in the composite score of COVID-19 worries was associated with over twice the increased probability of generalized anxiety and depression (odds ratio, 2.23; 95% confidence interval, 1.88-2.65; and OR, 1.67; 95% CI, 1.41-1.98, respectively; for both, P < .001).
On the other hand, for every 1-SD increase in the resilience score, there was a 64.9% decrease in the possibility of screening positive for generalized anxiety disorder and a 69.3% decrease in the possibility of screening positive for depression (for both, P < .0001).
Compared to participants from Israel, US participants were “more stressed” about contracting, dying from, and currently having COVID-19 themselves. Overall, Israeli participants scored higher than US participants on the resilience scale.
Rates of anxiety and depression did not differ significantly between healthcare providers and others. Health care providers worried more about contracting COVID-19 themselves and worried less about finances after COVID-19.
The authors propose that survey participants were more worried about others than about themselves because of “prosocial behavior under stress” and “tend-and-befriend,” whereby, “in response to threat, humans tend to protect their close ones (tending) and seek out their social group for mutual defense (befriending).”
This type of altruistic behavior has been “described in acute situations throughout history” and has been “linked to mechanisms of resilience for overcoming adversity,” the authors indicate.
Demographic biases
Commenting on the findings for Medscape Medical News, Golnaz Tabibnia, PhD, a neuroscientist at the University of California, Irvine, who was not involved in the research, suggested that although higher resilience scores were associated with lower COVID-related worries, it is possible, “as the authors suggest, that having more resilience resources makes you less worried, but the causality could go the other direction as well, and less worry/rumination may lead to more resilience.”
Also commenting on the study for Medscape Medical News, Christiaan Vinkers, MD, PhD, a psychiatrist at the Amsterdam University Medical Center, Amsterdam, the Netherlands, said it was noteworthy that healthcare providers reported similar levels of mood and anxiety symptoms, compared to others.
“This is encouraging, as it suggests adequate resilience levels in professionals who work in the front lines of the COVID-19 pandemic,” he said.
Resilience occurs not only at the individual level but also at the community level, which may help explain the striking differences in COVID-19-related worries and anxiety between participants from the United States and Israel, Vinkers added.
E. Alison Holman, PhD, professor, Sue and Bill Gross School of Nursing, University of California, Irvine, noted that respondents were predominantly white, female, and had relatively high incomes, “suggesting strong demographic biases in those who chose to participate.”
Holman, who was not involved with the study, told Medscape Medical News that the “findings do not address the real impact of COVID-19 on the hardest-hit communities in America – poor, Black, and Latinx communities, where a large proportion of essential workers live.”
Barzilay acknowledged that, “unfortunately, because of the way the study was circulated, it did not reach minorities, which is one of the things we want to improve.”
The study is ongoing and has been translated into Spanish, French, and Hebrew. The team plans to collect data on diverse populations.
The study was supported by grants from the National Institute of Mental Health, the Lifespan Brain Institute of Children’s Hospital of Philadelphia, Penn Medicine, the University of Pennsylvania, and in part by the Zuckerman STEM Leadership Program. Barzilay serves on the scientific board and reports stock ownership in Taliaz Health. The other authors, Golnaz, Vinkers, and Holman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
No one-size-fits-all approach to tissue-tightening devices
.
“There are many devices on the market, but their efficacy is not consistent,” Catherine M. DiGiorgio, MS, MD, said during the virtual annual Masters of Aesthetics Symposium. “The key to maximizing patient satisfaction is patient selection and setting realistic expectations.”
She avoids recommending the use of tissue-tightening devices for patients who require surgical correction and for those who find the idea of minimal improvement unacceptable. “These are not the treatments for them,” she said. “I also find that when a patient uses her fingers to pull her face back and says, ‘I want to look like this,’ this is not the right patient for these devices. They can get a good amount of improvement, but efficacy is not consistent.”
Still, patients favor noninvasive or minimally invasive procedures for skin tightening now more than ever before. “They are not willing to undergo surgical treatments, and they want something with low downtime,” she said.
Dr. DiGiorgio, who practices at the Boston Center for Facial Rejuvenation, began a review of tissue-tightening devices on the market by discussing the role of ablative fractional lasers such as the carbon dioxide 10,600-nm laser and the Erbium:YAG 2,940-nm laser, which carry risks and downtime. “I don’t view these lasers as a tissue-tightening devices, but they are included because they can provide a little bit of tightening,” she said.
The ideal candidate is someone with skin type I-II and mild skin laxity. “These lasers are really good at improving rhytides,” she noted. “The patient needs to be able to tolerate the discomfort and manage the healing process. Sometimes you can get blepharoplastylike results with some patients. This can be combined with vascular lasers and pigment-targeting lasers to improve the overall texture and tone of the skin. Many combine this with a face-lift or a blepharoplasty. You should wait at least 6-8 weeks after a face-lift before performing this procedure. Some plastic surgeons do combine this with blepharoplasty in the same visit.”
A less invasive option for skin tightening is the delivery of radiofrequency energy, which disrupts hydrogen bonds of the collagen triple helix. This occurs in temperatures greater than 60° C and results in collagen contraction and tightening and neocollagenesis. There are several devices available including transcutaneous monopolar radiofrequency (Thermage, TempSure), subsurface thermistor–controlled monopolar radiofrequency (ThermiTight), and fractional microneedling radiofrequency (Profound RF, Genius RF, Vivace, and Secret RF). The transcutaneous monopolar radiofrequency device delivers energy uniformly via a treatment tip that has contact cooling and coupling fluid. Collagen is denatured at 65° C and fibroblasts are stimulated to form new collagen. The healing process provides additional tightening.
“These treatments are noninvasive; there’s no downtime, and there’s mild discomfort,” Dr. DiGiorgio commented. “Treatments can be done around the eyes, on the face and body. When treating around the eyes with these devices you want to use a corneal plastic eye shield. Contraindications include having a pacemaker, defibrillator, or other electronic implantable device.”
In her opinion, the ideal patient for this device has mild skin laxity or is younger and seeking to maintain a youthful appearance. “It’s great for mild upper eyelid laxity and for temporary improvement of cellulite appearance,” she said. “The patient should not require surgical intervention and the patient should also agree to undergo multiple treatment sessions. Just one treatment session is not going to cut it.”
Another device in this class of technology is subsurface thermistor–controlled monopolar radiofrequency, “which is basically a probe that’s inserted into the skin, most commonly in the submental area,” Dr. DiGiorgio said. An external infrared camera monitors the epidermal temperature, which should not exceed 45°C. This results in a controlled deep dermal and subdermal delivery of thermal energy. “It requires light tumescent anesthesia, and it can be combined with liposuction,” she said. “Common side effects include erythema, edema, and bruising, and sometimes contour irregularities or nodules.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical correction. “You can combine this with liposuction, but you can achieve good results without it,” she said.
The next device in this class of technology that Dr. DiGiorgio discussed is fractional microneedling radiofrequency. Of several such devices on the market, some have adjustable depths up to 4 mm while others have fixed depths. The energy is adjustable, and the tips can be insulated or noninsulated. “Insulated tips make it safer to perform in darker skin types because the proximal portion of the needle is insulated and the epidermis is spared from damage,” she explained. “Some devices are a bit more painful than others. It does require topical anesthesia; some require local injection anesthesia. Patients have erythema for about 24 hours, and treatments are recommended monthly.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical intervention but who seeks to maintain a youthful appearance. “Patients should understand that multiple treatments will be required to achieve optimal results,” she said. “I find that there is less improvement in older patients. This can be combined with thread lifts, vascular lasers, pigment-targeting lasers, and CO2 lasers.”
