User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


PHM20 Virtual: Common incidental findings seen on pediatric imaging
PHM20 session title
The Incidentaloma: Common Incidental Findings Seen on Pediatric Imaging
Presenters
Jill Azok, MD; Amanda Lansell, MD; Allayne Stephans, MD; and Erin Frank, MD
Session summary
Dr. Azok, Dr. Lansell, and Dr. Frank of University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, described one to three common, incidentally noted findings in central nervous system, thoracic, abdominopelvic, and musculoskeletal imaging. The presenters explained the indications for further work-up and/or intervention of these findings, and the importance of judicious use of imaging in pediatric patients.
Dr. Frank discussed incidental findings seen on imaging of the central nervous system, using cases to focus on benign enlargement of the subarachnoid space, lipomas of the filum terminale, and pituitary abnormalities. Dr. Lansell continued by discussing possible clinical models for management of incidentally found pulmonary nodules and renal cysts. Dr. Azok completed the session with a discussion of the appearance and management of nonossifying fibromas and cortical fibrous defects. Common threads shared by all presenters were how frequent incidental findings are and the need for providers to be comfortable with a level of uncertainty.
Key takeaways
- Incidental findings are very common in pediatric imaging, occurring on up to one-third of CT scans, 25% of brain MRIs, and 21% of knee radiographs.
- An infant with personal and family history of macrocephaly, normal development, and increased extra-axial CSF on MRI likely has benign enlargement of the arachnoid space and does not need further evaluation.
- A hyperintensity of filum terminale on MRI is consistent with lipoma of the filum terminale and does not require follow-up unless symptoms of tethered cord are present.
- Pituitary abnormalities are common and call for dedicated history, physical exam, and an endocrine screening with imaging surveillance if screening is normal.
- Patient history and appearance of pulmonary nodules are important in determining appropriate follow-up.
- No single feature of renal lesions predicts future behavior, but larger lesions deserve more work-up.
- Nonossifying fibromas are well-demarcated intracortical radiolucencies of long bone metaphyses that do not require treatment or further evaluation unless they are large, painful, or occur in the proximal femur.
Dr. Miller is a second-year pediatric hospital medicine fellow at Cleveland Clinic Children’s. His academic interests include medical education, quality improvement, and high value care.
PHM20 session title
The Incidentaloma: Common Incidental Findings Seen on Pediatric Imaging
Presenters
Jill Azok, MD; Amanda Lansell, MD; Allayne Stephans, MD; and Erin Frank, MD
Session summary
Dr. Azok, Dr. Lansell, and Dr. Frank of University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, described one to three common, incidentally noted findings in central nervous system, thoracic, abdominopelvic, and musculoskeletal imaging. The presenters explained the indications for further work-up and/or intervention of these findings, and the importance of judicious use of imaging in pediatric patients.
Dr. Frank discussed incidental findings seen on imaging of the central nervous system, using cases to focus on benign enlargement of the subarachnoid space, lipomas of the filum terminale, and pituitary abnormalities. Dr. Lansell continued by discussing possible clinical models for management of incidentally found pulmonary nodules and renal cysts. Dr. Azok completed the session with a discussion of the appearance and management of nonossifying fibromas and cortical fibrous defects. Common threads shared by all presenters were how frequent incidental findings are and the need for providers to be comfortable with a level of uncertainty.
Key takeaways
- Incidental findings are very common in pediatric imaging, occurring on up to one-third of CT scans, 25% of brain MRIs, and 21% of knee radiographs.
- An infant with personal and family history of macrocephaly, normal development, and increased extra-axial CSF on MRI likely has benign enlargement of the arachnoid space and does not need further evaluation.
- A hyperintensity of filum terminale on MRI is consistent with lipoma of the filum terminale and does not require follow-up unless symptoms of tethered cord are present.
- Pituitary abnormalities are common and call for dedicated history, physical exam, and an endocrine screening with imaging surveillance if screening is normal.
- Patient history and appearance of pulmonary nodules are important in determining appropriate follow-up.
- No single feature of renal lesions predicts future behavior, but larger lesions deserve more work-up.
- Nonossifying fibromas are well-demarcated intracortical radiolucencies of long bone metaphyses that do not require treatment or further evaluation unless they are large, painful, or occur in the proximal femur.
Dr. Miller is a second-year pediatric hospital medicine fellow at Cleveland Clinic Children’s. His academic interests include medical education, quality improvement, and high value care.
PHM20 session title
The Incidentaloma: Common Incidental Findings Seen on Pediatric Imaging
Presenters
Jill Azok, MD; Amanda Lansell, MD; Allayne Stephans, MD; and Erin Frank, MD
Session summary
Dr. Azok, Dr. Lansell, and Dr. Frank of University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, described one to three common, incidentally noted findings in central nervous system, thoracic, abdominopelvic, and musculoskeletal imaging. The presenters explained the indications for further work-up and/or intervention of these findings, and the importance of judicious use of imaging in pediatric patients.
Dr. Frank discussed incidental findings seen on imaging of the central nervous system, using cases to focus on benign enlargement of the subarachnoid space, lipomas of the filum terminale, and pituitary abnormalities. Dr. Lansell continued by discussing possible clinical models for management of incidentally found pulmonary nodules and renal cysts. Dr. Azok completed the session with a discussion of the appearance and management of nonossifying fibromas and cortical fibrous defects. Common threads shared by all presenters were how frequent incidental findings are and the need for providers to be comfortable with a level of uncertainty.
Key takeaways
- Incidental findings are very common in pediatric imaging, occurring on up to one-third of CT scans, 25% of brain MRIs, and 21% of knee radiographs.
- An infant with personal and family history of macrocephaly, normal development, and increased extra-axial CSF on MRI likely has benign enlargement of the arachnoid space and does not need further evaluation.
- A hyperintensity of filum terminale on MRI is consistent with lipoma of the filum terminale and does not require follow-up unless symptoms of tethered cord are present.
- Pituitary abnormalities are common and call for dedicated history, physical exam, and an endocrine screening with imaging surveillance if screening is normal.
- Patient history and appearance of pulmonary nodules are important in determining appropriate follow-up.
- No single feature of renal lesions predicts future behavior, but larger lesions deserve more work-up.
- Nonossifying fibromas are well-demarcated intracortical radiolucencies of long bone metaphyses that do not require treatment or further evaluation unless they are large, painful, or occur in the proximal femur.
Dr. Miller is a second-year pediatric hospital medicine fellow at Cleveland Clinic Children’s. His academic interests include medical education, quality improvement, and high value care.
A ‘foolproof’ way to diagnose narrow complex tachycardias on EKGs
A hospitalist looking at an EKG showing a narrow complex tachycardia needs to be able to come up with an accurate diagnosis of the rhythm pronto. And hospitalist Meghan Mary Walsh, MD, MPH, has developed a simple and efficient method for doing so within a minute or two that she’s used with great success on the wards and in teaching medical students and residents for nearly a decade.
she promised at HM20 Virtual, hosted by the Society of Hospital Medicine.
Her method involves asking three questions about the 12-lead EKG:
1) What’s the rate?
A narrow complex tachycardia by definition needs to be both narrow and fast, with a QRS complex of less than 0.12 seconds and a heart rate above 100 bpm. Knowing how far above 100 bpm the rate is will help with the differential diagnosis.
2) Is the rhythm regular or irregular?
“If I put the EKG 10 feet away from you, you should still be able to look at it and say the QRS is either systematically marching out – boom, boom, boom – or there is an irregular sea of QRS complexes where the RR intervals are variable and inconsistent,” said Dr. Walsh, a hospitalist at the University of Minnesota, Minneapolis, and chief academic officer at Hennepin Healthcare, where she oversees all medical students and residents training in the health system.
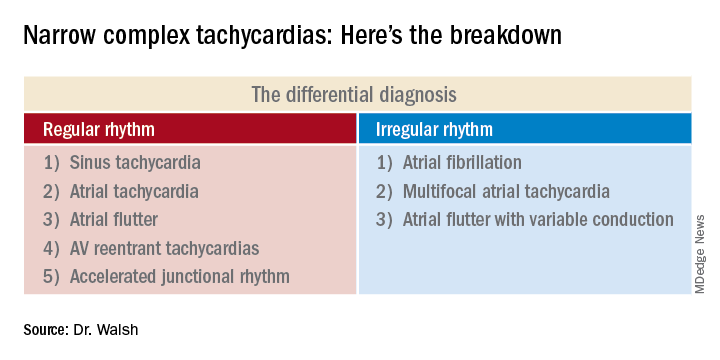
This distinction between a regular and irregular rhythm immediately narrows the differential by dividing the diagnostic possibilities into two columns (See chart). She urged her audience to commit the list to memory or keep it handy on their cell phone or in a notebook.
“If it’s irregular I’m going down the right column; if it’s regular I’m going down the left. And then I’m systematically running the drill,” she explained.
3) Are upright p waves present before each QRS complex in leads II and V1?
This information rules out some of the eight items in the differential diagnosis and rules in others.
Narrow complex tachycardias with an irregular rhythm
There are only three:
Atrial fibrillation: The heart rate is typically 110-160 bpm, although it can occasionally go higher. The rhythm is irregularly irregular: No two RR intervals on the EKG are exactly the same. And there are no p waves.
“If it’s faster than 100 bpm, irregularly irregular, and no p waves, the conclusion is very simple: It’s AFib,” Dr. Walsh said.
Multifocal atrial tachycardia (MAT): The heart rate is generally 100-150 bpm but can sometimes climb to about 180 bpm. The PP, PR, and RR intervals are varied, inconsistent, and don’t repeat. Most importantly, there are three or more different p wave morphologies in the same lead. One p wave might look like a tall mountain peak, another could be short and flat, and perhaps the next is big and broad.
MAT often occurs in patients with a structurally abnormal atrium – for example, in the setting of pulmonary hypertension leading to right atrial enlargement, with resultant depolarization occurring all over the atrium.
“Don’t confuse MAT with AFib: One has p waves, one does not. Otherwise they can look very similar,” she said.
Atrial flutter with variable conduction: A hallmark of this reentrant tachycardia is the atrial flutter waves occurring at about 300 bpm between each QRS complex.
“On board renewal exams, the question is often asked, ‘Which leads are the best identifiers of atrial flutter?’ And the answer is the inferior leads II, III, and aVF,” she said.
Another classic feature of atrial flutter with variable conduction is cluster beating attributable to a varied ventricular response. This results in a repeated pattern of irregular RR intervals: There might be a 2:1 block in AV conduction for several beats, then maybe a 4:1 block for several more, with resultant lengthening of the RR interval, then 3:1, with shortening of RR. This regularly irregular sequence is repeated throughout the EKG.
“Look for a pattern amidst the chaos,” the hospitalist advised.
The heart rate might be roughly 150 bpm with a 2:1 block, or 100 bpm with a 3:1 block. The p waves in atrial flutter with variable conduction can be either negatively or positively deflected.
Narrow complex tachycardias with a regular rhythm*
Sinus tachycardia: The heart rate is typically less than 160 bpm, the QRS complexes show a regular pattern, and upright p waves are clearly visible in leads II and V1.
The distinguishing feature of this arrhythmia is the ramping up and ramping down of the heart rate. The tachycardia is typically less than 160 bpm. But the rate doesn’t suddenly jump from, say, 70 to140 bpm in a flash while the patient is lying in the hospital bed. A trip to the telemetry room for a look at the telemetry strip will tell the tale: The heart rate will have progressively ramped up from 70, to 80, then 90, then 100, 110, 120, 130, to perhaps 140 bpm. And then it will similarly ramp back down in stages, with the up/down pattern being repeated.
Sinus tachycardia is generally a reflection of underlying significant systemic illness, such as sepsis, hypotension, or anemia.
Atrial tachycardia: The heart rate is generally 100-140 bpm, and p waves are present. But unlike in sinus tachycardia, the patient with atrial tachycardia lying in bed with a heart rate of 140 bpm is not in a state of profound neurohormonal activation and is not all that sick.
Another diagnostic clue is provided by a look at the telemonitoring strip. Unlike in sinus tachycardia, where the heart rate ramps up and then back down repeatedly, in atrial tachycardia the heart rate very quickly ramps up in stages to, say, 140 bpm, and then hangs there.
Atrial flutter: This is the only narrow complex tachycardia that appears in both the regular and irregular rhythm columns. It belongs in the irregular rhythm column when there is variable conduction and cluster beating, with a regularly irregular pattern of RR intervals. In contrast, when atrial flutter is in the regular rhythm column, it’s because the atrioventricular node is steadily conducting the atrial depolarizations at a rate of about 300 bpm. So there’s no cluster beating. As in atrial flutter with variable conduction, the flutter waves are visible most often in leads II, III, and aVF, where they can be either positively or negatively deflected.
AV reentrant tachycardias: These reentrant tachycardias can take two forms. In atrioventricular nodal reentrant tachycardia (AVnRT), the aberrant pathway is found entirely within the AV node, whereas in atrioventricular reentrant tachycardia (AVRT) the aberrant pathway is found outside the AV node. AVnRT is more common than AVRT. As in atrial flutter, there is no ramp up in heart rate. Patients will be lying in their hospital bed with a heart rate of, say, 80 bpm, and then suddenly it jumps to 180, 200, or even as high as 240 bpm “almost in a split second,” Dr. Walsh said.
No other narrow complex tachycardia reaches so high a heart rate. In both of these reentrant tachycardias the p waves are often buried in the QRS complex and can be tough to see. It’s very difficult to differentiate AVnRT from AVRT except by an electrophysiologic study.
Accelerated junctional tachycardia: This is most commonly the slowest of the narrow complex tachycardias, with a heart rate of less than 120 bpm.
“In the case of accelerated junctional tachycardia, think slow, think ‘regular,’ think of a rate often just over 100, usually with p waves after the QRS that are inverted because there’s retrograde conduction,” she advised.
She reported having no financial conflicts of interest regarding her presentation.
Correction, 8/19/20: An earlier version of this article mischaracterized the type of rhythm noted in this subhead.
A hospitalist looking at an EKG showing a narrow complex tachycardia needs to be able to come up with an accurate diagnosis of the rhythm pronto. And hospitalist Meghan Mary Walsh, MD, MPH, has developed a simple and efficient method for doing so within a minute or two that she’s used with great success on the wards and in teaching medical students and residents for nearly a decade.

she promised at HM20 Virtual, hosted by the Society of Hospital Medicine.
Her method involves asking three questions about the 12-lead EKG:
1) What’s the rate?
A narrow complex tachycardia by definition needs to be both narrow and fast, with a QRS complex of less than 0.12 seconds and a heart rate above 100 bpm. Knowing how far above 100 bpm the rate is will help with the differential diagnosis.
2) Is the rhythm regular or irregular?
“If I put the EKG 10 feet away from you, you should still be able to look at it and say the QRS is either systematically marching out – boom, boom, boom – or there is an irregular sea of QRS complexes where the RR intervals are variable and inconsistent,” said Dr. Walsh, a hospitalist at the University of Minnesota, Minneapolis, and chief academic officer at Hennepin Healthcare, where she oversees all medical students and residents training in the health system.
