User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Election gift for Florida? Trump poised to approve drug imports from Canada
Over the objections of drugmakers, the Trump administration is expected within weeks to finalize its plan that would allow states to import some prescription medicines from Canada.
Six states – Colorado, Florida, Maine, New Hampshire, New Mexico, and Vermont – have passed laws allowing them to seek federal approval to buy drugs from Canada to give their residents access to lower-cost medicines.
But industry observers say the drug importation proposal under review by the administration is squarely aimed at Florida – the most populous swing state in the November election. Trump’s support of the idea initially came at the urging of Florida Gov. Ron DeSantis, a close Republican ally.
The DeSantis administration is so confident Trump will move ahead with allowing drug importation that it put out a request June 30 for private companies to bid on a three-year, $30 million contract to run the program. It hopes to award the contract in December.
Industry experts say Florida is likely to be the first state to win federal approval for a drug importation plan – something that could occur before the November election.
“Approving Florida would feel like the politically astute thing to do,” said Mara Baer, a health consultant who has worked with Colorado on its importation proposal.
Ben England, CEO of FDAImports, a consulting firm in Glen Burnie, Maryland, said the OMB typically has 60 days to review final rules, although he expects this one could be completed before Nov. 3 and predicted there’s a small chance it could get finalized and Florida’s request approved by then. “It’s an election year, so I do see the current administration trying to use this as a talking point to say ‘Look what we’ve accomplished,’” he said.
Florida also makes sense because of the large number of retirees, who face high costs for medicines despite Medicare drug coverage.
The DeSantis administration did not respond to requests for comment.
Trump boasted about his importation plan during an October speech in The Villages, a large retirement community about 60 miles northwest of Orlando. “We will soon allow the safe and legal importation of prescription drugs from other countries, including the country of Canada, where, believe it or not, they pay much less money for the exact same drug,” Trump said, with DeSantis in attendance. “Stand up, Ron. Boy, he wants this so badly.”
The Food and Drug Administration released a detailed proposal last December and sought comments. A final plan was delivered Sept. 10 to the Office of Management and Budget for review, signaling it could be unveiled within weeks.
The proposal would regulate how states set up their own programs for importing drugs from Canada.
Prices are cheaper because Canada limits how much drugmakers can charge for medicines. The United States lets free markets dictate drug prices.
The pharmaceutical industry signaled it will likely sue the Trump administration if it goes forward with its importation plans, saying the plan violates several federal laws and the U.S. Constitution.
But the most stinging rebuke of the Trump importation plan came from the Canadian government, which said the proposal would make it harder for Canadian citizens to get drugs, putting their health at risk.
“Canada will employ all necessary measures to safeguard access for Canadians to needed drugs,” the Canadian government wrote in a letter to the FDA about the draft proposal. “The Canadian drug market and manufacturing capacity are too small to meet the demand of both Canadian and American consumers for prescription drugs.”
Without buy-in from Canada, any plan to import medicines is unlikely to succeed, officials said.
Ena Backus, director of Health Care Reform in Vermont, who has worked on setting up an importation program there, said states will need help from Canada. “Our state importation program relies on a willing partner in Canada,” she said.
For decades, Americans have been buying drugs from Canada for personal use — either by driving over the border, ordering medication on the Internet, or using storefronts that connect them to foreign pharmacies. Though illegal, the FDA has generally permitted purchases for individual use.
About 4 million Americans import lower-cost medicines for personal use each year, and about 20 million say they or someone in their household have done so because the prices are much lower in other countries, according to surveys.
The practice has been popular in Florida. More than a dozen storefronts across the state help consumers connect to pharmacies in Canada and other countries. Several cities, state and school districts in Florida help employees get drugs from Canada.
The administration’s proposal builds on a 2000 law that opened the door to allowing drug importation from Canada. But that provision could take effect only if the Health and Human Services secretary certified importation as safe, something that Democratic and Republican administrations have refused to do.
The drug industry for years has said allowing drugs to be imported from Canada would disrupt the nation’s supply chain and make it easier for unsafe or counterfeit medications to enter the market.
Trump, who made lowering prescription drug prices a signature promise in his 2016 campaign, has been eager to fulfill his pledge. In July 2019, at Trump’s direction, HHS Secretary Alex Azar said the federal government was “open for business” on drug importation, a year after calling drug importation a “gimmick.”
The administration envisions a system in which a Canadian-licensed wholesaler buys directly from a manufacturer for drugs approved for sale in Canada and exports the drugs to a U.S. wholesaler/importer under contract to a state.
Florida’s legislation – approved in 2019 – would set up two importation programs. The first would focus on getting drugs for state programs such as Medicaid, the Department of Corrections and county health departments. State officials said they expect the programs would save the state about $150 million annually.
The second program would be geared to the broader state population.
In response to the draft rule, the states seeking to start a drug importation program suggested changes to the administration’s proposal.
“Should the final rule not address these areas of concern, Colorado will struggle to find appropriate partners and realize significant savings for consumers,” Kim Bimestefer, executive director of the Colorado Department of Health Care Policy & Financing, told the FDA in March.
Among the state’s concerns is that it would be limited to using only one Canadian wholesaler, and without competition the state fears prices might not be as low as officials hoped. Bimestefer also noted that under the draft rule, the federal government would approve the importation program for only two years and states need a longer time frame to get buy-in from wholesalers and other partners.
Colorado officials estimate importing drugs from Canada could cut prices by 54% for cancer drugs and 75% for cardiac medicines. The state also noted the diabetes drug Jardiance costs $400 a month in the United States and sells for $85 in Canada.
Several states worry some of the most expensive drugs – including injectable and biologic medicines – were exempt from the federal rule. Those drug classes are not allowed to be imported under the 2000 law.
However, in an executive order in July, Trump said he would allow insulin to be imported if Azar determined it is required for emergency medical care. An HHS spokesman would not say whether Azar has done that.
Jane Horvath, a health policy consultant in College Park, Md., said the administration faces several challenges getting an importation program up and running, including possible opposition from the pharmaceutical industry and limits on classes of drugs that can be sold over the border.
“Despite the barriers, the programs are still quite worthwhile to pursue,” she said.
Maine’s top health official said the administration should work with the Canadian government to address Canada’s concerns. HHS officials refused to say whether such discussions have started.
Officials in Vermont, where the program would also include consumers covered by private insurance, remain hopeful.
“Given that we want to reduce the burden of health care costs on residents in our state, then it is important to pursue this option if there is a clear pathway forward,” Backus said.
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This story also ran on Miami Herald.
Over the objections of drugmakers, the Trump administration is expected within weeks to finalize its plan that would allow states to import some prescription medicines from Canada.
Six states – Colorado, Florida, Maine, New Hampshire, New Mexico, and Vermont – have passed laws allowing them to seek federal approval to buy drugs from Canada to give their residents access to lower-cost medicines.
But industry observers say the drug importation proposal under review by the administration is squarely aimed at Florida – the most populous swing state in the November election. Trump’s support of the idea initially came at the urging of Florida Gov. Ron DeSantis, a close Republican ally.
The DeSantis administration is so confident Trump will move ahead with allowing drug importation that it put out a request June 30 for private companies to bid on a three-year, $30 million contract to run the program. It hopes to award the contract in December.
Industry experts say Florida is likely to be the first state to win federal approval for a drug importation plan – something that could occur before the November election.
“Approving Florida would feel like the politically astute thing to do,” said Mara Baer, a health consultant who has worked with Colorado on its importation proposal.
Ben England, CEO of FDAImports, a consulting firm in Glen Burnie, Maryland, said the OMB typically has 60 days to review final rules, although he expects this one could be completed before Nov. 3 and predicted there’s a small chance it could get finalized and Florida’s request approved by then. “It’s an election year, so I do see the current administration trying to use this as a talking point to say ‘Look what we’ve accomplished,’” he said.
Florida also makes sense because of the large number of retirees, who face high costs for medicines despite Medicare drug coverage.
The DeSantis administration did not respond to requests for comment.
Trump boasted about his importation plan during an October speech in The Villages, a large retirement community about 60 miles northwest of Orlando. “We will soon allow the safe and legal importation of prescription drugs from other countries, including the country of Canada, where, believe it or not, they pay much less money for the exact same drug,” Trump said, with DeSantis in attendance. “Stand up, Ron. Boy, he wants this so badly.”
The Food and Drug Administration released a detailed proposal last December and sought comments. A final plan was delivered Sept. 10 to the Office of Management and Budget for review, signaling it could be unveiled within weeks.
The proposal would regulate how states set up their own programs for importing drugs from Canada.
Prices are cheaper because Canada limits how much drugmakers can charge for medicines. The United States lets free markets dictate drug prices.
The pharmaceutical industry signaled it will likely sue the Trump administration if it goes forward with its importation plans, saying the plan violates several federal laws and the U.S. Constitution.
But the most stinging rebuke of the Trump importation plan came from the Canadian government, which said the proposal would make it harder for Canadian citizens to get drugs, putting their health at risk.
“Canada will employ all necessary measures to safeguard access for Canadians to needed drugs,” the Canadian government wrote in a letter to the FDA about the draft proposal. “The Canadian drug market and manufacturing capacity are too small to meet the demand of both Canadian and American consumers for prescription drugs.”
Without buy-in from Canada, any plan to import medicines is unlikely to succeed, officials said.
Ena Backus, director of Health Care Reform in Vermont, who has worked on setting up an importation program there, said states will need help from Canada. “Our state importation program relies on a willing partner in Canada,” she said.
For decades, Americans have been buying drugs from Canada for personal use — either by driving over the border, ordering medication on the Internet, or using storefronts that connect them to foreign pharmacies. Though illegal, the FDA has generally permitted purchases for individual use.
About 4 million Americans import lower-cost medicines for personal use each year, and about 20 million say they or someone in their household have done so because the prices are much lower in other countries, according to surveys.
The practice has been popular in Florida. More than a dozen storefronts across the state help consumers connect to pharmacies in Canada and other countries. Several cities, state and school districts in Florida help employees get drugs from Canada.
The administration’s proposal builds on a 2000 law that opened the door to allowing drug importation from Canada. But that provision could take effect only if the Health and Human Services secretary certified importation as safe, something that Democratic and Republican administrations have refused to do.
The drug industry for years has said allowing drugs to be imported from Canada would disrupt the nation’s supply chain and make it easier for unsafe or counterfeit medications to enter the market.
Trump, who made lowering prescription drug prices a signature promise in his 2016 campaign, has been eager to fulfill his pledge. In July 2019, at Trump’s direction, HHS Secretary Alex Azar said the federal government was “open for business” on drug importation, a year after calling drug importation a “gimmick.”
The administration envisions a system in which a Canadian-licensed wholesaler buys directly from a manufacturer for drugs approved for sale in Canada and exports the drugs to a U.S. wholesaler/importer under contract to a state.
Florida’s legislation – approved in 2019 – would set up two importation programs. The first would focus on getting drugs for state programs such as Medicaid, the Department of Corrections and county health departments. State officials said they expect the programs would save the state about $150 million annually.
The second program would be geared to the broader state population.
In response to the draft rule, the states seeking to start a drug importation program suggested changes to the administration’s proposal.
“Should the final rule not address these areas of concern, Colorado will struggle to find appropriate partners and realize significant savings for consumers,” Kim Bimestefer, executive director of the Colorado Department of Health Care Policy & Financing, told the FDA in March.
Among the state’s concerns is that it would be limited to using only one Canadian wholesaler, and without competition the state fears prices might not be as low as officials hoped. Bimestefer also noted that under the draft rule, the federal government would approve the importation program for only two years and states need a longer time frame to get buy-in from wholesalers and other partners.
Colorado officials estimate importing drugs from Canada could cut prices by 54% for cancer drugs and 75% for cardiac medicines. The state also noted the diabetes drug Jardiance costs $400 a month in the United States and sells for $85 in Canada.
Several states worry some of the most expensive drugs – including injectable and biologic medicines – were exempt from the federal rule. Those drug classes are not allowed to be imported under the 2000 law.
However, in an executive order in July, Trump said he would allow insulin to be imported if Azar determined it is required for emergency medical care. An HHS spokesman would not say whether Azar has done that.
Jane Horvath, a health policy consultant in College Park, Md., said the administration faces several challenges getting an importation program up and running, including possible opposition from the pharmaceutical industry and limits on classes of drugs that can be sold over the border.
“Despite the barriers, the programs are still quite worthwhile to pursue,” she said.
Maine’s top health official said the administration should work with the Canadian government to address Canada’s concerns. HHS officials refused to say whether such discussions have started.
Officials in Vermont, where the program would also include consumers covered by private insurance, remain hopeful.
“Given that we want to reduce the burden of health care costs on residents in our state, then it is important to pursue this option if there is a clear pathway forward,” Backus said.
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This story also ran on Miami Herald.
Over the objections of drugmakers, the Trump administration is expected within weeks to finalize its plan that would allow states to import some prescription medicines from Canada.
Six states – Colorado, Florida, Maine, New Hampshire, New Mexico, and Vermont – have passed laws allowing them to seek federal approval to buy drugs from Canada to give their residents access to lower-cost medicines.
But industry observers say the drug importation proposal under review by the administration is squarely aimed at Florida – the most populous swing state in the November election. Trump’s support of the idea initially came at the urging of Florida Gov. Ron DeSantis, a close Republican ally.
