User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
FDA approves ixekizumab for nonradiographic axSpA
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
COVID-19: An opportunity to rehumanize psychiatry
Prior to the current crisis of COVID-19, I had a critical view of the direction of our psychiatric field. We have given up on complicated psychotherapies in favor of dispensing medications. We have given up on complicated diagnostic assessments in favor of simple self-rated symptoms questionnaires. Many of us even chose to give up on seeing patients face to face in favor of practicing telepsychiatry in the comfort of our homes. Some even promoted a future of psychiatry in which psychiatrists treated patients through large spreadsheets of evidence-based rating tools following evidence-based algorithms without even ever meeting the patients.
I do not view this problem as unique to psychiatry but rather as part of a larger trend in society. For the past couple of years, Vivek Murthy, MD, the former U.S. surgeon general, has popularized the idea that we are in a loneliness epidemic, saying, “We live in the most technologically connected age in the history of civilization, yet rates of loneliness have doubled since the 1980s.” Despite having enumerable means to reach other human beings, so many of us feel distant and out of touch with others. This loneliness has a measurable impact on our well-being with one study that states, “Actual and perceived social isolation are both associated with increased risk for early mortality.”
Then, seemingly out of nowhere, we were confronted with the largest challenge to our sense of connectedness in my lifetime. Throughout the past months, we have been asked to meet each other less frequently, do so through sterile means, and certainly not shake hands, hug, or embrace. The COVID-19 crisis has quickly made us all experts in telepsychiatry, remote work, and doing more with less. The COVID-19 crisis has asked many of us to put aside some of our human rituals like eating together, enjoying artistic experiences as a group, and touching, for the sake of saving lives.
For many, socially distancing has been a considerable added stressor – a stressor that continues to test humanity’s ability to be resilient. I am saddened by prior patients reaching out to seek comfort in these difficult times. I am touched by their desire to reconnect with someone they know, someone who feels familiar. I am surprised by the power of connection through phone and video calls. For some patients, despite the added burden, the current crisis has been an opportunity for their mental health and a reminder of the things that are important, including calling old friends and staying in touch with those who matter the most.
Yet, Checking in on others can become a chore. The social norm to partake in fashion, and self-care, become harder to find. In some cases, even hygiene and our health take a side role. The weekly phone visits with a therapist can feel just as mundane and repetitive as life. Sleep becomes harder to find, and food loses its taste. At this point, we realize the humanity that we lost in all this.
In the past couple of months, we have all become much more aware of the fragility of connectedness. However, we should recognize that the impact was well on its way before the COVID-19 crisis. It is my opinion that psychiatry should champion the issue of human relations. I do not think that we need to wait for a new DSM diagnosis, an evidence-based paradigm, or a Food and Drug Administration–approved medication to do so. The COVID-19 crisis has rendered us all cognizant of the importance of relationships.
While it may be that psychiatry continues to foray in electronic means of communication, use of impersonal scales and diagnosis, as well as anonymized algorithmic treatment plans, we should also promote as much humanity as society and public health safety will permit. Getting dressed to see your psychiatrist, face to face, to have an open-ended conversation about the nature of one’s life has clearly become something precious and powerful that should be cherished and protected. My hope is the rules and mandates we are required to use during the pandemic today do not become a continued habit that result in further loneliness and disconnect. If we chose to, the lessons we learn today can, in fact, strengthen our appreciation and pursuit of human connection.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Springer, 2019). He has no disclosures.
Prior to the current crisis of COVID-19, I had a critical view of the direction of our psychiatric field. We have given up on complicated psychotherapies in favor of dispensing medications. We have given up on complicated diagnostic assessments in favor of simple self-rated symptoms questionnaires. Many of us even chose to give up on seeing patients face to face in favor of practicing telepsychiatry in the comfort of our homes. Some even promoted a future of psychiatry in which psychiatrists treated patients through large spreadsheets of evidence-based rating tools following evidence-based algorithms without even ever meeting the patients.
I do not view this problem as unique to psychiatry but rather as part of a larger trend in society. For the past couple of years, Vivek Murthy, MD, the former U.S. surgeon general, has popularized the idea that we are in a loneliness epidemic, saying, “We live in the most technologically connected age in the history of civilization, yet rates of loneliness have doubled since the 1980s.” Despite having enumerable means to reach other human beings, so many of us feel distant and out of touch with others. This loneliness has a measurable impact on our well-being with one study that states, “Actual and perceived social isolation are both associated with increased risk for early mortality.”
Then, seemingly out of nowhere, we were confronted with the largest challenge to our sense of connectedness in my lifetime. Throughout the past months, we have been asked to meet each other less frequently, do so through sterile means, and certainly not shake hands, hug, or embrace. The COVID-19 crisis has quickly made us all experts in telepsychiatry, remote work, and doing more with less. The COVID-19 crisis has asked many of us to put aside some of our human rituals like eating together, enjoying artistic experiences as a group, and touching, for the sake of saving lives.
For many, socially distancing has been a considerable added stressor – a stressor that continues to test humanity’s ability to be resilient. I am saddened by prior patients reaching out to seek comfort in these difficult times. I am touched by their desire to reconnect with someone they know, someone who feels familiar. I am surprised by the power of connection through phone and video calls. For some patients, despite the added burden, the current crisis has been an opportunity for their mental health and a reminder of the things that are important, including calling old friends and staying in touch with those who matter the most.
Yet, Checking in on others can become a chore. The social norm to partake in fashion, and self-care, become harder to find. In some cases, even hygiene and our health take a side role. The weekly phone visits with a therapist can feel just as mundane and repetitive as life. Sleep becomes harder to find, and food loses its taste. At this point, we realize the humanity that we lost in all this.
In the past couple of months, we have all become much more aware of the fragility of connectedness. However, we should recognize that the impact was well on its way before the COVID-19 crisis. It is my opinion that psychiatry should champion the issue of human relations. I do not think that we need to wait for a new DSM diagnosis, an evidence-based paradigm, or a Food and Drug Administration–approved medication to do so. The COVID-19 crisis has rendered us all cognizant of the importance of relationships.
While it may be that psychiatry continues to foray in electronic means of communication, use of impersonal scales and diagnosis, as well as anonymized algorithmic treatment plans, we should also promote as much humanity as society and public health safety will permit. Getting dressed to see your psychiatrist, face to face, to have an open-ended conversation about the nature of one’s life has clearly become something precious and powerful that should be cherished and protected. My hope is the rules and mandates we are required to use during the pandemic today do not become a continued habit that result in further loneliness and disconnect. If we chose to, the lessons we learn today can, in fact, strengthen our appreciation and pursuit of human connection.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Springer, 2019). He has no disclosures.
Prior to the current crisis of COVID-19, I had a critical view of the direction of our psychiatric field. We have given up on complicated psychotherapies in favor of dispensing medications. We have given up on complicated diagnostic assessments in favor of simple self-rated symptoms questionnaires. Many of us even chose to give up on seeing patients face to face in favor of practicing telepsychiatry in the comfort of our homes. Some even promoted a future of psychiatry in which psychiatrists treated patients through large spreadsheets of evidence-based rating tools following evidence-based algorithms without even ever meeting the patients.
I do not view this problem as unique to psychiatry but rather as part of a larger trend in society. For the past couple of years, Vivek Murthy, MD, the former U.S. surgeon general, has popularized the idea that we are in a loneliness epidemic, saying, “We live in the most technologically connected age in the history of civilization, yet rates of loneliness have doubled since the 1980s.” Despite having enumerable means to reach other human beings, so many of us feel distant and out of touch with others. This loneliness has a measurable impact on our well-being with one study that states, “Actual and perceived social isolation are both associated with increased risk for early mortality.”
Then, seemingly out of nowhere, we were confronted with the largest challenge to our sense of connectedness in my lifetime. Throughout the past months, we have been asked to meet each other less frequently, do so through sterile means, and certainly not shake hands, hug, or embrace. The COVID-19 crisis has quickly made us all experts in telepsychiatry, remote work, and doing more with less. The COVID-19 crisis has asked many of us to put aside some of our human rituals like eating together, enjoying artistic experiences as a group, and touching, for the sake of saving lives.
For many, socially distancing has been a considerable added stressor – a stressor that continues to test humanity’s ability to be resilient. I am saddened by prior patients reaching out to seek comfort in these difficult times. I am touched by their desire to reconnect with someone they know, someone who feels familiar. I am surprised by the power of connection through phone and video calls. For some patients, despite the added burden, the current crisis has been an opportunity for their mental health and a reminder of the things that are important, including calling old friends and staying in touch with those who matter the most.
Yet, Checking in on others can become a chore. The social norm to partake in fashion, and self-care, become harder to find. In some cases, even hygiene and our health take a side role. The weekly phone visits with a therapist can feel just as mundane and repetitive as life. Sleep becomes harder to find, and food loses its taste. At this point, we realize the humanity that we lost in all this.
In the past couple of months, we have all become much more aware of the fragility of connectedness. However, we should recognize that the impact was well on its way before the COVID-19 crisis. It is my opinion that psychiatry should champion the issue of human relations. I do not think that we need to wait for a new DSM diagnosis, an evidence-based paradigm, or a Food and Drug Administration–approved medication to do so. The COVID-19 crisis has rendered us all cognizant of the importance of relationships.
While it may be that psychiatry continues to foray in electronic means of communication, use of impersonal scales and diagnosis, as well as anonymized algorithmic treatment plans, we should also promote as much humanity as society and public health safety will permit. Getting dressed to see your psychiatrist, face to face, to have an open-ended conversation about the nature of one’s life has clearly become something precious and powerful that should be cherished and protected. My hope is the rules and mandates we are required to use during the pandemic today do not become a continued habit that result in further loneliness and disconnect. If we chose to, the lessons we learn today can, in fact, strengthen our appreciation and pursuit of human connection.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the book “Critical Psychiatry: Controversies and Clinical Implications” (Springer, 2019). He has no disclosures.
Authors of picosecond laser review predict more widespread use of the technology
Ever since the first picosecond laser hit the market in 2012 as an option for treating unwanted tattoos and pigmented lesions, clinicians have used the technology to safely and effectively treat an expanding range of dermatologic conditions, from Nevus of Ota and melasma to rejuvenation.
. They called for further development of the technology and predicted that application of the devices will become more widespread.
“Future directions may include the development of even shorter pulse durations, improvements in fractionation method and delivery, and exploration of the utility of pulsing other laser wavelengths in the picosecond (or shorter) domain,” first author Douglas C. Wu, MD, PhD, of Cosmetic Laser Dermatology and colleagues wrote in the review. “The introduction of newer devices along with continued improvements in clinical technique and experience will drive the refinement and expansion of this technology.”
The authors evaluated medical literature on the topic published up to March 2020 and classified 78 studies into one of the following categories: discrete pigmented lesions, other nonmelasma pigmented conditions, rejuvenation, melasma, scar revision, and tattoo removal. They assessed the level of evidence for each indication according to modified criteria published by the Oxford Centre of Evidence-Based Medicine and proposed recommendations based on the medical literature in combination with the authors’ collective clinical experience with picosecond laser.
In the category of discrete pigmented lesions, the authors assigned level of evidence 1a to Nevus of Ota and Hori’s macules, level of evidence 2b to solar lentigines and freckles, level of evidence 3c to café au lait macules, and level of evidence 4 to all other benign pigmentary conditions. “Comparative studies utilizing clinical, histological, and microscopic endpoints further suggest that picosecond laser may be safer and more effective than nanosecond laser in some situations, with potentially reduced risk of inducing postinflammatory hyperpigmentation,” the authors wrote. “This increased safety level may be due to the reduction of non-specific photothermal damage of the melanocyte and dermal-epidermal junction,” they noted. They called for more robust clinical comparative data with a focus on shorter pulse durations and refined clinical endpoints “to further distinguish the differences between picosecond and nanosecond laser for the treatment of some benign pigmented lesions.”
Based on seven prospective open-label trials and three split-face comparison trials involving the use of picosecond lasers for photorejuvenation, the authors assigned a level of evidence 2a to this category. “The studies show a high level of safety associated with a moderate level of efficacy,” they wrote. “Indeed, when compared with traditional non-ablative fractional laser, fractionated picosecond laser may have an improved side effect profile without sacrificing treatment efficacy. This could be due to the unique mechanism of action of fractionated picosecond laser, which results in greater confinement of tissue injury to focal and precise points within the epidermis and papillary dermis.”
Clinical data on using picosecond lasers to treat melasma remains “mixed and unclear,” but it may have a role as an adjunctive treatment combined with rigorous photoprotection, topical melanin inhibitors, “and potentially other laser or systemic therapies as dictated by clinical circumstance,” the authors said. They do not recommend the picosecond laser as a monotherapy for melasma, and they assigned a level of evidence 2a to this category.
Although the fractionated picosecond laser is cleared by the Food and Drug Administration for the treatment of acne scars, Dr. Wu and his colleagues noted that rigorous clinical data on using the technology for this indication is limited. “Encouragingly, reports thus far seem to suggest that the risk of post-inflammatory pigmentary alteration is low when using fractionated picosecond laser, which has added significance due to the high prevalence of acne scarring in skin of color,” they wrote. They assigned a level of evidence 2b to this category. Meanwhile, clinical data on the use of picosecond lasers for non-acne scars are limited to cases series and retrospective reviews, reaching evidence level 3c. “Although the level of evidence is weak, there is likely an effective role for fractionated picosecond laser for the improvement of hyperpigmented scars given its more robust track record for the treatment of hyperpigmentation due to other causes such as benign pigmentary conditions and photodamage,” the authors wrote.
The manuscript concludes with a discussion of the picosecond laser’s role in tattoo removal, which represents the oldest and most established dermatologic indication for the technology. “The accumulated scientific and clinical evidence to date concludes that the shorter pulse duration confers a distinct advantage when other laser parameters remain equal,” the authors wrote. “The evidence also suggests that the shorter the pulse gets (within currently commercially available and tested devices), the greater becomes the efficacy for tattoo removal. There is no evidence to suggest that larger tattoo particles are more optimally targeted by longer nanosecond pulses.” They assigned a level of evidence 1a to this category and described using the picosecond laser for tattoo removal of almost any color as “the gold standard.”
In an interview, Arisa Ortiz, MD, described the manuscript as a thorough review of the clinical indications for picosecond lasers. “Overall, the review shows evidence for slightly better improvement of efficacy with picosecond lasers compared to nanosecond lasers,” said Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, who was not involved with the review. “They also show a slightly improved side effect profile with picosecond lasers [and] notably, less risk of postinflammatory hyperpigmentation in darker skin types compared to nanosecond lasers. One issue that was not addressed was the cost of picosecond lasers. The cost of a picosecond lasers remains substantially higher than the cost of a nanosecond laser. I am not sure that this extra cost justifies a slightly improved efficacy or slightly improved side effect profile.”
