User login
News and Views that Matter to Physicians
Common surgeries linked to chronic opioid use among opioid-naive patients
Common surgeries increase the risk of chronic opioid use in opioid-naive adults, especially those using antidepressants or benzodiazepines before their operations, and those with substance abuse histories, according to an insurance claims analysis from Stanford (Calif.) University.
The researchers reviewed opioid prescribing in the first postop year – excluding the first 90 days – for 641,941 patients and compared that information with opioid prescribing for 18,011,137 adult patients who did not have surgery. None of the subjects had filled an opioid prescription in the previous year (JAMA Intern Med. 2016 Jul 11. doi: 10.1001/jamainternmed.2016.3298).
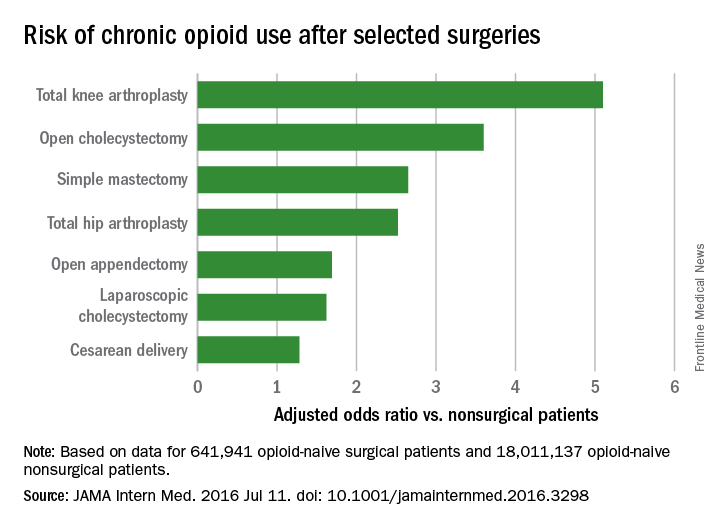
Chronic opioid use, defined as filling at least 120 days of opioid prescriptions within the first year of surgery, ranged up to 1.41% for total knee replacement, versus 0.136% in the nonsurgical controls. After adjustment for potential confounders, knee replacement increased the risk fivefold; open cholecystectomy almost fourfold; total hip replacement and simple mastectomy almost threefold; and laparoscopic cholecystectomy and open appendectomy almost twofold. Cesarean delivery increased the risk of chronic use by 28%.
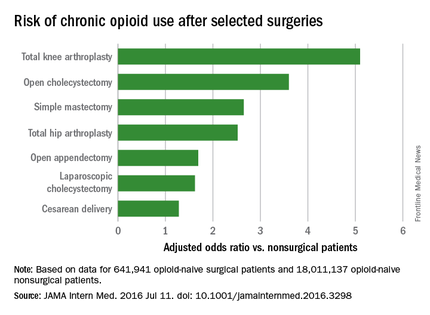
With the exception of knee and hip replacements, “these procedures are not indicated to relieve pain and are not thought to place patients at risk for long-term pain ... Our results suggest that primary care clinicians and surgeons should monitor opioid use closely in the postsurgical period,” wrote Eric C. Sun, MD, PhD, of the department of anesthesiology, perioperative and pain medicine at Stanford (Calif.) University, and his colleagues.
Preoperative antidepressants and benzodiazepines carried about the same risk of chronic use as alcohol abuse (odds ratio 1.83; P less than .001), while drug abuse history increased the risk even more (OR 3.15; P less than .001). Male sex, age over 50 years, and history of depression were also associated with chronic use on multivariate analysis. Meanwhile, transurethral prostatectomy, laparoscopic appendectomy, functional endoscopic sinus surgery, and cataract surgery did not increase chronic use risk.
“Surgical patients, particularly those at higher risk for chronic opioid use, may benefit from techniques to reduce the risk such as multimodal analgesia and regional anesthesia, particularly in light of literature suggesting that these interventions may improve other perioperative outcomes ... Patients may also benefit from other preoperative and postoperative interventions, such as evidence-based psychobehavioral pain management skills,” the investigators said.
It wasn’t clear until now that even opioid-naive patients are at risk for opioid problems after surgery. Stanford’s investigation is not the first to link surgery and opioid abuse, but previous studies tended to focus on patients with preexisting use and more painful operations.
The study included prescriptions for oral and patch fentanyl, hydrocodone, oral hydromorphone, methadone, morphine, oxymorphone, and oxycodone. Hydrocodone cough remedies and acetaminophen/codeine analgesics were excluded.
Nonsurgical patients tended to be younger than their surgical peers (mean 42 vs. 44 years) and more likely to be male (49% vs. 26%).
The authors had no disclosures. The work was funded in part by the Foundation for Anesthesia Education and Research and the Anesthesia Quality Institute. Claims data came from MarketScan (Truven Health Analytics).
Common surgeries increase the risk of chronic opioid use in opioid-naive adults, especially those using antidepressants or benzodiazepines before their operations, and those with substance abuse histories, according to an insurance claims analysis from Stanford (Calif.) University.
The researchers reviewed opioid prescribing in the first postop year – excluding the first 90 days – for 641,941 patients and compared that information with opioid prescribing for 18,011,137 adult patients who did not have surgery. None of the subjects had filled an opioid prescription in the previous year (JAMA Intern Med. 2016 Jul 11. doi: 10.1001/jamainternmed.2016.3298).
Chronic opioid use, defined as filling at least 120 days of opioid prescriptions within the first year of surgery, ranged up to 1.41% for total knee replacement, versus 0.136% in the nonsurgical controls. After adjustment for potential confounders, knee replacement increased the risk fivefold; open cholecystectomy almost fourfold; total hip replacement and simple mastectomy almost threefold; and laparoscopic cholecystectomy and open appendectomy almost twofold. Cesarean delivery increased the risk of chronic use by 28%.

With the exception of knee and hip replacements, “these procedures are not indicated to relieve pain and are not thought to place patients at risk for long-term pain ... Our results suggest that primary care clinicians and surgeons should monitor opioid use closely in the postsurgical period,” wrote Eric C. Sun, MD, PhD, of the department of anesthesiology, perioperative and pain medicine at Stanford (Calif.) University, and his colleagues.
Preoperative antidepressants and benzodiazepines carried about the same risk of chronic use as alcohol abuse (odds ratio 1.83; P less than .001), while drug abuse history increased the risk even more (OR 3.15; P less than .001). Male sex, age over 50 years, and history of depression were also associated with chronic use on multivariate analysis. Meanwhile, transurethral prostatectomy, laparoscopic appendectomy, functional endoscopic sinus surgery, and cataract surgery did not increase chronic use risk.
“Surgical patients, particularly those at higher risk for chronic opioid use, may benefit from techniques to reduce the risk such as multimodal analgesia and regional anesthesia, particularly in light of literature suggesting that these interventions may improve other perioperative outcomes ... Patients may also benefit from other preoperative and postoperative interventions, such as evidence-based psychobehavioral pain management skills,” the investigators said.
It wasn’t clear until now that even opioid-naive patients are at risk for opioid problems after surgery. Stanford’s investigation is not the first to link surgery and opioid abuse, but previous studies tended to focus on patients with preexisting use and more painful operations.
The study included prescriptions for oral and patch fentanyl, hydrocodone, oral hydromorphone, methadone, morphine, oxymorphone, and oxycodone. Hydrocodone cough remedies and acetaminophen/codeine analgesics were excluded.
Nonsurgical patients tended to be younger than their surgical peers (mean 42 vs. 44 years) and more likely to be male (49% vs. 26%).
The authors had no disclosures. The work was funded in part by the Foundation for Anesthesia Education and Research and the Anesthesia Quality Institute. Claims data came from MarketScan (Truven Health Analytics).
Common surgeries increase the risk of chronic opioid use in opioid-naive adults, especially those using antidepressants or benzodiazepines before their operations, and those with substance abuse histories, according to an insurance claims analysis from Stanford (Calif.) University.
The researchers reviewed opioid prescribing in the first postop year – excluding the first 90 days – for 641,941 patients and compared that information with opioid prescribing for 18,011,137 adult patients who did not have surgery. None of the subjects had filled an opioid prescription in the previous year (JAMA Intern Med. 2016 Jul 11. doi: 10.1001/jamainternmed.2016.3298).
Chronic opioid use, defined as filling at least 120 days of opioid prescriptions within the first year of surgery, ranged up to 1.41% for total knee replacement, versus 0.136% in the nonsurgical controls. After adjustment for potential confounders, knee replacement increased the risk fivefold; open cholecystectomy almost fourfold; total hip replacement and simple mastectomy almost threefold; and laparoscopic cholecystectomy and open appendectomy almost twofold. Cesarean delivery increased the risk of chronic use by 28%.

