User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Pandemic-related stress rising among ICU clinicians
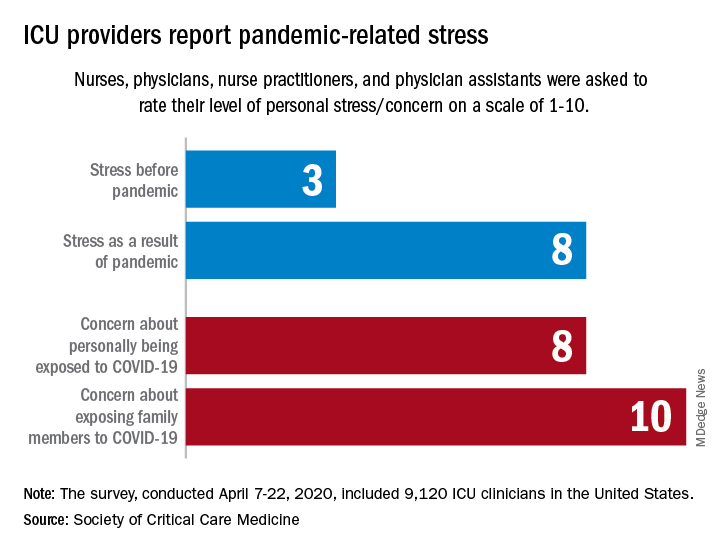
They are worried about getting infected, and they are even more worried about infecting family members, according to the Society for Critical Care Medicine, which surveyed members of four professional organizations – the American Association of Critical-Care Nurses, American College of Chest Physicians, American Thoracic Society, and the SCCM – April 7-22, 2020.
Four items in the survey assessed respondents’ level of stress or concern on a scale of 1-10:
- Personal stress before the COVID-19 pandemic.
- Personal stress as a result of COVID-19 pandemic.
- Concern about personally being exposed to COVID-19.
- Concern about exposing family members to COVID-19.
Personal stress rose from a median of 3 before the pandemic to a current 8, a level that was equaled by personal concerns about being exposed and surpassed (10) by concerns about exposing family members, the SCCM reported in a blog post.
Most of the respondents “are taking special measures to limit the potential spread of the virus to their loved ones, including implementing a decontamination routine before interacting with families,” the SCCM wrote.
The most common strategy, employed by 72% of ICU clinicians, is changing clothes before/after work. Showering before joining family was mentioned by 64% of providers, followed by limiting contact until decontamination (57%) and using hand sanitizer before entering home (51%), the SCCM said.
More extreme measures included self-isolating within their homes (16%) and staying in alternative housing away from their families (12%), the SCCM said, based on data for 9,120 clinicians in the United States.
Most of the respondents (88%) reported having cared for a patient with confirmed or presumed COVID-19. Nurses made up the majority (91%) of the sample, which also included nurse practitioners and physician assistants (4.5%) and physicians (2.9%), as well as smaller numbers of respiratory therapists, pharmacists, and emergency medicine flight personnel.
The results of the survey “underline the personal sacrifices of critical care clinicians during the COVID-19 response and suggest the need to help them proactively manage stress,” the SCCM wrote.

They are worried about getting infected, and they are even more worried about infecting family members, according to the Society for Critical Care Medicine, which surveyed members of four professional organizations – the American Association of Critical-Care Nurses, American College of Chest Physicians, American Thoracic Society, and the SCCM – April 7-22, 2020.
Four items in the survey assessed respondents’ level of stress or concern on a scale of 1-10:
- Personal stress before the COVID-19 pandemic.
- Personal stress as a result of COVID-19 pandemic.
- Concern about personally being exposed to COVID-19.
- Concern about exposing family members to COVID-19.
Personal stress rose from a median of 3 before the pandemic to a current 8, a level that was equaled by personal concerns about being exposed and surpassed (10) by concerns about exposing family members, the SCCM reported in a blog post.
Most of the respondents “are taking special measures to limit the potential spread of the virus to their loved ones, including implementing a decontamination routine before interacting with families,” the SCCM wrote.
The most common strategy, employed by 72% of ICU clinicians, is changing clothes before/after work. Showering before joining family was mentioned by 64% of providers, followed by limiting contact until decontamination (57%) and using hand sanitizer before entering home (51%), the SCCM said.
More extreme measures included self-isolating within their homes (16%) and staying in alternative housing away from their families (12%), the SCCM said, based on data for 9,120 clinicians in the United States.
Most of the respondents (88%) reported having cared for a patient with confirmed or presumed COVID-19. Nurses made up the majority (91%) of the sample, which also included nurse practitioners and physician assistants (4.5%) and physicians (2.9%), as well as smaller numbers of respiratory therapists, pharmacists, and emergency medicine flight personnel.
The results of the survey “underline the personal sacrifices of critical care clinicians during the COVID-19 response and suggest the need to help them proactively manage stress,” the SCCM wrote.

They are worried about getting infected, and they are even more worried about infecting family members, according to the Society for Critical Care Medicine, which surveyed members of four professional organizations – the American Association of Critical-Care Nurses, American College of Chest Physicians, American Thoracic Society, and the SCCM – April 7-22, 2020.
Four items in the survey assessed respondents’ level of stress or concern on a scale of 1-10:
- Personal stress before the COVID-19 pandemic.
- Personal stress as a result of COVID-19 pandemic.
- Concern about personally being exposed to COVID-19.
- Concern about exposing family members to COVID-19.
Personal stress rose from a median of 3 before the pandemic to a current 8, a level that was equaled by personal concerns about being exposed and surpassed (10) by concerns about exposing family members, the SCCM reported in a blog post.
Most of the respondents “are taking special measures to limit the potential spread of the virus to their loved ones, including implementing a decontamination routine before interacting with families,” the SCCM wrote.
The most common strategy, employed by 72% of ICU clinicians, is changing clothes before/after work. Showering before joining family was mentioned by 64% of providers, followed by limiting contact until decontamination (57%) and using hand sanitizer before entering home (51%), the SCCM said.
More extreme measures included self-isolating within their homes (16%) and staying in alternative housing away from their families (12%), the SCCM said, based on data for 9,120 clinicians in the United States.
Most of the respondents (88%) reported having cared for a patient with confirmed or presumed COVID-19. Nurses made up the majority (91%) of the sample, which also included nurse practitioners and physician assistants (4.5%) and physicians (2.9%), as well as smaller numbers of respiratory therapists, pharmacists, and emergency medicine flight personnel.
The results of the survey “underline the personal sacrifices of critical care clinicians during the COVID-19 response and suggest the need to help them proactively manage stress,” the SCCM wrote.
COVID-19: Eight steps for getting ready to see patients again
After COVID-19 hit the Denver area, internist Jean Kutner, MD, and her clinical colleagues drastically reduced the number of patients they saw and kept a minimum number of people in the office. A small team sees patients who still require in-person visits on one side of the clinic; on the other side, another team conducts clinic-based telehealth visits. A rotating schedule allows for social distancing.
The rest of the practice’s physicians are home, conducting more virtual visits.
Dr. Kutner said she is looking forward to reopening her practice completely at some point. She said she realizes that the practice probably won’t be exactly the same as before.
“We have to embrace the fact that the way we practice medicine has fundamentally changed,” said Dr. Kutner, professor of medicine at the University of Colorado at Denver, Aurora, and incoming president of the Society of General Internal Medicine. She anticipates keeping many of these changes in place for the foreseeable future.
Nearly half of 2,600 primary care physicians who responded to a recent national survey said they were struggling to remain open during the crisis. Most have had to limit wellness/chronic-disease management visits, and nearly half reported that physicians or staff were out sick. Layoffs, furloughs, and reduced hours are commonplace; some practices were forced to shut down entirely.
Social distancing helps reduce the rates of hospitalizations and deaths.
For example, remote monitoring capabilities have reduced the need for in-person checks of vital signs, such as respiratory rate oxygenation, blood glucose levels, and heart rate. “We can’t go back,” she said.
Dr. Kutner sees the pandemic as an opportunity to innovate, to think about how primary practices can best utilize their resources, face-to-face time with patients, and when and how to best leverage virtual visits in a way that improves patient health. The goal, of course, is to meet the needs of the patients while keeping everyone safe.
Like many physicians in private practice, Dr. Kutner is concerned about revenue. She hopes the Centers for Medicare & Medicaid Services makes its temporary waivers permanent.
What you need to consider when planning to reopen your office
Physicians say their post-COVID-19 practices will look very different from their prepandemic practices. Many plan to maintain guidelines, such as those from the AAFP, long after the pandemic has peaked.
If you are starting to think about reopening, here are some major considerations.
1. Develop procedures and practices that will keep your patients and staff safe.
“When we return, the first thing we need to do is limit the number of patients in the waiting room,” said Clinton Coleman, MD, who practices internal medicine and nephrology in Teaneck, N.J. “No one is comfortable in a waiting room any longer,” said Dr. Coleman, chief of internal medicine at Holy Name Medical Center in Teaneck.
Careful planning is required to resume in-person care of patients requiring non-COVID-19 care, as well as all aspects of care, according to the CMS. Adequate staff, testing, supplies, and support services, such as pathology services, are just a few considerations. The CMS recommends that physicians “evaluate the necessity of the care based on clinical needs. Providers should prioritize surgical/procedural care and high-complexity chronic disease management; however, select preventive services may also be highly necessary.”
The American Medical Association recently unveiled a checklist for reopening. One key recommendation was for practices to select a date for reopening the office, ideally preceded by a “soft” or incremental reopening to ensure that new procedures are working. The AMA also recommends opening incrementally, continuing telehealth while also inviting patients back into the office.
2. Figure out how to safely see patients, particularly in your waiting areas and common spaces.
Logistic factors, such as managing patient flow, will change. Waiting rooms will be emptier; in some locations, patients may be asked to wait in their cars until an exam room is available.
The AMA also suggests limiting nonpatient visitors by posting the practice’s policy at the entrance and on the practice’s website. If service calls for repairs are needed, have those visitors come outside of normal operating hours.
Commonly shared objects such magazines or toys in pediatric offices will likely disappear. Wipes, hand sanitizers, and the wearing of masks will become even more commonplace. Those who suspect they’re ill or who have respiratory symptoms may be relegated to specific “sick visit” appointment times or taken to designated exam rooms, which will be thoroughly sanitized between patients.
3. Prepare for routine screening of staff and other facility workers.
According to recent CMS guidelines, you and your staff will need to undergo routine screening, as will others who work in the facility (housekeeping, delivery personnel, and anyone else who enters the area). This may mean regularly stocking screening tests and setting guidelines for what to do if one of your staff tests positive.
You may need to hire temporary workers if your staff tests positive. The CDC recommends at the very least understanding the minimum staffing requirements to ensure good patient care and a safe work environment. Consider adjusting staff schedules and rotating clinical personnel to positions that support patient care activities. You may also want to look into cross-training your office staff so that they can fill in or help out with each other’s responsibilities if one or more persons are ill.
Dr. Kutner is on board with these changes. “We don’t want to get rid of social distancing right away, because it will give us a new spike in cases – how do we figure out patient flow while honoring that?”
4. Develop a strategy for triaging and caring for a potential backlog of patients.
“Many of my partners are scared right now because they have no income except for emergencies,” said Andrew Gonzalez, MD, JD, MPH, a vascular surgeon and assistant professor of surgery at Indiana University, Indianapolis. Almost all nonemergency surgery has been put on hold.
“If we don’t operate, the practice makes no money,” he said. He thinks revenue will continue to be a problem as long as patients fear in-person consultations or undergoing surgery for nonacute problems such as hernias.
As restrictions ease, most physicians will face an enormous backlog of patients and will need to find new ways of triaging the most serious cases, he says. Telehealth will help, but Dr. Gonzalez predicts many of his colleagues will be working longer hours and on weekends to catch up. “Physicians are going to have to really think about ways of optimizing their time and workflow to be very efficient, because the backlog is going to prodigious.”
5. Anticipate changes in patient expectations.
This may entail your reconsidering tests and procedures you previously performed and considering developing new sources for some services, phasing some others out, and revising your current approach. It will most likely also mean that you make telemedicine and televisits a greater part of your practice.
Carolyn Kaloostian, MD, a family medicine and geriatric practitioner in Los Angeles, points to increased reliance on community agencies for conducting common office-based procedures, such as performing blood tests and taking ECGs and x-rays. “A lot of patients are using telemedicine or telephone visits and get the lab work or x-rays somewhere that’s less congested,” she said. To become sustainable, many of these changes will hinge on economics – whether and how they are reimbursed.
The pandemic will leave lasting effects in our health care delivery, according to Dr. Kaloostian. She is sure many of her colleagues’ and patients’ current experiences will be infused into future care. “I can’t say we’ll ever be back to normal, necessarily.”
Even if the CMS rolls back its telehealth waivers, some physicians, like Dr. Coleman, plan to continue using the technology extensively. He’s confident about the level of care he’s currently providing patients in his practice. It allows him to better manage many low-income patients who can’t access his office regularly. Not only does splitting his time between the clinic and telehealth allow him to be more available for more patients, he says it also empowers patients to take better care of themselves.
6. Consider a new way to conduct “check-in visits.”
One thing that will likely go by the wayside are “check-in” visits, or so-called “social visits,” those interval appointments that can just as easily be completed virtually. “Patients are going to ask why they need to drive 3 hours so you can tell them their incision looks fine from an operation you did 5 years ago,” Dr. Gonzalez said.
He’s concerned that some people will remain so fearful of the health care system that a formerly busy practice may see the pendulum swing in the opposite direction. If an aneurysm patient skips a visit, that person may also decide not to undergo a CT scan – and something preventable will be missed. “Not everybody has the option to stay away until they feel comfortable. They’re basically playing hot potato. And at some point, the music’s going to stop,” Dr. Gonzalez said.
The pandemic has prompted some very honest conversations with his patients about what truly needs to get done and what may be optional. “Everyone has now become a hyper-rational user of health care,” he said.
7. If you haven’t yet, consider becoming more involved with technology.
In addition to greater use of telehealth, Dr. Kaloostian, assistant professor of clinical family medicine at the University of Southern California, Los Angeles, foresees continued reliance upon technology such as smartphone apps that connect with a user’s smartwatch. This allows for more proactive, remote monitoring.
“For example, any time a patient is having recurrent nighttime trips to the bathroom, I’ll get pinged and know that,” she explained. It means she can reach out and ask about any changes before a fall occurs or a condition worsens. “It provides reassurance to the provider and to the patient that you’re doing all you can to keep an eye on them from afar.”
8. Update or reformulate your business plans.
Some physicians in smaller practices may have to temporarily or permanently rethink their situation. Those who have struggled or who have closed down and are considering reopening need to update their business plans. It may be safer economically to become part of a bigger group that is affiliated with an academic center or join a larger health care system that has more funds or resources.
In addition, Dr. Kaloostian suggests that primary care physicians become more flexible in the short term, perhaps working part time in an urgent care clinic or larger organization to gain additional sources of revenue until their own practice finances pick back up.
For offices that reopen, the AMA recommends contacting medical malpractice insurance carriers to check on possible liability concerns. Congress has provided certain protections for clinicians during this time, but malpractice carriers may have more information and may offer more coverage.
Dr. Coleman said a hybrid model of fewer in-person and more telehealth visits “will allow me to practice in a different way.” If the CMS reimposes prior restrictions, reimbursement may be affected initially, but that will likely change once insurers see the increased cost-effectiveness of this approach. Patients with minor complaints, those who need to have medications refilled, and patients with chronic diseases that need managing won’t have to deal with crowded waiting rooms, and it will help mitigate problems with infection control.
If there’s any upside to the pandemic, it’s an increase in attention given to advanced care planning, said Dr. Kutner. It’s something she hopes continues after everyone stops being in crisis mode. “We’re realizing how important it is to have these conversations and document people’s goals and values and code status,” she said.
Are offices likely to open soon?
An assumption that may or may not be valid is that a practice will remain viable and can return to former capacity. Prior to passage of the CARES Act on March 27, a survey from Kareo, a company in Irvine, California, that makes a technology platform for independent physician practices, found that 9% of respondents reported practice closures. Many more reported concern about potential closures as patient office visits plummet because of stay-at-home orders and other concerns.
By mid-April, a survey from the Primary Care Collaborative and the Larry A. Green Center found that 42% of practices had experienced layoffs and had furloughed staff. Most (85%) have seen dramatic decreases in patient volume.
“Reopening the economy or loosening physical distancing restrictions will be difficult when 20% of primary care practices predict closure within 4 weeks,” the survey concluded.
For the practices and the doctors who make it through this, we’re going to probably be better, stronger, and more efficient, Dr. Gonzalez predicts. This shock has uncovered a lot of weaknesses in the American health care system that doctors have known about and have been complaining about for a long time. It will take an open mind and lots of continued flexibility on the part of physicians, hospitals, health care systems, and the government for these changes to stick.
A version of this article originally appeared on Medscape.com.
After COVID-19 hit the Denver area, internist Jean Kutner, MD, and her clinical colleagues drastically reduced the number of patients they saw and kept a minimum number of people in the office. A small team sees patients who still require in-person visits on one side of the clinic; on the other side, another team conducts clinic-based telehealth visits. A rotating schedule allows for social distancing.
The rest of the practice’s physicians are home, conducting more virtual visits.
Dr. Kutner said she is looking forward to reopening her practice completely at some point. She said she realizes that the practice probably won’t be exactly the same as before.
“We have to embrace the fact that the way we practice medicine has fundamentally changed,” said Dr. Kutner, professor of medicine at the University of Colorado at Denver, Aurora, and incoming president of the Society of General Internal Medicine. She anticipates keeping many of these changes in place for the foreseeable future.
Nearly half of 2,600 primary care physicians who responded to a recent national survey said they were struggling to remain open during the crisis. Most have had to limit wellness/chronic-disease management visits, and nearly half reported that physicians or staff were out sick. Layoffs, furloughs, and reduced hours are commonplace; some practices were forced to shut down entirely.
