User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


What’s driving burnout?
Working fewer hours but still struggling
According to a new survey report released by The Physicians Foundation, 80% of physicians across all specialties report being at full capacity or overextended, and 78% reported sometimes, often, or always experiencing feelings of burnout.
Sixty-two percent of U.S. doctors are pessimistic about the future of medicine, and 49% wouldn’t recommend medicine as a career to their children. This paints a pretty grim picture of medical practice in the United States in 2018.
The survey is conducted every other year by The Physicians Foundation with the assistance of Merritt Hawkins, and I wrote a blog post about the 2016 survey results, which showed alarming levels of disengagement and burnout. So, I thought it would be worthwhile looking over the 2018 report to see if anything has improved.
It appears that things haven’t changed much for doctors since 2016 regarding their attitudes toward their work. The biggest take-away from this year’s survey is that doctors overall are working fewer hours and seeing fewer patients but still struggling with morale and burnout. One important trend that was highlighted is the move toward employment by hospitals or integrated delivery systems; only 31% of physicians are independent practice owners or partners, vs. 49% in the first such survey conducted in 2012. Interestingly, employed doctors tend to work longer hours but see fewer patients compared with their practice-owner colleagues.
The 39-question survey is sent out via e-mail to more than 700,000 physicians (everyone the AMA or Merritt Hawkins has in their databases), and this year 8,774 physicians responded; the statistics geniuses at the University of Tennessee say the survey results have a margin of error of +/– 1.057%. Interestingly, that’s less than half of the 17,236 physicians who responded to the survey in 2016, and I wonder if the reduction in response rate itself indicates an increased level of disengagement among doctors.
Doctors expressed similar frustrations with specific aspects of their work this year, compared with 2016. The single biggest frustration cited by doctors was EHRs (39% this year vs. 27% in 2016), followed by regulatory/insurance requirements (down to 38% from 58% in 2016) and loss of clinical autonomy (37% vs. 32% in 2016). Survey respondents reported working an average of 51.4 hours per week, of which 11.4 hours (22%) are spent on nonclinical (paperwork) duties.
Read the full post at hospitalleader.org.
Also on The Hospital Leader
- I don’t want someone like you caring for me by Gopi Astik, MD
- CMS added care transition codes a few years back. How’s that goin’? by Brad Flansbaum, DO, MPH, MHM
- Sleepless in the hospital no more: Lessons learned during the SIESTA Study by Vineet Arora, MD, MAPP, MHM
- Are you committing malpractice by not treating opioid use disorder in the hospital? by Chris Moriates, MD
Working fewer hours but still struggling
Working fewer hours but still struggling
According to a new survey report released by The Physicians Foundation, 80% of physicians across all specialties report being at full capacity or overextended, and 78% reported sometimes, often, or always experiencing feelings of burnout.
Sixty-two percent of U.S. doctors are pessimistic about the future of medicine, and 49% wouldn’t recommend medicine as a career to their children. This paints a pretty grim picture of medical practice in the United States in 2018.
The survey is conducted every other year by The Physicians Foundation with the assistance of Merritt Hawkins, and I wrote a blog post about the 2016 survey results, which showed alarming levels of disengagement and burnout. So, I thought it would be worthwhile looking over the 2018 report to see if anything has improved.
It appears that things haven’t changed much for doctors since 2016 regarding their attitudes toward their work. The biggest take-away from this year’s survey is that doctors overall are working fewer hours and seeing fewer patients but still struggling with morale and burnout. One important trend that was highlighted is the move toward employment by hospitals or integrated delivery systems; only 31% of physicians are independent practice owners or partners, vs. 49% in the first such survey conducted in 2012. Interestingly, employed doctors tend to work longer hours but see fewer patients compared with their practice-owner colleagues.
The 39-question survey is sent out via e-mail to more than 700,000 physicians (everyone the AMA or Merritt Hawkins has in their databases), and this year 8,774 physicians responded; the statistics geniuses at the University of Tennessee say the survey results have a margin of error of +/– 1.057%. Interestingly, that’s less than half of the 17,236 physicians who responded to the survey in 2016, and I wonder if the reduction in response rate itself indicates an increased level of disengagement among doctors.
Doctors expressed similar frustrations with specific aspects of their work this year, compared with 2016. The single biggest frustration cited by doctors was EHRs (39% this year vs. 27% in 2016), followed by regulatory/insurance requirements (down to 38% from 58% in 2016) and loss of clinical autonomy (37% vs. 32% in 2016). Survey respondents reported working an average of 51.4 hours per week, of which 11.4 hours (22%) are spent on nonclinical (paperwork) duties.
Read the full post at hospitalleader.org.
Also on The Hospital Leader
- I don’t want someone like you caring for me by Gopi Astik, MD
- CMS added care transition codes a few years back. How’s that goin’? by Brad Flansbaum, DO, MPH, MHM
- Sleepless in the hospital no more: Lessons learned during the SIESTA Study by Vineet Arora, MD, MAPP, MHM
- Are you committing malpractice by not treating opioid use disorder in the hospital? by Chris Moriates, MD
According to a new survey report released by The Physicians Foundation, 80% of physicians across all specialties report being at full capacity or overextended, and 78% reported sometimes, often, or always experiencing feelings of burnout.
Sixty-two percent of U.S. doctors are pessimistic about the future of medicine, and 49% wouldn’t recommend medicine as a career to their children. This paints a pretty grim picture of medical practice in the United States in 2018.
The survey is conducted every other year by The Physicians Foundation with the assistance of Merritt Hawkins, and I wrote a blog post about the 2016 survey results, which showed alarming levels of disengagement and burnout. So, I thought it would be worthwhile looking over the 2018 report to see if anything has improved.
It appears that things haven’t changed much for doctors since 2016 regarding their attitudes toward their work. The biggest take-away from this year’s survey is that doctors overall are working fewer hours and seeing fewer patients but still struggling with morale and burnout. One important trend that was highlighted is the move toward employment by hospitals or integrated delivery systems; only 31% of physicians are independent practice owners or partners, vs. 49% in the first such survey conducted in 2012. Interestingly, employed doctors tend to work longer hours but see fewer patients compared with their practice-owner colleagues.
The 39-question survey is sent out via e-mail to more than 700,000 physicians (everyone the AMA or Merritt Hawkins has in their databases), and this year 8,774 physicians responded; the statistics geniuses at the University of Tennessee say the survey results have a margin of error of +/– 1.057%. Interestingly, that’s less than half of the 17,236 physicians who responded to the survey in 2016, and I wonder if the reduction in response rate itself indicates an increased level of disengagement among doctors.
Doctors expressed similar frustrations with specific aspects of their work this year, compared with 2016. The single biggest frustration cited by doctors was EHRs (39% this year vs. 27% in 2016), followed by regulatory/insurance requirements (down to 38% from 58% in 2016) and loss of clinical autonomy (37% vs. 32% in 2016). Survey respondents reported working an average of 51.4 hours per week, of which 11.4 hours (22%) are spent on nonclinical (paperwork) duties.
Read the full post at hospitalleader.org.
Also on The Hospital Leader
- I don’t want someone like you caring for me by Gopi Astik, MD
- CMS added care transition codes a few years back. How’s that goin’? by Brad Flansbaum, DO, MPH, MHM
- Sleepless in the hospital no more: Lessons learned during the SIESTA Study by Vineet Arora, MD, MAPP, MHM
- Are you committing malpractice by not treating opioid use disorder in the hospital? by Chris Moriates, MD
Five pitfalls in optimizing medical management of HFrEF
SNOWMASS, COLO. – Many of the abundant missed opportunities to optimize pharmacotherapy for heart failure with reduced ejection fraction revolve around not getting fully on board with the guideline-directed medical therapy shown to be highly effective at improving clinical outcomes, Akshay S. Desai, MD, asserted at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“If you take nothing else away from this talk, is really quite profound,” declared Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital, and a cardiologist at Harvard Medical School, Boston.
He highlighted five common traps or pitfalls for physicians with regard to medical therapy of patients with heart failure with reduced ejection fraction (HFrEF):
Underutilization of guideline-directed medical therapy
The current ACC/American Heart Association/Heart Failure Society of America guidelines on heart failure management (Circulation. 2017 Aug 8;136[6]:e137-61) reflect 20 years of impressive progress in improving heart failure outcomes through the use of increasingly effective guideline-directed medical therapy (GDMT). The magnitude of this improvement was nicely captured in a meta-analysis of 57 randomized controlled trials published during 1987-2015. The meta-analysis showed that, although ACE inhibitor therapy alone had no significant impact on all-cause mortality compared to placebo in patients with HFrEF, the sequential addition of guideline-directed drugs conferred stepwise improvements in survival. This approach culminated in a 56% reduction in all-cause mortality with the combination of an ACE inhibitor, beta-blocker, and mineralocorticoid receptor antagonist (MRA), compared with placebo, and a 63% reduction with an angiotensin receptor-neprilysin inhibitor (ARNI), beta-blocker, and MRA (Circ Heart Fail. 2017 Jan;10(1). pii: e003529).
Moreover, the benefits of contemporary GDMT extend beyond reductions in all-cause mortality, death due to heart failure, and heart failure–related hospitalizations into areas where one wouldn’t necessarily have expected to see much benefit. For example, an analysis of data on more than 40,000 HFrEF patients in 12 clinical trials showed a sharp decline in the rate of sudden death over the years as new agents were incorporated into GDMT. The cumulative incidence of sudden death within 90 days after randomization plunged from 2.4% in the earliest trial to 1.0% in the most recent one (N Engl J Med. 2017 Jul 6;377[1]:41-51).
“We’re at the point where we now question whether routine use of implantable cardioverter-defibrillators in primary prevention patients with nonischemic heart failure is really worthwhile on the backdrop of effective medical therapy,” Dr. Desai observed.
But there’s a problem: “We don’t do a great job with GDMT, even with this incredible evidence base that we have,” the cardiologist said.
He cited a report from the CHAMP-HF registry that scrutinized the use of GDMT in more than 3,500 ambulatory HFrEF patients in 150 U.S. primary care and cardiology practices. It found that 67% of patients deemed eligible for an MRA weren’t on one. Neither were 33% with no contraindications to beta-blocker therapy and 27% who were eligible for an ACE inhibitor, angiotensin receptor blocker (ARB), or ARNI (J Am Coll Cardiol. 2018 Jul 24;72[4]:351-66).
“This highlights a huge opportunity for further guideline-directed optimization of therapy,” he said.
Underdosing of GDMT
The CHAMP-HF registry contained further disappointing news regarding the state of treatment of patients with HFrEF in ambulatory settings: Among those patients who were on GDMT, very few were receiving the recommended target doses of the medications as established in major clinical trials and specified in the guidelines. Only 14% of patients on an ARNI were on the target dose, as were 28% on a beta-blocker, and 17% of those on an ACE inhibitor or ARB. And among patients who were eligible for all classes of GDMT, just 1% were simultaneously on the target doses of an MRA, beta-blocker, and ARNI, ACE inhibitor, or ARB. This despite solid evidence that, although some benefit is derived from initiating these medications, incremental benefit comes from dose titration.
“Even for those of us who feel like we do this quite well, if we examine our practices systematically – and we’ve done this in our own practices at Brigham and Women’s – you see that a lot of eligible patients aren’t on optimal therapy. And you might argue that many of them have contraindications, but even when you do a deep dive into the literature or the electronic medical record and ask the question – Why is this patient with normal renal function and normal potassium with class II HFrEF not on an MRA? – sometimes it’s hard to establish why that’s the case,” said Dr. Desai.
Interrupting GDMT during hospitalizations
This is common practice. But in fact, continuation of GDMT is generally well tolerated in the setting of acute decompensated heart failure in the absence of severe hypotension and cardiogenic shock. Moreover, in-hospital discontinuation or dose reduction is associated with increased risks of readmission and mortality.
And in treatment-naive HFrEF patients, what better place to introduce a medication and assess its tolerability than the hospital? Plus, medications prescribed at discharge are more likely to be continued in the outpatient setting, he noted.
Being seduced by the illusion of stability
The guidelines state that patients with NYHA class II or III HFrEF who tolerate an ACE inhibitor or ARB should be transitioned to an ARNI to further reduce their risk of morbidity and mortality. Yet many physicians wait to make the switch until clinical decompensation occurs. That’s a mistake, as was demonstrated in the landmark PARADIGM-HF trial. Twenty percent of study participants without a prior hospitalization for heart failure experienced cardiovascular death or heart failure hospitalization during the follow-up period. Patients who were clinically stable as defined by no prior heart failure hospitalization or none within 3 months prior to enrollment were as likely to benefit from ARNI therapy with sacubitril/valsartan (Entresto) as were those with a recent decompensation (JACC Heart Fail. 2016 Oct;4[10]:816-22). “A key message is that stability is an illusion in patients with symptomatic heart failure,”said Dr. Desai. “In PARADIGM-HF, the first event for about half of patients was not heralded by a worsening of symptoms or a heart failure hospitalization, it was an abrupt death at home. This may mean that a missed opportunity to optimize treatment may not come back to you down the road, so waiting until patients get worse in order to optimize their therapy may not be the best strategy.”
Inadequate laboratory monitoring
The MRAs, spironolactone and eplerenone (Inspra), are the GDMT drugs for which laboratory surveillance takes on the greatest importance because of their potential to induce hyperkalemia. The guidelines are clear that a potassium level and measurement of renal function should be obtained within a week of initiating therapy with an MRA, again at 4 weeks, and periodically thereafter.
“In general, this is done in clinical practice almost never,” Dr. Desai stressed.
These agents should be avoided in patients with prior hyperkalemia or advanced chronic kidney disease, and used with care in groups known to be at increased risk for hyperkalemia, including the elderly and patients with diabetes.
He considers spironolactone equivalent to eplerenone so long as the dosing is adequate. He generally reserves eplerenone for patients with poorly tolerated antiandrogenic effects on spironolactone.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
SNOWMASS, COLO. – Many of the abundant missed opportunities to optimize pharmacotherapy for heart failure with reduced ejection fraction revolve around not getting fully on board with the guideline-directed medical therapy shown to be highly effective at improving clinical outcomes, Akshay S. Desai, MD, asserted at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“If you take nothing else away from this talk, is really quite profound,” declared Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital, and a cardiologist at Harvard Medical School, Boston.
He highlighted five common traps or pitfalls for physicians with regard to medical therapy of patients with heart failure with reduced ejection fraction (HFrEF):
Underutilization of guideline-directed medical therapy
The current ACC/American Heart Association/Heart Failure Society of America guidelines on heart failure management (Circulation. 2017 Aug 8;136[6]:e137-61) reflect 20 years of impressive progress in improving heart failure outcomes through the use of increasingly effective guideline-directed medical therapy (GDMT). The magnitude of this improvement was nicely captured in a meta-analysis of 57 randomized controlled trials published during 1987-2015. The meta-analysis showed that, although ACE inhibitor therapy alone had no significant impact on all-cause mortality compared to placebo in patients with HFrEF, the sequential addition of guideline-directed drugs conferred stepwise improvements in survival. This approach culminated in a 56% reduction in all-cause mortality with the combination of an ACE inhibitor, beta-blocker, and mineralocorticoid receptor antagonist (MRA), compared with placebo, and a 63% reduction with an angiotensin receptor-neprilysin inhibitor (ARNI), beta-blocker, and MRA (Circ Heart Fail. 2017 Jan;10(1). pii: e003529).
Moreover, the benefits of contemporary GDMT extend beyond reductions in all-cause mortality, death due to heart failure, and heart failure–related hospitalizations into areas where one wouldn’t necessarily have expected to see much benefit. For example, an analysis of data on more than 40,000 HFrEF patients in 12 clinical trials showed a sharp decline in the rate of sudden death over the years as new agents were incorporated into GDMT. The cumulative incidence of sudden death within 90 days after randomization plunged from 2.4% in the earliest trial to 1.0% in the most recent one (N Engl J Med. 2017 Jul 6;377[1]:41-51).
“We’re at the point where we now question whether routine use of implantable cardioverter-defibrillators in primary prevention patients with nonischemic heart failure is really worthwhile on the backdrop of effective medical therapy,” Dr. Desai observed.
But there’s a problem: “We don’t do a great job with GDMT, even with this incredible evidence base that we have,” the cardiologist said.
He cited a report from the CHAMP-HF registry that scrutinized the use of GDMT in more than 3,500 ambulatory HFrEF patients in 150 U.S. primary care and cardiology practices. It found that 67% of patients deemed eligible for an MRA weren’t on one. Neither were 33% with no contraindications to beta-blocker therapy and 27% who were eligible for an ACE inhibitor, angiotensin receptor blocker (ARB), or ARNI (J Am Coll Cardiol. 2018 Jul 24;72[4]:351-66).
“This highlights a huge opportunity for further guideline-directed optimization of therapy,” he said.
Underdosing of GDMT
The CHAMP-HF registry contained further disappointing news regarding the state of treatment of patients with HFrEF in ambulatory settings: Among those patients who were on GDMT, very few were receiving the recommended target doses of the medications as established in major clinical trials and specified in the guidelines. Only 14% of patients on an ARNI were on the target dose, as were 28% on a beta-blocker, and 17% of those on an ACE inhibitor or ARB. And among patients who were eligible for all classes of GDMT, just 1% were simultaneously on the target doses of an MRA, beta-blocker, and ARNI, ACE inhibitor, or ARB. This despite solid evidence that, although some benefit is derived from initiating these medications, incremental benefit comes from dose titration.