With the exception of knee and hip replacements, “these procedures are not indicated to relieve pain and are not thought to place patients at risk for long-term pain ... Our results suggest that primary care clinicians and surgeons should monitor opioid use closely in the postsurgical period,” wrote Eric C. Sun, MD, PhD, of the department of anesthesiology, perioperative and pain medicine at Stanford (Calif.) University, and his colleagues.
Preoperative antidepressants and benzodiazepines carried about the same risk of chronic use as alcohol abuse (odds ratio 1.83; P less than .001), while drug abuse history increased the risk even more (OR 3.15; P less than .001). Male sex, age over 50 years, and history of depression were also associated with chronic use on multivariate analysis. Meanwhile, transurethral prostatectomy, laparoscopic appendectomy, functional endoscopic sinus surgery, and cataract surgery did not increase chronic use risk.
“Surgical patients, particularly those at higher risk for chronic opioid use, may benefit from techniques to reduce the risk such as multimodal analgesia and regional anesthesia, particularly in light of literature suggesting that these interventions may improve other perioperative outcomes ... Patients may also benefit from other preoperative and postoperative interventions, such as evidence-based psychobehavioral pain management skills,” the investigators said.
It wasn’t clear until now that even opioid-naive patients are at risk for opioid problems after surgery. Stanford’s investigation is not the first to link surgery and opioid abuse, but previous studies tended to focus on patients with preexisting use and more painful operations.
The study included prescriptions for oral and patch fentanyl, hydrocodone, oral hydromorphone, methadone, morphine, oxymorphone, and oxycodone. Hydrocodone cough remedies and acetaminophen/codeine analgesics were excluded.
Nonsurgical patients tended to be younger than their surgical peers (mean 42 vs. 44 years) and more likely to be male (49% vs. 26%).
The authors had no disclosures. The work was funded in part by the Foundation for Anesthesia Education and Research and the Anesthesia Quality Institute. Claims data came from MarketScan (Truven Health Analytics).
FROM JAMA INTERNAL MEDICINE
Key clinical point: Common surgeries increase the risk of chronic opioid use in opioid-naive adults, especially among those using antidepressants or benzodiazepines before their operations, and those with substance abuse histories.
Major finding: After adjustment for potential confounders, knee replacement increased the risk fivefold; open cholecystectomy almost fourfold; and total hip replacement and simple mastectomy almost threefold.
Data source: Insurance claims of more than 18 million people.
Disclosures: The authors had no disclosures. The work was funded in part by the Foundation for Anesthesia Education and Research and the Anesthesia Quality Institute. Claims data came from MarketScan (Truven Health Analytics).
Cost of end-of-life care peaks at age 73 years
The cost of end-of-life care for Americans on traditional Medicare is higher for those in their early 70s than for beneficiaries in their 80s or 90s, according to the Kaiser Family Foundation.
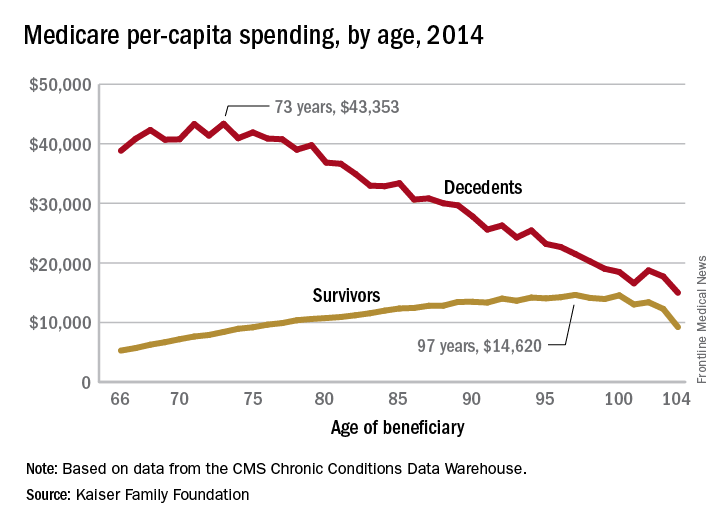
In 2014, the per-capita cost of care peaked at $43,353 for those who died at age 73, compared with $36,841 who died at age 80 and $27,779 for 90-year-old decedents, Kaiser found in its analysis of claims data from the Centers for Medicare & Medicaid Services Chronic Conditions Data Warehouse.
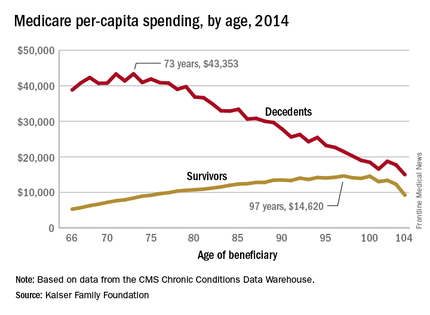
“This is a pattern we weren’t really expecting to see,” Juliette Cubanski, associate director of the program on Medicare policy for Kaiser, said in an article on the findings distributed by Kaiser Health News. “It kind of goes against the notion that doctors are throwing everything including the kitchen sink at people at the end of life regardless of how old they are,” she added.
The trend was quite different, and much less costly, for those who lived through the entire year. Their per-capita cost of care started at $5,271 for 66-year-olds and peaked at $14,620 for those aged 97. Medicare per capita spending for all decedents was nearly four times higher, at $34,529, than the $9,121 spent for each beneficiary who survived the year, the Kaiser report showed.
The largest share of that difference came from inpatient hospital care, which was 51% of decedents’ per-capita cost but only 27% for survivors. The cost for each group: $17,574 for decedents and $2,497 for survivors, according to Kaiser, which pointed out that its analysis covered only traditional Medicare beneficiaries during the calendar year in which they died and did not include spending in the full 12 months before their deaths.
The gap between decedents and survivors has narrowed in recent years. The growth rate from 2000 – when spending was $19,130 – to 2014 was 4.3% a year for decedents, while spending for survivors rose 5.5% annually from its $4,322 starting level at the turn of the century, the report noted.
The cost of end-of-life care for Americans on traditional Medicare is higher for those in their early 70s than for beneficiaries in their 80s or 90s, according to the Kaiser Family Foundation.
In 2014, the per-capita cost of care peaked at $43,353 for those who died at age 73, compared with $36,841 who died at age 80 and $27,779 for 90-year-old decedents, Kaiser found in its analysis of claims data from the Centers for Medicare & Medicaid Services Chronic Conditions Data Warehouse.

“This is a pattern we weren’t really expecting to see,” Juliette Cubanski, associate director of the program on Medicare policy for Kaiser, said in an article on the findings distributed by Kaiser Health News. “It kind of goes against the notion that doctors are throwing everything including the kitchen sink at people at the end of life regardless of how old they are,” she added.
The trend was quite different, and much less costly, for those who lived through the entire year. Their per-capita cost of care started at $5,271 for 66-year-olds and peaked at $14,620 for those aged 97. Medicare per capita spending for all decedents was nearly four times higher, at $34,529, than the $9,121 spent for each beneficiary who survived the year, the Kaiser report showed.
The largest share of that difference came from inpatient hospital care, which was 51% of decedents’ per-capita cost but only 27% for survivors. The cost for each group: $17,574 for decedents and $2,497 for survivors, according to Kaiser, which pointed out that its analysis covered only traditional Medicare beneficiaries during the calendar year in which they died and did not include spending in the full 12 months before their deaths.
The gap between decedents and survivors has narrowed in recent years. The growth rate from 2000 – when spending was $19,130 – to 2014 was 4.3% a year for decedents, while spending for survivors rose 5.5% annually from its $4,322 starting level at the turn of the century, the report noted.
The cost of end-of-life care for Americans on traditional Medicare is higher for those in their early 70s than for beneficiaries in their 80s or 90s, according to the Kaiser Family Foundation.
In 2014, the per-capita cost of care peaked at $43,353 for those who died at age 73, compared with $36,841 who died at age 80 and $27,779 for 90-year-old decedents, Kaiser found in its analysis of claims data from the Centers for Medicare & Medicaid Services Chronic Conditions Data Warehouse.

“This is a pattern we weren’t really expecting to see,” Juliette Cubanski, associate director of the program on Medicare policy for Kaiser, said in an article on the findings distributed by Kaiser Health News. “It kind of goes against the notion that doctors are throwing everything including the kitchen sink at people at the end of life regardless of how old they are,” she added.
The trend was quite different, and much less costly, for those who lived through the entire year. Their per-capita cost of care started at $5,271 for 66-year-olds and peaked at $14,620 for those aged 97. Medicare per capita spending for all decedents was nearly four times higher, at $34,529, than the $9,121 spent for each beneficiary who survived the year, the Kaiser report showed.
The largest share of that difference came from inpatient hospital care, which was 51% of decedents’ per-capita cost but only 27% for survivors. The cost for each group: $17,574 for decedents and $2,497 for survivors, according to Kaiser, which pointed out that its analysis covered only traditional Medicare beneficiaries during the calendar year in which they died and did not include spending in the full 12 months before their deaths.
