User login
News and Views that Matter to Physicians
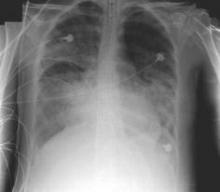
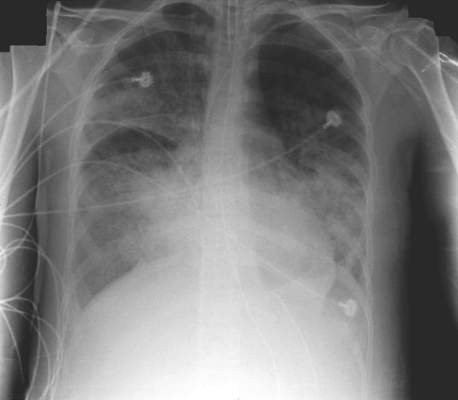
Diffuse alveolar damage ups death risk in acute respiratory distress syndrome
Among patients with acute respiratory distress syndrome (ARDS), those who are also diagnosed with diffuse alveolar damage (DAD) via an open lung biopsy face nearly twice as high a mortality risk as did those without DAD, according to a systematic review and a meta-analysis by Dr. Pablo Cardinal-Fernandez and his colleagues.
The results of this and other studies “support the hypothesis that ARDS with DAD is a specific clinicopathological entity different from ARDS without DAD,” said Dr. Cardinal-Fernandez of the department of genetic medicine, Cornell University, New York, and his colleagues (CHEST 2016 149:1155-64.).
“Our meta-analysis underscores the need for less-invasive approaches to individualize therapy for patients with ARDS, including the development of biomarkers for predicting responses to treatments.”
The researchers analyzed studies identified using MEDLINE, EMBASE, Cochrane Register of Controlled Trials, LILACS, and citation review from Jan. 1, 1967, to Sept. 1, 2015. Eight studies involving 350 patients satisfied the researchers’ criteria for inclusion in the review. All of such studies included patients who received an open lung biopsy after being diagnosed with ARDS, histologic results indicating the presence or absence of DAD based on the open lung biopsy, and the mortalities of both a group of patients diagnosed with DAD and a group of patients not diagnosed with DAD. Studies were excluded from the review if they included fewer than five participants.
The pooled odds ratio for mortality in patients who had ARDS with DAD, compared with patients with ARDs who did not have DAD was 1.81.
At the time of ARDS diagnosis, the meta-differences for sequential organ failure assessment scores and the index of hypoxemia (PaO2/FiO2) ratio between the patients who had DAD and those who did not were 0.26 and 4.36, respectively. On the day of open lung biopsy, the meta-differences for the sequential organ failure assessment score and the PaO2/FiO2 ratio between the two patient groups were also small; the meta-difference for sequential organ failure was 0.45 and the meta-difference for the PaO2/FiO2 ratio was –8.63.
The higher mortality risk in the group of patients with DAD – despite patients in both groups having similar severities of illness – suggests that “the presence of DAD confers additional prognostic information,” according to the researchers.
“The mortality heterogeneity of this meta-analysis was low, suggesting that no other variables affect the results (that is, the observed effect depends mainly on the presence of DAD). Conditions that were identified in patients without DAD included pneumonia, pulmonary embolism, edema, or no pathologic abnormality. It appears that these subsets of patients have a different and better prognosis than did patients with ARDS and DAD, perhaps as a result of favorable responses to specific treatments, with the possible exception of lower tidal volume, which appears to be beneficial in all subgroups,” the researchers said.
No financial or nonfinancial disclosures were declared.
Among patients with acute respiratory distress syndrome (ARDS), those who are also diagnosed with diffuse alveolar damage (DAD) via an open lung biopsy face nearly twice as high a mortality risk as did those without DAD, according to a systematic review and a meta-analysis by Dr. Pablo Cardinal-Fernandez and his colleagues.
The results of this and other studies “support the hypothesis that ARDS with DAD is a specific clinicopathological entity different from ARDS without DAD,” said Dr. Cardinal-Fernandez of the department of genetic medicine, Cornell University, New York, and his colleagues (CHEST 2016 149:1155-64.).
“Our meta-analysis underscores the need for less-invasive approaches to individualize therapy for patients with ARDS, including the development of biomarkers for predicting responses to treatments.”
The researchers analyzed studies identified using MEDLINE, EMBASE, Cochrane Register of Controlled Trials, LILACS, and citation review from Jan. 1, 1967, to Sept. 1, 2015. Eight studies involving 350 patients satisfied the researchers’ criteria for inclusion in the review. All of such studies included patients who received an open lung biopsy after being diagnosed with ARDS, histologic results indicating the presence or absence of DAD based on the open lung biopsy, and the mortalities of both a group of patients diagnosed with DAD and a group of patients not diagnosed with DAD. Studies were excluded from the review if they included fewer than five participants.
The pooled odds ratio for mortality in patients who had ARDS with DAD, compared with patients with ARDs who did not have DAD was 1.81.
At the time of ARDS diagnosis, the meta-differences for sequential organ failure assessment scores and the index of hypoxemia (PaO2/FiO2) ratio between the patients who had DAD and those who did not were 0.26 and 4.36, respectively. On the day of open lung biopsy, the meta-differences for the sequential organ failure assessment score and the PaO2/FiO2 ratio between the two patient groups were also small; the meta-difference for sequential organ failure was 0.45 and the meta-difference for the PaO2/FiO2 ratio was –8.63.
The higher mortality risk in the group of patients with DAD – despite patients in both groups having similar severities of illness – suggests that “the presence of DAD confers additional prognostic information,” according to the researchers.
“The mortality heterogeneity of this meta-analysis was low, suggesting that no other variables affect the results (that is, the observed effect depends mainly on the presence of DAD). Conditions that were identified in patients without DAD included pneumonia, pulmonary embolism, edema, or no pathologic abnormality. It appears that these subsets of patients have a different and better prognosis than did patients with ARDS and DAD, perhaps as a result of favorable responses to specific treatments, with the possible exception of lower tidal volume, which appears to be beneficial in all subgroups,” the researchers said.
No financial or nonfinancial disclosures were declared.
Among patients with acute respiratory distress syndrome (ARDS), those who are also diagnosed with diffuse alveolar damage (DAD) via an open lung biopsy face nearly twice as high a mortality risk as did those without DAD, according to a systematic review and a meta-analysis by Dr. Pablo Cardinal-Fernandez and his colleagues.
The results of this and other studies “support the hypothesis that ARDS with DAD is a specific clinicopathological entity different from ARDS without DAD,” said Dr. Cardinal-Fernandez of the department of genetic medicine, Cornell University, New York, and his colleagues (CHEST 2016 149:1155-64.).
“Our meta-analysis underscores the need for less-invasive approaches to individualize therapy for patients with ARDS, including the development of biomarkers for predicting responses to treatments.”
The researchers analyzed studies identified using MEDLINE, EMBASE, Cochrane Register of Controlled Trials, LILACS, and citation review from Jan. 1, 1967, to Sept. 1, 2015. Eight studies involving 350 patients satisfied the researchers’ criteria for inclusion in the review. All of such studies included patients who received an open lung biopsy after being diagnosed with ARDS, histologic results indicating the presence or absence of DAD based on the open lung biopsy, and the mortalities of both a group of patients diagnosed with DAD and a group of patients not diagnosed with DAD. Studies were excluded from the review if they included fewer than five participants.
The pooled odds ratio for mortality in patients who had ARDS with DAD, compared with patients with ARDs who did not have DAD was 1.81.
At the time of ARDS diagnosis, the meta-differences for sequential organ failure assessment scores and the index of hypoxemia (PaO2/FiO2) ratio between the patients who had DAD and those who did not were 0.26 and 4.36, respectively. On the day of open lung biopsy, the meta-differences for the sequential organ failure assessment score and the PaO2/FiO2 ratio between the two patient groups were also small; the meta-difference for sequential organ failure was 0.45 and the meta-difference for the PaO2/FiO2 ratio was –8.63.
The higher mortality risk in the group of patients with DAD – despite patients in both groups having similar severities of illness – suggests that “the presence of DAD confers additional prognostic information,” according to the researchers.
“The mortality heterogeneity of this meta-analysis was low, suggesting that no other variables affect the results (that is, the observed effect depends mainly on the presence of DAD). Conditions that were identified in patients without DAD included pneumonia, pulmonary embolism, edema, or no pathologic abnormality. It appears that these subsets of patients have a different and better prognosis than did patients with ARDS and DAD, perhaps as a result of favorable responses to specific treatments, with the possible exception of lower tidal volume, which appears to be beneficial in all subgroups,” the researchers said.
No financial or nonfinancial disclosures were declared.
FROM CHEST
Key clinical point: Among patients with ARDS, those who are also diagnosed with DAD faced nearly twice as high a mortality risk as did those without DAD.
Major finding: The pooled odds ratio for mortality in patients who had acute respiratory distress syndrome with diffuse alveolar damage, compared with patients with ARDs who did not have DAD was 1.81.
Data source: A systematic review and meta-analysis of eight studies, including 350 patients, published between 1967 and 2015.
Disclosures: No financial or nonfinancial disclosures were declared.
Recurrent C. diff infection much more costly to treat
The costs of treating patients with recurrent Clostridium difficile infection are approximately two to three times the treatment costs for patients with primary CDI, according to a study of hospitalized patients in a large tertiary care center.
Kevin W. Garey, Pharm.D., professor of pharmacy practice at the University of Houston College of Pharmacy, and his coauthors wrote in the Journal of Hospital Infection that the introduction of costly therapies, such as the narrow-spectrum macrocyclic antibiotic fidaxomicin and fecal microbiota transplantation, both of which reduce the risk of recurrent CDI, has increased interest in the economic burden of recurrent CDI (J Hosp Infect. 2016 Jul;93:286-9).
Between 2007-2013, Dr. Garey and his colleagues attempted to assess the additional costs of recurrent CDI by calculating total hospital length of stay (LOS), CDI-attributable LOS, and pharmacologic and hospitalization costs for 540 hospitalized adult patients (42% male) with a diagnosis of primary CDI. Of these patients, 95 (18%) experienced 101 recurrent CDI episodes. CDI-attributable hospital admissions occurred in 307 out of 540 (57%) primary CDI episodes, and 64 out of 101 (63%) recurrent CDI episodes.
The investigators estimated total and CDI-attributable hospitalization costs based on Healthcare Cost and Utilization Project data for average daily health care costs, multiplied by the total and CDI-attributable length of hospital stay. They then compared total hospital LOS, CDI-attributable LOS, and pharmacologic and hospitalization costs between patients with primary CDI only versus patients who experienced recurrent CDI.
Dr. Garey and his colleagues found that CDI-attributable median LOS and costs increased from 7 days and $13,168 for patients with primary CDI only, vs .15 days and $28,218 for patients with recurrent CDI (P less than .0001, each). Total hospital median LOS and costs increased from 11 days and $20,693 for patients with primary CDI only, vs. 24 days and $45,148 for patients with recurrent CDI (P less than .0001, each).
The median cost of pharmacologic treatment while hospitalized was $60 for patients with primary CDI only, and $140 for patients with recurrent CDI (P = .0013).
“Patients with CDI experience a significant health care economic burden that can be directly related to CDI,” the authors concluded.
The study was funded by a research grant from Merck. Dr. Garey reported receiving funding from Cubist, Summit, and Merck, while other coauthors reported research support from Merck, Cubist, and Actelion.
On Twitter @richpizzi
The costs of treating patients with recurrent Clostridium difficile infection are approximately two to three times the treatment costs for patients with primary CDI, according to a study of hospitalized patients in a large tertiary care center.
Kevin W. Garey, Pharm.D., professor of pharmacy practice at the University of Houston College of Pharmacy, and his coauthors wrote in the Journal of Hospital Infection that the introduction of costly therapies, such as the narrow-spectrum macrocyclic antibiotic fidaxomicin and fecal microbiota transplantation, both of which reduce the risk of recurrent CDI, has increased interest in the economic burden of recurrent CDI (J Hosp Infect. 2016 Jul;93:286-9).
