User login
EMERGENCY MEDICINE is a practical, peer-reviewed monthly publication and Web site that meets the educational needs of emergency clinicians and urgent care clinicians for their practice.
Vertebral fractures in COVID-19 linked to mortality
Vertebral fractures appear to be common in people with severe COVID-19, and also raise the mortality risk, findings from a retrospective cohort suggest.
Among 114 patients with COVID-19 who underwent lateral chest x-rays at the San Raffaele Hospital ED in Milan, more than a third were found to have thoracic vertebral fractures. And, those individuals were more than twice as likely to die as were those without vertebral fractures.
“Morphometric vertebral fractures are one of the most common comorbidities among adults hospitalized with COVID-19, and the presence of such fractures may predict the severity of disease outcomes,” lead investigator Andrea Giustina, MD, said in an interview.
This is the first study to examine vertebral fracture prevalence in any coronavirus disease, but such fractures have been linked to an increased risk of pneumonia and impaired respiratory function, including restrictive pulmonary dysfunction. One possible mechanism may be that they cause anatomical changes, such as kyphosis, which negatively impact respiratory function by decreasing vital capacity, forced expiratory volume in 1 second, and inspiratory time, explained Dr. Giustina, professor of endocrinology, San Raffaele Vita Salute University, Milan, and president of the European Society of Endocrinology. The results were published in the Journal of Clinical Endocrinology and Metabolism.
Clinically, the findings suggest that all patients with COVID-19 who are undergoing chest x-rays should have morphometric vertebral x-ray evaluation, said Dr. Giustina.
“One interesting aspect of the study is that without morphometry, approximatively two thirds of vertebral fractures [would have been] missed. Therefore, they are largely underestimated in clinical practice,” he noted.
Thoracic vertebral fractures assessed via lateral chest x-rays
The 114 study subjects included were those whose lateral chest x-rays allowed for a high-quality assessment and in which all the thoracic tract of T4-T12 were viewable and assessable. None had been using glucocorticoids and only 3% had a prior diagnosis of osteoporosis.
The majority (75%) were male, and median age was 57 years. Most (79%) were hospitalized after evaluation in the ED. Of those, 12% (13) were admitted to the ICU and 15% (16) died.
Thoracic vertebral fractures were detected on the lateral chest x-rays in 36% (41) of the patients. In contrast, in studies of women aged 50 years and older from the general European population, morphometric vertebral fracture prevalence ranged from 18% to 26%, the investigators noted.
Of the total 65 vertebral fractures detected, 60% were classified as mild (height ratio decrease <25%), 33.3% as moderate (25%-40% decrease) and 7.7% as severe (>40%). Patients with more than one vertebral fracture were classified by their most severe one.
Those with versus without vertebral fractures didn’t differ by sex, body mass index, or clinical or biological parameters evaluated in the ED. But, compared with those without vertebral fractures, those with them were significantly older (68 vs. 54 years) and were more likely to have arterial hypertension (56% vs. 30%) and coronary artery disease (22% vs. 7%).
In multivariate analysis, age was the only statistically significant predictor of vertebral fractures (odds ratio, 1.04; P < .001).
Mortality doubled, though not significantly
Those with vertebral fractures were more likely to be hospitalized, although not significantly (88% vs. 74%). There was no significant difference in ICU admission (11% vs. 12.5%).
However, those with vertebral fractures required noninvasive mechanical ventilation significantly more often (48.8% vs. 27.4%; P = .02), and were more than twice as likely to die (22% vs. 10%; P = .07). While the difference in overall mortality wasn’t quite statistically significant, those with severe vertebral fractures were significantly more likely to die, compared with those with mild or moderate fractures (60%, 7%, 24%, respectively, for severe, moderate, and mild; P = .04), despite no significant differences in clinical or laboratory parameters.
“Our data from the field reinforce the need of implementing previously published recommendations concerning the importance of bone fragility care during the COVID pandemic with at least those patients already treated with antiosteoporotic drugs maintaining their adherence to treatments including vitamin D, which have also been suggested very recently to have no relevant predisposing effect on COVID-19,” Dr. Giustina and colleagues wrote.
Moreover, they added, “continuity of care should also include bone density monitoring despite very restricted access to clinical facilities, during the COVID-19 pandemic. Finally, all patients with fractures should start antiresorptive treatment right away, even during hospital stay.”
The authors reported having no disclosures.
SOURCE: Giustina A et al. J Clin Endocrinol Metab. 2020 Oct 21. doi: 10.1210/clinem/dgaa738.
Vertebral fractures appear to be common in people with severe COVID-19, and also raise the mortality risk, findings from a retrospective cohort suggest.
Among 114 patients with COVID-19 who underwent lateral chest x-rays at the San Raffaele Hospital ED in Milan, more than a third were found to have thoracic vertebral fractures. And, those individuals were more than twice as likely to die as were those without vertebral fractures.
“Morphometric vertebral fractures are one of the most common comorbidities among adults hospitalized with COVID-19, and the presence of such fractures may predict the severity of disease outcomes,” lead investigator Andrea Giustina, MD, said in an interview.
This is the first study to examine vertebral fracture prevalence in any coronavirus disease, but such fractures have been linked to an increased risk of pneumonia and impaired respiratory function, including restrictive pulmonary dysfunction. One possible mechanism may be that they cause anatomical changes, such as kyphosis, which negatively impact respiratory function by decreasing vital capacity, forced expiratory volume in 1 second, and inspiratory time, explained Dr. Giustina, professor of endocrinology, San Raffaele Vita Salute University, Milan, and president of the European Society of Endocrinology. The results were published in the Journal of Clinical Endocrinology and Metabolism.
Clinically, the findings suggest that all patients with COVID-19 who are undergoing chest x-rays should have morphometric vertebral x-ray evaluation, said Dr. Giustina.
“One interesting aspect of the study is that without morphometry, approximatively two thirds of vertebral fractures [would have been] missed. Therefore, they are largely underestimated in clinical practice,” he noted.
Thoracic vertebral fractures assessed via lateral chest x-rays
The 114 study subjects included were those whose lateral chest x-rays allowed for a high-quality assessment and in which all the thoracic tract of T4-T12 were viewable and assessable. None had been using glucocorticoids and only 3% had a prior diagnosis of osteoporosis.
The majority (75%) were male, and median age was 57 years. Most (79%) were hospitalized after evaluation in the ED. Of those, 12% (13) were admitted to the ICU and 15% (16) died.
Thoracic vertebral fractures were detected on the lateral chest x-rays in 36% (41) of the patients. In contrast, in studies of women aged 50 years and older from the general European population, morphometric vertebral fracture prevalence ranged from 18% to 26%, the investigators noted.
Of the total 65 vertebral fractures detected, 60% were classified as mild (height ratio decrease <25%), 33.3% as moderate (25%-40% decrease) and 7.7% as severe (>40%). Patients with more than one vertebral fracture were classified by their most severe one.
Those with versus without vertebral fractures didn’t differ by sex, body mass index, or clinical or biological parameters evaluated in the ED. But, compared with those without vertebral fractures, those with them were significantly older (68 vs. 54 years) and were more likely to have arterial hypertension (56% vs. 30%) and coronary artery disease (22% vs. 7%).
In multivariate analysis, age was the only statistically significant predictor of vertebral fractures (odds ratio, 1.04; P < .001).
Mortality doubled, though not significantly
Those with vertebral fractures were more likely to be hospitalized, although not significantly (88% vs. 74%). There was no significant difference in ICU admission (11% vs. 12.5%).
However, those with vertebral fractures required noninvasive mechanical ventilation significantly more often (48.8% vs. 27.4%; P = .02), and were more than twice as likely to die (22% vs. 10%; P = .07). While the difference in overall mortality wasn’t quite statistically significant, those with severe vertebral fractures were significantly more likely to die, compared with those with mild or moderate fractures (60%, 7%, 24%, respectively, for severe, moderate, and mild; P = .04), despite no significant differences in clinical or laboratory parameters.
“Our data from the field reinforce the need of implementing previously published recommendations concerning the importance of bone fragility care during the COVID pandemic with at least those patients already treated with antiosteoporotic drugs maintaining their adherence to treatments including vitamin D, which have also been suggested very recently to have no relevant predisposing effect on COVID-19,” Dr. Giustina and colleagues wrote.
Moreover, they added, “continuity of care should also include bone density monitoring despite very restricted access to clinical facilities, during the COVID-19 pandemic. Finally, all patients with fractures should start antiresorptive treatment right away, even during hospital stay.”
The authors reported having no disclosures.
SOURCE: Giustina A et al. J Clin Endocrinol Metab. 2020 Oct 21. doi: 10.1210/clinem/dgaa738.
Vertebral fractures appear to be common in people with severe COVID-19, and also raise the mortality risk, findings from a retrospective cohort suggest.
Among 114 patients with COVID-19 who underwent lateral chest x-rays at the San Raffaele Hospital ED in Milan, more than a third were found to have thoracic vertebral fractures. And, those individuals were more than twice as likely to die as were those without vertebral fractures.
“Morphometric vertebral fractures are one of the most common comorbidities among adults hospitalized with COVID-19, and the presence of such fractures may predict the severity of disease outcomes,” lead investigator Andrea Giustina, MD, said in an interview.
This is the first study to examine vertebral fracture prevalence in any coronavirus disease, but such fractures have been linked to an increased risk of pneumonia and impaired respiratory function, including restrictive pulmonary dysfunction. One possible mechanism may be that they cause anatomical changes, such as kyphosis, which negatively impact respiratory function by decreasing vital capacity, forced expiratory volume in 1 second, and inspiratory time, explained Dr. Giustina, professor of endocrinology, San Raffaele Vita Salute University, Milan, and president of the European Society of Endocrinology. The results were published in the Journal of Clinical Endocrinology and Metabolism.
Clinically, the findings suggest that all patients with COVID-19 who are undergoing chest x-rays should have morphometric vertebral x-ray evaluation, said Dr. Giustina.
“One interesting aspect of the study is that without morphometry, approximatively two thirds of vertebral fractures [would have been] missed. Therefore, they are largely underestimated in clinical practice,” he noted.
Thoracic vertebral fractures assessed via lateral chest x-rays
The 114 study subjects included were those whose lateral chest x-rays allowed for a high-quality assessment and in which all the thoracic tract of T4-T12 were viewable and assessable. None had been using glucocorticoids and only 3% had a prior diagnosis of osteoporosis.
The majority (75%) were male, and median age was 57 years. Most (79%) were hospitalized after evaluation in the ED. Of those, 12% (13) were admitted to the ICU and 15% (16) died.
Thoracic vertebral fractures were detected on the lateral chest x-rays in 36% (41) of the patients. In contrast, in studies of women aged 50 years and older from the general European population, morphometric vertebral fracture prevalence ranged from 18% to 26%, the investigators noted.
Of the total 65 vertebral fractures detected, 60% were classified as mild (height ratio decrease <25%), 33.3% as moderate (25%-40% decrease) and 7.7% as severe (>40%). Patients with more than one vertebral fracture were classified by their most severe one.
Those with versus without vertebral fractures didn’t differ by sex, body mass index, or clinical or biological parameters evaluated in the ED. But, compared with those without vertebral fractures, those with them were significantly older (68 vs. 54 years) and were more likely to have arterial hypertension (56% vs. 30%) and coronary artery disease (22% vs. 7%).
In multivariate analysis, age was the only statistically significant predictor of vertebral fractures (odds ratio, 1.04; P < .001).
Mortality doubled, though not significantly
Those with vertebral fractures were more likely to be hospitalized, although not significantly (88% vs. 74%). There was no significant difference in ICU admission (11% vs. 12.5%).
However, those with vertebral fractures required noninvasive mechanical ventilation significantly more often (48.8% vs. 27.4%; P = .02), and were more than twice as likely to die (22% vs. 10%; P = .07). While the difference in overall mortality wasn’t quite statistically significant, those with severe vertebral fractures were significantly more likely to die, compared with those with mild or moderate fractures (60%, 7%, 24%, respectively, for severe, moderate, and mild; P = .04), despite no significant differences in clinical or laboratory parameters.
“Our data from the field reinforce the need of implementing previously published recommendations concerning the importance of bone fragility care during the COVID pandemic with at least those patients already treated with antiosteoporotic drugs maintaining their adherence to treatments including vitamin D, which have also been suggested very recently to have no relevant predisposing effect on COVID-19,” Dr. Giustina and colleagues wrote.
Moreover, they added, “continuity of care should also include bone density monitoring despite very restricted access to clinical facilities, during the COVID-19 pandemic. Finally, all patients with fractures should start antiresorptive treatment right away, even during hospital stay.”
The authors reported having no disclosures.
SOURCE: Giustina A et al. J Clin Endocrinol Metab. 2020 Oct 21. doi: 10.1210/clinem/dgaa738.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Valvular disease and COVID-19 are a deadly mix; don’t delay intervention
Danny Dvir, MD, has a message for physicians who have patients with severe valvular heart disease who are deferring valve replacement or repair until after the COVID-19 pandemic: Urge them not to wait.
Data from the Multicenter International Valve Disease Registry vividly demonstrate that clinical outcomes are poor in patients with uncorrected valve disease who become hospitalized with COVID-19. Indeed, the mortality rate within 30 days after hospital admission in 136 such patients enrolled in the registry from centers in Europe, North America, and Israel was 42%, Dr. Dvir reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
“That’s dramatically higher than for an age-matched population infected with COVID-19 without valvular heart disease, which is 10%-15%,” he noted at the meeting sponsored by the Cardiovascular Research Foundation.
The bright spot was that, in the small subgroup of 15 registry participants who underwent transcatheter or, much less frequently, surgical treatment of their failing valve while COVID-19 infected, 30-day mortality was far lower. In fact, it was comparable with the background rate in hospitalized COVID-19 patients without valve disease, according to Dr. Dvir, an interventional cardiologist at Shaare Zedek Medical Center, Hebrew University, Jerusalem.
He personally did several of the transcatheter aortic valve replacements.
“It’s doable. I truly believe that when you get a severe aortic stenosis patient who’s infected with the coronavirus, they get very unstable, but we can treat them. We can treat them even during the infection,” Dr. Dvir said.
The majority of patients in the registry had severe aortic stenosis. In the 42 such patients aged 80 years or more who didn’t undergo transcatheter aortic valve replacement (TAVR) or surgical valve replacement, 30-day mortality was 60%. In contrast, only one of the six patients in this advanced-age category who underwent valve replacement while infected died. Similarly, 30-day mortality was 24% among those younger than age 80 who valve remained untreated, but it dropped to 11% in those who received a prosthetic valve.
“We try our best to protect our patients through social distancing, but we have a treatment that can potentially reduce their mortality risk if they get infected later on. So I say to my patients: ‘Don’t wait at home. Do not wait! If you get infected when you have severe aortic stenosis, the clinical outcome is bad.’ But it seems reasonable that if they get infected when they’ve already been treated for their aortic stenosis or mitral regurgitation, they will do better.”
Dr. Dvir noted that, although the case numbers in the registry series were small and subject to potential bias, the data suggest this treatment approach may be lifesaving.
Session comoderator Timothy D. Henry, MD, commented that this registry study contains a great take-home point: “This is really consistent with what see in a lot of the other areas of COVID, that what we know to be best clinical care, we should do it, with or without the COVID.”
He asked Dr. Dvir about any special measures he takes while doing TAVR in this extreme setting. In the United States, for example, interventionalists are increasingly using transesophageal echocardiography to guide their procedures using conscious sedation, without intubation, noted Dr. Henry, medical director of the Carl and Edyth Lindner Center for Research at the Christ Hospital, Cincinnati.
“We try to minimize the procedure time; that’s one of the important things,” Dr. Dvir replied. “And you need to be protected during the procedure in a very cautious and meticulous way. You need many fans in the room because you sweat a lot.”
Discussant Renu Virmani, MD, president of the CVPath Institute in Gaithersburg, Md., commented: “The main thing I get from this presentation is the need for patients to be educated that if you’ve got valve disease, you’re better off getting it treated before you’ve got COVID. Obviously, try to prevent getting COVID – that’s the best thing you can do – but you can’t always control that.”
Discussant Mamas Mamas, MD, professor of cardiology at Keele University, Staffordshire, England, said deferred treatment of severe valvular heart disease during the pandemic has created a looming public health crisis in the United Kingdom.
“We’ve analyzed the U.K. management of aortic stenosis, and what we’ve found is that during the COVID pandemic there have been 2,500 fewer cases of aortic stenosis that have been treated. We’ve got 2,500 patients on the waiting list, and we’ve got to work out how we’re going to treat them. We estimate with simulations that about 300 of them are going to die before we can get them treated for their aortic stenosis,” according to Dr. Mamas.
Dr. Henry commented that deferral of valve procedures is “really challenging” for a couple of reasons: Not only are patients scared to come into the hospital because they fear getting COVID, but they don’t want to be hospitalized during the pandemic because their family can’t visit them there.
“These patients are mostly over 80 years old. No one wants to come in the hospital when the family won’t be around, especially when you’re 90 years old,” the interventional cardiologist said.
Dr. Dvir reported serving as a consultant to Medtronic, Edwards Lifesciences, Abbott, and Jena.
Danny Dvir, MD, has a message for physicians who have patients with severe valvular heart disease who are deferring valve replacement or repair until after the COVID-19 pandemic: Urge them not to wait.
Data from the Multicenter International Valve Disease Registry vividly demonstrate that clinical outcomes are poor in patients with uncorrected valve disease who become hospitalized with COVID-19. Indeed, the mortality rate within 30 days after hospital admission in 136 such patients enrolled in the registry from centers in Europe, North America, and Israel was 42%, Dr. Dvir reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
“That’s dramatically higher than for an age-matched population infected with COVID-19 without valvular heart disease, which is 10%-15%,” he noted at the meeting sponsored by the Cardiovascular Research Foundation.
The bright spot was that, in the small subgroup of 15 registry participants who underwent transcatheter or, much less frequently, surgical treatment of their failing valve while COVID-19 infected, 30-day mortality was far lower. In fact, it was comparable with the background rate in hospitalized COVID-19 patients without valve disease, according to Dr. Dvir, an interventional cardiologist at Shaare Zedek Medical Center, Hebrew University, Jerusalem.
He personally did several of the transcatheter aortic valve replacements.
“It’s doable. I truly believe that when you get a severe aortic stenosis patient who’s infected with the coronavirus, they get very unstable, but we can treat them. We can treat them even during the infection,” Dr. Dvir said.
The majority of patients in the registry had severe aortic stenosis. In the 42 such patients aged 80 years or more who didn’t undergo transcatheter aortic valve replacement (TAVR) or surgical valve replacement, 30-day mortality was 60%. In contrast, only one of the six patients in this advanced-age category who underwent valve replacement while infected died. Similarly, 30-day mortality was 24% among those younger than age 80 who valve remained untreated, but it dropped to 11% in those who received a prosthetic valve.
“We try our best to protect our patients through social distancing, but we have a treatment that can potentially reduce their mortality risk if they get infected later on. So I say to my patients: ‘Don’t wait at home. Do not wait! If you get infected when you have severe aortic stenosis, the clinical outcome is bad.’ But it seems reasonable that if they get infected when they’ve already been treated for their aortic stenosis or mitral regurgitation, they will do better.”
Dr. Dvir noted that, although the case numbers in the registry series were small and subject to potential bias, the data suggest this treatment approach may be lifesaving.
Session comoderator Timothy D. Henry, MD, commented that this registry study contains a great take-home point: “This is really consistent with what see in a lot of the other areas of COVID, that what we know to be best clinical care, we should do it, with or without the COVID.”
He asked Dr. Dvir about any special measures he takes while doing TAVR in this extreme setting. In the United States, for example, interventionalists are increasingly using transesophageal echocardiography to guide their procedures using conscious sedation, without intubation, noted Dr. Henry, medical director of the Carl and Edyth Lindner Center for Research at the Christ Hospital, Cincinnati.