The gap between decedents and survivors has narrowed in recent years. The growth rate from 2000 – when spending was $19,130 – to 2014 was 4.3% a year for decedents, while spending for survivors rose 5.5% annually from its $4,322 starting level at the turn of the century, the report noted.
Blood Aspergillus RNA a promising biomarker for invasive aspergillosis
Elevated Aspergillus RNA blood levels after 4-6 weeks of antifungal treatment predict poor response at week 12 in patients with proven or probable invasive aspergillosis, according to results of a small observational study of 41 evaluable patients.
The investigators are working to address the need for reliable biomarkers of early invasive aspergillosis (IA) treatment response. Standard clinical and radiological criteria are somewhat subjective, and serial biopsies and bronchoalveolar lavage are often impractical, reported Yanan Zhao, PhD, of the New Jersey Medical School–Rutgers Biomedical and Health Sciences, Newark, and her associates.
Study participants’ blood was checked for serum galactomannan (GM), 1, 3-beta-D-glucan (BG), and Aspergillus RNA within 24 hours of starting antifungal therapy, then twice per week during the first 2 weeks, then once during weeks 4, 6, and 12, the investigators reported (Med Mycol. 2016 Jun 22. pii: myw043).
Ribosomal Aspergillus RNA – like GM and BG, a marker of fungal load – was measured by nucleic acid sequence-based amplification (NASBA), a robust isothermal amplification technique more sensitive than polymerase chain reaction due largely “to increased starting target numbers (RNA versus DNA) and more robust amplification.” Although NASBA has been used before to diagnose IA, using it to monitor treatment “is still in its infancy,” the authors noted.
Eleven of 14 patients who did not respond to treatment at 12 weeks (79%) had Aspergillus RNA in their blood after 4 weeks of treatment, and 12 (86%) were positive at 6 weeks.
Among patients who did respond at 12 weeks, 11 of 27 (41%) had RNA in their blood at 4 weeks, and 14 (52%) at 6 weeks. The findings were statistically significant.
There was no correlation between Aspergillus RNA and serum GM levels in terms of outcomes, but the kinetics of circulating Aspergillus RNA correlated with BG in some patients, with an excellent match in three.
Serum GM responds fairly soon if treatment is working. Aspergillus RNA, however, responds more slowly, like BG. “This may explain ... the correlation between Aspergillus RNA and BG ... Therefore, the combination of Aspergillus RNA and BG might be useful to assess therapeutic response, particularly in GM negative cases,” the investigators said.
This work was funded by Merck. Four investigators are current or former employees.
Elevated Aspergillus RNA blood levels after 4-6 weeks of antifungal treatment predict poor response at week 12 in patients with proven or probable invasive aspergillosis, according to results of a small observational study of 41 evaluable patients.
The investigators are working to address the need for reliable biomarkers of early invasive aspergillosis (IA) treatment response. Standard clinical and radiological criteria are somewhat subjective, and serial biopsies and bronchoalveolar lavage are often impractical, reported Yanan Zhao, PhD, of the New Jersey Medical School–Rutgers Biomedical and Health Sciences, Newark, and her associates.
Study participants’ blood was checked for serum galactomannan (GM), 1, 3-beta-D-glucan (BG), and Aspergillus RNA within 24 hours of starting antifungal therapy, then twice per week during the first 2 weeks, then once during weeks 4, 6, and 12, the investigators reported (Med Mycol. 2016 Jun 22. pii: myw043).
Ribosomal Aspergillus RNA – like GM and BG, a marker of fungal load – was measured by nucleic acid sequence-based amplification (NASBA), a robust isothermal amplification technique more sensitive than polymerase chain reaction due largely “to increased starting target numbers (RNA versus DNA) and more robust amplification.” Although NASBA has been used before to diagnose IA, using it to monitor treatment “is still in its infancy,” the authors noted.
Eleven of 14 patients who did not respond to treatment at 12 weeks (79%) had Aspergillus RNA in their blood after 4 weeks of treatment, and 12 (86%) were positive at 6 weeks.
Among patients who did respond at 12 weeks, 11 of 27 (41%) had RNA in their blood at 4 weeks, and 14 (52%) at 6 weeks. The findings were statistically significant.
There was no correlation between Aspergillus RNA and serum GM levels in terms of outcomes, but the kinetics of circulating Aspergillus RNA correlated with BG in some patients, with an excellent match in three.
Serum GM responds fairly soon if treatment is working. Aspergillus RNA, however, responds more slowly, like BG. “This may explain ... the correlation between Aspergillus RNA and BG ... Therefore, the combination of Aspergillus RNA and BG might be useful to assess therapeutic response, particularly in GM negative cases,” the investigators said.
This work was funded by Merck. Four investigators are current or former employees.
Elevated Aspergillus RNA blood levels after 4-6 weeks of antifungal treatment predict poor response at week 12 in patients with proven or probable invasive aspergillosis, according to results of a small observational study of 41 evaluable patients.
The investigators are working to address the need for reliable biomarkers of early invasive aspergillosis (IA) treatment response. Standard clinical and radiological criteria are somewhat subjective, and serial biopsies and bronchoalveolar lavage are often impractical, reported Yanan Zhao, PhD, of the New Jersey Medical School–Rutgers Biomedical and Health Sciences, Newark, and her associates.
Study participants’ blood was checked for serum galactomannan (GM), 1, 3-beta-D-glucan (BG), and Aspergillus RNA within 24 hours of starting antifungal therapy, then twice per week during the first 2 weeks, then once during weeks 4, 6, and 12, the investigators reported (Med Mycol. 2016 Jun 22. pii: myw043).
Ribosomal Aspergillus RNA – like GM and BG, a marker of fungal load – was measured by nucleic acid sequence-based amplification (NASBA), a robust isothermal amplification technique more sensitive than polymerase chain reaction due largely “to increased starting target numbers (RNA versus DNA) and more robust amplification.” Although NASBA has been used before to diagnose IA, using it to monitor treatment “is still in its infancy,” the authors noted.
Eleven of 14 patients who did not respond to treatment at 12 weeks (79%) had Aspergillus RNA in their blood after 4 weeks of treatment, and 12 (86%) were positive at 6 weeks.
Among patients who did respond at 12 weeks, 11 of 27 (41%) had RNA in their blood at 4 weeks, and 14 (52%) at 6 weeks. The findings were statistically significant.
There was no correlation between Aspergillus RNA and serum GM levels in terms of outcomes, but the kinetics of circulating Aspergillus RNA correlated with BG in some patients, with an excellent match in three.
Serum GM responds fairly soon if treatment is working. Aspergillus RNA, however, responds more slowly, like BG. “This may explain ... the correlation between Aspergillus RNA and BG ... Therefore, the combination of Aspergillus RNA and BG might be useful to assess therapeutic response, particularly in GM negative cases,” the investigators said.
This work was funded by Merck. Four investigators are current or former employees.
FROM MEDICAL MYCOLOGY
Key clinical point: Elevated Aspergillus RNA blood levels during the first 4-6 weeks of antifungal treatment predicts poor response at week 12.
Major finding: Eleven of 14 patients who did not respond to antifungals at 12 weeks (79%) had Aspergillus RNA in their blood after 4 weeks of treatment, versus 11 of 27 (41%) who did respond (P = .046).
Data source: Small observational study of patients with proven or probable invasive aspergillosis.
Disclosures: This work was funded by Merck. Four investigators are current or former employees.
Infections, antibiotics more common in type 2 diabetes
People with type 2 diabetes are up to 55% more likely to experience hospital-treated infections and up to 30% more likely to receive an antibiotic prescription in the community setting, compared with the general population, but these associations moderated over an 8-year period – a phenomenon that could be at least partly related to better treatment of diabetes and an overall improvement in mean blood glucose levels, according to results of a large population-based study.
“These findings may be driven by earlier detection and treatment of milder type 2 diabetes cases over time,” or by improved therapy of hyperglycemia and other risk factors, wrote Anil Mor, MD, of Aarhus University Hospital, Denmark, and his associates (Clin Infect Dis. 2016 June 26. doi: 10.1093/cid/ciw345).
The study ran from 2004 to 2012 and used data from the Danish National Patient Registry. It tracked community- and hospital-treated infections in approximately 774,017 controls; of these, 155,158 had type 2 diabetes. Patients with diabetes were more likely to have serious medical comorbidities compared with controls (29% vs. 21%). These included myocardial infarction (5% vs. 3%), heart failure (4% vs. 2%), cerebrovascular diseases (7% vs. 5%), peripheral vascular diseases (4% vs. 2%), and chronic pulmonary disease (6% vs. 2%).
Over the study period, 62% of the diabetes patients received an antibiotic, compared with 55% of the controls – a 24% increased relative risk in a model that adjusted for factors such as alcohol use, Charlson comorbidity index, and cardiovascular and renal comorbidities. Cephalosporins were the most commonly prescribed drugs, followed by antimycobacterial agents, quinolones, and antibiotics commonly used for urinary tract and Staphylococcus aureus infections.
