User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


AGA and colleague societies issue clinical insights for COVID-19
Amid the growing SARS-CoV-2 pandemic, currently in its expansive growth phase in the United States, the American Gastroenterological Association (AGA), the American Association for the Study of Liver Diseases (AASLD), the American College of Gastroenterology (ACG), and the American Society for Gastrointestinal Endoscopy (ASGE) have jointly released “COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers,” which can be found on the websites of the various societies.
“The purpose of this communication is to jointly provide you with up to date COVID-19 information in order to maintain the highest level of health and safety for our patients, staff, community, and ourselves,” according to the AGA website announcement.
In particular, the societies point out that there is recent evidence suggesting the potential for coronavirus transmission through droplets and perhaps fecal shedding, which pose potential risks in particular during endoscopy and colonoscopy procedures to other patients, endoscopy personnel, and practitioners.
Relevant clinical factors related to COVID-19 are discussed, including the fact that asymptomatic spread can occur during the prodromal phase (the mean incubation period is approximately 5 days, with a range of 0-14 days), with viral shedding greatest when symptoms begin.
Between 20% and 30% of patients with COVID-19 infection show abnormal liver enzymes. In addition, COVID-19 patients show drops in their leukocyte counts, and elevated white blood cell counts is a poor prognostic sign, according to the release.
The Centers for Disease Control and Prevention lists vulnerable populations at the greatest risk for more serious outcomes; these include the elderly and those with severe chronic health conditions, such as heart disease, lung disease, diabetes, decompensated cirrhosis, HIV with low CD4 counts, and immunosuppression (including liver and other solid organ transplant recipients), are at higher risk of developing more serious illness. In addition pregnancy may provide added risk.
Specific advice for the gastroenterology profession
The joint statement urges that practitioners strongly consider rescheduling elective nonurgent endoscopic procedures, although some nonurgent procedures are higher priority and may need to be performed, including cancer evaluations, prosthetic removals, and evaluation of significant symptoms. “Of note, the Surgeon General on 3/14/20 advised hospitals to postpone all elective surgeries,” the document states.
Patient concerns
In all cases, patients should be prescreened for high-risk exposure or symptoms. This includes asking about history of fever or respiratory symptoms, family members or close contacts with similar symptoms, any contact with a confirmed case of COVID-19, and recent travel to a high-risk area. “Avoid bringing patients (or their escorts) into the medical facility who are over age 65 or have one of the CDC recognized risks listed above,” the societies advise.
Check body temperature of the patient upon arrival at endoscopy unit or clinic, and keep all patients at an appropriate distance from each other (6 feet is recommended) throughout the entire time in the endoscopy unit.
“For COVID-19 positive patients, or those awaiting test results, isolation precautions should be taken with procedures performed in negative pressure rooms,” according to the statement.
In addition, use telemedicine where possible in elective cases, and consider phone follow-up after any procedures at 7 and 14 days to ask about new diagnosis of COVID-19 or development of its symptoms, .
Those patients who are on immunosuppressive drugs for inflammatory bowel disease and autoimmune hepatitis should continue taking their medications because the risk of disease flare outweighs the chance of contracting coronavirus, according to the document. In addition, these patients should be advised to follow CDC guidelines for at-risk groups by avoiding crowds and limiting travel.
Protection of practitioners
Key factors in ensuring practitioner safety and maintaining practice functionality are discussed by the joint document. In particular, appropriate personal protective equipment (PPE) should be worn by all members of the endoscopy team: gloves, mask, eye shield/goggles, face shields, and gown, but practitioners should also be aware of how to put on and take off PPE appropriately.
“Conservation of PPE is critical. Only essential personnel should be present in cases. Consider extended use or reuse of surgical masks and eye protection in accordance with hospital policies,” the document recommends.
“It is important to address our collective staff needs and institute policies that protect our workforce.” To that end, the document recommends that centers should strategically assign available personnel in order to minimize concomitant exposure of those with similar or unique skill sets. This includes the use of nonphysician practitioners and fellows that cannot participate in cases for screening and triaging patients, or performing virtual visits.
Coming at a time of pandemic, when gastrointestinal symptoms have been recognized as a more common symptom of COVID-19 than previously expected and liver damage has been noted as a potential repercussion of SARS-CoV-2 infection, these clinical insights provide a template for gastroenterologists and related professionals for dealing with their patients and keeping themselves safe under dramatically changed circumstances.
The partnered organizations, AASLD, ACG, AGA, and ASGE, are committed to providing updated COVID-19 information as appropriate. However: “Given the evolving and fluid nature of the situation, institutions, hospitals and clinics have also been formulating their own local guidelines, so we urge you to follow the evolving CDC recommendations and your local requirements,” according to the AGA website announcement.
In addition to the joint communication, the society websites each offer additional COVID-19 information. The AGA practice updates on the COVID-19 webpage provides information about announcements, such as the cancellation of Digestive Disease Week® in May, a location for AGA members to discuss their COVID-19 experiences and share advice, and links to the CDC COVID-19 updates.
SOURCE: American Gastroenterological Association et al. March 2020, COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers.
Amid the growing SARS-CoV-2 pandemic, currently in its expansive growth phase in the United States, the American Gastroenterological Association (AGA), the American Association for the Study of Liver Diseases (AASLD), the American College of Gastroenterology (ACG), and the American Society for Gastrointestinal Endoscopy (ASGE) have jointly released “COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers,” which can be found on the websites of the various societies.
“The purpose of this communication is to jointly provide you with up to date COVID-19 information in order to maintain the highest level of health and safety for our patients, staff, community, and ourselves,” according to the AGA website announcement.
In particular, the societies point out that there is recent evidence suggesting the potential for coronavirus transmission through droplets and perhaps fecal shedding, which pose potential risks in particular during endoscopy and colonoscopy procedures to other patients, endoscopy personnel, and practitioners.
Relevant clinical factors related to COVID-19 are discussed, including the fact that asymptomatic spread can occur during the prodromal phase (the mean incubation period is approximately 5 days, with a range of 0-14 days), with viral shedding greatest when symptoms begin.
Between 20% and 30% of patients with COVID-19 infection show abnormal liver enzymes. In addition, COVID-19 patients show drops in their leukocyte counts, and elevated white blood cell counts is a poor prognostic sign, according to the release.
The Centers for Disease Control and Prevention lists vulnerable populations at the greatest risk for more serious outcomes; these include the elderly and those with severe chronic health conditions, such as heart disease, lung disease, diabetes, decompensated cirrhosis, HIV with low CD4 counts, and immunosuppression (including liver and other solid organ transplant recipients), are at higher risk of developing more serious illness. In addition pregnancy may provide added risk.
Specific advice for the gastroenterology profession
The joint statement urges that practitioners strongly consider rescheduling elective nonurgent endoscopic procedures, although some nonurgent procedures are higher priority and may need to be performed, including cancer evaluations, prosthetic removals, and evaluation of significant symptoms. “Of note, the Surgeon General on 3/14/20 advised hospitals to postpone all elective surgeries,” the document states.
Patient concerns
In all cases, patients should be prescreened for high-risk exposure or symptoms. This includes asking about history of fever or respiratory symptoms, family members or close contacts with similar symptoms, any contact with a confirmed case of COVID-19, and recent travel to a high-risk area. “Avoid bringing patients (or their escorts) into the medical facility who are over age 65 or have one of the CDC recognized risks listed above,” the societies advise.
Check body temperature of the patient upon arrival at endoscopy unit or clinic, and keep all patients at an appropriate distance from each other (6 feet is recommended) throughout the entire time in the endoscopy unit.
“For COVID-19 positive patients, or those awaiting test results, isolation precautions should be taken with procedures performed in negative pressure rooms,” according to the statement.
In addition, use telemedicine where possible in elective cases, and consider phone follow-up after any procedures at 7 and 14 days to ask about new diagnosis of COVID-19 or development of its symptoms, .
Those patients who are on immunosuppressive drugs for inflammatory bowel disease and autoimmune hepatitis should continue taking their medications because the risk of disease flare outweighs the chance of contracting coronavirus, according to the document. In addition, these patients should be advised to follow CDC guidelines for at-risk groups by avoiding crowds and limiting travel.
Protection of practitioners
Key factors in ensuring practitioner safety and maintaining practice functionality are discussed by the joint document. In particular, appropriate personal protective equipment (PPE) should be worn by all members of the endoscopy team: gloves, mask, eye shield/goggles, face shields, and gown, but practitioners should also be aware of how to put on and take off PPE appropriately.
“Conservation of PPE is critical. Only essential personnel should be present in cases. Consider extended use or reuse of surgical masks and eye protection in accordance with hospital policies,” the document recommends.
“It is important to address our collective staff needs and institute policies that protect our workforce.” To that end, the document recommends that centers should strategically assign available personnel in order to minimize concomitant exposure of those with similar or unique skill sets. This includes the use of nonphysician practitioners and fellows that cannot participate in cases for screening and triaging patients, or performing virtual visits.
Coming at a time of pandemic, when gastrointestinal symptoms have been recognized as a more common symptom of COVID-19 than previously expected and liver damage has been noted as a potential repercussion of SARS-CoV-2 infection, these clinical insights provide a template for gastroenterologists and related professionals for dealing with their patients and keeping themselves safe under dramatically changed circumstances.
The partnered organizations, AASLD, ACG, AGA, and ASGE, are committed to providing updated COVID-19 information as appropriate. However: “Given the evolving and fluid nature of the situation, institutions, hospitals and clinics have also been formulating their own local guidelines, so we urge you to follow the evolving CDC recommendations and your local requirements,” according to the AGA website announcement.
In addition to the joint communication, the society websites each offer additional COVID-19 information. The AGA practice updates on the COVID-19 webpage provides information about announcements, such as the cancellation of Digestive Disease Week® in May, a location for AGA members to discuss their COVID-19 experiences and share advice, and links to the CDC COVID-19 updates.
SOURCE: American Gastroenterological Association et al. March 2020, COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers.
Amid the growing SARS-CoV-2 pandemic, currently in its expansive growth phase in the United States, the American Gastroenterological Association (AGA), the American Association for the Study of Liver Diseases (AASLD), the American College of Gastroenterology (ACG), and the American Society for Gastrointestinal Endoscopy (ASGE) have jointly released “COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers,” which can be found on the websites of the various societies.
“The purpose of this communication is to jointly provide you with up to date COVID-19 information in order to maintain the highest level of health and safety for our patients, staff, community, and ourselves,” according to the AGA website announcement.
In particular, the societies point out that there is recent evidence suggesting the potential for coronavirus transmission through droplets and perhaps fecal shedding, which pose potential risks in particular during endoscopy and colonoscopy procedures to other patients, endoscopy personnel, and practitioners.
Relevant clinical factors related to COVID-19 are discussed, including the fact that asymptomatic spread can occur during the prodromal phase (the mean incubation period is approximately 5 days, with a range of 0-14 days), with viral shedding greatest when symptoms begin.
Between 20% and 30% of patients with COVID-19 infection show abnormal liver enzymes. In addition, COVID-19 patients show drops in their leukocyte counts, and elevated white blood cell counts is a poor prognostic sign, according to the release.
The Centers for Disease Control and Prevention lists vulnerable populations at the greatest risk for more serious outcomes; these include the elderly and those with severe chronic health conditions, such as heart disease, lung disease, diabetes, decompensated cirrhosis, HIV with low CD4 counts, and immunosuppression (including liver and other solid organ transplant recipients), are at higher risk of developing more serious illness. In addition pregnancy may provide added risk.
Specific advice for the gastroenterology profession
The joint statement urges that practitioners strongly consider rescheduling elective nonurgent endoscopic procedures, although some nonurgent procedures are higher priority and may need to be performed, including cancer evaluations, prosthetic removals, and evaluation of significant symptoms. “Of note, the Surgeon General on 3/14/20 advised hospitals to postpone all elective surgeries,” the document states.
Patient concerns
In all cases, patients should be prescreened for high-risk exposure or symptoms. This includes asking about history of fever or respiratory symptoms, family members or close contacts with similar symptoms, any contact with a confirmed case of COVID-19, and recent travel to a high-risk area. “Avoid bringing patients (or their escorts) into the medical facility who are over age 65 or have one of the CDC recognized risks listed above,” the societies advise.
Check body temperature of the patient upon arrival at endoscopy unit or clinic, and keep all patients at an appropriate distance from each other (6 feet is recommended) throughout the entire time in the endoscopy unit.
“For COVID-19 positive patients, or those awaiting test results, isolation precautions should be taken with procedures performed in negative pressure rooms,” according to the statement.
In addition, use telemedicine where possible in elective cases, and consider phone follow-up after any procedures at 7 and 14 days to ask about new diagnosis of COVID-19 or development of its symptoms, .
Those patients who are on immunosuppressive drugs for inflammatory bowel disease and autoimmune hepatitis should continue taking their medications because the risk of disease flare outweighs the chance of contracting coronavirus, according to the document. In addition, these patients should be advised to follow CDC guidelines for at-risk groups by avoiding crowds and limiting travel.
Protection of practitioners
Key factors in ensuring practitioner safety and maintaining practice functionality are discussed by the joint document. In particular, appropriate personal protective equipment (PPE) should be worn by all members of the endoscopy team: gloves, mask, eye shield/goggles, face shields, and gown, but practitioners should also be aware of how to put on and take off PPE appropriately.
“Conservation of PPE is critical. Only essential personnel should be present in cases. Consider extended use or reuse of surgical masks and eye protection in accordance with hospital policies,” the document recommends.
“It is important to address our collective staff needs and institute policies that protect our workforce.” To that end, the document recommends that centers should strategically assign available personnel in order to minimize concomitant exposure of those with similar or unique skill sets. This includes the use of nonphysician practitioners and fellows that cannot participate in cases for screening and triaging patients, or performing virtual visits.
Coming at a time of pandemic, when gastrointestinal symptoms have been recognized as a more common symptom of COVID-19 than previously expected and liver damage has been noted as a potential repercussion of SARS-CoV-2 infection, these clinical insights provide a template for gastroenterologists and related professionals for dealing with their patients and keeping themselves safe under dramatically changed circumstances.