The next device for skin tightening that she discussed is microfocused ultrasound (Ultherapy), which delivers millisecond domain pulses at three different depths that are determined by the transducer that you use. It can go as deep as 4.5 mm. “Each pulse delivers a focal zone of coagulation to achieve tissue contraction,” Dr. DiGiorgio said. “There’s an ultrasound-imaging device attached to it to ensure proper skin contact and the delivery of energy at an appropriate depth. Patients can have a little bit of pain and erythema and edema, sometime bruising. Usually there is not much downtime with these treatments.”
A newcomer in this class of technology is SoftWave, an intense ultrasound beam array (IUB), which delivers energy precisely to the middermis at a depth of 1.5 mm. “With each pulse, the hand piece has seven transducers that deliver energy in 3-dimensional cylindrical thermal zones,” Dr. DiGiorgio said. “You get greater than 25% tissue coverage in one treatment, and there is no injury to the epidermis or deeper structures. It has unique vectors that are along the lines of facial wrinkles, so you get tightening along those lines.”
The procedure takes about 30 minutes, there is no downtime, and it causes no pain, she said. Pretreatment, patients receive topical anesthesia. “This device has active skin cooling and has an ultrasound gel,” she added. “It does not have an imaging platform like the microfocused ultrasound does, because the depth is fixed. You get significant wrinkle reduction and decrease in submental fullness with improvement in jawline definition, eyebrow position, fine lines, and texture.” In her opinion, the ideal candidate for this device is a patient in the mid-40s to early 50s with mild to moderate elastosis, fullness, texture irregularities, laxity, rhytids, elastosis, and photoaging.
She reported having no financial disclosures.
.
“There are many devices on the market, but their efficacy is not consistent,” Catherine M. DiGiorgio, MS, MD, said during the virtual annual Masters of Aesthetics Symposium. “The key to maximizing patient satisfaction is patient selection and setting realistic expectations.”
She avoids recommending the use of tissue-tightening devices for patients who require surgical correction and for those who find the idea of minimal improvement unacceptable. “These are not the treatments for them,” she said. “I also find that when a patient uses her fingers to pull her face back and says, ‘I want to look like this,’ this is not the right patient for these devices. They can get a good amount of improvement, but efficacy is not consistent.”
Still, patients favor noninvasive or minimally invasive procedures for skin tightening now more than ever before. “They are not willing to undergo surgical treatments, and they want something with low downtime,” she said.
Dr. DiGiorgio, who practices at the Boston Center for Facial Rejuvenation, began a review of tissue-tightening devices on the market by discussing the role of ablative fractional lasers such as the carbon dioxide 10,600-nm laser and the Erbium:YAG 2,940-nm laser, which carry risks and downtime. “I don’t view these lasers as a tissue-tightening devices, but they are included because they can provide a little bit of tightening,” she said.
The ideal candidate is someone with skin type I-II and mild skin laxity. “These lasers are really good at improving rhytides,” she noted. “The patient needs to be able to tolerate the discomfort and manage the healing process. Sometimes you can get blepharoplastylike results with some patients. This can be combined with vascular lasers and pigment-targeting lasers to improve the overall texture and tone of the skin. Many combine this with a face-lift or a blepharoplasty. You should wait at least 6-8 weeks after a face-lift before performing this procedure. Some plastic surgeons do combine this with blepharoplasty in the same visit.”
A less invasive option for skin tightening is the delivery of radiofrequency energy, which disrupts hydrogen bonds of the collagen triple helix. This occurs in temperatures greater than 60° C and results in collagen contraction and tightening and neocollagenesis. There are several devices available including transcutaneous monopolar radiofrequency (Thermage, TempSure), subsurface thermistor–controlled monopolar radiofrequency (ThermiTight), and fractional microneedling radiofrequency (Profound RF, Genius RF, Vivace, and Secret RF). The transcutaneous monopolar radiofrequency device delivers energy uniformly via a treatment tip that has contact cooling and coupling fluid. Collagen is denatured at 65° C and fibroblasts are stimulated to form new collagen. The healing process provides additional tightening.
“These treatments are noninvasive; there’s no downtime, and there’s mild discomfort,” Dr. DiGiorgio commented. “Treatments can be done around the eyes, on the face and body. When treating around the eyes with these devices you want to use a corneal plastic eye shield. Contraindications include having a pacemaker, defibrillator, or other electronic implantable device.”
In her opinion, the ideal patient for this device has mild skin laxity or is younger and seeking to maintain a youthful appearance. “It’s great for mild upper eyelid laxity and for temporary improvement of cellulite appearance,” she said. “The patient should not require surgical intervention and the patient should also agree to undergo multiple treatment sessions. Just one treatment session is not going to cut it.”
Another device in this class of technology is subsurface thermistor–controlled monopolar radiofrequency, “which is basically a probe that’s inserted into the skin, most commonly in the submental area,” Dr. DiGiorgio said. An external infrared camera monitors the epidermal temperature, which should not exceed 45°C. This results in a controlled deep dermal and subdermal delivery of thermal energy. “It requires light tumescent anesthesia, and it can be combined with liposuction,” she said. “Common side effects include erythema, edema, and bruising, and sometimes contour irregularities or nodules.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical correction. “You can combine this with liposuction, but you can achieve good results without it,” she said.
The next device in this class of technology that Dr. DiGiorgio discussed is fractional microneedling radiofrequency. Of several such devices on the market, some have adjustable depths up to 4 mm while others have fixed depths. The energy is adjustable, and the tips can be insulated or noninsulated. “Insulated tips make it safer to perform in darker skin types because the proximal portion of the needle is insulated and the epidermis is spared from damage,” she explained. “Some devices are a bit more painful than others. It does require topical anesthesia; some require local injection anesthesia. Patients have erythema for about 24 hours, and treatments are recommended monthly.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical intervention but who seeks to maintain a youthful appearance. “Patients should understand that multiple treatments will be required to achieve optimal results,” she said. “I find that there is less improvement in older patients. This can be combined with thread lifts, vascular lasers, pigment-targeting lasers, and CO2 lasers.”
The next device for skin tightening that she discussed is microfocused ultrasound (Ultherapy), which delivers millisecond domain pulses at three different depths that are determined by the transducer that you use. It can go as deep as 4.5 mm. “Each pulse delivers a focal zone of coagulation to achieve tissue contraction,” Dr. DiGiorgio said. “There’s an ultrasound-imaging device attached to it to ensure proper skin contact and the delivery of energy at an appropriate depth. Patients can have a little bit of pain and erythema and edema, sometime bruising. Usually there is not much downtime with these treatments.”
A newcomer in this class of technology is SoftWave, an intense ultrasound beam array (IUB), which delivers energy precisely to the middermis at a depth of 1.5 mm. “With each pulse, the hand piece has seven transducers that deliver energy in 3-dimensional cylindrical thermal zones,” Dr. DiGiorgio said. “You get greater than 25% tissue coverage in one treatment, and there is no injury to the epidermis or deeper structures. It has unique vectors that are along the lines of facial wrinkles, so you get tightening along those lines.”
The procedure takes about 30 minutes, there is no downtime, and it causes no pain, she said. Pretreatment, patients receive topical anesthesia. “This device has active skin cooling and has an ultrasound gel,” she added. “It does not have an imaging platform like the microfocused ultrasound does, because the depth is fixed. You get significant wrinkle reduction and decrease in submental fullness with improvement in jawline definition, eyebrow position, fine lines, and texture.” In her opinion, the ideal candidate for this device is a patient in the mid-40s to early 50s with mild to moderate elastosis, fullness, texture irregularities, laxity, rhytids, elastosis, and photoaging.