Between 2007-2013, Dr. Garey and his colleagues attempted to assess the additional costs of recurrent CDI by calculating total hospital length of stay (LOS), CDI-attributable LOS, and pharmacologic and hospitalization costs for 540 hospitalized adult patients (42% male) with a diagnosis of primary CDI. Of these patients, 95 (18%) experienced 101 recurrent CDI episodes. CDI-attributable hospital admissions occurred in 307 out of 540 (57%) primary CDI episodes, and 64 out of 101 (63%) recurrent CDI episodes.
The investigators estimated total and CDI-attributable hospitalization costs based on Healthcare Cost and Utilization Project data for average daily health care costs, multiplied by the total and CDI-attributable length of hospital stay. They then compared total hospital LOS, CDI-attributable LOS, and pharmacologic and hospitalization costs between patients with primary CDI only versus patients who experienced recurrent CDI.
Dr. Garey and his colleagues found that CDI-attributable median LOS and costs increased from 7 days and $13,168 for patients with primary CDI only, vs .15 days and $28,218 for patients with recurrent CDI (P less than .0001, each). Total hospital median LOS and costs increased from 11 days and $20,693 for patients with primary CDI only, vs. 24 days and $45,148 for patients with recurrent CDI (P less than .0001, each).
The median cost of pharmacologic treatment while hospitalized was $60 for patients with primary CDI only, and $140 for patients with recurrent CDI (P = .0013).
“Patients with CDI experience a significant health care economic burden that can be directly related to CDI,” the authors concluded.
The study was funded by a research grant from Merck. Dr. Garey reported receiving funding from Cubist, Summit, and Merck, while other coauthors reported research support from Merck, Cubist, and Actelion.
On Twitter @richpizzi
The costs of treating patients with recurrent Clostridium difficile infection are approximately two to three times the treatment costs for patients with primary CDI, according to a study of hospitalized patients in a large tertiary care center.
Kevin W. Garey, Pharm.D., professor of pharmacy practice at the University of Houston College of Pharmacy, and his coauthors wrote in the Journal of Hospital Infection that the introduction of costly therapies, such as the narrow-spectrum macrocyclic antibiotic fidaxomicin and fecal microbiota transplantation, both of which reduce the risk of recurrent CDI, has increased interest in the economic burden of recurrent CDI (J Hosp Infect. 2016 Jul;93:286-9).
Between 2007-2013, Dr. Garey and his colleagues attempted to assess the additional costs of recurrent CDI by calculating total hospital length of stay (LOS), CDI-attributable LOS, and pharmacologic and hospitalization costs for 540 hospitalized adult patients (42% male) with a diagnosis of primary CDI. Of these patients, 95 (18%) experienced 101 recurrent CDI episodes. CDI-attributable hospital admissions occurred in 307 out of 540 (57%) primary CDI episodes, and 64 out of 101 (63%) recurrent CDI episodes.
The investigators estimated total and CDI-attributable hospitalization costs based on Healthcare Cost and Utilization Project data for average daily health care costs, multiplied by the total and CDI-attributable length of hospital stay. They then compared total hospital LOS, CDI-attributable LOS, and pharmacologic and hospitalization costs between patients with primary CDI only versus patients who experienced recurrent CDI.
Dr. Garey and his colleagues found that CDI-attributable median LOS and costs increased from 7 days and $13,168 for patients with primary CDI only, vs .15 days and $28,218 for patients with recurrent CDI (P less than .0001, each). Total hospital median LOS and costs increased from 11 days and $20,693 for patients with primary CDI only, vs. 24 days and $45,148 for patients with recurrent CDI (P less than .0001, each).
The median cost of pharmacologic treatment while hospitalized was $60 for patients with primary CDI only, and $140 for patients with recurrent CDI (P = .0013).
“Patients with CDI experience a significant health care economic burden that can be directly related to CDI,” the authors concluded.
The study was funded by a research grant from Merck. Dr. Garey reported receiving funding from Cubist, Summit, and Merck, while other coauthors reported research support from Merck, Cubist, and Actelion.
On Twitter @richpizzi
FROM THE JOURNAL OF HOSPITAL INFECTION
Key clinical point: The economic cost burden increased significantly for patients with recurrent C. difficile infections, compared with patients with primary CDI.
Major finding: Median hospital length of stay and costs attributable to C. difficile infections increased from 7 days and $13,168 for patients with primary CDI only, vs. 15 days and $28,218 for patients with recurrent CDI.
Data source: A prospective, observational cohort study of 540 hospitalized adult patients with primary CDI followed for 3 months to assess for recurrent CDI episodes.
Disclosures: The study was funded by a research grant from Merck. Dr. Garey reported receiving funding from Cubist, Summit, and Merck, while other coauthors reported research support from Merck, Cubist, and Actelion.
Enhanced recovery protocol speeds discharge, decreases readmissions for ventral hernia repair
A postsurgical recovery program featuring early feeding and multimodal pain management hastened the return of bowel function and shortened hospital stay by 2 days for patients undergoing complex ventral hernia repair.
Despite leaving the hospital sooner, however, patients were 75% less likely to be readmitted within 90 days, Dr. Arnab Majumder and his colleagues wrote in the June issue of the Journal of the American College of Surgeons (2016 Jun;222:1106-15). Most of those who did return had wound complications, a stark contrast to readmissions among patients who didn’t experience the enhanced recovery program. Among that group, 75% of the readmissions were caused by bowel obstruction/ileus, deep venous thrombosis or pulmonary embolism, pneumonia, and urinary tract infections – all of them “problems [that] could be related to prolonged hospitalizations,” said Dr. Majumder of the University Hospitals Case Medical Center, Cleveland.
The investigators created an Enhanced Recovery After Surgery (ERAS) pathway specifically for patients undergoing complex ventral hernia repairs using transversus abdominis release and sublay synthetic mesh placement. The patients “present formidable challenges to the surgeon, not only in the operating room but also during perioperative management,” the authors noted.
The ERAS the team preemptively addressed patient issues in the preoperative, intra- and perioperative, and postoperative periods. They compared the outcomes of 100 patients treated with the protocol to those in a control group of 100 who underwent the same surgery before the protocol was implemented. The main outcomes measures were time to diet advancement, time to return of bowel function, time to oral narcotics, length of stay, and 90-day readmissions.
The ERAS begins with preoperative optimization. This consists of weight loss, smoking cessation, and managing diabetes and obstructive sleep apnea. No surgery occurs until the HbA1c is less than 8 and patients have been tobacco free for at least 1 month.
All patients receive an arginine and omega-3 supplement thrice daily for 5 days before surgery. The night before surgery, they have a nasal swab screen for methicillin-resistant Staphylococcus aureus (MRSA), and decolonization with mupirocin ointment and a chlorhexidine bath.
Preoperatively, they receive 5,000 units of unfractionated heparin, along with sequential compression devices for deep vein thrombosis protection. Use of both continue postoperatively, with heparin given every 8 hours until the patient is ambulatory.
Those without a history of narcotic use receive a preoperative dose of alvimopan. Everyone also receives oral gabapentin and preoperative antibiotics.
Intraoperatively, intravenous fluids are given judiciously to minimize bowel edema and decrease the risk of respiratory side effects. The surgeon also employs a transversus abdominis plane (TAP) block with liposomal bupivacaine.
There is a multimodal pain management program, which includes intravenous hydromorphone by patient-controlled anesthesia pump and oral gabapentin three times daily. Patients switch to oral analgesics on postoperative day 2. These consist of acetaminophen and NSAIDs on an alternating schedule. Intravenous diazepam can be added as an antispasmodic. Alvimopan is continued twice daily until first bowel movement or hospital discharge.
Clear liquids are limited to 250 mL/8 hours on postoperative day 1, along with a clear protein drink. By day 2, patients are advanced to unrestricted volumes of clear liquids, and on day 3, to a regular solid diet. At that point, the clear protein drink is switched for a regular protein drink (Boost Plus).
Patients who vomit or require a nasogastric tube for decompression, and those with severe persistent nausea are made NPO, but can resume diet when these symptoms improve. Those with mild-moderate nausea receive intravenous ondansetron and are allowed to self-limit oral intake.
Patients were a mean of 57 years old with a mean body mass index of about 33 kg/m2. The hernia was recurrent for about 60% of each group, with a mean area of about 300 cm2, and a mean width of 14 cm. The mean mesh size was about 1,000 cm2. The mean operative time was significantly shorter in the control group: 197 vs. 245 minutes.
Diet was advanced significantly more quickly in the ERAS group than the control group: 1 vs. 2 days on a liquid diet and 3 vs. 4.8 days to regular diet. Emesis after diet advancement was similar (4% for ERAS and 5% for control).
Compared with the control group, the ERAS group experienced significantly shorter average times to flatus (3.1 vs. 3.9 days), bowel movement (3.6 vs. 5.2 days), and reaching GI-3 status (3.4 vs. 4.8 days). Those in the ERAS group switched to oral narcotics sooner (2.2 vs. 3.6 days) also.
The average length of stay was significantly lower in ERAS group as well (4 vs. 6 days). About 18% of those in the ERAS group had a stay of less than 3 days, compared with 2% of the control group.
Readmissions within 90 days occurred in 16% of the control group and 4% of the ERAS group – a significant difference. There were four surgical site complications in the control group and three in the ERAS group. Bowel obstruction occurred in three control patients and one ERAS patient. All other complications requiring readmission occurred in the control group: two pulmonary embolisms, two deep vein thromboses, one pneumonia, one urinary tract infection and three other unspecified causes.
The authors noted that the shift to multimodal pain management and shorter-term use of IV opiates is a large contributor to the protocol’s good bowel outcomes. “Introduction of preoperative and postoperative gabapentin, intraoperative surgeon-delivered TAP block with long-acting liposomal bupivacaine, postoperative use of acetaminophen, and nonsteroidal agents have all appeared to contribute to better pain control and likely decrease in opioid consumption,” they said.
The use of diazepam as a pain medication is unusual, they said, but effective.
“We believe a large component of postoperative pain in hernia patients is due to muscle spasms after myofascial release, irritation from mesh placement, and transabdominal suture fixation. Therefore, in the context of our frequent use of myofascial releases for large incisional hernias, we believe the antispasmodic effects of diazepam potentially alleviate some of the postoperative discomfort caused by major abdominal wall reconstruction.”
None of the investigators reported financial conflicts.
A postsurgical recovery program featuring early feeding and multimodal pain management hastened the return of bowel function and shortened hospital stay by 2 days for patients undergoing complex ventral hernia repair.
Despite leaving the hospital sooner, however, patients were 75% less likely to be readmitted within 90 days, Dr. Arnab Majumder and his colleagues wrote in the June issue of the Journal of the American College of Surgeons (2016 Jun;222:1106-15). Most of those who did return had wound complications, a stark contrast to readmissions among patients who didn’t experience the enhanced recovery program. Among that group, 75% of the readmissions were caused by bowel obstruction/ileus, deep venous thrombosis or pulmonary embolism, pneumonia, and urinary tract infections – all of them “problems [that] could be related to prolonged hospitalizations,” said Dr. Majumder of the University Hospitals Case Medical Center, Cleveland.
The investigators created an Enhanced Recovery After Surgery (ERAS) pathway specifically for patients undergoing complex ventral hernia repairs using transversus abdominis release and sublay synthetic mesh placement. The patients “present formidable challenges to the surgeon, not only in the operating room but also during perioperative management,” the authors noted.
The ERAS the team preemptively addressed patient issues in the preoperative, intra- and perioperative, and postoperative periods. They compared the outcomes of 100 patients treated with the protocol to those in a control group of 100 who underwent the same surgery before the protocol was implemented. The main outcomes measures were time to diet advancement, time to return of bowel function, time to oral narcotics, length of stay, and 90-day readmissions.