“We try to minimize the procedure time; that’s one of the important things,” Dr. Dvir replied. “And you need to be protected during the procedure in a very cautious and meticulous way. You need many fans in the room because you sweat a lot.”
Discussant Renu Virmani, MD, president of the CVPath Institute in Gaithersburg, Md., commented: “The main thing I get from this presentation is the need for patients to be educated that if you’ve got valve disease, you’re better off getting it treated before you’ve got COVID. Obviously, try to prevent getting COVID – that’s the best thing you can do – but you can’t always control that.”
Discussant Mamas Mamas, MD, professor of cardiology at Keele University, Staffordshire, England, said deferred treatment of severe valvular heart disease during the pandemic has created a looming public health crisis in the United Kingdom.
“We’ve analyzed the U.K. management of aortic stenosis, and what we’ve found is that during the COVID pandemic there have been 2,500 fewer cases of aortic stenosis that have been treated. We’ve got 2,500 patients on the waiting list, and we’ve got to work out how we’re going to treat them. We estimate with simulations that about 300 of them are going to die before we can get them treated for their aortic stenosis,” according to Dr. Mamas.
Dr. Henry commented that deferral of valve procedures is “really challenging” for a couple of reasons: Not only are patients scared to come into the hospital because they fear getting COVID, but they don’t want to be hospitalized during the pandemic because their family can’t visit them there.
“These patients are mostly over 80 years old. No one wants to come in the hospital when the family won’t be around, especially when you’re 90 years old,” the interventional cardiologist said.
Dr. Dvir reported serving as a consultant to Medtronic, Edwards Lifesciences, Abbott, and Jena.
Danny Dvir, MD, has a message for physicians who have patients with severe valvular heart disease who are deferring valve replacement or repair until after the COVID-19 pandemic: Urge them not to wait.
Data from the Multicenter International Valve Disease Registry vividly demonstrate that clinical outcomes are poor in patients with uncorrected valve disease who become hospitalized with COVID-19. Indeed, the mortality rate within 30 days after hospital admission in 136 such patients enrolled in the registry from centers in Europe, North America, and Israel was 42%, Dr. Dvir reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
“That’s dramatically higher than for an age-matched population infected with COVID-19 without valvular heart disease, which is 10%-15%,” he noted at the meeting sponsored by the Cardiovascular Research Foundation.
The bright spot was that, in the small subgroup of 15 registry participants who underwent transcatheter or, much less frequently, surgical treatment of their failing valve while COVID-19 infected, 30-day mortality was far lower. In fact, it was comparable with the background rate in hospitalized COVID-19 patients without valve disease, according to Dr. Dvir, an interventional cardiologist at Shaare Zedek Medical Center, Hebrew University, Jerusalem.
He personally did several of the transcatheter aortic valve replacements.
“It’s doable. I truly believe that when you get a severe aortic stenosis patient who’s infected with the coronavirus, they get very unstable, but we can treat them. We can treat them even during the infection,” Dr. Dvir said.
The majority of patients in the registry had severe aortic stenosis. In the 42 such patients aged 80 years or more who didn’t undergo transcatheter aortic valve replacement (TAVR) or surgical valve replacement, 30-day mortality was 60%. In contrast, only one of the six patients in this advanced-age category who underwent valve replacement while infected died. Similarly, 30-day mortality was 24% among those younger than age 80 who valve remained untreated, but it dropped to 11% in those who received a prosthetic valve.
“We try our best to protect our patients through social distancing, but we have a treatment that can potentially reduce their mortality risk if they get infected later on. So I say to my patients: ‘Don’t wait at home. Do not wait! If you get infected when you have severe aortic stenosis, the clinical outcome is bad.’ But it seems reasonable that if they get infected when they’ve already been treated for their aortic stenosis or mitral regurgitation, they will do better.”
Dr. Dvir noted that, although the case numbers in the registry series were small and subject to potential bias, the data suggest this treatment approach may be lifesaving.
Session comoderator Timothy D. Henry, MD, commented that this registry study contains a great take-home point: “This is really consistent with what see in a lot of the other areas of COVID, that what we know to be best clinical care, we should do it, with or without the COVID.”
He asked Dr. Dvir about any special measures he takes while doing TAVR in this extreme setting. In the United States, for example, interventionalists are increasingly using transesophageal echocardiography to guide their procedures using conscious sedation, without intubation, noted Dr. Henry, medical director of the Carl and Edyth Lindner Center for Research at the Christ Hospital, Cincinnati.
“We try to minimize the procedure time; that’s one of the important things,” Dr. Dvir replied. “And you need to be protected during the procedure in a very cautious and meticulous way. You need many fans in the room because you sweat a lot.”
Discussant Renu Virmani, MD, president of the CVPath Institute in Gaithersburg, Md., commented: “The main thing I get from this presentation is the need for patients to be educated that if you’ve got valve disease, you’re better off getting it treated before you’ve got COVID. Obviously, try to prevent getting COVID – that’s the best thing you can do – but you can’t always control that.”
Discussant Mamas Mamas, MD, professor of cardiology at Keele University, Staffordshire, England, said deferred treatment of severe valvular heart disease during the pandemic has created a looming public health crisis in the United Kingdom.
“We’ve analyzed the U.K. management of aortic stenosis, and what we’ve found is that during the COVID pandemic there have been 2,500 fewer cases of aortic stenosis that have been treated. We’ve got 2,500 patients on the waiting list, and we’ve got to work out how we’re going to treat them. We estimate with simulations that about 300 of them are going to die before we can get them treated for their aortic stenosis,” according to Dr. Mamas.
Dr. Henry commented that deferral of valve procedures is “really challenging” for a couple of reasons: Not only are patients scared to come into the hospital because they fear getting COVID, but they don’t want to be hospitalized during the pandemic because their family can’t visit them there.
“These patients are mostly over 80 years old. No one wants to come in the hospital when the family won’t be around, especially when you’re 90 years old,” the interventional cardiologist said.
Dr. Dvir reported serving as a consultant to Medtronic, Edwards Lifesciences, Abbott, and Jena.
FROM TCT 2020
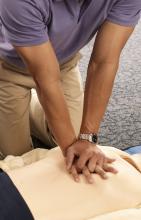
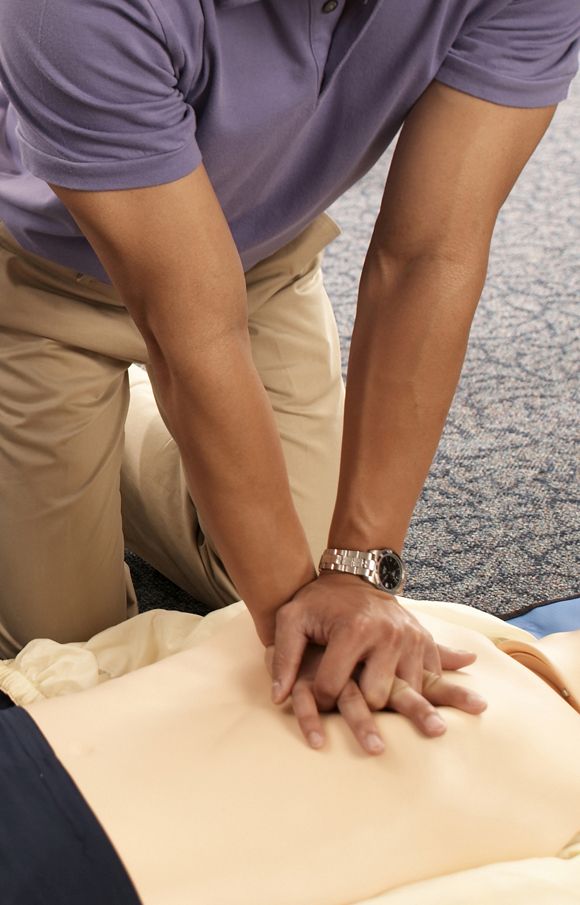
AHA adds recovery, emotional support to CPR guidelines
Highlights of new updated guidelines for cardiopulmonary resuscitation and emergency cardiovascular care from the American Heart Association include management of opioid-related emergencies; discussion of health disparities; and a new emphasis on physical, social, and emotional recovery after resuscitation.
The AHA is also exploring digital territory to improve CPR outcomes. The guidelines encourage use of mobile phone technology to summon trained laypeople to individuals requiring CPR, and an adaptive learning suite will be available online for personalized CPR instruction, with lessons catered to individual needs and knowledge levels.
These novel approaches reflect an ongoing effort by the AHA to ensure that the guidelines evolve rapidly with science and technology, reported Raina Merchant, MD, chair of the AHA Emergency Cardiovascular Care Committee and associate professor of emergency medicine at the University of Pennsylvania, Philadelphia, and colleagues. In 2015, the committee shifted from 5-year updates to a continuous online review process, citing a need for more immediate implementation of practice-altering data, they wrote in Circulation.
And new approaches do appear to save lives, at least in a hospital setting.
Since 2004, in-hospital cardiac arrest outcomes have been improving, but similar gains have yet to be realized for out-of-hospital cardiac arrest.
“Much of the variation in survival rates is thought to be due to the strength of the Chain of Survival, the [five] critical actions that must occur in rapid succession to maximize the chance of survival from cardiac arrest,” the committee wrote.
Update adds sixth link to Chains of Survival: Recovery
“Recovery expectations and survivorship plans that address treatment, surveillance, and rehabilitation need to be provided to cardiac arrest survivors and their caregivers at hospital discharge to address the sequelae of cardiac arrest and optimize transitions of care to independent physical, social, emotional, and role function,” the committee wrote.
Dr. Merchant and colleagues identified three “critically important” recommendations for both cardiac arrest survivors and caregivers during the recovery process: structured psychological assessment; multimodal rehabilitation assessment and treatment; and comprehensive, multidisciplinary discharge planning.
The recovery process is now part of all four Chains of Survival, which are specific to in-hospital and out-of-hospital arrest for adults and children.
New advice on opioid overdoses and bystander training
Among instances of out-of-hospital cardiac arrest, the committee noted that opioid overdoses are “sharply on the rise,” leading to new, scenario-specific recommendations. Among them, the committee encouraged lay rescuers and trained responders to activate emergency response systems immediately while awaiting improvements with naloxone and other interventions. They also suggested that, for individuals in known or suspected cardiac arrest, high-quality CPR, including compressions and ventilation, should be prioritized over naloxone administration.
In a broader discussion, the committee identified disparities in CPR training, which could explain lower rates of bystander CPR and poorer outcomes among certain demographics, such as black and Hispanic populations, as well as those with lower socioeconomic status.
“Targeting training efforts should consider barriers such as language, financial considerations, and poor access to information,” the committee wrote.
While low bystander CPR in these areas may be improved through mobile phone technology that alerts trained laypeople to individuals in need, the committee noted that this approach may be impacted by cultural and geographic factors. To date, use of mobile devices to improve bystander intervention rates has been demonstrated through “uniformly positive data,” but never in North America.
According to the guidelines, bystander intervention rates may also be improved through video-based learning, which is as effective as in-person, instructor-led training.
This led the AHA to create an online adaptive learning platform, which the organization describes as a “digital resuscitation portfolio” that connects programs and courses such as the Resuscitation Quality Improvement program and the HeartCode blended learning course.
“It will cover all of the guideline changes,” said Monica Sales, communications manager at the AHA. “It’s really groundbreaking because it’s the first time that we’re able to kind of close that gap between new science and new products.”
The online content also addresses CPR considerations for COVID-19, which were first addressed by interim CPR guidance published by the AHA in April.
According to Alexis Topjian, MD, coauthor of the present guidelines and pediatric critical care medicine physician at Children’s Hospital of Philadelphia, CPR awareness is more important now than ever.
“The major message [of the guidelines] is that high-quality CPR saves lives,” she said. “So push hard, and push fast. You have the power in your hands to make a difference, more so than ever during this pandemic.”
Concerning coronavirus precautions, Dr. Topjian noted that roughly 70% of out-of-hospital CPR events involve people who know each other, so most bystanders have already been exposed to the person in need, thereby reducing the concern of infection.
When asked about performing CPR on strangers, Dr. Topjian remained encouraging, though she noted that decision making may be informed by local coronavirus rates.
“It’s always a personal choice,” she said.
More for clinicians
For clinicians, Dr. Topjian highlighted several recommendations, including use of epinephrine as soon as possible during CPR, preferential use of a cuffed endotracheal tube, continuous EEG monitoring during and after cardiac arrest, and rapid intervention for clinical seizures and of nonconvulsive status epilepticus.
From a pediatric perspective, Dr. Topjian pointed out a change in breathing rate for infants and children who are receiving CPR or rescue breathing with a pulse, from 12-20 breaths/min to 20-30 breaths/min. While not a new recommendation, Dr. Topjian also pointed out the lifesaving benefit of early defibrillation among pediatric patients.
The guidelines were funded by the American Heart Association. The investigators disclosed additional relationships with BTG Pharmaceuticals, Zoll Foundation, the National Institutes of Health, and others.
SOURCE: American Heart Association. Circulation. 2020 Oct 20. Suppl 2.
Highlights of new updated guidelines for cardiopulmonary resuscitation and emergency cardiovascular care from the American Heart Association include management of opioid-related emergencies; discussion of health disparities; and a new emphasis on physical, social, and emotional recovery after resuscitation.
The AHA is also exploring digital territory to improve CPR outcomes. The guidelines encourage use of mobile phone technology to summon trained laypeople to individuals requiring CPR, and an adaptive learning suite will be available online for personalized CPR instruction, with lessons catered to individual needs and knowledge levels.
These novel approaches reflect an ongoing effort by the AHA to ensure that the guidelines evolve rapidly with science and technology, reported Raina Merchant, MD, chair of the AHA Emergency Cardiovascular Care Committee and associate professor of emergency medicine at the University of Pennsylvania, Philadelphia, and colleagues. In 2015, the committee shifted from 5-year updates to a continuous online review process, citing a need for more immediate implementation of practice-altering data, they wrote in Circulation.
And new approaches do appear to save lives, at least in a hospital setting.
Since 2004, in-hospital cardiac arrest outcomes have been improving, but similar gains have yet to be realized for out-of-hospital cardiac arrest.
“Much of the variation in survival rates is thought to be due to the strength of the Chain of Survival, the [five] critical actions that must occur in rapid succession to maximize the chance of survival from cardiac arrest,” the committee wrote.
Update adds sixth link to Chains of Survival: Recovery
“Recovery expectations and survivorship plans that address treatment, surveillance, and rehabilitation need to be provided to cardiac arrest survivors and their caregivers at hospital discharge to address the sequelae of cardiac arrest and optimize transitions of care to independent physical, social, emotional, and role function,” the committee wrote.
Dr. Merchant and colleagues identified three “critically important” recommendations for both cardiac arrest survivors and caregivers during the recovery process: structured psychological assessment; multimodal rehabilitation assessment and treatment; and comprehensive, multidisciplinary discharge planning.
The recovery process is now part of all four Chains of Survival, which are specific to in-hospital and out-of-hospital arrest for adults and children.
New advice on opioid overdoses and bystander training
Among instances of out-of-hospital cardiac arrest, the committee noted that opioid overdoses are “sharply on the rise,” leading to new, scenario-specific recommendations. Among them, the committee encouraged lay rescuers and trained responders to activate emergency response systems immediately while awaiting improvements with naloxone and other interventions. They also suggested that, for individuals in known or suspected cardiac arrest, high-quality CPR, including compressions and ventilation, should be prioritized over naloxone administration.
In a broader discussion, the committee identified disparities in CPR training, which could explain lower rates of bystander CPR and poorer outcomes among certain demographics, such as black and Hispanic populations, as well as those with lower socioeconomic status.
“Targeting training efforts should consider barriers such as language, financial considerations, and poor access to information,” the committee wrote.
While low bystander CPR in these areas may be improved through mobile phone technology that alerts trained laypeople to individuals in need, the committee noted that this approach may be impacted by cultural and geographic factors. To date, use of mobile devices to improve bystander intervention rates has been demonstrated through “uniformly positive data,” but never in North America.
According to the guidelines, bystander intervention rates may also be improved through video-based learning, which is as effective as in-person, instructor-led training.
This led the AHA to create an online adaptive learning platform, which the organization describes as a “digital resuscitation portfolio” that connects programs and courses such as the Resuscitation Quality Improvement program and the HeartCode blended learning course.
“It will cover all of the guideline changes,” said Monica Sales, communications manager at the AHA. “It’s really groundbreaking because it’s the first time that we’re able to kind of close that gap between new science and new products.”
The online content also addresses CPR considerations for COVID-19, which were first addressed by interim CPR guidance published by the AHA in April.
According to Alexis Topjian, MD, coauthor of the present guidelines and pediatric critical care medicine physician at Children’s Hospital of Philadelphia, CPR awareness is more important now than ever.
“The major message [of the guidelines] is that high-quality CPR saves lives,” she said. “So push hard, and push fast. You have the power in your hands to make a difference, more so than ever during this pandemic.”
Concerning coronavirus precautions, Dr. Topjian noted that roughly 70% of out-of-hospital CPR events involve people who know each other, so most bystanders have already been exposed to the person in need, thereby reducing the concern of infection.
When asked about performing CPR on strangers, Dr. Topjian remained encouraging, though she noted that decision making may be informed by local coronavirus rates.
“It’s always a personal choice,” she said.
More for clinicians
For clinicians, Dr. Topjian highlighted several recommendations, including use of epinephrine as soon as possible during CPR, preferential use of a cuffed endotracheal tube, continuous EEG monitoring during and after cardiac arrest, and rapid intervention for clinical seizures and of nonconvulsive status epilepticus.
From a pediatric perspective, Dr. Topjian pointed out a change in breathing rate for infants and children who are receiving CPR or rescue breathing with a pulse, from 12-20 breaths/min to 20-30 breaths/min. While not a new recommendation, Dr. Topjian also pointed out the lifesaving benefit of early defibrillation among pediatric patients.
The guidelines were funded by the American Heart Association. The investigators disclosed additional relationships with BTG Pharmaceuticals, Zoll Foundation, the National Institutes of Health, and others.
SOURCE: American Heart Association. Circulation. 2020 Oct 20. Suppl 2.
Highlights of new updated guidelines for cardiopulmonary resuscitation and emergency cardiovascular care from the American Heart Association include management of opioid-related emergencies; discussion of health disparities; and a new emphasis on physical, social, and emotional recovery after resuscitation.
The AHA is also exploring digital territory to improve CPR outcomes. The guidelines encourage use of mobile phone technology to summon trained laypeople to individuals requiring CPR, and an adaptive learning suite will be available online for personalized CPR instruction, with lessons catered to individual needs and knowledge levels.
These novel approaches reflect an ongoing effort by the AHA to ensure that the guidelines evolve rapidly with science and technology, reported Raina Merchant, MD, chair of the AHA Emergency Cardiovascular Care Committee and associate professor of emergency medicine at the University of Pennsylvania, Philadelphia, and colleagues. In 2015, the committee shifted from 5-year updates to a continuous online review process, citing a need for more immediate implementation of practice-altering data, they wrote in Circulation.
And new approaches do appear to save lives, at least in a hospital setting.
Since 2004, in-hospital cardiac arrest outcomes have been improving, but similar gains have yet to be realized for out-of-hospital cardiac arrest.
“Much of the variation in survival rates is thought to be due to the strength of the Chain of Survival, the [five] critical actions that must occur in rapid succession to maximize the chance of survival from cardiac arrest,” the committee wrote.
Update adds sixth link to Chains of Survival: Recovery
“Recovery expectations and survivorship plans that address treatment, surveillance, and rehabilitation need to be provided to cardiac arrest survivors and their caregivers at hospital discharge to address the sequelae of cardiac arrest and optimize transitions of care to independent physical, social, emotional, and role function,” the committee wrote.
Dr. Merchant and colleagues identified three “critically important” recommendations for both cardiac arrest survivors and caregivers during the recovery process: structured psychological assessment; multimodal rehabilitation assessment and treatment; and comprehensive, multidisciplinary discharge planning.
The recovery process is now part of all four Chains of Survival, which are specific to in-hospital and out-of-hospital arrest for adults and children.