She reported having no financial disclosures.
.
“There are many devices on the market, but their efficacy is not consistent,” Catherine M. DiGiorgio, MS, MD, said during the virtual annual Masters of Aesthetics Symposium. “The key to maximizing patient satisfaction is patient selection and setting realistic expectations.”
She avoids recommending the use of tissue-tightening devices for patients who require surgical correction and for those who find the idea of minimal improvement unacceptable. “These are not the treatments for them,” she said. “I also find that when a patient uses her fingers to pull her face back and says, ‘I want to look like this,’ this is not the right patient for these devices. They can get a good amount of improvement, but efficacy is not consistent.”
Still, patients favor noninvasive or minimally invasive procedures for skin tightening now more than ever before. “They are not willing to undergo surgical treatments, and they want something with low downtime,” she said.
Dr. DiGiorgio, who practices at the Boston Center for Facial Rejuvenation, began a review of tissue-tightening devices on the market by discussing the role of ablative fractional lasers such as the carbon dioxide 10,600-nm laser and the Erbium:YAG 2,940-nm laser, which carry risks and downtime. “I don’t view these lasers as a tissue-tightening devices, but they are included because they can provide a little bit of tightening,” she said.
The ideal candidate is someone with skin type I-II and mild skin laxity. “These lasers are really good at improving rhytides,” she noted. “The patient needs to be able to tolerate the discomfort and manage the healing process. Sometimes you can get blepharoplastylike results with some patients. This can be combined with vascular lasers and pigment-targeting lasers to improve the overall texture and tone of the skin. Many combine this with a face-lift or a blepharoplasty. You should wait at least 6-8 weeks after a face-lift before performing this procedure. Some plastic surgeons do combine this with blepharoplasty in the same visit.”
A less invasive option for skin tightening is the delivery of radiofrequency energy, which disrupts hydrogen bonds of the collagen triple helix. This occurs in temperatures greater than 60° C and results in collagen contraction and tightening and neocollagenesis. There are several devices available including transcutaneous monopolar radiofrequency (Thermage, TempSure), subsurface thermistor–controlled monopolar radiofrequency (ThermiTight), and fractional microneedling radiofrequency (Profound RF, Genius RF, Vivace, and Secret RF). The transcutaneous monopolar radiofrequency device delivers energy uniformly via a treatment tip that has contact cooling and coupling fluid. Collagen is denatured at 65° C and fibroblasts are stimulated to form new collagen. The healing process provides additional tightening.
“These treatments are noninvasive; there’s no downtime, and there’s mild discomfort,” Dr. DiGiorgio commented. “Treatments can be done around the eyes, on the face and body. When treating around the eyes with these devices you want to use a corneal plastic eye shield. Contraindications include having a pacemaker, defibrillator, or other electronic implantable device.”
In her opinion, the ideal patient for this device has mild skin laxity or is younger and seeking to maintain a youthful appearance. “It’s great for mild upper eyelid laxity and for temporary improvement of cellulite appearance,” she said. “The patient should not require surgical intervention and the patient should also agree to undergo multiple treatment sessions. Just one treatment session is not going to cut it.”
Another device in this class of technology is subsurface thermistor–controlled monopolar radiofrequency, “which is basically a probe that’s inserted into the skin, most commonly in the submental area,” Dr. DiGiorgio said. An external infrared camera monitors the epidermal temperature, which should not exceed 45°C. This results in a controlled deep dermal and subdermal delivery of thermal energy. “It requires light tumescent anesthesia, and it can be combined with liposuction,” she said. “Common side effects include erythema, edema, and bruising, and sometimes contour irregularities or nodules.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical correction. “You can combine this with liposuction, but you can achieve good results without it,” she said.
The next device in this class of technology that Dr. DiGiorgio discussed is fractional microneedling radiofrequency. Of several such devices on the market, some have adjustable depths up to 4 mm while others have fixed depths. The energy is adjustable, and the tips can be insulated or noninsulated. “Insulated tips make it safer to perform in darker skin types because the proximal portion of the needle is insulated and the epidermis is spared from damage,” she explained. “Some devices are a bit more painful than others. It does require topical anesthesia; some require local injection anesthesia. Patients have erythema for about 24 hours, and treatments are recommended monthly.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical intervention but who seeks to maintain a youthful appearance. “Patients should understand that multiple treatments will be required to achieve optimal results,” she said. “I find that there is less improvement in older patients. This can be combined with thread lifts, vascular lasers, pigment-targeting lasers, and CO2 lasers.”
The next device for skin tightening that she discussed is microfocused ultrasound (Ultherapy), which delivers millisecond domain pulses at three different depths that are determined by the transducer that you use. It can go as deep as 4.5 mm. “Each pulse delivers a focal zone of coagulation to achieve tissue contraction,” Dr. DiGiorgio said. “There’s an ultrasound-imaging device attached to it to ensure proper skin contact and the delivery of energy at an appropriate depth. Patients can have a little bit of pain and erythema and edema, sometime bruising. Usually there is not much downtime with these treatments.”
A newcomer in this class of technology is SoftWave, an intense ultrasound beam array (IUB), which delivers energy precisely to the middermis at a depth of 1.5 mm. “With each pulse, the hand piece has seven transducers that deliver energy in 3-dimensional cylindrical thermal zones,” Dr. DiGiorgio said. “You get greater than 25% tissue coverage in one treatment, and there is no injury to the epidermis or deeper structures. It has unique vectors that are along the lines of facial wrinkles, so you get tightening along those lines.”
The procedure takes about 30 minutes, there is no downtime, and it causes no pain, she said. Pretreatment, patients receive topical anesthesia. “This device has active skin cooling and has an ultrasound gel,” she added. “It does not have an imaging platform like the microfocused ultrasound does, because the depth is fixed. You get significant wrinkle reduction and decrease in submental fullness with improvement in jawline definition, eyebrow position, fine lines, and texture.” In her opinion, the ideal candidate for this device is a patient in the mid-40s to early 50s with mild to moderate elastosis, fullness, texture irregularities, laxity, rhytids, elastosis, and photoaging.
She reported having no financial disclosures.
REPORTING FROM MOA 2020
Innovator banks on ‘truly smart’ robotic lasers in dermatology
Dr. Anderson, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, conceived and developed many of the nonscarring laser treatments now widely used in dermatology. These include selective photothermolysis for birthmarks, microvascular and pigmented lesions, and tattoo and permanent hair removal. He also contributed to laser lithotripsy, laser angioplasty, photodynamic therapy, and optical diagnostics. The highest-resolution imaging device approved for human use, an infrared confocal microscope, came from his laboratory. Dr. Anderson has also contributed to basic knowledge of human photobiology, drug photosensitization mechanisms, tissue optics and laser-tissue interactions. In this Q&A with Doug Brunk, he reflects on his achievements and on the future of lasers in dermatology.
In published interviews you have described yourself as more of a problem solver than an inventor. How did your upbringing foster your affinity for problem solving?
I grew up in Central Illinois during the 1950s and early 1960s, an area known for corn, soybeans, and hogs. At an early age I learned to be interested in other things because it’s possible to die of boredom there. By the time I was 12 years old, I was an amateur radio operator, and I was building rockets to see how high they would go.