According to Eric F. Bernstein, MD, director of the Main Line Center for Laser Surgery in Ardmore, Penn., the versatility of picosecond lasers offers an advantage to dermatologists. “Most of them have three wavelengths at least,” said Dr. Bernstein, who was not involved with the systematic review. “That means you can treat skin types I-VI. I was never able to offer much for my patients with skin types V and VI for fractionated rejuvenation and treatment of acne scarring. But now, with these lasers, I have an option for them. That’s a huge advantage.”
He credited laser engineers as “the real heroes” in the success of picosecond lasers in dermatology. “They’re passionate, they’re brilliant, and they’re creative,” Dr. Bernstein said. “They’re the ones that build and produce these devices for multiple manufacturers. In our space, the innovation really comes from industry.”
The review authors and Dr. Ortiz reported having no relevant disclosures. Dr. Bernstein disclosed that he is head of Candela’s medical advisory board.
SOURCE: Wu DC et al. Lasers Surg Med. 2020. doi: 10.1002/lsm.23244.
Ever since the first picosecond laser hit the market in 2012 as an option for treating unwanted tattoos and pigmented lesions, clinicians have used the technology to safely and effectively treat an expanding range of dermatologic conditions, from Nevus of Ota and melasma to rejuvenation.
. They called for further development of the technology and predicted that application of the devices will become more widespread.
“Future directions may include the development of even shorter pulse durations, improvements in fractionation method and delivery, and exploration of the utility of pulsing other laser wavelengths in the picosecond (or shorter) domain,” first author Douglas C. Wu, MD, PhD, of Cosmetic Laser Dermatology and colleagues wrote in the review. “The introduction of newer devices along with continued improvements in clinical technique and experience will drive the refinement and expansion of this technology.”
The authors evaluated medical literature on the topic published up to March 2020 and classified 78 studies into one of the following categories: discrete pigmented lesions, other nonmelasma pigmented conditions, rejuvenation, melasma, scar revision, and tattoo removal. They assessed the level of evidence for each indication according to modified criteria published by the Oxford Centre of Evidence-Based Medicine and proposed recommendations based on the medical literature in combination with the authors’ collective clinical experience with picosecond laser.
In the category of discrete pigmented lesions, the authors assigned level of evidence 1a to Nevus of Ota and Hori’s macules, level of evidence 2b to solar lentigines and freckles, level of evidence 3c to café au lait macules, and level of evidence 4 to all other benign pigmentary conditions. “Comparative studies utilizing clinical, histological, and microscopic endpoints further suggest that picosecond laser may be safer and more effective than nanosecond laser in some situations, with potentially reduced risk of inducing postinflammatory hyperpigmentation,” the authors wrote. “This increased safety level may be due to the reduction of non-specific photothermal damage of the melanocyte and dermal-epidermal junction,” they noted. They called for more robust clinical comparative data with a focus on shorter pulse durations and refined clinical endpoints “to further distinguish the differences between picosecond and nanosecond laser for the treatment of some benign pigmented lesions.”
Based on seven prospective open-label trials and three split-face comparison trials involving the use of picosecond lasers for photorejuvenation, the authors assigned a level of evidence 2a to this category. “The studies show a high level of safety associated with a moderate level of efficacy,” they wrote. “Indeed, when compared with traditional non-ablative fractional laser, fractionated picosecond laser may have an improved side effect profile without sacrificing treatment efficacy. This could be due to the unique mechanism of action of fractionated picosecond laser, which results in greater confinement of tissue injury to focal and precise points within the epidermis and papillary dermis.”
Clinical data on using picosecond lasers to treat melasma remains “mixed and unclear,” but it may have a role as an adjunctive treatment combined with rigorous photoprotection, topical melanin inhibitors, “and potentially other laser or systemic therapies as dictated by clinical circumstance,” the authors said. They do not recommend the picosecond laser as a monotherapy for melasma, and they assigned a level of evidence 2a to this category.
Although the fractionated picosecond laser is cleared by the Food and Drug Administration for the treatment of acne scars, Dr. Wu and his colleagues noted that rigorous clinical data on using the technology for this indication is limited. “Encouragingly, reports thus far seem to suggest that the risk of post-inflammatory pigmentary alteration is low when using fractionated picosecond laser, which has added significance due to the high prevalence of acne scarring in skin of color,” they wrote. They assigned a level of evidence 2b to this category. Meanwhile, clinical data on the use of picosecond lasers for non-acne scars are limited to cases series and retrospective reviews, reaching evidence level 3c. “Although the level of evidence is weak, there is likely an effective role for fractionated picosecond laser for the improvement of hyperpigmented scars given its more robust track record for the treatment of hyperpigmentation due to other causes such as benign pigmentary conditions and photodamage,” the authors wrote.
The manuscript concludes with a discussion of the picosecond laser’s role in tattoo removal, which represents the oldest and most established dermatologic indication for the technology. “The accumulated scientific and clinical evidence to date concludes that the shorter pulse duration confers a distinct advantage when other laser parameters remain equal,” the authors wrote. “The evidence also suggests that the shorter the pulse gets (within currently commercially available and tested devices), the greater becomes the efficacy for tattoo removal. There is no evidence to suggest that larger tattoo particles are more optimally targeted by longer nanosecond pulses.” They assigned a level of evidence 1a to this category and described using the picosecond laser for tattoo removal of almost any color as “the gold standard.”
In an interview, Arisa Ortiz, MD, described the manuscript as a thorough review of the clinical indications for picosecond lasers. “Overall, the review shows evidence for slightly better improvement of efficacy with picosecond lasers compared to nanosecond lasers,” said Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, who was not involved with the review. “They also show a slightly improved side effect profile with picosecond lasers [and] notably, less risk of postinflammatory hyperpigmentation in darker skin types compared to nanosecond lasers. One issue that was not addressed was the cost of picosecond lasers. The cost of a picosecond lasers remains substantially higher than the cost of a nanosecond laser. I am not sure that this extra cost justifies a slightly improved efficacy or slightly improved side effect profile.”
According to Eric F. Bernstein, MD, director of the Main Line Center for Laser Surgery in Ardmore, Penn., the versatility of picosecond lasers offers an advantage to dermatologists. “Most of them have three wavelengths at least,” said Dr. Bernstein, who was not involved with the systematic review. “That means you can treat skin types I-VI. I was never able to offer much for my patients with skin types V and VI for fractionated rejuvenation and treatment of acne scarring. But now, with these lasers, I have an option for them. That’s a huge advantage.”
He credited laser engineers as “the real heroes” in the success of picosecond lasers in dermatology. “They’re passionate, they’re brilliant, and they’re creative,” Dr. Bernstein said. “They’re the ones that build and produce these devices for multiple manufacturers. In our space, the innovation really comes from industry.”
The review authors and Dr. Ortiz reported having no relevant disclosures. Dr. Bernstein disclosed that he is head of Candela’s medical advisory board.
SOURCE: Wu DC et al. Lasers Surg Med. 2020. doi: 10.1002/lsm.23244.
Ever since the first picosecond laser hit the market in 2012 as an option for treating unwanted tattoos and pigmented lesions, clinicians have used the technology to safely and effectively treat an expanding range of dermatologic conditions, from Nevus of Ota and melasma to rejuvenation.
. They called for further development of the technology and predicted that application of the devices will become more widespread.
“Future directions may include the development of even shorter pulse durations, improvements in fractionation method and delivery, and exploration of the utility of pulsing other laser wavelengths in the picosecond (or shorter) domain,” first author Douglas C. Wu, MD, PhD, of Cosmetic Laser Dermatology and colleagues wrote in the review. “The introduction of newer devices along with continued improvements in clinical technique and experience will drive the refinement and expansion of this technology.”
The authors evaluated medical literature on the topic published up to March 2020 and classified 78 studies into one of the following categories: discrete pigmented lesions, other nonmelasma pigmented conditions, rejuvenation, melasma, scar revision, and tattoo removal. They assessed the level of evidence for each indication according to modified criteria published by the Oxford Centre of Evidence-Based Medicine and proposed recommendations based on the medical literature in combination with the authors’ collective clinical experience with picosecond laser.
In the category of discrete pigmented lesions, the authors assigned level of evidence 1a to Nevus of Ota and Hori’s macules, level of evidence 2b to solar lentigines and freckles, level of evidence 3c to café au lait macules, and level of evidence 4 to all other benign pigmentary conditions. “Comparative studies utilizing clinical, histological, and microscopic endpoints further suggest that picosecond laser may be safer and more effective than nanosecond laser in some situations, with potentially reduced risk of inducing postinflammatory hyperpigmentation,” the authors wrote. “This increased safety level may be due to the reduction of non-specific photothermal damage of the melanocyte and dermal-epidermal junction,” they noted. They called for more robust clinical comparative data with a focus on shorter pulse durations and refined clinical endpoints “to further distinguish the differences between picosecond and nanosecond laser for the treatment of some benign pigmented lesions.”
Based on seven prospective open-label trials and three split-face comparison trials involving the use of picosecond lasers for photorejuvenation, the authors assigned a level of evidence 2a to this category. “The studies show a high level of safety associated with a moderate level of efficacy,” they wrote. “Indeed, when compared with traditional non-ablative fractional laser, fractionated picosecond laser may have an improved side effect profile without sacrificing treatment efficacy. This could be due to the unique mechanism of action of fractionated picosecond laser, which results in greater confinement of tissue injury to focal and precise points within the epidermis and papillary dermis.”
Clinical data on using picosecond lasers to treat melasma remains “mixed and unclear,” but it may have a role as an adjunctive treatment combined with rigorous photoprotection, topical melanin inhibitors, “and potentially other laser or systemic therapies as dictated by clinical circumstance,” the authors said. They do not recommend the picosecond laser as a monotherapy for melasma, and they assigned a level of evidence 2a to this category.
Although the fractionated picosecond laser is cleared by the Food and Drug Administration for the treatment of acne scars, Dr. Wu and his colleagues noted that rigorous clinical data on using the technology for this indication is limited. “Encouragingly, reports thus far seem to suggest that the risk of post-inflammatory pigmentary alteration is low when using fractionated picosecond laser, which has added significance due to the high prevalence of acne scarring in skin of color,” they wrote. They assigned a level of evidence 2b to this category. Meanwhile, clinical data on the use of picosecond lasers for non-acne scars are limited to cases series and retrospective reviews, reaching evidence level 3c. “Although the level of evidence is weak, there is likely an effective role for fractionated picosecond laser for the improvement of hyperpigmented scars given its more robust track record for the treatment of hyperpigmentation due to other causes such as benign pigmentary conditions and photodamage,” the authors wrote.
The manuscript concludes with a discussion of the picosecond laser’s role in tattoo removal, which represents the oldest and most established dermatologic indication for the technology. “The accumulated scientific and clinical evidence to date concludes that the shorter pulse duration confers a distinct advantage when other laser parameters remain equal,” the authors wrote. “The evidence also suggests that the shorter the pulse gets (within currently commercially available and tested devices), the greater becomes the efficacy for tattoo removal. There is no evidence to suggest that larger tattoo particles are more optimally targeted by longer nanosecond pulses.” They assigned a level of evidence 1a to this category and described using the picosecond laser for tattoo removal of almost any color as “the gold standard.”
In an interview, Arisa Ortiz, MD, described the manuscript as a thorough review of the clinical indications for picosecond lasers. “Overall, the review shows evidence for slightly better improvement of efficacy with picosecond lasers compared to nanosecond lasers,” said Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, who was not involved with the review. “They also show a slightly improved side effect profile with picosecond lasers [and] notably, less risk of postinflammatory hyperpigmentation in darker skin types compared to nanosecond lasers. One issue that was not addressed was the cost of picosecond lasers. The cost of a picosecond lasers remains substantially higher than the cost of a nanosecond laser. I am not sure that this extra cost justifies a slightly improved efficacy or slightly improved side effect profile.”
According to Eric F. Bernstein, MD, director of the Main Line Center for Laser Surgery in Ardmore, Penn., the versatility of picosecond lasers offers an advantage to dermatologists. “Most of them have three wavelengths at least,” said Dr. Bernstein, who was not involved with the systematic review. “That means you can treat skin types I-VI. I was never able to offer much for my patients with skin types V and VI for fractionated rejuvenation and treatment of acne scarring. But now, with these lasers, I have an option for them. That’s a huge advantage.”
He credited laser engineers as “the real heroes” in the success of picosecond lasers in dermatology. “They’re passionate, they’re brilliant, and they’re creative,” Dr. Bernstein said. “They’re the ones that build and produce these devices for multiple manufacturers. In our space, the innovation really comes from industry.”
The review authors and Dr. Ortiz reported having no relevant disclosures. Dr. Bernstein disclosed that he is head of Candela’s medical advisory board.
SOURCE: Wu DC et al. Lasers Surg Med. 2020. doi: 10.1002/lsm.23244.
FROM LASERS IN SURGERY AND MEDICINE
Despite guidelines, controversy remains over corticosteroids in COVID-19
Three main reasons have been put forth for using corticosteroids in critically ill patients with COVID-19, but only one – the hope of preventing lung fibrosis in patients with unresolved acute respiratory distress syndrome – is reasonable to employ now outside of formal randomized trials, Peter Pickkers, MD, PhD, asserted at a webinar on COVID-19 sponsored by the European Society of Intensive Care Medicine.
The most commonly invoked rationale for giving steroids in patients with severe COVID-19 is to modulate the destructive inflammatory immune response that occurs with advancing disease. Another justification cited for giving steroids is to treat suspected adrenal insufficiency in those with refractory shock. Of note, both practices are endorsed in the recent Surviving Sepsis Campaign guidelines on management of critically ill patients with COVID-19 (Intensive Care Med. 2020 May;46[5]:854-87).
But those recommendations – numbers 22 and 42 out of a total of 50 recommendations included in the guidelines – should never have been made, according to Dr. Pickkers, professor of experimental intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
Dueling guidelines
The Surviving Sepsis Campaign guidelines, which were developed by a panel comprising 36 experts in 12 countries, are quite frank in conceding that the guidance in favor of corticosteroids are weak recommendations based on low-quality evidence.
