User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


COVID-19 prompts ‘lifesaving’ policy change for opioid addiction
In the face of the US COVID-19 pandemic, the US Substance Abuse and Mental Health Services Administration (SAMHSA) has announced policy changes to allow some patients in opioid treatment programs (OTP) to take home their medication.
According to the agency, states may request “blanket exceptions” for all stable patients in an OTP to receive a 28-day supply of take-home doses of medications such as methadone and buprenorphine, which are used to treat opioid use disorder (OUD).
States may request up to 14 days of take-home medication for patients who are less stable but who can, in the judgment of OTP clinicians, safely handle this level of take-home medication.
“SAMHSA recognizes the evolving issues surrounding COVID-19 and the emerging needs OTPs continue to face,” the agency writes in its updated guidance.
“SAMHSA affirms its commitment to supporting OTPs in any way possible during this time. As such, we are expanding our previous guidance to provide increased flexibility,” the agency said.
A ‘Lifesaving’ Decision
Commenting on the SAMHSA policy change, Richard Saitz, MD, professor and chair of the department of community health sciences, Boston University School of Public Health, said, the policy “is not only a good idea, it is critical and lifesaving.”
“This approach had to be done now. With the reduction in face-to-face visits, patients with opioid use disorder need a way to access treatment. If they cannot get opioid agonists, they would withdraw and return to illicit opioid use and high overdose risk and it would be cruel,” said Saitz.
“It is possible that there will be some diversion and some risk of overdose or misuse, but even for less stable patients the benefit likely far outweighs the risk,” he told Medscape Medical News.
Saitz believes policy changes like this should have been made before a crisis.
“Honestly, this is perhaps a silver lining of the crisis” and could lead to permanent change in how OUD is treated in the US, he said.
“Just like we are learning what can be done without a medical in-person visit, we will learn that it is perfectly fine to treat patients with addiction more like we treat patients with other chronic diseases who take medication that has risks and benefits,” Saitz said.
in cases when a patient is quarantined because of coronavirus.
Typically, only licensed practitioners can dispense or administer OUD medications to patients, but during the COVID-19 crisis, treatment program staff members, law enforcement officers, and national guard personnel will be allowed to deliver OUD medications to an approved “lockbox” at the patient’s doorstep. The change applies only while the coronavirus public health emergency lasts.
“This is also an excellent idea,” Saitz said.
ASAM Also Responds
In addition, the American Society of Addiction Medicine (ASAM) released a focused update to its National Practice Guideline for the Treatment of Opioid Use Disorder (NPG).
The update is “especially critical in the context of the ongoing COVID-19 emergency, which threatens to curtail patient access to evidence-based treatment,” the organization said in a news release. The new document updates the 2015 NPG. It includes 13 new recommendations and major revisions to 35 existing recommendations.
One new recommendation states that comprehensive assessment of a patient is critical for treatment planning, but completing all assessments should not delay or preclude initiating pharmacotherapy for OUD. Another new recommendation states that there is no recommended time limit for pharmacotherapy.
ASAM continues to recommend that patients’ psychosocial needs be assessed and psychosocial treatment offered. However, if patients can’t access psychosocial treatment because they are in isolation or have other risk factors that preclude external interactions, clinicians should not delay initiation of medication for the treatment of addiction.
Expanding the use of telemedicine might also be appropriate for many patients, ASAM announced.
They note that the NPG is the first to address in a single document all medications currently approved by the US Food and Drug Administration to treat OUD and opioid withdrawal, including all available buprenorphine formulations.
“All of the updated recommendations are designed to both improve the quality and consistency of care and reduce barriers to access to care for Americans living with OUD. The updated recommendations aim to support initiation of buprenorphine treatment in the emergency department and other urgent care settings,” the society said in the release.
“In addition, [the recommendations] provide greater flexibility on dosing during the initiation of buprenorphine treatment and for initiation of buprenorphine at home (which is also an important change in the midst of the COVID-19 crisis).”
The full document is available online.
This article first appeared on Medscape.com.
In the face of the US COVID-19 pandemic, the US Substance Abuse and Mental Health Services Administration (SAMHSA) has announced policy changes to allow some patients in opioid treatment programs (OTP) to take home their medication.
According to the agency, states may request “blanket exceptions” for all stable patients in an OTP to receive a 28-day supply of take-home doses of medications such as methadone and buprenorphine, which are used to treat opioid use disorder (OUD).
States may request up to 14 days of take-home medication for patients who are less stable but who can, in the judgment of OTP clinicians, safely handle this level of take-home medication.
“SAMHSA recognizes the evolving issues surrounding COVID-19 and the emerging needs OTPs continue to face,” the agency writes in its updated guidance.
“SAMHSA affirms its commitment to supporting OTPs in any way possible during this time. As such, we are expanding our previous guidance to provide increased flexibility,” the agency said.
A ‘Lifesaving’ Decision
Commenting on the SAMHSA policy change, Richard Saitz, MD, professor and chair of the department of community health sciences, Boston University School of Public Health, said, the policy “is not only a good idea, it is critical and lifesaving.”
“This approach had to be done now. With the reduction in face-to-face visits, patients with opioid use disorder need a way to access treatment. If they cannot get opioid agonists, they would withdraw and return to illicit opioid use and high overdose risk and it would be cruel,” said Saitz.
“It is possible that there will be some diversion and some risk of overdose or misuse, but even for less stable patients the benefit likely far outweighs the risk,” he told Medscape Medical News.
Saitz believes policy changes like this should have been made before a crisis.
“Honestly, this is perhaps a silver lining of the crisis” and could lead to permanent change in how OUD is treated in the US, he said.
“Just like we are learning what can be done without a medical in-person visit, we will learn that it is perfectly fine to treat patients with addiction more like we treat patients with other chronic diseases who take medication that has risks and benefits,” Saitz said.
in cases when a patient is quarantined because of coronavirus.
Typically, only licensed practitioners can dispense or administer OUD medications to patients, but during the COVID-19 crisis, treatment program staff members, law enforcement officers, and national guard personnel will be allowed to deliver OUD medications to an approved “lockbox” at the patient’s doorstep. The change applies only while the coronavirus public health emergency lasts.
“This is also an excellent idea,” Saitz said.
ASAM Also Responds
In addition, the American Society of Addiction Medicine (ASAM) released a focused update to its National Practice Guideline for the Treatment of Opioid Use Disorder (NPG).
The update is “especially critical in the context of the ongoing COVID-19 emergency, which threatens to curtail patient access to evidence-based treatment,” the organization said in a news release. The new document updates the 2015 NPG. It includes 13 new recommendations and major revisions to 35 existing recommendations.
One new recommendation states that comprehensive assessment of a patient is critical for treatment planning, but completing all assessments should not delay or preclude initiating pharmacotherapy for OUD. Another new recommendation states that there is no recommended time limit for pharmacotherapy.
ASAM continues to recommend that patients’ psychosocial needs be assessed and psychosocial treatment offered. However, if patients can’t access psychosocial treatment because they are in isolation or have other risk factors that preclude external interactions, clinicians should not delay initiation of medication for the treatment of addiction.
Expanding the use of telemedicine might also be appropriate for many patients, ASAM announced.
They note that the NPG is the first to address in a single document all medications currently approved by the US Food and Drug Administration to treat OUD and opioid withdrawal, including all available buprenorphine formulations.
“All of the updated recommendations are designed to both improve the quality and consistency of care and reduce barriers to access to care for Americans living with OUD. The updated recommendations aim to support initiation of buprenorphine treatment in the emergency department and other urgent care settings,” the society said in the release.
“In addition, [the recommendations] provide greater flexibility on dosing during the initiation of buprenorphine treatment and for initiation of buprenorphine at home (which is also an important change in the midst of the COVID-19 crisis).”
The full document is available online.
This article first appeared on Medscape.com.
In the face of the US COVID-19 pandemic, the US Substance Abuse and Mental Health Services Administration (SAMHSA) has announced policy changes to allow some patients in opioid treatment programs (OTP) to take home their medication.
According to the agency, states may request “blanket exceptions” for all stable patients in an OTP to receive a 28-day supply of take-home doses of medications such as methadone and buprenorphine, which are used to treat opioid use disorder (OUD).
States may request up to 14 days of take-home medication for patients who are less stable but who can, in the judgment of OTP clinicians, safely handle this level of take-home medication.
“SAMHSA recognizes the evolving issues surrounding COVID-19 and the emerging needs OTPs continue to face,” the agency writes in its updated guidance.
“SAMHSA affirms its commitment to supporting OTPs in any way possible during this time. As such, we are expanding our previous guidance to provide increased flexibility,” the agency said.
A ‘Lifesaving’ Decision
Commenting on the SAMHSA policy change, Richard Saitz, MD, professor and chair of the department of community health sciences, Boston University School of Public Health, said, the policy “is not only a good idea, it is critical and lifesaving.”
“This approach had to be done now. With the reduction in face-to-face visits, patients with opioid use disorder need a way to access treatment. If they cannot get opioid agonists, they would withdraw and return to illicit opioid use and high overdose risk and it would be cruel,” said Saitz.
“It is possible that there will be some diversion and some risk of overdose or misuse, but even for less stable patients the benefit likely far outweighs the risk,” he told Medscape Medical News.
Saitz believes policy changes like this should have been made before a crisis.
“Honestly, this is perhaps a silver lining of the crisis” and could lead to permanent change in how OUD is treated in the US, he said.
“Just like we are learning what can be done without a medical in-person visit, we will learn that it is perfectly fine to treat patients with addiction more like we treat patients with other chronic diseases who take medication that has risks and benefits,” Saitz said.
in cases when a patient is quarantined because of coronavirus.
Typically, only licensed practitioners can dispense or administer OUD medications to patients, but during the COVID-19 crisis, treatment program staff members, law enforcement officers, and national guard personnel will be allowed to deliver OUD medications to an approved “lockbox” at the patient’s doorstep. The change applies only while the coronavirus public health emergency lasts.
“This is also an excellent idea,” Saitz said.
ASAM Also Responds
In addition, the American Society of Addiction Medicine (ASAM) released a focused update to its National Practice Guideline for the Treatment of Opioid Use Disorder (NPG).
The update is “especially critical in the context of the ongoing COVID-19 emergency, which threatens to curtail patient access to evidence-based treatment,” the organization said in a news release. The new document updates the 2015 NPG. It includes 13 new recommendations and major revisions to 35 existing recommendations.
One new recommendation states that comprehensive assessment of a patient is critical for treatment planning, but completing all assessments should not delay or preclude initiating pharmacotherapy for OUD. Another new recommendation states that there is no recommended time limit for pharmacotherapy.
ASAM continues to recommend that patients’ psychosocial needs be assessed and psychosocial treatment offered. However, if patients can’t access psychosocial treatment because they are in isolation or have other risk factors that preclude external interactions, clinicians should not delay initiation of medication for the treatment of addiction.
Expanding the use of telemedicine might also be appropriate for many patients, ASAM announced.
They note that the NPG is the first to address in a single document all medications currently approved by the US Food and Drug Administration to treat OUD and opioid withdrawal, including all available buprenorphine formulations.
“All of the updated recommendations are designed to both improve the quality and consistency of care and reduce barriers to access to care for Americans living with OUD. The updated recommendations aim to support initiation of buprenorphine treatment in the emergency department and other urgent care settings,” the society said in the release.
“In addition, [the recommendations] provide greater flexibility on dosing during the initiation of buprenorphine treatment and for initiation of buprenorphine at home (which is also an important change in the midst of the COVID-19 crisis).”
The full document is available online.
This article first appeared on Medscape.com.
Match Day 2020: Online announcements replace celebrations, champagne
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
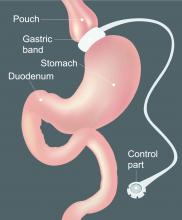
The hospitalized postbariatric surgery patient
What every hospitalist should know
With the prevalence of obesity worldwide topping 650 million people1 and nearly 40% of U.S. adults having obesity,2 bariatric surgery is increasingly used to treat this disease and its associated comorbidities.
The American Society for Metabolic & Bariatric Surgery estimates that 228,000 bariatric procedures were performed on Americans in 2017, up from 158,000 in 2011.3 Despite lowering the risks of diabetes, stroke, myocardial infarction, cancer, and all-cause mortality,4 bariatric surgery is associated with increased health care use. Neovius et al. found that people who underwent bariatric surgery used 54 mean cumulative hospital days in the 20 years following their procedures, compared with just 40 inpatient days used by controls.5
Although hospitalists are caring for increasing numbers of patients who have undergone bariatric surgery, many of us may not be aware of some of the things that can lead to hospitalization or otherwise affect inpatient medical care. Here are a few points to keep in mind the next time you care for an inpatient with prior bariatric surgery.
Pharmacokinetics change after surgery
Gastrointestinal anatomy necessarily changes after bariatric surgery and can affect the oral absorption of drugs. Because gastric motility may be impaired and the pH in the stomach is increased after bariatric surgery, the disintegration and dissolution of immediate-release solid pills or caps may be compromised.
It is therefore prudent to crush solid forms or switch to liquid or chewable formulations of immediate-release drugs for the first few weeks to months after surgery. Enteric-coated or long-acting drug formulations should not be crushed and should generally be avoided in patients who have undergone bypass procedures such as Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion with duodenal switch (BPD/DS), as they can demonstrate either enhanced or diminished absorption (depending on the drug).
Reduced intestinal transit times and changes in intestinal pH can alter the absorption of certain drugs as well, and the expression of some drug transporter proteins and enzymes such as the CYP3A4 variant of cytochrome P450 – which is estimated to metabolize up to half of currently available drugs – varies between the upper and the lower small intestine, potentially leading to increased bioavailability of medications metabolized by this enzyme in patients who have undergone bypass surgeries.
Interestingly, longer-term studies have reexamined drug absorption in patients 2-4 years after RYGB and found that initially-increased drug plasma levels often return to preoperative levels or even lower over time,6 likely because of adaptive changes in the GI tract. Because research on the pharmacokinetics of individual drugs after bariatric surgery is lacking, the hospitalist should be aware that the bioavailability of oral drugs is often altered and should monitor patients for the desired therapeutic effect as well as potential toxicities for any drug administered to postbariatric surgery patients.