Hospital-treated infections were significantly more common among patients with diabetes, with 19% having at least one such infection compared with 13% of controls (RR 1.55). On a larger scale, at a median follow-up of 2.8 years, the hospital-treated rate among diabetes patients was 58 per 1,000 person/years vs. 39 per 1,000 person/years among controls – a relative risk of 1.49.
Patients were at highest risk for emphysematous cholecystitis (adjusted rate ratio 1.74) and abscesses, tuberculosis, and meningococcal infections. Pneumonia was approximately 30% more likely among patients.
The risk of a hospital-treated infection was highest among younger patients aged 40-50 years (RR 1.77) and lowest among those older than 80 years (RR 1.29). It was also higher among those with higher comorbidity scores. Statin use appeared to attenuate some of the risk, the authors noted. The authors did not discuss the possible cause of this association.
The annual risk of a hospital-treated infection among patients declined from a high of 1.89 in 2004 to 1.59 in 2011. The risk of receiving a community-based antibiotic prescription declined as well, from 1.31 in 2004 to 1.26 in 2011.
These changes were not seen in the control group, suggesting that patients with diabetes were experiencing a unique improvement in infections – earlier detection and treatment of type 2 diabetes, and better comorbidity management could be explanations, the investigators speculated.
The study was sponsored by the Danish Centre for Strategic Research in Type 2 Diabetes and the Program for Clinical Research Infrastructure established by the Lundbeck Foundation and the Novo Nordisk Foundation. Several of the coauthors reported financial ties with various pharmaceutical companies.
On Twitter @Alz_Gal
People with type 2 diabetes are up to 55% more likely to experience hospital-treated infections and up to 30% more likely to receive an antibiotic prescription in the community setting, compared with the general population, but these associations moderated over an 8-year period – a phenomenon that could be at least partly related to better treatment of diabetes and an overall improvement in mean blood glucose levels, according to results of a large population-based study.
“These findings may be driven by earlier detection and treatment of milder type 2 diabetes cases over time,” or by improved therapy of hyperglycemia and other risk factors, wrote Anil Mor, MD, of Aarhus University Hospital, Denmark, and his associates (Clin Infect Dis. 2016 June 26. doi: 10.1093/cid/ciw345).
The study ran from 2004 to 2012 and used data from the Danish National Patient Registry. It tracked community- and hospital-treated infections in approximately 774,017 controls; of these, 155,158 had type 2 diabetes. Patients with diabetes were more likely to have serious medical comorbidities compared with controls (29% vs. 21%). These included myocardial infarction (5% vs. 3%), heart failure (4% vs. 2%), cerebrovascular diseases (7% vs. 5%), peripheral vascular diseases (4% vs. 2%), and chronic pulmonary disease (6% vs. 2%).
Over the study period, 62% of the diabetes patients received an antibiotic, compared with 55% of the controls – a 24% increased relative risk in a model that adjusted for factors such as alcohol use, Charlson comorbidity index, and cardiovascular and renal comorbidities. Cephalosporins were the most commonly prescribed drugs, followed by antimycobacterial agents, quinolones, and antibiotics commonly used for urinary tract and Staphylococcus aureus infections.
Hospital-treated infections were significantly more common among patients with diabetes, with 19% having at least one such infection compared with 13% of controls (RR 1.55). On a larger scale, at a median follow-up of 2.8 years, the hospital-treated rate among diabetes patients was 58 per 1,000 person/years vs. 39 per 1,000 person/years among controls – a relative risk of 1.49.
Patients were at highest risk for emphysematous cholecystitis (adjusted rate ratio 1.74) and abscesses, tuberculosis, and meningococcal infections. Pneumonia was approximately 30% more likely among patients.
The risk of a hospital-treated infection was highest among younger patients aged 40-50 years (RR 1.77) and lowest among those older than 80 years (RR 1.29). It was also higher among those with higher comorbidity scores. Statin use appeared to attenuate some of the risk, the authors noted. The authors did not discuss the possible cause of this association.
The annual risk of a hospital-treated infection among patients declined from a high of 1.89 in 2004 to 1.59 in 2011. The risk of receiving a community-based antibiotic prescription declined as well, from 1.31 in 2004 to 1.26 in 2011.
These changes were not seen in the control group, suggesting that patients with diabetes were experiencing a unique improvement in infections – earlier detection and treatment of type 2 diabetes, and better comorbidity management could be explanations, the investigators speculated.
The study was sponsored by the Danish Centre for Strategic Research in Type 2 Diabetes and the Program for Clinical Research Infrastructure established by the Lundbeck Foundation and the Novo Nordisk Foundation. Several of the coauthors reported financial ties with various pharmaceutical companies.
On Twitter @Alz_Gal
People with type 2 diabetes are up to 55% more likely to experience hospital-treated infections and up to 30% more likely to receive an antibiotic prescription in the community setting, compared with the general population, but these associations moderated over an 8-year period – a phenomenon that could be at least partly related to better treatment of diabetes and an overall improvement in mean blood glucose levels, according to results of a large population-based study.
“These findings may be driven by earlier detection and treatment of milder type 2 diabetes cases over time,” or by improved therapy of hyperglycemia and other risk factors, wrote Anil Mor, MD, of Aarhus University Hospital, Denmark, and his associates (Clin Infect Dis. 2016 June 26. doi: 10.1093/cid/ciw345).
The study ran from 2004 to 2012 and used data from the Danish National Patient Registry. It tracked community- and hospital-treated infections in approximately 774,017 controls; of these, 155,158 had type 2 diabetes. Patients with diabetes were more likely to have serious medical comorbidities compared with controls (29% vs. 21%). These included myocardial infarction (5% vs. 3%), heart failure (4% vs. 2%), cerebrovascular diseases (7% vs. 5%), peripheral vascular diseases (4% vs. 2%), and chronic pulmonary disease (6% vs. 2%).
Over the study period, 62% of the diabetes patients received an antibiotic, compared with 55% of the controls – a 24% increased relative risk in a model that adjusted for factors such as alcohol use, Charlson comorbidity index, and cardiovascular and renal comorbidities. Cephalosporins were the most commonly prescribed drugs, followed by antimycobacterial agents, quinolones, and antibiotics commonly used for urinary tract and Staphylococcus aureus infections.
Hospital-treated infections were significantly more common among patients with diabetes, with 19% having at least one such infection compared with 13% of controls (RR 1.55). On a larger scale, at a median follow-up of 2.8 years, the hospital-treated rate among diabetes patients was 58 per 1,000 person/years vs. 39 per 1,000 person/years among controls – a relative risk of 1.49.
Patients were at highest risk for emphysematous cholecystitis (adjusted rate ratio 1.74) and abscesses, tuberculosis, and meningococcal infections. Pneumonia was approximately 30% more likely among patients.
The risk of a hospital-treated infection was highest among younger patients aged 40-50 years (RR 1.77) and lowest among those older than 80 years (RR 1.29). It was also higher among those with higher comorbidity scores. Statin use appeared to attenuate some of the risk, the authors noted. The authors did not discuss the possible cause of this association.
The annual risk of a hospital-treated infection among patients declined from a high of 1.89 in 2004 to 1.59 in 2011. The risk of receiving a community-based antibiotic prescription declined as well, from 1.31 in 2004 to 1.26 in 2011.
These changes were not seen in the control group, suggesting that patients with diabetes were experiencing a unique improvement in infections – earlier detection and treatment of type 2 diabetes, and better comorbidity management could be explanations, the investigators speculated.
The study was sponsored by the Danish Centre for Strategic Research in Type 2 Diabetes and the Program for Clinical Research Infrastructure established by the Lundbeck Foundation and the Novo Nordisk Foundation. Several of the coauthors reported financial ties with various pharmaceutical companies.
On Twitter @Alz_Gal
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: Both hospital-treated infections and community-acquired antibiotics are more common among people with type 2 diabetes
Major finding: Hospital-treated infections were 55% more likely; community-dispensed antibiotics, 30% more common.
Data source: An observational study comprising almost 900,000 people in Denmark.
Disclosures: The study was sponsored by the Danish Centre for Strategic Research in Type 2 Diabetes and the Program for Clinical Research Infrastructure established by the Lundbeck Foundation and the Novo Nordisk Foundation. Several coauthors reported financial ties with various pharmaceutical companies.
Syndecan-1 may predict kidney injury after ped heart surgery
Acute kidney injury is a common complication after pediatric cardiac surgery, but measuring for a specific genetic protein immediately after cardiac surgery may improve cardiac surgeons’ ability to predict patients at higher risk of AKI, according to researchers from Brazil. The study results are in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152-178-86).
“Plasma syndecan-1 levels measured early in the postoperative period were independently associated with severe acute kidney injury,” wrote Candice Torres de Melo Bezerra Cavalcante, MD, of Heart Hospital of Messejana and Federal University of Ceará.
