User login
News and Views that Matter to Physicians
The drive to cut readmissions after bariatric surgery continues with DROP project
SAN DIEGO – John Morton, MD, started his bariatric surgery career about the same time that demand for gastric bypass and other bariatric procedures began to skyrocket. But a troubling trend emerged.
“About 10-15 years ago, bariatric surgery had a problem when it came to mortality,” Dr. Morton said at the American College of Surgeons/National Surgical Quality Improvement Program National Conference. “You can’t move forward without looking back.”
A 2005 study of early mortality among Medicare beneficiaries undergoing bariatric procedures found a 30-day mortality of 9% and a 1-year mortality of 21% (JAMA 2005 Oct. 19;294[15]:1903-8). Such data prompted Dr. Morton and other leaders in the field to push for accreditation in the field. In 2012, the ACS Bariatric Surgery Center Network program and the American Society for Metabolic and Bariatric Surgery (ASMBS) Bariatric Centers of Excellence program were extended accreditation in the joint Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP). As a result, the mortality rate among patients undergoing bariatric procedures has dropped nearly 10-fold and now stands at 1 out of 1,000, said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University. “That’s been a real success story for us,” he said. “Part of it has been the accreditation program, having the resources in place to accomplish those goals.”
Of the 802 participating centers in MBSAQIP, 647 are accredited. “One of the reasons we see such good results at accredited centers is the fact that they work as a multidisciplinary team, where you have the nutritionist, the psychologist, the internist, and the anesthesiologist working together,” said Dr. Morton, immediate past president of the American Society for Metabolic and Bariatric Surgery. “When you have that team, it allows you to marshal your resources, do appropriate risk assessment, and get those processes in place to have the very best outcomes.”
In an effort to reduce hospital readmissions among bariatric surgery patients, MBSAQIP launched a national project called Decreasing Readmissions through Opportunities Provided (DROP), which currently has 129 participating hospitals. “If you drill down on the reasons for bariatric surgery readmissions, many are preventable: dehydration, nausea, medication side effects, and patient expectations,” Dr. Morton said. “I have a formula called the Morton Formula: happiness equals reality divided by expectations. If you set expectations accordingly, you’ll get a happier patient. If my patients know they’re going to be discharged in 1 day, they can plan accordingly.”
These concepts were adopted from a study that Dr. Morton and his associates carried out at Stanford Health Care in an effort to reduce readmissions for complications within 30 days to below the national average. It involved “straightforward” strategy including improving patient education, discharge planning, and giving patients a direct phone number to call. “Anybody who has called a health center and has had to go through that phone tree knows how difficult that can be, so we provide a direct number,” he said. “The postop phone call is critical, because that’s a way to nip readmissions in the bud. We do same-day appointments so they come and see us in the clinic rather than going to the ER and getting the enormous workup. Infusion centers are our best friend, because many of these patients come in dehydrated.”
After implementing these strategies, the rate of readmission for complications at Stanford fell from 8% to 2.5%. This led to the creation of a readmission bundle for the DROP project with steps for preoperative, intraoperative, and postoperative aspects of care. For example, preoperatively, “we make sure that they have a postop appointment made [and] rather than waiting to give them a prescription when they get discharged, we make sure that they have those prescriptions earlier at the preoperative visit,” he said. “They are provided the clinic phone number and patients watch video vignettes from all members of the team: surgeon, nurse, nutritionist, pharmacist, and psychologist. Rather than the education being dependent on [the surgeon’s schedule], they can get the same dose of education and even watch these over and over again if they want to.”
Surgeons who participate in the DROP project also stratify high-risk patients by consulting with their primary care physicians and case managers to achieve optimal outcomes. They address modifiable risk factors. “Weight gain prior to bariatric surgery is not ideal, so we want to address that, and have a hemoglobin A1c of less than 10%,” Dr. Morton said. Patients receive a “HELP” card, which instructs them to contact the treating clinic if they have abdominal pain, dehydration, nausea and vomiting, diarrhea, and fatigue.
The inpatient part of the bundle includes a “clinical roadmap” with a fixed length of stay. “There are expectations every single day about what’s going to happen to their care,” Dr. Morton said. “We give them a water bottle with the logo of the hospital. It’s a reminder for them to stay hydrated. They have a nutritional consult and they go through a checklist before they get discharged.”
The postoperative component of the DROP bundle includes a phone call to the patient following discharge. “They also get an appointment with a nutritionist within a month of surgery,” he said. “We treat readmissions seriously, like a complication.”
Data from a study of 18,296 primary bariatric surgery patients gleaned from 2012 ACS-NSQIP Participant Use Data Files found a 30-day readmission rate of 5.2% (Am J Surg 2016 Jul;212[1]:76-80). Compared with the patients’ counterparts who did not require readmission within 30 days, risk factors for those who did included body mass index greater than 50 kg/m2 (30.2% vs. 24.6%, respectively; P = .001); longer operative time (132 vs. 115 minutes; P = .001); length of stay greater than 4 days (9.57% vs. 3.36%; P = .001); surgical site infection (15.5% vs. 1.15%; P less than .001); urinary tract infection (3.15% vs. .65%; P less than .001), and deep vein thrombosis (3.58% vs. .13%; P less than .001). Common reasons for readmissions were GI-related (45%), dietary (33.5%), and bleeding (6.57%). Dr. Morton went on to report preliminary findings from 19,648 cases included in the DROP project, which began collecting data in March 2015 and has a yearlong goal of reducing national admission rates by 20%. The preintervention readmission rate was 4.79%. By the end of October 2015 the readmission rate had dropped to 4.30%. “One of the things we realized is that the hospitals with the higher readmission rates were the ones who had the greatest improvement,” Dr. Morton said. “They went from about 8% down to about 5.51%. We anticipate that for each quarter that we do this, we’ll continue to see improvement.”
Individual center results were made available in late January 2016 and reviewed with mentors. “They also received aggregated reports to see how they stacked up others as a benchmark,” Dr. Morton said.
Final results from DROP are expected to be released later in 2016.
Dr. Morton reported having no financial disclosures.
SAN DIEGO – John Morton, MD, started his bariatric surgery career about the same time that demand for gastric bypass and other bariatric procedures began to skyrocket. But a troubling trend emerged.
“About 10-15 years ago, bariatric surgery had a problem when it came to mortality,” Dr. Morton said at the American College of Surgeons/National Surgical Quality Improvement Program National Conference. “You can’t move forward without looking back.”
A 2005 study of early mortality among Medicare beneficiaries undergoing bariatric procedures found a 30-day mortality of 9% and a 1-year mortality of 21% (JAMA 2005 Oct. 19;294[15]:1903-8). Such data prompted Dr. Morton and other leaders in the field to push for accreditation in the field. In 2012, the ACS Bariatric Surgery Center Network program and the American Society for Metabolic and Bariatric Surgery (ASMBS) Bariatric Centers of Excellence program were extended accreditation in the joint Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP). As a result, the mortality rate among patients undergoing bariatric procedures has dropped nearly 10-fold and now stands at 1 out of 1,000, said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University. “That’s been a real success story for us,” he said. “Part of it has been the accreditation program, having the resources in place to accomplish those goals.”
Of the 802 participating centers in MBSAQIP, 647 are accredited. “One of the reasons we see such good results at accredited centers is the fact that they work as a multidisciplinary team, where you have the nutritionist, the psychologist, the internist, and the anesthesiologist working together,” said Dr. Morton, immediate past president of the American Society for Metabolic and Bariatric Surgery. “When you have that team, it allows you to marshal your resources, do appropriate risk assessment, and get those processes in place to have the very best outcomes.”
In an effort to reduce hospital readmissions among bariatric surgery patients, MBSAQIP launched a national project called Decreasing Readmissions through Opportunities Provided (DROP), which currently has 129 participating hospitals. “If you drill down on the reasons for bariatric surgery readmissions, many are preventable: dehydration, nausea, medication side effects, and patient expectations,” Dr. Morton said. “I have a formula called the Morton Formula: happiness equals reality divided by expectations. If you set expectations accordingly, you’ll get a happier patient. If my patients know they’re going to be discharged in 1 day, they can plan accordingly.”
These concepts were adopted from a study that Dr. Morton and his associates carried out at Stanford Health Care in an effort to reduce readmissions for complications within 30 days to below the national average. It involved “straightforward” strategy including improving patient education, discharge planning, and giving patients a direct phone number to call. “Anybody who has called a health center and has had to go through that phone tree knows how difficult that can be, so we provide a direct number,” he said. “The postop phone call is critical, because that’s a way to nip readmissions in the bud. We do same-day appointments so they come and see us in the clinic rather than going to the ER and getting the enormous workup. Infusion centers are our best friend, because many of these patients come in dehydrated.”
After implementing these strategies, the rate of readmission for complications at Stanford fell from 8% to 2.5%. This led to the creation of a readmission bundle for the DROP project with steps for preoperative, intraoperative, and postoperative aspects of care. For example, preoperatively, “we make sure that they have a postop appointment made [and] rather than waiting to give them a prescription when they get discharged, we make sure that they have those prescriptions earlier at the preoperative visit,” he said. “They are provided the clinic phone number and patients watch video vignettes from all members of the team: surgeon, nurse, nutritionist, pharmacist, and psychologist. Rather than the education being dependent on [the surgeon’s schedule], they can get the same dose of education and even watch these over and over again if they want to.”
Surgeons who participate in the DROP project also stratify high-risk patients by consulting with their primary care physicians and case managers to achieve optimal outcomes. They address modifiable risk factors. “Weight gain prior to bariatric surgery is not ideal, so we want to address that, and have a hemoglobin A1c of less than 10%,” Dr. Morton said. Patients receive a “HELP” card, which instructs them to contact the treating clinic if they have abdominal pain, dehydration, nausea and vomiting, diarrhea, and fatigue.
The inpatient part of the bundle includes a “clinical roadmap” with a fixed length of stay. “There are expectations every single day about what’s going to happen to their care,” Dr. Morton said. “We give them a water bottle with the logo of the hospital. It’s a reminder for them to stay hydrated. They have a nutritional consult and they go through a checklist before they get discharged.”
The postoperative component of the DROP bundle includes a phone call to the patient following discharge. “They also get an appointment with a nutritionist within a month of surgery,” he said. “We treat readmissions seriously, like a complication.”
Data from a study of 18,296 primary bariatric surgery patients gleaned from 2012 ACS-NSQIP Participant Use Data Files found a 30-day readmission rate of 5.2% (Am J Surg 2016 Jul;212[1]:76-80). Compared with the patients’ counterparts who did not require readmission within 30 days, risk factors for those who did included body mass index greater than 50 kg/m2 (30.2% vs. 24.6%, respectively; P = .001); longer operative time (132 vs. 115 minutes; P = .001); length of stay greater than 4 days (9.57% vs. 3.36%; P = .001); surgical site infection (15.5% vs. 1.15%; P less than .001); urinary tract infection (3.15% vs. .65%; P less than .001), and deep vein thrombosis (3.58% vs. .13%; P less than .001). Common reasons for readmissions were GI-related (45%), dietary (33.5%), and bleeding (6.57%). Dr. Morton went on to report preliminary findings from 19,648 cases included in the DROP project, which began collecting data in March 2015 and has a yearlong goal of reducing national admission rates by 20%. The preintervention readmission rate was 4.79%. By the end of October 2015 the readmission rate had dropped to 4.30%. “One of the things we realized is that the hospitals with the higher readmission rates were the ones who had the greatest improvement,” Dr. Morton said. “They went from about 8% down to about 5.51%. We anticipate that for each quarter that we do this, we’ll continue to see improvement.”
Individual center results were made available in late January 2016 and reviewed with mentors. “They also received aggregated reports to see how they stacked up others as a benchmark,” Dr. Morton said.