Social distancing helps reduce the rates of hospitalizations and deaths.
For example, remote monitoring capabilities have reduced the need for in-person checks of vital signs, such as respiratory rate oxygenation, blood glucose levels, and heart rate. “We can’t go back,” she said.
Dr. Kutner sees the pandemic as an opportunity to innovate, to think about how primary practices can best utilize their resources, face-to-face time with patients, and when and how to best leverage virtual visits in a way that improves patient health. The goal, of course, is to meet the needs of the patients while keeping everyone safe.
Like many physicians in private practice, Dr. Kutner is concerned about revenue. She hopes the Centers for Medicare & Medicaid Services makes its temporary waivers permanent.
What you need to consider when planning to reopen your office
Physicians say their post-COVID-19 practices will look very different from their prepandemic practices. Many plan to maintain guidelines, such as those from the AAFP, long after the pandemic has peaked.
If you are starting to think about reopening, here are some major considerations.
1. Develop procedures and practices that will keep your patients and staff safe.
“When we return, the first thing we need to do is limit the number of patients in the waiting room,” said Clinton Coleman, MD, who practices internal medicine and nephrology in Teaneck, N.J. “No one is comfortable in a waiting room any longer,” said Dr. Coleman, chief of internal medicine at Holy Name Medical Center in Teaneck.
Careful planning is required to resume in-person care of patients requiring non-COVID-19 care, as well as all aspects of care, according to the CMS. Adequate staff, testing, supplies, and support services, such as pathology services, are just a few considerations. The CMS recommends that physicians “evaluate the necessity of the care based on clinical needs. Providers should prioritize surgical/procedural care and high-complexity chronic disease management; however, select preventive services may also be highly necessary.”
The American Medical Association recently unveiled a checklist for reopening. One key recommendation was for practices to select a date for reopening the office, ideally preceded by a “soft” or incremental reopening to ensure that new procedures are working. The AMA also recommends opening incrementally, continuing telehealth while also inviting patients back into the office.
2. Figure out how to safely see patients, particularly in your waiting areas and common spaces.
Logistic factors, such as managing patient flow, will change. Waiting rooms will be emptier; in some locations, patients may be asked to wait in their cars until an exam room is available.
The AMA also suggests limiting nonpatient visitors by posting the practice’s policy at the entrance and on the practice’s website. If service calls for repairs are needed, have those visitors come outside of normal operating hours.
Commonly shared objects such magazines or toys in pediatric offices will likely disappear. Wipes, hand sanitizers, and the wearing of masks will become even more commonplace. Those who suspect they’re ill or who have respiratory symptoms may be relegated to specific “sick visit” appointment times or taken to designated exam rooms, which will be thoroughly sanitized between patients.
3. Prepare for routine screening of staff and other facility workers.
According to recent CMS guidelines, you and your staff will need to undergo routine screening, as will others who work in the facility (housekeeping, delivery personnel, and anyone else who enters the area). This may mean regularly stocking screening tests and setting guidelines for what to do if one of your staff tests positive.
You may need to hire temporary workers if your staff tests positive. The CDC recommends at the very least understanding the minimum staffing requirements to ensure good patient care and a safe work environment. Consider adjusting staff schedules and rotating clinical personnel to positions that support patient care activities. You may also want to look into cross-training your office staff so that they can fill in or help out with each other’s responsibilities if one or more persons are ill.
Dr. Kutner is on board with these changes. “We don’t want to get rid of social distancing right away, because it will give us a new spike in cases – how do we figure out patient flow while honoring that?”
4. Develop a strategy for triaging and caring for a potential backlog of patients.
“Many of my partners are scared right now because they have no income except for emergencies,” said Andrew Gonzalez, MD, JD, MPH, a vascular surgeon and assistant professor of surgery at Indiana University, Indianapolis. Almost all nonemergency surgery has been put on hold.
“If we don’t operate, the practice makes no money,” he said. He thinks revenue will continue to be a problem as long as patients fear in-person consultations or undergoing surgery for nonacute problems such as hernias.
As restrictions ease, most physicians will face an enormous backlog of patients and will need to find new ways of triaging the most serious cases, he says. Telehealth will help, but Dr. Gonzalez predicts many of his colleagues will be working longer hours and on weekends to catch up. “Physicians are going to have to really think about ways of optimizing their time and workflow to be very efficient, because the backlog is going to prodigious.”
5. Anticipate changes in patient expectations.
This may entail your reconsidering tests and procedures you previously performed and considering developing new sources for some services, phasing some others out, and revising your current approach. It will most likely also mean that you make telemedicine and televisits a greater part of your practice.
Carolyn Kaloostian, MD, a family medicine and geriatric practitioner in Los Angeles, points to increased reliance on community agencies for conducting common office-based procedures, such as performing blood tests and taking ECGs and x-rays. “A lot of patients are using telemedicine or telephone visits and get the lab work or x-rays somewhere that’s less congested,” she said. To become sustainable, many of these changes will hinge on economics – whether and how they are reimbursed.
The pandemic will leave lasting effects in our health care delivery, according to Dr. Kaloostian. She is sure many of her colleagues’ and patients’ current experiences will be infused into future care. “I can’t say we’ll ever be back to normal, necessarily.”
Even if the CMS rolls back its telehealth waivers, some physicians, like Dr. Coleman, plan to continue using the technology extensively. He’s confident about the level of care he’s currently providing patients in his practice. It allows him to better manage many low-income patients who can’t access his office regularly. Not only does splitting his time between the clinic and telehealth allow him to be more available for more patients, he says it also empowers patients to take better care of themselves.
6. Consider a new way to conduct “check-in visits.”
One thing that will likely go by the wayside are “check-in” visits, or so-called “social visits,” those interval appointments that can just as easily be completed virtually. “Patients are going to ask why they need to drive 3 hours so you can tell them their incision looks fine from an operation you did 5 years ago,” Dr. Gonzalez said.
He’s concerned that some people will remain so fearful of the health care system that a formerly busy practice may see the pendulum swing in the opposite direction. If an aneurysm patient skips a visit, that person may also decide not to undergo a CT scan – and something preventable will be missed. “Not everybody has the option to stay away until they feel comfortable. They’re basically playing hot potato. And at some point, the music’s going to stop,” Dr. Gonzalez said.
The pandemic has prompted some very honest conversations with his patients about what truly needs to get done and what may be optional. “Everyone has now become a hyper-rational user of health care,” he said.
7. If you haven’t yet, consider becoming more involved with technology.
In addition to greater use of telehealth, Dr. Kaloostian, assistant professor of clinical family medicine at the University of Southern California, Los Angeles, foresees continued reliance upon technology such as smartphone apps that connect with a user’s smartwatch. This allows for more proactive, remote monitoring.
“For example, any time a patient is having recurrent nighttime trips to the bathroom, I’ll get pinged and know that,” she explained. It means she can reach out and ask about any changes before a fall occurs or a condition worsens. “It provides reassurance to the provider and to the patient that you’re doing all you can to keep an eye on them from afar.”
8. Update or reformulate your business plans.
Some physicians in smaller practices may have to temporarily or permanently rethink their situation. Those who have struggled or who have closed down and are considering reopening need to update their business plans. It may be safer economically to become part of a bigger group that is affiliated with an academic center or join a larger health care system that has more funds or resources.
In addition, Dr. Kaloostian suggests that primary care physicians become more flexible in the short term, perhaps working part time in an urgent care clinic or larger organization to gain additional sources of revenue until their own practice finances pick back up.
For offices that reopen, the AMA recommends contacting medical malpractice insurance carriers to check on possible liability concerns. Congress has provided certain protections for clinicians during this time, but malpractice carriers may have more information and may offer more coverage.
Dr. Coleman said a hybrid model of fewer in-person and more telehealth visits “will allow me to practice in a different way.” If the CMS reimposes prior restrictions, reimbursement may be affected initially, but that will likely change once insurers see the increased cost-effectiveness of this approach. Patients with minor complaints, those who need to have medications refilled, and patients with chronic diseases that need managing won’t have to deal with crowded waiting rooms, and it will help mitigate problems with infection control.
If there’s any upside to the pandemic, it’s an increase in attention given to advanced care planning, said Dr. Kutner. It’s something she hopes continues after everyone stops being in crisis mode. “We’re realizing how important it is to have these conversations and document people’s goals and values and code status,” she said.
Are offices likely to open soon?
An assumption that may or may not be valid is that a practice will remain viable and can return to former capacity. Prior to passage of the CARES Act on March 27, a survey from Kareo, a company in Irvine, California, that makes a technology platform for independent physician practices, found that 9% of respondents reported practice closures. Many more reported concern about potential closures as patient office visits plummet because of stay-at-home orders and other concerns.
By mid-April, a survey from the Primary Care Collaborative and the Larry A. Green Center found that 42% of practices had experienced layoffs and had furloughed staff. Most (85%) have seen dramatic decreases in patient volume.
“Reopening the economy or loosening physical distancing restrictions will be difficult when 20% of primary care practices predict closure within 4 weeks,” the survey concluded.
For the practices and the doctors who make it through this, we’re going to probably be better, stronger, and more efficient, Dr. Gonzalez predicts. This shock has uncovered a lot of weaknesses in the American health care system that doctors have known about and have been complaining about for a long time. It will take an open mind and lots of continued flexibility on the part of physicians, hospitals, health care systems, and the government for these changes to stick.
A version of this article originally appeared on Medscape.com.
After COVID-19 hit the Denver area, internist Jean Kutner, MD, and her clinical colleagues drastically reduced the number of patients they saw and kept a minimum number of people in the office. A small team sees patients who still require in-person visits on one side of the clinic; on the other side, another team conducts clinic-based telehealth visits. A rotating schedule allows for social distancing.
The rest of the practice’s physicians are home, conducting more virtual visits.
Dr. Kutner said she is looking forward to reopening her practice completely at some point. She said she realizes that the practice probably won’t be exactly the same as before.
“We have to embrace the fact that the way we practice medicine has fundamentally changed,” said Dr. Kutner, professor of medicine at the University of Colorado at Denver, Aurora, and incoming president of the Society of General Internal Medicine. She anticipates keeping many of these changes in place for the foreseeable future.
Nearly half of 2,600 primary care physicians who responded to a recent national survey said they were struggling to remain open during the crisis. Most have had to limit wellness/chronic-disease management visits, and nearly half reported that physicians or staff were out sick. Layoffs, furloughs, and reduced hours are commonplace; some practices were forced to shut down entirely.
Social distancing helps reduce the rates of hospitalizations and deaths.
For example, remote monitoring capabilities have reduced the need for in-person checks of vital signs, such as respiratory rate oxygenation, blood glucose levels, and heart rate. “We can’t go back,” she said.
Dr. Kutner sees the pandemic as an opportunity to innovate, to think about how primary practices can best utilize their resources, face-to-face time with patients, and when and how to best leverage virtual visits in a way that improves patient health. The goal, of course, is to meet the needs of the patients while keeping everyone safe.
Like many physicians in private practice, Dr. Kutner is concerned about revenue. She hopes the Centers for Medicare & Medicaid Services makes its temporary waivers permanent.
What you need to consider when planning to reopen your office
Physicians say their post-COVID-19 practices will look very different from their prepandemic practices. Many plan to maintain guidelines, such as those from the AAFP, long after the pandemic has peaked.
If you are starting to think about reopening, here are some major considerations.
1. Develop procedures and practices that will keep your patients and staff safe.
“When we return, the first thing we need to do is limit the number of patients in the waiting room,” said Clinton Coleman, MD, who practices internal medicine and nephrology in Teaneck, N.J. “No one is comfortable in a waiting room any longer,” said Dr. Coleman, chief of internal medicine at Holy Name Medical Center in Teaneck.
Careful planning is required to resume in-person care of patients requiring non-COVID-19 care, as well as all aspects of care, according to the CMS. Adequate staff, testing, supplies, and support services, such as pathology services, are just a few considerations. The CMS recommends that physicians “evaluate the necessity of the care based on clinical needs. Providers should prioritize surgical/procedural care and high-complexity chronic disease management; however, select preventive services may also be highly necessary.”
The American Medical Association recently unveiled a checklist for reopening. One key recommendation was for practices to select a date for reopening the office, ideally preceded by a “soft” or incremental reopening to ensure that new procedures are working. The AMA also recommends opening incrementally, continuing telehealth while also inviting patients back into the office.
2. Figure out how to safely see patients, particularly in your waiting areas and common spaces.
Logistic factors, such as managing patient flow, will change. Waiting rooms will be emptier; in some locations, patients may be asked to wait in their cars until an exam room is available.
The AMA also suggests limiting nonpatient visitors by posting the practice’s policy at the entrance and on the practice’s website. If service calls for repairs are needed, have those visitors come outside of normal operating hours.
Commonly shared objects such magazines or toys in pediatric offices will likely disappear. Wipes, hand sanitizers, and the wearing of masks will become even more commonplace. Those who suspect they’re ill or who have respiratory symptoms may be relegated to specific “sick visit” appointment times or taken to designated exam rooms, which will be thoroughly sanitized between patients.
3. Prepare for routine screening of staff and other facility workers.
According to recent CMS guidelines, you and your staff will need to undergo routine screening, as will others who work in the facility (housekeeping, delivery personnel, and anyone else who enters the area). This may mean regularly stocking screening tests and setting guidelines for what to do if one of your staff tests positive.
You may need to hire temporary workers if your staff tests positive. The CDC recommends at the very least understanding the minimum staffing requirements to ensure good patient care and a safe work environment. Consider adjusting staff schedules and rotating clinical personnel to positions that support patient care activities. You may also want to look into cross-training your office staff so that they can fill in or help out with each other’s responsibilities if one or more persons are ill.
Dr. Kutner is on board with these changes. “We don’t want to get rid of social distancing right away, because it will give us a new spike in cases – how do we figure out patient flow while honoring that?”
4. Develop a strategy for triaging and caring for a potential backlog of patients.
“Many of my partners are scared right now because they have no income except for emergencies,” said Andrew Gonzalez, MD, JD, MPH, a vascular surgeon and assistant professor of surgery at Indiana University, Indianapolis. Almost all nonemergency surgery has been put on hold.
“If we don’t operate, the practice makes no money,” he said. He thinks revenue will continue to be a problem as long as patients fear in-person consultations or undergoing surgery for nonacute problems such as hernias.
As restrictions ease, most physicians will face an enormous backlog of patients and will need to find new ways of triaging the most serious cases, he says. Telehealth will help, but Dr. Gonzalez predicts many of his colleagues will be working longer hours and on weekends to catch up. “Physicians are going to have to really think about ways of optimizing their time and workflow to be very efficient, because the backlog is going to prodigious.”
5. Anticipate changes in patient expectations.
This may entail your reconsidering tests and procedures you previously performed and considering developing new sources for some services, phasing some others out, and revising your current approach. It will most likely also mean that you make telemedicine and televisits a greater part of your practice.
Carolyn Kaloostian, MD, a family medicine and geriatric practitioner in Los Angeles, points to increased reliance on community agencies for conducting common office-based procedures, such as performing blood tests and taking ECGs and x-rays. “A lot of patients are using telemedicine or telephone visits and get the lab work or x-rays somewhere that’s less congested,” she said. To become sustainable, many of these changes will hinge on economics – whether and how they are reimbursed.
The pandemic will leave lasting effects in our health care delivery, according to Dr. Kaloostian. She is sure many of her colleagues’ and patients’ current experiences will be infused into future care. “I can’t say we’ll ever be back to normal, necessarily.”
Even if the CMS rolls back its telehealth waivers, some physicians, like Dr. Coleman, plan to continue using the technology extensively. He’s confident about the level of care he’s currently providing patients in his practice. It allows him to better manage many low-income patients who can’t access his office regularly. Not only does splitting his time between the clinic and telehealth allow him to be more available for more patients, he says it also empowers patients to take better care of themselves.
6. Consider a new way to conduct “check-in visits.”
One thing that will likely go by the wayside are “check-in” visits, or so-called “social visits,” those interval appointments that can just as easily be completed virtually. “Patients are going to ask why they need to drive 3 hours so you can tell them their incision looks fine from an operation you did 5 years ago,” Dr. Gonzalez said.
He’s concerned that some people will remain so fearful of the health care system that a formerly busy practice may see the pendulum swing in the opposite direction. If an aneurysm patient skips a visit, that person may also decide not to undergo a CT scan – and something preventable will be missed. “Not everybody has the option to stay away until they feel comfortable. They’re basically playing hot potato. And at some point, the music’s going to stop,” Dr. Gonzalez said.
The pandemic has prompted some very honest conversations with his patients about what truly needs to get done and what may be optional. “Everyone has now become a hyper-rational user of health care,” he said.
7. If you haven’t yet, consider becoming more involved with technology.
In addition to greater use of telehealth, Dr. Kaloostian, assistant professor of clinical family medicine at the University of Southern California, Los Angeles, foresees continued reliance upon technology such as smartphone apps that connect with a user’s smartwatch. This allows for more proactive, remote monitoring.
“For example, any time a patient is having recurrent nighttime trips to the bathroom, I’ll get pinged and know that,” she explained. It means she can reach out and ask about any changes before a fall occurs or a condition worsens. “It provides reassurance to the provider and to the patient that you’re doing all you can to keep an eye on them from afar.”
8. Update or reformulate your business plans.
Some physicians in smaller practices may have to temporarily or permanently rethink their situation. Those who have struggled or who have closed down and are considering reopening need to update their business plans. It may be safer economically to become part of a bigger group that is affiliated with an academic center or join a larger health care system that has more funds or resources.
In addition, Dr. Kaloostian suggests that primary care physicians become more flexible in the short term, perhaps working part time in an urgent care clinic or larger organization to gain additional sources of revenue until their own practice finances pick back up.
For offices that reopen, the AMA recommends contacting medical malpractice insurance carriers to check on possible liability concerns. Congress has provided certain protections for clinicians during this time, but malpractice carriers may have more information and may offer more coverage.