This distinction between a regular and irregular rhythm immediately narrows the differential by dividing the diagnostic possibilities into two columns (See chart). She urged her audience to commit the list to memory or keep it handy on their cell phone or in a notebook.
“If it’s irregular I’m going down the right column; if it’s regular I’m going down the left. And then I’m systematically running the drill,” she explained.
3) Are upright p waves present before each QRS complex in leads II and V1?
This information rules out some of the eight items in the differential diagnosis and rules in others.
Narrow complex tachycardias with an irregular rhythm
There are only three:
Atrial fibrillation: The heart rate is typically 110-160 bpm, although it can occasionally go higher. The rhythm is irregularly irregular: No two RR intervals on the EKG are exactly the same. And there are no p waves.
“If it’s faster than 100 bpm, irregularly irregular, and no p waves, the conclusion is very simple: It’s AFib,” Dr. Walsh said.
Multifocal atrial tachycardia (MAT): The heart rate is generally 100-150 bpm but can sometimes climb to about 180 bpm. The PP, PR, and RR intervals are varied, inconsistent, and don’t repeat. Most importantly, there are three or more different p wave morphologies in the same lead. One p wave might look like a tall mountain peak, another could be short and flat, and perhaps the next is big and broad.
MAT often occurs in patients with a structurally abnormal atrium – for example, in the setting of pulmonary hypertension leading to right atrial enlargement, with resultant depolarization occurring all over the atrium.
“Don’t confuse MAT with AFib: One has p waves, one does not. Otherwise they can look very similar,” she said.
Atrial flutter with variable conduction: A hallmark of this reentrant tachycardia is the atrial flutter waves occurring at about 300 bpm between each QRS complex.
“On board renewal exams, the question is often asked, ‘Which leads are the best identifiers of atrial flutter?’ And the answer is the inferior leads II, III, and aVF,” she said.
Another classic feature of atrial flutter with variable conduction is cluster beating attributable to a varied ventricular response. This results in a repeated pattern of irregular RR intervals: There might be a 2:1 block in AV conduction for several beats, then maybe a 4:1 block for several more, with resultant lengthening of the RR interval, then 3:1, with shortening of RR. This regularly irregular sequence is repeated throughout the EKG.
“Look for a pattern amidst the chaos,” the hospitalist advised.
The heart rate might be roughly 150 bpm with a 2:1 block, or 100 bpm with a 3:1 block. The p waves in atrial flutter with variable conduction can be either negatively or positively deflected.
Narrow complex tachycardias with a regular rhythm*
Sinus tachycardia: The heart rate is typically less than 160 bpm, the QRS complexes show a regular pattern, and upright p waves are clearly visible in leads II and V1.
The distinguishing feature of this arrhythmia is the ramping up and ramping down of the heart rate. The tachycardia is typically less than 160 bpm. But the rate doesn’t suddenly jump from, say, 70 to140 bpm in a flash while the patient is lying in the hospital bed. A trip to the telemetry room for a look at the telemetry strip will tell the tale: The heart rate will have progressively ramped up from 70, to 80, then 90, then 100, 110, 120, 130, to perhaps 140 bpm. And then it will similarly ramp back down in stages, with the up/down pattern being repeated.
Sinus tachycardia is generally a reflection of underlying significant systemic illness, such as sepsis, hypotension, or anemia.
Atrial tachycardia: The heart rate is generally 100-140 bpm, and p waves are present. But unlike in sinus tachycardia, the patient with atrial tachycardia lying in bed with a heart rate of 140 bpm is not in a state of profound neurohormonal activation and is not all that sick.
Another diagnostic clue is provided by a look at the telemonitoring strip. Unlike in sinus tachycardia, where the heart rate ramps up and then back down repeatedly, in atrial tachycardia the heart rate very quickly ramps up in stages to, say, 140 bpm, and then hangs there.
Atrial flutter: This is the only narrow complex tachycardia that appears in both the regular and irregular rhythm columns. It belongs in the irregular rhythm column when there is variable conduction and cluster beating, with a regularly irregular pattern of RR intervals. In contrast, when atrial flutter is in the regular rhythm column, it’s because the atrioventricular node is steadily conducting the atrial depolarizations at a rate of about 300 bpm. So there’s no cluster beating. As in atrial flutter with variable conduction, the flutter waves are visible most often in leads II, III, and aVF, where they can be either positively or negatively deflected.
AV reentrant tachycardias: These reentrant tachycardias can take two forms. In atrioventricular nodal reentrant tachycardia (AVnRT), the aberrant pathway is found entirely within the AV node, whereas in atrioventricular reentrant tachycardia (AVRT) the aberrant pathway is found outside the AV node. AVnRT is more common than AVRT. As in atrial flutter, there is no ramp up in heart rate. Patients will be lying in their hospital bed with a heart rate of, say, 80 bpm, and then suddenly it jumps to 180, 200, or even as high as 240 bpm “almost in a split second,” Dr. Walsh said.
No other narrow complex tachycardia reaches so high a heart rate. In both of these reentrant tachycardias the p waves are often buried in the QRS complex and can be tough to see. It’s very difficult to differentiate AVnRT from AVRT except by an electrophysiologic study.
Accelerated junctional tachycardia: This is most commonly the slowest of the narrow complex tachycardias, with a heart rate of less than 120 bpm.
“In the case of accelerated junctional tachycardia, think slow, think ‘regular,’ think of a rate often just over 100, usually with p waves after the QRS that are inverted because there’s retrograde conduction,” she advised.
She reported having no financial conflicts of interest regarding her presentation.
Correction, 8/19/20: An earlier version of this article mischaracterized the type of rhythm noted in this subhead.
A hospitalist looking at an EKG showing a narrow complex tachycardia needs to be able to come up with an accurate diagnosis of the rhythm pronto. And hospitalist Meghan Mary Walsh, MD, MPH, has developed a simple and efficient method for doing so within a minute or two that she’s used with great success on the wards and in teaching medical students and residents for nearly a decade.

she promised at HM20 Virtual, hosted by the Society of Hospital Medicine.
Her method involves asking three questions about the 12-lead EKG:
1) What’s the rate?
A narrow complex tachycardia by definition needs to be both narrow and fast, with a QRS complex of less than 0.12 seconds and a heart rate above 100 bpm. Knowing how far above 100 bpm the rate is will help with the differential diagnosis.
2) Is the rhythm regular or irregular?
“If I put the EKG 10 feet away from you, you should still be able to look at it and say the QRS is either systematically marching out – boom, boom, boom – or there is an irregular sea of QRS complexes where the RR intervals are variable and inconsistent,” said Dr. Walsh, a hospitalist at the University of Minnesota, Minneapolis, and chief academic officer at Hennepin Healthcare, where she oversees all medical students and residents training in the health system.
This distinction between a regular and irregular rhythm immediately narrows the differential by dividing the diagnostic possibilities into two columns (See chart). She urged her audience to commit the list to memory or keep it handy on their cell phone or in a notebook.
“If it’s irregular I’m going down the right column; if it’s regular I’m going down the left. And then I’m systematically running the drill,” she explained.
3) Are upright p waves present before each QRS complex in leads II and V1?
This information rules out some of the eight items in the differential diagnosis and rules in others.
Narrow complex tachycardias with an irregular rhythm
There are only three:
Atrial fibrillation: The heart rate is typically 110-160 bpm, although it can occasionally go higher. The rhythm is irregularly irregular: No two RR intervals on the EKG are exactly the same. And there are no p waves.
“If it’s faster than 100 bpm, irregularly irregular, and no p waves, the conclusion is very simple: It’s AFib,” Dr. Walsh said.
Multifocal atrial tachycardia (MAT): The heart rate is generally 100-150 bpm but can sometimes climb to about 180 bpm. The PP, PR, and RR intervals are varied, inconsistent, and don’t repeat. Most importantly, there are three or more different p wave morphologies in the same lead. One p wave might look like a tall mountain peak, another could be short and flat, and perhaps the next is big and broad.
MAT often occurs in patients with a structurally abnormal atrium – for example, in the setting of pulmonary hypertension leading to right atrial enlargement, with resultant depolarization occurring all over the atrium.
“Don’t confuse MAT with AFib: One has p waves, one does not. Otherwise they can look very similar,” she said.
Atrial flutter with variable conduction: A hallmark of this reentrant tachycardia is the atrial flutter waves occurring at about 300 bpm between each QRS complex.
“On board renewal exams, the question is often asked, ‘Which leads are the best identifiers of atrial flutter?’ And the answer is the inferior leads II, III, and aVF,” she said.
Another classic feature of atrial flutter with variable conduction is cluster beating attributable to a varied ventricular response. This results in a repeated pattern of irregular RR intervals: There might be a 2:1 block in AV conduction for several beats, then maybe a 4:1 block for several more, with resultant lengthening of the RR interval, then 3:1, with shortening of RR. This regularly irregular sequence is repeated throughout the EKG.
“Look for a pattern amidst the chaos,” the hospitalist advised.
The heart rate might be roughly 150 bpm with a 2:1 block, or 100 bpm with a 3:1 block. The p waves in atrial flutter with variable conduction can be either negatively or positively deflected.
Narrow complex tachycardias with a regular rhythm*
Sinus tachycardia: The heart rate is typically less than 160 bpm, the QRS complexes show a regular pattern, and upright p waves are clearly visible in leads II and V1.
The distinguishing feature of this arrhythmia is the ramping up and ramping down of the heart rate. The tachycardia is typically less than 160 bpm. But the rate doesn’t suddenly jump from, say, 70 to140 bpm in a flash while the patient is lying in the hospital bed. A trip to the telemetry room for a look at the telemetry strip will tell the tale: The heart rate will have progressively ramped up from 70, to 80, then 90, then 100, 110, 120, 130, to perhaps 140 bpm. And then it will similarly ramp back down in stages, with the up/down pattern being repeated.
Sinus tachycardia is generally a reflection of underlying significant systemic illness, such as sepsis, hypotension, or anemia.
Atrial tachycardia: The heart rate is generally 100-140 bpm, and p waves are present. But unlike in sinus tachycardia, the patient with atrial tachycardia lying in bed with a heart rate of 140 bpm is not in a state of profound neurohormonal activation and is not all that sick.
Another diagnostic clue is provided by a look at the telemonitoring strip. Unlike in sinus tachycardia, where the heart rate ramps up and then back down repeatedly, in atrial tachycardia the heart rate very quickly ramps up in stages to, say, 140 bpm, and then hangs there.
Atrial flutter: This is the only narrow complex tachycardia that appears in both the regular and irregular rhythm columns. It belongs in the irregular rhythm column when there is variable conduction and cluster beating, with a regularly irregular pattern of RR intervals. In contrast, when atrial flutter is in the regular rhythm column, it’s because the atrioventricular node is steadily conducting the atrial depolarizations at a rate of about 300 bpm. So there’s no cluster beating. As in atrial flutter with variable conduction, the flutter waves are visible most often in leads II, III, and aVF, where they can be either positively or negatively deflected.
AV reentrant tachycardias: These reentrant tachycardias can take two forms. In atrioventricular nodal reentrant tachycardia (AVnRT), the aberrant pathway is found entirely within the AV node, whereas in atrioventricular reentrant tachycardia (AVRT) the aberrant pathway is found outside the AV node. AVnRT is more common than AVRT. As in atrial flutter, there is no ramp up in heart rate. Patients will be lying in their hospital bed with a heart rate of, say, 80 bpm, and then suddenly it jumps to 180, 200, or even as high as 240 bpm “almost in a split second,” Dr. Walsh said.
No other narrow complex tachycardia reaches so high a heart rate. In both of these reentrant tachycardias the p waves are often buried in the QRS complex and can be tough to see. It’s very difficult to differentiate AVnRT from AVRT except by an electrophysiologic study.
Accelerated junctional tachycardia: This is most commonly the slowest of the narrow complex tachycardias, with a heart rate of less than 120 bpm.
“In the case of accelerated junctional tachycardia, think slow, think ‘regular,’ think of a rate often just over 100, usually with p waves after the QRS that are inverted because there’s retrograde conduction,” she advised.
She reported having no financial conflicts of interest regarding her presentation.
Correction, 8/19/20: An earlier version of this article mischaracterized the type of rhythm noted in this subhead.
FROM HM20 VIRTUAL
Determining cause of skin lesions in COVID-19 patients remains challenging
published in the Journal of the American Academy of Dermatology.
SARS-CoV-2 infection has been associated with a range of skin conditions, wrote Antonio Martinez-Lopez, MD, of Virgen de las Nieves University Hospital, Granada, Spain, and colleagues, who provided an overview of the cutaneous side effects associated with drugs used to treat COVID-19 infection.
“Cutaneous manifestations have recently been described in patients with the new coronavirus infection, similar to cutaneous involvement occurring in common viral infections,” they said. Infected individuals have experienced maculopapular eruption, pseudo-chilblain lesions, urticaria, monomorphic disseminated vesicular lesions, acral vesicular-pustulous lesions, and livedo or necrosis, they noted.
Diagnosing skin manifestations in patients with COVID-19 remains a challenge, because it is unclear whether the skin lesions are related to the virus, the authors said. “Skin diseases not related to coronavirus, other seasonal viral infections, and drug reactions should be considered in the differential diagnosis, especially in those patients suffering from nonspecific manifestations such as urticaria or maculopapular eruptions,” they wrote.
However, “urticarial lesions and maculopapular eruptions in SARS-CoV-2 infections usually appear at the same time as the systemic symptoms, while drug adverse reactions are likely to arise hours to days after the start of the treatment,” they said.
The reviewers noted several cutaneous side effects associated with several of the often-prescribed drugs for COVID-19 infection. The antimalarials hydroxychloroquine and chloroquine had been authorized for COVID-19 treatment by the Food and Drug Administration, but this emergency authorization was rescinded in June. They noted that up to 11.5% of patients on these drugs may experience cutaneous adverse effects, including some that “can be mistaken for skin manifestations of SARS-CoV-2, especially those with maculopapular rash or exanthematous reactions.” Another side effect is exacerbation of psoriasis, which has been described in patients with COVID-19, the authors said.
The oral antiretroviral combination lopinavir/ritonavir, under investigation in clinical trials for COVID-19, has been associated with skin rashes in as many as 5% of adults in HIV studies. Usually appearing after treatment is started, the maculopapular pruritic rash is “usually well tolerated,” they said, although there have been reports of Stevens-Johnson syndrome. Alopecia areata is among the other side effects reported.
Remdesivir also has been authorized for emergency treatment of COVID-19, and the small amount of data available suggest that cutaneous manifestations may be infrequent, the reviewers said. In a recent study of 53 patients treated with remdesivir for 10 days, approximately 8% developed a rash, but the study did not include any information “about rash morphology, distribution, or timeline in relation to remdesivir that may help clinicians differentiate from cutaneous manifestations of COVID-19,” they said.