Final results from DROP are expected to be released later in 2016.
Dr. Morton reported having no financial disclosures.
SAN DIEGO – John Morton, MD, started his bariatric surgery career about the same time that demand for gastric bypass and other bariatric procedures began to skyrocket. But a troubling trend emerged.
“About 10-15 years ago, bariatric surgery had a problem when it came to mortality,” Dr. Morton said at the American College of Surgeons/National Surgical Quality Improvement Program National Conference. “You can’t move forward without looking back.”
A 2005 study of early mortality among Medicare beneficiaries undergoing bariatric procedures found a 30-day mortality of 9% and a 1-year mortality of 21% (JAMA 2005 Oct. 19;294[15]:1903-8). Such data prompted Dr. Morton and other leaders in the field to push for accreditation in the field. In 2012, the ACS Bariatric Surgery Center Network program and the American Society for Metabolic and Bariatric Surgery (ASMBS) Bariatric Centers of Excellence program were extended accreditation in the joint Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP). As a result, the mortality rate among patients undergoing bariatric procedures has dropped nearly 10-fold and now stands at 1 out of 1,000, said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University. “That’s been a real success story for us,” he said. “Part of it has been the accreditation program, having the resources in place to accomplish those goals.”
Of the 802 participating centers in MBSAQIP, 647 are accredited. “One of the reasons we see such good results at accredited centers is the fact that they work as a multidisciplinary team, where you have the nutritionist, the psychologist, the internist, and the anesthesiologist working together,” said Dr. Morton, immediate past president of the American Society for Metabolic and Bariatric Surgery. “When you have that team, it allows you to marshal your resources, do appropriate risk assessment, and get those processes in place to have the very best outcomes.”
In an effort to reduce hospital readmissions among bariatric surgery patients, MBSAQIP launched a national project called Decreasing Readmissions through Opportunities Provided (DROP), which currently has 129 participating hospitals. “If you drill down on the reasons for bariatric surgery readmissions, many are preventable: dehydration, nausea, medication side effects, and patient expectations,” Dr. Morton said. “I have a formula called the Morton Formula: happiness equals reality divided by expectations. If you set expectations accordingly, you’ll get a happier patient. If my patients know they’re going to be discharged in 1 day, they can plan accordingly.”
These concepts were adopted from a study that Dr. Morton and his associates carried out at Stanford Health Care in an effort to reduce readmissions for complications within 30 days to below the national average. It involved “straightforward” strategy including improving patient education, discharge planning, and giving patients a direct phone number to call. “Anybody who has called a health center and has had to go through that phone tree knows how difficult that can be, so we provide a direct number,” he said. “The postop phone call is critical, because that’s a way to nip readmissions in the bud. We do same-day appointments so they come and see us in the clinic rather than going to the ER and getting the enormous workup. Infusion centers are our best friend, because many of these patients come in dehydrated.”
After implementing these strategies, the rate of readmission for complications at Stanford fell from 8% to 2.5%. This led to the creation of a readmission bundle for the DROP project with steps for preoperative, intraoperative, and postoperative aspects of care. For example, preoperatively, “we make sure that they have a postop appointment made [and] rather than waiting to give them a prescription when they get discharged, we make sure that they have those prescriptions earlier at the preoperative visit,” he said. “They are provided the clinic phone number and patients watch video vignettes from all members of the team: surgeon, nurse, nutritionist, pharmacist, and psychologist. Rather than the education being dependent on [the surgeon’s schedule], they can get the same dose of education and even watch these over and over again if they want to.”
Surgeons who participate in the DROP project also stratify high-risk patients by consulting with their primary care physicians and case managers to achieve optimal outcomes. They address modifiable risk factors. “Weight gain prior to bariatric surgery is not ideal, so we want to address that, and have a hemoglobin A1c of less than 10%,” Dr. Morton said. Patients receive a “HELP” card, which instructs them to contact the treating clinic if they have abdominal pain, dehydration, nausea and vomiting, diarrhea, and fatigue.
The inpatient part of the bundle includes a “clinical roadmap” with a fixed length of stay. “There are expectations every single day about what’s going to happen to their care,” Dr. Morton said. “We give them a water bottle with the logo of the hospital. It’s a reminder for them to stay hydrated. They have a nutritional consult and they go through a checklist before they get discharged.”
The postoperative component of the DROP bundle includes a phone call to the patient following discharge. “They also get an appointment with a nutritionist within a month of surgery,” he said. “We treat readmissions seriously, like a complication.”
Data from a study of 18,296 primary bariatric surgery patients gleaned from 2012 ACS-NSQIP Participant Use Data Files found a 30-day readmission rate of 5.2% (Am J Surg 2016 Jul;212[1]:76-80). Compared with the patients’ counterparts who did not require readmission within 30 days, risk factors for those who did included body mass index greater than 50 kg/m2 (30.2% vs. 24.6%, respectively; P = .001); longer operative time (132 vs. 115 minutes; P = .001); length of stay greater than 4 days (9.57% vs. 3.36%; P = .001); surgical site infection (15.5% vs. 1.15%; P less than .001); urinary tract infection (3.15% vs. .65%; P less than .001), and deep vein thrombosis (3.58% vs. .13%; P less than .001). Common reasons for readmissions were GI-related (45%), dietary (33.5%), and bleeding (6.57%). Dr. Morton went on to report preliminary findings from 19,648 cases included in the DROP project, which began collecting data in March 2015 and has a yearlong goal of reducing national admission rates by 20%. The preintervention readmission rate was 4.79%. By the end of October 2015 the readmission rate had dropped to 4.30%. “One of the things we realized is that the hospitals with the higher readmission rates were the ones who had the greatest improvement,” Dr. Morton said. “They went from about 8% down to about 5.51%. We anticipate that for each quarter that we do this, we’ll continue to see improvement.”
Individual center results were made available in late January 2016 and reviewed with mentors. “They also received aggregated reports to see how they stacked up others as a benchmark,” Dr. Morton said.
Final results from DROP are expected to be released later in 2016.
Dr. Morton reported having no financial disclosures.
EXPERT ANALYSIS AT THE ACS NSQIP NATIONAL CONFERENCE
Withdrawing risperidone spikes risk for psychotic relapse in hallucinating Alzheimer’s patients
TORONTO – Hallucinations – especially auditory hallucinations – triple the risk that a risperidone-controlled Alzheimer’s patient with psychoses will relapse if the drug is withdrawn.
“I think the clinical impact we see here is that for patients with hallucinations, and particularly auditory hallucinations, antipsychotic discontinuation should be done very, very cautiously because they do have a very high risk of relapse,” Anjali Patel, DO, said at the Alzheimer’s Association International Conference 2016. “Close monitoring will be necessary and antipsychotic medications promptly reinstated if relapse occurs.”
The findings come from a responder analysis of an open-label study of risperidone (Risperdal) use in Alzheimer’s patients who express neuropsychiatric symptoms. The primary results of the multicenter Antipsychotic Discontinuation in Alzheimer’s Disease (ADAD) trial were published in 2012 (N Engl J Med. 2012;367:1497-507). The 48-week study administered open-label, flexible-dose risperidone for 16 weeks to 180 patients with Alzheimer’s dementia, with agitation and/or aggression. The patients in this study had a mean age of 79 years at baseline. Most (62%) were taking a cholinesterase inhibitor, and many took memantine (35%). Patients commonly used anxiolytics (17%) and antidepressants (24%). The mean Neuropsychiatric Inventory (NPI) score at baseline was 36. Patients were moderately impaired, with a mean Mini Mental State Exam score of 14.
At 16 weeks, patients who had not responded left the study. After 16 weeks of open-label treatment, 110 patients who had responded well continued the dosing schedule for 32 weeks, continued risperidone for 16 weeks and then went on placebo for 16 weeks, or were switched to placebo for 32 weeks. Discontinuation of risperidone was associated with a two- to four-fold increased risk of relapse over 16-32 weeks.
Dr. Patel of Columbia University, New York, presented the preplanned post-hoc analysis that examined the association between the 12 NPI symptom domains and the likelihood of relapse at week 32. In a univariate analysis, only hallucinations posted a significant association with discontinuation of risperidone. Hallucinations of any severity at baseline were present in 43 patients (39%). The relapse rates were similar among patients without baseline hallucinations (35%) and those with mild baseline hallucinations (37%). But 78% of those with severe hallucinations relapsed when risperidone was withdrawn.
Baseline hallucinations remained a strong predictor of relapse in a multivariate model as well, with a risk ratio of 2.96 for relapse among patients who had severe baseline hallucinations, compared with those with mild or no hallucinations. Age, gender, race, and nursing home placement had no significant impact on relapse rate.
Of the 17 patients with any baseline hallucinations who were switched to placebo, 13 (77%) relapsed, compared with 38% of the patients with hallucinations who continued risperidone (risk ratio, 1.98).
The risk for relapse was particularly high when the hallucinations were primarily auditory, Dr. Patel said. “In fact, visual hallucinations were not predictive of relapse.”
Among the 11 patients with severe baseline hallucinations, 10 relapsed when risperidone was withdrawn (91%; RR, 2.88), compared with 57% of patients with severe hallucinations who stayed on the drug (RR, 1.59).
The ADAD trial was funded by the National Institutes of Health and the Department of Veterans Affairs. Dr. Patel had no financial disclosures for the subanalysis.
On Twitter @alz_gal
TORONTO – Hallucinations – especially auditory hallucinations – triple the risk that a risperidone-controlled Alzheimer’s patient with psychoses will relapse if the drug is withdrawn.
“I think the clinical impact we see here is that for patients with hallucinations, and particularly auditory hallucinations, antipsychotic discontinuation should be done very, very cautiously because they do have a very high risk of relapse,” Anjali Patel, DO, said at the Alzheimer’s Association International Conference 2016. “Close monitoring will be necessary and antipsychotic medications promptly reinstated if relapse occurs.”
The findings come from a responder analysis of an open-label study of risperidone (Risperdal) use in Alzheimer’s patients who express neuropsychiatric symptoms. The primary results of the multicenter Antipsychotic Discontinuation in Alzheimer’s Disease (ADAD) trial were published in 2012 (N Engl J Med. 2012;367:1497-507). The 48-week study administered open-label, flexible-dose risperidone for 16 weeks to 180 patients with Alzheimer’s dementia, with agitation and/or aggression. The patients in this study had a mean age of 79 years at baseline. Most (62%) were taking a cholinesterase inhibitor, and many took memantine (35%). Patients commonly used anxiolytics (17%) and antidepressants (24%). The mean Neuropsychiatric Inventory (NPI) score at baseline was 36. Patients were moderately impaired, with a mean Mini Mental State Exam score of 14.
At 16 weeks, patients who had not responded left the study. After 16 weeks of open-label treatment, 110 patients who had responded well continued the dosing schedule for 32 weeks, continued risperidone for 16 weeks and then went on placebo for 16 weeks, or were switched to placebo for 32 weeks. Discontinuation of risperidone was associated with a two- to four-fold increased risk of relapse over 16-32 weeks.
Dr. Patel of Columbia University, New York, presented the preplanned post-hoc analysis that examined the association between the 12 NPI symptom domains and the likelihood of relapse at week 32. In a univariate analysis, only hallucinations posted a significant association with discontinuation of risperidone. Hallucinations of any severity at baseline were present in 43 patients (39%). The relapse rates were similar among patients without baseline hallucinations (35%) and those with mild baseline hallucinations (37%). But 78% of those with severe hallucinations relapsed when risperidone was withdrawn.