Dr. Coleman said a hybrid model of fewer in-person and more telehealth visits “will allow me to practice in a different way.” If the CMS reimposes prior restrictions, reimbursement may be affected initially, but that will likely change once insurers see the increased cost-effectiveness of this approach. Patients with minor complaints, those who need to have medications refilled, and patients with chronic diseases that need managing won’t have to deal with crowded waiting rooms, and it will help mitigate problems with infection control.
If there’s any upside to the pandemic, it’s an increase in attention given to advanced care planning, said Dr. Kutner. It’s something she hopes continues after everyone stops being in crisis mode. “We’re realizing how important it is to have these conversations and document people’s goals and values and code status,” she said.
Are offices likely to open soon?
An assumption that may or may not be valid is that a practice will remain viable and can return to former capacity. Prior to passage of the CARES Act on March 27, a survey from Kareo, a company in Irvine, California, that makes a technology platform for independent physician practices, found that 9% of respondents reported practice closures. Many more reported concern about potential closures as patient office visits plummet because of stay-at-home orders and other concerns.
By mid-April, a survey from the Primary Care Collaborative and the Larry A. Green Center found that 42% of practices had experienced layoffs and had furloughed staff. Most (85%) have seen dramatic decreases in patient volume.
“Reopening the economy or loosening physical distancing restrictions will be difficult when 20% of primary care practices predict closure within 4 weeks,” the survey concluded.
For the practices and the doctors who make it through this, we’re going to probably be better, stronger, and more efficient, Dr. Gonzalez predicts. This shock has uncovered a lot of weaknesses in the American health care system that doctors have known about and have been complaining about for a long time. It will take an open mind and lots of continued flexibility on the part of physicians, hospitals, health care systems, and the government for these changes to stick.
A version of this article originally appeared on Medscape.com.
More guidance on inpatient management of blood glucose in COVID-19
“The glycemic management of many COVID-19–positive patients with diabetes is proving extremely complex, with huge fluctuations in glucose control and the need for very high doses of insulin,” says Diabetes UK’s National Diabetes Inpatient COVID Response Team.
“Intravenous infusion pumps, also required for inotropes, are at a premium and there may be the need to consider the use of subcutaneous or intramuscular insulin protocols,” they note.
Updated as of April 29, all of the information of the National Diabetes Inpatient COVID Response Team is available on the Diabetes UK website.
The new inpatient management graphic adds more detail to the previous “front-door” guidance, as reported by Medscape Medical News.
The document stressed that, as well as identifying patients with known diabetes, it is imperative that all newly admitted patients with COVID-19 are evaluated for diabetes, as the infection is known to cause new-onset diabetes.
Subcutaneous insulin dosing
The new graphic gives extensive details on subcutaneous insulin dosing in place of variable rate intravenous insulin when infusion pumps are not available, and when the patient has a glucose level above 12 mmol/L (216 mg/dL) but does not have DKA or hyperosmolar hyperglycemic state.
However, the advice is not intended for people with COVID-19 causing severe insulin resistance in the intensive care unit.
The other new guidance graphic on managing DKA or hyperosmolar state in people with COVID-19 using subcutaneous insulin is also intended for situations where intravenous infusion isn’t available.
Seek help from specialist diabetes team when needed
This is not to be used for mixed DKA/hyperosmolar state or for patients who are pregnant, have severe metabolic derangement, other significant comorbidity, or impaired consciousness, however.
For those situations, the advice is to seek help from a specialist diabetes team, says Diabetes UK.
Specialist teams will be available to answer diabetes queries, both by signposting to relevant existing local documents and also by providing patient-specific advice.
Indeed, NHS England recommends that such a team be available in every hospital, with a lead consultant designated each day to co-ordinate these services who must be free of other clinical duties when doing so. The role involves co-ordination of the whole service from the emergency department through to liaison with other specialties and managers.
Also newly updated is a page with extensive information for patients, including advice for staying at home, medication use, self-isolating, shielding, hospital and doctor appointments, need for urgent medical advice, and going to the hospital.
It also covers how coronavirus can affect people with diabetes, children and school, pregnancy, work situations, and tips for picking up prescriptions.
Another, shorter document with COVID-19 advice for patients has been posted by the JDRF and Beyond Type 1 Alliance.
It has also been endorsed by the American Diabetes Association, Harvard Medical School, and International Society for Pediatric and Adolescent Diabetes, in partnership with many other professional organizations, including the International Diabetes Federation, American Association of Clinical Endocrinologists, and Association of Diabetes Care & Education Specialists.
The shorter document covers topics such as personal hygiene, distancing, diabetes management, and seeking treatment, as well as links to other resources on what to do when health insurance is lost and legal rights.
This article first appeared on Medscape.com.
“The glycemic management of many COVID-19–positive patients with diabetes is proving extremely complex, with huge fluctuations in glucose control and the need for very high doses of insulin,” says Diabetes UK’s National Diabetes Inpatient COVID Response Team.
“Intravenous infusion pumps, also required for inotropes, are at a premium and there may be the need to consider the use of subcutaneous or intramuscular insulin protocols,” they note.
Updated as of April 29, all of the information of the National Diabetes Inpatient COVID Response Team is available on the Diabetes UK website.
The new inpatient management graphic adds more detail to the previous “front-door” guidance, as reported by Medscape Medical News.
The document stressed that, as well as identifying patients with known diabetes, it is imperative that all newly admitted patients with COVID-19 are evaluated for diabetes, as the infection is known to cause new-onset diabetes.
Subcutaneous insulin dosing
The new graphic gives extensive details on subcutaneous insulin dosing in place of variable rate intravenous insulin when infusion pumps are not available, and when the patient has a glucose level above 12 mmol/L (216 mg/dL) but does not have DKA or hyperosmolar hyperglycemic state.
However, the advice is not intended for people with COVID-19 causing severe insulin resistance in the intensive care unit.
The other new guidance graphic on managing DKA or hyperosmolar state in people with COVID-19 using subcutaneous insulin is also intended for situations where intravenous infusion isn’t available.
Seek help from specialist diabetes team when needed
This is not to be used for mixed DKA/hyperosmolar state or for patients who are pregnant, have severe metabolic derangement, other significant comorbidity, or impaired consciousness, however.
For those situations, the advice is to seek help from a specialist diabetes team, says Diabetes UK.
Specialist teams will be available to answer diabetes queries, both by signposting to relevant existing local documents and also by providing patient-specific advice.
Indeed, NHS England recommends that such a team be available in every hospital, with a lead consultant designated each day to co-ordinate these services who must be free of other clinical duties when doing so. The role involves co-ordination of the whole service from the emergency department through to liaison with other specialties and managers.
Also newly updated is a page with extensive information for patients, including advice for staying at home, medication use, self-isolating, shielding, hospital and doctor appointments, need for urgent medical advice, and going to the hospital.
It also covers how coronavirus can affect people with diabetes, children and school, pregnancy, work situations, and tips for picking up prescriptions.
Another, shorter document with COVID-19 advice for patients has been posted by the JDRF and Beyond Type 1 Alliance.
It has also been endorsed by the American Diabetes Association, Harvard Medical School, and International Society for Pediatric and Adolescent Diabetes, in partnership with many other professional organizations, including the International Diabetes Federation, American Association of Clinical Endocrinologists, and Association of Diabetes Care & Education Specialists.
The shorter document covers topics such as personal hygiene, distancing, diabetes management, and seeking treatment, as well as links to other resources on what to do when health insurance is lost and legal rights.
This article first appeared on Medscape.com.
“The glycemic management of many COVID-19–positive patients with diabetes is proving extremely complex, with huge fluctuations in glucose control and the need for very high doses of insulin,” says Diabetes UK’s National Diabetes Inpatient COVID Response Team.
“Intravenous infusion pumps, also required for inotropes, are at a premium and there may be the need to consider the use of subcutaneous or intramuscular insulin protocols,” they note.
Updated as of April 29, all of the information of the National Diabetes Inpatient COVID Response Team is available on the Diabetes UK website.
The new inpatient management graphic adds more detail to the previous “front-door” guidance, as reported by Medscape Medical News.
The document stressed that, as well as identifying patients with known diabetes, it is imperative that all newly admitted patients with COVID-19 are evaluated for diabetes, as the infection is known to cause new-onset diabetes.
Subcutaneous insulin dosing
The new graphic gives extensive details on subcutaneous insulin dosing in place of variable rate intravenous insulin when infusion pumps are not available, and when the patient has a glucose level above 12 mmol/L (216 mg/dL) but does not have DKA or hyperosmolar hyperglycemic state.
However, the advice is not intended for people with COVID-19 causing severe insulin resistance in the intensive care unit.
The other new guidance graphic on managing DKA or hyperosmolar state in people with COVID-19 using subcutaneous insulin is also intended for situations where intravenous infusion isn’t available.
Seek help from specialist diabetes team when needed
This is not to be used for mixed DKA/hyperosmolar state or for patients who are pregnant, have severe metabolic derangement, other significant comorbidity, or impaired consciousness, however.
For those situations, the advice is to seek help from a specialist diabetes team, says Diabetes UK.
Specialist teams will be available to answer diabetes queries, both by signposting to relevant existing local documents and also by providing patient-specific advice.
Indeed, NHS England recommends that such a team be available in every hospital, with a lead consultant designated each day to co-ordinate these services who must be free of other clinical duties when doing so. The role involves co-ordination of the whole service from the emergency department through to liaison with other specialties and managers.
Also newly updated is a page with extensive information for patients, including advice for staying at home, medication use, self-isolating, shielding, hospital and doctor appointments, need for urgent medical advice, and going to the hospital.
It also covers how coronavirus can affect people with diabetes, children and school, pregnancy, work situations, and tips for picking up prescriptions.
Another, shorter document with COVID-19 advice for patients has been posted by the JDRF and Beyond Type 1 Alliance.
It has also been endorsed by the American Diabetes Association, Harvard Medical School, and International Society for Pediatric and Adolescent Diabetes, in partnership with many other professional organizations, including the International Diabetes Federation, American Association of Clinical Endocrinologists, and Association of Diabetes Care & Education Specialists.
The shorter document covers topics such as personal hygiene, distancing, diabetes management, and seeking treatment, as well as links to other resources on what to do when health insurance is lost and legal rights.
This article first appeared on Medscape.com.
Results from 11 AHA-funded COVID-19 studies expected within months
Work is set to start in June, with findings reported in as few as 6 months. The Cleveland Clinic will coordinate the efforts, collecting and disseminating the findings.
There were more than 750 research proposals in less than a month after the association announced its COVID-19 and Its Cardiovascular Impact Rapid Response Grant initiative.
“We were just blown away and so impressed to see this level of interest and commitment from the teams submitting such thorough proposals so quickly,” AHA President Robert Harrington, MD, chair of the department of medicine at Stanford (Calif.) University, said in a press statement. “There’s so much we don’t know about this unique coronavirus, and we continue to see emerging complications affecting both heart and brain health for which we desperately need answers and we need them quickly.”
The projects include the following:
- A Comprehensive Assessment of Arterial and Venous Thrombotic Complications in Patients with COVID-19, led by Columbia University, New York City.
- Repurposing Drugs for Treatment of Cardiomyopathy Caused by Coronavirus-2 (SARS-CoV-2), led by Brigham and Women’s Hospital and Harvard Medical School, Boston.
- Risk of Severe Morbidity and Mortality of Coronavirus Disease 2019 (COVID-19) Among Patients Taking Antihypertensive Medications, led by Kaiser Permanente Southern California.
- Deep Learning Using Chest Radiographs to Predict COVID-19 Cardiopulmonary Risk, led by Massachusetts General Hospital, Boston.
- Cardiovascular Outcomes and Biomarker Titrated Corticosteroid Dosing for SARS COV-2 (COVID-19): A Randomized Controlled Trial, led by the Mayo Clinic, Rochester Minn.
- Outcomes for Patients With Hypertension, Diabetes, and Heart Disease in the Coronavirus Pandemic: Impact of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Treatment, led by Stanford University.
- Rapid COVID-19-on-A-Chip to Screen Competitive Targets for SARS-CoV-2 Spike Binding Sites, led by University of California, Los Angeles.
- COVID-19 Infection, African American Women and Cardiovascular Health, led by University of California, San Francisco.
- Myocardial Virus and Gene Expression in SARS CoV-2 Positive Patients with Clinically Important Myocardial Dysfunction, led by the University of Colorado, Aurora.
- The Role of the Platelet in Mediating Cardiovascular Disease in SARS-CoV-2 Infection, led by the University of Massachusetts, Worcester.
- Harnessing Glycomics to Understand Myocardial Injury in COVID-19, led by the University of Nebraska Medical Center, Omaha.
The AHA also awarded $800,000 for short-term projects to members of its new Health Technologies & Innovation Strategically Focused Research Network.
Cincinnati Children’s Hospital will assess the use of ejection fraction to triage COVID-19 patients; Johns Hopkins University, Baltimore, will assess smartphones for “virtual check-in” for stroke symptoms; Stanford will assess digital tracking of COVID-19 patients with cardiovascular complications; and the University of Michigan, Ann Arbor, will assess a system to track physiological and cardiovascular consequences of the infection.
Work is set to start in June, with findings reported in as few as 6 months. The Cleveland Clinic will coordinate the efforts, collecting and disseminating the findings.
There were more than 750 research proposals in less than a month after the association announced its COVID-19 and Its Cardiovascular Impact Rapid Response Grant initiative.
“We were just blown away and so impressed to see this level of interest and commitment from the teams submitting such thorough proposals so quickly,” AHA President Robert Harrington, MD, chair of the department of medicine at Stanford (Calif.) University, said in a press statement. “There’s so much we don’t know about this unique coronavirus, and we continue to see emerging complications affecting both heart and brain health for which we desperately need answers and we need them quickly.”
The projects include the following:
- A Comprehensive Assessment of Arterial and Venous Thrombotic Complications in Patients with COVID-19, led by Columbia University, New York City.
- Repurposing Drugs for Treatment of Cardiomyopathy Caused by Coronavirus-2 (SARS-CoV-2), led by Brigham and Women’s Hospital and Harvard Medical School, Boston.
- Risk of Severe Morbidity and Mortality of Coronavirus Disease 2019 (COVID-19) Among Patients Taking Antihypertensive Medications, led by Kaiser Permanente Southern California.
- Deep Learning Using Chest Radiographs to Predict COVID-19 Cardiopulmonary Risk, led by Massachusetts General Hospital, Boston.
- Cardiovascular Outcomes and Biomarker Titrated Corticosteroid Dosing for SARS COV-2 (COVID-19): A Randomized Controlled Trial, led by the Mayo Clinic, Rochester Minn.
- Outcomes for Patients With Hypertension, Diabetes, and Heart Disease in the Coronavirus Pandemic: Impact of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Treatment, led by Stanford University.
- Rapid COVID-19-on-A-Chip to Screen Competitive Targets for SARS-CoV-2 Spike Binding Sites, led by University of California, Los Angeles.
- COVID-19 Infection, African American Women and Cardiovascular Health, led by University of California, San Francisco.
- Myocardial Virus and Gene Expression in SARS CoV-2 Positive Patients with Clinically Important Myocardial Dysfunction, led by the University of Colorado, Aurora.
- The Role of the Platelet in Mediating Cardiovascular Disease in SARS-CoV-2 Infection, led by the University of Massachusetts, Worcester.
- Harnessing Glycomics to Understand Myocardial Injury in COVID-19, led by the University of Nebraska Medical Center, Omaha.
The AHA also awarded $800,000 for short-term projects to members of its new Health Technologies & Innovation Strategically Focused Research Network.
Cincinnati Children’s Hospital will assess the use of ejection fraction to triage COVID-19 patients; Johns Hopkins University, Baltimore, will assess smartphones for “virtual check-in” for stroke symptoms; Stanford will assess digital tracking of COVID-19 patients with cardiovascular complications; and the University of Michigan, Ann Arbor, will assess a system to track physiological and cardiovascular consequences of the infection.
Work is set to start in June, with findings reported in as few as 6 months. The Cleveland Clinic will coordinate the efforts, collecting and disseminating the findings.
There were more than 750 research proposals in less than a month after the association announced its COVID-19 and Its Cardiovascular Impact Rapid Response Grant initiative.
“We were just blown away and so impressed to see this level of interest and commitment from the teams submitting such thorough proposals so quickly,” AHA President Robert Harrington, MD, chair of the department of medicine at Stanford (Calif.) University, said in a press statement. “There’s so much we don’t know about this unique coronavirus, and we continue to see emerging complications affecting both heart and brain health for which we desperately need answers and we need them quickly.”
The projects include the following:
- A Comprehensive Assessment of Arterial and Venous Thrombotic Complications in Patients with COVID-19, led by Columbia University, New York City.
- Repurposing Drugs for Treatment of Cardiomyopathy Caused by Coronavirus-2 (SARS-CoV-2), led by Brigham and Women’s Hospital and Harvard Medical School, Boston.
- Risk of Severe Morbidity and Mortality of Coronavirus Disease 2019 (COVID-19) Among Patients Taking Antihypertensive Medications, led by Kaiser Permanente Southern California.
- Deep Learning Using Chest Radiographs to Predict COVID-19 Cardiopulmonary Risk, led by Massachusetts General Hospital, Boston.
- Cardiovascular Outcomes and Biomarker Titrated Corticosteroid Dosing for SARS COV-2 (COVID-19): A Randomized Controlled Trial, led by the Mayo Clinic, Rochester Minn.
- Outcomes for Patients With Hypertension, Diabetes, and Heart Disease in the Coronavirus Pandemic: Impact of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Treatment, led by Stanford University.
- Rapid COVID-19-on-A-Chip to Screen Competitive Targets for SARS-CoV-2 Spike Binding Sites, led by University of California, Los Angeles.
- COVID-19 Infection, African American Women and Cardiovascular Health, led by University of California, San Francisco.
- Myocardial Virus and Gene Expression in SARS CoV-2 Positive Patients with Clinically Important Myocardial Dysfunction, led by the University of Colorado, Aurora.
- The Role of the Platelet in Mediating Cardiovascular Disease in SARS-CoV-2 Infection, led by the University of Massachusetts, Worcester.
