User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Factor Xa inhibitors versus vitamin K antagonists for preventing embolism in AF patients
Clinical question: Do factor Xa inhibitors reduce the incidence of strokes and systemic embolic events, compared with warfarin, in people with atrial fibrillation?
Background: Factor Xa inhibitors, called DOACs or direct-acting anticoagulants, and vitamin K antagonists (VKAs) are part of treatment guidelines for preventing stroke and systemic embolic events in people with atrial fibrillation (AF). This study assessed the effectiveness and safety of treatment with factor Xa inhibitors versus VKAs for preventing cerebral or systemic embolic events in AF.
Study design: Cochrane Review update.
Setting: Data obtained from trial registers of the Cochrane Central Register of Controlled Trials (August 2017), the Cochrane Heart Group and the Cochrane Stroke Group (September 2016), Embase (1980 to April 2017), and MEDLINE (1950 to April 2017). Authors also screened reference lists and contacted pharmaceutical companies, authors, and sponsors of relevant published trials.
Synopsis: The study included 42,084 participants from 10 trials with a diagnosis of AF who were eligible for long-term anticoagulation with warfarin (target INR 2-3).
The trials directly compared dose-adjusted warfarin with factor Xa inhibitors. Median follow-up ranged from 12 weeks to 1.9 years, and composite primary endpoint was all strokes (both ischemic and hemorrhagic) and non–central nervous systemic embolic events. Factor Xa inhibitor significantly decreased the number of strokes and systemic embolic events, compared with dose-adjusted warfarin (odds ratio, 0.81; 95% confidence interval, 0.72-0.91), reduced the number of major bleeding events (OR, 0.92; 95% CI, 0.63-1.34), and significantly reduced the risk of intracranial hemorrhage (OR, 0.56; 95% CI, 0.45-0.70). They also significantly reduced the number of all-cause deaths (OR, 0.88; 95% CI, 0.81-0.97). One limitation of this study is the heterogeneity and hence lower quality of evidence. This study shows a small net clinical benefit of using factor Xa inhibitors in AF because of a reduction in strokes and systemic embolic events and also a lower risk of bleeding (including intracranial hemorrhages), compared with using warfarin.
Bottom line: Patients with AF have a lower incidence of strokes and systemic embolic events when treated with factor Xa inhibitors, compared with those treated with warfarin.
Citation: Bruins Slot KM et al. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev. 2018 Mar 6. doi: 10.1002/14651858.CD008980.pub3.
Dr. Veedu is a hospitalist and instructor in the division of hospital medicine at the University of Kentucky, Lexington.
Clinical question: Do factor Xa inhibitors reduce the incidence of strokes and systemic embolic events, compared with warfarin, in people with atrial fibrillation?
Background: Factor Xa inhibitors, called DOACs or direct-acting anticoagulants, and vitamin K antagonists (VKAs) are part of treatment guidelines for preventing stroke and systemic embolic events in people with atrial fibrillation (AF). This study assessed the effectiveness and safety of treatment with factor Xa inhibitors versus VKAs for preventing cerebral or systemic embolic events in AF.
Study design: Cochrane Review update.
Setting: Data obtained from trial registers of the Cochrane Central Register of Controlled Trials (August 2017), the Cochrane Heart Group and the Cochrane Stroke Group (September 2016), Embase (1980 to April 2017), and MEDLINE (1950 to April 2017). Authors also screened reference lists and contacted pharmaceutical companies, authors, and sponsors of relevant published trials.
Synopsis: The study included 42,084 participants from 10 trials with a diagnosis of AF who were eligible for long-term anticoagulation with warfarin (target INR 2-3).
The trials directly compared dose-adjusted warfarin with factor Xa inhibitors. Median follow-up ranged from 12 weeks to 1.9 years, and composite primary endpoint was all strokes (both ischemic and hemorrhagic) and non–central nervous systemic embolic events. Factor Xa inhibitor significantly decreased the number of strokes and systemic embolic events, compared with dose-adjusted warfarin (odds ratio, 0.81; 95% confidence interval, 0.72-0.91), reduced the number of major bleeding events (OR, 0.92; 95% CI, 0.63-1.34), and significantly reduced the risk of intracranial hemorrhage (OR, 0.56; 95% CI, 0.45-0.70). They also significantly reduced the number of all-cause deaths (OR, 0.88; 95% CI, 0.81-0.97). One limitation of this study is the heterogeneity and hence lower quality of evidence. This study shows a small net clinical benefit of using factor Xa inhibitors in AF because of a reduction in strokes and systemic embolic events and also a lower risk of bleeding (including intracranial hemorrhages), compared with using warfarin.
Bottom line: Patients with AF have a lower incidence of strokes and systemic embolic events when treated with factor Xa inhibitors, compared with those treated with warfarin.
Citation: Bruins Slot KM et al. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev. 2018 Mar 6. doi: 10.1002/14651858.CD008980.pub3.
Dr. Veedu is a hospitalist and instructor in the division of hospital medicine at the University of Kentucky, Lexington.
Clinical question: Do factor Xa inhibitors reduce the incidence of strokes and systemic embolic events, compared with warfarin, in people with atrial fibrillation?
Background: Factor Xa inhibitors, called DOACs or direct-acting anticoagulants, and vitamin K antagonists (VKAs) are part of treatment guidelines for preventing stroke and systemic embolic events in people with atrial fibrillation (AF). This study assessed the effectiveness and safety of treatment with factor Xa inhibitors versus VKAs for preventing cerebral or systemic embolic events in AF.
Study design: Cochrane Review update.
Setting: Data obtained from trial registers of the Cochrane Central Register of Controlled Trials (August 2017), the Cochrane Heart Group and the Cochrane Stroke Group (September 2016), Embase (1980 to April 2017), and MEDLINE (1950 to April 2017). Authors also screened reference lists and contacted pharmaceutical companies, authors, and sponsors of relevant published trials.
Synopsis: The study included 42,084 participants from 10 trials with a diagnosis of AF who were eligible for long-term anticoagulation with warfarin (target INR 2-3).
The trials directly compared dose-adjusted warfarin with factor Xa inhibitors. Median follow-up ranged from 12 weeks to 1.9 years, and composite primary endpoint was all strokes (both ischemic and hemorrhagic) and non–central nervous systemic embolic events. Factor Xa inhibitor significantly decreased the number of strokes and systemic embolic events, compared with dose-adjusted warfarin (odds ratio, 0.81; 95% confidence interval, 0.72-0.91), reduced the number of major bleeding events (OR, 0.92; 95% CI, 0.63-1.34), and significantly reduced the risk of intracranial hemorrhage (OR, 0.56; 95% CI, 0.45-0.70). They also significantly reduced the number of all-cause deaths (OR, 0.88; 95% CI, 0.81-0.97). One limitation of this study is the heterogeneity and hence lower quality of evidence. This study shows a small net clinical benefit of using factor Xa inhibitors in AF because of a reduction in strokes and systemic embolic events and also a lower risk of bleeding (including intracranial hemorrhages), compared with using warfarin.
Bottom line: Patients with AF have a lower incidence of strokes and systemic embolic events when treated with factor Xa inhibitors, compared with those treated with warfarin.
Citation: Bruins Slot KM et al. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev. 2018 Mar 6. doi: 10.1002/14651858.CD008980.pub3.
Dr. Veedu is a hospitalist and instructor in the division of hospital medicine at the University of Kentucky, Lexington.
How to handle anorexia in community hospitals
Food is nonnegotiable
ATLANTA – Everyone has to be on the same page when it comes to anorexia nervosa in a community hospital, according to pediatric hospitalists at Moses H. Cone Memorial Hospital in Greensboro, N.C.
Anorexia cases used to be rare there. When one came in, “everyone was anxious because we just didn’t know quite what to do,” said Suresh Nagappan, MD, a pediatrician and member of the teaching faculty at the hospital. Parents would hear one thing from one provider, something else from the next, and leave angry and confused. “Basically, it was a mess. We needed to standardize it,” he added.
So Dr. Nagappan and his colleagues created guidelines for treating patients with eating disorders about 3 years ago. “It was meeting after meeting for months, but well worth it,” he said at Pediatric Hospital Medicine.
Word of the hospital’s newfound expertise in anorexia has spread since then, and now it’s not unusual for Moses H. Cone to handle a few cases a week.
The pediatric hospitalist team has come to realize that, first and foremost, patients and families need to know why they are there; it’s about medical stabilization, not treating the eating disorder. That comes after discharge. Families need help sometimes to understand that it’s not a quick fix.
To make things clear, there’s strict criteria now for admission, based on American Academy of Pediatrics guidance. The main trigger is being under 75% of ideal body weight, but patients must also have systolic blood pressure below 90 mm Hg and other worrisome signs. “Sometimes, it feels like we’re splitting hairs” on who gets admitted, “but if we don’t have strict criteria on admission, we don’t have an end goal for discharge,” said pediatrician Maggie S. Hall, MD, also on the Moses H. Cone teaching faculty.