Finally, note that nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and corticosteroids should be avoided after bariatric surgery unless the benefit clearly outweighs the risk, as they increase the risk of ulcers even in patients without underlying surgical disruptions to the gastric mucosa.
Micronutrient deficiencies are common and can occur at any time
While many clinicians recognize that vitamin deficiencies can occur after weight loss surgeries which bypass the duodenum, such as the RYGB or the BPD/DS, it is important to note that vitamin and mineral deficiencies occur commonly even in patients with intact intestinal absorption such as those who underwent sleeve gastrectomy (SG) and even despite regained weight due to greater volumes of food (and micronutrient) intake over time.
The most common vitamin deficiencies include iron, vitamin B12, thiamine (vitamin B1), and vitamin D, but deficiencies in other vitamins and minerals may found as well. Anemia, bone fractures, heart failure, and encephalopathy can all be related to postoperative vitamin deficiencies. Most bariatric surgery patients should have micronutrient levels monitored on a yearly basis and should be taking at least a multivitamin with minerals (including zinc, copper, selenium and iron), a form of vitamin B12, and vitamin D with calcium supplementation. Additional supplements may be appropriate depending on the type of surgery the patient had or whether a deficiency is found.
The differential diagnosis for abdominal pain after bariatric surgery is unique
While the usual suspects such as diverticulitis or gastritis should be considered in postbariatric surgery patients just as in others, a few specific complications can arise after weight loss surgery.
Marginal ulcerations (ulcers at the surgical anastomotic sites) have been reported in up to a third of patients complaining of abdominal pain or dysphagia after RYGB, with tobacco, alcohol, or NSAID use conferring even greater risk.7 Early upper endoscopy may be warranted in symptomatic patients.
Small bowel obstruction (SBO) may occur due to surgical adhesions as in other patients, but catastrophic internal hernias with associated volvulus can occur due to specific anatomical defects that are created by the RYGB and BPD/DS procedures. CT imaging is insensitive and can miss up to 30% of these cases,8 and nasogastric tubes placed blindly for decompression of an SBO can lead to perforation of the end of the alimentary limb at the gastric pouch outlet, so post-RYGB or BPD/DS patients presenting with signs of small bowel obstruction should have an early surgical consult for expeditious surgical management rather than a trial of conservative medical management.9
Cholelithiasis is a very common postoperative complication, occurring in about 25% of SG patients and 32% of RYGB patients in the first year following surgery. The risk of gallstone formation can be significantly reduced with the postoperative use of ursodeoxycholic acid.10
Onset of abdominal cramping, nausea and diarrhea (sometimes accompanied by vasomotor symptoms) within 15-60 minutes of eating may be due to early dumping syndrome. Rapid delivery of food from the gastric pouch into the small intestine causes the release of gut peptides and an osmotic fluid shift into the intestinal lumen that can trigger these symptoms even in patients with a preserved pyloric sphincter, such as those who underwent SG. Simply eliminating sugars and simple carbohydrates from the diet usually resolves the problem, and eliminating lactose can often be helpful as well.
Postprandial hyperinsulinemic hypoglycemia (“late dumping syndrome”) can develop years after surgery
Vasomotor symptoms such as flushing/sweating, shaking, tachycardia/palpitations, lightheadedness, or difficulty concentrating occurring 1-3 hours after a meal should prompt blood glucose testing, as delayed hypoglycemia can occur after a large insulin surge.
Most commonly seen after RYGB, late dumping syndrome, like early dumping syndrome, can often be managed by eliminating sugars and simple carbohydrates from the diet. The onset of late dumping syndrome has been reported as late as 8 years after surgery,11 so the etiology of symptoms can be elusive. If the diagnosis is unclear, an oral glucose tolerance test may be helpful.
Alcohol use disorder is more prevalent after weight loss surgery
Changes to the gastrointestinal anatomy allow for more rapid absorption of ethanol into the bloodstream, making the drug more potent in postop patients. Simultaneously, many patients who undergo bariatric surgery have a history of using food to buffer negative emotions. Abruptly depriving them of that comfort in the context of the increased potency of alcohol could potentially leave bariatric surgery patients vulnerable to the development of alcohol use disorder, even when they did not misuse alcohol preoperatively.
Of note, alcohol misuse becomes more prevalent after the first postoperative year.12 Screening for alcohol misuse on admission to the hospital is wise in all cases, but perhaps even more so in the postbariatric surgery patient. If a patient does report excessive alcohol use, keep possible thiamine deficiency in mind.
The risk of suicide and self-harm increases after bariatric surgery
While all-cause mortality rates decrease after bariatric surgery compared with matched controls, the risk of suicide and nonfatal self-harm increases.
About half of bariatric surgery patients with nonfatal events have substance misuse.13 Notably, several studies have found reduced plasma levels of SSRIs in patients after RYGB,6 so pharmacotherapy for mood disorders could be less effective after bariatric surgery as well. The hospitalist could positively impact patients by screening for both substance misuse and depression and by having a low threshold for referral to a mental health professional.
As we see ever-increasing numbers of inpatients who have a history of bariatric surgery, being aware of these common and important complications can help today’s hospitalist provide the best care possible.
Dr. Kerns is a hospitalist and codirector of bariatric surgery at the Washington DC VA Medical Center.
References
1. Obesity and overweight. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Published Feb 16, 2018.
2. Hales CM et al. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS data brief, no 288. Hyattsville, MD: National Center for Health Statistics. 2017.
3. Estimate of Bariatric Surgery Numbers, 2011-2018. ASMBS.org. Published June 2018.
4. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013 Mar;273(3):219-34. doi: 10.1111/joim.12012.
5. Neovius M et al. Health care use during 20 years following bariatric surgery. JAMA. 2012 Sep 19; 308(11):1132-41. doi: 10.1001/2012.jama.11792.
6. Azran C. et al. Oral drug therapy following bariatric surgery: An overview of fundamentals, literature and clinical recommendations. Obes Rev. 2016 Nov;17(11):1050-66. doi: 10.1111/obr.12434.
7. El-hayek KM et al. Marginal ulcer after Roux-en-Y gastric bypass: What have we really learned? Surg Endosc. 2012 Oct;26(10):2789-96. Epub 2012 Apr 28. (Abstract presented at Society of American Gastrointestinal and Endoscopic Surgeons 2012 annual meeting, San Diego.) 8. Iannelli A et al. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2006;16:1265-71. doi: 10.1381/096089206778663689.
9. Lim R et al. Early and late complications of bariatric operation. Trauma Surg Acute Care Open. 2018 Oct 9;3(1): e000219. doi: 10.1136/tsaco-2018-000219.
10. Coupaye M et al. Evaluation of incidence of cholelithiasis after bariatric surgery in subjects treated or not treated with ursodeoxycholic acid. Surg Obes Relat Dis. 2017;13(4):681-5. doi: 10.1016/j.soard.2016.11.022.
11. Eisenberg D et al. ASMBS position statement on postprandial hyperinsulinemic hypoglycemia after bariatric surgery. Surg Obes Relat Dis. 2017 Mar;13(3):371-8. doi: 10.1016/j.soard.2016.12.005.
12. King WC et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012 Jun 20;307(23):2516-25. doi: 10.1001/jama.2012.6147.
13. Neovius M et al. Risk of suicide and non-fatal self-harm after bariatric surgery: Results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018 Mar;6(3):197-207. doi: 10.1016/S2213-8587(17)30437-0.
What every hospitalist should know
What every hospitalist should know
With the prevalence of obesity worldwide topping 650 million people1 and nearly 40% of U.S. adults having obesity,2 bariatric surgery is increasingly used to treat this disease and its associated comorbidities.
The American Society for Metabolic & Bariatric Surgery estimates that 228,000 bariatric procedures were performed on Americans in 2017, up from 158,000 in 2011.3 Despite lowering the risks of diabetes, stroke, myocardial infarction, cancer, and all-cause mortality,4 bariatric surgery is associated with increased health care use. Neovius et al. found that people who underwent bariatric surgery used 54 mean cumulative hospital days in the 20 years following their procedures, compared with just 40 inpatient days used by controls.5
Although hospitalists are caring for increasing numbers of patients who have undergone bariatric surgery, many of us may not be aware of some of the things that can lead to hospitalization or otherwise affect inpatient medical care. Here are a few points to keep in mind the next time you care for an inpatient with prior bariatric surgery.
Pharmacokinetics change after surgery
Gastrointestinal anatomy necessarily changes after bariatric surgery and can affect the oral absorption of drugs. Because gastric motility may be impaired and the pH in the stomach is increased after bariatric surgery, the disintegration and dissolution of immediate-release solid pills or caps may be compromised.
It is therefore prudent to crush solid forms or switch to liquid or chewable formulations of immediate-release drugs for the first few weeks to months after surgery. Enteric-coated or long-acting drug formulations should not be crushed and should generally be avoided in patients who have undergone bypass procedures such as Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion with duodenal switch (BPD/DS), as they can demonstrate either enhanced or diminished absorption (depending on the drug).
Reduced intestinal transit times and changes in intestinal pH can alter the absorption of certain drugs as well, and the expression of some drug transporter proteins and enzymes such as the CYP3A4 variant of cytochrome P450 – which is estimated to metabolize up to half of currently available drugs – varies between the upper and the lower small intestine, potentially leading to increased bioavailability of medications metabolized by this enzyme in patients who have undergone bypass surgeries.
Interestingly, longer-term studies have reexamined drug absorption in patients 2-4 years after RYGB and found that initially-increased drug plasma levels often return to preoperative levels or even lower over time,6 likely because of adaptive changes in the GI tract. Because research on the pharmacokinetics of individual drugs after bariatric surgery is lacking, the hospitalist should be aware that the bioavailability of oral drugs is often altered and should monitor patients for the desired therapeutic effect as well as potential toxicities for any drug administered to postbariatric surgery patients.
Finally, note that nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and corticosteroids should be avoided after bariatric surgery unless the benefit clearly outweighs the risk, as they increase the risk of ulcers even in patients without underlying surgical disruptions to the gastric mucosa.
Micronutrient deficiencies are common and can occur at any time
While many clinicians recognize that vitamin deficiencies can occur after weight loss surgeries which bypass the duodenum, such as the RYGB or the BPD/DS, it is important to note that vitamin and mineral deficiencies occur commonly even in patients with intact intestinal absorption such as those who underwent sleeve gastrectomy (SG) and even despite regained weight due to greater volumes of food (and micronutrient) intake over time.
The most common vitamin deficiencies include iron, vitamin B12, thiamine (vitamin B1), and vitamin D, but deficiencies in other vitamins and minerals may found as well. Anemia, bone fractures, heart failure, and encephalopathy can all be related to postoperative vitamin deficiencies. Most bariatric surgery patients should have micronutrient levels monitored on a yearly basis and should be taking at least a multivitamin with minerals (including zinc, copper, selenium and iron), a form of vitamin B12, and vitamin D with calcium supplementation. Additional supplements may be appropriate depending on the type of surgery the patient had or whether a deficiency is found.
The differential diagnosis for abdominal pain after bariatric surgery is unique
While the usual suspects such as diverticulitis or gastritis should be considered in postbariatric surgery patients just as in others, a few specific complications can arise after weight loss surgery.
Marginal ulcerations (ulcers at the surgical anastomotic sites) have been reported in up to a third of patients complaining of abdominal pain or dysphagia after RYGB, with tobacco, alcohol, or NSAID use conferring even greater risk.7 Early upper endoscopy may be warranted in symptomatic patients.
Small bowel obstruction (SBO) may occur due to surgical adhesions as in other patients, but catastrophic internal hernias with associated volvulus can occur due to specific anatomical defects that are created by the RYGB and BPD/DS procedures. CT imaging is insensitive and can miss up to 30% of these cases,8 and nasogastric tubes placed blindly for decompression of an SBO can lead to perforation of the end of the alimentary limb at the gastric pouch outlet, so post-RYGB or BPD/DS patients presenting with signs of small bowel obstruction should have an early surgical consult for expeditious surgical management rather than a trial of conservative medical management.9
Cholelithiasis is a very common postoperative complication, occurring in about 25% of SG patients and 32% of RYGB patients in the first year following surgery. The risk of gallstone formation can be significantly reduced with the postoperative use of ursodeoxycholic acid.10
Onset of abdominal cramping, nausea and diarrhea (sometimes accompanied by vasomotor symptoms) within 15-60 minutes of eating may be due to early dumping syndrome. Rapid delivery of food from the gastric pouch into the small intestine causes the release of gut peptides and an osmotic fluid shift into the intestinal lumen that can trigger these symptoms even in patients with a preserved pyloric sphincter, such as those who underwent SG. Simply eliminating sugars and simple carbohydrates from the diet usually resolves the problem, and eliminating lactose can often be helpful as well.
Postprandial hyperinsulinemic hypoglycemia (“late dumping syndrome”) can develop years after surgery
Vasomotor symptoms such as flushing/sweating, shaking, tachycardia/palpitations, lightheadedness, or difficulty concentrating occurring 1-3 hours after a meal should prompt blood glucose testing, as delayed hypoglycemia can occur after a large insulin surge.
Most commonly seen after RYGB, late dumping syndrome, like early dumping syndrome, can often be managed by eliminating sugars and simple carbohydrates from the diet. The onset of late dumping syndrome has been reported as late as 8 years after surgery,11 so the etiology of symptoms can be elusive. If the diagnosis is unclear, an oral glucose tolerance test may be helpful.
Alcohol use disorder is more prevalent after weight loss surgery
Changes to the gastrointestinal anatomy allow for more rapid absorption of ethanol into the bloodstream, making the drug more potent in postop patients. Simultaneously, many patients who undergo bariatric surgery have a history of using food to buffer negative emotions. Abruptly depriving them of that comfort in the context of the increased potency of alcohol could potentially leave bariatric surgery patients vulnerable to the development of alcohol use disorder, even when they did not misuse alcohol preoperatively.
Of note, alcohol misuse becomes more prevalent after the first postoperative year.12 Screening for alcohol misuse on admission to the hospital is wise in all cases, but perhaps even more so in the postbariatric surgery patient. If a patient does report excessive alcohol use, keep possible thiamine deficiency in mind.