New advice on opioid overdoses and bystander training
Among instances of out-of-hospital cardiac arrest, the committee noted that opioid overdoses are “sharply on the rise,” leading to new, scenario-specific recommendations. Among them, the committee encouraged lay rescuers and trained responders to activate emergency response systems immediately while awaiting improvements with naloxone and other interventions. They also suggested that, for individuals in known or suspected cardiac arrest, high-quality CPR, including compressions and ventilation, should be prioritized over naloxone administration.
In a broader discussion, the committee identified disparities in CPR training, which could explain lower rates of bystander CPR and poorer outcomes among certain demographics, such as black and Hispanic populations, as well as those with lower socioeconomic status.
“Targeting training efforts should consider barriers such as language, financial considerations, and poor access to information,” the committee wrote.
While low bystander CPR in these areas may be improved through mobile phone technology that alerts trained laypeople to individuals in need, the committee noted that this approach may be impacted by cultural and geographic factors. To date, use of mobile devices to improve bystander intervention rates has been demonstrated through “uniformly positive data,” but never in North America.
According to the guidelines, bystander intervention rates may also be improved through video-based learning, which is as effective as in-person, instructor-led training.
This led the AHA to create an online adaptive learning platform, which the organization describes as a “digital resuscitation portfolio” that connects programs and courses such as the Resuscitation Quality Improvement program and the HeartCode blended learning course.
“It will cover all of the guideline changes,” said Monica Sales, communications manager at the AHA. “It’s really groundbreaking because it’s the first time that we’re able to kind of close that gap between new science and new products.”
The online content also addresses CPR considerations for COVID-19, which were first addressed by interim CPR guidance published by the AHA in April.
According to Alexis Topjian, MD, coauthor of the present guidelines and pediatric critical care medicine physician at Children’s Hospital of Philadelphia, CPR awareness is more important now than ever.
“The major message [of the guidelines] is that high-quality CPR saves lives,” she said. “So push hard, and push fast. You have the power in your hands to make a difference, more so than ever during this pandemic.”
Concerning coronavirus precautions, Dr. Topjian noted that roughly 70% of out-of-hospital CPR events involve people who know each other, so most bystanders have already been exposed to the person in need, thereby reducing the concern of infection.
When asked about performing CPR on strangers, Dr. Topjian remained encouraging, though she noted that decision making may be informed by local coronavirus rates.
“It’s always a personal choice,” she said.
More for clinicians
For clinicians, Dr. Topjian highlighted several recommendations, including use of epinephrine as soon as possible during CPR, preferential use of a cuffed endotracheal tube, continuous EEG monitoring during and after cardiac arrest, and rapid intervention for clinical seizures and of nonconvulsive status epilepticus.
From a pediatric perspective, Dr. Topjian pointed out a change in breathing rate for infants and children who are receiving CPR or rescue breathing with a pulse, from 12-20 breaths/min to 20-30 breaths/min. While not a new recommendation, Dr. Topjian also pointed out the lifesaving benefit of early defibrillation among pediatric patients.
The guidelines were funded by the American Heart Association. The investigators disclosed additional relationships with BTG Pharmaceuticals, Zoll Foundation, the National Institutes of Health, and others.
SOURCE: American Heart Association. Circulation. 2020 Oct 20. Suppl 2.
FROM CIRCULATION
Cardiogenic shock rate soars in COVID-positive ACS
COVID-19–positive patients undergoing an invasive strategy for acute coronary syndrome presented hours later than uninfected historical controls, had a far higher incidence of cardiogenic shock, and their in-hospital mortality rate was four- to fivefold greater, according to data from the Global Multicenter Prospective COVID–ACS Registry. These phenomena are probably interrelated, according to Anthony Gershlick, MBBS, who presented the registry results at the Transcatheter Cardiovascular Therapeutics virtual annual meeting.
“We know that increasing ischemic time leads to bigger infarcts. And we know that bigger infarcts lead to cardiogenic shock, with its known higher mortality,” said Dr. Gershlick, professor of interventional cardiology at the University of Leicester (England).
“These data suggest that patients may have presented late, likely due to COVID concerns, and they had worse outcomes. If these data are borne out, future public information strategies need to be reassuring, proactive, simple, and more effective because we think patients stayed away,” the cardiologist added. “There are important public information messages to be taken from these data about getting patients to come to hospital during such pandemics.”
He presented prospectively collected registry data on 144 patients with confirmed ST-elevation MI (STEMI) and 122 with non-ST–elevation MI (NSTEMI), all COVID-19 positive on presentation at 85 hospitals in the United Kingdom, Europe, and North America during March through August of 2020. Since the initial message to the public early in the pandemic in many places was to try to avoid the hospital, the investigators selected for their no-COVID comparison group the data on more than 22,000 STEMI and NSTEMI patients included in two British national databases covering 2018-2019.
The COVID-positive STEMI patients were significantly younger, had more comorbidities, and had a higher mean heart rate and lower systolic blood pressure at admission than the non-COVID STEMI control group. Their median time from symptom onset to admission was 339 minutes, compared with 178 minutes in controls. Their door-to-balloon time averaged 83 minutes, versus 37 minutes in the era before the pandemic.
“I suspect that’s got something to do with the donning and doffing of personal protective equipment,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
The in-hospital mortality rates were strikingly different: 27.1% in COVID-positive STEMI patients versus 5.7% in controls. Bleeding Academic Research Consortium type 3-5 bleeding was increased as well, by a margin of 2.8% to 0.3%. So was stroke, with a 2.1% in-hospital incidence in COVID-positive STEMI patients and a 0.1% rate in the comparator arm.
“But the biggest headline here for me was that the cardiogenic shock rate was 20.1% in the COVID-positive patients versus 8.7% in the non-COVID STEMI patients,” the cardiologist continued.
The same pattern held true among the COVID-positive NSTEMI patients: They were younger, sicker, and slower to present to the hospital than the non-COVID group. The in-hospital mortality rate was 6.6% in the COVID-positive NSTEMI patients, compared with 1.2% in the reference group. The COVID-positive patients had a 2.5% bleeding rate versus 0.1% in the controls. And the incidence of cardiogenic shock was 5%, compared with 1.4% in the controls from before the pandemic.
“Even though NSTEMI is traditionally regarded as lower risk, this is really quite dramatic. These are sick patients,” Dr. Gershlick observed.
Nearly two-thirds of in-hospital deaths in COVID-positive ACS patients were cardiovascular, and three-quarters of those cardiovascular deaths occurred in patients with cardiogenic shock. Thirty-two percent of deaths in COVID-positive ACS patients were of respiratory causes, and 4.9% were neurologic.
Notably, the ischemic time of patients with cardiogenic shock who died – that is, the time from symptom onset to balloon deployment – averaged 1,271 minutes, compared with 441 minutes in those who died without being in cardiogenic shock.
Session comoderator Sahil A. Parikh, MD, director of endovascular services at Columbia University Medical Center in New York, commented, “One of the striking things that is resonating with me is the high incidence of cardiogenic shock and the mortality. It’s akin to what we’ve seen in New York.”
Discussant Valentin Fuster, MD, PhD, said he doubts that the increased in-hospital mortality in the COVID–ACS registry is related to the prolonged time to presentation at the hospital. More likely, it’s related to the greater thrombotic burden various studies have shown accompanies COVID-positive ACS. It might even be caused by a direct effect of the virus on the myocardium, added Dr. Fuster, director of the Zena and Michael A. Wiener Cardiovascular Institute and professor of medicine at the Icahn School of Medicine at Mount Sinai in New York.
“I have to say I absolutely disagree,” responded Dr. Gershlick. “I think it’s important that we try to understand all the mechanisms, but we know that patients with COVID are anxious, and I think one of the messages from this registry is patients took longer to come to hospital, they were sicker, they had more cardiogenic shock, and they died. And I don’t think it’s anything more complicated than that.”
Another discussant, Mamas Mamas, MD, is involved with a 500-patient U.K. pandemic ACS registry nearing publication. The findings, he said, are similar to what Dr. Gershlick reported in terms of the high rate of presentation with cardiogenic shock and elevated in-hospital mortality. The COVID-positive ACS patients were also more likely to present with out-of-hospital cardiac arrest. But like Dr. Fuster, he is skeptical that their worse outcomes can be explained by a delay in seeking care.
“I don’t think the delay in presentation is really associated with the high mortality rate that we see. The delay in our U.K. registry is maybe half an hour for STEMIs and maybe 2-3 hours for NSTEMIs. And I don’t think that can produce a 30%-40% increase in mortality,” asserted Dr. Mamas, professor of cardiology at Keele University in Staffordshire, England.
Dr. Gershlick reported having no financial conflicts regarding his presentation.
COVID-19–positive patients undergoing an invasive strategy for acute coronary syndrome presented hours later than uninfected historical controls, had a far higher incidence of cardiogenic shock, and their in-hospital mortality rate was four- to fivefold greater, according to data from the Global Multicenter Prospective COVID–ACS Registry. These phenomena are probably interrelated, according to Anthony Gershlick, MBBS, who presented the registry results at the Transcatheter Cardiovascular Therapeutics virtual annual meeting.
“We know that increasing ischemic time leads to bigger infarcts. And we know that bigger infarcts lead to cardiogenic shock, with its known higher mortality,” said Dr. Gershlick, professor of interventional cardiology at the University of Leicester (England).
“These data suggest that patients may have presented late, likely due to COVID concerns, and they had worse outcomes. If these data are borne out, future public information strategies need to be reassuring, proactive, simple, and more effective because we think patients stayed away,” the cardiologist added. “There are important public information messages to be taken from these data about getting patients to come to hospital during such pandemics.”
He presented prospectively collected registry data on 144 patients with confirmed ST-elevation MI (STEMI) and 122 with non-ST–elevation MI (NSTEMI), all COVID-19 positive on presentation at 85 hospitals in the United Kingdom, Europe, and North America during March through August of 2020. Since the initial message to the public early in the pandemic in many places was to try to avoid the hospital, the investigators selected for their no-COVID comparison group the data on more than 22,000 STEMI and NSTEMI patients included in two British national databases covering 2018-2019.
The COVID-positive STEMI patients were significantly younger, had more comorbidities, and had a higher mean heart rate and lower systolic blood pressure at admission than the non-COVID STEMI control group. Their median time from symptom onset to admission was 339 minutes, compared with 178 minutes in controls. Their door-to-balloon time averaged 83 minutes, versus 37 minutes in the era before the pandemic.
“I suspect that’s got something to do with the donning and doffing of personal protective equipment,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
The in-hospital mortality rates were strikingly different: 27.1% in COVID-positive STEMI patients versus 5.7% in controls. Bleeding Academic Research Consortium type 3-5 bleeding was increased as well, by a margin of 2.8% to 0.3%. So was stroke, with a 2.1% in-hospital incidence in COVID-positive STEMI patients and a 0.1% rate in the comparator arm.
“But the biggest headline here for me was that the cardiogenic shock rate was 20.1% in the COVID-positive patients versus 8.7% in the non-COVID STEMI patients,” the cardiologist continued.
The same pattern held true among the COVID-positive NSTEMI patients: They were younger, sicker, and slower to present to the hospital than the non-COVID group. The in-hospital mortality rate was 6.6% in the COVID-positive NSTEMI patients, compared with 1.2% in the reference group. The COVID-positive patients had a 2.5% bleeding rate versus 0.1% in the controls. And the incidence of cardiogenic shock was 5%, compared with 1.4% in the controls from before the pandemic.
“Even though NSTEMI is traditionally regarded as lower risk, this is really quite dramatic. These are sick patients,” Dr. Gershlick observed.
Nearly two-thirds of in-hospital deaths in COVID-positive ACS patients were cardiovascular, and three-quarters of those cardiovascular deaths occurred in patients with cardiogenic shock. Thirty-two percent of deaths in COVID-positive ACS patients were of respiratory causes, and 4.9% were neurologic.
Notably, the ischemic time of patients with cardiogenic shock who died – that is, the time from symptom onset to balloon deployment – averaged 1,271 minutes, compared with 441 minutes in those who died without being in cardiogenic shock.
Session comoderator Sahil A. Parikh, MD, director of endovascular services at Columbia University Medical Center in New York, commented, “One of the striking things that is resonating with me is the high incidence of cardiogenic shock and the mortality. It’s akin to what we’ve seen in New York.”
Discussant Valentin Fuster, MD, PhD, said he doubts that the increased in-hospital mortality in the COVID–ACS registry is related to the prolonged time to presentation at the hospital. More likely, it’s related to the greater thrombotic burden various studies have shown accompanies COVID-positive ACS. It might even be caused by a direct effect of the virus on the myocardium, added Dr. Fuster, director of the Zena and Michael A. Wiener Cardiovascular Institute and professor of medicine at the Icahn School of Medicine at Mount Sinai in New York.
“I have to say I absolutely disagree,” responded Dr. Gershlick. “I think it’s important that we try to understand all the mechanisms, but we know that patients with COVID are anxious, and I think one of the messages from this registry is patients took longer to come to hospital, they were sicker, they had more cardiogenic shock, and they died. And I don’t think it’s anything more complicated than that.”
Another discussant, Mamas Mamas, MD, is involved with a 500-patient U.K. pandemic ACS registry nearing publication. The findings, he said, are similar to what Dr. Gershlick reported in terms of the high rate of presentation with cardiogenic shock and elevated in-hospital mortality. The COVID-positive ACS patients were also more likely to present with out-of-hospital cardiac arrest. But like Dr. Fuster, he is skeptical that their worse outcomes can be explained by a delay in seeking care.
“I don’t think the delay in presentation is really associated with the high mortality rate that we see. The delay in our U.K. registry is maybe half an hour for STEMIs and maybe 2-3 hours for NSTEMIs. And I don’t think that can produce a 30%-40% increase in mortality,” asserted Dr. Mamas, professor of cardiology at Keele University in Staffordshire, England.
Dr. Gershlick reported having no financial conflicts regarding his presentation.
COVID-19–positive patients undergoing an invasive strategy for acute coronary syndrome presented hours later than uninfected historical controls, had a far higher incidence of cardiogenic shock, and their in-hospital mortality rate was four- to fivefold greater, according to data from the Global Multicenter Prospective COVID–ACS Registry. These phenomena are probably interrelated, according to Anthony Gershlick, MBBS, who presented the registry results at the Transcatheter Cardiovascular Therapeutics virtual annual meeting.
“We know that increasing ischemic time leads to bigger infarcts. And we know that bigger infarcts lead to cardiogenic shock, with its known higher mortality,” said Dr. Gershlick, professor of interventional cardiology at the University of Leicester (England).
“These data suggest that patients may have presented late, likely due to COVID concerns, and they had worse outcomes. If these data are borne out, future public information strategies need to be reassuring, proactive, simple, and more effective because we think patients stayed away,” the cardiologist added. “There are important public information messages to be taken from these data about getting patients to come to hospital during such pandemics.”
He presented prospectively collected registry data on 144 patients with confirmed ST-elevation MI (STEMI) and 122 with non-ST–elevation MI (NSTEMI), all COVID-19 positive on presentation at 85 hospitals in the United Kingdom, Europe, and North America during March through August of 2020. Since the initial message to the public early in the pandemic in many places was to try to avoid the hospital, the investigators selected for their no-COVID comparison group the data on more than 22,000 STEMI and NSTEMI patients included in two British national databases covering 2018-2019.
The COVID-positive STEMI patients were significantly younger, had more comorbidities, and had a higher mean heart rate and lower systolic blood pressure at admission than the non-COVID STEMI control group. Their median time from symptom onset to admission was 339 minutes, compared with 178 minutes in controls. Their door-to-balloon time averaged 83 minutes, versus 37 minutes in the era before the pandemic.
“I suspect that’s got something to do with the donning and doffing of personal protective equipment,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
The in-hospital mortality rates were strikingly different: 27.1% in COVID-positive STEMI patients versus 5.7% in controls. Bleeding Academic Research Consortium type 3-5 bleeding was increased as well, by a margin of 2.8% to 0.3%. So was stroke, with a 2.1% in-hospital incidence in COVID-positive STEMI patients and a 0.1% rate in the comparator arm.
“But the biggest headline here for me was that the cardiogenic shock rate was 20.1% in the COVID-positive patients versus 8.7% in the non-COVID STEMI patients,” the cardiologist continued.
The same pattern held true among the COVID-positive NSTEMI patients: They were younger, sicker, and slower to present to the hospital than the non-COVID group. The in-hospital mortality rate was 6.6% in the COVID-positive NSTEMI patients, compared with 1.2% in the reference group. The COVID-positive patients had a 2.5% bleeding rate versus 0.1% in the controls. And the incidence of cardiogenic shock was 5%, compared with 1.4% in the controls from before the pandemic.
“Even though NSTEMI is traditionally regarded as lower risk, this is really quite dramatic. These are sick patients,” Dr. Gershlick observed.
Nearly two-thirds of in-hospital deaths in COVID-positive ACS patients were cardiovascular, and three-quarters of those cardiovascular deaths occurred in patients with cardiogenic shock. Thirty-two percent of deaths in COVID-positive ACS patients were of respiratory causes, and 4.9% were neurologic.
Notably, the ischemic time of patients with cardiogenic shock who died – that is, the time from symptom onset to balloon deployment – averaged 1,271 minutes, compared with 441 minutes in those who died without being in cardiogenic shock.
Session comoderator Sahil A. Parikh, MD, director of endovascular services at Columbia University Medical Center in New York, commented, “One of the striking things that is resonating with me is the high incidence of cardiogenic shock and the mortality. It’s akin to what we’ve seen in New York.”
Discussant Valentin Fuster, MD, PhD, said he doubts that the increased in-hospital mortality in the COVID–ACS registry is related to the prolonged time to presentation at the hospital. More likely, it’s related to the greater thrombotic burden various studies have shown accompanies COVID-positive ACS. It might even be caused by a direct effect of the virus on the myocardium, added Dr. Fuster, director of the Zena and Michael A. Wiener Cardiovascular Institute and professor of medicine at the Icahn School of Medicine at Mount Sinai in New York.
“I have to say I absolutely disagree,” responded Dr. Gershlick. “I think it’s important that we try to understand all the mechanisms, but we know that patients with COVID are anxious, and I think one of the messages from this registry is patients took longer to come to hospital, they were sicker, they had more cardiogenic shock, and they died. And I don’t think it’s anything more complicated than that.”
Another discussant, Mamas Mamas, MD, is involved with a 500-patient U.K. pandemic ACS registry nearing publication. The findings, he said, are similar to what Dr. Gershlick reported in terms of the high rate of presentation with cardiogenic shock and elevated in-hospital mortality. The COVID-positive ACS patients were also more likely to present with out-of-hospital cardiac arrest. But like Dr. Fuster, he is skeptical that their worse outcomes can be explained by a delay in seeking care.
“I don’t think the delay in presentation is really associated with the high mortality rate that we see. The delay in our U.K. registry is maybe half an hour for STEMIs and maybe 2-3 hours for NSTEMIs. And I don’t think that can produce a 30%-40% increase in mortality,” asserted Dr. Mamas, professor of cardiology at Keele University in Staffordshire, England.
Dr. Gershlick reported having no financial conflicts regarding his presentation.
FROM TCT 2020
When the only clinical choices are ‘lose-lose’
Among the many tolls inflicted on health care workers by COVID-19 is one that is not as easily measured as rates of death or disease, but is no less tangible: moral injury. This is the term by which we describe the psychological, social, and spiritual impact of high-stakes situations that lead to the betrayal or transgression of our own deeply held moral beliefs and values.
The current pandemic has provided innumerable such situations that can increase the risk for moral injury, whether we deal directly with patients infected by the coronavirus or not. Telling family members they cannot visit critically ill loved ones. Delaying code activities, even momentarily, to get fully protected with personal protective equipment. Seeing patients who have delayed their necessary or preventive care. Using video rather than touch to reassure people.