Other potential treatments for complications of COVID-19 include imatinib, tocilizumab, anakinra, immunoglobulins, corticosteroids, colchicine, and low molecular weight heparins; all have the potential for association with skin reactions, but data on skin manifestations associated with COVID-19 are limited, the authors wrote.
Notably, data on the use of systemic corticosteroids for COVID-19 patients are controversial, although preliminary data showed some reduced mortality in COVID-19 patients who were on respiratory support, they noted. “With regard to differential diagnosis of cutaneous manifestations of COVID-19, the vascular fragility associated with corticosteroid use, especially in elderly patients, may be similar to the thrombotic complications of COVID-19 infection.”
Knowledge about the virology of COVID-19 continues to evolve rapidly, and the number of drugs being studied as treatments continues to expand, the authors pointed out.
“By considering adverse drug reactions in the differential diagnosis, dermatologists can be useful in assisting in the care of these patients,” they wrote. Drugs, rather than the infection, may be the cause of skin reactions in some COVID-19 patients, and “management is often symptomatic, but it is sometimes necessary to modify or discontinue the treatment, and some conditions can even be life-threatening,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Martinez-Lopez A et al. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.08.006.
published in the Journal of the American Academy of Dermatology.
SARS-CoV-2 infection has been associated with a range of skin conditions, wrote Antonio Martinez-Lopez, MD, of Virgen de las Nieves University Hospital, Granada, Spain, and colleagues, who provided an overview of the cutaneous side effects associated with drugs used to treat COVID-19 infection.
“Cutaneous manifestations have recently been described in patients with the new coronavirus infection, similar to cutaneous involvement occurring in common viral infections,” they said. Infected individuals have experienced maculopapular eruption, pseudo-chilblain lesions, urticaria, monomorphic disseminated vesicular lesions, acral vesicular-pustulous lesions, and livedo or necrosis, they noted.
Diagnosing skin manifestations in patients with COVID-19 remains a challenge, because it is unclear whether the skin lesions are related to the virus, the authors said. “Skin diseases not related to coronavirus, other seasonal viral infections, and drug reactions should be considered in the differential diagnosis, especially in those patients suffering from nonspecific manifestations such as urticaria or maculopapular eruptions,” they wrote.
However, “urticarial lesions and maculopapular eruptions in SARS-CoV-2 infections usually appear at the same time as the systemic symptoms, while drug adverse reactions are likely to arise hours to days after the start of the treatment,” they said.
The reviewers noted several cutaneous side effects associated with several of the often-prescribed drugs for COVID-19 infection. The antimalarials hydroxychloroquine and chloroquine had been authorized for COVID-19 treatment by the Food and Drug Administration, but this emergency authorization was rescinded in June. They noted that up to 11.5% of patients on these drugs may experience cutaneous adverse effects, including some that “can be mistaken for skin manifestations of SARS-CoV-2, especially those with maculopapular rash or exanthematous reactions.” Another side effect is exacerbation of psoriasis, which has been described in patients with COVID-19, the authors said.
The oral antiretroviral combination lopinavir/ritonavir, under investigation in clinical trials for COVID-19, has been associated with skin rashes in as many as 5% of adults in HIV studies. Usually appearing after treatment is started, the maculopapular pruritic rash is “usually well tolerated,” they said, although there have been reports of Stevens-Johnson syndrome. Alopecia areata is among the other side effects reported.
Remdesivir also has been authorized for emergency treatment of COVID-19, and the small amount of data available suggest that cutaneous manifestations may be infrequent, the reviewers said. In a recent study of 53 patients treated with remdesivir for 10 days, approximately 8% developed a rash, but the study did not include any information “about rash morphology, distribution, or timeline in relation to remdesivir that may help clinicians differentiate from cutaneous manifestations of COVID-19,” they said.
Other potential treatments for complications of COVID-19 include imatinib, tocilizumab, anakinra, immunoglobulins, corticosteroids, colchicine, and low molecular weight heparins; all have the potential for association with skin reactions, but data on skin manifestations associated with COVID-19 are limited, the authors wrote.
Notably, data on the use of systemic corticosteroids for COVID-19 patients are controversial, although preliminary data showed some reduced mortality in COVID-19 patients who were on respiratory support, they noted. “With regard to differential diagnosis of cutaneous manifestations of COVID-19, the vascular fragility associated with corticosteroid use, especially in elderly patients, may be similar to the thrombotic complications of COVID-19 infection.”
Knowledge about the virology of COVID-19 continues to evolve rapidly, and the number of drugs being studied as treatments continues to expand, the authors pointed out.
“By considering adverse drug reactions in the differential diagnosis, dermatologists can be useful in assisting in the care of these patients,” they wrote. Drugs, rather than the infection, may be the cause of skin reactions in some COVID-19 patients, and “management is often symptomatic, but it is sometimes necessary to modify or discontinue the treatment, and some conditions can even be life-threatening,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Martinez-Lopez A et al. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.08.006.
published in the Journal of the American Academy of Dermatology.
SARS-CoV-2 infection has been associated with a range of skin conditions, wrote Antonio Martinez-Lopez, MD, of Virgen de las Nieves University Hospital, Granada, Spain, and colleagues, who provided an overview of the cutaneous side effects associated with drugs used to treat COVID-19 infection.
“Cutaneous manifestations have recently been described in patients with the new coronavirus infection, similar to cutaneous involvement occurring in common viral infections,” they said. Infected individuals have experienced maculopapular eruption, pseudo-chilblain lesions, urticaria, monomorphic disseminated vesicular lesions, acral vesicular-pustulous lesions, and livedo or necrosis, they noted.
Diagnosing skin manifestations in patients with COVID-19 remains a challenge, because it is unclear whether the skin lesions are related to the virus, the authors said. “Skin diseases not related to coronavirus, other seasonal viral infections, and drug reactions should be considered in the differential diagnosis, especially in those patients suffering from nonspecific manifestations such as urticaria or maculopapular eruptions,” they wrote.
However, “urticarial lesions and maculopapular eruptions in SARS-CoV-2 infections usually appear at the same time as the systemic symptoms, while drug adverse reactions are likely to arise hours to days after the start of the treatment,” they said.
The reviewers noted several cutaneous side effects associated with several of the often-prescribed drugs for COVID-19 infection. The antimalarials hydroxychloroquine and chloroquine had been authorized for COVID-19 treatment by the Food and Drug Administration, but this emergency authorization was rescinded in June. They noted that up to 11.5% of patients on these drugs may experience cutaneous adverse effects, including some that “can be mistaken for skin manifestations of SARS-CoV-2, especially those with maculopapular rash or exanthematous reactions.” Another side effect is exacerbation of psoriasis, which has been described in patients with COVID-19, the authors said.
The oral antiretroviral combination lopinavir/ritonavir, under investigation in clinical trials for COVID-19, has been associated with skin rashes in as many as 5% of adults in HIV studies. Usually appearing after treatment is started, the maculopapular pruritic rash is “usually well tolerated,” they said, although there have been reports of Stevens-Johnson syndrome. Alopecia areata is among the other side effects reported.
Remdesivir also has been authorized for emergency treatment of COVID-19, and the small amount of data available suggest that cutaneous manifestations may be infrequent, the reviewers said. In a recent study of 53 patients treated with remdesivir for 10 days, approximately 8% developed a rash, but the study did not include any information “about rash morphology, distribution, or timeline in relation to remdesivir that may help clinicians differentiate from cutaneous manifestations of COVID-19,” they said.
Other potential treatments for complications of COVID-19 include imatinib, tocilizumab, anakinra, immunoglobulins, corticosteroids, colchicine, and low molecular weight heparins; all have the potential for association with skin reactions, but data on skin manifestations associated with COVID-19 are limited, the authors wrote.
Notably, data on the use of systemic corticosteroids for COVID-19 patients are controversial, although preliminary data showed some reduced mortality in COVID-19 patients who were on respiratory support, they noted. “With regard to differential diagnosis of cutaneous manifestations of COVID-19, the vascular fragility associated with corticosteroid use, especially in elderly patients, may be similar to the thrombotic complications of COVID-19 infection.”
Knowledge about the virology of COVID-19 continues to evolve rapidly, and the number of drugs being studied as treatments continues to expand, the authors pointed out.
“By considering adverse drug reactions in the differential diagnosis, dermatologists can be useful in assisting in the care of these patients,” they wrote. Drugs, rather than the infection, may be the cause of skin reactions in some COVID-19 patients, and “management is often symptomatic, but it is sometimes necessary to modify or discontinue the treatment, and some conditions can even be life-threatening,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Martinez-Lopez A et al. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.08.006.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Since COVID-19 onset, admissions for MI are down, mortality rates are up
A substantial decrease in hospital admissions for acute MI was accompanied by a rise in mortality, particularly for ST-segment elevation MI (STEMI), following the onset of the COVID-19 pandemic, according to a cross-sectional retrospective study.
Although it can’t be confirmed from these results that the observed increase in in-hospital acute MI (AMI) mortality are related to delays in seeking treatment, this is a reasonable working hypothesis until more is known, commented Harlan Krumholz, MD, who was not involved in the study.
The analysis, derived from data collected at 49 centers in a hospital system spread across six states, supports previous reports that patients with AMI were avoiding hospitalization, according to the investigators, who were led by Tyler J. Gluckman, MD, medical director of the Center for Cardiovascular Analytics, Providence Heart Institute, Portland, Ore.
When compared with a nearly 14-month period that preceded the COVID-19 pandemic, the rate of AMI-associated hospitalization fell by 19 cases per week (95% confidence interval, –29.0 to –9.0 cases) in the early COVID-19 period, which was defined by the investigators as spanning from Feb. 23, 2020 to March 28, 2020.
The case rate per week then increased by 10.5 (95% CI, 4.6-16.5 cases) in a subsequent 8-week period spanning between March 29, 2020, and May 16, 2020. Although a substantial increase from the early COVID-19 period, the case rate remained below the baseline established before COVID-19.
The analysis looked at 15,244 AMI hospitalizations among 14,724 patients treated in the Providence St. Joseph Hospital System, which has facilities in Alaska, California, Montana, Oregon, Texas, and Washington. The 1,915 AMI cases captured from Feb. 23, 2020, represented 13% of the total.
Differences in mortality, patients, treatment
In the early period, the ratio of observed-to-expected (O/E) mortality relative to the pre–COVID-19 baseline increased by 27% (odds ratio, 1.27; 95% CI, 1.07-1.48). When STEMI was analyzed separately, the O/E mortality was nearly double that of the baseline period (OR, 1.96; 95% CI, 1.22-2.70). In the latter post–COVID-19 period of observation, the overall increase in AMI-associated mortality on the basis of an O/E ratio was no longer significant relative to the baseline period (OR, 1.23; 95% CI, 0.98-1.47). However, the relative increase in STEMI-associated mortality on an O/E basis was even greater (OR, 2.40; 95% CI, 1.65-3.16) in the second COVID-19 period analyzed. Even after risk adjustment, the OR for STEMI mortality remained significantly elevated relative to baseline (1.52; 95% CI, 1.02-2.26).
The differences in AMI patients treated before the onset of the COVID-19 pandemic and those treated afterwards might be relevant, according to the investigators. Specifically, patients hospitalized after Feb. 23, 2020 were 1-3 years younger (P < .001) depending on type of AMI, and more likely to be Asian (P = .01).
The length of stay was 6 hours shorter in the early COVID-19 period and 7 hours shorter in the latter period relative to baseline, but an analysis of treatment approaches to non-STEMI and STEMI during the COVID-19 pandemic were not found to be significantly different from baseline.
Prior to the COVID-19 pandemic, 79% of STEMI patients and 77% of non-STEMI patients were discharged home, which was significantly lower than in the early COVID-19 period, when 83% (P = .02) of STEMI and 81% (P = .006) of non-STEMI patients were discharged home. In the latter period, discharge to home care was also significantly higher than in the baseline period.
More than fear of COVID-19?
One theory to account for the reduction in AMI hospitalizations and the increase in AMI-related mortality is the possibility that patients were slow to seek care at acute care hospitals because of concern about COVID-19 infection, according to Dr. Gluckman and coinvestigators.
“Given the time-sensitive nature of STEMI, any delay by patients, emergency medical services, the emergency department, or cardiac catheterization laboratory may have played a role,” they suggested.
In an interview, Dr. Gluckman said that further effort to identify the reasons for the increased AMI-related mortality is planned. Pulling data from the electronic medical records of the patients included in this retrospective analysis might be a “challenge,” but Dr. Gluckman reported that he and his coinvestigators plan to look at a different set of registry data that might provide information on sources of delay, particularly in the STEMI population.
“This includes looking at a number of time factors, such as symptom onset to first medical contact, first medical contact to device, and door-in-door-out times,” Dr. Gluckman said. The goal is to “better understand if delays [in treatment] occurred during the pandemic and, if so, how they may have contributed to increases in risk adjusted mortality.”
Dr. Krumholz, director of the Yale Center for Outcomes Research and Evaluation, New Haven, Conn., called this study a “useful” confirmation of changes in AMI-related care with the onset of the COVID-19 pandemic. As reported anecdotally, the study “indicates marked decreases in hospitalizations of patients with AMI even in areas that were not experiencing big outbreaks but did have some restrictions to limit spread,” he noted.
More data gathered by other centers might provide information about what it all means.
“There remain so many questions about what happened and what consequences accrued,” Dr. Krumholz observed. “In the meantime, we need to continue to send the message that people with symptoms that suggest a heart attack need to rapidly seek care.”
The investigators reported having no financial conflicts of interest.
SOURCE: Gluckman TJ et al. JAMA Cardiol. 2020 Aug 7. doi: 10.1001/jamacardio.2020.3629.
A substantial decrease in hospital admissions for acute MI was accompanied by a rise in mortality, particularly for ST-segment elevation MI (STEMI), following the onset of the COVID-19 pandemic, according to a cross-sectional retrospective study.
Although it can’t be confirmed from these results that the observed increase in in-hospital acute MI (AMI) mortality are related to delays in seeking treatment, this is a reasonable working hypothesis until more is known, commented Harlan Krumholz, MD, who was not involved in the study.
The analysis, derived from data collected at 49 centers in a hospital system spread across six states, supports previous reports that patients with AMI were avoiding hospitalization, according to the investigators, who were led by Tyler J. Gluckman, MD, medical director of the Center for Cardiovascular Analytics, Providence Heart Institute, Portland, Ore.
When compared with a nearly 14-month period that preceded the COVID-19 pandemic, the rate of AMI-associated hospitalization fell by 19 cases per week (95% confidence interval, –29.0 to –9.0 cases) in the early COVID-19 period, which was defined by the investigators as spanning from Feb. 23, 2020 to March 28, 2020.
The case rate per week then increased by 10.5 (95% CI, 4.6-16.5 cases) in a subsequent 8-week period spanning between March 29, 2020, and May 16, 2020. Although a substantial increase from the early COVID-19 period, the case rate remained below the baseline established before COVID-19.