The risk of suicide and self-harm increases after bariatric surgery
While all-cause mortality rates decrease after bariatric surgery compared with matched controls, the risk of suicide and nonfatal self-harm increases.
About half of bariatric surgery patients with nonfatal events have substance misuse.13 Notably, several studies have found reduced plasma levels of SSRIs in patients after RYGB,6 so pharmacotherapy for mood disorders could be less effective after bariatric surgery as well. The hospitalist could positively impact patients by screening for both substance misuse and depression and by having a low threshold for referral to a mental health professional.
As we see ever-increasing numbers of inpatients who have a history of bariatric surgery, being aware of these common and important complications can help today’s hospitalist provide the best care possible.
Dr. Kerns is a hospitalist and codirector of bariatric surgery at the Washington DC VA Medical Center.
References
1. Obesity and overweight. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Published Feb 16, 2018.
2. Hales CM et al. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS data brief, no 288. Hyattsville, MD: National Center for Health Statistics. 2017.
3. Estimate of Bariatric Surgery Numbers, 2011-2018. ASMBS.org. Published June 2018.
4. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013 Mar;273(3):219-34. doi: 10.1111/joim.12012.
5. Neovius M et al. Health care use during 20 years following bariatric surgery. JAMA. 2012 Sep 19; 308(11):1132-41. doi: 10.1001/2012.jama.11792.
6. Azran C. et al. Oral drug therapy following bariatric surgery: An overview of fundamentals, literature and clinical recommendations. Obes Rev. 2016 Nov;17(11):1050-66. doi: 10.1111/obr.12434.
7. El-hayek KM et al. Marginal ulcer after Roux-en-Y gastric bypass: What have we really learned? Surg Endosc. 2012 Oct;26(10):2789-96. Epub 2012 Apr 28. (Abstract presented at Society of American Gastrointestinal and Endoscopic Surgeons 2012 annual meeting, San Diego.) 8. Iannelli A et al. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2006;16:1265-71. doi: 10.1381/096089206778663689.
9. Lim R et al. Early and late complications of bariatric operation. Trauma Surg Acute Care Open. 2018 Oct 9;3(1): e000219. doi: 10.1136/tsaco-2018-000219.
10. Coupaye M et al. Evaluation of incidence of cholelithiasis after bariatric surgery in subjects treated or not treated with ursodeoxycholic acid. Surg Obes Relat Dis. 2017;13(4):681-5. doi: 10.1016/j.soard.2016.11.022.
11. Eisenberg D et al. ASMBS position statement on postprandial hyperinsulinemic hypoglycemia after bariatric surgery. Surg Obes Relat Dis. 2017 Mar;13(3):371-8. doi: 10.1016/j.soard.2016.12.005.
12. King WC et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012 Jun 20;307(23):2516-25. doi: 10.1001/jama.2012.6147.
13. Neovius M et al. Risk of suicide and non-fatal self-harm after bariatric surgery: Results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018 Mar;6(3):197-207. doi: 10.1016/S2213-8587(17)30437-0.
With the prevalence of obesity worldwide topping 650 million people1 and nearly 40% of U.S. adults having obesity,2 bariatric surgery is increasingly used to treat this disease and its associated comorbidities.
The American Society for Metabolic & Bariatric Surgery estimates that 228,000 bariatric procedures were performed on Americans in 2017, up from 158,000 in 2011.3 Despite lowering the risks of diabetes, stroke, myocardial infarction, cancer, and all-cause mortality,4 bariatric surgery is associated with increased health care use. Neovius et al. found that people who underwent bariatric surgery used 54 mean cumulative hospital days in the 20 years following their procedures, compared with just 40 inpatient days used by controls.5
Although hospitalists are caring for increasing numbers of patients who have undergone bariatric surgery, many of us may not be aware of some of the things that can lead to hospitalization or otherwise affect inpatient medical care. Here are a few points to keep in mind the next time you care for an inpatient with prior bariatric surgery.
Pharmacokinetics change after surgery
Gastrointestinal anatomy necessarily changes after bariatric surgery and can affect the oral absorption of drugs. Because gastric motility may be impaired and the pH in the stomach is increased after bariatric surgery, the disintegration and dissolution of immediate-release solid pills or caps may be compromised.
It is therefore prudent to crush solid forms or switch to liquid or chewable formulations of immediate-release drugs for the first few weeks to months after surgery. Enteric-coated or long-acting drug formulations should not be crushed and should generally be avoided in patients who have undergone bypass procedures such as Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion with duodenal switch (BPD/DS), as they can demonstrate either enhanced or diminished absorption (depending on the drug).
Reduced intestinal transit times and changes in intestinal pH can alter the absorption of certain drugs as well, and the expression of some drug transporter proteins and enzymes such as the CYP3A4 variant of cytochrome P450 – which is estimated to metabolize up to half of currently available drugs – varies between the upper and the lower small intestine, potentially leading to increased bioavailability of medications metabolized by this enzyme in patients who have undergone bypass surgeries.
Interestingly, longer-term studies have reexamined drug absorption in patients 2-4 years after RYGB and found that initially-increased drug plasma levels often return to preoperative levels or even lower over time,6 likely because of adaptive changes in the GI tract. Because research on the pharmacokinetics of individual drugs after bariatric surgery is lacking, the hospitalist should be aware that the bioavailability of oral drugs is often altered and should monitor patients for the desired therapeutic effect as well as potential toxicities for any drug administered to postbariatric surgery patients.
Finally, note that nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and corticosteroids should be avoided after bariatric surgery unless the benefit clearly outweighs the risk, as they increase the risk of ulcers even in patients without underlying surgical disruptions to the gastric mucosa.
Micronutrient deficiencies are common and can occur at any time
While many clinicians recognize that vitamin deficiencies can occur after weight loss surgeries which bypass the duodenum, such as the RYGB or the BPD/DS, it is important to note that vitamin and mineral deficiencies occur commonly even in patients with intact intestinal absorption such as those who underwent sleeve gastrectomy (SG) and even despite regained weight due to greater volumes of food (and micronutrient) intake over time.
The most common vitamin deficiencies include iron, vitamin B12, thiamine (vitamin B1), and vitamin D, but deficiencies in other vitamins and minerals may found as well. Anemia, bone fractures, heart failure, and encephalopathy can all be related to postoperative vitamin deficiencies. Most bariatric surgery patients should have micronutrient levels monitored on a yearly basis and should be taking at least a multivitamin with minerals (including zinc, copper, selenium and iron), a form of vitamin B12, and vitamin D with calcium supplementation. Additional supplements may be appropriate depending on the type of surgery the patient had or whether a deficiency is found.
The differential diagnosis for abdominal pain after bariatric surgery is unique
While the usual suspects such as diverticulitis or gastritis should be considered in postbariatric surgery patients just as in others, a few specific complications can arise after weight loss surgery.
Marginal ulcerations (ulcers at the surgical anastomotic sites) have been reported in up to a third of patients complaining of abdominal pain or dysphagia after RYGB, with tobacco, alcohol, or NSAID use conferring even greater risk.7 Early upper endoscopy may be warranted in symptomatic patients.
Small bowel obstruction (SBO) may occur due to surgical adhesions as in other patients, but catastrophic internal hernias with associated volvulus can occur due to specific anatomical defects that are created by the RYGB and BPD/DS procedures. CT imaging is insensitive and can miss up to 30% of these cases,8 and nasogastric tubes placed blindly for decompression of an SBO can lead to perforation of the end of the alimentary limb at the gastric pouch outlet, so post-RYGB or BPD/DS patients presenting with signs of small bowel obstruction should have an early surgical consult for expeditious surgical management rather than a trial of conservative medical management.9
Cholelithiasis is a very common postoperative complication, occurring in about 25% of SG patients and 32% of RYGB patients in the first year following surgery. The risk of gallstone formation can be significantly reduced with the postoperative use of ursodeoxycholic acid.10
Onset of abdominal cramping, nausea and diarrhea (sometimes accompanied by vasomotor symptoms) within 15-60 minutes of eating may be due to early dumping syndrome. Rapid delivery of food from the gastric pouch into the small intestine causes the release of gut peptides and an osmotic fluid shift into the intestinal lumen that can trigger these symptoms even in patients with a preserved pyloric sphincter, such as those who underwent SG. Simply eliminating sugars and simple carbohydrates from the diet usually resolves the problem, and eliminating lactose can often be helpful as well.
Postprandial hyperinsulinemic hypoglycemia (“late dumping syndrome”) can develop years after surgery
Vasomotor symptoms such as flushing/sweating, shaking, tachycardia/palpitations, lightheadedness, or difficulty concentrating occurring 1-3 hours after a meal should prompt blood glucose testing, as delayed hypoglycemia can occur after a large insulin surge.
Most commonly seen after RYGB, late dumping syndrome, like early dumping syndrome, can often be managed by eliminating sugars and simple carbohydrates from the diet. The onset of late dumping syndrome has been reported as late as 8 years after surgery,11 so the etiology of symptoms can be elusive. If the diagnosis is unclear, an oral glucose tolerance test may be helpful.
Alcohol use disorder is more prevalent after weight loss surgery
Changes to the gastrointestinal anatomy allow for more rapid absorption of ethanol into the bloodstream, making the drug more potent in postop patients. Simultaneously, many patients who undergo bariatric surgery have a history of using food to buffer negative emotions. Abruptly depriving them of that comfort in the context of the increased potency of alcohol could potentially leave bariatric surgery patients vulnerable to the development of alcohol use disorder, even when they did not misuse alcohol preoperatively.
Of note, alcohol misuse becomes more prevalent after the first postoperative year.12 Screening for alcohol misuse on admission to the hospital is wise in all cases, but perhaps even more so in the postbariatric surgery patient. If a patient does report excessive alcohol use, keep possible thiamine deficiency in mind.
The risk of suicide and self-harm increases after bariatric surgery
While all-cause mortality rates decrease after bariatric surgery compared with matched controls, the risk of suicide and nonfatal self-harm increases.
About half of bariatric surgery patients with nonfatal events have substance misuse.13 Notably, several studies have found reduced plasma levels of SSRIs in patients after RYGB,6 so pharmacotherapy for mood disorders could be less effective after bariatric surgery as well. The hospitalist could positively impact patients by screening for both substance misuse and depression and by having a low threshold for referral to a mental health professional.
As we see ever-increasing numbers of inpatients who have a history of bariatric surgery, being aware of these common and important complications can help today’s hospitalist provide the best care possible.
Dr. Kerns is a hospitalist and codirector of bariatric surgery at the Washington DC VA Medical Center.
References
1. Obesity and overweight. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Published Feb 16, 2018.
2. Hales CM et al. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS data brief, no 288. Hyattsville, MD: National Center for Health Statistics. 2017.
3. Estimate of Bariatric Surgery Numbers, 2011-2018. ASMBS.org. Published June 2018.
4. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013 Mar;273(3):219-34. doi: 10.1111/joim.12012.
5. Neovius M et al. Health care use during 20 years following bariatric surgery. JAMA. 2012 Sep 19; 308(11):1132-41. doi: 10.1001/2012.jama.11792.
6. Azran C. et al. Oral drug therapy following bariatric surgery: An overview of fundamentals, literature and clinical recommendations. Obes Rev. 2016 Nov;17(11):1050-66. doi: 10.1111/obr.12434.
7. El-hayek KM et al. Marginal ulcer after Roux-en-Y gastric bypass: What have we really learned? Surg Endosc. 2012 Oct;26(10):2789-96. Epub 2012 Apr 28. (Abstract presented at Society of American Gastrointestinal and Endoscopic Surgeons 2012 annual meeting, San Diego.) 8. Iannelli A et al. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2006;16:1265-71. doi: 10.1381/096089206778663689.
9. Lim R et al. Early and late complications of bariatric operation. Trauma Surg Acute Care Open. 2018 Oct 9;3(1): e000219. doi: 10.1136/tsaco-2018-000219.
10. Coupaye M et al. Evaluation of incidence of cholelithiasis after bariatric surgery in subjects treated or not treated with ursodeoxycholic acid. Surg Obes Relat Dis. 2017;13(4):681-5. doi: 10.1016/j.soard.2016.11.022.
11. Eisenberg D et al. ASMBS position statement on postprandial hyperinsulinemic hypoglycemia after bariatric surgery. Surg Obes Relat Dis. 2017 Mar;13(3):371-8. doi: 10.1016/j.soard.2016.12.005.
12. King WC et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012 Jun 20;307(23):2516-25. doi: 10.1001/jama.2012.6147.
13. Neovius M et al. Risk of suicide and non-fatal self-harm after bariatric surgery: Results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018 Mar;6(3):197-207. doi: 10.1016/S2213-8587(17)30437-0.
Feds tout drug candidates to treat COVID-19
Therapeutics could be available in the near term to help treat COVID-19 patients, according to President Donald Trump.
During a March 19 press briefing, the president highlighted two drugs that could be put into play in the battle against the virus.
The first product is hydroxychloroquine (Plaquenil), a drug used to treat malaria and severe arthritis, is showing promise as a possible treatment for COVID-19.
“The nice part is it’s been around for a long time, so we know that if things go as planned, it’s not going to kill anybody,” President Trump said. “When you go with a brand-new drug, you don’t know that that’s going to happen,” adding that it has shown “very, very encouraging” results as a potential treatment for COVID-19.
He said this drug will be made available “almost immediately.” During the press conference, Food and Drug Administration Commissioner Stephen M. Hahn, MD, suggested the drug would be made available in the context of a large pragmatic clinical trial, enabling the FDA to collect data on it and make a long-term decision on its viability to treat COVID-19.
Dr. Hahn also pointed to the Gilead drug remdesivir – a drug originally developed to fight Ebola and currently undergoing clinical trials – as another possible candidate for a near-term therapeutic to help treat patients while vaccine development occurs.
Dr. Hahn noted that, while the agency is striving to provide regulatory flexibility, safety is paramount. “Let me make one thing clear: FDA’s responsibility to the American people is to ensure that products are safe and effective and that we are continuing to do that.”
He noted that if these and other experimental drugs show promise, physicians can request them under “compassionate use” provisions.
“We have criteria for that, and very speedy approval for that,” Dr. Hahn said. “The important thing about compassionate use ... this is even beyond ‘right to try.’ [We] get to collect the information about that.”