“Even for those of us who feel like we do this quite well, if we examine our practices systematically – and we’ve done this in our own practices at Brigham and Women’s – you see that a lot of eligible patients aren’t on optimal therapy. And you might argue that many of them have contraindications, but even when you do a deep dive into the literature or the electronic medical record and ask the question – Why is this patient with normal renal function and normal potassium with class II HFrEF not on an MRA? – sometimes it’s hard to establish why that’s the case,” said Dr. Desai.
Interrupting GDMT during hospitalizations
This is common practice. But in fact, continuation of GDMT is generally well tolerated in the setting of acute decompensated heart failure in the absence of severe hypotension and cardiogenic shock. Moreover, in-hospital discontinuation or dose reduction is associated with increased risks of readmission and mortality.
And in treatment-naive HFrEF patients, what better place to introduce a medication and assess its tolerability than the hospital? Plus, medications prescribed at discharge are more likely to be continued in the outpatient setting, he noted.
Being seduced by the illusion of stability
The guidelines state that patients with NYHA class II or III HFrEF who tolerate an ACE inhibitor or ARB should be transitioned to an ARNI to further reduce their risk of morbidity and mortality. Yet many physicians wait to make the switch until clinical decompensation occurs. That’s a mistake, as was demonstrated in the landmark PARADIGM-HF trial. Twenty percent of study participants without a prior hospitalization for heart failure experienced cardiovascular death or heart failure hospitalization during the follow-up period. Patients who were clinically stable as defined by no prior heart failure hospitalization or none within 3 months prior to enrollment were as likely to benefit from ARNI therapy with sacubitril/valsartan (Entresto) as were those with a recent decompensation (JACC Heart Fail. 2016 Oct;4[10]:816-22). “A key message is that stability is an illusion in patients with symptomatic heart failure,”said Dr. Desai. “In PARADIGM-HF, the first event for about half of patients was not heralded by a worsening of symptoms or a heart failure hospitalization, it was an abrupt death at home. This may mean that a missed opportunity to optimize treatment may not come back to you down the road, so waiting until patients get worse in order to optimize their therapy may not be the best strategy.”
Inadequate laboratory monitoring
The MRAs, spironolactone and eplerenone (Inspra), are the GDMT drugs for which laboratory surveillance takes on the greatest importance because of their potential to induce hyperkalemia. The guidelines are clear that a potassium level and measurement of renal function should be obtained within a week of initiating therapy with an MRA, again at 4 weeks, and periodically thereafter.
“In general, this is done in clinical practice almost never,” Dr. Desai stressed.
These agents should be avoided in patients with prior hyperkalemia or advanced chronic kidney disease, and used with care in groups known to be at increased risk for hyperkalemia, including the elderly and patients with diabetes.
He considers spironolactone equivalent to eplerenone so long as the dosing is adequate. He generally reserves eplerenone for patients with poorly tolerated antiandrogenic effects on spironolactone.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
SNOWMASS, COLO. – Many of the abundant missed opportunities to optimize pharmacotherapy for heart failure with reduced ejection fraction revolve around not getting fully on board with the guideline-directed medical therapy shown to be highly effective at improving clinical outcomes, Akshay S. Desai, MD, asserted at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“If you take nothing else away from this talk, is really quite profound,” declared Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital, and a cardiologist at Harvard Medical School, Boston.
He highlighted five common traps or pitfalls for physicians with regard to medical therapy of patients with heart failure with reduced ejection fraction (HFrEF):
Underutilization of guideline-directed medical therapy
The current ACC/American Heart Association/Heart Failure Society of America guidelines on heart failure management (Circulation. 2017 Aug 8;136[6]:e137-61) reflect 20 years of impressive progress in improving heart failure outcomes through the use of increasingly effective guideline-directed medical therapy (GDMT). The magnitude of this improvement was nicely captured in a meta-analysis of 57 randomized controlled trials published during 1987-2015. The meta-analysis showed that, although ACE inhibitor therapy alone had no significant impact on all-cause mortality compared to placebo in patients with HFrEF, the sequential addition of guideline-directed drugs conferred stepwise improvements in survival. This approach culminated in a 56% reduction in all-cause mortality with the combination of an ACE inhibitor, beta-blocker, and mineralocorticoid receptor antagonist (MRA), compared with placebo, and a 63% reduction with an angiotensin receptor-neprilysin inhibitor (ARNI), beta-blocker, and MRA (Circ Heart Fail. 2017 Jan;10(1). pii: e003529).
Moreover, the benefits of contemporary GDMT extend beyond reductions in all-cause mortality, death due to heart failure, and heart failure–related hospitalizations into areas where one wouldn’t necessarily have expected to see much benefit. For example, an analysis of data on more than 40,000 HFrEF patients in 12 clinical trials showed a sharp decline in the rate of sudden death over the years as new agents were incorporated into GDMT. The cumulative incidence of sudden death within 90 days after randomization plunged from 2.4% in the earliest trial to 1.0% in the most recent one (N Engl J Med. 2017 Jul 6;377[1]:41-51).
“We’re at the point where we now question whether routine use of implantable cardioverter-defibrillators in primary prevention patients with nonischemic heart failure is really worthwhile on the backdrop of effective medical therapy,” Dr. Desai observed.
But there’s a problem: “We don’t do a great job with GDMT, even with this incredible evidence base that we have,” the cardiologist said.
He cited a report from the CHAMP-HF registry that scrutinized the use of GDMT in more than 3,500 ambulatory HFrEF patients in 150 U.S. primary care and cardiology practices. It found that 67% of patients deemed eligible for an MRA weren’t on one. Neither were 33% with no contraindications to beta-blocker therapy and 27% who were eligible for an ACE inhibitor, angiotensin receptor blocker (ARB), or ARNI (J Am Coll Cardiol. 2018 Jul 24;72[4]:351-66).
“This highlights a huge opportunity for further guideline-directed optimization of therapy,” he said.
Underdosing of GDMT
The CHAMP-HF registry contained further disappointing news regarding the state of treatment of patients with HFrEF in ambulatory settings: Among those patients who were on GDMT, very few were receiving the recommended target doses of the medications as established in major clinical trials and specified in the guidelines. Only 14% of patients on an ARNI were on the target dose, as were 28% on a beta-blocker, and 17% of those on an ACE inhibitor or ARB. And among patients who were eligible for all classes of GDMT, just 1% were simultaneously on the target doses of an MRA, beta-blocker, and ARNI, ACE inhibitor, or ARB. This despite solid evidence that, although some benefit is derived from initiating these medications, incremental benefit comes from dose titration.
“Even for those of us who feel like we do this quite well, if we examine our practices systematically – and we’ve done this in our own practices at Brigham and Women’s – you see that a lot of eligible patients aren’t on optimal therapy. And you might argue that many of them have contraindications, but even when you do a deep dive into the literature or the electronic medical record and ask the question – Why is this patient with normal renal function and normal potassium with class II HFrEF not on an MRA? – sometimes it’s hard to establish why that’s the case,” said Dr. Desai.
Interrupting GDMT during hospitalizations
This is common practice. But in fact, continuation of GDMT is generally well tolerated in the setting of acute decompensated heart failure in the absence of severe hypotension and cardiogenic shock. Moreover, in-hospital discontinuation or dose reduction is associated with increased risks of readmission and mortality.
And in treatment-naive HFrEF patients, what better place to introduce a medication and assess its tolerability than the hospital? Plus, medications prescribed at discharge are more likely to be continued in the outpatient setting, he noted.
Being seduced by the illusion of stability
The guidelines state that patients with NYHA class II or III HFrEF who tolerate an ACE inhibitor or ARB should be transitioned to an ARNI to further reduce their risk of morbidity and mortality. Yet many physicians wait to make the switch until clinical decompensation occurs. That’s a mistake, as was demonstrated in the landmark PARADIGM-HF trial. Twenty percent of study participants without a prior hospitalization for heart failure experienced cardiovascular death or heart failure hospitalization during the follow-up period. Patients who were clinically stable as defined by no prior heart failure hospitalization or none within 3 months prior to enrollment were as likely to benefit from ARNI therapy with sacubitril/valsartan (Entresto) as were those with a recent decompensation (JACC Heart Fail. 2016 Oct;4[10]:816-22). “A key message is that stability is an illusion in patients with symptomatic heart failure,”said Dr. Desai. “In PARADIGM-HF, the first event for about half of patients was not heralded by a worsening of symptoms or a heart failure hospitalization, it was an abrupt death at home. This may mean that a missed opportunity to optimize treatment may not come back to you down the road, so waiting until patients get worse in order to optimize their therapy may not be the best strategy.”
Inadequate laboratory monitoring
The MRAs, spironolactone and eplerenone (Inspra), are the GDMT drugs for which laboratory surveillance takes on the greatest importance because of their potential to induce hyperkalemia. The guidelines are clear that a potassium level and measurement of renal function should be obtained within a week of initiating therapy with an MRA, again at 4 weeks, and periodically thereafter.
“In general, this is done in clinical practice almost never,” Dr. Desai stressed.
These agents should be avoided in patients with prior hyperkalemia or advanced chronic kidney disease, and used with care in groups known to be at increased risk for hyperkalemia, including the elderly and patients with diabetes.
He considers spironolactone equivalent to eplerenone so long as the dosing is adequate. He generally reserves eplerenone for patients with poorly tolerated antiandrogenic effects on spironolactone.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
REPORTING FROM ACC SNOWMASS 2019
Best practices lower postsepsis risk, but only if implemented
SAN DIEGO – North Carolina health care workers often failed to provide best-practice follow-up to patients who were released after hospitalization for sepsis, a small study has found. There may be a cost to this gap:
“It’s disappointing to see that we are not providing these seemingly common-sense care processes to our sepsis patients at discharge,” said study lead author Stephanie Parks Taylor, MD, of Atrium Health’s Carolinas Medical Center in Charlotte, in an interview following the presentation of the study findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “We need to develop and implement strategies to improve outcomes for sepsis patients, not just while they are in the hospital, but after discharge as well.”
A 2017 report estimated that 1.7 million adults were hospitalized for sepsis in the United States in 2014, and 270,000 died (JAMA. 2017;318[13]:1241-9). Age-adjusted sepsis death rates in the United States are highest in states in the Eastern and Southern regions, a 2017 report from the Centers for Disease Control and Prevention suggested; North Carolina has the 32nd-worst sepsis death rate in the country (12.4 deaths per 100,000 population).
Dr. Taylor said some recent news about sepsis is promising. “We’ve seen decreasing mortality rates from initiatives that improve the early detection of sepsis and rapid delivery of antibiotics, fluids, and other treatment. However, there is growing evidence that patients who survive an episode of sepsis face residual health deficits. Many sepsis survivors are left with new functional, cognitive, or mental health declines or worsening of their underlying comorbidities. Unfortunately, these patients have high rates of mortality and hospital readmission that persist for multiple years after hospitalization.”
Indeed, a 2013 report linked sepsis to significantly higher mortality risk over 5 years, after accounting for comorbidities. Postsepsis patients were 13 times more likely to die over the first year after hospitalization than counterparts who didn’t have sepsis (BMJ Open. 2014;4:e004283).
For the new study, Dr. Taylor said, “we aimed to evaluate current care practices with the hope to identify a postsepsis management strategy that could help nudge these patients towards a more meaningful recovery.”
The researchers retrospectively tracked a random sample of 100 patients (median age, 63 years), who were discharged following an admission for sepsis in 2017. They were treated at eight acute care hospitals in western and central North Carolina and hospitalized for a median of 5 days; 75 were discharged to home (17 received home health services there), 17 went to skilled nursing or long-term care facilities, and 8 went to hospice or another location.
The researchers analyzed whether the patients received four kinds of postsepsis care within 90 days, as recommended by a 2018 review: screening for common functional impairments (53/100 patients received this screening); adjustment of medications as needed following discharge (53/100 patients); monitoring for common and preventable causes for health deterioration, such as infection, chronic lung disease, or heart failure exacerbation (37/100); and assessment for palliative care (25/100 patients) (JAMA. 2018;319[1]:62-75).
Within 90 days of discharge, 34 patients were readmitted and 17 died. The 32 patients who received at least two recommended kinds of postsepsis care were less likely to be readmitted or die (9/32) than those who got zero or one recommended kind of care (34/68; odds ratio, 0.26; 95% confidence ratio, 0.09-0.82).
In an interview, study coauthor Marc Kowalkowski, PhD, associate professor with Atrium Health’s Center for Outcomes Research and Evaluation, said he was hesitant to only allocate blame to hospitals or outpatient providers. “Transition out of the hospital is an extremely complex event, involving often fragmented care settings, and sepsis patients tend to be more complicated than other patients. It probably makes sense to provide an added layer of support during the transition out of the hospital for patients who are at high risk for poor outcomes.”
Overall, the findings are “a call for clinicians to realize sepsis is more than just an acute illness. The combination of a growing number of sepsis survivors and the increased health problems following an episode of sepsis creates an urgent public health challenge,” Dr. Taylor said.
Is more home health an important part of a solution? It may be helpful, Dr. Taylor said, but “our data suggest that there really needs to be better coordination to bridge between the inpatient and outpatient transition. We are currently conducting a randomized study to investigate whether these types of care processes can be delivered effectively through a nurse navigator to improve patient outcomes.”
Fortunately, she said, the findings suggest “we don’t have to reinvent the wheel. We just have to work on implementation of strategies for care processes that we are already familiar with.”
No funding was reported. None of the study authors reported relevant disclosures.
SOURCE: Taylor SP et al. CCC48, Abstract 1320.
SAN DIEGO – North Carolina health care workers often failed to provide best-practice follow-up to patients who were released after hospitalization for sepsis, a small study has found. There may be a cost to this gap:
“It’s disappointing to see that we are not providing these seemingly common-sense care processes to our sepsis patients at discharge,” said study lead author Stephanie Parks Taylor, MD, of Atrium Health’s Carolinas Medical Center in Charlotte, in an interview following the presentation of the study findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “We need to develop and implement strategies to improve outcomes for sepsis patients, not just while they are in the hospital, but after discharge as well.”
A 2017 report estimated that 1.7 million adults were hospitalized for sepsis in the United States in 2014, and 270,000 died (JAMA. 2017;318[13]:1241-9). Age-adjusted sepsis death rates in the United States are highest in states in the Eastern and Southern regions, a 2017 report from the Centers for Disease Control and Prevention suggested; North Carolina has the 32nd-worst sepsis death rate in the country (12.4 deaths per 100,000 population).
Dr. Taylor said some recent news about sepsis is promising. “We’ve seen decreasing mortality rates from initiatives that improve the early detection of sepsis and rapid delivery of antibiotics, fluids, and other treatment. However, there is growing evidence that patients who survive an episode of sepsis face residual health deficits. Many sepsis survivors are left with new functional, cognitive, or mental health declines or worsening of their underlying comorbidities. Unfortunately, these patients have high rates of mortality and hospital readmission that persist for multiple years after hospitalization.”
Indeed, a 2013 report linked sepsis to significantly higher mortality risk over 5 years, after accounting for comorbidities. Postsepsis patients were 13 times more likely to die over the first year after hospitalization than counterparts who didn’t have sepsis (BMJ Open. 2014;4:e004283).
For the new study, Dr. Taylor said, “we aimed to evaluate current care practices with the hope to identify a postsepsis management strategy that could help nudge these patients towards a more meaningful recovery.”
The researchers retrospectively tracked a random sample of 100 patients (median age, 63 years), who were discharged following an admission for sepsis in 2017. They were treated at eight acute care hospitals in western and central North Carolina and hospitalized for a median of 5 days; 75 were discharged to home (17 received home health services there), 17 went to skilled nursing or long-term care facilities, and 8 went to hospice or another location.
The researchers analyzed whether the patients received four kinds of postsepsis care within 90 days, as recommended by a 2018 review: screening for common functional impairments (53/100 patients received this screening); adjustment of medications as needed following discharge (53/100 patients); monitoring for common and preventable causes for health deterioration, such as infection, chronic lung disease, or heart failure exacerbation (37/100); and assessment for palliative care (25/100 patients) (JAMA. 2018;319[1]:62-75).
Within 90 days of discharge, 34 patients were readmitted and 17 died. The 32 patients who received at least two recommended kinds of postsepsis care were less likely to be readmitted or die (9/32) than those who got zero or one recommended kind of care (34/68; odds ratio, 0.26; 95% confidence ratio, 0.09-0.82).
In an interview, study coauthor Marc Kowalkowski, PhD, associate professor with Atrium Health’s Center for Outcomes Research and Evaluation, said he was hesitant to only allocate blame to hospitals or outpatient providers. “Transition out of the hospital is an extremely complex event, involving often fragmented care settings, and sepsis patients tend to be more complicated than other patients. It probably makes sense to provide an added layer of support during the transition out of the hospital for patients who are at high risk for poor outcomes.”