As for treatment, “food is medicine, and it’s not negotiable. We make that clear to everyone on day 1. If patients don’t eat their actual meal, they have 20 minutes to drink a supplement. If they can’t do that, they get a nasogastric tube,” Dr. Nagappan said. The tube is pulled after each meal, so that it remains an incentive to eat.
The team start patients with 1,600 calories a day and increase the intake by 200-250 calories a day. The goal is for a patient to gain 100-200 grams per day. Patients pick out what they want to eat with the help of a dietitian. When meals set off overwhelming anxiety, the Moses H. Cone team has learned that benzodiazepines can help.
Ironically, the initiation of regular meals is the most dangerous time for patients. As anorexic bodies switch from catabolic to anabolic metabolism, electrolytes can drop to dangerously low levels, causing arrhythmias, heart failure, and death. In general, “the reason these kids die is cardiac,” Dr. Nagappan said at the meeting, sponsored by the Society of Hospital Medicine, the AAP, and the Academic Pediatric Association.
Refeeding syndrome, as it’s known, is clinically significant in perhaps 6% of patients. The risk goes up if they are below 70% of their ideal body weight; have a prolonged QTc interval; or begin treatment with low phosphorous, magnesium, or potassium.
To counter the threat, electrolytes are measured twice a day at Moses H. Cone during the first week of treatment, and ECGs are taken daily for the first few days. “One thing to be really careful about is when you notice their heart rate beginning to creep up during rest. That can be a sign of developing cardiomyopathy; it’s an indication for us to get echocardiograms,” Dr. Hall said.
The Moses H. Cone team like to include families in meal times – it’s been shown to help – but family members need to be coached beforehand. They can’t be punitive. Mealtime talk has to be positive, and can’t focus on eating. Parents often need help handling their own anger and guilt before trying to eat with their child. Progress has to be monitored, but Dr. Nagappan cautioned that “you have to be really careful about how you get weights”; it should always be in the morning after the first void. Urine needs to be checked to make sure patients aren’t water loading.
Staff should be neutral about weight results, and keep them to themselves. Even something as benign as “good job” can be a problem. “You don’t want these patients focused on their weight. You want them focused on getting better and eating and taking it step by step,” he said.
The presenters had no disclosures to report.
Food is nonnegotiable
Food is nonnegotiable
ATLANTA – Everyone has to be on the same page when it comes to anorexia nervosa in a community hospital, according to pediatric hospitalists at Moses H. Cone Memorial Hospital in Greensboro, N.C.
Anorexia cases used to be rare there. When one came in, “everyone was anxious because we just didn’t know quite what to do,” said Suresh Nagappan, MD, a pediatrician and member of the teaching faculty at the hospital. Parents would hear one thing from one provider, something else from the next, and leave angry and confused. “Basically, it was a mess. We needed to standardize it,” he added.
So Dr. Nagappan and his colleagues created guidelines for treating patients with eating disorders about 3 years ago. “It was meeting after meeting for months, but well worth it,” he said at Pediatric Hospital Medicine.
Word of the hospital’s newfound expertise in anorexia has spread since then, and now it’s not unusual for Moses H. Cone to handle a few cases a week.
The pediatric hospitalist team has come to realize that, first and foremost, patients and families need to know why they are there; it’s about medical stabilization, not treating the eating disorder. That comes after discharge. Families need help sometimes to understand that it’s not a quick fix.
To make things clear, there’s strict criteria now for admission, based on American Academy of Pediatrics guidance. The main trigger is being under 75% of ideal body weight, but patients must also have systolic blood pressure below 90 mm Hg and other worrisome signs. “Sometimes, it feels like we’re splitting hairs” on who gets admitted, “but if we don’t have strict criteria on admission, we don’t have an end goal for discharge,” said pediatrician Maggie S. Hall, MD, also on the Moses H. Cone teaching faculty.
As for treatment, “food is medicine, and it’s not negotiable. We make that clear to everyone on day 1. If patients don’t eat their actual meal, they have 20 minutes to drink a supplement. If they can’t do that, they get a nasogastric tube,” Dr. Nagappan said. The tube is pulled after each meal, so that it remains an incentive to eat.
The team start patients with 1,600 calories a day and increase the intake by 200-250 calories a day. The goal is for a patient to gain 100-200 grams per day. Patients pick out what they want to eat with the help of a dietitian. When meals set off overwhelming anxiety, the Moses H. Cone team has learned that benzodiazepines can help.
Ironically, the initiation of regular meals is the most dangerous time for patients. As anorexic bodies switch from catabolic to anabolic metabolism, electrolytes can drop to dangerously low levels, causing arrhythmias, heart failure, and death. In general, “the reason these kids die is cardiac,” Dr. Nagappan said at the meeting, sponsored by the Society of Hospital Medicine, the AAP, and the Academic Pediatric Association.
Refeeding syndrome, as it’s known, is clinically significant in perhaps 6% of patients. The risk goes up if they are below 70% of their ideal body weight; have a prolonged QTc interval; or begin treatment with low phosphorous, magnesium, or potassium.
To counter the threat, electrolytes are measured twice a day at Moses H. Cone during the first week of treatment, and ECGs are taken daily for the first few days. “One thing to be really careful about is when you notice their heart rate beginning to creep up during rest. That can be a sign of developing cardiomyopathy; it’s an indication for us to get echocardiograms,” Dr. Hall said.
The Moses H. Cone team like to include families in meal times – it’s been shown to help – but family members need to be coached beforehand. They can’t be punitive. Mealtime talk has to be positive, and can’t focus on eating. Parents often need help handling their own anger and guilt before trying to eat with their child. Progress has to be monitored, but Dr. Nagappan cautioned that “you have to be really careful about how you get weights”; it should always be in the morning after the first void. Urine needs to be checked to make sure patients aren’t water loading.
Staff should be neutral about weight results, and keep them to themselves. Even something as benign as “good job” can be a problem. “You don’t want these patients focused on their weight. You want them focused on getting better and eating and taking it step by step,” he said.
The presenters had no disclosures to report.
ATLANTA – Everyone has to be on the same page when it comes to anorexia nervosa in a community hospital, according to pediatric hospitalists at Moses H. Cone Memorial Hospital in Greensboro, N.C.
Anorexia cases used to be rare there. When one came in, “everyone was anxious because we just didn’t know quite what to do,” said Suresh Nagappan, MD, a pediatrician and member of the teaching faculty at the hospital. Parents would hear one thing from one provider, something else from the next, and leave angry and confused. “Basically, it was a mess. We needed to standardize it,” he added.
So Dr. Nagappan and his colleagues created guidelines for treating patients with eating disorders about 3 years ago. “It was meeting after meeting for months, but well worth it,” he said at Pediatric Hospital Medicine.
Word of the hospital’s newfound expertise in anorexia has spread since then, and now it’s not unusual for Moses H. Cone to handle a few cases a week.
The pediatric hospitalist team has come to realize that, first and foremost, patients and families need to know why they are there; it’s about medical stabilization, not treating the eating disorder. That comes after discharge. Families need help sometimes to understand that it’s not a quick fix.
To make things clear, there’s strict criteria now for admission, based on American Academy of Pediatrics guidance. The main trigger is being under 75% of ideal body weight, but patients must also have systolic blood pressure below 90 mm Hg and other worrisome signs. “Sometimes, it feels like we’re splitting hairs” on who gets admitted, “but if we don’t have strict criteria on admission, we don’t have an end goal for discharge,” said pediatrician Maggie S. Hall, MD, also on the Moses H. Cone teaching faculty.
As for treatment, “food is medicine, and it’s not negotiable. We make that clear to everyone on day 1. If patients don’t eat their actual meal, they have 20 minutes to drink a supplement. If they can’t do that, they get a nasogastric tube,” Dr. Nagappan said. The tube is pulled after each meal, so that it remains an incentive to eat.
The team start patients with 1,600 calories a day and increase the intake by 200-250 calories a day. The goal is for a patient to gain 100-200 grams per day. Patients pick out what they want to eat with the help of a dietitian. When meals set off overwhelming anxiety, the Moses H. Cone team has learned that benzodiazepines can help.
Ironically, the initiation of regular meals is the most dangerous time for patients. As anorexic bodies switch from catabolic to anabolic metabolism, electrolytes can drop to dangerously low levels, causing arrhythmias, heart failure, and death. In general, “the reason these kids die is cardiac,” Dr. Nagappan said at the meeting, sponsored by the Society of Hospital Medicine, the AAP, and the Academic Pediatric Association.
Refeeding syndrome, as it’s known, is clinically significant in perhaps 6% of patients. The risk goes up if they are below 70% of their ideal body weight; have a prolonged QTc interval; or begin treatment with low phosphorous, magnesium, or potassium.
To counter the threat, electrolytes are measured twice a day at Moses H. Cone during the first week of treatment, and ECGs are taken daily for the first few days. “One thing to be really careful about is when you notice their heart rate beginning to creep up during rest. That can be a sign of developing cardiomyopathy; it’s an indication for us to get echocardiograms,” Dr. Hall said.