Knowing that we are following guidelines from the Centers for Disease Control and Prevention does not stop our feelings of guilt. The longer this pandemic goes on, the more likely it is that these situations will begin to take a toll on us.
For most of us, being exposed to moral injuries is new; they have historically been most associated with severe traumatic wartime experiences. Soldiers, philosophers, and writers have described the ethical dilemmas inherent in war for as long as recorded history. But the use of this term is a more recent development, which the Moral Injury Project at Syracuse (N.Y.) University describes as probably originating in the Vietnam War–era writings of veteran and peace activist Camillo “Mac” Bica and psychiatrist Jonathan Shay. Examples of wartime events that have been thought to lead to moral injury include: causing the harm or death of civilians, knowingly but without alternatives, or accidentally; failing to provide medical aid to an injured civilian or service member; and following orders that were illegal, immoral, and/or against the rules of engagement or the Geneva Conventions.
However, the occurrence of moral injuries in modern health care is increasingly being reported, primarily as an adverse effect of health care inefficiencies that can contribute to burnout. COVID-19 has now provided an array of additional stressors that can cause moral injuries among health care workers. A recent guidance document on moral injury published by the American Psychiatric Association noted that, in the context of a public health disaster, such as COVID-19, it is sometimes necessary to transition from ordinary standards of care to those more appropriate in a crisis, as in wartime. This forces us all to confront challenging questions for which there may be no clear answers, and to make “lose-lose” choices in which no one involved – patients, family, or clinicians – ends up feeling satisfied or even comfortable.
Our lives have been altered significantly, and for many, completely turned upside down by enormous sacrifices and tragic losses. Globally, physicians account for over half of healthcare worker deaths. In the United States alone, over 900 health care workers have died of COVID-19.
Most of us have felt the symptoms of moral injury: frustration, anger, disgust, guilt. A recent report describes three levels of stressors in health care occurring during the pandemic, which are not dissimilar to those wartime events described previously.
- Severe moral stressors, such as the denial of treatment to a COVID-19 patient owing to lack of resources, the inability to provide optimal care to non–COVID-19 patients for many reasons, and concern about passing COVID to loved ones.
- Moderate moral stressors, such as preventing visitors, especially to dying patients, triaging patients for healthcare services with inadequate information, and trying to solve the tension between the need for self-preservation and the need to treat.
- Lower-level but common moral challenges, especially in the community – for example, seeing others not protecting the community by hoarding food, gathering for large parties, and not social distancing or wearing masks. Such stressors lead to frustration and contempt, especially from healthcare workers making personal sacrifices and who may be at risk for infection caused by these behaviors.
Every one of us is affected by these stressors. I certainly am.
What are the outcomes? We know that moral injuries are a risk factor for the development of mental health problems and burnout, and not surprisingly we are seeing that mental health problems, suicidality, and substance use disorders have increased markedly during COVID-19, as recently detailed by the CDC.
Common emotions that occur in response to moral injuries are: feelings of guilt, shame, anger, sadness, anxiety, and disgust; intrapersonal outcomes, including lowered self-esteem, high self-criticism, and beliefs about being bad, damaged, unworthy, failing, or weak; interpersonal outcomes, including loss of faith in people, avoidance of intimacy, and lack of trust in authority figures; and existential and spiritual outcomes, including loss of faith in previous religious beliefs and no longer believing in a just world.
Moral injuries tend to originate primarily from systems-based problems, as we have seen with the lack of concerted national approaches to the pandemic. On the positive side, solutions typically also involve systems-based changes, which in this case may mean changes in leadership styles nationally and locally, as well as changes in the culture of medicine and the way healthcare is practiced and managed in the modern era. We are starting to see some of those changes with the increased use of telemedicine and health technologies, as well as more of a focus on the well-being of health care workers, now deemed “essential.”
As individuals, we are not helpless. There are things we can do in our workplaces to create change. I suggest:
- Acknowledge that you, like me, are affected by these stressors. This is not a secret, and you should not be ashamed of your feelings.
- Talk with your colleagues, loved ones, and friends about how you and they are affected. You are not alone. Encourage others to share their thoughts, stories, and feelings.
- Put this topic on your meeting and departmental agendas and discuss these moral issues openly with your colleagues. Allow sufficient time to engage in open dialogue.
- Work out ways of assisting those who are in high-risk situations, especially for moderate to severe injuries. Be supportive toward those affected.
- Modify policies and change rosters and rotate staff between high- and low-stress roles. Protect and support at-risk colleagues.
- Think about difficult ethical decisions in advance so they can be made by groups, not individuals, and certainly not “on the fly.”
- Keep everyone in your workplace constantly informed, especially of impending staff or equipment shortages.
- Maintain your inherent self-care and resilience with rest, good nutrition, sleep, exercise, love, caring, socialization, and work-life balance.
- Be prepared to access the many professional support services available in our community if you are intensely distressed or if the above suggestions are not enough.
Remember, we are in this together and will find strength in each other. This too will pass.
This article first appeared on Medscape.com.
Among the many tolls inflicted on health care workers by COVID-19 is one that is not as easily measured as rates of death or disease, but is no less tangible: moral injury. This is the term by which we describe the psychological, social, and spiritual impact of high-stakes situations that lead to the betrayal or transgression of our own deeply held moral beliefs and values.
The current pandemic has provided innumerable such situations that can increase the risk for moral injury, whether we deal directly with patients infected by the coronavirus or not. Telling family members they cannot visit critically ill loved ones. Delaying code activities, even momentarily, to get fully protected with personal protective equipment. Seeing patients who have delayed their necessary or preventive care. Using video rather than touch to reassure people.
Knowing that we are following guidelines from the Centers for Disease Control and Prevention does not stop our feelings of guilt. The longer this pandemic goes on, the more likely it is that these situations will begin to take a toll on us.
For most of us, being exposed to moral injuries is new; they have historically been most associated with severe traumatic wartime experiences. Soldiers, philosophers, and writers have described the ethical dilemmas inherent in war for as long as recorded history. But the use of this term is a more recent development, which the Moral Injury Project at Syracuse (N.Y.) University describes as probably originating in the Vietnam War–era writings of veteran and peace activist Camillo “Mac” Bica and psychiatrist Jonathan Shay. Examples of wartime events that have been thought to lead to moral injury include: causing the harm or death of civilians, knowingly but without alternatives, or accidentally; failing to provide medical aid to an injured civilian or service member; and following orders that were illegal, immoral, and/or against the rules of engagement or the Geneva Conventions.
However, the occurrence of moral injuries in modern health care is increasingly being reported, primarily as an adverse effect of health care inefficiencies that can contribute to burnout. COVID-19 has now provided an array of additional stressors that can cause moral injuries among health care workers. A recent guidance document on moral injury published by the American Psychiatric Association noted that, in the context of a public health disaster, such as COVID-19, it is sometimes necessary to transition from ordinary standards of care to those more appropriate in a crisis, as in wartime. This forces us all to confront challenging questions for which there may be no clear answers, and to make “lose-lose” choices in which no one involved – patients, family, or clinicians – ends up feeling satisfied or even comfortable.
Our lives have been altered significantly, and for many, completely turned upside down by enormous sacrifices and tragic losses. Globally, physicians account for over half of healthcare worker deaths. In the United States alone, over 900 health care workers have died of COVID-19.
Most of us have felt the symptoms of moral injury: frustration, anger, disgust, guilt. A recent report describes three levels of stressors in health care occurring during the pandemic, which are not dissimilar to those wartime events described previously.
- Severe moral stressors, such as the denial of treatment to a COVID-19 patient owing to lack of resources, the inability to provide optimal care to non–COVID-19 patients for many reasons, and concern about passing COVID to loved ones.
- Moderate moral stressors, such as preventing visitors, especially to dying patients, triaging patients for healthcare services with inadequate information, and trying to solve the tension between the need for self-preservation and the need to treat.
- Lower-level but common moral challenges, especially in the community – for example, seeing others not protecting the community by hoarding food, gathering for large parties, and not social distancing or wearing masks. Such stressors lead to frustration and contempt, especially from healthcare workers making personal sacrifices and who may be at risk for infection caused by these behaviors.
Every one of us is affected by these stressors. I certainly am.
What are the outcomes? We know that moral injuries are a risk factor for the development of mental health problems and burnout, and not surprisingly we are seeing that mental health problems, suicidality, and substance use disorders have increased markedly during COVID-19, as recently detailed by the CDC.
Common emotions that occur in response to moral injuries are: feelings of guilt, shame, anger, sadness, anxiety, and disgust; intrapersonal outcomes, including lowered self-esteem, high self-criticism, and beliefs about being bad, damaged, unworthy, failing, or weak; interpersonal outcomes, including loss of faith in people, avoidance of intimacy, and lack of trust in authority figures; and existential and spiritual outcomes, including loss of faith in previous religious beliefs and no longer believing in a just world.
Moral injuries tend to originate primarily from systems-based problems, as we have seen with the lack of concerted national approaches to the pandemic. On the positive side, solutions typically also involve systems-based changes, which in this case may mean changes in leadership styles nationally and locally, as well as changes in the culture of medicine and the way healthcare is practiced and managed in the modern era. We are starting to see some of those changes with the increased use of telemedicine and health technologies, as well as more of a focus on the well-being of health care workers, now deemed “essential.”
As individuals, we are not helpless. There are things we can do in our workplaces to create change. I suggest:
- Acknowledge that you, like me, are affected by these stressors. This is not a secret, and you should not be ashamed of your feelings.
- Talk with your colleagues, loved ones, and friends about how you and they are affected. You are not alone. Encourage others to share their thoughts, stories, and feelings.
- Put this topic on your meeting and departmental agendas and discuss these moral issues openly with your colleagues. Allow sufficient time to engage in open dialogue.
- Work out ways of assisting those who are in high-risk situations, especially for moderate to severe injuries. Be supportive toward those affected.
- Modify policies and change rosters and rotate staff between high- and low-stress roles. Protect and support at-risk colleagues.
- Think about difficult ethical decisions in advance so they can be made by groups, not individuals, and certainly not “on the fly.”
- Keep everyone in your workplace constantly informed, especially of impending staff or equipment shortages.
- Maintain your inherent self-care and resilience with rest, good nutrition, sleep, exercise, love, caring, socialization, and work-life balance.
- Be prepared to access the many professional support services available in our community if you are intensely distressed or if the above suggestions are not enough.
Remember, we are in this together and will find strength in each other. This too will pass.
This article first appeared on Medscape.com.
Among the many tolls inflicted on health care workers by COVID-19 is one that is not as easily measured as rates of death or disease, but is no less tangible: moral injury. This is the term by which we describe the psychological, social, and spiritual impact of high-stakes situations that lead to the betrayal or transgression of our own deeply held moral beliefs and values.
The current pandemic has provided innumerable such situations that can increase the risk for moral injury, whether we deal directly with patients infected by the coronavirus or not. Telling family members they cannot visit critically ill loved ones. Delaying code activities, even momentarily, to get fully protected with personal protective equipment. Seeing patients who have delayed their necessary or preventive care. Using video rather than touch to reassure people.
Knowing that we are following guidelines from the Centers for Disease Control and Prevention does not stop our feelings of guilt. The longer this pandemic goes on, the more likely it is that these situations will begin to take a toll on us.
For most of us, being exposed to moral injuries is new; they have historically been most associated with severe traumatic wartime experiences. Soldiers, philosophers, and writers have described the ethical dilemmas inherent in war for as long as recorded history. But the use of this term is a more recent development, which the Moral Injury Project at Syracuse (N.Y.) University describes as probably originating in the Vietnam War–era writings of veteran and peace activist Camillo “Mac” Bica and psychiatrist Jonathan Shay. Examples of wartime events that have been thought to lead to moral injury include: causing the harm or death of civilians, knowingly but without alternatives, or accidentally; failing to provide medical aid to an injured civilian or service member; and following orders that were illegal, immoral, and/or against the rules of engagement or the Geneva Conventions.
However, the occurrence of moral injuries in modern health care is increasingly being reported, primarily as an adverse effect of health care inefficiencies that can contribute to burnout. COVID-19 has now provided an array of additional stressors that can cause moral injuries among health care workers. A recent guidance document on moral injury published by the American Psychiatric Association noted that, in the context of a public health disaster, such as COVID-19, it is sometimes necessary to transition from ordinary standards of care to those more appropriate in a crisis, as in wartime. This forces us all to confront challenging questions for which there may be no clear answers, and to make “lose-lose” choices in which no one involved – patients, family, or clinicians – ends up feeling satisfied or even comfortable.
Our lives have been altered significantly, and for many, completely turned upside down by enormous sacrifices and tragic losses. Globally, physicians account for over half of healthcare worker deaths. In the United States alone, over 900 health care workers have died of COVID-19.
Most of us have felt the symptoms of moral injury: frustration, anger, disgust, guilt. A recent report describes three levels of stressors in health care occurring during the pandemic, which are not dissimilar to those wartime events described previously.
- Severe moral stressors, such as the denial of treatment to a COVID-19 patient owing to lack of resources, the inability to provide optimal care to non–COVID-19 patients for many reasons, and concern about passing COVID to loved ones.
- Moderate moral stressors, such as preventing visitors, especially to dying patients, triaging patients for healthcare services with inadequate information, and trying to solve the tension between the need for self-preservation and the need to treat.
- Lower-level but common moral challenges, especially in the community – for example, seeing others not protecting the community by hoarding food, gathering for large parties, and not social distancing or wearing masks. Such stressors lead to frustration and contempt, especially from healthcare workers making personal sacrifices and who may be at risk for infection caused by these behaviors.
Every one of us is affected by these stressors. I certainly am.
What are the outcomes? We know that moral injuries are a risk factor for the development of mental health problems and burnout, and not surprisingly we are seeing that mental health problems, suicidality, and substance use disorders have increased markedly during COVID-19, as recently detailed by the CDC.
Common emotions that occur in response to moral injuries are: feelings of guilt, shame, anger, sadness, anxiety, and disgust; intrapersonal outcomes, including lowered self-esteem, high self-criticism, and beliefs about being bad, damaged, unworthy, failing, or weak; interpersonal outcomes, including loss of faith in people, avoidance of intimacy, and lack of trust in authority figures; and existential and spiritual outcomes, including loss of faith in previous religious beliefs and no longer believing in a just world.
Moral injuries tend to originate primarily from systems-based problems, as we have seen with the lack of concerted national approaches to the pandemic. On the positive side, solutions typically also involve systems-based changes, which in this case may mean changes in leadership styles nationally and locally, as well as changes in the culture of medicine and the way healthcare is practiced and managed in the modern era. We are starting to see some of those changes with the increased use of telemedicine and health technologies, as well as more of a focus on the well-being of health care workers, now deemed “essential.”
As individuals, we are not helpless. There are things we can do in our workplaces to create change. I suggest:
- Acknowledge that you, like me, are affected by these stressors. This is not a secret, and you should not be ashamed of your feelings.
- Talk with your colleagues, loved ones, and friends about how you and they are affected. You are not alone. Encourage others to share their thoughts, stories, and feelings.
- Put this topic on your meeting and departmental agendas and discuss these moral issues openly with your colleagues. Allow sufficient time to engage in open dialogue.
- Work out ways of assisting those who are in high-risk situations, especially for moderate to severe injuries. Be supportive toward those affected.
- Modify policies and change rosters and rotate staff between high- and low-stress roles. Protect and support at-risk colleagues.
- Think about difficult ethical decisions in advance so they can be made by groups, not individuals, and certainly not “on the fly.”
- Keep everyone in your workplace constantly informed, especially of impending staff or equipment shortages.
- Maintain your inherent self-care and resilience with rest, good nutrition, sleep, exercise, love, caring, socialization, and work-life balance.
- Be prepared to access the many professional support services available in our community if you are intensely distressed or if the above suggestions are not enough.
Remember, we are in this together and will find strength in each other. This too will pass.
This article first appeared on Medscape.com.
Experts tout immediate quadruple therapy for HFrEF patients
Gregg C. Fonarow, MD, recommended.
Less than 2 months before Dr. Fonarow made that striking statement during the virtual annual meeting of the Heart Failure Society of America, investigators first reported results from the EMPEROR-Reduced trial at the European Society of Cardiology’s virtual annual meeting, showing that the sodium-glucose transporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) successfully cut events in patients with heart failure with reduced ejection fraction (HFrEF). That report, a year after results from a similar trial (DAPA-HF) showed the same outcome using a different drug from the same class, dapagliflozin (Farxiga), cemented the SGLT2 inhibitor drug class as the fourth pillar for treating HFrEF, joining the angiotensin receptor neprilysin inhibitor (ARNI) class (sacubitril valsartan), beta-blockers (like carvedilol), and mineralocorticoid receptor antagonists (like spironolactone).
This rejiggering of the consensus expert approach for treating HFrEF left cardiologists wondering what sequence to use when starting this quadruple therapy. Within weeks, the answer from heart failure opinion leaders was clear:
“Start all four pillars simultaneously. Most patients can tolerate, and will benefit from, a simultaneous start,” declared Dr. Fonarow, professor and chief of cardiology at the University of California, Los Angeles.
His rationale? Patients get benefits from each of these drug classes “surprisingly early,” with improved outcomes in clinical trials appearing within a few weeks, compared with patients in control arms. The consequence is that any delay in starting treatment denies patients time with improved health status, function, and survival.
Study results documented that the four foundational drug classes can produce rapid improvements in health status, left ventricular size and shape, and make clinically meaningful cuts in both first and recurrent hospitalizations for heart failure and in mortality, Dr. Fonarow said. After 30 days on quadruple treatment, a patient’s relative risk for death drops by more than three-quarters, compared with patients not on these medications.
The benefits from each of the four classes involve distinct physiologic pathways and hence are not diminished by concurrent treatment. And immediate initiation avoids the risk of clinical inertia and a negligence to prescribe one or more of the four important drug classes. Introducing the four classes in a sequential manner could mean spending as long as a year to get all four on board and up-titrated to optimal therapeutic levels, he noted.
“Overcome inertia by prescribing [all four drug classes] at the time of diagnosis,” Dr. Fonarow admonished his audience.
The challenge of prescribing inertia
The risk for inertia in prescribing heart failure medications is real. Data collected in the CHAMP-HF (Change the Management of Patients with Heart Failure) registry from more than 3,500 HFrEF patients managed at any of 150 U.S. primary care and cardiology practices starting in late 2015 and continuing through 2017 showed that, among patients eligible for treatment with renin-angiotensin system (RAS) inhibition (with either ARNI or a single RAS inhibiting drug), a beta-blocker, and a mineralocorticoid receptor antagonist (MRA), 22% received all three drug classes. A scant 1% were on target dosages of all three drug classes, noted Stephen J. Greene, MD, in a separate talk at the meeting when he cited his published findings.
The sole formulation currently in the ARNI class, sacubitril/valsartan (Entresto) has in recent years been the poster child for prescribing inertia in HFrEF patients after coming onto the U.S. market for routine use in 2015. A review run by Dr. Greene of more than 9,000 HFrEF patients who were at least 65 years old and discharged from a hospital participating in the Get With the Guidelines–Heart Failure registry during October 2015–September 2017 showed that 8% of eligible patients actually received a sacubitril/valsartan prescription. Separate assessment of outpatients with HFrEF from the same era showed 13% uptake, said D. Greene, a cardiologist at Duke University, Durham, N.C.
Substantial gaps in prescribing evidence-based treatments to HFrEF patients have existed for the past couple of decades, said Dr. Greene. “Even a blockbuster drug like sacubitril/valsartan has been slow to implement.”
Quadruple therapy adds an average of 6 years of life
One of the most strongest arguments favoring the start-four-at-once approach was detailed in what’s quickly become a widely cited analysis published in July 2020 by a team of researchers led by Muthiah Vaduganathan, MD. Using data from three key pivotal trials they estimated that timely treatment with all four drug classes would on average produce an extra 6 years of overall survival in a 55-year old HFrEF patient, and an added 8 years free from cardiovascular death or first hospitalization for heart failure, compared with less comprehensive treatment. The analysis also showed a significant 3-year average boost in overall survival among HFrEF patients who were 80 years old when using quadruple therapy compared with the “conventional medical therapy” used on control patients in the three trials examined.