Problem solving comes naturally to me. I enjoy very much finding a problem that is worth solving, which means getting passionate about it and brainstorming. Half the time you don’t come up with a potential route to solve the problem. I attended the Massachusetts Institute of Technology at the age of 17, which was a real eye-opener. I had never been east of the Wabash River prior to that. I studied physics for a while, then decided to flip into biology. That combination has served me well. My special sauce is to have some intuitive and academic rigorous feeling for physical processes. But we physicians have a front row seat to nature’s human drama. There is no lack of problems to solve. I can sit around and obsess about things theoretically, but at the end of the day I want to work on things that ultimately benefit people.
What inspired you most early in your career as a physician scientist?
After I made a commitment to medicine, Dr. John A. Parrish, and Dr. Thomas B. Fitzpatrick were key mentors to me. I was 30 years old when I started medical school, but they took me under their wing even before that. I took a part-time, temporary job with them, which turned into a permanent job. That turned into a love for the work they did. Instead of going into a graduate program in a laboratory and studying bacteria and genetics, the whole idea of working with people and on people was awesome. Dr. Parrish really mentored me. I won a lifetime achievement award from the American Academy of Dermatology a few years ago. I found myself on stage and it rolled out of my mouth that John Parrish believed in me before I believed in myself. It’s really true. He somehow recognized that I had some talents. I was very young and a combination of naive and humble, I guess.
What was the initial genesis for your idea of selective photothermolysis?
I was interested in going to medical school and working with Dr. Fitzpatrick and Dr. Parrish on things related to light. They were mostly interested in PUVA and UVB; it was the heyday of modern phototherapy. I attended a lecture at the Beth Israel Hospital in Boston given by a plastic surgeon, Dr. Joel Mark Noe. He was talking about using lasers to treat port-wine stains in children. The gist of the talk was that argon lasers were being used, and that the results were sometimes decent, but not great. Often children would have burn scars after the treatment. Dr. Noe was talking about how you had to choose the color of the wavelength of the laser to be absorbed by hemoglobin, but he wasn’t talking about what happens to the heat once it’s created. My background in physics led me to recognize that he wasn’t capturing the full picture. Selective disruption of a target in the skin by light is half of the story. The confinement of heat in the target is the other half of the story. Literally on a bus on the way home from that lecture to my apartment in Cambridge, I hatched the idea for selective photothermolysis and wrote down some equations. I also wrote down the ideal wavelength region, how much energy was needed, and what the pulse duration would have to be like to damage target vessels that small. I showed John Parrish what I had written. He took me seriously and said, “Let’s see if we can find a light source that can accomplish this.” We traveled around the country looking at various lasers, but we wound up building the first pulsed dye laser for treating port-wine stains. To me, the surprise was that we didn’t kill the skin. If you treat an area of skin with a laser and hurt all the blood vessels, you think, “Wait a minute. Are we going to kill the skin because it has no blood supply?” The questions of the day were so basic, and we just got lucky. It took 6-8 years before we ramped up the clinical studies showing efficacy and safety of this technology.
I presume that you experimented on your own skin while developing some of the nonscarring laser treatments now widely used in dermatology. What “war story” stands out to you most from that part of your work?
I’m right handed, so I’d grab a laser with my right hand and treat my left arm, so that arm sports a bit of history. In 1994, while working with Dr. Melanie Grossman on the development of laser hair removal, I used a ruby laser to self-treat a patch of hair on my left arm. I still have the world’s oldest laser-induced bald spot on that arm. It’s been 26 years now. I still look at it and count the hairs, because one of the big questions is, is laser hair removal permanent? In all these years I have grown two hairs.
What technology that you conceived of or developed has most surprised you, in term of its ultimate clinical impact?
I would say confocal microscopy. In the mid-1990s I worked with a physicist named Robert H. Webb, who invented an imaging system for the retina. We got together, noodled about it, and decided we would modify his ophthalmoscope system to see if we could get images from inside the skin. It worked pretty well. It was truly surprising from many points of view. First, it wasn’t clear at all that we’d get any images this way. Now, reflectance confocal microscopy is a standard tool in both clinical and research dermatology. But there were odd discoveries early on. For example, the darker your skin, the brighter it appeared in the microscope. You might think that melanin absorbs light and that you would get poor images in dark skin. It was the exact opposite; melanin acts as a natural contrast agent.
We worked with a small company to make the first confocal microscope. Initially, it had no clinical applications but what was fascinating to me was the incredible value of being able to see inside human skin harmlessly, and just see what’s going on. It became a potent research tool, and recently CPT codes were established for its use in evaluating skin cancer margins. I wouldn’t be surprised if 30 years from now, taking a skin biopsy is seemingly barbaric. A forerunner of all these new imaging tools for the skin was the confocal microscope developed in my lab in 1994.
During a 2011 TED talk, you said that nevus of Ota is your favorite thing to treat, because the outcome is usually perfect skin. Are there other technologies or devices you played a role in developing that make you proud at this stage in your career?
The reason I love treating nevus of Ota is that you have a lifelong facial disfigurement, and the only treatment for it is a laser we came up with, and it always works. How perfect could it be? The flip side of the same coin is, there are lesions of the skin that just don’t respond. One of the things we don’t know enough about is the connection between the biologic aspects of repair of various lesions and the treatments that we come up with. The most recent example of selective photothermolysis is a new laser we’re building right now for acne that is based on sebaceous gland injury. You’ll see this coming out in the next year or two. My heart goes out to people with nodular cystic acne. For young men it’s highly associated with suicide. So, I’m excited about optimizing and learning what happens when we target sebaceous glands.
One of the other big stories in laser dermatology is the fractional laser. I developed this with Dr. Dieter Manstein when he was a postdoc in my lab. One of the most pleasing things from this technology is how well you can rehabilitate scars, particularly burn scars in children. Over the last few years, I have trained plastic surgeons at the Shriners Hospital for Children in Boston on how to use fractional lasers to improve the lives of these kids. Another technology I developed with Dr. Manstein is cryolipolysis, which is removing fat from the body by cooling it. There are no lasers involved with this technology. I like to say that I’ve spent most of my career studying light and heat, and now we’ve come up with something that’s cold in the dark. We are now working on derivatives of cryolipolysis, to determine if what we’ve learned about targeting fat that might be applicable elsewhere.
Who inspires you most in your work today?
In addition to Dr. John Parrish and Dr. Thomas Fitzpatrick, the late Dr. Albert M. Kligman also influenced me. He never accepted dogma and he loved to ask questions, like, “What if?” as opposed to just accumulating a fund of knowledge. Understanding things is not just based on how much you know. It’s based on critical thinking and the ability to question. I also admire Albert Einstein, his ability to sit down with nothing more than pencil and paper and change our view of the universe. I love physics because it’s the science of everything. I also love poetry. My favorite poet is Stanley Kunitz. He had amazing insight and was named United States Poet Laureate in 1974 and in 2000. I have plenty of antiheroes as well, mostly politicians.
I understand that you play the banjo. How long have you been playing, and what do you enjoy about it?
You cannot sit down and play the banjo and have your mind on much else. It’s a wonderful moving meditation. Before my medical career, I was a schoolteacher in Vermont. There was a guy on the staff there who played banjo. He came from a small town in Georgia. I just picked it up and started plunking. It’s a happy instrument. It’s awfully hard to make the banjo sound melancholy.
What novel use of lasers and light in dermatology are you most excited about in the next 5 years?