The Moses H. Cone team like to include families in meal times – it’s been shown to help – but family members need to be coached beforehand. They can’t be punitive. Mealtime talk has to be positive, and can’t focus on eating. Parents often need help handling their own anger and guilt before trying to eat with their child. Progress has to be monitored, but Dr. Nagappan cautioned that “you have to be really careful about how you get weights”; it should always be in the morning after the first void. Urine needs to be checked to make sure patients aren’t water loading.
Staff should be neutral about weight results, and keep them to themselves. Even something as benign as “good job” can be a problem. “You don’t want these patients focused on their weight. You want them focused on getting better and eating and taking it step by step,” he said.
The presenters had no disclosures to report.
EXPERT ANALYSIS FROM PHM 2018
Short Takes
Digoxin and mortality in atrial fibrillation
Using propensity score-matched controls, post hoc subgroup analysis of the ARISTOTLE trial showed an independent dose-dependent association between serum digoxin levels and mortality in those receiving digoxin, with a 19% higher adjusted hazard of death for each increase of 0.5 ng/mL (P = .001). For those initiating digoxin there was an independent association with higher mortality, regardless of heart failure (adjusted hazard ratio, 1.78; 95% confidence interval, 1.37-2.31; P less than .0001).
Citation: Lopes RD et al. Digoxin and mortality in patients with atrial fibrillation. J Am Coll Cardiol. 2018 Mar 13;71(10):1063-74.
ED opioid overdoses
Prior studies have shown a recent increase in opioid overdose-related deaths, and this analysis of 136 million ED visits in 45 states showed a continued upward trend from July 2016 to September 2017 with average increases of 5.6% per quarter in all regions and across all demographic groups, but this increase was especially pronounced in urban areas. The authors of this analysis called for the medical community to use these data to educate providers and organize resources for the rapidly evolving opioid epidemic.
Citation: Vivolo-Kantor AM et al. Vital signs: Trends in emergency department visits for suspected opioid overdoses - United States, July 2016-September 2017. MMWR Morb Mortal Wkly Rep. 2018;67:279-85.
Routine oxygen therapy in patients with acute MI with normal oxygen saturation levels has no benefit
A large meta-analysis showed no decrease in all-cause mortality, recurrent ischemia, or myocardial infarction, heart failure, and arrhythmia from using routine oxygen therapy in patients with acute myocardial infarction with normal oxygen saturation levels. This meta-analysis confirmed prior studies and supported the changing trend in recommendations to avoid supplemental oxygen in patients with peripheral oxygen saturations greater than 90%.
Citation: Abuzaid A et al. Oxygen therapy in patients with acute myocardial infarction: A systemic review and meta-analysis. Am J Med. 2018 Jun;131(6):693-701.
Predicting hospitalization for acute kidney injury
Data from the 2000-2014 National Inpatient Sample and National Health Interview Surveys indicated that people with diabetes are nearly four times more likely to have acute kidney injury-related hospitalization than are people without. The analysis may have limitations for multiple admissions being counted several times, underestimation of prevalence of acute kidney injury, increased awareness regarding acute kidney injury, and unknown effects of type and duration of diabetes.
Citation: Pavkov ME et al. Trends in hospitalization of acute kidney injury - United States, 2000-2014. MMWR. 2018 Mar 16;67(10):289-93.
Digoxin and mortality in atrial fibrillation
Using propensity score-matched controls, post hoc subgroup analysis of the ARISTOTLE trial showed an independent dose-dependent association between serum digoxin levels and mortality in those receiving digoxin, with a 19% higher adjusted hazard of death for each increase of 0.5 ng/mL (P = .001). For those initiating digoxin there was an independent association with higher mortality, regardless of heart failure (adjusted hazard ratio, 1.78; 95% confidence interval, 1.37-2.31; P less than .0001).
Citation: Lopes RD et al. Digoxin and mortality in patients with atrial fibrillation. J Am Coll Cardiol. 2018 Mar 13;71(10):1063-74.
ED opioid overdoses
Prior studies have shown a recent increase in opioid overdose-related deaths, and this analysis of 136 million ED visits in 45 states showed a continued upward trend from July 2016 to September 2017 with average increases of 5.6% per quarter in all regions and across all demographic groups, but this increase was especially pronounced in urban areas. The authors of this analysis called for the medical community to use these data to educate providers and organize resources for the rapidly evolving opioid epidemic.
Citation: Vivolo-Kantor AM et al. Vital signs: Trends in emergency department visits for suspected opioid overdoses - United States, July 2016-September 2017. MMWR Morb Mortal Wkly Rep. 2018;67:279-85.
Routine oxygen therapy in patients with acute MI with normal oxygen saturation levels has no benefit
A large meta-analysis showed no decrease in all-cause mortality, recurrent ischemia, or myocardial infarction, heart failure, and arrhythmia from using routine oxygen therapy in patients with acute myocardial infarction with normal oxygen saturation levels. This meta-analysis confirmed prior studies and supported the changing trend in recommendations to avoid supplemental oxygen in patients with peripheral oxygen saturations greater than 90%.
Citation: Abuzaid A et al. Oxygen therapy in patients with acute myocardial infarction: A systemic review and meta-analysis. Am J Med. 2018 Jun;131(6):693-701.
Predicting hospitalization for acute kidney injury
Data from the 2000-2014 National Inpatient Sample and National Health Interview Surveys indicated that people with diabetes are nearly four times more likely to have acute kidney injury-related hospitalization than are people without. The analysis may have limitations for multiple admissions being counted several times, underestimation of prevalence of acute kidney injury, increased awareness regarding acute kidney injury, and unknown effects of type and duration of diabetes.
Citation: Pavkov ME et al. Trends in hospitalization of acute kidney injury - United States, 2000-2014. MMWR. 2018 Mar 16;67(10):289-93.
Digoxin and mortality in atrial fibrillation
Using propensity score-matched controls, post hoc subgroup analysis of the ARISTOTLE trial showed an independent dose-dependent association between serum digoxin levels and mortality in those receiving digoxin, with a 19% higher adjusted hazard of death for each increase of 0.5 ng/mL (P = .001). For those initiating digoxin there was an independent association with higher mortality, regardless of heart failure (adjusted hazard ratio, 1.78; 95% confidence interval, 1.37-2.31; P less than .0001).
Citation: Lopes RD et al. Digoxin and mortality in patients with atrial fibrillation. J Am Coll Cardiol. 2018 Mar 13;71(10):1063-74.
ED opioid overdoses
Prior studies have shown a recent increase in opioid overdose-related deaths, and this analysis of 136 million ED visits in 45 states showed a continued upward trend from July 2016 to September 2017 with average increases of 5.6% per quarter in all regions and across all demographic groups, but this increase was especially pronounced in urban areas. The authors of this analysis called for the medical community to use these data to educate providers and organize resources for the rapidly evolving opioid epidemic.
Citation: Vivolo-Kantor AM et al. Vital signs: Trends in emergency department visits for suspected opioid overdoses - United States, July 2016-September 2017. MMWR Morb Mortal Wkly Rep. 2018;67:279-85.
Routine oxygen therapy in patients with acute MI with normal oxygen saturation levels has no benefit
A large meta-analysis showed no decrease in all-cause mortality, recurrent ischemia, or myocardial infarction, heart failure, and arrhythmia from using routine oxygen therapy in patients with acute myocardial infarction with normal oxygen saturation levels. This meta-analysis confirmed prior studies and supported the changing trend in recommendations to avoid supplemental oxygen in patients with peripheral oxygen saturations greater than 90%.
Citation: Abuzaid A et al. Oxygen therapy in patients with acute myocardial infarction: A systemic review and meta-analysis. Am J Med. 2018 Jun;131(6):693-701.
Predicting hospitalization for acute kidney injury
Data from the 2000-2014 National Inpatient Sample and National Health Interview Surveys indicated that people with diabetes are nearly four times more likely to have acute kidney injury-related hospitalization than are people without. The analysis may have limitations for multiple admissions being counted several times, underestimation of prevalence of acute kidney injury, increased awareness regarding acute kidney injury, and unknown effects of type and duration of diabetes.
Citation: Pavkov ME et al. Trends in hospitalization of acute kidney injury - United States, 2000-2014. MMWR. 2018 Mar 16;67(10):289-93.
A new antiemetic
Clinical question: Can aromatherapy with isopropyl alcohol confer an adjunctive and lasting benefit as an antiemetic in ED patients who do not otherwise need IV access?
Background: Prior studies have shown a benefit of aromatherapy with isopropyl alcohol for postoperative nausea and vomiting, and it is both widely available and safe. Only one randomized, controlled study exists documenting use of aromatherapy with isopropyl alcohol in the ED, but this monitored for effects for only 10 minutes and did not compare it with other antiemetic therapies used in the emergency department.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Single urban tertiary care center emergency department.