Dr. Greene called these findings “remarkable.”
“Four drugs use five mechanistic pathways to produce 6 added years of survival,” summed up Dr. Vaduganathan during a separate talk at the virtual meeting.
In addition to this substantial potential for a meaningful impact on patents’ lives, he cited other factors that add to the case for early prescription of the pharmaceutical gauntlet: avoiding missed treatment opportunities that occur with slower, step-wise drug introduction; simplifying, streamlining, and standardizing the care pathway, which helps avoid care inequities and disrupts the potential for inertia; magnifying benefit when comprehensive treatment starts sooner; and providing additive benefits without drug-drug interactions.
“Upfront treatment at the time of [HFrEF] diagnosis or hospitalization is an approach that disrupts treatment inertia,” emphasized Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital in Boston.
New approaches needed to encourage quick uptake
“Efficacy alone has not been enough for efficient uptake in U.S. practice” of sacubitril/valsartan, other RAS inhibitors, beta-blockers, and MRAs, noted Dr. Greene.
He was more optimistic about prospects for relatively quick uptake of early SGLT2 inhibitor treatment as part of routine HFrEF management given all the positives that this new HFrEF treatment offers, including some “unique features” among HFrEF drugs. These include the simplicity of the regimen, which involves a single dosage for everyone that’s taken once daily; minimal blood pressure effects and no adverse renal effects while also producing substantial renal protection; and two SGLT2 inhibitors with proven HFrEF benefit (dapagliflozin and empagliflozin), which bodes well for an eventual price drop.
The SGLT2 inhibitors stack up as an “ideal” HFrEF treatment, concluded Dr. Greene, which should facilitate quick uptake. As far as getting clinicians to also add early on the other three members of the core four treatment classes in routine treatment, he conceded that “innovative and evidence-based approaches to improving real-world uptake of guideline-directed medical therapy are urgently needed.”
EMPEROR-Reduced was funded by Boehringer Ingelheim and Lilly, the companies that market empagliflozin (Jardiance). CHAMP-HF was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. Fonarow has been a consultant or adviser to Novartis, as well as to Abbott, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards, Janssen, Medtronic, and Merck. Dr. Greene has received research funding from Novartis, has been a consultant to Amgen and Merck, an adviser to Amgen and Cytokinetics, and has received research funding from Amgen, AstraZeneca, Bristol-Myers Squibb, and Merck. Dr. Vaduganathan has had financial relationships with Boehringer Ingelheim and Novartis, as well as with Amgen, AstraZeneca, Baxter Healthcare, Bayer, Cytokinetics, and Relypsa.
Gregg C. Fonarow, MD, recommended.
Less than 2 months before Dr. Fonarow made that striking statement during the virtual annual meeting of the Heart Failure Society of America, investigators first reported results from the EMPEROR-Reduced trial at the European Society of Cardiology’s virtual annual meeting, showing that the sodium-glucose transporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) successfully cut events in patients with heart failure with reduced ejection fraction (HFrEF). That report, a year after results from a similar trial (DAPA-HF) showed the same outcome using a different drug from the same class, dapagliflozin (Farxiga), cemented the SGLT2 inhibitor drug class as the fourth pillar for treating HFrEF, joining the angiotensin receptor neprilysin inhibitor (ARNI) class (sacubitril valsartan), beta-blockers (like carvedilol), and mineralocorticoid receptor antagonists (like spironolactone).
This rejiggering of the consensus expert approach for treating HFrEF left cardiologists wondering what sequence to use when starting this quadruple therapy. Within weeks, the answer from heart failure opinion leaders was clear:
“Start all four pillars simultaneously. Most patients can tolerate, and will benefit from, a simultaneous start,” declared Dr. Fonarow, professor and chief of cardiology at the University of California, Los Angeles.
His rationale? Patients get benefits from each of these drug classes “surprisingly early,” with improved outcomes in clinical trials appearing within a few weeks, compared with patients in control arms. The consequence is that any delay in starting treatment denies patients time with improved health status, function, and survival.
Study results documented that the four foundational drug classes can produce rapid improvements in health status, left ventricular size and shape, and make clinically meaningful cuts in both first and recurrent hospitalizations for heart failure and in mortality, Dr. Fonarow said. After 30 days on quadruple treatment, a patient’s relative risk for death drops by more than three-quarters, compared with patients not on these medications.
The benefits from each of the four classes involve distinct physiologic pathways and hence are not diminished by concurrent treatment. And immediate initiation avoids the risk of clinical inertia and a negligence to prescribe one or more of the four important drug classes. Introducing the four classes in a sequential manner could mean spending as long as a year to get all four on board and up-titrated to optimal therapeutic levels, he noted.
“Overcome inertia by prescribing [all four drug classes] at the time of diagnosis,” Dr. Fonarow admonished his audience.
The challenge of prescribing inertia
The risk for inertia in prescribing heart failure medications is real. Data collected in the CHAMP-HF (Change the Management of Patients with Heart Failure) registry from more than 3,500 HFrEF patients managed at any of 150 U.S. primary care and cardiology practices starting in late 2015 and continuing through 2017 showed that, among patients eligible for treatment with renin-angiotensin system (RAS) inhibition (with either ARNI or a single RAS inhibiting drug), a beta-blocker, and a mineralocorticoid receptor antagonist (MRA), 22% received all three drug classes. A scant 1% were on target dosages of all three drug classes, noted Stephen J. Greene, MD, in a separate talk at the meeting when he cited his published findings.
The sole formulation currently in the ARNI class, sacubitril/valsartan (Entresto) has in recent years been the poster child for prescribing inertia in HFrEF patients after coming onto the U.S. market for routine use in 2015. A review run by Dr. Greene of more than 9,000 HFrEF patients who were at least 65 years old and discharged from a hospital participating in the Get With the Guidelines–Heart Failure registry during October 2015–September 2017 showed that 8% of eligible patients actually received a sacubitril/valsartan prescription. Separate assessment of outpatients with HFrEF from the same era showed 13% uptake, said D. Greene, a cardiologist at Duke University, Durham, N.C.
Substantial gaps in prescribing evidence-based treatments to HFrEF patients have existed for the past couple of decades, said Dr. Greene. “Even a blockbuster drug like sacubitril/valsartan has been slow to implement.”
Quadruple therapy adds an average of 6 years of life
One of the most strongest arguments favoring the start-four-at-once approach was detailed in what’s quickly become a widely cited analysis published in July 2020 by a team of researchers led by Muthiah Vaduganathan, MD. Using data from three key pivotal trials they estimated that timely treatment with all four drug classes would on average produce an extra 6 years of overall survival in a 55-year old HFrEF patient, and an added 8 years free from cardiovascular death or first hospitalization for heart failure, compared with less comprehensive treatment. The analysis also showed a significant 3-year average boost in overall survival among HFrEF patients who were 80 years old when using quadruple therapy compared with the “conventional medical therapy” used on control patients in the three trials examined.
Dr. Greene called these findings “remarkable.”
“Four drugs use five mechanistic pathways to produce 6 added years of survival,” summed up Dr. Vaduganathan during a separate talk at the virtual meeting.
In addition to this substantial potential for a meaningful impact on patents’ lives, he cited other factors that add to the case for early prescription of the pharmaceutical gauntlet: avoiding missed treatment opportunities that occur with slower, step-wise drug introduction; simplifying, streamlining, and standardizing the care pathway, which helps avoid care inequities and disrupts the potential for inertia; magnifying benefit when comprehensive treatment starts sooner; and providing additive benefits without drug-drug interactions.
“Upfront treatment at the time of [HFrEF] diagnosis or hospitalization is an approach that disrupts treatment inertia,” emphasized Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital in Boston.
New approaches needed to encourage quick uptake
“Efficacy alone has not been enough for efficient uptake in U.S. practice” of sacubitril/valsartan, other RAS inhibitors, beta-blockers, and MRAs, noted Dr. Greene.
He was more optimistic about prospects for relatively quick uptake of early SGLT2 inhibitor treatment as part of routine HFrEF management given all the positives that this new HFrEF treatment offers, including some “unique features” among HFrEF drugs. These include the simplicity of the regimen, which involves a single dosage for everyone that’s taken once daily; minimal blood pressure effects and no adverse renal effects while also producing substantial renal protection; and two SGLT2 inhibitors with proven HFrEF benefit (dapagliflozin and empagliflozin), which bodes well for an eventual price drop.
The SGLT2 inhibitors stack up as an “ideal” HFrEF treatment, concluded Dr. Greene, which should facilitate quick uptake. As far as getting clinicians to also add early on the other three members of the core four treatment classes in routine treatment, he conceded that “innovative and evidence-based approaches to improving real-world uptake of guideline-directed medical therapy are urgently needed.”
EMPEROR-Reduced was funded by Boehringer Ingelheim and Lilly, the companies that market empagliflozin (Jardiance). CHAMP-HF was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. Fonarow has been a consultant or adviser to Novartis, as well as to Abbott, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards, Janssen, Medtronic, and Merck. Dr. Greene has received research funding from Novartis, has been a consultant to Amgen and Merck, an adviser to Amgen and Cytokinetics, and has received research funding from Amgen, AstraZeneca, Bristol-Myers Squibb, and Merck. Dr. Vaduganathan has had financial relationships with Boehringer Ingelheim and Novartis, as well as with Amgen, AstraZeneca, Baxter Healthcare, Bayer, Cytokinetics, and Relypsa.
Gregg C. Fonarow, MD, recommended.
Less than 2 months before Dr. Fonarow made that striking statement during the virtual annual meeting of the Heart Failure Society of America, investigators first reported results from the EMPEROR-Reduced trial at the European Society of Cardiology’s virtual annual meeting, showing that the sodium-glucose transporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) successfully cut events in patients with heart failure with reduced ejection fraction (HFrEF). That report, a year after results from a similar trial (DAPA-HF) showed the same outcome using a different drug from the same class, dapagliflozin (Farxiga), cemented the SGLT2 inhibitor drug class as the fourth pillar for treating HFrEF, joining the angiotensin receptor neprilysin inhibitor (ARNI) class (sacubitril valsartan), beta-blockers (like carvedilol), and mineralocorticoid receptor antagonists (like spironolactone).
This rejiggering of the consensus expert approach for treating HFrEF left cardiologists wondering what sequence to use when starting this quadruple therapy. Within weeks, the answer from heart failure opinion leaders was clear:
“Start all four pillars simultaneously. Most patients can tolerate, and will benefit from, a simultaneous start,” declared Dr. Fonarow, professor and chief of cardiology at the University of California, Los Angeles.
His rationale? Patients get benefits from each of these drug classes “surprisingly early,” with improved outcomes in clinical trials appearing within a few weeks, compared with patients in control arms. The consequence is that any delay in starting treatment denies patients time with improved health status, function, and survival.
Study results documented that the four foundational drug classes can produce rapid improvements in health status, left ventricular size and shape, and make clinically meaningful cuts in both first and recurrent hospitalizations for heart failure and in mortality, Dr. Fonarow said. After 30 days on quadruple treatment, a patient’s relative risk for death drops by more than three-quarters, compared with patients not on these medications.
The benefits from each of the four classes involve distinct physiologic pathways and hence are not diminished by concurrent treatment. And immediate initiation avoids the risk of clinical inertia and a negligence to prescribe one or more of the four important drug classes. Introducing the four classes in a sequential manner could mean spending as long as a year to get all four on board and up-titrated to optimal therapeutic levels, he noted.
“Overcome inertia by prescribing [all four drug classes] at the time of diagnosis,” Dr. Fonarow admonished his audience.
The challenge of prescribing inertia
The risk for inertia in prescribing heart failure medications is real. Data collected in the CHAMP-HF (Change the Management of Patients with Heart Failure) registry from more than 3,500 HFrEF patients managed at any of 150 U.S. primary care and cardiology practices starting in late 2015 and continuing through 2017 showed that, among patients eligible for treatment with renin-angiotensin system (RAS) inhibition (with either ARNI or a single RAS inhibiting drug), a beta-blocker, and a mineralocorticoid receptor antagonist (MRA), 22% received all three drug classes. A scant 1% were on target dosages of all three drug classes, noted Stephen J. Greene, MD, in a separate talk at the meeting when he cited his published findings.
The sole formulation currently in the ARNI class, sacubitril/valsartan (Entresto) has in recent years been the poster child for prescribing inertia in HFrEF patients after coming onto the U.S. market for routine use in 2015. A review run by Dr. Greene of more than 9,000 HFrEF patients who were at least 65 years old and discharged from a hospital participating in the Get With the Guidelines–Heart Failure registry during October 2015–September 2017 showed that 8% of eligible patients actually received a sacubitril/valsartan prescription. Separate assessment of outpatients with HFrEF from the same era showed 13% uptake, said D. Greene, a cardiologist at Duke University, Durham, N.C.
Substantial gaps in prescribing evidence-based treatments to HFrEF patients have existed for the past couple of decades, said Dr. Greene. “Even a blockbuster drug like sacubitril/valsartan has been slow to implement.”
Quadruple therapy adds an average of 6 years of life
One of the most strongest arguments favoring the start-four-at-once approach was detailed in what’s quickly become a widely cited analysis published in July 2020 by a team of researchers led by Muthiah Vaduganathan, MD. Using data from three key pivotal trials they estimated that timely treatment with all four drug classes would on average produce an extra 6 years of overall survival in a 55-year old HFrEF patient, and an added 8 years free from cardiovascular death or first hospitalization for heart failure, compared with less comprehensive treatment. The analysis also showed a significant 3-year average boost in overall survival among HFrEF patients who were 80 years old when using quadruple therapy compared with the “conventional medical therapy” used on control patients in the three trials examined.
Dr. Greene called these findings “remarkable.”
“Four drugs use five mechanistic pathways to produce 6 added years of survival,” summed up Dr. Vaduganathan during a separate talk at the virtual meeting.
In addition to this substantial potential for a meaningful impact on patents’ lives, he cited other factors that add to the case for early prescription of the pharmaceutical gauntlet: avoiding missed treatment opportunities that occur with slower, step-wise drug introduction; simplifying, streamlining, and standardizing the care pathway, which helps avoid care inequities and disrupts the potential for inertia; magnifying benefit when comprehensive treatment starts sooner; and providing additive benefits without drug-drug interactions.
“Upfront treatment at the time of [HFrEF] diagnosis or hospitalization is an approach that disrupts treatment inertia,” emphasized Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital in Boston.
New approaches needed to encourage quick uptake
“Efficacy alone has not been enough for efficient uptake in U.S. practice” of sacubitril/valsartan, other RAS inhibitors, beta-blockers, and MRAs, noted Dr. Greene.
He was more optimistic about prospects for relatively quick uptake of early SGLT2 inhibitor treatment as part of routine HFrEF management given all the positives that this new HFrEF treatment offers, including some “unique features” among HFrEF drugs. These include the simplicity of the regimen, which involves a single dosage for everyone that’s taken once daily; minimal blood pressure effects and no adverse renal effects while also producing substantial renal protection; and two SGLT2 inhibitors with proven HFrEF benefit (dapagliflozin and empagliflozin), which bodes well for an eventual price drop.
The SGLT2 inhibitors stack up as an “ideal” HFrEF treatment, concluded Dr. Greene, which should facilitate quick uptake. As far as getting clinicians to also add early on the other three members of the core four treatment classes in routine treatment, he conceded that “innovative and evidence-based approaches to improving real-world uptake of guideline-directed medical therapy are urgently needed.”
EMPEROR-Reduced was funded by Boehringer Ingelheim and Lilly, the companies that market empagliflozin (Jardiance). CHAMP-HF was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. Fonarow has been a consultant or adviser to Novartis, as well as to Abbott, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards, Janssen, Medtronic, and Merck. Dr. Greene has received research funding from Novartis, has been a consultant to Amgen and Merck, an adviser to Amgen and Cytokinetics, and has received research funding from Amgen, AstraZeneca, Bristol-Myers Squibb, and Merck. Dr. Vaduganathan has had financial relationships with Boehringer Ingelheim and Novartis, as well as with Amgen, AstraZeneca, Baxter Healthcare, Bayer, Cytokinetics, and Relypsa.
FROM HFSA 2020
‘Modest’ benefit for post-MI T2D glucose monitoring
Following a heart attack, there appears to be a “modest” benefit of using flash glucose monitoring over fingerstick testing to monitor blood glucose levels in patients with type 2 diabetes being treated with insulin or a sulfonylurea, according to investigators of the LIBERATES trial.
The results showed a nonsignificant increase in the time that subjects’ blood glucose was spent in the target range of 3.9-10.00 mmol/L (70-180 mg/dL) 3 months after experiencing an acute coronary syndrome (ACS).
At best, flash monitoring using Abbott’s Freestyle Libre system was associated with an increase in time spent in range (TIR) of 17-28 or 48 minutes per day over self-monitoring of blood glucose (SMBG), depending on the type of statistical analysis used. There was no difference in glycated hemoglobin A1c levels between the two groups, but there was a trend for less time spent in hypoglycemia in the flash monitoring arm.
Viewers underwhelmed
“My overall impression is that the effects were less pronounced than anticipated,” Kare Birkeland, MD, PhD, a specialist in internal medicine and endocrinology at Oslo University Hospital, Rikshospitalet, Norway, observed after the findings were presented at the virtual annual meeting of the European Association for the Study of Diabetes.
Others who had watched the live session seemed similarly underwhelmed by the findings, with one viewer questioning the value of devoting an hour-and-a-half session to the phase 2 trial.
However, the session chair Simon Heller, BA, MB, BChir, DM, professor of clinical diabetes at the University of Sheffield, and trial coinvestigator, defended the detailed look at the trial’s findings, noting that it was worthwhile to present the data from the trial as it “really helps explain why we do phase 2 and phase 3 trials.”
Strong rationale for monitoring post-MI
There is a strong rationale for ensuring that blood glucose is well controlled in type 2 diabetes patients who have experienced a myocardial infarction, observed Robert Storey, BSc, BM, DM, professor of cardiology at the University of Sheffield. One way to do that potentially is through improved glucose monitoring.
“There’s clearly a close link between diabetes and the risk of MI: Both high and low HbA1c are associated with adverse outcome, and high and low glucose levels following MI are also associated with adverse outcome,” he observed, noting also that hypoglycemia was not given enough attention in post-ACS patients.
“The hypothesis of the LIBERATES study was that a modern glycemic monitoring strategy can optimize blood glucose levels in type 2 diabetes patients following MI with the potential to reduce mortality and morbidity and improve quality of life,” Dr. Storey said. “The main research question of LIBERATES says, ‘Do new approaches in glucose monitoring increase the time in range and reduce hypoglycemia?’ ”
Pragmatic trial design
LIBERATES was a prospective, multicenter, parallel group, randomized controlled trial, explained the study’s statistician Deborah Stocken, PhD, professor of clinical trials research at the University of Leeds. There was “limited ability to blind the interventions,” so it was an open-label design.
“The patient population in LIBERATES was kept as inclusive and as pragmatic as possible to ensure that the results at the end of the trial are generalizable,” said Dr. Stocken. Patients with type 2 diabetes were recruited within 5 days of hospital admission for ACS, which could include both ST- and non-ST elevation MI. In all, 141 of a calculated 150 patients that would be needed were recruited and randomized to the flash monitoring (69) or SMBG (72) arm.
Dr. Stocken noted that early in the recruitment phase, the trials oversight committee recommended that Bayesian methodology should be used as the most robust analytical approach.
“Essentially, a Bayesian approach would avoid a hypothesis test, and instead would provide a probability of there being a treatment benefit for continuous monitoring. And if this probability was high enough, this would warrant further research in the phase 3 setting,” Dr. Stocken said.
What else was shown?
“We had a number of prespecified secondary endpoints, which to me are equally important,” said Ramzi Ajjan, MD, MMed.Sci, PhD, associate professor and consultant in diabetes and endocrinology at Leeds University and Leeds Teaching Hospitals Trust.