The ERAS begins with preoperative optimization. This consists of weight loss, smoking cessation, and managing diabetes and obstructive sleep apnea. No surgery occurs until the HbA1c is less than 8 and patients have been tobacco free for at least 1 month.
All patients receive an arginine and omega-3 supplement thrice daily for 5 days before surgery. The night before surgery, they have a nasal swab screen for methicillin-resistant Staphylococcus aureus (MRSA), and decolonization with mupirocin ointment and a chlorhexidine bath.
Preoperatively, they receive 5,000 units of unfractionated heparin, along with sequential compression devices for deep vein thrombosis protection. Use of both continue postoperatively, with heparin given every 8 hours until the patient is ambulatory.
Those without a history of narcotic use receive a preoperative dose of alvimopan. Everyone also receives oral gabapentin and preoperative antibiotics.
Intraoperatively, intravenous fluids are given judiciously to minimize bowel edema and decrease the risk of respiratory side effects. The surgeon also employs a transversus abdominis plane (TAP) block with liposomal bupivacaine.
There is a multimodal pain management program, which includes intravenous hydromorphone by patient-controlled anesthesia pump and oral gabapentin three times daily. Patients switch to oral analgesics on postoperative day 2. These consist of acetaminophen and NSAIDs on an alternating schedule. Intravenous diazepam can be added as an antispasmodic. Alvimopan is continued twice daily until first bowel movement or hospital discharge.
Clear liquids are limited to 250 mL/8 hours on postoperative day 1, along with a clear protein drink. By day 2, patients are advanced to unrestricted volumes of clear liquids, and on day 3, to a regular solid diet. At that point, the clear protein drink is switched for a regular protein drink (Boost Plus).
Patients who vomit or require a nasogastric tube for decompression, and those with severe persistent nausea are made NPO, but can resume diet when these symptoms improve. Those with mild-moderate nausea receive intravenous ondansetron and are allowed to self-limit oral intake.
Patients were a mean of 57 years old with a mean body mass index of about 33 kg/m2. The hernia was recurrent for about 60% of each group, with a mean area of about 300 cm2, and a mean width of 14 cm. The mean mesh size was about 1,000 cm2. The mean operative time was significantly shorter in the control group: 197 vs. 245 minutes.
Diet was advanced significantly more quickly in the ERAS group than the control group: 1 vs. 2 days on a liquid diet and 3 vs. 4.8 days to regular diet. Emesis after diet advancement was similar (4% for ERAS and 5% for control).
Compared with the control group, the ERAS group experienced significantly shorter average times to flatus (3.1 vs. 3.9 days), bowel movement (3.6 vs. 5.2 days), and reaching GI-3 status (3.4 vs. 4.8 days). Those in the ERAS group switched to oral narcotics sooner (2.2 vs. 3.6 days) also.
The average length of stay was significantly lower in ERAS group as well (4 vs. 6 days). About 18% of those in the ERAS group had a stay of less than 3 days, compared with 2% of the control group.
Readmissions within 90 days occurred in 16% of the control group and 4% of the ERAS group – a significant difference. There were four surgical site complications in the control group and three in the ERAS group. Bowel obstruction occurred in three control patients and one ERAS patient. All other complications requiring readmission occurred in the control group: two pulmonary embolisms, two deep vein thromboses, one pneumonia, one urinary tract infection and three other unspecified causes.
The authors noted that the shift to multimodal pain management and shorter-term use of IV opiates is a large contributor to the protocol’s good bowel outcomes. “Introduction of preoperative and postoperative gabapentin, intraoperative surgeon-delivered TAP block with long-acting liposomal bupivacaine, postoperative use of acetaminophen, and nonsteroidal agents have all appeared to contribute to better pain control and likely decrease in opioid consumption,” they said.
The use of diazepam as a pain medication is unusual, they said, but effective.
“We believe a large component of postoperative pain in hernia patients is due to muscle spasms after myofascial release, irritation from mesh placement, and transabdominal suture fixation. Therefore, in the context of our frequent use of myofascial releases for large incisional hernias, we believe the antispasmodic effects of diazepam potentially alleviate some of the postoperative discomfort caused by major abdominal wall reconstruction.”
None of the investigators reported financial conflicts.
A postsurgical recovery program featuring early feeding and multimodal pain management hastened the return of bowel function and shortened hospital stay by 2 days for patients undergoing complex ventral hernia repair.
Despite leaving the hospital sooner, however, patients were 75% less likely to be readmitted within 90 days, Dr. Arnab Majumder and his colleagues wrote in the June issue of the Journal of the American College of Surgeons (2016 Jun;222:1106-15). Most of those who did return had wound complications, a stark contrast to readmissions among patients who didn’t experience the enhanced recovery program. Among that group, 75% of the readmissions were caused by bowel obstruction/ileus, deep venous thrombosis or pulmonary embolism, pneumonia, and urinary tract infections – all of them “problems [that] could be related to prolonged hospitalizations,” said Dr. Majumder of the University Hospitals Case Medical Center, Cleveland.
The investigators created an Enhanced Recovery After Surgery (ERAS) pathway specifically for patients undergoing complex ventral hernia repairs using transversus abdominis release and sublay synthetic mesh placement. The patients “present formidable challenges to the surgeon, not only in the operating room but also during perioperative management,” the authors noted.
The ERAS the team preemptively addressed patient issues in the preoperative, intra- and perioperative, and postoperative periods. They compared the outcomes of 100 patients treated with the protocol to those in a control group of 100 who underwent the same surgery before the protocol was implemented. The main outcomes measures were time to diet advancement, time to return of bowel function, time to oral narcotics, length of stay, and 90-day readmissions.
The ERAS begins with preoperative optimization. This consists of weight loss, smoking cessation, and managing diabetes and obstructive sleep apnea. No surgery occurs until the HbA1c is less than 8 and patients have been tobacco free for at least 1 month.
All patients receive an arginine and omega-3 supplement thrice daily for 5 days before surgery. The night before surgery, they have a nasal swab screen for methicillin-resistant Staphylococcus aureus (MRSA), and decolonization with mupirocin ointment and a chlorhexidine bath.
Preoperatively, they receive 5,000 units of unfractionated heparin, along with sequential compression devices for deep vein thrombosis protection. Use of both continue postoperatively, with heparin given every 8 hours until the patient is ambulatory.
Those without a history of narcotic use receive a preoperative dose of alvimopan. Everyone also receives oral gabapentin and preoperative antibiotics.
Intraoperatively, intravenous fluids are given judiciously to minimize bowel edema and decrease the risk of respiratory side effects. The surgeon also employs a transversus abdominis plane (TAP) block with liposomal bupivacaine.
There is a multimodal pain management program, which includes intravenous hydromorphone by patient-controlled anesthesia pump and oral gabapentin three times daily. Patients switch to oral analgesics on postoperative day 2. These consist of acetaminophen and NSAIDs on an alternating schedule. Intravenous diazepam can be added as an antispasmodic. Alvimopan is continued twice daily until first bowel movement or hospital discharge.
Clear liquids are limited to 250 mL/8 hours on postoperative day 1, along with a clear protein drink. By day 2, patients are advanced to unrestricted volumes of clear liquids, and on day 3, to a regular solid diet. At that point, the clear protein drink is switched for a regular protein drink (Boost Plus).
Patients who vomit or require a nasogastric tube for decompression, and those with severe persistent nausea are made NPO, but can resume diet when these symptoms improve. Those with mild-moderate nausea receive intravenous ondansetron and are allowed to self-limit oral intake.
Patients were a mean of 57 years old with a mean body mass index of about 33 kg/m2. The hernia was recurrent for about 60% of each group, with a mean area of about 300 cm2, and a mean width of 14 cm. The mean mesh size was about 1,000 cm2. The mean operative time was significantly shorter in the control group: 197 vs. 245 minutes.
Diet was advanced significantly more quickly in the ERAS group than the control group: 1 vs. 2 days on a liquid diet and 3 vs. 4.8 days to regular diet. Emesis after diet advancement was similar (4% for ERAS and 5% for control).
Compared with the control group, the ERAS group experienced significantly shorter average times to flatus (3.1 vs. 3.9 days), bowel movement (3.6 vs. 5.2 days), and reaching GI-3 status (3.4 vs. 4.8 days). Those in the ERAS group switched to oral narcotics sooner (2.2 vs. 3.6 days) also.
The average length of stay was significantly lower in ERAS group as well (4 vs. 6 days). About 18% of those in the ERAS group had a stay of less than 3 days, compared with 2% of the control group.
Readmissions within 90 days occurred in 16% of the control group and 4% of the ERAS group – a significant difference. There were four surgical site complications in the control group and three in the ERAS group. Bowel obstruction occurred in three control patients and one ERAS patient. All other complications requiring readmission occurred in the control group: two pulmonary embolisms, two deep vein thromboses, one pneumonia, one urinary tract infection and three other unspecified causes.
The authors noted that the shift to multimodal pain management and shorter-term use of IV opiates is a large contributor to the protocol’s good bowel outcomes. “Introduction of preoperative and postoperative gabapentin, intraoperative surgeon-delivered TAP block with long-acting liposomal bupivacaine, postoperative use of acetaminophen, and nonsteroidal agents have all appeared to contribute to better pain control and likely decrease in opioid consumption,” they said.
The use of diazepam as a pain medication is unusual, they said, but effective.
“We believe a large component of postoperative pain in hernia patients is due to muscle spasms after myofascial release, irritation from mesh placement, and transabdominal suture fixation. Therefore, in the context of our frequent use of myofascial releases for large incisional hernias, we believe the antispasmodic effects of diazepam potentially alleviate some of the postoperative discomfort caused by major abdominal wall reconstruction.”
None of the investigators reported financial conflicts.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: Early enteral feeding and multimodal pain management both contributed to early return of bowel function.
Major finding: Length of stay was 2 days shorter, and readmissions decreased by 75%, compared with a control group.
Data source: The prospective study comprised 200 patients.
Disclosures: Neither Dr. Majumder nor his colleagues had any financial disclosures.
VIDEO: FDG-PET/CT useful for fever, inflammation of unknown origin
LONDON – The use of combined modality imaging with 18F-fluorodeoxyglucose-PET/CT may provide enough information to make a definitive diagnosis in patients who present with fever or inflammation of unknown origin, particularly in those who are aged 50 years or older, have elevated C-reactive protein, and have no fever, according to findings from a single-center study of 240 cases.
The retrospective study of patients seen at the University Clinic of Erlangen (Germany) during 2007-2015 found that 18F-FDG-PET/CT was helpful in finding a diagnosis for a majority of patients with fever of unknown origin (FUO) and inflammation of unknown origin (IUO).
In an interview prior to his presentation at the European Congress of Rheumatology, the study’s senior investigator Dr. Georg Schett said that “By implementing a single 18F-FDG-PET/CT scan in a structured diagnostic approach for patients with FUO or IUO we were able to catch the underlying disease in the majority (79%) of the 240 patients studied. In the FUO group the leading diagnosis was adult-onset Still’s disease, [and] in the IUO group it was large-vessel vasculitis and polymyalgia rheumatica.”
FUO was defined about 50 years ago as several episodes of temperature exceeding 38.3° C that accompany an illness lasting more than 3 weeks, with no diagnosis after a week of testing following hospital admittance. If inflammation but no fever is involved, the condition is termed IUO.
FUO and IUO are severe, sometimes even life-threatening conditions, in which the cause of fever and inflammation, respectively, has not been defined using standard diagnostic approaches. This makes diagnosis challenging and requires a costly and complicated work-up. A delayed diagnosis can be serious, resulting in severe organ damage in patients with FUO and IUO due to the underlying, and uncontrolled, inflammatory disease.
The current diagnostic approaches for FUO and IUO include a thorough medical history, physical examination, laboratory testing, and imaging. 18F-FDG-PET/CT imaging could be potentially useful for the diagnosis of FUO/IUO because of its high-resolution detection of inflammation and malignancy. Dr. Schett and his colleagues explored this potential and examined clinical markers that would increase the likelihood of accurate 18F-FDG-PET/CT-based diagnosis in patients presenting with FUO or IUO.