Synopsis: The study separated 120 patients with nausea who did not otherwise need intravenous access into three treatment groups. They were assessed for improvement in nausea using a visual analog scale 30 minutes after administration of either oral ondansetron 4 mg and inhaled isopropyl alcohol, oral placebo and inhaled isopropyl alcohol, or oral ondansetron and inhaled saline. The mean decrease in nausea visual analog scale score was 30 mm (95% confidence interval, 22-37 mm), 32 mm (95% CI, 25-39 mm), and 9 mm (95% CI, 5-14 mm), respectively. The need for rescue antiemetics was 27.5%, 25%, and 45%, respectively. This study is limited by its small size, its relatively healthy population with a predominant diagnosis of gastroenteritis, and that patients who required IV catheters were excluded; therefore, it may not be generalizable to sicker patients with alternative etiologies for nausea. Furthermore, many patients were able to distinguish isopropyl alcohol from placebo inhalant by smell so the blinding was possibly ineffective. However, since isopropyl alcohol is low risk, inexpensive, and readily available, it may be reasonable to consider this as a therapeutic option for some patients.
Bottom line: In ED patients who did not otherwise need intravenous access, aromatherapy with inhaled isopropyl alcohol alone or with ondansetron was superior for nausea relief when compared with ondansetron alone.
Citation: April MD et al. Aromatherapy versus oral ondansetron for antiemetic therapy among adult emergency department patients: A randomized controlled trial. Ann Emerg Med. 2018 Feb 17. doi: 10.1016/j.annemergmed.2018.01.016.
Dr. Sayers is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Clinical question: Can aromatherapy with isopropyl alcohol confer an adjunctive and lasting benefit as an antiemetic in ED patients who do not otherwise need IV access?
Background: Prior studies have shown a benefit of aromatherapy with isopropyl alcohol for postoperative nausea and vomiting, and it is both widely available and safe. Only one randomized, controlled study exists documenting use of aromatherapy with isopropyl alcohol in the ED, but this monitored for effects for only 10 minutes and did not compare it with other antiemetic therapies used in the emergency department.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Single urban tertiary care center emergency department.
Synopsis: The study separated 120 patients with nausea who did not otherwise need intravenous access into three treatment groups. They were assessed for improvement in nausea using a visual analog scale 30 minutes after administration of either oral ondansetron 4 mg and inhaled isopropyl alcohol, oral placebo and inhaled isopropyl alcohol, or oral ondansetron and inhaled saline. The mean decrease in nausea visual analog scale score was 30 mm (95% confidence interval, 22-37 mm), 32 mm (95% CI, 25-39 mm), and 9 mm (95% CI, 5-14 mm), respectively. The need for rescue antiemetics was 27.5%, 25%, and 45%, respectively. This study is limited by its small size, its relatively healthy population with a predominant diagnosis of gastroenteritis, and that patients who required IV catheters were excluded; therefore, it may not be generalizable to sicker patients with alternative etiologies for nausea. Furthermore, many patients were able to distinguish isopropyl alcohol from placebo inhalant by smell so the blinding was possibly ineffective. However, since isopropyl alcohol is low risk, inexpensive, and readily available, it may be reasonable to consider this as a therapeutic option for some patients.
Bottom line: In ED patients who did not otherwise need intravenous access, aromatherapy with inhaled isopropyl alcohol alone or with ondansetron was superior for nausea relief when compared with ondansetron alone.
Citation: April MD et al. Aromatherapy versus oral ondansetron for antiemetic therapy among adult emergency department patients: A randomized controlled trial. Ann Emerg Med. 2018 Feb 17. doi: 10.1016/j.annemergmed.2018.01.016.
Dr. Sayers is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Clinical question: Can aromatherapy with isopropyl alcohol confer an adjunctive and lasting benefit as an antiemetic in ED patients who do not otherwise need IV access?
Background: Prior studies have shown a benefit of aromatherapy with isopropyl alcohol for postoperative nausea and vomiting, and it is both widely available and safe. Only one randomized, controlled study exists documenting use of aromatherapy with isopropyl alcohol in the ED, but this monitored for effects for only 10 minutes and did not compare it with other antiemetic therapies used in the emergency department.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Single urban tertiary care center emergency department.
Synopsis: The study separated 120 patients with nausea who did not otherwise need intravenous access into three treatment groups. They were assessed for improvement in nausea using a visual analog scale 30 minutes after administration of either oral ondansetron 4 mg and inhaled isopropyl alcohol, oral placebo and inhaled isopropyl alcohol, or oral ondansetron and inhaled saline. The mean decrease in nausea visual analog scale score was 30 mm (95% confidence interval, 22-37 mm), 32 mm (95% CI, 25-39 mm), and 9 mm (95% CI, 5-14 mm), respectively. The need for rescue antiemetics was 27.5%, 25%, and 45%, respectively. This study is limited by its small size, its relatively healthy population with a predominant diagnosis of gastroenteritis, and that patients who required IV catheters were excluded; therefore, it may not be generalizable to sicker patients with alternative etiologies for nausea. Furthermore, many patients were able to distinguish isopropyl alcohol from placebo inhalant by smell so the blinding was possibly ineffective. However, since isopropyl alcohol is low risk, inexpensive, and readily available, it may be reasonable to consider this as a therapeutic option for some patients.
Bottom line: In ED patients who did not otherwise need intravenous access, aromatherapy with inhaled isopropyl alcohol alone or with ondansetron was superior for nausea relief when compared with ondansetron alone.
Citation: April MD et al. Aromatherapy versus oral ondansetron for antiemetic therapy among adult emergency department patients: A randomized controlled trial. Ann Emerg Med. 2018 Feb 17. doi: 10.1016/j.annemergmed.2018.01.016.
Dr. Sayers is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Acute biliary pancreatitis linked to poor outcomes in elderly
Mortality was almost three times as high in elderly patients after stringent matching for confounding variables, wrote researcher Kishan Patel, MD, of the Ohio State University, Columbus, and coauthors.
These findings represent a “current health care concern,” since the elderly population in the United States is expected to double within the next several decades and the prevalence of acute pancreatitis is on the rise, Dr. Patel and colleagues wrote in a report on the analysis in the Journal of Clinical Gastroenterology.
The analysis is the first, to the investigators’ knowledge, that addresses national-level outcomes associated with acute biliary pancreatitis in elderly patients.
To evaluate clinical outcomes of elderly patients with acute biliary pancreatitis, Dr. Patel and colleagues queried the Nationwide Readmissions Database, which is the largest inpatient readmission database in the United States.
The investigators looked at outcomes associated with index hospitalizations, defined as a patient’s first hospitalization in a calendar year, and found 184,763 adult patients who received a diagnosis of acute biliary pancreatitis between 2011 and 2014. Of those, 41% were elderly.
The mortality rate associated with the index admission was 1.96% (n = 356) for the elderly patients, compared with just 0.32% (n = 1,473) for nonelderly patients (P less than .001), according to the report.
Mortality was increased in the elderly versus nonelderly patients, with an odds ratio of 2.8 (95% CI, 2.2-3.5), according to results of a propensity score matched analysis. Likewise, severe acute pancreatitis was increased in the elderly, with an OR of 1.2 (95% CI: 1.1-1.3) in that analysis.
By contrast, patient age did not impact 30-day readmission rates, according to results of a multivariate analysis that adjusted for confounding factors.
Mortality and severe acute pancreatitis both increased with age within the elderly cohort, further multivariate analysis showed. For example, the ORs for mortality were 1.39 for patients aged 75-84 years and 2.21 for patients aged 85 years and older, the results show.
The elderly population in the United States is expected to almost double by 2050, rising from 48 to 88 million, Dr. Patel and colleagues said. The number of those aged 85 years or older is expected to increase from 5.9 to 18 million by 2050, at which time they will make up nearly 5% of the total U.S. population.
“This specific demographic is more susceptible to common medical ailments, more troubling is acute pancreatitis is one of the most frequent causes of hospitalization in gastroenterology,” Dr. Patel and colleagues wrote.
Dr. Patel and coauthors reported no conflicts of interest related to the analysis.
SOURCE: Patel K et al. J Clin Gastroenterol. 2018 Aug 28. doi: 10.1097/MCG.0000000000001108.
Mortality was almost three times as high in elderly patients after stringent matching for confounding variables, wrote researcher Kishan Patel, MD, of the Ohio State University, Columbus, and coauthors.
These findings represent a “current health care concern,” since the elderly population in the United States is expected to double within the next several decades and the prevalence of acute pancreatitis is on the rise, Dr. Patel and colleagues wrote in a report on the analysis in the Journal of Clinical Gastroenterology.
The analysis is the first, to the investigators’ knowledge, that addresses national-level outcomes associated with acute biliary pancreatitis in elderly patients.
To evaluate clinical outcomes of elderly patients with acute biliary pancreatitis, Dr. Patel and colleagues queried the Nationwide Readmissions Database, which is the largest inpatient readmission database in the United States.
The investigators looked at outcomes associated with index hospitalizations, defined as a patient’s first hospitalization in a calendar year, and found 184,763 adult patients who received a diagnosis of acute biliary pancreatitis between 2011 and 2014. Of those, 41% were elderly.
The mortality rate associated with the index admission was 1.96% (n = 356) for the elderly patients, compared with just 0.32% (n = 1,473) for nonelderly patients (P less than .001), according to the report.