Their prospective cohort study involved 289 pediatric patients who had cardiac surgery at their institution between September 2013 and December 2014.
Dr. Cavalcante and colleagues acknowledged that the traditional biomarker for renal function, serum creatinine, only increases appreciably after the glomerular filtration rate declines 50%, impairing physicians’ ability to detect AKI early enough to treat it. “This delay can explain, in part the, negative results in AKI therapeutic clinical trials,” they wrote.
They evaluated two different endothelial biomarkers in addition to syndecan-1 with regard to their capacity for predicting severe AKI: plasma ICAM-1, a marker of endothelial cell activation; and E-selectin, an endothelial cell adhesion molecule. Syndecan-1 works as a biomarker of injury to the glycocalyx protein that surrounds endothelial cell membranes that acts as a permeability barrier and prevents the cells from adhering to blood. They found that median syndecan-1 levels soon after surgery were higher in patients with severe AKI, 103.6 vs. 42.3 ng/mL.
“Although syndecan-1 is not a renal-specific biomarker, there has been recent increasing evidence that endothelial injury has an important role in AKI pathophysiology,” the researchers noted.
Study results showed the higher the level of syndecan-1, the greater the adjusted odds ratio (OR) for severe AKI. Levels of less than 17 ng/mL were considered normal; 17.1-46.7 ng/mL carried an adjusted OR of 1.42; 47.4-93.1 ng/mL had an adjusted OR of 2.05; and levels 96.3 or greater had an OR of 8.87.
“Maintenance of endothelial glycocalyx integrity can be a therapeutic target to reduce AKI in this setting,” the researchers wrote.
The authors acknowledged that the study was done at a single center that had dialysis and death rates three and five times higher, respectively, than those of developed countries; and it measured syndecan-1 at only one time point almost immediately after the operation.
“Adding postoperative syndecan-1, even when using a clinical model that already incorporates variables from renal angina index, results in significant improvement in the capacity to predict severe AKI,” Dr. Cavalcante and colleagues concluded.
They had no financial relationships to disclose.
Results of AKI in heart surgery patients have been “sobering,” with up to 56% of these patients being diagnosed with AKI, but research such as that by Dr. Cavalcante and colleagues represents a new approach to improving outcomes by combining clinical risk factors with specific biomarkers to identify patients at risk, Petros V. Anagnostopoulos, MD, of American Family Children’s Hospital, University of Wisconsin, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152[1]:187-8).
Dr. Anagnostopoulos acknowledged problems with traditional markers for renal function. “An ideal biomarker should be sensitive, easy to measure, reproducible, and inexpensive,” he said. “Finally, when combined with clinical prediction models, it should potentiate the discrimination of these models.”
Syndecan-1 answers that call, he said. “It peaks early and is cheap, fast, and easy to measure with readily available methods, which makes it an ideal early biomarker of AKI,” Dr. Anagnostopoulos said. Even so, he pointed out potential shortcomings of syndecan-1: It is not renal specific and it does not increase before the operation.
But he applauded Dr. Cavalcante and colleagues for pursuing research to combine clinical risk factors with specific biomarkers. “It is very likely that this type of clinical research will become prevalent in the near future and will hopefully produce results that will allow better individual patient-specific risk stratification,” Dr. Anagnostopoulos said.
He had no financial relationships to disclose.
Results of AKI in heart surgery patients have been “sobering,” with up to 56% of these patients being diagnosed with AKI, but research such as that by Dr. Cavalcante and colleagues represents a new approach to improving outcomes by combining clinical risk factors with specific biomarkers to identify patients at risk, Petros V. Anagnostopoulos, MD, of American Family Children’s Hospital, University of Wisconsin, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152[1]:187-8).
Dr. Anagnostopoulos acknowledged problems with traditional markers for renal function. “An ideal biomarker should be sensitive, easy to measure, reproducible, and inexpensive,” he said. “Finally, when combined with clinical prediction models, it should potentiate the discrimination of these models.”
Syndecan-1 answers that call, he said. “It peaks early and is cheap, fast, and easy to measure with readily available methods, which makes it an ideal early biomarker of AKI,” Dr. Anagnostopoulos said. Even so, he pointed out potential shortcomings of syndecan-1: It is not renal specific and it does not increase before the operation.
But he applauded Dr. Cavalcante and colleagues for pursuing research to combine clinical risk factors with specific biomarkers. “It is very likely that this type of clinical research will become prevalent in the near future and will hopefully produce results that will allow better individual patient-specific risk stratification,” Dr. Anagnostopoulos said.
He had no financial relationships to disclose.
Results of AKI in heart surgery patients have been “sobering,” with up to 56% of these patients being diagnosed with AKI, but research such as that by Dr. Cavalcante and colleagues represents a new approach to improving outcomes by combining clinical risk factors with specific biomarkers to identify patients at risk, Petros V. Anagnostopoulos, MD, of American Family Children’s Hospital, University of Wisconsin, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152[1]:187-8).
Dr. Anagnostopoulos acknowledged problems with traditional markers for renal function. “An ideal biomarker should be sensitive, easy to measure, reproducible, and inexpensive,” he said. “Finally, when combined with clinical prediction models, it should potentiate the discrimination of these models.”
Syndecan-1 answers that call, he said. “It peaks early and is cheap, fast, and easy to measure with readily available methods, which makes it an ideal early biomarker of AKI,” Dr. Anagnostopoulos said. Even so, he pointed out potential shortcomings of syndecan-1: It is not renal specific and it does not increase before the operation.
But he applauded Dr. Cavalcante and colleagues for pursuing research to combine clinical risk factors with specific biomarkers. “It is very likely that this type of clinical research will become prevalent in the near future and will hopefully produce results that will allow better individual patient-specific risk stratification,” Dr. Anagnostopoulos said.
He had no financial relationships to disclose.
Acute kidney injury is a common complication after pediatric cardiac surgery, but measuring for a specific genetic protein immediately after cardiac surgery may improve cardiac surgeons’ ability to predict patients at higher risk of AKI, according to researchers from Brazil. The study results are in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152-178-86).
“Plasma syndecan-1 levels measured early in the postoperative period were independently associated with severe acute kidney injury,” wrote Candice Torres de Melo Bezerra Cavalcante, MD, of Heart Hospital of Messejana and Federal University of Ceará.
Their prospective cohort study involved 289 pediatric patients who had cardiac surgery at their institution between September 2013 and December 2014.
Dr. Cavalcante and colleagues acknowledged that the traditional biomarker for renal function, serum creatinine, only increases appreciably after the glomerular filtration rate declines 50%, impairing physicians’ ability to detect AKI early enough to treat it. “This delay can explain, in part the, negative results in AKI therapeutic clinical trials,” they wrote.
They evaluated two different endothelial biomarkers in addition to syndecan-1 with regard to their capacity for predicting severe AKI: plasma ICAM-1, a marker of endothelial cell activation; and E-selectin, an endothelial cell adhesion molecule. Syndecan-1 works as a biomarker of injury to the glycocalyx protein that surrounds endothelial cell membranes that acts as a permeability barrier and prevents the cells from adhering to blood. They found that median syndecan-1 levels soon after surgery were higher in patients with severe AKI, 103.6 vs. 42.3 ng/mL.
“Although syndecan-1 is not a renal-specific biomarker, there has been recent increasing evidence that endothelial injury has an important role in AKI pathophysiology,” the researchers noted.
Study results showed the higher the level of syndecan-1, the greater the adjusted odds ratio (OR) for severe AKI. Levels of less than 17 ng/mL were considered normal; 17.1-46.7 ng/mL carried an adjusted OR of 1.42; 47.4-93.1 ng/mL had an adjusted OR of 2.05; and levels 96.3 or greater had an OR of 8.87.
“Maintenance of endothelial glycocalyx integrity can be a therapeutic target to reduce AKI in this setting,” the researchers wrote.
The authors acknowledged that the study was done at a single center that had dialysis and death rates three and five times higher, respectively, than those of developed countries; and it measured syndecan-1 at only one time point almost immediately after the operation.
“Adding postoperative syndecan-1, even when using a clinical model that already incorporates variables from renal angina index, results in significant improvement in the capacity to predict severe AKI,” Dr. Cavalcante and colleagues concluded.
They had no financial relationships to disclose.
Acute kidney injury is a common complication after pediatric cardiac surgery, but measuring for a specific genetic protein immediately after cardiac surgery may improve cardiac surgeons’ ability to predict patients at higher risk of AKI, according to researchers from Brazil. The study results are in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152-178-86).
“Plasma syndecan-1 levels measured early in the postoperative period were independently associated with severe acute kidney injury,” wrote Candice Torres de Melo Bezerra Cavalcante, MD, of Heart Hospital of Messejana and Federal University of Ceará.