- Harnessing Glycomics to Understand Myocardial Injury in COVID-19, led by the University of Nebraska Medical Center, Omaha.
The AHA also awarded $800,000 for short-term projects to members of its new Health Technologies & Innovation Strategically Focused Research Network.
Cincinnati Children’s Hospital will assess the use of ejection fraction to triage COVID-19 patients; Johns Hopkins University, Baltimore, will assess smartphones for “virtual check-in” for stroke symptoms; Stanford will assess digital tracking of COVID-19 patients with cardiovascular complications; and the University of Michigan, Ann Arbor, will assess a system to track physiological and cardiovascular consequences of the infection.
COVID-19–associated coagulopathy
Coronavirus disease 2019 (COVID-19) is a viral illness caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), currently causing a pandemic affecting many countries around the world, beginning in December 2019 and spreading rapidly on a global scale since. Globally, its burden has been increasing rapidly, with more than 1.2 million people testing positive for the illness and 123,000 people losing their lives, as per April 15th’s WHO COVID-19 Situation Report.1 These numbers are increasing with each passing day. Clinically, SARS-CoV-2 has a highly variable course, ranging from mild disease manifested as a self-limited illness (seen in younger and healthier patients) to severe pneumonia/ARDS and multiorgan failure with intravascular coagulopathy.2
In this article, we intend to investigate and establish a comprehensive review of COVID-19–associated coagulopathy mechanisms, laboratory findings, and current management guidelines put forth by various societies globally.
Mechanism of coagulopathy
COVID-19–associated coagulopathy has been shown to predispose to both arterial and venous thrombosis through excessive inflammation and hypoxia, leading to activation of the coagulation cascade and consumption of coagulation factors, resulting in microvascular thrombosis.3 Though the exact pathophysiology for the activation of this cascade is not known, the proposed mechanism has been: endothelial damage triggering platelet activation within the lung, leading to aggregation, thrombosis, and consumption of platelets in the lung.2,5,6
Fox et al. noted similar coagulopathy findings of four deceased COVID-19 patients. Autopsy results concluded that the dominant process was diffuse alveolar damage, notable CD4+ aggregates around thrombosed small vessels, significant associated hemorrhage, and thrombotic microangiopathy restricted to the lungs. The proposed mechanism was the activation of megakaryocytes, possibly native to the lung, with platelet aggregation, formation of platelet-rich clots, and fibrin deposition playing a major role.4
It has been noted that diabetic patients are at an increased risk of vascular events and hypercoagulability with COVID-19.7 COVID-19 can also cause livedo reticularis and acrocyanosis because of the microthrombosis in the cutaneous vasculature secondary to underlying coagulopathy, as reported in a case report of two U.S. patients with COVID-19.8
Clinical and laboratory abnormalities
A recent study reported from Netherlands by Klok et al. analyzed 184 ICU patients with COVID-19 pneumonia and concluded that the cumulative incidence of acute pulmonary embolism (PE), deep vein thrombosis (DVT), ischemic stroke, MI, or systemic arterial embolism was 31% (95% confidence interval, 20%-41%). PE was the most frequent thrombotic complication and was noted in 81% of patients. Coagulopathy, defined as spontaneous prolongation of prothrombin time (PT) > 3s or activated partial thromboplastin time (aPTT) > 5s, was reported as an independent predictor of thrombotic complications.3
Hematologic abnormalities that were noted in COVID-19 coagulopathy include: decreased platelet counts, decreased fibrinogen levels, elevated PT/INR, elevated partial thromboplastin time (PTT), and elevated d-dimer.9,10 In a retrospective analysis9 by Tang et al., 71.4% of nonsurvivors and 0.6% of survivors had met the criteria of disseminated intravascular coagulation (DIC) during their hospital stay. Nonsurvivors of COVID-19 had statistically significant elevation of d-dimer levels, FDP levels, PT, and aPTT, when compared to survivors (P < .05). The overall mortality in this study was reported as 11.5%.9 In addition, elevated d-dimer, fibrin and fibrinogen degradation product (FDP) levels and longer PT and aPTT were associated with poor prognosis.
Thus, d-dimer, PT, and platelet count should be measured in all patients who present with COVID-19 infection. We can also suggest that in patients with markedly elevated d-dimer (three- to fourfold increase), admission to hospital should be considered even in the absence of severe clinical symptoms.11
COVID-19 coagulopathy management
In a retrospective study9 of 449 patients with severe COVID-19 from Wuhan, China, by Tang et al., 99 patients mainly received low-weight molecular heparin (LMWH) for 7 days or longer. No difference in 28-day mortality was noted between heparin users and nonusers (30.3% vs. 29.7%; P = .910). A lower 28-day mortality rate was noted in heparin patients with sepsis-induced coagulopathy score of ≥4.0 (40.0% vs. 64.2%; P = .029) or a d-dimer level greater than sixfold of upper limit of normal, compared with nonusers of heparin.12
Another small study of seven COVID-19 patients with acroischemia in China demonstrated that administering LMWH was successful at decreasing the d-dimer and fibrinogen degradation product levels but noted no significant improvement in clinical symptoms.13
Recently, the International Society of Thrombosis and Hemostasis and American Society of Hematology published recommendations and guidelines regarding the recognition and management of coagulopathy in COVID-19.11 Prophylactic anticoagulation therapy with LMWH was recommended in all hospitalized patients with COVID-19, provided there was an absence of any contraindications (active bleeding, platelet count less than 25 x 109/L and fibrinogen less than 0.5 g/dL). Anticoagulation with LMWH was associated with better prognosis in severe COVID-19 patients and in COVID-19 patients with markedly elevated d-dimer, as it also has anti-inflammatory effects.12 This anti-inflammatory property of heparin has been documented in previous studies but the underlying mechanism is unknown and more research is required.14,15
Despite coagulopathy being noticed with cases of COVID-19, bleeding has been a rare finding in COVID-19 infections. If bleeding is noted, recommendations were made to keep platelet levels greater than 50 x109/L, fibrinogen less than 2.0 g/L, and INR [international normalized ratio] greater than 1.5.11 Mechanical thromboprophylaxis should be used when pharmacologic thromboprophylaxis is contraindicated.16
COVID-19 patients with new diagnoses of venous thromboembolism (VTE) or atrial fibrillation should be prescribed therapeutic anticoagulation. Patients who are already on anticoagulation for VTE or atrial fibrillation should continue their therapy unless the platelet count is less than 30-50x109/L or if the fibrinogen is less than 1.0 g/L.16
Conclusion
Coagulopathies associated with COVID-19 infections have been documented in several studies around the world, and it has been shown to be fatal in some cases. Despite documentation, the mechanism behind this coagulopathy is not well understood. Because of the potentially lethal complications associated with coagulopathies, early recognition and anticoagulation is imperative to improve clinical outcomes. These results are very preliminary: More studies are required to understand the role of anticoagulation and its effect on the morbidity and mortality associated with COVID-19–associated coagulopathy.
Dr. Yeruva is a board-certified hematologist/medical oncologist with WellSpan Health and clinical assistant professor of internal medicine, Penn State University, Hershey. Mr. Henderson is a third-year graduate-entry medical student at the Royal College of Surgeons in Ireland with interests in family medicine, dermatology, and tropical diseases. Dr. Al-Tawfiq is a consultant of internal medicine & infectious diseases, and the director of quality at Johns Hopkins Aramco Healthcare in Dhahran, Saudi Arabia, an adjunct associate professor of infectious diseases, molecular medicine and clinical pharmacology at Johns Hopkins University School of Medicine, and adjunct associate professor at Indiana University School of Medicine, Indianapolis. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals.
References
1. World Health Organization. Coronavirus disease (COVID-2019) situation reports.
2. Lippi G et al. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta. 2020 Mar 13. 506:145-8. doi: 10.1016/j.cca.2020.03.022.
3. Klok FA et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm Res. 2020;18(4):844-7. doi: 10.1016/j.thromres.2020.04.013.
4. Fox S et al. Pulmonary and cardiac pathology in Covid-19: The first autopsy series from New Orleans. MedRxiv. 2020 Apr 10. doi: 10.1101/2020.04.06.20050575.
5. Yang M et al. Thrombocytopenia in patients with severe acute respiratory syndrome (review). Hematology 2013 Sep 4. doi: 10.1080/1024533040002617.
6. Giannis D et al. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020 June. doi: 10.1016/j.jcv.2020.104362.
7. Guo W et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 Mar 31. doi: 10.1002/dmrr.3319.
8. Manalo IF et al. A dermatologic manifestation of COVID-19: Transient livedo reticularis. J Am Acad Dermat. 2020 Apr. doi: 10.1016/j.jaad.2020.04.018.
9. Tang N et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 Feb 19. doi: 10.1111/jth.14768, 18: 844-847.
10. Huang C et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
11. Thachil J et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020 Mar 25. doi: 10.1111/JTH.14810.
12. Tang N et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 Mar 27. doi: 10.1111/JTH.14817.
13. Zhang Y et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020 Mar 28. doi: 10.3760/cma.j.issn.0253-2727.2020.0006.
14. Poterucha TJ et al. More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb Haemost. 2017. doi: 10.1160/TH16-08-0620.
15. Mousavi S et al. Anti-inflammatory effects of heparin and its derivatives: A systematic review. Adv Pharmacol Pharm Sci. 2015 May 12. doi: 10.1155/2015/507151.
16. Kreuziger L et al. COVID-19 and VTE/anticoagulation: Frequently asked questions. American Society of Hematology. 2020 Apr 17.
Coronavirus disease 2019 (COVID-19) is a viral illness caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), currently causing a pandemic affecting many countries around the world, beginning in December 2019 and spreading rapidly on a global scale since. Globally, its burden has been increasing rapidly, with more than 1.2 million people testing positive for the illness and 123,000 people losing their lives, as per April 15th’s WHO COVID-19 Situation Report.1 These numbers are increasing with each passing day. Clinically, SARS-CoV-2 has a highly variable course, ranging from mild disease manifested as a self-limited illness (seen in younger and healthier patients) to severe pneumonia/ARDS and multiorgan failure with intravascular coagulopathy.2
In this article, we intend to investigate and establish a comprehensive review of COVID-19–associated coagulopathy mechanisms, laboratory findings, and current management guidelines put forth by various societies globally.
Mechanism of coagulopathy
COVID-19–associated coagulopathy has been shown to predispose to both arterial and venous thrombosis through excessive inflammation and hypoxia, leading to activation of the coagulation cascade and consumption of coagulation factors, resulting in microvascular thrombosis.3 Though the exact pathophysiology for the activation of this cascade is not known, the proposed mechanism has been: endothelial damage triggering platelet activation within the lung, leading to aggregation, thrombosis, and consumption of platelets in the lung.2,5,6
Fox et al. noted similar coagulopathy findings of four deceased COVID-19 patients. Autopsy results concluded that the dominant process was diffuse alveolar damage, notable CD4+ aggregates around thrombosed small vessels, significant associated hemorrhage, and thrombotic microangiopathy restricted to the lungs. The proposed mechanism was the activation of megakaryocytes, possibly native to the lung, with platelet aggregation, formation of platelet-rich clots, and fibrin deposition playing a major role.4
It has been noted that diabetic patients are at an increased risk of vascular events and hypercoagulability with COVID-19.7 COVID-19 can also cause livedo reticularis and acrocyanosis because of the microthrombosis in the cutaneous vasculature secondary to underlying coagulopathy, as reported in a case report of two U.S. patients with COVID-19.8
Clinical and laboratory abnormalities
A recent study reported from Netherlands by Klok et al. analyzed 184 ICU patients with COVID-19 pneumonia and concluded that the cumulative incidence of acute pulmonary embolism (PE), deep vein thrombosis (DVT), ischemic stroke, MI, or systemic arterial embolism was 31% (95% confidence interval, 20%-41%). PE was the most frequent thrombotic complication and was noted in 81% of patients. Coagulopathy, defined as spontaneous prolongation of prothrombin time (PT) > 3s or activated partial thromboplastin time (aPTT) > 5s, was reported as an independent predictor of thrombotic complications.3
Hematologic abnormalities that were noted in COVID-19 coagulopathy include: decreased platelet counts, decreased fibrinogen levels, elevated PT/INR, elevated partial thromboplastin time (PTT), and elevated d-dimer.9,10 In a retrospective analysis9 by Tang et al., 71.4% of nonsurvivors and 0.6% of survivors had met the criteria of disseminated intravascular coagulation (DIC) during their hospital stay. Nonsurvivors of COVID-19 had statistically significant elevation of d-dimer levels, FDP levels, PT, and aPTT, when compared to survivors (P < .05). The overall mortality in this study was reported as 11.5%.9 In addition, elevated d-dimer, fibrin and fibrinogen degradation product (FDP) levels and longer PT and aPTT were associated with poor prognosis.
Thus, d-dimer, PT, and platelet count should be measured in all patients who present with COVID-19 infection. We can also suggest that in patients with markedly elevated d-dimer (three- to fourfold increase), admission to hospital should be considered even in the absence of severe clinical symptoms.11
COVID-19 coagulopathy management
In a retrospective study9 of 449 patients with severe COVID-19 from Wuhan, China, by Tang et al., 99 patients mainly received low-weight molecular heparin (LMWH) for 7 days or longer. No difference in 28-day mortality was noted between heparin users and nonusers (30.3% vs. 29.7%; P = .910). A lower 28-day mortality rate was noted in heparin patients with sepsis-induced coagulopathy score of ≥4.0 (40.0% vs. 64.2%; P = .029) or a d-dimer level greater than sixfold of upper limit of normal, compared with nonusers of heparin.12
Another small study of seven COVID-19 patients with acroischemia in China demonstrated that administering LMWH was successful at decreasing the d-dimer and fibrinogen degradation product levels but noted no significant improvement in clinical symptoms.13
Recently, the International Society of Thrombosis and Hemostasis and American Society of Hematology published recommendations and guidelines regarding the recognition and management of coagulopathy in COVID-19.11 Prophylactic anticoagulation therapy with LMWH was recommended in all hospitalized patients with COVID-19, provided there was an absence of any contraindications (active bleeding, platelet count less than 25 x 109/L and fibrinogen less than 0.5 g/dL). Anticoagulation with LMWH was associated with better prognosis in severe COVID-19 patients and in COVID-19 patients with markedly elevated d-dimer, as it also has anti-inflammatory effects.12 This anti-inflammatory property of heparin has been documented in previous studies but the underlying mechanism is unknown and more research is required.14,15
Despite coagulopathy being noticed with cases of COVID-19, bleeding has been a rare finding in COVID-19 infections. If bleeding is noted, recommendations were made to keep platelet levels greater than 50 x109/L, fibrinogen less than 2.0 g/L, and INR [international normalized ratio] greater than 1.5.11 Mechanical thromboprophylaxis should be used when pharmacologic thromboprophylaxis is contraindicated.16
COVID-19 patients with new diagnoses of venous thromboembolism (VTE) or atrial fibrillation should be prescribed therapeutic anticoagulation. Patients who are already on anticoagulation for VTE or atrial fibrillation should continue their therapy unless the platelet count is less than 30-50x109/L or if the fibrinogen is less than 1.0 g/L.16
Conclusion
Coagulopathies associated with COVID-19 infections have been documented in several studies around the world, and it has been shown to be fatal in some cases. Despite documentation, the mechanism behind this coagulopathy is not well understood. Because of the potentially lethal complications associated with coagulopathies, early recognition and anticoagulation is imperative to improve clinical outcomes. These results are very preliminary: More studies are required to understand the role of anticoagulation and its effect on the morbidity and mortality associated with COVID-19–associated coagulopathy.
Dr. Yeruva is a board-certified hematologist/medical oncologist with WellSpan Health and clinical assistant professor of internal medicine, Penn State University, Hershey. Mr. Henderson is a third-year graduate-entry medical student at the Royal College of Surgeons in Ireland with interests in family medicine, dermatology, and tropical diseases. Dr. Al-Tawfiq is a consultant of internal medicine & infectious diseases, and the director of quality at Johns Hopkins Aramco Healthcare in Dhahran, Saudi Arabia, an adjunct associate professor of infectious diseases, molecular medicine and clinical pharmacology at Johns Hopkins University School of Medicine, and adjunct associate professor at Indiana University School of Medicine, Indianapolis. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals.
References
1. World Health Organization. Coronavirus disease (COVID-2019) situation reports.
2. Lippi G et al. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta. 2020 Mar 13. 506:145-8. doi: 10.1016/j.cca.2020.03.022.
3. Klok FA et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm Res. 2020;18(4):844-7. doi: 10.1016/j.thromres.2020.04.013.
4. Fox S et al. Pulmonary and cardiac pathology in Covid-19: The first autopsy series from New Orleans. MedRxiv. 2020 Apr 10. doi: 10.1101/2020.04.06.20050575.
5. Yang M et al. Thrombocytopenia in patients with severe acute respiratory syndrome (review). Hematology 2013 Sep 4. doi: 10.1080/1024533040002617.
6. Giannis D et al. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020 June. doi: 10.1016/j.jcv.2020.104362.
7. Guo W et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 Mar 31. doi: 10.1002/dmrr.3319.
8. Manalo IF et al. A dermatologic manifestation of COVID-19: Transient livedo reticularis. J Am Acad Dermat. 2020 Apr. doi: 10.1016/j.jaad.2020.04.018.
9. Tang N et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 Feb 19. doi: 10.1111/jth.14768, 18: 844-847.
10. Huang C et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
11. Thachil J et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020 Mar 25. doi: 10.1111/JTH.14810.
12. Tang N et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 Mar 27. doi: 10.1111/JTH.14817.
13. Zhang Y et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020 Mar 28. doi: 10.3760/cma.j.issn.0253-2727.2020.0006.
14. Poterucha TJ et al. More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb Haemost. 2017. doi: 10.1160/TH16-08-0620.
15. Mousavi S et al. Anti-inflammatory effects of heparin and its derivatives: A systematic review. Adv Pharmacol Pharm Sci. 2015 May 12. doi: 10.1155/2015/507151.