Baseline hallucinations remained a strong predictor of relapse in a multivariate model as well, with a risk ratio of 2.96 for relapse among patients who had severe baseline hallucinations, compared with those with mild or no hallucinations. Age, gender, race, and nursing home placement had no significant impact on relapse rate.
Of the 17 patients with any baseline hallucinations who were switched to placebo, 13 (77%) relapsed, compared with 38% of the patients with hallucinations who continued risperidone (risk ratio, 1.98).
The risk for relapse was particularly high when the hallucinations were primarily auditory, Dr. Patel said. “In fact, visual hallucinations were not predictive of relapse.”
Among the 11 patients with severe baseline hallucinations, 10 relapsed when risperidone was withdrawn (91%; RR, 2.88), compared with 57% of patients with severe hallucinations who stayed on the drug (RR, 1.59).
The ADAD trial was funded by the National Institutes of Health and the Department of Veterans Affairs. Dr. Patel had no financial disclosures for the subanalysis.
On Twitter @alz_gal
TORONTO – Hallucinations – especially auditory hallucinations – triple the risk that a risperidone-controlled Alzheimer’s patient with psychoses will relapse if the drug is withdrawn.
“I think the clinical impact we see here is that for patients with hallucinations, and particularly auditory hallucinations, antipsychotic discontinuation should be done very, very cautiously because they do have a very high risk of relapse,” Anjali Patel, DO, said at the Alzheimer’s Association International Conference 2016. “Close monitoring will be necessary and antipsychotic medications promptly reinstated if relapse occurs.”
The findings come from a responder analysis of an open-label study of risperidone (Risperdal) use in Alzheimer’s patients who express neuropsychiatric symptoms. The primary results of the multicenter Antipsychotic Discontinuation in Alzheimer’s Disease (ADAD) trial were published in 2012 (N Engl J Med. 2012;367:1497-507). The 48-week study administered open-label, flexible-dose risperidone for 16 weeks to 180 patients with Alzheimer’s dementia, with agitation and/or aggression. The patients in this study had a mean age of 79 years at baseline. Most (62%) were taking a cholinesterase inhibitor, and many took memantine (35%). Patients commonly used anxiolytics (17%) and antidepressants (24%). The mean Neuropsychiatric Inventory (NPI) score at baseline was 36. Patients were moderately impaired, with a mean Mini Mental State Exam score of 14.
At 16 weeks, patients who had not responded left the study. After 16 weeks of open-label treatment, 110 patients who had responded well continued the dosing schedule for 32 weeks, continued risperidone for 16 weeks and then went on placebo for 16 weeks, or were switched to placebo for 32 weeks. Discontinuation of risperidone was associated with a two- to four-fold increased risk of relapse over 16-32 weeks.
Dr. Patel of Columbia University, New York, presented the preplanned post-hoc analysis that examined the association between the 12 NPI symptom domains and the likelihood of relapse at week 32. In a univariate analysis, only hallucinations posted a significant association with discontinuation of risperidone. Hallucinations of any severity at baseline were present in 43 patients (39%). The relapse rates were similar among patients without baseline hallucinations (35%) and those with mild baseline hallucinations (37%). But 78% of those with severe hallucinations relapsed when risperidone was withdrawn.
Baseline hallucinations remained a strong predictor of relapse in a multivariate model as well, with a risk ratio of 2.96 for relapse among patients who had severe baseline hallucinations, compared with those with mild or no hallucinations. Age, gender, race, and nursing home placement had no significant impact on relapse rate.
Of the 17 patients with any baseline hallucinations who were switched to placebo, 13 (77%) relapsed, compared with 38% of the patients with hallucinations who continued risperidone (risk ratio, 1.98).
The risk for relapse was particularly high when the hallucinations were primarily auditory, Dr. Patel said. “In fact, visual hallucinations were not predictive of relapse.”
Among the 11 patients with severe baseline hallucinations, 10 relapsed when risperidone was withdrawn (91%; RR, 2.88), compared with 57% of patients with severe hallucinations who stayed on the drug (RR, 1.59).
The ADAD trial was funded by the National Institutes of Health and the Department of Veterans Affairs. Dr. Patel had no financial disclosures for the subanalysis.
On Twitter @alz_gal
AT AAIC 2016
Key clinical point: Risperidone-treated patients who reported severe hallucinations are likely to relapse if the drug is withdrawn.
Major finding: Patients with severe baseline hallucinations were almost three times as likely to relapse as those with mild or no hallucinations.
Data source: The study was a post-hoc responder analysis of the 2012 Antipsychotic Discontinuation in Alzheimer’s Disease (ADAD) trial.
Disclosures: The study was funded by the National Institutes of Health and the Department of Veterans Affairs. Dr. Patel had no financial disclosures on the substudy.
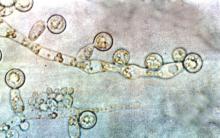
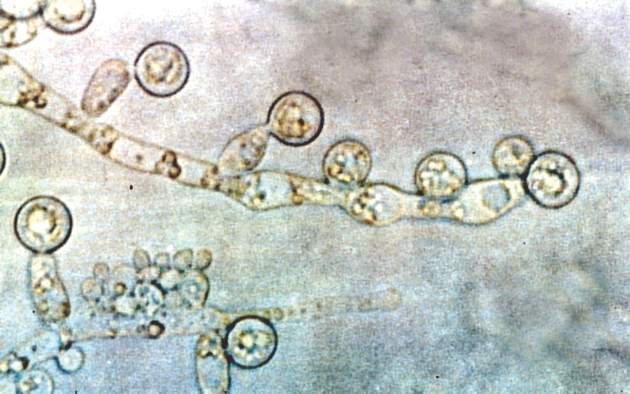
Candida colonization raises risk of Acinetobacter-based VAP
Acinetobacter baumanii was the most common cause of ventilator-associated pneumonia in ICU patients, and the risk of A. baumannii infection was significantly higher when airways were colonized with Candida species, based on data from 618 adults.
A. baumannii is a frequent cause of ventilator-associated pneumonia (VAP) in ICU patients, but its potential interactions with Candida species have not been well studied, wrote Dr. Xiaojiang Tan of Southern Medical University, Guangzhou, China, and colleagues (Med Mycol. 2016 Aug 1;54[6]:557-66. doi: 10.1093/mmy/myw009). The researchers reviewed data from 264 ICU patients on mechanical ventilation who had Candida species airway colonization and 354 who did not.
Overall, Candida was an independent risk factor for A. baumannii VAP; patients with Candida were significantly more likely than those without Candida to develop A. baumannii (23% vs. 15%). Other independent risk factors for A. baumannii VAP included the use of a central venous catheter and the use of mechanical ventilation for at least 7 days. Among patients on mechanical ventilation for at least 48 hours, Candida airway colonization occurred in 43%, and A. baumannii VAP occurred in 18%.
Candida albicans showed an especially strong association with A. baumannii VAP; it was identified in 38% of cases, compared with 21% caused by non-albicans species.
No significant differences in hospital stay or in-hospital mortality were noted between Candida-colonized and noncolonized patients, and antifungal treatment had no apparent impact on the development of A. baumannii VAP, but antifungals were associated with higher in-hospital mortality (53% vs. 39%, P = .037).
The results were limited by the retrospective nature of the study and the use of data from a single center; thus, the relationship between Candida and A. baumannii VAP may not be generalizable, the researchers noted. However, “the strong independent association between the two suggests that Candida spp. growth from the lower respiratory tract in intubated patients could be an important indicator of the risk for VAP, and even that C. albicans airway colonization may play a role in subsequent development of A. baumannii VAP,” they wrote.
The researchers reported having no relevant financial conflicts.
Acinetobacter baumanii was the most common cause of ventilator-associated pneumonia in ICU patients, and the risk of A. baumannii infection was significantly higher when airways were colonized with Candida species, based on data from 618 adults.
A. baumannii is a frequent cause of ventilator-associated pneumonia (VAP) in ICU patients, but its potential interactions with Candida species have not been well studied, wrote Dr. Xiaojiang Tan of Southern Medical University, Guangzhou, China, and colleagues (Med Mycol. 2016 Aug 1;54[6]:557-66. doi: 10.1093/mmy/myw009). The researchers reviewed data from 264 ICU patients on mechanical ventilation who had Candida species airway colonization and 354 who did not.
Overall, Candida was an independent risk factor for A. baumannii VAP; patients with Candida were significantly more likely than those without Candida to develop A. baumannii (23% vs. 15%). Other independent risk factors for A. baumannii VAP included the use of a central venous catheter and the use of mechanical ventilation for at least 7 days. Among patients on mechanical ventilation for at least 48 hours, Candida airway colonization occurred in 43%, and A. baumannii VAP occurred in 18%.
Candida albicans showed an especially strong association with A. baumannii VAP; it was identified in 38% of cases, compared with 21% caused by non-albicans species.
No significant differences in hospital stay or in-hospital mortality were noted between Candida-colonized and noncolonized patients, and antifungal treatment had no apparent impact on the development of A. baumannii VAP, but antifungals were associated with higher in-hospital mortality (53% vs. 39%, P = .037).
The results were limited by the retrospective nature of the study and the use of data from a single center; thus, the relationship between Candida and A. baumannii VAP may not be generalizable, the researchers noted. However, “the strong independent association between the two suggests that Candida spp. growth from the lower respiratory tract in intubated patients could be an important indicator of the risk for VAP, and even that C. albicans airway colonization may play a role in subsequent development of A. baumannii VAP,” they wrote.
The researchers reported having no relevant financial conflicts.
Acinetobacter baumanii was the most common cause of ventilator-associated pneumonia in ICU patients, and the risk of A. baumannii infection was significantly higher when airways were colonized with Candida species, based on data from 618 adults.
A. baumannii is a frequent cause of ventilator-associated pneumonia (VAP) in ICU patients, but its potential interactions with Candida species have not been well studied, wrote Dr. Xiaojiang Tan of Southern Medical University, Guangzhou, China, and colleagues (Med Mycol. 2016 Aug 1;54[6]:557-66. doi: 10.1093/mmy/myw009). The researchers reviewed data from 264 ICU patients on mechanical ventilation who had Candida species airway colonization and 354 who did not.
Overall, Candida was an independent risk factor for A. baumannii VAP; patients with Candida were significantly more likely than those without Candida to develop A. baumannii (23% vs. 15%). Other independent risk factors for A. baumannii VAP included the use of a central venous catheter and the use of mechanical ventilation for at least 7 days. Among patients on mechanical ventilation for at least 48 hours, Candida airway colonization occurred in 43%, and A. baumannii VAP occurred in 18%.
Candida albicans showed an especially strong association with A. baumannii VAP; it was identified in 38% of cases, compared with 21% caused by non-albicans species.
No significant differences in hospital stay or in-hospital mortality were noted between Candida-colonized and noncolonized patients, and antifungal treatment had no apparent impact on the development of A. baumannii VAP, but antifungals were associated with higher in-hospital mortality (53% vs. 39%, P = .037).
The results were limited by the retrospective nature of the study and the use of data from a single center; thus, the relationship between Candida and A. baumannii VAP may not be generalizable, the researchers noted. However, “the strong independent association between the two suggests that Candida spp. growth from the lower respiratory tract in intubated patients could be an important indicator of the risk for VAP, and even that C. albicans airway colonization may play a role in subsequent development of A. baumannii VAP,” they wrote.
The researchers reported having no relevant financial conflicts.
FROM MEDICAL MYCOLOGY
Key clinical point: Candida species colonization was an independent risk factor for ventilator-associated pneumonia caused by Acinetobacter baumannii among ICU patients.
Major finding: Hospitalized patients with Candida species airway colonization were significantly more likely to develop A. baumannii infection than were those without Candida (23% vs. 15%).
Data source: A retrospective case-control study of 618 ICU patients.