The guidelines recommend against using corticosteroids to try to modulate the immune system in mechanically ventilated COVID-19 patients without acute respiratory distress syndrome (ARDS), but do recommend steroids in those with COVID-19 and ARDS. However, the guidelines also note that, because of the very low quality of the evidence, some experts on the panel preferred not to issue a pro-steroids recommendation at all until higher-quality evidence becomes available. Dr. Pickkers said he believes that the minority view should have prevailed. Moreover, current COVID-19 guidance from the World Health Organization is at odds with the Surviving Sepsis Campaign recommendations; the WHO advises against corticosteroids unless the treatment is indicated for a reason other than immunomodulation, he noted.
The evidence in favor of steroids in an effort to blunt the immune response in COVID patients with ARDS is based largely upon a single small, retrospective, non–peer-reviewed report that 5-7 days of treatment with 1-2 mg/kg per day of methylprednisolone was associated with shortened fever duration and need for supplemental oxygen.
The evidence against steroids for immunomodulation comes mainly from earlier studies of the SARS and MERS novel coronaviruses. For example, in a multicenter study of 309 patients with the MERS (Middle East respiratory syndrome) virus, those who received corticosteroids received no benefit and experienced delayed viral clearance (Am J Respir Crit Care Med. 2018 Mar 15;197[6]:757-67).
“The thing is, virtually all COVID-19 patients in the ICU fulfill the criteria for ARDS, so following the Surviving Sepsis Campaign guidelines would have far-reaching consequences,” Dr. Pickkers said.
Those consequences include a theoretic potential for serious harm arising from dampening the immune response at a point in the course of COVID-19 when the virus is still present, which could result in slowed viral clearance and prolonged viral shedding. Moreover so far no one has been able to identify a sweet spot in the disease course where the viral load has waned and the immune response is sufficiently early that intervention with corticosteroids might have an optimal benefit/risk ratio, he continued.
“My opinion is that at this moment there is no benefit at all for corticosteroids for immunomodulation in patients with COVID-19,” Dr. Pickkers said. “My personal recommendation, in contrast to the Surviving Sepsis Campaign recommendation, is not to use this therapy outside of a study.”
He added that randomized, controlled trials of corticosteroid therapy in critically ill patients with COVID-19 are ongoing in Europe and the United States, including the large RECOVERY study of dexamethasone in the United Kingdom.
As for the Surviving Sepsis Campaign recommendation to use corticosteroids to treat refractory shock in COVID-19, Dr. Pickkers dismissed this guidance as largely irrelevant. That’s because few patients with COVID-19 who need mechanical ventilation have refractory shock as evidenced by the need for a high infusion rate of norepinephrine. Anyway, he noted, that Surviving Sepsis recommendation is based upon extrapolation from evidence of benefit in bacterial septic shock patients, which he deemed to be of questionable relevance to the COVID-19 pandemic.
Attacking the fibroproliferative phase of ARDS
First off, Dr. Pickkers conceded, there is no evidence that treatment with corticosteroids to prevent lung fibrosis in COVID-19 patients with nonresolving ARDS is an effective strategy; the pandemic is simply too new at this point for the appropriate studies to have been done. But this much is known: Postmortem pathologic studies show fibroplastic proliferation is present in the lungs of COVID-19 patients, as in those who die of ARDS of other causes. Also, COVID-19 patients typically aren’t admitted to the ICU until day 11 or 12 after developing their first symptoms, so by the time they display indications that their ARDS is not resolving, the virus has typically left the scene; thus, there is little risk at that point that corticosteroids will promote viral proliferation. Additionally, studies in critically ill patients with nonresolving ARDS of other causes show clinically meaningful benefits for corticosteroid therapy.
Dr. Pickkers cited as “must reading” an analysis of five randomized trials of corticosteroid therapy in a total of 518 patients with acute lung injury ARDS of non–COVID-19 origin. The analysis by investigators at the University of Tennessee, Memphis, concluded that treatment resulted in clinically meaningful reductions in duration of mechanical ventilation and ICU length of stay. Moreover, in the 400 patients whose steroid therapy commenced before day 14 of ARDS, there was a statistically significant 22% reduction in risk of death, compared with patients in whom corticosteroids were started later (Intensive Care Med. 2008 Jan;34[1]:61-9).
Session cochair Jan De Waele, MD, PhD, struck a cautious note, remarking, “It’s my perception that we’re using the evidence that we’ve gathered in other conditions and are now trying to apply it in COVID-19. But quality data on patients with COVID-19 itself is pretty scare, and it’s really hard to say whether this disease behaves similarly to bacterial septic shock or to other viral infections.”
“We need more information about the use of corticosteroids in COVID-19, although I think a lot of people are using it at this moment,” added Dr. De Waele, a surgical intensivist at Ghent (Belgium) University.
That being said, he asked Dr. Pickkers when he considers using corticosteroids to prevent pulmonary fibrosis.
Dr. Pickkers said that when he notices that a COVID-19 patient’s lung compliance is worsening, that stiff lung is a clue that fibrosis is occurring and is having clinical consequences. “We also measure blood procollagen, a not very sensitive but moderately specific marker of fibroproliferation. If we see an increase in this biomarker and the lung mechanics are changing, then we do treat these patients with corticosteroids,” Dr. Pickkers replied.
He and his colleagues try to start steroids before day 14 of ARDS, and they continue treatment for longer than 7 days in order to prevent a rebound inflammatory response upon treatment discontinuation. They also avoid using neuromuscular agents and engage in meticulous infection surveillance in order to minimize potential complications of corticosteroid therapy in the ICU.
Dr. Pickkers reported having no financial conflicts regarding his presentation.
Three main reasons have been put forth for using corticosteroids in critically ill patients with COVID-19, but only one – the hope of preventing lung fibrosis in patients with unresolved acute respiratory distress syndrome – is reasonable to employ now outside of formal randomized trials, Peter Pickkers, MD, PhD, asserted at a webinar on COVID-19 sponsored by the European Society of Intensive Care Medicine.
The most commonly invoked rationale for giving steroids in patients with severe COVID-19 is to modulate the destructive inflammatory immune response that occurs with advancing disease. Another justification cited for giving steroids is to treat suspected adrenal insufficiency in those with refractory shock. Of note, both practices are endorsed in the recent Surviving Sepsis Campaign guidelines on management of critically ill patients with COVID-19 (Intensive Care Med. 2020 May;46[5]:854-87).
But those recommendations – numbers 22 and 42 out of a total of 50 recommendations included in the guidelines – should never have been made, according to Dr. Pickkers, professor of experimental intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
Dueling guidelines
The Surviving Sepsis Campaign guidelines, which were developed by a panel comprising 36 experts in 12 countries, are quite frank in conceding that the guidance in favor of corticosteroids are weak recommendations based on low-quality evidence.
The guidelines recommend against using corticosteroids to try to modulate the immune system in mechanically ventilated COVID-19 patients without acute respiratory distress syndrome (ARDS), but do recommend steroids in those with COVID-19 and ARDS. However, the guidelines also note that, because of the very low quality of the evidence, some experts on the panel preferred not to issue a pro-steroids recommendation at all until higher-quality evidence becomes available. Dr. Pickkers said he believes that the minority view should have prevailed. Moreover, current COVID-19 guidance from the World Health Organization is at odds with the Surviving Sepsis Campaign recommendations; the WHO advises against corticosteroids unless the treatment is indicated for a reason other than immunomodulation, he noted.
The evidence in favor of steroids in an effort to blunt the immune response in COVID patients with ARDS is based largely upon a single small, retrospective, non–peer-reviewed report that 5-7 days of treatment with 1-2 mg/kg per day of methylprednisolone was associated with shortened fever duration and need for supplemental oxygen.
The evidence against steroids for immunomodulation comes mainly from earlier studies of the SARS and MERS novel coronaviruses. For example, in a multicenter study of 309 patients with the MERS (Middle East respiratory syndrome) virus, those who received corticosteroids received no benefit and experienced delayed viral clearance (Am J Respir Crit Care Med. 2018 Mar 15;197[6]:757-67).
“The thing is, virtually all COVID-19 patients in the ICU fulfill the criteria for ARDS, so following the Surviving Sepsis Campaign guidelines would have far-reaching consequences,” Dr. Pickkers said.
Those consequences include a theoretic potential for serious harm arising from dampening the immune response at a point in the course of COVID-19 when the virus is still present, which could result in slowed viral clearance and prolonged viral shedding. Moreover so far no one has been able to identify a sweet spot in the disease course where the viral load has waned and the immune response is sufficiently early that intervention with corticosteroids might have an optimal benefit/risk ratio, he continued.
“My opinion is that at this moment there is no benefit at all for corticosteroids for immunomodulation in patients with COVID-19,” Dr. Pickkers said. “My personal recommendation, in contrast to the Surviving Sepsis Campaign recommendation, is not to use this therapy outside of a study.”
He added that randomized, controlled trials of corticosteroid therapy in critically ill patients with COVID-19 are ongoing in Europe and the United States, including the large RECOVERY study of dexamethasone in the United Kingdom.
As for the Surviving Sepsis Campaign recommendation to use corticosteroids to treat refractory shock in COVID-19, Dr. Pickkers dismissed this guidance as largely irrelevant. That’s because few patients with COVID-19 who need mechanical ventilation have refractory shock as evidenced by the need for a high infusion rate of norepinephrine. Anyway, he noted, that Surviving Sepsis recommendation is based upon extrapolation from evidence of benefit in bacterial septic shock patients, which he deemed to be of questionable relevance to the COVID-19 pandemic.
Attacking the fibroproliferative phase of ARDS
First off, Dr. Pickkers conceded, there is no evidence that treatment with corticosteroids to prevent lung fibrosis in COVID-19 patients with nonresolving ARDS is an effective strategy; the pandemic is simply too new at this point for the appropriate studies to have been done. But this much is known: Postmortem pathologic studies show fibroplastic proliferation is present in the lungs of COVID-19 patients, as in those who die of ARDS of other causes. Also, COVID-19 patients typically aren’t admitted to the ICU until day 11 or 12 after developing their first symptoms, so by the time they display indications that their ARDS is not resolving, the virus has typically left the scene; thus, there is little risk at that point that corticosteroids will promote viral proliferation. Additionally, studies in critically ill patients with nonresolving ARDS of other causes show clinically meaningful benefits for corticosteroid therapy.
Dr. Pickkers cited as “must reading” an analysis of five randomized trials of corticosteroid therapy in a total of 518 patients with acute lung injury ARDS of non–COVID-19 origin. The analysis by investigators at the University of Tennessee, Memphis, concluded that treatment resulted in clinically meaningful reductions in duration of mechanical ventilation and ICU length of stay. Moreover, in the 400 patients whose steroid therapy commenced before day 14 of ARDS, there was a statistically significant 22% reduction in risk of death, compared with patients in whom corticosteroids were started later (Intensive Care Med. 2008 Jan;34[1]:61-9).
Session cochair Jan De Waele, MD, PhD, struck a cautious note, remarking, “It’s my perception that we’re using the evidence that we’ve gathered in other conditions and are now trying to apply it in COVID-19. But quality data on patients with COVID-19 itself is pretty scare, and it’s really hard to say whether this disease behaves similarly to bacterial septic shock or to other viral infections.”
“We need more information about the use of corticosteroids in COVID-19, although I think a lot of people are using it at this moment,” added Dr. De Waele, a surgical intensivist at Ghent (Belgium) University.
That being said, he asked Dr. Pickkers when he considers using corticosteroids to prevent pulmonary fibrosis.
Dr. Pickkers said that when he notices that a COVID-19 patient’s lung compliance is worsening, that stiff lung is a clue that fibrosis is occurring and is having clinical consequences. “We also measure blood procollagen, a not very sensitive but moderately specific marker of fibroproliferation. If we see an increase in this biomarker and the lung mechanics are changing, then we do treat these patients with corticosteroids,” Dr. Pickkers replied.
He and his colleagues try to start steroids before day 14 of ARDS, and they continue treatment for longer than 7 days in order to prevent a rebound inflammatory response upon treatment discontinuation. They also avoid using neuromuscular agents and engage in meticulous infection surveillance in order to minimize potential complications of corticosteroid therapy in the ICU.
Dr. Pickkers reported having no financial conflicts regarding his presentation.
Three main reasons have been put forth for using corticosteroids in critically ill patients with COVID-19, but only one – the hope of preventing lung fibrosis in patients with unresolved acute respiratory distress syndrome – is reasonable to employ now outside of formal randomized trials, Peter Pickkers, MD, PhD, asserted at a webinar on COVID-19 sponsored by the European Society of Intensive Care Medicine.
The most commonly invoked rationale for giving steroids in patients with severe COVID-19 is to modulate the destructive inflammatory immune response that occurs with advancing disease. Another justification cited for giving steroids is to treat suspected adrenal insufficiency in those with refractory shock. Of note, both practices are endorsed in the recent Surviving Sepsis Campaign guidelines on management of critically ill patients with COVID-19 (Intensive Care Med. 2020 May;46[5]:854-87).
But those recommendations – numbers 22 and 42 out of a total of 50 recommendations included in the guidelines – should never have been made, according to Dr. Pickkers, professor of experimental intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
Dueling guidelines
The Surviving Sepsis Campaign guidelines, which were developed by a panel comprising 36 experts in 12 countries, are quite frank in conceding that the guidance in favor of corticosteroids are weak recommendations based on low-quality evidence.
The guidelines recommend against using corticosteroids to try to modulate the immune system in mechanically ventilated COVID-19 patients without acute respiratory distress syndrome (ARDS), but do recommend steroids in those with COVID-19 and ARDS. However, the guidelines also note that, because of the very low quality of the evidence, some experts on the panel preferred not to issue a pro-steroids recommendation at all until higher-quality evidence becomes available. Dr. Pickkers said he believes that the minority view should have prevailed. Moreover, current COVID-19 guidance from the World Health Organization is at odds with the Surviving Sepsis Campaign recommendations; the WHO advises against corticosteroids unless the treatment is indicated for a reason other than immunomodulation, he noted.
The evidence in favor of steroids in an effort to blunt the immune response in COVID patients with ARDS is based largely upon a single small, retrospective, non–peer-reviewed report that 5-7 days of treatment with 1-2 mg/kg per day of methylprednisolone was associated with shortened fever duration and need for supplemental oxygen.
The evidence against steroids for immunomodulation comes mainly from earlier studies of the SARS and MERS novel coronaviruses. For example, in a multicenter study of 309 patients with the MERS (Middle East respiratory syndrome) virus, those who received corticosteroids received no benefit and experienced delayed viral clearance (Am J Respir Crit Care Med. 2018 Mar 15;197[6]:757-67).
“The thing is, virtually all COVID-19 patients in the ICU fulfill the criteria for ARDS, so following the Surviving Sepsis Campaign guidelines would have far-reaching consequences,” Dr. Pickkers said.