The partnered organizations, AASLD, ACG, AGA, and ASGE, are committed to providing updated COVID-19 information as appropriate. However: “Given the evolving and fluid nature of the situation, institutions, hospitals and clinics have also been formulating their own local guidelines, so we urge you to follow the evolving CDC recommendations and your local requirements,” according to the AGA website announcement.
In addition to the joint communication, the society websites each offer additional COVID-19 information. The AGA practice updates on the COVID-19 webpage provides information about announcements, such as the cancellation of Digestive Disease Week® in May, a location for AGA members to discuss their COVID-19 experiences and share advice, and links to the CDC COVID-19 updates.
SOURCE: American Gastroenterological Association et al. March 2020, COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers.
Patients with COVID-19 may face risk for liver injury
Patients with COVID-19 may be at risk for liver injury, but mechanisms of damage remain unclear, according to investigators.
Proposed mechanisms include direct virus-induced effects, immune-induced damage due to excessive inflammatory responses, and drug-induced injury, reported lead author Ling Xu of Huazhong University of Science and Technology, Wuhan, China, and colleagues.
“From a clinical perspective, in addition to actively dealing with the primary disease caused by coronavirus infection, attention should also be paid to monitor the occurrence of liver injury, and to the application of drugs which may induce liver damage,” the investigators wrote in Liver International. “Patients with liver damage are advised to be treated with drugs that could both protect liver functions and inhibit inflammatory responses, such as ammonium glycyrrhizinate, which may, in turn, accelerate the process of disease recovery.”
The review of liver injury associated with major pathogenic coronaviruses included severe acute respiratory syndrome coronavirus (SARS-CoV), the Middle East respiratory syndrome coronavirus (MERS-CoV), and the newly emergent SARS-CoV-2, which causes COVID-19.
In cases of COVID-19, reported incidence of liver injury ranges from 15% to 53%, based on elevations of alanine transaminase (ALT) and aspartate aminotransferase (AST), along with slightly elevated bilirubin levels. In severe cases, albumin decreases have also been documented.
Liver injury appears to be significantly more common among those with severe infection. In one cohort of 82 patients who died from COVID-19, the incidence of liver injury was 78%, while another study of 36 nonsurvivors reported a rate of 58%.
According to the investigators, both bile duct epithelial cells and liver cells express angiotensin converting enzyme II (ACE2), which is an entry receptor for SARS-CoV-2; however, expression of ACE2 in bile duct cells is “much higher” than in liver cells, and comparable with alveolar type 2 cells in the lungs.
“Bile duct epithelial cells are known to play important roles in liver regeneration and immune response,” the investigators noted.
Beyond direct- and immune-induced effects of COVID-19, postmortem findings suggest that drug-induced liver injury may also be a possibility, with a number of theoretical culprits, including antibiotics, steroids, and antivirals. Although the investigators emphasized that data are insufficient to pinpoint an exact agent, they highlighted a recent preprint study, which reported a significantly higher rate of lopinavir/ritonavir administration among patients with abnormal liver function, compared with those who had normal liver function (56.1% vs. 25%; P = .009).
“Drug-induced liver injury during the treatment of coronavirus infection should not be ignored and needs to be carefully investigated,” the investigators concluded.
Fundamental Research Funds for the Central Universities supported the work. The investigators reported no conflicts of interest.
SOURCE: Xu L et al. Liver Int. 2020 Mar 14. doi: 10.1111/liv.14435.
Patients with COVID-19 may be at risk for liver injury, but mechanisms of damage remain unclear, according to investigators.
Proposed mechanisms include direct virus-induced effects, immune-induced damage due to excessive inflammatory responses, and drug-induced injury, reported lead author Ling Xu of Huazhong University of Science and Technology, Wuhan, China, and colleagues.
“From a clinical perspective, in addition to actively dealing with the primary disease caused by coronavirus infection, attention should also be paid to monitor the occurrence of liver injury, and to the application of drugs which may induce liver damage,” the investigators wrote in Liver International. “Patients with liver damage are advised to be treated with drugs that could both protect liver functions and inhibit inflammatory responses, such as ammonium glycyrrhizinate, which may, in turn, accelerate the process of disease recovery.”
The review of liver injury associated with major pathogenic coronaviruses included severe acute respiratory syndrome coronavirus (SARS-CoV), the Middle East respiratory syndrome coronavirus (MERS-CoV), and the newly emergent SARS-CoV-2, which causes COVID-19.
In cases of COVID-19, reported incidence of liver injury ranges from 15% to 53%, based on elevations of alanine transaminase (ALT) and aspartate aminotransferase (AST), along with slightly elevated bilirubin levels. In severe cases, albumin decreases have also been documented.
Liver injury appears to be significantly more common among those with severe infection. In one cohort of 82 patients who died from COVID-19, the incidence of liver injury was 78%, while another study of 36 nonsurvivors reported a rate of 58%.
According to the investigators, both bile duct epithelial cells and liver cells express angiotensin converting enzyme II (ACE2), which is an entry receptor for SARS-CoV-2; however, expression of ACE2 in bile duct cells is “much higher” than in liver cells, and comparable with alveolar type 2 cells in the lungs.
“Bile duct epithelial cells are known to play important roles in liver regeneration and immune response,” the investigators noted.
Beyond direct- and immune-induced effects of COVID-19, postmortem findings suggest that drug-induced liver injury may also be a possibility, with a number of theoretical culprits, including antibiotics, steroids, and antivirals. Although the investigators emphasized that data are insufficient to pinpoint an exact agent, they highlighted a recent preprint study, which reported a significantly higher rate of lopinavir/ritonavir administration among patients with abnormal liver function, compared with those who had normal liver function (56.1% vs. 25%; P = .009).
“Drug-induced liver injury during the treatment of coronavirus infection should not be ignored and needs to be carefully investigated,” the investigators concluded.
Fundamental Research Funds for the Central Universities supported the work. The investigators reported no conflicts of interest.
SOURCE: Xu L et al. Liver Int. 2020 Mar 14. doi: 10.1111/liv.14435.
Patients with COVID-19 may be at risk for liver injury, but mechanisms of damage remain unclear, according to investigators.
Proposed mechanisms include direct virus-induced effects, immune-induced damage due to excessive inflammatory responses, and drug-induced injury, reported lead author Ling Xu of Huazhong University of Science and Technology, Wuhan, China, and colleagues.
“From a clinical perspective, in addition to actively dealing with the primary disease caused by coronavirus infection, attention should also be paid to monitor the occurrence of liver injury, and to the application of drugs which may induce liver damage,” the investigators wrote in Liver International. “Patients with liver damage are advised to be treated with drugs that could both protect liver functions and inhibit inflammatory responses, such as ammonium glycyrrhizinate, which may, in turn, accelerate the process of disease recovery.”
The review of liver injury associated with major pathogenic coronaviruses included severe acute respiratory syndrome coronavirus (SARS-CoV), the Middle East respiratory syndrome coronavirus (MERS-CoV), and the newly emergent SARS-CoV-2, which causes COVID-19.
In cases of COVID-19, reported incidence of liver injury ranges from 15% to 53%, based on elevations of alanine transaminase (ALT) and aspartate aminotransferase (AST), along with slightly elevated bilirubin levels. In severe cases, albumin decreases have also been documented.
Liver injury appears to be significantly more common among those with severe infection. In one cohort of 82 patients who died from COVID-19, the incidence of liver injury was 78%, while another study of 36 nonsurvivors reported a rate of 58%.
According to the investigators, both bile duct epithelial cells and liver cells express angiotensin converting enzyme II (ACE2), which is an entry receptor for SARS-CoV-2; however, expression of ACE2 in bile duct cells is “much higher” than in liver cells, and comparable with alveolar type 2 cells in the lungs.
“Bile duct epithelial cells are known to play important roles in liver regeneration and immune response,” the investigators noted.
Beyond direct- and immune-induced effects of COVID-19, postmortem findings suggest that drug-induced liver injury may also be a possibility, with a number of theoretical culprits, including antibiotics, steroids, and antivirals. Although the investigators emphasized that data are insufficient to pinpoint an exact agent, they highlighted a recent preprint study, which reported a significantly higher rate of lopinavir/ritonavir administration among patients with abnormal liver function, compared with those who had normal liver function (56.1% vs. 25%; P = .009).
“Drug-induced liver injury during the treatment of coronavirus infection should not be ignored and needs to be carefully investigated,” the investigators concluded.
Fundamental Research Funds for the Central Universities supported the work. The investigators reported no conflicts of interest.
SOURCE: Xu L et al. Liver Int. 2020 Mar 14. doi: 10.1111/liv.14435.
FROM LIVER INTERNATIONAL
Designing an effective onboarding program
It goes beyond welcoming and orientation
As I gear up to welcome and onboard new hires to our hospitalist group, I could not help but reflect on my first day as a hospitalist. Fresh out of residency, my orientation was a day and a half long.
The medical director gave me a brief overview of the program. The program administrator handed me a thick folder of policies followed by a quick tour of the hospital and an afternoon training for the computerized order entry system (that was a time before EHRs). The next morning, I was given my full panel of patients, my new lab coat, and sent off into the battlefield.
I can vividly remember feeling anxious, a bit confused, and quite overwhelmed as I went through my day. The days turned into a week and the next. I kept wondering if I was doing everything right. It took me a month to feel a little more comfortable. It all turned out fine. Since nobody told me otherwise, I assumed it did.
Quite a bit has changed since then in hospital medicine. Hospital medicine groups, nowadays, have to tackle the changing landscape of payment reform, take on responsibility for an increasing range of hospital quality metrics and juggle a swath of subspecialty comanagement agreements. Hospital medicine providers function from the inpatient to the post-acute care arena, all while continuing to demonstrate their value to the hospital administration. Simultaneously, they have to ensure their providers are engaged and functioning at their optimal level while battling the ever-increasing threat of burnout.
Thus, for new hires, all the above aspects of my orientation have become critical but alas terribly insufficient. Well into its third decade, the hospital medicine job market continues to boom but remains a revolving door. Hospital medicine groups continue to grow in size and integrate across hospitals in a given health system. The vast majority of the new hires tend to be fresh out of residency. The first year remains the most vulnerable period for a new hospitalist. Hospital medicine groups must design and implement a robust onboarding program for their new hires. It goes beyond welcoming and orientation of new hires to full integration and assimilation in order to transform them into highly efficient and productive team members. Effective onboarding is table stakes for a successful and thriving hospital medicine group.
The content
An effective onboarding program should focus on three key dimensions: the organizational, the technical, and the social.1
1. The organizational or administrative aspect: The most common aspect of onboarding is providing new hires with information on the group’s policies and procedures: what to do and how to do it. Equally essential is giving them the tools and contacts that will help them understand and navigate their first few months. Information on how to contact consultants, signing on and off shifts, and so on can be easily conveyed through documents. However, having peers and the critical administrative staff communicate other aspects such as a detailed tour of the hospital, scheduling, and vacation policies is far more effective. It provides an excellent opportunity to introduce new hires to the key personnel in the group and vice versa as new hires get familiar with the unofficial workplace language. Breaking down all this information into meaningful, absorbable boluses, spread over time, is key to avoiding information overload. Allowing new hires to assimilate and adapt to the group norms requires follow-up and reinforcement. Group leaders should plan to meet with them at predetermined intervals, such as at 30, 60, 90 days, to engage them in conversations about the group’s values, performance measurements, rewards, and the opportunities for growth that exist within the group and institution.
2. The technical or the clinical aspect: The majority of physicians and advanced providers hired to a hospital medicine group have come immediately from training. Transition into the autonomous role of an attending, or a semi-autonomous role for advanced providers, with a larger patient panel can be quite unnerving and stressful. It can be disorientating even for experienced providers transitioning into a new health system. A well-structured onboarding can allow providers to deploy their training and experience at your organization effectively. Many onboarding programs have a clinical ramp-up period. The providers begin with a limited patient panel and gradually acclimatize into a full patient load. Many programs pair a senior hospitalist with the new hire during this period – a ‘buddy.’ Buddies are available to help new hires navigate the health system and familiarize them with the stakeholders. They help new hires by providing context to understand their new role and how they can contribute to the group’s success. In many instances, buddies help outline the unspoken rules of the group.
3. The social aspect – enculturation and networking: This is probably the most important of the three elements. It is quite common for new hires to feel like a stranger in a new land. A well-designed onboarding program provides new hires the space to forge relationships with each other and existing members of the hospital medicine team. Groups can do this in myriad ways – an informal welcome social, a meet and greet breakfast or lunch, in-person orientation when designing the administrative onboarding, and assignment of buddies or mentors during their clinical ramp-up period. It is all about providing a space to establish and nurture lasting relationships between the new hires and the group. When done well, this helps transform a group into a community. It also lays the groundwork to avoid stress and loneliness, some of the culprits that lead to physician burnout. It is through these interpersonal connections that new hires adapt to a hospital medicine group’s prevailing culture.
The personnel
Effective onboarding should be more than mere orientation. Group leaders should make an active attempt at understanding the core values and needs of the group. A good onboarding process assists new hires to internalize and accept the norms of the group. This process is not just a result of what comes from top management but also what they see and hear from the rank and file providers in the group. Hence it is critical to have the right people who understand and embody these values at the planning table. It is equally essential that necessary time and resources are devoted to building a program that meets the needs of the group. The practice management committee at SHM interviewed five different programs across a spectrum of settings. All of them had a designated onboarding program leader with a planning committee that included the administrative staff and senior frontline hospitalists.
The costs
According to one estimate, the cost of physician turnover is $400,000-$600,000 per provider.2 Given such staggering costs, it is not difficult to justify the financial resources required to structure an effective onboarding program. Activities such as a detailed facility tour, a welcome breakfast, and a peer buddy system cost virtually nothing. They go a long way in building comradery, make new hires feel like they are part of a team, and reduce burnout and turnover. Costs of an onboarding program are typically related to wages during shadowing and clinical ramp-up. However, all the programs we interviewed acknowledged that the costs associated with onboarding, in the broader context, were small and necessary.
The bottom line
An effective onboarding program that is well planned, well structured, and well executed is inherently valuable. It sends a positive signal to new hires, reassuring them that they made a great decision by joining the group. It also reminds the existing providers why they want to be a part of the group and its culture.