He noted that the FDA is looking at other drugs that are approved for other indications. The examinations of existing therapies are meant to be a bridge as companies work to develop new therapeutics as well as vaccines.
Dr. Hahn also highlighted a cross-agency effort on convalescent plasma, which uses the plasma from a patient who has recovered from COVID-19 infection to help patients fight the virus. “This is a possible treatment; this is not a proven treatment, “ Dr. Hahn said.
Takeda is working on an immunoglobulin treatment based on its intravenous immunoglobulin product Gammagard Liquid.
Julie Kim, president of plasma-derived therapies at Takeda, said the company should be able to go straight into testing efficacy of this approach, given the known safety profile of the treatment. She made the comments during a March 18 press briefing hosted by Pharmaceutical Research and Manufacturers of America (PhRMA). Ms. Kim did caution that this would not be a mass market kind of treatment, as supply would depend on plasma donations from individuals who have fully recovered from a COVID-19 infection. She estimated that the treatment could be available to a targeted group of high-risk patients in 9-18 months.
PhRMA president and CEO Stephen Ubl said the industry is “literally working around the clock” on four key areas: development of new diagnostics, identification of potential existing treatments to make available through trials and compassionate use, development of novel therapies, and development of a vaccine.
There are more than 80 clinical trials underway on existing treatments that could have approval to treat COVID-19 in a matter of months, he said.
Mikael Dolsten, MD, PhD, chief scientific officer at Pfizer, said that the company is working with Germany-based BioNTech SE to develop an mRNA-based vaccine for COVID-19, with testing expected to begin in Germany, China, and the United States by the end of April. The company also is screening antiviral compounds that were previously in development against other coronavirus diseases.
Clement Lewin, PhD, associate vice president of R&D strategy for vaccines at Sanofi, said the company has partnered with Regeneron to launch a trial of sarilumab (Kevzara), a drug approved to treat moderate to severe rheumatoid arthritis, to help treat COVID-19.
Meanwhile, Lilly Chief Scientific Officer Daniel Skovronsky, MD, PhD, noted that his company is collaborating with AbCellera to develop therapeutics using monoclonal antibodies isolated from one of the first U.S. patients who recovered from COVID-19. He said the goal is to begin testing within the next 4 months.
Separately, World Health Organization Director General Tedros Adhanom Ghebreyesus announced during a March 18 press conference that it is spearheading a large international study examining a number of different treatments in what has been dubbed the SOLIDARITY trial. Argentina, Bahrain, Canada, France, Iran, Norway, South Africa, Spain, Switzerland, and Thailand have signed on to be a part of the trial, with more countries expected to participate.
“I continue to be inspired by the many demonstrations of solidarity from all over the world,” he said. “These and other efforts give me hope that together, we can and will prevail. This virus is presenting us with an unprecedented threat. But it’s also an unprecedented opportunity to come together as one against a common enemy, an enemy against humanity.”
Therapeutics could be available in the near term to help treat COVID-19 patients, according to President Donald Trump.
During a March 19 press briefing, the president highlighted two drugs that could be put into play in the battle against the virus.
The first product is hydroxychloroquine (Plaquenil), a drug used to treat malaria and severe arthritis, is showing promise as a possible treatment for COVID-19.
“The nice part is it’s been around for a long time, so we know that if things go as planned, it’s not going to kill anybody,” President Trump said. “When you go with a brand-new drug, you don’t know that that’s going to happen,” adding that it has shown “very, very encouraging” results as a potential treatment for COVID-19.
He said this drug will be made available “almost immediately.” During the press conference, Food and Drug Administration Commissioner Stephen M. Hahn, MD, suggested the drug would be made available in the context of a large pragmatic clinical trial, enabling the FDA to collect data on it and make a long-term decision on its viability to treat COVID-19.
Dr. Hahn also pointed to the Gilead drug remdesivir – a drug originally developed to fight Ebola and currently undergoing clinical trials – as another possible candidate for a near-term therapeutic to help treat patients while vaccine development occurs.
Dr. Hahn noted that, while the agency is striving to provide regulatory flexibility, safety is paramount. “Let me make one thing clear: FDA’s responsibility to the American people is to ensure that products are safe and effective and that we are continuing to do that.”
He noted that if these and other experimental drugs show promise, physicians can request them under “compassionate use” provisions.
“We have criteria for that, and very speedy approval for that,” Dr. Hahn said. “The important thing about compassionate use ... this is even beyond ‘right to try.’ [We] get to collect the information about that.”
He noted that the FDA is looking at other drugs that are approved for other indications. The examinations of existing therapies are meant to be a bridge as companies work to develop new therapeutics as well as vaccines.
Dr. Hahn also highlighted a cross-agency effort on convalescent plasma, which uses the plasma from a patient who has recovered from COVID-19 infection to help patients fight the virus. “This is a possible treatment; this is not a proven treatment, “ Dr. Hahn said.
Takeda is working on an immunoglobulin treatment based on its intravenous immunoglobulin product Gammagard Liquid.
Julie Kim, president of plasma-derived therapies at Takeda, said the company should be able to go straight into testing efficacy of this approach, given the known safety profile of the treatment. She made the comments during a March 18 press briefing hosted by Pharmaceutical Research and Manufacturers of America (PhRMA). Ms. Kim did caution that this would not be a mass market kind of treatment, as supply would depend on plasma donations from individuals who have fully recovered from a COVID-19 infection. She estimated that the treatment could be available to a targeted group of high-risk patients in 9-18 months.
PhRMA president and CEO Stephen Ubl said the industry is “literally working around the clock” on four key areas: development of new diagnostics, identification of potential existing treatments to make available through trials and compassionate use, development of novel therapies, and development of a vaccine.
There are more than 80 clinical trials underway on existing treatments that could have approval to treat COVID-19 in a matter of months, he said.
Mikael Dolsten, MD, PhD, chief scientific officer at Pfizer, said that the company is working with Germany-based BioNTech SE to develop an mRNA-based vaccine for COVID-19, with testing expected to begin in Germany, China, and the United States by the end of April. The company also is screening antiviral compounds that were previously in development against other coronavirus diseases.
Clement Lewin, PhD, associate vice president of R&D strategy for vaccines at Sanofi, said the company has partnered with Regeneron to launch a trial of sarilumab (Kevzara), a drug approved to treat moderate to severe rheumatoid arthritis, to help treat COVID-19.
Meanwhile, Lilly Chief Scientific Officer Daniel Skovronsky, MD, PhD, noted that his company is collaborating with AbCellera to develop therapeutics using monoclonal antibodies isolated from one of the first U.S. patients who recovered from COVID-19. He said the goal is to begin testing within the next 4 months.
Separately, World Health Organization Director General Tedros Adhanom Ghebreyesus announced during a March 18 press conference that it is spearheading a large international study examining a number of different treatments in what has been dubbed the SOLIDARITY trial. Argentina, Bahrain, Canada, France, Iran, Norway, South Africa, Spain, Switzerland, and Thailand have signed on to be a part of the trial, with more countries expected to participate.
“I continue to be inspired by the many demonstrations of solidarity from all over the world,” he said. “These and other efforts give me hope that together, we can and will prevail. This virus is presenting us with an unprecedented threat. But it’s also an unprecedented opportunity to come together as one against a common enemy, an enemy against humanity.”
Therapeutics could be available in the near term to help treat COVID-19 patients, according to President Donald Trump.
During a March 19 press briefing, the president highlighted two drugs that could be put into play in the battle against the virus.
The first product is hydroxychloroquine (Plaquenil), a drug used to treat malaria and severe arthritis, is showing promise as a possible treatment for COVID-19.
“The nice part is it’s been around for a long time, so we know that if things go as planned, it’s not going to kill anybody,” President Trump said. “When you go with a brand-new drug, you don’t know that that’s going to happen,” adding that it has shown “very, very encouraging” results as a potential treatment for COVID-19.
He said this drug will be made available “almost immediately.” During the press conference, Food and Drug Administration Commissioner Stephen M. Hahn, MD, suggested the drug would be made available in the context of a large pragmatic clinical trial, enabling the FDA to collect data on it and make a long-term decision on its viability to treat COVID-19.
Dr. Hahn also pointed to the Gilead drug remdesivir – a drug originally developed to fight Ebola and currently undergoing clinical trials – as another possible candidate for a near-term therapeutic to help treat patients while vaccine development occurs.
Dr. Hahn noted that, while the agency is striving to provide regulatory flexibility, safety is paramount. “Let me make one thing clear: FDA’s responsibility to the American people is to ensure that products are safe and effective and that we are continuing to do that.”
He noted that if these and other experimental drugs show promise, physicians can request them under “compassionate use” provisions.
“We have criteria for that, and very speedy approval for that,” Dr. Hahn said. “The important thing about compassionate use ... this is even beyond ‘right to try.’ [We] get to collect the information about that.”
He noted that the FDA is looking at other drugs that are approved for other indications. The examinations of existing therapies are meant to be a bridge as companies work to develop new therapeutics as well as vaccines.
Dr. Hahn also highlighted a cross-agency effort on convalescent plasma, which uses the plasma from a patient who has recovered from COVID-19 infection to help patients fight the virus. “This is a possible treatment; this is not a proven treatment, “ Dr. Hahn said.
Takeda is working on an immunoglobulin treatment based on its intravenous immunoglobulin product Gammagard Liquid.
Julie Kim, president of plasma-derived therapies at Takeda, said the company should be able to go straight into testing efficacy of this approach, given the known safety profile of the treatment. She made the comments during a March 18 press briefing hosted by Pharmaceutical Research and Manufacturers of America (PhRMA). Ms. Kim did caution that this would not be a mass market kind of treatment, as supply would depend on plasma donations from individuals who have fully recovered from a COVID-19 infection. She estimated that the treatment could be available to a targeted group of high-risk patients in 9-18 months.
PhRMA president and CEO Stephen Ubl said the industry is “literally working around the clock” on four key areas: development of new diagnostics, identification of potential existing treatments to make available through trials and compassionate use, development of novel therapies, and development of a vaccine.
There are more than 80 clinical trials underway on existing treatments that could have approval to treat COVID-19 in a matter of months, he said.
Mikael Dolsten, MD, PhD, chief scientific officer at Pfizer, said that the company is working with Germany-based BioNTech SE to develop an mRNA-based vaccine for COVID-19, with testing expected to begin in Germany, China, and the United States by the end of April. The company also is screening antiviral compounds that were previously in development against other coronavirus diseases.
Clement Lewin, PhD, associate vice president of R&D strategy for vaccines at Sanofi, said the company has partnered with Regeneron to launch a trial of sarilumab (Kevzara), a drug approved to treat moderate to severe rheumatoid arthritis, to help treat COVID-19.
Meanwhile, Lilly Chief Scientific Officer Daniel Skovronsky, MD, PhD, noted that his company is collaborating with AbCellera to develop therapeutics using monoclonal antibodies isolated from one of the first U.S. patients who recovered from COVID-19. He said the goal is to begin testing within the next 4 months.
Separately, World Health Organization Director General Tedros Adhanom Ghebreyesus announced during a March 18 press conference that it is spearheading a large international study examining a number of different treatments in what has been dubbed the SOLIDARITY trial. Argentina, Bahrain, Canada, France, Iran, Norway, South Africa, Spain, Switzerland, and Thailand have signed on to be a part of the trial, with more countries expected to participate.
“I continue to be inspired by the many demonstrations of solidarity from all over the world,” he said. “These and other efforts give me hope that together, we can and will prevail. This virus is presenting us with an unprecedented threat. But it’s also an unprecedented opportunity to come together as one against a common enemy, an enemy against humanity.”
20% of U.S. COVID-19 deaths were aged 20-64 years
*Correction, 3/20/2020: An earlier version of this story misstated the age range for COVID-19 deaths. The headline of this story was corrected to read "20% of COVID-19 deaths were aged 20-64 years" and the text was adjusted to reflect the correct age range.
A review of more than 4,000 U.S. patients who were diagnosed with novel coronavirus infection (COVID-19) shows that an unexpected 20% of deaths occurred among adults aged 20-64 years, and 20% of those hospitalized were aged 20-44 years.
The expectation has been that people over 65 are most vulnerable to COVID-19 infection, but this study indicates that, at least in the United States, a significant number of patients under 45 can land in the hospital and can even die of the disease.
To assess rates of hospitalization, admission to an ICU, and death among patients with COVID-19 by age group, the Centers for Disease Control and Prevention analyzed 4,226 COVID-19 cases in the United States that were reported between Feb. 12 and March 16.
Overall, older patients in this group were the most likely to be hospitalized, to be admitted to ICU, and to die of COVID-19. A total of 31% of the cases, 45% of hospitalizations, 53% of ICU admissions, and 80% of deaths occurred in patients aged 65 years and older. “Similar to reports from other countries, this finding suggests that the risk for serious disease and death from COVID-19 is higher in older age groups,” said the investigators. “In contrast, persons aged [19 years and younger] appear to have milder COVID-19 illness, with almost no hospitalizations or deaths reported to date in the United States in this age group.”
But compared with the under-19 group, patients aged 20-44 years appeared to be at higher risk for hospitalization and ICU admission, according to the data published March 18 in Morbidity and Mortality Weekly Report.
The researchers excluded from their analysis patients who repatriated to the United States from Wuhan, China, and from Japan, including patients repatriated from cruise ships. Data on serious underlying health conditions were not available, and many cases were missing key data, they noted.
Among 508 patients known to have been hospitalized, 9% were aged 85 years or older, 36% were aged 65-84 years, 17% were aged 55-64 years, 18% were 45-54 years, and 20% were aged 20-44 years.
Among 121 patients admitted to an ICU, 7% were aged 85 years or older, 46% were aged 65-84 years, 36% were aged 45-64 years, and 12% were aged 20-44 years. Between 11% and 31% of patients with COVID-19 aged 75-84 years were admitted to an ICU.
Of 44 deaths, more than a third occurred among adults aged 85 years and older, and 46% occurred among adults aged 65-84 years, and 20% occurred among adults aged 20-64 years.
More follow-up time is needed to determine outcomes among active cases, the researchers said. These results also might overestimate the prevalence of severe disease because the initial approach to testing for COVID-19 focused on people with more severe disease. “These preliminary data also demonstrate that severe illness leading to hospitalization, including ICU admission and death, can occur in adults of any age with COVID-19,” according to the CDC.