Overall, the findings are “a call for clinicians to realize sepsis is more than just an acute illness. The combination of a growing number of sepsis survivors and the increased health problems following an episode of sepsis creates an urgent public health challenge,” Dr. Taylor said.
Is more home health an important part of a solution? It may be helpful, Dr. Taylor said, but “our data suggest that there really needs to be better coordination to bridge between the inpatient and outpatient transition. We are currently conducting a randomized study to investigate whether these types of care processes can be delivered effectively through a nurse navigator to improve patient outcomes.”
Fortunately, she said, the findings suggest “we don’t have to reinvent the wheel. We just have to work on implementation of strategies for care processes that we are already familiar with.”
No funding was reported. None of the study authors reported relevant disclosures.
SOURCE: Taylor SP et al. CCC48, Abstract 1320.
SAN DIEGO – North Carolina health care workers often failed to provide best-practice follow-up to patients who were released after hospitalization for sepsis, a small study has found. There may be a cost to this gap:
“It’s disappointing to see that we are not providing these seemingly common-sense care processes to our sepsis patients at discharge,” said study lead author Stephanie Parks Taylor, MD, of Atrium Health’s Carolinas Medical Center in Charlotte, in an interview following the presentation of the study findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “We need to develop and implement strategies to improve outcomes for sepsis patients, not just while they are in the hospital, but after discharge as well.”
A 2017 report estimated that 1.7 million adults were hospitalized for sepsis in the United States in 2014, and 270,000 died (JAMA. 2017;318[13]:1241-9). Age-adjusted sepsis death rates in the United States are highest in states in the Eastern and Southern regions, a 2017 report from the Centers for Disease Control and Prevention suggested; North Carolina has the 32nd-worst sepsis death rate in the country (12.4 deaths per 100,000 population).
Dr. Taylor said some recent news about sepsis is promising. “We’ve seen decreasing mortality rates from initiatives that improve the early detection of sepsis and rapid delivery of antibiotics, fluids, and other treatment. However, there is growing evidence that patients who survive an episode of sepsis face residual health deficits. Many sepsis survivors are left with new functional, cognitive, or mental health declines or worsening of their underlying comorbidities. Unfortunately, these patients have high rates of mortality and hospital readmission that persist for multiple years after hospitalization.”
Indeed, a 2013 report linked sepsis to significantly higher mortality risk over 5 years, after accounting for comorbidities. Postsepsis patients were 13 times more likely to die over the first year after hospitalization than counterparts who didn’t have sepsis (BMJ Open. 2014;4:e004283).
For the new study, Dr. Taylor said, “we aimed to evaluate current care practices with the hope to identify a postsepsis management strategy that could help nudge these patients towards a more meaningful recovery.”
The researchers retrospectively tracked a random sample of 100 patients (median age, 63 years), who were discharged following an admission for sepsis in 2017. They were treated at eight acute care hospitals in western and central North Carolina and hospitalized for a median of 5 days; 75 were discharged to home (17 received home health services there), 17 went to skilled nursing or long-term care facilities, and 8 went to hospice or another location.
The researchers analyzed whether the patients received four kinds of postsepsis care within 90 days, as recommended by a 2018 review: screening for common functional impairments (53/100 patients received this screening); adjustment of medications as needed following discharge (53/100 patients); monitoring for common and preventable causes for health deterioration, such as infection, chronic lung disease, or heart failure exacerbation (37/100); and assessment for palliative care (25/100 patients) (JAMA. 2018;319[1]:62-75).
Within 90 days of discharge, 34 patients were readmitted and 17 died. The 32 patients who received at least two recommended kinds of postsepsis care were less likely to be readmitted or die (9/32) than those who got zero or one recommended kind of care (34/68; odds ratio, 0.26; 95% confidence ratio, 0.09-0.82).
In an interview, study coauthor Marc Kowalkowski, PhD, associate professor with Atrium Health’s Center for Outcomes Research and Evaluation, said he was hesitant to only allocate blame to hospitals or outpatient providers. “Transition out of the hospital is an extremely complex event, involving often fragmented care settings, and sepsis patients tend to be more complicated than other patients. It probably makes sense to provide an added layer of support during the transition out of the hospital for patients who are at high risk for poor outcomes.”
Overall, the findings are “a call for clinicians to realize sepsis is more than just an acute illness. The combination of a growing number of sepsis survivors and the increased health problems following an episode of sepsis creates an urgent public health challenge,” Dr. Taylor said.
Is more home health an important part of a solution? It may be helpful, Dr. Taylor said, but “our data suggest that there really needs to be better coordination to bridge between the inpatient and outpatient transition. We are currently conducting a randomized study to investigate whether these types of care processes can be delivered effectively through a nurse navigator to improve patient outcomes.”
Fortunately, she said, the findings suggest “we don’t have to reinvent the wheel. We just have to work on implementation of strategies for care processes that we are already familiar with.”
No funding was reported. None of the study authors reported relevant disclosures.
SOURCE: Taylor SP et al. CCC48, Abstract 1320.
REPORTING FROM CCC48
Sepsis survivors face ongoing immune system challenges
SAN DIEGO – Although it isn’t clear if such episodes result from incomplete resolution of the index infection, or they are due to lingering changes in immune function, they do suggest that physicians should engage sepsis patients in an effort to improve long-term outcomes.
It’s also an argument for biomarkers and precision immune modulation in these patients, Hallie Prescott, MD, a critical care physician at the VA Ann Arbor (Mich.) Healthcare System, said during a talk at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
When sepsis was first defined in 1992, physicians tended to focus on the inflammatory component, but it’s now understood that multiple pathways become dysregulated, and inflammation is no longer part of the most current definition of sepsis. “We now recognize that there is early activation of both pro- and anti-inflammatory pathways, but over the course of sepsis the balance tips from this proinflammatory state in the first few days toward, for most patients, an anti-inflammatory or immune-suppressed state in the later days,” said Dr. Prescott.
As advances in care have increased initial survival rates, more patients go on to the later stages, leaving clinicians to address nosocomial and other secondary infections. An autopsy study showed that many patients who die of sepsis in the ICU have evidence of immune suppression. A study of patients at the end of a pneumonia hospitalization found that many patients had elevated inflammatory markers even after hospital discharge, and that such elevation was associated with increased mortality as far out as 1 year. The relationship was significant even after adjustment for age, comorbidity, and acute illness. “It suggests that this isn’t just identification of patients who had a more severe septic episode,” said Dr. Prescott.
That study implies that some patients take a long time to return to homeostasis, and other work suggests that about two-thirds of sepsis deaths occur after day 5. A study by Dr. Prescott’s group showed about a 40% 2-year mortality after sepsis hospitalization. When they compared sepsis survivors to matched controls, they found about half the deaths could not be explained by presepsis health status. “Rather, it [seems to be] due to the last sequelae of sepsis, or perhaps this increased risk of secondary infections,” Dr. Prescott said.
Studies of the organisms causing secondary infections found increasing incidence of opportunistic infections, from 9% in the first 5 days of sepsis, to 18% in days 16 through 150. The frequency of Candida infection similarly increased, from 13% to 30%. “So in these later phases of sepsis, you’re more likely to see [pathogens] that are relatively rare as the initial cause of sepsis,” said Dr. Prescott.
Unfortunately, several studies showed that prophylaxis does not improve outcomes. “My suspicion is that it’s because these infections are one marker of a broader problem with immune dysfunction, and we probably need to boost or restore immune function more broadly as opposed to trying to prophylax against very specifically what the patient is at risk for,” said Dr. Prescott.
The problems appear to continue after hospital discharge. A study from Dr. Prescott’s group showed that about 40% of sepsis survivors were readmitted at least once within the next 90 days. The most common reason, at 6.4%, was another sepsis episode. Compared with matched controls, sepsis patients had about a 2.5-fold higher risk for sepsis, and about a 1.5-fold increased risk an infection. “So there seems to be this heightened risk among people surviving sepsis that’s not fully explained by the things that put them at risk for developing sepsis in the first place,” said Dr. Prescott.
Another study looking at the reason for recurring infections in sepsis survivors found that in about one in five cases, the readmission was due to the same infectious organism in the same site, suggesting incomplete resolution. In about half of patients, the infection was due to a different organism, or the same organism at a different site, and in about a third of patients, the results were ambiguous due to culture-negative infections.
“I think this suggests a complex picture. Some people perhaps fail to fully eradicate the initial infection, and a larger group of people come back with something else. There’s also a very high rate of infection in the same site – about 70% with a new bug have it in the same site as their initial sepsis. Some of this may be just be a reflection of the type of people who get sepsis the first time, but it still tells us that among the patients we care for who survive sepsis, that they are over the long haul at increased risk of recurrent infections and recurrent episodes of sepsis,” said Dr. Prescott.
The findings suggest a need for real-time assessment of immune function and the potential benefit of immune modulation in the later phases of sepsis. Such strategies are not likely to be implemented immediately, however. In the meantime, there are simple steps that clinicians can take, including screening of sepsis survivors and making sure they are up to date on vaccines, and then educating them about the risk of reinfection. “We know that the lay public awareness of sepsis is low. Even people who have sepsis are often unaware that they had it, and they are certainly unaware that they’re at risk for having another episode,” she said.
Dr. Prescott has no financial disclosures
SAN DIEGO – Although it isn’t clear if such episodes result from incomplete resolution of the index infection, or they are due to lingering changes in immune function, they do suggest that physicians should engage sepsis patients in an effort to improve long-term outcomes.
It’s also an argument for biomarkers and precision immune modulation in these patients, Hallie Prescott, MD, a critical care physician at the VA Ann Arbor (Mich.) Healthcare System, said during a talk at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
When sepsis was first defined in 1992, physicians tended to focus on the inflammatory component, but it’s now understood that multiple pathways become dysregulated, and inflammation is no longer part of the most current definition of sepsis. “We now recognize that there is early activation of both pro- and anti-inflammatory pathways, but over the course of sepsis the balance tips from this proinflammatory state in the first few days toward, for most patients, an anti-inflammatory or immune-suppressed state in the later days,” said Dr. Prescott.
As advances in care have increased initial survival rates, more patients go on to the later stages, leaving clinicians to address nosocomial and other secondary infections. An autopsy study showed that many patients who die of sepsis in the ICU have evidence of immune suppression. A study of patients at the end of a pneumonia hospitalization found that many patients had elevated inflammatory markers even after hospital discharge, and that such elevation was associated with increased mortality as far out as 1 year. The relationship was significant even after adjustment for age, comorbidity, and acute illness. “It suggests that this isn’t just identification of patients who had a more severe septic episode,” said Dr. Prescott.
That study implies that some patients take a long time to return to homeostasis, and other work suggests that about two-thirds of sepsis deaths occur after day 5. A study by Dr. Prescott’s group showed about a 40% 2-year mortality after sepsis hospitalization. When they compared sepsis survivors to matched controls, they found about half the deaths could not be explained by presepsis health status. “Rather, it [seems to be] due to the last sequelae of sepsis, or perhaps this increased risk of secondary infections,” Dr. Prescott said.
Studies of the organisms causing secondary infections found increasing incidence of opportunistic infections, from 9% in the first 5 days of sepsis, to 18% in days 16 through 150. The frequency of Candida infection similarly increased, from 13% to 30%. “So in these later phases of sepsis, you’re more likely to see [pathogens] that are relatively rare as the initial cause of sepsis,” said Dr. Prescott.
Unfortunately, several studies showed that prophylaxis does not improve outcomes. “My suspicion is that it’s because these infections are one marker of a broader problem with immune dysfunction, and we probably need to boost or restore immune function more broadly as opposed to trying to prophylax against very specifically what the patient is at risk for,” said Dr. Prescott.
The problems appear to continue after hospital discharge. A study from Dr. Prescott’s group showed that about 40% of sepsis survivors were readmitted at least once within the next 90 days. The most common reason, at 6.4%, was another sepsis episode. Compared with matched controls, sepsis patients had about a 2.5-fold higher risk for sepsis, and about a 1.5-fold increased risk an infection. “So there seems to be this heightened risk among people surviving sepsis that’s not fully explained by the things that put them at risk for developing sepsis in the first place,” said Dr. Prescott.
Another study looking at the reason for recurring infections in sepsis survivors found that in about one in five cases, the readmission was due to the same infectious organism in the same site, suggesting incomplete resolution. In about half of patients, the infection was due to a different organism, or the same organism at a different site, and in about a third of patients, the results were ambiguous due to culture-negative infections.
“I think this suggests a complex picture. Some people perhaps fail to fully eradicate the initial infection, and a larger group of people come back with something else. There’s also a very high rate of infection in the same site – about 70% with a new bug have it in the same site as their initial sepsis. Some of this may be just be a reflection of the type of people who get sepsis the first time, but it still tells us that among the patients we care for who survive sepsis, that they are over the long haul at increased risk of recurrent infections and recurrent episodes of sepsis,” said Dr. Prescott.
The findings suggest a need for real-time assessment of immune function and the potential benefit of immune modulation in the later phases of sepsis. Such strategies are not likely to be implemented immediately, however. In the meantime, there are simple steps that clinicians can take, including screening of sepsis survivors and making sure they are up to date on vaccines, and then educating them about the risk of reinfection. “We know that the lay public awareness of sepsis is low. Even people who have sepsis are often unaware that they had it, and they are certainly unaware that they’re at risk for having another episode,” she said.
Dr. Prescott has no financial disclosures
SAN DIEGO – Although it isn’t clear if such episodes result from incomplete resolution of the index infection, or they are due to lingering changes in immune function, they do suggest that physicians should engage sepsis patients in an effort to improve long-term outcomes.
It’s also an argument for biomarkers and precision immune modulation in these patients, Hallie Prescott, MD, a critical care physician at the VA Ann Arbor (Mich.) Healthcare System, said during a talk at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
When sepsis was first defined in 1992, physicians tended to focus on the inflammatory component, but it’s now understood that multiple pathways become dysregulated, and inflammation is no longer part of the most current definition of sepsis. “We now recognize that there is early activation of both pro- and anti-inflammatory pathways, but over the course of sepsis the balance tips from this proinflammatory state in the first few days toward, for most patients, an anti-inflammatory or immune-suppressed state in the later days,” said Dr. Prescott.
As advances in care have increased initial survival rates, more patients go on to the later stages, leaving clinicians to address nosocomial and other secondary infections. An autopsy study showed that many patients who die of sepsis in the ICU have evidence of immune suppression. A study of patients at the end of a pneumonia hospitalization found that many patients had elevated inflammatory markers even after hospital discharge, and that such elevation was associated with increased mortality as far out as 1 year. The relationship was significant even after adjustment for age, comorbidity, and acute illness. “It suggests that this isn’t just identification of patients who had a more severe septic episode,” said Dr. Prescott.
That study implies that some patients take a long time to return to homeostasis, and other work suggests that about two-thirds of sepsis deaths occur after day 5. A study by Dr. Prescott’s group showed about a 40% 2-year mortality after sepsis hospitalization. When they compared sepsis survivors to matched controls, they found about half the deaths could not be explained by presepsis health status. “Rather, it [seems to be] due to the last sequelae of sepsis, or perhaps this increased risk of secondary infections,” Dr. Prescott said.
Studies of the organisms causing secondary infections found increasing incidence of opportunistic infections, from 9% in the first 5 days of sepsis, to 18% in days 16 through 150. The frequency of Candida infection similarly increased, from 13% to 30%. “So in these later phases of sepsis, you’re more likely to see [pathogens] that are relatively rare as the initial cause of sepsis,” said Dr. Prescott.
Unfortunately, several studies showed that prophylaxis does not improve outcomes. “My suspicion is that it’s because these infections are one marker of a broader problem with immune dysfunction, and we probably need to boost or restore immune function more broadly as opposed to trying to prophylax against very specifically what the patient is at risk for,” said Dr. Prescott.
The problems appear to continue after hospital discharge. A study from Dr. Prescott’s group showed that about 40% of sepsis survivors were readmitted at least once within the next 90 days. The most common reason, at 6.4%, was another sepsis episode. Compared with matched controls, sepsis patients had about a 2.5-fold higher risk for sepsis, and about a 1.5-fold increased risk an infection. “So there seems to be this heightened risk among people surviving sepsis that’s not fully explained by the things that put them at risk for developing sepsis in the first place,” said Dr. Prescott.
Another study looking at the reason for recurring infections in sepsis survivors found that in about one in five cases, the readmission was due to the same infectious organism in the same site, suggesting incomplete resolution. In about half of patients, the infection was due to a different organism, or the same organism at a different site, and in about a third of patients, the results were ambiguous due to culture-negative infections.
“I think this suggests a complex picture. Some people perhaps fail to fully eradicate the initial infection, and a larger group of people come back with something else. There’s also a very high rate of infection in the same site – about 70% with a new bug have it in the same site as their initial sepsis. Some of this may be just be a reflection of the type of people who get sepsis the first time, but it still tells us that among the patients we care for who survive sepsis, that they are over the long haul at increased risk of recurrent infections and recurrent episodes of sepsis,” said Dr. Prescott.
The findings suggest a need for real-time assessment of immune function and the potential benefit of immune modulation in the later phases of sepsis. Such strategies are not likely to be implemented immediately, however. In the meantime, there are simple steps that clinicians can take, including screening of sepsis survivors and making sure they are up to date on vaccines, and then educating them about the risk of reinfection. “We know that the lay public awareness of sepsis is low. Even people who have sepsis are often unaware that they had it, and they are certainly unaware that they’re at risk for having another episode,” she said.