The analysis looked at 15,244 AMI hospitalizations among 14,724 patients treated in the Providence St. Joseph Hospital System, which has facilities in Alaska, California, Montana, Oregon, Texas, and Washington. The 1,915 AMI cases captured from Feb. 23, 2020, represented 13% of the total.
Differences in mortality, patients, treatment
In the early period, the ratio of observed-to-expected (O/E) mortality relative to the pre–COVID-19 baseline increased by 27% (odds ratio, 1.27; 95% CI, 1.07-1.48). When STEMI was analyzed separately, the O/E mortality was nearly double that of the baseline period (OR, 1.96; 95% CI, 1.22-2.70). In the latter post–COVID-19 period of observation, the overall increase in AMI-associated mortality on the basis of an O/E ratio was no longer significant relative to the baseline period (OR, 1.23; 95% CI, 0.98-1.47). However, the relative increase in STEMI-associated mortality on an O/E basis was even greater (OR, 2.40; 95% CI, 1.65-3.16) in the second COVID-19 period analyzed. Even after risk adjustment, the OR for STEMI mortality remained significantly elevated relative to baseline (1.52; 95% CI, 1.02-2.26).
The differences in AMI patients treated before the onset of the COVID-19 pandemic and those treated afterwards might be relevant, according to the investigators. Specifically, patients hospitalized after Feb. 23, 2020 were 1-3 years younger (P < .001) depending on type of AMI, and more likely to be Asian (P = .01).
The length of stay was 6 hours shorter in the early COVID-19 period and 7 hours shorter in the latter period relative to baseline, but an analysis of treatment approaches to non-STEMI and STEMI during the COVID-19 pandemic were not found to be significantly different from baseline.
Prior to the COVID-19 pandemic, 79% of STEMI patients and 77% of non-STEMI patients were discharged home, which was significantly lower than in the early COVID-19 period, when 83% (P = .02) of STEMI and 81% (P = .006) of non-STEMI patients were discharged home. In the latter period, discharge to home care was also significantly higher than in the baseline period.
More than fear of COVID-19?
One theory to account for the reduction in AMI hospitalizations and the increase in AMI-related mortality is the possibility that patients were slow to seek care at acute care hospitals because of concern about COVID-19 infection, according to Dr. Gluckman and coinvestigators.
“Given the time-sensitive nature of STEMI, any delay by patients, emergency medical services, the emergency department, or cardiac catheterization laboratory may have played a role,” they suggested.
In an interview, Dr. Gluckman said that further effort to identify the reasons for the increased AMI-related mortality is planned. Pulling data from the electronic medical records of the patients included in this retrospective analysis might be a “challenge,” but Dr. Gluckman reported that he and his coinvestigators plan to look at a different set of registry data that might provide information on sources of delay, particularly in the STEMI population.
“This includes looking at a number of time factors, such as symptom onset to first medical contact, first medical contact to device, and door-in-door-out times,” Dr. Gluckman said. The goal is to “better understand if delays [in treatment] occurred during the pandemic and, if so, how they may have contributed to increases in risk adjusted mortality.”
Dr. Krumholz, director of the Yale Center for Outcomes Research and Evaluation, New Haven, Conn., called this study a “useful” confirmation of changes in AMI-related care with the onset of the COVID-19 pandemic. As reported anecdotally, the study “indicates marked decreases in hospitalizations of patients with AMI even in areas that were not experiencing big outbreaks but did have some restrictions to limit spread,” he noted.
More data gathered by other centers might provide information about what it all means.
“There remain so many questions about what happened and what consequences accrued,” Dr. Krumholz observed. “In the meantime, we need to continue to send the message that people with symptoms that suggest a heart attack need to rapidly seek care.”
The investigators reported having no financial conflicts of interest.
SOURCE: Gluckman TJ et al. JAMA Cardiol. 2020 Aug 7. doi: 10.1001/jamacardio.2020.3629.
A substantial decrease in hospital admissions for acute MI was accompanied by a rise in mortality, particularly for ST-segment elevation MI (STEMI), following the onset of the COVID-19 pandemic, according to a cross-sectional retrospective study.
Although it can’t be confirmed from these results that the observed increase in in-hospital acute MI (AMI) mortality are related to delays in seeking treatment, this is a reasonable working hypothesis until more is known, commented Harlan Krumholz, MD, who was not involved in the study.
The analysis, derived from data collected at 49 centers in a hospital system spread across six states, supports previous reports that patients with AMI were avoiding hospitalization, according to the investigators, who were led by Tyler J. Gluckman, MD, medical director of the Center for Cardiovascular Analytics, Providence Heart Institute, Portland, Ore.
When compared with a nearly 14-month period that preceded the COVID-19 pandemic, the rate of AMI-associated hospitalization fell by 19 cases per week (95% confidence interval, –29.0 to –9.0 cases) in the early COVID-19 period, which was defined by the investigators as spanning from Feb. 23, 2020 to March 28, 2020.
The case rate per week then increased by 10.5 (95% CI, 4.6-16.5 cases) in a subsequent 8-week period spanning between March 29, 2020, and May 16, 2020. Although a substantial increase from the early COVID-19 period, the case rate remained below the baseline established before COVID-19.
The analysis looked at 15,244 AMI hospitalizations among 14,724 patients treated in the Providence St. Joseph Hospital System, which has facilities in Alaska, California, Montana, Oregon, Texas, and Washington. The 1,915 AMI cases captured from Feb. 23, 2020, represented 13% of the total.
Differences in mortality, patients, treatment
In the early period, the ratio of observed-to-expected (O/E) mortality relative to the pre–COVID-19 baseline increased by 27% (odds ratio, 1.27; 95% CI, 1.07-1.48). When STEMI was analyzed separately, the O/E mortality was nearly double that of the baseline period (OR, 1.96; 95% CI, 1.22-2.70). In the latter post–COVID-19 period of observation, the overall increase in AMI-associated mortality on the basis of an O/E ratio was no longer significant relative to the baseline period (OR, 1.23; 95% CI, 0.98-1.47). However, the relative increase in STEMI-associated mortality on an O/E basis was even greater (OR, 2.40; 95% CI, 1.65-3.16) in the second COVID-19 period analyzed. Even after risk adjustment, the OR for STEMI mortality remained significantly elevated relative to baseline (1.52; 95% CI, 1.02-2.26).
The differences in AMI patients treated before the onset of the COVID-19 pandemic and those treated afterwards might be relevant, according to the investigators. Specifically, patients hospitalized after Feb. 23, 2020 were 1-3 years younger (P < .001) depending on type of AMI, and more likely to be Asian (P = .01).
The length of stay was 6 hours shorter in the early COVID-19 period and 7 hours shorter in the latter period relative to baseline, but an analysis of treatment approaches to non-STEMI and STEMI during the COVID-19 pandemic were not found to be significantly different from baseline.
Prior to the COVID-19 pandemic, 79% of STEMI patients and 77% of non-STEMI patients were discharged home, which was significantly lower than in the early COVID-19 period, when 83% (P = .02) of STEMI and 81% (P = .006) of non-STEMI patients were discharged home. In the latter period, discharge to home care was also significantly higher than in the baseline period.
More than fear of COVID-19?
One theory to account for the reduction in AMI hospitalizations and the increase in AMI-related mortality is the possibility that patients were slow to seek care at acute care hospitals because of concern about COVID-19 infection, according to Dr. Gluckman and coinvestigators.
“Given the time-sensitive nature of STEMI, any delay by patients, emergency medical services, the emergency department, or cardiac catheterization laboratory may have played a role,” they suggested.
In an interview, Dr. Gluckman said that further effort to identify the reasons for the increased AMI-related mortality is planned. Pulling data from the electronic medical records of the patients included in this retrospective analysis might be a “challenge,” but Dr. Gluckman reported that he and his coinvestigators plan to look at a different set of registry data that might provide information on sources of delay, particularly in the STEMI population.
“This includes looking at a number of time factors, such as symptom onset to first medical contact, first medical contact to device, and door-in-door-out times,” Dr. Gluckman said. The goal is to “better understand if delays [in treatment] occurred during the pandemic and, if so, how they may have contributed to increases in risk adjusted mortality.”
Dr. Krumholz, director of the Yale Center for Outcomes Research and Evaluation, New Haven, Conn., called this study a “useful” confirmation of changes in AMI-related care with the onset of the COVID-19 pandemic. As reported anecdotally, the study “indicates marked decreases in hospitalizations of patients with AMI even in areas that were not experiencing big outbreaks but did have some restrictions to limit spread,” he noted.
More data gathered by other centers might provide information about what it all means.
“There remain so many questions about what happened and what consequences accrued,” Dr. Krumholz observed. “In the meantime, we need to continue to send the message that people with symptoms that suggest a heart attack need to rapidly seek care.”
The investigators reported having no financial conflicts of interest.
SOURCE: Gluckman TJ et al. JAMA Cardiol. 2020 Aug 7. doi: 10.1001/jamacardio.2020.3629.
FROM JAMA CARDIOLOGY
Pandemic effect: Telemedicine is now a ‘must-have’ service
If people try telemedicine, they’ll like telemedicine. And if they want to avoid a doctor’s office, as most people do these days, they’ll try telemedicine. That is the message coming from 1,000 people surveyed for DocASAP, a provider of online patient access and engagement systems.

Here are a couple of numbers: 92% of those who made a telemedicine visit said they were satisfied with the overall appointment experience, and 91% said that they are more likely to schedule a telemedicine visit instead of an in-person appointment. All of the survey respondents had visited a health care provider in the past year, and 40% already had made a telemedicine visit, DocASAP reported.
Puneet Maheshwari, DocASAP cofounder and CEO, said in a statement. “As providers continue to adopt innovative technology to power a more seamless, end-to-end digital consumer experience, I expect telehealth to become fully integrated into overall care management.”
For now, though, COVID-19 is an overriding concern and health care facilities are suspect. When respondents were asked to identify the types of public facilities where they felt safe, hospitals were named by 32%, doctors’ offices by 26%, and ED/urgent care by just 12%, the DocASAP report said. Even public transportation got 13%.
The safest place to be, according to 42% of the respondents? The grocery store.
Of those surveyed, 43% “indicated they will not feel safe entering any health care setting until at least the fall,” the company said. An even higher share of patients, 68%, canceled or postponed an in-person appointment during the pandemic.
“No longer are remote health services viewed as ‘nice to have’ – they are now a must-have care delivery option,” DocASAP said in their report.
Safety concerns involving COVID-19, named by 47% of the sample, were the leading factor that would influence patients’ decision to schedule a telemedicine visit. Insurance coverage was next at 43%, followed by “ease of accessing quality care” at 40%, the report said.
Among those who had made a telemedicine visit, scheduling the appointment was the most satisfying aspect of the experience, according to 54% of respondents, with day-of-appointment wait time next at 38% and quality of the video/audio technology tied with preappointment communication at almost 33%, the survey data show.
Conversely, scheduling the appointment also was declared the most frustrating aspect of the telemedicine experience, although the total in that category was a much lower 29%.
“The pandemic has thrust profound change on every aspect of life, particularly health care. … Innovations – like digital and telehealth solutions – designed to meet patient needs will likely become embedded into the health care delivery system,” DocASAP said.
The survey was commissioned by DocASAP and conducted by marketing research company OnePoll on June 29-30, 2020.
If people try telemedicine, they’ll like telemedicine. And if they want to avoid a doctor’s office, as most people do these days, they’ll try telemedicine. That is the message coming from 1,000 people surveyed for DocASAP, a provider of online patient access and engagement systems.

Here are a couple of numbers: 92% of those who made a telemedicine visit said they were satisfied with the overall appointment experience, and 91% said that they are more likely to schedule a telemedicine visit instead of an in-person appointment. All of the survey respondents had visited a health care provider in the past year, and 40% already had made a telemedicine visit, DocASAP reported.
Puneet Maheshwari, DocASAP cofounder and CEO, said in a statement. “As providers continue to adopt innovative technology to power a more seamless, end-to-end digital consumer experience, I expect telehealth to become fully integrated into overall care management.”
For now, though, COVID-19 is an overriding concern and health care facilities are suspect. When respondents were asked to identify the types of public facilities where they felt safe, hospitals were named by 32%, doctors’ offices by 26%, and ED/urgent care by just 12%, the DocASAP report said. Even public transportation got 13%.
The safest place to be, according to 42% of the respondents? The grocery store.
Of those surveyed, 43% “indicated they will not feel safe entering any health care setting until at least the fall,” the company said. An even higher share of patients, 68%, canceled or postponed an in-person appointment during the pandemic.
“No longer are remote health services viewed as ‘nice to have’ – they are now a must-have care delivery option,” DocASAP said in their report.
Safety concerns involving COVID-19, named by 47% of the sample, were the leading factor that would influence patients’ decision to schedule a telemedicine visit. Insurance coverage was next at 43%, followed by “ease of accessing quality care” at 40%, the report said.
Among those who had made a telemedicine visit, scheduling the appointment was the most satisfying aspect of the experience, according to 54% of respondents, with day-of-appointment wait time next at 38% and quality of the video/audio technology tied with preappointment communication at almost 33%, the survey data show.
Conversely, scheduling the appointment also was declared the most frustrating aspect of the telemedicine experience, although the total in that category was a much lower 29%.
“The pandemic has thrust profound change on every aspect of life, particularly health care. … Innovations – like digital and telehealth solutions – designed to meet patient needs will likely become embedded into the health care delivery system,” DocASAP said.
The survey was commissioned by DocASAP and conducted by marketing research company OnePoll on June 29-30, 2020.
If people try telemedicine, they’ll like telemedicine. And if they want to avoid a doctor’s office, as most people do these days, they’ll try telemedicine. That is the message coming from 1,000 people surveyed for DocASAP, a provider of online patient access and engagement systems.

Here are a couple of numbers: 92% of those who made a telemedicine visit said they were satisfied with the overall appointment experience, and 91% said that they are more likely to schedule a telemedicine visit instead of an in-person appointment. All of the survey respondents had visited a health care provider in the past year, and 40% already had made a telemedicine visit, DocASAP reported.
Puneet Maheshwari, DocASAP cofounder and CEO, said in a statement. “As providers continue to adopt innovative technology to power a more seamless, end-to-end digital consumer experience, I expect telehealth to become fully integrated into overall care management.”
For now, though, COVID-19 is an overriding concern and health care facilities are suspect. When respondents were asked to identify the types of public facilities where they felt safe, hospitals were named by 32%, doctors’ offices by 26%, and ED/urgent care by just 12%, the DocASAP report said. Even public transportation got 13%.
The safest place to be, according to 42% of the respondents? The grocery store.
Of those surveyed, 43% “indicated they will not feel safe entering any health care setting until at least the fall,” the company said. An even higher share of patients, 68%, canceled or postponed an in-person appointment during the pandemic.
“No longer are remote health services viewed as ‘nice to have’ – they are now a must-have care delivery option,” DocASAP said in their report.
Safety concerns involving COVID-19, named by 47% of the sample, were the leading factor that would influence patients’ decision to schedule a telemedicine visit. Insurance coverage was next at 43%, followed by “ease of accessing quality care” at 40%, the report said.