16. Kreuziger L et al. COVID-19 and VTE/anticoagulation: Frequently asked questions. American Society of Hematology. 2020 Apr 17.
Coronavirus disease 2019 (COVID-19) is a viral illness caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), currently causing a pandemic affecting many countries around the world, beginning in December 2019 and spreading rapidly on a global scale since. Globally, its burden has been increasing rapidly, with more than 1.2 million people testing positive for the illness and 123,000 people losing their lives, as per April 15th’s WHO COVID-19 Situation Report.1 These numbers are increasing with each passing day. Clinically, SARS-CoV-2 has a highly variable course, ranging from mild disease manifested as a self-limited illness (seen in younger and healthier patients) to severe pneumonia/ARDS and multiorgan failure with intravascular coagulopathy.2
In this article, we intend to investigate and establish a comprehensive review of COVID-19–associated coagulopathy mechanisms, laboratory findings, and current management guidelines put forth by various societies globally.
Mechanism of coagulopathy
COVID-19–associated coagulopathy has been shown to predispose to both arterial and venous thrombosis through excessive inflammation and hypoxia, leading to activation of the coagulation cascade and consumption of coagulation factors, resulting in microvascular thrombosis.3 Though the exact pathophysiology for the activation of this cascade is not known, the proposed mechanism has been: endothelial damage triggering platelet activation within the lung, leading to aggregation, thrombosis, and consumption of platelets in the lung.2,5,6
Fox et al. noted similar coagulopathy findings of four deceased COVID-19 patients. Autopsy results concluded that the dominant process was diffuse alveolar damage, notable CD4+ aggregates around thrombosed small vessels, significant associated hemorrhage, and thrombotic microangiopathy restricted to the lungs. The proposed mechanism was the activation of megakaryocytes, possibly native to the lung, with platelet aggregation, formation of platelet-rich clots, and fibrin deposition playing a major role.4
It has been noted that diabetic patients are at an increased risk of vascular events and hypercoagulability with COVID-19.7 COVID-19 can also cause livedo reticularis and acrocyanosis because of the microthrombosis in the cutaneous vasculature secondary to underlying coagulopathy, as reported in a case report of two U.S. patients with COVID-19.8
Clinical and laboratory abnormalities
A recent study reported from Netherlands by Klok et al. analyzed 184 ICU patients with COVID-19 pneumonia and concluded that the cumulative incidence of acute pulmonary embolism (PE), deep vein thrombosis (DVT), ischemic stroke, MI, or systemic arterial embolism was 31% (95% confidence interval, 20%-41%). PE was the most frequent thrombotic complication and was noted in 81% of patients. Coagulopathy, defined as spontaneous prolongation of prothrombin time (PT) > 3s or activated partial thromboplastin time (aPTT) > 5s, was reported as an independent predictor of thrombotic complications.3
Hematologic abnormalities that were noted in COVID-19 coagulopathy include: decreased platelet counts, decreased fibrinogen levels, elevated PT/INR, elevated partial thromboplastin time (PTT), and elevated d-dimer.9,10 In a retrospective analysis9 by Tang et al., 71.4% of nonsurvivors and 0.6% of survivors had met the criteria of disseminated intravascular coagulation (DIC) during their hospital stay. Nonsurvivors of COVID-19 had statistically significant elevation of d-dimer levels, FDP levels, PT, and aPTT, when compared to survivors (P < .05). The overall mortality in this study was reported as 11.5%.9 In addition, elevated d-dimer, fibrin and fibrinogen degradation product (FDP) levels and longer PT and aPTT were associated with poor prognosis.
Thus, d-dimer, PT, and platelet count should be measured in all patients who present with COVID-19 infection. We can also suggest that in patients with markedly elevated d-dimer (three- to fourfold increase), admission to hospital should be considered even in the absence of severe clinical symptoms.11
COVID-19 coagulopathy management
In a retrospective study9 of 449 patients with severe COVID-19 from Wuhan, China, by Tang et al., 99 patients mainly received low-weight molecular heparin (LMWH) for 7 days or longer. No difference in 28-day mortality was noted between heparin users and nonusers (30.3% vs. 29.7%; P = .910). A lower 28-day mortality rate was noted in heparin patients with sepsis-induced coagulopathy score of ≥4.0 (40.0% vs. 64.2%; P = .029) or a d-dimer level greater than sixfold of upper limit of normal, compared with nonusers of heparin.12
Another small study of seven COVID-19 patients with acroischemia in China demonstrated that administering LMWH was successful at decreasing the d-dimer and fibrinogen degradation product levels but noted no significant improvement in clinical symptoms.13
Recently, the International Society of Thrombosis and Hemostasis and American Society of Hematology published recommendations and guidelines regarding the recognition and management of coagulopathy in COVID-19.11 Prophylactic anticoagulation therapy with LMWH was recommended in all hospitalized patients with COVID-19, provided there was an absence of any contraindications (active bleeding, platelet count less than 25 x 109/L and fibrinogen less than 0.5 g/dL). Anticoagulation with LMWH was associated with better prognosis in severe COVID-19 patients and in COVID-19 patients with markedly elevated d-dimer, as it also has anti-inflammatory effects.12 This anti-inflammatory property of heparin has been documented in previous studies but the underlying mechanism is unknown and more research is required.14,15
Despite coagulopathy being noticed with cases of COVID-19, bleeding has been a rare finding in COVID-19 infections. If bleeding is noted, recommendations were made to keep platelet levels greater than 50 x109/L, fibrinogen less than 2.0 g/L, and INR [international normalized ratio] greater than 1.5.11 Mechanical thromboprophylaxis should be used when pharmacologic thromboprophylaxis is contraindicated.16
COVID-19 patients with new diagnoses of venous thromboembolism (VTE) or atrial fibrillation should be prescribed therapeutic anticoagulation. Patients who are already on anticoagulation for VTE or atrial fibrillation should continue their therapy unless the platelet count is less than 30-50x109/L or if the fibrinogen is less than 1.0 g/L.16
Conclusion
Coagulopathies associated with COVID-19 infections have been documented in several studies around the world, and it has been shown to be fatal in some cases. Despite documentation, the mechanism behind this coagulopathy is not well understood. Because of the potentially lethal complications associated with coagulopathies, early recognition and anticoagulation is imperative to improve clinical outcomes. These results are very preliminary: More studies are required to understand the role of anticoagulation and its effect on the morbidity and mortality associated with COVID-19–associated coagulopathy.
Dr. Yeruva is a board-certified hematologist/medical oncologist with WellSpan Health and clinical assistant professor of internal medicine, Penn State University, Hershey. Mr. Henderson is a third-year graduate-entry medical student at the Royal College of Surgeons in Ireland with interests in family medicine, dermatology, and tropical diseases. Dr. Al-Tawfiq is a consultant of internal medicine & infectious diseases, and the director of quality at Johns Hopkins Aramco Healthcare in Dhahran, Saudi Arabia, an adjunct associate professor of infectious diseases, molecular medicine and clinical pharmacology at Johns Hopkins University School of Medicine, and adjunct associate professor at Indiana University School of Medicine, Indianapolis. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals.
References
1. World Health Organization. Coronavirus disease (COVID-2019) situation reports.
2. Lippi G et al. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta. 2020 Mar 13. 506:145-8. doi: 10.1016/j.cca.2020.03.022.
3. Klok FA et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm Res. 2020;18(4):844-7. doi: 10.1016/j.thromres.2020.04.013.
4. Fox S et al. Pulmonary and cardiac pathology in Covid-19: The first autopsy series from New Orleans. MedRxiv. 2020 Apr 10. doi: 10.1101/2020.04.06.20050575.
5. Yang M et al. Thrombocytopenia in patients with severe acute respiratory syndrome (review). Hematology 2013 Sep 4. doi: 10.1080/1024533040002617.
6. Giannis D et al. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020 June. doi: 10.1016/j.jcv.2020.104362.
7. Guo W et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 Mar 31. doi: 10.1002/dmrr.3319.
8. Manalo IF et al. A dermatologic manifestation of COVID-19: Transient livedo reticularis. J Am Acad Dermat. 2020 Apr. doi: 10.1016/j.jaad.2020.04.018.
9. Tang N et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 Feb 19. doi: 10.1111/jth.14768, 18: 844-847.
10. Huang C et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
11. Thachil J et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020 Mar 25. doi: 10.1111/JTH.14810.
12. Tang N et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 Mar 27. doi: 10.1111/JTH.14817.
13. Zhang Y et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020 Mar 28. doi: 10.3760/cma.j.issn.0253-2727.2020.0006.
14. Poterucha TJ et al. More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb Haemost. 2017. doi: 10.1160/TH16-08-0620.
15. Mousavi S et al. Anti-inflammatory effects of heparin and its derivatives: A systematic review. Adv Pharmacol Pharm Sci. 2015 May 12. doi: 10.1155/2015/507151.
16. Kreuziger L et al. COVID-19 and VTE/anticoagulation: Frequently asked questions. American Society of Hematology. 2020 Apr 17.
IV-to-oral antibiotics can benefit patients with MRSA bloodstream infection
Background: Methicillin-resistant Staphylococcus aureus bloodstream infections carry a high risk of morbidity and relapse with most published guidelines recommending prolonged courses of IV antibiotics to ensure complete clearance of the infection. However, long-term IV antibiotic therapy may also be costly and is not without its own complications. An equally effective IV-to-oral antibiotic therapy would be welcome.
Study design: Retrospective cohort study.
Setting: A single academic center in the United States.
Synopsis: The investigators reviewed data from 492 adults with at least one positive blood culture for MRSA who had not yet completed their antibiotic course at the time of discharge during the index hospitalization but were sufficiently stable to complete outpatient antibiotic treatment. Of this cohort, 70 patients were switched to oral antibiotic therapy on discharge, while the rest received OPAT. The primary outcome was clinical failure, a 90-day composite measure of MRSA bloodstream infection recurrence, deep MRSA infection, or all-cause mortality. The most commonly used oral antibiotics were linezolid, trimethoprim/sulfamethoxazole, and clindamycin, all with high bioavailability. Endovascular infection was present in 21.5% of the study population. After propensity score adjustment for covariates, patients who received oral antibiotics had a nonsignificant reduction in the rate of clinical failure (hazard ratio, 0.379; 95% CI, 0.131-1.101).
Limitations of the study included its observational design with potential for significant residual confounding despite the propensity score–adjusted analysis, its single-center setting, the low frequency of endovascular infections, and the uncertainty in how the loss of patients to follow-up might have affected the results.
Bottom line: Selected patients with MRSA BSI may be successfully treated with sequential IV-to-oral antibiotic therapy.
Citation: Jorgensen SCJ et al. Sequential intravenous-to-oral outpatient antibiotic therapy for MRSA bacteraemia: One step closer. J Antimicrob Chemother. 2019 Feb;74(2):489-98.
Dr. Torres is a hospitalist at Massachusetts General Hospital.
Background: Methicillin-resistant Staphylococcus aureus bloodstream infections carry a high risk of morbidity and relapse with most published guidelines recommending prolonged courses of IV antibiotics to ensure complete clearance of the infection. However, long-term IV antibiotic therapy may also be costly and is not without its own complications. An equally effective IV-to-oral antibiotic therapy would be welcome.
Study design: Retrospective cohort study.
Setting: A single academic center in the United States.
Synopsis: The investigators reviewed data from 492 adults with at least one positive blood culture for MRSA who had not yet completed their antibiotic course at the time of discharge during the index hospitalization but were sufficiently stable to complete outpatient antibiotic treatment. Of this cohort, 70 patients were switched to oral antibiotic therapy on discharge, while the rest received OPAT. The primary outcome was clinical failure, a 90-day composite measure of MRSA bloodstream infection recurrence, deep MRSA infection, or all-cause mortality. The most commonly used oral antibiotics were linezolid, trimethoprim/sulfamethoxazole, and clindamycin, all with high bioavailability. Endovascular infection was present in 21.5% of the study population. After propensity score adjustment for covariates, patients who received oral antibiotics had a nonsignificant reduction in the rate of clinical failure (hazard ratio, 0.379; 95% CI, 0.131-1.101).
Limitations of the study included its observational design with potential for significant residual confounding despite the propensity score–adjusted analysis, its single-center setting, the low frequency of endovascular infections, and the uncertainty in how the loss of patients to follow-up might have affected the results.
Bottom line: Selected patients with MRSA BSI may be successfully treated with sequential IV-to-oral antibiotic therapy.
Citation: Jorgensen SCJ et al. Sequential intravenous-to-oral outpatient antibiotic therapy for MRSA bacteraemia: One step closer. J Antimicrob Chemother. 2019 Feb;74(2):489-98.
Dr. Torres is a hospitalist at Massachusetts General Hospital.
Background: Methicillin-resistant Staphylococcus aureus bloodstream infections carry a high risk of morbidity and relapse with most published guidelines recommending prolonged courses of IV antibiotics to ensure complete clearance of the infection. However, long-term IV antibiotic therapy may also be costly and is not without its own complications. An equally effective IV-to-oral antibiotic therapy would be welcome.
Study design: Retrospective cohort study.
Setting: A single academic center in the United States.
Synopsis: The investigators reviewed data from 492 adults with at least one positive blood culture for MRSA who had not yet completed their antibiotic course at the time of discharge during the index hospitalization but were sufficiently stable to complete outpatient antibiotic treatment. Of this cohort, 70 patients were switched to oral antibiotic therapy on discharge, while the rest received OPAT. The primary outcome was clinical failure, a 90-day composite measure of MRSA bloodstream infection recurrence, deep MRSA infection, or all-cause mortality. The most commonly used oral antibiotics were linezolid, trimethoprim/sulfamethoxazole, and clindamycin, all with high bioavailability. Endovascular infection was present in 21.5% of the study population. After propensity score adjustment for covariates, patients who received oral antibiotics had a nonsignificant reduction in the rate of clinical failure (hazard ratio, 0.379; 95% CI, 0.131-1.101).
Limitations of the study included its observational design with potential for significant residual confounding despite the propensity score–adjusted analysis, its single-center setting, the low frequency of endovascular infections, and the uncertainty in how the loss of patients to follow-up might have affected the results.
Bottom line: Selected patients with MRSA BSI may be successfully treated with sequential IV-to-oral antibiotic therapy.
Citation: Jorgensen SCJ et al. Sequential intravenous-to-oral outpatient antibiotic therapy for MRSA bacteraemia: One step closer. J Antimicrob Chemother. 2019 Feb;74(2):489-98.
Dr. Torres is a hospitalist at Massachusetts General Hospital.
FDA approves dapagliflozin for low-EF heart failure
The Food and Drug Administration has come through with the widely anticipated approval of dapagliflozin (Farxiga, AstraZeneca) for heart failure and reduced ejection fraction (HFrEF), adding to the rich array of medications lately available for this indication.
The approval follows the agency’s priority review of the sodium-glucose cotransporter 2 (SGLT2) inhibitor for reducing the risk of cardiovascular death and heart-failure hospitalization in adults with HFrEF following last year’s seminal results of the DAPA-HF trial.
In that study, treatment with dapagliflozin led to about a one-fourth reduction in risk of a primary endpoint consisting primarily of CV death or heart failure hospitalization in patients with chronic HFrEF, in both those with and without diabetes. The randomized, placebo-controlled trial had entered more than 4,700 patients.
Soon after, the FDA approved dapagliflozin for reducing the risk of heart failure hospitalization in adults with type 2 diabetes and other CV risk factors.
And of course, dapagliflozin – traditionally viewed only as an antidiabetic agent – has long been indicated for improvement of glycemic control in adults with type 2 diabetes.
The latest approval for patients with New York Heart Association functional class III-IV HFrEF makes dapagliflozin the only SGLT2 inhibitor to be indicated for heart failure in the absence of diabetes.
Soon after the DAPA-HF results had been unveiled at a major meeting, heart failure expert Christopher O’Connor, MD, expressed concern that dapagliflozin’s uptake for patients with HFrEF would be slow once it gained approval for patients without diabetes.
“We have to think of this as a drug that you would prescribe like an ACE inhibitor, or a beta-blocker, or a mineralocorticoid receptor antagonist, or sacubitril/valsartan [Entresto, Novartis],” Dr. O’Connor, of the Inova Heart and Vascular Institute, Falls Church, Va., said in an interview.
Dr. O’Connor was not associated with DAPA-HF and had previously disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has come through with the widely anticipated approval of dapagliflozin (Farxiga, AstraZeneca) for heart failure and reduced ejection fraction (HFrEF), adding to the rich array of medications lately available for this indication.
The approval follows the agency’s priority review of the sodium-glucose cotransporter 2 (SGLT2) inhibitor for reducing the risk of cardiovascular death and heart-failure hospitalization in adults with HFrEF following last year’s seminal results of the DAPA-HF trial.
In that study, treatment with dapagliflozin led to about a one-fourth reduction in risk of a primary endpoint consisting primarily of CV death or heart failure hospitalization in patients with chronic HFrEF, in both those with and without diabetes. The randomized, placebo-controlled trial had entered more than 4,700 patients.
Soon after, the FDA approved dapagliflozin for reducing the risk of heart failure hospitalization in adults with type 2 diabetes and other CV risk factors.
And of course, dapagliflozin – traditionally viewed only as an antidiabetic agent – has long been indicated for improvement of glycemic control in adults with type 2 diabetes.
The latest approval for patients with New York Heart Association functional class III-IV HFrEF makes dapagliflozin the only SGLT2 inhibitor to be indicated for heart failure in the absence of diabetes.
Soon after the DAPA-HF results had been unveiled at a major meeting, heart failure expert Christopher O’Connor, MD, expressed concern that dapagliflozin’s uptake for patients with HFrEF would be slow once it gained approval for patients without diabetes.
“We have to think of this as a drug that you would prescribe like an ACE inhibitor, or a beta-blocker, or a mineralocorticoid receptor antagonist, or sacubitril/valsartan [Entresto, Novartis],” Dr. O’Connor, of the Inova Heart and Vascular Institute, Falls Church, Va., said in an interview.