Disclosures: The researchers reported having no relevant financial conflicts.
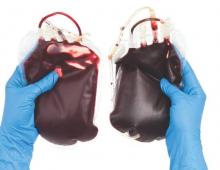
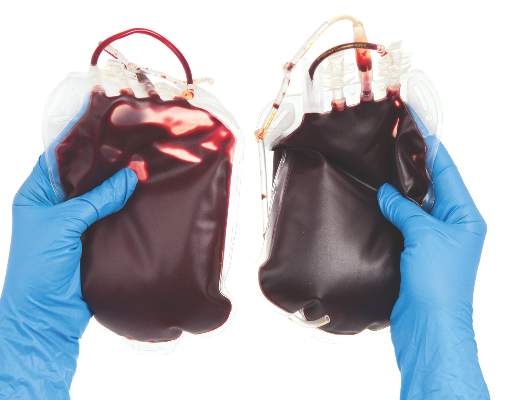
Blood management strategy leads to cost savings, less waste
SAN DIEGO – A process intended to optimize blood management led to a 30% reduction in blood use and a savings of $2 million, results from a single-center study showed.
“Blood is a limited resource and we have a responsibility as a health care provider to optimize the use of a resource that is difficult to get and only available through altruistic donations,” lead study author Barbara J. Martin, RN, said in a press release. The study was presented in a poster session at the American College of Surgeons/National Surgical Quality Improvement Program National Conference.
In an effort to evaluate how they could implement evidence-based guidelines around restrictive transfusion, Ms. Martin and her colleagues at Vanderbilt University Medical Center, Nashville, Tenn., first changed provider orders to support a single unit order and then follow-up order for more blood if necessary. The previous process was to order two units of blood, which was at times more blood than was needed. “The data on restrictive transfusion has been out for years documenting that patients have better outcomes with a more restrictive transfusion strategy,” Ms. Martin, of the Vanderbilt Center for Clinical Improvement, said in the press release. “We were looking at whether we could guide providers to treat symptomatic anemia with a single unit of blood rather than the usual two units.”
The researchers enhanced the Computerized Provider Order Entry (CPOE) system to allow blood ordering practices to be based on a specific assessment of each case rather than on a standard order of two units. As a result, red blood cell transfusions at Vanderbilt declined from 675 units per 1,000 discharges in 2011 to 432 units per 1,000 discharges in 2015, a decrease of more than 30%.
In an effort to reduce inefficiencies in the way blood is ordered, transported, and stored, Ms. Martin and her multidisciplinary team developed the following guidelines for perioperative handling:
• When more than one unit of blood is ordered, it is sent in a cooler rather than the pneumatic tube.
• Coolers are reconfigured to optimize temperature management.
• A specific staff member is tasked with “ownership” of the blood products, including returning unused product to the blood bank.
• Individual unit wastage is reported to clinical leaders for review; aggregate data are reported monthly.
After implementation of these practices, fewer than 80 units of blood were wasted at Vanderbilt in 2015, a drop from 300 in 2011. Collectively, the blood management strategies resulted in a savings of $2 million. Ms. Martin said that such guidelines can be implemented at other medical centers, but “you have to prioritize what your initiatives are. At Vanderbilt we had a lot of opportunities with blood transfusion and blood wastage and we made huge gains. Any incremental improvement would take additional resources.”
The researchers reported having no relevant disclosures.
SAN DIEGO – A process intended to optimize blood management led to a 30% reduction in blood use and a savings of $2 million, results from a single-center study showed.
“Blood is a limited resource and we have a responsibility as a health care provider to optimize the use of a resource that is difficult to get and only available through altruistic donations,” lead study author Barbara J. Martin, RN, said in a press release. The study was presented in a poster session at the American College of Surgeons/National Surgical Quality Improvement Program National Conference.
In an effort to evaluate how they could implement evidence-based guidelines around restrictive transfusion, Ms. Martin and her colleagues at Vanderbilt University Medical Center, Nashville, Tenn., first changed provider orders to support a single unit order and then follow-up order for more blood if necessary. The previous process was to order two units of blood, which was at times more blood than was needed. “The data on restrictive transfusion has been out for years documenting that patients have better outcomes with a more restrictive transfusion strategy,” Ms. Martin, of the Vanderbilt Center for Clinical Improvement, said in the press release. “We were looking at whether we could guide providers to treat symptomatic anemia with a single unit of blood rather than the usual two units.”
The researchers enhanced the Computerized Provider Order Entry (CPOE) system to allow blood ordering practices to be based on a specific assessment of each case rather than on a standard order of two units. As a result, red blood cell transfusions at Vanderbilt declined from 675 units per 1,000 discharges in 2011 to 432 units per 1,000 discharges in 2015, a decrease of more than 30%.
In an effort to reduce inefficiencies in the way blood is ordered, transported, and stored, Ms. Martin and her multidisciplinary team developed the following guidelines for perioperative handling:
• When more than one unit of blood is ordered, it is sent in a cooler rather than the pneumatic tube.
• Coolers are reconfigured to optimize temperature management.
• A specific staff member is tasked with “ownership” of the blood products, including returning unused product to the blood bank.
• Individual unit wastage is reported to clinical leaders for review; aggregate data are reported monthly.
After implementation of these practices, fewer than 80 units of blood were wasted at Vanderbilt in 2015, a drop from 300 in 2011. Collectively, the blood management strategies resulted in a savings of $2 million. Ms. Martin said that such guidelines can be implemented at other medical centers, but “you have to prioritize what your initiatives are. At Vanderbilt we had a lot of opportunities with blood transfusion and blood wastage and we made huge gains. Any incremental improvement would take additional resources.”
The researchers reported having no relevant disclosures.
SAN DIEGO – A process intended to optimize blood management led to a 30% reduction in blood use and a savings of $2 million, results from a single-center study showed.
“Blood is a limited resource and we have a responsibility as a health care provider to optimize the use of a resource that is difficult to get and only available through altruistic donations,” lead study author Barbara J. Martin, RN, said in a press release. The study was presented in a poster session at the American College of Surgeons/National Surgical Quality Improvement Program National Conference.
In an effort to evaluate how they could implement evidence-based guidelines around restrictive transfusion, Ms. Martin and her colleagues at Vanderbilt University Medical Center, Nashville, Tenn., first changed provider orders to support a single unit order and then follow-up order for more blood if necessary. The previous process was to order two units of blood, which was at times more blood than was needed. “The data on restrictive transfusion has been out for years documenting that patients have better outcomes with a more restrictive transfusion strategy,” Ms. Martin, of the Vanderbilt Center for Clinical Improvement, said in the press release. “We were looking at whether we could guide providers to treat symptomatic anemia with a single unit of blood rather than the usual two units.”
The researchers enhanced the Computerized Provider Order Entry (CPOE) system to allow blood ordering practices to be based on a specific assessment of each case rather than on a standard order of two units. As a result, red blood cell transfusions at Vanderbilt declined from 675 units per 1,000 discharges in 2011 to 432 units per 1,000 discharges in 2015, a decrease of more than 30%.
In an effort to reduce inefficiencies in the way blood is ordered, transported, and stored, Ms. Martin and her multidisciplinary team developed the following guidelines for perioperative handling:
• When more than one unit of blood is ordered, it is sent in a cooler rather than the pneumatic tube.
• Coolers are reconfigured to optimize temperature management.
• A specific staff member is tasked with “ownership” of the blood products, including returning unused product to the blood bank.
• Individual unit wastage is reported to clinical leaders for review; aggregate data are reported monthly.
After implementation of these practices, fewer than 80 units of blood were wasted at Vanderbilt in 2015, a drop from 300 in 2011. Collectively, the blood management strategies resulted in a savings of $2 million. Ms. Martin said that such guidelines can be implemented at other medical centers, but “you have to prioritize what your initiatives are. At Vanderbilt we had a lot of opportunities with blood transfusion and blood wastage and we made huge gains. Any incremental improvement would take additional resources.”
The researchers reported having no relevant disclosures.
AT THE ACS NSQIP NATIONAL CONFERENCE
Key clinical point: A multidisciplinary effort to improve the process of blood product management led to a significantly reduced use of blood products.
Major finding: Red blood cell transfusions declined from 675 units per 1,000 discharges in 2011 to 432 units per 1,000 discharges in 2015, a decrease of more than 30%.
Data source: An alteration of the Computerized Provider Order Entry (CPOE) system at Vanderbilt University Medical Center to allow blood ordering practices to be based on a specific assessment of each case rather than on a standard order of two units.
Disclosures: The researchers reported having no financial disclosures.
Hospitalization costs unaffected by Medicaid status for children with asthma
Medicaid status did not significantly affect costs for children who were hospitalized because of asthma, according to Jeffrey H. Silber, MD, and his associates.
In a study of 17,739 matched pairs of children with and without Medicaid who were hospitalized because of asthma, the median cost for Medicaid patients was $4,263; for non-Medicaid patients, it was $4,160. The median difference in cost between Medicaid and non-Medicaid patients was $84, and the mean difference in cost was $49.
Both Medicaid and non-Medicaid patients had similar lengths of stay, with a median of 1 day for both groups. Intensive care unit use was similar, with 10.1% of Medicaid patients visiting the ICU, compared with 10.6% of non-Medicaid patients.
“Our study should serve to provide potential benchmarks for use and reimbursement standards, with implications for care and payment even when children are hospitalized outside the [Pediatric Hospital Information System],” the investigators wrote.
Find the full study in Pediatrics (doi: 10.1542/peds.2016-0371).
Medicaid status did not significantly affect costs for children who were hospitalized because of asthma, according to Jeffrey H. Silber, MD, and his associates.
In a study of 17,739 matched pairs of children with and without Medicaid who were hospitalized because of asthma, the median cost for Medicaid patients was $4,263; for non-Medicaid patients, it was $4,160. The median difference in cost between Medicaid and non-Medicaid patients was $84, and the mean difference in cost was $49.
Both Medicaid and non-Medicaid patients had similar lengths of stay, with a median of 1 day for both groups. Intensive care unit use was similar, with 10.1% of Medicaid patients visiting the ICU, compared with 10.6% of non-Medicaid patients.
“Our study should serve to provide potential benchmarks for use and reimbursement standards, with implications for care and payment even when children are hospitalized outside the [Pediatric Hospital Information System],” the investigators wrote.
Find the full study in Pediatrics (doi: 10.1542/peds.2016-0371).
Medicaid status did not significantly affect costs for children who were hospitalized because of asthma, according to Jeffrey H. Silber, MD, and his associates.
In a study of 17,739 matched pairs of children with and without Medicaid who were hospitalized because of asthma, the median cost for Medicaid patients was $4,263; for non-Medicaid patients, it was $4,160. The median difference in cost between Medicaid and non-Medicaid patients was $84, and the mean difference in cost was $49.
Both Medicaid and non-Medicaid patients had similar lengths of stay, with a median of 1 day for both groups. Intensive care unit use was similar, with 10.1% of Medicaid patients visiting the ICU, compared with 10.6% of non-Medicaid patients.
“Our study should serve to provide potential benchmarks for use and reimbursement standards, with implications for care and payment even when children are hospitalized outside the [Pediatric Hospital Information System],” the investigators wrote.
Find the full study in Pediatrics (doi: 10.1542/peds.2016-0371).
FROM PEDIATRICS
CMS seeks input on future of Open Payments program
The Centers for Medicare & Medicaid Services is seeking physician input on the Open Payments program.
The agency signaled its intent to gather information in its proposed Medicare Physician Fee Schedule for 2017 and will host a conference call for that purpose on August 2.