Among those who had made a telemedicine visit, scheduling the appointment was the most satisfying aspect of the experience, according to 54% of respondents, with day-of-appointment wait time next at 38% and quality of the video/audio technology tied with preappointment communication at almost 33%, the survey data show.
Conversely, scheduling the appointment also was declared the most frustrating aspect of the telemedicine experience, although the total in that category was a much lower 29%.
“The pandemic has thrust profound change on every aspect of life, particularly health care. … Innovations – like digital and telehealth solutions – designed to meet patient needs will likely become embedded into the health care delivery system,” DocASAP said.
The survey was commissioned by DocASAP and conducted by marketing research company OnePoll on June 29-30, 2020.
Cancer treatments bring concerns for hospitalists
Advances in cancer treatment have brought a range of potential issues hospitalists are likely to see in admitted patients – many of which can escalate quickly into life-threatening emergencies if they’re not handled properly, an oncologist said in a presentation at HM20 Virtual, hosted by the Society of Hospital Medicine.
Checkpoint inhibitors and CAR T-cell therapy – revolutions in fighting cancer but potential instigators of serious side effects because of the way they set the immune system in motion – can have consequences throughout the body, said Megan Kruse, MD, an oncologist at the Cleveland Clinic.
Checkpoint inhibitors, which cause the body to essentially take its foot off the break of the immune system, in particular have diverse effects, Dr. Kruse said.
“Suffice it to say that any odd symptom in any organ system in a patient on immunotherapy, or with a history of immunotherapy, can be cause for concern,” she said. Most common are skin, gut, endocrine, lung, and musculoskeletal involvement. Cardiovascular, hematologic, renal, neurologic, and ophthalmological effects are less common, but when they happen, they’re often dramatic and need urgent management.
With these medications –which include anti–programmed death-1 agents pembrolizumab and nivolumab and anti–PD-ligand 1 agents atezolizumab and avelumab, among others – rash is often seen first, followed by diarrhea and colitis. Hypophysitis, which requires intervention, and liver toxicity, which usually tapers off on its own, often occur about 6-8 weeks into treatment. There are no rigid rules for the arrival of these symptoms, however, Dr. Kruse said.
“We must have a high index of suspicion. ... They really can occur at any point after a patient has had even one dose of an immunologic agent,” she said.
In more serious cases, steroids are typically the go-to treatment, she added, because they will quickly tamp down the immune activation brought on by the medications.
“When these drugs first came out, we were all very concerned about adding steroids,” she said. “In follow-up studies, it actually looks like we don’t attenuate the anticancer response very much by instituting steroids when clinically appropriate. And so you all should feel very comfortable adding steroids while waiting to talk to oncology.”
In these cases, the steroid taper is done very slowly, over weeks or even months.
With CAR T-cell therapy – in which patients receive T cells to target liquid tumors – cytokine release syndrome (CRS) can occur, often within 14 days after treatment. Dr. Kruse cautioned that it can present with symptoms similar to tumor lysis syndrome or sepsis.
“Patients are at a high risk of bacterial infection, so antibiotics are advised,” she said.
In these cases, fever is often a harbinger, often arriving at least a day before the rest of the symptoms of CRS.
Early treatment with the interleukin-6 inhibitor tocilizumab is recommended for these patients, she said. This agent has been shown to have a 69% response rate in severe CRS and has no known effect on the efficacy of the CAR T-cell treatment.
Dr. Kruse also touched on several other conditions that can rise to the level of emergencies in cancer treatment:
- In cases of neutropenic fever, patients should be treated as soon as possible with antibiotics, and some solid-tumor patients at lower risk can be treated as outpatients, she said. Those with hematologic cancer, however, will need inpatient care.
- For tumor lysis syndrome with renal failure, fluids should be started quickly. Rasburicase, a recombinant urate oxidase enzyme, can be considered in some cases, but requires caution.
- In cases of spinal cord compression, a full spine MRI should be completed because about a third of patients have multilevel involvement. Steroids should be started as soon as possible.
In a question-and-answer session, much of the discussion focused on when outpatient care for neutropenic fever was possible. Dr. Kruse said those who need to be admitted for neutropenic fever treatment tend to be those with hematologic malignancies because their treatment is so myelosuppressive.
“Their window of complications is longer,” she said. Solid tumor patients, on the other hand, will usually improve “fairly rapidly” in about 3-4 days.
Many session viewers expressed surprise at the possibility of outpatient neutropenic fever treatment. Dr. Kruse said that the Cleveland Clinic’s incorporation of this approach has included the input of neutropenic fever risk index scoring into their electronic medical record and a good deal of in-service training.
Asked about appropriate swabbing of patients for COVID-19 before chemotherapy, Dr. Kruse said that her center screens only patients who need to be hospitalized for the treatment – those with a high incidence of prolonged neutropenia.
“For our typical outpatients who are receiving chemotherapy,” she said, “we are not swabbing them.” But they have intense fever screening and distance measures in place.
Dr. Kruse reported advisory board involvement for Novartis Oncology and consulting for Puma Biotechnology.
Advances in cancer treatment have brought a range of potential issues hospitalists are likely to see in admitted patients – many of which can escalate quickly into life-threatening emergencies if they’re not handled properly, an oncologist said in a presentation at HM20 Virtual, hosted by the Society of Hospital Medicine.
Checkpoint inhibitors and CAR T-cell therapy – revolutions in fighting cancer but potential instigators of serious side effects because of the way they set the immune system in motion – can have consequences throughout the body, said Megan Kruse, MD, an oncologist at the Cleveland Clinic.
Checkpoint inhibitors, which cause the body to essentially take its foot off the break of the immune system, in particular have diverse effects, Dr. Kruse said.
“Suffice it to say that any odd symptom in any organ system in a patient on immunotherapy, or with a history of immunotherapy, can be cause for concern,” she said. Most common are skin, gut, endocrine, lung, and musculoskeletal involvement. Cardiovascular, hematologic, renal, neurologic, and ophthalmological effects are less common, but when they happen, they’re often dramatic and need urgent management.
With these medications –which include anti–programmed death-1 agents pembrolizumab and nivolumab and anti–PD-ligand 1 agents atezolizumab and avelumab, among others – rash is often seen first, followed by diarrhea and colitis. Hypophysitis, which requires intervention, and liver toxicity, which usually tapers off on its own, often occur about 6-8 weeks into treatment. There are no rigid rules for the arrival of these symptoms, however, Dr. Kruse said.
“We must have a high index of suspicion. ... They really can occur at any point after a patient has had even one dose of an immunologic agent,” she said.
In more serious cases, steroids are typically the go-to treatment, she added, because they will quickly tamp down the immune activation brought on by the medications.
“When these drugs first came out, we were all very concerned about adding steroids,” she said. “In follow-up studies, it actually looks like we don’t attenuate the anticancer response very much by instituting steroids when clinically appropriate. And so you all should feel very comfortable adding steroids while waiting to talk to oncology.”
In these cases, the steroid taper is done very slowly, over weeks or even months.
With CAR T-cell therapy – in which patients receive T cells to target liquid tumors – cytokine release syndrome (CRS) can occur, often within 14 days after treatment. Dr. Kruse cautioned that it can present with symptoms similar to tumor lysis syndrome or sepsis.
“Patients are at a high risk of bacterial infection, so antibiotics are advised,” she said.
In these cases, fever is often a harbinger, often arriving at least a day before the rest of the symptoms of CRS.
Early treatment with the interleukin-6 inhibitor tocilizumab is recommended for these patients, she said. This agent has been shown to have a 69% response rate in severe CRS and has no known effect on the efficacy of the CAR T-cell treatment.
Dr. Kruse also touched on several other conditions that can rise to the level of emergencies in cancer treatment:
- In cases of neutropenic fever, patients should be treated as soon as possible with antibiotics, and some solid-tumor patients at lower risk can be treated as outpatients, she said. Those with hematologic cancer, however, will need inpatient care.
- For tumor lysis syndrome with renal failure, fluids should be started quickly. Rasburicase, a recombinant urate oxidase enzyme, can be considered in some cases, but requires caution.
- In cases of spinal cord compression, a full spine MRI should be completed because about a third of patients have multilevel involvement. Steroids should be started as soon as possible.
In a question-and-answer session, much of the discussion focused on when outpatient care for neutropenic fever was possible. Dr. Kruse said those who need to be admitted for neutropenic fever treatment tend to be those with hematologic malignancies because their treatment is so myelosuppressive.
“Their window of complications is longer,” she said. Solid tumor patients, on the other hand, will usually improve “fairly rapidly” in about 3-4 days.
Many session viewers expressed surprise at the possibility of outpatient neutropenic fever treatment. Dr. Kruse said that the Cleveland Clinic’s incorporation of this approach has included the input of neutropenic fever risk index scoring into their electronic medical record and a good deal of in-service training.
Asked about appropriate swabbing of patients for COVID-19 before chemotherapy, Dr. Kruse said that her center screens only patients who need to be hospitalized for the treatment – those with a high incidence of prolonged neutropenia.
“For our typical outpatients who are receiving chemotherapy,” she said, “we are not swabbing them.” But they have intense fever screening and distance measures in place.
Dr. Kruse reported advisory board involvement for Novartis Oncology and consulting for Puma Biotechnology.
Advances in cancer treatment have brought a range of potential issues hospitalists are likely to see in admitted patients – many of which can escalate quickly into life-threatening emergencies if they’re not handled properly, an oncologist said in a presentation at HM20 Virtual, hosted by the Society of Hospital Medicine.
Checkpoint inhibitors and CAR T-cell therapy – revolutions in fighting cancer but potential instigators of serious side effects because of the way they set the immune system in motion – can have consequences throughout the body, said Megan Kruse, MD, an oncologist at the Cleveland Clinic.
Checkpoint inhibitors, which cause the body to essentially take its foot off the break of the immune system, in particular have diverse effects, Dr. Kruse said.
“Suffice it to say that any odd symptom in any organ system in a patient on immunotherapy, or with a history of immunotherapy, can be cause for concern,” she said. Most common are skin, gut, endocrine, lung, and musculoskeletal involvement. Cardiovascular, hematologic, renal, neurologic, and ophthalmological effects are less common, but when they happen, they’re often dramatic and need urgent management.
With these medications –which include anti–programmed death-1 agents pembrolizumab and nivolumab and anti–PD-ligand 1 agents atezolizumab and avelumab, among others – rash is often seen first, followed by diarrhea and colitis. Hypophysitis, which requires intervention, and liver toxicity, which usually tapers off on its own, often occur about 6-8 weeks into treatment. There are no rigid rules for the arrival of these symptoms, however, Dr. Kruse said.
“We must have a high index of suspicion. ... They really can occur at any point after a patient has had even one dose of an immunologic agent,” she said.
In more serious cases, steroids are typically the go-to treatment, she added, because they will quickly tamp down the immune activation brought on by the medications.
“When these drugs first came out, we were all very concerned about adding steroids,” she said. “In follow-up studies, it actually looks like we don’t attenuate the anticancer response very much by instituting steroids when clinically appropriate. And so you all should feel very comfortable adding steroids while waiting to talk to oncology.”
In these cases, the steroid taper is done very slowly, over weeks or even months.
With CAR T-cell therapy – in which patients receive T cells to target liquid tumors – cytokine release syndrome (CRS) can occur, often within 14 days after treatment. Dr. Kruse cautioned that it can present with symptoms similar to tumor lysis syndrome or sepsis.
“Patients are at a high risk of bacterial infection, so antibiotics are advised,” she said.
In these cases, fever is often a harbinger, often arriving at least a day before the rest of the symptoms of CRS.
Early treatment with the interleukin-6 inhibitor tocilizumab is recommended for these patients, she said. This agent has been shown to have a 69% response rate in severe CRS and has no known effect on the efficacy of the CAR T-cell treatment.
Dr. Kruse also touched on several other conditions that can rise to the level of emergencies in cancer treatment:
- In cases of neutropenic fever, patients should be treated as soon as possible with antibiotics, and some solid-tumor patients at lower risk can be treated as outpatients, she said. Those with hematologic cancer, however, will need inpatient care.
- For tumor lysis syndrome with renal failure, fluids should be started quickly. Rasburicase, a recombinant urate oxidase enzyme, can be considered in some cases, but requires caution.
- In cases of spinal cord compression, a full spine MRI should be completed because about a third of patients have multilevel involvement. Steroids should be started as soon as possible.
In a question-and-answer session, much of the discussion focused on when outpatient care for neutropenic fever was possible. Dr. Kruse said those who need to be admitted for neutropenic fever treatment tend to be those with hematologic malignancies because their treatment is so myelosuppressive.
“Their window of complications is longer,” she said. Solid tumor patients, on the other hand, will usually improve “fairly rapidly” in about 3-4 days.
Many session viewers expressed surprise at the possibility of outpatient neutropenic fever treatment. Dr. Kruse said that the Cleveland Clinic’s incorporation of this approach has included the input of neutropenic fever risk index scoring into their electronic medical record and a good deal of in-service training.
Asked about appropriate swabbing of patients for COVID-19 before chemotherapy, Dr. Kruse said that her center screens only patients who need to be hospitalized for the treatment – those with a high incidence of prolonged neutropenia.
“For our typical outpatients who are receiving chemotherapy,” she said, “we are not swabbing them.” But they have intense fever screening and distance measures in place.
Dr. Kruse reported advisory board involvement for Novartis Oncology and consulting for Puma Biotechnology.
FROM HM20 VIRTUAL
Welcome to week 2 of HM20 Virtual!
The Society of Hospital Medicine prides itself on bringing a broad range of experts together with the largest gathering of hospitalists at any conference – virtual or otherwise! Hospitalists, nurse practitioners, physician assistants, executives, pharmacists, educators, and practitioners of many hospital-based specialties make HM20 Virtual a unique educational experience.
We know that patients depend on you to have pertinent, updated, and timely information for their acute care needs. HM20 Virtual can provide the information you need to stay abreast in this complex and ever-changing year. From COVID-19 to common diagnosis, from racism/bias to blood glucose, from peds to pulmonary embolism, HM20 Virtual covers important topics for all acute care and hospital clinicians and professionals.
This year’s conference is something new. To meet the ever-changing challenges that the year 2020 has brought all of us, HM20 Virtual has addressed one of the limitations of an online conference: personal interactions. With Simulive sessions, you will have the opportunity to chat with fellow participants and interact with the expert faculty in real time! Of course, all Simulive sessions will be available on demand after the fact for those of you who need alternate times to watch.
Be sure to attend some (or all!) of this week’s Simulive sessions. There is something for everyone:
- On Tuesday, Aug. 18, Sam Brondfield, MD, will discuss oncologic work-ups, and James Kim, MD, will make antibiotics simple (where was Dr. Kim for my medical school training?).
- Wednesday, Aug. 19, circles back to another epidemic, the opioid crisis, presented by Theresa Vettese, MD. Dr. Alfred Burger updates us on Clinical Practice Guidelines, and Jeff Trost, MD, brings us up to speed on the effects of COVID-19 and the heart.