Dr. O’Connor was not associated with DAPA-HF and had previously disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has come through with the widely anticipated approval of dapagliflozin (Farxiga, AstraZeneca) for heart failure and reduced ejection fraction (HFrEF), adding to the rich array of medications lately available for this indication.
The approval follows the agency’s priority review of the sodium-glucose cotransporter 2 (SGLT2) inhibitor for reducing the risk of cardiovascular death and heart-failure hospitalization in adults with HFrEF following last year’s seminal results of the DAPA-HF trial.
In that study, treatment with dapagliflozin led to about a one-fourth reduction in risk of a primary endpoint consisting primarily of CV death or heart failure hospitalization in patients with chronic HFrEF, in both those with and without diabetes. The randomized, placebo-controlled trial had entered more than 4,700 patients.
Soon after, the FDA approved dapagliflozin for reducing the risk of heart failure hospitalization in adults with type 2 diabetes and other CV risk factors.
And of course, dapagliflozin – traditionally viewed only as an antidiabetic agent – has long been indicated for improvement of glycemic control in adults with type 2 diabetes.
The latest approval for patients with New York Heart Association functional class III-IV HFrEF makes dapagliflozin the only SGLT2 inhibitor to be indicated for heart failure in the absence of diabetes.
Soon after the DAPA-HF results had been unveiled at a major meeting, heart failure expert Christopher O’Connor, MD, expressed concern that dapagliflozin’s uptake for patients with HFrEF would be slow once it gained approval for patients without diabetes.
“We have to think of this as a drug that you would prescribe like an ACE inhibitor, or a beta-blocker, or a mineralocorticoid receptor antagonist, or sacubitril/valsartan [Entresto, Novartis],” Dr. O’Connor, of the Inova Heart and Vascular Institute, Falls Church, Va., said in an interview.
Dr. O’Connor was not associated with DAPA-HF and had previously disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 pulmonary severity ascribed to coagulation differences
Differences in COVID-19-related death rates between people of white and Asian ancestry may be partly explained by documented ethnic/racial differences in risk for blood clotting and pulmonary thrombotic events, investigators propose.
“Our novel findings demonstrate that COVID-19 is associated with a unique type of blood clotting disorder that is primarily focused within the lungs and which undoubtedly contributes to the high levels of mortality being seen in patients with COVID-19,” said James O’Donnell, MB, PhD, director of the Irish Centre for Vascular Biology at the Royal College of Surgeons in Ireland.
Dr. O’Donnell and colleagues studied pulmonary effects and outcomes of 83 patients admitted to St. James Hospital in Dublin, and found evidence to suggest that the diffuse, bilateral pulmonary inflammation seen in many patients with severe COVID-19 infections may be caused by a pulmonary-specific vasculopathy they label “pulmonary intravascular coagulopathy” (PIC), an entity distinct from disseminated intravascular coagulopathy (DIC).
“Given that thrombotic risk is significantly impacted by race, coupled with the accumulating evidence that coagulopathy is important in COVID-19 pathogenesis, our findings raise the intriguing possibility that pulmonary vasculopathy may contribute to the unexplained differences that are beginning to emerge highlighting racial susceptibility to COVID-19 mortality,” they wrote in a study published online in the British Journal of Haematology.
Study flaws harm conclusions
But critical care specialists who agreed to review and comment on the study for MDedge News said that it has significant flaws that affect the ability to interpret the findings and “undermine the conclusions reached by the authors.”
“The underlying premise of the study is that there are racial and ethnic differences in the development of venous thromboembolism that may explain the racial and ethnic differences in outcomes from COVID-19,” J. Daryl Thornton, MD, MPH, a fellow of the American Thoracic Society and associate professor of pulmonary, critical care, and sleep medicine at Case Western Reserve University, Cleveland, said in an interview. “This is an interesting hypothesis and one that could be easily tested in a well-designed study with sufficient representation from the relevant racial and ethnic groups. However, this study is neither well designed nor does it have sufficient racial and ethnic representation.”
Elliott R. Haut, MD, PhD, associate professor of surgery, anesthesiology and critical care medicine at Johns Hopkins Medicine, Baltimore, said in an interview that the study is “mediocre” and has the feel of a paper rushed to press.
“It talks about their theory that race, ethnicity, have an effect on venous thromboembolism, and that’s a pretty well-known fact. No one’s a hundred percent sure why that is, but certainly there are tons and tons of papers that show that there are groups that are at higher risk than others,” he said. “Their idea that this is caused by this pulmonary inflammation, that is totally a guess; there is no data in this paper to support that.”
Dr. Thornton and Dr. Haut both noted that the authors don’t define how race and ethnicity were determined and whether patients were asked to provide it, and although they mention the racial/ethnic breakdown once, subsequent references are to entire cohort are as “Caucasian.”
They also called into question the value of comparing laboratory data across continents in centers with different testing methods and parameters, especially in a time when the clinical picture changes so rapidly.
Coagulation differences
Dr. O’Donnell and colleagues noted that most studies of COVID-19-associated coagulopathy published to date have been with Chinese patients.
“This is important because race and ethnicity have major effects upon thrombotic risk. In particular, epidemiological studies have shown that the incidence of venous thromboembolism (VTE) is approximately three to fourfold lower in Chinese compared to Caucasian individuals. Conversely, VTE risk is significantly higher in African-Americans compared to Caucasians,” they wrote.
Because of the lower risk of VTE in the Chinese population, thromboprophylaxis with low-molecular-weight heparin (LMWH) or other agents is less frequently used in Chinese hospitals than in hospitals with predominantly non-Asian patients, they noted.
To see whether the were differences in coagulopathy between Chinese and white patients, the researchers enrolled 55 men and 28 women, median age 64, who were admitted to St. James Hospital with COVID-19 infections from March 13 through April 10, 2020. The cohort included 67 patients of white background, 10 of Asian ancestry, 5 of African ethnicity, and 1 of Latino/Hispanic ancestry.
Of the 83 patients, 67 had comorbidities at admission. At the time of the report, 50 patients had fully recovered and were discharged, 20 remained in the hospital, and 13 had died. In all, 50 patients were discharged without needing ICU care, 23 were admitted to the ICU, and 10 required ICU but were deemed “clinically unsuitable” for ICU admission.
Although the patients had normal prothrombin time (PT) and normal activated partial thromboplastin time (APTT), plasma d-dimer levels were significantly elevated and were above the range of normal in two-thirds of patients on admission.
Despite the increased d-dimer levels, however, there was no evidence of DIC as defined by the International Society of Thrombosis and Hemostasis Scientific and Standardization committee (ISTH SSC) guidelines. Platelet counts were in the normal range in 83.1% of patients, and only five had counts less than 100 x 109/L at admission. Fibrinogen levels were also elevated, as were C-reactive protein levels, both likely indicating an acute phase response.
“Thus, despite the fact that thrombotic risk is much higher in Caucasian patients and the significant elevated levels of d-dimers observed, overt DIC as defined according to the ISTH SSC DIC score was present in none of our COVID-19 patients at time of admission. Nevertheless, our data confirm that severe COVID-19 infection is associated with a significant coagulopathy in Caucasian patients that appears to be similar in magnitude to that previously reported in the original Chinese cohorts,” they wrote.
When they compared patients who required ICU admission for ventilator support and those who died with patients who were discharged without needing ICU support, they found that survivors were younger (median age 60.2 vs. 75.2 years), and that more critically ill patients were more likely to have comorbidities.
They also found that patients with abnormal coagulation parameters on admission were significantly more likely to have poor prognosis (P = .018), and that patients in the adverse outcomes group had significantly higher fibrinogen and CRP levels (P = .045 and .0005, respectively).
There was no significant difference in PT between the prognosis groups at admission, but by day 4 and beyond PT was a median of 13.1 vs. 12.5 seconds in the favorable outcomes groups (P = .007), and patients with poor prognosis continued to have significantly higher d-dimer levels. (P = .003)
“Cumulatively, these data support the hypothesis that COVID-19–associated coagulopathy probably contributes to the underlying pulmonary pathogenesis,” the researchers wrote.
They noted that the angiotensin converting enzyme 2 (ACE-2) receptor that COVID-19 uses to enter cells is expressed on both type II pneumocytes and vascular endothelial cells within the lung, suggesting that the coagulopathy may be related to direct pulmonary endothelial cell infection , activation, and/or damage, and to the documented cytokine storm that can affect thrombin generation and fibrin deposition within the lungs.
“In the context of this lung-centric vasculopathy, we hypothesize that the refractory acute respiratory distress syndrome phenotype observed in severe COVID-19 is due to concurrent ‘double-hit’ pathologies targeting both ventilation (V) and perfusion (Q) within the lungs where alveoli and pulmonary microvasculature exist in close anatomical juxtaposition,” they wrote.
The investigators noted that larger randomized trials will be needed to determine whether more aggressive anti-coagulation and/or targeted anti-inflammatory therapies could effectively treated PIC in patients with severe COVID-19.
The study was supported by the Wellcome Trust and the Health Research Board Health Service and the Research and Development Division, Northern Ireland. Dr. O’Donnell disclosed speakers bureau activities, advisory board participation, and research grants from multiple companies. The other doctors had no relevant conflicts of interest to disclose.
SOURCE: Fogarty H et al. Br J Haematol. 2020 Apr 24. doi: 10.1111/bjh.16749.
Differences in COVID-19-related death rates between people of white and Asian ancestry may be partly explained by documented ethnic/racial differences in risk for blood clotting and pulmonary thrombotic events, investigators propose.
“Our novel findings demonstrate that COVID-19 is associated with a unique type of blood clotting disorder that is primarily focused within the lungs and which undoubtedly contributes to the high levels of mortality being seen in patients with COVID-19,” said James O’Donnell, MB, PhD, director of the Irish Centre for Vascular Biology at the Royal College of Surgeons in Ireland.
Dr. O’Donnell and colleagues studied pulmonary effects and outcomes of 83 patients admitted to St. James Hospital in Dublin, and found evidence to suggest that the diffuse, bilateral pulmonary inflammation seen in many patients with severe COVID-19 infections may be caused by a pulmonary-specific vasculopathy they label “pulmonary intravascular coagulopathy” (PIC), an entity distinct from disseminated intravascular coagulopathy (DIC).
“Given that thrombotic risk is significantly impacted by race, coupled with the accumulating evidence that coagulopathy is important in COVID-19 pathogenesis, our findings raise the intriguing possibility that pulmonary vasculopathy may contribute to the unexplained differences that are beginning to emerge highlighting racial susceptibility to COVID-19 mortality,” they wrote in a study published online in the British Journal of Haematology.
Study flaws harm conclusions
But critical care specialists who agreed to review and comment on the study for MDedge News said that it has significant flaws that affect the ability to interpret the findings and “undermine the conclusions reached by the authors.”
“The underlying premise of the study is that there are racial and ethnic differences in the development of venous thromboembolism that may explain the racial and ethnic differences in outcomes from COVID-19,” J. Daryl Thornton, MD, MPH, a fellow of the American Thoracic Society and associate professor of pulmonary, critical care, and sleep medicine at Case Western Reserve University, Cleveland, said in an interview. “This is an interesting hypothesis and one that could be easily tested in a well-designed study with sufficient representation from the relevant racial and ethnic groups. However, this study is neither well designed nor does it have sufficient racial and ethnic representation.”
Elliott R. Haut, MD, PhD, associate professor of surgery, anesthesiology and critical care medicine at Johns Hopkins Medicine, Baltimore, said in an interview that the study is “mediocre” and has the feel of a paper rushed to press.
“It talks about their theory that race, ethnicity, have an effect on venous thromboembolism, and that’s a pretty well-known fact. No one’s a hundred percent sure why that is, but certainly there are tons and tons of papers that show that there are groups that are at higher risk than others,” he said. “Their idea that this is caused by this pulmonary inflammation, that is totally a guess; there is no data in this paper to support that.”
Dr. Thornton and Dr. Haut both noted that the authors don’t define how race and ethnicity were determined and whether patients were asked to provide it, and although they mention the racial/ethnic breakdown once, subsequent references are to entire cohort are as “Caucasian.”
They also called into question the value of comparing laboratory data across continents in centers with different testing methods and parameters, especially in a time when the clinical picture changes so rapidly.
Coagulation differences
Dr. O’Donnell and colleagues noted that most studies of COVID-19-associated coagulopathy published to date have been with Chinese patients.
“This is important because race and ethnicity have major effects upon thrombotic risk. In particular, epidemiological studies have shown that the incidence of venous thromboembolism (VTE) is approximately three to fourfold lower in Chinese compared to Caucasian individuals. Conversely, VTE risk is significantly higher in African-Americans compared to Caucasians,” they wrote.
Because of the lower risk of VTE in the Chinese population, thromboprophylaxis with low-molecular-weight heparin (LMWH) or other agents is less frequently used in Chinese hospitals than in hospitals with predominantly non-Asian patients, they noted.
To see whether the were differences in coagulopathy between Chinese and white patients, the researchers enrolled 55 men and 28 women, median age 64, who were admitted to St. James Hospital with COVID-19 infections from March 13 through April 10, 2020. The cohort included 67 patients of white background, 10 of Asian ancestry, 5 of African ethnicity, and 1 of Latino/Hispanic ancestry.
Of the 83 patients, 67 had comorbidities at admission. At the time of the report, 50 patients had fully recovered and were discharged, 20 remained in the hospital, and 13 had died. In all, 50 patients were discharged without needing ICU care, 23 were admitted to the ICU, and 10 required ICU but were deemed “clinically unsuitable” for ICU admission.
Although the patients had normal prothrombin time (PT) and normal activated partial thromboplastin time (APTT), plasma d-dimer levels were significantly elevated and were above the range of normal in two-thirds of patients on admission.
Despite the increased d-dimer levels, however, there was no evidence of DIC as defined by the International Society of Thrombosis and Hemostasis Scientific and Standardization committee (ISTH SSC) guidelines. Platelet counts were in the normal range in 83.1% of patients, and only five had counts less than 100 x 109/L at admission. Fibrinogen levels were also elevated, as were C-reactive protein levels, both likely indicating an acute phase response.
“Thus, despite the fact that thrombotic risk is much higher in Caucasian patients and the significant elevated levels of d-dimers observed, overt DIC as defined according to the ISTH SSC DIC score was present in none of our COVID-19 patients at time of admission. Nevertheless, our data confirm that severe COVID-19 infection is associated with a significant coagulopathy in Caucasian patients that appears to be similar in magnitude to that previously reported in the original Chinese cohorts,” they wrote.
When they compared patients who required ICU admission for ventilator support and those who died with patients who were discharged without needing ICU support, they found that survivors were younger (median age 60.2 vs. 75.2 years), and that more critically ill patients were more likely to have comorbidities.
They also found that patients with abnormal coagulation parameters on admission were significantly more likely to have poor prognosis (P = .018), and that patients in the adverse outcomes group had significantly higher fibrinogen and CRP levels (P = .045 and .0005, respectively).
There was no significant difference in PT between the prognosis groups at admission, but by day 4 and beyond PT was a median of 13.1 vs. 12.5 seconds in the favorable outcomes groups (P = .007), and patients with poor prognosis continued to have significantly higher d-dimer levels. (P = .003)
“Cumulatively, these data support the hypothesis that COVID-19–associated coagulopathy probably contributes to the underlying pulmonary pathogenesis,” the researchers wrote.
They noted that the angiotensin converting enzyme 2 (ACE-2) receptor that COVID-19 uses to enter cells is expressed on both type II pneumocytes and vascular endothelial cells within the lung, suggesting that the coagulopathy may be related to direct pulmonary endothelial cell infection , activation, and/or damage, and to the documented cytokine storm that can affect thrombin generation and fibrin deposition within the lungs.
“In the context of this lung-centric vasculopathy, we hypothesize that the refractory acute respiratory distress syndrome phenotype observed in severe COVID-19 is due to concurrent ‘double-hit’ pathologies targeting both ventilation (V) and perfusion (Q) within the lungs where alveoli and pulmonary microvasculature exist in close anatomical juxtaposition,” they wrote.
The investigators noted that larger randomized trials will be needed to determine whether more aggressive anti-coagulation and/or targeted anti-inflammatory therapies could effectively treated PIC in patients with severe COVID-19.
The study was supported by the Wellcome Trust and the Health Research Board Health Service and the Research and Development Division, Northern Ireland. Dr. O’Donnell disclosed speakers bureau activities, advisory board participation, and research grants from multiple companies. The other doctors had no relevant conflicts of interest to disclose.
SOURCE: Fogarty H et al. Br J Haematol. 2020 Apr 24. doi: 10.1111/bjh.16749.
Differences in COVID-19-related death rates between people of white and Asian ancestry may be partly explained by documented ethnic/racial differences in risk for blood clotting and pulmonary thrombotic events, investigators propose.
“Our novel findings demonstrate that COVID-19 is associated with a unique type of blood clotting disorder that is primarily focused within the lungs and which undoubtedly contributes to the high levels of mortality being seen in patients with COVID-19,” said James O’Donnell, MB, PhD, director of the Irish Centre for Vascular Biology at the Royal College of Surgeons in Ireland.
Dr. O’Donnell and colleagues studied pulmonary effects and outcomes of 83 patients admitted to St. James Hospital in Dublin, and found evidence to suggest that the diffuse, bilateral pulmonary inflammation seen in many patients with severe COVID-19 infections may be caused by a pulmonary-specific vasculopathy they label “pulmonary intravascular coagulopathy” (PIC), an entity distinct from disseminated intravascular coagulopathy (DIC).
“Given that thrombotic risk is significantly impacted by race, coupled with the accumulating evidence that coagulopathy is important in COVID-19 pathogenesis, our findings raise the intriguing possibility that pulmonary vasculopathy may contribute to the unexplained differences that are beginning to emerge highlighting racial susceptibility to COVID-19 mortality,” they wrote in a study published online in the British Journal of Haematology.