Dr. Prescott has no financial disclosures
REPORTING FROM CCC48
Hospitalist movers and shakers – March 2019
Flora Kisuule, MD, SFHM, has been awarded the 2018 Excellence in Service and Professionalism Award by Johns Hopkins Bayview Medical Center, Baltimore. Dr. Kisuule is associate director of the division of hospital medicine at Johns Hopkins Bayview, assistant professor at Johns Hopkins University School of Medicine, and assistant professor in the Johns Hopkins Bloomberg School of Public Health.
Dr. Kisuule codeveloped a hospitalist fellowship program for Johns Hopkins University, which she now directs. She has served on several Society of Hospital Medicine committees and consulted on hospitalist programs around the world. Dr. Kisuule is joining the SHM Board of Directors in March 2019.
Paul Hain, DO, was promoted recently to chief medical officer and senior vice president of market delivery for Blue Cross/Blue Shield of Texas. Dr. Hain has a wealth of experience in leadership, including helping create the pediatric hospitalist program at Vanderbilt Children’s Hospital in Nashville, Tenn.
With BC/BS, Dr. Hain will oversee the region’s public relations, community investments, government relations, and lobbying. He will help sponsor Medicare and Medicaid in the state, while also supporting enterprise and marketing efforts. Dr. Hain joins BC/BS after serving as vice president and medical director of population health and network development at the Children’s Medical Center of Dallas.
Laura M. Rosch, DO, recently was selected by Kansas City University as the new campus dean in the Joplin (Mo.) College of Osteopathic Medicine. Dr. Rosch is a practicing hospitalist in Illinois, where she was chair of internal medicine at the Chicago College of Osteopathic Medicine prior to taking her current position.
Dr. Rosch will manage daily operations for the Joplin medical school, streamlining the school with the main campus. She is a former president of the Illinois Osteopathic Medicine Society and holds a master’s degree in nutrition science.
Suzan Lowry, MD, has been named health officer for Charles County, Md., by the county Department of Health and Charles County Commissioners. A longtime pediatrician with 20 years’ experience, Dr. Lowry has served as a pediatric hospitalist educator at Children’s National Medical Center in Washington, D.C.
Dr. Lowry has spent a majority of her career working on behalf of public health. Most recently, she has worked at the United States Marine Corps Quantico Health Clinic in Virginia.
Robyn Chase, DO, a staff hospitalist at Yavapai Regional Medical Center (Prescott, Ariz.), recently was selected as the hospital’s Physician of the Year for 2018.
Dr. Chase has practiced at YRMC since 2010 and is a board-certified internist. She also serves as an associate professor at the University of Arizona, Phoenix.
Kevin Dishman, MD, has been elevated to senior vice president and chief medical officer at Stormont Vail Health (Topeka, Kan.). Dr. Dishman also will be president of Stormont medical services division’s medical staff.
Dr. Dishman came to Stormont in 2000 to work as a hospitalist. Most recently, he has served as the center’s vice president of acute care services. In his new role, Dr. Dishman will be charged with, among other duties, seeking out physicians to bring to Stormont’s Topeka location.
BUSINESS MOVES
The Hazel Hawkins Memorial Hospital Women’s Center (Hollister, Calif.) recently established a relationship with Pediatrix Medical Group (Sunrise, Fla.) to provide pediatric hospitalists to help with high-risk delivery of newborns. The hospitalists also will advise Hazel Hawkins staff with regards to critical care transport and assist with the care of newborns and the treatment of child and teen patients.
Hazel Hawkins has been in operation for the past 5 years. Pediatrix hospitalists will be used as consultants for attending staff and emergency physicians and will help treat patients in emergency situations.
American Physician Partners (Brentwood, Tenn.), a national hospital medicine management services company, has acquired private physician group Progressive Medical Associates (Mesa, Ariz.). Progressive’s 37 physicians and 21 private clinicians – working at Banner Health’s 28 nonprofit hospitals covering six states – join the APP team.
Flora Kisuule, MD, SFHM, has been awarded the 2018 Excellence in Service and Professionalism Award by Johns Hopkins Bayview Medical Center, Baltimore. Dr. Kisuule is associate director of the division of hospital medicine at Johns Hopkins Bayview, assistant professor at Johns Hopkins University School of Medicine, and assistant professor in the Johns Hopkins Bloomberg School of Public Health.
Dr. Kisuule codeveloped a hospitalist fellowship program for Johns Hopkins University, which she now directs. She has served on several Society of Hospital Medicine committees and consulted on hospitalist programs around the world. Dr. Kisuule is joining the SHM Board of Directors in March 2019.
Paul Hain, DO, was promoted recently to chief medical officer and senior vice president of market delivery for Blue Cross/Blue Shield of Texas. Dr. Hain has a wealth of experience in leadership, including helping create the pediatric hospitalist program at Vanderbilt Children’s Hospital in Nashville, Tenn.
With BC/BS, Dr. Hain will oversee the region’s public relations, community investments, government relations, and lobbying. He will help sponsor Medicare and Medicaid in the state, while also supporting enterprise and marketing efforts. Dr. Hain joins BC/BS after serving as vice president and medical director of population health and network development at the Children’s Medical Center of Dallas.
Laura M. Rosch, DO, recently was selected by Kansas City University as the new campus dean in the Joplin (Mo.) College of Osteopathic Medicine. Dr. Rosch is a practicing hospitalist in Illinois, where she was chair of internal medicine at the Chicago College of Osteopathic Medicine prior to taking her current position.
Dr. Rosch will manage daily operations for the Joplin medical school, streamlining the school with the main campus. She is a former president of the Illinois Osteopathic Medicine Society and holds a master’s degree in nutrition science.
Suzan Lowry, MD, has been named health officer for Charles County, Md., by the county Department of Health and Charles County Commissioners. A longtime pediatrician with 20 years’ experience, Dr. Lowry has served as a pediatric hospitalist educator at Children’s National Medical Center in Washington, D.C.
Dr. Lowry has spent a majority of her career working on behalf of public health. Most recently, she has worked at the United States Marine Corps Quantico Health Clinic in Virginia.
Robyn Chase, DO, a staff hospitalist at Yavapai Regional Medical Center (Prescott, Ariz.), recently was selected as the hospital’s Physician of the Year for 2018.
Dr. Chase has practiced at YRMC since 2010 and is a board-certified internist. She also serves as an associate professor at the University of Arizona, Phoenix.
Kevin Dishman, MD, has been elevated to senior vice president and chief medical officer at Stormont Vail Health (Topeka, Kan.). Dr. Dishman also will be president of Stormont medical services division’s medical staff.
Dr. Dishman came to Stormont in 2000 to work as a hospitalist. Most recently, he has served as the center’s vice president of acute care services. In his new role, Dr. Dishman will be charged with, among other duties, seeking out physicians to bring to Stormont’s Topeka location.
BUSINESS MOVES
The Hazel Hawkins Memorial Hospital Women’s Center (Hollister, Calif.) recently established a relationship with Pediatrix Medical Group (Sunrise, Fla.) to provide pediatric hospitalists to help with high-risk delivery of newborns. The hospitalists also will advise Hazel Hawkins staff with regards to critical care transport and assist with the care of newborns and the treatment of child and teen patients.
Hazel Hawkins has been in operation for the past 5 years. Pediatrix hospitalists will be used as consultants for attending staff and emergency physicians and will help treat patients in emergency situations.
American Physician Partners (Brentwood, Tenn.), a national hospital medicine management services company, has acquired private physician group Progressive Medical Associates (Mesa, Ariz.). Progressive’s 37 physicians and 21 private clinicians – working at Banner Health’s 28 nonprofit hospitals covering six states – join the APP team.
Flora Kisuule, MD, SFHM, has been awarded the 2018 Excellence in Service and Professionalism Award by Johns Hopkins Bayview Medical Center, Baltimore. Dr. Kisuule is associate director of the division of hospital medicine at Johns Hopkins Bayview, assistant professor at Johns Hopkins University School of Medicine, and assistant professor in the Johns Hopkins Bloomberg School of Public Health.
Dr. Kisuule codeveloped a hospitalist fellowship program for Johns Hopkins University, which she now directs. She has served on several Society of Hospital Medicine committees and consulted on hospitalist programs around the world. Dr. Kisuule is joining the SHM Board of Directors in March 2019.
Paul Hain, DO, was promoted recently to chief medical officer and senior vice president of market delivery for Blue Cross/Blue Shield of Texas. Dr. Hain has a wealth of experience in leadership, including helping create the pediatric hospitalist program at Vanderbilt Children’s Hospital in Nashville, Tenn.
With BC/BS, Dr. Hain will oversee the region’s public relations, community investments, government relations, and lobbying. He will help sponsor Medicare and Medicaid in the state, while also supporting enterprise and marketing efforts. Dr. Hain joins BC/BS after serving as vice president and medical director of population health and network development at the Children’s Medical Center of Dallas.
Laura M. Rosch, DO, recently was selected by Kansas City University as the new campus dean in the Joplin (Mo.) College of Osteopathic Medicine. Dr. Rosch is a practicing hospitalist in Illinois, where she was chair of internal medicine at the Chicago College of Osteopathic Medicine prior to taking her current position.
Dr. Rosch will manage daily operations for the Joplin medical school, streamlining the school with the main campus. She is a former president of the Illinois Osteopathic Medicine Society and holds a master’s degree in nutrition science.
Suzan Lowry, MD, has been named health officer for Charles County, Md., by the county Department of Health and Charles County Commissioners. A longtime pediatrician with 20 years’ experience, Dr. Lowry has served as a pediatric hospitalist educator at Children’s National Medical Center in Washington, D.C.
Dr. Lowry has spent a majority of her career working on behalf of public health. Most recently, she has worked at the United States Marine Corps Quantico Health Clinic in Virginia.
Robyn Chase, DO, a staff hospitalist at Yavapai Regional Medical Center (Prescott, Ariz.), recently was selected as the hospital’s Physician of the Year for 2018.
Dr. Chase has practiced at YRMC since 2010 and is a board-certified internist. She also serves as an associate professor at the University of Arizona, Phoenix.
Kevin Dishman, MD, has been elevated to senior vice president and chief medical officer at Stormont Vail Health (Topeka, Kan.). Dr. Dishman also will be president of Stormont medical services division’s medical staff.
Dr. Dishman came to Stormont in 2000 to work as a hospitalist. Most recently, he has served as the center’s vice president of acute care services. In his new role, Dr. Dishman will be charged with, among other duties, seeking out physicians to bring to Stormont’s Topeka location.
BUSINESS MOVES
The Hazel Hawkins Memorial Hospital Women’s Center (Hollister, Calif.) recently established a relationship with Pediatrix Medical Group (Sunrise, Fla.) to provide pediatric hospitalists to help with high-risk delivery of newborns. The hospitalists also will advise Hazel Hawkins staff with regards to critical care transport and assist with the care of newborns and the treatment of child and teen patients.
Hazel Hawkins has been in operation for the past 5 years. Pediatrix hospitalists will be used as consultants for attending staff and emergency physicians and will help treat patients in emergency situations.
American Physician Partners (Brentwood, Tenn.), a national hospital medicine management services company, has acquired private physician group Progressive Medical Associates (Mesa, Ariz.). Progressive’s 37 physicians and 21 private clinicians – working at Banner Health’s 28 nonprofit hospitals covering six states – join the APP team.
Rounding team boosts ICU liberation efforts
SAN DIEGO – A rounding team formed to oversee implementation of a bundle of ICU interventions reduced the incidence of ventilator-associated pneumonia (VAP) and the number of ventilation days, as well as the ICU and hospital length of stay, according to a new study conducted at a level 1 trauma center in California. The rounding team worked toward optimal implementation of the Society of Critical Care Medicine’s ABCDEF bundle, part of the society’s ICU liberation initiative.
ABCDEF stands for: Assessment, prevention, and management of pain; Both spontaneous awakening and breathing trials; Choice of analgesia and sedation; Delirium assessment, prevention, and management; Early mobility and exercise; and Family engagement and empowerment.
The Community Regional Medical Center in Fresno, Calif., where the study was conducted, was chosen in 2015 to participate in the ICU liberation initiative. The facility serves a population of 3.2 million and sees just under 4,000 trauma patients per year.
After a 6-month retrospective analysis, the team members at the medical center realized they needed to improve ABCDEF implementation with respect to evaluating sedation practices and improving delirium assessment.
Before the start of the 17-month collaborative period, they formed an ICU liberation team called SMART, short for Sedation, Mobilization, Assessment Rounding Team, which included representatives from ICU nursing, pharmacy, respiratory therapy, physical therapy, physicians, and administration. They developed a daily rounding tool to help the team implement procedures, with the goal of reducing the continuous infusion of benzodiazepines and increasing intermittent dosing, the use of short-acting medications, and conducting spontaneous awakening and breathing trials. The SMART team made daily rounds to ensure that the ABCDEF bundle was being implemented.
The researchers then continued the analysis for another 12 months after the end of the initiative. During this last phase, the benefits of the SMART team became evident.
“Stick with it. Don’t let up. Don’t quit,” Wade Veneman, a respiratory therapist at the medical center, said in an interview. He presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “It can be particularly difficult in the face of critical care providers who may be skeptical of new initiatives. They think it’s something new, and they hope that it goes away. But this is something we feel we’re going to keep for a long time,” he added.
Mr. Veneman hopes to implement the SMART program in the neurological critical care ICU. The medical director of that unit did not participate in the initial collaborative, but Mr. Veneman hopes to change that. “The data is going to show that his VAP and ventilator days are going up, and everywhere else they’re going down,” he said.
The researchers analyzed data on 1,127 mechanically ventilated patients in the ICU. At total of 197 patients were treated 6 months before the implementation of the collaborative, 519 during 17 months of collaborative implementation, and 411 in the 12 months after implementation. There were some differences between the populations: The before group was slightly younger than the after-implementation group (mean 41 vs. 44, P = .04), and the mean Injury Severity Score score was 24 in the before group, 22 during, and 20 after (P = .002). The researchers noted that the differences were clinically significant.
Benzodiazepine use declined, but the effect was statistically significant only in the after population. Continuous use declined from 87% before implementation to 83% during (P = .21) and 53% after (P less than .001). Intermittent use was 57% before implementation, increased to 61% during (P = .44), and fell to 44% after (P less than .001). Delirium assessment performance improved throughout, from 9% before implementation to 42% during (P less than .001) to 73% after implementation (P less than .001).
The VAP rate increased from 3.4% before the SMART program to 4.5% during implementation (P = .53), and then dropped to 0.9% afterward (P = .001). Ventilation days started at a mean of 10.5, then dropped to 9.5 during implementation (P = .30), and 8.2 after implementation (P = .027).
ICU length of stay improved from 10.7 before implementation to 9.3 afterward (P = .021), and overall hospital length of stay went from 17.3 days to 16.3 (P = .005).
The study was not funded. Mr. Veneman has no relevant financial disclosures.
SOURCE: Veneman W et al. CCC48, Abstract 63.
SAN DIEGO – A rounding team formed to oversee implementation of a bundle of ICU interventions reduced the incidence of ventilator-associated pneumonia (VAP) and the number of ventilation days, as well as the ICU and hospital length of stay, according to a new study conducted at a level 1 trauma center in California. The rounding team worked toward optimal implementation of the Society of Critical Care Medicine’s ABCDEF bundle, part of the society’s ICU liberation initiative.
ABCDEF stands for: Assessment, prevention, and management of pain; Both spontaneous awakening and breathing trials; Choice of analgesia and sedation; Delirium assessment, prevention, and management; Early mobility and exercise; and Family engagement and empowerment.
The Community Regional Medical Center in Fresno, Calif., where the study was conducted, was chosen in 2015 to participate in the ICU liberation initiative. The facility serves a population of 3.2 million and sees just under 4,000 trauma patients per year.
After a 6-month retrospective analysis, the team members at the medical center realized they needed to improve ABCDEF implementation with respect to evaluating sedation practices and improving delirium assessment.
Before the start of the 17-month collaborative period, they formed an ICU liberation team called SMART, short for Sedation, Mobilization, Assessment Rounding Team, which included representatives from ICU nursing, pharmacy, respiratory therapy, physical therapy, physicians, and administration. They developed a daily rounding tool to help the team implement procedures, with the goal of reducing the continuous infusion of benzodiazepines and increasing intermittent dosing, the use of short-acting medications, and conducting spontaneous awakening and breathing trials. The SMART team made daily rounds to ensure that the ABCDEF bundle was being implemented.
The researchers then continued the analysis for another 12 months after the end of the initiative. During this last phase, the benefits of the SMART team became evident.
“Stick with it. Don’t let up. Don’t quit,” Wade Veneman, a respiratory therapist at the medical center, said in an interview. He presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “It can be particularly difficult in the face of critical care providers who may be skeptical of new initiatives. They think it’s something new, and they hope that it goes away. But this is something we feel we’re going to keep for a long time,” he added.