The 240 patients in the study included 72 with FUO and 142 with IUO; the remaining 26 no longer fulfilled the criteria for either condition when they presented to the clinic (“ex-FUO/IUO” patients). The diagnostic work-up included 18F-FDG-PET/CT scans. Scans were considered to be positive when uptake of the tracer occurred at foci in addition to the other expected locations. The investigators explored whether the scans aided the final diagnosis, with multivariable regression analysis clarifying clinical parameters that aided the success of the scans in patients with and without FUO or IUO.
The mean age was 52 for FUO patients, 61 for IUO, and 51 for patients who no longer had IUO or FUO symptoms at presentation. These patients had mean C-reactive protein (CRP) levels of 95, 48, and 2 mg/L, respectively. Males comprised 64% of FUO, 40% of IUO, and 58% of ex-FUO/IUO patients.
18F-FDG-PET/CT was helpful in finding the diagnosis in 57% of all patients and 72% of the patients with a later diagnosis. A definitive diagnosis was not reached in 29% of patients with FUO and 17% of patients with IUO. Predictive markers for a diagnostic 18F-FDG-PET/CT for FUO and IUO were age over 50 years (P = .002 and P = .005, respectively), CRP level over 30 mg/L (P = .003 and P = .005, respectively), and the absence of fever (both P = .003). If all three parameters were fulfilled, 18F-FDG-PET/CT was diagnostic in nearly 80% of the cases, while it was successful in only 8% of cases where none of the three parameters was met.
The latter finding is particularly important, according to Dr. Schett, as it “indicates which patient subgroup is profiting the most from 18F-FDG-PET/CT.”
“FUO and IUO patients should be referred to specialized centers where 18F-FDG-PET/CT scanning is available to improve diagnosis. Simple clinical parameters such as age, CRP-level, and presence/absence of fever can guide targeted use of 18F-FDG-PET/CT,” said Dr. Schett, director of the department of internal medicine III and the Institute for Clinical Immunology at the University of Erlangen-Nuremberg (Germany).
False-positive results with 18F-FDG-PET/CT – when patients had tracer uptake that did not lead to diagnosis of the underlying diseases – are a challenge. “False-positives happen quite often due to activation of bone marrow and lymph node metabolism during inflammation, which does not support diagnosis,” Dr. Schett said. He added that, when tracer uptake associated with systemic inflammation was not considered, false positives were much less common. False-negative results – when 18F-FDG-PET/CT was negative but a diagnosis was made using other approaches – were rare, occurring in only 12 out of the 240 patients.
The research will support establishing recommendations for the use of 18F-FDG-PET/CT in FUO and IUO patients. Other patients could benefit as well. “It may be important to investigate also those patients who were referred for FUO or IUO but do not show fever or inflammation at time of admission,” Dr. Schett said. Of these ex-FUO/IUO patients, four were diagnosed with IgG4-related disease and three with familial Mediterranean syndrome by applying 18F-FDG-PET/CT.
Dr. Schett and the other authors had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – The use of combined modality imaging with 18F-fluorodeoxyglucose-PET/CT may provide enough information to make a definitive diagnosis in patients who present with fever or inflammation of unknown origin, particularly in those who are aged 50 years or older, have elevated C-reactive protein, and have no fever, according to findings from a single-center study of 240 cases.
The retrospective study of patients seen at the University Clinic of Erlangen (Germany) during 2007-2015 found that 18F-FDG-PET/CT was helpful in finding a diagnosis for a majority of patients with fever of unknown origin (FUO) and inflammation of unknown origin (IUO).
In an interview prior to his presentation at the European Congress of Rheumatology, the study’s senior investigator Dr. Georg Schett said that “By implementing a single 18F-FDG-PET/CT scan in a structured diagnostic approach for patients with FUO or IUO we were able to catch the underlying disease in the majority (79%) of the 240 patients studied. In the FUO group the leading diagnosis was adult-onset Still’s disease, [and] in the IUO group it was large-vessel vasculitis and polymyalgia rheumatica.”
FUO was defined about 50 years ago as several episodes of temperature exceeding 38.3° C that accompany an illness lasting more than 3 weeks, with no diagnosis after a week of testing following hospital admittance. If inflammation but no fever is involved, the condition is termed IUO.
FUO and IUO are severe, sometimes even life-threatening conditions, in which the cause of fever and inflammation, respectively, has not been defined using standard diagnostic approaches. This makes diagnosis challenging and requires a costly and complicated work-up. A delayed diagnosis can be serious, resulting in severe organ damage in patients with FUO and IUO due to the underlying, and uncontrolled, inflammatory disease.
The current diagnostic approaches for FUO and IUO include a thorough medical history, physical examination, laboratory testing, and imaging. 18F-FDG-PET/CT imaging could be potentially useful for the diagnosis of FUO/IUO because of its high-resolution detection of inflammation and malignancy. Dr. Schett and his colleagues explored this potential and examined clinical markers that would increase the likelihood of accurate 18F-FDG-PET/CT-based diagnosis in patients presenting with FUO or IUO.
The 240 patients in the study included 72 with FUO and 142 with IUO; the remaining 26 no longer fulfilled the criteria for either condition when they presented to the clinic (“ex-FUO/IUO” patients). The diagnostic work-up included 18F-FDG-PET/CT scans. Scans were considered to be positive when uptake of the tracer occurred at foci in addition to the other expected locations. The investigators explored whether the scans aided the final diagnosis, with multivariable regression analysis clarifying clinical parameters that aided the success of the scans in patients with and without FUO or IUO.
The mean age was 52 for FUO patients, 61 for IUO, and 51 for patients who no longer had IUO or FUO symptoms at presentation. These patients had mean C-reactive protein (CRP) levels of 95, 48, and 2 mg/L, respectively. Males comprised 64% of FUO, 40% of IUO, and 58% of ex-FUO/IUO patients.
18F-FDG-PET/CT was helpful in finding the diagnosis in 57% of all patients and 72% of the patients with a later diagnosis. A definitive diagnosis was not reached in 29% of patients with FUO and 17% of patients with IUO. Predictive markers for a diagnostic 18F-FDG-PET/CT for FUO and IUO were age over 50 years (P = .002 and P = .005, respectively), CRP level over 30 mg/L (P = .003 and P = .005, respectively), and the absence of fever (both P = .003). If all three parameters were fulfilled, 18F-FDG-PET/CT was diagnostic in nearly 80% of the cases, while it was successful in only 8% of cases where none of the three parameters was met.
The latter finding is particularly important, according to Dr. Schett, as it “indicates which patient subgroup is profiting the most from 18F-FDG-PET/CT.”
“FUO and IUO patients should be referred to specialized centers where 18F-FDG-PET/CT scanning is available to improve diagnosis. Simple clinical parameters such as age, CRP-level, and presence/absence of fever can guide targeted use of 18F-FDG-PET/CT,” said Dr. Schett, director of the department of internal medicine III and the Institute for Clinical Immunology at the University of Erlangen-Nuremberg (Germany).
False-positive results with 18F-FDG-PET/CT – when patients had tracer uptake that did not lead to diagnosis of the underlying diseases – are a challenge. “False-positives happen quite often due to activation of bone marrow and lymph node metabolism during inflammation, which does not support diagnosis,” Dr. Schett said. He added that, when tracer uptake associated with systemic inflammation was not considered, false positives were much less common. False-negative results – when 18F-FDG-PET/CT was negative but a diagnosis was made using other approaches – were rare, occurring in only 12 out of the 240 patients.
The research will support establishing recommendations for the use of 18F-FDG-PET/CT in FUO and IUO patients. Other patients could benefit as well. “It may be important to investigate also those patients who were referred for FUO or IUO but do not show fever or inflammation at time of admission,” Dr. Schett said. Of these ex-FUO/IUO patients, four were diagnosed with IgG4-related disease and three with familial Mediterranean syndrome by applying 18F-FDG-PET/CT.
Dr. Schett and the other authors had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – The use of combined modality imaging with 18F-fluorodeoxyglucose-PET/CT may provide enough information to make a definitive diagnosis in patients who present with fever or inflammation of unknown origin, particularly in those who are aged 50 years or older, have elevated C-reactive protein, and have no fever, according to findings from a single-center study of 240 cases.
The retrospective study of patients seen at the University Clinic of Erlangen (Germany) during 2007-2015 found that 18F-FDG-PET/CT was helpful in finding a diagnosis for a majority of patients with fever of unknown origin (FUO) and inflammation of unknown origin (IUO).
In an interview prior to his presentation at the European Congress of Rheumatology, the study’s senior investigator Dr. Georg Schett said that “By implementing a single 18F-FDG-PET/CT scan in a structured diagnostic approach for patients with FUO or IUO we were able to catch the underlying disease in the majority (79%) of the 240 patients studied. In the FUO group the leading diagnosis was adult-onset Still’s disease, [and] in the IUO group it was large-vessel vasculitis and polymyalgia rheumatica.”
FUO was defined about 50 years ago as several episodes of temperature exceeding 38.3° C that accompany an illness lasting more than 3 weeks, with no diagnosis after a week of testing following hospital admittance. If inflammation but no fever is involved, the condition is termed IUO.
FUO and IUO are severe, sometimes even life-threatening conditions, in which the cause of fever and inflammation, respectively, has not been defined using standard diagnostic approaches. This makes diagnosis challenging and requires a costly and complicated work-up. A delayed diagnosis can be serious, resulting in severe organ damage in patients with FUO and IUO due to the underlying, and uncontrolled, inflammatory disease.
The current diagnostic approaches for FUO and IUO include a thorough medical history, physical examination, laboratory testing, and imaging. 18F-FDG-PET/CT imaging could be potentially useful for the diagnosis of FUO/IUO because of its high-resolution detection of inflammation and malignancy. Dr. Schett and his colleagues explored this potential and examined clinical markers that would increase the likelihood of accurate 18F-FDG-PET/CT-based diagnosis in patients presenting with FUO or IUO.
The 240 patients in the study included 72 with FUO and 142 with IUO; the remaining 26 no longer fulfilled the criteria for either condition when they presented to the clinic (“ex-FUO/IUO” patients). The diagnostic work-up included 18F-FDG-PET/CT scans. Scans were considered to be positive when uptake of the tracer occurred at foci in addition to the other expected locations. The investigators explored whether the scans aided the final diagnosis, with multivariable regression analysis clarifying clinical parameters that aided the success of the scans in patients with and without FUO or IUO.
The mean age was 52 for FUO patients, 61 for IUO, and 51 for patients who no longer had IUO or FUO symptoms at presentation. These patients had mean C-reactive protein (CRP) levels of 95, 48, and 2 mg/L, respectively. Males comprised 64% of FUO, 40% of IUO, and 58% of ex-FUO/IUO patients.
18F-FDG-PET/CT was helpful in finding the diagnosis in 57% of all patients and 72% of the patients with a later diagnosis. A definitive diagnosis was not reached in 29% of patients with FUO and 17% of patients with IUO. Predictive markers for a diagnostic 18F-FDG-PET/CT for FUO and IUO were age over 50 years (P = .002 and P = .005, respectively), CRP level over 30 mg/L (P = .003 and P = .005, respectively), and the absence of fever (both P = .003). If all three parameters were fulfilled, 18F-FDG-PET/CT was diagnostic in nearly 80% of the cases, while it was successful in only 8% of cases where none of the three parameters was met.
The latter finding is particularly important, according to Dr. Schett, as it “indicates which patient subgroup is profiting the most from 18F-FDG-PET/CT.”
“FUO and IUO patients should be referred to specialized centers where 18F-FDG-PET/CT scanning is available to improve diagnosis. Simple clinical parameters such as age, CRP-level, and presence/absence of fever can guide targeted use of 18F-FDG-PET/CT,” said Dr. Schett, director of the department of internal medicine III and the Institute for Clinical Immunology at the University of Erlangen-Nuremberg (Germany).