Study flaws harm conclusions
But critical care specialists who agreed to review and comment on the study for MDedge News said that it has significant flaws that affect the ability to interpret the findings and “undermine the conclusions reached by the authors.”
“The underlying premise of the study is that there are racial and ethnic differences in the development of venous thromboembolism that may explain the racial and ethnic differences in outcomes from COVID-19,” J. Daryl Thornton, MD, MPH, a fellow of the American Thoracic Society and associate professor of pulmonary, critical care, and sleep medicine at Case Western Reserve University, Cleveland, said in an interview. “This is an interesting hypothesis and one that could be easily tested in a well-designed study with sufficient representation from the relevant racial and ethnic groups. However, this study is neither well designed nor does it have sufficient racial and ethnic representation.”
Elliott R. Haut, MD, PhD, associate professor of surgery, anesthesiology and critical care medicine at Johns Hopkins Medicine, Baltimore, said in an interview that the study is “mediocre” and has the feel of a paper rushed to press.
“It talks about their theory that race, ethnicity, have an effect on venous thromboembolism, and that’s a pretty well-known fact. No one’s a hundred percent sure why that is, but certainly there are tons and tons of papers that show that there are groups that are at higher risk than others,” he said. “Their idea that this is caused by this pulmonary inflammation, that is totally a guess; there is no data in this paper to support that.”
Dr. Thornton and Dr. Haut both noted that the authors don’t define how race and ethnicity were determined and whether patients were asked to provide it, and although they mention the racial/ethnic breakdown once, subsequent references are to entire cohort are as “Caucasian.”
They also called into question the value of comparing laboratory data across continents in centers with different testing methods and parameters, especially in a time when the clinical picture changes so rapidly.
Coagulation differences
Dr. O’Donnell and colleagues noted that most studies of COVID-19-associated coagulopathy published to date have been with Chinese patients.
“This is important because race and ethnicity have major effects upon thrombotic risk. In particular, epidemiological studies have shown that the incidence of venous thromboembolism (VTE) is approximately three to fourfold lower in Chinese compared to Caucasian individuals. Conversely, VTE risk is significantly higher in African-Americans compared to Caucasians,” they wrote.
Because of the lower risk of VTE in the Chinese population, thromboprophylaxis with low-molecular-weight heparin (LMWH) or other agents is less frequently used in Chinese hospitals than in hospitals with predominantly non-Asian patients, they noted.
To see whether the were differences in coagulopathy between Chinese and white patients, the researchers enrolled 55 men and 28 women, median age 64, who were admitted to St. James Hospital with COVID-19 infections from March 13 through April 10, 2020. The cohort included 67 patients of white background, 10 of Asian ancestry, 5 of African ethnicity, and 1 of Latino/Hispanic ancestry.
Of the 83 patients, 67 had comorbidities at admission. At the time of the report, 50 patients had fully recovered and were discharged, 20 remained in the hospital, and 13 had died. In all, 50 patients were discharged without needing ICU care, 23 were admitted to the ICU, and 10 required ICU but were deemed “clinically unsuitable” for ICU admission.
Although the patients had normal prothrombin time (PT) and normal activated partial thromboplastin time (APTT), plasma d-dimer levels were significantly elevated and were above the range of normal in two-thirds of patients on admission.
Despite the increased d-dimer levels, however, there was no evidence of DIC as defined by the International Society of Thrombosis and Hemostasis Scientific and Standardization committee (ISTH SSC) guidelines. Platelet counts were in the normal range in 83.1% of patients, and only five had counts less than 100 x 109/L at admission. Fibrinogen levels were also elevated, as were C-reactive protein levels, both likely indicating an acute phase response.
“Thus, despite the fact that thrombotic risk is much higher in Caucasian patients and the significant elevated levels of d-dimers observed, overt DIC as defined according to the ISTH SSC DIC score was present in none of our COVID-19 patients at time of admission. Nevertheless, our data confirm that severe COVID-19 infection is associated with a significant coagulopathy in Caucasian patients that appears to be similar in magnitude to that previously reported in the original Chinese cohorts,” they wrote.
When they compared patients who required ICU admission for ventilator support and those who died with patients who were discharged without needing ICU support, they found that survivors were younger (median age 60.2 vs. 75.2 years), and that more critically ill patients were more likely to have comorbidities.
They also found that patients with abnormal coagulation parameters on admission were significantly more likely to have poor prognosis (P = .018), and that patients in the adverse outcomes group had significantly higher fibrinogen and CRP levels (P = .045 and .0005, respectively).
There was no significant difference in PT between the prognosis groups at admission, but by day 4 and beyond PT was a median of 13.1 vs. 12.5 seconds in the favorable outcomes groups (P = .007), and patients with poor prognosis continued to have significantly higher d-dimer levels. (P = .003)
“Cumulatively, these data support the hypothesis that COVID-19–associated coagulopathy probably contributes to the underlying pulmonary pathogenesis,” the researchers wrote.
They noted that the angiotensin converting enzyme 2 (ACE-2) receptor that COVID-19 uses to enter cells is expressed on both type II pneumocytes and vascular endothelial cells within the lung, suggesting that the coagulopathy may be related to direct pulmonary endothelial cell infection , activation, and/or damage, and to the documented cytokine storm that can affect thrombin generation and fibrin deposition within the lungs.
“In the context of this lung-centric vasculopathy, we hypothesize that the refractory acute respiratory distress syndrome phenotype observed in severe COVID-19 is due to concurrent ‘double-hit’ pathologies targeting both ventilation (V) and perfusion (Q) within the lungs where alveoli and pulmonary microvasculature exist in close anatomical juxtaposition,” they wrote.
The investigators noted that larger randomized trials will be needed to determine whether more aggressive anti-coagulation and/or targeted anti-inflammatory therapies could effectively treated PIC in patients with severe COVID-19.
The study was supported by the Wellcome Trust and the Health Research Board Health Service and the Research and Development Division, Northern Ireland. Dr. O’Donnell disclosed speakers bureau activities, advisory board participation, and research grants from multiple companies. The other doctors had no relevant conflicts of interest to disclose.
SOURCE: Fogarty H et al. Br J Haematol. 2020 Apr 24. doi: 10.1111/bjh.16749.
FROM THE BRITISH JOURNAL OF HEMATOLOGY
New angiotensin studies in COVID-19 give more reassurance
Four more studies of the relationship of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) with COVID-19 have been published in the past few days in top-tier peer-reviewed journals, and on the whole, the data are reassuring.
Three of the new studies were published in the New England Journal of Medicine on May 1, and one study was published in JAMA Cardiology on May 5.
Although all the studies are observational in design and have some confounding factors, overall, However, there are some contradictory findings in secondary analyses regarding possible differences in the effects of the two drug classes.
Providing commentary, John McMurray, MD, professor of medical cardiology at the University of Glasgow, said: “The overall picture seems to suggest no increase in risk of adverse outcomes in patients taking renin-angiotensin system [RAS] blockers ― but with lots of caveats: These are all observational rather than randomized studies, and there may be residual or unmeasured confounding.”
Was it ‘Much ado about nothing’?
Franz Messerli, MD, professor of medicine at the University of Bern (Switzerland), added: “Given this state of the art, I am inclined to consider RAS blockade and COVID-19 – despite all the hype in the news media – as much ado about nothing.”
But both Dr. McMurray and Dr. Messerli said they were intrigued about possible differences in the effects of ACE inhibitors and ARBs that some of the new results suggest.
In one study, a team led by Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center, Boston, analyzed data from 8,910 patients with COVID-19 admitted to 169 hospitals in Asia, Europe, and North America who had either died in the hospital (5.8%) or survived to hospital discharge (94.2%).
In multivariate logistic-regression analysis, age greater than 65 years, coronary artery disease, congestive heart failure, history of cardiac arrhythmia, chronic obstructive pulmonary disease, and current smoking were associated with an increased risk for in-hospital death. Female sex was associated with a decreased risk. Neither ACE inhibitors nor ARBs were associated with an increased risk for in-hospital death.
In fact, ACE inhibitors were associated with a significant reduction in mortality (odds ratio, 0.33), as were statins (OR, 0.35).
The authors, however, stressed that these observations about reduced mortality with ACE inhibitors and statins “should be considered with extreme caution.”
“Because our study was not a randomized, controlled trial, we cannot exclude the possibility of confounding. In addition, we examined relationships between many variables and in-hospital death, and no primary hypothesis was prespecified; these factors increased the probability of chance associations being found. Therefore, a cause-and-effect relationship between drug therapy and survival should not be inferred,” they wrote.
A secondary analysis that was restricted to patients with hypertension (those for whom an ACE inhibitor or an ARB would be indicated) also did not show harm.
A second study published in the New England Journal of Medicine had a case-control design. The authors, led by Giuseppe Mancia, MD, of the University of Milano-Bicocca (Italy), compared 6,272 patients with confirmed COVID-19 (case patients) with 30,759 control persons who were matched according to age, sex, and municipality of residence.
In a conditional logistic-regression multivariate analysis, neither ACE inhibitors nor ARBs were associated with the likelihood of SARS-CoV-2 infection.
“Thus, our results do not provide evidence of an independent relationship between renin angiotensin aldosterone blockers and the susceptibility to COVID-19 in humans,” the authors concluded.
In addition, a second analysis that compared patients who had severe or fatal infections with matched control persons did not show an association between ACE inhibitors or ARBs and severe disease.
In the third study published in the New England Journal of Medicine, a group led by Harmony R. Reynolds, MD, of New York University, analyzed data from the health records of 12,594 patients in the NYU Langone Health system who had been tested for COVID-19. They found 5,894 patients whose test results were positive. Of these patients, 1,002 had severe illness, which was defined as illness requiring admission to the ICU, need for mechanical ventilation, or death.
Using Bayesian analysis and propensity score matching, the researchers assessed the relation between previous treatment with five different classes of antihypertensive drugs (ACE inhibitors, ARBs, beta blockers, calcium blockers, and thiazide diuretics) and the likelihood of a positive or negative result on COVID-19 testing, as well as the likelihood of severe illness among patients who tested positive.
Results showed no positive association between any of the analyzed drug classes and either a positive test result or severe illness.
In an accompanying editorial, a group led by John A. Jarcho, MD, of Harvard Medical School, Boston, and deputy editor of the New England Journal of Medicine, wrote: “Taken together, these three studies do not provide evidence to support the hypothesis that ACE inhibitor or ARB use is associated with the risk of SARS-CoV-2 infection, the risk of severe COVID-19 among those infected, or the risk of in-hospital death among those with a positive test.
“Each of these studies has weaknesses inherent in observational data, but we find it reassuring that three studies in different populations and with different designs arrive at the consistent message that the continued use of ACE inhibitors and ARBs is unlikely to be harmful in patients with COVID-19. Several other smaller studies from China and the United Kingdom have come to the same conclusion,” the authors of the editorial stated.
In the study published in JAMA Cardiology, a group led by Neil Mehta, MBBS, of the Cleveland Clinic, Ohio, analyzed data on 18,472 patients who had been tested for COVID-19 between March 8 and April 12 in the Cleveland Clinic Health System in Ohio and Florida. Of these patients, 9.4% tested positive.
After overlap propensity score weighting for both ACE inhibitors and ARBs to take into account relevant comorbidities, there was no difference in risk for testing positive among patients taking an ACE inhibitor or an ARB in comparison with those not taking such medication.
Are there different effects between ACE inhibitors and ARBs?
A secondary exploratory analysis showed a higher likelihood of hospital admission among patients who tested positive and who were taking either ACE inhibitors (OR, 1.84) or ARBs (OR, 1.61), and there was a higher likelihood of ICU admission among patients who tested positive and who were taking an ACE inhibitor (OR 1.77), but no such difference was observed among those taking ARBs.
Coauthor Ankur Kalra, MD, of the Cleveland Clinic, said in an interview that results of the exploratory analysis fit with the hypothesis that the two drugs classes may have different effects in patients with COVID-19.
“Angiotensin II promotes vasoconstriction, inflammation, and fibrosis in the lungs, and ARBs block the effects of angiotensin II more effectively than ACE inhibitors. In addition, ACE inhibitors (but not ARBs) increase levels of bradykinin, which may be one factor leading to acute respiratory distress syndrome,” he noted.
“However, these results should only be considered exploratory, as there is inherent bias in observational data,” Dr. Kalra stressed.
In an accompanying editorial in JAMA Cardiology, a group led by Laine E. Thomas, PhD, of Duke Clinical Research Institute, Durham, North Carolina, said that the results of this secondary exploratory analysis are limited by a small number of patients and “are likely explained by confounding and should not be inferred as causal.”
The New England Journal of Medicine editorialists reached a similar conclusion regarding the lower mortality in COVID-19 patients who took ACE inhibitors in the study by Dr. Mehra and colleagues. They say this unexpected result “may be due to unmeasured confounding and, in the absence of a randomized trial, should not be regarded as evidence to prescribe these drugs in patients with COVID-19.”
Providing further comment, Dr. McMurray said: “Normally, I would not read too much into the different effects of ACE inhibitors and ARBs suggested in the Cleveland study because of the small numbers (about 28 ACE inhibitor–treated patients admitted to ICU) and the limited information about matching and/or adjustment for potential differences between groups.
“I could also argue that the comparison that would best answer the question about risk related to type of RAS blocker would be the direct comparison of people taking an ACE inhibitor with those taking an ARB (and that doesn’t look very different). The only thing that makes me a little cautious about completely dismissing the possibility of a difference between ACE inhibitor and ARB here is the suggestion of a similar trend in another large study from the VA [Veterans Affairs] system,” he added.
He also noted that speculation about there being mechanisms that involve different effects of the two drug classes on bradykinin and angiotensin II was “plausible but unproven.”
Dr. Messerli added: “Before turning the page, I would like to see an analysis comparing ACE inhibitors and ARBs, since experimentally, their effect on ACE2 (the receptor to which the virus binds) seems to differ. The study of Mehta et al in JAMA Cardiology may be the first clinical hint indicating that ARBs are more protective than ACEIs. However even here, the looming possibility of confounding cannot be excluded.”
Dr. Messerli also pointed to a hypothesis that suggests that direct viral infection of endothelial cells expressing ACE2 receptors may explain worse outcomes in patients with cardiovascular comorbidities, which provides a rationale for therapies to stabilize the endothelium, particularly with anti-inflammatory anticytokine drugs, ACE inhibitors, and statins.
A version of this article originally appeared on Medscape.com.
Four more studies of the relationship of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) with COVID-19 have been published in the past few days in top-tier peer-reviewed journals, and on the whole, the data are reassuring.
Three of the new studies were published in the New England Journal of Medicine on May 1, and one study was published in JAMA Cardiology on May 5.
Although all the studies are observational in design and have some confounding factors, overall, However, there are some contradictory findings in secondary analyses regarding possible differences in the effects of the two drug classes.
Providing commentary, John McMurray, MD, professor of medical cardiology at the University of Glasgow, said: “The overall picture seems to suggest no increase in risk of adverse outcomes in patients taking renin-angiotensin system [RAS] blockers ― but with lots of caveats: These are all observational rather than randomized studies, and there may be residual or unmeasured confounding.”
Was it ‘Much ado about nothing’?
Franz Messerli, MD, professor of medicine at the University of Bern (Switzerland), added: “Given this state of the art, I am inclined to consider RAS blockade and COVID-19 – despite all the hype in the news media – as much ado about nothing.”
But both Dr. McMurray and Dr. Messerli said they were intrigued about possible differences in the effects of ACE inhibitors and ARBs that some of the new results suggest.
In one study, a team led by Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center, Boston, analyzed data from 8,910 patients with COVID-19 admitted to 169 hospitals in Asia, Europe, and North America who had either died in the hospital (5.8%) or survived to hospital discharge (94.2%).
In multivariate logistic-regression analysis, age greater than 65 years, coronary artery disease, congestive heart failure, history of cardiac arrhythmia, chronic obstructive pulmonary disease, and current smoking were associated with an increased risk for in-hospital death. Female sex was associated with a decreased risk. Neither ACE inhibitors nor ARBs were associated with an increased risk for in-hospital death.
In fact, ACE inhibitors were associated with a significant reduction in mortality (odds ratio, 0.33), as were statins (OR, 0.35).
The authors, however, stressed that these observations about reduced mortality with ACE inhibitors and statins “should be considered with extreme caution.”
“Because our study was not a randomized, controlled trial, we cannot exclude the possibility of confounding. In addition, we examined relationships between many variables and in-hospital death, and no primary hypothesis was prespecified; these factors increased the probability of chance associations being found. Therefore, a cause-and-effect relationship between drug therapy and survival should not be inferred,” they wrote.
A secondary analysis that was restricted to patients with hypertension (those for whom an ACE inhibitor or an ARB would be indicated) also did not show harm.
A second study published in the New England Journal of Medicine had a case-control design. The authors, led by Giuseppe Mancia, MD, of the University of Milano-Bicocca (Italy), compared 6,272 patients with confirmed COVID-19 (case patients) with 30,759 control persons who were matched according to age, sex, and municipality of residence.
In a conditional logistic-regression multivariate analysis, neither ACE inhibitors nor ARBs were associated with the likelihood of SARS-CoV-2 infection.
“Thus, our results do not provide evidence of an independent relationship between renin angiotensin aldosterone blockers and the susceptibility to COVID-19 in humans,” the authors concluded.
In addition, a second analysis that compared patients who had severe or fatal infections with matched control persons did not show an association between ACE inhibitors or ARBs and severe disease.
In the third study published in the New England Journal of Medicine, a group led by Harmony R. Reynolds, MD, of New York University, analyzed data from the health records of 12,594 patients in the NYU Langone Health system who had been tested for COVID-19. They found 5,894 patients whose test results were positive. Of these patients, 1,002 had severe illness, which was defined as illness requiring admission to the ICU, need for mechanical ventilation, or death.
Using Bayesian analysis and propensity score matching, the researchers assessed the relation between previous treatment with five different classes of antihypertensive drugs (ACE inhibitors, ARBs, beta blockers, calcium blockers, and thiazide diuretics) and the likelihood of a positive or negative result on COVID-19 testing, as well as the likelihood of severe illness among patients who tested positive.