Mortality was increased in the elderly versus nonelderly patients, with an odds ratio of 2.8 (95% CI, 2.2-3.5), according to results of a propensity score matched analysis. Likewise, severe acute pancreatitis was increased in the elderly, with an OR of 1.2 (95% CI: 1.1-1.3) in that analysis.
By contrast, patient age did not impact 30-day readmission rates, according to results of a multivariate analysis that adjusted for confounding factors.
Mortality and severe acute pancreatitis both increased with age within the elderly cohort, further multivariate analysis showed. For example, the ORs for mortality were 1.39 for patients aged 75-84 years and 2.21 for patients aged 85 years and older, the results show.
The elderly population in the United States is expected to almost double by 2050, rising from 48 to 88 million, Dr. Patel and colleagues said. The number of those aged 85 years or older is expected to increase from 5.9 to 18 million by 2050, at which time they will make up nearly 5% of the total U.S. population.
“This specific demographic is more susceptible to common medical ailments, more troubling is acute pancreatitis is one of the most frequent causes of hospitalization in gastroenterology,” Dr. Patel and colleagues wrote.
Dr. Patel and coauthors reported no conflicts of interest related to the analysis.
SOURCE: Patel K et al. J Clin Gastroenterol. 2018 Aug 28. doi: 10.1097/MCG.0000000000001108.
Mortality was almost three times as high in elderly patients after stringent matching for confounding variables, wrote researcher Kishan Patel, MD, of the Ohio State University, Columbus, and coauthors.
These findings represent a “current health care concern,” since the elderly population in the United States is expected to double within the next several decades and the prevalence of acute pancreatitis is on the rise, Dr. Patel and colleagues wrote in a report on the analysis in the Journal of Clinical Gastroenterology.
The analysis is the first, to the investigators’ knowledge, that addresses national-level outcomes associated with acute biliary pancreatitis in elderly patients.
To evaluate clinical outcomes of elderly patients with acute biliary pancreatitis, Dr. Patel and colleagues queried the Nationwide Readmissions Database, which is the largest inpatient readmission database in the United States.
The investigators looked at outcomes associated with index hospitalizations, defined as a patient’s first hospitalization in a calendar year, and found 184,763 adult patients who received a diagnosis of acute biliary pancreatitis between 2011 and 2014. Of those, 41% were elderly.
The mortality rate associated with the index admission was 1.96% (n = 356) for the elderly patients, compared with just 0.32% (n = 1,473) for nonelderly patients (P less than .001), according to the report.
Mortality was increased in the elderly versus nonelderly patients, with an odds ratio of 2.8 (95% CI, 2.2-3.5), according to results of a propensity score matched analysis. Likewise, severe acute pancreatitis was increased in the elderly, with an OR of 1.2 (95% CI: 1.1-1.3) in that analysis.
By contrast, patient age did not impact 30-day readmission rates, according to results of a multivariate analysis that adjusted for confounding factors.
Mortality and severe acute pancreatitis both increased with age within the elderly cohort, further multivariate analysis showed. For example, the ORs for mortality were 1.39 for patients aged 75-84 years and 2.21 for patients aged 85 years and older, the results show.
The elderly population in the United States is expected to almost double by 2050, rising from 48 to 88 million, Dr. Patel and colleagues said. The number of those aged 85 years or older is expected to increase from 5.9 to 18 million by 2050, at which time they will make up nearly 5% of the total U.S. population.
“This specific demographic is more susceptible to common medical ailments, more troubling is acute pancreatitis is one of the most frequent causes of hospitalization in gastroenterology,” Dr. Patel and colleagues wrote.
Dr. Patel and coauthors reported no conflicts of interest related to the analysis.
SOURCE: Patel K et al. J Clin Gastroenterol. 2018 Aug 28. doi: 10.1097/MCG.0000000000001108.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Key clinical point: Compared with younger patients, elderly patients admitted for acute biliary pancreatitis have increased rates of adverse outcomes.
Major finding: Elderly patients had increased mortality (odds ratio, 2.8; 95% confidence interval, 2.2-3.5) and severe acute pancreatitis (OR, 1.2; 95% CI: 1.1-1.3).
Study details: A propensity score matched analysis of a large, nationally representative database including nearly 185,000 adults with acute biliary pancreatitis.
Disclosures: The study authors reported no conflicts of interest related to the study.
Source: Patel K et al. J Clin Gastroenterol. 2018 Aug 28. doi: 10.1097/MCG.0000000000001108.
Penalties not necessary to save money in some Medicare ACOs
The Centers for Medicare & Medicaid Services may be able to reduce spending through the Medicare Shared Savings Program (MSSP) without asking for health care professionals and organizations to take on penalties or so-called downside risk, according to a study published in Sept. 5 in the New England Journal of Medicine.
Researchers, using fee-for-service claims from 2009 through 2015 and performing difference-in-difference analyses to compare changes in Medicare spending, found that Accountable Care Organizations (ACOs) formed from physician practices were able to save money while hospital-based ACOs were not.
“Our results also suggest that shared-savings contracts that do not impose a downside risk of financial losses for spending above benchmarks – which may appeal to smaller organizations without sufficient reserves to withstand potential losses – may be effective in lowering Medicare spending,” J. Michael McWilliams, MD, PhD, of Harvard Medical School, Boston, and his colleagues wrote.
Researchers found that by 2015, groups participating in MSSP, as compared with those who did not participate, were “associated with a mean differential reduction of $302 in total Medicare spending per beneficiary in the 2012 entry of cohorts of ACOs,” without accounting for bonus payments.
“Accounting for shared-savings bonus payments, we determined that the differential spending reductions in the entry cohorts of physician-group ACOs from 2012 through 2014 constituted a net savings to Medicare of $256.4 million in 2015,” Dr. McWilliams and his colleagues wrote. “For hospital-integrated ACOs, bonus payments more than offset annual spending reductions.”
Dr. McWilliams and his colleagues noted that their findings were limited by a narrow focus on organizational structure (financial independence from hospitals), so other factors could have held to differences in savings; changes in coding practices for ACOs coming in as of 2013; lack of data on costs to ACOs or efforts to lower spending or improve quality; and the inability to assess the effects of the MSSP on many aspects of quality of care because of the nature of using claims-based measures.
“Our results probably underestimate savings to Medicare because they do not account for spillover effects of ACO efforts on nonattributed patients or effects of lower fee-for-service Medicare spending on payments to Medicare Advantage plans,” the researchers added.
The study was funded by a grant from the National Institute on Aging. Dr. McWilliams and Michael Chernew, PhD, also of Harvard Medical School, both have received consulting fees related to ACO research.
SOURCE: McWilliams JM et al. N Engl J Med. 2018 Sep 5. doi: 10.1056/NEJMsa1803388.
The Centers for Medicare & Medicaid Services may be able to reduce spending through the Medicare Shared Savings Program (MSSP) without asking for health care professionals and organizations to take on penalties or so-called downside risk, according to a study published in Sept. 5 in the New England Journal of Medicine.
Researchers, using fee-for-service claims from 2009 through 2015 and performing difference-in-difference analyses to compare changes in Medicare spending, found that Accountable Care Organizations (ACOs) formed from physician practices were able to save money while hospital-based ACOs were not.
“Our results also suggest that shared-savings contracts that do not impose a downside risk of financial losses for spending above benchmarks – which may appeal to smaller organizations without sufficient reserves to withstand potential losses – may be effective in lowering Medicare spending,” J. Michael McWilliams, MD, PhD, of Harvard Medical School, Boston, and his colleagues wrote.
Researchers found that by 2015, groups participating in MSSP, as compared with those who did not participate, were “associated with a mean differential reduction of $302 in total Medicare spending per beneficiary in the 2012 entry of cohorts of ACOs,” without accounting for bonus payments.
“Accounting for shared-savings bonus payments, we determined that the differential spending reductions in the entry cohorts of physician-group ACOs from 2012 through 2014 constituted a net savings to Medicare of $256.4 million in 2015,” Dr. McWilliams and his colleagues wrote. “For hospital-integrated ACOs, bonus payments more than offset annual spending reductions.”
Dr. McWilliams and his colleagues noted that their findings were limited by a narrow focus on organizational structure (financial independence from hospitals), so other factors could have held to differences in savings; changes in coding practices for ACOs coming in as of 2013; lack of data on costs to ACOs or efforts to lower spending or improve quality; and the inability to assess the effects of the MSSP on many aspects of quality of care because of the nature of using claims-based measures.
“Our results probably underestimate savings to Medicare because they do not account for spillover effects of ACO efforts on nonattributed patients or effects of lower fee-for-service Medicare spending on payments to Medicare Advantage plans,” the researchers added.
The study was funded by a grant from the National Institute on Aging. Dr. McWilliams and Michael Chernew, PhD, also of Harvard Medical School, both have received consulting fees related to ACO research.
SOURCE: McWilliams JM et al. N Engl J Med. 2018 Sep 5. doi: 10.1056/NEJMsa1803388.
The Centers for Medicare & Medicaid Services may be able to reduce spending through the Medicare Shared Savings Program (MSSP) without asking for health care professionals and organizations to take on penalties or so-called downside risk, according to a study published in Sept. 5 in the New England Journal of Medicine.