Among these was the TIR at days 16-30, which showed a 90-minute increase per day in favor of flash monitoring over SMBG. This “seems to be driven by those who are an insulin,” Dr. Ajjan said, adding that “you get almost a 3-hour increase in time in range in people who are on insulin at baseline, and you don’t see that in people who are on sulfonylurea.”
Conversely, sulfonylurea treatment seemed to drive the reduction in the time spent in hypoglycemia defined as 3.9 mmol/L (70 g/dL) at 3 months. For the whole group, there was a 1.3-hour reduction in hypoglycemia per day with flash monitoring versus SMBG, which increased to 2 hours for those on sulfonylureas.
There also was a “pattern of reduction” in time spent in hypoglycemia defined as less than 3.0 mmol/L (54 g/dL) both early on and becoming more pronounced with time.
“Flash glucose monitoring is associated with higher treatment satisfaction score, compared with SMBG,” Dr. Ajjan said.
Although A1c dropped in both groups to a similar extent, he noted that the reduction seen in the flash monitoring group was associated with a decrease in hypoglycemia.
There was a huge amount of data collected during the trial and there are many more analyses that could be done, Dr. Ajjan said. The outcome of those may determine whether a phase 3 trial is likely, assuming sponsorship can be secured.
The LIBERATES Trial was funded by grants from the UK National Institute for Health Research and Abbott Diabetes Care. None of the investigators were additionally compensated for their work within the trial. Dr. Stocken had no disclosures in relation to this trial. Dr. Ajjan has received research funding and other financial support from Abbott, Bayer, Eli Lilly, Johnson & Johnson, and Novo Nordisk.
SOURCE: Ajjan R et al. EASD 2020. S11 – The LIBERATES Trial.
Following a heart attack, there appears to be a “modest” benefit of using flash glucose monitoring over fingerstick testing to monitor blood glucose levels in patients with type 2 diabetes being treated with insulin or a sulfonylurea, according to investigators of the LIBERATES trial.
The results showed a nonsignificant increase in the time that subjects’ blood glucose was spent in the target range of 3.9-10.00 mmol/L (70-180 mg/dL) 3 months after experiencing an acute coronary syndrome (ACS).
At best, flash monitoring using Abbott’s Freestyle Libre system was associated with an increase in time spent in range (TIR) of 17-28 or 48 minutes per day over self-monitoring of blood glucose (SMBG), depending on the type of statistical analysis used. There was no difference in glycated hemoglobin A1c levels between the two groups, but there was a trend for less time spent in hypoglycemia in the flash monitoring arm.
Viewers underwhelmed
“My overall impression is that the effects were less pronounced than anticipated,” Kare Birkeland, MD, PhD, a specialist in internal medicine and endocrinology at Oslo University Hospital, Rikshospitalet, Norway, observed after the findings were presented at the virtual annual meeting of the European Association for the Study of Diabetes.
Others who had watched the live session seemed similarly underwhelmed by the findings, with one viewer questioning the value of devoting an hour-and-a-half session to the phase 2 trial.
However, the session chair Simon Heller, BA, MB, BChir, DM, professor of clinical diabetes at the University of Sheffield, and trial coinvestigator, defended the detailed look at the trial’s findings, noting that it was worthwhile to present the data from the trial as it “really helps explain why we do phase 2 and phase 3 trials.”
Strong rationale for monitoring post-MI
There is a strong rationale for ensuring that blood glucose is well controlled in type 2 diabetes patients who have experienced a myocardial infarction, observed Robert Storey, BSc, BM, DM, professor of cardiology at the University of Sheffield. One way to do that potentially is through improved glucose monitoring.
“There’s clearly a close link between diabetes and the risk of MI: Both high and low HbA1c are associated with adverse outcome, and high and low glucose levels following MI are also associated with adverse outcome,” he observed, noting also that hypoglycemia was not given enough attention in post-ACS patients.
“The hypothesis of the LIBERATES study was that a modern glycemic monitoring strategy can optimize blood glucose levels in type 2 diabetes patients following MI with the potential to reduce mortality and morbidity and improve quality of life,” Dr. Storey said. “The main research question of LIBERATES says, ‘Do new approaches in glucose monitoring increase the time in range and reduce hypoglycemia?’ ”
Pragmatic trial design
LIBERATES was a prospective, multicenter, parallel group, randomized controlled trial, explained the study’s statistician Deborah Stocken, PhD, professor of clinical trials research at the University of Leeds. There was “limited ability to blind the interventions,” so it was an open-label design.
“The patient population in LIBERATES was kept as inclusive and as pragmatic as possible to ensure that the results at the end of the trial are generalizable,” said Dr. Stocken. Patients with type 2 diabetes were recruited within 5 days of hospital admission for ACS, which could include both ST- and non-ST elevation MI. In all, 141 of a calculated 150 patients that would be needed were recruited and randomized to the flash monitoring (69) or SMBG (72) arm.
Dr. Stocken noted that early in the recruitment phase, the trials oversight committee recommended that Bayesian methodology should be used as the most robust analytical approach.
“Essentially, a Bayesian approach would avoid a hypothesis test, and instead would provide a probability of there being a treatment benefit for continuous monitoring. And if this probability was high enough, this would warrant further research in the phase 3 setting,” Dr. Stocken said.
What else was shown?
“We had a number of prespecified secondary endpoints, which to me are equally important,” said Ramzi Ajjan, MD, MMed.Sci, PhD, associate professor and consultant in diabetes and endocrinology at Leeds University and Leeds Teaching Hospitals Trust.
Among these was the TIR at days 16-30, which showed a 90-minute increase per day in favor of flash monitoring over SMBG. This “seems to be driven by those who are an insulin,” Dr. Ajjan said, adding that “you get almost a 3-hour increase in time in range in people who are on insulin at baseline, and you don’t see that in people who are on sulfonylurea.”
Conversely, sulfonylurea treatment seemed to drive the reduction in the time spent in hypoglycemia defined as 3.9 mmol/L (70 g/dL) at 3 months. For the whole group, there was a 1.3-hour reduction in hypoglycemia per day with flash monitoring versus SMBG, which increased to 2 hours for those on sulfonylureas.
There also was a “pattern of reduction” in time spent in hypoglycemia defined as less than 3.0 mmol/L (54 g/dL) both early on and becoming more pronounced with time.
“Flash glucose monitoring is associated with higher treatment satisfaction score, compared with SMBG,” Dr. Ajjan said.
Although A1c dropped in both groups to a similar extent, he noted that the reduction seen in the flash monitoring group was associated with a decrease in hypoglycemia.
There was a huge amount of data collected during the trial and there are many more analyses that could be done, Dr. Ajjan said. The outcome of those may determine whether a phase 3 trial is likely, assuming sponsorship can be secured.
The LIBERATES Trial was funded by grants from the UK National Institute for Health Research and Abbott Diabetes Care. None of the investigators were additionally compensated for their work within the trial. Dr. Stocken had no disclosures in relation to this trial. Dr. Ajjan has received research funding and other financial support from Abbott, Bayer, Eli Lilly, Johnson & Johnson, and Novo Nordisk.
SOURCE: Ajjan R et al. EASD 2020. S11 – The LIBERATES Trial.
Following a heart attack, there appears to be a “modest” benefit of using flash glucose monitoring over fingerstick testing to monitor blood glucose levels in patients with type 2 diabetes being treated with insulin or a sulfonylurea, according to investigators of the LIBERATES trial.
The results showed a nonsignificant increase in the time that subjects’ blood glucose was spent in the target range of 3.9-10.00 mmol/L (70-180 mg/dL) 3 months after experiencing an acute coronary syndrome (ACS).
At best, flash monitoring using Abbott’s Freestyle Libre system was associated with an increase in time spent in range (TIR) of 17-28 or 48 minutes per day over self-monitoring of blood glucose (SMBG), depending on the type of statistical analysis used. There was no difference in glycated hemoglobin A1c levels between the two groups, but there was a trend for less time spent in hypoglycemia in the flash monitoring arm.
Viewers underwhelmed
“My overall impression is that the effects were less pronounced than anticipated,” Kare Birkeland, MD, PhD, a specialist in internal medicine and endocrinology at Oslo University Hospital, Rikshospitalet, Norway, observed after the findings were presented at the virtual annual meeting of the European Association for the Study of Diabetes.
Others who had watched the live session seemed similarly underwhelmed by the findings, with one viewer questioning the value of devoting an hour-and-a-half session to the phase 2 trial.
However, the session chair Simon Heller, BA, MB, BChir, DM, professor of clinical diabetes at the University of Sheffield, and trial coinvestigator, defended the detailed look at the trial’s findings, noting that it was worthwhile to present the data from the trial as it “really helps explain why we do phase 2 and phase 3 trials.”
Strong rationale for monitoring post-MI
There is a strong rationale for ensuring that blood glucose is well controlled in type 2 diabetes patients who have experienced a myocardial infarction, observed Robert Storey, BSc, BM, DM, professor of cardiology at the University of Sheffield. One way to do that potentially is through improved glucose monitoring.
“There’s clearly a close link between diabetes and the risk of MI: Both high and low HbA1c are associated with adverse outcome, and high and low glucose levels following MI are also associated with adverse outcome,” he observed, noting also that hypoglycemia was not given enough attention in post-ACS patients.
“The hypothesis of the LIBERATES study was that a modern glycemic monitoring strategy can optimize blood glucose levels in type 2 diabetes patients following MI with the potential to reduce mortality and morbidity and improve quality of life,” Dr. Storey said. “The main research question of LIBERATES says, ‘Do new approaches in glucose monitoring increase the time in range and reduce hypoglycemia?’ ”
Pragmatic trial design
LIBERATES was a prospective, multicenter, parallel group, randomized controlled trial, explained the study’s statistician Deborah Stocken, PhD, professor of clinical trials research at the University of Leeds. There was “limited ability to blind the interventions,” so it was an open-label design.
“The patient population in LIBERATES was kept as inclusive and as pragmatic as possible to ensure that the results at the end of the trial are generalizable,” said Dr. Stocken. Patients with type 2 diabetes were recruited within 5 days of hospital admission for ACS, which could include both ST- and non-ST elevation MI. In all, 141 of a calculated 150 patients that would be needed were recruited and randomized to the flash monitoring (69) or SMBG (72) arm.
Dr. Stocken noted that early in the recruitment phase, the trials oversight committee recommended that Bayesian methodology should be used as the most robust analytical approach.
“Essentially, a Bayesian approach would avoid a hypothesis test, and instead would provide a probability of there being a treatment benefit for continuous monitoring. And if this probability was high enough, this would warrant further research in the phase 3 setting,” Dr. Stocken said.
What else was shown?
“We had a number of prespecified secondary endpoints, which to me are equally important,” said Ramzi Ajjan, MD, MMed.Sci, PhD, associate professor and consultant in diabetes and endocrinology at Leeds University and Leeds Teaching Hospitals Trust.
Among these was the TIR at days 16-30, which showed a 90-minute increase per day in favor of flash monitoring over SMBG. This “seems to be driven by those who are an insulin,” Dr. Ajjan said, adding that “you get almost a 3-hour increase in time in range in people who are on insulin at baseline, and you don’t see that in people who are on sulfonylurea.”
Conversely, sulfonylurea treatment seemed to drive the reduction in the time spent in hypoglycemia defined as 3.9 mmol/L (70 g/dL) at 3 months. For the whole group, there was a 1.3-hour reduction in hypoglycemia per day with flash monitoring versus SMBG, which increased to 2 hours for those on sulfonylureas.
There also was a “pattern of reduction” in time spent in hypoglycemia defined as less than 3.0 mmol/L (54 g/dL) both early on and becoming more pronounced with time.
“Flash glucose monitoring is associated with higher treatment satisfaction score, compared with SMBG,” Dr. Ajjan said.
Although A1c dropped in both groups to a similar extent, he noted that the reduction seen in the flash monitoring group was associated with a decrease in hypoglycemia.
There was a huge amount of data collected during the trial and there are many more analyses that could be done, Dr. Ajjan said. The outcome of those may determine whether a phase 3 trial is likely, assuming sponsorship can be secured.
The LIBERATES Trial was funded by grants from the UK National Institute for Health Research and Abbott Diabetes Care. None of the investigators were additionally compensated for their work within the trial. Dr. Stocken had no disclosures in relation to this trial. Dr. Ajjan has received research funding and other financial support from Abbott, Bayer, Eli Lilly, Johnson & Johnson, and Novo Nordisk.
SOURCE: Ajjan R et al. EASD 2020. S11 – The LIBERATES Trial.
FROM EASD 2020
COVID-19 antibody response not reduced with diabetes
Neither diabetes per se nor hyperglycemia appear to impair the antibody response to SARS-CoV-2, suggesting that a COVID-19 vaccine would be just as effective in people with diabetes as in those without, new research finds.
Results from a study involving 480 patients with confirmed COVID-19 seen at an Italian hospital between February 25 and April 19 were published online October 8 in Diabetologia by Vito Lampasona, MD, and colleagues.
Antibody responses against multiple SARS-CoV-2 antigens among the 27% of patients with COVID-19 and diabetes (preexisting and newly diagnosed) were similar with regard to timing, titers, and classes to those of patients with COVID-19 and without diabetes, and the results did not differ by glucose levels.
Moreover, positivity for immunoglobulin G (IgG) against the SARS-CoV-2 spike receptor-binding domain (RBD) was associated with improved survival regardless of diabetes status.
And as previously shown, high blood glucose levels were strongly associated with greater COVID-19 mortality even in those without diabetes.
This is the first study of the immunologic humoral response against SARS-CoV-2 in patients with hyperglycemia, the authors say.
“The immunological response to a future SARS-CoV-2 vaccine will be assessed when the vaccine becomes available. However, our data allow a cautious optimism regarding effective immunization in individuals with diabetes, as well as in the general population,” wrote Dr. Lampasona of San Raffaele Diabetes Research Institute, IRCCS Ospedale San Raffaele in Milan, and colleagues.
Diabetes and hyperglycemia worsen COVID-19 outcomes
The investigators analyzed the presence of three types of antibody to multiple SARS-CoV-2 antigens in 509 participants: IgG, which is evidence of past infection; IgM, which indicates more recent or current infection; and IgA, which is involved in the mucosal immune response, for example, in the nose where the virus enters the body.
Overall, 452 (88.8%) patients were hospitalized, 79 (15.5%) patients were admitted to intensive care, and 93 (18.3%) patients died during follow-up.
Of the 139 patients with diabetes, 90 (17.7% of the study cohort) already had a diagnosis of diabetes, and 49 (9.6%) were newly diagnosed.
Those with diabetes were older, had a higher body mass index (BMI), and were more likely to have cardiovascular comorbidities, hypertension, and chronic kidney disease. As has been previously reported for diabetes and COVID-19, diabetes was also associated with increased levels of inflammatory biomarkers, hypercoagulopathy, leukocytosis, and neutrophilia.
In multivariate analysis, diabetes status (hazard ratio, 2.32; P = .001), mean fasting plasma glucose (P < .001), and glucose variability (P = .002) were all independently associated with increased mortality and ICU admission. And fasting plasma glucose was associated with increased mortality risk even among those without diabetes (P < .001).
Antibody response similar in patients with and without diabetes
The humoral response against SARS-CoV-2 in patients with diabetes was present and superimposable in terms of timing and antibody titers to that of patients without diabetes, with marginal differences, and was not influenced by glucose levels.
After adjustment for sex, age, and diabetes status and stratification by symptom duration at time of sampling, the development of SARS-CoV-2 RBD IgG antibodies was associated with improved survival, with an HR for time to death of 0.4 (P = .002).
“Of the measured antibody responses, positivity for IgG against the SARS-CoV-2 spike RBD was predictive of survival rate, both in the presence or absence of diabetes,” the authors stressed, with similar HRs for those with diabetes (0.37; P = .013) and without diabetes (0.43; P = .038).
These data confirm “the relevance for patient survival rate of the specific antigen response against spike RBD even in the presence of diabetes, and it underlines how the mechanism explaining the worse clinical outcome in patients with diabetes is unrelated to the antibody response,” they explain.
They added, “This, together with evidence that increased blood glucose levels do predict a poor prognosis even in nondiabetic individuals and the association with increased levels of inflammatory biomarkers and hypercoagulopathy, as well as leukocytosis and neutrophilia, support the speculation that glucose per se could be an independent biological negative factor, acting as a direct regulator of innate immunity.”
“The observed increased severity and mortality risk of COVID-19 pneumonia in patients with hyperglycemia was not the result of an impaired humoral response against SARS-CoV-2.”
“RBD IgG positivity was associated with a remarkable protective effect, allowing for a cautious optimism about the efficacy of future vaccines against SARS-COV-2 in people with diabetes,” they reiterated.
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Neither diabetes per se nor hyperglycemia appear to impair the antibody response to SARS-CoV-2, suggesting that a COVID-19 vaccine would be just as effective in people with diabetes as in those without, new research finds.
Results from a study involving 480 patients with confirmed COVID-19 seen at an Italian hospital between February 25 and April 19 were published online October 8 in Diabetologia by Vito Lampasona, MD, and colleagues.
Antibody responses against multiple SARS-CoV-2 antigens among the 27% of patients with COVID-19 and diabetes (preexisting and newly diagnosed) were similar with regard to timing, titers, and classes to those of patients with COVID-19 and without diabetes, and the results did not differ by glucose levels.
Moreover, positivity for immunoglobulin G (IgG) against the SARS-CoV-2 spike receptor-binding domain (RBD) was associated with improved survival regardless of diabetes status.
And as previously shown, high blood glucose levels were strongly associated with greater COVID-19 mortality even in those without diabetes.
This is the first study of the immunologic humoral response against SARS-CoV-2 in patients with hyperglycemia, the authors say.
“The immunological response to a future SARS-CoV-2 vaccine will be assessed when the vaccine becomes available. However, our data allow a cautious optimism regarding effective immunization in individuals with diabetes, as well as in the general population,” wrote Dr. Lampasona of San Raffaele Diabetes Research Institute, IRCCS Ospedale San Raffaele in Milan, and colleagues.
Diabetes and hyperglycemia worsen COVID-19 outcomes
The investigators analyzed the presence of three types of antibody to multiple SARS-CoV-2 antigens in 509 participants: IgG, which is evidence of past infection; IgM, which indicates more recent or current infection; and IgA, which is involved in the mucosal immune response, for example, in the nose where the virus enters the body.
Overall, 452 (88.8%) patients were hospitalized, 79 (15.5%) patients were admitted to intensive care, and 93 (18.3%) patients died during follow-up.
Of the 139 patients with diabetes, 90 (17.7% of the study cohort) already had a diagnosis of diabetes, and 49 (9.6%) were newly diagnosed.
Those with diabetes were older, had a higher body mass index (BMI), and were more likely to have cardiovascular comorbidities, hypertension, and chronic kidney disease. As has been previously reported for diabetes and COVID-19, diabetes was also associated with increased levels of inflammatory biomarkers, hypercoagulopathy, leukocytosis, and neutrophilia.
In multivariate analysis, diabetes status (hazard ratio, 2.32; P = .001), mean fasting plasma glucose (P < .001), and glucose variability (P = .002) were all independently associated with increased mortality and ICU admission. And fasting plasma glucose was associated with increased mortality risk even among those without diabetes (P < .001).
Antibody response similar in patients with and without diabetes
The humoral response against SARS-CoV-2 in patients with diabetes was present and superimposable in terms of timing and antibody titers to that of patients without diabetes, with marginal differences, and was not influenced by glucose levels.
After adjustment for sex, age, and diabetes status and stratification by symptom duration at time of sampling, the development of SARS-CoV-2 RBD IgG antibodies was associated with improved survival, with an HR for time to death of 0.4 (P = .002).
“Of the measured antibody responses, positivity for IgG against the SARS-CoV-2 spike RBD was predictive of survival rate, both in the presence or absence of diabetes,” the authors stressed, with similar HRs for those with diabetes (0.37; P = .013) and without diabetes (0.43; P = .038).