Their prospective cohort study involved 289 pediatric patients who had cardiac surgery at their institution between September 2013 and December 2014.
Dr. Cavalcante and colleagues acknowledged that the traditional biomarker for renal function, serum creatinine, only increases appreciably after the glomerular filtration rate declines 50%, impairing physicians’ ability to detect AKI early enough to treat it. “This delay can explain, in part the, negative results in AKI therapeutic clinical trials,” they wrote.
They evaluated two different endothelial biomarkers in addition to syndecan-1 with regard to their capacity for predicting severe AKI: plasma ICAM-1, a marker of endothelial cell activation; and E-selectin, an endothelial cell adhesion molecule. Syndecan-1 works as a biomarker of injury to the glycocalyx protein that surrounds endothelial cell membranes that acts as a permeability barrier and prevents the cells from adhering to blood. They found that median syndecan-1 levels soon after surgery were higher in patients with severe AKI, 103.6 vs. 42.3 ng/mL.
“Although syndecan-1 is not a renal-specific biomarker, there has been recent increasing evidence that endothelial injury has an important role in AKI pathophysiology,” the researchers noted.
Study results showed the higher the level of syndecan-1, the greater the adjusted odds ratio (OR) for severe AKI. Levels of less than 17 ng/mL were considered normal; 17.1-46.7 ng/mL carried an adjusted OR of 1.42; 47.4-93.1 ng/mL had an adjusted OR of 2.05; and levels 96.3 or greater had an OR of 8.87.
“Maintenance of endothelial glycocalyx integrity can be a therapeutic target to reduce AKI in this setting,” the researchers wrote.
The authors acknowledged that the study was done at a single center that had dialysis and death rates three and five times higher, respectively, than those of developed countries; and it measured syndecan-1 at only one time point almost immediately after the operation.
“Adding postoperative syndecan-1, even when using a clinical model that already incorporates variables from renal angina index, results in significant improvement in the capacity to predict severe AKI,” Dr. Cavalcante and colleagues concluded.
They had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: The biomarker syndecan-1 may aid in determining acute kidney injury risk for children having cardiac surgery.
Major finding: Children with elevated levels of syndecan-1 had a two- to ninefold greater risk of acute kidney injury.
Data source: Single-institution, prospective cohort study of 289 pediatric patients who had cardiac surgery from September 2013 to December 2014.
Disclosures: Dr. Cavalcante and coauthors had no financial relationships to disclose.
Prevention of postop GI disorders may reduce ‘failed discharges’ for laparoscopic hernia surgery
Postoperative hospital readmissions after ventral hernia repair may be both predictable and preventable if type of operation and patient characteristics are taken into account, a large retrospective study has found.
Early readmissions, or ‘failed discharges,’ are potentially more preventable than are later readmissions, according to Adam C. Celio, MD, of East Carolina University, Greenville, N.C., and his colleagues. Medical literature “demonstrates that patients readmitted within a few days of discharge return for different reasons than patients who are readmitted later; very early readmissions are likely due to poor coordination of care or inadequate recognition of postdischarge needs, while later readmissions (still within the 30-day window) are more likely due to patient disease or procedural complications.”
Dr. Celio and his coinvestigators examined data from the American College of Surgeons’ National Surgical Quality Improvement Program, looking for patients who underwent either open (n = 9,009) or laparoscopic (n = 3,360) ventral hernia repair (VHR) in 2012. The primary endpoint was defined as readmission within 30 days of discharge following a VHR procedure; early readmission was defined as occurring within 5 days of discharge for laparoscopic VHR and within 9 days of discharge for open VHR, while late readmission was defined as any readmission that occurred after the aforementioned time frames (Surgery. 2016;160[2]:413-17).
The researchers analyzed each readmission to determine its cause among nine classifications: bleeding, cardiovascular events, dehydration, gastrointestinal causes (nausea, emesis, ileus, and bowel obstruction), pain, venous thromboembolic event, wound occurrences (superficial site infection, deep operative site infection, wound disruptions), other infection, and other causes.
Of the 12,369 individuals included in the study, 1,057 (8.5%) were readmitted within 30 days of undergoing VHR. Generally, the patients who had open procedures had higher ASA scores than did those who had laparoscopic surgery, reflecting a greater likelihood of preoperative physical impairment and chronic disease. The researchers then analyzed which patients were mostly likely to be readmitted and the correlation between type of operation, cause of readmission, and timing of readmission (early or late).
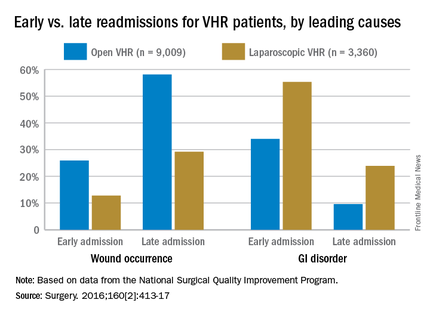
Open-VHR patients were more likely to be readmitted within 30 days than were those who underwent laparoscopic VHR: 9.2% vs. 6.9% readmission rates, respectively (OR 0.73, 95% CI 0.63-0.85). Among all the patients readmitted, wound complications were the most common cause (32.2%), followed by gastrointestinal disorders (14.3%), most which were attributed to emesis or nausea.
Wound complications were more highly associated with late readmission than with early ones, at 52% vs. 23%, respectively (OR 3.68, 95% CI 2.56-5.29). As might be expected, wound complications were more likely to occur in open-VHR patients than in laparoscopic-VHR patients: 49.6% vs. 24.4% (OR 3.05, 95% CI 2.06-4.52).
Of the entire cohort, 279 patients (2.3%) were classified as early readmissions, and gastrointestinal causes were more highly associated with early readmission than with late ones: 39% vs.13% (OR 4.45, 95% CI 3.06-6.47). Gastrointestinal issues occurred more often after laparoscopic VHR than after open VHR: 33% vs. 16% (OR 2.59, 95% CI 1.75-3.84). These early readmissions, occurring within days of discharge, are potentially preventable with a stepped up focus on control of nausea and emesis experienced by these patients, the researchers noted.
A funding source for this study was not disclosed. Dr. Celio and his coauthors did not report any relevant disclosures.
Postoperative hospital readmissions after ventral hernia repair may be both predictable and preventable if type of operation and patient characteristics are taken into account, a large retrospective study has found.
Early readmissions, or ‘failed discharges,’ are potentially more preventable than are later readmissions, according to Adam C. Celio, MD, of East Carolina University, Greenville, N.C., and his colleagues. Medical literature “demonstrates that patients readmitted within a few days of discharge return for different reasons than patients who are readmitted later; very early readmissions are likely due to poor coordination of care or inadequate recognition of postdischarge needs, while later readmissions (still within the 30-day window) are more likely due to patient disease or procedural complications.”
Dr. Celio and his coinvestigators examined data from the American College of Surgeons’ National Surgical Quality Improvement Program, looking for patients who underwent either open (n = 9,009) or laparoscopic (n = 3,360) ventral hernia repair (VHR) in 2012. The primary endpoint was defined as readmission within 30 days of discharge following a VHR procedure; early readmission was defined as occurring within 5 days of discharge for laparoscopic VHR and within 9 days of discharge for open VHR, while late readmission was defined as any readmission that occurred after the aforementioned time frames (Surgery. 2016;160[2]:413-17).
The researchers analyzed each readmission to determine its cause among nine classifications: bleeding, cardiovascular events, dehydration, gastrointestinal causes (nausea, emesis, ileus, and bowel obstruction), pain, venous thromboembolic event, wound occurrences (superficial site infection, deep operative site infection, wound disruptions), other infection, and other causes.
Of the 12,369 individuals included in the study, 1,057 (8.5%) were readmitted within 30 days of undergoing VHR. Generally, the patients who had open procedures had higher ASA scores than did those who had laparoscopic surgery, reflecting a greater likelihood of preoperative physical impairment and chronic disease. The researchers then analyzed which patients were mostly likely to be readmitted and the correlation between type of operation, cause of readmission, and timing of readmission (early or late).

Open-VHR patients were more likely to be readmitted within 30 days than were those who underwent laparoscopic VHR: 9.2% vs. 6.9% readmission rates, respectively (OR 0.73, 95% CI 0.63-0.85). Among all the patients readmitted, wound complications were the most common cause (32.2%), followed by gastrointestinal disorders (14.3%), most which were attributed to emesis or nausea.
Wound complications were more highly associated with late readmission than with early ones, at 52% vs. 23%, respectively (OR 3.68, 95% CI 2.56-5.29). As might be expected, wound complications were more likely to occur in open-VHR patients than in laparoscopic-VHR patients: 49.6% vs. 24.4% (OR 3.05, 95% CI 2.06-4.52).