False-positive results with 18F-FDG-PET/CT – when patients had tracer uptake that did not lead to diagnosis of the underlying diseases – are a challenge. “False-positives happen quite often due to activation of bone marrow and lymph node metabolism during inflammation, which does not support diagnosis,” Dr. Schett said. He added that, when tracer uptake associated with systemic inflammation was not considered, false positives were much less common. False-negative results – when 18F-FDG-PET/CT was negative but a diagnosis was made using other approaches – were rare, occurring in only 12 out of the 240 patients.
The research will support establishing recommendations for the use of 18F-FDG-PET/CT in FUO and IUO patients. Other patients could benefit as well. “It may be important to investigate also those patients who were referred for FUO or IUO but do not show fever or inflammation at time of admission,” Dr. Schett said. Of these ex-FUO/IUO patients, four were diagnosed with IgG4-related disease and three with familial Mediterranean syndrome by applying 18F-FDG-PET/CT.
Dr. Schett and the other authors had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2016 CONGRESS
Key clinical point: An 18F-FDG-PET/CT scan is most likely to aid diagnosis in patients who present with fever of unknown origin or inflammation of unknown origin if they are aged over 50 years, have elevated CRP level over 30 mg/L, and do not have fever.
Major finding: 18F-FDG-PET/CT was helpful in finding a diagnosis in 57% of all patients and 72% of the patients who eventually received a diagnosis.
Data source: A single-center study of 240 cases of fever of unknown origin or inflammation of unknown origin who underwent 18F-FDG-PET/CT scanning during 2007-2015.
Disclosures: Dr. Schett and the other authors had no disclosures.
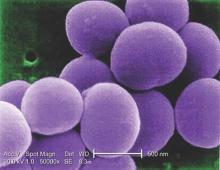
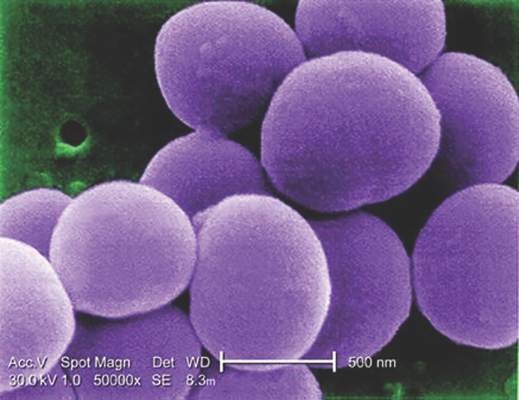
Glucocorticoids increase risk of S. aureus bacteremia
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
FROM MAYO CLINIC PROCEEDINGS
Key clinical point: Taking glucocorticoids can significantly increase the risk of contracting community-acquired Staphylococcus aureus bacteremia (CA-SAB).
Major finding: New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Data source: Retrospective, case-control study of all adults with first-time CA-SAB in Northern Denmark medical registries between 2000 and 2011.
Disclosures: Study supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
Genetic therapy lowers joint bleeding in hemophilia B
COPENHAGEN – It’s early days yet, but results look highly promising for the ability of an experimental gene-transfer therapy to improve coagulation parameters in patients with severe hemophilia B.
In a phase I/II dose-escalation study, a single 1-hour infusion of the gene-transfer product, labeled SPK-9001, resulted in factor IX activity levels ranging from 25% to 39% of normal in four men with severe or moderately severe hemophilia B, reported Dr. Katherine A High, president and chief scientific officer of Spark Therapeutics, maker of the product.
“One of the most remarkable features of the data in my mind has been a very consistent performance,” she said at a briefing at the annual congress of the European Hematology Association.
The product consists of a vector containing a novel bio-engineered adeno-associated virus (AAV) capsid with tropism for liver, and a factor IX cassette that carries a strong liver-specific promoter to drive the expression of the factor IX variant, dubbed factor IX Padua.
“The hypothesis of the work was that if we could engineer a vector efficient enough, we would be able to infuse it at a dose low enough that it would drive loads of expression greater than 12% of normal, which in previous work has been shown to be associated with an absence of joint bleeds in natural history studies of people with mild disease, and that infusion at a low dose would eliminate the need for any type of immune suppression with steroids,” Dr. High said.
Other attempts at genetic engineering in patients with hemophilia B have been hampered by the need to use high doses of vector that can induce an immune response, thereby negating the benefit of therapy.
In this ongoing study, conducted in Mississippi, Pennsylvania, and California, males 18 and older with a confirmed diagnosis of hemophilia B (defined as equal to or less than 2 IU/dL or 2% endogenous factor IX) who have received 50 or more days of exposure to factor IX products are enrolled. The patients must have a minimum average of four bleeding events per year requiring episodic treatment of factor IX infusions or prophylactic factor IX infusions, no measurable factor IX inhibitor as assessed by the central laboratory, and no prior history of inhibitors to factor IX protein.
In an oral session at the congress, Dr. Spencer Sullivan, assistant professor of pediatrics and medicine at the University of Mississippi Medical Center in Jackson, presented data on four men whose age ranges from 18 to 47 years.
In the dose-escalation phase of the study, the patients received single infusions of SPK-9001 at an initial starting dose of 5 x 1011 vector genomes of body weight. They were followed for 7-26 weeks after gene transfer for factor IX activity levels, liver enzymes, bleeding episode, and factor usage. As of May 22, 2016, the first four patients showed factor IX activity levels of 32%, 39%, 25%, and 27% of normal, respectively.
All subjects are currently off prophylactic factor IX infusions. During the course of follow-up, one patient infused himself with factor IX once, treating himself 2 days after vector infusion for a suspected ankle bleed.
Asked by a reporter how durable the effect was, Dr. High replied that in dog models of hemophilia B the effect of the gene transfer has been durable.
The best evidence to date of durability in humans, she said, comes from investigators at University College in London (England), who found that if patients can make it past the first 8-10 weeks without developing an immune response to the transfer product, they are likely to do well, and to have a durable effect, she said.
Dr. Anton Hagenbeek, from the Academic Medical Center, University of Amsterdam, who moderated the briefing but was not involved in the study, said that Dr. High was “to be congratulated for these best data ever seen.”
He asked, facetiously, whether she thought that “thousands of patients would buy a ticket to Philadelphia.” The “City of Brotherly Love” is home to one of the trial sites and to Spark headquarters.
The study is sponsored by Spark Therapeutics and Pfizer. Dr. High is president and chief scientific officer of Spark. Dr. Sullivan and Dr. Hagenbeek reported no relevant disclosures.
COPENHAGEN – It’s early days yet, but results look highly promising for the ability of an experimental gene-transfer therapy to improve coagulation parameters in patients with severe hemophilia B.
In a phase I/II dose-escalation study, a single 1-hour infusion of the gene-transfer product, labeled SPK-9001, resulted in factor IX activity levels ranging from 25% to 39% of normal in four men with severe or moderately severe hemophilia B, reported Dr. Katherine A High, president and chief scientific officer of Spark Therapeutics, maker of the product.
“One of the most remarkable features of the data in my mind has been a very consistent performance,” she said at a briefing at the annual congress of the European Hematology Association.
The product consists of a vector containing a novel bio-engineered adeno-associated virus (AAV) capsid with tropism for liver, and a factor IX cassette that carries a strong liver-specific promoter to drive the expression of the factor IX variant, dubbed factor IX Padua.
“The hypothesis of the work was that if we could engineer a vector efficient enough, we would be able to infuse it at a dose low enough that it would drive loads of expression greater than 12% of normal, which in previous work has been shown to be associated with an absence of joint bleeds in natural history studies of people with mild disease, and that infusion at a low dose would eliminate the need for any type of immune suppression with steroids,” Dr. High said.
Other attempts at genetic engineering in patients with hemophilia B have been hampered by the need to use high doses of vector that can induce an immune response, thereby negating the benefit of therapy.
In this ongoing study, conducted in Mississippi, Pennsylvania, and California, males 18 and older with a confirmed diagnosis of hemophilia B (defined as equal to or less than 2 IU/dL or 2% endogenous factor IX) who have received 50 or more days of exposure to factor IX products are enrolled. The patients must have a minimum average of four bleeding events per year requiring episodic treatment of factor IX infusions or prophylactic factor IX infusions, no measurable factor IX inhibitor as assessed by the central laboratory, and no prior history of inhibitors to factor IX protein.
In an oral session at the congress, Dr. Spencer Sullivan, assistant professor of pediatrics and medicine at the University of Mississippi Medical Center in Jackson, presented data on four men whose age ranges from 18 to 47 years.
In the dose-escalation phase of the study, the patients received single infusions of SPK-9001 at an initial starting dose of 5 x 1011 vector genomes of body weight. They were followed for 7-26 weeks after gene transfer for factor IX activity levels, liver enzymes, bleeding episode, and factor usage. As of May 22, 2016, the first four patients showed factor IX activity levels of 32%, 39%, 25%, and 27% of normal, respectively.
All subjects are currently off prophylactic factor IX infusions. During the course of follow-up, one patient infused himself with factor IX once, treating himself 2 days after vector infusion for a suspected ankle bleed.
Asked by a reporter how durable the effect was, Dr. High replied that in dog models of hemophilia B the effect of the gene transfer has been durable.
The best evidence to date of durability in humans, she said, comes from investigators at University College in London (England), who found that if patients can make it past the first 8-10 weeks without developing an immune response to the transfer product, they are likely to do well, and to have a durable effect, she said.
Dr. Anton Hagenbeek, from the Academic Medical Center, University of Amsterdam, who moderated the briefing but was not involved in the study, said that Dr. High was “to be congratulated for these best data ever seen.”
He asked, facetiously, whether she thought that “thousands of patients would buy a ticket to Philadelphia.” The “City of Brotherly Love” is home to one of the trial sites and to Spark headquarters.
The study is sponsored by Spark Therapeutics and Pfizer. Dr. High is president and chief scientific officer of Spark. Dr. Sullivan and Dr. Hagenbeek reported no relevant disclosures.
COPENHAGEN – It’s early days yet, but results look highly promising for the ability of an experimental gene-transfer therapy to improve coagulation parameters in patients with severe hemophilia B.
In a phase I/II dose-escalation study, a single 1-hour infusion of the gene-transfer product, labeled SPK-9001, resulted in factor IX activity levels ranging from 25% to 39% of normal in four men with severe or moderately severe hemophilia B, reported Dr. Katherine A High, president and chief scientific officer of Spark Therapeutics, maker of the product.
“One of the most remarkable features of the data in my mind has been a very consistent performance,” she said at a briefing at the annual congress of the European Hematology Association.
The product consists of a vector containing a novel bio-engineered adeno-associated virus (AAV) capsid with tropism for liver, and a factor IX cassette that carries a strong liver-specific promoter to drive the expression of the factor IX variant, dubbed factor IX Padua.
“The hypothesis of the work was that if we could engineer a vector efficient enough, we would be able to infuse it at a dose low enough that it would drive loads of expression greater than 12% of normal, which in previous work has been shown to be associated with an absence of joint bleeds in natural history studies of people with mild disease, and that infusion at a low dose would eliminate the need for any type of immune suppression with steroids,” Dr. High said.
Other attempts at genetic engineering in patients with hemophilia B have been hampered by the need to use high doses of vector that can induce an immune response, thereby negating the benefit of therapy.
In this ongoing study, conducted in Mississippi, Pennsylvania, and California, males 18 and older with a confirmed diagnosis of hemophilia B (defined as equal to or less than 2 IU/dL or 2% endogenous factor IX) who have received 50 or more days of exposure to factor IX products are enrolled. The patients must have a minimum average of four bleeding events per year requiring episodic treatment of factor IX infusions or prophylactic factor IX infusions, no measurable factor IX inhibitor as assessed by the central laboratory, and no prior history of inhibitors to factor IX protein.
In an oral session at the congress, Dr. Spencer Sullivan, assistant professor of pediatrics and medicine at the University of Mississippi Medical Center in Jackson, presented data on four men whose age ranges from 18 to 47 years.