Those consequences include a theoretic potential for serious harm arising from dampening the immune response at a point in the course of COVID-19 when the virus is still present, which could result in slowed viral clearance and prolonged viral shedding. Moreover so far no one has been able to identify a sweet spot in the disease course where the viral load has waned and the immune response is sufficiently early that intervention with corticosteroids might have an optimal benefit/risk ratio, he continued.
“My opinion is that at this moment there is no benefit at all for corticosteroids for immunomodulation in patients with COVID-19,” Dr. Pickkers said. “My personal recommendation, in contrast to the Surviving Sepsis Campaign recommendation, is not to use this therapy outside of a study.”
He added that randomized, controlled trials of corticosteroid therapy in critically ill patients with COVID-19 are ongoing in Europe and the United States, including the large RECOVERY study of dexamethasone in the United Kingdom.
As for the Surviving Sepsis Campaign recommendation to use corticosteroids to treat refractory shock in COVID-19, Dr. Pickkers dismissed this guidance as largely irrelevant. That’s because few patients with COVID-19 who need mechanical ventilation have refractory shock as evidenced by the need for a high infusion rate of norepinephrine. Anyway, he noted, that Surviving Sepsis recommendation is based upon extrapolation from evidence of benefit in bacterial septic shock patients, which he deemed to be of questionable relevance to the COVID-19 pandemic.
Attacking the fibroproliferative phase of ARDS
First off, Dr. Pickkers conceded, there is no evidence that treatment with corticosteroids to prevent lung fibrosis in COVID-19 patients with nonresolving ARDS is an effective strategy; the pandemic is simply too new at this point for the appropriate studies to have been done. But this much is known: Postmortem pathologic studies show fibroplastic proliferation is present in the lungs of COVID-19 patients, as in those who die of ARDS of other causes. Also, COVID-19 patients typically aren’t admitted to the ICU until day 11 or 12 after developing their first symptoms, so by the time they display indications that their ARDS is not resolving, the virus has typically left the scene; thus, there is little risk at that point that corticosteroids will promote viral proliferation. Additionally, studies in critically ill patients with nonresolving ARDS of other causes show clinically meaningful benefits for corticosteroid therapy.
Dr. Pickkers cited as “must reading” an analysis of five randomized trials of corticosteroid therapy in a total of 518 patients with acute lung injury ARDS of non–COVID-19 origin. The analysis by investigators at the University of Tennessee, Memphis, concluded that treatment resulted in clinically meaningful reductions in duration of mechanical ventilation and ICU length of stay. Moreover, in the 400 patients whose steroid therapy commenced before day 14 of ARDS, there was a statistically significant 22% reduction in risk of death, compared with patients in whom corticosteroids were started later (Intensive Care Med. 2008 Jan;34[1]:61-9).
Session cochair Jan De Waele, MD, PhD, struck a cautious note, remarking, “It’s my perception that we’re using the evidence that we’ve gathered in other conditions and are now trying to apply it in COVID-19. But quality data on patients with COVID-19 itself is pretty scare, and it’s really hard to say whether this disease behaves similarly to bacterial septic shock or to other viral infections.”
“We need more information about the use of corticosteroids in COVID-19, although I think a lot of people are using it at this moment,” added Dr. De Waele, a surgical intensivist at Ghent (Belgium) University.
That being said, he asked Dr. Pickkers when he considers using corticosteroids to prevent pulmonary fibrosis.
Dr. Pickkers said that when he notices that a COVID-19 patient’s lung compliance is worsening, that stiff lung is a clue that fibrosis is occurring and is having clinical consequences. “We also measure blood procollagen, a not very sensitive but moderately specific marker of fibroproliferation. If we see an increase in this biomarker and the lung mechanics are changing, then we do treat these patients with corticosteroids,” Dr. Pickkers replied.
He and his colleagues try to start steroids before day 14 of ARDS, and they continue treatment for longer than 7 days in order to prevent a rebound inflammatory response upon treatment discontinuation. They also avoid using neuromuscular agents and engage in meticulous infection surveillance in order to minimize potential complications of corticosteroid therapy in the ICU.
Dr. Pickkers reported having no financial conflicts regarding his presentation.
Today’s top news highlights: Cancer makes COVID-19 more dangerous, treatment for heavy menstrual bleeding, and more
Here are the stories our MDedge editors across specialties think you need to know about today:
Active cancer ups death risk for patients with COVID-19
New data show that patients with COVID-19 and progressing cancer had a significantly higher risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer. Interestingly, one of the independent risk factor for death in patients with COVID-19 and cancer was treatment with hydroxychloroquine plus azithromycin. This finding, however, was of “uncertain validity due to a high risk of residual confounding; for example, patients receiving this combination were more likely to have severe disease or more likely to be hospitalized,” said Jeremy L. Warner, MD, of Vanderbilt University Medical Center in Nashville. Read more.
Two new studies indicate that social distancing successfully flattened the curve on COVID-19 hospitalizations. One study, published in JAMA, showed significantly lower numbers of observed cases versus worst-case projections in four states: Colorado, Minnesota, Ohio, and Virginia. In Minnesota, 17 days after the order, there were 361 cumulative hospitalizations, compared with a projection of 988 had no such action been taken. In a separate study measuring COVID-19 patients occupying ICU beds in Ontario and deaths among those cases, hospitals “would have rapidly exceeded ICU capacity and observed substantially higher mortality” without any physical distancing intervention. Read more.
FDA approves treatment for heavy menstrual bleeding
The Food and Drug Administration has approved a medication for the management of heavy menstrual bleeding associated with uterine fibroids in premenopausal women. The medication, marketed as Oriahnn, is an estrogen and progestin combination product that consists of elagolix, estradiol, and norethindrone acetate capsules packaged together for oral use. The most common side effects of the drug included hot flushes, headache, fatigue, and irregular vaginal bleeding. The drug’s label includes a boxed warning about a risk of strokes and blood clots, especially in women at increased risk for these events. Read more.
Lessons from a drive-through COVID testing center
Chris Notte, MD, and Neil Skolnik, MD, were part of a team of clinicians charged with launching a drive-through COVID-19 testing center. Their task was to get the operation up and running in 2 days. It took them 3 days. While the launch was a success, the experience taught them some lessons about the limits of medical technology and the importance of personal protective equipment. “Prior to the coronavirus pandemic, I had never considered surgical masks, face shields, and nasal swabs to be critical components of medical technology. My opinion quickly changed after opening our drive-through COVID-19 site,” they wrote. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Active cancer ups death risk for patients with COVID-19
New data show that patients with COVID-19 and progressing cancer had a significantly higher risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer. Interestingly, one of the independent risk factor for death in patients with COVID-19 and cancer was treatment with hydroxychloroquine plus azithromycin. This finding, however, was of “uncertain validity due to a high risk of residual confounding; for example, patients receiving this combination were more likely to have severe disease or more likely to be hospitalized,” said Jeremy L. Warner, MD, of Vanderbilt University Medical Center in Nashville. Read more.
Two new studies indicate that social distancing successfully flattened the curve on COVID-19 hospitalizations. One study, published in JAMA, showed significantly lower numbers of observed cases versus worst-case projections in four states: Colorado, Minnesota, Ohio, and Virginia. In Minnesota, 17 days after the order, there were 361 cumulative hospitalizations, compared with a projection of 988 had no such action been taken. In a separate study measuring COVID-19 patients occupying ICU beds in Ontario and deaths among those cases, hospitals “would have rapidly exceeded ICU capacity and observed substantially higher mortality” without any physical distancing intervention. Read more.
FDA approves treatment for heavy menstrual bleeding
The Food and Drug Administration has approved a medication for the management of heavy menstrual bleeding associated with uterine fibroids in premenopausal women. The medication, marketed as Oriahnn, is an estrogen and progestin combination product that consists of elagolix, estradiol, and norethindrone acetate capsules packaged together for oral use. The most common side effects of the drug included hot flushes, headache, fatigue, and irregular vaginal bleeding. The drug’s label includes a boxed warning about a risk of strokes and blood clots, especially in women at increased risk for these events. Read more.
Lessons from a drive-through COVID testing center
Chris Notte, MD, and Neil Skolnik, MD, were part of a team of clinicians charged with launching a drive-through COVID-19 testing center. Their task was to get the operation up and running in 2 days. It took them 3 days. While the launch was a success, the experience taught them some lessons about the limits of medical technology and the importance of personal protective equipment. “Prior to the coronavirus pandemic, I had never considered surgical masks, face shields, and nasal swabs to be critical components of medical technology. My opinion quickly changed after opening our drive-through COVID-19 site,” they wrote. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Active cancer ups death risk for patients with COVID-19
New data show that patients with COVID-19 and progressing cancer had a significantly higher risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer. Interestingly, one of the independent risk factor for death in patients with COVID-19 and cancer was treatment with hydroxychloroquine plus azithromycin. This finding, however, was of “uncertain validity due to a high risk of residual confounding; for example, patients receiving this combination were more likely to have severe disease or more likely to be hospitalized,” said Jeremy L. Warner, MD, of Vanderbilt University Medical Center in Nashville. Read more.
Two new studies indicate that social distancing successfully flattened the curve on COVID-19 hospitalizations. One study, published in JAMA, showed significantly lower numbers of observed cases versus worst-case projections in four states: Colorado, Minnesota, Ohio, and Virginia. In Minnesota, 17 days after the order, there were 361 cumulative hospitalizations, compared with a projection of 988 had no such action been taken. In a separate study measuring COVID-19 patients occupying ICU beds in Ontario and deaths among those cases, hospitals “would have rapidly exceeded ICU capacity and observed substantially higher mortality” without any physical distancing intervention. Read more.
FDA approves treatment for heavy menstrual bleeding
The Food and Drug Administration has approved a medication for the management of heavy menstrual bleeding associated with uterine fibroids in premenopausal women. The medication, marketed as Oriahnn, is an estrogen and progestin combination product that consists of elagolix, estradiol, and norethindrone acetate capsules packaged together for oral use. The most common side effects of the drug included hot flushes, headache, fatigue, and irregular vaginal bleeding. The drug’s label includes a boxed warning about a risk of strokes and blood clots, especially in women at increased risk for these events. Read more.
Lessons from a drive-through COVID testing center
Chris Notte, MD, and Neil Skolnik, MD, were part of a team of clinicians charged with launching a drive-through COVID-19 testing center. Their task was to get the operation up and running in 2 days. It took them 3 days. While the launch was a success, the experience taught them some lessons about the limits of medical technology and the importance of personal protective equipment. “Prior to the coronavirus pandemic, I had never considered surgical masks, face shields, and nasal swabs to be critical components of medical technology. My opinion quickly changed after opening our drive-through COVID-19 site,” they wrote. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
COVID-19 effect: Prescription fills mostly down for leading drugs
Prescription fills for hydroxychloroquine/chloroquine spiked right after COVID-19 was declared a national emergency in March, but use of the drugs still remains well above 2019 levels, based on data from more than 58,000 U.S. pharmacies.
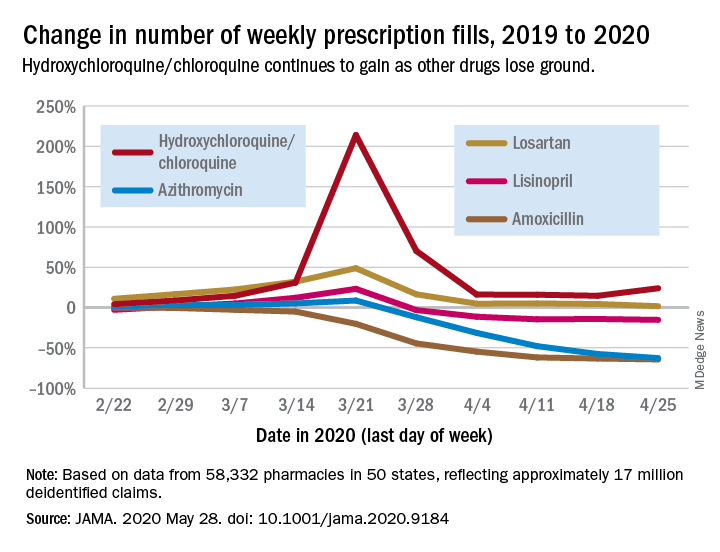
Hydroxychloroquine/chloroquine are also doing better than any of the prescription drugs in the top 10 based on total claims in 2019, Muthiah Vaduganathan, MD, MPH, of Brigham and Women’s Hospital, Boston, and associates reported May 28 in a research letter in JAMA.
Prescription fills for hydroxychloroquine/chloroquine have been above 2019 levels every week since the national emergency was declared on March 13, with the high occurring during the week of March 15-21, when fills were 214% higher than the corresponding week in 2019. The lowest level in that time came during the week of April 12-18, with growth of 14.6% over 2019, the investigators said.
The drugs occupying the top 10 – amlodipine, amoxicillin, atorvastatin, gabapentin, hydrocodone-acetaminophen, levothyroxine, lisinopril, losartan, omeprazole, and sertraline – have not done as well. Losartan, the only one that hasn’t lost ground in any week since March 13, rose by almost 49% during March 15-21, but was down to a 1.7% rise by the end of the study period, they reported.
Meanwhile, the other drug touted as a treatment for COVID-19, azithromycin, has fallen farther than most of the top 10. By April 19-25, the last week of the study period, fills for the antibiotic were down 62.7%, compared with last year, the analysis showed. Only amoxicillin had dropped more (64.4%).
“The modest decline for most common long-term therapies after peak could represent reduced contact with prescribing clinicians, restricted access to pharmacies, pharmacist rationing, loss of insurance from unemployment, or replete supplies from early stockpiling,” Dr. Vaduganathan and associates wrote.
The investigators “used all-payer U.S. pharmacy data from 58,332 chain, independent, and mail-order pharmacies across 14,421 zip codes in 50 states, reflecting approximately 17 million deidentified claims,” to estimate national prescription fills, they explained.
SOURCE: Vaduganathan M et al. JAMA 2020 May 28. doi: 10.1001/jama.2020.9184.
Prescription fills for hydroxychloroquine/chloroquine spiked right after COVID-19 was declared a national emergency in March, but use of the drugs still remains well above 2019 levels, based on data from more than 58,000 U.S. pharmacies.

Hydroxychloroquine/chloroquine are also doing better than any of the prescription drugs in the top 10 based on total claims in 2019, Muthiah Vaduganathan, MD, MPH, of Brigham and Women’s Hospital, Boston, and associates reported May 28 in a research letter in JAMA.