Researchers, using fee-for-service claims from 2009 through 2015 and performing difference-in-difference analyses to compare changes in Medicare spending, found that Accountable Care Organizations (ACOs) formed from physician practices were able to save money while hospital-based ACOs were not.
“Our results also suggest that shared-savings contracts that do not impose a downside risk of financial losses for spending above benchmarks – which may appeal to smaller organizations without sufficient reserves to withstand potential losses – may be effective in lowering Medicare spending,” J. Michael McWilliams, MD, PhD, of Harvard Medical School, Boston, and his colleagues wrote.
Researchers found that by 2015, groups participating in MSSP, as compared with those who did not participate, were “associated with a mean differential reduction of $302 in total Medicare spending per beneficiary in the 2012 entry of cohorts of ACOs,” without accounting for bonus payments.
“Accounting for shared-savings bonus payments, we determined that the differential spending reductions in the entry cohorts of physician-group ACOs from 2012 through 2014 constituted a net savings to Medicare of $256.4 million in 2015,” Dr. McWilliams and his colleagues wrote. “For hospital-integrated ACOs, bonus payments more than offset annual spending reductions.”
Dr. McWilliams and his colleagues noted that their findings were limited by a narrow focus on organizational structure (financial independence from hospitals), so other factors could have held to differences in savings; changes in coding practices for ACOs coming in as of 2013; lack of data on costs to ACOs or efforts to lower spending or improve quality; and the inability to assess the effects of the MSSP on many aspects of quality of care because of the nature of using claims-based measures.
“Our results probably underestimate savings to Medicare because they do not account for spillover effects of ACO efforts on nonattributed patients or effects of lower fee-for-service Medicare spending on payments to Medicare Advantage plans,” the researchers added.
The study was funded by a grant from the National Institute on Aging. Dr. McWilliams and Michael Chernew, PhD, also of Harvard Medical School, both have received consulting fees related to ACO research.
SOURCE: McWilliams JM et al. N Engl J Med. 2018 Sep 5. doi: 10.1056/NEJMsa1803388.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Physician group ACOs in the Medicare Shared Savings Program (MSSP) generated more savings than did hospital-led ACO groups.
Major finding: Physician group ACOs joining the MSSP in 2012-2014 generated $256.4 million in Medicare savings in 2015.
Study details: Analysis of fee-for-service Medicare claims during 2009-2015.
Disclosures: The study was funded by the National Institute on Aging. Dr. McWilliams and Dr. Chernew disclosed consulting fees related to ACO research.
Source: McWilliams JM et al. N Engl J Med. doi: 10.1056/NEJMsa1803388.
Single-dose influenza drug baloxavir similar to oseltamivir in efficacy
A new single-dose influenza antiviral drug appears significantly better than placebo at relieving the symptoms of infection, and reduces viral load faster than does oseltamivir, new research suggests.
Baloxavir marboxil – a selective inhibitor of influenza cap-dependent endonuclease – was tested in two randomized, double-blind, controlled trials. The first was a double-blind, placebo-controlled, dose-ranging, phase 2 randomized trial of 389 Japanese adults aged 20-64 years with acute uncomplicated influenza from December 2015 through March 2016. The second was a phase 3 randomized controlled trial of 1,366 patients comparing baloxavir with placebo and oseltamivir.
The phase 2 study showed patients treated with 10 mg, 20 mg or 40 mg oral dose of baloxavir experienced a significantly shorter median time to symptom alleviation compared with placebo (54.2, 51, 49.5, and 77.7 hours, respectively), according to a paper published in the Sept. 6 edition of the New England Journal of Medicine.
In addition, all three doses showed significantly greater reductions in influenza virus titers on days 2 and 3, compared with placebo.
The phase 3 trial CAPSTONE-1 (NCT02954354) was a double-blind, placebo- and oseltamivir-controlled, randomized trial that enrolled outpatients aged 12-64 years with influenza-like illness in the United States and Japan from December 2016 through March 2017. Patients aged 20-64 years received a single, weight-based oral dose of baloxavir (40 mg for patients weighing more than 80 kg, 80 mg for those weighing 80 kg or less) on day 1 only or oseltamivir at a dose of 75 mg twice daily or matching placebos on a 5-day regimen.
Patients aged 12-19 years were randomly assigned to receive either baloxavir or placebo on day 1 only, according to the researchers.
The median time to alleviation of symptoms was similar in the baloxavir (53.5 hours) and oseltamivir group (53.8 hours). However, patients taking baloxavir had significantly faster declines in infectious viral load compared with those taking oseltamivir, which was taken as a 75-mg dose twice daily for 5 days. In addition, patients who were treated with baloxavir within 24 hours of symptom onset showed significantly shorter time to alleviation of symptoms compared with placebo than did those who started treatment more than 24 hours after symptoms began.
Adverse events related to the study drug were more common among patients taking oseltamivir (8.4%) compared with those taking baloxavir (4.4%) or placebo (3.9%). In the phase 2 study, the adverse event rate was lower in the three baloxavir dosage groups compared with the placebo group. The study also showed a similar low frequency of complications requiring antibiotic treatment in both the baloxavir, oseltamivir, and placebo arms.
Some patients did show evidence of decreased susceptibility to baloxavir; for example, PA I38T/M amino acid substitutions were seen in 9.7% of the patients taking baloxavir but none of randomly selected patients in the placebo group of the phase 3 trial.
“These trials showed that single doses of the cap-dependent endonuclease inhibitor baloxavir were superior to placebo in alleviating influenza symptoms in patients with uncomplicated influenza, without clinically significant side effects,” wrote Dr. Frederick G. Hayden of the University of Virginia, Charlottesville, and his coauthors.
“The antiviral effects that were observed with baloxavir in patients with uncomplicated influenza provide encouragement with respect to its potential value in treating complicated or severe influenza infections,” they noted.
Because the treatment was inhibitory for influenza virus strains that were resistant to neuraminidase inhibitors or M2 ion-channel inhibitors, it could be a treatment option for patients infected with those viruses, the researchers added.
CAPSTONE-2, a randomized, controlled trial involving patients at high risk for influenza complications (NCT02949011) is in progress.
The study was supported by Shionogi, which developed baloxavir. Seven authors declared fees from the pharmaceutical industry, including Shionogi. Six authors were employees of Shionogi, one also holding stock. No other conflicts of interest were declared.
SOURCE: Hayden F et al. N Engl J Med. 2018;379:913-23. doi: 10.1056/NEJMoa1716197.
These two studies of baloxavir show that the drug has a clinical benefit similar to that of oseltamivir in individuals with uncomplicated influenza infection. As a single-dose treatment, baloxavir has the advantage in reducing concerns about adherence compared to the treatment regimen for oseltamivir, which requires 5 days of twice-daily dosing.
However, these studies should be viewed as the first step. While baloxavir showed significantly greater reductions in viral load at 24 hours and a shorter duration of infectious virus detection than did oseltamivir or placebo, it also induced the emergence of viral escape mutants with reduced susceptibility.
It’s not yet known whether these influenza viruses with reduced susceptibility are transmissible, and whether surveillance for I38T and other markers will be needed. We also need trials to identify which patients are most likely to benefit from baloxavir, and the timing for treatment.
Timothy M. Uyeki, MD, is with the Influenza Division at the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. These comments are taken from an editorial (N Engl J Med. 2018;397:975-7. doi: 10.1056/NEJMe1810815. No conflicts of interest were declared.
These two studies of baloxavir show that the drug has a clinical benefit similar to that of oseltamivir in individuals with uncomplicated influenza infection. As a single-dose treatment, baloxavir has the advantage in reducing concerns about adherence compared to the treatment regimen for oseltamivir, which requires 5 days of twice-daily dosing.
However, these studies should be viewed as the first step. While baloxavir showed significantly greater reductions in viral load at 24 hours and a shorter duration of infectious virus detection than did oseltamivir or placebo, it also induced the emergence of viral escape mutants with reduced susceptibility.
It’s not yet known whether these influenza viruses with reduced susceptibility are transmissible, and whether surveillance for I38T and other markers will be needed. We also need trials to identify which patients are most likely to benefit from baloxavir, and the timing for treatment.
Timothy M. Uyeki, MD, is with the Influenza Division at the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. These comments are taken from an editorial (N Engl J Med. 2018;397:975-7. doi: 10.1056/NEJMe1810815. No conflicts of interest were declared.
These two studies of baloxavir show that the drug has a clinical benefit similar to that of oseltamivir in individuals with uncomplicated influenza infection. As a single-dose treatment, baloxavir has the advantage in reducing concerns about adherence compared to the treatment regimen for oseltamivir, which requires 5 days of twice-daily dosing.
However, these studies should be viewed as the first step. While baloxavir showed significantly greater reductions in viral load at 24 hours and a shorter duration of infectious virus detection than did oseltamivir or placebo, it also induced the emergence of viral escape mutants with reduced susceptibility.
It’s not yet known whether these influenza viruses with reduced susceptibility are transmissible, and whether surveillance for I38T and other markers will be needed. We also need trials to identify which patients are most likely to benefit from baloxavir, and the timing for treatment.