These data confirm “the relevance for patient survival rate of the specific antigen response against spike RBD even in the presence of diabetes, and it underlines how the mechanism explaining the worse clinical outcome in patients with diabetes is unrelated to the antibody response,” they explain.
They added, “This, together with evidence that increased blood glucose levels do predict a poor prognosis even in nondiabetic individuals and the association with increased levels of inflammatory biomarkers and hypercoagulopathy, as well as leukocytosis and neutrophilia, support the speculation that glucose per se could be an independent biological negative factor, acting as a direct regulator of innate immunity.”
“The observed increased severity and mortality risk of COVID-19 pneumonia in patients with hyperglycemia was not the result of an impaired humoral response against SARS-CoV-2.”
“RBD IgG positivity was associated with a remarkable protective effect, allowing for a cautious optimism about the efficacy of future vaccines against SARS-COV-2 in people with diabetes,” they reiterated.
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Neither diabetes per se nor hyperglycemia appear to impair the antibody response to SARS-CoV-2, suggesting that a COVID-19 vaccine would be just as effective in people with diabetes as in those without, new research finds.
Results from a study involving 480 patients with confirmed COVID-19 seen at an Italian hospital between February 25 and April 19 were published online October 8 in Diabetologia by Vito Lampasona, MD, and colleagues.
Antibody responses against multiple SARS-CoV-2 antigens among the 27% of patients with COVID-19 and diabetes (preexisting and newly diagnosed) were similar with regard to timing, titers, and classes to those of patients with COVID-19 and without diabetes, and the results did not differ by glucose levels.
Moreover, positivity for immunoglobulin G (IgG) against the SARS-CoV-2 spike receptor-binding domain (RBD) was associated with improved survival regardless of diabetes status.
And as previously shown, high blood glucose levels were strongly associated with greater COVID-19 mortality even in those without diabetes.
This is the first study of the immunologic humoral response against SARS-CoV-2 in patients with hyperglycemia, the authors say.
“The immunological response to a future SARS-CoV-2 vaccine will be assessed when the vaccine becomes available. However, our data allow a cautious optimism regarding effective immunization in individuals with diabetes, as well as in the general population,” wrote Dr. Lampasona of San Raffaele Diabetes Research Institute, IRCCS Ospedale San Raffaele in Milan, and colleagues.
Diabetes and hyperglycemia worsen COVID-19 outcomes
The investigators analyzed the presence of three types of antibody to multiple SARS-CoV-2 antigens in 509 participants: IgG, which is evidence of past infection; IgM, which indicates more recent or current infection; and IgA, which is involved in the mucosal immune response, for example, in the nose where the virus enters the body.
Overall, 452 (88.8%) patients were hospitalized, 79 (15.5%) patients were admitted to intensive care, and 93 (18.3%) patients died during follow-up.
Of the 139 patients with diabetes, 90 (17.7% of the study cohort) already had a diagnosis of diabetes, and 49 (9.6%) were newly diagnosed.
Those with diabetes were older, had a higher body mass index (BMI), and were more likely to have cardiovascular comorbidities, hypertension, and chronic kidney disease. As has been previously reported for diabetes and COVID-19, diabetes was also associated with increased levels of inflammatory biomarkers, hypercoagulopathy, leukocytosis, and neutrophilia.
In multivariate analysis, diabetes status (hazard ratio, 2.32; P = .001), mean fasting plasma glucose (P < .001), and glucose variability (P = .002) were all independently associated with increased mortality and ICU admission. And fasting plasma glucose was associated with increased mortality risk even among those without diabetes (P < .001).
Antibody response similar in patients with and without diabetes
The humoral response against SARS-CoV-2 in patients with diabetes was present and superimposable in terms of timing and antibody titers to that of patients without diabetes, with marginal differences, and was not influenced by glucose levels.
After adjustment for sex, age, and diabetes status and stratification by symptom duration at time of sampling, the development of SARS-CoV-2 RBD IgG antibodies was associated with improved survival, with an HR for time to death of 0.4 (P = .002).
“Of the measured antibody responses, positivity for IgG against the SARS-CoV-2 spike RBD was predictive of survival rate, both in the presence or absence of diabetes,” the authors stressed, with similar HRs for those with diabetes (0.37; P = .013) and without diabetes (0.43; P = .038).
These data confirm “the relevance for patient survival rate of the specific antigen response against spike RBD even in the presence of diabetes, and it underlines how the mechanism explaining the worse clinical outcome in patients with diabetes is unrelated to the antibody response,” they explain.
They added, “This, together with evidence that increased blood glucose levels do predict a poor prognosis even in nondiabetic individuals and the association with increased levels of inflammatory biomarkers and hypercoagulopathy, as well as leukocytosis and neutrophilia, support the speculation that glucose per se could be an independent biological negative factor, acting as a direct regulator of innate immunity.”
“The observed increased severity and mortality risk of COVID-19 pneumonia in patients with hyperglycemia was not the result of an impaired humoral response against SARS-CoV-2.”
“RBD IgG positivity was associated with a remarkable protective effect, allowing for a cautious optimism about the efficacy of future vaccines against SARS-COV-2 in people with diabetes,” they reiterated.
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
MitraClip effective for post-MI acute mitral regurgitation with cardiogenic shock
Percutaneous mitral valve repair with the MitraClip appears to be a safe, effective, and life-saving new treatment for severe acute mitral regurgitation (MR) secondary to MI in surgical noncandidates, even when accompanied by cardiogenic shock, according to data from the international IREMMI registry.
“Cardiogenic shock, when adequately supported, does not seem to influence short- and mid-term outcomes, so the development of cardiogenic shock should not preclude percutaneous mitral valve repair in this scenario,” Rodrigo Estevez-Loureiro, MD, PhD, said in presenting the IREMMI (International Registry of MitraClip in Acute Myocardial Infarction) findings reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
Commentators hailed the prospective IREMMI data as potentially practice changing in light of the dire prognosis of such patients when surgery is deemed unacceptably high risk because medical management, the traditionally the only alternative, has a 30-day mortality of up to 50%.
Severe acute MR occurs in an estimated 3% of acute MIs, and in roughly 10% of patients who present with acute MI complicated by cardiogenic shock (CS). The impact of intervening with the MitraClip in an effort to correct the acute MR arising from MI with CS has previously been addressed only in sparse case reports. The new IREMMI study is easily the largest dataset to date detailing clinical and echocardiographic outcomes, Dr. Estevez-Loureiro of Alvaro Cunqueiro Hospital in Vigo, Spain, said at the meeting, sponsored by the Cardiovascular Research Foundation.
He reported on 93 consecutive patients who underwent MitraClip implantation for acute MR arising in the setting of MI, including 50 patients in CS at the time of the procedure. All 93 patients had been turned down by their surgical team because of extreme surgical risk. Three-quarters of the MIs showed ST-segment elevation. Only six patients had a papillary muscle rupture; in the rest, the mechanism of acute MR involved left ventricular global remodeling associated with mitral valve leaflet tethering. Percutaneous valve repair was performed at 18 expert valvular heart centers in the United States, Canada, Israel, and five European countries.
Procedural success
Time from MI to MitraClip implantation averaged 24 days in the CS patients and 33 days in the comparator arm without CS.
“These patients had been turned down for surgery, so the attending physicians generally followed a strategy of trying to cool them down with mechanical circulatory support and vasopressors. MitraClip wasn’t an option at the beginning, but after two or three failed weanings from all the possible therapies, then MitraClip becomes an option. This is one of the reasons why the time lapse between MI and the clip is so large,” the cardiologist explained.
Procedural success rates were similar in the two groups: 90% in those with CS and 93% in those without. However, average procedure time was significantly longer in the CS patients: 143 minutes versus 83 minutes in the patients without CS.
At baseline, 86% of the CS group had grade 4+ MR, similar to the 79% rate in the non-CS patients. Postprocedurally, 60% of the CS group were MR grade 0/1 and 34% were grade 2, comparable to the rates of 65% and 23% in the non-CS group.
At 3 months’ follow-up, 83.4% of the CS group had MR grade 2 or less, again not significantly different from the 90.5% rate in non-CS patients. Systolic pulmonary artery pressure was also similar: 39.6 mm Hg in the CS patients, 44 mm Hg in those without. While everyone was New York Heart Association functional class IV preprocedurally, 79.5% of the CS group were NYHA class I or II at 3 months, not significantly different from the 86.5% prevalence in the comparator arm.
Longer-term clinical outcomes
At a median follow-up of 7 months, the composite primary clinical outcome composed of all-cause mortality or heart failure rehospitalization did not differ between the two groups: a 28% rate in the CS group and 25.6% in non-CS patients. All-cause mortality occurred in 16% with CS and 9.3% without, again not a significant difference.
In a Cox regression analysis, neither surgical risk score, patient age, left ventricular geometry, nor CS was independently associated with the primary composite endpoint. Indeed, the only independent predictor of freedom from mortality or heart failure readmission at follow-up was procedural success, which is very much a function of the experience of the heart team, Dr. Estevez-Loureiro continued.
Michael A. Borger, MD, PhD, who comoderated the late-breaking clinical science session, was wowed by the IREMMI results.
“The mortality rates, I can tell you, compared to traditional surgical series of acute MR in the face of ACS [acute cardiogenic shock] are very, very respectable,” commented Dr. Borger, director of the cardiac surgery clinic at the Leipzig (Ger.) University Heart Center.
“Extremely impressive,” agreed discussant Vinayak N. Bapat, MD, a cardiothoracic surgeon and valve scientist at the Minneapolis Heart Institute Foundation. He posed a practical question: “Should we take from this presentation that patients should be stabilized with something like ECMO [extracorporeal membrane oxygenation] or Impella [left ventricular assist device], then transferred to an expert center for the procedure?”
“I think that the stabilization is essential in the patients with cardiogenic shock,” Dr. Estevez-Loureiro replied. “Unlike with surgery, it’s very difficult to establish a MitraClip procedure in a couple of hours in the middle of the night. You have to stabilize them and then treat for shock with ECMO, Impella, or both. I think they should be transferred to a center than can deliver the best treatment. In centers with less experience, patients can be put on mechanical support and transferred to an expert valve center, not only for MitraClip implantation, but for discussion of all the treatment possibilities, including surgery.”
At a press conference in which Dr. Estevez-Loureiro presented highlights of the IREMMI study, discussant Dee Dee Wang, MD, said the international coinvestigators “need to be applauded” for this study.
“Having these outcomes is incredible,” declared Dr. Wang, a structural heart disease specialist at the Henry Ford Health System, Detroit.
While this is an observational study, it’s a high-quality dataset with excellent methodology. And conducting a randomized trial in patients with such high surgical risk scores – the CS group had an average EuroSCORE II of 21 – would be extremely difficult, according to the cardiologist.
Dr. Estevez-Loureiro reported receiving research grants from Abbott and serving as a consultant to that company as well as Boston Scientific.
SOURCE: Estevez-Loureiro, R. TCT 2020, LBCS session IV.
Percutaneous mitral valve repair with the MitraClip appears to be a safe, effective, and life-saving new treatment for severe acute mitral regurgitation (MR) secondary to MI in surgical noncandidates, even when accompanied by cardiogenic shock, according to data from the international IREMMI registry.
“Cardiogenic shock, when adequately supported, does not seem to influence short- and mid-term outcomes, so the development of cardiogenic shock should not preclude percutaneous mitral valve repair in this scenario,” Rodrigo Estevez-Loureiro, MD, PhD, said in presenting the IREMMI (International Registry of MitraClip in Acute Myocardial Infarction) findings reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
Commentators hailed the prospective IREMMI data as potentially practice changing in light of the dire prognosis of such patients when surgery is deemed unacceptably high risk because medical management, the traditionally the only alternative, has a 30-day mortality of up to 50%.
Severe acute MR occurs in an estimated 3% of acute MIs, and in roughly 10% of patients who present with acute MI complicated by cardiogenic shock (CS). The impact of intervening with the MitraClip in an effort to correct the acute MR arising from MI with CS has previously been addressed only in sparse case reports. The new IREMMI study is easily the largest dataset to date detailing clinical and echocardiographic outcomes, Dr. Estevez-Loureiro of Alvaro Cunqueiro Hospital in Vigo, Spain, said at the meeting, sponsored by the Cardiovascular Research Foundation.
He reported on 93 consecutive patients who underwent MitraClip implantation for acute MR arising in the setting of MI, including 50 patients in CS at the time of the procedure. All 93 patients had been turned down by their surgical team because of extreme surgical risk. Three-quarters of the MIs showed ST-segment elevation. Only six patients had a papillary muscle rupture; in the rest, the mechanism of acute MR involved left ventricular global remodeling associated with mitral valve leaflet tethering. Percutaneous valve repair was performed at 18 expert valvular heart centers in the United States, Canada, Israel, and five European countries.
Procedural success
Time from MI to MitraClip implantation averaged 24 days in the CS patients and 33 days in the comparator arm without CS.
“These patients had been turned down for surgery, so the attending physicians generally followed a strategy of trying to cool them down with mechanical circulatory support and vasopressors. MitraClip wasn’t an option at the beginning, but after two or three failed weanings from all the possible therapies, then MitraClip becomes an option. This is one of the reasons why the time lapse between MI and the clip is so large,” the cardiologist explained.
Procedural success rates were similar in the two groups: 90% in those with CS and 93% in those without. However, average procedure time was significantly longer in the CS patients: 143 minutes versus 83 minutes in the patients without CS.
At baseline, 86% of the CS group had grade 4+ MR, similar to the 79% rate in the non-CS patients. Postprocedurally, 60% of the CS group were MR grade 0/1 and 34% were grade 2, comparable to the rates of 65% and 23% in the non-CS group.
At 3 months’ follow-up, 83.4% of the CS group had MR grade 2 or less, again not significantly different from the 90.5% rate in non-CS patients. Systolic pulmonary artery pressure was also similar: 39.6 mm Hg in the CS patients, 44 mm Hg in those without. While everyone was New York Heart Association functional class IV preprocedurally, 79.5% of the CS group were NYHA class I or II at 3 months, not significantly different from the 86.5% prevalence in the comparator arm.
Longer-term clinical outcomes
At a median follow-up of 7 months, the composite primary clinical outcome composed of all-cause mortality or heart failure rehospitalization did not differ between the two groups: a 28% rate in the CS group and 25.6% in non-CS patients. All-cause mortality occurred in 16% with CS and 9.3% without, again not a significant difference.
In a Cox regression analysis, neither surgical risk score, patient age, left ventricular geometry, nor CS was independently associated with the primary composite endpoint. Indeed, the only independent predictor of freedom from mortality or heart failure readmission at follow-up was procedural success, which is very much a function of the experience of the heart team, Dr. Estevez-Loureiro continued.
Michael A. Borger, MD, PhD, who comoderated the late-breaking clinical science session, was wowed by the IREMMI results.
“The mortality rates, I can tell you, compared to traditional surgical series of acute MR in the face of ACS [acute cardiogenic shock] are very, very respectable,” commented Dr. Borger, director of the cardiac surgery clinic at the Leipzig (Ger.) University Heart Center.
“Extremely impressive,” agreed discussant Vinayak N. Bapat, MD, a cardiothoracic surgeon and valve scientist at the Minneapolis Heart Institute Foundation. He posed a practical question: “Should we take from this presentation that patients should be stabilized with something like ECMO [extracorporeal membrane oxygenation] or Impella [left ventricular assist device], then transferred to an expert center for the procedure?”
“I think that the stabilization is essential in the patients with cardiogenic shock,” Dr. Estevez-Loureiro replied. “Unlike with surgery, it’s very difficult to establish a MitraClip procedure in a couple of hours in the middle of the night. You have to stabilize them and then treat for shock with ECMO, Impella, or both. I think they should be transferred to a center than can deliver the best treatment. In centers with less experience, patients can be put on mechanical support and transferred to an expert valve center, not only for MitraClip implantation, but for discussion of all the treatment possibilities, including surgery.”
At a press conference in which Dr. Estevez-Loureiro presented highlights of the IREMMI study, discussant Dee Dee Wang, MD, said the international coinvestigators “need to be applauded” for this study.
“Having these outcomes is incredible,” declared Dr. Wang, a structural heart disease specialist at the Henry Ford Health System, Detroit.
While this is an observational study, it’s a high-quality dataset with excellent methodology. And conducting a randomized trial in patients with such high surgical risk scores – the CS group had an average EuroSCORE II of 21 – would be extremely difficult, according to the cardiologist.
Dr. Estevez-Loureiro reported receiving research grants from Abbott and serving as a consultant to that company as well as Boston Scientific.
SOURCE: Estevez-Loureiro, R. TCT 2020, LBCS session IV.
Percutaneous mitral valve repair with the MitraClip appears to be a safe, effective, and life-saving new treatment for severe acute mitral regurgitation (MR) secondary to MI in surgical noncandidates, even when accompanied by cardiogenic shock, according to data from the international IREMMI registry.
“Cardiogenic shock, when adequately supported, does not seem to influence short- and mid-term outcomes, so the development of cardiogenic shock should not preclude percutaneous mitral valve repair in this scenario,” Rodrigo Estevez-Loureiro, MD, PhD, said in presenting the IREMMI (International Registry of MitraClip in Acute Myocardial Infarction) findings reported at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
Commentators hailed the prospective IREMMI data as potentially practice changing in light of the dire prognosis of such patients when surgery is deemed unacceptably high risk because medical management, the traditionally the only alternative, has a 30-day mortality of up to 50%.
Severe acute MR occurs in an estimated 3% of acute MIs, and in roughly 10% of patients who present with acute MI complicated by cardiogenic shock (CS). The impact of intervening with the MitraClip in an effort to correct the acute MR arising from MI with CS has previously been addressed only in sparse case reports. The new IREMMI study is easily the largest dataset to date detailing clinical and echocardiographic outcomes, Dr. Estevez-Loureiro of Alvaro Cunqueiro Hospital in Vigo, Spain, said at the meeting, sponsored by the Cardiovascular Research Foundation.
He reported on 93 consecutive patients who underwent MitraClip implantation for acute MR arising in the setting of MI, including 50 patients in CS at the time of the procedure. All 93 patients had been turned down by their surgical team because of extreme surgical risk. Three-quarters of the MIs showed ST-segment elevation. Only six patients had a papillary muscle rupture; in the rest, the mechanism of acute MR involved left ventricular global remodeling associated with mitral valve leaflet tethering. Percutaneous valve repair was performed at 18 expert valvular heart centers in the United States, Canada, Israel, and five European countries.
Procedural success
Time from MI to MitraClip implantation averaged 24 days in the CS patients and 33 days in the comparator arm without CS.
“These patients had been turned down for surgery, so the attending physicians generally followed a strategy of trying to cool them down with mechanical circulatory support and vasopressors. MitraClip wasn’t an option at the beginning, but after two or three failed weanings from all the possible therapies, then MitraClip becomes an option. This is one of the reasons why the time lapse between MI and the clip is so large,” the cardiologist explained.
Procedural success rates were similar in the two groups: 90% in those with CS and 93% in those without. However, average procedure time was significantly longer in the CS patients: 143 minutes versus 83 minutes in the patients without CS.
At baseline, 86% of the CS group had grade 4+ MR, similar to the 79% rate in the non-CS patients. Postprocedurally, 60% of the CS group were MR grade 0/1 and 34% were grade 2, comparable to the rates of 65% and 23% in the non-CS group.
At 3 months’ follow-up, 83.4% of the CS group had MR grade 2 or less, again not significantly different from the 90.5% rate in non-CS patients. Systolic pulmonary artery pressure was also similar: 39.6 mm Hg in the CS patients, 44 mm Hg in those without. While everyone was New York Heart Association functional class IV preprocedurally, 79.5% of the CS group were NYHA class I or II at 3 months, not significantly different from the 86.5% prevalence in the comparator arm.
Longer-term clinical outcomes
At a median follow-up of 7 months, the composite primary clinical outcome composed of all-cause mortality or heart failure rehospitalization did not differ between the two groups: a 28% rate in the CS group and 25.6% in non-CS patients. All-cause mortality occurred in 16% with CS and 9.3% without, again not a significant difference.