Prescription fills for hydroxychloroquine/chloroquine have been above 2019 levels every week since the national emergency was declared on March 13, with the high occurring during the week of March 15-21, when fills were 214% higher than the corresponding week in 2019. The lowest level in that time came during the week of April 12-18, with growth of 14.6% over 2019, the investigators said.
The drugs occupying the top 10 – amlodipine, amoxicillin, atorvastatin, gabapentin, hydrocodone-acetaminophen, levothyroxine, lisinopril, losartan, omeprazole, and sertraline – have not done as well. Losartan, the only one that hasn’t lost ground in any week since March 13, rose by almost 49% during March 15-21, but was down to a 1.7% rise by the end of the study period, they reported.
Meanwhile, the other drug touted as a treatment for COVID-19, azithromycin, has fallen farther than most of the top 10. By April 19-25, the last week of the study period, fills for the antibiotic were down 62.7%, compared with last year, the analysis showed. Only amoxicillin had dropped more (64.4%).
“The modest decline for most common long-term therapies after peak could represent reduced contact with prescribing clinicians, restricted access to pharmacies, pharmacist rationing, loss of insurance from unemployment, or replete supplies from early stockpiling,” Dr. Vaduganathan and associates wrote.
The investigators “used all-payer U.S. pharmacy data from 58,332 chain, independent, and mail-order pharmacies across 14,421 zip codes in 50 states, reflecting approximately 17 million deidentified claims,” to estimate national prescription fills, they explained.
SOURCE: Vaduganathan M et al. JAMA 2020 May 28. doi: 10.1001/jama.2020.9184.
Prescription fills for hydroxychloroquine/chloroquine spiked right after COVID-19 was declared a national emergency in March, but use of the drugs still remains well above 2019 levels, based on data from more than 58,000 U.S. pharmacies.

Hydroxychloroquine/chloroquine are also doing better than any of the prescription drugs in the top 10 based on total claims in 2019, Muthiah Vaduganathan, MD, MPH, of Brigham and Women’s Hospital, Boston, and associates reported May 28 in a research letter in JAMA.
Prescription fills for hydroxychloroquine/chloroquine have been above 2019 levels every week since the national emergency was declared on March 13, with the high occurring during the week of March 15-21, when fills were 214% higher than the corresponding week in 2019. The lowest level in that time came during the week of April 12-18, with growth of 14.6% over 2019, the investigators said.
The drugs occupying the top 10 – amlodipine, amoxicillin, atorvastatin, gabapentin, hydrocodone-acetaminophen, levothyroxine, lisinopril, losartan, omeprazole, and sertraline – have not done as well. Losartan, the only one that hasn’t lost ground in any week since March 13, rose by almost 49% during March 15-21, but was down to a 1.7% rise by the end of the study period, they reported.
Meanwhile, the other drug touted as a treatment for COVID-19, azithromycin, has fallen farther than most of the top 10. By April 19-25, the last week of the study period, fills for the antibiotic were down 62.7%, compared with last year, the analysis showed. Only amoxicillin had dropped more (64.4%).
“The modest decline for most common long-term therapies after peak could represent reduced contact with prescribing clinicians, restricted access to pharmacies, pharmacist rationing, loss of insurance from unemployment, or replete supplies from early stockpiling,” Dr. Vaduganathan and associates wrote.
The investigators “used all-payer U.S. pharmacy data from 58,332 chain, independent, and mail-order pharmacies across 14,421 zip codes in 50 states, reflecting approximately 17 million deidentified claims,” to estimate national prescription fills, they explained.
SOURCE: Vaduganathan M et al. JAMA 2020 May 28. doi: 10.1001/jama.2020.9184.
FROM JAMA
APA, others lobby to make COVID-19 telehealth waivers permanent
The American Psychiatric Association (APA) is calling on Congress to permanently lift restrictions that have allowed unfettered delivery of telehealth services during the COVID-19 pandemic, which experts say has been a boon to patients and physicians alike.
“We ask Congress to extend the telehealth waiver authority under COVID-19 beyond the emergency and to study its impact while doing so,” said APA President Jeffrey Geller, MD, in a May 27 video briefing with congressional staff and reporters.
The APA is also seeking to make permanent certain waivers granted by the Centers for Medicare & Medicaid Services on April 30, including elimination of geographic restrictions on behavioral health and allowing patients be seen at home, said Dr. Geller.
The APA also is asking for the elimination of the rule that requires clinicians to have an initial face-to-face meeting with patients before they can prescribe controlled substances, Dr. Geller said. The Drug Enforcement Administration waived that requirement, known as the Ryan Haight Act, on March 17 for the duration of the national emergency.
Telemedicine has supporters on both sides of the aisle in Congress, including Rep. Paul Tonko (D-N.Y.) who said at the APA briefing he would fight to make the waivers permanent.
“The expanded use of telehealth has enormous potential during normal times as well, especially in behavioral health,” said Mr. Tonko. “I am pushing fiercely for these current flexibilities to be extended for a reasonable time after the public health emergency so that we can have time to evaluate which should be made permanent,” he said.
Dr. Geller, other clinicians, and advocates in the briefing praised CMS for facilitating telepsychiatry for Medicare. That follows in the footsteps of most private insurers, who have also relaxed requirements into the summer, according to the Medical Group Management Association.
Game changer
The Medicare waivers “have dramatically changed the entire scene for someone like myself as a clinician to allow me to see my patients in a much easier way,” said Peter Yellowlees, MBBS, MD, chief wellness officer, University of California Davis Health. Within 2 weeks in March, the health system converted almost all of its regular outpatient visits to telemedicine, he said.
Dr. Yellowlees added government still needs to address, what he called, outdated HIPAA regulations that ban certain technologies.
“It makes no sense that I can talk to someone on an iPhone, but the moment I talk to them on FaceTime, it’s illegal,” said Dr. Yellowlees, a former president of the American Telemedicine Association.
Dr. Geller said that “psychiatric care provided by telehealth is as effective as in-person psychiatric services,” adding that “some patients prefer telepsychiatry because of its convenience and as a means of reducing stigma associated with seeking help for mental health.”
Shabana Khan, MD, a child psychiatrist and director of telepsychiatry at New York University Langone Health, said audio and video conferencing are helping address a shortage and maldistribution of child and adolescent psychiatrists.
Americans’ mental health is suffering during the pandemic. The U.S. Census Bureau recently released data showing that half of those surveyed reported depressed mood and that one-third are reporting anxiety, depression, or both, as reported by the Washington Post.
“At this very time that anxiety, depression, substance use, and other mental health problems are rising, our nation’s already strained mental health system is really being pushed to the brink,” said Jodi Kwarciany, manager for mental health policy for the National Alliance on Mental Illness, during the briefing.
Telemedicine can help “by connecting people to providers at the time and the place and using the technology that works best for them,” she said, adding that NAMI would press policymakers to address barriers to access.
The clinicians on the briefing said they’ve observed that some patients are more comfortable with video or audio interactions than with in-person visits.
Increased access to care
Telepsychiatry seems to be convincing some to reconsider therapy, since they can do it at home, said Dr. Yellowlees. he said.
For instance, he said, he has been able to consult by phone and video with several patients who receive care through the Indian Health Service who had not be able to get into the physical clinic.
Dr. Yellowlees said video sessions also may encourage patients to be more, not less, talkative. “Video is actually counterintuitively a very intimate experience,” he said, in part because of the perceived distance and people’s tendency to be less inhibited on technology platforms.“It’s less embarrassing,” he said. “If you’ve got really dramatic, difficult, traumatic things to talk about, it’s slightly easier to talk to someone who’s slightly further apart from you on video,” said Dr. Yellowlees.
“Individuals who have a significant amount of anxiety may actually feel more comfortable with the distance that this technology affords,” agreed Dr. Khan. She said telemedicine had made sessions more comfortable for some of her patients with autism spectrum disorder.
Dr. Geller said audio and video have been important to his practice during the pandemic. One of his patients never leaves the house and does not use computers. “He spends his time sequestered at home listening to records on his record player,” said Dr. Geller. But he’s been amenable to phone sessions. “What I’ve found with him, and I’ve found with several other patients, is that they actually talk more easily when they’re not face to face,” he said.
Far fewer no-shows
Another plus for his New England–based practice during the last few months: patients have not been anxious about missing sessions because of the weather. The clinicians all noted that telepsychiatry seemed to reduce missed visits.
Dr. Yellowlees said that no-show rates had decreased by half at UC Davis. “That means no significant loss of income,” during the pandemic, he said.
“The no-show rate is incredibly low, particularly because when you call the patients and they don’t remember they had an appointment, you have the appointment anyway, most of the time,” said Dr. Geller.
For Dr. Khan, being able to conduct audio and video sessions during the pandemic has meant keeping up continuity of care.
As a result of the pandemic, many college students in New York City had to go home – often to another state. The waivers granted by New York’s Medicaid program and other insurers have allowed Dr. Khan to continue care for these patients.
The NYU clinic also operates day programs in rural areas 5 hours from the city. Dr. Khan recently evaluated a 12-year-old girl with significant anxiety and low mood, both of which had worsened.
“She would not have been able to access care otherwise,” said Dr. Khan. And for rural patients who do not have access to broadband or smartphones, audio visits “have been immensely helpful,” she said.
Dr. Khan, Dr. Geller, and Dr. Yellowlees have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The American Psychiatric Association (APA) is calling on Congress to permanently lift restrictions that have allowed unfettered delivery of telehealth services during the COVID-19 pandemic, which experts say has been a boon to patients and physicians alike.
“We ask Congress to extend the telehealth waiver authority under COVID-19 beyond the emergency and to study its impact while doing so,” said APA President Jeffrey Geller, MD, in a May 27 video briefing with congressional staff and reporters.
The APA is also seeking to make permanent certain waivers granted by the Centers for Medicare & Medicaid Services on April 30, including elimination of geographic restrictions on behavioral health and allowing patients be seen at home, said Dr. Geller.
The APA also is asking for the elimination of the rule that requires clinicians to have an initial face-to-face meeting with patients before they can prescribe controlled substances, Dr. Geller said. The Drug Enforcement Administration waived that requirement, known as the Ryan Haight Act, on March 17 for the duration of the national emergency.
Telemedicine has supporters on both sides of the aisle in Congress, including Rep. Paul Tonko (D-N.Y.) who said at the APA briefing he would fight to make the waivers permanent.
“The expanded use of telehealth has enormous potential during normal times as well, especially in behavioral health,” said Mr. Tonko. “I am pushing fiercely for these current flexibilities to be extended for a reasonable time after the public health emergency so that we can have time to evaluate which should be made permanent,” he said.
Dr. Geller, other clinicians, and advocates in the briefing praised CMS for facilitating telepsychiatry for Medicare. That follows in the footsteps of most private insurers, who have also relaxed requirements into the summer, according to the Medical Group Management Association.
Game changer
The Medicare waivers “have dramatically changed the entire scene for someone like myself as a clinician to allow me to see my patients in a much easier way,” said Peter Yellowlees, MBBS, MD, chief wellness officer, University of California Davis Health. Within 2 weeks in March, the health system converted almost all of its regular outpatient visits to telemedicine, he said.
Dr. Yellowlees added government still needs to address, what he called, outdated HIPAA regulations that ban certain technologies.
“It makes no sense that I can talk to someone on an iPhone, but the moment I talk to them on FaceTime, it’s illegal,” said Dr. Yellowlees, a former president of the American Telemedicine Association.
Dr. Geller said that “psychiatric care provided by telehealth is as effective as in-person psychiatric services,” adding that “some patients prefer telepsychiatry because of its convenience and as a means of reducing stigma associated with seeking help for mental health.”
Shabana Khan, MD, a child psychiatrist and director of telepsychiatry at New York University Langone Health, said audio and video conferencing are helping address a shortage and maldistribution of child and adolescent psychiatrists.
Americans’ mental health is suffering during the pandemic. The U.S. Census Bureau recently released data showing that half of those surveyed reported depressed mood and that one-third are reporting anxiety, depression, or both, as reported by the Washington Post.
“At this very time that anxiety, depression, substance use, and other mental health problems are rising, our nation’s already strained mental health system is really being pushed to the brink,” said Jodi Kwarciany, manager for mental health policy for the National Alliance on Mental Illness, during the briefing.
Telemedicine can help “by connecting people to providers at the time and the place and using the technology that works best for them,” she said, adding that NAMI would press policymakers to address barriers to access.
The clinicians on the briefing said they’ve observed that some patients are more comfortable with video or audio interactions than with in-person visits.
Increased access to care
Telepsychiatry seems to be convincing some to reconsider therapy, since they can do it at home, said Dr. Yellowlees. he said.
For instance, he said, he has been able to consult by phone and video with several patients who receive care through the Indian Health Service who had not be able to get into the physical clinic.
Dr. Yellowlees said video sessions also may encourage patients to be more, not less, talkative. “Video is actually counterintuitively a very intimate experience,” he said, in part because of the perceived distance and people’s tendency to be less inhibited on technology platforms.“It’s less embarrassing,” he said. “If you’ve got really dramatic, difficult, traumatic things to talk about, it’s slightly easier to talk to someone who’s slightly further apart from you on video,” said Dr. Yellowlees.
“Individuals who have a significant amount of anxiety may actually feel more comfortable with the distance that this technology affords,” agreed Dr. Khan. She said telemedicine had made sessions more comfortable for some of her patients with autism spectrum disorder.
Dr. Geller said audio and video have been important to his practice during the pandemic. One of his patients never leaves the house and does not use computers. “He spends his time sequestered at home listening to records on his record player,” said Dr. Geller. But he’s been amenable to phone sessions. “What I’ve found with him, and I’ve found with several other patients, is that they actually talk more easily when they’re not face to face,” he said.
Far fewer no-shows
Another plus for his New England–based practice during the last few months: patients have not been anxious about missing sessions because of the weather. The clinicians all noted that telepsychiatry seemed to reduce missed visits.
Dr. Yellowlees said that no-show rates had decreased by half at UC Davis. “That means no significant loss of income,” during the pandemic, he said.
“The no-show rate is incredibly low, particularly because when you call the patients and they don’t remember they had an appointment, you have the appointment anyway, most of the time,” said Dr. Geller.
For Dr. Khan, being able to conduct audio and video sessions during the pandemic has meant keeping up continuity of care.
As a result of the pandemic, many college students in New York City had to go home – often to another state. The waivers granted by New York’s Medicaid program and other insurers have allowed Dr. Khan to continue care for these patients.