It is not about what is said or done during the onboarding process or how long it lasts. It need not be overly complicated. It is how the process makes everyone feel about the group. At the end of the day, like in all aspects of life, that is what ultimately matters.
The SHM Practice Management Committee has created a document that outlines the guiding principles for effective onboarding with attached case studies. Visit the SHM website for more information: https://www.hospitalmedicine.org.
Dr. Irani is a hospitalist affiliated with Baystate Health in Springfield, Mass. He would like to thank Joshua Lapps, Luke Heisenger, and all the members of the SHM Practice Management Committee for their assistance and input in drafting the guiding principles of onboarding and the case studies that have heavily inspired the above article.
References
1. Carucci R. To Retain New Hires, Spend More Time Onboarding Them. Harvard Busines Review. Dec 3, 2018. https://hbr.org/2018/12/to-retain-new-hires-spend-more-time-onboarding-them
2. Franz D. The staggering costs of physician turnover. Today’s Hospitalist. August 2016. https://www.todayshospitalist.com/staggering-costs-physician-turnover/
It goes beyond welcoming and orientation
It goes beyond welcoming and orientation
As I gear up to welcome and onboard new hires to our hospitalist group, I could not help but reflect on my first day as a hospitalist. Fresh out of residency, my orientation was a day and a half long.
The medical director gave me a brief overview of the program. The program administrator handed me a thick folder of policies followed by a quick tour of the hospital and an afternoon training for the computerized order entry system (that was a time before EHRs). The next morning, I was given my full panel of patients, my new lab coat, and sent off into the battlefield.
I can vividly remember feeling anxious, a bit confused, and quite overwhelmed as I went through my day. The days turned into a week and the next. I kept wondering if I was doing everything right. It took me a month to feel a little more comfortable. It all turned out fine. Since nobody told me otherwise, I assumed it did.
Quite a bit has changed since then in hospital medicine. Hospital medicine groups, nowadays, have to tackle the changing landscape of payment reform, take on responsibility for an increasing range of hospital quality metrics and juggle a swath of subspecialty comanagement agreements. Hospital medicine providers function from the inpatient to the post-acute care arena, all while continuing to demonstrate their value to the hospital administration. Simultaneously, they have to ensure their providers are engaged and functioning at their optimal level while battling the ever-increasing threat of burnout.
Thus, for new hires, all the above aspects of my orientation have become critical but alas terribly insufficient. Well into its third decade, the hospital medicine job market continues to boom but remains a revolving door. Hospital medicine groups continue to grow in size and integrate across hospitals in a given health system. The vast majority of the new hires tend to be fresh out of residency. The first year remains the most vulnerable period for a new hospitalist. Hospital medicine groups must design and implement a robust onboarding program for their new hires. It goes beyond welcoming and orientation of new hires to full integration and assimilation in order to transform them into highly efficient and productive team members. Effective onboarding is table stakes for a successful and thriving hospital medicine group.
The content
An effective onboarding program should focus on three key dimensions: the organizational, the technical, and the social.1
1. The organizational or administrative aspect: The most common aspect of onboarding is providing new hires with information on the group’s policies and procedures: what to do and how to do it. Equally essential is giving them the tools and contacts that will help them understand and navigate their first few months. Information on how to contact consultants, signing on and off shifts, and so on can be easily conveyed through documents. However, having peers and the critical administrative staff communicate other aspects such as a detailed tour of the hospital, scheduling, and vacation policies is far more effective. It provides an excellent opportunity to introduce new hires to the key personnel in the group and vice versa as new hires get familiar with the unofficial workplace language. Breaking down all this information into meaningful, absorbable boluses, spread over time, is key to avoiding information overload. Allowing new hires to assimilate and adapt to the group norms requires follow-up and reinforcement. Group leaders should plan to meet with them at predetermined intervals, such as at 30, 60, 90 days, to engage them in conversations about the group’s values, performance measurements, rewards, and the opportunities for growth that exist within the group and institution.
2. The technical or the clinical aspect: The majority of physicians and advanced providers hired to a hospital medicine group have come immediately from training. Transition into the autonomous role of an attending, or a semi-autonomous role for advanced providers, with a larger patient panel can be quite unnerving and stressful. It can be disorientating even for experienced providers transitioning into a new health system. A well-structured onboarding can allow providers to deploy their training and experience at your organization effectively. Many onboarding programs have a clinical ramp-up period. The providers begin with a limited patient panel and gradually acclimatize into a full patient load. Many programs pair a senior hospitalist with the new hire during this period – a ‘buddy.’ Buddies are available to help new hires navigate the health system and familiarize them with the stakeholders. They help new hires by providing context to understand their new role and how they can contribute to the group’s success. In many instances, buddies help outline the unspoken rules of the group.
3. The social aspect – enculturation and networking: This is probably the most important of the three elements. It is quite common for new hires to feel like a stranger in a new land. A well-designed onboarding program provides new hires the space to forge relationships with each other and existing members of the hospital medicine team. Groups can do this in myriad ways – an informal welcome social, a meet and greet breakfast or lunch, in-person orientation when designing the administrative onboarding, and assignment of buddies or mentors during their clinical ramp-up period. It is all about providing a space to establish and nurture lasting relationships between the new hires and the group. When done well, this helps transform a group into a community. It also lays the groundwork to avoid stress and loneliness, some of the culprits that lead to physician burnout. It is through these interpersonal connections that new hires adapt to a hospital medicine group’s prevailing culture.
The personnel
Effective onboarding should be more than mere orientation. Group leaders should make an active attempt at understanding the core values and needs of the group. A good onboarding process assists new hires to internalize and accept the norms of the group. This process is not just a result of what comes from top management but also what they see and hear from the rank and file providers in the group. Hence it is critical to have the right people who understand and embody these values at the planning table. It is equally essential that necessary time and resources are devoted to building a program that meets the needs of the group. The practice management committee at SHM interviewed five different programs across a spectrum of settings. All of them had a designated onboarding program leader with a planning committee that included the administrative staff and senior frontline hospitalists.
The costs
According to one estimate, the cost of physician turnover is $400,000-$600,000 per provider.2 Given such staggering costs, it is not difficult to justify the financial resources required to structure an effective onboarding program. Activities such as a detailed facility tour, a welcome breakfast, and a peer buddy system cost virtually nothing. They go a long way in building comradery, make new hires feel like they are part of a team, and reduce burnout and turnover. Costs of an onboarding program are typically related to wages during shadowing and clinical ramp-up. However, all the programs we interviewed acknowledged that the costs associated with onboarding, in the broader context, were small and necessary.
The bottom line
An effective onboarding program that is well planned, well structured, and well executed is inherently valuable. It sends a positive signal to new hires, reassuring them that they made a great decision by joining the group. It also reminds the existing providers why they want to be a part of the group and its culture.
It is not about what is said or done during the onboarding process or how long it lasts. It need not be overly complicated. It is how the process makes everyone feel about the group. At the end of the day, like in all aspects of life, that is what ultimately matters.
The SHM Practice Management Committee has created a document that outlines the guiding principles for effective onboarding with attached case studies. Visit the SHM website for more information: https://www.hospitalmedicine.org.
Dr. Irani is a hospitalist affiliated with Baystate Health in Springfield, Mass. He would like to thank Joshua Lapps, Luke Heisenger, and all the members of the SHM Practice Management Committee for their assistance and input in drafting the guiding principles of onboarding and the case studies that have heavily inspired the above article.
References
1. Carucci R. To Retain New Hires, Spend More Time Onboarding Them. Harvard Busines Review. Dec 3, 2018. https://hbr.org/2018/12/to-retain-new-hires-spend-more-time-onboarding-them
2. Franz D. The staggering costs of physician turnover. Today’s Hospitalist. August 2016. https://www.todayshospitalist.com/staggering-costs-physician-turnover/
As I gear up to welcome and onboard new hires to our hospitalist group, I could not help but reflect on my first day as a hospitalist. Fresh out of residency, my orientation was a day and a half long.
The medical director gave me a brief overview of the program. The program administrator handed me a thick folder of policies followed by a quick tour of the hospital and an afternoon training for the computerized order entry system (that was a time before EHRs). The next morning, I was given my full panel of patients, my new lab coat, and sent off into the battlefield.
I can vividly remember feeling anxious, a bit confused, and quite overwhelmed as I went through my day. The days turned into a week and the next. I kept wondering if I was doing everything right. It took me a month to feel a little more comfortable. It all turned out fine. Since nobody told me otherwise, I assumed it did.
Quite a bit has changed since then in hospital medicine. Hospital medicine groups, nowadays, have to tackle the changing landscape of payment reform, take on responsibility for an increasing range of hospital quality metrics and juggle a swath of subspecialty comanagement agreements. Hospital medicine providers function from the inpatient to the post-acute care arena, all while continuing to demonstrate their value to the hospital administration. Simultaneously, they have to ensure their providers are engaged and functioning at their optimal level while battling the ever-increasing threat of burnout.
Thus, for new hires, all the above aspects of my orientation have become critical but alas terribly insufficient. Well into its third decade, the hospital medicine job market continues to boom but remains a revolving door. Hospital medicine groups continue to grow in size and integrate across hospitals in a given health system. The vast majority of the new hires tend to be fresh out of residency. The first year remains the most vulnerable period for a new hospitalist. Hospital medicine groups must design and implement a robust onboarding program for their new hires. It goes beyond welcoming and orientation of new hires to full integration and assimilation in order to transform them into highly efficient and productive team members. Effective onboarding is table stakes for a successful and thriving hospital medicine group.
The content
An effective onboarding program should focus on three key dimensions: the organizational, the technical, and the social.1
1. The organizational or administrative aspect: The most common aspect of onboarding is providing new hires with information on the group’s policies and procedures: what to do and how to do it. Equally essential is giving them the tools and contacts that will help them understand and navigate their first few months. Information on how to contact consultants, signing on and off shifts, and so on can be easily conveyed through documents. However, having peers and the critical administrative staff communicate other aspects such as a detailed tour of the hospital, scheduling, and vacation policies is far more effective. It provides an excellent opportunity to introduce new hires to the key personnel in the group and vice versa as new hires get familiar with the unofficial workplace language. Breaking down all this information into meaningful, absorbable boluses, spread over time, is key to avoiding information overload. Allowing new hires to assimilate and adapt to the group norms requires follow-up and reinforcement. Group leaders should plan to meet with them at predetermined intervals, such as at 30, 60, 90 days, to engage them in conversations about the group’s values, performance measurements, rewards, and the opportunities for growth that exist within the group and institution.
2. The technical or the clinical aspect: The majority of physicians and advanced providers hired to a hospital medicine group have come immediately from training. Transition into the autonomous role of an attending, or a semi-autonomous role for advanced providers, with a larger patient panel can be quite unnerving and stressful. It can be disorientating even for experienced providers transitioning into a new health system. A well-structured onboarding can allow providers to deploy their training and experience at your organization effectively. Many onboarding programs have a clinical ramp-up period. The providers begin with a limited patient panel and gradually acclimatize into a full patient load. Many programs pair a senior hospitalist with the new hire during this period – a ‘buddy.’ Buddies are available to help new hires navigate the health system and familiarize them with the stakeholders. They help new hires by providing context to understand their new role and how they can contribute to the group’s success. In many instances, buddies help outline the unspoken rules of the group.
3. The social aspect – enculturation and networking: This is probably the most important of the three elements. It is quite common for new hires to feel like a stranger in a new land. A well-designed onboarding program provides new hires the space to forge relationships with each other and existing members of the hospital medicine team. Groups can do this in myriad ways – an informal welcome social, a meet and greet breakfast or lunch, in-person orientation when designing the administrative onboarding, and assignment of buddies or mentors during their clinical ramp-up period. It is all about providing a space to establish and nurture lasting relationships between the new hires and the group. When done well, this helps transform a group into a community. It also lays the groundwork to avoid stress and loneliness, some of the culprits that lead to physician burnout. It is through these interpersonal connections that new hires adapt to a hospital medicine group’s prevailing culture.
The personnel
Effective onboarding should be more than mere orientation. Group leaders should make an active attempt at understanding the core values and needs of the group. A good onboarding process assists new hires to internalize and accept the norms of the group. This process is not just a result of what comes from top management but also what they see and hear from the rank and file providers in the group. Hence it is critical to have the right people who understand and embody these values at the planning table. It is equally essential that necessary time and resources are devoted to building a program that meets the needs of the group. The practice management committee at SHM interviewed five different programs across a spectrum of settings. All of them had a designated onboarding program leader with a planning committee that included the administrative staff and senior frontline hospitalists.
The costs
According to one estimate, the cost of physician turnover is $400,000-$600,000 per provider.2 Given such staggering costs, it is not difficult to justify the financial resources required to structure an effective onboarding program. Activities such as a detailed facility tour, a welcome breakfast, and a peer buddy system cost virtually nothing. They go a long way in building comradery, make new hires feel like they are part of a team, and reduce burnout and turnover. Costs of an onboarding program are typically related to wages during shadowing and clinical ramp-up. However, all the programs we interviewed acknowledged that the costs associated with onboarding, in the broader context, were small and necessary.
The bottom line
An effective onboarding program that is well planned, well structured, and well executed is inherently valuable. It sends a positive signal to new hires, reassuring them that they made a great decision by joining the group. It also reminds the existing providers why they want to be a part of the group and its culture.
It is not about what is said or done during the onboarding process or how long it lasts. It need not be overly complicated. It is how the process makes everyone feel about the group. At the end of the day, like in all aspects of life, that is what ultimately matters.
The SHM Practice Management Committee has created a document that outlines the guiding principles for effective onboarding with attached case studies. Visit the SHM website for more information: https://www.hospitalmedicine.org.
Dr. Irani is a hospitalist affiliated with Baystate Health in Springfield, Mass. He would like to thank Joshua Lapps, Luke Heisenger, and all the members of the SHM Practice Management Committee for their assistance and input in drafting the guiding principles of onboarding and the case studies that have heavily inspired the above article.