In a Cox regression analysis, neither surgical risk score, patient age, left ventricular geometry, nor CS was independently associated with the primary composite endpoint. Indeed, the only independent predictor of freedom from mortality or heart failure readmission at follow-up was procedural success, which is very much a function of the experience of the heart team, Dr. Estevez-Loureiro continued.
Michael A. Borger, MD, PhD, who comoderated the late-breaking clinical science session, was wowed by the IREMMI results.
“The mortality rates, I can tell you, compared to traditional surgical series of acute MR in the face of ACS [acute cardiogenic shock] are very, very respectable,” commented Dr. Borger, director of the cardiac surgery clinic at the Leipzig (Ger.) University Heart Center.
“Extremely impressive,” agreed discussant Vinayak N. Bapat, MD, a cardiothoracic surgeon and valve scientist at the Minneapolis Heart Institute Foundation. He posed a practical question: “Should we take from this presentation that patients should be stabilized with something like ECMO [extracorporeal membrane oxygenation] or Impella [left ventricular assist device], then transferred to an expert center for the procedure?”
“I think that the stabilization is essential in the patients with cardiogenic shock,” Dr. Estevez-Loureiro replied. “Unlike with surgery, it’s very difficult to establish a MitraClip procedure in a couple of hours in the middle of the night. You have to stabilize them and then treat for shock with ECMO, Impella, or both. I think they should be transferred to a center than can deliver the best treatment. In centers with less experience, patients can be put on mechanical support and transferred to an expert valve center, not only for MitraClip implantation, but for discussion of all the treatment possibilities, including surgery.”
At a press conference in which Dr. Estevez-Loureiro presented highlights of the IREMMI study, discussant Dee Dee Wang, MD, said the international coinvestigators “need to be applauded” for this study.
“Having these outcomes is incredible,” declared Dr. Wang, a structural heart disease specialist at the Henry Ford Health System, Detroit.
While this is an observational study, it’s a high-quality dataset with excellent methodology. And conducting a randomized trial in patients with such high surgical risk scores – the CS group had an average EuroSCORE II of 21 – would be extremely difficult, according to the cardiologist.
Dr. Estevez-Loureiro reported receiving research grants from Abbott and serving as a consultant to that company as well as Boston Scientific.
SOURCE: Estevez-Loureiro, R. TCT 2020, LBCS session IV.
FROM TCT 2020
Intravascular lithotripsy hailed as ‘game changer’ for coronary calcification
aimed at gaining U.S. regulatory approval.
The technology is basically the same as in extracorporeal lithotripsy, used for the treatment of kidney stones for more than 30 years: namely, transmission of pulsed acoustic pressure waves in order to fracture calcium. For interventional cardiology purposes, however, the transmitter is located within a balloon angioplasty catheter, Dean J. Kereiakes, MD, explained in presenting the study results at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
In Disrupt CAD III, intravascular lithotripsy far exceeded the procedural success and 30-day freedom from major adverse cardiovascular event (MACE) performance targets set in conjunction with the Food and Drug Administration. In so doing, the intravascular lithotripsy device developed by Shockwave Medical successfully addressed one of the banes of contemporary interventional cardiology: heavily calcified coronary lesions.
Currently available technologies targeting such lesions, including noncompliant high-pressure balloons, intravascular lasers, cutting balloons, and orbital and rotational atherectomy, often yield suboptimal results, noted Dr. Kereiakes, medical director of the Christ Hospital Heart and Cardiovascular Center in Cincinnati.
Severe vascular calcifications are becoming more common, due in part to an aging population and the growing prevalence of hypertension, diabetes, and renal insufficiency. Severely calcified coronary lesions complicate percutaneous coronary intervention. They’re associated with increased risks of dissection, perforation, and periprocedural MI. Moreover, heavily calcified lesions impede stent delivery and expansion – and stent underexpansion is the leading predictor of restenosis and stent thrombosis, he observed at the meeting, sponsored by the Cardiovascular Research Foundation. Disrupt CAD III was a prospective single-arm study of 384 patients at 47 sites in the United States and several European countries. All participants had de novo coronary calcifications graded as severe by core laboratory assessment, with a mean calcified length of 47.9 mm by quantitative coronary angiography and a mean calcium angle and thickness of 292.5 degrees and 0.96 mm by optical coherence tomography.
“It’s staggering, the level of calcification these patients had. It’s jaw dropping,” Dr. Kereiakes observed.
Intravascular lithotripsy was used to prepare these severely calcified lesions for stenting. The intervention entailed transmission of acoustic waves circumferentially and transmurally at 1 pulse per second through tissue at an effective pressure of about 50 atm. Patients received an average of 69 pulses.
This was not a randomized trial; there was no sham-treated control arm. Instead, the comparator group selected under regulatory guidance was comprised of patients who had received orbital atherectomy for severe coronary calcifications in the earlier, similarly designed ORBIT II trial, which led to FDA marketing approval of that technology.
Key outcomes
The procedural success rate, defined as successful stent delivery with less than a 50% residual stenosis and no in-hospital MACE, was 92.4% in Disrupt CAD III, compared to 83.4% for orbital atherectomy in ORBIT II. The primary safety endpoint of freedom from cardiac death, MI, or target vessel revascularization at 30 days was achieved in 92.2% of patients in the intravascular lithotripsy trial, versus 84.4% in ORBIT II.
The 30-day MACE rate of 7.8% in Disrupt CAD III was primarily driven by periprocedural MIs, which occurred in 6.8% of participants. Only one-third of the MIs were clinically relevant by the Society for Coronary Angiography and Intervention definition. There were two cardiac deaths and three cases of stent thrombosis, all of which were associated with known predictors of the complication. There was 1 case each of dissection, abrupt closure, and perforation, but no instances of slow flow or no reflow at the procedure’s end. Transient lithotripsy-induced left ventricular capture occurred in 41% of patients, but they were benign events with no lasting consequences.
The device was able to cross and deliver acoustic pressure wave therapy to 98.2% of lesions. The mean diameter stenosis preprocedure was 65.1%, dropping to 37.2% post lithotripsy, with a final in-stent residual stenosis diameter of 11.9%, with a 1.7-mm acute gain. The average stent expansion at the site of maximum calcification was 102%, with a minimum stent area of 6.5 mm2.
Optical coherence imaging revealed that 67% of treated lesions had circumferential and transmural fractures of both deep and superficial calcium post lithotripsy. Yet outcomes were the same regardless of whether fractures were evident on imaging.
At 30-day follow-up, 72.9% of patients had no angina, up from just 12.6% of participants pre-PCI. Follow-up will continue for 2 years.
Outcomes were similar for the first case done at each participating center and all cases thereafter.
“The ease of use was remarkable,” Dr. Kereiakes recalled. “The learning curve is virtually nonexistent.”
The reaction
At a press conference where Dr. Kereiakes presented the Disrupt CAD III results, discussant Allen Jeremias, MD, said he found the results compelling.
“The success rate is high, I think it’s relatively easy to use, as demonstrated, and I think the results are spectacular,” said Dr. Jeremias, director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
Cardiologists “really don’t do a good job most of the time” with severely calcified coronary lesions, added Dr. Jeremias, who wasn’t involved in the trial.
“A lot of times these patients have inadequate stent outcomes when we do intravascular imaging. So to do something to try to basically crack the calcium and expand the stent is, I think, critically important in these patients, and this is an amazing technology that accomplishes that,” the cardiologist said.
Juan F. Granada, MD, of Columbia University, New York, who moderated the press conference, said, “Some of the debulking techniques used for calcified stenoses actually require a lot of training, knowledge, experience, and hospital infrastructure.
I really think having a technology that is easy to use and familiar to all interventional cardiologists, such as a balloon, could potentially be a disruptive change in our field.”
“It’s an absolute game changer,” agreed Dr. Jeremias.
Dr. Kereiakes reported serving as a consultant to a handful of medical device companies, including Shockwave Medical, which sponsored Disrupt CAD III.
SOURCE: Kereiakes DJ. TCT 2020. Late Breaking Clinical Science session 2.
aimed at gaining U.S. regulatory approval.
The technology is basically the same as in extracorporeal lithotripsy, used for the treatment of kidney stones for more than 30 years: namely, transmission of pulsed acoustic pressure waves in order to fracture calcium. For interventional cardiology purposes, however, the transmitter is located within a balloon angioplasty catheter, Dean J. Kereiakes, MD, explained in presenting the study results at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
In Disrupt CAD III, intravascular lithotripsy far exceeded the procedural success and 30-day freedom from major adverse cardiovascular event (MACE) performance targets set in conjunction with the Food and Drug Administration. In so doing, the intravascular lithotripsy device developed by Shockwave Medical successfully addressed one of the banes of contemporary interventional cardiology: heavily calcified coronary lesions.
Currently available technologies targeting such lesions, including noncompliant high-pressure balloons, intravascular lasers, cutting balloons, and orbital and rotational atherectomy, often yield suboptimal results, noted Dr. Kereiakes, medical director of the Christ Hospital Heart and Cardiovascular Center in Cincinnati.
Severe vascular calcifications are becoming more common, due in part to an aging population and the growing prevalence of hypertension, diabetes, and renal insufficiency. Severely calcified coronary lesions complicate percutaneous coronary intervention. They’re associated with increased risks of dissection, perforation, and periprocedural MI. Moreover, heavily calcified lesions impede stent delivery and expansion – and stent underexpansion is the leading predictor of restenosis and stent thrombosis, he observed at the meeting, sponsored by the Cardiovascular Research Foundation. Disrupt CAD III was a prospective single-arm study of 384 patients at 47 sites in the United States and several European countries. All participants had de novo coronary calcifications graded as severe by core laboratory assessment, with a mean calcified length of 47.9 mm by quantitative coronary angiography and a mean calcium angle and thickness of 292.5 degrees and 0.96 mm by optical coherence tomography.
“It’s staggering, the level of calcification these patients had. It’s jaw dropping,” Dr. Kereiakes observed.
Intravascular lithotripsy was used to prepare these severely calcified lesions for stenting. The intervention entailed transmission of acoustic waves circumferentially and transmurally at 1 pulse per second through tissue at an effective pressure of about 50 atm. Patients received an average of 69 pulses.
This was not a randomized trial; there was no sham-treated control arm. Instead, the comparator group selected under regulatory guidance was comprised of patients who had received orbital atherectomy for severe coronary calcifications in the earlier, similarly designed ORBIT II trial, which led to FDA marketing approval of that technology.
Key outcomes
The procedural success rate, defined as successful stent delivery with less than a 50% residual stenosis and no in-hospital MACE, was 92.4% in Disrupt CAD III, compared to 83.4% for orbital atherectomy in ORBIT II. The primary safety endpoint of freedom from cardiac death, MI, or target vessel revascularization at 30 days was achieved in 92.2% of patients in the intravascular lithotripsy trial, versus 84.4% in ORBIT II.
The 30-day MACE rate of 7.8% in Disrupt CAD III was primarily driven by periprocedural MIs, which occurred in 6.8% of participants. Only one-third of the MIs were clinically relevant by the Society for Coronary Angiography and Intervention definition. There were two cardiac deaths and three cases of stent thrombosis, all of which were associated with known predictors of the complication. There was 1 case each of dissection, abrupt closure, and perforation, but no instances of slow flow or no reflow at the procedure’s end. Transient lithotripsy-induced left ventricular capture occurred in 41% of patients, but they were benign events with no lasting consequences.
The device was able to cross and deliver acoustic pressure wave therapy to 98.2% of lesions. The mean diameter stenosis preprocedure was 65.1%, dropping to 37.2% post lithotripsy, with a final in-stent residual stenosis diameter of 11.9%, with a 1.7-mm acute gain. The average stent expansion at the site of maximum calcification was 102%, with a minimum stent area of 6.5 mm2.
Optical coherence imaging revealed that 67% of treated lesions had circumferential and transmural fractures of both deep and superficial calcium post lithotripsy. Yet outcomes were the same regardless of whether fractures were evident on imaging.
At 30-day follow-up, 72.9% of patients had no angina, up from just 12.6% of participants pre-PCI. Follow-up will continue for 2 years.
Outcomes were similar for the first case done at each participating center and all cases thereafter.
“The ease of use was remarkable,” Dr. Kereiakes recalled. “The learning curve is virtually nonexistent.”
The reaction
At a press conference where Dr. Kereiakes presented the Disrupt CAD III results, discussant Allen Jeremias, MD, said he found the results compelling.
“The success rate is high, I think it’s relatively easy to use, as demonstrated, and I think the results are spectacular,” said Dr. Jeremias, director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
Cardiologists “really don’t do a good job most of the time” with severely calcified coronary lesions, added Dr. Jeremias, who wasn’t involved in the trial.
“A lot of times these patients have inadequate stent outcomes when we do intravascular imaging. So to do something to try to basically crack the calcium and expand the stent is, I think, critically important in these patients, and this is an amazing technology that accomplishes that,” the cardiologist said.
Juan F. Granada, MD, of Columbia University, New York, who moderated the press conference, said, “Some of the debulking techniques used for calcified stenoses actually require a lot of training, knowledge, experience, and hospital infrastructure.
I really think having a technology that is easy to use and familiar to all interventional cardiologists, such as a balloon, could potentially be a disruptive change in our field.”
“It’s an absolute game changer,” agreed Dr. Jeremias.
Dr. Kereiakes reported serving as a consultant to a handful of medical device companies, including Shockwave Medical, which sponsored Disrupt CAD III.
SOURCE: Kereiakes DJ. TCT 2020. Late Breaking Clinical Science session 2.
aimed at gaining U.S. regulatory approval.
The technology is basically the same as in extracorporeal lithotripsy, used for the treatment of kidney stones for more than 30 years: namely, transmission of pulsed acoustic pressure waves in order to fracture calcium. For interventional cardiology purposes, however, the transmitter is located within a balloon angioplasty catheter, Dean J. Kereiakes, MD, explained in presenting the study results at the Transcatheter Cardiovascular Research Therapeutics virtual annual meeting.
In Disrupt CAD III, intravascular lithotripsy far exceeded the procedural success and 30-day freedom from major adverse cardiovascular event (MACE) performance targets set in conjunction with the Food and Drug Administration. In so doing, the intravascular lithotripsy device developed by Shockwave Medical successfully addressed one of the banes of contemporary interventional cardiology: heavily calcified coronary lesions.
Currently available technologies targeting such lesions, including noncompliant high-pressure balloons, intravascular lasers, cutting balloons, and orbital and rotational atherectomy, often yield suboptimal results, noted Dr. Kereiakes, medical director of the Christ Hospital Heart and Cardiovascular Center in Cincinnati.
Severe vascular calcifications are becoming more common, due in part to an aging population and the growing prevalence of hypertension, diabetes, and renal insufficiency. Severely calcified coronary lesions complicate percutaneous coronary intervention. They’re associated with increased risks of dissection, perforation, and periprocedural MI. Moreover, heavily calcified lesions impede stent delivery and expansion – and stent underexpansion is the leading predictor of restenosis and stent thrombosis, he observed at the meeting, sponsored by the Cardiovascular Research Foundation. Disrupt CAD III was a prospective single-arm study of 384 patients at 47 sites in the United States and several European countries. All participants had de novo coronary calcifications graded as severe by core laboratory assessment, with a mean calcified length of 47.9 mm by quantitative coronary angiography and a mean calcium angle and thickness of 292.5 degrees and 0.96 mm by optical coherence tomography.
“It’s staggering, the level of calcification these patients had. It’s jaw dropping,” Dr. Kereiakes observed.
Intravascular lithotripsy was used to prepare these severely calcified lesions for stenting. The intervention entailed transmission of acoustic waves circumferentially and transmurally at 1 pulse per second through tissue at an effective pressure of about 50 atm. Patients received an average of 69 pulses.
This was not a randomized trial; there was no sham-treated control arm. Instead, the comparator group selected under regulatory guidance was comprised of patients who had received orbital atherectomy for severe coronary calcifications in the earlier, similarly designed ORBIT II trial, which led to FDA marketing approval of that technology.
Key outcomes
The procedural success rate, defined as successful stent delivery with less than a 50% residual stenosis and no in-hospital MACE, was 92.4% in Disrupt CAD III, compared to 83.4% for orbital atherectomy in ORBIT II. The primary safety endpoint of freedom from cardiac death, MI, or target vessel revascularization at 30 days was achieved in 92.2% of patients in the intravascular lithotripsy trial, versus 84.4% in ORBIT II.
The 30-day MACE rate of 7.8% in Disrupt CAD III was primarily driven by periprocedural MIs, which occurred in 6.8% of participants. Only one-third of the MIs were clinically relevant by the Society for Coronary Angiography and Intervention definition. There were two cardiac deaths and three cases of stent thrombosis, all of which were associated with known predictors of the complication. There was 1 case each of dissection, abrupt closure, and perforation, but no instances of slow flow or no reflow at the procedure’s end. Transient lithotripsy-induced left ventricular capture occurred in 41% of patients, but they were benign events with no lasting consequences.
The device was able to cross and deliver acoustic pressure wave therapy to 98.2% of lesions. The mean diameter stenosis preprocedure was 65.1%, dropping to 37.2% post lithotripsy, with a final in-stent residual stenosis diameter of 11.9%, with a 1.7-mm acute gain. The average stent expansion at the site of maximum calcification was 102%, with a minimum stent area of 6.5 mm2.
Optical coherence imaging revealed that 67% of treated lesions had circumferential and transmural fractures of both deep and superficial calcium post lithotripsy. Yet outcomes were the same regardless of whether fractures were evident on imaging.
At 30-day follow-up, 72.9% of patients had no angina, up from just 12.6% of participants pre-PCI. Follow-up will continue for 2 years.
Outcomes were similar for the first case done at each participating center and all cases thereafter.
“The ease of use was remarkable,” Dr. Kereiakes recalled. “The learning curve is virtually nonexistent.”
The reaction
At a press conference where Dr. Kereiakes presented the Disrupt CAD III results, discussant Allen Jeremias, MD, said he found the results compelling.
“The success rate is high, I think it’s relatively easy to use, as demonstrated, and I think the results are spectacular,” said Dr. Jeremias, director of interventional cardiology research and associate director of the cardiac catheterization laboratory at St. Francis Hospital in Roslyn, N.Y.
Cardiologists “really don’t do a good job most of the time” with severely calcified coronary lesions, added Dr. Jeremias, who wasn’t involved in the trial.
“A lot of times these patients have inadequate stent outcomes when we do intravascular imaging. So to do something to try to basically crack the calcium and expand the stent is, I think, critically important in these patients, and this is an amazing technology that accomplishes that,” the cardiologist said.
Juan F. Granada, MD, of Columbia University, New York, who moderated the press conference, said, “Some of the debulking techniques used for calcified stenoses actually require a lot of training, knowledge, experience, and hospital infrastructure.
I really think having a technology that is easy to use and familiar to all interventional cardiologists, such as a balloon, could potentially be a disruptive change in our field.”
“It’s an absolute game changer,” agreed Dr. Jeremias.
Dr. Kereiakes reported serving as a consultant to a handful of medical device companies, including Shockwave Medical, which sponsored Disrupt CAD III.
SOURCE: Kereiakes DJ. TCT 2020. Late Breaking Clinical Science session 2.
FROM TCT 2020
Key clinical point: Intravascular lithotripsy was safe and effective for treatment of severely calcified coronary stenoses in a pivotal trial.
Major finding: The 30-day rate of freedom from major adverse cardiovascular events was 92.2%, well above the prespecified performance goal of 84.4%.
Study details: Disrupt CAD III study is a multicenter, single-arm, prospective study of intravascular lithotripsy in 384 patients with severe coronary calcification.
Disclosures: The presenter reported serving as a consultant to Shockwave Medical Inc., the study sponsor, as well as several other medical device companies.
Source: Kereiakes DJ. TCT 2020. Late Breaking Clinical Science session 2.