Timothy M. Uyeki, MD, is with the Influenza Division at the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. These comments are taken from an editorial (N Engl J Med. 2018;397:975-7. doi: 10.1056/NEJMe1810815. No conflicts of interest were declared.
A new single-dose influenza antiviral drug appears significantly better than placebo at relieving the symptoms of infection, and reduces viral load faster than does oseltamivir, new research suggests.
Baloxavir marboxil – a selective inhibitor of influenza cap-dependent endonuclease – was tested in two randomized, double-blind, controlled trials. The first was a double-blind, placebo-controlled, dose-ranging, phase 2 randomized trial of 389 Japanese adults aged 20-64 years with acute uncomplicated influenza from December 2015 through March 2016. The second was a phase 3 randomized controlled trial of 1,366 patients comparing baloxavir with placebo and oseltamivir.
The phase 2 study showed patients treated with 10 mg, 20 mg or 40 mg oral dose of baloxavir experienced a significantly shorter median time to symptom alleviation compared with placebo (54.2, 51, 49.5, and 77.7 hours, respectively), according to a paper published in the Sept. 6 edition of the New England Journal of Medicine.
In addition, all three doses showed significantly greater reductions in influenza virus titers on days 2 and 3, compared with placebo.
The phase 3 trial CAPSTONE-1 (NCT02954354) was a double-blind, placebo- and oseltamivir-controlled, randomized trial that enrolled outpatients aged 12-64 years with influenza-like illness in the United States and Japan from December 2016 through March 2017. Patients aged 20-64 years received a single, weight-based oral dose of baloxavir (40 mg for patients weighing more than 80 kg, 80 mg for those weighing 80 kg or less) on day 1 only or oseltamivir at a dose of 75 mg twice daily or matching placebos on a 5-day regimen.
Patients aged 12-19 years were randomly assigned to receive either baloxavir or placebo on day 1 only, according to the researchers.
The median time to alleviation of symptoms was similar in the baloxavir (53.5 hours) and oseltamivir group (53.8 hours). However, patients taking baloxavir had significantly faster declines in infectious viral load compared with those taking oseltamivir, which was taken as a 75-mg dose twice daily for 5 days. In addition, patients who were treated with baloxavir within 24 hours of symptom onset showed significantly shorter time to alleviation of symptoms compared with placebo than did those who started treatment more than 24 hours after symptoms began.
Adverse events related to the study drug were more common among patients taking oseltamivir (8.4%) compared with those taking baloxavir (4.4%) or placebo (3.9%). In the phase 2 study, the adverse event rate was lower in the three baloxavir dosage groups compared with the placebo group. The study also showed a similar low frequency of complications requiring antibiotic treatment in both the baloxavir, oseltamivir, and placebo arms.
Some patients did show evidence of decreased susceptibility to baloxavir; for example, PA I38T/M amino acid substitutions were seen in 9.7% of the patients taking baloxavir but none of randomly selected patients in the placebo group of the phase 3 trial.
“These trials showed that single doses of the cap-dependent endonuclease inhibitor baloxavir were superior to placebo in alleviating influenza symptoms in patients with uncomplicated influenza, without clinically significant side effects,” wrote Dr. Frederick G. Hayden of the University of Virginia, Charlottesville, and his coauthors.
“The antiviral effects that were observed with baloxavir in patients with uncomplicated influenza provide encouragement with respect to its potential value in treating complicated or severe influenza infections,” they noted.
Because the treatment was inhibitory for influenza virus strains that were resistant to neuraminidase inhibitors or M2 ion-channel inhibitors, it could be a treatment option for patients infected with those viruses, the researchers added.
CAPSTONE-2, a randomized, controlled trial involving patients at high risk for influenza complications (NCT02949011) is in progress.
The study was supported by Shionogi, which developed baloxavir. Seven authors declared fees from the pharmaceutical industry, including Shionogi. Six authors were employees of Shionogi, one also holding stock. No other conflicts of interest were declared.
SOURCE: Hayden F et al. N Engl J Med. 2018;379:913-23. doi: 10.1056/NEJMoa1716197.
A new single-dose influenza antiviral drug appears significantly better than placebo at relieving the symptoms of infection, and reduces viral load faster than does oseltamivir, new research suggests.
Baloxavir marboxil – a selective inhibitor of influenza cap-dependent endonuclease – was tested in two randomized, double-blind, controlled trials. The first was a double-blind, placebo-controlled, dose-ranging, phase 2 randomized trial of 389 Japanese adults aged 20-64 years with acute uncomplicated influenza from December 2015 through March 2016. The second was a phase 3 randomized controlled trial of 1,366 patients comparing baloxavir with placebo and oseltamivir.
The phase 2 study showed patients treated with 10 mg, 20 mg or 40 mg oral dose of baloxavir experienced a significantly shorter median time to symptom alleviation compared with placebo (54.2, 51, 49.5, and 77.7 hours, respectively), according to a paper published in the Sept. 6 edition of the New England Journal of Medicine.
In addition, all three doses showed significantly greater reductions in influenza virus titers on days 2 and 3, compared with placebo.
The phase 3 trial CAPSTONE-1 (NCT02954354) was a double-blind, placebo- and oseltamivir-controlled, randomized trial that enrolled outpatients aged 12-64 years with influenza-like illness in the United States and Japan from December 2016 through March 2017. Patients aged 20-64 years received a single, weight-based oral dose of baloxavir (40 mg for patients weighing more than 80 kg, 80 mg for those weighing 80 kg or less) on day 1 only or oseltamivir at a dose of 75 mg twice daily or matching placebos on a 5-day regimen.
Patients aged 12-19 years were randomly assigned to receive either baloxavir or placebo on day 1 only, according to the researchers.
The median time to alleviation of symptoms was similar in the baloxavir (53.5 hours) and oseltamivir group (53.8 hours). However, patients taking baloxavir had significantly faster declines in infectious viral load compared with those taking oseltamivir, which was taken as a 75-mg dose twice daily for 5 days. In addition, patients who were treated with baloxavir within 24 hours of symptom onset showed significantly shorter time to alleviation of symptoms compared with placebo than did those who started treatment more than 24 hours after symptoms began.
Adverse events related to the study drug were more common among patients taking oseltamivir (8.4%) compared with those taking baloxavir (4.4%) or placebo (3.9%). In the phase 2 study, the adverse event rate was lower in the three baloxavir dosage groups compared with the placebo group. The study also showed a similar low frequency of complications requiring antibiotic treatment in both the baloxavir, oseltamivir, and placebo arms.
Some patients did show evidence of decreased susceptibility to baloxavir; for example, PA I38T/M amino acid substitutions were seen in 9.7% of the patients taking baloxavir but none of randomly selected patients in the placebo group of the phase 3 trial.
“These trials showed that single doses of the cap-dependent endonuclease inhibitor baloxavir were superior to placebo in alleviating influenza symptoms in patients with uncomplicated influenza, without clinically significant side effects,” wrote Dr. Frederick G. Hayden of the University of Virginia, Charlottesville, and his coauthors.
“The antiviral effects that were observed with baloxavir in patients with uncomplicated influenza provide encouragement with respect to its potential value in treating complicated or severe influenza infections,” they noted.
Because the treatment was inhibitory for influenza virus strains that were resistant to neuraminidase inhibitors or M2 ion-channel inhibitors, it could be a treatment option for patients infected with those viruses, the researchers added.
CAPSTONE-2, a randomized, controlled trial involving patients at high risk for influenza complications (NCT02949011) is in progress.
The study was supported by Shionogi, which developed baloxavir. Seven authors declared fees from the pharmaceutical industry, including Shionogi. Six authors were employees of Shionogi, one also holding stock. No other conflicts of interest were declared.
SOURCE: Hayden F et al. N Engl J Med. 2018;379:913-23. doi: 10.1056/NEJMoa1716197.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Single-dose influenza antiviral baloxavir shows efficacy similar to that of oseltamivir.
Major finding: Baloxavir shows similar time to alleviation of influenza symptoms compared with oseltamivir, but greater reductions in viral load.
Study details: Phase 2 and phase 3 randomized controlled trials in 389 and 1,366 otherwise healthy patients with influenza.
Disclosures: The study was supported by Shionogi, which developed baloxavir. Seven authors declared fees from the pharmaceutical industry, including Shionogi. Six authors were employees of Shionogi, one also holding stock. No other conflicts of interest were declared.
Source: Hayden F et al. N Engl J Med 2018;379:913-23. doi: 10.1056/NEJMoa1716197.
Service, please: Hospital setting matters for pneumonia
the National Center for Health Statistics (NCHS) reported.