The DeSantis administration is so confident Trump will move ahead with allowing drug importation that it put out a request June 30 for private companies to bid on a three-year, $30 million contract to run the program. It hopes to award the contract in December.
Industry experts say Florida is likely to be the first state to win federal approval for a drug importation plan – something that could occur before the November election.
“Approving Florida would feel like the politically astute thing to do,” said Mara Baer, a health consultant who has worked with Colorado on its importation proposal.
Ben England, CEO of FDAImports, a consulting firm in Glen Burnie, Maryland, said the OMB typically has 60 days to review final rules, although he expects this one could be completed before Nov. 3 and predicted there’s a small chance it could get finalized and Florida’s request approved by then. “It’s an election year, so I do see the current administration trying to use this as a talking point to say ‘Look what we’ve accomplished,’” he said.
Florida also makes sense because of the large number of retirees, who face high costs for medicines despite Medicare drug coverage.
The DeSantis administration did not respond to requests for comment.
Trump boasted about his importation plan during an October speech in The Villages, a large retirement community about 60 miles northwest of Orlando. “We will soon allow the safe and legal importation of prescription drugs from other countries, including the country of Canada, where, believe it or not, they pay much less money for the exact same drug,” Trump said, with DeSantis in attendance. “Stand up, Ron. Boy, he wants this so badly.”
The Food and Drug Administration released a detailed proposal last December and sought comments. A final plan was delivered Sept. 10 to the Office of Management and Budget for review, signaling it could be unveiled within weeks.
The proposal would regulate how states set up their own programs for importing drugs from Canada.
Prices are cheaper because Canada limits how much drugmakers can charge for medicines. The United States lets free markets dictate drug prices.
The pharmaceutical industry signaled it will likely sue the Trump administration if it goes forward with its importation plans, saying the plan violates several federal laws and the U.S. Constitution.
But the most stinging rebuke of the Trump importation plan came from the Canadian government, which said the proposal would make it harder for Canadian citizens to get drugs, putting their health at risk.
“Canada will employ all necessary measures to safeguard access for Canadians to needed drugs,” the Canadian government wrote in a letter to the FDA about the draft proposal. “The Canadian drug market and manufacturing capacity are too small to meet the demand of both Canadian and American consumers for prescription drugs.”
Without buy-in from Canada, any plan to import medicines is unlikely to succeed, officials said.
Ena Backus, director of Health Care Reform in Vermont, who has worked on setting up an importation program there, said states will need help from Canada. “Our state importation program relies on a willing partner in Canada,” she said.
For decades, Americans have been buying drugs from Canada for personal use — either by driving over the border, ordering medication on the Internet, or using storefronts that connect them to foreign pharmacies. Though illegal, the FDA has generally permitted purchases for individual use.
About 4 million Americans import lower-cost medicines for personal use each year, and about 20 million say they or someone in their household have done so because the prices are much lower in other countries, according to surveys.
The practice has been popular in Florida. More than a dozen storefronts across the state help consumers connect to pharmacies in Canada and other countries. Several cities, state and school districts in Florida help employees get drugs from Canada.
The administration’s proposal builds on a 2000 law that opened the door to allowing drug importation from Canada. But that provision could take effect only if the Health and Human Services secretary certified importation as safe, something that Democratic and Republican administrations have refused to do.
The drug industry for years has said allowing drugs to be imported from Canada would disrupt the nation’s supply chain and make it easier for unsafe or counterfeit medications to enter the market.
Trump, who made lowering prescription drug prices a signature promise in his 2016 campaign, has been eager to fulfill his pledge. In July 2019, at Trump’s direction, HHS Secretary Alex Azar said the federal government was “open for business” on drug importation, a year after calling drug importation a “gimmick.”
The administration envisions a system in which a Canadian-licensed wholesaler buys directly from a manufacturer for drugs approved for sale in Canada and exports the drugs to a U.S. wholesaler/importer under contract to a state.
Florida’s legislation – approved in 2019 – would set up two importation programs. The first would focus on getting drugs for state programs such as Medicaid, the Department of Corrections and county health departments. State officials said they expect the programs would save the state about $150 million annually.
The second program would be geared to the broader state population.
In response to the draft rule, the states seeking to start a drug importation program suggested changes to the administration’s proposal.
“Should the final rule not address these areas of concern, Colorado will struggle to find appropriate partners and realize significant savings for consumers,” Kim Bimestefer, executive director of the Colorado Department of Health Care Policy & Financing, told the FDA in March.
Among the state’s concerns is that it would be limited to using only one Canadian wholesaler, and without competition the state fears prices might not be as low as officials hoped. Bimestefer also noted that under the draft rule, the federal government would approve the importation program for only two years and states need a longer time frame to get buy-in from wholesalers and other partners.
Colorado officials estimate importing drugs from Canada could cut prices by 54% for cancer drugs and 75% for cardiac medicines. The state also noted the diabetes drug Jardiance costs $400 a month in the United States and sells for $85 in Canada.
Several states worry some of the most expensive drugs – including injectable and biologic medicines – were exempt from the federal rule. Those drug classes are not allowed to be imported under the 2000 law.
However, in an executive order in July, Trump said he would allow insulin to be imported if Azar determined it is required for emergency medical care. An HHS spokesman would not say whether Azar has done that.
Jane Horvath, a health policy consultant in College Park, Md., said the administration faces several challenges getting an importation program up and running, including possible opposition from the pharmaceutical industry and limits on classes of drugs that can be sold over the border.
“Despite the barriers, the programs are still quite worthwhile to pursue,” she said.
Maine’s top health official said the administration should work with the Canadian government to address Canada’s concerns. HHS officials refused to say whether such discussions have started.
Officials in Vermont, where the program would also include consumers covered by private insurance, remain hopeful.
“Given that we want to reduce the burden of health care costs on residents in our state, then it is important to pursue this option if there is a clear pathway forward,” Backus said.
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This story also ran on Miami Herald.
Zeus’s thunderbolt and work-life balance
Like most of you, I had always thought I was invincible and would live forever, but the kids had gotten out of the house (at least for now) and Medicare and Social Security were looming, so it was time to redo the will and think about estate planning. And then I met Zeus’ thunderbolt!
For the last couple of years, I had been getting dizzy when pulling weeds, and 2 months ago, felt like I had been hit by a truck. One hour of yard work and I had to lie in the grass and pant like a dog, or pass out. My internist and I both had COVID-19 on the brain, but all tests were negative; however, the chest x-ray showed atelectasis. What? So I had good and bad days, and noticed that my pulse was 150 when I climbed two flights of stairs. Finally, my wife insisted I get an EKG, which showed atrial flutter/fibrillation and a new right-bundle branch block.
I was ruled out for infarction or a pulmonary embolism and after 6 days on heparin, confirmation that I had clean coronaries (yes, statins should be in the water), and no atrial clot, I got to meet Zeus. They all say, “oh you won’t remember it” or “it won’t hurt.” But let me tell you, the closest thing I recall to having 200 joules shot through you from front to back is being kicked across the milk barn by an angry cow. I hollered, I don’t recall exactly what, but hey, Mr. Sinus Rhythm, my new long-lost friend was back.
I feel great again and have since had an ablation (no big deal, but they poke a hole in your atrium!) and have been fitted with a sleep snorkel, and can once again work all day in the yard.
Personally, meeting Zeus has changed my perspective on life, retirement and estate planning, and personal perspective on work. I highly recommend the book, “Wealth Planning for the Modern Physician: Residency to Retirement.” It is a pretty easy read and an eye opener. If taxes increase, and I don’t see how they can’t considering pandemic expenses, careful planning will be more important than ever.
After careful consideration, we are going to give most of our money to charities (institutions my wife and I we figure we owe a debt), such as colleges and residency programs. I am also going to make an active effort to cut back on work I don’t enjoy (goodbye endless committee meetings!), though I do enjoy my patients very much and will continue to practice. I relish the conversation and interaction, but hate the human relations part of practice management. I’m not much of a golfer, but enjoy gardening, writing, and public speaking. I love my children a lot, but I am still waiting for them to make their personal orbit around the universe so they can come home and be close again. We may also build a house in Florida with a terrace where we can garden without the deer’s help.
There was a recent survey that showed a lot of physicians are reconsidering their practice environments amid COVID-19 and as many as 5% considering retirement. It is also the wrong time for physicians to be retiring when 10,000 baby boomers a day are turning 65, and some of them will need thunderbolts like me.
I also enjoy writing this column and sharing my life with you. It is an incredibly special thing to be able to openly share one’s loves, fears, and hopes with colleagues. So keep practicing, remember not all hoof beats are from COVID-19, try not to spoil the kids too much, keep one eye on the calendar, and beware Zeus’ thunderbolt!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Like most of you, I had always thought I was invincible and would live forever, but the kids had gotten out of the house (at least for now) and Medicare and Social Security were looming, so it was time to redo the will and think about estate planning. And then I met Zeus’ thunderbolt!
For the last couple of years, I had been getting dizzy when pulling weeds, and 2 months ago, felt like I had been hit by a truck. One hour of yard work and I had to lie in the grass and pant like a dog, or pass out. My internist and I both had COVID-19 on the brain, but all tests were negative; however, the chest x-ray showed atelectasis. What? So I had good and bad days, and noticed that my pulse was 150 when I climbed two flights of stairs. Finally, my wife insisted I get an EKG, which showed atrial flutter/fibrillation and a new right-bundle branch block.
I was ruled out for infarction or a pulmonary embolism and after 6 days on heparin, confirmation that I had clean coronaries (yes, statins should be in the water), and no atrial clot, I got to meet Zeus. They all say, “oh you won’t remember it” or “it won’t hurt.” But let me tell you, the closest thing I recall to having 200 joules shot through you from front to back is being kicked across the milk barn by an angry cow. I hollered, I don’t recall exactly what, but hey, Mr. Sinus Rhythm, my new long-lost friend was back.
I feel great again and have since had an ablation (no big deal, but they poke a hole in your atrium!) and have been fitted with a sleep snorkel, and can once again work all day in the yard.
Personally, meeting Zeus has changed my perspective on life, retirement and estate planning, and personal perspective on work. I highly recommend the book, “Wealth Planning for the Modern Physician: Residency to Retirement.” It is a pretty easy read and an eye opener. If taxes increase, and I don’t see how they can’t considering pandemic expenses, careful planning will be more important than ever.
After careful consideration, we are going to give most of our money to charities (institutions my wife and I we figure we owe a debt), such as colleges and residency programs. I am also going to make an active effort to cut back on work I don’t enjoy (goodbye endless committee meetings!), though I do enjoy my patients very much and will continue to practice. I relish the conversation and interaction, but hate the human relations part of practice management. I’m not much of a golfer, but enjoy gardening, writing, and public speaking. I love my children a lot, but I am still waiting for them to make their personal orbit around the universe so they can come home and be close again. We may also build a house in Florida with a terrace where we can garden without the deer’s help.
There was a recent survey that showed a lot of physicians are reconsidering their practice environments amid COVID-19 and as many as 5% considering retirement. It is also the wrong time for physicians to be retiring when 10,000 baby boomers a day are turning 65, and some of them will need thunderbolts like me.
I also enjoy writing this column and sharing my life with you. It is an incredibly special thing to be able to openly share one’s loves, fears, and hopes with colleagues. So keep practicing, remember not all hoof beats are from COVID-19, try not to spoil the kids too much, keep one eye on the calendar, and beware Zeus’ thunderbolt!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Like most of you, I had always thought I was invincible and would live forever, but the kids had gotten out of the house (at least for now) and Medicare and Social Security were looming, so it was time to redo the will and think about estate planning. And then I met Zeus’ thunderbolt!
For the last couple of years, I had been getting dizzy when pulling weeds, and 2 months ago, felt like I had been hit by a truck. One hour of yard work and I had to lie in the grass and pant like a dog, or pass out. My internist and I both had COVID-19 on the brain, but all tests were negative; however, the chest x-ray showed atelectasis. What? So I had good and bad days, and noticed that my pulse was 150 when I climbed two flights of stairs. Finally, my wife insisted I get an EKG, which showed atrial flutter/fibrillation and a new right-bundle branch block.
I was ruled out for infarction or a pulmonary embolism and after 6 days on heparin, confirmation that I had clean coronaries (yes, statins should be in the water), and no atrial clot, I got to meet Zeus. They all say, “oh you won’t remember it” or “it won’t hurt.” But let me tell you, the closest thing I recall to having 200 joules shot through you from front to back is being kicked across the milk barn by an angry cow. I hollered, I don’t recall exactly what, but hey, Mr. Sinus Rhythm, my new long-lost friend was back.
I feel great again and have since had an ablation (no big deal, but they poke a hole in your atrium!) and have been fitted with a sleep snorkel, and can once again work all day in the yard.
Personally, meeting Zeus has changed my perspective on life, retirement and estate planning, and personal perspective on work. I highly recommend the book, “Wealth Planning for the Modern Physician: Residency to Retirement.” It is a pretty easy read and an eye opener. If taxes increase, and I don’t see how they can’t considering pandemic expenses, careful planning will be more important than ever.