The marriage of therapeutic devices with diagnostic and imaging devices has not happened yet. They are not even in the honeymoon moment. But I think that having truly smart robotic systems in our hands for treating patients will become a reality. These days, dermatologists have to buy a certain type of laser to treat a certain type of lesion. For example, the Q-switched alexandrite laser you buy for treating Nevus of Ota won’t do anything for a port-wine stain; it’s the wrong pulse duration. This means that clinicians who practice a lot of laser dermatology end up with a dozen lasers in their practice. In the future, I think it will be possible to have a software laser, so when you want to acquire another target, you load an App as opposed to buying a new laser. This means that you would have software programmable targeting, and you would not have the requirement of having selective absorption. So, I’m excited by the idea of guided fractional lasers. None of them exist now. We have to start from scratch.
Dr. Anderson, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, conceived and developed many of the nonscarring laser treatments now widely used in dermatology. These include selective photothermolysis for birthmarks, microvascular and pigmented lesions, and tattoo and permanent hair removal. He also contributed to laser lithotripsy, laser angioplasty, photodynamic therapy, and optical diagnostics. The highest-resolution imaging device approved for human use, an infrared confocal microscope, came from his laboratory. Dr. Anderson has also contributed to basic knowledge of human photobiology, drug photosensitization mechanisms, tissue optics and laser-tissue interactions. In this Q&A with Doug Brunk, he reflects on his achievements and on the future of lasers in dermatology.
In published interviews you have described yourself as more of a problem solver than an inventor. How did your upbringing foster your affinity for problem solving?
I grew up in Central Illinois during the 1950s and early 1960s, an area known for corn, soybeans, and hogs. At an early age I learned to be interested in other things because it’s possible to die of boredom there. By the time I was 12 years old, I was an amateur radio operator, and I was building rockets to see how high they would go.
Problem solving comes naturally to me. I enjoy very much finding a problem that is worth solving, which means getting passionate about it and brainstorming. Half the time you don’t come up with a potential route to solve the problem. I attended the Massachusetts Institute of Technology at the age of 17, which was a real eye-opener. I had never been east of the Wabash River prior to that. I studied physics for a while, then decided to flip into biology. That combination has served me well. My special sauce is to have some intuitive and academic rigorous feeling for physical processes. But we physicians have a front row seat to nature’s human drama. There is no lack of problems to solve. I can sit around and obsess about things theoretically, but at the end of the day I want to work on things that ultimately benefit people.
What inspired you most early in your career as a physician scientist?
After I made a commitment to medicine, Dr. John A. Parrish, and Dr. Thomas B. Fitzpatrick were key mentors to me. I was 30 years old when I started medical school, but they took me under their wing even before that. I took a part-time, temporary job with them, which turned into a permanent job. That turned into a love for the work they did. Instead of going into a graduate program in a laboratory and studying bacteria and genetics, the whole idea of working with people and on people was awesome. Dr. Parrish really mentored me. I won a lifetime achievement award from the American Academy of Dermatology a few years ago. I found myself on stage and it rolled out of my mouth that John Parrish believed in me before I believed in myself. It’s really true. He somehow recognized that I had some talents. I was very young and a combination of naive and humble, I guess.
What was the initial genesis for your idea of selective photothermolysis?
I was interested in going to medical school and working with Dr. Fitzpatrick and Dr. Parrish on things related to light. They were mostly interested in PUVA and UVB; it was the heyday of modern phototherapy. I attended a lecture at the Beth Israel Hospital in Boston given by a plastic surgeon, Dr. Joel Mark Noe. He was talking about using lasers to treat port-wine stains in children. The gist of the talk was that argon lasers were being used, and that the results were sometimes decent, but not great. Often children would have burn scars after the treatment. Dr. Noe was talking about how you had to choose the color of the wavelength of the laser to be absorbed by hemoglobin, but he wasn’t talking about what happens to the heat once it’s created. My background in physics led me to recognize that he wasn’t capturing the full picture. Selective disruption of a target in the skin by light is half of the story. The confinement of heat in the target is the other half of the story. Literally on a bus on the way home from that lecture to my apartment in Cambridge, I hatched the idea for selective photothermolysis and wrote down some equations. I also wrote down the ideal wavelength region, how much energy was needed, and what the pulse duration would have to be like to damage target vessels that small. I showed John Parrish what I had written. He took me seriously and said, “Let’s see if we can find a light source that can accomplish this.” We traveled around the country looking at various lasers, but we wound up building the first pulsed dye laser for treating port-wine stains. To me, the surprise was that we didn’t kill the skin. If you treat an area of skin with a laser and hurt all the blood vessels, you think, “Wait a minute. Are we going to kill the skin because it has no blood supply?” The questions of the day were so basic, and we just got lucky. It took 6-8 years before we ramped up the clinical studies showing efficacy and safety of this technology.
I presume that you experimented on your own skin while developing some of the nonscarring laser treatments now widely used in dermatology. What “war story” stands out to you most from that part of your work?
I’m right handed, so I’d grab a laser with my right hand and treat my left arm, so that arm sports a bit of history. In 1994, while working with Dr. Melanie Grossman on the development of laser hair removal, I used a ruby laser to self-treat a patch of hair on my left arm. I still have the world’s oldest laser-induced bald spot on that arm. It’s been 26 years now. I still look at it and count the hairs, because one of the big questions is, is laser hair removal permanent? In all these years I have grown two hairs.
What technology that you conceived of or developed has most surprised you, in term of its ultimate clinical impact?
I would say confocal microscopy. In the mid-1990s I worked with a physicist named Robert H. Webb, who invented an imaging system for the retina. We got together, noodled about it, and decided we would modify his ophthalmoscope system to see if we could get images from inside the skin. It worked pretty well. It was truly surprising from many points of view. First, it wasn’t clear at all that we’d get any images this way. Now, reflectance confocal microscopy is a standard tool in both clinical and research dermatology. But there were odd discoveries early on. For example, the darker your skin, the brighter it appeared in the microscope. You might think that melanin absorbs light and that you would get poor images in dark skin. It was the exact opposite; melanin acts as a natural contrast agent.
We worked with a small company to make the first confocal microscope. Initially, it had no clinical applications but what was fascinating to me was the incredible value of being able to see inside human skin harmlessly, and just see what’s going on. It became a potent research tool, and recently CPT codes were established for its use in evaluating skin cancer margins. I wouldn’t be surprised if 30 years from now, taking a skin biopsy is seemingly barbaric. A forerunner of all these new imaging tools for the skin was the confocal microscope developed in my lab in 1994.
During a 2011 TED talk, you said that nevus of Ota is your favorite thing to treat, because the outcome is usually perfect skin. Are there other technologies or devices you played a role in developing that make you proud at this stage in your career?
The reason I love treating nevus of Ota is that you have a lifelong facial disfigurement, and the only treatment for it is a laser we came up with, and it always works. How perfect could it be? The flip side of the same coin is, there are lesions of the skin that just don’t respond. One of the things we don’t know enough about is the connection between the biologic aspects of repair of various lesions and the treatments that we come up with. The most recent example of selective photothermolysis is a new laser we’re building right now for acne that is based on sebaceous gland injury. You’ll see this coming out in the next year or two. My heart goes out to people with nodular cystic acne. For young men it’s highly associated with suicide. So, I’m excited about optimizing and learning what happens when we target sebaceous glands.