In the dose-escalation phase of the study, the patients received single infusions of SPK-9001 at an initial starting dose of 5 x 1011 vector genomes of body weight. They were followed for 7-26 weeks after gene transfer for factor IX activity levels, liver enzymes, bleeding episode, and factor usage. As of May 22, 2016, the first four patients showed factor IX activity levels of 32%, 39%, 25%, and 27% of normal, respectively.
All subjects are currently off prophylactic factor IX infusions. During the course of follow-up, one patient infused himself with factor IX once, treating himself 2 days after vector infusion for a suspected ankle bleed.
Asked by a reporter how durable the effect was, Dr. High replied that in dog models of hemophilia B the effect of the gene transfer has been durable.
The best evidence to date of durability in humans, she said, comes from investigators at University College in London (England), who found that if patients can make it past the first 8-10 weeks without developing an immune response to the transfer product, they are likely to do well, and to have a durable effect, she said.
Dr. Anton Hagenbeek, from the Academic Medical Center, University of Amsterdam, who moderated the briefing but was not involved in the study, said that Dr. High was “to be congratulated for these best data ever seen.”
He asked, facetiously, whether she thought that “thousands of patients would buy a ticket to Philadelphia.” The “City of Brotherly Love” is home to one of the trial sites and to Spark headquarters.
The study is sponsored by Spark Therapeutics and Pfizer. Dr. High is president and chief scientific officer of Spark. Dr. Sullivan and Dr. Hagenbeek reported no relevant disclosures.
At THE EHA CONGRESS
Key clinical point:. The experimental SPK-9001 is a gene transfer product carrying a strong factor IX promoter.
Major finding: A single one-hour infusion of SPK-9001 was associated with factor IX activity levels ranging from 25% to 39% of normal.
Data source: Dose-escalation cohort of four adult males in a phase I/II study.
Disclosures: The study is sponsored by Spark Therapeutics and Pfizer. Dr. High is president and chief scientific officer of Spark. Dr. Sullivan and Dr. Hagenbeek reported no relevant disclosures.
8 steps to avoid legal risks from your practice website
CHICAGO – An inadequately designed medical practice website can pose serious legal dangers, said Michael J. Sacopulos, a medical malpractice defense attorney based in Terre Haute, Ind.
Here is a list of website to-dos that can reduce your legal risks:
• Post emergency information on the website contact page. Unlike the practice’s phone system, the website may fail to include a disclaimer that the patient should call 911 if experiencing a medical emergency.
• Provide disclaimers about doctor-patient relationship. In addition, it’s important that the website includes a warning that communications through the website do not constitute a doctor-patient relationship, Mr. Sacopulos said during an American Bar Association conference. “Most [websites] have a box where you can leave comments. [People need to be told] that it does not create a physician-patient relationship when they describe their medical condition, sometimes even posting photographs.”
• Advise regarding comment security. Under the Health Information Technology for Economic and Clinical Health (HITECH) Act, medical information sent through electronic channels must be encrypted unless a patient consents otherwise. If information can be transmitted through a website’s comment box, patients should be advised that the transmission is not secure before they send their information, Mr. Sacopulos said.
• Secure any online appointment scheduling. Make sure that patients’ names and personal information are not visible to other patients when they schedule appointments, he said. A cardiology practice in Phoenix learned this the hard way when it had to pay the U.S. Department of Health & Human Services $100,000 for lack of HIPAA safeguards online. An investigation by the Office for Civil Rights found the practice was posting clinical and surgical appointments for patients on an Internet-based calendar that was publicly accessible.
• Ensure patient anonymity. The accidental release of private medical information occurred on the website of a St. Louis physician who obtained consent from her patients to include their before and after photos. No names were posted with the photos, but the computer file names of the photos included the patients’ names, and when a person scrolled over a photo with a cursor, the file name popped up. This allowed the public to view the patient name associated with each photo and caused serious problems for the practice, including litigation, he noted.
• Be aware of state board requirements that pertain to physician practice websites. Several state boards do not allow testimonials to be posted on websites. States also differ on the inclusion of before and after photos. New Jersey, for example, allows before and after photos on websites, while New York does not. Some state boards allow doctors to cite that they are board certified on a website without specifics, while states such as Louisiana require that physicians announce the specific certifying board.
“These are ethical and affirmative duties on behalf of physicians that oftentimes come up in websites,” he said.
• Adhere to the Americans With Disabilities Act (ADA). A website is considered real estate for purposes of the ADA, meaning it must include an accessible format to patients with disabilities, Mr. Sacupulos said. Problems arise when certain website features make sites difficult or incompatible with assistance devices that disabled patients require, such as a screen reader or voice interactive software. The National Federation for the Blind has been active in this area and has filed multiple class action lawsuits against companies that did not have compliant websites, he said. An ADA tool kit for best website practices can be found online.
• Hire an experienced Web designer to create the practice’s website. Too often, practices use a family member or friend to set up the company’s page, Mr. Sacupulos said. In one such instance, a young designer became angry at his doctor employer and set up a false website in his name, alleging abuses against patients. “Work with someone credible,” he advised. “Make sure you own your own domain. Many of these Web designers will purchase the domain name and build a site around it, which is great until you want to move to the next Web designer, and then you have to buy your domain back.”
On Twitter @legal_med
CHICAGO – An inadequately designed medical practice website can pose serious legal dangers, said Michael J. Sacopulos, a medical malpractice defense attorney based in Terre Haute, Ind.
Here is a list of website to-dos that can reduce your legal risks:
• Post emergency information on the website contact page. Unlike the practice’s phone system, the website may fail to include a disclaimer that the patient should call 911 if experiencing a medical emergency.
• Provide disclaimers about doctor-patient relationship. In addition, it’s important that the website includes a warning that communications through the website do not constitute a doctor-patient relationship, Mr. Sacopulos said during an American Bar Association conference. “Most [websites] have a box where you can leave comments. [People need to be told] that it does not create a physician-patient relationship when they describe their medical condition, sometimes even posting photographs.”
• Advise regarding comment security. Under the Health Information Technology for Economic and Clinical Health (HITECH) Act, medical information sent through electronic channels must be encrypted unless a patient consents otherwise. If information can be transmitted through a website’s comment box, patients should be advised that the transmission is not secure before they send their information, Mr. Sacopulos said.
• Secure any online appointment scheduling. Make sure that patients’ names and personal information are not visible to other patients when they schedule appointments, he said. A cardiology practice in Phoenix learned this the hard way when it had to pay the U.S. Department of Health & Human Services $100,000 for lack of HIPAA safeguards online. An investigation by the Office for Civil Rights found the practice was posting clinical and surgical appointments for patients on an Internet-based calendar that was publicly accessible.
• Ensure patient anonymity. The accidental release of private medical information occurred on the website of a St. Louis physician who obtained consent from her patients to include their before and after photos. No names were posted with the photos, but the computer file names of the photos included the patients’ names, and when a person scrolled over a photo with a cursor, the file name popped up. This allowed the public to view the patient name associated with each photo and caused serious problems for the practice, including litigation, he noted.
• Be aware of state board requirements that pertain to physician practice websites. Several state boards do not allow testimonials to be posted on websites. States also differ on the inclusion of before and after photos. New Jersey, for example, allows before and after photos on websites, while New York does not. Some state boards allow doctors to cite that they are board certified on a website without specifics, while states such as Louisiana require that physicians announce the specific certifying board.
“These are ethical and affirmative duties on behalf of physicians that oftentimes come up in websites,” he said.
• Adhere to the Americans With Disabilities Act (ADA). A website is considered real estate for purposes of the ADA, meaning it must include an accessible format to patients with disabilities, Mr. Sacupulos said. Problems arise when certain website features make sites difficult or incompatible with assistance devices that disabled patients require, such as a screen reader or voice interactive software. The National Federation for the Blind has been active in this area and has filed multiple class action lawsuits against companies that did not have compliant websites, he said. An ADA tool kit for best website practices can be found online.
• Hire an experienced Web designer to create the practice’s website. Too often, practices use a family member or friend to set up the company’s page, Mr. Sacupulos said. In one such instance, a young designer became angry at his doctor employer and set up a false website in his name, alleging abuses against patients. “Work with someone credible,” he advised. “Make sure you own your own domain. Many of these Web designers will purchase the domain name and build a site around it, which is great until you want to move to the next Web designer, and then you have to buy your domain back.”
On Twitter @legal_med
CHICAGO – An inadequately designed medical practice website can pose serious legal dangers, said Michael J. Sacopulos, a medical malpractice defense attorney based in Terre Haute, Ind.
Here is a list of website to-dos that can reduce your legal risks:
• Post emergency information on the website contact page. Unlike the practice’s phone system, the website may fail to include a disclaimer that the patient should call 911 if experiencing a medical emergency.
• Provide disclaimers about doctor-patient relationship. In addition, it’s important that the website includes a warning that communications through the website do not constitute a doctor-patient relationship, Mr. Sacopulos said during an American Bar Association conference. “Most [websites] have a box where you can leave comments. [People need to be told] that it does not create a physician-patient relationship when they describe their medical condition, sometimes even posting photographs.”
• Advise regarding comment security. Under the Health Information Technology for Economic and Clinical Health (HITECH) Act, medical information sent through electronic channels must be encrypted unless a patient consents otherwise. If information can be transmitted through a website’s comment box, patients should be advised that the transmission is not secure before they send their information, Mr. Sacopulos said.
• Secure any online appointment scheduling. Make sure that patients’ names and personal information are not visible to other patients when they schedule appointments, he said. A cardiology practice in Phoenix learned this the hard way when it had to pay the U.S. Department of Health & Human Services $100,000 for lack of HIPAA safeguards online. An investigation by the Office for Civil Rights found the practice was posting clinical and surgical appointments for patients on an Internet-based calendar that was publicly accessible.
• Ensure patient anonymity. The accidental release of private medical information occurred on the website of a St. Louis physician who obtained consent from her patients to include their before and after photos. No names were posted with the photos, but the computer file names of the photos included the patients’ names, and when a person scrolled over a photo with a cursor, the file name popped up. This allowed the public to view the patient name associated with each photo and caused serious problems for the practice, including litigation, he noted.
• Be aware of state board requirements that pertain to physician practice websites. Several state boards do not allow testimonials to be posted on websites. States also differ on the inclusion of before and after photos. New Jersey, for example, allows before and after photos on websites, while New York does not. Some state boards allow doctors to cite that they are board certified on a website without specifics, while states such as Louisiana require that physicians announce the specific certifying board.
“These are ethical and affirmative duties on behalf of physicians that oftentimes come up in websites,” he said.
• Adhere to the Americans With Disabilities Act (ADA). A website is considered real estate for purposes of the ADA, meaning it must include an accessible format to patients with disabilities, Mr. Sacupulos said. Problems arise when certain website features make sites difficult or incompatible with assistance devices that disabled patients require, such as a screen reader or voice interactive software. The National Federation for the Blind has been active in this area and has filed multiple class action lawsuits against companies that did not have compliant websites, he said. An ADA tool kit for best website practices can be found online.
• Hire an experienced Web designer to create the practice’s website. Too often, practices use a family member or friend to set up the company’s page, Mr. Sacupulos said. In one such instance, a young designer became angry at his doctor employer and set up a false website in his name, alleging abuses against patients. “Work with someone credible,” he advised. “Make sure you own your own domain. Many of these Web designers will purchase the domain name and build a site around it, which is great until you want to move to the next Web designer, and then you have to buy your domain back.”
On Twitter @legal_med
EXPERT ANALYSIS FROM THE PHYSICIANS LEGAL ISSUES CONFERENCE
Unanimous vote sends mental health reform to House floor
The much contested Helping Families in Mental Health Crisis Act is at last headed for the House floor. The bipartisan bill was passed 53-0 by members of the House Energy and Commerce Committee.
“The 53-0 vote marks another important milestone to delivering meaningful reforms to families in mental health crisis. Those suffering from mental illness need the attention of this Congress, and I hope the House will swiftly follow our lead,” Rep. Fred Upton (R-Mich.), chairman of the committee, said in a statement.