The NYU clinic also operates day programs in rural areas 5 hours from the city. Dr. Khan recently evaluated a 12-year-old girl with significant anxiety and low mood, both of which had worsened.
“She would not have been able to access care otherwise,” said Dr. Khan. And for rural patients who do not have access to broadband or smartphones, audio visits “have been immensely helpful,” she said.
Dr. Khan, Dr. Geller, and Dr. Yellowlees have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The American Psychiatric Association (APA) is calling on Congress to permanently lift restrictions that have allowed unfettered delivery of telehealth services during the COVID-19 pandemic, which experts say has been a boon to patients and physicians alike.
“We ask Congress to extend the telehealth waiver authority under COVID-19 beyond the emergency and to study its impact while doing so,” said APA President Jeffrey Geller, MD, in a May 27 video briefing with congressional staff and reporters.
The APA is also seeking to make permanent certain waivers granted by the Centers for Medicare & Medicaid Services on April 30, including elimination of geographic restrictions on behavioral health and allowing patients be seen at home, said Dr. Geller.
The APA also is asking for the elimination of the rule that requires clinicians to have an initial face-to-face meeting with patients before they can prescribe controlled substances, Dr. Geller said. The Drug Enforcement Administration waived that requirement, known as the Ryan Haight Act, on March 17 for the duration of the national emergency.
Telemedicine has supporters on both sides of the aisle in Congress, including Rep. Paul Tonko (D-N.Y.) who said at the APA briefing he would fight to make the waivers permanent.
“The expanded use of telehealth has enormous potential during normal times as well, especially in behavioral health,” said Mr. Tonko. “I am pushing fiercely for these current flexibilities to be extended for a reasonable time after the public health emergency so that we can have time to evaluate which should be made permanent,” he said.
Dr. Geller, other clinicians, and advocates in the briefing praised CMS for facilitating telepsychiatry for Medicare. That follows in the footsteps of most private insurers, who have also relaxed requirements into the summer, according to the Medical Group Management Association.
Game changer
The Medicare waivers “have dramatically changed the entire scene for someone like myself as a clinician to allow me to see my patients in a much easier way,” said Peter Yellowlees, MBBS, MD, chief wellness officer, University of California Davis Health. Within 2 weeks in March, the health system converted almost all of its regular outpatient visits to telemedicine, he said.
Dr. Yellowlees added government still needs to address, what he called, outdated HIPAA regulations that ban certain technologies.
“It makes no sense that I can talk to someone on an iPhone, but the moment I talk to them on FaceTime, it’s illegal,” said Dr. Yellowlees, a former president of the American Telemedicine Association.
Dr. Geller said that “psychiatric care provided by telehealth is as effective as in-person psychiatric services,” adding that “some patients prefer telepsychiatry because of its convenience and as a means of reducing stigma associated with seeking help for mental health.”
Shabana Khan, MD, a child psychiatrist and director of telepsychiatry at New York University Langone Health, said audio and video conferencing are helping address a shortage and maldistribution of child and adolescent psychiatrists.
Americans’ mental health is suffering during the pandemic. The U.S. Census Bureau recently released data showing that half of those surveyed reported depressed mood and that one-third are reporting anxiety, depression, or both, as reported by the Washington Post.
“At this very time that anxiety, depression, substance use, and other mental health problems are rising, our nation’s already strained mental health system is really being pushed to the brink,” said Jodi Kwarciany, manager for mental health policy for the National Alliance on Mental Illness, during the briefing.
Telemedicine can help “by connecting people to providers at the time and the place and using the technology that works best for them,” she said, adding that NAMI would press policymakers to address barriers to access.
The clinicians on the briefing said they’ve observed that some patients are more comfortable with video or audio interactions than with in-person visits.
Increased access to care
Telepsychiatry seems to be convincing some to reconsider therapy, since they can do it at home, said Dr. Yellowlees. he said.
For instance, he said, he has been able to consult by phone and video with several patients who receive care through the Indian Health Service who had not be able to get into the physical clinic.
Dr. Yellowlees said video sessions also may encourage patients to be more, not less, talkative. “Video is actually counterintuitively a very intimate experience,” he said, in part because of the perceived distance and people’s tendency to be less inhibited on technology platforms.“It’s less embarrassing,” he said. “If you’ve got really dramatic, difficult, traumatic things to talk about, it’s slightly easier to talk to someone who’s slightly further apart from you on video,” said Dr. Yellowlees.
“Individuals who have a significant amount of anxiety may actually feel more comfortable with the distance that this technology affords,” agreed Dr. Khan. She said telemedicine had made sessions more comfortable for some of her patients with autism spectrum disorder.
Dr. Geller said audio and video have been important to his practice during the pandemic. One of his patients never leaves the house and does not use computers. “He spends his time sequestered at home listening to records on his record player,” said Dr. Geller. But he’s been amenable to phone sessions. “What I’ve found with him, and I’ve found with several other patients, is that they actually talk more easily when they’re not face to face,” he said.
Far fewer no-shows
Another plus for his New England–based practice during the last few months: patients have not been anxious about missing sessions because of the weather. The clinicians all noted that telepsychiatry seemed to reduce missed visits.
Dr. Yellowlees said that no-show rates had decreased by half at UC Davis. “That means no significant loss of income,” during the pandemic, he said.
“The no-show rate is incredibly low, particularly because when you call the patients and they don’t remember they had an appointment, you have the appointment anyway, most of the time,” said Dr. Geller.
For Dr. Khan, being able to conduct audio and video sessions during the pandemic has meant keeping up continuity of care.
As a result of the pandemic, many college students in New York City had to go home – often to another state. The waivers granted by New York’s Medicaid program and other insurers have allowed Dr. Khan to continue care for these patients.
The NYU clinic also operates day programs in rural areas 5 hours from the city. Dr. Khan recently evaluated a 12-year-old girl with significant anxiety and low mood, both of which had worsened.
“She would not have been able to access care otherwise,” said Dr. Khan. And for rural patients who do not have access to broadband or smartphones, audio visits “have been immensely helpful,” she said.
Dr. Khan, Dr. Geller, and Dr. Yellowlees have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
‘Loss-frame’ approach makes psoriasis patients more agreeable to treatment
, held virtually.
“We typically explain to patients the benefits of treatment,” Ari A. Kassardjian, BS, of the University of Southern California, Los Angeles, said in his presentation. “However, explaining to them the harmful effects on their skin and joint diseases, such as exacerbation of psoriasis and/or psoriatic arthritis, could offer some patients a new perspective that may influence their treatment preferences; and ultimately, better communication may lead to better medication adherence in patients.”
In the study he presented, explaining to patients possible outcomes without treatment was more effective in getting them to agree to treatment than was messaging that focused on the positive effects of a therapy (reducing disease severity and pain, and improved health).
He noted that the impact of framing choices in terms of gain or loss on decision-making has been measured in other areas of medicine, including in patients with multiple sclerosis where medication adherence is an issue (J Health Commun. 2017 Jun;22[6]:523-31). “Gain-framed” messages focus on the benefits of taking a medication, while “loss-framed” messages highlight the potential consequences of not agreeing or adhering to treatment.
In the study, Mr. Kassardjian and coinvestigators evaluated 90 patients with psoriasis who were randomized to receive a gain-framed or loss-framed message about a hypothetical new biologic injectable medication for psoriasis and psoriatic arthritis (PsA). More than half were male (64.4%), white (53.3%), and non-Hispanic or Latino (55.6%); and about one-fourth of the participants (27.8%) also had psoriatic arthritis (PsA).
The gain-framed message emphasized “the chance to reduce psoriasis severity, reduce joint pain, and improve how you feel overall,” while the loss-framed message described the downsides of not taking medication – missing out “on the chance to improve your skin, your joints, and your overall health,” with the possibility that psoriasis may get worse, “with worsening pain in your joints from psoriatic arthritis,” and feeling “worse overall.” Both messages included the side effects of the theoretical injectable, a small risk of injection-site pain and skin infections. After receiving the message, participants ranked their likelihood of taking the medication on an 11-point Likert scale, with a score of 0 indicating that they would “definitely” not use the medication and a score of 10 indicating that they would “definitely” use the medication.
Scores among those who received the loss-framed message were a mean of 8.84, compared with 7.11 among patients who received the gain-framed message (between-group difference; 1.73; P less than .0001). When comparing patients with and without PsA, the between-group difference was 1.90 for patients with PsA (P less than .0001) and 1.08 for patients who did not have PsA (P = .002). Comparing the responses of those with PsA and those without PsA, the between-group difference was 1.08 (P = .03). While PsA and non-PsA patients favored the loss-framed messages, “regardless of the framing type, PsA patients always responded with a greater preference for the therapy,” Mr. Kassardjian said.
Gender also had an effect on responsiveness to gain-framed or loss-framed messaging. Both men and women ranked the loss-framed messaging as making them more likely to use the medication, but the between-group difference for women (2.00; P = .008) was higher than in men (1.49; P = .003). However, the total men compared with total women between-group differences were not significant.
“In clinical practice, physicians regularly weigh the benefits and risks of treatment. In order to communicate this information to patients, it is important to understand how framing these benefits and risks impacts patient preferences for therapy,” Mr. Kassardjian said. “While most available biologics are effective and have tolerable safety profiles, many psoriasis patients may be hesitant to initiate these therapies. Thus, it is important to convey the benefits and risks of these systemic agents in ways that resonate with patients.”
Mr. Kassardjian reports receiving the Dean’s Research Scholarship at the University of Southern California, funded by the Wright Foundation at the time of the study. Senior author April Armstrong, MD, disclosed serving as an investigator and/or consultant for AbbVie, BMS, Dermavant, Dermira, Eli Lilly, Janssen, Leo Pharma, Kyowa Hakko Kirin, Modernizing Medicine, Novartis, Ortho Dermatologics, Regeneron, Sanofi, Sun Pharma, and UCB.
SOURCE: Kassardjian A. SID 2020, Abstract 489.
, held virtually.
“We typically explain to patients the benefits of treatment,” Ari A. Kassardjian, BS, of the University of Southern California, Los Angeles, said in his presentation. “However, explaining to them the harmful effects on their skin and joint diseases, such as exacerbation of psoriasis and/or psoriatic arthritis, could offer some patients a new perspective that may influence their treatment preferences; and ultimately, better communication may lead to better medication adherence in patients.”
In the study he presented, explaining to patients possible outcomes without treatment was more effective in getting them to agree to treatment than was messaging that focused on the positive effects of a therapy (reducing disease severity and pain, and improved health).
He noted that the impact of framing choices in terms of gain or loss on decision-making has been measured in other areas of medicine, including in patients with multiple sclerosis where medication adherence is an issue (J Health Commun. 2017 Jun;22[6]:523-31). “Gain-framed” messages focus on the benefits of taking a medication, while “loss-framed” messages highlight the potential consequences of not agreeing or adhering to treatment.
In the study, Mr. Kassardjian and coinvestigators evaluated 90 patients with psoriasis who were randomized to receive a gain-framed or loss-framed message about a hypothetical new biologic injectable medication for psoriasis and psoriatic arthritis (PsA). More than half were male (64.4%), white (53.3%), and non-Hispanic or Latino (55.6%); and about one-fourth of the participants (27.8%) also had psoriatic arthritis (PsA).
The gain-framed message emphasized “the chance to reduce psoriasis severity, reduce joint pain, and improve how you feel overall,” while the loss-framed message described the downsides of not taking medication – missing out “on the chance to improve your skin, your joints, and your overall health,” with the possibility that psoriasis may get worse, “with worsening pain in your joints from psoriatic arthritis,” and feeling “worse overall.” Both messages included the side effects of the theoretical injectable, a small risk of injection-site pain and skin infections. After receiving the message, participants ranked their likelihood of taking the medication on an 11-point Likert scale, with a score of 0 indicating that they would “definitely” not use the medication and a score of 10 indicating that they would “definitely” use the medication.
Scores among those who received the loss-framed message were a mean of 8.84, compared with 7.11 among patients who received the gain-framed message (between-group difference; 1.73; P less than .0001). When comparing patients with and without PsA, the between-group difference was 1.90 for patients with PsA (P less than .0001) and 1.08 for patients who did not have PsA (P = .002). Comparing the responses of those with PsA and those without PsA, the between-group difference was 1.08 (P = .03). While PsA and non-PsA patients favored the loss-framed messages, “regardless of the framing type, PsA patients always responded with a greater preference for the therapy,” Mr. Kassardjian said.
Gender also had an effect on responsiveness to gain-framed or loss-framed messaging. Both men and women ranked the loss-framed messaging as making them more likely to use the medication, but the between-group difference for women (2.00; P = .008) was higher than in men (1.49; P = .003). However, the total men compared with total women between-group differences were not significant.
“In clinical practice, physicians regularly weigh the benefits and risks of treatment. In order to communicate this information to patients, it is important to understand how framing these benefits and risks impacts patient preferences for therapy,” Mr. Kassardjian said. “While most available biologics are effective and have tolerable safety profiles, many psoriasis patients may be hesitant to initiate these therapies. Thus, it is important to convey the benefits and risks of these systemic agents in ways that resonate with patients.”
Mr. Kassardjian reports receiving the Dean’s Research Scholarship at the University of Southern California, funded by the Wright Foundation at the time of the study. Senior author April Armstrong, MD, disclosed serving as an investigator and/or consultant for AbbVie, BMS, Dermavant, Dermira, Eli Lilly, Janssen, Leo Pharma, Kyowa Hakko Kirin, Modernizing Medicine, Novartis, Ortho Dermatologics, Regeneron, Sanofi, Sun Pharma, and UCB.
SOURCE: Kassardjian A. SID 2020, Abstract 489.
, held virtually.
“We typically explain to patients the benefits of treatment,” Ari A. Kassardjian, BS, of the University of Southern California, Los Angeles, said in his presentation. “However, explaining to them the harmful effects on their skin and joint diseases, such as exacerbation of psoriasis and/or psoriatic arthritis, could offer some patients a new perspective that may influence their treatment preferences; and ultimately, better communication may lead to better medication adherence in patients.”
In the study he presented, explaining to patients possible outcomes without treatment was more effective in getting them to agree to treatment than was messaging that focused on the positive effects of a therapy (reducing disease severity and pain, and improved health).
He noted that the impact of framing choices in terms of gain or loss on decision-making has been measured in other areas of medicine, including in patients with multiple sclerosis where medication adherence is an issue (J Health Commun. 2017 Jun;22[6]:523-31). “Gain-framed” messages focus on the benefits of taking a medication, while “loss-framed” messages highlight the potential consequences of not agreeing or adhering to treatment.