One of the other big stories in laser dermatology is the fractional laser. I developed this with Dr. Dieter Manstein when he was a postdoc in my lab. One of the most pleasing things from this technology is how well you can rehabilitate scars, particularly burn scars in children. Over the last few years, I have trained plastic surgeons at the Shriners Hospital for Children in Boston on how to use fractional lasers to improve the lives of these kids. Another technology I developed with Dr. Manstein is cryolipolysis, which is removing fat from the body by cooling it. There are no lasers involved with this technology. I like to say that I’ve spent most of my career studying light and heat, and now we’ve come up with something that’s cold in the dark. We are now working on derivatives of cryolipolysis, to determine if what we’ve learned about targeting fat that might be applicable elsewhere.
Who inspires you most in your work today?
In addition to Dr. John Parrish and Dr. Thomas Fitzpatrick, the late Dr. Albert M. Kligman also influenced me. He never accepted dogma and he loved to ask questions, like, “What if?” as opposed to just accumulating a fund of knowledge. Understanding things is not just based on how much you know. It’s based on critical thinking and the ability to question. I also admire Albert Einstein, his ability to sit down with nothing more than pencil and paper and change our view of the universe. I love physics because it’s the science of everything. I also love poetry. My favorite poet is Stanley Kunitz. He had amazing insight and was named United States Poet Laureate in 1974 and in 2000. I have plenty of antiheroes as well, mostly politicians.
I understand that you play the banjo. How long have you been playing, and what do you enjoy about it?
You cannot sit down and play the banjo and have your mind on much else. It’s a wonderful moving meditation. Before my medical career, I was a schoolteacher in Vermont. There was a guy on the staff there who played banjo. He came from a small town in Georgia. I just picked it up and started plunking. It’s a happy instrument. It’s awfully hard to make the banjo sound melancholy.
What novel use of lasers and light in dermatology are you most excited about in the next 5 years?
The marriage of therapeutic devices with diagnostic and imaging devices has not happened yet. They are not even in the honeymoon moment. But I think that having truly smart robotic systems in our hands for treating patients will become a reality. These days, dermatologists have to buy a certain type of laser to treat a certain type of lesion. For example, the Q-switched alexandrite laser you buy for treating Nevus of Ota won’t do anything for a port-wine stain; it’s the wrong pulse duration. This means that clinicians who practice a lot of laser dermatology end up with a dozen lasers in their practice. In the future, I think it will be possible to have a software laser, so when you want to acquire another target, you load an App as opposed to buying a new laser. This means that you would have software programmable targeting, and you would not have the requirement of having selective absorption. So, I’m excited by the idea of guided fractional lasers. None of them exist now. We have to start from scratch.
Dr. Anderson, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, conceived and developed many of the nonscarring laser treatments now widely used in dermatology. These include selective photothermolysis for birthmarks, microvascular and pigmented lesions, and tattoo and permanent hair removal. He also contributed to laser lithotripsy, laser angioplasty, photodynamic therapy, and optical diagnostics. The highest-resolution imaging device approved for human use, an infrared confocal microscope, came from his laboratory. Dr. Anderson has also contributed to basic knowledge of human photobiology, drug photosensitization mechanisms, tissue optics and laser-tissue interactions. In this Q&A with Doug Brunk, he reflects on his achievements and on the future of lasers in dermatology.
In published interviews you have described yourself as more of a problem solver than an inventor. How did your upbringing foster your affinity for problem solving?
I grew up in Central Illinois during the 1950s and early 1960s, an area known for corn, soybeans, and hogs. At an early age I learned to be interested in other things because it’s possible to die of boredom there. By the time I was 12 years old, I was an amateur radio operator, and I was building rockets to see how high they would go.
Problem solving comes naturally to me. I enjoy very much finding a problem that is worth solving, which means getting passionate about it and brainstorming. Half the time you don’t come up with a potential route to solve the problem. I attended the Massachusetts Institute of Technology at the age of 17, which was a real eye-opener. I had never been east of the Wabash River prior to that. I studied physics for a while, then decided to flip into biology. That combination has served me well. My special sauce is to have some intuitive and academic rigorous feeling for physical processes. But we physicians have a front row seat to nature’s human drama. There is no lack of problems to solve. I can sit around and obsess about things theoretically, but at the end of the day I want to work on things that ultimately benefit people.
What inspired you most early in your career as a physician scientist?
After I made a commitment to medicine, Dr. John A. Parrish, and Dr. Thomas B. Fitzpatrick were key mentors to me. I was 30 years old when I started medical school, but they took me under their wing even before that. I took a part-time, temporary job with them, which turned into a permanent job. That turned into a love for the work they did. Instead of going into a graduate program in a laboratory and studying bacteria and genetics, the whole idea of working with people and on people was awesome. Dr. Parrish really mentored me. I won a lifetime achievement award from the American Academy of Dermatology a few years ago. I found myself on stage and it rolled out of my mouth that John Parrish believed in me before I believed in myself. It’s really true. He somehow recognized that I had some talents. I was very young and a combination of naive and humble, I guess.
What was the initial genesis for your idea of selective photothermolysis?
I was interested in going to medical school and working with Dr. Fitzpatrick and Dr. Parrish on things related to light. They were mostly interested in PUVA and UVB; it was the heyday of modern phototherapy. I attended a lecture at the Beth Israel Hospital in Boston given by a plastic surgeon, Dr. Joel Mark Noe. He was talking about using lasers to treat port-wine stains in children. The gist of the talk was that argon lasers were being used, and that the results were sometimes decent, but not great. Often children would have burn scars after the treatment. Dr. Noe was talking about how you had to choose the color of the wavelength of the laser to be absorbed by hemoglobin, but he wasn’t talking about what happens to the heat once it’s created. My background in physics led me to recognize that he wasn’t capturing the full picture. Selective disruption of a target in the skin by light is half of the story. The confinement of heat in the target is the other half of the story. Literally on a bus on the way home from that lecture to my apartment in Cambridge, I hatched the idea for selective photothermolysis and wrote down some equations. I also wrote down the ideal wavelength region, how much energy was needed, and what the pulse duration would have to be like to damage target vessels that small. I showed John Parrish what I had written. He took me seriously and said, “Let’s see if we can find a light source that can accomplish this.” We traveled around the country looking at various lasers, but we wound up building the first pulsed dye laser for treating port-wine stains. To me, the surprise was that we didn’t kill the skin. If you treat an area of skin with a laser and hurt all the blood vessels, you think, “Wait a minute. Are we going to kill the skin because it has no blood supply?” The questions of the day were so basic, and we just got lucky. It took 6-8 years before we ramped up the clinical studies showing efficacy and safety of this technology.
I presume that you experimented on your own skin while developing some of the nonscarring laser treatments now widely used in dermatology. What “war story” stands out to you most from that part of your work?
I’m right handed, so I’d grab a laser with my right hand and treat my left arm, so that arm sports a bit of history. In 1994, while working with Dr. Melanie Grossman on the development of laser hair removal, I used a ruby laser to self-treat a patch of hair on my left arm. I still have the world’s oldest laser-induced bald spot on that arm. It’s been 26 years now. I still look at it and count the hairs, because one of the big questions is, is laser hair removal permanent? In all these years I have grown two hairs.
What technology that you conceived of or developed has most surprised you, in term of its ultimate clinical impact?
I would say confocal microscopy. In the mid-1990s I worked with a physicist named Robert H. Webb, who invented an imaging system for the retina. We got together, noodled about it, and decided we would modify his ophthalmoscope system to see if we could get images from inside the skin. It worked pretty well. It was truly surprising from many points of view. First, it wasn’t clear at all that we’d get any images this way. Now, reflectance confocal microscopy is a standard tool in both clinical and research dermatology. But there were odd discoveries early on. For example, the darker your skin, the brighter it appeared in the microscope. You might think that melanin absorbs light and that you would get poor images in dark skin. It was the exact opposite; melanin acts as a natural contrast agent.