The agency already has released a slide presentation to be used during the call that highlights the information being requested, including whether the payment categories are inclusive enough; how many years of payment data is relevant; whether reporting entities should pre-vet data before reporting to the Open Payment system; the adequacy of the definition of a covered recipient teaching hospital; whether reporting entities should be able to submit data continuously throughout the calendar year; how mergers affect reporting; clarity on reporting of ownership and investment interests; clarity on the definition of physician-owned distributors; and clarity on ways to streamline the reporting process.
Details for participating in the conference call can be found here.
The Centers for Medicare & Medicaid Services is seeking physician input on the Open Payments program.
The agency signaled its intent to gather information in its proposed Medicare Physician Fee Schedule for 2017 and will host a conference call for that purpose on August 2.
The agency already has released a slide presentation to be used during the call that highlights the information being requested, including whether the payment categories are inclusive enough; how many years of payment data is relevant; whether reporting entities should pre-vet data before reporting to the Open Payment system; the adequacy of the definition of a covered recipient teaching hospital; whether reporting entities should be able to submit data continuously throughout the calendar year; how mergers affect reporting; clarity on reporting of ownership and investment interests; clarity on the definition of physician-owned distributors; and clarity on ways to streamline the reporting process.
Details for participating in the conference call can be found here.
The Centers for Medicare & Medicaid Services is seeking physician input on the Open Payments program.
The agency signaled its intent to gather information in its proposed Medicare Physician Fee Schedule for 2017 and will host a conference call for that purpose on August 2.
The agency already has released a slide presentation to be used during the call that highlights the information being requested, including whether the payment categories are inclusive enough; how many years of payment data is relevant; whether reporting entities should pre-vet data before reporting to the Open Payment system; the adequacy of the definition of a covered recipient teaching hospital; whether reporting entities should be able to submit data continuously throughout the calendar year; how mergers affect reporting; clarity on reporting of ownership and investment interests; clarity on the definition of physician-owned distributors; and clarity on ways to streamline the reporting process.
Details for participating in the conference call can be found here.
Feds sue to block mega-mergers by health insurers
The U.S. Department of Justice (DOJ) is suing to block two mega-mergers between four of the largest health insurers in the nation, claiming the alignments will harm competition and reduce patient choice.
The DOJ and a number of state attorneys general filed legal challenges July 21 in the U.S. District Court for the District of Columbia seeking to ban Anthem’s proposed acquisition of Cigna and Aetna’s proposed acquisition of Humana. The lawsuits allege the two mergers – valued at $54 billion and $37 billion respectively – would negatively affect doctors, patients, and employers, by limiting price competition, reducing benefits, decreasing incentives to provide innovative wellness programs, and lowering quality of care.
“These mergers would restrict competition for health insurance products sold in markets across the country and would give tremendous power over the nation’s health insurance industry to just three large companies,” U.S. Attorney General Loretta E. Lynch said in a statement. “Our actions seek to preserve competition that keeps premiums down and drives insurers to collaborate with doctors and hospitals to provide better health care for all Americans.”
Anthem called the lawsuit “an unfortunate and misguided step backward for access to affordable health care.
“The DOJ’s action is based on a flawed analysis and misunderstanding of the dynamic, competitive, and highly regulated health care landscape and is inconsistent with the way that the DOJ has reviewed past health care transactions,” Anthem officials said in a statement. “Anthem has an unwavering commitment to enhancing access to affordable health care, and the benefits and efficiencies from its merger with Cigna is one way that Anthem will continue its mission of improving consumer choice, quality, and affordability.”
In a statement, Cigna officials said that company is evaluating its options given the nature of the concerns raised by the DOJ. Aetna and Humana meanwhile, vowed to vigorously defend their pending merger.
“A combined company will result in a broader choice of products, access to higher quality, and more affordable care, and a better overall experience for consumers,” according to a joint statement. “Aetna and Humana look forward to making this clear in court, where a judge will review the transaction based on its merits.”
Anthem’s proposed acquisition of Cigna would be the largest merger in the history of the health insurance industry, according to the DOJ. The companies began talks of a possible merger in early 2014 and Anthem agreed to acquire Cigna for $54 billion in 2015, according to court documents.
Meanwhile, Aetna began inquiring about a deal with Humana in March 2015, entering into a definitive agreement to acquire Humana for $37 billion later that year. The two proposed mergers have been closely watched by the DOJ and other regulatory agencies from the start.
The DOJ’s suit against Anthem and Cigna alleges the merger would substantially reduce competition for millions of patients who receive commercial health insurance coverage, from large-group employers in at least 35 metropolitan areas and from public exchanges created by the Affordable Care Act. The elimination of Cigna also threatens competition among commercial insurers for the purchase of health care services from hospitals, physicians, and other providers, the suit alleges. Eleven states and the District of Columbia joined the department’s challenge of the Cigna acquisition.
The government’s challenge against Aetna and Humana alleges the merger would greatly reduce Medicare Advantage competition in more than 350 counties in 21 states, affecting more than 1.5 million Medicare Advantage patients. The lawsuit also claims that Aetna’s purchase of Humana would substantially reduce competition to sell commercial health insurance to individuals and families on the public exchanges in 17 counties in Florida, Georgia, and Missouri. Eight states and the District of Columbia joined the department’s challenge of the Humana acquisition.
The American Medical Association voiced support for the lawsuit, condemning the proposed transactions as moves that will lessen competition and choice. In 2015, AMA issued special analyses showing that the combined impact of the proposed Anthem/Cigna and Aetna/Humana mergers and urged the federal government to block the transactions.
“The prospect of reducing five national health insurance carriers to just three is unacceptable,” AMA President Andrew W. Gurman, MD, said in a statement. “[The] action by the DOJ acknowledges the AMA’s concern that patients’ interests can be harmed when big insurers acquire rivals and develop strangleholds on local markets. Allowing commercial health insurers to become too big and exert control over the delivery of health care would be bad for patients and vitality of the nation’s health care system.”
On Twitter @legal_med
The U.S. Department of Justice (DOJ) is suing to block two mega-mergers between four of the largest health insurers in the nation, claiming the alignments will harm competition and reduce patient choice.
The DOJ and a number of state attorneys general filed legal challenges July 21 in the U.S. District Court for the District of Columbia seeking to ban Anthem’s proposed acquisition of Cigna and Aetna’s proposed acquisition of Humana. The lawsuits allege the two mergers – valued at $54 billion and $37 billion respectively – would negatively affect doctors, patients, and employers, by limiting price competition, reducing benefits, decreasing incentives to provide innovative wellness programs, and lowering quality of care.
“These mergers would restrict competition for health insurance products sold in markets across the country and would give tremendous power over the nation’s health insurance industry to just three large companies,” U.S. Attorney General Loretta E. Lynch said in a statement. “Our actions seek to preserve competition that keeps premiums down and drives insurers to collaborate with doctors and hospitals to provide better health care for all Americans.”
Anthem called the lawsuit “an unfortunate and misguided step backward for access to affordable health care.
“The DOJ’s action is based on a flawed analysis and misunderstanding of the dynamic, competitive, and highly regulated health care landscape and is inconsistent with the way that the DOJ has reviewed past health care transactions,” Anthem officials said in a statement. “Anthem has an unwavering commitment to enhancing access to affordable health care, and the benefits and efficiencies from its merger with Cigna is one way that Anthem will continue its mission of improving consumer choice, quality, and affordability.”
In a statement, Cigna officials said that company is evaluating its options given the nature of the concerns raised by the DOJ. Aetna and Humana meanwhile, vowed to vigorously defend their pending merger.
“A combined company will result in a broader choice of products, access to higher quality, and more affordable care, and a better overall experience for consumers,” according to a joint statement. “Aetna and Humana look forward to making this clear in court, where a judge will review the transaction based on its merits.”
Anthem’s proposed acquisition of Cigna would be the largest merger in the history of the health insurance industry, according to the DOJ. The companies began talks of a possible merger in early 2014 and Anthem agreed to acquire Cigna for $54 billion in 2015, according to court documents.
Meanwhile, Aetna began inquiring about a deal with Humana in March 2015, entering into a definitive agreement to acquire Humana for $37 billion later that year. The two proposed mergers have been closely watched by the DOJ and other regulatory agencies from the start.
The DOJ’s suit against Anthem and Cigna alleges the merger would substantially reduce competition for millions of patients who receive commercial health insurance coverage, from large-group employers in at least 35 metropolitan areas and from public exchanges created by the Affordable Care Act. The elimination of Cigna also threatens competition among commercial insurers for the purchase of health care services from hospitals, physicians, and other providers, the suit alleges. Eleven states and the District of Columbia joined the department’s challenge of the Cigna acquisition.
The government’s challenge against Aetna and Humana alleges the merger would greatly reduce Medicare Advantage competition in more than 350 counties in 21 states, affecting more than 1.5 million Medicare Advantage patients. The lawsuit also claims that Aetna’s purchase of Humana would substantially reduce competition to sell commercial health insurance to individuals and families on the public exchanges in 17 counties in Florida, Georgia, and Missouri. Eight states and the District of Columbia joined the department’s challenge of the Humana acquisition.
The American Medical Association voiced support for the lawsuit, condemning the proposed transactions as moves that will lessen competition and choice. In 2015, AMA issued special analyses showing that the combined impact of the proposed Anthem/Cigna and Aetna/Humana mergers and urged the federal government to block the transactions.
“The prospect of reducing five national health insurance carriers to just three is unacceptable,” AMA President Andrew W. Gurman, MD, said in a statement. “[The] action by the DOJ acknowledges the AMA’s concern that patients’ interests can be harmed when big insurers acquire rivals and develop strangleholds on local markets. Allowing commercial health insurers to become too big and exert control over the delivery of health care would be bad for patients and vitality of the nation’s health care system.”
On Twitter @legal_med
The U.S. Department of Justice (DOJ) is suing to block two mega-mergers between four of the largest health insurers in the nation, claiming the alignments will harm competition and reduce patient choice.
The DOJ and a number of state attorneys general filed legal challenges July 21 in the U.S. District Court for the District of Columbia seeking to ban Anthem’s proposed acquisition of Cigna and Aetna’s proposed acquisition of Humana. The lawsuits allege the two mergers – valued at $54 billion and $37 billion respectively – would negatively affect doctors, patients, and employers, by limiting price competition, reducing benefits, decreasing incentives to provide innovative wellness programs, and lowering quality of care.
“These mergers would restrict competition for health insurance products sold in markets across the country and would give tremendous power over the nation’s health insurance industry to just three large companies,” U.S. Attorney General Loretta E. Lynch said in a statement. “Our actions seek to preserve competition that keeps premiums down and drives insurers to collaborate with doctors and hospitals to provide better health care for all Americans.”
Anthem called the lawsuit “an unfortunate and misguided step backward for access to affordable health care.
“The DOJ’s action is based on a flawed analysis and misunderstanding of the dynamic, competitive, and highly regulated health care landscape and is inconsistent with the way that the DOJ has reviewed past health care transactions,” Anthem officials said in a statement. “Anthem has an unwavering commitment to enhancing access to affordable health care, and the benefits and efficiencies from its merger with Cigna is one way that Anthem will continue its mission of improving consumer choice, quality, and affordability.”
In a statement, Cigna officials said that company is evaluating its options given the nature of the concerns raised by the DOJ. Aetna and Humana meanwhile, vowed to vigorously defend their pending merger.
“A combined company will result in a broader choice of products, access to higher quality, and more affordable care, and a better overall experience for consumers,” according to a joint statement. “Aetna and Humana look forward to making this clear in court, where a judge will review the transaction based on its merits.”
Anthem’s proposed acquisition of Cigna would be the largest merger in the history of the health insurance industry, according to the DOJ. The companies began talks of a possible merger in early 2014 and Anthem agreed to acquire Cigna for $54 billion in 2015, according to court documents.