References
1. Carucci R. To Retain New Hires, Spend More Time Onboarding Them. Harvard Busines Review. Dec 3, 2018. https://hbr.org/2018/12/to-retain-new-hires-spend-more-time-onboarding-them
2. Franz D. The staggering costs of physician turnover. Today’s Hospitalist. August 2016. https://www.todayshospitalist.com/staggering-costs-physician-turnover/
COVID-19 will test medical supply stocks
In a JAMA Live Stream interview, in the United States.
Dr. Fauci got into the details of what is known, what is unknown, what is being done in laboratories, and what clinical elements are still not understood about this disease.
The next several weeks, he said, are likely to tell the tale of whether our health care system is up to the challenge of care for the most ill among those who will be affected by COVID-19.
“It shouldn’t panic or frighten us, but we have to know we’re dealing with a very serious problem that we have to address, and we have to deal with it in a very bold way,” said Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
Speaking in an interview with JAMA Editor in Chief Howard Bauchner, MD, Dr. Fauci said the situation favors action over fear. “Let’s apply that energy to doing the things that we know can mitigate this.”
He added that he heard the message loud and clear from health care leaders in Italy and France during a World Health Organization coronavirus call earlier in the day. Officials in those countries, he said, were “almost pleading with the rest of the world to please take this very seriously, because it happens all of a sudden – very abruptly. ... The best time to mitigate is before that happens, because if you wait until after it happens you’re playing catch-up.”
Dr. Bauchner, noting that strict social distancing has been underway in many parts of the United States for several days, posited that, by early April, “We’ll really have a sense if we can manage in terms of serious illness.” Seattle, New York, Boston, and the San Francisco Bay Area may experience demand that outstrips ICU capacity at that point, but the rest of the country, he said, “is doing relatively well.”
Stress test on the health care system
Dr. Fauci agreed with this statement and added: “We’re going to know – for better or worse – whether we have enough of what it takes to be able to practice the kind of medicine that we optimally would want to practice.
In the matter of a week or 2 ... I think we’ll get a feel for whether or not we really have enough of the supplies that it takes.”
The well-publicized regional shortages in personal protective equipment (PPE) are forcing tough choices in some areas. As expedited – and even drive-through – testing begins, some of the demand for testing-related PPE may abate, especially if protocols include self-administration of nasal swabs, he noted.
Dr. Fauci added that the strategic national stockpile of medical supplies and equipment has not yet been tapped, “but you need to backfill that as quickly as you can once you start drawing from the strategic national stockpile.”
Returning to work after COVID-19 infection
Regarding the thorny question of when health care workers should be permitted to return to work after coronavirus infection, “it’s an evolving story,” said Dr. Fauci. Current guidance advises that health care providers stay away from work until two negative tests after resolution of fever and improvement of respiratory symptoms, or 3 fever-free days.
“We are approaching a point where you’re going to get enough people who are getting infected that we aren’t going to be able to do that,” he said. Depending on the stress to the health care system in a given locality, he said that facilities are going to have to “decide with good judgment” when health care workers go back on the job after coronavirus infection.
Asked how soon an individual would reliably test positive for COVID-19 after exposure, Dr. Fauci said, “We don’t know the answer to that. ... We can surmise it ...” He noted that it’s a median of about 5 days with a range of 2 to 14 days, before an infected individual becomes symptomatic. “I can say it’s not going to happen immediately,” he added, noting that he wouldn’t expect to see a positive test until about 2 days after exposure at the earliest. “When you get to the point where you are symptomatic, you’re almost certainly going to be positive then. ... This is just an extrapolation,” rather than conclusions drawn from solid data, he emphasized.
Higher risk reported in cardiac patients
Dr. Bauchner, who was relaying questions sent in from physicians during the live-streamed interview, asked about a newly issued joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America, which on March 17 affirmed that individuals on ACE inhibitors and angiotensin receptor blockers (ARBs) continue that therapy if they should become ill with COVID-19. The European Society of Cardiology issued a similar recommendation a few days prior.
Despite these societies’ statements, Dr. Fauci pointed to population-level data in Italy as suggesting that the case isn’t yet closed. “We really need to get data, and we need to get data fast. There’s a mechanistic rationale for the concern. It’s there, and it’s firm,” he said. The theoretical concern is that ACE inhibitors can upregulate expression of the ACE-2 protein on cell membranes, which is the entry point for SARS-Cov-2 to enter cells.
He added that he remains concerned about the number of coronavirus fatalities of patients in Italy who had hypertension as their only, or primary, underlying health problem.“That to me was a bit of a red flag,” he said. “Patients with hypertension almost certainly had a physician, and the physician almost certainly treated that person with medication. Why should someone who has hypertension that was well controlled have a much greater chance of dying?” he asked, noting that “I look at a person with well-controlled hypertension as a relatively healthy person. I don’t know what the answer is, but somebody has to look very carefully,” ideally by means of a natural history study that identifies medications used by those who died from coronavirus.
Potential therapies
Regarding potential therapies for COVID-19, Dr. Fauci acknowledged the social media buzz and flurry of medical letters and case reports about the use of hydroxychloroquine (Plaquenil) to treat active infection. He said that he and other researchers are “in active discussion” about how best to study the efficacy and safety of hydroxychloroquine, but he also acknowledged that many treating clinicians will use hydroxychloroquine empirically in the absence of other treatments with proven efficacy.
Clinical trials underway in China for antiviral medication are facing some enrollment challenges currently “because people want to get the drug,” said Dr. Fauci. “They don’t want to be in the trial; they just want to get the drug.” Though each of two trials has targeted approximately 500 participants as the number needed for sufficient statistical power, Dr. Fauci urged Chinese data safety monitoring boards to “take a close look” at the data already accrued for the several hundred patients who have already enrolled for the studies “to see if there’s any hint of efficacy.”
In a JAMA Live Stream interview, in the United States.
Dr. Fauci got into the details of what is known, what is unknown, what is being done in laboratories, and what clinical elements are still not understood about this disease.
The next several weeks, he said, are likely to tell the tale of whether our health care system is up to the challenge of care for the most ill among those who will be affected by COVID-19.
“It shouldn’t panic or frighten us, but we have to know we’re dealing with a very serious problem that we have to address, and we have to deal with it in a very bold way,” said Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
Speaking in an interview with JAMA Editor in Chief Howard Bauchner, MD, Dr. Fauci said the situation favors action over fear. “Let’s apply that energy to doing the things that we know can mitigate this.”
He added that he heard the message loud and clear from health care leaders in Italy and France during a World Health Organization coronavirus call earlier in the day. Officials in those countries, he said, were “almost pleading with the rest of the world to please take this very seriously, because it happens all of a sudden – very abruptly. ... The best time to mitigate is before that happens, because if you wait until after it happens you’re playing catch-up.”
Dr. Bauchner, noting that strict social distancing has been underway in many parts of the United States for several days, posited that, by early April, “We’ll really have a sense if we can manage in terms of serious illness.” Seattle, New York, Boston, and the San Francisco Bay Area may experience demand that outstrips ICU capacity at that point, but the rest of the country, he said, “is doing relatively well.”
Stress test on the health care system
Dr. Fauci agreed with this statement and added: “We’re going to know – for better or worse – whether we have enough of what it takes to be able to practice the kind of medicine that we optimally would want to practice.
In the matter of a week or 2 ... I think we’ll get a feel for whether or not we really have enough of the supplies that it takes.”
The well-publicized regional shortages in personal protective equipment (PPE) are forcing tough choices in some areas. As expedited – and even drive-through – testing begins, some of the demand for testing-related PPE may abate, especially if protocols include self-administration of nasal swabs, he noted.
Dr. Fauci added that the strategic national stockpile of medical supplies and equipment has not yet been tapped, “but you need to backfill that as quickly as you can once you start drawing from the strategic national stockpile.”
Returning to work after COVID-19 infection
Regarding the thorny question of when health care workers should be permitted to return to work after coronavirus infection, “it’s an evolving story,” said Dr. Fauci. Current guidance advises that health care providers stay away from work until two negative tests after resolution of fever and improvement of respiratory symptoms, or 3 fever-free days.
“We are approaching a point where you’re going to get enough people who are getting infected that we aren’t going to be able to do that,” he said. Depending on the stress to the health care system in a given locality, he said that facilities are going to have to “decide with good judgment” when health care workers go back on the job after coronavirus infection.
Asked how soon an individual would reliably test positive for COVID-19 after exposure, Dr. Fauci said, “We don’t know the answer to that. ... We can surmise it ...” He noted that it’s a median of about 5 days with a range of 2 to 14 days, before an infected individual becomes symptomatic. “I can say it’s not going to happen immediately,” he added, noting that he wouldn’t expect to see a positive test until about 2 days after exposure at the earliest. “When you get to the point where you are symptomatic, you’re almost certainly going to be positive then. ... This is just an extrapolation,” rather than conclusions drawn from solid data, he emphasized.
Higher risk reported in cardiac patients
Dr. Bauchner, who was relaying questions sent in from physicians during the live-streamed interview, asked about a newly issued joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America, which on March 17 affirmed that individuals on ACE inhibitors and angiotensin receptor blockers (ARBs) continue that therapy if they should become ill with COVID-19. The European Society of Cardiology issued a similar recommendation a few days prior.
Despite these societies’ statements, Dr. Fauci pointed to population-level data in Italy as suggesting that the case isn’t yet closed. “We really need to get data, and we need to get data fast. There’s a mechanistic rationale for the concern. It’s there, and it’s firm,” he said. The theoretical concern is that ACE inhibitors can upregulate expression of the ACE-2 protein on cell membranes, which is the entry point for SARS-Cov-2 to enter cells.
He added that he remains concerned about the number of coronavirus fatalities of patients in Italy who had hypertension as their only, or primary, underlying health problem.“That to me was a bit of a red flag,” he said. “Patients with hypertension almost certainly had a physician, and the physician almost certainly treated that person with medication. Why should someone who has hypertension that was well controlled have a much greater chance of dying?” he asked, noting that “I look at a person with well-controlled hypertension as a relatively healthy person. I don’t know what the answer is, but somebody has to look very carefully,” ideally by means of a natural history study that identifies medications used by those who died from coronavirus.
Potential therapies
Regarding potential therapies for COVID-19, Dr. Fauci acknowledged the social media buzz and flurry of medical letters and case reports about the use of hydroxychloroquine (Plaquenil) to treat active infection. He said that he and other researchers are “in active discussion” about how best to study the efficacy and safety of hydroxychloroquine, but he also acknowledged that many treating clinicians will use hydroxychloroquine empirically in the absence of other treatments with proven efficacy.
Clinical trials underway in China for antiviral medication are facing some enrollment challenges currently “because people want to get the drug,” said Dr. Fauci. “They don’t want to be in the trial; they just want to get the drug.” Though each of two trials has targeted approximately 500 participants as the number needed for sufficient statistical power, Dr. Fauci urged Chinese data safety monitoring boards to “take a close look” at the data already accrued for the several hundred patients who have already enrolled for the studies “to see if there’s any hint of efficacy.”
In a JAMA Live Stream interview, in the United States.
Dr. Fauci got into the details of what is known, what is unknown, what is being done in laboratories, and what clinical elements are still not understood about this disease.
The next several weeks, he said, are likely to tell the tale of whether our health care system is up to the challenge of care for the most ill among those who will be affected by COVID-19.
“It shouldn’t panic or frighten us, but we have to know we’re dealing with a very serious problem that we have to address, and we have to deal with it in a very bold way,” said Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
Speaking in an interview with JAMA Editor in Chief Howard Bauchner, MD, Dr. Fauci said the situation favors action over fear. “Let’s apply that energy to doing the things that we know can mitigate this.”
He added that he heard the message loud and clear from health care leaders in Italy and France during a World Health Organization coronavirus call earlier in the day. Officials in those countries, he said, were “almost pleading with the rest of the world to please take this very seriously, because it happens all of a sudden – very abruptly. ... The best time to mitigate is before that happens, because if you wait until after it happens you’re playing catch-up.”
Dr. Bauchner, noting that strict social distancing has been underway in many parts of the United States for several days, posited that, by early April, “We’ll really have a sense if we can manage in terms of serious illness.” Seattle, New York, Boston, and the San Francisco Bay Area may experience demand that outstrips ICU capacity at that point, but the rest of the country, he said, “is doing relatively well.”
Stress test on the health care system
Dr. Fauci agreed with this statement and added: “We’re going to know – for better or worse – whether we have enough of what it takes to be able to practice the kind of medicine that we optimally would want to practice.
In the matter of a week or 2 ... I think we’ll get a feel for whether or not we really have enough of the supplies that it takes.”
The well-publicized regional shortages in personal protective equipment (PPE) are forcing tough choices in some areas. As expedited – and even drive-through – testing begins, some of the demand for testing-related PPE may abate, especially if protocols include self-administration of nasal swabs, he noted.
Dr. Fauci added that the strategic national stockpile of medical supplies and equipment has not yet been tapped, “but you need to backfill that as quickly as you can once you start drawing from the strategic national stockpile.”
Returning to work after COVID-19 infection
Regarding the thorny question of when health care workers should be permitted to return to work after coronavirus infection, “it’s an evolving story,” said Dr. Fauci. Current guidance advises that health care providers stay away from work until two negative tests after resolution of fever and improvement of respiratory symptoms, or 3 fever-free days.
“We are approaching a point where you’re going to get enough people who are getting infected that we aren’t going to be able to do that,” he said. Depending on the stress to the health care system in a given locality, he said that facilities are going to have to “decide with good judgment” when health care workers go back on the job after coronavirus infection.
Asked how soon an individual would reliably test positive for COVID-19 after exposure, Dr. Fauci said, “We don’t know the answer to that. ... We can surmise it ...” He noted that it’s a median of about 5 days with a range of 2 to 14 days, before an infected individual becomes symptomatic. “I can say it’s not going to happen immediately,” he added, noting that he wouldn’t expect to see a positive test until about 2 days after exposure at the earliest. “When you get to the point where you are symptomatic, you’re almost certainly going to be positive then. ... This is just an extrapolation,” rather than conclusions drawn from solid data, he emphasized.