Much of the debate on H.R. 2646, introduced last year by Rep. Tim Murphy (R-Pa.), has centered on its call for relaxation of HIPAA rules, tougher assisted outpatient treatment requirements, and changes to the structure of the Substance Abuse and Mental Health Services Administration (SAMHSA).
The bill originally called for relaxing HIPAA somewhat so that parents and caregivers could receive patient care information, excluding psychotherapy notes. Instead, the bill as passed by the committee calls upon the Department of Health and Human Services to clarify the law and better educate medical personnel as to what is and isn’t appropriate to share, particularly when a patient is considered incapacitated.
Rep. Murphy, who is a clinical child psychologist, also wanted to more widely implement assisted outpatient treatment (AOT) for patients with serious mental illness. In the end, the committee approved a 2-year extension for AOT block grant funding into 2020, at a cost of an additional $5 million.
The bill as passed by the committee entrusts the administration of SAMHSA to a cabinet-level appointee and calls for that individual to be a physician or clinical psychologist. Various checks and balances have also been added to ensure that if the bill becomes law, SAMHSA will focus on evidence-based practices.
The bill (which greatly mirrors the U.S. Senate bill the Mental Health Reform Act of 2016, S. 2680) now heads to the House floor where Speaker Paul Ryan (R-Wis.) has pledged to give it priority this summer.
“Here and now this committee jointly proclaims that the diagnosis and treatment of mental illness must come out of the shadows. We declare a new dawn of hope for the care of those with mental illness and we pledge our unwavering commitment to continued work to bring help and hope in the future,” Rep. Murphy said in a statement.
On Twitter @whitneymcknight
The much contested Helping Families in Mental Health Crisis Act is at last headed for the House floor. The bipartisan bill was passed 53-0 by members of the House Energy and Commerce Committee.
“The 53-0 vote marks another important milestone to delivering meaningful reforms to families in mental health crisis. Those suffering from mental illness need the attention of this Congress, and I hope the House will swiftly follow our lead,” Rep. Fred Upton (R-Mich.), chairman of the committee, said in a statement.
Much of the debate on H.R. 2646, introduced last year by Rep. Tim Murphy (R-Pa.), has centered on its call for relaxation of HIPAA rules, tougher assisted outpatient treatment requirements, and changes to the structure of the Substance Abuse and Mental Health Services Administration (SAMHSA).
The bill originally called for relaxing HIPAA somewhat so that parents and caregivers could receive patient care information, excluding psychotherapy notes. Instead, the bill as passed by the committee calls upon the Department of Health and Human Services to clarify the law and better educate medical personnel as to what is and isn’t appropriate to share, particularly when a patient is considered incapacitated.
Rep. Murphy, who is a clinical child psychologist, also wanted to more widely implement assisted outpatient treatment (AOT) for patients with serious mental illness. In the end, the committee approved a 2-year extension for AOT block grant funding into 2020, at a cost of an additional $5 million.
The bill as passed by the committee entrusts the administration of SAMHSA to a cabinet-level appointee and calls for that individual to be a physician or clinical psychologist. Various checks and balances have also been added to ensure that if the bill becomes law, SAMHSA will focus on evidence-based practices.
The bill (which greatly mirrors the U.S. Senate bill the Mental Health Reform Act of 2016, S. 2680) now heads to the House floor where Speaker Paul Ryan (R-Wis.) has pledged to give it priority this summer.
“Here and now this committee jointly proclaims that the diagnosis and treatment of mental illness must come out of the shadows. We declare a new dawn of hope for the care of those with mental illness and we pledge our unwavering commitment to continued work to bring help and hope in the future,” Rep. Murphy said in a statement.
On Twitter @whitneymcknight
The much contested Helping Families in Mental Health Crisis Act is at last headed for the House floor. The bipartisan bill was passed 53-0 by members of the House Energy and Commerce Committee.
“The 53-0 vote marks another important milestone to delivering meaningful reforms to families in mental health crisis. Those suffering from mental illness need the attention of this Congress, and I hope the House will swiftly follow our lead,” Rep. Fred Upton (R-Mich.), chairman of the committee, said in a statement.
Much of the debate on H.R. 2646, introduced last year by Rep. Tim Murphy (R-Pa.), has centered on its call for relaxation of HIPAA rules, tougher assisted outpatient treatment requirements, and changes to the structure of the Substance Abuse and Mental Health Services Administration (SAMHSA).
The bill originally called for relaxing HIPAA somewhat so that parents and caregivers could receive patient care information, excluding psychotherapy notes. Instead, the bill as passed by the committee calls upon the Department of Health and Human Services to clarify the law and better educate medical personnel as to what is and isn’t appropriate to share, particularly when a patient is considered incapacitated.
Rep. Murphy, who is a clinical child psychologist, also wanted to more widely implement assisted outpatient treatment (AOT) for patients with serious mental illness. In the end, the committee approved a 2-year extension for AOT block grant funding into 2020, at a cost of an additional $5 million.
The bill as passed by the committee entrusts the administration of SAMHSA to a cabinet-level appointee and calls for that individual to be a physician or clinical psychologist. Various checks and balances have also been added to ensure that if the bill becomes law, SAMHSA will focus on evidence-based practices.
The bill (which greatly mirrors the U.S. Senate bill the Mental Health Reform Act of 2016, S. 2680) now heads to the House floor where Speaker Paul Ryan (R-Wis.) has pledged to give it priority this summer.
“Here and now this committee jointly proclaims that the diagnosis and treatment of mental illness must come out of the shadows. We declare a new dawn of hope for the care of those with mental illness and we pledge our unwavering commitment to continued work to bring help and hope in the future,” Rep. Murphy said in a statement.
On Twitter @whitneymcknight
Ultrasound bests auscultation for ETT positioning
SAN DIEGO – Assessment of the trachea and pleura via point-of-care ultrasound is superior to auscultation in determining the exact location of the endotracheal tube, a randomized, single-center study found.
“It’s been reported that about 20% of the time the endotracheal tube is malpositioned,” study author Dr. Davinder S. Ramsingh said in an interview at the annual meeting of the American Society of Anesthesiologists. “Most of the time (the tube) is too deep, which can lead to severe complications.”
In a double-blinded, randomized study, Dr. Ramsingh and his associates assessed the accuracy of auscultation vs. point-of-care ultrasound in verifying the correct position of the endotracheal tube (ETT). They enrolled 42 adults who required general anesthesia with ETT and randomized them to right main bronchus, left main bronchus, or tracheal intubation, followed by fiber optically–guided visualization to place the ETT. Next, an anesthesiologist blinded to the ETT exact location used auscultation to assess the location of the ETT, while another anesthesiologist blinded to the ETT exact location used point-of-care ultrasound to assess the location of the ETT. The ultrasound exam consisted of assessing tracheal dilation via standard cuff inflation with air and evaluation of pleural lung sliding, explained Dr. Ramsingh of the department of anesthesiology and perioperative care at the University of California, Irvine.
Dr. Ramsingh reported that in differentiating tracheal versus bronchial intubations, auscultation demonstrated a sensitivity of 66% and a specificity of 59%, while ultrasound demonstrated a sensitivity of 93% and a specificity of 96%. Chi-square comparison showed a statistically significant improvement with ultrasound (P = .0005), while inter-observer agreement of the ultrasound findings was 100%.
Limitations of the study, he said, include the fact that “we don’t know the incidence of malpositioned endotracheal tubes in the operating room and that this study was evaluating patients undergoing elective surgical procedures.”
The researchers reported having no financial disclosures.
SAN DIEGO – Assessment of the trachea and pleura via point-of-care ultrasound is superior to auscultation in determining the exact location of the endotracheal tube, a randomized, single-center study found.
“It’s been reported that about 20% of the time the endotracheal tube is malpositioned,” study author Dr. Davinder S. Ramsingh said in an interview at the annual meeting of the American Society of Anesthesiologists. “Most of the time (the tube) is too deep, which can lead to severe complications.”
In a double-blinded, randomized study, Dr. Ramsingh and his associates assessed the accuracy of auscultation vs. point-of-care ultrasound in verifying the correct position of the endotracheal tube (ETT). They enrolled 42 adults who required general anesthesia with ETT and randomized them to right main bronchus, left main bronchus, or tracheal intubation, followed by fiber optically–guided visualization to place the ETT. Next, an anesthesiologist blinded to the ETT exact location used auscultation to assess the location of the ETT, while another anesthesiologist blinded to the ETT exact location used point-of-care ultrasound to assess the location of the ETT. The ultrasound exam consisted of assessing tracheal dilation via standard cuff inflation with air and evaluation of pleural lung sliding, explained Dr. Ramsingh of the department of anesthesiology and perioperative care at the University of California, Irvine.
Dr. Ramsingh reported that in differentiating tracheal versus bronchial intubations, auscultation demonstrated a sensitivity of 66% and a specificity of 59%, while ultrasound demonstrated a sensitivity of 93% and a specificity of 96%. Chi-square comparison showed a statistically significant improvement with ultrasound (P = .0005), while inter-observer agreement of the ultrasound findings was 100%.
Limitations of the study, he said, include the fact that “we don’t know the incidence of malpositioned endotracheal tubes in the operating room and that this study was evaluating patients undergoing elective surgical procedures.”
The researchers reported having no financial disclosures.
SAN DIEGO – Assessment of the trachea and pleura via point-of-care ultrasound is superior to auscultation in determining the exact location of the endotracheal tube, a randomized, single-center study found.
“It’s been reported that about 20% of the time the endotracheal tube is malpositioned,” study author Dr. Davinder S. Ramsingh said in an interview at the annual meeting of the American Society of Anesthesiologists. “Most of the time (the tube) is too deep, which can lead to severe complications.”
In a double-blinded, randomized study, Dr. Ramsingh and his associates assessed the accuracy of auscultation vs. point-of-care ultrasound in verifying the correct position of the endotracheal tube (ETT). They enrolled 42 adults who required general anesthesia with ETT and randomized them to right main bronchus, left main bronchus, or tracheal intubation, followed by fiber optically–guided visualization to place the ETT. Next, an anesthesiologist blinded to the ETT exact location used auscultation to assess the location of the ETT, while another anesthesiologist blinded to the ETT exact location used point-of-care ultrasound to assess the location of the ETT. The ultrasound exam consisted of assessing tracheal dilation via standard cuff inflation with air and evaluation of pleural lung sliding, explained Dr. Ramsingh of the department of anesthesiology and perioperative care at the University of California, Irvine.
Dr. Ramsingh reported that in differentiating tracheal versus bronchial intubations, auscultation demonstrated a sensitivity of 66% and a specificity of 59%, while ultrasound demonstrated a sensitivity of 93% and a specificity of 96%. Chi-square comparison showed a statistically significant improvement with ultrasound (P = .0005), while inter-observer agreement of the ultrasound findings was 100%.
Limitations of the study, he said, include the fact that “we don’t know the incidence of malpositioned endotracheal tubes in the operating room and that this study was evaluating patients undergoing elective surgical procedures.”
The researchers reported having no financial disclosures.
AT THE ASA ANNUAL MEETING
Key clinical point: Using point-of-care ultrasound was superior to auscultation in determining the exact location of the endotracheal tube.
Major finding: In differentiating tracheal versus bronchial intubations, auscultation demonstrated a sensitivity of 66% and a specificity of 59%, while ultrasound demonstrated a sensitivity of 93% and a specificity of 96%.
Data source: An randomized study of 42 adults who required general anesthesia with ETT.
Disclosures: The researchers reported having no financial disclosures.
Delaying renal replacement therapy in critically ill patients has advantages
SAN FRANCISCO – Delaying initiation of renal replacement therapy in critically ill patients with severe acute kidney injury appears to be not only safe but beneficial, according to a randomized controlled trial conducted in France.