Meanwhile, Aetna began inquiring about a deal with Humana in March 2015, entering into a definitive agreement to acquire Humana for $37 billion later that year. The two proposed mergers have been closely watched by the DOJ and other regulatory agencies from the start.
The DOJ’s suit against Anthem and Cigna alleges the merger would substantially reduce competition for millions of patients who receive commercial health insurance coverage, from large-group employers in at least 35 metropolitan areas and from public exchanges created by the Affordable Care Act. The elimination of Cigna also threatens competition among commercial insurers for the purchase of health care services from hospitals, physicians, and other providers, the suit alleges. Eleven states and the District of Columbia joined the department’s challenge of the Cigna acquisition.
The government’s challenge against Aetna and Humana alleges the merger would greatly reduce Medicare Advantage competition in more than 350 counties in 21 states, affecting more than 1.5 million Medicare Advantage patients. The lawsuit also claims that Aetna’s purchase of Humana would substantially reduce competition to sell commercial health insurance to individuals and families on the public exchanges in 17 counties in Florida, Georgia, and Missouri. Eight states and the District of Columbia joined the department’s challenge of the Humana acquisition.
The American Medical Association voiced support for the lawsuit, condemning the proposed transactions as moves that will lessen competition and choice. In 2015, AMA issued special analyses showing that the combined impact of the proposed Anthem/Cigna and Aetna/Humana mergers and urged the federal government to block the transactions.
“The prospect of reducing five national health insurance carriers to just three is unacceptable,” AMA President Andrew W. Gurman, MD, said in a statement. “[The] action by the DOJ acknowledges the AMA’s concern that patients’ interests can be harmed when big insurers acquire rivals and develop strangleholds on local markets. Allowing commercial health insurers to become too big and exert control over the delivery of health care would be bad for patients and vitality of the nation’s health care system.”
On Twitter @legal_med
Lessons learned from merging EHR systems
WASHINGTON – As practices merge, how hard is it to merge EHRs?
Even in what might seem to be the best circumstances, it can be a huge challenge, according to Jacqueline Fincher, MD, of McDuffie Medical Associates, Thompson, Ga.
One appealing aspect of the merger of Dr. Fincher’s practice with another was that each practice used an EHR from the same vendor and each was operating with the same updates.
“This is the perfect setup,” she said at the annual meeting of the American College of Physicians. “We don’t have to go to Epic. We can stay on the same EHR. In fact, this group had gone on the same EHR back in 2006 within a month of the time that we went on; we were on the exact same version and the same everything. We were totally even. The thought among the corporate and IT staff of the new entity was that this is going to be seamless. We’re just going to renumber the accounts and everything will be just fine.”
A call to the EHR vendor, whom she did not name, revealed that the process would be anything but seamless.
“Our IT staff contacted our common EHR vendor and said we want to merge this practice with our bigger practice and the EHR company said, ‘Wow, we’ve never done that before.’ What? In this day of consolidation and integration, they had never done it before? Nor did they have a business model to do so, much less a digital plan to do so. That was pretty shocking,” she said.
Dr. Fincher noted that the EHR vendor recommended a third party vendor to handle creating an interface between the two EHRs. “Most EHR companies do not, I say, do not have a dedicated service to migrate data. It’s almost always going out to a third-party conversion service that doesn’t know you, doesn’t know your work flow, and makes everything even more difficult.”
Two and a half months – and 10 interfaces – later, the launch of the combined EHR was a disaster, Dr. Fincher said.
Even though both practices were using the exact same version of the EHR, each had very different work flows, defaults, and other nuances that meant data didn’t transfer smoothly – cheaply.
Among the surprise expenses: about $55,000 for additional hardware, network cabling and interfaces; $35,000 for additional servers; and at least $100,000 for personnel expenses related to the data migration.
Given her experience, Dr. Fincher advised her peers “to start at least 6 months in advance to map and convert the data.”
And it is vital to get input and participation from all office stakeholders – both clinical and administrative staff – regarding how the data is migrated, she said. Be sure to completely understand all work flows from both practices so that you know how the data is going to migrate.
“Understanding work flow, that is absolutely critical. Every single office has a different work flow for every type of encounter by any method. How these work flows are the same or different between your office and the new practice or your EHR and the new EHR that you’re going to, they are different. You have to understand the differences,” she added.
“We didn’t perceive that there was that much difference but when everything crashed, we discovered there were because they had different work flows, they had different defaults in place, those types of things.”
Other key questions: Which data needs to be migrated? How long should the old system remain in place? Should the data be migrated manually or digitally? How much time will the EHR merger take?
“You want to establish that structured planning time,” Dr. Fincher said. “You’ve got to carve out scheduled time with the group in order to do this. Establish the tasks that need to be accomplished, so just making a to-do list every week and who’s going to be accountable to accomplish those parts of the list” is important.
WASHINGTON – As practices merge, how hard is it to merge EHRs?
Even in what might seem to be the best circumstances, it can be a huge challenge, according to Jacqueline Fincher, MD, of McDuffie Medical Associates, Thompson, Ga.
One appealing aspect of the merger of Dr. Fincher’s practice with another was that each practice used an EHR from the same vendor and each was operating with the same updates.
“This is the perfect setup,” she said at the annual meeting of the American College of Physicians. “We don’t have to go to Epic. We can stay on the same EHR. In fact, this group had gone on the same EHR back in 2006 within a month of the time that we went on; we were on the exact same version and the same everything. We were totally even. The thought among the corporate and IT staff of the new entity was that this is going to be seamless. We’re just going to renumber the accounts and everything will be just fine.”
A call to the EHR vendor, whom she did not name, revealed that the process would be anything but seamless.
“Our IT staff contacted our common EHR vendor and said we want to merge this practice with our bigger practice and the EHR company said, ‘Wow, we’ve never done that before.’ What? In this day of consolidation and integration, they had never done it before? Nor did they have a business model to do so, much less a digital plan to do so. That was pretty shocking,” she said.
Dr. Fincher noted that the EHR vendor recommended a third party vendor to handle creating an interface between the two EHRs. “Most EHR companies do not, I say, do not have a dedicated service to migrate data. It’s almost always going out to a third-party conversion service that doesn’t know you, doesn’t know your work flow, and makes everything even more difficult.”
Two and a half months – and 10 interfaces – later, the launch of the combined EHR was a disaster, Dr. Fincher said.
Even though both practices were using the exact same version of the EHR, each had very different work flows, defaults, and other nuances that meant data didn’t transfer smoothly – cheaply.
Among the surprise expenses: about $55,000 for additional hardware, network cabling and interfaces; $35,000 for additional servers; and at least $100,000 for personnel expenses related to the data migration.
Given her experience, Dr. Fincher advised her peers “to start at least 6 months in advance to map and convert the data.”
And it is vital to get input and participation from all office stakeholders – both clinical and administrative staff – regarding how the data is migrated, she said. Be sure to completely understand all work flows from both practices so that you know how the data is going to migrate.
“Understanding work flow, that is absolutely critical. Every single office has a different work flow for every type of encounter by any method. How these work flows are the same or different between your office and the new practice or your EHR and the new EHR that you’re going to, they are different. You have to understand the differences,” she added.
“We didn’t perceive that there was that much difference but when everything crashed, we discovered there were because they had different work flows, they had different defaults in place, those types of things.”
Other key questions: Which data needs to be migrated? How long should the old system remain in place? Should the data be migrated manually or digitally? How much time will the EHR merger take?
“You want to establish that structured planning time,” Dr. Fincher said. “You’ve got to carve out scheduled time with the group in order to do this. Establish the tasks that need to be accomplished, so just making a to-do list every week and who’s going to be accountable to accomplish those parts of the list” is important.
WASHINGTON – As practices merge, how hard is it to merge EHRs?
Even in what might seem to be the best circumstances, it can be a huge challenge, according to Jacqueline Fincher, MD, of McDuffie Medical Associates, Thompson, Ga.
One appealing aspect of the merger of Dr. Fincher’s practice with another was that each practice used an EHR from the same vendor and each was operating with the same updates.
“This is the perfect setup,” she said at the annual meeting of the American College of Physicians. “We don’t have to go to Epic. We can stay on the same EHR. In fact, this group had gone on the same EHR back in 2006 within a month of the time that we went on; we were on the exact same version and the same everything. We were totally even. The thought among the corporate and IT staff of the new entity was that this is going to be seamless. We’re just going to renumber the accounts and everything will be just fine.”
A call to the EHR vendor, whom she did not name, revealed that the process would be anything but seamless.
“Our IT staff contacted our common EHR vendor and said we want to merge this practice with our bigger practice and the EHR company said, ‘Wow, we’ve never done that before.’ What? In this day of consolidation and integration, they had never done it before? Nor did they have a business model to do so, much less a digital plan to do so. That was pretty shocking,” she said.
Dr. Fincher noted that the EHR vendor recommended a third party vendor to handle creating an interface between the two EHRs. “Most EHR companies do not, I say, do not have a dedicated service to migrate data. It’s almost always going out to a third-party conversion service that doesn’t know you, doesn’t know your work flow, and makes everything even more difficult.”
Two and a half months – and 10 interfaces – later, the launch of the combined EHR was a disaster, Dr. Fincher said.
Even though both practices were using the exact same version of the EHR, each had very different work flows, defaults, and other nuances that meant data didn’t transfer smoothly – cheaply.
Among the surprise expenses: about $55,000 for additional hardware, network cabling and interfaces; $35,000 for additional servers; and at least $100,000 for personnel expenses related to the data migration.
Given her experience, Dr. Fincher advised her peers “to start at least 6 months in advance to map and convert the data.”
And it is vital to get input and participation from all office stakeholders – both clinical and administrative staff – regarding how the data is migrated, she said. Be sure to completely understand all work flows from both practices so that you know how the data is going to migrate.
“Understanding work flow, that is absolutely critical. Every single office has a different work flow for every type of encounter by any method. How these work flows are the same or different between your office and the new practice or your EHR and the new EHR that you’re going to, they are different. You have to understand the differences,” she added.
“We didn’t perceive that there was that much difference but when everything crashed, we discovered there were because they had different work flows, they had different defaults in place, those types of things.”
Other key questions: Which data needs to be migrated? How long should the old system remain in place? Should the data be migrated manually or digitally? How much time will the EHR merger take?
“You want to establish that structured planning time,” Dr. Fincher said. “You’ve got to carve out scheduled time with the group in order to do this. Establish the tasks that need to be accomplished, so just making a to-do list every week and who’s going to be accountable to accomplish those parts of the list” is important.
EXPERT ANALYSIS FROM ACP INTERNAL MEDICINE 2016
New IDSA aspergillosis guidelines endorse galactomannan for diagnosis
New aspergillosis guidelines from the Infectious Diseases Society of America recommend serum and bronchoalveolar lavage galactomannan as a marker for the diagnosis of invasive Aspergillus in adult and pediatric patients who have hematologic malignancies or have undergone hematopoietic stem cell transplants.
Serial monitoring of serum galactomannan (GM) is also useful to monitor disease progression, therapeutic response, and prognosis in hematologic malignancy and hematopoietic stem cell transplant (HSCT) patients who have elevated baseline GM (Clin Infect Dis. 2016 Jun 29. doi: 10.1093/cid/ciw326).
Serum beta-D-glucan assays also are recommended for diagnosing invasive Aspergillus (IA) in high-risk hematologic malignancy and allogeneic HSCT patients, although these tests are not very specific for the infection.
The advice illustrates the Society’s emphasis on early diagnosis in its new guidelines, which supplant the group’s 2008 guidance. There are almost 100 recommendations covering – in depth – the management of invasive, allergic, and chronic Aspergillus infections in all their manifestations. It’s a step-by-step, how-to manual for handling the problem.