Results showed no positive association between any of the analyzed drug classes and either a positive test result or severe illness.
In an accompanying editorial, a group led by John A. Jarcho, MD, of Harvard Medical School, Boston, and deputy editor of the New England Journal of Medicine, wrote: “Taken together, these three studies do not provide evidence to support the hypothesis that ACE inhibitor or ARB use is associated with the risk of SARS-CoV-2 infection, the risk of severe COVID-19 among those infected, or the risk of in-hospital death among those with a positive test.
“Each of these studies has weaknesses inherent in observational data, but we find it reassuring that three studies in different populations and with different designs arrive at the consistent message that the continued use of ACE inhibitors and ARBs is unlikely to be harmful in patients with COVID-19. Several other smaller studies from China and the United Kingdom have come to the same conclusion,” the authors of the editorial stated.
In the study published in JAMA Cardiology, a group led by Neil Mehta, MBBS, of the Cleveland Clinic, Ohio, analyzed data on 18,472 patients who had been tested for COVID-19 between March 8 and April 12 in the Cleveland Clinic Health System in Ohio and Florida. Of these patients, 9.4% tested positive.
After overlap propensity score weighting for both ACE inhibitors and ARBs to take into account relevant comorbidities, there was no difference in risk for testing positive among patients taking an ACE inhibitor or an ARB in comparison with those not taking such medication.
Are there different effects between ACE inhibitors and ARBs?
A secondary exploratory analysis showed a higher likelihood of hospital admission among patients who tested positive and who were taking either ACE inhibitors (OR, 1.84) or ARBs (OR, 1.61), and there was a higher likelihood of ICU admission among patients who tested positive and who were taking an ACE inhibitor (OR 1.77), but no such difference was observed among those taking ARBs.
Coauthor Ankur Kalra, MD, of the Cleveland Clinic, said in an interview that results of the exploratory analysis fit with the hypothesis that the two drugs classes may have different effects in patients with COVID-19.
“Angiotensin II promotes vasoconstriction, inflammation, and fibrosis in the lungs, and ARBs block the effects of angiotensin II more effectively than ACE inhibitors. In addition, ACE inhibitors (but not ARBs) increase levels of bradykinin, which may be one factor leading to acute respiratory distress syndrome,” he noted.
“However, these results should only be considered exploratory, as there is inherent bias in observational data,” Dr. Kalra stressed.
In an accompanying editorial in JAMA Cardiology, a group led by Laine E. Thomas, PhD, of Duke Clinical Research Institute, Durham, North Carolina, said that the results of this secondary exploratory analysis are limited by a small number of patients and “are likely explained by confounding and should not be inferred as causal.”
The New England Journal of Medicine editorialists reached a similar conclusion regarding the lower mortality in COVID-19 patients who took ACE inhibitors in the study by Dr. Mehra and colleagues. They say this unexpected result “may be due to unmeasured confounding and, in the absence of a randomized trial, should not be regarded as evidence to prescribe these drugs in patients with COVID-19.”
Providing further comment, Dr. McMurray said: “Normally, I would not read too much into the different effects of ACE inhibitors and ARBs suggested in the Cleveland study because of the small numbers (about 28 ACE inhibitor–treated patients admitted to ICU) and the limited information about matching and/or adjustment for potential differences between groups.
“I could also argue that the comparison that would best answer the question about risk related to type of RAS blocker would be the direct comparison of people taking an ACE inhibitor with those taking an ARB (and that doesn’t look very different). The only thing that makes me a little cautious about completely dismissing the possibility of a difference between ACE inhibitor and ARB here is the suggestion of a similar trend in another large study from the VA [Veterans Affairs] system,” he added.
He also noted that speculation about there being mechanisms that involve different effects of the two drug classes on bradykinin and angiotensin II was “plausible but unproven.”
Dr. Messerli added: “Before turning the page, I would like to see an analysis comparing ACE inhibitors and ARBs, since experimentally, their effect on ACE2 (the receptor to which the virus binds) seems to differ. The study of Mehta et al in JAMA Cardiology may be the first clinical hint indicating that ARBs are more protective than ACEIs. However even here, the looming possibility of confounding cannot be excluded.”
Dr. Messerli also pointed to a hypothesis that suggests that direct viral infection of endothelial cells expressing ACE2 receptors may explain worse outcomes in patients with cardiovascular comorbidities, which provides a rationale for therapies to stabilize the endothelium, particularly with anti-inflammatory anticytokine drugs, ACE inhibitors, and statins.
A version of this article originally appeared on Medscape.com.
Four more studies of the relationship of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) with COVID-19 have been published in the past few days in top-tier peer-reviewed journals, and on the whole, the data are reassuring.
Three of the new studies were published in the New England Journal of Medicine on May 1, and one study was published in JAMA Cardiology on May 5.
Although all the studies are observational in design and have some confounding factors, overall, However, there are some contradictory findings in secondary analyses regarding possible differences in the effects of the two drug classes.
Providing commentary, John McMurray, MD, professor of medical cardiology at the University of Glasgow, said: “The overall picture seems to suggest no increase in risk of adverse outcomes in patients taking renin-angiotensin system [RAS] blockers ― but with lots of caveats: These are all observational rather than randomized studies, and there may be residual or unmeasured confounding.”
Was it ‘Much ado about nothing’?
Franz Messerli, MD, professor of medicine at the University of Bern (Switzerland), added: “Given this state of the art, I am inclined to consider RAS blockade and COVID-19 – despite all the hype in the news media – as much ado about nothing.”
But both Dr. McMurray and Dr. Messerli said they were intrigued about possible differences in the effects of ACE inhibitors and ARBs that some of the new results suggest.
In one study, a team led by Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center, Boston, analyzed data from 8,910 patients with COVID-19 admitted to 169 hospitals in Asia, Europe, and North America who had either died in the hospital (5.8%) or survived to hospital discharge (94.2%).
In multivariate logistic-regression analysis, age greater than 65 years, coronary artery disease, congestive heart failure, history of cardiac arrhythmia, chronic obstructive pulmonary disease, and current smoking were associated with an increased risk for in-hospital death. Female sex was associated with a decreased risk. Neither ACE inhibitors nor ARBs were associated with an increased risk for in-hospital death.
In fact, ACE inhibitors were associated with a significant reduction in mortality (odds ratio, 0.33), as were statins (OR, 0.35).
The authors, however, stressed that these observations about reduced mortality with ACE inhibitors and statins “should be considered with extreme caution.”
“Because our study was not a randomized, controlled trial, we cannot exclude the possibility of confounding. In addition, we examined relationships between many variables and in-hospital death, and no primary hypothesis was prespecified; these factors increased the probability of chance associations being found. Therefore, a cause-and-effect relationship between drug therapy and survival should not be inferred,” they wrote.
A secondary analysis that was restricted to patients with hypertension (those for whom an ACE inhibitor or an ARB would be indicated) also did not show harm.
A second study published in the New England Journal of Medicine had a case-control design. The authors, led by Giuseppe Mancia, MD, of the University of Milano-Bicocca (Italy), compared 6,272 patients with confirmed COVID-19 (case patients) with 30,759 control persons who were matched according to age, sex, and municipality of residence.
In a conditional logistic-regression multivariate analysis, neither ACE inhibitors nor ARBs were associated with the likelihood of SARS-CoV-2 infection.
“Thus, our results do not provide evidence of an independent relationship between renin angiotensin aldosterone blockers and the susceptibility to COVID-19 in humans,” the authors concluded.
In addition, a second analysis that compared patients who had severe or fatal infections with matched control persons did not show an association between ACE inhibitors or ARBs and severe disease.
In the third study published in the New England Journal of Medicine, a group led by Harmony R. Reynolds, MD, of New York University, analyzed data from the health records of 12,594 patients in the NYU Langone Health system who had been tested for COVID-19. They found 5,894 patients whose test results were positive. Of these patients, 1,002 had severe illness, which was defined as illness requiring admission to the ICU, need for mechanical ventilation, or death.
Using Bayesian analysis and propensity score matching, the researchers assessed the relation between previous treatment with five different classes of antihypertensive drugs (ACE inhibitors, ARBs, beta blockers, calcium blockers, and thiazide diuretics) and the likelihood of a positive or negative result on COVID-19 testing, as well as the likelihood of severe illness among patients who tested positive.
Results showed no positive association between any of the analyzed drug classes and either a positive test result or severe illness.
In an accompanying editorial, a group led by John A. Jarcho, MD, of Harvard Medical School, Boston, and deputy editor of the New England Journal of Medicine, wrote: “Taken together, these three studies do not provide evidence to support the hypothesis that ACE inhibitor or ARB use is associated with the risk of SARS-CoV-2 infection, the risk of severe COVID-19 among those infected, or the risk of in-hospital death among those with a positive test.
“Each of these studies has weaknesses inherent in observational data, but we find it reassuring that three studies in different populations and with different designs arrive at the consistent message that the continued use of ACE inhibitors and ARBs is unlikely to be harmful in patients with COVID-19. Several other smaller studies from China and the United Kingdom have come to the same conclusion,” the authors of the editorial stated.
In the study published in JAMA Cardiology, a group led by Neil Mehta, MBBS, of the Cleveland Clinic, Ohio, analyzed data on 18,472 patients who had been tested for COVID-19 between March 8 and April 12 in the Cleveland Clinic Health System in Ohio and Florida. Of these patients, 9.4% tested positive.
After overlap propensity score weighting for both ACE inhibitors and ARBs to take into account relevant comorbidities, there was no difference in risk for testing positive among patients taking an ACE inhibitor or an ARB in comparison with those not taking such medication.
Are there different effects between ACE inhibitors and ARBs?
A secondary exploratory analysis showed a higher likelihood of hospital admission among patients who tested positive and who were taking either ACE inhibitors (OR, 1.84) or ARBs (OR, 1.61), and there was a higher likelihood of ICU admission among patients who tested positive and who were taking an ACE inhibitor (OR 1.77), but no such difference was observed among those taking ARBs.
Coauthor Ankur Kalra, MD, of the Cleveland Clinic, said in an interview that results of the exploratory analysis fit with the hypothesis that the two drugs classes may have different effects in patients with COVID-19.
“Angiotensin II promotes vasoconstriction, inflammation, and fibrosis in the lungs, and ARBs block the effects of angiotensin II more effectively than ACE inhibitors. In addition, ACE inhibitors (but not ARBs) increase levels of bradykinin, which may be one factor leading to acute respiratory distress syndrome,” he noted.
“However, these results should only be considered exploratory, as there is inherent bias in observational data,” Dr. Kalra stressed.
In an accompanying editorial in JAMA Cardiology, a group led by Laine E. Thomas, PhD, of Duke Clinical Research Institute, Durham, North Carolina, said that the results of this secondary exploratory analysis are limited by a small number of patients and “are likely explained by confounding and should not be inferred as causal.”
The New England Journal of Medicine editorialists reached a similar conclusion regarding the lower mortality in COVID-19 patients who took ACE inhibitors in the study by Dr. Mehra and colleagues. They say this unexpected result “may be due to unmeasured confounding and, in the absence of a randomized trial, should not be regarded as evidence to prescribe these drugs in patients with COVID-19.”
Providing further comment, Dr. McMurray said: “Normally, I would not read too much into the different effects of ACE inhibitors and ARBs suggested in the Cleveland study because of the small numbers (about 28 ACE inhibitor–treated patients admitted to ICU) and the limited information about matching and/or adjustment for potential differences between groups.
“I could also argue that the comparison that would best answer the question about risk related to type of RAS blocker would be the direct comparison of people taking an ACE inhibitor with those taking an ARB (and that doesn’t look very different). The only thing that makes me a little cautious about completely dismissing the possibility of a difference between ACE inhibitor and ARB here is the suggestion of a similar trend in another large study from the VA [Veterans Affairs] system,” he added.
He also noted that speculation about there being mechanisms that involve different effects of the two drug classes on bradykinin and angiotensin II was “plausible but unproven.”
Dr. Messerli added: “Before turning the page, I would like to see an analysis comparing ACE inhibitors and ARBs, since experimentally, their effect on ACE2 (the receptor to which the virus binds) seems to differ. The study of Mehta et al in JAMA Cardiology may be the first clinical hint indicating that ARBs are more protective than ACEIs. However even here, the looming possibility of confounding cannot be excluded.”
Dr. Messerli also pointed to a hypothesis that suggests that direct viral infection of endothelial cells expressing ACE2 receptors may explain worse outcomes in patients with cardiovascular comorbidities, which provides a rationale for therapies to stabilize the endothelium, particularly with anti-inflammatory anticytokine drugs, ACE inhibitors, and statins.
A version of this article originally appeared on Medscape.com.
Don’t delay antibiotic treatment in elderly patients with UTI
Background: If left untreated, UTIs may lead to severe complications. Although campaigns aimed at decreasing unnecessary prescriptions have reduced the number of antibiotic prescriptions for UTI, a concurrent rise in the rates of gram-negative bloodstream infections (BSIs) has also been observed.
Study design: Retrospective, population-based cohort study with data compiled from primary care records from 2007 to 2015 linked to hospital episode statistics and death records.
Setting: General practices in England.
Synopsis: The investigators analyzed 312,896 UTI episodes among 157,264 unique patients (65 years of age or older) during the study period. Exclusion criteria included asymptomatic bacteriuria and complicated UTI. Of 271,070 patients who received antibiotics on the day of presentation with symptoms, 0.2% developed BSI within 60 days versus 2.2% of patients in whom antibiotics were delayed and 2.9% among patients not prescribed antibiotics. After adjustment for comorbidities, sex, and socioeconomic status, patients in whom antibiotics were deferred had a 7.12-fold greater odds of BSI, compared with the immediate-antibiotic group. BSIs were more common among men and older patients. All-cause mortality, a secondary outcome, was 1.16-fold higher with deferred antibiotics and 2.18 times higher with no antibiotics.
While the cohort studied was very large, a causal relationship cannot be firmly established in this observational study. Also, researchers were unable to include laboratory data, such as urinalysis and culture, in their analysis.
Bottom line: Delayed prescription of antibiotics for elderly patients presenting with UTI in primary care settings was associated with higher rates of BSI and death.
Citation: Gharbi M et al. Antibiotic management of urinary tract infection in elderly patients in primary care and its association with bloodstream infections and all-cause mortality: Population-based cohort study. BMJ. 2019 Feb;364:1525.
Dr. Torres is a hospitalist at Massachusetts General Hospital.
Background: If left untreated, UTIs may lead to severe complications. Although campaigns aimed at decreasing unnecessary prescriptions have reduced the number of antibiotic prescriptions for UTI, a concurrent rise in the rates of gram-negative bloodstream infections (BSIs) has also been observed.
Study design: Retrospective, population-based cohort study with data compiled from primary care records from 2007 to 2015 linked to hospital episode statistics and death records.
Setting: General practices in England.
Synopsis: The investigators analyzed 312,896 UTI episodes among 157,264 unique patients (65 years of age or older) during the study period. Exclusion criteria included asymptomatic bacteriuria and complicated UTI. Of 271,070 patients who received antibiotics on the day of presentation with symptoms, 0.2% developed BSI within 60 days versus 2.2% of patients in whom antibiotics were delayed and 2.9% among patients not prescribed antibiotics. After adjustment for comorbidities, sex, and socioeconomic status, patients in whom antibiotics were deferred had a 7.12-fold greater odds of BSI, compared with the immediate-antibiotic group. BSIs were more common among men and older patients. All-cause mortality, a secondary outcome, was 1.16-fold higher with deferred antibiotics and 2.18 times higher with no antibiotics.
While the cohort studied was very large, a causal relationship cannot be firmly established in this observational study. Also, researchers were unable to include laboratory data, such as urinalysis and culture, in their analysis.
Bottom line: Delayed prescription of antibiotics for elderly patients presenting with UTI in primary care settings was associated with higher rates of BSI and death.
Citation: Gharbi M et al. Antibiotic management of urinary tract infection in elderly patients in primary care and its association with bloodstream infections and all-cause mortality: Population-based cohort study. BMJ. 2019 Feb;364:1525.
Dr. Torres is a hospitalist at Massachusetts General Hospital.
Background: If left untreated, UTIs may lead to severe complications. Although campaigns aimed at decreasing unnecessary prescriptions have reduced the number of antibiotic prescriptions for UTI, a concurrent rise in the rates of gram-negative bloodstream infections (BSIs) has also been observed.
Study design: Retrospective, population-based cohort study with data compiled from primary care records from 2007 to 2015 linked to hospital episode statistics and death records.
Setting: General practices in England.
Synopsis: The investigators analyzed 312,896 UTI episodes among 157,264 unique patients (65 years of age or older) during the study period. Exclusion criteria included asymptomatic bacteriuria and complicated UTI. Of 271,070 patients who received antibiotics on the day of presentation with symptoms, 0.2% developed BSI within 60 days versus 2.2% of patients in whom antibiotics were delayed and 2.9% among patients not prescribed antibiotics. After adjustment for comorbidities, sex, and socioeconomic status, patients in whom antibiotics were deferred had a 7.12-fold greater odds of BSI, compared with the immediate-antibiotic group. BSIs were more common among men and older patients. All-cause mortality, a secondary outcome, was 1.16-fold higher with deferred antibiotics and 2.18 times higher with no antibiotics.
While the cohort studied was very large, a causal relationship cannot be firmly established in this observational study. Also, researchers were unable to include laboratory data, such as urinalysis and culture, in their analysis.
Bottom line: Delayed prescription of antibiotics for elderly patients presenting with UTI in primary care settings was associated with higher rates of BSI and death.
Citation: Gharbi M et al. Antibiotic management of urinary tract infection in elderly patients in primary care and its association with bloodstream infections and all-cause mortality: Population-based cohort study. BMJ. 2019 Feb;364:1525.
Dr. Torres is a hospitalist at Massachusetts General Hospital.