SOURCE: CDC COVID-19 Response Team. MMWR Morb Mortal Wkly Rep. 2020 Mar 18. doi: 10.15585/mmwr.mm6912e2.
*Correction, 3/20/2020: An earlier version of this story misstated the age range for COVID-19 deaths. The headline of this story was corrected to read "20% of COVID-19 deaths were aged 20-64 years" and the text was adjusted to reflect the correct age range.
A review of more than 4,000 U.S. patients who were diagnosed with novel coronavirus infection (COVID-19) shows that an unexpected 20% of deaths occurred among adults aged 20-64 years, and 20% of those hospitalized were aged 20-44 years.
The expectation has been that people over 65 are most vulnerable to COVID-19 infection, but this study indicates that, at least in the United States, a significant number of patients under 45 can land in the hospital and can even die of the disease.
To assess rates of hospitalization, admission to an ICU, and death among patients with COVID-19 by age group, the Centers for Disease Control and Prevention analyzed 4,226 COVID-19 cases in the United States that were reported between Feb. 12 and March 16.
Overall, older patients in this group were the most likely to be hospitalized, to be admitted to ICU, and to die of COVID-19. A total of 31% of the cases, 45% of hospitalizations, 53% of ICU admissions, and 80% of deaths occurred in patients aged 65 years and older. “Similar to reports from other countries, this finding suggests that the risk for serious disease and death from COVID-19 is higher in older age groups,” said the investigators. “In contrast, persons aged [19 years and younger] appear to have milder COVID-19 illness, with almost no hospitalizations or deaths reported to date in the United States in this age group.”
But compared with the under-19 group, patients aged 20-44 years appeared to be at higher risk for hospitalization and ICU admission, according to the data published March 18 in Morbidity and Mortality Weekly Report.
The researchers excluded from their analysis patients who repatriated to the United States from Wuhan, China, and from Japan, including patients repatriated from cruise ships. Data on serious underlying health conditions were not available, and many cases were missing key data, they noted.
Among 508 patients known to have been hospitalized, 9% were aged 85 years or older, 36% were aged 65-84 years, 17% were aged 55-64 years, 18% were 45-54 years, and 20% were aged 20-44 years.
Among 121 patients admitted to an ICU, 7% were aged 85 years or older, 46% were aged 65-84 years, 36% were aged 45-64 years, and 12% were aged 20-44 years. Between 11% and 31% of patients with COVID-19 aged 75-84 years were admitted to an ICU.
Of 44 deaths, more than a third occurred among adults aged 85 years and older, and 46% occurred among adults aged 65-84 years, and 20% occurred among adults aged 20-64 years.
More follow-up time is needed to determine outcomes among active cases, the researchers said. These results also might overestimate the prevalence of severe disease because the initial approach to testing for COVID-19 focused on people with more severe disease. “These preliminary data also demonstrate that severe illness leading to hospitalization, including ICU admission and death, can occur in adults of any age with COVID-19,” according to the CDC.
SOURCE: CDC COVID-19 Response Team. MMWR Morb Mortal Wkly Rep. 2020 Mar 18. doi: 10.15585/mmwr.mm6912e2.
*Correction, 3/20/2020: An earlier version of this story misstated the age range for COVID-19 deaths. The headline of this story was corrected to read "20% of COVID-19 deaths were aged 20-64 years" and the text was adjusted to reflect the correct age range.
A review of more than 4,000 U.S. patients who were diagnosed with novel coronavirus infection (COVID-19) shows that an unexpected 20% of deaths occurred among adults aged 20-64 years, and 20% of those hospitalized were aged 20-44 years.
The expectation has been that people over 65 are most vulnerable to COVID-19 infection, but this study indicates that, at least in the United States, a significant number of patients under 45 can land in the hospital and can even die of the disease.
To assess rates of hospitalization, admission to an ICU, and death among patients with COVID-19 by age group, the Centers for Disease Control and Prevention analyzed 4,226 COVID-19 cases in the United States that were reported between Feb. 12 and March 16.
Overall, older patients in this group were the most likely to be hospitalized, to be admitted to ICU, and to die of COVID-19. A total of 31% of the cases, 45% of hospitalizations, 53% of ICU admissions, and 80% of deaths occurred in patients aged 65 years and older. “Similar to reports from other countries, this finding suggests that the risk for serious disease and death from COVID-19 is higher in older age groups,” said the investigators. “In contrast, persons aged [19 years and younger] appear to have milder COVID-19 illness, with almost no hospitalizations or deaths reported to date in the United States in this age group.”
But compared with the under-19 group, patients aged 20-44 years appeared to be at higher risk for hospitalization and ICU admission, according to the data published March 18 in Morbidity and Mortality Weekly Report.
The researchers excluded from their analysis patients who repatriated to the United States from Wuhan, China, and from Japan, including patients repatriated from cruise ships. Data on serious underlying health conditions were not available, and many cases were missing key data, they noted.
Among 508 patients known to have been hospitalized, 9% were aged 85 years or older, 36% were aged 65-84 years, 17% were aged 55-64 years, 18% were 45-54 years, and 20% were aged 20-44 years.
Among 121 patients admitted to an ICU, 7% were aged 85 years or older, 46% were aged 65-84 years, 36% were aged 45-64 years, and 12% were aged 20-44 years. Between 11% and 31% of patients with COVID-19 aged 75-84 years were admitted to an ICU.
Of 44 deaths, more than a third occurred among adults aged 85 years and older, and 46% occurred among adults aged 65-84 years, and 20% occurred among adults aged 20-64 years.
More follow-up time is needed to determine outcomes among active cases, the researchers said. These results also might overestimate the prevalence of severe disease because the initial approach to testing for COVID-19 focused on people with more severe disease. “These preliminary data also demonstrate that severe illness leading to hospitalization, including ICU admission and death, can occur in adults of any age with COVID-19,” according to the CDC.
SOURCE: CDC COVID-19 Response Team. MMWR Morb Mortal Wkly Rep. 2020 Mar 18. doi: 10.15585/mmwr.mm6912e2.
Rheumatologists to share knowledge in COVID-19 patient-centered registry
Rheumatologists the world over are joining forces to create a COVID-19 rheumatology registry designed to help both patients and providers learn from each other regarding management of rheumatologic diseases and risk of infection among patients who are commonly on chronic immunosuppressive medications.
The COVID-19 Global Rheumatology Alliance, a consortium supported by more than 50 major clinical societies and foundations, quickly grew from messages on social media platforms to a multinational group focused on the common goal of helping to “guide rheumatology clinicians in assessing and treating patients with rheumatologic disease and in evaluating the risk of infection in patients on immunosuppression.”
As of this writing, the rheumatology registry is still being assembled, and organizers are currently seeking approvals from various authorities. As of March 17, 2020, the Institutional Review Board (IRB) at the University of California, San Francisco, has determined that the registry is exempt from IRB approval requirements, a finding that should apply elsewhere in the United States, according to the registry website.
When it is fully up and running, clinicians will be able to report to the secure website on any and all cases of patients with rheumatologic disorders who present with COVID-19 of any severity, including patients with mild disease or asymptomatic patients who test positive.
“We are aiming for 5 to 10 minutes to input the data. We don’t want to drag them away from their clinical duties too much, but if clinicians are able to spare a few minutes to put in details about a patient, then that’s going to help build our knowledge and it’s going to help them with other patients,” said Philip Robinson, MBChB, associate professor of medicine at the University of Queensland in Brisbane, Australia, and the chief architect of the registry.
The data will be deindentified, with no protected health care information required or included, and made available to the global rheumatology community, but the registry will not offer clinical advice, Dr. Robinson said in an interview.
“This is observational data, it’s not randomized, but our approach is that some data is better than no data,” he said.
He also cautioned that the data will need careful interpretation, because information about patients with mild symptoms may offer false reassurances about the severity or extent of infection.
“For example, the patients with severe cases may be in the ICU, and can’t tell their doctors that they’re on methotrexate, so you can see how we need to be really careful about the messages from that data and not misinterpret it,” he said.
The COVID-19 rheumatology registry was inspired by a similar effort in the gastroenterology community, the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE-IBD) registry. Patients with inflammatory bowel disease are often treated with immunosuppressive biologic agents familiar to the rheumatology community, such as infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars), and methotrexate.
Rheumatologists the world over are joining forces to create a COVID-19 rheumatology registry designed to help both patients and providers learn from each other regarding management of rheumatologic diseases and risk of infection among patients who are commonly on chronic immunosuppressive medications.
The COVID-19 Global Rheumatology Alliance, a consortium supported by more than 50 major clinical societies and foundations, quickly grew from messages on social media platforms to a multinational group focused on the common goal of helping to “guide rheumatology clinicians in assessing and treating patients with rheumatologic disease and in evaluating the risk of infection in patients on immunosuppression.”
As of this writing, the rheumatology registry is still being assembled, and organizers are currently seeking approvals from various authorities. As of March 17, 2020, the Institutional Review Board (IRB) at the University of California, San Francisco, has determined that the registry is exempt from IRB approval requirements, a finding that should apply elsewhere in the United States, according to the registry website.
When it is fully up and running, clinicians will be able to report to the secure website on any and all cases of patients with rheumatologic disorders who present with COVID-19 of any severity, including patients with mild disease or asymptomatic patients who test positive.
“We are aiming for 5 to 10 minutes to input the data. We don’t want to drag them away from their clinical duties too much, but if clinicians are able to spare a few minutes to put in details about a patient, then that’s going to help build our knowledge and it’s going to help them with other patients,” said Philip Robinson, MBChB, associate professor of medicine at the University of Queensland in Brisbane, Australia, and the chief architect of the registry.
The data will be deindentified, with no protected health care information required or included, and made available to the global rheumatology community, but the registry will not offer clinical advice, Dr. Robinson said in an interview.
“This is observational data, it’s not randomized, but our approach is that some data is better than no data,” he said.
He also cautioned that the data will need careful interpretation, because information about patients with mild symptoms may offer false reassurances about the severity or extent of infection.
“For example, the patients with severe cases may be in the ICU, and can’t tell their doctors that they’re on methotrexate, so you can see how we need to be really careful about the messages from that data and not misinterpret it,” he said.
The COVID-19 rheumatology registry was inspired by a similar effort in the gastroenterology community, the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE-IBD) registry. Patients with inflammatory bowel disease are often treated with immunosuppressive biologic agents familiar to the rheumatology community, such as infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars), and methotrexate.
Rheumatologists the world over are joining forces to create a COVID-19 rheumatology registry designed to help both patients and providers learn from each other regarding management of rheumatologic diseases and risk of infection among patients who are commonly on chronic immunosuppressive medications.
The COVID-19 Global Rheumatology Alliance, a consortium supported by more than 50 major clinical societies and foundations, quickly grew from messages on social media platforms to a multinational group focused on the common goal of helping to “guide rheumatology clinicians in assessing and treating patients with rheumatologic disease and in evaluating the risk of infection in patients on immunosuppression.”
As of this writing, the rheumatology registry is still being assembled, and organizers are currently seeking approvals from various authorities. As of March 17, 2020, the Institutional Review Board (IRB) at the University of California, San Francisco, has determined that the registry is exempt from IRB approval requirements, a finding that should apply elsewhere in the United States, according to the registry website.
When it is fully up and running, clinicians will be able to report to the secure website on any and all cases of patients with rheumatologic disorders who present with COVID-19 of any severity, including patients with mild disease or asymptomatic patients who test positive.
“We are aiming for 5 to 10 minutes to input the data. We don’t want to drag them away from their clinical duties too much, but if clinicians are able to spare a few minutes to put in details about a patient, then that’s going to help build our knowledge and it’s going to help them with other patients,” said Philip Robinson, MBChB, associate professor of medicine at the University of Queensland in Brisbane, Australia, and the chief architect of the registry.
The data will be deindentified, with no protected health care information required or included, and made available to the global rheumatology community, but the registry will not offer clinical advice, Dr. Robinson said in an interview.
“This is observational data, it’s not randomized, but our approach is that some data is better than no data,” he said.
He also cautioned that the data will need careful interpretation, because information about patients with mild symptoms may offer false reassurances about the severity or extent of infection.
“For example, the patients with severe cases may be in the ICU, and can’t tell their doctors that they’re on methotrexate, so you can see how we need to be really careful about the messages from that data and not misinterpret it,” he said.
The COVID-19 rheumatology registry was inspired by a similar effort in the gastroenterology community, the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE-IBD) registry. Patients with inflammatory bowel disease are often treated with immunosuppressive biologic agents familiar to the rheumatology community, such as infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars), and methotrexate.
Standing by and still open for business during COVID-19 pandemic
As of this morning, March 19, 2020, I’m still working.
Granted, there aren’t a lot of people who want to come in. My schedule has dropped to 3-5 follow-ups per day and no new patients.
I can understand people not wanting to expose themselves unnecessarily right now.
But, I’m still a doctor. What drove me to study for the MCAT, apply to med school 2 years in a row, and then survive medical school, internship, residency, and fellowship ... is still there.
Like I said in my 1987 personal statement, I still want to help people. I’d feel remiss if (provided I don’t have COVID-19) I didn’t show up for work each day, ready to care for any who need me. It’s part of who I am, what I do, and what I believe in.
I’m sure my colleagues in family practice, internal medicine, and pulmonology are swamped right now, but neurologists with primarily outpatient practices are taking a back seat except for a handful of patients.
My small office has been set up for my staff to work remotely in a pinch since 2016, so that was easy to enact. The three of us cover the phones the way we always have, and I see patients here.
With the relaxing of telehealth requirements for Medicare that were announced on March 17, I’m setting up to “see” patients remotely.
The whole situation seems bizarre and surreal.
It’s easy for anyone to read too much into anything. A brief tickle in my throat when I wake up, or a sneeze, or a few coughs, suddenly trigger a flurry of “could I have it?” thoughts. Fortunately, they fade when things quickly return to normal, but a few weeks ago I wouldn’t have thought anything of them at all.
Inevitably, I and pretty much everyone else will be exposed to or catch the virus. It’s what virions do. Unless you absolutely isolate yourself on a desert island, it will happen. When it does, you can only hope for the best.