The trial, known as Artificial Kidney Initiation in Kidney Injury (AKIKI), was conducted among 620 adult patients from 31 intensive care units. The investigators were led by Dr. Stéphane Gaudry of Assistance Publique–Hôpitaux de Paris.
The death rate did not differ significantly between groups assigned to an early versus a late initiation strategy, according to results presented at an international conference of the American Thoracic Society and simultaneously published (N Engl J Med. 2016 May 15. doi: 10.1056/NEJMoa1603017).
Moreover, nearly half of the patients in the delayed initiation group were able to avoid renal replacement therapy. And they were less likely to develop bloodstream infections and had more rapid onset of diuresis (heralding recovery of renal function) than did peers in whom the therapy was initiated early.
“Our study should not be interpreted as suggesting that a ‘wait and see’ approach is safe for all patients. Indeed, careful surveillance is mandatory when deciding to delay renal-replacement therapy in patients with severe acute kidney injury so that any complications will be detected and renal-replacement therapy initiated without delay,” the investigators concluded. “In our trial, delaying the initiation of therapy allowed many patients to recover from acute kidney injury without embarking on such a treatment course.”
Further, the “findings may not be generalizable, because more than 50% of the patients in our trial received intermittent hemodialysis as the first method of therapy and only 30% of the patients received continuous renal-replacement therapy as the sole method (with no intermittent dialysis at any time).”
The author of an accompanying editorial, Dr. Ravindra L. Mehta of the University of California, San Diego, lists some caveats in interpreting the trial’s findings as support for the delayed initiation strategy.
For example, he notes, the longer time to initiation with the delayed strategy contributed to worsening of metabolic and clinical status in the patients who ultimately did need therapy; the study did not assess the development of chronic kidney disease; and the types of renal replacement therapy selected for patients seem “surprising” as the majority put on this therapy needed vasopressors.
“The findings highlight a need for dynamic risk-stratification tools to identify patients who will not need renal-replacement therapy for management of their acute kidney injury,” Dr. Mehta concluded, noting that ongoing studies should help inform management in this area. “Meanwhile, we should focus on the timely application of renal-replacement therapy while considering individual patient characteristics, process-of-care elements, and logistics to achieve therapeutic goals …”
Patients were eligible for the trial if they had severe acute kidney injury, defined as Kidney Disease: Improving Global Outcomes (KDIGO) stage 3; required mechanical ventilation, catecholamine infusion, or both; and did not have a potentially life-threatening complication directly related to renal failure.
In those assigned to the early strategy, renal replacement therapy was started immediately after randomization. In those assigned to the delayed strategy, it was started if any of several criteria was met: severe hyperkalemia, metabolic acidosis, pulmonary edema, blood urea nitrogen level higher than 112 mg/dL, or oliguria for more than 72 hours after randomization. The specific type of renal replacement therapy was left up to each study site.
The median time between randomization and initiation of renal replacement therapy was 2 hours in the early strategy group and 57 hours in the delayed strategy group.
The estimated 60-day mortality rate – the trial’s primary outcome – was 48.5% with early initiation of therapy and 49.7% with delayed initiation, a nonsignificant difference.
Fully 49% of patients in the delayed strategy group never received renal replacement therapy. In addition, patients in this group were half as likely as were peers in the early initiation group to develop a bloodstream infection (5% vs. 10%), and they had more rapid onset of diuresis (P less than .001).
The groups were essentially the same with respect to the rate of gastrointestinal bleeding and the lengths of stay in the intensive care unit and in the hospital.
Dr. Gaudry disclosed that he received grant support from the French Ministry of Health during the study, and from XENIOS France outside the research. The trial was supported by a grant from Programme Hospitalier de Recherche Clinique National, 2012 (AOM12456), funded by the French Ministry of Health..
SAN FRANCISCO – Delaying initiation of renal replacement therapy in critically ill patients with severe acute kidney injury appears to be not only safe but beneficial, according to a randomized controlled trial conducted in France.
The trial, known as Artificial Kidney Initiation in Kidney Injury (AKIKI), was conducted among 620 adult patients from 31 intensive care units. The investigators were led by Dr. Stéphane Gaudry of Assistance Publique–Hôpitaux de Paris.
The death rate did not differ significantly between groups assigned to an early versus a late initiation strategy, according to results presented at an international conference of the American Thoracic Society and simultaneously published (N Engl J Med. 2016 May 15. doi: 10.1056/NEJMoa1603017).
Moreover, nearly half of the patients in the delayed initiation group were able to avoid renal replacement therapy. And they were less likely to develop bloodstream infections and had more rapid onset of diuresis (heralding recovery of renal function) than did peers in whom the therapy was initiated early.
“Our study should not be interpreted as suggesting that a ‘wait and see’ approach is safe for all patients. Indeed, careful surveillance is mandatory when deciding to delay renal-replacement therapy in patients with severe acute kidney injury so that any complications will be detected and renal-replacement therapy initiated without delay,” the investigators concluded. “In our trial, delaying the initiation of therapy allowed many patients to recover from acute kidney injury without embarking on such a treatment course.”
Further, the “findings may not be generalizable, because more than 50% of the patients in our trial received intermittent hemodialysis as the first method of therapy and only 30% of the patients received continuous renal-replacement therapy as the sole method (with no intermittent dialysis at any time).”
The author of an accompanying editorial, Dr. Ravindra L. Mehta of the University of California, San Diego, lists some caveats in interpreting the trial’s findings as support for the delayed initiation strategy.
For example, he notes, the longer time to initiation with the delayed strategy contributed to worsening of metabolic and clinical status in the patients who ultimately did need therapy; the study did not assess the development of chronic kidney disease; and the types of renal replacement therapy selected for patients seem “surprising” as the majority put on this therapy needed vasopressors.
“The findings highlight a need for dynamic risk-stratification tools to identify patients who will not need renal-replacement therapy for management of their acute kidney injury,” Dr. Mehta concluded, noting that ongoing studies should help inform management in this area. “Meanwhile, we should focus on the timely application of renal-replacement therapy while considering individual patient characteristics, process-of-care elements, and logistics to achieve therapeutic goals …”
Patients were eligible for the trial if they had severe acute kidney injury, defined as Kidney Disease: Improving Global Outcomes (KDIGO) stage 3; required mechanical ventilation, catecholamine infusion, or both; and did not have a potentially life-threatening complication directly related to renal failure.
In those assigned to the early strategy, renal replacement therapy was started immediately after randomization. In those assigned to the delayed strategy, it was started if any of several criteria was met: severe hyperkalemia, metabolic acidosis, pulmonary edema, blood urea nitrogen level higher than 112 mg/dL, or oliguria for more than 72 hours after randomization. The specific type of renal replacement therapy was left up to each study site.
The median time between randomization and initiation of renal replacement therapy was 2 hours in the early strategy group and 57 hours in the delayed strategy group.
The estimated 60-day mortality rate – the trial’s primary outcome – was 48.5% with early initiation of therapy and 49.7% with delayed initiation, a nonsignificant difference.
Fully 49% of patients in the delayed strategy group never received renal replacement therapy. In addition, patients in this group were half as likely as were peers in the early initiation group to develop a bloodstream infection (5% vs. 10%), and they had more rapid onset of diuresis (P less than .001).
The groups were essentially the same with respect to the rate of gastrointestinal bleeding and the lengths of stay in the intensive care unit and in the hospital.
Dr. Gaudry disclosed that he received grant support from the French Ministry of Health during the study, and from XENIOS France outside the research. The trial was supported by a grant from Programme Hospitalier de Recherche Clinique National, 2012 (AOM12456), funded by the French Ministry of Health..
SAN FRANCISCO – Delaying initiation of renal replacement therapy in critically ill patients with severe acute kidney injury appears to be not only safe but beneficial, according to a randomized controlled trial conducted in France.
The trial, known as Artificial Kidney Initiation in Kidney Injury (AKIKI), was conducted among 620 adult patients from 31 intensive care units. The investigators were led by Dr. Stéphane Gaudry of Assistance Publique–Hôpitaux de Paris.
The death rate did not differ significantly between groups assigned to an early versus a late initiation strategy, according to results presented at an international conference of the American Thoracic Society and simultaneously published (N Engl J Med. 2016 May 15. doi: 10.1056/NEJMoa1603017).
Moreover, nearly half of the patients in the delayed initiation group were able to avoid renal replacement therapy. And they were less likely to develop bloodstream infections and had more rapid onset of diuresis (heralding recovery of renal function) than did peers in whom the therapy was initiated early.
“Our study should not be interpreted as suggesting that a ‘wait and see’ approach is safe for all patients. Indeed, careful surveillance is mandatory when deciding to delay renal-replacement therapy in patients with severe acute kidney injury so that any complications will be detected and renal-replacement therapy initiated without delay,” the investigators concluded. “In our trial, delaying the initiation of therapy allowed many patients to recover from acute kidney injury without embarking on such a treatment course.”
Further, the “findings may not be generalizable, because more than 50% of the patients in our trial received intermittent hemodialysis as the first method of therapy and only 30% of the patients received continuous renal-replacement therapy as the sole method (with no intermittent dialysis at any time).”
The author of an accompanying editorial, Dr. Ravindra L. Mehta of the University of California, San Diego, lists some caveats in interpreting the trial’s findings as support for the delayed initiation strategy.
For example, he notes, the longer time to initiation with the delayed strategy contributed to worsening of metabolic and clinical status in the patients who ultimately did need therapy; the study did not assess the development of chronic kidney disease; and the types of renal replacement therapy selected for patients seem “surprising” as the majority put on this therapy needed vasopressors.
“The findings highlight a need for dynamic risk-stratification tools to identify patients who will not need renal-replacement therapy for management of their acute kidney injury,” Dr. Mehta concluded, noting that ongoing studies should help inform management in this area. “Meanwhile, we should focus on the timely application of renal-replacement therapy while considering individual patient characteristics, process-of-care elements, and logistics to achieve therapeutic goals …”
Patients were eligible for the trial if they had severe acute kidney injury, defined as Kidney Disease: Improving Global Outcomes (KDIGO) stage 3; required mechanical ventilation, catecholamine infusion, or both; and did not have a potentially life-threatening complication directly related to renal failure.
In those assigned to the early strategy, renal replacement therapy was started immediately after randomization. In those assigned to the delayed strategy, it was started if any of several criteria was met: severe hyperkalemia, metabolic acidosis, pulmonary edema, blood urea nitrogen level higher than 112 mg/dL, or oliguria for more than 72 hours after randomization. The specific type of renal replacement therapy was left up to each study site.
The median time between randomization and initiation of renal replacement therapy was 2 hours in the early strategy group and 57 hours in the delayed strategy group.
The estimated 60-day mortality rate – the trial’s primary outcome – was 48.5% with early initiation of therapy and 49.7% with delayed initiation, a nonsignificant difference.
Fully 49% of patients in the delayed strategy group never received renal replacement therapy. In addition, patients in this group were half as likely as were peers in the early initiation group to develop a bloodstream infection (5% vs. 10%), and they had more rapid onset of diuresis (P less than .001).
The groups were essentially the same with respect to the rate of gastrointestinal bleeding and the lengths of stay in the intensive care unit and in the hospital.
Dr. Gaudry disclosed that he received grant support from the French Ministry of Health during the study, and from XENIOS France outside the research. The trial was supported by a grant from Programme Hospitalier de Recherche Clinique National, 2012 (AOM12456), funded by the French Ministry of Health..
AT ATS 2016
Key clinical point: Delaying initiation of renal replacement therapy in critically ill patients with kidney injury appears safe and beneficial.
Major finding: The estimated 60-day mortality rate did not differ significantly between the early and delayed initiation strategies (48.5% vs. 49.7%).
Data source: A randomized controlled trial of 620 critically ill patients with severe acute kidney injury.
Disclosures: Dr. Gaudry disclosed that he received grant support from the French Ministry of Health during the study, and from XENIOS France outside the research. The trial was supported by a grant from Programme Hospitalier de Recherche Clinique National, 2012 (AOM12456), funded by the French Ministry of Health.