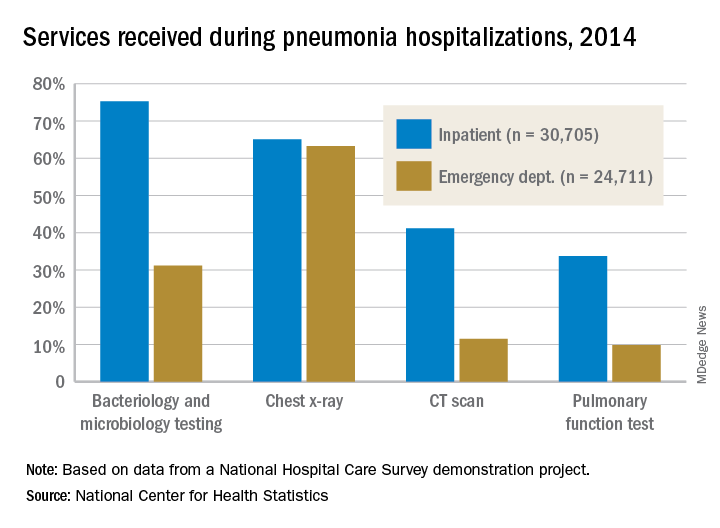
The percentages were not as close, however, for other diagnostic services. Inpatient stays were much more likely than ED encounters to involve bacteriology and microbiology testing (75.3% vs. 31.2%), CT scans (41.2% vs. 11.5%), and pulmonary function tests (33.7% vs. 9.8%), investigators from the NCHS said.
The age distribution of the two patient populations also were quite different, with those aged 65 years and older making up the largest share (46%) of pneumonia inpatients and the 15-and-under group representing the largest proportion (47%) of ED visits. For the inpatient setting, the smallest age group was those aged 15-44 years (10%), and for the ED it was those aged 65 years and older (14%), they reported.
The National Hospital Care Survey “is not yet nationally representative,” the NCHS investigators wrote – the overall sample for 2014 consisted of 581 hospitals – but “the number of encounters and the inclusion of [personally identifiable information] allow an example of analysis that was not previously possible.”
the National Center for Health Statistics (NCHS) reported.

The percentages were not as close, however, for other diagnostic services. Inpatient stays were much more likely than ED encounters to involve bacteriology and microbiology testing (75.3% vs. 31.2%), CT scans (41.2% vs. 11.5%), and pulmonary function tests (33.7% vs. 9.8%), investigators from the NCHS said.
The age distribution of the two patient populations also were quite different, with those aged 65 years and older making up the largest share (46%) of pneumonia inpatients and the 15-and-under group representing the largest proportion (47%) of ED visits. For the inpatient setting, the smallest age group was those aged 15-44 years (10%), and for the ED it was those aged 65 years and older (14%), they reported.
The National Hospital Care Survey “is not yet nationally representative,” the NCHS investigators wrote – the overall sample for 2014 consisted of 581 hospitals – but “the number of encounters and the inclusion of [personally identifiable information] allow an example of analysis that was not previously possible.”
the National Center for Health Statistics (NCHS) reported.

The percentages were not as close, however, for other diagnostic services. Inpatient stays were much more likely than ED encounters to involve bacteriology and microbiology testing (75.3% vs. 31.2%), CT scans (41.2% vs. 11.5%), and pulmonary function tests (33.7% vs. 9.8%), investigators from the NCHS said.
The age distribution of the two patient populations also were quite different, with those aged 65 years and older making up the largest share (46%) of pneumonia inpatients and the 15-and-under group representing the largest proportion (47%) of ED visits. For the inpatient setting, the smallest age group was those aged 15-44 years (10%), and for the ED it was those aged 65 years and older (14%), they reported.
The National Hospital Care Survey “is not yet nationally representative,” the NCHS investigators wrote – the overall sample for 2014 consisted of 581 hospitals – but “the number of encounters and the inclusion of [personally identifiable information] allow an example of analysis that was not previously possible.”
Hydrocortisone plus fludrocortisone for adults with septic shock
Background: Septic shock is a serious and common health problem, associated with a more than 50% mortality rate. It is characterized by a dysregulated patient response to infection, resulting in life-threatening circulatory, cellular, and metabolic abnormalities. The benefit of corticosteroid use in septic shock is still controversial.
Study design: Double-blinded, randomized, placebo-controlled trial.
Setting: The study was conducted in 34 centers in France.
Synopsis: All 1,241 septic shock patients received usual care. Of these patients, 614 patients received hydrocortisone and fludrocortisone (HF), while 627 patients received placebo. HF patients had a lower rate of 90-day mortality (43.0% vs. 49.1%; P = .03), mortality at ICU discharge (35.4% vs. 41.0%; P = .04), mortality at hospital discharge (39.0% vs. 45.3%; P = .02), and mortality at day 180 (46.6% vs. 52.5%; P = .04). There was no significant difference between mortality at day 28 (33.7% in the HF group versus 38.9% in the placebo group; P = .06), ventilator-free days (11 vs. 10 days; P = .07), and rate of serious adverse events (53.1% vs. 58.0%; P = .08). The number of vasopressor-free days to day 28 was significantly higher in the HF group (17 vs. 15 days; P less than .001), as were the number of organ failure–free days (14 vs. 12 days; P = .003). There was more hyperglycemia in the HF group (89.1% vs. 83.1%; P = .002).
Bottom line: Patients with septic shock who received hydrocortisone plus fludrocortisone have a lower rate of 90-day all-cause mortality, compared with placebo.
Citation: Annane D et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018 Mar 1. 378(9):809-18.
Dr. Salih is a hospitalist and instructor in the division of hospital medicine at the University of Kentucky, Lexington.
Background: Septic shock is a serious and common health problem, associated with a more than 50% mortality rate. It is characterized by a dysregulated patient response to infection, resulting in life-threatening circulatory, cellular, and metabolic abnormalities. The benefit of corticosteroid use in septic shock is still controversial.
Study design: Double-blinded, randomized, placebo-controlled trial.
Setting: The study was conducted in 34 centers in France.
Synopsis: All 1,241 septic shock patients received usual care. Of these patients, 614 patients received hydrocortisone and fludrocortisone (HF), while 627 patients received placebo. HF patients had a lower rate of 90-day mortality (43.0% vs. 49.1%; P = .03), mortality at ICU discharge (35.4% vs. 41.0%; P = .04), mortality at hospital discharge (39.0% vs. 45.3%; P = .02), and mortality at day 180 (46.6% vs. 52.5%; P = .04). There was no significant difference between mortality at day 28 (33.7% in the HF group versus 38.9% in the placebo group; P = .06), ventilator-free days (11 vs. 10 days; P = .07), and rate of serious adverse events (53.1% vs. 58.0%; P = .08). The number of vasopressor-free days to day 28 was significantly higher in the HF group (17 vs. 15 days; P less than .001), as were the number of organ failure–free days (14 vs. 12 days; P = .003). There was more hyperglycemia in the HF group (89.1% vs. 83.1%; P = .002).
Bottom line: Patients with septic shock who received hydrocortisone plus fludrocortisone have a lower rate of 90-day all-cause mortality, compared with placebo.
Citation: Annane D et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018 Mar 1. 378(9):809-18.
Dr. Salih is a hospitalist and instructor in the division of hospital medicine at the University of Kentucky, Lexington.
Background: Septic shock is a serious and common health problem, associated with a more than 50% mortality rate. It is characterized by a dysregulated patient response to infection, resulting in life-threatening circulatory, cellular, and metabolic abnormalities. The benefit of corticosteroid use in septic shock is still controversial.
Study design: Double-blinded, randomized, placebo-controlled trial.
Setting: The study was conducted in 34 centers in France.
Synopsis: All 1,241 septic shock patients received usual care. Of these patients, 614 patients received hydrocortisone and fludrocortisone (HF), while 627 patients received placebo. HF patients had a lower rate of 90-day mortality (43.0% vs. 49.1%; P = .03), mortality at ICU discharge (35.4% vs. 41.0%; P = .04), mortality at hospital discharge (39.0% vs. 45.3%; P = .02), and mortality at day 180 (46.6% vs. 52.5%; P = .04). There was no significant difference between mortality at day 28 (33.7% in the HF group versus 38.9% in the placebo group; P = .06), ventilator-free days (11 vs. 10 days; P = .07), and rate of serious adverse events (53.1% vs. 58.0%; P = .08). The number of vasopressor-free days to day 28 was significantly higher in the HF group (17 vs. 15 days; P less than .001), as were the number of organ failure–free days (14 vs. 12 days; P = .003). There was more hyperglycemia in the HF group (89.1% vs. 83.1%; P = .002).
Bottom line: Patients with septic shock who received hydrocortisone plus fludrocortisone have a lower rate of 90-day all-cause mortality, compared with placebo.
Citation: Annane D et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018 Mar 1. 378(9):809-18.
Dr. Salih is a hospitalist and instructor in the division of hospital medicine at the University of Kentucky, Lexington.
United Kingdom experience provides important lessons for controlling C. auris outbreaks
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at [email protected].”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at [email protected].”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at [email protected].”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
REPORTING FROM ICEID 2018
Key clinical point:
Major finding: Ten additional cases occurred in the 7 months after the axillary probes were removed from the environment.
Study details: A review of the epidemiology and control of a C. auris outbreak affecting 76 patients.
Disclosures: Dr. Eyre reported having no disclosures.
Source: Eyre D et al. ICEID 2018 Oral Abstract Presentation.