“Aspergillosis mortality rates have decreased significantly in recent years, but there is still significant mortality from the infection, and we have a ways to go. We felt that early diagnosis was key, which is why it’s such an important part of these guidelines,” said lead author Thomas Patterson, MD, chief of the Division of Infectious Diseases at the University of Texas Health Science Center, San Antonio. He highlighted the most important developments in a recent interview.
“We know a lot more since 2008 about the benefits of using biomarkers like GM in bronchoalveolar lavage samples, which could be highly useful for diagnosis. However, biomarkers have not been as well validated for biologic response and are not recommended” in most cases for monitoring how well patients are doing. Also, “biomarkers are not as useful in solid organ transplants; we discuss that” in the guidelines, Dr. Patterson said.
The society came out against routine polymerase chain reaction (PCR) testing of blood samples for diagnosis. Although there has been a lot of work on the technique, the evidence isn’t strong enough yet to establish overall clinical benefit, but there is emerging evidence for the diagnostic use of PCR in conjunction with radiologic findings.
For treatment, voriconazole remains the go-to drug, but the guidelines make room for more recently approved therapies. “We now have isavuconazole, which may be better tolerated,” but it’s recommended only as an alternative to voriconazole because evidence comes mostly from a single clinical trial, he said.
Posaconazole extended-release tablets are strongly recommended as prophylaxis based on high-quality evidence from studies in neutropenic patients. Posaconazole extended-release tablets result in significantly higher antifungal blood levels than those seen with voriconazole, and “it certainly has been useful in some patients”; however, posaconazole is not approved for primary therapy in the United States, Dr. Patterson said.
A large clinical trial that tested voriconazole plus an echinocandin against voriconazole alone found that in patients diagnosed using serum galactomannan – especially those with hematologic malignancies – outcomes were better with the combination. “The panel felt combinations could be considered in some patients” but didn’t recommend them for routine use because [again,] there’s not strong evidence,” he said.
For now, it seems that higher-risk patients with hematologic malignancies and those with more widespread disease might be the ones who benefit most from combination therapy.
“We also discussed allergic and saprophytic diseases. We know that some patients with allergic bronchopulmonary aspergillosis will respond to antifungal therapy, and perhaps reduce their need for steroids, so that’s now part of the suggestions, as well,” he said.
The IDSA funded the work. Dr. Patterson receives research funding from Astellas, Merck, and Revolution Medicines, and has been an adviser to numerous drug companies.
New aspergillosis guidelines from the Infectious Diseases Society of America recommend serum and bronchoalveolar lavage galactomannan as a marker for the diagnosis of invasive Aspergillus in adult and pediatric patients who have hematologic malignancies or have undergone hematopoietic stem cell transplants.
Serial monitoring of serum galactomannan (GM) is also useful to monitor disease progression, therapeutic response, and prognosis in hematologic malignancy and hematopoietic stem cell transplant (HSCT) patients who have elevated baseline GM (Clin Infect Dis. 2016 Jun 29. doi: 10.1093/cid/ciw326).
Serum beta-D-glucan assays also are recommended for diagnosing invasive Aspergillus (IA) in high-risk hematologic malignancy and allogeneic HSCT patients, although these tests are not very specific for the infection.
The advice illustrates the Society’s emphasis on early diagnosis in its new guidelines, which supplant the group’s 2008 guidance. There are almost 100 recommendations covering – in depth – the management of invasive, allergic, and chronic Aspergillus infections in all their manifestations. It’s a step-by-step, how-to manual for handling the problem.
“Aspergillosis mortality rates have decreased significantly in recent years, but there is still significant mortality from the infection, and we have a ways to go. We felt that early diagnosis was key, which is why it’s such an important part of these guidelines,” said lead author Thomas Patterson, MD, chief of the Division of Infectious Diseases at the University of Texas Health Science Center, San Antonio. He highlighted the most important developments in a recent interview.
“We know a lot more since 2008 about the benefits of using biomarkers like GM in bronchoalveolar lavage samples, which could be highly useful for diagnosis. However, biomarkers have not been as well validated for biologic response and are not recommended” in most cases for monitoring how well patients are doing. Also, “biomarkers are not as useful in solid organ transplants; we discuss that” in the guidelines, Dr. Patterson said.
The society came out against routine polymerase chain reaction (PCR) testing of blood samples for diagnosis. Although there has been a lot of work on the technique, the evidence isn’t strong enough yet to establish overall clinical benefit, but there is emerging evidence for the diagnostic use of PCR in conjunction with radiologic findings.
For treatment, voriconazole remains the go-to drug, but the guidelines make room for more recently approved therapies. “We now have isavuconazole, which may be better tolerated,” but it’s recommended only as an alternative to voriconazole because evidence comes mostly from a single clinical trial, he said.
Posaconazole extended-release tablets are strongly recommended as prophylaxis based on high-quality evidence from studies in neutropenic patients. Posaconazole extended-release tablets result in significantly higher antifungal blood levels than those seen with voriconazole, and “it certainly has been useful in some patients”; however, posaconazole is not approved for primary therapy in the United States, Dr. Patterson said.
A large clinical trial that tested voriconazole plus an echinocandin against voriconazole alone found that in patients diagnosed using serum galactomannan – especially those with hematologic malignancies – outcomes were better with the combination. “The panel felt combinations could be considered in some patients” but didn’t recommend them for routine use because [again,] there’s not strong evidence,” he said.
For now, it seems that higher-risk patients with hematologic malignancies and those with more widespread disease might be the ones who benefit most from combination therapy.
“We also discussed allergic and saprophytic diseases. We know that some patients with allergic bronchopulmonary aspergillosis will respond to antifungal therapy, and perhaps reduce their need for steroids, so that’s now part of the suggestions, as well,” he said.
The IDSA funded the work. Dr. Patterson receives research funding from Astellas, Merck, and Revolution Medicines, and has been an adviser to numerous drug companies.
New aspergillosis guidelines from the Infectious Diseases Society of America recommend serum and bronchoalveolar lavage galactomannan as a marker for the diagnosis of invasive Aspergillus in adult and pediatric patients who have hematologic malignancies or have undergone hematopoietic stem cell transplants.
Serial monitoring of serum galactomannan (GM) is also useful to monitor disease progression, therapeutic response, and prognosis in hematologic malignancy and hematopoietic stem cell transplant (HSCT) patients who have elevated baseline GM (Clin Infect Dis. 2016 Jun 29. doi: 10.1093/cid/ciw326).
Serum beta-D-glucan assays also are recommended for diagnosing invasive Aspergillus (IA) in high-risk hematologic malignancy and allogeneic HSCT patients, although these tests are not very specific for the infection.
The advice illustrates the Society’s emphasis on early diagnosis in its new guidelines, which supplant the group’s 2008 guidance. There are almost 100 recommendations covering – in depth – the management of invasive, allergic, and chronic Aspergillus infections in all their manifestations. It’s a step-by-step, how-to manual for handling the problem.
“Aspergillosis mortality rates have decreased significantly in recent years, but there is still significant mortality from the infection, and we have a ways to go. We felt that early diagnosis was key, which is why it’s such an important part of these guidelines,” said lead author Thomas Patterson, MD, chief of the Division of Infectious Diseases at the University of Texas Health Science Center, San Antonio. He highlighted the most important developments in a recent interview.
“We know a lot more since 2008 about the benefits of using biomarkers like GM in bronchoalveolar lavage samples, which could be highly useful for diagnosis. However, biomarkers have not been as well validated for biologic response and are not recommended” in most cases for monitoring how well patients are doing. Also, “biomarkers are not as useful in solid organ transplants; we discuss that” in the guidelines, Dr. Patterson said.
The society came out against routine polymerase chain reaction (PCR) testing of blood samples for diagnosis. Although there has been a lot of work on the technique, the evidence isn’t strong enough yet to establish overall clinical benefit, but there is emerging evidence for the diagnostic use of PCR in conjunction with radiologic findings.
For treatment, voriconazole remains the go-to drug, but the guidelines make room for more recently approved therapies. “We now have isavuconazole, which may be better tolerated,” but it’s recommended only as an alternative to voriconazole because evidence comes mostly from a single clinical trial, he said.
Posaconazole extended-release tablets are strongly recommended as prophylaxis based on high-quality evidence from studies in neutropenic patients. Posaconazole extended-release tablets result in significantly higher antifungal blood levels than those seen with voriconazole, and “it certainly has been useful in some patients”; however, posaconazole is not approved for primary therapy in the United States, Dr. Patterson said.
A large clinical trial that tested voriconazole plus an echinocandin against voriconazole alone found that in patients diagnosed using serum galactomannan – especially those with hematologic malignancies – outcomes were better with the combination. “The panel felt combinations could be considered in some patients” but didn’t recommend them for routine use because [again,] there’s not strong evidence,” he said.
For now, it seems that higher-risk patients with hematologic malignancies and those with more widespread disease might be the ones who benefit most from combination therapy.
“We also discussed allergic and saprophytic diseases. We know that some patients with allergic bronchopulmonary aspergillosis will respond to antifungal therapy, and perhaps reduce their need for steroids, so that’s now part of the suggestions, as well,” he said.
The IDSA funded the work. Dr. Patterson receives research funding from Astellas, Merck, and Revolution Medicines, and has been an adviser to numerous drug companies.
FROM CLINICAL INFECTIOUS DISEASES
Supreme Court offers mixed take on false claim liability
A U.S. Supreme Court ruling that expands liability under the federal False Claims Act (FCA) could have both positive and negative implications for physicians accused of submitting false claims to the government.
Justices ruled June 16 that health care providers can be held liable under the FCA if they bill for a service, but fail to comply with underlying regulations, even if the violation is not explicit in the claim. The decision upholds use of the “implied false certification theory” in FCA cases, which provides that any submission for government payment include an implicit certification of compliance with all applicable contract requirements, laws, and regulations.
The ruling allows a lawsuit by a patient’s family to continue against Universal Health Services, a national hospital management company. The plaintiff, Julio Escobar, claims that Universal presented false claims to Medicaid by seeking payments for services provided by unlicensed, unsupervised health care providers. Although the reimbursement claims submitted to the government accurately described the services provided and cited the correct charges, the plaintiffs alleged that because the clinic’s operations violated state requirements to participate in Medicaid, Universal had also violated the FCA. Universal argued the FCA suit was invalid because a reimbursement claim cannot be false unless its details are untrue or inaccurate.
The Supreme Court ruled in favor of the plaintiffs. By using National Provider Identification numbers that corresponded to specific job titles without disclosing many violations of staff and licensing requirements, Universal’s claims constituted misrepresentations, the justices said.
The opinion includes language that is both helpful and harmful to physicians, according to health law attorneys. On the one hand, the justices supported the implied certification theory, thus expanding the scope of potential liability under the FCA in certain circumstances, said George B. Breen, a Washington-based health law attorney.
“The court’s decision is significant for health care providers and suppliers that submit claims to federally funded health care programs, including Medicare and Medicaid, because the FCA remains one of the federal government’s primary enforcement tools,” Mr. Breen said in an interview. “In the court’s view, half-truths in a claim for reimbursement from a government program … [are] just as actionable as an outright lie if the failure to disclose noncompliance with a statute, regulation, or contract term makes those representations misleading half-truths.”
However, the Supreme Court rejected the government’s notion that every omission, regulatory violation, or contract breach can result in fines and damages under the FCA. The compliance violation must be material to the government’s payment decision, the justices said. This means an alleged regulatory or contractual violation must have mattered to the agency’s payment decision to be actionable under the FCA, said David L. Douglass, a Washington-based health law attorney.