In the study, Mr. Kassardjian and coinvestigators evaluated 90 patients with psoriasis who were randomized to receive a gain-framed or loss-framed message about a hypothetical new biologic injectable medication for psoriasis and psoriatic arthritis (PsA). More than half were male (64.4%), white (53.3%), and non-Hispanic or Latino (55.6%); and about one-fourth of the participants (27.8%) also had psoriatic arthritis (PsA).
The gain-framed message emphasized “the chance to reduce psoriasis severity, reduce joint pain, and improve how you feel overall,” while the loss-framed message described the downsides of not taking medication – missing out “on the chance to improve your skin, your joints, and your overall health,” with the possibility that psoriasis may get worse, “with worsening pain in your joints from psoriatic arthritis,” and feeling “worse overall.” Both messages included the side effects of the theoretical injectable, a small risk of injection-site pain and skin infections. After receiving the message, participants ranked their likelihood of taking the medication on an 11-point Likert scale, with a score of 0 indicating that they would “definitely” not use the medication and a score of 10 indicating that they would “definitely” use the medication.
Scores among those who received the loss-framed message were a mean of 8.84, compared with 7.11 among patients who received the gain-framed message (between-group difference; 1.73; P less than .0001). When comparing patients with and without PsA, the between-group difference was 1.90 for patients with PsA (P less than .0001) and 1.08 for patients who did not have PsA (P = .002). Comparing the responses of those with PsA and those without PsA, the between-group difference was 1.08 (P = .03). While PsA and non-PsA patients favored the loss-framed messages, “regardless of the framing type, PsA patients always responded with a greater preference for the therapy,” Mr. Kassardjian said.
Gender also had an effect on responsiveness to gain-framed or loss-framed messaging. Both men and women ranked the loss-framed messaging as making them more likely to use the medication, but the between-group difference for women (2.00; P = .008) was higher than in men (1.49; P = .003). However, the total men compared with total women between-group differences were not significant.
“In clinical practice, physicians regularly weigh the benefits and risks of treatment. In order to communicate this information to patients, it is important to understand how framing these benefits and risks impacts patient preferences for therapy,” Mr. Kassardjian said. “While most available biologics are effective and have tolerable safety profiles, many psoriasis patients may be hesitant to initiate these therapies. Thus, it is important to convey the benefits and risks of these systemic agents in ways that resonate with patients.”
Mr. Kassardjian reports receiving the Dean’s Research Scholarship at the University of Southern California, funded by the Wright Foundation at the time of the study. Senior author April Armstrong, MD, disclosed serving as an investigator and/or consultant for AbbVie, BMS, Dermavant, Dermira, Eli Lilly, Janssen, Leo Pharma, Kyowa Hakko Kirin, Modernizing Medicine, Novartis, Ortho Dermatologics, Regeneron, Sanofi, Sun Pharma, and UCB.
SOURCE: Kassardjian A. SID 2020, Abstract 489.
FROM SID 2020
COVID-19 may increase risk of preterm birth and cesarean delivery
Among 57 hospitalized patients with SARS-CoV-2 infection who underwent vaginal or cesarean delivery, 7 had spontaneous preterm or respiratory-indicated preterm delivery, a rate of 12%, according to a study published in Obstetrics & Gynecology. For comparison, 7% of patients had preterm delivery in 2019, researchers reported “We also noted a high cesarean delivery rate in the study population (39% vs. 27% in the same area in 2019), mainly as a result of maternal respiratory-indicated urgent delivery,” wrote Valeria M. Savasi, MD, PhD, of the University of Milan and Luigi Sacco Hospital, also in Milan, and colleagues.
Data do not indicate that pregnant women are more susceptible to severe COVID-19 infection, nor have studies suggested an increased risk of miscarriage, congenital anomalies, or early pregnancy loss in pregnant patients with COVID-19, the authors wrote. Studies have described an increased risk of preterm birth, however.
To study clinical features of maternal SARS-CoV-2 infection and potential factors associated with severe disease and iatrogenic delivery, Dr. Savasi and colleagues conducted a prospective study of 77 women with laboratory-confirmed SARS-CoV-2 infection who were admitted during pregnancy or the immediate postpartum period in 12 maternity hospitals in northern Italy between Feb. 23 and March 28, 2020.
The investigators classified patients as having severe disease if they underwent urgent delivery based on maternal respiratory function or if they were admitted to an ICU or subintensive care department. In all, 14 patients (18%) were classified as having severe disease.
“Three patients were intubated after emergency cesarean delivery performed for maternal deterioration, and one patient underwent extracorporeal membrane oxygenation,” Dr. Savasi and colleagues reported. The results are consistent with epidemiologic data in the nonpregnant population with COVID-19 disease.
Of 11 patients with severe disease who underwent urgent delivery for respiratory compromise, 6 had significant postpartum improvement in clinical conditions. No maternal deaths occurred.
“Increased BMI [body mass index] was a significant risk factor for severe disease,” Dr. Savasi and colleagues wrote. “Fever and dyspnea on admission were symptoms significantly associated with subsequent severe maternal respiratory deterioration.”
Most patients (65%) were admitted during the third trimester, and 20 patients were still pregnant at discharge.
“Nine newborns were admitted to the neonatal intensive care unit,” the authors wrote. “Interestingly, besides prematurity, fetal oxygenation and well-being at delivery were not apparently affected by the maternal acute conditions.” Three newborns with vaginal delivery and one with cesarean delivery tested positive for SARS-CoV-2. The newborns may have been infected after delivery, Dr. Savasi and colleagues added. For all newborns, rooming-in and breastfeeding were performed, and none developed respiratory symptoms.
Criteria for hospital admission and therapeutic protocols may have varied between hospitals, the authors noted. In addition, the study included 12 patients who were asymptomatic and admitted for obstetric indications. These patients were tested for SARS-CoV-2 because of contact with an infected individual. Most patients were symptomatic, however, which explains the high rate of maternal severe outcomes. Hospitals have since adopted a universal SARS-CoV-2 screening policy for hospitalized pregnant patients.
Kristina Adams Waldorf, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, commented in an interview that Savasi et al. describe one of the larger COVID-19 in pregnancy cohorts to date with rates of severe disease and delivery for respiratory compromise, which is remarkably similar to Washington state (severe disease, 18% vs. nearly 15%; delivery for respiratory compromise, 16% vs. 20%). As in Washington state, Italian women with a higher prepregnancy BMI were overrepresented in the severe disease group.
“Data are beginning to emerge that identify women who were overweight or obese prior to pregnancy as a high risk group for developing severe COVID-19. These data are similar to known associations between obesity and critical illness in pregnancy during the 2009 ‘swine flu’ (influenza A virus, H1N1) pandemic,” she said.
“This study and others indicate that the late second and third trimesters may be a time when women are more likely to be symptomatic from COVID-19. It remains unclear if women in the first trimester are protected from severe COVID-19 outcomes or have outcomes similar to nonpregnant women,” concluded Dr. Waldorf.
One study author disclosed receiving funds from Lo Li Pharma and Zambongroup. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Savasi VM et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003979.
Among 57 hospitalized patients with SARS-CoV-2 infection who underwent vaginal or cesarean delivery, 7 had spontaneous preterm or respiratory-indicated preterm delivery, a rate of 12%, according to a study published in Obstetrics & Gynecology. For comparison, 7% of patients had preterm delivery in 2019, researchers reported “We also noted a high cesarean delivery rate in the study population (39% vs. 27% in the same area in 2019), mainly as a result of maternal respiratory-indicated urgent delivery,” wrote Valeria M. Savasi, MD, PhD, of the University of Milan and Luigi Sacco Hospital, also in Milan, and colleagues.
Data do not indicate that pregnant women are more susceptible to severe COVID-19 infection, nor have studies suggested an increased risk of miscarriage, congenital anomalies, or early pregnancy loss in pregnant patients with COVID-19, the authors wrote. Studies have described an increased risk of preterm birth, however.
To study clinical features of maternal SARS-CoV-2 infection and potential factors associated with severe disease and iatrogenic delivery, Dr. Savasi and colleagues conducted a prospective study of 77 women with laboratory-confirmed SARS-CoV-2 infection who were admitted during pregnancy or the immediate postpartum period in 12 maternity hospitals in northern Italy between Feb. 23 and March 28, 2020.
The investigators classified patients as having severe disease if they underwent urgent delivery based on maternal respiratory function or if they were admitted to an ICU or subintensive care department. In all, 14 patients (18%) were classified as having severe disease.
“Three patients were intubated after emergency cesarean delivery performed for maternal deterioration, and one patient underwent extracorporeal membrane oxygenation,” Dr. Savasi and colleagues reported. The results are consistent with epidemiologic data in the nonpregnant population with COVID-19 disease.
Of 11 patients with severe disease who underwent urgent delivery for respiratory compromise, 6 had significant postpartum improvement in clinical conditions. No maternal deaths occurred.
“Increased BMI [body mass index] was a significant risk factor for severe disease,” Dr. Savasi and colleagues wrote. “Fever and dyspnea on admission were symptoms significantly associated with subsequent severe maternal respiratory deterioration.”
Most patients (65%) were admitted during the third trimester, and 20 patients were still pregnant at discharge.
“Nine newborns were admitted to the neonatal intensive care unit,” the authors wrote. “Interestingly, besides prematurity, fetal oxygenation and well-being at delivery were not apparently affected by the maternal acute conditions.” Three newborns with vaginal delivery and one with cesarean delivery tested positive for SARS-CoV-2. The newborns may have been infected after delivery, Dr. Savasi and colleagues added. For all newborns, rooming-in and breastfeeding were performed, and none developed respiratory symptoms.
Criteria for hospital admission and therapeutic protocols may have varied between hospitals, the authors noted. In addition, the study included 12 patients who were asymptomatic and admitted for obstetric indications. These patients were tested for SARS-CoV-2 because of contact with an infected individual. Most patients were symptomatic, however, which explains the high rate of maternal severe outcomes. Hospitals have since adopted a universal SARS-CoV-2 screening policy for hospitalized pregnant patients.
Kristina Adams Waldorf, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, commented in an interview that Savasi et al. describe one of the larger COVID-19 in pregnancy cohorts to date with rates of severe disease and delivery for respiratory compromise, which is remarkably similar to Washington state (severe disease, 18% vs. nearly 15%; delivery for respiratory compromise, 16% vs. 20%). As in Washington state, Italian women with a higher prepregnancy BMI were overrepresented in the severe disease group.
“Data are beginning to emerge that identify women who were overweight or obese prior to pregnancy as a high risk group for developing severe COVID-19. These data are similar to known associations between obesity and critical illness in pregnancy during the 2009 ‘swine flu’ (influenza A virus, H1N1) pandemic,” she said.
“This study and others indicate that the late second and third trimesters may be a time when women are more likely to be symptomatic from COVID-19. It remains unclear if women in the first trimester are protected from severe COVID-19 outcomes or have outcomes similar to nonpregnant women,” concluded Dr. Waldorf.
One study author disclosed receiving funds from Lo Li Pharma and Zambongroup. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Savasi VM et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003979.
Among 57 hospitalized patients with SARS-CoV-2 infection who underwent vaginal or cesarean delivery, 7 had spontaneous preterm or respiratory-indicated preterm delivery, a rate of 12%, according to a study published in Obstetrics & Gynecology. For comparison, 7% of patients had preterm delivery in 2019, researchers reported “We also noted a high cesarean delivery rate in the study population (39% vs. 27% in the same area in 2019), mainly as a result of maternal respiratory-indicated urgent delivery,” wrote Valeria M. Savasi, MD, PhD, of the University of Milan and Luigi Sacco Hospital, also in Milan, and colleagues.
Data do not indicate that pregnant women are more susceptible to severe COVID-19 infection, nor have studies suggested an increased risk of miscarriage, congenital anomalies, or early pregnancy loss in pregnant patients with COVID-19, the authors wrote. Studies have described an increased risk of preterm birth, however.
To study clinical features of maternal SARS-CoV-2 infection and potential factors associated with severe disease and iatrogenic delivery, Dr. Savasi and colleagues conducted a prospective study of 77 women with laboratory-confirmed SARS-CoV-2 infection who were admitted during pregnancy or the immediate postpartum period in 12 maternity hospitals in northern Italy between Feb. 23 and March 28, 2020.
The investigators classified patients as having severe disease if they underwent urgent delivery based on maternal respiratory function or if they were admitted to an ICU or subintensive care department. In all, 14 patients (18%) were classified as having severe disease.
“Three patients were intubated after emergency cesarean delivery performed for maternal deterioration, and one patient underwent extracorporeal membrane oxygenation,” Dr. Savasi and colleagues reported. The results are consistent with epidemiologic data in the nonpregnant population with COVID-19 disease.
Of 11 patients with severe disease who underwent urgent delivery for respiratory compromise, 6 had significant postpartum improvement in clinical conditions. No maternal deaths occurred.
“Increased BMI [body mass index] was a significant risk factor for severe disease,” Dr. Savasi and colleagues wrote. “Fever and dyspnea on admission were symptoms significantly associated with subsequent severe maternal respiratory deterioration.”
Most patients (65%) were admitted during the third trimester, and 20 patients were still pregnant at discharge.
“Nine newborns were admitted to the neonatal intensive care unit,” the authors wrote. “Interestingly, besides prematurity, fetal oxygenation and well-being at delivery were not apparently affected by the maternal acute conditions.” Three newborns with vaginal delivery and one with cesarean delivery tested positive for SARS-CoV-2. The newborns may have been infected after delivery, Dr. Savasi and colleagues added. For all newborns, rooming-in and breastfeeding were performed, and none developed respiratory symptoms.
Criteria for hospital admission and therapeutic protocols may have varied between hospitals, the authors noted. In addition, the study included 12 patients who were asymptomatic and admitted for obstetric indications. These patients were tested for SARS-CoV-2 because of contact with an infected individual. Most patients were symptomatic, however, which explains the high rate of maternal severe outcomes. Hospitals have since adopted a universal SARS-CoV-2 screening policy for hospitalized pregnant patients.
Kristina Adams Waldorf, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, commented in an interview that Savasi et al. describe one of the larger COVID-19 in pregnancy cohorts to date with rates of severe disease and delivery for respiratory compromise, which is remarkably similar to Washington state (severe disease, 18% vs. nearly 15%; delivery for respiratory compromise, 16% vs. 20%). As in Washington state, Italian women with a higher prepregnancy BMI were overrepresented in the severe disease group.
“Data are beginning to emerge that identify women who were overweight or obese prior to pregnancy as a high risk group for developing severe COVID-19. These data are similar to known associations between obesity and critical illness in pregnancy during the 2009 ‘swine flu’ (influenza A virus, H1N1) pandemic,” she said.