After careful consideration, we are going to give most of our money to charities (institutions my wife and I we figure we owe a debt), such as colleges and residency programs. I am also going to make an active effort to cut back on work I don’t enjoy (goodbye endless committee meetings!), though I do enjoy my patients very much and will continue to practice. I relish the conversation and interaction, but hate the human relations part of practice management. I’m not much of a golfer, but enjoy gardening, writing, and public speaking. I love my children a lot, but I am still waiting for them to make their personal orbit around the universe so they can come home and be close again. We may also build a house in Florida with a terrace where we can garden without the deer’s help.
There was a recent survey that showed a lot of physicians are reconsidering their practice environments amid COVID-19 and as many as 5% considering retirement. It is also the wrong time for physicians to be retiring when 10,000 baby boomers a day are turning 65, and some of them will need thunderbolts like me.
I also enjoy writing this column and sharing my life with you. It is an incredibly special thing to be able to openly share one’s loves, fears, and hopes with colleagues. So keep practicing, remember not all hoof beats are from COVID-19, try not to spoil the kids too much, keep one eye on the calendar, and beware Zeus’ thunderbolt!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Promising drugs line up for lupus treatment
Systemic lupus erythematosus remains a treatment challenge, but a variety of drugs in the pipeline are set to target type I interferons, cytokines, and B cells, according to Richard Furie, MD, chief of the division of rheumatology at Northwell Health and professor of medicine at Hofstra University, Hempstead, N.Y.
In general, when treating patients with systemic lupus erythematosus (SLE), “we just don’t see satisfactory response rates,” Dr. Furie said in an online presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
“I think the greatest unmet need is in lupus nephritis,” he said. The data show that not even one-third of patients are adequately responding to standard of care treatment. “We need to do better in lupus nephritis but also for those patients with moderate-severe manifestations outside the kidney.”
Patients with SLE have elevated levels of interferon-alpha, Dr. Furie noted. Data from recent studies show that interferon inhibitors can reduce clinical activity in SLE patients, he said.
“About two-thirds to three-quarters of lupus patients have evidence of interferon pathway activation,” he said. There are three types of interferons, and five subtypes of type I interferon, and all five subtypes of type I interferon bind to the same receptor, which is an important strategy for drug development.
In particular, recent phase 2 and 3 trials have focused on targeting type I interferons with anifrolumab, which blocks all five subtypes.
Dr. Furie cited “very robust results” from a phase 2 study. Results of two phase 3 trials of anifrolumab led to a split decision, but the totality of the data collected across the phase 2 and 3 studies points to a drug that is effective for patients with SLE. The two phase 3 studies were published in Lancet Rheumatology and the New England Journal of Medicine.
Dr. Furie also identified recent studies of baricitinib (Olumiant), which has the ability to target several different cytokines. A phase 2 study in 2018 showed a significant difference in SLE Responder Index between lupus patients who received 4 mg of baricitinib or placebo, and a phase 3 study is underway, he said.
For lupus nephritis, Dr. Furie cited the BLISS-LN trial, a 104-week, randomized trial of patients with active lupus nephritis. The group of patients who received belimumab (Benlysta), a monoclonal antibody that targets B-cell activating factor, in addition to standard therapy had significant improvements in renal responses, compared with standard therapy alone (43.0% vs. 32.3%). The outcome measure was Primary Efficacy Renal Response, defined as urinary protein/creatinine ratio <0.7, eGFR ≥60 mL/min per 1.73 m2, confirmation on consecutive visits, and required tapering of background glucocorticoids.
Although belimumab was approved for SLE in 2011, the BLISS-LN study focused on SLE patients with active kidney disease. “Neutralizing B-cell activating factor and down-regulating autoreactive B-cell function in kidneys represented a compelling therapeutic approach to lupus nephritis,” he explained.
Voclosporin, distinct from cyclosporine, has also been studied in lupus nephritis, Dr. Furie said. Voclosporin offers several benefits over cyclosporine, including greater potency and a lower drug and metabolite load, as well as a more consistent pharmacokinetic and pharmacodynamic relationship, he said. In the phase 3 AURORA study, presented at this year’s EULAR congress, 40% of patients with lupus nephritis met the primary endpoint of a renal response at 52 weeks, compared with 22.5% of placebo patients.
Looking ahead to the treatment of SLE in 2021, “I feel very strongly that voclosporin will be approved for lupus nephritis,” he said. He also predicted that the use of belimumab will be officially extended for lupus nephritis and that anifrolumab will receive an approval for SLE patients.
In addition, the future may witness the increased use of biomarkers and development of more individualized therapy. These breakthroughs will yield better outcomes for all lupus patients, he said.
Dr. Furie disclosed relationships with GlaxoSmithKline, Genentech/Roche, Aurinia Pharmaceuticals, AstraZeneca/MedImmune, and Eli Lilly. Global Academy for Medical Education and this news organization are owned by the same parent company.
Systemic lupus erythematosus remains a treatment challenge, but a variety of drugs in the pipeline are set to target type I interferons, cytokines, and B cells, according to Richard Furie, MD, chief of the division of rheumatology at Northwell Health and professor of medicine at Hofstra University, Hempstead, N.Y.
In general, when treating patients with systemic lupus erythematosus (SLE), “we just don’t see satisfactory response rates,” Dr. Furie said in an online presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
“I think the greatest unmet need is in lupus nephritis,” he said. The data show that not even one-third of patients are adequately responding to standard of care treatment. “We need to do better in lupus nephritis but also for those patients with moderate-severe manifestations outside the kidney.”
Patients with SLE have elevated levels of interferon-alpha, Dr. Furie noted. Data from recent studies show that interferon inhibitors can reduce clinical activity in SLE patients, he said.
“About two-thirds to three-quarters of lupus patients have evidence of interferon pathway activation,” he said. There are three types of interferons, and five subtypes of type I interferon, and all five subtypes of type I interferon bind to the same receptor, which is an important strategy for drug development.
In particular, recent phase 2 and 3 trials have focused on targeting type I interferons with anifrolumab, which blocks all five subtypes.
Dr. Furie cited “very robust results” from a phase 2 study. Results of two phase 3 trials of anifrolumab led to a split decision, but the totality of the data collected across the phase 2 and 3 studies points to a drug that is effective for patients with SLE. The two phase 3 studies were published in Lancet Rheumatology and the New England Journal of Medicine.
Dr. Furie also identified recent studies of baricitinib (Olumiant), which has the ability to target several different cytokines. A phase 2 study in 2018 showed a significant difference in SLE Responder Index between lupus patients who received 4 mg of baricitinib or placebo, and a phase 3 study is underway, he said.
For lupus nephritis, Dr. Furie cited the BLISS-LN trial, a 104-week, randomized trial of patients with active lupus nephritis. The group of patients who received belimumab (Benlysta), a monoclonal antibody that targets B-cell activating factor, in addition to standard therapy had significant improvements in renal responses, compared with standard therapy alone (43.0% vs. 32.3%). The outcome measure was Primary Efficacy Renal Response, defined as urinary protein/creatinine ratio <0.7, eGFR ≥60 mL/min per 1.73 m2, confirmation on consecutive visits, and required tapering of background glucocorticoids.
Although belimumab was approved for SLE in 2011, the BLISS-LN study focused on SLE patients with active kidney disease. “Neutralizing B-cell activating factor and down-regulating autoreactive B-cell function in kidneys represented a compelling therapeutic approach to lupus nephritis,” he explained.
Voclosporin, distinct from cyclosporine, has also been studied in lupus nephritis, Dr. Furie said. Voclosporin offers several benefits over cyclosporine, including greater potency and a lower drug and metabolite load, as well as a more consistent pharmacokinetic and pharmacodynamic relationship, he said. In the phase 3 AURORA study, presented at this year’s EULAR congress, 40% of patients with lupus nephritis met the primary endpoint of a renal response at 52 weeks, compared with 22.5% of placebo patients.
Looking ahead to the treatment of SLE in 2021, “I feel very strongly that voclosporin will be approved for lupus nephritis,” he said. He also predicted that the use of belimumab will be officially extended for lupus nephritis and that anifrolumab will receive an approval for SLE patients.
In addition, the future may witness the increased use of biomarkers and development of more individualized therapy. These breakthroughs will yield better outcomes for all lupus patients, he said.
Dr. Furie disclosed relationships with GlaxoSmithKline, Genentech/Roche, Aurinia Pharmaceuticals, AstraZeneca/MedImmune, and Eli Lilly. Global Academy for Medical Education and this news organization are owned by the same parent company.
Systemic lupus erythematosus remains a treatment challenge, but a variety of drugs in the pipeline are set to target type I interferons, cytokines, and B cells, according to Richard Furie, MD, chief of the division of rheumatology at Northwell Health and professor of medicine at Hofstra University, Hempstead, N.Y.
In general, when treating patients with systemic lupus erythematosus (SLE), “we just don’t see satisfactory response rates,” Dr. Furie said in an online presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
“I think the greatest unmet need is in lupus nephritis,” he said. The data show that not even one-third of patients are adequately responding to standard of care treatment. “We need to do better in lupus nephritis but also for those patients with moderate-severe manifestations outside the kidney.”
Patients with SLE have elevated levels of interferon-alpha, Dr. Furie noted. Data from recent studies show that interferon inhibitors can reduce clinical activity in SLE patients, he said.
“About two-thirds to three-quarters of lupus patients have evidence of interferon pathway activation,” he said. There are three types of interferons, and five subtypes of type I interferon, and all five subtypes of type I interferon bind to the same receptor, which is an important strategy for drug development.
In particular, recent phase 2 and 3 trials have focused on targeting type I interferons with anifrolumab, which blocks all five subtypes.
Dr. Furie cited “very robust results” from a phase 2 study. Results of two phase 3 trials of anifrolumab led to a split decision, but the totality of the data collected across the phase 2 and 3 studies points to a drug that is effective for patients with SLE. The two phase 3 studies were published in Lancet Rheumatology and the New England Journal of Medicine.
Dr. Furie also identified recent studies of baricitinib (Olumiant), which has the ability to target several different cytokines. A phase 2 study in 2018 showed a significant difference in SLE Responder Index between lupus patients who received 4 mg of baricitinib or placebo, and a phase 3 study is underway, he said.
For lupus nephritis, Dr. Furie cited the BLISS-LN trial, a 104-week, randomized trial of patients with active lupus nephritis. The group of patients who received belimumab (Benlysta), a monoclonal antibody that targets B-cell activating factor, in addition to standard therapy had significant improvements in renal responses, compared with standard therapy alone (43.0% vs. 32.3%). The outcome measure was Primary Efficacy Renal Response, defined as urinary protein/creatinine ratio <0.7, eGFR ≥60 mL/min per 1.73 m2, confirmation on consecutive visits, and required tapering of background glucocorticoids.
Although belimumab was approved for SLE in 2011, the BLISS-LN study focused on SLE patients with active kidney disease. “Neutralizing B-cell activating factor and down-regulating autoreactive B-cell function in kidneys represented a compelling therapeutic approach to lupus nephritis,” he explained.
Voclosporin, distinct from cyclosporine, has also been studied in lupus nephritis, Dr. Furie said. Voclosporin offers several benefits over cyclosporine, including greater potency and a lower drug and metabolite load, as well as a more consistent pharmacokinetic and pharmacodynamic relationship, he said. In the phase 3 AURORA study, presented at this year’s EULAR congress, 40% of patients with lupus nephritis met the primary endpoint of a renal response at 52 weeks, compared with 22.5% of placebo patients.
Looking ahead to the treatment of SLE in 2021, “I feel very strongly that voclosporin will be approved for lupus nephritis,” he said. He also predicted that the use of belimumab will be officially extended for lupus nephritis and that anifrolumab will receive an approval for SLE patients.
In addition, the future may witness the increased use of biomarkers and development of more individualized therapy. These breakthroughs will yield better outcomes for all lupus patients, he said.
Dr. Furie disclosed relationships with GlaxoSmithKline, Genentech/Roche, Aurinia Pharmaceuticals, AstraZeneca/MedImmune, and Eli Lilly. Global Academy for Medical Education and this news organization are owned by the same parent company.