I’m here for my patients today and will be as long as they need me. Unless I have to go into quarantine, of course. And even then, if able, I’ll do the best I can to treat them remotely.
That’s all I could ever want.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
As of this morning, March 19, 2020, I’m still working.
Granted, there aren’t a lot of people who want to come in. My schedule has dropped to 3-5 follow-ups per day and no new patients.
I can understand people not wanting to expose themselves unnecessarily right now.
But, I’m still a doctor. What drove me to study for the MCAT, apply to med school 2 years in a row, and then survive medical school, internship, residency, and fellowship ... is still there.
Like I said in my 1987 personal statement, I still want to help people. I’d feel remiss if (provided I don’t have COVID-19) I didn’t show up for work each day, ready to care for any who need me. It’s part of who I am, what I do, and what I believe in.
I’m sure my colleagues in family practice, internal medicine, and pulmonology are swamped right now, but neurologists with primarily outpatient practices are taking a back seat except for a handful of patients.
My small office has been set up for my staff to work remotely in a pinch since 2016, so that was easy to enact. The three of us cover the phones the way we always have, and I see patients here.
With the relaxing of telehealth requirements for Medicare that were announced on March 17, I’m setting up to “see” patients remotely.
The whole situation seems bizarre and surreal.
It’s easy for anyone to read too much into anything. A brief tickle in my throat when I wake up, or a sneeze, or a few coughs, suddenly trigger a flurry of “could I have it?” thoughts. Fortunately, they fade when things quickly return to normal, but a few weeks ago I wouldn’t have thought anything of them at all.
Inevitably, I and pretty much everyone else will be exposed to or catch the virus. It’s what virions do. Unless you absolutely isolate yourself on a desert island, it will happen. When it does, you can only hope for the best.
I’m here for my patients today and will be as long as they need me. Unless I have to go into quarantine, of course. And even then, if able, I’ll do the best I can to treat them remotely.
That’s all I could ever want.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
As of this morning, March 19, 2020, I’m still working.
Granted, there aren’t a lot of people who want to come in. My schedule has dropped to 3-5 follow-ups per day and no new patients.
I can understand people not wanting to expose themselves unnecessarily right now.
But, I’m still a doctor. What drove me to study for the MCAT, apply to med school 2 years in a row, and then survive medical school, internship, residency, and fellowship ... is still there.
Like I said in my 1987 personal statement, I still want to help people. I’d feel remiss if (provided I don’t have COVID-19) I didn’t show up for work each day, ready to care for any who need me. It’s part of who I am, what I do, and what I believe in.
I’m sure my colleagues in family practice, internal medicine, and pulmonology are swamped right now, but neurologists with primarily outpatient practices are taking a back seat except for a handful of patients.
My small office has been set up for my staff to work remotely in a pinch since 2016, so that was easy to enact. The three of us cover the phones the way we always have, and I see patients here.
With the relaxing of telehealth requirements for Medicare that were announced on March 17, I’m setting up to “see” patients remotely.
The whole situation seems bizarre and surreal.
It’s easy for anyone to read too much into anything. A brief tickle in my throat when I wake up, or a sneeze, or a few coughs, suddenly trigger a flurry of “could I have it?” thoughts. Fortunately, they fade when things quickly return to normal, but a few weeks ago I wouldn’t have thought anything of them at all.
Inevitably, I and pretty much everyone else will be exposed to or catch the virus. It’s what virions do. Unless you absolutely isolate yourself on a desert island, it will happen. When it does, you can only hope for the best.
I’m here for my patients today and will be as long as they need me. Unless I have to go into quarantine, of course. And even then, if able, I’ll do the best I can to treat them remotely.
That’s all I could ever want.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Real-world shortages not addressed in new COVID-19 guidance
Newly updated guidance on treating patients with the novel coronavirus (COVID-19) has been published by the World Health Organization.
While it can’t replace clinical judgment or specialist consultation, the new guidance may help strengthen the clinical management of patients when COVID-19 is suspected, according to its authors.
The guidance, adapted from an earlier edition focused on the management of suspected Middle East respiratory syndrome coronavirus (MERS-CoV), covers best practices for triage, infection prevention and control, and optimized supportive care for mild, severe, or critical coronavirus disease 2019 (COVID-19).
“This guidance should serve as a foundation for optimized supportive care to ensure the best possible chance for survival,” the authors wrote in the guidance.
While the WHO guidance does provide solid facts to support best practices for managing COVID-19, providers will also need to look beyond the document to tackle real-world issues, said David M. Ferraro, MD, FCCP, a pulmonary and critical care physician and associate professor of medicine at National Jewish Health in Denver.
For example, while the guidelines address the importance of screening and triage, limited COVID-19 testing may be a barrier to timely diagnoses that might compel more individuals to comply with social distancing recommendations, according to Dr. Ferraro, vice chair of the Fundamental Disaster Management Committee for the Society of Critical Care Medicine (SCCM).
“If we’re not providing people with confirmation that they have the virus, they may potentially continue to be spreaders of the disease, because they don’t have that absolute proof,” Dr. Ferraro said in an interview. “I think that’s where we are limited right now, because often we’re not able to tell the mild symptomatic people – or even the asymptomatic people – that they really need to play a role in preventing further spread.”
Likewise, the guidelines provide sound guidance on management of severe or critical COVID-19, according to Dr. Ferraro, yet they don’t address the potential for shortages of trained health care personnel to handle more severe cases requiring ventilation. That’s clearly an important issue, he said, especially with recent reports that the COVID-19 pandemic has pushed Italian intensive care units (ICUs) to the brink of collapse.
If the pandemic reaches crisis levels in the United States, nearly 1 million people would need ventilatory support, according to a recent report from SCCM on U.S. resource availability for COVID-19. And while there are an estimated 200,000 ventilators available in the United States, it’s estimated in that report that only 135,000 patients could be handled at a time, given the shortage of ICU physicians, advanced practice providers, nurses, and respiratory therapists with training in mechanical ventilation.
“If our ICUs get overwhelmed and swarmed, we may have the technology available, but we may not have enough resources and personnel to safely manage the number of patients,” Dr. Ferraro said.
The solution to that, according to the SCCM report, is to focus on expanding the pool of trained professionals who may be needed, not only to mechanically ventilate patients with COVID-19, but also to care for other critically ill patients routinely cared for in the ICU. They also suggest adopting a “tiered staffing strategy” in which non-ICU trained health care providers augment the capacity of experienced ICU staff.
With the prospect of untrained health care workers in mind, the WHO guidance could be a valuable resource for those who do have to jump into ICU roles, according to Dr. Ferraro.
The WHO also stresses immediate implementation of appropriate measures for infection prevention and control (IPC). According to their guidance, IPC needs to be initiated right at the point where the patient enters the hospital, with screening done at the first point of contact in the emergency department or outpatient clinics.
If patients are suspected to have COVID-19, they should receive a mask, and should be directed to a separate area where they are kept at least 1 meter apart from other individuals with suspected COVID-19, according to the WHO. (The Centers for Disease Control and Prevention recommends maintaining a distance of 6 feet to prevent spread of illness).
Beyond standard precautions such as hand washing and use of personal protective equipment, health care workers should do a point-of-care risk assessment at every patient contact to determine whether additional precautions are required.
Having standard IPC measures in place is “paramount,” according to Dr. Ferraro, for a disease that has no available vaccine, no proven treatments, and a stealthy spread fueled by asymptomatic carriers.
“Those are huge weapons against us, and the only thing we really have to knock this down is really infection prevention control, so that truly is at the cornerstone,” he said. “These are things that we must strictly follow.”
Newly updated guidance on treating patients with the novel coronavirus (COVID-19) has been published by the World Health Organization.
While it can’t replace clinical judgment or specialist consultation, the new guidance may help strengthen the clinical management of patients when COVID-19 is suspected, according to its authors.
The guidance, adapted from an earlier edition focused on the management of suspected Middle East respiratory syndrome coronavirus (MERS-CoV), covers best practices for triage, infection prevention and control, and optimized supportive care for mild, severe, or critical coronavirus disease 2019 (COVID-19).
“This guidance should serve as a foundation for optimized supportive care to ensure the best possible chance for survival,” the authors wrote in the guidance.
While the WHO guidance does provide solid facts to support best practices for managing COVID-19, providers will also need to look beyond the document to tackle real-world issues, said David M. Ferraro, MD, FCCP, a pulmonary and critical care physician and associate professor of medicine at National Jewish Health in Denver.
For example, while the guidelines address the importance of screening and triage, limited COVID-19 testing may be a barrier to timely diagnoses that might compel more individuals to comply with social distancing recommendations, according to Dr. Ferraro, vice chair of the Fundamental Disaster Management Committee for the Society of Critical Care Medicine (SCCM).
“If we’re not providing people with confirmation that they have the virus, they may potentially continue to be spreaders of the disease, because they don’t have that absolute proof,” Dr. Ferraro said in an interview. “I think that’s where we are limited right now, because often we’re not able to tell the mild symptomatic people – or even the asymptomatic people – that they really need to play a role in preventing further spread.”
Likewise, the guidelines provide sound guidance on management of severe or critical COVID-19, according to Dr. Ferraro, yet they don’t address the potential for shortages of trained health care personnel to handle more severe cases requiring ventilation. That’s clearly an important issue, he said, especially with recent reports that the COVID-19 pandemic has pushed Italian intensive care units (ICUs) to the brink of collapse.
If the pandemic reaches crisis levels in the United States, nearly 1 million people would need ventilatory support, according to a recent report from SCCM on U.S. resource availability for COVID-19. And while there are an estimated 200,000 ventilators available in the United States, it’s estimated in that report that only 135,000 patients could be handled at a time, given the shortage of ICU physicians, advanced practice providers, nurses, and respiratory therapists with training in mechanical ventilation.
“If our ICUs get overwhelmed and swarmed, we may have the technology available, but we may not have enough resources and personnel to safely manage the number of patients,” Dr. Ferraro said.
The solution to that, according to the SCCM report, is to focus on expanding the pool of trained professionals who may be needed, not only to mechanically ventilate patients with COVID-19, but also to care for other critically ill patients routinely cared for in the ICU. They also suggest adopting a “tiered staffing strategy” in which non-ICU trained health care providers augment the capacity of experienced ICU staff.
With the prospect of untrained health care workers in mind, the WHO guidance could be a valuable resource for those who do have to jump into ICU roles, according to Dr. Ferraro.
The WHO also stresses immediate implementation of appropriate measures for infection prevention and control (IPC). According to their guidance, IPC needs to be initiated right at the point where the patient enters the hospital, with screening done at the first point of contact in the emergency department or outpatient clinics.
If patients are suspected to have COVID-19, they should receive a mask, and should be directed to a separate area where they are kept at least 1 meter apart from other individuals with suspected COVID-19, according to the WHO. (The Centers for Disease Control and Prevention recommends maintaining a distance of 6 feet to prevent spread of illness).
Beyond standard precautions such as hand washing and use of personal protective equipment, health care workers should do a point-of-care risk assessment at every patient contact to determine whether additional precautions are required.
Having standard IPC measures in place is “paramount,” according to Dr. Ferraro, for a disease that has no available vaccine, no proven treatments, and a stealthy spread fueled by asymptomatic carriers.
“Those are huge weapons against us, and the only thing we really have to knock this down is really infection prevention control, so that truly is at the cornerstone,” he said. “These are things that we must strictly follow.”
Newly updated guidance on treating patients with the novel coronavirus (COVID-19) has been published by the World Health Organization.
While it can’t replace clinical judgment or specialist consultation, the new guidance may help strengthen the clinical management of patients when COVID-19 is suspected, according to its authors.
The guidance, adapted from an earlier edition focused on the management of suspected Middle East respiratory syndrome coronavirus (MERS-CoV), covers best practices for triage, infection prevention and control, and optimized supportive care for mild, severe, or critical coronavirus disease 2019 (COVID-19).
“This guidance should serve as a foundation for optimized supportive care to ensure the best possible chance for survival,” the authors wrote in the guidance.
While the WHO guidance does provide solid facts to support best practices for managing COVID-19, providers will also need to look beyond the document to tackle real-world issues, said David M. Ferraro, MD, FCCP, a pulmonary and critical care physician and associate professor of medicine at National Jewish Health in Denver.
For example, while the guidelines address the importance of screening and triage, limited COVID-19 testing may be a barrier to timely diagnoses that might compel more individuals to comply with social distancing recommendations, according to Dr. Ferraro, vice chair of the Fundamental Disaster Management Committee for the Society of Critical Care Medicine (SCCM).
“If we’re not providing people with confirmation that they have the virus, they may potentially continue to be spreaders of the disease, because they don’t have that absolute proof,” Dr. Ferraro said in an interview. “I think that’s where we are limited right now, because often we’re not able to tell the mild symptomatic people – or even the asymptomatic people – that they really need to play a role in preventing further spread.”
Likewise, the guidelines provide sound guidance on management of severe or critical COVID-19, according to Dr. Ferraro, yet they don’t address the potential for shortages of trained health care personnel to handle more severe cases requiring ventilation. That’s clearly an important issue, he said, especially with recent reports that the COVID-19 pandemic has pushed Italian intensive care units (ICUs) to the brink of collapse.
If the pandemic reaches crisis levels in the United States, nearly 1 million people would need ventilatory support, according to a recent report from SCCM on U.S. resource availability for COVID-19. And while there are an estimated 200,000 ventilators available in the United States, it’s estimated in that report that only 135,000 patients could be handled at a time, given the shortage of ICU physicians, advanced practice providers, nurses, and respiratory therapists with training in mechanical ventilation.
“If our ICUs get overwhelmed and swarmed, we may have the technology available, but we may not have enough resources and personnel to safely manage the number of patients,” Dr. Ferraro said.
The solution to that, according to the SCCM report, is to focus on expanding the pool of trained professionals who may be needed, not only to mechanically ventilate patients with COVID-19, but also to care for other critically ill patients routinely cared for in the ICU. They also suggest adopting a “tiered staffing strategy” in which non-ICU trained health care providers augment the capacity of experienced ICU staff.
With the prospect of untrained health care workers in mind, the WHO guidance could be a valuable resource for those who do have to jump into ICU roles, according to Dr. Ferraro.