In their written opinion, the justices offered this example: If the government adds a requirement that health providers who participate in Medicaid must buy American-made staplers, and a health provider submits a claim but fails to disclose the use of foreign staplers, that provider should not be held liable under the FCA.
“An undisclosed fact is material if, for instance, no one can say with reason that the plaintiff would have signed this contract if informed of the likelihood of the undisclosed fact,” the justices wrote. “A misrepresentation cannot be deemed material merely because the government designates compliance with a particular requirement as a condition of payment. … The False Claims Act does not adopt such an extraordinarily expansive view of liability.”
This aspect of the ruling is good news for defendants and potential FCA targets, Mr. Douglass said in an interview.
“The court has restored the role of the materiality element,” he said. “The materiality element is a stringent protection against the risk that technical instances of noncompliance can become the basis for punitive liability.”
The high court also noted that if the underlying violation is known to widely occur and, nonetheless, Medicare regularly pays such claims, the violation is likely not material to payment, added William W. Horton, a Birmingham, Ala.–based health law attorney and chair of the American Bar Association Health Law Section.
“What I think this does, from the standpoint of defense of these claims, is open up new possibilities for arguing about whether a technical violation of a legal requirement, in fact, satisfies this test,” Mr. Horton said in an interview. “Whether, if CMS had known the violation occurred, would that have been material to CMS’s decision to pay the claim or not?”
But attorneys for plaintiffs and whistle-blowers are also hailing the Supreme Court opinion as positive for their clients.
Stephen M. Kohn, executive director of the National Whistleblower Center, said the Supreme Court correctly ruled on the issue.
“The legal position taken by the Chamber of Commerce and health care industry in this case was dumbfounding,” Mr. Kohn said in a statement. “Had the court agreed with the chamber and its allies – that you can bill the taxpayers for the services of a so-called doctor who was in fact unlicensed, and whose degree came from an unaccredited Internet college – then the floodgates would be opened for fraud in government contracting.”
Houston-based plaintiffs’ attorney Joel Androphy, who represents whistle-blowers, called the opinion “very favorable” for implied certification claims.
“Eliminating barriers such as the mandated condition of payment language in statutes or regulations was a proper change in direction for evaluating pleadings,” Mr. Androphy said in an interview. “If the defendant cheats, there should not be a free pass because Congress did not include magic wording in statute or regulation to support a false claims case. Materiality may be a lingering area of dispute; however the court made clear that a commonsense approach should be applied.”
On Twitter @legal_med
A U.S. Supreme Court ruling that expands liability under the federal False Claims Act (FCA) could have both positive and negative implications for physicians accused of submitting false claims to the government.
Justices ruled June 16 that health care providers can be held liable under the FCA if they bill for a service, but fail to comply with underlying regulations, even if the violation is not explicit in the claim. The decision upholds use of the “implied false certification theory” in FCA cases, which provides that any submission for government payment include an implicit certification of compliance with all applicable contract requirements, laws, and regulations.
The ruling allows a lawsuit by a patient’s family to continue against Universal Health Services, a national hospital management company. The plaintiff, Julio Escobar, claims that Universal presented false claims to Medicaid by seeking payments for services provided by unlicensed, unsupervised health care providers. Although the reimbursement claims submitted to the government accurately described the services provided and cited the correct charges, the plaintiffs alleged that because the clinic’s operations violated state requirements to participate in Medicaid, Universal had also violated the FCA. Universal argued the FCA suit was invalid because a reimbursement claim cannot be false unless its details are untrue or inaccurate.
The Supreme Court ruled in favor of the plaintiffs. By using National Provider Identification numbers that corresponded to specific job titles without disclosing many violations of staff and licensing requirements, Universal’s claims constituted misrepresentations, the justices said.
The opinion includes language that is both helpful and harmful to physicians, according to health law attorneys. On the one hand, the justices supported the implied certification theory, thus expanding the scope of potential liability under the FCA in certain circumstances, said George B. Breen, a Washington-based health law attorney.
“The court’s decision is significant for health care providers and suppliers that submit claims to federally funded health care programs, including Medicare and Medicaid, because the FCA remains one of the federal government’s primary enforcement tools,” Mr. Breen said in an interview. “In the court’s view, half-truths in a claim for reimbursement from a government program … [are] just as actionable as an outright lie if the failure to disclose noncompliance with a statute, regulation, or contract term makes those representations misleading half-truths.”
However, the Supreme Court rejected the government’s notion that every omission, regulatory violation, or contract breach can result in fines and damages under the FCA. The compliance violation must be material to the government’s payment decision, the justices said. This means an alleged regulatory or contractual violation must have mattered to the agency’s payment decision to be actionable under the FCA, said David L. Douglass, a Washington-based health law attorney.
In their written opinion, the justices offered this example: If the government adds a requirement that health providers who participate in Medicaid must buy American-made staplers, and a health provider submits a claim but fails to disclose the use of foreign staplers, that provider should not be held liable under the FCA.
“An undisclosed fact is material if, for instance, no one can say with reason that the plaintiff would have signed this contract if informed of the likelihood of the undisclosed fact,” the justices wrote. “A misrepresentation cannot be deemed material merely because the government designates compliance with a particular requirement as a condition of payment. … The False Claims Act does not adopt such an extraordinarily expansive view of liability.”
This aspect of the ruling is good news for defendants and potential FCA targets, Mr. Douglass said in an interview.
“The court has restored the role of the materiality element,” he said. “The materiality element is a stringent protection against the risk that technical instances of noncompliance can become the basis for punitive liability.”
The high court also noted that if the underlying violation is known to widely occur and, nonetheless, Medicare regularly pays such claims, the violation is likely not material to payment, added William W. Horton, a Birmingham, Ala.–based health law attorney and chair of the American Bar Association Health Law Section.
“What I think this does, from the standpoint of defense of these claims, is open up new possibilities for arguing about whether a technical violation of a legal requirement, in fact, satisfies this test,” Mr. Horton said in an interview. “Whether, if CMS had known the violation occurred, would that have been material to CMS’s decision to pay the claim or not?”
But attorneys for plaintiffs and whistle-blowers are also hailing the Supreme Court opinion as positive for their clients.
Stephen M. Kohn, executive director of the National Whistleblower Center, said the Supreme Court correctly ruled on the issue.
“The legal position taken by the Chamber of Commerce and health care industry in this case was dumbfounding,” Mr. Kohn said in a statement. “Had the court agreed with the chamber and its allies – that you can bill the taxpayers for the services of a so-called doctor who was in fact unlicensed, and whose degree came from an unaccredited Internet college – then the floodgates would be opened for fraud in government contracting.”
Houston-based plaintiffs’ attorney Joel Androphy, who represents whistle-blowers, called the opinion “very favorable” for implied certification claims.
“Eliminating barriers such as the mandated condition of payment language in statutes or regulations was a proper change in direction for evaluating pleadings,” Mr. Androphy said in an interview. “If the defendant cheats, there should not be a free pass because Congress did not include magic wording in statute or regulation to support a false claims case. Materiality may be a lingering area of dispute; however the court made clear that a commonsense approach should be applied.”
On Twitter @legal_med
A U.S. Supreme Court ruling that expands liability under the federal False Claims Act (FCA) could have both positive and negative implications for physicians accused of submitting false claims to the government.
Justices ruled June 16 that health care providers can be held liable under the FCA if they bill for a service, but fail to comply with underlying regulations, even if the violation is not explicit in the claim. The decision upholds use of the “implied false certification theory” in FCA cases, which provides that any submission for government payment include an implicit certification of compliance with all applicable contract requirements, laws, and regulations.
The ruling allows a lawsuit by a patient’s family to continue against Universal Health Services, a national hospital management company. The plaintiff, Julio Escobar, claims that Universal presented false claims to Medicaid by seeking payments for services provided by unlicensed, unsupervised health care providers. Although the reimbursement claims submitted to the government accurately described the services provided and cited the correct charges, the plaintiffs alleged that because the clinic’s operations violated state requirements to participate in Medicaid, Universal had also violated the FCA. Universal argued the FCA suit was invalid because a reimbursement claim cannot be false unless its details are untrue or inaccurate.
The Supreme Court ruled in favor of the plaintiffs. By using National Provider Identification numbers that corresponded to specific job titles without disclosing many violations of staff and licensing requirements, Universal’s claims constituted misrepresentations, the justices said.
The opinion includes language that is both helpful and harmful to physicians, according to health law attorneys. On the one hand, the justices supported the implied certification theory, thus expanding the scope of potential liability under the FCA in certain circumstances, said George B. Breen, a Washington-based health law attorney.
“The court’s decision is significant for health care providers and suppliers that submit claims to federally funded health care programs, including Medicare and Medicaid, because the FCA remains one of the federal government’s primary enforcement tools,” Mr. Breen said in an interview. “In the court’s view, half-truths in a claim for reimbursement from a government program … [are] just as actionable as an outright lie if the failure to disclose noncompliance with a statute, regulation, or contract term makes those representations misleading half-truths.”
However, the Supreme Court rejected the government’s notion that every omission, regulatory violation, or contract breach can result in fines and damages under the FCA. The compliance violation must be material to the government’s payment decision, the justices said. This means an alleged regulatory or contractual violation must have mattered to the agency’s payment decision to be actionable under the FCA, said David L. Douglass, a Washington-based health law attorney.
In their written opinion, the justices offered this example: If the government adds a requirement that health providers who participate in Medicaid must buy American-made staplers, and a health provider submits a claim but fails to disclose the use of foreign staplers, that provider should not be held liable under the FCA.
“An undisclosed fact is material if, for instance, no one can say with reason that the plaintiff would have signed this contract if informed of the likelihood of the undisclosed fact,” the justices wrote. “A misrepresentation cannot be deemed material merely because the government designates compliance with a particular requirement as a condition of payment. … The False Claims Act does not adopt such an extraordinarily expansive view of liability.”
This aspect of the ruling is good news for defendants and potential FCA targets, Mr. Douglass said in an interview.
“The court has restored the role of the materiality element,” he said. “The materiality element is a stringent protection against the risk that technical instances of noncompliance can become the basis for punitive liability.”
The high court also noted that if the underlying violation is known to widely occur and, nonetheless, Medicare regularly pays such claims, the violation is likely not material to payment, added William W. Horton, a Birmingham, Ala.–based health law attorney and chair of the American Bar Association Health Law Section.
“What I think this does, from the standpoint of defense of these claims, is open up new possibilities for arguing about whether a technical violation of a legal requirement, in fact, satisfies this test,” Mr. Horton said in an interview. “Whether, if CMS had known the violation occurred, would that have been material to CMS’s decision to pay the claim or not?”
But attorneys for plaintiffs and whistle-blowers are also hailing the Supreme Court opinion as positive for their clients.
Stephen M. Kohn, executive director of the National Whistleblower Center, said the Supreme Court correctly ruled on the issue.
“The legal position taken by the Chamber of Commerce and health care industry in this case was dumbfounding,” Mr. Kohn said in a statement. “Had the court agreed with the chamber and its allies – that you can bill the taxpayers for the services of a so-called doctor who was in fact unlicensed, and whose degree came from an unaccredited Internet college – then the floodgates would be opened for fraud in government contracting.”
Houston-based plaintiffs’ attorney Joel Androphy, who represents whistle-blowers, called the opinion “very favorable” for implied certification claims.
“Eliminating barriers such as the mandated condition of payment language in statutes or regulations was a proper change in direction for evaluating pleadings,” Mr. Androphy said in an interview. “If the defendant cheats, there should not be a free pass because Congress did not include magic wording in statute or regulation to support a false claims case. Materiality may be a lingering area of dispute; however the court made clear that a commonsense approach should be applied.”
On Twitter @legal_med