FROM PRD 2020
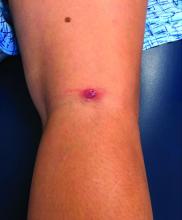
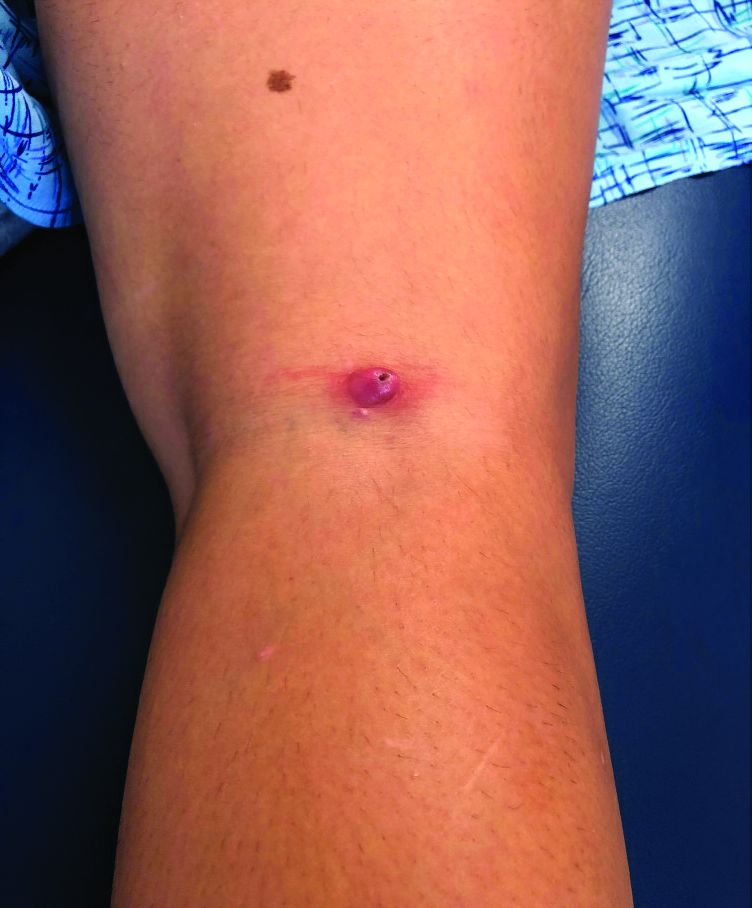
A teen girl presents with a pinkish-red bump on her right leg
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at [email protected].
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at [email protected].
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at [email protected].
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
Many Americans still concerned about access to health care
according to the results of a survey conducted Aug. 7-26.
Nationally, 23.8% of respondents said that they were very concerned about being able to receive care during the pandemic, and another 27.4% said that they were somewhat concerned. Just under a quarter, 24.3%, said they were not very concerned, while 20.4% were not at all concerned, the COVID-19 Consortium for Understanding the Public’s Policy Preferences Across States reported after surveying 21,196 adults.
At the state level, Mississippi had the most adults (35.5%) who were very concerned about their access to care, followed by Texas (32.7%) and Nevada (32.4%). The residents of Montana were least likely (10.5%) to be very concerned, with Vermont next at 11.6% and Wyoming slightly higher at 13.8%. Montana also had the highest proportion of adults, 30.2%, who were not at all concerned, the consortium’s data show.
When asked about getting the coronavirus themselves, 67.8% of U.S. adults came down on the concerned side (33.3% somewhat and 34.5% very concerned) versus 30.8% who were not concerned (18.6% were not very concerned; 12.2% were not concerned at all.). Respondents’ concern was higher for their family members’ risk of getting coronavirus: 30.2% were somewhat concerned and 47.6% were very concerned, the consortium said.
Among many other topics, respondents were asked how closely they had followed recommended health guidelines in the last week, with the two extremes shown here:
- Avoiding contact with other people: 49.3% very closely, 4.8% not at all closely.
- Frequently washing hands: 74.7% very, 1.6% not at all.
- Disinfecting often-touched surfaces: 54.4% very, 4.3% not at all.
- Wearing a face mask in public: 75.7% very, 3.5% not at all.
The consortium is a joint project of the Network Science Institute of Northeastern University; the Shorenstein Center on Media, Politics, and Public Policy of Harvard University; Harvard Medical School; the School of Communication and Information at Rutgers University; and the department of political science at Northwestern University. The project is supported by grants from the National Science Foundation.
according to the results of a survey conducted Aug. 7-26.
Nationally, 23.8% of respondents said that they were very concerned about being able to receive care during the pandemic, and another 27.4% said that they were somewhat concerned. Just under a quarter, 24.3%, said they were not very concerned, while 20.4% were not at all concerned, the COVID-19 Consortium for Understanding the Public’s Policy Preferences Across States reported after surveying 21,196 adults.
At the state level, Mississippi had the most adults (35.5%) who were very concerned about their access to care, followed by Texas (32.7%) and Nevada (32.4%). The residents of Montana were least likely (10.5%) to be very concerned, with Vermont next at 11.6% and Wyoming slightly higher at 13.8%. Montana also had the highest proportion of adults, 30.2%, who were not at all concerned, the consortium’s data show.
When asked about getting the coronavirus themselves, 67.8% of U.S. adults came down on the concerned side (33.3% somewhat and 34.5% very concerned) versus 30.8% who were not concerned (18.6% were not very concerned; 12.2% were not concerned at all.). Respondents’ concern was higher for their family members’ risk of getting coronavirus: 30.2% were somewhat concerned and 47.6% were very concerned, the consortium said.
Among many other topics, respondents were asked how closely they had followed recommended health guidelines in the last week, with the two extremes shown here:
- Avoiding contact with other people: 49.3% very closely, 4.8% not at all closely.
- Frequently washing hands: 74.7% very, 1.6% not at all.
- Disinfecting often-touched surfaces: 54.4% very, 4.3% not at all.
- Wearing a face mask in public: 75.7% very, 3.5% not at all.
The consortium is a joint project of the Network Science Institute of Northeastern University; the Shorenstein Center on Media, Politics, and Public Policy of Harvard University; Harvard Medical School; the School of Communication and Information at Rutgers University; and the department of political science at Northwestern University. The project is supported by grants from the National Science Foundation.
according to the results of a survey conducted Aug. 7-26.
Nationally, 23.8% of respondents said that they were very concerned about being able to receive care during the pandemic, and another 27.4% said that they were somewhat concerned. Just under a quarter, 24.3%, said they were not very concerned, while 20.4% were not at all concerned, the COVID-19 Consortium for Understanding the Public’s Policy Preferences Across States reported after surveying 21,196 adults.
At the state level, Mississippi had the most adults (35.5%) who were very concerned about their access to care, followed by Texas (32.7%) and Nevada (32.4%). The residents of Montana were least likely (10.5%) to be very concerned, with Vermont next at 11.6% and Wyoming slightly higher at 13.8%. Montana also had the highest proportion of adults, 30.2%, who were not at all concerned, the consortium’s data show.
When asked about getting the coronavirus themselves, 67.8% of U.S. adults came down on the concerned side (33.3% somewhat and 34.5% very concerned) versus 30.8% who were not concerned (18.6% were not very concerned; 12.2% were not concerned at all.). Respondents’ concern was higher for their family members’ risk of getting coronavirus: 30.2% were somewhat concerned and 47.6% were very concerned, the consortium said.
Among many other topics, respondents were asked how closely they had followed recommended health guidelines in the last week, with the two extremes shown here:
- Avoiding contact with other people: 49.3% very closely, 4.8% not at all closely.
- Frequently washing hands: 74.7% very, 1.6% not at all.
- Disinfecting often-touched surfaces: 54.4% very, 4.3% not at all.
- Wearing a face mask in public: 75.7% very, 3.5% not at all.
The consortium is a joint project of the Network Science Institute of Northeastern University; the Shorenstein Center on Media, Politics, and Public Policy of Harvard University; Harvard Medical School; the School of Communication and Information at Rutgers University; and the department of political science at Northwestern University. The project is supported by grants from the National Science Foundation.
2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic
Respiratory virus seasons usually follow a fairly well-known pattern. Enterovirus 68 (EV-D68) is a summer-to-early fall virus with biennial peak years. Rhinovirus (HRv) and adenovirus (Adv) occur nearly year-round but may have small upticks in the first month or so that children return to school. Early in the school year, upper respiratory infections from both HRv and Adv and viral sore throats from Adv are common, with conjunctivitis from Adv outbreaks in some years. October to November is human parainfluenza (HPiV) 1 and 2 season, often presenting as croup. Human metapneumovirus infections span October through April. In late November to December, influenza begins, usually with an A type, later transitioning to a B type in February through April. Also in December, respiratory syncytial virus (RSV) starts, characteristically with bronchiolitis presentations, peaking in February to March and tapering off in May. In late March to April, HPiV 3 also appears for 4-6 weeks.

Will 2020-2021 be different?
Summer was remarkably free of expected enterovirus activity, suggesting that the seasonal parade may differ this year. Remember that the 2019-2020 respiratory season suddenly and nearly completely stopped in March because of social distancing and lockdowns needed to address the SARS-CoV-2 pandemic.
The mild influenza season in the southern hemisphere suggests that our influenza season also could be mild. But perhaps not – most southern hemisphere countries that are surveyed for influenza activities had the most intense SARS-CoV-2 mitigations, making the observed mildness potentially related more to social mitigation than less virulent influenza strains. If so, southern hemisphere influenza data may not apply to the United States, where social distancing and masks are ignored or used inconsistently by almost half the population.
Further, the stop-and-go pattern of in-person school/college attendance adds to uncertainties for the usual orderly virus-specific seasonality. The result may be multiple stop-and-go “pop-up” or “mini” outbreaks for any given virus potentially reflected as exaggerated local or regional differences in circulation of various viruses. The erratic seasonality also would increase coinfections, which could present with more severe or different symptoms.
SARS-CoV-2’s potential interaction
Will the relatively mild presentations for most children with SARS-CoV-2 hold up in the setting of coinfections or sequential respiratory viral infections? Could SARS-CoV-2 cause worse/more prolonged symptoms or more sequelae if paired simultaneously or in tandem with a traditional respiratory virus? To date, data on the frequency and severity of SARS-CoV-2 coinfections are conflicting and sparse, but it appears that non-SARS-CoV-2 viruses can be involved in 15%-50% pediatric acute respiratory infections.1,2
However, it may not be important to know about coinfecting viruses other than influenza (can be treated) or SARS-CoV-2 (needs quarantine and contact tracing), unless symptoms are atypical or more severe than usual. For example, a young child with bronchiolitis is most likely infected with RSV, but HPiV, influenza, metapneumovirus, HRv, and even SARS-CoV-2 can cause bronchiolitis. Even so, testing outpatients for RSV or non-influenza is not routine or even clinically helpful. Supportive treatment and restriction from daycare attendance are sufficient management for outpatient ARIs whether presenting as bronchiolitis or not.
Considerations for SARS-CoV-2 testing: Outpatient bronchiolitis
If a child presents with classic bronchiolitis but has above moderate to severe symptoms, is SARS-CoV-2 a consideration? Perhaps, if SARS-CoV-2 acts similarly to non-SARS-CoV-2s.
A recent report from the 30th Multicenter Airway Research Collaboration (MARC-30) surveillance study (2007-2014) of children hospitalized with clinical bronchiolitis evaluated respiratory viruses, including RSV and the four common non-SARS coronaviruses using molecular testing.3 Among 1,880 subjects, a CoV (alpha CoV: NL63 or 229E, or beta CoV: KKU1 or OC43) was detected in 12%. Yet most had only RSV (n = 1,661); 32 had only CoV (n = 32). But note that 219 had both.
Bronchiolitis subjects with CoV were older – median 3.7 (1.4-5.8) vs. 2.8 (1.9-7.2) years – and more likely male than were RSV subjects (68% vs. 58%). OC43 was most frequent followed by equal numbers of HKU1 and NL63, while 229E was the least frequent. Medical utilization and severity did not differ among the CoVs, or between RSV+CoV vs. RSV alone, unless one considered CoV viral load as a variable. ICU use increased when the polymerase chain reaction cycle threshold result indicated a high CoV viral load.
These data suggest CoVs are not infrequent coinfectors with RSV in bronchiolitis – and that SARS-CoV-2 is the same. Therefore, a bronchiolitis presentation doesn’t necessarily take us off the hook for the need to consider SARS-CoV-2 testing, particularly in the somewhat older bronchiolitis patient with more than mild symptoms.
Considerations for SARS-CoV-2 testing: Outpatient influenza-like illness
In 2020-2021, the Centers for Disease Control and Prevention recommends considering empiric antiviral treatment for ILIs (fever plus either cough or sore throat) based upon our clinical judgement, even in non-high-risk children.4
While pediatric COVID-19 illnesses are predominantly asymptomatic or mild, a febrile ARI is also a SARS-CoV-2 compatible presentation. So, if all we use is our clinical judgment, how do we know if the febrile ARI is due to influenza or SARS-CoV-2 or both? At least one study used a highly sensitive and specific molecular influenza test to show that the accuracy of clinically diagnosing influenza in children is not much better than flipping a coin and would lead to potential antiviral overuse.5
So, it seems ideal to test for influenza when possible. Point-of-care (POC) tests are frequently used for outpatients. Eight POC Clinical Laboratory Improvement Amendments (CLIA)–waived kits, some also detecting RSV, are available but most have modest sensitivity (60%-80%) compared with lab-based molecular tests.6 That said, if supplies and kits for one of the POC tests are available to us during these SARS-CoV-2 stressed times (back orders seem more common this year), a positive influenza test in the first 48 hours of symptoms confirms the option to prescribe an antiviral. Yet how will we have confidence that the febrile ARI is not also partly due to SARS-CoV-2? Currently febrile ARIs usually are considered SARS-CoV-2 and the children are sent for SARS-CoV-2 testing. During influenza season, it seems we will need to continue to send febrile outpatients for SARS-CoV-2 testing, even if POC influenza positive, via whatever mechanisms are available as time goes on.