We worked with a small company to make the first confocal microscope. Initially, it had no clinical applications but what was fascinating to me was the incredible value of being able to see inside human skin harmlessly, and just see what’s going on. It became a potent research tool, and recently CPT codes were established for its use in evaluating skin cancer margins. I wouldn’t be surprised if 30 years from now, taking a skin biopsy is seemingly barbaric. A forerunner of all these new imaging tools for the skin was the confocal microscope developed in my lab in 1994.
During a 2011 TED talk, you said that nevus of Ota is your favorite thing to treat, because the outcome is usually perfect skin. Are there other technologies or devices you played a role in developing that make you proud at this stage in your career?
The reason I love treating nevus of Ota is that you have a lifelong facial disfigurement, and the only treatment for it is a laser we came up with, and it always works. How perfect could it be? The flip side of the same coin is, there are lesions of the skin that just don’t respond. One of the things we don’t know enough about is the connection between the biologic aspects of repair of various lesions and the treatments that we come up with. The most recent example of selective photothermolysis is a new laser we’re building right now for acne that is based on sebaceous gland injury. You’ll see this coming out in the next year or two. My heart goes out to people with nodular cystic acne. For young men it’s highly associated with suicide. So, I’m excited about optimizing and learning what happens when we target sebaceous glands.
One of the other big stories in laser dermatology is the fractional laser. I developed this with Dr. Dieter Manstein when he was a postdoc in my lab. One of the most pleasing things from this technology is how well you can rehabilitate scars, particularly burn scars in children. Over the last few years, I have trained plastic surgeons at the Shriners Hospital for Children in Boston on how to use fractional lasers to improve the lives of these kids. Another technology I developed with Dr. Manstein is cryolipolysis, which is removing fat from the body by cooling it. There are no lasers involved with this technology. I like to say that I’ve spent most of my career studying light and heat, and now we’ve come up with something that’s cold in the dark. We are now working on derivatives of cryolipolysis, to determine if what we’ve learned about targeting fat that might be applicable elsewhere.
Who inspires you most in your work today?
In addition to Dr. John Parrish and Dr. Thomas Fitzpatrick, the late Dr. Albert M. Kligman also influenced me. He never accepted dogma and he loved to ask questions, like, “What if?” as opposed to just accumulating a fund of knowledge. Understanding things is not just based on how much you know. It’s based on critical thinking and the ability to question. I also admire Albert Einstein, his ability to sit down with nothing more than pencil and paper and change our view of the universe. I love physics because it’s the science of everything. I also love poetry. My favorite poet is Stanley Kunitz. He had amazing insight and was named United States Poet Laureate in 1974 and in 2000. I have plenty of antiheroes as well, mostly politicians.
I understand that you play the banjo. How long have you been playing, and what do you enjoy about it?
You cannot sit down and play the banjo and have your mind on much else. It’s a wonderful moving meditation. Before my medical career, I was a schoolteacher in Vermont. There was a guy on the staff there who played banjo. He came from a small town in Georgia. I just picked it up and started plunking. It’s a happy instrument. It’s awfully hard to make the banjo sound melancholy.
What novel use of lasers and light in dermatology are you most excited about in the next 5 years?
The marriage of therapeutic devices with diagnostic and imaging devices has not happened yet. They are not even in the honeymoon moment. But I think that having truly smart robotic systems in our hands for treating patients will become a reality. These days, dermatologists have to buy a certain type of laser to treat a certain type of lesion. For example, the Q-switched alexandrite laser you buy for treating Nevus of Ota won’t do anything for a port-wine stain; it’s the wrong pulse duration. This means that clinicians who practice a lot of laser dermatology end up with a dozen lasers in their practice. In the future, I think it will be possible to have a software laser, so when you want to acquire another target, you load an App as opposed to buying a new laser. This means that you would have software programmable targeting, and you would not have the requirement of having selective absorption. So, I’m excited by the idea of guided fractional lasers. None of them exist now. We have to start from scratch.
Visionary reflects on the importance of teamwork in advancing technology
When John A. Parrish, MD, worked with R. Rox Anderson, MD, and a team of clinicians and scientists in the early 1980s to develop the first pulsed dye laser for dermatologic use, it became clear that the Food and Drug Administration required convincing that their prototype would be safe.
“Laser medicine was new, and lasers had some specific frightening risks like blindness and bleeding from laser suturing,” recalled Dr. Parrish, founder of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The main issue was eye risk. Because the operator and the patient were at risk for eye injury, the FDA was reluctant to press on with laser treatments of skin.”
To make the FDA more comfortable with their efforts, Dr. Parrish and his colleagues drew from the published work of ophthalmologists, who were ahead of dermatologists in the clinical use of lasers. “A lot of the animal experiments and the human understanding of laser-tissue interactions came from ophthalmologists,” he said. “We worked with a fellow named David H. Sliney, PhD. He was very interested in laser safety of the eye, so we worked closely with him to measure the boundary conditions that could be used without injuring the eye.”
To Dr. Parrish, forging that partnership illustrated a key principle in developing novel diagnostics and therapeutics that use lasers and light: You need a multidisciplinary team. “You need a pathologist, clinicians, physicists, technologists, and engineers, because all of the barriers to figure out how to deliver a new treatment safely often don’t rest in one person’s mind, so early on we had to be very collaborative and find experts who would help us solve problems,” he said. “That’s how the Wellman Labs got started. All of the new treatments were explored by multidisciplinary teams so that we didn’t have to hope that the expertise to get past all the barriers was in one person’s mind. That was often not the case.”
Dr. Parrish credits his mentor, the late Thomas B. Fitzpatrick, MD, PhD, who in 1975 devised the Fitzpatrick scale of skin phototypes, with inspiring his career path. Dr. Fitzpatrick, who is widely considered the father of modern academic dermatology, was professor and chief of dermatology at Harvard Medical School when Dr. Parrish began his dermatology training there. “He was a great clinician who loved patient care and he was a very curious investigator,” said Dr. Parrish, who cofounded the Consortia for Improving Medicine with Innovation and Technology (CIMIT). “He not only trained me, but I became his collaborator during my early faculty time. What I learned most from him was the joy of work, curiosity, and serious commitment to patient care. It was almost contagious.”
Of all the devices he’s played a role in developing in the past 50 years, Dr. Parrish said that he remains most surprised by the impact of pulsed lasers in dermatology. “It took us a while to understand the capabilities of pulsed lasers in that they could confine injury to small spots and treat multiple areas at once,” he said. “A lot of that did not come because we were so wise to think about that, but we did a lot of work in the early days with a free-electron laser, a pulsed laser which had a tunable wavelength and a tunable pulse duration. That gave us the capability of looking at very specific injuries and the host responses that heal without scarring.”
Dr. Parrish’s interest in dermatology was piqued in 1968, when he was assigned to Oak Knoll Naval Hospital in Oakland, Calif., after a year of serving in the U.S. Marine Corps as a battlefield doctor in Vietnam. (He wrote about his wartime experience in two books, most recently “Autopsy of War: A Personal History” [New York: Thomas Dunne Books, 2012].) Prior to serving in Vietnam he had completed early internal medicine training, but once at Oak Knoll he discovered that he had a propensity for diagnosing and treating disorders of the skin. “When I came back to resume my residency, I asked if I could train in dermatology,” he said. “It was by happenstance. I felt like I could understand skin disease and that I could make a difference. In internal medicine you often change blood pressure medicines around. I felt like I was a better diagnostician than in internal medicine and that I could most often make a difference. I liked seeing all ages of patients, and most of them got better, so it was more fun.”