- Thursday, Aug. 20, wraps up week 2 of HM20 Virtual with Population Health by Adam Myers, MD, and Updates in Pneumonia by Joanna Bonsall, MD.
The personal interactions don’t have to stop there! HM20 Virtual also features Special Interest Forums. Check out the list and find out how to join by visiting the HM20 Virtual website.
We look forward to “seeing” you at HM20 Virtual. We always want your feedback; however, in this socially distanced, travel-limited world, your input is more important now than ever. Be sure to let us know how this new format works for your learning, networking, and professional needs.
On behalf of the SHM board of directors, the SHM staff, and myself, we hope you enjoy HM20 Virtual. Through this meeting’s rich selection of educational opportunities – and the innovative approaches in a world dominated by the coronavirus – SHM continues to further its mission to promote excellence in the practice of hospital medicine. SHM remains at the forefront of health care today, empowering hospitalists and transforming patient care.
Dr. Howell is CEO of the Society of Hospital Medicine.
The Society of Hospital Medicine prides itself on bringing a broad range of experts together with the largest gathering of hospitalists at any conference – virtual or otherwise! Hospitalists, nurse practitioners, physician assistants, executives, pharmacists, educators, and practitioners of many hospital-based specialties make HM20 Virtual a unique educational experience.
We know that patients depend on you to have pertinent, updated, and timely information for their acute care needs. HM20 Virtual can provide the information you need to stay abreast in this complex and ever-changing year. From COVID-19 to common diagnosis, from racism/bias to blood glucose, from peds to pulmonary embolism, HM20 Virtual covers important topics for all acute care and hospital clinicians and professionals.
This year’s conference is something new. To meet the ever-changing challenges that the year 2020 has brought all of us, HM20 Virtual has addressed one of the limitations of an online conference: personal interactions. With Simulive sessions, you will have the opportunity to chat with fellow participants and interact with the expert faculty in real time! Of course, all Simulive sessions will be available on demand after the fact for those of you who need alternate times to watch.
Be sure to attend some (or all!) of this week’s Simulive sessions. There is something for everyone:
- On Tuesday, Aug. 18, Sam Brondfield, MD, will discuss oncologic work-ups, and James Kim, MD, will make antibiotics simple (where was Dr. Kim for my medical school training?).
- Wednesday, Aug. 19, circles back to another epidemic, the opioid crisis, presented by Theresa Vettese, MD. Dr. Alfred Burger updates us on Clinical Practice Guidelines, and Jeff Trost, MD, brings us up to speed on the effects of COVID-19 and the heart.
- Thursday, Aug. 20, wraps up week 2 of HM20 Virtual with Population Health by Adam Myers, MD, and Updates in Pneumonia by Joanna Bonsall, MD.
The personal interactions don’t have to stop there! HM20 Virtual also features Special Interest Forums. Check out the list and find out how to join by visiting the HM20 Virtual website.
We look forward to “seeing” you at HM20 Virtual. We always want your feedback; however, in this socially distanced, travel-limited world, your input is more important now than ever. Be sure to let us know how this new format works for your learning, networking, and professional needs.
On behalf of the SHM board of directors, the SHM staff, and myself, we hope you enjoy HM20 Virtual. Through this meeting’s rich selection of educational opportunities – and the innovative approaches in a world dominated by the coronavirus – SHM continues to further its mission to promote excellence in the practice of hospital medicine. SHM remains at the forefront of health care today, empowering hospitalists and transforming patient care.
Dr. Howell is CEO of the Society of Hospital Medicine.
The Society of Hospital Medicine prides itself on bringing a broad range of experts together with the largest gathering of hospitalists at any conference – virtual or otherwise! Hospitalists, nurse practitioners, physician assistants, executives, pharmacists, educators, and practitioners of many hospital-based specialties make HM20 Virtual a unique educational experience.
We know that patients depend on you to have pertinent, updated, and timely information for their acute care needs. HM20 Virtual can provide the information you need to stay abreast in this complex and ever-changing year. From COVID-19 to common diagnosis, from racism/bias to blood glucose, from peds to pulmonary embolism, HM20 Virtual covers important topics for all acute care and hospital clinicians and professionals.
This year’s conference is something new. To meet the ever-changing challenges that the year 2020 has brought all of us, HM20 Virtual has addressed one of the limitations of an online conference: personal interactions. With Simulive sessions, you will have the opportunity to chat with fellow participants and interact with the expert faculty in real time! Of course, all Simulive sessions will be available on demand after the fact for those of you who need alternate times to watch.
Be sure to attend some (or all!) of this week’s Simulive sessions. There is something for everyone:
- On Tuesday, Aug. 18, Sam Brondfield, MD, will discuss oncologic work-ups, and James Kim, MD, will make antibiotics simple (where was Dr. Kim for my medical school training?).
- Wednesday, Aug. 19, circles back to another epidemic, the opioid crisis, presented by Theresa Vettese, MD. Dr. Alfred Burger updates us on Clinical Practice Guidelines, and Jeff Trost, MD, brings us up to speed on the effects of COVID-19 and the heart.
- Thursday, Aug. 20, wraps up week 2 of HM20 Virtual with Population Health by Adam Myers, MD, and Updates in Pneumonia by Joanna Bonsall, MD.
The personal interactions don’t have to stop there! HM20 Virtual also features Special Interest Forums. Check out the list and find out how to join by visiting the HM20 Virtual website.
We look forward to “seeing” you at HM20 Virtual. We always want your feedback; however, in this socially distanced, travel-limited world, your input is more important now than ever. Be sure to let us know how this new format works for your learning, networking, and professional needs.
On behalf of the SHM board of directors, the SHM staff, and myself, we hope you enjoy HM20 Virtual. Through this meeting’s rich selection of educational opportunities – and the innovative approaches in a world dominated by the coronavirus – SHM continues to further its mission to promote excellence in the practice of hospital medicine. SHM remains at the forefront of health care today, empowering hospitalists and transforming patient care.
Dr. Howell is CEO of the Society of Hospital Medicine.
COVID-19/heart connection: What hospitalists need to know
The heart-related manifestations of COVID-19 are a serious matter, but no one should make the mistake of thinking of COVID-19 as primarily a cardiac disease, according to Jeffrey C. Trost, MD, a cardiologist at Johns Hopkins University, Baltimore.
he said in offering a preview of his upcoming clinical update at HM20 Virtual, hosted by the Society of Hospital Medicine.
For this reason, in his clinical update talk, titled “COVID-19 and the Heart: What Every Hospitalist Should Know,” he’ll urge hospitalists to be conservative in ordering cardiac biomarker tests such troponin and natriuretic peptide levels. The focus should appropriately be on the subset of COVID-19 patients having the same symptoms suggestive of acute coronary syndrome, heart failure, or new-onset cardiomyopathy that would trigger laboratory testing in non–COVID-19 patients.
“Be more selective. Definitely do not routinely monitor troponin or [N-terminal of the prohormone brain natriuretic peptide] in patients just because they have COVID-19. A lot of patients with COVID-19 have these labs drawn, especially in the emergency department. We see a high signal-to-noise ratio: not infrequently the values are abnormal, and yet we don’t really know what that means,” said Dr. Trost, who is also director of the cardiac catheterization laboratory at Johns Hopkins Bayview Medical Center.
COVID-19 patients with preexisting heart disease are clearly at increased risk of severe forms of the infectious illness. In his talk, Dr. Trost will review the epidemiology of this association. He’ll also discuss the varied cardiac manifestations of COVID-19, consisting of myocarditis or other forms of new-onset cardiomyopathy, acute coronary syndrome, heart failure, and arrhythmias.
Many questions regarding COVID-19 and the heart remain unanswered for now, such as the mechanism and long-term implications of the phenomenon of ST-elevation acute coronary syndrome with chest pain in the presence of unobstructed coronary arteries, which Dr. Trost and others have encountered. Or the extent to which COVID-19–associated myocarditis is directly virus mediated as opposed to an autoimmune process.
“We’re relying completely on case reports at this point,” according to the cardiologist.
But one major issue has, thankfully, been put to rest on the basis of persuasive evidence which Dr. Trost plans to highlight: Millions of patients on ACE inhibitors or angiotensin receptor blockers can now rest assured that taking those medications doesn’t place them at increased risk of becoming infected with the novel coronavirus or, if infected, developing severe complications of COVID-19. Earlier in the pandemic that had been a legitimate theoretic concern based upon a plausible mechanism.
“I think we as physicians can now confidently say that we don’t need to stop these medicines in folks,” Dr. Trost said.
COVID-19 and the Heart: What Every Hospitalist Should Know
Live Q&A: Wednesday, Aug. 19, 3:30 p.m. to 4:30 p.m. ET
The heart-related manifestations of COVID-19 are a serious matter, but no one should make the mistake of thinking of COVID-19 as primarily a cardiac disease, according to Jeffrey C. Trost, MD, a cardiologist at Johns Hopkins University, Baltimore.
he said in offering a preview of his upcoming clinical update at HM20 Virtual, hosted by the Society of Hospital Medicine.
For this reason, in his clinical update talk, titled “COVID-19 and the Heart: What Every Hospitalist Should Know,” he’ll urge hospitalists to be conservative in ordering cardiac biomarker tests such troponin and natriuretic peptide levels. The focus should appropriately be on the subset of COVID-19 patients having the same symptoms suggestive of acute coronary syndrome, heart failure, or new-onset cardiomyopathy that would trigger laboratory testing in non–COVID-19 patients.
“Be more selective. Definitely do not routinely monitor troponin or [N-terminal of the prohormone brain natriuretic peptide] in patients just because they have COVID-19. A lot of patients with COVID-19 have these labs drawn, especially in the emergency department. We see a high signal-to-noise ratio: not infrequently the values are abnormal, and yet we don’t really know what that means,” said Dr. Trost, who is also director of the cardiac catheterization laboratory at Johns Hopkins Bayview Medical Center.
COVID-19 patients with preexisting heart disease are clearly at increased risk of severe forms of the infectious illness. In his talk, Dr. Trost will review the epidemiology of this association. He’ll also discuss the varied cardiac manifestations of COVID-19, consisting of myocarditis or other forms of new-onset cardiomyopathy, acute coronary syndrome, heart failure, and arrhythmias.
Many questions regarding COVID-19 and the heart remain unanswered for now, such as the mechanism and long-term implications of the phenomenon of ST-elevation acute coronary syndrome with chest pain in the presence of unobstructed coronary arteries, which Dr. Trost and others have encountered. Or the extent to which COVID-19–associated myocarditis is directly virus mediated as opposed to an autoimmune process.
“We’re relying completely on case reports at this point,” according to the cardiologist.
But one major issue has, thankfully, been put to rest on the basis of persuasive evidence which Dr. Trost plans to highlight: Millions of patients on ACE inhibitors or angiotensin receptor blockers can now rest assured that taking those medications doesn’t place them at increased risk of becoming infected with the novel coronavirus or, if infected, developing severe complications of COVID-19. Earlier in the pandemic that had been a legitimate theoretic concern based upon a plausible mechanism.
“I think we as physicians can now confidently say that we don’t need to stop these medicines in folks,” Dr. Trost said.
COVID-19 and the Heart: What Every Hospitalist Should Know
Live Q&A: Wednesday, Aug. 19, 3:30 p.m. to 4:30 p.m. ET
The heart-related manifestations of COVID-19 are a serious matter, but no one should make the mistake of thinking of COVID-19 as primarily a cardiac disease, according to Jeffrey C. Trost, MD, a cardiologist at Johns Hopkins University, Baltimore.
he said in offering a preview of his upcoming clinical update at HM20 Virtual, hosted by the Society of Hospital Medicine.
For this reason, in his clinical update talk, titled “COVID-19 and the Heart: What Every Hospitalist Should Know,” he’ll urge hospitalists to be conservative in ordering cardiac biomarker tests such troponin and natriuretic peptide levels. The focus should appropriately be on the subset of COVID-19 patients having the same symptoms suggestive of acute coronary syndrome, heart failure, or new-onset cardiomyopathy that would trigger laboratory testing in non–COVID-19 patients.
“Be more selective. Definitely do not routinely monitor troponin or [N-terminal of the prohormone brain natriuretic peptide] in patients just because they have COVID-19. A lot of patients with COVID-19 have these labs drawn, especially in the emergency department. We see a high signal-to-noise ratio: not infrequently the values are abnormal, and yet we don’t really know what that means,” said Dr. Trost, who is also director of the cardiac catheterization laboratory at Johns Hopkins Bayview Medical Center.
COVID-19 patients with preexisting heart disease are clearly at increased risk of severe forms of the infectious illness. In his talk, Dr. Trost will review the epidemiology of this association. He’ll also discuss the varied cardiac manifestations of COVID-19, consisting of myocarditis or other forms of new-onset cardiomyopathy, acute coronary syndrome, heart failure, and arrhythmias.
Many questions regarding COVID-19 and the heart remain unanswered for now, such as the mechanism and long-term implications of the phenomenon of ST-elevation acute coronary syndrome with chest pain in the presence of unobstructed coronary arteries, which Dr. Trost and others have encountered. Or the extent to which COVID-19–associated myocarditis is directly virus mediated as opposed to an autoimmune process.
“We’re relying completely on case reports at this point,” according to the cardiologist.
But one major issue has, thankfully, been put to rest on the basis of persuasive evidence which Dr. Trost plans to highlight: Millions of patients on ACE inhibitors or angiotensin receptor blockers can now rest assured that taking those medications doesn’t place them at increased risk of becoming infected with the novel coronavirus or, if infected, developing severe complications of COVID-19. Earlier in the pandemic that had been a legitimate theoretic concern based upon a plausible mechanism.
“I think we as physicians can now confidently say that we don’t need to stop these medicines in folks,” Dr. Trost said.
COVID-19 and the Heart: What Every Hospitalist Should Know
Live Q&A: Wednesday, Aug. 19, 3:30 p.m. to 4:30 p.m. ET
Developing COVID-19 hospital protocols during the pandemic
As hospitalists and other physicians at the University of Texas at Austin considered how to treat COVID-19 patients in the early weeks of the pandemic, one question they had to consider was: What about convalescent plasma?
All they had to go on were small case series in Ebola, SARS, and MERS and a few small, nonrandomized COVID-19 studies showing a possible benefit and minimal risk, but the evidence was only “toward the middle or bottom” of the evidence pyramid, said Johanna Busch, MD, of the department of internal medicine at Dell Medical Center at the university.
The center’s COVID-19 committee asked a few of its members – infectious disease and internal medicine physicians – to analyze the literature and other factors. In the end, the committee – which meets regularly and also includes pulmonology–critical care experts, nursing experts, and others – recommended using convalescent plasma because of the evidence and the available supply. But in subsequent meetings, as the pandemic surged in the South and the supply dwindled, the committee changed its recommendation for convalescent plasma to more limited use, she said during the virtual annual meeting of the Society of Hospital Medicine.