We expect more rapid pediatric testing modalities for SARS-CoV-2 (maybe even saliva tests) to become available over the next months. Indeed, rapid antigen tests and rapid molecular tests are being evaluated in adults and seem destined for CLIA waivers as POC tests, and even home testing kits. Pediatric approvals hopefully also will occur. So, the pathways for SARS-CoV-2 testing available now will likely change over this winter. But be aware that supplies/kits will be prioritized to locations within high need areas and bulk purchase contracts. So POC kits may remain scarce for practices, meaning a reference laboratory still could be the way to go for SARS-CoV-2 for at least the rest of 2020. Reference labs are becoming creative as well; one combined detection of influenza A, influenza B, RSV, and SARS-CoV-2 into one test, and hopes to get approval for swab collection that can be done by families at home and mailed in.
Summary
Expect variations on the traditional parade of seasonal respiratory viruses, with increased numbers of coinfections. Choosing the outpatient who needs influenza testing is the same as in past years, although we have CDC permissive recommendations to prescribe antivirals for any outpatient ILI within the first 48 hours of symptoms. Still, POC testing for influenza remains potentially valuable in the ILI patient. The choice of whether and how to test for SARS-CoV-2 given its potential to be a primary or coinfecting agent in presentations linked more closely to a traditional virus (e.g. RSV bronchiolitis) will be a test of our clinical judgement until more data and easier testing are available. Further complicating coinfection recognition is the fact that many sick visits occur by telehealth and much testing is done at drive-through SARS-CoV-2 testing facilities with no clinician exam. Unless we are liberal in SARS-CoV-2 testing, detecting SARS-CoV-2 coinfections is easier said than done given its usually mild presentation being overshadowed by any coinfecting virus.
But understanding who has SARS-CoV-2, even as a coinfection, still is essential in controlling the pandemic. We will need to be vigilant for evolving approaches to SARS-CoV-2 testing in the context of symptomatic ARI presentations, knowing this will likely remain a moving target for the foreseeable future.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatrics. 2020;146(1):e20200961.
2. JAMA. 2020 May 26;323(20):2085-6.
3. Pediatrics. 2020. doi: 10.1542/peds.2020-1267.
4. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
5. J. Pediatr. 2020. doi: 10.1016/j.jpeds.2020.08.007.
6. www.cdc.gov/flu/professionals/diagnosis/table-nucleic-acid-detection.html.
Respiratory virus seasons usually follow a fairly well-known pattern. Enterovirus 68 (EV-D68) is a summer-to-early fall virus with biennial peak years. Rhinovirus (HRv) and adenovirus (Adv) occur nearly year-round but may have small upticks in the first month or so that children return to school. Early in the school year, upper respiratory infections from both HRv and Adv and viral sore throats from Adv are common, with conjunctivitis from Adv outbreaks in some years. October to November is human parainfluenza (HPiV) 1 and 2 season, often presenting as croup. Human metapneumovirus infections span October through April. In late November to December, influenza begins, usually with an A type, later transitioning to a B type in February through April. Also in December, respiratory syncytial virus (RSV) starts, characteristically with bronchiolitis presentations, peaking in February to March and tapering off in May. In late March to April, HPiV 3 also appears for 4-6 weeks.

Will 2020-2021 be different?
Summer was remarkably free of expected enterovirus activity, suggesting that the seasonal parade may differ this year. Remember that the 2019-2020 respiratory season suddenly and nearly completely stopped in March because of social distancing and lockdowns needed to address the SARS-CoV-2 pandemic.
The mild influenza season in the southern hemisphere suggests that our influenza season also could be mild. But perhaps not – most southern hemisphere countries that are surveyed for influenza activities had the most intense SARS-CoV-2 mitigations, making the observed mildness potentially related more to social mitigation than less virulent influenza strains. If so, southern hemisphere influenza data may not apply to the United States, where social distancing and masks are ignored or used inconsistently by almost half the population.
Further, the stop-and-go pattern of in-person school/college attendance adds to uncertainties for the usual orderly virus-specific seasonality. The result may be multiple stop-and-go “pop-up” or “mini” outbreaks for any given virus potentially reflected as exaggerated local or regional differences in circulation of various viruses. The erratic seasonality also would increase coinfections, which could present with more severe or different symptoms.
SARS-CoV-2’s potential interaction
Will the relatively mild presentations for most children with SARS-CoV-2 hold up in the setting of coinfections or sequential respiratory viral infections? Could SARS-CoV-2 cause worse/more prolonged symptoms or more sequelae if paired simultaneously or in tandem with a traditional respiratory virus? To date, data on the frequency and severity of SARS-CoV-2 coinfections are conflicting and sparse, but it appears that non-SARS-CoV-2 viruses can be involved in 15%-50% pediatric acute respiratory infections.1,2
However, it may not be important to know about coinfecting viruses other than influenza (can be treated) or SARS-CoV-2 (needs quarantine and contact tracing), unless symptoms are atypical or more severe than usual. For example, a young child with bronchiolitis is most likely infected with RSV, but HPiV, influenza, metapneumovirus, HRv, and even SARS-CoV-2 can cause bronchiolitis. Even so, testing outpatients for RSV or non-influenza is not routine or even clinically helpful. Supportive treatment and restriction from daycare attendance are sufficient management for outpatient ARIs whether presenting as bronchiolitis or not.
Considerations for SARS-CoV-2 testing: Outpatient bronchiolitis
If a child presents with classic bronchiolitis but has above moderate to severe symptoms, is SARS-CoV-2 a consideration? Perhaps, if SARS-CoV-2 acts similarly to non-SARS-CoV-2s.
A recent report from the 30th Multicenter Airway Research Collaboration (MARC-30) surveillance study (2007-2014) of children hospitalized with clinical bronchiolitis evaluated respiratory viruses, including RSV and the four common non-SARS coronaviruses using molecular testing.3 Among 1,880 subjects, a CoV (alpha CoV: NL63 or 229E, or beta CoV: KKU1 or OC43) was detected in 12%. Yet most had only RSV (n = 1,661); 32 had only CoV (n = 32). But note that 219 had both.
Bronchiolitis subjects with CoV were older – median 3.7 (1.4-5.8) vs. 2.8 (1.9-7.2) years – and more likely male than were RSV subjects (68% vs. 58%). OC43 was most frequent followed by equal numbers of HKU1 and NL63, while 229E was the least frequent. Medical utilization and severity did not differ among the CoVs, or between RSV+CoV vs. RSV alone, unless one considered CoV viral load as a variable. ICU use increased when the polymerase chain reaction cycle threshold result indicated a high CoV viral load.
These data suggest CoVs are not infrequent coinfectors with RSV in bronchiolitis – and that SARS-CoV-2 is the same. Therefore, a bronchiolitis presentation doesn’t necessarily take us off the hook for the need to consider SARS-CoV-2 testing, particularly in the somewhat older bronchiolitis patient with more than mild symptoms.
Considerations for SARS-CoV-2 testing: Outpatient influenza-like illness
In 2020-2021, the Centers for Disease Control and Prevention recommends considering empiric antiviral treatment for ILIs (fever plus either cough or sore throat) based upon our clinical judgement, even in non-high-risk children.4
While pediatric COVID-19 illnesses are predominantly asymptomatic or mild, a febrile ARI is also a SARS-CoV-2 compatible presentation. So, if all we use is our clinical judgment, how do we know if the febrile ARI is due to influenza or SARS-CoV-2 or both? At least one study used a highly sensitive and specific molecular influenza test to show that the accuracy of clinically diagnosing influenza in children is not much better than flipping a coin and would lead to potential antiviral overuse.5
So, it seems ideal to test for influenza when possible. Point-of-care (POC) tests are frequently used for outpatients. Eight POC Clinical Laboratory Improvement Amendments (CLIA)–waived kits, some also detecting RSV, are available but most have modest sensitivity (60%-80%) compared with lab-based molecular tests.6 That said, if supplies and kits for one of the POC tests are available to us during these SARS-CoV-2 stressed times (back orders seem more common this year), a positive influenza test in the first 48 hours of symptoms confirms the option to prescribe an antiviral. Yet how will we have confidence that the febrile ARI is not also partly due to SARS-CoV-2? Currently febrile ARIs usually are considered SARS-CoV-2 and the children are sent for SARS-CoV-2 testing. During influenza season, it seems we will need to continue to send febrile outpatients for SARS-CoV-2 testing, even if POC influenza positive, via whatever mechanisms are available as time goes on.
We expect more rapid pediatric testing modalities for SARS-CoV-2 (maybe even saliva tests) to become available over the next months. Indeed, rapid antigen tests and rapid molecular tests are being evaluated in adults and seem destined for CLIA waivers as POC tests, and even home testing kits. Pediatric approvals hopefully also will occur. So, the pathways for SARS-CoV-2 testing available now will likely change over this winter. But be aware that supplies/kits will be prioritized to locations within high need areas and bulk purchase contracts. So POC kits may remain scarce for practices, meaning a reference laboratory still could be the way to go for SARS-CoV-2 for at least the rest of 2020. Reference labs are becoming creative as well; one combined detection of influenza A, influenza B, RSV, and SARS-CoV-2 into one test, and hopes to get approval for swab collection that can be done by families at home and mailed in.
Summary
Expect variations on the traditional parade of seasonal respiratory viruses, with increased numbers of coinfections. Choosing the outpatient who needs influenza testing is the same as in past years, although we have CDC permissive recommendations to prescribe antivirals for any outpatient ILI within the first 48 hours of symptoms. Still, POC testing for influenza remains potentially valuable in the ILI patient. The choice of whether and how to test for SARS-CoV-2 given its potential to be a primary or coinfecting agent in presentations linked more closely to a traditional virus (e.g. RSV bronchiolitis) will be a test of our clinical judgement until more data and easier testing are available. Further complicating coinfection recognition is the fact that many sick visits occur by telehealth and much testing is done at drive-through SARS-CoV-2 testing facilities with no clinician exam. Unless we are liberal in SARS-CoV-2 testing, detecting SARS-CoV-2 coinfections is easier said than done given its usually mild presentation being overshadowed by any coinfecting virus.
But understanding who has SARS-CoV-2, even as a coinfection, still is essential in controlling the pandemic. We will need to be vigilant for evolving approaches to SARS-CoV-2 testing in the context of symptomatic ARI presentations, knowing this will likely remain a moving target for the foreseeable future.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatrics. 2020;146(1):e20200961.
2. JAMA. 2020 May 26;323(20):2085-6.
3. Pediatrics. 2020. doi: 10.1542/peds.2020-1267.
4. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
5. J. Pediatr. 2020. doi: 10.1016/j.jpeds.2020.08.007.
6. www.cdc.gov/flu/professionals/diagnosis/table-nucleic-acid-detection.html.
Respiratory virus seasons usually follow a fairly well-known pattern. Enterovirus 68 (EV-D68) is a summer-to-early fall virus with biennial peak years. Rhinovirus (HRv) and adenovirus (Adv) occur nearly year-round but may have small upticks in the first month or so that children return to school. Early in the school year, upper respiratory infections from both HRv and Adv and viral sore throats from Adv are common, with conjunctivitis from Adv outbreaks in some years. October to November is human parainfluenza (HPiV) 1 and 2 season, often presenting as croup. Human metapneumovirus infections span October through April. In late November to December, influenza begins, usually with an A type, later transitioning to a B type in February through April. Also in December, respiratory syncytial virus (RSV) starts, characteristically with bronchiolitis presentations, peaking in February to March and tapering off in May. In late March to April, HPiV 3 also appears for 4-6 weeks.

Will 2020-2021 be different?
Summer was remarkably free of expected enterovirus activity, suggesting that the seasonal parade may differ this year. Remember that the 2019-2020 respiratory season suddenly and nearly completely stopped in March because of social distancing and lockdowns needed to address the SARS-CoV-2 pandemic.
The mild influenza season in the southern hemisphere suggests that our influenza season also could be mild. But perhaps not – most southern hemisphere countries that are surveyed for influenza activities had the most intense SARS-CoV-2 mitigations, making the observed mildness potentially related more to social mitigation than less virulent influenza strains. If so, southern hemisphere influenza data may not apply to the United States, where social distancing and masks are ignored or used inconsistently by almost half the population.
Further, the stop-and-go pattern of in-person school/college attendance adds to uncertainties for the usual orderly virus-specific seasonality. The result may be multiple stop-and-go “pop-up” or “mini” outbreaks for any given virus potentially reflected as exaggerated local or regional differences in circulation of various viruses. The erratic seasonality also would increase coinfections, which could present with more severe or different symptoms.
SARS-CoV-2’s potential interaction
Will the relatively mild presentations for most children with SARS-CoV-2 hold up in the setting of coinfections or sequential respiratory viral infections? Could SARS-CoV-2 cause worse/more prolonged symptoms or more sequelae if paired simultaneously or in tandem with a traditional respiratory virus? To date, data on the frequency and severity of SARS-CoV-2 coinfections are conflicting and sparse, but it appears that non-SARS-CoV-2 viruses can be involved in 15%-50% pediatric acute respiratory infections.1,2
However, it may not be important to know about coinfecting viruses other than influenza (can be treated) or SARS-CoV-2 (needs quarantine and contact tracing), unless symptoms are atypical or more severe than usual. For example, a young child with bronchiolitis is most likely infected with RSV, but HPiV, influenza, metapneumovirus, HRv, and even SARS-CoV-2 can cause bronchiolitis. Even so, testing outpatients for RSV or non-influenza is not routine or even clinically helpful. Supportive treatment and restriction from daycare attendance are sufficient management for outpatient ARIs whether presenting as bronchiolitis or not.