Higher risk reported in cardiac patients
Dr. Bauchner, who was relaying questions sent in from physicians during the live-streamed interview, asked about a newly issued joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America, which on March 17 affirmed that individuals on ACE inhibitors and angiotensin receptor blockers (ARBs) continue that therapy if they should become ill with COVID-19. The European Society of Cardiology issued a similar recommendation a few days prior.
Despite these societies’ statements, Dr. Fauci pointed to population-level data in Italy as suggesting that the case isn’t yet closed. “We really need to get data, and we need to get data fast. There’s a mechanistic rationale for the concern. It’s there, and it’s firm,” he said. The theoretical concern is that ACE inhibitors can upregulate expression of the ACE-2 protein on cell membranes, which is the entry point for SARS-Cov-2 to enter cells.
He added that he remains concerned about the number of coronavirus fatalities of patients in Italy who had hypertension as their only, or primary, underlying health problem.“That to me was a bit of a red flag,” he said. “Patients with hypertension almost certainly had a physician, and the physician almost certainly treated that person with medication. Why should someone who has hypertension that was well controlled have a much greater chance of dying?” he asked, noting that “I look at a person with well-controlled hypertension as a relatively healthy person. I don’t know what the answer is, but somebody has to look very carefully,” ideally by means of a natural history study that identifies medications used by those who died from coronavirus.
Potential therapies
Regarding potential therapies for COVID-19, Dr. Fauci acknowledged the social media buzz and flurry of medical letters and case reports about the use of hydroxychloroquine (Plaquenil) to treat active infection. He said that he and other researchers are “in active discussion” about how best to study the efficacy and safety of hydroxychloroquine, but he also acknowledged that many treating clinicians will use hydroxychloroquine empirically in the absence of other treatments with proven efficacy.
Clinical trials underway in China for antiviral medication are facing some enrollment challenges currently “because people want to get the drug,” said Dr. Fauci. “They don’t want to be in the trial; they just want to get the drug.” Though each of two trials has targeted approximately 500 participants as the number needed for sufficient statistical power, Dr. Fauci urged Chinese data safety monitoring boards to “take a close look” at the data already accrued for the several hundred patients who have already enrolled for the studies “to see if there’s any hint of efficacy.”
REPORTING FROM JAMA LIVE STREAM
COVID-19 in China: Children have less severe disease, but are vulnerable
Clinical manifestations of COVID-19 infection among children in mainland China generally have been less severe than those among adults, but children of all ages – and infants in particular – are vulnerable to infection, according to a review of 2,143 cases.
Further, infection patterns in the nationwide series of all pediatric patients reported to the Chinese Center for Disease Control and Prevention from Jan. 16 to Feb. 8, 2020, provide strong evidence of human-to-human transmission, Yuanyuan Dong, MPH, a research assistant at Shanghai Children’s Medical Center, Shanghai Jiao Tong University, China, and colleagues reported in Pediatrics.
Of the 2,143 patients included in the review, 57% were boys and the median age was 7 years; 34% had laboratory-confirmed infection and 67% had suspected infection. More than 90% had asymptomatic, mild, or moderate disease (4%, 51%, and 39%, respectively), and 46% were from Hubei Province, where the first cases were reported, the investigators found.
The median time from illness onset to diagnosis was 2 days, and there was a trend of rapid increase of disease at the early stage of the epidemic – with rapid spread from Hubei Province to surrounding provinces – followed by a gradual and steady decrease, they noted.
“The total number of pediatric patients increased remarkably between mid-January and early February, peaked around February 1, and then declined since early February 2020,” they wrote. The proportion of severe and critical cases was 11% for infants under 1 year of age, compared with 7% for those aged 1-5 years; 4% for those aged 6-10 years; 4% for those 11-15 years; and 3% for those 16 years and older.
As of Feb. 8, 2020, only one child in this group of study patients died and most cases of COVID-19 symptoms were mild. There were many fewer severe and critical cases among the children (6%), compared with those reported in adult patients in other studies (19%). “It suggests that, compared with adult patients, clinical manifestations of children’s COVID-19 may be less severe,” the investigators suggested.
“As most of these children were likely to expose themselves to family members and/or other children with COVID-19, it clearly indicates person-to-person transmission ” of novel coronavirus 2019, they said, adding that similar evidence of such transmission also has been reported from studies of adult patients.
The reasons for reduced severity in children versus adults remain unclear, but may be related to both exposure and host factors, Ms. Dong and associates said. “Children were usually well cared for at home and might have relatively [fewer] opportunities to expose themselves to pathogens and/or sick patients.”
The findings demonstrate a pediatric distribution that varied across time and space, with most cases concentrated in the Hubei province and surrounding areas. No significant gender-related difference in infection rates was observed, and although the median patient age was 7 years, the range was 1 day to 18 years, suggesting that “all ages at childhood were susceptible” to the virus, they added.
The declining number of cases over time further suggests that disease control measures implemented by the government were effective, and that cases will “continue to decline, and finally stop in the near future unless sustained human-to-human transmissions occur,” Ms. Dong and associates concluded.
In an accompanying editorial, Andrea T. Cruz, MD, of Baylor College of Medicine, Houston, and Steven L. Zeichner, MD, PhD, of the University of Virginia, Charlottesville, said the findings regarding reduced severity among children versus adults with novel coronavirus 2019 infection are consistent with data on non-COVID-19 coronavirus.
They pointed out that Ms. Dong and associates did find that 13% of virologically-confirmed cases had asymptomatic infection, “a rate that almost certainly understates the true rate of asymptomatic infection, since many asymptomatic children are unlikely to be tested.”
Of the symptomatic children, “5% had dyspnea or hypoxemia (a substantially lower percentage than what has been reported for adults) and 0.6% progressed to acute respiratory distress syndrome (ARDS) or multiorgan system dysfunction”; this also is at a lower rate than seen in adults, they said.
Very young children –infants or children in preschool – were more likely to have severe clinical manifestations than children who were older.
Thus, it appears that certain subpopulations of children are at increased risk for more significant COVID-19 illness: “younger age, underlying pulmonary pathology, and immunocompromising conditions,” Dr. Cruz and Dr. Zeichner suggested.
The two editorialists said the findings suggest children “may play a major role in community-based viral transmission.” Evidence suggests that children may have more upper respiratory tract involvement and that fecal shedding may occur for several weeks after diagnosis; this raises concerns about fecal-oral transmission, particularly for infants and children, and about viral replication in the gastrointestinal tract, they said. This has substantial implications for community spread in day care centers, schools, and in the home.
A great deal has been learned about COVID-19 in a short time, but there still is much to learn about the effect of the virus on children, the impact of children on viral spread, and about possible vertical transmission, they said.
“Widespread availability of testing will allow for us to more accurately describe the spectrum of illness and may result in adjustment of the apparent morbidity and mortality rate as fewer ill individuals are diagnosed,” Dr. Cruz and Dr. Zeichner wrote, adding that “rigorously gauging the impact of COVID-19 on children will be important to accurately model the pandemic and to ensure that appropriate resources are allocated to children requiring care.”
They noted that understanding differences in children versus adults with COVID-19 “can yield important insights into disease pathogenesis, informing management and the development of therapeutics.”
This study was partially supported by the Science and Technology Commission of Shanghai Municipality. The authors reported having no disclosures. Dr. Cruz and Dr. Zeichner are associate editors for Pediatrics. Dr. Cruz reported having no disclosures. Dr. Zeichner is an inventor of new technologies for the rapid production of vaccines, for which the University of Virginia has filed patent applications.
SOURCE: Dong Y et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0702; Cruz A and Zeichner S. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0834.
Clinical manifestations of COVID-19 infection among children in mainland China generally have been less severe than those among adults, but children of all ages – and infants in particular – are vulnerable to infection, according to a review of 2,143 cases.
Further, infection patterns in the nationwide series of all pediatric patients reported to the Chinese Center for Disease Control and Prevention from Jan. 16 to Feb. 8, 2020, provide strong evidence of human-to-human transmission, Yuanyuan Dong, MPH, a research assistant at Shanghai Children’s Medical Center, Shanghai Jiao Tong University, China, and colleagues reported in Pediatrics.
Of the 2,143 patients included in the review, 57% were boys and the median age was 7 years; 34% had laboratory-confirmed infection and 67% had suspected infection. More than 90% had asymptomatic, mild, or moderate disease (4%, 51%, and 39%, respectively), and 46% were from Hubei Province, where the first cases were reported, the investigators found.
The median time from illness onset to diagnosis was 2 days, and there was a trend of rapid increase of disease at the early stage of the epidemic – with rapid spread from Hubei Province to surrounding provinces – followed by a gradual and steady decrease, they noted.
“The total number of pediatric patients increased remarkably between mid-January and early February, peaked around February 1, and then declined since early February 2020,” they wrote. The proportion of severe and critical cases was 11% for infants under 1 year of age, compared with 7% for those aged 1-5 years; 4% for those aged 6-10 years; 4% for those 11-15 years; and 3% for those 16 years and older.
As of Feb. 8, 2020, only one child in this group of study patients died and most cases of COVID-19 symptoms were mild. There were many fewer severe and critical cases among the children (6%), compared with those reported in adult patients in other studies (19%). “It suggests that, compared with adult patients, clinical manifestations of children’s COVID-19 may be less severe,” the investigators suggested.
“As most of these children were likely to expose themselves to family members and/or other children with COVID-19, it clearly indicates person-to-person transmission ” of novel coronavirus 2019, they said, adding that similar evidence of such transmission also has been reported from studies of adult patients.
The reasons for reduced severity in children versus adults remain unclear, but may be related to both exposure and host factors, Ms. Dong and associates said. “Children were usually well cared for at home and might have relatively [fewer] opportunities to expose themselves to pathogens and/or sick patients.”
The findings demonstrate a pediatric distribution that varied across time and space, with most cases concentrated in the Hubei province and surrounding areas. No significant gender-related difference in infection rates was observed, and although the median patient age was 7 years, the range was 1 day to 18 years, suggesting that “all ages at childhood were susceptible” to the virus, they added.
The declining number of cases over time further suggests that disease control measures implemented by the government were effective, and that cases will “continue to decline, and finally stop in the near future unless sustained human-to-human transmissions occur,” Ms. Dong and associates concluded.
In an accompanying editorial, Andrea T. Cruz, MD, of Baylor College of Medicine, Houston, and Steven L. Zeichner, MD, PhD, of the University of Virginia, Charlottesville, said the findings regarding reduced severity among children versus adults with novel coronavirus 2019 infection are consistent with data on non-COVID-19 coronavirus.
They pointed out that Ms. Dong and associates did find that 13% of virologically-confirmed cases had asymptomatic infection, “a rate that almost certainly understates the true rate of asymptomatic infection, since many asymptomatic children are unlikely to be tested.”
Of the symptomatic children, “5% had dyspnea or hypoxemia (a substantially lower percentage than what has been reported for adults) and 0.6% progressed to acute respiratory distress syndrome (ARDS) or multiorgan system dysfunction”; this also is at a lower rate than seen in adults, they said.
Very young children –infants or children in preschool – were more likely to have severe clinical manifestations than children who were older.
Thus, it appears that certain subpopulations of children are at increased risk for more significant COVID-19 illness: “younger age, underlying pulmonary pathology, and immunocompromising conditions,” Dr. Cruz and Dr. Zeichner suggested.
The two editorialists said the findings suggest children “may play a major role in community-based viral transmission.” Evidence suggests that children may have more upper respiratory tract involvement and that fecal shedding may occur for several weeks after diagnosis; this raises concerns about fecal-oral transmission, particularly for infants and children, and about viral replication in the gastrointestinal tract, they said. This has substantial implications for community spread in day care centers, schools, and in the home.
A great deal has been learned about COVID-19 in a short time, but there still is much to learn about the effect of the virus on children, the impact of children on viral spread, and about possible vertical transmission, they said.
“Widespread availability of testing will allow for us to more accurately describe the spectrum of illness and may result in adjustment of the apparent morbidity and mortality rate as fewer ill individuals are diagnosed,” Dr. Cruz and Dr. Zeichner wrote, adding that “rigorously gauging the impact of COVID-19 on children will be important to accurately model the pandemic and to ensure that appropriate resources are allocated to children requiring care.”
They noted that understanding differences in children versus adults with COVID-19 “can yield important insights into disease pathogenesis, informing management and the development of therapeutics.”
This study was partially supported by the Science and Technology Commission of Shanghai Municipality. The authors reported having no disclosures. Dr. Cruz and Dr. Zeichner are associate editors for Pediatrics. Dr. Cruz reported having no disclosures. Dr. Zeichner is an inventor of new technologies for the rapid production of vaccines, for which the University of Virginia has filed patent applications.
SOURCE: Dong Y et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0702; Cruz A and Zeichner S. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0834.
Clinical manifestations of COVID-19 infection among children in mainland China generally have been less severe than those among adults, but children of all ages – and infants in particular – are vulnerable to infection, according to a review of 2,143 cases.
Further, infection patterns in the nationwide series of all pediatric patients reported to the Chinese Center for Disease Control and Prevention from Jan. 16 to Feb. 8, 2020, provide strong evidence of human-to-human transmission, Yuanyuan Dong, MPH, a research assistant at Shanghai Children’s Medical Center, Shanghai Jiao Tong University, China, and colleagues reported in Pediatrics.
Of the 2,143 patients included in the review, 57% were boys and the median age was 7 years; 34% had laboratory-confirmed infection and 67% had suspected infection. More than 90% had asymptomatic, mild, or moderate disease (4%, 51%, and 39%, respectively), and 46% were from Hubei Province, where the first cases were reported, the investigators found.
The median time from illness onset to diagnosis was 2 days, and there was a trend of rapid increase of disease at the early stage of the epidemic – with rapid spread from Hubei Province to surrounding provinces – followed by a gradual and steady decrease, they noted.
“The total number of pediatric patients increased remarkably between mid-January and early February, peaked around February 1, and then declined since early February 2020,” they wrote. The proportion of severe and critical cases was 11% for infants under 1 year of age, compared with 7% for those aged 1-5 years; 4% for those aged 6-10 years; 4% for those 11-15 years; and 3% for those 16 years and older.
As of Feb. 8, 2020, only one child in this group of study patients died and most cases of COVID-19 symptoms were mild. There were many fewer severe and critical cases among the children (6%), compared with those reported in adult patients in other studies (19%). “It suggests that, compared with adult patients, clinical manifestations of children’s COVID-19 may be less severe,” the investigators suggested.