Mr. Veneman hopes to implement the SMART program in the neurological critical care ICU. The medical director of that unit did not participate in the initial collaborative, but Mr. Veneman hopes to change that. “The data is going to show that his VAP and ventilator days are going up, and everywhere else they’re going down,” he said.
The researchers analyzed data on 1,127 mechanically ventilated patients in the ICU. At total of 197 patients were treated 6 months before the implementation of the collaborative, 519 during 17 months of collaborative implementation, and 411 in the 12 months after implementation. There were some differences between the populations: The before group was slightly younger than the after-implementation group (mean 41 vs. 44, P = .04), and the mean Injury Severity Score score was 24 in the before group, 22 during, and 20 after (P = .002). The researchers noted that the differences were clinically significant.
Benzodiazepine use declined, but the effect was statistically significant only in the after population. Continuous use declined from 87% before implementation to 83% during (P = .21) and 53% after (P less than .001). Intermittent use was 57% before implementation, increased to 61% during (P = .44), and fell to 44% after (P less than .001). Delirium assessment performance improved throughout, from 9% before implementation to 42% during (P less than .001) to 73% after implementation (P less than .001).
The VAP rate increased from 3.4% before the SMART program to 4.5% during implementation (P = .53), and then dropped to 0.9% afterward (P = .001). Ventilation days started at a mean of 10.5, then dropped to 9.5 during implementation (P = .30), and 8.2 after implementation (P = .027).
ICU length of stay improved from 10.7 before implementation to 9.3 afterward (P = .021), and overall hospital length of stay went from 17.3 days to 16.3 (P = .005).
The study was not funded. Mr. Veneman has no relevant financial disclosures.
SOURCE: Veneman W et al. CCC48, Abstract 63.
SAN DIEGO – A rounding team formed to oversee implementation of a bundle of ICU interventions reduced the incidence of ventilator-associated pneumonia (VAP) and the number of ventilation days, as well as the ICU and hospital length of stay, according to a new study conducted at a level 1 trauma center in California. The rounding team worked toward optimal implementation of the Society of Critical Care Medicine’s ABCDEF bundle, part of the society’s ICU liberation initiative.
ABCDEF stands for: Assessment, prevention, and management of pain; Both spontaneous awakening and breathing trials; Choice of analgesia and sedation; Delirium assessment, prevention, and management; Early mobility and exercise; and Family engagement and empowerment.
The Community Regional Medical Center in Fresno, Calif., where the study was conducted, was chosen in 2015 to participate in the ICU liberation initiative. The facility serves a population of 3.2 million and sees just under 4,000 trauma patients per year.
After a 6-month retrospective analysis, the team members at the medical center realized they needed to improve ABCDEF implementation with respect to evaluating sedation practices and improving delirium assessment.
Before the start of the 17-month collaborative period, they formed an ICU liberation team called SMART, short for Sedation, Mobilization, Assessment Rounding Team, which included representatives from ICU nursing, pharmacy, respiratory therapy, physical therapy, physicians, and administration. They developed a daily rounding tool to help the team implement procedures, with the goal of reducing the continuous infusion of benzodiazepines and increasing intermittent dosing, the use of short-acting medications, and conducting spontaneous awakening and breathing trials. The SMART team made daily rounds to ensure that the ABCDEF bundle was being implemented.
The researchers then continued the analysis for another 12 months after the end of the initiative. During this last phase, the benefits of the SMART team became evident.
“Stick with it. Don’t let up. Don’t quit,” Wade Veneman, a respiratory therapist at the medical center, said in an interview. He presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “It can be particularly difficult in the face of critical care providers who may be skeptical of new initiatives. They think it’s something new, and they hope that it goes away. But this is something we feel we’re going to keep for a long time,” he added.
Mr. Veneman hopes to implement the SMART program in the neurological critical care ICU. The medical director of that unit did not participate in the initial collaborative, but Mr. Veneman hopes to change that. “The data is going to show that his VAP and ventilator days are going up, and everywhere else they’re going down,” he said.
The researchers analyzed data on 1,127 mechanically ventilated patients in the ICU. At total of 197 patients were treated 6 months before the implementation of the collaborative, 519 during 17 months of collaborative implementation, and 411 in the 12 months after implementation. There were some differences between the populations: The before group was slightly younger than the after-implementation group (mean 41 vs. 44, P = .04), and the mean Injury Severity Score score was 24 in the before group, 22 during, and 20 after (P = .002). The researchers noted that the differences were clinically significant.
Benzodiazepine use declined, but the effect was statistically significant only in the after population. Continuous use declined from 87% before implementation to 83% during (P = .21) and 53% after (P less than .001). Intermittent use was 57% before implementation, increased to 61% during (P = .44), and fell to 44% after (P less than .001). Delirium assessment performance improved throughout, from 9% before implementation to 42% during (P less than .001) to 73% after implementation (P less than .001).
The VAP rate increased from 3.4% before the SMART program to 4.5% during implementation (P = .53), and then dropped to 0.9% afterward (P = .001). Ventilation days started at a mean of 10.5, then dropped to 9.5 during implementation (P = .30), and 8.2 after implementation (P = .027).
ICU length of stay improved from 10.7 before implementation to 9.3 afterward (P = .021), and overall hospital length of stay went from 17.3 days to 16.3 (P = .005).
The study was not funded. Mr. Veneman has no relevant financial disclosures.
SOURCE: Veneman W et al. CCC48, Abstract 63.
REPORTING FROM CCC48
Glyceryl trinitrate does not improve outcomes of ischemic stroke
HONOLULU – , according to data presented at the International Stroke Conference sponsored by the American Heart Association. Results suggest that GTN causes adverse effects in patients with intracerebral hemorrhage (ICH), but this observation is not definitive, according to the researchers. Study results were published online ahead of print Feb. 6 in the Lancet.
Nitric oxide is a regulatory molecule that has vasoactive effects and promotes blood pressure reduction. Vascular levels of nitric oxide are low in stroke, which suggests that the molecule may be a target for stroke treatment. GTN, a nitric oxide donor, lowered blood pressure and improved functional outcome among patients with acute stroke in the phase 2 Rapid Intervention with GTN in Hypertensive Stroke Trial (RIGHT).
Philip Bath, MD, Stroke Association Professor of Stroke Medicine at the University of Nottingham (England), and colleagues conducted the RIGHT-2 study to evaluate the safety and efficacy of GTN when administered early after onset of suspected stroke. Paramedics randomized patients in equal groups to a GTN patch or a sham patch in the ambulance. Three more patches were administered in the hospital on the following days. Active and sham patches looked similar and had no writing on them, thus ensuring effective blinding upon administration. Investigators followed up patients by telephone at 90 days to assess the modified Rankin Scale score and markers of disability, mood, cognition, and quality of life.
Eligible participants were adults who had dialed emergency services, independently or with assistance, because of a possible stroke. They had a Face, Arm, Speech, Time (FAST) score of 2 or 3, were within 4 hours of onset, and had a systolic blood pressure greater than 120 mm Hg. Patients from nursing homes, those with hypoglycemia, those who were unconscious, and those with a witnessed seizure were excluded.
Dr. Bath and colleagues planned to enroll 850 patients from five ambulance services in 30 hospitals across the United Kingdom. Data were to be examined through an intention-to-treat analysis. During the trial, however, the investigators observed that the rate of stroke mimics was 26%, rather than the 12% that they had anticipated. To ensure the proper power for the study, the investigators increased the sample size to 1,149 patients. They also changed the planned data analysis from intention-to-treat to hierarchical analysis. Specifically, the researchers planned to perform the primary analysis in patients with stroke or TIA. If the results were positive, then they would perform a standard intention-to-treat analysis.
More than 99% of patients received the first patch. Approximately 57% of the population received the first two patches. One reason for this decrease in adherence was that many patients were discharged from the hospital with a TIA or a stroke mimic. Participants’ average age was 72. The median time from onset to randomization was 71 minutes, and the median time to treatment was 73 minutes. Participants’ mean systolic blood pressure was 162 mm Hg. Approximately 60% of the patients had a FAST score of 3. About 50% of participants had ischemic stroke, 13% had ICH, 10% had TIA, and 26% had stroke mimics.
At 1 hour after treatment initiation, systolic blood pressure decreased by 6.2 mm Hg and diastolic blood pressure decreased by 2.7 mm Hg among patients who received GTN, compared with controls. At one day, the differences were 5.2 mm Hg and 2.5 mm Hg, respectively, in treated patients, compared with controls. Blood pressure became similar between groups thereafter, “in part because of the tachyphylaxis that we know happens with GTN,” said Dr. Bath.
The researchers found no evidence of an effect of GTN on functional outcome at 90 days in participants with stroke or transient ischemic attack. The adjusted common odds ratio of poor outcome was 1.25 in the GTN group, compared with the control group (95 % confidence interval, 0.97-1.60; P = .083). “We were close to getting a negative trial,” said Dr. Bath.
Subgroup analyses revealed differences in outcome according to the time to randomization. GTN had a negative effect in patients treated within 1 hour of onset. Results were neutral, but tended to be negative, in patients treated between 1 and 2 hours of onset. Results were neutral, but tended to be positive, among patients treated at more than 2 hours after onset. There was no difference between groups in the rate of mortality.
One of the study’s limitations was its single-blind design. In addition, the trial was conducted in a single country, and the investigators changed the protocol after it was initiated. “We had a higher-than-expected [stroke] mimic rate, although I’m reassured by most experts that ... this is probably about right,” said Dr. Bath.
A potential reason for the neutral results is the negative effect that GTN had among patients with ICH, said Dr. Bath. “In that very early first hour, we are of course breaking a law that we learned in medical school, which is that the first part of hemostasis is spasm. We gave an antispasmodic: a vasodilator,” he added. “That is speculation.”
The trial was funded by the British Heart Foundation. Dr. Bath declared a modest ownership interest in Platelet Solutions and consultant or advisory board positions with Moleac, DiaMedica, Phagenesis, Nestle, and ReNeuron. The other investigators declared no conflicts of interest.
The RIGHT-2 trial shows the limitations of a prehospital enrollment model, wrote Karen C. Johnston, MD, professor of neurology at the University of Virginia in Charlottesville, and Valerie L. Durkalski-Mauldin, PhD, professor of medicine at Medical University of South Carolina in Charleston, in an editorial accompanying the RIGHT-2 trial results. The rate of nonstroke diagnoses was so high that it would have reduced the study’s power to assess the efficacy of glyceryl trinitrate (GTN), had the investigators not increased the sample size and changed the statistical analysis plan.
“Future prehospital trials need to consider the implications of enrolling, yet excluding, stroke mimics in the primary analysis,” said Dr. Johnston and Dr. Durkalski-Mauldin. Using telemedicine in the ambulance to facilitate direct contact between the stroke provider and the patient and emergency medical services provider could reduce the enrollment of patients with stroke mimics in clinical trials, they added. “Improved tools to exclude stroke mimics in the field have been difficult to develop and validate. The absence of imaging in most ambulances will continue to limit field personnel from definitively determining ischemic stroke from intracerebral hemorrhage, which will limit hyperacute trials to interventions presumed safe in both populations.”
In addition, the blood pressure reduction that GTN provided might not be clinically relevant, said Dr. Johnston and Dr. Durkalski-Mauldin. “The RIGHT-2 investigators report no difference in blood pressure at day 3 or day 4 of treatment, which might have been related to the very low adherence to study protocol by day 4.
“Regardless of these limitations, RIGHT-2 has provided high-level evidence that GTN given within 4 hours of onset does not significantly improve outcome in hyperacute patients presenting with possible stroke,” the authors concluded (Lancet. 2019 Feb 6. doi: 10.1016/
S0140-6736(19)30276-4). Dr. Johnston and Dr. Durkalski-Mauldin declared no conflicts of interest.
The RIGHT-2 trial shows the limitations of a prehospital enrollment model, wrote Karen C. Johnston, MD, professor of neurology at the University of Virginia in Charlottesville, and Valerie L. Durkalski-Mauldin, PhD, professor of medicine at Medical University of South Carolina in Charleston, in an editorial accompanying the RIGHT-2 trial results. The rate of nonstroke diagnoses was so high that it would have reduced the study’s power to assess the efficacy of glyceryl trinitrate (GTN), had the investigators not increased the sample size and changed the statistical analysis plan.
“Future prehospital trials need to consider the implications of enrolling, yet excluding, stroke mimics in the primary analysis,” said Dr. Johnston and Dr. Durkalski-Mauldin. Using telemedicine in the ambulance to facilitate direct contact between the stroke provider and the patient and emergency medical services provider could reduce the enrollment of patients with stroke mimics in clinical trials, they added. “Improved tools to exclude stroke mimics in the field have been difficult to develop and validate. The absence of imaging in most ambulances will continue to limit field personnel from definitively determining ischemic stroke from intracerebral hemorrhage, which will limit hyperacute trials to interventions presumed safe in both populations.”
In addition, the blood pressure reduction that GTN provided might not be clinically relevant, said Dr. Johnston and Dr. Durkalski-Mauldin. “The RIGHT-2 investigators report no difference in blood pressure at day 3 or day 4 of treatment, which might have been related to the very low adherence to study protocol by day 4.
“Regardless of these limitations, RIGHT-2 has provided high-level evidence that GTN given within 4 hours of onset does not significantly improve outcome in hyperacute patients presenting with possible stroke,” the authors concluded (Lancet. 2019 Feb 6. doi: 10.1016/
S0140-6736(19)30276-4). Dr. Johnston and Dr. Durkalski-Mauldin declared no conflicts of interest.
The RIGHT-2 trial shows the limitations of a prehospital enrollment model, wrote Karen C. Johnston, MD, professor of neurology at the University of Virginia in Charlottesville, and Valerie L. Durkalski-Mauldin, PhD, professor of medicine at Medical University of South Carolina in Charleston, in an editorial accompanying the RIGHT-2 trial results. The rate of nonstroke diagnoses was so high that it would have reduced the study’s power to assess the efficacy of glyceryl trinitrate (GTN), had the investigators not increased the sample size and changed the statistical analysis plan.
“Future prehospital trials need to consider the implications of enrolling, yet excluding, stroke mimics in the primary analysis,” said Dr. Johnston and Dr. Durkalski-Mauldin. Using telemedicine in the ambulance to facilitate direct contact between the stroke provider and the patient and emergency medical services provider could reduce the enrollment of patients with stroke mimics in clinical trials, they added. “Improved tools to exclude stroke mimics in the field have been difficult to develop and validate. The absence of imaging in most ambulances will continue to limit field personnel from definitively determining ischemic stroke from intracerebral hemorrhage, which will limit hyperacute trials to interventions presumed safe in both populations.”
In addition, the blood pressure reduction that GTN provided might not be clinically relevant, said Dr. Johnston and Dr. Durkalski-Mauldin. “The RIGHT-2 investigators report no difference in blood pressure at day 3 or day 4 of treatment, which might have been related to the very low adherence to study protocol by day 4.
“Regardless of these limitations, RIGHT-2 has provided high-level evidence that GTN given within 4 hours of onset does not significantly improve outcome in hyperacute patients presenting with possible stroke,” the authors concluded (Lancet. 2019 Feb 6. doi: 10.1016/
S0140-6736(19)30276-4). Dr. Johnston and Dr. Durkalski-Mauldin declared no conflicts of interest.
HONOLULU – , according to data presented at the International Stroke Conference sponsored by the American Heart Association. Results suggest that GTN causes adverse effects in patients with intracerebral hemorrhage (ICH), but this observation is not definitive, according to the researchers. Study results were published online ahead of print Feb. 6 in the Lancet.
Nitric oxide is a regulatory molecule that has vasoactive effects and promotes blood pressure reduction. Vascular levels of nitric oxide are low in stroke, which suggests that the molecule may be a target for stroke treatment. GTN, a nitric oxide donor, lowered blood pressure and improved functional outcome among patients with acute stroke in the phase 2 Rapid Intervention with GTN in Hypertensive Stroke Trial (RIGHT).
Philip Bath, MD, Stroke Association Professor of Stroke Medicine at the University of Nottingham (England), and colleagues conducted the RIGHT-2 study to evaluate the safety and efficacy of GTN when administered early after onset of suspected stroke. Paramedics randomized patients in equal groups to a GTN patch or a sham patch in the ambulance. Three more patches were administered in the hospital on the following days. Active and sham patches looked similar and had no writing on them, thus ensuring effective blinding upon administration. Investigators followed up patients by telephone at 90 days to assess the modified Rankin Scale score and markers of disability, mood, cognition, and quality of life.
Eligible participants were adults who had dialed emergency services, independently or with assistance, because of a possible stroke. They had a Face, Arm, Speech, Time (FAST) score of 2 or 3, were within 4 hours of onset, and had a systolic blood pressure greater than 120 mm Hg. Patients from nursing homes, those with hypoglycemia, those who were unconscious, and those with a witnessed seizure were excluded.
Dr. Bath and colleagues planned to enroll 850 patients from five ambulance services in 30 hospitals across the United Kingdom. Data were to be examined through an intention-to-treat analysis. During the trial, however, the investigators observed that the rate of stroke mimics was 26%, rather than the 12% that they had anticipated. To ensure the proper power for the study, the investigators increased the sample size to 1,149 patients. They also changed the planned data analysis from intention-to-treat to hierarchical analysis. Specifically, the researchers planned to perform the primary analysis in patients with stroke or TIA. If the results were positive, then they would perform a standard intention-to-treat analysis.