Of the entire cohort, 279 patients (2.3%) were classified as early readmissions, and gastrointestinal causes were more highly associated with early readmission than with late ones: 39% vs.13% (OR 4.45, 95% CI 3.06-6.47). Gastrointestinal issues occurred more often after laparoscopic VHR than after open VHR: 33% vs. 16% (OR 2.59, 95% CI 1.75-3.84). These early readmissions, occurring within days of discharge, are potentially preventable with a stepped up focus on control of nausea and emesis experienced by these patients, the researchers noted.
A funding source for this study was not disclosed. Dr. Celio and his coauthors did not report any relevant disclosures.
Postoperative hospital readmissions after ventral hernia repair may be both predictable and preventable if type of operation and patient characteristics are taken into account, a large retrospective study has found.
Early readmissions, or ‘failed discharges,’ are potentially more preventable than are later readmissions, according to Adam C. Celio, MD, of East Carolina University, Greenville, N.C., and his colleagues. Medical literature “demonstrates that patients readmitted within a few days of discharge return for different reasons than patients who are readmitted later; very early readmissions are likely due to poor coordination of care or inadequate recognition of postdischarge needs, while later readmissions (still within the 30-day window) are more likely due to patient disease or procedural complications.”
Dr. Celio and his coinvestigators examined data from the American College of Surgeons’ National Surgical Quality Improvement Program, looking for patients who underwent either open (n = 9,009) or laparoscopic (n = 3,360) ventral hernia repair (VHR) in 2012. The primary endpoint was defined as readmission within 30 days of discharge following a VHR procedure; early readmission was defined as occurring within 5 days of discharge for laparoscopic VHR and within 9 days of discharge for open VHR, while late readmission was defined as any readmission that occurred after the aforementioned time frames (Surgery. 2016;160[2]:413-17).
The researchers analyzed each readmission to determine its cause among nine classifications: bleeding, cardiovascular events, dehydration, gastrointestinal causes (nausea, emesis, ileus, and bowel obstruction), pain, venous thromboembolic event, wound occurrences (superficial site infection, deep operative site infection, wound disruptions), other infection, and other causes.
Of the 12,369 individuals included in the study, 1,057 (8.5%) were readmitted within 30 days of undergoing VHR. Generally, the patients who had open procedures had higher ASA scores than did those who had laparoscopic surgery, reflecting a greater likelihood of preoperative physical impairment and chronic disease. The researchers then analyzed which patients were mostly likely to be readmitted and the correlation between type of operation, cause of readmission, and timing of readmission (early or late).

Open-VHR patients were more likely to be readmitted within 30 days than were those who underwent laparoscopic VHR: 9.2% vs. 6.9% readmission rates, respectively (OR 0.73, 95% CI 0.63-0.85). Among all the patients readmitted, wound complications were the most common cause (32.2%), followed by gastrointestinal disorders (14.3%), most which were attributed to emesis or nausea.
Wound complications were more highly associated with late readmission than with early ones, at 52% vs. 23%, respectively (OR 3.68, 95% CI 2.56-5.29). As might be expected, wound complications were more likely to occur in open-VHR patients than in laparoscopic-VHR patients: 49.6% vs. 24.4% (OR 3.05, 95% CI 2.06-4.52).
Of the entire cohort, 279 patients (2.3%) were classified as early readmissions, and gastrointestinal causes were more highly associated with early readmission than with late ones: 39% vs.13% (OR 4.45, 95% CI 3.06-6.47). Gastrointestinal issues occurred more often after laparoscopic VHR than after open VHR: 33% vs. 16% (OR 2.59, 95% CI 1.75-3.84). These early readmissions, occurring within days of discharge, are potentially preventable with a stepped up focus on control of nausea and emesis experienced by these patients, the researchers noted.
A funding source for this study was not disclosed. Dr. Celio and his coauthors did not report any relevant disclosures.
FROM SURGERY
Key clinical point: Postoperative complications and likelihood of readmission differ according to the type of ventral hernia repair procedure (open or laparoscopic).
Major finding: Thirty-day readmissions occurred among 6.9% of patients who had laparoscopic surgery, compared with 9.2% of open-procedure patients; gastrointestinal disorders were more common in early readmissions but wound occurrences were more common in late readmissions.
Data source: Retrospective cohort study of 12,369 ventral hernia repair patients (open and laparoscopic) in 2012.
Disclosures: Funding source not disclosed. Authors did not report any relevant financial disclosures.
FDA advisory panel unanimously backs biosimilars for Humira, Enbrel
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.
On Twitter @mitchelzoler
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.
On Twitter @mitchelzoler
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.
On Twitter @mitchelzoler
CMS: Projected overall growth rate in health spending holding firm
Health spending is projected to grow on average 5.8% from 2015-2025, the same projected rate of grown as announced last year covering 2014-2024, according the CMS Office of the Actuary.
However, health care is projected to make up 20.1% of the economy at the end of the 10-year period, up from 17.5% in 2014, as health spending is projected to grow 1.3 percentage points faster than gross domestic product from 2015-2025. The analysis was published online July 13 in the journal Health Affairs.
Health spending for 2015 is projected to have grown 5.5%, up from 5.3% in 2014, and to have reached $3.2 trillion, driven in part by increased use of health services by newly insured patients. More than 9 in 10 (92%) of U.S. residents are projected to be insured by 2025, according to the actuary’s office.
While national spending per capita is projected to exceed $10,000 for the first time in 2016, spending growth is projected to slow to 4.8%, driven by slowdowns in Medicaid spending after 2 years of rapid growth.
Private health insurance expenditures are expected to grow at a similar rate (5.4%) through 2025, CMS actuaries said.
Medical price inflation slowed to 0.8% in 2015 from 1.4% in 2014. Hospital prices increased by 0.9% while prices for physician services dropped 1.1%, they noted.
Prescription drug spending is projected to grow an average of 6.7% from 2016 to 2025, slowing down from 12.2% in 2014 and 8.1% in 2015 when a number of high-priced specialty drugs, including those treating hepatitis C, were driving higher spending.
Health spending is projected to grow on average 5.8% from 2015-2025, the same projected rate of grown as announced last year covering 2014-2024, according the CMS Office of the Actuary.
However, health care is projected to make up 20.1% of the economy at the end of the 10-year period, up from 17.5% in 2014, as health spending is projected to grow 1.3 percentage points faster than gross domestic product from 2015-2025. The analysis was published online July 13 in the journal Health Affairs.
Health spending for 2015 is projected to have grown 5.5%, up from 5.3% in 2014, and to have reached $3.2 trillion, driven in part by increased use of health services by newly insured patients. More than 9 in 10 (92%) of U.S. residents are projected to be insured by 2025, according to the actuary’s office.
While national spending per capita is projected to exceed $10,000 for the first time in 2016, spending growth is projected to slow to 4.8%, driven by slowdowns in Medicaid spending after 2 years of rapid growth.
Private health insurance expenditures are expected to grow at a similar rate (5.4%) through 2025, CMS actuaries said.
Medical price inflation slowed to 0.8% in 2015 from 1.4% in 2014. Hospital prices increased by 0.9% while prices for physician services dropped 1.1%, they noted.
Prescription drug spending is projected to grow an average of 6.7% from 2016 to 2025, slowing down from 12.2% in 2014 and 8.1% in 2015 when a number of high-priced specialty drugs, including those treating hepatitis C, were driving higher spending.
Health spending is projected to grow on average 5.8% from 2015-2025, the same projected rate of grown as announced last year covering 2014-2024, according the CMS Office of the Actuary.
However, health care is projected to make up 20.1% of the economy at the end of the 10-year period, up from 17.5% in 2014, as health spending is projected to grow 1.3 percentage points faster than gross domestic product from 2015-2025. The analysis was published online July 13 in the journal Health Affairs.
Health spending for 2015 is projected to have grown 5.5%, up from 5.3% in 2014, and to have reached $3.2 trillion, driven in part by increased use of health services by newly insured patients. More than 9 in 10 (92%) of U.S. residents are projected to be insured by 2025, according to the actuary’s office.
While national spending per capita is projected to exceed $10,000 for the first time in 2016, spending growth is projected to slow to 4.8%, driven by slowdowns in Medicaid spending after 2 years of rapid growth.
Private health insurance expenditures are expected to grow at a similar rate (5.4%) through 2025, CMS actuaries said.
Medical price inflation slowed to 0.8% in 2015 from 1.4% in 2014. Hospital prices increased by 0.9% while prices for physician services dropped 1.1%, they noted.
Prescription drug spending is projected to grow an average of 6.7% from 2016 to 2025, slowing down from 12.2% in 2014 and 8.1% in 2015 when a number of high-priced specialty drugs, including those treating hepatitis C, were driving higher spending.
FROM HEALTH AFFAIRS
CMS to Congress: We might delay MACRA start
Implementation of some MACRA provisions could be delayed, CMS Acting Administrator Andy Slavitt told committee members at a July 13 hearing of the Senate Finance Committee.