“As most of these children were likely to expose themselves to family members and/or other children with COVID-19, it clearly indicates person-to-person transmission ” of novel coronavirus 2019, they said, adding that similar evidence of such transmission also has been reported from studies of adult patients.
The reasons for reduced severity in children versus adults remain unclear, but may be related to both exposure and host factors, Ms. Dong and associates said. “Children were usually well cared for at home and might have relatively [fewer] opportunities to expose themselves to pathogens and/or sick patients.”
The findings demonstrate a pediatric distribution that varied across time and space, with most cases concentrated in the Hubei province and surrounding areas. No significant gender-related difference in infection rates was observed, and although the median patient age was 7 years, the range was 1 day to 18 years, suggesting that “all ages at childhood were susceptible” to the virus, they added.
The declining number of cases over time further suggests that disease control measures implemented by the government were effective, and that cases will “continue to decline, and finally stop in the near future unless sustained human-to-human transmissions occur,” Ms. Dong and associates concluded.
In an accompanying editorial, Andrea T. Cruz, MD, of Baylor College of Medicine, Houston, and Steven L. Zeichner, MD, PhD, of the University of Virginia, Charlottesville, said the findings regarding reduced severity among children versus adults with novel coronavirus 2019 infection are consistent with data on non-COVID-19 coronavirus.
They pointed out that Ms. Dong and associates did find that 13% of virologically-confirmed cases had asymptomatic infection, “a rate that almost certainly understates the true rate of asymptomatic infection, since many asymptomatic children are unlikely to be tested.”
Of the symptomatic children, “5% had dyspnea or hypoxemia (a substantially lower percentage than what has been reported for adults) and 0.6% progressed to acute respiratory distress syndrome (ARDS) or multiorgan system dysfunction”; this also is at a lower rate than seen in adults, they said.
Very young children –infants or children in preschool – were more likely to have severe clinical manifestations than children who were older.
Thus, it appears that certain subpopulations of children are at increased risk for more significant COVID-19 illness: “younger age, underlying pulmonary pathology, and immunocompromising conditions,” Dr. Cruz and Dr. Zeichner suggested.
The two editorialists said the findings suggest children “may play a major role in community-based viral transmission.” Evidence suggests that children may have more upper respiratory tract involvement and that fecal shedding may occur for several weeks after diagnosis; this raises concerns about fecal-oral transmission, particularly for infants and children, and about viral replication in the gastrointestinal tract, they said. This has substantial implications for community spread in day care centers, schools, and in the home.
A great deal has been learned about COVID-19 in a short time, but there still is much to learn about the effect of the virus on children, the impact of children on viral spread, and about possible vertical transmission, they said.
“Widespread availability of testing will allow for us to more accurately describe the spectrum of illness and may result in adjustment of the apparent morbidity and mortality rate as fewer ill individuals are diagnosed,” Dr. Cruz and Dr. Zeichner wrote, adding that “rigorously gauging the impact of COVID-19 on children will be important to accurately model the pandemic and to ensure that appropriate resources are allocated to children requiring care.”
They noted that understanding differences in children versus adults with COVID-19 “can yield important insights into disease pathogenesis, informing management and the development of therapeutics.”
This study was partially supported by the Science and Technology Commission of Shanghai Municipality. The authors reported having no disclosures. Dr. Cruz and Dr. Zeichner are associate editors for Pediatrics. Dr. Cruz reported having no disclosures. Dr. Zeichner is an inventor of new technologies for the rapid production of vaccines, for which the University of Virginia has filed patent applications.
SOURCE: Dong Y et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0702; Cruz A and Zeichner S. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0834.
FROM PEDIATRICS
ACP outlines guide for COVID-19 telehealth coding, billing
and for handling clinician and staff absences due to illness or quarantine during the COVID-19 pandemic.
It strongly encourages practices to use telehealth, whenever possible, to mitigate exposure of patients who are sick or at risk because of other underlying conditions and to protect health care workers and the community from the spread of the disease.
The national organization of internists also recommends in the guidance that practices establish protocols and procedures for use by clinicians and all other staff in light of the pandemic.
The billing and coding tips are being offered to help practices deal with the rapidly changing situation surrounding the COVID-19 emergency, according to a statement from the ACP.
The coding-related guidance incorporates changes to a number of telehealth rules for Medicare beneficiaries, announced by the Centers for Medicare and Medicaid Services on March 17.
“Now in a full state of emergency, many Medicare restrictions related to telehealth have been lifted. Patients can be at home, and non-HIPAA compliant technology is allowed. There is no cost sharing for COVID-19 testing. In addition, to encourage use by patients, Medicare is allowing practices to waive cost sharing (copays and deductibles) for all telehealth services,” the organization said in the guidance. It notes, however, that the CMS does not currently reimburse for telephone calls.
The guidance includes details of the new ICD-10 codes, and stresses the importance of using the appropriate codes, given that some service cost-sharing has been waived for COVID-19 testing and treatment.
There is detailed coding guidance for virtual check-in, online evaluation and management, remote monitoring, originating site, and allowed technology and services.
In regard to clinician and staff absence due to illness or quarantine, the ACP says “practices may need to review emergency plans related to telework and to employee and clinician absence.” Among its recommendations are that practices and employers consider temporary adjustments to compensation formulas to accommodate those clinicians who experience a loss of income because they are paid based on production.
The organization emphasizes that, given the rapidly changing availability of testing for COVID-19, practices should contact their local health departments, hospitals, reference labs, or state health authorities to determine the status of their access to testing.
The full list of the ACP’s tips are available here.
Any new guidance for physicians will be posted on the ACP’s COVID-19 resource page.
and for handling clinician and staff absences due to illness or quarantine during the COVID-19 pandemic.
It strongly encourages practices to use telehealth, whenever possible, to mitigate exposure of patients who are sick or at risk because of other underlying conditions and to protect health care workers and the community from the spread of the disease.
The national organization of internists also recommends in the guidance that practices establish protocols and procedures for use by clinicians and all other staff in light of the pandemic.
The billing and coding tips are being offered to help practices deal with the rapidly changing situation surrounding the COVID-19 emergency, according to a statement from the ACP.
The coding-related guidance incorporates changes to a number of telehealth rules for Medicare beneficiaries, announced by the Centers for Medicare and Medicaid Services on March 17.
“Now in a full state of emergency, many Medicare restrictions related to telehealth have been lifted. Patients can be at home, and non-HIPAA compliant technology is allowed. There is no cost sharing for COVID-19 testing. In addition, to encourage use by patients, Medicare is allowing practices to waive cost sharing (copays and deductibles) for all telehealth services,” the organization said in the guidance. It notes, however, that the CMS does not currently reimburse for telephone calls.
The guidance includes details of the new ICD-10 codes, and stresses the importance of using the appropriate codes, given that some service cost-sharing has been waived for COVID-19 testing and treatment.
There is detailed coding guidance for virtual check-in, online evaluation and management, remote monitoring, originating site, and allowed technology and services.
In regard to clinician and staff absence due to illness or quarantine, the ACP says “practices may need to review emergency plans related to telework and to employee and clinician absence.” Among its recommendations are that practices and employers consider temporary adjustments to compensation formulas to accommodate those clinicians who experience a loss of income because they are paid based on production.
The organization emphasizes that, given the rapidly changing availability of testing for COVID-19, practices should contact their local health departments, hospitals, reference labs, or state health authorities to determine the status of their access to testing.
The full list of the ACP’s tips are available here.
Any new guidance for physicians will be posted on the ACP’s COVID-19 resource page.
and for handling clinician and staff absences due to illness or quarantine during the COVID-19 pandemic.
It strongly encourages practices to use telehealth, whenever possible, to mitigate exposure of patients who are sick or at risk because of other underlying conditions and to protect health care workers and the community from the spread of the disease.
The national organization of internists also recommends in the guidance that practices establish protocols and procedures for use by clinicians and all other staff in light of the pandemic.
The billing and coding tips are being offered to help practices deal with the rapidly changing situation surrounding the COVID-19 emergency, according to a statement from the ACP.
The coding-related guidance incorporates changes to a number of telehealth rules for Medicare beneficiaries, announced by the Centers for Medicare and Medicaid Services on March 17.
“Now in a full state of emergency, many Medicare restrictions related to telehealth have been lifted. Patients can be at home, and non-HIPAA compliant technology is allowed. There is no cost sharing for COVID-19 testing. In addition, to encourage use by patients, Medicare is allowing practices to waive cost sharing (copays and deductibles) for all telehealth services,” the organization said in the guidance. It notes, however, that the CMS does not currently reimburse for telephone calls.
The guidance includes details of the new ICD-10 codes, and stresses the importance of using the appropriate codes, given that some service cost-sharing has been waived for COVID-19 testing and treatment.
There is detailed coding guidance for virtual check-in, online evaluation and management, remote monitoring, originating site, and allowed technology and services.
In regard to clinician and staff absence due to illness or quarantine, the ACP says “practices may need to review emergency plans related to telework and to employee and clinician absence.” Among its recommendations are that practices and employers consider temporary adjustments to compensation formulas to accommodate those clinicians who experience a loss of income because they are paid based on production.
The organization emphasizes that, given the rapidly changing availability of testing for COVID-19, practices should contact their local health departments, hospitals, reference labs, or state health authorities to determine the status of their access to testing.
The full list of the ACP’s tips are available here.
Any new guidance for physicians will be posted on the ACP’s COVID-19 resource page.
Clinicians petition government for national quarantine
Clinicians across the United States are petitioning the federal government to follow the lead of South Korea, China, and other nations by imposing an immediate nationwide quarantine to slow the inevitable spread of COVID-19. Without federal action, the creators say, their lives and the lives of their colleagues, patients, and families are being put at increased risk.
In addition to the quarantine, the petition, posted on the website Change.org, calls on U.S. leaders to institute emergency production and distribution of personal protective equipment for healthcare workers and to rapidly increase access to testing.
The petition – which garnered more than 40,000 signatures in just 12 hours and as of this writing was approaching 94,000 – was started by an apolitical Facebook group to focus attention on what members see as the most critical issues for clinicians: slowing the spread of the virus through a coast-to-coast quarantine, protection of medical personnel with adequate supplies of essential equipment, and widespread testing.
“We started this group last Friday out of the realization that clinicians needed information about the outbreak and weren’t getting it,” said coadministrator Jessica McIntyre, MD, a pediatric hospitalist at Elliot Hospital in Manchester, N.H.
“We wanted to get ahead of it and connect with people before we were in the trenches experiencing it and to see what other programs were doing. From a local perspective, it has been really hard to see what people are doing in other states, especially when the protocols in our own states are changing every single day as we collect more information,” she said in an interview.
The Horse Has Bolted
A family medicine physician in Illinois helped launch the Facebook group. She asked that her name not be used but said in an interview that earlier actions may have prevented or at least delayed the need for the more draconian measures that her group is recommending.
“Clearly South Korea is one of the superstars as far as response has gone, but the concern we have in the United States is that we’re well beyond that point – we needed to be testing people over a month ago, in the hope of preventing a quarantine,” she said in an interview.
According to National Public Radio, as of March 13, South Korea had conducted 3,600 tests per million population, compared with five per million in the United States.
“I think the most concerning part is to see where Italy is now and where we are in comparison. Our ICUs have not yet overflowed, but I think we’re definitely looking at that in the next few weeks – hopefully longer, but I suspect that it will happen shortly,” she continued.
She cited work by Harvard University biostatistician Xihong Lin, PhD, that shows that when health authorities in Wuhan, China – widely cited as the epicenter of the global pandemic – cordoned off the city, the infection rate dropped from one person infecting 3.8 others to one infecting 1.25, thereby significantly slowing the rate of transmission.
“This is absolutely what we need to be doing,” she said.
Real News
Within 3 days of its creation, the online group had accrued more than 80,000 members with advanced medical training, including MDs, DOs, physician assistants, nurse practitioners, and certified registered nurse anesthetists.
“A lot of us were already very busy with our day-to-day work outside of COVID-19, and I think a lot of us felt unsure about where to get the best information,” said coadministrator David Janssen, MD, a family medicine physician in group practice in Sioux Center, Iowa,
“If you turn on the TV, there’s a lot of politicizing of the issue, and there’s a lot of good information, but also a lot of bad information. When health care providers talk to other health care providers, that’s often how we get our information and how we learn,” he said in an interview.
The COVID-19 U.S. Physicians/APP Facebook group includes 20 volunteer moderators who handle hundreds of posts per hour from persons seeking information on the novel coronavirus, what to tell patients, and how to protect themselves.
“It’s been wonderful to see how providers have been helping other providers sort through issues. Teaching hospitals have their hands on the latest research, but a lot of people like myself are at small community hospitals, critical-access hospitals, where we may have a lot of questions but don’t necessarily have the answers readily available to us,” Dr. Janssen said.
Dr. Janssen said that his community of about 8,000 residents initially had only four COVID-19 testing kits, or one for every 2,000 people. The situation has since improved, and more tests are now available, he added.
Dr. McIntyre, Dr. Janssen, and the Illinois family physician have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clinicians across the United States are petitioning the federal government to follow the lead of South Korea, China, and other nations by imposing an immediate nationwide quarantine to slow the inevitable spread of COVID-19. Without federal action, the creators say, their lives and the lives of their colleagues, patients, and families are being put at increased risk.
In addition to the quarantine, the petition, posted on the website Change.org, calls on U.S. leaders to institute emergency production and distribution of personal protective equipment for healthcare workers and to rapidly increase access to testing.
The petition – which garnered more than 40,000 signatures in just 12 hours and as of this writing was approaching 94,000 – was started by an apolitical Facebook group to focus attention on what members see as the most critical issues for clinicians: slowing the spread of the virus through a coast-to-coast quarantine, protection of medical personnel with adequate supplies of essential equipment, and widespread testing.
“We started this group last Friday out of the realization that clinicians needed information about the outbreak and weren’t getting it,” said coadministrator Jessica McIntyre, MD, a pediatric hospitalist at Elliot Hospital in Manchester, N.H.