More than 99% of patients received the first patch. Approximately 57% of the population received the first two patches. One reason for this decrease in adherence was that many patients were discharged from the hospital with a TIA or a stroke mimic. Participants’ average age was 72. The median time from onset to randomization was 71 minutes, and the median time to treatment was 73 minutes. Participants’ mean systolic blood pressure was 162 mm Hg. Approximately 60% of the patients had a FAST score of 3. About 50% of participants had ischemic stroke, 13% had ICH, 10% had TIA, and 26% had stroke mimics.
At 1 hour after treatment initiation, systolic blood pressure decreased by 6.2 mm Hg and diastolic blood pressure decreased by 2.7 mm Hg among patients who received GTN, compared with controls. At one day, the differences were 5.2 mm Hg and 2.5 mm Hg, respectively, in treated patients, compared with controls. Blood pressure became similar between groups thereafter, “in part because of the tachyphylaxis that we know happens with GTN,” said Dr. Bath.
The researchers found no evidence of an effect of GTN on functional outcome at 90 days in participants with stroke or transient ischemic attack. The adjusted common odds ratio of poor outcome was 1.25 in the GTN group, compared with the control group (95 % confidence interval, 0.97-1.60; P = .083). “We were close to getting a negative trial,” said Dr. Bath.
Subgroup analyses revealed differences in outcome according to the time to randomization. GTN had a negative effect in patients treated within 1 hour of onset. Results were neutral, but tended to be negative, in patients treated between 1 and 2 hours of onset. Results were neutral, but tended to be positive, among patients treated at more than 2 hours after onset. There was no difference between groups in the rate of mortality.
One of the study’s limitations was its single-blind design. In addition, the trial was conducted in a single country, and the investigators changed the protocol after it was initiated. “We had a higher-than-expected [stroke] mimic rate, although I’m reassured by most experts that ... this is probably about right,” said Dr. Bath.
A potential reason for the neutral results is the negative effect that GTN had among patients with ICH, said Dr. Bath. “In that very early first hour, we are of course breaking a law that we learned in medical school, which is that the first part of hemostasis is spasm. We gave an antispasmodic: a vasodilator,” he added. “That is speculation.”
The trial was funded by the British Heart Foundation. Dr. Bath declared a modest ownership interest in Platelet Solutions and consultant or advisory board positions with Moleac, DiaMedica, Phagenesis, Nestle, and ReNeuron. The other investigators declared no conflicts of interest.
HONOLULU – , according to data presented at the International Stroke Conference sponsored by the American Heart Association. Results suggest that GTN causes adverse effects in patients with intracerebral hemorrhage (ICH), but this observation is not definitive, according to the researchers. Study results were published online ahead of print Feb. 6 in the Lancet.
Nitric oxide is a regulatory molecule that has vasoactive effects and promotes blood pressure reduction. Vascular levels of nitric oxide are low in stroke, which suggests that the molecule may be a target for stroke treatment. GTN, a nitric oxide donor, lowered blood pressure and improved functional outcome among patients with acute stroke in the phase 2 Rapid Intervention with GTN in Hypertensive Stroke Trial (RIGHT).
Philip Bath, MD, Stroke Association Professor of Stroke Medicine at the University of Nottingham (England), and colleagues conducted the RIGHT-2 study to evaluate the safety and efficacy of GTN when administered early after onset of suspected stroke. Paramedics randomized patients in equal groups to a GTN patch or a sham patch in the ambulance. Three more patches were administered in the hospital on the following days. Active and sham patches looked similar and had no writing on them, thus ensuring effective blinding upon administration. Investigators followed up patients by telephone at 90 days to assess the modified Rankin Scale score and markers of disability, mood, cognition, and quality of life.
Eligible participants were adults who had dialed emergency services, independently or with assistance, because of a possible stroke. They had a Face, Arm, Speech, Time (FAST) score of 2 or 3, were within 4 hours of onset, and had a systolic blood pressure greater than 120 mm Hg. Patients from nursing homes, those with hypoglycemia, those who were unconscious, and those with a witnessed seizure were excluded.
Dr. Bath and colleagues planned to enroll 850 patients from five ambulance services in 30 hospitals across the United Kingdom. Data were to be examined through an intention-to-treat analysis. During the trial, however, the investigators observed that the rate of stroke mimics was 26%, rather than the 12% that they had anticipated. To ensure the proper power for the study, the investigators increased the sample size to 1,149 patients. They also changed the planned data analysis from intention-to-treat to hierarchical analysis. Specifically, the researchers planned to perform the primary analysis in patients with stroke or TIA. If the results were positive, then they would perform a standard intention-to-treat analysis.
More than 99% of patients received the first patch. Approximately 57% of the population received the first two patches. One reason for this decrease in adherence was that many patients were discharged from the hospital with a TIA or a stroke mimic. Participants’ average age was 72. The median time from onset to randomization was 71 minutes, and the median time to treatment was 73 minutes. Participants’ mean systolic blood pressure was 162 mm Hg. Approximately 60% of the patients had a FAST score of 3. About 50% of participants had ischemic stroke, 13% had ICH, 10% had TIA, and 26% had stroke mimics.
At 1 hour after treatment initiation, systolic blood pressure decreased by 6.2 mm Hg and diastolic blood pressure decreased by 2.7 mm Hg among patients who received GTN, compared with controls. At one day, the differences were 5.2 mm Hg and 2.5 mm Hg, respectively, in treated patients, compared with controls. Blood pressure became similar between groups thereafter, “in part because of the tachyphylaxis that we know happens with GTN,” said Dr. Bath.
The researchers found no evidence of an effect of GTN on functional outcome at 90 days in participants with stroke or transient ischemic attack. The adjusted common odds ratio of poor outcome was 1.25 in the GTN group, compared with the control group (95 % confidence interval, 0.97-1.60; P = .083). “We were close to getting a negative trial,” said Dr. Bath.
Subgroup analyses revealed differences in outcome according to the time to randomization. GTN had a negative effect in patients treated within 1 hour of onset. Results were neutral, but tended to be negative, in patients treated between 1 and 2 hours of onset. Results were neutral, but tended to be positive, among patients treated at more than 2 hours after onset. There was no difference between groups in the rate of mortality.
One of the study’s limitations was its single-blind design. In addition, the trial was conducted in a single country, and the investigators changed the protocol after it was initiated. “We had a higher-than-expected [stroke] mimic rate, although I’m reassured by most experts that ... this is probably about right,” said Dr. Bath.
A potential reason for the neutral results is the negative effect that GTN had among patients with ICH, said Dr. Bath. “In that very early first hour, we are of course breaking a law that we learned in medical school, which is that the first part of hemostasis is spasm. We gave an antispasmodic: a vasodilator,” he added. “That is speculation.”
The trial was funded by the British Heart Foundation. Dr. Bath declared a modest ownership interest in Platelet Solutions and consultant or advisory board positions with Moleac, DiaMedica, Phagenesis, Nestle, and ReNeuron. The other investigators declared no conflicts of interest.
REPORTING FROM ISC 2019
Hospitalist scheduling: A search for balance
Survey says ...
Scheduling. Has there ever been such a simple word that is so complex? A simple Internet search of hospitalist scheduling returns thousands of possible discussions, leaving readers to conclude that the possibilities are endless and the challenges great. The answer certainly is not a one-size-fits-all approach.
Hospitalist scheduling is one of the key sections in the 2018 State of Hospital Medicine (SoHM) report; the 2018 report delves deeper into hospitalist scheduling than ever before.
For those of you who have been regular users of prior SoHM reports, you should be pleasantly surprised to find new comparative values: There are nearly 50% more pages dedicated just to scheduling!
For those readers who have never subscribed to the SoHM Report, this is your chance to study how other groups approach hospitalist schedules.
Why is hospitalist scheduling such a hot topic? For one, flexible and sustainable scheduling is an important contributor to job satisfaction. It is important for hospitalists to have a high degree of input into managing and effecting change for personal work-life balance.
As John Nelson, MD, MHM, a cofounder of the Society of Hospital Medicine, wrote recently in The Hospitalist, “an optimal schedule alone isn’t the key to preventing it [burnout], but maybe a good schedule can reduce your risk you’ll suffer from it.”
Secondly, ensuring that the hospitalist team is right sized – that is, scheduling hospitalists in the right place at the right time – is an art. Using resources, such as the 2018 SoHM report, to identify quantifiable comparisons enables hospitalist groups to continuously ensure the hospitalist schedule meets the clinical demands while optimizing the hospitalist group’s schedule.
Unfilled positions
The 2018 SoHM report features a new section on unfilled positions that may provide insight and better understanding about how your group compares to others, as it relates to properly evaluating your recruitment pipeline.
For hospital medicine groups (HMGs) serving adults only, two out of three groups have unfilled positions, and about half of pediatric-only hospitalist groups have unfilled positions. Andrew White, MD, SFHM, associate professor of medicine at the University of Washington, Seattle, provided us with a deep-dive discussion of this topic in a recent article in The Hospitalist.
If your group has historically had more unfilled positions than the respondents, it might mean your group should consider different strategies to close the gap. It may also lead to conversations about how to rethink the schedule to better meet the demands of clinical care with limited resources.
So, with all these unfilled positions, how are hospitalist groups filling the gap? Not all groups are using locum tenens to fill those unfilled positions. About a third of hospitalist groups reported leaving those gaps uncovered.
The most commonly reported tactic to fill in the gaps was voluntary extra shifts by existing hospitalists (physicians and/or nurse practioners/physician assistants). This approach is used by 70% of hospitalist groups. The second most-used tactic was “moonlighters” or PRN physicians (57.4%). Thirdly, was use of locum tenens physicians.
With these baselines, we will be able to better track and trend the industry going forward.
Scheduling methodologies
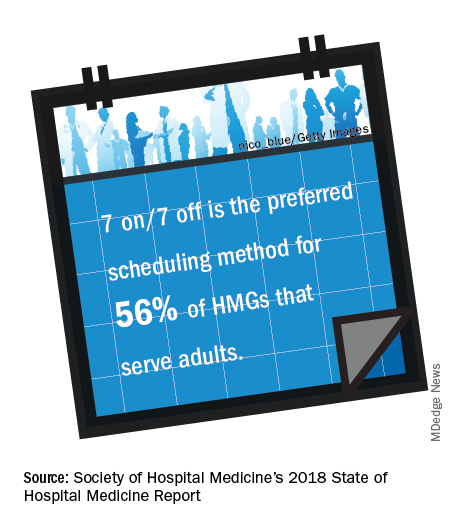
For pediatric practices, the fixed rotating block scheduling has decreased over the two survey periods (16.7% versus 6.7%).
Even though the 7-on/7-off schedule remains quite popular among adult-only HMGs, many seasoned hospitalists wonder whether this is sustainable through all seasons of life. Some hospitalists have said a 7-on/7-off schedule is like turning on and off your personal life and that it takes a day or 2 to recover from 7 consecutive 12-hour days.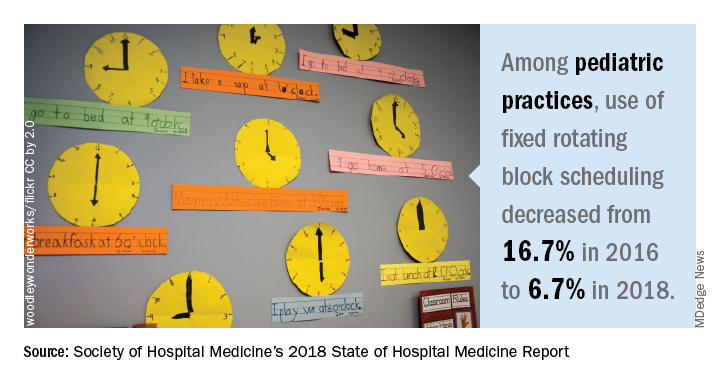
On the other hand, a fixed schedule is the easiest to explain, and many new hospitalists are requesting a fixed schedule. Even so, a fixed schedule may not allow for enough flexibility to adapt the schedule to the demands of patient care.
Nonetheless, a fixed schedule remains a very popular scheduling pattern. Does this scheduling model lead to burnout? Does this scheduling model increase or decrease elasticity? The debate of flexible versus fixed schedules continues!
Results by shift type
Very simply, the length of individual shifts has not changed much in prior years. For adult-only practices, most all day and night shifts are 12 hours in length. For pediatric-only HMGs, most day shifts are about 10 hours, and most night shifts are about 13 hours.
Most evening or swing shifts for adult-only practices are about 10 hours, which is a slight decrease from 2016. Pediatric-only practices’ evening shifts are about 8 hours in length.
A new question this year is about daytime admitters. For adult hospitalist groups, over half of groups have daytime admitters. For pediatric groups, nearly three out of four groups have daytime dedicated admitters. Also, the larger the group size, the more likely it is to have a dedicated daytime admitter.
Nocturnists remain in demand! Over 80% of adult hospitalist groups have on-site hospitalists at night. About a quarter of pediatric-only practices have nocturnists.
Scheduled workload distribution
One way of scheduling patient assignments is the phenomenon of unit-based assignments, or geographic rounding. As this has become more prevalent, the SHM Practice Analysis Committee recommended adding a question about unit-based assignments to the 2018 SoHM report.
The adoption of unit-based assignments is higher in academic groups (54.3%), as well as among hospitalists employed at a “hospital, health system or integrated delivery system” (47.4%), than in other group practice models.
Just as with the presence of daytime admitters, the larger the group the more likely it has some form of unit-based assignments. Further study would be needed to determine whether there is a link between the presence of daytime admitters and successful unit-based assignments for daytime rounders.
What’s the verdict?
Hospitalist scheduling will continue to evolve. It’s a never-ending balance of what’s best for patients and what’s best for hospitalists (and likely many other key stakeholders).
Scheduling is personal. Scheduling is an art form. The biggest question in this topic area is: Has anyone figured out the ‘secret sauce’ to hospitalist scheduling? Go online to SHM’s HMX to start the discussion!
Ms. Trask is national vice president of the Hospital Medicine Service Line at Catholic Health Initiatives in Englewood, Colo. She is also a member of The Hospitalist’s editorial advisory board.
Survey says ...
Survey says ...
Scheduling. Has there ever been such a simple word that is so complex? A simple Internet search of hospitalist scheduling returns thousands of possible discussions, leaving readers to conclude that the possibilities are endless and the challenges great. The answer certainly is not a one-size-fits-all approach.
Hospitalist scheduling is one of the key sections in the 2018 State of Hospital Medicine (SoHM) report; the 2018 report delves deeper into hospitalist scheduling than ever before.
For those of you who have been regular users of prior SoHM reports, you should be pleasantly surprised to find new comparative values: There are nearly 50% more pages dedicated just to scheduling!
For those readers who have never subscribed to the SoHM Report, this is your chance to study how other groups approach hospitalist schedules.
Why is hospitalist scheduling such a hot topic? For one, flexible and sustainable scheduling is an important contributor to job satisfaction. It is important for hospitalists to have a high degree of input into managing and effecting change for personal work-life balance.
As John Nelson, MD, MHM, a cofounder of the Society of Hospital Medicine, wrote recently in The Hospitalist, “an optimal schedule alone isn’t the key to preventing it [burnout], but maybe a good schedule can reduce your risk you’ll suffer from it.”
Secondly, ensuring that the hospitalist team is right sized – that is, scheduling hospitalists in the right place at the right time – is an art. Using resources, such as the 2018 SoHM report, to identify quantifiable comparisons enables hospitalist groups to continuously ensure the hospitalist schedule meets the clinical demands while optimizing the hospitalist group’s schedule.
Unfilled positions
The 2018 SoHM report features a new section on unfilled positions that may provide insight and better understanding about how your group compares to others, as it relates to properly evaluating your recruitment pipeline.
For hospital medicine groups (HMGs) serving adults only, two out of three groups have unfilled positions, and about half of pediatric-only hospitalist groups have unfilled positions. Andrew White, MD, SFHM, associate professor of medicine at the University of Washington, Seattle, provided us with a deep-dive discussion of this topic in a recent article in The Hospitalist.
If your group has historically had more unfilled positions than the respondents, it might mean your group should consider different strategies to close the gap. It may also lead to conversations about how to rethink the schedule to better meet the demands of clinical care with limited resources.
So, with all these unfilled positions, how are hospitalist groups filling the gap? Not all groups are using locum tenens to fill those unfilled positions. About a third of hospitalist groups reported leaving those gaps uncovered.
The most commonly reported tactic to fill in the gaps was voluntary extra shifts by existing hospitalists (physicians and/or nurse practioners/physician assistants). This approach is used by 70% of hospitalist groups. The second most-used tactic was “moonlighters” or PRN physicians (57.4%). Thirdly, was use of locum tenens physicians.
With these baselines, we will be able to better track and trend the industry going forward.
Scheduling methodologies

For pediatric practices, the fixed rotating block scheduling has decreased over the two survey periods (16.7% versus 6.7%).
Even though the 7-on/7-off schedule remains quite popular among adult-only HMGs, many seasoned hospitalists wonder whether this is sustainable through all seasons of life. Some hospitalists have said a 7-on/7-off schedule is like turning on and off your personal life and that it takes a day or 2 to recover from 7 consecutive 12-hour days.