The WHO also stresses immediate implementation of appropriate measures for infection prevention and control (IPC). According to their guidance, IPC needs to be initiated right at the point where the patient enters the hospital, with screening done at the first point of contact in the emergency department or outpatient clinics.
If patients are suspected to have COVID-19, they should receive a mask, and should be directed to a separate area where they are kept at least 1 meter apart from other individuals with suspected COVID-19, according to the WHO. (The Centers for Disease Control and Prevention recommends maintaining a distance of 6 feet to prevent spread of illness).
Beyond standard precautions such as hand washing and use of personal protective equipment, health care workers should do a point-of-care risk assessment at every patient contact to determine whether additional precautions are required.
Having standard IPC measures in place is “paramount,” according to Dr. Ferraro, for a disease that has no available vaccine, no proven treatments, and a stealthy spread fueled by asymptomatic carriers.
“Those are huge weapons against us, and the only thing we really have to knock this down is really infection prevention control, so that truly is at the cornerstone,” he said. “These are things that we must strictly follow.”
FROM THE WORLD HEALTH ORGANIZATION
Lopinavir-ritonavir trial results ‘disappointing’ for severe COVID-19
No difference in the primary endpoint of the time to clinical improvement was seen in an open-label trial of the antiretroviral drug lopinavir-ritonavir versus standard of care in adult patients hospitalized with severe COVID-19.
The median time to clinical improvement – defined as the time from randomization to either an improvement of two points on a seven-category ordinal scale or discharge from the hospital, whichever was first – was 16 days in both groups (hazard ratio, 1.31; 95% confidence interval 0.95-1.85; P = .09).
Although a numerically lower number of deaths were recorded at 28 days (19.2% vs. 25%) with the antiretroviral treatment versus standard of care, a similar percentage of patients had detectable levels of viral RNA in throat swabs taken at various time points during the study, Chinese researchers reported in the New England Journal of Medicine.
Bai Cao, M.D, from the China-Japan Friendship Hospital and Chinese Academy of Medical Sciences, both in Beijing, and associates, performed a randomized, controlled, trial of 199 adult patients hospitalized at the Jin Yin-Tan Hospital in Wuhan in Hubei Province, China.
For inclusion, patients had to have laboratory confirmed infection with SARS-Cov-2, the virus that causes COVID-19; pneumonia confirmed by chest imaging; and severely reduced oxygen saturation (94% or less while breathing ambient air or a ratio of the partial pressure of oxygen to the fraction of inspired oxygen of less than 300 mm Hg).
Patients were randomized to receive either a combination of lopinavir (400 mg) and ritonavir (100 mg) twice a day on top of standard care (n = 99) or to standard care alone (n = 100) for 14 days. “Because of the emergency nature of the trial, placebos of lopinavir-ritonavir were not prepared,” Dr. Cao et al. explained, noting that standard care consisted of supplemental oxygen, ventilation, antibiotic treatment, vasopressor support, renal-replacement therapy, and extracorporeal membrane oxygenation.
While the trial did not meet its primary endpoint, Dr. Cao et al. reported that patients in the lopinavir-ritonavir group had a shorter stay in the intensive care unit than did those in the standard-care group, at a median of 6 versus 11 days. They also reported that the duration from randomization to hospital discharge was numerically shorter, at a median of 12 versus 14 days. Furthermore, there was a higher percentage of patients with clinical improvement at day 14 in the lopinavir-ritonavir group than in the standard-care group (45.5% vs. 30.0%)
“The trial was initiated in rapid response to the COVID-19 public health emergency, at which time there was very limited information about clinical outcomes in hospitalized patients with COVID-19,” the researchers observed. “The question of whether earlier lopinavir-ritonavir treatment in COVID-19 could have clinical benefit is an important one that requires further study,” they wrote.
Among the trial’s limitations are it was not blinded and the researchers do not have data on the lopinavir exposure levels in the trial participants.
“This was a heroic effort” in a “particularly challenging population,” Lindsey R. Baden, MD, and Eric J. Rubin, MD, stated in an editorial accompanying the article (Baden LR and Rubin EJ. New Engl J Med. March 18, 2020. doi: 10.1056/NEJMe2005477). “Unfortunately, the trial results were disappointing,” they noted.
“The secondary end points provide both reason for hope and reason for discouragement,” wrote Dr. Baden and Dr. Rubin. The lack of effect on viral shedding, however, “strongly [suggests] that it did not have the activity desired” they observed.
Dr. Baden and Dr. Rubin commented that one of the important takeaways from the trial is that “the investigators appropriately prioritized speed, designing a trial that could rapidly produce an answer.” They continued that the investigators had shown that “rapidly initiated, high-quality randomized clinical trials are possible in epidemic conditions” and that results of such trials, whether they are positive or negative, “will be central to clinical care as the dangerous coronavirus outbreak continues.”
As it stands, more than 100 trials are listed in the ClinicalTrials.gov database as testing a wide range of different treatment approaches for COVID-19. These include trials investigating if sarilumab, hydroxychloroquine, fingolimod, bevacizumab, and losartan might have a role to play. There are also trials looking at the potential of other antiviral agents, such as Gilead’s investigational drug remdesivir, which has shown to have in vitro and in vivo activity against many emerging viral pathogens that cause Ebola, Middle Eastern Respiratory Syndrome, and Severe Acute Respiratory Syndrome.
Dr. Cao’s trial was supported by grants from Major Projects of National Science and Technology on New Drug Creation and Development and from the Chinese Academy of Medical Sciences (CAMS) Emergency Project of Covid-19, and a National Science Grant for Distinguished Young Scholars.
All authors had no financial conflicts of interest to disclose.
Dr. Baden is the director of clinical research in the Division of Infectious Diseases at the Brigham and Women’s Hospital and the director of infectious diseases at the Dana-Farber Cancer Institute. He is a deputy editor of the New England Journal of Medicine and chair of the FDA’s Antimicrobial Drug Advisory Committee. He is involved in HIV vaccine clinical trials and has received research grants from the Ragon Institute, the National Institutes of Health/National Institute of Allergy and Infectious Diseases, and the Gates Foundation.
Dr. Rubin is employed by the New England Journal of Medicine as editor-in-chief. He is an associate physician at Brigham and Women’s Hospital and is chair and Irene Heinz Given Professor of Immunology and Infectious Diseases at the Harvard T.H. Chan School of Public Health.
SOURCE: Cao B et al. New Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282.
No difference in the primary endpoint of the time to clinical improvement was seen in an open-label trial of the antiretroviral drug lopinavir-ritonavir versus standard of care in adult patients hospitalized with severe COVID-19.
The median time to clinical improvement – defined as the time from randomization to either an improvement of two points on a seven-category ordinal scale or discharge from the hospital, whichever was first – was 16 days in both groups (hazard ratio, 1.31; 95% confidence interval 0.95-1.85; P = .09).
Although a numerically lower number of deaths were recorded at 28 days (19.2% vs. 25%) with the antiretroviral treatment versus standard of care, a similar percentage of patients had detectable levels of viral RNA in throat swabs taken at various time points during the study, Chinese researchers reported in the New England Journal of Medicine.
Bai Cao, M.D, from the China-Japan Friendship Hospital and Chinese Academy of Medical Sciences, both in Beijing, and associates, performed a randomized, controlled, trial of 199 adult patients hospitalized at the Jin Yin-Tan Hospital in Wuhan in Hubei Province, China.
For inclusion, patients had to have laboratory confirmed infection with SARS-Cov-2, the virus that causes COVID-19; pneumonia confirmed by chest imaging; and severely reduced oxygen saturation (94% or less while breathing ambient air or a ratio of the partial pressure of oxygen to the fraction of inspired oxygen of less than 300 mm Hg).
Patients were randomized to receive either a combination of lopinavir (400 mg) and ritonavir (100 mg) twice a day on top of standard care (n = 99) or to standard care alone (n = 100) for 14 days. “Because of the emergency nature of the trial, placebos of lopinavir-ritonavir were not prepared,” Dr. Cao et al. explained, noting that standard care consisted of supplemental oxygen, ventilation, antibiotic treatment, vasopressor support, renal-replacement therapy, and extracorporeal membrane oxygenation.
While the trial did not meet its primary endpoint, Dr. Cao et al. reported that patients in the lopinavir-ritonavir group had a shorter stay in the intensive care unit than did those in the standard-care group, at a median of 6 versus 11 days. They also reported that the duration from randomization to hospital discharge was numerically shorter, at a median of 12 versus 14 days. Furthermore, there was a higher percentage of patients with clinical improvement at day 14 in the lopinavir-ritonavir group than in the standard-care group (45.5% vs. 30.0%)
“The trial was initiated in rapid response to the COVID-19 public health emergency, at which time there was very limited information about clinical outcomes in hospitalized patients with COVID-19,” the researchers observed. “The question of whether earlier lopinavir-ritonavir treatment in COVID-19 could have clinical benefit is an important one that requires further study,” they wrote.
Among the trial’s limitations are it was not blinded and the researchers do not have data on the lopinavir exposure levels in the trial participants.
“This was a heroic effort” in a “particularly challenging population,” Lindsey R. Baden, MD, and Eric J. Rubin, MD, stated in an editorial accompanying the article (Baden LR and Rubin EJ. New Engl J Med. March 18, 2020. doi: 10.1056/NEJMe2005477). “Unfortunately, the trial results were disappointing,” they noted.
“The secondary end points provide both reason for hope and reason for discouragement,” wrote Dr. Baden and Dr. Rubin. The lack of effect on viral shedding, however, “strongly [suggests] that it did not have the activity desired” they observed.
Dr. Baden and Dr. Rubin commented that one of the important takeaways from the trial is that “the investigators appropriately prioritized speed, designing a trial that could rapidly produce an answer.” They continued that the investigators had shown that “rapidly initiated, high-quality randomized clinical trials are possible in epidemic conditions” and that results of such trials, whether they are positive or negative, “will be central to clinical care as the dangerous coronavirus outbreak continues.”
As it stands, more than 100 trials are listed in the ClinicalTrials.gov database as testing a wide range of different treatment approaches for COVID-19. These include trials investigating if sarilumab, hydroxychloroquine, fingolimod, bevacizumab, and losartan might have a role to play. There are also trials looking at the potential of other antiviral agents, such as Gilead’s investigational drug remdesivir, which has shown to have in vitro and in vivo activity against many emerging viral pathogens that cause Ebola, Middle Eastern Respiratory Syndrome, and Severe Acute Respiratory Syndrome.
Dr. Cao’s trial was supported by grants from Major Projects of National Science and Technology on New Drug Creation and Development and from the Chinese Academy of Medical Sciences (CAMS) Emergency Project of Covid-19, and a National Science Grant for Distinguished Young Scholars.
All authors had no financial conflicts of interest to disclose.
Dr. Baden is the director of clinical research in the Division of Infectious Diseases at the Brigham and Women’s Hospital and the director of infectious diseases at the Dana-Farber Cancer Institute. He is a deputy editor of the New England Journal of Medicine and chair of the FDA’s Antimicrobial Drug Advisory Committee. He is involved in HIV vaccine clinical trials and has received research grants from the Ragon Institute, the National Institutes of Health/National Institute of Allergy and Infectious Diseases, and the Gates Foundation.
Dr. Rubin is employed by the New England Journal of Medicine as editor-in-chief. He is an associate physician at Brigham and Women’s Hospital and is chair and Irene Heinz Given Professor of Immunology and Infectious Diseases at the Harvard T.H. Chan School of Public Health.
SOURCE: Cao B et al. New Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282.
No difference in the primary endpoint of the time to clinical improvement was seen in an open-label trial of the antiretroviral drug lopinavir-ritonavir versus standard of care in adult patients hospitalized with severe COVID-19.
The median time to clinical improvement – defined as the time from randomization to either an improvement of two points on a seven-category ordinal scale or discharge from the hospital, whichever was first – was 16 days in both groups (hazard ratio, 1.31; 95% confidence interval 0.95-1.85; P = .09).
Although a numerically lower number of deaths were recorded at 28 days (19.2% vs. 25%) with the antiretroviral treatment versus standard of care, a similar percentage of patients had detectable levels of viral RNA in throat swabs taken at various time points during the study, Chinese researchers reported in the New England Journal of Medicine.
Bai Cao, M.D, from the China-Japan Friendship Hospital and Chinese Academy of Medical Sciences, both in Beijing, and associates, performed a randomized, controlled, trial of 199 adult patients hospitalized at the Jin Yin-Tan Hospital in Wuhan in Hubei Province, China.
For inclusion, patients had to have laboratory confirmed infection with SARS-Cov-2, the virus that causes COVID-19; pneumonia confirmed by chest imaging; and severely reduced oxygen saturation (94% or less while breathing ambient air or a ratio of the partial pressure of oxygen to the fraction of inspired oxygen of less than 300 mm Hg).
Patients were randomized to receive either a combination of lopinavir (400 mg) and ritonavir (100 mg) twice a day on top of standard care (n = 99) or to standard care alone (n = 100) for 14 days. “Because of the emergency nature of the trial, placebos of lopinavir-ritonavir were not prepared,” Dr. Cao et al. explained, noting that standard care consisted of supplemental oxygen, ventilation, antibiotic treatment, vasopressor support, renal-replacement therapy, and extracorporeal membrane oxygenation.
While the trial did not meet its primary endpoint, Dr. Cao et al. reported that patients in the lopinavir-ritonavir group had a shorter stay in the intensive care unit than did those in the standard-care group, at a median of 6 versus 11 days. They also reported that the duration from randomization to hospital discharge was numerically shorter, at a median of 12 versus 14 days. Furthermore, there was a higher percentage of patients with clinical improvement at day 14 in the lopinavir-ritonavir group than in the standard-care group (45.5% vs. 30.0%)
“The trial was initiated in rapid response to the COVID-19 public health emergency, at which time there was very limited information about clinical outcomes in hospitalized patients with COVID-19,” the researchers observed. “The question of whether earlier lopinavir-ritonavir treatment in COVID-19 could have clinical benefit is an important one that requires further study,” they wrote.
Among the trial’s limitations are it was not blinded and the researchers do not have data on the lopinavir exposure levels in the trial participants.