“We wanted to get ahead of it and connect with people before we were in the trenches experiencing it and to see what other programs were doing. From a local perspective, it has been really hard to see what people are doing in other states, especially when the protocols in our own states are changing every single day as we collect more information,” she said in an interview.
The Horse Has Bolted
A family medicine physician in Illinois helped launch the Facebook group. She asked that her name not be used but said in an interview that earlier actions may have prevented or at least delayed the need for the more draconian measures that her group is recommending.
“Clearly South Korea is one of the superstars as far as response has gone, but the concern we have in the United States is that we’re well beyond that point – we needed to be testing people over a month ago, in the hope of preventing a quarantine,” she said in an interview.
According to National Public Radio, as of March 13, South Korea had conducted 3,600 tests per million population, compared with five per million in the United States.
“I think the most concerning part is to see where Italy is now and where we are in comparison. Our ICUs have not yet overflowed, but I think we’re definitely looking at that in the next few weeks – hopefully longer, but I suspect that it will happen shortly,” she continued.
She cited work by Harvard University biostatistician Xihong Lin, PhD, that shows that when health authorities in Wuhan, China – widely cited as the epicenter of the global pandemic – cordoned off the city, the infection rate dropped from one person infecting 3.8 others to one infecting 1.25, thereby significantly slowing the rate of transmission.
“This is absolutely what we need to be doing,” she said.
Real News
Within 3 days of its creation, the online group had accrued more than 80,000 members with advanced medical training, including MDs, DOs, physician assistants, nurse practitioners, and certified registered nurse anesthetists.
“A lot of us were already very busy with our day-to-day work outside of COVID-19, and I think a lot of us felt unsure about where to get the best information,” said coadministrator David Janssen, MD, a family medicine physician in group practice in Sioux Center, Iowa,
“If you turn on the TV, there’s a lot of politicizing of the issue, and there’s a lot of good information, but also a lot of bad information. When health care providers talk to other health care providers, that’s often how we get our information and how we learn,” he said in an interview.
The COVID-19 U.S. Physicians/APP Facebook group includes 20 volunteer moderators who handle hundreds of posts per hour from persons seeking information on the novel coronavirus, what to tell patients, and how to protect themselves.
“It’s been wonderful to see how providers have been helping other providers sort through issues. Teaching hospitals have their hands on the latest research, but a lot of people like myself are at small community hospitals, critical-access hospitals, where we may have a lot of questions but don’t necessarily have the answers readily available to us,” Dr. Janssen said.
Dr. Janssen said that his community of about 8,000 residents initially had only four COVID-19 testing kits, or one for every 2,000 people. The situation has since improved, and more tests are now available, he added.
Dr. McIntyre, Dr. Janssen, and the Illinois family physician have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clinicians across the United States are petitioning the federal government to follow the lead of South Korea, China, and other nations by imposing an immediate nationwide quarantine to slow the inevitable spread of COVID-19. Without federal action, the creators say, their lives and the lives of their colleagues, patients, and families are being put at increased risk.
In addition to the quarantine, the petition, posted on the website Change.org, calls on U.S. leaders to institute emergency production and distribution of personal protective equipment for healthcare workers and to rapidly increase access to testing.
The petition – which garnered more than 40,000 signatures in just 12 hours and as of this writing was approaching 94,000 – was started by an apolitical Facebook group to focus attention on what members see as the most critical issues for clinicians: slowing the spread of the virus through a coast-to-coast quarantine, protection of medical personnel with adequate supplies of essential equipment, and widespread testing.
“We started this group last Friday out of the realization that clinicians needed information about the outbreak and weren’t getting it,” said coadministrator Jessica McIntyre, MD, a pediatric hospitalist at Elliot Hospital in Manchester, N.H.
“We wanted to get ahead of it and connect with people before we were in the trenches experiencing it and to see what other programs were doing. From a local perspective, it has been really hard to see what people are doing in other states, especially when the protocols in our own states are changing every single day as we collect more information,” she said in an interview.
The Horse Has Bolted
A family medicine physician in Illinois helped launch the Facebook group. She asked that her name not be used but said in an interview that earlier actions may have prevented or at least delayed the need for the more draconian measures that her group is recommending.
“Clearly South Korea is one of the superstars as far as response has gone, but the concern we have in the United States is that we’re well beyond that point – we needed to be testing people over a month ago, in the hope of preventing a quarantine,” she said in an interview.
According to National Public Radio, as of March 13, South Korea had conducted 3,600 tests per million population, compared with five per million in the United States.
“I think the most concerning part is to see where Italy is now and where we are in comparison. Our ICUs have not yet overflowed, but I think we’re definitely looking at that in the next few weeks – hopefully longer, but I suspect that it will happen shortly,” she continued.
She cited work by Harvard University biostatistician Xihong Lin, PhD, that shows that when health authorities in Wuhan, China – widely cited as the epicenter of the global pandemic – cordoned off the city, the infection rate dropped from one person infecting 3.8 others to one infecting 1.25, thereby significantly slowing the rate of transmission.
“This is absolutely what we need to be doing,” she said.
Real News
Within 3 days of its creation, the online group had accrued more than 80,000 members with advanced medical training, including MDs, DOs, physician assistants, nurse practitioners, and certified registered nurse anesthetists.
“A lot of us were already very busy with our day-to-day work outside of COVID-19, and I think a lot of us felt unsure about where to get the best information,” said coadministrator David Janssen, MD, a family medicine physician in group practice in Sioux Center, Iowa,
“If you turn on the TV, there’s a lot of politicizing of the issue, and there’s a lot of good information, but also a lot of bad information. When health care providers talk to other health care providers, that’s often how we get our information and how we learn,” he said in an interview.
The COVID-19 U.S. Physicians/APP Facebook group includes 20 volunteer moderators who handle hundreds of posts per hour from persons seeking information on the novel coronavirus, what to tell patients, and how to protect themselves.
“It’s been wonderful to see how providers have been helping other providers sort through issues. Teaching hospitals have their hands on the latest research, but a lot of people like myself are at small community hospitals, critical-access hospitals, where we may have a lot of questions but don’t necessarily have the answers readily available to us,” Dr. Janssen said.
Dr. Janssen said that his community of about 8,000 residents initially had only four COVID-19 testing kits, or one for every 2,000 people. The situation has since improved, and more tests are now available, he added.
Dr. McIntyre, Dr. Janssen, and the Illinois family physician have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
COVID-19 in pregnant women and the impact on newborns
Clinical question: How does infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in pregnant mothers affect their newborns?
Background: A novel coronavirus, now named SARS-CoV-2 by the World Health Organization (previously referred to as 2019-nCoV), is currently causing a worldwide pandemic. It is believed to have originated in Hubei province, China, but is now rapidly spreading in other countries. Although its effects are most severe in the elderly, SARS-CoV-2 has been infecting younger patients, including pregnant women. The effect of COVID-19, the disease caused by SARS-CoV-2, in pregnant women on their newborn children, is unknown, as is the nature of perinatal transmission of SARS-CoV-2.
Study design: Retrospective analysis.
Setting: Five hospitals in Hubei province, China.
Synopsis: Researchers retrospectively analyzed the clinical features and outcomes of 10 neonates (including two twins) born to nine mothers with confirmed SARS-CoV-2 infection in five hospitals in Hubei province, China, during Jan. 20–Feb. 5, 2020. The mothers were, on average, 30 years of age, but their prior state of health was not described. SARS-CoV-2 infection was confirmed in eight mothers by SARS-CoV-2 nucleic acid testing (NAT). The twins’ mother was diagnosed with COVID-19 based on chest CT scan showing viral interstitial pneumonia with other causes of fever and lung infection being “excluded,” despite a negative SARS-CoV-2 NAT test.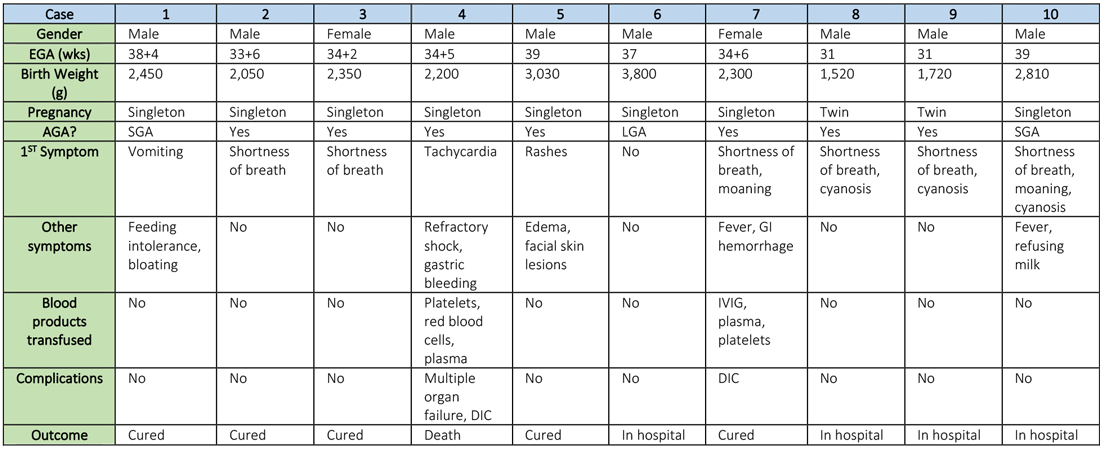
Symptoms occurred in the following:
- Before delivery in four mothers, three of whom were treated with oseltamivir (Tamiflu) after delivery.
- On the day of delivery in two mothers, one of whom was treated with oseltamivir and nebulized inhaled interferon after delivery.
- After delivery in three mothers.
Seven mothers delivered by cesarean section and two by vaginal delivery. Prenatal complications included intrauterine distress in six mothers, premature rupture of membranes in three (5-7 hours before onset of true labor), abnormal amniotic fluid in two, “abnormal” umbilical cord in two, and placenta previa in one.
The neonates born to these mothers included two females and eight males; four were full-term and six were premature (degree of prematurity not described). Symptoms first observed in these newborns included shortness of breath (six), fevers (two), tachycardia (one), and vomiting, feeding intolerance, “bloating,” refusing milk, and “gastric bleeding.” Chest radiographs were abnormal in seven newborns, including evidence of “infection” (four), neonatal respiratory distress syndrome (two), and pneumothorax (one). Two cases were described in detail:
- A neonate delivered at 34+5/7 weeks gestational age, was admitted due to shortness of breath and “moaning.” Eight days later, the neonate developed refractory shock, multiple organ failure, disseminated intravascular coagulation requiring transfusions of platelets, red blood cells, and plasma. He died on the ninth day.
- A neonate delivered at 34+6 weeks gestational age and was admitted 25 minutes after delivery due to shortness of breath and “moaning.” He required 2 days of noninvasive support/oxygen therapy and was observed to later develop “oxygen fluctuations” and thrombocytopenia at 3 days of life. The neonate was treated with “respiratory support,” intravenous immunoglobulin, transfusions of platelets and plasma, hydrocortisone (5 mg/kg per day for 6 days), low-dose heparin (2 units/kg per hr for 6 days), and low molecular weight heparin (2 units/kg per hr for 6 days). He was described to be “cured” 15 days later.
All nine neonates underwent pharyngeal swabs for SARS-CoV-2 NAT, and all were negative.
Bottom line: Although data are currently very limited, neonates born to mothers with COVID-19 appear to be at risk for adverse outcomes, including fetal distress, respiratory distress, thrombocytopenia associated with abnormal liver function, and death. There was no evidence of vertical transmission in this study.
Citation: Zhu H et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020 Feb;9(1):51-60.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
Clinical question: How does infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in pregnant mothers affect their newborns?
Background: A novel coronavirus, now named SARS-CoV-2 by the World Health Organization (previously referred to as 2019-nCoV), is currently causing a worldwide pandemic. It is believed to have originated in Hubei province, China, but is now rapidly spreading in other countries. Although its effects are most severe in the elderly, SARS-CoV-2 has been infecting younger patients, including pregnant women. The effect of COVID-19, the disease caused by SARS-CoV-2, in pregnant women on their newborn children, is unknown, as is the nature of perinatal transmission of SARS-CoV-2.
Study design: Retrospective analysis.
Setting: Five hospitals in Hubei province, China.
Synopsis: Researchers retrospectively analyzed the clinical features and outcomes of 10 neonates (including two twins) born to nine mothers with confirmed SARS-CoV-2 infection in five hospitals in Hubei province, China, during Jan. 20–Feb. 5, 2020. The mothers were, on average, 30 years of age, but their prior state of health was not described. SARS-CoV-2 infection was confirmed in eight mothers by SARS-CoV-2 nucleic acid testing (NAT). The twins’ mother was diagnosed with COVID-19 based on chest CT scan showing viral interstitial pneumonia with other causes of fever and lung infection being “excluded,” despite a negative SARS-CoV-2 NAT test.
Symptoms occurred in the following:
- Before delivery in four mothers, three of whom were treated with oseltamivir (Tamiflu) after delivery.
- On the day of delivery in two mothers, one of whom was treated with oseltamivir and nebulized inhaled interferon after delivery.
- After delivery in three mothers.
Seven mothers delivered by cesarean section and two by vaginal delivery. Prenatal complications included intrauterine distress in six mothers, premature rupture of membranes in three (5-7 hours before onset of true labor), abnormal amniotic fluid in two, “abnormal” umbilical cord in two, and placenta previa in one.
The neonates born to these mothers included two females and eight males; four were full-term and six were premature (degree of prematurity not described). Symptoms first observed in these newborns included shortness of breath (six), fevers (two), tachycardia (one), and vomiting, feeding intolerance, “bloating,” refusing milk, and “gastric bleeding.” Chest radiographs were abnormal in seven newborns, including evidence of “infection” (four), neonatal respiratory distress syndrome (two), and pneumothorax (one). Two cases were described in detail:
- A neonate delivered at 34+5/7 weeks gestational age, was admitted due to shortness of breath and “moaning.” Eight days later, the neonate developed refractory shock, multiple organ failure, disseminated intravascular coagulation requiring transfusions of platelets, red blood cells, and plasma. He died on the ninth day.