Considerations for SARS-CoV-2 testing: Outpatient bronchiolitis
If a child presents with classic bronchiolitis but has above moderate to severe symptoms, is SARS-CoV-2 a consideration? Perhaps, if SARS-CoV-2 acts similarly to non-SARS-CoV-2s.
A recent report from the 30th Multicenter Airway Research Collaboration (MARC-30) surveillance study (2007-2014) of children hospitalized with clinical bronchiolitis evaluated respiratory viruses, including RSV and the four common non-SARS coronaviruses using molecular testing.3 Among 1,880 subjects, a CoV (alpha CoV: NL63 or 229E, or beta CoV: KKU1 or OC43) was detected in 12%. Yet most had only RSV (n = 1,661); 32 had only CoV (n = 32). But note that 219 had both.
Bronchiolitis subjects with CoV were older – median 3.7 (1.4-5.8) vs. 2.8 (1.9-7.2) years – and more likely male than were RSV subjects (68% vs. 58%). OC43 was most frequent followed by equal numbers of HKU1 and NL63, while 229E was the least frequent. Medical utilization and severity did not differ among the CoVs, or between RSV+CoV vs. RSV alone, unless one considered CoV viral load as a variable. ICU use increased when the polymerase chain reaction cycle threshold result indicated a high CoV viral load.
These data suggest CoVs are not infrequent coinfectors with RSV in bronchiolitis – and that SARS-CoV-2 is the same. Therefore, a bronchiolitis presentation doesn’t necessarily take us off the hook for the need to consider SARS-CoV-2 testing, particularly in the somewhat older bronchiolitis patient with more than mild symptoms.
Considerations for SARS-CoV-2 testing: Outpatient influenza-like illness
In 2020-2021, the Centers for Disease Control and Prevention recommends considering empiric antiviral treatment for ILIs (fever plus either cough or sore throat) based upon our clinical judgement, even in non-high-risk children.4
While pediatric COVID-19 illnesses are predominantly asymptomatic or mild, a febrile ARI is also a SARS-CoV-2 compatible presentation. So, if all we use is our clinical judgment, how do we know if the febrile ARI is due to influenza or SARS-CoV-2 or both? At least one study used a highly sensitive and specific molecular influenza test to show that the accuracy of clinically diagnosing influenza in children is not much better than flipping a coin and would lead to potential antiviral overuse.5
So, it seems ideal to test for influenza when possible. Point-of-care (POC) tests are frequently used for outpatients. Eight POC Clinical Laboratory Improvement Amendments (CLIA)–waived kits, some also detecting RSV, are available but most have modest sensitivity (60%-80%) compared with lab-based molecular tests.6 That said, if supplies and kits for one of the POC tests are available to us during these SARS-CoV-2 stressed times (back orders seem more common this year), a positive influenza test in the first 48 hours of symptoms confirms the option to prescribe an antiviral. Yet how will we have confidence that the febrile ARI is not also partly due to SARS-CoV-2? Currently febrile ARIs usually are considered SARS-CoV-2 and the children are sent for SARS-CoV-2 testing. During influenza season, it seems we will need to continue to send febrile outpatients for SARS-CoV-2 testing, even if POC influenza positive, via whatever mechanisms are available as time goes on.
We expect more rapid pediatric testing modalities for SARS-CoV-2 (maybe even saliva tests) to become available over the next months. Indeed, rapid antigen tests and rapid molecular tests are being evaluated in adults and seem destined for CLIA waivers as POC tests, and even home testing kits. Pediatric approvals hopefully also will occur. So, the pathways for SARS-CoV-2 testing available now will likely change over this winter. But be aware that supplies/kits will be prioritized to locations within high need areas and bulk purchase contracts. So POC kits may remain scarce for practices, meaning a reference laboratory still could be the way to go for SARS-CoV-2 for at least the rest of 2020. Reference labs are becoming creative as well; one combined detection of influenza A, influenza B, RSV, and SARS-CoV-2 into one test, and hopes to get approval for swab collection that can be done by families at home and mailed in.
Summary
Expect variations on the traditional parade of seasonal respiratory viruses, with increased numbers of coinfections. Choosing the outpatient who needs influenza testing is the same as in past years, although we have CDC permissive recommendations to prescribe antivirals for any outpatient ILI within the first 48 hours of symptoms. Still, POC testing for influenza remains potentially valuable in the ILI patient. The choice of whether and how to test for SARS-CoV-2 given its potential to be a primary or coinfecting agent in presentations linked more closely to a traditional virus (e.g. RSV bronchiolitis) will be a test of our clinical judgement until more data and easier testing are available. Further complicating coinfection recognition is the fact that many sick visits occur by telehealth and much testing is done at drive-through SARS-CoV-2 testing facilities with no clinician exam. Unless we are liberal in SARS-CoV-2 testing, detecting SARS-CoV-2 coinfections is easier said than done given its usually mild presentation being overshadowed by any coinfecting virus.
But understanding who has SARS-CoV-2, even as a coinfection, still is essential in controlling the pandemic. We will need to be vigilant for evolving approaches to SARS-CoV-2 testing in the context of symptomatic ARI presentations, knowing this will likely remain a moving target for the foreseeable future.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatrics. 2020;146(1):e20200961.
2. JAMA. 2020 May 26;323(20):2085-6.
3. Pediatrics. 2020. doi: 10.1542/peds.2020-1267.
4. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
5. J. Pediatr. 2020. doi: 10.1016/j.jpeds.2020.08.007.
6. www.cdc.gov/flu/professionals/diagnosis/table-nucleic-acid-detection.html.
Dr. Fauci: ‘About 40%-45% of infections are asymptomatic’
Anthony Fauci, MD, highlighting the latest COVID-19 developments on Friday, said, “It is now clear that about 40%-45% of infections are asymptomatic.”
Asymptomatic carriers can account for a large proportion — up to 50% — of virus transmissions, Fauci, director of the National Institute of Allergy and Infectious Diseases, told a virtual crowd of critical care clinicians gathered by the Society of Critical Care Medicine.
Such transmissions have made response strategies, such as contact tracing, extremely difficult, he said.
Lew Kaplan, MD, president of SCCM, told Medscape Medical News after the presentation: “That really supports the universal wearing of masks and the capstone message from that – you should protect one another.
“That kind of social responsibility that sits within the public health domain to me is as important as the vaccine candidates and the science behind the receptors. It underpins the necessary relationship and the interdependence of the medical community with the public,” Kaplan added.
Fauci’s plenary led the SCCM’s conference, “COVID-19: What’s Next/Preparing for the Second Wave,” running today and Saturday.
Why U.S. response lags behind Spain and Italy
“This virus has literally exploded upon the planet in a pandemic manner which is unparalleled to anything we’ve seen in the last 102 years since the pandemic of 1918,” Fauci said.
“Unfortunately, the United States has been hit harder than any other country in the world, with 6 million reported cases.”
He explained that in the European Union countries the disease spiked early on and returned to a low baseline. “Unfortunately for them,” Fauci said, “as they’re trying to open up their economy, it’s coming back up.”
The United States, he explained, plateaued at about 20,000 cases a day, then a surge of cases in Florida, California, Texas, and Arizona brought the cases to 70,000 a day. Now cases have returned to 35,000-40,000 a day.
The difference in the trajectory of the response, he said, is that, compared with Spain and Italy for example, the United States has not shut down mobility in parks, outdoor spaces, and grocery stores nearly as much as some European countries did.
He pointed to numerous clusters of cases, spread from social or work gatherings, including the well-known Skagit County Washington state choir practice in March, in which a symptomatic choir member infected 87% of the 61 people rehearsing.
Vaccine by end of the year
As for a vaccine timeline, Fauci told SCCM members, “We project that by the end of this year, namely November/December, we will know if we have a safe and effective vaccine and we are cautiously optimistic that we will be successful, based on promising data in the animal model as well as good immunological data that we see from the phase 1 and phase 2 trials.”
However, also on Friday, Fauci told MSNBC’s Andrea Mitchell that a sense of normalcy is not likely before the middle of next year.
“By the time you mobilize the distribution of the vaccinations, and you get the majority, or more, of the population vaccinated and protected, that’s likely not going to happen [until] the mid- or end of 2021,” he said.
According to the Centers for Disease Control and Prevention (CDC) case tracker, as of Thursday, COVID-19 had resulted in more than 190,000 deaths overall and more than 256,000 new cases in the United States in the past 7 days.
Fauci has warned that the next few months will be critical in the virus’ trajectory, with the double onslaught of COVID-19 and the flu season.
On Thursday, Fauci said, “We need to hunker down and get through this fall and winter because it’s not going to be easy.”
Fauci remains a top trusted source in COVID-19 information, poll numbers show.
A Kaiser Family Foundation poll released Thursday found that 68% of US adults had a fair amount or a great deal of trust that Fauci would provide reliable information on COVID-19, just slightly more that the 67% who said they trust the CDC information. About half (53%) say they trust Deborah Birx, MD, the coordinator for the White House Coronavirus Task Force, as a reliable source of information.
The poll also found that 54% of Americans said they would not get a COVID-19 vaccine if one was approved by the US Food and Drug Administration before the November election and was made available and free to all who wanted it.
Kaplan and Fauci report no relevant financial relationships.
This article first appeared on Medscape.com.
Anthony Fauci, MD, highlighting the latest COVID-19 developments on Friday, said, “It is now clear that about 40%-45% of infections are asymptomatic.”
Asymptomatic carriers can account for a large proportion — up to 50% — of virus transmissions, Fauci, director of the National Institute of Allergy and Infectious Diseases, told a virtual crowd of critical care clinicians gathered by the Society of Critical Care Medicine.
Such transmissions have made response strategies, such as contact tracing, extremely difficult, he said.
Lew Kaplan, MD, president of SCCM, told Medscape Medical News after the presentation: “That really supports the universal wearing of masks and the capstone message from that – you should protect one another.
“That kind of social responsibility that sits within the public health domain to me is as important as the vaccine candidates and the science behind the receptors. It underpins the necessary relationship and the interdependence of the medical community with the public,” Kaplan added.
Fauci’s plenary led the SCCM’s conference, “COVID-19: What’s Next/Preparing for the Second Wave,” running today and Saturday.
Why U.S. response lags behind Spain and Italy
“This virus has literally exploded upon the planet in a pandemic manner which is unparalleled to anything we’ve seen in the last 102 years since the pandemic of 1918,” Fauci said.
“Unfortunately, the United States has been hit harder than any other country in the world, with 6 million reported cases.”
He explained that in the European Union countries the disease spiked early on and returned to a low baseline. “Unfortunately for them,” Fauci said, “as they’re trying to open up their economy, it’s coming back up.”
The United States, he explained, plateaued at about 20,000 cases a day, then a surge of cases in Florida, California, Texas, and Arizona brought the cases to 70,000 a day. Now cases have returned to 35,000-40,000 a day.
The difference in the trajectory of the response, he said, is that, compared with Spain and Italy for example, the United States has not shut down mobility in parks, outdoor spaces, and grocery stores nearly as much as some European countries did.
He pointed to numerous clusters of cases, spread from social or work gatherings, including the well-known Skagit County Washington state choir practice in March, in which a symptomatic choir member infected 87% of the 61 people rehearsing.
Vaccine by end of the year
As for a vaccine timeline, Fauci told SCCM members, “We project that by the end of this year, namely November/December, we will know if we have a safe and effective vaccine and we are cautiously optimistic that we will be successful, based on promising data in the animal model as well as good immunological data that we see from the phase 1 and phase 2 trials.”
However, also on Friday, Fauci told MSNBC’s Andrea Mitchell that a sense of normalcy is not likely before the middle of next year.
“By the time you mobilize the distribution of the vaccinations, and you get the majority, or more, of the population vaccinated and protected, that’s likely not going to happen [until] the mid- or end of 2021,” he said.
According to the Centers for Disease Control and Prevention (CDC) case tracker, as of Thursday, COVID-19 had resulted in more than 190,000 deaths overall and more than 256,000 new cases in the United States in the past 7 days.
Fauci has warned that the next few months will be critical in the virus’ trajectory, with the double onslaught of COVID-19 and the flu season.
On Thursday, Fauci said, “We need to hunker down and get through this fall and winter because it’s not going to be easy.”
Fauci remains a top trusted source in COVID-19 information, poll numbers show.
A Kaiser Family Foundation poll released Thursday found that 68% of US adults had a fair amount or a great deal of trust that Fauci would provide reliable information on COVID-19, just slightly more that the 67% who said they trust the CDC information. About half (53%) say they trust Deborah Birx, MD, the coordinator for the White House Coronavirus Task Force, as a reliable source of information.
The poll also found that 54% of Americans said they would not get a COVID-19 vaccine if one was approved by the US Food and Drug Administration before the November election and was made available and free to all who wanted it.
Kaplan and Fauci report no relevant financial relationships.
This article first appeared on Medscape.com.
Anthony Fauci, MD, highlighting the latest COVID-19 developments on Friday, said, “It is now clear that about 40%-45% of infections are asymptomatic.”
Asymptomatic carriers can account for a large proportion — up to 50% — of virus transmissions, Fauci, director of the National Institute of Allergy and Infectious Diseases, told a virtual crowd of critical care clinicians gathered by the Society of Critical Care Medicine.