“This study and others indicate that the late second and third trimesters may be a time when women are more likely to be symptomatic from COVID-19. It remains unclear if women in the first trimester are protected from severe COVID-19 outcomes or have outcomes similar to nonpregnant women,” concluded Dr. Waldorf.
One study author disclosed receiving funds from Lo Li Pharma and Zambongroup. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Savasi VM et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003979.
FROM OBSTETRICS & GYNECOLOGY
Testing the limits of medical technology
On March 9 my team was given a directive by the chief medical officer of our health system. It seemed like an impossible task, involving the mobilization of people, processes, and technology at a scale and speed we had never before achieved. It turned out getting this done was impossible. In spite of our best efforts, we failed to meet the deadline – it actually took us 3 days. Still, by March 12, we had opened the doors on the first community testing site in our area and gained the attention of local and national news outlets for our accomplishment.
Now more than 2 months later, I’m quite proud of what our team was able to achieve for the health system, but I’m still quite frustrated at the state of COVID-19 testing nationwide – there’s simply not enough available, and there is tremendous variability in the reliability of the tests. In this column, we’d like to highlight some of the challenges we’ve faced and reflect on how the shortcomings of modern technology have once again proven that medicine is both a science and an art.
Our dangerous lack of preparation
Prior to the coronavirus pandemic, I had never considered surgical masks, face shields, and nasal swabs to be critical components of medical technology. My opinion quickly changed after opening our drive-through COVID-19 site. I now have a much greater appreciation for the importance of personal protective equipment and basic testing supplies.
I was shocked by how difficult obtaining it has been during the past few months. It seems that no one anticipated the possibility of a pandemic on this grand a scale, so stockpiles of equipment were depleted quickly and couldn’t be replenished. Also, most manufacturing occurs outside the United States, which creates additional barriers to controlling the supply chain. One need not look far to find stories of widespread price-gouging, black market racketeering, and even hijackings that have stood in the way of accessing the necessary supplies. Sadly, the lack of equipment is far from the only challenge we’ve faced. In some cases, it has been a mistrust of results that has prevented widespread testing and mitigation.
The risks of flying blind
When President Trump touted the introduction of a rapid COVID-19 test at the end of March, many people were excited. Promising positive results in as few as 5 minutes, the assay was granted an Emergency Use Authorization (EUA) by the Food and Drug Administration in order to expedite its availability in the market. According to the FDA’s website, an EUA allows “unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions.” This rapid (though untested) approval was all that many health care providers needed to hear – immediately hospitals and physicians scrambled to get their hands on the testing devices. Unfortunately, on May 14th, the FDA issued a press release that raised concerns about that same test because it seemed to be reporting a high number of false-negative results. Just as quickly as the devices had been adopted, health care providers began backing away from them in favor of other assays, and a serious truth about COVID-19 testing was revealed: In many ways, we’re flying blind.
Laboratory manufacturers have been working overtime to create assays for SARS-CoV-2 (the coronavirus that causes COVID-19) and have used different technologies for detection. The most commonly used are polymerase chain reaction (PCR) tests. In these assays, viral RNA is converted to DNA by reverse transcriptase, then amplified through the addition of primers that enable detection. PCR technology has been available for years and is a reliable method for identifying DNA and RNA, but the required heating and cooling process takes time and results can take several hours to return. To address this and expedite testing, other methods of detection have been tried, such as the loop-mediated isothermal amplification (LAMP) technique employed by the rapid assay mentioned above. Regardless of methodology, all laboratory tests have one thing in common: None of them is perfect.
Every assay has a different level of reliability. When screening for a disease such as COVID-19, we are particularly interested in a test’s sensitivity (that is, it’s ability to detect disease); we’d love such a screening test to be 100% sensitive and thereby not miss a single case. In truth, no test’s sensitivity is 100%, and in this particular case even the best assays only score around 98%. This means that out of every 100 patients with COVID-19 who are evaluated, two might test negative for the virus. In a pandemic this can have dire consequences, so health care providers – unable to fully trust their instruments – must employ clinical acumen and years of experience to navigate these cloudy skies. We are hopeful that additional tools will complement our current methods, but with new assays also come new questions.
Is anyone safe?
We receive regular questions from physicians about the value of antibody testing, but it’s not yet clear how best to respond. While the assays seem to be reliable, the utility of the results are still ill defined. Antibodies to SARS-CoV-2 (both IgG and IgM) appear to peak about 2-3 weeks after symptom onset, but we don’t yet know if the presence of those antibodies confers long-term immunity. Therefore, patients should not use the information to change their masking or social-distancing practices, nor should they presume that they are safe from becoming reinfected with COVID-19. While new research looks promising, there are still too many unknowns to be able to confidently reassure providers or patients of the true value of antibody testing. This underscores our final point: Medicine remains an art.
As we are regularly reminded, we’ll never fully anticipate the challenges or barriers to success, and technology will never replace the value of clinical judgment and human experience. While the situation is unsettling in many ways, we are reassured and encouraged by the role we still get to play in keeping our patients healthy in this health care crisis, and we’ll continue to do so through whatever the future holds.
Dr. Notte is a family physician and chief medical officer of Abington Lansdale (Pa.) Hospital - Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
On March 9 my team was given a directive by the chief medical officer of our health system. It seemed like an impossible task, involving the mobilization of people, processes, and technology at a scale and speed we had never before achieved. It turned out getting this done was impossible. In spite of our best efforts, we failed to meet the deadline – it actually took us 3 days. Still, by March 12, we had opened the doors on the first community testing site in our area and gained the attention of local and national news outlets for our accomplishment.
Now more than 2 months later, I’m quite proud of what our team was able to achieve for the health system, but I’m still quite frustrated at the state of COVID-19 testing nationwide – there’s simply not enough available, and there is tremendous variability in the reliability of the tests. In this column, we’d like to highlight some of the challenges we’ve faced and reflect on how the shortcomings of modern technology have once again proven that medicine is both a science and an art.
Our dangerous lack of preparation
Prior to the coronavirus pandemic, I had never considered surgical masks, face shields, and nasal swabs to be critical components of medical technology. My opinion quickly changed after opening our drive-through COVID-19 site. I now have a much greater appreciation for the importance of personal protective equipment and basic testing supplies.
I was shocked by how difficult obtaining it has been during the past few months. It seems that no one anticipated the possibility of a pandemic on this grand a scale, so stockpiles of equipment were depleted quickly and couldn’t be replenished. Also, most manufacturing occurs outside the United States, which creates additional barriers to controlling the supply chain. One need not look far to find stories of widespread price-gouging, black market racketeering, and even hijackings that have stood in the way of accessing the necessary supplies. Sadly, the lack of equipment is far from the only challenge we’ve faced. In some cases, it has been a mistrust of results that has prevented widespread testing and mitigation.
The risks of flying blind
When President Trump touted the introduction of a rapid COVID-19 test at the end of March, many people were excited. Promising positive results in as few as 5 minutes, the assay was granted an Emergency Use Authorization (EUA) by the Food and Drug Administration in order to expedite its availability in the market. According to the FDA’s website, an EUA allows “unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions.” This rapid (though untested) approval was all that many health care providers needed to hear – immediately hospitals and physicians scrambled to get their hands on the testing devices. Unfortunately, on May 14th, the FDA issued a press release that raised concerns about that same test because it seemed to be reporting a high number of false-negative results. Just as quickly as the devices had been adopted, health care providers began backing away from them in favor of other assays, and a serious truth about COVID-19 testing was revealed: In many ways, we’re flying blind.
Laboratory manufacturers have been working overtime to create assays for SARS-CoV-2 (the coronavirus that causes COVID-19) and have used different technologies for detection. The most commonly used are polymerase chain reaction (PCR) tests. In these assays, viral RNA is converted to DNA by reverse transcriptase, then amplified through the addition of primers that enable detection. PCR technology has been available for years and is a reliable method for identifying DNA and RNA, but the required heating and cooling process takes time and results can take several hours to return. To address this and expedite testing, other methods of detection have been tried, such as the loop-mediated isothermal amplification (LAMP) technique employed by the rapid assay mentioned above. Regardless of methodology, all laboratory tests have one thing in common: None of them is perfect.
Every assay has a different level of reliability. When screening for a disease such as COVID-19, we are particularly interested in a test’s sensitivity (that is, it’s ability to detect disease); we’d love such a screening test to be 100% sensitive and thereby not miss a single case. In truth, no test’s sensitivity is 100%, and in this particular case even the best assays only score around 98%. This means that out of every 100 patients with COVID-19 who are evaluated, two might test negative for the virus. In a pandemic this can have dire consequences, so health care providers – unable to fully trust their instruments – must employ clinical acumen and years of experience to navigate these cloudy skies. We are hopeful that additional tools will complement our current methods, but with new assays also come new questions.
Is anyone safe?
We receive regular questions from physicians about the value of antibody testing, but it’s not yet clear how best to respond. While the assays seem to be reliable, the utility of the results are still ill defined. Antibodies to SARS-CoV-2 (both IgG and IgM) appear to peak about 2-3 weeks after symptom onset, but we don’t yet know if the presence of those antibodies confers long-term immunity. Therefore, patients should not use the information to change their masking or social-distancing practices, nor should they presume that they are safe from becoming reinfected with COVID-19. While new research looks promising, there are still too many unknowns to be able to confidently reassure providers or patients of the true value of antibody testing. This underscores our final point: Medicine remains an art.
As we are regularly reminded, we’ll never fully anticipate the challenges or barriers to success, and technology will never replace the value of clinical judgment and human experience. While the situation is unsettling in many ways, we are reassured and encouraged by the role we still get to play in keeping our patients healthy in this health care crisis, and we’ll continue to do so through whatever the future holds.
Dr. Notte is a family physician and chief medical officer of Abington Lansdale (Pa.) Hospital - Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
On March 9 my team was given a directive by the chief medical officer of our health system. It seemed like an impossible task, involving the mobilization of people, processes, and technology at a scale and speed we had never before achieved. It turned out getting this done was impossible. In spite of our best efforts, we failed to meet the deadline – it actually took us 3 days. Still, by March 12, we had opened the doors on the first community testing site in our area and gained the attention of local and national news outlets for our accomplishment.
Now more than 2 months later, I’m quite proud of what our team was able to achieve for the health system, but I’m still quite frustrated at the state of COVID-19 testing nationwide – there’s simply not enough available, and there is tremendous variability in the reliability of the tests. In this column, we’d like to highlight some of the challenges we’ve faced and reflect on how the shortcomings of modern technology have once again proven that medicine is both a science and an art.
Our dangerous lack of preparation
Prior to the coronavirus pandemic, I had never considered surgical masks, face shields, and nasal swabs to be critical components of medical technology. My opinion quickly changed after opening our drive-through COVID-19 site. I now have a much greater appreciation for the importance of personal protective equipment and basic testing supplies.
I was shocked by how difficult obtaining it has been during the past few months. It seems that no one anticipated the possibility of a pandemic on this grand a scale, so stockpiles of equipment were depleted quickly and couldn’t be replenished. Also, most manufacturing occurs outside the United States, which creates additional barriers to controlling the supply chain. One need not look far to find stories of widespread price-gouging, black market racketeering, and even hijackings that have stood in the way of accessing the necessary supplies. Sadly, the lack of equipment is far from the only challenge we’ve faced. In some cases, it has been a mistrust of results that has prevented widespread testing and mitigation.
The risks of flying blind
When President Trump touted the introduction of a rapid COVID-19 test at the end of March, many people were excited. Promising positive results in as few as 5 minutes, the assay was granted an Emergency Use Authorization (EUA) by the Food and Drug Administration in order to expedite its availability in the market. According to the FDA’s website, an EUA allows “unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions.” This rapid (though untested) approval was all that many health care providers needed to hear – immediately hospitals and physicians scrambled to get their hands on the testing devices. Unfortunately, on May 14th, the FDA issued a press release that raised concerns about that same test because it seemed to be reporting a high number of false-negative results. Just as quickly as the devices had been adopted, health care providers began backing away from them in favor of other assays, and a serious truth about COVID-19 testing was revealed: In many ways, we’re flying blind.
Laboratory manufacturers have been working overtime to create assays for SARS-CoV-2 (the coronavirus that causes COVID-19) and have used different technologies for detection. The most commonly used are polymerase chain reaction (PCR) tests. In these assays, viral RNA is converted to DNA by reverse transcriptase, then amplified through the addition of primers that enable detection. PCR technology has been available for years and is a reliable method for identifying DNA and RNA, but the required heating and cooling process takes time and results can take several hours to return. To address this and expedite testing, other methods of detection have been tried, such as the loop-mediated isothermal amplification (LAMP) technique employed by the rapid assay mentioned above. Regardless of methodology, all laboratory tests have one thing in common: None of them is perfect.
Every assay has a different level of reliability. When screening for a disease such as COVID-19, we are particularly interested in a test’s sensitivity (that is, it’s ability to detect disease); we’d love such a screening test to be 100% sensitive and thereby not miss a single case. In truth, no test’s sensitivity is 100%, and in this particular case even the best assays only score around 98%. This means that out of every 100 patients with COVID-19 who are evaluated, two might test negative for the virus. In a pandemic this can have dire consequences, so health care providers – unable to fully trust their instruments – must employ clinical acumen and years of experience to navigate these cloudy skies. We are hopeful that additional tools will complement our current methods, but with new assays also come new questions.
Is anyone safe?
We receive regular questions from physicians about the value of antibody testing, but it’s not yet clear how best to respond. While the assays seem to be reliable, the utility of the results are still ill defined. Antibodies to SARS-CoV-2 (both IgG and IgM) appear to peak about 2-3 weeks after symptom onset, but we don’t yet know if the presence of those antibodies confers long-term immunity. Therefore, patients should not use the information to change their masking or social-distancing practices, nor should they presume that they are safe from becoming reinfected with COVID-19. While new research looks promising, there are still too many unknowns to be able to confidently reassure providers or patients of the true value of antibody testing. This underscores our final point: Medicine remains an art.
As we are regularly reminded, we’ll never fully anticipate the challenges or barriers to success, and technology will never replace the value of clinical judgment and human experience. While the situation is unsettling in many ways, we are reassured and encouraged by the role we still get to play in keeping our patients healthy in this health care crisis, and we’ll continue to do so through whatever the future holds.
Dr. Notte is a family physician and chief medical officer of Abington Lansdale (Pa.) Hospital - Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. They have no conflicts related to the content of this piece.