“This was a heroic effort” in a “particularly challenging population,” Lindsey R. Baden, MD, and Eric J. Rubin, MD, stated in an editorial accompanying the article (Baden LR and Rubin EJ. New Engl J Med. March 18, 2020. doi: 10.1056/NEJMe2005477). “Unfortunately, the trial results were disappointing,” they noted.
“The secondary end points provide both reason for hope and reason for discouragement,” wrote Dr. Baden and Dr. Rubin. The lack of effect on viral shedding, however, “strongly [suggests] that it did not have the activity desired” they observed.
Dr. Baden and Dr. Rubin commented that one of the important takeaways from the trial is that “the investigators appropriately prioritized speed, designing a trial that could rapidly produce an answer.” They continued that the investigators had shown that “rapidly initiated, high-quality randomized clinical trials are possible in epidemic conditions” and that results of such trials, whether they are positive or negative, “will be central to clinical care as the dangerous coronavirus outbreak continues.”
As it stands, more than 100 trials are listed in the ClinicalTrials.gov database as testing a wide range of different treatment approaches for COVID-19. These include trials investigating if sarilumab, hydroxychloroquine, fingolimod, bevacizumab, and losartan might have a role to play. There are also trials looking at the potential of other antiviral agents, such as Gilead’s investigational drug remdesivir, which has shown to have in vitro and in vivo activity against many emerging viral pathogens that cause Ebola, Middle Eastern Respiratory Syndrome, and Severe Acute Respiratory Syndrome.
Dr. Cao’s trial was supported by grants from Major Projects of National Science and Technology on New Drug Creation and Development and from the Chinese Academy of Medical Sciences (CAMS) Emergency Project of Covid-19, and a National Science Grant for Distinguished Young Scholars.
All authors had no financial conflicts of interest to disclose.
Dr. Baden is the director of clinical research in the Division of Infectious Diseases at the Brigham and Women’s Hospital and the director of infectious diseases at the Dana-Farber Cancer Institute. He is a deputy editor of the New England Journal of Medicine and chair of the FDA’s Antimicrobial Drug Advisory Committee. He is involved in HIV vaccine clinical trials and has received research grants from the Ragon Institute, the National Institutes of Health/National Institute of Allergy and Infectious Diseases, and the Gates Foundation.
Dr. Rubin is employed by the New England Journal of Medicine as editor-in-chief. He is an associate physician at Brigham and Women’s Hospital and is chair and Irene Heinz Given Professor of Immunology and Infectious Diseases at the Harvard T.H. Chan School of Public Health.
SOURCE: Cao B et al. New Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
AFib-related cardiovascular deaths on the rise
PHOENIX, ARIZ. – Cardiovascular deaths and death rates related to atrial fibrillation have risen since 1999, with significant acceleration following 2009, results from a cross-sectional analysis of national data show.
“AFib is the most common arrhythmia disorder in the United States and it is estimated that it will effect more than 12 million Americans by 2030,” Yoshihiro Tanaka, MD, PhD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “The predicted lifetime risk ranges from 25% to 35%, and AFib is associated with an increased risk for heart failure, stroke, and death.”
A recent review reported that declines in total heart disease mortality rates in the United States have plateaued since 2011 (JAMA 2019;322[8]:780-2). However, it is not well understood what factors such as AFib contribute to this rate of plateau. In an effort to quantify U.S. trends in AFib-related CVD death rates, Dr. Tanaka and colleagues conducted a serial cross-sectional analysis of death certificate data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER) database during 1999-2017.
Outcomes included age-adjusted mortality per 100,000 based on the 2000 U.S. standard population. The researchers also used joinpoint regression to calculate the average annual percentage change over time and conducted subgroup analyses by race and sex and across two age groups: 35-64 years and 65-84 years.
In all, 522,104 AFib-related CVD deaths were identified during 1999-2017. Dr. Tanaka reported that age-adjusted mortality increased from 16.0 per 100,000 persons in 1999 to 22.2 per 100,000 person in 2017, with an acceleration following an inflection point in 2009. Specifically, the average annual percentage change in AFib-related CVD deaths rose from 0.4% in 2009 to 3.5% in 2017 (P < .001). “These increases were consistent across all race-sex subgroups,” said Dr. Tanaka, of the department of preventive medicine at Northwestern University, Chicago. “Relative increases were also greater in younger compared with older adults, although the absolute number of deaths in younger adults was less.”
The researchers observed that age-adjusted mortality increased across blacks and whites in both age groups, with a more pronounced increase among black and white men. Black men had the highest age-adjusted mortality among persons aged 35-64 (6.5 per 100,000 persons, compared with 4.2 among white men, 2.8 in black women, and 1.6 in white women 1.6 per 100,000). At the same time, white men had the highest age-adjusted mortality rate among those aged 65-84 years (112.5 per 100,000 persons, compared with 87.7 in black men, 77.4 in white women, and 61.3 in black women).
In an interview, one of the session’s moderators, Alvaro Alonso, MD, PhD, said that the study’s reliance on mortality data is a limitation. “You have to be careful with that, because it’s not the whole picture,” said Dr. Alonso, professor of epidemiology at the Rollins School of Public Health at Emory University, Atlanta. “It could be an underestimation of what is going on. The increase in recent years is probably due to a higher awareness of AFib as a risk factor for stroke; it’s more on the radar. Also, around 2009-2010, we started having new anticoagulants for AFib. It’s getting diagnosed more. When you look at coronary heart disease and stroke, there has been a decrease over time. In mortality and incidence of AFib, we don’t have that. That’s probably because we don’t know very much about what the risk factors for AFib are and how to prevent it.”
Dr. Tanaka said that the cause of increase in AFib-related CVD mortality can be classified into two major categories: a balance between case fatality of AFib and the prevalence of AFib. “The case fatality rate should have decreased over the last years,” he said at the meeting, which was sponsored by the American Heart Association. “In contrast, in the context of the aging of the population, the prevalence of AFib increased over the past years. Contributing factors include increasing awareness of AFib, a change in coding between ICD-9 and ICD-10, and a change in coding practices by physicians.”
Strengths of the study, he said, include its large sample size and the fact that the researchers were able to capture data from all death certificates filed in the United States. Limitations include the fact that the data “do not identify if changes in age-adjusted mortality rates are due to changing incidence or to case fatality rates,” he said. “CDC WONDER does not allow us to explore causes of these descriptive findings, but this would be an important next step.”
Dr. Tanaka reported having no financial disclosures.
SOURCE: Tanaka Y. EPI/Lifestyle 2020, Session 5, Abstract 15.
PHOENIX, ARIZ. – Cardiovascular deaths and death rates related to atrial fibrillation have risen since 1999, with significant acceleration following 2009, results from a cross-sectional analysis of national data show.
“AFib is the most common arrhythmia disorder in the United States and it is estimated that it will effect more than 12 million Americans by 2030,” Yoshihiro Tanaka, MD, PhD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “The predicted lifetime risk ranges from 25% to 35%, and AFib is associated with an increased risk for heart failure, stroke, and death.”
A recent review reported that declines in total heart disease mortality rates in the United States have plateaued since 2011 (JAMA 2019;322[8]:780-2). However, it is not well understood what factors such as AFib contribute to this rate of plateau. In an effort to quantify U.S. trends in AFib-related CVD death rates, Dr. Tanaka and colleagues conducted a serial cross-sectional analysis of death certificate data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER) database during 1999-2017.
Outcomes included age-adjusted mortality per 100,000 based on the 2000 U.S. standard population. The researchers also used joinpoint regression to calculate the average annual percentage change over time and conducted subgroup analyses by race and sex and across two age groups: 35-64 years and 65-84 years.
In all, 522,104 AFib-related CVD deaths were identified during 1999-2017. Dr. Tanaka reported that age-adjusted mortality increased from 16.0 per 100,000 persons in 1999 to 22.2 per 100,000 person in 2017, with an acceleration following an inflection point in 2009. Specifically, the average annual percentage change in AFib-related CVD deaths rose from 0.4% in 2009 to 3.5% in 2017 (P < .001). “These increases were consistent across all race-sex subgroups,” said Dr. Tanaka, of the department of preventive medicine at Northwestern University, Chicago. “Relative increases were also greater in younger compared with older adults, although the absolute number of deaths in younger adults was less.”
The researchers observed that age-adjusted mortality increased across blacks and whites in both age groups, with a more pronounced increase among black and white men. Black men had the highest age-adjusted mortality among persons aged 35-64 (6.5 per 100,000 persons, compared with 4.2 among white men, 2.8 in black women, and 1.6 in white women 1.6 per 100,000). At the same time, white men had the highest age-adjusted mortality rate among those aged 65-84 years (112.5 per 100,000 persons, compared with 87.7 in black men, 77.4 in white women, and 61.3 in black women).
In an interview, one of the session’s moderators, Alvaro Alonso, MD, PhD, said that the study’s reliance on mortality data is a limitation. “You have to be careful with that, because it’s not the whole picture,” said Dr. Alonso, professor of epidemiology at the Rollins School of Public Health at Emory University, Atlanta. “It could be an underestimation of what is going on. The increase in recent years is probably due to a higher awareness of AFib as a risk factor for stroke; it’s more on the radar. Also, around 2009-2010, we started having new anticoagulants for AFib. It’s getting diagnosed more. When you look at coronary heart disease and stroke, there has been a decrease over time. In mortality and incidence of AFib, we don’t have that. That’s probably because we don’t know very much about what the risk factors for AFib are and how to prevent it.”
Dr. Tanaka said that the cause of increase in AFib-related CVD mortality can be classified into two major categories: a balance between case fatality of AFib and the prevalence of AFib. “The case fatality rate should have decreased over the last years,” he said at the meeting, which was sponsored by the American Heart Association. “In contrast, in the context of the aging of the population, the prevalence of AFib increased over the past years. Contributing factors include increasing awareness of AFib, a change in coding between ICD-9 and ICD-10, and a change in coding practices by physicians.”
Strengths of the study, he said, include its large sample size and the fact that the researchers were able to capture data from all death certificates filed in the United States. Limitations include the fact that the data “do not identify if changes in age-adjusted mortality rates are due to changing incidence or to case fatality rates,” he said. “CDC WONDER does not allow us to explore causes of these descriptive findings, but this would be an important next step.”
Dr. Tanaka reported having no financial disclosures.
SOURCE: Tanaka Y. EPI/Lifestyle 2020, Session 5, Abstract 15.
PHOENIX, ARIZ. – Cardiovascular deaths and death rates related to atrial fibrillation have risen since 1999, with significant acceleration following 2009, results from a cross-sectional analysis of national data show.
“AFib is the most common arrhythmia disorder in the United States and it is estimated that it will effect more than 12 million Americans by 2030,” Yoshihiro Tanaka, MD, PhD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “The predicted lifetime risk ranges from 25% to 35%, and AFib is associated with an increased risk for heart failure, stroke, and death.”
A recent review reported that declines in total heart disease mortality rates in the United States have plateaued since 2011 (JAMA 2019;322[8]:780-2). However, it is not well understood what factors such as AFib contribute to this rate of plateau. In an effort to quantify U.S. trends in AFib-related CVD death rates, Dr. Tanaka and colleagues conducted a serial cross-sectional analysis of death certificate data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER) database during 1999-2017.
Outcomes included age-adjusted mortality per 100,000 based on the 2000 U.S. standard population. The researchers also used joinpoint regression to calculate the average annual percentage change over time and conducted subgroup analyses by race and sex and across two age groups: 35-64 years and 65-84 years.
In all, 522,104 AFib-related CVD deaths were identified during 1999-2017. Dr. Tanaka reported that age-adjusted mortality increased from 16.0 per 100,000 persons in 1999 to 22.2 per 100,000 person in 2017, with an acceleration following an inflection point in 2009. Specifically, the average annual percentage change in AFib-related CVD deaths rose from 0.4% in 2009 to 3.5% in 2017 (P < .001). “These increases were consistent across all race-sex subgroups,” said Dr. Tanaka, of the department of preventive medicine at Northwestern University, Chicago. “Relative increases were also greater in younger compared with older adults, although the absolute number of deaths in younger adults was less.”
The researchers observed that age-adjusted mortality increased across blacks and whites in both age groups, with a more pronounced increase among black and white men. Black men had the highest age-adjusted mortality among persons aged 35-64 (6.5 per 100,000 persons, compared with 4.2 among white men, 2.8 in black women, and 1.6 in white women 1.6 per 100,000). At the same time, white men had the highest age-adjusted mortality rate among those aged 65-84 years (112.5 per 100,000 persons, compared with 87.7 in black men, 77.4 in white women, and 61.3 in black women).
In an interview, one of the session’s moderators, Alvaro Alonso, MD, PhD, said that the study’s reliance on mortality data is a limitation. “You have to be careful with that, because it’s not the whole picture,” said Dr. Alonso, professor of epidemiology at the Rollins School of Public Health at Emory University, Atlanta. “It could be an underestimation of what is going on. The increase in recent years is probably due to a higher awareness of AFib as a risk factor for stroke; it’s more on the radar. Also, around 2009-2010, we started having new anticoagulants for AFib. It’s getting diagnosed more. When you look at coronary heart disease and stroke, there has been a decrease over time. In mortality and incidence of AFib, we don’t have that. That’s probably because we don’t know very much about what the risk factors for AFib are and how to prevent it.”
Dr. Tanaka said that the cause of increase in AFib-related CVD mortality can be classified into two major categories: a balance between case fatality of AFib and the prevalence of AFib. “The case fatality rate should have decreased over the last years,” he said at the meeting, which was sponsored by the American Heart Association. “In contrast, in the context of the aging of the population, the prevalence of AFib increased over the past years. Contributing factors include increasing awareness of AFib, a change in coding between ICD-9 and ICD-10, and a change in coding practices by physicians.”
Strengths of the study, he said, include its large sample size and the fact that the researchers were able to capture data from all death certificates filed in the United States. Limitations include the fact that the data “do not identify if changes in age-adjusted mortality rates are due to changing incidence or to case fatality rates,” he said. “CDC WONDER does not allow us to explore causes of these descriptive findings, but this would be an important next step.”
Dr. Tanaka reported having no financial disclosures.
SOURCE: Tanaka Y. EPI/Lifestyle 2020, Session 5, Abstract 15.
REPORTING FROM EPI/LIFESTYLE 2020