- A neonate delivered at 34+6 weeks gestational age and was admitted 25 minutes after delivery due to shortness of breath and “moaning.” He required 2 days of noninvasive support/oxygen therapy and was observed to later develop “oxygen fluctuations” and thrombocytopenia at 3 days of life. The neonate was treated with “respiratory support,” intravenous immunoglobulin, transfusions of platelets and plasma, hydrocortisone (5 mg/kg per day for 6 days), low-dose heparin (2 units/kg per hr for 6 days), and low molecular weight heparin (2 units/kg per hr for 6 days). He was described to be “cured” 15 days later.
All nine neonates underwent pharyngeal swabs for SARS-CoV-2 NAT, and all were negative.
Bottom line: Although data are currently very limited, neonates born to mothers with COVID-19 appear to be at risk for adverse outcomes, including fetal distress, respiratory distress, thrombocytopenia associated with abnormal liver function, and death. There was no evidence of vertical transmission in this study.
Citation: Zhu H et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020 Feb;9(1):51-60.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
Clinical question: How does infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in pregnant mothers affect their newborns?
Background: A novel coronavirus, now named SARS-CoV-2 by the World Health Organization (previously referred to as 2019-nCoV), is currently causing a worldwide pandemic. It is believed to have originated in Hubei province, China, but is now rapidly spreading in other countries. Although its effects are most severe in the elderly, SARS-CoV-2 has been infecting younger patients, including pregnant women. The effect of COVID-19, the disease caused by SARS-CoV-2, in pregnant women on their newborn children, is unknown, as is the nature of perinatal transmission of SARS-CoV-2.
Study design: Retrospective analysis.
Setting: Five hospitals in Hubei province, China.
Synopsis: Researchers retrospectively analyzed the clinical features and outcomes of 10 neonates (including two twins) born to nine mothers with confirmed SARS-CoV-2 infection in five hospitals in Hubei province, China, during Jan. 20–Feb. 5, 2020. The mothers were, on average, 30 years of age, but their prior state of health was not described. SARS-CoV-2 infection was confirmed in eight mothers by SARS-CoV-2 nucleic acid testing (NAT). The twins’ mother was diagnosed with COVID-19 based on chest CT scan showing viral interstitial pneumonia with other causes of fever and lung infection being “excluded,” despite a negative SARS-CoV-2 NAT test.
Symptoms occurred in the following:
- Before delivery in four mothers, three of whom were treated with oseltamivir (Tamiflu) after delivery.
- On the day of delivery in two mothers, one of whom was treated with oseltamivir and nebulized inhaled interferon after delivery.
- After delivery in three mothers.
Seven mothers delivered by cesarean section and two by vaginal delivery. Prenatal complications included intrauterine distress in six mothers, premature rupture of membranes in three (5-7 hours before onset of true labor), abnormal amniotic fluid in two, “abnormal” umbilical cord in two, and placenta previa in one.
The neonates born to these mothers included two females and eight males; four were full-term and six were premature (degree of prematurity not described). Symptoms first observed in these newborns included shortness of breath (six), fevers (two), tachycardia (one), and vomiting, feeding intolerance, “bloating,” refusing milk, and “gastric bleeding.” Chest radiographs were abnormal in seven newborns, including evidence of “infection” (four), neonatal respiratory distress syndrome (two), and pneumothorax (one). Two cases were described in detail:
- A neonate delivered at 34+5/7 weeks gestational age, was admitted due to shortness of breath and “moaning.” Eight days later, the neonate developed refractory shock, multiple organ failure, disseminated intravascular coagulation requiring transfusions of platelets, red blood cells, and plasma. He died on the ninth day.
- A neonate delivered at 34+6 weeks gestational age and was admitted 25 minutes after delivery due to shortness of breath and “moaning.” He required 2 days of noninvasive support/oxygen therapy and was observed to later develop “oxygen fluctuations” and thrombocytopenia at 3 days of life. The neonate was treated with “respiratory support,” intravenous immunoglobulin, transfusions of platelets and plasma, hydrocortisone (5 mg/kg per day for 6 days), low-dose heparin (2 units/kg per hr for 6 days), and low molecular weight heparin (2 units/kg per hr for 6 days). He was described to be “cured” 15 days later.
All nine neonates underwent pharyngeal swabs for SARS-CoV-2 NAT, and all were negative.
Bottom line: Although data are currently very limited, neonates born to mothers with COVID-19 appear to be at risk for adverse outcomes, including fetal distress, respiratory distress, thrombocytopenia associated with abnormal liver function, and death. There was no evidence of vertical transmission in this study.
Citation: Zhu H et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020 Feb;9(1):51-60.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
Hospitalist movers and shakers – March 2020
Swati Mehta, MD, recently was honored as the lone hospitalist on the National Executive Physician Council for Beryl Institute (Nashville, Tenn.). Only 24 total physicians were selected to the council. Dr. Mehta also was named the 2019 Distinguished Physician Award winner at Vituity (Emeryville, Calif.), where she is the executive director of quality and performance.
A nocturnist at Sequoia Hospital (Redwood City, Calif.), Dr. Mehta is a member of the Society of Hospital Medicine’s Patient Experience interest group.
Shannon Phillips, MD, SFHM, has been named to the National Quality Forum’s Board of Directors for 2020. The chief patient experience officer at Intermountain Healthcare (Salt Lake City, Utah), she also is a recent member of the Performance Measurement and Reporting Committee.
Dr. Phillips, whose focus at Intermountain is on catalyzing safety, quality, and experience of care, was named a 2018 Becker’s Hospital Review Hospital and Health System CXO to Know. Previously, she worked at the Cleveland Clinic, where she was its first patient safety officer and an associate chief quality officer.
Vineet Arora, MD, MHM, has been elected as a new member of the National Academy of Medicine, which honors pioneering scientific and professional achievements within the field.
An academic hospitalist at the University of Chicago, Dr. Arora specializes in improving the learning environment for her medical trainees, as well as maintaining a high level of quality, safety, and care for patients. She also is considered an expert in using social media and other new technology to enhance medical education.
The National Academy of Medicine stated that Dr. Arora’s honor was “for pioneering work to optimize resident fatigue and patient safety during long shifts.”
Edmondo Robinson, MD, SFHM, has been named senior vice president and chief digital innovation officer at Moffitt Cancer Center (Tampa, Fla.). The chief digital innovation officer position is a newly created position that the veteran physician has assumed. Dr. Robinson has 16 years’ experience in clinical and technological work.
In this new position, Dr. Robinson, a practicing academic hospitalist, will head Moffitt’s digital innovation while looking to create and test new services, programs, partnerships, and technologies.
Dr. Robinson comes to Moffitt after serving as chief transformation officer and senior vice president at ChristianaCare (Wilmington, Del.). A teacher at Sidney Kimmel Medical College, Philadelphia, Dr. Robinson was the founding medical director of ChristianaCare Hospitalist Partners.
Relias Healthcare (Tupelo, Miss.) has begun providing hospitalist and emergency medicine services for North Mississippi Health Services’ Gilmore-Amory Trauma Center. Relias, a multistate company that has partnered with more than 150 providers, now has a role at four different North Mississippi Health Services facilities.
Mednax (Sunrise, Fla.) has added Arcenio Chacon and Associated Pediatricians of Homestead, a pediatric critical care and hospital practice, as an affiliate.
Chacon and Associated Pediatricians are based out of Miami and have served Baptist Health South Florida for more than 25 years. The four-physician practice provides critical care and pediatric hospitalist services at Baptist Children’s Hospital (Miami) and hospitalist services at Miami Cancer Institute and Homestead (Fla.) Hospital.
Mednax is a health solutions company that provides subspecialty service in all 50 states. Established in 1979, Mednax partners with hospitals, health systems, and health care facilities to offer clinical services, as well as revenue cycle management, patient engagement, and perioperative improvement consulting services.
Swati Mehta, MD, recently was honored as the lone hospitalist on the National Executive Physician Council for Beryl Institute (Nashville, Tenn.). Only 24 total physicians were selected to the council. Dr. Mehta also was named the 2019 Distinguished Physician Award winner at Vituity (Emeryville, Calif.), where she is the executive director of quality and performance.
A nocturnist at Sequoia Hospital (Redwood City, Calif.), Dr. Mehta is a member of the Society of Hospital Medicine’s Patient Experience interest group.
Shannon Phillips, MD, SFHM, has been named to the National Quality Forum’s Board of Directors for 2020. The chief patient experience officer at Intermountain Healthcare (Salt Lake City, Utah), she also is a recent member of the Performance Measurement and Reporting Committee.
Dr. Phillips, whose focus at Intermountain is on catalyzing safety, quality, and experience of care, was named a 2018 Becker’s Hospital Review Hospital and Health System CXO to Know. Previously, she worked at the Cleveland Clinic, where she was its first patient safety officer and an associate chief quality officer.
Vineet Arora, MD, MHM, has been elected as a new member of the National Academy of Medicine, which honors pioneering scientific and professional achievements within the field.
An academic hospitalist at the University of Chicago, Dr. Arora specializes in improving the learning environment for her medical trainees, as well as maintaining a high level of quality, safety, and care for patients. She also is considered an expert in using social media and other new technology to enhance medical education.
The National Academy of Medicine stated that Dr. Arora’s honor was “for pioneering work to optimize resident fatigue and patient safety during long shifts.”
Edmondo Robinson, MD, SFHM, has been named senior vice president and chief digital innovation officer at Moffitt Cancer Center (Tampa, Fla.). The chief digital innovation officer position is a newly created position that the veteran physician has assumed. Dr. Robinson has 16 years’ experience in clinical and technological work.
In this new position, Dr. Robinson, a practicing academic hospitalist, will head Moffitt’s digital innovation while looking to create and test new services, programs, partnerships, and technologies.
Dr. Robinson comes to Moffitt after serving as chief transformation officer and senior vice president at ChristianaCare (Wilmington, Del.). A teacher at Sidney Kimmel Medical College, Philadelphia, Dr. Robinson was the founding medical director of ChristianaCare Hospitalist Partners.
Relias Healthcare (Tupelo, Miss.) has begun providing hospitalist and emergency medicine services for North Mississippi Health Services’ Gilmore-Amory Trauma Center. Relias, a multistate company that has partnered with more than 150 providers, now has a role at four different North Mississippi Health Services facilities.
Mednax (Sunrise, Fla.) has added Arcenio Chacon and Associated Pediatricians of Homestead, a pediatric critical care and hospital practice, as an affiliate.
Chacon and Associated Pediatricians are based out of Miami and have served Baptist Health South Florida for more than 25 years. The four-physician practice provides critical care and pediatric hospitalist services at Baptist Children’s Hospital (Miami) and hospitalist services at Miami Cancer Institute and Homestead (Fla.) Hospital.
Mednax is a health solutions company that provides subspecialty service in all 50 states. Established in 1979, Mednax partners with hospitals, health systems, and health care facilities to offer clinical services, as well as revenue cycle management, patient engagement, and perioperative improvement consulting services.
Swati Mehta, MD, recently was honored as the lone hospitalist on the National Executive Physician Council for Beryl Institute (Nashville, Tenn.). Only 24 total physicians were selected to the council. Dr. Mehta also was named the 2019 Distinguished Physician Award winner at Vituity (Emeryville, Calif.), where she is the executive director of quality and performance.
A nocturnist at Sequoia Hospital (Redwood City, Calif.), Dr. Mehta is a member of the Society of Hospital Medicine’s Patient Experience interest group.
Shannon Phillips, MD, SFHM, has been named to the National Quality Forum’s Board of Directors for 2020. The chief patient experience officer at Intermountain Healthcare (Salt Lake City, Utah), she also is a recent member of the Performance Measurement and Reporting Committee.
Dr. Phillips, whose focus at Intermountain is on catalyzing safety, quality, and experience of care, was named a 2018 Becker’s Hospital Review Hospital and Health System CXO to Know. Previously, she worked at the Cleveland Clinic, where she was its first patient safety officer and an associate chief quality officer.
Vineet Arora, MD, MHM, has been elected as a new member of the National Academy of Medicine, which honors pioneering scientific and professional achievements within the field.
An academic hospitalist at the University of Chicago, Dr. Arora specializes in improving the learning environment for her medical trainees, as well as maintaining a high level of quality, safety, and care for patients. She also is considered an expert in using social media and other new technology to enhance medical education.
The National Academy of Medicine stated that Dr. Arora’s honor was “for pioneering work to optimize resident fatigue and patient safety during long shifts.”
Edmondo Robinson, MD, SFHM, has been named senior vice president and chief digital innovation officer at Moffitt Cancer Center (Tampa, Fla.). The chief digital innovation officer position is a newly created position that the veteran physician has assumed. Dr. Robinson has 16 years’ experience in clinical and technological work.
In this new position, Dr. Robinson, a practicing academic hospitalist, will head Moffitt’s digital innovation while looking to create and test new services, programs, partnerships, and technologies.
Dr. Robinson comes to Moffitt after serving as chief transformation officer and senior vice president at ChristianaCare (Wilmington, Del.). A teacher at Sidney Kimmel Medical College, Philadelphia, Dr. Robinson was the founding medical director of ChristianaCare Hospitalist Partners.
Relias Healthcare (Tupelo, Miss.) has begun providing hospitalist and emergency medicine services for North Mississippi Health Services’ Gilmore-Amory Trauma Center. Relias, a multistate company that has partnered with more than 150 providers, now has a role at four different North Mississippi Health Services facilities.
Mednax (Sunrise, Fla.) has added Arcenio Chacon and Associated Pediatricians of Homestead, a pediatric critical care and hospital practice, as an affiliate.
Chacon and Associated Pediatricians are based out of Miami and have served Baptist Health South Florida for more than 25 years. The four-physician practice provides critical care and pediatric hospitalist services at Baptist Children’s Hospital (Miami) and hospitalist services at Miami Cancer Institute and Homestead (Fla.) Hospital.
Mednax is a health solutions company that provides subspecialty service in all 50 states. Established in 1979, Mednax partners with hospitals, health systems, and health care facilities to offer clinical services, as well as revenue cycle management, patient engagement, and perioperative improvement consulting services.
COVID-19: U.S. cardiology groups reaffirm continued use of RAAS-active drugs
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.