“It’s all about teamwork,” said W. Michael Brode, MD, of the department of internal medicine at Dell. “The interprofessional team members know their roles and have shared expectations because they have a common understanding of the protocol.” It’s okay to deviate from the protocol, he said, as long as the language exists to communicate these deviations.
“Maybe the approach is more important than the actual content,” he said.
What Dr. Brode and Dr. Busch described was in large part a fine-tuning of communication – being available to communicate in real time and being aware of when certain specialists should be contacted – for instance, to determine at what oxygenation level internal medicine staff should get in touch with the pulmonary–critical care team.
Dr. Brode said that the groundwork is laid for productive meetings, with agendas announced ahead of time and readings assigned and presenters ready with near-finished products at meeting time, “with a clear path for operationalizing it.”
“We don’t want people kind of riffing off the top of their heads,” he said.
Committee members are encouraged to be as specific as possible when giving input into COVID-19 care decisions, he said.
“We’re so used to dealing with uncertainty, but that doesn’t really help when we’re trying to make tough decisions,” Dr. Brode said. They might be asked, “What are you going to write in your consult note template?” or “It’s 1:00 a.m. and your intern’s panicked and calling you – what are you going to tell them to do over the phone?”
The recommendations have to go into writing and are incorporated into the electronic medical record, a process that required some workarounds, he said. He also noted that the committee learned early on that they should assume that no one reads the e-mails – especially after being off for a period of time – so they likely won’t digest updates on an email-by-email basis.
“We quickly learned,” Dr. Brode said, “that this information needs to live on a Web site or [be] linked to the most up-to-date version in a cloud-sharing platform.”
In a question-and-answer discussion, session viewers expressed enthusiasm for the presenters’ one-page summary of protocols – much more, they said, and it could feel overwhelming.
Dr. Busch and Dr. Brode were asked how standardized order sets for COVID patients could be justified without comparison to a control group that didn’t use the standard order set.
Dr. Busch responded that, while there was no controlled trial, the order sets they use have evolved based on experience.
“At the beginning, we were following every inflammatory marker known to mankind, and then we realized as we gained more experience with COVID and COVID patients that some of those markers were not really informing any of our clinical decisions,” she said. “Obviously, as literature comes out we may reevaluate what goes into that standard order set and how frequently we follow labs.”
Dr. Brode said the context – a pandemic – has to be considered.
“In an ideal world, we could show that the intervention is superior through a randomized fashion with a control group, but really our thought process behind it is just, what is the default?” he said. “I looked at the order sets [as] not that they’re going to be dictating care, but it’s really like the guardrails of what’s reasonable. And when you’re in the middle of a surge, what is usually reasonable and easiest is what is going to be done.”
Dr. Busch and Dr. Brode reported no relevant financial relationships.
As hospitalists and other physicians at the University of Texas at Austin considered how to treat COVID-19 patients in the early weeks of the pandemic, one question they had to consider was: What about convalescent plasma?
All they had to go on were small case series in Ebola, SARS, and MERS and a few small, nonrandomized COVID-19 studies showing a possible benefit and minimal risk, but the evidence was only “toward the middle or bottom” of the evidence pyramid, said Johanna Busch, MD, of the department of internal medicine at Dell Medical Center at the university.
The center’s COVID-19 committee asked a few of its members – infectious disease and internal medicine physicians – to analyze the literature and other factors. In the end, the committee – which meets regularly and also includes pulmonology–critical care experts, nursing experts, and others – recommended using convalescent plasma because of the evidence and the available supply. But in subsequent meetings, as the pandemic surged in the South and the supply dwindled, the committee changed its recommendation for convalescent plasma to more limited use, she said during the virtual annual meeting of the Society of Hospital Medicine.
“It’s all about teamwork,” said W. Michael Brode, MD, of the department of internal medicine at Dell. “The interprofessional team members know their roles and have shared expectations because they have a common understanding of the protocol.” It’s okay to deviate from the protocol, he said, as long as the language exists to communicate these deviations.
“Maybe the approach is more important than the actual content,” he said.
What Dr. Brode and Dr. Busch described was in large part a fine-tuning of communication – being available to communicate in real time and being aware of when certain specialists should be contacted – for instance, to determine at what oxygenation level internal medicine staff should get in touch with the pulmonary–critical care team.
Dr. Brode said that the groundwork is laid for productive meetings, with agendas announced ahead of time and readings assigned and presenters ready with near-finished products at meeting time, “with a clear path for operationalizing it.”
“We don’t want people kind of riffing off the top of their heads,” he said.
Committee members are encouraged to be as specific as possible when giving input into COVID-19 care decisions, he said.
“We’re so used to dealing with uncertainty, but that doesn’t really help when we’re trying to make tough decisions,” Dr. Brode said. They might be asked, “What are you going to write in your consult note template?” or “It’s 1:00 a.m. and your intern’s panicked and calling you – what are you going to tell them to do over the phone?”
The recommendations have to go into writing and are incorporated into the electronic medical record, a process that required some workarounds, he said. He also noted that the committee learned early on that they should assume that no one reads the e-mails – especially after being off for a period of time – so they likely won’t digest updates on an email-by-email basis.
“We quickly learned,” Dr. Brode said, “that this information needs to live on a Web site or [be] linked to the most up-to-date version in a cloud-sharing platform.”
In a question-and-answer discussion, session viewers expressed enthusiasm for the presenters’ one-page summary of protocols – much more, they said, and it could feel overwhelming.
Dr. Busch and Dr. Brode were asked how standardized order sets for COVID patients could be justified without comparison to a control group that didn’t use the standard order set.
Dr. Busch responded that, while there was no controlled trial, the order sets they use have evolved based on experience.
“At the beginning, we were following every inflammatory marker known to mankind, and then we realized as we gained more experience with COVID and COVID patients that some of those markers were not really informing any of our clinical decisions,” she said. “Obviously, as literature comes out we may reevaluate what goes into that standard order set and how frequently we follow labs.”
Dr. Brode said the context – a pandemic – has to be considered.
“In an ideal world, we could show that the intervention is superior through a randomized fashion with a control group, but really our thought process behind it is just, what is the default?” he said. “I looked at the order sets [as] not that they’re going to be dictating care, but it’s really like the guardrails of what’s reasonable. And when you’re in the middle of a surge, what is usually reasonable and easiest is what is going to be done.”
Dr. Busch and Dr. Brode reported no relevant financial relationships.
As hospitalists and other physicians at the University of Texas at Austin considered how to treat COVID-19 patients in the early weeks of the pandemic, one question they had to consider was: What about convalescent plasma?
All they had to go on were small case series in Ebola, SARS, and MERS and a few small, nonrandomized COVID-19 studies showing a possible benefit and minimal risk, but the evidence was only “toward the middle or bottom” of the evidence pyramid, said Johanna Busch, MD, of the department of internal medicine at Dell Medical Center at the university.
The center’s COVID-19 committee asked a few of its members – infectious disease and internal medicine physicians – to analyze the literature and other factors. In the end, the committee – which meets regularly and also includes pulmonology–critical care experts, nursing experts, and others – recommended using convalescent plasma because of the evidence and the available supply. But in subsequent meetings, as the pandemic surged in the South and the supply dwindled, the committee changed its recommendation for convalescent plasma to more limited use, she said during the virtual annual meeting of the Society of Hospital Medicine.
“It’s all about teamwork,” said W. Michael Brode, MD, of the department of internal medicine at Dell. “The interprofessional team members know their roles and have shared expectations because they have a common understanding of the protocol.” It’s okay to deviate from the protocol, he said, as long as the language exists to communicate these deviations.
“Maybe the approach is more important than the actual content,” he said.
What Dr. Brode and Dr. Busch described was in large part a fine-tuning of communication – being available to communicate in real time and being aware of when certain specialists should be contacted – for instance, to determine at what oxygenation level internal medicine staff should get in touch with the pulmonary–critical care team.
Dr. Brode said that the groundwork is laid for productive meetings, with agendas announced ahead of time and readings assigned and presenters ready with near-finished products at meeting time, “with a clear path for operationalizing it.”
“We don’t want people kind of riffing off the top of their heads,” he said.
Committee members are encouraged to be as specific as possible when giving input into COVID-19 care decisions, he said.
“We’re so used to dealing with uncertainty, but that doesn’t really help when we’re trying to make tough decisions,” Dr. Brode said. They might be asked, “What are you going to write in your consult note template?” or “It’s 1:00 a.m. and your intern’s panicked and calling you – what are you going to tell them to do over the phone?”
The recommendations have to go into writing and are incorporated into the electronic medical record, a process that required some workarounds, he said. He also noted that the committee learned early on that they should assume that no one reads the e-mails – especially after being off for a period of time – so they likely won’t digest updates on an email-by-email basis.
“We quickly learned,” Dr. Brode said, “that this information needs to live on a Web site or [be] linked to the most up-to-date version in a cloud-sharing platform.”
In a question-and-answer discussion, session viewers expressed enthusiasm for the presenters’ one-page summary of protocols – much more, they said, and it could feel overwhelming.
Dr. Busch and Dr. Brode were asked how standardized order sets for COVID patients could be justified without comparison to a control group that didn’t use the standard order set.
Dr. Busch responded that, while there was no controlled trial, the order sets they use have evolved based on experience.
“At the beginning, we were following every inflammatory marker known to mankind, and then we realized as we gained more experience with COVID and COVID patients that some of those markers were not really informing any of our clinical decisions,” she said. “Obviously, as literature comes out we may reevaluate what goes into that standard order set and how frequently we follow labs.”
Dr. Brode said the context – a pandemic – has to be considered.
“In an ideal world, we could show that the intervention is superior through a randomized fashion with a control group, but really our thought process behind it is just, what is the default?” he said. “I looked at the order sets [as] not that they’re going to be dictating care, but it’s really like the guardrails of what’s reasonable. And when you’re in the middle of a surge, what is usually reasonable and easiest is what is going to be done.”
Dr. Busch and Dr. Brode reported no relevant financial relationships.
FROM HM20 VIRTUAL
‘Doubling down’ on hydroxychloroquine QT prolongation in COVID-19
A new analysis from Michigan’s largest health system provides sobering verification of the risks for QT interval prolongation in COVID-19 patients treated with hydroxychloroquine and azithromycin (HCQ/AZM).
One in five patients (21%) had a corrected QT (QTc) interval of at least 500 msec, a value that increases the risk for torsade de pointes in the general population and at which cardiovascular leaders have suggested withholding HCQ/AZM in COVID-19 patients.
“One of the most striking findings was when we looked at the other drugs being administered to these patients; 61% were being administered drugs that had QT-prolonging effects concomitantly with the HCQ and AZM therapy. So they were inadvertently doubling down on the QT-prolonging effects of these drugs,” senior author David E. Haines, MD, director of the Heart Rhythm Center at William Beaumont Hospital, Royal Oak, Mich., said in an interview.
A total of 34 medications overlapped with HCQ/AZM therapy are known or suspected to increase the risk for torsade de pointes, a potentially life-threatening ventricular tachycardia. The most common of these were propofol coadministered in 123 patients, ondansetron in 114, dexmedetomidine in 54, haloperidol in 44, amiodarone in 43, and tramadol in 26.
“This speaks to the medical complexity of this patient population, but also suggests inadequate awareness of the QT-prolonging effects of many common medications,” the researchers say.
The study was published Aug. 5 in JACC Clinical Electrophysiology.
Both hydroxychloroquine and azithromycin increase the risk for QTc-interval prolongation by blocking the KCHN2-encoded hERG potassium channel. Several reports have linked the drugs to a triggering of QT prolongation in patients with COVID-19.
For the present study, Dr. Haines and colleagues examined data from 586 consecutive patients admitted with COVID-19 to the Beaumont Hospitals in Royal Oak and Troy, Mich., between March 13 and April 6. A baseline QTc interval was measured with 12-lead ECG prior to treatment initiation with hydroxychloroquine 400 mg twice daily for two doses, then 200 mg twice daily for 4 days, and azithromycin 500 mg once followed by 250 mg daily for 4 days.
Because of limited availability at the time, lead II ECG telemetry monitoring over the 5-day course of HCQ/AZM was recommended only in patients with baseline QTc intervals of at least 440 msec.
Patients without an interpretable baseline ECG or available telemetry/ECG monitoring for at least 1 day were also excluded, leaving 415 patients (mean age, 64 years; 45% female) in the study population. More than half (52%) were Black, 52% had hypertension, 30% had diabetes, and 14% had cancer.
As seen in previous studies, the QTc interval increased progressively and significantly after the administration of HCQ/AZM, from 443 msec to 473 msec.
The average time to maximum QTc was 2.9 days in a subset of 135 patients with QTc measurements prior to starting therapy and on days 1 through 5.
In multivariate analysis, independent predictors of a potentially hazardous QTc interval of at least 500 msec were:
- Age older than 65 years (odds ratio, 3.0; 95% confidence interval, 1.62-5.54).
- History of (OR, 4.65; 95% CI, 2.01-10.74).
- Admission of at least 1.5 mg/dL (OR, 2.22; 95% CI, 1.28-3.84).
- Peak troponin I level above 0.04 mg/mL (OR, 3.89; 95% CI, 2.22-6.83).
- Body mass index below 30 kg/m2 (OR for a BMI of 30 kg/m2 or higher, 0.45; 95% CI, 0.26-0.78).
Concomitant use of drugs with known risk for torsade de pointes was a significant risk factor in univariate analysis (OR, 1.73; P = .036), but fell out in the multivariate model.
No patients experienced high-grade arrhythmias during the study. In all, 112 of the 586 patients died during hospitalization, including 85 (21%) of the 415 study patients.
The change in QTc interval from baseline was greater in patients who died. Despite this, the only independent predictor of mortality was older age. One possible explanation is that the decision to monitor patients with baseline QTc intervals of at least 440 msec may have skewed the study population toward people with moderate or slightly long QTc intervals prior to the initiation of HCQ/AZM, Dr. Haines suggested. Monitoring and treatment duration were short, and clinicians also likely adjusted medications when excess QTc prolongation was observed.
Although it’s been months since data collection was completed in April, and the paper was written in record-breaking time, the study “is still very relevant because the drug is still out there,” observed Dr. Haines. “Even though it may not be used in as widespread a fashion as it had been when we first submitted the paper, it is still being used routinely by many hospitals and many practitioners.”
The use of hydroxychloroquine is “going through the roof” because of COVID-19, commented Dhanunjaya Lakkireddy, MD, medical director for the Kansas City Heart Rhythm Institute, HCA Midwest Health, Overland Park, Kan., who was not involved in the study.
“This study is very relevant, and I’m glad they shared their experience, and it’s pretty consistent with the data presented by other people. The question of whether hydroxychloroquine helps people with COVID is up for debate, but there is more evidence today that it is not as helpful as it was 3 months ago,” said Dr. Lakkireddy, who is also chair of the American College of Cardiology Electrophysiology Council.
He expressed concern for patients who may be taking HCQ with other medications that have QT-prolonging effects, and for the lack of long-term protocols in place for the drug.