Officials at the Centers for Medicare & Medicaid Services are considering “alternative start dates, looking at whether shorter periods could be used, and finding other ways for physicians to get experience with the program before the impact of it really hits them,” Mr. Slavitt testified.
Many of the comments that were submitted by physicians and other stakeholders on the proposed MACRA rule called for delayed implementation, Mr. Slavitt said. Other key themes included ensuring that patients are the focus, simplifying the rules, creating more pathways to advanced payment models, and providing a greater consideration of the needs of small and solo practices, particularly rural ones.
“We need to launch this program so that it begins on the right foot. That means that every physician in the country needs to feel like they are set up for success,” Mr. Slavitt said. He added that CMS may release an interim final rule to allow for more comment on changes stemming from the feedback received on the initial proposal.
Many Senators on the committee asked Mr. Slavitt how the proposed regulations would impact solo and small practices, especially ones in rural areas.
“The focus on small independent practices and their ability to continue to practice independently is a very high priority for us,” Mr. Slavitt said. “I would add that it’s not just small practices, it’s all also any physician that practices in a rural location. They have a very different set of dynamics than other physicians do. ... We need every physician set up for success and the challenges in small practices are far greater.”
He noted that for many small or solo practices, even a small amount of additional paperwork could affect how much time the physician can spend with patients.
Another area of concern in the MACRA regulation comments was the delay in the development of virtual groups that would allow physicians to virtually pool reporting requirements to allow for greater participation in value-based programs. The proposed MACRA rule calls for these groups to be delayed for 1 year .
“I think this going to be a high priority for us, and I think it’s going to be something that is going to need a lot more input from physicians to make sure we get it right,” Mr. Slavitt said. Virtual groups are “a whole new way of reporting, and we need to make a number of decisions, and physicians would need to make a number of decisions and they are not yet used to practicing that way.”
He added that CMS is asking physicians how they want to go about it, and the feedback so far is promising that physicians want to participate, but there needs to be operational and technology infrastructure to make it happen.
“I don’t think this is something that can’t be solved with just a little bit more time, but it’s certainly not something that’s ready to be launched in months,” he said, adding that the agency aims to launch virtual group reporting in the second year of the MACRA implementation.
Implementation of some MACRA provisions could be delayed, CMS Acting Administrator Andy Slavitt told committee members at a July 13 hearing of the Senate Finance Committee.
Officials at the Centers for Medicare & Medicaid Services are considering “alternative start dates, looking at whether shorter periods could be used, and finding other ways for physicians to get experience with the program before the impact of it really hits them,” Mr. Slavitt testified.
Many of the comments that were submitted by physicians and other stakeholders on the proposed MACRA rule called for delayed implementation, Mr. Slavitt said. Other key themes included ensuring that patients are the focus, simplifying the rules, creating more pathways to advanced payment models, and providing a greater consideration of the needs of small and solo practices, particularly rural ones.
“We need to launch this program so that it begins on the right foot. That means that every physician in the country needs to feel like they are set up for success,” Mr. Slavitt said. He added that CMS may release an interim final rule to allow for more comment on changes stemming from the feedback received on the initial proposal.
Many Senators on the committee asked Mr. Slavitt how the proposed regulations would impact solo and small practices, especially ones in rural areas.
“The focus on small independent practices and their ability to continue to practice independently is a very high priority for us,” Mr. Slavitt said. “I would add that it’s not just small practices, it’s all also any physician that practices in a rural location. They have a very different set of dynamics than other physicians do. ... We need every physician set up for success and the challenges in small practices are far greater.”
He noted that for many small or solo practices, even a small amount of additional paperwork could affect how much time the physician can spend with patients.
Another area of concern in the MACRA regulation comments was the delay in the development of virtual groups that would allow physicians to virtually pool reporting requirements to allow for greater participation in value-based programs. The proposed MACRA rule calls for these groups to be delayed for 1 year .
“I think this going to be a high priority for us, and I think it’s going to be something that is going to need a lot more input from physicians to make sure we get it right,” Mr. Slavitt said. Virtual groups are “a whole new way of reporting, and we need to make a number of decisions, and physicians would need to make a number of decisions and they are not yet used to practicing that way.”
He added that CMS is asking physicians how they want to go about it, and the feedback so far is promising that physicians want to participate, but there needs to be operational and technology infrastructure to make it happen.
“I don’t think this is something that can’t be solved with just a little bit more time, but it’s certainly not something that’s ready to be launched in months,” he said, adding that the agency aims to launch virtual group reporting in the second year of the MACRA implementation.
Implementation of some MACRA provisions could be delayed, CMS Acting Administrator Andy Slavitt told committee members at a July 13 hearing of the Senate Finance Committee.
Officials at the Centers for Medicare & Medicaid Services are considering “alternative start dates, looking at whether shorter periods could be used, and finding other ways for physicians to get experience with the program before the impact of it really hits them,” Mr. Slavitt testified.
Many of the comments that were submitted by physicians and other stakeholders on the proposed MACRA rule called for delayed implementation, Mr. Slavitt said. Other key themes included ensuring that patients are the focus, simplifying the rules, creating more pathways to advanced payment models, and providing a greater consideration of the needs of small and solo practices, particularly rural ones.
“We need to launch this program so that it begins on the right foot. That means that every physician in the country needs to feel like they are set up for success,” Mr. Slavitt said. He added that CMS may release an interim final rule to allow for more comment on changes stemming from the feedback received on the initial proposal.
Many Senators on the committee asked Mr. Slavitt how the proposed regulations would impact solo and small practices, especially ones in rural areas.
“The focus on small independent practices and their ability to continue to practice independently is a very high priority for us,” Mr. Slavitt said. “I would add that it’s not just small practices, it’s all also any physician that practices in a rural location. They have a very different set of dynamics than other physicians do. ... We need every physician set up for success and the challenges in small practices are far greater.”
He noted that for many small or solo practices, even a small amount of additional paperwork could affect how much time the physician can spend with patients.
Another area of concern in the MACRA regulation comments was the delay in the development of virtual groups that would allow physicians to virtually pool reporting requirements to allow for greater participation in value-based programs. The proposed MACRA rule calls for these groups to be delayed for 1 year .
“I think this going to be a high priority for us, and I think it’s going to be something that is going to need a lot more input from physicians to make sure we get it right,” Mr. Slavitt said. Virtual groups are “a whole new way of reporting, and we need to make a number of decisions, and physicians would need to make a number of decisions and they are not yet used to practicing that way.”
He added that CMS is asking physicians how they want to go about it, and the feedback so far is promising that physicians want to participate, but there needs to be operational and technology infrastructure to make it happen.
“I don’t think this is something that can’t be solved with just a little bit more time, but it’s certainly not something that’s ready to be launched in months,” he said, adding that the agency aims to launch virtual group reporting in the second year of the MACRA implementation.
FROM A SENATE FINANCE COMMITTEE HEARING
HHS issues guidance on ransomware attacks
The U.S. Department of Health & Human Services’ Office of Civil Rights has issued new guidance to help physicians and their practices combat a ransomware attack.
Ransomware – a type of malicious software designed to block access to a computer system until a sum of money is paid – is becoming a bigger problem for U.S. businesses in general. Daily ransomware attacks against all types of computer systems increased 300% in early 2016 to 4,000, from 1,000 daily attacks in 2015, according to the Department of Justice.
The HHS fact sheet offers information on how HIPAA compliance can help protect and recover infected systems; how to detect if systems are infected; and what to do if a system becomes infected, including what is reportable.
There are “measures known to be effective to prevent the introduction of ransomware and to recover from a ransomware attack,” according to HHS.
The U.S. Department of Health & Human Services’ Office of Civil Rights has issued new guidance to help physicians and their practices combat a ransomware attack.
Ransomware – a type of malicious software designed to block access to a computer system until a sum of money is paid – is becoming a bigger problem for U.S. businesses in general. Daily ransomware attacks against all types of computer systems increased 300% in early 2016 to 4,000, from 1,000 daily attacks in 2015, according to the Department of Justice.
The HHS fact sheet offers information on how HIPAA compliance can help protect and recover infected systems; how to detect if systems are infected; and what to do if a system becomes infected, including what is reportable.
There are “measures known to be effective to prevent the introduction of ransomware and to recover from a ransomware attack,” according to HHS.
The U.S. Department of Health & Human Services’ Office of Civil Rights has issued new guidance to help physicians and their practices combat a ransomware attack.
Ransomware – a type of malicious software designed to block access to a computer system until a sum of money is paid – is becoming a bigger problem for U.S. businesses in general. Daily ransomware attacks against all types of computer systems increased 300% in early 2016 to 4,000, from 1,000 daily attacks in 2015, according to the Department of Justice.
The HHS fact sheet offers information on how HIPAA compliance can help protect and recover infected systems; how to detect if systems are infected; and what to do if a system becomes infected, including what is reportable.
There are “measures known to be effective to prevent the introduction of ransomware and to recover from a ransomware attack,” according to HHS.