On the other hand, a fixed schedule is the easiest to explain, and many new hospitalists are requesting a fixed schedule. Even so, a fixed schedule may not allow for enough flexibility to adapt the schedule to the demands of patient care.
Nonetheless, a fixed schedule remains a very popular scheduling pattern. Does this scheduling model lead to burnout? Does this scheduling model increase or decrease elasticity? The debate of flexible versus fixed schedules continues!
Results by shift type
Very simply, the length of individual shifts has not changed much in prior years. For adult-only practices, most all day and night shifts are 12 hours in length. For pediatric-only HMGs, most day shifts are about 10 hours, and most night shifts are about 13 hours.
Most evening or swing shifts for adult-only practices are about 10 hours, which is a slight decrease from 2016. Pediatric-only practices’ evening shifts are about 8 hours in length.
A new question this year is about daytime admitters. For adult hospitalist groups, over half of groups have daytime admitters. For pediatric groups, nearly three out of four groups have daytime dedicated admitters. Also, the larger the group size, the more likely it is to have a dedicated daytime admitter.
Nocturnists remain in demand! Over 80% of adult hospitalist groups have on-site hospitalists at night. About a quarter of pediatric-only practices have nocturnists.
Scheduled workload distribution
One way of scheduling patient assignments is the phenomenon of unit-based assignments, or geographic rounding. As this has become more prevalent, the SHM Practice Analysis Committee recommended adding a question about unit-based assignments to the 2018 SoHM report.
The adoption of unit-based assignments is higher in academic groups (54.3%), as well as among hospitalists employed at a “hospital, health system or integrated delivery system” (47.4%), than in other group practice models.
Just as with the presence of daytime admitters, the larger the group the more likely it has some form of unit-based assignments. Further study would be needed to determine whether there is a link between the presence of daytime admitters and successful unit-based assignments for daytime rounders.
What’s the verdict?
Hospitalist scheduling will continue to evolve. It’s a never-ending balance of what’s best for patients and what’s best for hospitalists (and likely many other key stakeholders).
Scheduling is personal. Scheduling is an art form. The biggest question in this topic area is: Has anyone figured out the ‘secret sauce’ to hospitalist scheduling? Go online to SHM’s HMX to start the discussion!
Ms. Trask is national vice president of the Hospital Medicine Service Line at Catholic Health Initiatives in Englewood, Colo. She is also a member of The Hospitalist’s editorial advisory board.
Scheduling. Has there ever been such a simple word that is so complex? A simple Internet search of hospitalist scheduling returns thousands of possible discussions, leaving readers to conclude that the possibilities are endless and the challenges great. The answer certainly is not a one-size-fits-all approach.
Hospitalist scheduling is one of the key sections in the 2018 State of Hospital Medicine (SoHM) report; the 2018 report delves deeper into hospitalist scheduling than ever before.
For those of you who have been regular users of prior SoHM reports, you should be pleasantly surprised to find new comparative values: There are nearly 50% more pages dedicated just to scheduling!
For those readers who have never subscribed to the SoHM Report, this is your chance to study how other groups approach hospitalist schedules.
Why is hospitalist scheduling such a hot topic? For one, flexible and sustainable scheduling is an important contributor to job satisfaction. It is important for hospitalists to have a high degree of input into managing and effecting change for personal work-life balance.
As John Nelson, MD, MHM, a cofounder of the Society of Hospital Medicine, wrote recently in The Hospitalist, “an optimal schedule alone isn’t the key to preventing it [burnout], but maybe a good schedule can reduce your risk you’ll suffer from it.”
Secondly, ensuring that the hospitalist team is right sized – that is, scheduling hospitalists in the right place at the right time – is an art. Using resources, such as the 2018 SoHM report, to identify quantifiable comparisons enables hospitalist groups to continuously ensure the hospitalist schedule meets the clinical demands while optimizing the hospitalist group’s schedule.
Unfilled positions
The 2018 SoHM report features a new section on unfilled positions that may provide insight and better understanding about how your group compares to others, as it relates to properly evaluating your recruitment pipeline.
For hospital medicine groups (HMGs) serving adults only, two out of three groups have unfilled positions, and about half of pediatric-only hospitalist groups have unfilled positions. Andrew White, MD, SFHM, associate professor of medicine at the University of Washington, Seattle, provided us with a deep-dive discussion of this topic in a recent article in The Hospitalist.
If your group has historically had more unfilled positions than the respondents, it might mean your group should consider different strategies to close the gap. It may also lead to conversations about how to rethink the schedule to better meet the demands of clinical care with limited resources.
So, with all these unfilled positions, how are hospitalist groups filling the gap? Not all groups are using locum tenens to fill those unfilled positions. About a third of hospitalist groups reported leaving those gaps uncovered.
The most commonly reported tactic to fill in the gaps was voluntary extra shifts by existing hospitalists (physicians and/or nurse practioners/physician assistants). This approach is used by 70% of hospitalist groups. The second most-used tactic was “moonlighters” or PRN physicians (57.4%). Thirdly, was use of locum tenens physicians.
With these baselines, we will be able to better track and trend the industry going forward.
Scheduling methodologies

For pediatric practices, the fixed rotating block scheduling has decreased over the two survey periods (16.7% versus 6.7%).
Even though the 7-on/7-off schedule remains quite popular among adult-only HMGs, many seasoned hospitalists wonder whether this is sustainable through all seasons of life. Some hospitalists have said a 7-on/7-off schedule is like turning on and off your personal life and that it takes a day or 2 to recover from 7 consecutive 12-hour days.
On the other hand, a fixed schedule is the easiest to explain, and many new hospitalists are requesting a fixed schedule. Even so, a fixed schedule may not allow for enough flexibility to adapt the schedule to the demands of patient care.
Nonetheless, a fixed schedule remains a very popular scheduling pattern. Does this scheduling model lead to burnout? Does this scheduling model increase or decrease elasticity? The debate of flexible versus fixed schedules continues!
Results by shift type
Very simply, the length of individual shifts has not changed much in prior years. For adult-only practices, most all day and night shifts are 12 hours in length. For pediatric-only HMGs, most day shifts are about 10 hours, and most night shifts are about 13 hours.
Most evening or swing shifts for adult-only practices are about 10 hours, which is a slight decrease from 2016. Pediatric-only practices’ evening shifts are about 8 hours in length.
A new question this year is about daytime admitters. For adult hospitalist groups, over half of groups have daytime admitters. For pediatric groups, nearly three out of four groups have daytime dedicated admitters. Also, the larger the group size, the more likely it is to have a dedicated daytime admitter.
Nocturnists remain in demand! Over 80% of adult hospitalist groups have on-site hospitalists at night. About a quarter of pediatric-only practices have nocturnists.
Scheduled workload distribution
One way of scheduling patient assignments is the phenomenon of unit-based assignments, or geographic rounding. As this has become more prevalent, the SHM Practice Analysis Committee recommended adding a question about unit-based assignments to the 2018 SoHM report.
The adoption of unit-based assignments is higher in academic groups (54.3%), as well as among hospitalists employed at a “hospital, health system or integrated delivery system” (47.4%), than in other group practice models.
Just as with the presence of daytime admitters, the larger the group the more likely it has some form of unit-based assignments. Further study would be needed to determine whether there is a link between the presence of daytime admitters and successful unit-based assignments for daytime rounders.
What’s the verdict?
Hospitalist scheduling will continue to evolve. It’s a never-ending balance of what’s best for patients and what’s best for hospitalists (and likely many other key stakeholders).
Scheduling is personal. Scheduling is an art form. The biggest question in this topic area is: Has anyone figured out the ‘secret sauce’ to hospitalist scheduling? Go online to SHM’s HMX to start the discussion!
Ms. Trask is national vice president of the Hospital Medicine Service Line at Catholic Health Initiatives in Englewood, Colo. She is also a member of The Hospitalist’s editorial advisory board.
Vitamin C for sepsis? Experts take sides in sharp debate
SAN DIEGO –
“There is evidence supporting benefit, and ample evidence supporting safety,” Michael H. Hooper, MD, who practices in Norfolk, Va., said in a pro-and-con debate over the use of vitamin C in sepsis at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Dr. Hooper’s debate opponent countered by noting the lack of quality research into vitamin C in sepsis and declared that its time has not yet come. “We need more data to know the safety of this drug,” said Andre Kalil, MD, professor of internal medicine and director of Transplant Infectious Diseases at the University of Nebraska Medical Center, Omaha.
Dr. Hooper was part of a member of a team led by Paul E. Marik, MD, FCCP, of Eastern Virginia Medical School, Norfolk, that made waves in 2017 with a study in Chest suggesting IV vitamin C has tremendous potential as a treatment for sepsis (Chest. 2017 Jun;151[6]:1229-38).
The retrospective study compared two groups of 47 patients with sepsis – a control group and a group that received treatment with intravenous vitamin C, hydrocortisone, and thiamine. Remarkably, the team found that 9% (4 of 47) of those in the treatment group died in the hospital, compared with 40% (19 of 47) in the control group (P less than .001).
The findings make sense, Dr. Hooper said, in light of the fact that “our patients are remarkably deficient” in vitamin C. He pointed to a 2017 study that found nearly 40% of 24 patients with septic shock were deficient in vitamin C – despite getting recommended enteral nutrition, parenteral nutrition or both – compared with 25% of patients who were not septic. The study authors believe the difference is probably due to “increased metabolism due to the enhanced inflammatory response observed in septic shock” (Crit Care. 2017 Dec 11;21[1]:300).
“We’re dealing with a population of patients who need some sort of repletion of this vitamin,” Dr. Hooper said.
Why not try oral administration of vitamin C? “Oral administration at regular doses doesn’t work,” he said. “If you have normal volunteers who are made deficient, then you administer the recommended allowance, it takes days or weeks to return levels to normal.”
Dr. Hooper added that the goal of vitamin C therapy isn’t simply to restore proper levels in plasma. In addition, he said, “we’re trying to restore levels in crucial organs.”
He said the cost of treatment with IV vitamin C is low, and no serious adverse events have been seen in studies of the vitamin’s use in critical care.
In his comments at the debate, Dr. Kalil pointed to several weaknesses in the 2017 study of vitamin C in sepsis. According to him, it had many problems, including a sample size that lacked statistical power and imbalances in the two groups. He raised concerns about the study in a 2017 letter published in Chest titled “Vitamin C Is Not Ready for Prime Time in Sepsis but a Solution Is Close,” noting that the control group was sicker and none of those patients had their vitamin C levels measured (Chest. 2017 Sep;152[3]:676).
He added that “acute renal failure is associated with high doses of vitamin C.”
As of July 2018, several clinical trials into vitamin C, hydrocortisone, and thiamine for the treatment of septic shock were underway or planned, according to a report that described the current randomized, placebo-controlled, multicenter Ascorbic Acid, Corticosteroids, and Thiamine in Sepsis (ACTS) trial in the United States. The report notes that “robust evidence” for this approach is lacking, although “the potential effectiveness of this medication combination is rooted in biologic plausibility and supported by small clinical trials of the various individual components.” (Crit Care. 2018;22:283)
Dr. Hooper is an executive committee member and principal investigator with the Vitamin C, Thiamine And Steroids in Sepsis (VICTAS) study. Dr. Kalil reports no relevant disclosures.
SAN DIEGO –
“There is evidence supporting benefit, and ample evidence supporting safety,” Michael H. Hooper, MD, who practices in Norfolk, Va., said in a pro-and-con debate over the use of vitamin C in sepsis at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Dr. Hooper’s debate opponent countered by noting the lack of quality research into vitamin C in sepsis and declared that its time has not yet come. “We need more data to know the safety of this drug,” said Andre Kalil, MD, professor of internal medicine and director of Transplant Infectious Diseases at the University of Nebraska Medical Center, Omaha.
Dr. Hooper was part of a member of a team led by Paul E. Marik, MD, FCCP, of Eastern Virginia Medical School, Norfolk, that made waves in 2017 with a study in Chest suggesting IV vitamin C has tremendous potential as a treatment for sepsis (Chest. 2017 Jun;151[6]:1229-38).
The retrospective study compared two groups of 47 patients with sepsis – a control group and a group that received treatment with intravenous vitamin C, hydrocortisone, and thiamine. Remarkably, the team found that 9% (4 of 47) of those in the treatment group died in the hospital, compared with 40% (19 of 47) in the control group (P less than .001).
The findings make sense, Dr. Hooper said, in light of the fact that “our patients are remarkably deficient” in vitamin C. He pointed to a 2017 study that found nearly 40% of 24 patients with septic shock were deficient in vitamin C – despite getting recommended enteral nutrition, parenteral nutrition or both – compared with 25% of patients who were not septic. The study authors believe the difference is probably due to “increased metabolism due to the enhanced inflammatory response observed in septic shock” (Crit Care. 2017 Dec 11;21[1]:300).
“We’re dealing with a population of patients who need some sort of repletion of this vitamin,” Dr. Hooper said.
Why not try oral administration of vitamin C? “Oral administration at regular doses doesn’t work,” he said. “If you have normal volunteers who are made deficient, then you administer the recommended allowance, it takes days or weeks to return levels to normal.”
Dr. Hooper added that the goal of vitamin C therapy isn’t simply to restore proper levels in plasma. In addition, he said, “we’re trying to restore levels in crucial organs.”
He said the cost of treatment with IV vitamin C is low, and no serious adverse events have been seen in studies of the vitamin’s use in critical care.
In his comments at the debate, Dr. Kalil pointed to several weaknesses in the 2017 study of vitamin C in sepsis. According to him, it had many problems, including a sample size that lacked statistical power and imbalances in the two groups. He raised concerns about the study in a 2017 letter published in Chest titled “Vitamin C Is Not Ready for Prime Time in Sepsis but a Solution Is Close,” noting that the control group was sicker and none of those patients had their vitamin C levels measured (Chest. 2017 Sep;152[3]:676).
He added that “acute renal failure is associated with high doses of vitamin C.”
As of July 2018, several clinical trials into vitamin C, hydrocortisone, and thiamine for the treatment of septic shock were underway or planned, according to a report that described the current randomized, placebo-controlled, multicenter Ascorbic Acid, Corticosteroids, and Thiamine in Sepsis (ACTS) trial in the United States. The report notes that “robust evidence” for this approach is lacking, although “the potential effectiveness of this medication combination is rooted in biologic plausibility and supported by small clinical trials of the various individual components.” (Crit Care. 2018;22:283)
Dr. Hooper is an executive committee member and principal investigator with the Vitamin C, Thiamine And Steroids in Sepsis (VICTAS) study. Dr. Kalil reports no relevant disclosures.
SAN DIEGO –
“There is evidence supporting benefit, and ample evidence supporting safety,” Michael H. Hooper, MD, who practices in Norfolk, Va., said in a pro-and-con debate over the use of vitamin C in sepsis at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Dr. Hooper’s debate opponent countered by noting the lack of quality research into vitamin C in sepsis and declared that its time has not yet come. “We need more data to know the safety of this drug,” said Andre Kalil, MD, professor of internal medicine and director of Transplant Infectious Diseases at the University of Nebraska Medical Center, Omaha.
Dr. Hooper was part of a member of a team led by Paul E. Marik, MD, FCCP, of Eastern Virginia Medical School, Norfolk, that made waves in 2017 with a study in Chest suggesting IV vitamin C has tremendous potential as a treatment for sepsis (Chest. 2017 Jun;151[6]:1229-38).
The retrospective study compared two groups of 47 patients with sepsis – a control group and a group that received treatment with intravenous vitamin C, hydrocortisone, and thiamine. Remarkably, the team found that 9% (4 of 47) of those in the treatment group died in the hospital, compared with 40% (19 of 47) in the control group (P less than .001).
The findings make sense, Dr. Hooper said, in light of the fact that “our patients are remarkably deficient” in vitamin C. He pointed to a 2017 study that found nearly 40% of 24 patients with septic shock were deficient in vitamin C – despite getting recommended enteral nutrition, parenteral nutrition or both – compared with 25% of patients who were not septic. The study authors believe the difference is probably due to “increased metabolism due to the enhanced inflammatory response observed in septic shock” (Crit Care. 2017 Dec 11;21[1]:300).
“We’re dealing with a population of patients who need some sort of repletion of this vitamin,” Dr. Hooper said.
Why not try oral administration of vitamin C? “Oral administration at regular doses doesn’t work,” he said. “If you have normal volunteers who are made deficient, then you administer the recommended allowance, it takes days or weeks to return levels to normal.”
Dr. Hooper added that the goal of vitamin C therapy isn’t simply to restore proper levels in plasma. In addition, he said, “we’re trying to restore levels in crucial organs.”
He said the cost of treatment with IV vitamin C is low, and no serious adverse events have been seen in studies of the vitamin’s use in critical care.
In his comments at the debate, Dr. Kalil pointed to several weaknesses in the 2017 study of vitamin C in sepsis. According to him, it had many problems, including a sample size that lacked statistical power and imbalances in the two groups. He raised concerns about the study in a 2017 letter published in Chest titled “Vitamin C Is Not Ready for Prime Time in Sepsis but a Solution Is Close,” noting that the control group was sicker and none of those patients had their vitamin C levels measured (Chest. 2017 Sep;152[3]:676).