In the coming weeks, however, the ACC and rheumatology leaders will be publishing an expert consensus statement that addresses key issues, such as how to best to use HCQ, maintenance HCQ, electrolyte monitoring, the optimal timing of electrocardiography and cardiac magnetic imaging, and symptoms to look for if cardiac involvement is suspected, Dr. Lakkireddy said.
Asked whether HCQ and AZM should be used in COVID-19 patients, Dr. Haines said in an interview that the “QT-prolonging effects are real, the arrhythmogenic potential is real, and the benefit to patients is nil or marginal. So I think that use of these drugs is appropriate and reasonable if it is done in a setting of a controlled trial, and I support that. But the routine use of these drugs probably is not warranted based on the data that we have available.”
Still, hydroxychloroquine continues to be dragged into the spotlight in recent days as an effective treatment for COVID-19, despite discredited research and the U.S. Food and Drug Administration’s June 15 revocation of its emergency-use authorization to allow use of HCQ and chloroquine to treat certain hospitalized COVID-19 patients.
“The unfortunate politicization of this issue has really muddied the waters because the general public doesn’t know what to believe or who to believe. The fact that treatment for a disease as serious as COVID should be modulated by political affiliation is just crazy to me,” said Dr. Haines. “We should be using the best science and taking careful observations, and whatever the recommendations derived from that should be uniformly adopted by everybody, irrespective of your political affiliation.”
Dr. Haines has received honoraria from Biosense Webster, Farapulse, and Sagentia, and is a consultant for Affera, Boston Scientific, Integer, Medtronic, Philips Healthcare, and Zoll. Dr. Lakkireddy has served as a consultant to Abbott, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
A version of this article originally appeared on Medscape.com.
A new analysis from Michigan’s largest health system provides sobering verification of the risks for QT interval prolongation in COVID-19 patients treated with hydroxychloroquine and azithromycin (HCQ/AZM).
One in five patients (21%) had a corrected QT (QTc) interval of at least 500 msec, a value that increases the risk for torsade de pointes in the general population and at which cardiovascular leaders have suggested withholding HCQ/AZM in COVID-19 patients.
“One of the most striking findings was when we looked at the other drugs being administered to these patients; 61% were being administered drugs that had QT-prolonging effects concomitantly with the HCQ and AZM therapy. So they were inadvertently doubling down on the QT-prolonging effects of these drugs,” senior author David E. Haines, MD, director of the Heart Rhythm Center at William Beaumont Hospital, Royal Oak, Mich., said in an interview.
A total of 34 medications overlapped with HCQ/AZM therapy are known or suspected to increase the risk for torsade de pointes, a potentially life-threatening ventricular tachycardia. The most common of these were propofol coadministered in 123 patients, ondansetron in 114, dexmedetomidine in 54, haloperidol in 44, amiodarone in 43, and tramadol in 26.
“This speaks to the medical complexity of this patient population, but also suggests inadequate awareness of the QT-prolonging effects of many common medications,” the researchers say.
The study was published Aug. 5 in JACC Clinical Electrophysiology.
Both hydroxychloroquine and azithromycin increase the risk for QTc-interval prolongation by blocking the KCHN2-encoded hERG potassium channel. Several reports have linked the drugs to a triggering of QT prolongation in patients with COVID-19.
For the present study, Dr. Haines and colleagues examined data from 586 consecutive patients admitted with COVID-19 to the Beaumont Hospitals in Royal Oak and Troy, Mich., between March 13 and April 6. A baseline QTc interval was measured with 12-lead ECG prior to treatment initiation with hydroxychloroquine 400 mg twice daily for two doses, then 200 mg twice daily for 4 days, and azithromycin 500 mg once followed by 250 mg daily for 4 days.
Because of limited availability at the time, lead II ECG telemetry monitoring over the 5-day course of HCQ/AZM was recommended only in patients with baseline QTc intervals of at least 440 msec.
Patients without an interpretable baseline ECG or available telemetry/ECG monitoring for at least 1 day were also excluded, leaving 415 patients (mean age, 64 years; 45% female) in the study population. More than half (52%) were Black, 52% had hypertension, 30% had diabetes, and 14% had cancer.
As seen in previous studies, the QTc interval increased progressively and significantly after the administration of HCQ/AZM, from 443 msec to 473 msec.
The average time to maximum QTc was 2.9 days in a subset of 135 patients with QTc measurements prior to starting therapy and on days 1 through 5.
In multivariate analysis, independent predictors of a potentially hazardous QTc interval of at least 500 msec were:
- Age older than 65 years (odds ratio, 3.0; 95% confidence interval, 1.62-5.54).
- History of (OR, 4.65; 95% CI, 2.01-10.74).
- Admission of at least 1.5 mg/dL (OR, 2.22; 95% CI, 1.28-3.84).
- Peak troponin I level above 0.04 mg/mL (OR, 3.89; 95% CI, 2.22-6.83).
- Body mass index below 30 kg/m2 (OR for a BMI of 30 kg/m2 or higher, 0.45; 95% CI, 0.26-0.78).
Concomitant use of drugs with known risk for torsade de pointes was a significant risk factor in univariate analysis (OR, 1.73; P = .036), but fell out in the multivariate model.
No patients experienced high-grade arrhythmias during the study. In all, 112 of the 586 patients died during hospitalization, including 85 (21%) of the 415 study patients.
The change in QTc interval from baseline was greater in patients who died. Despite this, the only independent predictor of mortality was older age. One possible explanation is that the decision to monitor patients with baseline QTc intervals of at least 440 msec may have skewed the study population toward people with moderate or slightly long QTc intervals prior to the initiation of HCQ/AZM, Dr. Haines suggested. Monitoring and treatment duration were short, and clinicians also likely adjusted medications when excess QTc prolongation was observed.
Although it’s been months since data collection was completed in April, and the paper was written in record-breaking time, the study “is still very relevant because the drug is still out there,” observed Dr. Haines. “Even though it may not be used in as widespread a fashion as it had been when we first submitted the paper, it is still being used routinely by many hospitals and many practitioners.”
The use of hydroxychloroquine is “going through the roof” because of COVID-19, commented Dhanunjaya Lakkireddy, MD, medical director for the Kansas City Heart Rhythm Institute, HCA Midwest Health, Overland Park, Kan., who was not involved in the study.
“This study is very relevant, and I’m glad they shared their experience, and it’s pretty consistent with the data presented by other people. The question of whether hydroxychloroquine helps people with COVID is up for debate, but there is more evidence today that it is not as helpful as it was 3 months ago,” said Dr. Lakkireddy, who is also chair of the American College of Cardiology Electrophysiology Council.
He expressed concern for patients who may be taking HCQ with other medications that have QT-prolonging effects, and for the lack of long-term protocols in place for the drug.
In the coming weeks, however, the ACC and rheumatology leaders will be publishing an expert consensus statement that addresses key issues, such as how to best to use HCQ, maintenance HCQ, electrolyte monitoring, the optimal timing of electrocardiography and cardiac magnetic imaging, and symptoms to look for if cardiac involvement is suspected, Dr. Lakkireddy said.
Asked whether HCQ and AZM should be used in COVID-19 patients, Dr. Haines said in an interview that the “QT-prolonging effects are real, the arrhythmogenic potential is real, and the benefit to patients is nil or marginal. So I think that use of these drugs is appropriate and reasonable if it is done in a setting of a controlled trial, and I support that. But the routine use of these drugs probably is not warranted based on the data that we have available.”
Still, hydroxychloroquine continues to be dragged into the spotlight in recent days as an effective treatment for COVID-19, despite discredited research and the U.S. Food and Drug Administration’s June 15 revocation of its emergency-use authorization to allow use of HCQ and chloroquine to treat certain hospitalized COVID-19 patients.
“The unfortunate politicization of this issue has really muddied the waters because the general public doesn’t know what to believe or who to believe. The fact that treatment for a disease as serious as COVID should be modulated by political affiliation is just crazy to me,” said Dr. Haines. “We should be using the best science and taking careful observations, and whatever the recommendations derived from that should be uniformly adopted by everybody, irrespective of your political affiliation.”
Dr. Haines has received honoraria from Biosense Webster, Farapulse, and Sagentia, and is a consultant for Affera, Boston Scientific, Integer, Medtronic, Philips Healthcare, and Zoll. Dr. Lakkireddy has served as a consultant to Abbott, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
A version of this article originally appeared on Medscape.com.
A new analysis from Michigan’s largest health system provides sobering verification of the risks for QT interval prolongation in COVID-19 patients treated with hydroxychloroquine and azithromycin (HCQ/AZM).
One in five patients (21%) had a corrected QT (QTc) interval of at least 500 msec, a value that increases the risk for torsade de pointes in the general population and at which cardiovascular leaders have suggested withholding HCQ/AZM in COVID-19 patients.
“One of the most striking findings was when we looked at the other drugs being administered to these patients; 61% were being administered drugs that had QT-prolonging effects concomitantly with the HCQ and AZM therapy. So they were inadvertently doubling down on the QT-prolonging effects of these drugs,” senior author David E. Haines, MD, director of the Heart Rhythm Center at William Beaumont Hospital, Royal Oak, Mich., said in an interview.
A total of 34 medications overlapped with HCQ/AZM therapy are known or suspected to increase the risk for torsade de pointes, a potentially life-threatening ventricular tachycardia. The most common of these were propofol coadministered in 123 patients, ondansetron in 114, dexmedetomidine in 54, haloperidol in 44, amiodarone in 43, and tramadol in 26.
“This speaks to the medical complexity of this patient population, but also suggests inadequate awareness of the QT-prolonging effects of many common medications,” the researchers say.
The study was published Aug. 5 in JACC Clinical Electrophysiology.
Both hydroxychloroquine and azithromycin increase the risk for QTc-interval prolongation by blocking the KCHN2-encoded hERG potassium channel. Several reports have linked the drugs to a triggering of QT prolongation in patients with COVID-19.
For the present study, Dr. Haines and colleagues examined data from 586 consecutive patients admitted with COVID-19 to the Beaumont Hospitals in Royal Oak and Troy, Mich., between March 13 and April 6. A baseline QTc interval was measured with 12-lead ECG prior to treatment initiation with hydroxychloroquine 400 mg twice daily for two doses, then 200 mg twice daily for 4 days, and azithromycin 500 mg once followed by 250 mg daily for 4 days.
Because of limited availability at the time, lead II ECG telemetry monitoring over the 5-day course of HCQ/AZM was recommended only in patients with baseline QTc intervals of at least 440 msec.
Patients without an interpretable baseline ECG or available telemetry/ECG monitoring for at least 1 day were also excluded, leaving 415 patients (mean age, 64 years; 45% female) in the study population. More than half (52%) were Black, 52% had hypertension, 30% had diabetes, and 14% had cancer.
As seen in previous studies, the QTc interval increased progressively and significantly after the administration of HCQ/AZM, from 443 msec to 473 msec.
The average time to maximum QTc was 2.9 days in a subset of 135 patients with QTc measurements prior to starting therapy and on days 1 through 5.
In multivariate analysis, independent predictors of a potentially hazardous QTc interval of at least 500 msec were:
- Age older than 65 years (odds ratio, 3.0; 95% confidence interval, 1.62-5.54).
- History of (OR, 4.65; 95% CI, 2.01-10.74).
- Admission of at least 1.5 mg/dL (OR, 2.22; 95% CI, 1.28-3.84).
- Peak troponin I level above 0.04 mg/mL (OR, 3.89; 95% CI, 2.22-6.83).
- Body mass index below 30 kg/m2 (OR for a BMI of 30 kg/m2 or higher, 0.45; 95% CI, 0.26-0.78).
Concomitant use of drugs with known risk for torsade de pointes was a significant risk factor in univariate analysis (OR, 1.73; P = .036), but fell out in the multivariate model.
No patients experienced high-grade arrhythmias during the study. In all, 112 of the 586 patients died during hospitalization, including 85 (21%) of the 415 study patients.
The change in QTc interval from baseline was greater in patients who died. Despite this, the only independent predictor of mortality was older age. One possible explanation is that the decision to monitor patients with baseline QTc intervals of at least 440 msec may have skewed the study population toward people with moderate or slightly long QTc intervals prior to the initiation of HCQ/AZM, Dr. Haines suggested. Monitoring and treatment duration were short, and clinicians also likely adjusted medications when excess QTc prolongation was observed.
Although it’s been months since data collection was completed in April, and the paper was written in record-breaking time, the study “is still very relevant because the drug is still out there,” observed Dr. Haines. “Even though it may not be used in as widespread a fashion as it had been when we first submitted the paper, it is still being used routinely by many hospitals and many practitioners.”
The use of hydroxychloroquine is “going through the roof” because of COVID-19, commented Dhanunjaya Lakkireddy, MD, medical director for the Kansas City Heart Rhythm Institute, HCA Midwest Health, Overland Park, Kan., who was not involved in the study.
“This study is very relevant, and I’m glad they shared their experience, and it’s pretty consistent with the data presented by other people. The question of whether hydroxychloroquine helps people with COVID is up for debate, but there is more evidence today that it is not as helpful as it was 3 months ago,” said Dr. Lakkireddy, who is also chair of the American College of Cardiology Electrophysiology Council.
He expressed concern for patients who may be taking HCQ with other medications that have QT-prolonging effects, and for the lack of long-term protocols in place for the drug.
In the coming weeks, however, the ACC and rheumatology leaders will be publishing an expert consensus statement that addresses key issues, such as how to best to use HCQ, maintenance HCQ, electrolyte monitoring, the optimal timing of electrocardiography and cardiac magnetic imaging, and symptoms to look for if cardiac involvement is suspected, Dr. Lakkireddy said.
Asked whether HCQ and AZM should be used in COVID-19 patients, Dr. Haines said in an interview that the “QT-prolonging effects are real, the arrhythmogenic potential is real, and the benefit to patients is nil or marginal. So I think that use of these drugs is appropriate and reasonable if it is done in a setting of a controlled trial, and I support that. But the routine use of these drugs probably is not warranted based on the data that we have available.”
Still, hydroxychloroquine continues to be dragged into the spotlight in recent days as an effective treatment for COVID-19, despite discredited research and the U.S. Food and Drug Administration’s June 15 revocation of its emergency-use authorization to allow use of HCQ and chloroquine to treat certain hospitalized COVID-19 patients.
“The unfortunate politicization of this issue has really muddied the waters because the general public doesn’t know what to believe or who to believe. The fact that treatment for a disease as serious as COVID should be modulated by political affiliation is just crazy to me,” said Dr. Haines. “We should be using the best science and taking careful observations, and whatever the recommendations derived from that should be uniformly adopted by everybody, irrespective of your political affiliation.”
Dr. Haines has received honoraria from Biosense Webster, Farapulse, and Sagentia, and is a consultant for Affera, Boston Scientific, Integer, Medtronic, Philips Healthcare, and Zoll. Dr. Lakkireddy has served as a consultant to Abbott, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
A version of this article originally appeared on Medscape.com.