Such transmissions have made response strategies, such as contact tracing, extremely difficult, he said.
Lew Kaplan, MD, president of SCCM, told Medscape Medical News after the presentation: “That really supports the universal wearing of masks and the capstone message from that – you should protect one another.
“That kind of social responsibility that sits within the public health domain to me is as important as the vaccine candidates and the science behind the receptors. It underpins the necessary relationship and the interdependence of the medical community with the public,” Kaplan added.
Fauci’s plenary led the SCCM’s conference, “COVID-19: What’s Next/Preparing for the Second Wave,” running today and Saturday.
Why U.S. response lags behind Spain and Italy
“This virus has literally exploded upon the planet in a pandemic manner which is unparalleled to anything we’ve seen in the last 102 years since the pandemic of 1918,” Fauci said.
“Unfortunately, the United States has been hit harder than any other country in the world, with 6 million reported cases.”
He explained that in the European Union countries the disease spiked early on and returned to a low baseline. “Unfortunately for them,” Fauci said, “as they’re trying to open up their economy, it’s coming back up.”
The United States, he explained, plateaued at about 20,000 cases a day, then a surge of cases in Florida, California, Texas, and Arizona brought the cases to 70,000 a day. Now cases have returned to 35,000-40,000 a day.
The difference in the trajectory of the response, he said, is that, compared with Spain and Italy for example, the United States has not shut down mobility in parks, outdoor spaces, and grocery stores nearly as much as some European countries did.
He pointed to numerous clusters of cases, spread from social or work gatherings, including the well-known Skagit County Washington state choir practice in March, in which a symptomatic choir member infected 87% of the 61 people rehearsing.
Vaccine by end of the year
As for a vaccine timeline, Fauci told SCCM members, “We project that by the end of this year, namely November/December, we will know if we have a safe and effective vaccine and we are cautiously optimistic that we will be successful, based on promising data in the animal model as well as good immunological data that we see from the phase 1 and phase 2 trials.”
However, also on Friday, Fauci told MSNBC’s Andrea Mitchell that a sense of normalcy is not likely before the middle of next year.
“By the time you mobilize the distribution of the vaccinations, and you get the majority, or more, of the population vaccinated and protected, that’s likely not going to happen [until] the mid- or end of 2021,” he said.
According to the Centers for Disease Control and Prevention (CDC) case tracker, as of Thursday, COVID-19 had resulted in more than 190,000 deaths overall and more than 256,000 new cases in the United States in the past 7 days.
Fauci has warned that the next few months will be critical in the virus’ trajectory, with the double onslaught of COVID-19 and the flu season.
On Thursday, Fauci said, “We need to hunker down and get through this fall and winter because it’s not going to be easy.”
Fauci remains a top trusted source in COVID-19 information, poll numbers show.
A Kaiser Family Foundation poll released Thursday found that 68% of US adults had a fair amount or a great deal of trust that Fauci would provide reliable information on COVID-19, just slightly more that the 67% who said they trust the CDC information. About half (53%) say they trust Deborah Birx, MD, the coordinator for the White House Coronavirus Task Force, as a reliable source of information.
The poll also found that 54% of Americans said they would not get a COVID-19 vaccine if one was approved by the US Food and Drug Administration before the November election and was made available and free to all who wanted it.
Kaplan and Fauci report no relevant financial relationships.
This article first appeared on Medscape.com.
Biologics for psoriasis may also reduce coronary plaque
Biologics used as treatment for psoriasis may also help reduce lipid-rich necrotic core (LRNC), a high-risk plaque associated with cardiovascular events, recent research from a prospective, observational study suggests.
Cardiac CT scans performed on patients with psoriasis 1 year after starting biologic therapy revealed a reduction in LRNC, compared with patients who were not receiving biologics, according to Harry Choi, MD, of the National Heart, Lung, and Blood Institute at the National Institutes of Health and colleagues. The association with reduction in LRNC and biologic therapy remained significant when adjusted for type of biologic. “These findings demonstrate that LRNC may be modulated by the control of systemic inflammation,” the researchers wrote in their study, published Sept. 15 in Circulation: Cardiovascular Imaging.
Dr. Choi and colleagues evaluated 289 patients with psoriasis within the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative cohort. The patients had a mean age of 50 years and a mean body mass index of 29.4 kg/m2, as well as a mean Psoriasis Area and Severity Index (PASI) score of 6.0. At baseline, 29% of patients had hypertension, 41% had hyperlipidemia, their mean Framingham risk score was 1.9, and a three-quarters (212 of 289) had mild to moderate psoriasis.
Changes in LRNC were observed at 1 year, compared with baseline prior to and after receiving biologic therapy (124 patients) in comparison with patients who did not undergo biologic therapy (85 patients). Biologic therapies were grouped by type, which included anti–tumor necrosis factor (anti-TNF), anti–interleukin (IL)–12/23, and anti–IL-17 biologics.
There were a significant associations between LRNC and Framingham risk score (standardized beta coefficient, 0.12; 95% confidence interval, 0.00-0.15; P = .045) and severity of psoriasis (beta, 0.13; 95% CI, 0.01-0.26; P = .029) at baseline.
Key findings
The researchers found a significant reduction in LRNC 1 year after patients began biologic therapy (median, 2.97 mm2; interquartile range, 1.99-4.66), compared with baseline (median, 3.12 mm2; IQR, 1.84-4.35) (P = .028), while patients who did not receive biologic therapy had nonsignificantly higher LRNC after 1 year (median, 3.12 mm2; IQR, 1.82-4.60), compared with baseline measurements (median, 3.34 mm2; IQR, 2.04–4.74) (P = .06).
The results remained significant after the researchers adjusted for psoriasis severity, Framingham risk score, BMI, use of statins (beta, −0.09; 95% CI, −0.01 to −0.18; P = .033). Significant reductions in LRNC also remained when analyzing patients receiving anti-TNF, anti–IL-12/23, and anti–IL-17 biologics independently, and there were no significant between-group differences in reduction of LRNC.
The potential of biologics for improving vascular health
Discussing the study results in a press release from the American Heart Association, senior author Nehal N. Mehta, MD, MSCE, FAHA, chief of the Lab of Inflammation and Cardiometabolic Diseases at the NHLBI at NIH, compared the effect biologic therapy had on coronary plaque reduction with that of statins.
“There is approximately 6%-8% reduction in coronary plaque following therapy with statins. Similarly, our treatment with biologic therapy reduced coronary plaque by the same amount after one year. These findings suggest that biologic therapy to treat psoriasis may be just as beneficial as statin therapy on heart arteries,” Dr. Mehta said in the release.
In an interview, Nieca Goldberg, MD, medical director of NYU Women’s Heart Program at NYU Langone Health, echoed Dr. Mehta’s commments and said psoriasis carries the “potential to treat two conditions with the same drug.”
“We know conditions such as psoriatic arthritis and rheumatoid arthritis cause chronic inflammation. Chronic inflammation causes injury to blood vessels and high-risk coronary plaque. Individuals with these inflammatory conditions are at high risk for heart attack,” she said. “This study shows that biologic treatment for psoriatic arthritis can reduce the presence of high-risk plaque. It shows the potential to treat chronic inflammation and high-risk coronary plaque.”
While the results show an association between use of biologics and LRNC reduction, the study design was observational and patients had a short follow-up period. Dr. Goldberg noted more studies are needed to evaluate the effect of biologics on reducing cardiovascular events such as a myocardial infarction.
“We have never before been able to show healing of an inflamed plaque like this in humans. Biologic therapy reduces systemic inflammation and immune activation, and it has a favorable impact on improving overall vascular health,” Dr. Mehta said in the press release. “Imagine if we can treat both psoriasis and coronary heart disease with one therapy – that is the question to be asked in future studies.”
This study was funded with support from the NHLBI Intramural Research Program and the NIH Medical Research Scholars Program at the National Institutes of Health. One investigator reports financial relationships with numerous pharmaceutical companies. The other authors report no relevant conflicts of interest. Dr. Mehta also reports numerous such relationships. Dr. Goldberg reports no relevant conflicts of interest.
SOURCE: Choi H et al. Circ Cardiovasc Imaging. 2020 Sep;13(9):e011199.
Biologics used as treatment for psoriasis may also help reduce lipid-rich necrotic core (LRNC), a high-risk plaque associated with cardiovascular events, recent research from a prospective, observational study suggests.
Cardiac CT scans performed on patients with psoriasis 1 year after starting biologic therapy revealed a reduction in LRNC, compared with patients who were not receiving biologics, according to Harry Choi, MD, of the National Heart, Lung, and Blood Institute at the National Institutes of Health and colleagues. The association with reduction in LRNC and biologic therapy remained significant when adjusted for type of biologic. “These findings demonstrate that LRNC may be modulated by the control of systemic inflammation,” the researchers wrote in their study, published Sept. 15 in Circulation: Cardiovascular Imaging.
Dr. Choi and colleagues evaluated 289 patients with psoriasis within the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative cohort. The patients had a mean age of 50 years and a mean body mass index of 29.4 kg/m2, as well as a mean Psoriasis Area and Severity Index (PASI) score of 6.0. At baseline, 29% of patients had hypertension, 41% had hyperlipidemia, their mean Framingham risk score was 1.9, and a three-quarters (212 of 289) had mild to moderate psoriasis.
Changes in LRNC were observed at 1 year, compared with baseline prior to and after receiving biologic therapy (124 patients) in comparison with patients who did not undergo biologic therapy (85 patients). Biologic therapies were grouped by type, which included anti–tumor necrosis factor (anti-TNF), anti–interleukin (IL)–12/23, and anti–IL-17 biologics.
There were a significant associations between LRNC and Framingham risk score (standardized beta coefficient, 0.12; 95% confidence interval, 0.00-0.15; P = .045) and severity of psoriasis (beta, 0.13; 95% CI, 0.01-0.26; P = .029) at baseline.
Key findings
The researchers found a significant reduction in LRNC 1 year after patients began biologic therapy (median, 2.97 mm2; interquartile range, 1.99-4.66), compared with baseline (median, 3.12 mm2; IQR, 1.84-4.35) (P = .028), while patients who did not receive biologic therapy had nonsignificantly higher LRNC after 1 year (median, 3.12 mm2; IQR, 1.82-4.60), compared with baseline measurements (median, 3.34 mm2; IQR, 2.04–4.74) (P = .06).
The results remained significant after the researchers adjusted for psoriasis severity, Framingham risk score, BMI, use of statins (beta, −0.09; 95% CI, −0.01 to −0.18; P = .033). Significant reductions in LRNC also remained when analyzing patients receiving anti-TNF, anti–IL-12/23, and anti–IL-17 biologics independently, and there were no significant between-group differences in reduction of LRNC.
The potential of biologics for improving vascular health
Discussing the study results in a press release from the American Heart Association, senior author Nehal N. Mehta, MD, MSCE, FAHA, chief of the Lab of Inflammation and Cardiometabolic Diseases at the NHLBI at NIH, compared the effect biologic therapy had on coronary plaque reduction with that of statins.
“There is approximately 6%-8% reduction in coronary plaque following therapy with statins. Similarly, our treatment with biologic therapy reduced coronary plaque by the same amount after one year. These findings suggest that biologic therapy to treat psoriasis may be just as beneficial as statin therapy on heart arteries,” Dr. Mehta said in the release.
In an interview, Nieca Goldberg, MD, medical director of NYU Women’s Heart Program at NYU Langone Health, echoed Dr. Mehta’s commments and said psoriasis carries the “potential to treat two conditions with the same drug.”
“We know conditions such as psoriatic arthritis and rheumatoid arthritis cause chronic inflammation. Chronic inflammation causes injury to blood vessels and high-risk coronary plaque. Individuals with these inflammatory conditions are at high risk for heart attack,” she said. “This study shows that biologic treatment for psoriatic arthritis can reduce the presence of high-risk plaque. It shows the potential to treat chronic inflammation and high-risk coronary plaque.”
While the results show an association between use of biologics and LRNC reduction, the study design was observational and patients had a short follow-up period. Dr. Goldberg noted more studies are needed to evaluate the effect of biologics on reducing cardiovascular events such as a myocardial infarction.
“We have never before been able to show healing of an inflamed plaque like this in humans. Biologic therapy reduces systemic inflammation and immune activation, and it has a favorable impact on improving overall vascular health,” Dr. Mehta said in the press release. “Imagine if we can treat both psoriasis and coronary heart disease with one therapy – that is the question to be asked in future studies.”
This study was funded with support from the NHLBI Intramural Research Program and the NIH Medical Research Scholars Program at the National Institutes of Health. One investigator reports financial relationships with numerous pharmaceutical companies. The other authors report no relevant conflicts of interest. Dr. Mehta also reports numerous such relationships. Dr. Goldberg reports no relevant conflicts of interest.
SOURCE: Choi H et al. Circ Cardiovasc Imaging. 2020 Sep;13(9):e011199.
Biologics used as treatment for psoriasis may also help reduce lipid-rich necrotic core (LRNC), a high-risk plaque associated with cardiovascular events, recent research from a prospective, observational study suggests.