When John A. Parrish, MD, worked with R. Rox Anderson, MD, and a team of clinicians and scientists in the early 1980s to develop the first pulsed dye laser for dermatologic use, it became clear that the Food and Drug Administration required convincing that their prototype would be safe.
“Laser medicine was new, and lasers had some specific frightening risks like blindness and bleeding from laser suturing,” recalled Dr. Parrish, founder of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The main issue was eye risk. Because the operator and the patient were at risk for eye injury, the FDA was reluctant to press on with laser treatments of skin.”
To make the FDA more comfortable with their efforts, Dr. Parrish and his colleagues drew from the published work of ophthalmologists, who were ahead of dermatologists in the clinical use of lasers. “A lot of the animal experiments and the human understanding of laser-tissue interactions came from ophthalmologists,” he said. “We worked with a fellow named David H. Sliney, PhD. He was very interested in laser safety of the eye, so we worked closely with him to measure the boundary conditions that could be used without injuring the eye.”
To Dr. Parrish, forging that partnership illustrated a key principle in developing novel diagnostics and therapeutics that use lasers and light: You need a multidisciplinary team. “You need a pathologist, clinicians, physicists, technologists, and engineers, because all of the barriers to figure out how to deliver a new treatment safely often don’t rest in one person’s mind, so early on we had to be very collaborative and find experts who would help us solve problems,” he said. “That’s how the Wellman Labs got started. All of the new treatments were explored by multidisciplinary teams so that we didn’t have to hope that the expertise to get past all the barriers was in one person’s mind. That was often not the case.”
Dr. Parrish credits his mentor, the late Thomas B. Fitzpatrick, MD, PhD, who in 1975 devised the Fitzpatrick scale of skin phototypes, with inspiring his career path. Dr. Fitzpatrick, who is widely considered the father of modern academic dermatology, was professor and chief of dermatology at Harvard Medical School when Dr. Parrish began his dermatology training there. “He was a great clinician who loved patient care and he was a very curious investigator,” said Dr. Parrish, who cofounded the Consortia for Improving Medicine with Innovation and Technology (CIMIT). “He not only trained me, but I became his collaborator during my early faculty time. What I learned most from him was the joy of work, curiosity, and serious commitment to patient care. It was almost contagious.”
Of all the devices he’s played a role in developing in the past 50 years, Dr. Parrish said that he remains most surprised by the impact of pulsed lasers in dermatology. “It took us a while to understand the capabilities of pulsed lasers in that they could confine injury to small spots and treat multiple areas at once,” he said. “A lot of that did not come because we were so wise to think about that, but we did a lot of work in the early days with a free-electron laser, a pulsed laser which had a tunable wavelength and a tunable pulse duration. That gave us the capability of looking at very specific injuries and the host responses that heal without scarring.”
Dr. Parrish’s interest in dermatology was piqued in 1968, when he was assigned to Oak Knoll Naval Hospital in Oakland, Calif., after a year of serving in the U.S. Marine Corps as a battlefield doctor in Vietnam. (He wrote about his wartime experience in two books, most recently “Autopsy of War: A Personal History” [New York: Thomas Dunne Books, 2012].) Prior to serving in Vietnam he had completed early internal medicine training, but once at Oak Knoll he discovered that he had a propensity for diagnosing and treating disorders of the skin. “When I came back to resume my residency, I asked if I could train in dermatology,” he said. “It was by happenstance. I felt like I could understand skin disease and that I could make a difference. In internal medicine you often change blood pressure medicines around. I felt like I was a better diagnostician than in internal medicine and that I could most often make a difference. I liked seeing all ages of patients, and most of them got better, so it was more fun.”
When John A. Parrish, MD, worked with R. Rox Anderson, MD, and a team of clinicians and scientists in the early 1980s to develop the first pulsed dye laser for dermatologic use, it became clear that the Food and Drug Administration required convincing that their prototype would be safe.
“Laser medicine was new, and lasers had some specific frightening risks like blindness and bleeding from laser suturing,” recalled Dr. Parrish, founder of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The main issue was eye risk. Because the operator and the patient were at risk for eye injury, the FDA was reluctant to press on with laser treatments of skin.”
To make the FDA more comfortable with their efforts, Dr. Parrish and his colleagues drew from the published work of ophthalmologists, who were ahead of dermatologists in the clinical use of lasers. “A lot of the animal experiments and the human understanding of laser-tissue interactions came from ophthalmologists,” he said. “We worked with a fellow named David H. Sliney, PhD. He was very interested in laser safety of the eye, so we worked closely with him to measure the boundary conditions that could be used without injuring the eye.”
To Dr. Parrish, forging that partnership illustrated a key principle in developing novel diagnostics and therapeutics that use lasers and light: You need a multidisciplinary team. “You need a pathologist, clinicians, physicists, technologists, and engineers, because all of the barriers to figure out how to deliver a new treatment safely often don’t rest in one person’s mind, so early on we had to be very collaborative and find experts who would help us solve problems,” he said. “That’s how the Wellman Labs got started. All of the new treatments were explored by multidisciplinary teams so that we didn’t have to hope that the expertise to get past all the barriers was in one person’s mind. That was often not the case.”
Dr. Parrish credits his mentor, the late Thomas B. Fitzpatrick, MD, PhD, who in 1975 devised the Fitzpatrick scale of skin phototypes, with inspiring his career path. Dr. Fitzpatrick, who is widely considered the father of modern academic dermatology, was professor and chief of dermatology at Harvard Medical School when Dr. Parrish began his dermatology training there. “He was a great clinician who loved patient care and he was a very curious investigator,” said Dr. Parrish, who cofounded the Consortia for Improving Medicine with Innovation and Technology (CIMIT). “He not only trained me, but I became his collaborator during my early faculty time. What I learned most from him was the joy of work, curiosity, and serious commitment to patient care. It was almost contagious.”
Of all the devices he’s played a role in developing in the past 50 years, Dr. Parrish said that he remains most surprised by the impact of pulsed lasers in dermatology. “It took us a while to understand the capabilities of pulsed lasers in that they could confine injury to small spots and treat multiple areas at once,” he said. “A lot of that did not come because we were so wise to think about that, but we did a lot of work in the early days with a free-electron laser, a pulsed laser which had a tunable wavelength and a tunable pulse duration. That gave us the capability of looking at very specific injuries and the host responses that heal without scarring.”
Dr. Parrish’s interest in dermatology was piqued in 1968, when he was assigned to Oak Knoll Naval Hospital in Oakland, Calif., after a year of serving in the U.S. Marine Corps as a battlefield doctor in Vietnam. (He wrote about his wartime experience in two books, most recently “Autopsy of War: A Personal History” [New York: Thomas Dunne Books, 2012].) Prior to serving in Vietnam he had completed early internal medicine training, but once at Oak Knoll he discovered that he had a propensity for diagnosing and treating disorders of the skin. “When I came back to resume my residency, I asked if I could train in dermatology,” he said. “It was by happenstance. I felt like I could understand skin disease and that I could make a difference. In internal medicine you often change blood pressure medicines around. I felt like I was a better diagnostician than in internal medicine and that I could most often make a difference. I liked seeing all ages of patients, and most of them got better, so it was more fun.”