He added that “acute renal failure is associated with high doses of vitamin C.”
As of July 2018, several clinical trials into vitamin C, hydrocortisone, and thiamine for the treatment of septic shock were underway or planned, according to a report that described the current randomized, placebo-controlled, multicenter Ascorbic Acid, Corticosteroids, and Thiamine in Sepsis (ACTS) trial in the United States. The report notes that “robust evidence” for this approach is lacking, although “the potential effectiveness of this medication combination is rooted in biologic plausibility and supported by small clinical trials of the various individual components.” (Crit Care. 2018;22:283)
Dr. Hooper is an executive committee member and principal investigator with the Vitamin C, Thiamine And Steroids in Sepsis (VICTAS) study. Dr. Kalil reports no relevant disclosures.
EXPERT ANALYSIS FROM CCC48
Infective endocarditis isn’t what it used to be
SNOWMASS, COLO. – Infective endocarditis in 2019 is very different from the disease most physicians encountered in training, both in terms of epidemiology and clinical presentation, Patrick T. O’Gara, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The classic description of infective endocarditis provided by Sir William Osler, MD, was of a subacute bacterial infection characterized by a long latent phase of low-grade fever, back pain, weight loss, and night sweats. It was mainly a right-heart disease of younger individuals with an infected native valve, and the predominant pathogens were streptococci, Dr. O’Gara said.
“I think in the current era endocarditis is more often characterized by an acute illness with toxic features in the context of adults with a high burden of degenerative diseases – for example, patients with rheumatoid arthritis or psoriatic arthritis on immunosuppressive therapy, or diabetes, end-stage renal disease, and risk factors for hospital-acquired infection. Injectable drug use is through the roof, there’s a wider prevalence of cardiac implanted electronic devices, which are a wonderful place for bacteria to hide, and Staphylococcus aureus has certainly become the leading pathogen with regard to endocarditis in the United States, especially MRSA, often multidrug resistant,” said Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
“Also, no talk about endocarditis is sufficient without paying some attention to the opioid crisis in which we find ourselves. It’s one of the top three causes of death among young men in the United States, along with accidents and gun violence. No region of the country is spared. This has completely inundated our ER and hospitalist services and our inpatient cardiology services with folks who are often repeat offenders when it comes to the difficulty in being able to give up an injectable drug use habit. They have multiple infections and hospitalizations, tricuspid valve involvement, and depending upon the aggressiveness of the Staphylococcus organism, typically they have left-sided disease with multiple complications, including aortic regurgitation and heart failure,” the cardiologist continued.
This description underscored one of Dr. O’Gara’s major points about the challenges posed by infective endocarditis in contemporary practice: “Expect the unexpected,” he advised. “When you’ve seen one case of infective endocarditis, you’ve seen one case of infective endocarditis.”
Outcomes are ‘sobering’
In the current era, outcomes are “sobering,” the cardiologist noted. Infective endocarditis carries a 6-month mortality rate of 20%-25% despite early surgery being performed during the index hospitalization in up to 60% of patients, with a relatively high perioperative mortality rate of about 10%. However, the risk of reinfection occurring in a newly implanted cardiac valve is impressively low at about 2%.
Refer early for multimodality imaging and surgical consultation
Transesophageal echocardiography is valuable in assessment of the infected valve. However, when extravalvular extension of the infection is suspected and the echo assessment is nondiagnostic or indeterminate, it’s time to quickly move on to advanced imaging, such as PET-CT.
The ACC/American Heart Association class I recommendations for early surgery in infected native valves haven’t changed substantially in over a decade. Based largely on observational data, there is an association between early surgery and lower in-hospital mortality (Lancet. 2012 Mar 10;379[9819]:965-975).
Class IIa recommendations for native valve surgery include recurrent emboli and a persistent vegetation despite appropriate antibiotic therapy. A “very controversial” class IIb recommendation for surgery because of weak supporting data is the identification of a mobile vegetation larger than 10 mm, particularly if it’s located on an anterior mitral valve leaflet, he said.
If the decision is made to forgo early surgery, be sure to repeat transesophageal echocardiography on day 7-10 to reassess the size of the patient’s vegetation.
“There is an association between size of vegetation and 1-year mortality, with a cut point of greater than 15 mm. Some would argue this constitutes a reasonable indication for early surgery,” Dr. O’Gara noted.
The embolization rate in patients with infective endocarditis is highest during the day before presentation, the day of presentation, and through the first 2 days afterward. The rate drops precipitously within 2 weeks after initiation of appropriate antibiotic therapy. Thus, to utilize early surgery to maximum effect in order to decrease the risk of embolization, it makes sense to operate within the first several days following presentation, before antibiotics have had sufficient time to catch up with the evolving disease process.
Don’t use half measures when it comes to removal of cardiac implanted electronic devices
The guidelines are clear regarding infected pacemakers, implanted cardioverter-defibrillators, and cardiac resynchronization devices: “It all needs to come out,” Dr. O’Gara emphasized. That includes all leads and the generator in patients with documented infection of only one portion of the device system, as a class I, level of evidence B recommendation. Moreover, complete removal of a pacemaker or defibrillator system is deemed “reasonable” as a class IIa recommendation in all patients with valvular infection caused by S. aureus or fungi even in the absence of evidence of device infection.
“I think we as general cardiologists have become increasingly impressed about how sick and festering these kinds of patients can become, even when we’re not able to prove that the lead is infected. The lead looks okay on transesophageal echo or PET-CT, blood cultures are negative, the valvular heart disease is really not that advanced, but several days go by and the patient is just not responding. We should have a high index of suspicion that there’s an infection we cannot appreciate. But obviously, you make these difficult decisions in consultation with your electrophysiology colleagues,” he added.
Know when the cardiologist should say ‘no’ to early aggressive surgery
While an aggressive early surgical approach often pays off in terms of prevention of embolic sequelae and a reduction in heart failure, the timing of surgery in the 20%-40% of patients with infective endocarditis who present with stroke or other neurologic complications remains controversial. An international group of Canadian and French cardiac surgeons and neurologists developed a useful algorithm regarding the types of neurologic complications for which early cardiac surgery is a poor idea because of the high risk of neurologic exacerbation. For example, a mycotic neuroaneurysm is grounds for postponement of cardiac surgery for at least 4 weeks (Circulation. 2016 Oct 25;134[17]:1280-92).
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
SNOWMASS, COLO. – Infective endocarditis in 2019 is very different from the disease most physicians encountered in training, both in terms of epidemiology and clinical presentation, Patrick T. O’Gara, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The classic description of infective endocarditis provided by Sir William Osler, MD, was of a subacute bacterial infection characterized by a long latent phase of low-grade fever, back pain, weight loss, and night sweats. It was mainly a right-heart disease of younger individuals with an infected native valve, and the predominant pathogens were streptococci, Dr. O’Gara said.
“I think in the current era endocarditis is more often characterized by an acute illness with toxic features in the context of adults with a high burden of degenerative diseases – for example, patients with rheumatoid arthritis or psoriatic arthritis on immunosuppressive therapy, or diabetes, end-stage renal disease, and risk factors for hospital-acquired infection. Injectable drug use is through the roof, there’s a wider prevalence of cardiac implanted electronic devices, which are a wonderful place for bacteria to hide, and Staphylococcus aureus has certainly become the leading pathogen with regard to endocarditis in the United States, especially MRSA, often multidrug resistant,” said Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
“Also, no talk about endocarditis is sufficient without paying some attention to the opioid crisis in which we find ourselves. It’s one of the top three causes of death among young men in the United States, along with accidents and gun violence. No region of the country is spared. This has completely inundated our ER and hospitalist services and our inpatient cardiology services with folks who are often repeat offenders when it comes to the difficulty in being able to give up an injectable drug use habit. They have multiple infections and hospitalizations, tricuspid valve involvement, and depending upon the aggressiveness of the Staphylococcus organism, typically they have left-sided disease with multiple complications, including aortic regurgitation and heart failure,” the cardiologist continued.
This description underscored one of Dr. O’Gara’s major points about the challenges posed by infective endocarditis in contemporary practice: “Expect the unexpected,” he advised. “When you’ve seen one case of infective endocarditis, you’ve seen one case of infective endocarditis.”
Outcomes are ‘sobering’
In the current era, outcomes are “sobering,” the cardiologist noted. Infective endocarditis carries a 6-month mortality rate of 20%-25% despite early surgery being performed during the index hospitalization in up to 60% of patients, with a relatively high perioperative mortality rate of about 10%. However, the risk of reinfection occurring in a newly implanted cardiac valve is impressively low at about 2%.
Refer early for multimodality imaging and surgical consultation
Transesophageal echocardiography is valuable in assessment of the infected valve. However, when extravalvular extension of the infection is suspected and the echo assessment is nondiagnostic or indeterminate, it’s time to quickly move on to advanced imaging, such as PET-CT.
The ACC/American Heart Association class I recommendations for early surgery in infected native valves haven’t changed substantially in over a decade. Based largely on observational data, there is an association between early surgery and lower in-hospital mortality (Lancet. 2012 Mar 10;379[9819]:965-975).
Class IIa recommendations for native valve surgery include recurrent emboli and a persistent vegetation despite appropriate antibiotic therapy. A “very controversial” class IIb recommendation for surgery because of weak supporting data is the identification of a mobile vegetation larger than 10 mm, particularly if it’s located on an anterior mitral valve leaflet, he said.
If the decision is made to forgo early surgery, be sure to repeat transesophageal echocardiography on day 7-10 to reassess the size of the patient’s vegetation.
“There is an association between size of vegetation and 1-year mortality, with a cut point of greater than 15 mm. Some would argue this constitutes a reasonable indication for early surgery,” Dr. O’Gara noted.
The embolization rate in patients with infective endocarditis is highest during the day before presentation, the day of presentation, and through the first 2 days afterward. The rate drops precipitously within 2 weeks after initiation of appropriate antibiotic therapy. Thus, to utilize early surgery to maximum effect in order to decrease the risk of embolization, it makes sense to operate within the first several days following presentation, before antibiotics have had sufficient time to catch up with the evolving disease process.
Don’t use half measures when it comes to removal of cardiac implanted electronic devices
The guidelines are clear regarding infected pacemakers, implanted cardioverter-defibrillators, and cardiac resynchronization devices: “It all needs to come out,” Dr. O’Gara emphasized. That includes all leads and the generator in patients with documented infection of only one portion of the device system, as a class I, level of evidence B recommendation. Moreover, complete removal of a pacemaker or defibrillator system is deemed “reasonable” as a class IIa recommendation in all patients with valvular infection caused by S. aureus or fungi even in the absence of evidence of device infection.
“I think we as general cardiologists have become increasingly impressed about how sick and festering these kinds of patients can become, even when we’re not able to prove that the lead is infected. The lead looks okay on transesophageal echo or PET-CT, blood cultures are negative, the valvular heart disease is really not that advanced, but several days go by and the patient is just not responding. We should have a high index of suspicion that there’s an infection we cannot appreciate. But obviously, you make these difficult decisions in consultation with your electrophysiology colleagues,” he added.
Know when the cardiologist should say ‘no’ to early aggressive surgery
While an aggressive early surgical approach often pays off in terms of prevention of embolic sequelae and a reduction in heart failure, the timing of surgery in the 20%-40% of patients with infective endocarditis who present with stroke or other neurologic complications remains controversial. An international group of Canadian and French cardiac surgeons and neurologists developed a useful algorithm regarding the types of neurologic complications for which early cardiac surgery is a poor idea because of the high risk of neurologic exacerbation. For example, a mycotic neuroaneurysm is grounds for postponement of cardiac surgery for at least 4 weeks (Circulation. 2016 Oct 25;134[17]:1280-92).
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
SNOWMASS, COLO. – Infective endocarditis in 2019 is very different from the disease most physicians encountered in training, both in terms of epidemiology and clinical presentation, Patrick T. O’Gara, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The classic description of infective endocarditis provided by Sir William Osler, MD, was of a subacute bacterial infection characterized by a long latent phase of low-grade fever, back pain, weight loss, and night sweats. It was mainly a right-heart disease of younger individuals with an infected native valve, and the predominant pathogens were streptococci, Dr. O’Gara said.
“I think in the current era endocarditis is more often characterized by an acute illness with toxic features in the context of adults with a high burden of degenerative diseases – for example, patients with rheumatoid arthritis or psoriatic arthritis on immunosuppressive therapy, or diabetes, end-stage renal disease, and risk factors for hospital-acquired infection. Injectable drug use is through the roof, there’s a wider prevalence of cardiac implanted electronic devices, which are a wonderful place for bacteria to hide, and Staphylococcus aureus has certainly become the leading pathogen with regard to endocarditis in the United States, especially MRSA, often multidrug resistant,” said Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
“Also, no talk about endocarditis is sufficient without paying some attention to the opioid crisis in which we find ourselves. It’s one of the top three causes of death among young men in the United States, along with accidents and gun violence. No region of the country is spared. This has completely inundated our ER and hospitalist services and our inpatient cardiology services with folks who are often repeat offenders when it comes to the difficulty in being able to give up an injectable drug use habit. They have multiple infections and hospitalizations, tricuspid valve involvement, and depending upon the aggressiveness of the Staphylococcus organism, typically they have left-sided disease with multiple complications, including aortic regurgitation and heart failure,” the cardiologist continued.
This description underscored one of Dr. O’Gara’s major points about the challenges posed by infective endocarditis in contemporary practice: “Expect the unexpected,” he advised. “When you’ve seen one case of infective endocarditis, you’ve seen one case of infective endocarditis.”
Outcomes are ‘sobering’
In the current era, outcomes are “sobering,” the cardiologist noted. Infective endocarditis carries a 6-month mortality rate of 20%-25% despite early surgery being performed during the index hospitalization in up to 60% of patients, with a relatively high perioperative mortality rate of about 10%. However, the risk of reinfection occurring in a newly implanted cardiac valve is impressively low at about 2%.
Refer early for multimodality imaging and surgical consultation
Transesophageal echocardiography is valuable in assessment of the infected valve. However, when extravalvular extension of the infection is suspected and the echo assessment is nondiagnostic or indeterminate, it’s time to quickly move on to advanced imaging, such as PET-CT.
The ACC/American Heart Association class I recommendations for early surgery in infected native valves haven’t changed substantially in over a decade. Based largely on observational data, there is an association between early surgery and lower in-hospital mortality (Lancet. 2012 Mar 10;379[9819]:965-975).
Class IIa recommendations for native valve surgery include recurrent emboli and a persistent vegetation despite appropriate antibiotic therapy. A “very controversial” class IIb recommendation for surgery because of weak supporting data is the identification of a mobile vegetation larger than 10 mm, particularly if it’s located on an anterior mitral valve leaflet, he said.
If the decision is made to forgo early surgery, be sure to repeat transesophageal echocardiography on day 7-10 to reassess the size of the patient’s vegetation.
“There is an association between size of vegetation and 1-year mortality, with a cut point of greater than 15 mm. Some would argue this constitutes a reasonable indication for early surgery,” Dr. O’Gara noted.
The embolization rate in patients with infective endocarditis is highest during the day before presentation, the day of presentation, and through the first 2 days afterward. The rate drops precipitously within 2 weeks after initiation of appropriate antibiotic therapy. Thus, to utilize early surgery to maximum effect in order to decrease the risk of embolization, it makes sense to operate within the first several days following presentation, before antibiotics have had sufficient time to catch up with the evolving disease process.
Don’t use half measures when it comes to removal of cardiac implanted electronic devices
The guidelines are clear regarding infected pacemakers, implanted cardioverter-defibrillators, and cardiac resynchronization devices: “It all needs to come out,” Dr. O’Gara emphasized. That includes all leads and the generator in patients with documented infection of only one portion of the device system, as a class I, level of evidence B recommendation. Moreover, complete removal of a pacemaker or defibrillator system is deemed “reasonable” as a class IIa recommendation in all patients with valvular infection caused by S. aureus or fungi even in the absence of evidence of device infection.
“I think we as general cardiologists have become increasingly impressed about how sick and festering these kinds of patients can become, even when we’re not able to prove that the lead is infected. The lead looks okay on transesophageal echo or PET-CT, blood cultures are negative, the valvular heart disease is really not that advanced, but several days go by and the patient is just not responding. We should have a high index of suspicion that there’s an infection we cannot appreciate. But obviously, you make these difficult decisions in consultation with your electrophysiology colleagues,” he added.
Know when the cardiologist should say ‘no’ to early aggressive surgery
While an aggressive early surgical approach often pays off in terms of prevention of embolic sequelae and a reduction in heart failure, the timing of surgery in the 20%-40% of patients with infective endocarditis who present with stroke or other neurologic complications remains controversial. An international group of Canadian and French cardiac surgeons and neurologists developed a useful algorithm regarding the types of neurologic complications for which early cardiac surgery is a poor idea because of the high risk of neurologic exacerbation. For example, a mycotic neuroaneurysm is grounds for postponement of cardiac surgery for at least 4 weeks (Circulation. 2016 Oct 25;134[17]:1280-92).
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
REPORTING FROM ACC SNOWMASS 2019