Cardiac CT scans performed on patients with psoriasis 1 year after starting biologic therapy revealed a reduction in LRNC, compared with patients who were not receiving biologics, according to Harry Choi, MD, of the National Heart, Lung, and Blood Institute at the National Institutes of Health and colleagues. The association with reduction in LRNC and biologic therapy remained significant when adjusted for type of biologic. “These findings demonstrate that LRNC may be modulated by the control of systemic inflammation,” the researchers wrote in their study, published Sept. 15 in Circulation: Cardiovascular Imaging.
Dr. Choi and colleagues evaluated 289 patients with psoriasis within the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative cohort. The patients had a mean age of 50 years and a mean body mass index of 29.4 kg/m2, as well as a mean Psoriasis Area and Severity Index (PASI) score of 6.0. At baseline, 29% of patients had hypertension, 41% had hyperlipidemia, their mean Framingham risk score was 1.9, and a three-quarters (212 of 289) had mild to moderate psoriasis.
Changes in LRNC were observed at 1 year, compared with baseline prior to and after receiving biologic therapy (124 patients) in comparison with patients who did not undergo biologic therapy (85 patients). Biologic therapies were grouped by type, which included anti–tumor necrosis factor (anti-TNF), anti–interleukin (IL)–12/23, and anti–IL-17 biologics.
There were a significant associations between LRNC and Framingham risk score (standardized beta coefficient, 0.12; 95% confidence interval, 0.00-0.15; P = .045) and severity of psoriasis (beta, 0.13; 95% CI, 0.01-0.26; P = .029) at baseline.
Key findings
The researchers found a significant reduction in LRNC 1 year after patients began biologic therapy (median, 2.97 mm2; interquartile range, 1.99-4.66), compared with baseline (median, 3.12 mm2; IQR, 1.84-4.35) (P = .028), while patients who did not receive biologic therapy had nonsignificantly higher LRNC after 1 year (median, 3.12 mm2; IQR, 1.82-4.60), compared with baseline measurements (median, 3.34 mm2; IQR, 2.04–4.74) (P = .06).
The results remained significant after the researchers adjusted for psoriasis severity, Framingham risk score, BMI, use of statins (beta, −0.09; 95% CI, −0.01 to −0.18; P = .033). Significant reductions in LRNC also remained when analyzing patients receiving anti-TNF, anti–IL-12/23, and anti–IL-17 biologics independently, and there were no significant between-group differences in reduction of LRNC.
The potential of biologics for improving vascular health
Discussing the study results in a press release from the American Heart Association, senior author Nehal N. Mehta, MD, MSCE, FAHA, chief of the Lab of Inflammation and Cardiometabolic Diseases at the NHLBI at NIH, compared the effect biologic therapy had on coronary plaque reduction with that of statins.
“There is approximately 6%-8% reduction in coronary plaque following therapy with statins. Similarly, our treatment with biologic therapy reduced coronary plaque by the same amount after one year. These findings suggest that biologic therapy to treat psoriasis may be just as beneficial as statin therapy on heart arteries,” Dr. Mehta said in the release.
In an interview, Nieca Goldberg, MD, medical director of NYU Women’s Heart Program at NYU Langone Health, echoed Dr. Mehta’s commments and said psoriasis carries the “potential to treat two conditions with the same drug.”
“We know conditions such as psoriatic arthritis and rheumatoid arthritis cause chronic inflammation. Chronic inflammation causes injury to blood vessels and high-risk coronary plaque. Individuals with these inflammatory conditions are at high risk for heart attack,” she said. “This study shows that biologic treatment for psoriatic arthritis can reduce the presence of high-risk plaque. It shows the potential to treat chronic inflammation and high-risk coronary plaque.”
While the results show an association between use of biologics and LRNC reduction, the study design was observational and patients had a short follow-up period. Dr. Goldberg noted more studies are needed to evaluate the effect of biologics on reducing cardiovascular events such as a myocardial infarction.
“We have never before been able to show healing of an inflamed plaque like this in humans. Biologic therapy reduces systemic inflammation and immune activation, and it has a favorable impact on improving overall vascular health,” Dr. Mehta said in the press release. “Imagine if we can treat both psoriasis and coronary heart disease with one therapy – that is the question to be asked in future studies.”
This study was funded with support from the NHLBI Intramural Research Program and the NIH Medical Research Scholars Program at the National Institutes of Health. One investigator reports financial relationships with numerous pharmaceutical companies. The other authors report no relevant conflicts of interest. Dr. Mehta also reports numerous such relationships. Dr. Goldberg reports no relevant conflicts of interest.
SOURCE: Choi H et al. Circ Cardiovasc Imaging. 2020 Sep;13(9):e011199.
FROM CIRCULATION: CARDIOVASCULAR IMAGING
COVID-19 and the psychological side effects of PPE
A few months ago, I published a short thought piece on the use of “sitters” with patients who were COVID-19 positive, or patients under investigation. In it, I recommended the use of telesitters for those who normally would warrant a human sitter, to decrease the discomfort of sitting in full personal protective equipment (PPE) (gown, mask, gloves, etc.) while monitoring a suicidal patient.
I received several queries, which I want to address here. In addition, I want to draw from my Army days in terms of the claustrophobia often experienced with PPE.
The first of the questions was about evidence-based practices. The second was about the discomfort of having sitters sit for many hours in the full gear.
I do not know of any evidence-based practices, but I hope we will develop them.
I agree that spending many hours in full PPE can be discomforting, which is why I wrote the essay.
As far as lessons learned from the Army time, I briefly learned how to wear a “gas mask” or Mission-Oriented Protective Posture (MOPP gear) while at Fort Bragg. We were run through the “gas chamber,” where sergeants released tear gas while we had the mask on. We were then asked to lift it up, and then tearing and sputtering, we could leave the small wooden building.
We wore the mask as part of our Army gear, usually on the right leg. After that, I mainly used the protective mask in its bag as a pillow when I was in the field.
Fast forward to August 1990. I arrived at Camp Casey, near the Korean demilitarized zone. Four days later, Saddam Hussein invaded Kuwait. The gas mask moved from a pillow to something we had to wear while doing 12-mile road marches in “full ruck.” In full ruck, you have your uniform on, with TA-50, knapsack, and weapon. No, I do not remember any more what TA-50 stands for, but essentially it is the webbing that holds your bullets and bandages.
Many could not tolerate it. They developed claustrophobia – sweating, air hunger, and panic. If stationed in the Gulf for Operation Desert Storm, they were evacuated home.
I wrote a couple of short articles on treatment of gas mask phobia.1,2 I basically advised desensitization. Start by watching TV in it for 5 minutes. Graduate to ironing your uniform in the mask. Go then to shorter runs. Work up to the 12-mile road march.
In my second tour in Korea, we had exercises where we simulated being hit by nerve agents and had to operate the hospital for days at a time in partial or full PPE. It was tough but we did it, and felt more confident about surviving attacks from North Korea.
So back to the pandemic present. I have gotten more used to my constant wearing of a surgical mask. I get anxious when I see others with masks below their noses.
The pandemic is not going away anytime soon, in my opinion. Furthermore, there are other viruses that are worse, such as Ebola. It is only a matter of time.
So, let us train with our PPE. If health care workers cannot tolerate them, use desensitization- and anxiety-reducing techniques to help them.
There are no easy answers here, in the time of the COVID pandemic. However, we owe it to ourselves, our patients, and society to do the best we can.
References
1. Ritchie EC. Milit Med. 1992 Feb;157(2):104-6.
2. Ritchie EC. Milit Med. 2001 Dec;166. Suppl. 2(1)83-4.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures and can be reached at [email protected].
A few months ago, I published a short thought piece on the use of “sitters” with patients who were COVID-19 positive, or patients under investigation. In it, I recommended the use of telesitters for those who normally would warrant a human sitter, to decrease the discomfort of sitting in full personal protective equipment (PPE) (gown, mask, gloves, etc.) while monitoring a suicidal patient.
I received several queries, which I want to address here. In addition, I want to draw from my Army days in terms of the claustrophobia often experienced with PPE.
The first of the questions was about evidence-based practices. The second was about the discomfort of having sitters sit for many hours in the full gear.
I do not know of any evidence-based practices, but I hope we will develop them.
I agree that spending many hours in full PPE can be discomforting, which is why I wrote the essay.
As far as lessons learned from the Army time, I briefly learned how to wear a “gas mask” or Mission-Oriented Protective Posture (MOPP gear) while at Fort Bragg. We were run through the “gas chamber,” where sergeants released tear gas while we had the mask on. We were then asked to lift it up, and then tearing and sputtering, we could leave the small wooden building.
We wore the mask as part of our Army gear, usually on the right leg. After that, I mainly used the protective mask in its bag as a pillow when I was in the field.
Fast forward to August 1990. I arrived at Camp Casey, near the Korean demilitarized zone. Four days later, Saddam Hussein invaded Kuwait. The gas mask moved from a pillow to something we had to wear while doing 12-mile road marches in “full ruck.” In full ruck, you have your uniform on, with TA-50, knapsack, and weapon. No, I do not remember any more what TA-50 stands for, but essentially it is the webbing that holds your bullets and bandages.
Many could not tolerate it. They developed claustrophobia – sweating, air hunger, and panic. If stationed in the Gulf for Operation Desert Storm, they were evacuated home.
I wrote a couple of short articles on treatment of gas mask phobia.1,2 I basically advised desensitization. Start by watching TV in it for 5 minutes. Graduate to ironing your uniform in the mask. Go then to shorter runs. Work up to the 12-mile road march.
In my second tour in Korea, we had exercises where we simulated being hit by nerve agents and had to operate the hospital for days at a time in partial or full PPE. It was tough but we did it, and felt more confident about surviving attacks from North Korea.
So back to the pandemic present. I have gotten more used to my constant wearing of a surgical mask. I get anxious when I see others with masks below their noses.
The pandemic is not going away anytime soon, in my opinion. Furthermore, there are other viruses that are worse, such as Ebola. It is only a matter of time.
So, let us train with our PPE. If health care workers cannot tolerate them, use desensitization- and anxiety-reducing techniques to help them.
There are no easy answers here, in the time of the COVID pandemic. However, we owe it to ourselves, our patients, and society to do the best we can.
References
1. Ritchie EC. Milit Med. 1992 Feb;157(2):104-6.
2. Ritchie EC. Milit Med. 2001 Dec;166. Suppl. 2(1)83-4.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures and can be reached at [email protected].
A few months ago, I published a short thought piece on the use of “sitters” with patients who were COVID-19 positive, or patients under investigation. In it, I recommended the use of telesitters for those who normally would warrant a human sitter, to decrease the discomfort of sitting in full personal protective equipment (PPE) (gown, mask, gloves, etc.) while monitoring a suicidal patient.
I received several queries, which I want to address here. In addition, I want to draw from my Army days in terms of the claustrophobia often experienced with PPE.
The first of the questions was about evidence-based practices. The second was about the discomfort of having sitters sit for many hours in the full gear.
I do not know of any evidence-based practices, but I hope we will develop them.
I agree that spending many hours in full PPE can be discomforting, which is why I wrote the essay.
As far as lessons learned from the Army time, I briefly learned how to wear a “gas mask” or Mission-Oriented Protective Posture (MOPP gear) while at Fort Bragg. We were run through the “gas chamber,” where sergeants released tear gas while we had the mask on. We were then asked to lift it up, and then tearing and sputtering, we could leave the small wooden building.
We wore the mask as part of our Army gear, usually on the right leg. After that, I mainly used the protective mask in its bag as a pillow when I was in the field.
Fast forward to August 1990. I arrived at Camp Casey, near the Korean demilitarized zone. Four days later, Saddam Hussein invaded Kuwait. The gas mask moved from a pillow to something we had to wear while doing 12-mile road marches in “full ruck.” In full ruck, you have your uniform on, with TA-50, knapsack, and weapon. No, I do not remember any more what TA-50 stands for, but essentially it is the webbing that holds your bullets and bandages.
Many could not tolerate it. They developed claustrophobia – sweating, air hunger, and panic. If stationed in the Gulf for Operation Desert Storm, they were evacuated home.
I wrote a couple of short articles on treatment of gas mask phobia.1,2 I basically advised desensitization. Start by watching TV in it for 5 minutes. Graduate to ironing your uniform in the mask. Go then to shorter runs. Work up to the 12-mile road march.
In my second tour in Korea, we had exercises where we simulated being hit by nerve agents and had to operate the hospital for days at a time in partial or full PPE. It was tough but we did it, and felt more confident about surviving attacks from North Korea.
So back to the pandemic present. I have gotten more used to my constant wearing of a surgical mask. I get anxious when I see others with masks below their noses.
The pandemic is not going away anytime soon, in my opinion. Furthermore, there are other viruses that are worse, such as Ebola. It is only a matter of time.
So, let us train with our PPE. If health care workers cannot tolerate them, use desensitization- and anxiety-reducing techniques to help them.
There are no easy answers here, in the time of the COVID pandemic. However, we owe it to ourselves, our patients, and society to do the best we can.
References
1. Ritchie EC. Milit Med. 1992 Feb;157(2):104-6.
2. Ritchie EC. Milit Med. 2001 Dec;166. Suppl. 2(1)83-4.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures and can be reached at [email protected].
Conspiracy theories
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].